User login
Conjunctivitis and cervicitis
A 42 year-old African American female came to the emergency room complaining of fever, chills, and generalized pain in her joints, abdomen, and pelvis. Her symptoms began gradually over the previous 48 to 72 hours. She reported she had not been sexually active in the previous 3 weeks, and had no abnormal vaginal discharge, pharyngitis, or eye pain. She had only taken occasional acetamino-phen and denied illicit drug use.
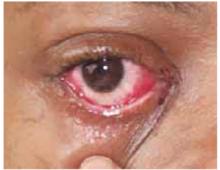
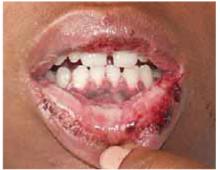
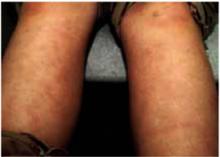
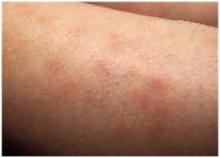
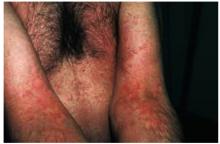
The physical exam revealed a temperature of 102.6°F orally but otherwise normal vital signs. Bilateral nonpurulent conjunctival infection (Figure 1) and oral mucosal inflammation (Figure 2) were plainly evident. Neck, chest, and cardiovascular examinations yielded no abnormal findings; however, her abdomen was diffusely ten-der without distension or organomegaly.
Pelvic exam revealed normal mucosa with thin white cervical discharge and cervical motion tenderness. Exam of the extremities showed no joint effusions or induration, but arthralgia was noted in both of her elbows, knees, and wrists. She had no rashes.
FIGURE 1
Conjunctivitis
FIGURE 2
Oral inflammation
What is the differential diagnosis?
What additional tests should be performed?
What is the initial treatment?
Differential diagnosis
This patient’s symptoms included pelvic inflammatory disease, conjunctivitis, mucositis, and arthralgias. Diagnoses that would tie these signs and symptoms together include disseminated gonococcemia, chlamydial infections, Reiter’s syndrome, and Stevens Johnson syndrome.
Lab tests: White blood cell count, chlamydia and other STDs
Her complete blood count revealed leukocytosis with a white blood cell count of 17,000 and a left shift of 6% bands. Endocervical DNA probe was positive for Chlamydia trachomatis. Blood cultures were negative.
Because many sexually transmitted diseases occur concurrently or are transmitted together, the patient was also tested for HIV, syphilis, and hepatitis B and C. The patient gave her consent for the HIV test, which came back negative. Serologies for syphilis and hepatitis B and C were drawn and found to be negative. A test for the human leukocyte antigen HLA-B27 was ordered because of the concern that this was Reiter’s syndrome; the test result was negative.
Diagnosis: Reiter’s syndrome
This patient was diagnosed with Reiter’s syndrome, based on her clinical syndromenjunctivitis, arthralgias, mucositis, and cervicitis. The widespread distribution of the symptoms in this syndrome may be due to activation of the immune system by a viral or bacterial agent.6,7
The fact that her HLA-B27 test result was negative did not change this diagnosis; only 85% of patients with the syndrome are positive for HLA-B27, and the test results are frequently negative in African Americans.4,5
Initial treatment: antibiotics and anti-inflammatory agents
The patient was hospitalized and started on intravenous ampicillin/sulbactam and oral azithromycin to cover for disseminated gonococ-cemia and chlamydia. Nonsteroidal anti-inflam-matory medications were given for pain control. The patient rapidly responded to therapy as evidenced by decreased arthralgias, normalization of temperatures and white blood cell count, and decreased abdominal pain. The patient was discharged after the third day in the hospital with instructions to take doxycycline twice daily (finishing a 14-day course).
An estimated 3 to 4 million cases of C trachomatis occur each year in the US, making it one of the most prevalent sexually transmitted diseases.1 This disease may be underreported, largely as a result of substantial numbers of asymptomatic persons whose infections are not identified because screening is not available.1 In addition, many females with mucopurulent cervicitis are asymptomatic. In some series, C trachomatis has been identified as the causative agent in up to 50% of women with pelvic inflammatory disease.
Reiter’s syndrome is a multisystem disease commonly triggered by a genitourinary or bacterial enteric infection.2 The incidence of Reiter’s syndrome is estimated to be 3.5 per 100,000 in the US population.3 Up to 85% of patients with Reiter’s syndrome possess the HLA-B27 antigen. 4 However, the true incidence of Reiter’s syndrome is debated because of the protean nature of its symptoms, as well as a lack of consensus in defining the syndrome.
The numbers of cases of Reiter’s syndrome caused from C trachomatis genitourinary infections are equally difficult to document. In some reports, silent cystitis and cervicitis without urethritis may be the only evidence of urogenital involvement.5
A positive DNA probe test for C trachomatis was helpful to guide therapy. As the availability of immunohistochemistry and polymerase chain reaction testing becomes more widely available, the number of cases of Reiter’s syndrome without a documented infectious cause will likely diminish.8
- Ampicillin/sulbactam • Unasyn
- Azithromycin • Zithromax
- Doxycycline • Doryx, Monodox, Periostat, Vibramycin
Corresponding author
Joseph M. Mazziotta, MD, Associate Director, Tallahassee Memorial Family Practice Residency Program, 1301 Hodges Drive, Tallahassee, FL 32308. E-mail: [email protected]
1. Centers for Disease Control and Prevention STD Prevention. Chlamydia in the United States. April 2001. Available at www.cdc.gov/nchstp/dstd/Fact_Sheets/chlamydia_facts.htm. Accessed on January 6, 2004.
2. Schneider JM, Matthews JH, Graham BS. Reiter’s syndrome. Cutis 2003;71:198-200.
3. Scoggins T. Reiter’s syndrome. Available at: Emedicine.com. Accessed on January 4, 2004.
4. Fauci AS, Braunwald E, Isselbacher KJ, et al, eds. Harrison’s Principles of Internal Medicine, Companion Handbook. 14th ed.New York, NY: McGraw-Hill; 1998;905-906.
5. Schumacher R, Klippel JH, Koopman WJ, et al, eds. Primer on the Rheumatic Diseases. 10th ed. Atlanta, Ga: Arthritis Foundation; 1993;160-161.
6. Parker CT, Thomas D. Reiter’s syndrome and reactive arthritis. J Am Osteopath Assoc 2000;100:101-104.
7. Prostatitis Foundation website. Reiter’s syndrome briefing. 2003. Available at:http://www.prostatitis.org/reit-ers.html. Accessed on January 6, 2004.
8. Rahman MU, Cheema MA, Schumacher HR, Hudson AP. Molecular evidence for the presence of chlamydia in the synovium of patients with Reiter’s syndrome. Arthritis Rheum 1992;35:521-529.
A 42 year-old African American female came to the emergency room complaining of fever, chills, and generalized pain in her joints, abdomen, and pelvis. Her symptoms began gradually over the previous 48 to 72 hours. She reported she had not been sexually active in the previous 3 weeks, and had no abnormal vaginal discharge, pharyngitis, or eye pain. She had only taken occasional acetamino-phen and denied illicit drug use.
The physical exam revealed a temperature of 102.6°F orally but otherwise normal vital signs. Bilateral nonpurulent conjunctival infection (Figure 1) and oral mucosal inflammation (Figure 2) were plainly evident. Neck, chest, and cardiovascular examinations yielded no abnormal findings; however, her abdomen was diffusely ten-der without distension or organomegaly.
Pelvic exam revealed normal mucosa with thin white cervical discharge and cervical motion tenderness. Exam of the extremities showed no joint effusions or induration, but arthralgia was noted in both of her elbows, knees, and wrists. She had no rashes.
FIGURE 1
Conjunctivitis
FIGURE 2
Oral inflammation
What is the differential diagnosis?
What additional tests should be performed?
What is the initial treatment?
Differential diagnosis
This patient’s symptoms included pelvic inflammatory disease, conjunctivitis, mucositis, and arthralgias. Diagnoses that would tie these signs and symptoms together include disseminated gonococcemia, chlamydial infections, Reiter’s syndrome, and Stevens Johnson syndrome.
Lab tests: White blood cell count, chlamydia and other STDs
Her complete blood count revealed leukocytosis with a white blood cell count of 17,000 and a left shift of 6% bands. Endocervical DNA probe was positive for Chlamydia trachomatis. Blood cultures were negative.
Because many sexually transmitted diseases occur concurrently or are transmitted together, the patient was also tested for HIV, syphilis, and hepatitis B and C. The patient gave her consent for the HIV test, which came back negative. Serologies for syphilis and hepatitis B and C were drawn and found to be negative. A test for the human leukocyte antigen HLA-B27 was ordered because of the concern that this was Reiter’s syndrome; the test result was negative.
Diagnosis: Reiter’s syndrome
This patient was diagnosed with Reiter’s syndrome, based on her clinical syndromenjunctivitis, arthralgias, mucositis, and cervicitis. The widespread distribution of the symptoms in this syndrome may be due to activation of the immune system by a viral or bacterial agent.6,7
The fact that her HLA-B27 test result was negative did not change this diagnosis; only 85% of patients with the syndrome are positive for HLA-B27, and the test results are frequently negative in African Americans.4,5
Initial treatment: antibiotics and anti-inflammatory agents
The patient was hospitalized and started on intravenous ampicillin/sulbactam and oral azithromycin to cover for disseminated gonococ-cemia and chlamydia. Nonsteroidal anti-inflam-matory medications were given for pain control. The patient rapidly responded to therapy as evidenced by decreased arthralgias, normalization of temperatures and white blood cell count, and decreased abdominal pain. The patient was discharged after the third day in the hospital with instructions to take doxycycline twice daily (finishing a 14-day course).
An estimated 3 to 4 million cases of C trachomatis occur each year in the US, making it one of the most prevalent sexually transmitted diseases.1 This disease may be underreported, largely as a result of substantial numbers of asymptomatic persons whose infections are not identified because screening is not available.1 In addition, many females with mucopurulent cervicitis are asymptomatic. In some series, C trachomatis has been identified as the causative agent in up to 50% of women with pelvic inflammatory disease.
Reiter’s syndrome is a multisystem disease commonly triggered by a genitourinary or bacterial enteric infection.2 The incidence of Reiter’s syndrome is estimated to be 3.5 per 100,000 in the US population.3 Up to 85% of patients with Reiter’s syndrome possess the HLA-B27 antigen. 4 However, the true incidence of Reiter’s syndrome is debated because of the protean nature of its symptoms, as well as a lack of consensus in defining the syndrome.
The numbers of cases of Reiter’s syndrome caused from C trachomatis genitourinary infections are equally difficult to document. In some reports, silent cystitis and cervicitis without urethritis may be the only evidence of urogenital involvement.5
A positive DNA probe test for C trachomatis was helpful to guide therapy. As the availability of immunohistochemistry and polymerase chain reaction testing becomes more widely available, the number of cases of Reiter’s syndrome without a documented infectious cause will likely diminish.8
- Ampicillin/sulbactam • Unasyn
- Azithromycin • Zithromax
- Doxycycline • Doryx, Monodox, Periostat, Vibramycin
Corresponding author
Joseph M. Mazziotta, MD, Associate Director, Tallahassee Memorial Family Practice Residency Program, 1301 Hodges Drive, Tallahassee, FL 32308. E-mail: [email protected]
A 42 year-old African American female came to the emergency room complaining of fever, chills, and generalized pain in her joints, abdomen, and pelvis. Her symptoms began gradually over the previous 48 to 72 hours. She reported she had not been sexually active in the previous 3 weeks, and had no abnormal vaginal discharge, pharyngitis, or eye pain. She had only taken occasional acetamino-phen and denied illicit drug use.
The physical exam revealed a temperature of 102.6°F orally but otherwise normal vital signs. Bilateral nonpurulent conjunctival infection (Figure 1) and oral mucosal inflammation (Figure 2) were plainly evident. Neck, chest, and cardiovascular examinations yielded no abnormal findings; however, her abdomen was diffusely ten-der without distension or organomegaly.
Pelvic exam revealed normal mucosa with thin white cervical discharge and cervical motion tenderness. Exam of the extremities showed no joint effusions or induration, but arthralgia was noted in both of her elbows, knees, and wrists. She had no rashes.
FIGURE 1
Conjunctivitis
FIGURE 2
Oral inflammation
What is the differential diagnosis?
What additional tests should be performed?
What is the initial treatment?
Differential diagnosis
This patient’s symptoms included pelvic inflammatory disease, conjunctivitis, mucositis, and arthralgias. Diagnoses that would tie these signs and symptoms together include disseminated gonococcemia, chlamydial infections, Reiter’s syndrome, and Stevens Johnson syndrome.
Lab tests: White blood cell count, chlamydia and other STDs
Her complete blood count revealed leukocytosis with a white blood cell count of 17,000 and a left shift of 6% bands. Endocervical DNA probe was positive for Chlamydia trachomatis. Blood cultures were negative.
Because many sexually transmitted diseases occur concurrently or are transmitted together, the patient was also tested for HIV, syphilis, and hepatitis B and C. The patient gave her consent for the HIV test, which came back negative. Serologies for syphilis and hepatitis B and C were drawn and found to be negative. A test for the human leukocyte antigen HLA-B27 was ordered because of the concern that this was Reiter’s syndrome; the test result was negative.
Diagnosis: Reiter’s syndrome
This patient was diagnosed with Reiter’s syndrome, based on her clinical syndromenjunctivitis, arthralgias, mucositis, and cervicitis. The widespread distribution of the symptoms in this syndrome may be due to activation of the immune system by a viral or bacterial agent.6,7
The fact that her HLA-B27 test result was negative did not change this diagnosis; only 85% of patients with the syndrome are positive for HLA-B27, and the test results are frequently negative in African Americans.4,5
Initial treatment: antibiotics and anti-inflammatory agents
The patient was hospitalized and started on intravenous ampicillin/sulbactam and oral azithromycin to cover for disseminated gonococ-cemia and chlamydia. Nonsteroidal anti-inflam-matory medications were given for pain control. The patient rapidly responded to therapy as evidenced by decreased arthralgias, normalization of temperatures and white blood cell count, and decreased abdominal pain. The patient was discharged after the third day in the hospital with instructions to take doxycycline twice daily (finishing a 14-day course).
An estimated 3 to 4 million cases of C trachomatis occur each year in the US, making it one of the most prevalent sexually transmitted diseases.1 This disease may be underreported, largely as a result of substantial numbers of asymptomatic persons whose infections are not identified because screening is not available.1 In addition, many females with mucopurulent cervicitis are asymptomatic. In some series, C trachomatis has been identified as the causative agent in up to 50% of women with pelvic inflammatory disease.
Reiter’s syndrome is a multisystem disease commonly triggered by a genitourinary or bacterial enteric infection.2 The incidence of Reiter’s syndrome is estimated to be 3.5 per 100,000 in the US population.3 Up to 85% of patients with Reiter’s syndrome possess the HLA-B27 antigen. 4 However, the true incidence of Reiter’s syndrome is debated because of the protean nature of its symptoms, as well as a lack of consensus in defining the syndrome.
The numbers of cases of Reiter’s syndrome caused from C trachomatis genitourinary infections are equally difficult to document. In some reports, silent cystitis and cervicitis without urethritis may be the only evidence of urogenital involvement.5
A positive DNA probe test for C trachomatis was helpful to guide therapy. As the availability of immunohistochemistry and polymerase chain reaction testing becomes more widely available, the number of cases of Reiter’s syndrome without a documented infectious cause will likely diminish.8
- Ampicillin/sulbactam • Unasyn
- Azithromycin • Zithromax
- Doxycycline • Doryx, Monodox, Periostat, Vibramycin
Corresponding author
Joseph M. Mazziotta, MD, Associate Director, Tallahassee Memorial Family Practice Residency Program, 1301 Hodges Drive, Tallahassee, FL 32308. E-mail: [email protected]
1. Centers for Disease Control and Prevention STD Prevention. Chlamydia in the United States. April 2001. Available at www.cdc.gov/nchstp/dstd/Fact_Sheets/chlamydia_facts.htm. Accessed on January 6, 2004.
2. Schneider JM, Matthews JH, Graham BS. Reiter’s syndrome. Cutis 2003;71:198-200.
3. Scoggins T. Reiter’s syndrome. Available at: Emedicine.com. Accessed on January 4, 2004.
4. Fauci AS, Braunwald E, Isselbacher KJ, et al, eds. Harrison’s Principles of Internal Medicine, Companion Handbook. 14th ed.New York, NY: McGraw-Hill; 1998;905-906.
5. Schumacher R, Klippel JH, Koopman WJ, et al, eds. Primer on the Rheumatic Diseases. 10th ed. Atlanta, Ga: Arthritis Foundation; 1993;160-161.
6. Parker CT, Thomas D. Reiter’s syndrome and reactive arthritis. J Am Osteopath Assoc 2000;100:101-104.
7. Prostatitis Foundation website. Reiter’s syndrome briefing. 2003. Available at:http://www.prostatitis.org/reit-ers.html. Accessed on January 6, 2004.
8. Rahman MU, Cheema MA, Schumacher HR, Hudson AP. Molecular evidence for the presence of chlamydia in the synovium of patients with Reiter’s syndrome. Arthritis Rheum 1992;35:521-529.
1. Centers for Disease Control and Prevention STD Prevention. Chlamydia in the United States. April 2001. Available at www.cdc.gov/nchstp/dstd/Fact_Sheets/chlamydia_facts.htm. Accessed on January 6, 2004.
2. Schneider JM, Matthews JH, Graham BS. Reiter’s syndrome. Cutis 2003;71:198-200.
3. Scoggins T. Reiter’s syndrome. Available at: Emedicine.com. Accessed on January 4, 2004.
4. Fauci AS, Braunwald E, Isselbacher KJ, et al, eds. Harrison’s Principles of Internal Medicine, Companion Handbook. 14th ed.New York, NY: McGraw-Hill; 1998;905-906.
5. Schumacher R, Klippel JH, Koopman WJ, et al, eds. Primer on the Rheumatic Diseases. 10th ed. Atlanta, Ga: Arthritis Foundation; 1993;160-161.
6. Parker CT, Thomas D. Reiter’s syndrome and reactive arthritis. J Am Osteopath Assoc 2000;100:101-104.
7. Prostatitis Foundation website. Reiter’s syndrome briefing. 2003. Available at:http://www.prostatitis.org/reit-ers.html. Accessed on January 6, 2004.
8. Rahman MU, Cheema MA, Schumacher HR, Hudson AP. Molecular evidence for the presence of chlamydia in the synovium of patients with Reiter’s syndrome. Arthritis Rheum 1992;35:521-529.
Bald spots on a young girl
An 8-year-old Hispanic girl was brought to see her family physician by her mother, who noticed 2 bald spots on the back of her daughter’s scalp while brushing her hair. The child had no itching or pain. No obvious precipitating events preceded the hair loss.
The mother was more worried than the child: she didn’t want to see her beautiful girl become bald. The girl was pleased that the bald spots could be completely covered with her long hair, but she didn’t want anyone to see them. The child was otherwise healthy. She did not have any chronic medical problems and was not taking any medications. No one else in the family had a similar problem.
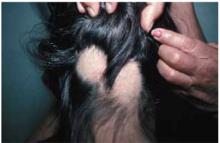
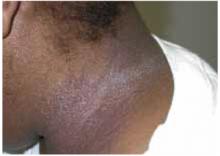
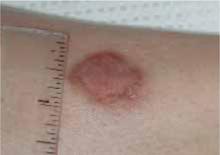
When the mother lifted the hair in the back, 2 round areas of hair loss were evident (Figure 1). On close inspection, there was no scaling or scarring. Her nails were normal. The child was afebrile, and the remainder of her exam was unremarkable.
FIGURE 1
Two round areas of hair loss
What is the diagnosis?
What are the management options?
Diagnosis: alopecia areata
This is the typical appearance of alopecia areata, a chronic inflammatory disease that affects the hair follicles, causing sudden hair loss. Sometimes it affects the nails as well.1 Alopecia areata occurs in both males and females of all ages and races.
Alopecia areata may be an autoimmune disease, though this is unproven. The affected skin may be slightly erythematous but otherwise appears normal. Short broken hairs (exclamation-mark hairs) may be seen around the margins of expanding patches of baldness. The nails are involved in about 10% of patients with severe enough alopecia to be referred to a specialist.1
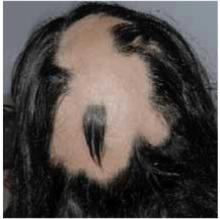
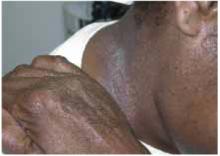
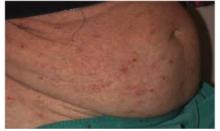
Many cases begin in childhood and can be psychologically devastating. Alopecia areata is part of a spectrum of diseases with mild to extensive hair loss (Figure 2), including alopecia totalis, in which all the hair on the scalp is lost, and alopecia universalis (Figure 3), in which all the hair on the body is lost. Extensive involvement, early age of onset, and Down syndrome are all poor prognostic factors for alopecia areata.
FIGURE 2
More extensive alopecia
FIGURE 3
Alopecia universalis
Differential diagnoses
The differential diagnosis for alopecia areata includes tinea capitis, trichotillomania, early scarring alopecia, telogen effluvium, anagen effluvium (drug-induced), systemic and discoid lupus erythematosus, and secondary syphilis. In most cases the history and physical exam are adequate to make the diagnosis.
This patient does not have the typical scalp scaling and inflammation seen with tinea capitis. Trichotillomania—hair loss caused by the purposeful pulling of hair by the patient—is likely to cause the most confusion because it coexists with alopecia areata in some cases. This child has shown no evidence of such behavior. She also has no evidence of scalp scarring as may be seen in lupus. Telogen effluvium and anagen effluvium cause a more even distribution of hair loss. The patient has no known risk factors for secondary syphilis.
Laboratory testing
No lab tests are needed in this case. If there were some scalp scaling or inflammation, a potassium hydroxide preparation of the involved area would be useful to look for fungal elements; a fungal culture might also be warranted. If needed, further investigations might include serological testing for lupus erythematosus and syphilis, and a skin biopsy if the diagnosis is still unknown.
Treatment: time, drug therapies, aromatherapy
Spontaneous remission occurs in up to 80% of patients with limited patchy hair loss of less than 1 year.1 Spontaneous remission rates are significantly lower with more extensive hair loss.
Treatments are potentially painful, expensive, or time-consuming, and few randomized controlled trials support their use. Often the best treatment is watching for spontaneous remission.
The only adverse health effect of alopecia areata is the psychological distress that it may cause. While this is not to be taken lightly, the lack of evidence for successful treatments needs to be weighed with the patient’s ability to cope with leaving the hair loss untreated over time. In cases of extensive hair loss, the best treatment may be a wig.
For patients that have more visible and extensive areas of hair loss, the psychological impact might prompt the patient to want any treatment available despite the lack of evidence. Alopecia totalis or universalis may cause considerable psychological and social disability. Patients can be referred to the National Alopecia Areata Foundation for support groups and additional information. Individual counseling may be needed for some patients.
National Alopecia Areata Foundation Web site: www.alopeciaareata.com. Patients can order a 7-minute video, This Weird Thing That Makes My Hair Fall Out: Alopecia Areata, which is available for any children who want a way to share their feelings about alopecia areata with friends, family, peers, schoolmates, principals, and teachers.
