User login
2016 Update on minimally invasive gynecologic surgery
Rightly so, the topics of mechanical tissue extraction and hysterectomy approach have dominated the field of obstetrics and gynecology over the past 12 months and more. A profusion of literature has been published on these subjects. However, there are 2 important topics within the field of minimally invasive gynecologic surgery that deserve our attention as well, and I have chosen to focus on these for this Update.
First, laparoscopic treatment of ovarian endometriomas is one of the most commonly performed gynecologic procedures worldwide. Many women undergoing such surgery are of childbearing age and have the desire for future pregnancy. What are best practices for preserving ovarian function in these women? Two studies recently published in the Journal of Minimally Invasive Gynecology addressed this question.
Second, until recently, the rate of bowel injury at laparoscopic gynecologic surgery has not been well established.1 Moreover, mechanical bowel preparation is commonly employed in case intestinal injury does occur, despite the lack of evidence that outcomes of these possible injuries can be improved.2 Understanding the rate of bowel injury can shed light on the overall value of the perceived benefits of bowel preparation. Therefore, I examine 2 recent systematic reviews that analyze the incidence of bowel injury and the value of bowel prep in gynecologic laparoscopic surgery.
bipolar coagulation inferior to suturing or hemostatic sealant for preserving ovarian function
Song T, Kim WY, Lee KW, Kim KH. Effect on ovarian reserve of hemostasis by bipolar coagulation versus suture during laparoendoscopic single-site cystectomy for ovarian endometriomas. J Minim Invasive Gynecol. 2015;22(3):415−420.
Ata B, Turkgeldi E, Seyhan A, Urman B. Effect of hemostatic method on ovarian reserve following laparoscopic endometrioma excision; comparison of suture, hemostatic sealant, and bipolar dessication. A systematic review and meta-analysis. J Minim Invasive Gynecol. 2015;22(3):363−372.
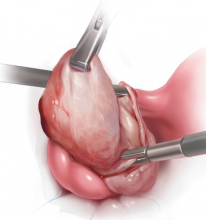
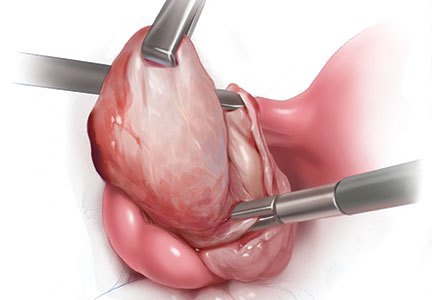
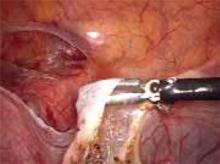
The customary surgical approach for laparoscopic cystectomy is by mechanical stripping of the cyst wall (FIGURE) and the use of bipolar desiccation for hemostasis. Stripping inevitably leads to removal of healthy ovarian cortex,3 especially in inexperienced hands,4 and ovarian follicles inevitably are destroyed during electrosurgical desiccation. When compared with the use of suturing or a hemostatic agent to control bleeding in the ovarian defect, the use of bipolar electrosurgery may harm more of the ovarian cortex, resulting in a comparatively diminished follicular cohort.
Possible deleterious effects on the ovarian reserve can be determined with a blood test to measure anti-Müllerian hormone (AMH) levels postoperatively. Produced by the granulosa cells of the ovary, this hormone directly reflects the remaining ovarian egg supply. Lower levels of AMH have been shown to significantly decrease the success rate of in vitro fertilization (IVF), especially in women older than age 35.5 Moreover, AMH levels in the late reproductive years can be used as a predictive marker of menopause, with lower levels predicting significantly earlier onset.6
Data from 2 recent studies, a quasi-randomized trial by Song and colleagues and a systematic review and meta-analysis by Ata and colleagues emphasize that bipolar desiccation for hemostasis may not be best practice for protecting ovarian reserve during laparoscopic ovarian cystectomy for an endometrioma.
AMH levels decline more significantly for women undergoing bipolar desiccation
Song and colleagues conducted a prospective quasi-randomized study of 125 women whose endometriomas were laparoscopically removed via a single-site approach and managed for hemostasis with either bipolar desiccation or suturing of the ovarian defect with a 2-0 barbed suture. All surgeries were conducted by a single surgeon.
At 3 months postsurgery, mean AMH levels had declined from baseline by 42.2% (interquartile range [IR], 16.5−53.0 ng/mL) in the desiccation group and by 24.6% (IR, 11.6−37.0 ng/mL) in the suture group (P = .001). Multivariate analysis showed that the method used for hemostasis was the only determinant for reduced ovarian reserve.
In their systematic review and meta-analysis, Ata and colleagues included 10 studies--6 qualitative and 4 quantitative. All studies examined the rate of change of serum AMH levels 3 months after laparoscopic removal of an endometrioma.
In their qualitative analysis, 5 of the 6 studies reported a significantly greater decrease in ovarian reserve after bipolar desiccation (varying from 13% to 44%) or a strong trend in the same direction. In the sixth study, the desiccation group had a lower decline in absolute AMH level than in the other 5 studies. The authors note that this 2.7% decline was much lower than the values reported for the bipolar desiccation group of any other study. (Those declines ranged between 19% and 58%.)
Although not significant, in all 3 of the included randomized controlled trials (RCTs), the desiccation groups had a greater loss in AMH level than the hemostatic sealant groups, and in 2 of these RCTs, bipolar desiccation groups had a greater loss than the suturing groups.
Among the 213 study participants in the 3 RCTs and the prospective cohort study included in the quantitative meta-analysis, alternative methods to bipolar desiccation were associated with a 6.95% lower decrease in AMH-level decline (95% confidence interval [CI], −13.0% to −0.9%; P = .02).
What this EVIDENCE means for practice
Compared with the use of bipolar electrosurgery to attain hemostasis, the use of a topical biosurgical agent or suturing could be significantly better for protection of the ovarian follicles during laparoscopic ovarian cystectomy for endometrioma. These alternative methods especially could benefit those women desiring future pregnancy who are demonstrated preoperatively to have a low ovarian reserve. As needed, electrosurgery should be sparingly employed for ovarian hemostasis.
Large Study identifies incidence of bowel injury during gynecologic laproscopy
Llarena NC, Shah AB, Milad MP. Bowel injury in gynecologic laparoscopy: a systematic review. Am J Obstet Gynecol. 2015;125(6):1407−1417.
In no aspect of laparoscopic surgery are preventive strategies more cautiously employed than during peritoneal access. Regardless of the applied technique, there is an irreducible risk of injury to the underlying viscera by either adhesions between the underlying bowel and abdominal wall or during the course of pilot error. Moreover, in the best of hands, bowel injury can occur whenever normal anatomic relationships need to be restored using intra-abdominal adhesiolysis. Given the ubiquity, these risks are never out of the surgeon's mind. Gynecologists are obliged to discuss these risks during the informed consent process.
Until recently, the rate of bowel injury has not been well established. Llarena and colleagues recently have conducted the largest systematic review of the medical literature to date for incidence, presentation, mortality, cause, and location of bowel injury associated with laparoscopic surgery while not necessarily distinguishing for the type of bowel injury. Sixty retrospective and 27 prospective studies met inclusion criteria.
The risk of bowel injury overall and defined
Among 474,063 laparoscopic surgeries conducted between 1972 and 2014, 604 bowel injuries were found, for an incidence of 1 in 769, or 0.13% (95% CI, 0.12−0.14%).
The rate of bowel injury varied by procedure, year, study type, and definition of bowel injury. The incidence of injury according to:
- definition, was 1 in 416 (0.24%) for studies that clearly included serosal injuries and enterotomies versus 1 in 833 (0.12%) for studies not clearly defining the type of bowel injury (relative risk [RR], 0.47; 95% CI, 0.38−0.59; P<.001)
- study type, was 1 in 666 (0.15%) for prospective studies versus 1 in 909 (0.11%) for retrospective studies (RR, 0.78; 95% CI, 0.63−0.96; P = .02)
- procedure, was 1 in 3,333 (0.03%; 95% CI, 0.01−0.03%) for sterilization and 1 in 256 (0.39%; 95% CI, 0.35−0.45%) for hysterectomy
- year, for laparoscopic hysterectomy only, was 1 in 222 (0.45%) before the year 2000 and 1 in 294 (0.34%) after 2000 (RR, 0.75; 95% CI, 0.57−0.98; P = .03).
How were injuries caused, found, and managed?
Thirty studies described the laparoscopic instrument used during 366 reported bowel injuries. The majority of injuries (55%) occurred during initial peritoneal access, with the Veress needle or trocar causing the damage. This was followed by electrosurgery (29%), dissection (11%), and forceps or scissors (4.1%).
According to 40 studies describing 307 injuries, bowel injuries most often were managed by converting to laparotomy (80%); only 8% of injuries were managed with laparoscopy and 2% expectantly.
Surgery to repair the bowel injury was delayed in 154 (41%) of 375 cases. The median time to injury discovery was 3 days (range, 1−13 days).
In only 19 cases were the presenting signs and symptoms of bowel injury recorded. Those reported from most to least often were: peritonitis, abdominal pain, fever, abdominal distention, leukocytosis, leukopenia, and septic shock.
Mortality
Mortality as an outcome was only reported in 29 of the total 90 studies; therefore, mortality may be underreported. Overall, however, death occurred in 1 (0.8%) of 125 bowel injuries.
The overall mortality rate from bowel injury--calculated from the only 42 studies that explicitly mentioned mortality as an outcome--was 1 in 125, or 0.8% (95% CI, 0.36%-1.9%). All 5 reported deaths occurred as a result of delayed recognition of bowel injury, which made the mortality rate for unrecognized bowel injury 1 in 31, or 3.2% (95% CI, 1%-7%). No deaths occurred when the bowel injury was noted intraoperatively.
What this EVIDENCE means for practice
In this review of 474,063 laparoscopic procedures, bowel injury occurred in 1 in 769, or 0.13% of procedures. Bowel injury is more apt to occur during more complicated laparoscopic procedures (compared with laparoscopic sterilization procedures, the risk during hysterectomy was greater than 10-fold).
Most of the injuries were managed by laparotomic surgery despite the potential to repair bowel injury by laparoscopy. Validating that peritoneal access is a high risk part of laparoscopic surgery, the majority of the injuries occurred during insufflation with a Veress needle or during abdominal access by trocar insertion. Nearly one-third of the injuries were from the use of electrosurgery, which are typically associated with a delay in presentation.
In this study, 41% of the injuries were unrecognized at the time of surgery. All 5 of the reported deaths were associated with a delay in diagnosis, with an overall mortality rate of 1 of 125, or 0.8%. Since all of these deaths were associated with a delay in diagnosis, the rate of mortality in unrecognized bowel injury was 5 of 154, or 3.2%. Among women who experienced delayed diagnosis, only 19 of 154 experienced signs or symptoms diagnostic for an underlying bowel injury, particularly when the small bowel was injured.
Can mechanical bowel prep positively affect outcomes in gynecologic laparoscopy, or should it be discarded?
Arnold A, Aitchison LP, Abbott J. Preoperative mechanical bowel preparation for abdominal, laparoscopic, and vaginal surgery: a systematic review. J Minim Invasive Gynecol. 2015;22(5):737−752.
Popularized for more than 4 decades, the practice of presurgical bowel preparation is predicated on the notion that the presence of less, versus more, feces can minimize bacterial count and thereby reduce peritoneal contamination. Logically then, surgical site infections (SSIs) should be reduced with bowel preparation. Moreover, the surgical view and bowel handling during laparoscopic surgery should be improved, with surgical times consequently reduced.
Surgeons must weigh the putative benefits of mechanical bowel preparation against the unpleasant experience it causes for patients, as well as the risks of dehydration or electrolyte disturbance it may cause. To this day, a considerable percentage of gynecologists and colorectal surgeons routinely prep the bowel after weighing all of these factors, despite the paucity of evidence for the practice's efficacy to reduce SSI and improve surgical outcomes.7
The results of this recent systematic review critically question the usefulness of preoperative bowel preparation for abdominal, laparoscopic, and vaginal surgery.
Details of the analysis
The authors evaluated high-quality studies on mechanical bowel preparation to assess evidence for:
- surgeon outcomes, including the surgical field and bowel handling
- operative outcomes, including intraoperative complications and operative times
- patient outcomes, including postoperative complications, overall morbidity, and length of stay.
The authors identified RCTs and prospective or retrospective cohort studies in various surgical specialties comparing preoperative bowel preparation to no such prep. Forty-three studies met inclusion criteria: 38 compared prep to no prep, and 5 compared prep to a single rectal enema. Five high-grade studies in gynecology were included (n = 795), with 4 of them RCTs of gynecologic laparoscopy (n = 645).
Operative field and duration
Of the studies comparing bowel prep with no prep, only the 5 gynecologic ones assessed operative field. Surgical view was perceived as improved in only 1 study. In another, surgeons only could guess allocation half the time.
Sixteen studies evaluated impact of mechanical bowel preparation on duration of surgery: 1 high-quality study found a significant reduction in OR time with bowel prep, and 1 moderate-quality study found longer operative time with bowel prep.
Patient outcomes
Of all studies assessing patient outcomes, 3 high-quality studies of colorectal patients (n = 490) found increased complications from prep versus no prep, including anastomotic dehiscence (P = .05), abdominal complications (P = .028), and infectious complications (P = .05).
Length of stay was assessed in 26 studies, with 4 reporting longer hospital stay with bowel prep and the remaining finding no difference between prep and no prep.
Across all specialties, only 2 studies reported improved outcomes with mechanical bowel preparation. One was a high-quality study reporting reduced 30-day morbidity (P = .018) and infectious complication rates (P = .018), and the other was a moderate-quality study that found reduced SSI (P = .0001) and organ space infection (P = .024) in patients undergoing bowel prep.
Mechanical bowel preparation vs enema
Bowel prep was compared with a single rectal enema in 5 studies. In 2 of these, patient outcomes were worse with enema. One high-quality study of 294 patients reported increased intra-abdominal fecal soiling (P = .008) in the enema group. (The surgeons believed that bowel preparation was more likely to be inadequate in this group, 25% compared with 6%, P<.05.) Whereas there was no statistical difference in the incidence of anastomotic leak between these groups, there was higher reoperation rate in the enema-only group where leakage was diagnosed (6 [4.1%] vs 0, respectively; P = .013).
Bowel prep and preoperative and postoperative symptoms
Six high-quality studies reported on the impact of mechanical bowel preparation on patient symptoms, such as nausea, weakness, abdominal distention, and satisfaction before and after surgery. In all but 1 study patients had significantly greater discomfort with bowel preparation. In 2 of the 6 studies, patients had more diarrhea (P = .0003), a delay in the first bowel movement (P = .001), and a slower return to normal diet (P = .004).
What this EVIDENCE means for practice
The theory behind mechanical bowel preparation is not supported by the evidence. Despite the fact that the bowel is not customarily entered, up to 50% of gynecologic surgeons employ bowel preparation, with the hope of improving visualization and decreasing risk of an anastomotic leak. The colorectal studies in this review demonstrate no evidence for decreased anastomotic leak or infectious complications. By extrapolation, there is no evidence that using preoperative bowel prep bestows any benefit if bowel injury occurs inadvertently and if resection or reanastomosis is then required.
Among the 7 studies examining bowel prep in laparoscopy (4 gynecology, 3 urology, and 1 colorectal), only data from 1 demonstrated an improved surgical field (and in this case only by 1 out of 10 on a Likert scale). The impact of mechanical bowel preparation on the visual field is the same for diagnostic or complex laparoscopic surgeries. One high-quality study with deep endometriosis resection demonstrated no change in the operative field as reflected by no practical differences in OR time or complications.
Preparing the bowel for surgery is an intrusive process that reduces patient satisfaction by inducing weakness, abdominal distention, nausea, vomiting, hunger, and thirst. Whereas this systematic analysis failed to confirm any benefit of the process, it provides evidence for the potential for harm. Mechanical bowel preparation should be discarded as a routine preoperative treatment for patients undergoing minimally invasive gynecologic surgery.
Share your thoughts! Send your Letter to the Editor to [email protected]. Please include your name and the city and state in which you practice.
Rightly so, the topics of mechanical tissue extraction and hysterectomy approach have dominated the field of obstetrics and gynecology over the past 12 months and more. A profusion of literature has been published on these subjects. However, there are 2 important topics within the field of minimally invasive gynecologic surgery that deserve our attention as well, and I have chosen to focus on these for this Update.
First, laparoscopic treatment of ovarian endometriomas is one of the most commonly performed gynecologic procedures worldwide. Many women undergoing such surgery are of childbearing age and have the desire for future pregnancy. What are best practices for preserving ovarian function in these women? Two studies recently published in the Journal of Minimally Invasive Gynecology addressed this question.
Second, until recently, the rate of bowel injury at laparoscopic gynecologic surgery has not been well established.1 Moreover, mechanical bowel preparation is commonly employed in case intestinal injury does occur, despite the lack of evidence that outcomes of these possible injuries can be improved.2 Understanding the rate of bowel injury can shed light on the overall value of the perceived benefits of bowel preparation. Therefore, I examine 2 recent systematic reviews that analyze the incidence of bowel injury and the value of bowel prep in gynecologic laparoscopic surgery.
bipolar coagulation inferior to suturing or hemostatic sealant for preserving ovarian function
Song T, Kim WY, Lee KW, Kim KH. Effect on ovarian reserve of hemostasis by bipolar coagulation versus suture during laparoendoscopic single-site cystectomy for ovarian endometriomas. J Minim Invasive Gynecol. 2015;22(3):415−420.
Ata B, Turkgeldi E, Seyhan A, Urman B. Effect of hemostatic method on ovarian reserve following laparoscopic endometrioma excision; comparison of suture, hemostatic sealant, and bipolar dessication. A systematic review and meta-analysis. J Minim Invasive Gynecol. 2015;22(3):363−372.
The customary surgical approach for laparoscopic cystectomy is by mechanical stripping of the cyst wall (FIGURE) and the use of bipolar desiccation for hemostasis. Stripping inevitably leads to removal of healthy ovarian cortex,3 especially in inexperienced hands,4 and ovarian follicles inevitably are destroyed during electrosurgical desiccation. When compared with the use of suturing or a hemostatic agent to control bleeding in the ovarian defect, the use of bipolar electrosurgery may harm more of the ovarian cortex, resulting in a comparatively diminished follicular cohort.
Possible deleterious effects on the ovarian reserve can be determined with a blood test to measure anti-Müllerian hormone (AMH) levels postoperatively. Produced by the granulosa cells of the ovary, this hormone directly reflects the remaining ovarian egg supply. Lower levels of AMH have been shown to significantly decrease the success rate of in vitro fertilization (IVF), especially in women older than age 35.5 Moreover, AMH levels in the late reproductive years can be used as a predictive marker of menopause, with lower levels predicting significantly earlier onset.6
Data from 2 recent studies, a quasi-randomized trial by Song and colleagues and a systematic review and meta-analysis by Ata and colleagues emphasize that bipolar desiccation for hemostasis may not be best practice for protecting ovarian reserve during laparoscopic ovarian cystectomy for an endometrioma.
AMH levels decline more significantly for women undergoing bipolar desiccation
Song and colleagues conducted a prospective quasi-randomized study of 125 women whose endometriomas were laparoscopically removed via a single-site approach and managed for hemostasis with either bipolar desiccation or suturing of the ovarian defect with a 2-0 barbed suture. All surgeries were conducted by a single surgeon.
At 3 months postsurgery, mean AMH levels had declined from baseline by 42.2% (interquartile range [IR], 16.5−53.0 ng/mL) in the desiccation group and by 24.6% (IR, 11.6−37.0 ng/mL) in the suture group (P = .001). Multivariate analysis showed that the method used for hemostasis was the only determinant for reduced ovarian reserve.
In their systematic review and meta-analysis, Ata and colleagues included 10 studies--6 qualitative and 4 quantitative. All studies examined the rate of change of serum AMH levels 3 months after laparoscopic removal of an endometrioma.
In their qualitative analysis, 5 of the 6 studies reported a significantly greater decrease in ovarian reserve after bipolar desiccation (varying from 13% to 44%) or a strong trend in the same direction. In the sixth study, the desiccation group had a lower decline in absolute AMH level than in the other 5 studies. The authors note that this 2.7% decline was much lower than the values reported for the bipolar desiccation group of any other study. (Those declines ranged between 19% and 58%.)
Although not significant, in all 3 of the included randomized controlled trials (RCTs), the desiccation groups had a greater loss in AMH level than the hemostatic sealant groups, and in 2 of these RCTs, bipolar desiccation groups had a greater loss than the suturing groups.
Among the 213 study participants in the 3 RCTs and the prospective cohort study included in the quantitative meta-analysis, alternative methods to bipolar desiccation were associated with a 6.95% lower decrease in AMH-level decline (95% confidence interval [CI], −13.0% to −0.9%; P = .02).
What this EVIDENCE means for practice
Compared with the use of bipolar electrosurgery to attain hemostasis, the use of a topical biosurgical agent or suturing could be significantly better for protection of the ovarian follicles during laparoscopic ovarian cystectomy for endometrioma. These alternative methods especially could benefit those women desiring future pregnancy who are demonstrated preoperatively to have a low ovarian reserve. As needed, electrosurgery should be sparingly employed for ovarian hemostasis.
Large Study identifies incidence of bowel injury during gynecologic laproscopy
Llarena NC, Shah AB, Milad MP. Bowel injury in gynecologic laparoscopy: a systematic review. Am J Obstet Gynecol. 2015;125(6):1407−1417.
In no aspect of laparoscopic surgery are preventive strategies more cautiously employed than during peritoneal access. Regardless of the applied technique, there is an irreducible risk of injury to the underlying viscera by either adhesions between the underlying bowel and abdominal wall or during the course of pilot error. Moreover, in the best of hands, bowel injury can occur whenever normal anatomic relationships need to be restored using intra-abdominal adhesiolysis. Given the ubiquity, these risks are never out of the surgeon's mind. Gynecologists are obliged to discuss these risks during the informed consent process.
Until recently, the rate of bowel injury has not been well established. Llarena and colleagues recently have conducted the largest systematic review of the medical literature to date for incidence, presentation, mortality, cause, and location of bowel injury associated with laparoscopic surgery while not necessarily distinguishing for the type of bowel injury. Sixty retrospective and 27 prospective studies met inclusion criteria.
The risk of bowel injury overall and defined
Among 474,063 laparoscopic surgeries conducted between 1972 and 2014, 604 bowel injuries were found, for an incidence of 1 in 769, or 0.13% (95% CI, 0.12−0.14%).
The rate of bowel injury varied by procedure, year, study type, and definition of bowel injury. The incidence of injury according to:
- definition, was 1 in 416 (0.24%) for studies that clearly included serosal injuries and enterotomies versus 1 in 833 (0.12%) for studies not clearly defining the type of bowel injury (relative risk [RR], 0.47; 95% CI, 0.38−0.59; P<.001)
- study type, was 1 in 666 (0.15%) for prospective studies versus 1 in 909 (0.11%) for retrospective studies (RR, 0.78; 95% CI, 0.63−0.96; P = .02)
- procedure, was 1 in 3,333 (0.03%; 95% CI, 0.01−0.03%) for sterilization and 1 in 256 (0.39%; 95% CI, 0.35−0.45%) for hysterectomy
- year, for laparoscopic hysterectomy only, was 1 in 222 (0.45%) before the year 2000 and 1 in 294 (0.34%) after 2000 (RR, 0.75; 95% CI, 0.57−0.98; P = .03).
How were injuries caused, found, and managed?
Thirty studies described the laparoscopic instrument used during 366 reported bowel injuries. The majority of injuries (55%) occurred during initial peritoneal access, with the Veress needle or trocar causing the damage. This was followed by electrosurgery (29%), dissection (11%), and forceps or scissors (4.1%).
According to 40 studies describing 307 injuries, bowel injuries most often were managed by converting to laparotomy (80%); only 8% of injuries were managed with laparoscopy and 2% expectantly.
