User login
An algorithmic approach to otitis media with effusion
› Perform a hearing test when otitis media with effusion is present for 3 months or longer, or whenever you suspect a language delay, learning problems, or a significant hearing loss. C
› Use the results of the hearing test to guide management decisions. C
Strength of recommendation (SOR)
A Good-quality patient-oriented evidence
B Inconsistent or limited-quality patient-oriented evidence
C Consensus, usual practice, opinion, disease-oriented evidence, case series
CASE › A mother brings in her 3-year-old son for a regular check-up. Her only concern is that for the past 2 weeks, he has not been sleeping through the night. She indicates that the sleeping problem began after he was diagnosed with and treated for an ear infection. Fortunately, this hasn’t affected his daily activity or energy, she says.
The child’s appetite is good and he speaks clearly, in 5-word sentences. He is meeting his developmental milestones, and appears well—sitting in his mother’s lap and playing with her smartphone. His head, eyes, ears, nose, and throat exam only turns up fluid behind his left tympanic membrane, which is not red or bulging. The right membrane appears normal, and he has no cervical lymphadenopathy. The rest of his exam is normal. How would you manage a patient like this?
"Glue ear" is often asymptomatic
Otitis media with effusion (OME) is defined as middle-ear effusion (MEE) in the absence of acute signs of infection. In children, OME—also referred to as “glue ear”—most often arises after acute otitis media (AOM). In adults, it often occurs in association with eustachian tube dysfunction, although OME is a separate diagnosis. (To learn more, see “What about OME in adults?”1,2.)
Experts have found it difficult to determine the exact incidence of OME because it is often asymptomatic. In addition, many cases quickly resolve on their own, making it challenging to diagnose. A 2-year prospective study of 2- to 6-year-old preschoolers revealed that MEE, diagnosed via monthly otoscopy and tympanometry, occurred at least once in 53% of the children in the first year and in 61% of the children in the second year.3 A second study followed 7-year-olds monthly for one year and found a 31% incidence of MEE using tympanometry.4 In the 25% of children found to have persistent MEE, the researchers noted spontaneous recovery after an average of 2 months.
We believe that nearly all children have experienced one episode of OME by the age of 3 years, but the prevalence of OME varies with age and the time of year. It is more prevalent in the winter than the summer months.5 OME is more common in Caucasian children than in African American or Asian children.6
Etiology remains elusive
Risk factors for children include a family history of OME, bottle-feeding, day care attendance, exposure to tobacco smoke, and a personal history of allergies.7,8 One study conducted on mice suggested that inherited structural abnormalities of the middle ear and eustachian tube may play a role as well.9 Some have suggested that effusions of OME in children result from chronic inflammation, for example, after AOM, and that the effusions are sterile; however, recent studies have demonstrated that a biofilm is formed by bacterial otopathogens in the effusion.10-12 The common pathogens found include nontypeable Haemophilus influenza, Streptococcus pneumoniae, and Moraxella catarrhalis. Inflammatory exudate or neutrophil infiltration is rare in the fluid, however.
The contribution of allergies to OME in children remains somewhat controversial. A retrospective review from the United Kingdom of 209 children with OME found a history of allergic rhinitis, asthma, and eczema in 89%, 36%, and 24%, respectively.13 However, this study was done at an allergy clinic, and it is possible that the data from the clinic’s specialized patient population are not generalizable. Gastroesophageal reflux may also be associated with OME in children. However, studies measuring the concentration of pepsin and pepsinogen in middle-ear fluid have provided conflicting results.14,15
Look for these signs and symptoms
OME is often asymptomatic. If a patient has clinical signs of an acute illness, including fever and an erythematous tympanic membrane, it’s important to evaluate for another cause. OME can present with hearing loss or a sense of fullness in the ear. While an infant cannot express the hearing loss, the parent may detect it when observing and interacting mwith the child. Parents are also likely to report that the child is experiencing sleep disturbances.16
Vertigo may occur with OME, although not often. It may manifest itself if the child stumbles or falls. An older child or adult with vertigo may say that it feels like the room is spinning.
Diagnosis relies on pneumatic otoscopy
On physical exam, the patient will likely appear well. Otoscopic examination reveals fluid behind a normal or retracted tympanic membrane; the fluid is often clear or yellowish in color.
A subcommittee comprised of members of the American Academy of Pediatrics, American Academy of Family Physicians, and the American Academy of Otolaryngology-Head and Neck Surgery (AAP/AAFP/AAOHNS) published a clinical practice guideline in 2004 that delineates the current diagnosis and management of children between 2 months and 12 years of age with OME.17
Pneumatic otoscopy, which can reveal decreased or absent movement of the tympanic membrane (the result of fluid behind the membrane), is the primary diagnostic method recommended by the guideline. Tympanometry and acoustic reflectometry may also be used to make the diagnosis, especially when the presence of MEE is difficult to determine using pneumatic otoscopy.
CASE › Upon further discussion with the patient’s mother, you learn that the boy goes to day care 3 days a week and stays with his grandmother 2 days a week. His grandmother smokes outside of the home when he is staying with her.
After reviewing these risk factors with the mother (including the importance of smoking cessation for the grandmother), the mother asks if he needs antibiotics or a referral to a specialist for treating his OME.
How best to approach treatment
There are several management options to choose from, including watchful waiting, medication, and/or surgery. (Another option, autoinflation, which has shown some short-term benefits, is described in “Should you recommend autoinflation?”17-19.)
The goals of management are to resolve the effusion, restore normal hearing (if diminished secondary to the effusion), and prevent future episodes or sequelae. The most significant complication of OME is permanent conductive hearing loss, but tinnitus, cholesteatoma, or tympanosclerosis may also occur.
In most patients, OME resolves without medical intervention. If additional action is required, however, the following options may be explored.
Medication. While the AAP/AAFP/AAOHNS guideline recommends against routine antibiotics for OME,17 it does note that a short course may provide short-term benefit to some patients (eg, those for whom a specialist referral or surgery is being considered).
A separate meta-analysis found that antibiotics improve clearance of the effusion within the first month after treatment (rate difference [RD]=0.16; 95% confidence interval [CI], 0.03-0.29 in 12 studies analyzed), but effusion relapses were common, and no significant benefit was noted past the first month (RD=0.06; 95% CI, -0.03 to 0.14 in 8 studies).20
If you do use antibiotics, a 10- to 14-day course is preferred.17 Amoxicillin, amoxicillin-clavulanate and ceftibuten have been evaluated in separate clinical trials, but none has been clearly shown to have significant advantage over any other.21,22
Antihistamines, decongestants, and oral and intranasal corticosteroids have little effect on OME in children and are not recommended.17 A Cochrane review including 16 studies found that children receiving antihistamines and decongestants are unlikely to see their symptoms improve significantly, and many patients experience adverse effects from the medications23 (number needed to harm=9).
A randomized, double-blind trial involving 144 children <9 years of age with OME for at least 2 months evaluated 4 regimens involving amoxicillin alone or in combination with prednisolone. Children in the amoxicillin+prednisolone arms were significantly more likely to clear their effusions at 2 weeks (number needed to treat=6; P=.03), but not at 4 weeks (P=.12). At 4-month follow-up, effusions had recurred in 68.4% and 69.2% of those receiving amoxicillin+prednisolone and those receiving amoxicillin alone, respectively (P= .94).24
Surgery—or not? The AAP/AAFP/AAOHNS guideline recommends physicians perform hearing testing when OME is present for 3 months or longer, or at any time if language delay, learning problems, or a significant hearing loss is suspected in a child with OME. The results of the hearing test can help determine how to proceed, based on the hearing level noted for the better hearing ear.
You can manage children with hearing loss ≤20 dB and without speech, language, or developmental problems with watchful waiting. Children with hearing loss of 21 to 39 dB can be managed with watchful waiting or referred for surgery. If watchful waiting is pursued, there are interventions at home and at school that can help. These include speaking near the child, facing the child when speaking, and providing accommodations in school so the child sits closer to the teacher. Consider re-examination and repeat hearing tests every 3 to 6 months until the effusion has resolved or the child develops symptoms indicating surgical referral.
When hearing loss is ≥40 dB, the AAP/AAFP/AAOHNS guideline recommends that you make a referral for surgical evaluation (ALGORITHM).17
Other indications for referral to a surgeon for evaluation of tympanostomy tube placement include situations in which there is:
• structural damage to the tympanic membrane or middle ear (prompt referral is recommended)
• OME of ≥4 months’ duration with persistent hearing loss (≥40 dB) or other signs or symptoms related to the effusion
• bilateral OME for ≥3 months, unilateral OME ≥6 months, or total duration of any degree of OME ≥12 months.17
Any decision regarding surgery should involve an otolaryngologist, the primary care provider, and the patient and/or family. The AAP/AAFP/AAOHNS guideline recommends against adenoidectomy in children with persistent OME without an indication for the procedure other than OME (eg, chronic sinusitis or nasal obstruction).17
Keep in mind that evidence of lasting benefit (>12 months) is limited for surgery in most patients, and the surgical and anesthetic risks must be considered before moving forward.17 (For more on the evidence regarding surgery, see “Cochrane weighs in on tympanostomy tubes”.25) Tonsillectomy also does not appear to affect outcomes and is not advised.17
When a referral is always needed. Regardless of hearing status, promptly refer children with recurrent or persistent OME who are at risk of speech, language, or learning problems (including those with autism spectrum disorder, developmental delay, Down’s syndrome, diagnosed speech or language delay, or craniofacial disorders such as cleft palate) to a specialist.17
CASE › You tell your young patient’s mother that watchful waiting is appropriate at this point, since his acute otitis media was only 2 weeks ago, and his OME likely started after the acute infection. Given that his speech is clear and he is otherwise meeting his milestones, you tell her that he does not need a referral at this time, but that she should bring him back in 4 weeks for reassessment. At the next visit, his effusion has resolved, and his mother reports he is sleeping well through the night again.
1. Lesinskas E. Factors affecting the results of nonsurgical treatment of secretory otitis media in adults. Auris Nasus Larynx. 2003:30:7-14.
