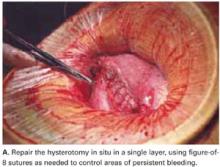
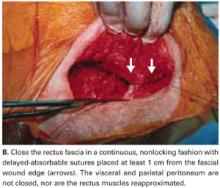
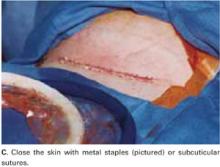
User login
Ovarian cancer: What can we expect of second-look laparotomy?
- Second-look laparotomy (SLL) is the only way to confirm complete pathologic response to ovarian cancer therapy.
- Offer SLL only to patients for whom results will affect decision-making—and only after discussion with the patient and a gynecologic oncologist.
- Although negative SLL findings confer improved prognosis, disease recurs in up to 60% of patients.
- Candidates should be in clinical remission as determined by physical examination, abdominopelvic imaging, and serum CA-125 determination.
Given the inability of noninvasive studies such as computed tomography, magnetic resonance imaging, and positron emission tomography to reliably detect small-volume and microscopic disease, second-look laparotomy (SLL) is the only technique capable of confirming a complete pathologic response to therapy.
Ob/Gyns involved in care of women with advanced ovarian cancer face the challenge of weighing the benefits of SLL against the potential morbidities of invasive surgery. This article describes those benefits, surgical technique, the prognostic significance of findings, and the status of salvage and consolidation therapies.
What SLL conveys
“Second look laparotomy” has rather loosely described many secondary surgeries for ovarian cancer, but we adopt the more rigorous definition: “a systematic surgical reexploration in asymptomatic patients who have no clinical evidence of tumor following initial surgery and completion of a planned program of chemotherapy.”1
Procedures to debulk recurrent or residual disease, relieve symptomatic tumor, or accomplish interval cytoreduction cannot be deemed second-look laparotomy.
Prognostic, therapeutic limitations complicate the decision
Although negative findings at SLL confer an improved prognosis, disease ultimately recurs in up to 60% of patients.2,3 Moreover, despite intensive research, consistently effective consolidation and salvage regimens remain elusive.
SLL may provide some information about prognosis, but that information is far from certain. Because of the cost and morbidity inherent in SLL, routine use has largely been limited to patients in clinical trials, where findings may serve as a surrogate endpoint for investigational therapies.
For these reasons, we strongly recommend careful discussion of this complex decision with patients prior to surgery, in consultation with a gynecologic oncologist.
Which patients are and are not candidates?
Candidates should be in clinical remission as determined by physical examination, abdominopelvic imaging, and serum CA-125 determination. Although SLL will detect residual disease in up to 50% of patients undergoing the procedure after primary chemotherapy, SLL is an imperfect method of determining the true response to therapy. Thus, it should be offered only to patients for whom results will influence clinical decision-making.
Patients with stage I disease treated with appropriate chemotherapy should not undergo SLL because of the low incidence of positive findings.4
Residual disease: 30% to 50%
Second-look laparotomy requires thorough inspection of the peritoneal cavity and retroperitoneum, but when properly performed on appropriate candidates, SLL detects residual disease in 30% to 50% of patients.2,5
Generally, stage and volume of residual disease at initial surgery are most closely correlated with findings. In a review of 31 series, patients with stage III and IV disease undergoing surgery had fewer negative SLLs (39% and 33%, respectively) than patients with stage I and II disease (81% and 69%, respectively).6 Similarly, in pooled data on 1,797 patients, 72% of those with no gross residual disease at the conclusion of primary surgery had negative findings at SLL, compared with 50% of those with optimal residual, and only 23% of those with suboptimal residual.6
Surgical technique
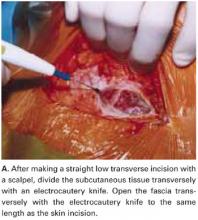
Surgery begins with a large vertical incision and involves the components listed in the TABLE.
If gross disease is apparent:
Consider surgical cytoreduction, which is typically performed at the surgeon’s discretion.
In the absence of gross tumor:
Use a 5-point strategy to search thoroughly for occult disease.
- Take washings for cytology from the abdomen and pelvis;
- lyse any adhesions to allow adequate examination of all peritoneal surfaces;
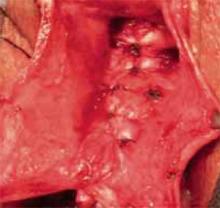
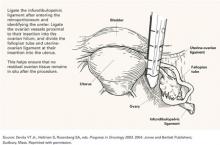
- obtain random biopsies from the pelvis, bladder serosa, vaginal cuff, culde-sac, paracolic gutters, and hemidiaphragms, as well as adhesions, sites of prior documented tumor, infundibulopelvic ligament pedicles, and areas suspicious for tumor recurrence;
- consider removing the uterus, adnexae, omentum, and appendix, if not done at the primary surgery; and
- sample any remaining pelvic and paraaortic lymph nodes.
Meticulous sampling is crucial
Although the number of biopsies performed at SLL varies widely by surgeon, meticulous sampling of peritoneal surfaces may be necessary to detect occult tumor. In cases of microscopic disease, fewer than 5% of biopsies may be positive for tumor.7 Not surprisingly, some studies have noted a significantly worse survival rate among patients deemed to be in complete pathologic remission who underwent fewer biopsies at the time of SLL.8
TABLE
Components of second-look laparotomy
| Vertical incision |
| Thorough inspection of abdomen and pelvis |
| Abdominopelvic washings for cytologic analysis |
| Complete adhesiolysis |
| Systematic biopsy of: |
|
| If necessary: Complete hysterectomy, salpingo-oophorectomy, omentectomy, and appendectomy |
| Pelvic and paraaortic lymph node sampling |
What do SLL findings predict?
Survival rates
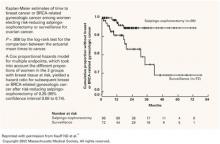
Women who achieve a complete pathologic response after primary chemotherapy have the greatest survival. Rubin et al3 noted a 10-year survival rate of 51% for 91 patients after negative SLL. Median survivals for patients with negative findings have been reported in excess of 70 months.7,9 Tuxen et al9 examined 242 patients after SLL and reported a median survival of 149 months for those with negative findings, versus 39 and 24 months for those with microscopic and gross disease, respectively (P<.0005>
Survival rates among women with negative findings were substantially higher than among patients with positive findings, even though the latter group received salvage chemotherapy.
Recurrence rates
In a review of 38 studies encompassing 1,511 patients, Barter and Barnes6 noted a 23% rate of recurrence among women with negative findings at SLL. Other studies from single institutions document recurrence rates approximating 50%.3,10 Patients experiencing recurrence after negative SLL have median survivals of 11 to 45 months.3,8,10-12
Gross versus microscopic disease
Studies comparing outcomes based on volume of disease detected at SLL have found statistically improved survival for patients without evidence of gross tumor.9,13-15 Podratz et al14 reported 4-year survival of 55% for women with microscopic findings versus 19% for those with gross disease (P<.01>
The presence of gross tumor at SLL indicates a grave prognosis; median survival ranges between 13 and 24 months.9,16,17 Nevertheless, several studies have shown that patients able to undergo debulking of all visible disease derive a survival benefit.4,15,18,19
Given the potential complications of extensive debulking surgery and lack of a proven survival benefit for patients unable to achieve complete cytoreduction, debulking should only be attempted if persistent disease is judged to be completely resectable.
When SLL is positive: Salvage therapy regimens
Many different salvage regimens for epithelial ovarian cancer have been investigated for use after positive second-look laparotomy, including intraperitoneal radioactive phosphorus (32P), systemic chemotherapy, whole abdominal radiation (WAR), hormonal therapy, and biologic response modifiers. Unfortunately, studies of salvage therapy tend to be retrospective, nonrandomized, and uncontrolled, and no proven regimen has yet been found.
Whole abdominal radiation
This modality appears to confer no definitive survival benefit and does produce toxicity. MacGibbon et al20 treated 51 patients with WAR for both salvage and consolidation. Of these, 27% could not complete treatment because of progressive disease, bowel perforation, myelosuppression, and bowel toxicity. An additional 24% required treatment delays because of hematologic and gastrointestinal toxicity. Among those completing the prescribed course of radiation, 6 developed late bowel symptoms, and 2 of these required surgical intervention to relieve bowel obstruction.
Other salvage therapies
Recently, Dowdy et al18 reported long-term follow-up for 145 patients with positive findings at SLL. Neither intraperitoneal 32P nor WAR provided a survival benefit. Multivariate analysis indicated that only grade and volume of residual disease following cytoreduction were associated with improved survival.
Other trials involving intraperitoneal interferon-alpha and carboplatin,21 and high-dose chemotherapy with autologous stem cell rescue22 have also failed to demonstrate any significant advantages in survival or rate of progression. An early phase II study of intraperitoneal paclitaxel showed promise: Markman et al23 noted a complete pathologic response in 61% of patients with microscopic disease at SLL, but only 4% of those with gross disease achieved a complete response.
Need for effective consolidation therapy
A critical component of cancer care is targeting patients at highest risk of recurrence for effective consolidation therapy. The factors most strongly correlated with disease progression are stage at diagnosis, tumor grade, and volume of residual disease after initial cytoreduction.
Many consolidation therapies have been described, including systemic and intraperitoneal chemotherapy, WAR, intraperitoneal 32P, and biologic response modifiers.
Significant risk of distant recurrences
Although most tumors recur in the abdomen and pelvis, approximately 30% recur at distant locations. For this reason, consider systemic treatment when planning the consolidation regimen. Bertucci et al22 studied systemic melphalan-based, high-dose chemotherapy with autologous stem cell rescue and noted a 5-year progression-free survival of 43% and overall survival of 75%.
Most therapies are localized. Most other studies have focused on therapies localized to the peritoneal cavity. Results for intraperitoneal 32P and WAR are mixed. Several different regimens of intraperitoneal chemotherapy have produced median disease-free survival rates of 18 to 41 months.24-27 In 1998, Barakat et al27 examined the use of intraperitoneal cisplatin and etoposide, reporting a statistically improved median disease-free survival for patients receiving intraperitoneal consolidation (median disease-free survival not yet reached), compared with patients treated by observation (28.5 months).
Bottom line
Second-look laparotomy reveals that approximately 50% of patients with a complete clinical response still harbor residual disease after primary chemotherapy. Even women who achieve a complete pathologic response have recurrence rates as high as 60%. While SLL can be a useful tool, the information it yields must be weighed against the potential morbidities of invasive surgery. Given its limited prognostic value, SLL should be offered only when results will influence clinical decision-making, or as part of a clinical trial.
Advocates of laparoscopy as a substitute for SLL report lower blood loss, shorter hospitalization, and decreased costs for laparoscopy.28
Clough et al29 performed the first study of second-look laparoscopy in 20 patients, using immediate laparotomy as a control. In 12 patients, adequate exploration was hindered by adhesions, and only 2 were able to undergo sufficient laparoscopic adhesiolysis. Overall, only 41% of patients could be completely explored at laparoscopy, versus 95% for laparotomy. However, obvious carcinomatosis was apparent in 3 patients at laparoscopy, rendering laparotomy unnecessary.
In general, laparoscopic second look has been reported to be a safe, feasible alternative to laparotomy. Although intraabdominal adhesions occur in as many as 70% of patients,30,31 complete laparoscopic evaluation may still be possible in up to 92%.32,33
How accurate?
Concerns remain about the accuracy of laparoscopic second look. Prior to 1985, several studies reported false-negative rates of 19% to 77%,33,34 although a 1999 study documented a false-negative rate of only 14%.29 The clinical impact of these false negatives is controversial. Some authors have reported no differences in clinical endpoints such as disease recurrence28 and overall survival35 for patients undergoing laparoscopy versus laparotomy. In contrast, a multivariate analysis by Gadducci et al10 showed a significantly prolonged disease-free interval for patients treated by laparotomy.
Switch to laparotomy for maximal cytoreduction
Laparoscopy may spare patients with obvious unresectable carcinomatosis a full laparotomy, though many patients will still require conversion to an open procedure to achieve maximal cytoreduction. Given these considerations, laparoscopy has only a limited role in second-look evaluation.
1. Rubin SC, Lewis JL, Jr. Second-look surgery in ovarian carcinoma. Crit Rev Oncol Hematol. 1988;8:75-91.
2. van der Burg ME. More than 20 years second-look surgery in advanced epithelial ovarian cancer: what did we learn? Ann Oncol. 1997;8:627-629.
3. Rubin SC, Randall TC, Armstrong KA, Chi DS, Hoskins WJ. Ten-year follow-up of ovarian cancer patients after second-look laparotomy with negative findings. Obstet Gynecol. 1999;93:21-24.
4. Hempling RE, Wesolowski JA, Piver MS. Second-look laparotomy in advanced ovarian cancer: a critical assessment of morbidity and impact on survival. Ann Surg Oncol. 1997;4:349-54.
5. Katsoulis M, Vorgias G, Panagiotides J, Dertimas B, Zis J. The prognostic significance of second-look laparotomy in advanced ovarian cancer. Eur J Gynaecol Oncol. 1997;18:200-202.
6. Barter JF, Barnes WA. Second-look laparotomy. In: Rubin SC, Sutton GP, eds. Ovarian Cancer. New York, NY: McGraw-Hill; 1993;269-300.
7. Friedman RL, Eisenkop SM, Wang HJ. Second-look laparotomy for ovarian cancer provides reliable prognostic information and improves survival. Gynecol Oncol. 1997;67:88-94.
8. Gershenson DM, Copeland LJ, Wharton JT, et al. Prognosis of surgically determined complete responders in advanced ovarian cancer. Cancer. 1985;55:1129-1135.
9. Tuxen MK, Strauss G, Lund B, Hansen M. The role of second-look laparotomy in the long-term survival in ovarian cancer. Ann Oncol. 1997;8:643-648.
10. Gadducci A, Sartori E, Maggino T, et al. Analysis of failures after negative second-look in patients with advanced ovarian cancer: an Italian multicenter study. Gynecol Oncol. 1998;68:150-155.
11. Rubin SC, Hoskins WJ, Hakes TB, Markman M, Cain JM, Lewis JL, Jr. Recurrence after negative second-look laparotomy for ovarian cancer: analysis of risk factors. Am J Obstet Gynecol. 1988;159:1094-1098.
12. Ghatage P, Krepart GV, Lotocki R. Factor analysis of false-negative second-look laparotomy. Gynecol Oncol. 1990;36:172-175.
13. Dauplat J, Ferriere JP, Gorbinet M, et al. Second-look laparotomy in managing epithelial ovarian carcinoma. Cancer. 1986;57:1627-1631.
14. Podratz KC, Schray MF, Wieand HS, et al. Evaluation of treatment and survival after positive second-look laparotomy. Gynecol Oncol. 1988;31:9-24.
15. Lippman SM, Alberts DS, Slymen DJ, et al. Second-look laparotomy in epithelial ovarian carcinoma. Prognostic factors associated with survival duration. Cancer. 1988;61:2571-2577.
16. Raju KS, McKinna JA, Barker GH, Wiltshaw E, Jones JM. Second-look operations in the planned management of advanced ovarian carcinoma. Am J Obstet Gynecol. 1982;144:650-654.
17. Williams L, Brunetto VL, Yordan E, DiSaia PJ, Creasman WT. Secondary cytoreductive surgery at second-look laparotomy in advanced ovarian cancer: a Gynecologic Oncology Group Study. Gynecol Oncol. 1997;66:171-178.
18. Dowdy SC, Constantinou CL, Hartmann LC, et al. Long-term follow-up of women with ovarian cancer after positive second-look laparotomy. Gynecol Oncol. 2003;91:563-568.
19. Schwartz PE, Smith JP. Second-look operations in ovarian cancer. Am J Obstet Gynecol. 1980;138:1124-1130.
20. MacGibbon A, Bucci J, MacLeod C, et al. Whole abdominal radiotherapy following second-look laparotomy for ovarian carcinoma. Gynecol Oncol. 1999;75:62-67.
21. Bruzzone M, Rubagotti A, Gadducci A, et al. Intraperitoneal carboplatin with or without interferonalpha in advanced ovarian cancer patients with minimal residual disease at second look: a prospective randomized trial of 111 patients. G.O.N.O. Gruppo Oncologic Nord Ovest. Gynecol Oncol. 1997;65:499-505.
22. Bertucci F, Viens P, Delpero JR, et al. High-dose melphalan-based chemotherapy and autologous stem cell transplantation after second look laparotomy in patients with chemosensitive advanced ovarian carcinoma: long-term results. Bone Marrow Transplant. 2000;26:61-67.
23. Markman M, Brady MF, Spirtos NM, Hanjani P, Rubin SC. Phase II trial of intraperitoneal paclitaxel in carcinoma of the ovary, tube, and peritoneum: a Gynecologic Oncology Group Study. J Clin Oncol. 1998;16:2620-2624.
24. Menczer J, Ben-Baruch G, Rizel S, Brenner H. Intraperitoneal cisplatin chemotherapy in ovarian carcinoma patients who are clinically in complete remission. Gynecol Oncol. 1992;46:222-225.
25. Tarraza HM,, Jr, Boyce CR, Smith WG, Jones MA. Consolidation intraperitoneal chemotherapy in epithelial ovarian cancer patients following negative second-look laparotomy. Gynecol Oncol. 1993;50:287-290.
26. Dufour P, Bergerat JP, Barats JC, et al. Intraperitoneal mitoxantrone as consolidation treatment for patients with ovarian carcinoma in pathologic complete remission. Cancer. 1994;73:1865-1869.
27. Barakat RR, Almadrones L, Venkatraman ES, et al. A phase II trial of intraperitoneal cisplatin and etoposide as consolidation therapy in patients with Stage II-IV epithelial ovarian cancer following negative surgical assessment. Gynecol Oncol. 1998;69:17-22.
28. Abu-Rustum NR, Barakat RR, Siegel PL, Venkatraman E, Curtin JP, Hoskins WJ. Second-look operation for epithelial ovarian cancer: laparoscopy or laparotomy? Obstet Gynecol. 1996;88:549-553.
29. Clough KB, Ladonne JM, Nos C, Renolleau C, Validire P, Durand JC. Second look for ovarian cancer: laparoscopy or laparotomy? A prospective comparative study. Gynecol Oncol. 1999;72:411-417.
30. Spinelli P, Luini A, Pizzetti P, de Palo GM. Laparoscopy in staging and restaging of 95 patients with ovarian carcinoma. Tumori. 1976;62:493-501.
31. Smith WG, Day TG, Jr, Smith JP. The use of laparoscopy to determine the results of chemotherapy for ovarian cancer. J Reprod Med. 1977;18:257-260.
32. Canis M, Chapron C, Mage G, et al. [Technique and preliminary results in second-look laparoscopy in epithelial malignant ovarian tumors]. J Gynecol Obstet Biol Reprod (Paris). 1992;21:655-663.
33. Quinn MA, Bishop GJ, Campbell JJ, Rodgerson J, Pepperell RJ. Laparoscopic follow-up of patients with ovarian carcinoma. Br J Obstet Gynaecol. 1980;87:1132-1139.
34. Xygakis AM, Politis GS, Michalas SP, Kaskarelis DB. Second-look laparoscopy in ovarian cancer. J Reprod Med. 1984;29:583-585.
35. Nicoletto MO, Tumolo S, Talamini R, et al. Surgical second look in ovarian cancer: a randomized study in patients with laparoscopic complete remission—a Northeastern Oncology Cooperative Group-Ovarian Cancer Cooperative Group Study. J Clin Oncol. 1997;15:994-999.
- Second-look laparotomy (SLL) is the only way to confirm complete pathologic response to ovarian cancer therapy.
- Offer SLL only to patients for whom results will affect decision-making—and only after discussion with the patient and a gynecologic oncologist.
- Although negative SLL findings confer improved prognosis, disease recurs in up to 60% of patients.
- Candidates should be in clinical remission as determined by physical examination, abdominopelvic imaging, and serum CA-125 determination.
Given the inability of noninvasive studies such as computed tomography, magnetic resonance imaging, and positron emission tomography to reliably detect small-volume and microscopic disease, second-look laparotomy (SLL) is the only technique capable of confirming a complete pathologic response to therapy.
Ob/Gyns involved in care of women with advanced ovarian cancer face the challenge of weighing the benefits of SLL against the potential morbidities of invasive surgery. This article describes those benefits, surgical technique, the prognostic significance of findings, and the status of salvage and consolidation therapies.
What SLL conveys
“Second look laparotomy” has rather loosely described many secondary surgeries for ovarian cancer, but we adopt the more rigorous definition: “a systematic surgical reexploration in asymptomatic patients who have no clinical evidence of tumor following initial surgery and completion of a planned program of chemotherapy.”1
Procedures to debulk recurrent or residual disease, relieve symptomatic tumor, or accomplish interval cytoreduction cannot be deemed second-look laparotomy.
Prognostic, therapeutic limitations complicate the decision
Although negative findings at SLL confer an improved prognosis, disease ultimately recurs in up to 60% of patients.2,3 Moreover, despite intensive research, consistently effective consolidation and salvage regimens remain elusive.
SLL may provide some information about prognosis, but that information is far from certain. Because of the cost and morbidity inherent in SLL, routine use has largely been limited to patients in clinical trials, where findings may serve as a surrogate endpoint for investigational therapies.
For these reasons, we strongly recommend careful discussion of this complex decision with patients prior to surgery, in consultation with a gynecologic oncologist.
Which patients are and are not candidates?
Candidates should be in clinical remission as determined by physical examination, abdominopelvic imaging, and serum CA-125 determination. Although SLL will detect residual disease in up to 50% of patients undergoing the procedure after primary chemotherapy, SLL is an imperfect method of determining the true response to therapy. Thus, it should be offered only to patients for whom results will influence clinical decision-making.
Patients with stage I disease treated with appropriate chemotherapy should not undergo SLL because of the low incidence of positive findings.4
Residual disease: 30% to 50%
Second-look laparotomy requires thorough inspection of the peritoneal cavity and retroperitoneum, but when properly performed on appropriate candidates, SLL detects residual disease in 30% to 50% of patients.2,5
Generally, stage and volume of residual disease at initial surgery are most closely correlated with findings. In a review of 31 series, patients with stage III and IV disease undergoing surgery had fewer negative SLLs (39% and 33%, respectively) than patients with stage I and II disease (81% and 69%, respectively).6 Similarly, in pooled data on 1,797 patients, 72% of those with no gross residual disease at the conclusion of primary surgery had negative findings at SLL, compared with 50% of those with optimal residual, and only 23% of those with suboptimal residual.6
Surgical technique
Surgery begins with a large vertical incision and involves the components listed in the TABLE.
If gross disease is apparent:
Consider surgical cytoreduction, which is typically performed at the surgeon’s discretion.
In the absence of gross tumor:
Use a 5-point strategy to search thoroughly for occult disease.
- Take washings for cytology from the abdomen and pelvis;
- lyse any adhesions to allow adequate examination of all peritoneal surfaces;
- obtain random biopsies from the pelvis, bladder serosa, vaginal cuff, culde-sac, paracolic gutters, and hemidiaphragms, as well as adhesions, sites of prior documented tumor, infundibulopelvic ligament pedicles, and areas suspicious for tumor recurrence;
- consider removing the uterus, adnexae, omentum, and appendix, if not done at the primary surgery; and
- sample any remaining pelvic and paraaortic lymph nodes.
Meticulous sampling is crucial
Although the number of biopsies performed at SLL varies widely by surgeon, meticulous sampling of peritoneal surfaces may be necessary to detect occult tumor. In cases of microscopic disease, fewer than 5% of biopsies may be positive for tumor.7 Not surprisingly, some studies have noted a significantly worse survival rate among patients deemed to be in complete pathologic remission who underwent fewer biopsies at the time of SLL.8
TABLE
Components of second-look laparotomy
| Vertical incision |
| Thorough inspection of abdomen and pelvis |
| Abdominopelvic washings for cytologic analysis |
| Complete adhesiolysis |
| Systematic biopsy of: |
|
| If necessary: Complete hysterectomy, salpingo-oophorectomy, omentectomy, and appendectomy |
| Pelvic and paraaortic lymph node sampling |
What do SLL findings predict?
Survival rates
Women who achieve a complete pathologic response after primary chemotherapy have the greatest survival. Rubin et al3 noted a 10-year survival rate of 51% for 91 patients after negative SLL. Median survivals for patients with negative findings have been reported in excess of 70 months.7,9 Tuxen et al9 examined 242 patients after SLL and reported a median survival of 149 months for those with negative findings, versus 39 and 24 months for those with microscopic and gross disease, respectively (P<.0005>
Survival rates among women with negative findings were substantially higher than among patients with positive findings, even though the latter group received salvage chemotherapy.
Recurrence rates
In a review of 38 studies encompassing 1,511 patients, Barter and Barnes6 noted a 23% rate of recurrence among women with negative findings at SLL. Other studies from single institutions document recurrence rates approximating 50%.3,10 Patients experiencing recurrence after negative SLL have median survivals of 11 to 45 months.3,8,10-12
Gross versus microscopic disease
Studies comparing outcomes based on volume of disease detected at SLL have found statistically improved survival for patients without evidence of gross tumor.9,13-15 Podratz et al14 reported 4-year survival of 55% for women with microscopic findings versus 19% for those with gross disease (P<.01>
The presence of gross tumor at SLL indicates a grave prognosis; median survival ranges between 13 and 24 months.9,16,17 Nevertheless, several studies have shown that patients able to undergo debulking of all visible disease derive a survival benefit.4,15,18,19
Given the potential complications of extensive debulking surgery and lack of a proven survival benefit for patients unable to achieve complete cytoreduction, debulking should only be attempted if persistent disease is judged to be completely resectable.
When SLL is positive: Salvage therapy regimens
Many different salvage regimens for epithelial ovarian cancer have been investigated for use after positive second-look laparotomy, including intraperitoneal radioactive phosphorus (32P), systemic chemotherapy, whole abdominal radiation (WAR), hormonal therapy, and biologic response modifiers. Unfortunately, studies of salvage therapy tend to be retrospective, nonrandomized, and uncontrolled, and no proven regimen has yet been found.
Whole abdominal radiation
This modality appears to confer no definitive survival benefit and does produce toxicity. MacGibbon et al20 treated 51 patients with WAR for both salvage and consolidation. Of these, 27% could not complete treatment because of progressive disease, bowel perforation, myelosuppression, and bowel toxicity. An additional 24% required treatment delays because of hematologic and gastrointestinal toxicity. Among those completing the prescribed course of radiation, 6 developed late bowel symptoms, and 2 of these required surgical intervention to relieve bowel obstruction.
Other salvage therapies
Recently, Dowdy et al18 reported long-term follow-up for 145 patients with positive findings at SLL. Neither intraperitoneal 32P nor WAR provided a survival benefit. Multivariate analysis indicated that only grade and volume of residual disease following cytoreduction were associated with improved survival.
Other trials involving intraperitoneal interferon-alpha and carboplatin,21 and high-dose chemotherapy with autologous stem cell rescue22 have also failed to demonstrate any significant advantages in survival or rate of progression. An early phase II study of intraperitoneal paclitaxel showed promise: Markman et al23 noted a complete pathologic response in 61% of patients with microscopic disease at SLL, but only 4% of those with gross disease achieved a complete response.
Need for effective consolidation therapy
A critical component of cancer care is targeting patients at highest risk of recurrence for effective consolidation therapy. The factors most strongly correlated with disease progression are stage at diagnosis, tumor grade, and volume of residual disease after initial cytoreduction.
Many consolidation therapies have been described, including systemic and intraperitoneal chemotherapy, WAR, intraperitoneal 32P, and biologic response modifiers.
Significant risk of distant recurrences
Although most tumors recur in the abdomen and pelvis, approximately 30% recur at distant locations. For this reason, consider systemic treatment when planning the consolidation regimen. Bertucci et al22 studied systemic melphalan-based, high-dose chemotherapy with autologous stem cell rescue and noted a 5-year progression-free survival of 43% and overall survival of 75%.
Most therapies are localized. Most other studies have focused on therapies localized to the peritoneal cavity. Results for intraperitoneal 32P and WAR are mixed. Several different regimens of intraperitoneal chemotherapy have produced median disease-free survival rates of 18 to 41 months.24-27 In 1998, Barakat et al27 examined the use of intraperitoneal cisplatin and etoposide, reporting a statistically improved median disease-free survival for patients receiving intraperitoneal consolidation (median disease-free survival not yet reached), compared with patients treated by observation (28.5 months).
Bottom line
Second-look laparotomy reveals that approximately 50% of patients with a complete clinical response still harbor residual disease after primary chemotherapy. Even women who achieve a complete pathologic response have recurrence rates as high as 60%. While SLL can be a useful tool, the information it yields must be weighed against the potential morbidities of invasive surgery. Given its limited prognostic value, SLL should be offered only when results will influence clinical decision-making, or as part of a clinical trial.
Advocates of laparoscopy as a substitute for SLL report lower blood loss, shorter hospitalization, and decreased costs for laparoscopy.28
Clough et al29 performed the first study of second-look laparoscopy in 20 patients, using immediate laparotomy as a control. In 12 patients, adequate exploration was hindered by adhesions, and only 2 were able to undergo sufficient laparoscopic adhesiolysis. Overall, only 41% of patients could be completely explored at laparoscopy, versus 95% for laparotomy. However, obvious carcinomatosis was apparent in 3 patients at laparoscopy, rendering laparotomy unnecessary.
In general, laparoscopic second look has been reported to be a safe, feasible alternative to laparotomy. Although intraabdominal adhesions occur in as many as 70% of patients,30,31 complete laparoscopic evaluation may still be possible in up to 92%.32,33
How accurate?
Concerns remain about the accuracy of laparoscopic second look. Prior to 1985, several studies reported false-negative rates of 19% to 77%,33,34 although a 1999 study documented a false-negative rate of only 14%.29 The clinical impact of these false negatives is controversial. Some authors have reported no differences in clinical endpoints such as disease recurrence28 and overall survival35 for patients undergoing laparoscopy versus laparotomy. In contrast, a multivariate analysis by Gadducci et al10 showed a significantly prolonged disease-free interval for patients treated by laparotomy.
Switch to laparotomy for maximal cytoreduction
Laparoscopy may spare patients with obvious unresectable carcinomatosis a full laparotomy, though many patients will still require conversion to an open procedure to achieve maximal cytoreduction. Given these considerations, laparoscopy has only a limited role in second-look evaluation.
- Second-look laparotomy (SLL) is the only way to confirm complete pathologic response to ovarian cancer therapy.
- Offer SLL only to patients for whom results will affect decision-making—and only after discussion with the patient and a gynecologic oncologist.
- Although negative SLL findings confer improved prognosis, disease recurs in up to 60% of patients.
- Candidates should be in clinical remission as determined by physical examination, abdominopelvic imaging, and serum CA-125 determination.
Given the inability of noninvasive studies such as computed tomography, magnetic resonance imaging, and positron emission tomography to reliably detect small-volume and microscopic disease, second-look laparotomy (SLL) is the only technique capable of confirming a complete pathologic response to therapy.
Ob/Gyns involved in care of women with advanced ovarian cancer face the challenge of weighing the benefits of SLL against the potential morbidities of invasive surgery. This article describes those benefits, surgical technique, the prognostic significance of findings, and the status of salvage and consolidation therapies.
What SLL conveys
“Second look laparotomy” has rather loosely described many secondary surgeries for ovarian cancer, but we adopt the more rigorous definition: “a systematic surgical reexploration in asymptomatic patients who have no clinical evidence of tumor following initial surgery and completion of a planned program of chemotherapy.”1
Procedures to debulk recurrent or residual disease, relieve symptomatic tumor, or accomplish interval cytoreduction cannot be deemed second-look laparotomy.
Prognostic, therapeutic limitations complicate the decision
Although negative findings at SLL confer an improved prognosis, disease ultimately recurs in up to 60% of patients.2,3 Moreover, despite intensive research, consistently effective consolidation and salvage regimens remain elusive.
SLL may provide some information about prognosis, but that information is far from certain. Because of the cost and morbidity inherent in SLL, routine use has largely been limited to patients in clinical trials, where findings may serve as a surrogate endpoint for investigational therapies.
For these reasons, we strongly recommend careful discussion of this complex decision with patients prior to surgery, in consultation with a gynecologic oncologist.
Which patients are and are not candidates?
Candidates should be in clinical remission as determined by physical examination, abdominopelvic imaging, and serum CA-125 determination. Although SLL will detect residual disease in up to 50% of patients undergoing the procedure after primary chemotherapy, SLL is an imperfect method of determining the true response to therapy. Thus, it should be offered only to patients for whom results will influence clinical decision-making.
Patients with stage I disease treated with appropriate chemotherapy should not undergo SLL because of the low incidence of positive findings.4
Residual disease: 30% to 50%
Second-look laparotomy requires thorough inspection of the peritoneal cavity and retroperitoneum, but when properly performed on appropriate candidates, SLL detects residual disease in 30% to 50% of patients.2,5
Generally, stage and volume of residual disease at initial surgery are most closely correlated with findings. In a review of 31 series, patients with stage III and IV disease undergoing surgery had fewer negative SLLs (39% and 33%, respectively) than patients with stage I and II disease (81% and 69%, respectively).6 Similarly, in pooled data on 1,797 patients, 72% of those with no gross residual disease at the conclusion of primary surgery had negative findings at SLL, compared with 50% of those with optimal residual, and only 23% of those with suboptimal residual.6
Surgical technique
Surgery begins with a large vertical incision and involves the components listed in the TABLE.
If gross disease is apparent:
Consider surgical cytoreduction, which is typically performed at the surgeon’s discretion.
In the absence of gross tumor:
Use a 5-point strategy to search thoroughly for occult disease.
- Take washings for cytology from the abdomen and pelvis;
- lyse any adhesions to allow adequate examination of all peritoneal surfaces;
- obtain random biopsies from the pelvis, bladder serosa, vaginal cuff, culde-sac, paracolic gutters, and hemidiaphragms, as well as adhesions, sites of prior documented tumor, infundibulopelvic ligament pedicles, and areas suspicious for tumor recurrence;
- consider removing the uterus, adnexae, omentum, and appendix, if not done at the primary surgery; and
- sample any remaining pelvic and paraaortic lymph nodes.
Meticulous sampling is crucial
Although the number of biopsies performed at SLL varies widely by surgeon, meticulous sampling of peritoneal surfaces may be necessary to detect occult tumor. In cases of microscopic disease, fewer than 5% of biopsies may be positive for tumor.7 Not surprisingly, some studies have noted a significantly worse survival rate among patients deemed to be in complete pathologic remission who underwent fewer biopsies at the time of SLL.8
TABLE
Components of second-look laparotomy
| Vertical incision |
| Thorough inspection of abdomen and pelvis |
| Abdominopelvic washings for cytologic analysis |
| Complete adhesiolysis |
| Systematic biopsy of: |
|
| If necessary: Complete hysterectomy, salpingo-oophorectomy, omentectomy, and appendectomy |
| Pelvic and paraaortic lymph node sampling |
What do SLL findings predict?
Survival rates
Women who achieve a complete pathologic response after primary chemotherapy have the greatest survival. Rubin et al3 noted a 10-year survival rate of 51% for 91 patients after negative SLL. Median survivals for patients with negative findings have been reported in excess of 70 months.7,9 Tuxen et al9 examined 242 patients after SLL and reported a median survival of 149 months for those with negative findings, versus 39 and 24 months for those with microscopic and gross disease, respectively (P<.0005>
Survival rates among women with negative findings were substantially higher than among patients with positive findings, even though the latter group received salvage chemotherapy.
Recurrence rates
In a review of 38 studies encompassing 1,511 patients, Barter and Barnes6 noted a 23% rate of recurrence among women with negative findings at SLL. Other studies from single institutions document recurrence rates approximating 50%.3,10 Patients experiencing recurrence after negative SLL have median survivals of 11 to 45 months.3,8,10-12
Gross versus microscopic disease
Studies comparing outcomes based on volume of disease detected at SLL have found statistically improved survival for patients without evidence of gross tumor.9,13-15 Podratz et al14 reported 4-year survival of 55% for women with microscopic findings versus 19% for those with gross disease (P<.01>
The presence of gross tumor at SLL indicates a grave prognosis; median survival ranges between 13 and 24 months.9,16,17 Nevertheless, several studies have shown that patients able to undergo debulking of all visible disease derive a survival benefit.4,15,18,19
Given the potential complications of extensive debulking surgery and lack of a proven survival benefit for patients unable to achieve complete cytoreduction, debulking should only be attempted if persistent disease is judged to be completely resectable.
When SLL is positive: Salvage therapy regimens
Many different salvage regimens for epithelial ovarian cancer have been investigated for use after positive second-look laparotomy, including intraperitoneal radioactive phosphorus (32P), systemic chemotherapy, whole abdominal radiation (WAR), hormonal therapy, and biologic response modifiers. Unfortunately, studies of salvage therapy tend to be retrospective, nonrandomized, and uncontrolled, and no proven regimen has yet been found.
Whole abdominal radiation
This modality appears to confer no definitive survival benefit and does produce toxicity. MacGibbon et al20 treated 51 patients with WAR for both salvage and consolidation. Of these, 27% could not complete treatment because of progressive disease, bowel perforation, myelosuppression, and bowel toxicity. An additional 24% required treatment delays because of hematologic and gastrointestinal toxicity. Among those completing the prescribed course of radiation, 6 developed late bowel symptoms, and 2 of these required surgical intervention to relieve bowel obstruction.
Other salvage therapies
Recently, Dowdy et al18 reported long-term follow-up for 145 patients with positive findings at SLL. Neither intraperitoneal 32P nor WAR provided a survival benefit. Multivariate analysis indicated that only grade and volume of residual disease following cytoreduction were associated with improved survival.
Other trials involving intraperitoneal interferon-alpha and carboplatin,21 and high-dose chemotherapy with autologous stem cell rescue22 have also failed to demonstrate any significant advantages in survival or rate of progression. An early phase II study of intraperitoneal paclitaxel showed promise: Markman et al23 noted a complete pathologic response in 61% of patients with microscopic disease at SLL, but only 4% of those with gross disease achieved a complete response.
Need for effective consolidation therapy
A critical component of cancer care is targeting patients at highest risk of recurrence for effective consolidation therapy. The factors most strongly correlated with disease progression are stage at diagnosis, tumor grade, and volume of residual disease after initial cytoreduction.
Many consolidation therapies have been described, including systemic and intraperitoneal chemotherapy, WAR, intraperitoneal 32P, and biologic response modifiers.
Significant risk of distant recurrences
Although most tumors recur in the abdomen and pelvis, approximately 30% recur at distant locations. For this reason, consider systemic treatment when planning the consolidation regimen. Bertucci et al22 studied systemic melphalan-based, high-dose chemotherapy with autologous stem cell rescue and noted a 5-year progression-free survival of 43% and overall survival of 75%.
