User login
- The goal of hysteroscopic myomectomy is complete removal of the fibroid without trauma to normal uterine tissue.
- Patients with Type 0 and Type I fibroids often require only 1 surgery; patients with Type II fibroids should be advised that 2 surgeries may be needed to remove the entire fibroid.
- Adjuvant preoperative hormonal therapy facilitates surgical scheduling, helps prevent further blood loss in patients already suffering from anemia, and reduces distention media intravasation.
- The monopolar loop electrode is the fibroid removal system that is used most often.
Hysteroscopic myomectomy should be offered to all patients with symptomatic submucous fibroids who desire to avoid hysterectomy. Although it is a highly effective, minimally invasive technique, it is underutilized.
Unfortunately, fewer than one third of US gynecologists perform this procedure. In a 1997 survey of members of the American Association of Gynecologic Laparoscopists—an organization committed to minimally invasive surgery—only half of the respondents reported that they perform this surgery.1
The reasons for learning to perform hysteroscopic myomectomy are compelling:
- A large cohort of patients could benefit, since most heavy vaginal bleeding from fibroids is due to the submucous location, and hysteroscopic resection is a much more benign approach than hysterectomy. Symptomatic fibroids account for 27% of all hysterectomies performed in the US (the largest single diagnostic category) and more than 100,000 are performed for fibroids that cause abnormal uterine bleeding.2
- Removing these lesions hysteroscopically greatly improves prognosis in women with recurrent pregnancy loss and infertility due to submucous fibroids.3 Up to 15% of patients presenting with infertility have otherwise asymptomatic uterine defects, including submucous fibroids. For example, a meta-analysis of patients undergoing in vitro fertilization determined that, compared with controls, the relative risk of pregnancy for women with submucous fibroids was 0.32 (95% confidence interval [CI], 0.13–0.70). When the submucous fibroids were resected, the relative risk of pregnancy rose to 1.72 (CI, 1.13–2.58).3
Preoperative evaluation of fibroids
Severity of menorrhagia (the most common symptom) is considered directly related to the volume of the myoma within the endometrial cavity. It is not uncommon to see large tortuous vessels covering the surface of the fibroids; although the exact mechanism of fibroid-related menorrhagia is undetermined, the fragility of these vessels is probably responsible, at least in part.
Additionally, fibroids involving the uterine mucosa or submucosa may interfere with the muscular contraction necessary for hemostasis.
Surgical options and pretreatment depend on fibroid type. Submucous fibroids are classified according to the percentage of the fibroid within the endometrial cavity:4
- Type 0: pedunculated; 100% within the cavity
- Type I: more than 50% within the cavity
- Type II: more than 50% within the myometrium
The type dictates surgical options, determines endometrial pretreatment, and shapes patient expectations. Type 0 and Type I submucosal fibroids are more successfully removed in a single surgery, whereas Type II submucous fibroids usually require 2 procedures for complete removal.5
My preference is office hysteroscopy to evaluate the endometrial cavity combined with vaginal ultrasound to assess intramural disease. The view with the office hysteroscope is the same view you will have during surgery.
Patients with Type II myomas should be informed of the potential need for 2 procedures, as this fact often influences their treatment decisions. In addition, whenever a patient with a Type II myoma is pretreated with a gonadotropin-releasing hormone (GnRH) agonist, the physician should reassess the fibroid preoperatively to ensure that it has not become completely intramural.
Office assessment: Hysteroscopy plus ultrasound. Preoperative assessment can be achieved with a hysterosalpingogram, vaginal ultrasound, hysterosonogram, or office hysteroscopy.
Method. My preference is office hysteroscopy to evaluate the endometrial cavity combined with vaginal ultrasound to assess intramural disease. The view with the office hysteroscope is the same view you will have during surgery. There will be no surprises. With a small flexible hysteroscope using saline for distention, the procedure is done with no tenaculum, no paracervical block, and can be completed with 60 to 100 cc of fluid in less than 1 minute.
Patients are often intrigued to view the myoma responsible for their heavy vaginal bleeding.
Several advantages of preoperative hormonal therapy
Preoperative hormonal therapy has several advantages:
- Since it is best to resect submucous myomas when the endometrium is thin, hormonal therapy facilitates surgical scheduling.
- Since preoperative therapy creates a state of amenorrhea, it enables patients suffering from menorrhagia and anemia to build up their blood counts, reducing the need for transfusion.
- Most importantly, preoperative therapy can reduce blood flow to the uterus, thereby reducing the rate of fluid intravasation.
Adjuvants used for these functions include oral contraceptives, progestogens, danazol, and GnRH agonists. However, a recent Cochrane review suggests that only GnRH agonists reduce fluid absorption during operative hysteroscopy.6
Method. Numerous protocols have been published, but my preference is to administer 1 intramuscular injection of depot leuprolide acetate (7.5 mg) 6 weeks prior to surgery.
A review of ‘systems’
Submucous myomas have been hysteroscopically removed using the neodymium/yttrium aluminum-garnet (Nd:YAG) laser, a monopolar resectoscope loop, a monopolar radiofrequency (RF) vaporizing electrode, a bipolar RF vaporizing electrode, and a bipolar RF loop.
Laser. The Nd:YAG laser is an expensive device that was popular in the late 1980s and early 1990s. It can be used as a cutting tool for pedunculated Type 0 myomas, or it can be used for myolysis by burning numerous holes in the myoma, causing devascularization and shrinkage.7,8
The sole advantage of the Nd:YAG laser is that it can be used in an isotonic-fluid-filled cavity. Now that accurate fluid-monitoring devices are available, the Nd:YAG laser is rarely used for this indication.
Vaporizing electrodes. Both the bipolar system and monopolar vaporizing electrodes utilize very high RF power (200 W to 300 W) to vaporize fibroids. The advantage is that the fibroids are eradicated very quickly, without any bothersome fibroid chips to remove. The bipolar system can be used with isotonic fluid, whereas the monopolar vaporizing electrodes require non-electrolyte-containing distention media.
The main disadvantages of vaporizing electrodes are:
- They do not produce a tissue sample for pathology. While uterine sarcomas are very rare, they are not homogeneous. Therefore, a simple sample prior to vaporization does not rule out the disease.
- Since vaporizing electrodes are used at high power, numerous gas bubbles are produced and enter the vascular system. Fortunately, these bubbles dissipate rapidly in the blood. As long as the rate of formation does not exceed the rate of dissipation, there are no significant clinical sequelae. The surgeon can avoid complications by monitoring the patient’s endtidal CO2 and maintaining communication with the anesthesiologist. If a sudden drop in endtidal CO2 is observed, the surgeon should stop the case until it resolves.9
Monopolar loop electrode. The instrument most commonly used to remove submucosal myomas is the monopolar loop electrode with a continuous-flow resectoscope. This is quite similar to the instrument used to resect the prostate; it has been slightly modified for gynecologic use to provide enhanced fluid inflow and outflow. A key instrument now considered standard is an accurate fluid-monitoring device that can be attached to the resectoscope.
Distention media for monopolar resection. Prior to the introduction of continuous high-flow resectoscopes and fluid-management systems, monopolar resectoscopy could only be performed using a solution of 32% dextran 70 and water because of that formulation’s viscosity and facilitation of visualization in the presence of blood. However, due to complications related to absorption, allergies, and the solution’s sticky residue, it is not used with today’s high-flow technology.
The instrument most commonly used to remove submucosal myomas is the monopolar loop electrode with a continuous-flow resectoscope.
When using nonviscous fluids with monopolar RF energy, a distention medium without electrolytes must be selected. Isotonic physiologic media such as normal saline or lactated Ringer’s solution would cause the energy at the electrode to disperse, eliminating the cutting effect. Contrary to popular belief, there would be no burning of the uterine cavity and no subsequent danger to the patient.