2. Chole RA, HH Sudhoff. Chronic otitis media, mastoiditis, and petrosis. In: Flint PW, Haughey BH, Lund VJ, et al, eds. Cummings Otolaryngology: Head and Neck Surgery. 5th ed. Maryland Heights, MO. Mosby;2010:chap 139.
3. Casselbrant ML, Brostoff LM, Cantekin EI, et al. Otitis media with effusion in preschool children. Laryngoscope. 1985;95:428-436.
4. Lous J, Fiellau-Nikolajsen M. Epidemiology of middle-ear effusion and tubal dysfunction: a one year prospective study comprising monthly tympanometry in 387 non-selected seven-yearold children. Int J Pediatr Otorhinolaryngol. 1981;3:303-317.
5. Tos M, Holm-Jensen S, Sørensen CH. Changes in prevalence of secretory otitis from summer to winter in four-year-old children. Am J Otol. 1981;2:324-327.
6. Vernacchio L, Lesko SM, Vezina RM, et al. Racial/ethnic disparities in the diagnosis of otitis media in infancy. Int J Pediatr Otorhinolaryngol. 2004;68:795-804.
7. Owen MJ, Baldwin CD, Swank PR, et al. Relation of infant feeding practices, cigarette smoke exposure, and group child care to the onset and duration of otitis media with effusion in the first two years of life. J Pediatr. 1993;123:702-711.
8. Gultekin E, Develio˘gu ON, Yener M, et al. Prevalence and risk factors for persistent otitis media with effusion in primary school children in Istanbul, Turkey. Auris Nasus Larynx. 2010;37:145-149.
9. Depreux FF, Darrow K, Conner DA, et al. Eya4-deficient mice are a model for heritable otitis media. J Clin Invest. 2008;118:651-658.
10. Poetker DM, Lindstrom DR, Edmiston CE, et al. Microbiology of middle ear effusions from 292 patients undergoing tympanostomy tube placement for middle ear disease. Int J Pediatr Otorhinolaryngol. 2005;69:799-804.
11. Hall-Stoodley L, Hu FZ, Gieseke A, et al. Direct detection of bacterial biofilms on the middle-ear mucosa of children with chronic otitis media. JAMA. 2006;296:202-211.
12. Brook I, Yocum P, Shah K, et al. Microbiology of serous otitis media in children: correlation with age and length of effusion. Ann Otol Rhinol Laryngol. 2001;110:87-90.
13. Alles R, Parikh A, Hawk L, et al. The prevalence of atopic disorders in children with chronic otitis media with effusion. Pediatr Allergy Immunol. 2001;12:102-106.
14. Lieu JE, Muthappan PG, Uppaluri R. Association of reflux with otitis media in children. Otolaryngol Head Neck Surg. 2005;133:357-361.
15. O’Reilly RC, He Z, Bloedon E, et al. The role of extraesophageal reflux in otitis media in infants and children. Laryngoscope. 2008;118 (7 part 2, suppl 116):S1-S9.
16. Rosenfeld RM, Goldsmith AJ, Tetlus L, et al. Quality of life for children with otitis media. Arch Otolaryngol Head Neck Surg. 1997;123:1049-1054.
17. American Academy of Family Physicians, American Academy of Otolaryngology-Head and Neck Surgery, American Academy of Pediatrics Subcommittee on Otitis Media With Effusion. Otitis media with effusion. Pediatrics. 2004;113:1412-1429.
18. Perera R, Glasziou PP, Heneghan CJ, et al Autoinflation for hearing loss associated with otitis media with effusion. Cochrane Database Syst Rev. 2013;5:CD006285.
19. Stangerup SE, Sederberg-Olsen J, Balle V. Autoinflation as a treatment of secretory otitis media. A randomized controlled study. Arch Otolaryngol Head Neck Surg. 1992;118:149-152.
20. Williams RL, Chalmers TC, Stange KC, et al. Use of antibiotics in preventing recurrent acute otitis media and in treating otitis media with effusion. A meta-analytic attempt to resolve the brouhaha. JAMA. 1993;270:1344-1351.
21. Mandel EM, Casselbrant ML, Kurs-Lasky M, et al. Efficacy of ceftibuten compared with amoxicillin for otitis media with effusion in infants and children. Pediatr Infect Dis J. 1996;15:409-414.
22. Chan KH, Mandel EM, Rockette HE, et al. A comparative study of amoxicillin-clavulanate and amoxicillin. Treatment of otitis media with effusion. Arch Otolaryngol Head Neck Surg. 1988; 114:142-146.
23. Griffin G, Flynn CA. Antihistamines and/or decongestants for otitis media with effusion (OME) in children. Cochrane Database Syst Rev. 2011;(9):CD003423.
24. Mandel EM, Casselbrant ML, Rockette HE, et al. Systemic steroid for chronic otitis media with effusion in children. Pediatrics. 2002;110:1071-1080.
25. Browning GG, Rovers MM, Williamson I, et al. Grommets (ventilation tubes) for hearing loss associated with otitis media with effusion in children. Cochrane Database Syst Rev. 2010;(10):CD001801.
› Perform a hearing test when otitis media with effusion is present for 3 months or longer, or whenever you suspect a language delay, learning problems, or a significant hearing loss. C
› Use the results of the hearing test to guide management decisions. C
Strength of recommendation (SOR)
A Good-quality patient-oriented evidence
B Inconsistent or limited-quality patient-oriented evidence
C Consensus, usual practice, opinion, disease-oriented evidence, case series
CASE › A mother brings in her 3-year-old son for a regular check-up. Her only concern is that for the past 2 weeks, he has not been sleeping through the night. She indicates that the sleeping problem began after he was diagnosed with and treated for an ear infection. Fortunately, this hasn’t affected his daily activity or energy, she says.
The child’s appetite is good and he speaks clearly, in 5-word sentences. He is meeting his developmental milestones, and appears well—sitting in his mother’s lap and playing with her smartphone. His head, eyes, ears, nose, and throat exam only turns up fluid behind his left tympanic membrane, which is not red or bulging. The right membrane appears normal, and he has no cervical lymphadenopathy. The rest of his exam is normal. How would you manage a patient like this?
"Glue ear" is often asymptomatic
Otitis media with effusion (OME) is defined as middle-ear effusion (MEE) in the absence of acute signs of infection. In children, OME—also referred to as “glue ear”—most often arises after acute otitis media (AOM). In adults, it often occurs in association with eustachian tube dysfunction, although OME is a separate diagnosis. (To learn more, see “What about OME in adults?”1,2.)
Experts have found it difficult to determine the exact incidence of OME because it is often asymptomatic. In addition, many cases quickly resolve on their own, making it challenging to diagnose. A 2-year prospective study of 2- to 6-year-old preschoolers revealed that MEE, diagnosed via monthly otoscopy and tympanometry, occurred at least once in 53% of the children in the first year and in 61% of the children in the second year.3 A second study followed 7-year-olds monthly for one year and found a 31% incidence of MEE using tympanometry.4 In the 25% of children found to have persistent MEE, the researchers noted spontaneous recovery after an average of 2 months.
We believe that nearly all children have experienced one episode of OME by the age of 3 years, but the prevalence of OME varies with age and the time of year. It is more prevalent in the winter than the summer months.5 OME is more common in Caucasian children than in African American or Asian children.6
Etiology remains elusive
Risk factors for children include a family history of OME, bottle-feeding, day care attendance, exposure to tobacco smoke, and a personal history of allergies.7,8 One study conducted on mice suggested that inherited structural abnormalities of the middle ear and eustachian tube may play a role as well.9 Some have suggested that effusions of OME in children result from chronic inflammation, for example, after AOM, and that the effusions are sterile; however, recent studies have demonstrated that a biofilm is formed by bacterial otopathogens in the effusion.10-12 The common pathogens found include nontypeable Haemophilus influenza, Streptococcus pneumoniae, and Moraxella catarrhalis. Inflammatory exudate or neutrophil infiltration is rare in the fluid, however.
The contribution of allergies to OME in children remains somewhat controversial. A retrospective review from the United Kingdom of 209 children with OME found a history of allergic rhinitis, asthma, and eczema in 89%, 36%, and 24%, respectively.13 However, this study was done at an allergy clinic, and it is possible that the data from the clinic’s specialized patient population are not generalizable. Gastroesophageal reflux may also be associated with OME in children. However, studies measuring the concentration of pepsin and pepsinogen in middle-ear fluid have provided conflicting results.14,15
Look for these signs and symptoms
OME is often asymptomatic. If a patient has clinical signs of an acute illness, including fever and an erythematous tympanic membrane, it’s important to evaluate for another cause. OME can present with hearing loss or a sense of fullness in the ear. While an infant cannot express the hearing loss, the parent may detect it when observing and interacting mwith the child. Parents are also likely to report that the child is experiencing sleep disturbances.16
Vertigo may occur with OME, although not often. It may manifest itself if the child stumbles or falls. An older child or adult with vertigo may say that it feels like the room is spinning.
Diagnosis relies on pneumatic otoscopy
On physical exam, the patient will likely appear well. Otoscopic examination reveals fluid behind a normal or retracted tympanic membrane; the fluid is often clear or yellowish in color.
A subcommittee comprised of members of the American Academy of Pediatrics, American Academy of Family Physicians, and the American Academy of Otolaryngology-Head and Neck Surgery (AAP/AAFP/AAOHNS) published a clinical practice guideline in 2004 that delineates the current diagnosis and management of children between 2 months and 12 years of age with OME.17
Pneumatic otoscopy, which can reveal decreased or absent movement of the tympanic membrane (the result of fluid behind the membrane), is the primary diagnostic method recommended by the guideline. Tympanometry and acoustic reflectometry may also be used to make the diagnosis, especially when the presence of MEE is difficult to determine using pneumatic otoscopy.
CASE › Upon further discussion with the patient’s mother, you learn that the boy goes to day care 3 days a week and stays with his grandmother 2 days a week. His grandmother smokes outside of the home when he is staying with her.
After reviewing these risk factors with the mother (including the importance of smoking cessation for the grandmother), the mother asks if he needs antibiotics or a referral to a specialist for treating his OME.
