User login
- Inspect all vascular sites with and without insufflation before assuming hemostasis is complete.
- In monopolar electrosurgery, electrode contact using low-voltage current leads to deeper, more effective penetration than higher-voltage current.
- To minimize unwanted thermal damage during bipolar electrosurgery, stop current flow at the end of the visible vapor phase, apply current in a pulsatile fashion, and secure pedicles by alternating between partial desiccation and incremental cutting.
- Since ultrasonic energy does not generate the high temperatures created by electrosurgery, it is less dependable for deep-tissue coagulation.
Compared with laparotomy, laparoscopic surgery achieves better hemostasis with less blood loss. Not only does this approach avoid an abdominal incision and the trauma associated with traction, manual manipulation, mechanical dissection, and larger tissue pedicles, but its illumination and magnification afford superior anatomical clarity, allowing the surgeon to seal a vessel before it is incised.
Still, keen surgical judgment remains critical—despite the availability of innovative electrosurgical, ultrasonic, and mechanical laparoscopic devices. Incomplete hemostasis or incision of an active vascular core can occur even with ideal application.
This article outlines the key ingredients of hemostasis during laparoscopy, focusing on the following modalities:
- monopolar electrosurgery
- bipolar electrosurgery
- ultrasonic energy
An orderly protocol minimizes risk
The art of surgical hemostasis is preventing vascular trauma while leaving the least-possible collateral tissue damage. When bleeding is encountered, the surgeon’s ability to attain hemostasis using a particular modality depends largely on how well he or she understands its technical aspects. Of course, thorough knowledge of anatomy also is crucial to prevent inadvertent damage to vital structures.
Surgical hemostasis should not be driven by reflex alone. Instead, surgeons should always follow this orderly sequence of steps to minimize risk:
Identify source of bleeding. Before taking any action, make every effort to accurately determine the source of bleeding and its proximity to vital anatomy. Even in the face of active hemorrhage, you can usually identify the bleeders by combining mechanical tamponade (using the jaws of a grasper or the side of a simple metallic probe) with active hydrolavage (using an irrigator-aspirator to break up and remove blood and clots).
Protect vital structures. If the bowel, bladder, or ureter is in close proximity to the bleeder, mobilize that structure sufficiently before applying energy. You can usually protect these entities by using a combination of countertraction and incremental tissue dissection. Whenever the peritoneum is involved, a relaxing incision parallel to the structure of concern also may be useful.
This protocol mandates withholding thermal energy until an orderly sequence of anatomical triage is carried out. Whenever a vital structure cannot be adequately mobilized, make every effort to control hemorrhage by using mechanical tamponade alone for up to 5 minutes. If access to the bleeding site or vessel caliber render pressure-alone unrealistic, employ either a carefully applied thermal energy or a suture ligature. If the surgeon is uncomfortable using either of these, conversion to laparotomy may be warranted.
Inspect vascular sites. Finally, because pneumoperitoneal pressure alone can tamponade venous bleeders—as well as small arterial ones—inspect all vascular sites with and without insufflation before assuming hemostasis is complete.
Monopolar electrosurgery
With conventional electrosurgery, tissue is coagulated when an electric field is applied across it using high-frequency alternating current. Whether cutting or coagulation occurs depends on the rate and extent of thermodynamic effects (FIGURE 1).
Mechanism of coagulation. When tissue comes into contact with the surface of an activated monopolar electrode, a relatively low-current circuit is completed.
- As the tissue is slowly heated to and maintained at temperatures above 50°C, irreversible cellular damage occurs. This is caused by deconfiguration of regulatory proteins and denaturation of cellular proteins.
- If the tissue is heated to 100°C, cellular water completely evaporates (desiccation), localized hemostasis occurs due to contraction of blood vessels and the surrounding tissues (coagulation), and collagens convert to glucose, which creates an adhesive effect between the tissue and electrode.
- Temperatures above 200°C cause carbonization and charring.
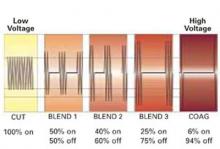
Select the best output voltage. Since the output voltage of “coag” current is very high (FIGURE 2), contact coagulation is generally limited to superficial layers. That is because of the accelerated buildup of tissue resistance from rapid desiccation and carbonization. Conversely, electrode contact using the lower-voltage “cut” current heats tissue more gradually, leading to deeper and more reliable penetration. Thus, both contact and coaptive coagulation with monopolar electrosurgery are more effectively performed using “cut” current.
Since superficial-appearing endometriotic implants may extend deeply into the retroperitoneal tissues, I thermally ablate these lesions using a broad-surface electrode in contact with “cut” current. In contrast, I treat superficial implants on the ovarian cortex with “coag” current to minimize unwanted thermal injury to adjacent follicular tissue.
Interrupt the blood flow. Coaptive vessel sealing using any type of monopolar current may be ineffective if the blood flow remains uninterrupted. Unless a vessel is sufficiently squeezed before electricity is applied, current density is dramatically reduced by conduction in blood, and luminal temperatures undergo little change, as any heat is dissipated by convection. Deceived by the appearance of well-coagulated tissue, a surgeon may discover an alarmingly viable core at the time of incision. Regardless of the selected output current (that is, “cut,” “blend,” or “coag”), coaptive desiccation with monopolar electrosurgery is usually insufficient to reliably secure the uterine or ovarian vessels during hysterectomy and oophorectomy.
Achieve the appropriate cutting arc. Electrosurgical cutting (vaporization/ablation) is possible whenever voltage is sufficient to create an electrical spark between an electrode and underlying tissue; tissue cutting is more apt to occur if the arcing remains unabated. When it does, cellular water is superheated to temperatures greater than 600°C, causing explosive vaporization secondary to the production of highly disruptive pressure (since steam occupies 6 times the volume of liquid water).
