User login
Hysteroscopic myomectomy: Fertility-preserving yet underutilized
- The goal of hysteroscopic myomectomy is complete removal of the fibroid without trauma to normal uterine tissue.
- Patients with Type 0 and Type I fibroids often require only 1 surgery; patients with Type II fibroids should be advised that 2 surgeries may be needed to remove the entire fibroid.
- Adjuvant preoperative hormonal therapy facilitates surgical scheduling, helps prevent further blood loss in patients already suffering from anemia, and reduces distention media intravasation.
- The monopolar loop electrode is the fibroid removal system that is used most often.
Hysteroscopic myomectomy should be offered to all patients with symptomatic submucous fibroids who desire to avoid hysterectomy. Although it is a highly effective, minimally invasive technique, it is underutilized.
Unfortunately, fewer than one third of US gynecologists perform this procedure. In a 1997 survey of members of the American Association of Gynecologic Laparoscopists—an organization committed to minimally invasive surgery—only half of the respondents reported that they perform this surgery.1
The reasons for learning to perform hysteroscopic myomectomy are compelling:
- A large cohort of patients could benefit, since most heavy vaginal bleeding from fibroids is due to the submucous location, and hysteroscopic resection is a much more benign approach than hysterectomy. Symptomatic fibroids account for 27% of all hysterectomies performed in the US (the largest single diagnostic category) and more than 100,000 are performed for fibroids that cause abnormal uterine bleeding.2
- Removing these lesions hysteroscopically greatly improves prognosis in women with recurrent pregnancy loss and infertility due to submucous fibroids.3 Up to 15% of patients presenting with infertility have otherwise asymptomatic uterine defects, including submucous fibroids. For example, a meta-analysis of patients undergoing in vitro fertilization determined that, compared with controls, the relative risk of pregnancy for women with submucous fibroids was 0.32 (95% confidence interval [CI], 0.13–0.70). When the submucous fibroids were resected, the relative risk of pregnancy rose to 1.72 (CI, 1.13–2.58).3
Preoperative evaluation of fibroids
Severity of menorrhagia (the most common symptom) is considered directly related to the volume of the myoma within the endometrial cavity. It is not uncommon to see large tortuous vessels covering the surface of the fibroids; although the exact mechanism of fibroid-related menorrhagia is undetermined, the fragility of these vessels is probably responsible, at least in part.
Additionally, fibroids involving the uterine mucosa or submucosa may interfere with the muscular contraction necessary for hemostasis.
Surgical options and pretreatment depend on fibroid type. Submucous fibroids are classified according to the percentage of the fibroid within the endometrial cavity:4
- Type 0: pedunculated; 100% within the cavity
- Type I: more than 50% within the cavity
- Type II: more than 50% within the myometrium
The type dictates surgical options, determines endometrial pretreatment, and shapes patient expectations. Type 0 and Type I submucosal fibroids are more successfully removed in a single surgery, whereas Type II submucous fibroids usually require 2 procedures for complete removal.5
My preference is office hysteroscopy to evaluate the endometrial cavity combined with vaginal ultrasound to assess intramural disease. The view with the office hysteroscope is the same view you will have during surgery.
Patients with Type II myomas should be informed of the potential need for 2 procedures, as this fact often influences their treatment decisions. In addition, whenever a patient with a Type II myoma is pretreated with a gonadotropin-releasing hormone (GnRH) agonist, the physician should reassess the fibroid preoperatively to ensure that it has not become completely intramural.
Office assessment: Hysteroscopy plus ultrasound. Preoperative assessment can be achieved with a hysterosalpingogram, vaginal ultrasound, hysterosonogram, or office hysteroscopy.
Method. My preference is office hysteroscopy to evaluate the endometrial cavity combined with vaginal ultrasound to assess intramural disease. The view with the office hysteroscope is the same view you will have during surgery. There will be no surprises. With a small flexible hysteroscope using saline for distention, the procedure is done with no tenaculum, no paracervical block, and can be completed with 60 to 100 cc of fluid in less than 1 minute.
Patients are often intrigued to view the myoma responsible for their heavy vaginal bleeding.
Several advantages of preoperative hormonal therapy
Preoperative hormonal therapy has several advantages:
- Since it is best to resect submucous myomas when the endometrium is thin, hormonal therapy facilitates surgical scheduling.
- Since preoperative therapy creates a state of amenorrhea, it enables patients suffering from menorrhagia and anemia to build up their blood counts, reducing the need for transfusion.
- Most importantly, preoperative therapy can reduce blood flow to the uterus, thereby reducing the rate of fluid intravasation.
Adjuvants used for these functions include oral contraceptives, progestogens, danazol, and GnRH agonists. However, a recent Cochrane review suggests that only GnRH agonists reduce fluid absorption during operative hysteroscopy.6
Method. Numerous protocols have been published, but my preference is to administer 1 intramuscular injection of depot leuprolide acetate (7.5 mg) 6 weeks prior to surgery.
A review of ‘systems’
Submucous myomas have been hysteroscopically removed using the neodymium/yttrium aluminum-garnet (Nd:YAG) laser, a monopolar resectoscope loop, a monopolar radiofrequency (RF) vaporizing electrode, a bipolar RF vaporizing electrode, and a bipolar RF loop.
Laser. The Nd:YAG laser is an expensive device that was popular in the late 1980s and early 1990s. It can be used as a cutting tool for pedunculated Type 0 myomas, or it can be used for myolysis by burning numerous holes in the myoma, causing devascularization and shrinkage.7,8
The sole advantage of the Nd:YAG laser is that it can be used in an isotonic-fluid-filled cavity. Now that accurate fluid-monitoring devices are available, the Nd:YAG laser is rarely used for this indication.
Vaporizing electrodes. Both the bipolar system and monopolar vaporizing electrodes utilize very high RF power (200 W to 300 W) to vaporize fibroids. The advantage is that the fibroids are eradicated very quickly, without any bothersome fibroid chips to remove. The bipolar system can be used with isotonic fluid, whereas the monopolar vaporizing electrodes require non-electrolyte-containing distention media.
The main disadvantages of vaporizing electrodes are:
- They do not produce a tissue sample for pathology. While uterine sarcomas are very rare, they are not homogeneous. Therefore, a simple sample prior to vaporization does not rule out the disease.
- Since vaporizing electrodes are used at high power, numerous gas bubbles are produced and enter the vascular system. Fortunately, these bubbles dissipate rapidly in the blood. As long as the rate of formation does not exceed the rate of dissipation, there are no significant clinical sequelae. The surgeon can avoid complications by monitoring the patient’s endtidal CO2 and maintaining communication with the anesthesiologist. If a sudden drop in endtidal CO2 is observed, the surgeon should stop the case until it resolves.9
Monopolar loop electrode. The instrument most commonly used to remove submucosal myomas is the monopolar loop electrode with a continuous-flow resectoscope. This is quite similar to the instrument used to resect the prostate; it has been slightly modified for gynecologic use to provide enhanced fluid inflow and outflow. A key instrument now considered standard is an accurate fluid-monitoring device that can be attached to the resectoscope.
Distention media for monopolar resection. Prior to the introduction of continuous high-flow resectoscopes and fluid-management systems, monopolar resectoscopy could only be performed using a solution of 32% dextran 70 and water because of that formulation’s viscosity and facilitation of visualization in the presence of blood. However, due to complications related to absorption, allergies, and the solution’s sticky residue, it is not used with today’s high-flow technology.
The instrument most commonly used to remove submucosal myomas is the monopolar loop electrode with a continuous-flow resectoscope.
When using nonviscous fluids with monopolar RF energy, a distention medium without electrolytes must be selected. Isotonic physiologic media such as normal saline or lactated Ringer’s solution would cause the energy at the electrode to disperse, eliminating the cutting effect. Contrary to popular belief, there would be no burning of the uterine cavity and no subsequent danger to the patient.
The 3 most commonly used fluids for uterine distention are 1.5% glycine, 3% sorbitol, and 5% mannitol. All 3 lack electrolytes. The glycine and sorbitol solutions are hypotonic, while 5% mannitol is isotonic. Although there is some debate over whether an isotonic non-electrolyte-containing medium is safer than a hypotonic one, the guidelines for fluid monitoring are the same for all 3.
The goal of a hysteroscopic myomectomy is to alleviate symptoms without causing a weak myometrium or intracavitary synechia. This is accomplished by removing the fibroid without traumatizing normal uterine tissue.
As a general rule, the serum sodium level will decrease approximately 10 meq for every liter absorbed in the blood stream. That is why, to predict hyponatremia, the gynecologist must rely on exact measurements of fluid deficits rather than operating time or total volume used.
Fluid management. Significant hyponatremia can result in pulmonary edema, transient blindness, cerebral edema, brainstem herniation, and death. Catastrophic as these complications are, they are almost 100% avoidable with accurate deficit measurements and adherence to sound fluid-management protocols.
At our institution, fluids are monitored using a weighted system, and the fluid deficit is displayed on the surgical screen in real time. When the deficit reaches 1 L, electrolytes are drawn, the patient is given 10 mg of intravenous (IV) furosemide, and attempts are made to finish the surgery as soon as possible. When the deficit reaches 1.5 L, the surgery is discontinued no matter how much or how little remains to complete it.
It is important that the protocol at each institution be agreed upon by the chief of service and anesthesia as well as the director of nursing so that there are no conflicts at the time of surgery.
Uterine distention methods
Numerous methods have been used to distend the uterus: gravity, gas pressure, and electronic pumps and pressure bags. The optimal intrauterine pressure is the minimum pressure that allows for flow, distention, and visualization. Most of the time this ranges from 40 mm Hg to 60 mm Hg. The higher the intrauterine pressure, the more rapid the fluid intravasation, particularly when intrauterine pressure exceeds the mean arterial pressure.
Rapid fluid loss can occur even with low intrauterine pressures. That is because a large venous sinus will have a pressure of 8 mm Hg to 10 mm Hg, while the minimum uterine distention pressure is much greater. Strict adherence to fluid-deficit protocols will minimize the risk of complications from fluid overload.
Surgical technique
The goal of a hysteroscopic myomectomy is to alleviate symptoms without causing a weak myometrium or intracavitary synechia. Ideally, this is accomplished by completely removing the fibroid without traumatizing normal uterine tissue. Removal of the entire myoma reduces the likelihood of recurrence and regrowth.
Surgical technique for removal of Types 0, I, and II fibroids is illustrated and explained on (FIGURES1-4).
Within a given range (40 W to 100 W), it makes little difference which cutting-current setting on the RF generator you use; the principles are the same. The loop must be in contact with the tissue to be resected. Energy is applied and, once the circuit is completed (through tissue, the dispersive electrode, the generator and back to the loop), the loop is able to cut with minimal tactile feedback.
The lower the wattage, the more contact necessary between the loop and the tissue and the longer it takes to complete the circuit. Conversely, the higher the power, the faster the circuit is completed and cutting occurs.
Though it does not matter what setting is used, the operator must choose a setting based on his or her comfort level. Lower power gives the operator more control. Higher power should be used only by the most experienced hysteroscopist, since it involves very little tactile feedback and a higher risk of uterine perforation. At our teaching institution, we use 60 W of pure cutting current.
The technique for removing a submucous fibroid depends on its type and location within the endometrial cavity.
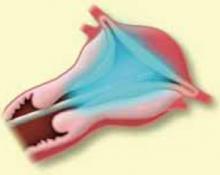
Type 0. Because these fibroids are pedunculated, the operator has the option of cutting the stalk (FIGURE 1) or shaving the myoma to remove it in pieces through the cervix. If the stalk is cut, it often is difficult to remove the fibroid through the cervix due to its large size. Nor can the fibroid be cut with monopolar energy because of the difficulty of completing the circuit. Type 0 myomas can be grabbed blindly with a Corson forceps or under direct visualization with an Isaacson optical tenaculum (Karl Storz Endoscopy, Culver City, Calif.). It also is acceptable to leave the fibroid in the cavity and let it degenerate and be expelled spontaneously. The risk of infection is very small.
My preference is to shave the fibroid down to the endometrium while it is attached to the stalk. I find it easier to remove when it is anchored rather than trying to pull out a single large specimen.
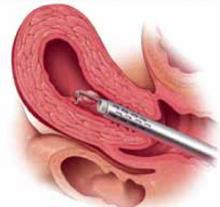
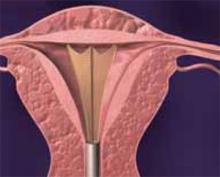
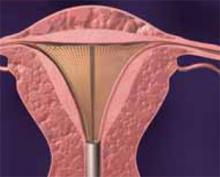
Type I. In a Type I myoma, more than 50% of the fibroid is within the uterine cavity, with a smaller portion embedded in the myometrium. To remove these fibroids, shave each to the level of the endometrium. To do so, place the loop behind the fibroid to be resected (FIGURE 5). Next, activate the RF energy using a foot pedal and, once the circuit is complete, draw the loop back into the resectoscope. Be careful to maintain visualization of the fibroid, loop, and cavity (FIGURE 6). This becomes difficult as the fibroid approaches the resectoscope and obstructs the hysteroscopic lens. To avoid this, bring the loop—which begins its cut fully extended—only halfway back to the resectoscope (FIGURE 7). Then retract the resectoscope itself, with the loop halfway extended, until the cut through the fibroid is complete (FIGURE 8). The loop then is retracted back into the resectoscope. The angle of the resectoscope must be adjusted to maintain loop contact with the fibroid tissue.