How best to approach treatment
There are several management options to choose from, including watchful waiting, medication, and/or surgery. (Another option, autoinflation, which has shown some short-term benefits, is described in “Should you recommend autoinflation?”17-19.)
The goals of management are to resolve the effusion, restore normal hearing (if diminished secondary to the effusion), and prevent future episodes or sequelae. The most significant complication of OME is permanent conductive hearing loss, but tinnitus, cholesteatoma, or tympanosclerosis may also occur.
In most patients, OME resolves without medical intervention. If additional action is required, however, the following options may be explored.
Medication. While the AAP/AAFP/AAOHNS guideline recommends against routine antibiotics for OME,17 it does note that a short course may provide short-term benefit to some patients (eg, those for whom a specialist referral or surgery is being considered).
A separate meta-analysis found that antibiotics improve clearance of the effusion within the first month after treatment (rate difference [RD]=0.16; 95% confidence interval [CI], 0.03-0.29 in 12 studies analyzed), but effusion relapses were common, and no significant benefit was noted past the first month (RD=0.06; 95% CI, -0.03 to 0.14 in 8 studies).20
If you do use antibiotics, a 10- to 14-day course is preferred.17 Amoxicillin, amoxicillin-clavulanate and ceftibuten have been evaluated in separate clinical trials, but none has been clearly shown to have significant advantage over any other.21,22
Antihistamines, decongestants, and oral and intranasal corticosteroids have little effect on OME in children and are not recommended.17 A Cochrane review including 16 studies found that children receiving antihistamines and decongestants are unlikely to see their symptoms improve significantly, and many patients experience adverse effects from the medications23 (number needed to harm=9).
A randomized, double-blind trial involving 144 children <9 years of age with OME for at least 2 months evaluated 4 regimens involving amoxicillin alone or in combination with prednisolone. Children in the amoxicillin+prednisolone arms were significantly more likely to clear their effusions at 2 weeks (number needed to treat=6; P=.03), but not at 4 weeks (P=.12). At 4-month follow-up, effusions had recurred in 68.4% and 69.2% of those receiving amoxicillin+prednisolone and those receiving amoxicillin alone, respectively (P= .94).24
Surgery—or not? The AAP/AAFP/AAOHNS guideline recommends physicians perform hearing testing when OME is present for 3 months or longer, or at any time if language delay, learning problems, or a significant hearing loss is suspected in a child with OME. The results of the hearing test can help determine how to proceed, based on the hearing level noted for the better hearing ear.
You can manage children with hearing loss ≤20 dB and without speech, language, or developmental problems with watchful waiting. Children with hearing loss of 21 to 39 dB can be managed with watchful waiting or referred for surgery. If watchful waiting is pursued, there are interventions at home and at school that can help. These include speaking near the child, facing the child when speaking, and providing accommodations in school so the child sits closer to the teacher. Consider re-examination and repeat hearing tests every 3 to 6 months until the effusion has resolved or the child develops symptoms indicating surgical referral.
When hearing loss is ≥40 dB, the AAP/AAFP/AAOHNS guideline recommends that you make a referral for surgical evaluation (ALGORITHM).17
Other indications for referral to a surgeon for evaluation of tympanostomy tube placement include situations in which there is:
• structural damage to the tympanic membrane or middle ear (prompt referral is recommended)
• OME of ≥4 months’ duration with persistent hearing loss (≥40 dB) or other signs or symptoms related to the effusion
• bilateral OME for ≥3 months, unilateral OME ≥6 months, or total duration of any degree of OME ≥12 months.17
Any decision regarding surgery should involve an otolaryngologist, the primary care provider, and the patient and/or family. The AAP/AAFP/AAOHNS guideline recommends against adenoidectomy in children with persistent OME without an indication for the procedure other than OME (eg, chronic sinusitis or nasal obstruction).17
Keep in mind that evidence of lasting benefit (>12 months) is limited for surgery in most patients, and the surgical and anesthetic risks must be considered before moving forward.17 (For more on the evidence regarding surgery, see “Cochrane weighs in on tympanostomy tubes”.25) Tonsillectomy also does not appear to affect outcomes and is not advised.17
When a referral is always needed. Regardless of hearing status, promptly refer children with recurrent or persistent OME who are at risk of speech, language, or learning problems (including those with autism spectrum disorder, developmental delay, Down’s syndrome, diagnosed speech or language delay, or craniofacial disorders such as cleft palate) to a specialist.17
CASE › You tell your young patient’s mother that watchful waiting is appropriate at this point, since his acute otitis media was only 2 weeks ago, and his OME likely started after the acute infection. Given that his speech is clear and he is otherwise meeting his milestones, you tell her that he does not need a referral at this time, but that she should bring him back in 4 weeks for reassessment. At the next visit, his effusion has resolved, and his mother reports he is sleeping well through the night again.
› Perform a hearing test when otitis media with effusion is present for 3 months or longer, or whenever you suspect a language delay, learning problems, or a significant hearing loss. C
› Use the results of the hearing test to guide management decisions. C
Strength of recommendation (SOR)
A Good-quality patient-oriented evidence
B Inconsistent or limited-quality patient-oriented evidence
C Consensus, usual practice, opinion, disease-oriented evidence, case series
CASE › A mother brings in her 3-year-old son for a regular check-up. Her only concern is that for the past 2 weeks, he has not been sleeping through the night. She indicates that the sleeping problem began after he was diagnosed with and treated for an ear infection. Fortunately, this hasn’t affected his daily activity or energy, she says.
The child’s appetite is good and he speaks clearly, in 5-word sentences. He is meeting his developmental milestones, and appears well—sitting in his mother’s lap and playing with her smartphone. His head, eyes, ears, nose, and throat exam only turns up fluid behind his left tympanic membrane, which is not red or bulging. The right membrane appears normal, and he has no cervical lymphadenopathy. The rest of his exam is normal. How would you manage a patient like this?
"Glue ear" is often asymptomatic
Otitis media with effusion (OME) is defined as middle-ear effusion (MEE) in the absence of acute signs of infection. In children, OME—also referred to as “glue ear”—most often arises after acute otitis media (AOM). In adults, it often occurs in association with eustachian tube dysfunction, although OME is a separate diagnosis. (To learn more, see “What about OME in adults?”1,2.)
Experts have found it difficult to determine the exact incidence of OME because it is often asymptomatic. In addition, many cases quickly resolve on their own, making it challenging to diagnose. A 2-year prospective study of 2- to 6-year-old preschoolers revealed that MEE, diagnosed via monthly otoscopy and tympanometry, occurred at least once in 53% of the children in the first year and in 61% of the children in the second year.3 A second study followed 7-year-olds monthly for one year and found a 31% incidence of MEE using tympanometry.4 In the 25% of children found to have persistent MEE, the researchers noted spontaneous recovery after an average of 2 months.
We believe that nearly all children have experienced one episode of OME by the age of 3 years, but the prevalence of OME varies with age and the time of year. It is more prevalent in the winter than the summer months.5 OME is more common in Caucasian children than in African American or Asian children.6
Etiology remains elusive
Risk factors for children include a family history of OME, bottle-feeding, day care attendance, exposure to tobacco smoke, and a personal history of allergies.7,8 One study conducted on mice suggested that inherited structural abnormalities of the middle ear and eustachian tube may play a role as well.9 Some have suggested that effusions of OME in children result from chronic inflammation, for example, after AOM, and that the effusions are sterile; however, recent studies have demonstrated that a biofilm is formed by bacterial otopathogens in the effusion.10-12 The common pathogens found include nontypeable Haemophilus influenza, Streptococcus pneumoniae, and Moraxella catarrhalis. Inflammatory exudate or neutrophil infiltration is rare in the fluid, however.
The contribution of allergies to OME in children remains somewhat controversial. A retrospective review from the United Kingdom of 209 children with OME found a history of allergic rhinitis, asthma, and eczema in 89%, 36%, and 24%, respectively.13 However, this study was done at an allergy clinic, and it is possible that the data from the clinic’s specialized patient population are not generalizable. Gastroesophageal reflux may also be associated with OME in children. However, studies measuring the concentration of pepsin and pepsinogen in middle-ear fluid have provided conflicting results.14,15
Look for these signs and symptoms
OME is often asymptomatic. If a patient has clinical signs of an acute illness, including fever and an erythematous tympanic membrane, it’s important to evaluate for another cause. OME can present with hearing loss or a sense of fullness in the ear. While an infant cannot express the hearing loss, the parent may detect it when observing and interacting mwith the child. Parents are also likely to report that the child is experiencing sleep disturbances.16
Vertigo may occur with OME, although not often. It may manifest itself if the child stumbles or falls. An older child or adult with vertigo may say that it feels like the room is spinning.
Diagnosis relies on pneumatic otoscopy
On physical exam, the patient will likely appear well. Otoscopic examination reveals fluid behind a normal or retracted tympanic membrane; the fluid is often clear or yellowish in color.
A subcommittee comprised of members of the American Academy of Pediatrics, American Academy of Family Physicians, and the American Academy of Otolaryngology-Head and Neck Surgery (AAP/AAFP/AAOHNS) published a clinical practice guideline in 2004 that delineates the current diagnosis and management of children between 2 months and 12 years of age with OME.17
Pneumatic otoscopy, which can reveal decreased or absent movement of the tympanic membrane (the result of fluid behind the membrane), is the primary diagnostic method recommended by the guideline. Tympanometry and acoustic reflectometry may also be used to make the diagnosis, especially when the presence of MEE is difficult to determine using pneumatic otoscopy.
CASE › Upon further discussion with the patient’s mother, you learn that the boy goes to day care 3 days a week and stays with his grandmother 2 days a week. His grandmother smokes outside of the home when he is staying with her.
After reviewing these risk factors with the mother (including the importance of smoking cessation for the grandmother), the mother asks if he needs antibiotics or a referral to a specialist for treating his OME.
How best to approach treatment
There are several management options to choose from, including watchful waiting, medication, and/or surgery. (Another option, autoinflation, which has shown some short-term benefits, is described in “Should you recommend autoinflation?”17-19.)