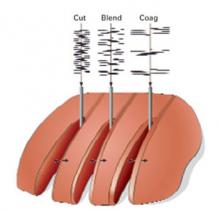
Although all of the typical output currents (“cut,” “blend,” and “coag”) provide sufficient voltage to ionize the air gap and arc to tissue, the higher voltages of “blend” and “coag” create progressively wider zones of thermal damage at the margins of the incision (FIGURE 3) These effects are amplified by using broad-surface electrodes.
Wide versus narrow hemostasis. Using “blend” or “coag” current to cut in order to provide wider hemostasis can be helpful during myomectomy, as well as when operating down the broad ligament and along the vaginal fornices during hysterectomy, across vascular adhesions, and to clarify the space of Retzius in preparation for colposuspension and paravaginal repair. Higher-voltage currents also facilitate incision of tissues that have greater impedance, such as fatty or desiccated pedicles and adhesions.
On the other hand, it is more prudent to utilize the lower-voltage “cut” current via the edge of an electrode for electrosurgical incision whenever lateral thermal spread may pose extra liability to adjacent tissues, such as the ovarian cortex during cystectomy and the ureter or rectum during excision of endometriosis from the lateral pelvic sidewall or cul-de-sac.
Deceived by the appearance of well-coagulated tissue, surgeons may discover an alarmingly viable core at the time of incision.
- Fulguration is the use of high-voltage sparking produced by “coag” current to coagulate a broad surface with open bleeders. As opposed to the continuous arcing produced by “cut” current, the highly interrupted output of “coag” current causes the arcs to strike the tissue surface in a widely dispersed and random fashion. This leads to more rapid thermal change, creating a zone of superficial coagulation.
I typically employ fulguration to control small bleeders and vessels cut on end along the undersurface of the ovarian cortex during cystectomy, atop the myometrial bed during myomectomy, and alongside Cooper’s ligament during colposuspension.
Protect vital structures with short bursts of “coag” current. If bleeding near the bowel, bladder, or ureter cannot be controlled with pressure alone, carefully directed short bursts of noncontact “coag” current with a broad-surface electrode may help you attain effective hemostasis with the least-possible amount of electrosurgical penetration.
Despite the high-voltage output involved, fulguration is useless in a wet, conductive surgical field due to the random diffusion of current.
FIGURE 2 Range of output voltages
Relative changes in voltage and average current related to “cut,” “blend,” and “coag” currents.
FIGURE 3Zones of thermal damage related to voltage
Using a conventional electrosurgical generator for tissue cutting, the margin of thermal necrosis expands with increasing voltage.
Bipolar electrosurgery
Mechanism of action. Bipolar electrosurgery consolidates an active electrode and return electrode into an instrument with 2 small poles. These poles can be the tines of a forceps, blades of a scissors, or an electrode matched to a more proximal conductive collar separated by an insulator. The output typically used is the low-voltage “cut” current.
Advantages of bipolar energy. Localization of current between the poles offers distinct advantages. Thermal damage is generally limited to a discrete volume of tissue. A bipolar forceps can be used to coapt and thermally weld blood vessels. The concentrated current and small distance between the poles also make it possible to desiccate tissue that is immersed in fluid. This modality is less useful, however, when open blood vessels are retracted or tissue pedicles are very thick.
Because it tends to promote the flow of energy well beyond desiccation, an ammeter should not be used to determine the treatment endpoint for coagulation of blood vessels in the vicinity of vital tissue.
Know when to terminate current to prevent vital-tissue damage. Although the flow of current and primary thermal effects are restricted to the tissue between the poles, this does not eliminate the risk of thermal injury to tissue that is distant from the site of directed hemostasis. As current is applied between the poles, the intervening tissue gradually desiccates until it becomes thoroughly dehydrated. Desiccation is complete when the tissue whitens and visible steam emission stops. If the application of current continues, the heat spreads well beyond the electrical limits of the instrument.
This secondary thermal bloom is caused by the bubbling of steam into the tissue parenchyma as heat is rapidly generated (due to dry tissue’s high resistance to the flow of electrical current). This explains why structures such as the ureter or bowel may suffer irreversible thermal damage despite being at some distance from an operative or bleeding site.
Use of an in-line ammeter does not prevent this problem. Rather, it tends to promote the flow of energy well beyond desiccation. Consequently, an ammeter should not be used to determine the treatment endpoint for coagulation of blood vessels in the vicinity of vital tissue.
Whenever bipolar electrosurgery is used for hemostasis, unwanted thermal damage can be minimized by:
- terminating the flow of current at the end of the visible vapor phase,
- applying current in a pulsatile fashion to permit tissue cooling,
- avoiding the use of an in-line ammeter to determine the coagulation endpoint, and
- securing pedicles by a stepwise process that alternates between partial desiccation and incremental cutting (TABLE 1).
Since the rate of temperature generation is a direct function of the volume of tissue being desiccated, thermal spread can also be reduced by using the sides or tips of a slightly open forceps to press or lift, rather than coapt for hemostasis (FIGURE 4).
Free adherent tissue gently. As with contact monopolar coagulation, tissue between the electrodes of a bipolar instrument may become adherent during desiccation. Repeated attempts to shake the tissue free may lead to traumatic avulsion of a key vascular pedicle. A stuck vascular pedicle can usually be unglued by energizing the opened device while immersing it in a conductive irrigant, such as saline. Once the solution is boiled by the high current density between the electrodes, the mechanical action of bubbling is usually sufficient to atraumatically free the pedicle.
FIGURE 4 Minimizing bipolar thermal damage
Using contact rather than coaptation is one way to limit thermal injury during bipolar electrosurgery.TABLE 1
Minimizing bipolar thermal damage
|
Ultrasonic energy
Mechanism of action. Ultrasonic shears produce mechanical energy to cut and coagulate tissue. Housed in the hand piece of this laparoscopic device is a piezoelectric crystal that vibrates a titanium blade 55,500 times per second over a variable excursion of 50 microns to 100 microns. As energy is transmitted to tissue, hydrogen bonds of tissue proteins are ruptured, leading to a denatured protein coagulum without significant charring. The tissue cutting that occurs is secondary to mechanical vibration and cavitational fragmentation of tissue parenchyma.
