User login
Energy-based techniques to ensure hemostasis and limit damage during laparoscopy
- Inspect all vascular sites with and without insufflation before assuming hemostasis is complete.
- In monopolar electrosurgery, electrode contact using low-voltage current leads to deeper, more effective penetration than higher-voltage current.
- To minimize unwanted thermal damage during bipolar electrosurgery, stop current flow at the end of the visible vapor phase, apply current in a pulsatile fashion, and secure pedicles by alternating between partial desiccation and incremental cutting.
- Since ultrasonic energy does not generate the high temperatures created by electrosurgery, it is less dependable for deep-tissue coagulation.
Compared with laparotomy, laparoscopic surgery achieves better hemostasis with less blood loss. Not only does this approach avoid an abdominal incision and the trauma associated with traction, manual manipulation, mechanical dissection, and larger tissue pedicles, but its illumination and magnification afford superior anatomical clarity, allowing the surgeon to seal a vessel before it is incised.
Still, keen surgical judgment remains critical—despite the availability of innovative electrosurgical, ultrasonic, and mechanical laparoscopic devices. Incomplete hemostasis or incision of an active vascular core can occur even with ideal application.
This article outlines the key ingredients of hemostasis during laparoscopy, focusing on the following modalities:
- monopolar electrosurgery
- bipolar electrosurgery
- ultrasonic energy
An orderly protocol minimizes risk
The art of surgical hemostasis is preventing vascular trauma while leaving the least-possible collateral tissue damage. When bleeding is encountered, the surgeon’s ability to attain hemostasis using a particular modality depends largely on how well he or she understands its technical aspects. Of course, thorough knowledge of anatomy also is crucial to prevent inadvertent damage to vital structures.
Surgical hemostasis should not be driven by reflex alone. Instead, surgeons should always follow this orderly sequence of steps to minimize risk:
Identify source of bleeding. Before taking any action, make every effort to accurately determine the source of bleeding and its proximity to vital anatomy. Even in the face of active hemorrhage, you can usually identify the bleeders by combining mechanical tamponade (using the jaws of a grasper or the side of a simple metallic probe) with active hydrolavage (using an irrigator-aspirator to break up and remove blood and clots).
Protect vital structures. If the bowel, bladder, or ureter is in close proximity to the bleeder, mobilize that structure sufficiently before applying energy. You can usually protect these entities by using a combination of countertraction and incremental tissue dissection. Whenever the peritoneum is involved, a relaxing incision parallel to the structure of concern also may be useful.
This protocol mandates withholding thermal energy until an orderly sequence of anatomical triage is carried out. Whenever a vital structure cannot be adequately mobilized, make every effort to control hemorrhage by using mechanical tamponade alone for up to 5 minutes. If access to the bleeding site or vessel caliber render pressure-alone unrealistic, employ either a carefully applied thermal energy or a suture ligature. If the surgeon is uncomfortable using either of these, conversion to laparotomy may be warranted.
Inspect vascular sites. Finally, because pneumoperitoneal pressure alone can tamponade venous bleeders—as well as small arterial ones—inspect all vascular sites with and without insufflation before assuming hemostasis is complete.
Monopolar electrosurgery
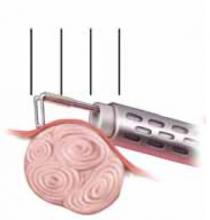
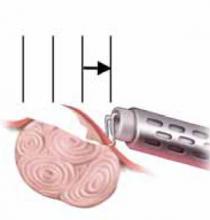
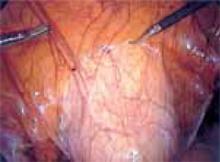
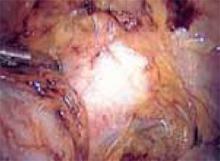
With conventional electrosurgery, tissue is coagulated when an electric field is applied across it using high-frequency alternating current. Whether cutting or coagulation occurs depends on the rate and extent of thermodynamic effects (FIGURE 1).
Mechanism of coagulation. When tissue comes into contact with the surface of an activated monopolar electrode, a relatively low-current circuit is completed.
- As the tissue is slowly heated to and maintained at temperatures above 50°C, irreversible cellular damage occurs. This is caused by deconfiguration of regulatory proteins and denaturation of cellular proteins.
- If the tissue is heated to 100°C, cellular water completely evaporates (desiccation), localized hemostasis occurs due to contraction of blood vessels and the surrounding tissues (coagulation), and collagens convert to glucose, which creates an adhesive effect between the tissue and electrode.
- Temperatures above 200°C cause carbonization and charring.
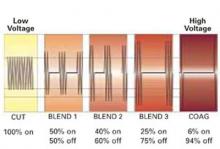
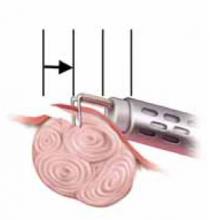
Select the best output voltage. Since the output voltage of “coag” current is very high (FIGURE 2), contact coagulation is generally limited to superficial layers. That is because of the accelerated buildup of tissue resistance from rapid desiccation and carbonization. Conversely, electrode contact using the lower-voltage “cut” current heats tissue more gradually, leading to deeper and more reliable penetration. Thus, both contact and coaptive coagulation with monopolar electrosurgery are more effectively performed using “cut” current.
Since superficial-appearing endometriotic implants may extend deeply into the retroperitoneal tissues, I thermally ablate these lesions using a broad-surface electrode in contact with “cut” current. In contrast, I treat superficial implants on the ovarian cortex with “coag” current to minimize unwanted thermal injury to adjacent follicular tissue.
Interrupt the blood flow. Coaptive vessel sealing using any type of monopolar current may be ineffective if the blood flow remains uninterrupted. Unless a vessel is sufficiently squeezed before electricity is applied, current density is dramatically reduced by conduction in blood, and luminal temperatures undergo little change, as any heat is dissipated by convection. Deceived by the appearance of well-coagulated tissue, a surgeon may discover an alarmingly viable core at the time of incision. Regardless of the selected output current (that is, “cut,” “blend,” or “coag”), coaptive desiccation with monopolar electrosurgery is usually insufficient to reliably secure the uterine or ovarian vessels during hysterectomy and oophorectomy.
Achieve the appropriate cutting arc. Electrosurgical cutting (vaporization/ablation) is possible whenever voltage is sufficient to create an electrical spark between an electrode and underlying tissue; tissue cutting is more apt to occur if the arcing remains unabated. When it does, cellular water is superheated to temperatures greater than 600°C, causing explosive vaporization secondary to the production of highly disruptive pressure (since steam occupies 6 times the volume of liquid water).
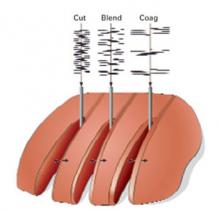
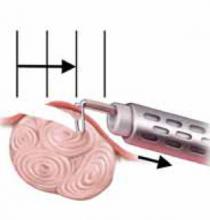
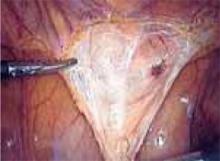
Although all of the typical output currents (“cut,” “blend,” and “coag”) provide sufficient voltage to ionize the air gap and arc to tissue, the higher voltages of “blend” and “coag” create progressively wider zones of thermal damage at the margins of the incision (FIGURE 3) These effects are amplified by using broad-surface electrodes.
Wide versus narrow hemostasis. Using “blend” or “coag” current to cut in order to provide wider hemostasis can be helpful during myomectomy, as well as when operating down the broad ligament and along the vaginal fornices during hysterectomy, across vascular adhesions, and to clarify the space of Retzius in preparation for colposuspension and paravaginal repair. Higher-voltage currents also facilitate incision of tissues that have greater impedance, such as fatty or desiccated pedicles and adhesions.
On the other hand, it is more prudent to utilize the lower-voltage “cut” current via the edge of an electrode for electrosurgical incision whenever lateral thermal spread may pose extra liability to adjacent tissues, such as the ovarian cortex during cystectomy and the ureter or rectum during excision of endometriosis from the lateral pelvic sidewall or cul-de-sac.
Deceived by the appearance of well-coagulated tissue, surgeons may discover an alarmingly viable core at the time of incision.
- Fulguration is the use of high-voltage sparking produced by “coag” current to coagulate a broad surface with open bleeders. As opposed to the continuous arcing produced by “cut” current, the highly interrupted output of “coag” current causes the arcs to strike the tissue surface in a widely dispersed and random fashion. This leads to more rapid thermal change, creating a zone of superficial coagulation.
I typically employ fulguration to control small bleeders and vessels cut on end along the undersurface of the ovarian cortex during cystectomy, atop the myometrial bed during myomectomy, and alongside Cooper’s ligament during colposuspension.
Protect vital structures with short bursts of “coag” current. If bleeding near the bowel, bladder, or ureter cannot be controlled with pressure alone, carefully directed short bursts of noncontact “coag” current with a broad-surface electrode may help you attain effective hemostasis with the least-possible amount of electrosurgical penetration.
Despite the high-voltage output involved, fulguration is useless in a wet, conductive surgical field due to the random diffusion of current.
FIGURE 2 Range of output voltages
Relative changes in voltage and average current related to “cut,” “blend,” and “coag” currents.
FIGURE 3Zones of thermal damage related to voltage
Using a conventional electrosurgical generator for tissue cutting, the margin of thermal necrosis expands with increasing voltage.
Bipolar electrosurgery
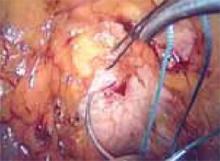
Mechanism of action. Bipolar electrosurgery consolidates an active electrode and return electrode into an instrument with 2 small poles. These poles can be the tines of a forceps, blades of a scissors, or an electrode matched to a more proximal conductive collar separated by an insulator. The output typically used is the low-voltage “cut” current.
Advantages of bipolar energy. Localization of current between the poles offers distinct advantages. Thermal damage is generally limited to a discrete volume of tissue. A bipolar forceps can be used to coapt and thermally weld blood vessels. The concentrated current and small distance between the poles also make it possible to desiccate tissue that is immersed in fluid. This modality is less useful, however, when open blood vessels are retracted or tissue pedicles are very thick.
Because it tends to promote the flow of energy well beyond desiccation, an ammeter should not be used to determine the treatment endpoint for coagulation of blood vessels in the vicinity of vital tissue.
Know when to terminate current to prevent vital-tissue damage. Although the flow of current and primary thermal effects are restricted to the tissue between the poles, this does not eliminate the risk of thermal injury to tissue that is distant from the site of directed hemostasis. As current is applied between the poles, the intervening tissue gradually desiccates until it becomes thoroughly dehydrated. Desiccation is complete when the tissue whitens and visible steam emission stops. If the application of current continues, the heat spreads well beyond the electrical limits of the instrument.
This secondary thermal bloom is caused by the bubbling of steam into the tissue parenchyma as heat is rapidly generated (due to dry tissue’s high resistance to the flow of electrical current). This explains why structures such as the ureter or bowel may suffer irreversible thermal damage despite being at some distance from an operative or bleeding site.
Use of an in-line ammeter does not prevent this problem. Rather, it tends to promote the flow of energy well beyond desiccation. Consequently, an ammeter should not be used to determine the treatment endpoint for coagulation of blood vessels in the vicinity of vital tissue.
Whenever bipolar electrosurgery is used for hemostasis, unwanted thermal damage can be minimized by:
- terminating the flow of current at the end of the visible vapor phase,
- applying current in a pulsatile fashion to permit tissue cooling,
- avoiding the use of an in-line ammeter to determine the coagulation endpoint, and
- securing pedicles by a stepwise process that alternates between partial desiccation and incremental cutting (TABLE 1).
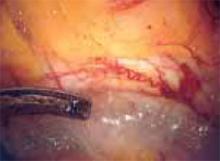
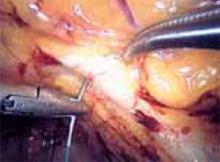
Since the rate of temperature generation is a direct function of the volume of tissue being desiccated, thermal spread can also be reduced by using the sides or tips of a slightly open forceps to press or lift, rather than coapt for hemostasis (FIGURE 4).
Free adherent tissue gently. As with contact monopolar coagulation, tissue between the electrodes of a bipolar instrument may become adherent during desiccation. Repeated attempts to shake the tissue free may lead to traumatic avulsion of a key vascular pedicle. A stuck vascular pedicle can usually be unglued by energizing the opened device while immersing it in a conductive irrigant, such as saline. Once the solution is boiled by the high current density between the electrodes, the mechanical action of bubbling is usually sufficient to atraumatically free the pedicle.
FIGURE 4 Minimizing bipolar thermal damage
Using contact rather than coaptation is one way to limit thermal injury during bipolar electrosurgery.TABLE 1
Minimizing bipolar thermal damage
|
Ultrasonic energy
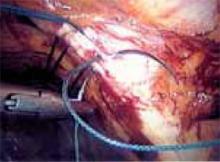
Mechanism of action. Ultrasonic shears produce mechanical energy to cut and coagulate tissue. Housed in the hand piece of this laparoscopic device is a piezoelectric crystal that vibrates a titanium blade 55,500 times per second over a variable excursion of 50 microns to 100 microns. As energy is transmitted to tissue, hydrogen bonds of tissue proteins are ruptured, leading to a denatured protein coagulum without significant charring. The tissue cutting that occurs is secondary to mechanical vibration and cavitational fragmentation of tissue parenchyma.
Since ultrasonic energy does not generate the high temperatures created by electrosurgery, it is less dependable for deep-tissue coagulation. Rather, thermal change is gradual, requiring a modicum of patience.
Available instrument configurations include 5-mm curved or hook blades. In addition, 5-mm or 10-mm ligating and cutting shears coaptively desiccate and cut the tissue by securing it between a grooved plastic pad and the vibrating blade.
Tissue effects depend on interplay of factors. By using various combinations of blade shapes, blade excursions, and tissue tensions, surgeons can accomplish a variety of specific effects. Cutting velocity is directly proportional to blade excursion, tissue traction, and blade surface area (energy density), and is inversely related to tissue density and elasticity. Thus, the fastest cutting with the least amount of coagulation occurs when tissue is placed on tension and firmly squeezed, lifted, or rotated with the sharpest side of a blade set at maximum excursion.
Coagulation is the obverse of cutting: It is inversely related to tissue tension, blade sharpness, blade excursion, and cutting speed. Therefore, coagulation is best achieved by relaxing tension, minimizing blade excursion, and using a blunt edge or flattened blade surface.
A stuck vascular pedicle can usually be unglued by energizing the opened bipolar device while immersing it in a conductive irrigant.
Avoid excessive traction and torsion. Be mindful of the potential for premature incision of an incompletely coagulated tissue pedicle when excessive traction or torsion is applied. When used to coaptively desiccate and incise a vascular pedicle, ultrasonic energy should be applied patiently, taking great care to minimize tissue tension while using the broadest blade surface set to the lowest excursion (TABLE 2). Hemostatic incision is best ensured by first coagulating several overlapping areas along an untracted pedicle, limiting each application to the point of tissue blanching and initial vapor emission. Only then should the pedicle be incised by gradually lifting, squeezing, or rotating the distal device. In this fashion, ultrasonic energy can be successfully used to secure both the ovarian and uterine vessels.
TABLE 2
Factors likely to cause premature incision with ultrasonic energy
|
Summary
The use of electrical and ultrasonic energy during operative laparoscopy poses several challenges, including the reduction of unwanted thermal injury and the elimination of incomplete hemostasis.
Since the depth of penetration during monopolar electrosurgery is proportional to both output voltage and surface area, unwanted thermal change can be reduced by using the smallest electrode surface with “cut” current for tissue cutting, and “coag” current with a broad-surface electrode for contact or noncontact (fulguration) coagulation.
Bipolar electrosurgery is the preferred modality for coaptive desiccation of a vascular pedicle with electricity. Despite the isolation of current to the intervening tissue, surgeons must take steps to reduce the lateral percolation of heat into adjacent tissues.
Ultrasonic energy provides reliable coaptive hemostasis and incision with little tissue damage. However, the surgeon must be mindful of the forces that promulgate premature incision. Knowledge of the biophysical behavior of electrical and ultrasonic energy is a prelude to safety and efficacy during laparoscopic dissection.
Dr. Brill reports no affiliations or financial arrangements with any of the manufacturers of products mentioned in this article.
1. Brill AI. Energy systems for operative laparoscopy. J Am Assoc Gynecol Laparosc. 1998;5:335-345.
2. Friedman J. The technical aspects of electrosurgery. Oral Surg. 1973;36:177-187.
3. Honig WM. The mechanism of cutting in electrosurgery. IEEE Trans Biomed Eng BME. 1975;22:55-58.
4. Sigel B, Dunn MR. The mechanism of blood vessel closure by high frequency electrocoagulation. Surg Gynecol Obstet. 1965;121:823-831.
5. Phipps JH. Thermometry studies with bipolar diathermy during hysterectomy. Gynecol Laparosc. 1994;1:146-149.
6. Ryder RM, Hulka JF. Bladder and bowel injury after electrodesiccation with Kleppinger bipolar forceps: A clinicopathologic study. J Reprod Med. 1993;3:595-598.
7. McCarus SD. Physiologic mechanism of the ultrasonically activated scalpel. J Am Assoc Gynecol Laparosc. 1996;3:601-608.
- Inspect all vascular sites with and without insufflation before assuming hemostasis is complete.
- In monopolar electrosurgery, electrode contact using low-voltage current leads to deeper, more effective penetration than higher-voltage current.
- To minimize unwanted thermal damage during bipolar electrosurgery, stop current flow at the end of the visible vapor phase, apply current in a pulsatile fashion, and secure pedicles by alternating between partial desiccation and incremental cutting.
- Since ultrasonic energy does not generate the high temperatures created by electrosurgery, it is less dependable for deep-tissue coagulation.
Compared with laparotomy, laparoscopic surgery achieves better hemostasis with less blood loss. Not only does this approach avoid an abdominal incision and the trauma associated with traction, manual manipulation, mechanical dissection, and larger tissue pedicles, but its illumination and magnification afford superior anatomical clarity, allowing the surgeon to seal a vessel before it is incised.
Still, keen surgical judgment remains critical—despite the availability of innovative electrosurgical, ultrasonic, and mechanical laparoscopic devices. Incomplete hemostasis or incision of an active vascular core can occur even with ideal application.
This article outlines the key ingredients of hemostasis during laparoscopy, focusing on the following modalities:
- monopolar electrosurgery
- bipolar electrosurgery
- ultrasonic energy
An orderly protocol minimizes risk
The art of surgical hemostasis is preventing vascular trauma while leaving the least-possible collateral tissue damage. When bleeding is encountered, the surgeon’s ability to attain hemostasis using a particular modality depends largely on how well he or she understands its technical aspects. Of course, thorough knowledge of anatomy also is crucial to prevent inadvertent damage to vital structures.
Surgical hemostasis should not be driven by reflex alone. Instead, surgeons should always follow this orderly sequence of steps to minimize risk:
Identify source of bleeding. Before taking any action, make every effort to accurately determine the source of bleeding and its proximity to vital anatomy. Even in the face of active hemorrhage, you can usually identify the bleeders by combining mechanical tamponade (using the jaws of a grasper or the side of a simple metallic probe) with active hydrolavage (using an irrigator-aspirator to break up and remove blood and clots).
Protect vital structures. If the bowel, bladder, or ureter is in close proximity to the bleeder, mobilize that structure sufficiently before applying energy. You can usually protect these entities by using a combination of countertraction and incremental tissue dissection. Whenever the peritoneum is involved, a relaxing incision parallel to the structure of concern also may be useful.
This protocol mandates withholding thermal energy until an orderly sequence of anatomical triage is carried out. Whenever a vital structure cannot be adequately mobilized, make every effort to control hemorrhage by using mechanical tamponade alone for up to 5 minutes. If access to the bleeding site or vessel caliber render pressure-alone unrealistic, employ either a carefully applied thermal energy or a suture ligature. If the surgeon is uncomfortable using either of these, conversion to laparotomy may be warranted.
Inspect vascular sites. Finally, because pneumoperitoneal pressure alone can tamponade venous bleeders—as well as small arterial ones—inspect all vascular sites with and without insufflation before assuming hemostasis is complete.
Monopolar electrosurgery
With conventional electrosurgery, tissue is coagulated when an electric field is applied across it using high-frequency alternating current. Whether cutting or coagulation occurs depends on the rate and extent of thermodynamic effects (FIGURE 1).
Mechanism of coagulation. When tissue comes into contact with the surface of an activated monopolar electrode, a relatively low-current circuit is completed.
- As the tissue is slowly heated to and maintained at temperatures above 50°C, irreversible cellular damage occurs. This is caused by deconfiguration of regulatory proteins and denaturation of cellular proteins.
- If the tissue is heated to 100°C, cellular water completely evaporates (desiccation), localized hemostasis occurs due to contraction of blood vessels and the surrounding tissues (coagulation), and collagens convert to glucose, which creates an adhesive effect between the tissue and electrode.
- Temperatures above 200°C cause carbonization and charring.
Select the best output voltage. Since the output voltage of “coag” current is very high (FIGURE 2), contact coagulation is generally limited to superficial layers. That is because of the accelerated buildup of tissue resistance from rapid desiccation and carbonization. Conversely, electrode contact using the lower-voltage “cut” current heats tissue more gradually, leading to deeper and more reliable penetration. Thus, both contact and coaptive coagulation with monopolar electrosurgery are more effectively performed using “cut” current.
Since superficial-appearing endometriotic implants may extend deeply into the retroperitoneal tissues, I thermally ablate these lesions using a broad-surface electrode in contact with “cut” current. In contrast, I treat superficial implants on the ovarian cortex with “coag” current to minimize unwanted thermal injury to adjacent follicular tissue.
Interrupt the blood flow. Coaptive vessel sealing using any type of monopolar current may be ineffective if the blood flow remains uninterrupted. Unless a vessel is sufficiently squeezed before electricity is applied, current density is dramatically reduced by conduction in blood, and luminal temperatures undergo little change, as any heat is dissipated by convection. Deceived by the appearance of well-coagulated tissue, a surgeon may discover an alarmingly viable core at the time of incision. Regardless of the selected output current (that is, “cut,” “blend,” or “coag”), coaptive desiccation with monopolar electrosurgery is usually insufficient to reliably secure the uterine or ovarian vessels during hysterectomy and oophorectomy.
Achieve the appropriate cutting arc. Electrosurgical cutting (vaporization/ablation) is possible whenever voltage is sufficient to create an electrical spark between an electrode and underlying tissue; tissue cutting is more apt to occur if the arcing remains unabated. When it does, cellular water is superheated to temperatures greater than 600°C, causing explosive vaporization secondary to the production of highly disruptive pressure (since steam occupies 6 times the volume of liquid water).
Although all of the typical output currents (“cut,” “blend,” and “coag”) provide sufficient voltage to ionize the air gap and arc to tissue, the higher voltages of “blend” and “coag” create progressively wider zones of thermal damage at the margins of the incision (FIGURE 3) These effects are amplified by using broad-surface electrodes.
Wide versus narrow hemostasis. Using “blend” or “coag” current to cut in order to provide wider hemostasis can be helpful during myomectomy, as well as when operating down the broad ligament and along the vaginal fornices during hysterectomy, across vascular adhesions, and to clarify the space of Retzius in preparation for colposuspension and paravaginal repair. Higher-voltage currents also facilitate incision of tissues that have greater impedance, such as fatty or desiccated pedicles and adhesions.
On the other hand, it is more prudent to utilize the lower-voltage “cut” current via the edge of an electrode for electrosurgical incision whenever lateral thermal spread may pose extra liability to adjacent tissues, such as the ovarian cortex during cystectomy and the ureter or rectum during excision of endometriosis from the lateral pelvic sidewall or cul-de-sac.
Deceived by the appearance of well-coagulated tissue, surgeons may discover an alarmingly viable core at the time of incision.
- Fulguration is the use of high-voltage sparking produced by “coag” current to coagulate a broad surface with open bleeders. As opposed to the continuous arcing produced by “cut” current, the highly interrupted output of “coag” current causes the arcs to strike the tissue surface in a widely dispersed and random fashion. This leads to more rapid thermal change, creating a zone of superficial coagulation.
I typically employ fulguration to control small bleeders and vessels cut on end along the undersurface of the ovarian cortex during cystectomy, atop the myometrial bed during myomectomy, and alongside Cooper’s ligament during colposuspension.
Protect vital structures with short bursts of “coag” current. If bleeding near the bowel, bladder, or ureter cannot be controlled with pressure alone, carefully directed short bursts of noncontact “coag” current with a broad-surface electrode may help you attain effective hemostasis with the least-possible amount of electrosurgical penetration.
Despite the high-voltage output involved, fulguration is useless in a wet, conductive surgical field due to the random diffusion of current.
FIGURE 2 Range of output voltages
Relative changes in voltage and average current related to “cut,” “blend,” and “coag” currents.
FIGURE 3Zones of thermal damage related to voltage
Using a conventional electrosurgical generator for tissue cutting, the margin of thermal necrosis expands with increasing voltage.
Bipolar electrosurgery
Mechanism of action. Bipolar electrosurgery consolidates an active electrode and return electrode into an instrument with 2 small poles. These poles can be the tines of a forceps, blades of a scissors, or an electrode matched to a more proximal conductive collar separated by an insulator. The output typically used is the low-voltage “cut” current.
Advantages of bipolar energy. Localization of current between the poles offers distinct advantages. Thermal damage is generally limited to a discrete volume of tissue. A bipolar forceps can be used to coapt and thermally weld blood vessels. The concentrated current and small distance between the poles also make it possible to desiccate tissue that is immersed in fluid. This modality is less useful, however, when open blood vessels are retracted or tissue pedicles are very thick.
Because it tends to promote the flow of energy well beyond desiccation, an ammeter should not be used to determine the treatment endpoint for coagulation of blood vessels in the vicinity of vital tissue.
Know when to terminate current to prevent vital-tissue damage. Although the flow of current and primary thermal effects are restricted to the tissue between the poles, this does not eliminate the risk of thermal injury to tissue that is distant from the site of directed hemostasis. As current is applied between the poles, the intervening tissue gradually desiccates until it becomes thoroughly dehydrated. Desiccation is complete when the tissue whitens and visible steam emission stops. If the application of current continues, the heat spreads well beyond the electrical limits of the instrument.
This secondary thermal bloom is caused by the bubbling of steam into the tissue parenchyma as heat is rapidly generated (due to dry tissue’s high resistance to the flow of electrical current). This explains why structures such as the ureter or bowel may suffer irreversible thermal damage despite being at some distance from an operative or bleeding site.
Use of an in-line ammeter does not prevent this problem. Rather, it tends to promote the flow of energy well beyond desiccation. Consequently, an ammeter should not be used to determine the treatment endpoint for coagulation of blood vessels in the vicinity of vital tissue.
Whenever bipolar electrosurgery is used for hemostasis, unwanted thermal damage can be minimized by:
- terminating the flow of current at the end of the visible vapor phase,
- applying current in a pulsatile fashion to permit tissue cooling,
- avoiding the use of an in-line ammeter to determine the coagulation endpoint, and
- securing pedicles by a stepwise process that alternates between partial desiccation and incremental cutting (TABLE 1).
Since the rate of temperature generation is a direct function of the volume of tissue being desiccated, thermal spread can also be reduced by using the sides or tips of a slightly open forceps to press or lift, rather than coapt for hemostasis (FIGURE 4).
Free adherent tissue gently. As with contact monopolar coagulation, tissue between the electrodes of a bipolar instrument may become adherent during desiccation. Repeated attempts to shake the tissue free may lead to traumatic avulsion of a key vascular pedicle. A stuck vascular pedicle can usually be unglued by energizing the opened device while immersing it in a conductive irrigant, such as saline. Once the solution is boiled by the high current density between the electrodes, the mechanical action of bubbling is usually sufficient to atraumatically free the pedicle.
FIGURE 4 Minimizing bipolar thermal damage
Using contact rather than coaptation is one way to limit thermal injury during bipolar electrosurgery.TABLE 1
Minimizing bipolar thermal damage
|
Ultrasonic energy
Mechanism of action. Ultrasonic shears produce mechanical energy to cut and coagulate tissue. Housed in the hand piece of this laparoscopic device is a piezoelectric crystal that vibrates a titanium blade 55,500 times per second over a variable excursion of 50 microns to 100 microns. As energy is transmitted to tissue, hydrogen bonds of tissue proteins are ruptured, leading to a denatured protein coagulum without significant charring. The tissue cutting that occurs is secondary to mechanical vibration and cavitational fragmentation of tissue parenchyma.
Since ultrasonic energy does not generate the high temperatures created by electrosurgery, it is less dependable for deep-tissue coagulation. Rather, thermal change is gradual, requiring a modicum of patience.
Available instrument configurations include 5-mm curved or hook blades. In addition, 5-mm or 10-mm ligating and cutting shears coaptively desiccate and cut the tissue by securing it between a grooved plastic pad and the vibrating blade.
Tissue effects depend on interplay of factors. By using various combinations of blade shapes, blade excursions, and tissue tensions, surgeons can accomplish a variety of specific effects. Cutting velocity is directly proportional to blade excursion, tissue traction, and blade surface area (energy density), and is inversely related to tissue density and elasticity. Thus, the fastest cutting with the least amount of coagulation occurs when tissue is placed on tension and firmly squeezed, lifted, or rotated with the sharpest side of a blade set at maximum excursion.
Coagulation is the obverse of cutting: It is inversely related to tissue tension, blade sharpness, blade excursion, and cutting speed. Therefore, coagulation is best achieved by relaxing tension, minimizing blade excursion, and using a blunt edge or flattened blade surface.
A stuck vascular pedicle can usually be unglued by energizing the opened bipolar device while immersing it in a conductive irrigant.
Avoid excessive traction and torsion. Be mindful of the potential for premature incision of an incompletely coagulated tissue pedicle when excessive traction or torsion is applied. When used to coaptively desiccate and incise a vascular pedicle, ultrasonic energy should be applied patiently, taking great care to minimize tissue tension while using the broadest blade surface set to the lowest excursion (TABLE 2). Hemostatic incision is best ensured by first coagulating several overlapping areas along an untracted pedicle, limiting each application to the point of tissue blanching and initial vapor emission. Only then should the pedicle be incised by gradually lifting, squeezing, or rotating the distal device. In this fashion, ultrasonic energy can be successfully used to secure both the ovarian and uterine vessels.
TABLE 2
Factors likely to cause premature incision with ultrasonic energy
|
Summary
The use of electrical and ultrasonic energy during operative laparoscopy poses several challenges, including the reduction of unwanted thermal injury and the elimination of incomplete hemostasis.
Since the depth of penetration during monopolar electrosurgery is proportional to both output voltage and surface area, unwanted thermal change can be reduced by using the smallest electrode surface with “cut” current for tissue cutting, and “coag” current with a broad-surface electrode for contact or noncontact (fulguration) coagulation.
Bipolar electrosurgery is the preferred modality for coaptive desiccation of a vascular pedicle with electricity. Despite the isolation of current to the intervening tissue, surgeons must take steps to reduce the lateral percolation of heat into adjacent tissues.
Ultrasonic energy provides reliable coaptive hemostasis and incision with little tissue damage. However, the surgeon must be mindful of the forces that promulgate premature incision. Knowledge of the biophysical behavior of electrical and ultrasonic energy is a prelude to safety and efficacy during laparoscopic dissection.
Dr. Brill reports no affiliations or financial arrangements with any of the manufacturers of products mentioned in this article.
- Inspect all vascular sites with and without insufflation before assuming hemostasis is complete.
- In monopolar electrosurgery, electrode contact using low-voltage current leads to deeper, more effective penetration than higher-voltage current.
- To minimize unwanted thermal damage during bipolar electrosurgery, stop current flow at the end of the visible vapor phase, apply current in a pulsatile fashion, and secure pedicles by alternating between partial desiccation and incremental cutting.
- Since ultrasonic energy does not generate the high temperatures created by electrosurgery, it is less dependable for deep-tissue coagulation.
Compared with laparotomy, laparoscopic surgery achieves better hemostasis with less blood loss. Not only does this approach avoid an abdominal incision and the trauma associated with traction, manual manipulation, mechanical dissection, and larger tissue pedicles, but its illumination and magnification afford superior anatomical clarity, allowing the surgeon to seal a vessel before it is incised.
Still, keen surgical judgment remains critical—despite the availability of innovative electrosurgical, ultrasonic, and mechanical laparoscopic devices. Incomplete hemostasis or incision of an active vascular core can occur even with ideal application.
This article outlines the key ingredients of hemostasis during laparoscopy, focusing on the following modalities:
- monopolar electrosurgery
- bipolar electrosurgery
- ultrasonic energy
An orderly protocol minimizes risk
The art of surgical hemostasis is preventing vascular trauma while leaving the least-possible collateral tissue damage. When bleeding is encountered, the surgeon’s ability to attain hemostasis using a particular modality depends largely on how well he or she understands its technical aspects. Of course, thorough knowledge of anatomy also is crucial to prevent inadvertent damage to vital structures.
Surgical hemostasis should not be driven by reflex alone. Instead, surgeons should always follow this orderly sequence of steps to minimize risk:
Identify source of bleeding. Before taking any action, make every effort to accurately determine the source of bleeding and its proximity to vital anatomy. Even in the face of active hemorrhage, you can usually identify the bleeders by combining mechanical tamponade (using the jaws of a grasper or the side of a simple metallic probe) with active hydrolavage (using an irrigator-aspirator to break up and remove blood and clots).
Protect vital structures. If the bowel, bladder, or ureter is in close proximity to the bleeder, mobilize that structure sufficiently before applying energy. You can usually protect these entities by using a combination of countertraction and incremental tissue dissection. Whenever the peritoneum is involved, a relaxing incision parallel to the structure of concern also may be useful.
This protocol mandates withholding thermal energy until an orderly sequence of anatomical triage is carried out. Whenever a vital structure cannot be adequately mobilized, make every effort to control hemorrhage by using mechanical tamponade alone for up to 5 minutes. If access to the bleeding site or vessel caliber render pressure-alone unrealistic, employ either a carefully applied thermal energy or a suture ligature. If the surgeon is uncomfortable using either of these, conversion to laparotomy may be warranted.
Inspect vascular sites. Finally, because pneumoperitoneal pressure alone can tamponade venous bleeders—as well as small arterial ones—inspect all vascular sites with and without insufflation before assuming hemostasis is complete.
Monopolar electrosurgery
With conventional electrosurgery, tissue is coagulated when an electric field is applied across it using high-frequency alternating current. Whether cutting or coagulation occurs depends on the rate and extent of thermodynamic effects (FIGURE 1).
Mechanism of coagulation. When tissue comes into contact with the surface of an activated monopolar electrode, a relatively low-current circuit is completed.
- As the tissue is slowly heated to and maintained at temperatures above 50°C, irreversible cellular damage occurs. This is caused by deconfiguration of regulatory proteins and denaturation of cellular proteins.
- If the tissue is heated to 100°C, cellular water completely evaporates (desiccation), localized hemostasis occurs due to contraction of blood vessels and the surrounding tissues (coagulation), and collagens convert to glucose, which creates an adhesive effect between the tissue and electrode.
- Temperatures above 200°C cause carbonization and charring.
Select the best output voltage. Since the output voltage of “coag” current is very high (FIGURE 2), contact coagulation is generally limited to superficial layers. That is because of the accelerated buildup of tissue resistance from rapid desiccation and carbonization. Conversely, electrode contact using the lower-voltage “cut” current heats tissue more gradually, leading to deeper and more reliable penetration. Thus, both contact and coaptive coagulation with monopolar electrosurgery are more effectively performed using “cut” current.
Since superficial-appearing endometriotic implants may extend deeply into the retroperitoneal tissues, I thermally ablate these lesions using a broad-surface electrode in contact with “cut” current. In contrast, I treat superficial implants on the ovarian cortex with “coag” current to minimize unwanted thermal injury to adjacent follicular tissue.
Interrupt the blood flow. Coaptive vessel sealing using any type of monopolar current may be ineffective if the blood flow remains uninterrupted. Unless a vessel is sufficiently squeezed before electricity is applied, current density is dramatically reduced by conduction in blood, and luminal temperatures undergo little change, as any heat is dissipated by convection. Deceived by the appearance of well-coagulated tissue, a surgeon may discover an alarmingly viable core at the time of incision. Regardless of the selected output current (that is, “cut,” “blend,” or “coag”), coaptive desiccation with monopolar electrosurgery is usually insufficient to reliably secure the uterine or ovarian vessels during hysterectomy and oophorectomy.
Achieve the appropriate cutting arc. Electrosurgical cutting (vaporization/ablation) is possible whenever voltage is sufficient to create an electrical spark between an electrode and underlying tissue; tissue cutting is more apt to occur if the arcing remains unabated. When it does, cellular water is superheated to temperatures greater than 600°C, causing explosive vaporization secondary to the production of highly disruptive pressure (since steam occupies 6 times the volume of liquid water).
Although all of the typical output currents (“cut,” “blend,” and “coag”) provide sufficient voltage to ionize the air gap and arc to tissue, the higher voltages of “blend” and “coag” create progressively wider zones of thermal damage at the margins of the incision (FIGURE 3) These effects are amplified by using broad-surface electrodes.
Wide versus narrow hemostasis. Using “blend” or “coag” current to cut in order to provide wider hemostasis can be helpful during myomectomy, as well as when operating down the broad ligament and along the vaginal fornices during hysterectomy, across vascular adhesions, and to clarify the space of Retzius in preparation for colposuspension and paravaginal repair. Higher-voltage currents also facilitate incision of tissues that have greater impedance, such as fatty or desiccated pedicles and adhesions.
On the other hand, it is more prudent to utilize the lower-voltage “cut” current via the edge of an electrode for electrosurgical incision whenever lateral thermal spread may pose extra liability to adjacent tissues, such as the ovarian cortex during cystectomy and the ureter or rectum during excision of endometriosis from the lateral pelvic sidewall or cul-de-sac.
Deceived by the appearance of well-coagulated tissue, surgeons may discover an alarmingly viable core at the time of incision.
- Fulguration is the use of high-voltage sparking produced by “coag” current to coagulate a broad surface with open bleeders. As opposed to the continuous arcing produced by “cut” current, the highly interrupted output of “coag” current causes the arcs to strike the tissue surface in a widely dispersed and random fashion. This leads to more rapid thermal change, creating a zone of superficial coagulation.
I typically employ fulguration to control small bleeders and vessels cut on end along the undersurface of the ovarian cortex during cystectomy, atop the myometrial bed during myomectomy, and alongside Cooper’s ligament during colposuspension.
Protect vital structures with short bursts of “coag” current. If bleeding near the bowel, bladder, or ureter cannot be controlled with pressure alone, carefully directed short bursts of noncontact “coag” current with a broad-surface electrode may help you attain effective hemostasis with the least-possible amount of electrosurgical penetration.
Despite the high-voltage output involved, fulguration is useless in a wet, conductive surgical field due to the random diffusion of current.
FIGURE 2 Range of output voltages
Relative changes in voltage and average current related to “cut,” “blend,” and “coag” currents.
FIGURE 3Zones of thermal damage related to voltage
Using a conventional electrosurgical generator for tissue cutting, the margin of thermal necrosis expands with increasing voltage.
Bipolar electrosurgery
Mechanism of action. Bipolar electrosurgery consolidates an active electrode and return electrode into an instrument with 2 small poles. These poles can be the tines of a forceps, blades of a scissors, or an electrode matched to a more proximal conductive collar separated by an insulator. The output typically used is the low-voltage “cut” current.
Advantages of bipolar energy. Localization of current between the poles offers distinct advantages. Thermal damage is generally limited to a discrete volume of tissue. A bipolar forceps can be used to coapt and thermally weld blood vessels. The concentrated current and small distance between the poles also make it possible to desiccate tissue that is immersed in fluid. This modality is less useful, however, when open blood vessels are retracted or tissue pedicles are very thick.
Because it tends to promote the flow of energy well beyond desiccation, an ammeter should not be used to determine the treatment endpoint for coagulation of blood vessels in the vicinity of vital tissue.
Know when to terminate current to prevent vital-tissue damage. Although the flow of current and primary thermal effects are restricted to the tissue between the poles, this does not eliminate the risk of thermal injury to tissue that is distant from the site of directed hemostasis. As current is applied between the poles, the intervening tissue gradually desiccates until it becomes thoroughly dehydrated. Desiccation is complete when the tissue whitens and visible steam emission stops. If the application of current continues, the heat spreads well beyond the electrical limits of the instrument.
This secondary thermal bloom is caused by the bubbling of steam into the tissue parenchyma as heat is rapidly generated (due to dry tissue’s high resistance to the flow of electrical current). This explains why structures such as the ureter or bowel may suffer irreversible thermal damage despite being at some distance from an operative or bleeding site.
Use of an in-line ammeter does not prevent this problem. Rather, it tends to promote the flow of energy well beyond desiccation. Consequently, an ammeter should not be used to determine the treatment endpoint for coagulation of blood vessels in the vicinity of vital tissue.
Whenever bipolar electrosurgery is used for hemostasis, unwanted thermal damage can be minimized by:
- terminating the flow of current at the end of the visible vapor phase,
- applying current in a pulsatile fashion to permit tissue cooling,
- avoiding the use of an in-line ammeter to determine the coagulation endpoint, and
- securing pedicles by a stepwise process that alternates between partial desiccation and incremental cutting (TABLE 1).
Since the rate of temperature generation is a direct function of the volume of tissue being desiccated, thermal spread can also be reduced by using the sides or tips of a slightly open forceps to press or lift, rather than coapt for hemostasis (FIGURE 4).
Free adherent tissue gently. As with contact monopolar coagulation, tissue between the electrodes of a bipolar instrument may become adherent during desiccation. Repeated attempts to shake the tissue free may lead to traumatic avulsion of a key vascular pedicle. A stuck vascular pedicle can usually be unglued by energizing the opened device while immersing it in a conductive irrigant, such as saline. Once the solution is boiled by the high current density between the electrodes, the mechanical action of bubbling is usually sufficient to atraumatically free the pedicle.
FIGURE 4 Minimizing bipolar thermal damage
Using contact rather than coaptation is one way to limit thermal injury during bipolar electrosurgery.TABLE 1
Minimizing bipolar thermal damage
|
Ultrasonic energy
Mechanism of action. Ultrasonic shears produce mechanical energy to cut and coagulate tissue. Housed in the hand piece of this laparoscopic device is a piezoelectric crystal that vibrates a titanium blade 55,500 times per second over a variable excursion of 50 microns to 100 microns. As energy is transmitted to tissue, hydrogen bonds of tissue proteins are ruptured, leading to a denatured protein coagulum without significant charring. The tissue cutting that occurs is secondary to mechanical vibration and cavitational fragmentation of tissue parenchyma.
Since ultrasonic energy does not generate the high temperatures created by electrosurgery, it is less dependable for deep-tissue coagulation. Rather, thermal change is gradual, requiring a modicum of patience.
Available instrument configurations include 5-mm curved or hook blades. In addition, 5-mm or 10-mm ligating and cutting shears coaptively desiccate and cut the tissue by securing it between a grooved plastic pad and the vibrating blade.
Tissue effects depend on interplay of factors. By using various combinations of blade shapes, blade excursions, and tissue tensions, surgeons can accomplish a variety of specific effects. Cutting velocity is directly proportional to blade excursion, tissue traction, and blade surface area (energy density), and is inversely related to tissue density and elasticity. Thus, the fastest cutting with the least amount of coagulation occurs when tissue is placed on tension and firmly squeezed, lifted, or rotated with the sharpest side of a blade set at maximum excursion.
Coagulation is the obverse of cutting: It is inversely related to tissue tension, blade sharpness, blade excursion, and cutting speed. Therefore, coagulation is best achieved by relaxing tension, minimizing blade excursion, and using a blunt edge or flattened blade surface.
A stuck vascular pedicle can usually be unglued by energizing the opened bipolar device while immersing it in a conductive irrigant.
Avoid excessive traction and torsion. Be mindful of the potential for premature incision of an incompletely coagulated tissue pedicle when excessive traction or torsion is applied. When used to coaptively desiccate and incise a vascular pedicle, ultrasonic energy should be applied patiently, taking great care to minimize tissue tension while using the broadest blade surface set to the lowest excursion (TABLE 2). Hemostatic incision is best ensured by first coagulating several overlapping areas along an untracted pedicle, limiting each application to the point of tissue blanching and initial vapor emission. Only then should the pedicle be incised by gradually lifting, squeezing, or rotating the distal device. In this fashion, ultrasonic energy can be successfully used to secure both the ovarian and uterine vessels.
TABLE 2
Factors likely to cause premature incision with ultrasonic energy
|
Summary
The use of electrical and ultrasonic energy during operative laparoscopy poses several challenges, including the reduction of unwanted thermal injury and the elimination of incomplete hemostasis.
Since the depth of penetration during monopolar electrosurgery is proportional to both output voltage and surface area, unwanted thermal change can be reduced by using the smallest electrode surface with “cut” current for tissue cutting, and “coag” current with a broad-surface electrode for contact or noncontact (fulguration) coagulation.
Bipolar electrosurgery is the preferred modality for coaptive desiccation of a vascular pedicle with electricity. Despite the isolation of current to the intervening tissue, surgeons must take steps to reduce the lateral percolation of heat into adjacent tissues.
Ultrasonic energy provides reliable coaptive hemostasis and incision with little tissue damage. However, the surgeon must be mindful of the forces that promulgate premature incision. Knowledge of the biophysical behavior of electrical and ultrasonic energy is a prelude to safety and efficacy during laparoscopic dissection.
Dr. Brill reports no affiliations or financial arrangements with any of the manufacturers of products mentioned in this article.
1. Brill AI. Energy systems for operative laparoscopy. J Am Assoc Gynecol Laparosc. 1998;5:335-345.
2. Friedman J. The technical aspects of electrosurgery. Oral Surg. 1973;36:177-187.
3. Honig WM. The mechanism of cutting in electrosurgery. IEEE Trans Biomed Eng BME. 1975;22:55-58.
4. Sigel B, Dunn MR. The mechanism of blood vessel closure by high frequency electrocoagulation. Surg Gynecol Obstet. 1965;121:823-831.
5. Phipps JH. Thermometry studies with bipolar diathermy during hysterectomy. Gynecol Laparosc. 1994;1:146-149.
6. Ryder RM, Hulka JF. Bladder and bowel injury after electrodesiccation with Kleppinger bipolar forceps: A clinicopathologic study. J Reprod Med. 1993;3:595-598.
7. McCarus SD. Physiologic mechanism of the ultrasonically activated scalpel. J Am Assoc Gynecol Laparosc. 1996;3:601-608.
1. Brill AI. Energy systems for operative laparoscopy. J Am Assoc Gynecol Laparosc. 1998;5:335-345.
2. Friedman J. The technical aspects of electrosurgery. Oral Surg. 1973;36:177-187.
3. Honig WM. The mechanism of cutting in electrosurgery. IEEE Trans Biomed Eng BME. 1975;22:55-58.
4. Sigel B, Dunn MR. The mechanism of blood vessel closure by high frequency electrocoagulation. Surg Gynecol Obstet. 1965;121:823-831.
5. Phipps JH. Thermometry studies with bipolar diathermy during hysterectomy. Gynecol Laparosc. 1994;1:146-149.
6. Ryder RM, Hulka JF. Bladder and bowel injury after electrodesiccation with Kleppinger bipolar forceps: A clinicopathologic study. J Reprod Med. 1993;3:595-598.
7. McCarus SD. Physiologic mechanism of the ultrasonically activated scalpel. J Am Assoc Gynecol Laparosc. 1996;3:601-608.
Pelosi minilaparotomy hysterectomy: Effective alternative to laparoscopy and laparotomy
Although laparoscopic hysterectomy offers a minimally invasive alternative to laparotomy when vaginal hysterectomy is contraindicated, it has its drawbacks. Among them: the cost of expensive equipment, the long learning curve, and prolonged operating time.
We describe another alternative to open surgery that is comparable to laparoscopic hysterectomy in postoperative pain, cosmetic results, and time to return to normal activities. Our procedure—a redesigned minilaparotomy hysterectomy—relies on traditional open techniques and inexpensive novel instrumentation, making it significantly faster than laparoscopy and easy to perform and teach.
For patients who cannot undergo vaginal hysterectomy, this new modality offers an expeditious, minimal-access option. Gynecologists reluctant to relinquish the routine use of standard laparotomy may find this approach an appealing, less-invasive alternative.
Position, incision, and retraction are crucial to success
Our minilaparotomy hysterectomy is a systemized approach with elements derived from both open and laparoscopic surgery. Three preparatory components are involved:
- position
- incision
- retraction
All are critical to a successful hysterectomy, ensuring that the procedure never becomes a haphazard struggle through an improvised, scaled-down, conventional Pfannenstiel or vertical incision. Our approach also avoids cumbersome traditional laparotomy exposure maneuvers and positioning.
Position: Modified lithotomy. After regional or general anesthesia is given, position the patient in a modified lithotomy with both arms tucked as for laparoscopic surgery. Place the legs in boot-type stirrups, with no hip flexion and sufficient thigh abduction to expose the vagina.
Next, perform a thorough pelvic examination and place an indwelling, transurethral catheter. A sturdy, hinged uterine manipulator is of paramount importance for the hysterectomy, as it facilitates exposure of the adnexa as well as elevation/rotation of the uterus and the uterine attachments. We recommend the Pelosi Uterine Manipulator (Apple Medical Corporation, Marlboro, Mass) or its equivalent (FIGURE 1).
Standard minilaparotomy
The use of standard minilaparotomy—which is nothing more than a conventional laparotomy of limited length (3 cm to 6 cm), performed either transversely or vertically—has been confined to the surgical treatment of benign pelvic pathology of limited extent.
To generate sufficient exposure to work effectively, surgeons using the standard minilaparotomy have relied on the length of the abdominal incision and, secondarily, bowel packing and metal handheld or self-retaining fixed retraction systems. When exposure is difficult to achieve or maintain, however, routine surgical maneuvers become frustrating and time-consuming—unless the clinician uses extensive traction force, extends the incision length, or performs muscle-splitting. These alternatives often result in an uncomfortable, slow recovery typical of most laparotomies, thereby negating the primary goal of minimally invasive surgery.
Use of traditional minilaparotomy for hysterectomy has been reported only rarely. Hoffman et al1 found the procedure safe and effective in nonobese women in whom a vaginal approach was precluded. Benedetti Panicci et al2,3 also have used minilaparotomy successfully in benign gynecologic disease and hysterectomy.
The Kustner incision
Originally reported in 1896,4 this incision is avoided by most surgeons in favor of complete transverse or complete vertical incisions—largely due to difficulties with exposure, troublesome seroma formation, and wound complications secondary to increased fluid accumulation in the large dead space that results from wide dissection of the subcutaneous flap.
In the early 1990s, we realized the potential benefits of a scaled-down Kustner’s incision (2 cm to 5 cm) when assistance was needed via minilaparotomy during such laparoscopic-assisted procedures as uterine morcellation, tubal reanastomosis, and extensive uterine suture and reconstruction following complex laparoscopic myomectomy.5 As a substitute for laparoscopy and laparotomy, we then tried a minilaparotomy Kustner’s incision (3 cm to 5 cm) as the sole means of surgical access, assessment, and treatment for benign pelvic conditions.
Benefits of this incision. When a sturdy uterine manipulator was used to facilitate exposure of the adnexa and uterine elevation/rotation, we found this technique more effective than similar procedures using a scaled-down Pfannenstiel or Maylard incision. In addition, because the incision was small and the extent of subcutaneous dissection required to expose the rectus fascia in a vertical fashion was limited, there was no need for incision drainage. Nor was the procedure associated with seroma formation, as the full-sized Kustner’s incision had been.3 However, the minilaparotomy Kustner’s incision still suffered from limited surgical exposure.
Adding the retractor
It became clear that a soft, self-retaining abdominal retractor that is capable of creating a rapid, effective, nontraumatic, and predictable circular area of abdominal retraction would be helpful, particularly one that could be placed through the minilaparotomy Kustner’s incision.6 Once this retractor system was developed, using technology borrowed from hand-assisted laparoscopy,7-10 the minilaparotomy hysterectomy became a much simpler, more useful surgical option.
REFERENCES
1. Hoffman MS, Lynch CM. Minilaparotomy hysterectomy. Am J Obstet Gynecol. 1998;179:316-320.
2. Benedetti Panicci P, Maneschi F, Cutillo G, et al. Surgery by minilaparotomy in benign gynecologic disease. Obstet Gynecol. 1996;87:456-459.
3. Benedetti Panicci P, Zullo MA, Casalino B, et al. Subcutaneous drainage versus no drainage after minilaparotomy in gynecologic benign conditions. Am J Obstet Gynecol. 2003;188:71-75.
4. Kustner O. Der suprasymphysare kruzschnitt, eine methode der coeliotomie bei wening umfanglichen affektionen der weiblichen beckenorgane. Monatsschr Geburtshilfe Gynakol. 1896;4:197-206.
5. Pelosi MA, II, Pelosi MA, III. The suprapubic cruciate incision for laparoscopic assisted microceliotomy. J Soc Laparoendosc Surg. 1997;1:269-272.
6. Pelosi MA, II, Pelosi MA, III. Self-retaining abdominal retractor for minilaparotomy. Obstet Gynecol. 2000;96:775-778.
7. Pelosi MA, II, Pelosi MA, III. Hand-assisted laparoscopy for complex hysterectomy. J Am Assoc Gynecol Laparosc. 1999;6:183-188.
8. Pelosi MA, II, Pelosi MA, III. Hand-assisted laparoscopic cholecystectomy at cesarean section. J Am Assoc Gynecol Laparosc. 1999;6:491-495.
9. Pelosi MA, II, Pelosi MA, III. Hand-assisted laparoscopy (handoscopy) for megamyomectomy: A case study. J Reprod Med. 2000;45:519-525.
10. Pelosi MA, II, Pelosi MA, III, Eim J. Hand-assisted laparoscopy for pelvic malignancy. J Laparoendosc Adv Surg Tech. 2000;10:143-150.
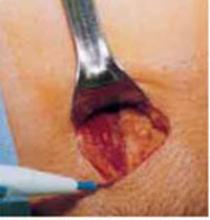
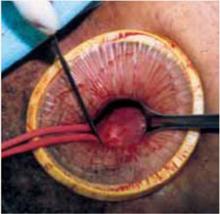
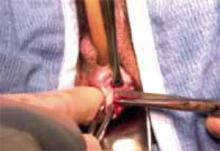
Incision: Modified Kustner’s. Open the abdomen with a cruciate incision. Using a conventional scalpel and the Bovie device, make a 2.5-cm to 5-cm transverse incision through the skin and subcutaneous fat until you reach the anterior rectus fascia (FIGURE 2A). Clear the fat from the midline superiorly and inferiorly to expose approximately 5 cm to 6 cm of fascia in the vertical axis. Then incise the anterior rectus fascia in a vertical direction through the full length of the cleared area (FIGURE 2B).
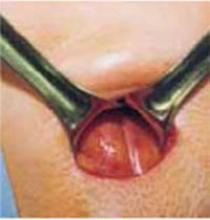
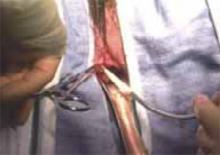
Retract the rectus muscles from the midline, exposing the transversalis fascia and the underlying peritoneum. Enter the peritoneum digitally or with scissors above the level of the bladder dome, incising vertically until the entrance extends the full length of the fascial incision (FIGURE 2C).
This modified Kustner’s incision is essentially a vertical midline incision in its deeper layers.1 The rapid surgical dissection of the fascia and rectus muscles and the intraperitoneal entry are relatively bloodless. This approach yields a surgical exposure superior to that of a small Pfannenstiel or Maylard incision.
Note that, in some patients, a vertical incision can be selected if there is a prior vertical incision or if the perioperative workup suggests a malignancy that may require a later extension of the original minilaparotomy incision.
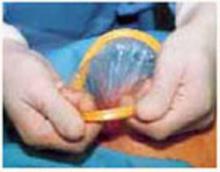
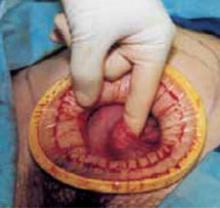
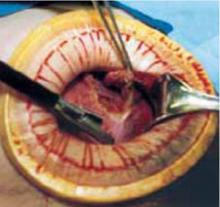
Retraction: Soft, sleeve-type, self-retaining abdominal retractor. This device consists of a flexible plastic inner ring and a firmer outer ring connected by a soft plastic sleeve (FIGURE 3A). Two models are available: the Mobius (Apple Medical Corporation) and the Protractor (Weck Closure Systems, Research Triangle Park, NC).
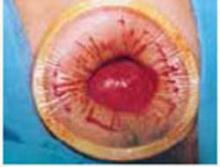
Squeeze the inner ring into the peritoneal cavity through the minilaparotomy incision, allowing it to spring open against the parietal peritoneum. Conduct a digital assessment to ensure that no viscera are trapped by elevating the outer ring. Next, roll the outer ring onto the sleeve, collecting excess length, until it sits firmly against the skin (FIGURES 3B and 3C). The result, when there is adequate tension within the sleeve, is a circular area of retraction offering excellent exposure of the pelvis. Note that during surgery you may need to adjust the outer ring if the sleeve loosens.
The soft, self-retaining abdominal retractor offers several advantages over traditional abdominal retraction:
- Atraumatic retraction. This device distributes retraction force evenly around the entire incision. Because standard retractors concentrate retraction force at only a few points, they often lead to tissue trauma, nerve damage, bruising, and postoperative pain.
- Incision protection. The retractor’s flexible material lines the incision, protecting the wound’s edges from contamination and potential implantation of malignant cells.
- Improved access. Because the continuous retraction force is delivered more effectively to the incision, exposure is maximized. As a result, the need for intensive surgical assistance is dramatically reduced.
- Adjustable height. The retractor’s design lets it adapt to wounds of varying depth—a feature that makes it ideal for obese patients. The device compresses the patient’s skin and peritoneum between the external and internal rings, keeping the full thickness of the abdominal incision constant throughout the surgery.
- Cost-effectiveness. The device, which costs under $100, is simple and fast to set up. In our experience, placement takes approximately 2 minutes; this compares favorably with table-mounted or self-retaining rigid retraction systems, which may require significant capital expenditures (cost may run in the thousands), repair costs, and complicated set-ups.
FIGURE 1 Hinged uterine manipulator
A sturdy hinged uterine manipulator facilitates exposure of the adnexa as well as elevation/rotation of the uterus.
FIGURE 2 Cruciate incision
A. Make a transverse incision suprapubically through the skin and the subcutaneous fat to reach the anterior rectus fascia.
FIGURE 2 Cruciate incision
B. Clear the fat from the midline to expose the rectus fascia in the vertical axis, then incise the fascia in a vertical direction through the full length of the previously cleared area. The rectus muscles are retracted, thereby exposing the peritoneum.
FIGURE 2 Cruciate incision
C. Incise the peritoneum vertically until it extends the full length of the fascial incision.
FIGURE 3 Soft, sleeve-type, self-retaining abdominal retractor
A. At left, the Protractor (Weck Closure Systems); at right, the Mobius (Apple Medical Corporation).
FIGURE 3 Soft, sleeve-type, self-retaining abdominal retractor
B. After inserting the inner ring into the peritoneal cavity, twist the outer ring downward until it inverts and rests snuggly against the skin.
FIGURE 3 Soft, sleeve-type, self-retaining abdominal retractor
C. An atraumatic, circular, self-retaining area of retraction is created.
Standard technique: Exteriorize the uterus; divide uterine attachments, vessels
Assess the anatomy. Using your index finger and the uterine manipulator to rotate and flex the uterus, carefully assess the uterus, adnexa, and pelvis, noting the location of the ureters. Determine the extent of any unexpected pelvic pathology or adhesions, using traditional small retractors or gentle packing to gain additional exposure. Perform any adhesiolysis that is necessary.
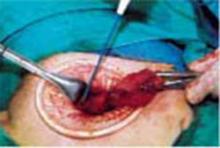
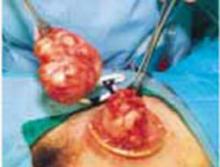
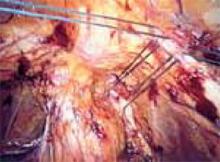
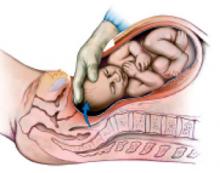
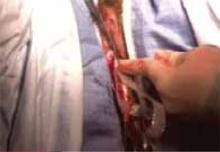
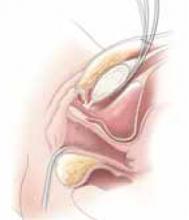
Exteriorize the uterus. Next, bring the uterus and the adnexa above the abdominal wall in order to perform as much of the hysterectomy extracorporeally as possible. Pass the uterus or adnexa through the incision with the upward assistance of the uterine manipulator, then divide the upper uterine attachments (FIGURE 4A).
Increase exposure. You can achieve additional uterine elevation and targeted exposure in several ways. For example, a strong traction suture can be placed in the uterine fundus, left long, and secured with a clamp. To achieve uterine elevation, place long clamps lateral to the corpus. Another effective approach is to place a heavy tenaculum on the uterine fundus.
When lateral exposure is limited, divide the proximal adnexal pedicles and round ligaments to begin the operation, and remove the adnexa separately following the completion of the hysterectomy.