Surgery to repair the bowel injury was delayed in 154 (41%) of 375 cases. The median time to injury discovery was 3 days (range, 1−13 days).
In only 19 cases were the presenting signs and symptoms of bowel injury recorded. Those reported from most to least often were: peritonitis, abdominal pain, fever, abdominal distention, leukocytosis, leukopenia, and septic shock.
Mortality
Mortality as an outcome was only reported in 29 of the total 90 studies; therefore, mortality may be underreported. Overall, however, death occurred in 1 (0.8%) of 125 bowel injuries.
The overall mortality rate from bowel injury--calculated from the only 42 studies that explicitly mentioned mortality as an outcome--was 1 in 125, or 0.8% (95% CI, 0.36%-1.9%). All 5 reported deaths occurred as a result of delayed recognition of bowel injury, which made the mortality rate for unrecognized bowel injury 1 in 31, or 3.2% (95% CI, 1%-7%). No deaths occurred when the bowel injury was noted intraoperatively.
What this EVIDENCE means for practice
In this review of 474,063 laparoscopic procedures, bowel injury occurred in 1 in 769, or 0.13% of procedures. Bowel injury is more apt to occur during more complicated laparoscopic procedures (compared with laparoscopic sterilization procedures, the risk during hysterectomy was greater than 10-fold).
Most of the injuries were managed by laparotomic surgery despite the potential to repair bowel injury by laparoscopy. Validating that peritoneal access is a high risk part of laparoscopic surgery, the majority of the injuries occurred during insufflation with a Veress needle or during abdominal access by trocar insertion. Nearly one-third of the injuries were from the use of electrosurgery, which are typically associated with a delay in presentation.
In this study, 41% of the injuries were unrecognized at the time of surgery. All 5 of the reported deaths were associated with a delay in diagnosis, with an overall mortality rate of 1 of 125, or 0.8%. Since all of these deaths were associated with a delay in diagnosis, the rate of mortality in unrecognized bowel injury was 5 of 154, or 3.2%. Among women who experienced delayed diagnosis, only 19 of 154 experienced signs or symptoms diagnostic for an underlying bowel injury, particularly when the small bowel was injured.
Can mechanical bowel prep positively affect outcomes in gynecologic laparoscopy, or should it be discarded?
Arnold A, Aitchison LP, Abbott J. Preoperative mechanical bowel preparation for abdominal, laparoscopic, and vaginal surgery: a systematic review. J Minim Invasive Gynecol. 2015;22(5):737−752.
Popularized for more than 4 decades, the practice of presurgical bowel preparation is predicated on the notion that the presence of less, versus more, feces can minimize bacterial count and thereby reduce peritoneal contamination. Logically then, surgical site infections (SSIs) should be reduced with bowel preparation. Moreover, the surgical view and bowel handling during laparoscopic surgery should be improved, with surgical times consequently reduced.
Surgeons must weigh the putative benefits of mechanical bowel preparation against the unpleasant experience it causes for patients, as well as the risks of dehydration or electrolyte disturbance it may cause. To this day, a considerable percentage of gynecologists and colorectal surgeons routinely prep the bowel after weighing all of these factors, despite the paucity of evidence for the practice's efficacy to reduce SSI and improve surgical outcomes.7
The results of this recent systematic review critically question the usefulness of preoperative bowel preparation for abdominal, laparoscopic, and vaginal surgery.
Details of the analysis
The authors evaluated high-quality studies on mechanical bowel preparation to assess evidence for:
- surgeon outcomes, including the surgical field and bowel handling
- operative outcomes, including intraoperative complications and operative times
- patient outcomes, including postoperative complications, overall morbidity, and length of stay.
The authors identified RCTs and prospective or retrospective cohort studies in various surgical specialties comparing preoperative bowel preparation to no such prep. Forty-three studies met inclusion criteria: 38 compared prep to no prep, and 5 compared prep to a single rectal enema. Five high-grade studies in gynecology were included (n = 795), with 4 of them RCTs of gynecologic laparoscopy (n = 645).
Operative field and duration
Of the studies comparing bowel prep with no prep, only the 5 gynecologic ones assessed operative field. Surgical view was perceived as improved in only 1 study. In another, surgeons only could guess allocation half the time.
Sixteen studies evaluated impact of mechanical bowel preparation on duration of surgery: 1 high-quality study found a significant reduction in OR time with bowel prep, and 1 moderate-quality study found longer operative time with bowel prep.
Patient outcomes
Of all studies assessing patient outcomes, 3 high-quality studies of colorectal patients (n = 490) found increased complications from prep versus no prep, including anastomotic dehiscence (P = .05), abdominal complications (P = .028), and infectious complications (P = .05).
Length of stay was assessed in 26 studies, with 4 reporting longer hospital stay with bowel prep and the remaining finding no difference between prep and no prep.
Across all specialties, only 2 studies reported improved outcomes with mechanical bowel preparation. One was a high-quality study reporting reduced 30-day morbidity (P = .018) and infectious complication rates (P = .018), and the other was a moderate-quality study that found reduced SSI (P = .0001) and organ space infection (P = .024) in patients undergoing bowel prep.
Mechanical bowel preparation vs enema
Bowel prep was compared with a single rectal enema in 5 studies. In 2 of these, patient outcomes were worse with enema. One high-quality study of 294 patients reported increased intra-abdominal fecal soiling (P = .008) in the enema group. (The surgeons believed that bowel preparation was more likely to be inadequate in this group, 25% compared with 6%, P<.05.) Whereas there was no statistical difference in the incidence of anastomotic leak between these groups, there was higher reoperation rate in the enema-only group where leakage was diagnosed (6 [4.1%] vs 0, respectively; P = .013).
Bowel prep and preoperative and postoperative symptoms
Six high-quality studies reported on the impact of mechanical bowel preparation on patient symptoms, such as nausea, weakness, abdominal distention, and satisfaction before and after surgery. In all but 1 study patients had significantly greater discomfort with bowel preparation. In 2 of the 6 studies, patients had more diarrhea (P = .0003), a delay in the first bowel movement (P = .001), and a slower return to normal diet (P = .004).
What this EVIDENCE means for practice
The theory behind mechanical bowel preparation is not supported by the evidence. Despite the fact that the bowel is not customarily entered, up to 50% of gynecologic surgeons employ bowel preparation, with the hope of improving visualization and decreasing risk of an anastomotic leak. The colorectal studies in this review demonstrate no evidence for decreased anastomotic leak or infectious complications. By extrapolation, there is no evidence that using preoperative bowel prep bestows any benefit if bowel injury occurs inadvertently and if resection or reanastomosis is then required.
Among the 7 studies examining bowel prep in laparoscopy (4 gynecology, 3 urology, and 1 colorectal), only data from 1 demonstrated an improved surgical field (and in this case only by 1 out of 10 on a Likert scale). The impact of mechanical bowel preparation on the visual field is the same for diagnostic or complex laparoscopic surgeries. One high-quality study with deep endometriosis resection demonstrated no change in the operative field as reflected by no practical differences in OR time or complications.
Preparing the bowel for surgery is an intrusive process that reduces patient satisfaction by inducing weakness, abdominal distention, nausea, vomiting, hunger, and thirst. Whereas this systematic analysis failed to confirm any benefit of the process, it provides evidence for the potential for harm. Mechanical bowel preparation should be discarded as a routine preoperative treatment for patients undergoing minimally invasive gynecologic surgery.
Share your thoughts! Send your Letter to the Editor to [email protected]. Please include your name and the city and state in which you practice.
Rightly so, the topics of mechanical tissue extraction and hysterectomy approach have dominated the field of obstetrics and gynecology over the past 12 months and more. A profusion of literature has been published on these subjects. However, there are 2 important topics within the field of minimally invasive gynecologic surgery that deserve our attention as well, and I have chosen to focus on these for this Update.
First, laparoscopic treatment of ovarian endometriomas is one of the most commonly performed gynecologic procedures worldwide. Many women undergoing such surgery are of childbearing age and have the desire for future pregnancy. What are best practices for preserving ovarian function in these women? Two studies recently published in the Journal of Minimally Invasive Gynecology addressed this question.
Second, until recently, the rate of bowel injury at laparoscopic gynecologic surgery has not been well established.1 Moreover, mechanical bowel preparation is commonly employed in case intestinal injury does occur, despite the lack of evidence that outcomes of these possible injuries can be improved.2 Understanding the rate of bowel injury can shed light on the overall value of the perceived benefits of bowel preparation. Therefore, I examine 2 recent systematic reviews that analyze the incidence of bowel injury and the value of bowel prep in gynecologic laparoscopic surgery.
bipolar coagulation inferior to suturing or hemostatic sealant for preserving ovarian function
Song T, Kim WY, Lee KW, Kim KH. Effect on ovarian reserve of hemostasis by bipolar coagulation versus suture during laparoendoscopic single-site cystectomy for ovarian endometriomas. J Minim Invasive Gynecol. 2015;22(3):415−420.
Ata B, Turkgeldi E, Seyhan A, Urman B. Effect of hemostatic method on ovarian reserve following laparoscopic endometrioma excision; comparison of suture, hemostatic sealant, and bipolar dessication. A systematic review and meta-analysis. J Minim Invasive Gynecol. 2015;22(3):363−372.
The customary surgical approach for laparoscopic cystectomy is by mechanical stripping of the cyst wall (FIGURE) and the use of bipolar desiccation for hemostasis. Stripping inevitably leads to removal of healthy ovarian cortex,3 especially in inexperienced hands,4 and ovarian follicles inevitably are destroyed during electrosurgical desiccation. When compared with the use of suturing or a hemostatic agent to control bleeding in the ovarian defect, the use of bipolar electrosurgery may harm more of the ovarian cortex, resulting in a comparatively diminished follicular cohort.
Possible deleterious effects on the ovarian reserve can be determined with a blood test to measure anti-Müllerian hormone (AMH) levels postoperatively. Produced by the granulosa cells of the ovary, this hormone directly reflects the remaining ovarian egg supply. Lower levels of AMH have been shown to significantly decrease the success rate of in vitro fertilization (IVF), especially in women older than age 35.5 Moreover, AMH levels in the late reproductive years can be used as a predictive marker of menopause, with lower levels predicting significantly earlier onset.6
Data from 2 recent studies, a quasi-randomized trial by Song and colleagues and a systematic review and meta-analysis by Ata and colleagues emphasize that bipolar desiccation for hemostasis may not be best practice for protecting ovarian reserve during laparoscopic ovarian cystectomy for an endometrioma.
AMH levels decline more significantly for women undergoing bipolar desiccation
Song and colleagues conducted a prospective quasi-randomized study of 125 women whose endometriomas were laparoscopically removed via a single-site approach and managed for hemostasis with either bipolar desiccation or suturing of the ovarian defect with a 2-0 barbed suture. All surgeries were conducted by a single surgeon.
At 3 months postsurgery, mean AMH levels had declined from baseline by 42.2% (interquartile range [IR], 16.5−53.0 ng/mL) in the desiccation group and by 24.6% (IR, 11.6−37.0 ng/mL) in the suture group (P = .001). Multivariate analysis showed that the method used for hemostasis was the only determinant for reduced ovarian reserve.
In their systematic review and meta-analysis, Ata and colleagues included 10 studies--6 qualitative and 4 quantitative. All studies examined the rate of change of serum AMH levels 3 months after laparoscopic removal of an endometrioma.
In their qualitative analysis, 5 of the 6 studies reported a significantly greater decrease in ovarian reserve after bipolar desiccation (varying from 13% to 44%) or a strong trend in the same direction. In the sixth study, the desiccation group had a lower decline in absolute AMH level than in the other 5 studies. The authors note that this 2.7% decline was much lower than the values reported for the bipolar desiccation group of any other study. (Those declines ranged between 19% and 58%.)
Although not significant, in all 3 of the included randomized controlled trials (RCTs), the desiccation groups had a greater loss in AMH level than the hemostatic sealant groups, and in 2 of these RCTs, bipolar desiccation groups had a greater loss than the suturing groups.
Among the 213 study participants in the 3 RCTs and the prospective cohort study included in the quantitative meta-analysis, alternative methods to bipolar desiccation were associated with a 6.95% lower decrease in AMH-level decline (95% confidence interval [CI], −13.0% to −0.9%; P = .02).
What this EVIDENCE means for practice
Compared with the use of bipolar electrosurgery to attain hemostasis, the use of a topical biosurgical agent or suturing could be significantly better for protection of the ovarian follicles during laparoscopic ovarian cystectomy for endometrioma. These alternative methods especially could benefit those women desiring future pregnancy who are demonstrated preoperatively to have a low ovarian reserve. As needed, electrosurgery should be sparingly employed for ovarian hemostasis.
Large Study identifies incidence of bowel injury during gynecologic laproscopy
Llarena NC, Shah AB, Milad MP. Bowel injury in gynecologic laparoscopy: a systematic review. Am J Obstet Gynecol. 2015;125(6):1407−1417.
In no aspect of laparoscopic surgery are preventive strategies more cautiously employed than during peritoneal access. Regardless of the applied technique, there is an irreducible risk of injury to the underlying viscera by either adhesions between the underlying bowel and abdominal wall or during the course of pilot error. Moreover, in the best of hands, bowel injury can occur whenever normal anatomic relationships need to be restored using intra-abdominal adhesiolysis. Given the ubiquity, these risks are never out of the surgeon's mind. Gynecologists are obliged to discuss these risks during the informed consent process.
Until recently, the rate of bowel injury has not been well established. Llarena and colleagues recently have conducted the largest systematic review of the medical literature to date for incidence, presentation, mortality, cause, and location of bowel injury associated with laparoscopic surgery while not necessarily distinguishing for the type of bowel injury. Sixty retrospective and 27 prospective studies met inclusion criteria.
The risk of bowel injury overall and defined
Among 474,063 laparoscopic surgeries conducted between 1972 and 2014, 604 bowel injuries were found, for an incidence of 1 in 769, or 0.13% (95% CI, 0.12−0.14%).
The rate of bowel injury varied by procedure, year, study type, and definition of bowel injury. The incidence of injury according to:
- definition, was 1 in 416 (0.24%) for studies that clearly included serosal injuries and enterotomies versus 1 in 833 (0.12%) for studies not clearly defining the type of bowel injury (relative risk [RR], 0.47; 95% CI, 0.38−0.59; P<.001)
- study type, was 1 in 666 (0.15%) for prospective studies versus 1 in 909 (0.11%) for retrospective studies (RR, 0.78; 95% CI, 0.63−0.96; P = .02)
- procedure, was 1 in 3,333 (0.03%; 95% CI, 0.01−0.03%) for sterilization and 1 in 256 (0.39%; 95% CI, 0.35−0.45%) for hysterectomy
- year, for laparoscopic hysterectomy only, was 1 in 222 (0.45%) before the year 2000 and 1 in 294 (0.34%) after 2000 (RR, 0.75; 95% CI, 0.57−0.98; P = .03).
How were injuries caused, found, and managed?
Thirty studies described the laparoscopic instrument used during 366 reported bowel injuries. The majority of injuries (55%) occurred during initial peritoneal access, with the Veress needle or trocar causing the damage. This was followed by electrosurgery (29%), dissection (11%), and forceps or scissors (4.1%).
According to 40 studies describing 307 injuries, bowel injuries most often were managed by converting to laparotomy (80%); only 8% of injuries were managed with laparoscopy and 2% expectantly.
Surgery to repair the bowel injury was delayed in 154 (41%) of 375 cases. The median time to injury discovery was 3 days (range, 1−13 days).
In only 19 cases were the presenting signs and symptoms of bowel injury recorded. Those reported from most to least often were: peritonitis, abdominal pain, fever, abdominal distention, leukocytosis, leukopenia, and septic shock.
Mortality
Mortality as an outcome was only reported in 29 of the total 90 studies; therefore, mortality may be underreported. Overall, however, death occurred in 1 (0.8%) of 125 bowel injuries.
The overall mortality rate from bowel injury--calculated from the only 42 studies that explicitly mentioned mortality as an outcome--was 1 in 125, or 0.8% (95% CI, 0.36%-1.9%). All 5 reported deaths occurred as a result of delayed recognition of bowel injury, which made the mortality rate for unrecognized bowel injury 1 in 31, or 3.2% (95% CI, 1%-7%). No deaths occurred when the bowel injury was noted intraoperatively.
What this EVIDENCE means for practice
In this review of 474,063 laparoscopic procedures, bowel injury occurred in 1 in 769, or 0.13% of procedures. Bowel injury is more apt to occur during more complicated laparoscopic procedures (compared with laparoscopic sterilization procedures, the risk during hysterectomy was greater than 10-fold).
Most of the injuries were managed by laparotomic surgery despite the potential to repair bowel injury by laparoscopy. Validating that peritoneal access is a high risk part of laparoscopic surgery, the majority of the injuries occurred during insufflation with a Veress needle or during abdominal access by trocar insertion. Nearly one-third of the injuries were from the use of electrosurgery, which are typically associated with a delay in presentation.
In this study, 41% of the injuries were unrecognized at the time of surgery. All 5 of the reported deaths were associated with a delay in diagnosis, with an overall mortality rate of 1 of 125, or 0.8%. Since all of these deaths were associated with a delay in diagnosis, the rate of mortality in unrecognized bowel injury was 5 of 154, or 3.2%. Among women who experienced delayed diagnosis, only 19 of 154 experienced signs or symptoms diagnostic for an underlying bowel injury, particularly when the small bowel was injured.
Can mechanical bowel prep positively affect outcomes in gynecologic laparoscopy, or should it be discarded?
Arnold A, Aitchison LP, Abbott J. Preoperative mechanical bowel preparation for abdominal, laparoscopic, and vaginal surgery: a systematic review. J Minim Invasive Gynecol. 2015;22(5):737−752.
Popularized for more than 4 decades, the practice of presurgical bowel preparation is predicated on the notion that the presence of less, versus more, feces can minimize bacterial count and thereby reduce peritoneal contamination. Logically then, surgical site infections (SSIs) should be reduced with bowel preparation. Moreover, the surgical view and bowel handling during laparoscopic surgery should be improved, with surgical times consequently reduced.
Surgeons must weigh the putative benefits of mechanical bowel preparation against the unpleasant experience it causes for patients, as well as the risks of dehydration or electrolyte disturbance it may cause. To this day, a considerable percentage of gynecologists and colorectal surgeons routinely prep the bowel after weighing all of these factors, despite the paucity of evidence for the practice's efficacy to reduce SSI and improve surgical outcomes.7
The results of this recent systematic review critically question the usefulness of preoperative bowel preparation for abdominal, laparoscopic, and vaginal surgery.
Details of the analysis
The authors evaluated high-quality studies on mechanical bowel preparation to assess evidence for:
- surgeon outcomes, including the surgical field and bowel handling
- operative outcomes, including intraoperative complications and operative times
- patient outcomes, including postoperative complications, overall morbidity, and length of stay.
The authors identified RCTs and prospective or retrospective cohort studies in various surgical specialties comparing preoperative bowel preparation to no such prep. Forty-three studies met inclusion criteria: 38 compared prep to no prep, and 5 compared prep to a single rectal enema. Five high-grade studies in gynecology were included (n = 795), with 4 of them RCTs of gynecologic laparoscopy (n = 645).
Operative field and duration
Of the studies comparing bowel prep with no prep, only the 5 gynecologic ones assessed operative field. Surgical view was perceived as improved in only 1 study. In another, surgeons only could guess allocation half the time.
Sixteen studies evaluated impact of mechanical bowel preparation on duration of surgery: 1 high-quality study found a significant reduction in OR time with bowel prep, and 1 moderate-quality study found longer operative time with bowel prep.
Patient outcomes
Of all studies assessing patient outcomes, 3 high-quality studies of colorectal patients (n = 490) found increased complications from prep versus no prep, including anastomotic dehiscence (P = .05), abdominal complications (P = .028), and infectious complications (P = .05).
Length of stay was assessed in 26 studies, with 4 reporting longer hospital stay with bowel prep and the remaining finding no difference between prep and no prep.
Across all specialties, only 2 studies reported improved outcomes with mechanical bowel preparation. One was a high-quality study reporting reduced 30-day morbidity (P = .018) and infectious complication rates (P = .018), and the other was a moderate-quality study that found reduced SSI (P = .0001) and organ space infection (P = .024) in patients undergoing bowel prep.
Mechanical bowel preparation vs enema
Bowel prep was compared with a single rectal enema in 5 studies. In 2 of these, patient outcomes were worse with enema. One high-quality study of 294 patients reported increased intra-abdominal fecal soiling (P = .008) in the enema group. (The surgeons believed that bowel preparation was more likely to be inadequate in this group, 25% compared with 6%, P<.05.) Whereas there was no statistical difference in the incidence of anastomotic leak between these groups, there was higher reoperation rate in the enema-only group where leakage was diagnosed (6 [4.1%] vs 0, respectively; P = .013).
Bowel prep and preoperative and postoperative symptoms
Six high-quality studies reported on the impact of mechanical bowel preparation on patient symptoms, such as nausea, weakness, abdominal distention, and satisfaction before and after surgery. In all but 1 study patients had significantly greater discomfort with bowel preparation. In 2 of the 6 studies, patients had more diarrhea (P = .0003), a delay in the first bowel movement (P = .001), and a slower return to normal diet (P = .004).
What this EVIDENCE means for practice
The theory behind mechanical bowel preparation is not supported by the evidence. Despite the fact that the bowel is not customarily entered, up to 50% of gynecologic surgeons employ bowel preparation, with the hope of improving visualization and decreasing risk of an anastomotic leak. The colorectal studies in this review demonstrate no evidence for decreased anastomotic leak or infectious complications. By extrapolation, there is no evidence that using preoperative bowel prep bestows any benefit if bowel injury occurs inadvertently and if resection or reanastomosis is then required.
Among the 7 studies examining bowel prep in laparoscopy (4 gynecology, 3 urology, and 1 colorectal), only data from 1 demonstrated an improved surgical field (and in this case only by 1 out of 10 on a Likert scale). The impact of mechanical bowel preparation on the visual field is the same for diagnostic or complex laparoscopic surgeries. One high-quality study with deep endometriosis resection demonstrated no change in the operative field as reflected by no practical differences in OR time or complications.
Preparing the bowel for surgery is an intrusive process that reduces patient satisfaction by inducing weakness, abdominal distention, nausea, vomiting, hunger, and thirst. Whereas this systematic analysis failed to confirm any benefit of the process, it provides evidence for the potential for harm. Mechanical bowel preparation should be discarded as a routine preoperative treatment for patients undergoing minimally invasive gynecologic surgery.
Share your thoughts! Send your Letter to the Editor to [email protected]. Please include your name and the city and state in which you practice.
In this article
- Preserving ovarian function at laparoscopic cystectomy
- Incidence of bowel injury during gyn surgery
- Usefulness and safety of mechanical bowel preparation
Is energy-based vessel sealing safer than suturing for vaginal hysterectomy?
Although the use of vaginal hysterectomy in the treatment of benign uterine conditions continues to decline, it is associated with better outcomes and fewer complications than the laparoscopic and abdominal approaches. Indeed, a 2009 Committee Opinion from ACOG recommends the vaginal route whenever it is feasible.1
Any instrumentation that facilitates or enables the vaginal approach would, therefore, be a welcome addition to the surgical armamentarium. The question of whether bipolar electrosurgical devices can significantly improve key outcomes is the subject of this systematic review and meta-analysis. These hand-held devices obviate the need for suturing to achieve hemostasis by providing rapid, sequential tissue and vessel sealing and coagulation, with or without transection.
The challenge of studying surgical devices
This is the first meta-analysis to estimate the effect on surgical outcomes of energy-based vessel sealing, compared with suturing, in women undergoing vaginal hysterectomy. Contemporary value analysis of new surgical instrumentation is a complex process that includes such variables as cost, education, ergonomics, efficiency, efficacy, and any influence on patient outcomes, including adverse events.
Energy-based surgical devices are more costly than conventional suturing and have a negative impact on the environment. They also carry a potential for harm through thermal injury. These devices are designed to save time and reduce blood loss, but these improvements may come at the cost of complications caused by unintended thermal injury.