Since ultrasonic energy does not generate the high temperatures created by electrosurgery, it is less dependable for deep-tissue coagulation. Rather, thermal change is gradual, requiring a modicum of patience.
Available instrument configurations include 5-mm curved or hook blades. In addition, 5-mm or 10-mm ligating and cutting shears coaptively desiccate and cut the tissue by securing it between a grooved plastic pad and the vibrating blade.
Tissue effects depend on interplay of factors. By using various combinations of blade shapes, blade excursions, and tissue tensions, surgeons can accomplish a variety of specific effects. Cutting velocity is directly proportional to blade excursion, tissue traction, and blade surface area (energy density), and is inversely related to tissue density and elasticity. Thus, the fastest cutting with the least amount of coagulation occurs when tissue is placed on tension and firmly squeezed, lifted, or rotated with the sharpest side of a blade set at maximum excursion.
Coagulation is the obverse of cutting: It is inversely related to tissue tension, blade sharpness, blade excursion, and cutting speed. Therefore, coagulation is best achieved by relaxing tension, minimizing blade excursion, and using a blunt edge or flattened blade surface.
A stuck vascular pedicle can usually be unglued by energizing the opened bipolar device while immersing it in a conductive irrigant.
Avoid excessive traction and torsion. Be mindful of the potential for premature incision of an incompletely coagulated tissue pedicle when excessive traction or torsion is applied. When used to coaptively desiccate and incise a vascular pedicle, ultrasonic energy should be applied patiently, taking great care to minimize tissue tension while using the broadest blade surface set to the lowest excursion (TABLE 2). Hemostatic incision is best ensured by first coagulating several overlapping areas along an untracted pedicle, limiting each application to the point of tissue blanching and initial vapor emission. Only then should the pedicle be incised by gradually lifting, squeezing, or rotating the distal device. In this fashion, ultrasonic energy can be successfully used to secure both the ovarian and uterine vessels.
TABLE 2
Factors likely to cause premature incision with ultrasonic energy
|
Summary
The use of electrical and ultrasonic energy during operative laparoscopy poses several challenges, including the reduction of unwanted thermal injury and the elimination of incomplete hemostasis.
Since the depth of penetration during monopolar electrosurgery is proportional to both output voltage and surface area, unwanted thermal change can be reduced by using the smallest electrode surface with “cut” current for tissue cutting, and “coag” current with a broad-surface electrode for contact or noncontact (fulguration) coagulation.
Bipolar electrosurgery is the preferred modality for coaptive desiccation of a vascular pedicle with electricity. Despite the isolation of current to the intervening tissue, surgeons must take steps to reduce the lateral percolation of heat into adjacent tissues.
Ultrasonic energy provides reliable coaptive hemostasis and incision with little tissue damage. However, the surgeon must be mindful of the forces that promulgate premature incision. Knowledge of the biophysical behavior of electrical and ultrasonic energy is a prelude to safety and efficacy during laparoscopic dissection.
Dr. Brill reports no affiliations or financial arrangements with any of the manufacturers of products mentioned in this article.
1. Brill AI. Energy systems for operative laparoscopy. J Am Assoc Gynecol Laparosc. 1998;5:335-345.
2. Friedman J. The technical aspects of electrosurgery. Oral Surg. 1973;36:177-187.
3. Honig WM. The mechanism of cutting in electrosurgery. IEEE Trans Biomed Eng BME. 1975;22:55-58.
4. Sigel B, Dunn MR. The mechanism of blood vessel closure by high frequency electrocoagulation. Surg Gynecol Obstet. 1965;121:823-831.
5. Phipps JH. Thermometry studies with bipolar diathermy during hysterectomy. Gynecol Laparosc. 1994;1:146-149.
6. Ryder RM, Hulka JF. Bladder and bowel injury after electrodesiccation with Kleppinger bipolar forceps: A clinicopathologic study. J Reprod Med. 1993;3:595-598.
7. McCarus SD. Physiologic mechanism of the ultrasonically activated scalpel. J Am Assoc Gynecol Laparosc. 1996;3:601-608.
- Inspect all vascular sites with and without insufflation before assuming hemostasis is complete.
- In monopolar electrosurgery, electrode contact using low-voltage current leads to deeper, more effective penetration than higher-voltage current.
- To minimize unwanted thermal damage during bipolar electrosurgery, stop current flow at the end of the visible vapor phase, apply current in a pulsatile fashion, and secure pedicles by alternating between partial desiccation and incremental cutting.
- Since ultrasonic energy does not generate the high temperatures created by electrosurgery, it is less dependable for deep-tissue coagulation.
Compared with laparotomy, laparoscopic surgery achieves better hemostasis with less blood loss. Not only does this approach avoid an abdominal incision and the trauma associated with traction, manual manipulation, mechanical dissection, and larger tissue pedicles, but its illumination and magnification afford superior anatomical clarity, allowing the surgeon to seal a vessel before it is incised.
Still, keen surgical judgment remains critical—despite the availability of innovative electrosurgical, ultrasonic, and mechanical laparoscopic devices. Incomplete hemostasis or incision of an active vascular core can occur even with ideal application.
This article outlines the key ingredients of hemostasis during laparoscopy, focusing on the following modalities:
- monopolar electrosurgery
- bipolar electrosurgery
- ultrasonic energy
An orderly protocol minimizes risk
The art of surgical hemostasis is preventing vascular trauma while leaving the least-possible collateral tissue damage. When bleeding is encountered, the surgeon’s ability to attain hemostasis using a particular modality depends largely on how well he or she understands its technical aspects. Of course, thorough knowledge of anatomy also is crucial to prevent inadvertent damage to vital structures.
