User login
A novel minilaparotomy approach for large ovarian cysts
- Make a cruciate incision by incising the skin transversely and the anterior rectus fascia vertically.
- Insert a soft, sleeved, self-retaining retractor.
- Using a surgical adhesive, glue a large plastic wound dressing to the surface of the cyst to prevent leakage of contents into the abdominal cavity.
- Aspirate the cyst until it collapses and can be delivered, with the ovary, through the abdominal incision.
- After performing an extracorporeal cystectomy and/or adnexectomy, return the repaired ovary to the abdominal cavity.
Although laparotomy is still considered the standard for ovarian cyst removal, over the past 15 years minimally invasive surgery has gained wider acceptance in cases where preoperative assessment suggests an adnexal mass is benign.
Unfortunately, minimally invasive management of a large ovarian cyst (greater than 10 cm) is particularly challenging for several reasons:
- The cyst can rupture and spill its contents into the peritoneum,
- the cyst’s size limits the surgical field, and
- an unexpected malignancy may be revealed.
An innovative minilaparotomy technique for the removal of benign ovarian cysts offers the advantages of laparoscopic and laparoscopic-assisted procedures while bypassing the major disadvantages: the necessity for specialized and expensive equipment, lengthy operative time, and long learning curves.1 (The minimally invasive procedures currently available for the treatment of ovarian cysts include laparoscopic cystectomy, laparoscopic-assisted minilaparotomy cystectomy, laparoscopic-assisted vaginal cystectomy, combined percutaneous ultrasound cyst aspiration and laparoscopic cystectomy, transvaginal cystectomy, and the traditional minilaparotomy cystectomy.2-10)
The procedure is faster, less expensive, carries fewer potential risks than traditional alternatives, and offers these advantages:
- can be performed under regional anesthesia
- relies on standard open techniques
- uses inexpensive instrumentation
- is easy to learn
- can be used for very large cysts
- eliminates the risk of intraperitoneal spillage of cyst contents
- offers similar postoperative convalescence and mean time to return to work as laparoscopic or laparoscopic-assisted management of large ovarian cysts
General Ob/Gyns—not gynecologic oncologists—perform most surgeries on patients with adnexal masses, since ovarian cancer is relatively uncommon in the absence of preoperative risk factors for malignancy. Our approach offers an appealing option to Ob/Gyns reluctant to abandon routine traditional laparotomy for such ovarian cysts.
Selecting the right patient
Adequate preoperative assessment diminishes the risk of unexpected malignancy in a patient undergoing surgery for an ovarian mass to less than 1%.4 At this time, the combination of menopausal status, cancer antigen (CA) 125 level, physical examination, and ultrasound is the best strategy for evaluating the patient with an ovarian cyst.11
Signs of malignancy. Ultrasound features that suggest malignancy include irregular borders, thick septa, solid areas, internal and external excrescences, matted bowel, and ascites. Benign cysts, on the other hand, are usually unilateral and have regular borders, thin septa, no solid areas, and no internal excrescences.4 The measurement of blood flow within the mass by color Doppler may improve the accuracy of ultrasound in differentiating benign from malignant cysts.4,12
On physical examination, an adnexal mass that is fixed, irregular, or solid also suggests a neoplasm. An elevated CA 125 combined with a complex adnexal mass is likely to be associated with malignancy. The test is even more specific in postmenopausal women with adnexal masses.4,12
However, plasma levels of CA 125 also can be elevated in several benign gynecologic conditions such as endometriosis, simple ovarian cysts, pelvic inflammatory disease, ovarian torsion, fibroids, and in physiologic conditions such as menstruation and pregnancy.13
Anticipate the need to convert to laparotomy. Every patient’s surgical consent should include a possible conversion to laparotomy. To avoid incomplete surgical treatment and significant delays in proper therapy, a gynecologic surgeon experienced in the management of ovarian cancer should be readily available, in the event an unexpected malignancy is encountered. Ideally, the staging surgery and definite treatment should be performed at the time of initial minilaparotomy. Comprehensive surgical staging and treatment include thorough exploration of the pelvis and abdomen, omentectomy, pelvic and paraaortic lymph node sampling, multiple peritoneal biopsies and washings, bilateral salpingo-oophorectomy, hysterectomy, and debulking, when indicated.
Prepare for surgery with position, incision, and retraction
Before beginning, it is crucial to correctly position the patient, make the appropriate incision, and insert the right retractor.1
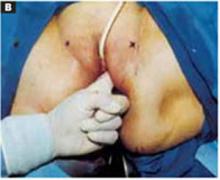
Position. After administering regional or general anesthesia, place the patient in a modified lithotomy, as for laparoscopic surgery. Tuck the arms alongside the torso and place the legs in boot stirrups. Avoid hip flexion and allow adequate thigh abduction to expose the vagina. Perform a careful pelvic examination to determine the size and mobility of the adnexal mass.
When properly placed, this retractor creates an atraumatic, circular area of self-retraction, enabling superior exposure
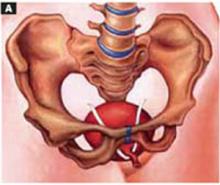
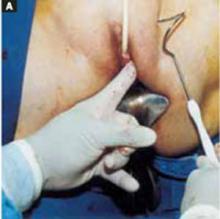
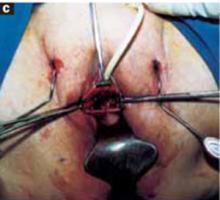
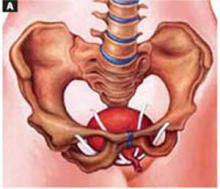
Place an indwelling transurethral catheter, and pass a sturdy, hinged uterine manipulator such as the Pelosi Uterine Manipulator (Apple Medical Corp, Marlboro, Mass) transcervically into the uterine cavity (FIGURE 1).
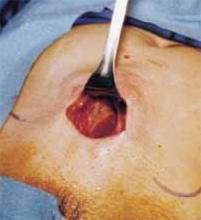
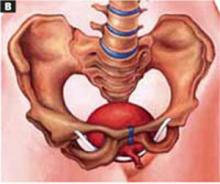
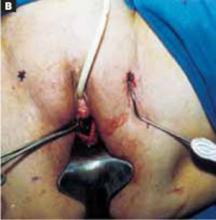
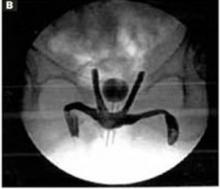
The cruciate incision. With a conventional scalpel make a small suprapubic transverse incision through the skin and subcutaneous tissue (FIGURE 2). After clearing subcutaneous fat from the midline, incise the rectus fascia and the peritoneum in a vertical direction.
A vertical skin incision can be selected if the preoperative workup suggests a later extension of the original minilaparotomy incision may be required, or if there is a prior vertical incision.
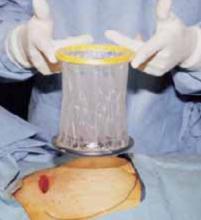
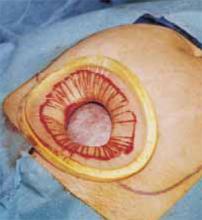
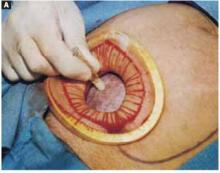
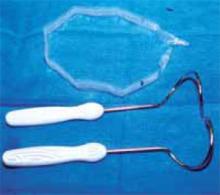
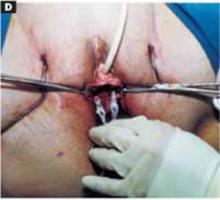
Retraction. Use a soft sleeve-type self-retaining plastic retractor, such as Mobius (Apple Medical Corp) (FIGURE 3 ). When properly placed, this retractor creates an atraumatic, circular area of self-retraction, enabling superior exposure of the pelvis (FIGURE 4).
During surgery it may be necessary to adjust the outer ring if the sleeve loosens. Narrow Deaver or Richardson retractors, if required, provide additional retraction. The bowel may be gently packed, if necessary, but typically it is adequately displaced by the large ovarian cyst.
The atraumatic retraction provided by the soft, self-retaining abdominal retractor minimizes the possibility of tissue trauma, nerve damage, bruising, and postoperative pain. At the same time, the continuous 360° retraction force on the incision maximizes surgical exposure, providing a significantly larger working area than conventional retractors. For example, when applied to a 6-cm incision, the self-retaining retractor creates a 28-cm2 exposed working area, compared with only 18 cm2 provided by a conventional 4-point metal retractor.
The adjustable height of the self-retaining retractor adapts to wounds of varying depth and works on virtually any tissue thickness—a feature that makes the device effective for obese patients. Further, by lining the abdominal incision, the retractor’s plastic sleeve protects the wound’s edges from contamination and potential implantation of malignant cells, making the device ideal for managing ovarian cysts.
FIGURE 1 Hinged uterine manipulator
The manipulator facilitates exposure of the cyst and contralateral adnexa, as well as uterine elevation/rotation.
FIGURE 2 Cruciate incision
Make a 2.5- to 5-cm suprapubic transverse skin incision. Using the Bovie device, incise the subcutaneous fat transversely along the full length of the skin incision down to the level of the anterior rectus fascia. Clear the subcutaneous fat from the midline superiorly and inferiorly to expose 5 to 6 cm of the rectus fascia in the vertical axis. Use blunt digital dissection to assist in mobilizing the subcutaneous fat. Incise the anterior rectus fascia vertically through the full length of the cleared area. Retract the rectus muscles from the midline to expose the underlying transversalis fascia and the peritoneum. Control small bleeding points with the Bovie device. Enter the peritoneum either digitally or with scissors above the bladder dome and extend the peritoneal incision to the full length of the fascial incision.
FIGURE 3 Self-retaining retractor
The retractor consists of a flexible plastic inner ring and a firmer outer ring connected by a soft plastic sleeve.
FIGURE 4360° retraction force
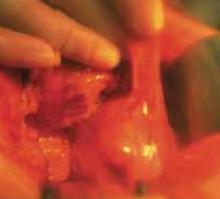
Squeeze the inner ring of the soft, sleeve-type, self-retaining retractor into the peritoneal cavity through the cruciate abdominal incision and allow it to spring open against the parietal peritoneum. Make a digital assessment to ensure that viscera is not trapped between the inner ring and the abdominal wall. Place the entire sleeve on traction by lifting the outer ring. Then roll the outer ring onto the sleeve, collecting excess length, until it sits firmly against the skin. The atraumatic retraction provided by the sleeve-type retractor maximizes exposure of the pelvis. Note how a portion of the large cyst is clearly seen through the atraumatic, circular area of retraction.
Cyst assessment
Visually and digitally inspect the cyst and carefully evaluate the uterus, pelvis, and contralateral adnexa. Determine the extent of adhesions and any unexpected pelvic pathology. When needed, use traditional small retractors or gentle packing to gain additional exposure. If the cystic mass appears suspicious (internal and external excrescences on the cyst, ovaries, or peritoneal surfaces, or ascites), obtain pelvic washings with a suction-irrigation cannula, send the fluid for cytologic examination, and convert the minilaparotomy to a standard exploratory laparotomy. Extensive adhesions to the bowel, broad ligament, or pelvic sidewall and unexpected extensive endometriosis may also require a conversion to standard laparotomy.
Reduce cyst size by decompression
Simple aspiration is inadvisable. To remove a large ovarian cyst using the Pelosi minilaparotomy, reduce the size of the cyst to permit safe and effective mobilization and resection through the small abdominal incision. Simple aspiration of the cyst, with its potential for spilling the contents, is not a wise strategy for several reasons. First, many ovarian cysts contain functional epithelium with a high recurrence rate (8% to 67%). Second, studies show that 10% to 66% of ovarian cyst fluid aspirates initially diagnosed as benign actually are malignant. Further, relying on negative cytology from the aspirate of an ovarian cyst without tissue biopsy may delay appropriate surgery, and the puncture of an unexpected malignant cyst may seed the peritoneal cavity and possibly worsen the patient’s prognosis.11,12
This technique, suitable for all ovarian cysts, makes it possible to aspirate a large cyst without leakage.
Whether spillage of cancer cells actually worsens the prognosis of a patient with a neoplastic cyst remains controversial because of conflicting study results. Nonetheless, the possibility of intraperitoneal dissemination of neoplastic cells from a ruptured cyst cannot be considered innocuous, and the potential negative effect on a patient’s prognosis should not be ignored.14 Make every attempt, therefore, to avoid rupturing the cyst and spilling the fluid into the peritoneal cavity.
A shared flaw plagues aspiration devices. Different devices are available for intraoperative cyst aspiration during laparoscopic, transvaginal, or laparotomy approaches.3,4,6 In addition to long needles, drainage trocars, suction cannulas, and suprapubic bladder catheters, special aspiration instruments have been developed.15 They include a metal vacuum system with an aspirator trocar that seals the surface of the cyst, and a catheter system that pinches the punctured cyst wall between double balloons to prevent spillage.16 In addition, several commercial bags are available to prevent intraperitoneal spillage during removal of ovarian cysts.
All these devices have a universal flaw, however: After a thin-walled cyst initially is punctured, none of these products can prevent the spontaneous dehiscence of the cyst and the resulting spillage of its contents into the abdominal cavity. Vacuum systems work well for large cysts with smooth, round surfaces, but in those with irregular surfaces, both application and maintaining the seal are difficult.17 Fortunately, our technique makes it possible to aspirate a large ovarian cyst without leakage, and the method is suitable for all ovarian cysts regardless of surface type or wall thickness.
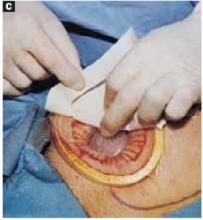
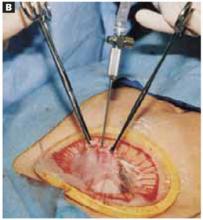
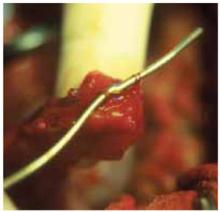
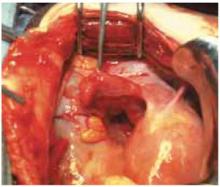
Glue the dressing to the cyst to capture leakage. Using a gauze pad, carefully dry the area of the ovarian cyst that is visible through the self-retaining retractor. Then generously spread sterile surgical glue such as Dermabond (Ethicon, Somerville, NJ) on the cyst wall surface (FIGURE 5A). Dermabond is the commercial name for 2-octyl cyanoacrylate, a sterile skin adhesive used as an alternative to stitches to close the edges of small wounds. It is similar to commercial adhesives such as Super Glue and Krazy Glue.
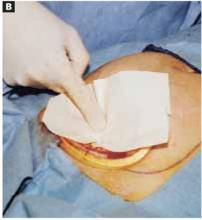
Remove the paper cover of a transparent plastic surgical dressing and place the adhesive side directly onto the glued cyst surface until you are sure the adhesive is completely fixed (FIGURES 5B AND 5C). The 35 cm x 35 cm Steri-Drape or the transparent Tegaderm (both from 3M Health Care, St. Paul, Minn) dressing is effective. A standard nonadhesive plastic dressing or a sterile plastic bag also can be used, as long as the free edges extend beyond the outer rim of the self-retaining retractor.
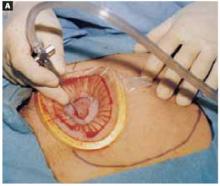
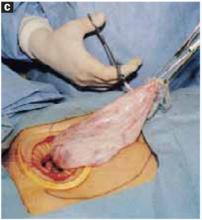
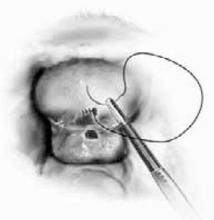
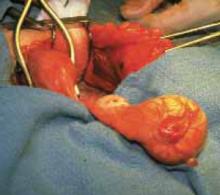
With a needle aspirator, pierce the cyst through the glued plastic dressing and carefully aspirate the fluid (FIGURES 6A AND 6B). Any leakage is trapped inside the plastic dressing rather than the abdominal cavity. Continue the aspiration until the collapsed cyst and ovary can be gradually delivered through the abdominal incision (FIGURE 6C). Note that the selfretaining retractor also protects the abdominal incision from potential contamination and implantation of neoplastic cells.
Once the cyst and ovary are extracted, you can readily perform an extracorporeal cystectomy, after which the repaired ovary is returned to the abdominal cavity. Be careful to avoid letting any fluid flow back into the peritoneal cavity during cyst removal. When indicated, an extracorporeal adnexectomy can readily be performed.
The presence of oily material or hairs in the aspirated fluid and on the needle tip readily identifies a dermoid cyst.
Dermoid cysts require extra care. Preventing intraperitoneal spillage is especially important when removing a large dermoid cyst, to avoid the possibility of chemical peritonitis, dense adhesions, and fistulas. The presence of oily material or hairs in the aspirated fluid and on the needle tip readily identifies a dermoid cyst. Quite frequently, a large-diameter suction cannula is required to remove the waxy contents. If the dermoid cyst is very large, aspiration alone will not empty it entirely. Complete emptying is not necessary, however. The primary goal of aspiration is to reduce the size and tension of the cyst to permit delivery through the abdominal incision.
Closing the cruciate incision is quicker and requires less exposure than a scaled-down Pfannenstiel’s incision.
Before concluding the procedure, irrigate the peritoneal cavity to remove any remnants of cyst contents that may have spilled—especially important with a dermoid cyst. Perform any additional indicated procedures through the minilaparotomy incision, such as a contralateral ovarian cystectomy, salpingo-oophorectomy, or hysterectomy. After the surgery is completed, remove the self-retaining retractor by hooking a finger through the bottom ring and pulling it gently out of the incision. Closing the cruciate incision is quicker and requires less exposure to complete than a scaled-down Pfannenstiel’s incision. Apply a vertical pressure dressing over the incision to prevent postoperative wound hematoma or seroma formation. Remove the dressing 24 hours later.
FIGURE 5A ‘Leak-proofing’ the aspiration
After drying the surface of the cyst, apply sterile surgical glue to the cyst wall (2 to 3 ampules are usually required to cover the cyst surface).
FIGURE 5B ‘Leak-proofing’ the aspiration
Apply the adhesive side of a clear plastic wound dressing directly onto the cyst’s surface, making sure that the dressing is large enough to fully cover the self-retaining retractor. Using either a piece of folded gauze or your fingers, press the plastic dressing against the adhesive-coated cyst for about 3 to 5 minutes until the adhesive is completely fixed.
FIGURE 5C ‘Leak-proofing’ the aspiration
Remove the paper cover of the wound dressing.
FIGURE 6A Aspirating the cyst
Pierce the dressing and carefully aspirate the fluid until the cyst is partially collapsed.
FIGURE 6B Aspirating the cyst
Place atraumatic clamps on the cyst wall to further control drainage. Any leakage is trapped inside the plastic dressing rather than draining into the abdominal cavity.
FIGURE 6C Aspirating the cyst
Continue the aspiration until the collapsed cyst and ovary can be delivered gradually through the abdominal incision, after detaching the adhesive plastic dressing from the edges of the self-retaining retractor. The portion of the plastic sleeve that is glued to the cyst surface remains attached to the cyst until the cyst is extracted. Following extraction, perform an extracorporeal cystectomy and return the repaired ovary to the abdominal cavity.
Good results
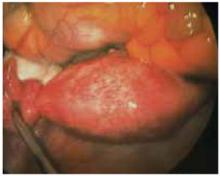
Using our approach, we have treated 38 patients with ovarian cysts of diameters greater than 20 cm thought to be benign by preoperative workup (FIGURE 7). We encountered no malignancies. All surgeries were successfully completed without laparoscopy or conversion to traditional laparotomy and with good cosmesis (FIGURE 8).
Other procedures performed in some of these patients using the same technique included contralateral cystectomy, salpingo-oophorectomy, subtotal and total hysterectomy, adhesiolysis, and appendectomy. We encountered no intraoperative or postoperative complications. Operating times ranged from 18 to 65 minutes. All patients were discharged home within 36 hours and returned to work in a mean of 12 days. Pathology findings of the ovarian cysts included endometrioma, dermoid cyst, serous cystadenoma, and mucinous cystadenoma.
FIGURE 7 Effective for large cysts
Following removal, the collapsed cyst was refilled with 1,300 mL of normal saline to demonstrate its actual size.
FIGURE 8 Good cosmetic results
Cosmetic appearance of the small cruciate abdominal incision 10 days after surgery.Dr. Pelosi II reports that he is a consultant for Apple Medical Corporation. Dr. Pelosi III reports no affiliations or financial arrangements with any companies whose products are mentioned in this article.
1. Pelosi MA, II, Pelosi MA, III. Pelosi minilaparotomy hysterectomy: Effective alternative to laparoscopy and laparotomy. OBG Management. 2003;15(4):16-33.
2. Havrilesky LJ, Peterson BL, Dryden DK, et al. Predictors of clinical outcomes in the laparoscopic management of adnexal masses. Obstet Gynecol. 2003;102:243-251.
3. Pelosi MA, II, Pelosi MA, III. Laparoscopic removal of a 103 pound ovarian tumor. J Am Assoc Gynecol Laparosc. 1996;3:413-417.
4. Eltabbaku GH. Laparoscopic management of ovarian cysts. Contemporary OB/GYN. 2003;48(8):37-50.
5. Ou C, Liu Y, Zabriskie V, et al. Alternate method for laparoscopic management of adnexal masses greater than 10 cm in diameter. J Laparoendosc Adv Surg Tech. 2001;11:125-132.
6. Jeong E, Kim H, Ahn C, et al. Successful laparoscopic removal of huge ovarian cysts. J Am Gynecol Laparosc. 1997;4:609-614.
7. Nagele F, Magos AL. Combined ultrasonographically guided drainage and laparoscopic excision of a large ovarian cyst. Am J Obstet Gynecol. 1996;175:1377-1378.
8. Sheth S. Adnexal pathology. In: Sheth S, Studd J, eds. Vaginal Hysterectomy. London, England: Martin Dunitz; 2002;165-178.
9. Flynn MK, Niloff JM. Outpatient minilaparotomy for ovarian cysts. J Reprod Med. 1999;44:399-404.
10. Benedetti P, Panicci P, Maneschi F, et al. Surgery by minilaparotomy in benign gynecological disease. Obstet Gynecol. 1996;87:456-459.
11. Jansen FW, Tanahotoe S, Veselie M, et al. Laparoscopic aspiration of ovarian cysts: an unreliable technique in primary diagnosis of sonographically benign ovarian lesions. Gynecol Endosc. 1997;6:363-367.
12. Parker WH. Laparoscopic management of the adnexal mass in postmenopausal women. J Gynecol Tech. 1995;1:3-5.
13. Guerriero S, Ajossa S, Mais V, et al. Prelaparoscopic assessment of ovarian cysts in reproductive-age women. Gynecol Endosc. 1997;6:157-167.
14. Fowler JM, Carter JR. Laparoscopic management of the adnexal mass in postmenopausal women. J Gynecol Tech. 1995;1:7-10.
15. McCormick JB, Fitzgibbons JP. Instrument for aspiration of large ovarian cysts. Obstet Gynecol. 1967;29:869-870.
16. Yamada T, Okamoto Y, Kasematsu H. Use of the Sand balloon catheter for the laparoscopic surgery of benign ovarian cysts. Gynecol Endosc. 1999;9:51-54.
17. Shozu M, Segawa T, Sumitani H, et al. Leak-proof puncture of ovarian cysts: instant mounting of plastic bag using cyanoacrylate adhesive. Obstet Gynecol. 2001;97:1007-1010.
- Make a cruciate incision by incising the skin transversely and the anterior rectus fascia vertically.
- Insert a soft, sleeved, self-retaining retractor.
- Using a surgical adhesive, glue a large plastic wound dressing to the surface of the cyst to prevent leakage of contents into the abdominal cavity.
- Aspirate the cyst until it collapses and can be delivered, with the ovary, through the abdominal incision.
- After performing an extracorporeal cystectomy and/or adnexectomy, return the repaired ovary to the abdominal cavity.
Although laparotomy is still considered the standard for ovarian cyst removal, over the past 15 years minimally invasive surgery has gained wider acceptance in cases where preoperative assessment suggests an adnexal mass is benign.
Unfortunately, minimally invasive management of a large ovarian cyst (greater than 10 cm) is particularly challenging for several reasons:
- The cyst can rupture and spill its contents into the peritoneum,
- the cyst’s size limits the surgical field, and
- an unexpected malignancy may be revealed.
An innovative minilaparotomy technique for the removal of benign ovarian cysts offers the advantages of laparoscopic and laparoscopic-assisted procedures while bypassing the major disadvantages: the necessity for specialized and expensive equipment, lengthy operative time, and long learning curves.1 (The minimally invasive procedures currently available for the treatment of ovarian cysts include laparoscopic cystectomy, laparoscopic-assisted minilaparotomy cystectomy, laparoscopic-assisted vaginal cystectomy, combined percutaneous ultrasound cyst aspiration and laparoscopic cystectomy, transvaginal cystectomy, and the traditional minilaparotomy cystectomy.2-10)
The procedure is faster, less expensive, carries fewer potential risks than traditional alternatives, and offers these advantages:
- can be performed under regional anesthesia
- relies on standard open techniques
- uses inexpensive instrumentation
- is easy to learn
- can be used for very large cysts
- eliminates the risk of intraperitoneal spillage of cyst contents
- offers similar postoperative convalescence and mean time to return to work as laparoscopic or laparoscopic-assisted management of large ovarian cysts
General Ob/Gyns—not gynecologic oncologists—perform most surgeries on patients with adnexal masses, since ovarian cancer is relatively uncommon in the absence of preoperative risk factors for malignancy. Our approach offers an appealing option to Ob/Gyns reluctant to abandon routine traditional laparotomy for such ovarian cysts.
Selecting the right patient
Adequate preoperative assessment diminishes the risk of unexpected malignancy in a patient undergoing surgery for an ovarian mass to less than 1%.4 At this time, the combination of menopausal status, cancer antigen (CA) 125 level, physical examination, and ultrasound is the best strategy for evaluating the patient with an ovarian cyst.11
Signs of malignancy. Ultrasound features that suggest malignancy include irregular borders, thick septa, solid areas, internal and external excrescences, matted bowel, and ascites. Benign cysts, on the other hand, are usually unilateral and have regular borders, thin septa, no solid areas, and no internal excrescences.4 The measurement of blood flow within the mass by color Doppler may improve the accuracy of ultrasound in differentiating benign from malignant cysts.4,12
On physical examination, an adnexal mass that is fixed, irregular, or solid also suggests a neoplasm. An elevated CA 125 combined with a complex adnexal mass is likely to be associated with malignancy. The test is even more specific in postmenopausal women with adnexal masses.4,12
However, plasma levels of CA 125 also can be elevated in several benign gynecologic conditions such as endometriosis, simple ovarian cysts, pelvic inflammatory disease, ovarian torsion, fibroids, and in physiologic conditions such as menstruation and pregnancy.13
Anticipate the need to convert to laparotomy. Every patient’s surgical consent should include a possible conversion to laparotomy. To avoid incomplete surgical treatment and significant delays in proper therapy, a gynecologic surgeon experienced in the management of ovarian cancer should be readily available, in the event an unexpected malignancy is encountered. Ideally, the staging surgery and definite treatment should be performed at the time of initial minilaparotomy. Comprehensive surgical staging and treatment include thorough exploration of the pelvis and abdomen, omentectomy, pelvic and paraaortic lymph node sampling, multiple peritoneal biopsies and washings, bilateral salpingo-oophorectomy, hysterectomy, and debulking, when indicated.
Prepare for surgery with position, incision, and retraction
Before beginning, it is crucial to correctly position the patient, make the appropriate incision, and insert the right retractor.1
Position. After administering regional or general anesthesia, place the patient in a modified lithotomy, as for laparoscopic surgery. Tuck the arms alongside the torso and place the legs in boot stirrups. Avoid hip flexion and allow adequate thigh abduction to expose the vagina. Perform a careful pelvic examination to determine the size and mobility of the adnexal mass.
When properly placed, this retractor creates an atraumatic, circular area of self-retraction, enabling superior exposure
Place an indwelling transurethral catheter, and pass a sturdy, hinged uterine manipulator such as the Pelosi Uterine Manipulator (Apple Medical Corp, Marlboro, Mass) transcervically into the uterine cavity (FIGURE 1).
The cruciate incision. With a conventional scalpel make a small suprapubic transverse incision through the skin and subcutaneous tissue (FIGURE 2). After clearing subcutaneous fat from the midline, incise the rectus fascia and the peritoneum in a vertical direction.
A vertical skin incision can be selected if the preoperative workup suggests a later extension of the original minilaparotomy incision may be required, or if there is a prior vertical incision.
Retraction. Use a soft sleeve-type self-retaining plastic retractor, such as Mobius (Apple Medical Corp) (FIGURE 3 ). When properly placed, this retractor creates an atraumatic, circular area of self-retraction, enabling superior exposure of the pelvis (FIGURE 4).
During surgery it may be necessary to adjust the outer ring if the sleeve loosens. Narrow Deaver or Richardson retractors, if required, provide additional retraction. The bowel may be gently packed, if necessary, but typically it is adequately displaced by the large ovarian cyst.
The atraumatic retraction provided by the soft, self-retaining abdominal retractor minimizes the possibility of tissue trauma, nerve damage, bruising, and postoperative pain. At the same time, the continuous 360° retraction force on the incision maximizes surgical exposure, providing a significantly larger working area than conventional retractors. For example, when applied to a 6-cm incision, the self-retaining retractor creates a 28-cm2 exposed working area, compared with only 18 cm2 provided by a conventional 4-point metal retractor.
The adjustable height of the self-retaining retractor adapts to wounds of varying depth and works on virtually any tissue thickness—a feature that makes the device effective for obese patients. Further, by lining the abdominal incision, the retractor’s plastic sleeve protects the wound’s edges from contamination and potential implantation of malignant cells, making the device ideal for managing ovarian cysts.
FIGURE 1 Hinged uterine manipulator
The manipulator facilitates exposure of the cyst and contralateral adnexa, as well as uterine elevation/rotation.
FIGURE 2 Cruciate incision
Make a 2.5- to 5-cm suprapubic transverse skin incision. Using the Bovie device, incise the subcutaneous fat transversely along the full length of the skin incision down to the level of the anterior rectus fascia. Clear the subcutaneous fat from the midline superiorly and inferiorly to expose 5 to 6 cm of the rectus fascia in the vertical axis. Use blunt digital dissection to assist in mobilizing the subcutaneous fat. Incise the anterior rectus fascia vertically through the full length of the cleared area. Retract the rectus muscles from the midline to expose the underlying transversalis fascia and the peritoneum. Control small bleeding points with the Bovie device. Enter the peritoneum either digitally or with scissors above the bladder dome and extend the peritoneal incision to the full length of the fascial incision.
FIGURE 3 Self-retaining retractor
The retractor consists of a flexible plastic inner ring and a firmer outer ring connected by a soft plastic sleeve.
FIGURE 4360° retraction force
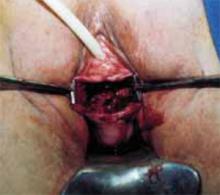
Squeeze the inner ring of the soft, sleeve-type, self-retaining retractor into the peritoneal cavity through the cruciate abdominal incision and allow it to spring open against the parietal peritoneum. Make a digital assessment to ensure that viscera is not trapped between the inner ring and the abdominal wall. Place the entire sleeve on traction by lifting the outer ring. Then roll the outer ring onto the sleeve, collecting excess length, until it sits firmly against the skin. The atraumatic retraction provided by the sleeve-type retractor maximizes exposure of the pelvis. Note how a portion of the large cyst is clearly seen through the atraumatic, circular area of retraction.
Cyst assessment
Visually and digitally inspect the cyst and carefully evaluate the uterus, pelvis, and contralateral adnexa. Determine the extent of adhesions and any unexpected pelvic pathology. When needed, use traditional small retractors or gentle packing to gain additional exposure. If the cystic mass appears suspicious (internal and external excrescences on the cyst, ovaries, or peritoneal surfaces, or ascites), obtain pelvic washings with a suction-irrigation cannula, send the fluid for cytologic examination, and convert the minilaparotomy to a standard exploratory laparotomy. Extensive adhesions to the bowel, broad ligament, or pelvic sidewall and unexpected extensive endometriosis may also require a conversion to standard laparotomy.
Reduce cyst size by decompression
Simple aspiration is inadvisable. To remove a large ovarian cyst using the Pelosi minilaparotomy, reduce the size of the cyst to permit safe and effective mobilization and resection through the small abdominal incision. Simple aspiration of the cyst, with its potential for spilling the contents, is not a wise strategy for several reasons. First, many ovarian cysts contain functional epithelium with a high recurrence rate (8% to 67%). Second, studies show that 10% to 66% of ovarian cyst fluid aspirates initially diagnosed as benign actually are malignant. Further, relying on negative cytology from the aspirate of an ovarian cyst without tissue biopsy may delay appropriate surgery, and the puncture of an unexpected malignant cyst may seed the peritoneal cavity and possibly worsen the patient’s prognosis.11,12
This technique, suitable for all ovarian cysts, makes it possible to aspirate a large cyst without leakage.
Whether spillage of cancer cells actually worsens the prognosis of a patient with a neoplastic cyst remains controversial because of conflicting study results. Nonetheless, the possibility of intraperitoneal dissemination of neoplastic cells from a ruptured cyst cannot be considered innocuous, and the potential negative effect on a patient’s prognosis should not be ignored.14 Make every attempt, therefore, to avoid rupturing the cyst and spilling the fluid into the peritoneal cavity.
A shared flaw plagues aspiration devices. Different devices are available for intraoperative cyst aspiration during laparoscopic, transvaginal, or laparotomy approaches.3,4,6 In addition to long needles, drainage trocars, suction cannulas, and suprapubic bladder catheters, special aspiration instruments have been developed.15 They include a metal vacuum system with an aspirator trocar that seals the surface of the cyst, and a catheter system that pinches the punctured cyst wall between double balloons to prevent spillage.16 In addition, several commercial bags are available to prevent intraperitoneal spillage during removal of ovarian cysts.
All these devices have a universal flaw, however: After a thin-walled cyst initially is punctured, none of these products can prevent the spontaneous dehiscence of the cyst and the resulting spillage of its contents into the abdominal cavity. Vacuum systems work well for large cysts with smooth, round surfaces, but in those with irregular surfaces, both application and maintaining the seal are difficult.17 Fortunately, our technique makes it possible to aspirate a large ovarian cyst without leakage, and the method is suitable for all ovarian cysts regardless of surface type or wall thickness.
Glue the dressing to the cyst to capture leakage. Using a gauze pad, carefully dry the area of the ovarian cyst that is visible through the self-retaining retractor. Then generously spread sterile surgical glue such as Dermabond (Ethicon, Somerville, NJ) on the cyst wall surface (FIGURE 5A). Dermabond is the commercial name for 2-octyl cyanoacrylate, a sterile skin adhesive used as an alternative to stitches to close the edges of small wounds. It is similar to commercial adhesives such as Super Glue and Krazy Glue.
Remove the paper cover of a transparent plastic surgical dressing and place the adhesive side directly onto the glued cyst surface until you are sure the adhesive is completely fixed (FIGURES 5B AND 5C). The 35 cm x 35 cm Steri-Drape or the transparent Tegaderm (both from 3M Health Care, St. Paul, Minn) dressing is effective. A standard nonadhesive plastic dressing or a sterile plastic bag also can be used, as long as the free edges extend beyond the outer rim of the self-retaining retractor.
With a needle aspirator, pierce the cyst through the glued plastic dressing and carefully aspirate the fluid (FIGURES 6A AND 6B). Any leakage is trapped inside the plastic dressing rather than the abdominal cavity. Continue the aspiration until the collapsed cyst and ovary can be gradually delivered through the abdominal incision (FIGURE 6C). Note that the selfretaining retractor also protects the abdominal incision from potential contamination and implantation of neoplastic cells.
Once the cyst and ovary are extracted, you can readily perform an extracorporeal cystectomy, after which the repaired ovary is returned to the abdominal cavity. Be careful to avoid letting any fluid flow back into the peritoneal cavity during cyst removal. When indicated, an extracorporeal adnexectomy can readily be performed.
The presence of oily material or hairs in the aspirated fluid and on the needle tip readily identifies a dermoid cyst.
Dermoid cysts require extra care. Preventing intraperitoneal spillage is especially important when removing a large dermoid cyst, to avoid the possibility of chemical peritonitis, dense adhesions, and fistulas. The presence of oily material or hairs in the aspirated fluid and on the needle tip readily identifies a dermoid cyst. Quite frequently, a large-diameter suction cannula is required to remove the waxy contents. If the dermoid cyst is very large, aspiration alone will not empty it entirely. Complete emptying is not necessary, however. The primary goal of aspiration is to reduce the size and tension of the cyst to permit delivery through the abdominal incision.
Closing the cruciate incision is quicker and requires less exposure than a scaled-down Pfannenstiel’s incision.
Before concluding the procedure, irrigate the peritoneal cavity to remove any remnants of cyst contents that may have spilled—especially important with a dermoid cyst. Perform any additional indicated procedures through the minilaparotomy incision, such as a contralateral ovarian cystectomy, salpingo-oophorectomy, or hysterectomy. After the surgery is completed, remove the self-retaining retractor by hooking a finger through the bottom ring and pulling it gently out of the incision. Closing the cruciate incision is quicker and requires less exposure to complete than a scaled-down Pfannenstiel’s incision. Apply a vertical pressure dressing over the incision to prevent postoperative wound hematoma or seroma formation. Remove the dressing 24 hours later.
FIGURE 5A ‘Leak-proofing’ the aspiration
After drying the surface of the cyst, apply sterile surgical glue to the cyst wall (2 to 3 ampules are usually required to cover the cyst surface).
FIGURE 5B ‘Leak-proofing’ the aspiration
Apply the adhesive side of a clear plastic wound dressing directly onto the cyst’s surface, making sure that the dressing is large enough to fully cover the self-retaining retractor. Using either a piece of folded gauze or your fingers, press the plastic dressing against the adhesive-coated cyst for about 3 to 5 minutes until the adhesive is completely fixed.
FIGURE 5C ‘Leak-proofing’ the aspiration
Remove the paper cover of the wound dressing.
FIGURE 6A Aspirating the cyst
Pierce the dressing and carefully aspirate the fluid until the cyst is partially collapsed.
FIGURE 6B Aspirating the cyst
Place atraumatic clamps on the cyst wall to further control drainage. Any leakage is trapped inside the plastic dressing rather than draining into the abdominal cavity.
FIGURE 6C Aspirating the cyst
Continue the aspiration until the collapsed cyst and ovary can be delivered gradually through the abdominal incision, after detaching the adhesive plastic dressing from the edges of the self-retaining retractor. The portion of the plastic sleeve that is glued to the cyst surface remains attached to the cyst until the cyst is extracted. Following extraction, perform an extracorporeal cystectomy and return the repaired ovary to the abdominal cavity.
Good results
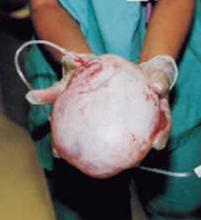
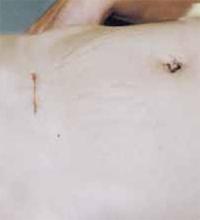
Using our approach, we have treated 38 patients with ovarian cysts of diameters greater than 20 cm thought to be benign by preoperative workup (FIGURE 7). We encountered no malignancies. All surgeries were successfully completed without laparoscopy or conversion to traditional laparotomy and with good cosmesis (FIGURE 8).
Other procedures performed in some of these patients using the same technique included contralateral cystectomy, salpingo-oophorectomy, subtotal and total hysterectomy, adhesiolysis, and appendectomy. We encountered no intraoperative or postoperative complications. Operating times ranged from 18 to 65 minutes. All patients were discharged home within 36 hours and returned to work in a mean of 12 days. Pathology findings of the ovarian cysts included endometrioma, dermoid cyst, serous cystadenoma, and mucinous cystadenoma.
FIGURE 7 Effective for large cysts
Following removal, the collapsed cyst was refilled with 1,300 mL of normal saline to demonstrate its actual size.
FIGURE 8 Good cosmetic results
Cosmetic appearance of the small cruciate abdominal incision 10 days after surgery.Dr. Pelosi II reports that he is a consultant for Apple Medical Corporation. Dr. Pelosi III reports no affiliations or financial arrangements with any companies whose products are mentioned in this article.
- Make a cruciate incision by incising the skin transversely and the anterior rectus fascia vertically.
- Insert a soft, sleeved, self-retaining retractor.
- Using a surgical adhesive, glue a large plastic wound dressing to the surface of the cyst to prevent leakage of contents into the abdominal cavity.
- Aspirate the cyst until it collapses and can be delivered, with the ovary, through the abdominal incision.
- After performing an extracorporeal cystectomy and/or adnexectomy, return the repaired ovary to the abdominal cavity.
Although laparotomy is still considered the standard for ovarian cyst removal, over the past 15 years minimally invasive surgery has gained wider acceptance in cases where preoperative assessment suggests an adnexal mass is benign.
Unfortunately, minimally invasive management of a large ovarian cyst (greater than 10 cm) is particularly challenging for several reasons:
- The cyst can rupture and spill its contents into the peritoneum,
- the cyst’s size limits the surgical field, and
- an unexpected malignancy may be revealed.
An innovative minilaparotomy technique for the removal of benign ovarian cysts offers the advantages of laparoscopic and laparoscopic-assisted procedures while bypassing the major disadvantages: the necessity for specialized and expensive equipment, lengthy operative time, and long learning curves.1 (The minimally invasive procedures currently available for the treatment of ovarian cysts include laparoscopic cystectomy, laparoscopic-assisted minilaparotomy cystectomy, laparoscopic-assisted vaginal cystectomy, combined percutaneous ultrasound cyst aspiration and laparoscopic cystectomy, transvaginal cystectomy, and the traditional minilaparotomy cystectomy.2-10)
The procedure is faster, less expensive, carries fewer potential risks than traditional alternatives, and offers these advantages:
- can be performed under regional anesthesia
- relies on standard open techniques
- uses inexpensive instrumentation
- is easy to learn
- can be used for very large cysts
- eliminates the risk of intraperitoneal spillage of cyst contents
- offers similar postoperative convalescence and mean time to return to work as laparoscopic or laparoscopic-assisted management of large ovarian cysts
General Ob/Gyns—not gynecologic oncologists—perform most surgeries on patients with adnexal masses, since ovarian cancer is relatively uncommon in the absence of preoperative risk factors for malignancy. Our approach offers an appealing option to Ob/Gyns reluctant to abandon routine traditional laparotomy for such ovarian cysts.
Selecting the right patient
Adequate preoperative assessment diminishes the risk of unexpected malignancy in a patient undergoing surgery for an ovarian mass to less than 1%.4 At this time, the combination of menopausal status, cancer antigen (CA) 125 level, physical examination, and ultrasound is the best strategy for evaluating the patient with an ovarian cyst.11
Signs of malignancy. Ultrasound features that suggest malignancy include irregular borders, thick septa, solid areas, internal and external excrescences, matted bowel, and ascites. Benign cysts, on the other hand, are usually unilateral and have regular borders, thin septa, no solid areas, and no internal excrescences.4 The measurement of blood flow within the mass by color Doppler may improve the accuracy of ultrasound in differentiating benign from malignant cysts.4,12
On physical examination, an adnexal mass that is fixed, irregular, or solid also suggests a neoplasm. An elevated CA 125 combined with a complex adnexal mass is likely to be associated with malignancy. The test is even more specific in postmenopausal women with adnexal masses.4,12
However, plasma levels of CA 125 also can be elevated in several benign gynecologic conditions such as endometriosis, simple ovarian cysts, pelvic inflammatory disease, ovarian torsion, fibroids, and in physiologic conditions such as menstruation and pregnancy.13
Anticipate the need to convert to laparotomy. Every patient’s surgical consent should include a possible conversion to laparotomy. To avoid incomplete surgical treatment and significant delays in proper therapy, a gynecologic surgeon experienced in the management of ovarian cancer should be readily available, in the event an unexpected malignancy is encountered. Ideally, the staging surgery and definite treatment should be performed at the time of initial minilaparotomy. Comprehensive surgical staging and treatment include thorough exploration of the pelvis and abdomen, omentectomy, pelvic and paraaortic lymph node sampling, multiple peritoneal biopsies and washings, bilateral salpingo-oophorectomy, hysterectomy, and debulking, when indicated.
Prepare for surgery with position, incision, and retraction
Before beginning, it is crucial to correctly position the patient, make the appropriate incision, and insert the right retractor.1
Position. After administering regional or general anesthesia, place the patient in a modified lithotomy, as for laparoscopic surgery. Tuck the arms alongside the torso and place the legs in boot stirrups. Avoid hip flexion and allow adequate thigh abduction to expose the vagina. Perform a careful pelvic examination to determine the size and mobility of the adnexal mass.
When properly placed, this retractor creates an atraumatic, circular area of self-retraction, enabling superior exposure
Place an indwelling transurethral catheter, and pass a sturdy, hinged uterine manipulator such as the Pelosi Uterine Manipulator (Apple Medical Corp, Marlboro, Mass) transcervically into the uterine cavity (FIGURE 1).
The cruciate incision. With a conventional scalpel make a small suprapubic transverse incision through the skin and subcutaneous tissue (FIGURE 2). After clearing subcutaneous fat from the midline, incise the rectus fascia and the peritoneum in a vertical direction.
A vertical skin incision can be selected if the preoperative workup suggests a later extension of the original minilaparotomy incision may be required, or if there is a prior vertical incision.
Retraction. Use a soft sleeve-type self-retaining plastic retractor, such as Mobius (Apple Medical Corp) (FIGURE 3 ). When properly placed, this retractor creates an atraumatic, circular area of self-retraction, enabling superior exposure of the pelvis (FIGURE 4).
During surgery it may be necessary to adjust the outer ring if the sleeve loosens. Narrow Deaver or Richardson retractors, if required, provide additional retraction. The bowel may be gently packed, if necessary, but typically it is adequately displaced by the large ovarian cyst.
The atraumatic retraction provided by the soft, self-retaining abdominal retractor minimizes the possibility of tissue trauma, nerve damage, bruising, and postoperative pain. At the same time, the continuous 360° retraction force on the incision maximizes surgical exposure, providing a significantly larger working area than conventional retractors. For example, when applied to a 6-cm incision, the self-retaining retractor creates a 28-cm2 exposed working area, compared with only 18 cm2 provided by a conventional 4-point metal retractor.
The adjustable height of the self-retaining retractor adapts to wounds of varying depth and works on virtually any tissue thickness—a feature that makes the device effective for obese patients. Further, by lining the abdominal incision, the retractor’s plastic sleeve protects the wound’s edges from contamination and potential implantation of malignant cells, making the device ideal for managing ovarian cysts.
FIGURE 1 Hinged uterine manipulator
The manipulator facilitates exposure of the cyst and contralateral adnexa, as well as uterine elevation/rotation.
FIGURE 2 Cruciate incision
Make a 2.5- to 5-cm suprapubic transverse skin incision. Using the Bovie device, incise the subcutaneous fat transversely along the full length of the skin incision down to the level of the anterior rectus fascia. Clear the subcutaneous fat from the midline superiorly and inferiorly to expose 5 to 6 cm of the rectus fascia in the vertical axis. Use blunt digital dissection to assist in mobilizing the subcutaneous fat. Incise the anterior rectus fascia vertically through the full length of the cleared area. Retract the rectus muscles from the midline to expose the underlying transversalis fascia and the peritoneum. Control small bleeding points with the Bovie device. Enter the peritoneum either digitally or with scissors above the bladder dome and extend the peritoneal incision to the full length of the fascial incision.
FIGURE 3 Self-retaining retractor
The retractor consists of a flexible plastic inner ring and a firmer outer ring connected by a soft plastic sleeve.
FIGURE 4360° retraction force
Squeeze the inner ring of the soft, sleeve-type, self-retaining retractor into the peritoneal cavity through the cruciate abdominal incision and allow it to spring open against the parietal peritoneum. Make a digital assessment to ensure that viscera is not trapped between the inner ring and the abdominal wall. Place the entire sleeve on traction by lifting the outer ring. Then roll the outer ring onto the sleeve, collecting excess length, until it sits firmly against the skin. The atraumatic retraction provided by the sleeve-type retractor maximizes exposure of the pelvis. Note how a portion of the large cyst is clearly seen through the atraumatic, circular area of retraction.
Cyst assessment
Visually and digitally inspect the cyst and carefully evaluate the uterus, pelvis, and contralateral adnexa. Determine the extent of adhesions and any unexpected pelvic pathology. When needed, use traditional small retractors or gentle packing to gain additional exposure. If the cystic mass appears suspicious (internal and external excrescences on the cyst, ovaries, or peritoneal surfaces, or ascites), obtain pelvic washings with a suction-irrigation cannula, send the fluid for cytologic examination, and convert the minilaparotomy to a standard exploratory laparotomy. Extensive adhesions to the bowel, broad ligament, or pelvic sidewall and unexpected extensive endometriosis may also require a conversion to standard laparotomy.
Reduce cyst size by decompression
Simple aspiration is inadvisable. To remove a large ovarian cyst using the Pelosi minilaparotomy, reduce the size of the cyst to permit safe and effective mobilization and resection through the small abdominal incision. Simple aspiration of the cyst, with its potential for spilling the contents, is not a wise strategy for several reasons. First, many ovarian cysts contain functional epithelium with a high recurrence rate (8% to 67%). Second, studies show that 10% to 66% of ovarian cyst fluid aspirates initially diagnosed as benign actually are malignant. Further, relying on negative cytology from the aspirate of an ovarian cyst without tissue biopsy may delay appropriate surgery, and the puncture of an unexpected malignant cyst may seed the peritoneal cavity and possibly worsen the patient’s prognosis.11,12
This technique, suitable for all ovarian cysts, makes it possible to aspirate a large cyst without leakage.
Whether spillage of cancer cells actually worsens the prognosis of a patient with a neoplastic cyst remains controversial because of conflicting study results. Nonetheless, the possibility of intraperitoneal dissemination of neoplastic cells from a ruptured cyst cannot be considered innocuous, and the potential negative effect on a patient’s prognosis should not be ignored.14 Make every attempt, therefore, to avoid rupturing the cyst and spilling the fluid into the peritoneal cavity.
A shared flaw plagues aspiration devices. Different devices are available for intraoperative cyst aspiration during laparoscopic, transvaginal, or laparotomy approaches.3,4,6 In addition to long needles, drainage trocars, suction cannulas, and suprapubic bladder catheters, special aspiration instruments have been developed.15 They include a metal vacuum system with an aspirator trocar that seals the surface of the cyst, and a catheter system that pinches the punctured cyst wall between double balloons to prevent spillage.16 In addition, several commercial bags are available to prevent intraperitoneal spillage during removal of ovarian cysts.
All these devices have a universal flaw, however: After a thin-walled cyst initially is punctured, none of these products can prevent the spontaneous dehiscence of the cyst and the resulting spillage of its contents into the abdominal cavity. Vacuum systems work well for large cysts with smooth, round surfaces, but in those with irregular surfaces, both application and maintaining the seal are difficult.17 Fortunately, our technique makes it possible to aspirate a large ovarian cyst without leakage, and the method is suitable for all ovarian cysts regardless of surface type or wall thickness.
Glue the dressing to the cyst to capture leakage. Using a gauze pad, carefully dry the area of the ovarian cyst that is visible through the self-retaining retractor. Then generously spread sterile surgical glue such as Dermabond (Ethicon, Somerville, NJ) on the cyst wall surface (FIGURE 5A). Dermabond is the commercial name for 2-octyl cyanoacrylate, a sterile skin adhesive used as an alternative to stitches to close the edges of small wounds. It is similar to commercial adhesives such as Super Glue and Krazy Glue.
Remove the paper cover of a transparent plastic surgical dressing and place the adhesive side directly onto the glued cyst surface until you are sure the adhesive is completely fixed (FIGURES 5B AND 5C). The 35 cm x 35 cm Steri-Drape or the transparent Tegaderm (both from 3M Health Care, St. Paul, Minn) dressing is effective. A standard nonadhesive plastic dressing or a sterile plastic bag also can be used, as long as the free edges extend beyond the outer rim of the self-retaining retractor.
With a needle aspirator, pierce the cyst through the glued plastic dressing and carefully aspirate the fluid (FIGURES 6A AND 6B). Any leakage is trapped inside the plastic dressing rather than the abdominal cavity. Continue the aspiration until the collapsed cyst and ovary can be gradually delivered through the abdominal incision (FIGURE 6C). Note that the selfretaining retractor also protects the abdominal incision from potential contamination and implantation of neoplastic cells.
Once the cyst and ovary are extracted, you can readily perform an extracorporeal cystectomy, after which the repaired ovary is returned to the abdominal cavity. Be careful to avoid letting any fluid flow back into the peritoneal cavity during cyst removal. When indicated, an extracorporeal adnexectomy can readily be performed.
The presence of oily material or hairs in the aspirated fluid and on the needle tip readily identifies a dermoid cyst.
Dermoid cysts require extra care. Preventing intraperitoneal spillage is especially important when removing a large dermoid cyst, to avoid the possibility of chemical peritonitis, dense adhesions, and fistulas. The presence of oily material or hairs in the aspirated fluid and on the needle tip readily identifies a dermoid cyst. Quite frequently, a large-diameter suction cannula is required to remove the waxy contents. If the dermoid cyst is very large, aspiration alone will not empty it entirely. Complete emptying is not necessary, however. The primary goal of aspiration is to reduce the size and tension of the cyst to permit delivery through the abdominal incision.
Closing the cruciate incision is quicker and requires less exposure than a scaled-down Pfannenstiel’s incision.
Before concluding the procedure, irrigate the peritoneal cavity to remove any remnants of cyst contents that may have spilled—especially important with a dermoid cyst. Perform any additional indicated procedures through the minilaparotomy incision, such as a contralateral ovarian cystectomy, salpingo-oophorectomy, or hysterectomy. After the surgery is completed, remove the self-retaining retractor by hooking a finger through the bottom ring and pulling it gently out of the incision. Closing the cruciate incision is quicker and requires less exposure to complete than a scaled-down Pfannenstiel’s incision. Apply a vertical pressure dressing over the incision to prevent postoperative wound hematoma or seroma formation. Remove the dressing 24 hours later.
FIGURE 5A ‘Leak-proofing’ the aspiration
After drying the surface of the cyst, apply sterile surgical glue to the cyst wall (2 to 3 ampules are usually required to cover the cyst surface).
FIGURE 5B ‘Leak-proofing’ the aspiration
Apply the adhesive side of a clear plastic wound dressing directly onto the cyst’s surface, making sure that the dressing is large enough to fully cover the self-retaining retractor. Using either a piece of folded gauze or your fingers, press the plastic dressing against the adhesive-coated cyst for about 3 to 5 minutes until the adhesive is completely fixed.
FIGURE 5C ‘Leak-proofing’ the aspiration
Remove the paper cover of the wound dressing.
FIGURE 6A Aspirating the cyst
Pierce the dressing and carefully aspirate the fluid until the cyst is partially collapsed.
FIGURE 6B Aspirating the cyst
Place atraumatic clamps on the cyst wall to further control drainage. Any leakage is trapped inside the plastic dressing rather than draining into the abdominal cavity.
FIGURE 6C Aspirating the cyst
Continue the aspiration until the collapsed cyst and ovary can be delivered gradually through the abdominal incision, after detaching the adhesive plastic dressing from the edges of the self-retaining retractor. The portion of the plastic sleeve that is glued to the cyst surface remains attached to the cyst until the cyst is extracted. Following extraction, perform an extracorporeal cystectomy and return the repaired ovary to the abdominal cavity.
Good results
Using our approach, we have treated 38 patients with ovarian cysts of diameters greater than 20 cm thought to be benign by preoperative workup (FIGURE 7). We encountered no malignancies. All surgeries were successfully completed without laparoscopy or conversion to traditional laparotomy and with good cosmesis (FIGURE 8).
Other procedures performed in some of these patients using the same technique included contralateral cystectomy, salpingo-oophorectomy, subtotal and total hysterectomy, adhesiolysis, and appendectomy. We encountered no intraoperative or postoperative complications. Operating times ranged from 18 to 65 minutes. All patients were discharged home within 36 hours and returned to work in a mean of 12 days. Pathology findings of the ovarian cysts included endometrioma, dermoid cyst, serous cystadenoma, and mucinous cystadenoma.
FIGURE 7 Effective for large cysts
Following removal, the collapsed cyst was refilled with 1,300 mL of normal saline to demonstrate its actual size.
FIGURE 8 Good cosmetic results
Cosmetic appearance of the small cruciate abdominal incision 10 days after surgery.Dr. Pelosi II reports that he is a consultant for Apple Medical Corporation. Dr. Pelosi III reports no affiliations or financial arrangements with any companies whose products are mentioned in this article.
1. Pelosi MA, II, Pelosi MA, III. Pelosi minilaparotomy hysterectomy: Effective alternative to laparoscopy and laparotomy. OBG Management. 2003;15(4):16-33.
2. Havrilesky LJ, Peterson BL, Dryden DK, et al. Predictors of clinical outcomes in the laparoscopic management of adnexal masses. Obstet Gynecol. 2003;102:243-251.
3. Pelosi MA, II, Pelosi MA, III. Laparoscopic removal of a 103 pound ovarian tumor. J Am Assoc Gynecol Laparosc. 1996;3:413-417.
4. Eltabbaku GH. Laparoscopic management of ovarian cysts. Contemporary OB/GYN. 2003;48(8):37-50.
5. Ou C, Liu Y, Zabriskie V, et al. Alternate method for laparoscopic management of adnexal masses greater than 10 cm in diameter. J Laparoendosc Adv Surg Tech. 2001;11:125-132.
6. Jeong E, Kim H, Ahn C, et al. Successful laparoscopic removal of huge ovarian cysts. J Am Gynecol Laparosc. 1997;4:609-614.
7. Nagele F, Magos AL. Combined ultrasonographically guided drainage and laparoscopic excision of a large ovarian cyst. Am J Obstet Gynecol. 1996;175:1377-1378.
8. Sheth S. Adnexal pathology. In: Sheth S, Studd J, eds. Vaginal Hysterectomy. London, England: Martin Dunitz; 2002;165-178.
9. Flynn MK, Niloff JM. Outpatient minilaparotomy for ovarian cysts. J Reprod Med. 1999;44:399-404.
10. Benedetti P, Panicci P, Maneschi F, et al. Surgery by minilaparotomy in benign gynecological disease. Obstet Gynecol. 1996;87:456-459.
11. Jansen FW, Tanahotoe S, Veselie M, et al. Laparoscopic aspiration of ovarian cysts: an unreliable technique in primary diagnosis of sonographically benign ovarian lesions. Gynecol Endosc. 1997;6:363-367.
12. Parker WH. Laparoscopic management of the adnexal mass in postmenopausal women. J Gynecol Tech. 1995;1:3-5.
13. Guerriero S, Ajossa S, Mais V, et al. Prelaparoscopic assessment of ovarian cysts in reproductive-age women. Gynecol Endosc. 1997;6:157-167.
14. Fowler JM, Carter JR. Laparoscopic management of the adnexal mass in postmenopausal women. J Gynecol Tech. 1995;1:7-10.
15. McCormick JB, Fitzgibbons JP. Instrument for aspiration of large ovarian cysts. Obstet Gynecol. 1967;29:869-870.
16. Yamada T, Okamoto Y, Kasematsu H. Use of the Sand balloon catheter for the laparoscopic surgery of benign ovarian cysts. Gynecol Endosc. 1999;9:51-54.
17. Shozu M, Segawa T, Sumitani H, et al. Leak-proof puncture of ovarian cysts: instant mounting of plastic bag using cyanoacrylate adhesive. Obstet Gynecol. 2001;97:1007-1010.
1. Pelosi MA, II, Pelosi MA, III. Pelosi minilaparotomy hysterectomy: Effective alternative to laparoscopy and laparotomy. OBG Management. 2003;15(4):16-33.
2. Havrilesky LJ, Peterson BL, Dryden DK, et al. Predictors of clinical outcomes in the laparoscopic management of adnexal masses. Obstet Gynecol. 2003;102:243-251.
3. Pelosi MA, II, Pelosi MA, III. Laparoscopic removal of a 103 pound ovarian tumor. J Am Assoc Gynecol Laparosc. 1996;3:413-417.
4. Eltabbaku GH. Laparoscopic management of ovarian cysts. Contemporary OB/GYN. 2003;48(8):37-50.
5. Ou C, Liu Y, Zabriskie V, et al. Alternate method for laparoscopic management of adnexal masses greater than 10 cm in diameter. J Laparoendosc Adv Surg Tech. 2001;11:125-132.
6. Jeong E, Kim H, Ahn C, et al. Successful laparoscopic removal of huge ovarian cysts. J Am Gynecol Laparosc. 1997;4:609-614.
7. Nagele F, Magos AL. Combined ultrasonographically guided drainage and laparoscopic excision of a large ovarian cyst. Am J Obstet Gynecol. 1996;175:1377-1378.
8. Sheth S. Adnexal pathology. In: Sheth S, Studd J, eds. Vaginal Hysterectomy. London, England: Martin Dunitz; 2002;165-178.
9. Flynn MK, Niloff JM. Outpatient minilaparotomy for ovarian cysts. J Reprod Med. 1999;44:399-404.
10. Benedetti P, Panicci P, Maneschi F, et al. Surgery by minilaparotomy in benign gynecological disease. Obstet Gynecol. 1996;87:456-459.
11. Jansen FW, Tanahotoe S, Veselie M, et al. Laparoscopic aspiration of ovarian cysts: an unreliable technique in primary diagnosis of sonographically benign ovarian lesions. Gynecol Endosc. 1997;6:363-367.
12. Parker WH. Laparoscopic management of the adnexal mass in postmenopausal women. J Gynecol Tech. 1995;1:3-5.
13. Guerriero S, Ajossa S, Mais V, et al. Prelaparoscopic assessment of ovarian cysts in reproductive-age women. Gynecol Endosc. 1997;6:157-167.
14. Fowler JM, Carter JR. Laparoscopic management of the adnexal mass in postmenopausal women. J Gynecol Tech. 1995;1:7-10.
15. McCormick JB, Fitzgibbons JP. Instrument for aspiration of large ovarian cysts. Obstet Gynecol. 1967;29:869-870.
16. Yamada T, Okamoto Y, Kasematsu H. Use of the Sand balloon catheter for the laparoscopic surgery of benign ovarian cysts. Gynecol Endosc. 1999;9:51-54.
17. Shozu M, Segawa T, Sumitani H, et al. Leak-proof puncture of ovarian cysts: instant mounting of plastic bag using cyanoacrylate adhesive. Obstet Gynecol. 2001;97:1007-1010.
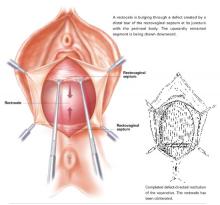
Defect-directed reconstruction: The common-sense technique for rectocele repair
- Tears can almost always be identified without difficulty with the aid of edge-grasping clamps at surgery and a rectal examining finger.
- We can restore the natural anatomic integrity of the rectovaginal septum by reuniting the torn edges, thus eliminating the rectocele.
- The most common tears are transverse. Others include U-shaped, linear, and combined tears.
Why did it take us 2 centuries to learn how to do it right? Posterior pelvic repair for the correction of rectocele combined with restoration of the perineal body has been on gynecologic OR schedules since time immemorial, so one would think we knew what we were doing. Yet only in the past 10 years has there been progressive general recognition of the true nature of the anatomic lesions responsible for rectocele formation, thereby finally pointing us in the right direction.
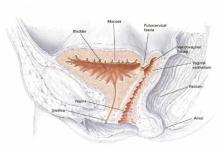
Although it was always assumed, correctly, that the major factor initiating rectoceles is tissue trauma sustained in vaginal birth, traditionally we were taught, and we believed, that the end result was attenuation and deterioration of the rectovaginal septum (RVS) connective tissue. Similar tissue changes also initiated at vaginal delivery were ascribed to the proximal and distal connective tissue attachments of the RVS, respectively; the cardinal-uterosacral ligament complex; and the perineal body—but tears were never entertained in our perceptions. We know, as emphasized by Nichols in the 1960s and 1970s,1-3 that all of these elements, together with the bilateral RVS connection to levator fascia plus the levator plate of the pelvic floor, constitute the normal vaginal axis when all elements are intact.
To be concise, the disrupted vaginal axis, including its 2 major anatomic abnormalities, rectocele and perineal body defects, was thought to be the direct result of disintegration over time after the initiating shock of vaginal delivery. Specific tissue tears were, until now, an unrealized concept. As a result, we routinely resorted to makeshift methods in attempts to restore posterior pelvic configuration. Today, thanks to A. Cullen Richardson, we are much better informed.4,5
Sutured reunion of RVS tears key to rectocele eradication
This article centers on today’s concepts of the defects isolated within the RVS, a critical part of the vaginal axis. No longer is there any question about the key role of sutured reunion of RVS tears in eradicating rectocele. We must acknowledge, however, that such anatomic restoration alone is insufficient without simultaneous fixed suspension of the posterior vaginal vault above and perineal body reconstitution below, as Nichols emphasized.3
The overall goal in corrective posterior pelvic surgery must always be restoration of the normal vaginal axis in order to preserve the pelvic valve mechanism, as described by Porges.6
The primary reason for connective tissue defects is tears, not attenuation. Richardson dramatically and conclusively demonstrated this fact in the early 1990s, after prolonged and intensive investigation of the anatomy.4,5,7 This astonishing disclosure was nowhere more obvious than in the defective RVS, where discrete separations in otherwise intact tissue could be uncovered and reattached by simple standard stitching technique. We had simply never looked for these torn segments (which were never obvious), so we never found them. Once we were convinced of Richardson’s concepts, the torn edges became easily identifiable in practically every case of rectocele. Suddenly, we were able to restore the natural anatomic integrity of the RVS by reuniting these torn edges. With absolute confidence, we eliminated the rectocele.8-16
The critical digital technique
The key to this logical operative technique was the rectal examining finger. Using this digital strategy, not only could we definitively outline the rectocele on outpatient examination, but we could delineate the exact extent of the rectocele intraoperatively. This is accomplished by performing the first rectal exam just before vaginal mucosal dissection, and then, most accurately, after full dissection and exposure of the entire operative field, thus clearly exposing the torn edges in the RVS. A final anorectal exam is performed to check the restored anatomic configuration of the RVS before vaginal mucosal reunification.8
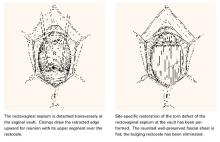
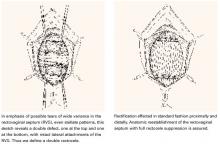
Transverse tears
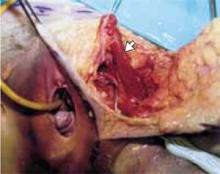
Transverse tears are the most common: either detachment from below, adjacent to the perineal body, or separation at the top in juxtaposition to the fibrous uterosacral extensions in the area of the cul-de-sac. FIGURES 1 AND 2 convey the “before and after” aspects, showing Allis clamps drawing the retracted edge to the site of the tear followed by the revelation of the actual repair accomplished by interrupted O-caliber sutures, either delayed absorbable or nonabsorbable. A rectally inserted index finger initially demonstrates that, except for scraps of areolar tissue, the anorectal wall is the only layer that prevents the examining finger from falling into the operative field as it produces rectocele simulation with forward pressure. When the clamps draw the long-torn RVS segments into normal anatomic positions, the anorectal finger cannot advance at all, despite strong effort, because of the natural firm barrier effect of the anatomically restored RVS.8
FIGURE 1 Lower transverse tear
FIGURE 2 Upper transverse tear
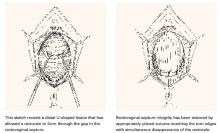
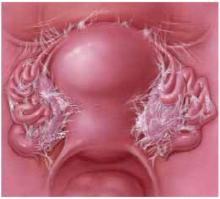
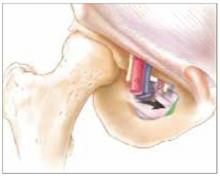
U-shaped tears
Another not uncommon type of defect can be U-shaped, either at the bottom, as depicted in FIGURE 3, or at the top of the posterior pelvic compartment. Similarly linear (longitudinal) tears near one side or the other directly adjacent to the pelvic sidewalls, rarely in the midline, are not unusual and can be repaired in the same manner.
FIGURE 3 U-shaped tear
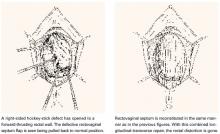
Hockey-stick tears
Occasionally, a hockey-stick lesion, combining a longitudinal tear and a transverse tear in continuity, as shown in FIGURE 4, is discovered.
FIGURE 4 Hockey-stick tear
Double defect
A more uncommon type of combined or double defect, in which the Denonvilliers fascia (RVS) has been torn both adjacent to the vault and in the perineal area but retains strong attachments bilaterally, is shown in FIGURE 5.
FIGURE 5 Double defect
A contented vaginal environment
The major breakthrough is the concept that tears in the Denonvilliers fascia, not attenuation, are the cause of rectoceles. These tears—transverse, longitudinal, U-shaped, multidirectional, even stellate—can be identified almost always without difficulty with the aid of a rectal examining finger and edge-grasping clamps at surgery. The repair itself is not only anatomically logical but also much easier and more confidence-inspiring than the traditional, now archaic, method of fishing around for scraps of levator fascia and muscle to approximate, under tension, in the midline.
This defect-directed repair, born of common sense (ie, comprehension and application of normal anatomy), is structurally nonconstrictive and functionally nonrestrictive—truly a contented vaginal environment.
All sketches from TeLinde’s Operative Gynecology, 9th edition, with permission from Lippincott, Williams, and Wilkins, Publishers.
Dr. Grody reports no financial relationship with any companies whose products are mentioned in this article.
1. Nichols DH. Posterior colporrhaphy and perineorrhaphy: separate and distinct operations. Am J Obstet Gynecol. 1991;164:714-721.
2. Nichols DH, Milley PS, Randall CL. Significance of restoration of normal vaginal depth and axis. Obstet Gynecol. 1970;36:251-256.
3. Nichols DH, Randall CS. Vaginal Surgery. 3rd ed. Baltimore, Md: Williams & Wilkins; 1989;21.-
4. Richardson AC. The rectovaginal septum revisited: its relationship to rectocele and its importance to rectocele repair. Clin Obstet Gynecol. 1993;36:976-983.
5. Richardson AC. The anatomic defects in rectocele and enterocele. J Pelv Surg. 1995;1:214.-
6. Porges RF, Sinden SW. Long-term analysis of the surgical management of pelvic support defects. Am J Obstet Gynecol. 1994;171:1518-1528.
7. Edmonds PB, Richardson AC. Anatomical Approach to Rectocele Repair [videotape]. St. Louis, Mo: Society of Gynecologic Surgeons; 1993.
8. Grody MHT. Posterior compartment defects. In: Rock JA, Jones III HW, eds. TeLinde’s Operative Gynecology. 9th ed. Philadelphia, Pa: Lippincott, Williams and Wilkins; 2003;966-985.
9. Cundiff GW, Weidner AC, Visco AG, Addison WA, Bump RC. An anatomic and functional assessment of the discrete defect rectocele repair. Am J Obstet Gynecol. 1998;179:1456-1457.
10. Kenton K, Shott S, Brubaker L. Outcome after rectovaginal fascia reattachment for rectocele repair. Am J Obstet Gynecol. 1999;181:360-364.
11. Porter WE, Steele A, Walsh P, Kohli N, Karram MM. The anatomic and functional outcomes of defect-specific rectocele repairs. Am J Obstet Gynecol. 1999;181:1353-13549.
12. Glavind K, Madsen H. A prospective study of the discrete fascial defect rectocele repair. Acta Obstet Gynecol Scand. 2000;79:145-147.
13. Cundiff GW. Defect Directed Rectocele Repairs: Restorative and Compensatory Techniques [videotape]. Washington, DC: American College of Obstetricians and Gynecologists; 2001.
14. Maccarone JL, Caraballo R, Holzberg A, Grody MHT. Innovative Defect-specific Posterior Pelvic Surgery: Triggered Ligament Sutures and Collagen Graft [videotape]. Washington, DC: American College of Obstetricians and Gynecologists; 2003.
15. Caraballo R, Maccarone JL, Holzberg AS, Grody MHT. New Concepts in Reconstructive Pelvic Surgery: Slings, Collagen Matrix Grafts, Triggered Sutures [videotape]. Washington, DC: American College of Obstetricians and Gynecologists; 2003.
16. Singh K, Cortes E, Reid WMN. Evaluation of the fascial technique for surgical repair of isolated posterior vaginal wall prolapse. Obstet Gynecol. 2003;101:320-324.
- Tears can almost always be identified without difficulty with the aid of edge-grasping clamps at surgery and a rectal examining finger.
- We can restore the natural anatomic integrity of the rectovaginal septum by reuniting the torn edges, thus eliminating the rectocele.
- The most common tears are transverse. Others include U-shaped, linear, and combined tears.
Why did it take us 2 centuries to learn how to do it right? Posterior pelvic repair for the correction of rectocele combined with restoration of the perineal body has been on gynecologic OR schedules since time immemorial, so one would think we knew what we were doing. Yet only in the past 10 years has there been progressive general recognition of the true nature of the anatomic lesions responsible for rectocele formation, thereby finally pointing us in the right direction.
Although it was always assumed, correctly, that the major factor initiating rectoceles is tissue trauma sustained in vaginal birth, traditionally we were taught, and we believed, that the end result was attenuation and deterioration of the rectovaginal septum (RVS) connective tissue. Similar tissue changes also initiated at vaginal delivery were ascribed to the proximal and distal connective tissue attachments of the RVS, respectively; the cardinal-uterosacral ligament complex; and the perineal body—but tears were never entertained in our perceptions. We know, as emphasized by Nichols in the 1960s and 1970s,1-3 that all of these elements, together with the bilateral RVS connection to levator fascia plus the levator plate of the pelvic floor, constitute the normal vaginal axis when all elements are intact.
To be concise, the disrupted vaginal axis, including its 2 major anatomic abnormalities, rectocele and perineal body defects, was thought to be the direct result of disintegration over time after the initiating shock of vaginal delivery. Specific tissue tears were, until now, an unrealized concept. As a result, we routinely resorted to makeshift methods in attempts to restore posterior pelvic configuration. Today, thanks to A. Cullen Richardson, we are much better informed.4,5
Sutured reunion of RVS tears key to rectocele eradication
This article centers on today’s concepts of the defects isolated within the RVS, a critical part of the vaginal axis. No longer is there any question about the key role of sutured reunion of RVS tears in eradicating rectocele. We must acknowledge, however, that such anatomic restoration alone is insufficient without simultaneous fixed suspension of the posterior vaginal vault above and perineal body reconstitution below, as Nichols emphasized.3
The overall goal in corrective posterior pelvic surgery must always be restoration of the normal vaginal axis in order to preserve the pelvic valve mechanism, as described by Porges.6
The primary reason for connective tissue defects is tears, not attenuation. Richardson dramatically and conclusively demonstrated this fact in the early 1990s, after prolonged and intensive investigation of the anatomy.4,5,7 This astonishing disclosure was nowhere more obvious than in the defective RVS, where discrete separations in otherwise intact tissue could be uncovered and reattached by simple standard stitching technique. We had simply never looked for these torn segments (which were never obvious), so we never found them. Once we were convinced of Richardson’s concepts, the torn edges became easily identifiable in practically every case of rectocele. Suddenly, we were able to restore the natural anatomic integrity of the RVS by reuniting these torn edges. With absolute confidence, we eliminated the rectocele.8-16
The critical digital technique
The key to this logical operative technique was the rectal examining finger. Using this digital strategy, not only could we definitively outline the rectocele on outpatient examination, but we could delineate the exact extent of the rectocele intraoperatively. This is accomplished by performing the first rectal exam just before vaginal mucosal dissection, and then, most accurately, after full dissection and exposure of the entire operative field, thus clearly exposing the torn edges in the RVS. A final anorectal exam is performed to check the restored anatomic configuration of the RVS before vaginal mucosal reunification.8
Transverse tears
Transverse tears are the most common: either detachment from below, adjacent to the perineal body, or separation at the top in juxtaposition to the fibrous uterosacral extensions in the area of the cul-de-sac. FIGURES 1 AND 2 convey the “before and after” aspects, showing Allis clamps drawing the retracted edge to the site of the tear followed by the revelation of the actual repair accomplished by interrupted O-caliber sutures, either delayed absorbable or nonabsorbable. A rectally inserted index finger initially demonstrates that, except for scraps of areolar tissue, the anorectal wall is the only layer that prevents the examining finger from falling into the operative field as it produces rectocele simulation with forward pressure. When the clamps draw the long-torn RVS segments into normal anatomic positions, the anorectal finger cannot advance at all, despite strong effort, because of the natural firm barrier effect of the anatomically restored RVS.8
FIGURE 1 Lower transverse tear
FIGURE 2 Upper transverse tear
U-shaped tears
Another not uncommon type of defect can be U-shaped, either at the bottom, as depicted in FIGURE 3, or at the top of the posterior pelvic compartment. Similarly linear (longitudinal) tears near one side or the other directly adjacent to the pelvic sidewalls, rarely in the midline, are not unusual and can be repaired in the same manner.
FIGURE 3 U-shaped tear
Hockey-stick tears
Occasionally, a hockey-stick lesion, combining a longitudinal tear and a transverse tear in continuity, as shown in FIGURE 4, is discovered.
FIGURE 4 Hockey-stick tear
Double defect
A more uncommon type of combined or double defect, in which the Denonvilliers fascia (RVS) has been torn both adjacent to the vault and in the perineal area but retains strong attachments bilaterally, is shown in FIGURE 5.
FIGURE 5 Double defect
A contented vaginal environment
The major breakthrough is the concept that tears in the Denonvilliers fascia, not attenuation, are the cause of rectoceles. These tears—transverse, longitudinal, U-shaped, multidirectional, even stellate—can be identified almost always without difficulty with the aid of a rectal examining finger and edge-grasping clamps at surgery. The repair itself is not only anatomically logical but also much easier and more confidence-inspiring than the traditional, now archaic, method of fishing around for scraps of levator fascia and muscle to approximate, under tension, in the midline.
This defect-directed repair, born of common sense (ie, comprehension and application of normal anatomy), is structurally nonconstrictive and functionally nonrestrictive—truly a contented vaginal environment.
All sketches from TeLinde’s Operative Gynecology, 9th edition, with permission from Lippincott, Williams, and Wilkins, Publishers.
Dr. Grody reports no financial relationship with any companies whose products are mentioned in this article.
- Tears can almost always be identified without difficulty with the aid of edge-grasping clamps at surgery and a rectal examining finger.
- We can restore the natural anatomic integrity of the rectovaginal septum by reuniting the torn edges, thus eliminating the rectocele.
- The most common tears are transverse. Others include U-shaped, linear, and combined tears.
Why did it take us 2 centuries to learn how to do it right? Posterior pelvic repair for the correction of rectocele combined with restoration of the perineal body has been on gynecologic OR schedules since time immemorial, so one would think we knew what we were doing. Yet only in the past 10 years has there been progressive general recognition of the true nature of the anatomic lesions responsible for rectocele formation, thereby finally pointing us in the right direction.
Although it was always assumed, correctly, that the major factor initiating rectoceles is tissue trauma sustained in vaginal birth, traditionally we were taught, and we believed, that the end result was attenuation and deterioration of the rectovaginal septum (RVS) connective tissue. Similar tissue changes also initiated at vaginal delivery were ascribed to the proximal and distal connective tissue attachments of the RVS, respectively; the cardinal-uterosacral ligament complex; and the perineal body—but tears were never entertained in our perceptions. We know, as emphasized by Nichols in the 1960s and 1970s,1-3 that all of these elements, together with the bilateral RVS connection to levator fascia plus the levator plate of the pelvic floor, constitute the normal vaginal axis when all elements are intact.
To be concise, the disrupted vaginal axis, including its 2 major anatomic abnormalities, rectocele and perineal body defects, was thought to be the direct result of disintegration over time after the initiating shock of vaginal delivery. Specific tissue tears were, until now, an unrealized concept. As a result, we routinely resorted to makeshift methods in attempts to restore posterior pelvic configuration. Today, thanks to A. Cullen Richardson, we are much better informed.4,5
Sutured reunion of RVS tears key to rectocele eradication
This article centers on today’s concepts of the defects isolated within the RVS, a critical part of the vaginal axis. No longer is there any question about the key role of sutured reunion of RVS tears in eradicating rectocele. We must acknowledge, however, that such anatomic restoration alone is insufficient without simultaneous fixed suspension of the posterior vaginal vault above and perineal body reconstitution below, as Nichols emphasized.3
The overall goal in corrective posterior pelvic surgery must always be restoration of the normal vaginal axis in order to preserve the pelvic valve mechanism, as described by Porges.6
The primary reason for connective tissue defects is tears, not attenuation. Richardson dramatically and conclusively demonstrated this fact in the early 1990s, after prolonged and intensive investigation of the anatomy.4,5,7 This astonishing disclosure was nowhere more obvious than in the defective RVS, where discrete separations in otherwise intact tissue could be uncovered and reattached by simple standard stitching technique. We had simply never looked for these torn segments (which were never obvious), so we never found them. Once we were convinced of Richardson’s concepts, the torn edges became easily identifiable in practically every case of rectocele. Suddenly, we were able to restore the natural anatomic integrity of the RVS by reuniting these torn edges. With absolute confidence, we eliminated the rectocele.8-16
The critical digital technique