The 3 most commonly used fluids for uterine distention are 1.5% glycine, 3% sorbitol, and 5% mannitol. All 3 lack electrolytes. The glycine and sorbitol solutions are hypotonic, while 5% mannitol is isotonic. Although there is some debate over whether an isotonic non-electrolyte-containing medium is safer than a hypotonic one, the guidelines for fluid monitoring are the same for all 3.
The goal of a hysteroscopic myomectomy is to alleviate symptoms without causing a weak myometrium or intracavitary synechia. This is accomplished by removing the fibroid without traumatizing normal uterine tissue.
As a general rule, the serum sodium level will decrease approximately 10 meq for every liter absorbed in the blood stream. That is why, to predict hyponatremia, the gynecologist must rely on exact measurements of fluid deficits rather than operating time or total volume used.
Fluid management. Significant hyponatremia can result in pulmonary edema, transient blindness, cerebral edema, brainstem herniation, and death. Catastrophic as these complications are, they are almost 100% avoidable with accurate deficit measurements and adherence to sound fluid-management protocols.
At our institution, fluids are monitored using a weighted system, and the fluid deficit is displayed on the surgical screen in real time. When the deficit reaches 1 L, electrolytes are drawn, the patient is given 10 mg of intravenous (IV) furosemide, and attempts are made to finish the surgery as soon as possible. When the deficit reaches 1.5 L, the surgery is discontinued no matter how much or how little remains to complete it.
It is important that the protocol at each institution be agreed upon by the chief of service and anesthesia as well as the director of nursing so that there are no conflicts at the time of surgery.
Uterine distention methods
Numerous methods have been used to distend the uterus: gravity, gas pressure, and electronic pumps and pressure bags. The optimal intrauterine pressure is the minimum pressure that allows for flow, distention, and visualization. Most of the time this ranges from 40 mm Hg to 60 mm Hg. The higher the intrauterine pressure, the more rapid the fluid intravasation, particularly when intrauterine pressure exceeds the mean arterial pressure.
Rapid fluid loss can occur even with low intrauterine pressures. That is because a large venous sinus will have a pressure of 8 mm Hg to 10 mm Hg, while the minimum uterine distention pressure is much greater. Strict adherence to fluid-deficit protocols will minimize the risk of complications from fluid overload.
Surgical technique
The goal of a hysteroscopic myomectomy is to alleviate symptoms without causing a weak myometrium or intracavitary synechia. Ideally, this is accomplished by completely removing the fibroid without traumatizing normal uterine tissue. Removal of the entire myoma reduces the likelihood of recurrence and regrowth.
Surgical technique for removal of Types 0, I, and II fibroids is illustrated and explained on (FIGURES1-4).
Within a given range (40 W to 100 W), it makes little difference which cutting-current setting on the RF generator you use; the principles are the same. The loop must be in contact with the tissue to be resected. Energy is applied and, once the circuit is completed (through tissue, the dispersive electrode, the generator and back to the loop), the loop is able to cut with minimal tactile feedback.
The lower the wattage, the more contact necessary between the loop and the tissue and the longer it takes to complete the circuit. Conversely, the higher the power, the faster the circuit is completed and cutting occurs.
Though it does not matter what setting is used, the operator must choose a setting based on his or her comfort level. Lower power gives the operator more control. Higher power should be used only by the most experienced hysteroscopist, since it involves very little tactile feedback and a higher risk of uterine perforation. At our teaching institution, we use 60 W of pure cutting current.
The technique for removing a submucous fibroid depends on its type and location within the endometrial cavity.
Type 0. Because these fibroids are pedunculated, the operator has the option of cutting the stalk (FIGURE 1) or shaving the myoma to remove it in pieces through the cervix. If the stalk is cut, it often is difficult to remove the fibroid through the cervix due to its large size. Nor can the fibroid be cut with monopolar energy because of the difficulty of completing the circuit. Type 0 myomas can be grabbed blindly with a Corson forceps or under direct visualization with an Isaacson optical tenaculum (Karl Storz Endoscopy, Culver City, Calif.). It also is acceptable to leave the fibroid in the cavity and let it degenerate and be expelled spontaneously. The risk of infection is very small.
My preference is to shave the fibroid down to the endometrium while it is attached to the stalk. I find it easier to remove when it is anchored rather than trying to pull out a single large specimen.
Type I. In a Type I myoma, more than 50% of the fibroid is within the uterine cavity, with a smaller portion embedded in the myometrium. To remove these fibroids, shave each to the level of the endometrium. To do so, place the loop behind the fibroid to be resected (FIGURE 5). Next, activate the RF energy using a foot pedal and, once the circuit is complete, draw the loop back into the resectoscope. Be careful to maintain visualization of the fibroid, loop, and cavity (FIGURE 6). This becomes difficult as the fibroid approaches the resectoscope and obstructs the hysteroscopic lens. To avoid this, bring the loop—which begins its cut fully extended—only halfway back to the resectoscope (FIGURE 7). Then retract the resectoscope itself, with the loop halfway extended, until the cut through the fibroid is complete (FIGURE 8). The loop then is retracted back into the resectoscope. The angle of the resectoscope must be adjusted to maintain loop contact with the fibroid tissue.
When more than 70% or 80% of the fibroid has been resected, the remainder will slough spontaneously over the next 2 to 4 months.
Once the fibroid is resected to the level of the endometrium, remove the fibroid chips (FIGURE 2). Often, when the hysteroscope is removed and uterine distention is reestablished, more of the fibroid will protrude into the cavity, facilitating safe resection. At some point, the fibroid will no longer protrude into the cavity; it will have been removed or only a small percentage will remain intramural.
Often, when more than 70% or 80% of the fibroid has been resected, the remainder will slough spontaneously over the next 2 to 4 months. The technique for removing the intramural fibroid is described on page 78 (FIGURE 3).
Type II. The first step in resecting a Type II myoma is shaving the intracavitary portion to the level of the endometrium, as in the resection of a Type I fibroid. Often, once a small Type II myoma is “unroofed,” the remainder will spontaneously fall into the cavity and can easily be removed.
Larger Type II myomas require a different approach. In these, the goal is to identify the pseudo-capsular plane that allows for blunt dissection. Once you have identified it, use the loop electrode to bluntly dissect (without RF activation) the fibroid from the myometrium. Place the electrode in the capsular plane and retract as much of the myoma into the cavity as possible (FIGURE 4). Another way to do this is to use a twisting motion—similar to those performed in open myomectomies—with the optical tenaculum. Once the fibroid is in the cavity, shave it to the level of the endometrium. Repeat this technique until you have removed as much of the fibroid as possible. Use minimal distention pressure when you perform this technique so that the fibroid will not be pressed back into the myometrium.
Underutilization of hysteroscopic myomectomy may be due to inadequate training in the technique or a misconception that it is difficult to learn. Fortunately, hysteroscope manufacturers are beginning to offer physicians and operating room personnel didactic and hands-on laboratory courses.
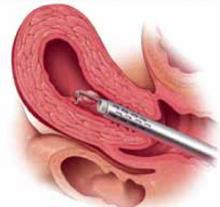
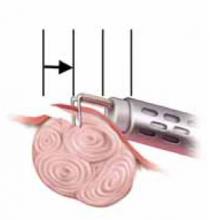
FIGURE 1 Surgical technique for removing Type 0, I, II fibroids
Type 0. The loop is placed behind the fibroid. Once activated, the loop is drawn toward the operator to resect the pedicle.