European Hair Research Society Web site: www.ehrs.org. Web site has links to several alopecia areata sites.
Steroid injections
Intralesional steroid injections may stimulate regrowth of hair at the site of injection (level of evidence [LOE]=5).1 The effect may last a few months, but there is no evidence that it improves the long-term outcome or increases the probability of a cure. New areas of alopecia can still develop.
Injections are typically performed with 5–10 mg/mL of triamcinolone acetonide using a small-gauge needle. Most children will not be able to tolerate the scalp injections and should not be forced to endure this type of therapy even if the parent is pushing for it.
Other medications
Topical diphenylcyclopropenone (DPCP) is a contact immunotherapy that has some proven benefit with extensive alopecia areata (LOE=2b).1,2 In 1 study, 56 patients with chronic, extensive alopecia areata (duration ranging from 1 to 10 years, involving 30% to 100% of the scalp) were treated with progressively higher concentrations of DPCP in a randomized crossover trial.2 Twenty-five of 56 patients had total hair regrowth at 6 months, and no relapse occurred in 60% of patients. Side effects included local inflammation, eczema, autosensitization reaction, and eyelid edema.
Unfortunately, contact immunotherapy involves multiple visits to the office over several months, and it stimulates cosmetically worthwhile hair regrowth in <50% of patients with extensive patchy hair loss.1 It is a reasonable alternative for patients who do not have spontaneous remission after 1 year.
While potent topical steroids and topical minoxidil are prescribed for limited patchy alopecia areata, no convincing evidence shows they are effective.1 Likewise, no evidence warrants the use systemic steroids or psoralen/ultraviolet light treatment (PUVA).1
Aromatherapy
The best evidence may be for aromatherapy. A single-blinded randomized controlled trial was performed with 86 patients.3 As Dr Ebell points out in his InfoPOEMs review of this study, aromatherapy involves rubbing scented essential oils into the skin to treat localized and systemic disease.4 Patients with alopecia areata were randomized to nightly aromatherapy—with Thymus vulgaris (thyme), Lavandula angustifolia (lavender), Rosmarinus officinalis (rosemary), and Cedrus atlantica (cedar)—or to a control consisting of carrier oils only.
Improvement was seen in 54% of the treatment group and 21% of the control group (P=.008; number needed to treat=3). Of the 19 patients in the active treatment group who reported improvement, 11 had “very good” or “excellent” improvement. The results show aromatherapy to be a safe and effective treatment for alopecia areata (LOE=1b).4
The main problem with this study is that the researchers did not describe the duration of the patients’ alopecia. However, in a reply to a letter, they described the patients as having had alopecia areata from less than 1 year to more than 9 years. This explains the low improvement rates in both groups but does not invalidate the statistically significant difference for those that received the essential oils.
Conclusion of visit, follow-up
For a mild case like this in which the hair loss can be hidden, the best treatment is reassurance and observation. The physician explained the natural history of the disease, including the fact that regrowth will take at least 3 months for any single patch. Therapeutic options were also discussed. The mother and child were reassured that the hair is likely to grow in on its own. Neither of them wanted intralesional injections or topical therapies.
One year later during a well-child check, it was noted that the girl’s hair had fully regrown.
1. MacDonald Hull SP, Wood ML, Hutchinson PE, Sladden M, Messenger AG. Guidelines for the management of alopecia areata. Br J Dermatol 2003;149:692-699.
2. Cotellessa C, Peris K, Caracciolo E, Mordenti C, Chimenti S. The use of topical diphenylcyclopropenone for the treatment of extensive alopecia areata. J Am Acad Dermatol 2001;44:73-76.
3. Hay IC, Jamieson M, Ormerod AD. Randomized trial of aromatherapy: successful treatment for alopecia areata. Arch Derm 1998;134:1349-1352.
4. Ebell M. Aromatherapy effective for alopecia areata. Review of Hay et al 1998. InfoPOEMs, Inc. March 1999.
An 8-year-old Hispanic girl was brought to see her family physician by her mother, who noticed 2 bald spots on the back of her daughter’s scalp while brushing her hair. The child had no itching or pain. No obvious precipitating events preceded the hair loss.
The mother was more worried than the child: she didn’t want to see her beautiful girl become bald. The girl was pleased that the bald spots could be completely covered with her long hair, but she didn’t want anyone to see them. The child was otherwise healthy. She did not have any chronic medical problems and was not taking any medications. No one else in the family had a similar problem.
When the mother lifted the hair in the back, 2 round areas of hair loss were evident (Figure 1). On close inspection, there was no scaling or scarring. Her nails were normal. The child was afebrile, and the remainder of her exam was unremarkable.
FIGURE 1
Two round areas of hair loss
What is the diagnosis?
What are the management options?
Diagnosis: alopecia areata
This is the typical appearance of alopecia areata, a chronic inflammatory disease that affects the hair follicles, causing sudden hair loss. Sometimes it affects the nails as well.1 Alopecia areata occurs in both males and females of all ages and races.
Alopecia areata may be an autoimmune disease, though this is unproven. The affected skin may be slightly erythematous but otherwise appears normal. Short broken hairs (exclamation-mark hairs) may be seen around the margins of expanding patches of baldness. The nails are involved in about 10% of patients with severe enough alopecia to be referred to a specialist.1
Many cases begin in childhood and can be psychologically devastating. Alopecia areata is part of a spectrum of diseases with mild to extensive hair loss (Figure 2), including alopecia totalis, in which all the hair on the scalp is lost, and alopecia universalis (Figure 3), in which all the hair on the body is lost. Extensive involvement, early age of onset, and Down syndrome are all poor prognostic factors for alopecia areata.
FIGURE 2
More extensive alopecia
FIGURE 3
Alopecia universalis
Differential diagnoses
The differential diagnosis for alopecia areata includes tinea capitis, trichotillomania, early scarring alopecia, telogen effluvium, anagen effluvium (drug-induced), systemic and discoid lupus erythematosus, and secondary syphilis. In most cases the history and physical exam are adequate to make the diagnosis.
This patient does not have the typical scalp scaling and inflammation seen with tinea capitis. Trichotillomania—hair loss caused by the purposeful pulling of hair by the patient—is likely to cause the most confusion because it coexists with alopecia areata in some cases. This child has shown no evidence of such behavior. She also has no evidence of scalp scarring as may be seen in lupus. Telogen effluvium and anagen effluvium cause a more even distribution of hair loss. The patient has no known risk factors for secondary syphilis.
Laboratory testing
No lab tests are needed in this case. If there were some scalp scaling or inflammation, a potassium hydroxide preparation of the involved area would be useful to look for fungal elements; a fungal culture might also be warranted. If needed, further investigations might include serological testing for lupus erythematosus and syphilis, and a skin biopsy if the diagnosis is still unknown.
Treatment: time, drug therapies, aromatherapy
Spontaneous remission occurs in up to 80% of patients with limited patchy hair loss of less than 1 year.1 Spontaneous remission rates are significantly lower with more extensive hair loss.
Treatments are potentially painful, expensive, or time-consuming, and few randomized controlled trials support their use. Often the best treatment is watching for spontaneous remission.
The only adverse health effect of alopecia areata is the psychological distress that it may cause. While this is not to be taken lightly, the lack of evidence for successful treatments needs to be weighed with the patient’s ability to cope with leaving the hair loss untreated over time. In cases of extensive hair loss, the best treatment may be a wig.
For patients that have more visible and extensive areas of hair loss, the psychological impact might prompt the patient to want any treatment available despite the lack of evidence. Alopecia totalis or universalis may cause considerable psychological and social disability. Patients can be referred to the National Alopecia Areata Foundation for support groups and additional information. Individual counseling may be needed for some patients.
National Alopecia Areata Foundation Web site: www.alopeciaareata.com. Patients can order a 7-minute video, This Weird Thing That Makes My Hair Fall Out: Alopecia Areata, which is available for any children who want a way to share their feelings about alopecia areata with friends, family, peers, schoolmates, principals, and teachers.
European Hair Research Society Web site: www.ehrs.org. Web site has links to several alopecia areata sites.
Steroid injections
Intralesional steroid injections may stimulate regrowth of hair at the site of injection (level of evidence [LOE]=5).1 The effect may last a few months, but there is no evidence that it improves the long-term outcome or increases the probability of a cure. New areas of alopecia can still develop.
Injections are typically performed with 5–10 mg/mL of triamcinolone acetonide using a small-gauge needle. Most children will not be able to tolerate the scalp injections and should not be forced to endure this type of therapy even if the parent is pushing for it.
Other medications
Topical diphenylcyclopropenone (DPCP) is a contact immunotherapy that has some proven benefit with extensive alopecia areata (LOE=2b).1,2 In 1 study, 56 patients with chronic, extensive alopecia areata (duration ranging from 1 to 10 years, involving 30% to 100% of the scalp) were treated with progressively higher concentrations of DPCP in a randomized crossover trial.2 Twenty-five of 56 patients had total hair regrowth at 6 months, and no relapse occurred in 60% of patients. Side effects included local inflammation, eczema, autosensitization reaction, and eyelid edema.
Unfortunately, contact immunotherapy involves multiple visits to the office over several months, and it stimulates cosmetically worthwhile hair regrowth in <50% of patients with extensive patchy hair loss.1 It is a reasonable alternative for patients who do not have spontaneous remission after 1 year.
While potent topical steroids and topical minoxidil are prescribed for limited patchy alopecia areata, no convincing evidence shows they are effective.1 Likewise, no evidence warrants the use systemic steroids or psoralen/ultraviolet light treatment (PUVA).1
Aromatherapy
The best evidence may be for aromatherapy. A single-blinded randomized controlled trial was performed with 86 patients.3 As Dr Ebell points out in his InfoPOEMs review of this study, aromatherapy involves rubbing scented essential oils into the skin to treat localized and systemic disease.4 Patients with alopecia areata were randomized to nightly aromatherapy—with Thymus vulgaris (thyme), Lavandula angustifolia (lavender), Rosmarinus officinalis (rosemary), and Cedrus atlantica (cedar)—or to a control consisting of carrier oils only.
Improvement was seen in 54% of the treatment group and 21% of the control group (P=.008; number needed to treat=3). Of the 19 patients in the active treatment group who reported improvement, 11 had “very good” or “excellent” improvement. The results show aromatherapy to be a safe and effective treatment for alopecia areata (LOE=1b).4
The main problem with this study is that the researchers did not describe the duration of the patients’ alopecia. However, in a reply to a letter, they described the patients as having had alopecia areata from less than 1 year to more than 9 years. This explains the low improvement rates in both groups but does not invalidate the statistically significant difference for those that received the essential oils.
Conclusion of visit, follow-up
For a mild case like this in which the hair loss can be hidden, the best treatment is reassurance and observation. The physician explained the natural history of the disease, including the fact that regrowth will take at least 3 months for any single patch. Therapeutic options were also discussed. The mother and child were reassured that the hair is likely to grow in on its own. Neither of them wanted intralesional injections or topical therapies.
One year later during a well-child check, it was noted that the girl’s hair had fully regrown.
An 8-year-old Hispanic girl was brought to see her family physician by her mother, who noticed 2 bald spots on the back of her daughter’s scalp while brushing her hair. The child had no itching or pain. No obvious precipitating events preceded the hair loss.
The mother was more worried than the child: she didn’t want to see her beautiful girl become bald. The girl was pleased that the bald spots could be completely covered with her long hair, but she didn’t want anyone to see them. The child was otherwise healthy. She did not have any chronic medical problems and was not taking any medications. No one else in the family had a similar problem.
When the mother lifted the hair in the back, 2 round areas of hair loss were evident (Figure 1). On close inspection, there was no scaling or scarring. Her nails were normal. The child was afebrile, and the remainder of her exam was unremarkable.
FIGURE 1
Two round areas of hair loss
What is the diagnosis?
What are the management options?
Diagnosis: alopecia areata
This is the typical appearance of alopecia areata, a chronic inflammatory disease that affects the hair follicles, causing sudden hair loss. Sometimes it affects the nails as well.1 Alopecia areata occurs in both males and females of all ages and races.
Alopecia areata may be an autoimmune disease, though this is unproven. The affected skin may be slightly erythematous but otherwise appears normal. Short broken hairs (exclamation-mark hairs) may be seen around the margins of expanding patches of baldness. The nails are involved in about 10% of patients with severe enough alopecia to be referred to a specialist.1
Many cases begin in childhood and can be psychologically devastating. Alopecia areata is part of a spectrum of diseases with mild to extensive hair loss (Figure 2), including alopecia totalis, in which all the hair on the scalp is lost, and alopecia universalis (Figure 3), in which all the hair on the body is lost. Extensive involvement, early age of onset, and Down syndrome are all poor prognostic factors for alopecia areata.
FIGURE 2
More extensive alopecia
FIGURE 3
Alopecia universalis
Differential diagnoses
The differential diagnosis for alopecia areata includes tinea capitis, trichotillomania, early scarring alopecia, telogen effluvium, anagen effluvium (drug-induced), systemic and discoid lupus erythematosus, and secondary syphilis. In most cases the history and physical exam are adequate to make the diagnosis.
This patient does not have the typical scalp scaling and inflammation seen with tinea capitis. Trichotillomania—hair loss caused by the purposeful pulling of hair by the patient—is likely to cause the most confusion because it coexists with alopecia areata in some cases. This child has shown no evidence of such behavior. She also has no evidence of scalp scarring as may be seen in lupus. Telogen effluvium and anagen effluvium cause a more even distribution of hair loss. The patient has no known risk factors for secondary syphilis.
Laboratory testing
No lab tests are needed in this case. If there were some scalp scaling or inflammation, a potassium hydroxide preparation of the involved area would be useful to look for fungal elements; a fungal culture might also be warranted. If needed, further investigations might include serological testing for lupus erythematosus and syphilis, and a skin biopsy if the diagnosis is still unknown.
Treatment: time, drug therapies, aromatherapy
Spontaneous remission occurs in up to 80% of patients with limited patchy hair loss of less than 1 year.1 Spontaneous remission rates are significantly lower with more extensive hair loss.
Treatments are potentially painful, expensive, or time-consuming, and few randomized controlled trials support their use. Often the best treatment is watching for spontaneous remission.
The only adverse health effect of alopecia areata is the psychological distress that it may cause. While this is not to be taken lightly, the lack of evidence for successful treatments needs to be weighed with the patient’s ability to cope with leaving the hair loss untreated over time. In cases of extensive hair loss, the best treatment may be a wig.
For patients that have more visible and extensive areas of hair loss, the psychological impact might prompt the patient to want any treatment available despite the lack of evidence. Alopecia totalis or universalis may cause considerable psychological and social disability. Patients can be referred to the National Alopecia Areata Foundation for support groups and additional information. Individual counseling may be needed for some patients.
National Alopecia Areata Foundation Web site: www.alopeciaareata.com. Patients can order a 7-minute video, This Weird Thing That Makes My Hair Fall Out: Alopecia Areata, which is available for any children who want a way to share their feelings about alopecia areata with friends, family, peers, schoolmates, principals, and teachers.
European Hair Research Society Web site: www.ehrs.org. Web site has links to several alopecia areata sites.
Steroid injections
Intralesional steroid injections may stimulate regrowth of hair at the site of injection (level of evidence [LOE]=5).1 The effect may last a few months, but there is no evidence that it improves the long-term outcome or increases the probability of a cure. New areas of alopecia can still develop.
Injections are typically performed with 5–10 mg/mL of triamcinolone acetonide using a small-gauge needle. Most children will not be able to tolerate the scalp injections and should not be forced to endure this type of therapy even if the parent is pushing for it.
Other medications
Topical diphenylcyclopropenone (DPCP) is a contact immunotherapy that has some proven benefit with extensive alopecia areata (LOE=2b).1,2 In 1 study, 56 patients with chronic, extensive alopecia areata (duration ranging from 1 to 10 years, involving 30% to 100% of the scalp) were treated with progressively higher concentrations of DPCP in a randomized crossover trial.2 Twenty-five of 56 patients had total hair regrowth at 6 months, and no relapse occurred in 60% of patients. Side effects included local inflammation, eczema, autosensitization reaction, and eyelid edema.
Unfortunately, contact immunotherapy involves multiple visits to the office over several months, and it stimulates cosmetically worthwhile hair regrowth in <50% of patients with extensive patchy hair loss.1 It is a reasonable alternative for patients who do not have spontaneous remission after 1 year.
While potent topical steroids and topical minoxidil are prescribed for limited patchy alopecia areata, no convincing evidence shows they are effective.1 Likewise, no evidence warrants the use systemic steroids or psoralen/ultraviolet light treatment (PUVA).1
Aromatherapy
The best evidence may be for aromatherapy. A single-blinded randomized controlled trial was performed with 86 patients.3 As Dr Ebell points out in his InfoPOEMs review of this study, aromatherapy involves rubbing scented essential oils into the skin to treat localized and systemic disease.4 Patients with alopecia areata were randomized to nightly aromatherapy—with Thymus vulgaris (thyme), Lavandula angustifolia (lavender), Rosmarinus officinalis (rosemary), and Cedrus atlantica (cedar)—or to a control consisting of carrier oils only.
Improvement was seen in 54% of the treatment group and 21% of the control group (P=.008; number needed to treat=3). Of the 19 patients in the active treatment group who reported improvement, 11 had “very good” or “excellent” improvement. The results show aromatherapy to be a safe and effective treatment for alopecia areata (LOE=1b).4
The main problem with this study is that the researchers did not describe the duration of the patients’ alopecia. However, in a reply to a letter, they described the patients as having had alopecia areata from less than 1 year to more than 9 years. This explains the low improvement rates in both groups but does not invalidate the statistically significant difference for those that received the essential oils.
Conclusion of visit, follow-up
For a mild case like this in which the hair loss can be hidden, the best treatment is reassurance and observation. The physician explained the natural history of the disease, including the fact that regrowth will take at least 3 months for any single patch. Therapeutic options were also discussed. The mother and child were reassured that the hair is likely to grow in on its own. Neither of them wanted intralesional injections or topical therapies.
One year later during a well-child check, it was noted that the girl’s hair had fully regrown.
1. MacDonald Hull SP, Wood ML, Hutchinson PE, Sladden M, Messenger AG. Guidelines for the management of alopecia areata. Br J Dermatol 2003;149:692-699.
2. Cotellessa C, Peris K, Caracciolo E, Mordenti C, Chimenti S. The use of topical diphenylcyclopropenone for the treatment of extensive alopecia areata. J Am Acad Dermatol 2001;44:73-76.
3. Hay IC, Jamieson M, Ormerod AD. Randomized trial of aromatherapy: successful treatment for alopecia areata. Arch Derm 1998;134:1349-1352.
4. Ebell M. Aromatherapy effective for alopecia areata. Review of Hay et al 1998. InfoPOEMs, Inc. March 1999.
1. MacDonald Hull SP, Wood ML, Hutchinson PE, Sladden M, Messenger AG. Guidelines for the management of alopecia areata. Br J Dermatol 2003;149:692-699.
2. Cotellessa C, Peris K, Caracciolo E, Mordenti C, Chimenti S. The use of topical diphenylcyclopropenone for the treatment of extensive alopecia areata. J Am Acad Dermatol 2001;44:73-76.
3. Hay IC, Jamieson M, Ormerod AD. Randomized trial of aromatherapy: successful treatment for alopecia areata. Arch Derm 1998;134:1349-1352.
4. Ebell M. Aromatherapy effective for alopecia areata. Review of Hay et al 1998. InfoPOEMs, Inc. March 1999.
Painful genital ulcers
A colleague came into the charting area and said he thought he had just seen his first case of chancroid. He asked me if I had a moment to see the patient, a 32-year-old African American man who noted the onset of painful sores on his penis 1 week ago. The patient consented to a second opinion. On further questioning, he remembered a tingling pain that started a few days prior to the sores. When asked about any previous outbreaks, he thought he may have had something like this 1 year ago. He did not remember seeing blisters before the sores appeared.
The last time the patient had sexual relations was 2 months ago, with someone he met at a party. He claimed he used a condom. He did not have any lesions at that time and had never had a sexually transmitted disease before.
He had recently fallen in love, and was concerned about these ulcers—he did not want to give her any diseases. They had only kissed so far and he wanted to know what he should tell her. He said he had never had sex with men or injected any drugs. He has had a number of serially monogamous relationships and reported no other human immunodeficiency virus (HIV) risk factors.
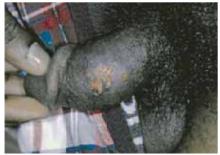
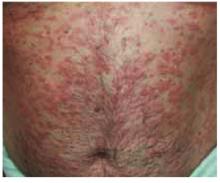
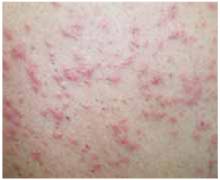
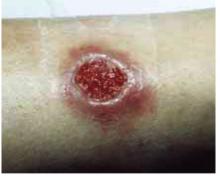
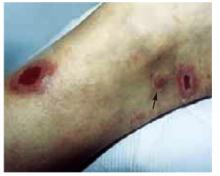
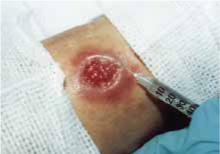
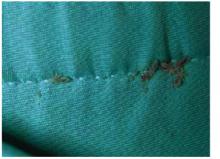
The patient was a healthy-looking young man. Examination of his penis (Figure 1) showed the ulcers clearly visible (Figure 2). He had only shotty inguinal adenopathy that was nontender.
FIGURE 1
Painful sores on the genitals
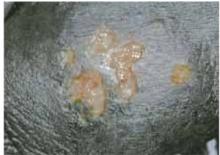
FIGURE 2
Close-up of ulcers on the penis
What is the diagnosis?
What is the treatment and prevention strategy?
Differential diagnosis
The most likely causes of painful genital ulcers in this case are herpes simplex, chancroid, and syphilis. Granuloma inguinale and lymphogranuloma venereum (LGV) are rare causes of genital ulceration in the United States. A zipper accident or other trauma can cause genital ulceration, but the patient should be able to give a clear history of such an event.
By epidemiology alone, the order of likelihood for the cause of any genital ulceration is herpes, syphilis, then chancroid.
This case points to herpes
Herpes simplex is by far the most common cause of painful genital ulcers in the United States; at least 50 million people have genital herpes simplex virus (HSV) infection1 The features of this case pointing to herpes are the appearance of multiple ulcers, the tingling pain that preceded the ulcers, and the history of a possible episode in the preceding year. While it would be helpful to have a history of blisters that preceded the ulcers, the evidence still points to herpes as the most likely diagnosis.
Could it be syphilis?
While the primary chancre of syphilis is classically described as painless, the patient with syphilis may experience pain. Syphilis tends to present as a single ulcer but may cause multiple ulcers.
Why not chancroid?
Chancroid may also cause multiple small painful ulcers. However, the ulcers of chancroid tend to be deeper than those of herpes and bleed more easily.
Other characteristics to look for
All of these sexually transmitted diseases can cause tender painful adenopathy, which is particularly characteristic of chancroid and LGV. Suppurative inguinal adenopathy with painful genital ulcers is almost pathognomonic of chancroid. With LGV, there may be a self-limited genital ulcer at the site of inoculation, which is often gone by the time a patient seeks care. Granuloma inguinale causes painless, progressive ulcerative lesions without regional lymphadenopathy. These lesions are highly vascular (with a characteristic beefy red appearance) and bleed easily on contact.1 While it would be helpful to have a history of blisters that preceded the ulcers, the evidence still points to herpes as the most likely diagnosis.