Surgical hemostasis should not be driven by reflex alone. Instead, surgeons should always follow this orderly sequence of steps to minimize risk:
Identify source of bleeding. Before taking any action, make every effort to accurately determine the source of bleeding and its proximity to vital anatomy. Even in the face of active hemorrhage, you can usually identify the bleeders by combining mechanical tamponade (using the jaws of a grasper or the side of a simple metallic probe) with active hydrolavage (using an irrigator-aspirator to break up and remove blood and clots).
Protect vital structures. If the bowel, bladder, or ureter is in close proximity to the bleeder, mobilize that structure sufficiently before applying energy. You can usually protect these entities by using a combination of countertraction and incremental tissue dissection. Whenever the peritoneum is involved, a relaxing incision parallel to the structure of concern also may be useful.
This protocol mandates withholding thermal energy until an orderly sequence of anatomical triage is carried out. Whenever a vital structure cannot be adequately mobilized, make every effort to control hemorrhage by using mechanical tamponade alone for up to 5 minutes. If access to the bleeding site or vessel caliber render pressure-alone unrealistic, employ either a carefully applied thermal energy or a suture ligature. If the surgeon is uncomfortable using either of these, conversion to laparotomy may be warranted.
Inspect vascular sites. Finally, because pneumoperitoneal pressure alone can tamponade venous bleeders—as well as small arterial ones—inspect all vascular sites with and without insufflation before assuming hemostasis is complete.
Monopolar electrosurgery
With conventional electrosurgery, tissue is coagulated when an electric field is applied across it using high-frequency alternating current. Whether cutting or coagulation occurs depends on the rate and extent of thermodynamic effects (FIGURE 1).
Mechanism of coagulation. When tissue comes into contact with the surface of an activated monopolar electrode, a relatively low-current circuit is completed.
- As the tissue is slowly heated to and maintained at temperatures above 50°C, irreversible cellular damage occurs. This is caused by deconfiguration of regulatory proteins and denaturation of cellular proteins.
- If the tissue is heated to 100°C, cellular water completely evaporates (desiccation), localized hemostasis occurs due to contraction of blood vessels and the surrounding tissues (coagulation), and collagens convert to glucose, which creates an adhesive effect between the tissue and electrode.
- Temperatures above 200°C cause carbonization and charring.
Select the best output voltage. Since the output voltage of “coag” current is very high (FIGURE 2), contact coagulation is generally limited to superficial layers. That is because of the accelerated buildup of tissue resistance from rapid desiccation and carbonization. Conversely, electrode contact using the lower-voltage “cut” current heats tissue more gradually, leading to deeper and more reliable penetration. Thus, both contact and coaptive coagulation with monopolar electrosurgery are more effectively performed using “cut” current.
Since superficial-appearing endometriotic implants may extend deeply into the retroperitoneal tissues, I thermally ablate these lesions using a broad-surface electrode in contact with “cut” current. In contrast, I treat superficial implants on the ovarian cortex with “coag” current to minimize unwanted thermal injury to adjacent follicular tissue.
Interrupt the blood flow. Coaptive vessel sealing using any type of monopolar current may be ineffective if the blood flow remains uninterrupted. Unless a vessel is sufficiently squeezed before electricity is applied, current density is dramatically reduced by conduction in blood, and luminal temperatures undergo little change, as any heat is dissipated by convection. Deceived by the appearance of well-coagulated tissue, a surgeon may discover an alarmingly viable core at the time of incision. Regardless of the selected output current (that is, “cut,” “blend,” or “coag”), coaptive desiccation with monopolar electrosurgery is usually insufficient to reliably secure the uterine or ovarian vessels during hysterectomy and oophorectomy.
Achieve the appropriate cutting arc. Electrosurgical cutting (vaporization/ablation) is possible whenever voltage is sufficient to create an electrical spark between an electrode and underlying tissue; tissue cutting is more apt to occur if the arcing remains unabated. When it does, cellular water is superheated to temperatures greater than 600°C, causing explosive vaporization secondary to the production of highly disruptive pressure (since steam occupies 6 times the volume of liquid water).
Although all of the typical output currents (“cut,” “blend,” and “coag”) provide sufficient voltage to ionize the air gap and arc to tissue, the higher voltages of “blend” and “coag” create progressively wider zones of thermal damage at the margins of the incision (FIGURE 3) These effects are amplified by using broad-surface electrodes.
Wide versus narrow hemostasis. Using “blend” or “coag” current to cut in order to provide wider hemostasis can be helpful during myomectomy, as well as when operating down the broad ligament and along the vaginal fornices during hysterectomy, across vascular adhesions, and to clarify the space of Retzius in preparation for colposuspension and paravaginal repair. Higher-voltage currents also facilitate incision of tissues that have greater impedance, such as fatty or desiccated pedicles and adhesions.
On the other hand, it is more prudent to utilize the lower-voltage “cut” current via the edge of an electrode for electrosurgical incision whenever lateral thermal spread may pose extra liability to adjacent tissues, such as the ovarian cortex during cystectomy and the ureter or rectum during excision of endometriosis from the lateral pelvic sidewall or cul-de-sac.
Deceived by the appearance of well-coagulated tissue, surgeons may discover an alarmingly viable core at the time of incision.
- Fulguration is the use of high-voltage sparking produced by “coag” current to coagulate a broad surface with open bleeders. As opposed to the continuous arcing produced by “cut” current, the highly interrupted output of “coag” current causes the arcs to strike the tissue surface in a widely dispersed and random fashion. This leads to more rapid thermal change, creating a zone of superficial coagulation.
I typically employ fulguration to control small bleeders and vessels cut on end along the undersurface of the ovarian cortex during cystectomy, atop the myometrial bed during myomectomy, and alongside Cooper’s ligament during colposuspension.
Protect vital structures with short bursts of “coag” current. If bleeding near the bowel, bladder, or ureter cannot be controlled with pressure alone, carefully directed short bursts of noncontact “coag” current with a broad-surface electrode may help you attain effective hemostasis with the least-possible amount of electrosurgical penetration.
Despite the high-voltage output involved, fulguration is useless in a wet, conductive surgical field due to the random diffusion of current.