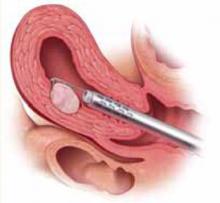
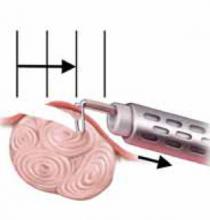
FIGURE 2 Surgical technique for removing Type 0, I, II fibroids
Type I. The myoma is resected to the level of the myometrium.
FIGURE 3 Surgical technique for removing Type 0, I, II fibroids
Resection of intramural portion of Type I and II. The resectoscopic loop is placed in the pseudocapsular lane between the fibroid and the myometrium to bluntly dissect the fibroid into the cavity for further resection.
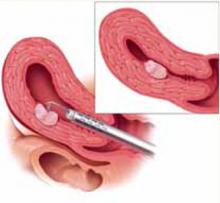
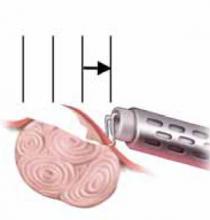
FIGURE 4 Surgical technique for removing Type 0, I, II fibroids
Type II. The roof of the myoma is shaved off to the level of the myometrium. The insert demonstrates the intramural portion of the myoma that will protrude into the cavity with a reduction in uterine distension pressure.
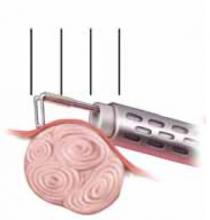
FIGURE 5 Resect technique—4 steps in resecting a submucous myoma
The loop is placed behind the fibroid with maximal contact with the tissue before RF activation.
FIGURE 6 Resect technique—4 steps in resecting a submucous myoma
The loop is withdrawn toward the operator (see arrow) while cutting the myoma but not so far as to obstruct the hysteroscopic view.
FIGURE 7 Resect technique—4 steps in resecting a submucous myoma
The loop is held in place while the hysteroscope is withdrawn (arrows indicate how to remain in direct contact with myoma) continuing to cut the myoma with visualization.
FIGURE 8 Resect technique—4 steps in resecting a submucous myoma
Once through the myoma, the loop is withdrawn into the hysteroscope.
Management of complications
The primary complications of hysteroscopic myomectomy are bleeding, infection, uterine perforation, and fluid overload.
Bleeding. If postoperative hemorrhage occurs, use a 30-cc Foley balloon to tamponade the vessels, or pack the uterus with gauze soaked in a vasopressin solution. Both can be removed any time from 4 hours to 24 hours following placement. If bleeding persists, uterine artery embolization should be considered prior to hysterectomy.
Most attempts at controlling bleeding with a rollerball are futile because the intrauterine distention pressure necessary to see the venous bleeders hides the actual bleeding. Arterial bleeders can be seen and controlled with the rollerball, but they are much less common.
Infection. Because infection is uncommon with hysteroscopic surgery, routine prophylaxis is unnecessary.
Antibiotics should be used if the patient had cervical laminaria placed preoperatively.
Uterine perforation. The management of uterine perforation depends on the site of perforation and whether energy was used during the perforation. For example, if the perforation was at the fundus and occurred with a blunt, non-energized instrument, observation without laparoscopy is sufficient. If the perforation was lateral or occurred with RF energy, laparoscopy is suggested to rule out a broad ligament hematoma or injury to intraperitoneal vessels and viscera. If the perforation is anterior, a cystoscopy is recommended; internal colon inspection is necessary if the perforation is posterior.
Fluid overload. Complications and prevention can be avoided and treated as previously discussed.
New training opportunities may include virtual reality
Underutilization of hysteroscopic myomectomy may be due to inadequate training in the technique during residency or the misconception that it is difficult to learn. (Training has been further hampered by the lack of a suitable animal model.)
Fortunately, hysteroscope manufacturers are beginning to offer physicians and operating room personnel didactic and hands-on laboratory courses. In the near future, we hope, these companies will hire gynecologic hysteroscopists to support physicians and nurses in the field, as has been done in many other specialties.
The creation of new tools such as virtual-reality simulators will undoubtedly enhance the teaching of operative hysteroscopy.
Dr. Isaacson teaches hysteroscopy courses sponsored by Karl Storz Endoscopy.
1. Hulka JF, Levy BS, Luciano AA, Parker WH, Phillips JM. 1997 AAGL membership survey: practice profiles. J Amer Assoc Gynecol Laparosc. 1998;5:93-96.
2. Carlson KJ, Nichols DH, Schiff I. Indications for hysterectomy. N Eng J Med. 1993;328:856-860.
3. Pritts E. Fibroids and infertility: a systematic review of the evidence. Obstet Gynecol Survey. 2001;56:483-491.
4. deBlok S, Dijkman AB, Hemrika DJ. Transcervical resection of fibroids (TCRM): results related to hysteroscopic classification. Gynaecol Endosc. 1995;4:243-246.
5. Istre O. Transcervical resection of endometrium and fibroids: the outcome of 412 operations performed over 5 years. Acta Obstet Gynecol Scand. 1996;75:567-574.
6. Sowter MC, Lethaby A, Singla AA. Preoperative endometrial thinning agents before hysteroscopic surgery for heavy menstrual bleeding. Cochrane Database Syst Rev. 2002;(3):CD001124.-
7. Baggish MS, Sze EM, Morgan G. Hysteroscopic treatment of symptomatic submucous myomas with the Nd:YAG laser. J Gynecol Surg. 1989;5:27-36.
8. Donnez J, Gillerot S, Bourgonjon D, et al. Neodymium:YAG laser hysteroscopy in large submucous fibroids. Fertil Steril. 1990;54:999-1003.
9. Bloomstone J, Chen C, Isselbacher E, Isaacson K. A pilot study examining the frequency and quantity of gas embolization during operative hysteroscopy using a monopolar resectoscope. J Am Assoc Gynecol Laparosc. 2002;9:9-14.
- The goal of hysteroscopic myomectomy is complete removal of the fibroid without trauma to normal uterine tissue.
- Patients with Type 0 and Type I fibroids often require only 1 surgery; patients with Type II fibroids should be advised that 2 surgeries may be needed to remove the entire fibroid.
- Adjuvant preoperative hormonal therapy facilitates surgical scheduling, helps prevent further blood loss in patients already suffering from anemia, and reduces distention media intravasation.
- The monopolar loop electrode is the fibroid removal system that is used most often.
Hysteroscopic myomectomy should be offered to all patients with symptomatic submucous fibroids who desire to avoid hysterectomy. Although it is a highly effective, minimally invasive technique, it is underutilized.
Unfortunately, fewer than one third of US gynecologists perform this procedure. In a 1997 survey of members of the American Association of Gynecologic Laparoscopists—an organization committed to minimally invasive surgery—only half of the respondents reported that they perform this surgery.1
The reasons for learning to perform hysteroscopic myomectomy are compelling:
- A large cohort of patients could benefit, since most heavy vaginal bleeding from fibroids is due to the submucous location, and hysteroscopic resection is a much more benign approach than hysterectomy. Symptomatic fibroids account for 27% of all hysterectomies performed in the US (the largest single diagnostic category) and more than 100,000 are performed for fibroids that cause abnormal uterine bleeding.2
- Removing these lesions hysteroscopically greatly improves prognosis in women with recurrent pregnancy loss and infertility due to submucous fibroids.3 Up to 15% of patients presenting with infertility have otherwise asymptomatic uterine defects, including submucous fibroids. For example, a meta-analysis of patients undergoing in vitro fertilization determined that, compared with controls, the relative risk of pregnancy for women with submucous fibroids was 0.32 (95% confidence interval [CI], 0.13–0.70). When the submucous fibroids were resected, the relative risk of pregnancy rose to 1.72 (CI, 1.13–2.58).3
Preoperative evaluation of fibroids
Severity of menorrhagia (the most common symptom) is considered directly related to the volume of the myoma within the endometrial cavity. It is not uncommon to see large tortuous vessels covering the surface of the fibroids; although the exact mechanism of fibroid-related menorrhagia is undetermined, the fragility of these vessels is probably responsible, at least in part.