Most therapies are localized. Most other studies have focused on therapies localized to the peritoneal cavity. Results for intraperitoneal 32P and WAR are mixed. Several different regimens of intraperitoneal chemotherapy have produced median disease-free survival rates of 18 to 41 months.24-27 In 1998, Barakat et al27 examined the use of intraperitoneal cisplatin and etoposide, reporting a statistically improved median disease-free survival for patients receiving intraperitoneal consolidation (median disease-free survival not yet reached), compared with patients treated by observation (28.5 months).
Bottom line
Second-look laparotomy reveals that approximately 50% of patients with a complete clinical response still harbor residual disease after primary chemotherapy. Even women who achieve a complete pathologic response have recurrence rates as high as 60%. While SLL can be a useful tool, the information it yields must be weighed against the potential morbidities of invasive surgery. Given its limited prognostic value, SLL should be offered only when results will influence clinical decision-making, or as part of a clinical trial.
Advocates of laparoscopy as a substitute for SLL report lower blood loss, shorter hospitalization, and decreased costs for laparoscopy.28
Clough et al29 performed the first study of second-look laparoscopy in 20 patients, using immediate laparotomy as a control. In 12 patients, adequate exploration was hindered by adhesions, and only 2 were able to undergo sufficient laparoscopic adhesiolysis. Overall, only 41% of patients could be completely explored at laparoscopy, versus 95% for laparotomy. However, obvious carcinomatosis was apparent in 3 patients at laparoscopy, rendering laparotomy unnecessary.
In general, laparoscopic second look has been reported to be a safe, feasible alternative to laparotomy. Although intraabdominal adhesions occur in as many as 70% of patients,30,31 complete laparoscopic evaluation may still be possible in up to 92%.32,33
How accurate?
Concerns remain about the accuracy of laparoscopic second look. Prior to 1985, several studies reported false-negative rates of 19% to 77%,33,34 although a 1999 study documented a false-negative rate of only 14%.29 The clinical impact of these false negatives is controversial. Some authors have reported no differences in clinical endpoints such as disease recurrence28 and overall survival35 for patients undergoing laparoscopy versus laparotomy. In contrast, a multivariate analysis by Gadducci et al10 showed a significantly prolonged disease-free interval for patients treated by laparotomy.
Switch to laparotomy for maximal cytoreduction
Laparoscopy may spare patients with obvious unresectable carcinomatosis a full laparotomy, though many patients will still require conversion to an open procedure to achieve maximal cytoreduction. Given these considerations, laparoscopy has only a limited role in second-look evaluation.
1. Rubin SC, Lewis JL, Jr. Second-look surgery in ovarian carcinoma. Crit Rev Oncol Hematol. 1988;8:75-91.
2. van der Burg ME. More than 20 years second-look surgery in advanced epithelial ovarian cancer: what did we learn? Ann Oncol. 1997;8:627-629.
3. Rubin SC, Randall TC, Armstrong KA, Chi DS, Hoskins WJ. Ten-year follow-up of ovarian cancer patients after second-look laparotomy with negative findings. Obstet Gynecol. 1999;93:21-24.
4. Hempling RE, Wesolowski JA, Piver MS. Second-look laparotomy in advanced ovarian cancer: a critical assessment of morbidity and impact on survival. Ann Surg Oncol. 1997;4:349-54.
5. Katsoulis M, Vorgias G, Panagiotides J, Dertimas B, Zis J. The prognostic significance of second-look laparotomy in advanced ovarian cancer. Eur J Gynaecol Oncol. 1997;18:200-202.
6. Barter JF, Barnes WA. Second-look laparotomy. In: Rubin SC, Sutton GP, eds. Ovarian Cancer. New York, NY: McGraw-Hill; 1993;269-300.
7. Friedman RL, Eisenkop SM, Wang HJ. Second-look laparotomy for ovarian cancer provides reliable prognostic information and improves survival. Gynecol Oncol. 1997;67:88-94.
8. Gershenson DM, Copeland LJ, Wharton JT, et al. Prognosis of surgically determined complete responders in advanced ovarian cancer. Cancer. 1985;55:1129-1135.
9. Tuxen MK, Strauss G, Lund B, Hansen M. The role of second-look laparotomy in the long-term survival in ovarian cancer. Ann Oncol. 1997;8:643-648.
10. Gadducci A, Sartori E, Maggino T, et al. Analysis of failures after negative second-look in patients with advanced ovarian cancer: an Italian multicenter study. Gynecol Oncol. 1998;68:150-155.
11. Rubin SC, Hoskins WJ, Hakes TB, Markman M, Cain JM, Lewis JL, Jr. Recurrence after negative second-look laparotomy for ovarian cancer: analysis of risk factors. Am J Obstet Gynecol. 1988;159:1094-1098.
12. Ghatage P, Krepart GV, Lotocki R. Factor analysis of false-negative second-look laparotomy. Gynecol Oncol. 1990;36:172-175.
13. Dauplat J, Ferriere JP, Gorbinet M, et al. Second-look laparotomy in managing epithelial ovarian carcinoma. Cancer. 1986;57:1627-1631.
14. Podratz KC, Schray MF, Wieand HS, et al. Evaluation of treatment and survival after positive second-look laparotomy. Gynecol Oncol. 1988;31:9-24.
15. Lippman SM, Alberts DS, Slymen DJ, et al. Second-look laparotomy in epithelial ovarian carcinoma. Prognostic factors associated with survival duration. Cancer. 1988;61:2571-2577.
16. Raju KS, McKinna JA, Barker GH, Wiltshaw E, Jones JM. Second-look operations in the planned management of advanced ovarian carcinoma. Am J Obstet Gynecol. 1982;144:650-654.
17. Williams L, Brunetto VL, Yordan E, DiSaia PJ, Creasman WT. Secondary cytoreductive surgery at second-look laparotomy in advanced ovarian cancer: a Gynecologic Oncology Group Study. Gynecol Oncol. 1997;66:171-178.
18. Dowdy SC, Constantinou CL, Hartmann LC, et al. Long-term follow-up of women with ovarian cancer after positive second-look laparotomy. Gynecol Oncol. 2003;91:563-568.
19. Schwartz PE, Smith JP. Second-look operations in ovarian cancer. Am J Obstet Gynecol. 1980;138:1124-1130.
20. MacGibbon A, Bucci J, MacLeod C, et al. Whole abdominal radiotherapy following second-look laparotomy for ovarian carcinoma. Gynecol Oncol. 1999;75:62-67.
21. Bruzzone M, Rubagotti A, Gadducci A, et al. Intraperitoneal carboplatin with or without interferonalpha in advanced ovarian cancer patients with minimal residual disease at second look: a prospective randomized trial of 111 patients. G.O.N.O. Gruppo Oncologic Nord Ovest. Gynecol Oncol. 1997;65:499-505.
22. Bertucci F, Viens P, Delpero JR, et al. High-dose melphalan-based chemotherapy and autologous stem cell transplantation after second look laparotomy in patients with chemosensitive advanced ovarian carcinoma: long-term results. Bone Marrow Transplant. 2000;26:61-67.
23. Markman M, Brady MF, Spirtos NM, Hanjani P, Rubin SC. Phase II trial of intraperitoneal paclitaxel in carcinoma of the ovary, tube, and peritoneum: a Gynecologic Oncology Group Study. J Clin Oncol. 1998;16:2620-2624.
24. Menczer J, Ben-Baruch G, Rizel S, Brenner H. Intraperitoneal cisplatin chemotherapy in ovarian carcinoma patients who are clinically in complete remission. Gynecol Oncol. 1992;46:222-225.
25. Tarraza HM,, Jr, Boyce CR, Smith WG, Jones MA. Consolidation intraperitoneal chemotherapy in epithelial ovarian cancer patients following negative second-look laparotomy. Gynecol Oncol. 1993;50:287-290.
26. Dufour P, Bergerat JP, Barats JC, et al. Intraperitoneal mitoxantrone as consolidation treatment for patients with ovarian carcinoma in pathologic complete remission. Cancer. 1994;73:1865-1869.
27. Barakat RR, Almadrones L, Venkatraman ES, et al. A phase II trial of intraperitoneal cisplatin and etoposide as consolidation therapy in patients with Stage II-IV epithelial ovarian cancer following negative surgical assessment. Gynecol Oncol. 1998;69:17-22.
28. Abu-Rustum NR, Barakat RR, Siegel PL, Venkatraman E, Curtin JP, Hoskins WJ. Second-look operation for epithelial ovarian cancer: laparoscopy or laparotomy? Obstet Gynecol. 1996;88:549-553.
29. Clough KB, Ladonne JM, Nos C, Renolleau C, Validire P, Durand JC. Second look for ovarian cancer: laparoscopy or laparotomy? A prospective comparative study. Gynecol Oncol. 1999;72:411-417.
30. Spinelli P, Luini A, Pizzetti P, de Palo GM. Laparoscopy in staging and restaging of 95 patients with ovarian carcinoma. Tumori. 1976;62:493-501.
31. Smith WG, Day TG, Jr, Smith JP. The use of laparoscopy to determine the results of chemotherapy for ovarian cancer. J Reprod Med. 1977;18:257-260.
32. Canis M, Chapron C, Mage G, et al. [Technique and preliminary results in second-look laparoscopy in epithelial malignant ovarian tumors]. J Gynecol Obstet Biol Reprod (Paris). 1992;21:655-663.
33. Quinn MA, Bishop GJ, Campbell JJ, Rodgerson J, Pepperell RJ. Laparoscopic follow-up of patients with ovarian carcinoma. Br J Obstet Gynaecol. 1980;87:1132-1139.
34. Xygakis AM, Politis GS, Michalas SP, Kaskarelis DB. Second-look laparoscopy in ovarian cancer. J Reprod Med. 1984;29:583-585.
35. Nicoletto MO, Tumolo S, Talamini R, et al. Surgical second look in ovarian cancer: a randomized study in patients with laparoscopic complete remission—a Northeastern Oncology Cooperative Group-Ovarian Cancer Cooperative Group Study. J Clin Oncol. 1997;15:994-999.
1. Rubin SC, Lewis JL, Jr. Second-look surgery in ovarian carcinoma. Crit Rev Oncol Hematol. 1988;8:75-91.
2. van der Burg ME. More than 20 years second-look surgery in advanced epithelial ovarian cancer: what did we learn? Ann Oncol. 1997;8:627-629.
3. Rubin SC, Randall TC, Armstrong KA, Chi DS, Hoskins WJ. Ten-year follow-up of ovarian cancer patients after second-look laparotomy with negative findings. Obstet Gynecol. 1999;93:21-24.
4. Hempling RE, Wesolowski JA, Piver MS. Second-look laparotomy in advanced ovarian cancer: a critical assessment of morbidity and impact on survival. Ann Surg Oncol. 1997;4:349-54.
5. Katsoulis M, Vorgias G, Panagiotides J, Dertimas B, Zis J. The prognostic significance of second-look laparotomy in advanced ovarian cancer. Eur J Gynaecol Oncol. 1997;18:200-202.
6. Barter JF, Barnes WA. Second-look laparotomy. In: Rubin SC, Sutton GP, eds. Ovarian Cancer. New York, NY: McGraw-Hill; 1993;269-300.
7. Friedman RL, Eisenkop SM, Wang HJ. Second-look laparotomy for ovarian cancer provides reliable prognostic information and improves survival. Gynecol Oncol. 1997;67:88-94.
8. Gershenson DM, Copeland LJ, Wharton JT, et al. Prognosis of surgically determined complete responders in advanced ovarian cancer. Cancer. 1985;55:1129-1135.
9. Tuxen MK, Strauss G, Lund B, Hansen M. The role of second-look laparotomy in the long-term survival in ovarian cancer. Ann Oncol. 1997;8:643-648.
10. Gadducci A, Sartori E, Maggino T, et al. Analysis of failures after negative second-look in patients with advanced ovarian cancer: an Italian multicenter study. Gynecol Oncol. 1998;68:150-155.
11. Rubin SC, Hoskins WJ, Hakes TB, Markman M, Cain JM, Lewis JL, Jr. Recurrence after negative second-look laparotomy for ovarian cancer: analysis of risk factors. Am J Obstet Gynecol. 1988;159:1094-1098.
12. Ghatage P, Krepart GV, Lotocki R. Factor analysis of false-negative second-look laparotomy. Gynecol Oncol. 1990;36:172-175.
13. Dauplat J, Ferriere JP, Gorbinet M, et al. Second-look laparotomy in managing epithelial ovarian carcinoma. Cancer. 1986;57:1627-1631.
14. Podratz KC, Schray MF, Wieand HS, et al. Evaluation of treatment and survival after positive second-look laparotomy. Gynecol Oncol. 1988;31:9-24.
15. Lippman SM, Alberts DS, Slymen DJ, et al. Second-look laparotomy in epithelial ovarian carcinoma. Prognostic factors associated with survival duration. Cancer. 1988;61:2571-2577.
16. Raju KS, McKinna JA, Barker GH, Wiltshaw E, Jones JM. Second-look operations in the planned management of advanced ovarian carcinoma. Am J Obstet Gynecol. 1982;144:650-654.
17. Williams L, Brunetto VL, Yordan E, DiSaia PJ, Creasman WT. Secondary cytoreductive surgery at second-look laparotomy in advanced ovarian cancer: a Gynecologic Oncology Group Study. Gynecol Oncol. 1997;66:171-178.
18. Dowdy SC, Constantinou CL, Hartmann LC, et al. Long-term follow-up of women with ovarian cancer after positive second-look laparotomy. Gynecol Oncol. 2003;91:563-568.
19. Schwartz PE, Smith JP. Second-look operations in ovarian cancer. Am J Obstet Gynecol. 1980;138:1124-1130.
20. MacGibbon A, Bucci J, MacLeod C, et al. Whole abdominal radiotherapy following second-look laparotomy for ovarian carcinoma. Gynecol Oncol. 1999;75:62-67.
21. Bruzzone M, Rubagotti A, Gadducci A, et al. Intraperitoneal carboplatin with or without interferonalpha in advanced ovarian cancer patients with minimal residual disease at second look: a prospective randomized trial of 111 patients. G.O.N.O. Gruppo Oncologic Nord Ovest. Gynecol Oncol. 1997;65:499-505.
22. Bertucci F, Viens P, Delpero JR, et al. High-dose melphalan-based chemotherapy and autologous stem cell transplantation after second look laparotomy in patients with chemosensitive advanced ovarian carcinoma: long-term results. Bone Marrow Transplant. 2000;26:61-67.
23. Markman M, Brady MF, Spirtos NM, Hanjani P, Rubin SC. Phase II trial of intraperitoneal paclitaxel in carcinoma of the ovary, tube, and peritoneum: a Gynecologic Oncology Group Study. J Clin Oncol. 1998;16:2620-2624.
24. Menczer J, Ben-Baruch G, Rizel S, Brenner H. Intraperitoneal cisplatin chemotherapy in ovarian carcinoma patients who are clinically in complete remission. Gynecol Oncol. 1992;46:222-225.
25. Tarraza HM,, Jr, Boyce CR, Smith WG, Jones MA. Consolidation intraperitoneal chemotherapy in epithelial ovarian cancer patients following negative second-look laparotomy. Gynecol Oncol. 1993;50:287-290.
26. Dufour P, Bergerat JP, Barats JC, et al. Intraperitoneal mitoxantrone as consolidation treatment for patients with ovarian carcinoma in pathologic complete remission. Cancer. 1994;73:1865-1869.
27. Barakat RR, Almadrones L, Venkatraman ES, et al. A phase II trial of intraperitoneal cisplatin and etoposide as consolidation therapy in patients with Stage II-IV epithelial ovarian cancer following negative surgical assessment. Gynecol Oncol. 1998;69:17-22.
28. Abu-Rustum NR, Barakat RR, Siegel PL, Venkatraman E, Curtin JP, Hoskins WJ. Second-look operation for epithelial ovarian cancer: laparoscopy or laparotomy? Obstet Gynecol. 1996;88:549-553.
29. Clough KB, Ladonne JM, Nos C, Renolleau C, Validire P, Durand JC. Second look for ovarian cancer: laparoscopy or laparotomy? A prospective comparative study. Gynecol Oncol. 1999;72:411-417.
30. Spinelli P, Luini A, Pizzetti P, de Palo GM. Laparoscopy in staging and restaging of 95 patients with ovarian carcinoma. Tumori. 1976;62:493-501.
31. Smith WG, Day TG, Jr, Smith JP. The use of laparoscopy to determine the results of chemotherapy for ovarian cancer. J Reprod Med. 1977;18:257-260.
32. Canis M, Chapron C, Mage G, et al. [Technique and preliminary results in second-look laparoscopy in epithelial malignant ovarian tumors]. J Gynecol Obstet Biol Reprod (Paris). 1992;21:655-663.
33. Quinn MA, Bishop GJ, Campbell JJ, Rodgerson J, Pepperell RJ. Laparoscopic follow-up of patients with ovarian carcinoma. Br J Obstet Gynaecol. 1980;87:1132-1139.
34. Xygakis AM, Politis GS, Michalas SP, Kaskarelis DB. Second-look laparoscopy in ovarian cancer. J Reprod Med. 1984;29:583-585.
35. Nicoletto MO, Tumolo S, Talamini R, et al. Surgical second look in ovarian cancer: a randomized study in patients with laparoscopic complete remission—a Northeastern Oncology Cooperative Group-Ovarian Cancer Cooperative Group Study. J Clin Oncol. 1997;15:994-999.
• New sling procedures • Correcting site-specific defects • Mesh augmentation
After several decades of slow progress, the field of urogynecology is experiencing dynamic change, including:
- new minimally invasive, tension-free, midurethral sling procedures, especially the transobturator approach,
- correction of size-specific defects to repair prolapse, and
- use of mesh/graft augmentation in pro lapse repair.
These developments are some of the most important since Kelly and Dunn first described suburethral fascial plication for stress incontinence and cystocele in 1914.1 They have come about through increased understanding of the pathophysiology of incontinence and prolapse, innovative technology and techniques, and improved communication and coordination among physicians worldwide.
New sling procedures, promising outcomes
The minimally invasive midurethral sling procedure spawned notable new approaches and is a mainstay of surgical treatment for stress urinary incontinence.
First described in Sweden by Ulmsten in 1995,2 the tension-free vaginal tape procedure is a revolutionary change in the suburethral sling procedure and is now the most widely performed surgery for stress incontinence worldwide.
In it, a tension-free vaginal tape (TVT) (Gynecare, Somerville, NJ) of synthetic polypropylene mesh is attached to 2 needles and passed through a vaginal incision and the retropubic space, exiting to small incisions in the suprapubic region to create a suburethral sling or hammock and provide urethral support during increased abdominal pressure. The sling remains fixed by friction and subsequent adhesions.
Although the traditional suburethral sling was less invasive than other abdominal incontinence procedures, it was associated with a steep learning curve and a high incidence of postoperative irritative bladder symptoms and voiding dysfunction.3
Tension-free vaginal tape: Excellent long-term cure
It can be performed routinely in under 30 minutes using local anesthesia, with minimal postoperative complications. Five-year cure rates approach 95%,4 and data presented at the 2003 International Urogynecology Association clinical meeting describe an objective 7-year cure rate of 82%.5
The incidence and severity of postoperative voiding dysfunction following the TVT procedure is significantly lower than that reported after traditional suburethral sling (2%–40%) or transvaginal needle suspension (2%–50%) procedures.6-9 Although bladder perforation has occurred in up to 10% of patients, reports of complications, including major hemorrhage, tape erosion, and bowel and nerve injury, are rare.10,11
Modifications
These products have a modified approach, materials, or refinements in technique to address various needs. For example, American Medical Systems (Minnetonka, Minn) introduced the SPARC procedure, which allows abdominal placement of the midurethral sling, similar to a needle suspension technique.
CR Bard (Murray Hill, NJ) introduced a midurethral sling of porcine dermis (Pelvicol) to address concerns physicians may have about using synthetic materials.
Minimally invasive midurethral slings include the Advantage (Boston Scientific, Natick, Mass), Centrasorb (Caldera Medical, Thousand Oaks, Calif), Stratasis TF (Cook, West Lafayette, Ind), and Uretex (CR Bard).
Newest approach: Transobturator sling
In this technique, the sling is placed in the midurethral position, but the insertion points are in the genital area lateral to the vagina, and the needle passes through the obturator membrane and paraurethral space. Because it avoids passage of the needles through the retropubic space, the transobturator approach theoretically should reduce the risk of bowel, bladder, and major blood vessel injury.
The procedure was initially described in Europe and introduced in the United States in 2003. Current product offerings include the outside-in approach of ObTape (Mentor, Santa Barbara, Calif), Monarc (American Medical Systems), and Uretex (CR Bard). Gynecare offers a variation of its TVT—the TVT-Obturator—which involves an inside-out approach to further minimize risk of vascular injury.
Shortage of long-term data, but good early results
In a 1-year follow-up of patients undergoing a sling procedure with the UraTape transobturator sling (Mentor), Delorme and colleagues12 reported that 29 of 32 patients (90.6%) were cured and 3 (9.4%) improved. De Leval13 described his inside-out approach with equally good results: no bladder or urethral injuries and no vascular (hematoma or bleeding) or neurological complications. A transobturator technique using porcine dermis has also been described.
Tension-free vaginal tape versus transobturator sling
In 2004, a small randomized, prospective trial of TVT (n = 29) versus transobturator tape (n = 27) with 1-year follow-up found the transobturator approach to be safer and easier to place with equivalent short-term results.14 Mean operative time was significantly shorter in the transobturator group (15±4 minutes versus 27±8 minutes, P <.001).
No bladder injury occurred in the transobturator group versus 9.7% (n = 3) in the TVT group (P >.05). The rate of postoperative urinary retention was 25.8% (n = 8) in the TVT group versus 13.3% (n = 4) in the transobturator group (P >.05). Cure rates were similar for the TVT and transobturator groups: 83.9% versus 90%, respectively), improvement (9.7% versus 3.3%), and failure (6.5% versus 6.7%). No vaginal erosion occurred in either group.
Additional investigations of the transobturator tape procedure are underway.
Correcting site-specific defects in prolapse repair
This repair rationale should become the standard, although it has yet to be widely adopted and procedural refinement and research are continuing.
As early as 1908, site-specific defects in the endopelvic fascia were identified as the likely cause of anterior vaginal segment prolapse. Like hernia repair, which requires closure of the fascial defect, the “cystocele hernia” repair advocated by George White involved reattaching the endopelvic fascia to the arcus tendineus fascia pelvis using a series of interrupted sutures through an abdominal retropubic approach.
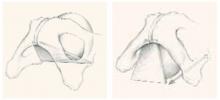
Although this view was later abandoned, it resurfaced in the 1980s, when Cullen Richardson described midline, lateral, and transverse defects (FIGURE 1) in the endopelvic fascia as the cause of cystocele and rectocele.15 Richardson advocated diagnosis that identified these fascial defects, along with treatment with site-specific repair.
(In the intervening decades, prolapse was thought to result from generalized weakening or attenuation of the endopelvic fascia that supports the bladder, rectum, or vagina, leading to cystocele, rectocele, or uterine prolapse, respectively. Traditional repairs, still widely performed by most gynecologists, consist of the anterior and posterior colporrhaphy, which involve midline plication of the endopelvic fascia to reduce the prolapse and recreate support by strengthening the weakened fascial layer.)
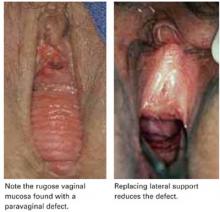
FIGURE 1 Identify the site of defect
Whether the defect that causes a rectocele or cystocele is transverse, central, or lateral determines the type of repair to be performed. Reprinted with permission from Richardson AC. The rectovaginal septum revisited: its relationship to rectocele and its importance in rectocele repair. Clin Obstet Gynecol.1993;36(4):976–983.
Excellent cure rates with fewer complications
Site-specific repair (FIGURE 2) has been adopted by many pelvic surgeons, who report excellent cure rates with fewer complications such as dyspareunia, vaginal narrowing, and increased blood loss—all of which are more common with traditional anterior and posterior colporrhaphy.
Traditional colporrhaphy does correct the underlying fascial defect when the “hernia” is in the midline. However, for lateral and transverse defects, traditional colporrhaphy leaves the defect uncorrected and may even create additional tension, resulting in recurrence.
Site-specific repair also is useful in the treatment of enterocele, which has been described as a herniation of the peritoneum through a defect of the anterior and posterior fascial planes at the vaginal apex.16
Widening but not broad acceptance
Although cure rates for site-specific prolapse repair range from 75% to 85%,17,18 the concept has yet to be widely adopted and continues to undergo procedural refinement and research, as well as physician education. With increasing experience and clinical data, it may become the standard for pelvic prolapse repair.
FIGURE 2 Repair the defect to correct the rectocele
Surgical technique for a transverse tear in the rectovaginal fascia: After identifying the defect, place a series of interrupted sutures to reapproximate the fascial edges (copyright Miklos/Kohli).
Mesh augmentation: Useful in selected patients
Although augmentation with mesh or biomaterials is easy to perform, it remains unclear which technique and materials are optimal.
The use of mesh and biomaterials to augment repair of a cystocele, rectocele, or enterocele is slowly increasing, although general surgeons have been utilizing these materials for many years in hernia repair.
Augmentation has been advocated for “pelvic hernias” because of poor long-term cure rates for traditional prolapse surgeries (which range from 40% to 80%—well behind rates of 90% or more for incontinence procedures).
A recent informal survey of the members of the American Urogynecologic Society and International Urogynecologic Association revealed that most pelvic surgeons are using some type of graft or mesh to augment repairs in selected patients.
Synthetic mesh versus autologous and heterologous grafts
Synthetic materials have the advantage of being readily available, cost-effective, and consistent in quality, but they may cause significant complications, including infection, stricture, and erosion. This is especially important in the vagina, which needs to stretch during intercourse and which alters in thickness and other properties during a woman’s lifetime.
In contrast, autologous and heterologous donor grafts provide naturally occurring biomaterials capable of remodeling. Unfortunately, the in vivo tissue response is not yet fully understood. Other disadvantages: Biomaterials may lack consistent tissue properties and can be expensive.
For these reasons, graft materials remain in an early period of evaluation, although their use is expected to rise steadily with increasing experience and new product development.
Limited data on safety and efficacy
Although many pelvic surgeons use graft/mesh materials in prolapse repair, data on their safety and efficacy are limited, partly due to the variety of surgical techniques and materials available. Another factor is the difficulty of obtaining good long-term data with large patient numbers. Most of the literature is comprised of case reports, with few prospective, randomized trials.
That said, initial data suggest a significant improvement in cure rates, compared with traditional techniques, with minimal short-term complications. Long-term results and complication rates are not yet available.
What existing studies show
A variety of synthetic materials have been used in surgical correction of cystocele. In the largest series to date, Flood and colleagues19 reported their 12-year experience with 142 women undergoing a modified anterior colporrhaphy reinforced with Marlex (Davol, Cranston, RI) mesh: 100% success in correcting cystocele and a 74% success rate for urinary stress incontinence, with a mean follow-up of 3.2 years and no significant intraoperative complications.
In a prospective randomized trial of 125 patients utilizing absorbable polyglactin 910 mesh (Vicryl) (Ethicon, Somerville, NJ) to augment standard anterior colporrhaphy, Koduri et al20 reported a failure rate of 13% in the colporrhaphy-alone group compared to 1% in the colporrhaphy-mesh group at the 1-year follow-up. Subjectively, both groups improved equally.
Clemons et al21 used a human dermis graft to treat advanced recurrent cystocele in 33 women, with a follow-up of 18 months. They noted 13 (41%) objective failures and 1 (3%) subjective failure. Complications included 1 case of febrile morbidity, 1 cystotomy, and 1 anterior wall breakdown secondary to hematoma formation caused by heparin therapy. No other erosions or rejections were seen.
Most effective applications
Graft/mesh augmentation may be most effective in posterior vaginal segment reconstruction and rectocele repair, as it obviates the need for levatorplasty in patients with poor rectovaginal fascia. A variety of synthetic materials have been used for posterior wall reconstruction in small series.
Mersilene mesh
Mersilene mesh (Ethicon) was used by Fox and Stanton22 to augment traditional rectocele repair in 29 women followed for 14 months. Most of the women had undergone previous rectocele repair with recurrence of their prolapse. All women with stage II and stage III vault prolapse were corrected, with an increase in stage I prolapse from 20% to 27%. All women with stage II and stage III rectocele were corrected, with a decrease in stage I prolapse from 36% to 7%. The only significant intraoperative complication was a cystotomy. One mesh became infected postoperatively, requiring removal.
Cadaveric dermal graft
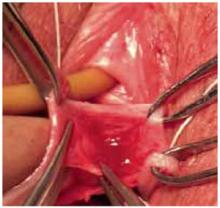
Recently, Kohli and Miklos23 reported their experience with 57 patients undergoing augmented rectocele repair using a cadaveric dermal graft (FIGURE 3) over a 2-year period. Average follow-up was 11 months. Average patient age in the follow-up group was 63.6±10.9 years (range: 33–79 years) and average parity was 2.8±1.5 (range: 0–7). No major intraoperative complications (hollow viscous injury, blood loss greater than 500 cc, or transfusion) or post-operative complications (infection, abscess, or hematoma) were noted. No graft-related complications such as rejection, erosion, infection, and fistula formation were noted during the follow-up period.
Using previously accepted Pelvic Organ Prolapse Quantification (POP-Q) parameters for success, Kohli and Miklos found 54 of 57 women (95%) to have surgical cure at follow-up. These authors have also described the use of a dermal graft in the repair of a complicated rectovaginal fistula.24
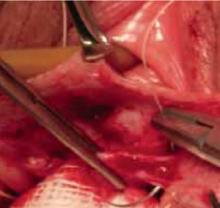
FIGURE 3 Placement of mesh augmentation
Augment enterocele/rectocele repair by attaching the mesh apically, laterally, and distally (copyright Miklos/Kohli).
Future outlook
Further data on the efficacy and safety of graft/mesh augmentation—including identification of the optimal technique and ideal material—are necessary before this approach can be widely adopted. However, in selected patients, it represents a significant advance in the surgical treatment of pelvic prolapse.
The author is a preceptor for American Medical Systems, Gynecare, CR Bard, and Mentor and a consultant for Boston Scientific.
1. Kelly HA, Dunn WM. Urinary incontinence in women without manifest injury to the bladder. Surg Gynecol Obstet. 1914;18:444.-
2. Ulmsten U, Petros P. Intravaginal slingplasty (IVS): an ambulatory surgical procedure for treatment of female urinary incontinence. Scand J Urol Nephrol. 1995;29:75-82.
3. Weinberger MW, Ostergard DR. Postoperative catheterization, urinary retention, and permanent voiding dysfunction after polytetrafluroethylene suburethral sling placement. Obstet Gynecol. 1996;87:50-54.
4. Nilsson CG, Kuuva N, Falconer C, Rezapour M, Ulmsten U. Long-term results of the tension-free vaginal tape (TVT) procedure for surgical treatment of female stress urinary incontinence. Int Urogynecol J Pelvic Floor Dysfunct. 2001;12(Suppl 2):S5-8.
5. Nilsson CG, Rezapour M, Falconer C. Seven years follow-up of the tension-free vaginal tape (TVT) procedure. Presented at the 2003 International Urogynecology Association Annual Meeting.
6. Horbach N. Suburethral sling procedures. In: Ostergard DR, Bent AE, eds. Urogynecology and Urodynamics: Theory and Practice. 3rd ed. Baltimore: Williams & Wilkins; 1991;449-458.
7. Stanton SL, Reynolds SF, Creighton SM. The modified Pereyra (Raz) procedure for genuine stress incontinence—a useful option in the elderly or frail patient? Int Urogynecol J. 1995;6:22-25.
8. Nygaard IE, Kreder KJ. Complications of incontinence surgery. Int Urogynecol J. 1994;5:353-360.
9. Lockhart vJL, Tirado A, Morillo G, Politano VA. Vesicourethral dysfunction following cystourethropexy. J Urol. 1982;128:943-945.
10. Leboeuf L, Tellez CA, Ead D, Gousse AE. Complication of bowel perforation during insertion of tension-free vaginal tape. J Urol. 2003;170(4 Pt 1):1310.-
11. Abouassaly R, Steinberg JR, Lemieux M, et al. Complications of tension-free vaginal tape surgery: a multi-institutional review. BJU Int. 2004;94:110-113.
12. Delorme E, Droupy S, de Tayrac R, Delmas V. [Transobturator tape (UraTape). A new minimally invasive method in the treatment of urinary incontinence in women.] Prog Urol. 2003;13:656-659.
13. de Leval J. Novel surgical technique for the treatment of female stress urinary incontinence: transobturator vaginal tape inside-out. Eur Urol. 2003;44:724-730.
14. De Tayrac R, Deffieux X, Droupy S, ChauveaudLambling A, Calvanese-Benamour L, Fernandez H. A prospective randomized trial comparing tensionfree vaginal tape and transobturator suburethral tape for surgical treatment of stress urinary incontinence. Am J Obstet Gynecol. 2004;190:602-608.
15. Richardson AC. The rectovaginal septum revisited: its relationship to rectocele and its importance in rectocele repair. Clin Obstet Gynecol. 1993;36:976-983.
16. Miklos JR, Kohli N, Lucente V, Saye WB. Site-specific fascial defects in the diagnosis and surgical management of enterocele. Am J Obstet Gynecol. 1998;179 (6 Pt 1):1418-1422; discussion 1422-823.
17. Porter WE, Steele A, Walsh P, Kohli N, Karram MM. The anatomic and functional outcomes of defectspecific rectocele repairs. Am J Obstet Gynecol. 1999;181:1353-1358; discussion 1358-1359.
18. Kenton K, Shott S, Brubaker L. Outcome after rectovaginal fascia reattachment for rectocele repair. Am J Obstet Gynecol. 1999;181:1360-1363; discussion 1363-1364.
19. Flood CG, Drutz HP, Waja L. Anterior colporrhaphy reinforced with Marlex mesh for the treatment of cystoceles. Int Urogynecol J Pelvic Floor Dysfunct. 1998;9:200-204.
20. Koduri S, Lobel RW, Winkler HA, Tomezsko J, Culligan PJ, Sand PK. Prospective randomized trial of polyglactin 910 mesh to prevent recurrence of cystoceles and rectoceles. Proceedings of the 25th Annual Meeting of the International Urogynecological Association, 2000. Int Urogynecol J Pelvic Floor Dysfunct. 2000;11:S80.-
21. Clemons JL, Myers DL, Aguilar VC, Arya LA. Vaginal paravaginal repair with an AlloDerm graft. Am J Obstet Gynecol. 2003;189:1612-1618; discussion 1618-1619.
22. Fox SD, Stanton SL. Vault prolapse and rectocele: assessment of repair using sacrocolpopexy with mesh interposition. BJOG. 2000;107:1371-1375.
23. Kohli N, Miklos JR. Site specific rectocele repair augmented with a cadaveric dermal graft. Proceedings of the 21st Annual Scientific Meeting of the American Urogynecologic Society, 2000. Int Urogynecol J Pelvic Floor Dysfunct. 2001;12:S9.-
24. Miklos JR, Kohli N. Rectovaginal fistula repair utilizing a cadaveric dermal allograft. Int Urogynecol J Pelvic Floor Dysfunct. 1999;10:405-406.
After several decades of slow progress, the field of urogynecology is experiencing dynamic change, including:
- new minimally invasive, tension-free, midurethral sling procedures, especially the transobturator approach,
- correction of size-specific defects to repair prolapse, and
- use of mesh/graft augmentation in pro lapse repair.
These developments are some of the most important since Kelly and Dunn first described suburethral fascial plication for stress incontinence and cystocele in 1914.1 They have come about through increased understanding of the pathophysiology of incontinence and prolapse, innovative technology and techniques, and improved communication and coordination among physicians worldwide.
New sling procedures, promising outcomes
The minimally invasive midurethral sling procedure spawned notable new approaches and is a mainstay of surgical treatment for stress urinary incontinence.
First described in Sweden by Ulmsten in 1995,2 the tension-free vaginal tape procedure is a revolutionary change in the suburethral sling procedure and is now the most widely performed surgery for stress incontinence worldwide.
In it, a tension-free vaginal tape (TVT) (Gynecare, Somerville, NJ) of synthetic polypropylene mesh is attached to 2 needles and passed through a vaginal incision and the retropubic space, exiting to small incisions in the suprapubic region to create a suburethral sling or hammock and provide urethral support during increased abdominal pressure. The sling remains fixed by friction and subsequent adhesions.
Although the traditional suburethral sling was less invasive than other abdominal incontinence procedures, it was associated with a steep learning curve and a high incidence of postoperative irritative bladder symptoms and voiding dysfunction.3
Tension-free vaginal tape: Excellent long-term cure
It can be performed routinely in under 30 minutes using local anesthesia, with minimal postoperative complications. Five-year cure rates approach 95%,4 and data presented at the 2003 International Urogynecology Association clinical meeting describe an objective 7-year cure rate of 82%.5
The incidence and severity of postoperative voiding dysfunction following the TVT procedure is significantly lower than that reported after traditional suburethral sling (2%–40%) or transvaginal needle suspension (2%–50%) procedures.6-9 Although bladder perforation has occurred in up to 10% of patients, reports of complications, including major hemorrhage, tape erosion, and bowel and nerve injury, are rare.10,11
Modifications
These products have a modified approach, materials, or refinements in technique to address various needs. For example, American Medical Systems (Minnetonka, Minn) introduced the SPARC procedure, which allows abdominal placement of the midurethral sling, similar to a needle suspension technique.
CR Bard (Murray Hill, NJ) introduced a midurethral sling of porcine dermis (Pelvicol) to address concerns physicians may have about using synthetic materials.
Minimally invasive midurethral slings include the Advantage (Boston Scientific, Natick, Mass), Centrasorb (Caldera Medical, Thousand Oaks, Calif), Stratasis TF (Cook, West Lafayette, Ind), and Uretex (CR Bard).
Newest approach: Transobturator sling
In this technique, the sling is placed in the midurethral position, but the insertion points are in the genital area lateral to the vagina, and the needle passes through the obturator membrane and paraurethral space. Because it avoids passage of the needles through the retropubic space, the transobturator approach theoretically should reduce the risk of bowel, bladder, and major blood vessel injury.
The procedure was initially described in Europe and introduced in the United States in 2003. Current product offerings include the outside-in approach of ObTape (Mentor, Santa Barbara, Calif), Monarc (American Medical Systems), and Uretex (CR Bard). Gynecare offers a variation of its TVT—the TVT-Obturator—which involves an inside-out approach to further minimize risk of vascular injury.