Details of the trial
Kroft and Selk reviewed published and unpublished resources to find randomized, controlled trials of women undergoing vaginal hysterectomy. Among the 979 databases and one registered trial so identified, they found only seven studies that met the eligibility criteria: Five studies compared the LigaSure device (Covidien) with suturing, and two compared the Biclamp device (a bipolar electrosurgical device without a cutting blade [ERBE]) with suturing. All seven studies (n = 662) reported operative time and complications; six measured blood loss; four recorded pain by visual analog scale; and five listed the length of stay. None of the studies reported on mortality, patient satisfaction, or quality of life.
Allocation concealment, a method used to prevent selection bias by concealing the allocation sequence from the investigators who assigned patients to intervention groups, was unclear or inadequate in all the studies identified.
Although 99% of patients were available for follow-up, none of the studies blinded outcome analysis or assessors.
Pooled analysis demonstrated that the bipolar electrosurgical devices reduced operative time related to suturing by 17.2 minutes (95% confidence interval [CI], 7.5–27.0). However, after subgroup analysis, this effect was found only for the LigaSure device.
When energy-based vessel sealing was used, estimated blood loss decreased by 47.7 mL (95% CI, 15.5–79.9), the postoperative pain score diminished by 1.25 (95% CI, 0.46–2.05), and the length of stay decreased by 0.25 day (95% CI, 0.13–0.37).
The quality of evidence for all these outcomes was either low or very low.
There were no differences demonstrated in the complication rate between groups. To detect a significant difference in the rate of complications between the two groups, this analysis would have required considerably more subjects in each arm of a prospective study.
Until a large, high-quality, randomized, controlled study is properly conducted to determine whether these novel surgical devices can improve outcomes and reduce costs, there is insufficient evidence to recommend the use of energy-based vessel-sealing devices for vaginal hysterectomy.
ANDREW I. BRILL, MD
We want to hear from you! Tell us what you think.
Reference
1. American College of Obstetricians and Gynecologists. ACOG Committee Opinion No. 444: Choosing the route of hysterectomy for benign disease. Obstet Gynecol. 2009;114(5):1156-1158.
Although the use of vaginal hysterectomy in the treatment of benign uterine conditions continues to decline, it is associated with better outcomes and fewer complications than the laparoscopic and abdominal approaches. Indeed, a 2009 Committee Opinion from ACOG recommends the vaginal route whenever it is feasible.1
Any instrumentation that facilitates or enables the vaginal approach would, therefore, be a welcome addition to the surgical armamentarium. The question of whether bipolar electrosurgical devices can significantly improve key outcomes is the subject of this systematic review and meta-analysis. These hand-held devices obviate the need for suturing to achieve hemostasis by providing rapid, sequential tissue and vessel sealing and coagulation, with or without transection.
The challenge of studying surgical devices
This is the first meta-analysis to estimate the effect on surgical outcomes of energy-based vessel sealing, compared with suturing, in women undergoing vaginal hysterectomy. Contemporary value analysis of new surgical instrumentation is a complex process that includes such variables as cost, education, ergonomics, efficiency, efficacy, and any influence on patient outcomes, including adverse events.
Energy-based surgical devices are more costly than conventional suturing and have a negative impact on the environment. They also carry a potential for harm through thermal injury. These devices are designed to save time and reduce blood loss, but these improvements may come at the cost of complications caused by unintended thermal injury.
Details of the trial
Kroft and Selk reviewed published and unpublished resources to find randomized, controlled trials of women undergoing vaginal hysterectomy. Among the 979 databases and one registered trial so identified, they found only seven studies that met the eligibility criteria: Five studies compared the LigaSure device (Covidien) with suturing, and two compared the Biclamp device (a bipolar electrosurgical device without a cutting blade [ERBE]) with suturing. All seven studies (n = 662) reported operative time and complications; six measured blood loss; four recorded pain by visual analog scale; and five listed the length of stay. None of the studies reported on mortality, patient satisfaction, or quality of life.
Allocation concealment, a method used to prevent selection bias by concealing the allocation sequence from the investigators who assigned patients to intervention groups, was unclear or inadequate in all the studies identified.
Although 99% of patients were available for follow-up, none of the studies blinded outcome analysis or assessors.
Pooled analysis demonstrated that the bipolar electrosurgical devices reduced operative time related to suturing by 17.2 minutes (95% confidence interval [CI], 7.5–27.0). However, after subgroup analysis, this effect was found only for the LigaSure device.
When energy-based vessel sealing was used, estimated blood loss decreased by 47.7 mL (95% CI, 15.5–79.9), the postoperative pain score diminished by 1.25 (95% CI, 0.46–2.05), and the length of stay decreased by 0.25 day (95% CI, 0.13–0.37).
The quality of evidence for all these outcomes was either low or very low.
There were no differences demonstrated in the complication rate between groups. To detect a significant difference in the rate of complications between the two groups, this analysis would have required considerably more subjects in each arm of a prospective study.
Until a large, high-quality, randomized, controlled study is properly conducted to determine whether these novel surgical devices can improve outcomes and reduce costs, there is insufficient evidence to recommend the use of energy-based vessel-sealing devices for vaginal hysterectomy.
ANDREW I. BRILL, MD
We want to hear from you! Tell us what you think.
Although the use of vaginal hysterectomy in the treatment of benign uterine conditions continues to decline, it is associated with better outcomes and fewer complications than the laparoscopic and abdominal approaches. Indeed, a 2009 Committee Opinion from ACOG recommends the vaginal route whenever it is feasible.1
Any instrumentation that facilitates or enables the vaginal approach would, therefore, be a welcome addition to the surgical armamentarium. The question of whether bipolar electrosurgical devices can significantly improve key outcomes is the subject of this systematic review and meta-analysis. These hand-held devices obviate the need for suturing to achieve hemostasis by providing rapid, sequential tissue and vessel sealing and coagulation, with or without transection.
The challenge of studying surgical devices
This is the first meta-analysis to estimate the effect on surgical outcomes of energy-based vessel sealing, compared with suturing, in women undergoing vaginal hysterectomy. Contemporary value analysis of new surgical instrumentation is a complex process that includes such variables as cost, education, ergonomics, efficiency, efficacy, and any influence on patient outcomes, including adverse events.
Energy-based surgical devices are more costly than conventional suturing and have a negative impact on the environment. They also carry a potential for harm through thermal injury. These devices are designed to save time and reduce blood loss, but these improvements may come at the cost of complications caused by unintended thermal injury.
Details of the trial
Kroft and Selk reviewed published and unpublished resources to find randomized, controlled trials of women undergoing vaginal hysterectomy. Among the 979 databases and one registered trial so identified, they found only seven studies that met the eligibility criteria: Five studies compared the LigaSure device (Covidien) with suturing, and two compared the Biclamp device (a bipolar electrosurgical device without a cutting blade [ERBE]) with suturing. All seven studies (n = 662) reported operative time and complications; six measured blood loss; four recorded pain by visual analog scale; and five listed the length of stay. None of the studies reported on mortality, patient satisfaction, or quality of life.
Allocation concealment, a method used to prevent selection bias by concealing the allocation sequence from the investigators who assigned patients to intervention groups, was unclear or inadequate in all the studies identified.
Although 99% of patients were available for follow-up, none of the studies blinded outcome analysis or assessors.
Pooled analysis demonstrated that the bipolar electrosurgical devices reduced operative time related to suturing by 17.2 minutes (95% confidence interval [CI], 7.5–27.0). However, after subgroup analysis, this effect was found only for the LigaSure device.
When energy-based vessel sealing was used, estimated blood loss decreased by 47.7 mL (95% CI, 15.5–79.9), the postoperative pain score diminished by 1.25 (95% CI, 0.46–2.05), and the length of stay decreased by 0.25 day (95% CI, 0.13–0.37).
The quality of evidence for all these outcomes was either low or very low.
There were no differences demonstrated in the complication rate between groups. To detect a significant difference in the rate of complications between the two groups, this analysis would have required considerably more subjects in each arm of a prospective study.
Until a large, high-quality, randomized, controlled study is properly conducted to determine whether these novel surgical devices can improve outcomes and reduce costs, there is insufficient evidence to recommend the use of energy-based vessel-sealing devices for vaginal hysterectomy.
ANDREW I. BRILL, MD
We want to hear from you! Tell us what you think.
Reference
1. American College of Obstetricians and Gynecologists. ACOG Committee Opinion No. 444: Choosing the route of hysterectomy for benign disease. Obstet Gynecol. 2009;114(5):1156-1158.
Reference
1. American College of Obstetricians and Gynecologists. ACOG Committee Opinion No. 444: Choosing the route of hysterectomy for benign disease. Obstet Gynecol. 2009;114(5):1156-1158.
Energy options in gynecologic surgery
Energy options in gynecologic surgery—a topic very dear to me and very often understudied for all of us in our basic training.
And as a signpost of today’s presentation is the review that we’ll partake of fundamental knowledge and technique. And for me, when I take fundamental knowledge and technique and reduce it to its ingredients, it definitely leads to being safer and also contributes to efficacy and efficiency of surgery. And for me, when I combine these 2 elements and I think about really how they contribute to my practice and the outcome of patients, it’s surgical outcome. So today is really dedicated to improving outcome in patients.
Now, in order to hopefully communicate as best as possible with you, I want to review both new concepts and also what I would term electrosurgery vernacular.
In order to do that, I’d like to create a model here and build a water tower. And just think of…in order to get the water into the tower, you have to have some sort of push or force to create that reservoir. In electrosurgical terms, voltage is what provides that push that pushes the current through something called resistance, which is a variable ability for something to pass through a circuit. Obviously, if the resistance goes up, the aperture get smaller. You have to push harder. If you push larger amounts of current, you have to push harder. But these are the 3 elements of electricity, and so we have current, we have resistance, and we have voltage. But…only when we use a battery and a direct current do we really call this resistance. The proper term in electrosurgery for resistance is impedance. So for the rest of this talk, I’ll be using the word impedance instead of resistance to talk about that difficulty by which electrons pass through a circuit. But most importantly, and fundamentally, voltage is force.
When we think about force and voltage, we think about surgery. Whether it’s physical or it’s electrical, greater force is greater risk. So whatever we can do to minimize voltage is going to minimize unpredictable behavior of thermal modalities, and it will maximize our safety in tissue.
Now what is electrosurgery? It’s…taking something hot and burning something with it. When, in fact, what we do is we take alternating current in very small frequencies—60 cycles per second. And it gets plugged into an electrosurgical generator, our boga unit, as we refer to it in slang terms in operating surgery. And that is simply a machine that accelerates the frequency to very high frequency—to up to 3 million, but typically around 500,000 to 700,000 cycles per second versus 60. As that current is transmitted through cells, because they have ions in them and water, this energy is now converted to kinetic energy, because you have alternating current that flows against the resistance. And in overcoming this resistance, you get what’s called resisted heating, or the production of heat. So in fact, as you see, you take something that really has no temperature whatsoever, and we create temperature with it by the nature of passing current through something of variable resistance, called resistive heating.
So the question comes up—What determines whether coagulation or cutting occurs? Does it have to do with the salic generator? No, in fact it has nothing to do with the generator. Think about it. If we take tissue and we heat it up slowly, and we percolate the water out, we coagulate or we desiccate it. If we take the same tissue and we now heat it up very rapidly, we now create steam, and that steam is very explosive—it ruptures the cell membranes and you get vaporization, or what’s called cutting. So what’s the difference between the two? The only difference we see here on this graph is the rate at which we heat up tissue. So, in fact, we completely moderate between cutting and coagulation, based on simply how fast or how slowly we heat up the water in tissue. And how do we do that intuitively?
Well, we all do it as surgeons intuitively because we manipulate energy density. And how do we do that? Well, think of the old model. I think at least all the males here watching this presentation remember taking a magnifying glass and taking sun and heating up different structures with the sunlight. And we know that if it passed our hand through this part of the beam, it’s actually relatively cool. And if we now go to where we have the beam focused, we end up having a much warmer spot. What’s the difference here? It’s just a difference of energy density, and this is what we do. That’s relatively cool and that’s relatively hot.
So now we take an electrosurgical instrument, and this instrument is universal—it’s a monopolar electrosurgical spoon. And we now look at…we have 2 possibilities with this instrument. One possibility is we have a large flat surface, a large surface area. The current density is going to be relatively low. So what do we expect it to do? It’s going to desiccate or coagulate the tissue. We now take the same instrument. We just turn it to the edge. We now have a small surface area. We have a much higher current density. What do you expect it to do? It’s going to vaporize or cut.
So now, let’s take a look and see if that’s actually true. Here we take a monopolar spoon. It vaporizes—we’re using a small surface area—and we take it and we turn it to a larger surface area that’s flat. It’s going to simply coagulate, desiccate the tissue—no cutting whatsoever. Now I’m going to take it and move it to the edge again, simply moderating the density of energy, and we have a very efficient cut. This is the moderation of current density having nothing to do with the generator settings.
Now, what do we need to get a cut? Well, we have to focus the energy. We have to spark. And so, what do we need to create a spark between the electrode and the surface of tissue? We need to create this spark with energy, and the minimum threshold for that is about 200 volts. So now, hopefully, you ask yourself the question, Where is there 200 volts coming out of your generator—the cutting, blend, or coag side? Well, it comes out of every side of the generator.
So we can cut and we can spark, regardless of where we are at the generator. And so we see this video and we know that this is being created by both a blend, cut, and coag current without difficulty whatsoever. So now, hopefully, we can ask the question, Does it matter? Do we care where we are on the side of the generator to create this cut, since it all works that way? And let’s take a look to see if there’s a basis for caring.
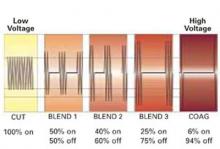
So now, for the first time, I’ll bring up the concept to you—What comes out of this box? This is essentially what comes out of the box.
You see on the cutting side of your generator, it’s an unmodulated…called simply uninterrupted, relatively low-voltage sign wave. And we start to blend and go to coag—if you notice, you have interruption of current, and you see a dramatic rise in the voltage. And finally, on the coag side of the generator, you see, in fact, the voltage is quite high and, in fact, the current’s off most of the time. It’s actually sparking.
So, look at where that voltage is. Here’s the lower voltage and there’s the higher voltage. Remember, voltage is risk, voltage is force. So, we know that the coag side of our generator is the least predictable and most thermally destructive part of the generator.
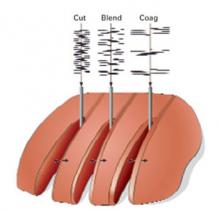
Now, let’s take a look at the cut edges of a piece of tissue. So look at the difference in the thermal margins of these cuts when you compare the cut blade form, the blend wave form, and the coag wave form. And let me help you understand this concept of blend. So, many people think blend means that we blend a little of this and we blend a little bit of that and we get some sort of a mixed wave out of the generator. No. The blend refers to a blended tissue effect. And what happens when you blend 1, blend 2, blend 3, and finally the coag, you’re starting to go from a pure cut to now a cut with more thermal margins. And notice on the coag side, you cut very efficiently, but you have a much wider swath of destruction. And so, if you see that and you think about it, your thermal spread or charring is much higher on the coag side. Your voltage is higher and, of course, the adverse is true on the cut in side of your generator. So we, essentially, as laparoscopic and also laparotomic surgeons, should learn how to live on the cutting, uninterrupted side of our generator, where there’s low voltage for virtually everything we do. And there are selective reasons to say, “I would want to be on the coag side of my generator,” and that’s—remember—bigger voltage, bigger push. If we need a bigger push because we have higher resistance or if we want to get more hemostasis because we’ve got wider thermal margins, we would want to be there. So think about it. Cutting through erectus muscle, cutting through myometrium, cutting through fat, cutting through adhesions—these are all situations where either you want more hemostasis at the edge or you need a bigger push to cut, as in cutting fat or cutting adhesions. So exemplary of this, let’s say you’re doing total laparoscopic hysterectomy. You’re going to incise the vaginal cuff off of the lower cervical tissue. And, of course, we would want to be somewhere on the blend side, if not coag, because we’ve got to hemostase the vessels in the vagina. So this is a perfect example. You would selectively use a certain side of your generator to get a certain desired effect because you expect the thermal margins, in this case, to be more vascular.
So, so far I’ve talked to you about sparking. I’ve said nothing about what happens when you take an electrode and you come in contact with tissue. The rules will change, as you see, when you do contact electrosurgery versus noncontact.
And think about it. Look at this slide and think about the difference in tissue effects just based on the current density. There’s your spark. Think the same electrode, same settings, and now instead of sparking noncontact, take that electrode and put it in contact with the tissue. Look what happens here—you have a much lower current density. You have different thermal effects on tissue. You have much deeper, wider thermal effects, and the rules are different.
So let’s look at a cross-section of liver. Let’s take a roller, sort of ball, electrode, and let’s do some desiccation, coagulation to the surface of the liver. There’s the coag side of the generator. There’s the cutting side of the generator. Remember—high voltage, low voltage—one’s highly interrupted, the others uninterrupted. And look at the visual features that you see here. Here you see cooked, carbonized, high-voltage, high-temperature phenomena, sticky, smoke, but look at the penetration. Relatively minimal, because when you contact with a very high voltage, you penetrate very superficially. On the other hand, look what happens when you use a lower voltage current, which heats the tissue more slowly. That tissue now heats more effectively and more deeply to get a more effective burn. Is this nuance? No, it’s more than nuance. If you happen to be a surgeon who treats endometriosis with electrosurgery and you do electrosurgical ablation of endometriosis with different types of electrodes, realizing that as a disease entity, endometriosis is a retroperitoneal disease. Very commonly you would want to have a depth of penetration that goes beyond the visible portion of the lesion. And you would be compelled to go to a cutting wave form if you wanted to have very thorough, deeper penetration of tissue. So high voltage, rapid desiccation, superficial penetration, lower voltage. Think about it—gradually cook the tissue, more effective, more water in the tissue, and deeper penetration.
And we’re reminded that, regardless of where you are and what kind of instrument you use, there’s one thing that’s always against you when it comes to thermal margins, and that is the longer you’re there, the more injury you produce. So that doesn’t mean that we are frenetic with our movements, but it means that we have a certain amount of alacrity and a certain amount of purposefulness. And we move with intent when we do surgery to minimize the chance for prolonged thermal injury.
So to summarize, we discussed how these variables might impact thermal margins and tissues and the behavior of electrodes. We can manipulate electrodes, which changes their surface area, which completely changes their behavior in the context of current density. We can choose—remember—cutting, blend, or coag waveforms and get different phenomena, different thermal margins. Because, in fact, we’re changing radically the height of the voltage, the output voltage that we’re utilizing. If we change the size of our electrode, you change the surface area, which changes the current density. And then remember, dwell time equals thermal margins. We want to know that we are purposeful with our surgical movements.
So using that information, I want to talk for the rest of this time about bipolar electrosurgery, what I call the basics of bipolar electrosurgery.
Now, not to confuse anyone, but all surgery is bipolar because alternating current means that the current alternates from one pole to the other. What’s really different about bipolar versus monopolar surgery, in the context of using that standard terminology, is the circuit. So think about this—in what we call bipolar electrosurgery, the circuit is the tissue and the 2 tips of the instrument or the 2 electrodes that are close to the tissue. That is the entire circuit. In monopolar electrosurgery, you have a dispersive or return electrode…on the thigh, or the leg, or possibly on the thorax, and you now have the energy going from the tip of the instrument all the way through the patient and back to the generator to complete the circuit.
Think about the energy requirements here. On one, you just have to have energy push through a small piece of tissue between the tips. On the other, you need energy to push all the way through the entire patient back to the generator to complete the circuit. One is much more power consuming. One requires a lot more voltage. Therefore, monopolar electrosurgery, on a power-for-power basis, has much greater requirements. And whether you know it or not, when you take your banana plugs and you plug into an electrosurgical generator—it doesn’t matter what the manufacturer is—and you hit your blue pedal, which is standard throughout the world for the coag output, and it’s set to bipolar, it always by default will put out a pure cut current even though you are putting a coag phenomenon in place. And that’s because, hopefully, it would make sense that if you used very high voltage on a small little area of tissue between 2 tips of an instrument, it literally would fuse and be dysfunctional for you in a surgical sense. So to minimize the chance of adverse thermal effects, it’s always a low-voltage cutting current that’s set off by default by bipolar electrosurgical devices.
Now, let’s take a look at this picture and focus in on the picture on the right. And when I first saw this picture, I really didn’t notice anything very significant until someone really educated me and pointed out what really happens when you take a vessel of substantial size, you grasp it, and you compress it with a conventional Kleppinger bipolar forceps. And then take a look, in fact, how hemostasis is attained in that vessel. Well, the first thing you notice is that it’s shrunken, and it shrinks because when you heat collagen and elastin, it’s like an accordion—it shrinks up about 40% to 50% of volume. When you heat collagen, it just shrinks up like a Slinky. So you have shrinkage of the vessel wall. But you’ll also notice there’s a large proximal thrombus there. But if you’re really a careful observer—and as I’ve said, I missed this the first time I looked at it—the lumen is still apparent. In fact, the lumen is patent. And when we coagulate and desiccate vessels of significant size with a Kleppinger bipolar forceps, regardless of what your generator settings are, you have a vessel that essentially has a patent lumen. Now, as surgeons in the pelvis, we can get away with this because our pulse pressers in general are very low, because we’re down the vascular tree from the central pump. But you are a general surgeon, and you saw this and you realized you have to work on vessels like the gastric artery that has significant high systolic pulse pressures, you would realize you could not rely upon this technology, and we can understanding looking at this, for our general surgeon colleagues really didn’t become interested in bipolar electrosurgery until they had further assurances that it would be more effective.
Now, this slide here is a summary of what are my experiences with conventional bipolar electrosurgery. And I think every surgeon who’s watching this and is honest about their work experiences these on a daily basis using conventional bipolar electrosurgery. I don’t know how many times I’m going to overlap. I’m not sure you do, either. On 1 pedicle, 2 pedicles, right side, left side. We have variable numbers of compressions to attain the task. No automation, no regulation of what we’re doing. How long do I apply the energy? How long do you apply the energy? How long do I apply the energy on the right? And then how much do I apply on the left? And then you know and I know, we look at the thermal margins—we have spread. We don’t have total control over our thermal margins, especially using conventional bipolar electrosurgery. And most importantly, you’re satisfied, I’m satisfied, our vessel looks like a rope, it’s shrunken down, it looks completely desiccated. We now take our scissors and we cut it and it bleeds. And you have your little red dot that pulsates, and you realize that after all that work, you’ve got to go and work on it again. So these, I think, are generic, universal problems we have with conventional bipolar electrosurgery.
And I also ask you the question, What’s the margin of safety? Yes, we know that the current flows between the 2 jaws. No question the current goes nowhere else. There’s no diversion of current that occurs with bipolar electrosurgery. It cannot happen. But there’s something else with bipolar that’s problematic—and that is the production of steam and thermal effects.
So when you see this picture, you have to ask yourself the question, If the electricity just goes from one jaw to the other, what is this white that is blooming? And all of you who have done tubal coagulation procedures for a female sterilization have seen, on the application of energy with Kleppinger bipolar forceps, a little puff of steam and then, all of a sudden, the whole mesosalpinx rises and drops. And, of course, that’s the filling of the broad ligament with steam. The steam rises, and it gets absorbed, it drops. So we create a huge plume of steam. Remember what steam does to cells? Steam is very percussive—it explodes the cells and it spreads heat. So it’s very destructive.
So one way we avoid making too much steam, if we’re doing conventional bipolar electrosurgery, is we never use ammeters to direct the surgical endpoint. We use ammeters to direct the surgical endpoint for tubal coagulation because we want napalm to be delivered to the mesosalpinx. That’s the opposite of what we want, of course, when we’re doing surgical dissection of the pelvis. So if you use an ammeter to direct your endpoint in bipolar electrosurgery, you are maximizing steam production and therefore maximizing lateral thermal injury.