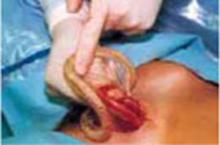
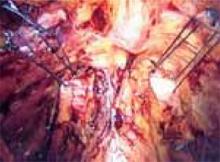
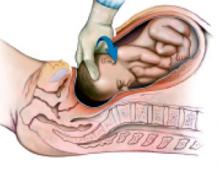
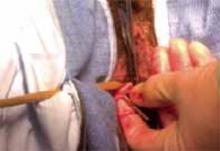
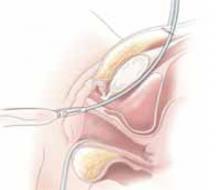
Divide the uterine vessels through the small incision using clamping, division, and ligation. Unless you intend to preserve the cervix, mobilize the bladder to the level of the anterior vaginal fornix. Inward pressure on the uterine manipulator provides additional elevation of the lower uterine vasculature and the cardinal and uterosacral ligaments as these structures are ligated and divided. Amputate the uterine specimen from the vaginal cuff using the uterine manipulator to guide the vaginal circumcision (FIGURE 4B). Close the vaginal cuff using standard closure.
If the cervix is to be preserved, amputate the uterus supracervically following division of the uterine vessels. Then suture the cervical stump in the traditional fashion. Upward elevation of the cervix using the uterine manipulator expedites this step.
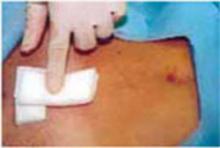
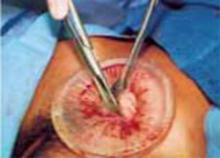
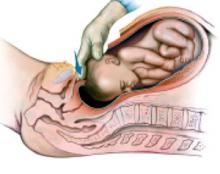
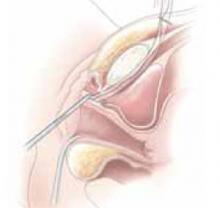
Complete the procedure. Once the surgery is completed, remove the retractor, hooking the bottom ring by inserting a finger into it and pulling it up and out of the incision (FIGURE 4C). Closing a cruciate incision is faster and requires less exposure than closing a mini-Pfannenstiel incision. Eliminate the possibility of postoperative wound hematoma or seroma formation by applying a vertical pressure dressing over the incision (FIGURE 4D). Remove the dressing 24 hours later.
FIGURE 4 Hysterectomy for the normal to moderately enlarged uterus
A. Exteriorize the uterus as much as possible with both upward assistance of the manipulator and uterine fundal elevation (using clamps lateral to the uterus, a heavy tenaculum, or a traction suture). Then conduct a standard hysterectomy.
FIGURE 4 Hysterectomy for the normal to moderately enlarged uterus
B. Separate the uterus from the vagina using the manipulator to guide the vaginal circumcision. (If the cervix is to be preserved, a supracervical amputation is performed instead.)
FIGURE 4 Hysterectomy for the normal to moderately enlarged uterus
C. After completing the surgery, remove the retractor from the incision.
FIGURE 4 Hysterectomy for the normal to moderately enlarged uterus
D. Apply a vertical pressure dressing over the incision.
Variations for abnormal uteri
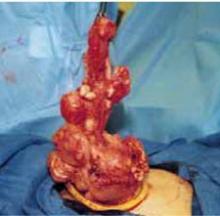
The large fibroid uterus: Begin with the dominant myoma. A large fibroid uterus can be easily removed with our minilaparotomy technique using 3 basic steps:
- Reduce size by selective myomectomy.
- Deliver the debulked uterus through the abdominal incision.
- Perform extracorporeal hysterectomy.
First, you must conduct a thorough assessment of the number, size, and location of the myomas. Begin the myomectomy on the largest tumor of those closest to the minilaparotomy incision. (Minimize bleeding by injecting diluted vasopressin subserosally prior to the procedure.)
Incise the uterine serosa, myometrium, and pseudocapsule of the myoma via scalpel or Bovie electrocautery until the whorly appearance of the myoma is apparent. Next, grasp the myoma with claw-toothed forceps to stabilize it and place it under traction. Then, using a combination of sharp and digital dissection, develop a plane of dissection between the fibroid and the myometrium (FIGURE 5A).
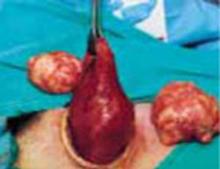
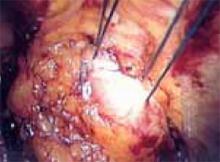
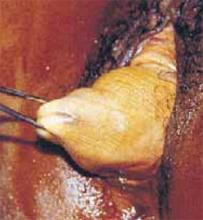
After securing the dominant myoma, deliver it through the abdominal incision. If the myoma is too large to be removed intact from the abdominal cavity, morcellate it using a scalpel or scissors (FIGURE 5B). Then continue systematic removal of the remaining myomas using the same approach. It is not necessary to remove all myomas—the goal of this process is merely to permit delivery of the uterine body for subsequent hysterectomy.
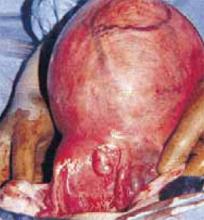
Once the uterus is debulked, deliver it through the abdominal incision. Hysterectomy then is easily completed (FIGURE 5C).
The ‘solid’ uterus: In situ supracervical hysterectomy and uterine morcellation.
Very large uteri are sometimes homogeneous and solid in nature, possessing few or no individual myomas. This so-called cannonball fibroid uterus is the most challenging type of uterus to remove. The selective-myomectomy approach cannot be used because of the potential for massive bleeding and the technical anatomical difficulties that arise when operating through such a small abdominal incision.
Instead, manage this type of uterus by performing a deliberate in situ supracervical hysterectomy through the minilaparotomy incision. At the end of this procedure, morcellate the amputated fibroid uterus.
Begin the surgery by dividing the upper uterine attachments. Regardless of uterine size, the origins of the round and adnexal ligaments will always be lateral to and within easy reach of a transverse minilaparotomy incision. (Access to these areas is the only factor that determines the feasibility of this procedure; uterine size is completely irrelevant.) We have found that these elongated ligaments are quite lax. Thus, in most cases it is relatively simple to navigate your index finger laterally and, using digital traction, elevate these structures into the minilaparotomy incision (FIGURE 6A). You can then clamp, cut, and suture the ligaments in the standard fashion in whatever sequence is most efficient.
Thanks to the retractor, minimal assistance is necessary during the surgery. You can create additional exposure by deflecting the uterus toward the opposite side of the pelvis using external abdominal pressure and the uterine manipulator.
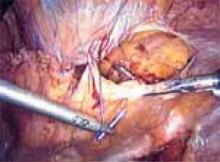
Once both round ligaments and adnexal pedicles are divided, dissect the bladder flap to expose the uterine arteries (inward pressure on the uterine manipulator provides helpful countertraction). Then clamp, divide, and ligate the uterine arteries (FIGURE 6B).
The uterus is now ready for supracervical amputation. Upward traction on the isthmus by means of a rubber tourniquet facilitates uterine division (FIGURE 6C). After the uterus is amputated, push it toward the upper abdomen to increase exposure for suturing of the cervical stump (if the cervix is preserved) or for cervical excision and vaginal cuff closure (when total hysterectomy is chosen).
Next, remove the uterine specimen by morcellation through the minilaparotomy incision. Using the Doyen ladder-shaped uterine morcellation technique (originally described in the early 1920s) grasp an area of the uterus and, alternating right and left, make deep but incomplete incisions on the uterus, creating a ladder shape.2 Because of its elasticity, the retractor can stretch quite significantly without tearing the edges of the abdominal incision (FIGURE 6D). This allows the easy exteriorization of uteri with diameters considerably larger than that of the retractor, mimicking the stretching of the perineum during the crowning of the fetal head.
When the surgery is complete, remove the retractor, close the minilaparotomy incision, and apply a vertical pressure dressing over the incision. Neither vaginal packing nor bladder catheterization is required.
FIGURE 5 Hysterectomy for the fibroid uterus
A. Develop a plane of dissection between the myoma and myometrium.
FIGURE 5 Hysterectomy for the fibroid uterus
B. Deliver the myoma through the incision; if it is too large to remove intact, morcellate it with a scalpel or scissors.
FIGURE 5 Hysterectomy for the fibroid uterus
C. After reducing the uterine size by selective myomectomy, deliver the debulked uterus through the abdominal incision. Then proceed with an extracorporeal total or subtotal hysterectomy.
A short learning curve
Since it uses conventional open techniques and traditional instrumentation, this method can be learned and mastered quickly.
We tend to think of this procedure as a transabdominal “vaginal” hysterectomy, since the average diameter of the minilaparotomy opening is approximately the same as the vaginal canal. Further, as in vaginal hysterectomy, only 1 portion of the uterus, adnexa, or ligaments must be exteriorized at a given time. Thus, this approach requires less general exposure but offers effective targeted exposure.
The technique also removes the need for frequent use of traumatic metal retractors, extensive bowel packing, and extended incision exposure. The benefits: diminished postoperative discomfort and bowel dysfunction.
High success rates. We have performed more than 100 minilaparotomy procedures using this technique in patients in whom vaginal hysterectomy was contraindicated. Uterine weight ranged from 80 g to 2,500 g. Mean operating time was 50 minutes. All patients were discharged within 36 hours. Mean return to work time was 12 days, and there have been no intraoperative or postoperative complications. All surgeries were successfully completed without laparoscopy or conversion to traditional laparotomy.
FIGURE 6 Hysterectomy for the large, solid, fibroid uterus
A. Draw the upper uterine attachments to the surgical field with finger traction.
FIGURE 6 Hysterectomy for the large, solid, fibroid uterus
B. Divide the round ligaments and proximal adnexal pedicles, then carry out division of the uterine vessels bilaterally.
FIGURE 6 Hysterectomy for the large, solid, fibroid uterus
C. After dividing both round ligaments, adnexal pedicles, and uterine vessels, place the uterine isthmus in traction with a rubber tourniquet and perform an in situ supracervical amputation.
FIGURE 6 Hysterectomy for the large, solid, fibroid uterus
D. After trachelectomy, the large uterine specimen is removed by morcellation. Notice that the retractor is able to stretch significantly, allowing the exteriorization of uteri with diameters considerably larger than that of the retractor.
Devices that simplify the procedure
Occasionally, hysterectomy using traditional clamp, division, and suture ligation can be tedious, frustrating, and time-consuming, especially when exposure is limited or difficult. Several devices developed for laparoscopic surgery can ease suture ligation and the division of blood vessels, ligaments, and tissue bundles during minilaparotomy hysterectomy. They include the Hem-o-lok ligating clip (Weck Closure Systems); the LigaSure Atlas, a vessel sealer-divider (Valleylab, Tyco Healthcare, Boulder, Colo); the ETS 45-Flex endoscopic linear cutter (Ethicon Endo-Surgery, Cincinnati, Ohio); and the PK bipolar cutting forceps (Gyrus Medical, Maple Grove, Minn).
Additional concerns
Is the incision too large? Fears that incisions over 5 cm might nullify minimally invasive surgery’s benefits have proven unfounded.
Laparoscopic procedures that use a 7-cm to 8-cm incision to introduce the hand into the abdomen, as well as those performed in conjunction with minilaparotomy, have consistently failed to identify a link between these combinations and morbidity or lengthy recovery. A “large” minilaparotomy incision is still superior to a standard abdominal hysterectomy in terms of convalescence, and it is significantly faster and more cost-effective than a prolonged laparoscopic or laparoscopic-assisted vaginal hysterectomy.3-10
Do any conditions contraindicate minilaparotomy? In patients with documented or strongly suspected severe pelvic conditions (for example, advanced endometriosis, pelvic inflammatory disease, bowel disease, or malignancy), preliminary laparoscopic evaluation to determine the pathologic condition’s severity and extent is strongly recommended.
If during this assessment you detect pathology that is not appropriate for laparoscopic surgery or minilaparotomy, perform a traditional laparotomy. If, however, this evaluation demonstrates that pelvic pathology is amenable to laparoscopic surgery, a laparoscopic hysterectomy or laparoscopic-assisted minilaparotomy hysterectomy is indicated.
Dr. Pelosi II reports that he is a consultant for Apple Medical Corporation. Dr. Pelosi III reports no affiliations or financial arrangements with any of the manufacturers of products mentioned in this article or their competitors.
1. Kustner O. Der suprasymphysare kruzschnitt, eine methode der coeliotomie bei wening umfanglichen affektionen der weiblichen beckenorgane. Monatsschr Geburtshilfe Gynakol. 1896;4:197-206.
2. Doyen E. Surgical Therapeutics and Operative Technique. Vol. III. Spencer-Browne H, translator. London, England: Bailliere, Tindal and Cox; 1920.
3. Benedetti Panicci P, Maneschi F, Cutillo G, et al. Surgery by minilaparotomy in benign gynecologic disease. Obstet Gynecol. 1996;87:456-459.
4. Benedetti Panicci P, Zullo MA, Casalino B, et al. Subcutaneous drainage versus no drainage after minilaparotomy in gynecologic benign conditions. Am J Obstet Gynecol. 2003;188:71-75.
5. Pelosi MA, II, Pelosi MA, III. The suprapubic cruciate incision for laparoscopic assisted microceliotomy. J Soc Laparoendosc Surg. 1997;1:269-272.
6. Pelosi MA, II, Pelosi MA, III. Self-retaining abdominal retractor for minilaparotomy. Obstet Gynecol. 2000;96:775-778.
7. Pelosi MA, II, Pelosi MA, III. Hand-assisted laparoscopy for complex hysterectomy. J Am Assoc Gynecol Laparosc. 1999;6:183-188.
8. Pelosi MA, II, Pelosi MA, III. Hand-assisted laparoscopic cholecystectomy at cesarean section. J Am Assoc Gynecol Laparosc. 1999;6:491-495.
9. Pelosi MA, II, Pelosi MA, III. Hand-assisted laparoscopy (handoscopy) for megamyomectomy: A case study. J Reprod Med. 2000;45:519-525.
10. Pelosi MA, II, Pelosi MA, III, Eim J. Hand-assisted laparoscopy for pelvic malignancy. J Laparoendosc Adv Surg Tech. 2000;10:143-150.
Although laparoscopic hysterectomy offers a minimally invasive alternative to laparotomy when vaginal hysterectomy is contraindicated, it has its drawbacks. Among them: the cost of expensive equipment, the long learning curve, and prolonged operating time.
We describe another alternative to open surgery that is comparable to laparoscopic hysterectomy in postoperative pain, cosmetic results, and time to return to normal activities. Our procedure—a redesigned minilaparotomy hysterectomy—relies on traditional open techniques and inexpensive novel instrumentation, making it significantly faster than laparoscopy and easy to perform and teach.
For patients who cannot undergo vaginal hysterectomy, this new modality offers an expeditious, minimal-access option. Gynecologists reluctant to relinquish the routine use of standard laparotomy may find this approach an appealing, less-invasive alternative.
Position, incision, and retraction are crucial to success
Our minilaparotomy hysterectomy is a systemized approach with elements derived from both open and laparoscopic surgery. Three preparatory components are involved:
- position
- incision
- retraction
All are critical to a successful hysterectomy, ensuring that the procedure never becomes a haphazard struggle through an improvised, scaled-down, conventional Pfannenstiel or vertical incision. Our approach also avoids cumbersome traditional laparotomy exposure maneuvers and positioning.
Position: Modified lithotomy. After regional or general anesthesia is given, position the patient in a modified lithotomy with both arms tucked as for laparoscopic surgery. Place the legs in boot-type stirrups, with no hip flexion and sufficient thigh abduction to expose the vagina.
Next, perform a thorough pelvic examination and place an indwelling, transurethral catheter. A sturdy, hinged uterine manipulator is of paramount importance for the hysterectomy, as it facilitates exposure of the adnexa as well as elevation/rotation of the uterus and the uterine attachments. We recommend the Pelosi Uterine Manipulator (Apple Medical Corporation, Marlboro, Mass) or its equivalent (FIGURE 1).
Standard minilaparotomy
The use of standard minilaparotomy—which is nothing more than a conventional laparotomy of limited length (3 cm to 6 cm), performed either transversely or vertically—has been confined to the surgical treatment of benign pelvic pathology of limited extent.
To generate sufficient exposure to work effectively, surgeons using the standard minilaparotomy have relied on the length of the abdominal incision and, secondarily, bowel packing and metal handheld or self-retaining fixed retraction systems. When exposure is difficult to achieve or maintain, however, routine surgical maneuvers become frustrating and time-consuming—unless the clinician uses extensive traction force, extends the incision length, or performs muscle-splitting. These alternatives often result in an uncomfortable, slow recovery typical of most laparotomies, thereby negating the primary goal of minimally invasive surgery.
Use of traditional minilaparotomy for hysterectomy has been reported only rarely. Hoffman et al1 found the procedure safe and effective in nonobese women in whom a vaginal approach was precluded. Benedetti Panicci et al2,3 also have used minilaparotomy successfully in benign gynecologic disease and hysterectomy.
The Kustner incision
Originally reported in 1896,4 this incision is avoided by most surgeons in favor of complete transverse or complete vertical incisions—largely due to difficulties with exposure, troublesome seroma formation, and wound complications secondary to increased fluid accumulation in the large dead space that results from wide dissection of the subcutaneous flap.
In the early 1990s, we realized the potential benefits of a scaled-down Kustner’s incision (2 cm to 5 cm) when assistance was needed via minilaparotomy during such laparoscopic-assisted procedures as uterine morcellation, tubal reanastomosis, and extensive uterine suture and reconstruction following complex laparoscopic myomectomy.5 As a substitute for laparoscopy and laparotomy, we then tried a minilaparotomy Kustner’s incision (3 cm to 5 cm) as the sole means of surgical access, assessment, and treatment for benign pelvic conditions.
Benefits of this incision. When a sturdy uterine manipulator was used to facilitate exposure of the adnexa and uterine elevation/rotation, we found this technique more effective than similar procedures using a scaled-down Pfannenstiel or Maylard incision. In addition, because the incision was small and the extent of subcutaneous dissection required to expose the rectus fascia in a vertical fashion was limited, there was no need for incision drainage. Nor was the procedure associated with seroma formation, as the full-sized Kustner’s incision had been.3 However, the minilaparotomy Kustner’s incision still suffered from limited surgical exposure.
Adding the retractor
It became clear that a soft, self-retaining abdominal retractor that is capable of creating a rapid, effective, nontraumatic, and predictable circular area of abdominal retraction would be helpful, particularly one that could be placed through the minilaparotomy Kustner’s incision.6 Once this retractor system was developed, using technology borrowed from hand-assisted laparoscopy,7-10 the minilaparotomy hysterectomy became a much simpler, more useful surgical option.
REFERENCES
1. Hoffman MS, Lynch CM. Minilaparotomy hysterectomy. Am J Obstet Gynecol. 1998;179:316-320.
2. Benedetti Panicci P, Maneschi F, Cutillo G, et al. Surgery by minilaparotomy in benign gynecologic disease. Obstet Gynecol. 1996;87:456-459.
3. Benedetti Panicci P, Zullo MA, Casalino B, et al. Subcutaneous drainage versus no drainage after minilaparotomy in gynecologic benign conditions. Am J Obstet Gynecol. 2003;188:71-75.
4. Kustner O. Der suprasymphysare kruzschnitt, eine methode der coeliotomie bei wening umfanglichen affektionen der weiblichen beckenorgane. Monatsschr Geburtshilfe Gynakol. 1896;4:197-206.
5. Pelosi MA, II, Pelosi MA, III. The suprapubic cruciate incision for laparoscopic assisted microceliotomy. J Soc Laparoendosc Surg. 1997;1:269-272.
6. Pelosi MA, II, Pelosi MA, III. Self-retaining abdominal retractor for minilaparotomy. Obstet Gynecol. 2000;96:775-778.
7. Pelosi MA, II, Pelosi MA, III. Hand-assisted laparoscopy for complex hysterectomy. J Am Assoc Gynecol Laparosc. 1999;6:183-188.
8. Pelosi MA, II, Pelosi MA, III. Hand-assisted laparoscopic cholecystectomy at cesarean section. J Am Assoc Gynecol Laparosc. 1999;6:491-495.
9. Pelosi MA, II, Pelosi MA, III. Hand-assisted laparoscopy (handoscopy) for megamyomectomy: A case study. J Reprod Med. 2000;45:519-525.
10. Pelosi MA, II, Pelosi MA, III, Eim J. Hand-assisted laparoscopy for pelvic malignancy. J Laparoendosc Adv Surg Tech. 2000;10:143-150.
Incision: Modified Kustner’s. Open the abdomen with a cruciate incision. Using a conventional scalpel and the Bovie device, make a 2.5-cm to 5-cm transverse incision through the skin and subcutaneous fat until you reach the anterior rectus fascia (FIGURE 2A). Clear the fat from the midline superiorly and inferiorly to expose approximately 5 cm to 6 cm of fascia in the vertical axis. Then incise the anterior rectus fascia in a vertical direction through the full length of the cleared area (FIGURE 2B).
Retract the rectus muscles from the midline, exposing the transversalis fascia and the underlying peritoneum. Enter the peritoneum digitally or with scissors above the level of the bladder dome, incising vertically until the entrance extends the full length of the fascial incision (FIGURE 2C).
This modified Kustner’s incision is essentially a vertical midline incision in its deeper layers.1 The rapid surgical dissection of the fascia and rectus muscles and the intraperitoneal entry are relatively bloodless. This approach yields a surgical exposure superior to that of a small Pfannenstiel or Maylard incision.
Note that, in some patients, a vertical incision can be selected if there is a prior vertical incision or if the perioperative workup suggests a malignancy that may require a later extension of the original minilaparotomy incision.
Retraction: Soft, sleeve-type, self-retaining abdominal retractor. This device consists of a flexible plastic inner ring and a firmer outer ring connected by a soft plastic sleeve (FIGURE 3A). Two models are available: the Mobius (Apple Medical Corporation) and the Protractor (Weck Closure Systems, Research Triangle Park, NC).
Squeeze the inner ring into the peritoneal cavity through the minilaparotomy incision, allowing it to spring open against the parietal peritoneum. Conduct a digital assessment to ensure that no viscera are trapped by elevating the outer ring. Next, roll the outer ring onto the sleeve, collecting excess length, until it sits firmly against the skin (FIGURES 3B and 3C). The result, when there is adequate tension within the sleeve, is a circular area of retraction offering excellent exposure of the pelvis. Note that during surgery you may need to adjust the outer ring if the sleeve loosens.
The soft, self-retaining abdominal retractor offers several advantages over traditional abdominal retraction:
- Atraumatic retraction. This device distributes retraction force evenly around the entire incision. Because standard retractors concentrate retraction force at only a few points, they often lead to tissue trauma, nerve damage, bruising, and postoperative pain.
- Incision protection. The retractor’s flexible material lines the incision, protecting the wound’s edges from contamination and potential implantation of malignant cells.
- Improved access. Because the continuous retraction force is delivered more effectively to the incision, exposure is maximized. As a result, the need for intensive surgical assistance is dramatically reduced.
- Adjustable height. The retractor’s design lets it adapt to wounds of varying depth—a feature that makes it ideal for obese patients. The device compresses the patient’s skin and peritoneum between the external and internal rings, keeping the full thickness of the abdominal incision constant throughout the surgery.
- Cost-effectiveness. The device, which costs under $100, is simple and fast to set up. In our experience, placement takes approximately 2 minutes; this compares favorably with table-mounted or self-retaining rigid retraction systems, which may require significant capital expenditures (cost may run in the thousands), repair costs, and complicated set-ups.
FIGURE 1 Hinged uterine manipulator
A sturdy hinged uterine manipulator facilitates exposure of the adnexa as well as elevation/rotation of the uterus.
FIGURE 2 Cruciate incision
A. Make a transverse incision suprapubically through the skin and the subcutaneous fat to reach the anterior rectus fascia.
FIGURE 2 Cruciate incision
B. Clear the fat from the midline to expose the rectus fascia in the vertical axis, then incise the fascia in a vertical direction through the full length of the previously cleared area. The rectus muscles are retracted, thereby exposing the peritoneum.
FIGURE 2 Cruciate incision
C. Incise the peritoneum vertically until it extends the full length of the fascial incision.
FIGURE 3 Soft, sleeve-type, self-retaining abdominal retractor
A. At left, the Protractor (Weck Closure Systems); at right, the Mobius (Apple Medical Corporation).
FIGURE 3 Soft, sleeve-type, self-retaining abdominal retractor
B. After inserting the inner ring into the peritoneal cavity, twist the outer ring downward until it inverts and rests snuggly against the skin.
FIGURE 3 Soft, sleeve-type, self-retaining abdominal retractor
C. An atraumatic, circular, self-retaining area of retraction is created.
Standard technique: Exteriorize the uterus; divide uterine attachments, vessels
Assess the anatomy. Using your index finger and the uterine manipulator to rotate and flex the uterus, carefully assess the uterus, adnexa, and pelvis, noting the location of the ureters. Determine the extent of any unexpected pelvic pathology or adhesions, using traditional small retractors or gentle packing to gain additional exposure. Perform any adhesiolysis that is necessary.
Exteriorize the uterus. Next, bring the uterus and the adnexa above the abdominal wall in order to perform as much of the hysterectomy extracorporeally as possible. Pass the uterus or adnexa through the incision with the upward assistance of the uterine manipulator, then divide the upper uterine attachments (FIGURE 4A).
Increase exposure. You can achieve additional uterine elevation and targeted exposure in several ways. For example, a strong traction suture can be placed in the uterine fundus, left long, and secured with a clamp. To achieve uterine elevation, place long clamps lateral to the corpus. Another effective approach is to place a heavy tenaculum on the uterine fundus.
When lateral exposure is limited, divide the proximal adnexal pedicles and round ligaments to begin the operation, and remove the adnexa separately following the completion of the hysterectomy.
Divide the uterine vessels through the small incision using clamping, division, and ligation. Unless you intend to preserve the cervix, mobilize the bladder to the level of the anterior vaginal fornix. Inward pressure on the uterine manipulator provides additional elevation of the lower uterine vasculature and the cardinal and uterosacral ligaments as these structures are ligated and divided. Amputate the uterine specimen from the vaginal cuff using the uterine manipulator to guide the vaginal circumcision (FIGURE 4B). Close the vaginal cuff using standard closure.
If the cervix is to be preserved, amputate the uterus supracervically following division of the uterine vessels. Then suture the cervical stump in the traditional fashion. Upward elevation of the cervix using the uterine manipulator expedites this step.
Complete the procedure. Once the surgery is completed, remove the retractor, hooking the bottom ring by inserting a finger into it and pulling it up and out of the incision (FIGURE 4C). Closing a cruciate incision is faster and requires less exposure than closing a mini-Pfannenstiel incision. Eliminate the possibility of postoperative wound hematoma or seroma formation by applying a vertical pressure dressing over the incision (FIGURE 4D). Remove the dressing 24 hours later.
FIGURE 4 Hysterectomy for the normal to moderately enlarged uterus
A. Exteriorize the uterus as much as possible with both upward assistance of the manipulator and uterine fundal elevation (using clamps lateral to the uterus, a heavy tenaculum, or a traction suture). Then conduct a standard hysterectomy.
FIGURE 4 Hysterectomy for the normal to moderately enlarged uterus
B. Separate the uterus from the vagina using the manipulator to guide the vaginal circumcision. (If the cervix is to be preserved, a supracervical amputation is performed instead.)
FIGURE 4 Hysterectomy for the normal to moderately enlarged uterus
C. After completing the surgery, remove the retractor from the incision.
FIGURE 4 Hysterectomy for the normal to moderately enlarged uterus
D. Apply a vertical pressure dressing over the incision.
Variations for abnormal uteri
The large fibroid uterus: Begin with the dominant myoma. A large fibroid uterus can be easily removed with our minilaparotomy technique using 3 basic steps:
- Reduce size by selective myomectomy.
- Deliver the debulked uterus through the abdominal incision.
- Perform extracorporeal hysterectomy.
First, you must conduct a thorough assessment of the number, size, and location of the myomas. Begin the myomectomy on the largest tumor of those closest to the minilaparotomy incision. (Minimize bleeding by injecting diluted vasopressin subserosally prior to the procedure.)
Incise the uterine serosa, myometrium, and pseudocapsule of the myoma via scalpel or Bovie electrocautery until the whorly appearance of the myoma is apparent. Next, grasp the myoma with claw-toothed forceps to stabilize it and place it under traction. Then, using a combination of sharp and digital dissection, develop a plane of dissection between the fibroid and the myometrium (FIGURE 5A).
After securing the dominant myoma, deliver it through the abdominal incision. If the myoma is too large to be removed intact from the abdominal cavity, morcellate it using a scalpel or scissors (FIGURE 5B). Then continue systematic removal of the remaining myomas using the same approach. It is not necessary to remove all myomas—the goal of this process is merely to permit delivery of the uterine body for subsequent hysterectomy.
Once the uterus is debulked, deliver it through the abdominal incision. Hysterectomy then is easily completed (FIGURE 5C).
The ‘solid’ uterus: In situ supracervical hysterectomy and uterine morcellation.
Very large uteri are sometimes homogeneous and solid in nature, possessing few or no individual myomas. This so-called cannonball fibroid uterus is the most challenging type of uterus to remove. The selective-myomectomy approach cannot be used because of the potential for massive bleeding and the technical anatomical difficulties that arise when operating through such a small abdominal incision.
Instead, manage this type of uterus by performing a deliberate in situ supracervical hysterectomy through the minilaparotomy incision. At the end of this procedure, morcellate the amputated fibroid uterus.
Begin the surgery by dividing the upper uterine attachments. Regardless of uterine size, the origins of the round and adnexal ligaments will always be lateral to and within easy reach of a transverse minilaparotomy incision. (Access to these areas is the only factor that determines the feasibility of this procedure; uterine size is completely irrelevant.) We have found that these elongated ligaments are quite lax. Thus, in most cases it is relatively simple to navigate your index finger laterally and, using digital traction, elevate these structures into the minilaparotomy incision (FIGURE 6A). You can then clamp, cut, and suture the ligaments in the standard fashion in whatever sequence is most efficient.
Thanks to the retractor, minimal assistance is necessary during the surgery. You can create additional exposure by deflecting the uterus toward the opposite side of the pelvis using external abdominal pressure and the uterine manipulator.
Once both round ligaments and adnexal pedicles are divided, dissect the bladder flap to expose the uterine arteries (inward pressure on the uterine manipulator provides helpful countertraction). Then clamp, divide, and ligate the uterine arteries (FIGURE 6B).
The uterus is now ready for supracervical amputation. Upward traction on the isthmus by means of a rubber tourniquet facilitates uterine division (FIGURE 6C). After the uterus is amputated, push it toward the upper abdomen to increase exposure for suturing of the cervical stump (if the cervix is preserved) or for cervical excision and vaginal cuff closure (when total hysterectomy is chosen).
Next, remove the uterine specimen by morcellation through the minilaparotomy incision. Using the Doyen ladder-shaped uterine morcellation technique (originally described in the early 1920s) grasp an area of the uterus and, alternating right and left, make deep but incomplete incisions on the uterus, creating a ladder shape.2 Because of its elasticity, the retractor can stretch quite significantly without tearing the edges of the abdominal incision (FIGURE 6D). This allows the easy exteriorization of uteri with diameters considerably larger than that of the retractor, mimicking the stretching of the perineum during the crowning of the fetal head.
When the surgery is complete, remove the retractor, close the minilaparotomy incision, and apply a vertical pressure dressing over the incision. Neither vaginal packing nor bladder catheterization is required.
FIGURE 5 Hysterectomy for the fibroid uterus
A. Develop a plane of dissection between the myoma and myometrium.
FIGURE 5 Hysterectomy for the fibroid uterus
B. Deliver the myoma through the incision; if it is too large to remove intact, morcellate it with a scalpel or scissors.
FIGURE 5 Hysterectomy for the fibroid uterus
C. After reducing the uterine size by selective myomectomy, deliver the debulked uterus through the abdominal incision. Then proceed with an extracorporeal total or subtotal hysterectomy.
A short learning curve
Since it uses conventional open techniques and traditional instrumentation, this method can be learned and mastered quickly.
We tend to think of this procedure as a transabdominal “vaginal” hysterectomy, since the average diameter of the minilaparotomy opening is approximately the same as the vaginal canal. Further, as in vaginal hysterectomy, only 1 portion of the uterus, adnexa, or ligaments must be exteriorized at a given time. Thus, this approach requires less general exposure but offers effective targeted exposure.
The technique also removes the need for frequent use of traumatic metal retractors, extensive bowel packing, and extended incision exposure. The benefits: diminished postoperative discomfort and bowel dysfunction.
High success rates. We have performed more than 100 minilaparotomy procedures using this technique in patients in whom vaginal hysterectomy was contraindicated. Uterine weight ranged from 80 g to 2,500 g. Mean operating time was 50 minutes. All patients were discharged within 36 hours. Mean return to work time was 12 days, and there have been no intraoperative or postoperative complications. All surgeries were successfully completed without laparoscopy or conversion to traditional laparotomy.
FIGURE 6 Hysterectomy for the large, solid, fibroid uterus
A. Draw the upper uterine attachments to the surgical field with finger traction.
FIGURE 6 Hysterectomy for the large, solid, fibroid uterus
B. Divide the round ligaments and proximal adnexal pedicles, then carry out division of the uterine vessels bilaterally.
FIGURE 6 Hysterectomy for the large, solid, fibroid uterus
C. After dividing both round ligaments, adnexal pedicles, and uterine vessels, place the uterine isthmus in traction with a rubber tourniquet and perform an in situ supracervical amputation.
FIGURE 6 Hysterectomy for the large, solid, fibroid uterus
D. After trachelectomy, the large uterine specimen is removed by morcellation. Notice that the retractor is able to stretch significantly, allowing the exteriorization of uteri with diameters considerably larger than that of the retractor.
Devices that simplify the procedure
Occasionally, hysterectomy using traditional clamp, division, and suture ligation can be tedious, frustrating, and time-consuming, especially when exposure is limited or difficult. Several devices developed for laparoscopic surgery can ease suture ligation and the division of blood vessels, ligaments, and tissue bundles during minilaparotomy hysterectomy. They include the Hem-o-lok ligating clip (Weck Closure Systems); the LigaSure Atlas, a vessel sealer-divider (Valleylab, Tyco Healthcare, Boulder, Colo); the ETS 45-Flex endoscopic linear cutter (Ethicon Endo-Surgery, Cincinnati, Ohio); and the PK bipolar cutting forceps (Gyrus Medical, Maple Grove, Minn).
Additional concerns
Is the incision too large? Fears that incisions over 5 cm might nullify minimally invasive surgery’s benefits have proven unfounded.
Laparoscopic procedures that use a 7-cm to 8-cm incision to introduce the hand into the abdomen, as well as those performed in conjunction with minilaparotomy, have consistently failed to identify a link between these combinations and morbidity or lengthy recovery. A “large” minilaparotomy incision is still superior to a standard abdominal hysterectomy in terms of convalescence, and it is significantly faster and more cost-effective than a prolonged laparoscopic or laparoscopic-assisted vaginal hysterectomy.3-10
Do any conditions contraindicate minilaparotomy? In patients with documented or strongly suspected severe pelvic conditions (for example, advanced endometriosis, pelvic inflammatory disease, bowel disease, or malignancy), preliminary laparoscopic evaluation to determine the pathologic condition’s severity and extent is strongly recommended.
If during this assessment you detect pathology that is not appropriate for laparoscopic surgery or minilaparotomy, perform a traditional laparotomy. If, however, this evaluation demonstrates that pelvic pathology is amenable to laparoscopic surgery, a laparoscopic hysterectomy or laparoscopic-assisted minilaparotomy hysterectomy is indicated.
Dr. Pelosi II reports that he is a consultant for Apple Medical Corporation. Dr. Pelosi III reports no affiliations or financial arrangements with any of the manufacturers of products mentioned in this article or their competitors.
Although laparoscopic hysterectomy offers a minimally invasive alternative to laparotomy when vaginal hysterectomy is contraindicated, it has its drawbacks. Among them: the cost of expensive equipment, the long learning curve, and prolonged operating time.
We describe another alternative to open surgery that is comparable to laparoscopic hysterectomy in postoperative pain, cosmetic results, and time to return to normal activities. Our procedure—a redesigned minilaparotomy hysterectomy—relies on traditional open techniques and inexpensive novel instrumentation, making it significantly faster than laparoscopy and easy to perform and teach.
For patients who cannot undergo vaginal hysterectomy, this new modality offers an expeditious, minimal-access option. Gynecologists reluctant to relinquish the routine use of standard laparotomy may find this approach an appealing, less-invasive alternative.
Position, incision, and retraction are crucial to success
Our minilaparotomy hysterectomy is a systemized approach with elements derived from both open and laparoscopic surgery. Three preparatory components are involved:
- position
- incision
- retraction
All are critical to a successful hysterectomy, ensuring that the procedure never becomes a haphazard struggle through an improvised, scaled-down, conventional Pfannenstiel or vertical incision. Our approach also avoids cumbersome traditional laparotomy exposure maneuvers and positioning.
Position: Modified lithotomy. After regional or general anesthesia is given, position the patient in a modified lithotomy with both arms tucked as for laparoscopic surgery. Place the legs in boot-type stirrups, with no hip flexion and sufficient thigh abduction to expose the vagina.
Next, perform a thorough pelvic examination and place an indwelling, transurethral catheter. A sturdy, hinged uterine manipulator is of paramount importance for the hysterectomy, as it facilitates exposure of the adnexa as well as elevation/rotation of the uterus and the uterine attachments. We recommend the Pelosi Uterine Manipulator (Apple Medical Corporation, Marlboro, Mass) or its equivalent (FIGURE 1).
Standard minilaparotomy
The use of standard minilaparotomy—which is nothing more than a conventional laparotomy of limited length (3 cm to 6 cm), performed either transversely or vertically—has been confined to the surgical treatment of benign pelvic pathology of limited extent.
To generate sufficient exposure to work effectively, surgeons using the standard minilaparotomy have relied on the length of the abdominal incision and, secondarily, bowel packing and metal handheld or self-retaining fixed retraction systems. When exposure is difficult to achieve or maintain, however, routine surgical maneuvers become frustrating and time-consuming—unless the clinician uses extensive traction force, extends the incision length, or performs muscle-splitting. These alternatives often result in an uncomfortable, slow recovery typical of most laparotomies, thereby negating the primary goal of minimally invasive surgery.
Use of traditional minilaparotomy for hysterectomy has been reported only rarely. Hoffman et al1 found the procedure safe and effective in nonobese women in whom a vaginal approach was precluded. Benedetti Panicci et al2,3 also have used minilaparotomy successfully in benign gynecologic disease and hysterectomy.
The Kustner incision
Originally reported in 1896,4 this incision is avoided by most surgeons in favor of complete transverse or complete vertical incisions—largely due to difficulties with exposure, troublesome seroma formation, and wound complications secondary to increased fluid accumulation in the large dead space that results from wide dissection of the subcutaneous flap.
In the early 1990s, we realized the potential benefits of a scaled-down Kustner’s incision (2 cm to 5 cm) when assistance was needed via minilaparotomy during such laparoscopic-assisted procedures as uterine morcellation, tubal reanastomosis, and extensive uterine suture and reconstruction following complex laparoscopic myomectomy.5 As a substitute for laparoscopy and laparotomy, we then tried a minilaparotomy Kustner’s incision (3 cm to 5 cm) as the sole means of surgical access, assessment, and treatment for benign pelvic conditions.
Benefits of this incision. When a sturdy uterine manipulator was used to facilitate exposure of the adnexa and uterine elevation/rotation, we found this technique more effective than similar procedures using a scaled-down Pfannenstiel or Maylard incision. In addition, because the incision was small and the extent of subcutaneous dissection required to expose the rectus fascia in a vertical fashion was limited, there was no need for incision drainage. Nor was the procedure associated with seroma formation, as the full-sized Kustner’s incision had been.3 However, the minilaparotomy Kustner’s incision still suffered from limited surgical exposure.
Adding the retractor
It became clear that a soft, self-retaining abdominal retractor that is capable of creating a rapid, effective, nontraumatic, and predictable circular area of abdominal retraction would be helpful, particularly one that could be placed through the minilaparotomy Kustner’s incision.6 Once this retractor system was developed, using technology borrowed from hand-assisted laparoscopy,7-10 the minilaparotomy hysterectomy became a much simpler, more useful surgical option.
REFERENCES
1. Hoffman MS, Lynch CM. Minilaparotomy hysterectomy. Am J Obstet Gynecol. 1998;179:316-320.
2. Benedetti Panicci P, Maneschi F, Cutillo G, et al. Surgery by minilaparotomy in benign gynecologic disease. Obstet Gynecol. 1996;87:456-459.
3. Benedetti Panicci P, Zullo MA, Casalino B, et al. Subcutaneous drainage versus no drainage after minilaparotomy in gynecologic benign conditions. Am J Obstet Gynecol. 2003;188:71-75.
4. Kustner O. Der suprasymphysare kruzschnitt, eine methode der coeliotomie bei wening umfanglichen affektionen der weiblichen beckenorgane. Monatsschr Geburtshilfe Gynakol. 1896;4:197-206.
5. Pelosi MA, II, Pelosi MA, III. The suprapubic cruciate incision for laparoscopic assisted microceliotomy. J Soc Laparoendosc Surg. 1997;1:269-272.
6. Pelosi MA, II, Pelosi MA, III. Self-retaining abdominal retractor for minilaparotomy. Obstet Gynecol. 2000;96:775-778.
7. Pelosi MA, II, Pelosi MA, III. Hand-assisted laparoscopy for complex hysterectomy. J Am Assoc Gynecol Laparosc. 1999;6:183-188.
8. Pelosi MA, II, Pelosi MA, III. Hand-assisted laparoscopic cholecystectomy at cesarean section. J Am Assoc Gynecol Laparosc. 1999;6:491-495.
9. Pelosi MA, II, Pelosi MA, III. Hand-assisted laparoscopy (handoscopy) for megamyomectomy: A case study. J Reprod Med. 2000;45:519-525.
10. Pelosi MA, II, Pelosi MA, III, Eim J. Hand-assisted laparoscopy for pelvic malignancy. J Laparoendosc Adv Surg Tech. 2000;10:143-150.
Incision: Modified Kustner’s. Open the abdomen with a cruciate incision. Using a conventional scalpel and the Bovie device, make a 2.5-cm to 5-cm transverse incision through the skin and subcutaneous fat until you reach the anterior rectus fascia (FIGURE 2A). Clear the fat from the midline superiorly and inferiorly to expose approximately 5 cm to 6 cm of fascia in the vertical axis. Then incise the anterior rectus fascia in a vertical direction through the full length of the cleared area (FIGURE 2B).
Retract the rectus muscles from the midline, exposing the transversalis fascia and the underlying peritoneum. Enter the peritoneum digitally or with scissors above the level of the bladder dome, incising vertically until the entrance extends the full length of the fascial incision (FIGURE 2C).
This modified Kustner’s incision is essentially a vertical midline incision in its deeper layers.1 The rapid surgical dissection of the fascia and rectus muscles and the intraperitoneal entry are relatively bloodless. This approach yields a surgical exposure superior to that of a small Pfannenstiel or Maylard incision.
Note that, in some patients, a vertical incision can be selected if there is a prior vertical incision or if the perioperative workup suggests a malignancy that may require a later extension of the original minilaparotomy incision.
Retraction: Soft, sleeve-type, self-retaining abdominal retractor. This device consists of a flexible plastic inner ring and a firmer outer ring connected by a soft plastic sleeve (FIGURE 3A). Two models are available: the Mobius (Apple Medical Corporation) and the Protractor (Weck Closure Systems, Research Triangle Park, NC).
Squeeze the inner ring into the peritoneal cavity through the minilaparotomy incision, allowing it to spring open against the parietal peritoneum. Conduct a digital assessment to ensure that no viscera are trapped by elevating the outer ring. Next, roll the outer ring onto the sleeve, collecting excess length, until it sits firmly against the skin (FIGURES 3B and 3C). The result, when there is adequate tension within the sleeve, is a circular area of retraction offering excellent exposure of the pelvis. Note that during surgery you may need to adjust the outer ring if the sleeve loosens.
The soft, self-retaining abdominal retractor offers several advantages over traditional abdominal retraction:
- Atraumatic retraction. This device distributes retraction force evenly around the entire incision. Because standard retractors concentrate retraction force at only a few points, they often lead to tissue trauma, nerve damage, bruising, and postoperative pain.
- Incision protection. The retractor’s flexible material lines the incision, protecting the wound’s edges from contamination and potential implantation of malignant cells.
- Improved access. Because the continuous retraction force is delivered more effectively to the incision, exposure is maximized. As a result, the need for intensive surgical assistance is dramatically reduced.
- Adjustable height. The retractor’s design lets it adapt to wounds of varying depth—a feature that makes it ideal for obese patients. The device compresses the patient’s skin and peritoneum between the external and internal rings, keeping the full thickness of the abdominal incision constant throughout the surgery.
- Cost-effectiveness. The device, which costs under $100, is simple and fast to set up. In our experience, placement takes approximately 2 minutes; this compares favorably with table-mounted or self-retaining rigid retraction systems, which may require significant capital expenditures (cost may run in the thousands), repair costs, and complicated set-ups.
FIGURE 1 Hinged uterine manipulator
A sturdy hinged uterine manipulator facilitates exposure of the adnexa as well as elevation/rotation of the uterus.
FIGURE 2 Cruciate incision
A. Make a transverse incision suprapubically through the skin and the subcutaneous fat to reach the anterior rectus fascia.
FIGURE 2 Cruciate incision
B. Clear the fat from the midline to expose the rectus fascia in the vertical axis, then incise the fascia in a vertical direction through the full length of the previously cleared area. The rectus muscles are retracted, thereby exposing the peritoneum.
FIGURE 2 Cruciate incision
C. Incise the peritoneum vertically until it extends the full length of the fascial incision.
FIGURE 3 Soft, sleeve-type, self-retaining abdominal retractor
A. At left, the Protractor (Weck Closure Systems); at right, the Mobius (Apple Medical Corporation).
FIGURE 3 Soft, sleeve-type, self-retaining abdominal retractor
B. After inserting the inner ring into the peritoneal cavity, twist the outer ring downward until it inverts and rests snuggly against the skin.
FIGURE 3 Soft, sleeve-type, self-retaining abdominal retractor
C. An atraumatic, circular, self-retaining area of retraction is created.
Standard technique: Exteriorize the uterus; divide uterine attachments, vessels
Assess the anatomy. Using your index finger and the uterine manipulator to rotate and flex the uterus, carefully assess the uterus, adnexa, and pelvis, noting the location of the ureters. Determine the extent of any unexpected pelvic pathology or adhesions, using traditional small retractors or gentle packing to gain additional exposure. Perform any adhesiolysis that is necessary.
Exteriorize the uterus. Next, bring the uterus and the adnexa above the abdominal wall in order to perform as much of the hysterectomy extracorporeally as possible. Pass the uterus or adnexa through the incision with the upward assistance of the uterine manipulator, then divide the upper uterine attachments (FIGURE 4A).
Increase exposure. You can achieve additional uterine elevation and targeted exposure in several ways. For example, a strong traction suture can be placed in the uterine fundus, left long, and secured with a clamp. To achieve uterine elevation, place long clamps lateral to the corpus. Another effective approach is to place a heavy tenaculum on the uterine fundus.
When lateral exposure is limited, divide the proximal adnexal pedicles and round ligaments to begin the operation, and remove the adnexa separately following the completion of the hysterectomy.
Divide the uterine vessels through the small incision using clamping, division, and ligation. Unless you intend to preserve the cervix, mobilize the bladder to the level of the anterior vaginal fornix. Inward pressure on the uterine manipulator provides additional elevation of the lower uterine vasculature and the cardinal and uterosacral ligaments as these structures are ligated and divided. Amputate the uterine specimen from the vaginal cuff using the uterine manipulator to guide the vaginal circumcision (FIGURE 4B). Close the vaginal cuff using standard closure.
If the cervix is to be preserved, amputate the uterus supracervically following division of the uterine vessels. Then suture the cervical stump in the traditional fashion. Upward elevation of the cervix using the uterine manipulator expedites this step.
Complete the procedure. Once the surgery is completed, remove the retractor, hooking the bottom ring by inserting a finger into it and pulling it up and out of the incision (FIGURE 4C). Closing a cruciate incision is faster and requires less exposure than closing a mini-Pfannenstiel incision. Eliminate the possibility of postoperative wound hematoma or seroma formation by applying a vertical pressure dressing over the incision (FIGURE 4D). Remove the dressing 24 hours later.
FIGURE 4 Hysterectomy for the normal to moderately enlarged uterus
A. Exteriorize the uterus as much as possible with both upward assistance of the manipulator and uterine fundal elevation (using clamps lateral to the uterus, a heavy tenaculum, or a traction suture). Then conduct a standard hysterectomy.
FIGURE 4 Hysterectomy for the normal to moderately enlarged uterus
B. Separate the uterus from the vagina using the manipulator to guide the vaginal circumcision. (If the cervix is to be preserved, a supracervical amputation is performed instead.)
FIGURE 4 Hysterectomy for the normal to moderately enlarged uterus
C. After completing the surgery, remove the retractor from the incision.
FIGURE 4 Hysterectomy for the normal to moderately enlarged uterus
D. Apply a vertical pressure dressing over the incision.
Variations for abnormal uteri
The large fibroid uterus: Begin with the dominant myoma. A large fibroid uterus can be easily removed with our minilaparotomy technique using 3 basic steps:
- Reduce size by selective myomectomy.
- Deliver the debulked uterus through the abdominal incision.
- Perform extracorporeal hysterectomy.
First, you must conduct a thorough assessment of the number, size, and location of the myomas. Begin the myomectomy on the largest tumor of those closest to the minilaparotomy incision. (Minimize bleeding by injecting diluted vasopressin subserosally prior to the procedure.)
Incise the uterine serosa, myometrium, and pseudocapsule of the myoma via scalpel or Bovie electrocautery until the whorly appearance of the myoma is apparent. Next, grasp the myoma with claw-toothed forceps to stabilize it and place it under traction. Then, using a combination of sharp and digital dissection, develop a plane of dissection between the fibroid and the myometrium (FIGURE 5A).
After securing the dominant myoma, deliver it through the abdominal incision. If the myoma is too large to be removed intact from the abdominal cavity, morcellate it using a scalpel or scissors (FIGURE 5B). Then continue systematic removal of the remaining myomas using the same approach. It is not necessary to remove all myomas—the goal of this process is merely to permit delivery of the uterine body for subsequent hysterectomy.
Once the uterus is debulked, deliver it through the abdominal incision. Hysterectomy then is easily completed (FIGURE 5C).
The ‘solid’ uterus: In situ supracervical hysterectomy and uterine morcellation.
Very large uteri are sometimes homogeneous and solid in nature, possessing few or no individual myomas. This so-called cannonball fibroid uterus is the most challenging type of uterus to remove. The selective-myomectomy approach cannot be used because of the potential for massive bleeding and the technical anatomical difficulties that arise when operating through such a small abdominal incision.
Instead, manage this type of uterus by performing a deliberate in situ supracervical hysterectomy through the minilaparotomy incision. At the end of this procedure, morcellate the amputated fibroid uterus.
Begin the surgery by dividing the upper uterine attachments. Regardless of uterine size, the origins of the round and adnexal ligaments will always be lateral to and within easy reach of a transverse minilaparotomy incision. (Access to these areas is the only factor that determines the feasibility of this procedure; uterine size is completely irrelevant.) We have found that these elongated ligaments are quite lax. Thus, in most cases it is relatively simple to navigate your index finger laterally and, using digital traction, elevate these structures into the minilaparotomy incision (FIGURE 6A). You can then clamp, cut, and suture the ligaments in the standard fashion in whatever sequence is most efficient.
Thanks to the retractor, minimal assistance is necessary during the surgery. You can create additional exposure by deflecting the uterus toward the opposite side of the pelvis using external abdominal pressure and the uterine manipulator.
Once both round ligaments and adnexal pedicles are divided, dissect the bladder flap to expose the uterine arteries (inward pressure on the uterine manipulator provides helpful countertraction). Then clamp, divide, and ligate the uterine arteries (FIGURE 6B).
The uterus is now ready for supracervical amputation. Upward traction on the isthmus by means of a rubber tourniquet facilitates uterine division (FIGURE 6C). After the uterus is amputated, push it toward the upper abdomen to increase exposure for suturing of the cervical stump (if the cervix is preserved) or for cervical excision and vaginal cuff closure (when total hysterectomy is chosen).
Next, remove the uterine specimen by morcellation through the minilaparotomy incision. Using the Doyen ladder-shaped uterine morcellation technique (originally described in the early 1920s) grasp an area of the uterus and, alternating right and left, make deep but incomplete incisions on the uterus, creating a ladder shape.2 Because of its elasticity, the retractor can stretch quite significantly without tearing the edges of the abdominal incision (FIGURE 6D). This allows the easy exteriorization of uteri with diameters considerably larger than that of the retractor, mimicking the stretching of the perineum during the crowning of the fetal head.
When the surgery is complete, remove the retractor, close the minilaparotomy incision, and apply a vertical pressure dressing over the incision. Neither vaginal packing nor bladder catheterization is required.
FIGURE 5 Hysterectomy for the fibroid uterus
A. Develop a plane of dissection between the myoma and myometrium.
FIGURE 5 Hysterectomy for the fibroid uterus
B. Deliver the myoma through the incision; if it is too large to remove intact, morcellate it with a scalpel or scissors.
FIGURE 5 Hysterectomy for the fibroid uterus
C. After reducing the uterine size by selective myomectomy, deliver the debulked uterus through the abdominal incision. Then proceed with an extracorporeal total or subtotal hysterectomy.
A short learning curve
Since it uses conventional open techniques and traditional instrumentation, this method can be learned and mastered quickly.
We tend to think of this procedure as a transabdominal “vaginal” hysterectomy, since the average diameter of the minilaparotomy opening is approximately the same as the vaginal canal. Further, as in vaginal hysterectomy, only 1 portion of the uterus, adnexa, or ligaments must be exteriorized at a given time. Thus, this approach requires less general exposure but offers effective targeted exposure.
The technique also removes the need for frequent use of traumatic metal retractors, extensive bowel packing, and extended incision exposure. The benefits: diminished postoperative discomfort and bowel dysfunction.
High success rates. We have performed more than 100 minilaparotomy procedures using this technique in patients in whom vaginal hysterectomy was contraindicated. Uterine weight ranged from 80 g to 2,500 g. Mean operating time was 50 minutes. All patients were discharged within 36 hours. Mean return to work time was 12 days, and there have been no intraoperative or postoperative complications. All surgeries were successfully completed without laparoscopy or conversion to traditional laparotomy.
FIGURE 6 Hysterectomy for the large, solid, fibroid uterus
A. Draw the upper uterine attachments to the surgical field with finger traction.
FIGURE 6 Hysterectomy for the large, solid, fibroid uterus
B. Divide the round ligaments and proximal adnexal pedicles, then carry out division of the uterine vessels bilaterally.
FIGURE 6 Hysterectomy for the large, solid, fibroid uterus
C. After dividing both round ligaments, adnexal pedicles, and uterine vessels, place the uterine isthmus in traction with a rubber tourniquet and perform an in situ supracervical amputation.
FIGURE 6 Hysterectomy for the large, solid, fibroid uterus
D. After trachelectomy, the large uterine specimen is removed by morcellation. Notice that the retractor is able to stretch significantly, allowing the exteriorization of uteri with diameters considerably larger than that of the retractor.
Devices that simplify the procedure
Occasionally, hysterectomy using traditional clamp, division, and suture ligation can be tedious, frustrating, and time-consuming, especially when exposure is limited or difficult. Several devices developed for laparoscopic surgery can ease suture ligation and the division of blood vessels, ligaments, and tissue bundles during minilaparotomy hysterectomy. They include the Hem-o-lok ligating clip (Weck Closure Systems); the LigaSure Atlas, a vessel sealer-divider (Valleylab, Tyco Healthcare, Boulder, Colo); the ETS 45-Flex endoscopic linear cutter (Ethicon Endo-Surgery, Cincinnati, Ohio); and the PK bipolar cutting forceps (Gyrus Medical, Maple Grove, Minn).
Additional concerns
Is the incision too large? Fears that incisions over 5 cm might nullify minimally invasive surgery’s benefits have proven unfounded.
Laparoscopic procedures that use a 7-cm to 8-cm incision to introduce the hand into the abdomen, as well as those performed in conjunction with minilaparotomy, have consistently failed to identify a link between these combinations and morbidity or lengthy recovery. A “large” minilaparotomy incision is still superior to a standard abdominal hysterectomy in terms of convalescence, and it is significantly faster and more cost-effective than a prolonged laparoscopic or laparoscopic-assisted vaginal hysterectomy.3-10
Do any conditions contraindicate minilaparotomy? In patients with documented or strongly suspected severe pelvic conditions (for example, advanced endometriosis, pelvic inflammatory disease, bowel disease, or malignancy), preliminary laparoscopic evaluation to determine the pathologic condition’s severity and extent is strongly recommended.
If during this assessment you detect pathology that is not appropriate for laparoscopic surgery or minilaparotomy, perform a traditional laparotomy. If, however, this evaluation demonstrates that pelvic pathology is amenable to laparoscopic surgery, a laparoscopic hysterectomy or laparoscopic-assisted minilaparotomy hysterectomy is indicated.
Dr. Pelosi II reports that he is a consultant for Apple Medical Corporation. Dr. Pelosi III reports no affiliations or financial arrangements with any of the manufacturers of products mentioned in this article or their competitors.
1. Kustner O. Der suprasymphysare kruzschnitt, eine methode der coeliotomie bei wening umfanglichen affektionen der weiblichen beckenorgane. Monatsschr Geburtshilfe Gynakol. 1896;4:197-206.
2. Doyen E. Surgical Therapeutics and Operative Technique. Vol. III. Spencer-Browne H, translator. London, England: Bailliere, Tindal and Cox; 1920.
3. Benedetti Panicci P, Maneschi F, Cutillo G, et al. Surgery by minilaparotomy in benign gynecologic disease. Obstet Gynecol. 1996;87:456-459.
4. Benedetti Panicci P, Zullo MA, Casalino B, et al. Subcutaneous drainage versus no drainage after minilaparotomy in gynecologic benign conditions. Am J Obstet Gynecol. 2003;188:71-75.
5. Pelosi MA, II, Pelosi MA, III. The suprapubic cruciate incision for laparoscopic assisted microceliotomy. J Soc Laparoendosc Surg. 1997;1:269-272.
6. Pelosi MA, II, Pelosi MA, III. Self-retaining abdominal retractor for minilaparotomy. Obstet Gynecol. 2000;96:775-778.
7. Pelosi MA, II, Pelosi MA, III. Hand-assisted laparoscopy for complex hysterectomy. J Am Assoc Gynecol Laparosc. 1999;6:183-188.
8. Pelosi MA, II, Pelosi MA, III. Hand-assisted laparoscopic cholecystectomy at cesarean section. J Am Assoc Gynecol Laparosc. 1999;6:491-495.
9. Pelosi MA, II, Pelosi MA, III. Hand-assisted laparoscopy (handoscopy) for megamyomectomy: A case study. J Reprod Med. 2000;45:519-525.
10. Pelosi MA, II, Pelosi MA, III, Eim J. Hand-assisted laparoscopy for pelvic malignancy. J Laparoendosc Adv Surg Tech. 2000;10:143-150.
1. Kustner O. Der suprasymphysare kruzschnitt, eine methode der coeliotomie bei wening umfanglichen affektionen der weiblichen beckenorgane. Monatsschr Geburtshilfe Gynakol. 1896;4:197-206.
2. Doyen E. Surgical Therapeutics and Operative Technique. Vol. III. Spencer-Browne H, translator. London, England: Bailliere, Tindal and Cox; 1920.
3. Benedetti Panicci P, Maneschi F, Cutillo G, et al. Surgery by minilaparotomy in benign gynecologic disease. Obstet Gynecol. 1996;87:456-459.
4. Benedetti Panicci P, Zullo MA, Casalino B, et al. Subcutaneous drainage versus no drainage after minilaparotomy in gynecologic benign conditions. Am J Obstet Gynecol. 2003;188:71-75.
5. Pelosi MA, II, Pelosi MA, III. The suprapubic cruciate incision for laparoscopic assisted microceliotomy. J Soc Laparoendosc Surg. 1997;1:269-272.
6. Pelosi MA, II, Pelosi MA, III. Self-retaining abdominal retractor for minilaparotomy. Obstet Gynecol. 2000;96:775-778.
7. Pelosi MA, II, Pelosi MA, III. Hand-assisted laparoscopy for complex hysterectomy. J Am Assoc Gynecol Laparosc. 1999;6:183-188.
8. Pelosi MA, II, Pelosi MA, III. Hand-assisted laparoscopic cholecystectomy at cesarean section. J Am Assoc Gynecol Laparosc. 1999;6:491-495.
9. Pelosi MA, II, Pelosi MA, III. Hand-assisted laparoscopy (handoscopy) for megamyomectomy: A case study. J Reprod Med. 2000;45:519-525.
10. Pelosi MA, II, Pelosi MA, III, Eim J. Hand-assisted laparoscopy for pelvic malignancy. J Laparoendosc Adv Surg Tech. 2000;10:143-150.
Uterine artery embolization for symptomatic fibroids: Pros and cons
- Uterine artery embolization (UAE) may be especially useful in women who are poor surgical candidates or have extensive adhesive disease, or who refuse blood products or are perimenopausal.
- The average reported symptom improvement is 87%; the mean reduction in fibroid volume is 46%.
- Most patients are discharged within 24 hours of the procedure and experience an average recovery period of 8 days.
- Women undergoing UAE for fibroids are more likely than those undergoing myomectomy to need further invasive treatment within 3 to 5 years.
- Although several series and case reports have noted successful pregnancies following UAE, desire for fertility is considered a relative contraindication by some authorities.
With the increasing demand for nonsurgical alternatives to hysterectomy or myomectomy for fibroids, uterine artery embolization (UAE) has grown in use and popularity—and most patients report a high level of satisfaction after the procedure.
