User login
A 25-year-old woman visited her family doctor for treatment of skin lesions on her left leg, which had developed over the previous 8 weeks. The patient had just returned to the US after working with the Peace Corps in the Amazon rain forest for 3 months. During that time, she slept outside in the jungle without any skin protection—no mosquito netting, no insect repellent, no protective clothing on her extremities. She reported no recent history of trauma, burns, or chemical exposure to the leg, and she had been healthy prior to this skin condition. She reported that she was taking no medications, and was not using alcohol or tobacco.
The lesions started with the development of small pustules on her left leg and ankle. Over the next 3 weeks, these grew into larger, ulcerated lesions. The ulcers persisted for 5 more weeks despite multiple attempts at local topical treatments in Ecuador. No pain or itching were associated with the lesions.
The physical examination revealed a generally healthy woman, remarkable only for the 2 skin ulcers on her left lower leg and ankle (Figure 1 and Figure 2). The patient was afebrile, and had no lymphadenopathy or hepatosplenomegaly.
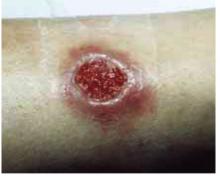
FIGURE 1
Cutaneous ulceration with indurated border.
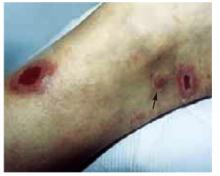
Two active ulcers with small healed satellite lesion near the medial malleolus (see arrow).
How would you treat this condition?
Differential diagnosis of tropical skin ulcers
The woman’s family physician used the Internet to determine that the differential diagnosis of tropical skin ulcers includes localized cutaneous leishmaniasis, foreign body reaction, impetigo, pyoderma gangrenosum, bacterial or deep fungal infection, atypical mycobacterial infection, sarcoidosis, squamous and basal cell carcinoma, and superinfected insect bites. The combination of the patient’s history of potential exposure, clinical presentation, and epidemiology of leishmaniasis within the region in which she was working supports a presumptive diagnosis of localized cutaneous leishmaniasis.
Leishmaniasis
Leishmaniasis is comprised of several diverse diseases with varying degrees of severity, ranging from spontaneously healing skin ulcers to a disfiguring mucocutaneous disease, and even to fatal visceral illness. Endemic worldwide except Australia, Oceania, and Antarctica, leishmaniasis is found in the tropics and subtropics.
Approximately 2 million new cases occur each year, with markedly increasing incidence in several parts of the world resulting from international travel, immigration, military deployment, and human immunodeficiency virus coinfection.1
An estimated 10% of the world’s population is at risk for contracting leishmaniasis. Most cases reported in the US are in travelers to countries where the disease is endemic, although sporadic cases have been reported in Texas and Oklahoma.2
Characteristics
Leishmaniasis is a vector-borne disease, caused by obligate intracellular protozoa of the genus Leishmaniaand transmitted by the bite of infected female sandflies. The disease may take several forms, including cutaneous, mucocutaneous, and visceral leishmaniasis, depending on the particular Leishmaniaspecies and host response.
Localized cutaneous leishmaniasis is characterized clinically by the appearance of skin lesions at the site of the sandfly bite, which typically present as inflammatory papules before progressing to nodules and ulcers. Local lymphadenopathy may occur. Sores may be painless or painful.
Both cutaneous and mucocutaneous forms generally yield normal laboratory values, although complete blood counts may show mild anemia, leukopenia, or thrombocytopenia.3 US travelers may consult numerous physicians before leishmaniasis is diagnosed. The median time from recognition of skin lesions to drug treatment is 112 days.4
Lab results
The patient’s complete blood count with differential, electrolytes, creatinine, liver function tests, and electrocardiogram were within normal limits. An infectious disease consultant suggested direct microscopic visualization of skin biopsies, dermal scrapings, and needle aspirates using Giemsa and Leishman stains, but these failed to reveal any parasitic organisms. It was thought these results were most likely false negatives.
In the best situation, the diagnosis of leishmaniasis is confirmed by isolating, visualizing, and culturing the parasite from infected tissue. Dermal scrapings or needle aspirates may reveal Leishmania amastigotes using a Giemsa stain. Early in the course of localized disease, Leishmania organisms may be numerous and found readily within the cytoplasm of macrophages. However, biopsy specimens from old lesions (>6 months), partially or incompletely treated, are frequently negative.