The key to this logical operative technique was the rectal examining finger. Using this digital strategy, not only could we definitively outline the rectocele on outpatient examination, but we could delineate the exact extent of the rectocele intraoperatively. This is accomplished by performing the first rectal exam just before vaginal mucosal dissection, and then, most accurately, after full dissection and exposure of the entire operative field, thus clearly exposing the torn edges in the RVS. A final anorectal exam is performed to check the restored anatomic configuration of the RVS before vaginal mucosal reunification.8
Transverse tears
Transverse tears are the most common: either detachment from below, adjacent to the perineal body, or separation at the top in juxtaposition to the fibrous uterosacral extensions in the area of the cul-de-sac. FIGURES 1 AND 2 convey the “before and after” aspects, showing Allis clamps drawing the retracted edge to the site of the tear followed by the revelation of the actual repair accomplished by interrupted O-caliber sutures, either delayed absorbable or nonabsorbable. A rectally inserted index finger initially demonstrates that, except for scraps of areolar tissue, the anorectal wall is the only layer that prevents the examining finger from falling into the operative field as it produces rectocele simulation with forward pressure. When the clamps draw the long-torn RVS segments into normal anatomic positions, the anorectal finger cannot advance at all, despite strong effort, because of the natural firm barrier effect of the anatomically restored RVS.8
FIGURE 1 Lower transverse tear
FIGURE 2 Upper transverse tear
U-shaped tears
Another not uncommon type of defect can be U-shaped, either at the bottom, as depicted in FIGURE 3, or at the top of the posterior pelvic compartment. Similarly linear (longitudinal) tears near one side or the other directly adjacent to the pelvic sidewalls, rarely in the midline, are not unusual and can be repaired in the same manner.
FIGURE 3 U-shaped tear
Hockey-stick tears
Occasionally, a hockey-stick lesion, combining a longitudinal tear and a transverse tear in continuity, as shown in FIGURE 4, is discovered.
FIGURE 4 Hockey-stick tear
Double defect
A more uncommon type of combined or double defect, in which the Denonvilliers fascia (RVS) has been torn both adjacent to the vault and in the perineal area but retains strong attachments bilaterally, is shown in FIGURE 5.
FIGURE 5 Double defect
A contented vaginal environment
The major breakthrough is the concept that tears in the Denonvilliers fascia, not attenuation, are the cause of rectoceles. These tears—transverse, longitudinal, U-shaped, multidirectional, even stellate—can be identified almost always without difficulty with the aid of a rectal examining finger and edge-grasping clamps at surgery. The repair itself is not only anatomically logical but also much easier and more confidence-inspiring than the traditional, now archaic, method of fishing around for scraps of levator fascia and muscle to approximate, under tension, in the midline.
This defect-directed repair, born of common sense (ie, comprehension and application of normal anatomy), is structurally nonconstrictive and functionally nonrestrictive—truly a contented vaginal environment.
All sketches from TeLinde’s Operative Gynecology, 9th edition, with permission from Lippincott, Williams, and Wilkins, Publishers.
Dr. Grody reports no financial relationship with any companies whose products are mentioned in this article.
1. Nichols DH. Posterior colporrhaphy and perineorrhaphy: separate and distinct operations. Am J Obstet Gynecol. 1991;164:714-721.
2. Nichols DH, Milley PS, Randall CL. Significance of restoration of normal vaginal depth and axis. Obstet Gynecol. 1970;36:251-256.
3. Nichols DH, Randall CS. Vaginal Surgery. 3rd ed. Baltimore, Md: Williams & Wilkins; 1989;21.-
4. Richardson AC. The rectovaginal septum revisited: its relationship to rectocele and its importance to rectocele repair. Clin Obstet Gynecol. 1993;36:976-983.
5. Richardson AC. The anatomic defects in rectocele and enterocele. J Pelv Surg. 1995;1:214.-
6. Porges RF, Sinden SW. Long-term analysis of the surgical management of pelvic support defects. Am J Obstet Gynecol. 1994;171:1518-1528.
7. Edmonds PB, Richardson AC. Anatomical Approach to Rectocele Repair [videotape]. St. Louis, Mo: Society of Gynecologic Surgeons; 1993.
8. Grody MHT. Posterior compartment defects. In: Rock JA, Jones III HW, eds. TeLinde’s Operative Gynecology. 9th ed. Philadelphia, Pa: Lippincott, Williams and Wilkins; 2003;966-985.
9. Cundiff GW, Weidner AC, Visco AG, Addison WA, Bump RC. An anatomic and functional assessment of the discrete defect rectocele repair. Am J Obstet Gynecol. 1998;179:1456-1457.
10. Kenton K, Shott S, Brubaker L. Outcome after rectovaginal fascia reattachment for rectocele repair. Am J Obstet Gynecol. 1999;181:360-364.
11. Porter WE, Steele A, Walsh P, Kohli N, Karram MM. The anatomic and functional outcomes of defect-specific rectocele repairs. Am J Obstet Gynecol. 1999;181:1353-13549.
12. Glavind K, Madsen H. A prospective study of the discrete fascial defect rectocele repair. Acta Obstet Gynecol Scand. 2000;79:145-147.
13. Cundiff GW. Defect Directed Rectocele Repairs: Restorative and Compensatory Techniques [videotape]. Washington, DC: American College of Obstetricians and Gynecologists; 2001.
14. Maccarone JL, Caraballo R, Holzberg A, Grody MHT. Innovative Defect-specific Posterior Pelvic Surgery: Triggered Ligament Sutures and Collagen Graft [videotape]. Washington, DC: American College of Obstetricians and Gynecologists; 2003.
15. Caraballo R, Maccarone JL, Holzberg AS, Grody MHT. New Concepts in Reconstructive Pelvic Surgery: Slings, Collagen Matrix Grafts, Triggered Sutures [videotape]. Washington, DC: American College of Obstetricians and Gynecologists; 2003.
16. Singh K, Cortes E, Reid WMN. Evaluation of the fascial technique for surgical repair of isolated posterior vaginal wall prolapse. Obstet Gynecol. 2003;101:320-324.
1. Nichols DH. Posterior colporrhaphy and perineorrhaphy: separate and distinct operations. Am J Obstet Gynecol. 1991;164:714-721.
2. Nichols DH, Milley PS, Randall CL. Significance of restoration of normal vaginal depth and axis. Obstet Gynecol. 1970;36:251-256.
3. Nichols DH, Randall CS. Vaginal Surgery. 3rd ed. Baltimore, Md: Williams & Wilkins; 1989;21.-
4. Richardson AC. The rectovaginal septum revisited: its relationship to rectocele and its importance to rectocele repair. Clin Obstet Gynecol. 1993;36:976-983.
5. Richardson AC. The anatomic defects in rectocele and enterocele. J Pelv Surg. 1995;1:214.-
6. Porges RF, Sinden SW. Long-term analysis of the surgical management of pelvic support defects. Am J Obstet Gynecol. 1994;171:1518-1528.
7. Edmonds PB, Richardson AC. Anatomical Approach to Rectocele Repair [videotape]. St. Louis, Mo: Society of Gynecologic Surgeons; 1993.
8. Grody MHT. Posterior compartment defects. In: Rock JA, Jones III HW, eds. TeLinde’s Operative Gynecology. 9th ed. Philadelphia, Pa: Lippincott, Williams and Wilkins; 2003;966-985.
9. Cundiff GW, Weidner AC, Visco AG, Addison WA, Bump RC. An anatomic and functional assessment of the discrete defect rectocele repair. Am J Obstet Gynecol. 1998;179:1456-1457.
10. Kenton K, Shott S, Brubaker L. Outcome after rectovaginal fascia reattachment for rectocele repair. Am J Obstet Gynecol. 1999;181:360-364.
11. Porter WE, Steele A, Walsh P, Kohli N, Karram MM. The anatomic and functional outcomes of defect-specific rectocele repairs. Am J Obstet Gynecol. 1999;181:1353-13549.
12. Glavind K, Madsen H. A prospective study of the discrete fascial defect rectocele repair. Acta Obstet Gynecol Scand. 2000;79:145-147.
13. Cundiff GW. Defect Directed Rectocele Repairs: Restorative and Compensatory Techniques [videotape]. Washington, DC: American College of Obstetricians and Gynecologists; 2001.
14. Maccarone JL, Caraballo R, Holzberg A, Grody MHT. Innovative Defect-specific Posterior Pelvic Surgery: Triggered Ligament Sutures and Collagen Graft [videotape]. Washington, DC: American College of Obstetricians and Gynecologists; 2003.
15. Caraballo R, Maccarone JL, Holzberg AS, Grody MHT. New Concepts in Reconstructive Pelvic Surgery: Slings, Collagen Matrix Grafts, Triggered Sutures [videotape]. Washington, DC: American College of Obstetricians and Gynecologists; 2003.
16. Singh K, Cortes E, Reid WMN. Evaluation of the fascial technique for surgical repair of isolated posterior vaginal wall prolapse. Obstet Gynecol. 2003;101:320-324.
Cutting the risk of hysteroscopic complications
- Preoperative treatment with a gonadotropinreleasing hormone agonist increases the odds of operative complications by a factor of 4 to 7.
- Preoperative cervical ripening reduced the need for cervical dilation, minimized cervical complications, and reduced operative time.
- CO2 should never be used for operative hysteroscopic procedures because of the high risk of CO2 embolism.
- Ultrasound guidance may improve outcomes in selected hysteroscopic procedures.
It is a valuable tool in the evaluation and treatment of infertility, recurrent pregnancy loss, and abnormal and postmenopausal uterine bleeding, and is useful when saline infusion sonography findings are equivocal.
Further, if a global ablation device fails, the surgeon can convert to hysteroscopic ablation rather than abandon the procedure altogether. This is not as unusual as it might appear: In US Food and Drug Administration trials, there was a staggering 10% to 22% malfunction of global ablation technology.1
Safe, easily learned
Although gynecologists are beginning to embrace this modality, many physicians avoid it because of inadequate training or exaggerated fears of complications. In reality, hysteroscopy is one of the safest and most easily acquired surgical skills in gynecology. For example, in a prospective evaluation of 13,600 diagnostic and operative hysteroscopic procedures performed at 63 hospitals in the Netherlands—which involved both established surgeons and residents—Jansen et al2 found an astonishingly low complication rate of 0.28%, with no deaths.
Proper selection and treatment of patients and prompt intervention minimize complications as well as legal risks. Surgical misadventures and lawsuits occur with delayed intervention, failure to recognize pathology or risky conditions, and inadequate preventive maneuvers.
Overall, emphasis on safety is vital to success, and thorough awareness of potential complications is just as important.
Three types of complications
Complications fall into 3 categories (TABLE):
- Procedure-related
- Media-related
- Postoperative
PROCEDURE-RELATED COMPLICATIONS
Complication rates
In a retrospective investigation, Propst et al3 determined the rate of complications associated with specific hysteroscopic procedures. Demographic data and medical histories were collected for 925 women who had operative hysteroscopy in 1995 and 1996. The overall complication rate was 2.7%. Myomectomy and resection of uterine septa carried the greatest odds of complications; polypectomy and endometrial ablation had the lowest. Preoperative treatment with a gonadotropinreleasing hormone (GnRH) agonist increased the odds of complications by a factor of 4 to 7. Women under age 50 were more likely to experience complications than those over 50.
In the study by Jansen et al,2 38 complica-tions occurred in the 13,600 procedures. The greatest risk of complications occurred with adhesiolysis (4.48%), followed by endometrial resection (0.81%), myomectomy (0.75%), and polypectomy (0.38%).
Cervical entry requires special attention
Almost half of the complications in the Jansen study were related to cervical entry, so caution and, perhaps, preoperative cervical ripening are advised. Many premenopausal subjects were given GnRH analogues, which may render the cervix more resistant to dilation. Complications associated with a stenotic cervix include a cervical tear, creation of a false cervical passage, and uterine perforation.
Cervical ripening may help prevent uterine perforation. The most common complication, occurring in 14.2 cases per thousand, is uterine perforation.2 The risk of this is highest in postpartum procedures, followed by procedures in postmenopausal, then perimenopausal, women. Patients with endometrial cancer also have a higher rate of perforation.
Risk factors for uterine perforation include:
- nulliparity
- menopause
- use of GnRH agonists
- prior cone biopsy
- markedly retroverted uterus
- undue force
Vaginal or oral misoprostol for cervical ripening prior to operative hysteroscopy was evaluated in a randomized trial.4 Researchers found a reduced need for cervical dilation, a minimum of cervical complications, and reduced operative time in study patients compared with controls.
When 400 μg oral misoprostol is given 12 and 24 hours before surgery, it also softens the cervix and eases dilation.5 Although misoprostol has several bothersome side effects (such as lower abdominal pain and slight vaginal bleeding), few if any prevent its use.
Signs of perforation. Patients who sustain uterine perforation with subsequent intraperitoneal bleeding often complain of pain in the abdomen and shoulder, and experience hemodynamic instability. A quick sonographic survey of the abdomen will demonstrate free intraperitoneal fluid. (It is rare for much intraperitoneal fluid to accumulate by transtubal regurgitation during operative hysteroscopy, despite the quantity of fluid used.)
If perforation is suspected, laparoscopy or laparotomy is necessary to clarify the cause of pain, unstable vital signs, or free fluid visualized by ultrasound.6
Exercise extra care and precautions in women who have had a prior cesarean section, myomectomy, or uterine perforation. Complete visualization of uterine landmarks is necessary during operative hysteroscopy to exclude uterine dehiscence, sacculation, and perforation. Prior uterine surgery may cause myometrial weakness and lead to possible perforation. Do not proceed if abnormal uterine morphology is detected. If uterine perforation occurs, injury to bladder and bowel is possible when electrical energy is applied to a uterine wall compromised by prior surgery. Strict visualization of uterine anatomy is critical in this population so that bowel or bladder burns can be avoided.
MEDIA-RELATED COMPLICATIONS
Notorious complications and several recent lawsuits have stemmed from fluid overload. A common element has been the physician’s lack of awareness of how rapidly complications can arise, and what signs and symptoms are specific to the fluid used.
Monitor fluids vigilantly
Operative hysteroscopy must be performed in a fluid medium. The type of fluid depends on the surgeon’s preference and the instrument utilized, but any fluid can be associated with complications. Fluid choices with monopolar instruments include glycine 1.5%, a mixture of sorbitol 3% and mannitol 0.54%, and mannitol 5%. These are frequently used with the continuous-flow resectoscope. Bipolar operative hysteroscopy can be performed using saline.
The solution to media-related complications is basic: vigilant monitoring of fluids. A cavalier attitude, poor fluid documentation, and failure to respond to complications can lead to trouble. If fluid overload occurs, comanagement and consultation with an intensive care specialist is advised.
Distention media
Among the options for distention media in operative and diagnostic hysteroscopy are high-viscosity dextran 70 and low-viscosity fluids such as hypotonic, electrolyte-free and isotonic, electrolyte-containing solutions. The popularity of dextran 70 is waning, however. While it is immiscible with blood, significant complications have been reported.
Signs of anaphylactic reactions to dextran 70 include acute hypotension, hypoxia, pulmonary edema, fluid overload, fulminant coagulopathies, and anemia. The surgeon must operate quickly, minimize endometrial trauma, use continuous pulse oximetry, and obtain a preoperative coagulation panel.
Dextran 70 also can ruin operative hysteroscopes if they are not cleaned promptly and thoroughly after use.
Hypotonic, electrolyte-free solutions. With hypotonic, electrolyte-free solutions such as glycine 1.5%, early recognition of possible complications, including hyponatremic hypervolemia, is vital. For example, when glycine and sorbitol are metabolized, free water accumulates and the body attempts to achieve homeostasis through compensatory mechanisms such as osmosis, which moves free water into extracellular and intracellular spaces. This can lead to increased free water in the brain, resulting in cerebral edema, rising intracranial pressure, and cellular necrosis.
The cerebral cation pump normally pumps osmotically active cations into the extracellular space, thereby minimizing cerebral edema. However, this pump is inhibited by estrogen, so the compensatory mechanism is diminished.
Classic clinical features of hyponatremic hypervolemia include apprehension, confusion, fatigue, headache, mental agitation, nausea, visual disturbances (including blindness), vomiting, and weakness. These complications are more readily apparent when regional anesthesia is used rather than general anesthesia.
If hyponatremic hypervolemia goes unrecognized, bradycardia and hypertension can ensue, followed rapidly by cerebral and pulmonary edema and cardiovascular collapse. In addition, glycine 1.5% is metabolized to glycolic acid and ammonia. Free ammonia is associated with central nervous system disorders. Recognition and prompt treatment by an intensivist may prevent permanent neurologic sequelae, death, and lawsuits.7
Isotonic, electrolyte-containing solutions. Mannitol 5% is electrolyte poor but isotonic, creating less risk for hypo-osmolality. However, dilutional hyponatremia (ie, low sodium levels) can still occur.
Advantages of bipolar instruments. To minimize complications from hypotonic, electrolyte-free solutions, manufacturers developed operative hysteroscopes that can function in a bipolar environment. Bipolar instruments can operate in isotonic, physiologic, electrolyte-containing media. Hyponatremia and hypo-osmolality cannot occur with normal saline or Ringer’s lactate, but fluid overload can. (Fluid overload with saline can cause pulmonary edema and congestive heart failure.)
How much fluid will be absorbed? The answer depends on factors including surface area of the surgical field, duration of surgery, opened venous channels, type of irrigation fluid used, and pressure of the delivery system. Modern gynecologic suites employ fluid irrigation systems that continuously measure input and output, with alarms that signal a predetermined fluid deficit. The alarm indicates the need to halt the procedure and quickly evaluate the patient. Careful attention to the recommendations of Loffer et al8 would lead to fewer complications from fluid mismanagement.
Appropriate use of CO2
High risk of embolism with CO2 in operative procedures. Although diagnostic hysteroscopic procedures often are performed with carbon dioxide (CO2), operative procedures never should be. The reason: the high risk of CO2 embolism that occurs with open venous channels and vascular endometrium. The choice between CO2 and fluid medium for diagnostic hysteroscopy often is determined by physician preference and the presence of uterine bleeding. Many gynecologists prefer CO2 for its optical clarity and patient comfort during insufflation.9
Purge tubing of room air before each procedure. Embolic complications with CO2 have been recorded with use of the neodymium: yttrium aluminum garnet (Nd:YAG) laser and during operative procedures. Less well known are the adverse sequelae that can occur with room air prior to beginning the procedure. It is critical to purge the entire tubal system with CO2 prior to instrumentation, since up to 40 cm3 of room air may be insufflated into a patient when 200 cm of connective tubing with a 0.5-cm lumen is used.10 Wait for several minutes before starting the procedure so that the whole system is purged.
One of the greatest concerns about endometrial ablation is that diagnosis of endometrial cancer will be delayed because the endometrial cavity has been obliterated. Vilos19 recently reviewed the salient characteristics and findings in women treated by endometrial ablation who subsequently developed endometrial cancer. A review of the individual cases revealed that most of these patients had numerous risk factors for endometrial cancer.
Review risk factors, chronic conditions
Many patients with abnormal bleeding also have risk factors for endometrial cancer, as well as medical conditions that increase the likelihood of morbidity with surgery, such as obesity, hypertension, diabetes, and advanced age. In these cases, hysterectomy may be a better option than endometrial ablation. It would be far better to have such high-risk patients cleared for hysterectomy than to chance their becoming an endometrial-ablation “statistic.” If endometrial ablation is performed in these cases, we prevent the egress of blood, foster development of synechiae, render endometrial biopsy difficult or impossible and, potentially, “bury” endometrial cells deeper within the myometrium—all of which contribute to a delayed and “upstaged” diagnosis of endometrial cancer.
Patients at risk of endometrial cancer should undergo a scrupulous and unambiguous work-up and evaluation. Indeterminate endometrial echo and office evaluation that generates biopsy samples designated as “insufficient for diagnosis,” “no endometrial tissue seen,” or “atrophy” should raise suspicion. These patients require full visualization of the endometrium.
Heightened risk during perimenopause
Newer ablation techniques that utilize global therapy make it paramount that perimenopausal women undergo scrupulous evaluation. Until much more information is available, endometrial ablation should be avoided in patients with endometrial hyperplasia, particularly with atypia. While some gynecologists may be persuaded to consider endometrial ablation as a minimally invasive procedure compared to hysterectomy, the risk of delayed diagnosis of endometrial cancer is of paramount concern. The treatment of choice for these patients remains medical therapy with oral progesterone and, possibly, longterm use of a levonorgestrel-releasing intrauterine system. If this fails, hysterectomy is advisable. As Cooper20 aptly states, “Conservative, nonextirpative procedures offer no life raft” compared with hysterectomy, which covers many missed diagnoses.
No risk of spreading cancer cells
Some gynecologists have worried about the risk of disseminating endometrial cancer cells during hysteroscopy. However, Kudela and Pilka21 studied the true risk in women undergoing blind dilation and curettage and hysteroscopy performed with a fluid medium. Cul-de-sac aspiration prior to instrumentation and at the conclusion of the procedure demonstrated no increased risk of positive cytology. They are continuing a Phase II trial comparing outcomes of both groups over 5 years.
6 most common symptoms of venous or air emboli. Anesthesiologists and gynecologists must be vigilant to prevent venous or air emboli. Munro et al12 succinctly outline the 6 most common symptoms:
- pulmonary hypertension
- hypercarbia
- hypoxia
- arrhythmias
- tachypnea
- systemic hypotension
Beware of a drop in end-tidal CO2. The most common sign of impending cardiovascular collapse is a sudden decrease in end-tidal CO2, when the right cardiac outflow tract is obstructed by CO2, which leads to arterial oxygen (O2) desaturation. If such a decrease is suspected, stop the procedure immediately and administer 100% O2. (Also stop nitrous oxide, if used.) Turn the patient to the left lateral decubitis position and use a central venous catheter to aspirate gas, if necessary. Cardiac massage and a precordial thump may dislodge CO2; unfortunately, high false-positive rates of pre-cordial Doppler make its use impractical.
How to minimize risks
- Avoid coaxial gas cooling tips associated with Nd:YAG crystal lasers
- Avoid a steep Trendelenburg position
- Keep cervix covered with sponge or dilator when operative hysteroscope is removed to minimize air embolism
- Deaerate the equipment prior to surgery Use a low-pressure hysteroscopic CO2 insufflator
- Carefully monitor the patient
- Be highly suspicious when vital signs are unstable
POSTOPERATIVE COMPLICATIONS
Some complications of hysteroscopy may not become clinically evident for months or even years. The most common complications of hysteroscopic endometrial ablation include pregnancy, postablation tubal sterilization syndrome, new or worsening dysmenorrhea, hematometra, endometrial cancer, and failure to completely treat symptoms.
Patients scheduled for hysteroscopy must be informed of potential delayed risks of the procedure. In addition, all reproductive-aged women should be advised that pregnancy is possible after endometrial ablation or operative removal of an intracavitary mass; thus, contraception is crucial. The endometrial tissue is resilient and may regenerate after ablation.
Hematometra: Avoid cervical canal
Hematometra is an infrequent late complication of operative hysteroscopy. If menstruating women or those taking hormone replacement therapy experience cyclic or chronic lower pelvic pain after surgery, scarring or narrowing of the endometrial cavity may be the cause. Approximately 1% to 2% of women who undergo operative hysteroscopy experience this phenomenon. Most cases can be treated with cervical dilation alone.
Since the cervical canal contains no endometrial glands, there is no need to treat this area in women undergoing endometrial ablation. In fact, avoiding this area during treatment is a critical component of successful surgery.
Tubal sterilization syndrome possible after endometrial ablation
Consider this syndrome when a patient undergoing endometrial ablation complains of crampy, cyclic, unilateral or bilateral pelvic pain, possibly accompanied by vaginal spotting. Sometimes a unilateral mass can be palpated, but more commonly tenderness is elicited on pelvic examination.
Ultrasound may demonstrate fluid near the cornual region. Laparoscopy confirms the diagnosis by visualizing a swollen, edematous proximal fallopian tube. Salpingectomy may confirm hematosalpinx, chronic or acute inflammation, or hemosiderin deposits.
Treatment includes bilateral cornual resection and reablation of proximal endometrium, or hysterectomy.14
Pregnancy complications
Endometrial ablation is not to be regarded as a method of contraception. Patients needing birth control should consider concurrent tubal ligation or other reliable methods after this procedure.
The frequency of pregnancy after endometrial ablation ranges from 0.2% to 1.6%, though this data may represent underreporting. Pregnancy outcomes have been dismal in women conceiving after endometrial ablation. Complications include preterm labor, premature delivery, intrauterine growth retardation, prenatal death, postpartum hemorrhage, and placentation problems such as placenta accreta, increta, or percreta, as well as placental abruption.15
Uterine dehiscence and sacculation and extremely thin myometrium have been reported after uterine adhesiolysis, uterine perforation during operative hysteroscopy, and with myoma resections. A high index of suspicion is vital when a gravida presents with pelvic pain, decreased fetal movement, vaginal bleeding, or abnormal uterine masses detected ultrasonographically.
Signs of uterine rupture. Pregnancyrelated complications of operative hysteroscopy can be dramatic and fatal if not recognized quickly, as in the case of uterine rupture. Kerimis et al16 describe uterine rupture in a term pregnancy after hysteroscopic resection of a uterine septum. Severe fetal distress, maternal shoulder pain, and abdominal pain led to an emergency cesarean section. Intraoperative findings included a 7-cm tear from left cornua to right cornua. The original metroplasty, performed with cutting diathermy and laparoscopy, was not accompanied by complications or perforation.
Patients who experience intraoperative complications during metroplasty or deep resection of intramural fibroids should be informed of the risk of uterine rupture so they may consider elective cesarean. Regardless of the mode of delivery, prompt attention is vital if fetal distress is suspected.
Postablation warning signs
Patients undergoing endometrial ablation generally have a quick postoperative return to activity, minimal need for postoperative pain medication, and limited complaints. Beware of patients who make frequent postoperative phone calls and have escalating requirements for pain medication. While bowel and bladder injuries are infrequent—as is postoperative endometritis—these must be vigilantly considered and evaluated when patients complain of persistent pain, fever, and general malaise. Office evaluation is necessary, including thorough abdominal and pelvic examinations. Laboratory testing should include electrolytes, complete blood count, sedimentation rate, ultrasound, and a flat plate of the abdomen (kidneys, ureter, and bladder; upright) may be required. Sometimes a computed tomography scan of the pelvis/abdomen may be needed if perforation with bowel or bladder injury is suspected.
Hysteroscopic fibroid removal may be necessary after UAE
Uterine artery embolization (UAE) is gaining popularity for the treatment of symptomatic uterine fibroids. Transcatheter embolization of the uterine artery leads to occlusion of the fibroid, ischemic shrinkage of the fibroid, and shrinkage of residual myometrial tissue. Fibroids may migrate weeks to months after the procedure as the myometrium contracts and the treated fibroid degenerates, leading to delayed discharge, passage of necrotic fibroids, cramps, and heavy bleeding if the fibroid migrates to a submucosal location. Hysteroscopic removal is an obvious option.
Recently, De Iaco et al17 reported the development of a uterine fistula and discontinuity of the myometrium after hysteroscopic resection of an embolized migrated fibroid. They speculated this was due to the development of an avascular myometrium after UAE. The patient was asymptomatic, but routine diagnostic hysteroscopy revealed a 2-cm discontinuity of the uterine wall at the site of the previous resectoscopic myomectomy. The myometrium was white and less than the full thickness.
Ultrasound guidance improves outcomes
Coccia et al18 described the benefits of intraoperative ultrasound guidance during operative hysteroscopy in fibroid treatment and uterine septum removal. Prospective evaluation of 81 patients involved an experienced ultrasonographer who mapped the limits of treatment. Patients were compared to 45 historical controls who had been similarly treated with laparoscopic monitoring. Satisfactory outcomes included relief of menorrhagia, complete resection of fibroids (including full resection of intramural fibroids), and thorough metroplasty of uterine septum.
Ultrasound guidance made it possible to extend the resection beyond the limit conventionally defined by hysteroscopy; none of the patients in the ultrasound group required reintervention. Among controls, a second operation was necessary in 4 cases. Investigators concluded that a wider resection (10 to 15 mm distance from the external surface of the uterus) of fibroids was achieved using ultrasound guidance.
Dr. Bradley reports that she serves as a consultant to Karl Storz, ACMI, Olympus, and Gynecare, and as a lecturer for Novacept.
1. Gurtcheff SE, Sharp HT. Complications associated with global endometrial ablation: the utility of the MAUDE database. Obstet Gynecol. 2003;102:1278-1282.
2. Jansen FW, Vredevoogd CB, Ulzen K, et al. Complications of hysteroscopy: a prospective, multicenter study. Obstet Gynecol. 2000;96:266-270.
3. Propst AM, Liberman RF, Harlow BL, Ginsburg ES. Complications of hysteroscopic surgery: predicting patients at risk. Obstet Gynecol. 2000;96:517-520.
4. Preutthipan S, Herabutya Y. Vaginal misoprostol for cervical priming before operative hysteroscopy: a randomized controlled trial. Obstet Gynecol. 2000;96:890-894.
5. Thomas JA, Leyland N, Durand N, Windrim RD. The use of oral misoprostol as a cervical ripening agent in operative hysteroscopy: a double-blind, placebo-controlled trial. Am J Obstet Gynecol. 2002;186:876-879.
6. Stotz M, Lampart A, Kochli OR, Schneider M. Intraabdominal bleeding masked by hemodilution after hysteroscopy. Anesthesiology. 2000;93:569-570.
7. Cooper JM, Brady RM. Intraoperative and early postoperative complications of operative hysteroscopy. Obstet Gynecol Clin North Am. 2000;27:347-366.
8. Loffer FD, Bradley LD, Brill AL, Brooks PG, Cooper JM. Hysteroscopic fluid monitoring guidelines. J Am Assoc Gynecol Laparosc. 2000;7:167-168.
9. Bradley LD, Widrich T. Flexible hysteroscopy: a state-of-the-art procedure for gynecologic evaluation. J Am Assoc Gynecol Laparosc. 1995;2:263-267.
10. Neis KJ, Brandner P, Lindemann HJ. Room air as a cause of gas embolism in diagnostic CO2hysteroscopy. Zentralbl Gynakol. 2000;122:222-225.
11. Bradner P, Neis KJ, Ehmer C. The etiology, frequency, and prevention of gas embolism during CO2hysteroscopy. J Am Assoc Gynecol Laparosc. 1999;6:421-428.
12. Munro MG, Weisberg M, Rubinstein E. Gas and air embolization during hysteroscopic electrosurgical vaporization: comparison of gas generation using bipolar and monopolar electrodes in an experimental model. J Am Assoc Gynecol Laparosc. 2001;8:488-494.
13. Murdoch JAC, Gan TJ. Anesthesia for hysteroscopy. Anesthesiol Clin North Am. 2001;1:125-140.
14. Cooper JM, Brady RM. Late complications of operative hysteroscopy. Obstet Gynecol Clin North Am. 2000;27:367-374.
15. Rogerson L, Gannon B, O’Donovan P. Outcome of pregnancy following endometrial ablation. J Gynecol Surg. 1997;13:155-160.
16. Kerimis P, Zolti M, Sinwany G, Mashiach S, Carp H. Uterine rupture after hysteroscopic resection of uterine septum. Fertil Steril. 2002;77:618-620.
17. De Iaco P, Golfieri R, Ghi T, Muzzupapa G, Ceccarini M, Bovicelli L. Uterine fistula induced by hysteroscopic resection of an embolized migrated fibroid: a rare complication after embolization of uterine fibroids. Fertil Steril. 2001;75:818-820.
18. Coccia ME, Becattini C, Bracco GL, et al. Intraoperative ultrasound guidance for operative hysteroscopy. J Reprod Med. 2000;45:413-418.
19. Brooks-Carter GN, Killackey MA, Neuwirth RS. Adenocarcinoma of the endometrium after endometrial ablation. Obstet Gynecol. 2000;96:836-837.
20. Cooper JM. Swimming lessons: check the water before jumping in. J Am Assoc Gynecol Laparosc. 1998;5:87-90.
21. Kudela M, Pilka R. Is there a real risk in patients with endometrial carcinoma undergoing diagnostic hysteroscopy (HSC)? Eur J Gynecol Oncol. 2001;22:342-344.
- Preoperative treatment with a gonadotropinreleasing hormone agonist increases the odds of operative complications by a factor of 4 to 7.
- Preoperative cervical ripening reduced the need for cervical dilation, minimized cervical complications, and reduced operative time.
- CO2 should never be used for operative hysteroscopic procedures because of the high risk of CO2 embolism.
- Ultrasound guidance may improve outcomes in selected hysteroscopic procedures.
It is a valuable tool in the evaluation and treatment of infertility, recurrent pregnancy loss, and abnormal and postmenopausal uterine bleeding, and is useful when saline infusion sonography findings are equivocal.
Further, if a global ablation device fails, the surgeon can convert to hysteroscopic ablation rather than abandon the procedure altogether. This is not as unusual as it might appear: In US Food and Drug Administration trials, there was a staggering 10% to 22% malfunction of global ablation technology.1
Safe, easily learned
Although gynecologists are beginning to embrace this modality, many physicians avoid it because of inadequate training or exaggerated fears of complications. In reality, hysteroscopy is one of the safest and most easily acquired surgical skills in gynecology. For example, in a prospective evaluation of 13,600 diagnostic and operative hysteroscopic procedures performed at 63 hospitals in the Netherlands—which involved both established surgeons and residents—Jansen et al2 found an astonishingly low complication rate of 0.28%, with no deaths.
Proper selection and treatment of patients and prompt intervention minimize complications as well as legal risks. Surgical misadventures and lawsuits occur with delayed intervention, failure to recognize pathology or risky conditions, and inadequate preventive maneuvers.
Overall, emphasis on safety is vital to success, and thorough awareness of potential complications is just as important.
Three types of complications
Complications fall into 3 categories (TABLE):
- Procedure-related
- Media-related
- Postoperative
PROCEDURE-RELATED COMPLICATIONS
Complication rates
In a retrospective investigation, Propst et al3 determined the rate of complications associated with specific hysteroscopic procedures. Demographic data and medical histories were collected for 925 women who had operative hysteroscopy in 1995 and 1996. The overall complication rate was 2.7%. Myomectomy and resection of uterine septa carried the greatest odds of complications; polypectomy and endometrial ablation had the lowest. Preoperative treatment with a gonadotropinreleasing hormone (GnRH) agonist increased the odds of complications by a factor of 4 to 7. Women under age 50 were more likely to experience complications than those over 50.
In the study by Jansen et al,2 38 complica-tions occurred in the 13,600 procedures. The greatest risk of complications occurred with adhesiolysis (4.48%), followed by endometrial resection (0.81%), myomectomy (0.75%), and polypectomy (0.38%).
Cervical entry requires special attention
Almost half of the complications in the Jansen study were related to cervical entry, so caution and, perhaps, preoperative cervical ripening are advised. Many premenopausal subjects were given GnRH analogues, which may render the cervix more resistant to dilation. Complications associated with a stenotic cervix include a cervical tear, creation of a false cervical passage, and uterine perforation.
Cervical ripening may help prevent uterine perforation. The most common complication, occurring in 14.2 cases per thousand, is uterine perforation.2 The risk of this is highest in postpartum procedures, followed by procedures in postmenopausal, then perimenopausal, women. Patients with endometrial cancer also have a higher rate of perforation.
Risk factors for uterine perforation include:
- nulliparity
- menopause
- use of GnRH agonists
- prior cone biopsy
- markedly retroverted uterus
- undue force
Vaginal or oral misoprostol for cervical ripening prior to operative hysteroscopy was evaluated in a randomized trial.4 Researchers found a reduced need for cervical dilation, a minimum of cervical complications, and reduced operative time in study patients compared with controls.
When 400 μg oral misoprostol is given 12 and 24 hours before surgery, it also softens the cervix and eases dilation.5 Although misoprostol has several bothersome side effects (such as lower abdominal pain and slight vaginal bleeding), few if any prevent its use.
Signs of perforation. Patients who sustain uterine perforation with subsequent intraperitoneal bleeding often complain of pain in the abdomen and shoulder, and experience hemodynamic instability. A quick sonographic survey of the abdomen will demonstrate free intraperitoneal fluid. (It is rare for much intraperitoneal fluid to accumulate by transtubal regurgitation during operative hysteroscopy, despite the quantity of fluid used.)
If perforation is suspected, laparoscopy or laparotomy is necessary to clarify the cause of pain, unstable vital signs, or free fluid visualized by ultrasound.6
Exercise extra care and precautions in women who have had a prior cesarean section, myomectomy, or uterine perforation. Complete visualization of uterine landmarks is necessary during operative hysteroscopy to exclude uterine dehiscence, sacculation, and perforation. Prior uterine surgery may cause myometrial weakness and lead to possible perforation. Do not proceed if abnormal uterine morphology is detected. If uterine perforation occurs, injury to bladder and bowel is possible when electrical energy is applied to a uterine wall compromised by prior surgery. Strict visualization of uterine anatomy is critical in this population so that bowel or bladder burns can be avoided.
MEDIA-RELATED COMPLICATIONS
Notorious complications and several recent lawsuits have stemmed from fluid overload. A common element has been the physician’s lack of awareness of how rapidly complications can arise, and what signs and symptoms are specific to the fluid used.
Monitor fluids vigilantly
Operative hysteroscopy must be performed in a fluid medium. The type of fluid depends on the surgeon’s preference and the instrument utilized, but any fluid can be associated with complications. Fluid choices with monopolar instruments include glycine 1.5%, a mixture of sorbitol 3% and mannitol 0.54%, and mannitol 5%. These are frequently used with the continuous-flow resectoscope. Bipolar operative hysteroscopy can be performed using saline.
The solution to media-related complications is basic: vigilant monitoring of fluids. A cavalier attitude, poor fluid documentation, and failure to respond to complications can lead to trouble. If fluid overload occurs, comanagement and consultation with an intensive care specialist is advised.
Distention media
Among the options for distention media in operative and diagnostic hysteroscopy are high-viscosity dextran 70 and low-viscosity fluids such as hypotonic, electrolyte-free and isotonic, electrolyte-containing solutions. The popularity of dextran 70 is waning, however. While it is immiscible with blood, significant complications have been reported.
Signs of anaphylactic reactions to dextran 70 include acute hypotension, hypoxia, pulmonary edema, fluid overload, fulminant coagulopathies, and anemia. The surgeon must operate quickly, minimize endometrial trauma, use continuous pulse oximetry, and obtain a preoperative coagulation panel.
Dextran 70 also can ruin operative hysteroscopes if they are not cleaned promptly and thoroughly after use.
Hypotonic, electrolyte-free solutions. With hypotonic, electrolyte-free solutions such as glycine 1.5%, early recognition of possible complications, including hyponatremic hypervolemia, is vital. For example, when glycine and sorbitol are metabolized, free water accumulates and the body attempts to achieve homeostasis through compensatory mechanisms such as osmosis, which moves free water into extracellular and intracellular spaces. This can lead to increased free water in the brain, resulting in cerebral edema, rising intracranial pressure, and cellular necrosis.
The cerebral cation pump normally pumps osmotically active cations into the extracellular space, thereby minimizing cerebral edema. However, this pump is inhibited by estrogen, so the compensatory mechanism is diminished.
Classic clinical features of hyponatremic hypervolemia include apprehension, confusion, fatigue, headache, mental agitation, nausea, visual disturbances (including blindness), vomiting, and weakness. These complications are more readily apparent when regional anesthesia is used rather than general anesthesia.
If hyponatremic hypervolemia goes unrecognized, bradycardia and hypertension can ensue, followed rapidly by cerebral and pulmonary edema and cardiovascular collapse. In addition, glycine 1.5% is metabolized to glycolic acid and ammonia. Free ammonia is associated with central nervous system disorders. Recognition and prompt treatment by an intensivist may prevent permanent neurologic sequelae, death, and lawsuits.7
Isotonic, electrolyte-containing solutions. Mannitol 5% is electrolyte poor but isotonic, creating less risk for hypo-osmolality. However, dilutional hyponatremia (ie, low sodium levels) can still occur.
Advantages of bipolar instruments. To minimize complications from hypotonic, electrolyte-free solutions, manufacturers developed operative hysteroscopes that can function in a bipolar environment. Bipolar instruments can operate in isotonic, physiologic, electrolyte-containing media. Hyponatremia and hypo-osmolality cannot occur with normal saline or Ringer’s lactate, but fluid overload can. (Fluid overload with saline can cause pulmonary edema and congestive heart failure.)
How much fluid will be absorbed? The answer depends on factors including surface area of the surgical field, duration of surgery, opened venous channels, type of irrigation fluid used, and pressure of the delivery system. Modern gynecologic suites employ fluid irrigation systems that continuously measure input and output, with alarms that signal a predetermined fluid deficit. The alarm indicates the need to halt the procedure and quickly evaluate the patient. Careful attention to the recommendations of Loffer et al8 would lead to fewer complications from fluid mismanagement.
Appropriate use of CO2
High risk of embolism with CO2 in operative procedures. Although diagnostic hysteroscopic procedures often are performed with carbon dioxide (CO2), operative procedures never should be. The reason: the high risk of CO2 embolism that occurs with open venous channels and vascular endometrium. The choice between CO2 and fluid medium for diagnostic hysteroscopy often is determined by physician preference and the presence of uterine bleeding. Many gynecologists prefer CO2 for its optical clarity and patient comfort during insufflation.9
Purge tubing of room air before each procedure. Embolic complications with CO2 have been recorded with use of the neodymium: yttrium aluminum garnet (Nd:YAG) laser and during operative procedures. Less well known are the adverse sequelae that can occur with room air prior to beginning the procedure. It is critical to purge the entire tubal system with CO2 prior to instrumentation, since up to 40 cm3 of room air may be insufflated into a patient when 200 cm of connective tubing with a 0.5-cm lumen is used.10 Wait for several minutes before starting the procedure so that the whole system is purged.
One of the greatest concerns about endometrial ablation is that diagnosis of endometrial cancer will be delayed because the endometrial cavity has been obliterated. Vilos19 recently reviewed the salient characteristics and findings in women treated by endometrial ablation who subsequently developed endometrial cancer. A review of the individual cases revealed that most of these patients had numerous risk factors for endometrial cancer.
Review risk factors, chronic conditions
Many patients with abnormal bleeding also have risk factors for endometrial cancer, as well as medical conditions that increase the likelihood of morbidity with surgery, such as obesity, hypertension, diabetes, and advanced age. In these cases, hysterectomy may be a better option than endometrial ablation. It would be far better to have such high-risk patients cleared for hysterectomy than to chance their becoming an endometrial-ablation “statistic.” If endometrial ablation is performed in these cases, we prevent the egress of blood, foster development of synechiae, render endometrial biopsy difficult or impossible and, potentially, “bury” endometrial cells deeper within the myometrium—all of which contribute to a delayed and “upstaged” diagnosis of endometrial cancer.
Patients at risk of endometrial cancer should undergo a scrupulous and unambiguous work-up and evaluation. Indeterminate endometrial echo and office evaluation that generates biopsy samples designated as “insufficient for diagnosis,” “no endometrial tissue seen,” or “atrophy” should raise suspicion. These patients require full visualization of the endometrium.
Heightened risk during perimenopause
Newer ablation techniques that utilize global therapy make it paramount that perimenopausal women undergo scrupulous evaluation. Until much more information is available, endometrial ablation should be avoided in patients with endometrial hyperplasia, particularly with atypia. While some gynecologists may be persuaded to consider endometrial ablation as a minimally invasive procedure compared to hysterectomy, the risk of delayed diagnosis of endometrial cancer is of paramount concern. The treatment of choice for these patients remains medical therapy with oral progesterone and, possibly, longterm use of a levonorgestrel-releasing intrauterine system. If this fails, hysterectomy is advisable. As Cooper20 aptly states, “Conservative, nonextirpative procedures offer no life raft” compared with hysterectomy, which covers many missed diagnoses.
No risk of spreading cancer cells
Some gynecologists have worried about the risk of disseminating endometrial cancer cells during hysteroscopy. However, Kudela and Pilka21 studied the true risk in women undergoing blind dilation and curettage and hysteroscopy performed with a fluid medium. Cul-de-sac aspiration prior to instrumentation and at the conclusion of the procedure demonstrated no increased risk of positive cytology. They are continuing a Phase II trial comparing outcomes of both groups over 5 years.
6 most common symptoms of venous or air emboli. Anesthesiologists and gynecologists must be vigilant to prevent venous or air emboli. Munro et al12 succinctly outline the 6 most common symptoms:
- pulmonary hypertension
- hypercarbia
- hypoxia
- arrhythmias
- tachypnea
- systemic hypotension
Beware of a drop in end-tidal CO2. The most common sign of impending cardiovascular collapse is a sudden decrease in end-tidal CO2, when the right cardiac outflow tract is obstructed by CO2, which leads to arterial oxygen (O2) desaturation. If such a decrease is suspected, stop the procedure immediately and administer 100% O2. (Also stop nitrous oxide, if used.) Turn the patient to the left lateral decubitis position and use a central venous catheter to aspirate gas, if necessary. Cardiac massage and a precordial thump may dislodge CO2; unfortunately, high false-positive rates of pre-cordial Doppler make its use impractical.
How to minimize risks
- Avoid coaxial gas cooling tips associated with Nd:YAG crystal lasers
- Avoid a steep Trendelenburg position
- Keep cervix covered with sponge or dilator when operative hysteroscope is removed to minimize air embolism
- Deaerate the equipment prior to surgery Use a low-pressure hysteroscopic CO2 insufflator
- Carefully monitor the patient
- Be highly suspicious when vital signs are unstable
POSTOPERATIVE COMPLICATIONS
Some complications of hysteroscopy may not become clinically evident for months or even years. The most common complications of hysteroscopic endometrial ablation include pregnancy, postablation tubal sterilization syndrome, new or worsening dysmenorrhea, hematometra, endometrial cancer, and failure to completely treat symptoms.
Patients scheduled for hysteroscopy must be informed of potential delayed risks of the procedure. In addition, all reproductive-aged women should be advised that pregnancy is possible after endometrial ablation or operative removal of an intracavitary mass; thus, contraception is crucial. The endometrial tissue is resilient and may regenerate after ablation.
Hematometra: Avoid cervical canal
Hematometra is an infrequent late complication of operative hysteroscopy. If menstruating women or those taking hormone replacement therapy experience cyclic or chronic lower pelvic pain after surgery, scarring or narrowing of the endometrial cavity may be the cause. Approximately 1% to 2% of women who undergo operative hysteroscopy experience this phenomenon. Most cases can be treated with cervical dilation alone.
Since the cervical canal contains no endometrial glands, there is no need to treat this area in women undergoing endometrial ablation. In fact, avoiding this area during treatment is a critical component of successful surgery.
Tubal sterilization syndrome possible after endometrial ablation
Consider this syndrome when a patient undergoing endometrial ablation complains of crampy, cyclic, unilateral or bilateral pelvic pain, possibly accompanied by vaginal spotting. Sometimes a unilateral mass can be palpated, but more commonly tenderness is elicited on pelvic examination.
Ultrasound may demonstrate fluid near the cornual region. Laparoscopy confirms the diagnosis by visualizing a swollen, edematous proximal fallopian tube. Salpingectomy may confirm hematosalpinx, chronic or acute inflammation, or hemosiderin deposits.
Treatment includes bilateral cornual resection and reablation of proximal endometrium, or hysterectomy.14
Pregnancy complications
Endometrial ablation is not to be regarded as a method of contraception. Patients needing birth control should consider concurrent tubal ligation or other reliable methods after this procedure.
The frequency of pregnancy after endometrial ablation ranges from 0.2% to 1.6%, though this data may represent underreporting. Pregnancy outcomes have been dismal in women conceiving after endometrial ablation. Complications include preterm labor, premature delivery, intrauterine growth retardation, prenatal death, postpartum hemorrhage, and placentation problems such as placenta accreta, increta, or percreta, as well as placental abruption.15
Uterine dehiscence and sacculation and extremely thin myometrium have been reported after uterine adhesiolysis, uterine perforation during operative hysteroscopy, and with myoma resections. A high index of suspicion is vital when a gravida presents with pelvic pain, decreased fetal movement, vaginal bleeding, or abnormal uterine masses detected ultrasonographically.
Signs of uterine rupture. Pregnancyrelated complications of operative hysteroscopy can be dramatic and fatal if not recognized quickly, as in the case of uterine rupture. Kerimis et al16 describe uterine rupture in a term pregnancy after hysteroscopic resection of a uterine septum. Severe fetal distress, maternal shoulder pain, and abdominal pain led to an emergency cesarean section. Intraoperative findings included a 7-cm tear from left cornua to right cornua. The original metroplasty, performed with cutting diathermy and laparoscopy, was not accompanied by complications or perforation.
Patients who experience intraoperative complications during metroplasty or deep resection of intramural fibroids should be informed of the risk of uterine rupture so they may consider elective cesarean. Regardless of the mode of delivery, prompt attention is vital if fetal distress is suspected.
Postablation warning signs
Patients undergoing endometrial ablation generally have a quick postoperative return to activity, minimal need for postoperative pain medication, and limited complaints. Beware of patients who make frequent postoperative phone calls and have escalating requirements for pain medication. While bowel and bladder injuries are infrequent—as is postoperative endometritis—these must be vigilantly considered and evaluated when patients complain of persistent pain, fever, and general malaise. Office evaluation is necessary, including thorough abdominal and pelvic examinations. Laboratory testing should include electrolytes, complete blood count, sedimentation rate, ultrasound, and a flat plate of the abdomen (kidneys, ureter, and bladder; upright) may be required. Sometimes a computed tomography scan of the pelvis/abdomen may be needed if perforation with bowel or bladder injury is suspected.
Hysteroscopic fibroid removal may be necessary after UAE
Uterine artery embolization (UAE) is gaining popularity for the treatment of symptomatic uterine fibroids. Transcatheter embolization of the uterine artery leads to occlusion of the fibroid, ischemic shrinkage of the fibroid, and shrinkage of residual myometrial tissue. Fibroids may migrate weeks to months after the procedure as the myometrium contracts and the treated fibroid degenerates, leading to delayed discharge, passage of necrotic fibroids, cramps, and heavy bleeding if the fibroid migrates to a submucosal location. Hysteroscopic removal is an obvious option.
Recently, De Iaco et al17 reported the development of a uterine fistula and discontinuity of the myometrium after hysteroscopic resection of an embolized migrated fibroid. They speculated this was due to the development of an avascular myometrium after UAE. The patient was asymptomatic, but routine diagnostic hysteroscopy revealed a 2-cm discontinuity of the uterine wall at the site of the previous resectoscopic myomectomy. The myometrium was white and less than the full thickness.
Ultrasound guidance improves outcomes
Coccia et al18 described the benefits of intraoperative ultrasound guidance during operative hysteroscopy in fibroid treatment and uterine septum removal. Prospective evaluation of 81 patients involved an experienced ultrasonographer who mapped the limits of treatment. Patients were compared to 45 historical controls who had been similarly treated with laparoscopic monitoring. Satisfactory outcomes included relief of menorrhagia, complete resection of fibroids (including full resection of intramural fibroids), and thorough metroplasty of uterine septum.
Ultrasound guidance made it possible to extend the resection beyond the limit conventionally defined by hysteroscopy; none of the patients in the ultrasound group required reintervention. Among controls, a second operation was necessary in 4 cases. Investigators concluded that a wider resection (10 to 15 mm distance from the external surface of the uterus) of fibroids was achieved using ultrasound guidance.
Dr. Bradley reports that she serves as a consultant to Karl Storz, ACMI, Olympus, and Gynecare, and as a lecturer for Novacept.
- Preoperative treatment with a gonadotropinreleasing hormone agonist increases the odds of operative complications by a factor of 4 to 7.
- Preoperative cervical ripening reduced the need for cervical dilation, minimized cervical complications, and reduced operative time.
- CO2 should never be used for operative hysteroscopic procedures because of the high risk of CO2 embolism.
- Ultrasound guidance may improve outcomes in selected hysteroscopic procedures.
It is a valuable tool in the evaluation and treatment of infertility, recurrent pregnancy loss, and abnormal and postmenopausal uterine bleeding, and is useful when saline infusion sonography findings are equivocal.
Further, if a global ablation device fails, the surgeon can convert to hysteroscopic ablation rather than abandon the procedure altogether. This is not as unusual as it might appear: In US Food and Drug Administration trials, there was a staggering 10% to 22% malfunction of global ablation technology.1
Safe, easily learned
Although gynecologists are beginning to embrace this modality, many physicians avoid it because of inadequate training or exaggerated fears of complications. In reality, hysteroscopy is one of the safest and most easily acquired surgical skills in gynecology. For example, in a prospective evaluation of 13,600 diagnostic and operative hysteroscopic procedures performed at 63 hospitals in the Netherlands—which involved both established surgeons and residents—Jansen et al2 found an astonishingly low complication rate of 0.28%, with no deaths.
Proper selection and treatment of patients and prompt intervention minimize complications as well as legal risks. Surgical misadventures and lawsuits occur with delayed intervention, failure to recognize pathology or risky conditions, and inadequate preventive maneuvers.
Overall, emphasis on safety is vital to success, and thorough awareness of potential complications is just as important.
Three types of complications
Complications fall into 3 categories (TABLE):
- Procedure-related
- Media-related
- Postoperative
PROCEDURE-RELATED COMPLICATIONS
Complication rates
In a retrospective investigation, Propst et al3 determined the rate of complications associated with specific hysteroscopic procedures. Demographic data and medical histories were collected for 925 women who had operative hysteroscopy in 1995 and 1996. The overall complication rate was 2.7%. Myomectomy and resection of uterine septa carried the greatest odds of complications; polypectomy and endometrial ablation had the lowest. Preoperative treatment with a gonadotropinreleasing hormone (GnRH) agonist increased the odds of complications by a factor of 4 to 7. Women under age 50 were more likely to experience complications than those over 50.
In the study by Jansen et al,2 38 complica-tions occurred in the 13,600 procedures. The greatest risk of complications occurred with adhesiolysis (4.48%), followed by endometrial resection (0.81%), myomectomy (0.75%), and polypectomy (0.38%).
Cervical entry requires special attention
Almost half of the complications in the Jansen study were related to cervical entry, so caution and, perhaps, preoperative cervical ripening are advised. Many premenopausal subjects were given GnRH analogues, which may render the cervix more resistant to dilation. Complications associated with a stenotic cervix include a cervical tear, creation of a false cervical passage, and uterine perforation.
Cervical ripening may help prevent uterine perforation. The most common complication, occurring in 14.2 cases per thousand, is uterine perforation.2 The risk of this is highest in postpartum procedures, followed by procedures in postmenopausal, then perimenopausal, women. Patients with endometrial cancer also have a higher rate of perforation.
Risk factors for uterine perforation include:
- nulliparity
- menopause
- use of GnRH agonists
- prior cone biopsy
- markedly retroverted uterus
- undue force
Vaginal or oral misoprostol for cervical ripening prior to operative hysteroscopy was evaluated in a randomized trial.4 Researchers found a reduced need for cervical dilation, a minimum of cervical complications, and reduced operative time in study patients compared with controls.
When 400 μg oral misoprostol is given 12 and 24 hours before surgery, it also softens the cervix and eases dilation.5 Although misoprostol has several bothersome side effects (such as lower abdominal pain and slight vaginal bleeding), few if any prevent its use.
Signs of perforation. Patients who sustain uterine perforation with subsequent intraperitoneal bleeding often complain of pain in the abdomen and shoulder, and experience hemodynamic instability. A quick sonographic survey of the abdomen will demonstrate free intraperitoneal fluid. (It is rare for much intraperitoneal fluid to accumulate by transtubal regurgitation during operative hysteroscopy, despite the quantity of fluid used.)
If perforation is suspected, laparoscopy or laparotomy is necessary to clarify the cause of pain, unstable vital signs, or free fluid visualized by ultrasound.6
Exercise extra care and precautions in women who have had a prior cesarean section, myomectomy, or uterine perforation. Complete visualization of uterine landmarks is necessary during operative hysteroscopy to exclude uterine dehiscence, sacculation, and perforation. Prior uterine surgery may cause myometrial weakness and lead to possible perforation. Do not proceed if abnormal uterine morphology is detected. If uterine perforation occurs, injury to bladder and bowel is possible when electrical energy is applied to a uterine wall compromised by prior surgery. Strict visualization of uterine anatomy is critical in this population so that bowel or bladder burns can be avoided.
MEDIA-RELATED COMPLICATIONS
Notorious complications and several recent lawsuits have stemmed from fluid overload. A common element has been the physician’s lack of awareness of how rapidly complications can arise, and what signs and symptoms are specific to the fluid used.
Monitor fluids vigilantly
Operative hysteroscopy must be performed in a fluid medium. The type of fluid depends on the surgeon’s preference and the instrument utilized, but any fluid can be associated with complications. Fluid choices with monopolar instruments include glycine 1.5%, a mixture of sorbitol 3% and mannitol 0.54%, and mannitol 5%. These are frequently used with the continuous-flow resectoscope. Bipolar operative hysteroscopy can be performed using saline.
The solution to media-related complications is basic: vigilant monitoring of fluids. A cavalier attitude, poor fluid documentation, and failure to respond to complications can lead to trouble. If fluid overload occurs, comanagement and consultation with an intensive care specialist is advised.
Distention media
Among the options for distention media in operative and diagnostic hysteroscopy are high-viscosity dextran 70 and low-viscosity fluids such as hypotonic, electrolyte-free and isotonic, electrolyte-containing solutions. The popularity of dextran 70 is waning, however. While it is immiscible with blood, significant complications have been reported.
Signs of anaphylactic reactions to dextran 70 include acute hypotension, hypoxia, pulmonary edema, fluid overload, fulminant coagulopathies, and anemia. The surgeon must operate quickly, minimize endometrial trauma, use continuous pulse oximetry, and obtain a preoperative coagulation panel.
Dextran 70 also can ruin operative hysteroscopes if they are not cleaned promptly and thoroughly after use.
Hypotonic, electrolyte-free solutions. With hypotonic, electrolyte-free solutions such as glycine 1.5%, early recognition of possible complications, including hyponatremic hypervolemia, is vital. For example, when glycine and sorbitol are metabolized, free water accumulates and the body attempts to achieve homeostasis through compensatory mechanisms such as osmosis, which moves free water into extracellular and intracellular spaces. This can lead to increased free water in the brain, resulting in cerebral edema, rising intracranial pressure, and cellular necrosis.
The cerebral cation pump normally pumps osmotically active cations into the extracellular space, thereby minimizing cerebral edema. However, this pump is inhibited by estrogen, so the compensatory mechanism is diminished.
Classic clinical features of hyponatremic hypervolemia include apprehension, confusion, fatigue, headache, mental agitation, nausea, visual disturbances (including blindness), vomiting, and weakness. These complications are more readily apparent when regional anesthesia is used rather than general anesthesia.
If hyponatremic hypervolemia goes unrecognized, bradycardia and hypertension can ensue, followed rapidly by cerebral and pulmonary edema and cardiovascular collapse. In addition, glycine 1.5% is metabolized to glycolic acid and ammonia. Free ammonia is associated with central nervous system disorders. Recognition and prompt treatment by an intensivist may prevent permanent neurologic sequelae, death, and lawsuits.7
Isotonic, electrolyte-containing solutions. Mannitol 5% is electrolyte poor but isotonic, creating less risk for hypo-osmolality. However, dilutional hyponatremia (ie, low sodium levels) can still occur.
Advantages of bipolar instruments. To minimize complications from hypotonic, electrolyte-free solutions, manufacturers developed operative hysteroscopes that can function in a bipolar environment. Bipolar instruments can operate in isotonic, physiologic, electrolyte-containing media. Hyponatremia and hypo-osmolality cannot occur with normal saline or Ringer’s lactate, but fluid overload can. (Fluid overload with saline can cause pulmonary edema and congestive heart failure.)
How much fluid will be absorbed? The answer depends on factors including surface area of the surgical field, duration of surgery, opened venous channels, type of irrigation fluid used, and pressure of the delivery system. Modern gynecologic suites employ fluid irrigation systems that continuously measure input and output, with alarms that signal a predetermined fluid deficit. The alarm indicates the need to halt the procedure and quickly evaluate the patient. Careful attention to the recommendations of Loffer et al8 would lead to fewer complications from fluid mismanagement.
Appropriate use of CO2
High risk of embolism with CO2 in operative procedures. Although diagnostic hysteroscopic procedures often are performed with carbon dioxide (CO2), operative procedures never should be. The reason: the high risk of CO2 embolism that occurs with open venous channels and vascular endometrium. The choice between CO2 and fluid medium for diagnostic hysteroscopy often is determined by physician preference and the presence of uterine bleeding. Many gynecologists prefer CO2 for its optical clarity and patient comfort during insufflation.9
Purge tubing of room air before each procedure. Embolic complications with CO2 have been recorded with use of the neodymium: yttrium aluminum garnet (Nd:YAG) laser and during operative procedures. Less well known are the adverse sequelae that can occur with room air prior to beginning the procedure. It is critical to purge the entire tubal system with CO2 prior to instrumentation, since up to 40 cm3 of room air may be insufflated into a patient when 200 cm of connective tubing with a 0.5-cm lumen is used.10 Wait for several minutes before starting the procedure so that the whole system is purged.
One of the greatest concerns about endometrial ablation is that diagnosis of endometrial cancer will be delayed because the endometrial cavity has been obliterated. Vilos19 recently reviewed the salient characteristics and findings in women treated by endometrial ablation who subsequently developed endometrial cancer. A review of the individual cases revealed that most of these patients had numerous risk factors for endometrial cancer.
Review risk factors, chronic conditions
Many patients with abnormal bleeding also have risk factors for endometrial cancer, as well as medical conditions that increase the likelihood of morbidity with surgery, such as obesity, hypertension, diabetes, and advanced age. In these cases, hysterectomy may be a better option than endometrial ablation. It would be far better to have such high-risk patients cleared for hysterectomy than to chance their becoming an endometrial-ablation “statistic.” If endometrial ablation is performed in these cases, we prevent the egress of blood, foster development of synechiae, render endometrial biopsy difficult or impossible and, potentially, “bury” endometrial cells deeper within the myometrium—all of which contribute to a delayed and “upstaged” diagnosis of endometrial cancer.
Patients at risk of endometrial cancer should undergo a scrupulous and unambiguous work-up and evaluation. Indeterminate endometrial echo and office evaluation that generates biopsy samples designated as “insufficient for diagnosis,” “no endometrial tissue seen,” or “atrophy” should raise suspicion. These patients require full visualization of the endometrium.
Heightened risk during perimenopause
Newer ablation techniques that utilize global therapy make it paramount that perimenopausal women undergo scrupulous evaluation. Until much more information is available, endometrial ablation should be avoided in patients with endometrial hyperplasia, particularly with atypia. While some gynecologists may be persuaded to consider endometrial ablation as a minimally invasive procedure compared to hysterectomy, the risk of delayed diagnosis of endometrial cancer is of paramount concern. The treatment of choice for these patients remains medical therapy with oral progesterone and, possibly, longterm use of a levonorgestrel-releasing intrauterine system. If this fails, hysterectomy is advisable. As Cooper20 aptly states, “Conservative, nonextirpative procedures offer no life raft” compared with hysterectomy, which covers many missed diagnoses.
No risk of spreading cancer cells
Some gynecologists have worried about the risk of disseminating endometrial cancer cells during hysteroscopy. However, Kudela and Pilka21 studied the true risk in women undergoing blind dilation and curettage and hysteroscopy performed with a fluid medium. Cul-de-sac aspiration prior to instrumentation and at the conclusion of the procedure demonstrated no increased risk of positive cytology. They are continuing a Phase II trial comparing outcomes of both groups over 5 years.
6 most common symptoms of venous or air emboli. Anesthesiologists and gynecologists must be vigilant to prevent venous or air emboli. Munro et al12 succinctly outline the 6 most common symptoms:
- pulmonary hypertension
- hypercarbia
- hypoxia
- arrhythmias
- tachypnea
- systemic hypotension
Beware of a drop in end-tidal CO2. The most common sign of impending cardiovascular collapse is a sudden decrease in end-tidal CO2, when the right cardiac outflow tract is obstructed by CO2, which leads to arterial oxygen (O2) desaturation. If such a decrease is suspected, stop the procedure immediately and administer 100% O2. (Also stop nitrous oxide, if used.) Turn the patient to the left lateral decubitis position and use a central venous catheter to aspirate gas, if necessary. Cardiac massage and a precordial thump may dislodge CO2; unfortunately, high false-positive rates of pre-cordial Doppler make its use impractical.
How to minimize risks
- Avoid coaxial gas cooling tips associated with Nd:YAG crystal lasers
- Avoid a steep Trendelenburg position
- Keep cervix covered with sponge or dilator when operative hysteroscope is removed to minimize air embolism
- Deaerate the equipment prior to surgery Use a low-pressure hysteroscopic CO2 insufflator
- Carefully monitor the patient
- Be highly suspicious when vital signs are unstable
POSTOPERATIVE COMPLICATIONS
Some complications of hysteroscopy may not become clinically evident for months or even years. The most common complications of hysteroscopic endometrial ablation include pregnancy, postablation tubal sterilization syndrome, new or worsening dysmenorrhea, hematometra, endometrial cancer, and failure to completely treat symptoms.
Patients scheduled for hysteroscopy must be informed of potential delayed risks of the procedure. In addition, all reproductive-aged women should be advised that pregnancy is possible after endometrial ablation or operative removal of an intracavitary mass; thus, contraception is crucial. The endometrial tissue is resilient and may regenerate after ablation.
Hematometra: Avoid cervical canal
Hematometra is an infrequent late complication of operative hysteroscopy. If menstruating women or those taking hormone replacement therapy experience cyclic or chronic lower pelvic pain after surgery, scarring or narrowing of the endometrial cavity may be the cause. Approximately 1% to 2% of women who undergo operative hysteroscopy experience this phenomenon. Most cases can be treated with cervical dilation alone.
Since the cervical canal contains no endometrial glands, there is no need to treat this area in women undergoing endometrial ablation. In fact, avoiding this area during treatment is a critical component of successful surgery.
Tubal sterilization syndrome possible after endometrial ablation
Consider this syndrome when a patient undergoing endometrial ablation complains of crampy, cyclic, unilateral or bilateral pelvic pain, possibly accompanied by vaginal spotting. Sometimes a unilateral mass can be palpated, but more commonly tenderness is elicited on pelvic examination.
Ultrasound may demonstrate fluid near the cornual region. Laparoscopy confirms the diagnosis by visualizing a swollen, edematous proximal fallopian tube. Salpingectomy may confirm hematosalpinx, chronic or acute inflammation, or hemosiderin deposits.
Treatment includes bilateral cornual resection and reablation of proximal endometrium, or hysterectomy.14
Pregnancy complications
Endometrial ablation is not to be regarded as a method of contraception. Patients needing birth control should consider concurrent tubal ligation or other reliable methods after this procedure.
The frequency of pregnancy after endometrial ablation ranges from 0.2% to 1.6%, though this data may represent underreporting. Pregnancy outcomes have been dismal in women conceiving after endometrial ablation. Complications include preterm labor, premature delivery, intrauterine growth retardation, prenatal death, postpartum hemorrhage, and placentation problems such as placenta accreta, increta, or percreta, as well as placental abruption.15
Uterine dehiscence and sacculation and extremely thin myometrium have been reported after uterine adhesiolysis, uterine perforation during operative hysteroscopy, and with myoma resections. A high index of suspicion is vital when a gravida presents with pelvic pain, decreased fetal movement, vaginal bleeding, or abnormal uterine masses detected ultrasonographically.
Signs of uterine rupture. Pregnancyrelated complications of operative hysteroscopy can be dramatic and fatal if not recognized quickly, as in the case of uterine rupture. Kerimis et al16 describe uterine rupture in a term pregnancy after hysteroscopic resection of a uterine septum. Severe fetal distress, maternal shoulder pain, and abdominal pain led to an emergency cesarean section. Intraoperative findings included a 7-cm tear from left cornua to right cornua. The original metroplasty, performed with cutting diathermy and laparoscopy, was not accompanied by complications or perforation.
Patients who experience intraoperative complications during metroplasty or deep resection of intramural fibroids should be informed of the risk of uterine rupture so they may consider elective cesarean. Regardless of the mode of delivery, prompt attention is vital if fetal distress is suspected.
Postablation warning signs
Patients undergoing endometrial ablation generally have a quick postoperative return to activity, minimal need for postoperative pain medication, and limited complaints. Beware of patients who make frequent postoperative phone calls and have escalating requirements for pain medication. While bowel and bladder injuries are infrequent—as is postoperative endometritis—these must be vigilantly considered and evaluated when patients complain of persistent pain, fever, and general malaise. Office evaluation is necessary, including thorough abdominal and pelvic examinations. Laboratory testing should include electrolytes, complete blood count, sedimentation rate, ultrasound, and a flat plate of the abdomen (kidneys, ureter, and bladder; upright) may be required. Sometimes a computed tomography scan of the pelvis/abdomen may be needed if perforation with bowel or bladder injury is suspected.
Hysteroscopic fibroid removal may be necessary after UAE
Uterine artery embolization (UAE) is gaining popularity for the treatment of symptomatic uterine fibroids. Transcatheter embolization of the uterine artery leads to occlusion of the fibroid, ischemic shrinkage of the fibroid, and shrinkage of residual myometrial tissue. Fibroids may migrate weeks to months after the procedure as the myometrium contracts and the treated fibroid degenerates, leading to delayed discharge, passage of necrotic fibroids, cramps, and heavy bleeding if the fibroid migrates to a submucosal location. Hysteroscopic removal is an obvious option.
Recently, De Iaco et al17 reported the development of a uterine fistula and discontinuity of the myometrium after hysteroscopic resection of an embolized migrated fibroid. They speculated this was due to the development of an avascular myometrium after UAE. The patient was asymptomatic, but routine diagnostic hysteroscopy revealed a 2-cm discontinuity of the uterine wall at the site of the previous resectoscopic myomectomy. The myometrium was white and less than the full thickness.
Ultrasound guidance improves outcomes
Coccia et al18 described the benefits of intraoperative ultrasound guidance during operative hysteroscopy in fibroid treatment and uterine septum removal. Prospective evaluation of 81 patients involved an experienced ultrasonographer who mapped the limits of treatment. Patients were compared to 45 historical controls who had been similarly treated with laparoscopic monitoring. Satisfactory outcomes included relief of menorrhagia, complete resection of fibroids (including full resection of intramural fibroids), and thorough metroplasty of uterine septum.
Ultrasound guidance made it possible to extend the resection beyond the limit conventionally defined by hysteroscopy; none of the patients in the ultrasound group required reintervention. Among controls, a second operation was necessary in 4 cases. Investigators concluded that a wider resection (10 to 15 mm distance from the external surface of the uterus) of fibroids was achieved using ultrasound guidance.
Dr. Bradley reports that she serves as a consultant to Karl Storz, ACMI, Olympus, and Gynecare, and as a lecturer for Novacept.
1. Gurtcheff SE, Sharp HT. Complications associated with global endometrial ablation: the utility of the MAUDE database. Obstet Gynecol. 2003;102:1278-1282.
2. Jansen FW, Vredevoogd CB, Ulzen K, et al. Complications of hysteroscopy: a prospective, multicenter study. Obstet Gynecol. 2000;96:266-270.
3. Propst AM, Liberman RF, Harlow BL, Ginsburg ES. Complications of hysteroscopic surgery: predicting patients at risk. Obstet Gynecol. 2000;96:517-520.
4. Preutthipan S, Herabutya Y. Vaginal misoprostol for cervical priming before operative hysteroscopy: a randomized controlled trial. Obstet Gynecol. 2000;96:890-894.
5. Thomas JA, Leyland N, Durand N, Windrim RD. The use of oral misoprostol as a cervical ripening agent in operative hysteroscopy: a double-blind, placebo-controlled trial. Am J Obstet Gynecol. 2002;186:876-879.
6. Stotz M, Lampart A, Kochli OR, Schneider M. Intraabdominal bleeding masked by hemodilution after hysteroscopy. Anesthesiology. 2000;93:569-570.
7. Cooper JM, Brady RM. Intraoperative and early postoperative complications of operative hysteroscopy. Obstet Gynecol Clin North Am. 2000;27:347-366.
8. Loffer FD, Bradley LD, Brill AL, Brooks PG, Cooper JM. Hysteroscopic fluid monitoring guidelines. J Am Assoc Gynecol Laparosc. 2000;7:167-168.
9. Bradley LD, Widrich T. Flexible hysteroscopy: a state-of-the-art procedure for gynecologic evaluation. J Am Assoc Gynecol Laparosc. 1995;2:263-267.
10. Neis KJ, Brandner P, Lindemann HJ. Room air as a cause of gas embolism in diagnostic CO2hysteroscopy. Zentralbl Gynakol. 2000;122:222-225.
11. Bradner P, Neis KJ, Ehmer C. The etiology, frequency, and prevention of gas embolism during CO2hysteroscopy. J Am Assoc Gynecol Laparosc. 1999;6:421-428.
12. Munro MG, Weisberg M, Rubinstein E. Gas and air embolization during hysteroscopic electrosurgical vaporization: comparison of gas generation using bipolar and monopolar electrodes in an experimental model. J Am Assoc Gynecol Laparosc. 2001;8:488-494.
13. Murdoch JAC, Gan TJ. Anesthesia for hysteroscopy. Anesthesiol Clin North Am. 2001;1:125-140.
14. Cooper JM, Brady RM. Late complications of operative hysteroscopy. Obstet Gynecol Clin North Am. 2000;27:367-374.
15. Rogerson L, Gannon B, O’Donovan P. Outcome of pregnancy following endometrial ablation. J Gynecol Surg. 1997;13:155-160.
16. Kerimis P, Zolti M, Sinwany G, Mashiach S, Carp H. Uterine rupture after hysteroscopic resection of uterine septum. Fertil Steril. 2002;77:618-620.
17. De Iaco P, Golfieri R, Ghi T, Muzzupapa G, Ceccarini M, Bovicelli L. Uterine fistula induced by hysteroscopic resection of an embolized migrated fibroid: a rare complication after embolization of uterine fibroids. Fertil Steril. 2001;75:818-820.
18. Coccia ME, Becattini C, Bracco GL, et al. Intraoperative ultrasound guidance for operative hysteroscopy. J Reprod Med. 2000;45:413-418.
19. Brooks-Carter GN, Killackey MA, Neuwirth RS. Adenocarcinoma of the endometrium after endometrial ablation. Obstet Gynecol. 2000;96:836-837.
20. Cooper JM. Swimming lessons: check the water before jumping in. J Am Assoc Gynecol Laparosc. 1998;5:87-90.
21. Kudela M, Pilka R. Is there a real risk in patients with endometrial carcinoma undergoing diagnostic hysteroscopy (HSC)? Eur J Gynecol Oncol. 2001;22:342-344.
1. Gurtcheff SE, Sharp HT. Complications associated with global endometrial ablation: the utility of the MAUDE database. Obstet Gynecol. 2003;102:1278-1282.
2. Jansen FW, Vredevoogd CB, Ulzen K, et al. Complications of hysteroscopy: a prospective, multicenter study. Obstet Gynecol. 2000;96:266-270.
3. Propst AM, Liberman RF, Harlow BL, Ginsburg ES. Complications of hysteroscopic surgery: predicting patients at risk. Obstet Gynecol. 2000;96:517-520.
4. Preutthipan S, Herabutya Y. Vaginal misoprostol for cervical priming before operative hysteroscopy: a randomized controlled trial. Obstet Gynecol. 2000;96:890-894.
5. Thomas JA, Leyland N, Durand N, Windrim RD. The use of oral misoprostol as a cervical ripening agent in operative hysteroscopy: a double-blind, placebo-controlled trial. Am J Obstet Gynecol. 2002;186:876-879.
6. Stotz M, Lampart A, Kochli OR, Schneider M. Intraabdominal bleeding masked by hemodilution after hysteroscopy. Anesthesiology. 2000;93:569-570.
7. Cooper JM, Brady RM. Intraoperative and early postoperative complications of operative hysteroscopy. Obstet Gynecol Clin North Am. 2000;27:347-366.
8. Loffer FD, Bradley LD, Brill AL, Brooks PG, Cooper JM. Hysteroscopic fluid monitoring guidelines. J Am Assoc Gynecol Laparosc. 2000;7:167-168.
9. Bradley LD, Widrich T. Flexible hysteroscopy: a state-of-the-art procedure for gynecologic evaluation. J Am Assoc Gynecol Laparosc. 1995;2:263-267.
10. Neis KJ, Brandner P, Lindemann HJ. Room air as a cause of gas embolism in diagnostic CO2hysteroscopy. Zentralbl Gynakol. 2000;122:222-225.
11. Bradner P, Neis KJ, Ehmer C. The etiology, frequency, and prevention of gas embolism during CO2hysteroscopy. J Am Assoc Gynecol Laparosc. 1999;6:421-428.
12. Munro MG, Weisberg M, Rubinstein E. Gas and air embolization during hysteroscopic electrosurgical vaporization: comparison of gas generation using bipolar and monopolar electrodes in an experimental model. J Am Assoc Gynecol Laparosc. 2001;8:488-494.
13. Murdoch JAC, Gan TJ. Anesthesia for hysteroscopy. Anesthesiol Clin North Am. 2001;1:125-140.
14. Cooper JM, Brady RM. Late complications of operative hysteroscopy. Obstet Gynecol Clin North Am. 2000;27:367-374.
15. Rogerson L, Gannon B, O’Donovan P. Outcome of pregnancy following endometrial ablation. J Gynecol Surg. 1997;13:155-160.
16. Kerimis P, Zolti M, Sinwany G, Mashiach S, Carp H. Uterine rupture after hysteroscopic resection of uterine septum. Fertil Steril. 2002;77:618-620.
17. De Iaco P, Golfieri R, Ghi T, Muzzupapa G, Ceccarini M, Bovicelli L. Uterine fistula induced by hysteroscopic resection of an embolized migrated fibroid: a rare complication after embolization of uterine fibroids. Fertil Steril. 2001;75:818-820.
18. Coccia ME, Becattini C, Bracco GL, et al. Intraoperative ultrasound guidance for operative hysteroscopy. J Reprod Med. 2000;45:413-418.
19. Brooks-Carter GN, Killackey MA, Neuwirth RS. Adenocarcinoma of the endometrium after endometrial ablation. Obstet Gynecol. 2000;96:836-837.
20. Cooper JM. Swimming lessons: check the water before jumping in. J Am Assoc Gynecol Laparosc. 1998;5:87-90.
21. Kudela M, Pilka R. Is there a real risk in patients with endometrial carcinoma undergoing diagnostic hysteroscopy (HSC)? Eur J Gynecol Oncol. 2001;22:342-344.
Expert Panel: Techniques and tools to prevent pelvic adhesions