When more than 70% or 80% of the fibroid has been resected, the remainder will slough spontaneously over the next 2 to 4 months.
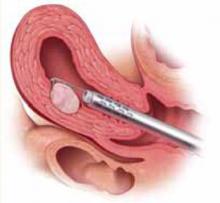
Once the fibroid is resected to the level of the endometrium, remove the fibroid chips (FIGURE 2). Often, when the hysteroscope is removed and uterine distention is reestablished, more of the fibroid will protrude into the cavity, facilitating safe resection. At some point, the fibroid will no longer protrude into the cavity; it will have been removed or only a small percentage will remain intramural.
Often, when more than 70% or 80% of the fibroid has been resected, the remainder will slough spontaneously over the next 2 to 4 months. The technique for removing the intramural fibroid is described on page 78 (FIGURE 3).
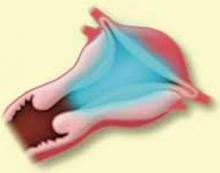
Type II. The first step in resecting a Type II myoma is shaving the intracavitary portion to the level of the endometrium, as in the resection of a Type I fibroid. Often, once a small Type II myoma is “unroofed,” the remainder will spontaneously fall into the cavity and can easily be removed.
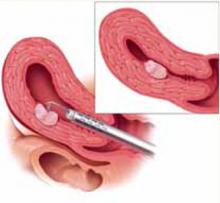
Larger Type II myomas require a different approach. In these, the goal is to identify the pseudo-capsular plane that allows for blunt dissection. Once you have identified it, use the loop electrode to bluntly dissect (without RF activation) the fibroid from the myometrium. Place the electrode in the capsular plane and retract as much of the myoma into the cavity as possible (FIGURE 4). Another way to do this is to use a twisting motion—similar to those performed in open myomectomies—with the optical tenaculum. Once the fibroid is in the cavity, shave it to the level of the endometrium. Repeat this technique until you have removed as much of the fibroid as possible. Use minimal distention pressure when you perform this technique so that the fibroid will not be pressed back into the myometrium.
Underutilization of hysteroscopic myomectomy may be due to inadequate training in the technique or a misconception that it is difficult to learn. Fortunately, hysteroscope manufacturers are beginning to offer physicians and operating room personnel didactic and hands-on laboratory courses.
FIGURE 1 Surgical technique for removing Type 0, I, II fibroids
Type 0. The loop is placed behind the fibroid. Once activated, the loop is drawn toward the operator to resect the pedicle.
FIGURE 2 Surgical technique for removing Type 0, I, II fibroids
Type I. The myoma is resected to the level of the myometrium.
FIGURE 3 Surgical technique for removing Type 0, I, II fibroids
Resection of intramural portion of Type I and II. The resectoscopic loop is placed in the pseudocapsular lane between the fibroid and the myometrium to bluntly dissect the fibroid into the cavity for further resection.
FIGURE 4 Surgical technique for removing Type 0, I, II fibroids
Type II. The roof of the myoma is shaved off to the level of the myometrium. The insert demonstrates the intramural portion of the myoma that will protrude into the cavity with a reduction in uterine distension pressure.
FIGURE 5 Resect technique—4 steps in resecting a submucous myoma
The loop is placed behind the fibroid with maximal contact with the tissue before RF activation.
FIGURE 6 Resect technique—4 steps in resecting a submucous myoma
The loop is withdrawn toward the operator (see arrow) while cutting the myoma but not so far as to obstruct the hysteroscopic view.
FIGURE 7 Resect technique—4 steps in resecting a submucous myoma
The loop is held in place while the hysteroscope is withdrawn (arrows indicate how to remain in direct contact with myoma) continuing to cut the myoma with visualization.
FIGURE 8 Resect technique—4 steps in resecting a submucous myoma
Once through the myoma, the loop is withdrawn into the hysteroscope.
Management of complications
The primary complications of hysteroscopic myomectomy are bleeding, infection, uterine perforation, and fluid overload.
Bleeding. If postoperative hemorrhage occurs, use a 30-cc Foley balloon to tamponade the vessels, or pack the uterus with gauze soaked in a vasopressin solution. Both can be removed any time from 4 hours to 24 hours following placement. If bleeding persists, uterine artery embolization should be considered prior to hysterectomy.
Most attempts at controlling bleeding with a rollerball are futile because the intrauterine distention pressure necessary to see the venous bleeders hides the actual bleeding. Arterial bleeders can be seen and controlled with the rollerball, but they are much less common.
Infection. Because infection is uncommon with hysteroscopic surgery, routine prophylaxis is unnecessary.
Antibiotics should be used if the patient had cervical laminaria placed preoperatively.
Uterine perforation. The management of uterine perforation depends on the site of perforation and whether energy was used during the perforation. For example, if the perforation was at the fundus and occurred with a blunt, non-energized instrument, observation without laparoscopy is sufficient. If the perforation was lateral or occurred with RF energy, laparoscopy is suggested to rule out a broad ligament hematoma or injury to intraperitoneal vessels and viscera. If the perforation is anterior, a cystoscopy is recommended; internal colon inspection is necessary if the perforation is posterior.
Fluid overload. Complications and prevention can be avoided and treated as previously discussed.
New training opportunities may include virtual reality
Underutilization of hysteroscopic myomectomy may be due to inadequate training in the technique during residency or the misconception that it is difficult to learn. (Training has been further hampered by the lack of a suitable animal model.)
Fortunately, hysteroscope manufacturers are beginning to offer physicians and operating room personnel didactic and hands-on laboratory courses. In the near future, we hope, these companies will hire gynecologic hysteroscopists to support physicians and nurses in the field, as has been done in many other specialties.
The creation of new tools such as virtual-reality simulators will undoubtedly enhance the teaching of operative hysteroscopy.
Dr. Isaacson teaches hysteroscopy courses sponsored by Karl Storz Endoscopy.
1. Hulka JF, Levy BS, Luciano AA, Parker WH, Phillips JM. 1997 AAGL membership survey: practice profiles. J Amer Assoc Gynecol Laparosc. 1998;5:93-96.
2. Carlson KJ, Nichols DH, Schiff I. Indications for hysterectomy. N Eng J Med. 1993;328:856-860.
3. Pritts E. Fibroids and infertility: a systematic review of the evidence. Obstet Gynecol Survey. 2001;56:483-491.
4. deBlok S, Dijkman AB, Hemrika DJ. Transcervical resection of fibroids (TCRM): results related to hysteroscopic classification. Gynaecol Endosc. 1995;4:243-246.
5. Istre O. Transcervical resection of endometrium and fibroids: the outcome of 412 operations performed over 5 years. Acta Obstet Gynecol Scand. 1996;75:567-574.
6. Sowter MC, Lethaby A, Singla AA. Preoperative endometrial thinning agents before hysteroscopic surgery for heavy menstrual bleeding. Cochrane Database Syst Rev. 2002;(3):CD001124.-
7. Baggish MS, Sze EM, Morgan G. Hysteroscopic treatment of symptomatic submucous myomas with the Nd:YAG laser. J Gynecol Surg. 1989;5:27-36.
8. Donnez J, Gillerot S, Bourgonjon D, et al. Neodymium:YAG laser hysteroscopy in large submucous fibroids. Fertil Steril. 1990;54:999-1003.
9. Bloomstone J, Chen C, Isselbacher E, Isaacson K. A pilot study examining the frequency and quantity of gas embolization during operative hysteroscopy using a monopolar resectoscope. J Am Assoc Gynecol Laparosc. 2002;9:9-14.
- The goal of hysteroscopic myomectomy is complete removal of the fibroid without trauma to normal uterine tissue.
- Patients with Type 0 and Type I fibroids often require only 1 surgery; patients with Type II fibroids should be advised that 2 surgeries may be needed to remove the entire fibroid.
- Adjuvant preoperative hormonal therapy facilitates surgical scheduling, helps prevent further blood loss in patients already suffering from anemia, and reduces distention media intravasation.
- The monopolar loop electrode is the fibroid removal system that is used most often.
Hysteroscopic myomectomy should be offered to all patients with symptomatic submucous fibroids who desire to avoid hysterectomy. Although it is a highly effective, minimally invasive technique, it is underutilized.
Unfortunately, fewer than one third of US gynecologists perform this procedure. In a 1997 survey of members of the American Association of Gynecologic Laparoscopists—an organization committed to minimally invasive surgery—only half of the respondents reported that they perform this surgery.1
The reasons for learning to perform hysteroscopic myomectomy are compelling:
- A large cohort of patients could benefit, since most heavy vaginal bleeding from fibroids is due to the submucous location, and hysteroscopic resection is a much more benign approach than hysterectomy. Symptomatic fibroids account for 27% of all hysterectomies performed in the US (the largest single diagnostic category) and more than 100,000 are performed for fibroids that cause abnormal uterine bleeding.2
- Removing these lesions hysteroscopically greatly improves prognosis in women with recurrent pregnancy loss and infertility due to submucous fibroids.3 Up to 15% of patients presenting with infertility have otherwise asymptomatic uterine defects, including submucous fibroids. For example, a meta-analysis of patients undergoing in vitro fertilization determined that, compared with controls, the relative risk of pregnancy for women with submucous fibroids was 0.32 (95% confidence interval [CI], 0.13–0.70). When the submucous fibroids were resected, the relative risk of pregnancy rose to 1.72 (CI, 1.13–2.58).3
Preoperative evaluation of fibroids
Severity of menorrhagia (the most common symptom) is considered directly related to the volume of the myoma within the endometrial cavity. It is not uncommon to see large tortuous vessels covering the surface of the fibroids; although the exact mechanism of fibroid-related menorrhagia is undetermined, the fragility of these vessels is probably responsible, at least in part.
Additionally, fibroids involving the uterine mucosa or submucosa may interfere with the muscular contraction necessary for hemostasis.
Surgical options and pretreatment depend on fibroid type. Submucous fibroids are classified according to the percentage of the fibroid within the endometrial cavity:4
- Type 0: pedunculated; 100% within the cavity
- Type I: more than 50% within the cavity
- Type II: more than 50% within the myometrium
The type dictates surgical options, determines endometrial pretreatment, and shapes patient expectations. Type 0 and Type I submucosal fibroids are more successfully removed in a single surgery, whereas Type II submucous fibroids usually require 2 procedures for complete removal.5
My preference is office hysteroscopy to evaluate the endometrial cavity combined with vaginal ultrasound to assess intramural disease. The view with the office hysteroscope is the same view you will have during surgery.
Patients with Type II myomas should be informed of the potential need for 2 procedures, as this fact often influences their treatment decisions. In addition, whenever a patient with a Type II myoma is pretreated with a gonadotropin-releasing hormone (GnRH) agonist, the physician should reassess the fibroid preoperatively to ensure that it has not become completely intramural.
Office assessment: Hysteroscopy plus ultrasound. Preoperative assessment can be achieved with a hysterosalpingogram, vaginal ultrasound, hysterosonogram, or office hysteroscopy.
Method. My preference is office hysteroscopy to evaluate the endometrial cavity combined with vaginal ultrasound to assess intramural disease. The view with the office hysteroscope is the same view you will have during surgery. There will be no surprises. With a small flexible hysteroscope using saline for distention, the procedure is done with no tenaculum, no paracervical block, and can be completed with 60 to 100 cc of fluid in less than 1 minute.
Patients are often intrigued to view the myoma responsible for their heavy vaginal bleeding.
Several advantages of preoperative hormonal therapy
Preoperative hormonal therapy has several advantages:
- Since it is best to resect submucous myomas when the endometrium is thin, hormonal therapy facilitates surgical scheduling.
- Since preoperative therapy creates a state of amenorrhea, it enables patients suffering from menorrhagia and anemia to build up their blood counts, reducing the need for transfusion.
- Most importantly, preoperative therapy can reduce blood flow to the uterus, thereby reducing the rate of fluid intravasation.
Adjuvants used for these functions include oral contraceptives, progestogens, danazol, and GnRH agonists. However, a recent Cochrane review suggests that only GnRH agonists reduce fluid absorption during operative hysteroscopy.6
Method. Numerous protocols have been published, but my preference is to administer 1 intramuscular injection of depot leuprolide acetate (7.5 mg) 6 weeks prior to surgery.
A review of ‘systems’
Submucous myomas have been hysteroscopically removed using the neodymium/yttrium aluminum-garnet (Nd:YAG) laser, a monopolar resectoscope loop, a monopolar radiofrequency (RF) vaporizing electrode, a bipolar RF vaporizing electrode, and a bipolar RF loop.
Laser. The Nd:YAG laser is an expensive device that was popular in the late 1980s and early 1990s. It can be used as a cutting tool for pedunculated Type 0 myomas, or it can be used for myolysis by burning numerous holes in the myoma, causing devascularization and shrinkage.7,8
The sole advantage of the Nd:YAG laser is that it can be used in an isotonic-fluid-filled cavity. Now that accurate fluid-monitoring devices are available, the Nd:YAG laser is rarely used for this indication.