UAE has been shown to be safe and effective in selected patients with symptomatic fibroids unresponsive to medical treatment. If they are not contemplating pregnancy and do not have additional pelvic pathology, these women may elect UAE as an appropriate alternative to hysterectomy or myomectomy. This article reviews the indications, contraindications, technique, complications, and outcomes of UAE.
Limits of primary surgeries increase demand for UAE
Most of the 590,000 hysterectomies performed each year in the United States are for symptomatic fibroids, the most common tumors of the female reproductive tract.1 Although hysterectomy is the definitive treatment, increasing numbers of patients express a desire for alternatives, primarily to preserve the uterus. While myomectomy spares the uterus, as many as 25% of women who undergo this procedure require another surgery for recurrent symptoms.2 These limitations of the primary surgeries for fibroids have increased the demand for UAE.
Embolization of the uterine arteries has been utilized for more than 20 years to treat pelvic hemorrhage following delivery or abortion, ectopic or cervical pregnancy, gestational trophoblastic disease, or malignancy.3,4 It was first reported as an effective intervention for fibroids in 1995, when Ravina et al5 noted that several women with symptomatic leiomyomata who underwent UAE as a pre-hysterectomy treatment had such significant clinical improvement that hysterectoMy was no longer required.
In a study involving 200 patients undergoing UAE for leiomyomata, Spies et al6 noted improvement in heavy bleeding in 90% (95% confidence interval [CI], 86%, 95%) and a reduction in bulk-related symptoms in 91% (95% CI, 86%, 95%) at 1 year.
Because data are limited on the safety of pregnancy following uterine artery embolization, some authorities consider the desire for future fertility a relative contraindication.
On a global level, more than 30,000 UAE procedures have been performed for symptomatic uterine fibroids.
The economic considerations surrounding uterine artery embolization (UAE) have led to a turf war of sorts between gynecologists and interventional radiologists. In Philadelphia, Pa, the average reimbursement to an interventional radiologist for a UAE is approximately $1,650; for a gynecologist performing a hysterectomy or myomectomy, it is approximately $1,000.
Some Ob/Gyns are reluctant to recommend UAE for their patients for a variety of reasons. Because of this reluctance, UAE is increasingly marketed directly to the consumer over the Internet and in print media. Large proportions of women undergoing UAE are self-referrals or are referred by their gynecologist after specifically requesting the procedure.
UAE may represent a societal savings in terms of direct and indirect costs. For example, a Canadian cost analysis found that UAE was associated with significantly lower hospital costs ($1007.44 Canadian) than abdominal myomectomy ($1,781.73 Canadian).1
Reference
1. Al-Fozan H, Dufort J, Kaplow M, Valenti D, Tulandi T. Cost analysis of myomectomy, hysterectomy, and uterine artery embolization. Am J Obstet Gynecol. 2002;187:1401-1404.
Technique
UAE is a radiologic procedure performed with either local or regional anesthesia. Most commonly, an approach through the right femoral artery is used, after a preliminary arteriogram (FIGURE 1) has been made to visualize the pelvic vasculature.
Fluoroscopic guidance enables a catheter to be passed into the right femoral artery and through the right external iliac artery to the aorta, then down the left common iliac artery to the left internal iliac, down the anterior division, and finally to the left uterine artery. When the catheter is properly positioned, polyvinyl alcohol particles or acrylic copolymer beads (300 microns to 700 microns) are infused until slow flow or stasis occurs in the uterine artery and the fibroid vasculature is occluded (FIGURE 2). The catheter is then pulled back and manipulated into the right uterine artery, which is similarly embolized. Procedure time ranges from 15 to 120 minutes, depending on the patient’s anatomy and the skill of the operator.1,6,7
FIGURE 1 Pre—uterine-artery-embolization arteriograms
Preprocedure right and left arteriograms for vasculature of a 12-week size fibroid uterus.
Preprocedure right and left arteriograms for vasculature of a 24-week size fibroid uterus.
FIGURE 2 Embolization catheter infusing microparticles within uterine artery
A catheter is passed into the right femoral artery, through the right external iliac artery to the aorta, then down the left common iliac artery to the left internal iliac, down the anterior division to the left uterine artery. Embolization material—polyvinyl alcohol particles or acrylic copolymer beads—is then delivered via catheter in the uterine artery until slow flow or stasis occurs in the artery.
Indications
Like hysterectomy and myomectomy, the indications for UAE are symptomatic fibroids that are unresponsive to medical management (with hormonal agents or analgesics). Common symptoms of fibroids include abdominal or pelvic pain, abnormal menstrual bleeding, anemia, urinary frequency, dyspareunia, and infertility.
UAE may be an especially useful option for women who are poor surgical candidates or have extensive adhesive disease, as well as for those who refuse blood products or are perimenopausal.6,8,9
Contraindications
Pelvic infection, severe contrast allergy, arteriovenous shunting, the presence of an undiagnosed pelvic mass, coagulopathy, renal insufficiency, a history of pelvic radiation, and genital tract malignancy all are contraindications.
Because data are limited on the safety of pregnancy following UAE, some authorities consider the desire for future fertility a relative contraindication.4,7,10
Preoperative evaluation
The preoperative workup should include a thorough history and physical examination by both an interventional radiologist and a gynecologist, a pregnancy test, pelvic imaging via ultrasound or magnetic resonance imaging, and endometrial biopsy to exclude endometrial hyperplasia or cancer. (Patients without abnormal bleeding may not require an endometrial biopsy.)
Outcomes
The average reported symptom improvement is 87%, and the mean reduction in fibroid volume is 46%.1 Most patients see improvement within 3 months of the procedure, with control over the symptoms lasting at least 2 years. At 1 year, 90% of patients report improvement in heavy menstrual bleeding.4
Most patients are discharged within 24 hours of the procedure, compared with 48 to 72 hours for abdominal hysterectomy or myomectomy. They also experience an average recovery period of 8 days, compared with 4 to 6 weeks for abdominal hysterectomy or myomectomy.6
However, a recent study found that women undergoing embolization for fibroids were more likely than those undergoing myomectomy to need further invasive treatment (i.e., repeat embolization or surgery) within 3 to 5 years (29% versus 3%).11
Complications
Most patients report some degree of “post-embolization syndrome,” which is characterized by low-grade fever, pain, malaise, nausea, and leukocytosis, generally within the first 4 days.4 This may be caused by the systemic effects of transient fibroid and uterine ischemia. Although the condition is usually self-limiting and observable on an outpatient basis, these patients are often admitted for antibiotic therapy.
“Post-embolization syndrome” is characterized by low-grade fever, pain, malaise, nausea, and leukocytosis, generally within the first 4 days.
In the most recent series published,12 major complications occurred in 0.5% of embolizations performed for symptomatic fibroids. They include pulmonary embolism, arterial thrombosis, groin hematomas, local infection, guide-wire perforation of arteries, allergic reaction to contrast medium, endometritis, ischemia of pelvic organs, sepsis, and death. Among more than 30,000 procedures performed to date worldwide, there have been 4 related fatalities. In 2 cases, pulmonary embolism occurred within a few days of the procedure; the 2 other deaths occurred within 2 weeks and were related to septicemia and disseminated intravascular coagulation.
There have been reports of total uterine necrosis, transient and permanent ovarian failure, and external sexual dysfunction. These complications may occur up to 2 years after the procedure.1,4,7,10,13-16 Nontarget vascular embolizations of the gluteus muscle, ovaries, labia minora, and bladder wall also have been noted.17,18
When viewed in the context of the large number of procedures performed—and considering the complications associated with myomectomy and hysterectomy—these rare complications show that, overall, UAE is a very safe procedure.
Pregnancy after embolization
Because we lack controlled studies and abundant data, the role of UAE in women contemplating childbearing is unclear. More studies are needed before UAE can be confidently recommended for these women. Premature ovarian failure is an uncommon but recognized complication of UAE.13
Several series and case reports have noted successful pregnancies following UAE.19,20 Our recent report21 on 50 pregnancies following UAE for leiomyomata noted higher rates of cesarean delivery, preterm delivery, malpresentation, spontaneous abortion, and postpartum hemorrhage than in the general population (TABLE). It is unclear whether the increased rate of premature delivery and malpresentation is due to residual fibroids, changes in myometrial vascularity or elasticity, or other unknown labor-associated processes.
Pregnancy outcomes following myomectomy have been reported in several case series, with rates of premature delivery and other complications similar to those for the general population.22,23 Confounding factors (for example, residual fibroids) and the absence of randomized controlled trials, however, make well-founded comparisons of pregnancy outcomes following UAE and myomectomy difficult.
Theoretical concerns about risk of growth restriction and preeclampsia following UAE have been raised. In our study, we did not observe an increase of small-for-gestational-age infants following embolization.21 Uterine rupture during pregnancy after UAE also has been reported.24
TABLE
Rates of pregnancy complications after UAE and in the general population
| PREGNANCY | COMPLICATIONS OF PREGNANCY % (NUMBER AFFECTED/NUMBER OF SUBJECTS STUDIED) | |||||
|---|---|---|---|---|---|---|
| SPONTANEOUS ABORTION | POSTPARTUM HEMORRHAGE | PREMATURE DELIVERY | CESAREAN DELIVERY | SMALL FOR GESTATIONAL AGE | MAL.* | |
| After UAE for leiomyomata | 32 (11/34) | 9(2/23) | 22 (5/23) | 65 (15/23) | 9 (2/22) | 22 (5/23) |
| In the general population | 10-15 | 4-6 | 5-10 | 22 | 10 | 5 |
| UAE=uterine artery embolization | ||||||
| *MAL=malpresentation | ||||||
| Reprinted from the American Journal of Obstetrics and Gynecology, 100, Goldberg J, Pereira L, Berghella V, Pregnancy after uterine artery embolization, 869-872, 2002, with permission from Elsevier. | ||||||
Internet Resources
- Society of Cardiovascular and Interventional Radiologists (www.SIRweb.org)
- Fibroid Uterine Artery Embolization Registry (www.fibroidregistry.org)
- American College of Obstetricians and Gynecologists (www.acog.org)
The authors report no financial relationships with any companies whose products are mentioned in this article.
1. Floridon C, Lund N, Thomsen SG. Alternative treatment for symptomatic fibroids. Curr Opin Obstet Gynecol. 2001;13:491-495.
2. Stenchever MA, Droegemueller W, Herbst AL, Mishell DR. Comprehensive Gynecology. 4th ed. St. Louis, Mo: Mosby; 2001.
3. Goodwin SC, Walker WJ. Uterine artery embolization for the treatment of uterine fibroids. Curr Opin Obstet Gynecol. 1998;10:315-320.
4. Schwartz ML, Klein A, McLucas B. Using uterine artery embolization to treat fibroids. Contemporary OB/GYN. 2001;8:14-37.
5. Ravina J, Herbreteau D, Ciraru-Vigneron N, et al. Arterial embolization to treat uterine myomata. Lancet. 1995;346:671-672.
6. Spies JB, Ascher SA, Roth AR, Kim J, Levy EB, Gomez-Jorge J. Uterine artery embolization for leiomyomata. Obstet Gynecol. 2001;98:29-34.
7. Demello AB. Uterine artery embolization. AORN J. 2001;73:788-814.
8. Bradley EA, Reidy JF, Forman RG, Jarosz J, Braude PR. Transcatheter uterine artery embolization to treat large uterine fibroids. Br J Obstet Gynaecol. 1998;15:235-240.
9. Goodwin SC, Vedantham S, McLucas B, Forno A, Perrella R. Preliminary experience with uterine artery embolization for uterine fibroids. J Vasc Interv Radiol. 1997;8:517-520.
10. Godfrey CD, Zbella EA. Uterine necrosis after uterine artery embolization for leiomyoma. Obstet Gynecol. 2001;98:950-952.
11. Broder MS, Goodwin S, Chen G, et al. Comparison of long-term outcomes of myomectomy and uterine artery embolization. Obstet Gynecol. 2002;100:864-868.
12. Spies JB, Spector A, Roth A, Baker C, Mauro L, Murphy-Skyrynarz K. Complications after uterine artery embolization for leiomyomas. Obstet Gynecol. 2002;100:873-880.
13. Amato P, Roberts AC. Transient ovarian failure: a complication of uterine artery embolization. Fertil Steril. 2001;75:438-439.
14. Lai AC, Goodwin SC, Bonilla SM, et al. Sexual dysfunction after uterine artery embolization. J Vasc Interv Radiol. 2000;11:755-758.
15. Stringer N. Diagnosis and management of long-term complications of uterine artery embolization. Female Patient. 2002;27(11):13-24.
16. Vashisht A, Studd J, Carey A, Burn P. Fatal septicaemia after fibroid embolisation. Lancet. 1999;354:307-308.
17. Sultana CJ, Goldberg J, Aizenman L, Chon JK. Vesicouterine fistula after uterine artery embolization: a case report. Am J Obstet Gynecol. 2002;187:1726-1727.
18. Yeagley T, Goldberg J, Klein T, Bonn J. Labial necrosis after uterine artery embolization for leiomyomas. Obstet Gynecol. 2002;100:881.-
19. Vashisht A, Smith JR, Thorpe-Beeston G, McCall J. Pregnancy subsequent to uterine artery embolization. Fertil Steril. 2001;75:1246-1247.
20. Ravina JH, Vigneron NC, Aymard A, Le Dref O, Merland JJ. Pregnancy after embolization of uterine myoma: Report of 12 cases. Fertil Steril. 2000;73:1241-1243.
21. Goldberg J, Pereira L, Berghella V. Pregnancy after uterine artery embolization. Obstet Gynecol. 2002;100:869-872.
22. Seracchioli R, Rossi S, Govoni F, et al. Fertility and obstetric outcome after laparoscopic myomectomy of large myomata: A randomized comparison with abdominal myomectomy. Hum Reprod. 2000;15:2663-2668.
23. Dessolle L, Soriano D, Poncelet C, Benifla JL, Madelenat P, Darai E. Determinants of pregnancy rate and obstetric outcome after laparoscopic myomectomy for infertility. Fertil Steril. 2001;76:370-374.
24. Walker W, Green A, Sutton C. Bilateral uterine artery embolization for myomata: Results, complications, and failures. Min Invas Ther Allied Technol. 1999;8:449-454.
- Uterine artery embolization (UAE) may be especially useful in women who are poor surgical candidates or have extensive adhesive disease, or who refuse blood products or are perimenopausal.
- The average reported symptom improvement is 87%; the mean reduction in fibroid volume is 46%.
- Most patients are discharged within 24 hours of the procedure and experience an average recovery period of 8 days.
- Women undergoing UAE for fibroids are more likely than those undergoing myomectomy to need further invasive treatment within 3 to 5 years.
- Although several series and case reports have noted successful pregnancies following UAE, desire for fertility is considered a relative contraindication by some authorities.
With the increasing demand for nonsurgical alternatives to hysterectomy or myomectomy for fibroids, uterine artery embolization (UAE) has grown in use and popularity—and most patients report a high level of satisfaction after the procedure.
UAE has been shown to be safe and effective in selected patients with symptomatic fibroids unresponsive to medical treatment. If they are not contemplating pregnancy and do not have additional pelvic pathology, these women may elect UAE as an appropriate alternative to hysterectomy or myomectomy. This article reviews the indications, contraindications, technique, complications, and outcomes of UAE.
Limits of primary surgeries increase demand for UAE
Most of the 590,000 hysterectomies performed each year in the United States are for symptomatic fibroids, the most common tumors of the female reproductive tract.1 Although hysterectomy is the definitive treatment, increasing numbers of patients express a desire for alternatives, primarily to preserve the uterus. While myomectomy spares the uterus, as many as 25% of women who undergo this procedure require another surgery for recurrent symptoms.2 These limitations of the primary surgeries for fibroids have increased the demand for UAE.
Embolization of the uterine arteries has been utilized for more than 20 years to treat pelvic hemorrhage following delivery or abortion, ectopic or cervical pregnancy, gestational trophoblastic disease, or malignancy.3,4 It was first reported as an effective intervention for fibroids in 1995, when Ravina et al5 noted that several women with symptomatic leiomyomata who underwent UAE as a pre-hysterectomy treatment had such significant clinical improvement that hysterectoMy was no longer required.
In a study involving 200 patients undergoing UAE for leiomyomata, Spies et al6 noted improvement in heavy bleeding in 90% (95% confidence interval [CI], 86%, 95%) and a reduction in bulk-related symptoms in 91% (95% CI, 86%, 95%) at 1 year.
Because data are limited on the safety of pregnancy following uterine artery embolization, some authorities consider the desire for future fertility a relative contraindication.
On a global level, more than 30,000 UAE procedures have been performed for symptomatic uterine fibroids.
The economic considerations surrounding uterine artery embolization (UAE) have led to a turf war of sorts between gynecologists and interventional radiologists. In Philadelphia, Pa, the average reimbursement to an interventional radiologist for a UAE is approximately $1,650; for a gynecologist performing a hysterectomy or myomectomy, it is approximately $1,000.
Some Ob/Gyns are reluctant to recommend UAE for their patients for a variety of reasons. Because of this reluctance, UAE is increasingly marketed directly to the consumer over the Internet and in print media. Large proportions of women undergoing UAE are self-referrals or are referred by their gynecologist after specifically requesting the procedure.
UAE may represent a societal savings in terms of direct and indirect costs. For example, a Canadian cost analysis found that UAE was associated with significantly lower hospital costs ($1007.44 Canadian) than abdominal myomectomy ($1,781.73 Canadian).1
Reference
1. Al-Fozan H, Dufort J, Kaplow M, Valenti D, Tulandi T. Cost analysis of myomectomy, hysterectomy, and uterine artery embolization. Am J Obstet Gynecol. 2002;187:1401-1404.
Technique
UAE is a radiologic procedure performed with either local or regional anesthesia. Most commonly, an approach through the right femoral artery is used, after a preliminary arteriogram (FIGURE 1) has been made to visualize the pelvic vasculature.
Fluoroscopic guidance enables a catheter to be passed into the right femoral artery and through the right external iliac artery to the aorta, then down the left common iliac artery to the left internal iliac, down the anterior division, and finally to the left uterine artery. When the catheter is properly positioned, polyvinyl alcohol particles or acrylic copolymer beads (300 microns to 700 microns) are infused until slow flow or stasis occurs in the uterine artery and the fibroid vasculature is occluded (FIGURE 2). The catheter is then pulled back and manipulated into the right uterine artery, which is similarly embolized. Procedure time ranges from 15 to 120 minutes, depending on the patient’s anatomy and the skill of the operator.1,6,7
FIGURE 1 Pre—uterine-artery-embolization arteriograms
Preprocedure right and left arteriograms for vasculature of a 12-week size fibroid uterus.
Preprocedure right and left arteriograms for vasculature of a 24-week size fibroid uterus.
FIGURE 2 Embolization catheter infusing microparticles within uterine artery
A catheter is passed into the right femoral artery, through the right external iliac artery to the aorta, then down the left common iliac artery to the left internal iliac, down the anterior division to the left uterine artery. Embolization material—polyvinyl alcohol particles or acrylic copolymer beads—is then delivered via catheter in the uterine artery until slow flow or stasis occurs in the artery.
Indications
Like hysterectomy and myomectomy, the indications for UAE are symptomatic fibroids that are unresponsive to medical management (with hormonal agents or analgesics). Common symptoms of fibroids include abdominal or pelvic pain, abnormal menstrual bleeding, anemia, urinary frequency, dyspareunia, and infertility.
UAE may be an especially useful option for women who are poor surgical candidates or have extensive adhesive disease, as well as for those who refuse blood products or are perimenopausal.6,8,9
Contraindications
Pelvic infection, severe contrast allergy, arteriovenous shunting, the presence of an undiagnosed pelvic mass, coagulopathy, renal insufficiency, a history of pelvic radiation, and genital tract malignancy all are contraindications.
Because data are limited on the safety of pregnancy following UAE, some authorities consider the desire for future fertility a relative contraindication.4,7,10
Preoperative evaluation
The preoperative workup should include a thorough history and physical examination by both an interventional radiologist and a gynecologist, a pregnancy test, pelvic imaging via ultrasound or magnetic resonance imaging, and endometrial biopsy to exclude endometrial hyperplasia or cancer. (Patients without abnormal bleeding may not require an endometrial biopsy.)
Outcomes
The average reported symptom improvement is 87%, and the mean reduction in fibroid volume is 46%.1 Most patients see improvement within 3 months of the procedure, with control over the symptoms lasting at least 2 years. At 1 year, 90% of patients report improvement in heavy menstrual bleeding.4
Most patients are discharged within 24 hours of the procedure, compared with 48 to 72 hours for abdominal hysterectomy or myomectomy. They also experience an average recovery period of 8 days, compared with 4 to 6 weeks for abdominal hysterectomy or myomectomy.6
However, a recent study found that women undergoing embolization for fibroids were more likely than those undergoing myomectomy to need further invasive treatment (i.e., repeat embolization or surgery) within 3 to 5 years (29% versus 3%).11
Complications
Most patients report some degree of “post-embolization syndrome,” which is characterized by low-grade fever, pain, malaise, nausea, and leukocytosis, generally within the first 4 days.4 This may be caused by the systemic effects of transient fibroid and uterine ischemia. Although the condition is usually self-limiting and observable on an outpatient basis, these patients are often admitted for antibiotic therapy.
“Post-embolization syndrome” is characterized by low-grade fever, pain, malaise, nausea, and leukocytosis, generally within the first 4 days.
In the most recent series published,12 major complications occurred in 0.5% of embolizations performed for symptomatic fibroids. They include pulmonary embolism, arterial thrombosis, groin hematomas, local infection, guide-wire perforation of arteries, allergic reaction to contrast medium, endometritis, ischemia of pelvic organs, sepsis, and death. Among more than 30,000 procedures performed to date worldwide, there have been 4 related fatalities. In 2 cases, pulmonary embolism occurred within a few days of the procedure; the 2 other deaths occurred within 2 weeks and were related to septicemia and disseminated intravascular coagulation.
There have been reports of total uterine necrosis, transient and permanent ovarian failure, and external sexual dysfunction. These complications may occur up to 2 years after the procedure.1,4,7,10,13-16 Nontarget vascular embolizations of the gluteus muscle, ovaries, labia minora, and bladder wall also have been noted.17,18
When viewed in the context of the large number of procedures performed—and considering the complications associated with myomectomy and hysterectomy—these rare complications show that, overall, UAE is a very safe procedure.
Pregnancy after embolization
Because we lack controlled studies and abundant data, the role of UAE in women contemplating childbearing is unclear. More studies are needed before UAE can be confidently recommended for these women. Premature ovarian failure is an uncommon but recognized complication of UAE.13
Several series and case reports have noted successful pregnancies following UAE.19,20 Our recent report21 on 50 pregnancies following UAE for leiomyomata noted higher rates of cesarean delivery, preterm delivery, malpresentation, spontaneous abortion, and postpartum hemorrhage than in the general population (TABLE). It is unclear whether the increased rate of premature delivery and malpresentation is due to residual fibroids, changes in myometrial vascularity or elasticity, or other unknown labor-associated processes.
Pregnancy outcomes following myomectomy have been reported in several case series, with rates of premature delivery and other complications similar to those for the general population.22,23 Confounding factors (for example, residual fibroids) and the absence of randomized controlled trials, however, make well-founded comparisons of pregnancy outcomes following UAE and myomectomy difficult.
Theoretical concerns about risk of growth restriction and preeclampsia following UAE have been raised. In our study, we did not observe an increase of small-for-gestational-age infants following embolization.21 Uterine rupture during pregnancy after UAE also has been reported.24
TABLE
Rates of pregnancy complications after UAE and in the general population
| PREGNANCY | COMPLICATIONS OF PREGNANCY % (NUMBER AFFECTED/NUMBER OF SUBJECTS STUDIED) | |||||
|---|---|---|---|---|---|---|
| SPONTANEOUS ABORTION | POSTPARTUM HEMORRHAGE | PREMATURE DELIVERY | CESAREAN DELIVERY | SMALL FOR GESTATIONAL AGE | MAL.* | |
| After UAE for leiomyomata | 32 (11/34) | 9(2/23) | 22 (5/23) | 65 (15/23) | 9 (2/22) | 22 (5/23) |
| In the general population | 10-15 | 4-6 | 5-10 | 22 | 10 | 5 |
| UAE=uterine artery embolization | ||||||
| *MAL=malpresentation | ||||||
| Reprinted from the American Journal of Obstetrics and Gynecology, 100, Goldberg J, Pereira L, Berghella V, Pregnancy after uterine artery embolization, 869-872, 2002, with permission from Elsevier. | ||||||
Internet Resources
- Society of Cardiovascular and Interventional Radiologists (www.SIRweb.org)
- Fibroid Uterine Artery Embolization Registry (www.fibroidregistry.org)
- American College of Obstetricians and Gynecologists (www.acog.org)
The authors report no financial relationships with any companies whose products are mentioned in this article.
- Uterine artery embolization (UAE) may be especially useful in women who are poor surgical candidates or have extensive adhesive disease, or who refuse blood products or are perimenopausal.
- The average reported symptom improvement is 87%; the mean reduction in fibroid volume is 46%.
- Most patients are discharged within 24 hours of the procedure and experience an average recovery period of 8 days.
- Women undergoing UAE for fibroids are more likely than those undergoing myomectomy to need further invasive treatment within 3 to 5 years.
- Although several series and case reports have noted successful pregnancies following UAE, desire for fertility is considered a relative contraindication by some authorities.
With the increasing demand for nonsurgical alternatives to hysterectomy or myomectomy for fibroids, uterine artery embolization (UAE) has grown in use and popularity—and most patients report a high level of satisfaction after the procedure.
UAE has been shown to be safe and effective in selected patients with symptomatic fibroids unresponsive to medical treatment. If they are not contemplating pregnancy and do not have additional pelvic pathology, these women may elect UAE as an appropriate alternative to hysterectomy or myomectomy. This article reviews the indications, contraindications, technique, complications, and outcomes of UAE.
Limits of primary surgeries increase demand for UAE
Most of the 590,000 hysterectomies performed each year in the United States are for symptomatic fibroids, the most common tumors of the female reproductive tract.1 Although hysterectomy is the definitive treatment, increasing numbers of patients express a desire for alternatives, primarily to preserve the uterus. While myomectomy spares the uterus, as many as 25% of women who undergo this procedure require another surgery for recurrent symptoms.2 These limitations of the primary surgeries for fibroids have increased the demand for UAE.
Embolization of the uterine arteries has been utilized for more than 20 years to treat pelvic hemorrhage following delivery or abortion, ectopic or cervical pregnancy, gestational trophoblastic disease, or malignancy.3,4 It was first reported as an effective intervention for fibroids in 1995, when Ravina et al5 noted that several women with symptomatic leiomyomata who underwent UAE as a pre-hysterectomy treatment had such significant clinical improvement that hysterectoMy was no longer required.
In a study involving 200 patients undergoing UAE for leiomyomata, Spies et al6 noted improvement in heavy bleeding in 90% (95% confidence interval [CI], 86%, 95%) and a reduction in bulk-related symptoms in 91% (95% CI, 86%, 95%) at 1 year.
Because data are limited on the safety of pregnancy following uterine artery embolization, some authorities consider the desire for future fertility a relative contraindication.
On a global level, more than 30,000 UAE procedures have been performed for symptomatic uterine fibroids.
The economic considerations surrounding uterine artery embolization (UAE) have led to a turf war of sorts between gynecologists and interventional radiologists. In Philadelphia, Pa, the average reimbursement to an interventional radiologist for a UAE is approximately $1,650; for a gynecologist performing a hysterectomy or myomectomy, it is approximately $1,000.
Some Ob/Gyns are reluctant to recommend UAE for their patients for a variety of reasons. Because of this reluctance, UAE is increasingly marketed directly to the consumer over the Internet and in print media. Large proportions of women undergoing UAE are self-referrals or are referred by their gynecologist after specifically requesting the procedure.
UAE may represent a societal savings in terms of direct and indirect costs. For example, a Canadian cost analysis found that UAE was associated with significantly lower hospital costs ($1007.44 Canadian) than abdominal myomectomy ($1,781.73 Canadian).1
Reference
1. Al-Fozan H, Dufort J, Kaplow M, Valenti D, Tulandi T. Cost analysis of myomectomy, hysterectomy, and uterine artery embolization. Am J Obstet Gynecol. 2002;187:1401-1404.
Technique
UAE is a radiologic procedure performed with either local or regional anesthesia. Most commonly, an approach through the right femoral artery is used, after a preliminary arteriogram (FIGURE 1) has been made to visualize the pelvic vasculature.
Fluoroscopic guidance enables a catheter to be passed into the right femoral artery and through the right external iliac artery to the aorta, then down the left common iliac artery to the left internal iliac, down the anterior division, and finally to the left uterine artery. When the catheter is properly positioned, polyvinyl alcohol particles or acrylic copolymer beads (300 microns to 700 microns) are infused until slow flow or stasis occurs in the uterine artery and the fibroid vasculature is occluded (FIGURE 2). The catheter is then pulled back and manipulated into the right uterine artery, which is similarly embolized. Procedure time ranges from 15 to 120 minutes, depending on the patient’s anatomy and the skill of the operator.1,6,7
FIGURE 1 Pre—uterine-artery-embolization arteriograms
Preprocedure right and left arteriograms for vasculature of a 12-week size fibroid uterus.
Preprocedure right and left arteriograms for vasculature of a 24-week size fibroid uterus.
FIGURE 2 Embolization catheter infusing microparticles within uterine artery
A catheter is passed into the right femoral artery, through the right external iliac artery to the aorta, then down the left common iliac artery to the left internal iliac, down the anterior division to the left uterine artery. Embolization material—polyvinyl alcohol particles or acrylic copolymer beads—is then delivered via catheter in the uterine artery until slow flow or stasis occurs in the artery.
Indications
Like hysterectomy and myomectomy, the indications for UAE are symptomatic fibroids that are unresponsive to medical management (with hormonal agents or analgesics). Common symptoms of fibroids include abdominal or pelvic pain, abnormal menstrual bleeding, anemia, urinary frequency, dyspareunia, and infertility.
UAE may be an especially useful option for women who are poor surgical candidates or have extensive adhesive disease, as well as for those who refuse blood products or are perimenopausal.6,8,9
Contraindications
Pelvic infection, severe contrast allergy, arteriovenous shunting, the presence of an undiagnosed pelvic mass, coagulopathy, renal insufficiency, a history of pelvic radiation, and genital tract malignancy all are contraindications.
Because data are limited on the safety of pregnancy following UAE, some authorities consider the desire for future fertility a relative contraindication.4,7,10
Preoperative evaluation
The preoperative workup should include a thorough history and physical examination by both an interventional radiologist and a gynecologist, a pregnancy test, pelvic imaging via ultrasound or magnetic resonance imaging, and endometrial biopsy to exclude endometrial hyperplasia or cancer. (Patients without abnormal bleeding may not require an endometrial biopsy.)
Outcomes
The average reported symptom improvement is 87%, and the mean reduction in fibroid volume is 46%.1 Most patients see improvement within 3 months of the procedure, with control over the symptoms lasting at least 2 years. At 1 year, 90% of patients report improvement in heavy menstrual bleeding.4
Most patients are discharged within 24 hours of the procedure, compared with 48 to 72 hours for abdominal hysterectomy or myomectomy. They also experience an average recovery period of 8 days, compared with 4 to 6 weeks for abdominal hysterectomy or myomectomy.6
However, a recent study found that women undergoing embolization for fibroids were more likely than those undergoing myomectomy to need further invasive treatment (i.e., repeat embolization or surgery) within 3 to 5 years (29% versus 3%).11
Complications
Most patients report some degree of “post-embolization syndrome,” which is characterized by low-grade fever, pain, malaise, nausea, and leukocytosis, generally within the first 4 days.4 This may be caused by the systemic effects of transient fibroid and uterine ischemia. Although the condition is usually self-limiting and observable on an outpatient basis, these patients are often admitted for antibiotic therapy.
“Post-embolization syndrome” is characterized by low-grade fever, pain, malaise, nausea, and leukocytosis, generally within the first 4 days.
In the most recent series published,12 major complications occurred in 0.5% of embolizations performed for symptomatic fibroids. They include pulmonary embolism, arterial thrombosis, groin hematomas, local infection, guide-wire perforation of arteries, allergic reaction to contrast medium, endometritis, ischemia of pelvic organs, sepsis, and death. Among more than 30,000 procedures performed to date worldwide, there have been 4 related fatalities. In 2 cases, pulmonary embolism occurred within a few days of the procedure; the 2 other deaths occurred within 2 weeks and were related to septicemia and disseminated intravascular coagulation.
There have been reports of total uterine necrosis, transient and permanent ovarian failure, and external sexual dysfunction. These complications may occur up to 2 years after the procedure.1,4,7,10,13-16 Nontarget vascular embolizations of the gluteus muscle, ovaries, labia minora, and bladder wall also have been noted.17,18
When viewed in the context of the large number of procedures performed—and considering the complications associated with myomectomy and hysterectomy—these rare complications show that, overall, UAE is a very safe procedure.
Pregnancy after embolization
Because we lack controlled studies and abundant data, the role of UAE in women contemplating childbearing is unclear. More studies are needed before UAE can be confidently recommended for these women. Premature ovarian failure is an uncommon but recognized complication of UAE.13
Several series and case reports have noted successful pregnancies following UAE.19,20 Our recent report21 on 50 pregnancies following UAE for leiomyomata noted higher rates of cesarean delivery, preterm delivery, malpresentation, spontaneous abortion, and postpartum hemorrhage than in the general population (TABLE). It is unclear whether the increased rate of premature delivery and malpresentation is due to residual fibroids, changes in myometrial vascularity or elasticity, or other unknown labor-associated processes.
Pregnancy outcomes following myomectomy have been reported in several case series, with rates of premature delivery and other complications similar to those for the general population.22,23 Confounding factors (for example, residual fibroids) and the absence of randomized controlled trials, however, make well-founded comparisons of pregnancy outcomes following UAE and myomectomy difficult.
Theoretical concerns about risk of growth restriction and preeclampsia following UAE have been raised. In our study, we did not observe an increase of small-for-gestational-age infants following embolization.21 Uterine rupture during pregnancy after UAE also has been reported.24
TABLE
Rates of pregnancy complications after UAE and in the general population
| PREGNANCY | COMPLICATIONS OF PREGNANCY % (NUMBER AFFECTED/NUMBER OF SUBJECTS STUDIED) | |||||
|---|---|---|---|---|---|---|
| SPONTANEOUS ABORTION | POSTPARTUM HEMORRHAGE | PREMATURE DELIVERY | CESAREAN DELIVERY | SMALL FOR GESTATIONAL AGE | MAL.* | |
| After UAE for leiomyomata | 32 (11/34) | 9(2/23) | 22 (5/23) | 65 (15/23) | 9 (2/22) | 22 (5/23) |
| In the general population | 10-15 | 4-6 | 5-10 | 22 | 10 | 5 |
| UAE=uterine artery embolization | ||||||
| *MAL=malpresentation | ||||||
| Reprinted from the American Journal of Obstetrics and Gynecology, 100, Goldberg J, Pereira L, Berghella V, Pregnancy after uterine artery embolization, 869-872, 2002, with permission from Elsevier. | ||||||
Internet Resources
- Society of Cardiovascular and Interventional Radiologists (www.SIRweb.org)
- Fibroid Uterine Artery Embolization Registry (www.fibroidregistry.org)
- American College of Obstetricians and Gynecologists (www.acog.org)
The authors report no financial relationships with any companies whose products are mentioned in this article.
1. Floridon C, Lund N, Thomsen SG. Alternative treatment for symptomatic fibroids. Curr Opin Obstet Gynecol. 2001;13:491-495.
2. Stenchever MA, Droegemueller W, Herbst AL, Mishell DR. Comprehensive Gynecology. 4th ed. St. Louis, Mo: Mosby; 2001.
3. Goodwin SC, Walker WJ. Uterine artery embolization for the treatment of uterine fibroids. Curr Opin Obstet Gynecol. 1998;10:315-320.
4. Schwartz ML, Klein A, McLucas B. Using uterine artery embolization to treat fibroids. Contemporary OB/GYN. 2001;8:14-37.
5. Ravina J, Herbreteau D, Ciraru-Vigneron N, et al. Arterial embolization to treat uterine myomata. Lancet. 1995;346:671-672.
6. Spies JB, Ascher SA, Roth AR, Kim J, Levy EB, Gomez-Jorge J. Uterine artery embolization for leiomyomata. Obstet Gynecol. 2001;98:29-34.
7. Demello AB. Uterine artery embolization. AORN J. 2001;73:788-814.
8. Bradley EA, Reidy JF, Forman RG, Jarosz J, Braude PR. Transcatheter uterine artery embolization to treat large uterine fibroids. Br J Obstet Gynaecol. 1998;15:235-240.
9. Goodwin SC, Vedantham S, McLucas B, Forno A, Perrella R. Preliminary experience with uterine artery embolization for uterine fibroids. J Vasc Interv Radiol. 1997;8:517-520.
10. Godfrey CD, Zbella EA. Uterine necrosis after uterine artery embolization for leiomyoma. Obstet Gynecol. 2001;98:950-952.
11. Broder MS, Goodwin S, Chen G, et al. Comparison of long-term outcomes of myomectomy and uterine artery embolization. Obstet Gynecol. 2002;100:864-868.
12. Spies JB, Spector A, Roth A, Baker C, Mauro L, Murphy-Skyrynarz K. Complications after uterine artery embolization for leiomyomas. Obstet Gynecol. 2002;100:873-880.
13. Amato P, Roberts AC. Transient ovarian failure: a complication of uterine artery embolization. Fertil Steril. 2001;75:438-439.
14. Lai AC, Goodwin SC, Bonilla SM, et al. Sexual dysfunction after uterine artery embolization. J Vasc Interv Radiol. 2000;11:755-758.
15. Stringer N. Diagnosis and management of long-term complications of uterine artery embolization. Female Patient. 2002;27(11):13-24.
16. Vashisht A, Studd J, Carey A, Burn P. Fatal septicaemia after fibroid embolisation. Lancet. 1999;354:307-308.
17. Sultana CJ, Goldberg J, Aizenman L, Chon JK. Vesicouterine fistula after uterine artery embolization: a case report. Am J Obstet Gynecol. 2002;187:1726-1727.
18. Yeagley T, Goldberg J, Klein T, Bonn J. Labial necrosis after uterine artery embolization for leiomyomas. Obstet Gynecol. 2002;100:881.-
19. Vashisht A, Smith JR, Thorpe-Beeston G, McCall J. Pregnancy subsequent to uterine artery embolization. Fertil Steril. 2001;75:1246-1247.
20. Ravina JH, Vigneron NC, Aymard A, Le Dref O, Merland JJ. Pregnancy after embolization of uterine myoma: Report of 12 cases. Fertil Steril. 2000;73:1241-1243.
21. Goldberg J, Pereira L, Berghella V. Pregnancy after uterine artery embolization. Obstet Gynecol. 2002;100:869-872.
22. Seracchioli R, Rossi S, Govoni F, et al. Fertility and obstetric outcome after laparoscopic myomectomy of large myomata: A randomized comparison with abdominal myomectomy. Hum Reprod. 2000;15:2663-2668.
23. Dessolle L, Soriano D, Poncelet C, Benifla JL, Madelenat P, Darai E. Determinants of pregnancy rate and obstetric outcome after laparoscopic myomectomy for infertility. Fertil Steril. 2001;76:370-374.
24. Walker W, Green A, Sutton C. Bilateral uterine artery embolization for myomata: Results, complications, and failures. Min Invas Ther Allied Technol. 1999;8:449-454.
1. Floridon C, Lund N, Thomsen SG. Alternative treatment for symptomatic fibroids. Curr Opin Obstet Gynecol. 2001;13:491-495.
2. Stenchever MA, Droegemueller W, Herbst AL, Mishell DR. Comprehensive Gynecology. 4th ed. St. Louis, Mo: Mosby; 2001.
3. Goodwin SC, Walker WJ. Uterine artery embolization for the treatment of uterine fibroids. Curr Opin Obstet Gynecol. 1998;10:315-320.
4. Schwartz ML, Klein A, McLucas B. Using uterine artery embolization to treat fibroids. Contemporary OB/GYN. 2001;8:14-37.
5. Ravina J, Herbreteau D, Ciraru-Vigneron N, et al. Arterial embolization to treat uterine myomata. Lancet. 1995;346:671-672.
6. Spies JB, Ascher SA, Roth AR, Kim J, Levy EB, Gomez-Jorge J. Uterine artery embolization for leiomyomata. Obstet Gynecol. 2001;98:29-34.
7. Demello AB. Uterine artery embolization. AORN J. 2001;73:788-814.
8. Bradley EA, Reidy JF, Forman RG, Jarosz J, Braude PR. Transcatheter uterine artery embolization to treat large uterine fibroids. Br J Obstet Gynaecol. 1998;15:235-240.
9. Goodwin SC, Vedantham S, McLucas B, Forno A, Perrella R. Preliminary experience with uterine artery embolization for uterine fibroids. J Vasc Interv Radiol. 1997;8:517-520.
10. Godfrey CD, Zbella EA. Uterine necrosis after uterine artery embolization for leiomyoma. Obstet Gynecol. 2001;98:950-952.
11. Broder MS, Goodwin S, Chen G, et al. Comparison of long-term outcomes of myomectomy and uterine artery embolization. Obstet Gynecol. 2002;100:864-868.
12. Spies JB, Spector A, Roth A, Baker C, Mauro L, Murphy-Skyrynarz K. Complications after uterine artery embolization for leiomyomas. Obstet Gynecol. 2002;100:873-880.
13. Amato P, Roberts AC. Transient ovarian failure: a complication of uterine artery embolization. Fertil Steril. 2001;75:438-439.
14. Lai AC, Goodwin SC, Bonilla SM, et al. Sexual dysfunction after uterine artery embolization. J Vasc Interv Radiol. 2000;11:755-758.
15. Stringer N. Diagnosis and management of long-term complications of uterine artery embolization. Female Patient. 2002;27(11):13-24.
16. Vashisht A, Studd J, Carey A, Burn P. Fatal septicaemia after fibroid embolisation. Lancet. 1999;354:307-308.
17. Sultana CJ, Goldberg J, Aizenman L, Chon JK. Vesicouterine fistula after uterine artery embolization: a case report. Am J Obstet Gynecol. 2002;187:1726-1727.
18. Yeagley T, Goldberg J, Klein T, Bonn J. Labial necrosis after uterine artery embolization for leiomyomas. Obstet Gynecol. 2002;100:881.-
19. Vashisht A, Smith JR, Thorpe-Beeston G, McCall J. Pregnancy subsequent to uterine artery embolization. Fertil Steril. 2001;75:1246-1247.
20. Ravina JH, Vigneron NC, Aymard A, Le Dref O, Merland JJ. Pregnancy after embolization of uterine myoma: Report of 12 cases. Fertil Steril. 2000;73:1241-1243.
21. Goldberg J, Pereira L, Berghella V. Pregnancy after uterine artery embolization. Obstet Gynecol. 2002;100:869-872.
22. Seracchioli R, Rossi S, Govoni F, et al. Fertility and obstetric outcome after laparoscopic myomectomy of large myomata: A randomized comparison with abdominal myomectomy. Hum Reprod. 2000;15:2663-2668.
23. Dessolle L, Soriano D, Poncelet C, Benifla JL, Madelenat P, Darai E. Determinants of pregnancy rate and obstetric outcome after laparoscopic myomectomy for infertility. Fertil Steril. 2001;76:370-374.
24. Walker W, Green A, Sutton C. Bilateral uterine artery embolization for myomata: Results, complications, and failures. Min Invas Ther Allied Technol. 1999;8:449-454.
Hysteroscopic myomectomy: Fertility-preserving yet underutilized
- The goal of hysteroscopic myomectomy is complete removal of the fibroid without trauma to normal uterine tissue.
- Patients with Type 0 and Type I fibroids often require only 1 surgery; patients with Type II fibroids should be advised that 2 surgeries may be needed to remove the entire fibroid.
- Adjuvant preoperative hormonal therapy facilitates surgical scheduling, helps prevent further blood loss in patients already suffering from anemia, and reduces distention media intravasation.
- The monopolar loop electrode is the fibroid removal system that is used most often.
Hysteroscopic myomectomy should be offered to all patients with symptomatic submucous fibroids who desire to avoid hysterectomy. Although it is a highly effective, minimally invasive technique, it is underutilized.
Unfortunately, fewer than one third of US gynecologists perform this procedure. In a 1997 survey of members of the American Association of Gynecologic Laparoscopists—an organization committed to minimally invasive surgery—only half of the respondents reported that they perform this surgery.1
The reasons for learning to perform hysteroscopic myomectomy are compelling:
- A large cohort of patients could benefit, since most heavy vaginal bleeding from fibroids is due to the submucous location, and hysteroscopic resection is a much more benign approach than hysterectomy. Symptomatic fibroids account for 27% of all hysterectomies performed in the US (the largest single diagnostic category) and more than 100,000 are performed for fibroids that cause abnormal uterine bleeding.2
- Removing these lesions hysteroscopically greatly improves prognosis in women with recurrent pregnancy loss and infertility due to submucous fibroids.3 Up to 15% of patients presenting with infertility have otherwise asymptomatic uterine defects, including submucous fibroids. For example, a meta-analysis of patients undergoing in vitro fertilization determined that, compared with controls, the relative risk of pregnancy for women with submucous fibroids was 0.32 (95% confidence interval [CI], 0.13–0.70). When the submucous fibroids were resected, the relative risk of pregnancy rose to 1.72 (CI, 1.13–2.58).3
Preoperative evaluation of fibroids
Severity of menorrhagia (the most common symptom) is considered directly related to the volume of the myoma within the endometrial cavity. It is not uncommon to see large tortuous vessels covering the surface of the fibroids; although the exact mechanism of fibroid-related menorrhagia is undetermined, the fragility of these vessels is probably responsible, at least in part.
Additionally, fibroids involving the uterine mucosa or submucosa may interfere with the muscular contraction necessary for hemostasis.
Surgical options and pretreatment depend on fibroid type. Submucous fibroids are classified according to the percentage of the fibroid within the endometrial cavity:4
- Type 0: pedunculated; 100% within the cavity
- Type I: more than 50% within the cavity
- Type II: more than 50% within the myometrium
The type dictates surgical options, determines endometrial pretreatment, and shapes patient expectations. Type 0 and Type I submucosal fibroids are more successfully removed in a single surgery, whereas Type II submucous fibroids usually require 2 procedures for complete removal.5
My preference is office hysteroscopy to evaluate the endometrial cavity combined with vaginal ultrasound to assess intramural disease. The view with the office hysteroscope is the same view you will have during surgery.
Patients with Type II myomas should be informed of the potential need for 2 procedures, as this fact often influences their treatment decisions. In addition, whenever a patient with a Type II myoma is pretreated with a gonadotropin-releasing hormone (GnRH) agonist, the physician should reassess the fibroid preoperatively to ensure that it has not become completely intramural.
Office assessment: Hysteroscopy plus ultrasound. Preoperative assessment can be achieved with a hysterosalpingogram, vaginal ultrasound, hysterosonogram, or office hysteroscopy.
Method. My preference is office hysteroscopy to evaluate the endometrial cavity combined with vaginal ultrasound to assess intramural disease. The view with the office hysteroscope is the same view you will have during surgery. There will be no surprises. With a small flexible hysteroscope using saline for distention, the procedure is done with no tenaculum, no paracervical block, and can be completed with 60 to 100 cc of fluid in less than 1 minute.
Patients are often intrigued to view the myoma responsible for their heavy vaginal bleeding.
Several advantages of preoperative hormonal therapy
Preoperative hormonal therapy has several advantages:
- Since it is best to resect submucous myomas when the endometrium is thin, hormonal therapy facilitates surgical scheduling.
- Since preoperative therapy creates a state of amenorrhea, it enables patients suffering from menorrhagia and anemia to build up their blood counts, reducing the need for transfusion.
- Most importantly, preoperative therapy can reduce blood flow to the uterus, thereby reducing the rate of fluid intravasation.
Adjuvants used for these functions include oral contraceptives, progestogens, danazol, and GnRH agonists. However, a recent Cochrane review suggests that only GnRH agonists reduce fluid absorption during operative hysteroscopy.6
Method. Numerous protocols have been published, but my preference is to administer 1 intramuscular injection of depot leuprolide acetate (7.5 mg) 6 weeks prior to surgery.
A review of ‘systems’
Submucous myomas have been hysteroscopically removed using the neodymium/yttrium aluminum-garnet (Nd:YAG) laser, a monopolar resectoscope loop, a monopolar radiofrequency (RF) vaporizing electrode, a bipolar RF vaporizing electrode, and a bipolar RF loop.
Laser. The Nd:YAG laser is an expensive device that was popular in the late 1980s and early 1990s. It can be used as a cutting tool for pedunculated Type 0 myomas, or it can be used for myolysis by burning numerous holes in the myoma, causing devascularization and shrinkage.7,8
The sole advantage of the Nd:YAG laser is that it can be used in an isotonic-fluid-filled cavity. Now that accurate fluid-monitoring devices are available, the Nd:YAG laser is rarely used for this indication.
Vaporizing electrodes. Both the bipolar system and monopolar vaporizing electrodes utilize very high RF power (200 W to 300 W) to vaporize fibroids. The advantage is that the fibroids are eradicated very quickly, without any bothersome fibroid chips to remove. The bipolar system can be used with isotonic fluid, whereas the monopolar vaporizing electrodes require non-electrolyte-containing distention media.
The main disadvantages of vaporizing electrodes are:
- They do not produce a tissue sample for pathology. While uterine sarcomas are very rare, they are not homogeneous. Therefore, a simple sample prior to vaporization does not rule out the disease.
- Since vaporizing electrodes are used at high power, numerous gas bubbles are produced and enter the vascular system. Fortunately, these bubbles dissipate rapidly in the blood. As long as the rate of formation does not exceed the rate of dissipation, there are no significant clinical sequelae. The surgeon can avoid complications by monitoring the patient’s endtidal CO2 and maintaining communication with the anesthesiologist. If a sudden drop in endtidal CO2 is observed, the surgeon should stop the case until it resolves.9
Monopolar loop electrode. The instrument most commonly used to remove submucosal myomas is the monopolar loop electrode with a continuous-flow resectoscope. This is quite similar to the instrument used to resect the prostate; it has been slightly modified for gynecologic use to provide enhanced fluid inflow and outflow. A key instrument now considered standard is an accurate fluid-monitoring device that can be attached to the resectoscope.
Distention media for monopolar resection. Prior to the introduction of continuous high-flow resectoscopes and fluid-management systems, monopolar resectoscopy could only be performed using a solution of 32% dextran 70 and water because of that formulation’s viscosity and facilitation of visualization in the presence of blood. However, due to complications related to absorption, allergies, and the solution’s sticky residue, it is not used with today’s high-flow technology.
The instrument most commonly used to remove submucosal myomas is the monopolar loop electrode with a continuous-flow resectoscope.
When using nonviscous fluids with monopolar RF energy, a distention medium without electrolytes must be selected. Isotonic physiologic media such as normal saline or lactated Ringer’s solution would cause the energy at the electrode to disperse, eliminating the cutting effect. Contrary to popular belief, there would be no burning of the uterine cavity and no subsequent danger to the patient.
The 3 most commonly used fluids for uterine distention are 1.5% glycine, 3% sorbitol, and 5% mannitol. All 3 lack electrolytes. The glycine and sorbitol solutions are hypotonic, while 5% mannitol is isotonic. Although there is some debate over whether an isotonic non-electrolyte-containing medium is safer than a hypotonic one, the guidelines for fluid monitoring are the same for all 3.
The goal of a hysteroscopic myomectomy is to alleviate symptoms without causing a weak myometrium or intracavitary synechia. This is accomplished by removing the fibroid without traumatizing normal uterine tissue.
As a general rule, the serum sodium level will decrease approximately 10 meq for every liter absorbed in the blood stream. That is why, to predict hyponatremia, the gynecologist must rely on exact measurements of fluid deficits rather than operating time or total volume used.
Fluid management. Significant hyponatremia can result in pulmonary edema, transient blindness, cerebral edema, brainstem herniation, and death. Catastrophic as these complications are, they are almost 100% avoidable with accurate deficit measurements and adherence to sound fluid-management protocols.
At our institution, fluids are monitored using a weighted system, and the fluid deficit is displayed on the surgical screen in real time. When the deficit reaches 1 L, electrolytes are drawn, the patient is given 10 mg of intravenous (IV) furosemide, and attempts are made to finish the surgery as soon as possible. When the deficit reaches 1.5 L, the surgery is discontinued no matter how much or how little remains to complete it.
It is important that the protocol at each institution be agreed upon by the chief of service and anesthesia as well as the director of nursing so that there are no conflicts at the time of surgery.
Uterine distention methods
Numerous methods have been used to distend the uterus: gravity, gas pressure, and electronic pumps and pressure bags. The optimal intrauterine pressure is the minimum pressure that allows for flow, distention, and visualization. Most of the time this ranges from 40 mm Hg to 60 mm Hg. The higher the intrauterine pressure, the more rapid the fluid intravasation, particularly when intrauterine pressure exceeds the mean arterial pressure.
Rapid fluid loss can occur even with low intrauterine pressures. That is because a large venous sinus will have a pressure of 8 mm Hg to 10 mm Hg, while the minimum uterine distention pressure is much greater. Strict adherence to fluid-deficit protocols will minimize the risk of complications from fluid overload.
Surgical technique
The goal of a hysteroscopic myomectomy is to alleviate symptoms without causing a weak myometrium or intracavitary synechia. Ideally, this is accomplished by completely removing the fibroid without traumatizing normal uterine tissue. Removal of the entire myoma reduces the likelihood of recurrence and regrowth.
Surgical technique for removal of Types 0, I, and II fibroids is illustrated and explained on (FIGURES1-4).
Within a given range (40 W to 100 W), it makes little difference which cutting-current setting on the RF generator you use; the principles are the same. The loop must be in contact with the tissue to be resected. Energy is applied and, once the circuit is completed (through tissue, the dispersive electrode, the generator and back to the loop), the loop is able to cut with minimal tactile feedback.
The lower the wattage, the more contact necessary between the loop and the tissue and the longer it takes to complete the circuit. Conversely, the higher the power, the faster the circuit is completed and cutting occurs.
Though it does not matter what setting is used, the operator must choose a setting based on his or her comfort level. Lower power gives the operator more control. Higher power should be used only by the most experienced hysteroscopist, since it involves very little tactile feedback and a higher risk of uterine perforation. At our teaching institution, we use 60 W of pure cutting current.
The technique for removing a submucous fibroid depends on its type and location within the endometrial cavity.
Type 0. Because these fibroids are pedunculated, the operator has the option of cutting the stalk (FIGURE 1) or shaving the myoma to remove it in pieces through the cervix. If the stalk is cut, it often is difficult to remove the fibroid through the cervix due to its large size. Nor can the fibroid be cut with monopolar energy because of the difficulty of completing the circuit. Type 0 myomas can be grabbed blindly with a Corson forceps or under direct visualization with an Isaacson optical tenaculum (Karl Storz Endoscopy, Culver City, Calif.). It also is acceptable to leave the fibroid in the cavity and let it degenerate and be expelled spontaneously. The risk of infection is very small.
My preference is to shave the fibroid down to the endometrium while it is attached to the stalk. I find it easier to remove when it is anchored rather than trying to pull out a single large specimen.
Type I. In a Type I myoma, more than 50% of the fibroid is within the uterine cavity, with a smaller portion embedded in the myometrium. To remove these fibroids, shave each to the level of the endometrium. To do so, place the loop behind the fibroid to be resected (FIGURE 5). Next, activate the RF energy using a foot pedal and, once the circuit is complete, draw the loop back into the resectoscope. Be careful to maintain visualization of the fibroid, loop, and cavity (FIGURE 6). This becomes difficult as the fibroid approaches the resectoscope and obstructs the hysteroscopic lens. To avoid this, bring the loop—which begins its cut fully extended—only halfway back to the resectoscope (FIGURE 7). Then retract the resectoscope itself, with the loop halfway extended, until the cut through the fibroid is complete (FIGURE 8). The loop then is retracted back into the resectoscope. The angle of the resectoscope must be adjusted to maintain loop contact with the fibroid tissue.
When more than 70% or 80% of the fibroid has been resected, the remainder will slough spontaneously over the next 2 to 4 months.
Once the fibroid is resected to the level of the endometrium, remove the fibroid chips (FIGURE 2). Often, when the hysteroscope is removed and uterine distention is reestablished, more of the fibroid will protrude into the cavity, facilitating safe resection. At some point, the fibroid will no longer protrude into the cavity; it will have been removed or only a small percentage will remain intramural.
Often, when more than 70% or 80% of the fibroid has been resected, the remainder will slough spontaneously over the next 2 to 4 months. The technique for removing the intramural fibroid is described on page 78 (FIGURE 3).
Type II. The first step in resecting a Type II myoma is shaving the intracavitary portion to the level of the endometrium, as in the resection of a Type I fibroid. Often, once a small Type II myoma is “unroofed,” the remainder will spontaneously fall into the cavity and can easily be removed.
Larger Type II myomas require a different approach. In these, the goal is to identify the pseudo-capsular plane that allows for blunt dissection. Once you have identified it, use the loop electrode to bluntly dissect (without RF activation) the fibroid from the myometrium. Place the electrode in the capsular plane and retract as much of the myoma into the cavity as possible (FIGURE 4). Another way to do this is to use a twisting motion—similar to those performed in open myomectomies—with the optical tenaculum. Once the fibroid is in the cavity, shave it to the level of the endometrium. Repeat this technique until you have removed as much of the fibroid as possible. Use minimal distention pressure when you perform this technique so that the fibroid will not be pressed back into the myometrium.
Underutilization of hysteroscopic myomectomy may be due to inadequate training in the technique or a misconception that it is difficult to learn. Fortunately, hysteroscope manufacturers are beginning to offer physicians and operating room personnel didactic and hands-on laboratory courses.
FIGURE 1 Surgical technique for removing Type 0, I, II fibroids
Type 0. The loop is placed behind the fibroid. Once activated, the loop is drawn toward the operator to resect the pedicle.
FIGURE 2 Surgical technique for removing Type 0, I, II fibroids
Type I. The myoma is resected to the level of the myometrium.
FIGURE 3 Surgical technique for removing Type 0, I, II fibroids
Resection of intramural portion of Type I and II. The resectoscopic loop is placed in the pseudocapsular lane between the fibroid and the myometrium to bluntly dissect the fibroid into the cavity for further resection.
FIGURE 4 Surgical technique for removing Type 0, I, II fibroids
Type II. The roof of the myoma is shaved off to the level of the myometrium. The insert demonstrates the intramural portion of the myoma that will protrude into the cavity with a reduction in uterine distension pressure.
FIGURE 5 Resect technique—4 steps in resecting a submucous myoma
The loop is placed behind the fibroid with maximal contact with the tissue before RF activation.
FIGURE 6 Resect technique—4 steps in resecting a submucous myoma
The loop is withdrawn toward the operator (see arrow) while cutting the myoma but not so far as to obstruct the hysteroscopic view.
FIGURE 7 Resect technique—4 steps in resecting a submucous myoma
The loop is held in place while the hysteroscope is withdrawn (arrows indicate how to remain in direct contact with myoma) continuing to cut the myoma with visualization.
FIGURE 8 Resect technique—4 steps in resecting a submucous myoma
Once through the myoma, the loop is withdrawn into the hysteroscope.
Management of complications
The primary complications of hysteroscopic myomectomy are bleeding, infection, uterine perforation, and fluid overload.
Bleeding. If postoperative hemorrhage occurs, use a 30-cc Foley balloon to tamponade the vessels, or pack the uterus with gauze soaked in a vasopressin solution. Both can be removed any time from 4 hours to 24 hours following placement. If bleeding persists, uterine artery embolization should be considered prior to hysterectomy.
Most attempts at controlling bleeding with a rollerball are futile because the intrauterine distention pressure necessary to see the venous bleeders hides the actual bleeding. Arterial bleeders can be seen and controlled with the rollerball, but they are much less common.
Infection. Because infection is uncommon with hysteroscopic surgery, routine prophylaxis is unnecessary.
Antibiotics should be used if the patient had cervical laminaria placed preoperatively.
Uterine perforation. The management of uterine perforation depends on the site of perforation and whether energy was used during the perforation. For example, if the perforation was at the fundus and occurred with a blunt, non-energized instrument, observation without laparoscopy is sufficient. If the perforation was lateral or occurred with RF energy, laparoscopy is suggested to rule out a broad ligament hematoma or injury to intraperitoneal vessels and viscera. If the perforation is anterior, a cystoscopy is recommended; internal colon inspection is necessary if the perforation is posterior.
Fluid overload. Complications and prevention can be avoided and treated as previously discussed.
New training opportunities may include virtual reality
Underutilization of hysteroscopic myomectomy may be due to inadequate training in the technique during residency or the misconception that it is difficult to learn. (Training has been further hampered by the lack of a suitable animal model.)
Fortunately, hysteroscope manufacturers are beginning to offer physicians and operating room personnel didactic and hands-on laboratory courses. In the near future, we hope, these companies will hire gynecologic hysteroscopists to support physicians and nurses in the field, as has been done in many other specialties.
The creation of new tools such as virtual-reality simulators will undoubtedly enhance the teaching of operative hysteroscopy.
Dr. Isaacson teaches hysteroscopy courses sponsored by Karl Storz Endoscopy.
1. Hulka JF, Levy BS, Luciano AA, Parker WH, Phillips JM. 1997 AAGL membership survey: practice profiles. J Amer Assoc Gynecol Laparosc. 1998;5:93-96.
2. Carlson KJ, Nichols DH, Schiff I. Indications for hysterectomy. N Eng J Med. 1993;328:856-860.
3. Pritts E. Fibroids and infertility: a systematic review of the evidence. Obstet Gynecol Survey. 2001;56:483-491.
4. deBlok S, Dijkman AB, Hemrika DJ. Transcervical resection of fibroids (TCRM): results related to hysteroscopic classification. Gynaecol Endosc. 1995;4:243-246.