-

-

- Approximately 40% of people who undergo primary surgery develop adhesions and reformation occurs in 80% to 90% of cases.
- Microsurgical techniques such as gentle handling of tissues, careful hemostasis, and avoidance of heat may help reduce the incidence.
- Laparoscopy appears to be less likely to produce adhesions than laparotomy.
- Ob/Gyns should be aware of the potential for adhesion-related bowel obstruction and take steps to prevent it.
Are adhesions a pathologic response to injury or a normal aspect of healing? Can they be avoided, or are preventive efforts part of the problem? How useful are the different barriers in gynecologic surgery? What is the ideal adjuvant?
OBG Management convened a panel of experts to explore these and other questions.
Common problem, high recurrence rate
DECHERNEY: Adhesion formation is serious because it is associated with clinical entities such as infertility, pelvic pain, and bowel obstruction. We all agree that approximately 40% of people who undergo primary surgery develop adhesions and that 80% to 90% of patients who undergo lysis develop recurrent adhesions.
SANFILIPPO: One study several years ago explored adhesion formation.1 Unfortunately, no matter how meticulous the surgeon is, adhesions will form, even with microsurgical techniques and carefully ensured hemostasis.
HURD: The number of patients with significant adhesion formation after some gynecologic procedures has been reported to be greater than 90%.2
DECHERNEY: That higher incidence usually occurs after general surgery—and there’s a reason it is so high: General surgeons don’t use adjunctive therapy. They are critical of it. It is to our credit as gynecologic surgeons that we adopted adjunctive therapies about 15 years ago with the introduction of dextran 70 (Hyskon; Medisan Pharmaceuticals, Parsippany, NJ).
PAGIDAS: If anything, the pelvis seems to have even more of a predilection for adhesion formation than the abdomen, probably because of the close proximity of structures.
How and why adhesions form
DECHERNEY: What is the pathophysiology of adhesion formation? Let’s say you have 2 raw surface areas. What happens?
The process
PAGIDAS: The increase in leukotrienes and prostaglandins and the decrease in plasminogen activity (which actually initiates the inflammation) appear to be significant.
HURD: Vessel permeability also increases, and inflammatory cells leak through the vessels and set up a matrix for adhesion formation.
DECHERNEY: So we have 2 raw surface areas with fibrin leaking out and forming bridges between them.
PAGIDAS: The key is that it takes 2 surfaces to form these bridges. As I mentioned, the greater proximity of pelvic structures—particularly around the tube and ovary—probably contributes to adhesion formation.
DECHERNEY: Macrophage activity also is important. The macrophage “migrates” along these fibrin bridges and lays down collagen over a period of time. Then the collagen becomes organized and, eventually, vascular.
Window of opportunity
SANFILIPPO: Adhesion formation probably occurs and is pretty well established within 5 to 7 days of the precipitating event—usually surgery. Once that process is under way, attempts to halt it yield diminishing returns. Unfortunately, we don’t know how to interfere with it in a positive way.
HURD: Under normal conditions, there seems to be a balance between fibrin deposition and fibrinolysis. In some tissues, however, these functions become imbalanced. This disparity may contribute more to adhesions than the actual laying down of fibrin—especially in tissue that is hypoxic.
DECHERNEY: Would you say that adhesion formation represents normal or abnormal healing?
HURD: It is one of the body’s normal protective mechanisms and an important part of healing. Without it, any abdominal injury would likely result in death.
SANFILIPPO: I don’t think it differs that much from processes that occur externally. For example, if you get cut deeply enough, you develop a scar. Is that scar part of the normal healing process? It is.
PAGIDAS: Right. It is a normal process of tissue remodeling. The question is: What allows it to go astray?
What surgical techniques help prevent adhesions?
DECHERNEY: Let’s review the aspects of surgical technique that are important for adhesion prevention.
PAGIDAS: I emphasize the value of microsurgical techniques, which help to minimize severe tissue handling. It also is important to keep surfaces moistened so they don’t desiccate.
SANFILIPPO: I agree with Dr. Pagidas about microsurgical techniques such as gentle tissue handling, careful hemostasis, and keeping tissues moist. If we follow these principles, we create an environment that minimizes the potential for adhesion formation.
HURD: The findings of many well-controlled animal studies have been surprising. For example, it is difficult to demonstrate that drying of tissue increases adhesions.3 Probably the greatest contributor to adhesions in these models was abrasion.4 One way that laparoscopic surgery decreases adhesions is by avoiding abrasion of the bowel mucosa, which occurs specifically with packing.
A 38-year-old mother of 2 undergoes myomectomy for menorrhagia.
SANFILIPPO: The initial question is: Can this case be managed laparoscopically? I do myomectomies laparoscopically whenever possible, although I do close the uterus with a minilaparotomy incision. The reason is my strong concern about reapproximating the myometrium, since wound dehiscence sometimes occurs at the site of myoma removal.
In this case, depending on the size of the myomas, I would do as much as possible laparoscopically and then reapproximate the myometrium. I would plan my incisions carefully, to maximize the number of myomas that can be removed. I would end with meticulous hemostasis and, assuming it is successful, use a barrier over the incision—in this case, Interceed.
HURD: Does the patient desire future childbearing? If so, I would avoid the laparoscopic approach because of the possibly increased risk of uterine rupture during pregnancy. If she isn’t planning pregnancy, there are more options.
The next question is: How many myomas are there, and where are they located? If they are intrauterine, a hysteroscopic approach would avoid extrauterine adhesions. If they are multiple and large, I am pretty much limited to laparotomy. If there is 1 or only a few myomas, a laparoscopic approach would be best.
I have not used Interceed. In laparoscopic cases, I worry that it would create more problems because, as you allow the carbon dioxide to decrease at the end of a case, oozing begins. Instead of a barrier, I would use limited hydroflotation.
SANFILIPPO: That’s a good point. At the end of the myomectomy, with the laparoscope in place, I decrease the insufflation, eliminating the tamponade effect. Then, assuming good hemostasis, I apply Interceed.
HURD: With open cases, I use Seprafilm, which takes practice because, as it gets wet, it sticks to anything, including gloves and instruments. But if you can put it down dry on the uterus, it sticks and stays in place. If oozing occurs, it seems to block or stop it.
PAGIDAS: I want to reiterate the importance of determining whether childbearing is an issue. In this case, the biggest concern is the risk of adhesions developing on our incision, so I would use a barrier. My preference would be Seprafilm or GoreTex. If we can limit adhesions at the incision site, then hopefully we can minimize bowel and tuboovarian adhesions, too.
In open cases, one thing we can do to minimize the risk of adhesions is to pack gently when needed. Also, we should avoid using packing to reposition the bowel.
Another factor frequently overlooked is the application of heat, which appears to be a very effective way to create adhesions. This probably isn’t an issue for laparoscopic cases, but when you use irrigation fluid in an open case, watch the temperature. If it feels hot to you, you need to worry about potential injury to the bowel surfaces.
PAGIDAS: That is critical. In abdominal cases you want to make sure irrigation fluid is warm, but not too warm, because heat increases the vascular permeability of vessels and leads to more macrophages, more prostaglandins, and more leukotrienes.
HURD: Another important element is the type of suture material used.
DECHERNEY: Overall, we need to minimize the use of sutures. For example, when I am operating laparoscopically on an ovarian cyst, I try to apply bipolar energy to the edges so that they will coapt without a stitch.
HURD: When it first became clear that suturing ovaries increased adhesion formation, we conducted a controlled trial of different kinds of sutures in animals. Not surprisingly, we found that the less reactive the suture, the fewer adhesions.5 Sutures that are absorbed more slowly, such as polydioxanone, seem to be less reactive.
Obviously, inert sutures like nylon are the least reactive, but they are permanent. It is assumed that animal-protein sutures such as chromic and plain gut are the most reactive, although I am not sure there are sufficient data to support that conclusion.
Multiple clinical studies have shown laparoscopy to be associated with a lower adhesion rate, although it isn’t clear why. It may be related to decreased suturing.
DECHERNEY: Bulk is important, too—that is, the number of throws in the suture. When Vicryl (polyglactin 910) became available, we conducted a study in mice using proportionately small Vicryl plaques to determine whether this would be good a barrier (A. DeCherney, MD, unpublished data). It caused a tremendous amount of adhesion because so much foreign matter was applied.
We also did a study using human-size titanium clips in rats (A. DeCherney, MD, unpublished data). Not surprisingly, there was a lot of adhesion formation.
FIGURE 1 Pelvic adhesions: How they develop, problems they cause
Adhesions occur when 2 or more raw surfaces are exposed to leaking fibrin, which forms a bridge between the surfaces. Macrophages “migrate” along these bridges, depositing collagen.
The pelvis has a greater predilection for adhesions than the abdomen because of the close proximity of structures.
Although adhesions represent one of the body’s protective mechanisms, they may also cause pain or interfere with fertility, bowel function, or other processes.
Laparoscopy versus laparotomy: More adhesions in open cases?
DECHERNEY: Based on all the techniques we have learned from microsurgery—with the exception of magnification—it appears that laparoscopic procedures are less likely to cause adhesions than laparotomy. Do you agree?
PAGIDAS: I think so. As Dr. Hurd noted, a main reason is the diminished tissue handling, because there is no packing.
HURD: Multiple clinical studies have shown laparoscopy to be associated with a lower adhesion rate, although it isn’t clear why.6,7 It may be related to decreased suturing.
DECHERNEY: Less bleeding occurs because surgeons are less aggressive laparoscopically than in laparotomy.
A 15-year-old undergoes removal of a dermoid cyst, which was shelled out laparoscopically.
PAGIDAS: This case is easier because the cyst has been successfully shelled out. It is not the spill of a cyst’s contents at surgery that creates adhesions, but a chronic leak, which can occur if you do not remove the cyst in its entirety.
Once the cyst has been excised completely, I would ensure hemostasis with bipolar cautery and reapproximate the edges. I would not suture. There seems to be no clear advantage to suturing. I would use hydroflotation. Although Ringer’s lactate solution has not been shown to be effective, it is safe and has no toxicity.
DECHERNEY: Would you remove the cyst via laparotomy?
PAGIDAS: I would do it laparoscopically, using the endobag to minimize spillage, even though we know that a spill doesn’t necessarily change the outcome. If spillage does occur, I would perform copious irrigation to ensure that nothing is left behind.
HURD: My priority would be minimizing the use of power on the ovary. Studies of ovarian drilling have demonstrated that burning an ovary stimulates adhesion formation.21,22 If the dermoid cyst spills, as happens occasionally, I perform copious rinsing until no more oil is visible on the surface of the peritoneal fluid.
I also would minimize the amount of ovarian capsule that is removed. Good studies of endometriomas have shown that the more capsulate that is removed, the more adhesions. Even if the capsule looks redundant and floppy, the concern should be to achieve hemostasis with bipolar cautery and then leave it alone.
DECHERNEY: Would you use crystalloids in this case?
HURD: Yes.
SANFILIPPO: If spillage occurs, I would ensure that the patient is taken out of the Trendelenburg position. I want to emphasize the importance of thorough irrigation to eliminate any material that could produce chemical peritonitis.
DECHERNEY: Over the years, I have seen a fair number of cases of Fitz-Hugh and Curtis syndrome. You rigorously lavage a ruptured dermoid cyst, which sometimes presents with low-grade fever, but always with pain.
I’m surprised that none of you would use Interceed, since wrapping the ovary is the only thing for which it has been clearly shown to be effective. Since the cortex is relatively avascular, you don’t get a lot of bleeding. Unfortunately, it is not technically easy to wrap the ovary.
HURD: Since we do not know the effect on future fertility of changing the ovarian surface, less would seem to be better in patients this young.
I have stopped doing difficult cases laparoscopically. For example, it is rare for me to operate laparoscopically on a patient with stage IV endometriosis, at least when it comes to infertility—I might consider laparoscopy for pain.
I think case selection plays a role as well, although there are few data to back that up. It is purely clinical opinion.
SANFILIPPO: We need a well-designed prospective study to explore the effects of laparotomy versus laparoscopy. Existing data are not clear. You would assume laparoscopy would be associated with less adhesion formation. But genetic or other factors may explain why patient A is more prone to adhesions than patient B.
Does anybody think carbon dioxide plays a role in adhesion formation?
HURD: In the laboratory, carbon dioxide increases cell growth.8 Without an increased carbon dioxide concentration in the atmosphere, cell cultures don’t grow well. This might suggest that the carbon dioxide used for laparoscopy could actually enhance adhesion growth. Fortunately, this does not appear to be the case clinically.
With myomectomy, the surgeon needs to plan ahead to maximize the number of myomas removed from a single incision.
DECHERNEY: What about second-look laparoscopies? Do any of you perform them after a patient has undergone lysis of adhesions?
SANFILIPPO: Only as part of a research protocol. It amazes me how rapidly adhesions can form and how dense they are 2 or more weeks after the initial laparoscopic surgery.
PAGIDAS: We tend to limit second-look laparoscopy to a research protocol, although it is sometimes valuable after laparoscopic or abdominal myomectomy, which has the highest incidence of adhesions. If the surgeon can perform a second look and lyse adhesions, he or she may potentially alter the reproductive outcome. However, with assisted reproductive techniques becoming integral to every infertility case, that approach has begun to go out of style.
SANFILIPPO: That’s a good point. With myomectomy, the surgeon needs to plan ahead to maximize the number of myomas removed from a single incision. If adhesions do occur, it is best if they occur toward the bladder rather than in the area of the tubes and ovaries.
HURD: For second-look laparoscopy, we must keep in mind the cost and the small but real risks of surgery. Until good controlled studies show a reasonable clinical advantage, this approach probably should remain a research protocol.
SANFILIPPO: The literature suggests it is helpful, but does not help fertility, so second-look laparoscopy is used mainly to evaluate adjunctive therapies. I don’t think anybody uses it as a primary therapy anymore.
Bowel obstruction still a risk, though rarely seen by Ob/Gyns
DECHERNEY: Although bowel obstruction is fairly common, Ob/Gyns do not often encounter it because it occurs relatively distant from the index surgery. Even though a patient may not experience bowel obstruction in the first year, an obstruction related to the index surgery is just as likely to develop 20 years later as 2 years later. These patients usually are treated by general surgeons. Still, we should beware of the potential for bowel obstruction and take steps to prevent it, if at all possible. Do you agree?
PAGIDAS: Yes. We tend to forget about bowel obstruction because we rarely follow patients past pregnancy or the first trimester if they are seeking infertility treatment.
HURD: The primary problem seems to be the abdominal wall incision. Fortunately, cesarean section seems to carry a decreased risk of abdominal wall adhesions, probably because the uterus serves as a splint over the incision.
The Pfannenstiel incision also appears to have some advantage. Both human and animal models suggest little advantage or disadvantage when peritoneal closure is compared to nonclosure.9,10
SANFILIPPO: I’m curious about how the panelists manage loose clips. If you are using an EndoGIA (US Surgical, Norwalk, Conn) or other stapling device and you have free-floating clips, do you make a concerted effort to find them? In some cases, they have been implicated in bowel adhesion and obstruction. I try to retrieve loose clips, whether open or closed.
HURD: The advantage of those devices is minimal tissue damage, and the clips are inert. In general, inert, nonreactive clips have not been implicated as much in adhesion formation. I retrieve them if I see them, but I don’t search them out.
PAGIDAS: I do the same. If the clips are visible, I remove them. But I would not repack the bowel or do anything more heroic than look in locations where they might be.
What drugs may inhibit inflammatory response?
DECHERNEY: What about use of pharmacologic agents to prevent adhesions? Is there reason to think research should focus on inhibiting the inflammatory response? How important is polymorphonuclear cell infiltration?
Cyclooxygenase (COX) 2 agents could be helpful for inhibition of platelet function, since they are low in side effects. Thus, high doses of these drugs might be effective. At one time, aspirin was proposed, but you’d have to give a human so much aspirin that her ears would ring.
PAGIDAS: Pharmacologic agents have a role, especially for dampening the inflammatory immune response. But you don’t want to dampen it completely because, as we observed, it is an important part of healing. The difficulty is finding a balance between allowing the tissue to heal and preventing adhesions.
HURD: We studied the ability of a water-soluble prostaglandin inhibitor to prevent postoperative adhesion formation. Like many other agents, we found only a partial response.11
DECHERNEY: With current options, the best you can aim for is a 50% reduction.
Adjunctive therapy likely to limit adhesion rate Hydroflotation
DECHERNEY: The original adjunctive therapy was 20 mg dexamethasone and 25 mg femergin in 200 cc of Ringer’s lactate, with an equivalent amount of dexamethasone and femergin every 4 hours for a total of 6 doses. I prescribed that regimen because I was trained to do so. I stopped after it became clear that hydroflotation from the fluid—not the medication itself—was responsible for the improvement.
I must admit I gave it up reluctantly; patients felt fabulous with those higher load doses of glucocorticoids after surgery.
Do any of you use crystalloids as adjunctive therapy?
SANFILIPPO: In the animal model, they are so rapidly absorbed that they aren’t effective. I was a strong advocate, but now I don’t use them at all.
HURD: A lot depends on the kind of case. For instance, at the end of an open myomectomy, the patient often is oozing, so you want to use a barrier that blood won’t affect.
For ovarian surgery, you might want to specifically target the ovaries with some kind of coverage. But when you are doing a broad lysis of adhesions, you have few choices to cover the pelvis. In those cases I use hydroflotation with Ringer’s lactate. Both human and animal studies have shown some benefit in preventing adhesions, and it appears to have little risk.12,13
It’s better than nothing, in my opinion.
DECHERNEY: Do you use dextran 70 or crystalloids?
HURD: I use Ringer’s lactate solution. I was trained in the dextran 70 era, and there were certain problems with that approach. Since studies have failed to show a consistent effect of dextran 70, I no longer use this solution.14,15
DECHERNEY: Another problem with crystalloids is that they leak, which is disconcerting to the patient.
HURD: They also can mask an injury to the bladder in difficult cases.
DECHERNEY: I agree that dextran 70 is only appropriate in certain cases, but it is a good hydroflotation agent. Every cubic centimeter of dextran 70 brings in 1.2 cc of transudate, so it hangs around for at least 4 days.
It is appropriate only for certain surface areas—mainly the cul-de-sac. It is harmful on raw surface areas on the lateral pelvic sidewall because it tends to push the ovary and tube to those areas. Unless you are doing a lot of work in the deep pelvis, I would avoid dextran 70.
A 45-year-old woman undergoes an abdominal hysterectomy. The cuff is closed and the ovaries are intact.
PAGIDAS: I would do nothing other than ensure adequate hemostasis, check that I have left no round surfaces and, probably, use hydroflotation. I see no advantage to barriers.
HURD: One of the main causes of adhesions is devascularized tissue, and the perfect devascularized tissue might be the vaginal cuff. Re-“peritonealizing” the cuff might be advantageous. Thus, I would use minimal sutures—probably a slowly absorbable, light polydioxanone suture to place the peritoneum over the cuff so there are no pedicles.
DECHERNEY: All the pedicles are exteriorized.
HURD: Yes, that could be. We don’t bring all of it down like we used to years ago, but we do cover the cuff.
PAGIDAS: I agree that closing the cuff and reperitonealizing may actually minimize formation of hematomas—clearly an advantage.
SANFILIPPO: I agree. I guess I’m old fashioned. If it looks good, then hopefully it will stimulate less adhesion formation, so peritonealization is important.
As far as the abdominal incision is concerned, I would not close that peritoneum. I’m convinced now that there is no advantage.
DECHERNEY: Reperitonealizing the cuff is controversial. Most gynecologic surgeons do not do it, the theory being that the peritoneum is being stretched, attenuating the vessels that go through it and thereby creating an ischemic barrier that contributes to adhesions. Personally, I like to do it because it looks better—and that is certainly an important aspect of a surgery. No evidence shows that it is bad or good, either way.
In addition, there have been allergic reactions, most of which seem to occur in patients with fluid overload; a lot of the dextran 70 is absorbed.
With infertility patients, even if you lyse dense adhesions, you do not render the ovarian surface normal.
SANFILIPPO: Dextran 70 is not recommended for patients with sugar beet allergy, either.
We completed a study in a rabbit model, in which the peritoneal cavity was lavaged with either chlorhexidine or iodine.16 At the time of second-look surgery, the rate of adhesion formation was decreased, especially with the iodine preparation. I would hope that this has potential in humans.
DECHERNEY: In your lavage procedures to prevent adhesions, do any of you use heparin?
HURD: No.
PAGIDAS: I don’t think any evidence suggests that local administration changes the outcome.
DECHERNEY: I agree. When heparin has been used, the doses have been so low that it was not terribly helpful. And when you consider that hemorrhage can be a problem, heparin is probably deleterious rather than helpful.
Barriers
DECHERNEY: What about barriers? The first to become available, Interceed (Gynecare, a division of Ethicon, Somerville, NJ), is oxidized cellulose, similar to Surgicell (Johnson & Johnson, New Brunswick, NJ). Since it gelates quickly, there is no fenestration, so the fibrin is unable to penetrate. However, if the patient has bleeding by capillary action, the raw surface just moves from one side of the Interceed barrier to the other.
What has been your experience? Do you use it?
PAGIDAS: I do not use Interceed, although prospective randomized trials and a meta-analysis confirmed its benefits in de novo formation and reformation.17-19 I don’t use it because it requires complete hemostasis. Also, with the surfaces we work on—notably, the ovary and tube—it is difficult to apply to just 1 surface area. From a clinical perspective, I appreciate the data, but it is hard to ensure a good application to optimize its effectiveness.
A 29-year-old woman is undergoing her third cesarean section, although adhesions cause difficulty getting through anteriorly.
DECHERNEY: In this case, will you use barriers or re-peritonealize the surface? Will you do a 1- or a 2-layered closure?
HURD: In these cases, I have not been doing anything, since there is no peritoneum to reperitonealize—just old scar tissue. I assume that the uterus will immediately readhere to the anterior peritoneum where it was before.
DECHERNEY: You would use a barrier?
HURD: No. I would not.
PAGIDAS: I would take the same approach, although I have not performed cesarean sections in about 4 years. I don’t think any intervention would change the outcome. And, as Dr. Hurd mentioned, that is pretty much old scar tissue anyway.
SANFILIPPO: I use Interceed, but I agree with you about its limitations. Meticulous hemostasis is a prerequisite.
HURD: With infertility patients, even if you lyse dense adhesions, you do not render the ovarian surface normal. If those patients have dense adhesions of the ovary or the sidewall, I generally leave them alone, and I try to avoid putting Interceed around the ovaries. No study has shown that using Interceed improves pregnancy.
In contrast, when a chronic pain patient’s ovaries are densely adherent to the cul-de-sac, which appears to be highly associated with dyspareunia, I lyse the adhesions, achieve meticulous hemostasis, and then use Interceed. It is hard to demonstrate in a study that this approach decreases the chance of pain. Even so, it certainly does decrease the chance of the ovaries being adherent.
DECHERNEY: One issue with Interceed is that we don’t know what happens to it once the abdomen is closed. It may migrate significantly.
Psychological issues may also be involved. For example, patients with multiple somatic complaints may be less likely to benefit from lysis of adhesions.
PAGIDAS: Right. Interestingly, a meta-analysis of all the randomized trials involving mechanical barriers found no correlation to pregnancy outcome or pelvic pain.18 If we were to consider new trials, the psychological aspect would be worth looking into.
Seprafilm
DECHERNEY: Let’s move on to Seprafilm (Genzyme, Cambridge, Mass). What is it and how useful is it?
HURD: Seprafilm is modified hyaluronic acid, which forms a brittle, thin plastic layer. It is somewhat difficult to work with but, once it is in place, seems to adhere well. The presence of blood does not appear to be a problem, since the Seprafilm forms an impermeable barrier—unless it breaks. I have found it especially useful in myomectomies, which produce postoperative oozing through the incisions no matter how hard you try to prevent it.
In addition, in open cases, surfaces can easily be covered with this material. Unfortunately, it can’t be used laparoscopically because it is so brittle.
DECHERNEY: In my opinion, that is its major drawback.
PAGIDAS: In cardiac surgery, Seprafilm appears to work quite effectively.
SANFILIPPO: The manufacturer initially focused on surgeons in the context of sigmoid colon surgery, and it seems to work well in that setting.
Intergel
DECHERNEY: That brings us to the current state of the art: gels. I’m sure you all are familiar with Intergel (Lifecore Biomedical, Chaska, Minn), which is a ferrous derivative of hyaluronic acid that works by coating the raw surface areas. It also has the theoretical advantage of ease of use. Have any of you used Intergel?
HURD: As you know it was only recently approved by the US Food and Drug Administration, but not for laparoscopic use. It may work best on abraded bowel, which is avoided by laparoscopic surgery.
As you are probably also aware, the manufacturer recently took it off the market because of unusual side effects, namely a chemical peritonitis. Although peritonitis was cited as being rare, we encountered it in probably half the patients we operated on.
PAGIDAS: When we used it on hospitalized patients, the peritonitis wasn’t that obvious, since there was an expectation of significant pain. However, when we used Intergel on short-stay patients, we had to readmit them and do a full workup because we were concerned about bowel perforations. I’m surprised the manufacturer didn’t take it off the market sooner.
DECHERNEY: It seems strange, since Intergel has been used in Europe for a while now. I’ve used it in only 1 case and didn’t have adverse effects. It seemed to work well.
SANFILIPPO: It had all the right ingredients for success. It is unfortunate that these side effects have prohibited its use.
There is no question that adhesion prevention is one of the unmet challenges in all surgeries, especially reproductive surgery.
Gels and the cost factor
DECHERNEY: Other gels are in the pipeline. I’m reminded of plasminogen activator, which is a powerful antiadhesive agent that lyses fibrin effectively. Unfortunately, it is prohibitively expensive.
The next phase likely will involve the so-called polymers. If you spray them on your hand, they are activated by light or another chemical and become a cellophane-like substance. The problem is viscosity. If sprayed on the sidewall, for instance, they run halfway down before they are activated, so the entire surface does not get covered.
PAGIDAS: One concern with polymers is that they could actually bring surfaces together when they polymerize. We still have a lot to learn.
DECHERNEY: Let’s say a new gel comes on the market that takes reformation adhesions from 90% to 10%, as opposed to 40% recurrence. Would you use it in all 4 of the cases we discuss here?
HURD: If it was that effective and had no adverse effects, it would be wonderful.
The cesarean-delivery case is different, as healing in a pregnant patient is 1 concern; the size of the uterus also has an effect. If the patient is breastfeeding, you would want to make sure the gel didn’t interfere.
PAGIDAS: We desperately need a product that can minimize adhesions regardless of the route of access or type of procedure. Even though we lack data on outcomes, I predict wide use of such a product, assuming it is nontoxic and effective.
DECHERNEY: What if it costs $1,000 a case?
SANFILIPPO: If it prevents 1 bowel obstruction, it still would be cost-effective.
DECHERNEY: The incidence of bowel obstruction for total abdominal hysterectomy is 2%, and 5% for radical hysterectomy.
HURD: We must be careful of the cost-benefit ratio. Bowel obstruction after gynecologic surgery is uncommon.
Is the gel worth $100? $1,000? $3,000? It’s difficult to say, but the more expensive it is, the less likely it will find widespread use.
PAGIDAS: I agree. We should remember that we still need to maintain microsurgical techniques and appropriate tissue handling, as well as avoid ischemia and infection.
Looking for the magic bullet
DECHERNEY: What is the future of adjunctive therapy?
SANFILIPPO: I would focus on noxythiolin; it has potential. Calcium channel blockers for adhesion prevention have also been studied.20 In 1 investigation involving a rat model, the calcium channel blocker verapamil as well as several other agents—including vitamin E, carboxymethylcellulose, cyclosporin, aprotinin, and tenoxicam—were compared with respect to tissue effects. A beneficial effect was noted with all agents except cyclosporin and carboxymethylcellulose.
Whoever succeeds in manufacturing an effective preventive will be a winner.
HURD: There is no question that adhesion prevention is one of the unmet challenges in all surgeries, especially reproductive surgery. The most effective agent would be applied intraabdominally, since any systemic agent that stops adhesion formation would probably decrease wound healing as well.
I hope the most effective agents can be used in both laparoscopy and laparotomy, and that they will decrease the adhesion-formation rate by more than 50%. We need to find the magic bullet that can cover the entire pelvis—if not the entire abdomen.
Dr. DeCherney reports small holdings with Lifecore Biomedical and Johnson & Johnson. Drs. Hurd and Pagidas report no financial relationship with any companies whose products are mentioned in this article. Dr. Sanfilippo serves on the speaker’s bureau for Berlex, Ortho Pharmaceuticals, and Wyeth, and receives grant support from Eli Lilly and Wyeth.
1. Operative Laparoscopy Study Group Postoperative adhesion development after operative laparoscopy: evaluation at early second look procedures. Fertil Steril. 1991;55:700-704.
2. Tulandi T, Murray C, Guralnick M. Adhesion formation and reproductive outcome after myomectomy and second-look laparoscopy. Obstet Gynecol. 1993;82:213-215.
3. Larsson B, Perbeck L. The possible advantage of keeping the uterine and intestinal serosa irrigated with saline to prevent intraabdominal adhesions in operations for infertility. An experimental study in rats. Acta Chir Scand Suppl. 1985;530:15-18.
4. Zamir G, Bloom AI, Reissman P. Prevention of intestinal adhesions after laparotomy in a rat model—a randomized prospective study. Res Exp Med (Berl). 1998;197:349-353.
5. Hurd WW, Himebaugh KS, Cofer KF, Gauvin JM, Elkins TE. The etiology of closure-related adhesion formation after wedge resection of the rabbit ovary. J Reprod Med. 1993;38:465-468.
6. Chen MD, Teigen GA, Reynolds HT, Johnson PR, Fowler JM. Laparoscopy versus laparotomy: an evaluation of adhesion formation after pelvic and paraaortic lymphadenectomy in a porcine model. Am J Obstet Gynecol. 1998;178:499-503.
7. Polymeneas G, Theodosopoulos T, Stamatiadis A, Kourias E. A comparative study of postoperative adhesion formation after laparoscopic vs. open cholecystectomy. Surg Endosc. 2001;15:41-43.
8. Smidt VJ, Singh DM, Hurteau JA, Hurd WW. Effect of carbon dioxide on human ovarian carcinoma cell growth. Am J Obstet Gynecol. 2001;185:1314-1317.
9. Tulandi T, Hum HS, Gelfand MM. Closure of laparotomy incisions with or without peritoneal suturing and second look laparoscopy. Am J Obstet Gynecol. 1988;158:536-537.
10. Kapur ML, Daneswar A, Chopra P. Evaluation of peritoneal closure at laparotomy. Am J Surg. 1979;137:650-652.
11. Cofer KF, Himebaugh KS, Gauvin JM, Hurd WW. Inhibition of adhesion reformation in the rabbit model by meclofenamate: an inhibitor of both prostaglandin and leukotriene production. Fertil Steril. 1994;62:1262-1265.
12. Elkelani OA, Molinas CR, Mynbaev O, Koninckx PR. Prevention of adhesions with crystalloids during laparoscopic surgery in mice. J Am Assoc Gynecol Laparosc. 2002;9:447-452.
13. Chan KL, Marino T, Qu WM, Tulandi T. Effects of intraperitoneal Ringer’s lactate instillation and infusion on postsurgical adhesion formation. J Gynecol Surg. 1995;11:241-243.
14. Watson A, Vandekerckhove P, Lilford R. Pharmacological adjuvants during infertility surgery: a systematic review of evidence derived from randomized controlled trial. Hum Fertil (Camb). 1999;2:149-157.
15. Larson B. Dextran—later clinical studies. clinical and experimental evaluation of different adjuvant therapies. Prog Clin Biol Res. 1990;358:165-175.
16. Roberts L, Sanfilippo JS, Ehrlich G, Raab S. Adhesion formation, peritoneal effects and the presence of bacterial biofilms after laparoscopic lavage in an animal model of pelvic inflammatory disease. J Am Assoc Gynecol Laparosc. 2002;9:4.-
17. Barrier agents for preventing adhesions after surgery for subfertility Cochrane Database Syst Rev. 2000;CD000475.-
18. Wiseman DM, Trout JR, Franklin RR, Diamond MP. Metaanalysis of the safety and efficacy of an adhesion barrier (Interceed TC7) in laparotomy. J Reprod Med. 1999;44:325-331.
19. Sawada T, Nishizawa H, Nishio E, Kadowaki M. Postoperative adhesion prevention with an oxidized regenerated cellulose adhesion barrier in infertile women. J Reprod. Med. 2000;45:387-389.
20. Uzunkoy A, Akinci OF, Coskun A, Aslan O, Kocyigit A. Effects of antiadhesive agents on the healing of intestinal anastomosis. Dis Colon Rectum. 2000;43:370-375.
21. Farquhar C, Vandekerckhove P, Arnot M, Lilford R. Laparoscopic “drilling” by diathermy or laser for ovulation induction in anovulatory polycystic ovary syndrome. Cochrane Database Syst Rev. 2000(2);CD001122.-
22. Greenblatt EM, Casper RF. Laparoscopic ovarian drilling in women with polycystic ovarian syndrome. Prog Clin Biol Res. 1993;381:129-138.


-

-

- Approximately 40% of people who undergo primary surgery develop adhesions and reformation occurs in 80% to 90% of cases.
- Microsurgical techniques such as gentle handling of tissues, careful hemostasis, and avoidance of heat may help reduce the incidence.
- Laparoscopy appears to be less likely to produce adhesions than laparotomy.
- Ob/Gyns should be aware of the potential for adhesion-related bowel obstruction and take steps to prevent it.
Are adhesions a pathologic response to injury or a normal aspect of healing? Can they be avoided, or are preventive efforts part of the problem? How useful are the different barriers in gynecologic surgery? What is the ideal adjuvant?
OBG Management convened a panel of experts to explore these and other questions.
Common problem, high recurrence rate
DECHERNEY: Adhesion formation is serious because it is associated with clinical entities such as infertility, pelvic pain, and bowel obstruction. We all agree that approximately 40% of people who undergo primary surgery develop adhesions and that 80% to 90% of patients who undergo lysis develop recurrent adhesions.
SANFILIPPO: One study several years ago explored adhesion formation.1 Unfortunately, no matter how meticulous the surgeon is, adhesions will form, even with microsurgical techniques and carefully ensured hemostasis.
HURD: The number of patients with significant adhesion formation after some gynecologic procedures has been reported to be greater than 90%.2
DECHERNEY: That higher incidence usually occurs after general surgery—and there’s a reason it is so high: General surgeons don’t use adjunctive therapy. They are critical of it. It is to our credit as gynecologic surgeons that we adopted adjunctive therapies about 15 years ago with the introduction of dextran 70 (Hyskon; Medisan Pharmaceuticals, Parsippany, NJ).
PAGIDAS: If anything, the pelvis seems to have even more of a predilection for adhesion formation than the abdomen, probably because of the close proximity of structures.
How and why adhesions form
DECHERNEY: What is the pathophysiology of adhesion formation? Let’s say you have 2 raw surface areas. What happens?
The process
PAGIDAS: The increase in leukotrienes and prostaglandins and the decrease in plasminogen activity (which actually initiates the inflammation) appear to be significant.
HURD: Vessel permeability also increases, and inflammatory cells leak through the vessels and set up a matrix for adhesion formation.
DECHERNEY: So we have 2 raw surface areas with fibrin leaking out and forming bridges between them.
PAGIDAS: The key is that it takes 2 surfaces to form these bridges. As I mentioned, the greater proximity of pelvic structures—particularly around the tube and ovary—probably contributes to adhesion formation.
DECHERNEY: Macrophage activity also is important. The macrophage “migrates” along these fibrin bridges and lays down collagen over a period of time. Then the collagen becomes organized and, eventually, vascular.
Window of opportunity
SANFILIPPO: Adhesion formation probably occurs and is pretty well established within 5 to 7 days of the precipitating event—usually surgery. Once that process is under way, attempts to halt it yield diminishing returns. Unfortunately, we don’t know how to interfere with it in a positive way.
HURD: Under normal conditions, there seems to be a balance between fibrin deposition and fibrinolysis. In some tissues, however, these functions become imbalanced. This disparity may contribute more to adhesions than the actual laying down of fibrin—especially in tissue that is hypoxic.
DECHERNEY: Would you say that adhesion formation represents normal or abnormal healing?
HURD: It is one of the body’s normal protective mechanisms and an important part of healing. Without it, any abdominal injury would likely result in death.
SANFILIPPO: I don’t think it differs that much from processes that occur externally. For example, if you get cut deeply enough, you develop a scar. Is that scar part of the normal healing process? It is.
PAGIDAS: Right. It is a normal process of tissue remodeling. The question is: What allows it to go astray?
What surgical techniques help prevent adhesions?
DECHERNEY: Let’s review the aspects of surgical technique that are important for adhesion prevention.
PAGIDAS: I emphasize the value of microsurgical techniques, which help to minimize severe tissue handling. It also is important to keep surfaces moistened so they don’t desiccate.
SANFILIPPO: I agree with Dr. Pagidas about microsurgical techniques such as gentle tissue handling, careful hemostasis, and keeping tissues moist. If we follow these principles, we create an environment that minimizes the potential for adhesion formation.
HURD: The findings of many well-controlled animal studies have been surprising. For example, it is difficult to demonstrate that drying of tissue increases adhesions.3 Probably the greatest contributor to adhesions in these models was abrasion.4 One way that laparoscopic surgery decreases adhesions is by avoiding abrasion of the bowel mucosa, which occurs specifically with packing.
A 38-year-old mother of 2 undergoes myomectomy for menorrhagia.
SANFILIPPO: The initial question is: Can this case be managed laparoscopically? I do myomectomies laparoscopically whenever possible, although I do close the uterus with a minilaparotomy incision. The reason is my strong concern about reapproximating the myometrium, since wound dehiscence sometimes occurs at the site of myoma removal.
In this case, depending on the size of the myomas, I would do as much as possible laparoscopically and then reapproximate the myometrium. I would plan my incisions carefully, to maximize the number of myomas that can be removed. I would end with meticulous hemostasis and, assuming it is successful, use a barrier over the incision—in this case, Interceed.
HURD: Does the patient desire future childbearing? If so, I would avoid the laparoscopic approach because of the possibly increased risk of uterine rupture during pregnancy. If she isn’t planning pregnancy, there are more options.
The next question is: How many myomas are there, and where are they located? If they are intrauterine, a hysteroscopic approach would avoid extrauterine adhesions. If they are multiple and large, I am pretty much limited to laparotomy. If there is 1 or only a few myomas, a laparoscopic approach would be best.
I have not used Interceed. In laparoscopic cases, I worry that it would create more problems because, as you allow the carbon dioxide to decrease at the end of a case, oozing begins. Instead of a barrier, I would use limited hydroflotation.
SANFILIPPO: That’s a good point. At the end of the myomectomy, with the laparoscope in place, I decrease the insufflation, eliminating the tamponade effect. Then, assuming good hemostasis, I apply Interceed.
HURD: With open cases, I use Seprafilm, which takes practice because, as it gets wet, it sticks to anything, including gloves and instruments. But if you can put it down dry on the uterus, it sticks and stays in place. If oozing occurs, it seems to block or stop it.
PAGIDAS: I want to reiterate the importance of determining whether childbearing is an issue. In this case, the biggest concern is the risk of adhesions developing on our incision, so I would use a barrier. My preference would be Seprafilm or GoreTex. If we can limit adhesions at the incision site, then hopefully we can minimize bowel and tuboovarian adhesions, too.
In open cases, one thing we can do to minimize the risk of adhesions is to pack gently when needed. Also, we should avoid using packing to reposition the bowel.
Another factor frequently overlooked is the application of heat, which appears to be a very effective way to create adhesions. This probably isn’t an issue for laparoscopic cases, but when you use irrigation fluid in an open case, watch the temperature. If it feels hot to you, you need to worry about potential injury to the bowel surfaces.
PAGIDAS: That is critical. In abdominal cases you want to make sure irrigation fluid is warm, but not too warm, because heat increases the vascular permeability of vessels and leads to more macrophages, more prostaglandins, and more leukotrienes.
HURD: Another important element is the type of suture material used.
DECHERNEY: Overall, we need to minimize the use of sutures. For example, when I am operating laparoscopically on an ovarian cyst, I try to apply bipolar energy to the edges so that they will coapt without a stitch.
HURD: When it first became clear that suturing ovaries increased adhesion formation, we conducted a controlled trial of different kinds of sutures in animals. Not surprisingly, we found that the less reactive the suture, the fewer adhesions.5 Sutures that are absorbed more slowly, such as polydioxanone, seem to be less reactive.
Obviously, inert sutures like nylon are the least reactive, but they are permanent. It is assumed that animal-protein sutures such as chromic and plain gut are the most reactive, although I am not sure there are sufficient data to support that conclusion.
Multiple clinical studies have shown laparoscopy to be associated with a lower adhesion rate, although it isn’t clear why. It may be related to decreased suturing.
DECHERNEY: Bulk is important, too—that is, the number of throws in the suture. When Vicryl (polyglactin 910) became available, we conducted a study in mice using proportionately small Vicryl plaques to determine whether this would be good a barrier (A. DeCherney, MD, unpublished data). It caused a tremendous amount of adhesion because so much foreign matter was applied.
We also did a study using human-size titanium clips in rats (A. DeCherney, MD, unpublished data). Not surprisingly, there was a lot of adhesion formation.
FIGURE 1 Pelvic adhesions: How they develop, problems they cause
Adhesions occur when 2 or more raw surfaces are exposed to leaking fibrin, which forms a bridge between the surfaces. Macrophages “migrate” along these bridges, depositing collagen.
The pelvis has a greater predilection for adhesions than the abdomen because of the close proximity of structures.
Although adhesions represent one of the body’s protective mechanisms, they may also cause pain or interfere with fertility, bowel function, or other processes.
Laparoscopy versus laparotomy: More adhesions in open cases?
DECHERNEY: Based on all the techniques we have learned from microsurgery—with the exception of magnification—it appears that laparoscopic procedures are less likely to cause adhesions than laparotomy. Do you agree?
PAGIDAS: I think so. As Dr. Hurd noted, a main reason is the diminished tissue handling, because there is no packing.
HURD: Multiple clinical studies have shown laparoscopy to be associated with a lower adhesion rate, although it isn’t clear why.6,7 It may be related to decreased suturing.
DECHERNEY: Less bleeding occurs because surgeons are less aggressive laparoscopically than in laparotomy.
A 15-year-old undergoes removal of a dermoid cyst, which was shelled out laparoscopically.
PAGIDAS: This case is easier because the cyst has been successfully shelled out. It is not the spill of a cyst’s contents at surgery that creates adhesions, but a chronic leak, which can occur if you do not remove the cyst in its entirety.
Once the cyst has been excised completely, I would ensure hemostasis with bipolar cautery and reapproximate the edges. I would not suture. There seems to be no clear advantage to suturing. I would use hydroflotation. Although Ringer’s lactate solution has not been shown to be effective, it is safe and has no toxicity.
DECHERNEY: Would you remove the cyst via laparotomy?
PAGIDAS: I would do it laparoscopically, using the endobag to minimize spillage, even though we know that a spill doesn’t necessarily change the outcome. If spillage does occur, I would perform copious irrigation to ensure that nothing is left behind.
HURD: My priority would be minimizing the use of power on the ovary. Studies of ovarian drilling have demonstrated that burning an ovary stimulates adhesion formation.21,22 If the dermoid cyst spills, as happens occasionally, I perform copious rinsing until no more oil is visible on the surface of the peritoneal fluid.
I also would minimize the amount of ovarian capsule that is removed. Good studies of endometriomas have shown that the more capsulate that is removed, the more adhesions. Even if the capsule looks redundant and floppy, the concern should be to achieve hemostasis with bipolar cautery and then leave it alone.
DECHERNEY: Would you use crystalloids in this case?
HURD: Yes.
SANFILIPPO: If spillage occurs, I would ensure that the patient is taken out of the Trendelenburg position. I want to emphasize the importance of thorough irrigation to eliminate any material that could produce chemical peritonitis.
DECHERNEY: Over the years, I have seen a fair number of cases of Fitz-Hugh and Curtis syndrome. You rigorously lavage a ruptured dermoid cyst, which sometimes presents with low-grade fever, but always with pain.
I’m surprised that none of you would use Interceed, since wrapping the ovary is the only thing for which it has been clearly shown to be effective. Since the cortex is relatively avascular, you don’t get a lot of bleeding. Unfortunately, it is not technically easy to wrap the ovary.
HURD: Since we do not know the effect on future fertility of changing the ovarian surface, less would seem to be better in patients this young.
I have stopped doing difficult cases laparoscopically. For example, it is rare for me to operate laparoscopically on a patient with stage IV endometriosis, at least when it comes to infertility—I might consider laparoscopy for pain.
I think case selection plays a role as well, although there are few data to back that up. It is purely clinical opinion.
SANFILIPPO: We need a well-designed prospective study to explore the effects of laparotomy versus laparoscopy. Existing data are not clear. You would assume laparoscopy would be associated with less adhesion formation. But genetic or other factors may explain why patient A is more prone to adhesions than patient B.
Does anybody think carbon dioxide plays a role in adhesion formation?
HURD: In the laboratory, carbon dioxide increases cell growth.8 Without an increased carbon dioxide concentration in the atmosphere, cell cultures don’t grow well. This might suggest that the carbon dioxide used for laparoscopy could actually enhance adhesion growth. Fortunately, this does not appear to be the case clinically.
With myomectomy, the surgeon needs to plan ahead to maximize the number of myomas removed from a single incision.
DECHERNEY: What about second-look laparoscopies? Do any of you perform them after a patient has undergone lysis of adhesions?
SANFILIPPO: Only as part of a research protocol. It amazes me how rapidly adhesions can form and how dense they are 2 or more weeks after the initial laparoscopic surgery.
PAGIDAS: We tend to limit second-look laparoscopy to a research protocol, although it is sometimes valuable after laparoscopic or abdominal myomectomy, which has the highest incidence of adhesions. If the surgeon can perform a second look and lyse adhesions, he or she may potentially alter the reproductive outcome. However, with assisted reproductive techniques becoming integral to every infertility case, that approach has begun to go out of style.
SANFILIPPO: That’s a good point. With myomectomy, the surgeon needs to plan ahead to maximize the number of myomas removed from a single incision. If adhesions do occur, it is best if they occur toward the bladder rather than in the area of the tubes and ovaries.
HURD: For second-look laparoscopy, we must keep in mind the cost and the small but real risks of surgery. Until good controlled studies show a reasonable clinical advantage, this approach probably should remain a research protocol.
SANFILIPPO: The literature suggests it is helpful, but does not help fertility, so second-look laparoscopy is used mainly to evaluate adjunctive therapies. I don’t think anybody uses it as a primary therapy anymore.
Bowel obstruction still a risk, though rarely seen by Ob/Gyns
DECHERNEY: Although bowel obstruction is fairly common, Ob/Gyns do not often encounter it because it occurs relatively distant from the index surgery. Even though a patient may not experience bowel obstruction in the first year, an obstruction related to the index surgery is just as likely to develop 20 years later as 2 years later. These patients usually are treated by general surgeons. Still, we should beware of the potential for bowel obstruction and take steps to prevent it, if at all possible. Do you agree?
PAGIDAS: Yes. We tend to forget about bowel obstruction because we rarely follow patients past pregnancy or the first trimester if they are seeking infertility treatment.
HURD: The primary problem seems to be the abdominal wall incision. Fortunately, cesarean section seems to carry a decreased risk of abdominal wall adhesions, probably because the uterus serves as a splint over the incision.
The Pfannenstiel incision also appears to have some advantage. Both human and animal models suggest little advantage or disadvantage when peritoneal closure is compared to nonclosure.9,10
SANFILIPPO: I’m curious about how the panelists manage loose clips. If you are using an EndoGIA (US Surgical, Norwalk, Conn) or other stapling device and you have free-floating clips, do you make a concerted effort to find them? In some cases, they have been implicated in bowel adhesion and obstruction. I try to retrieve loose clips, whether open or closed.
HURD: The advantage of those devices is minimal tissue damage, and the clips are inert. In general, inert, nonreactive clips have not been implicated as much in adhesion formation. I retrieve them if I see them, but I don’t search them out.
PAGIDAS: I do the same. If the clips are visible, I remove them. But I would not repack the bowel or do anything more heroic than look in locations where they might be.
What drugs may inhibit inflammatory response?
DECHERNEY: What about use of pharmacologic agents to prevent adhesions? Is there reason to think research should focus on inhibiting the inflammatory response? How important is polymorphonuclear cell infiltration?
Cyclooxygenase (COX) 2 agents could be helpful for inhibition of platelet function, since they are low in side effects. Thus, high doses of these drugs might be effective. At one time, aspirin was proposed, but you’d have to give a human so much aspirin that her ears would ring.
PAGIDAS: Pharmacologic agents have a role, especially for dampening the inflammatory immune response. But you don’t want to dampen it completely because, as we observed, it is an important part of healing. The difficulty is finding a balance between allowing the tissue to heal and preventing adhesions.
HURD: We studied the ability of a water-soluble prostaglandin inhibitor to prevent postoperative adhesion formation. Like many other agents, we found only a partial response.11
DECHERNEY: With current options, the best you can aim for is a 50% reduction.
Adjunctive therapy likely to limit adhesion rate Hydroflotation
DECHERNEY: The original adjunctive therapy was 20 mg dexamethasone and 25 mg femergin in 200 cc of Ringer’s lactate, with an equivalent amount of dexamethasone and femergin every 4 hours for a total of 6 doses. I prescribed that regimen because I was trained to do so. I stopped after it became clear that hydroflotation from the fluid—not the medication itself—was responsible for the improvement.
I must admit I gave it up reluctantly; patients felt fabulous with those higher load doses of glucocorticoids after surgery.
Do any of you use crystalloids as adjunctive therapy?
SANFILIPPO: In the animal model, they are so rapidly absorbed that they aren’t effective. I was a strong advocate, but now I don’t use them at all.
HURD: A lot depends on the kind of case. For instance, at the end of an open myomectomy, the patient often is oozing, so you want to use a barrier that blood won’t affect.
For ovarian surgery, you might want to specifically target the ovaries with some kind of coverage. But when you are doing a broad lysis of adhesions, you have few choices to cover the pelvis. In those cases I use hydroflotation with Ringer’s lactate. Both human and animal studies have shown some benefit in preventing adhesions, and it appears to have little risk.12,13
It’s better than nothing, in my opinion.
DECHERNEY: Do you use dextran 70 or crystalloids?
HURD: I use Ringer’s lactate solution. I was trained in the dextran 70 era, and there were certain problems with that approach. Since studies have failed to show a consistent effect of dextran 70, I no longer use this solution.14,15
DECHERNEY: Another problem with crystalloids is that they leak, which is disconcerting to the patient.
HURD: They also can mask an injury to the bladder in difficult cases.
DECHERNEY: I agree that dextran 70 is only appropriate in certain cases, but it is a good hydroflotation agent. Every cubic centimeter of dextran 70 brings in 1.2 cc of transudate, so it hangs around for at least 4 days.
It is appropriate only for certain surface areas—mainly the cul-de-sac. It is harmful on raw surface areas on the lateral pelvic sidewall because it tends to push the ovary and tube to those areas. Unless you are doing a lot of work in the deep pelvis, I would avoid dextran 70.
A 45-year-old woman undergoes an abdominal hysterectomy. The cuff is closed and the ovaries are intact.
PAGIDAS: I would do nothing other than ensure adequate hemostasis, check that I have left no round surfaces and, probably, use hydroflotation. I see no advantage to barriers.
HURD: One of the main causes of adhesions is devascularized tissue, and the perfect devascularized tissue might be the vaginal cuff. Re-“peritonealizing” the cuff might be advantageous. Thus, I would use minimal sutures—probably a slowly absorbable, light polydioxanone suture to place the peritoneum over the cuff so there are no pedicles.
DECHERNEY: All the pedicles are exteriorized.
HURD: Yes, that could be. We don’t bring all of it down like we used to years ago, but we do cover the cuff.
PAGIDAS: I agree that closing the cuff and reperitonealizing may actually minimize formation of hematomas—clearly an advantage.
SANFILIPPO: I agree. I guess I’m old fashioned. If it looks good, then hopefully it will stimulate less adhesion formation, so peritonealization is important.
As far as the abdominal incision is concerned, I would not close that peritoneum. I’m convinced now that there is no advantage.
DECHERNEY: Reperitonealizing the cuff is controversial. Most gynecologic surgeons do not do it, the theory being that the peritoneum is being stretched, attenuating the vessels that go through it and thereby creating an ischemic barrier that contributes to adhesions. Personally, I like to do it because it looks better—and that is certainly an important aspect of a surgery. No evidence shows that it is bad or good, either way.
In addition, there have been allergic reactions, most of which seem to occur in patients with fluid overload; a lot of the dextran 70 is absorbed.
With infertility patients, even if you lyse dense adhesions, you do not render the ovarian surface normal.
SANFILIPPO: Dextran 70 is not recommended for patients with sugar beet allergy, either.
We completed a study in a rabbit model, in which the peritoneal cavity was lavaged with either chlorhexidine or iodine.16 At the time of second-look surgery, the rate of adhesion formation was decreased, especially with the iodine preparation. I would hope that this has potential in humans.
DECHERNEY: In your lavage procedures to prevent adhesions, do any of you use heparin?
HURD: No.
PAGIDAS: I don’t think any evidence suggests that local administration changes the outcome.
DECHERNEY: I agree. When heparin has been used, the doses have been so low that it was not terribly helpful. And when you consider that hemorrhage can be a problem, heparin is probably deleterious rather than helpful.
Barriers
DECHERNEY: What about barriers? The first to become available, Interceed (Gynecare, a division of Ethicon, Somerville, NJ), is oxidized cellulose, similar to Surgicell (Johnson & Johnson, New Brunswick, NJ). Since it gelates quickly, there is no fenestration, so the fibrin is unable to penetrate. However, if the patient has bleeding by capillary action, the raw surface just moves from one side of the Interceed barrier to the other.
What has been your experience? Do you use it?
PAGIDAS: I do not use Interceed, although prospective randomized trials and a meta-analysis confirmed its benefits in de novo formation and reformation.17-19 I don’t use it because it requires complete hemostasis. Also, with the surfaces we work on—notably, the ovary and tube—it is difficult to apply to just 1 surface area. From a clinical perspective, I appreciate the data, but it is hard to ensure a good application to optimize its effectiveness.
A 29-year-old woman is undergoing her third cesarean section, although adhesions cause difficulty getting through anteriorly.
DECHERNEY: In this case, will you use barriers or re-peritonealize the surface? Will you do a 1- or a 2-layered closure?
HURD: In these cases, I have not been doing anything, since there is no peritoneum to reperitonealize—just old scar tissue. I assume that the uterus will immediately readhere to the anterior peritoneum where it was before.
DECHERNEY: You would use a barrier?
HURD: No. I would not.
PAGIDAS: I would take the same approach, although I have not performed cesarean sections in about 4 years. I don’t think any intervention would change the outcome. And, as Dr. Hurd mentioned, that is pretty much old scar tissue anyway.
SANFILIPPO: I use Interceed, but I agree with you about its limitations. Meticulous hemostasis is a prerequisite.
HURD: With infertility patients, even if you lyse dense adhesions, you do not render the ovarian surface normal. If those patients have dense adhesions of the ovary or the sidewall, I generally leave them alone, and I try to avoid putting Interceed around the ovaries. No study has shown that using Interceed improves pregnancy.
In contrast, when a chronic pain patient’s ovaries are densely adherent to the cul-de-sac, which appears to be highly associated with dyspareunia, I lyse the adhesions, achieve meticulous hemostasis, and then use Interceed. It is hard to demonstrate in a study that this approach decreases the chance of pain. Even so, it certainly does decrease the chance of the ovaries being adherent.
DECHERNEY: One issue with Interceed is that we don’t know what happens to it once the abdomen is closed. It may migrate significantly.
Psychological issues may also be involved. For example, patients with multiple somatic complaints may be less likely to benefit from lysis of adhesions.
PAGIDAS: Right. Interestingly, a meta-analysis of all the randomized trials involving mechanical barriers found no correlation to pregnancy outcome or pelvic pain.18 If we were to consider new trials, the psychological aspect would be worth looking into.
Seprafilm
DECHERNEY: Let’s move on to Seprafilm (Genzyme, Cambridge, Mass). What is it and how useful is it?
HURD: Seprafilm is modified hyaluronic acid, which forms a brittle, thin plastic layer. It is somewhat difficult to work with but, once it is in place, seems to adhere well. The presence of blood does not appear to be a problem, since the Seprafilm forms an impermeable barrier—unless it breaks. I have found it especially useful in myomectomies, which produce postoperative oozing through the incisions no matter how hard you try to prevent it.
In addition, in open cases, surfaces can easily be covered with this material. Unfortunately, it can’t be used laparoscopically because it is so brittle.
DECHERNEY: In my opinion, that is its major drawback.
PAGIDAS: In cardiac surgery, Seprafilm appears to work quite effectively.
SANFILIPPO: The manufacturer initially focused on surgeons in the context of sigmoid colon surgery, and it seems to work well in that setting.
Intergel
DECHERNEY: That brings us to the current state of the art: gels. I’m sure you all are familiar with Intergel (Lifecore Biomedical, Chaska, Minn), which is a ferrous derivative of hyaluronic acid that works by coating the raw surface areas. It also has the theoretical advantage of ease of use. Have any of you used Intergel?
HURD: As you know it was only recently approved by the US Food and Drug Administration, but not for laparoscopic use. It may work best on abraded bowel, which is avoided by laparoscopic surgery.
As you are probably also aware, the manufacturer recently took it off the market because of unusual side effects, namely a chemical peritonitis. Although peritonitis was cited as being rare, we encountered it in probably half the patients we operated on.
PAGIDAS: When we used it on hospitalized patients, the peritonitis wasn’t that obvious, since there was an expectation of significant pain. However, when we used Intergel on short-stay patients, we had to readmit them and do a full workup because we were concerned about bowel perforations. I’m surprised the manufacturer didn’t take it off the market sooner.
DECHERNEY: It seems strange, since Intergel has been used in Europe for a while now. I’ve used it in only 1 case and didn’t have adverse effects. It seemed to work well.
SANFILIPPO: It had all the right ingredients for success. It is unfortunate that these side effects have prohibited its use.
There is no question that adhesion prevention is one of the unmet challenges in all surgeries, especially reproductive surgery.
Gels and the cost factor
DECHERNEY: Other gels are in the pipeline. I’m reminded of plasminogen activator, which is a powerful antiadhesive agent that lyses fibrin effectively. Unfortunately, it is prohibitively expensive.
The next phase likely will involve the so-called polymers. If you spray them on your hand, they are activated by light or another chemical and become a cellophane-like substance. The problem is viscosity. If sprayed on the sidewall, for instance, they run halfway down before they are activated, so the entire surface does not get covered.
PAGIDAS: One concern with polymers is that they could actually bring surfaces together when they polymerize. We still have a lot to learn.
DECHERNEY: Let’s say a new gel comes on the market that takes reformation adhesions from 90% to 10%, as opposed to 40% recurrence. Would you use it in all 4 of the cases we discuss here?
HURD: If it was that effective and had no adverse effects, it would be wonderful.
The cesarean-delivery case is different, as healing in a pregnant patient is 1 concern; the size of the uterus also has an effect. If the patient is breastfeeding, you would want to make sure the gel didn’t interfere.
PAGIDAS: We desperately need a product that can minimize adhesions regardless of the route of access or type of procedure. Even though we lack data on outcomes, I predict wide use of such a product, assuming it is nontoxic and effective.
DECHERNEY: What if it costs $1,000 a case?
SANFILIPPO: If it prevents 1 bowel obstruction, it still would be cost-effective.
DECHERNEY: The incidence of bowel obstruction for total abdominal hysterectomy is 2%, and 5% for radical hysterectomy.
HURD: We must be careful of the cost-benefit ratio. Bowel obstruction after gynecologic surgery is uncommon.
Is the gel worth $100? $1,000? $3,000? It’s difficult to say, but the more expensive it is, the less likely it will find widespread use.
PAGIDAS: I agree. We should remember that we still need to maintain microsurgical techniques and appropriate tissue handling, as well as avoid ischemia and infection.
Looking for the magic bullet
DECHERNEY: What is the future of adjunctive therapy?
SANFILIPPO: I would focus on noxythiolin; it has potential. Calcium channel blockers for adhesion prevention have also been studied.20 In 1 investigation involving a rat model, the calcium channel blocker verapamil as well as several other agents—including vitamin E, carboxymethylcellulose, cyclosporin, aprotinin, and tenoxicam—were compared with respect to tissue effects. A beneficial effect was noted with all agents except cyclosporin and carboxymethylcellulose.
Whoever succeeds in manufacturing an effective preventive will be a winner.
HURD: There is no question that adhesion prevention is one of the unmet challenges in all surgeries, especially reproductive surgery. The most effective agent would be applied intraabdominally, since any systemic agent that stops adhesion formation would probably decrease wound healing as well.
I hope the most effective agents can be used in both laparoscopy and laparotomy, and that they will decrease the adhesion-formation rate by more than 50%. We need to find the magic bullet that can cover the entire pelvis—if not the entire abdomen.
Dr. DeCherney reports small holdings with Lifecore Biomedical and Johnson & Johnson. Drs. Hurd and Pagidas report no financial relationship with any companies whose products are mentioned in this article. Dr. Sanfilippo serves on the speaker’s bureau for Berlex, Ortho Pharmaceuticals, and Wyeth, and receives grant support from Eli Lilly and Wyeth.