In vitro culture of the parasite from tissue samples using Nicolle-Noy-McNeal medium is often obtained to aid in diagnosis and to identify Leishmania species. With successful culture, the parasite can be sent to the Centers for Disease Control for speciation. New, rapid tests for leishmaniasis are being developed.
Treatment
Although 90% of skin lesions caused by cutaneous leishmaniasis heal spontaneously to form atrophic scars, the infectious disease consultant recommended treatment to prevent development of disfiguring mucocutaneous disease (level of evidence [LOE]=5)—otherwise, immunity may not be complete and skin ulcers can recur.
Experts believe it is important to treat cutaneous leishmaniasis if a species known or suspected of being capable of converting to the mucocutaneous disease form—eg, New World L braziliensis—is present. These lesions may appear months or even years after the initial exposure, and can be refractory to further treatment. However, surgical excision usually is not recommended because of the risk of relapse and further cosmetic disfigurement.3
Yearly follow-up to evaluate for recurrence or evolution of mucocutaneous leishmaniasis is crucial; early treatment of this form of the disease is more efficacious and can yield more favorable outcomes by limiting potential facial involvement (LOE=5).
Meglumine antimonate
The patient’s initial treatment included gluteal injections of meglumine antimonate at a dosage of 20 mg antimony/kg/d intramuscularly for a total course of 21 days (LOE=5). In addition, one tenth of each dose was injected directly into skin lesions under the peripheral margins, as shown in Figure 3 (LOE=5).
Side effects. The patient’s side effects from the meglumine antimonate therapy included insomnia, lightheadedness, increased fatigue, pain at the gluteal injection sites, bone aches, painful splenomegaly, and left leg myoclonic spasms. These signs and symptoms resolved shortly after completion of the course of therapy with no apparent sequelae. Monitoring cardiac, renal, and hepatic function before and during treatment is important, as meglumine antimonate has significant potential to cause renal and hepatic toxicity.
Outcome
The patient’s skin lesions began to show signs of healing after a few days of treatment. Healed lesions can be seen in Figure 4 Of course, we don’t know that these lesions would not have healed without treatment.
Follow-up diagnostic tests including electrocardiograms, liver function tests, and renal function showed normal values at the conclusion of the 21-day course of therapy.
Fluconazole studied
Since this patient was treated, a Saudi Arabian study was published on use of oral fluconazole 200 mg/d for 6 weeks in patients with cutaneous leishmaniasis (L major).5 At the 3-month followup, healing of lesions was complete for 79% of patients in the fluconazole group and 34% patients in the placebo group (LOE=2b).
The toxicity of this treatment is much lower than the antimonials; we should be looking for further evidence of its benefits in other countries with other species of Leishmania.
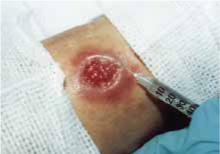
FIGURE 3
Perilesional infiltration of leishmaniasis lesion using meglumine antimonate.
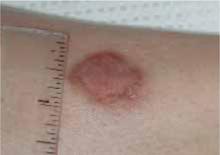
FIGURE 4
Healed cutaneous leishmaniasis lesions on left leg.
Preventing sandfly bites
Leishmaniasis is preventable by avoiding contact with the vector—the sandfly—while living or traveling in endemic areas. Sandflies are most active from dusk to dawn. Though relatively poor fliers, they are small enough to fit through standard mosquito netting and make no audible noise. Effective prevention may be achieved by avoiding nighttime outdoor activities, using topical insecticides (eg, N, N diethyl-m-toluamide [DEET]) on exposed skin surfaces, using insecticide-impregnated clothing (permethrin stays in material for many washings), using fine-mesh mosquito netting, and sleeping with a fan.3
In this time of the globalization of infectious diseases, family doctors in the US should be aware of the tropical diseases that our patients may bring to their offices.
1. Roberts LJ, Handman E, Foote SJ. Science, medicine and the future: leishmaniasis. BMJ 2000;321:801-804.
2. Melby PC, Kreutzer RD, McMahon-Pratt D, Gam AA, Neva FA. Cutaneous leishmaniasis: review of 59 cases seen at the National Institutes of Health. Clin Infect Dis 1992;15:924-937.
3. Kenner JR, Kaugh YC. Leishmaniasis. eMedicine J. 2001 2(11). Available at http://www.emedicine.com/derm/topic219.htm Accessed on May 29, 2003.