Laboratory examination
Herpes
All patients with genital ulcers thought to be from an STD should be tested for syphilis and HIV regardless of other risk factors.1 This patient should additionally be tested for herpes simplex. A bacteriologic test for chancroid is not necessary, but the clinician who first saw the patient asked that we conduct the test for chancroid—a culture for the Haemophilus ducreyi bacterium.
Isolation of HSV in cell culture is the preferred virologic test for patients with genital ulcers.1Unfortunately, the sensitivity of culture declines rapidly as lesions begin to heal, usually within a few days of onset. Direct fluorescent antibody tests are also available. Both herpes culture and the direct fluorescent antibody test distinguish HSV-1 from HSV-2. Polymerase chain reaction assays for HSV DNA are highly sensitive, but their role in the diagnosis of genital ulcer disease has not been well-defined.
Most cases of recurrent genital herpes are caused by HSV-2. Specific serologic testing can be expensive, and is not needed at the time of the initial virologic screening. However, consider ordering the test at a subsequent visit, because the distinction between HSV serotypes influences prognosis and counseling. Also, because false-negative HSV cultures are common—especially with recurrent infection or healing lesions—type-specific serologic tests are useful for confirming a diagnosis of genital herpes.1 Herpes serologies can also be used to help manage sexual partners of persons with genital herpes.
Syphilis
The Venereal Disease Research Laboratory (VDRL) test or rapid plasma reagin (RPR) test should be used to detect syphilis. Both tests are used for nonspecific screening only, because they measure anticardiolipin antibodies. A positive result should be confirmed with a specific treponemal test such as a fluorescent treponemal antibody absorption test (FTA-ABS).
The results of these laboratory tests are not available immediately during the patient’s visit. If there was a high suspicion for syphilis, a dark field examination from the ulcer exudate could be used to look for spirochetes while the patient was still in the office. In this case, the suspicion for syphilis was low.
Treatment: Antivirals
The major question is whether the patient should be treated empirically with medication. The most likely diagnosis is herpes simplex. Randomized trials indicate that 3 antiviral medications— acyclovir, famciclovir, and valacyclovir—provide clinical benefit for genital herpes (level of evidence [LOE]=1a).1
The Centers for Disease Control and Prevention (CDC) 2002 treatment guidelines for STDs recommend the following medications for the first clinical episode of genital herpes:
- Acyclovir 400 mg orally, 3 times daily for 7–10 days or until clinically resolved, OR
- Acyclovir 200 mg orally, 5 times daily for 7–10 days or until clinically resolved, OR
- Famciclovir 250 mg orally, 3 times daily for 7–10 days or until clinically resolved, OR
- Valacyclovir 1 g orally, twice daily for 7–10 days or until clinically resolved.
Topical acyclovir is less effective than the oral formulaton and its use is discouraged.
The suspicion for syphilis is too low to warrant an intramuscular shot of penicillin, which is painful and can cause anaphylaxis in some patients. The likelihood of chancroid is too low to prescribe an oral antibiotic such as erythromycin.
The patient wanted empirical treatment for herpes. He was given valacyclovir, 1 gm for 7 days, taken twice daily, with the option to call in for more if the ulcers did not resolve by day 7. He was told he might apply petrolatum and clean gauze to the ulcers to diminish the pain when open ulcers rub against underwear. Acetaminophen or other analgesics were recommended for pain, and he was advised to avoid sexual activity until the ulcers had fully healed.
Preventing transmission
The patient is appropriately concerned about the transmission of this condition to a new partner. Not having a firm diagnosis makes definitive counseling more difficult. However, general principles of safe sex and condom use were discussed. On the follow-up visit the patient was told that the result of his herpes test was positive for HSV-2. Results of his RPR, HIV antibody test, and H ducreyiculture were all negative.
Information about condom use was reinforced, and the patient was told there is definitive evidence that condom use does diminish the risk of transmission of herpes from a man to a woman (LOE=1b).2 That same study did not show that condom use prevents transmission from women to men. Also, changes in sexual behavior, correlated with counseling about avoiding sex when a partner has lesions, were associated with reduction in HSV-2 acquisition over time (LOE=1b).2
One study showed that the overall risk of genital HSV transmission in couples is low (10%/year). The risk may be significantly increased in women and in seronegative individuals.3 This speaks for serologic testing for the potential partner of this patient.
When recurrences are frequent, antiviral agents can decrease the frequency (LOE=1a).1 If this patient has frequent recurrences, antiviral agents would be appropriate and would decrease the times when the patient is shedding virus asymptomatically.
Herpes is transmitted between sexual partners during asymptomatic shedding.1 Acyclovir 400 mg twice daily can reduce asymptomatic viral shedding significantly among women with recurrent herpes simplex (LOE=1b).4 While it is likely this will decrease transmission from women to men, this has not been proven. Data on decreasing viral transmission from men to women by antiviral therapy is not available. At some point, the Glycoprotein-D-adjuvant vaccine may be an option to prevent genital herpes transmission to his partner.5
Note. The CDC 2002 sexually transmitted diseases treatment guidelines are available for download and use on a Palm handheld computer at www.cdcnpin.org/scripts/std/pda.asp.
1. Centers for Disease Control and Prevention. 2002 sexually transmitted diseases treatment guidelines. MMWR Recomm Rep 2002;51(RR-6).-Available online at: http://www.cdc.gov/std/treatment/. Accessed on November 4, 2003.
2. Wald A, Langenberg AG, Link K, et al. Effect of condoms on reducing the transmission of herpes simplex virus type 2 from men to women. JAMA 2001;285:31003106.
3. Bryson Y, Dillon M, Bernstein DI, Radolf J, Zakowski P, Garratty E. Risk of acquisition of genital herpes simplex virus type 2 in sex partners of persons with genital herpes: a prospective couple study. J Infect Dis 1993;167:942-946.
4. Wald A, Zeh J, Barnum G, et al. Suppression of subclinical shedding of herpes simplex virus type 2 with acyclovir. Ann Intern Med 1996;124:8-15.
5. Stanberry LR, Spruance SL, Cunningham AL, et al. GlaxoSmithKline Herpes Vaccine Efficacy Study Group. Glycoprotein-D-adjuvant vaccine to prevent genital herpes. N Engl J Med 2002;347:1652-1661.
A colleague came into the charting area and said he thought he had just seen his first case of chancroid. He asked me if I had a moment to see the patient, a 32-year-old African American man who noted the onset of painful sores on his penis 1 week ago. The patient consented to a second opinion. On further questioning, he remembered a tingling pain that started a few days prior to the sores. When asked about any previous outbreaks, he thought he may have had something like this 1 year ago. He did not remember seeing blisters before the sores appeared.
The last time the patient had sexual relations was 2 months ago, with someone he met at a party. He claimed he used a condom. He did not have any lesions at that time and had never had a sexually transmitted disease before.
He had recently fallen in love, and was concerned about these ulcers—he did not want to give her any diseases. They had only kissed so far and he wanted to know what he should tell her. He said he had never had sex with men or injected any drugs. He has had a number of serially monogamous relationships and reported no other human immunodeficiency virus (HIV) risk factors.
The patient was a healthy-looking young man. Examination of his penis (Figure 1) showed the ulcers clearly visible (Figure 2). He had only shotty inguinal adenopathy that was nontender.
FIGURE 1
Painful sores on the genitals
FIGURE 2
Close-up of ulcers on the penis
What is the diagnosis?
What is the treatment and prevention strategy?
Differential diagnosis
The most likely causes of painful genital ulcers in this case are herpes simplex, chancroid, and syphilis. Granuloma inguinale and lymphogranuloma venereum (LGV) are rare causes of genital ulceration in the United States. A zipper accident or other trauma can cause genital ulceration, but the patient should be able to give a clear history of such an event.
By epidemiology alone, the order of likelihood for the cause of any genital ulceration is herpes, syphilis, then chancroid.
This case points to herpes
Herpes simplex is by far the most common cause of painful genital ulcers in the United States; at least 50 million people have genital herpes simplex virus (HSV) infection1 The features of this case pointing to herpes are the appearance of multiple ulcers, the tingling pain that preceded the ulcers, and the history of a possible episode in the preceding year. While it would be helpful to have a history of blisters that preceded the ulcers, the evidence still points to herpes as the most likely diagnosis.
Could it be syphilis?
While the primary chancre of syphilis is classically described as painless, the patient with syphilis may experience pain. Syphilis tends to present as a single ulcer but may cause multiple ulcers.
Why not chancroid?
Chancroid may also cause multiple small painful ulcers. However, the ulcers of chancroid tend to be deeper than those of herpes and bleed more easily.
Other characteristics to look for
All of these sexually transmitted diseases can cause tender painful adenopathy, which is particularly characteristic of chancroid and LGV. Suppurative inguinal adenopathy with painful genital ulcers is almost pathognomonic of chancroid. With LGV, there may be a self-limited genital ulcer at the site of inoculation, which is often gone by the time a patient seeks care. Granuloma inguinale causes painless, progressive ulcerative lesions without regional lymphadenopathy. These lesions are highly vascular (with a characteristic beefy red appearance) and bleed easily on contact.1 While it would be helpful to have a history of blisters that preceded the ulcers, the evidence still points to herpes as the most likely diagnosis.
Laboratory examination
Herpes
All patients with genital ulcers thought to be from an STD should be tested for syphilis and HIV regardless of other risk factors.1 This patient should additionally be tested for herpes simplex. A bacteriologic test for chancroid is not necessary, but the clinician who first saw the patient asked that we conduct the test for chancroid—a culture for the Haemophilus ducreyi bacterium.
Isolation of HSV in cell culture is the preferred virologic test for patients with genital ulcers.1Unfortunately, the sensitivity of culture declines rapidly as lesions begin to heal, usually within a few days of onset. Direct fluorescent antibody tests are also available. Both herpes culture and the direct fluorescent antibody test distinguish HSV-1 from HSV-2. Polymerase chain reaction assays for HSV DNA are highly sensitive, but their role in the diagnosis of genital ulcer disease has not been well-defined.
Most cases of recurrent genital herpes are caused by HSV-2. Specific serologic testing can be expensive, and is not needed at the time of the initial virologic screening. However, consider ordering the test at a subsequent visit, because the distinction between HSV serotypes influences prognosis and counseling. Also, because false-negative HSV cultures are common—especially with recurrent infection or healing lesions—type-specific serologic tests are useful for confirming a diagnosis of genital herpes.1 Herpes serologies can also be used to help manage sexual partners of persons with genital herpes.
Syphilis
The Venereal Disease Research Laboratory (VDRL) test or rapid plasma reagin (RPR) test should be used to detect syphilis. Both tests are used for nonspecific screening only, because they measure anticardiolipin antibodies. A positive result should be confirmed with a specific treponemal test such as a fluorescent treponemal antibody absorption test (FTA-ABS).
The results of these laboratory tests are not available immediately during the patient’s visit. If there was a high suspicion for syphilis, a dark field examination from the ulcer exudate could be used to look for spirochetes while the patient was still in the office. In this case, the suspicion for syphilis was low.
Treatment: Antivirals
The major question is whether the patient should be treated empirically with medication. The most likely diagnosis is herpes simplex. Randomized trials indicate that 3 antiviral medications— acyclovir, famciclovir, and valacyclovir—provide clinical benefit for genital herpes (level of evidence [LOE]=1a).1
The Centers for Disease Control and Prevention (CDC) 2002 treatment guidelines for STDs recommend the following medications for the first clinical episode of genital herpes:
- Acyclovir 400 mg orally, 3 times daily for 7–10 days or until clinically resolved, OR
- Acyclovir 200 mg orally, 5 times daily for 7–10 days or until clinically resolved, OR
- Famciclovir 250 mg orally, 3 times daily for 7–10 days or until clinically resolved, OR
- Valacyclovir 1 g orally, twice daily for 7–10 days or until clinically resolved.
Topical acyclovir is less effective than the oral formulaton and its use is discouraged.
The suspicion for syphilis is too low to warrant an intramuscular shot of penicillin, which is painful and can cause anaphylaxis in some patients. The likelihood of chancroid is too low to prescribe an oral antibiotic such as erythromycin.
The patient wanted empirical treatment for herpes. He was given valacyclovir, 1 gm for 7 days, taken twice daily, with the option to call in for more if the ulcers did not resolve by day 7. He was told he might apply petrolatum and clean gauze to the ulcers to diminish the pain when open ulcers rub against underwear. Acetaminophen or other analgesics were recommended for pain, and he was advised to avoid sexual activity until the ulcers had fully healed.
Preventing transmission
The patient is appropriately concerned about the transmission of this condition to a new partner. Not having a firm diagnosis makes definitive counseling more difficult. However, general principles of safe sex and condom use were discussed. On the follow-up visit the patient was told that the result of his herpes test was positive for HSV-2. Results of his RPR, HIV antibody test, and H ducreyiculture were all negative.
Information about condom use was reinforced, and the patient was told there is definitive evidence that condom use does diminish the risk of transmission of herpes from a man to a woman (LOE=1b).2 That same study did not show that condom use prevents transmission from women to men. Also, changes in sexual behavior, correlated with counseling about avoiding sex when a partner has lesions, were associated with reduction in HSV-2 acquisition over time (LOE=1b).2
One study showed that the overall risk of genital HSV transmission in couples is low (10%/year). The risk may be significantly increased in women and in seronegative individuals.3 This speaks for serologic testing for the potential partner of this patient.
When recurrences are frequent, antiviral agents can decrease the frequency (LOE=1a).1 If this patient has frequent recurrences, antiviral agents would be appropriate and would decrease the times when the patient is shedding virus asymptomatically.
Herpes is transmitted between sexual partners during asymptomatic shedding.1 Acyclovir 400 mg twice daily can reduce asymptomatic viral shedding significantly among women with recurrent herpes simplex (LOE=1b).4 While it is likely this will decrease transmission from women to men, this has not been proven. Data on decreasing viral transmission from men to women by antiviral therapy is not available. At some point, the Glycoprotein-D-adjuvant vaccine may be an option to prevent genital herpes transmission to his partner.5
Note. The CDC 2002 sexually transmitted diseases treatment guidelines are available for download and use on a Palm handheld computer at www.cdcnpin.org/scripts/std/pda.asp.
A colleague came into the charting area and said he thought he had just seen his first case of chancroid. He asked me if I had a moment to see the patient, a 32-year-old African American man who noted the onset of painful sores on his penis 1 week ago. The patient consented to a second opinion. On further questioning, he remembered a tingling pain that started a few days prior to the sores. When asked about any previous outbreaks, he thought he may have had something like this 1 year ago. He did not remember seeing blisters before the sores appeared.
The last time the patient had sexual relations was 2 months ago, with someone he met at a party. He claimed he used a condom. He did not have any lesions at that time and had never had a sexually transmitted disease before.
He had recently fallen in love, and was concerned about these ulcers—he did not want to give her any diseases. They had only kissed so far and he wanted to know what he should tell her. He said he had never had sex with men or injected any drugs. He has had a number of serially monogamous relationships and reported no other human immunodeficiency virus (HIV) risk factors.
The patient was a healthy-looking young man. Examination of his penis (Figure 1) showed the ulcers clearly visible (Figure 2). He had only shotty inguinal adenopathy that was nontender.
FIGURE 1
Painful sores on the genitals
FIGURE 2
Close-up of ulcers on the penis
What is the diagnosis?
What is the treatment and prevention strategy?
Differential diagnosis
The most likely causes of painful genital ulcers in this case are herpes simplex, chancroid, and syphilis. Granuloma inguinale and lymphogranuloma venereum (LGV) are rare causes of genital ulceration in the United States. A zipper accident or other trauma can cause genital ulceration, but the patient should be able to give a clear history of such an event.
By epidemiology alone, the order of likelihood for the cause of any genital ulceration is herpes, syphilis, then chancroid.
This case points to herpes
Herpes simplex is by far the most common cause of painful genital ulcers in the United States; at least 50 million people have genital herpes simplex virus (HSV) infection1 The features of this case pointing to herpes are the appearance of multiple ulcers, the tingling pain that preceded the ulcers, and the history of a possible episode in the preceding year. While it would be helpful to have a history of blisters that preceded the ulcers, the evidence still points to herpes as the most likely diagnosis.
Could it be syphilis?
While the primary chancre of syphilis is classically described as painless, the patient with syphilis may experience pain. Syphilis tends to present as a single ulcer but may cause multiple ulcers.
Why not chancroid?
Chancroid may also cause multiple small painful ulcers. However, the ulcers of chancroid tend to be deeper than those of herpes and bleed more easily.
Other characteristics to look for
All of these sexually transmitted diseases can cause tender painful adenopathy, which is particularly characteristic of chancroid and LGV. Suppurative inguinal adenopathy with painful genital ulcers is almost pathognomonic of chancroid. With LGV, there may be a self-limited genital ulcer at the site of inoculation, which is often gone by the time a patient seeks care. Granuloma inguinale causes painless, progressive ulcerative lesions without regional lymphadenopathy. These lesions are highly vascular (with a characteristic beefy red appearance) and bleed easily on contact.1 While it would be helpful to have a history of blisters that preceded the ulcers, the evidence still points to herpes as the most likely diagnosis.
Laboratory examination
Herpes
All patients with genital ulcers thought to be from an STD should be tested for syphilis and HIV regardless of other risk factors.1 This patient should additionally be tested for herpes simplex. A bacteriologic test for chancroid is not necessary, but the clinician who first saw the patient asked that we conduct the test for chancroid—a culture for the Haemophilus ducreyi bacterium.
Isolation of HSV in cell culture is the preferred virologic test for patients with genital ulcers.1Unfortunately, the sensitivity of culture declines rapidly as lesions begin to heal, usually within a few days of onset. Direct fluorescent antibody tests are also available. Both herpes culture and the direct fluorescent antibody test distinguish HSV-1 from HSV-2. Polymerase chain reaction assays for HSV DNA are highly sensitive, but their role in the diagnosis of genital ulcer disease has not been well-defined.
Most cases of recurrent genital herpes are caused by HSV-2. Specific serologic testing can be expensive, and is not needed at the time of the initial virologic screening. However, consider ordering the test at a subsequent visit, because the distinction between HSV serotypes influences prognosis and counseling. Also, because false-negative HSV cultures are common—especially with recurrent infection or healing lesions—type-specific serologic tests are useful for confirming a diagnosis of genital herpes.1 Herpes serologies can also be used to help manage sexual partners of persons with genital herpes.
Syphilis
The Venereal Disease Research Laboratory (VDRL) test or rapid plasma reagin (RPR) test should be used to detect syphilis. Both tests are used for nonspecific screening only, because they measure anticardiolipin antibodies. A positive result should be confirmed with a specific treponemal test such as a fluorescent treponemal antibody absorption test (FTA-ABS).
The results of these laboratory tests are not available immediately during the patient’s visit. If there was a high suspicion for syphilis, a dark field examination from the ulcer exudate could be used to look for spirochetes while the patient was still in the office. In this case, the suspicion for syphilis was low.
Treatment: Antivirals
The major question is whether the patient should be treated empirically with medication. The most likely diagnosis is herpes simplex. Randomized trials indicate that 3 antiviral medications— acyclovir, famciclovir, and valacyclovir—provide clinical benefit for genital herpes (level of evidence [LOE]=1a).1
The Centers for Disease Control and Prevention (CDC) 2002 treatment guidelines for STDs recommend the following medications for the first clinical episode of genital herpes:
- Acyclovir 400 mg orally, 3 times daily for 7–10 days or until clinically resolved, OR
- Acyclovir 200 mg orally, 5 times daily for 7–10 days or until clinically resolved, OR
- Famciclovir 250 mg orally, 3 times daily for 7–10 days or until clinically resolved, OR
- Valacyclovir 1 g orally, twice daily for 7–10 days or until clinically resolved.
Topical acyclovir is less effective than the oral formulaton and its use is discouraged.
The suspicion for syphilis is too low to warrant an intramuscular shot of penicillin, which is painful and can cause anaphylaxis in some patients. The likelihood of chancroid is too low to prescribe an oral antibiotic such as erythromycin.
The patient wanted empirical treatment for herpes. He was given valacyclovir, 1 gm for 7 days, taken twice daily, with the option to call in for more if the ulcers did not resolve by day 7. He was told he might apply petrolatum and clean gauze to the ulcers to diminish the pain when open ulcers rub against underwear. Acetaminophen or other analgesics were recommended for pain, and he was advised to avoid sexual activity until the ulcers had fully healed.
Preventing transmission
The patient is appropriately concerned about the transmission of this condition to a new partner. Not having a firm diagnosis makes definitive counseling more difficult. However, general principles of safe sex and condom use were discussed. On the follow-up visit the patient was told that the result of his herpes test was positive for HSV-2. Results of his RPR, HIV antibody test, and H ducreyiculture were all negative.
Information about condom use was reinforced, and the patient was told there is definitive evidence that condom use does diminish the risk of transmission of herpes from a man to a woman (LOE=1b).2 That same study did not show that condom use prevents transmission from women to men. Also, changes in sexual behavior, correlated with counseling about avoiding sex when a partner has lesions, were associated with reduction in HSV-2 acquisition over time (LOE=1b).2
One study showed that the overall risk of genital HSV transmission in couples is low (10%/year). The risk may be significantly increased in women and in seronegative individuals.3 This speaks for serologic testing for the potential partner of this patient.
When recurrences are frequent, antiviral agents can decrease the frequency (LOE=1a).1 If this patient has frequent recurrences, antiviral agents would be appropriate and would decrease the times when the patient is shedding virus asymptomatically.
Herpes is transmitted between sexual partners during asymptomatic shedding.1 Acyclovir 400 mg twice daily can reduce asymptomatic viral shedding significantly among women with recurrent herpes simplex (LOE=1b).4 While it is likely this will decrease transmission from women to men, this has not been proven. Data on decreasing viral transmission from men to women by antiviral therapy is not available. At some point, the Glycoprotein-D-adjuvant vaccine may be an option to prevent genital herpes transmission to his partner.5
Note. The CDC 2002 sexually transmitted diseases treatment guidelines are available for download and use on a Palm handheld computer at www.cdcnpin.org/scripts/std/pda.asp.
1. Centers for Disease Control and Prevention. 2002 sexually transmitted diseases treatment guidelines. MMWR Recomm Rep 2002;51(RR-6).-Available online at: http://www.cdc.gov/std/treatment/. Accessed on November 4, 2003.
2. Wald A, Langenberg AG, Link K, et al. Effect of condoms on reducing the transmission of herpes simplex virus type 2 from men to women. JAMA 2001;285:31003106.
3. Bryson Y, Dillon M, Bernstein DI, Radolf J, Zakowski P, Garratty E. Risk of acquisition of genital herpes simplex virus type 2 in sex partners of persons with genital herpes: a prospective couple study. J Infect Dis 1993;167:942-946.
4. Wald A, Zeh J, Barnum G, et al. Suppression of subclinical shedding of herpes simplex virus type 2 with acyclovir. Ann Intern Med 1996;124:8-15.
5. Stanberry LR, Spruance SL, Cunningham AL, et al. GlaxoSmithKline Herpes Vaccine Efficacy Study Group. Glycoprotein-D-adjuvant vaccine to prevent genital herpes. N Engl J Med 2002;347:1652-1661.
1. Centers for Disease Control and Prevention. 2002 sexually transmitted diseases treatment guidelines. MMWR Recomm Rep 2002;51(RR-6).-Available online at: http://www.cdc.gov/std/treatment/. Accessed on November 4, 2003.