-

-

- Approximately 40% of people who undergo primary surgery develop adhesions and reformation occurs in 80% to 90% of cases.
- Microsurgical techniques such as gentle handling of tissues, careful hemostasis, and avoidance of heat may help reduce the incidence.
- Laparoscopy appears to be less likely to produce adhesions than laparotomy.
- Ob/Gyns should be aware of the potential for adhesion-related bowel obstruction and take steps to prevent it.
Are adhesions a pathologic response to injury or a normal aspect of healing? Can they be avoided, or are preventive efforts part of the problem? How useful are the different barriers in gynecologic surgery? What is the ideal adjuvant?
OBG Management convened a panel of experts to explore these and other questions.
Common problem, high recurrence rate
DECHERNEY: Adhesion formation is serious because it is associated with clinical entities such as infertility, pelvic pain, and bowel obstruction. We all agree that approximately 40% of people who undergo primary surgery develop adhesions and that 80% to 90% of patients who undergo lysis develop recurrent adhesions.
SANFILIPPO: One study several years ago explored adhesion formation.1 Unfortunately, no matter how meticulous the surgeon is, adhesions will form, even with microsurgical techniques and carefully ensured hemostasis.
HURD: The number of patients with significant adhesion formation after some gynecologic procedures has been reported to be greater than 90%.2
DECHERNEY: That higher incidence usually occurs after general surgery—and there’s a reason it is so high: General surgeons don’t use adjunctive therapy. They are critical of it. It is to our credit as gynecologic surgeons that we adopted adjunctive therapies about 15 years ago with the introduction of dextran 70 (Hyskon; Medisan Pharmaceuticals, Parsippany, NJ).
PAGIDAS: If anything, the pelvis seems to have even more of a predilection for adhesion formation than the abdomen, probably because of the close proximity of structures.
How and why adhesions form
DECHERNEY: What is the pathophysiology of adhesion formation? Let’s say you have 2 raw surface areas. What happens?
The process
PAGIDAS: The increase in leukotrienes and prostaglandins and the decrease in plasminogen activity (which actually initiates the inflammation) appear to be significant.
HURD: Vessel permeability also increases, and inflammatory cells leak through the vessels and set up a matrix for adhesion formation.
DECHERNEY: So we have 2 raw surface areas with fibrin leaking out and forming bridges between them.
PAGIDAS: The key is that it takes 2 surfaces to form these bridges. As I mentioned, the greater proximity of pelvic structures—particularly around the tube and ovary—probably contributes to adhesion formation.
DECHERNEY: Macrophage activity also is important. The macrophage “migrates” along these fibrin bridges and lays down collagen over a period of time. Then the collagen becomes organized and, eventually, vascular.
Window of opportunity
SANFILIPPO: Adhesion formation probably occurs and is pretty well established within 5 to 7 days of the precipitating event—usually surgery. Once that process is under way, attempts to halt it yield diminishing returns. Unfortunately, we don’t know how to interfere with it in a positive way.
HURD: Under normal conditions, there seems to be a balance between fibrin deposition and fibrinolysis. In some tissues, however, these functions become imbalanced. This disparity may contribute more to adhesions than the actual laying down of fibrin—especially in tissue that is hypoxic.
DECHERNEY: Would you say that adhesion formation represents normal or abnormal healing?
HURD: It is one of the body’s normal protective mechanisms and an important part of healing. Without it, any abdominal injury would likely result in death.
SANFILIPPO: I don’t think it differs that much from processes that occur externally. For example, if you get cut deeply enough, you develop a scar. Is that scar part of the normal healing process? It is.
PAGIDAS: Right. It is a normal process of tissue remodeling. The question is: What allows it to go astray?
What surgical techniques help prevent adhesions?
DECHERNEY: Let’s review the aspects of surgical technique that are important for adhesion prevention.
PAGIDAS: I emphasize the value of microsurgical techniques, which help to minimize severe tissue handling. It also is important to keep surfaces moistened so they don’t desiccate.
SANFILIPPO: I agree with Dr. Pagidas about microsurgical techniques such as gentle tissue handling, careful hemostasis, and keeping tissues moist. If we follow these principles, we create an environment that minimizes the potential for adhesion formation.
HURD: The findings of many well-controlled animal studies have been surprising. For example, it is difficult to demonstrate that drying of tissue increases adhesions.3 Probably the greatest contributor to adhesions in these models was abrasion.4 One way that laparoscopic surgery decreases adhesions is by avoiding abrasion of the bowel mucosa, which occurs specifically with packing.
A 38-year-old mother of 2 undergoes myomectomy for menorrhagia.
SANFILIPPO: The initial question is: Can this case be managed laparoscopically? I do myomectomies laparoscopically whenever possible, although I do close the uterus with a minilaparotomy incision. The reason is my strong concern about reapproximating the myometrium, since wound dehiscence sometimes occurs at the site of myoma removal.
In this case, depending on the size of the myomas, I would do as much as possible laparoscopically and then reapproximate the myometrium. I would plan my incisions carefully, to maximize the number of myomas that can be removed. I would end with meticulous hemostasis and, assuming it is successful, use a barrier over the incision—in this case, Interceed.
HURD: Does the patient desire future childbearing? If so, I would avoid the laparoscopic approach because of the possibly increased risk of uterine rupture during pregnancy. If she isn’t planning pregnancy, there are more options.
The next question is: How many myomas are there, and where are they located? If they are intrauterine, a hysteroscopic approach would avoid extrauterine adhesions. If they are multiple and large, I am pretty much limited to laparotomy. If there is 1 or only a few myomas, a laparoscopic approach would be best.
I have not used Interceed. In laparoscopic cases, I worry that it would create more problems because, as you allow the carbon dioxide to decrease at the end of a case, oozing begins. Instead of a barrier, I would use limited hydroflotation.
SANFILIPPO: That’s a good point. At the end of the myomectomy, with the laparoscope in place, I decrease the insufflation, eliminating the tamponade effect. Then, assuming good hemostasis, I apply Interceed.
HURD: With open cases, I use Seprafilm, which takes practice because, as it gets wet, it sticks to anything, including gloves and instruments. But if you can put it down dry on the uterus, it sticks and stays in place. If oozing occurs, it seems to block or stop it.
PAGIDAS: I want to reiterate the importance of determining whether childbearing is an issue. In this case, the biggest concern is the risk of adhesions developing on our incision, so I would use a barrier. My preference would be Seprafilm or GoreTex. If we can limit adhesions at the incision site, then hopefully we can minimize bowel and tuboovarian adhesions, too.
In open cases, one thing we can do to minimize the risk of adhesions is to pack gently when needed. Also, we should avoid using packing to reposition the bowel.
Another factor frequently overlooked is the application of heat, which appears to be a very effective way to create adhesions. This probably isn’t an issue for laparoscopic cases, but when you use irrigation fluid in an open case, watch the temperature. If it feels hot to you, you need to worry about potential injury to the bowel surfaces.
PAGIDAS: That is critical. In abdominal cases you want to make sure irrigation fluid is warm, but not too warm, because heat increases the vascular permeability of vessels and leads to more macrophages, more prostaglandins, and more leukotrienes.
HURD: Another important element is the type of suture material used.
DECHERNEY: Overall, we need to minimize the use of sutures. For example, when I am operating laparoscopically on an ovarian cyst, I try to apply bipolar energy to the edges so that they will coapt without a stitch.
HURD: When it first became clear that suturing ovaries increased adhesion formation, we conducted a controlled trial of different kinds of sutures in animals. Not surprisingly, we found that the less reactive the suture, the fewer adhesions.5 Sutures that are absorbed more slowly, such as polydioxanone, seem to be less reactive.
Obviously, inert sutures like nylon are the least reactive, but they are permanent. It is assumed that animal-protein sutures such as chromic and plain gut are the most reactive, although I am not sure there are sufficient data to support that conclusion.
Multiple clinical studies have shown laparoscopy to be associated with a lower adhesion rate, although it isn’t clear why. It may be related to decreased suturing.
DECHERNEY: Bulk is important, too—that is, the number of throws in the suture. When Vicryl (polyglactin 910) became available, we conducted a study in mice using proportionately small Vicryl plaques to determine whether this would be good a barrier (A. DeCherney, MD, unpublished data). It caused a tremendous amount of adhesion because so much foreign matter was applied.
We also did a study using human-size titanium clips in rats (A. DeCherney, MD, unpublished data). Not surprisingly, there was a lot of adhesion formation.
FIGURE 1 Pelvic adhesions: How they develop, problems they cause
Adhesions occur when 2 or more raw surfaces are exposed to leaking fibrin, which forms a bridge between the surfaces. Macrophages “migrate” along these bridges, depositing collagen.
The pelvis has a greater predilection for adhesions than the abdomen because of the close proximity of structures.
Although adhesions represent one of the body’s protective mechanisms, they may also cause pain or interfere with fertility, bowel function, or other processes.
Laparoscopy versus laparotomy: More adhesions in open cases?
DECHERNEY: Based on all the techniques we have learned from microsurgery—with the exception of magnification—it appears that laparoscopic procedures are less likely to cause adhesions than laparotomy. Do you agree?
PAGIDAS: I think so. As Dr. Hurd noted, a main reason is the diminished tissue handling, because there is no packing.
HURD: Multiple clinical studies have shown laparoscopy to be associated with a lower adhesion rate, although it isn’t clear why.6,7 It may be related to decreased suturing.
DECHERNEY: Less bleeding occurs because surgeons are less aggressive laparoscopically than in laparotomy.
A 15-year-old undergoes removal of a dermoid cyst, which was shelled out laparoscopically.
PAGIDAS: This case is easier because the cyst has been successfully shelled out. It is not the spill of a cyst’s contents at surgery that creates adhesions, but a chronic leak, which can occur if you do not remove the cyst in its entirety.
Once the cyst has been excised completely, I would ensure hemostasis with bipolar cautery and reapproximate the edges. I would not suture. There seems to be no clear advantage to suturing. I would use hydroflotation. Although Ringer’s lactate solution has not been shown to be effective, it is safe and has no toxicity.
DECHERNEY: Would you remove the cyst via laparotomy?
PAGIDAS: I would do it laparoscopically, using the endobag to minimize spillage, even though we know that a spill doesn’t necessarily change the outcome. If spillage does occur, I would perform copious irrigation to ensure that nothing is left behind.
HURD: My priority would be minimizing the use of power on the ovary. Studies of ovarian drilling have demonstrated that burning an ovary stimulates adhesion formation.21,22 If the dermoid cyst spills, as happens occasionally, I perform copious rinsing until no more oil is visible on the surface of the peritoneal fluid.
I also would minimize the amount of ovarian capsule that is removed. Good studies of endometriomas have shown that the more capsulate that is removed, the more adhesions. Even if the capsule looks redundant and floppy, the concern should be to achieve hemostasis with bipolar cautery and then leave it alone.
DECHERNEY: Would you use crystalloids in this case?
HURD: Yes.
SANFILIPPO: If spillage occurs, I would ensure that the patient is taken out of the Trendelenburg position. I want to emphasize the importance of thorough irrigation to eliminate any material that could produce chemical peritonitis.
DECHERNEY: Over the years, I have seen a fair number of cases of Fitz-Hugh and Curtis syndrome. You rigorously lavage a ruptured dermoid cyst, which sometimes presents with low-grade fever, but always with pain.
I’m surprised that none of you would use Interceed, since wrapping the ovary is the only thing for which it has been clearly shown to be effective. Since the cortex is relatively avascular, you don’t get a lot of bleeding. Unfortunately, it is not technically easy to wrap the ovary.
HURD: Since we do not know the effect on future fertility of changing the ovarian surface, less would seem to be better in patients this young.
I have stopped doing difficult cases laparoscopically. For example, it is rare for me to operate laparoscopically on a patient with stage IV endometriosis, at least when it comes to infertility—I might consider laparoscopy for pain.
I think case selection plays a role as well, although there are few data to back that up. It is purely clinical opinion.
SANFILIPPO: We need a well-designed prospective study to explore the effects of laparotomy versus laparoscopy. Existing data are not clear. You would assume laparoscopy would be associated with less adhesion formation. But genetic or other factors may explain why patient A is more prone to adhesions than patient B.
Does anybody think carbon dioxide plays a role in adhesion formation?
HURD: In the laboratory, carbon dioxide increases cell growth.8 Without an increased carbon dioxide concentration in the atmosphere, cell cultures don’t grow well. This might suggest that the carbon dioxide used for laparoscopy could actually enhance adhesion growth. Fortunately, this does not appear to be the case clinically.
With myomectomy, the surgeon needs to plan ahead to maximize the number of myomas removed from a single incision.
DECHERNEY: What about second-look laparoscopies? Do any of you perform them after a patient has undergone lysis of adhesions?
SANFILIPPO: Only as part of a research protocol. It amazes me how rapidly adhesions can form and how dense they are 2 or more weeks after the initial laparoscopic surgery.
PAGIDAS: We tend to limit second-look laparoscopy to a research protocol, although it is sometimes valuable after laparoscopic or abdominal myomectomy, which has the highest incidence of adhesions. If the surgeon can perform a second look and lyse adhesions, he or she may potentially alter the reproductive outcome. However, with assisted reproductive techniques becoming integral to every infertility case, that approach has begun to go out of style.
SANFILIPPO: That’s a good point. With myomectomy, the surgeon needs to plan ahead to maximize the number of myomas removed from a single incision. If adhesions do occur, it is best if they occur toward the bladder rather than in the area of the tubes and ovaries.
HURD: For second-look laparoscopy, we must keep in mind the cost and the small but real risks of surgery. Until good controlled studies show a reasonable clinical advantage, this approach probably should remain a research protocol.
SANFILIPPO: The literature suggests it is helpful, but does not help fertility, so second-look laparoscopy is used mainly to evaluate adjunctive therapies. I don’t think anybody uses it as a primary therapy anymore.
Bowel obstruction still a risk, though rarely seen by Ob/Gyns
DECHERNEY: Although bowel obstruction is fairly common, Ob/Gyns do not often encounter it because it occurs relatively distant from the index surgery. Even though a patient may not experience bowel obstruction in the first year, an obstruction related to the index surgery is just as likely to develop 20 years later as 2 years later. These patients usually are treated by general surgeons. Still, we should beware of the potential for bowel obstruction and take steps to prevent it, if at all possible. Do you agree?
PAGIDAS: Yes. We tend to forget about bowel obstruction because we rarely follow patients past pregnancy or the first trimester if they are seeking infertility treatment.
HURD: The primary problem seems to be the abdominal wall incision. Fortunately, cesarean section seems to carry a decreased risk of abdominal wall adhesions, probably because the uterus serves as a splint over the incision.
The Pfannenstiel incision also appears to have some advantage. Both human and animal models suggest little advantage or disadvantage when peritoneal closure is compared to nonclosure.9,10
SANFILIPPO: I’m curious about how the panelists manage loose clips. If you are using an EndoGIA (US Surgical, Norwalk, Conn) or other stapling device and you have free-floating clips, do you make a concerted effort to find them? In some cases, they have been implicated in bowel adhesion and obstruction. I try to retrieve loose clips, whether open or closed.
HURD: The advantage of those devices is minimal tissue damage, and the clips are inert. In general, inert, nonreactive clips have not been implicated as much in adhesion formation. I retrieve them if I see them, but I don’t search them out.
PAGIDAS: I do the same. If the clips are visible, I remove them. But I would not repack the bowel or do anything more heroic than look in locations where they might be.
What drugs may inhibit inflammatory response?
DECHERNEY: What about use of pharmacologic agents to prevent adhesions? Is there reason to think research should focus on inhibiting the inflammatory response? How important is polymorphonuclear cell infiltration?
Cyclooxygenase (COX) 2 agents could be helpful for inhibition of platelet function, since they are low in side effects. Thus, high doses of these drugs might be effective. At one time, aspirin was proposed, but you’d have to give a human so much aspirin that her ears would ring.
PAGIDAS: Pharmacologic agents have a role, especially for dampening the inflammatory immune response. But you don’t want to dampen it completely because, as we observed, it is an important part of healing. The difficulty is finding a balance between allowing the tissue to heal and preventing adhesions.
HURD: We studied the ability of a water-soluble prostaglandin inhibitor to prevent postoperative adhesion formation. Like many other agents, we found only a partial response.11
DECHERNEY: With current options, the best you can aim for is a 50% reduction.
Adjunctive therapy likely to limit adhesion rate Hydroflotation
DECHERNEY: The original adjunctive therapy was 20 mg dexamethasone and 25 mg femergin in 200 cc of Ringer’s lactate, with an equivalent amount of dexamethasone and femergin every 4 hours for a total of 6 doses. I prescribed that regimen because I was trained to do so. I stopped after it became clear that hydroflotation from the fluid—not the medication itself—was responsible for the improvement.
I must admit I gave it up reluctantly; patients felt fabulous with those higher load doses of glucocorticoids after surgery.
Do any of you use crystalloids as adjunctive therapy?
SANFILIPPO: In the animal model, they are so rapidly absorbed that they aren’t effective. I was a strong advocate, but now I don’t use them at all.
HURD: A lot depends on the kind of case. For instance, at the end of an open myomectomy, the patient often is oozing, so you want to use a barrier that blood won’t affect.
For ovarian surgery, you might want to specifically target the ovaries with some kind of coverage. But when you are doing a broad lysis of adhesions, you have few choices to cover the pelvis. In those cases I use hydroflotation with Ringer’s lactate. Both human and animal studies have shown some benefit in preventing adhesions, and it appears to have little risk.12,13
It’s better than nothing, in my opinion.
DECHERNEY: Do you use dextran 70 or crystalloids?
HURD: I use Ringer’s lactate solution. I was trained in the dextran 70 era, and there were certain problems with that approach. Since studies have failed to show a consistent effect of dextran 70, I no longer use this solution.14,15
DECHERNEY: Another problem with crystalloids is that they leak, which is disconcerting to the patient.
HURD: They also can mask an injury to the bladder in difficult cases.
DECHERNEY: I agree that dextran 70 is only appropriate in certain cases, but it is a good hydroflotation agent. Every cubic centimeter of dextran 70 brings in 1.2 cc of transudate, so it hangs around for at least 4 days.
It is appropriate only for certain surface areas—mainly the cul-de-sac. It is harmful on raw surface areas on the lateral pelvic sidewall because it tends to push the ovary and tube to those areas. Unless you are doing a lot of work in the deep pelvis, I would avoid dextran 70.
A 45-year-old woman undergoes an abdominal hysterectomy. The cuff is closed and the ovaries are intact.
PAGIDAS: I would do nothing other than ensure adequate hemostasis, check that I have left no round surfaces and, probably, use hydroflotation. I see no advantage to barriers.
HURD: One of the main causes of adhesions is devascularized tissue, and the perfect devascularized tissue might be the vaginal cuff. Re-“peritonealizing” the cuff might be advantageous. Thus, I would use minimal sutures—probably a slowly absorbable, light polydioxanone suture to place the peritoneum over the cuff so there are no pedicles.
DECHERNEY: All the pedicles are exteriorized.
HURD: Yes, that could be. We don’t bring all of it down like we used to years ago, but we do cover the cuff.
PAGIDAS: I agree that closing the cuff and reperitonealizing may actually minimize formation of hematomas—clearly an advantage.
SANFILIPPO: I agree. I guess I’m old fashioned. If it looks good, then hopefully it will stimulate less adhesion formation, so peritonealization is important.
As far as the abdominal incision is concerned, I would not close that peritoneum. I’m convinced now that there is no advantage.
DECHERNEY: Reperitonealizing the cuff is controversial. Most gynecologic surgeons do not do it, the theory being that the peritoneum is being stretched, attenuating the vessels that go through it and thereby creating an ischemic barrier that contributes to adhesions. Personally, I like to do it because it looks better—and that is certainly an important aspect of a surgery. No evidence shows that it is bad or good, either way.
In addition, there have been allergic reactions, most of which seem to occur in patients with fluid overload; a lot of the dextran 70 is absorbed.
With infertility patients, even if you lyse dense adhesions, you do not render the ovarian surface normal.
SANFILIPPO: Dextran 70 is not recommended for patients with sugar beet allergy, either.
We completed a study in a rabbit model, in which the peritoneal cavity was lavaged with either chlorhexidine or iodine.16 At the time of second-look surgery, the rate of adhesion formation was decreased, especially with the iodine preparation. I would hope that this has potential in humans.
DECHERNEY: In your lavage procedures to prevent adhesions, do any of you use heparin?
HURD: No.
PAGIDAS: I don’t think any evidence suggests that local administration changes the outcome.
DECHERNEY: I agree. When heparin has been used, the doses have been so low that it was not terribly helpful. And when you consider that hemorrhage can be a problem, heparin is probably deleterious rather than helpful.
Barriers
DECHERNEY: What about barriers? The first to become available, Interceed (Gynecare, a division of Ethicon, Somerville, NJ), is oxidized cellulose, similar to Surgicell (Johnson & Johnson, New Brunswick, NJ). Since it gelates quickly, there is no fenestration, so the fibrin is unable to penetrate. However, if the patient has bleeding by capillary action, the raw surface just moves from one side of the Interceed barrier to the other.
What has been your experience? Do you use it?
PAGIDAS: I do not use Interceed, although prospective randomized trials and a meta-analysis confirmed its benefits in de novo formation and reformation.17-19 I don’t use it because it requires complete hemostasis. Also, with the surfaces we work on—notably, the ovary and tube—it is difficult to apply to just 1 surface area. From a clinical perspective, I appreciate the data, but it is hard to ensure a good application to optimize its effectiveness.
A 29-year-old woman is undergoing her third cesarean section, although adhesions cause difficulty getting through anteriorly.
DECHERNEY: In this case, will you use barriers or re-peritonealize the surface? Will you do a 1- or a 2-layered closure?
HURD: In these cases, I have not been doing anything, since there is no peritoneum to reperitonealize—just old scar tissue. I assume that the uterus will immediately readhere to the anterior peritoneum where it was before.
DECHERNEY: You would use a barrier?
HURD: No. I would not.
PAGIDAS: I would take the same approach, although I have not performed cesarean sections in about 4 years. I don’t think any intervention would change the outcome. And, as Dr. Hurd mentioned, that is pretty much old scar tissue anyway.
SANFILIPPO: I use Interceed, but I agree with you about its limitations. Meticulous hemostasis is a prerequisite.
HURD: With infertility patients, even if you lyse dense adhesions, you do not render the ovarian surface normal. If those patients have dense adhesions of the ovary or the sidewall, I generally leave them alone, and I try to avoid putting Interceed around the ovaries. No study has shown that using Interceed improves pregnancy.
In contrast, when a chronic pain patient’s ovaries are densely adherent to the cul-de-sac, which appears to be highly associated with dyspareunia, I lyse the adhesions, achieve meticulous hemostasis, and then use Interceed. It is hard to demonstrate in a study that this approach decreases the chance of pain. Even so, it certainly does decrease the chance of the ovaries being adherent.
DECHERNEY: One issue with Interceed is that we don’t know what happens to it once the abdomen is closed. It may migrate significantly.
Psychological issues may also be involved. For example, patients with multiple somatic complaints may be less likely to benefit from lysis of adhesions.
PAGIDAS: Right. Interestingly, a meta-analysis of all the randomized trials involving mechanical barriers found no correlation to pregnancy outcome or pelvic pain.18 If we were to consider new trials, the psychological aspect would be worth looking into.
Seprafilm
DECHERNEY: Let’s move on to Seprafilm (Genzyme, Cambridge, Mass). What is it and how useful is it?
HURD: Seprafilm is modified hyaluronic acid, which forms a brittle, thin plastic layer. It is somewhat difficult to work with but, once it is in place, seems to adhere well. The presence of blood does not appear to be a problem, since the Seprafilm forms an impermeable barrier—unless it breaks. I have found it especially useful in myomectomies, which produce postoperative oozing through the incisions no matter how hard you try to prevent it.
In addition, in open cases, surfaces can easily be covered with this material. Unfortunately, it can’t be used laparoscopically because it is so brittle.
DECHERNEY: In my opinion, that is its major drawback.
PAGIDAS: In cardiac surgery, Seprafilm appears to work quite effectively.
SANFILIPPO: The manufacturer initially focused on surgeons in the context of sigmoid colon surgery, and it seems to work well in that setting.
Intergel
DECHERNEY: That brings us to the current state of the art: gels. I’m sure you all are familiar with Intergel (Lifecore Biomedical, Chaska, Minn), which is a ferrous derivative of hyaluronic acid that works by coating the raw surface areas. It also has the theoretical advantage of ease of use. Have any of you used Intergel?
HURD: As you know it was only recently approved by the US Food and Drug Administration, but not for laparoscopic use. It may work best on abraded bowel, which is avoided by laparoscopic surgery.
As you are probably also aware, the manufacturer recently took it off the market because of unusual side effects, namely a chemical peritonitis. Although peritonitis was cited as being rare, we encountered it in probably half the patients we operated on.
PAGIDAS: When we used it on hospitalized patients, the peritonitis wasn’t that obvious, since there was an expectation of significant pain. However, when we used Intergel on short-stay patients, we had to readmit them and do a full workup because we were concerned about bowel perforations. I’m surprised the manufacturer didn’t take it off the market sooner.
DECHERNEY: It seems strange, since Intergel has been used in Europe for a while now. I’ve used it in only 1 case and didn’t have adverse effects. It seemed to work well.
SANFILIPPO: It had all the right ingredients for success. It is unfortunate that these side effects have prohibited its use.
There is no question that adhesion prevention is one of the unmet challenges in all surgeries, especially reproductive surgery.
Gels and the cost factor
DECHERNEY: Other gels are in the pipeline. I’m reminded of plasminogen activator, which is a powerful antiadhesive agent that lyses fibrin effectively. Unfortunately, it is prohibitively expensive.
The next phase likely will involve the so-called polymers. If you spray them on your hand, they are activated by light or another chemical and become a cellophane-like substance. The problem is viscosity. If sprayed on the sidewall, for instance, they run halfway down before they are activated, so the entire surface does not get covered.
PAGIDAS: One concern with polymers is that they could actually bring surfaces together when they polymerize. We still have a lot to learn.
DECHERNEY: Let’s say a new gel comes on the market that takes reformation adhesions from 90% to 10%, as opposed to 40% recurrence. Would you use it in all 4 of the cases we discuss here?
HURD: If it was that effective and had no adverse effects, it would be wonderful.
The cesarean-delivery case is different, as healing in a pregnant patient is 1 concern; the size of the uterus also has an effect. If the patient is breastfeeding, you would want to make sure the gel didn’t interfere.
PAGIDAS: We desperately need a product that can minimize adhesions regardless of the route of access or type of procedure. Even though we lack data on outcomes, I predict wide use of such a product, assuming it is nontoxic and effective.
DECHERNEY: What if it costs $1,000 a case?
SANFILIPPO: If it prevents 1 bowel obstruction, it still would be cost-effective.
DECHERNEY: The incidence of bowel obstruction for total abdominal hysterectomy is 2%, and 5% for radical hysterectomy.
HURD: We must be careful of the cost-benefit ratio. Bowel obstruction after gynecologic surgery is uncommon.
Is the gel worth $100? $1,000? $3,000? It’s difficult to say, but the more expensive it is, the less likely it will find widespread use.
PAGIDAS: I agree. We should remember that we still need to maintain microsurgical techniques and appropriate tissue handling, as well as avoid ischemia and infection.
Looking for the magic bullet
DECHERNEY: What is the future of adjunctive therapy?
SANFILIPPO: I would focus on noxythiolin; it has potential. Calcium channel blockers for adhesion prevention have also been studied.20 In 1 investigation involving a rat model, the calcium channel blocker verapamil as well as several other agents—including vitamin E, carboxymethylcellulose, cyclosporin, aprotinin, and tenoxicam—were compared with respect to tissue effects. A beneficial effect was noted with all agents except cyclosporin and carboxymethylcellulose.
Whoever succeeds in manufacturing an effective preventive will be a winner.
HURD: There is no question that adhesion prevention is one of the unmet challenges in all surgeries, especially reproductive surgery. The most effective agent would be applied intraabdominally, since any systemic agent that stops adhesion formation would probably decrease wound healing as well.
I hope the most effective agents can be used in both laparoscopy and laparotomy, and that they will decrease the adhesion-formation rate by more than 50%. We need to find the magic bullet that can cover the entire pelvis—if not the entire abdomen.
Dr. DeCherney reports small holdings with Lifecore Biomedical and Johnson & Johnson. Drs. Hurd and Pagidas report no financial relationship with any companies whose products are mentioned in this article. Dr. Sanfilippo serves on the speaker’s bureau for Berlex, Ortho Pharmaceuticals, and Wyeth, and receives grant support from Eli Lilly and Wyeth.
1. Operative Laparoscopy Study Group Postoperative adhesion development after operative laparoscopy: evaluation at early second look procedures. Fertil Steril. 1991;55:700-704.
2. Tulandi T, Murray C, Guralnick M. Adhesion formation and reproductive outcome after myomectomy and second-look laparoscopy. Obstet Gynecol. 1993;82:213-215.
3. Larsson B, Perbeck L. The possible advantage of keeping the uterine and intestinal serosa irrigated with saline to prevent intraabdominal adhesions in operations for infertility. An experimental study in rats. Acta Chir Scand Suppl. 1985;530:15-18.
4. Zamir G, Bloom AI, Reissman P. Prevention of intestinal adhesions after laparotomy in a rat model—a randomized prospective study. Res Exp Med (Berl). 1998;197:349-353.
5. Hurd WW, Himebaugh KS, Cofer KF, Gauvin JM, Elkins TE. The etiology of closure-related adhesion formation after wedge resection of the rabbit ovary. J Reprod Med. 1993;38:465-468.
6. Chen MD, Teigen GA, Reynolds HT, Johnson PR, Fowler JM. Laparoscopy versus laparotomy: an evaluation of adhesion formation after pelvic and paraaortic lymphadenectomy in a porcine model. Am J Obstet Gynecol. 1998;178:499-503.
7. Polymeneas G, Theodosopoulos T, Stamatiadis A, Kourias E. A comparative study of postoperative adhesion formation after laparoscopic vs. open cholecystectomy. Surg Endosc. 2001;15:41-43.
8. Smidt VJ, Singh DM, Hurteau JA, Hurd WW. Effect of carbon dioxide on human ovarian carcinoma cell growth. Am J Obstet Gynecol. 2001;185:1314-1317.
9. Tulandi T, Hum HS, Gelfand MM. Closure of laparotomy incisions with or without peritoneal suturing and second look laparoscopy. Am J Obstet Gynecol. 1988;158:536-537.
10. Kapur ML, Daneswar A, Chopra P. Evaluation of peritoneal closure at laparotomy. Am J Surg. 1979;137:650-652.
11. Cofer KF, Himebaugh KS, Gauvin JM, Hurd WW. Inhibition of adhesion reformation in the rabbit model by meclofenamate: an inhibitor of both prostaglandin and leukotriene production. Fertil Steril. 1994;62:1262-1265.
12. Elkelani OA, Molinas CR, Mynbaev O, Koninckx PR. Prevention of adhesions with crystalloids during laparoscopic surgery in mice. J Am Assoc Gynecol Laparosc. 2002;9:447-452.
13. Chan KL, Marino T, Qu WM, Tulandi T. Effects of intraperitoneal Ringer’s lactate instillation and infusion on postsurgical adhesion formation. J Gynecol Surg. 1995;11:241-243.
14. Watson A, Vandekerckhove P, Lilford R. Pharmacological adjuvants during infertility surgery: a systematic review of evidence derived from randomized controlled trial. Hum Fertil (Camb). 1999;2:149-157.
15. Larson B. Dextran—later clinical studies. clinical and experimental evaluation of different adjuvant therapies. Prog Clin Biol Res. 1990;358:165-175.
16. Roberts L, Sanfilippo JS, Ehrlich G, Raab S. Adhesion formation, peritoneal effects and the presence of bacterial biofilms after laparoscopic lavage in an animal model of pelvic inflammatory disease. J Am Assoc Gynecol Laparosc. 2002;9:4.-
17. Barrier agents for preventing adhesions after surgery for subfertility Cochrane Database Syst Rev. 2000;CD000475.-
18. Wiseman DM, Trout JR, Franklin RR, Diamond MP. Metaanalysis of the safety and efficacy of an adhesion barrier (Interceed TC7) in laparotomy. J Reprod Med. 1999;44:325-331.
19. Sawada T, Nishizawa H, Nishio E, Kadowaki M. Postoperative adhesion prevention with an oxidized regenerated cellulose adhesion barrier in infertile women. J Reprod. Med. 2000;45:387-389.
20. Uzunkoy A, Akinci OF, Coskun A, Aslan O, Kocyigit A. Effects of antiadhesive agents on the healing of intestinal anastomosis. Dis Colon Rectum. 2000;43:370-375.
21. Farquhar C, Vandekerckhove P, Arnot M, Lilford R. Laparoscopic “drilling” by diathermy or laser for ovulation induction in anovulatory polycystic ovary syndrome. Cochrane Database Syst Rev. 2000(2);CD001122.-
22. Greenblatt EM, Casper RF. Laparoscopic ovarian drilling in women with polycystic ovarian syndrome. Prog Clin Biol Res. 1993;381:129-138.
1. Operative Laparoscopy Study Group Postoperative adhesion development after operative laparoscopy: evaluation at early second look procedures. Fertil Steril. 1991;55:700-704.
2. Tulandi T, Murray C, Guralnick M. Adhesion formation and reproductive outcome after myomectomy and second-look laparoscopy. Obstet Gynecol. 1993;82:213-215.
3. Larsson B, Perbeck L. The possible advantage of keeping the uterine and intestinal serosa irrigated with saline to prevent intraabdominal adhesions in operations for infertility. An experimental study in rats. Acta Chir Scand Suppl. 1985;530:15-18.
4. Zamir G, Bloom AI, Reissman P. Prevention of intestinal adhesions after laparotomy in a rat model—a randomized prospective study. Res Exp Med (Berl). 1998;197:349-353.
5. Hurd WW, Himebaugh KS, Cofer KF, Gauvin JM, Elkins TE. The etiology of closure-related adhesion formation after wedge resection of the rabbit ovary. J Reprod Med. 1993;38:465-468.
6. Chen MD, Teigen GA, Reynolds HT, Johnson PR, Fowler JM. Laparoscopy versus laparotomy: an evaluation of adhesion formation after pelvic and paraaortic lymphadenectomy in a porcine model. Am J Obstet Gynecol. 1998;178:499-503.
7. Polymeneas G, Theodosopoulos T, Stamatiadis A, Kourias E. A comparative study of postoperative adhesion formation after laparoscopic vs. open cholecystectomy. Surg Endosc. 2001;15:41-43.
8. Smidt VJ, Singh DM, Hurteau JA, Hurd WW. Effect of carbon dioxide on human ovarian carcinoma cell growth. Am J Obstet Gynecol. 2001;185:1314-1317.
9. Tulandi T, Hum HS, Gelfand MM. Closure of laparotomy incisions with or without peritoneal suturing and second look laparoscopy. Am J Obstet Gynecol. 1988;158:536-537.
10. Kapur ML, Daneswar A, Chopra P. Evaluation of peritoneal closure at laparotomy. Am J Surg. 1979;137:650-652.
11. Cofer KF, Himebaugh KS, Gauvin JM, Hurd WW. Inhibition of adhesion reformation in the rabbit model by meclofenamate: an inhibitor of both prostaglandin and leukotriene production. Fertil Steril. 1994;62:1262-1265.
12. Elkelani OA, Molinas CR, Mynbaev O, Koninckx PR. Prevention of adhesions with crystalloids during laparoscopic surgery in mice. J Am Assoc Gynecol Laparosc. 2002;9:447-452.
13. Chan KL, Marino T, Qu WM, Tulandi T. Effects of intraperitoneal Ringer’s lactate instillation and infusion on postsurgical adhesion formation. J Gynecol Surg. 1995;11:241-243.
14. Watson A, Vandekerckhove P, Lilford R. Pharmacological adjuvants during infertility surgery: a systematic review of evidence derived from randomized controlled trial. Hum Fertil (Camb). 1999;2:149-157.
15. Larson B. Dextran—later clinical studies. clinical and experimental evaluation of different adjuvant therapies. Prog Clin Biol Res. 1990;358:165-175.
16. Roberts L, Sanfilippo JS, Ehrlich G, Raab S. Adhesion formation, peritoneal effects and the presence of bacterial biofilms after laparoscopic lavage in an animal model of pelvic inflammatory disease. J Am Assoc Gynecol Laparosc. 2002;9:4.-
17. Barrier agents for preventing adhesions after surgery for subfertility Cochrane Database Syst Rev. 2000;CD000475.-
18. Wiseman DM, Trout JR, Franklin RR, Diamond MP. Metaanalysis of the safety and efficacy of an adhesion barrier (Interceed TC7) in laparotomy. J Reprod Med. 1999;44:325-331.
19. Sawada T, Nishizawa H, Nishio E, Kadowaki M. Postoperative adhesion prevention with an oxidized regenerated cellulose adhesion barrier in infertile women. J Reprod. Med. 2000;45:387-389.
20. Uzunkoy A, Akinci OF, Coskun A, Aslan O, Kocyigit A. Effects of antiadhesive agents on the healing of intestinal anastomosis. Dis Colon Rectum. 2000;43:370-375.
21. Farquhar C, Vandekerckhove P, Arnot M, Lilford R. Laparoscopic “drilling” by diathermy or laser for ovulation induction in anovulatory polycystic ovary syndrome. Cochrane Database Syst Rev. 2000(2);CD001122.-
22. Greenblatt EM, Casper RF. Laparoscopic ovarian drilling in women with polycystic ovarian syndrome. Prog Clin Biol Res. 1993;381:129-138.
When is episiotomy warranted? What the evidence shows
- A Cochrane Database review concluded that restrictive episiotomy utilization is preferable to routine utilization. The review of 6 randomized trials found no differences in vaginal or perineal trauma, dyspareunia, or urinary incontinence between patients with and without episiotomy. Patients who had an episiotomy had less risk of anterior perineal trauma but an overall greater risk of posterior perineal trauma and other complications.
- Shoulder dystocia, operative vaginal delivery, and a “short” perineal body have been presumed indications for episiotomy, although data are inadequate to support these claims.
- The rationale for routine prophylactic episiotomy is to protect the pelvic floor, thereby minimizing the risk of urinary incontinence and pelvic floor dysfunction; however, episiotomy’s role in preventing such dysfunction remains to be established.
Although a large body of evidence indicates reassessment is in order, prophylactic episiotomy is a contentious issue. Indeed, it has been controversial ever since the procedure first became “routine” in the United States, in 1920. Still, advocates and dissenters share the same goal: to prevent severe perineal tears and their potential for urinary and fecal incontinence and sexual dysfunction.
This article reviews research findings that indicate:
- Data are inadequate to recommend one method of episiotomy over another.
- Timing of episiotomy to shorten the second stage of labor may be less relevant in an era of decreasing forceps utilization and without evidence of improved neonatal outcomes.5
- Episiotomy, particularly midline episiotomy, remains the single greatest risk that a patient will sustain a third- or fourth-degree laceration. When such lacerations occur spontaneously, recovery is equivalent to episiotomy extension or deliberate proctoepisiotomy.
TABLE
Incidence of third- or fourth-degree laceration with and without episiotomy
| NO. STUDIES COMPILED | NO. PATIENTS | % WITH 3RD- OR 4TH-DEGREE LACERATION | |
|---|---|---|---|
| Midline episiotomy | 12 | 49,395 | 6.5 |
| No episiotomy | 13 | 38,961 | 1.4 |
| Adapted from Thorp JM.3 | |||
Research does not support presumed indications
Episiotomy was first described in 1742 as a procedure that could assist the obstetrician in difficult vaginal deliveries.3 It was not until the work of DeLee6 and Pomeroy7 was published in 1920—coincident with deliveries beginning to move from home to hospital—that the procedure became “routine.” Still, some leaders in the field—specifically, J. Whitridge Williams of Johns Hopkins—vigorously dissented.8
Historically, episiotomy has been used to facilitate delivery in cases of protracted second stage, instrumented vaginal delivery, and suspected fetal compromise. However, data supporting episiotomy as a facilitating procedure are sparse, and evidence endorsing prophylactic episiotomy is largely anecdotal or descriptive.
Agreement is widespread that episiotomy is warranted under certain circumstances: Shoulder dystocia, operative vaginal delivery, and a “short” perineal body have been presumed indications. Data are inadequate to support these claims, however.
Shoulder dystocia. While it might seem to make sense to perform an episiotomy (or more likely, a proctoepisiotomy) in cases of shoulder dystocia, no data from controlled trials support this theory. Given the relative rarity of severe shoulder dystocia and the inability to conduct a truly randomized trial, physicians are left with only their clinical judgment as a guide in this circumstance.
Operative delivery. Many clinicians have advocated routine episiotomy before operative vaginal delivery, particularly with forceps. The intent is to increase the space available for delivery that has been diminished by the introduction of forceps. This rationale does not hold up as well for vacuum extraction; 1 study noted that when episiotomy is performed in cases of vacuum extraction, the likelihood of severe perineal trauma is increased.9
It has been reported10 that the greatest risk factor for both perineal trauma and third- or fourth-degree perineal laceration is episiotomy itself (TABLE), independent of mode of delivery (spontaneous or operative).
Short perineum. Many physicians, myself included, have performed episiotomies because they perceived that the perineum was short and that even a controlled delivery with optimal use of the Ritgen maneuver probably would not prevent a perineal laceration. That said, data on anal and flatus incontinence and postpartum sexual functioning suggest that spontaneous recovery from second-degree lacerations is no worse than recovery from midline episiotomy11,12 and, as stated, episiotomy itself is the leading risk factor for incurring a third- or fourth-degree extension—which imposes significantly greater recovery problems.
Two recent studies11,12 identified episiotomy as a specific, independent risk factor for fecal incontinence and delayed return of sexual activity postpartum. When matched for degree of perineal trauma, episiotomy without extension still resulted in poorer outcomes at 3 and 6 months postpartum than did spontaneous second-degree lacerations, suggesting that routine episiotomy not only fails to prevent, but may actually increase risk of perineal injury and impaired function.
‘Prophylactic’ episiotomy is not preventive
Much debate has centered on optimal utilization of so-called prophylactic episiotomy. The intent of routine prophylactic episiotomy is to protect the pelvic floor, thus minimizing the risk of urinary incontinence and pelvic floor dysfunction. Data have suggested that absence of labor and cesarean delivery may protect against pelvic floor dysfunction; however, the role of episiotomy in preventing such dysfunction remains to be determined.
Cochrane Database review. This review1 found no differences in vaginal or perineal trauma, dyspareunia, or urinary incontinence between patients with and without episiotomy. Patients who had an episiotomy had less risk of anterior perineal trauma but an overall greater risk of posterior perineal trauma and other complications. The reviewers concluded that restrictive episiotomy utilization is preferable to routine utilization.
The reviewers selected a total of 6 randomized trials; these examined:
- restrictive versus routine use of episiotomy;
- restrictive versus mediolateral episiotomy;
- restrictive versus routine midline episiotomy; and
- midline versus mediolateral episiotomy.
Compared with routine use, restrictive episiotomy involved less posterior perineal trauma (relative risk [RR], 0.88; 95% confidence interval [CI], 0.84 to 0.920), less suturing (RR, 0.74; 95% CI, 0.71 to 0.77), and fewer healing complications (RR, 0.69; 95% CI, 0.56 to 0.85). Restrictive episiotomy was associated with more anterior perineal trauma (RR, 1.79; 95% CI, 1.55 to 2.07).
There was no difference in severe vaginal or perineal trauma (RR, 1.11; 95% CI, 0.83 to 1.50), dyspareunia (RR, 1.02; 95% CI, 0.90 to 1.16), urinary incontinence (RR 0.98; 95% CI, 0.79 to 1.20), or several pain measures.
Results for restrictive versus routine mediolateral and midline episiotomies were similar to the overall comparison.
Reviewers concluded that a policy of restrictive episiotomy appears to have several benefits over routine episiotomy: less posterior perineal trauma, less suturing, fewer complications, and no difference for most pain measures and severe vaginal or perineal trauma.
Risk of anterior perineal trauma with restrictive episiotomy was increased, however. Restrictive-use protocols, likely to be institution-specific, essentially curb episiotomy use by stating that the procedure should not be “routinely performed.” Instead, episiotomy is restricted to cases in which the clinician believes it is warranted. Examples of such situations include use of forceps, shoulder dystocia, and an estimated fetal weight above 4,000 g. As discussed, the data cannot support the value of episiotomy use even in these circumstances; however, simply discouraging routine episiotomy would effectively lower the rate to the desired 30% range.
Midline versus mediolateral incision. The most vocal debates focus on which type of episiotomy to perform and whether it should be performed earlier or later in the second stage of labor.
It has been proposed that by abandoning midline episiotomies in favor of the mediolateral technique, physicians can avoid injury to the sphincter and improve immediate birth outcome without compromising long-term function—though pros and cons of this approach are a subject of debate (see “Comparison of mid-line versus mediolateral episiotomy”).
Still, the data suggest that, when properly performed, median and mediolateral episiotomy have equivalent rates of satisfactory recovery,13 though the latter technique may require more technical skill for both its performance and repair.
Early versus late incision. Proponents argue that an episiotomy at the time the presenting part is crowning is “too little, too late.” They maintain that for the procedure to be truly protective, it should be utilized earlier in the second stage of labor.
Data are insufficient to confirm or refute the efficacy of early episiotomy. One would do well to remember, however, that early episiotomy was endorsed as a method to help shorten the second stage of labor when used in conjunction with prophylactic forceps delivery—a method that is now less prevalent in obstetric practice.
The category of obstetric provider—midwife, faculty, or private provider—may be the most reliable predictor of episiotomy. Interestingly, use of episiotomy increased in the 1920s as delivery moved from home to hospital and birth attendants shifted from midwives to physicians.
In a study of demographic variables and obstetric factors associated with episiotomy in spontaneous vaginal delivery, researchers examined 1,576 term, singleton, spontaneous vaginal deliveries in nulliparas. They found that midwives had the lowest episiotomy rate (21.4%), compared with residents and full-time faculty (33.3%) and private physicians (55.6%).15
After controlling for confounding factors with logistic regression, the authors determined that private practice provider was the strongest predictor of episiotomy, followed by faculty provider, prolonged second stage of labor, fetal macrosomia, and epidural analgesia.
The study concluded that the obstetric and demographic factors evaluated did not readily explain the link between type of provider and episiotomy rate. Numerous theories have been proposed, but factors that would clearly explain the differences have yet to be identified.
Does vaginal birth trauma cause pelvic floor dysfunction?
The relationship between vaginal birth trauma, irrespective of episiotomy, and pelvic floor dysfunction remains a topic of investigation. A recent report generated much interest in the potentially protective role of prophylactic cesarean section, particularly if performed prior to the onset of labor.14
Dr. Repke reports no financial relationship with any companies whose products are mentioned in this article.
1. Carroli G, Belizan J. Episiotomy for vaginal birth [Cochrane Review]. In: The Cochrane Library Issue 3, 2003. Oxford: Update Software.
2. Lede RL, Belizan JM, Caroli G. Is routine use of episiotomy justified? Am J Obstet Gynecol. 1996;174:1399-1402.
3. Thorp JM. Episiotomy. In: Repke JT, ed. Intrapartum Obstetrics. New York, NY: Churchill Livingstone; 1996;489-499.
4. Thacker SB, Banta HD. Benefits and risks of episiotomy: an interpretive review of the English language literature, 1860-1980. Obstet Gynecol Surv. 1983;38:322-338.
5. Eason E, Feldman P. Much ado about a little cut: is episiotomy worthwhile? Obstet Gynecol. 2000;95:616-618.
6. DeLee JB. The prophylactic forceps operation. Am J Obstet Gynecol. 1920;1:34-44.
7. Pomeroy RH. Shall we cut and reconstruct the perineum for every primipara? Am J Obstet Dis Women Child. 1918;78:211-219.
8. Taylor ES. Comment on episiotomy and third degree tears. Obstet Gynecol Surg. 1985;41:229.-
9. Robinson JN, Norwitz ER, Cohen AP, McElrath TF, Lieberman ES. Episiotomy, operative vaginal delivery and significant perinatal trauma in nulliparous women. Am J Obstet Gynecol. 1999;181:1180-1184.
10. Robinson JN, Norwitz ER, Cohen AP, McElrath TF, Lieberman ES. Epidural analgesia and the occurrence of third and fourth degree laceration in nulliparas. Obstet Gynecol. 1999;94:259-262.
11. Signorello LB, Harlow BL, Chekos AK, Repke JT. Midline episiotomy and anal incontinence: a retrospective cohort study. BMJ. 2000;320:86-90.
12. Signorello LB, Harlow BL, Chekos AK, Repke JT. Postpartum sexual functioning and its relationship to perineal trauma: a retrospective cohort study of primiparous women. Am J Obstet Gynecol. 2001;184:881-890.
13. Coats PM, Chan KK, Wilkins M, Beard RJ. A comparison between midline and mediolateral episiotomies. Br J Obstet Gynaecol. 1980;87:408-412.
14. Dietz HP, Bennett MJ. The effect of childbirth on pelvic organ mobility. Obstet Gynecol. 2003;102:223-228.
15. Robinson JN, Norwitz ER, Cohen AP, Lieberman E. Predictors of episiotomy use at first spontaneous vaginal delivery. Obstet Gynecol. 2000;96:214-218
- A Cochrane Database review concluded that restrictive episiotomy utilization is preferable to routine utilization. The review of 6 randomized trials found no differences in vaginal or perineal trauma, dyspareunia, or urinary incontinence between patients with and without episiotomy. Patients who had an episiotomy had less risk of anterior perineal trauma but an overall greater risk of posterior perineal trauma and other complications.
- Shoulder dystocia, operative vaginal delivery, and a “short” perineal body have been presumed indications for episiotomy, although data are inadequate to support these claims.
- The rationale for routine prophylactic episiotomy is to protect the pelvic floor, thereby minimizing the risk of urinary incontinence and pelvic floor dysfunction; however, episiotomy’s role in preventing such dysfunction remains to be established.
Although a large body of evidence indicates reassessment is in order, prophylactic episiotomy is a contentious issue. Indeed, it has been controversial ever since the procedure first became “routine” in the United States, in 1920. Still, advocates and dissenters share the same goal: to prevent severe perineal tears and their potential for urinary and fecal incontinence and sexual dysfunction.
This article reviews research findings that indicate:
- Data are inadequate to recommend one method of episiotomy over another.
- Timing of episiotomy to shorten the second stage of labor may be less relevant in an era of decreasing forceps utilization and without evidence of improved neonatal outcomes.5
- Episiotomy, particularly midline episiotomy, remains the single greatest risk that a patient will sustain a third- or fourth-degree laceration. When such lacerations occur spontaneously, recovery is equivalent to episiotomy extension or deliberate proctoepisiotomy.
TABLE
Incidence of third- or fourth-degree laceration with and without episiotomy
| NO. STUDIES COMPILED | NO. PATIENTS | % WITH 3RD- OR 4TH-DEGREE LACERATION | |
|---|---|---|---|
| Midline episiotomy | 12 | 49,395 | 6.5 |
| No episiotomy | 13 | 38,961 | 1.4 |
| Adapted from Thorp JM.3 | |||
Research does not support presumed indications
Episiotomy was first described in 1742 as a procedure that could assist the obstetrician in difficult vaginal deliveries.3 It was not until the work of DeLee6 and Pomeroy7 was published in 1920—coincident with deliveries beginning to move from home to hospital—that the procedure became “routine.” Still, some leaders in the field—specifically, J. Whitridge Williams of Johns Hopkins—vigorously dissented.8
Historically, episiotomy has been used to facilitate delivery in cases of protracted second stage, instrumented vaginal delivery, and suspected fetal compromise. However, data supporting episiotomy as a facilitating procedure are sparse, and evidence endorsing prophylactic episiotomy is largely anecdotal or descriptive.
Agreement is widespread that episiotomy is warranted under certain circumstances: Shoulder dystocia, operative vaginal delivery, and a “short” perineal body have been presumed indications. Data are inadequate to support these claims, however.
Shoulder dystocia. While it might seem to make sense to perform an episiotomy (or more likely, a proctoepisiotomy) in cases of shoulder dystocia, no data from controlled trials support this theory. Given the relative rarity of severe shoulder dystocia and the inability to conduct a truly randomized trial, physicians are left with only their clinical judgment as a guide in this circumstance.
Operative delivery. Many clinicians have advocated routine episiotomy before operative vaginal delivery, particularly with forceps. The intent is to increase the space available for delivery that has been diminished by the introduction of forceps. This rationale does not hold up as well for vacuum extraction; 1 study noted that when episiotomy is performed in cases of vacuum extraction, the likelihood of severe perineal trauma is increased.9
It has been reported10 that the greatest risk factor for both perineal trauma and third- or fourth-degree perineal laceration is episiotomy itself (TABLE), independent of mode of delivery (spontaneous or operative).
Short perineum. Many physicians, myself included, have performed episiotomies because they perceived that the perineum was short and that even a controlled delivery with optimal use of the Ritgen maneuver probably would not prevent a perineal laceration. That said, data on anal and flatus incontinence and postpartum sexual functioning suggest that spontaneous recovery from second-degree lacerations is no worse than recovery from midline episiotomy11,12 and, as stated, episiotomy itself is the leading risk factor for incurring a third- or fourth-degree extension—which imposes significantly greater recovery problems.
Two recent studies11,12 identified episiotomy as a specific, independent risk factor for fecal incontinence and delayed return of sexual activity postpartum. When matched for degree of perineal trauma, episiotomy without extension still resulted in poorer outcomes at 3 and 6 months postpartum than did spontaneous second-degree lacerations, suggesting that routine episiotomy not only fails to prevent, but may actually increase risk of perineal injury and impaired function.
‘Prophylactic’ episiotomy is not preventive
Much debate has centered on optimal utilization of so-called prophylactic episiotomy. The intent of routine prophylactic episiotomy is to protect the pelvic floor, thus minimizing the risk of urinary incontinence and pelvic floor dysfunction. Data have suggested that absence of labor and cesarean delivery may protect against pelvic floor dysfunction; however, the role of episiotomy in preventing such dysfunction remains to be determined.
Cochrane Database review. This review1 found no differences in vaginal or perineal trauma, dyspareunia, or urinary incontinence between patients with and without episiotomy. Patients who had an episiotomy had less risk of anterior perineal trauma but an overall greater risk of posterior perineal trauma and other complications. The reviewers concluded that restrictive episiotomy utilization is preferable to routine utilization.
The reviewers selected a total of 6 randomized trials; these examined:
- restrictive versus routine use of episiotomy;
- restrictive versus mediolateral episiotomy;
- restrictive versus routine midline episiotomy; and
- midline versus mediolateral episiotomy.
Compared with routine use, restrictive episiotomy involved less posterior perineal trauma (relative risk [RR], 0.88; 95% confidence interval [CI], 0.84 to 0.920), less suturing (RR, 0.74; 95% CI, 0.71 to 0.77), and fewer healing complications (RR, 0.69; 95% CI, 0.56 to 0.85). Restrictive episiotomy was associated with more anterior perineal trauma (RR, 1.79; 95% CI, 1.55 to 2.07).
There was no difference in severe vaginal or perineal trauma (RR, 1.11; 95% CI, 0.83 to 1.50), dyspareunia (RR, 1.02; 95% CI, 0.90 to 1.16), urinary incontinence (RR 0.98; 95% CI, 0.79 to 1.20), or several pain measures.
Results for restrictive versus routine mediolateral and midline episiotomies were similar to the overall comparison.
Reviewers concluded that a policy of restrictive episiotomy appears to have several benefits over routine episiotomy: less posterior perineal trauma, less suturing, fewer complications, and no difference for most pain measures and severe vaginal or perineal trauma.
Risk of anterior perineal trauma with restrictive episiotomy was increased, however. Restrictive-use protocols, likely to be institution-specific, essentially curb episiotomy use by stating that the procedure should not be “routinely performed.” Instead, episiotomy is restricted to cases in which the clinician believes it is warranted. Examples of such situations include use of forceps, shoulder dystocia, and an estimated fetal weight above 4,000 g. As discussed, the data cannot support the value of episiotomy use even in these circumstances; however, simply discouraging routine episiotomy would effectively lower the rate to the desired 30% range.
Midline versus mediolateral incision. The most vocal debates focus on which type of episiotomy to perform and whether it should be performed earlier or later in the second stage of labor.
It has been proposed that by abandoning midline episiotomies in favor of the mediolateral technique, physicians can avoid injury to the sphincter and improve immediate birth outcome without compromising long-term function—though pros and cons of this approach are a subject of debate (see “Comparison of mid-line versus mediolateral episiotomy”).
Still, the data suggest that, when properly performed, median and mediolateral episiotomy have equivalent rates of satisfactory recovery,13 though the latter technique may require more technical skill for both its performance and repair.
Early versus late incision. Proponents argue that an episiotomy at the time the presenting part is crowning is “too little, too late.” They maintain that for the procedure to be truly protective, it should be utilized earlier in the second stage of labor.
Data are insufficient to confirm or refute the efficacy of early episiotomy. One would do well to remember, however, that early episiotomy was endorsed as a method to help shorten the second stage of labor when used in conjunction with prophylactic forceps delivery—a method that is now less prevalent in obstetric practice.
The category of obstetric provider—midwife, faculty, or private provider—may be the most reliable predictor of episiotomy. Interestingly, use of episiotomy increased in the 1920s as delivery moved from home to hospital and birth attendants shifted from midwives to physicians.
In a study of demographic variables and obstetric factors associated with episiotomy in spontaneous vaginal delivery, researchers examined 1,576 term, singleton, spontaneous vaginal deliveries in nulliparas. They found that midwives had the lowest episiotomy rate (21.4%), compared with residents and full-time faculty (33.3%) and private physicians (55.6%).15
After controlling for confounding factors with logistic regression, the authors determined that private practice provider was the strongest predictor of episiotomy, followed by faculty provider, prolonged second stage of labor, fetal macrosomia, and epidural analgesia.
The study concluded that the obstetric and demographic factors evaluated did not readily explain the link between type of provider and episiotomy rate. Numerous theories have been proposed, but factors that would clearly explain the differences have yet to be identified.
Does vaginal birth trauma cause pelvic floor dysfunction?
The relationship between vaginal birth trauma, irrespective of episiotomy, and pelvic floor dysfunction remains a topic of investigation. A recent report generated much interest in the potentially protective role of prophylactic cesarean section, particularly if performed prior to the onset of labor.14
Dr. Repke reports no financial relationship with any companies whose products are mentioned in this article.
- A Cochrane Database review concluded that restrictive episiotomy utilization is preferable to routine utilization. The review of 6 randomized trials found no differences in vaginal or perineal trauma, dyspareunia, or urinary incontinence between patients with and without episiotomy. Patients who had an episiotomy had less risk of anterior perineal trauma but an overall greater risk of posterior perineal trauma and other complications.
- Shoulder dystocia, operative vaginal delivery, and a “short” perineal body have been presumed indications for episiotomy, although data are inadequate to support these claims.
- The rationale for routine prophylactic episiotomy is to protect the pelvic floor, thereby minimizing the risk of urinary incontinence and pelvic floor dysfunction; however, episiotomy’s role in preventing such dysfunction remains to be established.
Although a large body of evidence indicates reassessment is in order, prophylactic episiotomy is a contentious issue. Indeed, it has been controversial ever since the procedure first became “routine” in the United States, in 1920. Still, advocates and dissenters share the same goal: to prevent severe perineal tears and their potential for urinary and fecal incontinence and sexual dysfunction.
This article reviews research findings that indicate:
- Data are inadequate to recommend one method of episiotomy over another.
- Timing of episiotomy to shorten the second stage of labor may be less relevant in an era of decreasing forceps utilization and without evidence of improved neonatal outcomes.5
- Episiotomy, particularly midline episiotomy, remains the single greatest risk that a patient will sustain a third- or fourth-degree laceration. When such lacerations occur spontaneously, recovery is equivalent to episiotomy extension or deliberate proctoepisiotomy.
TABLE
Incidence of third- or fourth-degree laceration with and without episiotomy
| NO. STUDIES COMPILED | NO. PATIENTS | % WITH 3RD- OR 4TH-DEGREE LACERATION | |
|---|---|---|---|
| Midline episiotomy | 12 | 49,395 | 6.5 |
| No episiotomy | 13 | 38,961 | 1.4 |
| Adapted from Thorp JM.3 | |||
Research does not support presumed indications
Episiotomy was first described in 1742 as a procedure that could assist the obstetrician in difficult vaginal deliveries.3 It was not until the work of DeLee6 and Pomeroy7 was published in 1920—coincident with deliveries beginning to move from home to hospital—that the procedure became “routine.” Still, some leaders in the field—specifically, J. Whitridge Williams of Johns Hopkins—vigorously dissented.8
Historically, episiotomy has been used to facilitate delivery in cases of protracted second stage, instrumented vaginal delivery, and suspected fetal compromise. However, data supporting episiotomy as a facilitating procedure are sparse, and evidence endorsing prophylactic episiotomy is largely anecdotal or descriptive.
Agreement is widespread that episiotomy is warranted under certain circumstances: Shoulder dystocia, operative vaginal delivery, and a “short” perineal body have been presumed indications. Data are inadequate to support these claims, however.
Shoulder dystocia. While it might seem to make sense to perform an episiotomy (or more likely, a proctoepisiotomy) in cases of shoulder dystocia, no data from controlled trials support this theory. Given the relative rarity of severe shoulder dystocia and the inability to conduct a truly randomized trial, physicians are left with only their clinical judgment as a guide in this circumstance.
Operative delivery. Many clinicians have advocated routine episiotomy before operative vaginal delivery, particularly with forceps. The intent is to increase the space available for delivery that has been diminished by the introduction of forceps. This rationale does not hold up as well for vacuum extraction; 1 study noted that when episiotomy is performed in cases of vacuum extraction, the likelihood of severe perineal trauma is increased.9
It has been reported10 that the greatest risk factor for both perineal trauma and third- or fourth-degree perineal laceration is episiotomy itself (TABLE), independent of mode of delivery (spontaneous or operative).
Short perineum. Many physicians, myself included, have performed episiotomies because they perceived that the perineum was short and that even a controlled delivery with optimal use of the Ritgen maneuver probably would not prevent a perineal laceration. That said, data on anal and flatus incontinence and postpartum sexual functioning suggest that spontaneous recovery from second-degree lacerations is no worse than recovery from midline episiotomy11,12 and, as stated, episiotomy itself is the leading risk factor for incurring a third- or fourth-degree extension—which imposes significantly greater recovery problems.
Two recent studies11,12 identified episiotomy as a specific, independent risk factor for fecal incontinence and delayed return of sexual activity postpartum. When matched for degree of perineal trauma, episiotomy without extension still resulted in poorer outcomes at 3 and 6 months postpartum than did spontaneous second-degree lacerations, suggesting that routine episiotomy not only fails to prevent, but may actually increase risk of perineal injury and impaired function.
‘Prophylactic’ episiotomy is not preventive
Much debate has centered on optimal utilization of so-called prophylactic episiotomy. The intent of routine prophylactic episiotomy is to protect the pelvic floor, thus minimizing the risk of urinary incontinence and pelvic floor dysfunction. Data have suggested that absence of labor and cesarean delivery may protect against pelvic floor dysfunction; however, the role of episiotomy in preventing such dysfunction remains to be determined.
Cochrane Database review. This review1 found no differences in vaginal or perineal trauma, dyspareunia, or urinary incontinence between patients with and without episiotomy. Patients who had an episiotomy had less risk of anterior perineal trauma but an overall greater risk of posterior perineal trauma and other complications. The reviewers concluded that restrictive episiotomy utilization is preferable to routine utilization.
The reviewers selected a total of 6 randomized trials; these examined:
- restrictive versus routine use of episiotomy;
- restrictive versus mediolateral episiotomy;
- restrictive versus routine midline episiotomy; and
- midline versus mediolateral episiotomy.
Compared with routine use, restrictive episiotomy involved less posterior perineal trauma (relative risk [RR], 0.88; 95% confidence interval [CI], 0.84 to 0.920), less suturing (RR, 0.74; 95% CI, 0.71 to 0.77), and fewer healing complications (RR, 0.69; 95% CI, 0.56 to 0.85). Restrictive episiotomy was associated with more anterior perineal trauma (RR, 1.79; 95% CI, 1.55 to 2.07).
There was no difference in severe vaginal or perineal trauma (RR, 1.11; 95% CI, 0.83 to 1.50), dyspareunia (RR, 1.02; 95% CI, 0.90 to 1.16), urinary incontinence (RR 0.98; 95% CI, 0.79 to 1.20), or several pain measures.
Results for restrictive versus routine mediolateral and midline episiotomies were similar to the overall comparison.
Reviewers concluded that a policy of restrictive episiotomy appears to have several benefits over routine episiotomy: less posterior perineal trauma, less suturing, fewer complications, and no difference for most pain measures and severe vaginal or perineal trauma.
Risk of anterior perineal trauma with restrictive episiotomy was increased, however. Restrictive-use protocols, likely to be institution-specific, essentially curb episiotomy use by stating that the procedure should not be “routinely performed.” Instead, episiotomy is restricted to cases in which the clinician believes it is warranted. Examples of such situations include use of forceps, shoulder dystocia, and an estimated fetal weight above 4,000 g. As discussed, the data cannot support the value of episiotomy use even in these circumstances; however, simply discouraging routine episiotomy would effectively lower the rate to the desired 30% range.
Midline versus mediolateral incision. The most vocal debates focus on which type of episiotomy to perform and whether it should be performed earlier or later in the second stage of labor.
It has been proposed that by abandoning midline episiotomies in favor of the mediolateral technique, physicians can avoid injury to the sphincter and improve immediate birth outcome without compromising long-term function—though pros and cons of this approach are a subject of debate (see “Comparison of mid-line versus mediolateral episiotomy”).
Still, the data suggest that, when properly performed, median and mediolateral episiotomy have equivalent rates of satisfactory recovery,13 though the latter technique may require more technical skill for both its performance and repair.
Early versus late incision. Proponents argue that an episiotomy at the time the presenting part is crowning is “too little, too late.” They maintain that for the procedure to be truly protective, it should be utilized earlier in the second stage of labor.
Data are insufficient to confirm or refute the efficacy of early episiotomy. One would do well to remember, however, that early episiotomy was endorsed as a method to help shorten the second stage of labor when used in conjunction with prophylactic forceps delivery—a method that is now less prevalent in obstetric practice.
The category of obstetric provider—midwife, faculty, or private provider—may be the most reliable predictor of episiotomy. Interestingly, use of episiotomy increased in the 1920s as delivery moved from home to hospital and birth attendants shifted from midwives to physicians.
In a study of demographic variables and obstetric factors associated with episiotomy in spontaneous vaginal delivery, researchers examined 1,576 term, singleton, spontaneous vaginal deliveries in nulliparas. They found that midwives had the lowest episiotomy rate (21.4%), compared with residents and full-time faculty (33.3%) and private physicians (55.6%).15
After controlling for confounding factors with logistic regression, the authors determined that private practice provider was the strongest predictor of episiotomy, followed by faculty provider, prolonged second stage of labor, fetal macrosomia, and epidural analgesia.
The study concluded that the obstetric and demographic factors evaluated did not readily explain the link between type of provider and episiotomy rate. Numerous theories have been proposed, but factors that would clearly explain the differences have yet to be identified.
Does vaginal birth trauma cause pelvic floor dysfunction?
The relationship between vaginal birth trauma, irrespective of episiotomy, and pelvic floor dysfunction remains a topic of investigation. A recent report generated much interest in the potentially protective role of prophylactic cesarean section, particularly if performed prior to the onset of labor.14
Dr. Repke reports no financial relationship with any companies whose products are mentioned in this article.
1. Carroli G, Belizan J. Episiotomy for vaginal birth [Cochrane Review]. In: The Cochrane Library Issue 3, 2003. Oxford: Update Software.
2. Lede RL, Belizan JM, Caroli G. Is routine use of episiotomy justified? Am J Obstet Gynecol. 1996;174:1399-1402.
3. Thorp JM. Episiotomy. In: Repke JT, ed. Intrapartum Obstetrics. New York, NY: Churchill Livingstone; 1996;489-499.
4. Thacker SB, Banta HD. Benefits and risks of episiotomy: an interpretive review of the English language literature, 1860-1980. Obstet Gynecol Surv. 1983;38:322-338.
5. Eason E, Feldman P. Much ado about a little cut: is episiotomy worthwhile? Obstet Gynecol. 2000;95:616-618.
6. DeLee JB. The prophylactic forceps operation. Am J Obstet Gynecol. 1920;1:34-44.
7. Pomeroy RH. Shall we cut and reconstruct the perineum for every primipara? Am J Obstet Dis Women Child. 1918;78:211-219.
8. Taylor ES. Comment on episiotomy and third degree tears. Obstet Gynecol Surg. 1985;41:229.-
9. Robinson JN, Norwitz ER, Cohen AP, McElrath TF, Lieberman ES. Episiotomy, operative vaginal delivery and significant perinatal trauma in nulliparous women. Am J Obstet Gynecol. 1999;181:1180-1184.
10. Robinson JN, Norwitz ER, Cohen AP, McElrath TF, Lieberman ES. Epidural analgesia and the occurrence of third and fourth degree laceration in nulliparas. Obstet Gynecol. 1999;94:259-262.
11. Signorello LB, Harlow BL, Chekos AK, Repke JT. Midline episiotomy and anal incontinence: a retrospective cohort study. BMJ. 2000;320:86-90.
12. Signorello LB, Harlow BL, Chekos AK, Repke JT. Postpartum sexual functioning and its relationship to perineal trauma: a retrospective cohort study of primiparous women. Am J Obstet Gynecol. 2001;184:881-890.
13. Coats PM, Chan KK, Wilkins M, Beard RJ. A comparison between midline and mediolateral episiotomies. Br J Obstet Gynaecol. 1980;87:408-412.
14. Dietz HP, Bennett MJ. The effect of childbirth on pelvic organ mobility. Obstet Gynecol. 2003;102:223-228.
15. Robinson JN, Norwitz ER, Cohen AP, Lieberman E. Predictors of episiotomy use at first spontaneous vaginal delivery. Obstet Gynecol. 2000;96:214-218
1. Carroli G, Belizan J. Episiotomy for vaginal birth [Cochrane Review]. In: The Cochrane Library Issue 3, 2003. Oxford: Update Software.
2. Lede RL, Belizan JM, Caroli G. Is routine use of episiotomy justified? Am J Obstet Gynecol. 1996;174:1399-1402.
3. Thorp JM. Episiotomy. In: Repke JT, ed. Intrapartum Obstetrics. New York, NY: Churchill Livingstone; 1996;489-499.
4. Thacker SB, Banta HD. Benefits and risks of episiotomy: an interpretive review of the English language literature, 1860-1980. Obstet Gynecol Surv. 1983;38:322-338.
5. Eason E, Feldman P. Much ado about a little cut: is episiotomy worthwhile? Obstet Gynecol. 2000;95:616-618.
6. DeLee JB. The prophylactic forceps operation. Am J Obstet Gynecol. 1920;1:34-44.
7. Pomeroy RH. Shall we cut and reconstruct the perineum for every primipara? Am J Obstet Dis Women Child. 1918;78:211-219.
8. Taylor ES. Comment on episiotomy and third degree tears. Obstet Gynecol Surg. 1985;41:229.-
9. Robinson JN, Norwitz ER, Cohen AP, McElrath TF, Lieberman ES. Episiotomy, operative vaginal delivery and significant perinatal trauma in nulliparous women. Am J Obstet Gynecol. 1999;181:1180-1184.
10. Robinson JN, Norwitz ER, Cohen AP, McElrath TF, Lieberman ES. Epidural analgesia and the occurrence of third and fourth degree laceration in nulliparas. Obstet Gynecol. 1999;94:259-262.
11. Signorello LB, Harlow BL, Chekos AK, Repke JT. Midline episiotomy and anal incontinence: a retrospective cohort study. BMJ. 2000;320:86-90.
12. Signorello LB, Harlow BL, Chekos AK, Repke JT. Postpartum sexual functioning and its relationship to perineal trauma: a retrospective cohort study of primiparous women. Am J Obstet Gynecol. 2001;184:881-890.
13. Coats PM, Chan KK, Wilkins M, Beard RJ. A comparison between midline and mediolateral episiotomies. Br J Obstet Gynaecol. 1980;87:408-412.
14. Dietz HP, Bennett MJ. The effect of childbirth on pelvic organ mobility. Obstet Gynecol. 2003;102:223-228.
15. Robinson JN, Norwitz ER, Cohen AP, Lieberman E. Predictors of episiotomy use at first spontaneous vaginal delivery. Obstet Gynecol. 2000;96:214-218
3 steps to reduce postoperative ileus
- Rather than contributing to ileus, early postoperative feeding now appears to help diminish its occurrence.
- There is no justification for routine postoperative placement of nasogastric tubes in asymptomatic patients.
- Thoracic epidurals block the reflex that causes postoperative ileus and can be used to prevent its occurrence.
Traditionally, the routine approach to avoiding this complication consisted of placing a nasogastric (NG) tube to decompress the bowel and delaying feeding until bowel function resumed.
More recent studies indicate that a different tactic may be preferable. These suggest that postoperative ileus—which has an estimated annual cost of $750 million1—can be significantly reduced with a simple 3-step process:
- withholding the nasogastric (NG) tube,
- feeding the patient early in the recovery process, and
- continuing epidural local anesthesia postoperatively.
Pathogenesis of ileus
We now know that the return of bowel function following surgery is an orderly event. The return of the small intestine’s action begins first, usually 4 to 8 hours postoperatively, and generally becomes complete around 24 hours. The colon resumes its function between 48 and 72 hours postoperatively.2
Very little has been written about the pathogenesis of postoperative ileus, but multiple causes have been suggested: sympathetic reflexes; inhibitory humoral agents; release of norepinephrine from the bowel wall; and the effects of anesthesia agents, opiates, and inflammation.3 The 2 most frequently mentioned etiologies are:
- the inhibitory neural reflex and
- inflammatory mediators released from the site of injury. (Inflammation is thought to trigger the release of macrophages, cytokines, and other inflammatory mediators, causing neutrophil infiltration.4)
Controversy remains as to what actually initiates the ileus. Is it manipulation of the bowel or the rigors of surgery and anesthesia? Kalff et al6 conducted bowel muscle studies in rats and concluded that manipulation of the bowel—and not the laparotomy per se—causes a failure of gut circular muscle function 24 hours later. They also noted an increase of phagocytes and mast cells. Their data support the hypothesis that abdominal surgery initiates a cascade of inflammatory events that leads to postoperative ileus.2
The case for early feeding
In the past 10 years, several studies have demonstrated that—rather than reduce the incidence of ileus—inserting an NG tube and withholding regular feeding following abdominal surgery can cause an ileus or prolong a preexisting one. Other trials have shown that feeding a patient early in the postoperative period can actually prevent ileus.7,8
Physiologic studies have shown that neither electrical activity of the bowel nor motor activities in the stomach are affected by surgery.8 Schilder et al9 reported bowel activity before the passage of flatus, indicating that the bowel is on its way to recovery much earlier than had been assumed. Thus, early postoperative feeding is well tolerated in most patients and associated with reduced discomfort and a more rapid recovery.7
For example, MacMillan et al studied 139 women undergoing gynecologic surgery for benign conditions; 67 were randomized to “early” feeding and 72 to traditional management. Early feeding involved a low-residue diet given 6 hours postoperatively, while traditional feeding consisted of clear liquids, which were withheld until the return of normal bowel sounds. Patients progressed to a regular diet with the passage of flatus. No increase in gastrointestinal complaints occurred in the early feeding group.7
Pearl et al8 compared similar groups of patients (TABLE 1). Patients in the first group were fed a clear liquid diet on the first postoperative day and progressed to a regular diet as soon as it could be tolerated. The traditional group was not fed until the return of bowel function, which was defined as the passage of flatus and no abdominal distension or vomiting; they were then started on clear liquids and, later, solid foods. While the incidence of complications was the same in both groups, hospitalization was shorter in the early feeding group.
A Cochrane review compared early versus delayed oral fluids and food after cesarean delivery. Of 12 studies considered, 6 were included in the review. No evidence was found to justify a policy of withholding oral fluids after uncomplicated cesarean sections.5
Simple versus complex procedures. Early feeding is not only safe in standardized surgeries such as cesarean section, but extends to complicated surgeries as well, as demonstrated in the trials by MacMillan et al7 and Pearl et al,8 which involved major gynecologic surgery. Trials with gynecologic oncology patients have shown the same result.2,8 Even patients undergoing colorectal surgery can tolerate oral feeding very early in their postoperative course without bowel complications.2,8
Not all studies have reported similar findings. Several concluded that other, nonmedical reasons, such as insurance requirements, accounted for the shorter hospital stays in many patients receiving early feeding.
TABLE 1
Complication rates associated with early feeding versus traditional management
| COMPLICATION | EARLY FEEDING* (N=92) | TRADITIONAL MANAGEMENT· (N= 103) |
|---|---|---|
| Nausea | 43.5% | 24.3% |
| Nasogastric tube use | 3.3% | 6.7% |
| Febrile morbidity | 54.3% | 55.3% |
| Pneumonia | 0% | 1.9% |
| Wound complications | 21.7% | 21.4% |
| Atelectasis | 8.7% | 10.7% |
| Length of hospital stay (mean±standard deviation) | 4.6±2.1 | 5.8±2.7 |
| *Clear liquid diet on postoperative day 1 | ||
| †Feeding delayed until return of bowel function | ||
| Reprinted from Obstet Gyn; vol 92; Pearl ML, Valea FA, Fischer M, Mahler L, Chalas E. A randomized controlled trial of early postoperative feeding in gynecologic oncology patients undergoing intraabdominal surgery; pages 94-97; copyright 1998; with permission from American College of Obstetricians and Gynecologists. | ||
Nasogastric tubes only when indicated
The use of NG tubes after laparotomy has been studied extensively. A review of the literature suggests that routine placement of the tubes in asymptomatic patients is not justified and may possibly be harmful.10
In their meta-analysis of the issue, Cheatham et al11 showed that although abdominal distension and vomiting are more frequent in patients who forgo NG tubes postoperatively, fever, atelectasis, and pneumonia are less common, and the interval between surgery and oral feeding is reduced (TABLE 2).
The authors concluded that for every NG tube inserted after abdominal surgery, at least 20 patients can be managed without it.
Forgoing an NG tube also lowers the risk of pulmonary complications, which increases 10-fold when a tube is inserted.12
TABLE 2
Complications associated with selective versus routine NG tube placement
| SELECTIVE PLACEMENT (N) | ROUTINE PLACEMENT (N) | P VALUE | RELATIVE RISK | |
|---|---|---|---|---|
| Patients | 1,986 | 1,978 | ||
| Tubes placed/replaced | 103 | 36 | .001> | 2.9 |
| Complications | 833 | 1,084 | .03 | 0.76 |
| Deaths | 13 | 25 | .22 | 0.36 |
| Pneumonia | 53 | 119 | .0001> | 0.49 |
| Atelectasis | 44 | 94 | .001 | 0.46 |
| Fever | 108 | 212 | .02 | 0.51 |
| Vomiting | 201 | 168 | .11 | 1.19 |
| Oral feedings (days) | 3.53 | 4.59 | .04 | |
| Length of stay (days) | 9.32 | 10.1 | .22 | |
| NG=nasogastric | ||||
| Reprinted with permission from Cheatham ML, Chapman WC, Key SP, Sawyer JL. A meta-analysis of selective versus routine nasogastric decompression after elective laparotomy. Ann Surg. 1995:221:469-478. | ||||
Continue the epidural anesthetic
A number of experts believe that postoperative ileus is caused by stimulation of neural reflexes, which appear to be of 2 kinds: afferent stimuli to the spinal cord and efferent stimuli to the intestines through the sympathetic nervous system. The latter inhibits motility of the intestinal tract. Numerous studies demonstrate that this sympathetic reflex can be blocked by the use of epidural anesthesia.10
For example, Holte et al4 found that postoperative administration of thoracic epidural blockade with local anesthesia significantly reduced both ileus and pulmonary complications. They concluded that continuous epidural anesthesia with local anesthesia and minimally invasive surgery are the 2 most critical events in reducing postoperative ileus.
In a Cochrane review, Jorgensen and colleagues12 compared the effects of epidural local anesthesia and opioid-based analgesic regimens on postoperative gastrointestinal paralysis, nausea and vomiting, and pain after abdominal surgery (TABLE 3). Epidural local anesthetics reduced gastrointestinal paralysis, as compared with systemic or epidural opioids, but provided the same postoperative pain relief. They also found that the addition of opioids to local epidural anesthesia provided superior postoperative analgesia—compared with epidural local anesthetics alone—without increasing the likelihood of ileus.
A study10 of patients undergoing colectomy found postoperative ileus was prevented or decreased with a 2-day regimen that included:
- continuous thoracic epidural anesthesia for 48 hours;
- withholding NG tubes;
- having the patient drink a liter of fluid on the day of surgery;
- initiating feeding after 24 hours;
- administering milk of magnesia; and
- mobilization after 8 hours, if possible.
Physicians in this study also used transverse surgical incisions to reduce pain and pulmonary problems.
TABLE 3
Anesthetic effect on GI function, postoperative pain, and nausea and vomiting: A comparison
| ANESTHETIC | GI FUNCTION RETURNS | PAIN RELIEF | NAUSEA AND VOMITING |
|---|---|---|---|
| Epidural plus local | 24 hr | Comparable | No significant difference |
| Epidural plus opioids | 37 hr | Comparable | No significant difference |
| Systemic plus opioids | 37 hr | Comparable | No significant difference |
| GI=gastrointestinal | |||
| Data from Jorgensen H, et al12 | |||
Clinical recommendations
The deregulation of the autonomic nervous system during surgery alters the gastrointestinal tract postoperatively, with neurotransmitters, local factors, and hormones playing a large role. Some forms of anesthesia also contribute to postoperative ileus, as does the use of narcotic analgesia after surgery.
The most efficient ways to activate the bowel postoperatively are:
- Continuing the thoracic epidural from 24 to 48 hours, which increases the splanchnic blood flow and blocks afferent and efferent sympathetic inhibitory nerve impulses. Note, however, that comparative studies of thoracic epidural anesthesia with local anesthesia are needed to quantify its impact.
- Hydrating the patient with a large amount of fluid in the first 24 hours after surgery.
Following these steps routinely can significantly decrease the risk of postoperative ileus and thus its resulting complications.
Dr. Rosenman reports no financial relationship with any companies whose products are mentioned in this article.
1. Groudine S. Use of inexpensive anesthesia during surgery may shorten hospital stay. Anesth Analg. 1998;87:1212-1213.
2. Cutillo G, Maneschi F, Franchi M, Giannice R, Scambia G, Benedetti-Panici P. Early feeding compared with nasogastric decompression after major gynecologic surgery: a randomized study. Obstet Gynecol. 1999;93:41-45.
3. Luckey A, Livingston E, Tache Y. Mechanisms and treatment of postoperative ileus. Arch Surg. 2000;138:206-214.
4. Holte K, Kehlet H. Postoperative ileus: a preventable event. Br J Surg. 2000;87:1480-1493.
5. Mangesi L, Hofmeyr GJ. Early compared with delayed oral fluids and food after caesarean section. Cochrane Database Syst Rev. 2002;(3):CD003516.-
6. Kalff JC, Schraut WH, Simmons RL, Bauer AJ. Surgical manipulation of the gut elicits an intestinal muscularis inflammatory response resulting in postsurgical ileus. Ann Surg. 1998;228,5:652-663.
7. MacMillan SL, Kammerer-Doak D, Rogers RG, Parker KM. Early feeding and the incidence of gastrointestinal symptoms after major gynecologic surgery. Obstet Gynecol. 2000;96:604-608.
8. Pearl ML, Valea FA, Fischer M, Mahler L, Chalas E. A randomized controlled trial of early postoperative feeding in gynecologic oncology patients undergoing intraabdominal surgery. Obstet Gynecol. 1998;92:94-97.
9. Schilder JM, Hurteau JA, Look KY, Moore DH, Raff G, Stehman FB, Sutton GP. A prospective controlled trial of early postoperative oral intake following major abdominal gynecologic surgery. Gynecol Oncol. 1997;67:235-240.
10. Kehlet H, Holte K. Review of postoperative ileus. Am J Surg. 2001;182(5a Suppl):3S-10S.
11. Cheatham ML, Chapman WC, Key SP, Sawyer JL. A meta-analysis of selective versus routine nasogastric decompression after elective laparotomy. Ann Surg. 1995;221:469-478.
12. Jorgensen H, Wetterslev J, Moiniche S, Dahl JB. Epidural local anesthetics versus opioid-based analgesic regimens on postoperative gastrointestinal paralysis, PONV and pain after abdominal surgery. Cochrane Database Syst Rev. 2000;(4):CD001893.-
- Rather than contributing to ileus, early postoperative feeding now appears to help diminish its occurrence.
- There is no justification for routine postoperative placement of nasogastric tubes in asymptomatic patients.
- Thoracic epidurals block the reflex that causes postoperative ileus and can be used to prevent its occurrence.
Traditionally, the routine approach to avoiding this complication consisted of placing a nasogastric (NG) tube to decompress the bowel and delaying feeding until bowel function resumed.
More recent studies indicate that a different tactic may be preferable. These suggest that postoperative ileus—which has an estimated annual cost of $750 million1—can be significantly reduced with a simple 3-step process:
- withholding the nasogastric (NG) tube,
- feeding the patient early in the recovery process, and
- continuing epidural local anesthesia postoperatively.
Pathogenesis of ileus
We now know that the return of bowel function following surgery is an orderly event. The return of the small intestine’s action begins first, usually 4 to 8 hours postoperatively, and generally becomes complete around 24 hours. The colon resumes its function between 48 and 72 hours postoperatively.2
Very little has been written about the pathogenesis of postoperative ileus, but multiple causes have been suggested: sympathetic reflexes; inhibitory humoral agents; release of norepinephrine from the bowel wall; and the effects of anesthesia agents, opiates, and inflammation.3 The 2 most frequently mentioned etiologies are:
- the inhibitory neural reflex and
- inflammatory mediators released from the site of injury. (Inflammation is thought to trigger the release of macrophages, cytokines, and other inflammatory mediators, causing neutrophil infiltration.4)
Controversy remains as to what actually initiates the ileus. Is it manipulation of the bowel or the rigors of surgery and anesthesia? Kalff et al6 conducted bowel muscle studies in rats and concluded that manipulation of the bowel—and not the laparotomy per se—causes a failure of gut circular muscle function 24 hours later. They also noted an increase of phagocytes and mast cells. Their data support the hypothesis that abdominal surgery initiates a cascade of inflammatory events that leads to postoperative ileus.2
The case for early feeding
In the past 10 years, several studies have demonstrated that—rather than reduce the incidence of ileus—inserting an NG tube and withholding regular feeding following abdominal surgery can cause an ileus or prolong a preexisting one. Other trials have shown that feeding a patient early in the postoperative period can actually prevent ileus.7,8
Physiologic studies have shown that neither electrical activity of the bowel nor motor activities in the stomach are affected by surgery.8 Schilder et al9 reported bowel activity before the passage of flatus, indicating that the bowel is on its way to recovery much earlier than had been assumed. Thus, early postoperative feeding is well tolerated in most patients and associated with reduced discomfort and a more rapid recovery.7
For example, MacMillan et al studied 139 women undergoing gynecologic surgery for benign conditions; 67 were randomized to “early” feeding and 72 to traditional management. Early feeding involved a low-residue diet given 6 hours postoperatively, while traditional feeding consisted of clear liquids, which were withheld until the return of normal bowel sounds. Patients progressed to a regular diet with the passage of flatus. No increase in gastrointestinal complaints occurred in the early feeding group.7
Pearl et al8 compared similar groups of patients (TABLE 1). Patients in the first group were fed a clear liquid diet on the first postoperative day and progressed to a regular diet as soon as it could be tolerated. The traditional group was not fed until the return of bowel function, which was defined as the passage of flatus and no abdominal distension or vomiting; they were then started on clear liquids and, later, solid foods. While the incidence of complications was the same in both groups, hospitalization was shorter in the early feeding group.
A Cochrane review compared early versus delayed oral fluids and food after cesarean delivery. Of 12 studies considered, 6 were included in the review. No evidence was found to justify a policy of withholding oral fluids after uncomplicated cesarean sections.5
Simple versus complex procedures. Early feeding is not only safe in standardized surgeries such as cesarean section, but extends to complicated surgeries as well, as demonstrated in the trials by MacMillan et al7 and Pearl et al,8 which involved major gynecologic surgery. Trials with gynecologic oncology patients have shown the same result.2,8 Even patients undergoing colorectal surgery can tolerate oral feeding very early in their postoperative course without bowel complications.2,8
Not all studies have reported similar findings. Several concluded that other, nonmedical reasons, such as insurance requirements, accounted for the shorter hospital stays in many patients receiving early feeding.
TABLE 1
Complication rates associated with early feeding versus traditional management
| COMPLICATION | EARLY FEEDING* (N=92) | TRADITIONAL MANAGEMENT· (N= 103) |
|---|---|---|
| Nausea | 43.5% | 24.3% |
| Nasogastric tube use | 3.3% | 6.7% |
| Febrile morbidity | 54.3% | 55.3% |
| Pneumonia | 0% | 1.9% |
| Wound complications | 21.7% | 21.4% |
| Atelectasis | 8.7% | 10.7% |
| Length of hospital stay (mean±standard deviation) | 4.6±2.1 | 5.8±2.7 |
| *Clear liquid diet on postoperative day 1 | ||
| †Feeding delayed until return of bowel function | ||
| Reprinted from Obstet Gyn; vol 92; Pearl ML, Valea FA, Fischer M, Mahler L, Chalas E. A randomized controlled trial of early postoperative feeding in gynecologic oncology patients undergoing intraabdominal surgery; pages 94-97; copyright 1998; with permission from American College of Obstetricians and Gynecologists. | ||
Nasogastric tubes only when indicated
The use of NG tubes after laparotomy has been studied extensively. A review of the literature suggests that routine placement of the tubes in asymptomatic patients is not justified and may possibly be harmful.10
In their meta-analysis of the issue, Cheatham et al11 showed that although abdominal distension and vomiting are more frequent in patients who forgo NG tubes postoperatively, fever, atelectasis, and pneumonia are less common, and the interval between surgery and oral feeding is reduced (TABLE 2).
The authors concluded that for every NG tube inserted after abdominal surgery, at least 20 patients can be managed without it.
Forgoing an NG tube also lowers the risk of pulmonary complications, which increases 10-fold when a tube is inserted.12
TABLE 2
Complications associated with selective versus routine NG tube placement
| SELECTIVE PLACEMENT (N) | ROUTINE PLACEMENT (N) | P VALUE | RELATIVE RISK | |
|---|---|---|---|---|
| Patients | 1,986 | 1,978 | ||
| Tubes placed/replaced | 103 | 36 | .001> | 2.9 |
| Complications | 833 | 1,084 | .03 | 0.76 |
| Deaths | 13 | 25 | .22 | 0.36 |
| Pneumonia | 53 | 119 | .0001> | 0.49 |
| Atelectasis | 44 | 94 | .001 | 0.46 |
| Fever | 108 | 212 | .02 | 0.51 |
| Vomiting | 201 | 168 | .11 | 1.19 |
| Oral feedings (days) | 3.53 | 4.59 | .04 | |
| Length of stay (days) | 9.32 | 10.1 | .22 | |
| NG=nasogastric | ||||
| Reprinted with permission from Cheatham ML, Chapman WC, Key SP, Sawyer JL. A meta-analysis of selective versus routine nasogastric decompression after elective laparotomy. Ann Surg. 1995:221:469-478. | ||||
Continue the epidural anesthetic
A number of experts believe that postoperative ileus is caused by stimulation of neural reflexes, which appear to be of 2 kinds: afferent stimuli to the spinal cord and efferent stimuli to the intestines through the sympathetic nervous system. The latter inhibits motility of the intestinal tract. Numerous studies demonstrate that this sympathetic reflex can be blocked by the use of epidural anesthesia.10
For example, Holte et al4 found that postoperative administration of thoracic epidural blockade with local anesthesia significantly reduced both ileus and pulmonary complications. They concluded that continuous epidural anesthesia with local anesthesia and minimally invasive surgery are the 2 most critical events in reducing postoperative ileus.
In a Cochrane review, Jorgensen and colleagues12 compared the effects of epidural local anesthesia and opioid-based analgesic regimens on postoperative gastrointestinal paralysis, nausea and vomiting, and pain after abdominal surgery (TABLE 3). Epidural local anesthetics reduced gastrointestinal paralysis, as compared with systemic or epidural opioids, but provided the same postoperative pain relief. They also found that the addition of opioids to local epidural anesthesia provided superior postoperative analgesia—compared with epidural local anesthetics alone—without increasing the likelihood of ileus.
A study10 of patients undergoing colectomy found postoperative ileus was prevented or decreased with a 2-day regimen that included:
- continuous thoracic epidural anesthesia for 48 hours;
- withholding NG tubes;
- having the patient drink a liter of fluid on the day of surgery;
- initiating feeding after 24 hours;
- administering milk of magnesia; and
- mobilization after 8 hours, if possible.
Physicians in this study also used transverse surgical incisions to reduce pain and pulmonary problems.
TABLE 3
Anesthetic effect on GI function, postoperative pain, and nausea and vomiting: A comparison
| ANESTHETIC | GI FUNCTION RETURNS | PAIN RELIEF | NAUSEA AND VOMITING |
|---|---|---|---|
| Epidural plus local | 24 hr | Comparable | No significant difference |
| Epidural plus opioids | 37 hr | Comparable | No significant difference |
| Systemic plus opioids | 37 hr | Comparable | No significant difference |
| GI=gastrointestinal | |||
| Data from Jorgensen H, et al12 | |||
Clinical recommendations
The deregulation of the autonomic nervous system during surgery alters the gastrointestinal tract postoperatively, with neurotransmitters, local factors, and hormones playing a large role. Some forms of anesthesia also contribute to postoperative ileus, as does the use of narcotic analgesia after surgery.
The most efficient ways to activate the bowel postoperatively are:
- Continuing the thoracic epidural from 24 to 48 hours, which increases the splanchnic blood flow and blocks afferent and efferent sympathetic inhibitory nerve impulses. Note, however, that comparative studies of thoracic epidural anesthesia with local anesthesia are needed to quantify its impact.
- Hydrating the patient with a large amount of fluid in the first 24 hours after surgery.
Following these steps routinely can significantly decrease the risk of postoperative ileus and thus its resulting complications.
Dr. Rosenman reports no financial relationship with any companies whose products are mentioned in this article.
- Rather than contributing to ileus, early postoperative feeding now appears to help diminish its occurrence.
- There is no justification for routine postoperative placement of nasogastric tubes in asymptomatic patients.
- Thoracic epidurals block the reflex that causes postoperative ileus and can be used to prevent its occurrence.
Traditionally, the routine approach to avoiding this complication consisted of placing a nasogastric (NG) tube to decompress the bowel and delaying feeding until bowel function resumed.
More recent studies indicate that a different tactic may be preferable. These suggest that postoperative ileus—which has an estimated annual cost of $750 million1—can be significantly reduced with a simple 3-step process:
- withholding the nasogastric (NG) tube,
- feeding the patient early in the recovery process, and
- continuing epidural local anesthesia postoperatively.
Pathogenesis of ileus
We now know that the return of bowel function following surgery is an orderly event. The return of the small intestine’s action begins first, usually 4 to 8 hours postoperatively, and generally becomes complete around 24 hours. The colon resumes its function between 48 and 72 hours postoperatively.2
Very little has been written about the pathogenesis of postoperative ileus, but multiple causes have been suggested: sympathetic reflexes; inhibitory humoral agents; release of norepinephrine from the bowel wall; and the effects of anesthesia agents, opiates, and inflammation.3 The 2 most frequently mentioned etiologies are:
- the inhibitory neural reflex and
- inflammatory mediators released from the site of injury. (Inflammation is thought to trigger the release of macrophages, cytokines, and other inflammatory mediators, causing neutrophil infiltration.4)
Controversy remains as to what actually initiates the ileus. Is it manipulation of the bowel or the rigors of surgery and anesthesia? Kalff et al6 conducted bowel muscle studies in rats and concluded that manipulation of the bowel—and not the laparotomy per se—causes a failure of gut circular muscle function 24 hours later. They also noted an increase of phagocytes and mast cells. Their data support the hypothesis that abdominal surgery initiates a cascade of inflammatory events that leads to postoperative ileus.2
The case for early feeding
In the past 10 years, several studies have demonstrated that—rather than reduce the incidence of ileus—inserting an NG tube and withholding regular feeding following abdominal surgery can cause an ileus or prolong a preexisting one. Other trials have shown that feeding a patient early in the postoperative period can actually prevent ileus.7,8
Physiologic studies have shown that neither electrical activity of the bowel nor motor activities in the stomach are affected by surgery.8 Schilder et al9 reported bowel activity before the passage of flatus, indicating that the bowel is on its way to recovery much earlier than had been assumed. Thus, early postoperative feeding is well tolerated in most patients and associated with reduced discomfort and a more rapid recovery.7
For example, MacMillan et al studied 139 women undergoing gynecologic surgery for benign conditions; 67 were randomized to “early” feeding and 72 to traditional management. Early feeding involved a low-residue diet given 6 hours postoperatively, while traditional feeding consisted of clear liquids, which were withheld until the return of normal bowel sounds. Patients progressed to a regular diet with the passage of flatus. No increase in gastrointestinal complaints occurred in the early feeding group.7
Pearl et al8 compared similar groups of patients (TABLE 1). Patients in the first group were fed a clear liquid diet on the first postoperative day and progressed to a regular diet as soon as it could be tolerated. The traditional group was not fed until the return of bowel function, which was defined as the passage of flatus and no abdominal distension or vomiting; they were then started on clear liquids and, later, solid foods. While the incidence of complications was the same in both groups, hospitalization was shorter in the early feeding group.
A Cochrane review compared early versus delayed oral fluids and food after cesarean delivery. Of 12 studies considered, 6 were included in the review. No evidence was found to justify a policy of withholding oral fluids after uncomplicated cesarean sections.5
Simple versus complex procedures. Early feeding is not only safe in standardized surgeries such as cesarean section, but extends to complicated surgeries as well, as demonstrated in the trials by MacMillan et al7 and Pearl et al,8 which involved major gynecologic surgery. Trials with gynecologic oncology patients have shown the same result.2,8 Even patients undergoing colorectal surgery can tolerate oral feeding very early in their postoperative course without bowel complications.2,8
Not all studies have reported similar findings. Several concluded that other, nonmedical reasons, such as insurance requirements, accounted for the shorter hospital stays in many patients receiving early feeding.
TABLE 1
Complication rates associated with early feeding versus traditional management
| COMPLICATION | EARLY FEEDING* (N=92) | TRADITIONAL MANAGEMENT· (N= 103) |
|---|---|---|
| Nausea | 43.5% | 24.3% |
| Nasogastric tube use | 3.3% | 6.7% |
| Febrile morbidity | 54.3% | 55.3% |
| Pneumonia | 0% | 1.9% |
| Wound complications | 21.7% | 21.4% |
| Atelectasis | 8.7% | 10.7% |
| Length of hospital stay (mean±standard deviation) | 4.6±2.1 | 5.8±2.7 |
| *Clear liquid diet on postoperative day 1 | ||
| †Feeding delayed until return of bowel function | ||
| Reprinted from Obstet Gyn; vol 92; Pearl ML, Valea FA, Fischer M, Mahler L, Chalas E. A randomized controlled trial of early postoperative feeding in gynecologic oncology patients undergoing intraabdominal surgery; pages 94-97; copyright 1998; with permission from American College of Obstetricians and Gynecologists. | ||
Nasogastric tubes only when indicated
The use of NG tubes after laparotomy has been studied extensively. A review of the literature suggests that routine placement of the tubes in asymptomatic patients is not justified and may possibly be harmful.10
In their meta-analysis of the issue, Cheatham et al11 showed that although abdominal distension and vomiting are more frequent in patients who forgo NG tubes postoperatively, fever, atelectasis, and pneumonia are less common, and the interval between surgery and oral feeding is reduced (TABLE 2).
The authors concluded that for every NG tube inserted after abdominal surgery, at least 20 patients can be managed without it.
Forgoing an NG tube also lowers the risk of pulmonary complications, which increases 10-fold when a tube is inserted.12
TABLE 2
Complications associated with selective versus routine NG tube placement
| SELECTIVE PLACEMENT (N) | ROUTINE PLACEMENT (N) | P VALUE | RELATIVE RISK | |
|---|---|---|---|---|
| Patients | 1,986 | 1,978 | ||
| Tubes placed/replaced | 103 | 36 | .001> | 2.9 |
| Complications | 833 | 1,084 | .03 | 0.76 |
| Deaths | 13 | 25 | .22 | 0.36 |
| Pneumonia | 53 | 119 | .0001> | 0.49 |
| Atelectasis | 44 | 94 | .001 | 0.46 |
| Fever | 108 | 212 | .02 | 0.51 |
| Vomiting | 201 | 168 | .11 | 1.19 |
| Oral feedings (days) | 3.53 | 4.59 | .04 | |
| Length of stay (days) | 9.32 | 10.1 | .22 | |
| NG=nasogastric | ||||
| Reprinted with permission from Cheatham ML, Chapman WC, Key SP, Sawyer JL. A meta-analysis of selective versus routine nasogastric decompression after elective laparotomy. Ann Surg. 1995:221:469-478. | ||||
Continue the epidural anesthetic
A number of experts believe that postoperative ileus is caused by stimulation of neural reflexes, which appear to be of 2 kinds: afferent stimuli to the spinal cord and efferent stimuli to the intestines through the sympathetic nervous system. The latter inhibits motility of the intestinal tract. Numerous studies demonstrate that this sympathetic reflex can be blocked by the use of epidural anesthesia.10
For example, Holte et al4 found that postoperative administration of thoracic epidural blockade with local anesthesia significantly reduced both ileus and pulmonary complications. They concluded that continuous epidural anesthesia with local anesthesia and minimally invasive surgery are the 2 most critical events in reducing postoperative ileus.
In a Cochrane review, Jorgensen and colleagues12 compared the effects of epidural local anesthesia and opioid-based analgesic regimens on postoperative gastrointestinal paralysis, nausea and vomiting, and pain after abdominal surgery (TABLE 3). Epidural local anesthetics reduced gastrointestinal paralysis, as compared with systemic or epidural opioids, but provided the same postoperative pain relief. They also found that the addition of opioids to local epidural anesthesia provided superior postoperative analgesia—compared with epidural local anesthetics alone—without increasing the likelihood of ileus.
A study10 of patients undergoing colectomy found postoperative ileus was prevented or decreased with a 2-day regimen that included:
- continuous thoracic epidural anesthesia for 48 hours;
- withholding NG tubes;
- having the patient drink a liter of fluid on the day of surgery;
- initiating feeding after 24 hours;
- administering milk of magnesia; and
- mobilization after 8 hours, if possible.
Physicians in this study also used transverse surgical incisions to reduce pain and pulmonary problems.
TABLE 3
Anesthetic effect on GI function, postoperative pain, and nausea and vomiting: A comparison
| ANESTHETIC | GI FUNCTION RETURNS | PAIN RELIEF | NAUSEA AND VOMITING |
|---|---|---|---|
| Epidural plus local | 24 hr | Comparable | No significant difference |
| Epidural plus opioids | 37 hr | Comparable | No significant difference |
| Systemic plus opioids | 37 hr | Comparable | No significant difference |
| GI=gastrointestinal | |||
| Data from Jorgensen H, et al12 | |||
Clinical recommendations
The deregulation of the autonomic nervous system during surgery alters the gastrointestinal tract postoperatively, with neurotransmitters, local factors, and hormones playing a large role. Some forms of anesthesia also contribute to postoperative ileus, as does the use of narcotic analgesia after surgery.
The most efficient ways to activate the bowel postoperatively are:
- Continuing the thoracic epidural from 24 to 48 hours, which increases the splanchnic blood flow and blocks afferent and efferent sympathetic inhibitory nerve impulses. Note, however, that comparative studies of thoracic epidural anesthesia with local anesthesia are needed to quantify its impact.
- Hydrating the patient with a large amount of fluid in the first 24 hours after surgery.
Following these steps routinely can significantly decrease the risk of postoperative ileus and thus its resulting complications.
Dr. Rosenman reports no financial relationship with any companies whose products are mentioned in this article.
1. Groudine S. Use of inexpensive anesthesia during surgery may shorten hospital stay. Anesth Analg. 1998;87:1212-1213.
2. Cutillo G, Maneschi F, Franchi M, Giannice R, Scambia G, Benedetti-Panici P. Early feeding compared with nasogastric decompression after major gynecologic surgery: a randomized study. Obstet Gynecol. 1999;93:41-45.
3. Luckey A, Livingston E, Tache Y. Mechanisms and treatment of postoperative ileus. Arch Surg. 2000;138:206-214.
4. Holte K, Kehlet H. Postoperative ileus: a preventable event. Br J Surg. 2000;87:1480-1493.
5. Mangesi L, Hofmeyr GJ. Early compared with delayed oral fluids and food after caesarean section. Cochrane Database Syst Rev. 2002;(3):CD003516.-
6. Kalff JC, Schraut WH, Simmons RL, Bauer AJ. Surgical manipulation of the gut elicits an intestinal muscularis inflammatory response resulting in postsurgical ileus. Ann Surg. 1998;228,5:652-663.
7. MacMillan SL, Kammerer-Doak D, Rogers RG, Parker KM. Early feeding and the incidence of gastrointestinal symptoms after major gynecologic surgery. Obstet Gynecol. 2000;96:604-608.
8. Pearl ML, Valea FA, Fischer M, Mahler L, Chalas E. A randomized controlled trial of early postoperative feeding in gynecologic oncology patients undergoing intraabdominal surgery. Obstet Gynecol. 1998;92:94-97.
9. Schilder JM, Hurteau JA, Look KY, Moore DH, Raff G, Stehman FB, Sutton GP. A prospective controlled trial of early postoperative oral intake following major abdominal gynecologic surgery. Gynecol Oncol. 1997;67:235-240.
10. Kehlet H, Holte K. Review of postoperative ileus. Am J Surg. 2001;182(5a Suppl):3S-10S.
11. Cheatham ML, Chapman WC, Key SP, Sawyer JL. A meta-analysis of selective versus routine nasogastric decompression after elective laparotomy. Ann Surg. 1995;221:469-478.
12. Jorgensen H, Wetterslev J, Moiniche S, Dahl JB. Epidural local anesthetics versus opioid-based analgesic regimens on postoperative gastrointestinal paralysis, PONV and pain after abdominal surgery. Cochrane Database Syst Rev. 2000;(4):CD001893.-
1. Groudine S. Use of inexpensive anesthesia during surgery may shorten hospital stay. Anesth Analg. 1998;87:1212-1213.
2. Cutillo G, Maneschi F, Franchi M, Giannice R, Scambia G, Benedetti-Panici P. Early feeding compared with nasogastric decompression after major gynecologic surgery: a randomized study. Obstet Gynecol. 1999;93:41-45.
3. Luckey A, Livingston E, Tache Y. Mechanisms and treatment of postoperative ileus. Arch Surg. 2000;138:206-214.
4. Holte K, Kehlet H. Postoperative ileus: a preventable event. Br J Surg. 2000;87:1480-1493.
5. Mangesi L, Hofmeyr GJ. Early compared with delayed oral fluids and food after caesarean section. Cochrane Database Syst Rev. 2002;(3):CD003516.-
6. Kalff JC, Schraut WH, Simmons RL, Bauer AJ. Surgical manipulation of the gut elicits an intestinal muscularis inflammatory response resulting in postsurgical ileus. Ann Surg. 1998;228,5:652-663.
7. MacMillan SL, Kammerer-Doak D, Rogers RG, Parker KM. Early feeding and the incidence of gastrointestinal symptoms after major gynecologic surgery. Obstet Gynecol. 2000;96:604-608.
8. Pearl ML, Valea FA, Fischer M, Mahler L, Chalas E. A randomized controlled trial of early postoperative feeding in gynecologic oncology patients undergoing intraabdominal surgery. Obstet Gynecol. 1998;92:94-97.
9. Schilder JM, Hurteau JA, Look KY, Moore DH, Raff G, Stehman FB, Sutton GP. A prospective controlled trial of early postoperative oral intake following major abdominal gynecologic surgery. Gynecol Oncol. 1997;67:235-240.
10. Kehlet H, Holte K. Review of postoperative ileus. Am J Surg. 2001;182(5a Suppl):3S-10S.
11. Cheatham ML, Chapman WC, Key SP, Sawyer JL. A meta-analysis of selective versus routine nasogastric decompression after elective laparotomy. Ann Surg. 1995;221:469-478.
12. Jorgensen H, Wetterslev J, Moiniche S, Dahl JB. Epidural local anesthetics versus opioid-based analgesic regimens on postoperative gastrointestinal paralysis, PONV and pain after abdominal surgery. Cochrane Database Syst Rev. 2000;(4):CD001893.-
Surgery for stress incontinence: Which technique for which patient?
-