Additionally, fibroids involving the uterine mucosa or submucosa may interfere with the muscular contraction necessary for hemostasis.
Surgical options and pretreatment depend on fibroid type. Submucous fibroids are classified according to the percentage of the fibroid within the endometrial cavity:4
- Type 0: pedunculated; 100% within the cavity
- Type I: more than 50% within the cavity
- Type II: more than 50% within the myometrium
The type dictates surgical options, determines endometrial pretreatment, and shapes patient expectations. Type 0 and Type I submucosal fibroids are more successfully removed in a single surgery, whereas Type II submucous fibroids usually require 2 procedures for complete removal.5
My preference is office hysteroscopy to evaluate the endometrial cavity combined with vaginal ultrasound to assess intramural disease. The view with the office hysteroscope is the same view you will have during surgery.
Patients with Type II myomas should be informed of the potential need for 2 procedures, as this fact often influences their treatment decisions. In addition, whenever a patient with a Type II myoma is pretreated with a gonadotropin-releasing hormone (GnRH) agonist, the physician should reassess the fibroid preoperatively to ensure that it has not become completely intramural.
Office assessment: Hysteroscopy plus ultrasound. Preoperative assessment can be achieved with a hysterosalpingogram, vaginal ultrasound, hysterosonogram, or office hysteroscopy.
Method. My preference is office hysteroscopy to evaluate the endometrial cavity combined with vaginal ultrasound to assess intramural disease. The view with the office hysteroscope is the same view you will have during surgery. There will be no surprises. With a small flexible hysteroscope using saline for distention, the procedure is done with no tenaculum, no paracervical block, and can be completed with 60 to 100 cc of fluid in less than 1 minute.
Patients are often intrigued to view the myoma responsible for their heavy vaginal bleeding.
Several advantages of preoperative hormonal therapy
Preoperative hormonal therapy has several advantages:
- Since it is best to resect submucous myomas when the endometrium is thin, hormonal therapy facilitates surgical scheduling.
- Since preoperative therapy creates a state of amenorrhea, it enables patients suffering from menorrhagia and anemia to build up their blood counts, reducing the need for transfusion.
- Most importantly, preoperative therapy can reduce blood flow to the uterus, thereby reducing the rate of fluid intravasation.
Adjuvants used for these functions include oral contraceptives, progestogens, danazol, and GnRH agonists. However, a recent Cochrane review suggests that only GnRH agonists reduce fluid absorption during operative hysteroscopy.6
Method. Numerous protocols have been published, but my preference is to administer 1 intramuscular injection of depot leuprolide acetate (7.5 mg) 6 weeks prior to surgery.
A review of ‘systems’
Submucous myomas have been hysteroscopically removed using the neodymium/yttrium aluminum-garnet (Nd:YAG) laser, a monopolar resectoscope loop, a monopolar radiofrequency (RF) vaporizing electrode, a bipolar RF vaporizing electrode, and a bipolar RF loop.
Laser. The Nd:YAG laser is an expensive device that was popular in the late 1980s and early 1990s. It can be used as a cutting tool for pedunculated Type 0 myomas, or it can be used for myolysis by burning numerous holes in the myoma, causing devascularization and shrinkage.7,8
The sole advantage of the Nd:YAG laser is that it can be used in an isotonic-fluid-filled cavity. Now that accurate fluid-monitoring devices are available, the Nd:YAG laser is rarely used for this indication.
Vaporizing electrodes. Both the bipolar system and monopolar vaporizing electrodes utilize very high RF power (200 W to 300 W) to vaporize fibroids. The advantage is that the fibroids are eradicated very quickly, without any bothersome fibroid chips to remove. The bipolar system can be used with isotonic fluid, whereas the monopolar vaporizing electrodes require non-electrolyte-containing distention media.
The main disadvantages of vaporizing electrodes are:
- They do not produce a tissue sample for pathology. While uterine sarcomas are very rare, they are not homogeneous. Therefore, a simple sample prior to vaporization does not rule out the disease.
- Since vaporizing electrodes are used at high power, numerous gas bubbles are produced and enter the vascular system. Fortunately, these bubbles dissipate rapidly in the blood. As long as the rate of formation does not exceed the rate of dissipation, there are no significant clinical sequelae. The surgeon can avoid complications by monitoring the patient’s endtidal CO2 and maintaining communication with the anesthesiologist. If a sudden drop in endtidal CO2 is observed, the surgeon should stop the case until it resolves.9
Monopolar loop electrode. The instrument most commonly used to remove submucosal myomas is the monopolar loop electrode with a continuous-flow resectoscope. This is quite similar to the instrument used to resect the prostate; it has been slightly modified for gynecologic use to provide enhanced fluid inflow and outflow. A key instrument now considered standard is an accurate fluid-monitoring device that can be attached to the resectoscope.
Distention media for monopolar resection. Prior to the introduction of continuous high-flow resectoscopes and fluid-management systems, monopolar resectoscopy could only be performed using a solution of 32% dextran 70 and water because of that formulation’s viscosity and facilitation of visualization in the presence of blood. However, due to complications related to absorption, allergies, and the solution’s sticky residue, it is not used with today’s high-flow technology.
The instrument most commonly used to remove submucosal myomas is the monopolar loop electrode with a continuous-flow resectoscope.
When using nonviscous fluids with monopolar RF energy, a distention medium without electrolytes must be selected. Isotonic physiologic media such as normal saline or lactated Ringer’s solution would cause the energy at the electrode to disperse, eliminating the cutting effect. Contrary to popular belief, there would be no burning of the uterine cavity and no subsequent danger to the patient.
The 3 most commonly used fluids for uterine distention are 1.5% glycine, 3% sorbitol, and 5% mannitol. All 3 lack electrolytes. The glycine and sorbitol solutions are hypotonic, while 5% mannitol is isotonic. Although there is some debate over whether an isotonic non-electrolyte-containing medium is safer than a hypotonic one, the guidelines for fluid monitoring are the same for all 3.
The goal of a hysteroscopic myomectomy is to alleviate symptoms without causing a weak myometrium or intracavitary synechia. This is accomplished by removing the fibroid without traumatizing normal uterine tissue.
As a general rule, the serum sodium level will decrease approximately 10 meq for every liter absorbed in the blood stream. That is why, to predict hyponatremia, the gynecologist must rely on exact measurements of fluid deficits rather than operating time or total volume used.
Fluid management. Significant hyponatremia can result in pulmonary edema, transient blindness, cerebral edema, brainstem herniation, and death. Catastrophic as these complications are, they are almost 100% avoidable with accurate deficit measurements and adherence to sound fluid-management protocols.
At our institution, fluids are monitored using a weighted system, and the fluid deficit is displayed on the surgical screen in real time. When the deficit reaches 1 L, electrolytes are drawn, the patient is given 10 mg of intravenous (IV) furosemide, and attempts are made to finish the surgery as soon as possible. When the deficit reaches 1.5 L, the surgery is discontinued no matter how much or how little remains to complete it.
It is important that the protocol at each institution be agreed upon by the chief of service and anesthesia as well as the director of nursing so that there are no conflicts at the time of surgery.