FIGURE 2 Range of output voltages
Relative changes in voltage and average current related to “cut,” “blend,” and “coag” currents.
FIGURE 3Zones of thermal damage related to voltage
Using a conventional electrosurgical generator for tissue cutting, the margin of thermal necrosis expands with increasing voltage.
Bipolar electrosurgery
Mechanism of action. Bipolar electrosurgery consolidates an active electrode and return electrode into an instrument with 2 small poles. These poles can be the tines of a forceps, blades of a scissors, or an electrode matched to a more proximal conductive collar separated by an insulator. The output typically used is the low-voltage “cut” current.
Advantages of bipolar energy. Localization of current between the poles offers distinct advantages. Thermal damage is generally limited to a discrete volume of tissue. A bipolar forceps can be used to coapt and thermally weld blood vessels. The concentrated current and small distance between the poles also make it possible to desiccate tissue that is immersed in fluid. This modality is less useful, however, when open blood vessels are retracted or tissue pedicles are very thick.
Because it tends to promote the flow of energy well beyond desiccation, an ammeter should not be used to determine the treatment endpoint for coagulation of blood vessels in the vicinity of vital tissue.
Know when to terminate current to prevent vital-tissue damage. Although the flow of current and primary thermal effects are restricted to the tissue between the poles, this does not eliminate the risk of thermal injury to tissue that is distant from the site of directed hemostasis. As current is applied between the poles, the intervening tissue gradually desiccates until it becomes thoroughly dehydrated. Desiccation is complete when the tissue whitens and visible steam emission stops. If the application of current continues, the heat spreads well beyond the electrical limits of the instrument.
This secondary thermal bloom is caused by the bubbling of steam into the tissue parenchyma as heat is rapidly generated (due to dry tissue’s high resistance to the flow of electrical current). This explains why structures such as the ureter or bowel may suffer irreversible thermal damage despite being at some distance from an operative or bleeding site.
Use of an in-line ammeter does not prevent this problem. Rather, it tends to promote the flow of energy well beyond desiccation. Consequently, an ammeter should not be used to determine the treatment endpoint for coagulation of blood vessels in the vicinity of vital tissue.
Whenever bipolar electrosurgery is used for hemostasis, unwanted thermal damage can be minimized by:
- terminating the flow of current at the end of the visible vapor phase,
- applying current in a pulsatile fashion to permit tissue cooling,
- avoiding the use of an in-line ammeter to determine the coagulation endpoint, and
- securing pedicles by a stepwise process that alternates between partial desiccation and incremental cutting (TABLE 1).
Since the rate of temperature generation is a direct function of the volume of tissue being desiccated, thermal spread can also be reduced by using the sides or tips of a slightly open forceps to press or lift, rather than coapt for hemostasis (FIGURE 4).
Free adherent tissue gently. As with contact monopolar coagulation, tissue between the electrodes of a bipolar instrument may become adherent during desiccation. Repeated attempts to shake the tissue free may lead to traumatic avulsion of a key vascular pedicle. A stuck vascular pedicle can usually be unglued by energizing the opened device while immersing it in a conductive irrigant, such as saline. Once the solution is boiled by the high current density between the electrodes, the mechanical action of bubbling is usually sufficient to atraumatically free the pedicle.
FIGURE 4 Minimizing bipolar thermal damage
Using contact rather than coaptation is one way to limit thermal injury during bipolar electrosurgery.TABLE 1
Minimizing bipolar thermal damage
|
Ultrasonic energy
Mechanism of action. Ultrasonic shears produce mechanical energy to cut and coagulate tissue. Housed in the hand piece of this laparoscopic device is a piezoelectric crystal that vibrates a titanium blade 55,500 times per second over a variable excursion of 50 microns to 100 microns. As energy is transmitted to tissue, hydrogen bonds of tissue proteins are ruptured, leading to a denatured protein coagulum without significant charring. The tissue cutting that occurs is secondary to mechanical vibration and cavitational fragmentation of tissue parenchyma.
Since ultrasonic energy does not generate the high temperatures created by electrosurgery, it is less dependable for deep-tissue coagulation. Rather, thermal change is gradual, requiring a modicum of patience.
Available instrument configurations include 5-mm curved or hook blades. In addition, 5-mm or 10-mm ligating and cutting shears coaptively desiccate and cut the tissue by securing it between a grooved plastic pad and the vibrating blade.
Tissue effects depend on interplay of factors. By using various combinations of blade shapes, blade excursions, and tissue tensions, surgeons can accomplish a variety of specific effects. Cutting velocity is directly proportional to blade excursion, tissue traction, and blade surface area (energy density), and is inversely related to tissue density and elasticity. Thus, the fastest cutting with the least amount of coagulation occurs when tissue is placed on tension and firmly squeezed, lifted, or rotated with the sharpest side of a blade set at maximum excursion.
Coagulation is the obverse of cutting: It is inversely related to tissue tension, blade sharpness, blade excursion, and cutting speed. Therefore, coagulation is best achieved by relaxing tension, minimizing blade excursion, and using a blunt edge or flattened blade surface.
A stuck vascular pedicle can usually be unglued by energizing the opened bipolar device while immersing it in a conductive irrigant.
Avoid excessive traction and torsion. Be mindful of the potential for premature incision of an incompletely coagulated tissue pedicle when excessive traction or torsion is applied. When used to coaptively desiccate and incise a vascular pedicle, ultrasonic energy should be applied patiently, taking great care to minimize tissue tension while using the broadest blade surface set to the lowest excursion (TABLE 2). Hemostatic incision is best ensured by first coagulating several overlapping areas along an untracted pedicle, limiting each application to the point of tissue blanching and initial vapor emission. Only then should the pedicle be incised by gradually lifting, squeezing, or rotating the distal device. In this fashion, ultrasonic energy can be successfully used to secure both the ovarian and uterine vessels.