-

-


Choosing appropriate surgical interventions is the focus of the second of our 2-part panel on stress urinary incontinence (SUI). The panelists discuss:
- how to weigh the factors that influence choice of technique, including Burch retropubic urethropexy and the various sling operations;
- the challenges of treating “mixed” stress and urge incontinence; and
- when to use bulking agents for intrinsic sphincteric deficiency.
The panelists also share tips on:
- how to help patients accurately describe their symptoms, and
- what issues to review with patients as they consider their options.
Part 1 covered medical therapies such as pelvic floor muscle rehabilitation, occlusive devices, and drugs. (Stress urinary incontinence: A closer look at nonsurgical therapies. OBG Management. 2003;15(9):40-51.)
Review surgical options with the patient
SAND: How do you counsel patients about surgical treatments for stress urinary incontinence?
MYERS: After the initial evaluation and diagnosis, I review the conservative options, and I also offer surgery. At this time, I discuss whether an operation is appropriate.
I work with the patient, going over her diagnosis as well as the different types of operations that are performed. Some patients are fairly well educated about their options, having looked up information on the Internet.
Next I explore whether other types of procedures need to be done concomitantly. For instance, does the patient need abdominal hysterectomy for some other reason? That would prompt me to offer an abdominal approach to the SUI. Do other types of vaginal surgery need to be done? Then I would probably opt for a vaginal approach.
I also look at the patient’s health status. Is she healthy and physically active? Or is she sedentary with comorbidities? In a woman who is a poor surgical candidate, I would consider less invasive procedures or procedures with less operative risk, such as urethral injections or the newer tape slings.
LUBER: When it comes to surgery for urinary incontinence, I like to reinforce the reconstructive nature of the repair, since patients tend to view surgical procedures as definitive. For example, when the uterus and ovaries are removed, they never bother that patient again. Incontinence procedures are different. Their effect is potentially time-limited, so it’s important to reinforce the patient’s understanding of their reconstructive and fallible nature.
At the first surgical consultation, I basically go through an informed consent. I do so again preoperatively, but I think it is a very important initial step for a patient who is considering surgery.
SAND: What if a patient isn’t sure she wants surgery?
LUBER: When a patient asks, “What should I do, Dr. Luber? Should I have an operation?” I like to use the example of standing in front of the refrigerator and asking, “Gee, am I hungry?” If you have to ask, you probably aren’t.
Potential surgical patients should feel extremely comfortable that they have exhausted all the nonsurgical options. Even if they have decided against nonsurgical therapy, they should feel very comfortable with that choice. Then I am confident we can work through any potential problems of surgery.
As for the operation itself, history has demonstrated the irresistible impulse to innovate during surgery for female stress incontinence. Literally hundreds of operations have been described, and dozens are currently in use; this reinforces the supposition that our techniques are imperfect, and the importance of basing what we do upon the available data. The 1997 American Urological Association guidelines are an excellent example. Looking forward, the National Institutes of Health are sponsoring studies comparing, for example, the goldstandard Burch to the goldstandard sling operation. In the next few years, we should have better evidence-based guidance.
Consider patient characteristics when choosing treatment
SAND: How do you select a surgical procedure for a particular patient?
MYERS: Because of all the different variables, I use various treatment arms. Since my institution does a large number of sling procedures, I am very comfortable performing those operations. I still do retropubic urethropexy. I also do the newer vaginal-tape procedures, and I use bulking agents for patients who have a demonstrated sphincter deficiency with no obvious support problems. Basically, I try to tailor my procedure to the patient.
For example, in a woman who requires a total abdominal hysterectomy for fibroids as well as an anti-incontinence procedure, I would do a Burch operation. For a woman who needed a vaginal hysterectomy, I probably would perform a sling procedure.
DAVILA: Ob/Gyns may be a bit unsure how to proceed at this point. For example, we formerly considered the Burch procedure the gold standard against which other procedures should be judged. Although I continue to view it as the standard, the Burch procedure increasingly is overlooked in favor of tension-free slings—due to increased marketing of the latter—for any form of stress incontinence. I think that has led us down a path that is not entirely beneficial for many of our patients.
In contrast to that approach, I use a basic evaluation of the patient to construct a treatment algorithm. In simple terms, 2 factors are taken into account: urethral sphincter function and bladder neck or urethral support. Using those 2 factors, I create a 2×2 table to select patients who do or don’t have urethral hypermobility and who do or don’t have sphincteric deficiency (TABLE).
For example, a patient with hypermobility and normal sphincter function has what we might consider “garden-variety” stress incontinence. Such patients do well with any form of treatment, whether it’s conservative therapy, a vaginal device, or a Burch procedure or tension-free sling.
I am more concerned when the patient has hypermobility with a significant degree of sphincteric deficiency. In recent years, the tendency has been to treat such patients with a tension-free sling. Although the literature is not absolutely clear, the success rate of tension-free slings in a patient with intrinsic sphincteric deficiency (ISD) is not as high as in a woman without ISD.1 So in these patients, I do a traditional sling.
Atrophy can cause significant urgency and nocturia symptoms.—Dr. Davila
The other 2 groups of patients have no hypermobility. I think most of us would agree that a woman with ISD and no hypermobility would best be treated with a bulking agent such as Contigen (C.R. Bard, Murray Hill, NJ) or Durasphere (Advanced Uroscience, St. Paul, Minn).
I have had good success rates with bulking agents. I do not think current data would support a tension-free sling in these patients.
Finally, there is the patient without hypermobility who has normal sphincter function. These patients do fairly well with conservative therapy, including pelvic floor exercises. They usually have mild forms of stress incontinence to begin with.
TABLE 1
Stress urinary incontinence treatment choices based on urethral support and urethral sphincteric function
| URETHRAL SUPPORT | URETHRAL SPHINCTERIC FUNCTION | |
|---|---|---|
| Bladder neck mobility (Q-tip test) | Normal urethral | Poor urethral function function |
| MUCP >20 cm H20 | LPP <20 cm H20 | |
| VLPP <60 cm H20 | VLPP >60 cm H20 | |
| Negative EBST | Positive EBST | |
| >30 degrees (hypermobility) | Kegel exercises | Traditional sling |
| Biofeedback | ||
| Vaginal device | ||
| Tension-free vaginal tape | ||
| Burch urethropexy | ||
| <30 degrees | Kegel exercises | Bulking agents |
| Biofeedback | ||
| Source: GW Davila, MD | ||
| EBST = empty bladder stress test; | ||
| MUCP = maximal urethral closure pressure; | ||
| VLPP = Valsalva leak point pressure | ||
| NOTE: Urethral plugs may function in all categories | ||
Simple method to assess sphincter function
DAVILA: This is the algorithm I tend to follow. It does entail evaluation of the urethral sphincter mechanism, but there are simpler ways to do that than with multi-channel urodynamics. For example, if the patient leaks with a Valsalva maneuver, after voiding, in a supine position, that suggests she has ISD and therefore is likely to have a low-pressure urethra or a low leak-point pressure. Multiple centers have reported on this.2,3
Role of urethral function in choice of treatment
LUBER: There seems to be 2 schools. The first dichotomizes urethral function to reasonable (“good” urethral function) versus unreasonable (“poor” urethral function or ISD) and selects the operation based on that. Thus, a Burch or supportive operation would be used for good urethral function with hypermobility, and a sling operation would be selected for poor urethral function or ISD.
More recently, some experts have preached an inclusive approach, whereby all patients undergo sling operations. That strategy evolved out of frustration over the difficulty of identifying which patients have poor urethral function. Unfortunately, we lack good long-term data on the potential downside of performing sling procedures on all patients with incontinence. Hopefully, over the next 3 years, the National Institutes of Health data will help clarify whether we need to dichotomize patients in terms of urethral function.
Meanwhile, at our center, we continue to consider urethral function the deciding factor as to whether patients will undergo a gold-standard Burch procedure or a sling. We steer toward a sling procedure when the patient clearly has poor urethral function or ISD. Of course in cases of the fixed immobile and poorly functioning urethra, we also make bulking agents available.
Additional factors in the choice of treatment
SAND: We throw 2 other things into the algorithm at our center: One is detrusor overactivity, which is very important when considering surgical treatment of stress urinary incontinence. The second is voiding function.
Activity level of the patient is an additional measure, as Dr. Myers commented on earlier. I’m not as concerned about age as I am about the patient’s physical activity and expectations for the operation over time. For example, for a woman who is relatively homebound and not physically active and has poor voiding function (underactive detrusor) and prolapse with normal intrinsic urethral function, a Kelly Kennedy procedure at the time of an anterior colporrhaphy may be more appropriate than a Burch procedure.
Detrusor overactivity is important because, in the trial that we performed, the Burch retropubic urethropexy had a 55% objective cure rate of concurrent detrusor overactivity and a 70% subjective cure rate of the symptom of urge urinary incontinence. In contrast, over the last 12 years, the sling procedure has had a resolution rate of between 20% and 28% for recurrent detrusor overactivity. Recent subjective data at 1 year for midurethral slings fall into the same range: 20% to 30% resolution of recurrent urge incontinence.
Another factor is de novo detrusor overactivity. The rate of de novo detrusor overactivity and urge incontinence in our sling patients seems consistently higher, compared with our retropubic urethropexy patients. We all know that the patient with urge incontinence is far more upset about her condition than the patient who has predictable stress incontinence, because urge incontinence can be far more destructive to quality of life. I try to encourage gynecologists to consider this factor.
Voiding function is less clear-cut. Basically, because intrinsic urethral function declines with age, it is not uncommon to see a woman in her 70s or 80s with ISD who also has absent detrusor contractions during voiding studies. The physician can assess this function by ultrasound or urodynamic testing, or by measuring the postvoid residual volume, which usually falls in the range of 100 to 200 mL, especially if no prolapse is present. Thus, even in cases in which I normally would want to do a sling for ISD, I opt against it if the patient has poor voiding function. That’s because the risk of permanent retention may rise as high as 15% to 20% in some of these patients.
Evaluation and treatment of mixed incontinence
DAVILA: I think we all agree that incontinence is easier to address than “hypercontinence” resulting from postoperative urethral obstruction, urinary retention, and irritative voiding symptoms. But what about patients with mixed incontinence? How do you evaluate them? Is there a role for surgical procedures in patients with primary urge incontinence?
I believe in offering all patients both nonsurgical and surgical options.—Dr. Sand
SAND: Many centers offer nonsurgical treatment of mixed incontinence, especially if urge incontinence predominates. But I believe in offering all patients both nonsurgical and surgical options. I end up triaging based on what patients select first, regardless of whether they have pure SUI or mixed symptoms, as long as they have been counseled appropriately about the expected outcomes of the various options.
MYERS: In my practice, I treat the urge symptoms first with anticholinergics or other medications. Then, if the stress incontinence continues, I offer a procedure.
This may be difficult in some cases, such as a patient with severe ISD. It is hard to treat detrusor overactivity when the urethral sphincter is weak, as the woman cannot hold increasing volumes of urine in the bladder.
Thus, I approach these cases by treating the stress incontinence first and then the urgency symptoms if intervention is still necessary—which is a 180-degree shift from my previous statement. In these cases, I treat the ISD first. Then, after the stress incontinence resolves, I offer medications for the urgency.
LUBER: I want to throw a little cold water on surgical treatment of overactive bladder. Clearly, this is an enormous issue. Probably 40% of women who come to my office complaining of urinary incontinence have mixed symptoms or mixed disease as determined by urodynamics. For the doctors out there caring for these patients daily, I think it is important to remember, as Dr. Sand mentioned, that in some cases, anti-incontinence operations can provoke detrusor overactivity in patients who were relatively asymptomatic previously. Dr. Myers’ suggestion that surgery may simply unmask the detrusor overactivity is also possible, of course.
Probably 40% of women who come to my office complaining of urinary incontinence have mixed symptoms.—Dr. Luber
Thus, I think it is reasonable to ask patients reporting mixed symptoms to characterize their urine loss. I usually have them pick a percentage (which isn’t always easy). I ask, “Is your urge incontinence 10%, 50% or 90% of your problem?” If urgency is 90% of the problem and stress incontinence is minimal, then naturally that patient’s care should be focused on the urge incontinence, and vice versa.
It becomes more problematic in the middle, with that 50/50 group. But I’m old-fashioned in that I like to treat the urge incontinence first and get that under control. Of course, in women with poor sphincter function, this distinction becomes more difficult because of the inevitable overlap of symptoms. But this is a small subset of the whole population.
DAVILA: Even if you operate on these patients to correct sphincter function, you must follow them closely. You shouldn’t be saying, “We’ll see you in 6 months.”
In addition, the cofactor of urogenital atrophy should be addressed. Atrophy can cause significant urinary urgency and nocturia symptoms, although most women may not report vaginal atrophy symptoms.4 Local estrogen cream at a low dosage can be used pre- and postoperatively without concern about systemic absorption.5
Which patients benefit from bulking agents?
SAND: We touched on the use of periurethral injections of bulking agents. How do you determine when bulking agents are appropriate?
MYERS: I use periurethral injections for demonstrated sphincter deficiency. The ideal patient has a supported bladder neck and true sphincter deficiency—for example, patients whose sphincter deficiency is caused by pelvic radiation or significant surgical scarring.
In recent years, I have loosened the guidelines slightly, in that a number of my patients are elderly women with urethral hypermobility who are not healthy enough to undergo a major operation. In these patients, I use a pessary to support the bladder neck before performing an injection, and I make sure the patient understands that she will need to use the pessary even after the injection. I have better results when there is no hypermobility.
LUBER: The concept of ISD as the sole cause of urinary incontinence is less mysterious than it at first appears. In the typical patient with a fixed poorly functioning urethra, a Qtip test will be 0 degrees at rest, and will still be 0 degrees with straining. The patient will leak readily with any kind of provocative maneuver, be it coughing or slight straining. With or without urodynamics, we know that patient’s urethra is not functioning properly. Such a patient, for me, is the ideal candidate for bulking agents.
Unfortunately, bulking agents tend to have short half-lives of around 2 years. Still, you can improve the quality of life of these patients tremendously in that time by periurethrally injecting bulking agents during a very simple office or outpatient visit.
I have had less satisfaction and success using bulking agents in patients with urethral hypermobility, although I do like Dr. Myers’ idea of correcting the hypermobility with an intravaginal support device and then using collagen to improve their urethral coaptation. That’s a nice concept.
Improvements being studied
SAND: Currently, we have 2 injectables approved by the US Food and Drug Administration: collagen and carbon-coated microspheres. Are new agents coming out? Do you expect improvements in the current agents?
DAVILA: The number of bulking agents being studied right now is huge. I think the future will bring one that can be implanted without the need for cystoscopy. A couple of trials under way use a conical urethral template for needle placement, and the injection is performed without cystoscopy. Although we’re a number of years away from having enough data to support the widespread implementation of this approach, the momentum is certainly in that direction.
The half-lives of bulking agents also are increasing. Collagen was an excellent start, but we are moving toward permanence. In addition, most of the agents being studied are simpler to inject than collagen. Biotechnology is coming to the forefront with polymers that are either temperature-sensitive or able to reconfigure themselves over time. Thus, they should serve as a nidus for collagen deposition or remain in place longer, enhancing urethral sphincteric function.
It isn’t clear which agent will take a leading role in the next few years, but a number of them have great promise. This is an exciting time in the management of stress incontinence as we move from invasive procedures such as retropubic urethropexy to minimally invasive surgery—as well as from the operating room to the office.
Radiofrequency technologies
SAND: What do you think about the new radiofrequency technologies being used to create support in periurethral tissues to correct hypermobility? Is there a role for this evolving technology?
LUBER: The concept of developing scar tissue adjacent to the urethra to provide better support underlies much of what we already do surgically. So there is some logic in the use of radiofrequency technologies to accomplish the same thing.
Still, there is the theoretical risk of further denervating the urethra, which is probably already denervated. Having looked at outcome studies for radiofrequency therapy, I think it’s a modality that can be embraced, but that should be done under the auspices of clinical research. Again, I’m old fashioned and am not comfortable integrating untested approaches into routine clinical care until we have adequate evidence of their effectiveness. In caring for incontinence, it is important that this effectiveness be looked at over a reasonable period of time, for example, 48 months.
It is hard to treat detrusor overactivity when the urethral sphincter is weak.—Dr. Myers
DAVILA: I have had some experience with radiofrequency therapy, and the tissue changes it stimulates are fairly impressive. I share your concern about the issue of denervation. In fact, my colleagues and I are hoping to initiate a trial in which we plan to look at pudendal latencies to the urethral sphincter in these patients. As of now, with limited experience, it appears that there is no worsening of urethral sphincter function with the therapy.
Tension-free vaginal tape
SAND: What about other new treatments on the horizon?
DAVILA: Like bulking agents, new minimally invasive surgical techniques are also increasing in number. The advent of the tension-free vaginal tape (TVT), a technique that moved from Sweden to the United States a couple of years ago, truly has revolutionized what we do in anti-incontinence surgery. I think we all can agree that it has changed our practice patterns quite significantly, and the modifications or theoretical improvements by different companies are likely to have a further impact.
Polypropylene mesh appears to be very well received by the body. It doesn’t get rejected or infected at a significant rate, so most physicians are comfortable with it. But I think it has become the surgeon’s preference as to which product works better.
For the Ob/Gyn, the primary issue remains the small yet well-recognized risks of retropubic needle placement, beginning with bladder perforation and including vascular or bowel injury. As a result, many Ob/Gyns have probably been hesitant to perform these procedures.
What the future holds is very exciting: the transobturator approach to surgery for stress incontinence. Instead of bringing the needle superiorly behind the pubic bone, the surgeon maneuvers it laterally beneath the pubic ramus and through the obturator membrane. This is an anti-incontinence procedure a gynecologist can embrace. It may not be necessary to perform cystoscopy afterward because the surgery is nowhere near the bladder.
The French have taken the lead in developing this technique and recently presented approximately 2 years of data.6,7 A US trial also is being initiated.
LUBER: TVT is billed as a midurethral procedure. However, when you consider where those tapes actually end up after a few months, it probably functions much like a traditional sling. Recent studies are in conflict: Some demonstrate the sling remains at the midurethra while others show that it readily migrates to the bladder neck. So the quest for a less obstructive procedure has not been fulfilled with the TVT.
In fact, in our recent TVT series that Drs. Lukacz and Nager are meticulously following, the voiding time increases and the maximum flow decreases postoperatively in roughly 30% of patients. So it definitely has some obstructive characteristics.
Quest for the Holy Grail continues
LUBER: I think we need to continue to explore these newer, less invasive procedures that offer wonderful potential. At this point, I might employ them in patients who are not able to tolerate the more invasive procedures or who flatly state that they want a less invasive operation. But I am not ready to embrace them as first-line therapy for all of my patients with stress incontinence.
Over the years, we have all seen various waves of surgical innovation, from the noincision urethropexies of the mid-1990s to the laparoscopic techniques prominent later in the decade. Now, more minimally invasive techniques are coming to the fore. At some point, we may even find the Holy Grail. But for the most part, we continue to evolve, examining new approaches until we are forced to reconcile with their limitations.
SAND: It is clear that this is a very exciting time to be treating SUI, with continuing innovation and an increased awareness of the problem by the public. While we all treat these women differently, there is a surprising consensus. We all seem to concur that the operation should be tailored to the individual, considering the relative balance of any concurrent urinary urge incontinence, urethral hypermobility, voiding dysfunction, and the woman’s health and activity level. We all use midurethral slings and periurethral bulking agents in selected women, but still also rely on Burch procedures and bladder-neck slings.
The authors report no financial relationship with any companies whose products are mentioned in this article.
1. Mutone N, Mastropietro M, Brizendine E, Hale D. Effect of tension-free vaginal tape procedure on urodynamic continence indices. Obstet Gynecol. 2001;98:638-645.
2. McLennan MT, Bent AE. Supine empty stress test as a predictor of low valsalva leak pressure. Neurourol Urodynamics. 1998;17:121-127.
3. Lobel RW, Sand PK. The empty supine stress test as a predictor of intrinsic urethral sphincter dysfunction. Obstet Gynecol. 1996;88:128-132.
4. Davila GW, Singh A, Karapanagiotou I, et al. Are women with urogenital atrophy symptomatic? Am J Obstet Gynecol. 2003;188:382-388.
5. Handa VL, Bachus KE, Johnston WW, Robboy SJ, Hammond CP. Vaginal administration of low-dose conjugated estrogens: systemic absorption and effects on the endometrium. Obstet Gynecol. 1994;84:215-218.
6. Delorme E. Transobturator sling: a minimally invasive procedure to treat female stress urinary incontinence. Prog Urol. 2001;11:1306-1313.
7. Dargent D, Bretones S, George P, Mellier G. Insertion of a suburethral sling through the obturator membrane in the treatment of female urinary incontinence. Gynecol Obstet Fertil. 2002;30:576-582.
-