Vaporizing electrodes. Both the bipolar system and monopolar vaporizing electrodes utilize very high RF power (200 W to 300 W) to vaporize fibroids. The advantage is that the fibroids are eradicated very quickly, without any bothersome fibroid chips to remove. The bipolar system can be used with isotonic fluid, whereas the monopolar vaporizing electrodes require non-electrolyte-containing distention media.
The main disadvantages of vaporizing electrodes are:
- They do not produce a tissue sample for pathology. While uterine sarcomas are very rare, they are not homogeneous. Therefore, a simple sample prior to vaporization does not rule out the disease.
- Since vaporizing electrodes are used at high power, numerous gas bubbles are produced and enter the vascular system. Fortunately, these bubbles dissipate rapidly in the blood. As long as the rate of formation does not exceed the rate of dissipation, there are no significant clinical sequelae. The surgeon can avoid complications by monitoring the patient’s endtidal CO2 and maintaining communication with the anesthesiologist. If a sudden drop in endtidal CO2 is observed, the surgeon should stop the case until it resolves.9
Monopolar loop electrode. The instrument most commonly used to remove submucosal myomas is the monopolar loop electrode with a continuous-flow resectoscope. This is quite similar to the instrument used to resect the prostate; it has been slightly modified for gynecologic use to provide enhanced fluid inflow and outflow. A key instrument now considered standard is an accurate fluid-monitoring device that can be attached to the resectoscope.
Distention media for monopolar resection. Prior to the introduction of continuous high-flow resectoscopes and fluid-management systems, monopolar resectoscopy could only be performed using a solution of 32% dextran 70 and water because of that formulation’s viscosity and facilitation of visualization in the presence of blood. However, due to complications related to absorption, allergies, and the solution’s sticky residue, it is not used with today’s high-flow technology.
The instrument most commonly used to remove submucosal myomas is the monopolar loop electrode with a continuous-flow resectoscope.
When using nonviscous fluids with monopolar RF energy, a distention medium without electrolytes must be selected. Isotonic physiologic media such as normal saline or lactated Ringer’s solution would cause the energy at the electrode to disperse, eliminating the cutting effect. Contrary to popular belief, there would be no burning of the uterine cavity and no subsequent danger to the patient.
The 3 most commonly used fluids for uterine distention are 1.5% glycine, 3% sorbitol, and 5% mannitol. All 3 lack electrolytes. The glycine and sorbitol solutions are hypotonic, while 5% mannitol is isotonic. Although there is some debate over whether an isotonic non-electrolyte-containing medium is safer than a hypotonic one, the guidelines for fluid monitoring are the same for all 3.
The goal of a hysteroscopic myomectomy is to alleviate symptoms without causing a weak myometrium or intracavitary synechia. This is accomplished by removing the fibroid without traumatizing normal uterine tissue.
As a general rule, the serum sodium level will decrease approximately 10 meq for every liter absorbed in the blood stream. That is why, to predict hyponatremia, the gynecologist must rely on exact measurements of fluid deficits rather than operating time or total volume used.
Fluid management. Significant hyponatremia can result in pulmonary edema, transient blindness, cerebral edema, brainstem herniation, and death. Catastrophic as these complications are, they are almost 100% avoidable with accurate deficit measurements and adherence to sound fluid-management protocols.
At our institution, fluids are monitored using a weighted system, and the fluid deficit is displayed on the surgical screen in real time. When the deficit reaches 1 L, electrolytes are drawn, the patient is given 10 mg of intravenous (IV) furosemide, and attempts are made to finish the surgery as soon as possible. When the deficit reaches 1.5 L, the surgery is discontinued no matter how much or how little remains to complete it.
It is important that the protocol at each institution be agreed upon by the chief of service and anesthesia as well as the director of nursing so that there are no conflicts at the time of surgery.
Uterine distention methods
Numerous methods have been used to distend the uterus: gravity, gas pressure, and electronic pumps and pressure bags. The optimal intrauterine pressure is the minimum pressure that allows for flow, distention, and visualization. Most of the time this ranges from 40 mm Hg to 60 mm Hg. The higher the intrauterine pressure, the more rapid the fluid intravasation, particularly when intrauterine pressure exceeds the mean arterial pressure.
Rapid fluid loss can occur even with low intrauterine pressures. That is because a large venous sinus will have a pressure of 8 mm Hg to 10 mm Hg, while the minimum uterine distention pressure is much greater. Strict adherence to fluid-deficit protocols will minimize the risk of complications from fluid overload.
Surgical technique
The goal of a hysteroscopic myomectomy is to alleviate symptoms without causing a weak myometrium or intracavitary synechia. Ideally, this is accomplished by completely removing the fibroid without traumatizing normal uterine tissue. Removal of the entire myoma reduces the likelihood of recurrence and regrowth.
Surgical technique for removal of Types 0, I, and II fibroids is illustrated and explained on (FIGURES1-4).
Within a given range (40 W to 100 W), it makes little difference which cutting-current setting on the RF generator you use; the principles are the same. The loop must be in contact with the tissue to be resected. Energy is applied and, once the circuit is completed (through tissue, the dispersive electrode, the generator and back to the loop), the loop is able to cut with minimal tactile feedback.
The lower the wattage, the more contact necessary between the loop and the tissue and the longer it takes to complete the circuit. Conversely, the higher the power, the faster the circuit is completed and cutting occurs.
Though it does not matter what setting is used, the operator must choose a setting based on his or her comfort level. Lower power gives the operator more control. Higher power should be used only by the most experienced hysteroscopist, since it involves very little tactile feedback and a higher risk of uterine perforation. At our teaching institution, we use 60 W of pure cutting current.
The technique for removing a submucous fibroid depends on its type and location within the endometrial cavity.
Type 0. Because these fibroids are pedunculated, the operator has the option of cutting the stalk (FIGURE 1) or shaving the myoma to remove it in pieces through the cervix. If the stalk is cut, it often is difficult to remove the fibroid through the cervix due to its large size. Nor can the fibroid be cut with monopolar energy because of the difficulty of completing the circuit. Type 0 myomas can be grabbed blindly with a Corson forceps or under direct visualization with an Isaacson optical tenaculum (Karl Storz Endoscopy, Culver City, Calif.). It also is acceptable to leave the fibroid in the cavity and let it degenerate and be expelled spontaneously. The risk of infection is very small.
My preference is to shave the fibroid down to the endometrium while it is attached to the stalk. I find it easier to remove when it is anchored rather than trying to pull out a single large specimen.
Type I. In a Type I myoma, more than 50% of the fibroid is within the uterine cavity, with a smaller portion embedded in the myometrium. To remove these fibroids, shave each to the level of the endometrium. To do so, place the loop behind the fibroid to be resected (FIGURE 5). Next, activate the RF energy using a foot pedal and, once the circuit is complete, draw the loop back into the resectoscope. Be careful to maintain visualization of the fibroid, loop, and cavity (FIGURE 6). This becomes difficult as the fibroid approaches the resectoscope and obstructs the hysteroscopic lens. To avoid this, bring the loop—which begins its cut fully extended—only halfway back to the resectoscope (FIGURE 7). Then retract the resectoscope itself, with the loop halfway extended, until the cut through the fibroid is complete (FIGURE 8). The loop then is retracted back into the resectoscope. The angle of the resectoscope must be adjusted to maintain loop contact with the fibroid tissue.
When more than 70% or 80% of the fibroid has been resected, the remainder will slough spontaneously over the next 2 to 4 months.
Once the fibroid is resected to the level of the endometrium, remove the fibroid chips (FIGURE 2). Often, when the hysteroscope is removed and uterine distention is reestablished, more of the fibroid will protrude into the cavity, facilitating safe resection. At some point, the fibroid will no longer protrude into the cavity; it will have been removed or only a small percentage will remain intramural.
Often, when more than 70% or 80% of the fibroid has been resected, the remainder will slough spontaneously over the next 2 to 4 months. The technique for removing the intramural fibroid is described on page 78 (FIGURE 3).
Type II. The first step in resecting a Type II myoma is shaving the intracavitary portion to the level of the endometrium, as in the resection of a Type I fibroid. Often, once a small Type II myoma is “unroofed,” the remainder will spontaneously fall into the cavity and can easily be removed.
Larger Type II myomas require a different approach. In these, the goal is to identify the pseudo-capsular plane that allows for blunt dissection. Once you have identified it, use the loop electrode to bluntly dissect (without RF activation) the fibroid from the myometrium. Place the electrode in the capsular plane and retract as much of the myoma into the cavity as possible (FIGURE 4). Another way to do this is to use a twisting motion—similar to those performed in open myomectomies—with the optical tenaculum. Once the fibroid is in the cavity, shave it to the level of the endometrium. Repeat this technique until you have removed as much of the fibroid as possible. Use minimal distention pressure when you perform this technique so that the fibroid will not be pressed back into the myometrium.
Underutilization of hysteroscopic myomectomy may be due to inadequate training in the technique or a misconception that it is difficult to learn. Fortunately, hysteroscope manufacturers are beginning to offer physicians and operating room personnel didactic and hands-on laboratory courses.
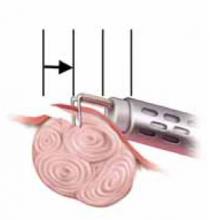
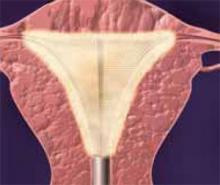
FIGURE 1 Surgical technique for removing Type 0, I, II fibroids
Type 0. The loop is placed behind the fibroid. Once activated, the loop is drawn toward the operator to resect the pedicle.
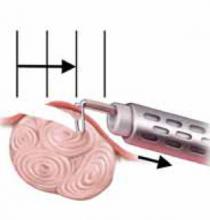
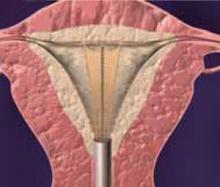
FIGURE 2 Surgical technique for removing Type 0, I, II fibroids
Type I. The myoma is resected to the level of the myometrium.
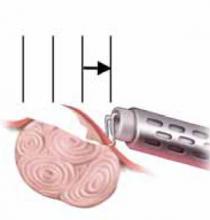
FIGURE 3 Surgical technique for removing Type 0, I, II fibroids
Resection of intramural portion of Type I and II. The resectoscopic loop is placed in the pseudocapsular lane between the fibroid and the myometrium to bluntly dissect the fibroid into the cavity for further resection.
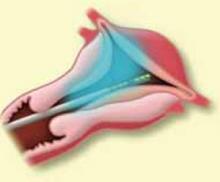
FIGURE 4 Surgical technique for removing Type 0, I, II fibroids
Type II. The roof of the myoma is shaved off to the level of the myometrium. The insert demonstrates the intramural portion of the myoma that will protrude into the cavity with a reduction in uterine distension pressure.
FIGURE 5 Resect technique—4 steps in resecting a submucous myoma
The loop is placed behind the fibroid with maximal contact with the tissue before RF activation.
FIGURE 6 Resect technique—4 steps in resecting a submucous myoma
The loop is withdrawn toward the operator (see arrow) while cutting the myoma but not so far as to obstruct the hysteroscopic view.
FIGURE 7 Resect technique—4 steps in resecting a submucous myoma
The loop is held in place while the hysteroscope is withdrawn (arrows indicate how to remain in direct contact with myoma) continuing to cut the myoma with visualization.
FIGURE 8 Resect technique—4 steps in resecting a submucous myoma
Once through the myoma, the loop is withdrawn into the hysteroscope.
Management of complications
The primary complications of hysteroscopic myomectomy are bleeding, infection, uterine perforation, and fluid overload.
Bleeding. If postoperative hemorrhage occurs, use a 30-cc Foley balloon to tamponade the vessels, or pack the uterus with gauze soaked in a vasopressin solution. Both can be removed any time from 4 hours to 24 hours following placement. If bleeding persists, uterine artery embolization should be considered prior to hysterectomy.
Most attempts at controlling bleeding with a rollerball are futile because the intrauterine distention pressure necessary to see the venous bleeders hides the actual bleeding. Arterial bleeders can be seen and controlled with the rollerball, but they are much less common.
Infection. Because infection is uncommon with hysteroscopic surgery, routine prophylaxis is unnecessary.
Antibiotics should be used if the patient had cervical laminaria placed preoperatively.
Uterine perforation. The management of uterine perforation depends on the site of perforation and whether energy was used during the perforation. For example, if the perforation was at the fundus and occurred with a blunt, non-energized instrument, observation without laparoscopy is sufficient. If the perforation was lateral or occurred with RF energy, laparoscopy is suggested to rule out a broad ligament hematoma or injury to intraperitoneal vessels and viscera. If the perforation is anterior, a cystoscopy is recommended; internal colon inspection is necessary if the perforation is posterior.
Fluid overload. Complications and prevention can be avoided and treated as previously discussed.
New training opportunities may include virtual reality
Underutilization of hysteroscopic myomectomy may be due to inadequate training in the technique during residency or the misconception that it is difficult to learn. (Training has been further hampered by the lack of a suitable animal model.)