5. Istre O. Transcervical resection of endometrium and fibroids: the outcome of 412 operations performed over 5 years. Acta Obstet Gynecol Scand. 1996;75:567-574.
6. Sowter MC, Lethaby A, Singla AA. Preoperative endometrial thinning agents before hysteroscopic surgery for heavy menstrual bleeding. Cochrane Database Syst Rev. 2002;(3):CD001124.-
7. Baggish MS, Sze EM, Morgan G. Hysteroscopic treatment of symptomatic submucous myomas with the Nd:YAG laser. J Gynecol Surg. 1989;5:27-36.
8. Donnez J, Gillerot S, Bourgonjon D, et al. Neodymium:YAG laser hysteroscopy in large submucous fibroids. Fertil Steril. 1990;54:999-1003.
9. Bloomstone J, Chen C, Isselbacher E, Isaacson K. A pilot study examining the frequency and quantity of gas embolization during operative hysteroscopy using a monopolar resectoscope. J Am Assoc Gynecol Laparosc. 2002;9:9-14.
- The goal of hysteroscopic myomectomy is complete removal of the fibroid without trauma to normal uterine tissue.
- Patients with Type 0 and Type I fibroids often require only 1 surgery; patients with Type II fibroids should be advised that 2 surgeries may be needed to remove the entire fibroid.
- Adjuvant preoperative hormonal therapy facilitates surgical scheduling, helps prevent further blood loss in patients already suffering from anemia, and reduces distention media intravasation.
- The monopolar loop electrode is the fibroid removal system that is used most often.
Hysteroscopic myomectomy should be offered to all patients with symptomatic submucous fibroids who desire to avoid hysterectomy. Although it is a highly effective, minimally invasive technique, it is underutilized.
Unfortunately, fewer than one third of US gynecologists perform this procedure. In a 1997 survey of members of the American Association of Gynecologic Laparoscopists—an organization committed to minimally invasive surgery—only half of the respondents reported that they perform this surgery.1
The reasons for learning to perform hysteroscopic myomectomy are compelling:
- A large cohort of patients could benefit, since most heavy vaginal bleeding from fibroids is due to the submucous location, and hysteroscopic resection is a much more benign approach than hysterectomy. Symptomatic fibroids account for 27% of all hysterectomies performed in the US (the largest single diagnostic category) and more than 100,000 are performed for fibroids that cause abnormal uterine bleeding.2
- Removing these lesions hysteroscopically greatly improves prognosis in women with recurrent pregnancy loss and infertility due to submucous fibroids.3 Up to 15% of patients presenting with infertility have otherwise asymptomatic uterine defects, including submucous fibroids. For example, a meta-analysis of patients undergoing in vitro fertilization determined that, compared with controls, the relative risk of pregnancy for women with submucous fibroids was 0.32 (95% confidence interval [CI], 0.13–0.70). When the submucous fibroids were resected, the relative risk of pregnancy rose to 1.72 (CI, 1.13–2.58).3
Preoperative evaluation of fibroids
Severity of menorrhagia (the most common symptom) is considered directly related to the volume of the myoma within the endometrial cavity. It is not uncommon to see large tortuous vessels covering the surface of the fibroids; although the exact mechanism of fibroid-related menorrhagia is undetermined, the fragility of these vessels is probably responsible, at least in part.
Additionally, fibroids involving the uterine mucosa or submucosa may interfere with the muscular contraction necessary for hemostasis.
Surgical options and pretreatment depend on fibroid type. Submucous fibroids are classified according to the percentage of the fibroid within the endometrial cavity:4
- Type 0: pedunculated; 100% within the cavity
- Type I: more than 50% within the cavity
- Type II: more than 50% within the myometrium
The type dictates surgical options, determines endometrial pretreatment, and shapes patient expectations. Type 0 and Type I submucosal fibroids are more successfully removed in a single surgery, whereas Type II submucous fibroids usually require 2 procedures for complete removal.5
My preference is office hysteroscopy to evaluate the endometrial cavity combined with vaginal ultrasound to assess intramural disease. The view with the office hysteroscope is the same view you will have during surgery.
Patients with Type II myomas should be informed of the potential need for 2 procedures, as this fact often influences their treatment decisions. In addition, whenever a patient with a Type II myoma is pretreated with a gonadotropin-releasing hormone (GnRH) agonist, the physician should reassess the fibroid preoperatively to ensure that it has not become completely intramural.
Office assessment: Hysteroscopy plus ultrasound. Preoperative assessment can be achieved with a hysterosalpingogram, vaginal ultrasound, hysterosonogram, or office hysteroscopy.
Method. My preference is office hysteroscopy to evaluate the endometrial cavity combined with vaginal ultrasound to assess intramural disease. The view with the office hysteroscope is the same view you will have during surgery. There will be no surprises. With a small flexible hysteroscope using saline for distention, the procedure is done with no tenaculum, no paracervical block, and can be completed with 60 to 100 cc of fluid in less than 1 minute.
Patients are often intrigued to view the myoma responsible for their heavy vaginal bleeding.
Several advantages of preoperative hormonal therapy
Preoperative hormonal therapy has several advantages:
- Since it is best to resect submucous myomas when the endometrium is thin, hormonal therapy facilitates surgical scheduling.
- Since preoperative therapy creates a state of amenorrhea, it enables patients suffering from menorrhagia and anemia to build up their blood counts, reducing the need for transfusion.
- Most importantly, preoperative therapy can reduce blood flow to the uterus, thereby reducing the rate of fluid intravasation.
Adjuvants used for these functions include oral contraceptives, progestogens, danazol, and GnRH agonists. However, a recent Cochrane review suggests that only GnRH agonists reduce fluid absorption during operative hysteroscopy.6
Method. Numerous protocols have been published, but my preference is to administer 1 intramuscular injection of depot leuprolide acetate (7.5 mg) 6 weeks prior to surgery.
A review of ‘systems’
Submucous myomas have been hysteroscopically removed using the neodymium/yttrium aluminum-garnet (Nd:YAG) laser, a monopolar resectoscope loop, a monopolar radiofrequency (RF) vaporizing electrode, a bipolar RF vaporizing electrode, and a bipolar RF loop.
Laser. The Nd:YAG laser is an expensive device that was popular in the late 1980s and early 1990s. It can be used as a cutting tool for pedunculated Type 0 myomas, or it can be used for myolysis by burning numerous holes in the myoma, causing devascularization and shrinkage.7,8
The sole advantage of the Nd:YAG laser is that it can be used in an isotonic-fluid-filled cavity. Now that accurate fluid-monitoring devices are available, the Nd:YAG laser is rarely used for this indication.
Vaporizing electrodes. Both the bipolar system and monopolar vaporizing electrodes utilize very high RF power (200 W to 300 W) to vaporize fibroids. The advantage is that the fibroids are eradicated very quickly, without any bothersome fibroid chips to remove. The bipolar system can be used with isotonic fluid, whereas the monopolar vaporizing electrodes require non-electrolyte-containing distention media.
The main disadvantages of vaporizing electrodes are:
- They do not produce a tissue sample for pathology. While uterine sarcomas are very rare, they are not homogeneous. Therefore, a simple sample prior to vaporization does not rule out the disease.
- Since vaporizing electrodes are used at high power, numerous gas bubbles are produced and enter the vascular system. Fortunately, these bubbles dissipate rapidly in the blood. As long as the rate of formation does not exceed the rate of dissipation, there are no significant clinical sequelae. The surgeon can avoid complications by monitoring the patient’s endtidal CO2 and maintaining communication with the anesthesiologist. If a sudden drop in endtidal CO2 is observed, the surgeon should stop the case until it resolves.9
Monopolar loop electrode. The instrument most commonly used to remove submucosal myomas is the monopolar loop electrode with a continuous-flow resectoscope. This is quite similar to the instrument used to resect the prostate; it has been slightly modified for gynecologic use to provide enhanced fluid inflow and outflow. A key instrument now considered standard is an accurate fluid-monitoring device that can be attached to the resectoscope.
Distention media for monopolar resection. Prior to the introduction of continuous high-flow resectoscopes and fluid-management systems, monopolar resectoscopy could only be performed using a solution of 32% dextran 70 and water because of that formulation’s viscosity and facilitation of visualization in the presence of blood. However, due to complications related to absorption, allergies, and the solution’s sticky residue, it is not used with today’s high-flow technology.
The instrument most commonly used to remove submucosal myomas is the monopolar loop electrode with a continuous-flow resectoscope.
When using nonviscous fluids with monopolar RF energy, a distention medium without electrolytes must be selected. Isotonic physiologic media such as normal saline or lactated Ringer’s solution would cause the energy at the electrode to disperse, eliminating the cutting effect. Contrary to popular belief, there would be no burning of the uterine cavity and no subsequent danger to the patient.
The 3 most commonly used fluids for uterine distention are 1.5% glycine, 3% sorbitol, and 5% mannitol. All 3 lack electrolytes. The glycine and sorbitol solutions are hypotonic, while 5% mannitol is isotonic. Although there is some debate over whether an isotonic non-electrolyte-containing medium is safer than a hypotonic one, the guidelines for fluid monitoring are the same for all 3.
The goal of a hysteroscopic myomectomy is to alleviate symptoms without causing a weak myometrium or intracavitary synechia. This is accomplished by removing the fibroid without traumatizing normal uterine tissue.
As a general rule, the serum sodium level will decrease approximately 10 meq for every liter absorbed in the blood stream. That is why, to predict hyponatremia, the gynecologist must rely on exact measurements of fluid deficits rather than operating time or total volume used.
Fluid management. Significant hyponatremia can result in pulmonary edema, transient blindness, cerebral edema, brainstem herniation, and death. Catastrophic as these complications are, they are almost 100% avoidable with accurate deficit measurements and adherence to sound fluid-management protocols.
At our institution, fluids are monitored using a weighted system, and the fluid deficit is displayed on the surgical screen in real time. When the deficit reaches 1 L, electrolytes are drawn, the patient is given 10 mg of intravenous (IV) furosemide, and attempts are made to finish the surgery as soon as possible. When the deficit reaches 1.5 L, the surgery is discontinued no matter how much or how little remains to complete it.
It is important that the protocol at each institution be agreed upon by the chief of service and anesthesia as well as the director of nursing so that there are no conflicts at the time of surgery.
Uterine distention methods
Numerous methods have been used to distend the uterus: gravity, gas pressure, and electronic pumps and pressure bags. The optimal intrauterine pressure is the minimum pressure that allows for flow, distention, and visualization. Most of the time this ranges from 40 mm Hg to 60 mm Hg. The higher the intrauterine pressure, the more rapid the fluid intravasation, particularly when intrauterine pressure exceeds the mean arterial pressure.
Rapid fluid loss can occur even with low intrauterine pressures. That is because a large venous sinus will have a pressure of 8 mm Hg to 10 mm Hg, while the minimum uterine distention pressure is much greater. Strict adherence to fluid-deficit protocols will minimize the risk of complications from fluid overload.
Surgical technique
The goal of a hysteroscopic myomectomy is to alleviate symptoms without causing a weak myometrium or intracavitary synechia. Ideally, this is accomplished by completely removing the fibroid without traumatizing normal uterine tissue. Removal of the entire myoma reduces the likelihood of recurrence and regrowth.
Surgical technique for removal of Types 0, I, and II fibroids is illustrated and explained on (FIGURES1-4).
Within a given range (40 W to 100 W), it makes little difference which cutting-current setting on the RF generator you use; the principles are the same. The loop must be in contact with the tissue to be resected. Energy is applied and, once the circuit is completed (through tissue, the dispersive electrode, the generator and back to the loop), the loop is able to cut with minimal tactile feedback.
The lower the wattage, the more contact necessary between the loop and the tissue and the longer it takes to complete the circuit. Conversely, the higher the power, the faster the circuit is completed and cutting occurs.
Though it does not matter what setting is used, the operator must choose a setting based on his or her comfort level. Lower power gives the operator more control. Higher power should be used only by the most experienced hysteroscopist, since it involves very little tactile feedback and a higher risk of uterine perforation. At our teaching institution, we use 60 W of pure cutting current.
The technique for removing a submucous fibroid depends on its type and location within the endometrial cavity.
Type 0. Because these fibroids are pedunculated, the operator has the option of cutting the stalk (FIGURE 1) or shaving the myoma to remove it in pieces through the cervix. If the stalk is cut, it often is difficult to remove the fibroid through the cervix due to its large size. Nor can the fibroid be cut with monopolar energy because of the difficulty of completing the circuit. Type 0 myomas can be grabbed blindly with a Corson forceps or under direct visualization with an Isaacson optical tenaculum (Karl Storz Endoscopy, Culver City, Calif.). It also is acceptable to leave the fibroid in the cavity and let it degenerate and be expelled spontaneously. The risk of infection is very small.
My preference is to shave the fibroid down to the endometrium while it is attached to the stalk. I find it easier to remove when it is anchored rather than trying to pull out a single large specimen.
Type I. In a Type I myoma, more than 50% of the fibroid is within the uterine cavity, with a smaller portion embedded in the myometrium. To remove these fibroids, shave each to the level of the endometrium. To do so, place the loop behind the fibroid to be resected (FIGURE 5). Next, activate the RF energy using a foot pedal and, once the circuit is complete, draw the loop back into the resectoscope. Be careful to maintain visualization of the fibroid, loop, and cavity (FIGURE 6). This becomes difficult as the fibroid approaches the resectoscope and obstructs the hysteroscopic lens. To avoid this, bring the loop—which begins its cut fully extended—only halfway back to the resectoscope (FIGURE 7). Then retract the resectoscope itself, with the loop halfway extended, until the cut through the fibroid is complete (FIGURE 8). The loop then is retracted back into the resectoscope. The angle of the resectoscope must be adjusted to maintain loop contact with the fibroid tissue.
When more than 70% or 80% of the fibroid has been resected, the remainder will slough spontaneously over the next 2 to 4 months.
Once the fibroid is resected to the level of the endometrium, remove the fibroid chips (FIGURE 2). Often, when the hysteroscope is removed and uterine distention is reestablished, more of the fibroid will protrude into the cavity, facilitating safe resection. At some point, the fibroid will no longer protrude into the cavity; it will have been removed or only a small percentage will remain intramural.
Often, when more than 70% or 80% of the fibroid has been resected, the remainder will slough spontaneously over the next 2 to 4 months. The technique for removing the intramural fibroid is described on page 78 (FIGURE 3).
Type II. The first step in resecting a Type II myoma is shaving the intracavitary portion to the level of the endometrium, as in the resection of a Type I fibroid. Often, once a small Type II myoma is “unroofed,” the remainder will spontaneously fall into the cavity and can easily be removed.
Larger Type II myomas require a different approach. In these, the goal is to identify the pseudo-capsular plane that allows for blunt dissection. Once you have identified it, use the loop electrode to bluntly dissect (without RF activation) the fibroid from the myometrium. Place the electrode in the capsular plane and retract as much of the myoma into the cavity as possible (FIGURE 4). Another way to do this is to use a twisting motion—similar to those performed in open myomectomies—with the optical tenaculum. Once the fibroid is in the cavity, shave it to the level of the endometrium. Repeat this technique until you have removed as much of the fibroid as possible. Use minimal distention pressure when you perform this technique so that the fibroid will not be pressed back into the myometrium.
Underutilization of hysteroscopic myomectomy may be due to inadequate training in the technique or a misconception that it is difficult to learn. Fortunately, hysteroscope manufacturers are beginning to offer physicians and operating room personnel didactic and hands-on laboratory courses.
FIGURE 1 Surgical technique for removing Type 0, I, II fibroids
Type 0. The loop is placed behind the fibroid. Once activated, the loop is drawn toward the operator to resect the pedicle.
FIGURE 2 Surgical technique for removing Type 0, I, II fibroids
Type I. The myoma is resected to the level of the myometrium.
FIGURE 3 Surgical technique for removing Type 0, I, II fibroids
Resection of intramural portion of Type I and II. The resectoscopic loop is placed in the pseudocapsular lane between the fibroid and the myometrium to bluntly dissect the fibroid into the cavity for further resection.
FIGURE 4 Surgical technique for removing Type 0, I, II fibroids
Type II. The roof of the myoma is shaved off to the level of the myometrium. The insert demonstrates the intramural portion of the myoma that will protrude into the cavity with a reduction in uterine distension pressure.
FIGURE 5 Resect technique—4 steps in resecting a submucous myoma
The loop is placed behind the fibroid with maximal contact with the tissue before RF activation.
FIGURE 6 Resect technique—4 steps in resecting a submucous myoma
The loop is withdrawn toward the operator (see arrow) while cutting the myoma but not so far as to obstruct the hysteroscopic view.
FIGURE 7 Resect technique—4 steps in resecting a submucous myoma
The loop is held in place while the hysteroscope is withdrawn (arrows indicate how to remain in direct contact with myoma) continuing to cut the myoma with visualization.
FIGURE 8 Resect technique—4 steps in resecting a submucous myoma
Once through the myoma, the loop is withdrawn into the hysteroscope.
Management of complications
The primary complications of hysteroscopic myomectomy are bleeding, infection, uterine perforation, and fluid overload.
Bleeding. If postoperative hemorrhage occurs, use a 30-cc Foley balloon to tamponade the vessels, or pack the uterus with gauze soaked in a vasopressin solution. Both can be removed any time from 4 hours to 24 hours following placement. If bleeding persists, uterine artery embolization should be considered prior to hysterectomy.
Most attempts at controlling bleeding with a rollerball are futile because the intrauterine distention pressure necessary to see the venous bleeders hides the actual bleeding. Arterial bleeders can be seen and controlled with the rollerball, but they are much less common.
Infection. Because infection is uncommon with hysteroscopic surgery, routine prophylaxis is unnecessary.
Antibiotics should be used if the patient had cervical laminaria placed preoperatively.
Uterine perforation. The management of uterine perforation depends on the site of perforation and whether energy was used during the perforation. For example, if the perforation was at the fundus and occurred with a blunt, non-energized instrument, observation without laparoscopy is sufficient. If the perforation was lateral or occurred with RF energy, laparoscopy is suggested to rule out a broad ligament hematoma or injury to intraperitoneal vessels and viscera. If the perforation is anterior, a cystoscopy is recommended; internal colon inspection is necessary if the perforation is posterior.
Fluid overload. Complications and prevention can be avoided and treated as previously discussed.
New training opportunities may include virtual reality
Underutilization of hysteroscopic myomectomy may be due to inadequate training in the technique during residency or the misconception that it is difficult to learn. (Training has been further hampered by the lack of a suitable animal model.)
Fortunately, hysteroscope manufacturers are beginning to offer physicians and operating room personnel didactic and hands-on laboratory courses. In the near future, we hope, these companies will hire gynecologic hysteroscopists to support physicians and nurses in the field, as has been done in many other specialties.
The creation of new tools such as virtual-reality simulators will undoubtedly enhance the teaching of operative hysteroscopy.
Dr. Isaacson teaches hysteroscopy courses sponsored by Karl Storz Endoscopy.
- The goal of hysteroscopic myomectomy is complete removal of the fibroid without trauma to normal uterine tissue.
- Patients with Type 0 and Type I fibroids often require only 1 surgery; patients with Type II fibroids should be advised that 2 surgeries may be needed to remove the entire fibroid.
- Adjuvant preoperative hormonal therapy facilitates surgical scheduling, helps prevent further blood loss in patients already suffering from anemia, and reduces distention media intravasation.
- The monopolar loop electrode is the fibroid removal system that is used most often.
Hysteroscopic myomectomy should be offered to all patients with symptomatic submucous fibroids who desire to avoid hysterectomy. Although it is a highly effective, minimally invasive technique, it is underutilized.
Unfortunately, fewer than one third of US gynecologists perform this procedure. In a 1997 survey of members of the American Association of Gynecologic Laparoscopists—an organization committed to minimally invasive surgery—only half of the respondents reported that they perform this surgery.1
The reasons for learning to perform hysteroscopic myomectomy are compelling:
- A large cohort of patients could benefit, since most heavy vaginal bleeding from fibroids is due to the submucous location, and hysteroscopic resection is a much more benign approach than hysterectomy. Symptomatic fibroids account for 27% of all hysterectomies performed in the US (the largest single diagnostic category) and more than 100,000 are performed for fibroids that cause abnormal uterine bleeding.2
- Removing these lesions hysteroscopically greatly improves prognosis in women with recurrent pregnancy loss and infertility due to submucous fibroids.3 Up to 15% of patients presenting with infertility have otherwise asymptomatic uterine defects, including submucous fibroids. For example, a meta-analysis of patients undergoing in vitro fertilization determined that, compared with controls, the relative risk of pregnancy for women with submucous fibroids was 0.32 (95% confidence interval [CI], 0.13–0.70). When the submucous fibroids were resected, the relative risk of pregnancy rose to 1.72 (CI, 1.13–2.58).3
Preoperative evaluation of fibroids
Severity of menorrhagia (the most common symptom) is considered directly related to the volume of the myoma within the endometrial cavity. It is not uncommon to see large tortuous vessels covering the surface of the fibroids; although the exact mechanism of fibroid-related menorrhagia is undetermined, the fragility of these vessels is probably responsible, at least in part.
Additionally, fibroids involving the uterine mucosa or submucosa may interfere with the muscular contraction necessary for hemostasis.
Surgical options and pretreatment depend on fibroid type. Submucous fibroids are classified according to the percentage of the fibroid within the endometrial cavity:4
- Type 0: pedunculated; 100% within the cavity
- Type I: more than 50% within the cavity
- Type II: more than 50% within the myometrium
The type dictates surgical options, determines endometrial pretreatment, and shapes patient expectations. Type 0 and Type I submucosal fibroids are more successfully removed in a single surgery, whereas Type II submucous fibroids usually require 2 procedures for complete removal.5
My preference is office hysteroscopy to evaluate the endometrial cavity combined with vaginal ultrasound to assess intramural disease. The view with the office hysteroscope is the same view you will have during surgery.
Patients with Type II myomas should be informed of the potential need for 2 procedures, as this fact often influences their treatment decisions. In addition, whenever a patient with a Type II myoma is pretreated with a gonadotropin-releasing hormone (GnRH) agonist, the physician should reassess the fibroid preoperatively to ensure that it has not become completely intramural.
Office assessment: Hysteroscopy plus ultrasound. Preoperative assessment can be achieved with a hysterosalpingogram, vaginal ultrasound, hysterosonogram, or office hysteroscopy.
Method. My preference is office hysteroscopy to evaluate the endometrial cavity combined with vaginal ultrasound to assess intramural disease. The view with the office hysteroscope is the same view you will have during surgery. There will be no surprises. With a small flexible hysteroscope using saline for distention, the procedure is done with no tenaculum, no paracervical block, and can be completed with 60 to 100 cc of fluid in less than 1 minute.
Patients are often intrigued to view the myoma responsible for their heavy vaginal bleeding.
Several advantages of preoperative hormonal therapy
Preoperative hormonal therapy has several advantages:
- Since it is best to resect submucous myomas when the endometrium is thin, hormonal therapy facilitates surgical scheduling.
- Since preoperative therapy creates a state of amenorrhea, it enables patients suffering from menorrhagia and anemia to build up their blood counts, reducing the need for transfusion.
- Most importantly, preoperative therapy can reduce blood flow to the uterus, thereby reducing the rate of fluid intravasation.
Adjuvants used for these functions include oral contraceptives, progestogens, danazol, and GnRH agonists. However, a recent Cochrane review suggests that only GnRH agonists reduce fluid absorption during operative hysteroscopy.6
Method. Numerous protocols have been published, but my preference is to administer 1 intramuscular injection of depot leuprolide acetate (7.5 mg) 6 weeks prior to surgery.
A review of ‘systems’
Submucous myomas have been hysteroscopically removed using the neodymium/yttrium aluminum-garnet (Nd:YAG) laser, a monopolar resectoscope loop, a monopolar radiofrequency (RF) vaporizing electrode, a bipolar RF vaporizing electrode, and a bipolar RF loop.
Laser. The Nd:YAG laser is an expensive device that was popular in the late 1980s and early 1990s. It can be used as a cutting tool for pedunculated Type 0 myomas, or it can be used for myolysis by burning numerous holes in the myoma, causing devascularization and shrinkage.7,8
The sole advantage of the Nd:YAG laser is that it can be used in an isotonic-fluid-filled cavity. Now that accurate fluid-monitoring devices are available, the Nd:YAG laser is rarely used for this indication.
Vaporizing electrodes. Both the bipolar system and monopolar vaporizing electrodes utilize very high RF power (200 W to 300 W) to vaporize fibroids. The advantage is that the fibroids are eradicated very quickly, without any bothersome fibroid chips to remove. The bipolar system can be used with isotonic fluid, whereas the monopolar vaporizing electrodes require non-electrolyte-containing distention media.
The main disadvantages of vaporizing electrodes are:
- They do not produce a tissue sample for pathology. While uterine sarcomas are very rare, they are not homogeneous. Therefore, a simple sample prior to vaporization does not rule out the disease.
- Since vaporizing electrodes are used at high power, numerous gas bubbles are produced and enter the vascular system. Fortunately, these bubbles dissipate rapidly in the blood. As long as the rate of formation does not exceed the rate of dissipation, there are no significant clinical sequelae. The surgeon can avoid complications by monitoring the patient’s endtidal CO2 and maintaining communication with the anesthesiologist. If a sudden drop in endtidal CO2 is observed, the surgeon should stop the case until it resolves.9
Monopolar loop electrode. The instrument most commonly used to remove submucosal myomas is the monopolar loop electrode with a continuous-flow resectoscope. This is quite similar to the instrument used to resect the prostate; it has been slightly modified for gynecologic use to provide enhanced fluid inflow and outflow. A key instrument now considered standard is an accurate fluid-monitoring device that can be attached to the resectoscope.
Distention media for monopolar resection. Prior to the introduction of continuous high-flow resectoscopes and fluid-management systems, monopolar resectoscopy could only be performed using a solution of 32% dextran 70 and water because of that formulation’s viscosity and facilitation of visualization in the presence of blood. However, due to complications related to absorption, allergies, and the solution’s sticky residue, it is not used with today’s high-flow technology.
The instrument most commonly used to remove submucosal myomas is the monopolar loop electrode with a continuous-flow resectoscope.
When using nonviscous fluids with monopolar RF energy, a distention medium without electrolytes must be selected. Isotonic physiologic media such as normal saline or lactated Ringer’s solution would cause the energy at the electrode to disperse, eliminating the cutting effect. Contrary to popular belief, there would be no burning of the uterine cavity and no subsequent danger to the patient.
The 3 most commonly used fluids for uterine distention are 1.5% glycine, 3% sorbitol, and 5% mannitol. All 3 lack electrolytes. The glycine and sorbitol solutions are hypotonic, while 5% mannitol is isotonic. Although there is some debate over whether an isotonic non-electrolyte-containing medium is safer than a hypotonic one, the guidelines for fluid monitoring are the same for all 3.
The goal of a hysteroscopic myomectomy is to alleviate symptoms without causing a weak myometrium or intracavitary synechia. This is accomplished by removing the fibroid without traumatizing normal uterine tissue.
As a general rule, the serum sodium level will decrease approximately 10 meq for every liter absorbed in the blood stream. That is why, to predict hyponatremia, the gynecologist must rely on exact measurements of fluid deficits rather than operating time or total volume used.
Fluid management. Significant hyponatremia can result in pulmonary edema, transient blindness, cerebral edema, brainstem herniation, and death. Catastrophic as these complications are, they are almost 100% avoidable with accurate deficit measurements and adherence to sound fluid-management protocols.
At our institution, fluids are monitored using a weighted system, and the fluid deficit is displayed on the surgical screen in real time. When the deficit reaches 1 L, electrolytes are drawn, the patient is given 10 mg of intravenous (IV) furosemide, and attempts are made to finish the surgery as soon as possible. When the deficit reaches 1.5 L, the surgery is discontinued no matter how much or how little remains to complete it.
It is important that the protocol at each institution be agreed upon by the chief of service and anesthesia as well as the director of nursing so that there are no conflicts at the time of surgery.
Uterine distention methods
Numerous methods have been used to distend the uterus: gravity, gas pressure, and electronic pumps and pressure bags. The optimal intrauterine pressure is the minimum pressure that allows for flow, distention, and visualization. Most of the time this ranges from 40 mm Hg to 60 mm Hg. The higher the intrauterine pressure, the more rapid the fluid intravasation, particularly when intrauterine pressure exceeds the mean arterial pressure.
Rapid fluid loss can occur even with low intrauterine pressures. That is because a large venous sinus will have a pressure of 8 mm Hg to 10 mm Hg, while the minimum uterine distention pressure is much greater. Strict adherence to fluid-deficit protocols will minimize the risk of complications from fluid overload.
Surgical technique
The goal of a hysteroscopic myomectomy is to alleviate symptoms without causing a weak myometrium or intracavitary synechia. Ideally, this is accomplished by completely removing the fibroid without traumatizing normal uterine tissue. Removal of the entire myoma reduces the likelihood of recurrence and regrowth.
Surgical technique for removal of Types 0, I, and II fibroids is illustrated and explained on (FIGURES1-4).
Within a given range (40 W to 100 W), it makes little difference which cutting-current setting on the RF generator you use; the principles are the same. The loop must be in contact with the tissue to be resected. Energy is applied and, once the circuit is completed (through tissue, the dispersive electrode, the generator and back to the loop), the loop is able to cut with minimal tactile feedback.
The lower the wattage, the more contact necessary between the loop and the tissue and the longer it takes to complete the circuit. Conversely, the higher the power, the faster the circuit is completed and cutting occurs.
Though it does not matter what setting is used, the operator must choose a setting based on his or her comfort level. Lower power gives the operator more control. Higher power should be used only by the most experienced hysteroscopist, since it involves very little tactile feedback and a higher risk of uterine perforation. At our teaching institution, we use 60 W of pure cutting current.
The technique for removing a submucous fibroid depends on its type and location within the endometrial cavity.
Type 0. Because these fibroids are pedunculated, the operator has the option of cutting the stalk (FIGURE 1) or shaving the myoma to remove it in pieces through the cervix. If the stalk is cut, it often is difficult to remove the fibroid through the cervix due to its large size. Nor can the fibroid be cut with monopolar energy because of the difficulty of completing the circuit. Type 0 myomas can be grabbed blindly with a Corson forceps or under direct visualization with an Isaacson optical tenaculum (Karl Storz Endoscopy, Culver City, Calif.). It also is acceptable to leave the fibroid in the cavity and let it degenerate and be expelled spontaneously. The risk of infection is very small.
My preference is to shave the fibroid down to the endometrium while it is attached to the stalk. I find it easier to remove when it is anchored rather than trying to pull out a single large specimen.
Type I. In a Type I myoma, more than 50% of the fibroid is within the uterine cavity, with a smaller portion embedded in the myometrium. To remove these fibroids, shave each to the level of the endometrium. To do so, place the loop behind the fibroid to be resected (FIGURE 5). Next, activate the RF energy using a foot pedal and, once the circuit is complete, draw the loop back into the resectoscope. Be careful to maintain visualization of the fibroid, loop, and cavity (FIGURE 6). This becomes difficult as the fibroid approaches the resectoscope and obstructs the hysteroscopic lens. To avoid this, bring the loop—which begins its cut fully extended—only halfway back to the resectoscope (FIGURE 7). Then retract the resectoscope itself, with the loop halfway extended, until the cut through the fibroid is complete (FIGURE 8). The loop then is retracted back into the resectoscope. The angle of the resectoscope must be adjusted to maintain loop contact with the fibroid tissue.
When more than 70% or 80% of the fibroid has been resected, the remainder will slough spontaneously over the next 2 to 4 months.
Once the fibroid is resected to the level of the endometrium, remove the fibroid chips (FIGURE 2). Often, when the hysteroscope is removed and uterine distention is reestablished, more of the fibroid will protrude into the cavity, facilitating safe resection. At some point, the fibroid will no longer protrude into the cavity; it will have been removed or only a small percentage will remain intramural.
Often, when more than 70% or 80% of the fibroid has been resected, the remainder will slough spontaneously over the next 2 to 4 months. The technique for removing the intramural fibroid is described on page 78 (FIGURE 3).
Type II. The first step in resecting a Type II myoma is shaving the intracavitary portion to the level of the endometrium, as in the resection of a Type I fibroid. Often, once a small Type II myoma is “unroofed,” the remainder will spontaneously fall into the cavity and can easily be removed.
Larger Type II myomas require a different approach. In these, the goal is to identify the pseudo-capsular plane that allows for blunt dissection. Once you have identified it, use the loop electrode to bluntly dissect (without RF activation) the fibroid from the myometrium. Place the electrode in the capsular plane and retract as much of the myoma into the cavity as possible (FIGURE 4). Another way to do this is to use a twisting motion—similar to those performed in open myomectomies—with the optical tenaculum. Once the fibroid is in the cavity, shave it to the level of the endometrium. Repeat this technique until you have removed as much of the fibroid as possible. Use minimal distention pressure when you perform this technique so that the fibroid will not be pressed back into the myometrium.
Underutilization of hysteroscopic myomectomy may be due to inadequate training in the technique or a misconception that it is difficult to learn. Fortunately, hysteroscope manufacturers are beginning to offer physicians and operating room personnel didactic and hands-on laboratory courses.
FIGURE 1 Surgical technique for removing Type 0, I, II fibroids
Type 0. The loop is placed behind the fibroid. Once activated, the loop is drawn toward the operator to resect the pedicle.
FIGURE 2 Surgical technique for removing Type 0, I, II fibroids
Type I. The myoma is resected to the level of the myometrium.
FIGURE 3 Surgical technique for removing Type 0, I, II fibroids
Resection of intramural portion of Type I and II. The resectoscopic loop is placed in the pseudocapsular lane between the fibroid and the myometrium to bluntly dissect the fibroid into the cavity for further resection.
FIGURE 4 Surgical technique for removing Type 0, I, II fibroids
Type II. The roof of the myoma is shaved off to the level of the myometrium. The insert demonstrates the intramural portion of the myoma that will protrude into the cavity with a reduction in uterine distension pressure.
FIGURE 5 Resect technique—4 steps in resecting a submucous myoma
The loop is placed behind the fibroid with maximal contact with the tissue before RF activation.
FIGURE 6 Resect technique—4 steps in resecting a submucous myoma
The loop is withdrawn toward the operator (see arrow) while cutting the myoma but not so far as to obstruct the hysteroscopic view.
FIGURE 7 Resect technique—4 steps in resecting a submucous myoma
The loop is held in place while the hysteroscope is withdrawn (arrows indicate how to remain in direct contact with myoma) continuing to cut the myoma with visualization.
FIGURE 8 Resect technique—4 steps in resecting a submucous myoma
Once through the myoma, the loop is withdrawn into the hysteroscope.
Management of complications
The primary complications of hysteroscopic myomectomy are bleeding, infection, uterine perforation, and fluid overload.
Bleeding. If postoperative hemorrhage occurs, use a 30-cc Foley balloon to tamponade the vessels, or pack the uterus with gauze soaked in a vasopressin solution. Both can be removed any time from 4 hours to 24 hours following placement. If bleeding persists, uterine artery embolization should be considered prior to hysterectomy.
Most attempts at controlling bleeding with a rollerball are futile because the intrauterine distention pressure necessary to see the venous bleeders hides the actual bleeding. Arterial bleeders can be seen and controlled with the rollerball, but they are much less common.
Infection. Because infection is uncommon with hysteroscopic surgery, routine prophylaxis is unnecessary.
Antibiotics should be used if the patient had cervical laminaria placed preoperatively.
Uterine perforation. The management of uterine perforation depends on the site of perforation and whether energy was used during the perforation. For example, if the perforation was at the fundus and occurred with a blunt, non-energized instrument, observation without laparoscopy is sufficient. If the perforation was lateral or occurred with RF energy, laparoscopy is suggested to rule out a broad ligament hematoma or injury to intraperitoneal vessels and viscera. If the perforation is anterior, a cystoscopy is recommended; internal colon inspection is necessary if the perforation is posterior.
Fluid overload. Complications and prevention can be avoided and treated as previously discussed.
New training opportunities may include virtual reality
Underutilization of hysteroscopic myomectomy may be due to inadequate training in the technique during residency or the misconception that it is difficult to learn. (Training has been further hampered by the lack of a suitable animal model.)
Fortunately, hysteroscope manufacturers are beginning to offer physicians and operating room personnel didactic and hands-on laboratory courses. In the near future, we hope, these companies will hire gynecologic hysteroscopists to support physicians and nurses in the field, as has been done in many other specialties.
The creation of new tools such as virtual-reality simulators will undoubtedly enhance the teaching of operative hysteroscopy.
Dr. Isaacson teaches hysteroscopy courses sponsored by Karl Storz Endoscopy.
1. Hulka JF, Levy BS, Luciano AA, Parker WH, Phillips JM. 1997 AAGL membership survey: practice profiles. J Amer Assoc Gynecol Laparosc. 1998;5:93-96.
2. Carlson KJ, Nichols DH, Schiff I. Indications for hysterectomy. N Eng J Med. 1993;328:856-860.
3. Pritts E. Fibroids and infertility: a systematic review of the evidence. Obstet Gynecol Survey. 2001;56:483-491.
4. deBlok S, Dijkman AB, Hemrika DJ. Transcervical resection of fibroids (TCRM): results related to hysteroscopic classification. Gynaecol Endosc. 1995;4:243-246.
5. Istre O. Transcervical resection of endometrium and fibroids: the outcome of 412 operations performed over 5 years. Acta Obstet Gynecol Scand. 1996;75:567-574.
6. Sowter MC, Lethaby A, Singla AA. Preoperative endometrial thinning agents before hysteroscopic surgery for heavy menstrual bleeding. Cochrane Database Syst Rev. 2002;(3):CD001124.-
7. Baggish MS, Sze EM, Morgan G. Hysteroscopic treatment of symptomatic submucous myomas with the Nd:YAG laser. J Gynecol Surg. 1989;5:27-36.
8. Donnez J, Gillerot S, Bourgonjon D, et al. Neodymium:YAG laser hysteroscopy in large submucous fibroids. Fertil Steril. 1990;54:999-1003.
9. Bloomstone J, Chen C, Isselbacher E, Isaacson K. A pilot study examining the frequency and quantity of gas embolization during operative hysteroscopy using a monopolar resectoscope. J Am Assoc Gynecol Laparosc. 2002;9:9-14.
1. Hulka JF, Levy BS, Luciano AA, Parker WH, Phillips JM. 1997 AAGL membership survey: practice profiles. J Amer Assoc Gynecol Laparosc. 1998;5:93-96.
2. Carlson KJ, Nichols DH, Schiff I. Indications for hysterectomy. N Eng J Med. 1993;328:856-860.
3. Pritts E. Fibroids and infertility: a systematic review of the evidence. Obstet Gynecol Survey. 2001;56:483-491.
4. deBlok S, Dijkman AB, Hemrika DJ. Transcervical resection of fibroids (TCRM): results related to hysteroscopic classification. Gynaecol Endosc. 1995;4:243-246.
5. Istre O. Transcervical resection of endometrium and fibroids: the outcome of 412 operations performed over 5 years. Acta Obstet Gynecol Scand. 1996;75:567-574.
6. Sowter MC, Lethaby A, Singla AA. Preoperative endometrial thinning agents before hysteroscopic surgery for heavy menstrual bleeding. Cochrane Database Syst Rev. 2002;(3):CD001124.-
7. Baggish MS, Sze EM, Morgan G. Hysteroscopic treatment of symptomatic submucous myomas with the Nd:YAG laser. J Gynecol Surg. 1989;5:27-36.
8. Donnez J, Gillerot S, Bourgonjon D, et al. Neodymium:YAG laser hysteroscopy in large submucous fibroids. Fertil Steril. 1990;54:999-1003.
9. Bloomstone J, Chen C, Isselbacher E, Isaacson K. A pilot study examining the frequency and quantity of gas embolization during operative hysteroscopy using a monopolar resectoscope. J Am Assoc Gynecol Laparosc. 2002;9:9-14.
Laparoscopic Burch colposuspension for stress urinary incontinence: When, how, and why?
- Laparoscopic Burch colposuspension provides high long-term success rates, reduced morbidity, and accelerated convalescence.
- A growing number of studies have shown the laparoscopic Burch to have results similar to traditional laparotomy when conventional surgical techniques and suture materials are used.
- When we limit the discussion to 2 comparable techniques—a laparoscopic versus open 2-suture procedure—there is moderately strong evidence that the laparoscopic approach maintains efficacy while modestly reducing morbidity.
- The selection of suture material and the total number and placement of sutures are crucial to the long-term cure rate.
Despite the growing body of medical knowledge on stress urinary incontinence (SUI), controversies over its management remain.
SUI is the most common type of inconti-nence and occurs almost exclusively in females. A recent survey by the National Association for Continence revealed that SUI affects approximately 16.5 million women in the United States.1 Nearly two thirds of these women are under 50 years of age.
Still, there is no surgical procedure of choice for women with this condition. In fact, a recent systematic review of the literature by Black and Downs could not determine the “best procedure” based on scientific clinical evidence.2
Among the large number of surgical options for SUI treatment is bladder-neck suspension via a laparoscopic Burch colposus-pension. When it is properly executed, this procedure offers high long-term success rates, reduced morbidity, and accelerated convalescence. In this article, we describe how to perform the laparoscopic Burch procedure (reviewing both conventional suturing techniques and the Tanagho modification)3 and discuss when to consider a laparoscopic approach. In addition, we explain why the procedure should be part of your surgical options for female SUI.
Retropubic versus transvaginal suspensions
With so many surgeries to choose from, determining which procedure would be best for a woman with genuine stress urinary incontinence is a challenge. In 1997, the American Urological Association published a report designed to offer some guidance.
An 8-member panel reviewed data from 282 articles, all of which followed patients for a minimum of 12 months for short-term cure/dry results, and 48 months for long-term results. The Report on the Surgical Management of Female Stress Urinary Incontinence—based on expert opinion and evidence from the literature (as determined by probability estimates)—stated that retropubic suspensions and slings are the most efficacious procedures for long-term success.
Still, the panel noted that these interventions are associated with slightly higher complication rates—including an increased incidence of voiding dysfunction—and longer convalescence than other SUI procedures. For patients willing to accept these complication rates for the sake of improved long-term success, the panel concluded, retropubic suspension and slings are appropriate. However, for patients valuing a decreased hospital stay, reduced morbidity, and an earlier return to normal activity, transvaginal suspensions—the only minimally invasive option widely offered at that time—were the better option.4
The Burch procedure
In the classic Burch colposuspension, a physician places 2 bilateral nonabsorbable sutures through the pubocervical fascia—1 at the level of the midurethra, the other at the urethrovesical junction (UVJ)—and fixes them to Cooper’s ligament. But since 1991, when Vancaille and Schuessler introduced a laparoscopic approach to a retropubic colposuspension (MMK technique),5 a growing number of studies have shown the laparoscopic Burch to have results similar to traditional laparotomy when conventional surgical techniques and suture materials are used.6-24
A number of reports have also described modifications or alternatives to the classic laparoscopic Burch.25-28 These variations—which use stapling devices, mesh placement, bone anchors, and even fibrin glue—avoid laparoscopic suturing, thereby reducing the surgical complexity and shortening the learning curve. They also may lower the cost per procedure by decreasing time in the operating room.29-32 Still, an experienced laparoscopist who has mastered endoscopic suturing can perform a laparoscopic Burch using “standard” suturing in a time frame comparable to that of one of the modifications.33
Retropubic suspensions and slings are associated with slightly higher complication rates than other SUI procedures.
As far as outcomes go, it is the selection of suture material, the total number of sutures used, and their proper placement that are crucial to an optimal long-term cure rate—regardless of the surgical access to the space of Retzius.34-36 In fact, if a surgeon laparoscopically employs the identical operative technique, “suture for suture,” that he or she would use via laparotomy, there is no biological reason why the continence cure rates would be any different.
When? Burch procedure versus the TVT sling
Several studies have demonstrated that for patients with intrinsic sphincter deficiency (ISD) the Burch colposuspension cure/dry rate is less than that of a standard sling procedure.37 We therefore obtain urodynamic studies on all patients presenting with SUI who we feel are at risk for ISD ( TABLE). For patients with ISD and urethral hypermobility, we recommend the minimally invasive pubovaginal sling tension-free vaginal tape (TVT) procedure. In the absence of urethral hypermobility, we first utilize periurethral bulking agents to correct the ISD. If this is not successful, we proceed with urethrolysis and a traditional sling procedure.
For women who have concomitant pelvic-support defects such as uterine prolapse, vaginal vault inversion, or lateral cystoceles, we routinely perform laparoscopic reconstructive surgery, including the laparoscopic Burch for correction of the SUI.
Still, the difficult question remains: Which minimally invasive procedure—a laparoscopic Burch or a TVT—is preferable for the patient with genuine SUI without ISD or any additional pelvic-support defects aside from urethral hypermobility? Only a few studies comparing the clinical outcomes of the TVT and laparoscopic Burch procedures have reported preliminary findings. One retrospective study of 74 women followed for at least 1 year demonstrated an overall objective cure rate of 88% for the laparoscopic Burch versus 92% for the TVT procedure.38 There were no significant differences in time to resumption of normal voiding or in irritative symptoms such as frequency, urgency, and urge incontinence. The TVT group, however, was noted to have a shorter operative time and hospital stay.
Another study reported a higher cure or improvement rate (94%) among patients undergoing the laparoscopic Burch than the TVT (82%). Postoperative voiding difficulty was also significantly less in the laparoscopic group (0% versus 18%).39
Although these early studies suggest that laparoscopic Burch and TVT are comparable, we anxiously await the results of welldesigned, prospective, randomized clinical trials currently under way. One recent report (level I evidence) has demonstrated that the open Burch and the TVT procedure have equivalent results.40
TABLE
Patients at risk for intrinsic sphincter deficiency
|
How? The laparoscopic Burch technique
Preparing the patient. As always, obtain informed consent prior to the procedure. Beyond the usual surgical risks of blood loss, infection, surgical injury, failure rate, and thromboembolic complications, patients also face potential postoperative voiding dysfunction, as mentioned earlier, as well as de novo detrusor instability. Also inform your patients of the possible conversion to laparotomy.
Administer a single intravenous dose of an appropriate broad-spectrum antibiotic no more than 1 hour prior to surgery. For patients undergoing additional laparoscopic reconstructive surgery, we recommend a modified bowel preparation to improve visualization by decompressing the sigmoid colon.
Administer general anesthesia and place the patient in a dorsal lithotomy position with both arms tucked. Support the patient’s lower extremities with Allen Universal Stirrups (Allen Medical Systems, Mayfield, Ohio) and avoid excessive flexion of the knees or hips. Insert a 16F 3-way Foley catheter into the bladder—this allows intermittent bladder filling during the procedure—and inflate the bulb to 10 cc to facilitate identification of the UVJ throughout surgery.
Entering the space of Retzius. We routinely perform operative laparoscopy after Veress needle insertion and insufflation through an umbilical incision. (Use open laparoscopy for patients with prior abdominal surgery and paraumbilical scarring.)
Under direct visualization, place 2 additional accessory 10-mm trocars in the lower quadrants, just lateral to the inferior epigastric arteries. Brief insufflation to greater than 20 mm Hg intra-abdominal pressure facilitates safe entry for these secondary trocars. Although you may opt for smaller trocars, the 10-mm size allows unhindered passage of suture, thus providing more options for maximizing favorable ergonomics with future suture placement.
Although a preperitoneal, or extraperitoneal, approach has been described, we favor a transperitoneal entrance into the space of Retzius. The extraperitoneal approach allows the use of regional anesthesia, avoids intraabdominal adhesions, and eliminates the associated risks of peritoneal entry.31 The disadvantages, however, are significant, including failure to enter the retropubic space secondary to abdominal wall scarring, the inability to perform concomitant vault suspension, and the cost of commercially available dissecting balloons. With experience, a transperitoneal approach will not prolong operative time.
With experience, a transperitoneal approach into the space of Retzius will not prolong operative time.
Approaching the bladder. Distend the bladder in a retrograde fashion with 300 mL to 400 mL of normal saline. This allows identification of the superior margin of the bladder dome and provides mass traction posteriorly. Use the urachus to identify the midline; then, grasp the anterior abdominal wall peritoneum and apply downward traction ( FIGURE 1). Next, create a transverse incision 3 cm to 4 cm above the bladder reflection, using monopolar endoscopic scissors on a 70-watt pure-cut setting ( FIGURE 2). The incision should be within the obliterated umbilical ligaments, but can be extended slightly beyond for patients undergoing a combined laparoscopic Burch-paravaginal repair.41 Using a combination of blunt and electrocautery dissection, you then can easily dissect the loose areolar tissue of the prevesicle space down to the level of the pubic symphysis and ramus ( FIGURE 3).
FIGURE 1
Pictured is the distended bladder, forceps (right) at the bladder margin, endoshears (left) at level of incision, and urachus.
FIGURE 2
Create a transverse incision using monopolar scissors. Note the loose areolar tissue of prevesicle space.
FIGURE 3
Locate the pubic symphysis and ramus using the pelvic brim as a landmark.As the paravesical space is further developed, the pubocervical fascia will become exposed at the level of the UVJ. You must carefully protect the urethra, avoiding aggressive midline dissection as well as the obturator neurovascular bundle laterally. Medial traction on the bladder, perpendicular to the slope of the pubic ramus, encourages identification of the proper surgical plane. Use electrocautery to maintain meticulous hemostasis at all times. Identify Cooper’s ligament, and bluntly dissect away any obstructing fat or areolar tissue (FIGURE 4). To encourage scarification, gently remove excessive overlying periurethral and perivesical fat from pubocervical fascia at the level of the bladder neck, while avoiding any dissection within 1 cm lateral to the urethra ( FIGURE 5).
Placing the sutures. Using an extra-long (36-in), doubled-armed, nonabsorbable suture on an SH needle, place the sutures in the consistent sequence outlined below (note that sturdy needle drivers will facilitate secure needle placement):
First, introduce a needle from the contralateral port and pass it through the pubocervical fascia at the level of the midurethra, using your index finger for transvaginal guidance. If you think the tissue bite will not purchase nearly the entire thickness of the anterior vaginal wall, place a second helical throw (FIGURE 6). Next, bring the suture up through Cooper’s ligament and “store” it by hooking the anterior wall peritoneum (FIGURE 7). Bring up the second arm (needle) of the suture through Cooper’s ligament, but at a different depth than the first pass so ligament fibers are truly encircled by the suture (FIGURE 8). Retrieve both needles, bringing them out through the same port, but do not yet tie the suture.
Introduce the second suture through the ipsilateral port and place it in the same fashion at the level of the UVJ. Again, use helical throws as necessary. Once both sutures have been placed, tie them extracorporeally in sequence using a closed-loop knot pusher. (Waiting until both sutures are placed before tying allows exposure for easy placement of the second suture [FIGURE 9].) The appropriate tension should create a small, localized “knuckle” of pubocervical fascia that approximates laterally to the obturator internus fascia ( FIGURE 10).
Repeat this procedure in the same sequence on the opposite side of the pelvis ( FIGURE 11), then close the retropubic space using a running continuous 2-0 suture reapproximating the peritoneum (FIGURE 12). Close the laparoscopic ports at the fascia level using a Veress needle threaded with a 0-Vicryl. Both ends of the suture are passed on either side of the fascial incision. Using a contralateral grasping forceps, the suture end is freed from the Veress needle, then retrieved using an ipsilateral forceps. This port closure technique is easy to perform as well as cost-effective.
Postoperative care. Place a suprapubic catheter with a 2-way stop clock; this makes postoperative voiding trials easier for both patients and nursing staff.
Most patients will be discharged the day after surgery. If the patient still has an elevated postvoid residual, she’ll likely find going home with the suprapubic catheter more acceptable than intermittent self-catheterization or an indwelling Foley running to a leg bag. For postoperative discomfort, acetaminophen and nonsteroidal anti-inflammatory preparations are generally sufficient. Patients can resume normal living activities within days, but should be cautioned to delay strenuous work or exercise for at least 8 weeks.
FIGURE 4
Apply medial traction to the bladder as the paravesical space is developed down to the level of pubocervical fascia. Note Cooper’s ligament, seen anteriorly.
FIGURE 5
Gently remove the overlying periurethral and perivesical fat to expose white pubocervical fascia.
FIGURE 6
Apply counter traction on the suture from the first pass to facilitate better tissue purchase on the second pass of the helical suture.
FIGURE 7
Place the needle through Cooper’s ligament.
FIGURE 8
Pass the second needle at a deeper depth through Cooper’s ligament to encircle fibers within the suture. This will minimize the risk of “pull out.”
FIGURE 9
Place both sutures before extracorporeal tying.
FIGURE 10
Suture tension should create only a small knuckle of pubocervical fascia, approximating obturator fascia laterally.
FIGURE 11
Both sides completed.
FIGURE 12
Close the retropubic space.
Why? A look at the evidence
The learning curve for laparoscopic Burch is steep and somewhat long—approximately 20 cases. The real question, therefore, is whether the benefits justify the time needed to master this procedure. In other words, is there clinical evidence that, once the plateau of this curve has been reached, we can reduce patient morbidity while maintaining efficacy compared to the traditional open technique? If not, there’s little reason for surgeons to learn the technique. If there is, however, more physicians should include the laparoscopic Burch in their surgical arsenal.
The real question is whether the benefits of the laparoscopic Burch justify the time needed to master the procedure.
When we limit the discussion to 2 comparable techniques, a laparoscopic versus open 2-suture procedure, there is moderately strong evidence that the laparoscopic approach maintains efficacy while modestly reducing morbidity.42 The strength of this evidence is established in 6 studies, including 3 randomized trials (level I evidence).8,18,23,24,43,44
Currently, data are insufficient regarding a laparoscopic approach to make concomitant site-specific defect repairs.45-51 However, if the procedures are performed in the same fashion, “suture for suture,” as their abdominal counterparts, we should expect to see, as we have with the laparoscopic Burch, similar efficacy rates between the laparoscopic and open approaches.
So, in closing, we encourage our colleagues to reignite their interest in learning laparoscopic reconstructive surgery and recommit to reaching that learning curve plateau. Why? We owe it to our patients.
Dr. Lucente is a consultant for GyneCare. Dr. Murphy reports no financial relationship with any companies whose products are mentioned in this article.
1. National Association for Incontinence Quarterly Report. Summer 2002, Vol 20 No 3.
2. Black NA, Down SH. The effectiveness of surgery for stress incontinence in women: A systematic review. Br J Urol. 1996;78:497.-
3. Tanagho E. Colpocystourethropexy: The way we do it. J Urol. 1976;116:751-753.
4. Leach GE, Dinochowski RR, Appell RA, et al. Female stress urinary incontinence clinical guidelines panel: Report on the surgical management of female stress urinary incontinence. Baltimore, Md: American Urological Association, Inc.; 1997.
5. Vancaille TG, Schuessler W. Laparoscopic bladder neck suspension. J Laparoendosc Surg. 1991;1:169-173.
6. Albala DM, Schuessler WW, Vancaillie TG. Laparoscopic bladder suspension for the treatment of stress incontinence. Semin Urol. 1992;10:222-225.
7. Burton G. A randomized comparison of laparoscopic and open colposuspension [abstract]. Neurourol Urodyn. 1993;16:353-354.
8. Polascik TJ, Moore RG, Roseberg MT, Kavoussi LR. Comparison of laparoscopic and open retropubic colposuspension for treatment of stress urinary incontinence. Urology. 1995;45:647-652.
9. Liu CY. Laparoscopic treatment of genuine urinary stress incontinence. Clin Obstet Gynecol. 1994;8:789-798.
10. Gunn GC, Cooper RP, Gordon NS, Gragnon L. Use of a new device for endoscopic suturing in the laparoscopic Burch procedure. J Am Assoc Gynecol Laparosc. 1994;2:65-70.
11. Nezhat CH, Nezhat F, Nezhat CR, et al. Laparoscopic retropubic cystocolposuspension. J Am Assoc Gynecol Laparosc. 1994;1:339-349.
12. Lyons TL, Winer WK. Clinical outcomes with laparoscopic approaches and open Burch procedures for urinary stress incontinence. J Am Assoc Gynecol Laparosc. 1995;2:193-197.
13. McDougal EM, Klutke CG, Cornell T. Comparison of transvaginal versus laparoscopic bladder neck suspension for stress urinary incontinence. Adult Urol. 1995;45:641-645.
14. Ross JW. Laparoscopic Burch repair compared to laparotomy Burch for cure of urinary stress incontinence. Int Urogynecol. 1995;6:323-328.
15. Langebrekke A, Dahlstrom B, Eraker R, Urnes A. The laparoscopic Burch procedure: a preliminary report. Acta Obstet Gynecol Scand. 1995;74:153-155.
16. Radomski SB, Herschorn S. Laparoscopic Burch bladder neck suspension: early results. J Urol. 1996;155:515-518.
17. Ross JW. Two techniques of laparoscopic Burch repair for stress incontinence: a prospective randomized study. J Am Assoc Gynecol Laparoscopists. 1996;3:351-357.
18. Lam AM, Jenkins GJ, Hyslop RS. Laparoscopic results. Med J Aust. 1995;162:18-22.
19. Su TH, Wang KG, Hsu CY, Wei H, Hong BK. Prospective comparison of laparoscopic and traditional colposuspensions in the treatment of genuine stress inconti-nence. Acta Obstet Gynecol. 1997;76:576-582.
20. Papasakelariou C, Papasakelariou B. Laparoscopic bladder neck suspension. J Am Assoc Gynecol Laparosc. 1997;4:185-188.
21. Lobel RW, Davis GD. Long-term results of laparoscopic Burch colposuspension. J Am Assoc Gynecol Laparosc. 1997;4:341-345.
22. Ross JW. Multichannel urodynamic evaluation of laparoscopic Burch colposuspension for genuine stress incontinence. Obstet Gynecol. 1998;91:55-59.
23. Miannay E, Cosson M, Querleu D, et al. [Comparison of laparoscopic and laparotomy colposuspension in the treatment of urinary stress incontinence. Comparative study of 72 matched cases][French]. Contracept Fertil Sex. 1998;26:376-385.
24. Saidi MH, Gallagher MS, Skop IP, et al. Extrperitoneal laparoscopic colposuspension: short-term cure rate, complications and duration of hospital stay in comparison with Burch colposuspension. Obstet Gynecol. 1998;92:619-625.
25. Henley C. The Henley staple-suture technique for laparoscopic Burch colposuspension. J Am Assoc Gynecol Laparosc. 1995;2:441-444.
26. Das S, Palmer JK. Laparoscopic colposuspension. J Urol. 1995;154:1119-1121.
27. Ou CS, Presthus J, Beadle E. Laparoscopic bladder neck suspension using hernia mesh and surgical staples. J Laparoendosc Surg. 1993;3:563-566.
28. Kiilholma P, Haarala M, Polvi II, Makinen J, Chancellor MB. Sutureless colposuspension with fibrin sealant. Tech Urol. 1995;1:81-83.
29. Lose G. Laparoscopic Burch colposuspension. Acta Obstet Gynecol Scand Suppl. 1998;168:29-33.
30. Miannay E, Cosson M, Lanvin D, et al. Comparison of open retropubic and laparoscopic colposuspension for treatment of stress urinary incontinence. Eur J Obstet Gynecol Reprod Biol. 1998;79:159-166.
31. Saidi MH, Sadler RK, Saidi JA. Extraperitoneal laparoscopic colposuspension for genuine urinary stress incontinence. J Am Assoc Gynecol Laparosc. 1998;9:249-252.
32. Ou CS, Rowbotham R. Five-year follow-up of a laparoscopic bladder neck suspension using synthetic mesh and surgical staples. J Laparoendosc Adv Surg Techn A. 1999;9:249-252.
33. Zullo F, Morelli M, Russo T, et al. Two techniques of laparoscopic retropubic urethropexy. J Am Assoc Gynecol Laparosc. 2001;12:323-327.
34. Langer R, Lipshitz Y, Halperin R, et al. Long-term (10-15 years) follow-up after Burch colposuspension for urinary stress incontinence. Int Urogynecol J. 2001;12:323-327.
35. Herbertsson G, Iosif CS. Surgical results and urodynamic studies 10 years after retropubic colpurethropexy. Acta Obstet Gynecol Scand. 1993;72:298-301.
36. Parasio M, Falcone T, Walters M. Laparoscopic surgery for genuine stress incontinence. Int Urogynecol J. 1999;10:237-247.
37. Horbach NS. Suburethral sling procedures. Urogynecology and Urodynamics: Theory and Practice. 4th ed. Baltimore, Md: Williams and Wilkins 1996;569-579.
38. Vassallo B, Murphy M, Lucente V, Karram M. TVT versus Laparoscopic Burch: a retrospective review at 1-4 years. 27th Scientific Meeting of the Society of Gynecologic Surgeons. March 5-7, 2001.
39. Fotte A. Which is the best minimally invasive procedure? TVT versus laparoscopic colposuspension. Presented at the 31st annual meeting of the International Continence Society; September 18-21, 2001; Seoul, Korea.
40. Ward KL, Hilton P. Prospective multicentre randomized trial of tension-free vaginal tape and colposuspension as primary treatment for stress incontinence. BMJ. 2002;326:67.-
41. Miklos J, Kohli N. Laparoscopic paravaginal repair plus Burch colposuspension: review and descriptive technique. Urology. 2000;56:64-68.
42. Walter A. Laparoscopic repairs for stress urinary incontinence and pelvic organ prolapse: “show me the evidence.” Presented at the annual meeting of the American Urogynecologic Society; 2002; San Diego, Calif.
43. Summitt RL, Lucente V, Karram MM, Shull BL, Bent AE. Randomized comparison of laparoscopic and transabdominal Burch urethropexy for the treatment of genuine stress incontinence. Obstet Gynecol. 2000;95:(suppl):2.-
44. Fatthy H, El Hao M, Samaha I, Abdallah K. Modified Burch colposuspension: laparoscopy versus laparotomy. J Am Assoc Gynecol Laparosc. 2001;8:99-106.