Now, we can use common-sense methods to minimize destruction from conventional bipolar electrosurgery. And we can watch tissue, and when it becomes white and when it becomes dry and doesn’t emit steam any more, we can feel confident it’s ready to cut. So we use color and we use tissue behavior to dictate when we’re done, rather than to overcook tissue through fear and anxiety that we may have a patent lumen. We can also pulse current because current can be applied and, in between the pulses, the energy can be dissipated by the circulation of the blood, by convection and conduction of heat. We also can take the sides of our instrument and realize that when you take tissue and you grasp it and you crush it, you end up having wide thermal margins.
So look at this little bleeding pedicle after salpingo-oophorectomy. And the attack is to take a tack from the right side contralateral and use a tamponade desiccate, no grasping of tissue. This is an entirely different method of hemostasis than grabbing a pedicle, crushing it, and desiccating it. So whenever we can tamponade desiccate, almost like a trampoline—tamponade desiccate, tamponade desiccate—we basically have almost no thermal penetration and no thermal margins.
And, of course, both you and I get panicked when we see active bleeding. And what we must do is we must resist this immediate desire to stop bleeding with energy. And so when we have active bleeding, first and foremost as surgeons, we have to find out where we are and then we’ve got to stop everything mechanically.
And if you see any advanced laparoscopic surgeon deal with adversity in hemostasis, you will see every one of them grasp or put tension on a bleeder to tamponade, time out, take a breath, find out where you are, and then, and only then proceed. And if you see there’s something relevant that’s near, whether it’s uterosacral ligament, ureter, bladder, bowel, you will mobilize that tissue with the appropriate relaxant incisions. And then and only then do you bring in the heat. And even then, you might be conservative and be pulsatile desiccating versus pure desiccating.
Now, this is the background of where we’ve been. And I and a number of colleagues have been talking about this, writing about this, for many years. And to our pleasure, I don’t think in response to anything we’ve done, industry has come forward with amazing innovation and research outputs that have changed the face of bipolar electrosurgery. And we’re going to discuss a few of these evolutionary steps as they contrast to traditional bipolar electrosurgery to help you understand what’s available today.
We all remember a number of years ago that we had a 10-mm device that finally came out that was like a waffle iron and we had a blade that came down. So now for the first time, we had a multifunctional, multiple-purpose instrument to be placed on a trocar that could coagulate and cut with a mechanical blade. Many things followed that innovation. In response to everything we know about voltage—you remember voltage is destructive, is force; voltage is the wrong side to be on the generator, if you can help it. These all became now relatively constant, low-voltage devices. So the high-voltage equation has been erased from bipolar electrosurgery with use of these devices. And for the first time, rather than a generator autonomously just delivering energy as you push on the pedal, now the generator is talking to the tissue and the instruments all send back information about the resistance impedance of the tissue. So now, the amount of energy that’s created, the amount of current that’s delivered by the instrument, is completely moderated to what’s needed to desiccate the tissue, so you get more uniform effects. And as we’ve advocated for pulsing, for cooling, pulsing does 2 things. Not only does it help the tissue cool, it also allows the generator in between pulses to register the information and now respond. So the introduction of pulsing gives the generator a chance to communicate with the tissue. And with this tissue cooling, you also get delivery of energy that’s more uniform. And finally, if you remember the last point on my conventional slide, which was we’re all happy, everything’s wonderful, now we cut the vessel and we’ve got the little red course spurting in our face. We now have technology, that when we even get audible feedback, or we are integrated with the device, and we get visual proprioceptive feedback and we decide to push the button, push the blade, we in fact have reliable, consistent hemostasis. We’ve changed the whole equation with the evolution of bipolar electrosurgery. So look at this vessel now. We’re talking about intima-to-intima fusion from vessel sealing.
Now, one of the pieces of these devices is that they’re all approved for vessels up to 7 mm. And this is well beyond anything that you or I are going to experience and need in the pelvis.
Another nuance is when you take a blood vessel and you compress it with low energy, ie, low voltage and high current. You need to do a little trick if you want a great seal. And a great seal is also to release your tension just a little bit on your pedicle and then to apply the energy. So we allow that lower energy to do an intima-to-intima fusion, which sometimes is physically overcome by too much torsion or traction on your pedicle.
So you would expect to see some promises here. And the promises are, hopefully, natural and you’ll see them each time. We don’t have tissue sticking, because that was from high-voltage carbon. We don’t have it anymore. Overheat the tissue, get plume and smoke. Your environment gets muddled. Reduced thermal spread, because now we’re just delivering the right amount of energy that’s needed to attain the task. And, ultimately, we have consistent vessel sealing. This is almost the adverse of that slide that I showed with the generic concerns that we have as laparoscopic and laparotomic surgeons.
Now, one of the advents that was very important in the last several decades was an understanding of the whole idea of tissue welding. And then, in fact, it realized that you could use a certain amount of pressure—very, very high compressive force. If you could deliver that with a small amount of voltage and a small amount of energy, temperatures less than 100°, for the right amount of time you could literally transform collagen and elastin into a tissue glue that had tremendous sustaining powers that could overcome any high or superphysiologic systolic pressures.
So we look at this vessel, which is vessel seal. Incredible difference from the one that we saw before. And here we see true intima-to-intima sealing. And look at the margins, the heat thermal affects how dramatically they differ in these 2 vessels.
So I want to finish this up by talking to you about the 2 devices that are in my armamentarium, because they’re complementary, and especially about this new technology called ENSEAL®. And, as I said, there’s been an evolution of devices. But with this particular dedicated advanced bipolar device, there are 3 unique features that, to me, are very exciting because they open up new possibilities and new promises in the context of delivery of energy to the tissue and regulating the thermal effect. And I’m going to tell you about each one separately. One is basically an intrinsic control that’s in the device that actually determines the temperature at which the tissue is heated. And we’ll talk about that. And it regulates the energy delivery. Another is a unique configuration. And I think you’re all going to be immediately excited about this when you see this invention that was thought of by the inventor. It gives you this, for the first time, a uniform compression from the heel to the tip of the instrument. And lastly, there’s a jaw design with the electrodes that help keep the current from spreading laterally into the tissue that gives you a consistent effect.
So let me try the first one with you, and it may be the most difficult to explain, but I think it’s the most interesting. As I told you, there was no way to moderate output in conventional bipolar electrosurgery. Push your foot on the pedal, deliver the energy, and if it was a blow torch of voltage, it was a blow torch of voltage. That’s what you get with the Kleppinger. Now you use more advanced devices. The generators are communicating with the tissue and only putting out what it needs. Now for the first time, it’s regulated at the jaw.
So, in fact, the thermal effects in the ENSEAL device are regulated by the compositional electrical characteristics of the jaw. And I’ll explain to you how this is. The jaw basically is plastic, and plastic is a nonconductor. And the reason that this conducts electricity is because it’s been impregnated with small spears of conductive particles. And these conductive particles can control energy delivery through a very, very unique mechanism. So look at the picture on the left and then look at the one on the right in this lower diagram. You’ll notice on the left that the particles are lined up and they’re close together. They’re in the plastic, and this plastic very comfortably is going to conduct the electricity. As the plastic heats up, the spirals, these conductive particles are going to move farther apart. And as they move farther apart, guess what happens? It becomes nonconductive. So if you can take this other step and realize that the jaw is not uniformly one temperature, there may be several hundred zones along the jaw but at different temperatures, in fact, like thermostats, are turning on, turning off, turning on, turning off, in response. And the end result is that you get uniform temperature because it’s going to turn off at approximately less than 100°C.
So look at this real-time thermography here of how literally the thermal margins intrinsically plateau at about 100°. And that is determined by the construction of the jaw, having nothing to do with generator output.
Now, take a look here at an animation that would help us. And so here we have your conductive particles close together, conducting electricity. As the plastic starts to heat up, what’s going to happen to these particles? The particles are going to spread apart and, naturally, certain zones of the jaws are going to stop. And when they then cool off, these spirals will come closer together and they’ll start conducting again. So here we have a reconfiguration. You see 100° becomes nonconductive. Now, of course, as they cool, they come closer together again and they’re going to conduct again. So you have this mirror infinite number of microcircuits occurring until finally the current is self-regulated and turns off, based on where the jaw is in impedance of the tissue. Secondly, if you look at a Kelly clamp, a Heaney clamp, or any bipolar electrosurgical device, and you measure the pressure, and you know this intuitively, as surgeons, pressure is greatest at the heel and is least at the tip. We’ve got to change that—that’s the physics of the device. And unless you increase the girth of a device, as you increase the length this becomes even dramatically more problematic. So of all the devices up to this point, you’ve got high pressure at the heel, lower pressure at the tip. So for vessel sealing—think about it—you want high compressive forces—remember, low energy a certain amount of time—the tip of the instrument in certain cases does not get sealed very well in certain devices, because we can’t get the same compressive pressure from the heel to the tip by the physics. So the maximum compression is at the heel of the clamp.
Now take a look at an I-Blade™, I-Beam configuration, that now is part of the ENSEAL device, which to me is one of the most innovative features.
So here you have a blade coming down, but it’s an I-Beam, and since it’s an I-beam, it pulls across, and the pressure is absolutely the same at the tip as it is on the heel and, as a result your seal, is going to be absolutely the same histologically whether you’re looking at the heel, midway in the clamp, or at the tip histologically, and you’re going to get a uniform cut. A very unique discipline here.
And you can see this cut, and you can see, in fact, it is highly predictable, and minimal thermal margins.
And lastly, very interesting. And there’s a background to explain this third feature.
Think about the 2 fundamental principles of electricity that can’t be changed. One is that electricity always seeks to ground, which is the earth. We cannot change this reality. And the other reality you cannot change in electricity is electricity is always going to follow the path of least resistance. You cannot change this.
So think about a conventional bipolar electrosurgical device. Conventional bipolar electrosurgical devices have a positive and negative pull—this is the way they’re set up.
So look at the way the current flows. The current initially is going to flow from positive to negative, but as the tissue heats up and gets drier, the current is going to try to seek other paths to make the connection of the circuit. And it moves the current more laterally, in a progressive fashion, until finally the resistance is so high it can’t go more laterally. But this literally tends to push the current lateral to the jaws to complete the circuit. Electricity seeks the path of least resistance. Now, there is an offset jaw configuration of the electrodes on the ENSEAL device that, in fact, keeps the current within. Because if you look here carefully, you see a positive/negative, but the positive is surrounded by negative poles. So as a result, what this does is this isolates the current to within the material that’s between jaws and keeps it from spreading laterally, as compared to the other portion of the device.
So to recap, I talked to you about electrode configuration, which are the conductive elements in there, the nano spiral technology, that expand and contract and keep the temperature at about 100°. It doesn’t matter how long you’re there, it’s going to stay at that temperature. You have an I-Blade that overcomes all of the intrinsic problems we have in the context of heel versus tip pressures on vascular and hemostatic clamps. And we have an interesting design of the electrodes that tends to keep the current within the jaws rather than making it spread laterally to complete the circuit through the path of least resistance. So that’s not the only device that we use for surgery. And, of course, there are many devices that are complementary. And, for me, the most complementary device because it’s so specifically designed for soft-tissue dissection, we’re talking dissection instrument, is the Harmonic® Ace™ device.
And, of course, the Harmonic Ace device has nothing to do with electricity, and I want to dispel that right away. Harmonic energy has to do with mechanical energy. It’s very different.
And here, we have high-frequency ultrasound in tissue creating a number of predictable events. And the most predictable event is that it basically denatures proteins by deconfiguring them, by breaking up the hydrogen bonds that keep their structure, and they get denatured—a very low temperature phenomenon. And, ultimately, if you have ultrasound and tissue you form steam—and, remember, steam is percussive, you can’t take that away from steam. And steam that’s in the tissue will start to cavitate it and it will make it fragment, and that’s what’s called cutting. Ultrasonic energy attains cutting by cavitational fragmentation of tissue with the production of steam from high-frequency ultrasound.
This is the anatomy of a Harmonic Ace. And we have a tissue-holding device on top, which is plastic, nonmoving, nonthermal. And we have a blade that’s oscillating 55,500 times a cycle. Think about how low-frequency this is. You can use 3 million cycles per second when you’re doing ultrasound, vaginal ultrasound in the office. This is 55,500 times a cycle. And that’s also why it has such thermal effects.
And you can vary how far this blade goes out per cycle. And that’s what’s called your levels. And, in fact, this has nothing to do with power; it has to do with how much your blade comes out per cycle, which is called excursion. And, essentially, a Harmonic scalpel…you can see how different settings—you know, I’ll just run that through again, settings 1, 2, 3, 4, 5—affect how far the blade comes out per cycle. And as a result of this, since the energy that’s being produced is being delivered in the same amount of time, whether you come out a short distance or a long distance, you can predict the following algorithm based on those excursions. In general, if you want to coagulate with a Harmonic ACE, you want to be in the lowest setting so the energy is delivered over the smallest surface area, which is the smaller blade excursion. And on the other hand, just the opposite—if you want to cut, you’re obviously going to just use the opposite algorithm, and you’re going to want to have the largest surface area with the same amount of energy, and that’s going to promote cutting. So our variable features on the Harmonic scalpel totally relate to what we intend on doing—or not doing—with our Harmonic ACE.
Now, what’s really different to me between ultrasonic energy and thermal energy from electrosurgery is the volumetric spread of energy. So let’s take a look at the Harmonic scalpel. Remember, it’s an ultrasound, it’s a blade that’s oscillating 55,500 times a second. Where’s most of the energy? It’s at the tip of the instrument, in fact, and the most active portion of a Harmonic scalpel is at the tip of the blade because it is linearly propagating. As a result, the lateral margins are that much smaller because you don’t have the propagation of energy in an ellipsoid 3-dimensional fashion as you do with electrosurgery. It also makes your thermal margins much more predictable.
And so, of course, what we do is we make sure, as I told you, low-energy devices—we’re getting back to that central principle—low-energy devices get the best hemostasis based on our vessel compression. You want to release tension. So when you do the Harmonic ACE for vessels, you grasp, you release tension, you then desiccate. And then, after you see the visual signs of desiccation—which, of course, is a plume of steam—you now will wait, let the cutter curve, and then you will remove. And you always remove in a linear fashion in the same track that you applied your device.
So I want to finish up and thank you for being here today. I hope that was helpful for all who might want to know more about electrosurgery, ultrasonic energy, to remind you and myself that it really has nothing to do with the wand, and it has to do with the mirror and my understanding of fundamentals of electricity and ultrasonic. And it’s really up to you, it’s not up to the wand. And I’d be more than happy to take any questions or comments from anybody here who’s been nice enough to listen to today’s talk. Okay, thank you very much.
Energy options in gynecologic surgery—a topic very dear to me and very often understudied for all of us in our basic training.
And as a signpost of today’s presentation is the review that we’ll partake of fundamental knowledge and technique. And for me, when I take fundamental knowledge and technique and reduce it to its ingredients, it definitely leads to being safer and also contributes to efficacy and efficiency of surgery. And for me, when I combine these 2 elements and I think about really how they contribute to my practice and the outcome of patients, it’s surgical outcome. So today is really dedicated to improving outcome in patients.
Now, in order to hopefully communicate as best as possible with you, I want to review both new concepts and also what I would term electrosurgery vernacular.
In order to do that, I’d like to create a model here and build a water tower. And just think of…in order to get the water into the tower, you have to have some sort of push or force to create that reservoir. In electrosurgical terms, voltage is what provides that push that pushes the current through something called resistance, which is a variable ability for something to pass through a circuit. Obviously, if the resistance goes up, the aperture get smaller. You have to push harder. If you push larger amounts of current, you have to push harder. But these are the 3 elements of electricity, and so we have current, we have resistance, and we have voltage. But…only when we use a battery and a direct current do we really call this resistance. The proper term in electrosurgery for resistance is impedance. So for the rest of this talk, I’ll be using the word impedance instead of resistance to talk about that difficulty by which electrons pass through a circuit. But most importantly, and fundamentally, voltage is force.
When we think about force and voltage, we think about surgery. Whether it’s physical or it’s electrical, greater force is greater risk. So whatever we can do to minimize voltage is going to minimize unpredictable behavior of thermal modalities, and it will maximize our safety in tissue.
Now what is electrosurgery? It’s…taking something hot and burning something with it. When, in fact, what we do is we take alternating current in very small frequencies—60 cycles per second. And it gets plugged into an electrosurgical generator, our boga unit, as we refer to it in slang terms in operating surgery. And that is simply a machine that accelerates the frequency to very high frequency—to up to 3 million, but typically around 500,000 to 700,000 cycles per second versus 60. As that current is transmitted through cells, because they have ions in them and water, this energy is now converted to kinetic energy, because you have alternating current that flows against the resistance. And in overcoming this resistance, you get what’s called resisted heating, or the production of heat. So in fact, as you see, you take something that really has no temperature whatsoever, and we create temperature with it by the nature of passing current through something of variable resistance, called resistive heating.
So the question comes up—What determines whether coagulation or cutting occurs? Does it have to do with the salic generator? No, in fact it has nothing to do with the generator. Think about it. If we take tissue and we heat it up slowly, and we percolate the water out, we coagulate or we desiccate it. If we take the same tissue and we now heat it up very rapidly, we now create steam, and that steam is very explosive—it ruptures the cell membranes and you get vaporization, or what’s called cutting. So what’s the difference between the two? The only difference we see here on this graph is the rate at which we heat up tissue. So, in fact, we completely moderate between cutting and coagulation, based on simply how fast or how slowly we heat up the water in tissue. And how do we do that intuitively?
Well, we all do it as surgeons intuitively because we manipulate energy density. And how do we do that? Well, think of the old model. I think at least all the males here watching this presentation remember taking a magnifying glass and taking sun and heating up different structures with the sunlight. And we know that if it passed our hand through this part of the beam, it’s actually relatively cool. And if we now go to where we have the beam focused, we end up having a much warmer spot. What’s the difference here? It’s just a difference of energy density, and this is what we do. That’s relatively cool and that’s relatively hot.
So now we take an electrosurgical instrument, and this instrument is universal—it’s a monopolar electrosurgical spoon. And we now look at…we have 2 possibilities with this instrument. One possibility is we have a large flat surface, a large surface area. The current density is going to be relatively low. So what do we expect it to do? It’s going to desiccate or coagulate the tissue. We now take the same instrument. We just turn it to the edge. We now have a small surface area. We have a much higher current density. What do you expect it to do? It’s going to vaporize or cut.
So now, let’s take a look and see if that’s actually true. Here we take a monopolar spoon. It vaporizes—we’re using a small surface area—and we take it and we turn it to a larger surface area that’s flat. It’s going to simply coagulate, desiccate the tissue—no cutting whatsoever. Now I’m going to take it and move it to the edge again, simply moderating the density of energy, and we have a very efficient cut. This is the moderation of current density having nothing to do with the generator settings.
Now, what do we need to get a cut? Well, we have to focus the energy. We have to spark. And so, what do we need to create a spark between the electrode and the surface of tissue? We need to create this spark with energy, and the minimum threshold for that is about 200 volts. So now, hopefully, you ask yourself the question, Where is there 200 volts coming out of your generator—the cutting, blend, or coag side? Well, it comes out of every side of the generator.
So we can cut and we can spark, regardless of where we are at the generator. And so we see this video and we know that this is being created by both a blend, cut, and coag current without difficulty whatsoever. So now, hopefully, we can ask the question, Does it matter? Do we care where we are on the side of the generator to create this cut, since it all works that way? And let’s take a look to see if there’s a basis for caring.
So now, for the first time, I’ll bring up the concept to you—What comes out of this box? This is essentially what comes out of the box.
You see on the cutting side of your generator, it’s an unmodulated…called simply uninterrupted, relatively low-voltage sign wave. And we start to blend and go to coag—if you notice, you have interruption of current, and you see a dramatic rise in the voltage. And finally, on the coag side of the generator, you see, in fact, the voltage is quite high and, in fact, the current’s off most of the time. It’s actually sparking.
So, look at where that voltage is. Here’s the lower voltage and there’s the higher voltage. Remember, voltage is risk, voltage is force. So, we know that the coag side of our generator is the least predictable and most thermally destructive part of the generator.
Now, let’s take a look at the cut edges of a piece of tissue. So look at the difference in the thermal margins of these cuts when you compare the cut blade form, the blend wave form, and the coag wave form. And let me help you understand this concept of blend. So, many people think blend means that we blend a little of this and we blend a little bit of that and we get some sort of a mixed wave out of the generator. No. The blend refers to a blended tissue effect. And what happens when you blend 1, blend 2, blend 3, and finally the coag, you’re starting to go from a pure cut to now a cut with more thermal margins. And notice on the coag side, you cut very efficiently, but you have a much wider swath of destruction. And so, if you see that and you think about it, your thermal spread or charring is much higher on the coag side. Your voltage is higher and, of course, the adverse is true on the cut in side of your generator. So we, essentially, as laparoscopic and also laparotomic surgeons, should learn how to live on the cutting, uninterrupted side of our generator, where there’s low voltage for virtually everything we do. And there are selective reasons to say, “I would want to be on the coag side of my generator,” and that’s—remember—bigger voltage, bigger push. If we need a bigger push because we have higher resistance or if we want to get more hemostasis because we’ve got wider thermal margins, we would want to be there. So think about it. Cutting through erectus muscle, cutting through myometrium, cutting through fat, cutting through adhesions—these are all situations where either you want more hemostasis at the edge or you need a bigger push to cut, as in cutting fat or cutting adhesions. So exemplary of this, let’s say you’re doing total laparoscopic hysterectomy. You’re going to incise the vaginal cuff off of the lower cervical tissue. And, of course, we would want to be somewhere on the blend side, if not coag, because we’ve got to hemostase the vessels in the vagina. So this is a perfect example. You would selectively use a certain side of your generator to get a certain desired effect because you expect the thermal margins, in this case, to be more vascular.
So, so far I’ve talked to you about sparking. I’ve said nothing about what happens when you take an electrode and you come in contact with tissue. The rules will change, as you see, when you do contact electrosurgery versus noncontact.
And think about it. Look at this slide and think about the difference in tissue effects just based on the current density. There’s your spark. Think the same electrode, same settings, and now instead of sparking noncontact, take that electrode and put it in contact with the tissue. Look what happens here—you have a much lower current density. You have different thermal effects on tissue. You have much deeper, wider thermal effects, and the rules are different.
So let’s look at a cross-section of liver. Let’s take a roller, sort of ball, electrode, and let’s do some desiccation, coagulation to the surface of the liver. There’s the coag side of the generator. There’s the cutting side of the generator. Remember—high voltage, low voltage—one’s highly interrupted, the others uninterrupted. And look at the visual features that you see here. Here you see cooked, carbonized, high-voltage, high-temperature phenomena, sticky, smoke, but look at the penetration. Relatively minimal, because when you contact with a very high voltage, you penetrate very superficially. On the other hand, look what happens when you use a lower voltage current, which heats the tissue more slowly. That tissue now heats more effectively and more deeply to get a more effective burn. Is this nuance? No, it’s more than nuance. If you happen to be a surgeon who treats endometriosis with electrosurgery and you do electrosurgical ablation of endometriosis with different types of electrodes, realizing that as a disease entity, endometriosis is a retroperitoneal disease. Very commonly you would want to have a depth of penetration that goes beyond the visible portion of the lesion. And you would be compelled to go to a cutting wave form if you wanted to have very thorough, deeper penetration of tissue. So high voltage, rapid desiccation, superficial penetration, lower voltage. Think about it—gradually cook the tissue, more effective, more water in the tissue, and deeper penetration.
And we’re reminded that, regardless of where you are and what kind of instrument you use, there’s one thing that’s always against you when it comes to thermal margins, and that is the longer you’re there, the more injury you produce. So that doesn’t mean that we are frenetic with our movements, but it means that we have a certain amount of alacrity and a certain amount of purposefulness. And we move with intent when we do surgery to minimize the chance for prolonged thermal injury.