Uterine distention methods
Numerous methods have been used to distend the uterus: gravity, gas pressure, and electronic pumps and pressure bags. The optimal intrauterine pressure is the minimum pressure that allows for flow, distention, and visualization. Most of the time this ranges from 40 mm Hg to 60 mm Hg. The higher the intrauterine pressure, the more rapid the fluid intravasation, particularly when intrauterine pressure exceeds the mean arterial pressure.
Rapid fluid loss can occur even with low intrauterine pressures. That is because a large venous sinus will have a pressure of 8 mm Hg to 10 mm Hg, while the minimum uterine distention pressure is much greater. Strict adherence to fluid-deficit protocols will minimize the risk of complications from fluid overload.
Surgical technique
The goal of a hysteroscopic myomectomy is to alleviate symptoms without causing a weak myometrium or intracavitary synechia. Ideally, this is accomplished by completely removing the fibroid without traumatizing normal uterine tissue. Removal of the entire myoma reduces the likelihood of recurrence and regrowth.
Surgical technique for removal of Types 0, I, and II fibroids is illustrated and explained on (FIGURES1-4).
Within a given range (40 W to 100 W), it makes little difference which cutting-current setting on the RF generator you use; the principles are the same. The loop must be in contact with the tissue to be resected. Energy is applied and, once the circuit is completed (through tissue, the dispersive electrode, the generator and back to the loop), the loop is able to cut with minimal tactile feedback.
The lower the wattage, the more contact necessary between the loop and the tissue and the longer it takes to complete the circuit. Conversely, the higher the power, the faster the circuit is completed and cutting occurs.
Though it does not matter what setting is used, the operator must choose a setting based on his or her comfort level. Lower power gives the operator more control. Higher power should be used only by the most experienced hysteroscopist, since it involves very little tactile feedback and a higher risk of uterine perforation. At our teaching institution, we use 60 W of pure cutting current.
The technique for removing a submucous fibroid depends on its type and location within the endometrial cavity.
Type 0. Because these fibroids are pedunculated, the operator has the option of cutting the stalk (FIGURE 1) or shaving the myoma to remove it in pieces through the cervix. If the stalk is cut, it often is difficult to remove the fibroid through the cervix due to its large size. Nor can the fibroid be cut with monopolar energy because of the difficulty of completing the circuit. Type 0 myomas can be grabbed blindly with a Corson forceps or under direct visualization with an Isaacson optical tenaculum (Karl Storz Endoscopy, Culver City, Calif.). It also is acceptable to leave the fibroid in the cavity and let it degenerate and be expelled spontaneously. The risk of infection is very small.
My preference is to shave the fibroid down to the endometrium while it is attached to the stalk. I find it easier to remove when it is anchored rather than trying to pull out a single large specimen.
Type I. In a Type I myoma, more than 50% of the fibroid is within the uterine cavity, with a smaller portion embedded in the myometrium. To remove these fibroids, shave each to the level of the endometrium. To do so, place the loop behind the fibroid to be resected (FIGURE 5). Next, activate the RF energy using a foot pedal and, once the circuit is complete, draw the loop back into the resectoscope. Be careful to maintain visualization of the fibroid, loop, and cavity (FIGURE 6). This becomes difficult as the fibroid approaches the resectoscope and obstructs the hysteroscopic lens. To avoid this, bring the loop—which begins its cut fully extended—only halfway back to the resectoscope (FIGURE 7). Then retract the resectoscope itself, with the loop halfway extended, until the cut through the fibroid is complete (FIGURE 8). The loop then is retracted back into the resectoscope. The angle of the resectoscope must be adjusted to maintain loop contact with the fibroid tissue.
When more than 70% or 80% of the fibroid has been resected, the remainder will slough spontaneously over the next 2 to 4 months.
Once the fibroid is resected to the level of the endometrium, remove the fibroid chips (FIGURE 2). Often, when the hysteroscope is removed and uterine distention is reestablished, more of the fibroid will protrude into the cavity, facilitating safe resection. At some point, the fibroid will no longer protrude into the cavity; it will have been removed or only a small percentage will remain intramural.
Often, when more than 70% or 80% of the fibroid has been resected, the remainder will slough spontaneously over the next 2 to 4 months. The technique for removing the intramural fibroid is described on page 78 (FIGURE 3).
Type II. The first step in resecting a Type II myoma is shaving the intracavitary portion to the level of the endometrium, as in the resection of a Type I fibroid. Often, once a small Type II myoma is “unroofed,” the remainder will spontaneously fall into the cavity and can easily be removed.
Larger Type II myomas require a different approach. In these, the goal is to identify the pseudo-capsular plane that allows for blunt dissection. Once you have identified it, use the loop electrode to bluntly dissect (without RF activation) the fibroid from the myometrium. Place the electrode in the capsular plane and retract as much of the myoma into the cavity as possible (FIGURE 4). Another way to do this is to use a twisting motion—similar to those performed in open myomectomies—with the optical tenaculum. Once the fibroid is in the cavity, shave it to the level of the endometrium. Repeat this technique until you have removed as much of the fibroid as possible. Use minimal distention pressure when you perform this technique so that the fibroid will not be pressed back into the myometrium.
Underutilization of hysteroscopic myomectomy may be due to inadequate training in the technique or a misconception that it is difficult to learn. Fortunately, hysteroscope manufacturers are beginning to offer physicians and operating room personnel didactic and hands-on laboratory courses.
FIGURE 1 Surgical technique for removing Type 0, I, II fibroids
Type 0. The loop is placed behind the fibroid. Once activated, the loop is drawn toward the operator to resect the pedicle.
FIGURE 2 Surgical technique for removing Type 0, I, II fibroids
Type I. The myoma is resected to the level of the myometrium.
FIGURE 3 Surgical technique for removing Type 0, I, II fibroids
Resection of intramural portion of Type I and II. The resectoscopic loop is placed in the pseudocapsular lane between the fibroid and the myometrium to bluntly dissect the fibroid into the cavity for further resection.
FIGURE 4 Surgical technique for removing Type 0, I, II fibroids
Type II. The roof of the myoma is shaved off to the level of the myometrium. The insert demonstrates the intramural portion of the myoma that will protrude into the cavity with a reduction in uterine distension pressure.
FIGURE 5 Resect technique—4 steps in resecting a submucous myoma
The loop is placed behind the fibroid with maximal contact with the tissue before RF activation.
FIGURE 6 Resect technique—4 steps in resecting a submucous myoma
The loop is withdrawn toward the operator (see arrow) while cutting the myoma but not so far as to obstruct the hysteroscopic view.
FIGURE 7 Resect technique—4 steps in resecting a submucous myoma
The loop is held in place while the hysteroscope is withdrawn (arrows indicate how to remain in direct contact with myoma) continuing to cut the myoma with visualization.
FIGURE 8 Resect technique—4 steps in resecting a submucous myoma
Once through the myoma, the loop is withdrawn into the hysteroscope.
Management of complications
The primary complications of hysteroscopic myomectomy are bleeding, infection, uterine perforation, and fluid overload.
Bleeding. If postoperative hemorrhage occurs, use a 30-cc Foley balloon to tamponade the vessels, or pack the uterus with gauze soaked in a vasopressin solution. Both can be removed any time from 4 hours to 24 hours following placement. If bleeding persists, uterine artery embolization should be considered prior to hysterectomy.
Most attempts at controlling bleeding with a rollerball are futile because the intrauterine distention pressure necessary to see the venous bleeders hides the actual bleeding. Arterial bleeders can be seen and controlled with the rollerball, but they are much less common.
Infection. Because infection is uncommon with hysteroscopic surgery, routine prophylaxis is unnecessary.
Antibiotics should be used if the patient had cervical laminaria placed preoperatively.