TABLE 2
Factors likely to cause premature incision with ultrasonic energy
|
Summary
The use of electrical and ultrasonic energy during operative laparoscopy poses several challenges, including the reduction of unwanted thermal injury and the elimination of incomplete hemostasis.
Since the depth of penetration during monopolar electrosurgery is proportional to both output voltage and surface area, unwanted thermal change can be reduced by using the smallest electrode surface with “cut” current for tissue cutting, and “coag” current with a broad-surface electrode for contact or noncontact (fulguration) coagulation.
Bipolar electrosurgery is the preferred modality for coaptive desiccation of a vascular pedicle with electricity. Despite the isolation of current to the intervening tissue, surgeons must take steps to reduce the lateral percolation of heat into adjacent tissues.
Ultrasonic energy provides reliable coaptive hemostasis and incision with little tissue damage. However, the surgeon must be mindful of the forces that promulgate premature incision. Knowledge of the biophysical behavior of electrical and ultrasonic energy is a prelude to safety and efficacy during laparoscopic dissection.
Dr. Brill reports no affiliations or financial arrangements with any of the manufacturers of products mentioned in this article.
- Inspect all vascular sites with and without insufflation before assuming hemostasis is complete.
- In monopolar electrosurgery, electrode contact using low-voltage current leads to deeper, more effective penetration than higher-voltage current.
- To minimize unwanted thermal damage during bipolar electrosurgery, stop current flow at the end of the visible vapor phase, apply current in a pulsatile fashion, and secure pedicles by alternating between partial desiccation and incremental cutting.
- Since ultrasonic energy does not generate the high temperatures created by electrosurgery, it is less dependable for deep-tissue coagulation.
Compared with laparotomy, laparoscopic surgery achieves better hemostasis with less blood loss. Not only does this approach avoid an abdominal incision and the trauma associated with traction, manual manipulation, mechanical dissection, and larger tissue pedicles, but its illumination and magnification afford superior anatomical clarity, allowing the surgeon to seal a vessel before it is incised.
Still, keen surgical judgment remains critical—despite the availability of innovative electrosurgical, ultrasonic, and mechanical laparoscopic devices. Incomplete hemostasis or incision of an active vascular core can occur even with ideal application.
This article outlines the key ingredients of hemostasis during laparoscopy, focusing on the following modalities:
- monopolar electrosurgery
- bipolar electrosurgery
- ultrasonic energy
An orderly protocol minimizes risk
The art of surgical hemostasis is preventing vascular trauma while leaving the least-possible collateral tissue damage. When bleeding is encountered, the surgeon’s ability to attain hemostasis using a particular modality depends largely on how well he or she understands its technical aspects. Of course, thorough knowledge of anatomy also is crucial to prevent inadvertent damage to vital structures.
Surgical hemostasis should not be driven by reflex alone. Instead, surgeons should always follow this orderly sequence of steps to minimize risk:
Identify source of bleeding. Before taking any action, make every effort to accurately determine the source of bleeding and its proximity to vital anatomy. Even in the face of active hemorrhage, you can usually identify the bleeders by combining mechanical tamponade (using the jaws of a grasper or the side of a simple metallic probe) with active hydrolavage (using an irrigator-aspirator to break up and remove blood and clots).
Protect vital structures. If the bowel, bladder, or ureter is in close proximity to the bleeder, mobilize that structure sufficiently before applying energy. You can usually protect these entities by using a combination of countertraction and incremental tissue dissection. Whenever the peritoneum is involved, a relaxing incision parallel to the structure of concern also may be useful.
This protocol mandates withholding thermal energy until an orderly sequence of anatomical triage is carried out. Whenever a vital structure cannot be adequately mobilized, make every effort to control hemorrhage by using mechanical tamponade alone for up to 5 minutes. If access to the bleeding site or vessel caliber render pressure-alone unrealistic, employ either a carefully applied thermal energy or a suture ligature. If the surgeon is uncomfortable using either of these, conversion to laparotomy may be warranted.
Inspect vascular sites. Finally, because pneumoperitoneal pressure alone can tamponade venous bleeders—as well as small arterial ones—inspect all vascular sites with and without insufflation before assuming hemostasis is complete.
Monopolar electrosurgery
With conventional electrosurgery, tissue is coagulated when an electric field is applied across it using high-frequency alternating current. Whether cutting or coagulation occurs depends on the rate and extent of thermodynamic effects (FIGURE 1).
Mechanism of coagulation. When tissue comes into contact with the surface of an activated monopolar electrode, a relatively low-current circuit is completed.
- As the tissue is slowly heated to and maintained at temperatures above 50°C, irreversible cellular damage occurs. This is caused by deconfiguration of regulatory proteins and denaturation of cellular proteins.
- If the tissue is heated to 100°C, cellular water completely evaporates (desiccation), localized hemostasis occurs due to contraction of blood vessels and the surrounding tissues (coagulation), and collagens convert to glucose, which creates an adhesive effect between the tissue and electrode.
- Temperatures above 200°C cause carbonization and charring.
Select the best output voltage. Since the output voltage of “coag” current is very high (FIGURE 2), contact coagulation is generally limited to superficial layers. That is because of the accelerated buildup of tissue resistance from rapid desiccation and carbonization. Conversely, electrode contact using the lower-voltage “cut” current heats tissue more gradually, leading to deeper and more reliable penetration. Thus, both contact and coaptive coagulation with monopolar electrosurgery are more effectively performed using “cut” current.
Since superficial-appearing endometriotic implants may extend deeply into the retroperitoneal tissues, I thermally ablate these lesions using a broad-surface electrode in contact with “cut” current. In contrast, I treat superficial implants on the ovarian cortex with “coag” current to minimize unwanted thermal injury to adjacent follicular tissue.