45. Ross JW. Techniques of laparoscopic repair for total vault eversion after hysterectomy. J Am Assoc Gynecol Laparosc. 1997;4:173-183.
46. Ross JW. Laparoscopic approach for severe pelvic vault prolapse. J Am Assoc Gynecol Laparosc. 1996;3(suppl):43.-
47. Lyons TL, Winer WK. Laparoscopic rectocele repair using polyglactin mesh. J Am Assoc Gynecol Laparosc. 1997;4:381-384.
48. Ostrzenski A. Laparoscopic colposuspension for total vaginal prolapse. Int J Gynaecol Obstet. 1996;55:147-152.
49. Carter JE, Winter M, Mendehlsohn S, Saye W, Richardson AC. Vaginal vault suspension and enterocele repair by Richardson-Saye laparoscopic technique: description of training technique and results. JSLS. 2001;5:29-36.
50. Cosson M, Rajabally R, Bogaert E, Querleu D, Crepin G. Laparoscopic sacrocolpopexy, hysterectomy and Burch colposuspension: feasibility and short-term complications of 77 procedures. JSLS. 2002;6:115-119.
51. Miklos JR, Moore RD, Kohli N. Laparoscopic surgery for pelvic support defects. Curr Opin Obstet Gynecol. 2002;14:387-395.
- Laparoscopic Burch colposuspension provides high long-term success rates, reduced morbidity, and accelerated convalescence.
- A growing number of studies have shown the laparoscopic Burch to have results similar to traditional laparotomy when conventional surgical techniques and suture materials are used.
- When we limit the discussion to 2 comparable techniques—a laparoscopic versus open 2-suture procedure—there is moderately strong evidence that the laparoscopic approach maintains efficacy while modestly reducing morbidity.
- The selection of suture material and the total number and placement of sutures are crucial to the long-term cure rate.
Despite the growing body of medical knowledge on stress urinary incontinence (SUI), controversies over its management remain.
SUI is the most common type of inconti-nence and occurs almost exclusively in females. A recent survey by the National Association for Continence revealed that SUI affects approximately 16.5 million women in the United States.1 Nearly two thirds of these women are under 50 years of age.
Still, there is no surgical procedure of choice for women with this condition. In fact, a recent systematic review of the literature by Black and Downs could not determine the “best procedure” based on scientific clinical evidence.2
Among the large number of surgical options for SUI treatment is bladder-neck suspension via a laparoscopic Burch colposus-pension. When it is properly executed, this procedure offers high long-term success rates, reduced morbidity, and accelerated convalescence. In this article, we describe how to perform the laparoscopic Burch procedure (reviewing both conventional suturing techniques and the Tanagho modification)3 and discuss when to consider a laparoscopic approach. In addition, we explain why the procedure should be part of your surgical options for female SUI.
Retropubic versus transvaginal suspensions
With so many surgeries to choose from, determining which procedure would be best for a woman with genuine stress urinary incontinence is a challenge. In 1997, the American Urological Association published a report designed to offer some guidance.
An 8-member panel reviewed data from 282 articles, all of which followed patients for a minimum of 12 months for short-term cure/dry results, and 48 months for long-term results. The Report on the Surgical Management of Female Stress Urinary Incontinence—based on expert opinion and evidence from the literature (as determined by probability estimates)—stated that retropubic suspensions and slings are the most efficacious procedures for long-term success.
Still, the panel noted that these interventions are associated with slightly higher complication rates—including an increased incidence of voiding dysfunction—and longer convalescence than other SUI procedures. For patients willing to accept these complication rates for the sake of improved long-term success, the panel concluded, retropubic suspension and slings are appropriate. However, for patients valuing a decreased hospital stay, reduced morbidity, and an earlier return to normal activity, transvaginal suspensions—the only minimally invasive option widely offered at that time—were the better option.4
The Burch procedure
In the classic Burch colposuspension, a physician places 2 bilateral nonabsorbable sutures through the pubocervical fascia—1 at the level of the midurethra, the other at the urethrovesical junction (UVJ)—and fixes them to Cooper’s ligament. But since 1991, when Vancaille and Schuessler introduced a laparoscopic approach to a retropubic colposuspension (MMK technique),5 a growing number of studies have shown the laparoscopic Burch to have results similar to traditional laparotomy when conventional surgical techniques and suture materials are used.6-24
A number of reports have also described modifications or alternatives to the classic laparoscopic Burch.25-28 These variations—which use stapling devices, mesh placement, bone anchors, and even fibrin glue—avoid laparoscopic suturing, thereby reducing the surgical complexity and shortening the learning curve. They also may lower the cost per procedure by decreasing time in the operating room.29-32 Still, an experienced laparoscopist who has mastered endoscopic suturing can perform a laparoscopic Burch using “standard” suturing in a time frame comparable to that of one of the modifications.33
Retropubic suspensions and slings are associated with slightly higher complication rates than other SUI procedures.
As far as outcomes go, it is the selection of suture material, the total number of sutures used, and their proper placement that are crucial to an optimal long-term cure rate—regardless of the surgical access to the space of Retzius.34-36 In fact, if a surgeon laparoscopically employs the identical operative technique, “suture for suture,” that he or she would use via laparotomy, there is no biological reason why the continence cure rates would be any different.
When? Burch procedure versus the TVT sling
Several studies have demonstrated that for patients with intrinsic sphincter deficiency (ISD) the Burch colposuspension cure/dry rate is less than that of a standard sling procedure.37 We therefore obtain urodynamic studies on all patients presenting with SUI who we feel are at risk for ISD ( TABLE). For patients with ISD and urethral hypermobility, we recommend the minimally invasive pubovaginal sling tension-free vaginal tape (TVT) procedure. In the absence of urethral hypermobility, we first utilize periurethral bulking agents to correct the ISD. If this is not successful, we proceed with urethrolysis and a traditional sling procedure.
For women who have concomitant pelvic-support defects such as uterine prolapse, vaginal vault inversion, or lateral cystoceles, we routinely perform laparoscopic reconstructive surgery, including the laparoscopic Burch for correction of the SUI.
Still, the difficult question remains: Which minimally invasive procedure—a laparoscopic Burch or a TVT—is preferable for the patient with genuine SUI without ISD or any additional pelvic-support defects aside from urethral hypermobility? Only a few studies comparing the clinical outcomes of the TVT and laparoscopic Burch procedures have reported preliminary findings. One retrospective study of 74 women followed for at least 1 year demonstrated an overall objective cure rate of 88% for the laparoscopic Burch versus 92% for the TVT procedure.38 There were no significant differences in time to resumption of normal voiding or in irritative symptoms such as frequency, urgency, and urge incontinence. The TVT group, however, was noted to have a shorter operative time and hospital stay.
Another study reported a higher cure or improvement rate (94%) among patients undergoing the laparoscopic Burch than the TVT (82%). Postoperative voiding difficulty was also significantly less in the laparoscopic group (0% versus 18%).39
Although these early studies suggest that laparoscopic Burch and TVT are comparable, we anxiously await the results of welldesigned, prospective, randomized clinical trials currently under way. One recent report (level I evidence) has demonstrated that the open Burch and the TVT procedure have equivalent results.40
TABLE
Patients at risk for intrinsic sphincter deficiency
|
How? The laparoscopic Burch technique
Preparing the patient. As always, obtain informed consent prior to the procedure. Beyond the usual surgical risks of blood loss, infection, surgical injury, failure rate, and thromboembolic complications, patients also face potential postoperative voiding dysfunction, as mentioned earlier, as well as de novo detrusor instability. Also inform your patients of the possible conversion to laparotomy.
Administer a single intravenous dose of an appropriate broad-spectrum antibiotic no more than 1 hour prior to surgery. For patients undergoing additional laparoscopic reconstructive surgery, we recommend a modified bowel preparation to improve visualization by decompressing the sigmoid colon.
Administer general anesthesia and place the patient in a dorsal lithotomy position with both arms tucked. Support the patient’s lower extremities with Allen Universal Stirrups (Allen Medical Systems, Mayfield, Ohio) and avoid excessive flexion of the knees or hips. Insert a 16F 3-way Foley catheter into the bladder—this allows intermittent bladder filling during the procedure—and inflate the bulb to 10 cc to facilitate identification of the UVJ throughout surgery.
Entering the space of Retzius. We routinely perform operative laparoscopy after Veress needle insertion and insufflation through an umbilical incision. (Use open laparoscopy for patients with prior abdominal surgery and paraumbilical scarring.)
Under direct visualization, place 2 additional accessory 10-mm trocars in the lower quadrants, just lateral to the inferior epigastric arteries. Brief insufflation to greater than 20 mm Hg intra-abdominal pressure facilitates safe entry for these secondary trocars. Although you may opt for smaller trocars, the 10-mm size allows unhindered passage of suture, thus providing more options for maximizing favorable ergonomics with future suture placement.
Although a preperitoneal, or extraperitoneal, approach has been described, we favor a transperitoneal entrance into the space of Retzius. The extraperitoneal approach allows the use of regional anesthesia, avoids intraabdominal adhesions, and eliminates the associated risks of peritoneal entry.31 The disadvantages, however, are significant, including failure to enter the retropubic space secondary to abdominal wall scarring, the inability to perform concomitant vault suspension, and the cost of commercially available dissecting balloons. With experience, a transperitoneal approach will not prolong operative time.
With experience, a transperitoneal approach into the space of Retzius will not prolong operative time.
Approaching the bladder. Distend the bladder in a retrograde fashion with 300 mL to 400 mL of normal saline. This allows identification of the superior margin of the bladder dome and provides mass traction posteriorly. Use the urachus to identify the midline; then, grasp the anterior abdominal wall peritoneum and apply downward traction ( FIGURE 1). Next, create a transverse incision 3 cm to 4 cm above the bladder reflection, using monopolar endoscopic scissors on a 70-watt pure-cut setting ( FIGURE 2). The incision should be within the obliterated umbilical ligaments, but can be extended slightly beyond for patients undergoing a combined laparoscopic Burch-paravaginal repair.41 Using a combination of blunt and electrocautery dissection, you then can easily dissect the loose areolar tissue of the prevesicle space down to the level of the pubic symphysis and ramus ( FIGURE 3).
FIGURE 1
Pictured is the distended bladder, forceps (right) at the bladder margin, endoshears (left) at level of incision, and urachus.
FIGURE 2
Create a transverse incision using monopolar scissors. Note the loose areolar tissue of prevesicle space.
FIGURE 3
Locate the pubic symphysis and ramus using the pelvic brim as a landmark.As the paravesical space is further developed, the pubocervical fascia will become exposed at the level of the UVJ. You must carefully protect the urethra, avoiding aggressive midline dissection as well as the obturator neurovascular bundle laterally. Medial traction on the bladder, perpendicular to the slope of the pubic ramus, encourages identification of the proper surgical plane. Use electrocautery to maintain meticulous hemostasis at all times. Identify Cooper’s ligament, and bluntly dissect away any obstructing fat or areolar tissue (FIGURE 4). To encourage scarification, gently remove excessive overlying periurethral and perivesical fat from pubocervical fascia at the level of the bladder neck, while avoiding any dissection within 1 cm lateral to the urethra ( FIGURE 5).
Placing the sutures. Using an extra-long (36-in), doubled-armed, nonabsorbable suture on an SH needle, place the sutures in the consistent sequence outlined below (note that sturdy needle drivers will facilitate secure needle placement):
First, introduce a needle from the contralateral port and pass it through the pubocervical fascia at the level of the midurethra, using your index finger for transvaginal guidance. If you think the tissue bite will not purchase nearly the entire thickness of the anterior vaginal wall, place a second helical throw (FIGURE 6). Next, bring the suture up through Cooper’s ligament and “store” it by hooking the anterior wall peritoneum (FIGURE 7). Bring up the second arm (needle) of the suture through Cooper’s ligament, but at a different depth than the first pass so ligament fibers are truly encircled by the suture (FIGURE 8). Retrieve both needles, bringing them out through the same port, but do not yet tie the suture.
Introduce the second suture through the ipsilateral port and place it in the same fashion at the level of the UVJ. Again, use helical throws as necessary. Once both sutures have been placed, tie them extracorporeally in sequence using a closed-loop knot pusher. (Waiting until both sutures are placed before tying allows exposure for easy placement of the second suture [FIGURE 9].) The appropriate tension should create a small, localized “knuckle” of pubocervical fascia that approximates laterally to the obturator internus fascia ( FIGURE 10).
Repeat this procedure in the same sequence on the opposite side of the pelvis ( FIGURE 11), then close the retropubic space using a running continuous 2-0 suture reapproximating the peritoneum (FIGURE 12). Close the laparoscopic ports at the fascia level using a Veress needle threaded with a 0-Vicryl. Both ends of the suture are passed on either side of the fascial incision. Using a contralateral grasping forceps, the suture end is freed from the Veress needle, then retrieved using an ipsilateral forceps. This port closure technique is easy to perform as well as cost-effective.
Postoperative care. Place a suprapubic catheter with a 2-way stop clock; this makes postoperative voiding trials easier for both patients and nursing staff.
Most patients will be discharged the day after surgery. If the patient still has an elevated postvoid residual, she’ll likely find going home with the suprapubic catheter more acceptable than intermittent self-catheterization or an indwelling Foley running to a leg bag. For postoperative discomfort, acetaminophen and nonsteroidal anti-inflammatory preparations are generally sufficient. Patients can resume normal living activities within days, but should be cautioned to delay strenuous work or exercise for at least 8 weeks.
FIGURE 4
Apply medial traction to the bladder as the paravesical space is developed down to the level of pubocervical fascia. Note Cooper’s ligament, seen anteriorly.
FIGURE 5
Gently remove the overlying periurethral and perivesical fat to expose white pubocervical fascia.
FIGURE 6
Apply counter traction on the suture from the first pass to facilitate better tissue purchase on the second pass of the helical suture.
FIGURE 7
Place the needle through Cooper’s ligament.
FIGURE 8
Pass the second needle at a deeper depth through Cooper’s ligament to encircle fibers within the suture. This will minimize the risk of “pull out.”
FIGURE 9
Place both sutures before extracorporeal tying.
FIGURE 10
Suture tension should create only a small knuckle of pubocervical fascia, approximating obturator fascia laterally.
FIGURE 11
Both sides completed.
FIGURE 12
Close the retropubic space.
Why? A look at the evidence
The learning curve for laparoscopic Burch is steep and somewhat long—approximately 20 cases. The real question, therefore, is whether the benefits justify the time needed to master this procedure. In other words, is there clinical evidence that, once the plateau of this curve has been reached, we can reduce patient morbidity while maintaining efficacy compared to the traditional open technique? If not, there’s little reason for surgeons to learn the technique. If there is, however, more physicians should include the laparoscopic Burch in their surgical arsenal.
The real question is whether the benefits of the laparoscopic Burch justify the time needed to master the procedure.
When we limit the discussion to 2 comparable techniques, a laparoscopic versus open 2-suture procedure, there is moderately strong evidence that the laparoscopic approach maintains efficacy while modestly reducing morbidity.42 The strength of this evidence is established in 6 studies, including 3 randomized trials (level I evidence).8,18,23,24,43,44
Currently, data are insufficient regarding a laparoscopic approach to make concomitant site-specific defect repairs.45-51 However, if the procedures are performed in the same fashion, “suture for suture,” as their abdominal counterparts, we should expect to see, as we have with the laparoscopic Burch, similar efficacy rates between the laparoscopic and open approaches.
So, in closing, we encourage our colleagues to reignite their interest in learning laparoscopic reconstructive surgery and recommit to reaching that learning curve plateau. Why? We owe it to our patients.
Dr. Lucente is a consultant for GyneCare. Dr. Murphy reports no financial relationship with any companies whose products are mentioned in this article.
- Laparoscopic Burch colposuspension provides high long-term success rates, reduced morbidity, and accelerated convalescence.
- A growing number of studies have shown the laparoscopic Burch to have results similar to traditional laparotomy when conventional surgical techniques and suture materials are used.
- When we limit the discussion to 2 comparable techniques—a laparoscopic versus open 2-suture procedure—there is moderately strong evidence that the laparoscopic approach maintains efficacy while modestly reducing morbidity.
- The selection of suture material and the total number and placement of sutures are crucial to the long-term cure rate.
Despite the growing body of medical knowledge on stress urinary incontinence (SUI), controversies over its management remain.
SUI is the most common type of inconti-nence and occurs almost exclusively in females. A recent survey by the National Association for Continence revealed that SUI affects approximately 16.5 million women in the United States.1 Nearly two thirds of these women are under 50 years of age.
Still, there is no surgical procedure of choice for women with this condition. In fact, a recent systematic review of the literature by Black and Downs could not determine the “best procedure” based on scientific clinical evidence.2
Among the large number of surgical options for SUI treatment is bladder-neck suspension via a laparoscopic Burch colposus-pension. When it is properly executed, this procedure offers high long-term success rates, reduced morbidity, and accelerated convalescence. In this article, we describe how to perform the laparoscopic Burch procedure (reviewing both conventional suturing techniques and the Tanagho modification)3 and discuss when to consider a laparoscopic approach. In addition, we explain why the procedure should be part of your surgical options for female SUI.
Retropubic versus transvaginal suspensions
With so many surgeries to choose from, determining which procedure would be best for a woman with genuine stress urinary incontinence is a challenge. In 1997, the American Urological Association published a report designed to offer some guidance.
An 8-member panel reviewed data from 282 articles, all of which followed patients for a minimum of 12 months for short-term cure/dry results, and 48 months for long-term results. The Report on the Surgical Management of Female Stress Urinary Incontinence—based on expert opinion and evidence from the literature (as determined by probability estimates)—stated that retropubic suspensions and slings are the most efficacious procedures for long-term success.
Still, the panel noted that these interventions are associated with slightly higher complication rates—including an increased incidence of voiding dysfunction—and longer convalescence than other SUI procedures. For patients willing to accept these complication rates for the sake of improved long-term success, the panel concluded, retropubic suspension and slings are appropriate. However, for patients valuing a decreased hospital stay, reduced morbidity, and an earlier return to normal activity, transvaginal suspensions—the only minimally invasive option widely offered at that time—were the better option.4
The Burch procedure
In the classic Burch colposuspension, a physician places 2 bilateral nonabsorbable sutures through the pubocervical fascia—1 at the level of the midurethra, the other at the urethrovesical junction (UVJ)—and fixes them to Cooper’s ligament. But since 1991, when Vancaille and Schuessler introduced a laparoscopic approach to a retropubic colposuspension (MMK technique),5 a growing number of studies have shown the laparoscopic Burch to have results similar to traditional laparotomy when conventional surgical techniques and suture materials are used.6-24
A number of reports have also described modifications or alternatives to the classic laparoscopic Burch.25-28 These variations—which use stapling devices, mesh placement, bone anchors, and even fibrin glue—avoid laparoscopic suturing, thereby reducing the surgical complexity and shortening the learning curve. They also may lower the cost per procedure by decreasing time in the operating room.29-32 Still, an experienced laparoscopist who has mastered endoscopic suturing can perform a laparoscopic Burch using “standard” suturing in a time frame comparable to that of one of the modifications.33
Retropubic suspensions and slings are associated with slightly higher complication rates than other SUI procedures.
As far as outcomes go, it is the selection of suture material, the total number of sutures used, and their proper placement that are crucial to an optimal long-term cure rate—regardless of the surgical access to the space of Retzius.34-36 In fact, if a surgeon laparoscopically employs the identical operative technique, “suture for suture,” that he or she would use via laparotomy, there is no biological reason why the continence cure rates would be any different.
When? Burch procedure versus the TVT sling
Several studies have demonstrated that for patients with intrinsic sphincter deficiency (ISD) the Burch colposuspension cure/dry rate is less than that of a standard sling procedure.37 We therefore obtain urodynamic studies on all patients presenting with SUI who we feel are at risk for ISD ( TABLE). For patients with ISD and urethral hypermobility, we recommend the minimally invasive pubovaginal sling tension-free vaginal tape (TVT) procedure. In the absence of urethral hypermobility, we first utilize periurethral bulking agents to correct the ISD. If this is not successful, we proceed with urethrolysis and a traditional sling procedure.
For women who have concomitant pelvic-support defects such as uterine prolapse, vaginal vault inversion, or lateral cystoceles, we routinely perform laparoscopic reconstructive surgery, including the laparoscopic Burch for correction of the SUI.
Still, the difficult question remains: Which minimally invasive procedure—a laparoscopic Burch or a TVT—is preferable for the patient with genuine SUI without ISD or any additional pelvic-support defects aside from urethral hypermobility? Only a few studies comparing the clinical outcomes of the TVT and laparoscopic Burch procedures have reported preliminary findings. One retrospective study of 74 women followed for at least 1 year demonstrated an overall objective cure rate of 88% for the laparoscopic Burch versus 92% for the TVT procedure.38 There were no significant differences in time to resumption of normal voiding or in irritative symptoms such as frequency, urgency, and urge incontinence. The TVT group, however, was noted to have a shorter operative time and hospital stay.
Another study reported a higher cure or improvement rate (94%) among patients undergoing the laparoscopic Burch than the TVT (82%). Postoperative voiding difficulty was also significantly less in the laparoscopic group (0% versus 18%).39
Although these early studies suggest that laparoscopic Burch and TVT are comparable, we anxiously await the results of welldesigned, prospective, randomized clinical trials currently under way. One recent report (level I evidence) has demonstrated that the open Burch and the TVT procedure have equivalent results.40
TABLE
Patients at risk for intrinsic sphincter deficiency
|
How? The laparoscopic Burch technique
Preparing the patient. As always, obtain informed consent prior to the procedure. Beyond the usual surgical risks of blood loss, infection, surgical injury, failure rate, and thromboembolic complications, patients also face potential postoperative voiding dysfunction, as mentioned earlier, as well as de novo detrusor instability. Also inform your patients of the possible conversion to laparotomy.
Administer a single intravenous dose of an appropriate broad-spectrum antibiotic no more than 1 hour prior to surgery. For patients undergoing additional laparoscopic reconstructive surgery, we recommend a modified bowel preparation to improve visualization by decompressing the sigmoid colon.
Administer general anesthesia and place the patient in a dorsal lithotomy position with both arms tucked. Support the patient’s lower extremities with Allen Universal Stirrups (Allen Medical Systems, Mayfield, Ohio) and avoid excessive flexion of the knees or hips. Insert a 16F 3-way Foley catheter into the bladder—this allows intermittent bladder filling during the procedure—and inflate the bulb to 10 cc to facilitate identification of the UVJ throughout surgery.
Entering the space of Retzius. We routinely perform operative laparoscopy after Veress needle insertion and insufflation through an umbilical incision. (Use open laparoscopy for patients with prior abdominal surgery and paraumbilical scarring.)
Under direct visualization, place 2 additional accessory 10-mm trocars in the lower quadrants, just lateral to the inferior epigastric arteries. Brief insufflation to greater than 20 mm Hg intra-abdominal pressure facilitates safe entry for these secondary trocars. Although you may opt for smaller trocars, the 10-mm size allows unhindered passage of suture, thus providing more options for maximizing favorable ergonomics with future suture placement.
Although a preperitoneal, or extraperitoneal, approach has been described, we favor a transperitoneal entrance into the space of Retzius. The extraperitoneal approach allows the use of regional anesthesia, avoids intraabdominal adhesions, and eliminates the associated risks of peritoneal entry.31 The disadvantages, however, are significant, including failure to enter the retropubic space secondary to abdominal wall scarring, the inability to perform concomitant vault suspension, and the cost of commercially available dissecting balloons. With experience, a transperitoneal approach will not prolong operative time.
With experience, a transperitoneal approach into the space of Retzius will not prolong operative time.
Approaching the bladder. Distend the bladder in a retrograde fashion with 300 mL to 400 mL of normal saline. This allows identification of the superior margin of the bladder dome and provides mass traction posteriorly. Use the urachus to identify the midline; then, grasp the anterior abdominal wall peritoneum and apply downward traction ( FIGURE 1). Next, create a transverse incision 3 cm to 4 cm above the bladder reflection, using monopolar endoscopic scissors on a 70-watt pure-cut setting ( FIGURE 2). The incision should be within the obliterated umbilical ligaments, but can be extended slightly beyond for patients undergoing a combined laparoscopic Burch-paravaginal repair.41 Using a combination of blunt and electrocautery dissection, you then can easily dissect the loose areolar tissue of the prevesicle space down to the level of the pubic symphysis and ramus ( FIGURE 3).
FIGURE 1
Pictured is the distended bladder, forceps (right) at the bladder margin, endoshears (left) at level of incision, and urachus.
FIGURE 2
Create a transverse incision using monopolar scissors. Note the loose areolar tissue of prevesicle space.
FIGURE 3
Locate the pubic symphysis and ramus using the pelvic brim as a landmark.As the paravesical space is further developed, the pubocervical fascia will become exposed at the level of the UVJ. You must carefully protect the urethra, avoiding aggressive midline dissection as well as the obturator neurovascular bundle laterally. Medial traction on the bladder, perpendicular to the slope of the pubic ramus, encourages identification of the proper surgical plane. Use electrocautery to maintain meticulous hemostasis at all times. Identify Cooper’s ligament, and bluntly dissect away any obstructing fat or areolar tissue (FIGURE 4). To encourage scarification, gently remove excessive overlying periurethral and perivesical fat from pubocervical fascia at the level of the bladder neck, while avoiding any dissection within 1 cm lateral to the urethra ( FIGURE 5).
Placing the sutures. Using an extra-long (36-in), doubled-armed, nonabsorbable suture on an SH needle, place the sutures in the consistent sequence outlined below (note that sturdy needle drivers will facilitate secure needle placement):
First, introduce a needle from the contralateral port and pass it through the pubocervical fascia at the level of the midurethra, using your index finger for transvaginal guidance. If you think the tissue bite will not purchase nearly the entire thickness of the anterior vaginal wall, place a second helical throw (FIGURE 6). Next, bring the suture up through Cooper’s ligament and “store” it by hooking the anterior wall peritoneum (FIGURE 7). Bring up the second arm (needle) of the suture through Cooper’s ligament, but at a different depth than the first pass so ligament fibers are truly encircled by the suture (FIGURE 8). Retrieve both needles, bringing them out through the same port, but do not yet tie the suture.
Introduce the second suture through the ipsilateral port and place it in the same fashion at the level of the UVJ. Again, use helical throws as necessary. Once both sutures have been placed, tie them extracorporeally in sequence using a closed-loop knot pusher. (Waiting until both sutures are placed before tying allows exposure for easy placement of the second suture [FIGURE 9].) The appropriate tension should create a small, localized “knuckle” of pubocervical fascia that approximates laterally to the obturator internus fascia ( FIGURE 10).
Repeat this procedure in the same sequence on the opposite side of the pelvis ( FIGURE 11), then close the retropubic space using a running continuous 2-0 suture reapproximating the peritoneum (FIGURE 12). Close the laparoscopic ports at the fascia level using a Veress needle threaded with a 0-Vicryl. Both ends of the suture are passed on either side of the fascial incision. Using a contralateral grasping forceps, the suture end is freed from the Veress needle, then retrieved using an ipsilateral forceps. This port closure technique is easy to perform as well as cost-effective.
Postoperative care. Place a suprapubic catheter with a 2-way stop clock; this makes postoperative voiding trials easier for both patients and nursing staff.
Most patients will be discharged the day after surgery. If the patient still has an elevated postvoid residual, she’ll likely find going home with the suprapubic catheter more acceptable than intermittent self-catheterization or an indwelling Foley running to a leg bag. For postoperative discomfort, acetaminophen and nonsteroidal anti-inflammatory preparations are generally sufficient. Patients can resume normal living activities within days, but should be cautioned to delay strenuous work or exercise for at least 8 weeks.
FIGURE 4
Apply medial traction to the bladder as the paravesical space is developed down to the level of pubocervical fascia. Note Cooper’s ligament, seen anteriorly.
FIGURE 5
Gently remove the overlying periurethral and perivesical fat to expose white pubocervical fascia.
FIGURE 6
Apply counter traction on the suture from the first pass to facilitate better tissue purchase on the second pass of the helical suture.
FIGURE 7
Place the needle through Cooper’s ligament.
FIGURE 8
Pass the second needle at a deeper depth through Cooper’s ligament to encircle fibers within the suture. This will minimize the risk of “pull out.”
FIGURE 9
Place both sutures before extracorporeal tying.
FIGURE 10
Suture tension should create only a small knuckle of pubocervical fascia, approximating obturator fascia laterally.
FIGURE 11
Both sides completed.
FIGURE 12
Close the retropubic space.
Why? A look at the evidence
The learning curve for laparoscopic Burch is steep and somewhat long—approximately 20 cases. The real question, therefore, is whether the benefits justify the time needed to master this procedure. In other words, is there clinical evidence that, once the plateau of this curve has been reached, we can reduce patient morbidity while maintaining efficacy compared to the traditional open technique? If not, there’s little reason for surgeons to learn the technique. If there is, however, more physicians should include the laparoscopic Burch in their surgical arsenal.
The real question is whether the benefits of the laparoscopic Burch justify the time needed to master the procedure.
When we limit the discussion to 2 comparable techniques, a laparoscopic versus open 2-suture procedure, there is moderately strong evidence that the laparoscopic approach maintains efficacy while modestly reducing morbidity.42 The strength of this evidence is established in 6 studies, including 3 randomized trials (level I evidence).8,18,23,24,43,44
Currently, data are insufficient regarding a laparoscopic approach to make concomitant site-specific defect repairs.45-51 However, if the procedures are performed in the same fashion, “suture for suture,” as their abdominal counterparts, we should expect to see, as we have with the laparoscopic Burch, similar efficacy rates between the laparoscopic and open approaches.
So, in closing, we encourage our colleagues to reignite their interest in learning laparoscopic reconstructive surgery and recommit to reaching that learning curve plateau. Why? We owe it to our patients.
Dr. Lucente is a consultant for GyneCare. Dr. Murphy reports no financial relationship with any companies whose products are mentioned in this article.
1. National Association for Incontinence Quarterly Report. Summer 2002, Vol 20 No 3.
2. Black NA, Down SH. The effectiveness of surgery for stress incontinence in women: A systematic review. Br J Urol. 1996;78:497.-
3. Tanagho E. Colpocystourethropexy: The way we do it. J Urol. 1976;116:751-753.
4. Leach GE, Dinochowski RR, Appell RA, et al. Female stress urinary incontinence clinical guidelines panel: Report on the surgical management of female stress urinary incontinence. Baltimore, Md: American Urological Association, Inc.; 1997.
5. Vancaille TG, Schuessler W. Laparoscopic bladder neck suspension. J Laparoendosc Surg. 1991;1:169-173.
6. Albala DM, Schuessler WW, Vancaillie TG. Laparoscopic bladder suspension for the treatment of stress incontinence. Semin Urol. 1992;10:222-225.
7. Burton G. A randomized comparison of laparoscopic and open colposuspension [abstract]. Neurourol Urodyn. 1993;16:353-354.
8. Polascik TJ, Moore RG, Roseberg MT, Kavoussi LR. Comparison of laparoscopic and open retropubic colposuspension for treatment of stress urinary incontinence. Urology. 1995;45:647-652.
9. Liu CY. Laparoscopic treatment of genuine urinary stress incontinence. Clin Obstet Gynecol. 1994;8:789-798.
10. Gunn GC, Cooper RP, Gordon NS, Gragnon L. Use of a new device for endoscopic suturing in the laparoscopic Burch procedure. J Am Assoc Gynecol Laparosc. 1994;2:65-70.
11. Nezhat CH, Nezhat F, Nezhat CR, et al. Laparoscopic retropubic cystocolposuspension. J Am Assoc Gynecol Laparosc. 1994;1:339-349.
12. Lyons TL, Winer WK. Clinical outcomes with laparoscopic approaches and open Burch procedures for urinary stress incontinence. J Am Assoc Gynecol Laparosc. 1995;2:193-197.
13. McDougal EM, Klutke CG, Cornell T. Comparison of transvaginal versus laparoscopic bladder neck suspension for stress urinary incontinence. Adult Urol. 1995;45:641-645.
14. Ross JW. Laparoscopic Burch repair compared to laparotomy Burch for cure of urinary stress incontinence. Int Urogynecol. 1995;6:323-328.
15. Langebrekke A, Dahlstrom B, Eraker R, Urnes A. The laparoscopic Burch procedure: a preliminary report. Acta Obstet Gynecol Scand. 1995;74:153-155.
16. Radomski SB, Herschorn S. Laparoscopic Burch bladder neck suspension: early results. J Urol. 1996;155:515-518.
17. Ross JW. Two techniques of laparoscopic Burch repair for stress incontinence: a prospective randomized study. J Am Assoc Gynecol Laparoscopists. 1996;3:351-357.
18. Lam AM, Jenkins GJ, Hyslop RS. Laparoscopic results. Med J Aust. 1995;162:18-22.
19. Su TH, Wang KG, Hsu CY, Wei H, Hong BK. Prospective comparison of laparoscopic and traditional colposuspensions in the treatment of genuine stress inconti-nence. Acta Obstet Gynecol. 1997;76:576-582.
20. Papasakelariou C, Papasakelariou B. Laparoscopic bladder neck suspension. J Am Assoc Gynecol Laparosc. 1997;4:185-188.
21. Lobel RW, Davis GD. Long-term results of laparoscopic Burch colposuspension. J Am Assoc Gynecol Laparosc. 1997;4:341-345.
22. Ross JW. Multichannel urodynamic evaluation of laparoscopic Burch colposuspension for genuine stress incontinence. Obstet Gynecol. 1998;91:55-59.
23. Miannay E, Cosson M, Querleu D, et al. [Comparison of laparoscopic and laparotomy colposuspension in the treatment of urinary stress incontinence. Comparative study of 72 matched cases][French]. Contracept Fertil Sex. 1998;26:376-385.
24. Saidi MH, Gallagher MS, Skop IP, et al. Extrperitoneal laparoscopic colposuspension: short-term cure rate, complications and duration of hospital stay in comparison with Burch colposuspension. Obstet Gynecol. 1998;92:619-625.
25. Henley C. The Henley staple-suture technique for laparoscopic Burch colposuspension. J Am Assoc Gynecol Laparosc. 1995;2:441-444.
26. Das S, Palmer JK. Laparoscopic colposuspension. J Urol. 1995;154:1119-1121.
27. Ou CS, Presthus J, Beadle E. Laparoscopic bladder neck suspension using hernia mesh and surgical staples. J Laparoendosc Surg. 1993;3:563-566.
28. Kiilholma P, Haarala M, Polvi II, Makinen J, Chancellor MB. Sutureless colposuspension with fibrin sealant. Tech Urol. 1995;1:81-83.
29. Lose G. Laparoscopic Burch colposuspension. Acta Obstet Gynecol Scand Suppl. 1998;168:29-33.
30. Miannay E, Cosson M, Lanvin D, et al. Comparison of open retropubic and laparoscopic colposuspension for treatment of stress urinary incontinence. Eur J Obstet Gynecol Reprod Biol. 1998;79:159-166.
31. Saidi MH, Sadler RK, Saidi JA. Extraperitoneal laparoscopic colposuspension for genuine urinary stress incontinence. J Am Assoc Gynecol Laparosc. 1998;9:249-252.
32. Ou CS, Rowbotham R. Five-year follow-up of a laparoscopic bladder neck suspension using synthetic mesh and surgical staples. J Laparoendosc Adv Surg Techn A. 1999;9:249-252.
33. Zullo F, Morelli M, Russo T, et al. Two techniques of laparoscopic retropubic urethropexy. J Am Assoc Gynecol Laparosc. 2001;12:323-327.
34. Langer R, Lipshitz Y, Halperin R, et al. Long-term (10-15 years) follow-up after Burch colposuspension for urinary stress incontinence. Int Urogynecol J. 2001;12:323-327.
35. Herbertsson G, Iosif CS. Surgical results and urodynamic studies 10 years after retropubic colpurethropexy. Acta Obstet Gynecol Scand. 1993;72:298-301.
36. Parasio M, Falcone T, Walters M. Laparoscopic surgery for genuine stress incontinence. Int Urogynecol J. 1999;10:237-247.
37. Horbach NS. Suburethral sling procedures. Urogynecology and Urodynamics: Theory and Practice. 4th ed. Baltimore, Md: Williams and Wilkins 1996;569-579.
38. Vassallo B, Murphy M, Lucente V, Karram M. TVT versus Laparoscopic Burch: a retrospective review at 1-4 years. 27th Scientific Meeting of the Society of Gynecologic Surgeons. March 5-7, 2001.
39. Fotte A. Which is the best minimally invasive procedure? TVT versus laparoscopic colposuspension. Presented at the 31st annual meeting of the International Continence Society; September 18-21, 2001; Seoul, Korea.
40. Ward KL, Hilton P. Prospective multicentre randomized trial of tension-free vaginal tape and colposuspension as primary treatment for stress incontinence. BMJ. 2002;326:67.-
41. Miklos J, Kohli N. Laparoscopic paravaginal repair plus Burch colposuspension: review and descriptive technique. Urology. 2000;56:64-68.
42. Walter A. Laparoscopic repairs for stress urinary incontinence and pelvic organ prolapse: “show me the evidence.” Presented at the annual meeting of the American Urogynecologic Society; 2002; San Diego, Calif.
43. Summitt RL, Lucente V, Karram MM, Shull BL, Bent AE. Randomized comparison of laparoscopic and transabdominal Burch urethropexy for the treatment of genuine stress incontinence. Obstet Gynecol. 2000;95:(suppl):2.-
44. Fatthy H, El Hao M, Samaha I, Abdallah K. Modified Burch colposuspension: laparoscopy versus laparotomy. J Am Assoc Gynecol Laparosc. 2001;8:99-106.
45. Ross JW. Techniques of laparoscopic repair for total vault eversion after hysterectomy. J Am Assoc Gynecol Laparosc. 1997;4:173-183.
46. Ross JW. Laparoscopic approach for severe pelvic vault prolapse. J Am Assoc Gynecol Laparosc. 1996;3(suppl):43.-
47. Lyons TL, Winer WK. Laparoscopic rectocele repair using polyglactin mesh. J Am Assoc Gynecol Laparosc. 1997;4:381-384.
48. Ostrzenski A. Laparoscopic colposuspension for total vaginal prolapse. Int J Gynaecol Obstet. 1996;55:147-152.
49. Carter JE, Winter M, Mendehlsohn S, Saye W, Richardson AC. Vaginal vault suspension and enterocele repair by Richardson-Saye laparoscopic technique: description of training technique and results. JSLS. 2001;5:29-36.
50. Cosson M, Rajabally R, Bogaert E, Querleu D, Crepin G. Laparoscopic sacrocolpopexy, hysterectomy and Burch colposuspension: feasibility and short-term complications of 77 procedures. JSLS. 2002;6:115-119.
51. Miklos JR, Moore RD, Kohli N. Laparoscopic surgery for pelvic support defects. Curr Opin Obstet Gynecol. 2002;14:387-395.
1. National Association for Incontinence Quarterly Report. Summer 2002, Vol 20 No 3.
2. Black NA, Down SH. The effectiveness of surgery for stress incontinence in women: A systematic review. Br J Urol. 1996;78:497.-
3. Tanagho E. Colpocystourethropexy: The way we do it. J Urol. 1976;116:751-753.
4. Leach GE, Dinochowski RR, Appell RA, et al. Female stress urinary incontinence clinical guidelines panel: Report on the surgical management of female stress urinary incontinence. Baltimore, Md: American Urological Association, Inc.; 1997.
5. Vancaille TG, Schuessler W. Laparoscopic bladder neck suspension. J Laparoendosc Surg. 1991;1:169-173.
6. Albala DM, Schuessler WW, Vancaillie TG. Laparoscopic bladder suspension for the treatment of stress incontinence. Semin Urol. 1992;10:222-225.
7. Burton G. A randomized comparison of laparoscopic and open colposuspension [abstract]. Neurourol Urodyn. 1993;16:353-354.
8. Polascik TJ, Moore RG, Roseberg MT, Kavoussi LR. Comparison of laparoscopic and open retropubic colposuspension for treatment of stress urinary incontinence. Urology. 1995;45:647-652.
9. Liu CY. Laparoscopic treatment of genuine urinary stress incontinence. Clin Obstet Gynecol. 1994;8:789-798.
10. Gunn GC, Cooper RP, Gordon NS, Gragnon L. Use of a new device for endoscopic suturing in the laparoscopic Burch procedure. J Am Assoc Gynecol Laparosc. 1994;2:65-70.
11. Nezhat CH, Nezhat F, Nezhat CR, et al. Laparoscopic retropubic cystocolposuspension. J Am Assoc Gynecol Laparosc. 1994;1:339-349.
12. Lyons TL, Winer WK. Clinical outcomes with laparoscopic approaches and open Burch procedures for urinary stress incontinence. J Am Assoc Gynecol Laparosc. 1995;2:193-197.
13. McDougal EM, Klutke CG, Cornell T. Comparison of transvaginal versus laparoscopic bladder neck suspension for stress urinary incontinence. Adult Urol. 1995;45:641-645.
14. Ross JW. Laparoscopic Burch repair compared to laparotomy Burch for cure of urinary stress incontinence. Int Urogynecol. 1995;6:323-328.
15. Langebrekke A, Dahlstrom B, Eraker R, Urnes A. The laparoscopic Burch procedure: a preliminary report. Acta Obstet Gynecol Scand. 1995;74:153-155.
16. Radomski SB, Herschorn S. Laparoscopic Burch bladder neck suspension: early results. J Urol. 1996;155:515-518.
17. Ross JW. Two techniques of laparoscopic Burch repair for stress incontinence: a prospective randomized study. J Am Assoc Gynecol Laparoscopists. 1996;3:351-357.
18. Lam AM, Jenkins GJ, Hyslop RS. Laparoscopic results. Med J Aust. 1995;162:18-22.
19. Su TH, Wang KG, Hsu CY, Wei H, Hong BK. Prospective comparison of laparoscopic and traditional colposuspensions in the treatment of genuine stress inconti-nence. Acta Obstet Gynecol. 1997;76:576-582.
20. Papasakelariou C, Papasakelariou B. Laparoscopic bladder neck suspension. J Am Assoc Gynecol Laparosc. 1997;4:185-188.
21. Lobel RW, Davis GD. Long-term results of laparoscopic Burch colposuspension. J Am Assoc Gynecol Laparosc. 1997;4:341-345.
22. Ross JW. Multichannel urodynamic evaluation of laparoscopic Burch colposuspension for genuine stress incontinence. Obstet Gynecol. 1998;91:55-59.
23. Miannay E, Cosson M, Querleu D, et al. [Comparison of laparoscopic and laparotomy colposuspension in the treatment of urinary stress incontinence. Comparative study of 72 matched cases][French]. Contracept Fertil Sex. 1998;26:376-385.
24. Saidi MH, Gallagher MS, Skop IP, et al. Extrperitoneal laparoscopic colposuspension: short-term cure rate, complications and duration of hospital stay in comparison with Burch colposuspension. Obstet Gynecol. 1998;92:619-625.
25. Henley C. The Henley staple-suture technique for laparoscopic Burch colposuspension. J Am Assoc Gynecol Laparosc. 1995;2:441-444.
26. Das S, Palmer JK. Laparoscopic colposuspension. J Urol. 1995;154:1119-1121.
27. Ou CS, Presthus J, Beadle E. Laparoscopic bladder neck suspension using hernia mesh and surgical staples. J Laparoendosc Surg. 1993;3:563-566.
28. Kiilholma P, Haarala M, Polvi II, Makinen J, Chancellor MB. Sutureless colposuspension with fibrin sealant. Tech Urol. 1995;1:81-83.
29. Lose G. Laparoscopic Burch colposuspension. Acta Obstet Gynecol Scand Suppl. 1998;168:29-33.
30. Miannay E, Cosson M, Lanvin D, et al. Comparison of open retropubic and laparoscopic colposuspension for treatment of stress urinary incontinence. Eur J Obstet Gynecol Reprod Biol. 1998;79:159-166.
31. Saidi MH, Sadler RK, Saidi JA. Extraperitoneal laparoscopic colposuspension for genuine urinary stress incontinence. J Am Assoc Gynecol Laparosc. 1998;9:249-252.
32. Ou CS, Rowbotham R. Five-year follow-up of a laparoscopic bladder neck suspension using synthetic mesh and surgical staples. J Laparoendosc Adv Surg Techn A. 1999;9:249-252.
33. Zullo F, Morelli M, Russo T, et al. Two techniques of laparoscopic retropubic urethropexy. J Am Assoc Gynecol Laparosc. 2001;12:323-327.
34. Langer R, Lipshitz Y, Halperin R, et al. Long-term (10-15 years) follow-up after Burch colposuspension for urinary stress incontinence. Int Urogynecol J. 2001;12:323-327.
35. Herbertsson G, Iosif CS. Surgical results and urodynamic studies 10 years after retropubic colpurethropexy. Acta Obstet Gynecol Scand. 1993;72:298-301.
36. Parasio M, Falcone T, Walters M. Laparoscopic surgery for genuine stress incontinence. Int Urogynecol J. 1999;10:237-247.
37. Horbach NS. Suburethral sling procedures. Urogynecology and Urodynamics: Theory and Practice. 4th ed. Baltimore, Md: Williams and Wilkins 1996;569-579.
38. Vassallo B, Murphy M, Lucente V, Karram M. TVT versus Laparoscopic Burch: a retrospective review at 1-4 years. 27th Scientific Meeting of the Society of Gynecologic Surgeons. March 5-7, 2001.
39. Fotte A. Which is the best minimally invasive procedure? TVT versus laparoscopic colposuspension. Presented at the 31st annual meeting of the International Continence Society; September 18-21, 2001; Seoul, Korea.
40. Ward KL, Hilton P. Prospective multicentre randomized trial of tension-free vaginal tape and colposuspension as primary treatment for stress incontinence. BMJ. 2002;326:67.-
41. Miklos J, Kohli N. Laparoscopic paravaginal repair plus Burch colposuspension: review and descriptive technique. Urology. 2000;56:64-68.
42. Walter A. Laparoscopic repairs for stress urinary incontinence and pelvic organ prolapse: “show me the evidence.” Presented at the annual meeting of the American Urogynecologic Society; 2002; San Diego, Calif.
43. Summitt RL, Lucente V, Karram MM, Shull BL, Bent AE. Randomized comparison of laparoscopic and transabdominal Burch urethropexy for the treatment of genuine stress incontinence. Obstet Gynecol. 2000;95:(suppl):2.-
44. Fatthy H, El Hao M, Samaha I, Abdallah K. Modified Burch colposuspension: laparoscopy versus laparotomy. J Am Assoc Gynecol Laparosc. 2001;8:99-106.
45. Ross JW. Techniques of laparoscopic repair for total vault eversion after hysterectomy. J Am Assoc Gynecol Laparosc. 1997;4:173-183.
46. Ross JW. Laparoscopic approach for severe pelvic vault prolapse. J Am Assoc Gynecol Laparosc. 1996;3(suppl):43.-
47. Lyons TL, Winer WK. Laparoscopic rectocele repair using polyglactin mesh. J Am Assoc Gynecol Laparosc. 1997;4:381-384.
48. Ostrzenski A. Laparoscopic colposuspension for total vaginal prolapse. Int J Gynaecol Obstet. 1996;55:147-152.
49. Carter JE, Winter M, Mendehlsohn S, Saye W, Richardson AC. Vaginal vault suspension and enterocele repair by Richardson-Saye laparoscopic technique: description of training technique and results. JSLS. 2001;5:29-36.
50. Cosson M, Rajabally R, Bogaert E, Querleu D, Crepin G. Laparoscopic sacrocolpopexy, hysterectomy and Burch colposuspension: feasibility and short-term complications of 77 procedures. JSLS. 2002;6:115-119.
51. Miklos JR, Moore RD, Kohli N. Laparoscopic surgery for pelvic support defects. Curr Opin Obstet Gynecol. 2002;14:387-395.
Safe delivery of the fetal head during cesarean section
- Position yourself so your upper trunk, arm, and hand move as a unit to elevate the head.
- Elevate the head to the level of the uterine incision, rather than bringing the incision down to the head.
- Rotate the occiput anteriorly to present the shortest fetal head diameters to the incision.
- Reduce the lower lip of the uterine incision beneath the fetal head, as you would reduce a posterior cervical lip at a vaginal delivery.
Although it’s a skill vital for cesarean birth, manual delivery of the fetal head from a low pelvic station is given only cursory coverage in standard obstetrics texts and reviews.1-4 To learn successful methods, therefore, physicians are forced to rely on observation, anecdotal experience, and—least desirably—trial and error.
Possibly the most frequently employed maneuver in this scenario is to have an assistant elevate the head with his or her hand.1,3,4 However, this technique may not provide sufficient elevation. Furthermore, subsequent manipulation by the clinician may extend the uterine incision and risk injury to the uterine vessels and bladder. It is an untested assumption that such extensions increase the risk of uterine rupture during subsequent trials of labor.
With the goals of minimizing delay, head compression, and strain on the uterine incision, I developed the elevate, rotate, and reduce (ERR) technique for expeditious delivery of the head from a deep pelvic station.
Approaching the head
Begin the procedure by identifying the fetal position; if it is occiput posterior, you may wish to have a disposable vacuum extractor available. Then stand so that your dominant hand is closest to the patient’s pelvis.
When making the abdominal incision, consider fetal size and maternal body habitus. Be sure all layers of the incision are long enough to permit manipulation and delivery—don’t let surgical pride force you to struggle with an inadequate Pfannenstiel incision. Be prepared to identify and extend any layer of the incision that gives considerable resistance at the time of delivery.
Before proceeding to the uterine incision, make sure there is adequate rectus separation. Check the clearance by separating the right and left bodies of the rectus muscle with manual traction. If more room is needed, create a “partial Maylard incision” by cutting bilateral transverse incisions with Mayo scissors across the medial third of each body. This gives the fetus more room while avoiding damage to the inferior epigastric vessels.
The uterine incision should be as wide as the mean fetal head diameter—usually the same 10 cm that we expect of the fully dilated cervix at term. In patients with an undeveloped lower uterine segment, varices, adhesions, or leiomyomata, there may be insufficient width for a low-transverse incision. In these cases you may need to convert to a U, J, or vertical incision.
Take advantage of a well-developed lower uterine segment and avoid tight U- or V-shaped transverse incisions. Identify the uterine vessels with adequate retraction, and incise laterally right up to them. An alternate technique would be to extend the initial incision by pulling apart the ends with your index fingers, laterally and cephalad (towards the mother’s axillae). Blunt dissection may cause less bleeding from the uterine vessels.5
The ERR technique
“Don’t break the wrist” is a classic admonition, familiar to generations of obstetricians. It warns against a practice that begins by reaching for the head with extended wrist, not elevating the head enough, and delivering the head from whatever position it presents in. The head is delivered with firm wrist flexion (breaking, cocking, or levering the wrist) and anterior traction. This puts caudal leverage upon the lower edge of the uterine incision, which may cause an extension down to the bladder or into the cervix. The ERR technique corrects these errors and minimizes strain on the incision.
Elevate. The first goal of ERR is to elevate the fetal vertex to the lower edge of the uterine incision. Bringing the incision down to the presenting part may strain it. Start by holding the fingers of your dominant hand together, with the thumb tucked against the lateral base Safe delivery of the fetal head during cesarean section of the index finger and the fingers extended straight.
Next, insert your fingertips into the uterine incision and advance them around the fetal head—but not while standing erect with your wrist extended. Such a posture would cause 2 problems: First, you would have insufficient leverage and strength to advance your fingers deep into the pelvis. Second, the angle of your wrist would be obstructed by the uterine incision, the lower edge of the abdominal incision, and the pubis.
Instead, try 1 of the following 2 approaches: The first option is to extend your arm in a straight line from shoulder to fingertip, keeping it almost parallel to the patient’s longitudinal axis. This is accomplished by flexing forward at the waist while facing cross-table, and abducting your arm out from your shoulder. Another option is to turn and face the head of the table, flex at the waist, and extend your arm behind you while pronating your hand. Both of these positions keep your wrist angle neutral and allow you to use the strength and leverage of your entire upper trunk to move your hand.
In order to elevate the head, your fingers need to achieve at least a quarter-circle grip around the vertex (FIGURE 1). If the head is so deep or tightly applied that you cannot achieve this in 1 continuous movement, proceed in stages. First, have an assistant apply transvaginal digital pressure to elevate the head from below. Then, advance your fingers as far as you can around the fetal head while flexing and extending the fingers in a worm-like wiggling movement. Flex and lock your fingers against the fetal cranium, and lean your entire upper trunk and arm away from the maternal pelvis. This generally elevates the head by roughly a centimeter, enough to advance your fingers a little further. Repeat these motions until your fingers have achieved the desired quarter-circle, at which point your assistant may withdraw from below.
Now lock your arm from shoulder to fingertip, and lean out of the patient’s pelvis 1 more time. You may note the sensation and sound of breaking suction as the fetal head escapes the pelvic grip. Maintain this steady traction for several seconds. When the vertex has reached the level of the lower margin of the uterine incision, you can rotate the fetus.
Rotate. Before you begin rotation, confirm the fetal position (the fetal ears are useful as a landmark). If the head is not already in occiput anterior, grasp it and rotate the occiput into the incision (FIGURE 2). As in vaginal delivery, this presents the shortest fetal-head diameters to the birth orifice. If the head has been markedly molded by labor, rotation will bring the narrow end of a dilating cone into the uterine incision. This helps direct the head anteriorly, rather than back into the pelvis, when fundal pressure is applied.
Reduce. Using your hand like a pair of forceps may cause uterine extensions or lacerations if the uterine incision is not large enough to accommodate both your hand and the fetal head. Instead, use 4 fingers in shoehorn fashion to guide the head out of the incision. First, however, there is usually another obstacle to overcome: Fundal pressure may merely force the head back toward the pelvis. Your fingers cannot present a shallow enough angle to direct the head anteriorly, unless your hand is in so deep that your palm fills the uterine incision.
You may substitute instruments for the guiding fingers, using the Murless head extractor, the Torpin vectis blade, or 1 blade of a short-shank Simpson forceps. However, a lack of availability may limit your choices. Although you may use a disposable vacuum extractor system, consider saving that expense with this alternative: Using your fingertips, reduce the lower lip of the uterine incision beneath the fetal head, as you would reduce a posterior cervical lip at a vaginal delivery. (FIGURE 3). The reduced lip tends to extend the head anteriorly and direct it away from the pelvis. Withdraw your hand so only your fingertips remain to guide the head, while the back of your fingers retract the lower abdominal wall. With fundal pressure the head should move anteriorly out of the abdomen.
A head in the occiput-posterior position may resist attempts to rotate to occiput anterior. Should this occur, try following the incision reduction with direct manual flexion of the head out of the abdomen. If reduction flexion puts excess strain on the incision, abandon the attempt. Instead, use a disposable vacuum extractor cup—placed on the fetal brow as close to the vertex as possible—to flex the head out of the incision. Should this fail as well, extend the low-transverse uterine incision cranially into a generous U-incision, and repeat the vacuum procedure.
FIGURE 1 THE ERR SEQUENCE
Elevate. Lock the fingers into a quarter-circle around the vertex. Apply traction out of the pelvis with the hand and the entire extended arm.
FIGURE 2 THE ERR SEQUENCE
Rotate. Grasp the fetal head between the thumb and fingers and rotate it so the occiput faces the incision.
FIGURE 3 THE ERR SEQUENCE
Reduce. Push the lower edge of the uterine incision down until it is posterior to the fetal head.
Conclusion
This technique for delivering the fetal head offers minimal risk of uterine laceration and lends itself to instruction and recollection.
I have not evaluated ERR by clinical trial, both due to absence of a gold-standard technique for the control group and because ERR has yielded satisfactory outcomes in the 20 years over which I have developed, applied, and taught it. Still, the technique raises opportunities for clinical investigation. For example, residency training programs concerned over their incidence of uterine extensions and lacerations may wish to adopt this method and subsequently produce a historical-controlled, retrospective cohort study as a quality-improvement project.
Dr. Chao reports no financial relationship with any companies whose products are mentioned in this article.
1. Cunningham FG, MacDonald PC, Gant NF, et al. Williams Obstetrics. 20th ed. Stamford, Conn: Appleton & Lange; 1997;518.-
2. Scott JR. Cesarean delivery. In: Scott JR, Disaia PJ, Hammond CB, Spellacy WN, eds. Danforth’s Obstetrics and Gynecology. 8th ed. Philadelphia: Lippincott Williams and Wilkins; 1999;462.-
3. Depp R. Cesarean delivery. In: Gabbe SG, Niebyl JR, Simpson JL. Obstetrics: Normal and Problem Pregnancies. 4th ed. New York: Churchill Livingstone, 2002;554.-
4. Field CS. Surgical techniques for cesarean section. Obstet Gynecol Clin NA. 1988;15:664-665.
5. Magann EF, Chauhan SP, Bufkin L, Field K, Roberts WE, Martin JN, Jr. Intraoperative hemorrhage by blunt versus sharp expansion of the uterine incision at caesarean delivery: a randomized clinical trial. BJOG. 2002;109:448-452.
- Position yourself so your upper trunk, arm, and hand move as a unit to elevate the head.
- Elevate the head to the level of the uterine incision, rather than bringing the incision down to the head.
- Rotate the occiput anteriorly to present the shortest fetal head diameters to the incision.
- Reduce the lower lip of the uterine incision beneath the fetal head, as you would reduce a posterior cervical lip at a vaginal delivery.
Although it’s a skill vital for cesarean birth, manual delivery of the fetal head from a low pelvic station is given only cursory coverage in standard obstetrics texts and reviews.1-4 To learn successful methods, therefore, physicians are forced to rely on observation, anecdotal experience, and—least desirably—trial and error.
Possibly the most frequently employed maneuver in this scenario is to have an assistant elevate the head with his or her hand.1,3,4 However, this technique may not provide sufficient elevation. Furthermore, subsequent manipulation by the clinician may extend the uterine incision and risk injury to the uterine vessels and bladder. It is an untested assumption that such extensions increase the risk of uterine rupture during subsequent trials of labor.
With the goals of minimizing delay, head compression, and strain on the uterine incision, I developed the elevate, rotate, and reduce (ERR) technique for expeditious delivery of the head from a deep pelvic station.
Approaching the head
Begin the procedure by identifying the fetal position; if it is occiput posterior, you may wish to have a disposable vacuum extractor available. Then stand so that your dominant hand is closest to the patient’s pelvis.
When making the abdominal incision, consider fetal size and maternal body habitus. Be sure all layers of the incision are long enough to permit manipulation and delivery—don’t let surgical pride force you to struggle with an inadequate Pfannenstiel incision. Be prepared to identify and extend any layer of the incision that gives considerable resistance at the time of delivery.
Before proceeding to the uterine incision, make sure there is adequate rectus separation. Check the clearance by separating the right and left bodies of the rectus muscle with manual traction. If more room is needed, create a “partial Maylard incision” by cutting bilateral transverse incisions with Mayo scissors across the medial third of each body. This gives the fetus more room while avoiding damage to the inferior epigastric vessels.
The uterine incision should be as wide as the mean fetal head diameter—usually the same 10 cm that we expect of the fully dilated cervix at term. In patients with an undeveloped lower uterine segment, varices, adhesions, or leiomyomata, there may be insufficient width for a low-transverse incision. In these cases you may need to convert to a U, J, or vertical incision.
Take advantage of a well-developed lower uterine segment and avoid tight U- or V-shaped transverse incisions. Identify the uterine vessels with adequate retraction, and incise laterally right up to them. An alternate technique would be to extend the initial incision by pulling apart the ends with your index fingers, laterally and cephalad (towards the mother’s axillae). Blunt dissection may cause less bleeding from the uterine vessels.5
The ERR technique
“Don’t break the wrist” is a classic admonition, familiar to generations of obstetricians. It warns against a practice that begins by reaching for the head with extended wrist, not elevating the head enough, and delivering the head from whatever position it presents in. The head is delivered with firm wrist flexion (breaking, cocking, or levering the wrist) and anterior traction. This puts caudal leverage upon the lower edge of the uterine incision, which may cause an extension down to the bladder or into the cervix. The ERR technique corrects these errors and minimizes strain on the incision.
Elevate. The first goal of ERR is to elevate the fetal vertex to the lower edge of the uterine incision. Bringing the incision down to the presenting part may strain it. Start by holding the fingers of your dominant hand together, with the thumb tucked against the lateral base Safe delivery of the fetal head during cesarean section of the index finger and the fingers extended straight.
Next, insert your fingertips into the uterine incision and advance them around the fetal head—but not while standing erect with your wrist extended. Such a posture would cause 2 problems: First, you would have insufficient leverage and strength to advance your fingers deep into the pelvis. Second, the angle of your wrist would be obstructed by the uterine incision, the lower edge of the abdominal incision, and the pubis.
Instead, try 1 of the following 2 approaches: The first option is to extend your arm in a straight line from shoulder to fingertip, keeping it almost parallel to the patient’s longitudinal axis. This is accomplished by flexing forward at the waist while facing cross-table, and abducting your arm out from your shoulder. Another option is to turn and face the head of the table, flex at the waist, and extend your arm behind you while pronating your hand. Both of these positions keep your wrist angle neutral and allow you to use the strength and leverage of your entire upper trunk to move your hand.
In order to elevate the head, your fingers need to achieve at least a quarter-circle grip around the vertex (FIGURE 1). If the head is so deep or tightly applied that you cannot achieve this in 1 continuous movement, proceed in stages. First, have an assistant apply transvaginal digital pressure to elevate the head from below. Then, advance your fingers as far as you can around the fetal head while flexing and extending the fingers in a worm-like wiggling movement. Flex and lock your fingers against the fetal cranium, and lean your entire upper trunk and arm away from the maternal pelvis. This generally elevates the head by roughly a centimeter, enough to advance your fingers a little further. Repeat these motions until your fingers have achieved the desired quarter-circle, at which point your assistant may withdraw from below.