Fortunately, hysteroscope manufacturers are beginning to offer physicians and operating room personnel didactic and hands-on laboratory courses. In the near future, we hope, these companies will hire gynecologic hysteroscopists to support physicians and nurses in the field, as has been done in many other specialties.
The creation of new tools such as virtual-reality simulators will undoubtedly enhance the teaching of operative hysteroscopy.
Dr. Isaacson teaches hysteroscopy courses sponsored by Karl Storz Endoscopy.
- The goal of hysteroscopic myomectomy is complete removal of the fibroid without trauma to normal uterine tissue.
- Patients with Type 0 and Type I fibroids often require only 1 surgery; patients with Type II fibroids should be advised that 2 surgeries may be needed to remove the entire fibroid.
- Adjuvant preoperative hormonal therapy facilitates surgical scheduling, helps prevent further blood loss in patients already suffering from anemia, and reduces distention media intravasation.
- The monopolar loop electrode is the fibroid removal system that is used most often.
Hysteroscopic myomectomy should be offered to all patients with symptomatic submucous fibroids who desire to avoid hysterectomy. Although it is a highly effective, minimally invasive technique, it is underutilized.
Unfortunately, fewer than one third of US gynecologists perform this procedure. In a 1997 survey of members of the American Association of Gynecologic Laparoscopists—an organization committed to minimally invasive surgery—only half of the respondents reported that they perform this surgery.1
The reasons for learning to perform hysteroscopic myomectomy are compelling:
- A large cohort of patients could benefit, since most heavy vaginal bleeding from fibroids is due to the submucous location, and hysteroscopic resection is a much more benign approach than hysterectomy. Symptomatic fibroids account for 27% of all hysterectomies performed in the US (the largest single diagnostic category) and more than 100,000 are performed for fibroids that cause abnormal uterine bleeding.2
- Removing these lesions hysteroscopically greatly improves prognosis in women with recurrent pregnancy loss and infertility due to submucous fibroids.3 Up to 15% of patients presenting with infertility have otherwise asymptomatic uterine defects, including submucous fibroids. For example, a meta-analysis of patients undergoing in vitro fertilization determined that, compared with controls, the relative risk of pregnancy for women with submucous fibroids was 0.32 (95% confidence interval [CI], 0.13–0.70). When the submucous fibroids were resected, the relative risk of pregnancy rose to 1.72 (CI, 1.13–2.58).3
Preoperative evaluation of fibroids
Severity of menorrhagia (the most common symptom) is considered directly related to the volume of the myoma within the endometrial cavity. It is not uncommon to see large tortuous vessels covering the surface of the fibroids; although the exact mechanism of fibroid-related menorrhagia is undetermined, the fragility of these vessels is probably responsible, at least in part.
Additionally, fibroids involving the uterine mucosa or submucosa may interfere with the muscular contraction necessary for hemostasis.
Surgical options and pretreatment depend on fibroid type. Submucous fibroids are classified according to the percentage of the fibroid within the endometrial cavity:4
- Type 0: pedunculated; 100% within the cavity
- Type I: more than 50% within the cavity
- Type II: more than 50% within the myometrium
The type dictates surgical options, determines endometrial pretreatment, and shapes patient expectations. Type 0 and Type I submucosal fibroids are more successfully removed in a single surgery, whereas Type II submucous fibroids usually require 2 procedures for complete removal.5
My preference is office hysteroscopy to evaluate the endometrial cavity combined with vaginal ultrasound to assess intramural disease. The view with the office hysteroscope is the same view you will have during surgery.
Patients with Type II myomas should be informed of the potential need for 2 procedures, as this fact often influences their treatment decisions. In addition, whenever a patient with a Type II myoma is pretreated with a gonadotropin-releasing hormone (GnRH) agonist, the physician should reassess the fibroid preoperatively to ensure that it has not become completely intramural.
Office assessment: Hysteroscopy plus ultrasound. Preoperative assessment can be achieved with a hysterosalpingogram, vaginal ultrasound, hysterosonogram, or office hysteroscopy.
Method. My preference is office hysteroscopy to evaluate the endometrial cavity combined with vaginal ultrasound to assess intramural disease. The view with the office hysteroscope is the same view you will have during surgery. There will be no surprises. With a small flexible hysteroscope using saline for distention, the procedure is done with no tenaculum, no paracervical block, and can be completed with 60 to 100 cc of fluid in less than 1 minute.
Patients are often intrigued to view the myoma responsible for their heavy vaginal bleeding.
Several advantages of preoperative hormonal therapy
Preoperative hormonal therapy has several advantages:
- Since it is best to resect submucous myomas when the endometrium is thin, hormonal therapy facilitates surgical scheduling.
- Since preoperative therapy creates a state of amenorrhea, it enables patients suffering from menorrhagia and anemia to build up their blood counts, reducing the need for transfusion.
- Most importantly, preoperative therapy can reduce blood flow to the uterus, thereby reducing the rate of fluid intravasation.
Adjuvants used for these functions include oral contraceptives, progestogens, danazol, and GnRH agonists. However, a recent Cochrane review suggests that only GnRH agonists reduce fluid absorption during operative hysteroscopy.6
Method. Numerous protocols have been published, but my preference is to administer 1 intramuscular injection of depot leuprolide acetate (7.5 mg) 6 weeks prior to surgery.
A review of ‘systems’
Submucous myomas have been hysteroscopically removed using the neodymium/yttrium aluminum-garnet (Nd:YAG) laser, a monopolar resectoscope loop, a monopolar radiofrequency (RF) vaporizing electrode, a bipolar RF vaporizing electrode, and a bipolar RF loop.
Laser. The Nd:YAG laser is an expensive device that was popular in the late 1980s and early 1990s. It can be used as a cutting tool for pedunculated Type 0 myomas, or it can be used for myolysis by burning numerous holes in the myoma, causing devascularization and shrinkage.7,8
The sole advantage of the Nd:YAG laser is that it can be used in an isotonic-fluid-filled cavity. Now that accurate fluid-monitoring devices are available, the Nd:YAG laser is rarely used for this indication.
Vaporizing electrodes. Both the bipolar system and monopolar vaporizing electrodes utilize very high RF power (200 W to 300 W) to vaporize fibroids. The advantage is that the fibroids are eradicated very quickly, without any bothersome fibroid chips to remove. The bipolar system can be used with isotonic fluid, whereas the monopolar vaporizing electrodes require non-electrolyte-containing distention media.
The main disadvantages of vaporizing electrodes are:
- They do not produce a tissue sample for pathology. While uterine sarcomas are very rare, they are not homogeneous. Therefore, a simple sample prior to vaporization does not rule out the disease.
- Since vaporizing electrodes are used at high power, numerous gas bubbles are produced and enter the vascular system. Fortunately, these bubbles dissipate rapidly in the blood. As long as the rate of formation does not exceed the rate of dissipation, there are no significant clinical sequelae. The surgeon can avoid complications by monitoring the patient’s endtidal CO2 and maintaining communication with the anesthesiologist. If a sudden drop in endtidal CO2 is observed, the surgeon should stop the case until it resolves.9
Monopolar loop electrode. The instrument most commonly used to remove submucosal myomas is the monopolar loop electrode with a continuous-flow resectoscope. This is quite similar to the instrument used to resect the prostate; it has been slightly modified for gynecologic use to provide enhanced fluid inflow and outflow. A key instrument now considered standard is an accurate fluid-monitoring device that can be attached to the resectoscope.
Distention media for monopolar resection. Prior to the introduction of continuous high-flow resectoscopes and fluid-management systems, monopolar resectoscopy could only be performed using a solution of 32% dextran 70 and water because of that formulation’s viscosity and facilitation of visualization in the presence of blood. However, due to complications related to absorption, allergies, and the solution’s sticky residue, it is not used with today’s high-flow technology.
The instrument most commonly used to remove submucosal myomas is the monopolar loop electrode with a continuous-flow resectoscope.
When using nonviscous fluids with monopolar RF energy, a distention medium without electrolytes must be selected. Isotonic physiologic media such as normal saline or lactated Ringer’s solution would cause the energy at the electrode to disperse, eliminating the cutting effect. Contrary to popular belief, there would be no burning of the uterine cavity and no subsequent danger to the patient.
The 3 most commonly used fluids for uterine distention are 1.5% glycine, 3% sorbitol, and 5% mannitol. All 3 lack electrolytes. The glycine and sorbitol solutions are hypotonic, while 5% mannitol is isotonic. Although there is some debate over whether an isotonic non-electrolyte-containing medium is safer than a hypotonic one, the guidelines for fluid monitoring are the same for all 3.
The goal of a hysteroscopic myomectomy is to alleviate symptoms without causing a weak myometrium or intracavitary synechia. This is accomplished by removing the fibroid without traumatizing normal uterine tissue.
As a general rule, the serum sodium level will decrease approximately 10 meq for every liter absorbed in the blood stream. That is why, to predict hyponatremia, the gynecologist must rely on exact measurements of fluid deficits rather than operating time or total volume used.
Fluid management. Significant hyponatremia can result in pulmonary edema, transient blindness, cerebral edema, brainstem herniation, and death. Catastrophic as these complications are, they are almost 100% avoidable with accurate deficit measurements and adherence to sound fluid-management protocols.
At our institution, fluids are monitored using a weighted system, and the fluid deficit is displayed on the surgical screen in real time. When the deficit reaches 1 L, electrolytes are drawn, the patient is given 10 mg of intravenous (IV) furosemide, and attempts are made to finish the surgery as soon as possible. When the deficit reaches 1.5 L, the surgery is discontinued no matter how much or how little remains to complete it.
It is important that the protocol at each institution be agreed upon by the chief of service and anesthesia as well as the director of nursing so that there are no conflicts at the time of surgery.
Uterine distention methods
Numerous methods have been used to distend the uterus: gravity, gas pressure, and electronic pumps and pressure bags. The optimal intrauterine pressure is the minimum pressure that allows for flow, distention, and visualization. Most of the time this ranges from 40 mm Hg to 60 mm Hg. The higher the intrauterine pressure, the more rapid the fluid intravasation, particularly when intrauterine pressure exceeds the mean arterial pressure.
Rapid fluid loss can occur even with low intrauterine pressures. That is because a large venous sinus will have a pressure of 8 mm Hg to 10 mm Hg, while the minimum uterine distention pressure is much greater. Strict adherence to fluid-deficit protocols will minimize the risk of complications from fluid overload.
Surgical technique
The goal of a hysteroscopic myomectomy is to alleviate symptoms without causing a weak myometrium or intracavitary synechia. Ideally, this is accomplished by completely removing the fibroid without traumatizing normal uterine tissue. Removal of the entire myoma reduces the likelihood of recurrence and regrowth.
Surgical technique for removal of Types 0, I, and II fibroids is illustrated and explained on (FIGURES1-4).
Within a given range (40 W to 100 W), it makes little difference which cutting-current setting on the RF generator you use; the principles are the same. The loop must be in contact with the tissue to be resected. Energy is applied and, once the circuit is completed (through tissue, the dispersive electrode, the generator and back to the loop), the loop is able to cut with minimal tactile feedback.
The lower the wattage, the more contact necessary between the loop and the tissue and the longer it takes to complete the circuit. Conversely, the higher the power, the faster the circuit is completed and cutting occurs.
Though it does not matter what setting is used, the operator must choose a setting based on his or her comfort level. Lower power gives the operator more control. Higher power should be used only by the most experienced hysteroscopist, since it involves very little tactile feedback and a higher risk of uterine perforation. At our teaching institution, we use 60 W of pure cutting current.
The technique for removing a submucous fibroid depends on its type and location within the endometrial cavity.
Type 0. Because these fibroids are pedunculated, the operator has the option of cutting the stalk (FIGURE 1) or shaving the myoma to remove it in pieces through the cervix. If the stalk is cut, it often is difficult to remove the fibroid through the cervix due to its large size. Nor can the fibroid be cut with monopolar energy because of the difficulty of completing the circuit. Type 0 myomas can be grabbed blindly with a Corson forceps or under direct visualization with an Isaacson optical tenaculum (Karl Storz Endoscopy, Culver City, Calif.). It also is acceptable to leave the fibroid in the cavity and let it degenerate and be expelled spontaneously. The risk of infection is very small.
My preference is to shave the fibroid down to the endometrium while it is attached to the stalk. I find it easier to remove when it is anchored rather than trying to pull out a single large specimen.
Type I. In a Type I myoma, more than 50% of the fibroid is within the uterine cavity, with a smaller portion embedded in the myometrium. To remove these fibroids, shave each to the level of the endometrium. To do so, place the loop behind the fibroid to be resected (FIGURE 5). Next, activate the RF energy using a foot pedal and, once the circuit is complete, draw the loop back into the resectoscope. Be careful to maintain visualization of the fibroid, loop, and cavity (FIGURE 6). This becomes difficult as the fibroid approaches the resectoscope and obstructs the hysteroscopic lens. To avoid this, bring the loop—which begins its cut fully extended—only halfway back to the resectoscope (FIGURE 7). Then retract the resectoscope itself, with the loop halfway extended, until the cut through the fibroid is complete (FIGURE 8). The loop then is retracted back into the resectoscope. The angle of the resectoscope must be adjusted to maintain loop contact with the fibroid tissue.