So to summarize, we discussed how these variables might impact thermal margins and tissues and the behavior of electrodes. We can manipulate electrodes, which changes their surface area, which completely changes their behavior in the context of current density. We can choose—remember—cutting, blend, or coag waveforms and get different phenomena, different thermal margins. Because, in fact, we’re changing radically the height of the voltage, the output voltage that we’re utilizing. If we change the size of our electrode, you change the surface area, which changes the current density. And then remember, dwell time equals thermal margins. We want to know that we are purposeful with our surgical movements.
So using that information, I want to talk for the rest of this time about bipolar electrosurgery, what I call the basics of bipolar electrosurgery.
Now, not to confuse anyone, but all surgery is bipolar because alternating current means that the current alternates from one pole to the other. What’s really different about bipolar versus monopolar surgery, in the context of using that standard terminology, is the circuit. So think about this—in what we call bipolar electrosurgery, the circuit is the tissue and the 2 tips of the instrument or the 2 electrodes that are close to the tissue. That is the entire circuit. In monopolar electrosurgery, you have a dispersive or return electrode…on the thigh, or the leg, or possibly on the thorax, and you now have the energy going from the tip of the instrument all the way through the patient and back to the generator to complete the circuit.
Think about the energy requirements here. On one, you just have to have energy push through a small piece of tissue between the tips. On the other, you need energy to push all the way through the entire patient back to the generator to complete the circuit. One is much more power consuming. One requires a lot more voltage. Therefore, monopolar electrosurgery, on a power-for-power basis, has much greater requirements. And whether you know it or not, when you take your banana plugs and you plug into an electrosurgical generator—it doesn’t matter what the manufacturer is—and you hit your blue pedal, which is standard throughout the world for the coag output, and it’s set to bipolar, it always by default will put out a pure cut current even though you are putting a coag phenomenon in place. And that’s because, hopefully, it would make sense that if you used very high voltage on a small little area of tissue between 2 tips of an instrument, it literally would fuse and be dysfunctional for you in a surgical sense. So to minimize the chance of adverse thermal effects, it’s always a low-voltage cutting current that’s set off by default by bipolar electrosurgical devices.
Now, let’s take a look at this picture and focus in on the picture on the right. And when I first saw this picture, I really didn’t notice anything very significant until someone really educated me and pointed out what really happens when you take a vessel of substantial size, you grasp it, and you compress it with a conventional Kleppinger bipolar forceps. And then take a look, in fact, how hemostasis is attained in that vessel. Well, the first thing you notice is that it’s shrunken, and it shrinks because when you heat collagen and elastin, it’s like an accordion—it shrinks up about 40% to 50% of volume. When you heat collagen, it just shrinks up like a Slinky. So you have shrinkage of the vessel wall. But you’ll also notice there’s a large proximal thrombus there. But if you’re really a careful observer—and as I’ve said, I missed this the first time I looked at it—the lumen is still apparent. In fact, the lumen is patent. And when we coagulate and desiccate vessels of significant size with a Kleppinger bipolar forceps, regardless of what your generator settings are, you have a vessel that essentially has a patent lumen. Now, as surgeons in the pelvis, we can get away with this because our pulse pressers in general are very low, because we’re down the vascular tree from the central pump. But you are a general surgeon, and you saw this and you realized you have to work on vessels like the gastric artery that has significant high systolic pulse pressures, you would realize you could not rely upon this technology, and we can understanding looking at this, for our general surgeon colleagues really didn’t become interested in bipolar electrosurgery until they had further assurances that it would be more effective.
Now, this slide here is a summary of what are my experiences with conventional bipolar electrosurgery. And I think every surgeon who’s watching this and is honest about their work experiences these on a daily basis using conventional bipolar electrosurgery. I don’t know how many times I’m going to overlap. I’m not sure you do, either. On 1 pedicle, 2 pedicles, right side, left side. We have variable numbers of compressions to attain the task. No automation, no regulation of what we’re doing. How long do I apply the energy? How long do you apply the energy? How long do I apply the energy on the right? And then how much do I apply on the left? And then you know and I know, we look at the thermal margins—we have spread. We don’t have total control over our thermal margins, especially using conventional bipolar electrosurgery. And most importantly, you’re satisfied, I’m satisfied, our vessel looks like a rope, it’s shrunken down, it looks completely desiccated. We now take our scissors and we cut it and it bleeds. And you have your little red dot that pulsates, and you realize that after all that work, you’ve got to go and work on it again. So these, I think, are generic, universal problems we have with conventional bipolar electrosurgery.
And I also ask you the question, What’s the margin of safety? Yes, we know that the current flows between the 2 jaws. No question the current goes nowhere else. There’s no diversion of current that occurs with bipolar electrosurgery. It cannot happen. But there’s something else with bipolar that’s problematic—and that is the production of steam and thermal effects.
So when you see this picture, you have to ask yourself the question, If the electricity just goes from one jaw to the other, what is this white that is blooming? And all of you who have done tubal coagulation procedures for a female sterilization have seen, on the application of energy with Kleppinger bipolar forceps, a little puff of steam and then, all of a sudden, the whole mesosalpinx rises and drops. And, of course, that’s the filling of the broad ligament with steam. The steam rises, and it gets absorbed, it drops. So we create a huge plume of steam. Remember what steam does to cells? Steam is very percussive—it explodes the cells and it spreads heat. So it’s very destructive.
So one way we avoid making too much steam, if we’re doing conventional bipolar electrosurgery, is we never use ammeters to direct the surgical endpoint. We use ammeters to direct the surgical endpoint for tubal coagulation because we want napalm to be delivered to the mesosalpinx. That’s the opposite of what we want, of course, when we’re doing surgical dissection of the pelvis. So if you use an ammeter to direct your endpoint in bipolar electrosurgery, you are maximizing steam production and therefore maximizing lateral thermal injury.
Now, we can use common-sense methods to minimize destruction from conventional bipolar electrosurgery. And we can watch tissue, and when it becomes white and when it becomes dry and doesn’t emit steam any more, we can feel confident it’s ready to cut. So we use color and we use tissue behavior to dictate when we’re done, rather than to overcook tissue through fear and anxiety that we may have a patent lumen. We can also pulse current because current can be applied and, in between the pulses, the energy can be dissipated by the circulation of the blood, by convection and conduction of heat. We also can take the sides of our instrument and realize that when you take tissue and you grasp it and you crush it, you end up having wide thermal margins.
So look at this little bleeding pedicle after salpingo-oophorectomy. And the attack is to take a tack from the right side contralateral and use a tamponade desiccate, no grasping of tissue. This is an entirely different method of hemostasis than grabbing a pedicle, crushing it, and desiccating it. So whenever we can tamponade desiccate, almost like a trampoline—tamponade desiccate, tamponade desiccate—we basically have almost no thermal penetration and no thermal margins.
And, of course, both you and I get panicked when we see active bleeding. And what we must do is we must resist this immediate desire to stop bleeding with energy. And so when we have active bleeding, first and foremost as surgeons, we have to find out where we are and then we’ve got to stop everything mechanically.
And if you see any advanced laparoscopic surgeon deal with adversity in hemostasis, you will see every one of them grasp or put tension on a bleeder to tamponade, time out, take a breath, find out where you are, and then, and only then proceed. And if you see there’s something relevant that’s near, whether it’s uterosacral ligament, ureter, bladder, bowel, you will mobilize that tissue with the appropriate relaxant incisions. And then and only then do you bring in the heat. And even then, you might be conservative and be pulsatile desiccating versus pure desiccating.
Now, this is the background of where we’ve been. And I and a number of colleagues have been talking about this, writing about this, for many years. And to our pleasure, I don’t think in response to anything we’ve done, industry has come forward with amazing innovation and research outputs that have changed the face of bipolar electrosurgery. And we’re going to discuss a few of these evolutionary steps as they contrast to traditional bipolar electrosurgery to help you understand what’s available today.
We all remember a number of years ago that we had a 10-mm device that finally came out that was like a waffle iron and we had a blade that came down. So now for the first time, we had a multifunctional, multiple-purpose instrument to be placed on a trocar that could coagulate and cut with a mechanical blade. Many things followed that innovation. In response to everything we know about voltage—you remember voltage is destructive, is force; voltage is the wrong side to be on the generator, if you can help it. These all became now relatively constant, low-voltage devices. So the high-voltage equation has been erased from bipolar electrosurgery with use of these devices. And for the first time, rather than a generator autonomously just delivering energy as you push on the pedal, now the generator is talking to the tissue and the instruments all send back information about the resistance impedance of the tissue. So now, the amount of energy that’s created, the amount of current that’s delivered by the instrument, is completely moderated to what’s needed to desiccate the tissue, so you get more uniform effects. And as we’ve advocated for pulsing, for cooling, pulsing does 2 things. Not only does it help the tissue cool, it also allows the generator in between pulses to register the information and now respond. So the introduction of pulsing gives the generator a chance to communicate with the tissue. And with this tissue cooling, you also get delivery of energy that’s more uniform. And finally, if you remember the last point on my conventional slide, which was we’re all happy, everything’s wonderful, now we cut the vessel and we’ve got the little red course spurting in our face. We now have technology, that when we even get audible feedback, or we are integrated with the device, and we get visual proprioceptive feedback and we decide to push the button, push the blade, we in fact have reliable, consistent hemostasis. We’ve changed the whole equation with the evolution of bipolar electrosurgery. So look at this vessel now. We’re talking about intima-to-intima fusion from vessel sealing.
Now, one of the pieces of these devices is that they’re all approved for vessels up to 7 mm. And this is well beyond anything that you or I are going to experience and need in the pelvis.
Another nuance is when you take a blood vessel and you compress it with low energy, ie, low voltage and high current. You need to do a little trick if you want a great seal. And a great seal is also to release your tension just a little bit on your pedicle and then to apply the energy. So we allow that lower energy to do an intima-to-intima fusion, which sometimes is physically overcome by too much torsion or traction on your pedicle.
So you would expect to see some promises here. And the promises are, hopefully, natural and you’ll see them each time. We don’t have tissue sticking, because that was from high-voltage carbon. We don’t have it anymore. Overheat the tissue, get plume and smoke. Your environment gets muddled. Reduced thermal spread, because now we’re just delivering the right amount of energy that’s needed to attain the task. And, ultimately, we have consistent vessel sealing. This is almost the adverse of that slide that I showed with the generic concerns that we have as laparoscopic and laparotomic surgeons.
Now, one of the advents that was very important in the last several decades was an understanding of the whole idea of tissue welding. And then, in fact, it realized that you could use a certain amount of pressure—very, very high compressive force. If you could deliver that with a small amount of voltage and a small amount of energy, temperatures less than 100°, for the right amount of time you could literally transform collagen and elastin into a tissue glue that had tremendous sustaining powers that could overcome any high or superphysiologic systolic pressures.
So we look at this vessel, which is vessel seal. Incredible difference from the one that we saw before. And here we see true intima-to-intima sealing. And look at the margins, the heat thermal affects how dramatically they differ in these 2 vessels.
So I want to finish this up by talking to you about the 2 devices that are in my armamentarium, because they’re complementary, and especially about this new technology called ENSEAL®. And, as I said, there’s been an evolution of devices. But with this particular dedicated advanced bipolar device, there are 3 unique features that, to me, are very exciting because they open up new possibilities and new promises in the context of delivery of energy to the tissue and regulating the thermal effect. And I’m going to tell you about each one separately. One is basically an intrinsic control that’s in the device that actually determines the temperature at which the tissue is heated. And we’ll talk about that. And it regulates the energy delivery. Another is a unique configuration. And I think you’re all going to be immediately excited about this when you see this invention that was thought of by the inventor. It gives you this, for the first time, a uniform compression from the heel to the tip of the instrument. And lastly, there’s a jaw design with the electrodes that help keep the current from spreading laterally into the tissue that gives you a consistent effect.
So let me try the first one with you, and it may be the most difficult to explain, but I think it’s the most interesting. As I told you, there was no way to moderate output in conventional bipolar electrosurgery. Push your foot on the pedal, deliver the energy, and if it was a blow torch of voltage, it was a blow torch of voltage. That’s what you get with the Kleppinger. Now you use more advanced devices. The generators are communicating with the tissue and only putting out what it needs. Now for the first time, it’s regulated at the jaw.
So, in fact, the thermal effects in the ENSEAL device are regulated by the compositional electrical characteristics of the jaw. And I’ll explain to you how this is. The jaw basically is plastic, and plastic is a nonconductor. And the reason that this conducts electricity is because it’s been impregnated with small spears of conductive particles. And these conductive particles can control energy delivery through a very, very unique mechanism. So look at the picture on the left and then look at the one on the right in this lower diagram. You’ll notice on the left that the particles are lined up and they’re close together. They’re in the plastic, and this plastic very comfortably is going to conduct the electricity. As the plastic heats up, the spirals, these conductive particles are going to move farther apart. And as they move farther apart, guess what happens? It becomes nonconductive. So if you can take this other step and realize that the jaw is not uniformly one temperature, there may be several hundred zones along the jaw but at different temperatures, in fact, like thermostats, are turning on, turning off, turning on, turning off, in response. And the end result is that you get uniform temperature because it’s going to turn off at approximately less than 100°C.
So look at this real-time thermography here of how literally the thermal margins intrinsically plateau at about 100°. And that is determined by the construction of the jaw, having nothing to do with generator output.
Now, take a look here at an animation that would help us. And so here we have your conductive particles close together, conducting electricity. As the plastic starts to heat up, what’s going to happen to these particles? The particles are going to spread apart and, naturally, certain zones of the jaws are going to stop. And when they then cool off, these spirals will come closer together and they’ll start conducting again. So here we have a reconfiguration. You see 100° becomes nonconductive. Now, of course, as they cool, they come closer together again and they’re going to conduct again. So you have this mirror infinite number of microcircuits occurring until finally the current is self-regulated and turns off, based on where the jaw is in impedance of the tissue. Secondly, if you look at a Kelly clamp, a Heaney clamp, or any bipolar electrosurgical device, and you measure the pressure, and you know this intuitively, as surgeons, pressure is greatest at the heel and is least at the tip. We’ve got to change that—that’s the physics of the device. And unless you increase the girth of a device, as you increase the length this becomes even dramatically more problematic. So of all the devices up to this point, you’ve got high pressure at the heel, lower pressure at the tip. So for vessel sealing—think about it—you want high compressive forces—remember, low energy a certain amount of time—the tip of the instrument in certain cases does not get sealed very well in certain devices, because we can’t get the same compressive pressure from the heel to the tip by the physics. So the maximum compression is at the heel of the clamp.
Now take a look at an I-Blade™, I-Beam configuration, that now is part of the ENSEAL device, which to me is one of the most innovative features.
So here you have a blade coming down, but it’s an I-Beam, and since it’s an I-beam, it pulls across, and the pressure is absolutely the same at the tip as it is on the heel and, as a result your seal, is going to be absolutely the same histologically whether you’re looking at the heel, midway in the clamp, or at the tip histologically, and you’re going to get a uniform cut. A very unique discipline here.
And you can see this cut, and you can see, in fact, it is highly predictable, and minimal thermal margins.
And lastly, very interesting. And there’s a background to explain this third feature.
Think about the 2 fundamental principles of electricity that can’t be changed. One is that electricity always seeks to ground, which is the earth. We cannot change this reality. And the other reality you cannot change in electricity is electricity is always going to follow the path of least resistance. You cannot change this.
So think about a conventional bipolar electrosurgical device. Conventional bipolar electrosurgical devices have a positive and negative pull—this is the way they’re set up.
So look at the way the current flows. The current initially is going to flow from positive to negative, but as the tissue heats up and gets drier, the current is going to try to seek other paths to make the connection of the circuit. And it moves the current more laterally, in a progressive fashion, until finally the resistance is so high it can’t go more laterally. But this literally tends to push the current lateral to the jaws to complete the circuit. Electricity seeks the path of least resistance. Now, there is an offset jaw configuration of the electrodes on the ENSEAL device that, in fact, keeps the current within. Because if you look here carefully, you see a positive/negative, but the positive is surrounded by negative poles. So as a result, what this does is this isolates the current to within the material that’s between jaws and keeps it from spreading laterally, as compared to the other portion of the device.
So to recap, I talked to you about electrode configuration, which are the conductive elements in there, the nano spiral technology, that expand and contract and keep the temperature at about 100°. It doesn’t matter how long you’re there, it’s going to stay at that temperature. You have an I-Blade that overcomes all of the intrinsic problems we have in the context of heel versus tip pressures on vascular and hemostatic clamps. And we have an interesting design of the electrodes that tends to keep the current within the jaws rather than making it spread laterally to complete the circuit through the path of least resistance. So that’s not the only device that we use for surgery. And, of course, there are many devices that are complementary. And, for me, the most complementary device because it’s so specifically designed for soft-tissue dissection, we’re talking dissection instrument, is the Harmonic® Ace™ device.
And, of course, the Harmonic Ace device has nothing to do with electricity, and I want to dispel that right away. Harmonic energy has to do with mechanical energy. It’s very different.
And here, we have high-frequency ultrasound in tissue creating a number of predictable events. And the most predictable event is that it basically denatures proteins by deconfiguring them, by breaking up the hydrogen bonds that keep their structure, and they get denatured—a very low temperature phenomenon. And, ultimately, if you have ultrasound and tissue you form steam—and, remember, steam is percussive, you can’t take that away from steam. And steam that’s in the tissue will start to cavitate it and it will make it fragment, and that’s what’s called cutting. Ultrasonic energy attains cutting by cavitational fragmentation of tissue with the production of steam from high-frequency ultrasound.
This is the anatomy of a Harmonic Ace. And we have a tissue-holding device on top, which is plastic, nonmoving, nonthermal. And we have a blade that’s oscillating 55,500 times a cycle. Think about how low-frequency this is. You can use 3 million cycles per second when you’re doing ultrasound, vaginal ultrasound in the office. This is 55,500 times a cycle. And that’s also why it has such thermal effects.
And you can vary how far this blade goes out per cycle. And that’s what’s called your levels. And, in fact, this has nothing to do with power; it has to do with how much your blade comes out per cycle, which is called excursion. And, essentially, a Harmonic scalpel…you can see how different settings—you know, I’ll just run that through again, settings 1, 2, 3, 4, 5—affect how far the blade comes out per cycle. And as a result of this, since the energy that’s being produced is being delivered in the same amount of time, whether you come out a short distance or a long distance, you can predict the following algorithm based on those excursions. In general, if you want to coagulate with a Harmonic ACE, you want to be in the lowest setting so the energy is delivered over the smallest surface area, which is the smaller blade excursion. And on the other hand, just the opposite—if you want to cut, you’re obviously going to just use the opposite algorithm, and you’re going to want to have the largest surface area with the same amount of energy, and that’s going to promote cutting. So our variable features on the Harmonic scalpel totally relate to what we intend on doing—or not doing—with our Harmonic ACE.
Now, what’s really different to me between ultrasonic energy and thermal energy from electrosurgery is the volumetric spread of energy. So let’s take a look at the Harmonic scalpel. Remember, it’s an ultrasound, it’s a blade that’s oscillating 55,500 times a second. Where’s most of the energy? It’s at the tip of the instrument, in fact, and the most active portion of a Harmonic scalpel is at the tip of the blade because it is linearly propagating. As a result, the lateral margins are that much smaller because you don’t have the propagation of energy in an ellipsoid 3-dimensional fashion as you do with electrosurgery. It also makes your thermal margins much more predictable.
And so, of course, what we do is we make sure, as I told you, low-energy devices—we’re getting back to that central principle—low-energy devices get the best hemostasis based on our vessel compression. You want to release tension. So when you do the Harmonic ACE for vessels, you grasp, you release tension, you then desiccate. And then, after you see the visual signs of desiccation—which, of course, is a plume of steam—you now will wait, let the cutter curve, and then you will remove. And you always remove in a linear fashion in the same track that you applied your device.
So I want to finish up and thank you for being here today. I hope that was helpful for all who might want to know more about electrosurgery, ultrasonic energy, to remind you and myself that it really has nothing to do with the wand, and it has to do with the mirror and my understanding of fundamentals of electricity and ultrasonic. And it’s really up to you, it’s not up to the wand. And I’d be more than happy to take any questions or comments from anybody here who’s been nice enough to listen to today’s talk. Okay, thank you very much.
Energy options in gynecologic surgery—a topic very dear to me and very often understudied for all of us in our basic training.
And as a signpost of today’s presentation is the review that we’ll partake of fundamental knowledge and technique. And for me, when I take fundamental knowledge and technique and reduce it to its ingredients, it definitely leads to being safer and also contributes to efficacy and efficiency of surgery. And for me, when I combine these 2 elements and I think about really how they contribute to my practice and the outcome of patients, it’s surgical outcome. So today is really dedicated to improving outcome in patients.
Now, in order to hopefully communicate as best as possible with you, I want to review both new concepts and also what I would term electrosurgery vernacular.
In order to do that, I’d like to create a model here and build a water tower. And just think of…in order to get the water into the tower, you have to have some sort of push or force to create that reservoir. In electrosurgical terms, voltage is what provides that push that pushes the current through something called resistance, which is a variable ability for something to pass through a circuit. Obviously, if the resistance goes up, the aperture get smaller. You have to push harder. If you push larger amounts of current, you have to push harder. But these are the 3 elements of electricity, and so we have current, we have resistance, and we have voltage. But…only when we use a battery and a direct current do we really call this resistance. The proper term in electrosurgery for resistance is impedance. So for the rest of this talk, I’ll be using the word impedance instead of resistance to talk about that difficulty by which electrons pass through a circuit. But most importantly, and fundamentally, voltage is force.
When we think about force and voltage, we think about surgery. Whether it’s physical or it’s electrical, greater force is greater risk. So whatever we can do to minimize voltage is going to minimize unpredictable behavior of thermal modalities, and it will maximize our safety in tissue.
Now what is electrosurgery? It’s…taking something hot and burning something with it. When, in fact, what we do is we take alternating current in very small frequencies—60 cycles per second. And it gets plugged into an electrosurgical generator, our boga unit, as we refer to it in slang terms in operating surgery. And that is simply a machine that accelerates the frequency to very high frequency—to up to 3 million, but typically around 500,000 to 700,000 cycles per second versus 60. As that current is transmitted through cells, because they have ions in them and water, this energy is now converted to kinetic energy, because you have alternating current that flows against the resistance. And in overcoming this resistance, you get what’s called resisted heating, or the production of heat. So in fact, as you see, you take something that really has no temperature whatsoever, and we create temperature with it by the nature of passing current through something of variable resistance, called resistive heating.
So the question comes up—What determines whether coagulation or cutting occurs? Does it have to do with the salic generator? No, in fact it has nothing to do with the generator. Think about it. If we take tissue and we heat it up slowly, and we percolate the water out, we coagulate or we desiccate it. If we take the same tissue and we now heat it up very rapidly, we now create steam, and that steam is very explosive—it ruptures the cell membranes and you get vaporization, or what’s called cutting. So what’s the difference between the two? The only difference we see here on this graph is the rate at which we heat up tissue. So, in fact, we completely moderate between cutting and coagulation, based on simply how fast or how slowly we heat up the water in tissue. And how do we do that intuitively?
Well, we all do it as surgeons intuitively because we manipulate energy density. And how do we do that? Well, think of the old model. I think at least all the males here watching this presentation remember taking a magnifying glass and taking sun and heating up different structures with the sunlight. And we know that if it passed our hand through this part of the beam, it’s actually relatively cool. And if we now go to where we have the beam focused, we end up having a much warmer spot. What’s the difference here? It’s just a difference of energy density, and this is what we do. That’s relatively cool and that’s relatively hot.
So now we take an electrosurgical instrument, and this instrument is universal—it’s a monopolar electrosurgical spoon. And we now look at…we have 2 possibilities with this instrument. One possibility is we have a large flat surface, a large surface area. The current density is going to be relatively low. So what do we expect it to do? It’s going to desiccate or coagulate the tissue. We now take the same instrument. We just turn it to the edge. We now have a small surface area. We have a much higher current density. What do you expect it to do? It’s going to vaporize or cut.