Shortage of long-term data, but good early results
In a 1-year follow-up of patients undergoing a sling procedure with the UraTape transobturator sling (Mentor), Delorme and colleagues12 reported that 29 of 32 patients (90.6%) were cured and 3 (9.4%) improved. De Leval13 described his inside-out approach with equally good results: no bladder or urethral injuries and no vascular (hematoma or bleeding) or neurological complications. A transobturator technique using porcine dermis has also been described.
Tension-free vaginal tape versus transobturator sling
In 2004, a small randomized, prospective trial of TVT (n = 29) versus transobturator tape (n = 27) with 1-year follow-up found the transobturator approach to be safer and easier to place with equivalent short-term results.14 Mean operative time was significantly shorter in the transobturator group (15±4 minutes versus 27±8 minutes, P <.001).
No bladder injury occurred in the transobturator group versus 9.7% (n = 3) in the TVT group (P >.05). The rate of postoperative urinary retention was 25.8% (n = 8) in the TVT group versus 13.3% (n = 4) in the transobturator group (P >.05). Cure rates were similar for the TVT and transobturator groups: 83.9% versus 90%, respectively), improvement (9.7% versus 3.3%), and failure (6.5% versus 6.7%). No vaginal erosion occurred in either group.
Additional investigations of the transobturator tape procedure are underway.
Correcting site-specific defects in prolapse repair
This repair rationale should become the standard, although it has yet to be widely adopted and procedural refinement and research are continuing.
As early as 1908, site-specific defects in the endopelvic fascia were identified as the likely cause of anterior vaginal segment prolapse. Like hernia repair, which requires closure of the fascial defect, the “cystocele hernia” repair advocated by George White involved reattaching the endopelvic fascia to the arcus tendineus fascia pelvis using a series of interrupted sutures through an abdominal retropubic approach.
Although this view was later abandoned, it resurfaced in the 1980s, when Cullen Richardson described midline, lateral, and transverse defects (FIGURE 1) in the endopelvic fascia as the cause of cystocele and rectocele.15 Richardson advocated diagnosis that identified these fascial defects, along with treatment with site-specific repair.
(In the intervening decades, prolapse was thought to result from generalized weakening or attenuation of the endopelvic fascia that supports the bladder, rectum, or vagina, leading to cystocele, rectocele, or uterine prolapse, respectively. Traditional repairs, still widely performed by most gynecologists, consist of the anterior and posterior colporrhaphy, which involve midline plication of the endopelvic fascia to reduce the prolapse and recreate support by strengthening the weakened fascial layer.)
FIGURE 1 Identify the site of defect
Whether the defect that causes a rectocele or cystocele is transverse, central, or lateral determines the type of repair to be performed. Reprinted with permission from Richardson AC. The rectovaginal septum revisited: its relationship to rectocele and its importance in rectocele repair. Clin Obstet Gynecol.1993;36(4):976–983.
Excellent cure rates with fewer complications
Site-specific repair (FIGURE 2) has been adopted by many pelvic surgeons, who report excellent cure rates with fewer complications such as dyspareunia, vaginal narrowing, and increased blood loss—all of which are more common with traditional anterior and posterior colporrhaphy.
Traditional colporrhaphy does correct the underlying fascial defect when the “hernia” is in the midline. However, for lateral and transverse defects, traditional colporrhaphy leaves the defect uncorrected and may even create additional tension, resulting in recurrence.
Site-specific repair also is useful in the treatment of enterocele, which has been described as a herniation of the peritoneum through a defect of the anterior and posterior fascial planes at the vaginal apex.16
Widening but not broad acceptance
Although cure rates for site-specific prolapse repair range from 75% to 85%,17,18 the concept has yet to be widely adopted and continues to undergo procedural refinement and research, as well as physician education. With increasing experience and clinical data, it may become the standard for pelvic prolapse repair.
FIGURE 2 Repair the defect to correct the rectocele
Surgical technique for a transverse tear in the rectovaginal fascia: After identifying the defect, place a series of interrupted sutures to reapproximate the fascial edges (copyright Miklos/Kohli).
Mesh augmentation: Useful in selected patients
Although augmentation with mesh or biomaterials is easy to perform, it remains unclear which technique and materials are optimal.
The use of mesh and biomaterials to augment repair of a cystocele, rectocele, or enterocele is slowly increasing, although general surgeons have been utilizing these materials for many years in hernia repair.
Augmentation has been advocated for “pelvic hernias” because of poor long-term cure rates for traditional prolapse surgeries (which range from 40% to 80%—well behind rates of 90% or more for incontinence procedures).
A recent informal survey of the members of the American Urogynecologic Society and International Urogynecologic Association revealed that most pelvic surgeons are using some type of graft or mesh to augment repairs in selected patients.
Synthetic mesh versus autologous and heterologous grafts
Synthetic materials have the advantage of being readily available, cost-effective, and consistent in quality, but they may cause significant complications, including infection, stricture, and erosion. This is especially important in the vagina, which needs to stretch during intercourse and which alters in thickness and other properties during a woman’s lifetime.
In contrast, autologous and heterologous donor grafts provide naturally occurring biomaterials capable of remodeling. Unfortunately, the in vivo tissue response is not yet fully understood. Other disadvantages: Biomaterials may lack consistent tissue properties and can be expensive.
For these reasons, graft materials remain in an early period of evaluation, although their use is expected to rise steadily with increasing experience and new product development.
Limited data on safety and efficacy
Although many pelvic surgeons use graft/mesh materials in prolapse repair, data on their safety and efficacy are limited, partly due to the variety of surgical techniques and materials available. Another factor is the difficulty of obtaining good long-term data with large patient numbers. Most of the literature is comprised of case reports, with few prospective, randomized trials.
That said, initial data suggest a significant improvement in cure rates, compared with traditional techniques, with minimal short-term complications. Long-term results and complication rates are not yet available.
What existing studies show
A variety of synthetic materials have been used in surgical correction of cystocele. In the largest series to date, Flood and colleagues19 reported their 12-year experience with 142 women undergoing a modified anterior colporrhaphy reinforced with Marlex (Davol, Cranston, RI) mesh: 100% success in correcting cystocele and a 74% success rate for urinary stress incontinence, with a mean follow-up of 3.2 years and no significant intraoperative complications.
In a prospective randomized trial of 125 patients utilizing absorbable polyglactin 910 mesh (Vicryl) (Ethicon, Somerville, NJ) to augment standard anterior colporrhaphy, Koduri et al20 reported a failure rate of 13% in the colporrhaphy-alone group compared to 1% in the colporrhaphy-mesh group at the 1-year follow-up. Subjectively, both groups improved equally.
Clemons et al21 used a human dermis graft to treat advanced recurrent cystocele in 33 women, with a follow-up of 18 months. They noted 13 (41%) objective failures and 1 (3%) subjective failure. Complications included 1 case of febrile morbidity, 1 cystotomy, and 1 anterior wall breakdown secondary to hematoma formation caused by heparin therapy. No other erosions or rejections were seen.
Most effective applications
Graft/mesh augmentation may be most effective in posterior vaginal segment reconstruction and rectocele repair, as it obviates the need for levatorplasty in patients with poor rectovaginal fascia. A variety of synthetic materials have been used for posterior wall reconstruction in small series.
Mersilene mesh
Mersilene mesh (Ethicon) was used by Fox and Stanton22 to augment traditional rectocele repair in 29 women followed for 14 months. Most of the women had undergone previous rectocele repair with recurrence of their prolapse. All women with stage II and stage III vault prolapse were corrected, with an increase in stage I prolapse from 20% to 27%. All women with stage II and stage III rectocele were corrected, with a decrease in stage I prolapse from 36% to 7%. The only significant intraoperative complication was a cystotomy. One mesh became infected postoperatively, requiring removal.
Cadaveric dermal graft
Recently, Kohli and Miklos23 reported their experience with 57 patients undergoing augmented rectocele repair using a cadaveric dermal graft (FIGURE 3) over a 2-year period. Average follow-up was 11 months. Average patient age in the follow-up group was 63.6±10.9 years (range: 33–79 years) and average parity was 2.8±1.5 (range: 0–7). No major intraoperative complications (hollow viscous injury, blood loss greater than 500 cc, or transfusion) or post-operative complications (infection, abscess, or hematoma) were noted. No graft-related complications such as rejection, erosion, infection, and fistula formation were noted during the follow-up period.
Using previously accepted Pelvic Organ Prolapse Quantification (POP-Q) parameters for success, Kohli and Miklos found 54 of 57 women (95%) to have surgical cure at follow-up. These authors have also described the use of a dermal graft in the repair of a complicated rectovaginal fistula.24
FIGURE 3 Placement of mesh augmentation
Augment enterocele/rectocele repair by attaching the mesh apically, laterally, and distally (copyright Miklos/Kohli).
Future outlook
Further data on the efficacy and safety of graft/mesh augmentation—including identification of the optimal technique and ideal material—are necessary before this approach can be widely adopted. However, in selected patients, it represents a significant advance in the surgical treatment of pelvic prolapse.
The author is a preceptor for American Medical Systems, Gynecare, CR Bard, and Mentor and a consultant for Boston Scientific.
After several decades of slow progress, the field of urogynecology is experiencing dynamic change, including:
- new minimally invasive, tension-free, midurethral sling procedures, especially the transobturator approach,
- correction of size-specific defects to repair prolapse, and
- use of mesh/graft augmentation in pro lapse repair.
These developments are some of the most important since Kelly and Dunn first described suburethral fascial plication for stress incontinence and cystocele in 1914.1 They have come about through increased understanding of the pathophysiology of incontinence and prolapse, innovative technology and techniques, and improved communication and coordination among physicians worldwide.
New sling procedures, promising outcomes
The minimally invasive midurethral sling procedure spawned notable new approaches and is a mainstay of surgical treatment for stress urinary incontinence.
First described in Sweden by Ulmsten in 1995,2 the tension-free vaginal tape procedure is a revolutionary change in the suburethral sling procedure and is now the most widely performed surgery for stress incontinence worldwide.
In it, a tension-free vaginal tape (TVT) (Gynecare, Somerville, NJ) of synthetic polypropylene mesh is attached to 2 needles and passed through a vaginal incision and the retropubic space, exiting to small incisions in the suprapubic region to create a suburethral sling or hammock and provide urethral support during increased abdominal pressure. The sling remains fixed by friction and subsequent adhesions.
Although the traditional suburethral sling was less invasive than other abdominal incontinence procedures, it was associated with a steep learning curve and a high incidence of postoperative irritative bladder symptoms and voiding dysfunction.3
Tension-free vaginal tape: Excellent long-term cure
It can be performed routinely in under 30 minutes using local anesthesia, with minimal postoperative complications. Five-year cure rates approach 95%,4 and data presented at the 2003 International Urogynecology Association clinical meeting describe an objective 7-year cure rate of 82%.5
The incidence and severity of postoperative voiding dysfunction following the TVT procedure is significantly lower than that reported after traditional suburethral sling (2%–40%) or transvaginal needle suspension (2%–50%) procedures.6-9 Although bladder perforation has occurred in up to 10% of patients, reports of complications, including major hemorrhage, tape erosion, and bowel and nerve injury, are rare.10,11
Modifications
These products have a modified approach, materials, or refinements in technique to address various needs. For example, American Medical Systems (Minnetonka, Minn) introduced the SPARC procedure, which allows abdominal placement of the midurethral sling, similar to a needle suspension technique.
CR Bard (Murray Hill, NJ) introduced a midurethral sling of porcine dermis (Pelvicol) to address concerns physicians may have about using synthetic materials.
Minimally invasive midurethral slings include the Advantage (Boston Scientific, Natick, Mass), Centrasorb (Caldera Medical, Thousand Oaks, Calif), Stratasis TF (Cook, West Lafayette, Ind), and Uretex (CR Bard).
Newest approach: Transobturator sling
In this technique, the sling is placed in the midurethral position, but the insertion points are in the genital area lateral to the vagina, and the needle passes through the obturator membrane and paraurethral space. Because it avoids passage of the needles through the retropubic space, the transobturator approach theoretically should reduce the risk of bowel, bladder, and major blood vessel injury.
The procedure was initially described in Europe and introduced in the United States in 2003. Current product offerings include the outside-in approach of ObTape (Mentor, Santa Barbara, Calif), Monarc (American Medical Systems), and Uretex (CR Bard). Gynecare offers a variation of its TVT—the TVT-Obturator—which involves an inside-out approach to further minimize risk of vascular injury.
Shortage of long-term data, but good early results
In a 1-year follow-up of patients undergoing a sling procedure with the UraTape transobturator sling (Mentor), Delorme and colleagues12 reported that 29 of 32 patients (90.6%) were cured and 3 (9.4%) improved. De Leval13 described his inside-out approach with equally good results: no bladder or urethral injuries and no vascular (hematoma or bleeding) or neurological complications. A transobturator technique using porcine dermis has also been described.
Tension-free vaginal tape versus transobturator sling
In 2004, a small randomized, prospective trial of TVT (n = 29) versus transobturator tape (n = 27) with 1-year follow-up found the transobturator approach to be safer and easier to place with equivalent short-term results.14 Mean operative time was significantly shorter in the transobturator group (15±4 minutes versus 27±8 minutes, P <.001).
No bladder injury occurred in the transobturator group versus 9.7% (n = 3) in the TVT group (P >.05). The rate of postoperative urinary retention was 25.8% (n = 8) in the TVT group versus 13.3% (n = 4) in the transobturator group (P >.05). Cure rates were similar for the TVT and transobturator groups: 83.9% versus 90%, respectively), improvement (9.7% versus 3.3%), and failure (6.5% versus 6.7%). No vaginal erosion occurred in either group.
Additional investigations of the transobturator tape procedure are underway.
Correcting site-specific defects in prolapse repair
This repair rationale should become the standard, although it has yet to be widely adopted and procedural refinement and research are continuing.
As early as 1908, site-specific defects in the endopelvic fascia were identified as the likely cause of anterior vaginal segment prolapse. Like hernia repair, which requires closure of the fascial defect, the “cystocele hernia” repair advocated by George White involved reattaching the endopelvic fascia to the arcus tendineus fascia pelvis using a series of interrupted sutures through an abdominal retropubic approach.
Although this view was later abandoned, it resurfaced in the 1980s, when Cullen Richardson described midline, lateral, and transverse defects (FIGURE 1) in the endopelvic fascia as the cause of cystocele and rectocele.15 Richardson advocated diagnosis that identified these fascial defects, along with treatment with site-specific repair.
(In the intervening decades, prolapse was thought to result from generalized weakening or attenuation of the endopelvic fascia that supports the bladder, rectum, or vagina, leading to cystocele, rectocele, or uterine prolapse, respectively. Traditional repairs, still widely performed by most gynecologists, consist of the anterior and posterior colporrhaphy, which involve midline plication of the endopelvic fascia to reduce the prolapse and recreate support by strengthening the weakened fascial layer.)
FIGURE 1 Identify the site of defect
Whether the defect that causes a rectocele or cystocele is transverse, central, or lateral determines the type of repair to be performed. Reprinted with permission from Richardson AC. The rectovaginal septum revisited: its relationship to rectocele and its importance in rectocele repair. Clin Obstet Gynecol.1993;36(4):976–983.
Excellent cure rates with fewer complications
Site-specific repair (FIGURE 2) has been adopted by many pelvic surgeons, who report excellent cure rates with fewer complications such as dyspareunia, vaginal narrowing, and increased blood loss—all of which are more common with traditional anterior and posterior colporrhaphy.
Traditional colporrhaphy does correct the underlying fascial defect when the “hernia” is in the midline. However, for lateral and transverse defects, traditional colporrhaphy leaves the defect uncorrected and may even create additional tension, resulting in recurrence.
Site-specific repair also is useful in the treatment of enterocele, which has been described as a herniation of the peritoneum through a defect of the anterior and posterior fascial planes at the vaginal apex.16
Widening but not broad acceptance
Although cure rates for site-specific prolapse repair range from 75% to 85%,17,18 the concept has yet to be widely adopted and continues to undergo procedural refinement and research, as well as physician education. With increasing experience and clinical data, it may become the standard for pelvic prolapse repair.
FIGURE 2 Repair the defect to correct the rectocele
Surgical technique for a transverse tear in the rectovaginal fascia: After identifying the defect, place a series of interrupted sutures to reapproximate the fascial edges (copyright Miklos/Kohli).
Mesh augmentation: Useful in selected patients
Although augmentation with mesh or biomaterials is easy to perform, it remains unclear which technique and materials are optimal.
The use of mesh and biomaterials to augment repair of a cystocele, rectocele, or enterocele is slowly increasing, although general surgeons have been utilizing these materials for many years in hernia repair.
Augmentation has been advocated for “pelvic hernias” because of poor long-term cure rates for traditional prolapse surgeries (which range from 40% to 80%—well behind rates of 90% or more for incontinence procedures).
A recent informal survey of the members of the American Urogynecologic Society and International Urogynecologic Association revealed that most pelvic surgeons are using some type of graft or mesh to augment repairs in selected patients.
Synthetic mesh versus autologous and heterologous grafts
Synthetic materials have the advantage of being readily available, cost-effective, and consistent in quality, but they may cause significant complications, including infection, stricture, and erosion. This is especially important in the vagina, which needs to stretch during intercourse and which alters in thickness and other properties during a woman’s lifetime.
In contrast, autologous and heterologous donor grafts provide naturally occurring biomaterials capable of remodeling. Unfortunately, the in vivo tissue response is not yet fully understood. Other disadvantages: Biomaterials may lack consistent tissue properties and can be expensive.
For these reasons, graft materials remain in an early period of evaluation, although their use is expected to rise steadily with increasing experience and new product development.
Limited data on safety and efficacy
Although many pelvic surgeons use graft/mesh materials in prolapse repair, data on their safety and efficacy are limited, partly due to the variety of surgical techniques and materials available. Another factor is the difficulty of obtaining good long-term data with large patient numbers. Most of the literature is comprised of case reports, with few prospective, randomized trials.
That said, initial data suggest a significant improvement in cure rates, compared with traditional techniques, with minimal short-term complications. Long-term results and complication rates are not yet available.
What existing studies show
A variety of synthetic materials have been used in surgical correction of cystocele. In the largest series to date, Flood and colleagues19 reported their 12-year experience with 142 women undergoing a modified anterior colporrhaphy reinforced with Marlex (Davol, Cranston, RI) mesh: 100% success in correcting cystocele and a 74% success rate for urinary stress incontinence, with a mean follow-up of 3.2 years and no significant intraoperative complications.
In a prospective randomized trial of 125 patients utilizing absorbable polyglactin 910 mesh (Vicryl) (Ethicon, Somerville, NJ) to augment standard anterior colporrhaphy, Koduri et al20 reported a failure rate of 13% in the colporrhaphy-alone group compared to 1% in the colporrhaphy-mesh group at the 1-year follow-up. Subjectively, both groups improved equally.
Clemons et al21 used a human dermis graft to treat advanced recurrent cystocele in 33 women, with a follow-up of 18 months. They noted 13 (41%) objective failures and 1 (3%) subjective failure. Complications included 1 case of febrile morbidity, 1 cystotomy, and 1 anterior wall breakdown secondary to hematoma formation caused by heparin therapy. No other erosions or rejections were seen.
Most effective applications
Graft/mesh augmentation may be most effective in posterior vaginal segment reconstruction and rectocele repair, as it obviates the need for levatorplasty in patients with poor rectovaginal fascia. A variety of synthetic materials have been used for posterior wall reconstruction in small series.
Mersilene mesh
Mersilene mesh (Ethicon) was used by Fox and Stanton22 to augment traditional rectocele repair in 29 women followed for 14 months. Most of the women had undergone previous rectocele repair with recurrence of their prolapse. All women with stage II and stage III vault prolapse were corrected, with an increase in stage I prolapse from 20% to 27%. All women with stage II and stage III rectocele were corrected, with a decrease in stage I prolapse from 36% to 7%. The only significant intraoperative complication was a cystotomy. One mesh became infected postoperatively, requiring removal.
Cadaveric dermal graft
Recently, Kohli and Miklos23 reported their experience with 57 patients undergoing augmented rectocele repair using a cadaveric dermal graft (FIGURE 3) over a 2-year period. Average follow-up was 11 months. Average patient age in the follow-up group was 63.6±10.9 years (range: 33–79 years) and average parity was 2.8±1.5 (range: 0–7). No major intraoperative complications (hollow viscous injury, blood loss greater than 500 cc, or transfusion) or post-operative complications (infection, abscess, or hematoma) were noted. No graft-related complications such as rejection, erosion, infection, and fistula formation were noted during the follow-up period.
Using previously accepted Pelvic Organ Prolapse Quantification (POP-Q) parameters for success, Kohli and Miklos found 54 of 57 women (95%) to have surgical cure at follow-up. These authors have also described the use of a dermal graft in the repair of a complicated rectovaginal fistula.24
FIGURE 3 Placement of mesh augmentation
Augment enterocele/rectocele repair by attaching the mesh apically, laterally, and distally (copyright Miklos/Kohli).
Future outlook
Further data on the efficacy and safety of graft/mesh augmentation—including identification of the optimal technique and ideal material—are necessary before this approach can be widely adopted. However, in selected patients, it represents a significant advance in the surgical treatment of pelvic prolapse.
The author is a preceptor for American Medical Systems, Gynecare, CR Bard, and Mentor and a consultant for Boston Scientific.
1. Kelly HA, Dunn WM. Urinary incontinence in women without manifest injury to the bladder. Surg Gynecol Obstet. 1914;18:444.-
2. Ulmsten U, Petros P. Intravaginal slingplasty (IVS): an ambulatory surgical procedure for treatment of female urinary incontinence. Scand J Urol Nephrol. 1995;29:75-82.
3. Weinberger MW, Ostergard DR. Postoperative catheterization, urinary retention, and permanent voiding dysfunction after polytetrafluroethylene suburethral sling placement. Obstet Gynecol. 1996;87:50-54.
4. Nilsson CG, Kuuva N, Falconer C, Rezapour M, Ulmsten U. Long-term results of the tension-free vaginal tape (TVT) procedure for surgical treatment of female stress urinary incontinence. Int Urogynecol J Pelvic Floor Dysfunct. 2001;12(Suppl 2):S5-8.
5. Nilsson CG, Rezapour M, Falconer C. Seven years follow-up of the tension-free vaginal tape (TVT) procedure. Presented at the 2003 International Urogynecology Association Annual Meeting.
6. Horbach N. Suburethral sling procedures. In: Ostergard DR, Bent AE, eds. Urogynecology and Urodynamics: Theory and Practice. 3rd ed. Baltimore: Williams & Wilkins; 1991;449-458.
7. Stanton SL, Reynolds SF, Creighton SM. The modified Pereyra (Raz) procedure for genuine stress incontinence—a useful option in the elderly or frail patient? Int Urogynecol J. 1995;6:22-25.
8. Nygaard IE, Kreder KJ. Complications of incontinence surgery. Int Urogynecol J. 1994;5:353-360.
9. Lockhart vJL, Tirado A, Morillo G, Politano VA. Vesicourethral dysfunction following cystourethropexy. J Urol. 1982;128:943-945.
10. Leboeuf L, Tellez CA, Ead D, Gousse AE. Complication of bowel perforation during insertion of tension-free vaginal tape. J Urol. 2003;170(4 Pt 1):1310.-
11. Abouassaly R, Steinberg JR, Lemieux M, et al. Complications of tension-free vaginal tape surgery: a multi-institutional review. BJU Int. 2004;94:110-113.
12. Delorme E, Droupy S, de Tayrac R, Delmas V. [Transobturator tape (UraTape). A new minimally invasive method in the treatment of urinary incontinence in women.] Prog Urol. 2003;13:656-659.
13. de Leval J. Novel surgical technique for the treatment of female stress urinary incontinence: transobturator vaginal tape inside-out. Eur Urol. 2003;44:724-730.
14. De Tayrac R, Deffieux X, Droupy S, ChauveaudLambling A, Calvanese-Benamour L, Fernandez H. A prospective randomized trial comparing tensionfree vaginal tape and transobturator suburethral tape for surgical treatment of stress urinary incontinence. Am J Obstet Gynecol. 2004;190:602-608.
15. Richardson AC. The rectovaginal septum revisited: its relationship to rectocele and its importance in rectocele repair. Clin Obstet Gynecol. 1993;36:976-983.
16. Miklos JR, Kohli N, Lucente V, Saye WB. Site-specific fascial defects in the diagnosis and surgical management of enterocele. Am J Obstet Gynecol. 1998;179 (6 Pt 1):1418-1422; discussion 1422-823.
17. Porter WE, Steele A, Walsh P, Kohli N, Karram MM. The anatomic and functional outcomes of defectspecific rectocele repairs. Am J Obstet Gynecol. 1999;181:1353-1358; discussion 1358-1359.
18. Kenton K, Shott S, Brubaker L. Outcome after rectovaginal fascia reattachment for rectocele repair. Am J Obstet Gynecol. 1999;181:1360-1363; discussion 1363-1364.
19. Flood CG, Drutz HP, Waja L. Anterior colporrhaphy reinforced with Marlex mesh for the treatment of cystoceles. Int Urogynecol J Pelvic Floor Dysfunct. 1998;9:200-204.
20. Koduri S, Lobel RW, Winkler HA, Tomezsko J, Culligan PJ, Sand PK. Prospective randomized trial of polyglactin 910 mesh to prevent recurrence of cystoceles and rectoceles. Proceedings of the 25th Annual Meeting of the International Urogynecological Association, 2000. Int Urogynecol J Pelvic Floor Dysfunct. 2000;11:S80.-
21. Clemons JL, Myers DL, Aguilar VC, Arya LA. Vaginal paravaginal repair with an AlloDerm graft. Am J Obstet Gynecol. 2003;189:1612-1618; discussion 1618-1619.
22. Fox SD, Stanton SL. Vault prolapse and rectocele: assessment of repair using sacrocolpopexy with mesh interposition. BJOG. 2000;107:1371-1375.
23. Kohli N, Miklos JR. Site specific rectocele repair augmented with a cadaveric dermal graft. Proceedings of the 21st Annual Scientific Meeting of the American Urogynecologic Society, 2000. Int Urogynecol J Pelvic Floor Dysfunct. 2001;12:S9.-
24. Miklos JR, Kohli N. Rectovaginal fistula repair utilizing a cadaveric dermal allograft. Int Urogynecol J Pelvic Floor Dysfunct. 1999;10:405-406.
1. Kelly HA, Dunn WM. Urinary incontinence in women without manifest injury to the bladder. Surg Gynecol Obstet. 1914;18:444.-
2. Ulmsten U, Petros P. Intravaginal slingplasty (IVS): an ambulatory surgical procedure for treatment of female urinary incontinence. Scand J Urol Nephrol. 1995;29:75-82.
3. Weinberger MW, Ostergard DR. Postoperative catheterization, urinary retention, and permanent voiding dysfunction after polytetrafluroethylene suburethral sling placement. Obstet Gynecol. 1996;87:50-54.
4. Nilsson CG, Kuuva N, Falconer C, Rezapour M, Ulmsten U. Long-term results of the tension-free vaginal tape (TVT) procedure for surgical treatment of female stress urinary incontinence. Int Urogynecol J Pelvic Floor Dysfunct. 2001;12(Suppl 2):S5-8.
5. Nilsson CG, Rezapour M, Falconer C. Seven years follow-up of the tension-free vaginal tape (TVT) procedure. Presented at the 2003 International Urogynecology Association Annual Meeting.
6. Horbach N. Suburethral sling procedures. In: Ostergard DR, Bent AE, eds. Urogynecology and Urodynamics: Theory and Practice. 3rd ed. Baltimore: Williams & Wilkins; 1991;449-458.
7. Stanton SL, Reynolds SF, Creighton SM. The modified Pereyra (Raz) procedure for genuine stress incontinence—a useful option in the elderly or frail patient? Int Urogynecol J. 1995;6:22-25.
8. Nygaard IE, Kreder KJ. Complications of incontinence surgery. Int Urogynecol J. 1994;5:353-360.
9. Lockhart vJL, Tirado A, Morillo G, Politano VA. Vesicourethral dysfunction following cystourethropexy. J Urol. 1982;128:943-945.
10. Leboeuf L, Tellez CA, Ead D, Gousse AE. Complication of bowel perforation during insertion of tension-free vaginal tape. J Urol. 2003;170(4 Pt 1):1310.-
11. Abouassaly R, Steinberg JR, Lemieux M, et al. Complications of tension-free vaginal tape surgery: a multi-institutional review. BJU Int. 2004;94:110-113.
12. Delorme E, Droupy S, de Tayrac R, Delmas V. [Transobturator tape (UraTape). A new minimally invasive method in the treatment of urinary incontinence in women.] Prog Urol. 2003;13:656-659.
13. de Leval J. Novel surgical technique for the treatment of female stress urinary incontinence: transobturator vaginal tape inside-out. Eur Urol. 2003;44:724-730.
14. De Tayrac R, Deffieux X, Droupy S, ChauveaudLambling A, Calvanese-Benamour L, Fernandez H. A prospective randomized trial comparing tensionfree vaginal tape and transobturator suburethral tape for surgical treatment of stress urinary incontinence. Am J Obstet Gynecol. 2004;190:602-608.
15. Richardson AC. The rectovaginal septum revisited: its relationship to rectocele and its importance in rectocele repair. Clin Obstet Gynecol. 1993;36:976-983.
16. Miklos JR, Kohli N, Lucente V, Saye WB. Site-specific fascial defects in the diagnosis and surgical management of enterocele. Am J Obstet Gynecol. 1998;179 (6 Pt 1):1418-1422; discussion 1422-823.
17. Porter WE, Steele A, Walsh P, Kohli N, Karram MM. The anatomic and functional outcomes of defectspecific rectocele repairs. Am J Obstet Gynecol. 1999;181:1353-1358; discussion 1358-1359.
18. Kenton K, Shott S, Brubaker L. Outcome after rectovaginal fascia reattachment for rectocele repair. Am J Obstet Gynecol. 1999;181:1360-1363; discussion 1363-1364.
19. Flood CG, Drutz HP, Waja L. Anterior colporrhaphy reinforced with Marlex mesh for the treatment of cystoceles. Int Urogynecol J Pelvic Floor Dysfunct. 1998;9:200-204.
20. Koduri S, Lobel RW, Winkler HA, Tomezsko J, Culligan PJ, Sand PK. Prospective randomized trial of polyglactin 910 mesh to prevent recurrence of cystoceles and rectoceles. Proceedings of the 25th Annual Meeting of the International Urogynecological Association, 2000. Int Urogynecol J Pelvic Floor Dysfunct. 2000;11:S80.-
21. Clemons JL, Myers DL, Aguilar VC, Arya LA. Vaginal paravaginal repair with an AlloDerm graft. Am J Obstet Gynecol. 2003;189:1612-1618; discussion 1618-1619.
22. Fox SD, Stanton SL. Vault prolapse and rectocele: assessment of repair using sacrocolpopexy with mesh interposition. BJOG. 2000;107:1371-1375.
23. Kohli N, Miklos JR. Site specific rectocele repair augmented with a cadaveric dermal graft. Proceedings of the 21st Annual Scientific Meeting of the American Urogynecologic Society, 2000. Int Urogynecol J Pelvic Floor Dysfunct. 2001;12:S9.-
24. Miklos JR, Kohli N. Rectovaginal fistula repair utilizing a cadaveric dermal allograft. Int Urogynecol J Pelvic Floor Dysfunct. 1999;10:405-406.
Avoiding vascular injury at laparoscopy
- Distances between the entry trocar and the aorta bifurcation increase directly with body mass index, mainly because of the commensurate increase in abdominal wall thickness.
- The mean thrusting force for insertion of a disposable trocar is 10.2 lb versus 17.53 lb for a reusable device, and the time to penetrate is shorter for the disposable trocar: mean of 3.54 seconds versus 11.64 seconds. Thus, greater caution is warranted when inserting a disposable trocar.
- Thrust the primary trocar into the midline of the abdomen at a 45° to 60° angle relative to the plane of the abdominal wall, with the trocar pointing toward the uterus, to avoid injuring the iliac vessels.
- When injury occurs, call for a vascular surgeon immediately, perform a laparotomy using a vertical incision, and get accurate inputs, outputs, and blood-loss estimates.
Major vessel injury is a two-sided coin: It can occur with alarming speed, but it is preventable.
Fortunately, the laparoscopic surgeon can avoid the problem by following simple precautions and steering clear of scenarios that increase the risk of injury. This article tells how to accomplish both objectives.
In the process, it reviews the evidence, details management for any injuries that occur, and includes a comprehensive table listing typical distances between the entry trocar and vascular structures, to help the surgeon adjust entry strategy.
Adequate prevention depends on:
- familiarity with the vascular anatomy, particularly in relation to the umbilicus, presacral space, infundibulopelvic ligament, and ovarian fossa.
- creating a proper pneumoperitoneum, especially when using disposable trocars.
- careful attention to primary trocar thrusting techniques to ensure midline insertion at the proper angle. Also exercise caution when placing secondary trocars. Specifically, during far lateral insertion, avoid cleaving the inferior epigastric artery from the external iliac or directly hitting the external artery or vein.
- avoiding long trocars, which are unnecessary to penetrate the peritoneal cavity.
- reliance on laparotomy if trocar insertion proves too difficult, vision is obscured, or appropriate anatomic dissection planes cannot be developed.
- when injury occurs, performing laparotomy using a vertical incision.
A 36-year-old woman with a body mass index of 38.2, indicating severe obesity, is scheduled to undergo hysteroscopy and dilatation and curettage for irregular bleeding, as well as laparoscopic bilateral partial salpingectomy for elective sterilization. The setting is an outpatient surgery center without a blood bank.
After general anesthesia, the surgeon makes a 1.5-cm incision just below the umbilicus, inserts a Verres needle, and insufflates carbon dioxide gas to a volume of approximately 3.4 L. He then inserts a disposable trocar and places a laparoscope, but views fat. Unbeknownst to him, he has insufflated the properitoneal fat space rather than the peritoneum.
The surgeon finally enters the peritoneum with a “long” trocar after several more attempts. Since the uterus and adnexa appear to be normal, he inserts a second trocar and places a probe. As he is moving the intestines, however, he observes blood, and the field suddenly becomes unclear. He removes the probe and, when the gas-pressure valve of the secondary trocar is opened, blood spews from the site.
The surgeon removes all trocars and performs an emergency laparotomy using a Pfannenstiel incision. He and 2 general surgeons, who arrive within 20 to 30 minutes, work for 2 hours to repair what they believe is a hole in the inferior vena cava. The woman is brought out of anesthesia and transferred to the local community hospital, where she goes into cardiac arrest and dies. A postmortem reveals injury to the right common iliac artery and vein. No sutures were observed in either vessel. Cause of death: exsanguination.
What went wrong?
Three serious errors contributed to the patient’s death:
- He made multiple attempts to insert the trocar without considering the possibility that the wrong space had been insufflated.
- He inserted the trocar off the midline and at the wrong angle relative to the abdominal wall.
- In his frustration, he switched to a “long” trocar, which made it more likely that vascular structures would be injured.
Operating on an obese patient in a center without a blood bank also was unwise, as obese women of short stature are at greatest risk for vascular injury.
How big is the problem?
A French study1 of 103,852 laparoscopic procedures—of which 15.7%, or 16,000 operations, were gynecologic—reported 47 cases of major vascular injury for an incidence of 0.5 per 1,000 cases and a mortality rate of 17%. Several additional articles2-8 reported a range of vascular complications of between 0.1 and 6.4 per 1,000 laparoscopies.
In a study9 conducted in 7 gynecologic laparoscopy surgery centers in France over 9 years and involving 29,966 diagnostic and operative cases, the overall complication rate was 4.64 per 1,000 laparoscopies (n = 139). Of the 21 major vascular injuries associated with gynecologic surgery, the majority occurred during set-up, and 84.6% during insertion of the primary trocar. Two patients died from their injuries.
Bhoyrul and colleagues10 analyzed data reported to the US Food and Drug Administration and found that 408 of 629 trocar-related injuries involved major blood vessels, as did 26 of 32 deaths (81%). Most of the deaths (87%) were linked to the use of disposable trocars equipped with safety shields; 9% with direct-view trocars. Although surgeons asserted that the trocar malfunctioned in 41 cases, that claim was confirmed in only 1 case (2%).
Another study found that 37 of 79 (46.8%) serious complications involving optical-access trocars between 1994 and 2002 involved major vessels, injuring the aorta, iliac vessels, or vena cava.11
A study12 carried out in the Netherlands in 1994 evaluated the relative number of complications that occurred within a total of 25,764 laparoscopic procedures. The study divided complications into those occurring as the result of the laparoscopic approach (eg, trocar insertion) versus those happening during the performance of the operation. Fifty-seven percent of the 145 complications were caused by the laparoscopic approach; the 2 reported deaths also were secondary to that approach.
Snapshot of vascular injury: A series of 31 patients
In 2003, I published data13 on 31 cases of major vessel injury associated with gynecologic laparoscopy (see). These cases were collected from a variety of sources: medicolegal case files, hospital morbidity-mortality presentations, and quality-assurance departments. Eight cases involved diagnostic procedures, while 23 involved operative laparoscopy.
The medical records of these cases provided details on the nature of the injury. The cases were categorized by body mass index (BMI) and cause, ie, whether they occurred as the result of the laparoscopic approach (ie, entry-related) or arose during surgery.
Of the 31 cases, 22 (71%) involved women with BMIs from 25 to more than 30 (overweight or obese). A large majority—28 cases (90%)—were related to entry. Only 3 injuries occurred during surgery.
In several women, more than 1 vessel was damaged. Of the 49 total injuries, 38 (78%) involved the iliac vessels. Seven (23%) women died as a result of their injuries, all of which involved venous trauma.
Damage to structures in the vicinity of the injured vessels was substantial in 16 cases. Major morbidity included ureteral, nerve, and intestinal injury; arterial and venous thrombosis; compartment syndrome; and suturing of the wrong vessel.
Some patients also experienced edema or pain in an extremity (vascular insufficiency); infection; diffuse intravascular coagulation and/or adult respiratory distress; cardiac arrest; central nervous system injury (stroke); or hospitalization of more than 1 week. Cases also were categorized as early or late diagnosis, depending on whether shock had supervened. Diagnosis was early in 8 cases (26%) and late in 21 (68%). Two patients were diagnosed postoperatively; ie, they had gone to the recovery room prior to developing shock.
The volume of blood loss ranged from 1,000 mL to 7,000 mL, with a mean loss of 3,400 mL. All patients received packed red blood cells and/or a mixture of other blood products. The time required for cross-matching and receiving blood ranged from 10 to 120 minutes.
In all cases, a vascular or general surgeon was called to consult on the case.