2. Wald A, Langenberg AG, Link K, et al. Effect of condoms on reducing the transmission of herpes simplex virus type 2 from men to women. JAMA 2001;285:31003106.
3. Bryson Y, Dillon M, Bernstein DI, Radolf J, Zakowski P, Garratty E. Risk of acquisition of genital herpes simplex virus type 2 in sex partners of persons with genital herpes: a prospective couple study. J Infect Dis 1993;167:942-946.
4. Wald A, Zeh J, Barnum G, et al. Suppression of subclinical shedding of herpes simplex virus type 2 with acyclovir. Ann Intern Med 1996;124:8-15.
5. Stanberry LR, Spruance SL, Cunningham AL, et al. GlaxoSmithKline Herpes Vaccine Efficacy Study Group. Glycoprotein-D-adjuvant vaccine to prevent genital herpes. N Engl J Med 2002;347:1652-1661.
New rash on the right hand and neck
A 43-year-old African-American woman was seen for a rash on her right hand and neck. The rash appeared 2 weeks earlier, and was itchy. The patient had been applying alcohol directly to the affected skin.
The patient said she had experienced rashes on and off during her adult life. She has not worn necklaces for years because they irritate the skin around her neck. She also cannot wear wool clothing or any shirts with bright colors. She had not discussed this with a physician before, and she did not know why her skin is so sensitive.
The patient was not taking any new medications, using new bath products, or wearing new clothing before the rash appeared. However, she did remember getting new perfume 2 weeks earlier. She did not have asthma, but did have a mild hay fever. She was adopted and therefore did not know her biologic family’s medical history.
On close inspection, prominent erythema was seen around her neck and on the dorsum of her right hand (Figures 1 and 2).
FIGURE 1
Itchy rash on the neck…
FIGURE 2
…and the right hand
What is the diagnosis?
What are the management options?
Diagnosis: contact dermatitis
The patient demonstrated how she applied perfume to her neck using the back of her right hand. She has acute contact dermatitis on top of previously undiagnosed eczema. Her history is positive for contact dermatitis in reaction to metal (probably nickel) in necklaces and to various fabrics.
Contact dermatitis is a common inflammatory skin condition in which erythematous and pruritic skin lesions result from contact with a foreign substance. The term describes 2 diagnostic categories—allergic contact dermatitis and irritant contact dermatitis. In this case, the chemicals in the perfume may have acted as irritants. The alcohol she used to treat the rash was probably an irritant as well.
A hypersensitivity reaction to irritants and allergens
The mechanism of allergic contact dermatitis is a delayed hypersensitivity reaction. A foreign substance comes into contact with skin and links to skin protein, forming an antigen complex recognized by the immune system. In the skin, the major antigen-presenting cell is the Langerhans cell. When the epidermis is re-exposed to the antigen, the sensitized T-cells initiate an inflammatory cascade leading to changes in the skin. The reaction generally occurs within 12 to 48 hours in a sensitized person. The differential diagnoses for this patient include atopic dermatitis, psoriasis, and fungal infection.
Cosmetics, perfumes, and chemicals in cleaning solutions are known to cause irritant contact dermatitis. The most typical locations are hands or feet, with the dorsum affected more often than the palm or the sole. The lesions are usually erythematous and not well demarcated. The lesions may be macular, papular, or vesicular.
Allergic contact dermatitis often occurs after contact with allergens such as nickel in jewelry, or resins in plants such as poison ivy or poison oak. Vesicles may appear, and the hands are most commonly affected. The lesions may form a linear pattern, as in the case of plant dermatitis.
Evaluation: tests usually not needed
A diagnosis of contact dermatitis usually requires nothing more than findings in the patient’s history and physical exam. If a fungal infection is suspected, use a potassium hydroxide (KOH) preparation to look for hyphae or spores (see page 854 in this issue). Patch testing—placing common antigens on the patient’s skin and observing for reactions—is used to confirm the diagnosis of contact dermatitis and to determine the offending agent.
In this case, neither test was necessary or warranted because the history and physical exam led to a clear diagnosis that was treatable without having to analyze the chemicals in the perfume.
Treatment: remove the irritant, stop the swelling
Whether this is irritant or allergic contact dermatitis, the first line of treatment is to stop further exposure to the offending substance. A mid- to high-potency topical corticosteroid is recommended to stopping the inflammatory process (level of evidence [LOE]=5). (For an explanation of evidence-based medicine ratings, see page 000.) Ointments are more potent and particularly useful when the affected skin is dry and scaly.
Oral steroids are often used for severe local dermatitis, such as that caused by poison ivy (LOE=5). Topical tacrolimus ointment 0.1% is a more expensive option for the treatment of allergic contact dermatitis induced by nickel (LOE=2b).1 Oral H1 blocking sedating anti-histamines (such as diphenhydramine and hydroxyzine) provide relief from itching and help with sleep. Sedating antihistamines may be more effective for itching, but are probably inadvisable for persons who must drive or operate machinery. Newer nonsedating anti-histamines are an option when sedating agents should be avoided.
Conclusion of visit
The patient was given 0.1% triamcinolone ointment (a generic mid-potency steroid) to apply 2 to 3 times a day to the affected areas. She did not want any oral medications for the itching, but understood that diphenhydramine is available without a prescription if needed. She planned to give the perfume away and understood it was now on her list of items to avoid. Her physician explained her underlying eczema and what could be done to prevent rashes.
1. Saripalli YV, Gadzia JE, Belsito DV. Tacrolimus ointment 0.1% in the treatment of nickel-induced allergic contact dermatitis. J Am Acad Dermatol 2003;49:477-482.
A 43-year-old African-American woman was seen for a rash on her right hand and neck. The rash appeared 2 weeks earlier, and was itchy. The patient had been applying alcohol directly to the affected skin.
The patient said she had experienced rashes on and off during her adult life. She has not worn necklaces for years because they irritate the skin around her neck. She also cannot wear wool clothing or any shirts with bright colors. She had not discussed this with a physician before, and she did not know why her skin is so sensitive.
The patient was not taking any new medications, using new bath products, or wearing new clothing before the rash appeared. However, she did remember getting new perfume 2 weeks earlier. She did not have asthma, but did have a mild hay fever. She was adopted and therefore did not know her biologic family’s medical history.
On close inspection, prominent erythema was seen around her neck and on the dorsum of her right hand (Figures 1 and 2).
FIGURE 1
Itchy rash on the neck…
FIGURE 2
…and the right hand
What is the diagnosis?
What are the management options?
Diagnosis: contact dermatitis
The patient demonstrated how she applied perfume to her neck using the back of her right hand. She has acute contact dermatitis on top of previously undiagnosed eczema. Her history is positive for contact dermatitis in reaction to metal (probably nickel) in necklaces and to various fabrics.
Contact dermatitis is a common inflammatory skin condition in which erythematous and pruritic skin lesions result from contact with a foreign substance. The term describes 2 diagnostic categories—allergic contact dermatitis and irritant contact dermatitis. In this case, the chemicals in the perfume may have acted as irritants. The alcohol she used to treat the rash was probably an irritant as well.
A hypersensitivity reaction to irritants and allergens
The mechanism of allergic contact dermatitis is a delayed hypersensitivity reaction. A foreign substance comes into contact with skin and links to skin protein, forming an antigen complex recognized by the immune system. In the skin, the major antigen-presenting cell is the Langerhans cell. When the epidermis is re-exposed to the antigen, the sensitized T-cells initiate an inflammatory cascade leading to changes in the skin. The reaction generally occurs within 12 to 48 hours in a sensitized person. The differential diagnoses for this patient include atopic dermatitis, psoriasis, and fungal infection.
Cosmetics, perfumes, and chemicals in cleaning solutions are known to cause irritant contact dermatitis. The most typical locations are hands or feet, with the dorsum affected more often than the palm or the sole. The lesions are usually erythematous and not well demarcated. The lesions may be macular, papular, or vesicular.
Allergic contact dermatitis often occurs after contact with allergens such as nickel in jewelry, or resins in plants such as poison ivy or poison oak. Vesicles may appear, and the hands are most commonly affected. The lesions may form a linear pattern, as in the case of plant dermatitis.
Evaluation: tests usually not needed
A diagnosis of contact dermatitis usually requires nothing more than findings in the patient’s history and physical exam. If a fungal infection is suspected, use a potassium hydroxide (KOH) preparation to look for hyphae or spores (see page 854 in this issue). Patch testing—placing common antigens on the patient’s skin and observing for reactions—is used to confirm the diagnosis of contact dermatitis and to determine the offending agent.
In this case, neither test was necessary or warranted because the history and physical exam led to a clear diagnosis that was treatable without having to analyze the chemicals in the perfume.
Treatment: remove the irritant, stop the swelling
Whether this is irritant or allergic contact dermatitis, the first line of treatment is to stop further exposure to the offending substance. A mid- to high-potency topical corticosteroid is recommended to stopping the inflammatory process (level of evidence [LOE]=5). (For an explanation of evidence-based medicine ratings, see page 000.) Ointments are more potent and particularly useful when the affected skin is dry and scaly.
Oral steroids are often used for severe local dermatitis, such as that caused by poison ivy (LOE=5). Topical tacrolimus ointment 0.1% is a more expensive option for the treatment of allergic contact dermatitis induced by nickel (LOE=2b).1 Oral H1 blocking sedating anti-histamines (such as diphenhydramine and hydroxyzine) provide relief from itching and help with sleep. Sedating antihistamines may be more effective for itching, but are probably inadvisable for persons who must drive or operate machinery. Newer nonsedating anti-histamines are an option when sedating agents should be avoided.
Conclusion of visit
The patient was given 0.1% triamcinolone ointment (a generic mid-potency steroid) to apply 2 to 3 times a day to the affected areas. She did not want any oral medications for the itching, but understood that diphenhydramine is available without a prescription if needed. She planned to give the perfume away and understood it was now on her list of items to avoid. Her physician explained her underlying eczema and what could be done to prevent rashes.
A 43-year-old African-American woman was seen for a rash on her right hand and neck. The rash appeared 2 weeks earlier, and was itchy. The patient had been applying alcohol directly to the affected skin.
The patient said she had experienced rashes on and off during her adult life. She has not worn necklaces for years because they irritate the skin around her neck. She also cannot wear wool clothing or any shirts with bright colors. She had not discussed this with a physician before, and she did not know why her skin is so sensitive.
The patient was not taking any new medications, using new bath products, or wearing new clothing before the rash appeared. However, she did remember getting new perfume 2 weeks earlier. She did not have asthma, but did have a mild hay fever. She was adopted and therefore did not know her biologic family’s medical history.
On close inspection, prominent erythema was seen around her neck and on the dorsum of her right hand (Figures 1 and 2).
FIGURE 1
Itchy rash on the neck…
FIGURE 2
…and the right hand
What is the diagnosis?
What are the management options?
Diagnosis: contact dermatitis
The patient demonstrated how she applied perfume to her neck using the back of her right hand. She has acute contact dermatitis on top of previously undiagnosed eczema. Her history is positive for contact dermatitis in reaction to metal (probably nickel) in necklaces and to various fabrics.
Contact dermatitis is a common inflammatory skin condition in which erythematous and pruritic skin lesions result from contact with a foreign substance. The term describes 2 diagnostic categories—allergic contact dermatitis and irritant contact dermatitis. In this case, the chemicals in the perfume may have acted as irritants. The alcohol she used to treat the rash was probably an irritant as well.
A hypersensitivity reaction to irritants and allergens
The mechanism of allergic contact dermatitis is a delayed hypersensitivity reaction. A foreign substance comes into contact with skin and links to skin protein, forming an antigen complex recognized by the immune system. In the skin, the major antigen-presenting cell is the Langerhans cell. When the epidermis is re-exposed to the antigen, the sensitized T-cells initiate an inflammatory cascade leading to changes in the skin. The reaction generally occurs within 12 to 48 hours in a sensitized person. The differential diagnoses for this patient include atopic dermatitis, psoriasis, and fungal infection.
Cosmetics, perfumes, and chemicals in cleaning solutions are known to cause irritant contact dermatitis. The most typical locations are hands or feet, with the dorsum affected more often than the palm or the sole. The lesions are usually erythematous and not well demarcated. The lesions may be macular, papular, or vesicular.
Allergic contact dermatitis often occurs after contact with allergens such as nickel in jewelry, or resins in plants such as poison ivy or poison oak. Vesicles may appear, and the hands are most commonly affected. The lesions may form a linear pattern, as in the case of plant dermatitis.
Evaluation: tests usually not needed
A diagnosis of contact dermatitis usually requires nothing more than findings in the patient’s history and physical exam. If a fungal infection is suspected, use a potassium hydroxide (KOH) preparation to look for hyphae or spores (see page 854 in this issue). Patch testing—placing common antigens on the patient’s skin and observing for reactions—is used to confirm the diagnosis of contact dermatitis and to determine the offending agent.
In this case, neither test was necessary or warranted because the history and physical exam led to a clear diagnosis that was treatable without having to analyze the chemicals in the perfume.
Treatment: remove the irritant, stop the swelling
Whether this is irritant or allergic contact dermatitis, the first line of treatment is to stop further exposure to the offending substance. A mid- to high-potency topical corticosteroid is recommended to stopping the inflammatory process (level of evidence [LOE]=5). (For an explanation of evidence-based medicine ratings, see page 000.) Ointments are more potent and particularly useful when the affected skin is dry and scaly.
Oral steroids are often used for severe local dermatitis, such as that caused by poison ivy (LOE=5). Topical tacrolimus ointment 0.1% is a more expensive option for the treatment of allergic contact dermatitis induced by nickel (LOE=2b).1 Oral H1 blocking sedating anti-histamines (such as diphenhydramine and hydroxyzine) provide relief from itching and help with sleep. Sedating antihistamines may be more effective for itching, but are probably inadvisable for persons who must drive or operate machinery. Newer nonsedating anti-histamines are an option when sedating agents should be avoided.
Conclusion of visit
The patient was given 0.1% triamcinolone ointment (a generic mid-potency steroid) to apply 2 to 3 times a day to the affected areas. She did not want any oral medications for the itching, but understood that diphenhydramine is available without a prescription if needed. She planned to give the perfume away and understood it was now on her list of items to avoid. Her physician explained her underlying eczema and what could be done to prevent rashes.
1. Saripalli YV, Gadzia JE, Belsito DV. Tacrolimus ointment 0.1% in the treatment of nickel-induced allergic contact dermatitis. J Am Acad Dermatol 2003;49:477-482.
1. Saripalli YV, Gadzia JE, Belsito DV. Tacrolimus ointment 0.1% in the treatment of nickel-induced allergic contact dermatitis. J Am Acad Dermatol 2003;49:477-482.
Painful, swollen lower legs
A 25-year-old female came to the clinic reporting a 1-day history of painful red nodules on her lower legs. She also said that her lower legs felt swollen (Figure 1). She had just started taking mefloquine 2 days before, for malaria prophylaxis prior to anticipated travel with the United States military. She had been seen in the clinic about 1 week before for fever and general joint aches, and she was diagnosed with a probable viral syndrome from which she had completely recovered. She reported no other symptoms.
She started taking sertraline for depression several weeks prior to the onset of her symptoms. She was taking no other medications and had no other medical or surgical history.
Although she had been in the Horn of Africa region, she had not gone ashore before the onset of her symptoms. She had also not traveled recently to any other foreign country.
Upon examination, the patient had tender erythematous nodular areas of varying sizes and irregular borders on both shins ( Figure 2). The lesions had no scales or other noteworthy epidermal changes. Her calves and ankles were symmetrically swollen, though without pitting edema. The rest of her examination was unremarkable. The patient was on active duty and had received a variety of immunizations and screening testing in the preceding 12 months, including recent human immunodeficiency virus and tuberculosis testing, which proved negative.
FIGURE 1
Swollen lower legs
FIGURE 2
Erythematous nodules
What is the diagnosis?
WHAT ARE THE MANAGEMENT OPTIONS?
Diagnosis: erythema nodosum, a hypersensitivity reaction
The patient’s presentation and clinical findings suggest erythema nodosum. Erythema nodosum is one of several hypersensitivity syndromes. It is likely a delayed hypersensitivity reaction and may be triggered by a number of antigens.
Painful, erythematous nodules on both shins are characteristic of erythema nodosum, though similar lesions occur on other extensor surfaces.1Panniculitis is often used to describe this condition, as pathologic evaluation demonstrates inflammation within the subcutaneous fat.
The nodules generally resolve over several weeks with possible desquamation on the lesion’s surface. Patients may have a prodrome of fever, arthralgias, and often symptoms of an upper respiratory infection occurring 2 to 8 weeks before the eruptive phase.1,2
Erythema nodosum may occur as a result of bacterial and fungal infections. Many medications have also been known to cause erythema nodosum, with sulfa drugs and oral contraceptives among the most common. The condition may also herald systemic disease, such as sarcoidosis or inflammatory bowel disease.
The photographs show the characteristic erythematous eruptive phase in its early stage. The lesions progress from tender erythematous areas with a nodular texture to become yellowish-purple and bruiselike. The lesions generally resolve over several weeks.
Approximately 50% of cases are idiopathic. Erythema nodosum can affect persons at any age but appears most often in those in their twenties and thirties. It affects women 3 to 6 times more frequently than men.1,2
Differential diagnosis and laboratory investigations
The differential diagnosis includes erythema multiforme, Hodgkin’s disease, Sweet’s syndrome, tuberculosis, sarcoidosis, and streptococcal infection.1,2
Erythema multiforme most often forms a characteristic target lesion. It can also present as an urticarial lesion or in a vesiculobullous form. While patients with a history of Hodgkin’s disease may exhibit erythema nodosum heralding an impending relapse, it is not generally a sign of primary disease.
Sweet’s syndrome (acute febrile neutrophilic dermatosis) is a reactive disorder generally considered to be a dermatologic manifestation of a systemic disease. In many cases an underlying systemic disease is discovered, such as myelodysplastic syndrome, nonlymphocytic leukemia, or inflammatory bowel disease. The mean age at presentation is about 56 years, with lesions well demarcated and more widespread than those of erythema nodosum.
Tuberculosis and streptococcal infections are 2 of the most common causes of erythema nodosum in children. In adults, erythema nodosum may result from streptococcal infection or sarcoidosis. Tuberculin skin testing, chest radiography, throat culture/rapid streptococcal antigen testing, and an antistreptolysin O titer should be included in a patient’s evaluation.
Treatment: disease is self-limited
Erythema nodosum is usually a self-limited disease. It may help to remove the offending medicine or allergen, if identified. Nonsteroidal anti-inflammatory drugs (NSAIDs) usually relieve symptoms.
In more severe or recurrent cases, potassium iodide 360–900 mg/d may be helpful, but the best effect is seen when it is used early in the course of the condition (level of evidence: 4).3
Systemic corticosteroids may help in the short term, but erythema nodosum may recur after discontinuing the medication. In addition, if there is an infectious cause, the use of corticosteroids may exacerbate the infection.
Conclusion: pain and swelling resolved
The patient began taking ibuprofen prior to the office visit and had achieved good pain control. This treatment was continued and the patient’s nodules resolved after approximately 4 weeks. Her leg swelling resolved 2 to 3 weeks after the clearance of her nodules. She resumed both the sertraline and mefloquine without problems.
Correspondence
Donald W. Shenenberger, MD, 1609 Emberhill Court, Chesapeake, VA 23321-1807. E-mail: [email protected].
1. Habif TP. Clinical Dermatology: A Color Guide to Diagnosis and Ttherapy. 3d ed. St. Louis, Mo: Mosby; 1996;566-596.
2. Parker F. Skin diseases of general importance. In: Bennett JC, Plum F, eds. Cecil Textbook of Internal Medicine. 20th ed. Philadelphia, Pa: W. B. Saunders, 1996(2):2211.
3. Schulz EJ, Whiting DA. Treatment of erythema nodosum and nodular vasculitis with potassium iodide. Br J Dermatol 1976;94:75-78.
A 25-year-old female came to the clinic reporting a 1-day history of painful red nodules on her lower legs. She also said that her lower legs felt swollen (Figure 1). She had just started taking mefloquine 2 days before, for malaria prophylaxis prior to anticipated travel with the United States military. She had been seen in the clinic about 1 week before for fever and general joint aches, and she was diagnosed with a probable viral syndrome from which she had completely recovered. She reported no other symptoms.
She started taking sertraline for depression several weeks prior to the onset of her symptoms. She was taking no other medications and had no other medical or surgical history.
Although she had been in the Horn of Africa region, she had not gone ashore before the onset of her symptoms. She had also not traveled recently to any other foreign country.
Upon examination, the patient had tender erythematous nodular areas of varying sizes and irregular borders on both shins ( Figure 2). The lesions had no scales or other noteworthy epidermal changes. Her calves and ankles were symmetrically swollen, though without pitting edema. The rest of her examination was unremarkable. The patient was on active duty and had received a variety of immunizations and screening testing in the preceding 12 months, including recent human immunodeficiency virus and tuberculosis testing, which proved negative.
FIGURE 1
Swollen lower legs
FIGURE 2
Erythematous nodules
What is the diagnosis?
WHAT ARE THE MANAGEMENT OPTIONS?
Diagnosis: erythema nodosum, a hypersensitivity reaction
The patient’s presentation and clinical findings suggest erythema nodosum. Erythema nodosum is one of several hypersensitivity syndromes. It is likely a delayed hypersensitivity reaction and may be triggered by a number of antigens.
Painful, erythematous nodules on both shins are characteristic of erythema nodosum, though similar lesions occur on other extensor surfaces.1Panniculitis is often used to describe this condition, as pathologic evaluation demonstrates inflammation within the subcutaneous fat.
The nodules generally resolve over several weeks with possible desquamation on the lesion’s surface. Patients may have a prodrome of fever, arthralgias, and often symptoms of an upper respiratory infection occurring 2 to 8 weeks before the eruptive phase.1,2
Erythema nodosum may occur as a result of bacterial and fungal infections. Many medications have also been known to cause erythema nodosum, with sulfa drugs and oral contraceptives among the most common. The condition may also herald systemic disease, such as sarcoidosis or inflammatory bowel disease.
The photographs show the characteristic erythematous eruptive phase in its early stage. The lesions progress from tender erythematous areas with a nodular texture to become yellowish-purple and bruiselike. The lesions generally resolve over several weeks.
Approximately 50% of cases are idiopathic. Erythema nodosum can affect persons at any age but appears most often in those in their twenties and thirties. It affects women 3 to 6 times more frequently than men.1,2
Differential diagnosis and laboratory investigations
The differential diagnosis includes erythema multiforme, Hodgkin’s disease, Sweet’s syndrome, tuberculosis, sarcoidosis, and streptococcal infection.1,2
Erythema multiforme most often forms a characteristic target lesion. It can also present as an urticarial lesion or in a vesiculobullous form. While patients with a history of Hodgkin’s disease may exhibit erythema nodosum heralding an impending relapse, it is not generally a sign of primary disease.
Sweet’s syndrome (acute febrile neutrophilic dermatosis) is a reactive disorder generally considered to be a dermatologic manifestation of a systemic disease. In many cases an underlying systemic disease is discovered, such as myelodysplastic syndrome, nonlymphocytic leukemia, or inflammatory bowel disease. The mean age at presentation is about 56 years, with lesions well demarcated and more widespread than those of erythema nodosum.
Tuberculosis and streptococcal infections are 2 of the most common causes of erythema nodosum in children. In adults, erythema nodosum may result from streptococcal infection or sarcoidosis. Tuberculin skin testing, chest radiography, throat culture/rapid streptococcal antigen testing, and an antistreptolysin O titer should be included in a patient’s evaluation.