-

-


Choosing appropriate surgical interventions is the focus of the second of our 2-part panel on stress urinary incontinence (SUI). The panelists discuss:
- how to weigh the factors that influence choice of technique, including Burch retropubic urethropexy and the various sling operations;
- the challenges of treating “mixed” stress and urge incontinence; and
- when to use bulking agents for intrinsic sphincteric deficiency.
The panelists also share tips on:
- how to help patients accurately describe their symptoms, and
- what issues to review with patients as they consider their options.
Part 1 covered medical therapies such as pelvic floor muscle rehabilitation, occlusive devices, and drugs. (Stress urinary incontinence: A closer look at nonsurgical therapies. OBG Management. 2003;15(9):40-51.)
Review surgical options with the patient
SAND: How do you counsel patients about surgical treatments for stress urinary incontinence?
MYERS: After the initial evaluation and diagnosis, I review the conservative options, and I also offer surgery. At this time, I discuss whether an operation is appropriate.
I work with the patient, going over her diagnosis as well as the different types of operations that are performed. Some patients are fairly well educated about their options, having looked up information on the Internet.
Next I explore whether other types of procedures need to be done concomitantly. For instance, does the patient need abdominal hysterectomy for some other reason? That would prompt me to offer an abdominal approach to the SUI. Do other types of vaginal surgery need to be done? Then I would probably opt for a vaginal approach.
I also look at the patient’s health status. Is she healthy and physically active? Or is she sedentary with comorbidities? In a woman who is a poor surgical candidate, I would consider less invasive procedures or procedures with less operative risk, such as urethral injections or the newer tape slings.
LUBER: When it comes to surgery for urinary incontinence, I like to reinforce the reconstructive nature of the repair, since patients tend to view surgical procedures as definitive. For example, when the uterus and ovaries are removed, they never bother that patient again. Incontinence procedures are different. Their effect is potentially time-limited, so it’s important to reinforce the patient’s understanding of their reconstructive and fallible nature.
At the first surgical consultation, I basically go through an informed consent. I do so again preoperatively, but I think it is a very important initial step for a patient who is considering surgery.
SAND: What if a patient isn’t sure she wants surgery?
LUBER: When a patient asks, “What should I do, Dr. Luber? Should I have an operation?” I like to use the example of standing in front of the refrigerator and asking, “Gee, am I hungry?” If you have to ask, you probably aren’t.
Potential surgical patients should feel extremely comfortable that they have exhausted all the nonsurgical options. Even if they have decided against nonsurgical therapy, they should feel very comfortable with that choice. Then I am confident we can work through any potential problems of surgery.
As for the operation itself, history has demonstrated the irresistible impulse to innovate during surgery for female stress incontinence. Literally hundreds of operations have been described, and dozens are currently in use; this reinforces the supposition that our techniques are imperfect, and the importance of basing what we do upon the available data. The 1997 American Urological Association guidelines are an excellent example. Looking forward, the National Institutes of Health are sponsoring studies comparing, for example, the goldstandard Burch to the goldstandard sling operation. In the next few years, we should have better evidence-based guidance.
Consider patient characteristics when choosing treatment
SAND: How do you select a surgical procedure for a particular patient?
MYERS: Because of all the different variables, I use various treatment arms. Since my institution does a large number of sling procedures, I am very comfortable performing those operations. I still do retropubic urethropexy. I also do the newer vaginal-tape procedures, and I use bulking agents for patients who have a demonstrated sphincter deficiency with no obvious support problems. Basically, I try to tailor my procedure to the patient.
For example, in a woman who requires a total abdominal hysterectomy for fibroids as well as an anti-incontinence procedure, I would do a Burch operation. For a woman who needed a vaginal hysterectomy, I probably would perform a sling procedure.
DAVILA: Ob/Gyns may be a bit unsure how to proceed at this point. For example, we formerly considered the Burch procedure the gold standard against which other procedures should be judged. Although I continue to view it as the standard, the Burch procedure increasingly is overlooked in favor of tension-free slings—due to increased marketing of the latter—for any form of stress incontinence. I think that has led us down a path that is not entirely beneficial for many of our patients.
In contrast to that approach, I use a basic evaluation of the patient to construct a treatment algorithm. In simple terms, 2 factors are taken into account: urethral sphincter function and bladder neck or urethral support. Using those 2 factors, I create a 2×2 table to select patients who do or don’t have urethral hypermobility and who do or don’t have sphincteric deficiency (TABLE).
For example, a patient with hypermobility and normal sphincter function has what we might consider “garden-variety” stress incontinence. Such patients do well with any form of treatment, whether it’s conservative therapy, a vaginal device, or a Burch procedure or tension-free sling.
I am more concerned when the patient has hypermobility with a significant degree of sphincteric deficiency. In recent years, the tendency has been to treat such patients with a tension-free sling. Although the literature is not absolutely clear, the success rate of tension-free slings in a patient with intrinsic sphincteric deficiency (ISD) is not as high as in a woman without ISD.1 So in these patients, I do a traditional sling.
Atrophy can cause significant urgency and nocturia symptoms.—Dr. Davila
The other 2 groups of patients have no hypermobility. I think most of us would agree that a woman with ISD and no hypermobility would best be treated with a bulking agent such as Contigen (C.R. Bard, Murray Hill, NJ) or Durasphere (Advanced Uroscience, St. Paul, Minn).
I have had good success rates with bulking agents. I do not think current data would support a tension-free sling in these patients.
Finally, there is the patient without hypermobility who has normal sphincter function. These patients do fairly well with conservative therapy, including pelvic floor exercises. They usually have mild forms of stress incontinence to begin with.
TABLE 1
Stress urinary incontinence treatment choices based on urethral support and urethral sphincteric function
| URETHRAL SUPPORT | URETHRAL SPHINCTERIC FUNCTION | |
|---|---|---|
| Bladder neck mobility (Q-tip test) | Normal urethral | Poor urethral function function |
| MUCP >20 cm H20 | LPP <20 cm H20 | |
| VLPP <60 cm H20 | VLPP >60 cm H20 | |
| Negative EBST | Positive EBST | |
| >30 degrees (hypermobility) | Kegel exercises | Traditional sling |
| Biofeedback | ||
| Vaginal device | ||
| Tension-free vaginal tape | ||
| Burch urethropexy | ||
| <30 degrees | Kegel exercises | Bulking agents |
| Biofeedback | ||
| Source: GW Davila, MD | ||
| EBST = empty bladder stress test; | ||
| MUCP = maximal urethral closure pressure; | ||
| VLPP = Valsalva leak point pressure | ||
| NOTE: Urethral plugs may function in all categories | ||
Simple method to assess sphincter function
DAVILA: This is the algorithm I tend to follow. It does entail evaluation of the urethral sphincter mechanism, but there are simpler ways to do that than with multi-channel urodynamics. For example, if the patient leaks with a Valsalva maneuver, after voiding, in a supine position, that suggests she has ISD and therefore is likely to have a low-pressure urethra or a low leak-point pressure. Multiple centers have reported on this.2,3
Role of urethral function in choice of treatment
LUBER: There seems to be 2 schools. The first dichotomizes urethral function to reasonable (“good” urethral function) versus unreasonable (“poor” urethral function or ISD) and selects the operation based on that. Thus, a Burch or supportive operation would be used for good urethral function with hypermobility, and a sling operation would be selected for poor urethral function or ISD.
More recently, some experts have preached an inclusive approach, whereby all patients undergo sling operations. That strategy evolved out of frustration over the difficulty of identifying which patients have poor urethral function. Unfortunately, we lack good long-term data on the potential downside of performing sling procedures on all patients with incontinence. Hopefully, over the next 3 years, the National Institutes of Health data will help clarify whether we need to dichotomize patients in terms of urethral function.
Meanwhile, at our center, we continue to consider urethral function the deciding factor as to whether patients will undergo a gold-standard Burch procedure or a sling. We steer toward a sling procedure when the patient clearly has poor urethral function or ISD. Of course in cases of the fixed immobile and poorly functioning urethra, we also make bulking agents available.
Additional factors in the choice of treatment
SAND: We throw 2 other things into the algorithm at our center: One is detrusor overactivity, which is very important when considering surgical treatment of stress urinary incontinence. The second is voiding function.
Activity level of the patient is an additional measure, as Dr. Myers commented on earlier. I’m not as concerned about age as I am about the patient’s physical activity and expectations for the operation over time. For example, for a woman who is relatively homebound and not physically active and has poor voiding function (underactive detrusor) and prolapse with normal intrinsic urethral function, a Kelly Kennedy procedure at the time of an anterior colporrhaphy may be more appropriate than a Burch procedure.
Detrusor overactivity is important because, in the trial that we performed, the Burch retropubic urethropexy had a 55% objective cure rate of concurrent detrusor overactivity and a 70% subjective cure rate of the symptom of urge urinary incontinence. In contrast, over the last 12 years, the sling procedure has had a resolution rate of between 20% and 28% for recurrent detrusor overactivity. Recent subjective data at 1 year for midurethral slings fall into the same range: 20% to 30% resolution of recurrent urge incontinence.
Another factor is de novo detrusor overactivity. The rate of de novo detrusor overactivity and urge incontinence in our sling patients seems consistently higher, compared with our retropubic urethropexy patients. We all know that the patient with urge incontinence is far more upset about her condition than the patient who has predictable stress incontinence, because urge incontinence can be far more destructive to quality of life. I try to encourage gynecologists to consider this factor.
Voiding function is less clear-cut. Basically, because intrinsic urethral function declines with age, it is not uncommon to see a woman in her 70s or 80s with ISD who also has absent detrusor contractions during voiding studies. The physician can assess this function by ultrasound or urodynamic testing, or by measuring the postvoid residual volume, which usually falls in the range of 100 to 200 mL, especially if no prolapse is present. Thus, even in cases in which I normally would want to do a sling for ISD, I opt against it if the patient has poor voiding function. That’s because the risk of permanent retention may rise as high as 15% to 20% in some of these patients.
Evaluation and treatment of mixed incontinence
DAVILA: I think we all agree that incontinence is easier to address than “hypercontinence” resulting from postoperative urethral obstruction, urinary retention, and irritative voiding symptoms. But what about patients with mixed incontinence? How do you evaluate them? Is there a role for surgical procedures in patients with primary urge incontinence?
I believe in offering all patients both nonsurgical and surgical options.—Dr. Sand
SAND: Many centers offer nonsurgical treatment of mixed incontinence, especially if urge incontinence predominates. But I believe in offering all patients both nonsurgical and surgical options. I end up triaging based on what patients select first, regardless of whether they have pure SUI or mixed symptoms, as long as they have been counseled appropriately about the expected outcomes of the various options.
MYERS: In my practice, I treat the urge symptoms first with anticholinergics or other medications. Then, if the stress incontinence continues, I offer a procedure.
This may be difficult in some cases, such as a patient with severe ISD. It is hard to treat detrusor overactivity when the urethral sphincter is weak, as the woman cannot hold increasing volumes of urine in the bladder.
Thus, I approach these cases by treating the stress incontinence first and then the urgency symptoms if intervention is still necessary—which is a 180-degree shift from my previous statement. In these cases, I treat the ISD first. Then, after the stress incontinence resolves, I offer medications for the urgency.
LUBER: I want to throw a little cold water on surgical treatment of overactive bladder. Clearly, this is an enormous issue. Probably 40% of women who come to my office complaining of urinary incontinence have mixed symptoms or mixed disease as determined by urodynamics. For the doctors out there caring for these patients daily, I think it is important to remember, as Dr. Sand mentioned, that in some cases, anti-incontinence operations can provoke detrusor overactivity in patients who were relatively asymptomatic previously. Dr. Myers’ suggestion that surgery may simply unmask the detrusor overactivity is also possible, of course.
Probably 40% of women who come to my office complaining of urinary incontinence have mixed symptoms.—Dr. Luber
Thus, I think it is reasonable to ask patients reporting mixed symptoms to characterize their urine loss. I usually have them pick a percentage (which isn’t always easy). I ask, “Is your urge incontinence 10%, 50% or 90% of your problem?” If urgency is 90% of the problem and stress incontinence is minimal, then naturally that patient’s care should be focused on the urge incontinence, and vice versa.
It becomes more problematic in the middle, with that 50/50 group. But I’m old-fashioned in that I like to treat the urge incontinence first and get that under control. Of course, in women with poor sphincter function, this distinction becomes more difficult because of the inevitable overlap of symptoms. But this is a small subset of the whole population.
DAVILA: Even if you operate on these patients to correct sphincter function, you must follow them closely. You shouldn’t be saying, “We’ll see you in 6 months.”
In addition, the cofactor of urogenital atrophy should be addressed. Atrophy can cause significant urinary urgency and nocturia symptoms, although most women may not report vaginal atrophy symptoms.4 Local estrogen cream at a low dosage can be used pre- and postoperatively without concern about systemic absorption.5
Which patients benefit from bulking agents?
SAND: We touched on the use of periurethral injections of bulking agents. How do you determine when bulking agents are appropriate?
MYERS: I use periurethral injections for demonstrated sphincter deficiency. The ideal patient has a supported bladder neck and true sphincter deficiency—for example, patients whose sphincter deficiency is caused by pelvic radiation or significant surgical scarring.
In recent years, I have loosened the guidelines slightly, in that a number of my patients are elderly women with urethral hypermobility who are not healthy enough to undergo a major operation. In these patients, I use a pessary to support the bladder neck before performing an injection, and I make sure the patient understands that she will need to use the pessary even after the injection. I have better results when there is no hypermobility.
LUBER: The concept of ISD as the sole cause of urinary incontinence is less mysterious than it at first appears. In the typical patient with a fixed poorly functioning urethra, a Qtip test will be 0 degrees at rest, and will still be 0 degrees with straining. The patient will leak readily with any kind of provocative maneuver, be it coughing or slight straining. With or without urodynamics, we know that patient’s urethra is not functioning properly. Such a patient, for me, is the ideal candidate for bulking agents.
Unfortunately, bulking agents tend to have short half-lives of around 2 years. Still, you can improve the quality of life of these patients tremendously in that time by periurethrally injecting bulking agents during a very simple office or outpatient visit.
I have had less satisfaction and success using bulking agents in patients with urethral hypermobility, although I do like Dr. Myers’ idea of correcting the hypermobility with an intravaginal support device and then using collagen to improve their urethral coaptation. That’s a nice concept.
Improvements being studied
SAND: Currently, we have 2 injectables approved by the US Food and Drug Administration: collagen and carbon-coated microspheres. Are new agents coming out? Do you expect improvements in the current agents?
DAVILA: The number of bulking agents being studied right now is huge. I think the future will bring one that can be implanted without the need for cystoscopy. A couple of trials under way use a conical urethral template for needle placement, and the injection is performed without cystoscopy. Although we’re a number of years away from having enough data to support the widespread implementation of this approach, the momentum is certainly in that direction.
The half-lives of bulking agents also are increasing. Collagen was an excellent start, but we are moving toward permanence. In addition, most of the agents being studied are simpler to inject than collagen. Biotechnology is coming to the forefront with polymers that are either temperature-sensitive or able to reconfigure themselves over time. Thus, they should serve as a nidus for collagen deposition or remain in place longer, enhancing urethral sphincteric function.
It isn’t clear which agent will take a leading role in the next few years, but a number of them have great promise. This is an exciting time in the management of stress incontinence as we move from invasive procedures such as retropubic urethropexy to minimally invasive surgery—as well as from the operating room to the office.
Radiofrequency technologies
SAND: What do you think about the new radiofrequency technologies being used to create support in periurethral tissues to correct hypermobility? Is there a role for this evolving technology?
LUBER: The concept of developing scar tissue adjacent to the urethra to provide better support underlies much of what we already do surgically. So there is some logic in the use of radiofrequency technologies to accomplish the same thing.
Still, there is the theoretical risk of further denervating the urethra, which is probably already denervated. Having looked at outcome studies for radiofrequency therapy, I think it’s a modality that can be embraced, but that should be done under the auspices of clinical research. Again, I’m old fashioned and am not comfortable integrating untested approaches into routine clinical care until we have adequate evidence of their effectiveness. In caring for incontinence, it is important that this effectiveness be looked at over a reasonable period of time, for example, 48 months.
It is hard to treat detrusor overactivity when the urethral sphincter is weak.—Dr. Myers
DAVILA: I have had some experience with radiofrequency therapy, and the tissue changes it stimulates are fairly impressive. I share your concern about the issue of denervation. In fact, my colleagues and I are hoping to initiate a trial in which we plan to look at pudendal latencies to the urethral sphincter in these patients. As of now, with limited experience, it appears that there is no worsening of urethral sphincter function with the therapy.
Tension-free vaginal tape
SAND: What about other new treatments on the horizon?
DAVILA: Like bulking agents, new minimally invasive surgical techniques are also increasing in number. The advent of the tension-free vaginal tape (TVT), a technique that moved from Sweden to the United States a couple of years ago, truly has revolutionized what we do in anti-incontinence surgery. I think we all can agree that it has changed our practice patterns quite significantly, and the modifications or theoretical improvements by different companies are likely to have a further impact.
Polypropylene mesh appears to be very well received by the body. It doesn’t get rejected or infected at a significant rate, so most physicians are comfortable with it. But I think it has become the surgeon’s preference as to which product works better.
For the Ob/Gyn, the primary issue remains the small yet well-recognized risks of retropubic needle placement, beginning with bladder perforation and including vascular or bowel injury. As a result, many Ob/Gyns have probably been hesitant to perform these procedures.
What the future holds is very exciting: the transobturator approach to surgery for stress incontinence. Instead of bringing the needle superiorly behind the pubic bone, the surgeon maneuvers it laterally beneath the pubic ramus and through the obturator membrane. This is an anti-incontinence procedure a gynecologist can embrace. It may not be necessary to perform cystoscopy afterward because the surgery is nowhere near the bladder.
The French have taken the lead in developing this technique and recently presented approximately 2 years of data.6,7 A US trial also is being initiated.
LUBER: TVT is billed as a midurethral procedure. However, when you consider where those tapes actually end up after a few months, it probably functions much like a traditional sling. Recent studies are in conflict: Some demonstrate the sling remains at the midurethra while others show that it readily migrates to the bladder neck. So the quest for a less obstructive procedure has not been fulfilled with the TVT.
In fact, in our recent TVT series that Drs. Lukacz and Nager are meticulously following, the voiding time increases and the maximum flow decreases postoperatively in roughly 30% of patients. So it definitely has some obstructive characteristics.
Quest for the Holy Grail continues
LUBER: I think we need to continue to explore these newer, less invasive procedures that offer wonderful potential. At this point, I might employ them in patients who are not able to tolerate the more invasive procedures or who flatly state that they want a less invasive operation. But I am not ready to embrace them as first-line therapy for all of my patients with stress incontinence.
Over the years, we have all seen various waves of surgical innovation, from the noincision urethropexies of the mid-1990s to the laparoscopic techniques prominent later in the decade. Now, more minimally invasive techniques are coming to the fore. At some point, we may even find the Holy Grail. But for the most part, we continue to evolve, examining new approaches until we are forced to reconcile with their limitations.
SAND: It is clear that this is a very exciting time to be treating SUI, with continuing innovation and an increased awareness of the problem by the public. While we all treat these women differently, there is a surprising consensus. We all seem to concur that the operation should be tailored to the individual, considering the relative balance of any concurrent urinary urge incontinence, urethral hypermobility, voiding dysfunction, and the woman’s health and activity level. We all use midurethral slings and periurethral bulking agents in selected women, but still also rely on Burch procedures and bladder-neck slings.
The authors report no financial relationship with any companies whose products are mentioned in this article.
-