Interrupt the blood flow. Coaptive vessel sealing using any type of monopolar current may be ineffective if the blood flow remains uninterrupted. Unless a vessel is sufficiently squeezed before electricity is applied, current density is dramatically reduced by conduction in blood, and luminal temperatures undergo little change, as any heat is dissipated by convection. Deceived by the appearance of well-coagulated tissue, a surgeon may discover an alarmingly viable core at the time of incision. Regardless of the selected output current (that is, “cut,” “blend,” or “coag”), coaptive desiccation with monopolar electrosurgery is usually insufficient to reliably secure the uterine or ovarian vessels during hysterectomy and oophorectomy.
Achieve the appropriate cutting arc. Electrosurgical cutting (vaporization/ablation) is possible whenever voltage is sufficient to create an electrical spark between an electrode and underlying tissue; tissue cutting is more apt to occur if the arcing remains unabated. When it does, cellular water is superheated to temperatures greater than 600°C, causing explosive vaporization secondary to the production of highly disruptive pressure (since steam occupies 6 times the volume of liquid water).
Although all of the typical output currents (“cut,” “blend,” and “coag”) provide sufficient voltage to ionize the air gap and arc to tissue, the higher voltages of “blend” and “coag” create progressively wider zones of thermal damage at the margins of the incision (FIGURE 3) These effects are amplified by using broad-surface electrodes.
Wide versus narrow hemostasis. Using “blend” or “coag” current to cut in order to provide wider hemostasis can be helpful during myomectomy, as well as when operating down the broad ligament and along the vaginal fornices during hysterectomy, across vascular adhesions, and to clarify the space of Retzius in preparation for colposuspension and paravaginal repair. Higher-voltage currents also facilitate incision of tissues that have greater impedance, such as fatty or desiccated pedicles and adhesions.
On the other hand, it is more prudent to utilize the lower-voltage “cut” current via the edge of an electrode for electrosurgical incision whenever lateral thermal spread may pose extra liability to adjacent tissues, such as the ovarian cortex during cystectomy and the ureter or rectum during excision of endometriosis from the lateral pelvic sidewall or cul-de-sac.
Deceived by the appearance of well-coagulated tissue, surgeons may discover an alarmingly viable core at the time of incision.
- Fulguration is the use of high-voltage sparking produced by “coag” current to coagulate a broad surface with open bleeders. As opposed to the continuous arcing produced by “cut” current, the highly interrupted output of “coag” current causes the arcs to strike the tissue surface in a widely dispersed and random fashion. This leads to more rapid thermal change, creating a zone of superficial coagulation.
I typically employ fulguration to control small bleeders and vessels cut on end along the undersurface of the ovarian cortex during cystectomy, atop the myometrial bed during myomectomy, and alongside Cooper’s ligament during colposuspension.
Protect vital structures with short bursts of “coag” current. If bleeding near the bowel, bladder, or ureter cannot be controlled with pressure alone, carefully directed short bursts of noncontact “coag” current with a broad-surface electrode may help you attain effective hemostasis with the least-possible amount of electrosurgical penetration.
Despite the high-voltage output involved, fulguration is useless in a wet, conductive surgical field due to the random diffusion of current.
FIGURE 2 Range of output voltages
Relative changes in voltage and average current related to “cut,” “blend,” and “coag” currents.
FIGURE 3Zones of thermal damage related to voltage
Using a conventional electrosurgical generator for tissue cutting, the margin of thermal necrosis expands with increasing voltage.
Bipolar electrosurgery
Mechanism of action. Bipolar electrosurgery consolidates an active electrode and return electrode into an instrument with 2 small poles. These poles can be the tines of a forceps, blades of a scissors, or an electrode matched to a more proximal conductive collar separated by an insulator. The output typically used is the low-voltage “cut” current.
Advantages of bipolar energy. Localization of current between the poles offers distinct advantages. Thermal damage is generally limited to a discrete volume of tissue. A bipolar forceps can be used to coapt and thermally weld blood vessels. The concentrated current and small distance between the poles also make it possible to desiccate tissue that is immersed in fluid. This modality is less useful, however, when open blood vessels are retracted or tissue pedicles are very thick.
Because it tends to promote the flow of energy well beyond desiccation, an ammeter should not be used to determine the treatment endpoint for coagulation of blood vessels in the vicinity of vital tissue.
Know when to terminate current to prevent vital-tissue damage. Although the flow of current and primary thermal effects are restricted to the tissue between the poles, this does not eliminate the risk of thermal injury to tissue that is distant from the site of directed hemostasis. As current is applied between the poles, the intervening tissue gradually desiccates until it becomes thoroughly dehydrated. Desiccation is complete when the tissue whitens and visible steam emission stops. If the application of current continues, the heat spreads well beyond the electrical limits of the instrument.
This secondary thermal bloom is caused by the bubbling of steam into the tissue parenchyma as heat is rapidly generated (due to dry tissue’s high resistance to the flow of electrical current). This explains why structures such as the ureter or bowel may suffer irreversible thermal damage despite being at some distance from an operative or bleeding site.
Use of an in-line ammeter does not prevent this problem. Rather, it tends to promote the flow of energy well beyond desiccation. Consequently, an ammeter should not be used to determine the treatment endpoint for coagulation of blood vessels in the vicinity of vital tissue.
Whenever bipolar electrosurgery is used for hemostasis, unwanted thermal damage can be minimized by:
- terminating the flow of current at the end of the visible vapor phase,
- applying current in a pulsatile fashion to permit tissue cooling,
- avoiding the use of an in-line ammeter to determine the coagulation endpoint, and
- securing pedicles by a stepwise process that alternates between partial desiccation and incremental cutting (TABLE 1).
Since the rate of temperature generation is a direct function of the volume of tissue being desiccated, thermal spread can also be reduced by using the sides or tips of a slightly open forceps to press or lift, rather than coapt for hemostasis (FIGURE 4).
Free adherent tissue gently. As with contact monopolar coagulation, tissue between the electrodes of a bipolar instrument may become adherent during desiccation. Repeated attempts to shake the tissue free may lead to traumatic avulsion of a key vascular pedicle. A stuck vascular pedicle can usually be unglued by energizing the opened device while immersing it in a conductive irrigant, such as saline. Once the solution is boiled by the high current density between the electrodes, the mechanical action of bubbling is usually sufficient to atraumatically free the pedicle.