4. Herwaldt BL, Stokes SL, Juranek DD. American cutaneous leishmaniasis in U.S. travelers. Ann Intern Med 1993;118:779-784.
5. Alrajhi AA, Ibrahim EA, De Vol EB, Khairat M, Faris RM, Maguire JH. Fluconazole for the treatment of cutaneous leishmaniasis caused by Leishmania major. N Engl J Med 2002;346:891-895.
A 25-year-old woman visited her family doctor for treatment of skin lesions on her left leg, which had developed over the previous 8 weeks. The patient had just returned to the US after working with the Peace Corps in the Amazon rain forest for 3 months. During that time, she slept outside in the jungle without any skin protection—no mosquito netting, no insect repellent, no protective clothing on her extremities. She reported no recent history of trauma, burns, or chemical exposure to the leg, and she had been healthy prior to this skin condition. She reported that she was taking no medications, and was not using alcohol or tobacco.
The lesions started with the development of small pustules on her left leg and ankle. Over the next 3 weeks, these grew into larger, ulcerated lesions. The ulcers persisted for 5 more weeks despite multiple attempts at local topical treatments in Ecuador. No pain or itching were associated with the lesions.
The physical examination revealed a generally healthy woman, remarkable only for the 2 skin ulcers on her left lower leg and ankle (Figure 1 and Figure 2). The patient was afebrile, and had no lymphadenopathy or hepatosplenomegaly.
FIGURE 1
Cutaneous ulceration with indurated border.
Two active ulcers with small healed satellite lesion near the medial malleolus (see arrow).
How would you treat this condition?
Differential diagnosis of tropical skin ulcers
The woman’s family physician used the Internet to determine that the differential diagnosis of tropical skin ulcers includes localized cutaneous leishmaniasis, foreign body reaction, impetigo, pyoderma gangrenosum, bacterial or deep fungal infection, atypical mycobacterial infection, sarcoidosis, squamous and basal cell carcinoma, and superinfected insect bites. The combination of the patient’s history of potential exposure, clinical presentation, and epidemiology of leishmaniasis within the region in which she was working supports a presumptive diagnosis of localized cutaneous leishmaniasis.
Leishmaniasis
Leishmaniasis is comprised of several diverse diseases with varying degrees of severity, ranging from spontaneously healing skin ulcers to a disfiguring mucocutaneous disease, and even to fatal visceral illness. Endemic worldwide except Australia, Oceania, and Antarctica, leishmaniasis is found in the tropics and subtropics.
Approximately 2 million new cases occur each year, with markedly increasing incidence in several parts of the world resulting from international travel, immigration, military deployment, and human immunodeficiency virus coinfection.1
An estimated 10% of the world’s population is at risk for contracting leishmaniasis. Most cases reported in the US are in travelers to countries where the disease is endemic, although sporadic cases have been reported in Texas and Oklahoma.2
Characteristics
Leishmaniasis is a vector-borne disease, caused by obligate intracellular protozoa of the genus Leishmaniaand transmitted by the bite of infected female sandflies. The disease may take several forms, including cutaneous, mucocutaneous, and visceral leishmaniasis, depending on the particular Leishmaniaspecies and host response.
Localized cutaneous leishmaniasis is characterized clinically by the appearance of skin lesions at the site of the sandfly bite, which typically present as inflammatory papules before progressing to nodules and ulcers. Local lymphadenopathy may occur. Sores may be painless or painful.
Both cutaneous and mucocutaneous forms generally yield normal laboratory values, although complete blood counts may show mild anemia, leukopenia, or thrombocytopenia.3 US travelers may consult numerous physicians before leishmaniasis is diagnosed. The median time from recognition of skin lesions to drug treatment is 112 days.4
Lab results
The patient’s complete blood count with differential, electrolytes, creatinine, liver function tests, and electrocardiogram were within normal limits. An infectious disease consultant suggested direct microscopic visualization of skin biopsies, dermal scrapings, and needle aspirates using Giemsa and Leishman stains, but these failed to reveal any parasitic organisms. It was thought these results were most likely false negatives.
In the best situation, the diagnosis of leishmaniasis is confirmed by isolating, visualizing, and culturing the parasite from infected tissue. Dermal scrapings or needle aspirates may reveal Leishmania amastigotes using a Giemsa stain. Early in the course of localized disease, Leishmania organisms may be numerous and found readily within the cytoplasm of macrophages. However, biopsy specimens from old lesions (>6 months), partially or incompletely treated, are frequently negative.