The goals of management are to resolve the effusion, restore normal hearing (if diminished secondary to the effusion), and prevent future episodes or sequelae. The most significant complication of OME is permanent conductive hearing loss, but tinnitus, cholesteatoma, or tympanosclerosis may also occur.
In most patients, OME resolves without medical intervention. If additional action is required, however, the following options may be explored.
Medication. While the AAP/AAFP/AAOHNS guideline recommends against routine antibiotics for OME,17 it does note that a short course may provide short-term benefit to some patients (eg, those for whom a specialist referral or surgery is being considered).
A separate meta-analysis found that antibiotics improve clearance of the effusion within the first month after treatment (rate difference [RD]=0.16; 95% confidence interval [CI], 0.03-0.29 in 12 studies analyzed), but effusion relapses were common, and no significant benefit was noted past the first month (RD=0.06; 95% CI, -0.03 to 0.14 in 8 studies).20
If you do use antibiotics, a 10- to 14-day course is preferred.17 Amoxicillin, amoxicillin-clavulanate and ceftibuten have been evaluated in separate clinical trials, but none has been clearly shown to have significant advantage over any other.21,22
Antihistamines, decongestants, and oral and intranasal corticosteroids have little effect on OME in children and are not recommended.17 A Cochrane review including 16 studies found that children receiving antihistamines and decongestants are unlikely to see their symptoms improve significantly, and many patients experience adverse effects from the medications23 (number needed to harm=9).
A randomized, double-blind trial involving 144 children <9 years of age with OME for at least 2 months evaluated 4 regimens involving amoxicillin alone or in combination with prednisolone. Children in the amoxicillin+prednisolone arms were significantly more likely to clear their effusions at 2 weeks (number needed to treat=6; P=.03), but not at 4 weeks (P=.12). At 4-month follow-up, effusions had recurred in 68.4% and 69.2% of those receiving amoxicillin+prednisolone and those receiving amoxicillin alone, respectively (P= .94).24
Surgery—or not? The AAP/AAFP/AAOHNS guideline recommends physicians perform hearing testing when OME is present for 3 months or longer, or at any time if language delay, learning problems, or a significant hearing loss is suspected in a child with OME. The results of the hearing test can help determine how to proceed, based on the hearing level noted for the better hearing ear.
You can manage children with hearing loss ≤20 dB and without speech, language, or developmental problems with watchful waiting. Children with hearing loss of 21 to 39 dB can be managed with watchful waiting or referred for surgery. If watchful waiting is pursued, there are interventions at home and at school that can help. These include speaking near the child, facing the child when speaking, and providing accommodations in school so the child sits closer to the teacher. Consider re-examination and repeat hearing tests every 3 to 6 months until the effusion has resolved or the child develops symptoms indicating surgical referral.
When hearing loss is ≥40 dB, the AAP/AAFP/AAOHNS guideline recommends that you make a referral for surgical evaluation (ALGORITHM).17
Other indications for referral to a surgeon for evaluation of tympanostomy tube placement include situations in which there is:
• structural damage to the tympanic membrane or middle ear (prompt referral is recommended)
• OME of ≥4 months’ duration with persistent hearing loss (≥40 dB) or other signs or symptoms related to the effusion
• bilateral OME for ≥3 months, unilateral OME ≥6 months, or total duration of any degree of OME ≥12 months.17
Any decision regarding surgery should involve an otolaryngologist, the primary care provider, and the patient and/or family. The AAP/AAFP/AAOHNS guideline recommends against adenoidectomy in children with persistent OME without an indication for the procedure other than OME (eg, chronic sinusitis or nasal obstruction).17
Keep in mind that evidence of lasting benefit (>12 months) is limited for surgery in most patients, and the surgical and anesthetic risks must be considered before moving forward.17 (For more on the evidence regarding surgery, see “Cochrane weighs in on tympanostomy tubes”.25) Tonsillectomy also does not appear to affect outcomes and is not advised.17
When a referral is always needed. Regardless of hearing status, promptly refer children with recurrent or persistent OME who are at risk of speech, language, or learning problems (including those with autism spectrum disorder, developmental delay, Down’s syndrome, diagnosed speech or language delay, or craniofacial disorders such as cleft palate) to a specialist.17
CASE › You tell your young patient’s mother that watchful waiting is appropriate at this point, since his acute otitis media was only 2 weeks ago, and his OME likely started after the acute infection. Given that his speech is clear and he is otherwise meeting his milestones, you tell her that he does not need a referral at this time, but that she should bring him back in 4 weeks for reassessment. At the next visit, his effusion has resolved, and his mother reports he is sleeping well through the night again.
1. Lesinskas E. Factors affecting the results of nonsurgical treatment of secretory otitis media in adults. Auris Nasus Larynx. 2003:30:7-14.
2. Chole RA, HH Sudhoff. Chronic otitis media, mastoiditis, and petrosis. In: Flint PW, Haughey BH, Lund VJ, et al, eds. Cummings Otolaryngology: Head and Neck Surgery. 5th ed. Maryland Heights, MO. Mosby;2010:chap 139.
3. Casselbrant ML, Brostoff LM, Cantekin EI, et al. Otitis media with effusion in preschool children. Laryngoscope. 1985;95:428-436.
4. Lous J, Fiellau-Nikolajsen M. Epidemiology of middle-ear effusion and tubal dysfunction: a one year prospective study comprising monthly tympanometry in 387 non-selected seven-yearold children. Int J Pediatr Otorhinolaryngol. 1981;3:303-317.
5. Tos M, Holm-Jensen S, Sørensen CH. Changes in prevalence of secretory otitis from summer to winter in four-year-old children. Am J Otol. 1981;2:324-327.
6. Vernacchio L, Lesko SM, Vezina RM, et al. Racial/ethnic disparities in the diagnosis of otitis media in infancy. Int J Pediatr Otorhinolaryngol. 2004;68:795-804.
7. Owen MJ, Baldwin CD, Swank PR, et al. Relation of infant feeding practices, cigarette smoke exposure, and group child care to the onset and duration of otitis media with effusion in the first two years of life. J Pediatr. 1993;123:702-711.
8. Gultekin E, Develio˘gu ON, Yener M, et al. Prevalence and risk factors for persistent otitis media with effusion in primary school children in Istanbul, Turkey. Auris Nasus Larynx. 2010;37:145-149.
9. Depreux FF, Darrow K, Conner DA, et al. Eya4-deficient mice are a model for heritable otitis media. J Clin Invest. 2008;118:651-658.
10. Poetker DM, Lindstrom DR, Edmiston CE, et al. Microbiology of middle ear effusions from 292 patients undergoing tympanostomy tube placement for middle ear disease. Int J Pediatr Otorhinolaryngol. 2005;69:799-804.
11. Hall-Stoodley L, Hu FZ, Gieseke A, et al. Direct detection of bacterial biofilms on the middle-ear mucosa of children with chronic otitis media. JAMA. 2006;296:202-211.
12. Brook I, Yocum P, Shah K, et al. Microbiology of serous otitis media in children: correlation with age and length of effusion. Ann Otol Rhinol Laryngol. 2001;110:87-90.
13. Alles R, Parikh A, Hawk L, et al. The prevalence of atopic disorders in children with chronic otitis media with effusion. Pediatr Allergy Immunol. 2001;12:102-106.
14. Lieu JE, Muthappan PG, Uppaluri R. Association of reflux with otitis media in children. Otolaryngol Head Neck Surg. 2005;133:357-361.
15. O’Reilly RC, He Z, Bloedon E, et al. The role of extraesophageal reflux in otitis media in infants and children. Laryngoscope. 2008;118 (7 part 2, suppl 116):S1-S9.
16. Rosenfeld RM, Goldsmith AJ, Tetlus L, et al. Quality of life for children with otitis media. Arch Otolaryngol Head Neck Surg. 1997;123:1049-1054.
17. American Academy of Family Physicians, American Academy of Otolaryngology-Head and Neck Surgery, American Academy of Pediatrics Subcommittee on Otitis Media With Effusion. Otitis media with effusion. Pediatrics. 2004;113:1412-1429.
18. Perera R, Glasziou PP, Heneghan CJ, et al Autoinflation for hearing loss associated with otitis media with effusion. Cochrane Database Syst Rev. 2013;5:CD006285.
19. Stangerup SE, Sederberg-Olsen J, Balle V. Autoinflation as a treatment of secretory otitis media. A randomized controlled study. Arch Otolaryngol Head Neck Surg. 1992;118:149-152.
20. Williams RL, Chalmers TC, Stange KC, et al. Use of antibiotics in preventing recurrent acute otitis media and in treating otitis media with effusion. A meta-analytic attempt to resolve the brouhaha. JAMA. 1993;270:1344-1351.
21. Mandel EM, Casselbrant ML, Kurs-Lasky M, et al. Efficacy of ceftibuten compared with amoxicillin for otitis media with effusion in infants and children. Pediatr Infect Dis J. 1996;15:409-414.
22. Chan KH, Mandel EM, Rockette HE, et al. A comparative study of amoxicillin-clavulanate and amoxicillin. Treatment of otitis media with effusion. Arch Otolaryngol Head Neck Surg. 1988; 114:142-146.
23. Griffin G, Flynn CA. Antihistamines and/or decongestants for otitis media with effusion (OME) in children. Cochrane Database Syst Rev. 2011;(9):CD003423.
24. Mandel EM, Casselbrant ML, Rockette HE, et al. Systemic steroid for chronic otitis media with effusion in children. Pediatrics. 2002;110:1071-1080.
25. Browning GG, Rovers MM, Williamson I, et al. Grommets (ventilation tubes) for hearing loss associated with otitis media with effusion in children. Cochrane Database Syst Rev. 2010;(10):CD001801.
1. Lesinskas E. Factors affecting the results of nonsurgical treatment of secretory otitis media in adults. Auris Nasus Larynx. 2003:30:7-14.
2. Chole RA, HH Sudhoff. Chronic otitis media, mastoiditis, and petrosis. In: Flint PW, Haughey BH, Lund VJ, et al, eds. Cummings Otolaryngology: Head and Neck Surgery. 5th ed. Maryland Heights, MO. Mosby;2010:chap 139.