-

-


Choosing appropriate surgical interventions is the focus of the second of our 2-part panel on stress urinary incontinence (SUI). The panelists discuss:
- how to weigh the factors that influence choice of technique, including Burch retropubic urethropexy and the various sling operations;
- the challenges of treating “mixed” stress and urge incontinence; and
- when to use bulking agents for intrinsic sphincteric deficiency.
The panelists also share tips on:
- how to help patients accurately describe their symptoms, and
- what issues to review with patients as they consider their options.
Part 1 covered medical therapies such as pelvic floor muscle rehabilitation, occlusive devices, and drugs. (Stress urinary incontinence: A closer look at nonsurgical therapies. OBG Management. 2003;15(9):40-51.)
Review surgical options with the patient
SAND: How do you counsel patients about surgical treatments for stress urinary incontinence?
MYERS: After the initial evaluation and diagnosis, I review the conservative options, and I also offer surgery. At this time, I discuss whether an operation is appropriate.
I work with the patient, going over her diagnosis as well as the different types of operations that are performed. Some patients are fairly well educated about their options, having looked up information on the Internet.
Next I explore whether other types of procedures need to be done concomitantly. For instance, does the patient need abdominal hysterectomy for some other reason? That would prompt me to offer an abdominal approach to the SUI. Do other types of vaginal surgery need to be done? Then I would probably opt for a vaginal approach.
I also look at the patient’s health status. Is she healthy and physically active? Or is she sedentary with comorbidities? In a woman who is a poor surgical candidate, I would consider less invasive procedures or procedures with less operative risk, such as urethral injections or the newer tape slings.
LUBER: When it comes to surgery for urinary incontinence, I like to reinforce the reconstructive nature of the repair, since patients tend to view surgical procedures as definitive. For example, when the uterus and ovaries are removed, they never bother that patient again. Incontinence procedures are different. Their effect is potentially time-limited, so it’s important to reinforce the patient’s understanding of their reconstructive and fallible nature.
At the first surgical consultation, I basically go through an informed consent. I do so again preoperatively, but I think it is a very important initial step for a patient who is considering surgery.
SAND: What if a patient isn’t sure she wants surgery?
LUBER: When a patient asks, “What should I do, Dr. Luber? Should I have an operation?” I like to use the example of standing in front of the refrigerator and asking, “Gee, am I hungry?” If you have to ask, you probably aren’t.
Potential surgical patients should feel extremely comfortable that they have exhausted all the nonsurgical options. Even if they have decided against nonsurgical therapy, they should feel very comfortable with that choice. Then I am confident we can work through any potential problems of surgery.
As for the operation itself, history has demonstrated the irresistible impulse to innovate during surgery for female stress incontinence. Literally hundreds of operations have been described, and dozens are currently in use; this reinforces the supposition that our techniques are imperfect, and the importance of basing what we do upon the available data. The 1997 American Urological Association guidelines are an excellent example. Looking forward, the National Institutes of Health are sponsoring studies comparing, for example, the goldstandard Burch to the goldstandard sling operation. In the next few years, we should have better evidence-based guidance.
Consider patient characteristics when choosing treatment
SAND: How do you select a surgical procedure for a particular patient?
MYERS: Because of all the different variables, I use various treatment arms. Since my institution does a large number of sling procedures, I am very comfortable performing those operations. I still do retropubic urethropexy. I also do the newer vaginal-tape procedures, and I use bulking agents for patients who have a demonstrated sphincter deficiency with no obvious support problems. Basically, I try to tailor my procedure to the patient.
For example, in a woman who requires a total abdominal hysterectomy for fibroids as well as an anti-incontinence procedure, I would do a Burch operation. For a woman who needed a vaginal hysterectomy, I probably would perform a sling procedure.
DAVILA: Ob/Gyns may be a bit unsure how to proceed at this point. For example, we formerly considered the Burch procedure the gold standard against which other procedures should be judged. Although I continue to view it as the standard, the Burch procedure increasingly is overlooked in favor of tension-free slings—due to increased marketing of the latter—for any form of stress incontinence. I think that has led us down a path that is not entirely beneficial for many of our patients.
In contrast to that approach, I use a basic evaluation of the patient to construct a treatment algorithm. In simple terms, 2 factors are taken into account: urethral sphincter function and bladder neck or urethral support. Using those 2 factors, I create a 2×2 table to select patients who do or don’t have urethral hypermobility and who do or don’t have sphincteric deficiency (TABLE).
For example, a patient with hypermobility and normal sphincter function has what we might consider “garden-variety” stress incontinence. Such patients do well with any form of treatment, whether it’s conservative therapy, a vaginal device, or a Burch procedure or tension-free sling.
I am more concerned when the patient has hypermobility with a significant degree of sphincteric deficiency. In recent years, the tendency has been to treat such patients with a tension-free sling. Although the literature is not absolutely clear, the success rate of tension-free slings in a patient with intrinsic sphincteric deficiency (ISD) is not as high as in a woman without ISD.1 So in these patients, I do a traditional sling.
Atrophy can cause significant urgency and nocturia symptoms.—Dr. Davila
The other 2 groups of patients have no hypermobility. I think most of us would agree that a woman with ISD and no hypermobility would best be treated with a bulking agent such as Contigen (C.R. Bard, Murray Hill, NJ) or Durasphere (Advanced Uroscience, St. Paul, Minn).
I have had good success rates with bulking agents. I do not think current data would support a tension-free sling in these patients.
Finally, there is the patient without hypermobility who has normal sphincter function. These patients do fairly well with conservative therapy, including pelvic floor exercises. They usually have mild forms of stress incontinence to begin with.
TABLE 1
Stress urinary incontinence treatment choices based on urethral support and urethral sphincteric function
| URETHRAL SUPPORT | URETHRAL SPHINCTERIC FUNCTION | |
|---|---|---|
| Bladder neck mobility (Q-tip test) | Normal urethral | Poor urethral function function |
| MUCP >20 cm H20 | LPP <20 cm H20 | |
| VLPP <60 cm H20 | VLPP >60 cm H20 | |
| Negative EBST | Positive EBST | |
| >30 degrees (hypermobility) | Kegel exercises | Traditional sling |
| Biofeedback | ||
| Vaginal device | ||
| Tension-free vaginal tape | ||
| Burch urethropexy | ||
| <30 degrees | Kegel exercises | Bulking agents |
| Biofeedback | ||
| Source: GW Davila, MD | ||
| EBST = empty bladder stress test; | ||
| MUCP = maximal urethral closure pressure; | ||
| VLPP = Valsalva leak point pressure | ||
| NOTE: Urethral plugs may function in all categories | ||
Simple method to assess sphincter function
DAVILA: This is the algorithm I tend to follow. It does entail evaluation of the urethral sphincter mechanism, but there are simpler ways to do that than with multi-channel urodynamics. For example, if the patient leaks with a Valsalva maneuver, after voiding, in a supine position, that suggests she has ISD and therefore is likely to have a low-pressure urethra or a low leak-point pressure. Multiple centers have reported on this.2,3
Role of urethral function in choice of treatment
LUBER: There seems to be 2 schools. The first dichotomizes urethral function to reasonable (“good” urethral function) versus unreasonable (“poor” urethral function or ISD) and selects the operation based on that. Thus, a Burch or supportive operation would be used for good urethral function with hypermobility, and a sling operation would be selected for poor urethral function or ISD.
More recently, some experts have preached an inclusive approach, whereby all patients undergo sling operations. That strategy evolved out of frustration over the difficulty of identifying which patients have poor urethral function. Unfortunately, we lack good long-term data on the potential downside of performing sling procedures on all patients with incontinence. Hopefully, over the next 3 years, the National Institutes of Health data will help clarify whether we need to dichotomize patients in terms of urethral function.
Meanwhile, at our center, we continue to consider urethral function the deciding factor as to whether patients will undergo a gold-standard Burch procedure or a sling. We steer toward a sling procedure when the patient clearly has poor urethral function or ISD. Of course in cases of the fixed immobile and poorly functioning urethra, we also make bulking agents available.
Additional factors in the choice of treatment
SAND: We throw 2 other things into the algorithm at our center: One is detrusor overactivity, which is very important when considering surgical treatment of stress urinary incontinence. The second is voiding function.
Activity level of the patient is an additional measure, as Dr. Myers commented on earlier. I’m not as concerned about age as I am about the patient’s physical activity and expectations for the operation over time. For example, for a woman who is relatively homebound and not physically active and has poor voiding function (underactive detrusor) and prolapse with normal intrinsic urethral function, a Kelly Kennedy procedure at the time of an anterior colporrhaphy may be more appropriate than a Burch procedure.
Detrusor overactivity is important because, in the trial that we performed, the Burch retropubic urethropexy had a 55% objective cure rate of concurrent detrusor overactivity and a 70% subjective cure rate of the symptom of urge urinary incontinence. In contrast, over the last 12 years, the sling procedure has had a resolution rate of between 20% and 28% for recurrent detrusor overactivity. Recent subjective data at 1 year for midurethral slings fall into the same range: 20% to 30% resolution of recurrent urge incontinence.
Another factor is de novo detrusor overactivity. The rate of de novo detrusor overactivity and urge incontinence in our sling patients seems consistently higher, compared with our retropubic urethropexy patients. We all know that the patient with urge incontinence is far more upset about her condition than the patient who has predictable stress incontinence, because urge incontinence can be far more destructive to quality of life. I try to encourage gynecologists to consider this factor.
Voiding function is less clear-cut. Basically, because intrinsic urethral function declines with age, it is not uncommon to see a woman in her 70s or 80s with ISD who also has absent detrusor contractions during voiding studies. The physician can assess this function by ultrasound or urodynamic testing, or by measuring the postvoid residual volume, which usually falls in the range of 100 to 200 mL, especially if no prolapse is present. Thus, even in cases in which I normally would want to do a sling for ISD, I opt against it if the patient has poor voiding function. That’s because the risk of permanent retention may rise as high as 15% to 20% in some of these patients.
Evaluation and treatment of mixed incontinence
DAVILA: I think we all agree that incontinence is easier to address than “hypercontinence” resulting from postoperative urethral obstruction, urinary retention, and irritative voiding symptoms. But what about patients with mixed incontinence? How do you evaluate them? Is there a role for surgical procedures in patients with primary urge incontinence?
I believe in offering all patients both nonsurgical and surgical options.—Dr. Sand
SAND: Many centers offer nonsurgical treatment of mixed incontinence, especially if urge incontinence predominates. But I believe in offering all patients both nonsurgical and surgical options. I end up triaging based on what patients select first, regardless of whether they have pure SUI or mixed symptoms, as long as they have been counseled appropriately about the expected outcomes of the various options.
MYERS: In my practice, I treat the urge symptoms first with anticholinergics or other medications. Then, if the stress incontinence continues, I offer a procedure.
This may be difficult in some cases, such as a patient with severe ISD. It is hard to treat detrusor overactivity when the urethral sphincter is weak, as the woman cannot hold increasing volumes of urine in the bladder.
Thus, I approach these cases by treating the stress incontinence first and then the urgency symptoms if intervention is still necessary—which is a 180-degree shift from my previous statement. In these cases, I treat the ISD first. Then, after the stress incontinence resolves, I offer medications for the urgency.
LUBER: I want to throw a little cold water on surgical treatment of overactive bladder. Clearly, this is an enormous issue. Probably 40% of women who come to my office complaining of urinary incontinence have mixed symptoms or mixed disease as determined by urodynamics. For the doctors out there caring for these patients daily, I think it is important to remember, as Dr. Sand mentioned, that in some cases, anti-incontinence operations can provoke detrusor overactivity in patients who were relatively asymptomatic previously. Dr. Myers’ suggestion that surgery may simply unmask the detrusor overactivity is also possible, of course.
Probably 40% of women who come to my office complaining of urinary incontinence have mixed symptoms.—Dr. Luber
Thus, I think it is reasonable to ask patients reporting mixed symptoms to characterize their urine loss. I usually have them pick a percentage (which isn’t always easy). I ask, “Is your urge incontinence 10%, 50% or 90% of your problem?” If urgency is 90% of the problem and stress incontinence is minimal, then naturally that patient’s care should be focused on the urge incontinence, and vice versa.
It becomes more problematic in the middle, with that 50/50 group. But I’m old-fashioned in that I like to treat the urge incontinence first and get that under control. Of course, in women with poor sphincter function, this distinction becomes more difficult because of the inevitable overlap of symptoms. But this is a small subset of the whole population.
DAVILA: Even if you operate on these patients to correct sphincter function, you must follow them closely. You shouldn’t be saying, “We’ll see you in 6 months.”
In addition, the cofactor of urogenital atrophy should be addressed. Atrophy can cause significant urinary urgency and nocturia symptoms, although most women may not report vaginal atrophy symptoms.4 Local estrogen cream at a low dosage can be used pre- and postoperatively without concern about systemic absorption.5
Which patients benefit from bulking agents?
SAND: We touched on the use of periurethral injections of bulking agents. How do you determine when bulking agents are appropriate?
MYERS: I use periurethral injections for demonstrated sphincter deficiency. The ideal patient has a supported bladder neck and true sphincter deficiency—for example, patients whose sphincter deficiency is caused by pelvic radiation or significant surgical scarring.
In recent years, I have loosened the guidelines slightly, in that a number of my patients are elderly women with urethral hypermobility who are not healthy enough to undergo a major operation. In these patients, I use a pessary to support the bladder neck before performing an injection, and I make sure the patient understands that she will need to use the pessary even after the injection. I have better results when there is no hypermobility.
LUBER: The concept of ISD as the sole cause of urinary incontinence is less mysterious than it at first appears. In the typical patient with a fixed poorly functioning urethra, a Qtip test will be 0 degrees at rest, and will still be 0 degrees with straining. The patient will leak readily with any kind of provocative maneuver, be it coughing or slight straining. With or without urodynamics, we know that patient’s urethra is not functioning properly. Such a patient, for me, is the ideal candidate for bulking agents.
Unfortunately, bulking agents tend to have short half-lives of around 2 years. Still, you can improve the quality of life of these patients tremendously in that time by periurethrally injecting bulking agents during a very simple office or outpatient visit.
I have had less satisfaction and success using bulking agents in patients with urethral hypermobility, although I do like Dr. Myers’ idea of correcting the hypermobility with an intravaginal support device and then using collagen to improve their urethral coaptation. That’s a nice concept.
Improvements being studied
SAND: Currently, we have 2 injectables approved by the US Food and Drug Administration: collagen and carbon-coated microspheres. Are new agents coming out? Do you expect improvements in the current agents?
DAVILA: The number of bulking agents being studied right now is huge. I think the future will bring one that can be implanted without the need for cystoscopy. A couple of trials under way use a conical urethral template for needle placement, and the injection is performed without cystoscopy. Although we’re a number of years away from having enough data to support the widespread implementation of this approach, the momentum is certainly in that direction.
The half-lives of bulking agents also are increasing. Collagen was an excellent start, but we are moving toward permanence. In addition, most of the agents being studied are simpler to inject than collagen. Biotechnology is coming to the forefront with polymers that are either temperature-sensitive or able to reconfigure themselves over time. Thus, they should serve as a nidus for collagen deposition or remain in place longer, enhancing urethral sphincteric function.
It isn’t clear which agent will take a leading role in the next few years, but a number of them have great promise. This is an exciting time in the management of stress incontinence as we move from invasive procedures such as retropubic urethropexy to minimally invasive surgery—as well as from the operating room to the office.
Radiofrequency technologies
SAND: What do you think about the new radiofrequency technologies being used to create support in periurethral tissues to correct hypermobility? Is there a role for this evolving technology?
LUBER: The concept of developing scar tissue adjacent to the urethra to provide better support underlies much of what we already do surgically. So there is some logic in the use of radiofrequency technologies to accomplish the same thing.
Still, there is the theoretical risk of further denervating the urethra, which is probably already denervated. Having looked at outcome studies for radiofrequency therapy, I think it’s a modality that can be embraced, but that should be done under the auspices of clinical research. Again, I’m old fashioned and am not comfortable integrating untested approaches into routine clinical care until we have adequate evidence of their effectiveness. In caring for incontinence, it is important that this effectiveness be looked at over a reasonable period of time, for example, 48 months.
It is hard to treat detrusor overactivity when the urethral sphincter is weak.—Dr. Myers
DAVILA: I have had some experience with radiofrequency therapy, and the tissue changes it stimulates are fairly impressive. I share your concern about the issue of denervation. In fact, my colleagues and I are hoping to initiate a trial in which we plan to look at pudendal latencies to the urethral sphincter in these patients. As of now, with limited experience, it appears that there is no worsening of urethral sphincter function with the therapy.
Tension-free vaginal tape
SAND: What about other new treatments on the horizon?
DAVILA: Like bulking agents, new minimally invasive surgical techniques are also increasing in number. The advent of the tension-free vaginal tape (TVT), a technique that moved from Sweden to the United States a couple of years ago, truly has revolutionized what we do in anti-incontinence surgery. I think we all can agree that it has changed our practice patterns quite significantly, and the modifications or theoretical improvements by different companies are likely to have a further impact.
Polypropylene mesh appears to be very well received by the body. It doesn’t get rejected or infected at a significant rate, so most physicians are comfortable with it. But I think it has become the surgeon’s preference as to which product works better.
For the Ob/Gyn, the primary issue remains the small yet well-recognized risks of retropubic needle placement, beginning with bladder perforation and including vascular or bowel injury. As a result, many Ob/Gyns have probably been hesitant to perform these procedures.
What the future holds is very exciting: the transobturator approach to surgery for stress incontinence. Instead of bringing the needle superiorly behind the pubic bone, the surgeon maneuvers it laterally beneath the pubic ramus and through the obturator membrane. This is an anti-incontinence procedure a gynecologist can embrace. It may not be necessary to perform cystoscopy afterward because the surgery is nowhere near the bladder.
The French have taken the lead in developing this technique and recently presented approximately 2 years of data.6,7 A US trial also is being initiated.
LUBER: TVT is billed as a midurethral procedure. However, when you consider where those tapes actually end up after a few months, it probably functions much like a traditional sling. Recent studies are in conflict: Some demonstrate the sling remains at the midurethra while others show that it readily migrates to the bladder neck. So the quest for a less obstructive procedure has not been fulfilled with the TVT.
In fact, in our recent TVT series that Drs. Lukacz and Nager are meticulously following, the voiding time increases and the maximum flow decreases postoperatively in roughly 30% of patients. So it definitely has some obstructive characteristics.
Quest for the Holy Grail continues
LUBER: I think we need to continue to explore these newer, less invasive procedures that offer wonderful potential. At this point, I might employ them in patients who are not able to tolerate the more invasive procedures or who flatly state that they want a less invasive operation. But I am not ready to embrace them as first-line therapy for all of my patients with stress incontinence.
Over the years, we have all seen various waves of surgical innovation, from the noincision urethropexies of the mid-1990s to the laparoscopic techniques prominent later in the decade. Now, more minimally invasive techniques are coming to the fore. At some point, we may even find the Holy Grail. But for the most part, we continue to evolve, examining new approaches until we are forced to reconcile with their limitations.
SAND: It is clear that this is a very exciting time to be treating SUI, with continuing innovation and an increased awareness of the problem by the public. While we all treat these women differently, there is a surprising consensus. We all seem to concur that the operation should be tailored to the individual, considering the relative balance of any concurrent urinary urge incontinence, urethral hypermobility, voiding dysfunction, and the woman’s health and activity level. We all use midurethral slings and periurethral bulking agents in selected women, but still also rely on Burch procedures and bladder-neck slings.
The authors report no financial relationship with any companies whose products are mentioned in this article.
1. Mutone N, Mastropietro M, Brizendine E, Hale D. Effect of tension-free vaginal tape procedure on urodynamic continence indices. Obstet Gynecol. 2001;98:638-645.
2. McLennan MT, Bent AE. Supine empty stress test as a predictor of low valsalva leak pressure. Neurourol Urodynamics. 1998;17:121-127.
3. Lobel RW, Sand PK. The empty supine stress test as a predictor of intrinsic urethral sphincter dysfunction. Obstet Gynecol. 1996;88:128-132.
4. Davila GW, Singh A, Karapanagiotou I, et al. Are women with urogenital atrophy symptomatic? Am J Obstet Gynecol. 2003;188:382-388.
5. Handa VL, Bachus KE, Johnston WW, Robboy SJ, Hammond CP. Vaginal administration of low-dose conjugated estrogens: systemic absorption and effects on the endometrium. Obstet Gynecol. 1994;84:215-218.
6. Delorme E. Transobturator sling: a minimally invasive procedure to treat female stress urinary incontinence. Prog Urol. 2001;11:1306-1313.
7. Dargent D, Bretones S, George P, Mellier G. Insertion of a suburethral sling through the obturator membrane in the treatment of female urinary incontinence. Gynecol Obstet Fertil. 2002;30:576-582.
1. Mutone N, Mastropietro M, Brizendine E, Hale D. Effect of tension-free vaginal tape procedure on urodynamic continence indices. Obstet Gynecol. 2001;98:638-645.
2. McLennan MT, Bent AE. Supine empty stress test as a predictor of low valsalva leak pressure. Neurourol Urodynamics. 1998;17:121-127.
3. Lobel RW, Sand PK. The empty supine stress test as a predictor of intrinsic urethral sphincter dysfunction. Obstet Gynecol. 1996;88:128-132.
4. Davila GW, Singh A, Karapanagiotou I, et al. Are women with urogenital atrophy symptomatic? Am J Obstet Gynecol. 2003;188:382-388.
5. Handa VL, Bachus KE, Johnston WW, Robboy SJ, Hammond CP. Vaginal administration of low-dose conjugated estrogens: systemic absorption and effects on the endometrium. Obstet Gynecol. 1994;84:215-218.
6. Delorme E. Transobturator sling: a minimally invasive procedure to treat female stress urinary incontinence. Prog Urol. 2001;11:1306-1313.
7. Dargent D, Bretones S, George P, Mellier G. Insertion of a suburethral sling through the obturator membrane in the treatment of female urinary incontinence. Gynecol Obstet Fertil. 2002;30:576-582.
Meeting the challenge of vesicovaginal fistula repair: Conservative and surgical measures
- Surgical risk factors include prior pelvic surgery, history of pelvic inflammatory disease, pelvic malignancy, endometriosis, infection, diabetes, and anatomic distortion.
- Conservative therapy should be reserved for simple fistulae that are less than 1 cm in size, diagnosed within 7 days of the index surgery, lacking associated carcinoma or radiation, and subject to at least 4 weeks of constant bladder drainage.
- In surgical repair, the Latzko partial colpocleisis or fistulectomy with flap-splitting closure is preferred.
Recent advances have improved the success of vesicovaginal fistula (VVF) repair—a challenge that can test even the most experienced gynecologic surgeon. For example, it now is apparent that some small uncomplicated fistulae respond to conservative treatment. Further, in selected cases, laparoscopic repair can eliminate the need for complicated laparotomy.
In addition, timing of fistula repair no longer requires long periods of observation, and good surgical technique for identifying and repairing bladder injuries at the time of the index surgery can often prevent the development or reduce the severity of VVF.
Vesicovaginal fistula is the most common type of urogenital fistula. Presentation and prognosis vary, depending on location and size of the defect, as well as coexisting factors such as tissue devascularization and previous radiation. However, surgical repair is associated with a high cure rate if it is performed by an experienced surgeon.
Most US cases follow gynecologic surgery
Vesicovaginal fistula was first documented in the mummified remains of Egyptian Queen Henhenit (11th Dynasty, 2050 BC), which were examined in 1923 by Derry.1 Although the exact incidence of VVF in the United States is unknown, the primary cause is gynecologic surgery, especially hysterectomy. The defect is estimated to occur in 0.01% to 0.04% of gynecologic procedures.
A study of 303 women with genitourinary fistula found that the defect was related to gynecologic surgery in 82% of cases, obstetric events in 8%, radiation therapy in 6%, and trauma or fulguration in 4%.2 Rare causes of VVF include lymphogranuloma venereum, tuberculosis, syphilis, bladder stones, and a retained foreign body in the vagina. In rare instances, spontaneous vesicouterine fistulae were reported following uncomplicated vaginal birth after cesarean section.3
Gynecologic surgery may lead to VVF due to extensive dissection between the bladder and the uterus, unrecognized bladder laceration, inappropriate stitch placement, and/or devascularization injury to the tissue planes. Concurrent ureteric involvement has been reported in as many as 10% to 15% of vesicovaginal fistula cases.
In developing countries, vesicovaginal fistulae are far more common and generally related to obstetric factors such as obstructed labor (due to unattended deliveries), small pelvic dimensions, malpresentation, poor uterine contractions, and introital stenosis.
Risk factors. Conditions that may predispose patients to VVF include prior pelvic surgery, a history of pelvic inflammatory disease, pelvic malignancy, endometriosis, infection, diabetes, and anatomic distortion. If these risk factors are present, the patient should be counseled accordingly prior to gynecologic surgery.
Correct classification crucial to surgical success
Proper classification of VVF can help the gynecologic surgeon plan operative intervention. Obstetric vesicovaginal fistulae usually are categorized according to their cause, complexity, and site of obstruction. In contrast, gynecologic fistulae are generally classified as simple or complicated (TABLE).
These levels may have important implications for the surgical approach and prognosis.4 For example, simple vesicovaginal fistulae are usually uncomplicated surgical cases with good prognosis. Complicated vesicovaginal fistulae, on the other hand, can challenge even highly practiced and skilled gynecologic surgeons and are associated with a high rate of recurrence.
Women typically present within specific intervals after the various antecedent events (pelvic surgery, childbirth, radiation therapy) with a primary complaint of constant, painless urinary incontinence. If the fistula is related to traumatic childbirth, most patients experience urine leakage within the first 24 to 48 hours. Following pelvic surgery, symptoms usually occur within the first 30 days. In contrast, radiation-induced fistulae develop over a much longer interval secondary to progressive devascularization necrosis, and may present 30 days to 30 years after the antecedent event.
Some patients report exacerbation during physical activities, which can sometimes lead to erroneous diagnosis of uncomplicated stress incontinence. If the fistula is small, intermittent leakage with increased bladder distention or physical activity may be noted.
Other patients may complain of vaginal discharge or hematuria.
If there is concurrent ureteric involvement, the patient may experience constitutional symptoms (such as fever, chills, and flank pain) or even gastrointestinal symptoms.
Physical findings. Any pooling of fluid in the vagina that is noted should be sent for analysis if the diagnosis is unclear. Next, perform a careful speculum exam that allows visualization of the entire anterior vaginal wall to identify the fistula tract (FIGURE 1). In many cases, the fistula is grossly visible.
Determine the location of the fistula in relation to the vaginal apex and bladder trigone and assess the quality of surrounding tissue (eg, presence of inflammation, edema, or infection). Fistulas near the vaginal apex may require a more complicated abdominal approach, and those close to the trigone may be associated with increased risk of ureteral injury during repair.
If the fistula is particularly small, no tract may be apparent. In such cases, bimanual exam with careful palpation of the anterior wall may help isolate the fistula (eg, when there is a surrounding zone of induration).
Office tests. If no fistula is noted despite highly suspicious signs and symptoms and careful examination, a simple office test can be performed. Using a catheter, fill the bladder with a dyed solution such as normal saline with indigo carmine and repeat the pelvic exam with a half-speculum to visualize the anterior wall. Ask the patient to cough and bear down, and identify the fistula by visualizing urine leakage.
If this test fails to locate the fistula, insert a tampon and ask the patient to perform 10 to 15 minutes of exertional maneuvers, including stair climbing and jumping in place. Then remove the tampon. Visualization of dye beyond the most distal edge of the tampon confirms the presence of a fistula.
A variation of this technique is the double-dye test: Give the patient oral phenazopyridine (Pyridium), fill the bladder with the blue-tinted solution, and insert a tampon. The presence of blue staining suggests vesicovaginal or urethrovaginal fistula, while red staining (Pyridium) suggests ureterovaginal fistula.
Other testing. Further assessment is recommended to rule out concurrent pathology and formulate an appropriate treatment plan. Routine testing should include a urinalysis and culture to exclude coexisting urinary tract infection, an electrolyte panel to evaluate renal function, and a complete blood cell count to rule out systemic infection.
Cystoscopy should be performed to visualize the fistulous tract, assess its location in relation to the ureters and trigone, assure bilateral ureteral patency, and exclude the presence of a foreign body or suture in the bladder.
In patients with a history of urogenital malignancy, biopsy of the fistula tract and urine cytology is warranted.
Comparable success rates have been reported for early and late repair of surgery-induced fistulae.
Radiologic studies are recommended prior to surgical repair of a vesicovaginal fistula to fully assess the defect and exclude the presence of multiple fistulae. An intravenous pyelogram is helpful to exclude concurrent ureterovaginal fistulae or ureteral obstruction. A targeted fistulogram may be indicated if conservative therapy is planned, including expectant management, continuous bladder drainage, fulguration, or fibrin occlusion.
FIGURE 1 Vesicovaginal fistula
Pelvic cross section depicting high vesicovaginal fistula.TABLE
Classification of vesicovaginal fistulae17
| CLASSIFICATION | DESCRIPTION |
|---|---|
| Simple |
|
| Complicated |
|
Indications for conservative management
Because spontaneous closure is uncommon, symptomatic VVF merits treatment. Appropriate therapy depends on various factors, including fistula size and location, timing from the antecedent event, severity of symptoms, quality of surrounding tissue, and clinician experience and surgical skills.
Occasionally, a fistula heals following prolonged bladder drainage through a transurethral or suprapubic catheter—provided it is diagnosed within a few days of surgery. Zimmern5 recommends a conservative approach to small fistulae if the patient’s complaints of urinary incontinence are resolved with insertion of a Foley catheter. In this case, bladder drainage should be continued for 3 weeks, followed by reevaluation of the fistula. If the fistula has diminished in size, an additional 3 weeks of catheter drainage is associated with a high rate of spontaneous closure; if there is no change, the fistula is unlikely to resolve spontaneously.5
Varying success rates have been reported for conservative management, ranging from 2% to 80%.4,6 The chances of success are apparently greater if the fistula is:
- diagnosed within 7 days of the index surgery,
- less than 1 cm in size,
- simple, without associated carcinoma or radiation, and
- subject to at least 4 weeks of constant bladder drainage.
Persistent, large, or complex fistulae are best treated surgically.
Indications for surgical management
The basic principles of fistula closure apply. They are:
- adequate exposure,
- good hemostasis,
- wide mobilization of the bladder and vagina,
- resection of devascularized tissue,
- removal of any foreign bodies,
- tension-free closure,
- nonopposition of suture lines,
- confirmation of a water-tight seal on bladder closure, and
- bladder drainage for 10 to 14 days following the repair.
Timing of the repair has been the subject of controversy and can pose a dilemma to physician and patient alike. Traditionally, an interval of 3 months was recommended between the index surgery and fistula repair, with a delay of up to 1 year when the fistula was radiation-induced. However, little data support these recommendations.
Today most experts recommend an individualized approach, delaying the surgery until inflammation and infection of the surrounding tissue have resolved. The use of estrogen, antibiotics, or steroids to facilitate healing during this period also has been recommended.7 Comparable success rates have been reported for early and late repair of surgery-induced fistulae based on these principles.8-10
Vaginal approach. Most vesicovaginal fistulae can be surgically corrected using a vaginal approach. Traditionally, a Latzko partial colpocleisis or fistulectomy with flap-splitting closure has been advocated.
Debate continues about whether resection of the fistulous tract is necessary. Some experts believe that wide resection increases the size of the fistula and, therefore, the risk of recurrence. They also maintain that the fibrous tissue surrounding the fistula helps to reinforce the surgical repair. Proponents of fistulectomy counter that resection of the fistula and exposure of healthy tissue optimizes wound healing and improves surgical success rates. Comparable success has been reported for both techniques.11,12
We prefer an individualized approach, with minimal resection of the fistulous tract to simplify the procedure and minimize associated complications, including recurrence.
- Latzko partial colpocleisis. This technique, first reported in 1942, remains a common procedure, with success rates of 90% to 100%.13 Advantages include a short operative time, low intraoperative and postoperative morbidity, and low risk of ureteral injury.
- Fistulectomy technique. Alternatively, to perform fistulectomy with a flap-splitting closure, begin by resecting the fistulous tract to expose healthy tissue at the wound margins. Then close the defect in a multilayer fashion, beginning with the bladder mucosa, bladder serosa, pubocervical fascia, and vaginal mucosa. Be careful to avoid tension on suture lines. In addition, create a fascial flap to prevent opposition of the incision planes and reduce the risk of recurrence.
- Grafts. In cases with a high risk of recurrence, such as complex or large fistulae, a Martius fat-pad graft should be interposed between the closure layers to promote vascularization and reduce the risk of recurrence.14 Placement of a cadaveric biomaterial graft also has been reported, reducing the need for complicated flap procedures.15
Abdominal approach. Although most vesicovaginal fistulae can be surgically corrected via the vaginal approach, the abdominal route may be preferred when the fistula is high and inaccessible, large and complex, multiple in number, or when there is concurrent uterine or bowel involvement or a need for ureteral reimplantation. The abdominal approach may be facilitated by cystoscopically guided placement of a catheter through the fistulous tract to assist in subsequent identification and dissection.
To begin, make a vertical skin incision to optimize visualization and allow mobilization of an omental flap, if necessary. Expose the bladder and perform a high extraperitoneal cystotomy to visualize the fistulous tract. Place ureteral stents if the fistula is in close proximity to the ureteral orifice.
Extend the bladder incision to the fistulous tract and completely excise it following mobilization of the vagina. Then close the vagina and bladder with interrupted, delayed absorbable suture in a double layer.
Transpose an omental flap between the vaginal and bladder incisions to promote vascularization, minimize opposition of suture lines, and reduce the risk of recurrence (FIGURE 2).
Laparoscopic approach. A similar laparoscopic repair has been reported with comparable results, but requires advanced skills with endoscopic suturing and knot tying (FIGURE 3).16
FIGURE 2 Abdominal repair
Abdominal repair of vesicovaginal fistula, with closure of bladder defect and posterior cystotomy and separate closure of vaginal defect. Note the omental flap pictured in the insert.
FIGURE 3 Laparoscopic repair
Laparoscopic repair of vesicovaginal fistula.The authors report no financial relationship with any companies whose products are mentioned in this article.
1. Kremling H. Labor-induced bladder injuries: historical observations. Gynakol Geburtshilfliche Rundsch. 1996;36:197-200.
2. Lee RA, Symmonds RE, Williams TJ. Current state of genitourinary fistula. Obstet Gynecol. 1998;72:313-315.
3. Miklos JR, Sze EHM, Parobeck D, et al. Vesicouterine fistula: a rare complication of vaginal birth after cesarean. Obstet Gynecol. 1995;86:638-639.
4. Elkins TE, Thompson JR. Lower urinary tract fistulas. In: Walters M, Karram MM, eds. Urogynecology and Reconstructive Pelvic Surgery. 2nd ed. St. Louis, Mo: Mosby; 1999;355-365.
5. Zimmern PE, Hadley HR, Staskin D. Genitourinary fistulas: vaginal approach for repair of vesicovaginal fistulas. Clin Obstet Gynaecol. 1985;12:403-413.
6. Davits RJ, Miranda SI. Conservative treatment of vesicovaginal fistulas by bladder drainage alone. Br J Urol. 1991;68:155-156.
7. Margolis T, Mercer LJ. Vesicovaginal fistula. Obstet Gynecol Surv. 1995;49:840-847.
8. Blaivas JG, Heritz DM, Romanzi LJ. Early versus late repair of vesicovaginal fistulas: vaginal and abdominal approaches. J Urol. 1995;153:1110-1112.
9. Blandy JP, Badenoch DF, Fowler CG. Early repair of iatrogenic injury to the ureter or bladder after gynecological surgery. J Urol. 1991;146:761-765.
10. Cruikshank SH. Early closure of posthysterectomy vesicovaginal fistulas. South Med J. 1988;81:1525-1528.
11. Raz S, Bregg KJ, Nitti VW. Transvaginal repair of vesicovaginal fistula using a peritoneal flap. J Urol. 1993;150:56-59.
12. Iselin CE, Aslan P, Webster GD. Transvaginal repair of vesicovaginal fistulas after hysterectomy by vaginal cuff excision. J Urol. 1998;160(3 Pt 1):728-730.
13. Latzko W. Postoperative vesicovaginal fistulas: genesis and therapy. Am J Surg. 1942;58:211-218.
14. Punekar SV, Buch DN, Soni AB. Martius’ labial fat pad interposition and its modification in complex lower urinary fistulae. J Postgrad Med. 1999;10:405-406.
15. Miklos JR, Kohli N. Rectovaginal fistula repair utilizing a cadaveric dermal allograft. Int Urogynecol J Pelvic Floor Dysfunct. 1999;10:405-406.
16. Nezhat CH, Nezhat F, Nezhat C. Laparoscopic repair of a vesicovaginal fistula: a case report. Obstet Gynecol. 1994;83(5 Pt 2):899-901.
17. Walters MD, Karram MM, eds. Urogynecology and Reconstructive Pelvic Surgery. 2nd ed. St. Louis, Mo: Mosby; 1999.
- Surgical risk factors include prior pelvic surgery, history of pelvic inflammatory disease, pelvic malignancy, endometriosis, infection, diabetes, and anatomic distortion.
- Conservative therapy should be reserved for simple fistulae that are less than 1 cm in size, diagnosed within 7 days of the index surgery, lacking associated carcinoma or radiation, and subject to at least 4 weeks of constant bladder drainage.
- In surgical repair, the Latzko partial colpocleisis or fistulectomy with flap-splitting closure is preferred.
Recent advances have improved the success of vesicovaginal fistula (VVF) repair—a challenge that can test even the most experienced gynecologic surgeon. For example, it now is apparent that some small uncomplicated fistulae respond to conservative treatment. Further, in selected cases, laparoscopic repair can eliminate the need for complicated laparotomy.
In addition, timing of fistula repair no longer requires long periods of observation, and good surgical technique for identifying and repairing bladder injuries at the time of the index surgery can often prevent the development or reduce the severity of VVF.
Vesicovaginal fistula is the most common type of urogenital fistula. Presentation and prognosis vary, depending on location and size of the defect, as well as coexisting factors such as tissue devascularization and previous radiation. However, surgical repair is associated with a high cure rate if it is performed by an experienced surgeon.
Most US cases follow gynecologic surgery
Vesicovaginal fistula was first documented in the mummified remains of Egyptian Queen Henhenit (11th Dynasty, 2050 BC), which were examined in 1923 by Derry.1 Although the exact incidence of VVF in the United States is unknown, the primary cause is gynecologic surgery, especially hysterectomy. The defect is estimated to occur in 0.01% to 0.04% of gynecologic procedures.
A study of 303 women with genitourinary fistula found that the defect was related to gynecologic surgery in 82% of cases, obstetric events in 8%, radiation therapy in 6%, and trauma or fulguration in 4%.2 Rare causes of VVF include lymphogranuloma venereum, tuberculosis, syphilis, bladder stones, and a retained foreign body in the vagina. In rare instances, spontaneous vesicouterine fistulae were reported following uncomplicated vaginal birth after cesarean section.3
Gynecologic surgery may lead to VVF due to extensive dissection between the bladder and the uterus, unrecognized bladder laceration, inappropriate stitch placement, and/or devascularization injury to the tissue planes. Concurrent ureteric involvement has been reported in as many as 10% to 15% of vesicovaginal fistula cases.
In developing countries, vesicovaginal fistulae are far more common and generally related to obstetric factors such as obstructed labor (due to unattended deliveries), small pelvic dimensions, malpresentation, poor uterine contractions, and introital stenosis.
Risk factors. Conditions that may predispose patients to VVF include prior pelvic surgery, a history of pelvic inflammatory disease, pelvic malignancy, endometriosis, infection, diabetes, and anatomic distortion. If these risk factors are present, the patient should be counseled accordingly prior to gynecologic surgery.
Correct classification crucial to surgical success
Proper classification of VVF can help the gynecologic surgeon plan operative intervention. Obstetric vesicovaginal fistulae usually are categorized according to their cause, complexity, and site of obstruction. In contrast, gynecologic fistulae are generally classified as simple or complicated (TABLE).
These levels may have important implications for the surgical approach and prognosis.4 For example, simple vesicovaginal fistulae are usually uncomplicated surgical cases with good prognosis. Complicated vesicovaginal fistulae, on the other hand, can challenge even highly practiced and skilled gynecologic surgeons and are associated with a high rate of recurrence.
Women typically present within specific intervals after the various antecedent events (pelvic surgery, childbirth, radiation therapy) with a primary complaint of constant, painless urinary incontinence. If the fistula is related to traumatic childbirth, most patients experience urine leakage within the first 24 to 48 hours. Following pelvic surgery, symptoms usually occur within the first 30 days. In contrast, radiation-induced fistulae develop over a much longer interval secondary to progressive devascularization necrosis, and may present 30 days to 30 years after the antecedent event.
Some patients report exacerbation during physical activities, which can sometimes lead to erroneous diagnosis of uncomplicated stress incontinence. If the fistula is small, intermittent leakage with increased bladder distention or physical activity may be noted.
Other patients may complain of vaginal discharge or hematuria.
If there is concurrent ureteric involvement, the patient may experience constitutional symptoms (such as fever, chills, and flank pain) or even gastrointestinal symptoms.
Physical findings. Any pooling of fluid in the vagina that is noted should be sent for analysis if the diagnosis is unclear. Next, perform a careful speculum exam that allows visualization of the entire anterior vaginal wall to identify the fistula tract (FIGURE 1). In many cases, the fistula is grossly visible.
Determine the location of the fistula in relation to the vaginal apex and bladder trigone and assess the quality of surrounding tissue (eg, presence of inflammation, edema, or infection). Fistulas near the vaginal apex may require a more complicated abdominal approach, and those close to the trigone may be associated with increased risk of ureteral injury during repair.
If the fistula is particularly small, no tract may be apparent. In such cases, bimanual exam with careful palpation of the anterior wall may help isolate the fistula (eg, when there is a surrounding zone of induration).
Office tests. If no fistula is noted despite highly suspicious signs and symptoms and careful examination, a simple office test can be performed. Using a catheter, fill the bladder with a dyed solution such as normal saline with indigo carmine and repeat the pelvic exam with a half-speculum to visualize the anterior wall. Ask the patient to cough and bear down, and identify the fistula by visualizing urine leakage.
If this test fails to locate the fistula, insert a tampon and ask the patient to perform 10 to 15 minutes of exertional maneuvers, including stair climbing and jumping in place. Then remove the tampon. Visualization of dye beyond the most distal edge of the tampon confirms the presence of a fistula.
A variation of this technique is the double-dye test: Give the patient oral phenazopyridine (Pyridium), fill the bladder with the blue-tinted solution, and insert a tampon. The presence of blue staining suggests vesicovaginal or urethrovaginal fistula, while red staining (Pyridium) suggests ureterovaginal fistula.
Other testing. Further assessment is recommended to rule out concurrent pathology and formulate an appropriate treatment plan. Routine testing should include a urinalysis and culture to exclude coexisting urinary tract infection, an electrolyte panel to evaluate renal function, and a complete blood cell count to rule out systemic infection.
Cystoscopy should be performed to visualize the fistulous tract, assess its location in relation to the ureters and trigone, assure bilateral ureteral patency, and exclude the presence of a foreign body or suture in the bladder.
In patients with a history of urogenital malignancy, biopsy of the fistula tract and urine cytology is warranted.
Comparable success rates have been reported for early and late repair of surgery-induced fistulae.
Radiologic studies are recommended prior to surgical repair of a vesicovaginal fistula to fully assess the defect and exclude the presence of multiple fistulae. An intravenous pyelogram is helpful to exclude concurrent ureterovaginal fistulae or ureteral obstruction. A targeted fistulogram may be indicated if conservative therapy is planned, including expectant management, continuous bladder drainage, fulguration, or fibrin occlusion.
FIGURE 1 Vesicovaginal fistula
Pelvic cross section depicting high vesicovaginal fistula.TABLE
Classification of vesicovaginal fistulae17
| CLASSIFICATION | DESCRIPTION |
|---|---|
| Simple |
|
| Complicated |
|
Indications for conservative management
Because spontaneous closure is uncommon, symptomatic VVF merits treatment. Appropriate therapy depends on various factors, including fistula size and location, timing from the antecedent event, severity of symptoms, quality of surrounding tissue, and clinician experience and surgical skills.
Occasionally, a fistula heals following prolonged bladder drainage through a transurethral or suprapubic catheter—provided it is diagnosed within a few days of surgery. Zimmern5 recommends a conservative approach to small fistulae if the patient’s complaints of urinary incontinence are resolved with insertion of a Foley catheter. In this case, bladder drainage should be continued for 3 weeks, followed by reevaluation of the fistula. If the fistula has diminished in size, an additional 3 weeks of catheter drainage is associated with a high rate of spontaneous closure; if there is no change, the fistula is unlikely to resolve spontaneously.5
Varying success rates have been reported for conservative management, ranging from 2% to 80%.4,6 The chances of success are apparently greater if the fistula is:
- diagnosed within 7 days of the index surgery,
- less than 1 cm in size,
- simple, without associated carcinoma or radiation, and
- subject to at least 4 weeks of constant bladder drainage.
Persistent, large, or complex fistulae are best treated surgically.
Indications for surgical management
The basic principles of fistula closure apply. They are:
- adequate exposure,
- good hemostasis,
- wide mobilization of the bladder and vagina,
- resection of devascularized tissue,
- removal of any foreign bodies,
- tension-free closure,
- nonopposition of suture lines,
- confirmation of a water-tight seal on bladder closure, and
- bladder drainage for 10 to 14 days following the repair.
Timing of the repair has been the subject of controversy and can pose a dilemma to physician and patient alike. Traditionally, an interval of 3 months was recommended between the index surgery and fistula repair, with a delay of up to 1 year when the fistula was radiation-induced. However, little data support these recommendations.
Today most experts recommend an individualized approach, delaying the surgery until inflammation and infection of the surrounding tissue have resolved. The use of estrogen, antibiotics, or steroids to facilitate healing during this period also has been recommended.7 Comparable success rates have been reported for early and late repair of surgery-induced fistulae based on these principles.8-10
Vaginal approach. Most vesicovaginal fistulae can be surgically corrected using a vaginal approach. Traditionally, a Latzko partial colpocleisis or fistulectomy with flap-splitting closure has been advocated.
Debate continues about whether resection of the fistulous tract is necessary. Some experts believe that wide resection increases the size of the fistula and, therefore, the risk of recurrence. They also maintain that the fibrous tissue surrounding the fistula helps to reinforce the surgical repair. Proponents of fistulectomy counter that resection of the fistula and exposure of healthy tissue optimizes wound healing and improves surgical success rates. Comparable success has been reported for both techniques.11,12
We prefer an individualized approach, with minimal resection of the fistulous tract to simplify the procedure and minimize associated complications, including recurrence.
- Latzko partial colpocleisis. This technique, first reported in 1942, remains a common procedure, with success rates of 90% to 100%.13 Advantages include a short operative time, low intraoperative and postoperative morbidity, and low risk of ureteral injury.
- Fistulectomy technique. Alternatively, to perform fistulectomy with a flap-splitting closure, begin by resecting the fistulous tract to expose healthy tissue at the wound margins. Then close the defect in a multilayer fashion, beginning with the bladder mucosa, bladder serosa, pubocervical fascia, and vaginal mucosa. Be careful to avoid tension on suture lines. In addition, create a fascial flap to prevent opposition of the incision planes and reduce the risk of recurrence.
- Grafts. In cases with a high risk of recurrence, such as complex or large fistulae, a Martius fat-pad graft should be interposed between the closure layers to promote vascularization and reduce the risk of recurrence.14 Placement of a cadaveric biomaterial graft also has been reported, reducing the need for complicated flap procedures.15
Abdominal approach. Although most vesicovaginal fistulae can be surgically corrected via the vaginal approach, the abdominal route may be preferred when the fistula is high and inaccessible, large and complex, multiple in number, or when there is concurrent uterine or bowel involvement or a need for ureteral reimplantation. The abdominal approach may be facilitated by cystoscopically guided placement of a catheter through the fistulous tract to assist in subsequent identification and dissection.
To begin, make a vertical skin incision to optimize visualization and allow mobilization of an omental flap, if necessary. Expose the bladder and perform a high extraperitoneal cystotomy to visualize the fistulous tract. Place ureteral stents if the fistula is in close proximity to the ureteral orifice.
Extend the bladder incision to the fistulous tract and completely excise it following mobilization of the vagina. Then close the vagina and bladder with interrupted, delayed absorbable suture in a double layer.
Transpose an omental flap between the vaginal and bladder incisions to promote vascularization, minimize opposition of suture lines, and reduce the risk of recurrence (FIGURE 2).
Laparoscopic approach. A similar laparoscopic repair has been reported with comparable results, but requires advanced skills with endoscopic suturing and knot tying (FIGURE 3).16
FIGURE 2 Abdominal repair
Abdominal repair of vesicovaginal fistula, with closure of bladder defect and posterior cystotomy and separate closure of vaginal defect. Note the omental flap pictured in the insert.
FIGURE 3 Laparoscopic repair
Laparoscopic repair of vesicovaginal fistula.The authors report no financial relationship with any companies whose products are mentioned in this article.
- Surgical risk factors include prior pelvic surgery, history of pelvic inflammatory disease, pelvic malignancy, endometriosis, infection, diabetes, and anatomic distortion.
- Conservative therapy should be reserved for simple fistulae that are less than 1 cm in size, diagnosed within 7 days of the index surgery, lacking associated carcinoma or radiation, and subject to at least 4 weeks of constant bladder drainage.
- In surgical repair, the Latzko partial colpocleisis or fistulectomy with flap-splitting closure is preferred.
Recent advances have improved the success of vesicovaginal fistula (VVF) repair—a challenge that can test even the most experienced gynecologic surgeon. For example, it now is apparent that some small uncomplicated fistulae respond to conservative treatment. Further, in selected cases, laparoscopic repair can eliminate the need for complicated laparotomy.
In addition, timing of fistula repair no longer requires long periods of observation, and good surgical technique for identifying and repairing bladder injuries at the time of the index surgery can often prevent the development or reduce the severity of VVF.
Vesicovaginal fistula is the most common type of urogenital fistula. Presentation and prognosis vary, depending on location and size of the defect, as well as coexisting factors such as tissue devascularization and previous radiation. However, surgical repair is associated with a high cure rate if it is performed by an experienced surgeon.
Most US cases follow gynecologic surgery
Vesicovaginal fistula was first documented in the mummified remains of Egyptian Queen Henhenit (11th Dynasty, 2050 BC), which were examined in 1923 by Derry.1 Although the exact incidence of VVF in the United States is unknown, the primary cause is gynecologic surgery, especially hysterectomy. The defect is estimated to occur in 0.01% to 0.04% of gynecologic procedures.
A study of 303 women with genitourinary fistula found that the defect was related to gynecologic surgery in 82% of cases, obstetric events in 8%, radiation therapy in 6%, and trauma or fulguration in 4%.2 Rare causes of VVF include lymphogranuloma venereum, tuberculosis, syphilis, bladder stones, and a retained foreign body in the vagina. In rare instances, spontaneous vesicouterine fistulae were reported following uncomplicated vaginal birth after cesarean section.3
Gynecologic surgery may lead to VVF due to extensive dissection between the bladder and the uterus, unrecognized bladder laceration, inappropriate stitch placement, and/or devascularization injury to the tissue planes. Concurrent ureteric involvement has been reported in as many as 10% to 15% of vesicovaginal fistula cases.
In developing countries, vesicovaginal fistulae are far more common and generally related to obstetric factors such as obstructed labor (due to unattended deliveries), small pelvic dimensions, malpresentation, poor uterine contractions, and introital stenosis.
Risk factors. Conditions that may predispose patients to VVF include prior pelvic surgery, a history of pelvic inflammatory disease, pelvic malignancy, endometriosis, infection, diabetes, and anatomic distortion. If these risk factors are present, the patient should be counseled accordingly prior to gynecologic surgery.
Correct classification crucial to surgical success
Proper classification of VVF can help the gynecologic surgeon plan operative intervention. Obstetric vesicovaginal fistulae usually are categorized according to their cause, complexity, and site of obstruction. In contrast, gynecologic fistulae are generally classified as simple or complicated (TABLE).
These levels may have important implications for the surgical approach and prognosis.4 For example, simple vesicovaginal fistulae are usually uncomplicated surgical cases with good prognosis. Complicated vesicovaginal fistulae, on the other hand, can challenge even highly practiced and skilled gynecologic surgeons and are associated with a high rate of recurrence.
Women typically present within specific intervals after the various antecedent events (pelvic surgery, childbirth, radiation therapy) with a primary complaint of constant, painless urinary incontinence. If the fistula is related to traumatic childbirth, most patients experience urine leakage within the first 24 to 48 hours. Following pelvic surgery, symptoms usually occur within the first 30 days. In contrast, radiation-induced fistulae develop over a much longer interval secondary to progressive devascularization necrosis, and may present 30 days to 30 years after the antecedent event.
Some patients report exacerbation during physical activities, which can sometimes lead to erroneous diagnosis of uncomplicated stress incontinence. If the fistula is small, intermittent leakage with increased bladder distention or physical activity may be noted.
Other patients may complain of vaginal discharge or hematuria.
If there is concurrent ureteric involvement, the patient may experience constitutional symptoms (such as fever, chills, and flank pain) or even gastrointestinal symptoms.
Physical findings. Any pooling of fluid in the vagina that is noted should be sent for analysis if the diagnosis is unclear. Next, perform a careful speculum exam that allows visualization of the entire anterior vaginal wall to identify the fistula tract (FIGURE 1). In many cases, the fistula is grossly visible.
Determine the location of the fistula in relation to the vaginal apex and bladder trigone and assess the quality of surrounding tissue (eg, presence of inflammation, edema, or infection). Fistulas near the vaginal apex may require a more complicated abdominal approach, and those close to the trigone may be associated with increased risk of ureteral injury during repair.
If the fistula is particularly small, no tract may be apparent. In such cases, bimanual exam with careful palpation of the anterior wall may help isolate the fistula (eg, when there is a surrounding zone of induration).
Office tests. If no fistula is noted despite highly suspicious signs and symptoms and careful examination, a simple office test can be performed. Using a catheter, fill the bladder with a dyed solution such as normal saline with indigo carmine and repeat the pelvic exam with a half-speculum to visualize the anterior wall. Ask the patient to cough and bear down, and identify the fistula by visualizing urine leakage.
If this test fails to locate the fistula, insert a tampon and ask the patient to perform 10 to 15 minutes of exertional maneuvers, including stair climbing and jumping in place. Then remove the tampon. Visualization of dye beyond the most distal edge of the tampon confirms the presence of a fistula.
A variation of this technique is the double-dye test: Give the patient oral phenazopyridine (Pyridium), fill the bladder with the blue-tinted solution, and insert a tampon. The presence of blue staining suggests vesicovaginal or urethrovaginal fistula, while red staining (Pyridium) suggests ureterovaginal fistula.
Other testing. Further assessment is recommended to rule out concurrent pathology and formulate an appropriate treatment plan. Routine testing should include a urinalysis and culture to exclude coexisting urinary tract infection, an electrolyte panel to evaluate renal function, and a complete blood cell count to rule out systemic infection.
Cystoscopy should be performed to visualize the fistulous tract, assess its location in relation to the ureters and trigone, assure bilateral ureteral patency, and exclude the presence of a foreign body or suture in the bladder.
In patients with a history of urogenital malignancy, biopsy of the fistula tract and urine cytology is warranted.
Comparable success rates have been reported for early and late repair of surgery-induced fistulae.
Radiologic studies are recommended prior to surgical repair of a vesicovaginal fistula to fully assess the defect and exclude the presence of multiple fistulae. An intravenous pyelogram is helpful to exclude concurrent ureterovaginal fistulae or ureteral obstruction. A targeted fistulogram may be indicated if conservative therapy is planned, including expectant management, continuous bladder drainage, fulguration, or fibrin occlusion.
FIGURE 1 Vesicovaginal fistula
Pelvic cross section depicting high vesicovaginal fistula.TABLE
Classification of vesicovaginal fistulae17
| CLASSIFICATION | DESCRIPTION |
|---|---|
| Simple |
|
| Complicated |
|
Indications for conservative management
Because spontaneous closure is uncommon, symptomatic VVF merits treatment. Appropriate therapy depends on various factors, including fistula size and location, timing from the antecedent event, severity of symptoms, quality of surrounding tissue, and clinician experience and surgical skills.
Occasionally, a fistula heals following prolonged bladder drainage through a transurethral or suprapubic catheter—provided it is diagnosed within a few days of surgery. Zimmern5 recommends a conservative approach to small fistulae if the patient’s complaints of urinary incontinence are resolved with insertion of a Foley catheter. In this case, bladder drainage should be continued for 3 weeks, followed by reevaluation of the fistula. If the fistula has diminished in size, an additional 3 weeks of catheter drainage is associated with a high rate of spontaneous closure; if there is no change, the fistula is unlikely to resolve spontaneously.5
Varying success rates have been reported for conservative management, ranging from 2% to 80%.4,6 The chances of success are apparently greater if the fistula is:
- diagnosed within 7 days of the index surgery,
- less than 1 cm in size,
- simple, without associated carcinoma or radiation, and
- subject to at least 4 weeks of constant bladder drainage.
Persistent, large, or complex fistulae are best treated surgically.
Indications for surgical management
The basic principles of fistula closure apply. They are:
- adequate exposure,
- good hemostasis,
- wide mobilization of the bladder and vagina,
- resection of devascularized tissue,
- removal of any foreign bodies,
- tension-free closure,
- nonopposition of suture lines,
- confirmation of a water-tight seal on bladder closure, and
- bladder drainage for 10 to 14 days following the repair.
Timing of the repair has been the subject of controversy and can pose a dilemma to physician and patient alike. Traditionally, an interval of 3 months was recommended between the index surgery and fistula repair, with a delay of up to 1 year when the fistula was radiation-induced. However, little data support these recommendations.
Today most experts recommend an individualized approach, delaying the surgery until inflammation and infection of the surrounding tissue have resolved. The use of estrogen, antibiotics, or steroids to facilitate healing during this period also has been recommended.7 Comparable success rates have been reported for early and late repair of surgery-induced fistulae based on these principles.8-10
Vaginal approach. Most vesicovaginal fistulae can be surgically corrected using a vaginal approach. Traditionally, a Latzko partial colpocleisis or fistulectomy with flap-splitting closure has been advocated.
Debate continues about whether resection of the fistulous tract is necessary. Some experts believe that wide resection increases the size of the fistula and, therefore, the risk of recurrence. They also maintain that the fibrous tissue surrounding the fistula helps to reinforce the surgical repair. Proponents of fistulectomy counter that resection of the fistula and exposure of healthy tissue optimizes wound healing and improves surgical success rates. Comparable success has been reported for both techniques.11,12
We prefer an individualized approach, with minimal resection of the fistulous tract to simplify the procedure and minimize associated complications, including recurrence.
- Latzko partial colpocleisis. This technique, first reported in 1942, remains a common procedure, with success rates of 90% to 100%.13 Advantages include a short operative time, low intraoperative and postoperative morbidity, and low risk of ureteral injury.
- Fistulectomy technique. Alternatively, to perform fistulectomy with a flap-splitting closure, begin by resecting the fistulous tract to expose healthy tissue at the wound margins. Then close the defect in a multilayer fashion, beginning with the bladder mucosa, bladder serosa, pubocervical fascia, and vaginal mucosa. Be careful to avoid tension on suture lines. In addition, create a fascial flap to prevent opposition of the incision planes and reduce the risk of recurrence.
- Grafts. In cases with a high risk of recurrence, such as complex or large fistulae, a Martius fat-pad graft should be interposed between the closure layers to promote vascularization and reduce the risk of recurrence.14 Placement of a cadaveric biomaterial graft also has been reported, reducing the need for complicated flap procedures.15
Abdominal approach. Although most vesicovaginal fistulae can be surgically corrected via the vaginal approach, the abdominal route may be preferred when the fistula is high and inaccessible, large and complex, multiple in number, or when there is concurrent uterine or bowel involvement or a need for ureteral reimplantation. The abdominal approach may be facilitated by cystoscopically guided placement of a catheter through the fistulous tract to assist in subsequent identification and dissection.
To begin, make a vertical skin incision to optimize visualization and allow mobilization of an omental flap, if necessary. Expose the bladder and perform a high extraperitoneal cystotomy to visualize the fistulous tract. Place ureteral stents if the fistula is in close proximity to the ureteral orifice.
Extend the bladder incision to the fistulous tract and completely excise it following mobilization of the vagina. Then close the vagina and bladder with interrupted, delayed absorbable suture in a double layer.
Transpose an omental flap between the vaginal and bladder incisions to promote vascularization, minimize opposition of suture lines, and reduce the risk of recurrence (FIGURE 2).
Laparoscopic approach. A similar laparoscopic repair has been reported with comparable results, but requires advanced skills with endoscopic suturing and knot tying (FIGURE 3).16
FIGURE 2 Abdominal repair
Abdominal repair of vesicovaginal fistula, with closure of bladder defect and posterior cystotomy and separate closure of vaginal defect. Note the omental flap pictured in the insert.
FIGURE 3 Laparoscopic repair
Laparoscopic repair of vesicovaginal fistula.The authors report no financial relationship with any companies whose products are mentioned in this article.
1. Kremling H. Labor-induced bladder injuries: historical observations. Gynakol Geburtshilfliche Rundsch. 1996;36:197-200.
2. Lee RA, Symmonds RE, Williams TJ. Current state of genitourinary fistula. Obstet Gynecol. 1998;72:313-315.
3. Miklos JR, Sze EHM, Parobeck D, et al. Vesicouterine fistula: a rare complication of vaginal birth after cesarean. Obstet Gynecol. 1995;86:638-639.
4. Elkins TE, Thompson JR. Lower urinary tract fistulas. In: Walters M, Karram MM, eds. Urogynecology and Reconstructive Pelvic Surgery. 2nd ed. St. Louis, Mo: Mosby; 1999;355-365.
5. Zimmern PE, Hadley HR, Staskin D. Genitourinary fistulas: vaginal approach for repair of vesicovaginal fistulas. Clin Obstet Gynaecol. 1985;12:403-413.
6. Davits RJ, Miranda SI. Conservative treatment of vesicovaginal fistulas by bladder drainage alone. Br J Urol. 1991;68:155-156.
7. Margolis T, Mercer LJ. Vesicovaginal fistula. Obstet Gynecol Surv. 1995;49:840-847.
8. Blaivas JG, Heritz DM, Romanzi LJ. Early versus late repair of vesicovaginal fistulas: vaginal and abdominal approaches. J Urol. 1995;153:1110-1112.
9. Blandy JP, Badenoch DF, Fowler CG. Early repair of iatrogenic injury to the ureter or bladder after gynecological surgery. J Urol. 1991;146:761-765.
10. Cruikshank SH. Early closure of posthysterectomy vesicovaginal fistulas. South Med J. 1988;81:1525-1528.
11. Raz S, Bregg KJ, Nitti VW. Transvaginal repair of vesicovaginal fistula using a peritoneal flap. J Urol. 1993;150:56-59.
12. Iselin CE, Aslan P, Webster GD. Transvaginal repair of vesicovaginal fistulas after hysterectomy by vaginal cuff excision. J Urol. 1998;160(3 Pt 1):728-730.
13. Latzko W. Postoperative vesicovaginal fistulas: genesis and therapy. Am J Surg. 1942;58:211-218.
14. Punekar SV, Buch DN, Soni AB. Martius’ labial fat pad interposition and its modification in complex lower urinary fistulae. J Postgrad Med. 1999;10:405-406.
15. Miklos JR, Kohli N. Rectovaginal fistula repair utilizing a cadaveric dermal allograft. Int Urogynecol J Pelvic Floor Dysfunct. 1999;10:405-406.
16. Nezhat CH, Nezhat F, Nezhat C. Laparoscopic repair of a vesicovaginal fistula: a case report. Obstet Gynecol. 1994;83(5 Pt 2):899-901.
17. Walters MD, Karram MM, eds. Urogynecology and Reconstructive Pelvic Surgery. 2nd ed. St. Louis, Mo: Mosby; 1999.
1. Kremling H. Labor-induced bladder injuries: historical observations. Gynakol Geburtshilfliche Rundsch. 1996;36:197-200.
2. Lee RA, Symmonds RE, Williams TJ. Current state of genitourinary fistula. Obstet Gynecol. 1998;72:313-315.
3. Miklos JR, Sze EHM, Parobeck D, et al. Vesicouterine fistula: a rare complication of vaginal birth after cesarean. Obstet Gynecol. 1995;86:638-639.
4. Elkins TE, Thompson JR. Lower urinary tract fistulas. In: Walters M, Karram MM, eds. Urogynecology and Reconstructive Pelvic Surgery. 2nd ed. St. Louis, Mo: Mosby; 1999;355-365.
5. Zimmern PE, Hadley HR, Staskin D. Genitourinary fistulas: vaginal approach for repair of vesicovaginal fistulas. Clin Obstet Gynaecol. 1985;12:403-413.
6. Davits RJ, Miranda SI. Conservative treatment of vesicovaginal fistulas by bladder drainage alone. Br J Urol. 1991;68:155-156.
7. Margolis T, Mercer LJ. Vesicovaginal fistula. Obstet Gynecol Surv. 1995;49:840-847.
8. Blaivas JG, Heritz DM, Romanzi LJ. Early versus late repair of vesicovaginal fistulas: vaginal and abdominal approaches. J Urol. 1995;153:1110-1112.
9. Blandy JP, Badenoch DF, Fowler CG. Early repair of iatrogenic injury to the ureter or bladder after gynecological surgery. J Urol. 1991;146:761-765.
10. Cruikshank SH. Early closure of posthysterectomy vesicovaginal fistulas. South Med J. 1988;81:1525-1528.
11. Raz S, Bregg KJ, Nitti VW. Transvaginal repair of vesicovaginal fistula using a peritoneal flap. J Urol. 1993;150:56-59.
12. Iselin CE, Aslan P, Webster GD. Transvaginal repair of vesicovaginal fistulas after hysterectomy by vaginal cuff excision. J Urol. 1998;160(3 Pt 1):728-730.
13. Latzko W. Postoperative vesicovaginal fistulas: genesis and therapy. Am J Surg. 1942;58:211-218.
14. Punekar SV, Buch DN, Soni AB. Martius’ labial fat pad interposition and its modification in complex lower urinary fistulae. J Postgrad Med. 1999;10:405-406.
15. Miklos JR, Kohli N. Rectovaginal fistula repair utilizing a cadaveric dermal allograft. Int Urogynecol J Pelvic Floor Dysfunct. 1999;10:405-406.
16. Nezhat CH, Nezhat F, Nezhat C. Laparoscopic repair of a vesicovaginal fistula: a case report. Obstet Gynecol. 1994;83(5 Pt 2):899-901.
17. Walters MD, Karram MM, eds. Urogynecology and Reconstructive Pelvic Surgery. 2nd ed. St. Louis, Mo: Mosby; 1999.
New transobturator sling reduces risk of injury
- The route of the transobturator sling is strictly perineal, with a very short, blind passage through the crural region. This pathway ensures that the retropubic space is not entered and that the anatomic structures crossed by the needle passer and mesh are either muscle or fascia.
- The transobturator approach eliminates the need for routine cystoscopy in most patients.
- Short-term efficacy of the transobturator sling is similar to that of other suburethral tension-free slings.
- In our experience, the transobturator approach reduces average operating time to 17 minutes.
Potential complications associated with passing needle carriers through the retropubic space are eliminated, and cystoscopy is not routinely required with the use of a transobturator sling.
Although it is effective and easy to perform,1-6 retropubic placement of suburethral tension-free vaginal tape (TVT) for the treatment of stress urinary incontinence has been associated with a number of bowel, vascular, nerve, and bladder injuries (TABLE).7-13
Such complications appear to be related to the unique upward vaginal passage of the metallic sling trocars through the retropubic space. Newer slings eliminate the upward approach, but still require routine cystoscopy to confirm an intact bladder and urethra.
A new tension-free suburethral sling addresses these shortcomings using an innovative transobturator route.14 In the new procedure, the surgeon uses needle passers to run the sling from one obturator foramen to the other. The perineal approach reproduces the natural suspension of the urethral fascia while preserving an intact retropubic space (FIGURE 1).
TABLE
Advantages of the transobturator sling
|
FIGURE 1 Suburethral slings: Retropubic and transobturator pathways
Traditional suburethral tension-free slings require the needle passers and sling to be placed through the retropubic space.
The newer transobturator sling uses a perineal approach. The needle passer and sling are passed from one obturator foramen to the other, preserving an intact retropubic space.
What does ‘tension-free’ mean?
Tension-free slings are used to treat stress urinary incontinence caused by urethral hypermobility and intrinsic sphincter deficiency. In this approach, a synthetic transvaginal suburethral sling is placed through the retropubic space without using suspension sutures. The sling is held in place by the friction between the mesh and the tissue canals created by the metallic needle passers. Scar tissue later fixes the mesh, preventing migration.
Short-term efficacy of the Monarc transobturator sling is similar to that of the SPARC and tension-free vaginal slings.
Because the sling is not anchored to the pubic bone, ligaments, or rectus fascia, it is considered “free of tension.” The result is a midcomplex urethral support that limits urethral descent, improves the stabilization mechanism generated by pubourethral ligaments and levator ani muscles, and reinforces support of the backboard vaginal hammock.
A tension-free sling designed to reduce injury
Since its introduction in the mid 1990s, retropubic placement of suburethral tension-free tape has continued to gain in popularity.15-22 The technique has several advantages: It is simple to perform under local, regional, or general anesthesia, and has proved to be a safe treatment for stress urinary incontinence, with minimal complications in experienced hands. Still, injuries have occurred7-13 —likely due to the blind passing of metal trocars from the vagina upward through the retropubic space.9,23
Downward placement introduced. In an effort to reduce these complications, tension-free sling systems that rely on suprapubic, downward needle-carrier placement through the retropubic space were developed, such as the SPARC Female Sling System (American Medical Systems, Minnetonka, Minn), introduced in 2001.24 The manufacturer of the traditional TVT (Gynecare, a division of Ethicon, Somerville, NJ) also recently introduced suprapubic needle passers. However, because these slings still require passage through the retropubic space, their use may lead to vascular, bowel, and bladder injury. Routine cystoscopy is therefore required.
The transobturator sling. In the Netherlands in 1998, Nickel et al25 reported a successful sling procedure using a polyester ribbon passed through the obturator foramen and around the urethra for treatment of refractory urethral sphincter incompetence in female dogs. In France in 2001, Delorme14 introduced the transobturator sling procedure in humans. Dargent et al26 then performed the operation in 71 patients using a technique inspired by Delorme, and found the shortterm results similar to those of the TVT.
The Monarc transobturator sling (American Medical Systems), introduced in Europe in January 2003, has been used successfully in more than 1,000 procedures ( FIGURE 2). This new system offers significant technical improvements over the original transobturator sling devices.
The Monarc transobturator sling, introduced in Europe in January 2003, has been used successfully in more than 1,000 procedures.
Specifically, the Monarc system simplifies rotation of the needle passer during insertion and extraction around the inferior aspect of the ischiopubic ramus. The 3-mm–diameter passers are specifically designed for right-side or left-side use. They are attached to fixed handles to create a natural wrist rotation. The sling connectors, meanwhile, facilitate rapid, effortless, and secure mesh placement and fixation.
FIGURE 2 Monarc transobturator sling system
The Monarc system consists of 2 stainless steel helical-shaped needle passers and a 1-cm by 35-cm polypropylene sling mesh with a protected sheath, attached connectors, and a reabsorbable tensioning suture.
Easy to learn and perform
Anesthesia may be local, regional, or general, and prophylactic antibiotics are routinely given. FIGURES 3 through 6 offer a detailed description of how to surgically place the Monarc transobturator sling for treatment of stress urinary incontinence. What follows is a brief overview:
After making a small vertical incision along the anterior vaginal mucosa, use a finger to locate the upper-inner corner of the obturator foramen—the safe zone for needle insertion. You will incise the skin at this point and introduce the needle tip perpendicularly. Rotate the needle, with the tip following the posterior surface of the pubic ramus, until the tip exits the vaginal incision. Repeat this process on the other side.
Attach the sling and its plastic sheath, then draw both out through the skin incisions by pulling back on the needle passers. Cut the mesh, adjust the tension, and close the incisions.
A Foley catheter and vaginal packing with metronidazole gel may be used at the surgeon’s discretion.
Route minimizes anatomical hazards. The pathway described ensures that there tropubic space is not entered and that muscle or fascia are the only anatomic structures crossed by the needle passer and mesh. No major vessels or nerves come in contact with the sling (FIGURES 7 and 8).
Our fluoroscopic observations of the transobturator sling in cadavers show that insertion of the needle passer through the obturator foramen with exit through the vaginal incision under the mid-urethra is precise and simple (FIGURE 9).
Our comparative studies in cadavers revealed that, despite the different shape and placement, lengths for the SPARC and Monarc transobturator slings in the same cadaver are almost equivalent—ranging from 12 to 14 cm, depending on the patient’s height and weight (FIGURE 10).
The transobturator sling involves fewer potential anatomical hazards than retropubic placement.
These considerations strongly suggest that the transobturator sling offers a simpler approach with fewer potential anatomic hazards than retropubic tension-free slings.
FIGURE 3 Vaginal incision
Inject a diluted vasoconstrictive solution into the anterior vaginal wall, and make a small vertical incision along the anterior vaginal mucosa 0.5 cm below the urethral meatus. Separate the vaginal epithelium from the underlying periurethral fascia using sharp and digital dissection, advancing bilaterally to the inferior pubic ramus. Insert a finger into the vaginal dissection and palpate the internal edge of the ischiopubic ramus and the upper-inner corner of the obturator foramen.
FIGURE 4 Needle entry: Transobturator sling procedure
The safe zone for needle insertion is the upper-inner corner of the obturator foramen (red line). The red dot indicates the recommended insertion site (upper inner corner).
The insertion area corresponds to the point where a horizontal line at the level of the clitoris crosses a vertical line at the level of the genitofemoral fold. Make a small skin incision at this point.
FIGURE 5 Needle passage: Transobturator sling procedure
Insert your left index finger into the vaginal incision on the patient’s left side. Holding the right-side Monarc needle, introduce the needle tip perpendicularly through the skin incision.
Place the thumb of your left hand onto the outside curve of the needle and gently push until its tip penetrates the obturator membrane and muscle. Use the needle’s tip to locate the pubic ramus, and rotate the needle so that the tip follows the posterior surface of this structure.
Continue this rotation, using your left index finger to guide the needle tip to exit the vaginal incision.
Repeat steps A and B on the contralateral side.
With the needle-passer tips extending out of the vaginal incision, attach the sling and its plastic sheath using the connectors. Make sure the mesh lies flat and is not twisted before attaching the connector to the second needle.
FIGURE 7 Route of transobturator sling minimizes anatomical hazards
The needle travels around the ischiopubic ramus. Transobturator needle passage begins with penetration of the skin and subcutaneous tissue. After traversing the superficial perineal fascia, the needle crosses the adductor muscles of the thigh near their pubic bone origin and below the insertion point of the adductor longus tendon (arrow). It then penetrates the obturator membrane, obturator internus muscle, and periurethral endopelvic fascia. After passing the level of the white line—either over or under the puborectalis section of the levator—the needle exits through the vaginal incision.
FIGURE 8 The obturator foramen
The tough obturator membrane covers the foramen. The obturator canal is 2 to 3 cm long and is located at the anterolateral upper margin of the membrane. The distance between the safe zone for needle insertion and the obturator canal is approximately 3.5 to 4 cm. At the safe entry zone (green area), the anterior branch of the obturator artery is reduced to a capillary diameter and no significant arteries, veins, or nerve pedicles are present.
FIGURE 10 Routes of transobturator and retropubic slings
Graphic depiction of both the transobturator sling and the retropubic tension-free sling in place.
Fluoroscopic image of both slings in a cadaver. The transobturator sling runs horizontally while the retropubic tension-free sling forms a tighter “U.”
Clinical experience yields positive results
Early European series and our initial experience suggest that the short-term efficacy of the Monarc transobturator sling is similar to that of the SPARC and TVT slings. Average operating time is 17 minutes. The technique involves a short learning curve (approximately 4 cases).
Cystoscopy is not routinely required; we perform it only in women with significant prolapse or suspected urethral injury, and have encountered no intraoperative or post-operative complications.
No major vessels or nerves come in contact with the transobturator tension-free sling.
No patients have complained of postoperative pain in the area of the adductor muscles of the thigh, and no sling erosions have occurred. We also have found this approach useful in obese patients and women with retropubic scarring, in whom retropubic needle passage can be a challenge.
In addition to its application in the treatment of female stress urinary incontinence, the transobturator foramen route was recently used to insert the anterior wings of a large mesh in the repair of severe cystocele.27
Although long-term follow-up data are not available for the transobturator approach, short-term results are encouraging. Large comparative studies with other anti-incontinent procedures are needed.
Dr. Pelosi II reports that he is a consultant for American Medical Systems. Dr. Pelosi III reports no affiliations or financial arrangements with any companies whose products are mentioned in this article.
1. Reschers UM, Tunn R, Buczkowski M, et al. Tension-free vaginal tape for the treatment of stress urinary incontinence. Clin Obstet Gynecol. 2000;43:670-675.
2. Tamussino KF, Hanzal E, Kolle D, et al. Tension-free vaginal tape operation: results of the Austrian Registry. Obstet Gynecol. 2001;98:732-736.
3. Haab F, Sananes S, Amarenco G, et al. Results of the tension-free vaginal tape procedure for the treatment of type II stress urinary incontinence at a minimum follow up of one year. J Urol. 2001;165:159-162.
4. Rardin CR, Kohli N, Rosenblatt PL, et al. Tension-free vaginal tape: outcomes among women with primary versus recurrent stress urinary incontinence. Obstet Gynecol. 2002;100:893-897.
5. Vassallo BJ, Kleeman SD, Segal JL, et al. Tension-free vaginal tape: a quality of life assessment. Obstet Gynecol. 2002;100:518-524.
6. Karram MM, Segal JL, Vassallo BJ, et al. Complications and untoward effects of the tension-free vaginal tape procedure. Obstet Gynecol. 2003;101:929-932.
7. MAUDE: Food and Drug Administration Manufacturer and User Facility Device Experience Database. Available at http://www.fda.gov/cdrh/maude.html. Accessed June 7, 2003.
8. Peyrat L, Boutin JM, Bruyere F, et al. Intestinal perforation is a complication of TVT procedure for UI. Eur Urol. 2001;39:603-605.
9. Walters MD, Tulikangas PR, LaSala C, et al. Vascular injury during tension-free vaginal tape procedure for stress urinary incontinence. Obstet Gynecol. 2001;98:957-959.
10. Zilbert AW, Farrell SA. External iliac artery laceration during tension-free vaginal tape procedure. Int Urogynecol J Pelvic Floor Dysfunct. 2001;12:141-143.
11. Vierhout ME. Severe hemorrhage complicating TVT: a case report. Int Urogynecol J Pelvic Floor Dysfunct. 2000;12:139-140.
12. Brink DM. Bowel injury following insertion of TVT. S Afr Med J. 2000;90:450-452.
13. Shobeiri SA, Garely AD, Chesson RR. Recognition of occult bladder injury during the tension-free vaginal tape procedure. Obstet Gynecol. 2002;99:1067-1072.
14. Delorme E. Transobturator urethral suspension: mini-invasive procedure in the treatment of stress urinary incontinence in women. Prog Urol. 2001;11:1306-1313.
15. Ulmstein V, Petros P. Intravaginal slingplasty (IVS): an ambulatory surgical procedure for treatment of female urinary incontinence. Scand J Urol Neprhol. 1995;29:75-82.
16. Chung MK, Chung RP. Comparison of laparoscopic Burch and tension-free vaginal tape in treating stress urinary incontinence in obese patients. JSLS. 2002;6:17-21.
17. Pelosi MA, III, Pelosi MA, II. Pubic bone suburethral stabilization sling: laparoscopic assessment of a transvaginal operation for the treatment of stress urinary incontinence. J Laparoendosc Adv Surg Tech. 1999;9:45-50.
18. Pelosi MA, II, Pelosi MA, III, Pelekanos M. The YAMA uropatch sling for treatment of female stress urinary incontinence: a pilot study. J Laparoendosc Adv Surg Tech. 2002;12:27-33.
19. Pelosi MA, II, Pelosi MA, III. Laparoscopic assessment of transvaginal suture fixation to Cooper’s ligament for suburethral slings. J Am Assoc Gynecol Laparosc. 2001;8:S53.-
20. Cundiff GW, Wright JE. A review of surgical management of genuine stress incontinence in women with a discussion of innovative procedures. J Pelvic Surg. 1998;4:271-278.
21. Sarver R, Govier FE. Pubovaginal slings: past, present, and future. Int Urogynecol J. 1997;8:358-368.
22. Kovac RS, Cruikshank SH. Pubic bone suburethral stabilization sling for recurrent urinary incontinence. Obstet Gynecol. 1997;89:624-627.
23. Muir TW, Tulikangos PK, Paraiso MF, et al. The relationship of tension-free vaginal tape insertion and the vascular anatomy. Obstet Gynecol. 2003;101:933-936.
24. Pelosi MA, II, Pelosi MA, III. Laparoscopic evaluation of a new tension-free vaginal sling (SPARC) for stress urinary incontinence. Proceedings of the 31st annual meeting of the American Association of Gynecologic Laparoscopists. November 2002; Miami, Fla.
25. Nickel RF, Wiegand U, van den Brom WE. Evaluation of a transpelvic sling procedure with and without colposuspension for treatment of female dogs with refractory urethral sphincter mechanism incompetence. Vet Surg. 1998;27:94-104.
26. Dargent D, Bretones S, George P, et al. Insertion of a suburethral sling through the obturator membrane in the treatment of female urinary incontinence. Gynecol Obstet Fertil. 2002;30:576-582.
27. Mouly P, Soulie M, Seguin P, et al. Vaginal reconstruction of a complete vaginal prolapse: the transobturator repair. J Urol. 2003;169:S182.-
- The route of the transobturator sling is strictly perineal, with a very short, blind passage through the crural region. This pathway ensures that the retropubic space is not entered and that the anatomic structures crossed by the needle passer and mesh are either muscle or fascia.
- The transobturator approach eliminates the need for routine cystoscopy in most patients.
- Short-term efficacy of the transobturator sling is similar to that of other suburethral tension-free slings.
- In our experience, the transobturator approach reduces average operating time to 17 minutes.
Potential complications associated with passing needle carriers through the retropubic space are eliminated, and cystoscopy is not routinely required with the use of a transobturator sling.
Although it is effective and easy to perform,1-6 retropubic placement of suburethral tension-free vaginal tape (TVT) for the treatment of stress urinary incontinence has been associated with a number of bowel, vascular, nerve, and bladder injuries (TABLE).7-13
Such complications appear to be related to the unique upward vaginal passage of the metallic sling trocars through the retropubic space. Newer slings eliminate the upward approach, but still require routine cystoscopy to confirm an intact bladder and urethra.
A new tension-free suburethral sling addresses these shortcomings using an innovative transobturator route.14 In the new procedure, the surgeon uses needle passers to run the sling from one obturator foramen to the other. The perineal approach reproduces the natural suspension of the urethral fascia while preserving an intact retropubic space (FIGURE 1).
TABLE
Advantages of the transobturator sling
|
FIGURE 1 Suburethral slings: Retropubic and transobturator pathways
Traditional suburethral tension-free slings require the needle passers and sling to be placed through the retropubic space.
The newer transobturator sling uses a perineal approach. The needle passer and sling are passed from one obturator foramen to the other, preserving an intact retropubic space.
What does ‘tension-free’ mean?
Tension-free slings are used to treat stress urinary incontinence caused by urethral hypermobility and intrinsic sphincter deficiency. In this approach, a synthetic transvaginal suburethral sling is placed through the retropubic space without using suspension sutures. The sling is held in place by the friction between the mesh and the tissue canals created by the metallic needle passers. Scar tissue later fixes the mesh, preventing migration.
Short-term efficacy of the Monarc transobturator sling is similar to that of the SPARC and tension-free vaginal slings.
Because the sling is not anchored to the pubic bone, ligaments, or rectus fascia, it is considered “free of tension.” The result is a midcomplex urethral support that limits urethral descent, improves the stabilization mechanism generated by pubourethral ligaments and levator ani muscles, and reinforces support of the backboard vaginal hammock.
A tension-free sling designed to reduce injury
Since its introduction in the mid 1990s, retropubic placement of suburethral tension-free tape has continued to gain in popularity.15-22 The technique has several advantages: It is simple to perform under local, regional, or general anesthesia, and has proved to be a safe treatment for stress urinary incontinence, with minimal complications in experienced hands. Still, injuries have occurred7-13 —likely due to the blind passing of metal trocars from the vagina upward through the retropubic space.9,23
Downward placement introduced. In an effort to reduce these complications, tension-free sling systems that rely on suprapubic, downward needle-carrier placement through the retropubic space were developed, such as the SPARC Female Sling System (American Medical Systems, Minnetonka, Minn), introduced in 2001.24 The manufacturer of the traditional TVT (Gynecare, a division of Ethicon, Somerville, NJ) also recently introduced suprapubic needle passers. However, because these slings still require passage through the retropubic space, their use may lead to vascular, bowel, and bladder injury. Routine cystoscopy is therefore required.
The transobturator sling. In the Netherlands in 1998, Nickel et al25 reported a successful sling procedure using a polyester ribbon passed through the obturator foramen and around the urethra for treatment of refractory urethral sphincter incompetence in female dogs. In France in 2001, Delorme14 introduced the transobturator sling procedure in humans. Dargent et al26 then performed the operation in 71 patients using a technique inspired by Delorme, and found the shortterm results similar to those of the TVT.
The Monarc transobturator sling (American Medical Systems), introduced in Europe in January 2003, has been used successfully in more than 1,000 procedures ( FIGURE 2). This new system offers significant technical improvements over the original transobturator sling devices.
The Monarc transobturator sling, introduced in Europe in January 2003, has been used successfully in more than 1,000 procedures.
Specifically, the Monarc system simplifies rotation of the needle passer during insertion and extraction around the inferior aspect of the ischiopubic ramus. The 3-mm–diameter passers are specifically designed for right-side or left-side use. They are attached to fixed handles to create a natural wrist rotation. The sling connectors, meanwhile, facilitate rapid, effortless, and secure mesh placement and fixation.
FIGURE 2 Monarc transobturator sling system
The Monarc system consists of 2 stainless steel helical-shaped needle passers and a 1-cm by 35-cm polypropylene sling mesh with a protected sheath, attached connectors, and a reabsorbable tensioning suture.
Easy to learn and perform
Anesthesia may be local, regional, or general, and prophylactic antibiotics are routinely given. FIGURES 3 through 6 offer a detailed description of how to surgically place the Monarc transobturator sling for treatment of stress urinary incontinence. What follows is a brief overview:
After making a small vertical incision along the anterior vaginal mucosa, use a finger to locate the upper-inner corner of the obturator foramen—the safe zone for needle insertion. You will incise the skin at this point and introduce the needle tip perpendicularly. Rotate the needle, with the tip following the posterior surface of the pubic ramus, until the tip exits the vaginal incision. Repeat this process on the other side.
Attach the sling and its plastic sheath, then draw both out through the skin incisions by pulling back on the needle passers. Cut the mesh, adjust the tension, and close the incisions.
A Foley catheter and vaginal packing with metronidazole gel may be used at the surgeon’s discretion.
Route minimizes anatomical hazards. The pathway described ensures that there tropubic space is not entered and that muscle or fascia are the only anatomic structures crossed by the needle passer and mesh. No major vessels or nerves come in contact with the sling (FIGURES 7 and 8).
Our fluoroscopic observations of the transobturator sling in cadavers show that insertion of the needle passer through the obturator foramen with exit through the vaginal incision under the mid-urethra is precise and simple (FIGURE 9).
Our comparative studies in cadavers revealed that, despite the different shape and placement, lengths for the SPARC and Monarc transobturator slings in the same cadaver are almost equivalent—ranging from 12 to 14 cm, depending on the patient’s height and weight (FIGURE 10).
The transobturator sling involves fewer potential anatomical hazards than retropubic placement.
These considerations strongly suggest that the transobturator sling offers a simpler approach with fewer potential anatomic hazards than retropubic tension-free slings.
FIGURE 3 Vaginal incision
Inject a diluted vasoconstrictive solution into the anterior vaginal wall, and make a small vertical incision along the anterior vaginal mucosa 0.5 cm below the urethral meatus. Separate the vaginal epithelium from the underlying periurethral fascia using sharp and digital dissection, advancing bilaterally to the inferior pubic ramus. Insert a finger into the vaginal dissection and palpate the internal edge of the ischiopubic ramus and the upper-inner corner of the obturator foramen.
FIGURE 4 Needle entry: Transobturator sling procedure
The safe zone for needle insertion is the upper-inner corner of the obturator foramen (red line). The red dot indicates the recommended insertion site (upper inner corner).
The insertion area corresponds to the point where a horizontal line at the level of the clitoris crosses a vertical line at the level of the genitofemoral fold. Make a small skin incision at this point.
FIGURE 5 Needle passage: Transobturator sling procedure
Insert your left index finger into the vaginal incision on the patient’s left side. Holding the right-side Monarc needle, introduce the needle tip perpendicularly through the skin incision.
Place the thumb of your left hand onto the outside curve of the needle and gently push until its tip penetrates the obturator membrane and muscle. Use the needle’s tip to locate the pubic ramus, and rotate the needle so that the tip follows the posterior surface of this structure.
Continue this rotation, using your left index finger to guide the needle tip to exit the vaginal incision.
Repeat steps A and B on the contralateral side.
With the needle-passer tips extending out of the vaginal incision, attach the sling and its plastic sheath using the connectors. Make sure the mesh lies flat and is not twisted before attaching the connector to the second needle.
FIGURE 7 Route of transobturator sling minimizes anatomical hazards
The needle travels around the ischiopubic ramus. Transobturator needle passage begins with penetration of the skin and subcutaneous tissue. After traversing the superficial perineal fascia, the needle crosses the adductor muscles of the thigh near their pubic bone origin and below the insertion point of the adductor longus tendon (arrow). It then penetrates the obturator membrane, obturator internus muscle, and periurethral endopelvic fascia. After passing the level of the white line—either over or under the puborectalis section of the levator—the needle exits through the vaginal incision.