Mapping vascular structures to ensure safe trocar entry
Knowing the distances between blood vessels and laparoscopic entry trocars is critical if injury is to be avoided. In pursuit of this goal, Hurd and colleagues14 performed a retrospective study involving women who had undergone magnetic resonance imaging or computed tomography scans of the abdomen. Investigators measured the distance between the lower abdominal wall and the aortic bifurcation in these women, who were all unanesthetized and in the supine position.
Distances increased with BMI
This occurred in the study by Hurd et al,14 as well as in a prospective study by Narendran and Baggish,15 who calculated body mass index in 101 consecutive women who were undergoing diagnostic or operative laparoscopy. These women were anesthetized, with pneumoperitoneum established and a laparoscope inserted; all were in the lithotomy position.
In this study, Narendran and Baggish measured the following distances from the entry trocar:
- perpendicular distance to aortic bifurcation,
- oblique distance to the right and left common iliac vessels,
- oblique distance to the superior margin of the bladder,
- perpendicular distance from the peritoneum to skin at the umbilicus (abdominal wall thickness), and
- oblique distance from the subumbilical peritoneal opening to the right and left common iliac vessels.
Wide range of BMIs
In the study by Narendran and Baggish, successful measurement panels were created for 99 of the 101 cases. Of these, 49 women had a BMI of less than 25 (normal), 29 had a BMI greater than 25 but less than 30 (overweight), and 21 had a BMI greater than 30 (obese).
A significant difference was observed in the perpendicular distance from the entry trocar to aortic bifurcation (TABLE 1). Specifically, as the BMI increased, so did the distance. The only other significant BMI-related increase was the abdominal wall thickness, which also varied directly with the BMI.
Other distances increase with height
The distance between the primary trocar and the iliac vessels and urinary bladder consistently increased with the patient’s height.
However, no significant change in distance between the great vessels and the primary trocar site occurred when the patient’s position changed from level to Trendelenburg.
Trocar insertion: Disposable devices require less force
Laparoscopic trocar thrusting is a dynamic process, and we observed that process in our study.15 When force is applied via trocar to the anterior abdominal wall, that structure is displaced toward the abdominal cavity in the direction of the posterior abdominal wall—even when countertraction is taken into consideration. The movement is more apparent in obese women because of greater elasticity created by the larger mass of properitoneal and subcutaneous fat. We measured the distortion and determined that the depression can be 5 cm or more.
In contrast, thin women have rigid, relatively unyielding anterior abdominal walls and therefore experience minimal displacement. In thin women, the greater risk is the shorter passive distance between the anterior abdominal wall and the great vessels.
Comparing force curves
We16 calculated the force required to thrust a disposable or reusable trocar through the anterior abdominal wall during actual laparoscopic surgery. We used a 25-lb compression load cell connected to the trocar by an Ultem handle, which could be sterilized between cases. A linear variable displacement transducer detected displacement, and the measuring apparata fed data into a computer. Ten women were randomized to a disposable trocar and 10 to a reusable device.
The mean thrusting force for disposable trocars was 10.2 lb versus 17.53 lb for the reusable device. The time to penetrate was likewise significantly shortened for disposable trocars: mean time of 3.54 seconds versus 11.64 seconds. Overall work tilted in favor of disposable trocars: 14.34 pound-seconds versus 103.88 pound-seconds.
The disposable trocar has the advantage for 2 reasons: its razor-sharp cutting edge and streamlined design.
FIGURE 1 shows typical force curves of disposable and reusable trocars.
FIGURE 1 Reusable trocar requires more force than disposable trocar
A considerable difference in force is required for insertion, depending on type of device, as this graph of typical force curves shows. The reusable trocar requires 18 to 20 lb of force over 12 seconds; the disposable, only 5 lb over 2 seconds.
Safe trocar insertion begins with pneumoperitoneum
McDougall et al17 demonstrated that adequate pneumoperitoneum lessens the force required to drive a trocar through the anterior abdominal wall. Although the differences were small, the forces required with an intraperitoneal gas pressure of 30 mm Hg were smaller than those required with a pressure of 15 mm Hg.17
Manufacturers of disposable trocars also recommend creating an adequate pneumoperitoneum prior to aiming and inserting the razor-sharp device. The goal is creating a carbon dioxide gas pocket large enough to permit rapid deployment of the “safety shield” after the trocar tip clears the properitoneal fat and peritoneal membrane.
Slow-motion video sequences of disposable trocar entry show the sharp trocar tip penetrating the parietal peritoneum of the anterior abdominal wall for 1 cm before the spring-loaded shield advances and locks over the blade. During this insertion, the anterior abdominal wall has an elastic reaction to the applied force; this reaction pushes it toward the posterior abdominal wall.
Direct insertions (ie, without adequate pneumoperitoneum) involve less space for the trocar’s safety shield to deploy. Thus, there is a greater risk of the armed trocar tip coming into direct contact with underlying viscera and blood vessels.
Delayed diagnosis
The earlier a major vessel incident can be diagnosed, the better for patient, physician, and hospital. Diagnosis after the onset of hypotension, tachycardia, or tachypnia constitutes “late” diagnosis. Dark venous blood pooling in the abdomen, bright red pulsatile blood emitting from a trocar sleeve, or a retroperitoneal hematoma lateral to the iliacs or at the level of the presacral space suggests major vessel injury. Signs of hypovolemic shock or sudden appearance of profound shock places the possibility of major vessel injury at the top of the differential diagnosis.
Relying on observation when a retroperitoneal hematoma develops
Unfortunately, with observation, the surgeon cannot determine the identity or nature of the damaged vessel, know whether the hematoma is expanding beyond the view of the laparoscope, or predict when the patient will go into shock.
Leaving an armed trocar in place in a vessel
Assuming that the trocar is plugging a hole and preventing hemorrhage is a recipe for disaster. The movement of the sharp device against a vessel wall is most likely to create greater trauma to the vessel. In the case of partial penetration, the device may cut the rest of the way through the vessel.
Laparoscopic exploration
Attempts to locate the injury via laparoscopy usually are unsuccessful, and laparoscopic attempts to sew up the injury limit accuracy and efficacy.
Use of the Pfannenstiel incision during emergency laparotomy
Unfortunately, in 1 study,13 27 of 31 women with vascular injuries received this incision. A vertical incision is preferred because it affords greater access and visibility.
Underestimating blood loss
In the case of a major vessel injury, underestimation of blood volume requirements can be fatal. In 1 study,13 19 of 31 women were under-transfused and/or inadequately cross-matched.
Clamping injured vessels
This can lead to arterial or venous thrombosis. Nonvascular clamps can tear large vessels, adding to the damage and complicating the vascular surgeon’s attempt at repair. Rather, apply direct pressure with a sponge stick.
Delay in calling for help
This translates into greater blood loss and a less stable patient. In 1 study,13 the mean time for a vascular surgeon to intercede was 23 minutes.
Use a shorter insufflation needle
Our data on women with a BMI greater than 30 (obese range) indicate that the mean thickness of the anterior abdominal wall is 5.05 cm and the distance to the aorta is 15.14 cm.15 A standard Verres needle measures 12.5 cm from the tip of the shaft to the point where the shaft joins the hub of the needle. This is clearly excessive length, since women with a BMI above 30 have an abdominal wall thickness of approximately 5.05 cm and women with BMIs between 25 and 30 have a thickness of only 3.85 cm.
I prefer a Touhy epidural needle for subumbilical insertion and creation of the pneumoperitoneum, since it is a relatively short 8.5 cm. Thus, it is less hazardous than the Verres needle. It also is less likely to clog with tissue fragments because of its curved tip, and more likely to create a successful pneumoperitoneum on the first try.
Fortunately, large-vessel injuries caused by the insufflation needle are rare.
Proper insertion technique
I have residents draw a straight line with a marking pen from the lower margin of the umbilicus to the superior margin of the pubic symphysis. This serves as a guide to keep the trocar pointing toward the middle of the abdomen, away from the iliac vessels. I also teach residents to thrust the trocar in the midline at a 45° to 60° angle in relation to the plane of the abdominal wall, with the trocar pointing toward the uterus (FIGURES 2 AND 3).
Many residents twist disposable trocars during insertion. This “door knob” movement works against the design of the trocar and traumatizes tissue. The correct approach is thrusting the device into the abdominal cavity, or holding the trocar (only for disposable trocar devices) like a dart and thrusting it into the abdomen as though throwing a dart. The only trocar designed for twisting is the conical reusable device; the sharp pyramidal reusable trocar should be thrust rather than twisted.
Avoid “long” trocars
These are a full 5 cm longer than the 20-cm standard device (hub of handle to tip of shaft). Abdominal wall measurements indicate that these devices are never required to simply penetrate the anterior abdominal wall; these trocars also carry the risk of hitting the iliac vessels.
Open laparoscopy is not foolproof
Although open laparoscopy would seem to guarantee safe entry of the primary trocar, reports of aortic injuries have recently been published. Similar data have been reported for optical access trocars.11,18
FIGURE 2 Insert the trocar at 45° to 60° angle
At insertion, the trocar should be at a 45°to 60°angle relative to the abdominal wall, with the tip of the device tilted in the direction of the uterus and bladder. A 90°angle of insertion is dangerous.
FIGURE 3 Midline insertion is safest
Insert the primary trocar in the midline pointing toward the uterus; deviation to the right or left is dangerous. Also avoid injuring the inferior epigastric and external iliac vessels with far lateral trocar insertion.
Body habitus and vascular injury
The obese patient of short stature is at the greatest risk for vascular injury. Although the relative distances between the anterior abdominal wall and the aorta are greater at the highest BMI levels, short stature means that the iliac vessels are closer. Significantly, large vessel injuries in the series cited herein were associated with the use of disposable trocars 90% of the time.
I believe high-risk conditions are created when carbon dioxide gas is inadvertently infused into the properitoneal fat space (FIGURE 4). As the volume of gas grows, the anterior wall parietal peritoneum dissects free from the remainder of the anterior abdominal fat, creating a pseudo-pneumoperitoneum. The operator fails to realize that the true peritoneal cavity has not been entered and, in fact, has paradoxically constricted in size because of the enlarging pseudoperitoneal space. Careful attention to the pressure gauges would have aroused suspicion that gas was being infused into the wrong space, since pressures tend to be higher and flow erratic in such situations.
Nevertheless, the surgeon places a trocar into the space, looks through the laparoscope, sees red or yellow, and realizes that the peritoneal cavity has not been entered. More gas is insufflated and the trocar is tried again.
Typically, the duller, reusable trocar pushes the leading edge of the peritoneum rather than penetrating it, further enlarging the properitoneal space and bringing the anterior and posterior peritoneal walls very close together.
In another scenario, the same set of circumstances exists except, rather than employing a reusable trocar, the surgeon selects a disposable device or even, after 2 failures to enter the peritoneal cavity with the reusable device, an extra-long (11-inch) disposable trocar (FIGURE 4).
In this scenario, an armed trocar enters the pseudospace—without the safety shield deployed—because no resistance was encountered during penetration of the incision, owing to the fact that two 10–12-mm trocars have previously traversed the same skin incision.
As the tip of the trocar comes into contact with the leading edge of the peritoneum, it encounters resistance, and the razor-sharp blade cuts through the anterior peritoneum, traverses the narrow peritoneal space, and cuts through the posterior peritoneum and the underlying great vessel.
Often, the trocar’s knife edge injures an artery by glancing off the curved surface of the vessels and embedding itself in the neighboring or underlying vein.
The best technique to manage a pseudo-pneumoperitoneal pocket is to abandon the subumbilical site, insert a Touhy needle in the left upper quadrant, and enter and overinflate the peritoneal cavity, thereby obliterating the properitoneal gas space.
When injury occurs: 7 recommended management steps
In the event of a vascular injury, early diagnosis and treatment are vital. Do not observe retroperitoneal hematomas. The following steps are recommended:
- Call for a vascular surgeon immediately and indicate that the situation is an emergency. Do not waste time trying to locate the injury before calling for help.
- Get emergency type and cross-match for at least 6 U of whole blood.
- Obtain baseline lab measurements, including hemoglobin, hematocrit, platelets, fibrinogen, and fibrin split products.
- Open the abdomen using a vertical incision for maximum access and visibility.
- Get accurate outputs and blood-loss estimates and have anesthesia keep careful records of fluids given.
- Advise anesthesia staff to obtain additional help. This will facilitate starting additional IV sites, rapidly infusing blood products, obtaining key samples for laboratory data, and maintaining accurate and detailed records of blood gases, blood loss, replacement fluids, and blood products.
- Use a circulator to manage urgent medications or laboratory tests.
The author reports no financial relationships relevant to this article.
1. Champault G, Cazacu F, Taffinader N. Serious trocar accidents in laparoscopic surgery: a French survey of 103,852 operations. Surg Laparosc Endosc. 1996;6:367.
2. Baadsgaard SE, Bille S, Egelblad K. Major vascular injury during gynecologic laparoscopy: report of a case and review of published cases. Acta Obstet Gynecol Scand. 1989;68:283.
3. Chamberlain G, Brown JD. Gynecologic laparoscopy: report of the working party of the confidential enquiry into gynecologic laparoscopy. Br J Obstet Gynaecol. 1978;85:401.
4. Mintz M. Risk and prophylaxis in laparoscopy: a survey of 100,000 cases. J Reprod Med. 1977;18:269.
5. Phillips JM, Hulka JF, Peterson HB. American Association of Gynecologic Laparoscopists’ 1982 Membership Survey. J Reprod Med. 1984;29:592.
6. Saidi MH, Vancaillie TG, White AJ, et al. Complications of major operative laparoscopy: a review of 452 cases. J Reprod Med. 1996;41:471.
7. Loffer F, Pent D. Indications, contraindications, and complications of laparoscopy. Obstet Gynecol Surv. 1975;30:407.
8. Härkki-Sirén P, Kurki T. A nationwide analysis of laparoscopic complications. Obstet Gynecol. 1997;89:108.
9. Chapron C, Querleu D, Bruhat MA, et al. Surgical complications of diagnostic and operative gynecologic laparoscopy: a series of 29,966 cases. Hum Reprod. 1998;13:867-872.
10. Bhoyrul S, Vierra MA, Nezhat CR, Krummel TM, Way LW. Trocar injuries in laparoscopic surgery. J Am Coll Surg. 2001;192:677-683.
11. Sharp HT, Dodson MK, Draper ML, Watts DA, Doucette RC, Hurd WW. Complications associated with optical-access laparoscopic trocars. Obstet Gynecol. 2002;99:553-555.
12. Jansen FW, Kapiteyn K, Trimbos-Kemper T, Herman J, Trimbos JB. Complications of laparoscopy: a prospective multi-center observational study. Br J Obstet Gynaecol. 1997;104:595-600.
13. Baggish MS. Analysis of 31 cases of major vessel injury associated with gynecologic laparoscopy operations. J Gynecol Surg. 2003;19:63-73.
14. Hurd WW, Bude RO, DeLancey JOL, Gauvin JM, Aisen AM. Abdominal wall characterization by MRI and CT imaging: the effect of obesity on laparoscopic approach. J Reprod Med. 1991;36:473.
15. Narendran M, Baggish MS. Mean distance between primary trocar insertion site and major retroperitoneal vessels during routine laparoscopy. J Gynecol Surg. 2002;18:121-127.
16. Baggish MS, Gandhi S, Kasper G. Force required by laparoscopic trocar devices to penetrate the human female’s anterior abdominal wall. J Gynecol Surg. 2003;19:1-11.
17. McDougall EM, Figenshau RS, Clayman RV, Monk TG, Smith DS. Laparoscopic pneumoperitoneum: impact of body habitus. J Laparosc Endosc Surg. 1994;4:385-391.
18. Hanney RM, Carmalt HL, Merrett N, Tait N. Use of Hassan cannula producing major vascular injury at laparoscopy. Surg Endosc. 1999;13:1238-1240.
- Distances between the entry trocar and the aorta bifurcation increase directly with body mass index, mainly because of the commensurate increase in abdominal wall thickness.
- The mean thrusting force for insertion of a disposable trocar is 10.2 lb versus 17.53 lb for a reusable device, and the time to penetrate is shorter for the disposable trocar: mean of 3.54 seconds versus 11.64 seconds. Thus, greater caution is warranted when inserting a disposable trocar.
- Thrust the primary trocar into the midline of the abdomen at a 45° to 60° angle relative to the plane of the abdominal wall, with the trocar pointing toward the uterus, to avoid injuring the iliac vessels.
- When injury occurs, call for a vascular surgeon immediately, perform a laparotomy using a vertical incision, and get accurate inputs, outputs, and blood-loss estimates.
Major vessel injury is a two-sided coin: It can occur with alarming speed, but it is preventable.
Fortunately, the laparoscopic surgeon can avoid the problem by following simple precautions and steering clear of scenarios that increase the risk of injury. This article tells how to accomplish both objectives.
In the process, it reviews the evidence, details management for any injuries that occur, and includes a comprehensive table listing typical distances between the entry trocar and vascular structures, to help the surgeon adjust entry strategy.
Adequate prevention depends on:
- familiarity with the vascular anatomy, particularly in relation to the umbilicus, presacral space, infundibulopelvic ligament, and ovarian fossa.
- creating a proper pneumoperitoneum, especially when using disposable trocars.
- careful attention to primary trocar thrusting techniques to ensure midline insertion at the proper angle. Also exercise caution when placing secondary trocars. Specifically, during far lateral insertion, avoid cleaving the inferior epigastric artery from the external iliac or directly hitting the external artery or vein.
- avoiding long trocars, which are unnecessary to penetrate the peritoneal cavity.
- reliance on laparotomy if trocar insertion proves too difficult, vision is obscured, or appropriate anatomic dissection planes cannot be developed.
- when injury occurs, performing laparotomy using a vertical incision.
A 36-year-old woman with a body mass index of 38.2, indicating severe obesity, is scheduled to undergo hysteroscopy and dilatation and curettage for irregular bleeding, as well as laparoscopic bilateral partial salpingectomy for elective sterilization. The setting is an outpatient surgery center without a blood bank.
After general anesthesia, the surgeon makes a 1.5-cm incision just below the umbilicus, inserts a Verres needle, and insufflates carbon dioxide gas to a volume of approximately 3.4 L. He then inserts a disposable trocar and places a laparoscope, but views fat. Unbeknownst to him, he has insufflated the properitoneal fat space rather than the peritoneum.
The surgeon finally enters the peritoneum with a “long” trocar after several more attempts. Since the uterus and adnexa appear to be normal, he inserts a second trocar and places a probe. As he is moving the intestines, however, he observes blood, and the field suddenly becomes unclear. He removes the probe and, when the gas-pressure valve of the secondary trocar is opened, blood spews from the site.
The surgeon removes all trocars and performs an emergency laparotomy using a Pfannenstiel incision. He and 2 general surgeons, who arrive within 20 to 30 minutes, work for 2 hours to repair what they believe is a hole in the inferior vena cava. The woman is brought out of anesthesia and transferred to the local community hospital, where she goes into cardiac arrest and dies. A postmortem reveals injury to the right common iliac artery and vein. No sutures were observed in either vessel. Cause of death: exsanguination.
What went wrong?
Three serious errors contributed to the patient’s death:
- He made multiple attempts to insert the trocar without considering the possibility that the wrong space had been insufflated.
- He inserted the trocar off the midline and at the wrong angle relative to the abdominal wall.
- In his frustration, he switched to a “long” trocar, which made it more likely that vascular structures would be injured.
Operating on an obese patient in a center without a blood bank also was unwise, as obese women of short stature are at greatest risk for vascular injury.
How big is the problem?
A French study1 of 103,852 laparoscopic procedures—of which 15.7%, or 16,000 operations, were gynecologic—reported 47 cases of major vascular injury for an incidence of 0.5 per 1,000 cases and a mortality rate of 17%. Several additional articles2-8 reported a range of vascular complications of between 0.1 and 6.4 per 1,000 laparoscopies.
In a study9 conducted in 7 gynecologic laparoscopy surgery centers in France over 9 years and involving 29,966 diagnostic and operative cases, the overall complication rate was 4.64 per 1,000 laparoscopies (n = 139). Of the 21 major vascular injuries associated with gynecologic surgery, the majority occurred during set-up, and 84.6% during insertion of the primary trocar. Two patients died from their injuries.
Bhoyrul and colleagues10 analyzed data reported to the US Food and Drug Administration and found that 408 of 629 trocar-related injuries involved major blood vessels, as did 26 of 32 deaths (81%). Most of the deaths (87%) were linked to the use of disposable trocars equipped with safety shields; 9% with direct-view trocars. Although surgeons asserted that the trocar malfunctioned in 41 cases, that claim was confirmed in only 1 case (2%).
Another study found that 37 of 79 (46.8%) serious complications involving optical-access trocars between 1994 and 2002 involved major vessels, injuring the aorta, iliac vessels, or vena cava.11
A study12 carried out in the Netherlands in 1994 evaluated the relative number of complications that occurred within a total of 25,764 laparoscopic procedures. The study divided complications into those occurring as the result of the laparoscopic approach (eg, trocar insertion) versus those happening during the performance of the operation. Fifty-seven percent of the 145 complications were caused by the laparoscopic approach; the 2 reported deaths also were secondary to that approach.
Snapshot of vascular injury: A series of 31 patients
In 2003, I published data13 on 31 cases of major vessel injury associated with gynecologic laparoscopy (see). These cases were collected from a variety of sources: medicolegal case files, hospital morbidity-mortality presentations, and quality-assurance departments. Eight cases involved diagnostic procedures, while 23 involved operative laparoscopy.
The medical records of these cases provided details on the nature of the injury. The cases were categorized by body mass index (BMI) and cause, ie, whether they occurred as the result of the laparoscopic approach (ie, entry-related) or arose during surgery.
Of the 31 cases, 22 (71%) involved women with BMIs from 25 to more than 30 (overweight or obese). A large majority—28 cases (90%)—were related to entry. Only 3 injuries occurred during surgery.
In several women, more than 1 vessel was damaged. Of the 49 total injuries, 38 (78%) involved the iliac vessels. Seven (23%) women died as a result of their injuries, all of which involved venous trauma.
Damage to structures in the vicinity of the injured vessels was substantial in 16 cases. Major morbidity included ureteral, nerve, and intestinal injury; arterial and venous thrombosis; compartment syndrome; and suturing of the wrong vessel.
Some patients also experienced edema or pain in an extremity (vascular insufficiency); infection; diffuse intravascular coagulation and/or adult respiratory distress; cardiac arrest; central nervous system injury (stroke); or hospitalization of more than 1 week. Cases also were categorized as early or late diagnosis, depending on whether shock had supervened. Diagnosis was early in 8 cases (26%) and late in 21 (68%). Two patients were diagnosed postoperatively; ie, they had gone to the recovery room prior to developing shock.
The volume of blood loss ranged from 1,000 mL to 7,000 mL, with a mean loss of 3,400 mL. All patients received packed red blood cells and/or a mixture of other blood products. The time required for cross-matching and receiving blood ranged from 10 to 120 minutes.
In all cases, a vascular or general surgeon was called to consult on the case.
Mapping vascular structures to ensure safe trocar entry
Knowing the distances between blood vessels and laparoscopic entry trocars is critical if injury is to be avoided. In pursuit of this goal, Hurd and colleagues14 performed a retrospective study involving women who had undergone magnetic resonance imaging or computed tomography scans of the abdomen. Investigators measured the distance between the lower abdominal wall and the aortic bifurcation in these women, who were all unanesthetized and in the supine position.
Distances increased with BMI
This occurred in the study by Hurd et al,14 as well as in a prospective study by Narendran and Baggish,15 who calculated body mass index in 101 consecutive women who were undergoing diagnostic or operative laparoscopy. These women were anesthetized, with pneumoperitoneum established and a laparoscope inserted; all were in the lithotomy position.
In this study, Narendran and Baggish measured the following distances from the entry trocar:
- perpendicular distance to aortic bifurcation,
- oblique distance to the right and left common iliac vessels,
- oblique distance to the superior margin of the bladder,
- perpendicular distance from the peritoneum to skin at the umbilicus (abdominal wall thickness), and
- oblique distance from the subumbilical peritoneal opening to the right and left common iliac vessels.
Wide range of BMIs
In the study by Narendran and Baggish, successful measurement panels were created for 99 of the 101 cases. Of these, 49 women had a BMI of less than 25 (normal), 29 had a BMI greater than 25 but less than 30 (overweight), and 21 had a BMI greater than 30 (obese).
A significant difference was observed in the perpendicular distance from the entry trocar to aortic bifurcation (TABLE 1). Specifically, as the BMI increased, so did the distance. The only other significant BMI-related increase was the abdominal wall thickness, which also varied directly with the BMI.
Other distances increase with height
The distance between the primary trocar and the iliac vessels and urinary bladder consistently increased with the patient’s height.
However, no significant change in distance between the great vessels and the primary trocar site occurred when the patient’s position changed from level to Trendelenburg.
Trocar insertion: Disposable devices require less force
Laparoscopic trocar thrusting is a dynamic process, and we observed that process in our study.15 When force is applied via trocar to the anterior abdominal wall, that structure is displaced toward the abdominal cavity in the direction of the posterior abdominal wall—even when countertraction is taken into consideration. The movement is more apparent in obese women because of greater elasticity created by the larger mass of properitoneal and subcutaneous fat. We measured the distortion and determined that the depression can be 5 cm or more.
In contrast, thin women have rigid, relatively unyielding anterior abdominal walls and therefore experience minimal displacement. In thin women, the greater risk is the shorter passive distance between the anterior abdominal wall and the great vessels.
Comparing force curves
We16 calculated the force required to thrust a disposable or reusable trocar through the anterior abdominal wall during actual laparoscopic surgery. We used a 25-lb compression load cell connected to the trocar by an Ultem handle, which could be sterilized between cases. A linear variable displacement transducer detected displacement, and the measuring apparata fed data into a computer. Ten women were randomized to a disposable trocar and 10 to a reusable device.
The mean thrusting force for disposable trocars was 10.2 lb versus 17.53 lb for the reusable device. The time to penetrate was likewise significantly shortened for disposable trocars: mean time of 3.54 seconds versus 11.64 seconds. Overall work tilted in favor of disposable trocars: 14.34 pound-seconds versus 103.88 pound-seconds.
The disposable trocar has the advantage for 2 reasons: its razor-sharp cutting edge and streamlined design.
FIGURE 1 shows typical force curves of disposable and reusable trocars.
FIGURE 1 Reusable trocar requires more force than disposable trocar
A considerable difference in force is required for insertion, depending on type of device, as this graph of typical force curves shows. The reusable trocar requires 18 to 20 lb of force over 12 seconds; the disposable, only 5 lb over 2 seconds.
Safe trocar insertion begins with pneumoperitoneum
McDougall et al17 demonstrated that adequate pneumoperitoneum lessens the force required to drive a trocar through the anterior abdominal wall. Although the differences were small, the forces required with an intraperitoneal gas pressure of 30 mm Hg were smaller than those required with a pressure of 15 mm Hg.17
Manufacturers of disposable trocars also recommend creating an adequate pneumoperitoneum prior to aiming and inserting the razor-sharp device. The goal is creating a carbon dioxide gas pocket large enough to permit rapid deployment of the “safety shield” after the trocar tip clears the properitoneal fat and peritoneal membrane.
Slow-motion video sequences of disposable trocar entry show the sharp trocar tip penetrating the parietal peritoneum of the anterior abdominal wall for 1 cm before the spring-loaded shield advances and locks over the blade. During this insertion, the anterior abdominal wall has an elastic reaction to the applied force; this reaction pushes it toward the posterior abdominal wall.
Direct insertions (ie, without adequate pneumoperitoneum) involve less space for the trocar’s safety shield to deploy. Thus, there is a greater risk of the armed trocar tip coming into direct contact with underlying viscera and blood vessels.
Delayed diagnosis
The earlier a major vessel incident can be diagnosed, the better for patient, physician, and hospital. Diagnosis after the onset of hypotension, tachycardia, or tachypnia constitutes “late” diagnosis. Dark venous blood pooling in the abdomen, bright red pulsatile blood emitting from a trocar sleeve, or a retroperitoneal hematoma lateral to the iliacs or at the level of the presacral space suggests major vessel injury. Signs of hypovolemic shock or sudden appearance of profound shock places the possibility of major vessel injury at the top of the differential diagnosis.
Relying on observation when a retroperitoneal hematoma develops
Unfortunately, with observation, the surgeon cannot determine the identity or nature of the damaged vessel, know whether the hematoma is expanding beyond the view of the laparoscope, or predict when the patient will go into shock.
Leaving an armed trocar in place in a vessel
Assuming that the trocar is plugging a hole and preventing hemorrhage is a recipe for disaster. The movement of the sharp device against a vessel wall is most likely to create greater trauma to the vessel. In the case of partial penetration, the device may cut the rest of the way through the vessel.
Laparoscopic exploration
Attempts to locate the injury via laparoscopy usually are unsuccessful, and laparoscopic attempts to sew up the injury limit accuracy and efficacy.
Use of the Pfannenstiel incision during emergency laparotomy
Unfortunately, in 1 study,13 27 of 31 women with vascular injuries received this incision. A vertical incision is preferred because it affords greater access and visibility.
Underestimating blood loss
In the case of a major vessel injury, underestimation of blood volume requirements can be fatal. In 1 study,13 19 of 31 women were under-transfused and/or inadequately cross-matched.
Clamping injured vessels
This can lead to arterial or venous thrombosis. Nonvascular clamps can tear large vessels, adding to the damage and complicating the vascular surgeon’s attempt at repair. Rather, apply direct pressure with a sponge stick.
Delay in calling for help
This translates into greater blood loss and a less stable patient. In 1 study,13 the mean time for a vascular surgeon to intercede was 23 minutes.
Use a shorter insufflation needle
Our data on women with a BMI greater than 30 (obese range) indicate that the mean thickness of the anterior abdominal wall is 5.05 cm and the distance to the aorta is 15.14 cm.15 A standard Verres needle measures 12.5 cm from the tip of the shaft to the point where the shaft joins the hub of the needle. This is clearly excessive length, since women with a BMI above 30 have an abdominal wall thickness of approximately 5.05 cm and women with BMIs between 25 and 30 have a thickness of only 3.85 cm.
I prefer a Touhy epidural needle for subumbilical insertion and creation of the pneumoperitoneum, since it is a relatively short 8.5 cm. Thus, it is less hazardous than the Verres needle. It also is less likely to clog with tissue fragments because of its curved tip, and more likely to create a successful pneumoperitoneum on the first try.
Fortunately, large-vessel injuries caused by the insufflation needle are rare.
Proper insertion technique
I have residents draw a straight line with a marking pen from the lower margin of the umbilicus to the superior margin of the pubic symphysis. This serves as a guide to keep the trocar pointing toward the middle of the abdomen, away from the iliac vessels. I also teach residents to thrust the trocar in the midline at a 45° to 60° angle in relation to the plane of the abdominal wall, with the trocar pointing toward the uterus (FIGURES 2 AND 3).
Many residents twist disposable trocars during insertion. This “door knob” movement works against the design of the trocar and traumatizes tissue. The correct approach is thrusting the device into the abdominal cavity, or holding the trocar (only for disposable trocar devices) like a dart and thrusting it into the abdomen as though throwing a dart. The only trocar designed for twisting is the conical reusable device; the sharp pyramidal reusable trocar should be thrust rather than twisted.
Avoid “long” trocars
These are a full 5 cm longer than the 20-cm standard device (hub of handle to tip of shaft). Abdominal wall measurements indicate that these devices are never required to simply penetrate the anterior abdominal wall; these trocars also carry the risk of hitting the iliac vessels.
Open laparoscopy is not foolproof
Although open laparoscopy would seem to guarantee safe entry of the primary trocar, reports of aortic injuries have recently been published. Similar data have been reported for optical access trocars.11,18
FIGURE 2 Insert the trocar at 45° to 60° angle
At insertion, the trocar should be at a 45°to 60°angle relative to the abdominal wall, with the tip of the device tilted in the direction of the uterus and bladder. A 90°angle of insertion is dangerous.
FIGURE 3 Midline insertion is safest
Insert the primary trocar in the midline pointing toward the uterus; deviation to the right or left is dangerous. Also avoid injuring the inferior epigastric and external iliac vessels with far lateral trocar insertion.
Body habitus and vascular injury
The obese patient of short stature is at the greatest risk for vascular injury. Although the relative distances between the anterior abdominal wall and the aorta are greater at the highest BMI levels, short stature means that the iliac vessels are closer. Significantly, large vessel injuries in the series cited herein were associated with the use of disposable trocars 90% of the time.
I believe high-risk conditions are created when carbon dioxide gas is inadvertently infused into the properitoneal fat space (FIGURE 4). As the volume of gas grows, the anterior wall parietal peritoneum dissects free from the remainder of the anterior abdominal fat, creating a pseudo-pneumoperitoneum. The operator fails to realize that the true peritoneal cavity has not been entered and, in fact, has paradoxically constricted in size because of the enlarging pseudoperitoneal space. Careful attention to the pressure gauges would have aroused suspicion that gas was being infused into the wrong space, since pressures tend to be higher and flow erratic in such situations.
Nevertheless, the surgeon places a trocar into the space, looks through the laparoscope, sees red or yellow, and realizes that the peritoneal cavity has not been entered. More gas is insufflated and the trocar is tried again.
Typically, the duller, reusable trocar pushes the leading edge of the peritoneum rather than penetrating it, further enlarging the properitoneal space and bringing the anterior and posterior peritoneal walls very close together.
In another scenario, the same set of circumstances exists except, rather than employing a reusable trocar, the surgeon selects a disposable device or even, after 2 failures to enter the peritoneal cavity with the reusable device, an extra-long (11-inch) disposable trocar (FIGURE 4).
In this scenario, an armed trocar enters the pseudospace—without the safety shield deployed—because no resistance was encountered during penetration of the incision, owing to the fact that two 10–12-mm trocars have previously traversed the same skin incision.
As the tip of the trocar comes into contact with the leading edge of the peritoneum, it encounters resistance, and the razor-sharp blade cuts through the anterior peritoneum, traverses the narrow peritoneal space, and cuts through the posterior peritoneum and the underlying great vessel.
Often, the trocar’s knife edge injures an artery by glancing off the curved surface of the vessels and embedding itself in the neighboring or underlying vein.
The best technique to manage a pseudo-pneumoperitoneal pocket is to abandon the subumbilical site, insert a Touhy needle in the left upper quadrant, and enter and overinflate the peritoneal cavity, thereby obliterating the properitoneal gas space.
When injury occurs: 7 recommended management steps
In the event of a vascular injury, early diagnosis and treatment are vital. Do not observe retroperitoneal hematomas. The following steps are recommended:
- Call for a vascular surgeon immediately and indicate that the situation is an emergency. Do not waste time trying to locate the injury before calling for help.
- Get emergency type and cross-match for at least 6 U of whole blood.
- Obtain baseline lab measurements, including hemoglobin, hematocrit, platelets, fibrinogen, and fibrin split products.
- Open the abdomen using a vertical incision for maximum access and visibility.
- Get accurate outputs and blood-loss estimates and have anesthesia keep careful records of fluids given.
- Advise anesthesia staff to obtain additional help. This will facilitate starting additional IV sites, rapidly infusing blood products, obtaining key samples for laboratory data, and maintaining accurate and detailed records of blood gases, blood loss, replacement fluids, and blood products.
- Use a circulator to manage urgent medications or laboratory tests.
The author reports no financial relationships relevant to this article.
- Distances between the entry trocar and the aorta bifurcation increase directly with body mass index, mainly because of the commensurate increase in abdominal wall thickness.
- The mean thrusting force for insertion of a disposable trocar is 10.2 lb versus 17.53 lb for a reusable device, and the time to penetrate is shorter for the disposable trocar: mean of 3.54 seconds versus 11.64 seconds. Thus, greater caution is warranted when inserting a disposable trocar.
- Thrust the primary trocar into the midline of the abdomen at a 45° to 60° angle relative to the plane of the abdominal wall, with the trocar pointing toward the uterus, to avoid injuring the iliac vessels.
- When injury occurs, call for a vascular surgeon immediately, perform a laparotomy using a vertical incision, and get accurate inputs, outputs, and blood-loss estimates.
Major vessel injury is a two-sided coin: It can occur with alarming speed, but it is preventable.
Fortunately, the laparoscopic surgeon can avoid the problem by following simple precautions and steering clear of scenarios that increase the risk of injury. This article tells how to accomplish both objectives.
In the process, it reviews the evidence, details management for any injuries that occur, and includes a comprehensive table listing typical distances between the entry trocar and vascular structures, to help the surgeon adjust entry strategy.
Adequate prevention depends on:
- familiarity with the vascular anatomy, particularly in relation to the umbilicus, presacral space, infundibulopelvic ligament, and ovarian fossa.
- creating a proper pneumoperitoneum, especially when using disposable trocars.
- careful attention to primary trocar thrusting techniques to ensure midline insertion at the proper angle. Also exercise caution when placing secondary trocars. Specifically, during far lateral insertion, avoid cleaving the inferior epigastric artery from the external iliac or directly hitting the external artery or vein.
- avoiding long trocars, which are unnecessary to penetrate the peritoneal cavity.
- reliance on laparotomy if trocar insertion proves too difficult, vision is obscured, or appropriate anatomic dissection planes cannot be developed.
- when injury occurs, performing laparotomy using a vertical incision.
A 36-year-old woman with a body mass index of 38.2, indicating severe obesity, is scheduled to undergo hysteroscopy and dilatation and curettage for irregular bleeding, as well as laparoscopic bilateral partial salpingectomy for elective sterilization. The setting is an outpatient surgery center without a blood bank.
After general anesthesia, the surgeon makes a 1.5-cm incision just below the umbilicus, inserts a Verres needle, and insufflates carbon dioxide gas to a volume of approximately 3.4 L. He then inserts a disposable trocar and places a laparoscope, but views fat. Unbeknownst to him, he has insufflated the properitoneal fat space rather than the peritoneum.
The surgeon finally enters the peritoneum with a “long” trocar after several more attempts. Since the uterus and adnexa appear to be normal, he inserts a second trocar and places a probe. As he is moving the intestines, however, he observes blood, and the field suddenly becomes unclear. He removes the probe and, when the gas-pressure valve of the secondary trocar is opened, blood spews from the site.
The surgeon removes all trocars and performs an emergency laparotomy using a Pfannenstiel incision. He and 2 general surgeons, who arrive within 20 to 30 minutes, work for 2 hours to repair what they believe is a hole in the inferior vena cava. The woman is brought out of anesthesia and transferred to the local community hospital, where she goes into cardiac arrest and dies. A postmortem reveals injury to the right common iliac artery and vein. No sutures were observed in either vessel. Cause of death: exsanguination.
What went wrong?
Three serious errors contributed to the patient’s death:
- He made multiple attempts to insert the trocar without considering the possibility that the wrong space had been insufflated.