Now lock your arm from shoulder to fingertip, and lean out of the patient’s pelvis 1 more time. You may note the sensation and sound of breaking suction as the fetal head escapes the pelvic grip. Maintain this steady traction for several seconds. When the vertex has reached the level of the lower margin of the uterine incision, you can rotate the fetus.
Rotate. Before you begin rotation, confirm the fetal position (the fetal ears are useful as a landmark). If the head is not already in occiput anterior, grasp it and rotate the occiput into the incision (FIGURE 2). As in vaginal delivery, this presents the shortest fetal-head diameters to the birth orifice. If the head has been markedly molded by labor, rotation will bring the narrow end of a dilating cone into the uterine incision. This helps direct the head anteriorly, rather than back into the pelvis, when fundal pressure is applied.
Reduce. Using your hand like a pair of forceps may cause uterine extensions or lacerations if the uterine incision is not large enough to accommodate both your hand and the fetal head. Instead, use 4 fingers in shoehorn fashion to guide the head out of the incision. First, however, there is usually another obstacle to overcome: Fundal pressure may merely force the head back toward the pelvis. Your fingers cannot present a shallow enough angle to direct the head anteriorly, unless your hand is in so deep that your palm fills the uterine incision.
You may substitute instruments for the guiding fingers, using the Murless head extractor, the Torpin vectis blade, or 1 blade of a short-shank Simpson forceps. However, a lack of availability may limit your choices. Although you may use a disposable vacuum extractor system, consider saving that expense with this alternative: Using your fingertips, reduce the lower lip of the uterine incision beneath the fetal head, as you would reduce a posterior cervical lip at a vaginal delivery. (FIGURE 3). The reduced lip tends to extend the head anteriorly and direct it away from the pelvis. Withdraw your hand so only your fingertips remain to guide the head, while the back of your fingers retract the lower abdominal wall. With fundal pressure the head should move anteriorly out of the abdomen.
A head in the occiput-posterior position may resist attempts to rotate to occiput anterior. Should this occur, try following the incision reduction with direct manual flexion of the head out of the abdomen. If reduction flexion puts excess strain on the incision, abandon the attempt. Instead, use a disposable vacuum extractor cup—placed on the fetal brow as close to the vertex as possible—to flex the head out of the incision. Should this fail as well, extend the low-transverse uterine incision cranially into a generous U-incision, and repeat the vacuum procedure.
FIGURE 1 THE ERR SEQUENCE
Elevate. Lock the fingers into a quarter-circle around the vertex. Apply traction out of the pelvis with the hand and the entire extended arm.
FIGURE 2 THE ERR SEQUENCE
Rotate. Grasp the fetal head between the thumb and fingers and rotate it so the occiput faces the incision.
FIGURE 3 THE ERR SEQUENCE
Reduce. Push the lower edge of the uterine incision down until it is posterior to the fetal head.
Conclusion
This technique for delivering the fetal head offers minimal risk of uterine laceration and lends itself to instruction and recollection.
I have not evaluated ERR by clinical trial, both due to absence of a gold-standard technique for the control group and because ERR has yielded satisfactory outcomes in the 20 years over which I have developed, applied, and taught it. Still, the technique raises opportunities for clinical investigation. For example, residency training programs concerned over their incidence of uterine extensions and lacerations may wish to adopt this method and subsequently produce a historical-controlled, retrospective cohort study as a quality-improvement project.
Dr. Chao reports no financial relationship with any companies whose products are mentioned in this article.
- Position yourself so your upper trunk, arm, and hand move as a unit to elevate the head.
- Elevate the head to the level of the uterine incision, rather than bringing the incision down to the head.
- Rotate the occiput anteriorly to present the shortest fetal head diameters to the incision.
- Reduce the lower lip of the uterine incision beneath the fetal head, as you would reduce a posterior cervical lip at a vaginal delivery.
Although it’s a skill vital for cesarean birth, manual delivery of the fetal head from a low pelvic station is given only cursory coverage in standard obstetrics texts and reviews.1-4 To learn successful methods, therefore, physicians are forced to rely on observation, anecdotal experience, and—least desirably—trial and error.
Possibly the most frequently employed maneuver in this scenario is to have an assistant elevate the head with his or her hand.1,3,4 However, this technique may not provide sufficient elevation. Furthermore, subsequent manipulation by the clinician may extend the uterine incision and risk injury to the uterine vessels and bladder. It is an untested assumption that such extensions increase the risk of uterine rupture during subsequent trials of labor.
With the goals of minimizing delay, head compression, and strain on the uterine incision, I developed the elevate, rotate, and reduce (ERR) technique for expeditious delivery of the head from a deep pelvic station.
Approaching the head
Begin the procedure by identifying the fetal position; if it is occiput posterior, you may wish to have a disposable vacuum extractor available. Then stand so that your dominant hand is closest to the patient’s pelvis.
When making the abdominal incision, consider fetal size and maternal body habitus. Be sure all layers of the incision are long enough to permit manipulation and delivery—don’t let surgical pride force you to struggle with an inadequate Pfannenstiel incision. Be prepared to identify and extend any layer of the incision that gives considerable resistance at the time of delivery.
Before proceeding to the uterine incision, make sure there is adequate rectus separation. Check the clearance by separating the right and left bodies of the rectus muscle with manual traction. If more room is needed, create a “partial Maylard incision” by cutting bilateral transverse incisions with Mayo scissors across the medial third of each body. This gives the fetus more room while avoiding damage to the inferior epigastric vessels.
The uterine incision should be as wide as the mean fetal head diameter—usually the same 10 cm that we expect of the fully dilated cervix at term. In patients with an undeveloped lower uterine segment, varices, adhesions, or leiomyomata, there may be insufficient width for a low-transverse incision. In these cases you may need to convert to a U, J, or vertical incision.
Take advantage of a well-developed lower uterine segment and avoid tight U- or V-shaped transverse incisions. Identify the uterine vessels with adequate retraction, and incise laterally right up to them. An alternate technique would be to extend the initial incision by pulling apart the ends with your index fingers, laterally and cephalad (towards the mother’s axillae). Blunt dissection may cause less bleeding from the uterine vessels.5
The ERR technique
“Don’t break the wrist” is a classic admonition, familiar to generations of obstetricians. It warns against a practice that begins by reaching for the head with extended wrist, not elevating the head enough, and delivering the head from whatever position it presents in. The head is delivered with firm wrist flexion (breaking, cocking, or levering the wrist) and anterior traction. This puts caudal leverage upon the lower edge of the uterine incision, which may cause an extension down to the bladder or into the cervix. The ERR technique corrects these errors and minimizes strain on the incision.
Elevate. The first goal of ERR is to elevate the fetal vertex to the lower edge of the uterine incision. Bringing the incision down to the presenting part may strain it. Start by holding the fingers of your dominant hand together, with the thumb tucked against the lateral base Safe delivery of the fetal head during cesarean section of the index finger and the fingers extended straight.
Next, insert your fingertips into the uterine incision and advance them around the fetal head—but not while standing erect with your wrist extended. Such a posture would cause 2 problems: First, you would have insufficient leverage and strength to advance your fingers deep into the pelvis. Second, the angle of your wrist would be obstructed by the uterine incision, the lower edge of the abdominal incision, and the pubis.
Instead, try 1 of the following 2 approaches: The first option is to extend your arm in a straight line from shoulder to fingertip, keeping it almost parallel to the patient’s longitudinal axis. This is accomplished by flexing forward at the waist while facing cross-table, and abducting your arm out from your shoulder. Another option is to turn and face the head of the table, flex at the waist, and extend your arm behind you while pronating your hand. Both of these positions keep your wrist angle neutral and allow you to use the strength and leverage of your entire upper trunk to move your hand.
In order to elevate the head, your fingers need to achieve at least a quarter-circle grip around the vertex (FIGURE 1). If the head is so deep or tightly applied that you cannot achieve this in 1 continuous movement, proceed in stages. First, have an assistant apply transvaginal digital pressure to elevate the head from below. Then, advance your fingers as far as you can around the fetal head while flexing and extending the fingers in a worm-like wiggling movement. Flex and lock your fingers against the fetal cranium, and lean your entire upper trunk and arm away from the maternal pelvis. This generally elevates the head by roughly a centimeter, enough to advance your fingers a little further. Repeat these motions until your fingers have achieved the desired quarter-circle, at which point your assistant may withdraw from below.
Now lock your arm from shoulder to fingertip, and lean out of the patient’s pelvis 1 more time. You may note the sensation and sound of breaking suction as the fetal head escapes the pelvic grip. Maintain this steady traction for several seconds. When the vertex has reached the level of the lower margin of the uterine incision, you can rotate the fetus.
Rotate. Before you begin rotation, confirm the fetal position (the fetal ears are useful as a landmark). If the head is not already in occiput anterior, grasp it and rotate the occiput into the incision (FIGURE 2). As in vaginal delivery, this presents the shortest fetal-head diameters to the birth orifice. If the head has been markedly molded by labor, rotation will bring the narrow end of a dilating cone into the uterine incision. This helps direct the head anteriorly, rather than back into the pelvis, when fundal pressure is applied.
Reduce. Using your hand like a pair of forceps may cause uterine extensions or lacerations if the uterine incision is not large enough to accommodate both your hand and the fetal head. Instead, use 4 fingers in shoehorn fashion to guide the head out of the incision. First, however, there is usually another obstacle to overcome: Fundal pressure may merely force the head back toward the pelvis. Your fingers cannot present a shallow enough angle to direct the head anteriorly, unless your hand is in so deep that your palm fills the uterine incision.
You may substitute instruments for the guiding fingers, using the Murless head extractor, the Torpin vectis blade, or 1 blade of a short-shank Simpson forceps. However, a lack of availability may limit your choices. Although you may use a disposable vacuum extractor system, consider saving that expense with this alternative: Using your fingertips, reduce the lower lip of the uterine incision beneath the fetal head, as you would reduce a posterior cervical lip at a vaginal delivery. (FIGURE 3). The reduced lip tends to extend the head anteriorly and direct it away from the pelvis. Withdraw your hand so only your fingertips remain to guide the head, while the back of your fingers retract the lower abdominal wall. With fundal pressure the head should move anteriorly out of the abdomen.
A head in the occiput-posterior position may resist attempts to rotate to occiput anterior. Should this occur, try following the incision reduction with direct manual flexion of the head out of the abdomen. If reduction flexion puts excess strain on the incision, abandon the attempt. Instead, use a disposable vacuum extractor cup—placed on the fetal brow as close to the vertex as possible—to flex the head out of the incision. Should this fail as well, extend the low-transverse uterine incision cranially into a generous U-incision, and repeat the vacuum procedure.
FIGURE 1 THE ERR SEQUENCE
Elevate. Lock the fingers into a quarter-circle around the vertex. Apply traction out of the pelvis with the hand and the entire extended arm.
FIGURE 2 THE ERR SEQUENCE
Rotate. Grasp the fetal head between the thumb and fingers and rotate it so the occiput faces the incision.
FIGURE 3 THE ERR SEQUENCE
Reduce. Push the lower edge of the uterine incision down until it is posterior to the fetal head.
Conclusion
This technique for delivering the fetal head offers minimal risk of uterine laceration and lends itself to instruction and recollection.
I have not evaluated ERR by clinical trial, both due to absence of a gold-standard technique for the control group and because ERR has yielded satisfactory outcomes in the 20 years over which I have developed, applied, and taught it. Still, the technique raises opportunities for clinical investigation. For example, residency training programs concerned over their incidence of uterine extensions and lacerations may wish to adopt this method and subsequently produce a historical-controlled, retrospective cohort study as a quality-improvement project.
Dr. Chao reports no financial relationship with any companies whose products are mentioned in this article.
1. Cunningham FG, MacDonald PC, Gant NF, et al. Williams Obstetrics. 20th ed. Stamford, Conn: Appleton & Lange; 1997;518.-
2. Scott JR. Cesarean delivery. In: Scott JR, Disaia PJ, Hammond CB, Spellacy WN, eds. Danforth’s Obstetrics and Gynecology. 8th ed. Philadelphia: Lippincott Williams and Wilkins; 1999;462.-
3. Depp R. Cesarean delivery. In: Gabbe SG, Niebyl JR, Simpson JL. Obstetrics: Normal and Problem Pregnancies. 4th ed. New York: Churchill Livingstone, 2002;554.-
4. Field CS. Surgical techniques for cesarean section. Obstet Gynecol Clin NA. 1988;15:664-665.
5. Magann EF, Chauhan SP, Bufkin L, Field K, Roberts WE, Martin JN, Jr. Intraoperative hemorrhage by blunt versus sharp expansion of the uterine incision at caesarean delivery: a randomized clinical trial. BJOG. 2002;109:448-452.
1. Cunningham FG, MacDonald PC, Gant NF, et al. Williams Obstetrics. 20th ed. Stamford, Conn: Appleton & Lange; 1997;518.-
2. Scott JR. Cesarean delivery. In: Scott JR, Disaia PJ, Hammond CB, Spellacy WN, eds. Danforth’s Obstetrics and Gynecology. 8th ed. Philadelphia: Lippincott Williams and Wilkins; 1999;462.-
3. Depp R. Cesarean delivery. In: Gabbe SG, Niebyl JR, Simpson JL. Obstetrics: Normal and Problem Pregnancies. 4th ed. New York: Churchill Livingstone, 2002;554.-
4. Field CS. Surgical techniques for cesarean section. Obstet Gynecol Clin NA. 1988;15:664-665.
5. Magann EF, Chauhan SP, Bufkin L, Field K, Roberts WE, Martin JN, Jr. Intraoperative hemorrhage by blunt versus sharp expansion of the uterine incision at caesarean delivery: a randomized clinical trial. BJOG. 2002;109:448-452.
Treating stress urinary incontinence with suburethral slings
- Suburethral sling procedures are effective in treating patients with urethral hypermobility, intrinsic sphincter deficiency, low-pressure urethras, and increased intra-abdominal pressure.
- Autologous slings may be a better choice in cases of severe urogenital atrophy, previous radiation, or extensive scarring from previous repairs.
- For both the tension-free vaginal tape and SPARC slings, mark the suprapubic region 1 cm above and 1 cm lateral to the pubic symphsis on the left and right sides and inject 20 cc of a 1:1 mixture of local anesthetic and normal saline into the marked regions.
- Once the trocars are in place, fill the bladder with 250 cc of water and perform a cough stress test to confirm continence.
When the suburethral sling was first described in 1907 by von Giordano, it entailed placing autologous tissue underneath the bladder neck and suspending it superiorly. Complications including urethral erosion, infection, bleeding, and fistula formation led many surgeons to use it sparingly.
Fast forward to the 21st century: Synthetic materials and new techniques were introduced, simplifying the sling procedures and raising the long-term success rates to 84%.1 As a result, slings now stand at the forefront of stress urinary incontinence (SUI) treatment. Among advances are the tension-free vaginal tape (TVT) sling (Gynecare, a division of Ethicon Inc., Somerville, NJ) and the SPARC sling (American Medical Systems, Inc., Minnetonka, Minn). The former, approved in the U.S. in 1998, calls for another look due to of the recent publication of a Cochrane review of outcomes studies, while the latter, approved by the FDA in August 2001, is the newest technique deserving examination. Clearly, with 83,010 incontinence procedures performed in the U.S. in 1999,2 a detailed look at the suburethral sling is warranted. Here, we review materials, indications, techniques, complications, and outcomes.
Materials
The choice of material—either organic or synthetic—depends on several factors: availability, cost, patient and surgeon preference, and clinical variables. (TABLE 1) outlines the advantages and disadvantages of each material type. Organic slings include autologous tissues (rectus fascia and fascia lata graft), and allografts or xenografts (cadaveric fascia lata graft, human dermal graft, or porcine small intestine and dermal graft). Synthetic slings are made of polyethylene terephthalate, expanded polytetrafluoroethylene, and polypropylene.
While sling procedures utilizing organic materials do have their benefits, synthetic slings, particularly the polypropylene mesh used in TVT and SPARC, have proven to be a stable material unlikely to deteriorate with time. Further, increased collagen metabolism around this synthetic sling promotes an ingrowth of tissue through the mesh.
TABLE 1
Slings: advantages and disadvantages of various materials
| SLING MATERIAL | ADVANTAGES | DISADVANTAGES |
|---|---|---|
| Autologous tissues (rectus fascia, fascia lata, or vaginal wall) |
|
|
| Allografts (cadaveric fascia lata or dermis) |
|
|
| Xenografts (porcine dermis or small intestine) | ||
| Synthetic mesh (polyethylene terephthalate, expanded polytetrafluoroethylene, or polypropylene) |
|
|
Indications
Suburethral sling procedures are typically used for the treatment of genuine stress urinary incontinence (GSUI), in which the urethra becomes either hypermobile and unstable or its intrinsic sphincter becomes incompetent. In fact, slings are technically easier to place in patients with anatomic urethrovesical junction hypermobility compared to those with fixed urethras. Several authors also have suggested the sling’s advantage in patients with low-pressure urethras.3
Use urodynamic criteria to diagnose intrinsic sphincter deficiency (ISD), which is defined as a Valsalva leak point pressure of less than 60 cm water or maximal urethral closure pressure of less than 20 cm water. (Bear in mind, however, that these cut-off criteria are controversial.4,5)
Also, consider slings in patients with recurrent GSUI, inherited collagen deficiency, and increased abdominal pressure (e.g., women with chronic obstructive pulmonary disease, obesity, or high-impact physical activity). The sling also can be used as an adjunct to other transvaginal surgeries (e.g., hysterectomy or prolapse repair).
Autologous slings may be a better choice than synthetic slings in cases of severe urogenital atrophy, previous radiation, or extensive scarring from previous repairs. In these instances, the patient may be at-risk for postoperative vaginal necrosis or erosion.6 Due to their biocompatibility, autologous slings are more likely to heal over a vaginal erosion and less likely to infect or erode into the urethra. In any event, urogenital atrophy should be treated with local estrogen preoperatively to prevent some of these complications.
Technique
Conventionally, suburethral slings were placed via a combined vaginal and abdominal approach into the retropubic space of Retzius. Alternatively, the procedure could be performed abdominally by creating a suburethral tunnel via pelvic incisions, but this is the most difficult route.
Most recently, technological advances have simplified the vaginal approach, which utilizes minimal suburethral dissection and small suprapubic incisions. This technique is subdivided into “bottom-up” and “top-down” approaches. In the bottom-up TVT, the sling is inserted into a vaginal incision and threaded up through the patient’s pelvis, exiting from a small suprapubic incision. The topdown SPARC entails a reverse approach, starting from a suprapubic incision and exiting from a vaginal incision. New modifications allow for an abdominal TVT approach, as well, which we describe in detail in a later section.
Surgeons who are familiar with traditional needle suspensions may be more comfortable with the top-down approach. The need for concomitant surgery (e.g., hysterectomy or prolapse repair) not only determines the type of incontinence procedure, but also dictates the approach.
Preparing the patient. Place the patient under regional or local anesthesia with sedation so that an intraoperative cough stress test can be performed. Then administer an intravenous dose of a broad-spectrum antibiotic. Insert a 16 to 18 French Foley catheter into the urethra. Mark the suprapubic region 1 cm above and 1 cm lateral to the pubic symphsis on the left and right sides of the patient. Inject approximately 20 cc of a 1:1 mixture of local anesthetic and normal saline into the marked areas. We typically use 60 cc of 0.25% bupivicaine with epinephrine, diluted 1:1 with 60 cc of normal saline. After administering the local anesthetic suprapubically, inject a similar solution into the anterior vaginal wall suburethrally in the midline and laterally toward the retropubic tunnels.
Making the incisions. Both the TVT and SPARC techniques utilize the same type and location of incisions. As such, make a 0.5-cm incision into the abdominal skin on each side of the midline, approximately 1 cm lateral to midline and 1 cm above the pubic symphsis. Next, make a 1.5- to 2-cm vertical incision in the vaginal mucosa, starting 1.5 cm from the urethral meatus (FIGURE 1). Use Metzenbaum scissors to dissect the vaginal mucosa from the pubocervical fascia sub- and para-urethrally on both sides (FIGURE 2). Insert a Foley catheter guide (similar to the Lowsley retractor) into the catheter and deviate it to the ipsilateral side, thereby retracting the bladder neck to the contralateral side. Proceed with the placement of either the TVT or SPARC sling.
Placing the TVT sling. Attach the TVT introducer to the curved needle trocar on 1 end of the polypropylene sling. Insert the trocar with the tape attached into the vaginal incision and push through the retropubic space, keeping the trocar in close contact with the posterior surface of the pubic bone (FIGURES 3 and 4). Continue pushing the trocar through the urogenital diaphragm until its tip comes through the suprapubic incision on the ipsilateral side (FIGURE 5). It is important to not deviate too laterally, medially, or cephalad during trocar insertion to prevent vessel, bladder, or bowel injury. Perform a cystoscopy to rule out cystotomy. Place the second trocar in a similar manner on the opposite side. After both trocars have been pulled through their respective incisions, perform a tension test.
Placing the SPARC/abdominal TVT sling. Guide the abdominal needles through the previously marked suprapubic incision and the patient’s retropubic cavity (keeping the needle behind the pubic bone), to a finger placed in the vaginal incision (FIGURE 6). Snap the abdominal needle guides with the attached polypropylene mesh to the sling connectors (FIGURE 7). Bring the abdominal needles through the suprapubic incisions. Perform a tension test. As with the TVT sling, perform a cystoscopy after each needle placement to rule out cystotomy. Then pass the sling through the tunnel.
Testing for continence. Once the sling is in place, fill the bladder with 250 cc of water and perform a cough stress test. Adjust sling tension by pulling up on both sling arms until only a few drops of leakage are noted. It is important not to secure the sling too tightly as this may lead to urinary retention, detrusor instability, or urethral erosion. We prefer placing a hemostat between the sling tape and the urethra to avoid over tightening.
Suspending the sling arms. Remove the plastic sheaths after tension adjustment and cut the sling flush with the skin (FIGURE 8). Compared to the conventional bone-anchored slings, the newer tension-free sling devices are not anchored but instead suspended through the retropubic space. At first, the sling is held in place by friction from the opposing tissues. Over time, collagen formation fixes the mesh more strongly within the suburethral and paravaginal tissues.
Finally, close the suprapubic and vaginal incisions with absorbable sutures.
Placing autologous or allogenic slings. Fashion the graft, typically 2 cm wide and 10 to 12 cm long, with permanent sutures at the edges. Make a 1-cm incision into the suprapubic rectus fascia. Use either a Stamey-type needle trocar or uterine packing forceps and guide the instrument “top down” from the retropubic incision to the vaginal tunnel. The tunnel is made directly into the retropubic space from the vaginal incision. Bring the sling arms up on each side. Attach the arms to the rectus fascia and tie them down once cystoscopy and the tension test are complete.
The sling also can be performed with bone anchors placed through the vaginal incision into the pubic bone. Placement requires vaginal dissection into the retropubic space with no suprapubic incision. Once anchored, the sutures are then passed through the chosen graft materials and tied down. Bear in mind that anchoring into the periosteum of the pubic bone may cause severe osteomyelitis or osteitis pubis, though the actual incidence is unknown.7
FIGURE 1 Surgical steps for tension-free vaginal tape (TVT)
Place a Sims speculum into the vagina and make a vertical incision 1.5 cm from the external urethra meatus.
FIGURE 2
Use Metzenbaum scissors to dissect the vaginal mucosa from the underlying fascia bilaterally. Insert a Foley catheter with guide.
FIGURE 3
Place TVT trocars through the vaginal incision. Place abdominal guides suprapubically and attach them to the TVT trocar tip.
FIGURE 4
Push the tape through the retropubic space, keeping the trocar in close contact with the posterior surface of the pubic bone.
FIGURE 5
Continue pushing the trocar through the urogenital diaphragm until its tip comes through the suprapubic incision on the ipsilateral side.
FIGURE 6 Surgical steps for SPARC
Guide the needle down the posterior side of the pubic bone, keeping the needle tip in contact with the pubic bone.
FIGURE 7
Snap the needle guide and sling onto the sling connectors and pull through the suprapubic incision.
FIGURE 8
Perform a tension test. Remove the plastic sheaths and cut the sling flush with the skin.
Complications
Intraoperative and immediate postoperative complications include bladder perforation, vaginal or retropubic bleeding, wound or urinary tract infection (UTI), and short-term urinary retention. Possible long-term problems include urethral or vaginal erosion, mesh infection, prolonged voiding dysfunction, fistula formation, or de novo urge incontinence (TABLE 2).
Specifically, the TVT sling, which has been placed in more than 50,000 women in the U.S. and 200,000 worldwide, carries the potential for significant vascular injury and bowel perforation. In addition, the Food and Drug Administration (FDA) reported 4 deaths (2 from unrecognized bowel injuries, 1 from retropubic bleeding in a patient with a bleeding disorder, and 1 from a heart attack more than 1 week after an incontinence repair procedure complicated by a vascular injury); 168 device malfunctions (mostly tape or sheath detachment from the trocar); and 128 other injuries, including bowel perforations and major vascular injuries to the obturator, external iliac, femoral, or inferior epigastrics (TABLE 3).8 In a review of 1,455 TVT sling cases at 38 hospitals, Kuuva and Nilsson9 found bladder perforation in 3.8% of the patients and retropubic hematoma in 1.9%, along with 1 case of vesicovaginal fistula, 1 obturator nerve injury, and 1 epigastric vessel injury.
According to the FDA, there have been 2 complications reported with the SPARC sling system. Both involved vaginal erosion subsequently repaired by oversewing the vaginal mucosa. One of these complications occurred in a woman undergoing her fourth vaginal procedure who was therefore deemed to have “poor tissue.”8
TABLE 2
Suburethral sling complications1
| COMPLICATION | 1,715 AUTOLOGOUS | 1,515 SYNTHETIC |
|---|---|---|
| Vaginal erosion | 1 (.0001%) | 10 (.007%) |
| Urethral erosion | 5 (.003%) | 27 (.02%) |
| Fistula | 6 (.003%) | 4 (.002%) |
| Wound sinus | 3 (.002%) | 11 (.007%) |
| Wound infection | 11 (.006%) | 15 (.009%) |
| Seroma | 6 (.003%) | 1 (.0007%) |
TABLE 3
TVT complications in 200,000 procedures worldwide
| COMPLICATION | U.S. | WORLD | TOTAL |
|---|---|---|---|
| Vascular injury | 3 | 25 | 28 |
| Vaginal mesh exposure | 15 | 2 | 17 |
| Urethral erosion | 8 | 0 | 8 |
| Bowel perforation | 4 | 6 | 10 |
| Nerve injury | 1 | 0 | 1 |
Outcomes studies
Unfortunately, most of the published clinical studies on the surgical management of stress urinary incontinence suffer from inadequate follow-up and sample size, unclear patient selection criteria, and poor postoperative documentation, especially with respect to quality of life. However, multiple studies to assess the effectiveness and safety of TVT slings have been published. The following is an outline of these preliminary yet important findings.
In 2002, the Cochrane Database evaluated 7 randomized and quasi-randomized trials of suburethral slings for the treatment of urinary incontinence.10 Of 682 women evaluated, 457 had some type of suburethral sling procedure. Four trials compared slings to retropubic urethropexies, 1 compared slings to Stamey needle suspensions, and 2 compared the use of different sling materials. The results indicated that the data were insufficient to suggest that slings were more effective than other incontinence procedures or that slings were associated with fewer postoperative complications. While TVT slings did provide similar cure rates as open retropubic urethropexy, research is still lacking with respect to other types of slings. More studies comparing TVT slings to traditional pubovaginal slings also are needed before the 2 can be deemed equivalent.
In Sweden and Finland, where the TVT procedure was developed,11 85 patients who had undergone the procedure were evaluated at 48 to 70 months. Of those, 84.7% were completely cured of stress incontinence, 10.6% had significantly improved symptoms, and 4.7% were regarded as failures.
A recent well-designed, multicenter, randomized, prospective trial in the U.K. and Ireland compared 146 open Burch colposuspensions to 170 TVTs. Similar cure rates (57% and 66%, respectively) were reported.12 Although these rates are low compared to the Nordic nonrandomized TVT studies mentioned, the U.K./Ireland outcome criteria were particularly stringent and included a negative cystometrogram for stress incontinence and negative pad test. These differences in reported success rates highlight the importance of clearly defining objective outcomes criteria from randomized trials.
Nonetheless, the U.K./Ireland study showed that TVT is less invasive than the Burch procedure and is associated with shorter recovery periods and greater cost savings. Follow-up on complications (bladder perforation and hematoma in TVTs and incisional hernia formation in Burch colposuspensions) will be the most crucial aspect of this study.13
Clearly, the question of whether a Burch retropubic urethropexy or a suburethral sling procedure is better for SUI needs to be further investigated. Weber and Walters sought to answer this question by developing a decision analytical model (without the aid of randomized, controlled trials) and discovered similar cure rates.14 However, there were higher rates of urinary retention and detrusor instability associated with the traditional pubovaginal sling. But, most importantly, sensitivity analyses proved that if the rate of permanent urinary retention after a sling procedure was less than 9%—as in most sling series—the overall effectiveness of slings was higher than that of the Burch.
Conclusion
The suburethral sling procedure has undergone many modifications since its first description nearly a century ago. As such, Ob/Gyns need to familiarize themselves with the current options. Typically, we perform up to 6 suburethral sling procedures per month. Of those, 50% are referrals from failed incontinence procedures. Recently, we have made the switch from using autologous slings to tension-free type slings due to ease and good outcomes. While more data from randomized, prospective, multicenter trials are needed to determine the best approach for individual patients, surgeons should become comfortable with the technique that works best for them.
The authors report no financial relationship with any companies whose products are mentioned in this article.
1. Leach GE, Dmochowski RR, Appell RA, et al. Female Stress Urinary Incontinence Clinical Guidelines Panel summary report on surgical management of female stress urinary incontinence. J Urol. 1997;875-880.
2. Nihira MA, Schaffer JI. Surgical procedures for stress urinary incontinence in 1999. Presented at: 28th Annual Scientific Meeting of the Society of Gynecologic Surgeons; March 6, 2002; Dallas, Texas.
3. Kobashi KC, Leach GE. Stress urinary incontinence. Curr Opin Urol. 1999;9:285-290.
4. Bowen LW, Sand PK, Ostergard DR, Franti CE. Unsuccessful Burch retropubic urethropexy: a case-controlled urodynamic study. Am J Obstet Gynecol. 1989;160:452-458.
5. Bump RC, Coates KW, Cundiff GW, Harris RL, Weidner AC. Diagnosing intrinsic sphincteric deficiency: Comparing urethral closure pressure, urethral axis, and Valsalva leak point pressures. Am J Obstet Gynecol. 1997;177:303-310.
6. Nichols DH, Randal CL. Operations for urinary stress incontinence. In: Vaginal Surgery. 4th ed. Baltimore, Md: Williams & Wilkins; 1996;402-415.
7. Rackley RR, Abdelmalak JB, Madjar S, Yanilmaz A, Appell RA, Tchetgen MB. Bone anchor infections in female pelvic reconstructive procedures: a literature review of series and case reports. J Urol. 2001;165:1975-1978.
8. Food and Drug Administration manufacturer and user facility device experience database. Available at: www.fda.gov/cdrh/maude.html. Accessed November 12, 2002.
9. Kuuva N, Nilsson CG. A nationwide analysis of complications associated with the tension-free vaginal tape (TVT) procedure. Neurourol Urodyn. 2000;19:394.-
10. Bezerra CA, Bruschini H. Suburethral sling operations for urinary incontinence in women. Cochrane Database Syst Rev. 2001;(3):CD001754.-
11. Nilsson CG, Kuuva N, Falconer C, Rezapour M, Ulmsten U. Long-term results of the tension-free vaginal tape procedure (TVT) for surgical treatment of female stress urinary incontinence. Int Urogynecol J Pelvic Floor Dysfunct. 2001;12(suppl 2):S5-S8.
12. Ward KL, Hilton P, Browning J. A randomized trial of colposuspension and tension-free vaginal tape (TVT) for primary genuine stress incontinence. Neurourol Urodyn. 2000;19:386.-
13. Ward K, Hilton P. Prospective multicenter randomized trial of tension-free vaginal tape and colposuspension as primary treatment for stress incontinence. BMJ. 2002;325:67.-
14. Weber A, Walters M. Burch procedure compared with sling for stress urinary incontinence: a decision analysis. Obstet Gynecol. 2000;96:867-873.
- Suburethral sling procedures are effective in treating patients with urethral hypermobility, intrinsic sphincter deficiency, low-pressure urethras, and increased intra-abdominal pressure.
- Autologous slings may be a better choice in cases of severe urogenital atrophy, previous radiation, or extensive scarring from previous repairs.
- For both the tension-free vaginal tape and SPARC slings, mark the suprapubic region 1 cm above and 1 cm lateral to the pubic symphsis on the left and right sides and inject 20 cc of a 1:1 mixture of local anesthetic and normal saline into the marked regions.
- Once the trocars are in place, fill the bladder with 250 cc of water and perform a cough stress test to confirm continence.
When the suburethral sling was first described in 1907 by von Giordano, it entailed placing autologous tissue underneath the bladder neck and suspending it superiorly. Complications including urethral erosion, infection, bleeding, and fistula formation led many surgeons to use it sparingly.
Fast forward to the 21st century: Synthetic materials and new techniques were introduced, simplifying the sling procedures and raising the long-term success rates to 84%.1 As a result, slings now stand at the forefront of stress urinary incontinence (SUI) treatment. Among advances are the tension-free vaginal tape (TVT) sling (Gynecare, a division of Ethicon Inc., Somerville, NJ) and the SPARC sling (American Medical Systems, Inc., Minnetonka, Minn). The former, approved in the U.S. in 1998, calls for another look due to of the recent publication of a Cochrane review of outcomes studies, while the latter, approved by the FDA in August 2001, is the newest technique deserving examination. Clearly, with 83,010 incontinence procedures performed in the U.S. in 1999,2 a detailed look at the suburethral sling is warranted. Here, we review materials, indications, techniques, complications, and outcomes.
Materials
The choice of material—either organic or synthetic—depends on several factors: availability, cost, patient and surgeon preference, and clinical variables. (TABLE 1) outlines the advantages and disadvantages of each material type. Organic slings include autologous tissues (rectus fascia and fascia lata graft), and allografts or xenografts (cadaveric fascia lata graft, human dermal graft, or porcine small intestine and dermal graft). Synthetic slings are made of polyethylene terephthalate, expanded polytetrafluoroethylene, and polypropylene.
While sling procedures utilizing organic materials do have their benefits, synthetic slings, particularly the polypropylene mesh used in TVT and SPARC, have proven to be a stable material unlikely to deteriorate with time. Further, increased collagen metabolism around this synthetic sling promotes an ingrowth of tissue through the mesh.
TABLE 1
Slings: advantages and disadvantages of various materials
| SLING MATERIAL | ADVANTAGES | DISADVANTAGES |
|---|---|---|
| Autologous tissues (rectus fascia, fascia lata, or vaginal wall) |
|
|
| Allografts (cadaveric fascia lata or dermis) |
|
|
| Xenografts (porcine dermis or small intestine) | ||
| Synthetic mesh (polyethylene terephthalate, expanded polytetrafluoroethylene, or polypropylene) |
|
|
Indications
Suburethral sling procedures are typically used for the treatment of genuine stress urinary incontinence (GSUI), in which the urethra becomes either hypermobile and unstable or its intrinsic sphincter becomes incompetent. In fact, slings are technically easier to place in patients with anatomic urethrovesical junction hypermobility compared to those with fixed urethras. Several authors also have suggested the sling’s advantage in patients with low-pressure urethras.3
Use urodynamic criteria to diagnose intrinsic sphincter deficiency (ISD), which is defined as a Valsalva leak point pressure of less than 60 cm water or maximal urethral closure pressure of less than 20 cm water. (Bear in mind, however, that these cut-off criteria are controversial.4,5)
Also, consider slings in patients with recurrent GSUI, inherited collagen deficiency, and increased abdominal pressure (e.g., women with chronic obstructive pulmonary disease, obesity, or high-impact physical activity). The sling also can be used as an adjunct to other transvaginal surgeries (e.g., hysterectomy or prolapse repair).
Autologous slings may be a better choice than synthetic slings in cases of severe urogenital atrophy, previous radiation, or extensive scarring from previous repairs. In these instances, the patient may be at-risk for postoperative vaginal necrosis or erosion.6 Due to their biocompatibility, autologous slings are more likely to heal over a vaginal erosion and less likely to infect or erode into the urethra. In any event, urogenital atrophy should be treated with local estrogen preoperatively to prevent some of these complications.
Technique
Conventionally, suburethral slings were placed via a combined vaginal and abdominal approach into the retropubic space of Retzius. Alternatively, the procedure could be performed abdominally by creating a suburethral tunnel via pelvic incisions, but this is the most difficult route.
Most recently, technological advances have simplified the vaginal approach, which utilizes minimal suburethral dissection and small suprapubic incisions. This technique is subdivided into “bottom-up” and “top-down” approaches. In the bottom-up TVT, the sling is inserted into a vaginal incision and threaded up through the patient’s pelvis, exiting from a small suprapubic incision. The topdown SPARC entails a reverse approach, starting from a suprapubic incision and exiting from a vaginal incision. New modifications allow for an abdominal TVT approach, as well, which we describe in detail in a later section.
Surgeons who are familiar with traditional needle suspensions may be more comfortable with the top-down approach. The need for concomitant surgery (e.g., hysterectomy or prolapse repair) not only determines the type of incontinence procedure, but also dictates the approach.
Preparing the patient. Place the patient under regional or local anesthesia with sedation so that an intraoperative cough stress test can be performed. Then administer an intravenous dose of a broad-spectrum antibiotic. Insert a 16 to 18 French Foley catheter into the urethra. Mark the suprapubic region 1 cm above and 1 cm lateral to the pubic symphsis on the left and right sides of the patient. Inject approximately 20 cc of a 1:1 mixture of local anesthetic and normal saline into the marked areas. We typically use 60 cc of 0.25% bupivicaine with epinephrine, diluted 1:1 with 60 cc of normal saline. After administering the local anesthetic suprapubically, inject a similar solution into the anterior vaginal wall suburethrally in the midline and laterally toward the retropubic tunnels.
Making the incisions. Both the TVT and SPARC techniques utilize the same type and location of incisions. As such, make a 0.5-cm incision into the abdominal skin on each side of the midline, approximately 1 cm lateral to midline and 1 cm above the pubic symphsis. Next, make a 1.5- to 2-cm vertical incision in the vaginal mucosa, starting 1.5 cm from the urethral meatus (FIGURE 1). Use Metzenbaum scissors to dissect the vaginal mucosa from the pubocervical fascia sub- and para-urethrally on both sides (FIGURE 2). Insert a Foley catheter guide (similar to the Lowsley retractor) into the catheter and deviate it to the ipsilateral side, thereby retracting the bladder neck to the contralateral side. Proceed with the placement of either the TVT or SPARC sling.
Placing the TVT sling. Attach the TVT introducer to the curved needle trocar on 1 end of the polypropylene sling. Insert the trocar with the tape attached into the vaginal incision and push through the retropubic space, keeping the trocar in close contact with the posterior surface of the pubic bone (FIGURES 3 and 4). Continue pushing the trocar through the urogenital diaphragm until its tip comes through the suprapubic incision on the ipsilateral side (FIGURE 5). It is important to not deviate too laterally, medially, or cephalad during trocar insertion to prevent vessel, bladder, or bowel injury. Perform a cystoscopy to rule out cystotomy. Place the second trocar in a similar manner on the opposite side. After both trocars have been pulled through their respective incisions, perform a tension test.
Placing the SPARC/abdominal TVT sling. Guide the abdominal needles through the previously marked suprapubic incision and the patient’s retropubic cavity (keeping the needle behind the pubic bone), to a finger placed in the vaginal incision (FIGURE 6). Snap the abdominal needle guides with the attached polypropylene mesh to the sling connectors (FIGURE 7). Bring the abdominal needles through the suprapubic incisions. Perform a tension test. As with the TVT sling, perform a cystoscopy after each needle placement to rule out cystotomy. Then pass the sling through the tunnel.
Testing for continence. Once the sling is in place, fill the bladder with 250 cc of water and perform a cough stress test. Adjust sling tension by pulling up on both sling arms until only a few drops of leakage are noted. It is important not to secure the sling too tightly as this may lead to urinary retention, detrusor instability, or urethral erosion. We prefer placing a hemostat between the sling tape and the urethra to avoid over tightening.
Suspending the sling arms. Remove the plastic sheaths after tension adjustment and cut the sling flush with the skin (FIGURE 8). Compared to the conventional bone-anchored slings, the newer tension-free sling devices are not anchored but instead suspended through the retropubic space. At first, the sling is held in place by friction from the opposing tissues. Over time, collagen formation fixes the mesh more strongly within the suburethral and paravaginal tissues.
Finally, close the suprapubic and vaginal incisions with absorbable sutures.
Placing autologous or allogenic slings. Fashion the graft, typically 2 cm wide and 10 to 12 cm long, with permanent sutures at the edges. Make a 1-cm incision into the suprapubic rectus fascia. Use either a Stamey-type needle trocar or uterine packing forceps and guide the instrument “top down” from the retropubic incision to the vaginal tunnel. The tunnel is made directly into the retropubic space from the vaginal incision. Bring the sling arms up on each side. Attach the arms to the rectus fascia and tie them down once cystoscopy and the tension test are complete.
The sling also can be performed with bone anchors placed through the vaginal incision into the pubic bone. Placement requires vaginal dissection into the retropubic space with no suprapubic incision. Once anchored, the sutures are then passed through the chosen graft materials and tied down. Bear in mind that anchoring into the periosteum of the pubic bone may cause severe osteomyelitis or osteitis pubis, though the actual incidence is unknown.7
FIGURE 1 Surgical steps for tension-free vaginal tape (TVT)
Place a Sims speculum into the vagina and make a vertical incision 1.5 cm from the external urethra meatus.
FIGURE 2
Use Metzenbaum scissors to dissect the vaginal mucosa from the underlying fascia bilaterally. Insert a Foley catheter with guide.
FIGURE 3
Place TVT trocars through the vaginal incision. Place abdominal guides suprapubically and attach them to the TVT trocar tip.
FIGURE 4
Push the tape through the retropubic space, keeping the trocar in close contact with the posterior surface of the pubic bone.
FIGURE 5
Continue pushing the trocar through the urogenital diaphragm until its tip comes through the suprapubic incision on the ipsilateral side.
FIGURE 6 Surgical steps for SPARC
Guide the needle down the posterior side of the pubic bone, keeping the needle tip in contact with the pubic bone.
FIGURE 7
Snap the needle guide and sling onto the sling connectors and pull through the suprapubic incision.
FIGURE 8
Perform a tension test. Remove the plastic sheaths and cut the sling flush with the skin.
Complications
Intraoperative and immediate postoperative complications include bladder perforation, vaginal or retropubic bleeding, wound or urinary tract infection (UTI), and short-term urinary retention. Possible long-term problems include urethral or vaginal erosion, mesh infection, prolonged voiding dysfunction, fistula formation, or de novo urge incontinence (TABLE 2).
Specifically, the TVT sling, which has been placed in more than 50,000 women in the U.S. and 200,000 worldwide, carries the potential for significant vascular injury and bowel perforation. In addition, the Food and Drug Administration (FDA) reported 4 deaths (2 from unrecognized bowel injuries, 1 from retropubic bleeding in a patient with a bleeding disorder, and 1 from a heart attack more than 1 week after an incontinence repair procedure complicated by a vascular injury); 168 device malfunctions (mostly tape or sheath detachment from the trocar); and 128 other injuries, including bowel perforations and major vascular injuries to the obturator, external iliac, femoral, or inferior epigastrics (TABLE 3).8 In a review of 1,455 TVT sling cases at 38 hospitals, Kuuva and Nilsson9 found bladder perforation in 3.8% of the patients and retropubic hematoma in 1.9%, along with 1 case of vesicovaginal fistula, 1 obturator nerve injury, and 1 epigastric vessel injury.
According to the FDA, there have been 2 complications reported with the SPARC sling system. Both involved vaginal erosion subsequently repaired by oversewing the vaginal mucosa. One of these complications occurred in a woman undergoing her fourth vaginal procedure who was therefore deemed to have “poor tissue.”8
TABLE 2
Suburethral sling complications1
| COMPLICATION | 1,715 AUTOLOGOUS | 1,515 SYNTHETIC |
|---|---|---|
| Vaginal erosion | 1 (.0001%) | 10 (.007%) |
| Urethral erosion | 5 (.003%) | 27 (.02%) |
| Fistula | 6 (.003%) | 4 (.002%) |
| Wound sinus | 3 (.002%) | 11 (.007%) |
| Wound infection | 11 (.006%) | 15 (.009%) |
| Seroma | 6 (.003%) | 1 (.0007%) |
TABLE 3
TVT complications in 200,000 procedures worldwide
| COMPLICATION | U.S. | WORLD | TOTAL |
|---|---|---|---|
| Vascular injury | 3 | 25 | 28 |
| Vaginal mesh exposure | 15 | 2 | 17 |
| Urethral erosion | 8 | 0 | 8 |
| Bowel perforation | 4 | 6 | 10 |
| Nerve injury | 1 | 0 | 1 |
Outcomes studies
Unfortunately, most of the published clinical studies on the surgical management of stress urinary incontinence suffer from inadequate follow-up and sample size, unclear patient selection criteria, and poor postoperative documentation, especially with respect to quality of life. However, multiple studies to assess the effectiveness and safety of TVT slings have been published. The following is an outline of these preliminary yet important findings.
In 2002, the Cochrane Database evaluated 7 randomized and quasi-randomized trials of suburethral slings for the treatment of urinary incontinence.10 Of 682 women evaluated, 457 had some type of suburethral sling procedure. Four trials compared slings to retropubic urethropexies, 1 compared slings to Stamey needle suspensions, and 2 compared the use of different sling materials. The results indicated that the data were insufficient to suggest that slings were more effective than other incontinence procedures or that slings were associated with fewer postoperative complications. While TVT slings did provide similar cure rates as open retropubic urethropexy, research is still lacking with respect to other types of slings. More studies comparing TVT slings to traditional pubovaginal slings also are needed before the 2 can be deemed equivalent.
In Sweden and Finland, where the TVT procedure was developed,11 85 patients who had undergone the procedure were evaluated at 48 to 70 months. Of those, 84.7% were completely cured of stress incontinence, 10.6% had significantly improved symptoms, and 4.7% were regarded as failures.
A recent well-designed, multicenter, randomized, prospective trial in the U.K. and Ireland compared 146 open Burch colposuspensions to 170 TVTs. Similar cure rates (57% and 66%, respectively) were reported.12 Although these rates are low compared to the Nordic nonrandomized TVT studies mentioned, the U.K./Ireland outcome criteria were particularly stringent and included a negative cystometrogram for stress incontinence and negative pad test. These differences in reported success rates highlight the importance of clearly defining objective outcomes criteria from randomized trials.
Nonetheless, the U.K./Ireland study showed that TVT is less invasive than the Burch procedure and is associated with shorter recovery periods and greater cost savings. Follow-up on complications (bladder perforation and hematoma in TVTs and incisional hernia formation in Burch colposuspensions) will be the most crucial aspect of this study.13
Clearly, the question of whether a Burch retropubic urethropexy or a suburethral sling procedure is better for SUI needs to be further investigated. Weber and Walters sought to answer this question by developing a decision analytical model (without the aid of randomized, controlled trials) and discovered similar cure rates.14 However, there were higher rates of urinary retention and detrusor instability associated with the traditional pubovaginal sling. But, most importantly, sensitivity analyses proved that if the rate of permanent urinary retention after a sling procedure was less than 9%—as in most sling series—the overall effectiveness of slings was higher than that of the Burch.
Conclusion
The suburethral sling procedure has undergone many modifications since its first description nearly a century ago. As such, Ob/Gyns need to familiarize themselves with the current options. Typically, we perform up to 6 suburethral sling procedures per month. Of those, 50% are referrals from failed incontinence procedures. Recently, we have made the switch from using autologous slings to tension-free type slings due to ease and good outcomes. While more data from randomized, prospective, multicenter trials are needed to determine the best approach for individual patients, surgeons should become comfortable with the technique that works best for them.
The authors report no financial relationship with any companies whose products are mentioned in this article.
- Suburethral sling procedures are effective in treating patients with urethral hypermobility, intrinsic sphincter deficiency, low-pressure urethras, and increased intra-abdominal pressure.
- Autologous slings may be a better choice in cases of severe urogenital atrophy, previous radiation, or extensive scarring from previous repairs.
- For both the tension-free vaginal tape and SPARC slings, mark the suprapubic region 1 cm above and 1 cm lateral to the pubic symphsis on the left and right sides and inject 20 cc of a 1:1 mixture of local anesthetic and normal saline into the marked regions.
- Once the trocars are in place, fill the bladder with 250 cc of water and perform a cough stress test to confirm continence.
When the suburethral sling was first described in 1907 by von Giordano, it entailed placing autologous tissue underneath the bladder neck and suspending it superiorly. Complications including urethral erosion, infection, bleeding, and fistula formation led many surgeons to use it sparingly.
Fast forward to the 21st century: Synthetic materials and new techniques were introduced, simplifying the sling procedures and raising the long-term success rates to 84%.1 As a result, slings now stand at the forefront of stress urinary incontinence (SUI) treatment. Among advances are the tension-free vaginal tape (TVT) sling (Gynecare, a division of Ethicon Inc., Somerville, NJ) and the SPARC sling (American Medical Systems, Inc., Minnetonka, Minn). The former, approved in the U.S. in 1998, calls for another look due to of the recent publication of a Cochrane review of outcomes studies, while the latter, approved by the FDA in August 2001, is the newest technique deserving examination. Clearly, with 83,010 incontinence procedures performed in the U.S. in 1999,2 a detailed look at the suburethral sling is warranted. Here, we review materials, indications, techniques, complications, and outcomes.
Materials
The choice of material—either organic or synthetic—depends on several factors: availability, cost, patient and surgeon preference, and clinical variables. (TABLE 1) outlines the advantages and disadvantages of each material type. Organic slings include autologous tissues (rectus fascia and fascia lata graft), and allografts or xenografts (cadaveric fascia lata graft, human dermal graft, or porcine small intestine and dermal graft). Synthetic slings are made of polyethylene terephthalate, expanded polytetrafluoroethylene, and polypropylene.
While sling procedures utilizing organic materials do have their benefits, synthetic slings, particularly the polypropylene mesh used in TVT and SPARC, have proven to be a stable material unlikely to deteriorate with time. Further, increased collagen metabolism around this synthetic sling promotes an ingrowth of tissue through the mesh.
TABLE 1
Slings: advantages and disadvantages of various materials
| SLING MATERIAL | ADVANTAGES | DISADVANTAGES |
|---|---|---|
| Autologous tissues (rectus fascia, fascia lata, or vaginal wall) |
|
|
| Allografts (cadaveric fascia lata or dermis) |
|
|
| Xenografts (porcine dermis or small intestine) | ||
| Synthetic mesh (polyethylene terephthalate, expanded polytetrafluoroethylene, or polypropylene) |
|
|
Indications
Suburethral sling procedures are typically used for the treatment of genuine stress urinary incontinence (GSUI), in which the urethra becomes either hypermobile and unstable or its intrinsic sphincter becomes incompetent. In fact, slings are technically easier to place in patients with anatomic urethrovesical junction hypermobility compared to those with fixed urethras. Several authors also have suggested the sling’s advantage in patients with low-pressure urethras.3
Use urodynamic criteria to diagnose intrinsic sphincter deficiency (ISD), which is defined as a Valsalva leak point pressure of less than 60 cm water or maximal urethral closure pressure of less than 20 cm water. (Bear in mind, however, that these cut-off criteria are controversial.4,5)
Also, consider slings in patients with recurrent GSUI, inherited collagen deficiency, and increased abdominal pressure (e.g., women with chronic obstructive pulmonary disease, obesity, or high-impact physical activity). The sling also can be used as an adjunct to other transvaginal surgeries (e.g., hysterectomy or prolapse repair).
Autologous slings may be a better choice than synthetic slings in cases of severe urogenital atrophy, previous radiation, or extensive scarring from previous repairs. In these instances, the patient may be at-risk for postoperative vaginal necrosis or erosion.6 Due to their biocompatibility, autologous slings are more likely to heal over a vaginal erosion and less likely to infect or erode into the urethra. In any event, urogenital atrophy should be treated with local estrogen preoperatively to prevent some of these complications.
Technique
Conventionally, suburethral slings were placed via a combined vaginal and abdominal approach into the retropubic space of Retzius. Alternatively, the procedure could be performed abdominally by creating a suburethral tunnel via pelvic incisions, but this is the most difficult route.
Most recently, technological advances have simplified the vaginal approach, which utilizes minimal suburethral dissection and small suprapubic incisions. This technique is subdivided into “bottom-up” and “top-down” approaches. In the bottom-up TVT, the sling is inserted into a vaginal incision and threaded up through the patient’s pelvis, exiting from a small suprapubic incision. The topdown SPARC entails a reverse approach, starting from a suprapubic incision and exiting from a vaginal incision. New modifications allow for an abdominal TVT approach, as well, which we describe in detail in a later section.
Surgeons who are familiar with traditional needle suspensions may be more comfortable with the top-down approach. The need for concomitant surgery (e.g., hysterectomy or prolapse repair) not only determines the type of incontinence procedure, but also dictates the approach.
Preparing the patient. Place the patient under regional or local anesthesia with sedation so that an intraoperative cough stress test can be performed. Then administer an intravenous dose of a broad-spectrum antibiotic. Insert a 16 to 18 French Foley catheter into the urethra. Mark the suprapubic region 1 cm above and 1 cm lateral to the pubic symphsis on the left and right sides of the patient. Inject approximately 20 cc of a 1:1 mixture of local anesthetic and normal saline into the marked areas. We typically use 60 cc of 0.25% bupivicaine with epinephrine, diluted 1:1 with 60 cc of normal saline. After administering the local anesthetic suprapubically, inject a similar solution into the anterior vaginal wall suburethrally in the midline and laterally toward the retropubic tunnels.
Making the incisions. Both the TVT and SPARC techniques utilize the same type and location of incisions. As such, make a 0.5-cm incision into the abdominal skin on each side of the midline, approximately 1 cm lateral to midline and 1 cm above the pubic symphsis. Next, make a 1.5- to 2-cm vertical incision in the vaginal mucosa, starting 1.5 cm from the urethral meatus (FIGURE 1). Use Metzenbaum scissors to dissect the vaginal mucosa from the pubocervical fascia sub- and para-urethrally on both sides (FIGURE 2). Insert a Foley catheter guide (similar to the Lowsley retractor) into the catheter and deviate it to the ipsilateral side, thereby retracting the bladder neck to the contralateral side. Proceed with the placement of either the TVT or SPARC sling.
Placing the TVT sling. Attach the TVT introducer to the curved needle trocar on 1 end of the polypropylene sling. Insert the trocar with the tape attached into the vaginal incision and push through the retropubic space, keeping the trocar in close contact with the posterior surface of the pubic bone (FIGURES 3 and 4). Continue pushing the trocar through the urogenital diaphragm until its tip comes through the suprapubic incision on the ipsilateral side (FIGURE 5). It is important to not deviate too laterally, medially, or cephalad during trocar insertion to prevent vessel, bladder, or bowel injury. Perform a cystoscopy to rule out cystotomy. Place the second trocar in a similar manner on the opposite side. After both trocars have been pulled through their respective incisions, perform a tension test.
Placing the SPARC/abdominal TVT sling. Guide the abdominal needles through the previously marked suprapubic incision and the patient’s retropubic cavity (keeping the needle behind the pubic bone), to a finger placed in the vaginal incision (FIGURE 6). Snap the abdominal needle guides with the attached polypropylene mesh to the sling connectors (FIGURE 7). Bring the abdominal needles through the suprapubic incisions. Perform a tension test. As with the TVT sling, perform a cystoscopy after each needle placement to rule out cystotomy. Then pass the sling through the tunnel.
Testing for continence. Once the sling is in place, fill the bladder with 250 cc of water and perform a cough stress test. Adjust sling tension by pulling up on both sling arms until only a few drops of leakage are noted. It is important not to secure the sling too tightly as this may lead to urinary retention, detrusor instability, or urethral erosion. We prefer placing a hemostat between the sling tape and the urethra to avoid over tightening.
Suspending the sling arms. Remove the plastic sheaths after tension adjustment and cut the sling flush with the skin (FIGURE 8). Compared to the conventional bone-anchored slings, the newer tension-free sling devices are not anchored but instead suspended through the retropubic space. At first, the sling is held in place by friction from the opposing tissues. Over time, collagen formation fixes the mesh more strongly within the suburethral and paravaginal tissues.
Finally, close the suprapubic and vaginal incisions with absorbable sutures.
Placing autologous or allogenic slings. Fashion the graft, typically 2 cm wide and 10 to 12 cm long, with permanent sutures at the edges. Make a 1-cm incision into the suprapubic rectus fascia. Use either a Stamey-type needle trocar or uterine packing forceps and guide the instrument “top down” from the retropubic incision to the vaginal tunnel. The tunnel is made directly into the retropubic space from the vaginal incision. Bring the sling arms up on each side. Attach the arms to the rectus fascia and tie them down once cystoscopy and the tension test are complete.
The sling also can be performed with bone anchors placed through the vaginal incision into the pubic bone. Placement requires vaginal dissection into the retropubic space with no suprapubic incision. Once anchored, the sutures are then passed through the chosen graft materials and tied down. Bear in mind that anchoring into the periosteum of the pubic bone may cause severe osteomyelitis or osteitis pubis, though the actual incidence is unknown.7
FIGURE 1 Surgical steps for tension-free vaginal tape (TVT)
Place a Sims speculum into the vagina and make a vertical incision 1.5 cm from the external urethra meatus.
FIGURE 2
Use Metzenbaum scissors to dissect the vaginal mucosa from the underlying fascia bilaterally. Insert a Foley catheter with guide.
FIGURE 3
Place TVT trocars through the vaginal incision. Place abdominal guides suprapubically and attach them to the TVT trocar tip.
FIGURE 4
Push the tape through the retropubic space, keeping the trocar in close contact with the posterior surface of the pubic bone.
FIGURE 5
Continue pushing the trocar through the urogenital diaphragm until its tip comes through the suprapubic incision on the ipsilateral side.
FIGURE 6 Surgical steps for SPARC
Guide the needle down the posterior side of the pubic bone, keeping the needle tip in contact with the pubic bone.
FIGURE 7
Snap the needle guide and sling onto the sling connectors and pull through the suprapubic incision.
FIGURE 8
Perform a tension test. Remove the plastic sheaths and cut the sling flush with the skin.
Complications
Intraoperative and immediate postoperative complications include bladder perforation, vaginal or retropubic bleeding, wound or urinary tract infection (UTI), and short-term urinary retention. Possible long-term problems include urethral or vaginal erosion, mesh infection, prolonged voiding dysfunction, fistula formation, or de novo urge incontinence (TABLE 2).
Specifically, the TVT sling, which has been placed in more than 50,000 women in the U.S. and 200,000 worldwide, carries the potential for significant vascular injury and bowel perforation. In addition, the Food and Drug Administration (FDA) reported 4 deaths (2 from unrecognized bowel injuries, 1 from retropubic bleeding in a patient with a bleeding disorder, and 1 from a heart attack more than 1 week after an incontinence repair procedure complicated by a vascular injury); 168 device malfunctions (mostly tape or sheath detachment from the trocar); and 128 other injuries, including bowel perforations and major vascular injuries to the obturator, external iliac, femoral, or inferior epigastrics (TABLE 3).8 In a review of 1,455 TVT sling cases at 38 hospitals, Kuuva and Nilsson9 found bladder perforation in 3.8% of the patients and retropubic hematoma in 1.9%, along with 1 case of vesicovaginal fistula, 1 obturator nerve injury, and 1 epigastric vessel injury.
According to the FDA, there have been 2 complications reported with the SPARC sling system. Both involved vaginal erosion subsequently repaired by oversewing the vaginal mucosa. One of these complications occurred in a woman undergoing her fourth vaginal procedure who was therefore deemed to have “poor tissue.”8
TABLE 2
Suburethral sling complications1
| COMPLICATION | 1,715 AUTOLOGOUS | 1,515 SYNTHETIC |
|---|---|---|
| Vaginal erosion | 1 (.0001%) | 10 (.007%) |
| Urethral erosion | 5 (.003%) | 27 (.02%) |
| Fistula | 6 (.003%) | 4 (.002%) |
| Wound sinus | 3 (.002%) | 11 (.007%) |
| Wound infection | 11 (.006%) | 15 (.009%) |
| Seroma | 6 (.003%) | 1 (.0007%) |
TABLE 3
TVT complications in 200,000 procedures worldwide
| COMPLICATION | U.S. | WORLD | TOTAL |
|---|---|---|---|
| Vascular injury | 3 | 25 | 28 |
| Vaginal mesh exposure | 15 | 2 | 17 |
| Urethral erosion | 8 | 0 | 8 |
| Bowel perforation | 4 | 6 | 10 |
| Nerve injury | 1 | 0 | 1 |
Outcomes studies
Unfortunately, most of the published clinical studies on the surgical management of stress urinary incontinence suffer from inadequate follow-up and sample size, unclear patient selection criteria, and poor postoperative documentation, especially with respect to quality of life. However, multiple studies to assess the effectiveness and safety of TVT slings have been published. The following is an outline of these preliminary yet important findings.