Treatment: disease is self-limited
Erythema nodosum is usually a self-limited disease. It may help to remove the offending medicine or allergen, if identified. Nonsteroidal anti-inflammatory drugs (NSAIDs) usually relieve symptoms.
In more severe or recurrent cases, potassium iodide 360–900 mg/d may be helpful, but the best effect is seen when it is used early in the course of the condition (level of evidence: 4).3
Systemic corticosteroids may help in the short term, but erythema nodosum may recur after discontinuing the medication. In addition, if there is an infectious cause, the use of corticosteroids may exacerbate the infection.
Conclusion: pain and swelling resolved
The patient began taking ibuprofen prior to the office visit and had achieved good pain control. This treatment was continued and the patient’s nodules resolved after approximately 4 weeks. Her leg swelling resolved 2 to 3 weeks after the clearance of her nodules. She resumed both the sertraline and mefloquine without problems.
Correspondence
Donald W. Shenenberger, MD, 1609 Emberhill Court, Chesapeake, VA 23321-1807. E-mail: [email protected].
A 25-year-old female came to the clinic reporting a 1-day history of painful red nodules on her lower legs. She also said that her lower legs felt swollen (Figure 1). She had just started taking mefloquine 2 days before, for malaria prophylaxis prior to anticipated travel with the United States military. She had been seen in the clinic about 1 week before for fever and general joint aches, and she was diagnosed with a probable viral syndrome from which she had completely recovered. She reported no other symptoms.
She started taking sertraline for depression several weeks prior to the onset of her symptoms. She was taking no other medications and had no other medical or surgical history.
Although she had been in the Horn of Africa region, she had not gone ashore before the onset of her symptoms. She had also not traveled recently to any other foreign country.
Upon examination, the patient had tender erythematous nodular areas of varying sizes and irregular borders on both shins ( Figure 2). The lesions had no scales or other noteworthy epidermal changes. Her calves and ankles were symmetrically swollen, though without pitting edema. The rest of her examination was unremarkable. The patient was on active duty and had received a variety of immunizations and screening testing in the preceding 12 months, including recent human immunodeficiency virus and tuberculosis testing, which proved negative.
FIGURE 1
Swollen lower legs
FIGURE 2
Erythematous nodules
What is the diagnosis?
WHAT ARE THE MANAGEMENT OPTIONS?
Diagnosis: erythema nodosum, a hypersensitivity reaction
The patient’s presentation and clinical findings suggest erythema nodosum. Erythema nodosum is one of several hypersensitivity syndromes. It is likely a delayed hypersensitivity reaction and may be triggered by a number of antigens.
Painful, erythematous nodules on both shins are characteristic of erythema nodosum, though similar lesions occur on other extensor surfaces.1Panniculitis is often used to describe this condition, as pathologic evaluation demonstrates inflammation within the subcutaneous fat.
The nodules generally resolve over several weeks with possible desquamation on the lesion’s surface. Patients may have a prodrome of fever, arthralgias, and often symptoms of an upper respiratory infection occurring 2 to 8 weeks before the eruptive phase.1,2
Erythema nodosum may occur as a result of bacterial and fungal infections. Many medications have also been known to cause erythema nodosum, with sulfa drugs and oral contraceptives among the most common. The condition may also herald systemic disease, such as sarcoidosis or inflammatory bowel disease.
The photographs show the characteristic erythematous eruptive phase in its early stage. The lesions progress from tender erythematous areas with a nodular texture to become yellowish-purple and bruiselike. The lesions generally resolve over several weeks.
Approximately 50% of cases are idiopathic. Erythema nodosum can affect persons at any age but appears most often in those in their twenties and thirties. It affects women 3 to 6 times more frequently than men.1,2
Differential diagnosis and laboratory investigations
The differential diagnosis includes erythema multiforme, Hodgkin’s disease, Sweet’s syndrome, tuberculosis, sarcoidosis, and streptococcal infection.1,2
Erythema multiforme most often forms a characteristic target lesion. It can also present as an urticarial lesion or in a vesiculobullous form. While patients with a history of Hodgkin’s disease may exhibit erythema nodosum heralding an impending relapse, it is not generally a sign of primary disease.
Sweet’s syndrome (acute febrile neutrophilic dermatosis) is a reactive disorder generally considered to be a dermatologic manifestation of a systemic disease. In many cases an underlying systemic disease is discovered, such as myelodysplastic syndrome, nonlymphocytic leukemia, or inflammatory bowel disease. The mean age at presentation is about 56 years, with lesions well demarcated and more widespread than those of erythema nodosum.
Tuberculosis and streptococcal infections are 2 of the most common causes of erythema nodosum in children. In adults, erythema nodosum may result from streptococcal infection or sarcoidosis. Tuberculin skin testing, chest radiography, throat culture/rapid streptococcal antigen testing, and an antistreptolysin O titer should be included in a patient’s evaluation.
Treatment: disease is self-limited
Erythema nodosum is usually a self-limited disease. It may help to remove the offending medicine or allergen, if identified. Nonsteroidal anti-inflammatory drugs (NSAIDs) usually relieve symptoms.
In more severe or recurrent cases, potassium iodide 360–900 mg/d may be helpful, but the best effect is seen when it is used early in the course of the condition (level of evidence: 4).3
Systemic corticosteroids may help in the short term, but erythema nodosum may recur after discontinuing the medication. In addition, if there is an infectious cause, the use of corticosteroids may exacerbate the infection.
Conclusion: pain and swelling resolved
The patient began taking ibuprofen prior to the office visit and had achieved good pain control. This treatment was continued and the patient’s nodules resolved after approximately 4 weeks. Her leg swelling resolved 2 to 3 weeks after the clearance of her nodules. She resumed both the sertraline and mefloquine without problems.
Correspondence
Donald W. Shenenberger, MD, 1609 Emberhill Court, Chesapeake, VA 23321-1807. E-mail: [email protected].
1. Habif TP. Clinical Dermatology: A Color Guide to Diagnosis and Ttherapy. 3d ed. St. Louis, Mo: Mosby; 1996;566-596.
2. Parker F. Skin diseases of general importance. In: Bennett JC, Plum F, eds. Cecil Textbook of Internal Medicine. 20th ed. Philadelphia, Pa: W. B. Saunders, 1996(2):2211.
3. Schulz EJ, Whiting DA. Treatment of erythema nodosum and nodular vasculitis with potassium iodide. Br J Dermatol 1976;94:75-78.
1. Habif TP. Clinical Dermatology: A Color Guide to Diagnosis and Ttherapy. 3d ed. St. Louis, Mo: Mosby; 1996;566-596.
2. Parker F. Skin diseases of general importance. In: Bennett JC, Plum F, eds. Cecil Textbook of Internal Medicine. 20th ed. Philadelphia, Pa: W. B. Saunders, 1996(2):2211.
3. Schulz EJ, Whiting DA. Treatment of erythema nodosum and nodular vasculitis with potassium iodide. Br J Dermatol 1976;94:75-78.
A red rash on the face
A 28-year-old man came to the office with a rash on his face. He reported he has had this rash on and off for 3 years. The rash sometimes itches around his moustache, and it gets worse with stress—he has been under increasing stress for the past 2 months. He is in good health and does not have any other symptoms, and he denies any risk factors for human immunodeficiency virus (HIV).
Physical examination reveals erythema and scale across his eyebrows, cheeks, and near his moustache. On close inspection, the scale is visible under his moustache and eyebrows as well.
FIGURE 1
Erythema on the face
FIGURE 2
Close-up of erythema
What is the diagnosis?
What are the management options?
DIAGNOSIS
This young man has seborrhea (seborrheic dermatitis), a superficial inflammatory dermatitis. It is a common condition characterized by patches of erythema and scaling, usually on the scalp (ie, dandruff), eyebrows, nasolabial creases, forehead, cheeks, around the nose, behind the ears, and under facial hair. Seborrhea can also occur over the sternum and in the axillae, submammary folds, umbilicus, groin, and gluteal creases. These areas are regions with a greater number of pilosebaceous units, which produce sebum.
Seborrhea is thought to be caused by an inflammatory hypersensitivity to epidermal, bacterial, or yeast antigens. Persons with seborrhea have a profusion of Pityrosporum yeast on the skin. This yeast can be a normal part of skin flora; seborrhea is an inflammatory reaction to its presence. Seborrhea is characterized by remissions and exacerbations. The most common precipitating factors are stress, immuno suppression, and cold weather. The treatment of seborrhea should be directed at the inflammation and the Pityrosporum.
Epidemiology
Seborrhea is most commonly seen in patients aged 20 and 50 years, and mostly in males. The prevalence of seborrhea is approximately 3% to 5% in young adults who are HIV-negative. The prevalence of seborrhea is as high as 36% in HIV-positive persons, although the vast majority of persons with seborrhea have a normal immune system. No lab tests are required to make the diagnosis.
Treatment: antifungals and topical steroids
Treat seborrhea with a 2-pronged plan: antifungal agents for the yeast and topical corticosteroids for the inflammation. The antifungals can also be used to prevent exacerbations; steroids should only be applied to active areas of inflammation.
Fortunately, seborrhea on the face responds well to low-potency topical corticosteroids, such as 1% hydrocortisone cream or lotion. Still, corticosteroids should not be used on the face for prolonged periods to avoid skin atrophy and other side effects. The lotion is better for hair-covered areas as it is less messy to apply than a cream.
Antifungal shampoos
Over-the-counter dandruff shampoos have been the mainstay of therapy for seborrhea of the scalp. These products often contain selenium (ie, Selsun Blue) or zinc (ie, Head and Shoulders), both of which are toxic to Pityrosporum. Often patients have both seborrhea on the scalp and the face, and using these shampoos can cut down the amount of Pityrosporumon both. Instruct patients with facial hair to lather their beards and moustache with shampoo as well.
Both ketoconazole (Nizoral) 2% shampoo and selenium sulfide 2.5% shampoo are effective in the treatment of moderate to severe dandruff (level of evidence [LOE]: 1b).1 Ketoconazole 2% shampoo is highly effective not only for clearing seborrheic dermatitis on the scalp but also for preventing relapse when used prophylactically once weekly (LOE: 1b).2 Ketoconazole has become available in a 1% over-the-counter dandruff shampoo, but the 2% shampoo still requires a prescription.
Treating severe cases
When seborrhea of the scalp becomes more severe, add a higher-potency steroid solution or lotion to the treatment until the exacerbation is under control. Ketoconazole cream is also a good treatment for seborrheic dermatitis in areas other than the scalp. Other antifungal creams such as miconazole can be used to treat seborrhea of the face.
One trial demonstrated the effectiveness of topical 1% metronidazole gel in seborrheic dermatitis (LOE: 1b). At the 8-week follow-up, 14 patients in the metronidazole group showed a marked to complete improvement compared with 2 in the placebo group (P<.001; number needed to treat=2).3 This is not an approved indication for metronidazole gel, but it may be considered when other topical medications fail. In a randomized controlled trial using crossover design, treatment with a low-dose homeopathic preparation provided significant improvement in seborrheic dermatitis and dandruff after 10 weeks of dosing (LOE: 2b).4
Most seborrheic dermatitis is fully treatable with topical agents. When topical medications are not providing adequate results, oral antifungal agents may be considered. In 1 study, oral terbinafine was found to be effective in the treatment of moderate to severe seborrheic dermatitis. Clinical improvement following 4 weeks treatment with terbinafine was maintained 8 weeks after completing treatment (LOE: 1b).5
Conclusion of visit
The patient was given a prescription for 1% hydrocortisone lotion and 2% ketoconazole cream, both to be applied twice daily to the affected areas. He also planned to investigate the homeopathy option for the future. It was explained to the patient that these treatments may not be curative, and seborrhea may come back when he is under stress.
1. Danby FW, Maddin WS, Margesson LJ, Rosenthal D. A randomized, double-blind, placebo-controlled trial of ketoconazole 2% shampoo versus selenium sulfide 2.5% shampoo in the treatment of moderate to severe dandruff. J Am Acad Dermatol 1993;29:1008-1012.
2. Peter RU, Richarz-Barthauer U. Successful treatment and prophylaxis of scalp seborrhoeic dermatitis and dandruff with 2% ketoconazole shampoo: results of a multicentre, double-blind, placebo-controlled trial. Br J Dermatol 1995;132:441-445.
3. Parsad D, Pandhi R, Negi KS, Kumar B. Topical metronidazole in seborrheic dermatitis—a double-blind study. Dermatology 2001;202:35-37.
4. Smith SA, Baker AE, Williams JH. Effective treatment of seborrheic dermatitis using a low dose, oral homeopathic medication consisting of potassium bromide, sodium bromide, nickel sulfate, and sodium chloride in a double-blind, placebo-controlled study. Altern Med Rev 2002;7:59-67.
5. Scaparro E, Quadri G, Virno G, Orifici C, Milani M. Evaluation of the efficacy and tolerability of oral terbinafine (Daskil) in patients with seborrhoeic dermatitis. A multicentre, randomized, investigator-blinded, placebo-controlled trial. Br J Dermatol 2001;144:854-857.
A 28-year-old man came to the office with a rash on his face. He reported he has had this rash on and off for 3 years. The rash sometimes itches around his moustache, and it gets worse with stress—he has been under increasing stress for the past 2 months. He is in good health and does not have any other symptoms, and he denies any risk factors for human immunodeficiency virus (HIV).
Physical examination reveals erythema and scale across his eyebrows, cheeks, and near his moustache. On close inspection, the scale is visible under his moustache and eyebrows as well.
FIGURE 1
Erythema on the face
FIGURE 2
Close-up of erythema
What is the diagnosis?
What are the management options?
DIAGNOSIS
This young man has seborrhea (seborrheic dermatitis), a superficial inflammatory dermatitis. It is a common condition characterized by patches of erythema and scaling, usually on the scalp (ie, dandruff), eyebrows, nasolabial creases, forehead, cheeks, around the nose, behind the ears, and under facial hair. Seborrhea can also occur over the sternum and in the axillae, submammary folds, umbilicus, groin, and gluteal creases. These areas are regions with a greater number of pilosebaceous units, which produce sebum.
Seborrhea is thought to be caused by an inflammatory hypersensitivity to epidermal, bacterial, or yeast antigens. Persons with seborrhea have a profusion of Pityrosporum yeast on the skin. This yeast can be a normal part of skin flora; seborrhea is an inflammatory reaction to its presence. Seborrhea is characterized by remissions and exacerbations. The most common precipitating factors are stress, immuno suppression, and cold weather. The treatment of seborrhea should be directed at the inflammation and the Pityrosporum.
Epidemiology
Seborrhea is most commonly seen in patients aged 20 and 50 years, and mostly in males. The prevalence of seborrhea is approximately 3% to 5% in young adults who are HIV-negative. The prevalence of seborrhea is as high as 36% in HIV-positive persons, although the vast majority of persons with seborrhea have a normal immune system. No lab tests are required to make the diagnosis.
Treatment: antifungals and topical steroids
Treat seborrhea with a 2-pronged plan: antifungal agents for the yeast and topical corticosteroids for the inflammation. The antifungals can also be used to prevent exacerbations; steroids should only be applied to active areas of inflammation.
Fortunately, seborrhea on the face responds well to low-potency topical corticosteroids, such as 1% hydrocortisone cream or lotion. Still, corticosteroids should not be used on the face for prolonged periods to avoid skin atrophy and other side effects. The lotion is better for hair-covered areas as it is less messy to apply than a cream.
Antifungal shampoos
Over-the-counter dandruff shampoos have been the mainstay of therapy for seborrhea of the scalp. These products often contain selenium (ie, Selsun Blue) or zinc (ie, Head and Shoulders), both of which are toxic to Pityrosporum. Often patients have both seborrhea on the scalp and the face, and using these shampoos can cut down the amount of Pityrosporumon both. Instruct patients with facial hair to lather their beards and moustache with shampoo as well.
Both ketoconazole (Nizoral) 2% shampoo and selenium sulfide 2.5% shampoo are effective in the treatment of moderate to severe dandruff (level of evidence [LOE]: 1b).1 Ketoconazole 2% shampoo is highly effective not only for clearing seborrheic dermatitis on the scalp but also for preventing relapse when used prophylactically once weekly (LOE: 1b).2 Ketoconazole has become available in a 1% over-the-counter dandruff shampoo, but the 2% shampoo still requires a prescription.
Treating severe cases
When seborrhea of the scalp becomes more severe, add a higher-potency steroid solution or lotion to the treatment until the exacerbation is under control. Ketoconazole cream is also a good treatment for seborrheic dermatitis in areas other than the scalp. Other antifungal creams such as miconazole can be used to treat seborrhea of the face.
One trial demonstrated the effectiveness of topical 1% metronidazole gel in seborrheic dermatitis (LOE: 1b). At the 8-week follow-up, 14 patients in the metronidazole group showed a marked to complete improvement compared with 2 in the placebo group (P<.001; number needed to treat=2).3 This is not an approved indication for metronidazole gel, but it may be considered when other topical medications fail. In a randomized controlled trial using crossover design, treatment with a low-dose homeopathic preparation provided significant improvement in seborrheic dermatitis and dandruff after 10 weeks of dosing (LOE: 2b).4
Most seborrheic dermatitis is fully treatable with topical agents. When topical medications are not providing adequate results, oral antifungal agents may be considered. In 1 study, oral terbinafine was found to be effective in the treatment of moderate to severe seborrheic dermatitis. Clinical improvement following 4 weeks treatment with terbinafine was maintained 8 weeks after completing treatment (LOE: 1b).5
Conclusion of visit
The patient was given a prescription for 1% hydrocortisone lotion and 2% ketoconazole cream, both to be applied twice daily to the affected areas. He also planned to investigate the homeopathy option for the future. It was explained to the patient that these treatments may not be curative, and seborrhea may come back when he is under stress.
A 28-year-old man came to the office with a rash on his face. He reported he has had this rash on and off for 3 years. The rash sometimes itches around his moustache, and it gets worse with stress—he has been under increasing stress for the past 2 months. He is in good health and does not have any other symptoms, and he denies any risk factors for human immunodeficiency virus (HIV).
Physical examination reveals erythema and scale across his eyebrows, cheeks, and near his moustache. On close inspection, the scale is visible under his moustache and eyebrows as well.
FIGURE 1
Erythema on the face
FIGURE 2
Close-up of erythema
What is the diagnosis?
What are the management options?
DIAGNOSIS
This young man has seborrhea (seborrheic dermatitis), a superficial inflammatory dermatitis. It is a common condition characterized by patches of erythema and scaling, usually on the scalp (ie, dandruff), eyebrows, nasolabial creases, forehead, cheeks, around the nose, behind the ears, and under facial hair. Seborrhea can also occur over the sternum and in the axillae, submammary folds, umbilicus, groin, and gluteal creases. These areas are regions with a greater number of pilosebaceous units, which produce sebum.
Seborrhea is thought to be caused by an inflammatory hypersensitivity to epidermal, bacterial, or yeast antigens. Persons with seborrhea have a profusion of Pityrosporum yeast on the skin. This yeast can be a normal part of skin flora; seborrhea is an inflammatory reaction to its presence. Seborrhea is characterized by remissions and exacerbations. The most common precipitating factors are stress, immuno suppression, and cold weather. The treatment of seborrhea should be directed at the inflammation and the Pityrosporum.
Epidemiology
Seborrhea is most commonly seen in patients aged 20 and 50 years, and mostly in males. The prevalence of seborrhea is approximately 3% to 5% in young adults who are HIV-negative. The prevalence of seborrhea is as high as 36% in HIV-positive persons, although the vast majority of persons with seborrhea have a normal immune system. No lab tests are required to make the diagnosis.
Treatment: antifungals and topical steroids
Treat seborrhea with a 2-pronged plan: antifungal agents for the yeast and topical corticosteroids for the inflammation. The antifungals can also be used to prevent exacerbations; steroids should only be applied to active areas of inflammation.
Fortunately, seborrhea on the face responds well to low-potency topical corticosteroids, such as 1% hydrocortisone cream or lotion. Still, corticosteroids should not be used on the face for prolonged periods to avoid skin atrophy and other side effects. The lotion is better for hair-covered areas as it is less messy to apply than a cream.
Antifungal shampoos
Over-the-counter dandruff shampoos have been the mainstay of therapy for seborrhea of the scalp. These products often contain selenium (ie, Selsun Blue) or zinc (ie, Head and Shoulders), both of which are toxic to Pityrosporum. Often patients have both seborrhea on the scalp and the face, and using these shampoos can cut down the amount of Pityrosporumon both. Instruct patients with facial hair to lather their beards and moustache with shampoo as well.
Both ketoconazole (Nizoral) 2% shampoo and selenium sulfide 2.5% shampoo are effective in the treatment of moderate to severe dandruff (level of evidence [LOE]: 1b).1 Ketoconazole 2% shampoo is highly effective not only for clearing seborrheic dermatitis on the scalp but also for preventing relapse when used prophylactically once weekly (LOE: 1b).2 Ketoconazole has become available in a 1% over-the-counter dandruff shampoo, but the 2% shampoo still requires a prescription.
Treating severe cases
When seborrhea of the scalp becomes more severe, add a higher-potency steroid solution or lotion to the treatment until the exacerbation is under control. Ketoconazole cream is also a good treatment for seborrheic dermatitis in areas other than the scalp. Other antifungal creams such as miconazole can be used to treat seborrhea of the face.
One trial demonstrated the effectiveness of topical 1% metronidazole gel in seborrheic dermatitis (LOE: 1b). At the 8-week follow-up, 14 patients in the metronidazole group showed a marked to complete improvement compared with 2 in the placebo group (P<.001; number needed to treat=2).3 This is not an approved indication for metronidazole gel, but it may be considered when other topical medications fail. In a randomized controlled trial using crossover design, treatment with a low-dose homeopathic preparation provided significant improvement in seborrheic dermatitis and dandruff after 10 weeks of dosing (LOE: 2b).4
Most seborrheic dermatitis is fully treatable with topical agents. When topical medications are not providing adequate results, oral antifungal agents may be considered. In 1 study, oral terbinafine was found to be effective in the treatment of moderate to severe seborrheic dermatitis. Clinical improvement following 4 weeks treatment with terbinafine was maintained 8 weeks after completing treatment (LOE: 1b).5
Conclusion of visit
The patient was given a prescription for 1% hydrocortisone lotion and 2% ketoconazole cream, both to be applied twice daily to the affected areas. He also planned to investigate the homeopathy option for the future. It was explained to the patient that these treatments may not be curative, and seborrhea may come back when he is under stress.
1. Danby FW, Maddin WS, Margesson LJ, Rosenthal D. A randomized, double-blind, placebo-controlled trial of ketoconazole 2% shampoo versus selenium sulfide 2.5% shampoo in the treatment of moderate to severe dandruff. J Am Acad Dermatol 1993;29:1008-1012.
2. Peter RU, Richarz-Barthauer U. Successful treatment and prophylaxis of scalp seborrhoeic dermatitis and dandruff with 2% ketoconazole shampoo: results of a multicentre, double-blind, placebo-controlled trial. Br J Dermatol 1995;132:441-445.
3. Parsad D, Pandhi R, Negi KS, Kumar B. Topical metronidazole in seborrheic dermatitis—a double-blind study. Dermatology 2001;202:35-37.