3. Casselbrant ML, Brostoff LM, Cantekin EI, et al. Otitis media with effusion in preschool children. Laryngoscope. 1985;95:428-436.
4. Lous J, Fiellau-Nikolajsen M. Epidemiology of middle-ear effusion and tubal dysfunction: a one year prospective study comprising monthly tympanometry in 387 non-selected seven-yearold children. Int J Pediatr Otorhinolaryngol. 1981;3:303-317.
5. Tos M, Holm-Jensen S, Sørensen CH. Changes in prevalence of secretory otitis from summer to winter in four-year-old children. Am J Otol. 1981;2:324-327.
6. Vernacchio L, Lesko SM, Vezina RM, et al. Racial/ethnic disparities in the diagnosis of otitis media in infancy. Int J Pediatr Otorhinolaryngol. 2004;68:795-804.
7. Owen MJ, Baldwin CD, Swank PR, et al. Relation of infant feeding practices, cigarette smoke exposure, and group child care to the onset and duration of otitis media with effusion in the first two years of life. J Pediatr. 1993;123:702-711.
8. Gultekin E, Develio˘gu ON, Yener M, et al. Prevalence and risk factors for persistent otitis media with effusion in primary school children in Istanbul, Turkey. Auris Nasus Larynx. 2010;37:145-149.
9. Depreux FF, Darrow K, Conner DA, et al. Eya4-deficient mice are a model for heritable otitis media. J Clin Invest. 2008;118:651-658.
10. Poetker DM, Lindstrom DR, Edmiston CE, et al. Microbiology of middle ear effusions from 292 patients undergoing tympanostomy tube placement for middle ear disease. Int J Pediatr Otorhinolaryngol. 2005;69:799-804.
11. Hall-Stoodley L, Hu FZ, Gieseke A, et al. Direct detection of bacterial biofilms on the middle-ear mucosa of children with chronic otitis media. JAMA. 2006;296:202-211.
12. Brook I, Yocum P, Shah K, et al. Microbiology of serous otitis media in children: correlation with age and length of effusion. Ann Otol Rhinol Laryngol. 2001;110:87-90.
13. Alles R, Parikh A, Hawk L, et al. The prevalence of atopic disorders in children with chronic otitis media with effusion. Pediatr Allergy Immunol. 2001;12:102-106.
14. Lieu JE, Muthappan PG, Uppaluri R. Association of reflux with otitis media in children. Otolaryngol Head Neck Surg. 2005;133:357-361.
15. O’Reilly RC, He Z, Bloedon E, et al. The role of extraesophageal reflux in otitis media in infants and children. Laryngoscope. 2008;118 (7 part 2, suppl 116):S1-S9.
16. Rosenfeld RM, Goldsmith AJ, Tetlus L, et al. Quality of life for children with otitis media. Arch Otolaryngol Head Neck Surg. 1997;123:1049-1054.
17. American Academy of Family Physicians, American Academy of Otolaryngology-Head and Neck Surgery, American Academy of Pediatrics Subcommittee on Otitis Media With Effusion. Otitis media with effusion. Pediatrics. 2004;113:1412-1429.
18. Perera R, Glasziou PP, Heneghan CJ, et al Autoinflation for hearing loss associated with otitis media with effusion. Cochrane Database Syst Rev. 2013;5:CD006285.
19. Stangerup SE, Sederberg-Olsen J, Balle V. Autoinflation as a treatment of secretory otitis media. A randomized controlled study. Arch Otolaryngol Head Neck Surg. 1992;118:149-152.
20. Williams RL, Chalmers TC, Stange KC, et al. Use of antibiotics in preventing recurrent acute otitis media and in treating otitis media with effusion. A meta-analytic attempt to resolve the brouhaha. JAMA. 1993;270:1344-1351.
21. Mandel EM, Casselbrant ML, Kurs-Lasky M, et al. Efficacy of ceftibuten compared with amoxicillin for otitis media with effusion in infants and children. Pediatr Infect Dis J. 1996;15:409-414.
22. Chan KH, Mandel EM, Rockette HE, et al. A comparative study of amoxicillin-clavulanate and amoxicillin. Treatment of otitis media with effusion. Arch Otolaryngol Head Neck Surg. 1988; 114:142-146.
23. Griffin G, Flynn CA. Antihistamines and/or decongestants for otitis media with effusion (OME) in children. Cochrane Database Syst Rev. 2011;(9):CD003423.
24. Mandel EM, Casselbrant ML, Rockette HE, et al. Systemic steroid for chronic otitis media with effusion in children. Pediatrics. 2002;110:1071-1080.
25. Browning GG, Rovers MM, Williamson I, et al. Grommets (ventilation tubes) for hearing loss associated with otitis media with effusion in children. Cochrane Database Syst Rev. 2010;(10):CD001801.
Incidental splenic lesions
A 43-year-old Caucasian male presented to the emergency department complaining of left lower quadrant pain that had lasted 1 day. He described the pain as “a wrench twisting my insides,” and he rated it as 8 out of 10. He also complained of nausea and non-bloody, nonbilious emesis. He did not have fever, chills, or weight loss. His medical history included diverticulitis, sigmoid colectomy, and nephrolithiasis.
The physical exam revealed a heart rate of 102 beats per minute, blood pressure of 153/89 mm Hg, respiratory rate of 20, and a temperature of 99.2°F. Abdominal exam demonstrated mild left lower quadrant tenderness to palpation without rebound or guarding. The remainder of the physical exam was unremarkable. Laboratory studies demonstrated a normal blood count and differential, and a normal comprehensive metabolic profile. The urinalysis showed 60–80 red blood cells per high-power field (RBC/HPF). A computed tomography (CT) scan of his abdomen revealed a 3-mm calculus in the mid-left ureter without hydronephrosis, and a few small descending colon diverticuli. The patient was treated conservatively with hydration, analgesics, and antiemetic medications.
Radiographic findings
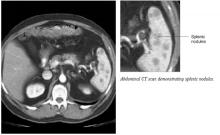
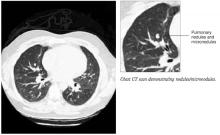
The following day, the radiologist called the patient’s primary care physician to report that upon rereading the abdominal CT scan there appeared to be “patchy enhancement of the spleen” (Figure 1). Follow-up abdominal ultrasound revealed multiple irregular areas of low density within the spleen. A CT-guided spleen biopsy discovered multiple noncaseating, non-necrotizing granulomas. Subsequent chest CT revealed multiple bilateral micronodules and nodules (Figure 2).
FIGURE 1
Abdominal CT scan
Chest CT scan
What are the management options?
What is the prognosis?
Diagnosis: Sarcoidosis
Sarcoidosis is an idiopathic systemic disorder identified by noncaseating granulomas in affected organs. Clinical presentation is variable and can involve virtually any organ system. Because of this, sarcoidosis is often misdiagnosed. The diagnosis of sarcoidosis requires clinical and radiographic features known to be associated with sarcoidosis, noncaseating granulomas found on tissue biopsy, and exclusion of known causes of granulomatous diseases such as tuberculosis or berylliosis.1
Sarcoidosis most commonly affects adults in their third decade of life.2 It is unusual to see the initial presentation of sarcoidosis after the age of 40 years—except in Scandinavia and Japan, where a second peak occurs among women after age 50 years.3,4,5 There seems to be a slightly higher rate of sarcoidosis in women.1 The incidence of sarcoidosis is higher in Swedes, Danes, and African Americans. The lifetime risk of sarcoidosis is 0.85% for US whites and 2.4% for US blacks.6
Possible causes of sarcoidosis
Although the cause of sarcoidosis is unknown, there is evidence of spatial, seasonal (winter and early spring), and familial clustering. Multiple theories have been proposed suggesting possible transmissible agents as the cause of sarcoidosis, including infectious agents such as Mycoplasma and Borrelia burgdorferi.
Other proposed agents include metals and inorganic substances such as clay and pine tree pollen.10 Despite extensive research, these hypotheses have not been validated. None-theless, there seems to be an emerging pattern of immunologic abnormalities in patients with sarcoidosis, suggesting an abnormal host response.
Clinical presentation of sarcoidosis
The local Th1-type T lymphocyte is now believed to be the central cell responsible for granuloma formation.10 It is hypothesized that sarcoid granulomas are formed in response to chronic antigenic stimulation of genetically susceptible Th-1 T cells and subsequent macrocyte-mediated inflammation.10,11 Certain HLA haplotypes have been implicated in the pathogenesis of sarcoidosis, yet there is no consistent data linking any one haplotype to the disease.11
The clinical presentation of sarcoidosis is related to the organ(s) involved. Specifically, symptoms may be a result of granuloma mass-effect, immune complex vasculitis, metabolically active granulomas, or fibrotic organ destruction. The lungs are the most commonly affected organ and lung involvement is seen in 88% of patients diagnosed with sarcoidosis.1
The most common presentation of intrathoracic sarcoidosis is bilateral hilar lymphadenopathy with or without diffuse pulmonary infiltration. This may present clinically as cough, wheeze, shortness of breath, or dyspnea on exertion.
Appearance on the skin
Skin manifestations of sarcoidosis are also relatively common, affecting up to one third of patients.1,7 Cutaneous sarcoidosis is divided into nonspecific and specific lesions. Nonspecific skin lesions do not contain granulomas on biopsy.
Erythema nodosum, the most common nonspecific skin lesion of sarcoidosis, is commonly seen in European, Puerto Rican, and Mexican patients.8 It presents as tender, subcutaneous erythematous nodules on the lower legs.
Lofgren’s syndrome is the combination of erythema nodosum, bilateral hilar lympha-denopathy, fever, and polyarthritis.
Specific sarcoid lesions may present as cutaneous papules, plaques, or enlarging scars.
Lupus pernio is another specific skin lesion characteristic of sarcoidosis. It presents as chronic, indurated, violaceous plaques on the nose, cheeks, lips, ears, or nasal mucosa. Lupus pernio is more common in African American women.8
Other manifestations
Ocular manifestations of sarcoidosis include uveitis or chorioretinitis and are present in 27% of patients with sarcoidosis.1
Sarcoidosis in peripheral lymph nodes, spleen, and liver is seen in 10% to 30% of patients with sarcoidosis; however, these tend to be incidental findings.1 Occasionally, hepatomegaly and splenomegaly are present.