In 2002, the Cochrane Database evaluated 7 randomized and quasi-randomized trials of suburethral slings for the treatment of urinary incontinence.10 Of 682 women evaluated, 457 had some type of suburethral sling procedure. Four trials compared slings to retropubic urethropexies, 1 compared slings to Stamey needle suspensions, and 2 compared the use of different sling materials. The results indicated that the data were insufficient to suggest that slings were more effective than other incontinence procedures or that slings were associated with fewer postoperative complications. While TVT slings did provide similar cure rates as open retropubic urethropexy, research is still lacking with respect to other types of slings. More studies comparing TVT slings to traditional pubovaginal slings also are needed before the 2 can be deemed equivalent.
In Sweden and Finland, where the TVT procedure was developed,11 85 patients who had undergone the procedure were evaluated at 48 to 70 months. Of those, 84.7% were completely cured of stress incontinence, 10.6% had significantly improved symptoms, and 4.7% were regarded as failures.
A recent well-designed, multicenter, randomized, prospective trial in the U.K. and Ireland compared 146 open Burch colposuspensions to 170 TVTs. Similar cure rates (57% and 66%, respectively) were reported.12 Although these rates are low compared to the Nordic nonrandomized TVT studies mentioned, the U.K./Ireland outcome criteria were particularly stringent and included a negative cystometrogram for stress incontinence and negative pad test. These differences in reported success rates highlight the importance of clearly defining objective outcomes criteria from randomized trials.
Nonetheless, the U.K./Ireland study showed that TVT is less invasive than the Burch procedure and is associated with shorter recovery periods and greater cost savings. Follow-up on complications (bladder perforation and hematoma in TVTs and incisional hernia formation in Burch colposuspensions) will be the most crucial aspect of this study.13
Clearly, the question of whether a Burch retropubic urethropexy or a suburethral sling procedure is better for SUI needs to be further investigated. Weber and Walters sought to answer this question by developing a decision analytical model (without the aid of randomized, controlled trials) and discovered similar cure rates.14 However, there were higher rates of urinary retention and detrusor instability associated with the traditional pubovaginal sling. But, most importantly, sensitivity analyses proved that if the rate of permanent urinary retention after a sling procedure was less than 9%—as in most sling series—the overall effectiveness of slings was higher than that of the Burch.
Conclusion
The suburethral sling procedure has undergone many modifications since its first description nearly a century ago. As such, Ob/Gyns need to familiarize themselves with the current options. Typically, we perform up to 6 suburethral sling procedures per month. Of those, 50% are referrals from failed incontinence procedures. Recently, we have made the switch from using autologous slings to tension-free type slings due to ease and good outcomes. While more data from randomized, prospective, multicenter trials are needed to determine the best approach for individual patients, surgeons should become comfortable with the technique that works best for them.
The authors report no financial relationship with any companies whose products are mentioned in this article.
1. Leach GE, Dmochowski RR, Appell RA, et al. Female Stress Urinary Incontinence Clinical Guidelines Panel summary report on surgical management of female stress urinary incontinence. J Urol. 1997;875-880.
2. Nihira MA, Schaffer JI. Surgical procedures for stress urinary incontinence in 1999. Presented at: 28th Annual Scientific Meeting of the Society of Gynecologic Surgeons; March 6, 2002; Dallas, Texas.
3. Kobashi KC, Leach GE. Stress urinary incontinence. Curr Opin Urol. 1999;9:285-290.
4. Bowen LW, Sand PK, Ostergard DR, Franti CE. Unsuccessful Burch retropubic urethropexy: a case-controlled urodynamic study. Am J Obstet Gynecol. 1989;160:452-458.
5. Bump RC, Coates KW, Cundiff GW, Harris RL, Weidner AC. Diagnosing intrinsic sphincteric deficiency: Comparing urethral closure pressure, urethral axis, and Valsalva leak point pressures. Am J Obstet Gynecol. 1997;177:303-310.
6. Nichols DH, Randal CL. Operations for urinary stress incontinence. In: Vaginal Surgery. 4th ed. Baltimore, Md: Williams & Wilkins; 1996;402-415.
7. Rackley RR, Abdelmalak JB, Madjar S, Yanilmaz A, Appell RA, Tchetgen MB. Bone anchor infections in female pelvic reconstructive procedures: a literature review of series and case reports. J Urol. 2001;165:1975-1978.
8. Food and Drug Administration manufacturer and user facility device experience database. Available at: www.fda.gov/cdrh/maude.html. Accessed November 12, 2002.
9. Kuuva N, Nilsson CG. A nationwide analysis of complications associated with the tension-free vaginal tape (TVT) procedure. Neurourol Urodyn. 2000;19:394.-
10. Bezerra CA, Bruschini H. Suburethral sling operations for urinary incontinence in women. Cochrane Database Syst Rev. 2001;(3):CD001754.-
11. Nilsson CG, Kuuva N, Falconer C, Rezapour M, Ulmsten U. Long-term results of the tension-free vaginal tape procedure (TVT) for surgical treatment of female stress urinary incontinence. Int Urogynecol J Pelvic Floor Dysfunct. 2001;12(suppl 2):S5-S8.
12. Ward KL, Hilton P, Browning J. A randomized trial of colposuspension and tension-free vaginal tape (TVT) for primary genuine stress incontinence. Neurourol Urodyn. 2000;19:386.-
13. Ward K, Hilton P. Prospective multicenter randomized trial of tension-free vaginal tape and colposuspension as primary treatment for stress incontinence. BMJ. 2002;325:67.-
14. Weber A, Walters M. Burch procedure compared with sling for stress urinary incontinence: a decision analysis. Obstet Gynecol. 2000;96:867-873.
1. Leach GE, Dmochowski RR, Appell RA, et al. Female Stress Urinary Incontinence Clinical Guidelines Panel summary report on surgical management of female stress urinary incontinence. J Urol. 1997;875-880.
2. Nihira MA, Schaffer JI. Surgical procedures for stress urinary incontinence in 1999. Presented at: 28th Annual Scientific Meeting of the Society of Gynecologic Surgeons; March 6, 2002; Dallas, Texas.
3. Kobashi KC, Leach GE. Stress urinary incontinence. Curr Opin Urol. 1999;9:285-290.
4. Bowen LW, Sand PK, Ostergard DR, Franti CE. Unsuccessful Burch retropubic urethropexy: a case-controlled urodynamic study. Am J Obstet Gynecol. 1989;160:452-458.
5. Bump RC, Coates KW, Cundiff GW, Harris RL, Weidner AC. Diagnosing intrinsic sphincteric deficiency: Comparing urethral closure pressure, urethral axis, and Valsalva leak point pressures. Am J Obstet Gynecol. 1997;177:303-310.
6. Nichols DH, Randal CL. Operations for urinary stress incontinence. In: Vaginal Surgery. 4th ed. Baltimore, Md: Williams & Wilkins; 1996;402-415.
7. Rackley RR, Abdelmalak JB, Madjar S, Yanilmaz A, Appell RA, Tchetgen MB. Bone anchor infections in female pelvic reconstructive procedures: a literature review of series and case reports. J Urol. 2001;165:1975-1978.
8. Food and Drug Administration manufacturer and user facility device experience database. Available at: www.fda.gov/cdrh/maude.html. Accessed November 12, 2002.
9. Kuuva N, Nilsson CG. A nationwide analysis of complications associated with the tension-free vaginal tape (TVT) procedure. Neurourol Urodyn. 2000;19:394.-
10. Bezerra CA, Bruschini H. Suburethral sling operations for urinary incontinence in women. Cochrane Database Syst Rev. 2001;(3):CD001754.-
11. Nilsson CG, Kuuva N, Falconer C, Rezapour M, Ulmsten U. Long-term results of the tension-free vaginal tape procedure (TVT) for surgical treatment of female stress urinary incontinence. Int Urogynecol J Pelvic Floor Dysfunct. 2001;12(suppl 2):S5-S8.
12. Ward KL, Hilton P, Browning J. A randomized trial of colposuspension and tension-free vaginal tape (TVT) for primary genuine stress incontinence. Neurourol Urodyn. 2000;19:386.-
13. Ward K, Hilton P. Prospective multicenter randomized trial of tension-free vaginal tape and colposuspension as primary treatment for stress incontinence. BMJ. 2002;325:67.-
14. Weber A, Walters M. Burch procedure compared with sling for stress urinary incontinence: a decision analysis. Obstet Gynecol. 2000;96:867-873.
Pearls on cesarean
Even with its recent decline in occurrence, cesarean delivery remains the most frequently performed surgical procedure in the United States, accounting for approximately 21.5% of all deliveries. Here, obstetricians from across the country share pearls on various aspects of cesarean birth.
The difficult cesarean. When a difficult cesarean is predicted and the threat of obstetrical hemorrhage is imminent, I recommend that clinicians position the patient in low Allen stirrups, using a drape that provides vaginal access. This allows for proper elevation of the fetal head and better assessment of potential blood loss. Make sure a hysterectomy instrument tray is nearby and an experienced gynecologic scrub nurse is available. These steps should eliminate occult hemorrhage and allow for rapid response to any changes in the patient’s clinical status. —Richard Hill, MD, Kansas City, Mo
Cranial dystocia. A cesarean is often required when labor-arrest disorders occur. Cranial dystocia is sometimes found in these cases, making for a difficult delivery. When manual extraction of the fetal head fails, the surgeon typically has an assistant place a hand in the vagina to elevate and disengage the head. In these cases, I instead suggest surgeons remove their delivering hand from the uterine incision, cup the fetal head outside the lower uterine segment and bladder, and then pull upward. This will break the vacuum that spontaneously occurs, liberating the fetal head and allowing for successful cesarean delivery. —Peter Napolitano, MD, Tacoma, Wash
Intact membranes. When a patient with unruptured membranes presents for a cesarean, avoid breaching the amniotic sac sharply or with a clamp. Instead, first approach the uterine muscle sharply through the outer layers, then bluntly dissect the remaining fibers with your fingertip, allowing the amniotic sac to bulge out through the incision. Complete the uterine incision sharply with bandage scissors prior to rupturing the membranes, then open the bulging membranes either bluntly or with an Allis clamp. These steps ensure that the fetus will not experience trauma from sharp surgical objects. —Scott Resnick, MD, Taos, NM
Lowering the cesarean rate. To reduce the number of cesarean deliveries, try inducing labor of the unscarred uterus as follows:
- 8 AM: Insert 50 μg of misoprostol deep in the vagina.
- Noon: If the cervix is dilated, rupture membranes regardless of the Bishop score. Labor will almost invariably ensue within 1 to 2 hours. If the cervix is still closed at this time, insert another dose of misoprostol.
- Noon to 5 PM: Observe for cervical dilation. If this does not occur, augment labor with oxytocin.
Some patients will achieve vaginal delivery by 5 PM, and most will deliver by midnight.
With this technique, selecting patients for induction prior to their due date and before macrosomia or fetal stress develop is key to successfully keeping the cesarean rate low. —Susan Vicente, MD, Kailua, Hawaii
Even with its recent decline in occurrence, cesarean delivery remains the most frequently performed surgical procedure in the United States, accounting for approximately 21.5% of all deliveries. Here, obstetricians from across the country share pearls on various aspects of cesarean birth.
The difficult cesarean. When a difficult cesarean is predicted and the threat of obstetrical hemorrhage is imminent, I recommend that clinicians position the patient in low Allen stirrups, using a drape that provides vaginal access. This allows for proper elevation of the fetal head and better assessment of potential blood loss. Make sure a hysterectomy instrument tray is nearby and an experienced gynecologic scrub nurse is available. These steps should eliminate occult hemorrhage and allow for rapid response to any changes in the patient’s clinical status. —Richard Hill, MD, Kansas City, Mo
Cranial dystocia. A cesarean is often required when labor-arrest disorders occur. Cranial dystocia is sometimes found in these cases, making for a difficult delivery. When manual extraction of the fetal head fails, the surgeon typically has an assistant place a hand in the vagina to elevate and disengage the head. In these cases, I instead suggest surgeons remove their delivering hand from the uterine incision, cup the fetal head outside the lower uterine segment and bladder, and then pull upward. This will break the vacuum that spontaneously occurs, liberating the fetal head and allowing for successful cesarean delivery. —Peter Napolitano, MD, Tacoma, Wash
Intact membranes. When a patient with unruptured membranes presents for a cesarean, avoid breaching the amniotic sac sharply or with a clamp. Instead, first approach the uterine muscle sharply through the outer layers, then bluntly dissect the remaining fibers with your fingertip, allowing the amniotic sac to bulge out through the incision. Complete the uterine incision sharply with bandage scissors prior to rupturing the membranes, then open the bulging membranes either bluntly or with an Allis clamp. These steps ensure that the fetus will not experience trauma from sharp surgical objects. —Scott Resnick, MD, Taos, NM
Lowering the cesarean rate. To reduce the number of cesarean deliveries, try inducing labor of the unscarred uterus as follows:
- 8 AM: Insert 50 μg of misoprostol deep in the vagina.
- Noon: If the cervix is dilated, rupture membranes regardless of the Bishop score. Labor will almost invariably ensue within 1 to 2 hours. If the cervix is still closed at this time, insert another dose of misoprostol.
- Noon to 5 PM: Observe for cervical dilation. If this does not occur, augment labor with oxytocin.
Some patients will achieve vaginal delivery by 5 PM, and most will deliver by midnight.
With this technique, selecting patients for induction prior to their due date and before macrosomia or fetal stress develop is key to successfully keeping the cesarean rate low. —Susan Vicente, MD, Kailua, Hawaii
Even with its recent decline in occurrence, cesarean delivery remains the most frequently performed surgical procedure in the United States, accounting for approximately 21.5% of all deliveries. Here, obstetricians from across the country share pearls on various aspects of cesarean birth.
The difficult cesarean. When a difficult cesarean is predicted and the threat of obstetrical hemorrhage is imminent, I recommend that clinicians position the patient in low Allen stirrups, using a drape that provides vaginal access. This allows for proper elevation of the fetal head and better assessment of potential blood loss. Make sure a hysterectomy instrument tray is nearby and an experienced gynecologic scrub nurse is available. These steps should eliminate occult hemorrhage and allow for rapid response to any changes in the patient’s clinical status. —Richard Hill, MD, Kansas City, Mo
Cranial dystocia. A cesarean is often required when labor-arrest disorders occur. Cranial dystocia is sometimes found in these cases, making for a difficult delivery. When manual extraction of the fetal head fails, the surgeon typically has an assistant place a hand in the vagina to elevate and disengage the head. In these cases, I instead suggest surgeons remove their delivering hand from the uterine incision, cup the fetal head outside the lower uterine segment and bladder, and then pull upward. This will break the vacuum that spontaneously occurs, liberating the fetal head and allowing for successful cesarean delivery. —Peter Napolitano, MD, Tacoma, Wash
Intact membranes. When a patient with unruptured membranes presents for a cesarean, avoid breaching the amniotic sac sharply or with a clamp. Instead, first approach the uterine muscle sharply through the outer layers, then bluntly dissect the remaining fibers with your fingertip, allowing the amniotic sac to bulge out through the incision. Complete the uterine incision sharply with bandage scissors prior to rupturing the membranes, then open the bulging membranes either bluntly or with an Allis clamp. These steps ensure that the fetus will not experience trauma from sharp surgical objects. —Scott Resnick, MD, Taos, NM
Lowering the cesarean rate. To reduce the number of cesarean deliveries, try inducing labor of the unscarred uterus as follows:
- 8 AM: Insert 50 μg of misoprostol deep in the vagina.
- Noon: If the cervix is dilated, rupture membranes regardless of the Bishop score. Labor will almost invariably ensue within 1 to 2 hours. If the cervix is still closed at this time, insert another dose of misoprostol.
- Noon to 5 PM: Observe for cervical dilation. If this does not occur, augment labor with oxytocin.
Some patients will achieve vaginal delivery by 5 PM, and most will deliver by midnight.
With this technique, selecting patients for induction prior to their due date and before macrosomia or fetal stress develop is key to successfully keeping the cesarean rate low. —Susan Vicente, MD, Kailua, Hawaii
Excisional biopsy for CIN
- In most cases, loop electrosurgical excision procedure (LEEP) and cold-knife conization (CKC) result in equivalent success rates and margin status.
- CKC is preferred in cases of adenocarcinoma in situ or squamous microinvasion.
- Conservative follow-up is generally possible in adenocarcinoma in situ and squamous microinvasion when margins are negative.
- Colposcopy, endocervical curettage, and biopsies should be part of the follow-up strategy for patients with positive margins.
When cervical intraepithelial neoplasia (CIN) requires treatment, loop electrosurgical excision procedure (LEEP) is the most frequently used modality, although cold-knife conization (CKC) of the cervical transformation zone still is preferred in select cases. Since excisional techniques are used with CKC, margin status is known and clinical decisions may be based on this information.
This article reviews current indications for excisional biopsy and presents evidence to direct management and follow-up of patients with positive and negative margins.
Selecting a technique
Several large randomized and prospective studies have demonstrated that LEEP is similar in efficacy to CKC and may even remove less of the normal cervical stroma.1-3 In general, LEEP is used to excise high-grade and recurrent squamous dysplasia, as it is similar to CKC in its rates of incomplete excision and residual disease.1,4 However, when adenocarcinoma in situ (AIS) is present, CKC is preferred for diagnosis and treatment, since it results in a lower incidence of involved margins and a lower recurrence rate.3,5-7
CKC also is preferred when histologic confirmation of the margin status is crucial, such as when invasion with squamous lesions is suspected. This is because thermal artifacts that may result from LEEP will interfere with interpretation, further complicating treatment planning.1,4,8,9 In cases of microinvasion, the ability to confirm margins makes conservative treatment possible; if the depth of invasion cannot be determined from the specimen, radical surgery may be necessary.
In the hands of an experienced clinician, however, thermal artifact from the LEEP technique generally is not a significant problem. Series reporting high rates of uninterpretable margins have been attributed to operator inexperience.10 In general, thermal artifact is reduced by limiting the number of sections taken. In ideal cases, only a single-piece specimen is obtained, similar to that achieved using CKC.8,10,11 Newer loops, such as the cone biopsy excision loop, may decrease the number of sections and further improve margin interpretation.8
I use CKC in cases of suspected squamous invasion and in the evaluation of glandular lesions, but feel that in other cases LEEP is efficacious and quicker.
Identifying residual disease
When both the endocervical margin and endocervical curettage (ECC) are positive for squamous dysplasia at the time of excision, there is an increased risk of residual disease (TABLE 1).12-14 However, it is not clear whether ECC alone is an independent predictor of residual disease. Although 1 study suggests an increased risk of invasive cancer in women over 50 years of age with a positive ECC at the time of conization, other series have failed to demonstrate a difference in treatment failure rates based on ECC.15-17 Thus, the utility of ECC in directing further therapy at the time of excisional biopsy is unclear. I generally do not perform an ECC with LEEP for squamous dysplasia.
In AIS, a positive ECC is a strong predictor of residual disease, while a negative ECC is of limited significance.18 I am more likely to perform an ECC with glandular lesions. If it is negative, close follow-up still is indicated. If it is positive, repeat excision may be necessary.
TABLE 1
Residual disease and margin status: squamous dysplasia
| AUTHOR | PROPORTION WITH RESIDUAL DISEASE | |||
|---|---|---|---|---|
| NEGATIVE MARGINS | POSITIVE MARGINS | |||
| NUMBER | % | NUMBER | % | |
| SQUAMOUS LESIONS | ||||
| Bertelsen et al, 199923 | 44/485 | 9 | 29/76 | 38.2 |
| Moore et al, 199524 | 170/523 | 32.5 | 37/91 | 40.7 |
| Lopes et al, 199325 | 0/176 | 0 | 11/131 | 8.4 |
| Murdoch et al, 199226 | 7/405 | 1.7 | 22/160 | 13.8 |
| Andersen et al, 199019 | 0/411 | 0 | 6/469 | 1.3 |
| TOTALS | 221/2,000 | 11 | 105/927 | 11.3 |
| SQUAMOUS MICROINVASION | ||||
| Gurgel et al, 199727 | 6/74 | 8.1 | 45/76 | 59.2 |
| Roman et al, 199722 | 1/30 | 3.3 | 7/50 | 14 |
| TOTALS | 7/104 | 6.7 | 52/126 | 41.3 |
Follow-up of squamous lesions
Considerable clinical uncertainty remains over the relative strengths of cytologic, colposcopic, and histologic evaluation for residual or recurrent disease following excisional biopsy. Conservative management includes Papanicolaou smears alone or in combination with ECC and/or colposcopy.
Negative margins. If margins are negative, the success rate of excisional biopsy is high (90%-100%), and careful observation is the preferred follow-up. Repeat cytologic testing will identify the majority of patients with residual high-grade disease. A prospective randomized trial of cytologic surveillance showed that the detection rate was only 1.9% higher for histology.19 Although 1 report suggests that colposcopy can expedite the diagnosis of recurrent dysplasia, it is unclear from this report whether colposcopy identified significant high-grade dysplasia that cytology missed.20
Positive margins. In most series involving CIN with positive margins, treatment success rates do not differ significantly between patients who undergo repeat surgical procedures and those who have close follow-up. However, endocervical margin involvement appears to carry a higher treatment failure rate than ectocervical margin involvement. Data are conflicting on the proper method of conservative follow-up. Some authors propose only frequent cytologic testing, while others recommend that colposcopy be performed.19-21
For patients with positive margins, I perform both cytology and colposcopy in 4 to 6 months. If an endocervical margin was positive, I also perform an ECC. If this evaluation is negative, I repeat cytologic testing every 6 months until 3 consecutive Pap smears are normal and satisfactory. In cases of recurrent high-grade dysplasia with positive margins, hysterectomy may be indicated, depending on the patient’s age and desire for continued fertility.14,21
Squamous microinvasion
There is a significant risk (50%-80%) of residual disease or true invasion when margins or an ECC are positive and microinvasion is present.2,5-7 In these cases, a repeat excisional biopsy should be performed to determine the true extent of disease prior to deciding on definitive therapy.22 As previously mentioned, CKC is preferred to limit artifact that could obscure interpretation. If the patient has completed her childbearing, hysterectomy remains the standard treatment for microinvasive squamous cell carcinoma. When the woman wishes to preserve fertility, conservative follow-up appears to be safe if the final pathologic specimen has negative margins. A follow-up protocol including cytologic, colposcopic, and ECC monitoring in the first post-conization visit is recommended.2,6
Glandular lesions
AIS with involved margins requires further surgery due to the possibility of residual AIS or invasion (TABLES 2 and 3).2,5-7 In these cases, CKC is preferable to LEEP.3,5-7 Hysterectomy remains the standard therapy for AIS. Conservative management is an option if fertility is desired and margin status is negative. Patients should be informed that persistent disease or recurrence is possible and that there is a risk of invasive disease.15
Counseling, thorough documentation, and second surgical and pathologic opinions may be helpful in conservative management. Colposcopy, ECC, and cytology are indicated at the first follow-up visit, with cytology repeated every 6 months until 4 consecutive Paps are normal. More frequent colposcopy and liberal use of ECC also may be considered.
TABLE 2
Residual disease and margin status: adenocarcinoma in situ
| AUTHOR | PROPORTION WITH RESIDUAL DISEASE | |||
|---|---|---|---|---|
| NEGATIVE MARGINS | POSITIVE MARGINS | |||
| NUMBER | % | NUMBER | % | |
| Azodi et al, 199928 | 5/16 | 31.3 | 9/16 | 56.3 |
| Goldstein et al, 199828 | 13/43 | 30.2 | 8/18 | 44.4 |
| Denehy et al, 19976 | 2/7 | 28.6 | 7/10 | 70 |
| Widrich et al, 19965 | 0/3 | 0 | 9/14 | 64.3 |
| Wolf et al, 199629 | 7/21 | 33.3 | 10/19 | 52.6 |
| TOTAL | 27/90 | 30 | 43/77 | 55.8 |
TABLE 3
Follow-up recommendations
| Pathology | Margin status | Recommendation |
|---|---|---|
| High-grade squamous lesion | Negative |
|
| Positive, endocervical |
| |
| Positive, ectocervical |
| |
| Squamous microinvasion | Negative, fertility desired |
|
| Negative, no desire for fertility | Hysterectomy | |
| Positive | CKC | |
| Adenocarcinoma in situ | Negative, fertility desired |
|
| Negative, no desire for fertility | Hysterectomy | |
| Positive | CKC | |
| CKC = cold-knife conization; ECC = endocervical curettage | ||
When further excision is necessary
As noted earlier, repeat excision is necessary in cases of squamous microinvasion or AIS with positive margins. CKC is generally preferred to allow for optimal pathologic interpretation. For squamous intraepithelial lesions, excisional biopsy with close follow-up has a significant cure rate, and hysterectomy usually is not indicated. However, hysterectomy still should be considered part of the treatment continuum for CIN, particularly for patients who have completed childbearing.
Conclusion
Although the risk of recurrence is correlated with a patient’s margin status in cases of squamous dysplasia, conservative follow-up is possible and has a high success rate. Cytology is sufficient surveillance for cases involving negative margins. When margins are positive, colposcopy and ECC also may be useful. Microinvasive lesions with positive margins require further surgical evaluation to determine treatment. For glandular lesions, CKC is preferred for both diagnosis and treatment.
Dr. Dunton reports no affiliation or financial arrangement with any of the companies that manufacture drugs or devices in any of the product classes mentioned in this article.
1. Mathevet P, Dargent D, Roy M, Beau G. A randomized prospective study comparing three techniques of conization: cold knife, laser, and LEEP. Gynecol Oncol. 1994;54(2):175-179.
2. Duggan BD, Felix JC, Muderspach LI, et al. Cold-knife conization versus conization by the loop electrosurgical excision procedure: a randomized, prospective study. Am J Obstet Gynecol. 1999;180:276-282.
3. Girardi F, Heydarfadi M, Koroschetz F, et al. Cold-knife conization versus loop excision: histopathologic and clinical results of a randomized trial. Gynecol Oncol. 1994;55:368-370.
4. Gold M, Dunton CJ, Murray J, et al. Loop electrocautery excisional procedure: therapeutic effectiveness as an ablation and a conization equivalent. Gynecol Oncol. 1996;61:241-244.
5. Widrich T, Kennedy AW, Myers TM, et al. Adenocarcinoma in situ of the uterine cervix: management and outcome. Gynecol Oncol. 1996;61:304-308.
6. Denehy TR, Gregori CA, Breen JL. Endocervical curettage, cone margins, and residual adenocarcinoma in situ of the cervix. Obstet Gynecol. 1997;90:1-6.
7. Muntz HG, Bell DA, Lage JM, et al. Adenocarcinoma in situ of the uterine cervix. Obstet Gynecol. 1992;80:935-939.
8. Naumann RW, Bell MC, Alvarez RD, et al. LLETZ is an acceptable alternative to diagnostic cold-knife conization. Gynecol Oncol. 1994;55:224-228.
9. Eddy GL, Spiegel GW, Creasman WT. Adverse effect of electrosurgical loop excision on assignment of FIGO stage in cervical cancer: report of two cases. Gynecol Oncol. 1994;55:313-317.
10. Montz FJ, Holschneider CH, Thompson LDR. Large-loop excision of the transformation zone: effect on the pathologic interpretation of resection margins. Obstet Gynecol. 1993;81:976-982.
11. Gardeil F, Barry-Walsh C, Prendiville W, et al. Persistent intraepithelial neoplasia after excision for cervical intraepithelial neoplasia grade III. Obstet Gynecol. 1987;89:419-422.
12. Felix JC, Muderspach LI, Duggan BD, Roman LD. The significance of positive margins in loop electrosurgical cone biopsies. Obstet Gynecol. 1994;84:996-1000.
13. Kobak WH, Roman LD, Felix JC, et al. The role of endocervical curettage at cervical conization for high-grade dysplasia. Obstet Gynecol. 1995;85:197-201.
14. Husseinzadeh N, Shbara I, Wessler T. Predictive value of cone margins and post-cone endocervical curettage with residual disease in subsequent hysterectomy. Gynecol Oncol. 1989;33:198-200.
15. Poyner EA, Barakat RR, Hoskins WJ. Management and follow-up of patients with adenocarcinoma in situ of the uterine cervix. Gynecol Oncol. 1995;57:158-164.
16. Frauchiger WL, De Frias DVS, Cajulis RS, Yu GH. The immediate postconization endocervical smear: evaluation of its utility in the detection of residual dysplasia. Acta Cytol. 1998;42:1139-1143.
17. Wright TC, Gagnon S, Richart RM, Ferenczy A. Treatment of cervical intraepithelial neoplasia using the loop electrosurgical excision procedure. Obstet Gynecol. 1992;79:173-178.
18. Goldstein NS, Mani A. The status and distance of cone biopsy margins as a predictor of excision adequacy for endocervical adenocarcinoma in situ. Am J Clin Pathol. 1998;109:727-732.
19. Andersen ES, Nielsen K, Larsen G. Laser conization: follow-up in patients with cervical intraepithelial neoplasia in the cone margin. Gynecol Oncol. 1990;39:328-331.
20. Paraskevaidis E, Jandial L, Mann EMF, Fisher PM. Pattern of treatment failure following laser for cervical intraepithelial neoplasia: implications for follow-up protocol. Obstet Gynecol. 1991;78:80-83.
21. Lapaquette TK, Dinh TV, Hannigan EV, et al. Management of patients with positive margins after cervical conization. Obstet Gynecol. 1993;82:440-443.
22. Roman LD, Felix JC, Muderspach LI, et al. Risk of residual invasive disease in women with microinvasive squamous cancer in a conization specimen. Obstet Gynecol. 1997;90:759-764.
23. Bertelsen B, Tande T, Sandvei, Hartveit F. Laser conization of cervical intraepithelial neoplasia grade 3. Acta Obstet Gynecol Scand. 1999;78:54-59.
24. Moore BC, Higgins RV, Laurent SL, et al. Predictive factors from cold knife conization for residual cervical intraepithelial neoplasia in subsequent hysterectomy. Am J Obstet Gynecol. 1995;173:361-368.
25. Lopes A, Morgan P, Murdoch J, et al. The case for conservative management of “incomplete excision” of CIN after laser conization. Gynecol Oncol. 1993;49:247-249.
26. Murdoch JB, Morgan PR, Lopes A, Monaghan JM. Histological incomplete excision of CIN after large loop excision of the transformation zone (LLETZ) merits careful follow up, not retreatment. Br J Obstet Gynaecol. 1992;99:990-993.
27. Gurgel MSC, Bedone AJ, Andrade LA, et al. Microinvasive carcinoma of the uterine cervix: histological findings on cone specimens related to residual neoplasia on hysterectomy. Gynecol Oncol. 1997;65:437-440.
28. Azodi M, Chambers SK, Rutherford TJ, et al. Adenocarcinoma in situ of the cervix: management and outcome. Gynecol Oncol. 1999;73:348-353.
29. Wolf JK, Levenback C, Malpica A, et al. Adenocarcinoma in situ of the cervix: significance of cone biopsy margins. Obstet Gynecol. 1996;88:82-86.
- In most cases, loop electrosurgical excision procedure (LEEP) and cold-knife conization (CKC) result in equivalent success rates and margin status.
- CKC is preferred in cases of adenocarcinoma in situ or squamous microinvasion.
- Conservative follow-up is generally possible in adenocarcinoma in situ and squamous microinvasion when margins are negative.
- Colposcopy, endocervical curettage, and biopsies should be part of the follow-up strategy for patients with positive margins.
When cervical intraepithelial neoplasia (CIN) requires treatment, loop electrosurgical excision procedure (LEEP) is the most frequently used modality, although cold-knife conization (CKC) of the cervical transformation zone still is preferred in select cases. Since excisional techniques are used with CKC, margin status is known and clinical decisions may be based on this information.
This article reviews current indications for excisional biopsy and presents evidence to direct management and follow-up of patients with positive and negative margins.
Selecting a technique
Several large randomized and prospective studies have demonstrated that LEEP is similar in efficacy to CKC and may even remove less of the normal cervical stroma.1-3 In general, LEEP is used to excise high-grade and recurrent squamous dysplasia, as it is similar to CKC in its rates of incomplete excision and residual disease.1,4 However, when adenocarcinoma in situ (AIS) is present, CKC is preferred for diagnosis and treatment, since it results in a lower incidence of involved margins and a lower recurrence rate.3,5-7
CKC also is preferred when histologic confirmation of the margin status is crucial, such as when invasion with squamous lesions is suspected. This is because thermal artifacts that may result from LEEP will interfere with interpretation, further complicating treatment planning.1,4,8,9 In cases of microinvasion, the ability to confirm margins makes conservative treatment possible; if the depth of invasion cannot be determined from the specimen, radical surgery may be necessary.
In the hands of an experienced clinician, however, thermal artifact from the LEEP technique generally is not a significant problem. Series reporting high rates of uninterpretable margins have been attributed to operator inexperience.10 In general, thermal artifact is reduced by limiting the number of sections taken. In ideal cases, only a single-piece specimen is obtained, similar to that achieved using CKC.8,10,11 Newer loops, such as the cone biopsy excision loop, may decrease the number of sections and further improve margin interpretation.8
I use CKC in cases of suspected squamous invasion and in the evaluation of glandular lesions, but feel that in other cases LEEP is efficacious and quicker.
Identifying residual disease
When both the endocervical margin and endocervical curettage (ECC) are positive for squamous dysplasia at the time of excision, there is an increased risk of residual disease (TABLE 1).12-14 However, it is not clear whether ECC alone is an independent predictor of residual disease. Although 1 study suggests an increased risk of invasive cancer in women over 50 years of age with a positive ECC at the time of conization, other series have failed to demonstrate a difference in treatment failure rates based on ECC.15-17 Thus, the utility of ECC in directing further therapy at the time of excisional biopsy is unclear. I generally do not perform an ECC with LEEP for squamous dysplasia.
In AIS, a positive ECC is a strong predictor of residual disease, while a negative ECC is of limited significance.18 I am more likely to perform an ECC with glandular lesions. If it is negative, close follow-up still is indicated. If it is positive, repeat excision may be necessary.
TABLE 1
Residual disease and margin status: squamous dysplasia
| AUTHOR | PROPORTION WITH RESIDUAL DISEASE | |||
|---|---|---|---|---|
| NEGATIVE MARGINS | POSITIVE MARGINS | |||
| NUMBER | % | NUMBER | % | |
| SQUAMOUS LESIONS | ||||
| Bertelsen et al, 199923 | 44/485 | 9 | 29/76 | 38.2 |
| Moore et al, 199524 | 170/523 | 32.5 | 37/91 | 40.7 |
| Lopes et al, 199325 | 0/176 | 0 | 11/131 | 8.4 |
| Murdoch et al, 199226 | 7/405 | 1.7 | 22/160 | 13.8 |
| Andersen et al, 199019 | 0/411 | 0 | 6/469 | 1.3 |
| TOTALS | 221/2,000 | 11 | 105/927 | 11.3 |
| SQUAMOUS MICROINVASION | ||||
| Gurgel et al, 199727 | 6/74 | 8.1 | 45/76 | 59.2 |
| Roman et al, 199722 | 1/30 | 3.3 | 7/50 | 14 |
| TOTALS | 7/104 | 6.7 | 52/126 | 41.3 |
Follow-up of squamous lesions
Considerable clinical uncertainty remains over the relative strengths of cytologic, colposcopic, and histologic evaluation for residual or recurrent disease following excisional biopsy. Conservative management includes Papanicolaou smears alone or in combination with ECC and/or colposcopy.
Negative margins. If margins are negative, the success rate of excisional biopsy is high (90%-100%), and careful observation is the preferred follow-up. Repeat cytologic testing will identify the majority of patients with residual high-grade disease. A prospective randomized trial of cytologic surveillance showed that the detection rate was only 1.9% higher for histology.19 Although 1 report suggests that colposcopy can expedite the diagnosis of recurrent dysplasia, it is unclear from this report whether colposcopy identified significant high-grade dysplasia that cytology missed.20
Positive margins. In most series involving CIN with positive margins, treatment success rates do not differ significantly between patients who undergo repeat surgical procedures and those who have close follow-up. However, endocervical margin involvement appears to carry a higher treatment failure rate than ectocervical margin involvement. Data are conflicting on the proper method of conservative follow-up. Some authors propose only frequent cytologic testing, while others recommend that colposcopy be performed.19-21
For patients with positive margins, I perform both cytology and colposcopy in 4 to 6 months. If an endocervical margin was positive, I also perform an ECC. If this evaluation is negative, I repeat cytologic testing every 6 months until 3 consecutive Pap smears are normal and satisfactory. In cases of recurrent high-grade dysplasia with positive margins, hysterectomy may be indicated, depending on the patient’s age and desire for continued fertility.14,21
Squamous microinvasion
There is a significant risk (50%-80%) of residual disease or true invasion when margins or an ECC are positive and microinvasion is present.2,5-7 In these cases, a repeat excisional biopsy should be performed to determine the true extent of disease prior to deciding on definitive therapy.22 As previously mentioned, CKC is preferred to limit artifact that could obscure interpretation. If the patient has completed her childbearing, hysterectomy remains the standard treatment for microinvasive squamous cell carcinoma. When the woman wishes to preserve fertility, conservative follow-up appears to be safe if the final pathologic specimen has negative margins. A follow-up protocol including cytologic, colposcopic, and ECC monitoring in the first post-conization visit is recommended.2,6
Glandular lesions
AIS with involved margins requires further surgery due to the possibility of residual AIS or invasion (TABLES 2 and 3).2,5-7 In these cases, CKC is preferable to LEEP.3,5-7 Hysterectomy remains the standard therapy for AIS. Conservative management is an option if fertility is desired and margin status is negative. Patients should be informed that persistent disease or recurrence is possible and that there is a risk of invasive disease.15
Counseling, thorough documentation, and second surgical and pathologic opinions may be helpful in conservative management. Colposcopy, ECC, and cytology are indicated at the first follow-up visit, with cytology repeated every 6 months until 4 consecutive Paps are normal. More frequent colposcopy and liberal use of ECC also may be considered.
TABLE 2
Residual disease and margin status: adenocarcinoma in situ
| AUTHOR | PROPORTION WITH RESIDUAL DISEASE | |||
|---|---|---|---|---|
| NEGATIVE MARGINS | POSITIVE MARGINS | |||
| NUMBER | % | NUMBER | % | |
| Azodi et al, 199928 | 5/16 | 31.3 | 9/16 | 56.3 |
| Goldstein et al, 199828 | 13/43 | 30.2 | 8/18 | 44.4 |
| Denehy et al, 19976 | 2/7 | 28.6 | 7/10 | 70 |
| Widrich et al, 19965 | 0/3 | 0 | 9/14 | 64.3 |
| Wolf et al, 199629 | 7/21 | 33.3 | 10/19 | 52.6 |
| TOTAL | 27/90 | 30 | 43/77 | 55.8 |
TABLE 3
Follow-up recommendations
| Pathology | Margin status | Recommendation |
|---|---|---|
| High-grade squamous lesion | Negative |
|
| Positive, endocervical |
| |
| Positive, ectocervical |
| |
| Squamous microinvasion | Negative, fertility desired |
|
| Negative, no desire for fertility | Hysterectomy | |
| Positive | CKC | |
| Adenocarcinoma in situ | Negative, fertility desired |
|
| Negative, no desire for fertility | Hysterectomy | |
| Positive | CKC | |
| CKC = cold-knife conization; ECC = endocervical curettage | ||
When further excision is necessary
As noted earlier, repeat excision is necessary in cases of squamous microinvasion or AIS with positive margins. CKC is generally preferred to allow for optimal pathologic interpretation. For squamous intraepithelial lesions, excisional biopsy with close follow-up has a significant cure rate, and hysterectomy usually is not indicated. However, hysterectomy still should be considered part of the treatment continuum for CIN, particularly for patients who have completed childbearing.
Conclusion
Although the risk of recurrence is correlated with a patient’s margin status in cases of squamous dysplasia, conservative follow-up is possible and has a high success rate. Cytology is sufficient surveillance for cases involving negative margins. When margins are positive, colposcopy and ECC also may be useful. Microinvasive lesions with positive margins require further surgical evaluation to determine treatment. For glandular lesions, CKC is preferred for both diagnosis and treatment.
Dr. Dunton reports no affiliation or financial arrangement with any of the companies that manufacture drugs or devices in any of the product classes mentioned in this article.
- In most cases, loop electrosurgical excision procedure (LEEP) and cold-knife conization (CKC) result in equivalent success rates and margin status.
- CKC is preferred in cases of adenocarcinoma in situ or squamous microinvasion.
- Conservative follow-up is generally possible in adenocarcinoma in situ and squamous microinvasion when margins are negative.
- Colposcopy, endocervical curettage, and biopsies should be part of the follow-up strategy for patients with positive margins.
When cervical intraepithelial neoplasia (CIN) requires treatment, loop electrosurgical excision procedure (LEEP) is the most frequently used modality, although cold-knife conization (CKC) of the cervical transformation zone still is preferred in select cases. Since excisional techniques are used with CKC, margin status is known and clinical decisions may be based on this information.
This article reviews current indications for excisional biopsy and presents evidence to direct management and follow-up of patients with positive and negative margins.
Selecting a technique
Several large randomized and prospective studies have demonstrated that LEEP is similar in efficacy to CKC and may even remove less of the normal cervical stroma.1-3 In general, LEEP is used to excise high-grade and recurrent squamous dysplasia, as it is similar to CKC in its rates of incomplete excision and residual disease.1,4 However, when adenocarcinoma in situ (AIS) is present, CKC is preferred for diagnosis and treatment, since it results in a lower incidence of involved margins and a lower recurrence rate.3,5-7
CKC also is preferred when histologic confirmation of the margin status is crucial, such as when invasion with squamous lesions is suspected. This is because thermal artifacts that may result from LEEP will interfere with interpretation, further complicating treatment planning.1,4,8,9 In cases of microinvasion, the ability to confirm margins makes conservative treatment possible; if the depth of invasion cannot be determined from the specimen, radical surgery may be necessary.
In the hands of an experienced clinician, however, thermal artifact from the LEEP technique generally is not a significant problem. Series reporting high rates of uninterpretable margins have been attributed to operator inexperience.10 In general, thermal artifact is reduced by limiting the number of sections taken. In ideal cases, only a single-piece specimen is obtained, similar to that achieved using CKC.8,10,11 Newer loops, such as the cone biopsy excision loop, may decrease the number of sections and further improve margin interpretation.8
I use CKC in cases of suspected squamous invasion and in the evaluation of glandular lesions, but feel that in other cases LEEP is efficacious and quicker.
Identifying residual disease
When both the endocervical margin and endocervical curettage (ECC) are positive for squamous dysplasia at the time of excision, there is an increased risk of residual disease (TABLE 1).12-14 However, it is not clear whether ECC alone is an independent predictor of residual disease. Although 1 study suggests an increased risk of invasive cancer in women over 50 years of age with a positive ECC at the time of conization, other series have failed to demonstrate a difference in treatment failure rates based on ECC.15-17 Thus, the utility of ECC in directing further therapy at the time of excisional biopsy is unclear. I generally do not perform an ECC with LEEP for squamous dysplasia.
In AIS, a positive ECC is a strong predictor of residual disease, while a negative ECC is of limited significance.18 I am more likely to perform an ECC with glandular lesions. If it is negative, close follow-up still is indicated. If it is positive, repeat excision may be necessary.
TABLE 1
Residual disease and margin status: squamous dysplasia
| AUTHOR | PROPORTION WITH RESIDUAL DISEASE | |||
|---|---|---|---|---|
| NEGATIVE MARGINS | POSITIVE MARGINS | |||
| NUMBER | % | NUMBER | % | |
| SQUAMOUS LESIONS | ||||
| Bertelsen et al, 199923 | 44/485 | 9 | 29/76 | 38.2 |
| Moore et al, 199524 | 170/523 | 32.5 | 37/91 | 40.7 |
| Lopes et al, 199325 | 0/176 | 0 | 11/131 | 8.4 |
| Murdoch et al, 199226 | 7/405 | 1.7 | 22/160 | 13.8 |
| Andersen et al, 199019 | 0/411 | 0 | 6/469 | 1.3 |
| TOTALS | 221/2,000 | 11 | 105/927 | 11.3 |
| SQUAMOUS MICROINVASION | ||||
| Gurgel et al, 199727 | 6/74 | 8.1 | 45/76 | 59.2 |
| Roman et al, 199722 | 1/30 | 3.3 | 7/50 | 14 |
| TOTALS | 7/104 | 6.7 | 52/126 | 41.3 |
Follow-up of squamous lesions
Considerable clinical uncertainty remains over the relative strengths of cytologic, colposcopic, and histologic evaluation for residual or recurrent disease following excisional biopsy. Conservative management includes Papanicolaou smears alone or in combination with ECC and/or colposcopy.
Negative margins. If margins are negative, the success rate of excisional biopsy is high (90%-100%), and careful observation is the preferred follow-up. Repeat cytologic testing will identify the majority of patients with residual high-grade disease. A prospective randomized trial of cytologic surveillance showed that the detection rate was only 1.9% higher for histology.19 Although 1 report suggests that colposcopy can expedite the diagnosis of recurrent dysplasia, it is unclear from this report whether colposcopy identified significant high-grade dysplasia that cytology missed.20
Positive margins. In most series involving CIN with positive margins, treatment success rates do not differ significantly between patients who undergo repeat surgical procedures and those who have close follow-up. However, endocervical margin involvement appears to carry a higher treatment failure rate than ectocervical margin involvement. Data are conflicting on the proper method of conservative follow-up. Some authors propose only frequent cytologic testing, while others recommend that colposcopy be performed.19-21
For patients with positive margins, I perform both cytology and colposcopy in 4 to 6 months. If an endocervical margin was positive, I also perform an ECC. If this evaluation is negative, I repeat cytologic testing every 6 months until 3 consecutive Pap smears are normal and satisfactory. In cases of recurrent high-grade dysplasia with positive margins, hysterectomy may be indicated, depending on the patient’s age and desire for continued fertility.14,21
Squamous microinvasion
There is a significant risk (50%-80%) of residual disease or true invasion when margins or an ECC are positive and microinvasion is present.2,5-7 In these cases, a repeat excisional biopsy should be performed to determine the true extent of disease prior to deciding on definitive therapy.22 As previously mentioned, CKC is preferred to limit artifact that could obscure interpretation. If the patient has completed her childbearing, hysterectomy remains the standard treatment for microinvasive squamous cell carcinoma. When the woman wishes to preserve fertility, conservative follow-up appears to be safe if the final pathologic specimen has negative margins. A follow-up protocol including cytologic, colposcopic, and ECC monitoring in the first post-conization visit is recommended.2,6
Glandular lesions
AIS with involved margins requires further surgery due to the possibility of residual AIS or invasion (TABLES 2 and 3).2,5-7 In these cases, CKC is preferable to LEEP.3,5-7 Hysterectomy remains the standard therapy for AIS. Conservative management is an option if fertility is desired and margin status is negative. Patients should be informed that persistent disease or recurrence is possible and that there is a risk of invasive disease.15
Counseling, thorough documentation, and second surgical and pathologic opinions may be helpful in conservative management. Colposcopy, ECC, and cytology are indicated at the first follow-up visit, with cytology repeated every 6 months until 4 consecutive Paps are normal. More frequent colposcopy and liberal use of ECC also may be considered.
TABLE 2
Residual disease and margin status: adenocarcinoma in situ
| AUTHOR | PROPORTION WITH RESIDUAL DISEASE | |||
|---|---|---|---|---|
| NEGATIVE MARGINS | POSITIVE MARGINS | |||
| NUMBER | % | NUMBER | % | |
| Azodi et al, 199928 | 5/16 | 31.3 | 9/16 | 56.3 |
| Goldstein et al, 199828 | 13/43 | 30.2 | 8/18 | 44.4 |
| Denehy et al, 19976 | 2/7 | 28.6 | 7/10 | 70 |
| Widrich et al, 19965 | 0/3 | 0 | 9/14 | 64.3 |
| Wolf et al, 199629 | 7/21 | 33.3 | 10/19 | 52.6 |
| TOTAL | 27/90 | 30 | 43/77 | 55.8 |
TABLE 3
Follow-up recommendations
| Pathology | Margin status | Recommendation |
|---|---|---|
| High-grade squamous lesion | Negative |
|
| Positive, endocervical |
| |
| Positive, ectocervical |
| |
| Squamous microinvasion | Negative, fertility desired |
|
| Negative, no desire for fertility | Hysterectomy | |
| Positive | CKC | |
| Adenocarcinoma in situ | Negative, fertility desired |
|
| Negative, no desire for fertility | Hysterectomy | |
| Positive | CKC | |
| CKC = cold-knife conization; ECC = endocervical curettage | ||
When further excision is necessary
As noted earlier, repeat excision is necessary in cases of squamous microinvasion or AIS with positive margins. CKC is generally preferred to allow for optimal pathologic interpretation. For squamous intraepithelial lesions, excisional biopsy with close follow-up has a significant cure rate, and hysterectomy usually is not indicated. However, hysterectomy still should be considered part of the treatment continuum for CIN, particularly for patients who have completed childbearing.
Conclusion
Although the risk of recurrence is correlated with a patient’s margin status in cases of squamous dysplasia, conservative follow-up is possible and has a high success rate. Cytology is sufficient surveillance for cases involving negative margins. When margins are positive, colposcopy and ECC also may be useful. Microinvasive lesions with positive margins require further surgical evaluation to determine treatment. For glandular lesions, CKC is preferred for both diagnosis and treatment.
Dr. Dunton reports no affiliation or financial arrangement with any of the companies that manufacture drugs or devices in any of the product classes mentioned in this article.
1. Mathevet P, Dargent D, Roy M, Beau G. A randomized prospective study comparing three techniques of conization: cold knife, laser, and LEEP. Gynecol Oncol. 1994;54(2):175-179.
2. Duggan BD, Felix JC, Muderspach LI, et al. Cold-knife conization versus conization by the loop electrosurgical excision procedure: a randomized, prospective study. Am J Obstet Gynecol. 1999;180:276-282.
3. Girardi F, Heydarfadi M, Koroschetz F, et al. Cold-knife conization versus loop excision: histopathologic and clinical results of a randomized trial. Gynecol Oncol. 1994;55:368-370.
4. Gold M, Dunton CJ, Murray J, et al. Loop electrocautery excisional procedure: therapeutic effectiveness as an ablation and a conization equivalent. Gynecol Oncol. 1996;61:241-244.
5. Widrich T, Kennedy AW, Myers TM, et al. Adenocarcinoma in situ of the uterine cervix: management and outcome. Gynecol Oncol. 1996;61:304-308.
6. Denehy TR, Gregori CA, Breen JL. Endocervical curettage, cone margins, and residual adenocarcinoma in situ of the cervix. Obstet Gynecol. 1997;90:1-6.
7. Muntz HG, Bell DA, Lage JM, et al. Adenocarcinoma in situ of the uterine cervix. Obstet Gynecol. 1992;80:935-939.
8. Naumann RW, Bell MC, Alvarez RD, et al. LLETZ is an acceptable alternative to diagnostic cold-knife conization. Gynecol Oncol. 1994;55:224-228.
9. Eddy GL, Spiegel GW, Creasman WT. Adverse effect of electrosurgical loop excision on assignment of FIGO stage in cervical cancer: report of two cases. Gynecol Oncol. 1994;55:313-317.
10. Montz FJ, Holschneider CH, Thompson LDR. Large-loop excision of the transformation zone: effect on the pathologic interpretation of resection margins. Obstet Gynecol. 1993;81:976-982.
11. Gardeil F, Barry-Walsh C, Prendiville W, et al. Persistent intraepithelial neoplasia after excision for cervical intraepithelial neoplasia grade III. Obstet Gynecol. 1987;89:419-422.
12. Felix JC, Muderspach LI, Duggan BD, Roman LD. The significance of positive margins in loop electrosurgical cone biopsies. Obstet Gynecol. 1994;84:996-1000.
13. Kobak WH, Roman LD, Felix JC, et al. The role of endocervical curettage at cervical conization for high-grade dysplasia. Obstet Gynecol. 1995;85:197-201.
14. Husseinzadeh N, Shbara I, Wessler T. Predictive value of cone margins and post-cone endocervical curettage with residual disease in subsequent hysterectomy. Gynecol Oncol. 1989;33:198-200.
15. Poyner EA, Barakat RR, Hoskins WJ. Management and follow-up of patients with adenocarcinoma in situ of the uterine cervix. Gynecol Oncol. 1995;57:158-164.
16. Frauchiger WL, De Frias DVS, Cajulis RS, Yu GH. The immediate postconization endocervical smear: evaluation of its utility in the detection of residual dysplasia. Acta Cytol. 1998;42:1139-1143.
17. Wright TC, Gagnon S, Richart RM, Ferenczy A. Treatment of cervical intraepithelial neoplasia using the loop electrosurgical excision procedure. Obstet Gynecol. 1992;79:173-178.
18. Goldstein NS, Mani A. The status and distance of cone biopsy margins as a predictor of excision adequacy for endocervical adenocarcinoma in situ. Am J Clin Pathol. 1998;109:727-732.
19. Andersen ES, Nielsen K, Larsen G. Laser conization: follow-up in patients with cervical intraepithelial neoplasia in the cone margin. Gynecol Oncol. 1990;39:328-331.
20. Paraskevaidis E, Jandial L, Mann EMF, Fisher PM. Pattern of treatment failure following laser for cervical intraepithelial neoplasia: implications for follow-up protocol. Obstet Gynecol. 1991;78:80-83.
21. Lapaquette TK, Dinh TV, Hannigan EV, et al. Management of patients with positive margins after cervical conization. Obstet Gynecol. 1993;82:440-443.
22. Roman LD, Felix JC, Muderspach LI, et al. Risk of residual invasive disease in women with microinvasive squamous cancer in a conization specimen. Obstet Gynecol. 1997;90:759-764.
23. Bertelsen B, Tande T, Sandvei, Hartveit F. Laser conization of cervical intraepithelial neoplasia grade 3. Acta Obstet Gynecol Scand. 1999;78:54-59.
24. Moore BC, Higgins RV, Laurent SL, et al. Predictive factors from cold knife conization for residual cervical intraepithelial neoplasia in subsequent hysterectomy. Am J Obstet Gynecol. 1995;173:361-368.
25. Lopes A, Morgan P, Murdoch J, et al. The case for conservative management of “incomplete excision” of CIN after laser conization. Gynecol Oncol. 1993;49:247-249.
26. Murdoch JB, Morgan PR, Lopes A, Monaghan JM. Histological incomplete excision of CIN after large loop excision of the transformation zone (LLETZ) merits careful follow up, not retreatment. Br J Obstet Gynaecol. 1992;99:990-993.
27. Gurgel MSC, Bedone AJ, Andrade LA, et al. Microinvasive carcinoma of the uterine cervix: histological findings on cone specimens related to residual neoplasia on hysterectomy. Gynecol Oncol. 1997;65:437-440.
28. Azodi M, Chambers SK, Rutherford TJ, et al. Adenocarcinoma in situ of the cervix: management and outcome. Gynecol Oncol. 1999;73:348-353.
29. Wolf JK, Levenback C, Malpica A, et al. Adenocarcinoma in situ of the cervix: significance of cone biopsy margins. Obstet Gynecol. 1996;88:82-86.
1. Mathevet P, Dargent D, Roy M, Beau G. A randomized prospective study comparing three techniques of conization: cold knife, laser, and LEEP. Gynecol Oncol. 1994;54(2):175-179.
2. Duggan BD, Felix JC, Muderspach LI, et al. Cold-knife conization versus conization by the loop electrosurgical excision procedure: a randomized, prospective study. Am J Obstet Gynecol. 1999;180:276-282.
3. Girardi F, Heydarfadi M, Koroschetz F, et al. Cold-knife conization versus loop excision: histopathologic and clinical results of a randomized trial. Gynecol Oncol. 1994;55:368-370.
4. Gold M, Dunton CJ, Murray J, et al. Loop electrocautery excisional procedure: therapeutic effectiveness as an ablation and a conization equivalent. Gynecol Oncol. 1996;61:241-244.
5. Widrich T, Kennedy AW, Myers TM, et al. Adenocarcinoma in situ of the uterine cervix: management and outcome. Gynecol Oncol. 1996;61:304-308.
6. Denehy TR, Gregori CA, Breen JL. Endocervical curettage, cone margins, and residual adenocarcinoma in situ of the cervix. Obstet Gynecol. 1997;90:1-6.
7. Muntz HG, Bell DA, Lage JM, et al. Adenocarcinoma in situ of the uterine cervix. Obstet Gynecol. 1992;80:935-939.
8. Naumann RW, Bell MC, Alvarez RD, et al. LLETZ is an acceptable alternative to diagnostic cold-knife conization. Gynecol Oncol. 1994;55:224-228.
9. Eddy GL, Spiegel GW, Creasman WT. Adverse effect of electrosurgical loop excision on assignment of FIGO stage in cervical cancer: report of two cases. Gynecol Oncol. 1994;55:313-317.
10. Montz FJ, Holschneider CH, Thompson LDR. Large-loop excision of the transformation zone: effect on the pathologic interpretation of resection margins. Obstet Gynecol. 1993;81:976-982.
11. Gardeil F, Barry-Walsh C, Prendiville W, et al. Persistent intraepithelial neoplasia after excision for cervical intraepithelial neoplasia grade III. Obstet Gynecol. 1987;89:419-422.
12. Felix JC, Muderspach LI, Duggan BD, Roman LD. The significance of positive margins in loop electrosurgical cone biopsies. Obstet Gynecol. 1994;84:996-1000.
13. Kobak WH, Roman LD, Felix JC, et al. The role of endocervical curettage at cervical conization for high-grade dysplasia. Obstet Gynecol. 1995;85:197-201.
14. Husseinzadeh N, Shbara I, Wessler T. Predictive value of cone margins and post-cone endocervical curettage with residual disease in subsequent hysterectomy. Gynecol Oncol. 1989;33:198-200.
15. Poyner EA, Barakat RR, Hoskins WJ. Management and follow-up of patients with adenocarcinoma in situ of the uterine cervix. Gynecol Oncol. 1995;57:158-164.
16. Frauchiger WL, De Frias DVS, Cajulis RS, Yu GH. The immediate postconization endocervical smear: evaluation of its utility in the detection of residual dysplasia. Acta Cytol. 1998;42:1139-1143.
17. Wright TC, Gagnon S, Richart RM, Ferenczy A. Treatment of cervical intraepithelial neoplasia using the loop electrosurgical excision procedure. Obstet Gynecol. 1992;79:173-178.
18. Goldstein NS, Mani A. The status and distance of cone biopsy margins as a predictor of excision adequacy for endocervical adenocarcinoma in situ. Am J Clin Pathol. 1998;109:727-732.
19. Andersen ES, Nielsen K, Larsen G. Laser conization: follow-up in patients with cervical intraepithelial neoplasia in the cone margin. Gynecol Oncol. 1990;39:328-331.
20. Paraskevaidis E, Jandial L, Mann EMF, Fisher PM. Pattern of treatment failure following laser for cervical intraepithelial neoplasia: implications for follow-up protocol. Obstet Gynecol. 1991;78:80-83.
21. Lapaquette TK, Dinh TV, Hannigan EV, et al. Management of patients with positive margins after cervical conization. Obstet Gynecol. 1993;82:440-443.
22. Roman LD, Felix JC, Muderspach LI, et al. Risk of residual invasive disease in women with microinvasive squamous cancer in a conization specimen. Obstet Gynecol. 1997;90:759-764.
23. Bertelsen B, Tande T, Sandvei, Hartveit F. Laser conization of cervical intraepithelial neoplasia grade 3. Acta Obstet Gynecol Scand. 1999;78:54-59.
24. Moore BC, Higgins RV, Laurent SL, et al. Predictive factors from cold knife conization for residual cervical intraepithelial neoplasia in subsequent hysterectomy. Am J Obstet Gynecol. 1995;173:361-368.
25. Lopes A, Morgan P, Murdoch J, et al. The case for conservative management of “incomplete excision” of CIN after laser conization. Gynecol Oncol. 1993;49:247-249.
26. Murdoch JB, Morgan PR, Lopes A, Monaghan JM. Histological incomplete excision of CIN after large loop excision of the transformation zone (LLETZ) merits careful follow up, not retreatment. Br J Obstet Gynaecol. 1992;99:990-993.
27. Gurgel MSC, Bedone AJ, Andrade LA, et al. Microinvasive carcinoma of the uterine cervix: histological findings on cone specimens related to residual neoplasia on hysterectomy. Gynecol Oncol. 1997;65:437-440.
28. Azodi M, Chambers SK, Rutherford TJ, et al. Adenocarcinoma in situ of the cervix: management and outcome. Gynecol Oncol. 1999;73:348-353.
29. Wolf JK, Levenback C, Malpica A, et al. Adenocarcinoma in situ of the cervix: significance of cone biopsy margins. Obstet Gynecol. 1996;88:82-86.
Determining the best route for hysterectomy
- Choose the abdominal route when extensive intraperitoneal surgery and/or exploration are required in addition to the hysterectomy, i.e., in cases of pelvic organ carcinoma.
- Use a combination of uterine morcellation techniques to accomplish a vaginal hysterectomy, as researchers have found morcellation of an enlarged uterus to be safer than removing it abdominally.
- For laparoscopic-assisted vaginal hysterectomy, use 3 ports and avoid most disposable instruments by using cautery on vascular pedicles to help minimize costs.
- Seek alternatives to abdominal hysterectomy given its less favorable outcome in terms of morbidity and recovery.
While hysterectomy is one of the most frequently performed operations in gynecology, how to perform it—abdominally, vaginally, or laparoscopically—is less evident. Numerous studies have been published in an attempt to shed some light on this controversy.
Individualize the approach for each patient rather than rely on a dogmatic assignment of technique.
Prior to the introduction of the laparoscopic-assisted vaginal hysterectomy (LAVH) by Reich et al in 1989,1 several large studies were published that compared the abdominal and vaginal routes for hysterectomy. The largest was the Collaborative Review of Sterilization (CREST) study conducted by the Centers for Disease Control (CDC).2 This report included 1,856 women aged 15 to 44 who underwent non-emergency, non-radical hysterectomies at 9 institutions between 1978 and 1981. Fewer complications were associated with vaginal hysterectomy (VH) than abdominal hysterectomy (AH) (Table 1).