In vitro culture of the parasite from tissue samples using Nicolle-Noy-McNeal medium is often obtained to aid in diagnosis and to identify Leishmania species. With successful culture, the parasite can be sent to the Centers for Disease Control for speciation. New, rapid tests for leishmaniasis are being developed.
Treatment
Although 90% of skin lesions caused by cutaneous leishmaniasis heal spontaneously to form atrophic scars, the infectious disease consultant recommended treatment to prevent development of disfiguring mucocutaneous disease (level of evidence [LOE]=5)—otherwise, immunity may not be complete and skin ulcers can recur.
Experts believe it is important to treat cutaneous leishmaniasis if a species known or suspected of being capable of converting to the mucocutaneous disease form—eg, New World L braziliensis—is present. These lesions may appear months or even years after the initial exposure, and can be refractory to further treatment. However, surgical excision usually is not recommended because of the risk of relapse and further cosmetic disfigurement.3
Yearly follow-up to evaluate for recurrence or evolution of mucocutaneous leishmaniasis is crucial; early treatment of this form of the disease is more efficacious and can yield more favorable outcomes by limiting potential facial involvement (LOE=5).
Meglumine antimonate
The patient’s initial treatment included gluteal injections of meglumine antimonate at a dosage of 20 mg antimony/kg/d intramuscularly for a total course of 21 days (LOE=5). In addition, one tenth of each dose was injected directly into skin lesions under the peripheral margins, as shown in Figure 3 (LOE=5).
Side effects. The patient’s side effects from the meglumine antimonate therapy included insomnia, lightheadedness, increased fatigue, pain at the gluteal injection sites, bone aches, painful splenomegaly, and left leg myoclonic spasms. These signs and symptoms resolved shortly after completion of the course of therapy with no apparent sequelae. Monitoring cardiac, renal, and hepatic function before and during treatment is important, as meglumine antimonate has significant potential to cause renal and hepatic toxicity.
Outcome
The patient’s skin lesions began to show signs of healing after a few days of treatment. Healed lesions can be seen in Figure 4 Of course, we don’t know that these lesions would not have healed without treatment.
Follow-up diagnostic tests including electrocardiograms, liver function tests, and renal function showed normal values at the conclusion of the 21-day course of therapy.
Fluconazole studied
Since this patient was treated, a Saudi Arabian study was published on use of oral fluconazole 200 mg/d for 6 weeks in patients with cutaneous leishmaniasis (L major).5 At the 3-month followup, healing of lesions was complete for 79% of patients in the fluconazole group and 34% patients in the placebo group (LOE=2b).
The toxicity of this treatment is much lower than the antimonials; we should be looking for further evidence of its benefits in other countries with other species of Leishmania.
FIGURE 3
Perilesional infiltration of leishmaniasis lesion using meglumine antimonate.
FIGURE 4
Healed cutaneous leishmaniasis lesions on left leg.
Preventing sandfly bites
Leishmaniasis is preventable by avoiding contact with the vector—the sandfly—while living or traveling in endemic areas. Sandflies are most active from dusk to dawn. Though relatively poor fliers, they are small enough to fit through standard mosquito netting and make no audible noise. Effective prevention may be achieved by avoiding nighttime outdoor activities, using topical insecticides (eg, N, N diethyl-m-toluamide [DEET]) on exposed skin surfaces, using insecticide-impregnated clothing (permethrin stays in material for many washings), using fine-mesh mosquito netting, and sleeping with a fan.3
In this time of the globalization of infectious diseases, family doctors in the US should be aware of the tropical diseases that our patients may bring to their offices.
A 25-year-old woman visited her family doctor for treatment of skin lesions on her left leg, which had developed over the previous 8 weeks. The patient had just returned to the US after working with the Peace Corps in the Amazon rain forest for 3 months. During that time, she slept outside in the jungle without any skin protection—no mosquito netting, no insect repellent, no protective clothing on her extremities. She reported no recent history of trauma, burns, or chemical exposure to the leg, and she had been healthy prior to this skin condition. She reported that she was taking no medications, and was not using alcohol or tobacco.
The lesions started with the development of small pustules on her left leg and ankle. Over the next 3 weeks, these grew into larger, ulcerated lesions. The ulcers persisted for 5 more weeks despite multiple attempts at local topical treatments in Ecuador. No pain or itching were associated with the lesions.