So now, let’s take a look and see if that’s actually true. Here we take a monopolar spoon. It vaporizes—we’re using a small surface area—and we take it and we turn it to a larger surface area that’s flat. It’s going to simply coagulate, desiccate the tissue—no cutting whatsoever. Now I’m going to take it and move it to the edge again, simply moderating the density of energy, and we have a very efficient cut. This is the moderation of current density having nothing to do with the generator settings.
Now, what do we need to get a cut? Well, we have to focus the energy. We have to spark. And so, what do we need to create a spark between the electrode and the surface of tissue? We need to create this spark with energy, and the minimum threshold for that is about 200 volts. So now, hopefully, you ask yourself the question, Where is there 200 volts coming out of your generator—the cutting, blend, or coag side? Well, it comes out of every side of the generator.
So we can cut and we can spark, regardless of where we are at the generator. And so we see this video and we know that this is being created by both a blend, cut, and coag current without difficulty whatsoever. So now, hopefully, we can ask the question, Does it matter? Do we care where we are on the side of the generator to create this cut, since it all works that way? And let’s take a look to see if there’s a basis for caring.
So now, for the first time, I’ll bring up the concept to you—What comes out of this box? This is essentially what comes out of the box.
You see on the cutting side of your generator, it’s an unmodulated…called simply uninterrupted, relatively low-voltage sign wave. And we start to blend and go to coag—if you notice, you have interruption of current, and you see a dramatic rise in the voltage. And finally, on the coag side of the generator, you see, in fact, the voltage is quite high and, in fact, the current’s off most of the time. It’s actually sparking.
So, look at where that voltage is. Here’s the lower voltage and there’s the higher voltage. Remember, voltage is risk, voltage is force. So, we know that the coag side of our generator is the least predictable and most thermally destructive part of the generator.
Now, let’s take a look at the cut edges of a piece of tissue. So look at the difference in the thermal margins of these cuts when you compare the cut blade form, the blend wave form, and the coag wave form. And let me help you understand this concept of blend. So, many people think blend means that we blend a little of this and we blend a little bit of that and we get some sort of a mixed wave out of the generator. No. The blend refers to a blended tissue effect. And what happens when you blend 1, blend 2, blend 3, and finally the coag, you’re starting to go from a pure cut to now a cut with more thermal margins. And notice on the coag side, you cut very efficiently, but you have a much wider swath of destruction. And so, if you see that and you think about it, your thermal spread or charring is much higher on the coag side. Your voltage is higher and, of course, the adverse is true on the cut in side of your generator. So we, essentially, as laparoscopic and also laparotomic surgeons, should learn how to live on the cutting, uninterrupted side of our generator, where there’s low voltage for virtually everything we do. And there are selective reasons to say, “I would want to be on the coag side of my generator,” and that’s—remember—bigger voltage, bigger push. If we need a bigger push because we have higher resistance or if we want to get more hemostasis because we’ve got wider thermal margins, we would want to be there. So think about it. Cutting through erectus muscle, cutting through myometrium, cutting through fat, cutting through adhesions—these are all situations where either you want more hemostasis at the edge or you need a bigger push to cut, as in cutting fat or cutting adhesions. So exemplary of this, let’s say you’re doing total laparoscopic hysterectomy. You’re going to incise the vaginal cuff off of the lower cervical tissue. And, of course, we would want to be somewhere on the blend side, if not coag, because we’ve got to hemostase the vessels in the vagina. So this is a perfect example. You would selectively use a certain side of your generator to get a certain desired effect because you expect the thermal margins, in this case, to be more vascular.
So, so far I’ve talked to you about sparking. I’ve said nothing about what happens when you take an electrode and you come in contact with tissue. The rules will change, as you see, when you do contact electrosurgery versus noncontact.
And think about it. Look at this slide and think about the difference in tissue effects just based on the current density. There’s your spark. Think the same electrode, same settings, and now instead of sparking noncontact, take that electrode and put it in contact with the tissue. Look what happens here—you have a much lower current density. You have different thermal effects on tissue. You have much deeper, wider thermal effects, and the rules are different.
So let’s look at a cross-section of liver. Let’s take a roller, sort of ball, electrode, and let’s do some desiccation, coagulation to the surface of the liver. There’s the coag side of the generator. There’s the cutting side of the generator. Remember—high voltage, low voltage—one’s highly interrupted, the others uninterrupted. And look at the visual features that you see here. Here you see cooked, carbonized, high-voltage, high-temperature phenomena, sticky, smoke, but look at the penetration. Relatively minimal, because when you contact with a very high voltage, you penetrate very superficially. On the other hand, look what happens when you use a lower voltage current, which heats the tissue more slowly. That tissue now heats more effectively and more deeply to get a more effective burn. Is this nuance? No, it’s more than nuance. If you happen to be a surgeon who treats endometriosis with electrosurgery and you do electrosurgical ablation of endometriosis with different types of electrodes, realizing that as a disease entity, endometriosis is a retroperitoneal disease. Very commonly you would want to have a depth of penetration that goes beyond the visible portion of the lesion. And you would be compelled to go to a cutting wave form if you wanted to have very thorough, deeper penetration of tissue. So high voltage, rapid desiccation, superficial penetration, lower voltage. Think about it—gradually cook the tissue, more effective, more water in the tissue, and deeper penetration.
And we’re reminded that, regardless of where you are and what kind of instrument you use, there’s one thing that’s always against you when it comes to thermal margins, and that is the longer you’re there, the more injury you produce. So that doesn’t mean that we are frenetic with our movements, but it means that we have a certain amount of alacrity and a certain amount of purposefulness. And we move with intent when we do surgery to minimize the chance for prolonged thermal injury.
So to summarize, we discussed how these variables might impact thermal margins and tissues and the behavior of electrodes. We can manipulate electrodes, which changes their surface area, which completely changes their behavior in the context of current density. We can choose—remember—cutting, blend, or coag waveforms and get different phenomena, different thermal margins. Because, in fact, we’re changing radically the height of the voltage, the output voltage that we’re utilizing. If we change the size of our electrode, you change the surface area, which changes the current density. And then remember, dwell time equals thermal margins. We want to know that we are purposeful with our surgical movements.
So using that information, I want to talk for the rest of this time about bipolar electrosurgery, what I call the basics of bipolar electrosurgery.
Now, not to confuse anyone, but all surgery is bipolar because alternating current means that the current alternates from one pole to the other. What’s really different about bipolar versus monopolar surgery, in the context of using that standard terminology, is the circuit. So think about this—in what we call bipolar electrosurgery, the circuit is the tissue and the 2 tips of the instrument or the 2 electrodes that are close to the tissue. That is the entire circuit. In monopolar electrosurgery, you have a dispersive or return electrode…on the thigh, or the leg, or possibly on the thorax, and you now have the energy going from the tip of the instrument all the way through the patient and back to the generator to complete the circuit.
Think about the energy requirements here. On one, you just have to have energy push through a small piece of tissue between the tips. On the other, you need energy to push all the way through the entire patient back to the generator to complete the circuit. One is much more power consuming. One requires a lot more voltage. Therefore, monopolar electrosurgery, on a power-for-power basis, has much greater requirements. And whether you know it or not, when you take your banana plugs and you plug into an electrosurgical generator—it doesn’t matter what the manufacturer is—and you hit your blue pedal, which is standard throughout the world for the coag output, and it’s set to bipolar, it always by default will put out a pure cut current even though you are putting a coag phenomenon in place. And that’s because, hopefully, it would make sense that if you used very high voltage on a small little area of tissue between 2 tips of an instrument, it literally would fuse and be dysfunctional for you in a surgical sense. So to minimize the chance of adverse thermal effects, it’s always a low-voltage cutting current that’s set off by default by bipolar electrosurgical devices.
Now, let’s take a look at this picture and focus in on the picture on the right. And when I first saw this picture, I really didn’t notice anything very significant until someone really educated me and pointed out what really happens when you take a vessel of substantial size, you grasp it, and you compress it with a conventional Kleppinger bipolar forceps. And then take a look, in fact, how hemostasis is attained in that vessel. Well, the first thing you notice is that it’s shrunken, and it shrinks because when you heat collagen and elastin, it’s like an accordion—it shrinks up about 40% to 50% of volume. When you heat collagen, it just shrinks up like a Slinky. So you have shrinkage of the vessel wall. But you’ll also notice there’s a large proximal thrombus there. But if you’re really a careful observer—and as I’ve said, I missed this the first time I looked at it—the lumen is still apparent. In fact, the lumen is patent. And when we coagulate and desiccate vessels of significant size with a Kleppinger bipolar forceps, regardless of what your generator settings are, you have a vessel that essentially has a patent lumen. Now, as surgeons in the pelvis, we can get away with this because our pulse pressers in general are very low, because we’re down the vascular tree from the central pump. But you are a general surgeon, and you saw this and you realized you have to work on vessels like the gastric artery that has significant high systolic pulse pressures, you would realize you could not rely upon this technology, and we can understanding looking at this, for our general surgeon colleagues really didn’t become interested in bipolar electrosurgery until they had further assurances that it would be more effective.
Now, this slide here is a summary of what are my experiences with conventional bipolar electrosurgery. And I think every surgeon who’s watching this and is honest about their work experiences these on a daily basis using conventional bipolar electrosurgery. I don’t know how many times I’m going to overlap. I’m not sure you do, either. On 1 pedicle, 2 pedicles, right side, left side. We have variable numbers of compressions to attain the task. No automation, no regulation of what we’re doing. How long do I apply the energy? How long do you apply the energy? How long do I apply the energy on the right? And then how much do I apply on the left? And then you know and I know, we look at the thermal margins—we have spread. We don’t have total control over our thermal margins, especially using conventional bipolar electrosurgery. And most importantly, you’re satisfied, I’m satisfied, our vessel looks like a rope, it’s shrunken down, it looks completely desiccated. We now take our scissors and we cut it and it bleeds. And you have your little red dot that pulsates, and you realize that after all that work, you’ve got to go and work on it again. So these, I think, are generic, universal problems we have with conventional bipolar electrosurgery.
And I also ask you the question, What’s the margin of safety? Yes, we know that the current flows between the 2 jaws. No question the current goes nowhere else. There’s no diversion of current that occurs with bipolar electrosurgery. It cannot happen. But there’s something else with bipolar that’s problematic—and that is the production of steam and thermal effects.
So when you see this picture, you have to ask yourself the question, If the electricity just goes from one jaw to the other, what is this white that is blooming? And all of you who have done tubal coagulation procedures for a female sterilization have seen, on the application of energy with Kleppinger bipolar forceps, a little puff of steam and then, all of a sudden, the whole mesosalpinx rises and drops. And, of course, that’s the filling of the broad ligament with steam. The steam rises, and it gets absorbed, it drops. So we create a huge plume of steam. Remember what steam does to cells? Steam is very percussive—it explodes the cells and it spreads heat. So it’s very destructive.
So one way we avoid making too much steam, if we’re doing conventional bipolar electrosurgery, is we never use ammeters to direct the surgical endpoint. We use ammeters to direct the surgical endpoint for tubal coagulation because we want napalm to be delivered to the mesosalpinx. That’s the opposite of what we want, of course, when we’re doing surgical dissection of the pelvis. So if you use an ammeter to direct your endpoint in bipolar electrosurgery, you are maximizing steam production and therefore maximizing lateral thermal injury.
Now, we can use common-sense methods to minimize destruction from conventional bipolar electrosurgery. And we can watch tissue, and when it becomes white and when it becomes dry and doesn’t emit steam any more, we can feel confident it’s ready to cut. So we use color and we use tissue behavior to dictate when we’re done, rather than to overcook tissue through fear and anxiety that we may have a patent lumen. We can also pulse current because current can be applied and, in between the pulses, the energy can be dissipated by the circulation of the blood, by convection and conduction of heat. We also can take the sides of our instrument and realize that when you take tissue and you grasp it and you crush it, you end up having wide thermal margins.
So look at this little bleeding pedicle after salpingo-oophorectomy. And the attack is to take a tack from the right side contralateral and use a tamponade desiccate, no grasping of tissue. This is an entirely different method of hemostasis than grabbing a pedicle, crushing it, and desiccating it. So whenever we can tamponade desiccate, almost like a trampoline—tamponade desiccate, tamponade desiccate—we basically have almost no thermal penetration and no thermal margins.
And, of course, both you and I get panicked when we see active bleeding. And what we must do is we must resist this immediate desire to stop bleeding with energy. And so when we have active bleeding, first and foremost as surgeons, we have to find out where we are and then we’ve got to stop everything mechanically.
And if you see any advanced laparoscopic surgeon deal with adversity in hemostasis, you will see every one of them grasp or put tension on a bleeder to tamponade, time out, take a breath, find out where you are, and then, and only then proceed. And if you see there’s something relevant that’s near, whether it’s uterosacral ligament, ureter, bladder, bowel, you will mobilize that tissue with the appropriate relaxant incisions. And then and only then do you bring in the heat. And even then, you might be conservative and be pulsatile desiccating versus pure desiccating.
Now, this is the background of where we’ve been. And I and a number of colleagues have been talking about this, writing about this, for many years. And to our pleasure, I don’t think in response to anything we’ve done, industry has come forward with amazing innovation and research outputs that have changed the face of bipolar electrosurgery. And we’re going to discuss a few of these evolutionary steps as they contrast to traditional bipolar electrosurgery to help you understand what’s available today.
We all remember a number of years ago that we had a 10-mm device that finally came out that was like a waffle iron and we had a blade that came down. So now for the first time, we had a multifunctional, multiple-purpose instrument to be placed on a trocar that could coagulate and cut with a mechanical blade. Many things followed that innovation. In response to everything we know about voltage—you remember voltage is destructive, is force; voltage is the wrong side to be on the generator, if you can help it. These all became now relatively constant, low-voltage devices. So the high-voltage equation has been erased from bipolar electrosurgery with use of these devices. And for the first time, rather than a generator autonomously just delivering energy as you push on the pedal, now the generator is talking to the tissue and the instruments all send back information about the resistance impedance of the tissue. So now, the amount of energy that’s created, the amount of current that’s delivered by the instrument, is completely moderated to what’s needed to desiccate the tissue, so you get more uniform effects. And as we’ve advocated for pulsing, for cooling, pulsing does 2 things. Not only does it help the tissue cool, it also allows the generator in between pulses to register the information and now respond. So the introduction of pulsing gives the generator a chance to communicate with the tissue. And with this tissue cooling, you also get delivery of energy that’s more uniform. And finally, if you remember the last point on my conventional slide, which was we’re all happy, everything’s wonderful, now we cut the vessel and we’ve got the little red course spurting in our face. We now have technology, that when we even get audible feedback, or we are integrated with the device, and we get visual proprioceptive feedback and we decide to push the button, push the blade, we in fact have reliable, consistent hemostasis. We’ve changed the whole equation with the evolution of bipolar electrosurgery. So look at this vessel now. We’re talking about intima-to-intima fusion from vessel sealing.
Now, one of the pieces of these devices is that they’re all approved for vessels up to 7 mm. And this is well beyond anything that you or I are going to experience and need in the pelvis.
Another nuance is when you take a blood vessel and you compress it with low energy, ie, low voltage and high current. You need to do a little trick if you want a great seal. And a great seal is also to release your tension just a little bit on your pedicle and then to apply the energy. So we allow that lower energy to do an intima-to-intima fusion, which sometimes is physically overcome by too much torsion or traction on your pedicle.
So you would expect to see some promises here. And the promises are, hopefully, natural and you’ll see them each time. We don’t have tissue sticking, because that was from high-voltage carbon. We don’t have it anymore. Overheat the tissue, get plume and smoke. Your environment gets muddled. Reduced thermal spread, because now we’re just delivering the right amount of energy that’s needed to attain the task. And, ultimately, we have consistent vessel sealing. This is almost the adverse of that slide that I showed with the generic concerns that we have as laparoscopic and laparotomic surgeons.
Now, one of the advents that was very important in the last several decades was an understanding of the whole idea of tissue welding. And then, in fact, it realized that you could use a certain amount of pressure—very, very high compressive force. If you could deliver that with a small amount of voltage and a small amount of energy, temperatures less than 100°, for the right amount of time you could literally transform collagen and elastin into a tissue glue that had tremendous sustaining powers that could overcome any high or superphysiologic systolic pressures.
So we look at this vessel, which is vessel seal. Incredible difference from the one that we saw before. And here we see true intima-to-intima sealing. And look at the margins, the heat thermal affects how dramatically they differ in these 2 vessels.
So I want to finish this up by talking to you about the 2 devices that are in my armamentarium, because they’re complementary, and especially about this new technology called ENSEAL®. And, as I said, there’s been an evolution of devices. But with this particular dedicated advanced bipolar device, there are 3 unique features that, to me, are very exciting because they open up new possibilities and new promises in the context of delivery of energy to the tissue and regulating the thermal effect. And I’m going to tell you about each one separately. One is basically an intrinsic control that’s in the device that actually determines the temperature at which the tissue is heated. And we’ll talk about that. And it regulates the energy delivery. Another is a unique configuration. And I think you’re all going to be immediately excited about this when you see this invention that was thought of by the inventor. It gives you this, for the first time, a uniform compression from the heel to the tip of the instrument. And lastly, there’s a jaw design with the electrodes that help keep the current from spreading laterally into the tissue that gives you a consistent effect.
So let me try the first one with you, and it may be the most difficult to explain, but I think it’s the most interesting. As I told you, there was no way to moderate output in conventional bipolar electrosurgery. Push your foot on the pedal, deliver the energy, and if it was a blow torch of voltage, it was a blow torch of voltage. That’s what you get with the Kleppinger. Now you use more advanced devices. The generators are communicating with the tissue and only putting out what it needs. Now for the first time, it’s regulated at the jaw.
So, in fact, the thermal effects in the ENSEAL device are regulated by the compositional electrical characteristics of the jaw. And I’ll explain to you how this is. The jaw basically is plastic, and plastic is a nonconductor. And the reason that this conducts electricity is because it’s been impregnated with small spears of conductive particles. And these conductive particles can control energy delivery through a very, very unique mechanism. So look at the picture on the left and then look at the one on the right in this lower diagram. You’ll notice on the left that the particles are lined up and they’re close together. They’re in the plastic, and this plastic very comfortably is going to conduct the electricity. As the plastic heats up, the spirals, these conductive particles are going to move farther apart. And as they move farther apart, guess what happens? It becomes nonconductive. So if you can take this other step and realize that the jaw is not uniformly one temperature, there may be several hundred zones along the jaw but at different temperatures, in fact, like thermostats, are turning on, turning off, turning on, turning off, in response. And the end result is that you get uniform temperature because it’s going to turn off at approximately less than 100°C.
So look at this real-time thermography here of how literally the thermal margins intrinsically plateau at about 100°. And that is determined by the construction of the jaw, having nothing to do with generator output.
Now, take a look here at an animation that would help us. And so here we have your conductive particles close together, conducting electricity. As the plastic starts to heat up, what’s going to happen to these particles? The particles are going to spread apart and, naturally, certain zones of the jaws are going to stop. And when they then cool off, these spirals will come closer together and they’ll start conducting again. So here we have a reconfiguration. You see 100° becomes nonconductive. Now, of course, as they cool, they come closer together again and they’re going to conduct again. So you have this mirror infinite number of microcircuits occurring until finally the current is self-regulated and turns off, based on where the jaw is in impedance of the tissue. Secondly, if you look at a Kelly clamp, a Heaney clamp, or any bipolar electrosurgical device, and you measure the pressure, and you know this intuitively, as surgeons, pressure is greatest at the heel and is least at the tip. We’ve got to change that—that’s the physics of the device. And unless you increase the girth of a device, as you increase the length this becomes even dramatically more problematic. So of all the devices up to this point, you’ve got high pressure at the heel, lower pressure at the tip. So for vessel sealing—think about it—you want high compressive forces—remember, low energy a certain amount of time—the tip of the instrument in certain cases does not get sealed very well in certain devices, because we can’t get the same compressive pressure from the heel to the tip by the physics. So the maximum compression is at the heel of the clamp.
Now take a look at an I-Blade™, I-Beam configuration, that now is part of the ENSEAL device, which to me is one of the most innovative features.
So here you have a blade coming down, but it’s an I-Beam, and since it’s an I-beam, it pulls across, and the pressure is absolutely the same at the tip as it is on the heel and, as a result your seal, is going to be absolutely the same histologically whether you’re looking at the heel, midway in the clamp, or at the tip histologically, and you’re going to get a uniform cut. A very unique discipline here.
And you can see this cut, and you can see, in fact, it is highly predictable, and minimal thermal margins.
And lastly, very interesting. And there’s a background to explain this third feature.
Think about the 2 fundamental principles of electricity that can’t be changed. One is that electricity always seeks to ground, which is the earth. We cannot change this reality. And the other reality you cannot change in electricity is electricity is always going to follow the path of least resistance. You cannot change this.
So think about a conventional bipolar electrosurgical device. Conventional bipolar electrosurgical devices have a positive and negative pull—this is the way they’re set up.
So look at the way the current flows. The current initially is going to flow from positive to negative, but as the tissue heats up and gets drier, the current is going to try to seek other paths to make the connection of the circuit. And it moves the current more laterally, in a progressive fashion, until finally the resistance is so high it can’t go more laterally. But this literally tends to push the current lateral to the jaws to complete the circuit. Electricity seeks the path of least resistance. Now, there is an offset jaw configuration of the electrodes on the ENSEAL device that, in fact, keeps the current within. Because if you look here carefully, you see a positive/negative, but the positive is surrounded by negative poles. So as a result, what this does is this isolates the current to within the material that’s between jaws and keeps it from spreading laterally, as compared to the other portion of the device.
So to recap, I talked to you about electrode configuration, which are the conductive elements in there, the nano spiral technology, that expand and contract and keep the temperature at about 100°. It doesn’t matter how long you’re there, it’s going to stay at that temperature. You have an I-Blade that overcomes all of the intrinsic problems we have in the context of heel versus tip pressures on vascular and hemostatic clamps. And we have an interesting design of the electrodes that tends to keep the current within the jaws rather than making it spread laterally to complete the circuit through the path of least resistance. So that’s not the only device that we use for surgery. And, of course, there are many devices that are complementary. And, for me, the most complementary device because it’s so specifically designed for soft-tissue dissection, we’re talking dissection instrument, is the Harmonic® Ace™ device.
And, of course, the Harmonic Ace device has nothing to do with electricity, and I want to dispel that right away. Harmonic energy has to do with mechanical energy. It’s very different.
And here, we have high-frequency ultrasound in tissue creating a number of predictable events. And the most predictable event is that it basically denatures proteins by deconfiguring them, by breaking up the hydrogen bonds that keep their structure, and they get denatured—a very low temperature phenomenon. And, ultimately, if you have ultrasound and tissue you form steam—and, remember, steam is percussive, you can’t take that away from steam. And steam that’s in the tissue will start to cavitate it and it will make it fragment, and that’s what’s called cutting. Ultrasonic energy attains cutting by cavitational fragmentation of tissue with the production of steam from high-frequency ultrasound.
This is the anatomy of a Harmonic Ace. And we have a tissue-holding device on top, which is plastic, nonmoving, nonthermal. And we have a blade that’s oscillating 55,500 times a cycle. Think about how low-frequency this is. You can use 3 million cycles per second when you’re doing ultrasound, vaginal ultrasound in the office. This is 55,500 times a cycle. And that’s also why it has such thermal effects.
And you can vary how far this blade goes out per cycle. And that’s what’s called your levels. And, in fact, this has nothing to do with power; it has to do with how much your blade comes out per cycle, which is called excursion. And, essentially, a Harmonic scalpel…you can see how different settings—you know, I’ll just run that through again, settings 1, 2, 3, 4, 5—affect how far the blade comes out per cycle. And as a result of this, since the energy that’s being produced is being delivered in the same amount of time, whether you come out a short distance or a long distance, you can predict the following algorithm based on those excursions. In general, if you want to coagulate with a Harmonic ACE, you want to be in the lowest setting so the energy is delivered over the smallest surface area, which is the smaller blade excursion. And on the other hand, just the opposite—if you want to cut, you’re obviously going to just use the opposite algorithm, and you’re going to want to have the largest surface area with the same amount of energy, and that’s going to promote cutting. So our variable features on the Harmonic scalpel totally relate to what we intend on doing—or not doing—with our Harmonic ACE.