4. Smith SA, Baker AE, Williams JH. Effective treatment of seborrheic dermatitis using a low dose, oral homeopathic medication consisting of potassium bromide, sodium bromide, nickel sulfate, and sodium chloride in a double-blind, placebo-controlled study. Altern Med Rev 2002;7:59-67.
5. Scaparro E, Quadri G, Virno G, Orifici C, Milani M. Evaluation of the efficacy and tolerability of oral terbinafine (Daskil) in patients with seborrhoeic dermatitis. A multicentre, randomized, investigator-blinded, placebo-controlled trial. Br J Dermatol 2001;144:854-857.
1. Danby FW, Maddin WS, Margesson LJ, Rosenthal D. A randomized, double-blind, placebo-controlled trial of ketoconazole 2% shampoo versus selenium sulfide 2.5% shampoo in the treatment of moderate to severe dandruff. J Am Acad Dermatol 1993;29:1008-1012.
2. Peter RU, Richarz-Barthauer U. Successful treatment and prophylaxis of scalp seborrhoeic dermatitis and dandruff with 2% ketoconazole shampoo: results of a multicentre, double-blind, placebo-controlled trial. Br J Dermatol 1995;132:441-445.
3. Parsad D, Pandhi R, Negi KS, Kumar B. Topical metronidazole in seborrheic dermatitis—a double-blind study. Dermatology 2001;202:35-37.
4. Smith SA, Baker AE, Williams JH. Effective treatment of seborrheic dermatitis using a low dose, oral homeopathic medication consisting of potassium bromide, sodium bromide, nickel sulfate, and sodium chloride in a double-blind, placebo-controlled study. Altern Med Rev 2002;7:59-67.
5. Scaparro E, Quadri G, Virno G, Orifici C, Milani M. Evaluation of the efficacy and tolerability of oral terbinafine (Daskil) in patients with seborrhoeic dermatitis. A multicentre, randomized, investigator-blinded, placebo-controlled trial. Br J Dermatol 2001;144:854-857.
Caribbean itch
A 25-year-old man came to the office in July after awakening with a pruritic rash on his chest, back, and abdomen. He reported feeling feverish with chills and malaise, but had no symptoms of respiratory distress and his vital signs were normal. He mentioned that he had gone snorkeling in the Caribbean the previous day, but did not remember being stung by any marine life. He said he was wearing swimming trunks and a t-shirt while in the ocean.
The patient had a diffuse erythematous maculopapular rash on his neck, chest, back, abdomen, shoulders, and axillae (Figure 1 and Figure 2).
FIGURE 1
Rash on abdomen
FIGURE 2
Macules
What is the most likely diagnosis?
- Listen to local beach reports and observe posted beach messages in affected areas.
- Avoid ocean activities in areas where cases have been reported, especially during the peak season, April through July.
- Avoid wearing t-shirts while swimming. Women should consider wearing 2-piece bathing suits.These measures decrease the surface area over which larvae can be trapped. (Of course, swimmers should use potent sunscreens and carefully limit sun exposure to avoid sunburns when more skin is exposed to the sun. Some evidence suggests that the use of a sunscreen may actually protect skin from penetration by the nematocysts.)8
- Wetsuits with restrictive cuffs may be of benefit when surfing or diving.
- When swimming in areas where recent cases have occurred, change swimwear quickly after exiting the water. Do not shower with fresh water while still wearing the suit, because this may cause nematocysts trapped inside to discharge.Wash swimwear with detergent and heat-dry before wearing again. Air-dried nematocysts may still be able to fire.4,8
Diagnosis: Seabather’s eruption
Seabather’s eruption is a pruritic dermatitis that occurs predominantly on areas covered by a bathing suit or shirt after swimming in saltwater. Cases have been reported in Florida, Bermuda, the Caribbean, and as far north as Long Island, New York.
Larvae of members of the phylum Cnidaria are believed to be the cause of this dermatitis.1,2 Cnidaria include hydrozoans (fire coral and Portuguese man-of-war), scyphozoans (true jellyfish), and anthozoans (sea anemones). The condition is sometimes called sea lice, although the organisms have no relationship to lice.
All Cnidaria have microscopic envenomation capsules called nematocysts. Each capsule contains a folded, eversible tubule containing a variety of toxins. Skin contact or chemical stimulation of the triggering apparatus leads to a build-up of hydrostatic force, resulting in eversion of the tubule. Toxins pass across the tubule membrane and are deposited into the skin.
Clinical Course
Onset of seabather’s eruption generally occurs within a few minutes to several hours after the swimmer leaves the water. A pruritic maculopapular rash, occasionally with urticaria, spreads across the skin of the chest, abdomen, neck, axillae, and the flexor surfaces of the arms and legs. The rash may be accompanied by systemic symptoms of malaise, headache, fever, chills, nausea, and vomiting. The dermatitis often lasts 3 to 7 days, but more severe reactions can last up to 6 weeks.3
Are immunological mechanisms responsible?
Tomchik et al4 observed several cases of secondary eruptions that occurred 5 to 10 days after initial exposure. They also reported cases in which urticarial lesions developed 3 weeks after onset of illness, following a disease-free interval during which there was no re-exposure to ocean water. The lesions were seen in the same areas as the initial eruption.
This suggests that immunological mechanisms are responsible for the various clinical courses observed, which would explain the lack of symptoms immediately after exposure, delayed onset of the dermatitis, urticaria among sensitized individuals, and the ability of steroids to suppress the reaction.
A prospective cohort study of cases in Palm Beach County, Florida, concluded that persons with a history of exposure to the condition, children, and surfers were at greatest risk for seabather’s eruption.5
Culprit: the thimble jellyfish
The cause of seabather’s eruption in South Florida and the Caribbean has been identified as the larvae of Linuche unguiculata, or the thimble jellyfish. The adult Linuche (Figure 3) measure 5 mm to 20 mm. They breed between March and September, and the larvae measure about 0.5 mm in diameter, making them invisible to the naked eye.4
These larvae are washed toward the shore by high tides or strong winds. They are small enough to pass through the weave of most swimwear, becoming trapped against the skin. Pressure applied to the skin or changes in osmotic pressure—by evaporation of seawater or showering in fresh water—cause the larvae to discharge their toxin into the skin.6
In a study of cases of seabather’s eruption in the Mexican Caribbean, Segura-Puertas et al7 found that all 3 swimming stages of Linuche (ephyrae, medusae, and larvae) to cause the eruption.
Treatment: antihistamines, topical steroids
Seabather’s eruption can be treated with sedating or nonsedating antihistamines (level of evidence [LOE]=5). While sedating antihistamines may be more antipruritic and less expensive, they should be used with caution if sedation may pose a danger to the patient.
Topical steroids, such as hydrocortisone, may be beneficial. Systemic steroids are reserved for only the most severe reactions. Comfort measures, such as oatmeal baths, may provide some relief of the itching (LOE=5).4
FIGURE 3
Thimble jellyfish
1. Habif TB. Clinical Dermatology: A Color Guide to Diagnosis and Therapy. 3rd ed. St Louis, Mo: Mosby–Year Book; 1996;486-494.
2. Basler RSW, Basler GC, Palmer AH, Garcia MA. Special skin symptoms seen in swimmers. J Am Acad Dermatol 2000;43:299-305.
3. Freudenthal AR, Joseph PR. Seabather’s eruption. N Engl J Med 1993;329:542-544.
4. Tomchik RS, Russell MT, Szmant AM, Black NA. Clinical perspectives on seabather’s eruption, also known as “sea lice.” JAMA 1993;269:1669-1672.
5. Kumar S, Hlady WG, Malecki JM. Risk factors for seabather’s eruption: a prospective cohort study. Public Health Rep 1997;112:59-62.
6. MacSween RM, Williams HC. Lesson of the week: seabather’s eruption-a case of Caribbean itch. Br Med J 1996;312:957-958.
7. Segura-Puertas L, Ramos ME, Aramburo C, Heimer De La Cotera EP, Burnett JW. One Linuche mystery solved: all 3 stages of the coronate scyphomedusa Linuche unguiculata cause seabather’s eruption. J Am Acad Dermatol 2001;44:624-628.
8. Russell MT, Tomchik RS. Clinical articles seabather’s eruption or “sea lice”: New findings and clinical implications [Florida Atlantic University Web site].1995. Available at: www.fau.edu/safe/sea-lice.html. Accessed on July 1, 2003.
A 25-year-old man came to the office in July after awakening with a pruritic rash on his chest, back, and abdomen. He reported feeling feverish with chills and malaise, but had no symptoms of respiratory distress and his vital signs were normal. He mentioned that he had gone snorkeling in the Caribbean the previous day, but did not remember being stung by any marine life. He said he was wearing swimming trunks and a t-shirt while in the ocean.
The patient had a diffuse erythematous maculopapular rash on his neck, chest, back, abdomen, shoulders, and axillae (Figure 1 and Figure 2).
FIGURE 1
Rash on abdomen
FIGURE 2
Macules
What is the most likely diagnosis?
- Listen to local beach reports and observe posted beach messages in affected areas.
- Avoid ocean activities in areas where cases have been reported, especially during the peak season, April through July.
- Avoid wearing t-shirts while swimming. Women should consider wearing 2-piece bathing suits.These measures decrease the surface area over which larvae can be trapped. (Of course, swimmers should use potent sunscreens and carefully limit sun exposure to avoid sunburns when more skin is exposed to the sun. Some evidence suggests that the use of a sunscreen may actually protect skin from penetration by the nematocysts.)8
- Wetsuits with restrictive cuffs may be of benefit when surfing or diving.
- When swimming in areas where recent cases have occurred, change swimwear quickly after exiting the water. Do not shower with fresh water while still wearing the suit, because this may cause nematocysts trapped inside to discharge.Wash swimwear with detergent and heat-dry before wearing again. Air-dried nematocysts may still be able to fire.4,8
Diagnosis: Seabather’s eruption
Seabather’s eruption is a pruritic dermatitis that occurs predominantly on areas covered by a bathing suit or shirt after swimming in saltwater. Cases have been reported in Florida, Bermuda, the Caribbean, and as far north as Long Island, New York.
Larvae of members of the phylum Cnidaria are believed to be the cause of this dermatitis.1,2 Cnidaria include hydrozoans (fire coral and Portuguese man-of-war), scyphozoans (true jellyfish), and anthozoans (sea anemones). The condition is sometimes called sea lice, although the organisms have no relationship to lice.
All Cnidaria have microscopic envenomation capsules called nematocysts. Each capsule contains a folded, eversible tubule containing a variety of toxins. Skin contact or chemical stimulation of the triggering apparatus leads to a build-up of hydrostatic force, resulting in eversion of the tubule. Toxins pass across the tubule membrane and are deposited into the skin.
Clinical Course
Onset of seabather’s eruption generally occurs within a few minutes to several hours after the swimmer leaves the water. A pruritic maculopapular rash, occasionally with urticaria, spreads across the skin of the chest, abdomen, neck, axillae, and the flexor surfaces of the arms and legs. The rash may be accompanied by systemic symptoms of malaise, headache, fever, chills, nausea, and vomiting. The dermatitis often lasts 3 to 7 days, but more severe reactions can last up to 6 weeks.3
Are immunological mechanisms responsible?
Tomchik et al4 observed several cases of secondary eruptions that occurred 5 to 10 days after initial exposure. They also reported cases in which urticarial lesions developed 3 weeks after onset of illness, following a disease-free interval during which there was no re-exposure to ocean water. The lesions were seen in the same areas as the initial eruption.
This suggests that immunological mechanisms are responsible for the various clinical courses observed, which would explain the lack of symptoms immediately after exposure, delayed onset of the dermatitis, urticaria among sensitized individuals, and the ability of steroids to suppress the reaction.
A prospective cohort study of cases in Palm Beach County, Florida, concluded that persons with a history of exposure to the condition, children, and surfers were at greatest risk for seabather’s eruption.5
Culprit: the thimble jellyfish
The cause of seabather’s eruption in South Florida and the Caribbean has been identified as the larvae of Linuche unguiculata, or the thimble jellyfish. The adult Linuche (Figure 3) measure 5 mm to 20 mm. They breed between March and September, and the larvae measure about 0.5 mm in diameter, making them invisible to the naked eye.4
These larvae are washed toward the shore by high tides or strong winds. They are small enough to pass through the weave of most swimwear, becoming trapped against the skin. Pressure applied to the skin or changes in osmotic pressure—by evaporation of seawater or showering in fresh water—cause the larvae to discharge their toxin into the skin.6
In a study of cases of seabather’s eruption in the Mexican Caribbean, Segura-Puertas et al7 found that all 3 swimming stages of Linuche (ephyrae, medusae, and larvae) to cause the eruption.
Treatment: antihistamines, topical steroids
Seabather’s eruption can be treated with sedating or nonsedating antihistamines (level of evidence [LOE]=5). While sedating antihistamines may be more antipruritic and less expensive, they should be used with caution if sedation may pose a danger to the patient.
Topical steroids, such as hydrocortisone, may be beneficial. Systemic steroids are reserved for only the most severe reactions. Comfort measures, such as oatmeal baths, may provide some relief of the itching (LOE=5).4
FIGURE 3
Thimble jellyfish
A 25-year-old man came to the office in July after awakening with a pruritic rash on his chest, back, and abdomen. He reported feeling feverish with chills and malaise, but had no symptoms of respiratory distress and his vital signs were normal. He mentioned that he had gone snorkeling in the Caribbean the previous day, but did not remember being stung by any marine life. He said he was wearing swimming trunks and a t-shirt while in the ocean.
The patient had a diffuse erythematous maculopapular rash on his neck, chest, back, abdomen, shoulders, and axillae (Figure 1 and Figure 2).
FIGURE 1
Rash on abdomen
FIGURE 2
Macules
What is the most likely diagnosis?
- Listen to local beach reports and observe posted beach messages in affected areas.
- Avoid ocean activities in areas where cases have been reported, especially during the peak season, April through July.
- Avoid wearing t-shirts while swimming. Women should consider wearing 2-piece bathing suits.These measures decrease the surface area over which larvae can be trapped. (Of course, swimmers should use potent sunscreens and carefully limit sun exposure to avoid sunburns when more skin is exposed to the sun. Some evidence suggests that the use of a sunscreen may actually protect skin from penetration by the nematocysts.)8
- Wetsuits with restrictive cuffs may be of benefit when surfing or diving.
- When swimming in areas where recent cases have occurred, change swimwear quickly after exiting the water. Do not shower with fresh water while still wearing the suit, because this may cause nematocysts trapped inside to discharge.Wash swimwear with detergent and heat-dry before wearing again. Air-dried nematocysts may still be able to fire.4,8
Diagnosis: Seabather’s eruption
Seabather’s eruption is a pruritic dermatitis that occurs predominantly on areas covered by a bathing suit or shirt after swimming in saltwater. Cases have been reported in Florida, Bermuda, the Caribbean, and as far north as Long Island, New York.
Larvae of members of the phylum Cnidaria are believed to be the cause of this dermatitis.1,2 Cnidaria include hydrozoans (fire coral and Portuguese man-of-war), scyphozoans (true jellyfish), and anthozoans (sea anemones). The condition is sometimes called sea lice, although the organisms have no relationship to lice.
All Cnidaria have microscopic envenomation capsules called nematocysts. Each capsule contains a folded, eversible tubule containing a variety of toxins. Skin contact or chemical stimulation of the triggering apparatus leads to a build-up of hydrostatic force, resulting in eversion of the tubule. Toxins pass across the tubule membrane and are deposited into the skin.
Clinical Course
Onset of seabather’s eruption generally occurs within a few minutes to several hours after the swimmer leaves the water. A pruritic maculopapular rash, occasionally with urticaria, spreads across the skin of the chest, abdomen, neck, axillae, and the flexor surfaces of the arms and legs. The rash may be accompanied by systemic symptoms of malaise, headache, fever, chills, nausea, and vomiting. The dermatitis often lasts 3 to 7 days, but more severe reactions can last up to 6 weeks.3
Are immunological mechanisms responsible?
Tomchik et al4 observed several cases of secondary eruptions that occurred 5 to 10 days after initial exposure. They also reported cases in which urticarial lesions developed 3 weeks after onset of illness, following a disease-free interval during which there was no re-exposure to ocean water. The lesions were seen in the same areas as the initial eruption.
This suggests that immunological mechanisms are responsible for the various clinical courses observed, which would explain the lack of symptoms immediately after exposure, delayed onset of the dermatitis, urticaria among sensitized individuals, and the ability of steroids to suppress the reaction.
A prospective cohort study of cases in Palm Beach County, Florida, concluded that persons with a history of exposure to the condition, children, and surfers were at greatest risk for seabather’s eruption.5
Culprit: the thimble jellyfish
The cause of seabather’s eruption in South Florida and the Caribbean has been identified as the larvae of Linuche unguiculata, or the thimble jellyfish. The adult Linuche (Figure 3) measure 5 mm to 20 mm. They breed between March and September, and the larvae measure about 0.5 mm in diameter, making them invisible to the naked eye.4
These larvae are washed toward the shore by high tides or strong winds. They are small enough to pass through the weave of most swimwear, becoming trapped against the skin. Pressure applied to the skin or changes in osmotic pressure—by evaporation of seawater or showering in fresh water—cause the larvae to discharge their toxin into the skin.6
In a study of cases of seabather’s eruption in the Mexican Caribbean, Segura-Puertas et al7 found that all 3 swimming stages of Linuche (ephyrae, medusae, and larvae) to cause the eruption.
Treatment: antihistamines, topical steroids
Seabather’s eruption can be treated with sedating or nonsedating antihistamines (level of evidence [LOE]=5). While sedating antihistamines may be more antipruritic and less expensive, they should be used with caution if sedation may pose a danger to the patient.
Topical steroids, such as hydrocortisone, may be beneficial. Systemic steroids are reserved for only the most severe reactions. Comfort measures, such as oatmeal baths, may provide some relief of the itching (LOE=5).4
FIGURE 3
Thimble jellyfish
1. Habif TB. Clinical Dermatology: A Color Guide to Diagnosis and Therapy. 3rd ed. St Louis, Mo: Mosby–Year Book; 1996;486-494.
2. Basler RSW, Basler GC, Palmer AH, Garcia MA. Special skin symptoms seen in swimmers. J Am Acad Dermatol 2000;43:299-305.
3. Freudenthal AR, Joseph PR. Seabather’s eruption. N Engl J Med 1993;329:542-544.
4. Tomchik RS, Russell MT, Szmant AM, Black NA. Clinical perspectives on seabather’s eruption, also known as “sea lice.” JAMA 1993;269:1669-1672.
5. Kumar S, Hlady WG, Malecki JM. Risk factors for seabather’s eruption: a prospective cohort study. Public Health Rep 1997;112:59-62.
6. MacSween RM, Williams HC. Lesson of the week: seabather’s eruption-a case of Caribbean itch. Br Med J 1996;312:957-958.
7. Segura-Puertas L, Ramos ME, Aramburo C, Heimer De La Cotera EP, Burnett JW. One Linuche mystery solved: all 3 stages of the coronate scyphomedusa Linuche unguiculata cause seabather’s eruption. J Am Acad Dermatol 2001;44:624-628.
8. Russell MT, Tomchik RS. Clinical articles seabather’s eruption or “sea lice”: New findings and clinical implications [Florida Atlantic University Web site].1995. Available at: www.fau.edu/safe/sea-lice.html. Accessed on July 1, 2003.
1. Habif TB. Clinical Dermatology: A Color Guide to Diagnosis and Therapy. 3rd ed. St Louis, Mo: Mosby–Year Book; 1996;486-494.
2. Basler RSW, Basler GC, Palmer AH, Garcia MA. Special skin symptoms seen in swimmers. J Am Acad Dermatol 2000;43:299-305.
3. Freudenthal AR, Joseph PR. Seabather’s eruption. N Engl J Med 1993;329:542-544.
4. Tomchik RS, Russell MT, Szmant AM, Black NA. Clinical perspectives on seabather’s eruption, also known as “sea lice.” JAMA 1993;269:1669-1672.
5. Kumar S, Hlady WG, Malecki JM. Risk factors for seabather’s eruption: a prospective cohort study. Public Health Rep 1997;112:59-62.
6. MacSween RM, Williams HC. Lesson of the week: seabather’s eruption-a case of Caribbean itch. Br Med J 1996;312:957-958.
7. Segura-Puertas L, Ramos ME, Aramburo C, Heimer De La Cotera EP, Burnett JW. One Linuche mystery solved: all 3 stages of the coronate scyphomedusa Linuche unguiculata cause seabather’s eruption. J Am Acad Dermatol 2001;44:624-628.
8. Russell MT, Tomchik RS. Clinical articles seabather’s eruption or “sea lice”: New findings and clinical implications [Florida Atlantic University Web site].1995. Available at: www.fau.edu/safe/sea-lice.html. Accessed on July 1, 2003.
Back from the Peace Corps— with skin lesions from South America
A 25-year-old woman visited her family doctor for treatment of skin lesions on her left leg, which had developed over the previous 8 weeks. The patient had just returned to the US after working with the Peace Corps in the Amazon rain forest for 3 months. During that time, she slept outside in the jungle without any skin protection—no mosquito netting, no insect repellent, no protective clothing on her extremities. She reported no recent history of trauma, burns, or chemical exposure to the leg, and she had been healthy prior to this skin condition. She reported that she was taking no medications, and was not using alcohol or tobacco.
The lesions started with the development of small pustules on her left leg and ankle. Over the next 3 weeks, these grew into larger, ulcerated lesions. The ulcers persisted for 5 more weeks despite multiple attempts at local topical treatments in Ecuador. No pain or itching were associated with the lesions.
The physical examination revealed a generally healthy woman, remarkable only for the 2 skin ulcers on her left lower leg and ankle (Figure 1 and Figure 2). The patient was afebrile, and had no lymphadenopathy or hepatosplenomegaly.
FIGURE 1
Cutaneous ulceration with indurated border.
Two active ulcers with small healed satellite lesion near the medial malleolus (see arrow).
How would you treat this condition?
Differential diagnosis of tropical skin ulcers
The woman’s family physician used the Internet to determine that the differential diagnosis of tropical skin ulcers includes localized cutaneous leishmaniasis, foreign body reaction, impetigo, pyoderma gangrenosum, bacterial or deep fungal infection, atypical mycobacterial infection, sarcoidosis, squamous and basal cell carcinoma, and superinfected insect bites. The combination of the patient’s history of potential exposure, clinical presentation, and epidemiology of leishmaniasis within the region in which she was working supports a presumptive diagnosis of localized cutaneous leishmaniasis.
Leishmaniasis
Leishmaniasis is comprised of several diverse diseases with varying degrees of severity, ranging from spontaneously healing skin ulcers to a disfiguring mucocutaneous disease, and even to fatal visceral illness. Endemic worldwide except Australia, Oceania, and Antarctica, leishmaniasis is found in the tropics and subtropics.
Approximately 2 million new cases occur each year, with markedly increasing incidence in several parts of the world resulting from international travel, immigration, military deployment, and human immunodeficiency virus coinfection.1
An estimated 10% of the world’s population is at risk for contracting leishmaniasis. Most cases reported in the US are in travelers to countries where the disease is endemic, although sporadic cases have been reported in Texas and Oklahoma.2
Characteristics
Leishmaniasis is a vector-borne disease, caused by obligate intracellular protozoa of the genus Leishmaniaand transmitted by the bite of infected female sandflies. The disease may take several forms, including cutaneous, mucocutaneous, and visceral leishmaniasis, depending on the particular Leishmaniaspecies and host response.