The physical examination revealed a generally healthy woman, remarkable only for the 2 skin ulcers on her left lower leg and ankle (Figure 1 and Figure 2). The patient was afebrile, and had no lymphadenopathy or hepatosplenomegaly.
FIGURE 1
Cutaneous ulceration with indurated border.
Two active ulcers with small healed satellite lesion near the medial malleolus (see arrow).
How would you treat this condition?
Differential diagnosis of tropical skin ulcers
The woman’s family physician used the Internet to determine that the differential diagnosis of tropical skin ulcers includes localized cutaneous leishmaniasis, foreign body reaction, impetigo, pyoderma gangrenosum, bacterial or deep fungal infection, atypical mycobacterial infection, sarcoidosis, squamous and basal cell carcinoma, and superinfected insect bites. The combination of the patient’s history of potential exposure, clinical presentation, and epidemiology of leishmaniasis within the region in which she was working supports a presumptive diagnosis of localized cutaneous leishmaniasis.
Leishmaniasis
Leishmaniasis is comprised of several diverse diseases with varying degrees of severity, ranging from spontaneously healing skin ulcers to a disfiguring mucocutaneous disease, and even to fatal visceral illness. Endemic worldwide except Australia, Oceania, and Antarctica, leishmaniasis is found in the tropics and subtropics.
Approximately 2 million new cases occur each year, with markedly increasing incidence in several parts of the world resulting from international travel, immigration, military deployment, and human immunodeficiency virus coinfection.1
An estimated 10% of the world’s population is at risk for contracting leishmaniasis. Most cases reported in the US are in travelers to countries where the disease is endemic, although sporadic cases have been reported in Texas and Oklahoma.2
Characteristics
Leishmaniasis is a vector-borne disease, caused by obligate intracellular protozoa of the genus Leishmaniaand transmitted by the bite of infected female sandflies. The disease may take several forms, including cutaneous, mucocutaneous, and visceral leishmaniasis, depending on the particular Leishmaniaspecies and host response.
Localized cutaneous leishmaniasis is characterized clinically by the appearance of skin lesions at the site of the sandfly bite, which typically present as inflammatory papules before progressing to nodules and ulcers. Local lymphadenopathy may occur. Sores may be painless or painful.
Both cutaneous and mucocutaneous forms generally yield normal laboratory values, although complete blood counts may show mild anemia, leukopenia, or thrombocytopenia.3 US travelers may consult numerous physicians before leishmaniasis is diagnosed. The median time from recognition of skin lesions to drug treatment is 112 days.4
Lab results
The patient’s complete blood count with differential, electrolytes, creatinine, liver function tests, and electrocardiogram were within normal limits. An infectious disease consultant suggested direct microscopic visualization of skin biopsies, dermal scrapings, and needle aspirates using Giemsa and Leishman stains, but these failed to reveal any parasitic organisms. It was thought these results were most likely false negatives.
In the best situation, the diagnosis of leishmaniasis is confirmed by isolating, visualizing, and culturing the parasite from infected tissue. Dermal scrapings or needle aspirates may reveal Leishmania amastigotes using a Giemsa stain. Early in the course of localized disease, Leishmania organisms may be numerous and found readily within the cytoplasm of macrophages. However, biopsy specimens from old lesions (>6 months), partially or incompletely treated, are frequently negative.
In vitro culture of the parasite from tissue samples using Nicolle-Noy-McNeal medium is often obtained to aid in diagnosis and to identify Leishmania species. With successful culture, the parasite can be sent to the Centers for Disease Control for speciation. New, rapid tests for leishmaniasis are being developed.
Treatment
Although 90% of skin lesions caused by cutaneous leishmaniasis heal spontaneously to form atrophic scars, the infectious disease consultant recommended treatment to prevent development of disfiguring mucocutaneous disease (level of evidence [LOE]=5)—otherwise, immunity may not be complete and skin ulcers can recur.
Experts believe it is important to treat cutaneous leishmaniasis if a species known or suspected of being capable of converting to the mucocutaneous disease form—eg, New World L braziliensis—is present. These lesions may appear months or even years after the initial exposure, and can be refractory to further treatment. However, surgical excision usually is not recommended because of the risk of relapse and further cosmetic disfigurement.3
Yearly follow-up to evaluate for recurrence or evolution of mucocutaneous leishmaniasis is crucial; early treatment of this form of the disease is more efficacious and can yield more favorable outcomes by limiting potential facial involvement (LOE=5).