- He inserted the trocar off the midline and at the wrong angle relative to the abdominal wall.
- In his frustration, he switched to a “long” trocar, which made it more likely that vascular structures would be injured.
Operating on an obese patient in a center without a blood bank also was unwise, as obese women of short stature are at greatest risk for vascular injury.
How big is the problem?
A French study1 of 103,852 laparoscopic procedures—of which 15.7%, or 16,000 operations, were gynecologic—reported 47 cases of major vascular injury for an incidence of 0.5 per 1,000 cases and a mortality rate of 17%. Several additional articles2-8 reported a range of vascular complications of between 0.1 and 6.4 per 1,000 laparoscopies.
In a study9 conducted in 7 gynecologic laparoscopy surgery centers in France over 9 years and involving 29,966 diagnostic and operative cases, the overall complication rate was 4.64 per 1,000 laparoscopies (n = 139). Of the 21 major vascular injuries associated with gynecologic surgery, the majority occurred during set-up, and 84.6% during insertion of the primary trocar. Two patients died from their injuries.
Bhoyrul and colleagues10 analyzed data reported to the US Food and Drug Administration and found that 408 of 629 trocar-related injuries involved major blood vessels, as did 26 of 32 deaths (81%). Most of the deaths (87%) were linked to the use of disposable trocars equipped with safety shields; 9% with direct-view trocars. Although surgeons asserted that the trocar malfunctioned in 41 cases, that claim was confirmed in only 1 case (2%).
Another study found that 37 of 79 (46.8%) serious complications involving optical-access trocars between 1994 and 2002 involved major vessels, injuring the aorta, iliac vessels, or vena cava.11
A study12 carried out in the Netherlands in 1994 evaluated the relative number of complications that occurred within a total of 25,764 laparoscopic procedures. The study divided complications into those occurring as the result of the laparoscopic approach (eg, trocar insertion) versus those happening during the performance of the operation. Fifty-seven percent of the 145 complications were caused by the laparoscopic approach; the 2 reported deaths also were secondary to that approach.
Snapshot of vascular injury: A series of 31 patients
In 2003, I published data13 on 31 cases of major vessel injury associated with gynecologic laparoscopy (see). These cases were collected from a variety of sources: medicolegal case files, hospital morbidity-mortality presentations, and quality-assurance departments. Eight cases involved diagnostic procedures, while 23 involved operative laparoscopy.
The medical records of these cases provided details on the nature of the injury. The cases were categorized by body mass index (BMI) and cause, ie, whether they occurred as the result of the laparoscopic approach (ie, entry-related) or arose during surgery.
Of the 31 cases, 22 (71%) involved women with BMIs from 25 to more than 30 (overweight or obese). A large majority—28 cases (90%)—were related to entry. Only 3 injuries occurred during surgery.
In several women, more than 1 vessel was damaged. Of the 49 total injuries, 38 (78%) involved the iliac vessels. Seven (23%) women died as a result of their injuries, all of which involved venous trauma.
Damage to structures in the vicinity of the injured vessels was substantial in 16 cases. Major morbidity included ureteral, nerve, and intestinal injury; arterial and venous thrombosis; compartment syndrome; and suturing of the wrong vessel.
Some patients also experienced edema or pain in an extremity (vascular insufficiency); infection; diffuse intravascular coagulation and/or adult respiratory distress; cardiac arrest; central nervous system injury (stroke); or hospitalization of more than 1 week. Cases also were categorized as early or late diagnosis, depending on whether shock had supervened. Diagnosis was early in 8 cases (26%) and late in 21 (68%). Two patients were diagnosed postoperatively; ie, they had gone to the recovery room prior to developing shock.
The volume of blood loss ranged from 1,000 mL to 7,000 mL, with a mean loss of 3,400 mL. All patients received packed red blood cells and/or a mixture of other blood products. The time required for cross-matching and receiving blood ranged from 10 to 120 minutes.
In all cases, a vascular or general surgeon was called to consult on the case.
Mapping vascular structures to ensure safe trocar entry
Knowing the distances between blood vessels and laparoscopic entry trocars is critical if injury is to be avoided. In pursuit of this goal, Hurd and colleagues14 performed a retrospective study involving women who had undergone magnetic resonance imaging or computed tomography scans of the abdomen. Investigators measured the distance between the lower abdominal wall and the aortic bifurcation in these women, who were all unanesthetized and in the supine position.
Distances increased with BMI
This occurred in the study by Hurd et al,14 as well as in a prospective study by Narendran and Baggish,15 who calculated body mass index in 101 consecutive women who were undergoing diagnostic or operative laparoscopy. These women were anesthetized, with pneumoperitoneum established and a laparoscope inserted; all were in the lithotomy position.
In this study, Narendran and Baggish measured the following distances from the entry trocar:
- perpendicular distance to aortic bifurcation,
- oblique distance to the right and left common iliac vessels,
- oblique distance to the superior margin of the bladder,
- perpendicular distance from the peritoneum to skin at the umbilicus (abdominal wall thickness), and
- oblique distance from the subumbilical peritoneal opening to the right and left common iliac vessels.
Wide range of BMIs
In the study by Narendran and Baggish, successful measurement panels were created for 99 of the 101 cases. Of these, 49 women had a BMI of less than 25 (normal), 29 had a BMI greater than 25 but less than 30 (overweight), and 21 had a BMI greater than 30 (obese).
A significant difference was observed in the perpendicular distance from the entry trocar to aortic bifurcation (TABLE 1). Specifically, as the BMI increased, so did the distance. The only other significant BMI-related increase was the abdominal wall thickness, which also varied directly with the BMI.
Other distances increase with height
The distance between the primary trocar and the iliac vessels and urinary bladder consistently increased with the patient’s height.
However, no significant change in distance between the great vessels and the primary trocar site occurred when the patient’s position changed from level to Trendelenburg.
Trocar insertion: Disposable devices require less force
Laparoscopic trocar thrusting is a dynamic process, and we observed that process in our study.15 When force is applied via trocar to the anterior abdominal wall, that structure is displaced toward the abdominal cavity in the direction of the posterior abdominal wall—even when countertraction is taken into consideration. The movement is more apparent in obese women because of greater elasticity created by the larger mass of properitoneal and subcutaneous fat. We measured the distortion and determined that the depression can be 5 cm or more.
In contrast, thin women have rigid, relatively unyielding anterior abdominal walls and therefore experience minimal displacement. In thin women, the greater risk is the shorter passive distance between the anterior abdominal wall and the great vessels.
Comparing force curves
We16 calculated the force required to thrust a disposable or reusable trocar through the anterior abdominal wall during actual laparoscopic surgery. We used a 25-lb compression load cell connected to the trocar by an Ultem handle, which could be sterilized between cases. A linear variable displacement transducer detected displacement, and the measuring apparata fed data into a computer. Ten women were randomized to a disposable trocar and 10 to a reusable device.
The mean thrusting force for disposable trocars was 10.2 lb versus 17.53 lb for the reusable device. The time to penetrate was likewise significantly shortened for disposable trocars: mean time of 3.54 seconds versus 11.64 seconds. Overall work tilted in favor of disposable trocars: 14.34 pound-seconds versus 103.88 pound-seconds.
The disposable trocar has the advantage for 2 reasons: its razor-sharp cutting edge and streamlined design.
FIGURE 1 shows typical force curves of disposable and reusable trocars.
FIGURE 1 Reusable trocar requires more force than disposable trocar
A considerable difference in force is required for insertion, depending on type of device, as this graph of typical force curves shows. The reusable trocar requires 18 to 20 lb of force over 12 seconds; the disposable, only 5 lb over 2 seconds.
Safe trocar insertion begins with pneumoperitoneum
McDougall et al17 demonstrated that adequate pneumoperitoneum lessens the force required to drive a trocar through the anterior abdominal wall. Although the differences were small, the forces required with an intraperitoneal gas pressure of 30 mm Hg were smaller than those required with a pressure of 15 mm Hg.17
Manufacturers of disposable trocars also recommend creating an adequate pneumoperitoneum prior to aiming and inserting the razor-sharp device. The goal is creating a carbon dioxide gas pocket large enough to permit rapid deployment of the “safety shield” after the trocar tip clears the properitoneal fat and peritoneal membrane.
Slow-motion video sequences of disposable trocar entry show the sharp trocar tip penetrating the parietal peritoneum of the anterior abdominal wall for 1 cm before the spring-loaded shield advances and locks over the blade. During this insertion, the anterior abdominal wall has an elastic reaction to the applied force; this reaction pushes it toward the posterior abdominal wall.
Direct insertions (ie, without adequate pneumoperitoneum) involve less space for the trocar’s safety shield to deploy. Thus, there is a greater risk of the armed trocar tip coming into direct contact with underlying viscera and blood vessels.
Delayed diagnosis
The earlier a major vessel incident can be diagnosed, the better for patient, physician, and hospital. Diagnosis after the onset of hypotension, tachycardia, or tachypnia constitutes “late” diagnosis. Dark venous blood pooling in the abdomen, bright red pulsatile blood emitting from a trocar sleeve, or a retroperitoneal hematoma lateral to the iliacs or at the level of the presacral space suggests major vessel injury. Signs of hypovolemic shock or sudden appearance of profound shock places the possibility of major vessel injury at the top of the differential diagnosis.
Relying on observation when a retroperitoneal hematoma develops
Unfortunately, with observation, the surgeon cannot determine the identity or nature of the damaged vessel, know whether the hematoma is expanding beyond the view of the laparoscope, or predict when the patient will go into shock.
Leaving an armed trocar in place in a vessel
Assuming that the trocar is plugging a hole and preventing hemorrhage is a recipe for disaster. The movement of the sharp device against a vessel wall is most likely to create greater trauma to the vessel. In the case of partial penetration, the device may cut the rest of the way through the vessel.
Laparoscopic exploration
Attempts to locate the injury via laparoscopy usually are unsuccessful, and laparoscopic attempts to sew up the injury limit accuracy and efficacy.
Use of the Pfannenstiel incision during emergency laparotomy
Unfortunately, in 1 study,13 27 of 31 women with vascular injuries received this incision. A vertical incision is preferred because it affords greater access and visibility.
Underestimating blood loss
In the case of a major vessel injury, underestimation of blood volume requirements can be fatal. In 1 study,13 19 of 31 women were under-transfused and/or inadequately cross-matched.
Clamping injured vessels
This can lead to arterial or venous thrombosis. Nonvascular clamps can tear large vessels, adding to the damage and complicating the vascular surgeon’s attempt at repair. Rather, apply direct pressure with a sponge stick.
Delay in calling for help
This translates into greater blood loss and a less stable patient. In 1 study,13 the mean time for a vascular surgeon to intercede was 23 minutes.
Use a shorter insufflation needle
Our data on women with a BMI greater than 30 (obese range) indicate that the mean thickness of the anterior abdominal wall is 5.05 cm and the distance to the aorta is 15.14 cm.15 A standard Verres needle measures 12.5 cm from the tip of the shaft to the point where the shaft joins the hub of the needle. This is clearly excessive length, since women with a BMI above 30 have an abdominal wall thickness of approximately 5.05 cm and women with BMIs between 25 and 30 have a thickness of only 3.85 cm.
I prefer a Touhy epidural needle for subumbilical insertion and creation of the pneumoperitoneum, since it is a relatively short 8.5 cm. Thus, it is less hazardous than the Verres needle. It also is less likely to clog with tissue fragments because of its curved tip, and more likely to create a successful pneumoperitoneum on the first try.
Fortunately, large-vessel injuries caused by the insufflation needle are rare.
Proper insertion technique
I have residents draw a straight line with a marking pen from the lower margin of the umbilicus to the superior margin of the pubic symphysis. This serves as a guide to keep the trocar pointing toward the middle of the abdomen, away from the iliac vessels. I also teach residents to thrust the trocar in the midline at a 45° to 60° angle in relation to the plane of the abdominal wall, with the trocar pointing toward the uterus (FIGURES 2 AND 3).
Many residents twist disposable trocars during insertion. This “door knob” movement works against the design of the trocar and traumatizes tissue. The correct approach is thrusting the device into the abdominal cavity, or holding the trocar (only for disposable trocar devices) like a dart and thrusting it into the abdomen as though throwing a dart. The only trocar designed for twisting is the conical reusable device; the sharp pyramidal reusable trocar should be thrust rather than twisted.
Avoid “long” trocars
These are a full 5 cm longer than the 20-cm standard device (hub of handle to tip of shaft). Abdominal wall measurements indicate that these devices are never required to simply penetrate the anterior abdominal wall; these trocars also carry the risk of hitting the iliac vessels.
Open laparoscopy is not foolproof
Although open laparoscopy would seem to guarantee safe entry of the primary trocar, reports of aortic injuries have recently been published. Similar data have been reported for optical access trocars.11,18
FIGURE 2 Insert the trocar at 45° to 60° angle
At insertion, the trocar should be at a 45°to 60°angle relative to the abdominal wall, with the tip of the device tilted in the direction of the uterus and bladder. A 90°angle of insertion is dangerous.
FIGURE 3 Midline insertion is safest
Insert the primary trocar in the midline pointing toward the uterus; deviation to the right or left is dangerous. Also avoid injuring the inferior epigastric and external iliac vessels with far lateral trocar insertion.
Body habitus and vascular injury
The obese patient of short stature is at the greatest risk for vascular injury. Although the relative distances between the anterior abdominal wall and the aorta are greater at the highest BMI levels, short stature means that the iliac vessels are closer. Significantly, large vessel injuries in the series cited herein were associated with the use of disposable trocars 90% of the time.
I believe high-risk conditions are created when carbon dioxide gas is inadvertently infused into the properitoneal fat space (FIGURE 4). As the volume of gas grows, the anterior wall parietal peritoneum dissects free from the remainder of the anterior abdominal fat, creating a pseudo-pneumoperitoneum. The operator fails to realize that the true peritoneal cavity has not been entered and, in fact, has paradoxically constricted in size because of the enlarging pseudoperitoneal space. Careful attention to the pressure gauges would have aroused suspicion that gas was being infused into the wrong space, since pressures tend to be higher and flow erratic in such situations.
Nevertheless, the surgeon places a trocar into the space, looks through the laparoscope, sees red or yellow, and realizes that the peritoneal cavity has not been entered. More gas is insufflated and the trocar is tried again.
Typically, the duller, reusable trocar pushes the leading edge of the peritoneum rather than penetrating it, further enlarging the properitoneal space and bringing the anterior and posterior peritoneal walls very close together.
In another scenario, the same set of circumstances exists except, rather than employing a reusable trocar, the surgeon selects a disposable device or even, after 2 failures to enter the peritoneal cavity with the reusable device, an extra-long (11-inch) disposable trocar (FIGURE 4).
In this scenario, an armed trocar enters the pseudospace—without the safety shield deployed—because no resistance was encountered during penetration of the incision, owing to the fact that two 10–12-mm trocars have previously traversed the same skin incision.
As the tip of the trocar comes into contact with the leading edge of the peritoneum, it encounters resistance, and the razor-sharp blade cuts through the anterior peritoneum, traverses the narrow peritoneal space, and cuts through the posterior peritoneum and the underlying great vessel.
Often, the trocar’s knife edge injures an artery by glancing off the curved surface of the vessels and embedding itself in the neighboring or underlying vein.
The best technique to manage a pseudo-pneumoperitoneal pocket is to abandon the subumbilical site, insert a Touhy needle in the left upper quadrant, and enter and overinflate the peritoneal cavity, thereby obliterating the properitoneal gas space.
When injury occurs: 7 recommended management steps
In the event of a vascular injury, early diagnosis and treatment are vital. Do not observe retroperitoneal hematomas. The following steps are recommended:
- Call for a vascular surgeon immediately and indicate that the situation is an emergency. Do not waste time trying to locate the injury before calling for help.
- Get emergency type and cross-match for at least 6 U of whole blood.
- Obtain baseline lab measurements, including hemoglobin, hematocrit, platelets, fibrinogen, and fibrin split products.
- Open the abdomen using a vertical incision for maximum access and visibility.
- Get accurate outputs and blood-loss estimates and have anesthesia keep careful records of fluids given.
- Advise anesthesia staff to obtain additional help. This will facilitate starting additional IV sites, rapidly infusing blood products, obtaining key samples for laboratory data, and maintaining accurate and detailed records of blood gases, blood loss, replacement fluids, and blood products.
- Use a circulator to manage urgent medications or laboratory tests.
The author reports no financial relationships relevant to this article.
1. Champault G, Cazacu F, Taffinader N. Serious trocar accidents in laparoscopic surgery: a French survey of 103,852 operations. Surg Laparosc Endosc. 1996;6:367.
2. Baadsgaard SE, Bille S, Egelblad K. Major vascular injury during gynecologic laparoscopy: report of a case and review of published cases. Acta Obstet Gynecol Scand. 1989;68:283.
3. Chamberlain G, Brown JD. Gynecologic laparoscopy: report of the working party of the confidential enquiry into gynecologic laparoscopy. Br J Obstet Gynaecol. 1978;85:401.
4. Mintz M. Risk and prophylaxis in laparoscopy: a survey of 100,000 cases. J Reprod Med. 1977;18:269.
5. Phillips JM, Hulka JF, Peterson HB. American Association of Gynecologic Laparoscopists’ 1982 Membership Survey. J Reprod Med. 1984;29:592.
6. Saidi MH, Vancaillie TG, White AJ, et al. Complications of major operative laparoscopy: a review of 452 cases. J Reprod Med. 1996;41:471.
7. Loffer F, Pent D. Indications, contraindications, and complications of laparoscopy. Obstet Gynecol Surv. 1975;30:407.
8. Härkki-Sirén P, Kurki T. A nationwide analysis of laparoscopic complications. Obstet Gynecol. 1997;89:108.
9. Chapron C, Querleu D, Bruhat MA, et al. Surgical complications of diagnostic and operative gynecologic laparoscopy: a series of 29,966 cases. Hum Reprod. 1998;13:867-872.
10. Bhoyrul S, Vierra MA, Nezhat CR, Krummel TM, Way LW. Trocar injuries in laparoscopic surgery. J Am Coll Surg. 2001;192:677-683.
11. Sharp HT, Dodson MK, Draper ML, Watts DA, Doucette RC, Hurd WW. Complications associated with optical-access laparoscopic trocars. Obstet Gynecol. 2002;99:553-555.
12. Jansen FW, Kapiteyn K, Trimbos-Kemper T, Herman J, Trimbos JB. Complications of laparoscopy: a prospective multi-center observational study. Br J Obstet Gynaecol. 1997;104:595-600.
13. Baggish MS. Analysis of 31 cases of major vessel injury associated with gynecologic laparoscopy operations. J Gynecol Surg. 2003;19:63-73.
14. Hurd WW, Bude RO, DeLancey JOL, Gauvin JM, Aisen AM. Abdominal wall characterization by MRI and CT imaging: the effect of obesity on laparoscopic approach. J Reprod Med. 1991;36:473.
15. Narendran M, Baggish MS. Mean distance between primary trocar insertion site and major retroperitoneal vessels during routine laparoscopy. J Gynecol Surg. 2002;18:121-127.
16. Baggish MS, Gandhi S, Kasper G. Force required by laparoscopic trocar devices to penetrate the human female’s anterior abdominal wall. J Gynecol Surg. 2003;19:1-11.
17. McDougall EM, Figenshau RS, Clayman RV, Monk TG, Smith DS. Laparoscopic pneumoperitoneum: impact of body habitus. J Laparosc Endosc Surg. 1994;4:385-391.
18. Hanney RM, Carmalt HL, Merrett N, Tait N. Use of Hassan cannula producing major vascular injury at laparoscopy. Surg Endosc. 1999;13:1238-1240.
1. Champault G, Cazacu F, Taffinader N. Serious trocar accidents in laparoscopic surgery: a French survey of 103,852 operations. Surg Laparosc Endosc. 1996;6:367.
2. Baadsgaard SE, Bille S, Egelblad K. Major vascular injury during gynecologic laparoscopy: report of a case and review of published cases. Acta Obstet Gynecol Scand. 1989;68:283.
3. Chamberlain G, Brown JD. Gynecologic laparoscopy: report of the working party of the confidential enquiry into gynecologic laparoscopy. Br J Obstet Gynaecol. 1978;85:401.
4. Mintz M. Risk and prophylaxis in laparoscopy: a survey of 100,000 cases. J Reprod Med. 1977;18:269.
5. Phillips JM, Hulka JF, Peterson HB. American Association of Gynecologic Laparoscopists’ 1982 Membership Survey. J Reprod Med. 1984;29:592.
6. Saidi MH, Vancaillie TG, White AJ, et al. Complications of major operative laparoscopy: a review of 452 cases. J Reprod Med. 1996;41:471.
7. Loffer F, Pent D. Indications, contraindications, and complications of laparoscopy. Obstet Gynecol Surv. 1975;30:407.
8. Härkki-Sirén P, Kurki T. A nationwide analysis of laparoscopic complications. Obstet Gynecol. 1997;89:108.
9. Chapron C, Querleu D, Bruhat MA, et al. Surgical complications of diagnostic and operative gynecologic laparoscopy: a series of 29,966 cases. Hum Reprod. 1998;13:867-872.
10. Bhoyrul S, Vierra MA, Nezhat CR, Krummel TM, Way LW. Trocar injuries in laparoscopic surgery. J Am Coll Surg. 2001;192:677-683.
11. Sharp HT, Dodson MK, Draper ML, Watts DA, Doucette RC, Hurd WW. Complications associated with optical-access laparoscopic trocars. Obstet Gynecol. 2002;99:553-555.
12. Jansen FW, Kapiteyn K, Trimbos-Kemper T, Herman J, Trimbos JB. Complications of laparoscopy: a prospective multi-center observational study. Br J Obstet Gynaecol. 1997;104:595-600.
13. Baggish MS. Analysis of 31 cases of major vessel injury associated with gynecologic laparoscopy operations. J Gynecol Surg. 2003;19:63-73.
14. Hurd WW, Bude RO, DeLancey JOL, Gauvin JM, Aisen AM. Abdominal wall characterization by MRI and CT imaging: the effect of obesity on laparoscopic approach. J Reprod Med. 1991;36:473.
15. Narendran M, Baggish MS. Mean distance between primary trocar insertion site and major retroperitoneal vessels during routine laparoscopy. J Gynecol Surg. 2002;18:121-127.
16. Baggish MS, Gandhi S, Kasper G. Force required by laparoscopic trocar devices to penetrate the human female’s anterior abdominal wall. J Gynecol Surg. 2003;19:1-11.
17. McDougall EM, Figenshau RS, Clayman RV, Monk TG, Smith DS. Laparoscopic pneumoperitoneum: impact of body habitus. J Laparosc Endosc Surg. 1994;4:385-391.
18. Hanney RM, Carmalt HL, Merrett N, Tait N. Use of Hassan cannula producing major vascular injury at laparoscopy. Surg Endosc. 1999;13:1238-1240.
Subtotal vs total hysterectomy: Does the evidence support saving the cervix?
- Sexual function is not improved more with supracervical than with total hysterectomy.
- Operative morbidity for supracervical and total hysterectomy are similar.
- Pelvic-floor support and urinary incontinence do not seem to be improved with the supracervical approach.
- Cyclic bleeding occurs in 5% to 20% of women after supracervical hysterectomy.
- Reoperation rates for symptoms related to the retained cervix are significant—over 20% in the hands of highly skilled surgeons.
Hysterectomy is an obvious target. The number of hysterectomies performed has not declined substantially since these technologies were introduced, and persists at more than 550,000 per year in the United States. It is still the most widely performed major gynecological procedure.
Technological advances have made possible the use of laparoscopy to facilitate removal of the uterus without a major abdominal incision, with its inherent hazards. Many surgeons, seeking to make the most of new technology, have revisited laparoscopic subtotal hysterectomy, advocating preservation of the cervix to reduce surgical complications, sexual dysfunction, and pelvic-floor defects after hysterectomy.
New data, however—much of it released only in the last 12 to 18 months—tell us there is no difference in sexual function, pelvic floor support, or return to normal activities when the cervix is retained. What’s more, leaving the cervix in place puts the patient at greater risk of reoperation related to hysterectomy.
THEORY
Improved sexual function
EVIDENCE
Recent prospective analyses using validated measures of female sexual function have failed to demonstrate any advantage for supracervical hysterectomy.
Scientific study of sexual function is difficult at best. Many factors influence sexual behavior, and all must be considered when analyzing the effects of hysterectomy. To clearly understand the impact of hysterectomy on female sexual function, prospective studies in which women serve as their own controls provide the best quality evidence. That said, the contention that supracervical hysterectomy results in better sexual function than total hysterectomy stems from the research of a single group, which in 1983 retrospectively compared coital frequency, dyspareunia, libido, and orgasm after “supravaginal uterine amputation” with total hysterectomy.1,2
Simple hysterectomy causes minimal disruption of Frankenhauser’s plexus of autonomic nerves.
They theorized that supracervical operation preserves Frankenhauser’s plexus of autonomic nerves, resulting in better sexual function. However, careful anatomic assessment of the nerve content in the ligaments supporting the uterus has since demonstrated that the rich nerve supply to the uterosacral and cardinal ligaments occurs in the lateral two thirds of these structures. Simple hysterectomy causes minimal disruption of these autonomic nerves, ganglia, and extensions of the inferior hypogastric plexus.3
Thakar et al,4 in a pivotal multicenter, double-blind, randomized trial conducted in the United Kingdom, randomized 279 patients with benign disease to supracervical or total hysterectomy and followed them for 12 months. Surgical technique was standardized and the endocervix was coagulated in all patients.
The 2 groups were similar in measures of sexual function, including frequency of intercourse, orgasm, and rating of relationship with partner.
The Danish Hysterectomy Group5 randomized 319 patients with benign disease to total abdominal hysterectomy or subtotal abdominal hysterectomy, of whom 276 completed validated mailed questionnaires preoperatively, and at 2, 6, and 12 months postoperatively.
There was no change in sexual satisfaction in either group from their prehysterectomy levels. Overall quality of life improved significantly in both groups, in both mental and physical measures.
Roovers et al,6 in a multicenter, nonrandomized trial—powered well to detect 20% differences—compared vaginal hysterectomy, subtotal abdominal hysterectomy, and total abdominal hysterectomy (technique chosen by the surgeon).
Of the 379 patients recruited (from 13 teaching and nonteaching hospitals in the Netherlands) who had a male partner, 93% completed a validated questionnaire before and 6 months after surgery.
The questionnaire—used to assess sexual pleasure, activity, and problems—specifically addressed lubrication, orgasm, pain, and arousal on a 5-point scale (“not bothered” to “severely bothered”). Their findings:
- Sexual pleasure increased significantly in all groups regardless of type of hysterectomy.
- There was no difference in the incidence of bothersome sexual problems, but a significant number were reported: 43% after vaginal, 41% after subtotal, and 39% after total abdominal hysterectomy (P =.88).
- New sexual problems were reported in 9 patients (23%) after vaginal hysterectomy, 8 patients (24%) after subtotal hysterectomy, and 12 patients (19%) after total abdominal hysterectomy.
- There was a nonsignificant trend toward higher prevalence of arousal and lubrication problems after subtotal hysterectomy and total abdominal hysterectomy, compared with vaginal hysterectomy.
Theory
Improved pelvic floor support, less incontinence
Evidence
Pelvic floor support and urinary incontinence do not seem to be improved with the supracervical approach.
Proponents of supracervical hysterectomy argue that preservation of the cardinal and uterosacral ligaments will reduce the incidence of apical prolapse. In addition, maintenance of the pubocervical ring should lead to less posthysterectomy urinary incontinence.
Our newfound understanding that the nerves are, for the most part, spared at simple hysterectomy should argue against allegations that bowel and urinary function are better preserved by retaining the cervix.
Clearly, long-term outcome studies are required to assess these issues. The randomized trials thus far comparing supracervical with total hysterectomy have not followed patients beyond 2 years.
Nevertheless, at 12 to 24 months, published trials5,7 report an increased incidence of urinary incontinence in patients randomized to supracervical hysterectomy. Prolapse was also reported in a larger number of the patients undergoing subtotal as compared with total hysterectomy (1 to 2% versus 0% at 12 months).
The Total or Supracervical Hysterectomy (TOSH) Research Group7 conducted a multi-center randomized trial with a diverse patient population (78% of women were African American). From January 1998 through April 2000, 135 patients at 4 centers were randomized to supracervical hysterectomy or total abdominal hysterectomy. All patients had benign disease. Surgical technique varied by surgeon as in the general community. Patients and researchers were not blinded as to the technique performed. Subjects were followed for 2 years.
Women undergoing supracervical hysterectomy had a greater incidence of urinary incontinence after surgery.
Both techniques resulted in significant decreases in complaints of urinary incontinence and voiding dysfunction
The Danish Hysterectomy Group5 found that patients who had supracervical hysterectomies had a statistically greater incidence of urinary incontinence after surgery than those undergoing total abdominal hysterectomy (18% versus 9% P = .04). The incidence of new incontinence symptoms was 2.1% in the total abdominal hysterectomy group compared with 7.6% in the subtotal group. There was no change in bowel function in either group.
Thakar et al4 found urinary frequency declined significantly in both groups.
Theory
Fewer injuries and complications, less blood loss
Evidence
Randomized trials have offered no evidence to support a reduction in complication rates or bleeding requiring transfusion.
Since the creation of a bladder flap and division of the cardinal ligaments is not required in supracervical hysterectomy, we might theoretically expect to see reduced rates of injury to the ureters and bladder. Without the need for colpotomy, blood loss should also be reduced.
Cyclic bleeding may occur after supracervical hysterectomy even when residual endocervical tissue is cored or coagulated.
Given the low incidence of these complications at total hysterectomy, however, a meta-analysis of published randomized trials would be required to properly evaluate this issue. Thus far, randomized trials have offered no evidence to support a reduction in complication rates or bleeding requiring transfusion.
Thakar et al4 found a significant reduction in operating time as well as a reduction in blood loss (422 mL versus 320 mL) for the supracervical group compared with the total hysterectomy group; however there were no differences in the need for transfusion (5% in each group).
Women who underwent total abdominal hysterectomy had a higher incidence of fever while in the hospital (27% versus 10%), but there was no difference in the rate of infectious morbidity.
Within 1 year of discharge, more patients undergoing supracervical hysterectomy experienced complications: 7% had cyclic bleeding; 2% had cervical prolapse.
The TOSH Research Group7 found no difference in the rate of complications, activity limitations, or symptom improvement between groups. During the first 3 months, there was no difference in missed work, restricting activities, or bed rest. Both techniques resulted in significant decreases in complaints of pelvic pain, pressure, and back pain.
Of women undergoing supracervical hysterectomy, 5% had cyclic vaginal bleeding (only half of the patients in this series had the endocervix ablated).
Further, there were more readmissions related to the hysterectomy in the supracervical group, though this was not statistically significant (relative risk 1.99; 95% confidence interval, 0.58 to 6.8).
Twenty percent of women in the Danish Hysterectomy Group study 5 had persistent vaginal bleeding after subtotal hysterectomy; 2 went on to have trachelectomy for cyclic bleeding.
Prolapse of the cervical stump occurred in 3/136 patients after subtotal hysterectomy, versus no prolapse after total abdominal hysterectomy.
Outcomes after total versus subtotal abdominal hysterectomy. Thakar R, et al. N Engl J Med. 2002;347:1318–1325.4 Level I evidence
CONCLUSION Neither subtotal nor total abdominal hysterectomy adversely affected pelvic organ function at 12 months. Subtotal abdominal hysterectomy resulted in more rapid recovery and fewer short-term complications but infrequently caused cyclical bleeding or cervical prolapse.
- Pivotal multicenter, double-blind, randomized trial conducted in the United Kingdom.
- Randomized 279 patients with benign disease to supracervical or total hysterectomy and followed them for 12 months.
- Surgical technique was standardized and the endocervix coagulated in all patients.
Randomized controlled trial of total compared with subtotal hysterectomy with 1-year follow-up results. The Danish Hysterectomy Group. Br J Obstet Gynaecol. 2003;110:1088–1098.5 Level I evidence
CONCLUSION A smaller proportion of women suffered from urinary incontinence after total abdominal hysterectomy than after subtotal abdominal hysterectomy 1 year postoperatively.
- Multicenter, unblinded randomized trial conducted in Denmark.
- Randomized 319 patients with benign disease to total abdominal hysterectomy or subtotal abdominal hysterectomy, of whom 276 completed validated mailed questionnaires preoperatively, and at 2, 6, and 12 months postoperatively.
Hysterectomy and sexual well being: Prospective observational study of vaginal hysterectomy, subtotal abdominal hysterectomy, and total abdominal hysterectomy. Roovers JP, et al. Br Med J. 2003;327:774–779.6 Level II-1 evidence
CONCLUSION Sexual pleasure improved after vaginal hysterectomy, subtotal abdominal hysterectomy, and total abdominal hysterectomy. The persistence and development of bothersome problems during sexual activity were similar for all 3 techniques.
- Multicenter, nonrandomized trial conducted in the Netherlands.
- Investigated sexual function only.
- Compared vaginal, subtotal abdominal, and total abdominal hysterectomy (technique chosen by the surgeon).
- Of the 379 patients with a male partner, 93% completed a validated questionnaire before and 6 months after surgery.
A randomized comparison of total or supracervical hysterectomy: Surgical complications and clinical outcomes. Total or Supracervical Hysterectomy Research Group, Obstet Gynecol. 2003;102:453–462.7 Level I evidence
CONCLUSION We found no statistically significant differences between supracervical hysterectomy and total abdominal hysterectomy in surgical complications and clinical outcomes during 2 years of follow-up.
- Multicenter, unblinded randomized trial conducted in the United States.
- Randomized 135 patients with benign disease to supracervical hysterectomy or total abdominal hysterectomy and followed them for 2 years.
- Surgical technique varied by surgeon as in the general community.
- Only half of the patients had the endocervix ablated.
Long term outcome following laparoscopic supracervical hysterectomy. Okaro EO, et al. Br J Obstet Gynecol. 2001;108:1017–1020.8 Level II-3 evidence
CONCLUSION Symptoms related to the cervical stump requiring further surgery frequently occur following a laparoscopic supracervical hysterectomy.
- Retrospective analysis of case records for 70 patients.
- All subjects were women who would have otherwise undergone abdominal hysterectomy, but agreed to laparoscopic supracervical hysterectomy.
- All surgeries were performed by the same surgeon.
Long-term outcomes: the downside
For a mean of 66 months (range: 4 to 7 years), Okaro et al8 followed 70 patients undergoing laparoscopic supracervical hysterectomy by a single, highly skilled laparoscopic surgeon.
Their findings point out the downside of cervical preservation. Although all patients had the endocervical canal and transition zone cored out, over 24% reported symptoms related to the cervical stump—and all required further surgery. Further, cyclic vaginal bleeding occurred in 11% of women.
One patient developed cervical intraepithelial neoplasia. Dyspareunia and pelvic pain were significant complaints in 19% of the patients. (These women were more likely to have had hysterectomy for endometriosis.) Sixteen of the 17 patients with cervical complaints required trachelectomy within the follow-up time period.
“How empty is theory in the presence of fact!”
Mark Twain, A Connecticut Yankee in King Arthur’s Court
Practice recommendations
We, as clinicians, must accumulate evidence from basic science as well as clinical research, put it all together, and make recommendations based on these data. The data, tell us, in fact, that there is no difference in sexual dysfunction, pelvic floor support, or return to normal activity levels when the cervix is retained, and no evidence to support an advantage to supracervical hysterectomy.
My recommendation is for vaginal hysterectomy when possible. The theoretical advantages of retention of the cervix have driven many clinicians to abandon the vaginal approach in favor of laparoscopic supracervical hysterectomy; no data support this theory.
While not the focus of this article, ample data tell us that the vaginal approach, when technically feasible, is less invasive and carries fewer risks for our patients than laparoscopic or abdominal hysterectomy, and permits excellent access for support of the pelvic floor. I do think that patients who truly believe that their sex lives will be ruined after total hysterectomy or that they will do dramatically better if the cervix remains, may experience this self-fulfilling prophecy.
What I tell patients. I carefully review all the facts with patients in helping them select the appropriate surgical procedure.
I tell patients:
- That overwhelming evidence suggests that sexual function improves in the vast majority of women after hysterectomy, whether or not the cervix is left.
- That there is a real possibility that cyclic bleeding may occur after supracervical hysterectomy, even when the residual endocervical tissue is cored or coagulated. I stress this point to all women who elect hysterectomy.
- That randomized trials demonstrate a significant incidence of reoperation for persistent bleeding.
1. Kikku P. Supravaginal uterine amputation vs. hysterectomy: effects on coital frequency and dyspareunia. Acta Obstet Gynecol Scand. 1983;63:141-145.
2. Kikku P, Gronroos M, Hirvonen T, et al. Supravaginal uterine amputation vs. hysterectomy: effect on libido and orgasm. Acta Obstet Gynecol Scand. 1983;62:147-152.
3. Butler-Manuel SA, Buttery LD, A’Hern RP, et al. Pelvic nerve plexus trauma at radical hysterectomy and simple hysterectomy: the nerve content of the uterine supporting ligaments. Cancer. 2000;89:834-841.
4. Thakar R, Ayers S, Clarkson P, et al. Outcomes after total versus subtotal abdominal hysterectomy. N Engl J Med. 2002;347:1318-1325.
5. Gimbel H, Zobbe V, Andersen BM, et al. Randomized controlled trial of total compared with subtotal hysterectomy with 1-year follow-up results. Br J Obstet Gynaecol. 2003;110:1088-1098.
6. Roovers JP, van der Bom JG, van der Vaart CH, et al. Hysterectomy and sexual wellbeing: prospective observational study of vaginal hysterectomy, subtotal abdominal hysterectomy, and total abdominal hysterectomy. Br Med J. 2003;327:774-779.
7. Learman LA, Summitt RL, Jr, Varner RE, et al. A randomized comparison of total or supracervical hysterectomy: surgical complications and clinical outcomes. Obstet Gynecol. 2003;102:453-462.
8. Okaro EO, Jones KD, Sutton C. Long term outcome following laparoscopic supracervical hysterectomy. Br J Obstet Gynecol. 2001;108:1017-1020.
- Sexual function is not improved more with supracervical than with total hysterectomy.
- Operative morbidity for supracervical and total hysterectomy are similar.
- Pelvic-floor support and urinary incontinence do not seem to be improved with the supracervical approach.
- Cyclic bleeding occurs in 5% to 20% of women after supracervical hysterectomy.
- Reoperation rates for symptoms related to the retained cervix are significant—over 20% in the hands of highly skilled surgeons.
Hysterectomy is an obvious target. The number of hysterectomies performed has not declined substantially since these technologies were introduced, and persists at more than 550,000 per year in the United States. It is still the most widely performed major gynecological procedure.