Now, what’s really different to me between ultrasonic energy and thermal energy from electrosurgery is the volumetric spread of energy. So let’s take a look at the Harmonic scalpel. Remember, it’s an ultrasound, it’s a blade that’s oscillating 55,500 times a second. Where’s most of the energy? It’s at the tip of the instrument, in fact, and the most active portion of a Harmonic scalpel is at the tip of the blade because it is linearly propagating. As a result, the lateral margins are that much smaller because you don’t have the propagation of energy in an ellipsoid 3-dimensional fashion as you do with electrosurgery. It also makes your thermal margins much more predictable.
And so, of course, what we do is we make sure, as I told you, low-energy devices—we’re getting back to that central principle—low-energy devices get the best hemostasis based on our vessel compression. You want to release tension. So when you do the Harmonic ACE for vessels, you grasp, you release tension, you then desiccate. And then, after you see the visual signs of desiccation—which, of course, is a plume of steam—you now will wait, let the cutter curve, and then you will remove. And you always remove in a linear fashion in the same track that you applied your device.
So I want to finish up and thank you for being here today. I hope that was helpful for all who might want to know more about electrosurgery, ultrasonic energy, to remind you and myself that it really has nothing to do with the wand, and it has to do with the mirror and my understanding of fundamentals of electricity and ultrasonic. And it’s really up to you, it’s not up to the wand. And I’d be more than happy to take any questions or comments from anybody here who’s been nice enough to listen to today’s talk. Okay, thank you very much.
Energy-based techniques to ensure hemostasis and limit damage during laparoscopy
- Inspect all vascular sites with and without insufflation before assuming hemostasis is complete.
- In monopolar electrosurgery, electrode contact using low-voltage current leads to deeper, more effective penetration than higher-voltage current.
- To minimize unwanted thermal damage during bipolar electrosurgery, stop current flow at the end of the visible vapor phase, apply current in a pulsatile fashion, and secure pedicles by alternating between partial desiccation and incremental cutting.
- Since ultrasonic energy does not generate the high temperatures created by electrosurgery, it is less dependable for deep-tissue coagulation.
Compared with laparotomy, laparoscopic surgery achieves better hemostasis with less blood loss. Not only does this approach avoid an abdominal incision and the trauma associated with traction, manual manipulation, mechanical dissection, and larger tissue pedicles, but its illumination and magnification afford superior anatomical clarity, allowing the surgeon to seal a vessel before it is incised.
Still, keen surgical judgment remains critical—despite the availability of innovative electrosurgical, ultrasonic, and mechanical laparoscopic devices. Incomplete hemostasis or incision of an active vascular core can occur even with ideal application.
This article outlines the key ingredients of hemostasis during laparoscopy, focusing on the following modalities:
- monopolar electrosurgery
- bipolar electrosurgery
- ultrasonic energy
An orderly protocol minimizes risk
The art of surgical hemostasis is preventing vascular trauma while leaving the least-possible collateral tissue damage. When bleeding is encountered, the surgeon’s ability to attain hemostasis using a particular modality depends largely on how well he or she understands its technical aspects. Of course, thorough knowledge of anatomy also is crucial to prevent inadvertent damage to vital structures.
Surgical hemostasis should not be driven by reflex alone. Instead, surgeons should always follow this orderly sequence of steps to minimize risk:
Identify source of bleeding. Before taking any action, make every effort to accurately determine the source of bleeding and its proximity to vital anatomy. Even in the face of active hemorrhage, you can usually identify the bleeders by combining mechanical tamponade (using the jaws of a grasper or the side of a simple metallic probe) with active hydrolavage (using an irrigator-aspirator to break up and remove blood and clots).
Protect vital structures. If the bowel, bladder, or ureter is in close proximity to the bleeder, mobilize that structure sufficiently before applying energy. You can usually protect these entities by using a combination of countertraction and incremental tissue dissection. Whenever the peritoneum is involved, a relaxing incision parallel to the structure of concern also may be useful.
This protocol mandates withholding thermal energy until an orderly sequence of anatomical triage is carried out. Whenever a vital structure cannot be adequately mobilized, make every effort to control hemorrhage by using mechanical tamponade alone for up to 5 minutes. If access to the bleeding site or vessel caliber render pressure-alone unrealistic, employ either a carefully applied thermal energy or a suture ligature. If the surgeon is uncomfortable using either of these, conversion to laparotomy may be warranted.
Inspect vascular sites. Finally, because pneumoperitoneal pressure alone can tamponade venous bleeders—as well as small arterial ones—inspect all vascular sites with and without insufflation before assuming hemostasis is complete.
Monopolar electrosurgery
With conventional electrosurgery, tissue is coagulated when an electric field is applied across it using high-frequency alternating current. Whether cutting or coagulation occurs depends on the rate and extent of thermodynamic effects (FIGURE 1).
Mechanism of coagulation. When tissue comes into contact with the surface of an activated monopolar electrode, a relatively low-current circuit is completed.
- As the tissue is slowly heated to and maintained at temperatures above 50°C, irreversible cellular damage occurs. This is caused by deconfiguration of regulatory proteins and denaturation of cellular proteins.
- If the tissue is heated to 100°C, cellular water completely evaporates (desiccation), localized hemostasis occurs due to contraction of blood vessels and the surrounding tissues (coagulation), and collagens convert to glucose, which creates an adhesive effect between the tissue and electrode.
- Temperatures above 200°C cause carbonization and charring.
Select the best output voltage. Since the output voltage of “coag” current is very high (FIGURE 2), contact coagulation is generally limited to superficial layers. That is because of the accelerated buildup of tissue resistance from rapid desiccation and carbonization. Conversely, electrode contact using the lower-voltage “cut” current heats tissue more gradually, leading to deeper and more reliable penetration. Thus, both contact and coaptive coagulation with monopolar electrosurgery are more effectively performed using “cut” current.
Since superficial-appearing endometriotic implants may extend deeply into the retroperitoneal tissues, I thermally ablate these lesions using a broad-surface electrode in contact with “cut” current. In contrast, I treat superficial implants on the ovarian cortex with “coag” current to minimize unwanted thermal injury to adjacent follicular tissue.
Interrupt the blood flow. Coaptive vessel sealing using any type of monopolar current may be ineffective if the blood flow remains uninterrupted. Unless a vessel is sufficiently squeezed before electricity is applied, current density is dramatically reduced by conduction in blood, and luminal temperatures undergo little change, as any heat is dissipated by convection. Deceived by the appearance of well-coagulated tissue, a surgeon may discover an alarmingly viable core at the time of incision. Regardless of the selected output current (that is, “cut,” “blend,” or “coag”), coaptive desiccation with monopolar electrosurgery is usually insufficient to reliably secure the uterine or ovarian vessels during hysterectomy and oophorectomy.
Achieve the appropriate cutting arc. Electrosurgical cutting (vaporization/ablation) is possible whenever voltage is sufficient to create an electrical spark between an electrode and underlying tissue; tissue cutting is more apt to occur if the arcing remains unabated. When it does, cellular water is superheated to temperatures greater than 600°C, causing explosive vaporization secondary to the production of highly disruptive pressure (since steam occupies 6 times the volume of liquid water).
Although all of the typical output currents (“cut,” “blend,” and “coag”) provide sufficient voltage to ionize the air gap and arc to tissue, the higher voltages of “blend” and “coag” create progressively wider zones of thermal damage at the margins of the incision (FIGURE 3) These effects are amplified by using broad-surface electrodes.
Wide versus narrow hemostasis. Using “blend” or “coag” current to cut in order to provide wider hemostasis can be helpful during myomectomy, as well as when operating down the broad ligament and along the vaginal fornices during hysterectomy, across vascular adhesions, and to clarify the space of Retzius in preparation for colposuspension and paravaginal repair. Higher-voltage currents also facilitate incision of tissues that have greater impedance, such as fatty or desiccated pedicles and adhesions.
On the other hand, it is more prudent to utilize the lower-voltage “cut” current via the edge of an electrode for electrosurgical incision whenever lateral thermal spread may pose extra liability to adjacent tissues, such as the ovarian cortex during cystectomy and the ureter or rectum during excision of endometriosis from the lateral pelvic sidewall or cul-de-sac.
Deceived by the appearance of well-coagulated tissue, surgeons may discover an alarmingly viable core at the time of incision.
- Fulguration is the use of high-voltage sparking produced by “coag” current to coagulate a broad surface with open bleeders. As opposed to the continuous arcing produced by “cut” current, the highly interrupted output of “coag” current causes the arcs to strike the tissue surface in a widely dispersed and random fashion. This leads to more rapid thermal change, creating a zone of superficial coagulation.
I typically employ fulguration to control small bleeders and vessels cut on end along the undersurface of the ovarian cortex during cystectomy, atop the myometrial bed during myomectomy, and alongside Cooper’s ligament during colposuspension.
Protect vital structures with short bursts of “coag” current. If bleeding near the bowel, bladder, or ureter cannot be controlled with pressure alone, carefully directed short bursts of noncontact “coag” current with a broad-surface electrode may help you attain effective hemostasis with the least-possible amount of electrosurgical penetration.
Despite the high-voltage output involved, fulguration is useless in a wet, conductive surgical field due to the random diffusion of current.
FIGURE 2 Range of output voltages
Relative changes in voltage and average current related to “cut,” “blend,” and “coag” currents.
FIGURE 3Zones of thermal damage related to voltage
Using a conventional electrosurgical generator for tissue cutting, the margin of thermal necrosis expands with increasing voltage.
Bipolar electrosurgery
Mechanism of action. Bipolar electrosurgery consolidates an active electrode and return electrode into an instrument with 2 small poles. These poles can be the tines of a forceps, blades of a scissors, or an electrode matched to a more proximal conductive collar separated by an insulator. The output typically used is the low-voltage “cut” current.
Advantages of bipolar energy. Localization of current between the poles offers distinct advantages. Thermal damage is generally limited to a discrete volume of tissue. A bipolar forceps can be used to coapt and thermally weld blood vessels. The concentrated current and small distance between the poles also make it possible to desiccate tissue that is immersed in fluid. This modality is less useful, however, when open blood vessels are retracted or tissue pedicles are very thick.
Because it tends to promote the flow of energy well beyond desiccation, an ammeter should not be used to determine the treatment endpoint for coagulation of blood vessels in the vicinity of vital tissue.
Know when to terminate current to prevent vital-tissue damage. Although the flow of current and primary thermal effects are restricted to the tissue between the poles, this does not eliminate the risk of thermal injury to tissue that is distant from the site of directed hemostasis. As current is applied between the poles, the intervening tissue gradually desiccates until it becomes thoroughly dehydrated. Desiccation is complete when the tissue whitens and visible steam emission stops. If the application of current continues, the heat spreads well beyond the electrical limits of the instrument.
This secondary thermal bloom is caused by the bubbling of steam into the tissue parenchyma as heat is rapidly generated (due to dry tissue’s high resistance to the flow of electrical current). This explains why structures such as the ureter or bowel may suffer irreversible thermal damage despite being at some distance from an operative or bleeding site.
Use of an in-line ammeter does not prevent this problem. Rather, it tends to promote the flow of energy well beyond desiccation. Consequently, an ammeter should not be used to determine the treatment endpoint for coagulation of blood vessels in the vicinity of vital tissue.
Whenever bipolar electrosurgery is used for hemostasis, unwanted thermal damage can be minimized by:
- terminating the flow of current at the end of the visible vapor phase,
- applying current in a pulsatile fashion to permit tissue cooling,
- avoiding the use of an in-line ammeter to determine the coagulation endpoint, and
- securing pedicles by a stepwise process that alternates between partial desiccation and incremental cutting (TABLE 1).
Since the rate of temperature generation is a direct function of the volume of tissue being desiccated, thermal spread can also be reduced by using the sides or tips of a slightly open forceps to press or lift, rather than coapt for hemostasis (FIGURE 4).
Free adherent tissue gently. As with contact monopolar coagulation, tissue between the electrodes of a bipolar instrument may become adherent during desiccation. Repeated attempts to shake the tissue free may lead to traumatic avulsion of a key vascular pedicle. A stuck vascular pedicle can usually be unglued by energizing the opened device while immersing it in a conductive irrigant, such as saline. Once the solution is boiled by the high current density between the electrodes, the mechanical action of bubbling is usually sufficient to atraumatically free the pedicle.
FIGURE 4 Minimizing bipolar thermal damage
Using contact rather than coaptation is one way to limit thermal injury during bipolar electrosurgery.TABLE 1
Minimizing bipolar thermal damage
|
Ultrasonic energy
Mechanism of action. Ultrasonic shears produce mechanical energy to cut and coagulate tissue. Housed in the hand piece of this laparoscopic device is a piezoelectric crystal that vibrates a titanium blade 55,500 times per second over a variable excursion of 50 microns to 100 microns. As energy is transmitted to tissue, hydrogen bonds of tissue proteins are ruptured, leading to a denatured protein coagulum without significant charring. The tissue cutting that occurs is secondary to mechanical vibration and cavitational fragmentation of tissue parenchyma.
Since ultrasonic energy does not generate the high temperatures created by electrosurgery, it is less dependable for deep-tissue coagulation. Rather, thermal change is gradual, requiring a modicum of patience.
Available instrument configurations include 5-mm curved or hook blades. In addition, 5-mm or 10-mm ligating and cutting shears coaptively desiccate and cut the tissue by securing it between a grooved plastic pad and the vibrating blade.
Tissue effects depend on interplay of factors. By using various combinations of blade shapes, blade excursions, and tissue tensions, surgeons can accomplish a variety of specific effects. Cutting velocity is directly proportional to blade excursion, tissue traction, and blade surface area (energy density), and is inversely related to tissue density and elasticity. Thus, the fastest cutting with the least amount of coagulation occurs when tissue is placed on tension and firmly squeezed, lifted, or rotated with the sharpest side of a blade set at maximum excursion.
Coagulation is the obverse of cutting: It is inversely related to tissue tension, blade sharpness, blade excursion, and cutting speed. Therefore, coagulation is best achieved by relaxing tension, minimizing blade excursion, and using a blunt edge or flattened blade surface.
A stuck vascular pedicle can usually be unglued by energizing the opened bipolar device while immersing it in a conductive irrigant.
Avoid excessive traction and torsion. Be mindful of the potential for premature incision of an incompletely coagulated tissue pedicle when excessive traction or torsion is applied. When used to coaptively desiccate and incise a vascular pedicle, ultrasonic energy should be applied patiently, taking great care to minimize tissue tension while using the broadest blade surface set to the lowest excursion (TABLE 2). Hemostatic incision is best ensured by first coagulating several overlapping areas along an untracted pedicle, limiting each application to the point of tissue blanching and initial vapor emission. Only then should the pedicle be incised by gradually lifting, squeezing, or rotating the distal device. In this fashion, ultrasonic energy can be successfully used to secure both the ovarian and uterine vessels.
TABLE 2
Factors likely to cause premature incision with ultrasonic energy
|
Summary
The use of electrical and ultrasonic energy during operative laparoscopy poses several challenges, including the reduction of unwanted thermal injury and the elimination of incomplete hemostasis.
Since the depth of penetration during monopolar electrosurgery is proportional to both output voltage and surface area, unwanted thermal change can be reduced by using the smallest electrode surface with “cut” current for tissue cutting, and “coag” current with a broad-surface electrode for contact or noncontact (fulguration) coagulation.
Bipolar electrosurgery is the preferred modality for coaptive desiccation of a vascular pedicle with electricity. Despite the isolation of current to the intervening tissue, surgeons must take steps to reduce the lateral percolation of heat into adjacent tissues.
Ultrasonic energy provides reliable coaptive hemostasis and incision with little tissue damage. However, the surgeon must be mindful of the forces that promulgate premature incision. Knowledge of the biophysical behavior of electrical and ultrasonic energy is a prelude to safety and efficacy during laparoscopic dissection.
Dr. Brill reports no affiliations or financial arrangements with any of the manufacturers of products mentioned in this article.
1. Brill AI. Energy systems for operative laparoscopy. J Am Assoc Gynecol Laparosc. 1998;5:335-345.
2. Friedman J. The technical aspects of electrosurgery. Oral Surg. 1973;36:177-187.
3. Honig WM. The mechanism of cutting in electrosurgery. IEEE Trans Biomed Eng BME. 1975;22:55-58.
4. Sigel B, Dunn MR. The mechanism of blood vessel closure by high frequency electrocoagulation. Surg Gynecol Obstet. 1965;121:823-831.
5. Phipps JH. Thermometry studies with bipolar diathermy during hysterectomy. Gynecol Laparosc. 1994;1:146-149.
6. Ryder RM, Hulka JF. Bladder and bowel injury after electrodesiccation with Kleppinger bipolar forceps: A clinicopathologic study. J Reprod Med. 1993;3:595-598.
7. McCarus SD. Physiologic mechanism of the ultrasonically activated scalpel. J Am Assoc Gynecol Laparosc. 1996;3:601-608.
- Inspect all vascular sites with and without insufflation before assuming hemostasis is complete.
- In monopolar electrosurgery, electrode contact using low-voltage current leads to deeper, more effective penetration than higher-voltage current.
- To minimize unwanted thermal damage during bipolar electrosurgery, stop current flow at the end of the visible vapor phase, apply current in a pulsatile fashion, and secure pedicles by alternating between partial desiccation and incremental cutting.
- Since ultrasonic energy does not generate the high temperatures created by electrosurgery, it is less dependable for deep-tissue coagulation.
Compared with laparotomy, laparoscopic surgery achieves better hemostasis with less blood loss. Not only does this approach avoid an abdominal incision and the trauma associated with traction, manual manipulation, mechanical dissection, and larger tissue pedicles, but its illumination and magnification afford superior anatomical clarity, allowing the surgeon to seal a vessel before it is incised.
Still, keen surgical judgment remains critical—despite the availability of innovative electrosurgical, ultrasonic, and mechanical laparoscopic devices. Incomplete hemostasis or incision of an active vascular core can occur even with ideal application.
This article outlines the key ingredients of hemostasis during laparoscopy, focusing on the following modalities:
- monopolar electrosurgery
- bipolar electrosurgery
- ultrasonic energy
An orderly protocol minimizes risk
The art of surgical hemostasis is preventing vascular trauma while leaving the least-possible collateral tissue damage. When bleeding is encountered, the surgeon’s ability to attain hemostasis using a particular modality depends largely on how well he or she understands its technical aspects. Of course, thorough knowledge of anatomy also is crucial to prevent inadvertent damage to vital structures.
Surgical hemostasis should not be driven by reflex alone. Instead, surgeons should always follow this orderly sequence of steps to minimize risk:
Identify source of bleeding. Before taking any action, make every effort to accurately determine the source of bleeding and its proximity to vital anatomy. Even in the face of active hemorrhage, you can usually identify the bleeders by combining mechanical tamponade (using the jaws of a grasper or the side of a simple metallic probe) with active hydrolavage (using an irrigator-aspirator to break up and remove blood and clots).
Protect vital structures. If the bowel, bladder, or ureter is in close proximity to the bleeder, mobilize that structure sufficiently before applying energy. You can usually protect these entities by using a combination of countertraction and incremental tissue dissection. Whenever the peritoneum is involved, a relaxing incision parallel to the structure of concern also may be useful.
This protocol mandates withholding thermal energy until an orderly sequence of anatomical triage is carried out. Whenever a vital structure cannot be adequately mobilized, make every effort to control hemorrhage by using mechanical tamponade alone for up to 5 minutes. If access to the bleeding site or vessel caliber render pressure-alone unrealistic, employ either a carefully applied thermal energy or a suture ligature. If the surgeon is uncomfortable using either of these, conversion to laparotomy may be warranted.
Inspect vascular sites. Finally, because pneumoperitoneal pressure alone can tamponade venous bleeders—as well as small arterial ones—inspect all vascular sites with and without insufflation before assuming hemostasis is complete.
Monopolar electrosurgery
With conventional electrosurgery, tissue is coagulated when an electric field is applied across it using high-frequency alternating current. Whether cutting or coagulation occurs depends on the rate and extent of thermodynamic effects (FIGURE 1).
Mechanism of coagulation. When tissue comes into contact with the surface of an activated monopolar electrode, a relatively low-current circuit is completed.
- As the tissue is slowly heated to and maintained at temperatures above 50°C, irreversible cellular damage occurs. This is caused by deconfiguration of regulatory proteins and denaturation of cellular proteins.
- If the tissue is heated to 100°C, cellular water completely evaporates (desiccation), localized hemostasis occurs due to contraction of blood vessels and the surrounding tissues (coagulation), and collagens convert to glucose, which creates an adhesive effect between the tissue and electrode.
- Temperatures above 200°C cause carbonization and charring.
Select the best output voltage. Since the output voltage of “coag” current is very high (FIGURE 2), contact coagulation is generally limited to superficial layers. That is because of the accelerated buildup of tissue resistance from rapid desiccation and carbonization. Conversely, electrode contact using the lower-voltage “cut” current heats tissue more gradually, leading to deeper and more reliable penetration. Thus, both contact and coaptive coagulation with monopolar electrosurgery are more effectively performed using “cut” current.
Since superficial-appearing endometriotic implants may extend deeply into the retroperitoneal tissues, I thermally ablate these lesions using a broad-surface electrode in contact with “cut” current. In contrast, I treat superficial implants on the ovarian cortex with “coag” current to minimize unwanted thermal injury to adjacent follicular tissue.
Interrupt the blood flow. Coaptive vessel sealing using any type of monopolar current may be ineffective if the blood flow remains uninterrupted. Unless a vessel is sufficiently squeezed before electricity is applied, current density is dramatically reduced by conduction in blood, and luminal temperatures undergo little change, as any heat is dissipated by convection. Deceived by the appearance of well-coagulated tissue, a surgeon may discover an alarmingly viable core at the time of incision. Regardless of the selected output current (that is, “cut,” “blend,” or “coag”), coaptive desiccation with monopolar electrosurgery is usually insufficient to reliably secure the uterine or ovarian vessels during hysterectomy and oophorectomy.
Achieve the appropriate cutting arc. Electrosurgical cutting (vaporization/ablation) is possible whenever voltage is sufficient to create an electrical spark between an electrode and underlying tissue; tissue cutting is more apt to occur if the arcing remains unabated. When it does, cellular water is superheated to temperatures greater than 600°C, causing explosive vaporization secondary to the production of highly disruptive pressure (since steam occupies 6 times the volume of liquid water).
Although all of the typical output currents (“cut,” “blend,” and “coag”) provide sufficient voltage to ionize the air gap and arc to tissue, the higher voltages of “blend” and “coag” create progressively wider zones of thermal damage at the margins of the incision (FIGURE 3) These effects are amplified by using broad-surface electrodes.
Wide versus narrow hemostasis. Using “blend” or “coag” current to cut in order to provide wider hemostasis can be helpful during myomectomy, as well as when operating down the broad ligament and along the vaginal fornices during hysterectomy, across vascular adhesions, and to clarify the space of Retzius in preparation for colposuspension and paravaginal repair. Higher-voltage currents also facilitate incision of tissues that have greater impedance, such as fatty or desiccated pedicles and adhesions.
On the other hand, it is more prudent to utilize the lower-voltage “cut” current via the edge of an electrode for electrosurgical incision whenever lateral thermal spread may pose extra liability to adjacent tissues, such as the ovarian cortex during cystectomy and the ureter or rectum during excision of endometriosis from the lateral pelvic sidewall or cul-de-sac.
Deceived by the appearance of well-coagulated tissue, surgeons may discover an alarmingly viable core at the time of incision.