Liver enzymes are typically normal, however a mildly elevated alkaline phosphatase is sometimes seen.1 Neurological, cardiac, and renal involvement are uncommon, affecting less than 5% of patients with sarcoidosis.1,13 Hypercalciuria with or without hypercalcemia is commonly seen secondary to overproduction of 1,25-vitamin D.8
Differential diagnosis
The differential diagnoses of solid splenic lesions can be divided into 3 categories: granulomatous disease, metastasis, and primary masses. Granulomatous diseases include tuberculosis, histoplasmosis, and sarcoid.
Metastasis is most commonly from melanoma, lymphoma, breast, or lung cancer, although prostate, colon, stomach, ovarian, and pancreatic metastasis have occurred. Primary masses include hemangioma, hemangiosarcoma, lymphangioma, and splenic infarction. Occasionally, regenerating nodules of hematopoeisis are seen in the spleen.
Testing: A Diagnosis Of Exclusion
The diagnosis of sarcoidosis is ultimately a diagnosis of exclusion. No laboratory tests can confirm the diagnosis of sarcoid. However, serum levels of angiotensin-converting enzyme are elevated in 50% to 80% of patients with sarcoidosis. Also, cutaneous anergy to common antigens (mumps, candida) is seen in 50% of patients with sarcoidosis.1,9
Once the diagnosis of sarcoidosis is made, additional clinical information is needed. In addition to a detailed history and physical, a chest x-ray, pulmonary function tests (PFT), electrocardiogram, comprehensive metabolic profile, and slit lamp evaluation should be obtained.
Some clinicians follow angiotensin-converting enzyme levels to monitor disease activity; however, treatment is ultimately based on the patient’s symptoms.
Treatment: Corticosteroids Treat The Symptoms
Corticosteroids are the cornerstone of treatment for sarcoidosis. Asymptomatic, early disease can be observed with routine follow-up. Oral corticosteroids should be prescribed for symptomatic cardiac, neurologic, and ocular sarcoidosis. Oral corticosteroids should also be prescribed for any symptomatic pulmonary disease, especially in the setting of worsening PFTs, severe hypercalcemia, and painful or disfiguring skin lesions (LOE: 2a).7,8,10
For symptomatic or worsening pulmonary sarcoidosis, patients are prescribed prednisone 20 to 40 mg daily for 3 months. If the patient responses to prednisone, it should slowly be tapered to 5 to 10 mg and continued for a period of 12 months.7,11,12
Topical steroids can be used for uveitis and skin lesions;1,8 the latter also respond well to intralesional steroids.7 Inhaled corticosteroids are now being used to treat pulmonary disease, but their efficacy is uncertain.1,8,10
Of patients being treated with corticosteroids, 25% to 40% will relapse and require further treatment.1,8 The addition of methotrexate, azathioprine, or hydroychloroquine is sometimes required in recalcitrant cases of sarcoidosis (LOE: 2b).1,8,11
Prognosis: Most Experience Spontaneous Remission
Overall, 70% of patients with sarcoidosis will experience a spontaneous remission.1,8,11 Serious extrapulmonary involvement is seen in 4% to 7% of patients at presentation and 1% to 5% of patients with sarcoidosis will die from respiratory, cardiac, or neurological sequela of sarcoidosis.1,8
Higher rates of chronic and more serious sarcoidosis are found in patients of African heritage or who are older than 40 years at the onset of disease, and in those who have lupus pernio, chronic uveitis, chronic hypercalcemia, progressive pulmonary sarcoidosis, nasal mucosal involvement, cystic bone lesions, neu-rosarcoidosis, cardiac involvement, and chronic respiratory insufficiency.8
Patient follow-up
All of the recommended tests were done for this patient. Pulmonary function testing was normal, and he never developed any respiratory symptoms. Therefore, treatment with cortico-steroids was deferred. He is now being seen every 4 to 6 months for follow-up PFTs and laboratory testing.
- Azathioprine • Imuran
- Hydroxychloroquine • Plaquenil
- Methotrexate • Folex
Corresponding author
Rade Nicholas Pejic, MD, Santa Monica/UCLA Medical Center Family Medicine Residency, 1920 Colorado Avenue, Santa Monica, CA 90404. E-mail: [email protected]. Submissions: Richard P. Usatine, Editor, Photo Rounds, University of Texas Health Sciences Center at San Antonio, Dept of Family and Community Medicine, MC 7794, 7703 Floyd Curl Drive, San Antonio, TX 78229-3900. E-mail: [email protected].
1. Belfer MH, Stevens RW. Sarcoidosis: a primary care review. Am Fam Physician 1998;58:2041-2058.
2. Thomas KW, Hunninghake GW. Sarcoidosis. JAMA 2003;289:3300-3303.
3. Alsbirk PH. Epidemiologic studies of sarcoidosis in Denmark based on a nation wide central register. A preliminary report. Acta Med Scand Suppl 1964;176:106-109.
4. Iwai K, Sekiguti M, Hosoda Y, et al. Racial difference in cardiac sarcoidosis incidence observed at autopsy. Sarcoidosis 1994;11:26-31.
5. Milman N, Selroos O. Pulmonary sarcoidosis in the Nordic countries 1950-1982. Epidemiology and clinical picture. Sarcoidosis 1990;7:50-57.
6. Rybicki BA, Major M, Popovich J, Jr, Maliarik MJ, Iannuzzi MC. Racial differences in sarcoidosis incidence: a 5-year study in a health maintenance organization. Am J Epidemiol 997;145:234-241.
7. Katta R. Cutaneous sarcoidosis: A dermatologic masquer-ader. Am Fam Physician 2002;65:1581-1584.
8. Statement on sarcoidosis. Joint Statement of the American Thoracic Society (ATS), the European Respiratory Society (ERS) and the World Association of Sarcoidosis and Other Granulomatous Disorders (WASOG) adopted by the ATS Board of Directors and by the ERS Executive Committee, February 1999. Am J Respir Crit Care Med 1999;160:736-755.
9. Chesnutt AN. Enigmas in sarcoidosis. West J Med 1995;162:519-526.
10. Paramothayan S, Jones PW. Corticosteroid therapy in pulmonary sarcoidosis: a systematic review. JAMA 2002;287:1301-1307.
11. Newman LS, Rose CS, Maier LA. Sarcoidosis. N Engl J Med 1997;336:1224-1234.
12. Baughman RP, Lower EE, Lynch JP. Treatment modalities for sarcoidosis. Clin Pulmonol Med 1994;1:223-231.
A 43-year-old Caucasian male presented to the emergency department complaining of left lower quadrant pain that had lasted 1 day. He described the pain as “a wrench twisting my insides,” and he rated it as 8 out of 10. He also complained of nausea and non-bloody, nonbilious emesis. He did not have fever, chills, or weight loss. His medical history included diverticulitis, sigmoid colectomy, and nephrolithiasis.
The physical exam revealed a heart rate of 102 beats per minute, blood pressure of 153/89 mm Hg, respiratory rate of 20, and a temperature of 99.2°F. Abdominal exam demonstrated mild left lower quadrant tenderness to palpation without rebound or guarding. The remainder of the physical exam was unremarkable. Laboratory studies demonstrated a normal blood count and differential, and a normal comprehensive metabolic profile. The urinalysis showed 60–80 red blood cells per high-power field (RBC/HPF). A computed tomography (CT) scan of his abdomen revealed a 3-mm calculus in the mid-left ureter without hydronephrosis, and a few small descending colon diverticuli. The patient was treated conservatively with hydration, analgesics, and antiemetic medications.
Radiographic findings
The following day, the radiologist called the patient’s primary care physician to report that upon rereading the abdominal CT scan there appeared to be “patchy enhancement of the spleen” (Figure 1). Follow-up abdominal ultrasound revealed multiple irregular areas of low density within the spleen. A CT-guided spleen biopsy discovered multiple noncaseating, non-necrotizing granulomas. Subsequent chest CT revealed multiple bilateral micronodules and nodules (Figure 2).
FIGURE 1
Abdominal CT scan
Chest CT scan
What are the management options?
What is the prognosis?
Diagnosis: Sarcoidosis
Sarcoidosis is an idiopathic systemic disorder identified by noncaseating granulomas in affected organs. Clinical presentation is variable and can involve virtually any organ system. Because of this, sarcoidosis is often misdiagnosed. The diagnosis of sarcoidosis requires clinical and radiographic features known to be associated with sarcoidosis, noncaseating granulomas found on tissue biopsy, and exclusion of known causes of granulomatous diseases such as tuberculosis or berylliosis.1
Sarcoidosis most commonly affects adults in their third decade of life.2 It is unusual to see the initial presentation of sarcoidosis after the age of 40 years—except in Scandinavia and Japan, where a second peak occurs among women after age 50 years.3,4,5 There seems to be a slightly higher rate of sarcoidosis in women.1 The incidence of sarcoidosis is higher in Swedes, Danes, and African Americans. The lifetime risk of sarcoidosis is 0.85% for US whites and 2.4% for US blacks.6
Possible causes of sarcoidosis
Although the cause of sarcoidosis is unknown, there is evidence of spatial, seasonal (winter and early spring), and familial clustering. Multiple theories have been proposed suggesting possible transmissible agents as the cause of sarcoidosis, including infectious agents such as Mycoplasma and Borrelia burgdorferi.
Other proposed agents include metals and inorganic substances such as clay and pine tree pollen.10 Despite extensive research, these hypotheses have not been validated. None-theless, there seems to be an emerging pattern of immunologic abnormalities in patients with sarcoidosis, suggesting an abnormal host response.