Technological advances have made possible the use of laparoscopy to facilitate removal of the uterus without a major abdominal incision, with its inherent hazards. Many surgeons, seeking to make the most of new technology, have revisited laparoscopic subtotal hysterectomy, advocating preservation of the cervix to reduce surgical complications, sexual dysfunction, and pelvic-floor defects after hysterectomy.
New data, however—much of it released only in the last 12 to 18 months—tell us there is no difference in sexual function, pelvic floor support, or return to normal activities when the cervix is retained. What’s more, leaving the cervix in place puts the patient at greater risk of reoperation related to hysterectomy.
THEORY
Improved sexual function
EVIDENCE
Recent prospective analyses using validated measures of female sexual function have failed to demonstrate any advantage for supracervical hysterectomy.
Scientific study of sexual function is difficult at best. Many factors influence sexual behavior, and all must be considered when analyzing the effects of hysterectomy. To clearly understand the impact of hysterectomy on female sexual function, prospective studies in which women serve as their own controls provide the best quality evidence. That said, the contention that supracervical hysterectomy results in better sexual function than total hysterectomy stems from the research of a single group, which in 1983 retrospectively compared coital frequency, dyspareunia, libido, and orgasm after “supravaginal uterine amputation” with total hysterectomy.1,2
Simple hysterectomy causes minimal disruption of Frankenhauser’s plexus of autonomic nerves.
They theorized that supracervical operation preserves Frankenhauser’s plexus of autonomic nerves, resulting in better sexual function. However, careful anatomic assessment of the nerve content in the ligaments supporting the uterus has since demonstrated that the rich nerve supply to the uterosacral and cardinal ligaments occurs in the lateral two thirds of these structures. Simple hysterectomy causes minimal disruption of these autonomic nerves, ganglia, and extensions of the inferior hypogastric plexus.3
Thakar et al,4 in a pivotal multicenter, double-blind, randomized trial conducted in the United Kingdom, randomized 279 patients with benign disease to supracervical or total hysterectomy and followed them for 12 months. Surgical technique was standardized and the endocervix was coagulated in all patients.
The 2 groups were similar in measures of sexual function, including frequency of intercourse, orgasm, and rating of relationship with partner.
The Danish Hysterectomy Group5 randomized 319 patients with benign disease to total abdominal hysterectomy or subtotal abdominal hysterectomy, of whom 276 completed validated mailed questionnaires preoperatively, and at 2, 6, and 12 months postoperatively.
There was no change in sexual satisfaction in either group from their prehysterectomy levels. Overall quality of life improved significantly in both groups, in both mental and physical measures.
Roovers et al,6 in a multicenter, nonrandomized trial—powered well to detect 20% differences—compared vaginal hysterectomy, subtotal abdominal hysterectomy, and total abdominal hysterectomy (technique chosen by the surgeon).
Of the 379 patients recruited (from 13 teaching and nonteaching hospitals in the Netherlands) who had a male partner, 93% completed a validated questionnaire before and 6 months after surgery.
The questionnaire—used to assess sexual pleasure, activity, and problems—specifically addressed lubrication, orgasm, pain, and arousal on a 5-point scale (“not bothered” to “severely bothered”). Their findings:
- Sexual pleasure increased significantly in all groups regardless of type of hysterectomy.
- There was no difference in the incidence of bothersome sexual problems, but a significant number were reported: 43% after vaginal, 41% after subtotal, and 39% after total abdominal hysterectomy (P =.88).
- New sexual problems were reported in 9 patients (23%) after vaginal hysterectomy, 8 patients (24%) after subtotal hysterectomy, and 12 patients (19%) after total abdominal hysterectomy.
- There was a nonsignificant trend toward higher prevalence of arousal and lubrication problems after subtotal hysterectomy and total abdominal hysterectomy, compared with vaginal hysterectomy.
Theory
Improved pelvic floor support, less incontinence
Evidence
Pelvic floor support and urinary incontinence do not seem to be improved with the supracervical approach.
Proponents of supracervical hysterectomy argue that preservation of the cardinal and uterosacral ligaments will reduce the incidence of apical prolapse. In addition, maintenance of the pubocervical ring should lead to less posthysterectomy urinary incontinence.
Our newfound understanding that the nerves are, for the most part, spared at simple hysterectomy should argue against allegations that bowel and urinary function are better preserved by retaining the cervix.
Clearly, long-term outcome studies are required to assess these issues. The randomized trials thus far comparing supracervical with total hysterectomy have not followed patients beyond 2 years.
Nevertheless, at 12 to 24 months, published trials5,7 report an increased incidence of urinary incontinence in patients randomized to supracervical hysterectomy. Prolapse was also reported in a larger number of the patients undergoing subtotal as compared with total hysterectomy (1 to 2% versus 0% at 12 months).
The Total or Supracervical Hysterectomy (TOSH) Research Group7 conducted a multi-center randomized trial with a diverse patient population (78% of women were African American). From January 1998 through April 2000, 135 patients at 4 centers were randomized to supracervical hysterectomy or total abdominal hysterectomy. All patients had benign disease. Surgical technique varied by surgeon as in the general community. Patients and researchers were not blinded as to the technique performed. Subjects were followed for 2 years.
Women undergoing supracervical hysterectomy had a greater incidence of urinary incontinence after surgery.
Both techniques resulted in significant decreases in complaints of urinary incontinence and voiding dysfunction
The Danish Hysterectomy Group5 found that patients who had supracervical hysterectomies had a statistically greater incidence of urinary incontinence after surgery than those undergoing total abdominal hysterectomy (18% versus 9% P = .04). The incidence of new incontinence symptoms was 2.1% in the total abdominal hysterectomy group compared with 7.6% in the subtotal group. There was no change in bowel function in either group.
Thakar et al4 found urinary frequency declined significantly in both groups.
Theory
Fewer injuries and complications, less blood loss
Evidence
Randomized trials have offered no evidence to support a reduction in complication rates or bleeding requiring transfusion.
Since the creation of a bladder flap and division of the cardinal ligaments is not required in supracervical hysterectomy, we might theoretically expect to see reduced rates of injury to the ureters and bladder. Without the need for colpotomy, blood loss should also be reduced.
Cyclic bleeding may occur after supracervical hysterectomy even when residual endocervical tissue is cored or coagulated.
Given the low incidence of these complications at total hysterectomy, however, a meta-analysis of published randomized trials would be required to properly evaluate this issue. Thus far, randomized trials have offered no evidence to support a reduction in complication rates or bleeding requiring transfusion.
Thakar et al4 found a significant reduction in operating time as well as a reduction in blood loss (422 mL versus 320 mL) for the supracervical group compared with the total hysterectomy group; however there were no differences in the need for transfusion (5% in each group).
Women who underwent total abdominal hysterectomy had a higher incidence of fever while in the hospital (27% versus 10%), but there was no difference in the rate of infectious morbidity.
Within 1 year of discharge, more patients undergoing supracervical hysterectomy experienced complications: 7% had cyclic bleeding; 2% had cervical prolapse.
The TOSH Research Group7 found no difference in the rate of complications, activity limitations, or symptom improvement between groups. During the first 3 months, there was no difference in missed work, restricting activities, or bed rest. Both techniques resulted in significant decreases in complaints of pelvic pain, pressure, and back pain.
Of women undergoing supracervical hysterectomy, 5% had cyclic vaginal bleeding (only half of the patients in this series had the endocervix ablated).
Further, there were more readmissions related to the hysterectomy in the supracervical group, though this was not statistically significant (relative risk 1.99; 95% confidence interval, 0.58 to 6.8).
Twenty percent of women in the Danish Hysterectomy Group study 5 had persistent vaginal bleeding after subtotal hysterectomy; 2 went on to have trachelectomy for cyclic bleeding.
Prolapse of the cervical stump occurred in 3/136 patients after subtotal hysterectomy, versus no prolapse after total abdominal hysterectomy.
Outcomes after total versus subtotal abdominal hysterectomy. Thakar R, et al. N Engl J Med. 2002;347:1318–1325.4 Level I evidence
CONCLUSION Neither subtotal nor total abdominal hysterectomy adversely affected pelvic organ function at 12 months. Subtotal abdominal hysterectomy resulted in more rapid recovery and fewer short-term complications but infrequently caused cyclical bleeding or cervical prolapse.
- Pivotal multicenter, double-blind, randomized trial conducted in the United Kingdom.
- Randomized 279 patients with benign disease to supracervical or total hysterectomy and followed them for 12 months.
- Surgical technique was standardized and the endocervix coagulated in all patients.
Randomized controlled trial of total compared with subtotal hysterectomy with 1-year follow-up results. The Danish Hysterectomy Group. Br J Obstet Gynaecol. 2003;110:1088–1098.5 Level I evidence
CONCLUSION A smaller proportion of women suffered from urinary incontinence after total abdominal hysterectomy than after subtotal abdominal hysterectomy 1 year postoperatively.
- Multicenter, unblinded randomized trial conducted in Denmark.
- Randomized 319 patients with benign disease to total abdominal hysterectomy or subtotal abdominal hysterectomy, of whom 276 completed validated mailed questionnaires preoperatively, and at 2, 6, and 12 months postoperatively.
Hysterectomy and sexual well being: Prospective observational study of vaginal hysterectomy, subtotal abdominal hysterectomy, and total abdominal hysterectomy. Roovers JP, et al. Br Med J. 2003;327:774–779.6 Level II-1 evidence
CONCLUSION Sexual pleasure improved after vaginal hysterectomy, subtotal abdominal hysterectomy, and total abdominal hysterectomy. The persistence and development of bothersome problems during sexual activity were similar for all 3 techniques.
- Multicenter, nonrandomized trial conducted in the Netherlands.
- Investigated sexual function only.
- Compared vaginal, subtotal abdominal, and total abdominal hysterectomy (technique chosen by the surgeon).
- Of the 379 patients with a male partner, 93% completed a validated questionnaire before and 6 months after surgery.
A randomized comparison of total or supracervical hysterectomy: Surgical complications and clinical outcomes. Total or Supracervical Hysterectomy Research Group, Obstet Gynecol. 2003;102:453–462.7 Level I evidence
CONCLUSION We found no statistically significant differences between supracervical hysterectomy and total abdominal hysterectomy in surgical complications and clinical outcomes during 2 years of follow-up.
- Multicenter, unblinded randomized trial conducted in the United States.
- Randomized 135 patients with benign disease to supracervical hysterectomy or total abdominal hysterectomy and followed them for 2 years.
- Surgical technique varied by surgeon as in the general community.
- Only half of the patients had the endocervix ablated.
Long term outcome following laparoscopic supracervical hysterectomy. Okaro EO, et al. Br J Obstet Gynecol. 2001;108:1017–1020.8 Level II-3 evidence
CONCLUSION Symptoms related to the cervical stump requiring further surgery frequently occur following a laparoscopic supracervical hysterectomy.
- Retrospective analysis of case records for 70 patients.
- All subjects were women who would have otherwise undergone abdominal hysterectomy, but agreed to laparoscopic supracervical hysterectomy.
- All surgeries were performed by the same surgeon.
Long-term outcomes: the downside
For a mean of 66 months (range: 4 to 7 years), Okaro et al8 followed 70 patients undergoing laparoscopic supracervical hysterectomy by a single, highly skilled laparoscopic surgeon.
Their findings point out the downside of cervical preservation. Although all patients had the endocervical canal and transition zone cored out, over 24% reported symptoms related to the cervical stump—and all required further surgery. Further, cyclic vaginal bleeding occurred in 11% of women.
One patient developed cervical intraepithelial neoplasia. Dyspareunia and pelvic pain were significant complaints in 19% of the patients. (These women were more likely to have had hysterectomy for endometriosis.) Sixteen of the 17 patients with cervical complaints required trachelectomy within the follow-up time period.
“How empty is theory in the presence of fact!”
Mark Twain, A Connecticut Yankee in King Arthur’s Court
Practice recommendations
We, as clinicians, must accumulate evidence from basic science as well as clinical research, put it all together, and make recommendations based on these data. The data, tell us, in fact, that there is no difference in sexual dysfunction, pelvic floor support, or return to normal activity levels when the cervix is retained, and no evidence to support an advantage to supracervical hysterectomy.
My recommendation is for vaginal hysterectomy when possible. The theoretical advantages of retention of the cervix have driven many clinicians to abandon the vaginal approach in favor of laparoscopic supracervical hysterectomy; no data support this theory.
While not the focus of this article, ample data tell us that the vaginal approach, when technically feasible, is less invasive and carries fewer risks for our patients than laparoscopic or abdominal hysterectomy, and permits excellent access for support of the pelvic floor. I do think that patients who truly believe that their sex lives will be ruined after total hysterectomy or that they will do dramatically better if the cervix remains, may experience this self-fulfilling prophecy.
What I tell patients. I carefully review all the facts with patients in helping them select the appropriate surgical procedure.
I tell patients:
- That overwhelming evidence suggests that sexual function improves in the vast majority of women after hysterectomy, whether or not the cervix is left.
- That there is a real possibility that cyclic bleeding may occur after supracervical hysterectomy, even when the residual endocervical tissue is cored or coagulated. I stress this point to all women who elect hysterectomy.
- That randomized trials demonstrate a significant incidence of reoperation for persistent bleeding.
- Sexual function is not improved more with supracervical than with total hysterectomy.
- Operative morbidity for supracervical and total hysterectomy are similar.
- Pelvic-floor support and urinary incontinence do not seem to be improved with the supracervical approach.
- Cyclic bleeding occurs in 5% to 20% of women after supracervical hysterectomy.
- Reoperation rates for symptoms related to the retained cervix are significant—over 20% in the hands of highly skilled surgeons.
Hysterectomy is an obvious target. The number of hysterectomies performed has not declined substantially since these technologies were introduced, and persists at more than 550,000 per year in the United States. It is still the most widely performed major gynecological procedure.
Technological advances have made possible the use of laparoscopy to facilitate removal of the uterus without a major abdominal incision, with its inherent hazards. Many surgeons, seeking to make the most of new technology, have revisited laparoscopic subtotal hysterectomy, advocating preservation of the cervix to reduce surgical complications, sexual dysfunction, and pelvic-floor defects after hysterectomy.
New data, however—much of it released only in the last 12 to 18 months—tell us there is no difference in sexual function, pelvic floor support, or return to normal activities when the cervix is retained. What’s more, leaving the cervix in place puts the patient at greater risk of reoperation related to hysterectomy.
THEORY
Improved sexual function
EVIDENCE
Recent prospective analyses using validated measures of female sexual function have failed to demonstrate any advantage for supracervical hysterectomy.
Scientific study of sexual function is difficult at best. Many factors influence sexual behavior, and all must be considered when analyzing the effects of hysterectomy. To clearly understand the impact of hysterectomy on female sexual function, prospective studies in which women serve as their own controls provide the best quality evidence. That said, the contention that supracervical hysterectomy results in better sexual function than total hysterectomy stems from the research of a single group, which in 1983 retrospectively compared coital frequency, dyspareunia, libido, and orgasm after “supravaginal uterine amputation” with total hysterectomy.1,2
Simple hysterectomy causes minimal disruption of Frankenhauser’s plexus of autonomic nerves.
They theorized that supracervical operation preserves Frankenhauser’s plexus of autonomic nerves, resulting in better sexual function. However, careful anatomic assessment of the nerve content in the ligaments supporting the uterus has since demonstrated that the rich nerve supply to the uterosacral and cardinal ligaments occurs in the lateral two thirds of these structures. Simple hysterectomy causes minimal disruption of these autonomic nerves, ganglia, and extensions of the inferior hypogastric plexus.3
Thakar et al,4 in a pivotal multicenter, double-blind, randomized trial conducted in the United Kingdom, randomized 279 patients with benign disease to supracervical or total hysterectomy and followed them for 12 months. Surgical technique was standardized and the endocervix was coagulated in all patients.
The 2 groups were similar in measures of sexual function, including frequency of intercourse, orgasm, and rating of relationship with partner.
The Danish Hysterectomy Group5 randomized 319 patients with benign disease to total abdominal hysterectomy or subtotal abdominal hysterectomy, of whom 276 completed validated mailed questionnaires preoperatively, and at 2, 6, and 12 months postoperatively.
There was no change in sexual satisfaction in either group from their prehysterectomy levels. Overall quality of life improved significantly in both groups, in both mental and physical measures.
Roovers et al,6 in a multicenter, nonrandomized trial—powered well to detect 20% differences—compared vaginal hysterectomy, subtotal abdominal hysterectomy, and total abdominal hysterectomy (technique chosen by the surgeon).
Of the 379 patients recruited (from 13 teaching and nonteaching hospitals in the Netherlands) who had a male partner, 93% completed a validated questionnaire before and 6 months after surgery.
The questionnaire—used to assess sexual pleasure, activity, and problems—specifically addressed lubrication, orgasm, pain, and arousal on a 5-point scale (“not bothered” to “severely bothered”). Their findings:
- Sexual pleasure increased significantly in all groups regardless of type of hysterectomy.
- There was no difference in the incidence of bothersome sexual problems, but a significant number were reported: 43% after vaginal, 41% after subtotal, and 39% after total abdominal hysterectomy (P =.88).
- New sexual problems were reported in 9 patients (23%) after vaginal hysterectomy, 8 patients (24%) after subtotal hysterectomy, and 12 patients (19%) after total abdominal hysterectomy.
- There was a nonsignificant trend toward higher prevalence of arousal and lubrication problems after subtotal hysterectomy and total abdominal hysterectomy, compared with vaginal hysterectomy.
Theory
Improved pelvic floor support, less incontinence
Evidence
Pelvic floor support and urinary incontinence do not seem to be improved with the supracervical approach.
Proponents of supracervical hysterectomy argue that preservation of the cardinal and uterosacral ligaments will reduce the incidence of apical prolapse. In addition, maintenance of the pubocervical ring should lead to less posthysterectomy urinary incontinence.
Our newfound understanding that the nerves are, for the most part, spared at simple hysterectomy should argue against allegations that bowel and urinary function are better preserved by retaining the cervix.
Clearly, long-term outcome studies are required to assess these issues. The randomized trials thus far comparing supracervical with total hysterectomy have not followed patients beyond 2 years.
Nevertheless, at 12 to 24 months, published trials5,7 report an increased incidence of urinary incontinence in patients randomized to supracervical hysterectomy. Prolapse was also reported in a larger number of the patients undergoing subtotal as compared with total hysterectomy (1 to 2% versus 0% at 12 months).
The Total or Supracervical Hysterectomy (TOSH) Research Group7 conducted a multi-center randomized trial with a diverse patient population (78% of women were African American). From January 1998 through April 2000, 135 patients at 4 centers were randomized to supracervical hysterectomy or total abdominal hysterectomy. All patients had benign disease. Surgical technique varied by surgeon as in the general community. Patients and researchers were not blinded as to the technique performed. Subjects were followed for 2 years.
Women undergoing supracervical hysterectomy had a greater incidence of urinary incontinence after surgery.
Both techniques resulted in significant decreases in complaints of urinary incontinence and voiding dysfunction
The Danish Hysterectomy Group5 found that patients who had supracervical hysterectomies had a statistically greater incidence of urinary incontinence after surgery than those undergoing total abdominal hysterectomy (18% versus 9% P = .04). The incidence of new incontinence symptoms was 2.1% in the total abdominal hysterectomy group compared with 7.6% in the subtotal group. There was no change in bowel function in either group.
Thakar et al4 found urinary frequency declined significantly in both groups.
Theory
Fewer injuries and complications, less blood loss
Evidence
Randomized trials have offered no evidence to support a reduction in complication rates or bleeding requiring transfusion.
Since the creation of a bladder flap and division of the cardinal ligaments is not required in supracervical hysterectomy, we might theoretically expect to see reduced rates of injury to the ureters and bladder. Without the need for colpotomy, blood loss should also be reduced.
Cyclic bleeding may occur after supracervical hysterectomy even when residual endocervical tissue is cored or coagulated.
Given the low incidence of these complications at total hysterectomy, however, a meta-analysis of published randomized trials would be required to properly evaluate this issue. Thus far, randomized trials have offered no evidence to support a reduction in complication rates or bleeding requiring transfusion.
Thakar et al4 found a significant reduction in operating time as well as a reduction in blood loss (422 mL versus 320 mL) for the supracervical group compared with the total hysterectomy group; however there were no differences in the need for transfusion (5% in each group).
Women who underwent total abdominal hysterectomy had a higher incidence of fever while in the hospital (27% versus 10%), but there was no difference in the rate of infectious morbidity.
Within 1 year of discharge, more patients undergoing supracervical hysterectomy experienced complications: 7% had cyclic bleeding; 2% had cervical prolapse.
The TOSH Research Group7 found no difference in the rate of complications, activity limitations, or symptom improvement between groups. During the first 3 months, there was no difference in missed work, restricting activities, or bed rest. Both techniques resulted in significant decreases in complaints of pelvic pain, pressure, and back pain.
Of women undergoing supracervical hysterectomy, 5% had cyclic vaginal bleeding (only half of the patients in this series had the endocervix ablated).
Further, there were more readmissions related to the hysterectomy in the supracervical group, though this was not statistically significant (relative risk 1.99; 95% confidence interval, 0.58 to 6.8).
Twenty percent of women in the Danish Hysterectomy Group study 5 had persistent vaginal bleeding after subtotal hysterectomy; 2 went on to have trachelectomy for cyclic bleeding.
Prolapse of the cervical stump occurred in 3/136 patients after subtotal hysterectomy, versus no prolapse after total abdominal hysterectomy.
Outcomes after total versus subtotal abdominal hysterectomy. Thakar R, et al. N Engl J Med. 2002;347:1318–1325.4 Level I evidence
CONCLUSION Neither subtotal nor total abdominal hysterectomy adversely affected pelvic organ function at 12 months. Subtotal abdominal hysterectomy resulted in more rapid recovery and fewer short-term complications but infrequently caused cyclical bleeding or cervical prolapse.
- Pivotal multicenter, double-blind, randomized trial conducted in the United Kingdom.
- Randomized 279 patients with benign disease to supracervical or total hysterectomy and followed them for 12 months.
- Surgical technique was standardized and the endocervix coagulated in all patients.
Randomized controlled trial of total compared with subtotal hysterectomy with 1-year follow-up results. The Danish Hysterectomy Group. Br J Obstet Gynaecol. 2003;110:1088–1098.5 Level I evidence
CONCLUSION A smaller proportion of women suffered from urinary incontinence after total abdominal hysterectomy than after subtotal abdominal hysterectomy 1 year postoperatively.
- Multicenter, unblinded randomized trial conducted in Denmark.
- Randomized 319 patients with benign disease to total abdominal hysterectomy or subtotal abdominal hysterectomy, of whom 276 completed validated mailed questionnaires preoperatively, and at 2, 6, and 12 months postoperatively.
Hysterectomy and sexual well being: Prospective observational study of vaginal hysterectomy, subtotal abdominal hysterectomy, and total abdominal hysterectomy. Roovers JP, et al. Br Med J. 2003;327:774–779.6 Level II-1 evidence
CONCLUSION Sexual pleasure improved after vaginal hysterectomy, subtotal abdominal hysterectomy, and total abdominal hysterectomy. The persistence and development of bothersome problems during sexual activity were similar for all 3 techniques.
- Multicenter, nonrandomized trial conducted in the Netherlands.
- Investigated sexual function only.
- Compared vaginal, subtotal abdominal, and total abdominal hysterectomy (technique chosen by the surgeon).
- Of the 379 patients with a male partner, 93% completed a validated questionnaire before and 6 months after surgery.
A randomized comparison of total or supracervical hysterectomy: Surgical complications and clinical outcomes. Total or Supracervical Hysterectomy Research Group, Obstet Gynecol. 2003;102:453–462.7 Level I evidence
CONCLUSION We found no statistically significant differences between supracervical hysterectomy and total abdominal hysterectomy in surgical complications and clinical outcomes during 2 years of follow-up.
- Multicenter, unblinded randomized trial conducted in the United States.
- Randomized 135 patients with benign disease to supracervical hysterectomy or total abdominal hysterectomy and followed them for 2 years.
- Surgical technique varied by surgeon as in the general community.
- Only half of the patients had the endocervix ablated.
Long term outcome following laparoscopic supracervical hysterectomy. Okaro EO, et al. Br J Obstet Gynecol. 2001;108:1017–1020.8 Level II-3 evidence
CONCLUSION Symptoms related to the cervical stump requiring further surgery frequently occur following a laparoscopic supracervical hysterectomy.
- Retrospective analysis of case records for 70 patients.
- All subjects were women who would have otherwise undergone abdominal hysterectomy, but agreed to laparoscopic supracervical hysterectomy.
- All surgeries were performed by the same surgeon.
Long-term outcomes: the downside
For a mean of 66 months (range: 4 to 7 years), Okaro et al8 followed 70 patients undergoing laparoscopic supracervical hysterectomy by a single, highly skilled laparoscopic surgeon.
Their findings point out the downside of cervical preservation. Although all patients had the endocervical canal and transition zone cored out, over 24% reported symptoms related to the cervical stump—and all required further surgery. Further, cyclic vaginal bleeding occurred in 11% of women.
One patient developed cervical intraepithelial neoplasia. Dyspareunia and pelvic pain were significant complaints in 19% of the patients. (These women were more likely to have had hysterectomy for endometriosis.) Sixteen of the 17 patients with cervical complaints required trachelectomy within the follow-up time period.
“How empty is theory in the presence of fact!”
Mark Twain, A Connecticut Yankee in King Arthur’s Court
Practice recommendations
We, as clinicians, must accumulate evidence from basic science as well as clinical research, put it all together, and make recommendations based on these data. The data, tell us, in fact, that there is no difference in sexual dysfunction, pelvic floor support, or return to normal activity levels when the cervix is retained, and no evidence to support an advantage to supracervical hysterectomy.
My recommendation is for vaginal hysterectomy when possible. The theoretical advantages of retention of the cervix have driven many clinicians to abandon the vaginal approach in favor of laparoscopic supracervical hysterectomy; no data support this theory.
While not the focus of this article, ample data tell us that the vaginal approach, when technically feasible, is less invasive and carries fewer risks for our patients than laparoscopic or abdominal hysterectomy, and permits excellent access for support of the pelvic floor. I do think that patients who truly believe that their sex lives will be ruined after total hysterectomy or that they will do dramatically better if the cervix remains, may experience this self-fulfilling prophecy.
What I tell patients. I carefully review all the facts with patients in helping them select the appropriate surgical procedure.
I tell patients:
- That overwhelming evidence suggests that sexual function improves in the vast majority of women after hysterectomy, whether or not the cervix is left.
- That there is a real possibility that cyclic bleeding may occur after supracervical hysterectomy, even when the residual endocervical tissue is cored or coagulated. I stress this point to all women who elect hysterectomy.
- That randomized trials demonstrate a significant incidence of reoperation for persistent bleeding.
1. Kikku P. Supravaginal uterine amputation vs. hysterectomy: effects on coital frequency and dyspareunia. Acta Obstet Gynecol Scand. 1983;63:141-145.
2. Kikku P, Gronroos M, Hirvonen T, et al. Supravaginal uterine amputation vs. hysterectomy: effect on libido and orgasm. Acta Obstet Gynecol Scand. 1983;62:147-152.
3. Butler-Manuel SA, Buttery LD, A’Hern RP, et al. Pelvic nerve plexus trauma at radical hysterectomy and simple hysterectomy: the nerve content of the uterine supporting ligaments. Cancer. 2000;89:834-841.
4. Thakar R, Ayers S, Clarkson P, et al. Outcomes after total versus subtotal abdominal hysterectomy. N Engl J Med. 2002;347:1318-1325.
5. Gimbel H, Zobbe V, Andersen BM, et al. Randomized controlled trial of total compared with subtotal hysterectomy with 1-year follow-up results. Br J Obstet Gynaecol. 2003;110:1088-1098.
6. Roovers JP, van der Bom JG, van der Vaart CH, et al. Hysterectomy and sexual wellbeing: prospective observational study of vaginal hysterectomy, subtotal abdominal hysterectomy, and total abdominal hysterectomy. Br Med J. 2003;327:774-779.
7. Learman LA, Summitt RL, Jr, Varner RE, et al. A randomized comparison of total or supracervical hysterectomy: surgical complications and clinical outcomes. Obstet Gynecol. 2003;102:453-462.
8. Okaro EO, Jones KD, Sutton C. Long term outcome following laparoscopic supracervical hysterectomy. Br J Obstet Gynecol. 2001;108:1017-1020.
1. Kikku P. Supravaginal uterine amputation vs. hysterectomy: effects on coital frequency and dyspareunia. Acta Obstet Gynecol Scand. 1983;63:141-145.
2. Kikku P, Gronroos M, Hirvonen T, et al. Supravaginal uterine amputation vs. hysterectomy: effect on libido and orgasm. Acta Obstet Gynecol Scand. 1983;62:147-152.
3. Butler-Manuel SA, Buttery LD, A’Hern RP, et al. Pelvic nerve plexus trauma at radical hysterectomy and simple hysterectomy: the nerve content of the uterine supporting ligaments. Cancer. 2000;89:834-841.
4. Thakar R, Ayers S, Clarkson P, et al. Outcomes after total versus subtotal abdominal hysterectomy. N Engl J Med. 2002;347:1318-1325.
5. Gimbel H, Zobbe V, Andersen BM, et al. Randomized controlled trial of total compared with subtotal hysterectomy with 1-year follow-up results. Br J Obstet Gynaecol. 2003;110:1088-1098.
6. Roovers JP, van der Bom JG, van der Vaart CH, et al. Hysterectomy and sexual wellbeing: prospective observational study of vaginal hysterectomy, subtotal abdominal hysterectomy, and total abdominal hysterectomy. Br Med J. 2003;327:774-779.
7. Learman LA, Summitt RL, Jr, Varner RE, et al. A randomized comparison of total or supracervical hysterectomy: surgical complications and clinical outcomes. Obstet Gynecol. 2003;102:453-462.
8. Okaro EO, Jones KD, Sutton C. Long term outcome following laparoscopic supracervical hysterectomy. Br J Obstet Gynecol. 2001;108:1017-1020.
Avoiding and repairing bowel injury in gynecologic surgery
- Although the optimal method is a matter of choice, preoperative bowel preparation is recommended to reduce bacteria, stool bulk, and infectious complications.
- When entering the peritoneal cavity in patients with prior surgery, watch for adhesions between a loop of bowel and the abdominal wall.
- In high-risk patients, enter the peritoneal cavity by extending the previous abdominal scar superiorly and inferiorly to minimize risk of injury.
- Close small perforations in 2 layers, with the suture line always perpendicular to the long axis of the bowel.
- For more extensive injury or compromised blood supply to the bowel wall, resection and anastomosis may be necessary. Obtain intraoperative general surgical consultation if not trained to perform this kind of repair.
This dreaded complication requires vigilance and skill to avoid, and adequate training and experience to manage and repair. In a perfect world, every gynecologist would be trained in techniques to prevent and repair inadvertent bowel injuries. Unfortunately, residency programs often do not provide such training.
Gynecologists routinely operate on patients with risk factors for bowel injury—obesity, endometriosis, multiple abdominal procedures, pelvic inflammatory disease, history of malignancy, and advanced age. A general surgeon is often called, however, for bowel repairs that can be performed by a gynecologist with sufficient training and experience. (There are instances, however, in which a general surgical consultation may not be readily available—another reason to master repair of bowel injuries encountered during gynecologic surgery.)
This article describes techniques to avert and manage intestinal injury. Topics include adhesiolysis, repair of bowel perforations, segmental bowel resection, and pre- and postoperative management. Vascular anatomy of the bowel is illustrated.
We emphasize the need for direct supervision by an experienced surgeon while mastering these techniques.
Bowel preparation: A useful tool to reduce infection, leakage
Isolated reports have questioned the need for mechanical bowel preparation,1,2 and some experts point to the recent success of primary repairs of gunshot and stab wounds to the colon as evidence that bowel preparation and preoperative oral antibiotics are unnecessary.
Other studies indicate potential benefits, namely reducing infectious complications and anastomotic leakage following repair of inadvertent enterotomy. Indeed, the vast majority of North American surgeons continue to use some form of bowel preparation,3,4 and it is the standard of care for elective intestinal surgery. For these reasons, bowel preparation is strongly encouraged for the gynecologic surgeon operating on a pelvic mass, endometriosis, or malignancy, or when difficult dissection is anticipated with the potential for inadvertent enterotomy and spillage of intestinal contents.
Bowel preparation consists of 2 phases: mechanical cleansing and antibiotic administration (TABLE). The postoperative infection rate can be reduced to well below 10% when these are properly performed.
Mechanical cleansing reduces the bulk of stool content within the lumen of the bowel, which also decreases the absolute amount of bacteria.5 Anaerobes are the predominant flora in the colon, with an estimated density of 1010 organisms per gram of stool. Perforation and spillage of colon contents contaminates the peritoneal cavity with more than 400 species of bacteria.
Perforation and spillage of colon contents contaminates the peritoneal cavity with more than 400 species of bacteria.
In the past, stool bulk was reduced via a low-residue or liquid diet combined with cathartics, enemas, or other agents given over 2 to 3 days. This regimen was time-consuming, patient compliance was poor, and nutritional intake was severely restricted prior to major surgery.
Today, polyethylene glycol and sodium phosphate are the 2 most popular methods of bowel preparation.
- Polyethylene glycol (Golytely, Braintree Labs, Braintree, Mass) is a balanced electrolyte solution dispensed in a 4-L quantity that must be taken over 4 hours. Some patients find this volume difficult to consume; one option is administering the solution via a small nasogastric tube. Complications may include nausea/vomiting, abdominal cramping, and, rarely, fluid overload and electrolyte disturbances.
- Sodium phosphate (Fleet Phosphosoda, C.B. Fleet Co, Lynchburg, Va) is administered in two 45-mL increments several hours apart. There is no consensus on which bowel-prep method is superior3,4; most surgeons prefer one or the other. Due to potential electrolyte abnormalities with the use of sodium phosphate, polyethylene glycol is favored for patients with significant renal, cardiac, or hepatic disease.
- We recommend minimally absorbed oral antibiotics (1 g each of neomycin and erythromycin, given at 1 PM, 2 PM, and 11 PM the day before surgery) in combination with an intravenous second-generation cephalosporin (1 g if using cefotetan, 2 g if using cefoxitin; given immediately before surgery and continued postoperatively for 3 doses).
- Timing of antibiotic administration is important, since postoperative antibiotics alone do not appear to be effective. If significant spillage occurs intraoperatively, parenteral antibiotics should be continued for 5 days.
TABLE
Bowel prep regimen
| DAY BEFORE SURGERY |
| Morning |
| Light breakfast |
| Noon |
| Clear liquids |
| Polyethylene glycol, 4L, to be consumed over 4 to 6 hours |
| 1 PM |
| Neomycin, 1 g orally |
| Erythromycin, 1 g orally |
| 2 PM |
| Neomycin, 1 g orally |
| Erythromycin, 1 g orally |
| Evening |
| Clear liquids |
| 11 PM |
| Neomycin, 1 g orally |
| Erythromycin, 1 g orally |
| DAY OF SURGERY |
| Morning |
| Intravenous cephalosporin (1 g cefotetan or 2 g cefoxitin); 1 hour before incision, continued postoperatively for 3 doses |
When injuries are most likely
Intestinal injuries during gynecologic surgery usually involve the small bowel and can be minor, such as a serosal tear or a small, full-thickness laceration—or major, involving a devitalized bowel loop or its mesentery.
Bowel injury may occur during a variety of surgical procedures. One study showed that most injuries occur during adhesiolysis or entry into the peritoneal cavity. A smaller but substantial number of cases occur during “less extensive” procedures such as uterine curettage and laparoscopy.6
Upon entering the peritoneal cavity, keep in mind the possibility of injuring an adherent loop of bowel. Because of its anatomical relationships to the pelvic viscera, portions of the bowel may become involved in adhesions, which can lead to extremely challenging pelvic dissections in conditions such as endometriosis or severe pelvic infection. Dissection of pelvic adhesions is a common cause of bowel injury, because bowel loops are retracted deeply downward by adhesive bands, and the limited pelvic space hampers visualization and gentle adhesiolysis.
At special risk for bowel injury are women who have undergone prior abdominal operations or who are obese. In a series of 270 general surgery patients undergoing reoperation,7 52 (19%) sustained inadvertent enterotomy. These patients had undergone a mean of 3.3 previous laparotomies and had a higher body mass index (mean of 25.5 versus 21.9).
Age may be another risk factor, since patients with enterotomies were 60 years or older.7
Injury during laparoscopy. Inadvertent bowel injuries may occur during laparoscopic procedures, especially at the time of trocar insertion or manipulation of pelvic structures.5 One device that helps prevent these injuries is the optical trocar (Visiport, US Surgical, Norwalk, Conn), which allows physicians to visualize the layers of the abdominal wall as penetration occurs.
We also routinely direct anesthesia personnel to insert a nasogastric tube at the beginning of laparoscopic procedures to facilitate decompression of the stomach and small bowel.
The risks of electrosurgery. Electrocautery used for tubal ligation, pelvic dissection, or hemostasis may injure the bowel if the surgeon is not careful. Thermal injury due to unipolar cautery is particularly ominous because the extent of injury is greater than what can be grossly observed. The incidence of this type of injury can be reduced using bipolar cautery devices, as well as clips or bands for tubal ligation.
Injury as a result of uterine perforation is unlikely, but can occur. If perforation occurs during dilation and curettage, bowel laceration may result, particularly adhesions are present between the uterus and bowel loops. In extremely rare instances, a loop of bowel may be pulled through a perforation into the uterine cavity or vagina, requiring laparotomy for reduction and repair. Caution is advised during curettage, especially in a gravid uterus, to prevent this potentially catastrophic event.
Avoid the temptation to lyse opaque adhesions using blunt dissection, as serosal tears and enterotomies may occur.
Adhesiolysis: Plan on a lengthy, meticulous procedure
Adhesions are a common cause of pelvic pain, infertility, and bowel obstruction, and their presence may make it difficult to carry out the intended surgical procedure. Adhesiolysis may be necessary to mobilize loops of bowel tightly adherent to pelvic structures, to provide sufficient exposure of the surgical field and prevent subsequent bowel obstruction.
The extent of adhesions does not necessarily correlate with clinical symptoms.
Adhesions may be of the thin, filmy, “friendly” variety or dense, thick bands.
How adhesions occur. When tissue is injured, fibrin is deposited on the peritoneal and serosal surfaces. The extent to which this fibrin is infiltrated with fibroblasts and the degree of subsequent fibrosis determine adhesion density. Any process that impairs fibrinolysis tends to delay resolution of adhesions.
Contributing factors. Adhesions are commonly encountered in pelvic surgery and may be observed in 50% to 90% of patients who have undergone previous surgery.8
Obese patients also are more susceptible to adhesions. Other contributing factors include pelvic infection, bleeding, irradiation, chemical irritants, and conditions such as endometriosis.