Localized cutaneous leishmaniasis is characterized clinically by the appearance of skin lesions at the site of the sandfly bite, which typically present as inflammatory papules before progressing to nodules and ulcers. Local lymphadenopathy may occur. Sores may be painless or painful.
Both cutaneous and mucocutaneous forms generally yield normal laboratory values, although complete blood counts may show mild anemia, leukopenia, or thrombocytopenia.3 US travelers may consult numerous physicians before leishmaniasis is diagnosed. The median time from recognition of skin lesions to drug treatment is 112 days.4
Lab results
The patient’s complete blood count with differential, electrolytes, creatinine, liver function tests, and electrocardiogram were within normal limits. An infectious disease consultant suggested direct microscopic visualization of skin biopsies, dermal scrapings, and needle aspirates using Giemsa and Leishman stains, but these failed to reveal any parasitic organisms. It was thought these results were most likely false negatives.
In the best situation, the diagnosis of leishmaniasis is confirmed by isolating, visualizing, and culturing the parasite from infected tissue. Dermal scrapings or needle aspirates may reveal Leishmania amastigotes using a Giemsa stain. Early in the course of localized disease, Leishmania organisms may be numerous and found readily within the cytoplasm of macrophages. However, biopsy specimens from old lesions (>6 months), partially or incompletely treated, are frequently negative.
In vitro culture of the parasite from tissue samples using Nicolle-Noy-McNeal medium is often obtained to aid in diagnosis and to identify Leishmania species. With successful culture, the parasite can be sent to the Centers for Disease Control for speciation. New, rapid tests for leishmaniasis are being developed.
Treatment
Although 90% of skin lesions caused by cutaneous leishmaniasis heal spontaneously to form atrophic scars, the infectious disease consultant recommended treatment to prevent development of disfiguring mucocutaneous disease (level of evidence [LOE]=5)—otherwise, immunity may not be complete and skin ulcers can recur.
Experts believe it is important to treat cutaneous leishmaniasis if a species known or suspected of being capable of converting to the mucocutaneous disease form—eg, New World L braziliensis—is present. These lesions may appear months or even years after the initial exposure, and can be refractory to further treatment. However, surgical excision usually is not recommended because of the risk of relapse and further cosmetic disfigurement.3
Yearly follow-up to evaluate for recurrence or evolution of mucocutaneous leishmaniasis is crucial; early treatment of this form of the disease is more efficacious and can yield more favorable outcomes by limiting potential facial involvement (LOE=5).
Meglumine antimonate
The patient’s initial treatment included gluteal injections of meglumine antimonate at a dosage of 20 mg antimony/kg/d intramuscularly for a total course of 21 days (LOE=5). In addition, one tenth of each dose was injected directly into skin lesions under the peripheral margins, as shown in Figure 3 (LOE=5).
Side effects. The patient’s side effects from the meglumine antimonate therapy included insomnia, lightheadedness, increased fatigue, pain at the gluteal injection sites, bone aches, painful splenomegaly, and left leg myoclonic spasms. These signs and symptoms resolved shortly after completion of the course of therapy with no apparent sequelae. Monitoring cardiac, renal, and hepatic function before and during treatment is important, as meglumine antimonate has significant potential to cause renal and hepatic toxicity.
Outcome
The patient’s skin lesions began to show signs of healing after a few days of treatment. Healed lesions can be seen in Figure 4 Of course, we don’t know that these lesions would not have healed without treatment.
Follow-up diagnostic tests including electrocardiograms, liver function tests, and renal function showed normal values at the conclusion of the 21-day course of therapy.
Fluconazole studied
Since this patient was treated, a Saudi Arabian study was published on use of oral fluconazole 200 mg/d for 6 weeks in patients with cutaneous leishmaniasis (L major).5 At the 3-month followup, healing of lesions was complete for 79% of patients in the fluconazole group and 34% patients in the placebo group (LOE=2b).
The toxicity of this treatment is much lower than the antimonials; we should be looking for further evidence of its benefits in other countries with other species of Leishmania.
FIGURE 3
Perilesional infiltration of leishmaniasis lesion using meglumine antimonate.
FIGURE 4
Healed cutaneous leishmaniasis lesions on left leg.
Preventing sandfly bites
Leishmaniasis is preventable by avoiding contact with the vector—the sandfly—while living or traveling in endemic areas. Sandflies are most active from dusk to dawn. Though relatively poor fliers, they are small enough to fit through standard mosquito netting and make no audible noise. Effective prevention may be achieved by avoiding nighttime outdoor activities, using topical insecticides (eg, N, N diethyl-m-toluamide [DEET]) on exposed skin surfaces, using insecticide-impregnated clothing (permethrin stays in material for many washings), using fine-mesh mosquito netting, and sleeping with a fan.3
In this time of the globalization of infectious diseases, family doctors in the US should be aware of the tropical diseases that our patients may bring to their offices.
1. Roberts LJ, Handman E, Foote SJ. Science, medicine and the future: leishmaniasis. BMJ 2000;321:801-804.
2. Melby PC, Kreutzer RD, McMahon-Pratt D, Gam AA, Neva FA. Cutaneous leishmaniasis: review of 59 cases seen at the National Institutes of Health. Clin Infect Dis 1992;15:924-937.
3. Kenner JR, Kaugh YC. Leishmaniasis. eMedicine J. 2001 2(11). Available at http://www.emedicine.com/derm/topic219.htm Accessed on May 29, 2003.
4. Herwaldt BL, Stokes SL, Juranek DD. American cutaneous leishmaniasis in U.S. travelers. Ann Intern Med 1993;118:779-784.
5. Alrajhi AA, Ibrahim EA, De Vol EB, Khairat M, Faris RM, Maguire JH. Fluconazole for the treatment of cutaneous leishmaniasis caused by Leishmania major. N Engl J Med 2002;346:891-895.
A 25-year-old woman visited her family doctor for treatment of skin lesions on her left leg, which had developed over the previous 8 weeks. The patient had just returned to the US after working with the Peace Corps in the Amazon rain forest for 3 months. During that time, she slept outside in the jungle without any skin protection—no mosquito netting, no insect repellent, no protective clothing on her extremities. She reported no recent history of trauma, burns, or chemical exposure to the leg, and she had been healthy prior to this skin condition. She reported that she was taking no medications, and was not using alcohol or tobacco.
The lesions started with the development of small pustules on her left leg and ankle. Over the next 3 weeks, these grew into larger, ulcerated lesions. The ulcers persisted for 5 more weeks despite multiple attempts at local topical treatments in Ecuador. No pain or itching were associated with the lesions.
The physical examination revealed a generally healthy woman, remarkable only for the 2 skin ulcers on her left lower leg and ankle (Figure 1 and Figure 2). The patient was afebrile, and had no lymphadenopathy or hepatosplenomegaly.
FIGURE 1
Cutaneous ulceration with indurated border.
Two active ulcers with small healed satellite lesion near the medial malleolus (see arrow).
How would you treat this condition?
Differential diagnosis of tropical skin ulcers
The woman’s family physician used the Internet to determine that the differential diagnosis of tropical skin ulcers includes localized cutaneous leishmaniasis, foreign body reaction, impetigo, pyoderma gangrenosum, bacterial or deep fungal infection, atypical mycobacterial infection, sarcoidosis, squamous and basal cell carcinoma, and superinfected insect bites. The combination of the patient’s history of potential exposure, clinical presentation, and epidemiology of leishmaniasis within the region in which she was working supports a presumptive diagnosis of localized cutaneous leishmaniasis.
Leishmaniasis
Leishmaniasis is comprised of several diverse diseases with varying degrees of severity, ranging from spontaneously healing skin ulcers to a disfiguring mucocutaneous disease, and even to fatal visceral illness. Endemic worldwide except Australia, Oceania, and Antarctica, leishmaniasis is found in the tropics and subtropics.
Approximately 2 million new cases occur each year, with markedly increasing incidence in several parts of the world resulting from international travel, immigration, military deployment, and human immunodeficiency virus coinfection.1
An estimated 10% of the world’s population is at risk for contracting leishmaniasis. Most cases reported in the US are in travelers to countries where the disease is endemic, although sporadic cases have been reported in Texas and Oklahoma.2
Characteristics
Leishmaniasis is a vector-borne disease, caused by obligate intracellular protozoa of the genus Leishmaniaand transmitted by the bite of infected female sandflies. The disease may take several forms, including cutaneous, mucocutaneous, and visceral leishmaniasis, depending on the particular Leishmaniaspecies and host response.
Localized cutaneous leishmaniasis is characterized clinically by the appearance of skin lesions at the site of the sandfly bite, which typically present as inflammatory papules before progressing to nodules and ulcers. Local lymphadenopathy may occur. Sores may be painless or painful.
Both cutaneous and mucocutaneous forms generally yield normal laboratory values, although complete blood counts may show mild anemia, leukopenia, or thrombocytopenia.3 US travelers may consult numerous physicians before leishmaniasis is diagnosed. The median time from recognition of skin lesions to drug treatment is 112 days.4
Lab results
The patient’s complete blood count with differential, electrolytes, creatinine, liver function tests, and electrocardiogram were within normal limits. An infectious disease consultant suggested direct microscopic visualization of skin biopsies, dermal scrapings, and needle aspirates using Giemsa and Leishman stains, but these failed to reveal any parasitic organisms. It was thought these results were most likely false negatives.
In the best situation, the diagnosis of leishmaniasis is confirmed by isolating, visualizing, and culturing the parasite from infected tissue. Dermal scrapings or needle aspirates may reveal Leishmania amastigotes using a Giemsa stain. Early in the course of localized disease, Leishmania organisms may be numerous and found readily within the cytoplasm of macrophages. However, biopsy specimens from old lesions (>6 months), partially or incompletely treated, are frequently negative.
In vitro culture of the parasite from tissue samples using Nicolle-Noy-McNeal medium is often obtained to aid in diagnosis and to identify Leishmania species. With successful culture, the parasite can be sent to the Centers for Disease Control for speciation. New, rapid tests for leishmaniasis are being developed.
Treatment
Although 90% of skin lesions caused by cutaneous leishmaniasis heal spontaneously to form atrophic scars, the infectious disease consultant recommended treatment to prevent development of disfiguring mucocutaneous disease (level of evidence [LOE]=5)—otherwise, immunity may not be complete and skin ulcers can recur.
Experts believe it is important to treat cutaneous leishmaniasis if a species known or suspected of being capable of converting to the mucocutaneous disease form—eg, New World L braziliensis—is present. These lesions may appear months or even years after the initial exposure, and can be refractory to further treatment. However, surgical excision usually is not recommended because of the risk of relapse and further cosmetic disfigurement.3
Yearly follow-up to evaluate for recurrence or evolution of mucocutaneous leishmaniasis is crucial; early treatment of this form of the disease is more efficacious and can yield more favorable outcomes by limiting potential facial involvement (LOE=5).
Meglumine antimonate
The patient’s initial treatment included gluteal injections of meglumine antimonate at a dosage of 20 mg antimony/kg/d intramuscularly for a total course of 21 days (LOE=5). In addition, one tenth of each dose was injected directly into skin lesions under the peripheral margins, as shown in Figure 3 (LOE=5).
Side effects. The patient’s side effects from the meglumine antimonate therapy included insomnia, lightheadedness, increased fatigue, pain at the gluteal injection sites, bone aches, painful splenomegaly, and left leg myoclonic spasms. These signs and symptoms resolved shortly after completion of the course of therapy with no apparent sequelae. Monitoring cardiac, renal, and hepatic function before and during treatment is important, as meglumine antimonate has significant potential to cause renal and hepatic toxicity.
Outcome
The patient’s skin lesions began to show signs of healing after a few days of treatment. Healed lesions can be seen in Figure 4 Of course, we don’t know that these lesions would not have healed without treatment.
Follow-up diagnostic tests including electrocardiograms, liver function tests, and renal function showed normal values at the conclusion of the 21-day course of therapy.
Fluconazole studied
Since this patient was treated, a Saudi Arabian study was published on use of oral fluconazole 200 mg/d for 6 weeks in patients with cutaneous leishmaniasis (L major).5 At the 3-month followup, healing of lesions was complete for 79% of patients in the fluconazole group and 34% patients in the placebo group (LOE=2b).
The toxicity of this treatment is much lower than the antimonials; we should be looking for further evidence of its benefits in other countries with other species of Leishmania.
FIGURE 3
Perilesional infiltration of leishmaniasis lesion using meglumine antimonate.
FIGURE 4
Healed cutaneous leishmaniasis lesions on left leg.
Preventing sandfly bites
Leishmaniasis is preventable by avoiding contact with the vector—the sandfly—while living or traveling in endemic areas. Sandflies are most active from dusk to dawn. Though relatively poor fliers, they are small enough to fit through standard mosquito netting and make no audible noise. Effective prevention may be achieved by avoiding nighttime outdoor activities, using topical insecticides (eg, N, N diethyl-m-toluamide [DEET]) on exposed skin surfaces, using insecticide-impregnated clothing (permethrin stays in material for many washings), using fine-mesh mosquito netting, and sleeping with a fan.3
In this time of the globalization of infectious diseases, family doctors in the US should be aware of the tropical diseases that our patients may bring to their offices.
A 25-year-old woman visited her family doctor for treatment of skin lesions on her left leg, which had developed over the previous 8 weeks. The patient had just returned to the US after working with the Peace Corps in the Amazon rain forest for 3 months. During that time, she slept outside in the jungle without any skin protection—no mosquito netting, no insect repellent, no protective clothing on her extremities. She reported no recent history of trauma, burns, or chemical exposure to the leg, and she had been healthy prior to this skin condition. She reported that she was taking no medications, and was not using alcohol or tobacco.
The lesions started with the development of small pustules on her left leg and ankle. Over the next 3 weeks, these grew into larger, ulcerated lesions. The ulcers persisted for 5 more weeks despite multiple attempts at local topical treatments in Ecuador. No pain or itching were associated with the lesions.
The physical examination revealed a generally healthy woman, remarkable only for the 2 skin ulcers on her left lower leg and ankle (Figure 1 and Figure 2). The patient was afebrile, and had no lymphadenopathy or hepatosplenomegaly.
FIGURE 1
Cutaneous ulceration with indurated border.
Two active ulcers with small healed satellite lesion near the medial malleolus (see arrow).
How would you treat this condition?
Differential diagnosis of tropical skin ulcers
The woman’s family physician used the Internet to determine that the differential diagnosis of tropical skin ulcers includes localized cutaneous leishmaniasis, foreign body reaction, impetigo, pyoderma gangrenosum, bacterial or deep fungal infection, atypical mycobacterial infection, sarcoidosis, squamous and basal cell carcinoma, and superinfected insect bites. The combination of the patient’s history of potential exposure, clinical presentation, and epidemiology of leishmaniasis within the region in which she was working supports a presumptive diagnosis of localized cutaneous leishmaniasis.
Leishmaniasis
Leishmaniasis is comprised of several diverse diseases with varying degrees of severity, ranging from spontaneously healing skin ulcers to a disfiguring mucocutaneous disease, and even to fatal visceral illness. Endemic worldwide except Australia, Oceania, and Antarctica, leishmaniasis is found in the tropics and subtropics.
Approximately 2 million new cases occur each year, with markedly increasing incidence in several parts of the world resulting from international travel, immigration, military deployment, and human immunodeficiency virus coinfection.1
An estimated 10% of the world’s population is at risk for contracting leishmaniasis. Most cases reported in the US are in travelers to countries where the disease is endemic, although sporadic cases have been reported in Texas and Oklahoma.2
Characteristics
Leishmaniasis is a vector-borne disease, caused by obligate intracellular protozoa of the genus Leishmaniaand transmitted by the bite of infected female sandflies. The disease may take several forms, including cutaneous, mucocutaneous, and visceral leishmaniasis, depending on the particular Leishmaniaspecies and host response.
Localized cutaneous leishmaniasis is characterized clinically by the appearance of skin lesions at the site of the sandfly bite, which typically present as inflammatory papules before progressing to nodules and ulcers. Local lymphadenopathy may occur. Sores may be painless or painful.
Both cutaneous and mucocutaneous forms generally yield normal laboratory values, although complete blood counts may show mild anemia, leukopenia, or thrombocytopenia.3 US travelers may consult numerous physicians before leishmaniasis is diagnosed. The median time from recognition of skin lesions to drug treatment is 112 days.4
Lab results
The patient’s complete blood count with differential, electrolytes, creatinine, liver function tests, and electrocardiogram were within normal limits. An infectious disease consultant suggested direct microscopic visualization of skin biopsies, dermal scrapings, and needle aspirates using Giemsa and Leishman stains, but these failed to reveal any parasitic organisms. It was thought these results were most likely false negatives.
In the best situation, the diagnosis of leishmaniasis is confirmed by isolating, visualizing, and culturing the parasite from infected tissue. Dermal scrapings or needle aspirates may reveal Leishmania amastigotes using a Giemsa stain. Early in the course of localized disease, Leishmania organisms may be numerous and found readily within the cytoplasm of macrophages. However, biopsy specimens from old lesions (>6 months), partially or incompletely treated, are frequently negative.
In vitro culture of the parasite from tissue samples using Nicolle-Noy-McNeal medium is often obtained to aid in diagnosis and to identify Leishmania species. With successful culture, the parasite can be sent to the Centers for Disease Control for speciation. New, rapid tests for leishmaniasis are being developed.
Treatment
Although 90% of skin lesions caused by cutaneous leishmaniasis heal spontaneously to form atrophic scars, the infectious disease consultant recommended treatment to prevent development of disfiguring mucocutaneous disease (level of evidence [LOE]=5)—otherwise, immunity may not be complete and skin ulcers can recur.
Experts believe it is important to treat cutaneous leishmaniasis if a species known or suspected of being capable of converting to the mucocutaneous disease form—eg, New World L braziliensis—is present. These lesions may appear months or even years after the initial exposure, and can be refractory to further treatment. However, surgical excision usually is not recommended because of the risk of relapse and further cosmetic disfigurement.3
Yearly follow-up to evaluate for recurrence or evolution of mucocutaneous leishmaniasis is crucial; early treatment of this form of the disease is more efficacious and can yield more favorable outcomes by limiting potential facial involvement (LOE=5).
Meglumine antimonate
The patient’s initial treatment included gluteal injections of meglumine antimonate at a dosage of 20 mg antimony/kg/d intramuscularly for a total course of 21 days (LOE=5). In addition, one tenth of each dose was injected directly into skin lesions under the peripheral margins, as shown in Figure 3 (LOE=5).
Side effects. The patient’s side effects from the meglumine antimonate therapy included insomnia, lightheadedness, increased fatigue, pain at the gluteal injection sites, bone aches, painful splenomegaly, and left leg myoclonic spasms. These signs and symptoms resolved shortly after completion of the course of therapy with no apparent sequelae. Monitoring cardiac, renal, and hepatic function before and during treatment is important, as meglumine antimonate has significant potential to cause renal and hepatic toxicity.
Outcome
The patient’s skin lesions began to show signs of healing after a few days of treatment. Healed lesions can be seen in Figure 4 Of course, we don’t know that these lesions would not have healed without treatment.
Follow-up diagnostic tests including electrocardiograms, liver function tests, and renal function showed normal values at the conclusion of the 21-day course of therapy.
Fluconazole studied
Since this patient was treated, a Saudi Arabian study was published on use of oral fluconazole 200 mg/d for 6 weeks in patients with cutaneous leishmaniasis (L major).5 At the 3-month followup, healing of lesions was complete for 79% of patients in the fluconazole group and 34% patients in the placebo group (LOE=2b).
The toxicity of this treatment is much lower than the antimonials; we should be looking for further evidence of its benefits in other countries with other species of Leishmania.
FIGURE 3
Perilesional infiltration of leishmaniasis lesion using meglumine antimonate.
FIGURE 4
Healed cutaneous leishmaniasis lesions on left leg.
Preventing sandfly bites
Leishmaniasis is preventable by avoiding contact with the vector—the sandfly—while living or traveling in endemic areas. Sandflies are most active from dusk to dawn. Though relatively poor fliers, they are small enough to fit through standard mosquito netting and make no audible noise. Effective prevention may be achieved by avoiding nighttime outdoor activities, using topical insecticides (eg, N, N diethyl-m-toluamide [DEET]) on exposed skin surfaces, using insecticide-impregnated clothing (permethrin stays in material for many washings), using fine-mesh mosquito netting, and sleeping with a fan.3
In this time of the globalization of infectious diseases, family doctors in the US should be aware of the tropical diseases that our patients may bring to their offices.
1. Roberts LJ, Handman E, Foote SJ. Science, medicine and the future: leishmaniasis. BMJ 2000;321:801-804.
2. Melby PC, Kreutzer RD, McMahon-Pratt D, Gam AA, Neva FA. Cutaneous leishmaniasis: review of 59 cases seen at the National Institutes of Health. Clin Infect Dis 1992;15:924-937.
3. Kenner JR, Kaugh YC. Leishmaniasis. eMedicine J. 2001 2(11). Available at http://www.emedicine.com/derm/topic219.htm Accessed on May 29, 2003.
4. Herwaldt BL, Stokes SL, Juranek DD. American cutaneous leishmaniasis in U.S. travelers. Ann Intern Med 1993;118:779-784.
5. Alrajhi AA, Ibrahim EA, De Vol EB, Khairat M, Faris RM, Maguire JH. Fluconazole for the treatment of cutaneous leishmaniasis caused by Leishmania major. N Engl J Med 2002;346:891-895.
1. Roberts LJ, Handman E, Foote SJ. Science, medicine and the future: leishmaniasis. BMJ 2000;321:801-804.
2. Melby PC, Kreutzer RD, McMahon-Pratt D, Gam AA, Neva FA. Cutaneous leishmaniasis: review of 59 cases seen at the National Institutes of Health. Clin Infect Dis 1992;15:924-937.
3. Kenner JR, Kaugh YC. Leishmaniasis. eMedicine J. 2001 2(11). Available at http://www.emedicine.com/derm/topic219.htm Accessed on May 29, 2003.
4. Herwaldt BL, Stokes SL, Juranek DD. American cutaneous leishmaniasis in U.S. travelers. Ann Intern Med 1993;118:779-784.
5. Alrajhi AA, Ibrahim EA, De Vol EB, Khairat M, Faris RM, Maguire JH. Fluconazole for the treatment of cutaneous leishmaniasis caused by Leishmania major. N Engl J Med 2002;346:891-895.
A terrible itch
A 74-year-old Caucasian woman visited an outpatient clinic at a homeless shelter. Her chief concern was itching all over her body. Her chart revealed a history of chronic scabies and mental illness. She had been seen in the clinic on 4 separate occasions over a 2-year period for scabies. She claimed on this visit that she could not get rid of the scabies because people kept taking away her medication. She also stated she could see the creatures feed on her and move in and out of her skin.
The physical exam revealed that she was unwashed and disheveled. She had multiple excoriations on her extremities, hands, abdomen, back, and the nape of her neck (Figure 1).
FIGURE 1
Excoriations on the abdomen
FIGURE 2
Adult body lice and nymphs on clothing seams
Diagnosis
The woman’s appearance and mental status are consistent with schizophrenia; however, a diagnosis of scabies is not possible if she is really seeing the “bugs” on her body, as opposed to just imagining them. The scabies mite can only be seen with the aid of a microscope.
We looked for the bugs to confirm her story. While this could have been a case of delusions of parasitosis, on close inspection small “bugs” were indeed visible on her abdomen and along the seams of her pants. Multiple nits appeared attached to the hairs of her head, and a “bug” is noted to be feeding on the nape of her neck. This homeless woman has a massive infestation of body and head lice (Figure 2).