Uterine perforation. The management of uterine perforation depends on the site of perforation and whether energy was used during the perforation. For example, if the perforation was at the fundus and occurred with a blunt, non-energized instrument, observation without laparoscopy is sufficient. If the perforation was lateral or occurred with RF energy, laparoscopy is suggested to rule out a broad ligament hematoma or injury to intraperitoneal vessels and viscera. If the perforation is anterior, a cystoscopy is recommended; internal colon inspection is necessary if the perforation is posterior.
Fluid overload. Complications and prevention can be avoided and treated as previously discussed.
New training opportunities may include virtual reality
Underutilization of hysteroscopic myomectomy may be due to inadequate training in the technique during residency or the misconception that it is difficult to learn. (Training has been further hampered by the lack of a suitable animal model.)
Fortunately, hysteroscope manufacturers are beginning to offer physicians and operating room personnel didactic and hands-on laboratory courses. In the near future, we hope, these companies will hire gynecologic hysteroscopists to support physicians and nurses in the field, as has been done in many other specialties.
The creation of new tools such as virtual-reality simulators will undoubtedly enhance the teaching of operative hysteroscopy.
Dr. Isaacson teaches hysteroscopy courses sponsored by Karl Storz Endoscopy.
- The goal of hysteroscopic myomectomy is complete removal of the fibroid without trauma to normal uterine tissue.
- Patients with Type 0 and Type I fibroids often require only 1 surgery; patients with Type II fibroids should be advised that 2 surgeries may be needed to remove the entire fibroid.
- Adjuvant preoperative hormonal therapy facilitates surgical scheduling, helps prevent further blood loss in patients already suffering from anemia, and reduces distention media intravasation.
- The monopolar loop electrode is the fibroid removal system that is used most often.
Hysteroscopic myomectomy should be offered to all patients with symptomatic submucous fibroids who desire to avoid hysterectomy. Although it is a highly effective, minimally invasive technique, it is underutilized.
Unfortunately, fewer than one third of US gynecologists perform this procedure. In a 1997 survey of members of the American Association of Gynecologic Laparoscopists—an organization committed to minimally invasive surgery—only half of the respondents reported that they perform this surgery.1
The reasons for learning to perform hysteroscopic myomectomy are compelling:
- A large cohort of patients could benefit, since most heavy vaginal bleeding from fibroids is due to the submucous location, and hysteroscopic resection is a much more benign approach than hysterectomy. Symptomatic fibroids account for 27% of all hysterectomies performed in the US (the largest single diagnostic category) and more than 100,000 are performed for fibroids that cause abnormal uterine bleeding.2
- Removing these lesions hysteroscopically greatly improves prognosis in women with recurrent pregnancy loss and infertility due to submucous fibroids.3 Up to 15% of patients presenting with infertility have otherwise asymptomatic uterine defects, including submucous fibroids. For example, a meta-analysis of patients undergoing in vitro fertilization determined that, compared with controls, the relative risk of pregnancy for women with submucous fibroids was 0.32 (95% confidence interval [CI], 0.13–0.70). When the submucous fibroids were resected, the relative risk of pregnancy rose to 1.72 (CI, 1.13–2.58).3
Preoperative evaluation of fibroids
Severity of menorrhagia (the most common symptom) is considered directly related to the volume of the myoma within the endometrial cavity. It is not uncommon to see large tortuous vessels covering the surface of the fibroids; although the exact mechanism of fibroid-related menorrhagia is undetermined, the fragility of these vessels is probably responsible, at least in part.
Additionally, fibroids involving the uterine mucosa or submucosa may interfere with the muscular contraction necessary for hemostasis.
Surgical options and pretreatment depend on fibroid type. Submucous fibroids are classified according to the percentage of the fibroid within the endometrial cavity:4
- Type 0: pedunculated; 100% within the cavity
- Type I: more than 50% within the cavity
- Type II: more than 50% within the myometrium
The type dictates surgical options, determines endometrial pretreatment, and shapes patient expectations. Type 0 and Type I submucosal fibroids are more successfully removed in a single surgery, whereas Type II submucous fibroids usually require 2 procedures for complete removal.5
My preference is office hysteroscopy to evaluate the endometrial cavity combined with vaginal ultrasound to assess intramural disease. The view with the office hysteroscope is the same view you will have during surgery.
Patients with Type II myomas should be informed of the potential need for 2 procedures, as this fact often influences their treatment decisions. In addition, whenever a patient with a Type II myoma is pretreated with a gonadotropin-releasing hormone (GnRH) agonist, the physician should reassess the fibroid preoperatively to ensure that it has not become completely intramural.
Office assessment: Hysteroscopy plus ultrasound. Preoperative assessment can be achieved with a hysterosalpingogram, vaginal ultrasound, hysterosonogram, or office hysteroscopy.
Method. My preference is office hysteroscopy to evaluate the endometrial cavity combined with vaginal ultrasound to assess intramural disease. The view with the office hysteroscope is the same view you will have during surgery. There will be no surprises. With a small flexible hysteroscope using saline for distention, the procedure is done with no tenaculum, no paracervical block, and can be completed with 60 to 100 cc of fluid in less than 1 minute.
Patients are often intrigued to view the myoma responsible for their heavy vaginal bleeding.
Several advantages of preoperative hormonal therapy
Preoperative hormonal therapy has several advantages:
- Since it is best to resect submucous myomas when the endometrium is thin, hormonal therapy facilitates surgical scheduling.
- Since preoperative therapy creates a state of amenorrhea, it enables patients suffering from menorrhagia and anemia to build up their blood counts, reducing the need for transfusion.
- Most importantly, preoperative therapy can reduce blood flow to the uterus, thereby reducing the rate of fluid intravasation.
Adjuvants used for these functions include oral contraceptives, progestogens, danazol, and GnRH agonists. However, a recent Cochrane review suggests that only GnRH agonists reduce fluid absorption during operative hysteroscopy.6
Method. Numerous protocols have been published, but my preference is to administer 1 intramuscular injection of depot leuprolide acetate (7.5 mg) 6 weeks prior to surgery.
A review of ‘systems’
Submucous myomas have been hysteroscopically removed using the neodymium/yttrium aluminum-garnet (Nd:YAG) laser, a monopolar resectoscope loop, a monopolar radiofrequency (RF) vaporizing electrode, a bipolar RF vaporizing electrode, and a bipolar RF loop.
Laser. The Nd:YAG laser is an expensive device that was popular in the late 1980s and early 1990s. It can be used as a cutting tool for pedunculated Type 0 myomas, or it can be used for myolysis by burning numerous holes in the myoma, causing devascularization and shrinkage.7,8
The sole advantage of the Nd:YAG laser is that it can be used in an isotonic-fluid-filled cavity. Now that accurate fluid-monitoring devices are available, the Nd:YAG laser is rarely used for this indication.
Vaporizing electrodes. Both the bipolar system and monopolar vaporizing electrodes utilize very high RF power (200 W to 300 W) to vaporize fibroids. The advantage is that the fibroids are eradicated very quickly, without any bothersome fibroid chips to remove. The bipolar system can be used with isotonic fluid, whereas the monopolar vaporizing electrodes require non-electrolyte-containing distention media.
The main disadvantages of vaporizing electrodes are:
- They do not produce a tissue sample for pathology. While uterine sarcomas are very rare, they are not homogeneous. Therefore, a simple sample prior to vaporization does not rule out the disease.