FIGURE 4 Minimizing bipolar thermal damage
Using contact rather than coaptation is one way to limit thermal injury during bipolar electrosurgery.TABLE 1
Minimizing bipolar thermal damage
|
Ultrasonic energy
Mechanism of action. Ultrasonic shears produce mechanical energy to cut and coagulate tissue. Housed in the hand piece of this laparoscopic device is a piezoelectric crystal that vibrates a titanium blade 55,500 times per second over a variable excursion of 50 microns to 100 microns. As energy is transmitted to tissue, hydrogen bonds of tissue proteins are ruptured, leading to a denatured protein coagulum without significant charring. The tissue cutting that occurs is secondary to mechanical vibration and cavitational fragmentation of tissue parenchyma.
Since ultrasonic energy does not generate the high temperatures created by electrosurgery, it is less dependable for deep-tissue coagulation. Rather, thermal change is gradual, requiring a modicum of patience.
Available instrument configurations include 5-mm curved or hook blades. In addition, 5-mm or 10-mm ligating and cutting shears coaptively desiccate and cut the tissue by securing it between a grooved plastic pad and the vibrating blade.
Tissue effects depend on interplay of factors. By using various combinations of blade shapes, blade excursions, and tissue tensions, surgeons can accomplish a variety of specific effects. Cutting velocity is directly proportional to blade excursion, tissue traction, and blade surface area (energy density), and is inversely related to tissue density and elasticity. Thus, the fastest cutting with the least amount of coagulation occurs when tissue is placed on tension and firmly squeezed, lifted, or rotated with the sharpest side of a blade set at maximum excursion.
Coagulation is the obverse of cutting: It is inversely related to tissue tension, blade sharpness, blade excursion, and cutting speed. Therefore, coagulation is best achieved by relaxing tension, minimizing blade excursion, and using a blunt edge or flattened blade surface.
A stuck vascular pedicle can usually be unglued by energizing the opened bipolar device while immersing it in a conductive irrigant.
Avoid excessive traction and torsion. Be mindful of the potential for premature incision of an incompletely coagulated tissue pedicle when excessive traction or torsion is applied. When used to coaptively desiccate and incise a vascular pedicle, ultrasonic energy should be applied patiently, taking great care to minimize tissue tension while using the broadest blade surface set to the lowest excursion (TABLE 2). Hemostatic incision is best ensured by first coagulating several overlapping areas along an untracted pedicle, limiting each application to the point of tissue blanching and initial vapor emission. Only then should the pedicle be incised by gradually lifting, squeezing, or rotating the distal device. In this fashion, ultrasonic energy can be successfully used to secure both the ovarian and uterine vessels.
TABLE 2
Factors likely to cause premature incision with ultrasonic energy
|
Summary
The use of electrical and ultrasonic energy during operative laparoscopy poses several challenges, including the reduction of unwanted thermal injury and the elimination of incomplete hemostasis.
Since the depth of penetration during monopolar electrosurgery is proportional to both output voltage and surface area, unwanted thermal change can be reduced by using the smallest electrode surface with “cut” current for tissue cutting, and “coag” current with a broad-surface electrode for contact or noncontact (fulguration) coagulation.
Bipolar electrosurgery is the preferred modality for coaptive desiccation of a vascular pedicle with electricity. Despite the isolation of current to the intervening tissue, surgeons must take steps to reduce the lateral percolation of heat into adjacent tissues.
Ultrasonic energy provides reliable coaptive hemostasis and incision with little tissue damage. However, the surgeon must be mindful of the forces that promulgate premature incision. Knowledge of the biophysical behavior of electrical and ultrasonic energy is a prelude to safety and efficacy during laparoscopic dissection.
Dr. Brill reports no affiliations or financial arrangements with any of the manufacturers of products mentioned in this article.
1. Brill AI. Energy systems for operative laparoscopy. J Am Assoc Gynecol Laparosc. 1998;5:335-345.
2. Friedman J. The technical aspects of electrosurgery. Oral Surg. 1973;36:177-187.
3. Honig WM. The mechanism of cutting in electrosurgery. IEEE Trans Biomed Eng BME. 1975;22:55-58.
4. Sigel B, Dunn MR. The mechanism of blood vessel closure by high frequency electrocoagulation. Surg Gynecol Obstet. 1965;121:823-831.
5. Phipps JH. Thermometry studies with bipolar diathermy during hysterectomy. Gynecol Laparosc. 1994;1:146-149.
6. Ryder RM, Hulka JF. Bladder and bowel injury after electrodesiccation with Kleppinger bipolar forceps: A clinicopathologic study. J Reprod Med. 1993;3:595-598.
7. McCarus SD. Physiologic mechanism of the ultrasonically activated scalpel. J Am Assoc Gynecol Laparosc. 1996;3:601-608.
1. Brill AI. Energy systems for operative laparoscopy. J Am Assoc Gynecol Laparosc. 1998;5:335-345.
2. Friedman J. The technical aspects of electrosurgery. Oral Surg. 1973;36:177-187.
3. Honig WM. The mechanism of cutting in electrosurgery. IEEE Trans Biomed Eng BME. 1975;22:55-58.
4. Sigel B, Dunn MR. The mechanism of blood vessel closure by high frequency electrocoagulation. Surg Gynecol Obstet. 1965;121:823-831.
5. Phipps JH. Thermometry studies with bipolar diathermy during hysterectomy. Gynecol Laparosc. 1994;1:146-149.
6. Ryder RM, Hulka JF. Bladder and bowel injury after electrodesiccation with Kleppinger bipolar forceps: A clinicopathologic study. J Reprod Med. 1993;3:595-598.
7. McCarus SD. Physiologic mechanism of the ultrasonically activated scalpel. J Am Assoc Gynecol Laparosc. 1996;3:601-608.