Lysis technique. Apply gentle, controlled traction—as well as countertraction—on the bowel loops to facilitate isolation and dissection with sharp Metzenbaum scissors or a scalpel. (Forceful traction or rough handling of bowel loops may cause a breach in the bowel wall with subsequent spillage of intestinal contents.)
Avoid the temptation to lyse adhesions using blunt dissection (serosal tears and enterotomies may occur)—except in the case of translucent adhesions. These may be lysed via gentle, blunt dissection by rubbing the index finger and thumb back and forth over tissue. They also may be sharply cut using the tip of the scissors to form a “window” in a portion of the adhesion and cutting the adhesive segments in increments.
A characteristic line of demarcation often appears between adhesions and their peritoneal attachment, denoting a safe dissection plane.
Technique for special challenges: Chronic pelvic disease, prior laparotomies. When operating on these patients, be prepared for a long, meticulous procedure. A hasty approach in such cases is perilous and increases the likelihood of postoperative complications.
First, dissect the anterior abdominal wall from the adherent bowel on either side of the incision. Then extend the dissection laterally on both sides until the ascending and descending colon are identified. Next, dissect the small bowel free and mobilize it out of the pelvis.
It often is helpful to move to another area when dissection becomes too difficult; dissection through easier planes often will clarify the relationship of pelvic structures and adherent bowel loops.
Once the small bowel has been mobilized from the pelvis, lyse adhesions between loops of bowel that are causing kinking or narrowing of the lumen, to reduce the risk of postoperative bowel obstruction. Next, carefully dissect pelvic structures from the sigmoid colon and rectum.
How and when to repair serosal injury
Serosal injury is a breach of integrity of the visceral peritoneum, the outermost covering of the bowel wall. This may occur when the serosa is cut during entry into the abdomen or when it is torn during blunt dissection of dense adhesions.
If the underlying muscular and mucosal layers remain intact, these small areas of “denuded” serosa need not be repaired, since most experts believe that suture placement increases the likelihood of future adhesions. The serosal and muscular layers should be repaired if the mucosa is exposed, however. Otherwise the bowel wall will weaken at the site, making it vulnerable to perforation. The seromuscular layers can be approximated easily using interrupted 4-0 silk on a small tapered needle. Be careful to avoid placing the stitch through the mucosa, which would violate the bowel lumen.
When the defect of the seromuscular layer is large (when a more extensive area is denuded during dissection of densely adherent bowel away from a tumor or endometriotic lesion), repair becomes more involved. This may require resection of the injured area with primary anastomosis.
Intestinal perforations: Early recognition is essential
This critical serious complication can become disastrous if not immediately recognized and repaired. Perforation of the small intestine (enterotomy) or large bowel (colotomy) often occurs upon entry into the peritoneal cavity or during a difficult dissection, particularly when extensive adhesions are present.
Exercise special caution when operating on patients who have undergone prior surgery, who are advanced in age, or both.
Reoperation technique. When entering the abdomen through an old scar, reduce the likelihood of bowel injury by extending the new incision to either side of the old scar. Then enter the peritoneal cavity in a virgin area of the abdominal wall, where adherent loops of bowel are less likely.
Carefully open the fascia and dissect the preperitoneal fat down to the peritoneum. Before entering the abdominal cavity, retract the peritoneum upward with smooth forceps and palpate it between the thumb and index finger to ensure that a bowel loop is not in harm’s way.
If the underlying muscular and mucosal layers remain intact, small areas of “denuded” serosa need not be repaired.
Examine the entire small and large bowel carefully after surgery, to rule out injury. It is not uncommon for more than 1 perforation to occur in a bowel segment during a difficult dissection.
Begin at the ligament of Treitz and continue to the ileocecal junction. This is “running” the bowel—ie, inspecting in hand-over-hand fashion.
In the small bowel, the division between the jejunum and ileum is arbitrary, with no sharply defined line of demarcation. However, the diameter of the lumen decreases as one moves from jejunum to ileum, the number of vascular arcades increases, and the number of windows of Deaver diminishes. Also, the wall of the jejunum is generally thicker than that of the ileum.
In addition, inspect the colon in its entirety, with special emphasis on the sigmoid and rectum. Besides its larger lumen, the large bowel is distinguished by 3 longitudinal muscular bands called taenia coli, out-pouching of the wall (sacculations), and epiploic appendages.
Also examine the mesentery to exclude vascular compromise to the bowel wall.
Repair perforations immediately to limit contamination of the peritoneal cavity. Prior to closure, inspect wound edges for devitalized tissue and, if found, promptly debride it.
If colotomy occurs in the setting of an unprepared bowel with significant spillage, follow closure with copious irrigation.
Small perforations can usually be closed in 2 layers, with an inner layer of 3-0 delayed synthetic absorbable suture (Dexon, Vicryl) through the full thickness of the bowel wall, ensuring mucosal approximation. It is vital that this layer be “waterproof,” allowing no leakage of intestinal contents. Then place a second row of suture in the seromuscular layer using 4-0 silk to imbricate the first suture line.
General surgical consultation is needed whenever the gynecologist is inexperienced with bowel resection and anastomosis.
It also is essential that the suture line be perpendicular to the long axis of the bowel, rather than parallel; otherwise, the bowel lumen would narrow. Even perforations extending along the longitudinal axis for several centimeters should be repaired in transverse fashion to provide a lumen of adequate diameter.
Resecting the small bowel: If inexperienced, obtain general surgery consultation
Bowel resection and anastomosis require a greater degree of skill than is attained in a typical gynecologic training program. For that reason, resection is addressed here only superficially. Our primary caveat: A general surgical consultation should be obtained whenever the gynecologist is inexperienced with bowel resection and anastomosis.
Indications for resection. Strongly consider resection and anastomosis if the perforation involves more than 50% of the circumference of the bowel wall, if multiple perforations occur in a short segment of bowel, or if there is vascular compromise to a segment of bowel. Adequate perfusion to the bowel usually is indicated by a pink serosal surface. If the serosa remains dark or dusky and fails to become pink after several minutes of observation, vascular compromise is likely and resection is preferred.
If there is doubt about the blood supply to the bowel, give 1 g fluorescein intravenously and inspect the bowel under ultraviolet light (Wood’s lamp). Normal vascularized bowel will have a homogenous yellow-green appearance. Patchy fluorescence or areas without any fluorescence are evidence of ischemia.
To drain or not to drain
Because perforation and resection both involve entry into the bowel lumen, some degree of spillage is inevitable. This is of greater concern when the large bowel is involved, because of the increased likelihood of bacterial contamination. Immediate copious irrigation of the peritoneal cavity is indicated. Also consider a pelvic drain, especially when dissection has been extensive or raw surfaces are oozing.
The combination of bacterial contamination and free peritoneal blood in the pelvis increases the risk for infection. A strategically placed, half-inch Jackson Pratt drain (or a similar device) may help prevent abscess. In the event of anastomotic leakage, a drain often allows for a controlled enterocutaneous fistula to be managed without reoperation.
Some surgeons have satisfactory results without these drainage techniques.
When to begin postop feeding: Depends on type of repair
Opinion varies about the appropriate time to commence feeding after major abdominal surgery, particularly bowel surgery. Over the past decade, with the pressure to discharge patients earlier, many physicians have opted for earlier timing.
Traditionally, feeding was withheld until bowel sounds were auscultated; then it progressed slowly. Today many surgeons advance the diet much more quickly, with little or no delay in recovery. Fanning and Andrews9 demonstrated that early feeding does not increase the incidence of anastomotic leakage, dehiscence, or aspiration pneumonia—although it is associated with increased emesis.
Patients undergoing surgery for relatively minor injuries can have their diet advanced as if there were no intestinal involvement.
Feeding after minor repairs. When the surgery has involved relatively minor injuries, such as isolated serosal tears and adhesiolysis, nasogastric tube placement is not required. These patients can have their diet advanced as if there were no intestinal involvement. Give clear liquids when bowel sounds are heard and, if tolerated, advance to solids. It is probably not necessary to await a bowel movement before discharging the patient; she can be released once flatus is passed.
Substantial repairs. When major injuries have been repaired, such as with a large perforation repair or bowel resection, it is prudent to proceed more slowly.
Place a nasogastric tube to minimize bowel distention and subsequent leakage from the repair site. Give the patient nothing by mouth until bowel sounds are clearly present and flatus is passed. Then clamp the nasogastric tube for 24 hours, remove it, and institute clear liquids, provided there is no nausea, vomiting, or distension. Advance to full liquids and then solids, tailoring this process to the patient. When she can tolerate a regular diet, with substantial passage of flatus or bowel movement, recovery is signaled.
Need for additional training
The techniques necessary to manage simple bowel injury are not difficult to master. However, Ob/Gyn residency programs need to extend training in this area. Additional rotations on the general surgery or trauma services as second- or third-year residents would be ideal, but the use of animal laboratories is a good alternative.
The authors report no financial relationships relevant to this article.
1. Burke P, Mealy K, Gillen P, Joyce W, Traynor O, Hyland J. Requirement for bowel preparation in colorectal surgery. Br J Surg. 1994;81:907-910.
2. Miettinen RPJ, Laitinen ST, Makela JT, Paakkonen ME. Bowel preparation with oral polyethylene glycol electrolyte solution vs. no preparation in elective open colorectal surgery. Dis Colon Rectum. 2000;43:669-677.
3. Zmora O, Pikarsky AJ, Wexner SD. Bowel preparation for colorectal surgery. Dis Colon Rectum. 2001;44:1537-1547.
4. Nichols RL, Smith JW, Garcia RY, Waterman RS, Holmes JWC. Current practices of preoperative bowel preparation among North American colorectal surgeons. Clin Infect Dis. 1997;24:609-619.
5. Rock JA, Jones HW. Intestinal tract in gynecologic surgery. In: TeLinde RW, Rock JA, Jones HW, eds. Telinde’s Operative Gynecology. 9th ed. Philadelphia: Lippincott Williams & Wilkins; 2003;1239-1272.
6. Krebs H. Intestinal injury in gynecologic surgery: a ten-year experience. Am J Obstet Gynecol. 1986;155:509-514.
7. Van Der Krabben AA, Dukstra FR, Nieuwenhuijzen M, Reijnen M, Schaapveld M, Van Der Goor H. Morbidity and mortality of inadvertent enterotomy during adhesiotomy. Br J Surg. 2000;87:467-471.
8. Monk BJ, Berman ML, Montz FJ. Adhesions after extensive gynecologic surgery: clinical significance, etiology, and prevention. Am J Obstet Gynecol. 1994;170:1396-1403.
9. Fanning J, Andrews S. Early postoperative feeding after major gynecologic surgery: evidence-based scientific medicine. Am J Obstet Gynecol. 2001;185:1-4.
- Although the optimal method is a matter of choice, preoperative bowel preparation is recommended to reduce bacteria, stool bulk, and infectious complications.
- When entering the peritoneal cavity in patients with prior surgery, watch for adhesions between a loop of bowel and the abdominal wall.
- In high-risk patients, enter the peritoneal cavity by extending the previous abdominal scar superiorly and inferiorly to minimize risk of injury.
- Close small perforations in 2 layers, with the suture line always perpendicular to the long axis of the bowel.
- For more extensive injury or compromised blood supply to the bowel wall, resection and anastomosis may be necessary. Obtain intraoperative general surgical consultation if not trained to perform this kind of repair.
This dreaded complication requires vigilance and skill to avoid, and adequate training and experience to manage and repair. In a perfect world, every gynecologist would be trained in techniques to prevent and repair inadvertent bowel injuries. Unfortunately, residency programs often do not provide such training.
Gynecologists routinely operate on patients with risk factors for bowel injury—obesity, endometriosis, multiple abdominal procedures, pelvic inflammatory disease, history of malignancy, and advanced age. A general surgeon is often called, however, for bowel repairs that can be performed by a gynecologist with sufficient training and experience. (There are instances, however, in which a general surgical consultation may not be readily available—another reason to master repair of bowel injuries encountered during gynecologic surgery.)
This article describes techniques to avert and manage intestinal injury. Topics include adhesiolysis, repair of bowel perforations, segmental bowel resection, and pre- and postoperative management. Vascular anatomy of the bowel is illustrated.
We emphasize the need for direct supervision by an experienced surgeon while mastering these techniques.
Bowel preparation: A useful tool to reduce infection, leakage
Isolated reports have questioned the need for mechanical bowel preparation,1,2 and some experts point to the recent success of primary repairs of gunshot and stab wounds to the colon as evidence that bowel preparation and preoperative oral antibiotics are unnecessary.
Other studies indicate potential benefits, namely reducing infectious complications and anastomotic leakage following repair of inadvertent enterotomy. Indeed, the vast majority of North American surgeons continue to use some form of bowel preparation,3,4 and it is the standard of care for elective intestinal surgery. For these reasons, bowel preparation is strongly encouraged for the gynecologic surgeon operating on a pelvic mass, endometriosis, or malignancy, or when difficult dissection is anticipated with the potential for inadvertent enterotomy and spillage of intestinal contents.
Bowel preparation consists of 2 phases: mechanical cleansing and antibiotic administration (TABLE). The postoperative infection rate can be reduced to well below 10% when these are properly performed.
Mechanical cleansing reduces the bulk of stool content within the lumen of the bowel, which also decreases the absolute amount of bacteria.5 Anaerobes are the predominant flora in the colon, with an estimated density of 1010 organisms per gram of stool. Perforation and spillage of colon contents contaminates the peritoneal cavity with more than 400 species of bacteria.
Perforation and spillage of colon contents contaminates the peritoneal cavity with more than 400 species of bacteria.
In the past, stool bulk was reduced via a low-residue or liquid diet combined with cathartics, enemas, or other agents given over 2 to 3 days. This regimen was time-consuming, patient compliance was poor, and nutritional intake was severely restricted prior to major surgery.
Today, polyethylene glycol and sodium phosphate are the 2 most popular methods of bowel preparation.
- Polyethylene glycol (Golytely, Braintree Labs, Braintree, Mass) is a balanced electrolyte solution dispensed in a 4-L quantity that must be taken over 4 hours. Some patients find this volume difficult to consume; one option is administering the solution via a small nasogastric tube. Complications may include nausea/vomiting, abdominal cramping, and, rarely, fluid overload and electrolyte disturbances.
- Sodium phosphate (Fleet Phosphosoda, C.B. Fleet Co, Lynchburg, Va) is administered in two 45-mL increments several hours apart. There is no consensus on which bowel-prep method is superior3,4; most surgeons prefer one or the other. Due to potential electrolyte abnormalities with the use of sodium phosphate, polyethylene glycol is favored for patients with significant renal, cardiac, or hepatic disease.
- We recommend minimally absorbed oral antibiotics (1 g each of neomycin and erythromycin, given at 1 PM, 2 PM, and 11 PM the day before surgery) in combination with an intravenous second-generation cephalosporin (1 g if using cefotetan, 2 g if using cefoxitin; given immediately before surgery and continued postoperatively for 3 doses).
- Timing of antibiotic administration is important, since postoperative antibiotics alone do not appear to be effective. If significant spillage occurs intraoperatively, parenteral antibiotics should be continued for 5 days.
TABLE
Bowel prep regimen
| DAY BEFORE SURGERY |
| Morning |
| Light breakfast |
| Noon |
| Clear liquids |
| Polyethylene glycol, 4L, to be consumed over 4 to 6 hours |
| 1 PM |
| Neomycin, 1 g orally |
| Erythromycin, 1 g orally |
| 2 PM |
| Neomycin, 1 g orally |
| Erythromycin, 1 g orally |
| Evening |
| Clear liquids |
| 11 PM |
| Neomycin, 1 g orally |
| Erythromycin, 1 g orally |
| DAY OF SURGERY |
| Morning |
| Intravenous cephalosporin (1 g cefotetan or 2 g cefoxitin); 1 hour before incision, continued postoperatively for 3 doses |
When injuries are most likely
Intestinal injuries during gynecologic surgery usually involve the small bowel and can be minor, such as a serosal tear or a small, full-thickness laceration—or major, involving a devitalized bowel loop or its mesentery.
Bowel injury may occur during a variety of surgical procedures. One study showed that most injuries occur during adhesiolysis or entry into the peritoneal cavity. A smaller but substantial number of cases occur during “less extensive” procedures such as uterine curettage and laparoscopy.6
Upon entering the peritoneal cavity, keep in mind the possibility of injuring an adherent loop of bowel. Because of its anatomical relationships to the pelvic viscera, portions of the bowel may become involved in adhesions, which can lead to extremely challenging pelvic dissections in conditions such as endometriosis or severe pelvic infection. Dissection of pelvic adhesions is a common cause of bowel injury, because bowel loops are retracted deeply downward by adhesive bands, and the limited pelvic space hampers visualization and gentle adhesiolysis.
At special risk for bowel injury are women who have undergone prior abdominal operations or who are obese. In a series of 270 general surgery patients undergoing reoperation,7 52 (19%) sustained inadvertent enterotomy. These patients had undergone a mean of 3.3 previous laparotomies and had a higher body mass index (mean of 25.5 versus 21.9).
Age may be another risk factor, since patients with enterotomies were 60 years or older.7
Injury during laparoscopy. Inadvertent bowel injuries may occur during laparoscopic procedures, especially at the time of trocar insertion or manipulation of pelvic structures.5 One device that helps prevent these injuries is the optical trocar (Visiport, US Surgical, Norwalk, Conn), which allows physicians to visualize the layers of the abdominal wall as penetration occurs.
We also routinely direct anesthesia personnel to insert a nasogastric tube at the beginning of laparoscopic procedures to facilitate decompression of the stomach and small bowel.
The risks of electrosurgery. Electrocautery used for tubal ligation, pelvic dissection, or hemostasis may injure the bowel if the surgeon is not careful. Thermal injury due to unipolar cautery is particularly ominous because the extent of injury is greater than what can be grossly observed. The incidence of this type of injury can be reduced using bipolar cautery devices, as well as clips or bands for tubal ligation.
Injury as a result of uterine perforation is unlikely, but can occur. If perforation occurs during dilation and curettage, bowel laceration may result, particularly adhesions are present between the uterus and bowel loops. In extremely rare instances, a loop of bowel may be pulled through a perforation into the uterine cavity or vagina, requiring laparotomy for reduction and repair. Caution is advised during curettage, especially in a gravid uterus, to prevent this potentially catastrophic event.
Avoid the temptation to lyse opaque adhesions using blunt dissection, as serosal tears and enterotomies may occur.
Adhesiolysis: Plan on a lengthy, meticulous procedure
Adhesions are a common cause of pelvic pain, infertility, and bowel obstruction, and their presence may make it difficult to carry out the intended surgical procedure. Adhesiolysis may be necessary to mobilize loops of bowel tightly adherent to pelvic structures, to provide sufficient exposure of the surgical field and prevent subsequent bowel obstruction.
The extent of adhesions does not necessarily correlate with clinical symptoms.
Adhesions may be of the thin, filmy, “friendly” variety or dense, thick bands.
How adhesions occur. When tissue is injured, fibrin is deposited on the peritoneal and serosal surfaces. The extent to which this fibrin is infiltrated with fibroblasts and the degree of subsequent fibrosis determine adhesion density. Any process that impairs fibrinolysis tends to delay resolution of adhesions.
Contributing factors. Adhesions are commonly encountered in pelvic surgery and may be observed in 50% to 90% of patients who have undergone previous surgery.8
Obese patients also are more susceptible to adhesions. Other contributing factors include pelvic infection, bleeding, irradiation, chemical irritants, and conditions such as endometriosis.
Lysis technique. Apply gentle, controlled traction—as well as countertraction—on the bowel loops to facilitate isolation and dissection with sharp Metzenbaum scissors or a scalpel. (Forceful traction or rough handling of bowel loops may cause a breach in the bowel wall with subsequent spillage of intestinal contents.)
Avoid the temptation to lyse adhesions using blunt dissection (serosal tears and enterotomies may occur)—except in the case of translucent adhesions. These may be lysed via gentle, blunt dissection by rubbing the index finger and thumb back and forth over tissue. They also may be sharply cut using the tip of the scissors to form a “window” in a portion of the adhesion and cutting the adhesive segments in increments.
A characteristic line of demarcation often appears between adhesions and their peritoneal attachment, denoting a safe dissection plane.
Technique for special challenges: Chronic pelvic disease, prior laparotomies. When operating on these patients, be prepared for a long, meticulous procedure. A hasty approach in such cases is perilous and increases the likelihood of postoperative complications.
First, dissect the anterior abdominal wall from the adherent bowel on either side of the incision. Then extend the dissection laterally on both sides until the ascending and descending colon are identified. Next, dissect the small bowel free and mobilize it out of the pelvis.
It often is helpful to move to another area when dissection becomes too difficult; dissection through easier planes often will clarify the relationship of pelvic structures and adherent bowel loops.
Once the small bowel has been mobilized from the pelvis, lyse adhesions between loops of bowel that are causing kinking or narrowing of the lumen, to reduce the risk of postoperative bowel obstruction. Next, carefully dissect pelvic structures from the sigmoid colon and rectum.
How and when to repair serosal injury
Serosal injury is a breach of integrity of the visceral peritoneum, the outermost covering of the bowel wall. This may occur when the serosa is cut during entry into the abdomen or when it is torn during blunt dissection of dense adhesions.
If the underlying muscular and mucosal layers remain intact, these small areas of “denuded” serosa need not be repaired, since most experts believe that suture placement increases the likelihood of future adhesions. The serosal and muscular layers should be repaired if the mucosa is exposed, however. Otherwise the bowel wall will weaken at the site, making it vulnerable to perforation. The seromuscular layers can be approximated easily using interrupted 4-0 silk on a small tapered needle. Be careful to avoid placing the stitch through the mucosa, which would violate the bowel lumen.
When the defect of the seromuscular layer is large (when a more extensive area is denuded during dissection of densely adherent bowel away from a tumor or endometriotic lesion), repair becomes more involved. This may require resection of the injured area with primary anastomosis.
Intestinal perforations: Early recognition is essential
This critical serious complication can become disastrous if not immediately recognized and repaired. Perforation of the small intestine (enterotomy) or large bowel (colotomy) often occurs upon entry into the peritoneal cavity or during a difficult dissection, particularly when extensive adhesions are present.
Exercise special caution when operating on patients who have undergone prior surgery, who are advanced in age, or both.
Reoperation technique. When entering the abdomen through an old scar, reduce the likelihood of bowel injury by extending the new incision to either side of the old scar. Then enter the peritoneal cavity in a virgin area of the abdominal wall, where adherent loops of bowel are less likely.
Carefully open the fascia and dissect the preperitoneal fat down to the peritoneum. Before entering the abdominal cavity, retract the peritoneum upward with smooth forceps and palpate it between the thumb and index finger to ensure that a bowel loop is not in harm’s way.
If the underlying muscular and mucosal layers remain intact, small areas of “denuded” serosa need not be repaired.
Examine the entire small and large bowel carefully after surgery, to rule out injury. It is not uncommon for more than 1 perforation to occur in a bowel segment during a difficult dissection.
Begin at the ligament of Treitz and continue to the ileocecal junction. This is “running” the bowel—ie, inspecting in hand-over-hand fashion.
In the small bowel, the division between the jejunum and ileum is arbitrary, with no sharply defined line of demarcation. However, the diameter of the lumen decreases as one moves from jejunum to ileum, the number of vascular arcades increases, and the number of windows of Deaver diminishes. Also, the wall of the jejunum is generally thicker than that of the ileum.
In addition, inspect the colon in its entirety, with special emphasis on the sigmoid and rectum. Besides its larger lumen, the large bowel is distinguished by 3 longitudinal muscular bands called taenia coli, out-pouching of the wall (sacculations), and epiploic appendages.
Also examine the mesentery to exclude vascular compromise to the bowel wall.
Repair perforations immediately to limit contamination of the peritoneal cavity. Prior to closure, inspect wound edges for devitalized tissue and, if found, promptly debride it.
If colotomy occurs in the setting of an unprepared bowel with significant spillage, follow closure with copious irrigation.
Small perforations can usually be closed in 2 layers, with an inner layer of 3-0 delayed synthetic absorbable suture (Dexon, Vicryl) through the full thickness of the bowel wall, ensuring mucosal approximation. It is vital that this layer be “waterproof,” allowing no leakage of intestinal contents. Then place a second row of suture in the seromuscular layer using 4-0 silk to imbricate the first suture line.
General surgical consultation is needed whenever the gynecologist is inexperienced with bowel resection and anastomosis.
It also is essential that the suture line be perpendicular to the long axis of the bowel, rather than parallel; otherwise, the bowel lumen would narrow. Even perforations extending along the longitudinal axis for several centimeters should be repaired in transverse fashion to provide a lumen of adequate diameter.
Resecting the small bowel: If inexperienced, obtain general surgery consultation
Bowel resection and anastomosis require a greater degree of skill than is attained in a typical gynecologic training program. For that reason, resection is addressed here only superficially. Our primary caveat: A general surgical consultation should be obtained whenever the gynecologist is inexperienced with bowel resection and anastomosis.
Indications for resection. Strongly consider resection and anastomosis if the perforation involves more than 50% of the circumference of the bowel wall, if multiple perforations occur in a short segment of bowel, or if there is vascular compromise to a segment of bowel. Adequate perfusion to the bowel usually is indicated by a pink serosal surface. If the serosa remains dark or dusky and fails to become pink after several minutes of observation, vascular compromise is likely and resection is preferred.
If there is doubt about the blood supply to the bowel, give 1 g fluorescein intravenously and inspect the bowel under ultraviolet light (Wood’s lamp). Normal vascularized bowel will have a homogenous yellow-green appearance. Patchy fluorescence or areas without any fluorescence are evidence of ischemia.
To drain or not to drain
Because perforation and resection both involve entry into the bowel lumen, some degree of spillage is inevitable. This is of greater concern when the large bowel is involved, because of the increased likelihood of bacterial contamination. Immediate copious irrigation of the peritoneal cavity is indicated. Also consider a pelvic drain, especially when dissection has been extensive or raw surfaces are oozing.
The combination of bacterial contamination and free peritoneal blood in the pelvis increases the risk for infection. A strategically placed, half-inch Jackson Pratt drain (or a similar device) may help prevent abscess. In the event of anastomotic leakage, a drain often allows for a controlled enterocutaneous fistula to be managed without reoperation.
Some surgeons have satisfactory results without these drainage techniques.
When to begin postop feeding: Depends on type of repair
Opinion varies about the appropriate time to commence feeding after major abdominal surgery, particularly bowel surgery. Over the past decade, with the pressure to discharge patients earlier, many physicians have opted for earlier timing.
Traditionally, feeding was withheld until bowel sounds were auscultated; then it progressed slowly. Today many surgeons advance the diet much more quickly, with little or no delay in recovery. Fanning and Andrews9 demonstrated that early feeding does not increase the incidence of anastomotic leakage, dehiscence, or aspiration pneumonia—although it is associated with increased emesis.
Patients undergoing surgery for relatively minor injuries can have their diet advanced as if there were no intestinal involvement.
Feeding after minor repairs. When the surgery has involved relatively minor injuries, such as isolated serosal tears and adhesiolysis, nasogastric tube placement is not required. These patients can have their diet advanced as if there were no intestinal involvement. Give clear liquids when bowel sounds are heard and, if tolerated, advance to solids. It is probably not necessary to await a bowel movement before discharging the patient; she can be released once flatus is passed.
Substantial repairs. When major injuries have been repaired, such as with a large perforation repair or bowel resection, it is prudent to proceed more slowly.
Place a nasogastric tube to minimize bowel distention and subsequent leakage from the repair site. Give the patient nothing by mouth until bowel sounds are clearly present and flatus is passed. Then clamp the nasogastric tube for 24 hours, remove it, and institute clear liquids, provided there is no nausea, vomiting, or distension. Advance to full liquids and then solids, tailoring this process to the patient. When she can tolerate a regular diet, with substantial passage of flatus or bowel movement, recovery is signaled.
Need for additional training
The techniques necessary to manage simple bowel injury are not difficult to master. However, Ob/Gyn residency programs need to extend training in this area. Additional rotations on the general surgery or trauma services as second- or third-year residents would be ideal, but the use of animal laboratories is a good alternative.
The authors report no financial relationships relevant to this article.
- Although the optimal method is a matter of choice, preoperative bowel preparation is recommended to reduce bacteria, stool bulk, and infectious complications.
- When entering the peritoneal cavity in patients with prior surgery, watch for adhesions between a loop of bowel and the abdominal wall.
- In high-risk patients, enter the peritoneal cavity by extending the previous abdominal scar superiorly and inferiorly to minimize risk of injury.
- Close small perforations in 2 layers, with the suture line always perpendicular to the long axis of the bowel.
- For more extensive injury or compromised blood supply to the bowel wall, resection and anastomosis may be necessary. Obtain intraoperative general surgical consultation if not trained to perform this kind of repair.
This dreaded complication requires vigilance and skill to avoid, and adequate training and experience to manage and repair. In a perfect world, every gynecologist would be trained in techniques to prevent and repair inadvertent bowel injuries. Unfortunately, residency programs often do not provide such training.
Gynecologists routinely operate on patients with risk factors for bowel injury—obesity, endometriosis, multiple abdominal procedures, pelvic inflammatory disease, history of malignancy, and advanced age. A general surgeon is often called, however, for bowel repairs that can be performed by a gynecologist with sufficient training and experience. (There are instances, however, in which a general surgical consultation may not be readily available—another reason to master repair of bowel injuries encountered during gynecologic surgery.)
This article describes techniques to avert and manage intestinal injury. Topics include adhesiolysis, repair of bowel perforations, segmental bowel resection, and pre- and postoperative management. Vascular anatomy of the bowel is illustrated.
We emphasize the need for direct supervision by an experienced surgeon while mastering these techniques.
Bowel preparation: A useful tool to reduce infection, leakage
Isolated reports have questioned the need for mechanical bowel preparation,1,2 and some experts point to the recent success of primary repairs of gunshot and stab wounds to the colon as evidence that bowel preparation and preoperative oral antibiotics are unnecessary.
Other studies indicate potential benefits, namely reducing infectious complications and anastomotic leakage following repair of inadvertent enterotomy. Indeed, the vast majority of North American surgeons continue to use some form of bowel preparation,3,4 and it is the standard of care for elective intestinal surgery. For these reasons, bowel preparation is strongly encouraged for the gynecologic surgeon operating on a pelvic mass, endometriosis, or malignancy, or when difficult dissection is anticipated with the potential for inadvertent enterotomy and spillage of intestinal contents.
Bowel preparation consists of 2 phases: mechanical cleansing and antibiotic administration (TABLE). The postoperative infection rate can be reduced to well below 10% when these are properly performed.
Mechanical cleansing reduces the bulk of stool content within the lumen of the bowel, which also decreases the absolute amount of bacteria.5 Anaerobes are the predominant flora in the colon, with an estimated density of 1010 organisms per gram of stool. Perforation and spillage of colon contents contaminates the peritoneal cavity with more than 400 species of bacteria.
Perforation and spillage of colon contents contaminates the peritoneal cavity with more than 400 species of bacteria.
In the past, stool bulk was reduced via a low-residue or liquid diet combined with cathartics, enemas, or other agents given over 2 to 3 days. This regimen was time-consuming, patient compliance was poor, and nutritional intake was severely restricted prior to major surgery.
Today, polyethylene glycol and sodium phosphate are the 2 most popular methods of bowel preparation.
- Polyethylene glycol (Golytely, Braintree Labs, Braintree, Mass) is a balanced electrolyte solution dispensed in a 4-L quantity that must be taken over 4 hours. Some patients find this volume difficult to consume; one option is administering the solution via a small nasogastric tube. Complications may include nausea/vomiting, abdominal cramping, and, rarely, fluid overload and electrolyte disturbances.
- Sodium phosphate (Fleet Phosphosoda, C.B. Fleet Co, Lynchburg, Va) is administered in two 45-mL increments several hours apart. There is no consensus on which bowel-prep method is superior3,4; most surgeons prefer one or the other. Due to potential electrolyte abnormalities with the use of sodium phosphate, polyethylene glycol is favored for patients with significant renal, cardiac, or hepatic disease.
- We recommend minimally absorbed oral antibiotics (1 g each of neomycin and erythromycin, given at 1 PM, 2 PM, and 11 PM the day before surgery) in combination with an intravenous second-generation cephalosporin (1 g if using cefotetan, 2 g if using cefoxitin; given immediately before surgery and continued postoperatively for 3 doses).
- Timing of antibiotic administration is important, since postoperative antibiotics alone do not appear to be effective. If significant spillage occurs intraoperatively, parenteral antibiotics should be continued for 5 days.
TABLE
Bowel prep regimen
| DAY BEFORE SURGERY |
| Morning |
| Light breakfast |
| Noon |
| Clear liquids |
| Polyethylene glycol, 4L, to be consumed over 4 to 6 hours |
| 1 PM |
| Neomycin, 1 g orally |
| Erythromycin, 1 g orally |
| 2 PM |
| Neomycin, 1 g orally |
| Erythromycin, 1 g orally |
| Evening |
| Clear liquids |
| 11 PM |
| Neomycin, 1 g orally |
| Erythromycin, 1 g orally |
| DAY OF SURGERY |
| Morning |
| Intravenous cephalosporin (1 g cefotetan or 2 g cefoxitin); 1 hour before incision, continued postoperatively for 3 doses |
When injuries are most likely
Intestinal injuries during gynecologic surgery usually involve the small bowel and can be minor, such as a serosal tear or a small, full-thickness laceration—or major, involving a devitalized bowel loop or its mesentery.
Bowel injury may occur during a variety of surgical procedures. One study showed that most injuries occur during adhesiolysis or entry into the peritoneal cavity. A smaller but substantial number of cases occur during “less extensive” procedures such as uterine curettage and laparoscopy.6
Upon entering the peritoneal cavity, keep in mind the possibility of injuring an adherent loop of bowel. Because of its anatomical relationships to the pelvic viscera, portions of the bowel may become involved in adhesions, which can lead to extremely challenging pelvic dissections in conditions such as endometriosis or severe pelvic infection. Dissection of pelvic adhesions is a common cause of bowel injury, because bowel loops are retracted deeply downward by adhesive bands, and the limited pelvic space hampers visualization and gentle adhesiolysis.
At special risk for bowel injury are women who have undergone prior abdominal operations or who are obese. In a series of 270 general surgery patients undergoing reoperation,7 52 (19%) sustained inadvertent enterotomy. These patients had undergone a mean of 3.3 previous laparotomies and had a higher body mass index (mean of 25.5 versus 21.9).
Age may be another risk factor, since patients with enterotomies were 60 years or older.7
Injury during laparoscopy. Inadvertent bowel injuries may occur during laparoscopic procedures, especially at the time of trocar insertion or manipulation of pelvic structures.5 One device that helps prevent these injuries is the optical trocar (Visiport, US Surgical, Norwalk, Conn), which allows physicians to visualize the layers of the abdominal wall as penetration occurs.
We also routinely direct anesthesia personnel to insert a nasogastric tube at the beginning of laparoscopic procedures to facilitate decompression of the stomach and small bowel.
The risks of electrosurgery. Electrocautery used for tubal ligation, pelvic dissection, or hemostasis may injure the bowel if the surgeon is not careful. Thermal injury due to unipolar cautery is particularly ominous because the extent of injury is greater than what can be grossly observed. The incidence of this type of injury can be reduced using bipolar cautery devices, as well as clips or bands for tubal ligation.
Injury as a result of uterine perforation is unlikely, but can occur. If perforation occurs during dilation and curettage, bowel laceration may result, particularly adhesions are present between the uterus and bowel loops. In extremely rare instances, a loop of bowel may be pulled through a perforation into the uterine cavity or vagina, requiring laparotomy for reduction and repair. Caution is advised during curettage, especially in a gravid uterus, to prevent this potentially catastrophic event.
Avoid the temptation to lyse opaque adhesions using blunt dissection, as serosal tears and enterotomies may occur.
Adhesiolysis: Plan on a lengthy, meticulous procedure
Adhesions are a common cause of pelvic pain, infertility, and bowel obstruction, and their presence may make it difficult to carry out the intended surgical procedure. Adhesiolysis may be necessary to mobilize loops of bowel tightly adherent to pelvic structures, to provide sufficient exposure of the surgical field and prevent subsequent bowel obstruction.
The extent of adhesions does not necessarily correlate with clinical symptoms.
Adhesions may be of the thin, filmy, “friendly” variety or dense, thick bands.
How adhesions occur. When tissue is injured, fibrin is deposited on the peritoneal and serosal surfaces. The extent to which this fibrin is infiltrated with fibroblasts and the degree of subsequent fibrosis determine adhesion density. Any process that impairs fibrinolysis tends to delay resolution of adhesions.
Contributing factors. Adhesions are commonly encountered in pelvic surgery and may be observed in 50% to 90% of patients who have undergone previous surgery.8
Obese patients also are more susceptible to adhesions. Other contributing factors include pelvic infection, bleeding, irradiation, chemical irritants, and conditions such as endometriosis.
Lysis technique. Apply gentle, controlled traction—as well as countertraction—on the bowel loops to facilitate isolation and dissection with sharp Metzenbaum scissors or a scalpel. (Forceful traction or rough handling of bowel loops may cause a breach in the bowel wall with subsequent spillage of intestinal contents.)
Avoid the temptation to lyse adhesions using blunt dissection (serosal tears and enterotomies may occur)—except in the case of translucent adhesions. These may be lysed via gentle, blunt dissection by rubbing the index finger and thumb back and forth over tissue. They also may be sharply cut using the tip of the scissors to form a “window” in a portion of the adhesion and cutting the adhesive segments in increments.
A characteristic line of demarcation often appears between adhesions and their peritoneal attachment, denoting a safe dissection plane.
Technique for special challenges: Chronic pelvic disease, prior laparotomies. When operating on these patients, be prepared for a long, meticulous procedure. A hasty approach in such cases is perilous and increases the likelihood of postoperative complications.
First, dissect the anterior abdominal wall from the adherent bowel on either side of the incision. Then extend the dissection laterally on both sides until the ascending and descending colon are identified. Next, dissect the small bowel free and mobilize it out of the pelvis.
It often is helpful to move to another area when dissection becomes too difficult; dissection through easier planes often will clarify the relationship of pelvic structures and adherent bowel loops.
Once the small bowel has been mobilized from the pelvis, lyse adhesions between loops of bowel that are causing kinking or narrowing of the lumen, to reduce the risk of postoperative bowel obstruction. Next, carefully dissect pelvic structures from the sigmoid colon and rectum.
How and when to repair serosal injury
Serosal injury is a breach of integrity of the visceral peritoneum, the outermost covering of the bowel wall. This may occur when the serosa is cut during entry into the abdomen or when it is torn during blunt dissection of dense adhesions.