- Since vaporizing electrodes are used at high power, numerous gas bubbles are produced and enter the vascular system. Fortunately, these bubbles dissipate rapidly in the blood. As long as the rate of formation does not exceed the rate of dissipation, there are no significant clinical sequelae. The surgeon can avoid complications by monitoring the patient’s endtidal CO2 and maintaining communication with the anesthesiologist. If a sudden drop in endtidal CO2 is observed, the surgeon should stop the case until it resolves.9
Monopolar loop electrode. The instrument most commonly used to remove submucosal myomas is the monopolar loop electrode with a continuous-flow resectoscope. This is quite similar to the instrument used to resect the prostate; it has been slightly modified for gynecologic use to provide enhanced fluid inflow and outflow. A key instrument now considered standard is an accurate fluid-monitoring device that can be attached to the resectoscope.
Distention media for monopolar resection. Prior to the introduction of continuous high-flow resectoscopes and fluid-management systems, monopolar resectoscopy could only be performed using a solution of 32% dextran 70 and water because of that formulation’s viscosity and facilitation of visualization in the presence of blood. However, due to complications related to absorption, allergies, and the solution’s sticky residue, it is not used with today’s high-flow technology.
The instrument most commonly used to remove submucosal myomas is the monopolar loop electrode with a continuous-flow resectoscope.
When using nonviscous fluids with monopolar RF energy, a distention medium without electrolytes must be selected. Isotonic physiologic media such as normal saline or lactated Ringer’s solution would cause the energy at the electrode to disperse, eliminating the cutting effect. Contrary to popular belief, there would be no burning of the uterine cavity and no subsequent danger to the patient.
The 3 most commonly used fluids for uterine distention are 1.5% glycine, 3% sorbitol, and 5% mannitol. All 3 lack electrolytes. The glycine and sorbitol solutions are hypotonic, while 5% mannitol is isotonic. Although there is some debate over whether an isotonic non-electrolyte-containing medium is safer than a hypotonic one, the guidelines for fluid monitoring are the same for all 3.
The goal of a hysteroscopic myomectomy is to alleviate symptoms without causing a weak myometrium or intracavitary synechia. This is accomplished by removing the fibroid without traumatizing normal uterine tissue.
As a general rule, the serum sodium level will decrease approximately 10 meq for every liter absorbed in the blood stream. That is why, to predict hyponatremia, the gynecologist must rely on exact measurements of fluid deficits rather than operating time or total volume used.
Fluid management. Significant hyponatremia can result in pulmonary edema, transient blindness, cerebral edema, brainstem herniation, and death. Catastrophic as these complications are, they are almost 100% avoidable with accurate deficit measurements and adherence to sound fluid-management protocols.
At our institution, fluids are monitored using a weighted system, and the fluid deficit is displayed on the surgical screen in real time. When the deficit reaches 1 L, electrolytes are drawn, the patient is given 10 mg of intravenous (IV) furosemide, and attempts are made to finish the surgery as soon as possible. When the deficit reaches 1.5 L, the surgery is discontinued no matter how much or how little remains to complete it.
It is important that the protocol at each institution be agreed upon by the chief of service and anesthesia as well as the director of nursing so that there are no conflicts at the time of surgery.
Uterine distention methods
Numerous methods have been used to distend the uterus: gravity, gas pressure, and electronic pumps and pressure bags. The optimal intrauterine pressure is the minimum pressure that allows for flow, distention, and visualization. Most of the time this ranges from 40 mm Hg to 60 mm Hg. The higher the intrauterine pressure, the more rapid the fluid intravasation, particularly when intrauterine pressure exceeds the mean arterial pressure.
Rapid fluid loss can occur even with low intrauterine pressures. That is because a large venous sinus will have a pressure of 8 mm Hg to 10 mm Hg, while the minimum uterine distention pressure is much greater. Strict adherence to fluid-deficit protocols will minimize the risk of complications from fluid overload.
Surgical technique
The goal of a hysteroscopic myomectomy is to alleviate symptoms without causing a weak myometrium or intracavitary synechia. Ideally, this is accomplished by completely removing the fibroid without traumatizing normal uterine tissue. Removal of the entire myoma reduces the likelihood of recurrence and regrowth.
Surgical technique for removal of Types 0, I, and II fibroids is illustrated and explained on (FIGURES1-4).
Within a given range (40 W to 100 W), it makes little difference which cutting-current setting on the RF generator you use; the principles are the same. The loop must be in contact with the tissue to be resected. Energy is applied and, once the circuit is completed (through tissue, the dispersive electrode, the generator and back to the loop), the loop is able to cut with minimal tactile feedback.
The lower the wattage, the more contact necessary between the loop and the tissue and the longer it takes to complete the circuit. Conversely, the higher the power, the faster the circuit is completed and cutting occurs.
Though it does not matter what setting is used, the operator must choose a setting based on his or her comfort level. Lower power gives the operator more control. Higher power should be used only by the most experienced hysteroscopist, since it involves very little tactile feedback and a higher risk of uterine perforation. At our teaching institution, we use 60 W of pure cutting current.
The technique for removing a submucous fibroid depends on its type and location within the endometrial cavity.
Type 0. Because these fibroids are pedunculated, the operator has the option of cutting the stalk (FIGURE 1) or shaving the myoma to remove it in pieces through the cervix. If the stalk is cut, it often is difficult to remove the fibroid through the cervix due to its large size. Nor can the fibroid be cut with monopolar energy because of the difficulty of completing the circuit. Type 0 myomas can be grabbed blindly with a Corson forceps or under direct visualization with an Isaacson optical tenaculum (Karl Storz Endoscopy, Culver City, Calif.). It also is acceptable to leave the fibroid in the cavity and let it degenerate and be expelled spontaneously. The risk of infection is very small.
My preference is to shave the fibroid down to the endometrium while it is attached to the stalk. I find it easier to remove when it is anchored rather than trying to pull out a single large specimen.
Type I. In a Type I myoma, more than 50% of the fibroid is within the uterine cavity, with a smaller portion embedded in the myometrium. To remove these fibroids, shave each to the level of the endometrium. To do so, place the loop behind the fibroid to be resected (FIGURE 5). Next, activate the RF energy using a foot pedal and, once the circuit is complete, draw the loop back into the resectoscope. Be careful to maintain visualization of the fibroid, loop, and cavity (FIGURE 6). This becomes difficult as the fibroid approaches the resectoscope and obstructs the hysteroscopic lens. To avoid this, bring the loop—which begins its cut fully extended—only halfway back to the resectoscope (FIGURE 7). Then retract the resectoscope itself, with the loop halfway extended, until the cut through the fibroid is complete (FIGURE 8). The loop then is retracted back into the resectoscope. The angle of the resectoscope must be adjusted to maintain loop contact with the fibroid tissue.
When more than 70% or 80% of the fibroid has been resected, the remainder will slough spontaneously over the next 2 to 4 months.
Once the fibroid is resected to the level of the endometrium, remove the fibroid chips (FIGURE 2). Often, when the hysteroscope is removed and uterine distention is reestablished, more of the fibroid will protrude into the cavity, facilitating safe resection. At some point, the fibroid will no longer protrude into the cavity; it will have been removed or only a small percentage will remain intramural.
Often, when more than 70% or 80% of the fibroid has been resected, the remainder will slough spontaneously over the next 2 to 4 months. The technique for removing the intramural fibroid is described on page 78 (FIGURE 3).
Type II. The first step in resecting a Type II myoma is shaving the intracavitary portion to the level of the endometrium, as in the resection of a Type I fibroid. Often, once a small Type II myoma is “unroofed,” the remainder will spontaneously fall into the cavity and can easily be removed.