Clinical presentation of sarcoidosis
The local Th1-type T lymphocyte is now believed to be the central cell responsible for granuloma formation.10 It is hypothesized that sarcoid granulomas are formed in response to chronic antigenic stimulation of genetically susceptible Th-1 T cells and subsequent macrocyte-mediated inflammation.10,11 Certain HLA haplotypes have been implicated in the pathogenesis of sarcoidosis, yet there is no consistent data linking any one haplotype to the disease.11
The clinical presentation of sarcoidosis is related to the organ(s) involved. Specifically, symptoms may be a result of granuloma mass-effect, immune complex vasculitis, metabolically active granulomas, or fibrotic organ destruction. The lungs are the most commonly affected organ and lung involvement is seen in 88% of patients diagnosed with sarcoidosis.1
The most common presentation of intrathoracic sarcoidosis is bilateral hilar lymphadenopathy with or without diffuse pulmonary infiltration. This may present clinically as cough, wheeze, shortness of breath, or dyspnea on exertion.
Appearance on the skin
Skin manifestations of sarcoidosis are also relatively common, affecting up to one third of patients.1,7 Cutaneous sarcoidosis is divided into nonspecific and specific lesions. Nonspecific skin lesions do not contain granulomas on biopsy.
Erythema nodosum, the most common nonspecific skin lesion of sarcoidosis, is commonly seen in European, Puerto Rican, and Mexican patients.8 It presents as tender, subcutaneous erythematous nodules on the lower legs.
Lofgren’s syndrome is the combination of erythema nodosum, bilateral hilar lympha-denopathy, fever, and polyarthritis.
Specific sarcoid lesions may present as cutaneous papules, plaques, or enlarging scars.
Lupus pernio is another specific skin lesion characteristic of sarcoidosis. It presents as chronic, indurated, violaceous plaques on the nose, cheeks, lips, ears, or nasal mucosa. Lupus pernio is more common in African American women.8
Other manifestations
Ocular manifestations of sarcoidosis include uveitis or chorioretinitis and are present in 27% of patients with sarcoidosis.1
Sarcoidosis in peripheral lymph nodes, spleen, and liver is seen in 10% to 30% of patients with sarcoidosis; however, these tend to be incidental findings.1 Occasionally, hepatomegaly and splenomegaly are present.
Liver enzymes are typically normal, however a mildly elevated alkaline phosphatase is sometimes seen.1 Neurological, cardiac, and renal involvement are uncommon, affecting less than 5% of patients with sarcoidosis.1,13 Hypercalciuria with or without hypercalcemia is commonly seen secondary to overproduction of 1,25-vitamin D.8
Differential diagnosis
The differential diagnoses of solid splenic lesions can be divided into 3 categories: granulomatous disease, metastasis, and primary masses. Granulomatous diseases include tuberculosis, histoplasmosis, and sarcoid.
Metastasis is most commonly from melanoma, lymphoma, breast, or lung cancer, although prostate, colon, stomach, ovarian, and pancreatic metastasis have occurred. Primary masses include hemangioma, hemangiosarcoma, lymphangioma, and splenic infarction. Occasionally, regenerating nodules of hematopoeisis are seen in the spleen.
Testing: A Diagnosis Of Exclusion
The diagnosis of sarcoidosis is ultimately a diagnosis of exclusion. No laboratory tests can confirm the diagnosis of sarcoid. However, serum levels of angiotensin-converting enzyme are elevated in 50% to 80% of patients with sarcoidosis. Also, cutaneous anergy to common antigens (mumps, candida) is seen in 50% of patients with sarcoidosis.1,9
Once the diagnosis of sarcoidosis is made, additional clinical information is needed. In addition to a detailed history and physical, a chest x-ray, pulmonary function tests (PFT), electrocardiogram, comprehensive metabolic profile, and slit lamp evaluation should be obtained.
Some clinicians follow angiotensin-converting enzyme levels to monitor disease activity; however, treatment is ultimately based on the patient’s symptoms.
Treatment: Corticosteroids Treat The Symptoms
Corticosteroids are the cornerstone of treatment for sarcoidosis. Asymptomatic, early disease can be observed with routine follow-up. Oral corticosteroids should be prescribed for symptomatic cardiac, neurologic, and ocular sarcoidosis. Oral corticosteroids should also be prescribed for any symptomatic pulmonary disease, especially in the setting of worsening PFTs, severe hypercalcemia, and painful or disfiguring skin lesions (LOE: 2a).7,8,10
For symptomatic or worsening pulmonary sarcoidosis, patients are prescribed prednisone 20 to 40 mg daily for 3 months. If the patient responses to prednisone, it should slowly be tapered to 5 to 10 mg and continued for a period of 12 months.7,11,12
Topical steroids can be used for uveitis and skin lesions;1,8 the latter also respond well to intralesional steroids.7 Inhaled corticosteroids are now being used to treat pulmonary disease, but their efficacy is uncertain.1,8,10
Of patients being treated with corticosteroids, 25% to 40% will relapse and require further treatment.1,8 The addition of methotrexate, azathioprine, or hydroychloroquine is sometimes required in recalcitrant cases of sarcoidosis (LOE: 2b).1,8,11
Prognosis: Most Experience Spontaneous Remission
Overall, 70% of patients with sarcoidosis will experience a spontaneous remission.1,8,11 Serious extrapulmonary involvement is seen in 4% to 7% of patients at presentation and 1% to 5% of patients with sarcoidosis will die from respiratory, cardiac, or neurological sequela of sarcoidosis.1,8
Higher rates of chronic and more serious sarcoidosis are found in patients of African heritage or who are older than 40 years at the onset of disease, and in those who have lupus pernio, chronic uveitis, chronic hypercalcemia, progressive pulmonary sarcoidosis, nasal mucosal involvement, cystic bone lesions, neu-rosarcoidosis, cardiac involvement, and chronic respiratory insufficiency.8
Patient follow-up
All of the recommended tests were done for this patient. Pulmonary function testing was normal, and he never developed any respiratory symptoms. Therefore, treatment with cortico-steroids was deferred. He is now being seen every 4 to 6 months for follow-up PFTs and laboratory testing.
- Azathioprine • Imuran
- Hydroxychloroquine • Plaquenil
- Methotrexate • Folex
Corresponding author
Rade Nicholas Pejic, MD, Santa Monica/UCLA Medical Center Family Medicine Residency, 1920 Colorado Avenue, Santa Monica, CA 90404. E-mail: [email protected]. Submissions: Richard P. Usatine, Editor, Photo Rounds, University of Texas Health Sciences Center at San Antonio, Dept of Family and Community Medicine, MC 7794, 7703 Floyd Curl Drive, San Antonio, TX 78229-3900. E-mail: [email protected].
A 43-year-old Caucasian male presented to the emergency department complaining of left lower quadrant pain that had lasted 1 day. He described the pain as “a wrench twisting my insides,” and he rated it as 8 out of 10. He also complained of nausea and non-bloody, nonbilious emesis. He did not have fever, chills, or weight loss. His medical history included diverticulitis, sigmoid colectomy, and nephrolithiasis.
The physical exam revealed a heart rate of 102 beats per minute, blood pressure of 153/89 mm Hg, respiratory rate of 20, and a temperature of 99.2°F. Abdominal exam demonstrated mild left lower quadrant tenderness to palpation without rebound or guarding. The remainder of the physical exam was unremarkable. Laboratory studies demonstrated a normal blood count and differential, and a normal comprehensive metabolic profile. The urinalysis showed 60–80 red blood cells per high-power field (RBC/HPF). A computed tomography (CT) scan of his abdomen revealed a 3-mm calculus in the mid-left ureter without hydronephrosis, and a few small descending colon diverticuli. The patient was treated conservatively with hydration, analgesics, and antiemetic medications.
Radiographic findings
The following day, the radiologist called the patient’s primary care physician to report that upon rereading the abdominal CT scan there appeared to be “patchy enhancement of the spleen” (Figure 1). Follow-up abdominal ultrasound revealed multiple irregular areas of low density within the spleen. A CT-guided spleen biopsy discovered multiple noncaseating, non-necrotizing granulomas. Subsequent chest CT revealed multiple bilateral micronodules and nodules (Figure 2).
FIGURE 1
Abdominal CT scan
Chest CT scan
What are the management options?
What is the prognosis?
Diagnosis: Sarcoidosis
Sarcoidosis is an idiopathic systemic disorder identified by noncaseating granulomas in affected organs. Clinical presentation is variable and can involve virtually any organ system. Because of this, sarcoidosis is often misdiagnosed. The diagnosis of sarcoidosis requires clinical and radiographic features known to be associated with sarcoidosis, noncaseating granulomas found on tissue biopsy, and exclusion of known causes of granulomatous diseases such as tuberculosis or berylliosis.1
Sarcoidosis most commonly affects adults in their third decade of life.2 It is unusual to see the initial presentation of sarcoidosis after the age of 40 years—except in Scandinavia and Japan, where a second peak occurs among women after age 50 years.3,4,5 There seems to be a slightly higher rate of sarcoidosis in women.1 The incidence of sarcoidosis is higher in Swedes, Danes, and African Americans. The lifetime risk of sarcoidosis is 0.85% for US whites and 2.4% for US blacks.6
Possible causes of sarcoidosis
Although the cause of sarcoidosis is unknown, there is evidence of spatial, seasonal (winter and early spring), and familial clustering. Multiple theories have been proposed suggesting possible transmissible agents as the cause of sarcoidosis, including infectious agents such as Mycoplasma and Borrelia burgdorferi.
Other proposed agents include metals and inorganic substances such as clay and pine tree pollen.10 Despite extensive research, these hypotheses have not been validated. None-theless, there seems to be an emerging pattern of immunologic abnormalities in patients with sarcoidosis, suggesting an abnormal host response.
Clinical presentation of sarcoidosis
The local Th1-type T lymphocyte is now believed to be the central cell responsible for granuloma formation.10 It is hypothesized that sarcoid granulomas are formed in response to chronic antigenic stimulation of genetically susceptible Th-1 T cells and subsequent macrocyte-mediated inflammation.10,11 Certain HLA haplotypes have been implicated in the pathogenesis of sarcoidosis, yet there is no consistent data linking any one haplotype to the disease.11
The clinical presentation of sarcoidosis is related to the organ(s) involved. Specifically, symptoms may be a result of granuloma mass-effect, immune complex vasculitis, metabolically active granulomas, or fibrotic organ destruction. The lungs are the most commonly affected organ and lung involvement is seen in 88% of patients diagnosed with sarcoidosis.1
The most common presentation of intrathoracic sarcoidosis is bilateral hilar lymphadenopathy with or without diffuse pulmonary infiltration. This may present clinically as cough, wheeze, shortness of breath, or dyspnea on exertion.