- Fulguration is the use of high-voltage sparking produced by “coag” current to coagulate a broad surface with open bleeders. As opposed to the continuous arcing produced by “cut” current, the highly interrupted output of “coag” current causes the arcs to strike the tissue surface in a widely dispersed and random fashion. This leads to more rapid thermal change, creating a zone of superficial coagulation.
I typically employ fulguration to control small bleeders and vessels cut on end along the undersurface of the ovarian cortex during cystectomy, atop the myometrial bed during myomectomy, and alongside Cooper’s ligament during colposuspension.
Protect vital structures with short bursts of “coag” current. If bleeding near the bowel, bladder, or ureter cannot be controlled with pressure alone, carefully directed short bursts of noncontact “coag” current with a broad-surface electrode may help you attain effective hemostasis with the least-possible amount of electrosurgical penetration.
Despite the high-voltage output involved, fulguration is useless in a wet, conductive surgical field due to the random diffusion of current.
FIGURE 2 Range of output voltages
Relative changes in voltage and average current related to “cut,” “blend,” and “coag” currents.
FIGURE 3Zones of thermal damage related to voltage
Using a conventional electrosurgical generator for tissue cutting, the margin of thermal necrosis expands with increasing voltage.
Bipolar electrosurgery
Mechanism of action. Bipolar electrosurgery consolidates an active electrode and return electrode into an instrument with 2 small poles. These poles can be the tines of a forceps, blades of a scissors, or an electrode matched to a more proximal conductive collar separated by an insulator. The output typically used is the low-voltage “cut” current.
Advantages of bipolar energy. Localization of current between the poles offers distinct advantages. Thermal damage is generally limited to a discrete volume of tissue. A bipolar forceps can be used to coapt and thermally weld blood vessels. The concentrated current and small distance between the poles also make it possible to desiccate tissue that is immersed in fluid. This modality is less useful, however, when open blood vessels are retracted or tissue pedicles are very thick.
Because it tends to promote the flow of energy well beyond desiccation, an ammeter should not be used to determine the treatment endpoint for coagulation of blood vessels in the vicinity of vital tissue.
Know when to terminate current to prevent vital-tissue damage. Although the flow of current and primary thermal effects are restricted to the tissue between the poles, this does not eliminate the risk of thermal injury to tissue that is distant from the site of directed hemostasis. As current is applied between the poles, the intervening tissue gradually desiccates until it becomes thoroughly dehydrated. Desiccation is complete when the tissue whitens and visible steam emission stops. If the application of current continues, the heat spreads well beyond the electrical limits of the instrument.
This secondary thermal bloom is caused by the bubbling of steam into the tissue parenchyma as heat is rapidly generated (due to dry tissue’s high resistance to the flow of electrical current). This explains why structures such as the ureter or bowel may suffer irreversible thermal damage despite being at some distance from an operative or bleeding site.
Use of an in-line ammeter does not prevent this problem. Rather, it tends to promote the flow of energy well beyond desiccation. Consequently, an ammeter should not be used to determine the treatment endpoint for coagulation of blood vessels in the vicinity of vital tissue.
Whenever bipolar electrosurgery is used for hemostasis, unwanted thermal damage can be minimized by:
- terminating the flow of current at the end of the visible vapor phase,
- applying current in a pulsatile fashion to permit tissue cooling,
- avoiding the use of an in-line ammeter to determine the coagulation endpoint, and
- securing pedicles by a stepwise process that alternates between partial desiccation and incremental cutting (TABLE 1).
Since the rate of temperature generation is a direct function of the volume of tissue being desiccated, thermal spread can also be reduced by using the sides or tips of a slightly open forceps to press or lift, rather than coapt for hemostasis (FIGURE 4).
Free adherent tissue gently. As with contact monopolar coagulation, tissue between the electrodes of a bipolar instrument may become adherent during desiccation. Repeated attempts to shake the tissue free may lead to traumatic avulsion of a key vascular pedicle. A stuck vascular pedicle can usually be unglued by energizing the opened device while immersing it in a conductive irrigant, such as saline. Once the solution is boiled by the high current density between the electrodes, the mechanical action of bubbling is usually sufficient to atraumatically free the pedicle.
FIGURE 4 Minimizing bipolar thermal damage
Using contact rather than coaptation is one way to limit thermal injury during bipolar electrosurgery.TABLE 1
Minimizing bipolar thermal damage
|
Ultrasonic energy
Mechanism of action. Ultrasonic shears produce mechanical energy to cut and coagulate tissue. Housed in the hand piece of this laparoscopic device is a piezoelectric crystal that vibrates a titanium blade 55,500 times per second over a variable excursion of 50 microns to 100 microns. As energy is transmitted to tissue, hydrogen bonds of tissue proteins are ruptured, leading to a denatured protein coagulum without significant charring. The tissue cutting that occurs is secondary to mechanical vibration and cavitational fragmentation of tissue parenchyma.
Since ultrasonic energy does not generate the high temperatures created by electrosurgery, it is less dependable for deep-tissue coagulation. Rather, thermal change is gradual, requiring a modicum of patience.
Available instrument configurations include 5-mm curved or hook blades. In addition, 5-mm or 10-mm ligating and cutting shears coaptively desiccate and cut the tissue by securing it between a grooved plastic pad and the vibrating blade.
Tissue effects depend on interplay of factors. By using various combinations of blade shapes, blade excursions, and tissue tensions, surgeons can accomplish a variety of specific effects. Cutting velocity is directly proportional to blade excursion, tissue traction, and blade surface area (energy density), and is inversely related to tissue density and elasticity. Thus, the fastest cutting with the least amount of coagulation occurs when tissue is placed on tension and firmly squeezed, lifted, or rotated with the sharpest side of a blade set at maximum excursion.
Coagulation is the obverse of cutting: It is inversely related to tissue tension, blade sharpness, blade excursion, and cutting speed. Therefore, coagulation is best achieved by relaxing tension, minimizing blade excursion, and using a blunt edge or flattened blade surface.
A stuck vascular pedicle can usually be unglued by energizing the opened bipolar device while immersing it in a conductive irrigant.
Avoid excessive traction and torsion. Be mindful of the potential for premature incision of an incompletely coagulated tissue pedicle when excessive traction or torsion is applied. When used to coaptively desiccate and incise a vascular pedicle, ultrasonic energy should be applied patiently, taking great care to minimize tissue tension while using the broadest blade surface set to the lowest excursion (TABLE 2). Hemostatic incision is best ensured by first coagulating several overlapping areas along an untracted pedicle, limiting each application to the point of tissue blanching and initial vapor emission. Only then should the pedicle be incised by gradually lifting, squeezing, or rotating the distal device. In this fashion, ultrasonic energy can be successfully used to secure both the ovarian and uterine vessels.
TABLE 2
Factors likely to cause premature incision with ultrasonic energy
|
Summary
The use of electrical and ultrasonic energy during operative laparoscopy poses several challenges, including the reduction of unwanted thermal injury and the elimination of incomplete hemostasis.
Since the depth of penetration during monopolar electrosurgery is proportional to both output voltage and surface area, unwanted thermal change can be reduced by using the smallest electrode surface with “cut” current for tissue cutting, and “coag” current with a broad-surface electrode for contact or noncontact (fulguration) coagulation.
Bipolar electrosurgery is the preferred modality for coaptive desiccation of a vascular pedicle with electricity. Despite the isolation of current to the intervening tissue, surgeons must take steps to reduce the lateral percolation of heat into adjacent tissues.
Ultrasonic energy provides reliable coaptive hemostasis and incision with little tissue damage. However, the surgeon must be mindful of the forces that promulgate premature incision. Knowledge of the biophysical behavior of electrical and ultrasonic energy is a prelude to safety and efficacy during laparoscopic dissection.
Dr. Brill reports no affiliations or financial arrangements with any of the manufacturers of products mentioned in this article.
- Inspect all vascular sites with and without insufflation before assuming hemostasis is complete.
- In monopolar electrosurgery, electrode contact using low-voltage current leads to deeper, more effective penetration than higher-voltage current.
- To minimize unwanted thermal damage during bipolar electrosurgery, stop current flow at the end of the visible vapor phase, apply current in a pulsatile fashion, and secure pedicles by alternating between partial desiccation and incremental cutting.
- Since ultrasonic energy does not generate the high temperatures created by electrosurgery, it is less dependable for deep-tissue coagulation.
Compared with laparotomy, laparoscopic surgery achieves better hemostasis with less blood loss. Not only does this approach avoid an abdominal incision and the trauma associated with traction, manual manipulation, mechanical dissection, and larger tissue pedicles, but its illumination and magnification afford superior anatomical clarity, allowing the surgeon to seal a vessel before it is incised.
Still, keen surgical judgment remains critical—despite the availability of innovative electrosurgical, ultrasonic, and mechanical laparoscopic devices. Incomplete hemostasis or incision of an active vascular core can occur even with ideal application.
This article outlines the key ingredients of hemostasis during laparoscopy, focusing on the following modalities:
- monopolar electrosurgery
- bipolar electrosurgery
- ultrasonic energy
An orderly protocol minimizes risk
The art of surgical hemostasis is preventing vascular trauma while leaving the least-possible collateral tissue damage. When bleeding is encountered, the surgeon’s ability to attain hemostasis using a particular modality depends largely on how well he or she understands its technical aspects. Of course, thorough knowledge of anatomy also is crucial to prevent inadvertent damage to vital structures.
Surgical hemostasis should not be driven by reflex alone. Instead, surgeons should always follow this orderly sequence of steps to minimize risk:
Identify source of bleeding. Before taking any action, make every effort to accurately determine the source of bleeding and its proximity to vital anatomy. Even in the face of active hemorrhage, you can usually identify the bleeders by combining mechanical tamponade (using the jaws of a grasper or the side of a simple metallic probe) with active hydrolavage (using an irrigator-aspirator to break up and remove blood and clots).
Protect vital structures. If the bowel, bladder, or ureter is in close proximity to the bleeder, mobilize that structure sufficiently before applying energy. You can usually protect these entities by using a combination of countertraction and incremental tissue dissection. Whenever the peritoneum is involved, a relaxing incision parallel to the structure of concern also may be useful.
This protocol mandates withholding thermal energy until an orderly sequence of anatomical triage is carried out. Whenever a vital structure cannot be adequately mobilized, make every effort to control hemorrhage by using mechanical tamponade alone for up to 5 minutes. If access to the bleeding site or vessel caliber render pressure-alone unrealistic, employ either a carefully applied thermal energy or a suture ligature. If the surgeon is uncomfortable using either of these, conversion to laparotomy may be warranted.
Inspect vascular sites. Finally, because pneumoperitoneal pressure alone can tamponade venous bleeders—as well as small arterial ones—inspect all vascular sites with and without insufflation before assuming hemostasis is complete.
Monopolar electrosurgery
With conventional electrosurgery, tissue is coagulated when an electric field is applied across it using high-frequency alternating current. Whether cutting or coagulation occurs depends on the rate and extent of thermodynamic effects (FIGURE 1).
Mechanism of coagulation. When tissue comes into contact with the surface of an activated monopolar electrode, a relatively low-current circuit is completed.
- As the tissue is slowly heated to and maintained at temperatures above 50°C, irreversible cellular damage occurs. This is caused by deconfiguration of regulatory proteins and denaturation of cellular proteins.
- If the tissue is heated to 100°C, cellular water completely evaporates (desiccation), localized hemostasis occurs due to contraction of blood vessels and the surrounding tissues (coagulation), and collagens convert to glucose, which creates an adhesive effect between the tissue and electrode.
- Temperatures above 200°C cause carbonization and charring.
Select the best output voltage. Since the output voltage of “coag” current is very high (FIGURE 2), contact coagulation is generally limited to superficial layers. That is because of the accelerated buildup of tissue resistance from rapid desiccation and carbonization. Conversely, electrode contact using the lower-voltage “cut” current heats tissue more gradually, leading to deeper and more reliable penetration. Thus, both contact and coaptive coagulation with monopolar electrosurgery are more effectively performed using “cut” current.
Since superficial-appearing endometriotic implants may extend deeply into the retroperitoneal tissues, I thermally ablate these lesions using a broad-surface electrode in contact with “cut” current. In contrast, I treat superficial implants on the ovarian cortex with “coag” current to minimize unwanted thermal injury to adjacent follicular tissue.
Interrupt the blood flow. Coaptive vessel sealing using any type of monopolar current may be ineffective if the blood flow remains uninterrupted. Unless a vessel is sufficiently squeezed before electricity is applied, current density is dramatically reduced by conduction in blood, and luminal temperatures undergo little change, as any heat is dissipated by convection. Deceived by the appearance of well-coagulated tissue, a surgeon may discover an alarmingly viable core at the time of incision. Regardless of the selected output current (that is, “cut,” “blend,” or “coag”), coaptive desiccation with monopolar electrosurgery is usually insufficient to reliably secure the uterine or ovarian vessels during hysterectomy and oophorectomy.
Achieve the appropriate cutting arc. Electrosurgical cutting (vaporization/ablation) is possible whenever voltage is sufficient to create an electrical spark between an electrode and underlying tissue; tissue cutting is more apt to occur if the arcing remains unabated. When it does, cellular water is superheated to temperatures greater than 600°C, causing explosive vaporization secondary to the production of highly disruptive pressure (since steam occupies 6 times the volume of liquid water).
Although all of the typical output currents (“cut,” “blend,” and “coag”) provide sufficient voltage to ionize the air gap and arc to tissue, the higher voltages of “blend” and “coag” create progressively wider zones of thermal damage at the margins of the incision (FIGURE 3) These effects are amplified by using broad-surface electrodes.
Wide versus narrow hemostasis. Using “blend” or “coag” current to cut in order to provide wider hemostasis can be helpful during myomectomy, as well as when operating down the broad ligament and along the vaginal fornices during hysterectomy, across vascular adhesions, and to clarify the space of Retzius in preparation for colposuspension and paravaginal repair. Higher-voltage currents also facilitate incision of tissues that have greater impedance, such as fatty or desiccated pedicles and adhesions.
On the other hand, it is more prudent to utilize the lower-voltage “cut” current via the edge of an electrode for electrosurgical incision whenever lateral thermal spread may pose extra liability to adjacent tissues, such as the ovarian cortex during cystectomy and the ureter or rectum during excision of endometriosis from the lateral pelvic sidewall or cul-de-sac.
Deceived by the appearance of well-coagulated tissue, surgeons may discover an alarmingly viable core at the time of incision.
- Fulguration is the use of high-voltage sparking produced by “coag” current to coagulate a broad surface with open bleeders. As opposed to the continuous arcing produced by “cut” current, the highly interrupted output of “coag” current causes the arcs to strike the tissue surface in a widely dispersed and random fashion. This leads to more rapid thermal change, creating a zone of superficial coagulation.
I typically employ fulguration to control small bleeders and vessels cut on end along the undersurface of the ovarian cortex during cystectomy, atop the myometrial bed during myomectomy, and alongside Cooper’s ligament during colposuspension.
Protect vital structures with short bursts of “coag” current. If bleeding near the bowel, bladder, or ureter cannot be controlled with pressure alone, carefully directed short bursts of noncontact “coag” current with a broad-surface electrode may help you attain effective hemostasis with the least-possible amount of electrosurgical penetration.
Despite the high-voltage output involved, fulguration is useless in a wet, conductive surgical field due to the random diffusion of current.
FIGURE 2 Range of output voltages
Relative changes in voltage and average current related to “cut,” “blend,” and “coag” currents.
FIGURE 3Zones of thermal damage related to voltage
Using a conventional electrosurgical generator for tissue cutting, the margin of thermal necrosis expands with increasing voltage.
Bipolar electrosurgery
Mechanism of action. Bipolar electrosurgery consolidates an active electrode and return electrode into an instrument with 2 small poles. These poles can be the tines of a forceps, blades of a scissors, or an electrode matched to a more proximal conductive collar separated by an insulator. The output typically used is the low-voltage “cut” current.
Advantages of bipolar energy. Localization of current between the poles offers distinct advantages. Thermal damage is generally limited to a discrete volume of tissue. A bipolar forceps can be used to coapt and thermally weld blood vessels. The concentrated current and small distance between the poles also make it possible to desiccate tissue that is immersed in fluid. This modality is less useful, however, when open blood vessels are retracted or tissue pedicles are very thick.
Because it tends to promote the flow of energy well beyond desiccation, an ammeter should not be used to determine the treatment endpoint for coagulation of blood vessels in the vicinity of vital tissue.
Know when to terminate current to prevent vital-tissue damage. Although the flow of current and primary thermal effects are restricted to the tissue between the poles, this does not eliminate the risk of thermal injury to tissue that is distant from the site of directed hemostasis. As current is applied between the poles, the intervening tissue gradually desiccates until it becomes thoroughly dehydrated. Desiccation is complete when the tissue whitens and visible steam emission stops. If the application of current continues, the heat spreads well beyond the electrical limits of the instrument.
This secondary thermal bloom is caused by the bubbling of steam into the tissue parenchyma as heat is rapidly generated (due to dry tissue’s high resistance to the flow of electrical current). This explains why structures such as the ureter or bowel may suffer irreversible thermal damage despite being at some distance from an operative or bleeding site.
Use of an in-line ammeter does not prevent this problem. Rather, it tends to promote the flow of energy well beyond desiccation. Consequently, an ammeter should not be used to determine the treatment endpoint for coagulation of blood vessels in the vicinity of vital tissue.
Whenever bipolar electrosurgery is used for hemostasis, unwanted thermal damage can be minimized by:
- terminating the flow of current at the end of the visible vapor phase,
- applying current in a pulsatile fashion to permit tissue cooling,
- avoiding the use of an in-line ammeter to determine the coagulation endpoint, and
- securing pedicles by a stepwise process that alternates between partial desiccation and incremental cutting (TABLE 1).
Since the rate of temperature generation is a direct function of the volume of tissue being desiccated, thermal spread can also be reduced by using the sides or tips of a slightly open forceps to press or lift, rather than coapt for hemostasis (FIGURE 4).
Free adherent tissue gently. As with contact monopolar coagulation, tissue between the electrodes of a bipolar instrument may become adherent during desiccation. Repeated attempts to shake the tissue free may lead to traumatic avulsion of a key vascular pedicle. A stuck vascular pedicle can usually be unglued by energizing the opened device while immersing it in a conductive irrigant, such as saline. Once the solution is boiled by the high current density between the electrodes, the mechanical action of bubbling is usually sufficient to atraumatically free the pedicle.
FIGURE 4 Minimizing bipolar thermal damage
Using contact rather than coaptation is one way to limit thermal injury during bipolar electrosurgery.TABLE 1
Minimizing bipolar thermal damage
|
Ultrasonic energy
Mechanism of action. Ultrasonic shears produce mechanical energy to cut and coagulate tissue. Housed in the hand piece of this laparoscopic device is a piezoelectric crystal that vibrates a titanium blade 55,500 times per second over a variable excursion of 50 microns to 100 microns. As energy is transmitted to tissue, hydrogen bonds of tissue proteins are ruptured, leading to a denatured protein coagulum without significant charring. The tissue cutting that occurs is secondary to mechanical vibration and cavitational fragmentation of tissue parenchyma.
Since ultrasonic energy does not generate the high temperatures created by electrosurgery, it is less dependable for deep-tissue coagulation. Rather, thermal change is gradual, requiring a modicum of patience.
Available instrument configurations include 5-mm curved or hook blades. In addition, 5-mm or 10-mm ligating and cutting shears coaptively desiccate and cut the tissue by securing it between a grooved plastic pad and the vibrating blade.
Tissue effects depend on interplay of factors. By using various combinations of blade shapes, blade excursions, and tissue tensions, surgeons can accomplish a variety of specific effects. Cutting velocity is directly proportional to blade excursion, tissue traction, and blade surface area (energy density), and is inversely related to tissue density and elasticity. Thus, the fastest cutting with the least amount of coagulation occurs when tissue is placed on tension and firmly squeezed, lifted, or rotated with the sharpest side of a blade set at maximum excursion.
Coagulation is the obverse of cutting: It is inversely related to tissue tension, blade sharpness, blade excursion, and cutting speed. Therefore, coagulation is best achieved by relaxing tension, minimizing blade excursion, and using a blunt edge or flattened blade surface.
A stuck vascular pedicle can usually be unglued by energizing the opened bipolar device while immersing it in a conductive irrigant.
Avoid excessive traction and torsion. Be mindful of the potential for premature incision of an incompletely coagulated tissue pedicle when excessive traction or torsion is applied. When used to coaptively desiccate and incise a vascular pedicle, ultrasonic energy should be applied patiently, taking great care to minimize tissue tension while using the broadest blade surface set to the lowest excursion (TABLE 2). Hemostatic incision is best ensured by first coagulating several overlapping areas along an untracted pedicle, limiting each application to the point of tissue blanching and initial vapor emission. Only then should the pedicle be incised by gradually lifting, squeezing, or rotating the distal device. In this fashion, ultrasonic energy can be successfully used to secure both the ovarian and uterine vessels.
TABLE 2
Factors likely to cause premature incision with ultrasonic energy
|
Summary
The use of electrical and ultrasonic energy during operative laparoscopy poses several challenges, including the reduction of unwanted thermal injury and the elimination of incomplete hemostasis.
Since the depth of penetration during monopolar electrosurgery is proportional to both output voltage and surface area, unwanted thermal change can be reduced by using the smallest electrode surface with “cut” current for tissue cutting, and “coag” current with a broad-surface electrode for contact or noncontact (fulguration) coagulation.
Bipolar electrosurgery is the preferred modality for coaptive desiccation of a vascular pedicle with electricity. Despite the isolation of current to the intervening tissue, surgeons must take steps to reduce the lateral percolation of heat into adjacent tissues.
Ultrasonic energy provides reliable coaptive hemostasis and incision with little tissue damage. However, the surgeon must be mindful of the forces that promulgate premature incision. Knowledge of the biophysical behavior of electrical and ultrasonic energy is a prelude to safety and efficacy during laparoscopic dissection.
Dr. Brill reports no affiliations or financial arrangements with any of the manufacturers of products mentioned in this article.
1. Brill AI. Energy systems for operative laparoscopy. J Am Assoc Gynecol Laparosc. 1998;5:335-345.
2. Friedman J. The technical aspects of electrosurgery. Oral Surg. 1973;36:177-187.
3. Honig WM. The mechanism of cutting in electrosurgery. IEEE Trans Biomed Eng BME. 1975;22:55-58.
4. Sigel B, Dunn MR. The mechanism of blood vessel closure by high frequency electrocoagulation. Surg Gynecol Obstet. 1965;121:823-831.
5. Phipps JH. Thermometry studies with bipolar diathermy during hysterectomy. Gynecol Laparosc. 1994;1:146-149.
6. Ryder RM, Hulka JF. Bladder and bowel injury after electrodesiccation with Kleppinger bipolar forceps: A clinicopathologic study. J Reprod Med. 1993;3:595-598.
7. McCarus SD. Physiologic mechanism of the ultrasonically activated scalpel. J Am Assoc Gynecol Laparosc. 1996;3:601-608.
1. Brill AI. Energy systems for operative laparoscopy. J Am Assoc Gynecol Laparosc. 1998;5:335-345.
2. Friedman J. The technical aspects of electrosurgery. Oral Surg. 1973;36:177-187.
3. Honig WM. The mechanism of cutting in electrosurgery. IEEE Trans Biomed Eng BME. 1975;22:55-58.
4. Sigel B, Dunn MR. The mechanism of blood vessel closure by high frequency electrocoagulation. Surg Gynecol Obstet. 1965;121:823-831.
5. Phipps JH. Thermometry studies with bipolar diathermy during hysterectomy. Gynecol Laparosc. 1994;1:146-149.
6. Ryder RM, Hulka JF. Bladder and bowel injury after electrodesiccation with Kleppinger bipolar forceps: A clinicopathologic study. J Reprod Med. 1993;3:595-598.
7. McCarus SD. Physiologic mechanism of the ultrasonically activated scalpel. J Am Assoc Gynecol Laparosc. 1996;3:601-608.