Larger Type II myomas require a different approach. In these, the goal is to identify the pseudo-capsular plane that allows for blunt dissection. Once you have identified it, use the loop electrode to bluntly dissect (without RF activation) the fibroid from the myometrium. Place the electrode in the capsular plane and retract as much of the myoma into the cavity as possible (FIGURE 4). Another way to do this is to use a twisting motion—similar to those performed in open myomectomies—with the optical tenaculum. Once the fibroid is in the cavity, shave it to the level of the endometrium. Repeat this technique until you have removed as much of the fibroid as possible. Use minimal distention pressure when you perform this technique so that the fibroid will not be pressed back into the myometrium.
Underutilization of hysteroscopic myomectomy may be due to inadequate training in the technique or a misconception that it is difficult to learn. Fortunately, hysteroscope manufacturers are beginning to offer physicians and operating room personnel didactic and hands-on laboratory courses.
FIGURE 1 Surgical technique for removing Type 0, I, II fibroids
Type 0. The loop is placed behind the fibroid. Once activated, the loop is drawn toward the operator to resect the pedicle.
FIGURE 2 Surgical technique for removing Type 0, I, II fibroids
Type I. The myoma is resected to the level of the myometrium.
FIGURE 3 Surgical technique for removing Type 0, I, II fibroids
Resection of intramural portion of Type I and II. The resectoscopic loop is placed in the pseudocapsular lane between the fibroid and the myometrium to bluntly dissect the fibroid into the cavity for further resection.
FIGURE 4 Surgical technique for removing Type 0, I, II fibroids
Type II. The roof of the myoma is shaved off to the level of the myometrium. The insert demonstrates the intramural portion of the myoma that will protrude into the cavity with a reduction in uterine distension pressure.
FIGURE 5 Resect technique—4 steps in resecting a submucous myoma
The loop is placed behind the fibroid with maximal contact with the tissue before RF activation.
FIGURE 6 Resect technique—4 steps in resecting a submucous myoma
The loop is withdrawn toward the operator (see arrow) while cutting the myoma but not so far as to obstruct the hysteroscopic view.
FIGURE 7 Resect technique—4 steps in resecting a submucous myoma
The loop is held in place while the hysteroscope is withdrawn (arrows indicate how to remain in direct contact with myoma) continuing to cut the myoma with visualization.
FIGURE 8 Resect technique—4 steps in resecting a submucous myoma
Once through the myoma, the loop is withdrawn into the hysteroscope.
Management of complications
The primary complications of hysteroscopic myomectomy are bleeding, infection, uterine perforation, and fluid overload.
Bleeding. If postoperative hemorrhage occurs, use a 30-cc Foley balloon to tamponade the vessels, or pack the uterus with gauze soaked in a vasopressin solution. Both can be removed any time from 4 hours to 24 hours following placement. If bleeding persists, uterine artery embolization should be considered prior to hysterectomy.
Most attempts at controlling bleeding with a rollerball are futile because the intrauterine distention pressure necessary to see the venous bleeders hides the actual bleeding. Arterial bleeders can be seen and controlled with the rollerball, but they are much less common.
Infection. Because infection is uncommon with hysteroscopic surgery, routine prophylaxis is unnecessary.
Antibiotics should be used if the patient had cervical laminaria placed preoperatively.
Uterine perforation. The management of uterine perforation depends on the site of perforation and whether energy was used during the perforation. For example, if the perforation was at the fundus and occurred with a blunt, non-energized instrument, observation without laparoscopy is sufficient. If the perforation was lateral or occurred with RF energy, laparoscopy is suggested to rule out a broad ligament hematoma or injury to intraperitoneal vessels and viscera. If the perforation is anterior, a cystoscopy is recommended; internal colon inspection is necessary if the perforation is posterior.
Fluid overload. Complications and prevention can be avoided and treated as previously discussed.
New training opportunities may include virtual reality
Underutilization of hysteroscopic myomectomy may be due to inadequate training in the technique during residency or the misconception that it is difficult to learn. (Training has been further hampered by the lack of a suitable animal model.)
Fortunately, hysteroscope manufacturers are beginning to offer physicians and operating room personnel didactic and hands-on laboratory courses. In the near future, we hope, these companies will hire gynecologic hysteroscopists to support physicians and nurses in the field, as has been done in many other specialties.
The creation of new tools such as virtual-reality simulators will undoubtedly enhance the teaching of operative hysteroscopy.
Dr. Isaacson teaches hysteroscopy courses sponsored by Karl Storz Endoscopy.
1. Hulka JF, Levy BS, Luciano AA, Parker WH, Phillips JM. 1997 AAGL membership survey: practice profiles. J Amer Assoc Gynecol Laparosc. 1998;5:93-96.
2. Carlson KJ, Nichols DH, Schiff I. Indications for hysterectomy. N Eng J Med. 1993;328:856-860.
3. Pritts E. Fibroids and infertility: a systematic review of the evidence. Obstet Gynecol Survey. 2001;56:483-491.
4. deBlok S, Dijkman AB, Hemrika DJ. Transcervical resection of fibroids (TCRM): results related to hysteroscopic classification. Gynaecol Endosc. 1995;4:243-246.
5. Istre O. Transcervical resection of endometrium and fibroids: the outcome of 412 operations performed over 5 years. Acta Obstet Gynecol Scand. 1996;75:567-574.
6. Sowter MC, Lethaby A, Singla AA. Preoperative endometrial thinning agents before hysteroscopic surgery for heavy menstrual bleeding. Cochrane Database Syst Rev. 2002;(3):CD001124.-
7. Baggish MS, Sze EM, Morgan G. Hysteroscopic treatment of symptomatic submucous myomas with the Nd:YAG laser. J Gynecol Surg. 1989;5:27-36.
8. Donnez J, Gillerot S, Bourgonjon D, et al. Neodymium:YAG laser hysteroscopy in large submucous fibroids. Fertil Steril. 1990;54:999-1003.
9. Bloomstone J, Chen C, Isselbacher E, Isaacson K. A pilot study examining the frequency and quantity of gas embolization during operative hysteroscopy using a monopolar resectoscope. J Am Assoc Gynecol Laparosc. 2002;9:9-14.
1. Hulka JF, Levy BS, Luciano AA, Parker WH, Phillips JM. 1997 AAGL membership survey: practice profiles. J Amer Assoc Gynecol Laparosc. 1998;5:93-96.
2. Carlson KJ, Nichols DH, Schiff I. Indications for hysterectomy. N Eng J Med. 1993;328:856-860.
3. Pritts E. Fibroids and infertility: a systematic review of the evidence. Obstet Gynecol Survey. 2001;56:483-491.
4. deBlok S, Dijkman AB, Hemrika DJ. Transcervical resection of fibroids (TCRM): results related to hysteroscopic classification. Gynaecol Endosc. 1995;4:243-246.
5. Istre O. Transcervical resection of endometrium and fibroids: the outcome of 412 operations performed over 5 years. Acta Obstet Gynecol Scand. 1996;75:567-574.
6. Sowter MC, Lethaby A, Singla AA. Preoperative endometrial thinning agents before hysteroscopic surgery for heavy menstrual bleeding. Cochrane Database Syst Rev. 2002;(3):CD001124.-
7. Baggish MS, Sze EM, Morgan G. Hysteroscopic treatment of symptomatic submucous myomas with the Nd:YAG laser. J Gynecol Surg. 1989;5:27-36.
8. Donnez J, Gillerot S, Bourgonjon D, et al. Neodymium:YAG laser hysteroscopy in large submucous fibroids. Fertil Steril. 1990;54:999-1003.
9. Bloomstone J, Chen C, Isselbacher E, Isaacson K. A pilot study examining the frequency and quantity of gas embolization during operative hysteroscopy using a monopolar resectoscope. J Am Assoc Gynecol Laparosc. 2002;9:9-14.