In the past she may have had a scabies infestation, but there was no documentation in the chart of any skin scrapings looked at under the microscope for diagnosis. It is more likely that she has been chronically infested with lice. Due to her mental illness, this patient may have been misdiagnosed with scabies based on the assumption that her claim to see the bugs was a hallucination. A close exam may not have been performed due to her mental illness and poor hygiene, along with the health care providers’ fears of catching scabies. Repeated documentation of scabies may have discouraged further investigation into another cause.
Pediculosis
Lice are bloodsucking obligate parasites. There are hundreds of millions of cases of pediculosis worldwide affecting men, women, and children from all socioeconomic classes.
Three types of lice infest humans: the head louse (Pediculus humanus capitis), the body louse (Pediculus humanus corporis), and the pubic louse (Phthirus pubis). Lice cling to hairs with their clawlike legs and pierce the skin, inject saliva, and then defecate while obtaining their blood meal. When exposed to lice, people clinically experience little irritation from the first bite, but after a short period they become sensitized. A hypersensitivity reaction—producing reddening of the skin, itching, and overall inflammation— subsequently develops.
Body lice are similar to head lice, except they are slightly larger. They are primarily seen in the homeless and indigent populations, with transmission occurring with direct body contact or sharing of contaminated clothes or bedding.
The body louse, contrary to popular belief, does not live on the body. It lives in the seams of clothing, clinging to the fibers. It will feed, remaining on the clothes, and only in massive infestations are they typically seen moving about the body. The axillae, groin, and truncal areas are the most severely affected. Patients have severe itching and tend to excoriate these regions. In chronically infested patients a postinflammatory hyperpigmentation can be observed.
Treatment
Persons infested with body lice need to discard or launder their clothing using hot water, and then bathe themselves. In cases of massive infestation—such as this case, in which head lice were also found—a pediculicide should be applied to the hair and entire body from head to toe. This can then be washed off in the shower (level of evidence [LOE]=1a, from Cochrane Review). This same Cochrane systematic review of the treatment of head lice found no evidence that any one pediculicide has greater effect than another (LOE=1a).1
In this case, the patient did not want to get rid of her green pants because she was able to find the “bugs” easily in its seams, which she thought helped keep them under control. When we explained that the full treatment of this condition required her to be given new clothes, she finally accepted this course of action.
Arrangements were made for her to shower at the shelter and obtain new clothes, and she was given permethrin shampoo to apply over her entire body from head to toe. She was scheduled to follow up in 1 week but unfortunately never returned to the clinic.
1. Dodd CS. Interventions for treating headlice (Cochrane Review). In: The Cochrane Library, Issue 2, 2002. Oxford.
A 74-year-old Caucasian woman visited an outpatient clinic at a homeless shelter. Her chief concern was itching all over her body. Her chart revealed a history of chronic scabies and mental illness. She had been seen in the clinic on 4 separate occasions over a 2-year period for scabies. She claimed on this visit that she could not get rid of the scabies because people kept taking away her medication. She also stated she could see the creatures feed on her and move in and out of her skin.
The physical exam revealed that she was unwashed and disheveled. She had multiple excoriations on her extremities, hands, abdomen, back, and the nape of her neck (Figure 1).
FIGURE 1
Excoriations on the abdomen
FIGURE 2
Adult body lice and nymphs on clothing seams
Diagnosis
The woman’s appearance and mental status are consistent with schizophrenia; however, a diagnosis of scabies is not possible if she is really seeing the “bugs” on her body, as opposed to just imagining them. The scabies mite can only be seen with the aid of a microscope.
We looked for the bugs to confirm her story. While this could have been a case of delusions of parasitosis, on close inspection small “bugs” were indeed visible on her abdomen and along the seams of her pants. Multiple nits appeared attached to the hairs of her head, and a “bug” is noted to be feeding on the nape of her neck. This homeless woman has a massive infestation of body and head lice (Figure 2).
In the past she may have had a scabies infestation, but there was no documentation in the chart of any skin scrapings looked at under the microscope for diagnosis. It is more likely that she has been chronically infested with lice. Due to her mental illness, this patient may have been misdiagnosed with scabies based on the assumption that her claim to see the bugs was a hallucination. A close exam may not have been performed due to her mental illness and poor hygiene, along with the health care providers’ fears of catching scabies. Repeated documentation of scabies may have discouraged further investigation into another cause.
Pediculosis
Lice are bloodsucking obligate parasites. There are hundreds of millions of cases of pediculosis worldwide affecting men, women, and children from all socioeconomic classes.
Three types of lice infest humans: the head louse (Pediculus humanus capitis), the body louse (Pediculus humanus corporis), and the pubic louse (Phthirus pubis). Lice cling to hairs with their clawlike legs and pierce the skin, inject saliva, and then defecate while obtaining their blood meal. When exposed to lice, people clinically experience little irritation from the first bite, but after a short period they become sensitized. A hypersensitivity reaction—producing reddening of the skin, itching, and overall inflammation— subsequently develops.
Body lice are similar to head lice, except they are slightly larger. They are primarily seen in the homeless and indigent populations, with transmission occurring with direct body contact or sharing of contaminated clothes or bedding.
The body louse, contrary to popular belief, does not live on the body. It lives in the seams of clothing, clinging to the fibers. It will feed, remaining on the clothes, and only in massive infestations are they typically seen moving about the body. The axillae, groin, and truncal areas are the most severely affected. Patients have severe itching and tend to excoriate these regions. In chronically infested patients a postinflammatory hyperpigmentation can be observed.
Treatment
Persons infested with body lice need to discard or launder their clothing using hot water, and then bathe themselves. In cases of massive infestation—such as this case, in which head lice were also found—a pediculicide should be applied to the hair and entire body from head to toe. This can then be washed off in the shower (level of evidence [LOE]=1a, from Cochrane Review). This same Cochrane systematic review of the treatment of head lice found no evidence that any one pediculicide has greater effect than another (LOE=1a).1
In this case, the patient did not want to get rid of her green pants because she was able to find the “bugs” easily in its seams, which she thought helped keep them under control. When we explained that the full treatment of this condition required her to be given new clothes, she finally accepted this course of action.
Arrangements were made for her to shower at the shelter and obtain new clothes, and she was given permethrin shampoo to apply over her entire body from head to toe. She was scheduled to follow up in 1 week but unfortunately never returned to the clinic.
A 74-year-old Caucasian woman visited an outpatient clinic at a homeless shelter. Her chief concern was itching all over her body. Her chart revealed a history of chronic scabies and mental illness. She had been seen in the clinic on 4 separate occasions over a 2-year period for scabies. She claimed on this visit that she could not get rid of the scabies because people kept taking away her medication. She also stated she could see the creatures feed on her and move in and out of her skin.
The physical exam revealed that she was unwashed and disheveled. She had multiple excoriations on her extremities, hands, abdomen, back, and the nape of her neck (Figure 1).
FIGURE 1
Excoriations on the abdomen
FIGURE 2
Adult body lice and nymphs on clothing seams
Diagnosis
The woman’s appearance and mental status are consistent with schizophrenia; however, a diagnosis of scabies is not possible if she is really seeing the “bugs” on her body, as opposed to just imagining them. The scabies mite can only be seen with the aid of a microscope.
We looked for the bugs to confirm her story. While this could have been a case of delusions of parasitosis, on close inspection small “bugs” were indeed visible on her abdomen and along the seams of her pants. Multiple nits appeared attached to the hairs of her head, and a “bug” is noted to be feeding on the nape of her neck. This homeless woman has a massive infestation of body and head lice (Figure 2).
In the past she may have had a scabies infestation, but there was no documentation in the chart of any skin scrapings looked at under the microscope for diagnosis. It is more likely that she has been chronically infested with lice. Due to her mental illness, this patient may have been misdiagnosed with scabies based on the assumption that her claim to see the bugs was a hallucination. A close exam may not have been performed due to her mental illness and poor hygiene, along with the health care providers’ fears of catching scabies. Repeated documentation of scabies may have discouraged further investigation into another cause.
Pediculosis
Lice are bloodsucking obligate parasites. There are hundreds of millions of cases of pediculosis worldwide affecting men, women, and children from all socioeconomic classes.
Three types of lice infest humans: the head louse (Pediculus humanus capitis), the body louse (Pediculus humanus corporis), and the pubic louse (Phthirus pubis). Lice cling to hairs with their clawlike legs and pierce the skin, inject saliva, and then defecate while obtaining their blood meal. When exposed to lice, people clinically experience little irritation from the first bite, but after a short period they become sensitized. A hypersensitivity reaction—producing reddening of the skin, itching, and overall inflammation— subsequently develops.
Body lice are similar to head lice, except they are slightly larger. They are primarily seen in the homeless and indigent populations, with transmission occurring with direct body contact or sharing of contaminated clothes or bedding.
The body louse, contrary to popular belief, does not live on the body. It lives in the seams of clothing, clinging to the fibers. It will feed, remaining on the clothes, and only in massive infestations are they typically seen moving about the body. The axillae, groin, and truncal areas are the most severely affected. Patients have severe itching and tend to excoriate these regions. In chronically infested patients a postinflammatory hyperpigmentation can be observed.
Treatment
Persons infested with body lice need to discard or launder their clothing using hot water, and then bathe themselves. In cases of massive infestation—such as this case, in which head lice were also found—a pediculicide should be applied to the hair and entire body from head to toe. This can then be washed off in the shower (level of evidence [LOE]=1a, from Cochrane Review). This same Cochrane systematic review of the treatment of head lice found no evidence that any one pediculicide has greater effect than another (LOE=1a).1
In this case, the patient did not want to get rid of her green pants because she was able to find the “bugs” easily in its seams, which she thought helped keep them under control. When we explained that the full treatment of this condition required her to be given new clothes, she finally accepted this course of action.
Arrangements were made for her to shower at the shelter and obtain new clothes, and she was given permethrin shampoo to apply over her entire body from head to toe. She was scheduled to follow up in 1 week but unfortunately never returned to the clinic.
1. Dodd CS. Interventions for treating headlice (Cochrane Review). In: The Cochrane Library, Issue 2, 2002. Oxford.
1. Dodd CS. Interventions for treating headlice (Cochrane Review). In: The Cochrane Library, Issue 2, 2002. Oxford.
Prostatitis and pruritus
A 47-year-old man had severe itching that started the previous day and kept him up all night. We examined his rash (Figure ) as we inquired about his history. He had no previous skin problems and no known allergies. He had no fever, pain, or malaise.
We discovered that a month ago he had started taking 1 trimethoprim-sulfamethoxazole double-strength tablet twice daily for chronic prostatitis. He was told he would need to take the medication for 3 months. Other than prostatitis, he had no other medical problems and was not taking any other medications.
FIGURE
What is the diagnosis?
What is the best course of management?
Diagnosis
This patient has urticaria (hives), presenting as variously shaped wheals on his chest and arms, resulting from a drug allergy.
These wheals are erythematous, nonpitting, edematous plaques that change size and shape by peripheral extension or regression. Urticaria is a dynamic process in which new wheals evolve as old ones resolve. Wheals result from localized capillary vasodilation, followed by transudation of protein-rich fluid into the surrounding skin. The wheals resolve when the fluid is slowly reabsorbed.
Urticaria symptoms and signs
Itching is the hallmark symptom of urticaria. Patients may also experience burning or stinging. Acute urticaria may exhibit a rapid or gradual onset. The onset and resolution of wheals vary with the cause, and vary even among persons who have the same underlying cause.
It is easier to determine the precipitating factor—a drug allergy in this case—with acute urticaria than chronic urticaria (lasting 6 or more weeks). Angioedema causes a deeper edematous area that involves transudation of fluid into the dermis and subcutaneous tissue.
Wheals vary in size from the small, 2-mm papules of cholinergic urticaria to giant hives that may cover an extremity or part of the abdomen in a single wheal. The wheal may be all red or white, or the border may be red with the remainder of the surface white. Wheals may be surrounded by a red halo. The larger lesions (over 5 mm in diameter) are called plaques. In patients with darker skin, the wheals may be skin-colored only, with no visible erythema.
Differential diagnosis
The full differential diagnosis of urticaria includes angioedema, insect bites, food allergies, erythema multiforme, bullous pemphigoid, dermatitis herpetiformis, urticarial contact dermatitis, pruritic urticarial papules and plaques of pregnancy (known as PUPPP), mast cell releasability syndromes, and urticarial vasculitis.
Food allergies and insect bites can sometimes cause urticarial reactions. Angioedema is seen more often on the face and is especially found around the mouth and eyes.
Management
First-generation antihistamines
H1 antihistamines, which compete with histamine for the H1 receptor sites, are the first-line therapy for urticaria. First-generation antihistamines—such as diphenhydramine, chlorpheniramine, and hydroxyzine—can be very effective, particularly in acute cases. Diphenhydramine and chlorpheniramine are available over-the-counter and are relatively inexpensive. Hydroxyzine still requires a prescription, and it is thought to be more potent than diphenhydramine and chlorpheniramine.
The sedation experienced with these agents may help reduce pruritus, but it may also be a danger when a patient is driving or operating machinery. Because people respond to these medicines differently, you must weigh the bene fits and risks for each person based on their response to the medicine.
The pathophysiology of urticaria and angioedema can be mediated by immunoglobulin E, complement, physical stimuli, or autoantibodies, or it may be idiopathic. These mechanisms lead to mast cell degranulation, resulting in the release of histamine. Histamine and other inflammatory mediators produce the wheals, edema, and pruritus.
Second-generation antihistamines
Second-generation H1 antihistamines—such as astemizole, loratadine, desloratadine, and cetirizine—cause less sedation and are better for long-term daytime use. While more expensive, they are valuable in the management of chronic urticaria.
In the most refractory cases, combinations of various antihistamines may be useful in suppressing symptomatology. A nonsedating H1 antihistamine in the daytime can be combined with a sedating H1antihistamine and doxepin at night. An H2antihistamine can be added to this regimen before starting oral prednisone (level of evidence=5 for all the treatment regimes cited, based on expert opinion without explicit critical appraisal and based on physiology).
This patient’s treatment
The patient understood that he must stop taking the trimethoprim-sulfamethoxazole tablets and was given a fluoroquinolone for his chronic prostatitis. He took 1 dose of diphenhydramine (Benadryl, in this case) in the office, and the itching and wheals began to subside.
He was also told the could purchase diphenhydramine over the counter to continue to relieve his itching and wheals. He was advised that if it made him sleepy, he could call the office for a prescription for a nonsedating antihistamine.
This patient’s outcome
The patient was significantly better the next day and never needed additional medications for urticaria. His chronic prostatitis did resolve with a 2-month course of fluoroquinolone.
A 47-year-old man had severe itching that started the previous day and kept him up all night. We examined his rash (Figure ) as we inquired about his history. He had no previous skin problems and no known allergies. He had no fever, pain, or malaise.
We discovered that a month ago he had started taking 1 trimethoprim-sulfamethoxazole double-strength tablet twice daily for chronic prostatitis. He was told he would need to take the medication for 3 months. Other than prostatitis, he had no other medical problems and was not taking any other medications.
FIGURE
What is the diagnosis?
What is the best course of management?
Diagnosis
This patient has urticaria (hives), presenting as variously shaped wheals on his chest and arms, resulting from a drug allergy.
These wheals are erythematous, nonpitting, edematous plaques that change size and shape by peripheral extension or regression. Urticaria is a dynamic process in which new wheals evolve as old ones resolve. Wheals result from localized capillary vasodilation, followed by transudation of protein-rich fluid into the surrounding skin. The wheals resolve when the fluid is slowly reabsorbed.
Urticaria symptoms and signs
Itching is the hallmark symptom of urticaria. Patients may also experience burning or stinging. Acute urticaria may exhibit a rapid or gradual onset. The onset and resolution of wheals vary with the cause, and vary even among persons who have the same underlying cause.
It is easier to determine the precipitating factor—a drug allergy in this case—with acute urticaria than chronic urticaria (lasting 6 or more weeks). Angioedema causes a deeper edematous area that involves transudation of fluid into the dermis and subcutaneous tissue.
Wheals vary in size from the small, 2-mm papules of cholinergic urticaria to giant hives that may cover an extremity or part of the abdomen in a single wheal. The wheal may be all red or white, or the border may be red with the remainder of the surface white. Wheals may be surrounded by a red halo. The larger lesions (over 5 mm in diameter) are called plaques. In patients with darker skin, the wheals may be skin-colored only, with no visible erythema.
Differential diagnosis
The full differential diagnosis of urticaria includes angioedema, insect bites, food allergies, erythema multiforme, bullous pemphigoid, dermatitis herpetiformis, urticarial contact dermatitis, pruritic urticarial papules and plaques of pregnancy (known as PUPPP), mast cell releasability syndromes, and urticarial vasculitis.
Food allergies and insect bites can sometimes cause urticarial reactions. Angioedema is seen more often on the face and is especially found around the mouth and eyes.
Management
First-generation antihistamines
H1 antihistamines, which compete with histamine for the H1 receptor sites, are the first-line therapy for urticaria. First-generation antihistamines—such as diphenhydramine, chlorpheniramine, and hydroxyzine—can be very effective, particularly in acute cases. Diphenhydramine and chlorpheniramine are available over-the-counter and are relatively inexpensive. Hydroxyzine still requires a prescription, and it is thought to be more potent than diphenhydramine and chlorpheniramine.
The sedation experienced with these agents may help reduce pruritus, but it may also be a danger when a patient is driving or operating machinery. Because people respond to these medicines differently, you must weigh the bene fits and risks for each person based on their response to the medicine.
The pathophysiology of urticaria and angioedema can be mediated by immunoglobulin E, complement, physical stimuli, or autoantibodies, or it may be idiopathic. These mechanisms lead to mast cell degranulation, resulting in the release of histamine. Histamine and other inflammatory mediators produce the wheals, edema, and pruritus.
Second-generation antihistamines
Second-generation H1 antihistamines—such as astemizole, loratadine, desloratadine, and cetirizine—cause less sedation and are better for long-term daytime use. While more expensive, they are valuable in the management of chronic urticaria.
In the most refractory cases, combinations of various antihistamines may be useful in suppressing symptomatology. A nonsedating H1 antihistamine in the daytime can be combined with a sedating H1antihistamine and doxepin at night. An H2antihistamine can be added to this regimen before starting oral prednisone (level of evidence=5 for all the treatment regimes cited, based on expert opinion without explicit critical appraisal and based on physiology).
This patient’s treatment
The patient understood that he must stop taking the trimethoprim-sulfamethoxazole tablets and was given a fluoroquinolone for his chronic prostatitis. He took 1 dose of diphenhydramine (Benadryl, in this case) in the office, and the itching and wheals began to subside.
He was also told the could purchase diphenhydramine over the counter to continue to relieve his itching and wheals. He was advised that if it made him sleepy, he could call the office for a prescription for a nonsedating antihistamine.
This patient’s outcome
The patient was significantly better the next day and never needed additional medications for urticaria. His chronic prostatitis did resolve with a 2-month course of fluoroquinolone.
A 47-year-old man had severe itching that started the previous day and kept him up all night. We examined his rash (Figure ) as we inquired about his history. He had no previous skin problems and no known allergies. He had no fever, pain, or malaise.
We discovered that a month ago he had started taking 1 trimethoprim-sulfamethoxazole double-strength tablet twice daily for chronic prostatitis. He was told he would need to take the medication for 3 months. Other than prostatitis, he had no other medical problems and was not taking any other medications.
FIGURE
What is the diagnosis?
What is the best course of management?
Diagnosis
This patient has urticaria (hives), presenting as variously shaped wheals on his chest and arms, resulting from a drug allergy.
These wheals are erythematous, nonpitting, edematous plaques that change size and shape by peripheral extension or regression. Urticaria is a dynamic process in which new wheals evolve as old ones resolve. Wheals result from localized capillary vasodilation, followed by transudation of protein-rich fluid into the surrounding skin. The wheals resolve when the fluid is slowly reabsorbed.
Urticaria symptoms and signs
Itching is the hallmark symptom of urticaria. Patients may also experience burning or stinging. Acute urticaria may exhibit a rapid or gradual onset. The onset and resolution of wheals vary with the cause, and vary even among persons who have the same underlying cause.
It is easier to determine the precipitating factor—a drug allergy in this case—with acute urticaria than chronic urticaria (lasting 6 or more weeks). Angioedema causes a deeper edematous area that involves transudation of fluid into the dermis and subcutaneous tissue.
Wheals vary in size from the small, 2-mm papules of cholinergic urticaria to giant hives that may cover an extremity or part of the abdomen in a single wheal. The wheal may be all red or white, or the border may be red with the remainder of the surface white. Wheals may be surrounded by a red halo. The larger lesions (over 5 mm in diameter) are called plaques. In patients with darker skin, the wheals may be skin-colored only, with no visible erythema.
Differential diagnosis
The full differential diagnosis of urticaria includes angioedema, insect bites, food allergies, erythema multiforme, bullous pemphigoid, dermatitis herpetiformis, urticarial contact dermatitis, pruritic urticarial papules and plaques of pregnancy (known as PUPPP), mast cell releasability syndromes, and urticarial vasculitis.
Food allergies and insect bites can sometimes cause urticarial reactions. Angioedema is seen more often on the face and is especially found around the mouth and eyes.
Management
First-generation antihistamines
H1 antihistamines, which compete with histamine for the H1 receptor sites, are the first-line therapy for urticaria. First-generation antihistamines—such as diphenhydramine, chlorpheniramine, and hydroxyzine—can be very effective, particularly in acute cases. Diphenhydramine and chlorpheniramine are available over-the-counter and are relatively inexpensive. Hydroxyzine still requires a prescription, and it is thought to be more potent than diphenhydramine and chlorpheniramine.
The sedation experienced with these agents may help reduce pruritus, but it may also be a danger when a patient is driving or operating machinery. Because people respond to these medicines differently, you must weigh the bene fits and risks for each person based on their response to the medicine.
The pathophysiology of urticaria and angioedema can be mediated by immunoglobulin E, complement, physical stimuli, or autoantibodies, or it may be idiopathic. These mechanisms lead to mast cell degranulation, resulting in the release of histamine. Histamine and other inflammatory mediators produce the wheals, edema, and pruritus.
Second-generation antihistamines
Second-generation H1 antihistamines—such as astemizole, loratadine, desloratadine, and cetirizine—cause less sedation and are better for long-term daytime use. While more expensive, they are valuable in the management of chronic urticaria.
In the most refractory cases, combinations of various antihistamines may be useful in suppressing symptomatology. A nonsedating H1 antihistamine in the daytime can be combined with a sedating H1antihistamine and doxepin at night. An H2antihistamine can be added to this regimen before starting oral prednisone (level of evidence=5 for all the treatment regimes cited, based on expert opinion without explicit critical appraisal and based on physiology).
This patient’s treatment
The patient understood that he must stop taking the trimethoprim-sulfamethoxazole tablets and was given a fluoroquinolone for his chronic prostatitis. He took 1 dose of diphenhydramine (Benadryl, in this case) in the office, and the itching and wheals began to subside.
He was also told the could purchase diphenhydramine over the counter to continue to relieve his itching and wheals. He was advised that if it made him sleepy, he could call the office for a prescription for a nonsedating antihistamine.
This patient’s outcome
The patient was significantly better the next day and never needed additional medications for urticaria. His chronic prostatitis did resolve with a 2-month course of fluoroquinolone.