Now, several trials have included LAVH in the comparison of hysterectomy routes. In the most comprehensive study to date, Johns et al reviewed 2,563 hysterectomies performed for nonmalignant indications by 37 private gynecologists from a single institution.3 The researchers found that bowel, bladder, and ureteral injuries were uncommon, and the rates of each were similar among LAVH, abdominal hysterectomy, and vaginal hysterectomy (Table 2). In addition, a review of the literature between 1989 and 1995 revealed that LAVH is associated with a shorter hospital stay, decreased recovery time, and less analgesia compared with AH.4
However, since most of the data on route for hysterectomy are from retrospective and uncontrolled trials, one must interpret the findings carefully. For example, many studies do not control for additional procedures performed at the time of hysterectomy (e.g., enterocele, rectocele, and cystocele repairs). In addition, information on how researchers categorized unsuccessful attempts at VH or LAVH—which then had to be converted to AH—often is excluded. Also, physicians usually select the technique based on personal preference, practice style, and traditional dogma such as uterine size rather than a standard protocol.5,6 Therefore, the increased incidence of postoperative morbidity associated with AH is difficult to decipher. Is it due to the increased number of obese and nulliparous women undergoing AH, the surgeon’s experience, pelvic pathology or operative indication, or is it related to the actual opening of the abdomen and intraperitoneal manipulation? Most likely, it is a combination of these factors.
Overall, hysterectomy is a relatively safe procedure with a mortality rate of 1 to 2 per 1,000.7 Morbidity, however, remains high. Fortunately, most complications are minor and easily remedied with little clinical consequence. Since certain aspects of postoperative morbidity are related to the route for hysterectomy, the surgeon must individualize the approach for each patient and not rely on a dogmatic assignment of technique. Here, we will review the patient selection for and provide pearls on abdominal, vaginal, and laparoscopic-assisted vaginal hysterectomy, as well as look at the advantages and disadvantages of each method.
TABLE 1
CREST*study results
| VH | AH | |
|---|---|---|
| Mean age (yrs) | 34.4 | 35.8 |
| Nulliparous (%) | 1.4 | 13.3 |
| Prior cesarean section (%) | 4.8 | 10.1 |
| Obese** (%) | 38.0 | 44.7 |
| Febrile morbidity (%) | 15.3 | 32.3 |
| Required transfusion (%) | 8.3 | 15.4 |
| Death (%) | 0.2 | 0.1 |
| *The Collaborative Review of Sterilization | ||
| **Greater than 120% ideal body weight | ||
| VH=vaginal hysterectomy | ||
| AH=abdominal hysterectomy | ||
| Source: Dicker RC, Greenspan JR, Strauss LT, Cowart MR, Scally MJ, et al. Complications of abdominal and vaginal hysterectomy among women of reproductive age in the United States. Am J Obstet Gynecol. 1982;144:841-848. | ||
TABLE 2
Complications at hysterectomy
| AH | VH | LAVH | |
|---|---|---|---|
| Operating time (minutes) | 82 | 63 | 102 |
| Uterine weight (grams) | 216 | 113 | 129 |
| Febrile morbidity (%) | 9.1 | 3.2 | 2.0 |
| Required transfusion (%) | 2.5 | 1.0 | 0.06 |
| Bowel, bladder, or ureteral injury (%) | 1.0 | 0.9 | 1.1 |
| Death (%) | 0 | 0.2 | 0 |
| AH=abdominal hysterectomy | |||
| VH=vaginal hysterectomy | |||
| LAVH=laparoscopic-assisted vaginal hysterectomy | |||
| Source: Johns DA, et al. The medical and economic impact of laparoscopically assisted vaginal hysterectomy in a large, metropolitan, not-for-profit hospital. Am J Obstet Gynecol. 1995;172:1709-1719. | |||
Abdominal hysterectomy
Patient selection. Choose the abdominal route when extensive intraperitoneal surgery and/or exploration are required in addition to the hysterectomy, i.e., in cases of pelvic organ carcinoma. Severe pelvic adhesive disease from documented severe endometriosis, salpingitis, or significant adnexal pathology and a considerably enlarged uterus also are best approached abdominally (Figure 1). In addition, use the abdominal route for obstetric emergencies such as postpartum hemorrhage.
Technique. First, determine the type of incision based on the following factors: Which one will allow completion of the procedure in a safe and efficient manner, minimize complications, expedite recovery, and offer a favorable cosmetic result? A vertical midline incision is the most adaptable to unsuspected pathology, especially if found in the upper abdomen. It is the quickest to perform and is associated with the least blood loss, an important advantage when operating emergently on an anemic or hemorrhaging patient. However, bear in mind that vertical incisions that have been extended into the upper abdomen are accompanied by increased postoperative pulmonary morbidity, pain, and risk of hernia formation.8,9
Transverse incisions such as the commonly used Pfannenstiel, Cherney, and Maylard compromise upper abdominal exposure but are more cosmetically appealing than vertical incisions. Each of these involves a lower abdominal transverse skin incision and a transverse fascial incision. With the Pfannenstiel technique, the rectus fascia is separated from the rectus muscles, and the muscles are split vertically in the midline, allowing access to the peritoneal cavity. This is the quickest transverse incision to make but provides the least exposure.
If a Pfannenstiel incision has been made and additional lateral pelvic exposure is needed, convert the incision to a Cherney. Both the Cherney and Maylard incisions provide additional exposure to the pelvis, especially laterally, which is advantageous when complex pathology is limited to the pelvis. The Cherney incision involves dividing the rectus muscles from the pubic symphysis, whereas the Maylard incision entails horizontal transection of the rectus muscles at the level of the fascial incision. With the Maylard technique, the rectus fascia is not separated from the underlying muscles as it is in the Cherney incision. If upper abdominal exposure becomes necessary, all of these incisions can be extended upward from their lateral margin, creating a “J.” Alternatively, a second incision can be made in the upper abdomen.
Another option is mini-laparotomy (an incision less than 6 cm). Hoffman and Lynch reported success with this technique for hysterectomy in select patients.10 Most of the procedures were completed with minimal use of retractors by exteriorizing the uterus via one of the aforementioned incisions. They found this approach to be safe and effective in nonobese women in whom a vaginal approach was precluded due to anatomy.
Use the mini-laparotomy approach when there is a suspicious adnexal mass that can be removed through a small abdominal incision.11 In addition, a large benign-appearing cystic adnexal mass can be drained, and the procedure then completed through this incision. Proceed cautiously, however, since an occasional unanticipated carcinoma will be encountered, and the act of drainage will result in the need for chemotherapy in a woman with otherwise early-stage disease. If cancer is suspected, consider a vertical minilaparotomy, which allows easy extension into the upper abdomen should surgical staging or debulking become necessary.
Advantages and disadvantages. The abdominal approach offers the best exposure of the pelvic and upper abdominal cavity but is associated with a high rate of complications, including fever and excessive blood loss. We surmise that postoperative recovery with a mini-laparotomy is likely to be improved compared with the traditional abdominal technique.
Figure 1
Opt for abdominal hysterectomy when a considerably enlarged uterus due to numerous fibroids is encountered.
Vaginal hysterectomy
Patient selection. Choose the vaginal route when pelvic support defects are present (Figure 2), but bear in mind that the course of the ureter changes with worsening degrees of uterine prolapse. We have found the ureter to be palpable in the bladder pillar in most women undergoing VH for prolapse, which helps avoid intraoperative ureteral injury.
The ovaries can be safely removed through the vagina in a large percentage of patients.
Obese, elderly, and otherwise medically debilitated patients also will benefit from the vaginal approach. Avoiding an abdominal wound in these women has obvious advantages. Also, vaginal surgery is associated with a reduction in postoperative pulmonary complications when compared with AH.6,12
Vaginal accessibility inevitably influences the decision to proceed vaginally with a hysterectomy. An inadequate bony pelvis is reason to forgo a VH. Orthopedic conditions and muscular contractures of the lower extremities, which prevent safe positioning, also inhibit this approach. Some surgeons also consider prior abdominal or pelvic surgery a reason to avoid vaginal hysterectomy, while others find this route a means of eluding potential adhesions.
We are strong advocates of vaginal surgery and do not consider nulliparity, lack of uterine descent, or prior abdominopelvic surgery to be strict contraindications for VH. A Schuchardt incision may overcome a small introitus, and a mobile uterus often will descend as the uterosacral and cardinal ligaments are divided.
In women with a prior cesarean delivery, it may be easier to enter the vesicouterine space by approaching it from the less-scarred vaginal side. In a study of more than 200 patients with a previous cesarean who underwent VH, Sheth and Malpani found no increase in complications and concluded that VH is the route of choice in this patient population.13 When unsuspected pelvic adhesions are encountered, carefully dissect them transvaginally and complete the procedure.
Besides accessibility, uterine size must be considered. However, size alone should present a dilemma in only about 15% of patients since most hysterectomy specimens are 12 weeks’ gestational size or smaller.14
Technique. Large uteri can be removed vaginally, depending on the surgeon’s technical ability and experience. A familiarity with the various methods of morcellation—hemisection, posterior fundal morcellation, intramyometrial coring, and myomectomy—is mandatory. It often is beneficial to use a combination of these techniques to accomplish the procedure vaginally, as opposed to a single means of morcellation. With any morcellation procedure, maintain a midline orientation to avoid dissection into the broad ligament. Continued caudal traction also is helpful in eliminating excessive blood loss during this prolonged procedure. Do not morcellate a uterus when endometrial carcinoma is suspected.
In a prospective observational study comparing vaginal morcellation to AH, Hoffman et al15 found the former to be safer. Other researchers also have demonstrated the procedure’s safety in retrospective reports16-18 and as subgroups in other series.14 Alternatively, preoperative treatment with a gonadotropin-releasing hor-Surgical Techniques mone (GnRH) agonist can reduce uterine size and result in a technically less challenging VH in some women.19,20
Also, whether or not the ovaries will be resected may influence the decision to perform a VH. While some surgeons believe that oophorectomy precludes the vaginal approach, the ovaries can be safely removed through the vagina in a large percentage of patients.21-23 In fact, Sheth24 and Kovac14 have published 95% and 97% success rates in 2 separate series in which vaginal oophorectomy was attempted in 740 and 142 women, respectively. However, do not attempt adnexal removal without adequate transvaginal access and visibility, as it increases the risk of hemorrhage and ureteral injury. In these cases, LAVH or mini-laparotomy AH may be preferable if oophorectomy is mandatory, i.e., in women who are predisposed to ovarian malignancies because of genetic mutations. However, when patients are appropriately selected, there are essentially no disadvantages to VH.
Advantages and disadvantages.VH has many advantages over AH. Minimal intraperitoneal manipulation and the avoidance of an abdominal wound lead to a shorter hospital stay and decreased recovery time, which is coupled with less cost. Patients also find the lack of an abdominal scar to be an attractive feature of VH. A disadvantage is reduced operative exposure, making it difficult to manipulate pelvic pathology and resect the adnexa.
Figure 2
Choose the vaginal route when pelvic support defects are present, but bear in mind that the course of the ureter changes due to prolapse.
Laparoscopic-assisted vaginal hysterectomy
Patient selection. When in doubt about which approach to use, turn to LAVH. The laparoscope allows the surgeon to thoroughly assess intra-abdominal pathology, which aids in the appropriate selection of a hysterectomy route. For example, if cancer is suspected based on the visual inspection, opt for an AH. On the other hand, if only extensive adhesions are noted, laparoscopic adhesiolysis can be performed to allow the hysterectomy to be completed vaginally. As previously noted, LAVH is appropriate in patients undergoing hysterectomy with prophylactic oophorectomy due to genetic or familial risk factors.
Technique. Bear in mind that the laparoscope does not permit all patients to undergo a VH. In fact, about 10% of attempted LAVHs will be unsuccessful.25,26 Some authors have reported failure during the laparoscopic portion,25,26 while others have attributed the lack of success to the vaginal portion of the procedure.27 Thus, it is important to be skilled at both laparoscopic and vaginal surgery in order to be successful with an LAVH.
LAVH requires three to five 5-to 10-mm abdominal incisions for port sites. An umbilical port is commonly used for the camera, and the remaining ports are used for operating. We typically use 3 ports and avoid most disposable instruments by using cautery on vascular pedicles, which helps minimize costs.3 Other alternatives include the use of endoscopic staplers, the Harmonic Scalpel (Ethicon Endo-Surgery, a Johnson & Johnson company, Cincinnati, Ohio), and suture ligatures with extra-or intracorporeal knot tying. Whichever instrument is used, familiarize yourself with all available equipment given the occasional malfunction encountered during laparoscopy.
Advantages and disadvantages. LAVH requires special equipment and expertise beyond that needed for an abdominal or vaginal hysterectomy. Additionally, this procedure increases operative time, cost, and morbidity (depending on the surgeon’s experience),4,27,28 while the postoperative recovery is similar to that of VH.
Although we use the laparoscope infrequently and believe there is little need to perform excessive laparoscopy at the time of routine hysterectomy, we do not wish to understate the role of the laparoscope. Its use in specific instances can certainly avoid a laparotomy or help determine when an AH is more appropriate.
Conclusion
Emergent situations and patients with excessively enlarged uteri, significant pelvic pathology, or cancer are obvious candidates for AH. On the other hand, VH is frequently chosen for the small uterus in a multiparous woman with a large pelvis and no prior pelvic inflammatory disease or surgery. The dilemma arises when determining the approach for those patients with moderately enlarged uteri or presumptive risk factors for serious pelvic disease.
Kovac reported a standard protocol for selecting the route for hysterectomy. Uterine size, other pelvic pathology (endometriosis, adnexal disease, chronic pain, etc.), and uterine and adnexal accessibility (bony architecture, uterine support, and vaginal diameter) were each considered in the decision-making. A simplification of Kovac’s guidelines applicable to women undergoing hysterectomy for benign indications is summarized in Figure 3. Using these guidelines, Kovac reported a 99% (608/611) success rate for women assigned to VH or LAVH. The laparoscope was deemed necessary in only 19% of those assigned to the LAVH group, and ultimately only 9 patients required AH, yielding a 1:68 ratio for AH to VH.14
Gynecologists should seek alternatives to AH given its less favorable outcome in terms of morbidity and recovery. However, the surgeon who is competent with AH better serves the patient by performing the procedure via this route than by attempting an alternative procedure without the necessary proficiency. In other words, pelvic surgeons must be cognizant of their abilities and practice within that realm. Ultimately, the final selection of hysterectomy route should be based on the surgeon’s experience, the indication for surgery, and the patient’s anatomy.
FIGURE 3Guidelines to determine route of hysterectomy.
The authors report no financial relationship with any companies whose products are mentioned in this article.
1. Reich H, DeCaprio J, McGlynn F. Laparoscopic hysterectomy. J Gynecol Surg. 1989;5:213-216.
2. Dicker RC, Greenspan JR, Strauss LT, Cowart MR, Scally MJ, et al. Complications of abdominal and vaginal hysterectomy among women of reproductive age in the United States. Am J Obstet Gynecol. 1982;144:841-848.
3. Johns DA, Carrera B, Jones J, DeLeon F, Vincent R, et al. The medical and economic impact of laparoscopic-assisted vaginal hysterectomy in a large, metropolitan, not-for-profit hospital. Am J Obstet Gynecol. 1995;172:1709-719.
4. Meikle SF, Nugent EW, Orleans M. Complication and recovery for laparoscopic-assisted vaginal hysterectomy compared with abdominal and vaginal hysterectomy. Obstet Gynecol. 1997;89:304-311.
5. Kovac SR, Christie SJ, Bindbeutel GA. Abdominal versus vaginal hysterectomy: a statistical model for determining physician decision making and patient outcomes. Med Decis Making. 1991;11:19-28.
6. Dorsey JH, Steinberg EP, Holtz PM. Clinical indications for hysterectomy route: patient characteristics or physician preference. Am J Obstet Gynecol. 1995;173:1452-1460.
7. Wingo PA, Huezo CM, Rubin GL, Ory HW, Peterson HB. The mortality risk associated with hysterectomy. Am J Obstet Gynecol. 1985;152:803-808.
8. Tollefson DG, Russell KP. The transverse incision in pelvic surgery. Am J Obstet Gynecol. 1954;68:410-422.
9. Helmkamp BF. Abdominal wound dehiscence. Am J Obstet Gynecol. 1977;128:803-807.
10. Hoffman MS, Lynch CM. Minilaparotomy hysterectomy. Am J Obstet Gynecol. 1998;179:316-320.
11. Flynn M, Niloff JM. Minilaparotomy for the ambulatory management of ovarian cysts. Am J Obstet Gynecol. 1995;173:1727-1730.
12. White SC, Wartel LJ, Wade ME. Comparison of abdominal and vaginal hysterectomies. Obstet Gynecol. 1971;37:530-537.
13. Sheth SS, Malpani AN. Vaginal hysterectomy following previous cesarean section. Int J Gynaecol Obstet. 1995;50:65-69.
14. Kovac SR. Guidelines to determine route of hysterectomy. Obstet Gynecol. 1995;85:18-23.
15. Hoffman MS, DeCesare S, Kalter C. Abdominal hysterectomy versus transvaginal morcellation for the removal of enlarged uteri. Am J Obstet Gynecol. 1994;171:309-315.
16. Mazdisnian F, Kurzel RB, Coe S, Bosuk M, Montz F. Vaginal hysterectomy by uterine morcellation: an efficient, non-morbid procedure. Obstet Gynecol. 1995;86:60-64.
17. Kudo R, Yamauchi O, Okazaki T, Sagac S, Ito E, Hashimoto M. Vaginal hysterectomy without ligation of the ligaments of the cervix uteri. Surg Gynecol Obstet. 1990;70:299-305.
18. Kovac SR. Intramyometrial coring as an adjunct to vaginal hysterectomy. Obstet Gynecol. 1986;67:131-136.
19. Stovall TG, Summit RL, Washburn SA, Ling FW. Gonadotropin-releasing hormone agonist use before hysterectomy. Am J Obstet Gynecol. 1994;170:1744-1748.
20. Crosignani PG, Vercellini P, Meschia M, Oldani S, Bramante T. GnRH agonists before surgery for uterine leiomyomas. J Reprod Med. 1996;41:415-421.
21. Hoffman MS. Transvaginal removal of ovaries with endoloop sutures at the time of transvaginal hysterectomy. Am J Obstet Gynecol. 1991;165:407-408.
22. Sheth SS. Ovarian clamp for oophorectomy during vaginal hysterectomy. J Am Assoc Gynecol Laparosc. 1995;2(4):S76-S77.
23. Hefni MA, Davies AE. Vaginal endoscopic oophorectomy with vaginal hysterectomy: a simple minimal access surgery technique. Br J Obstet Gynaecol. 1997;104:621-622.
24. Sheth SS. The place of oophorectomy at vaginal hysterectomy. Br J Obstet Gynaecol. 1991;98:662-666.
25. Cristoforoni PM, Palmieri A, Gerbaldo D, Montz FJ. Frequency and cause of aborted laparoscopic-assisted vaginal hysterectomy. J Am Assoc Gynecol Laparosc. 1995;3:33-37.
26. Summitt RL, Stovall TG, Steege JF, Lipscomb GH. A multicenter randomized comparison of laparoscopic-assisted vaginal hysterectomy and abdominal hysterectomy in abdominal hysterectomy candidates. Obstet Gynecol. 1998;92:321-326.
27. Dorsey JH, Holtz PM, Griffiths RI, McGrath MM, Steinberg EP. Costs and charges associated with three alternative techniques of hysterectomy. N Engl J Med. 1996;335:476-482.
28. Summitt RL, Stovall TG, Lipscomb GH, Ling FW. Randomized comparison of laparoscopic-assisted vaginal hysterectomy with standard vaginal hysterectomy in an outpatient setting. Obstet Gynecol. 1992;80:895-901.
- Choose the abdominal route when extensive intraperitoneal surgery and/or exploration are required in addition to the hysterectomy, i.e., in cases of pelvic organ carcinoma.
- Use a combination of uterine morcellation techniques to accomplish a vaginal hysterectomy, as researchers have found morcellation of an enlarged uterus to be safer than removing it abdominally.
- For laparoscopic-assisted vaginal hysterectomy, use 3 ports and avoid most disposable instruments by using cautery on vascular pedicles to help minimize costs.
- Seek alternatives to abdominal hysterectomy given its less favorable outcome in terms of morbidity and recovery.
While hysterectomy is one of the most frequently performed operations in gynecology, how to perform it—abdominally, vaginally, or laparoscopically—is less evident. Numerous studies have been published in an attempt to shed some light on this controversy.
Individualize the approach for each patient rather than rely on a dogmatic assignment of technique.
Prior to the introduction of the laparoscopic-assisted vaginal hysterectomy (LAVH) by Reich et al in 1989,1 several large studies were published that compared the abdominal and vaginal routes for hysterectomy. The largest was the Collaborative Review of Sterilization (CREST) study conducted by the Centers for Disease Control (CDC).2 This report included 1,856 women aged 15 to 44 who underwent non-emergency, non-radical hysterectomies at 9 institutions between 1978 and 1981. Fewer complications were associated with vaginal hysterectomy (VH) than abdominal hysterectomy (AH) (Table 1).
Now, several trials have included LAVH in the comparison of hysterectomy routes. In the most comprehensive study to date, Johns et al reviewed 2,563 hysterectomies performed for nonmalignant indications by 37 private gynecologists from a single institution.3 The researchers found that bowel, bladder, and ureteral injuries were uncommon, and the rates of each were similar among LAVH, abdominal hysterectomy, and vaginal hysterectomy (Table 2). In addition, a review of the literature between 1989 and 1995 revealed that LAVH is associated with a shorter hospital stay, decreased recovery time, and less analgesia compared with AH.4
However, since most of the data on route for hysterectomy are from retrospective and uncontrolled trials, one must interpret the findings carefully. For example, many studies do not control for additional procedures performed at the time of hysterectomy (e.g., enterocele, rectocele, and cystocele repairs). In addition, information on how researchers categorized unsuccessful attempts at VH or LAVH—which then had to be converted to AH—often is excluded. Also, physicians usually select the technique based on personal preference, practice style, and traditional dogma such as uterine size rather than a standard protocol.5,6 Therefore, the increased incidence of postoperative morbidity associated with AH is difficult to decipher. Is it due to the increased number of obese and nulliparous women undergoing AH, the surgeon’s experience, pelvic pathology or operative indication, or is it related to the actual opening of the abdomen and intraperitoneal manipulation? Most likely, it is a combination of these factors.
Overall, hysterectomy is a relatively safe procedure with a mortality rate of 1 to 2 per 1,000.7 Morbidity, however, remains high. Fortunately, most complications are minor and easily remedied with little clinical consequence. Since certain aspects of postoperative morbidity are related to the route for hysterectomy, the surgeon must individualize the approach for each patient and not rely on a dogmatic assignment of technique. Here, we will review the patient selection for and provide pearls on abdominal, vaginal, and laparoscopic-assisted vaginal hysterectomy, as well as look at the advantages and disadvantages of each method.
TABLE 1
CREST*study results
| VH | AH | |
|---|---|---|
| Mean age (yrs) | 34.4 | 35.8 |
| Nulliparous (%) | 1.4 | 13.3 |
| Prior cesarean section (%) | 4.8 | 10.1 |
| Obese** (%) | 38.0 | 44.7 |
| Febrile morbidity (%) | 15.3 | 32.3 |
| Required transfusion (%) | 8.3 | 15.4 |
| Death (%) | 0.2 | 0.1 |
| *The Collaborative Review of Sterilization | ||
| **Greater than 120% ideal body weight | ||
| VH=vaginal hysterectomy | ||
| AH=abdominal hysterectomy | ||
| Source: Dicker RC, Greenspan JR, Strauss LT, Cowart MR, Scally MJ, et al. Complications of abdominal and vaginal hysterectomy among women of reproductive age in the United States. Am J Obstet Gynecol. 1982;144:841-848. | ||
TABLE 2
Complications at hysterectomy
| AH | VH | LAVH | |
|---|---|---|---|
| Operating time (minutes) | 82 | 63 | 102 |
| Uterine weight (grams) | 216 | 113 | 129 |
| Febrile morbidity (%) | 9.1 | 3.2 | 2.0 |
| Required transfusion (%) | 2.5 | 1.0 | 0.06 |
| Bowel, bladder, or ureteral injury (%) | 1.0 | 0.9 | 1.1 |
| Death (%) | 0 | 0.2 | 0 |
| AH=abdominal hysterectomy | |||
| VH=vaginal hysterectomy | |||
| LAVH=laparoscopic-assisted vaginal hysterectomy | |||
| Source: Johns DA, et al. The medical and economic impact of laparoscopically assisted vaginal hysterectomy in a large, metropolitan, not-for-profit hospital. Am J Obstet Gynecol. 1995;172:1709-1719. | |||
Abdominal hysterectomy
Patient selection. Choose the abdominal route when extensive intraperitoneal surgery and/or exploration are required in addition to the hysterectomy, i.e., in cases of pelvic organ carcinoma. Severe pelvic adhesive disease from documented severe endometriosis, salpingitis, or significant adnexal pathology and a considerably enlarged uterus also are best approached abdominally (Figure 1). In addition, use the abdominal route for obstetric emergencies such as postpartum hemorrhage.
Technique. First, determine the type of incision based on the following factors: Which one will allow completion of the procedure in a safe and efficient manner, minimize complications, expedite recovery, and offer a favorable cosmetic result? A vertical midline incision is the most adaptable to unsuspected pathology, especially if found in the upper abdomen. It is the quickest to perform and is associated with the least blood loss, an important advantage when operating emergently on an anemic or hemorrhaging patient. However, bear in mind that vertical incisions that have been extended into the upper abdomen are accompanied by increased postoperative pulmonary morbidity, pain, and risk of hernia formation.8,9
Transverse incisions such as the commonly used Pfannenstiel, Cherney, and Maylard compromise upper abdominal exposure but are more cosmetically appealing than vertical incisions. Each of these involves a lower abdominal transverse skin incision and a transverse fascial incision. With the Pfannenstiel technique, the rectus fascia is separated from the rectus muscles, and the muscles are split vertically in the midline, allowing access to the peritoneal cavity. This is the quickest transverse incision to make but provides the least exposure.
If a Pfannenstiel incision has been made and additional lateral pelvic exposure is needed, convert the incision to a Cherney. Both the Cherney and Maylard incisions provide additional exposure to the pelvis, especially laterally, which is advantageous when complex pathology is limited to the pelvis. The Cherney incision involves dividing the rectus muscles from the pubic symphysis, whereas the Maylard incision entails horizontal transection of the rectus muscles at the level of the fascial incision. With the Maylard technique, the rectus fascia is not separated from the underlying muscles as it is in the Cherney incision. If upper abdominal exposure becomes necessary, all of these incisions can be extended upward from their lateral margin, creating a “J.” Alternatively, a second incision can be made in the upper abdomen.
Another option is mini-laparotomy (an incision less than 6 cm). Hoffman and Lynch reported success with this technique for hysterectomy in select patients.10 Most of the procedures were completed with minimal use of retractors by exteriorizing the uterus via one of the aforementioned incisions. They found this approach to be safe and effective in nonobese women in whom a vaginal approach was precluded due to anatomy.
Use the mini-laparotomy approach when there is a suspicious adnexal mass that can be removed through a small abdominal incision.11 In addition, a large benign-appearing cystic adnexal mass can be drained, and the procedure then completed through this incision. Proceed cautiously, however, since an occasional unanticipated carcinoma will be encountered, and the act of drainage will result in the need for chemotherapy in a woman with otherwise early-stage disease. If cancer is suspected, consider a vertical minilaparotomy, which allows easy extension into the upper abdomen should surgical staging or debulking become necessary.
Advantages and disadvantages. The abdominal approach offers the best exposure of the pelvic and upper abdominal cavity but is associated with a high rate of complications, including fever and excessive blood loss. We surmise that postoperative recovery with a mini-laparotomy is likely to be improved compared with the traditional abdominal technique.
Figure 1
Opt for abdominal hysterectomy when a considerably enlarged uterus due to numerous fibroids is encountered.
Vaginal hysterectomy
Patient selection. Choose the vaginal route when pelvic support defects are present (Figure 2), but bear in mind that the course of the ureter changes with worsening degrees of uterine prolapse. We have found the ureter to be palpable in the bladder pillar in most women undergoing VH for prolapse, which helps avoid intraoperative ureteral injury.
The ovaries can be safely removed through the vagina in a large percentage of patients.
Obese, elderly, and otherwise medically debilitated patients also will benefit from the vaginal approach. Avoiding an abdominal wound in these women has obvious advantages. Also, vaginal surgery is associated with a reduction in postoperative pulmonary complications when compared with AH.6,12
Vaginal accessibility inevitably influences the decision to proceed vaginally with a hysterectomy. An inadequate bony pelvis is reason to forgo a VH. Orthopedic conditions and muscular contractures of the lower extremities, which prevent safe positioning, also inhibit this approach. Some surgeons also consider prior abdominal or pelvic surgery a reason to avoid vaginal hysterectomy, while others find this route a means of eluding potential adhesions.
We are strong advocates of vaginal surgery and do not consider nulliparity, lack of uterine descent, or prior abdominopelvic surgery to be strict contraindications for VH. A Schuchardt incision may overcome a small introitus, and a mobile uterus often will descend as the uterosacral and cardinal ligaments are divided.
In women with a prior cesarean delivery, it may be easier to enter the vesicouterine space by approaching it from the less-scarred vaginal side. In a study of more than 200 patients with a previous cesarean who underwent VH, Sheth and Malpani found no increase in complications and concluded that VH is the route of choice in this patient population.13 When unsuspected pelvic adhesions are encountered, carefully dissect them transvaginally and complete the procedure.
Besides accessibility, uterine size must be considered. However, size alone should present a dilemma in only about 15% of patients since most hysterectomy specimens are 12 weeks’ gestational size or smaller.14
Technique. Large uteri can be removed vaginally, depending on the surgeon’s technical ability and experience. A familiarity with the various methods of morcellation—hemisection, posterior fundal morcellation, intramyometrial coring, and myomectomy—is mandatory. It often is beneficial to use a combination of these techniques to accomplish the procedure vaginally, as opposed to a single means of morcellation. With any morcellation procedure, maintain a midline orientation to avoid dissection into the broad ligament. Continued caudal traction also is helpful in eliminating excessive blood loss during this prolonged procedure. Do not morcellate a uterus when endometrial carcinoma is suspected.
In a prospective observational study comparing vaginal morcellation to AH, Hoffman et al15 found the former to be safer. Other researchers also have demonstrated the procedure’s safety in retrospective reports16-18 and as subgroups in other series.14 Alternatively, preoperative treatment with a gonadotropin-releasing hor-Surgical Techniques mone (GnRH) agonist can reduce uterine size and result in a technically less challenging VH in some women.19,20
Also, whether or not the ovaries will be resected may influence the decision to perform a VH. While some surgeons believe that oophorectomy precludes the vaginal approach, the ovaries can be safely removed through the vagina in a large percentage of patients.21-23 In fact, Sheth24 and Kovac14 have published 95% and 97% success rates in 2 separate series in which vaginal oophorectomy was attempted in 740 and 142 women, respectively. However, do not attempt adnexal removal without adequate transvaginal access and visibility, as it increases the risk of hemorrhage and ureteral injury. In these cases, LAVH or mini-laparotomy AH may be preferable if oophorectomy is mandatory, i.e., in women who are predisposed to ovarian malignancies because of genetic mutations. However, when patients are appropriately selected, there are essentially no disadvantages to VH.
Advantages and disadvantages.VH has many advantages over AH. Minimal intraperitoneal manipulation and the avoidance of an abdominal wound lead to a shorter hospital stay and decreased recovery time, which is coupled with less cost. Patients also find the lack of an abdominal scar to be an attractive feature of VH. A disadvantage is reduced operative exposure, making it difficult to manipulate pelvic pathology and resect the adnexa.
Figure 2
Choose the vaginal route when pelvic support defects are present, but bear in mind that the course of the ureter changes due to prolapse.
Laparoscopic-assisted vaginal hysterectomy
Patient selection. When in doubt about which approach to use, turn to LAVH. The laparoscope allows the surgeon to thoroughly assess intra-abdominal pathology, which aids in the appropriate selection of a hysterectomy route. For example, if cancer is suspected based on the visual inspection, opt for an AH. On the other hand, if only extensive adhesions are noted, laparoscopic adhesiolysis can be performed to allow the hysterectomy to be completed vaginally. As previously noted, LAVH is appropriate in patients undergoing hysterectomy with prophylactic oophorectomy due to genetic or familial risk factors.
Technique. Bear in mind that the laparoscope does not permit all patients to undergo a VH. In fact, about 10% of attempted LAVHs will be unsuccessful.25,26 Some authors have reported failure during the laparoscopic portion,25,26 while others have attributed the lack of success to the vaginal portion of the procedure.27 Thus, it is important to be skilled at both laparoscopic and vaginal surgery in order to be successful with an LAVH.
LAVH requires three to five 5-to 10-mm abdominal incisions for port sites. An umbilical port is commonly used for the camera, and the remaining ports are used for operating. We typically use 3 ports and avoid most disposable instruments by using cautery on vascular pedicles, which helps minimize costs.3 Other alternatives include the use of endoscopic staplers, the Harmonic Scalpel (Ethicon Endo-Surgery, a Johnson & Johnson company, Cincinnati, Ohio), and suture ligatures with extra-or intracorporeal knot tying. Whichever instrument is used, familiarize yourself with all available equipment given the occasional malfunction encountered during laparoscopy.
Advantages and disadvantages. LAVH requires special equipment and expertise beyond that needed for an abdominal or vaginal hysterectomy. Additionally, this procedure increases operative time, cost, and morbidity (depending on the surgeon’s experience),4,27,28 while the postoperative recovery is similar to that of VH.
Although we use the laparoscope infrequently and believe there is little need to perform excessive laparoscopy at the time of routine hysterectomy, we do not wish to understate the role of the laparoscope. Its use in specific instances can certainly avoid a laparotomy or help determine when an AH is more appropriate.
Conclusion
Emergent situations and patients with excessively enlarged uteri, significant pelvic pathology, or cancer are obvious candidates for AH. On the other hand, VH is frequently chosen for the small uterus in a multiparous woman with a large pelvis and no prior pelvic inflammatory disease or surgery. The dilemma arises when determining the approach for those patients with moderately enlarged uteri or presumptive risk factors for serious pelvic disease.
Kovac reported a standard protocol for selecting the route for hysterectomy. Uterine size, other pelvic pathology (endometriosis, adnexal disease, chronic pain, etc.), and uterine and adnexal accessibility (bony architecture, uterine support, and vaginal diameter) were each considered in the decision-making. A simplification of Kovac’s guidelines applicable to women undergoing hysterectomy for benign indications is summarized in Figure 3. Using these guidelines, Kovac reported a 99% (608/611) success rate for women assigned to VH or LAVH. The laparoscope was deemed necessary in only 19% of those assigned to the LAVH group, and ultimately only 9 patients required AH, yielding a 1:68 ratio for AH to VH.14
Gynecologists should seek alternatives to AH given its less favorable outcome in terms of morbidity and recovery. However, the surgeon who is competent with AH better serves the patient by performing the procedure via this route than by attempting an alternative procedure without the necessary proficiency. In other words, pelvic surgeons must be cognizant of their abilities and practice within that realm. Ultimately, the final selection of hysterectomy route should be based on the surgeon’s experience, the indication for surgery, and the patient’s anatomy.
FIGURE 3Guidelines to determine route of hysterectomy.
The authors report no financial relationship with any companies whose products are mentioned in this article.
- Choose the abdominal route when extensive intraperitoneal surgery and/or exploration are required in addition to the hysterectomy, i.e., in cases of pelvic organ carcinoma.
- Use a combination of uterine morcellation techniques to accomplish a vaginal hysterectomy, as researchers have found morcellation of an enlarged uterus to be safer than removing it abdominally.
- For laparoscopic-assisted vaginal hysterectomy, use 3 ports and avoid most disposable instruments by using cautery on vascular pedicles to help minimize costs.
- Seek alternatives to abdominal hysterectomy given its less favorable outcome in terms of morbidity and recovery.
While hysterectomy is one of the most frequently performed operations in gynecology, how to perform it—abdominally, vaginally, or laparoscopically—is less evident. Numerous studies have been published in an attempt to shed some light on this controversy.
Individualize the approach for each patient rather than rely on a dogmatic assignment of technique.
Prior to the introduction of the laparoscopic-assisted vaginal hysterectomy (LAVH) by Reich et al in 1989,1 several large studies were published that compared the abdominal and vaginal routes for hysterectomy. The largest was the Collaborative Review of Sterilization (CREST) study conducted by the Centers for Disease Control (CDC).2 This report included 1,856 women aged 15 to 44 who underwent non-emergency, non-radical hysterectomies at 9 institutions between 1978 and 1981. Fewer complications were associated with vaginal hysterectomy (VH) than abdominal hysterectomy (AH) (Table 1).
Now, several trials have included LAVH in the comparison of hysterectomy routes. In the most comprehensive study to date, Johns et al reviewed 2,563 hysterectomies performed for nonmalignant indications by 37 private gynecologists from a single institution.3 The researchers found that bowel, bladder, and ureteral injuries were uncommon, and the rates of each were similar among LAVH, abdominal hysterectomy, and vaginal hysterectomy (Table 2). In addition, a review of the literature between 1989 and 1995 revealed that LAVH is associated with a shorter hospital stay, decreased recovery time, and less analgesia compared with AH.4
However, since most of the data on route for hysterectomy are from retrospective and uncontrolled trials, one must interpret the findings carefully. For example, many studies do not control for additional procedures performed at the time of hysterectomy (e.g., enterocele, rectocele, and cystocele repairs). In addition, information on how researchers categorized unsuccessful attempts at VH or LAVH—which then had to be converted to AH—often is excluded. Also, physicians usually select the technique based on personal preference, practice style, and traditional dogma such as uterine size rather than a standard protocol.5,6 Therefore, the increased incidence of postoperative morbidity associated with AH is difficult to decipher. Is it due to the increased number of obese and nulliparous women undergoing AH, the surgeon’s experience, pelvic pathology or operative indication, or is it related to the actual opening of the abdomen and intraperitoneal manipulation? Most likely, it is a combination of these factors.
Overall, hysterectomy is a relatively safe procedure with a mortality rate of 1 to 2 per 1,000.7 Morbidity, however, remains high. Fortunately, most complications are minor and easily remedied with little clinical consequence. Since certain aspects of postoperative morbidity are related to the route for hysterectomy, the surgeon must individualize the approach for each patient and not rely on a dogmatic assignment of technique. Here, we will review the patient selection for and provide pearls on abdominal, vaginal, and laparoscopic-assisted vaginal hysterectomy, as well as look at the advantages and disadvantages of each method.
TABLE 1
CREST*study results
| VH | AH | |
|---|---|---|
| Mean age (yrs) | 34.4 | 35.8 |
| Nulliparous (%) | 1.4 | 13.3 |
| Prior cesarean section (%) | 4.8 | 10.1 |
| Obese** (%) | 38.0 | 44.7 |
| Febrile morbidity (%) | 15.3 | 32.3 |
| Required transfusion (%) | 8.3 | 15.4 |
| Death (%) | 0.2 | 0.1 |
| *The Collaborative Review of Sterilization | ||
| **Greater than 120% ideal body weight | ||
| VH=vaginal hysterectomy | ||
| AH=abdominal hysterectomy | ||
| Source: Dicker RC, Greenspan JR, Strauss LT, Cowart MR, Scally MJ, et al. Complications of abdominal and vaginal hysterectomy among women of reproductive age in the United States. Am J Obstet Gynecol. 1982;144:841-848. | ||
TABLE 2
Complications at hysterectomy
| AH | VH | LAVH | |
|---|---|---|---|
| Operating time (minutes) | 82 | 63 | 102 |
| Uterine weight (grams) | 216 | 113 | 129 |
| Febrile morbidity (%) | 9.1 | 3.2 | 2.0 |
| Required transfusion (%) | 2.5 | 1.0 | 0.06 |
| Bowel, bladder, or ureteral injury (%) | 1.0 | 0.9 | 1.1 |
| Death (%) | 0 | 0.2 | 0 |
| AH=abdominal hysterectomy | |||
| VH=vaginal hysterectomy | |||
| LAVH=laparoscopic-assisted vaginal hysterectomy | |||
| Source: Johns DA, et al. The medical and economic impact of laparoscopically assisted vaginal hysterectomy in a large, metropolitan, not-for-profit hospital. Am J Obstet Gynecol. 1995;172:1709-1719. | |||
Abdominal hysterectomy
Patient selection. Choose the abdominal route when extensive intraperitoneal surgery and/or exploration are required in addition to the hysterectomy, i.e., in cases of pelvic organ carcinoma. Severe pelvic adhesive disease from documented severe endometriosis, salpingitis, or significant adnexal pathology and a considerably enlarged uterus also are best approached abdominally (Figure 1). In addition, use the abdominal route for obstetric emergencies such as postpartum hemorrhage.
Technique. First, determine the type of incision based on the following factors: Which one will allow completion of the procedure in a safe and efficient manner, minimize complications, expedite recovery, and offer a favorable cosmetic result? A vertical midline incision is the most adaptable to unsuspected pathology, especially if found in the upper abdomen. It is the quickest to perform and is associated with the least blood loss, an important advantage when operating emergently on an anemic or hemorrhaging patient. However, bear in mind that vertical incisions that have been extended into the upper abdomen are accompanied by increased postoperative pulmonary morbidity, pain, and risk of hernia formation.8,9
Transverse incisions such as the commonly used Pfannenstiel, Cherney, and Maylard compromise upper abdominal exposure but are more cosmetically appealing than vertical incisions. Each of these involves a lower abdominal transverse skin incision and a transverse fascial incision. With the Pfannenstiel technique, the rectus fascia is separated from the rectus muscles, and the muscles are split vertically in the midline, allowing access to the peritoneal cavity. This is the quickest transverse incision to make but provides the least exposure.
If a Pfannenstiel incision has been made and additional lateral pelvic exposure is needed, convert the incision to a Cherney. Both the Cherney and Maylard incisions provide additional exposure to the pelvis, especially laterally, which is advantageous when complex pathology is limited to the pelvis. The Cherney incision involves dividing the rectus muscles from the pubic symphysis, whereas the Maylard incision entails horizontal transection of the rectus muscles at the level of the fascial incision. With the Maylard technique, the rectus fascia is not separated from the underlying muscles as it is in the Cherney incision. If upper abdominal exposure becomes necessary, all of these incisions can be extended upward from their lateral margin, creating a “J.” Alternatively, a second incision can be made in the upper abdomen.
Another option is mini-laparotomy (an incision less than 6 cm). Hoffman and Lynch reported success with this technique for hysterectomy in select patients.10 Most of the procedures were completed with minimal use of retractors by exteriorizing the uterus via one of the aforementioned incisions. They found this approach to be safe and effective in nonobese women in whom a vaginal approach was precluded due to anatomy.
Use the mini-laparotomy approach when there is a suspicious adnexal mass that can be removed through a small abdominal incision.11 In addition, a large benign-appearing cystic adnexal mass can be drained, and the procedure then completed through this incision. Proceed cautiously, however, since an occasional unanticipated carcinoma will be encountered, and the act of drainage will result in the need for chemotherapy in a woman with otherwise early-stage disease. If cancer is suspected, consider a vertical minilaparotomy, which allows easy extension into the upper abdomen should surgical staging or debulking become necessary.
Advantages and disadvantages. The abdominal approach offers the best exposure of the pelvic and upper abdominal cavity but is associated with a high rate of complications, including fever and excessive blood loss. We surmise that postoperative recovery with a mini-laparotomy is likely to be improved compared with the traditional abdominal technique.
Figure 1
Opt for abdominal hysterectomy when a considerably enlarged uterus due to numerous fibroids is encountered.
Vaginal hysterectomy
Patient selection. Choose the vaginal route when pelvic support defects are present (Figure 2), but bear in mind that the course of the ureter changes with worsening degrees of uterine prolapse. We have found the ureter to be palpable in the bladder pillar in most women undergoing VH for prolapse, which helps avoid intraoperative ureteral injury.
The ovaries can be safely removed through the vagina in a large percentage of patients.
Obese, elderly, and otherwise medically debilitated patients also will benefit from the vaginal approach. Avoiding an abdominal wound in these women has obvious advantages. Also, vaginal surgery is associated with a reduction in postoperative pulmonary complications when compared with AH.6,12
Vaginal accessibility inevitably influences the decision to proceed vaginally with a hysterectomy. An inadequate bony pelvis is reason to forgo a VH. Orthopedic conditions and muscular contractures of the lower extremities, which prevent safe positioning, also inhibit this approach. Some surgeons also consider prior abdominal or pelvic surgery a reason to avoid vaginal hysterectomy, while others find this route a means of eluding potential adhesions.
We are strong advocates of vaginal surgery and do not consider nulliparity, lack of uterine descent, or prior abdominopelvic surgery to be strict contraindications for VH. A Schuchardt incision may overcome a small introitus, and a mobile uterus often will descend as the uterosacral and cardinal ligaments are divided.
In women with a prior cesarean delivery, it may be easier to enter the vesicouterine space by approaching it from the less-scarred vaginal side. In a study of more than 200 patients with a previous cesarean who underwent VH, Sheth and Malpani found no increase in complications and concluded that VH is the route of choice in this patient population.13 When unsuspected pelvic adhesions are encountered, carefully dissect them transvaginally and complete the procedure.
Besides accessibility, uterine size must be considered. However, size alone should present a dilemma in only about 15% of patients since most hysterectomy specimens are 12 weeks’ gestational size or smaller.14
Technique. Large uteri can be removed vaginally, depending on the surgeon’s technical ability and experience. A familiarity with the various methods of morcellation—hemisection, posterior fundal morcellation, intramyometrial coring, and myomectomy—is mandatory. It often is beneficial to use a combination of these techniques to accomplish the procedure vaginally, as opposed to a single means of morcellation. With any morcellation procedure, maintain a midline orientation to avoid dissection into the broad ligament. Continued caudal traction also is helpful in eliminating excessive blood loss during this prolonged procedure. Do not morcellate a uterus when endometrial carcinoma is suspected.
In a prospective observational study comparing vaginal morcellation to AH, Hoffman et al15 found the former to be safer. Other researchers also have demonstrated the procedure’s safety in retrospective reports16-18 and as subgroups in other series.14 Alternatively, preoperative treatment with a gonadotropin-releasing hor-Surgical Techniques mone (GnRH) agonist can reduce uterine size and result in a technically less challenging VH in some women.19,20
Also, whether or not the ovaries will be resected may influence the decision to perform a VH. While some surgeons believe that oophorectomy precludes the vaginal approach, the ovaries can be safely removed through the vagina in a large percentage of patients.21-23 In fact, Sheth24 and Kovac14 have published 95% and 97% success rates in 2 separate series in which vaginal oophorectomy was attempted in 740 and 142 women, respectively. However, do not attempt adnexal removal without adequate transvaginal access and visibility, as it increases the risk of hemorrhage and ureteral injury. In these cases, LAVH or mini-laparotomy AH may be preferable if oophorectomy is mandatory, i.e., in women who are predisposed to ovarian malignancies because of genetic mutations. However, when patients are appropriately selected, there are essentially no disadvantages to VH.
Advantages and disadvantages.VH has many advantages over AH. Minimal intraperitoneal manipulation and the avoidance of an abdominal wound lead to a shorter hospital stay and decreased recovery time, which is coupled with less cost. Patients also find the lack of an abdominal scar to be an attractive feature of VH. A disadvantage is reduced operative exposure, making it difficult to manipulate pelvic pathology and resect the adnexa.
Figure 2
Choose the vaginal route when pelvic support defects are present, but bear in mind that the course of the ureter changes due to prolapse.
Laparoscopic-assisted vaginal hysterectomy
Patient selection. When in doubt about which approach to use, turn to LAVH. The laparoscope allows the surgeon to thoroughly assess intra-abdominal pathology, which aids in the appropriate selection of a hysterectomy route. For example, if cancer is suspected based on the visual inspection, opt for an AH. On the other hand, if only extensive adhesions are noted, laparoscopic adhesiolysis can be performed to allow the hysterectomy to be completed vaginally. As previously noted, LAVH is appropriate in patients undergoing hysterectomy with prophylactic oophorectomy due to genetic or familial risk factors.
Technique. Bear in mind that the laparoscope does not permit all patients to undergo a VH. In fact, about 10% of attempted LAVHs will be unsuccessful.25,26 Some authors have reported failure during the laparoscopic portion,25,26 while others have attributed the lack of success to the vaginal portion of the procedure.27 Thus, it is important to be skilled at both laparoscopic and vaginal surgery in order to be successful with an LAVH.
LAVH requires three to five 5-to 10-mm abdominal incisions for port sites. An umbilical port is commonly used for the camera, and the remaining ports are used for operating. We typically use 3 ports and avoid most disposable instruments by using cautery on vascular pedicles, which helps minimize costs.3 Other alternatives include the use of endoscopic staplers, the Harmonic Scalpel (Ethicon Endo-Surgery, a Johnson & Johnson company, Cincinnati, Ohio), and suture ligatures with extra-or intracorporeal knot tying. Whichever instrument is used, familiarize yourself with all available equipment given the occasional malfunction encountered during laparoscopy.
Advantages and disadvantages. LAVH requires special equipment and expertise beyond that needed for an abdominal or vaginal hysterectomy. Additionally, this procedure increases operative time, cost, and morbidity (depending on the surgeon’s experience),4,27,28 while the postoperative recovery is similar to that of VH.
Although we use the laparoscope infrequently and believe there is little need to perform excessive laparoscopy at the time of routine hysterectomy, we do not wish to understate the role of the laparoscope. Its use in specific instances can certainly avoid a laparotomy or help determine when an AH is more appropriate.
Conclusion
Emergent situations and patients with excessively enlarged uteri, significant pelvic pathology, or cancer are obvious candidates for AH. On the other hand, VH is frequently chosen for the small uterus in a multiparous woman with a large pelvis and no prior pelvic inflammatory disease or surgery. The dilemma arises when determining the approach for those patients with moderately enlarged uteri or presumptive risk factors for serious pelvic disease.
Kovac reported a standard protocol for selecting the route for hysterectomy. Uterine size, other pelvic pathology (endometriosis, adnexal disease, chronic pain, etc.), and uterine and adnexal accessibility (bony architecture, uterine support, and vaginal diameter) were each considered in the decision-making. A simplification of Kovac’s guidelines applicable to women undergoing hysterectomy for benign indications is summarized in Figure 3. Using these guidelines, Kovac reported a 99% (608/611) success rate for women assigned to VH or LAVH. The laparoscope was deemed necessary in only 19% of those assigned to the LAVH group, and ultimately only 9 patients required AH, yielding a 1:68 ratio for AH to VH.14
Gynecologists should seek alternatives to AH given its less favorable outcome in terms of morbidity and recovery. However, the surgeon who is competent with AH better serves the patient by performing the procedure via this route than by attempting an alternative procedure without the necessary proficiency. In other words, pelvic surgeons must be cognizant of their abilities and practice within that realm. Ultimately, the final selection of hysterectomy route should be based on the surgeon’s experience, the indication for surgery, and the patient’s anatomy.
FIGURE 3Guidelines to determine route of hysterectomy.
The authors report no financial relationship with any companies whose products are mentioned in this article.
1. Reich H, DeCaprio J, McGlynn F. Laparoscopic hysterectomy. J Gynecol Surg. 1989;5:213-216.
2. Dicker RC, Greenspan JR, Strauss LT, Cowart MR, Scally MJ, et al. Complications of abdominal and vaginal hysterectomy among women of reproductive age in the United States. Am J Obstet Gynecol. 1982;144:841-848.
3. Johns DA, Carrera B, Jones J, DeLeon F, Vincent R, et al. The medical and economic impact of laparoscopic-assisted vaginal hysterectomy in a large, metropolitan, not-for-profit hospital. Am J Obstet Gynecol. 1995;172:1709-719.
4. Meikle SF, Nugent EW, Orleans M. Complication and recovery for laparoscopic-assisted vaginal hysterectomy compared with abdominal and vaginal hysterectomy. Obstet Gynecol. 1997;89:304-311.
5. Kovac SR, Christie SJ, Bindbeutel GA. Abdominal versus vaginal hysterectomy: a statistical model for determining physician decision making and patient outcomes. Med Decis Making. 1991;11:19-28.
6. Dorsey JH, Steinberg EP, Holtz PM. Clinical indications for hysterectomy route: patient characteristics or physician preference. Am J Obstet Gynecol. 1995;173:1452-1460.
7. Wingo PA, Huezo CM, Rubin GL, Ory HW, Peterson HB. The mortality risk associated with hysterectomy. Am J Obstet Gynecol. 1985;152:803-808.
8. Tollefson DG, Russell KP. The transverse incision in pelvic surgery. Am J Obstet Gynecol. 1954;68:410-422.
9. Helmkamp BF. Abdominal wound dehiscence. Am J Obstet Gynecol. 1977;128:803-807.
10. Hoffman MS, Lynch CM. Minilaparotomy hysterectomy. Am J Obstet Gynecol. 1998;179:316-320.
11. Flynn M, Niloff JM. Minilaparotomy for the ambulatory management of ovarian cysts. Am J Obstet Gynecol. 1995;173:1727-1730.
12. White SC, Wartel LJ, Wade ME. Comparison of abdominal and vaginal hysterectomies. Obstet Gynecol. 1971;37:530-537.
13. Sheth SS, Malpani AN. Vaginal hysterectomy following previous cesarean section. Int J Gynaecol Obstet. 1995;50:65-69.
14. Kovac SR. Guidelines to determine route of hysterectomy. Obstet Gynecol. 1995;85:18-23.
15. Hoffman MS, DeCesare S, Kalter C. Abdominal hysterectomy versus transvaginal morcellation for the removal of enlarged uteri. Am J Obstet Gynecol. 1994;171:309-315.
16. Mazdisnian F, Kurzel RB, Coe S, Bosuk M, Montz F. Vaginal hysterectomy by uterine morcellation: an efficient, non-morbid procedure. Obstet Gynecol. 1995;86:60-64.
17. Kudo R, Yamauchi O, Okazaki T, Sagac S, Ito E, Hashimoto M. Vaginal hysterectomy without ligation of the ligaments of the cervix uteri. Surg Gynecol Obstet. 1990;70:299-305.
18. Kovac SR. Intramyometrial coring as an adjunct to vaginal hysterectomy. Obstet Gynecol. 1986;67:131-136.
19. Stovall TG, Summit RL, Washburn SA, Ling FW. Gonadotropin-releasing hormone agonist use before hysterectomy. Am J Obstet Gynecol. 1994;170:1744-1748.
20. Crosignani PG, Vercellini P, Meschia M, Oldani S, Bramante T. GnRH agonists before surgery for uterine leiomyomas. J Reprod Med. 1996;41:415-421.
21. Hoffman MS. Transvaginal removal of ovaries with endoloop sutures at the time of transvaginal hysterectomy. Am J Obstet Gynecol. 1991;165:407-408.
22. Sheth SS. Ovarian clamp for oophorectomy during vaginal hysterectomy. J Am Assoc Gynecol Laparosc. 1995;2(4):S76-S77.
23. Hefni MA, Davies AE. Vaginal endoscopic oophorectomy with vaginal hysterectomy: a simple minimal access surgery technique. Br J Obstet Gynaecol. 1997;104:621-622.
24. Sheth SS. The place of oophorectomy at vaginal hysterectomy. Br J Obstet Gynaecol. 1991;98:662-666.
25. Cristoforoni PM, Palmieri A, Gerbaldo D, Montz FJ. Frequency and cause of aborted laparoscopic-assisted vaginal hysterectomy. J Am Assoc Gynecol Laparosc. 1995;3:33-37.
26. Summitt RL, Stovall TG, Steege JF, Lipscomb GH. A multicenter randomized comparison of laparoscopic-assisted vaginal hysterectomy and abdominal hysterectomy in abdominal hysterectomy candidates. Obstet Gynecol. 1998;92:321-326.
27. Dorsey JH, Holtz PM, Griffiths RI, McGrath MM, Steinberg EP. Costs and charges associated with three alternative techniques of hysterectomy. N Engl J Med. 1996;335:476-482.
28. Summitt RL, Stovall TG, Lipscomb GH, Ling FW. Randomized comparison of laparoscopic-assisted vaginal hysterectomy with standard vaginal hysterectomy in an outpatient setting. Obstet Gynecol. 1992;80:895-901.
1. Reich H, DeCaprio J, McGlynn F. Laparoscopic hysterectomy. J Gynecol Surg. 1989;5:213-216.
2. Dicker RC, Greenspan JR, Strauss LT, Cowart MR, Scally MJ, et al. Complications of abdominal and vaginal hysterectomy among women of reproductive age in the United States. Am J Obstet Gynecol. 1982;144:841-848.
3. Johns DA, Carrera B, Jones J, DeLeon F, Vincent R, et al. The medical and economic impact of laparoscopic-assisted vaginal hysterectomy in a large, metropolitan, not-for-profit hospital. Am J Obstet Gynecol. 1995;172:1709-719.
4. Meikle SF, Nugent EW, Orleans M. Complication and recovery for laparoscopic-assisted vaginal hysterectomy compared with abdominal and vaginal hysterectomy. Obstet Gynecol. 1997;89:304-311.
5. Kovac SR, Christie SJ, Bindbeutel GA. Abdominal versus vaginal hysterectomy: a statistical model for determining physician decision making and patient outcomes. Med Decis Making. 1991;11:19-28.
6. Dorsey JH, Steinberg EP, Holtz PM. Clinical indications for hysterectomy route: patient characteristics or physician preference. Am J Obstet Gynecol. 1995;173:1452-1460.
7. Wingo PA, Huezo CM, Rubin GL, Ory HW, Peterson HB. The mortality risk associated with hysterectomy. Am J Obstet Gynecol. 1985;152:803-808.
8. Tollefson DG, Russell KP. The transverse incision in pelvic surgery. Am J Obstet Gynecol. 1954;68:410-422.
9. Helmkamp BF. Abdominal wound dehiscence. Am J Obstet Gynecol. 1977;128:803-807.
10. Hoffman MS, Lynch CM. Minilaparotomy hysterectomy. Am J Obstet Gynecol. 1998;179:316-320.
11. Flynn M, Niloff JM. Minilaparotomy for the ambulatory management of ovarian cysts. Am J Obstet Gynecol. 1995;173:1727-1730.
12. White SC, Wartel LJ, Wade ME. Comparison of abdominal and vaginal hysterectomies. Obstet Gynecol. 1971;37:530-537.
13. Sheth SS, Malpani AN. Vaginal hysterectomy following previous cesarean section. Int J Gynaecol Obstet. 1995;50:65-69.
14. Kovac SR. Guidelines to determine route of hysterectomy. Obstet Gynecol. 1995;85:18-23.
15. Hoffman MS, DeCesare S, Kalter C. Abdominal hysterectomy versus transvaginal morcellation for the removal of enlarged uteri. Am J Obstet Gynecol. 1994;171:309-315.
16. Mazdisnian F, Kurzel RB, Coe S, Bosuk M, Montz F. Vaginal hysterectomy by uterine morcellation: an efficient, non-morbid procedure. Obstet Gynecol. 1995;86:60-64.
17. Kudo R, Yamauchi O, Okazaki T, Sagac S, Ito E, Hashimoto M. Vaginal hysterectomy without ligation of the ligaments of the cervix uteri. Surg Gynecol Obstet. 1990;70:299-305.
18. Kovac SR. Intramyometrial coring as an adjunct to vaginal hysterectomy. Obstet Gynecol. 1986;67:131-136.
19. Stovall TG, Summit RL, Washburn SA, Ling FW. Gonadotropin-releasing hormone agonist use before hysterectomy. Am J Obstet Gynecol. 1994;170:1744-1748.
20. Crosignani PG, Vercellini P, Meschia M, Oldani S, Bramante T. GnRH agonists before surgery for uterine leiomyomas. J Reprod Med. 1996;41:415-421.
21. Hoffman MS. Transvaginal removal of ovaries with endoloop sutures at the time of transvaginal hysterectomy. Am J Obstet Gynecol. 1991;165:407-408.
22. Sheth SS. Ovarian clamp for oophorectomy during vaginal hysterectomy. J Am Assoc Gynecol Laparosc. 1995;2(4):S76-S77.
23. Hefni MA, Davies AE. Vaginal endoscopic oophorectomy with vaginal hysterectomy: a simple minimal access surgery technique. Br J Obstet Gynaecol. 1997;104:621-622.
24. Sheth SS. The place of oophorectomy at vaginal hysterectomy. Br J Obstet Gynaecol. 1991;98:662-666.
25. Cristoforoni PM, Palmieri A, Gerbaldo D, Montz FJ. Frequency and cause of aborted laparoscopic-assisted vaginal hysterectomy. J Am Assoc Gynecol Laparosc. 1995;3:33-37.
26. Summitt RL, Stovall TG, Steege JF, Lipscomb GH. A multicenter randomized comparison of laparoscopic-assisted vaginal hysterectomy and abdominal hysterectomy in abdominal hysterectomy candidates. Obstet Gynecol. 1998;92:321-326.
27. Dorsey JH, Holtz PM, Griffiths RI, McGrath MM, Steinberg EP. Costs and charges associated with three alternative techniques of hysterectomy. N Engl J Med. 1996;335:476-482.
28. Summitt RL, Stovall TG, Lipscomb GH, Ling FW. Randomized comparison of laparoscopic-assisted vaginal hysterectomy with standard vaginal hysterectomy in an outpatient setting. Obstet Gynecol. 1992;80:895-901.