When more than 70% or 80% of the fibroid has been resected, the remainder will slough spontaneously over the next 2 to 4 months.
Once the fibroid is resected to the level of the endometrium, remove the fibroid chips (FIGURE 2). Often, when the hysteroscope is removed and uterine distention is reestablished, more of the fibroid will protrude into the cavity, facilitating safe resection. At some point, the fibroid will no longer protrude into the cavity; it will have been removed or only a small percentage will remain intramural.
Often, when more than 70% or 80% of the fibroid has been resected, the remainder will slough spontaneously over the next 2 to 4 months. The technique for removing the intramural fibroid is described on page 78 (FIGURE 3).
Type II. The first step in resecting a Type II myoma is shaving the intracavitary portion to the level of the endometrium, as in the resection of a Type I fibroid. Often, once a small Type II myoma is “unroofed,” the remainder will spontaneously fall into the cavity and can easily be removed.
Larger Type II myomas require a different approach. In these, the goal is to identify the pseudo-capsular plane that allows for blunt dissection. Once you have identified it, use the loop electrode to bluntly dissect (without RF activation) the fibroid from the myometrium. Place the electrode in the capsular plane and retract as much of the myoma into the cavity as possible (FIGURE 4). Another way to do this is to use a twisting motion—similar to those performed in open myomectomies—with the optical tenaculum. Once the fibroid is in the cavity, shave it to the level of the endometrium. Repeat this technique until you have removed as much of the fibroid as possible. Use minimal distention pressure when you perform this technique so that the fibroid will not be pressed back into the myometrium.
Underutilization of hysteroscopic myomectomy may be due to inadequate training in the technique or a misconception that it is difficult to learn. Fortunately, hysteroscope manufacturers are beginning to offer physicians and operating room personnel didactic and hands-on laboratory courses.
FIGURE 1 Surgical technique for removing Type 0, I, II fibroids
Type 0. The loop is placed behind the fibroid. Once activated, the loop is drawn toward the operator to resect the pedicle.
FIGURE 2 Surgical technique for removing Type 0, I, II fibroids
Type I. The myoma is resected to the level of the myometrium.
FIGURE 3 Surgical technique for removing Type 0, I, II fibroids
Resection of intramural portion of Type I and II. The resectoscopic loop is placed in the pseudocapsular lane between the fibroid and the myometrium to bluntly dissect the fibroid into the cavity for further resection.
FIGURE 4 Surgical technique for removing Type 0, I, II fibroids
Type II. The roof of the myoma is shaved off to the level of the myometrium. The insert demonstrates the intramural portion of the myoma that will protrude into the cavity with a reduction in uterine distension pressure.
FIGURE 5 Resect technique—4 steps in resecting a submucous myoma
The loop is placed behind the fibroid with maximal contact with the tissue before RF activation.
FIGURE 6 Resect technique—4 steps in resecting a submucous myoma
The loop is withdrawn toward the operator (see arrow) while cutting the myoma but not so far as to obstruct the hysteroscopic view.
FIGURE 7 Resect technique—4 steps in resecting a submucous myoma
The loop is held in place while the hysteroscope is withdrawn (arrows indicate how to remain in direct contact with myoma) continuing to cut the myoma with visualization.
FIGURE 8 Resect technique—4 steps in resecting a submucous myoma
Once through the myoma, the loop is withdrawn into the hysteroscope.
Management of complications
The primary complications of hysteroscopic myomectomy are bleeding, infection, uterine perforation, and fluid overload.
Bleeding. If postoperative hemorrhage occurs, use a 30-cc Foley balloon to tamponade the vessels, or pack the uterus with gauze soaked in a vasopressin solution. Both can be removed any time from 4 hours to 24 hours following placement. If bleeding persists, uterine artery embolization should be considered prior to hysterectomy.
Most attempts at controlling bleeding with a rollerball are futile because the intrauterine distention pressure necessary to see the venous bleeders hides the actual bleeding. Arterial bleeders can be seen and controlled with the rollerball, but they are much less common.
Infection. Because infection is uncommon with hysteroscopic surgery, routine prophylaxis is unnecessary.
Antibiotics should be used if the patient had cervical laminaria placed preoperatively.
Uterine perforation. The management of uterine perforation depends on the site of perforation and whether energy was used during the perforation. For example, if the perforation was at the fundus and occurred with a blunt, non-energized instrument, observation without laparoscopy is sufficient. If the perforation was lateral or occurred with RF energy, laparoscopy is suggested to rule out a broad ligament hematoma or injury to intraperitoneal vessels and viscera. If the perforation is anterior, a cystoscopy is recommended; internal colon inspection is necessary if the perforation is posterior.
Fluid overload. Complications and prevention can be avoided and treated as previously discussed.
New training opportunities may include virtual reality
Underutilization of hysteroscopic myomectomy may be due to inadequate training in the technique during residency or the misconception that it is difficult to learn. (Training has been further hampered by the lack of a suitable animal model.)
Fortunately, hysteroscope manufacturers are beginning to offer physicians and operating room personnel didactic and hands-on laboratory courses. In the near future, we hope, these companies will hire gynecologic hysteroscopists to support physicians and nurses in the field, as has been done in many other specialties.
The creation of new tools such as virtual-reality simulators will undoubtedly enhance the teaching of operative hysteroscopy.
Dr. Isaacson teaches hysteroscopy courses sponsored by Karl Storz Endoscopy.
1. Hulka JF, Levy BS, Luciano AA, Parker WH, Phillips JM. 1997 AAGL membership survey: practice profiles. J Amer Assoc Gynecol Laparosc. 1998;5:93-96.
2. Carlson KJ, Nichols DH, Schiff I. Indications for hysterectomy. N Eng J Med. 1993;328:856-860.
3. Pritts E. Fibroids and infertility: a systematic review of the evidence. Obstet Gynecol Survey. 2001;56:483-491.
4. deBlok S, Dijkman AB, Hemrika DJ. Transcervical resection of fibroids (TCRM): results related to hysteroscopic classification. Gynaecol Endosc. 1995;4:243-246.
5. Istre O. Transcervical resection of endometrium and fibroids: the outcome of 412 operations performed over 5 years. Acta Obstet Gynecol Scand. 1996;75:567-574.
6. Sowter MC, Lethaby A, Singla AA. Preoperative endometrial thinning agents before hysteroscopic surgery for heavy menstrual bleeding. Cochrane Database Syst Rev. 2002;(3):CD001124.-
7. Baggish MS, Sze EM, Morgan G. Hysteroscopic treatment of symptomatic submucous myomas with the Nd:YAG laser. J Gynecol Surg. 1989;5:27-36.
8. Donnez J, Gillerot S, Bourgonjon D, et al. Neodymium:YAG laser hysteroscopy in large submucous fibroids. Fertil Steril. 1990;54:999-1003.
9. Bloomstone J, Chen C, Isselbacher E, Isaacson K. A pilot study examining the frequency and quantity of gas embolization during operative hysteroscopy using a monopolar resectoscope. J Am Assoc Gynecol Laparosc. 2002;9:9-14.
1. Hulka JF, Levy BS, Luciano AA, Parker WH, Phillips JM. 1997 AAGL membership survey: practice profiles. J Amer Assoc Gynecol Laparosc. 1998;5:93-96.
2. Carlson KJ, Nichols DH, Schiff I. Indications for hysterectomy. N Eng J Med. 1993;328:856-860.
3. Pritts E. Fibroids and infertility: a systematic review of the evidence. Obstet Gynecol Survey. 2001;56:483-491.
4. deBlok S, Dijkman AB, Hemrika DJ. Transcervical resection of fibroids (TCRM): results related to hysteroscopic classification. Gynaecol Endosc. 1995;4:243-246.
5. Istre O. Transcervical resection of endometrium and fibroids: the outcome of 412 operations performed over 5 years. Acta Obstet Gynecol Scand. 1996;75:567-574.
6. Sowter MC, Lethaby A, Singla AA. Preoperative endometrial thinning agents before hysteroscopic surgery for heavy menstrual bleeding. Cochrane Database Syst Rev. 2002;(3):CD001124.-
7. Baggish MS, Sze EM, Morgan G. Hysteroscopic treatment of symptomatic submucous myomas with the Nd:YAG laser. J Gynecol Surg. 1989;5:27-36.
8. Donnez J, Gillerot S, Bourgonjon D, et al. Neodymium:YAG laser hysteroscopy in large submucous fibroids. Fertil Steril. 1990;54:999-1003.
9. Bloomstone J, Chen C, Isselbacher E, Isaacson K. A pilot study examining the frequency and quantity of gas embolization during operative hysteroscopy using a monopolar resectoscope. J Am Assoc Gynecol Laparosc. 2002;9:9-14.
Endometrial ablation: a look at the newest global procedures
- Global ablation methods involve destroying the endometrium using specialized devices that do not require an operative hysteroscope or resectoscope.
- The endometrium should be destroyed or resected to the level of the basalis, which is approximately 4 to 6 mm deep.
- In cryoablation, freezing the tissue causes less pain than the heat energy associated with other ablation devices. The procedure typically takes 10 to 20 minutes.
- Not only is bipolar desiccation quick and simple, no endometrial pretreatment is required because the system allows for consistent depths of ablation regardless of endometrial thickness.
- The advantage of hydrothermal ablation is that the circulating hot saline solution contacts the entire endometrial surface regardless of the shape or size of the cavity.
Traditionally, physicians have preferred hysterectomy for the treatment of abnormal uterine bleeding not related to endometrial cancer, representing about 20% of the 590,000 hysterectomie performed annually in the United States.1 The advent of standard endometrial ablation, e.g., surgical resection and rollerball desiccation, offered women less radical alternatives to hysterectomy. However, these “classic” methods are considered by some physicians to be technically difficult because they require the use of an operative hysteroscope—and all of its attendant risks. As a result, many Ob/Gyns continued to opt for hysterectomy when given a choice.
Now there are simpler alternatives: global ablation methods, including the newest—cryoablation, bipolar desiccation, and hydrothermal ablation. These procedures involve destroying the endometrium using specialized devices that do not require an operative hysteroscope or resectoscope. The advantage of these methods is that they are simple, rapid procedures that are easier to perform than standard endometrial ablation. Many surgeons who were uncomfortable utilizing the standard resectoscope can offer this treatment option to women with menorrhagia. In fact, it generally takes clinicians only 5 to 10 procedures to become proficient in endometrial ablation.
While global ablation is not intended to replace hysterectomy—the definitive treatment for most uterine pathology2—it gives patients a choice. Women who want permanent cessation of menses can choose hys-terectomy. Those who want to preserve the uterus or desire an outpatient procedure with minimal morbidity may opt for endometrial ablation. Regardless of the method they choose, patients who participate in the deci-sion-making process are more likely to be satisfied with their outcome.
The thinner the endometrial lining, the more likely ablation will be successful.
Part of this process includes an informed-consent discussion with the patient, including a review of each technique, its risks and complications, and outcomes. The long-term consequences of endometrial ablation remain unknown. The reason: Follow-up data beyond 24 months are minimal. And another question remains unanswered: How many women who have undergone endometrial ablation will develop endometrial carcinoma without symptomatic bleeding? There has been at least 1 report of this phenomenon occurring in a woman who ultimately underwent a hysterectomy for other reasons.3 Other case reports have noted uterine bleeding as the presenting symptom of endometrial carcinoma in women who had undergone ablation.4-6 The bottom line: The risk of asymptomatic endometrial cancer appears to be small in properly selected patients, i.e.,women without a history of endometrial hyperplasia or carcinoma (Table 1).
TABLE 1
Patient selection
INDICATIONS
|
CONTRAINDICATIONS
|
Preparation
Prior to performing any endometrial ablation, obtain an endometrial sample to rule out premalignant or malignant disease. Also, assess the endometrial cavity using either office hysteroscopy or sonohysterography to exclude the possibility of submucous myomata or polyps, which can be treated with simple resection. These imaging techniques also can reveal abnormally shaped uterine cavities, thus eliminating certain women as candidates for global techniques such as balloon ablation.
For all methods, destroy or resect the endometrium to the level of the basalis, which is approximately 4 to 6 mm deep, depending upon the stage of the menstrual cycle or cycle suppression. It is reasonable to assume that the thinner the endometrial lining, the more likely ablation will be successful. Several methods of producing endometrial atrophy have been used, including dilatation and curettage (D&C) and hormonal suppression with medroxyprogesterone acetate, oral contraceptives (OCs), danocrine, or gonadotropin releasing hormone ago-nists (GnRH-a). I prefer to use leuprolide acetate (7.5 mg for 1 dose) 4 to 5 weeks prior to performing cryoablation or hydrothermal ablation. (Pretreatment of the endometrial lining is not necessary with bipolar desiccation.)
Many patients who undergo endometrial ablation via either standard or global techniques can tolerate the procedure well with local anesthesia and intravenous sedation. This is especially true for global procedures because they are of short duration, i.e., about 5 to 20 minutes, depending on the method. Much of the pain results from cervical dilation and manipulation, as well as trauma to the endometrium. Therefore, the global ablation techniques that utilize the smallest probe (5.5 mm or smaller) and freeze the uterus, i.e., cryoablation, require the least anesthesia.