FIGURE 8 The obturator foramen
The tough obturator membrane covers the foramen. The obturator canal is 2 to 3 cm long and is located at the anterolateral upper margin of the membrane. The distance between the safe zone for needle insertion and the obturator canal is approximately 3.5 to 4 cm. At the safe entry zone (green area), the anterior branch of the obturator artery is reduced to a capillary diameter and no significant arteries, veins, or nerve pedicles are present.
FIGURE 10 Routes of transobturator and retropubic slings
Graphic depiction of both the transobturator sling and the retropubic tension-free sling in place.
Fluoroscopic image of both slings in a cadaver. The transobturator sling runs horizontally while the retropubic tension-free sling forms a tighter “U.”
Clinical experience yields positive results
Early European series and our initial experience suggest that the short-term efficacy of the Monarc transobturator sling is similar to that of the SPARC and TVT slings. Average operating time is 17 minutes. The technique involves a short learning curve (approximately 4 cases).
Cystoscopy is not routinely required; we perform it only in women with significant prolapse or suspected urethral injury, and have encountered no intraoperative or post-operative complications.
No major vessels or nerves come in contact with the transobturator tension-free sling.
No patients have complained of postoperative pain in the area of the adductor muscles of the thigh, and no sling erosions have occurred. We also have found this approach useful in obese patients and women with retropubic scarring, in whom retropubic needle passage can be a challenge.
In addition to its application in the treatment of female stress urinary incontinence, the transobturator foramen route was recently used to insert the anterior wings of a large mesh in the repair of severe cystocele.27
Although long-term follow-up data are not available for the transobturator approach, short-term results are encouraging. Large comparative studies with other anti-incontinent procedures are needed.
Dr. Pelosi II reports that he is a consultant for American Medical Systems. Dr. Pelosi III reports no affiliations or financial arrangements with any companies whose products are mentioned in this article.
- The route of the transobturator sling is strictly perineal, with a very short, blind passage through the crural region. This pathway ensures that the retropubic space is not entered and that the anatomic structures crossed by the needle passer and mesh are either muscle or fascia.
- The transobturator approach eliminates the need for routine cystoscopy in most patients.
- Short-term efficacy of the transobturator sling is similar to that of other suburethral tension-free slings.
- In our experience, the transobturator approach reduces average operating time to 17 minutes.
Potential complications associated with passing needle carriers through the retropubic space are eliminated, and cystoscopy is not routinely required with the use of a transobturator sling.
Although it is effective and easy to perform,1-6 retropubic placement of suburethral tension-free vaginal tape (TVT) for the treatment of stress urinary incontinence has been associated with a number of bowel, vascular, nerve, and bladder injuries (TABLE).7-13
Such complications appear to be related to the unique upward vaginal passage of the metallic sling trocars through the retropubic space. Newer slings eliminate the upward approach, but still require routine cystoscopy to confirm an intact bladder and urethra.
A new tension-free suburethral sling addresses these shortcomings using an innovative transobturator route.14 In the new procedure, the surgeon uses needle passers to run the sling from one obturator foramen to the other. The perineal approach reproduces the natural suspension of the urethral fascia while preserving an intact retropubic space (FIGURE 1).
TABLE
Advantages of the transobturator sling
|
FIGURE 1 Suburethral slings: Retropubic and transobturator pathways
Traditional suburethral tension-free slings require the needle passers and sling to be placed through the retropubic space.
The newer transobturator sling uses a perineal approach. The needle passer and sling are passed from one obturator foramen to the other, preserving an intact retropubic space.
What does ‘tension-free’ mean?
Tension-free slings are used to treat stress urinary incontinence caused by urethral hypermobility and intrinsic sphincter deficiency. In this approach, a synthetic transvaginal suburethral sling is placed through the retropubic space without using suspension sutures. The sling is held in place by the friction between the mesh and the tissue canals created by the metallic needle passers. Scar tissue later fixes the mesh, preventing migration.
Short-term efficacy of the Monarc transobturator sling is similar to that of the SPARC and tension-free vaginal slings.
Because the sling is not anchored to the pubic bone, ligaments, or rectus fascia, it is considered “free of tension.” The result is a midcomplex urethral support that limits urethral descent, improves the stabilization mechanism generated by pubourethral ligaments and levator ani muscles, and reinforces support of the backboard vaginal hammock.
A tension-free sling designed to reduce injury
Since its introduction in the mid 1990s, retropubic placement of suburethral tension-free tape has continued to gain in popularity.15-22 The technique has several advantages: It is simple to perform under local, regional, or general anesthesia, and has proved to be a safe treatment for stress urinary incontinence, with minimal complications in experienced hands. Still, injuries have occurred7-13 —likely due to the blind passing of metal trocars from the vagina upward through the retropubic space.9,23
Downward placement introduced. In an effort to reduce these complications, tension-free sling systems that rely on suprapubic, downward needle-carrier placement through the retropubic space were developed, such as the SPARC Female Sling System (American Medical Systems, Minnetonka, Minn), introduced in 2001.24 The manufacturer of the traditional TVT (Gynecare, a division of Ethicon, Somerville, NJ) also recently introduced suprapubic needle passers. However, because these slings still require passage through the retropubic space, their use may lead to vascular, bowel, and bladder injury. Routine cystoscopy is therefore required.
The transobturator sling. In the Netherlands in 1998, Nickel et al25 reported a successful sling procedure using a polyester ribbon passed through the obturator foramen and around the urethra for treatment of refractory urethral sphincter incompetence in female dogs. In France in 2001, Delorme14 introduced the transobturator sling procedure in humans. Dargent et al26 then performed the operation in 71 patients using a technique inspired by Delorme, and found the shortterm results similar to those of the TVT.
The Monarc transobturator sling (American Medical Systems), introduced in Europe in January 2003, has been used successfully in more than 1,000 procedures ( FIGURE 2). This new system offers significant technical improvements over the original transobturator sling devices.
The Monarc transobturator sling, introduced in Europe in January 2003, has been used successfully in more than 1,000 procedures.
Specifically, the Monarc system simplifies rotation of the needle passer during insertion and extraction around the inferior aspect of the ischiopubic ramus. The 3-mm–diameter passers are specifically designed for right-side or left-side use. They are attached to fixed handles to create a natural wrist rotation. The sling connectors, meanwhile, facilitate rapid, effortless, and secure mesh placement and fixation.
FIGURE 2 Monarc transobturator sling system
The Monarc system consists of 2 stainless steel helical-shaped needle passers and a 1-cm by 35-cm polypropylene sling mesh with a protected sheath, attached connectors, and a reabsorbable tensioning suture.
Easy to learn and perform
Anesthesia may be local, regional, or general, and prophylactic antibiotics are routinely given. FIGURES 3 through 6 offer a detailed description of how to surgically place the Monarc transobturator sling for treatment of stress urinary incontinence. What follows is a brief overview:
After making a small vertical incision along the anterior vaginal mucosa, use a finger to locate the upper-inner corner of the obturator foramen—the safe zone for needle insertion. You will incise the skin at this point and introduce the needle tip perpendicularly. Rotate the needle, with the tip following the posterior surface of the pubic ramus, until the tip exits the vaginal incision. Repeat this process on the other side.
Attach the sling and its plastic sheath, then draw both out through the skin incisions by pulling back on the needle passers. Cut the mesh, adjust the tension, and close the incisions.
A Foley catheter and vaginal packing with metronidazole gel may be used at the surgeon’s discretion.
Route minimizes anatomical hazards. The pathway described ensures that there tropubic space is not entered and that muscle or fascia are the only anatomic structures crossed by the needle passer and mesh. No major vessels or nerves come in contact with the sling (FIGURES 7 and 8).
Our fluoroscopic observations of the transobturator sling in cadavers show that insertion of the needle passer through the obturator foramen with exit through the vaginal incision under the mid-urethra is precise and simple (FIGURE 9).
Our comparative studies in cadavers revealed that, despite the different shape and placement, lengths for the SPARC and Monarc transobturator slings in the same cadaver are almost equivalent—ranging from 12 to 14 cm, depending on the patient’s height and weight (FIGURE 10).
The transobturator sling involves fewer potential anatomical hazards than retropubic placement.
These considerations strongly suggest that the transobturator sling offers a simpler approach with fewer potential anatomic hazards than retropubic tension-free slings.
FIGURE 3 Vaginal incision
Inject a diluted vasoconstrictive solution into the anterior vaginal wall, and make a small vertical incision along the anterior vaginal mucosa 0.5 cm below the urethral meatus. Separate the vaginal epithelium from the underlying periurethral fascia using sharp and digital dissection, advancing bilaterally to the inferior pubic ramus. Insert a finger into the vaginal dissection and palpate the internal edge of the ischiopubic ramus and the upper-inner corner of the obturator foramen.
FIGURE 4 Needle entry: Transobturator sling procedure
The safe zone for needle insertion is the upper-inner corner of the obturator foramen (red line). The red dot indicates the recommended insertion site (upper inner corner).
The insertion area corresponds to the point where a horizontal line at the level of the clitoris crosses a vertical line at the level of the genitofemoral fold. Make a small skin incision at this point.
FIGURE 5 Needle passage: Transobturator sling procedure
Insert your left index finger into the vaginal incision on the patient’s left side. Holding the right-side Monarc needle, introduce the needle tip perpendicularly through the skin incision.
Place the thumb of your left hand onto the outside curve of the needle and gently push until its tip penetrates the obturator membrane and muscle. Use the needle’s tip to locate the pubic ramus, and rotate the needle so that the tip follows the posterior surface of this structure.
Continue this rotation, using your left index finger to guide the needle tip to exit the vaginal incision.
Repeat steps A and B on the contralateral side.
With the needle-passer tips extending out of the vaginal incision, attach the sling and its plastic sheath using the connectors. Make sure the mesh lies flat and is not twisted before attaching the connector to the second needle.
FIGURE 7 Route of transobturator sling minimizes anatomical hazards
The needle travels around the ischiopubic ramus. Transobturator needle passage begins with penetration of the skin and subcutaneous tissue. After traversing the superficial perineal fascia, the needle crosses the adductor muscles of the thigh near their pubic bone origin and below the insertion point of the adductor longus tendon (arrow). It then penetrates the obturator membrane, obturator internus muscle, and periurethral endopelvic fascia. After passing the level of the white line—either over or under the puborectalis section of the levator—the needle exits through the vaginal incision.
FIGURE 8 The obturator foramen
The tough obturator membrane covers the foramen. The obturator canal is 2 to 3 cm long and is located at the anterolateral upper margin of the membrane. The distance between the safe zone for needle insertion and the obturator canal is approximately 3.5 to 4 cm. At the safe entry zone (green area), the anterior branch of the obturator artery is reduced to a capillary diameter and no significant arteries, veins, or nerve pedicles are present.
FIGURE 10 Routes of transobturator and retropubic slings
Graphic depiction of both the transobturator sling and the retropubic tension-free sling in place.
Fluoroscopic image of both slings in a cadaver. The transobturator sling runs horizontally while the retropubic tension-free sling forms a tighter “U.”
Clinical experience yields positive results
Early European series and our initial experience suggest that the short-term efficacy of the Monarc transobturator sling is similar to that of the SPARC and TVT slings. Average operating time is 17 minutes. The technique involves a short learning curve (approximately 4 cases).
Cystoscopy is not routinely required; we perform it only in women with significant prolapse or suspected urethral injury, and have encountered no intraoperative or post-operative complications.
No major vessels or nerves come in contact with the transobturator tension-free sling.
No patients have complained of postoperative pain in the area of the adductor muscles of the thigh, and no sling erosions have occurred. We also have found this approach useful in obese patients and women with retropubic scarring, in whom retropubic needle passage can be a challenge.
In addition to its application in the treatment of female stress urinary incontinence, the transobturator foramen route was recently used to insert the anterior wings of a large mesh in the repair of severe cystocele.27
Although long-term follow-up data are not available for the transobturator approach, short-term results are encouraging. Large comparative studies with other anti-incontinent procedures are needed.
Dr. Pelosi II reports that he is a consultant for American Medical Systems. Dr. Pelosi III reports no affiliations or financial arrangements with any companies whose products are mentioned in this article.
1. Reschers UM, Tunn R, Buczkowski M, et al. Tension-free vaginal tape for the treatment of stress urinary incontinence. Clin Obstet Gynecol. 2000;43:670-675.
2. Tamussino KF, Hanzal E, Kolle D, et al. Tension-free vaginal tape operation: results of the Austrian Registry. Obstet Gynecol. 2001;98:732-736.
3. Haab F, Sananes S, Amarenco G, et al. Results of the tension-free vaginal tape procedure for the treatment of type II stress urinary incontinence at a minimum follow up of one year. J Urol. 2001;165:159-162.
4. Rardin CR, Kohli N, Rosenblatt PL, et al. Tension-free vaginal tape: outcomes among women with primary versus recurrent stress urinary incontinence. Obstet Gynecol. 2002;100:893-897.
5. Vassallo BJ, Kleeman SD, Segal JL, et al. Tension-free vaginal tape: a quality of life assessment. Obstet Gynecol. 2002;100:518-524.
6. Karram MM, Segal JL, Vassallo BJ, et al. Complications and untoward effects of the tension-free vaginal tape procedure. Obstet Gynecol. 2003;101:929-932.
7. MAUDE: Food and Drug Administration Manufacturer and User Facility Device Experience Database. Available at http://www.fda.gov/cdrh/maude.html. Accessed June 7, 2003.
8. Peyrat L, Boutin JM, Bruyere F, et al. Intestinal perforation is a complication of TVT procedure for UI. Eur Urol. 2001;39:603-605.
9. Walters MD, Tulikangas PR, LaSala C, et al. Vascular injury during tension-free vaginal tape procedure for stress urinary incontinence. Obstet Gynecol. 2001;98:957-959.
10. Zilbert AW, Farrell SA. External iliac artery laceration during tension-free vaginal tape procedure. Int Urogynecol J Pelvic Floor Dysfunct. 2001;12:141-143.
11. Vierhout ME. Severe hemorrhage complicating TVT: a case report. Int Urogynecol J Pelvic Floor Dysfunct. 2000;12:139-140.
12. Brink DM. Bowel injury following insertion of TVT. S Afr Med J. 2000;90:450-452.
13. Shobeiri SA, Garely AD, Chesson RR. Recognition of occult bladder injury during the tension-free vaginal tape procedure. Obstet Gynecol. 2002;99:1067-1072.
14. Delorme E. Transobturator urethral suspension: mini-invasive procedure in the treatment of stress urinary incontinence in women. Prog Urol. 2001;11:1306-1313.
15. Ulmstein V, Petros P. Intravaginal slingplasty (IVS): an ambulatory surgical procedure for treatment of female urinary incontinence. Scand J Urol Neprhol. 1995;29:75-82.
16. Chung MK, Chung RP. Comparison of laparoscopic Burch and tension-free vaginal tape in treating stress urinary incontinence in obese patients. JSLS. 2002;6:17-21.
17. Pelosi MA, III, Pelosi MA, II. Pubic bone suburethral stabilization sling: laparoscopic assessment of a transvaginal operation for the treatment of stress urinary incontinence. J Laparoendosc Adv Surg Tech. 1999;9:45-50.
18. Pelosi MA, II, Pelosi MA, III, Pelekanos M. The YAMA uropatch sling for treatment of female stress urinary incontinence: a pilot study. J Laparoendosc Adv Surg Tech. 2002;12:27-33.
19. Pelosi MA, II, Pelosi MA, III. Laparoscopic assessment of transvaginal suture fixation to Cooper’s ligament for suburethral slings. J Am Assoc Gynecol Laparosc. 2001;8:S53.-
20. Cundiff GW, Wright JE. A review of surgical management of genuine stress incontinence in women with a discussion of innovative procedures. J Pelvic Surg. 1998;4:271-278.
21. Sarver R, Govier FE. Pubovaginal slings: past, present, and future. Int Urogynecol J. 1997;8:358-368.
22. Kovac RS, Cruikshank SH. Pubic bone suburethral stabilization sling for recurrent urinary incontinence. Obstet Gynecol. 1997;89:624-627.
23. Muir TW, Tulikangos PK, Paraiso MF, et al. The relationship of tension-free vaginal tape insertion and the vascular anatomy. Obstet Gynecol. 2003;101:933-936.
24. Pelosi MA, II, Pelosi MA, III. Laparoscopic evaluation of a new tension-free vaginal sling (SPARC) for stress urinary incontinence. Proceedings of the 31st annual meeting of the American Association of Gynecologic Laparoscopists. November 2002; Miami, Fla.
25. Nickel RF, Wiegand U, van den Brom WE. Evaluation of a transpelvic sling procedure with and without colposuspension for treatment of female dogs with refractory urethral sphincter mechanism incompetence. Vet Surg. 1998;27:94-104.
26. Dargent D, Bretones S, George P, et al. Insertion of a suburethral sling through the obturator membrane in the treatment of female urinary incontinence. Gynecol Obstet Fertil. 2002;30:576-582.
27. Mouly P, Soulie M, Seguin P, et al. Vaginal reconstruction of a complete vaginal prolapse: the transobturator repair. J Urol. 2003;169:S182.-
1. Reschers UM, Tunn R, Buczkowski M, et al. Tension-free vaginal tape for the treatment of stress urinary incontinence. Clin Obstet Gynecol. 2000;43:670-675.
2. Tamussino KF, Hanzal E, Kolle D, et al. Tension-free vaginal tape operation: results of the Austrian Registry. Obstet Gynecol. 2001;98:732-736.
3. Haab F, Sananes S, Amarenco G, et al. Results of the tension-free vaginal tape procedure for the treatment of type II stress urinary incontinence at a minimum follow up of one year. J Urol. 2001;165:159-162.
4. Rardin CR, Kohli N, Rosenblatt PL, et al. Tension-free vaginal tape: outcomes among women with primary versus recurrent stress urinary incontinence. Obstet Gynecol. 2002;100:893-897.
5. Vassallo BJ, Kleeman SD, Segal JL, et al. Tension-free vaginal tape: a quality of life assessment. Obstet Gynecol. 2002;100:518-524.
6. Karram MM, Segal JL, Vassallo BJ, et al. Complications and untoward effects of the tension-free vaginal tape procedure. Obstet Gynecol. 2003;101:929-932.
7. MAUDE: Food and Drug Administration Manufacturer and User Facility Device Experience Database. Available at http://www.fda.gov/cdrh/maude.html. Accessed June 7, 2003.
8. Peyrat L, Boutin JM, Bruyere F, et al. Intestinal perforation is a complication of TVT procedure for UI. Eur Urol. 2001;39:603-605.
9. Walters MD, Tulikangas PR, LaSala C, et al. Vascular injury during tension-free vaginal tape procedure for stress urinary incontinence. Obstet Gynecol. 2001;98:957-959.
10. Zilbert AW, Farrell SA. External iliac artery laceration during tension-free vaginal tape procedure. Int Urogynecol J Pelvic Floor Dysfunct. 2001;12:141-143.
11. Vierhout ME. Severe hemorrhage complicating TVT: a case report. Int Urogynecol J Pelvic Floor Dysfunct. 2000;12:139-140.
12. Brink DM. Bowel injury following insertion of TVT. S Afr Med J. 2000;90:450-452.
13. Shobeiri SA, Garely AD, Chesson RR. Recognition of occult bladder injury during the tension-free vaginal tape procedure. Obstet Gynecol. 2002;99:1067-1072.
14. Delorme E. Transobturator urethral suspension: mini-invasive procedure in the treatment of stress urinary incontinence in women. Prog Urol. 2001;11:1306-1313.
15. Ulmstein V, Petros P. Intravaginal slingplasty (IVS): an ambulatory surgical procedure for treatment of female urinary incontinence. Scand J Urol Neprhol. 1995;29:75-82.
16. Chung MK, Chung RP. Comparison of laparoscopic Burch and tension-free vaginal tape in treating stress urinary incontinence in obese patients. JSLS. 2002;6:17-21.
17. Pelosi MA, III, Pelosi MA, II. Pubic bone suburethral stabilization sling: laparoscopic assessment of a transvaginal operation for the treatment of stress urinary incontinence. J Laparoendosc Adv Surg Tech. 1999;9:45-50.
18. Pelosi MA, II, Pelosi MA, III, Pelekanos M. The YAMA uropatch sling for treatment of female stress urinary incontinence: a pilot study. J Laparoendosc Adv Surg Tech. 2002;12:27-33.
19. Pelosi MA, II, Pelosi MA, III. Laparoscopic assessment of transvaginal suture fixation to Cooper’s ligament for suburethral slings. J Am Assoc Gynecol Laparosc. 2001;8:S53.-
20. Cundiff GW, Wright JE. A review of surgical management of genuine stress incontinence in women with a discussion of innovative procedures. J Pelvic Surg. 1998;4:271-278.
21. Sarver R, Govier FE. Pubovaginal slings: past, present, and future. Int Urogynecol J. 1997;8:358-368.
22. Kovac RS, Cruikshank SH. Pubic bone suburethral stabilization sling for recurrent urinary incontinence. Obstet Gynecol. 1997;89:624-627.
23. Muir TW, Tulikangos PK, Paraiso MF, et al. The relationship of tension-free vaginal tape insertion and the vascular anatomy. Obstet Gynecol. 2003;101:933-936.
24. Pelosi MA, II, Pelosi MA, III. Laparoscopic evaluation of a new tension-free vaginal sling (SPARC) for stress urinary incontinence. Proceedings of the 31st annual meeting of the American Association of Gynecologic Laparoscopists. November 2002; Miami, Fla.
25. Nickel RF, Wiegand U, van den Brom WE. Evaluation of a transpelvic sling procedure with and without colposuspension for treatment of female dogs with refractory urethral sphincter mechanism incompetence. Vet Surg. 1998;27:94-104.
26. Dargent D, Bretones S, George P, et al. Insertion of a suburethral sling through the obturator membrane in the treatment of female urinary incontinence. Gynecol Obstet Fertil. 2002;30:576-582.
27. Mouly P, Soulie M, Seguin P, et al. Vaginal reconstruction of a complete vaginal prolapse: the transobturator repair. J Urol. 2003;169:S182.-
Preventing adhesions after abdominal myomectomy: Tools and techniques
- To reduce the incidence of postoperative adhesions, follow basic principles of microsurgery: Minimize the number and extent of incisions, handle all tissue gently, strive for absolute hemostasis, and use small, nonreactive suture.
- Despite limited data from prospective, randomized studies, both fluid and barrier adjuvants have proved effective in reducing the incidence and extent of adhesions after abdominal myomectomy.
Abdominal myomectomy is the preferred treatment in women with large or numerous intramural myomas, especially in the setting of infertility, recurrent pregnancy loss, and preservation of future fertility.1,2 However, postoperative adhesions are distressingly common following this procedure, resulting in significant potential morbidity. Fortunately, a number of products can reduce their occurrence. Proper surgical techniques and a thorough knowledge of these products are invaluable in helping reduce the incidence of adhesions.
The association between adhesions and diminished fertility is well-established,3,4 particularly when peritubal involvement is present (FIGURES 1-3). Abdominopelvic adhesions also contribute to significant chronic pelvic pain, bowel obstruction, and technical difficulty in subsequent surgical or assisted-reproduction procedures.5 Unfortunately, most attempts at adhesiolysis meet with less than complete success, since adhesions recur in 55% to 100% of patients (FIGURE 4).6 Thus, preventing adhesions in the first place would seem to be key to successful outcomes in abdominal myomectomy.
This article reviews the evidence on various approaches and products. While the number of studies examining each adjuvant in the setting of abdominal myomectomy is limited, the overall evidence supports the safety and efficacy of both liquid and barrier adjuvants.
Adhesions present a significant clinical dilemma after abdominal myomectomy, occurring in 50% to 90% of patients.5,7 In 1 prospective series of women undergoing second-look laparoscopy (SLL) after myomectomy, adnexal adhesions were noted in 94% of patients with posterior uterine incisions and in 56% of patients with only anterior or fundal incisions; adhesions between the uterus and omentum or bowel occurred in 88% of all patients.5
In another study of early SLL following abdominal myomectomy, 83% of patients had adhesions between the surgical site and the bowel or omentum, and 65% had adhesions involving the adnexae.2 Removal of large, bulky fibroids (with uterine mass exceeding 13 weeks’ gestational size) resulted in higher adhesion scores than did small myomas. Again, the incidence and severity of adhesions also correlated with location of the uterine incision: Adnexal adhesions were more common after posterior uterine incisions (76%) than after anterior or fundal entries (45%).2
FIGURE 1 Peritubal adhesion
Adhesion at distal end of the fallopian tube. Peritubal involvement often leads to diminished fertility.
FIGURE 2 Adherent structures
Postoperative adhesion between fallopian tube and the uterus.
FIGURE 3 Cyst dissection
Adhesions can follow common surgeries such as paratubal cyst dissection.
FIGURE 4 Recurrent adhesions
Adhesions may recur following adhesiolysis.
Causes of pelvic adhesions
Adhesion prevention requires an understanding of risk factors and maneuvers that increase the likelihood of injury (see “Pathophysiology of adhesion formation”). A number of causes have been proposed, most of them centering on tissue and peritoneal trauma (TABLE 1).
Injury can arise from excessive or rough manipulation of tissue and peritoneal surfaces or from common effects such as cutting, abrasion, and denudation (FIGURE 5). Tissue desiccation or manual blotting may lead to peritoneal desquamation and fibrin deposition.
Exposure of surfaces to intraperitoneal blood in the setting of tissue hypoxia—virtually unavoidable during abdominal myomectomy—disrupts normal fibrinolytic activity, resulting in stimulation of angiogenesis.8
Introduction of reactive foreign bodies such as talc powder, residual suture material, and even lint from laparotomy pads can favor adhesion formation. These serve as substrates or niduses of fibrin deposition.
FIGURE 5 Conducive conditions
Raw surface area can become a potent substrate for adhesions.TABLE 1
Proposed causes of adhesion formation
| Tissue hypoxia or ischemia |
| Tissue desiccation |
| Intra-abdominal infection |
| Introduction of reactive foreign body |
| Presence of intraperitoneal blood |
| Dissection of adhesions |
Techniques that may help prevent adhesions
Based on findings from studies of second-look procedures, most physicians advocate avoiding posterior uterine incisions, as well as minimizing the number and extent of incisions, to help reduce the likelihood of adhesion formation. Further, many Ob/Gyns favor removing myomas through as few uterine incisions as possible.9 We select anterior hysterotomy sites that enable removal of multiple fibroids, avoiding posterior incisions and the uterotubal junction whenever possible.
Interestingly, reapproximation of peritoneal defects after reproductive surgery (and, probably, myomectomy) does not appear to help prevent adhesions. Tulandi and colleagues10 examined the clinical and SLL outcomes of peritoneal closure in patients undergoing Pfannenstiel incisions with or without peritoneal closure at the end of the procedure. There was no difference in postoperative complications or wound healing in the 2 groups. At the time of SLL, there was no significant difference in the incidence of adhesion formation at the anterior abdominal wall.
Principles of microsurgery have been adopted by reproductive surgeons to minimize the likelihood of adhesions after myomectomy and gynecologic surgery in general. The basic techniques reflect respect for tissue integrity:
- Gentle handling of tissue, with minimal manipulation of all peritoneal surfaces.
- Meticulous hemostasis. Examine all myomectomy sites to ensure adequate hemostasis prior to closure.
- Continuous irrigation to prevent tissue desiccation. We favor continuous saline irrigation throughout the procedure. We also use only moistened laparotomy sponges and pads, and avoid applying dry gauze to any tissue surface.
- Avoidance of foreign-body introduction. We use only talc-free gloves and remove all residual suture fragments and tissue debris before closure. We also perform copious saline suction-irrigation at the end of the procedure to remove as much residue as possible.
- Use of fine, nonreactive suture. We favor fine, resorbable sutures that incite as little tissue reactivity as possible. For closure of large myomectomy defects, we use braided multifilaments such as Vicryl (polyglactine 910) (Ethicon, Somerville, NJ) or Dexon (polyglycolic acid) (Davis and Geck, Danbury, Conn) for strength and ease of handling. These sutures are absorbed through simple hydrolysis and stimulate less tissue reactivity than do chromic or catgut sutures.
The idea is appealing, but a randomized, blinded comparison of “good” and “bad” microsurgical technique is unlikely, since no one would wish to perform “bad” technique.
With barrier adjuvants, optimal benefit is obtained when the physician can predict potential sites of adhesions.
Wide range of prevention tools has been studied
Many types of agents have been studied in an attempt to reduce postsurgical adhesions after gynecologic surgery. Although most offer little or no benefit (TABLE 2), a few have potential in myomectomy procedures.
Barriers that form mechanical separation (TABLE 3) theoretically physically separate damaged tissues during early peritoneal wound healing, when adhesions form.11 The original adhesion barriers consisted of omental and peritoneal grafts that were placed over surgical sites. However, studies demonstrated that devitalized tissue positioned on damaged peritoneum serves as a potent substrate—not inhibitor—for adhesions.12 More recent trials have examined the adhesion-prevention benefit of other types of absorbable and nonabsorbable barriers.
TABLE 2
Pharmacologic agents studied for adhesion prevention in reproductive surgery
| AGENT | THEORETICAL ACTION | EXAMPLES | MODE OF USE/APPLICATION | RISKS/PROBLEMS |
|---|---|---|---|---|
| Antibiotics | Prevent infection or inflammation | Cephalosporins Tetracyclines | Intraperitoneal irrigation with antibiotic fluid Hydrotubation fluid with antibiotic | Theoretical reaction to antibiotic |
| Anticoagulants | Clot prevention Fibrin prevention | Heparin | In conjunction with Interceed | Risk of postoperative bleeding |
| Anti-inflammatory agents | Decrease permeability and histamine release | Nonsteroidal anti-inflammatory drugs Corticosteroids | Awaiting further investigation | Theoretical reaction to agent |
| Crystalloid solutions | Hydroflotation effect, decrease surface contact between pelvic organs | Normal saline Ringer’s lactate | Intra-abdominal instillation | Possible volume overload from intravascular absorption |
| Fibrinolytic agents | Fibrinolysis Plasminogen activation | Streptokinase Trypsin Fibrinolysin | Awaiting further investigation | Theoretical risk of postoperative bleeding |
| Steroids | Decrease inflammatory response | Dexamethasone | Systemic and/or intraperitoneal | Possible suppression of hypothalamic-pituitary axis |
| Polysaccharide polymer | "Siliconizing" effect to coat raw surfaces | Dextran 70 (Hyskon) | 200 mL placed in posterior cul-de-sac or coating surgical site surfaces | Abdominal bloating, anaphylaxis, pleural effusion, liver function abnormalities, wound separation, rare diffuse intravascular coagulation |
| Other fluid and barrier agents* | Peritoneal surface separation Hydroflotation | Absorbable and nonabsorbable barriers (see Table 3) | See Table 3 | See Table 3 |
| *Adjuvants studied in the setting of abdominal myomectomy | ||||
TABLE 3
Fluid and barrier adjuvants studied for adhesion reduction after myomectomy
| AGENT | THEORETICAL ACTION | MODE OF USE/APPLICATION | PROBLEMS | EVIDENCE FOR ADHESION REDUCTION |
|---|---|---|---|---|
| ABSORBABLE (BARRIER) | ||||
| Oxidized regenerated cellulose (Interceed) | Protective layer over surgical sites to prevent surface contact | Direct placement onto surface of uterus; no suturing required | Requires hemostasis | Prospective studies and meta-analysis support benefit in reproductive surgery including abdominal myomectomy |
| Hyaluronatecarboxymethycellulose derivative film (Seprafilm) | Protective layer over surgical sites to prevent surface contact | Direct placement around entire uterine surface; no suturing required | Requires hemostasis | Multicenter prospective, randomized study supports benefit in reducing adhesions after abdominal myomectomy |
| NONABSORBABLE (BARRIER) | ||||
| Expanded polytetrafluoroethylene (GoreTex) | Prevent contact between surgical surfaces | Patch sutured onto surface of uterus | Usually must be removed Report of fistula formation when left in situ | Multicenter prospective studies support adhesion-preventive benefit after abdominal myomectomy |
| Pericardial patch (Shelhigh No-React) | Prevent contact between surgical surfaces | Patch sutured onto surface of uterus | Early clinical use in myomectomy Proven safety as pericardial patch in humans | Preliminary study (case series data only) shows potential benefit |
| FLUID | ||||
| Hyaluronic acid-coat (Sepracoat) | Diffuse coating on surgical sites and potential sites of contact | 100-mL to 250-mL aliquots injected into peritoneal cavity | Limited data on efficacy in abdominal myomectomy | Small studies (multicenter, prospective, randomized, controlled trials) demonstrated reduced postoperative adhesions after reproductive surgery via laparotomy, including myomectomy |
| Hyaluronate-carboxymethycellulose derivative gel (Intergel) | Diffuse coating on surgical sites and potential sites of contact | 300-mL aliquot into peritoneal cavity | Withdrawn from market for reports of postoperative pain, complications | Reduced adhesion formation in animal studies and in preliminary human studies |
Adhesions are fibrous or fibrovascular bands that connect tissue surfaces in abnormal locations.1,2 Their development likely results from an imbalance in inflammatory mediators or fibrin degradation during peritoneal wound healing.
Peritoneal injury initiates the release of histamine and vasoactive kinins that mediate increased capillary permeability and outpouring of serosanguineous fluid.3 This proteinaceous exudate coagulates, depositing fibrinous bands between areas of denuded tissue.
Under normal circumstances, the fibrinolytic system is activated to lyse these bands within 72 hours. Peritoneal healing occurs when mesothelial cells migrate from the underlying mesenchyme to reepithelialize the injured site.4 Disequilibrium of the fibrin deposition-fibrinolysis system results in a persistent band that will eventually undergo fibroblast and vascular invasion.
REFERENCES
1. Diamond MP, DeCherney AH. Pathogenesis of adhesion formation/reformation: application to reproductive pelvic surgery. Microsurgery. 1987;8:103-107.
2. Diamond MP, Freeman ML. Clinical implications of postsurgical adhesions. Hum Reprod. 2001;7:567-576.
3. Diamond MP, El-Mowafi DM. Pelvic adhesions. Surg Technol Int. 1998;VII:273-283.
4. Farquhar C, Vandekerckhove P, Watson A, Vail A, Wiseman D. Barrier agents for preventing adhesions after surgery for subfertility. Cochrane Database Systematic Rev. 2002;(4):1-34.
Absorbable barriers
These are largely derivatives of organic materials. Their application to myomectomy may be limited by the requirement for absolute hemostasis at the site of application.
Interceed (Gynecare, a division of Ethicon), which is derived from oxidized regenerated cellulose, is one of the first and most extensively evaluated barriers. A mesh synthetic designed to be placed over injured tissue, it is a derivative of the hemostatic agent Surgicell (Johnson & Johnson, New Brunswick, NJ), with modifications in weave and pore size.
Interceed offers ease of application: It can be cut to the size or shape necessary and requires no suturing. It forms a gelatinous protective layer within 8 hours of placement, and is degraded into monosaccharides and absorbed within 2 weeks.13
The use of Interceed has been shown to reduce adhesions following adhesiolysis and ovarian surgery. In a large, multicenter, prospective, randomized trial, it significantly decreased the incidence of adhesion reformation after adnexal adhesiolysis in infertility patients with bilateral tubal disease.14
In a retrospective series of 38 infertility patients, including 19 patients after myomectomy (13 with the barrier, 6 without), reproductive outcomes were significantly better in the Interceed group, and adhesion development was reduced.15 Pregnancy rates in the 2 years following surgery were 78% in the Interceed group, compared with 47% in controls. In addition, among 23 patients who had second-look procedures, postoperative adhesions were noted in 38% of the Interceed group, compared with 86% in controls.15
Seprafilm (hyaluronic acid-film) (Genzyme Corp, Cambridge, Mass) is a bioresorbable membrane derived from sodium hyaluronate and carboxymethylcellulose. It is absorbed from the peritoneal cavity within 1 week and is completely excreted within 1 month.
Its potential for adhesion prevention after abdominal myomectomy was examined by SLL in a multicenter, prospective, randomized, blinded study11 in which 127 women undergoing abdominal myomectomy at 19 institutions were randomized to either Seprafilm or no barrier. In the Seprafilm group, the barrier was wrapped circumferentially around the uterus, covering all uterine defects, at the time of abdominal closure. Clinical outcomes—including vital signs, adverse events (pain, fever, nausea), and abdominal wound complications—were similar in the control and Seprafilm groups.
In this study, the incidence (mean number of sites), severity, and extent (mean area) of uterine adhesions were significantly lower in the Seprafilm-treated patients. The proportion of patients undergoing anterior hysterotomies who were found to have no anterior uterine adhesions was 39% in the Seprafilm group, compared with 6% in the control group. The percentage of patients with at least 1 adnexa totally free of adhesions to the posterior uterus also was higher in the Seprafilm group—48% versus 31%.11
Nonabsorbable barriers
GoreTex Surgical Membrane (W.L. Gore and Associates, Newark, Del) is an inert expanded polytetrafluoroethylene (PTFE) derivative that must be sutured in place. It is of potential utility in myomectomy because its application does not require absolute hemostasis. However, its usefulness and application are limited by the need for later removal.
In a multicenter, randomized, controlled trial exploring its adhesion-preventive properties after myomectomy, the GoreTex membrane significantly outperformed the barrierfree group.16
In a separate multicenter, randomized clinical trial, the GoreTex membrane was more effective than Interceed in preventing adhesions after pelvic/tubal reconstructive surgery.17
Yet another multicenter, randomized trial—this one involving 27 women undergoing abdominal myomectomy—used SLL to determine the extent of adhesions and to remove the barrier. The percentage of adhesion-free surgical sites was significantly higher in the GoreTex group: 56% compared with 7% in no-barrier controls.16
The adhesion scores, determined by the extent (area of involvement), nature (filmy versus opaque), and tenacity (ease of lysis or dissection) of the adhesions, were significantly lower in the GoreTex group.16
Despite the few prospective, randomized trials, the evidence supports the efficacy of various fluid and barrier adjuvants.
Histologic examination of the removed barriers demonstrated no tissue attachment to the PTFE. Despite these positive findings, however, both fistula formation and graft infection have been reported after placement of a GoreTex barrier.18
The Shelhigh Pericardial No-React Patch (Herzog Surgical, Sacramento, Calif) is a new nonabsorbable adhesion barrier that was first described in gynecologic use by Pelosi and Pelosi in a case series of 20 patients.19 Consisting of a 12-cm-diameter patch of glutaraldehyde-treated bovine/porcine pericardium, this product has an excellent safety record as a permanent pericardial substitute in the cardiovascular literature and has been shown to resist calcification and adhesions.
In the Pelosi series, the patch was placed over the uterine fundus and secured by 4 monofilament sutures at the dome of the uterus and the parietal peritoneum.19 All patients underwent SLL at 6 weeks, and 3 patients also underwent third-look laparoscopy. None of the 20 women had adhesions between the abdominal wall and the bladder, bowel, uterus, or adnexae at SLL. However, minimal adhesions of the ovaries and tubes were found in 7 patients who had undergone posterior hysterotomy. Clinical trials are likely to follow this small pilot study.
Absorbable fluid adjuvants
With barrier adjuvants, optimal benefit is obtained when the physician can predict potential sites of adhesions. This is not a strict requirement with fluid barriers, which is their chief advantage. Among the agents described below, Sepracoat (Genzyme Corp) is available in the United States, while Intergel (Lifecore Biomedical, Chaska, Minn) was withdrawn from the market in March.
Intergel, a 0.5% ferric hyaluronade formulation, is a sterile nonpyrogenic gel of highly purified sodium hyaluronate, which is ionically cross-linked with ferric ion and adjusted to isotonicity with sodium chloride.11 (Hyaluronic acid is a major component of body tissues and fluids such as peritoneal fluid, where it performs physically supportive and mechanically protective roles.)
Johns and colleagues20 studied Intergel in a randomized, multicenter, third-party– blinded, placebo-controlled study. Of the 265 patients who completed the study, 131 were given 300 mL of Intergel and 134 were given lactated Ringer’s solution (the placebo) at the time of their surgery, through the laparoscopic port. When SLL was performed 6 to 12 weeks after surgery, the mean number and severity of adhesions—overall and at the surgical site—were significantly lower in the Intergel group. Adhesions reformed in 91% of those in the control group, compared with 63% in the Intergel group.
In myomectomy patients, the modified American Fertility Society Score, which uses 24 potential adhesion sites, was reduced by 42% in the Intergel group—a statistically significant improvement.20
One major advantage of Intergel is that it reduces adhesion formation at sites distant from the area of application, secondary to its wide intra-abdominal circulation. However, as mentioned above, sales were voluntarily suspended due to post-market reports of tissue adherence, sterile foreign-body reaction, and late-onset pain that sometimes required surgical intervention. In some patients, persistent residual material was noted at the time of subsequent surgery.
Sepracoat (hyaluronic acid-coat) is a dilute solution of 0.4% hyaluronic acid in phosphate-buffered saline. It is bioresorbable, persists at the application site less than 24 hours, and is completely cleared in less than 5 days.
In a prospective, randomized, blinded, placebo-controlled, multicenter study in 1998, Diamond and colleagues compared Sepracoat with a pure phosphate-buffered saline solution in 227 women undergoing gynecologic procedures via laparotomy.21 The aim of the study was to assess the efficacy and safety of the fluid at sites without direct surgical trauma or adhesiolysis. Both solutions were warmed to room temperature and injected into the abdominal cavity before the procedure began (250 mL after skin incision). The solution was reapplied after irrigation or every 30 minutes (100 mL), and at the end of the procedure (250 mL) before closure. The maximum volume used was 1,000 mL. After application, the fluid was left 1 minute before suctioning. Patients underwent SLL 40 days later, and adhesions were identified at the initial procedure and at SLL.
In the Sepracoat group, there was a reduction in de novo adhesions at nonsurgical sites by a factor of 2.8. The proportion of sites with de novo adhesions also decreased, and 80% of Sepracoat patients had at least 1 ovary that was adhesion-free compared with 58% of placebo-treated patients. These findings occurred in areas of indirect trauma, demonstrating that Sepracoat limited trauma at tissue injury.21
Conclusion
Adhesion prevention is of utmost importance after abdominal myomectomy, especially in patients who desire future fertility. Despite the limited number of prospective, randomized studies, the literature does support the efficacy of various fluid and barrier adjuvants.
Our practice is to use an adjuvant in all abdominal myomectomies. We have long relied on Interceed, which enjoys both ease of application and an excellent safety record. However, as reviewed above, other potentially useful products are available or in development. As ever, the reproductive surgeon should adhere to principles of microsurgery, strive for meticulous hemostasis, and demonstrate respect for tissue integrity.
Dr. DeCherney reports small holdings with Lifecore Biomedical. Drs. Chang and Marin report no affiliations or financial arrangements with any of the manufacturers of products mentioned in this article.
1. Stewart EA. Treatment of uterine leiomyomas. UpToDate (Version 10.3) 2002; 1-8. Available at: www.uptodate.com.
2. Ugur M, Tura C, Mungan T, Aydogdu T, Sahin Y, Gokmen O. Laparoscopy for adhesion prevention following myomectomy. Int J Gynecol Obstet. 1996;53:145-149.
3. Berkeley AS, DeCherney AH, Plan ML. Abdominal myomectomy and subsequent fertility. Surg Gynecol Obstet. 1983;153:319-322.
4. Tulandi T, Collin JA, Burrows E, et al. Treatment-dependent and treatment-independent pregnancy among women with periadnexal adhesions. Am J Obstet Gynecol. 1990;162:354-357.
5. Tulandi T, Murria C, Guralnick M. Adhesion formation and reproductive outcome after myomectomy and second-look laparoscopy. Obstet Gynecol. 1993;82:213-215.
6. Diamond MP, Freeman ML. Clinical implications of postsurgical adhesions. Hum Reprod. 2001;7:567-576.
7. Lau S, Tulandi T. Myomectomy and adhesion formation. In: diZerega GS, ed. Peritoneal Surgery. New York: Springer-Verlag 1999;289-290.
8. Diamond MP, El-Mowafi DM. Pelvic adhesions. Surg Technol Int. 1998;VII:273-283.
9. Guarnaccia MM, Rein MS. Traditional surgical approaches to uterine fibroids: abdominal myomectomy and hysterectomy. Clin Obstet Gynecol. 2001;44:385-400.
10. Tulandi T, Hum HS, Gelfand MM. Closure of laparotomy incisions with or without peritoneal suturing and second-look laparoscopy. Am J Obstet Gynecol. 1988;158:536-537.
11. Diamond MP. Reduction of adhesions after uterine myomectomy by Seprafilm membrane (HAL-F): a blinded, prospective, randomized, multicenter clinical study. Fertil Steril. 1996;66:904-910.
12. Johns D. Reduction of postsurgical adhesions with Intergel adhesion prevention solution: a multicenter study of safety and efficacy after conservative gynecologic surgery. Fertil Steril. 2001;76:595-604.
13. Farquhar C, vandekerckhove P, Watson A, Vail A, Wiseman D. Barrier agents for preventing adhesions after surgery for subfertility. Cochrane Database Systematic Rev. 2002(4);1-34.
14. Nordic Adhesion Prevention Study Group. The efficacy of Interceed (TC7) for prevention of reformation of postoperative adhesions on ovaries, fallopian tubes, and fimbriae in microsurgical operations for fertility: a multicenter study. Fertil Steril. 1995;63:709-714.
15. Sawada T, Nishizawa H, Nishio E, Kadowaki M. Postoperative adhesion prevention with an oxidized regenerated cellulose adhesion barrier in infertile women. J Reprod Med. 2000;45:387-389.
16. The Myomectomy Adhesions Multicenter Study Group. An expanded polytetrafluoroethylene barrier (Gore-Tex Surgical Membrane) reduces post-myomectomy adhesion formation. Fertil Steril. 1995;63:491-493.
17. Haney AF, Hesla J, Hurst BS, et al. Expanded polytetrafluoroethylene (Gore-Tex Surgical Membrane) is superior to oxidized regenerated cellulose (Interceed TC7) in preventing adhesions. Fertil Steril. 1995;63:1021-1026.
18. Monteforte CA, Queirazza R, Francescetting P, Herbst TJ. Fistula formation after implanting an ePTFE membrane. A case report. J Reprod Med. 1997;42:184-187.
19. Pelosi MA, II, Pelosi MA, III. A new nonabsorbable adhesion barrier for myomectomy. Am J Surg. 2002;184:428-432.
20. Johns D. Reduction of postsurgical adhesions with Intergel adhesion prevention solution: a multicenter study of safety and efficacy after conservative gynecologic surgery. Fertil Steril. 2001;76:596-604.
21. Diamond MP and The Sepracoat Adhesion Study Group. Reduction of de novo postsurgical adhesions by intraoperative precoating with Sepracoat (HAL-C) solution: a prospective, randomized, blinded, placebo-controlled multicenter study. Fertil Steril. 1998;69:1067-1074.
- To reduce the incidence of postoperative adhesions, follow basic principles of microsurgery: Minimize the number and extent of incisions, handle all tissue gently, strive for absolute hemostasis, and use small, nonreactive suture.
- Despite limited data from prospective, randomized studies, both fluid and barrier adjuvants have proved effective in reducing the incidence and extent of adhesions after abdominal myomectomy.
Abdominal myomectomy is the preferred treatment in women with large or numerous intramural myomas, especially in the setting of infertility, recurrent pregnancy loss, and preservation of future fertility.1,2 However, postoperative adhesions are distressingly common following this procedure, resulting in significant potential morbidity. Fortunately, a number of products can reduce their occurrence. Proper surgical techniques and a thorough knowledge of these products are invaluable in helping reduce the incidence of adhesions.
The association between adhesions and diminished fertility is well-established,3,4 particularly when peritubal involvement is present (FIGURES 1-3). Abdominopelvic adhesions also contribute to significant chronic pelvic pain, bowel obstruction, and technical difficulty in subsequent surgical or assisted-reproduction procedures.5 Unfortunately, most attempts at adhesiolysis meet with less than complete success, since adhesions recur in 55% to 100% of patients (FIGURE 4).6 Thus, preventing adhesions in the first place would seem to be key to successful outcomes in abdominal myomectomy.
This article reviews the evidence on various approaches and products. While the number of studies examining each adjuvant in the setting of abdominal myomectomy is limited, the overall evidence supports the safety and efficacy of both liquid and barrier adjuvants.
Adhesions present a significant clinical dilemma after abdominal myomectomy, occurring in 50% to 90% of patients.5,7 In 1 prospective series of women undergoing second-look laparoscopy (SLL) after myomectomy, adnexal adhesions were noted in 94% of patients with posterior uterine incisions and in 56% of patients with only anterior or fundal incisions; adhesions between the uterus and omentum or bowel occurred in 88% of all patients.5
In another study of early SLL following abdominal myomectomy, 83% of patients had adhesions between the surgical site and the bowel or omentum, and 65% had adhesions involving the adnexae.2 Removal of large, bulky fibroids (with uterine mass exceeding 13 weeks’ gestational size) resulted in higher adhesion scores than did small myomas. Again, the incidence and severity of adhesions also correlated with location of the uterine incision: Adnexal adhesions were more common after posterior uterine incisions (76%) than after anterior or fundal entries (45%).2
FIGURE 1 Peritubal adhesion
Adhesion at distal end of the fallopian tube. Peritubal involvement often leads to diminished fertility.
FIGURE 2 Adherent structures
Postoperative adhesion between fallopian tube and the uterus.
FIGURE 3 Cyst dissection
Adhesions can follow common surgeries such as paratubal cyst dissection.
FIGURE 4 Recurrent adhesions
Adhesions may recur following adhesiolysis.
Causes of pelvic adhesions
Adhesion prevention requires an understanding of risk factors and maneuvers that increase the likelihood of injury (see “Pathophysiology of adhesion formation”). A number of causes have been proposed, most of them centering on tissue and peritoneal trauma (TABLE 1).
Injury can arise from excessive or rough manipulation of tissue and peritoneal surfaces or from common effects such as cutting, abrasion, and denudation (FIGURE 5). Tissue desiccation or manual blotting may lead to peritoneal desquamation and fibrin deposition.
Exposure of surfaces to intraperitoneal blood in the setting of tissue hypoxia—virtually unavoidable during abdominal myomectomy—disrupts normal fibrinolytic activity, resulting in stimulation of angiogenesis.8
Introduction of reactive foreign bodies such as talc powder, residual suture material, and even lint from laparotomy pads can favor adhesion formation. These serve as substrates or niduses of fibrin deposition.
FIGURE 5 Conducive conditions
Raw surface area can become a potent substrate for adhesions.TABLE 1
Proposed causes of adhesion formation
| Tissue hypoxia or ischemia |
| Tissue desiccation |
| Intra-abdominal infection |
| Introduction of reactive foreign body |
| Presence of intraperitoneal blood |
| Dissection of adhesions |
Techniques that may help prevent adhesions
Based on findings from studies of second-look procedures, most physicians advocate avoiding posterior uterine incisions, as well as minimizing the number and extent of incisions, to help reduce the likelihood of adhesion formation. Further, many Ob/Gyns favor removing myomas through as few uterine incisions as possible.9 We select anterior hysterotomy sites that enable removal of multiple fibroids, avoiding posterior incisions and the uterotubal junction whenever possible.
Interestingly, reapproximation of peritoneal defects after reproductive surgery (and, probably, myomectomy) does not appear to help prevent adhesions. Tulandi and colleagues10 examined the clinical and SLL outcomes of peritoneal closure in patients undergoing Pfannenstiel incisions with or without peritoneal closure at the end of the procedure. There was no difference in postoperative complications or wound healing in the 2 groups. At the time of SLL, there was no significant difference in the incidence of adhesion formation at the anterior abdominal wall.
Principles of microsurgery have been adopted by reproductive surgeons to minimize the likelihood of adhesions after myomectomy and gynecologic surgery in general. The basic techniques reflect respect for tissue integrity:
- Gentle handling of tissue, with minimal manipulation of all peritoneal surfaces.
- Meticulous hemostasis. Examine all myomectomy sites to ensure adequate hemostasis prior to closure.
- Continuous irrigation to prevent tissue desiccation. We favor continuous saline irrigation throughout the procedure. We also use only moistened laparotomy sponges and pads, and avoid applying dry gauze to any tissue surface.
- Avoidance of foreign-body introduction. We use only talc-free gloves and remove all residual suture fragments and tissue debris before closure. We also perform copious saline suction-irrigation at the end of the procedure to remove as much residue as possible.
- Use of fine, nonreactive suture. We favor fine, resorbable sutures that incite as little tissue reactivity as possible. For closure of large myomectomy defects, we use braided multifilaments such as Vicryl (polyglactine 910) (Ethicon, Somerville, NJ) or Dexon (polyglycolic acid) (Davis and Geck, Danbury, Conn) for strength and ease of handling. These sutures are absorbed through simple hydrolysis and stimulate less tissue reactivity than do chromic or catgut sutures.
The idea is appealing, but a randomized, blinded comparison of “good” and “bad” microsurgical technique is unlikely, since no one would wish to perform “bad” technique.
With barrier adjuvants, optimal benefit is obtained when the physician can predict potential sites of adhesions.
Wide range of prevention tools has been studied
Many types of agents have been studied in an attempt to reduce postsurgical adhesions after gynecologic surgery. Although most offer little or no benefit (TABLE 2), a few have potential in myomectomy procedures.
Barriers that form mechanical separation (TABLE 3) theoretically physically separate damaged tissues during early peritoneal wound healing, when adhesions form.11 The original adhesion barriers consisted of omental and peritoneal grafts that were placed over surgical sites. However, studies demonstrated that devitalized tissue positioned on damaged peritoneum serves as a potent substrate—not inhibitor—for adhesions.12 More recent trials have examined the adhesion-prevention benefit of other types of absorbable and nonabsorbable barriers.
TABLE 2
Pharmacologic agents studied for adhesion prevention in reproductive surgery
| AGENT | THEORETICAL ACTION | EXAMPLES | MODE OF USE/APPLICATION | RISKS/PROBLEMS |
|---|---|---|---|---|
| Antibiotics | Prevent infection or inflammation | Cephalosporins Tetracyclines | Intraperitoneal irrigation with antibiotic fluid Hydrotubation fluid with antibiotic | Theoretical reaction to antibiotic |
| Anticoagulants | Clot prevention Fibrin prevention | Heparin | In conjunction with Interceed | Risk of postoperative bleeding |
| Anti-inflammatory agents | Decrease permeability and histamine release | Nonsteroidal anti-inflammatory drugs Corticosteroids | Awaiting further investigation | Theoretical reaction to agent |
| Crystalloid solutions | Hydroflotation effect, decrease surface contact between pelvic organs | Normal saline Ringer’s lactate | Intra-abdominal instillation | Possible volume overload from intravascular absorption |
| Fibrinolytic agents | Fibrinolysis Plasminogen activation | Streptokinase Trypsin Fibrinolysin | Awaiting further investigation | Theoretical risk of postoperative bleeding |
| Steroids | Decrease inflammatory response | Dexamethasone | Systemic and/or intraperitoneal | Possible suppression of hypothalamic-pituitary axis |
| Polysaccharide polymer | "Siliconizing" effect to coat raw surfaces | Dextran 70 (Hyskon) | 200 mL placed in posterior cul-de-sac or coating surgical site surfaces | Abdominal bloating, anaphylaxis, pleural effusion, liver function abnormalities, wound separation, rare diffuse intravascular coagulation |
| Other fluid and barrier agents* | Peritoneal surface separation Hydroflotation | Absorbable and nonabsorbable barriers (see Table 3) | See Table 3 | See Table 3 |
| *Adjuvants studied in the setting of abdominal myomectomy | ||||
TABLE 3
Fluid and barrier adjuvants studied for adhesion reduction after myomectomy
| AGENT | THEORETICAL ACTION | MODE OF USE/APPLICATION | PROBLEMS | EVIDENCE FOR ADHESION REDUCTION |
|---|---|---|---|---|
| ABSORBABLE (BARRIER) | ||||
| Oxidized regenerated cellulose (Interceed) | Protective layer over surgical sites to prevent surface contact | Direct placement onto surface of uterus; no suturing required | Requires hemostasis | Prospective studies and meta-analysis support benefit in reproductive surgery including abdominal myomectomy |
| Hyaluronatecarboxymethycellulose derivative film (Seprafilm) | Protective layer over surgical sites to prevent surface contact | Direct placement around entire uterine surface; no suturing required | Requires hemostasis | Multicenter prospective, randomized study supports benefit in reducing adhesions after abdominal myomectomy |
| NONABSORBABLE (BARRIER) | ||||
| Expanded polytetrafluoroethylene (GoreTex) | Prevent contact between surgical surfaces | Patch sutured onto surface of uterus | Usually must be removed Report of fistula formation when left in situ | Multicenter prospective studies support adhesion-preventive benefit after abdominal myomectomy |
| Pericardial patch (Shelhigh No-React) | Prevent contact between surgical surfaces | Patch sutured onto surface of uterus | Early clinical use in myomectomy Proven safety as pericardial patch in humans | Preliminary study (case series data only) shows potential benefit |
| FLUID | ||||
| Hyaluronic acid-coat (Sepracoat) | Diffuse coating on surgical sites and potential sites of contact | 100-mL to 250-mL aliquots injected into peritoneal cavity | Limited data on efficacy in abdominal myomectomy | Small studies (multicenter, prospective, randomized, controlled trials) demonstrated reduced postoperative adhesions after reproductive surgery via laparotomy, including myomectomy |
| Hyaluronate-carboxymethycellulose derivative gel (Intergel) | Diffuse coating on surgical sites and potential sites of contact | 300-mL aliquot into peritoneal cavity | Withdrawn from market for reports of postoperative pain, complications | Reduced adhesion formation in animal studies and in preliminary human studies |
Adhesions are fibrous or fibrovascular bands that connect tissue surfaces in abnormal locations.1,2 Their development likely results from an imbalance in inflammatory mediators or fibrin degradation during peritoneal wound healing.
Peritoneal injury initiates the release of histamine and vasoactive kinins that mediate increased capillary permeability and outpouring of serosanguineous fluid.3 This proteinaceous exudate coagulates, depositing fibrinous bands between areas of denuded tissue.
Under normal circumstances, the fibrinolytic system is activated to lyse these bands within 72 hours. Peritoneal healing occurs when mesothelial cells migrate from the underlying mesenchyme to reepithelialize the injured site.4 Disequilibrium of the fibrin deposition-fibrinolysis system results in a persistent band that will eventually undergo fibroblast and vascular invasion.
REFERENCES
1. Diamond MP, DeCherney AH. Pathogenesis of adhesion formation/reformation: application to reproductive pelvic surgery. Microsurgery. 1987;8:103-107.
2. Diamond MP, Freeman ML. Clinical implications of postsurgical adhesions. Hum Reprod. 2001;7:567-576.
3. Diamond MP, El-Mowafi DM. Pelvic adhesions. Surg Technol Int. 1998;VII:273-283.
4. Farquhar C, Vandekerckhove P, Watson A, Vail A, Wiseman D. Barrier agents for preventing adhesions after surgery for subfertility. Cochrane Database Systematic Rev. 2002;(4):1-34.
Absorbable barriers
These are largely derivatives of organic materials. Their application to myomectomy may be limited by the requirement for absolute hemostasis at the site of application.
Interceed (Gynecare, a division of Ethicon), which is derived from oxidized regenerated cellulose, is one of the first and most extensively evaluated barriers. A mesh synthetic designed to be placed over injured tissue, it is a derivative of the hemostatic agent Surgicell (Johnson & Johnson, New Brunswick, NJ), with modifications in weave and pore size.
Interceed offers ease of application: It can be cut to the size or shape necessary and requires no suturing. It forms a gelatinous protective layer within 8 hours of placement, and is degraded into monosaccharides and absorbed within 2 weeks.13
The use of Interceed has been shown to reduce adhesions following adhesiolysis and ovarian surgery. In a large, multicenter, prospective, randomized trial, it significantly decreased the incidence of adhesion reformation after adnexal adhesiolysis in infertility patients with bilateral tubal disease.14
In a retrospective series of 38 infertility patients, including 19 patients after myomectomy (13 with the barrier, 6 without), reproductive outcomes were significantly better in the Interceed group, and adhesion development was reduced.15 Pregnancy rates in the 2 years following surgery were 78% in the Interceed group, compared with 47% in controls. In addition, among 23 patients who had second-look procedures, postoperative adhesions were noted in 38% of the Interceed group, compared with 86% in controls.15
Seprafilm (hyaluronic acid-film) (Genzyme Corp, Cambridge, Mass) is a bioresorbable membrane derived from sodium hyaluronate and carboxymethylcellulose. It is absorbed from the peritoneal cavity within 1 week and is completely excreted within 1 month.
Its potential for adhesion prevention after abdominal myomectomy was examined by SLL in a multicenter, prospective, randomized, blinded study11 in which 127 women undergoing abdominal myomectomy at 19 institutions were randomized to either Seprafilm or no barrier. In the Seprafilm group, the barrier was wrapped circumferentially around the uterus, covering all uterine defects, at the time of abdominal closure. Clinical outcomes—including vital signs, adverse events (pain, fever, nausea), and abdominal wound complications—were similar in the control and Seprafilm groups.
In this study, the incidence (mean number of sites), severity, and extent (mean area) of uterine adhesions were significantly lower in the Seprafilm-treated patients. The proportion of patients undergoing anterior hysterotomies who were found to have no anterior uterine adhesions was 39% in the Seprafilm group, compared with 6% in the control group. The percentage of patients with at least 1 adnexa totally free of adhesions to the posterior uterus also was higher in the Seprafilm group—48% versus 31%.11
Nonabsorbable barriers
GoreTex Surgical Membrane (W.L. Gore and Associates, Newark, Del) is an inert expanded polytetrafluoroethylene (PTFE) derivative that must be sutured in place. It is of potential utility in myomectomy because its application does not require absolute hemostasis. However, its usefulness and application are limited by the need for later removal.
In a multicenter, randomized, controlled trial exploring its adhesion-preventive properties after myomectomy, the GoreTex membrane significantly outperformed the barrierfree group.16
In a separate multicenter, randomized clinical trial, the GoreTex membrane was more effective than Interceed in preventing adhesions after pelvic/tubal reconstructive surgery.17
Yet another multicenter, randomized trial—this one involving 27 women undergoing abdominal myomectomy—used SLL to determine the extent of adhesions and to remove the barrier. The percentage of adhesion-free surgical sites was significantly higher in the GoreTex group: 56% compared with 7% in no-barrier controls.16
The adhesion scores, determined by the extent (area of involvement), nature (filmy versus opaque), and tenacity (ease of lysis or dissection) of the adhesions, were significantly lower in the GoreTex group.16
Despite the few prospective, randomized trials, the evidence supports the efficacy of various fluid and barrier adjuvants.
Histologic examination of the removed barriers demonstrated no tissue attachment to the PTFE. Despite these positive findings, however, both fistula formation and graft infection have been reported after placement of a GoreTex barrier.18
The Shelhigh Pericardial No-React Patch (Herzog Surgical, Sacramento, Calif) is a new nonabsorbable adhesion barrier that was first described in gynecologic use by Pelosi and Pelosi in a case series of 20 patients.19 Consisting of a 12-cm-diameter patch of glutaraldehyde-treated bovine/porcine pericardium, this product has an excellent safety record as a permanent pericardial substitute in the cardiovascular literature and has been shown to resist calcification and adhesions.
In the Pelosi series, the patch was placed over the uterine fundus and secured by 4 monofilament sutures at the dome of the uterus and the parietal peritoneum.19 All patients underwent SLL at 6 weeks, and 3 patients also underwent third-look laparoscopy. None of the 20 women had adhesions between the abdominal wall and the bladder, bowel, uterus, or adnexae at SLL. However, minimal adhesions of the ovaries and tubes were found in 7 patients who had undergone posterior hysterotomy. Clinical trials are likely to follow this small pilot study.
Absorbable fluid adjuvants
With barrier adjuvants, optimal benefit is obtained when the physician can predict potential sites of adhesions. This is not a strict requirement with fluid barriers, which is their chief advantage. Among the agents described below, Sepracoat (Genzyme Corp) is available in the United States, while Intergel (Lifecore Biomedical, Chaska, Minn) was withdrawn from the market in March.
Intergel, a 0.5% ferric hyaluronade formulation, is a sterile nonpyrogenic gel of highly purified sodium hyaluronate, which is ionically cross-linked with ferric ion and adjusted to isotonicity with sodium chloride.11 (Hyaluronic acid is a major component of body tissues and fluids such as peritoneal fluid, where it performs physically supportive and mechanically protective roles.)
Johns and colleagues20 studied Intergel in a randomized, multicenter, third-party– blinded, placebo-controlled study. Of the 265 patients who completed the study, 131 were given 300 mL of Intergel and 134 were given lactated Ringer’s solution (the placebo) at the time of their surgery, through the laparoscopic port. When SLL was performed 6 to 12 weeks after surgery, the mean number and severity of adhesions—overall and at the surgical site—were significantly lower in the Intergel group. Adhesions reformed in 91% of those in the control group, compared with 63% in the Intergel group.
In myomectomy patients, the modified American Fertility Society Score, which uses 24 potential adhesion sites, was reduced by 42% in the Intergel group—a statistically significant improvement.20
One major advantage of Intergel is that it reduces adhesion formation at sites distant from the area of application, secondary to its wide intra-abdominal circulation. However, as mentioned above, sales were voluntarily suspended due to post-market reports of tissue adherence, sterile foreign-body reaction, and late-onset pain that sometimes required surgical intervention. In some patients, persistent residual material was noted at the time of subsequent surgery.
Sepracoat (hyaluronic acid-coat) is a dilute solution of 0.4% hyaluronic acid in phosphate-buffered saline. It is bioresorbable, persists at the application site less than 24 hours, and is completely cleared in less than 5 days.
In a prospective, randomized, blinded, placebo-controlled, multicenter study in 1998, Diamond and colleagues compared Sepracoat with a pure phosphate-buffered saline solution in 227 women undergoing gynecologic procedures via laparotomy.21 The aim of the study was to assess the efficacy and safety of the fluid at sites without direct surgical trauma or adhesiolysis. Both solutions were warmed to room temperature and injected into the abdominal cavity before the procedure began (250 mL after skin incision). The solution was reapplied after irrigation or every 30 minutes (100 mL), and at the end of the procedure (250 mL) before closure. The maximum volume used was 1,000 mL. After application, the fluid was left 1 minute before suctioning. Patients underwent SLL 40 days later, and adhesions were identified at the initial procedure and at SLL.
In the Sepracoat group, there was a reduction in de novo adhesions at nonsurgical sites by a factor of 2.8. The proportion of sites with de novo adhesions also decreased, and 80% of Sepracoat patients had at least 1 ovary that was adhesion-free compared with 58% of placebo-treated patients. These findings occurred in areas of indirect trauma, demonstrating that Sepracoat limited trauma at tissue injury.21
Conclusion
Adhesion prevention is of utmost importance after abdominal myomectomy, especially in patients who desire future fertility. Despite the limited number of prospective, randomized studies, the literature does support the efficacy of various fluid and barrier adjuvants.
Our practice is to use an adjuvant in all abdominal myomectomies. We have long relied on Interceed, which enjoys both ease of application and an excellent safety record. However, as reviewed above, other potentially useful products are available or in development. As ever, the reproductive surgeon should adhere to principles of microsurgery, strive for meticulous hemostasis, and demonstrate respect for tissue integrity.
Dr. DeCherney reports small holdings with Lifecore Biomedical. Drs. Chang and Marin report no affiliations or financial arrangements with any of the manufacturers of products mentioned in this article.
- To reduce the incidence of postoperative adhesions, follow basic principles of microsurgery: Minimize the number and extent of incisions, handle all tissue gently, strive for absolute hemostasis, and use small, nonreactive suture.
- Despite limited data from prospective, randomized studies, both fluid and barrier adjuvants have proved effective in reducing the incidence and extent of adhesions after abdominal myomectomy.
Abdominal myomectomy is the preferred treatment in women with large or numerous intramural myomas, especially in the setting of infertility, recurrent pregnancy loss, and preservation of future fertility.1,2 However, postoperative adhesions are distressingly common following this procedure, resulting in significant potential morbidity. Fortunately, a number of products can reduce their occurrence. Proper surgical techniques and a thorough knowledge of these products are invaluable in helping reduce the incidence of adhesions.
The association between adhesions and diminished fertility is well-established,3,4 particularly when peritubal involvement is present (FIGURES 1-3). Abdominopelvic adhesions also contribute to significant chronic pelvic pain, bowel obstruction, and technical difficulty in subsequent surgical or assisted-reproduction procedures.5 Unfortunately, most attempts at adhesiolysis meet with less than complete success, since adhesions recur in 55% to 100% of patients (FIGURE 4).6 Thus, preventing adhesions in the first place would seem to be key to successful outcomes in abdominal myomectomy.
This article reviews the evidence on various approaches and products. While the number of studies examining each adjuvant in the setting of abdominal myomectomy is limited, the overall evidence supports the safety and efficacy of both liquid and barrier adjuvants.
Adhesions present a significant clinical dilemma after abdominal myomectomy, occurring in 50% to 90% of patients.5,7 In 1 prospective series of women undergoing second-look laparoscopy (SLL) after myomectomy, adnexal adhesions were noted in 94% of patients with posterior uterine incisions and in 56% of patients with only anterior or fundal incisions; adhesions between the uterus and omentum or bowel occurred in 88% of all patients.5
In another study of early SLL following abdominal myomectomy, 83% of patients had adhesions between the surgical site and the bowel or omentum, and 65% had adhesions involving the adnexae.2 Removal of large, bulky fibroids (with uterine mass exceeding 13 weeks’ gestational size) resulted in higher adhesion scores than did small myomas. Again, the incidence and severity of adhesions also correlated with location of the uterine incision: Adnexal adhesions were more common after posterior uterine incisions (76%) than after anterior or fundal entries (45%).2
FIGURE 1 Peritubal adhesion
Adhesion at distal end of the fallopian tube. Peritubal involvement often leads to diminished fertility.
FIGURE 2 Adherent structures
Postoperative adhesion between fallopian tube and the uterus.
FIGURE 3 Cyst dissection
Adhesions can follow common surgeries such as paratubal cyst dissection.
FIGURE 4 Recurrent adhesions
Adhesions may recur following adhesiolysis.
Causes of pelvic adhesions
Adhesion prevention requires an understanding of risk factors and maneuvers that increase the likelihood of injury (see “Pathophysiology of adhesion formation”). A number of causes have been proposed, most of them centering on tissue and peritoneal trauma (TABLE 1).
Injury can arise from excessive or rough manipulation of tissue and peritoneal surfaces or from common effects such as cutting, abrasion, and denudation (FIGURE 5). Tissue desiccation or manual blotting may lead to peritoneal desquamation and fibrin deposition.
Exposure of surfaces to intraperitoneal blood in the setting of tissue hypoxia—virtually unavoidable during abdominal myomectomy—disrupts normal fibrinolytic activity, resulting in stimulation of angiogenesis.8
Introduction of reactive foreign bodies such as talc powder, residual suture material, and even lint from laparotomy pads can favor adhesion formation. These serve as substrates or niduses of fibrin deposition.
FIGURE 5 Conducive conditions
Raw surface area can become a potent substrate for adhesions.TABLE 1
Proposed causes of adhesion formation
| Tissue hypoxia or ischemia |
| Tissue desiccation |
| Intra-abdominal infection |
| Introduction of reactive foreign body |
| Presence of intraperitoneal blood |
| Dissection of adhesions |
Techniques that may help prevent adhesions
Based on findings from studies of second-look procedures, most physicians advocate avoiding posterior uterine incisions, as well as minimizing the number and extent of incisions, to help reduce the likelihood of adhesion formation. Further, many Ob/Gyns favor removing myomas through as few uterine incisions as possible.9 We select anterior hysterotomy sites that enable removal of multiple fibroids, avoiding posterior incisions and the uterotubal junction whenever possible.
Interestingly, reapproximation of peritoneal defects after reproductive surgery (and, probably, myomectomy) does not appear to help prevent adhesions. Tulandi and colleagues10 examined the clinical and SLL outcomes of peritoneal closure in patients undergoing Pfannenstiel incisions with or without peritoneal closure at the end of the procedure. There was no difference in postoperative complications or wound healing in the 2 groups. At the time of SLL, there was no significant difference in the incidence of adhesion formation at the anterior abdominal wall.
Principles of microsurgery have been adopted by reproductive surgeons to minimize the likelihood of adhesions after myomectomy and gynecologic surgery in general. The basic techniques reflect respect for tissue integrity:
- Gentle handling of tissue, with minimal manipulation of all peritoneal surfaces.
- Meticulous hemostasis. Examine all myomectomy sites to ensure adequate hemostasis prior to closure.
- Continuous irrigation to prevent tissue desiccation. We favor continuous saline irrigation throughout the procedure. We also use only moistened laparotomy sponges and pads, and avoid applying dry gauze to any tissue surface.
- Avoidance of foreign-body introduction. We use only talc-free gloves and remove all residual suture fragments and tissue debris before closure. We also perform copious saline suction-irrigation at the end of the procedure to remove as much residue as possible.
- Use of fine, nonreactive suture. We favor fine, resorbable sutures that incite as little tissue reactivity as possible. For closure of large myomectomy defects, we use braided multifilaments such as Vicryl (polyglactine 910) (Ethicon, Somerville, NJ) or Dexon (polyglycolic acid) (Davis and Geck, Danbury, Conn) for strength and ease of handling. These sutures are absorbed through simple hydrolysis and stimulate less tissue reactivity than do chromic or catgut sutures.
The idea is appealing, but a randomized, blinded comparison of “good” and “bad” microsurgical technique is unlikely, since no one would wish to perform “bad” technique.
With barrier adjuvants, optimal benefit is obtained when the physician can predict potential sites of adhesions.
Wide range of prevention tools has been studied
Many types of agents have been studied in an attempt to reduce postsurgical adhesions after gynecologic surgery. Although most offer little or no benefit (TABLE 2), a few have potential in myomectomy procedures.
Barriers that form mechanical separation (TABLE 3) theoretically physically separate damaged tissues during early peritoneal wound healing, when adhesions form.11 The original adhesion barriers consisted of omental and peritoneal grafts that were placed over surgical sites. However, studies demonstrated that devitalized tissue positioned on damaged peritoneum serves as a potent substrate—not inhibitor—for adhesions.12 More recent trials have examined the adhesion-prevention benefit of other types of absorbable and nonabsorbable barriers.
TABLE 2
Pharmacologic agents studied for adhesion prevention in reproductive surgery
| AGENT | THEORETICAL ACTION | EXAMPLES | MODE OF USE/APPLICATION | RISKS/PROBLEMS |
|---|---|---|---|---|
| Antibiotics | Prevent infection or inflammation | Cephalosporins Tetracyclines | Intraperitoneal irrigation with antibiotic fluid Hydrotubation fluid with antibiotic | Theoretical reaction to antibiotic |
| Anticoagulants | Clot prevention Fibrin prevention | Heparin | In conjunction with Interceed | Risk of postoperative bleeding |
| Anti-inflammatory agents | Decrease permeability and histamine release | Nonsteroidal anti-inflammatory drugs Corticosteroids | Awaiting further investigation | Theoretical reaction to agent |
| Crystalloid solutions | Hydroflotation effect, decrease surface contact between pelvic organs | Normal saline Ringer’s lactate | Intra-abdominal instillation | Possible volume overload from intravascular absorption |
| Fibrinolytic agents | Fibrinolysis Plasminogen activation | Streptokinase Trypsin Fibrinolysin | Awaiting further investigation | Theoretical risk of postoperative bleeding |
| Steroids | Decrease inflammatory response | Dexamethasone | Systemic and/or intraperitoneal | Possible suppression of hypothalamic-pituitary axis |
| Polysaccharide polymer | "Siliconizing" effect to coat raw surfaces | Dextran 70 (Hyskon) | 200 mL placed in posterior cul-de-sac or coating surgical site surfaces | Abdominal bloating, anaphylaxis, pleural effusion, liver function abnormalities, wound separation, rare diffuse intravascular coagulation |
| Other fluid and barrier agents* | Peritoneal surface separation Hydroflotation | Absorbable and nonabsorbable barriers (see Table 3) | See Table 3 | See Table 3 |
| *Adjuvants studied in the setting of abdominal myomectomy | ||||
TABLE 3
Fluid and barrier adjuvants studied for adhesion reduction after myomectomy
| AGENT | THEORETICAL ACTION | MODE OF USE/APPLICATION | PROBLEMS | EVIDENCE FOR ADHESION REDUCTION |
|---|---|---|---|---|
| ABSORBABLE (BARRIER) | ||||
| Oxidized regenerated cellulose (Interceed) | Protective layer over surgical sites to prevent surface contact | Direct placement onto surface of uterus; no suturing required | Requires hemostasis | Prospective studies and meta-analysis support benefit in reproductive surgery including abdominal myomectomy |
| Hyaluronatecarboxymethycellulose derivative film (Seprafilm) | Protective layer over surgical sites to prevent surface contact | Direct placement around entire uterine surface; no suturing required | Requires hemostasis | Multicenter prospective, randomized study supports benefit in reducing adhesions after abdominal myomectomy |
| NONABSORBABLE (BARRIER) | ||||
| Expanded polytetrafluoroethylene (GoreTex) | Prevent contact between surgical surfaces | Patch sutured onto surface of uterus | Usually must be removed Report of fistula formation when left in situ | Multicenter prospective studies support adhesion-preventive benefit after abdominal myomectomy |
| Pericardial patch (Shelhigh No-React) | Prevent contact between surgical surfaces | Patch sutured onto surface of uterus | Early clinical use in myomectomy Proven safety as pericardial patch in humans | Preliminary study (case series data only) shows potential benefit |
| FLUID | ||||
| Hyaluronic acid-coat (Sepracoat) | Diffuse coating on surgical sites and potential sites of contact | 100-mL to 250-mL aliquots injected into peritoneal cavity | Limited data on efficacy in abdominal myomectomy | Small studies (multicenter, prospective, randomized, controlled trials) demonstrated reduced postoperative adhesions after reproductive surgery via laparotomy, including myomectomy |
| Hyaluronate-carboxymethycellulose derivative gel (Intergel) | Diffuse coating on surgical sites and potential sites of contact | 300-mL aliquot into peritoneal cavity | Withdrawn from market for reports of postoperative pain, complications | Reduced adhesion formation in animal studies and in preliminary human studies |
Adhesions are fibrous or fibrovascular bands that connect tissue surfaces in abnormal locations.1,2 Their development likely results from an imbalance in inflammatory mediators or fibrin degradation during peritoneal wound healing.
Peritoneal injury initiates the release of histamine and vasoactive kinins that mediate increased capillary permeability and outpouring of serosanguineous fluid.3 This proteinaceous exudate coagulates, depositing fibrinous bands between areas of denuded tissue.
Under normal circumstances, the fibrinolytic system is activated to lyse these bands within 72 hours. Peritoneal healing occurs when mesothelial cells migrate from the underlying mesenchyme to reepithelialize the injured site.4 Disequilibrium of the fibrin deposition-fibrinolysis system results in a persistent band that will eventually undergo fibroblast and vascular invasion.
REFERENCES
1. Diamond MP, DeCherney AH. Pathogenesis of adhesion formation/reformation: application to reproductive pelvic surgery. Microsurgery. 1987;8:103-107.
2. Diamond MP, Freeman ML. Clinical implications of postsurgical adhesions. Hum Reprod. 2001;7:567-576.
3. Diamond MP, El-Mowafi DM. Pelvic adhesions. Surg Technol Int. 1998;VII:273-283.
4. Farquhar C, Vandekerckhove P, Watson A, Vail A, Wiseman D. Barrier agents for preventing adhesions after surgery for subfertility. Cochrane Database Systematic Rev. 2002;(4):1-34.
Absorbable barriers
These are largely derivatives of organic materials. Their application to myomectomy may be limited by the requirement for absolute hemostasis at the site of application.
Interceed (Gynecare, a division of Ethicon), which is derived from oxidized regenerated cellulose, is one of the first and most extensively evaluated barriers. A mesh synthetic designed to be placed over injured tissue, it is a derivative of the hemostatic agent Surgicell (Johnson & Johnson, New Brunswick, NJ), with modifications in weave and pore size.
Interceed offers ease of application: It can be cut to the size or shape necessary and requires no suturing. It forms a gelatinous protective layer within 8 hours of placement, and is degraded into monosaccharides and absorbed within 2 weeks.13
The use of Interceed has been shown to reduce adhesions following adhesiolysis and ovarian surgery. In a large, multicenter, prospective, randomized trial, it significantly decreased the incidence of adhesion reformation after adnexal adhesiolysis in infertility patients with bilateral tubal disease.14
In a retrospective series of 38 infertility patients, including 19 patients after myomectomy (13 with the barrier, 6 without), reproductive outcomes were significantly better in the Interceed group, and adhesion development was reduced.15 Pregnancy rates in the 2 years following surgery were 78% in the Interceed group, compared with 47% in controls. In addition, among 23 patients who had second-look procedures, postoperative adhesions were noted in 38% of the Interceed group, compared with 86% in controls.15
Seprafilm (hyaluronic acid-film) (Genzyme Corp, Cambridge, Mass) is a bioresorbable membrane derived from sodium hyaluronate and carboxymethylcellulose. It is absorbed from the peritoneal cavity within 1 week and is completely excreted within 1 month.
Its potential for adhesion prevention after abdominal myomectomy was examined by SLL in a multicenter, prospective, randomized, blinded study11 in which 127 women undergoing abdominal myomectomy at 19 institutions were randomized to either Seprafilm or no barrier. In the Seprafilm group, the barrier was wrapped circumferentially around the uterus, covering all uterine defects, at the time of abdominal closure. Clinical outcomes—including vital signs, adverse events (pain, fever, nausea), and abdominal wound complications—were similar in the control and Seprafilm groups.
In this study, the incidence (mean number of sites), severity, and extent (mean area) of uterine adhesions were significantly lower in the Seprafilm-treated patients. The proportion of patients undergoing anterior hysterotomies who were found to have no anterior uterine adhesions was 39% in the Seprafilm group, compared with 6% in the control group. The percentage of patients with at least 1 adnexa totally free of adhesions to the posterior uterus also was higher in the Seprafilm group—48% versus 31%.11
Nonabsorbable barriers
GoreTex Surgical Membrane (W.L. Gore and Associates, Newark, Del) is an inert expanded polytetrafluoroethylene (PTFE) derivative that must be sutured in place. It is of potential utility in myomectomy because its application does not require absolute hemostasis. However, its usefulness and application are limited by the need for later removal.
In a multicenter, randomized, controlled trial exploring its adhesion-preventive properties after myomectomy, the GoreTex membrane significantly outperformed the barrierfree group.16
In a separate multicenter, randomized clinical trial, the GoreTex membrane was more effective than Interceed in preventing adhesions after pelvic/tubal reconstructive surgery.17
Yet another multicenter, randomized trial—this one involving 27 women undergoing abdominal myomectomy—used SLL to determine the extent of adhesions and to remove the barrier. The percentage of adhesion-free surgical sites was significantly higher in the GoreTex group: 56% compared with 7% in no-barrier controls.16
The adhesion scores, determined by the extent (area of involvement), nature (filmy versus opaque), and tenacity (ease of lysis or dissection) of the adhesions, were significantly lower in the GoreTex group.16
Despite the few prospective, randomized trials, the evidence supports the efficacy of various fluid and barrier adjuvants.
Histologic examination of the removed barriers demonstrated no tissue attachment to the PTFE. Despite these positive findings, however, both fistula formation and graft infection have been reported after placement of a GoreTex barrier.18
The Shelhigh Pericardial No-React Patch (Herzog Surgical, Sacramento, Calif) is a new nonabsorbable adhesion barrier that was first described in gynecologic use by Pelosi and Pelosi in a case series of 20 patients.19 Consisting of a 12-cm-diameter patch of glutaraldehyde-treated bovine/porcine pericardium, this product has an excellent safety record as a permanent pericardial substitute in the cardiovascular literature and has been shown to resist calcification and adhesions.
In the Pelosi series, the patch was placed over the uterine fundus and secured by 4 monofilament sutures at the dome of the uterus and the parietal peritoneum.19 All patients underwent SLL at 6 weeks, and 3 patients also underwent third-look laparoscopy. None of the 20 women had adhesions between the abdominal wall and the bladder, bowel, uterus, or adnexae at SLL. However, minimal adhesions of the ovaries and tubes were found in 7 patients who had undergone posterior hysterotomy. Clinical trials are likely to follow this small pilot study.
Absorbable fluid adjuvants
With barrier adjuvants, optimal benefit is obtained when the physician can predict potential sites of adhesions. This is not a strict requirement with fluid barriers, which is their chief advantage. Among the agents described below, Sepracoat (Genzyme Corp) is available in the United States, while Intergel (Lifecore Biomedical, Chaska, Minn) was withdrawn from the market in March.
Intergel, a 0.5% ferric hyaluronade formulation, is a sterile nonpyrogenic gel of highly purified sodium hyaluronate, which is ionically cross-linked with ferric ion and adjusted to isotonicity with sodium chloride.11 (Hyaluronic acid is a major component of body tissues and fluids such as peritoneal fluid, where it performs physically supportive and mechanically protective roles.)
Johns and colleagues20 studied Intergel in a randomized, multicenter, third-party– blinded, placebo-controlled study. Of the 265 patients who completed the study, 131 were given 300 mL of Intergel and 134 were given lactated Ringer’s solution (the placebo) at the time of their surgery, through the laparoscopic port. When SLL was performed 6 to 12 weeks after surgery, the mean number and severity of adhesions—overall and at the surgical site—were significantly lower in the Intergel group. Adhesions reformed in 91% of those in the control group, compared with 63% in the Intergel group.
In myomectomy patients, the modified American Fertility Society Score, which uses 24 potential adhesion sites, was reduced by 42% in the Intergel group—a statistically significant improvement.20
One major advantage of Intergel is that it reduces adhesion formation at sites distant from the area of application, secondary to its wide intra-abdominal circulation. However, as mentioned above, sales were voluntarily suspended due to post-market reports of tissue adherence, sterile foreign-body reaction, and late-onset pain that sometimes required surgical intervention. In some patients, persistent residual material was noted at the time of subsequent surgery.
Sepracoat (hyaluronic acid-coat) is a dilute solution of 0.4% hyaluronic acid in phosphate-buffered saline. It is bioresorbable, persists at the application site less than 24 hours, and is completely cleared in less than 5 days.
In a prospective, randomized, blinded, placebo-controlled, multicenter study in 1998, Diamond and colleagues compared Sepracoat with a pure phosphate-buffered saline solution in 227 women undergoing gynecologic procedures via laparotomy.21 The aim of the study was to assess the efficacy and safety of the fluid at sites without direct surgical trauma or adhesiolysis. Both solutions were warmed to room temperature and injected into the abdominal cavity before the procedure began (250 mL after skin incision). The solution was reapplied after irrigation or every 30 minutes (100 mL), and at the end of the procedure (250 mL) before closure. The maximum volume used was 1,000 mL. After application, the fluid was left 1 minute before suctioning. Patients underwent SLL 40 days later, and adhesions were identified at the initial procedure and at SLL.
In the Sepracoat group, there was a reduction in de novo adhesions at nonsurgical sites by a factor of 2.8. The proportion of sites with de novo adhesions also decreased, and 80% of Sepracoat patients had at least 1 ovary that was adhesion-free compared with 58% of placebo-treated patients. These findings occurred in areas of indirect trauma, demonstrating that Sepracoat limited trauma at tissue injury.21
Conclusion
Adhesion prevention is of utmost importance after abdominal myomectomy, especially in patients who desire future fertility. Despite the limited number of prospective, randomized studies, the literature does support the efficacy of various fluid and barrier adjuvants.
Our practice is to use an adjuvant in all abdominal myomectomies. We have long relied on Interceed, which enjoys both ease of application and an excellent safety record. However, as reviewed above, other potentially useful products are available or in development. As ever, the reproductive surgeon should adhere to principles of microsurgery, strive for meticulous hemostasis, and demonstrate respect for tissue integrity.
Dr. DeCherney reports small holdings with Lifecore Biomedical. Drs. Chang and Marin report no affiliations or financial arrangements with any of the manufacturers of products mentioned in this article.
1. Stewart EA. Treatment of uterine leiomyomas. UpToDate (Version 10.3) 2002; 1-8. Available at: www.uptodate.com.
2. Ugur M, Tura C, Mungan T, Aydogdu T, Sahin Y, Gokmen O. Laparoscopy for adhesion prevention following myomectomy. Int J Gynecol Obstet. 1996;53:145-149.
3. Berkeley AS, DeCherney AH, Plan ML. Abdominal myomectomy and subsequent fertility. Surg Gynecol Obstet. 1983;153:319-322.
4. Tulandi T, Collin JA, Burrows E, et al. Treatment-dependent and treatment-independent pregnancy among women with periadnexal adhesions. Am J Obstet Gynecol. 1990;162:354-357.
5. Tulandi T, Murria C, Guralnick M. Adhesion formation and reproductive outcome after myomectomy and second-look laparoscopy. Obstet Gynecol. 1993;82:213-215.
6. Diamond MP, Freeman ML. Clinical implications of postsurgical adhesions. Hum Reprod. 2001;7:567-576.
7. Lau S, Tulandi T. Myomectomy and adhesion formation. In: diZerega GS, ed. Peritoneal Surgery. New York: Springer-Verlag 1999;289-290.
8. Diamond MP, El-Mowafi DM. Pelvic adhesions. Surg Technol Int. 1998;VII:273-283.
9. Guarnaccia MM, Rein MS. Traditional surgical approaches to uterine fibroids: abdominal myomectomy and hysterectomy. Clin Obstet Gynecol. 2001;44:385-400.
10. Tulandi T, Hum HS, Gelfand MM. Closure of laparotomy incisions with or without peritoneal suturing and second-look laparoscopy. Am J Obstet Gynecol. 1988;158:536-537.
11. Diamond MP. Reduction of adhesions after uterine myomectomy by Seprafilm membrane (HAL-F): a blinded, prospective, randomized, multicenter clinical study. Fertil Steril. 1996;66:904-910.
12. Johns D. Reduction of postsurgical adhesions with Intergel adhesion prevention solution: a multicenter study of safety and efficacy after conservative gynecologic surgery. Fertil Steril. 2001;76:595-604.
13. Farquhar C, vandekerckhove P, Watson A, Vail A, Wiseman D. Barrier agents for preventing adhesions after surgery for subfertility. Cochrane Database Systematic Rev. 2002(4);1-34.
14. Nordic Adhesion Prevention Study Group. The efficacy of Interceed (TC7) for prevention of reformation of postoperative adhesions on ovaries, fallopian tubes, and fimbriae in microsurgical operations for fertility: a multicenter study. Fertil Steril. 1995;63:709-714.
15. Sawada T, Nishizawa H, Nishio E, Kadowaki M. Postoperative adhesion prevention with an oxidized regenerated cellulose adhesion barrier in infertile women. J Reprod Med. 2000;45:387-389.
16. The Myomectomy Adhesions Multicenter Study Group. An expanded polytetrafluoroethylene barrier (Gore-Tex Surgical Membrane) reduces post-myomectomy adhesion formation. Fertil Steril. 1995;63:491-493.
17. Haney AF, Hesla J, Hurst BS, et al. Expanded polytetrafluoroethylene (Gore-Tex Surgical Membrane) is superior to oxidized regenerated cellulose (Interceed TC7) in preventing adhesions. Fertil Steril. 1995;63:1021-1026.
18. Monteforte CA, Queirazza R, Francescetting P, Herbst TJ. Fistula formation after implanting an ePTFE membrane. A case report. J Reprod Med. 1997;42:184-187.
19. Pelosi MA, II, Pelosi MA, III. A new nonabsorbable adhesion barrier for myomectomy. Am J Surg. 2002;184:428-432.
20. Johns D. Reduction of postsurgical adhesions with Intergel adhesion prevention solution: a multicenter study of safety and efficacy after conservative gynecologic surgery. Fertil Steril. 2001;76:596-604.
21. Diamond MP and The Sepracoat Adhesion Study Group. Reduction of de novo postsurgical adhesions by intraoperative precoating with Sepracoat (HAL-C) solution: a prospective, randomized, blinded, placebo-controlled multicenter study. Fertil Steril. 1998;69:1067-1074.
1. Stewart EA. Treatment of uterine leiomyomas. UpToDate (Version 10.3) 2002; 1-8. Available at: www.uptodate.com.
2. Ugur M, Tura C, Mungan T, Aydogdu T, Sahin Y, Gokmen O. Laparoscopy for adhesion prevention following myomectomy. Int J Gynecol Obstet. 1996;53:145-149.
3. Berkeley AS, DeCherney AH, Plan ML. Abdominal myomectomy and subsequent fertility. Surg Gynecol Obstet. 1983;153:319-322.
4. Tulandi T, Collin JA, Burrows E, et al. Treatment-dependent and treatment-independent pregnancy among women with periadnexal adhesions. Am J Obstet Gynecol. 1990;162:354-357.
5. Tulandi T, Murria C, Guralnick M. Adhesion formation and reproductive outcome after myomectomy and second-look laparoscopy. Obstet Gynecol. 1993;82:213-215.
6. Diamond MP, Freeman ML. Clinical implications of postsurgical adhesions. Hum Reprod. 2001;7:567-576.
7. Lau S, Tulandi T. Myomectomy and adhesion formation. In: diZerega GS, ed. Peritoneal Surgery. New York: Springer-Verlag 1999;289-290.
8. Diamond MP, El-Mowafi DM. Pelvic adhesions. Surg Technol Int. 1998;VII:273-283.
9. Guarnaccia MM, Rein MS. Traditional surgical approaches to uterine fibroids: abdominal myomectomy and hysterectomy. Clin Obstet Gynecol. 2001;44:385-400.
10. Tulandi T, Hum HS, Gelfand MM. Closure of laparotomy incisions with or without peritoneal suturing and second-look laparoscopy. Am J Obstet Gynecol. 1988;158:536-537.
11. Diamond MP. Reduction of adhesions after uterine myomectomy by Seprafilm membrane (HAL-F): a blinded, prospective, randomized, multicenter clinical study. Fertil Steril. 1996;66:904-910.
12. Johns D. Reduction of postsurgical adhesions with Intergel adhesion prevention solution: a multicenter study of safety and efficacy after conservative gynecologic surgery. Fertil Steril. 2001;76:595-604.
13. Farquhar C, vandekerckhove P, Watson A, Vail A, Wiseman D. Barrier agents for preventing adhesions after surgery for subfertility. Cochrane Database Systematic Rev. 2002(4);1-34.
14. Nordic Adhesion Prevention Study Group. The efficacy of Interceed (TC7) for prevention of reformation of postoperative adhesions on ovaries, fallopian tubes, and fimbriae in microsurgical operations for fertility: a multicenter study. Fertil Steril. 1995;63:709-714.
15. Sawada T, Nishizawa H, Nishio E, Kadowaki M. Postoperative adhesion prevention with an oxidized regenerated cellulose adhesion barrier in infertile women. J Reprod Med. 2000;45:387-389.
16. The Myomectomy Adhesions Multicenter Study Group. An expanded polytetrafluoroethylene barrier (Gore-Tex Surgical Membrane) reduces post-myomectomy adhesion formation. Fertil Steril. 1995;63:491-493.
17. Haney AF, Hesla J, Hurst BS, et al. Expanded polytetrafluoroethylene (Gore-Tex Surgical Membrane) is superior to oxidized regenerated cellulose (Interceed TC7) in preventing adhesions. Fertil Steril. 1995;63:1021-1026.
18. Monteforte CA, Queirazza R, Francescetting P, Herbst TJ. Fistula formation after implanting an ePTFE membrane. A case report. J Reprod Med. 1997;42:184-187.
19. Pelosi MA, II, Pelosi MA, III. A new nonabsorbable adhesion barrier for myomectomy. Am J Surg. 2002;184:428-432.
20. Johns D. Reduction of postsurgical adhesions with Intergel adhesion prevention solution: a multicenter study of safety and efficacy after conservative gynecologic surgery. Fertil Steril. 2001;76:596-604.
21. Diamond MP and The Sepracoat Adhesion Study Group. Reduction of de novo postsurgical adhesions by intraoperative precoating with Sepracoat (HAL-C) solution: a prospective, randomized, blinded, placebo-controlled multicenter study. Fertil Steril. 1998;69:1067-1074.