Meglumine antimonate
The patient’s initial treatment included gluteal injections of meglumine antimonate at a dosage of 20 mg antimony/kg/d intramuscularly for a total course of 21 days (LOE=5). In addition, one tenth of each dose was injected directly into skin lesions under the peripheral margins, as shown in Figure 3 (LOE=5).
Side effects. The patient’s side effects from the meglumine antimonate therapy included insomnia, lightheadedness, increased fatigue, pain at the gluteal injection sites, bone aches, painful splenomegaly, and left leg myoclonic spasms. These signs and symptoms resolved shortly after completion of the course of therapy with no apparent sequelae. Monitoring cardiac, renal, and hepatic function before and during treatment is important, as meglumine antimonate has significant potential to cause renal and hepatic toxicity.
Outcome
The patient’s skin lesions began to show signs of healing after a few days of treatment. Healed lesions can be seen in Figure 4 Of course, we don’t know that these lesions would not have healed without treatment.
Follow-up diagnostic tests including electrocardiograms, liver function tests, and renal function showed normal values at the conclusion of the 21-day course of therapy.
Fluconazole studied
Since this patient was treated, a Saudi Arabian study was published on use of oral fluconazole 200 mg/d for 6 weeks in patients with cutaneous leishmaniasis (L major).5 At the 3-month followup, healing of lesions was complete for 79% of patients in the fluconazole group and 34% patients in the placebo group (LOE=2b).
The toxicity of this treatment is much lower than the antimonials; we should be looking for further evidence of its benefits in other countries with other species of Leishmania.
FIGURE 3
Perilesional infiltration of leishmaniasis lesion using meglumine antimonate.
FIGURE 4
Healed cutaneous leishmaniasis lesions on left leg.
Preventing sandfly bites
Leishmaniasis is preventable by avoiding contact with the vector—the sandfly—while living or traveling in endemic areas. Sandflies are most active from dusk to dawn. Though relatively poor fliers, they are small enough to fit through standard mosquito netting and make no audible noise. Effective prevention may be achieved by avoiding nighttime outdoor activities, using topical insecticides (eg, N, N diethyl-m-toluamide [DEET]) on exposed skin surfaces, using insecticide-impregnated clothing (permethrin stays in material for many washings), using fine-mesh mosquito netting, and sleeping with a fan.3
In this time of the globalization of infectious diseases, family doctors in the US should be aware of the tropical diseases that our patients may bring to their offices.
1. Roberts LJ, Handman E, Foote SJ. Science, medicine and the future: leishmaniasis. BMJ 2000;321:801-804.
2. Melby PC, Kreutzer RD, McMahon-Pratt D, Gam AA, Neva FA. Cutaneous leishmaniasis: review of 59 cases seen at the National Institutes of Health. Clin Infect Dis 1992;15:924-937.
3. Kenner JR, Kaugh YC. Leishmaniasis. eMedicine J. 2001 2(11). Available at http://www.emedicine.com/derm/topic219.htm Accessed on May 29, 2003.
4. Herwaldt BL, Stokes SL, Juranek DD. American cutaneous leishmaniasis in U.S. travelers. Ann Intern Med 1993;118:779-784.
5. Alrajhi AA, Ibrahim EA, De Vol EB, Khairat M, Faris RM, Maguire JH. Fluconazole for the treatment of cutaneous leishmaniasis caused by Leishmania major. N Engl J Med 2002;346:891-895.
1. Roberts LJ, Handman E, Foote SJ. Science, medicine and the future: leishmaniasis. BMJ 2000;321:801-804.
2. Melby PC, Kreutzer RD, McMahon-Pratt D, Gam AA, Neva FA. Cutaneous leishmaniasis: review of 59 cases seen at the National Institutes of Health. Clin Infect Dis 1992;15:924-937.
3. Kenner JR, Kaugh YC. Leishmaniasis. eMedicine J. 2001 2(11). Available at http://www.emedicine.com/derm/topic219.htm Accessed on May 29, 2003.
4. Herwaldt BL, Stokes SL, Juranek DD. American cutaneous leishmaniasis in U.S. travelers. Ann Intern Med 1993;118:779-784.
5. Alrajhi AA, Ibrahim EA, De Vol EB, Khairat M, Faris RM, Maguire JH. Fluconazole for the treatment of cutaneous leishmaniasis caused by Leishmania major. N Engl J Med 2002;346:891-895.