After the patient has been properly selected and prepared, proceed with one the global techniques—cryoablation, bipolar desiccation, or hydrothermal ablation—outlined in this article.
Cryoablation
Technique. Dilate the cervix and insert a 5.5-mm cryoprobe into the uterine cavity. Cool the probe to less than-80 degrees Celsius by liquid differential gas exchange, described by Joule-Thompson, utilizing the cooling unit (Her Option; CryoGen, San Diego, Calif). An elliptical ice ball approximately 3.5 by 5 cm will then form around the probe.
Using abdominal ultrasound guidance, follow the edge of the ice ball into the uterine muscle at 1 cornu. (While the edge of the ice ball reaches 0 degrees Celsius, which is nondestructive to surrounding tissue, the endometrium is permanently destroyed approximately 1.5 cm from the edge of the ice ball, where a temperature of-20 degrees Celsius is reached.) Before the outer edge of the ice ball approaches the serosa of the uterus, stop the procedure (Figure 1). Heat the probe to body temperature and remove it from the cornu. Repeat the process in the contralateral cornu (Figure 2) and, in large uteri (greater than 10 cm sound), in the lower uterine segment (Figure 3). Each freeze cycle takes about 5 to 6 minutes.
FIGURE 1
Using ultrasound guidance, follow the edge of the ice ball into the uterine muscle at 1 cornu. Stop before the edge of the ice ball approaches the serosa.
FIGURE 2
Heat the probe to body temperature, remove it from the first cornu, and repeat the process in the contralateral cornu.
FIGURE 3
For large uteri (greater than 10 cm sound), perform another freeze cycle in the lower uterine segment. Each cycle takes about 5 to 6 minutes.The number of ice balls needed to destroy the entire uterine cavity and the length of time required to perform the procedure depend on the size of the cavity. In general, 2 to 3 ice balls are sufficient, and the entire procedure takes 10 to 20 minutes.
In a recent randomized multicenter study, researchers investigated whether a freeze cycle longer than recommended would result in better outcomes. At 12-month follow-up, 7 (64%) of the 12 patients who underwent treatments of 5 to 7 minutes rather than 4 minutes were amenorrheic/spotting, with a 91% success rate.7 Larger studies are underway to confirm these data. Further, repeat treatment can contribute to better outcomes, especially when the patient receives leuprolide acetate prior to the procedure.8
Advantages. The primary advantage of cryoab-lation is that it is not a totally blind procedure. The visual feedback provided by the ultrasound facilitates complete ablation of the entire uterine cavity, regardless of size. Further, while the hysteroscope used in standard ablation allows the physician to see only the destruction of the surface epithelium, ultrasound enables the surgeon to visualize the depth of treatment during cryoablation.
Another advantage: Freezing tissue causes less pain (cryoanesthesia) than the heat energy associated with other ablation devices.
Disadvantages. While ultrasound visualization has its benefits, it also can complicate matters because accurate imaging and interpretation require a certain level of skill. For Ob/Gyns not experienced in ultrasound, there is a potential for poor positioning of the probe, which could lead to bowel and bladder injuries. Further, physicians can mistakenly place the cryoprobe back into the treated cornu instead of the contralateral side, leading to ablation of the uterine serosa. Lastly, the technology involved in this procedure increases the cost of the equipment and disposable instruments.
What the evidence shows. According to an unpublished study conducted by Cryogen, 54% of 222 patients experienced amenorrhea or staining only at 6-month follow-up. Ninety-three percent of the patients had a Patient Bleeding Assessment Card (PBAC) score of less than 75, and 75% of the patients had a greater than 90% reduction in their PBAC scores. (A score of greater than 150 was needed to enter the study; the score is reached by assigning a value to the amount of staining on a validated pad and adding these points based on the number of pads used per cycle.)
Cost. The disposable cryoprobes are $1,250 per case, and the unit costs $29,995. Since most ablation procedures currently are performed in the operating room, ancillary costs of the techniques are similar.
Bipolar desiccation
Technique. After dilating the cervix, insert the 8-mm NovaSure System (Novacept, Palo Alto, Calif) catheter into the uterus. To rule out auterine perforation prior to beginning the procedure, insert carbon dioxide (CO2) into the cavity (Figure 4).The machine will not turn on if a seal of CO2 has not been created, unless the surgeon overrides the system.
Then expand the 3-dimensional gold-plated bipolar mesh electrode, which imitates the shape of the uterine cavity, out of the catheter. Gently push the probe toward the fundus so that the mesh electrode is accurately positioned within the uterine cavity (Figure 5). Once the electrode is activated with up to 180 W of bipolar power, gentle suction brings the endometrium into close contact with the mesh (Figure 6). This suction also removes debris and allows for more complete desiccation.
The entire bipolar desiccation procedure takes less than 5 minutes to perform.
The system shuts down automatically when complete desiccation (calculated at 50 ohms of resistance) has occurred. The average treatment time is just over 1 minute, and the average depth of ablation is 4 to 5 mm.9 Once ablation is complete, retract the mesh electrode and remove the catheter (Figure 7). The entire procedure takes less than 5 minutes.
Advantages. Not only is the technique quick and simple, no endometrial pretreatment is required because the system allows for consistent depths of ablation regardless of endometrial thickness.
Disadvantages. There are limited data on the 1-year success rates for this completely blind procedure. In addition, the uterus needs to be of normal shape to successfully ablate the endometrium.
What the evidence shows. Unpublished data from randomized, controlled clinical trials comparing bipolar desiccation with balloon ablation have demonstrated that the former achieves higher amenorrhea and success (a return to normal menstrual bleeding) rates than the latter. Further, patients treated with bipolar desiccation experienced less pain, both intra-operatively and postoperatively, than women who underwent balloon ablation.
Cost. Each disposable probe costs $850, and the bipolar controller lists for $15,000.
FIGURE 4
Dilate the cervix and insert the 8-mm catheter. To rule out a uterine perforation prior to beginning the procedure, place carbon dioxide in the cavity.
FIGURE 5
Expand the bipolar mesh electrode. Gently push the probe toward the fundus to accurately position the electrode within the uterine cavity.
FIGURE 6
Activate the electrode with up to 180 W of bipolar power. A gentle suction brings the endometrium into close contact with the mesh.
FIGURE 7
The system shuts down automatically when complete desiccation has occurred. Retract the bipolar mesh electrode and remove the catheter.
Hydrothermal ablation/circulating heated saline
Technique. Begin by dilating the cervix and inserting the sheath, which includes an 8-mm hysteroscope (Hydro ThermAblator; BEI Medical Systems, Teterboro, NJ), into the uter-ine cavity. Using gravity rather than a pump, introduce saline solution through the sheath into the uterus. As the saline circulates, gradually heat it until the solution reaches and maintains 90 degrees Celsius for 10 minutes, completely destroying the endometrium. (The total time from insertion of the sheath to completion of the procedure is 17 minutes.)
A safety feature detects whether any fluid has escaped from the closed system; a shutoff mechanism activates if more than 10 cc of fluid are lost. The system is controlled by software, so even physicians who are inexperienced with hysteroscopy can easily perform this technique.
Advantages. The primary advantage of hydrothermal ablation (HTA) is that the circulating hot saline solution contacts the entire endometrial surface regardless of the size or shape of the uterine cavity. In addition, since the procedure is performed under direct visualization, the surgeon can ensure that the entire cavity has been thoroughly ablated.
Disadvantages. One drawback is that it can be a painful procedure because the hysteroscope is large, necessitating wide dilation of the cervix, and hot water stimulates pain. Further, there have been unpublished reports of vaginal burns when the cervix created a poor seal around the sheath.
What the evidence shows. Based on their research of 276 women (187 women in the HTA group and 89 patients in the rollerball group), investigators have found that HTA has a treatment success rate of 77% compared to 82% for rollerball ablation. (The treatment success rate is defined as a PBAC score of 75 or lower; eumenorrhea is a score of less than 100.) Amenorrhea rates at 12-month follow-up were 40% and 51%, respectively. The researchers concluded that HTA offers an advantage over rollerball ablation in that it reduces anesthesia requirements and eliminates the problem of hypotonic fluid absorption.10
Cost. Disposable hysteroscopic tubing is $625 per case. The machine costs $10,900 plus $395 for the heater canister.
The author reports no financial relationship with any companies whose products are mentioned in this article.
1. Carlson KJ, Nichols DH, Schiff I. Indications for hysterectomy. N Engl J Med. 1993;Mar 25 328(12):856-860.
2. Carlson KJ, Miller BA, Fowler FJ, Jr. The Maine Women’s Health Study: I. Outcomes of hysterectomy. Obstet Gynecol. 1994;83-556.
3. Margolis MT, et al. Asymptomatic endometrial carcinoma after endometrial ablation. Int J Gynaecol Obstet. 1995;51-255.
4. Gimpelson RJ. Not so benign endometrial hyperplasia: endometrial cancer after endometrial ablation. J Am Assoc Gynecol Laparosc. 1997;4-507.
5. Horowitz IR, Copas PR, Aaronoff, et al. Endometrial adenocarcinoma following endometrial ablation for postmenopausal bleeding. Gynecol Oncol. 1995;56:460.-
6. Valle RF, Baggish MS. Endometrial carcinoma after endometrial ablation: high-risk factors predicting its occurrence. Am J Obstet Gynecol. 1998;179:569.-
7. Heppard M, et al. Data on 222 patients from a multicenter study using Cryogen First Option uterine cryoablation therapy in women with abnormal uterine bleeding. Obstet Gynecol. 2001;97(4 Suppl 1):S21.-
8. Sanders B. Preliminary outcomes of longer freeze cycles in the treatment of women with abnormal uterine bleeding using Cryogen First Option uterine cryoablation therapy. Obstet Gynecol. 2001;97(4 Suppl 1):S14.-
9. Cooper JM, Erickson ML. Global endometrial ablation technologies. Obstet Gynecol Clin North Am. 2000;27-385.
10. Corson SL. A multicenter evaluation of endometrial ablation by Hydro ThermAblator and rollerball for treatment of menorrhagia. J Am Assoc Gynecol Laparosc. 2001;8(3):359-367.
- Global ablation methods involve destroying the endometrium using specialized devices that do not require an operative hysteroscope or resectoscope.
- The endometrium should be destroyed or resected to the level of the basalis, which is approximately 4 to 6 mm deep.
- In cryoablation, freezing the tissue causes less pain than the heat energy associated with other ablation devices. The procedure typically takes 10 to 20 minutes.
- Not only is bipolar desiccation quick and simple, no endometrial pretreatment is required because the system allows for consistent depths of ablation regardless of endometrial thickness.
- The advantage of hydrothermal ablation is that the circulating hot saline solution contacts the entire endometrial surface regardless of the shape or size of the cavity.
Traditionally, physicians have preferred hysterectomy for the treatment of abnormal uterine bleeding not related to endometrial cancer, representing about 20% of the 590,000 hysterectomie performed annually in the United States.1 The advent of standard endometrial ablation, e.g., surgical resection and rollerball desiccation, offered women less radical alternatives to hysterectomy. However, these “classic” methods are considered by some physicians to be technically difficult because they require the use of an operative hysteroscope—and all of its attendant risks. As a result, many Ob/Gyns continued to opt for hysterectomy when given a choice.
Now there are simpler alternatives: global ablation methods, including the newest—cryoablation, bipolar desiccation, and hydrothermal ablation. These procedures involve destroying the endometrium using specialized devices that do not require an operative hysteroscope or resectoscope. The advantage of these methods is that they are simple, rapid procedures that are easier to perform than standard endometrial ablation. Many surgeons who were uncomfortable utilizing the standard resectoscope can offer this treatment option to women with menorrhagia. In fact, it generally takes clinicians only 5 to 10 procedures to become proficient in endometrial ablation.
While global ablation is not intended to replace hysterectomy—the definitive treatment for most uterine pathology2—it gives patients a choice. Women who want permanent cessation of menses can choose hys-terectomy. Those who want to preserve the uterus or desire an outpatient procedure with minimal morbidity may opt for endometrial ablation. Regardless of the method they choose, patients who participate in the deci-sion-making process are more likely to be satisfied with their outcome.
The thinner the endometrial lining, the more likely ablation will be successful.
Part of this process includes an informed-consent discussion with the patient, including a review of each technique, its risks and complications, and outcomes. The long-term consequences of endometrial ablation remain unknown. The reason: Follow-up data beyond 24 months are minimal. And another question remains unanswered: How many women who have undergone endometrial ablation will develop endometrial carcinoma without symptomatic bleeding? There has been at least 1 report of this phenomenon occurring in a woman who ultimately underwent a hysterectomy for other reasons.3 Other case reports have noted uterine bleeding as the presenting symptom of endometrial carcinoma in women who had undergone ablation.4-6 The bottom line: The risk of asymptomatic endometrial cancer appears to be small in properly selected patients, i.e.,women without a history of endometrial hyperplasia or carcinoma (Table 1).