Appearance on the skin
Skin manifestations of sarcoidosis are also relatively common, affecting up to one third of patients.1,7 Cutaneous sarcoidosis is divided into nonspecific and specific lesions. Nonspecific skin lesions do not contain granulomas on biopsy.
Erythema nodosum, the most common nonspecific skin lesion of sarcoidosis, is commonly seen in European, Puerto Rican, and Mexican patients.8 It presents as tender, subcutaneous erythematous nodules on the lower legs.
Lofgren’s syndrome is the combination of erythema nodosum, bilateral hilar lympha-denopathy, fever, and polyarthritis.
Specific sarcoid lesions may present as cutaneous papules, plaques, or enlarging scars.
Lupus pernio is another specific skin lesion characteristic of sarcoidosis. It presents as chronic, indurated, violaceous plaques on the nose, cheeks, lips, ears, or nasal mucosa. Lupus pernio is more common in African American women.8
Other manifestations
Ocular manifestations of sarcoidosis include uveitis or chorioretinitis and are present in 27% of patients with sarcoidosis.1
Sarcoidosis in peripheral lymph nodes, spleen, and liver is seen in 10% to 30% of patients with sarcoidosis; however, these tend to be incidental findings.1 Occasionally, hepatomegaly and splenomegaly are present.
Liver enzymes are typically normal, however a mildly elevated alkaline phosphatase is sometimes seen.1 Neurological, cardiac, and renal involvement are uncommon, affecting less than 5% of patients with sarcoidosis.1,13 Hypercalciuria with or without hypercalcemia is commonly seen secondary to overproduction of 1,25-vitamin D.8
Differential diagnosis
The differential diagnoses of solid splenic lesions can be divided into 3 categories: granulomatous disease, metastasis, and primary masses. Granulomatous diseases include tuberculosis, histoplasmosis, and sarcoid.
Metastasis is most commonly from melanoma, lymphoma, breast, or lung cancer, although prostate, colon, stomach, ovarian, and pancreatic metastasis have occurred. Primary masses include hemangioma, hemangiosarcoma, lymphangioma, and splenic infarction. Occasionally, regenerating nodules of hematopoeisis are seen in the spleen.
Testing: A Diagnosis Of Exclusion
The diagnosis of sarcoidosis is ultimately a diagnosis of exclusion. No laboratory tests can confirm the diagnosis of sarcoid. However, serum levels of angiotensin-converting enzyme are elevated in 50% to 80% of patients with sarcoidosis. Also, cutaneous anergy to common antigens (mumps, candida) is seen in 50% of patients with sarcoidosis.1,9
Once the diagnosis of sarcoidosis is made, additional clinical information is needed. In addition to a detailed history and physical, a chest x-ray, pulmonary function tests (PFT), electrocardiogram, comprehensive metabolic profile, and slit lamp evaluation should be obtained.
Some clinicians follow angiotensin-converting enzyme levels to monitor disease activity; however, treatment is ultimately based on the patient’s symptoms.
Treatment: Corticosteroids Treat The Symptoms
Corticosteroids are the cornerstone of treatment for sarcoidosis. Asymptomatic, early disease can be observed with routine follow-up. Oral corticosteroids should be prescribed for symptomatic cardiac, neurologic, and ocular sarcoidosis. Oral corticosteroids should also be prescribed for any symptomatic pulmonary disease, especially in the setting of worsening PFTs, severe hypercalcemia, and painful or disfiguring skin lesions (LOE: 2a).7,8,10
For symptomatic or worsening pulmonary sarcoidosis, patients are prescribed prednisone 20 to 40 mg daily for 3 months. If the patient responses to prednisone, it should slowly be tapered to 5 to 10 mg and continued for a period of 12 months.7,11,12
Topical steroids can be used for uveitis and skin lesions;1,8 the latter also respond well to intralesional steroids.7 Inhaled corticosteroids are now being used to treat pulmonary disease, but their efficacy is uncertain.1,8,10
Of patients being treated with corticosteroids, 25% to 40% will relapse and require further treatment.1,8 The addition of methotrexate, azathioprine, or hydroychloroquine is sometimes required in recalcitrant cases of sarcoidosis (LOE: 2b).1,8,11
Prognosis: Most Experience Spontaneous Remission
Overall, 70% of patients with sarcoidosis will experience a spontaneous remission.1,8,11 Serious extrapulmonary involvement is seen in 4% to 7% of patients at presentation and 1% to 5% of patients with sarcoidosis will die from respiratory, cardiac, or neurological sequela of sarcoidosis.1,8
Higher rates of chronic and more serious sarcoidosis are found in patients of African heritage or who are older than 40 years at the onset of disease, and in those who have lupus pernio, chronic uveitis, chronic hypercalcemia, progressive pulmonary sarcoidosis, nasal mucosal involvement, cystic bone lesions, neu-rosarcoidosis, cardiac involvement, and chronic respiratory insufficiency.8
Patient follow-up
All of the recommended tests were done for this patient. Pulmonary function testing was normal, and he never developed any respiratory symptoms. Therefore, treatment with cortico-steroids was deferred. He is now being seen every 4 to 6 months for follow-up PFTs and laboratory testing.
- Azathioprine • Imuran
- Hydroxychloroquine • Plaquenil
- Methotrexate • Folex
Corresponding author
Rade Nicholas Pejic, MD, Santa Monica/UCLA Medical Center Family Medicine Residency, 1920 Colorado Avenue, Santa Monica, CA 90404. E-mail: [email protected]. Submissions: Richard P. Usatine, Editor, Photo Rounds, University of Texas Health Sciences Center at San Antonio, Dept of Family and Community Medicine, MC 7794, 7703 Floyd Curl Drive, San Antonio, TX 78229-3900. E-mail: [email protected].
1. Belfer MH, Stevens RW. Sarcoidosis: a primary care review. Am Fam Physician 1998;58:2041-2058.
2. Thomas KW, Hunninghake GW. Sarcoidosis. JAMA 2003;289:3300-3303.
3. Alsbirk PH. Epidemiologic studies of sarcoidosis in Denmark based on a nation wide central register. A preliminary report. Acta Med Scand Suppl 1964;176:106-109.
4. Iwai K, Sekiguti M, Hosoda Y, et al. Racial difference in cardiac sarcoidosis incidence observed at autopsy. Sarcoidosis 1994;11:26-31.
5. Milman N, Selroos O. Pulmonary sarcoidosis in the Nordic countries 1950-1982. Epidemiology and clinical picture. Sarcoidosis 1990;7:50-57.
6. Rybicki BA, Major M, Popovich J, Jr, Maliarik MJ, Iannuzzi MC. Racial differences in sarcoidosis incidence: a 5-year study in a health maintenance organization. Am J Epidemiol 997;145:234-241.
7. Katta R. Cutaneous sarcoidosis: A dermatologic masquer-ader. Am Fam Physician 2002;65:1581-1584.
8. Statement on sarcoidosis. Joint Statement of the American Thoracic Society (ATS), the European Respiratory Society (ERS) and the World Association of Sarcoidosis and Other Granulomatous Disorders (WASOG) adopted by the ATS Board of Directors and by the ERS Executive Committee, February 1999. Am J Respir Crit Care Med 1999;160:736-755.
9. Chesnutt AN. Enigmas in sarcoidosis. West J Med 1995;162:519-526.
10. Paramothayan S, Jones PW. Corticosteroid therapy in pulmonary sarcoidosis: a systematic review. JAMA 2002;287:1301-1307.
11. Newman LS, Rose CS, Maier LA. Sarcoidosis. N Engl J Med 1997;336:1224-1234.
12. Baughman RP, Lower EE, Lynch JP. Treatment modalities for sarcoidosis. Clin Pulmonol Med 1994;1:223-231.
1. Belfer MH, Stevens RW. Sarcoidosis: a primary care review. Am Fam Physician 1998;58:2041-2058.
2. Thomas KW, Hunninghake GW. Sarcoidosis. JAMA 2003;289:3300-3303.
3. Alsbirk PH. Epidemiologic studies of sarcoidosis in Denmark based on a nation wide central register. A preliminary report. Acta Med Scand Suppl 1964;176:106-109.
4. Iwai K, Sekiguti M, Hosoda Y, et al. Racial difference in cardiac sarcoidosis incidence observed at autopsy. Sarcoidosis 1994;11:26-31.
5. Milman N, Selroos O. Pulmonary sarcoidosis in the Nordic countries 1950-1982. Epidemiology and clinical picture. Sarcoidosis 1990;7:50-57.
6. Rybicki BA, Major M, Popovich J, Jr, Maliarik MJ, Iannuzzi MC. Racial differences in sarcoidosis incidence: a 5-year study in a health maintenance organization. Am J Epidemiol 997;145:234-241.
7. Katta R. Cutaneous sarcoidosis: A dermatologic masquer-ader. Am Fam Physician 2002;65:1581-1584.
8. Statement on sarcoidosis. Joint Statement of the American Thoracic Society (ATS), the European Respiratory Society (ERS) and the World Association of Sarcoidosis and Other Granulomatous Disorders (WASOG) adopted by the ATS Board of Directors and by the ERS Executive Committee, February 1999. Am J Respir Crit Care Med 1999;160:736-755.
9. Chesnutt AN. Enigmas in sarcoidosis. West J Med 1995;162:519-526.
10. Paramothayan S, Jones PW. Corticosteroid therapy in pulmonary sarcoidosis: a systematic review. JAMA 2002;287:1301-1307.
11. Newman LS, Rose CS, Maier LA. Sarcoidosis. N Engl J Med 1997;336:1224-1234.
12. Baughman RP, Lower EE, Lynch JP. Treatment modalities for sarcoidosis. Clin Pulmonol Med 1994;1:223-231.