TABLE 1
Patient selection
INDICATIONS
|
CONTRAINDICATIONS
|
Preparation
Prior to performing any endometrial ablation, obtain an endometrial sample to rule out premalignant or malignant disease. Also, assess the endometrial cavity using either office hysteroscopy or sonohysterography to exclude the possibility of submucous myomata or polyps, which can be treated with simple resection. These imaging techniques also can reveal abnormally shaped uterine cavities, thus eliminating certain women as candidates for global techniques such as balloon ablation.
For all methods, destroy or resect the endometrium to the level of the basalis, which is approximately 4 to 6 mm deep, depending upon the stage of the menstrual cycle or cycle suppression. It is reasonable to assume that the thinner the endometrial lining, the more likely ablation will be successful. Several methods of producing endometrial atrophy have been used, including dilatation and curettage (D&C) and hormonal suppression with medroxyprogesterone acetate, oral contraceptives (OCs), danocrine, or gonadotropin releasing hormone ago-nists (GnRH-a). I prefer to use leuprolide acetate (7.5 mg for 1 dose) 4 to 5 weeks prior to performing cryoablation or hydrothermal ablation. (Pretreatment of the endometrial lining is not necessary with bipolar desiccation.)
Many patients who undergo endometrial ablation via either standard or global techniques can tolerate the procedure well with local anesthesia and intravenous sedation. This is especially true for global procedures because they are of short duration, i.e., about 5 to 20 minutes, depending on the method. Much of the pain results from cervical dilation and manipulation, as well as trauma to the endometrium. Therefore, the global ablation techniques that utilize the smallest probe (5.5 mm or smaller) and freeze the uterus, i.e., cryoablation, require the least anesthesia.
After the patient has been properly selected and prepared, proceed with one the global techniques—cryoablation, bipolar desiccation, or hydrothermal ablation—outlined in this article.
Cryoablation
Technique. Dilate the cervix and insert a 5.5-mm cryoprobe into the uterine cavity. Cool the probe to less than-80 degrees Celsius by liquid differential gas exchange, described by Joule-Thompson, utilizing the cooling unit (Her Option; CryoGen, San Diego, Calif). An elliptical ice ball approximately 3.5 by 5 cm will then form around the probe.
Using abdominal ultrasound guidance, follow the edge of the ice ball into the uterine muscle at 1 cornu. (While the edge of the ice ball reaches 0 degrees Celsius, which is nondestructive to surrounding tissue, the endometrium is permanently destroyed approximately 1.5 cm from the edge of the ice ball, where a temperature of-20 degrees Celsius is reached.) Before the outer edge of the ice ball approaches the serosa of the uterus, stop the procedure (Figure 1). Heat the probe to body temperature and remove it from the cornu. Repeat the process in the contralateral cornu (Figure 2) and, in large uteri (greater than 10 cm sound), in the lower uterine segment (Figure 3). Each freeze cycle takes about 5 to 6 minutes.
FIGURE 1
Using ultrasound guidance, follow the edge of the ice ball into the uterine muscle at 1 cornu. Stop before the edge of the ice ball approaches the serosa.
FIGURE 2
Heat the probe to body temperature, remove it from the first cornu, and repeat the process in the contralateral cornu.
FIGURE 3
For large uteri (greater than 10 cm sound), perform another freeze cycle in the lower uterine segment. Each cycle takes about 5 to 6 minutes.The number of ice balls needed to destroy the entire uterine cavity and the length of time required to perform the procedure depend on the size of the cavity. In general, 2 to 3 ice balls are sufficient, and the entire procedure takes 10 to 20 minutes.
In a recent randomized multicenter study, researchers investigated whether a freeze cycle longer than recommended would result in better outcomes. At 12-month follow-up, 7 (64%) of the 12 patients who underwent treatments of 5 to 7 minutes rather than 4 minutes were amenorrheic/spotting, with a 91% success rate.7 Larger studies are underway to confirm these data. Further, repeat treatment can contribute to better outcomes, especially when the patient receives leuprolide acetate prior to the procedure.8
Advantages. The primary advantage of cryoab-lation is that it is not a totally blind procedure. The visual feedback provided by the ultrasound facilitates complete ablation of the entire uterine cavity, regardless of size. Further, while the hysteroscope used in standard ablation allows the physician to see only the destruction of the surface epithelium, ultrasound enables the surgeon to visualize the depth of treatment during cryoablation.
Another advantage: Freezing tissue causes less pain (cryoanesthesia) than the heat energy associated with other ablation devices.
Disadvantages. While ultrasound visualization has its benefits, it also can complicate matters because accurate imaging and interpretation require a certain level of skill. For Ob/Gyns not experienced in ultrasound, there is a potential for poor positioning of the probe, which could lead to bowel and bladder injuries. Further, physicians can mistakenly place the cryoprobe back into the treated cornu instead of the contralateral side, leading to ablation of the uterine serosa. Lastly, the technology involved in this procedure increases the cost of the equipment and disposable instruments.
What the evidence shows. According to an unpublished study conducted by Cryogen, 54% of 222 patients experienced amenorrhea or staining only at 6-month follow-up. Ninety-three percent of the patients had a Patient Bleeding Assessment Card (PBAC) score of less than 75, and 75% of the patients had a greater than 90% reduction in their PBAC scores. (A score of greater than 150 was needed to enter the study; the score is reached by assigning a value to the amount of staining on a validated pad and adding these points based on the number of pads used per cycle.)
Cost. The disposable cryoprobes are $1,250 per case, and the unit costs $29,995. Since most ablation procedures currently are performed in the operating room, ancillary costs of the techniques are similar.
Bipolar desiccation
Technique. After dilating the cervix, insert the 8-mm NovaSure System (Novacept, Palo Alto, Calif) catheter into the uterus. To rule out auterine perforation prior to beginning the procedure, insert carbon dioxide (CO2) into the cavity (Figure 4).The machine will not turn on if a seal of CO2 has not been created, unless the surgeon overrides the system.
Then expand the 3-dimensional gold-plated bipolar mesh electrode, which imitates the shape of the uterine cavity, out of the catheter. Gently push the probe toward the fundus so that the mesh electrode is accurately positioned within the uterine cavity (Figure 5). Once the electrode is activated with up to 180 W of bipolar power, gentle suction brings the endometrium into close contact with the mesh (Figure 6). This suction also removes debris and allows for more complete desiccation.
The entire bipolar desiccation procedure takes less than 5 minutes to perform.
The system shuts down automatically when complete desiccation (calculated at 50 ohms of resistance) has occurred. The average treatment time is just over 1 minute, and the average depth of ablation is 4 to 5 mm.9 Once ablation is complete, retract the mesh electrode and remove the catheter (Figure 7). The entire procedure takes less than 5 minutes.
Advantages. Not only is the technique quick and simple, no endometrial pretreatment is required because the system allows for consistent depths of ablation regardless of endometrial thickness.
Disadvantages. There are limited data on the 1-year success rates for this completely blind procedure. In addition, the uterus needs to be of normal shape to successfully ablate the endometrium.
What the evidence shows. Unpublished data from randomized, controlled clinical trials comparing bipolar desiccation with balloon ablation have demonstrated that the former achieves higher amenorrhea and success (a return to normal menstrual bleeding) rates than the latter. Further, patients treated with bipolar desiccation experienced less pain, both intra-operatively and postoperatively, than women who underwent balloon ablation.
Cost. Each disposable probe costs $850, and the bipolar controller lists for $15,000.
FIGURE 4
Dilate the cervix and insert the 8-mm catheter. To rule out a uterine perforation prior to beginning the procedure, place carbon dioxide in the cavity.
FIGURE 5
Expand the bipolar mesh electrode. Gently push the probe toward the fundus to accurately position the electrode within the uterine cavity.
FIGURE 6
Activate the electrode with up to 180 W of bipolar power. A gentle suction brings the endometrium into close contact with the mesh.
FIGURE 7
The system shuts down automatically when complete desiccation has occurred. Retract the bipolar mesh electrode and remove the catheter.
Hydrothermal ablation/circulating heated saline
Technique. Begin by dilating the cervix and inserting the sheath, which includes an 8-mm hysteroscope (Hydro ThermAblator; BEI Medical Systems, Teterboro, NJ), into the uter-ine cavity. Using gravity rather than a pump, introduce saline solution through the sheath into the uterus. As the saline circulates, gradually heat it until the solution reaches and maintains 90 degrees Celsius for 10 minutes, completely destroying the endometrium. (The total time from insertion of the sheath to completion of the procedure is 17 minutes.)
A safety feature detects whether any fluid has escaped from the closed system; a shutoff mechanism activates if more than 10 cc of fluid are lost. The system is controlled by software, so even physicians who are inexperienced with hysteroscopy can easily perform this technique.
Advantages. The primary advantage of hydrothermal ablation (HTA) is that the circulating hot saline solution contacts the entire endometrial surface regardless of the size or shape of the uterine cavity. In addition, since the procedure is performed under direct visualization, the surgeon can ensure that the entire cavity has been thoroughly ablated.
Disadvantages. One drawback is that it can be a painful procedure because the hysteroscope is large, necessitating wide dilation of the cervix, and hot water stimulates pain. Further, there have been unpublished reports of vaginal burns when the cervix created a poor seal around the sheath.
What the evidence shows. Based on their research of 276 women (187 women in the HTA group and 89 patients in the rollerball group), investigators have found that HTA has a treatment success rate of 77% compared to 82% for rollerball ablation. (The treatment success rate is defined as a PBAC score of 75 or lower; eumenorrhea is a score of less than 100.) Amenorrhea rates at 12-month follow-up were 40% and 51%, respectively. The researchers concluded that HTA offers an advantage over rollerball ablation in that it reduces anesthesia requirements and eliminates the problem of hypotonic fluid absorption.10
Cost. Disposable hysteroscopic tubing is $625 per case. The machine costs $10,900 plus $395 for the heater canister.
The author reports no financial relationship with any companies whose products are mentioned in this article.
- Global ablation methods involve destroying the endometrium using specialized devices that do not require an operative hysteroscope or resectoscope.
- The endometrium should be destroyed or resected to the level of the basalis, which is approximately 4 to 6 mm deep.
- In cryoablation, freezing the tissue causes less pain than the heat energy associated with other ablation devices. The procedure typically takes 10 to 20 minutes.
- Not only is bipolar desiccation quick and simple, no endometrial pretreatment is required because the system allows for consistent depths of ablation regardless of endometrial thickness.
- The advantage of hydrothermal ablation is that the circulating hot saline solution contacts the entire endometrial surface regardless of the shape or size of the cavity.
Traditionally, physicians have preferred hysterectomy for the treatment of abnormal uterine bleeding not related to endometrial cancer, representing about 20% of the 590,000 hysterectomie performed annually in the United States.1 The advent of standard endometrial ablation, e.g., surgical resection and rollerball desiccation, offered women less radical alternatives to hysterectomy. However, these “classic” methods are considered by some physicians to be technically difficult because they require the use of an operative hysteroscope—and all of its attendant risks. As a result, many Ob/Gyns continued to opt for hysterectomy when given a choice.
Now there are simpler alternatives: global ablation methods, including the newest—cryoablation, bipolar desiccation, and hydrothermal ablation. These procedures involve destroying the endometrium using specialized devices that do not require an operative hysteroscope or resectoscope. The advantage of these methods is that they are simple, rapid procedures that are easier to perform than standard endometrial ablation. Many surgeons who were uncomfortable utilizing the standard resectoscope can offer this treatment option to women with menorrhagia. In fact, it generally takes clinicians only 5 to 10 procedures to become proficient in endometrial ablation.
While global ablation is not intended to replace hysterectomy—the definitive treatment for most uterine pathology2—it gives patients a choice. Women who want permanent cessation of menses can choose hys-terectomy. Those who want to preserve the uterus or desire an outpatient procedure with minimal morbidity may opt for endometrial ablation. Regardless of the method they choose, patients who participate in the deci-sion-making process are more likely to be satisfied with their outcome.
The thinner the endometrial lining, the more likely ablation will be successful.
Part of this process includes an informed-consent discussion with the patient, including a review of each technique, its risks and complications, and outcomes. The long-term consequences of endometrial ablation remain unknown. The reason: Follow-up data beyond 24 months are minimal. And another question remains unanswered: How many women who have undergone endometrial ablation will develop endometrial carcinoma without symptomatic bleeding? There has been at least 1 report of this phenomenon occurring in a woman who ultimately underwent a hysterectomy for other reasons.3 Other case reports have noted uterine bleeding as the presenting symptom of endometrial carcinoma in women who had undergone ablation.4-6 The bottom line: The risk of asymptomatic endometrial cancer appears to be small in properly selected patients, i.e.,women without a history of endometrial hyperplasia or carcinoma (Table 1).
TABLE 1
Patient selection
INDICATIONS
|
CONTRAINDICATIONS
|
Preparation
Prior to performing any endometrial ablation, obtain an endometrial sample to rule out premalignant or malignant disease. Also, assess the endometrial cavity using either office hysteroscopy or sonohysterography to exclude the possibility of submucous myomata or polyps, which can be treated with simple resection. These imaging techniques also can reveal abnormally shaped uterine cavities, thus eliminating certain women as candidates for global techniques such as balloon ablation.
For all methods, destroy or resect the endometrium to the level of the basalis, which is approximately 4 to 6 mm deep, depending upon the stage of the menstrual cycle or cycle suppression. It is reasonable to assume that the thinner the endometrial lining, the more likely ablation will be successful. Several methods of producing endometrial atrophy have been used, including dilatation and curettage (D&C) and hormonal suppression with medroxyprogesterone acetate, oral contraceptives (OCs), danocrine, or gonadotropin releasing hormone ago-nists (GnRH-a). I prefer to use leuprolide acetate (7.5 mg for 1 dose) 4 to 5 weeks prior to performing cryoablation or hydrothermal ablation. (Pretreatment of the endometrial lining is not necessary with bipolar desiccation.)
Many patients who undergo endometrial ablation via either standard or global techniques can tolerate the procedure well with local anesthesia and intravenous sedation. This is especially true for global procedures because they are of short duration, i.e., about 5 to 20 minutes, depending on the method. Much of the pain results from cervical dilation and manipulation, as well as trauma to the endometrium. Therefore, the global ablation techniques that utilize the smallest probe (5.5 mm or smaller) and freeze the uterus, i.e., cryoablation, require the least anesthesia.
After the patient has been properly selected and prepared, proceed with one the global techniques—cryoablation, bipolar desiccation, or hydrothermal ablation—outlined in this article.
Cryoablation
Technique. Dilate the cervix and insert a 5.5-mm cryoprobe into the uterine cavity. Cool the probe to less than-80 degrees Celsius by liquid differential gas exchange, described by Joule-Thompson, utilizing the cooling unit (Her Option; CryoGen, San Diego, Calif). An elliptical ice ball approximately 3.5 by 5 cm will then form around the probe.
Using abdominal ultrasound guidance, follow the edge of the ice ball into the uterine muscle at 1 cornu. (While the edge of the ice ball reaches 0 degrees Celsius, which is nondestructive to surrounding tissue, the endometrium is permanently destroyed approximately 1.5 cm from the edge of the ice ball, where a temperature of-20 degrees Celsius is reached.) Before the outer edge of the ice ball approaches the serosa of the uterus, stop the procedure (Figure 1). Heat the probe to body temperature and remove it from the cornu. Repeat the process in the contralateral cornu (Figure 2) and, in large uteri (greater than 10 cm sound), in the lower uterine segment (Figure 3). Each freeze cycle takes about 5 to 6 minutes.
FIGURE 1
Using ultrasound guidance, follow the edge of the ice ball into the uterine muscle at 1 cornu. Stop before the edge of the ice ball approaches the serosa.
FIGURE 2
Heat the probe to body temperature, remove it from the first cornu, and repeat the process in the contralateral cornu.
FIGURE 3
For large uteri (greater than 10 cm sound), perform another freeze cycle in the lower uterine segment. Each cycle takes about 5 to 6 minutes.The number of ice balls needed to destroy the entire uterine cavity and the length of time required to perform the procedure depend on the size of the cavity. In general, 2 to 3 ice balls are sufficient, and the entire procedure takes 10 to 20 minutes.
In a recent randomized multicenter study, researchers investigated whether a freeze cycle longer than recommended would result in better outcomes. At 12-month follow-up, 7 (64%) of the 12 patients who underwent treatments of 5 to 7 minutes rather than 4 minutes were amenorrheic/spotting, with a 91% success rate.7 Larger studies are underway to confirm these data. Further, repeat treatment can contribute to better outcomes, especially when the patient receives leuprolide acetate prior to the procedure.8
Advantages. The primary advantage of cryoab-lation is that it is not a totally blind procedure. The visual feedback provided by the ultrasound facilitates complete ablation of the entire uterine cavity, regardless of size. Further, while the hysteroscope used in standard ablation allows the physician to see only the destruction of the surface epithelium, ultrasound enables the surgeon to visualize the depth of treatment during cryoablation.
Another advantage: Freezing tissue causes less pain (cryoanesthesia) than the heat energy associated with other ablation devices.
Disadvantages. While ultrasound visualization has its benefits, it also can complicate matters because accurate imaging and interpretation require a certain level of skill. For Ob/Gyns not experienced in ultrasound, there is a potential for poor positioning of the probe, which could lead to bowel and bladder injuries. Further, physicians can mistakenly place the cryoprobe back into the treated cornu instead of the contralateral side, leading to ablation of the uterine serosa. Lastly, the technology involved in this procedure increases the cost of the equipment and disposable instruments.
What the evidence shows. According to an unpublished study conducted by Cryogen, 54% of 222 patients experienced amenorrhea or staining only at 6-month follow-up. Ninety-three percent of the patients had a Patient Bleeding Assessment Card (PBAC) score of less than 75, and 75% of the patients had a greater than 90% reduction in their PBAC scores. (A score of greater than 150 was needed to enter the study; the score is reached by assigning a value to the amount of staining on a validated pad and adding these points based on the number of pads used per cycle.)
Cost. The disposable cryoprobes are $1,250 per case, and the unit costs $29,995. Since most ablation procedures currently are performed in the operating room, ancillary costs of the techniques are similar.
Bipolar desiccation
Technique. After dilating the cervix, insert the 8-mm NovaSure System (Novacept, Palo Alto, Calif) catheter into the uterus. To rule out auterine perforation prior to beginning the procedure, insert carbon dioxide (CO2) into the cavity (Figure 4).The machine will not turn on if a seal of CO2 has not been created, unless the surgeon overrides the system.
Then expand the 3-dimensional gold-plated bipolar mesh electrode, which imitates the shape of the uterine cavity, out of the catheter. Gently push the probe toward the fundus so that the mesh electrode is accurately positioned within the uterine cavity (Figure 5). Once the electrode is activated with up to 180 W of bipolar power, gentle suction brings the endometrium into close contact with the mesh (Figure 6). This suction also removes debris and allows for more complete desiccation.
The entire bipolar desiccation procedure takes less than 5 minutes to perform.
The system shuts down automatically when complete desiccation (calculated at 50 ohms of resistance) has occurred. The average treatment time is just over 1 minute, and the average depth of ablation is 4 to 5 mm.9 Once ablation is complete, retract the mesh electrode and remove the catheter (Figure 7). The entire procedure takes less than 5 minutes.
Advantages. Not only is the technique quick and simple, no endometrial pretreatment is required because the system allows for consistent depths of ablation regardless of endometrial thickness.
Disadvantages. There are limited data on the 1-year success rates for this completely blind procedure. In addition, the uterus needs to be of normal shape to successfully ablate the endometrium.
What the evidence shows. Unpublished data from randomized, controlled clinical trials comparing bipolar desiccation with balloon ablation have demonstrated that the former achieves higher amenorrhea and success (a return to normal menstrual bleeding) rates than the latter. Further, patients treated with bipolar desiccation experienced less pain, both intra-operatively and postoperatively, than women who underwent balloon ablation.
Cost. Each disposable probe costs $850, and the bipolar controller lists for $15,000.
FIGURE 4
Dilate the cervix and insert the 8-mm catheter. To rule out a uterine perforation prior to beginning the procedure, place carbon dioxide in the cavity.
FIGURE 5
Expand the bipolar mesh electrode. Gently push the probe toward the fundus to accurately position the electrode within the uterine cavity.
FIGURE 6
Activate the electrode with up to 180 W of bipolar power. A gentle suction brings the endometrium into close contact with the mesh.
FIGURE 7
The system shuts down automatically when complete desiccation has occurred. Retract the bipolar mesh electrode and remove the catheter.
Hydrothermal ablation/circulating heated saline
Technique. Begin by dilating the cervix and inserting the sheath, which includes an 8-mm hysteroscope (Hydro ThermAblator; BEI Medical Systems, Teterboro, NJ), into the uter-ine cavity. Using gravity rather than a pump, introduce saline solution through the sheath into the uterus. As the saline circulates, gradually heat it until the solution reaches and maintains 90 degrees Celsius for 10 minutes, completely destroying the endometrium. (The total time from insertion of the sheath to completion of the procedure is 17 minutes.)
A safety feature detects whether any fluid has escaped from the closed system; a shutoff mechanism activates if more than 10 cc of fluid are lost. The system is controlled by software, so even physicians who are inexperienced with hysteroscopy can easily perform this technique.
Advantages. The primary advantage of hydrothermal ablation (HTA) is that the circulating hot saline solution contacts the entire endometrial surface regardless of the size or shape of the uterine cavity. In addition, since the procedure is performed under direct visualization, the surgeon can ensure that the entire cavity has been thoroughly ablated.
Disadvantages. One drawback is that it can be a painful procedure because the hysteroscope is large, necessitating wide dilation of the cervix, and hot water stimulates pain. Further, there have been unpublished reports of vaginal burns when the cervix created a poor seal around the sheath.
What the evidence shows. Based on their research of 276 women (187 women in the HTA group and 89 patients in the rollerball group), investigators have found that HTA has a treatment success rate of 77% compared to 82% for rollerball ablation. (The treatment success rate is defined as a PBAC score of 75 or lower; eumenorrhea is a score of less than 100.) Amenorrhea rates at 12-month follow-up were 40% and 51%, respectively. The researchers concluded that HTA offers an advantage over rollerball ablation in that it reduces anesthesia requirements and eliminates the problem of hypotonic fluid absorption.10
Cost. Disposable hysteroscopic tubing is $625 per case. The machine costs $10,900 plus $395 for the heater canister.
The author reports no financial relationship with any companies whose products are mentioned in this article.
1. Carlson KJ, Nichols DH, Schiff I. Indications for hysterectomy. N Engl J Med. 1993;Mar 25 328(12):856-860.
2. Carlson KJ, Miller BA, Fowler FJ, Jr. The Maine Women’s Health Study: I. Outcomes of hysterectomy. Obstet Gynecol. 1994;83-556.
3. Margolis MT, et al. Asymptomatic endometrial carcinoma after endometrial ablation. Int J Gynaecol Obstet. 1995;51-255.
4. Gimpelson RJ. Not so benign endometrial hyperplasia: endometrial cancer after endometrial ablation. J Am Assoc Gynecol Laparosc. 1997;4-507.
5. Horowitz IR, Copas PR, Aaronoff, et al. Endometrial adenocarcinoma following endometrial ablation for postmenopausal bleeding. Gynecol Oncol. 1995;56:460.-
6. Valle RF, Baggish MS. Endometrial carcinoma after endometrial ablation: high-risk factors predicting its occurrence. Am J Obstet Gynecol. 1998;179:569.-
7. Heppard M, et al. Data on 222 patients from a multicenter study using Cryogen First Option uterine cryoablation therapy in women with abnormal uterine bleeding. Obstet Gynecol. 2001;97(4 Suppl 1):S21.-
8. Sanders B. Preliminary outcomes of longer freeze cycles in the treatment of women with abnormal uterine bleeding using Cryogen First Option uterine cryoablation therapy. Obstet Gynecol. 2001;97(4 Suppl 1):S14.-
9. Cooper JM, Erickson ML. Global endometrial ablation technologies. Obstet Gynecol Clin North Am. 2000;27-385.
10. Corson SL. A multicenter evaluation of endometrial ablation by Hydro ThermAblator and rollerball for treatment of menorrhagia. J Am Assoc Gynecol Laparosc. 2001;8(3):359-367.
1. Carlson KJ, Nichols DH, Schiff I. Indications for hysterectomy. N Engl J Med. 1993;Mar 25 328(12):856-860.
2. Carlson KJ, Miller BA, Fowler FJ, Jr. The Maine Women’s Health Study: I. Outcomes of hysterectomy. Obstet Gynecol. 1994;83-556.
3. Margolis MT, et al. Asymptomatic endometrial carcinoma after endometrial ablation. Int J Gynaecol Obstet. 1995;51-255.
4. Gimpelson RJ. Not so benign endometrial hyperplasia: endometrial cancer after endometrial ablation. J Am Assoc Gynecol Laparosc. 1997;4-507.
5. Horowitz IR, Copas PR, Aaronoff, et al. Endometrial adenocarcinoma following endometrial ablation for postmenopausal bleeding. Gynecol Oncol. 1995;56:460.-
6. Valle RF, Baggish MS. Endometrial carcinoma after endometrial ablation: high-risk factors predicting its occurrence. Am J Obstet Gynecol. 1998;179:569.-
7. Heppard M, et al. Data on 222 patients from a multicenter study using Cryogen First Option uterine cryoablation therapy in women with abnormal uterine bleeding. Obstet Gynecol. 2001;97(4 Suppl 1):S21.-
8. Sanders B. Preliminary outcomes of longer freeze cycles in the treatment of women with abnormal uterine bleeding using Cryogen First Option uterine cryoablation therapy. Obstet Gynecol. 2001;97(4 Suppl 1):S14.-
9. Cooper JM, Erickson ML. Global endometrial ablation technologies. Obstet Gynecol Clin North Am. 2000;27-385.
10. Corson SL. A multicenter evaluation of endometrial ablation by Hydro ThermAblator and rollerball for treatment of menorrhagia. J Am Assoc Gynecol Laparosc. 2001;8(3):359-367.