If the underlying muscular and mucosal layers remain intact, these small areas of “denuded” serosa need not be repaired, since most experts believe that suture placement increases the likelihood of future adhesions. The serosal and muscular layers should be repaired if the mucosa is exposed, however. Otherwise the bowel wall will weaken at the site, making it vulnerable to perforation. The seromuscular layers can be approximated easily using interrupted 4-0 silk on a small tapered needle. Be careful to avoid placing the stitch through the mucosa, which would violate the bowel lumen.
When the defect of the seromuscular layer is large (when a more extensive area is denuded during dissection of densely adherent bowel away from a tumor or endometriotic lesion), repair becomes more involved. This may require resection of the injured area with primary anastomosis.
Intestinal perforations: Early recognition is essential
This critical serious complication can become disastrous if not immediately recognized and repaired. Perforation of the small intestine (enterotomy) or large bowel (colotomy) often occurs upon entry into the peritoneal cavity or during a difficult dissection, particularly when extensive adhesions are present.
Exercise special caution when operating on patients who have undergone prior surgery, who are advanced in age, or both.
Reoperation technique. When entering the abdomen through an old scar, reduce the likelihood of bowel injury by extending the new incision to either side of the old scar. Then enter the peritoneal cavity in a virgin area of the abdominal wall, where adherent loops of bowel are less likely.
Carefully open the fascia and dissect the preperitoneal fat down to the peritoneum. Before entering the abdominal cavity, retract the peritoneum upward with smooth forceps and palpate it between the thumb and index finger to ensure that a bowel loop is not in harm’s way.
If the underlying muscular and mucosal layers remain intact, small areas of “denuded” serosa need not be repaired.
Examine the entire small and large bowel carefully after surgery, to rule out injury. It is not uncommon for more than 1 perforation to occur in a bowel segment during a difficult dissection.
Begin at the ligament of Treitz and continue to the ileocecal junction. This is “running” the bowel—ie, inspecting in hand-over-hand fashion.
In the small bowel, the division between the jejunum and ileum is arbitrary, with no sharply defined line of demarcation. However, the diameter of the lumen decreases as one moves from jejunum to ileum, the number of vascular arcades increases, and the number of windows of Deaver diminishes. Also, the wall of the jejunum is generally thicker than that of the ileum.
In addition, inspect the colon in its entirety, with special emphasis on the sigmoid and rectum. Besides its larger lumen, the large bowel is distinguished by 3 longitudinal muscular bands called taenia coli, out-pouching of the wall (sacculations), and epiploic appendages.
Also examine the mesentery to exclude vascular compromise to the bowel wall.
Repair perforations immediately to limit contamination of the peritoneal cavity. Prior to closure, inspect wound edges for devitalized tissue and, if found, promptly debride it.
If colotomy occurs in the setting of an unprepared bowel with significant spillage, follow closure with copious irrigation.
Small perforations can usually be closed in 2 layers, with an inner layer of 3-0 delayed synthetic absorbable suture (Dexon, Vicryl) through the full thickness of the bowel wall, ensuring mucosal approximation. It is vital that this layer be “waterproof,” allowing no leakage of intestinal contents. Then place a second row of suture in the seromuscular layer using 4-0 silk to imbricate the first suture line.
General surgical consultation is needed whenever the gynecologist is inexperienced with bowel resection and anastomosis.
It also is essential that the suture line be perpendicular to the long axis of the bowel, rather than parallel; otherwise, the bowel lumen would narrow. Even perforations extending along the longitudinal axis for several centimeters should be repaired in transverse fashion to provide a lumen of adequate diameter.
Resecting the small bowel: If inexperienced, obtain general surgery consultation
Bowel resection and anastomosis require a greater degree of skill than is attained in a typical gynecologic training program. For that reason, resection is addressed here only superficially. Our primary caveat: A general surgical consultation should be obtained whenever the gynecologist is inexperienced with bowel resection and anastomosis.
Indications for resection. Strongly consider resection and anastomosis if the perforation involves more than 50% of the circumference of the bowel wall, if multiple perforations occur in a short segment of bowel, or if there is vascular compromise to a segment of bowel. Adequate perfusion to the bowel usually is indicated by a pink serosal surface. If the serosa remains dark or dusky and fails to become pink after several minutes of observation, vascular compromise is likely and resection is preferred.
If there is doubt about the blood supply to the bowel, give 1 g fluorescein intravenously and inspect the bowel under ultraviolet light (Wood’s lamp). Normal vascularized bowel will have a homogenous yellow-green appearance. Patchy fluorescence or areas without any fluorescence are evidence of ischemia.
To drain or not to drain
Because perforation and resection both involve entry into the bowel lumen, some degree of spillage is inevitable. This is of greater concern when the large bowel is involved, because of the increased likelihood of bacterial contamination. Immediate copious irrigation of the peritoneal cavity is indicated. Also consider a pelvic drain, especially when dissection has been extensive or raw surfaces are oozing.
The combination of bacterial contamination and free peritoneal blood in the pelvis increases the risk for infection. A strategically placed, half-inch Jackson Pratt drain (or a similar device) may help prevent abscess. In the event of anastomotic leakage, a drain often allows for a controlled enterocutaneous fistula to be managed without reoperation.
Some surgeons have satisfactory results without these drainage techniques.
When to begin postop feeding: Depends on type of repair
Opinion varies about the appropriate time to commence feeding after major abdominal surgery, particularly bowel surgery. Over the past decade, with the pressure to discharge patients earlier, many physicians have opted for earlier timing.
Traditionally, feeding was withheld until bowel sounds were auscultated; then it progressed slowly. Today many surgeons advance the diet much more quickly, with little or no delay in recovery. Fanning and Andrews9 demonstrated that early feeding does not increase the incidence of anastomotic leakage, dehiscence, or aspiration pneumonia—although it is associated with increased emesis.
Patients undergoing surgery for relatively minor injuries can have their diet advanced as if there were no intestinal involvement.
Feeding after minor repairs. When the surgery has involved relatively minor injuries, such as isolated serosal tears and adhesiolysis, nasogastric tube placement is not required. These patients can have their diet advanced as if there were no intestinal involvement. Give clear liquids when bowel sounds are heard and, if tolerated, advance to solids. It is probably not necessary to await a bowel movement before discharging the patient; she can be released once flatus is passed.
Substantial repairs. When major injuries have been repaired, such as with a large perforation repair or bowel resection, it is prudent to proceed more slowly.
Place a nasogastric tube to minimize bowel distention and subsequent leakage from the repair site. Give the patient nothing by mouth until bowel sounds are clearly present and flatus is passed. Then clamp the nasogastric tube for 24 hours, remove it, and institute clear liquids, provided there is no nausea, vomiting, or distension. Advance to full liquids and then solids, tailoring this process to the patient. When she can tolerate a regular diet, with substantial passage of flatus or bowel movement, recovery is signaled.
Need for additional training
The techniques necessary to manage simple bowel injury are not difficult to master. However, Ob/Gyn residency programs need to extend training in this area. Additional rotations on the general surgery or trauma services as second- or third-year residents would be ideal, but the use of animal laboratories is a good alternative.
The authors report no financial relationships relevant to this article.
1. Burke P, Mealy K, Gillen P, Joyce W, Traynor O, Hyland J. Requirement for bowel preparation in colorectal surgery. Br J Surg. 1994;81:907-910.
2. Miettinen RPJ, Laitinen ST, Makela JT, Paakkonen ME. Bowel preparation with oral polyethylene glycol electrolyte solution vs. no preparation in elective open colorectal surgery. Dis Colon Rectum. 2000;43:669-677.
3. Zmora O, Pikarsky AJ, Wexner SD. Bowel preparation for colorectal surgery. Dis Colon Rectum. 2001;44:1537-1547.
4. Nichols RL, Smith JW, Garcia RY, Waterman RS, Holmes JWC. Current practices of preoperative bowel preparation among North American colorectal surgeons. Clin Infect Dis. 1997;24:609-619.
5. Rock JA, Jones HW. Intestinal tract in gynecologic surgery. In: TeLinde RW, Rock JA, Jones HW, eds. Telinde’s Operative Gynecology. 9th ed. Philadelphia: Lippincott Williams & Wilkins; 2003;1239-1272.
6. Krebs H. Intestinal injury in gynecologic surgery: a ten-year experience. Am J Obstet Gynecol. 1986;155:509-514.
7. Van Der Krabben AA, Dukstra FR, Nieuwenhuijzen M, Reijnen M, Schaapveld M, Van Der Goor H. Morbidity and mortality of inadvertent enterotomy during adhesiotomy. Br J Surg. 2000;87:467-471.
8. Monk BJ, Berman ML, Montz FJ. Adhesions after extensive gynecologic surgery: clinical significance, etiology, and prevention. Am J Obstet Gynecol. 1994;170:1396-1403.
9. Fanning J, Andrews S. Early postoperative feeding after major gynecologic surgery: evidence-based scientific medicine. Am J Obstet Gynecol. 2001;185:1-4.
1. Burke P, Mealy K, Gillen P, Joyce W, Traynor O, Hyland J. Requirement for bowel preparation in colorectal surgery. Br J Surg. 1994;81:907-910.
2. Miettinen RPJ, Laitinen ST, Makela JT, Paakkonen ME. Bowel preparation with oral polyethylene glycol electrolyte solution vs. no preparation in elective open colorectal surgery. Dis Colon Rectum. 2000;43:669-677.
3. Zmora O, Pikarsky AJ, Wexner SD. Bowel preparation for colorectal surgery. Dis Colon Rectum. 2001;44:1537-1547.
4. Nichols RL, Smith JW, Garcia RY, Waterman RS, Holmes JWC. Current practices of preoperative bowel preparation among North American colorectal surgeons. Clin Infect Dis. 1997;24:609-619.
5. Rock JA, Jones HW. Intestinal tract in gynecologic surgery. In: TeLinde RW, Rock JA, Jones HW, eds. Telinde’s Operative Gynecology. 9th ed. Philadelphia: Lippincott Williams & Wilkins; 2003;1239-1272.
6. Krebs H. Intestinal injury in gynecologic surgery: a ten-year experience. Am J Obstet Gynecol. 1986;155:509-514.
7. Van Der Krabben AA, Dukstra FR, Nieuwenhuijzen M, Reijnen M, Schaapveld M, Van Der Goor H. Morbidity and mortality of inadvertent enterotomy during adhesiotomy. Br J Surg. 2000;87:467-471.
8. Monk BJ, Berman ML, Montz FJ. Adhesions after extensive gynecologic surgery: clinical significance, etiology, and prevention. Am J Obstet Gynecol. 1994;170:1396-1403.
9. Fanning J, Andrews S. Early postoperative feeding after major gynecologic surgery: evidence-based scientific medicine. Am J Obstet Gynecol. 2001;185:1-4.
4 global ablation devices: Efficacy, indications, and technique
- These techniques are easy to learn and use, and offer results comparable to rollerball procedures. Selected patients can be treated successfully in the office setting.
- It is is vital that the patient have a reliable and permanent form of contraception, such as tubal ligation or vasectomy.
- Long-term complications, though rare, include endometrial hyperplasia and occult endometrial carcinoma.
Thus, attempts to destroy it and achieve amenorrhea have met with limited success.
Hysterectomy is still the definitive treatment for excessive uterine bleeding, but a more conservative treatment, ablation, uses surgical or chemical means to obliterate the endometrial surface. Newer devices (FDA approved since 1997) allow office-based or same-day surgery; recovery time is shorter, and complication rates are lower than for hysterectomy.
This approach has gained popularity as instrumentation has improved; yet, because the endometrial surface is so resilient, success rates fall well shy of 100%. This article summaries the data on efficacy, and describes the indications, preoperative evaluation, and technique for 4 ablation options:
- thermal balloon ablation
- thermal fluid ablation
- cryotherapy
- impedance-controlled ablation
Other modalities include microwave, laser, and a progestin-releasing intrauterine contraception system.
Each uses a different energy-transfer technique to destroy the endometrium ( TABLE).
TABLE
4 global ablation devices at a glance
| THERMAL BALLOON (THERMACHOICE) | THERMAL FLUID (HYDROTHERMABLATOR) | CRYOTHERAPY (HER OPTION) | IMPEDANCE-CONTROLLED (NOVASURE) | |
|---|---|---|---|---|
| Pretreatment | Yes | Yes | Yes | No |
| Time of energy delivery | 8 minutes | 10 minutes | 10–12 minutes | 90–120 seconds |
| Cornual ablation | No | Yes | User-dependent | Yes |
| Principle | Balloon filled with fluid (5% dextrose in water) at 87°C | Hydrothermal circulation of saline at 90°C | Probe with transfer media creates ice ball at –100 to –120°C | Bipolar, radiofrequency ablation at 100°C |
| Direct visualization | None | Hysteroscopy | Ultrasound guidance | None |
| Safety features | Pressure and temperature-sensing cutoffs | Fluid loss detection system | Ultrasound guidance | Uterine cavity integrity assessment system |
Indications
The typical candidate for endometrial ablation has heavy menses requiring excessive sanitary protection (eg, tampon and pad simultaneously); her daily activity frequently is limited. The patient may have tried such management as nonsteroidal anti-inflammatory agents, oral contraceptives, or surgical dilatation and curettage (D&C) without success.
Excessive or abnormal uterine bleeding is defined as blood loss exceeding 80 mL per menses or a menstrual flow longer than 7 days. Abnormal uterine bleeding affects 22% of women of reproductive age.1 Each year in the United States, approximately 180,000 women undergo hysterectomy for this indication.2
The optimal patient for endometrial ablation has a history of regular menses without excessive dysmenorrhea, which could suggest an underlying diagnosis of adenomyosis. (Findings suggestive of this difficult-to-diagnose condition include a tender, soft, boggy uterus at the time of menses.) Many women with adenomyosis fail to achieve adequate pain relief with endometrial ablation alone and eventually require a hysterectomy.
The patient should have completed childbearing and have a permanent method of contraception in place—endometrial ablation only reduces fertility, it does not eliminate it.
Preoperative evaluation
Laboratory studies include a complete blood count and urine human chorionic gonadotropin level, as well as screening for bleeding disorders when indicated.
A bleeding diary helps quantify symptoms. Its use should be encouraged.
Other tests and examinations. Also recommended are endometrial biopsy, a Pap test, and assessment of the endometrial cavity via hysteroscopy or sonohysterography.
Biopsy should reflect histologically normal tissue. The patient should have:
- regular menstrual cycles lasting 25 to 34 days
- no uterine anomaly or potential myometrial wall defect from a previous classical cesarean or transmural myomectomy
Contraindications
Anovulatory patients may not be good candidates because islands of endometrial tissue can remain after ablation. These tissue “nests” may spontaneously change to hyperplasia or endometrial carcinoma (due to unopposed estrogen). Further, uterine bleeding may not always occur when hyperplasia is present after endometrial ablation, delaying this serious diagnosis.
Relative contraindications include intramural or submucosal uterine myomas.
Earlier techniques: Hysteroscopic ablation
Techniques developed earlier require expert operative hysteroscopic skills and should be performed by experienced gynecologic surgeons to minimize complications.
For example, the first treatment of menorrhagia using a hysteroscopic approach with a Nd:YAG laser power source, reported in 1981 by Goldrath et al,3 utilized 55 watts of power with a 600-micron fiber dragged across the endometrium. Later, other surgeons used a rollerball unipolar electrode that coagulated the surface with continuous contact.4,5
Modifications of this technique included a loop electrode that used monopolar electrical energy to “shave” the thicker portions of endometrium. In some reports, the rollerball electrode was used to reach the uterine cornu and endocoagulate the lower uterine segments. The most successful reports of this approach used a loop electrode to shave the endometrium followed by rollerball coagulation of the shaved areas. Amenorrhea rates with these techniques approached 60%.
Thermal balloon ablation
ThermaChoice (Gynecare, Somerville, NJ), the first global-ablation device to be marketed, was FDA-approved in 1997.6,7 It is a single-use balloon that is filled with fluid (5% dextrose and water) and inflated to a pressure of 180 mm Hg.
Technique. After general or regional anesthesia and prior to balloon insertion, remove the superficial endometrium by suction curettage.
The balloon contains a central heating element that warms the fluid to 87°C for 8 minutes via electronic control. Pressure within the balloon must be stabilized within the uterine cavity.
Safety features include a pressure shut-off device that activates at 210 mm Hg or higher and 45 mm Hg or below. The procedure is terminated if the temperature exceeds 95°C or falls below 75°C.
Caveats. The device may not function optimally if the cavity is irregular. In addition, it may not destroy residual and endometrial tissue in cornual regions of the uterus.
Postoperative response. Patients have reported increased uterine pain secondary to release of prostaglandins and other tissue factors that may increase uterine contractility.
What the data show. In a series of 296 patients followed for 1 year, 88% reported decreased flow and 14% achieved amenorrhea.6 Meyer et al7 compared thermal balloon ablation with the rollerball technique and found an amenorrhea rate of 27% with rollerball and 15% with the balloon. Patient satisfaction remained high in both groups: 87% for rollerball versus 86% with the balloon.
More recently, 5- and 7-year follow-up studies have been published. At 5-year follow-up, Loffer and Grainger8 concluded that thermal balloon ablation therapy was an effective treatment of menorrhagia in premenopausal women, with clinical outcomes similar to rollerball ablation. Patient satisfaction was noted in 93% of women treated with thermal balloon ablation and 100% of those treated with rollerball ablation. A 7-year multicenter follow-up study of thermal balloon therapy defined avoidance of hysterectomy as the primary outcome.9 Overall, the probability of avoiding any surgery was 75% at 6.5 or 7 years.
Thermal fluid ablation
The HydroThermAblator (Boston Scientific, Natick, Mass) is similar to the balloon. It delivers heated saline at 90°C directly to the uterine cavity under hysteroscopic guidance.10 This solution is circulated at gravity pressure so that it remains in the uterine cavity and does not flow out the fallopian tubes into the peritoneal cavity. Approximately 10 minutes is required for the procedure.
Preparation is via GnRH-agonist hormonal suppression or a suction D&C.
Technique. Following regional or general anesthesia, the uterus is sounded and the endocervical canal dilated sufficiently to insert the operative hysteroscope. After inspection of the uterine cavity by direct visualization via the hysteroscope/TV monitor, the tubing that delivers the heated saline is connected to the operative hysteroscope to perform the ablation. The procedure takes place under direct visualization.
Safety features include automatic shutdown if there is a 10-mL fluid loss or an increase in fluid accumulation in excess of 20 mL.
What the data show. At 12 months, 1 trial reported an amenorrhea rate of 50%, hypomenorrhea of 39%, and eumenorrhea of 5.5%.11
Cryotherapy
The Her Option cryoablation system (American Medical Systems, Minnetonka, Minn) involves insertion of a cryoprobe into the uterine cavity, cooling it to –100 to –120°C to form an ice ball, and destroying adjacent endometrium.
Preparation is via preoperative hormonal suppression with a GnRH agonist.
Abdominal ultrasound monitoring is necessary for insertion of the cryoprobe and ice ball formation.
Technique. In some patients, multiple ice balls may be needed to thoroughly ablate the endometrial cavity, which can prolong the procedure.
What the data show. A multicenter randomized trial comparing durability of treatment effects after endometrial cryoablation versus rollerball electroablation for abnormal uterine bleeding found 94% of patients (n = 94) free of abnormal uterine bleeding at 24 months of follow-up, compared to 93% of rollerball electroablation patients (n = 43).12
Impedance-controlled endometrial ablation
The NovaSure device (Novacept, Palo Alto, Calif) consists of a hand-held, disposable, 3-dimensional ablation wand that functions as a bipolar electrode. It is constructed of gold-plated fabric mesh mounted on a metal wire frame.
Treatment time. The procedure can be completed in less than 120 seconds. Because it is so quick, this technique can be accomplished with paracervical blockade and conscious sedation in suitable patients.
Pretreatment is not necessary. The procedure can be performed any time during the menstrual cycle.
Technique. After measuring the uterine cavity with a sound, insert and deploy the wand. Because it is flexible, it will make contact with and conform to the shape of the uterine cavity. Ablation depth is controlled by tissue impedance (electrical resistance).
As the wand makes contact with the endometrial surface, tissue is vaporized, and vapors are evacuated from the uterine cavity by continuous suction—which also brings additional endometrial tissue layers into contact with the bipolar electrode. As the device reaches myometrial tissue, resistance increases to a preset threshold and the device automatically shuts down.
The ablation electrode is configured so that the ablation zone in the lower uterine segment and corneal region will not exceed 2 mm; in the miduterine cavity, meanwhile, it reaches a depth of 5 to 7 mm.
Safety features. If inadvertent perforation occurs before the treatment cycle begins, the device will not activate.
What the data show. In a large multicenter clinical trial of 265 patients followed for 12 months, 41% reported amenorrhea and 88% eumenorrhea or hypomenorrhea.13
Other techniques
Microwave. Novel endometrial ablation techniques include use of microwave energies delivered to the uterine cavity via an 8-mm probe (Microsoulis, Waterloo, UK).14
Laser. A procedure known as endometrial laser intrauterine thermal therapy, or ELITT,15 delivers laser energy via a tri-fibershaped intrauterine device.
Progestin-releasing intrauterine system. Recently, the medical treatment of excessive uterine bleeding has been advanced by the levonorgestrel-releasing intrauterine device, approved by the FDA in 2000 for intrauterine contraception. The Mirena device (Berlex, Montville, NJ) has a Pearl index of 0.11 and is more reliable than tubal ligation. It can induce endometrial thinning and reduce menstrual blood loss by as much as 90%. When Mirena was compared with rollerball endometrial ablation, it was more effective in reducing menstrual blood loss and had similar satisfaction rates.16 No doubt future trials will compare Mirena with the newer ablation devices.
Complications and long-term considerations
Short-term complications, which are rare, include uterine perforation, low-grade endometritis, cervical stenosis, hematometra, and pelvic infection. These problems can be minimized by giving preoperative antibiotics and reducing tissue destruction in the lower uterine segment and cervix.
Long-term complications. Development of occult endometrial carcinoma in islands of endometrial tissue is a remote possibility. The likelihood of this rare occurrence remains low if the patient is ovulatory. Once a woman transitions into menopause and desires hormone therapy, a progestin should be included in treatment to reduce the risk of endometrial hyperplasia.
Pregnancy after endometrial ablation has been reported even in the absence of significant amounts of normal endometrial tissue.17 Thus, it is vital that the patient have a reliable and permanent form of contraception, such as tubal ligation or vasectomy.
Failure rates. Long-term failure rates in women undergoing ablation are not known, but clinical trials exploring the issue are under way.18
Dr. Brzozowski is a speaker for Novasure. Dr. Liu reports no financial relationships relevant to this article.
1. Shah A, Grainger D. Contemporary concepts in managing menorrhagia. Medscape General Medicine. December 24, 1996. Available at http://www.medscape.com/Medscape/viewarticle/408831. Accessed June 16, 2004.
2. Sculpher M, Bryan S, Dwyer N, Hutton J, Stirrat G. An economic evaluation of transcervical endometrial resection versus abdominal hysterectomy for the treatment of menorrhagia. Br J Obstet Gynecol. 1993;100:244-252.
3. Goldrath MH, Fuller TA, Segal S. Laser photo vaporization of the endometrium for the treatment of menorrhagia. Am J Obstet Gynecol. 1981;140:14-19.
4. Vancaillie TG. Electrocoagulation of the endometrium with the ball-end resectoscope. Obstet Gynecol. 1989;74:425-427.
5. Townsend DE, Richart RM, Paskowitz RA, Woolfork RE. “Rollerball” coagulation of the endometrium. Obstet Gynecol. 1990;76:310-313.
6. Amso NN, Stabinsky SA, McFaul P, Blanc B, Pendley L, Neuwirth R. Uterine thermal balloon therapy for the treatment of menorrhagia: the first 300 patients from a multi-centered study. Br J Obstet Gynaecol. 1998;105:517-523.
7. Meyer WR, Walsh BW, Grainger DA, Peacock LM, Loffer FD, Steege JF. Thermal balloon and rollerball to treat menorrhagia: a multicenter comparison. Obstet Gynecol. 1998;92:98-103.
8. Loffer FD, Grainger D. Five-year follow-up of patients participating in a randomized trial of uterine balloon therapy versus rollerball ablation for treatment of menorrhagia. J Am Assoc Gynecol Laparosc. 2002;9:429-435.
9. Amso NN, Fernandez H, Vilos G, et al. Uterine endometrial thermal balloon therapy for the treatment of menorrhagia: long-term multicenter follow-up study. Hum Reprod. 2003;18:1082-1087.
10. Corson S. A multicenter evaluation of endometrial ablation by HydroThermAblator and rollerball for treatment of menorrhagia. J Am Assoc Gynecol Laparosc. 2001;8:359-367.
11. Romer T, Muller J. A simple method of coagulating endometrium in patients with therapy-resistant recurring hypermenorrhea. J Am Assoc Gynecol Laparosc. 1999;6:265-268.
12. Townsend DE, Duleba AJ, Wilkes MM. Durability of treatment effects after endometrial cryoablation versus rollerball electroablation for abnormal uterine bleeding: two-year results of a multicenter randomized trial. Am J Obstet Gynecol. 2003;188:699-701.
13. Cooper J, Gimpelson R, Laberge P, et al. A randomized, multicenter trial of safety and efficacy of the NovaSure System in the treatment of menorrhagia. J Am Assoc Gynecol Laparosc. 2002;9:418-428.
14. Bain C, Cooper K, Parkin D. Microwave endometrial ablation versus endometrial resection: a randomized controlled trial. Obstet Gynecol. 2002;99:983-987.
15. Donnez J, Polet R, Rabinovitz R, Ak M, Squiffler J, Nisolle M. Endometrial laser intrauterine thermotherapy: the first series of 100 patients observed for 1 year. Fertil Steril. 2000;74:791-796.
16. Lethaby A, Cooke I, Rees M. Progesterone/progestogen releasing intrauterine systems versus either placebo or any other medications for heavy menstrual bleeding. Cochrane Database System Rev. 2000;CD002126.-
17. Cook JR, Seman EI. Pregnancy following endometrial ablation: case history and literature review. Obstet Gynecol Survey. 2003;58:551-556.
18. Dickersin K, Munro M, Langenberg P, et al. Surgical treatments outcomes project for dysfunctional uterine bleeding (STOP-DUB): design and methods. Clinical Trials. 2003;24:591-609.
- These techniques are easy to learn and use, and offer results comparable to rollerball procedures. Selected patients can be treated successfully in the office setting.
- It is is vital that the patient have a reliable and permanent form of contraception, such as tubal ligation or vasectomy.
- Long-term complications, though rare, include endometrial hyperplasia and occult endometrial carcinoma.
Thus, attempts to destroy it and achieve amenorrhea have met with limited success.
Hysterectomy is still the definitive treatment for excessive uterine bleeding, but a more conservative treatment, ablation, uses surgical or chemical means to obliterate the endometrial surface. Newer devices (FDA approved since 1997) allow office-based or same-day surgery; recovery time is shorter, and complication rates are lower than for hysterectomy.
This approach has gained popularity as instrumentation has improved; yet, because the endometrial surface is so resilient, success rates fall well shy of 100%. This article summaries the data on efficacy, and describes the indications, preoperative evaluation, and technique for 4 ablation options:
- thermal balloon ablation
- thermal fluid ablation
- cryotherapy
- impedance-controlled ablation
Other modalities include microwave, laser, and a progestin-releasing intrauterine contraception system.
Each uses a different energy-transfer technique to destroy the endometrium ( TABLE).
TABLE
4 global ablation devices at a glance
| THERMAL BALLOON (THERMACHOICE) | THERMAL FLUID (HYDROTHERMABLATOR) | CRYOTHERAPY (HER OPTION) | IMPEDANCE-CONTROLLED (NOVASURE) | |
|---|---|---|---|---|
| Pretreatment | Yes | Yes | Yes | No |
| Time of energy delivery | 8 minutes | 10 minutes | 10–12 minutes | 90–120 seconds |
| Cornual ablation | No | Yes | User-dependent | Yes |
| Principle | Balloon filled with fluid (5% dextrose in water) at 87°C | Hydrothermal circulation of saline at 90°C | Probe with transfer media creates ice ball at –100 to –120°C | Bipolar, radiofrequency ablation at 100°C |
| Direct visualization | None | Hysteroscopy | Ultrasound guidance | None |
| Safety features | Pressure and temperature-sensing cutoffs | Fluid loss detection system | Ultrasound guidance | Uterine cavity integrity assessment system |
Indications
The typical candidate for endometrial ablation has heavy menses requiring excessive sanitary protection (eg, tampon and pad simultaneously); her daily activity frequently is limited. The patient may have tried such management as nonsteroidal anti-inflammatory agents, oral contraceptives, or surgical dilatation and curettage (D&C) without success.
Excessive or abnormal uterine bleeding is defined as blood loss exceeding 80 mL per menses or a menstrual flow longer than 7 days. Abnormal uterine bleeding affects 22% of women of reproductive age.1 Each year in the United States, approximately 180,000 women undergo hysterectomy for this indication.2
The optimal patient for endometrial ablation has a history of regular menses without excessive dysmenorrhea, which could suggest an underlying diagnosis of adenomyosis. (Findings suggestive of this difficult-to-diagnose condition include a tender, soft, boggy uterus at the time of menses.) Many women with adenomyosis fail to achieve adequate pain relief with endometrial ablation alone and eventually require a hysterectomy.
The patient should have completed childbearing and have a permanent method of contraception in place—endometrial ablation only reduces fertility, it does not eliminate it.
Preoperative evaluation
Laboratory studies include a complete blood count and urine human chorionic gonadotropin level, as well as screening for bleeding disorders when indicated.
A bleeding diary helps quantify symptoms. Its use should be encouraged.
Other tests and examinations. Also recommended are endometrial biopsy, a Pap test, and assessment of the endometrial cavity via hysteroscopy or sonohysterography.
Biopsy should reflect histologically normal tissue. The patient should have:
- regular menstrual cycles lasting 25 to 34 days
- no uterine anomaly or potential myometrial wall defect from a previous classical cesarean or transmural myomectomy
Contraindications
Anovulatory patients may not be good candidates because islands of endometrial tissue can remain after ablation. These tissue “nests” may spontaneously change to hyperplasia or endometrial carcinoma (due to unopposed estrogen). Further, uterine bleeding may not always occur when hyperplasia is present after endometrial ablation, delaying this serious diagnosis.
Relative contraindications include intramural or submucosal uterine myomas.
Earlier techniques: Hysteroscopic ablation
Techniques developed earlier require expert operative hysteroscopic skills and should be performed by experienced gynecologic surgeons to minimize complications.
For example, the first treatment of menorrhagia using a hysteroscopic approach with a Nd:YAG laser power source, reported in 1981 by Goldrath et al,3 utilized 55 watts of power with a 600-micron fiber dragged across the endometrium. Later, other surgeons used a rollerball unipolar electrode that coagulated the surface with continuous contact.4,5
Modifications of this technique included a loop electrode that used monopolar electrical energy to “shave” the thicker portions of endometrium. In some reports, the rollerball electrode was used to reach the uterine cornu and endocoagulate the lower uterine segments. The most successful reports of this approach used a loop electrode to shave the endometrium followed by rollerball coagulation of the shaved areas. Amenorrhea rates with these techniques approached 60%.
Thermal balloon ablation
ThermaChoice (Gynecare, Somerville, NJ), the first global-ablation device to be marketed, was FDA-approved in 1997.6,7 It is a single-use balloon that is filled with fluid (5% dextrose and water) and inflated to a pressure of 180 mm Hg.
Technique. After general or regional anesthesia and prior to balloon insertion, remove the superficial endometrium by suction curettage.
The balloon contains a central heating element that warms the fluid to 87°C for 8 minutes via electronic control. Pressure within the balloon must be stabilized within the uterine cavity.
Safety features include a pressure shut-off device that activates at 210 mm Hg or higher and 45 mm Hg or below. The procedure is terminated if the temperature exceeds 95°C or falls below 75°C.
Caveats. The device may not function optimally if the cavity is irregular. In addition, it may not destroy residual and endometrial tissue in cornual regions of the uterus.
Postoperative response. Patients have reported increased uterine pain secondary to release of prostaglandins and other tissue factors that may increase uterine contractility.
What the data show. In a series of 296 patients followed for 1 year, 88% reported decreased flow and 14% achieved amenorrhea.6 Meyer et al7 compared thermal balloon ablation with the rollerball technique and found an amenorrhea rate of 27% with rollerball and 15% with the balloon. Patient satisfaction remained high in both groups: 87% for rollerball versus 86% with the balloon.
More recently, 5- and 7-year follow-up studies have been published. At 5-year follow-up, Loffer and Grainger8 concluded that thermal balloon ablation therapy was an effective treatment of menorrhagia in premenopausal women, with clinical outcomes similar to rollerball ablation. Patient satisfaction was noted in 93% of women treated with thermal balloon ablation and 100% of those treated with rollerball ablation. A 7-year multicenter follow-up study of thermal balloon therapy defined avoidance of hysterectomy as the primary outcome.9 Overall, the probability of avoiding any surgery was 75% at 6.5 or 7 years.
Thermal fluid ablation
The HydroThermAblator (Boston Scientific, Natick, Mass) is similar to the balloon. It delivers heated saline at 90°C directly to the uterine cavity under hysteroscopic guidance.10 This solution is circulated at gravity pressure so that it remains in the uterine cavity and does not flow out the fallopian tubes into the peritoneal cavity. Approximately 10 minutes is required for the procedure.
Preparation is via GnRH-agonist hormonal suppression or a suction D&C.
Technique. Following regional or general anesthesia, the uterus is sounded and the endocervical canal dilated sufficiently to insert the operative hysteroscope. After inspection of the uterine cavity by direct visualization via the hysteroscope/TV monitor, the tubing that delivers the heated saline is connected to the operative hysteroscope to perform the ablation. The procedure takes place under direct visualization.
Safety features include automatic shutdown if there is a 10-mL fluid loss or an increase in fluid accumulation in excess of 20 mL.
What the data show. At 12 months, 1 trial reported an amenorrhea rate of 50%, hypomenorrhea of 39%, and eumenorrhea of 5.5%.11
Cryotherapy
The Her Option cryoablation system (American Medical Systems, Minnetonka, Minn) involves insertion of a cryoprobe into the uterine cavity, cooling it to –100 to –120°C to form an ice ball, and destroying adjacent endometrium.
Preparation is via preoperative hormonal suppression with a GnRH agonist.
Abdominal ultrasound monitoring is necessary for insertion of the cryoprobe and ice ball formation.
Technique. In some patients, multiple ice balls may be needed to thoroughly ablate the endometrial cavity, which can prolong the procedure.
What the data show. A multicenter randomized trial comparing durability of treatment effects after endometrial cryoablation versus rollerball electroablation for abnormal uterine bleeding found 94% of patients (n = 94) free of abnormal uterine bleeding at 24 months of follow-up, compared to 93% of rollerball electroablation patients (n = 43).12
Impedance-controlled endometrial ablation
The NovaSure device (Novacept, Palo Alto, Calif) consists of a hand-held, disposable, 3-dimensional ablation wand that functions as a bipolar electrode. It is constructed of gold-plated fabric mesh mounted on a metal wire frame.
Treatment time. The procedure can be completed in less than 120 seconds. Because it is so quick, this technique can be accomplished with paracervical blockade and conscious sedation in suitable patients.
Pretreatment is not necessary. The procedure can be performed any time during the menstrual cycle.
Technique. After measuring the uterine cavity with a sound, insert and deploy the wand. Because it is flexible, it will make contact with and conform to the shape of the uterine cavity. Ablation depth is controlled by tissue impedance (electrical resistance).
As the wand makes contact with the endometrial surface, tissue is vaporized, and vapors are evacuated from the uterine cavity by continuous suction—which also brings additional endometrial tissue layers into contact with the bipolar electrode. As the device reaches myometrial tissue, resistance increases to a preset threshold and the device automatically shuts down.
The ablation electrode is configured so that the ablation zone in the lower uterine segment and corneal region will not exceed 2 mm; in the miduterine cavity, meanwhile, it reaches a depth of 5 to 7 mm.
Safety features. If inadvertent perforation occurs before the treatment cycle begins, the device will not activate.
What the data show. In a large multicenter clinical trial of 265 patients followed for 12 months, 41% reported amenorrhea and 88% eumenorrhea or hypomenorrhea.13
Other techniques
Microwave. Novel endometrial ablation techniques include use of microwave energies delivered to the uterine cavity via an 8-mm probe (Microsoulis, Waterloo, UK).14
Laser. A procedure known as endometrial laser intrauterine thermal therapy, or ELITT,15 delivers laser energy via a tri-fibershaped intrauterine device.
Progestin-releasing intrauterine system. Recently, the medical treatment of excessive uterine bleeding has been advanced by the levonorgestrel-releasing intrauterine device, approved by the FDA in 2000 for intrauterine contraception. The Mirena device (Berlex, Montville, NJ) has a Pearl index of 0.11 and is more reliable than tubal ligation. It can induce endometrial thinning and reduce menstrual blood loss by as much as 90%. When Mirena was compared with rollerball endometrial ablation, it was more effective in reducing menstrual blood loss and had similar satisfaction rates.16 No doubt future trials will compare Mirena with the newer ablation devices.
Complications and long-term considerations
Short-term complications, which are rare, include uterine perforation, low-grade endometritis, cervical stenosis, hematometra, and pelvic infection. These problems can be minimized by giving preoperative antibiotics and reducing tissue destruction in the lower uterine segment and cervix.
Long-term complications. Development of occult endometrial carcinoma in islands of endometrial tissue is a remote possibility. The likelihood of this rare occurrence remains low if the patient is ovulatory. Once a woman transitions into menopause and desires hormone therapy, a progestin should be included in treatment to reduce the risk of endometrial hyperplasia.
Pregnancy after endometrial ablation has been reported even in the absence of significant amounts of normal endometrial tissue.17 Thus, it is vital that the patient have a reliable and permanent form of contraception, such as tubal ligation or vasectomy.
Failure rates. Long-term failure rates in women undergoing ablation are not known, but clinical trials exploring the issue are under way.18
Dr. Brzozowski is a speaker for Novasure. Dr. Liu reports no financial relationships relevant to this article.
- These techniques are easy to learn and use, and offer results comparable to rollerball procedures. Selected patients can be treated successfully in the office setting.
- It is is vital that the patient have a reliable and permanent form of contraception, such as tubal ligation or vasectomy.
- Long-term complications, though rare, include endometrial hyperplasia and occult endometrial carcinoma.
Thus, attempts to destroy it and achieve amenorrhea have met with limited success.
Hysterectomy is still the definitive treatment for excessive uterine bleeding, but a more conservative treatment, ablation, uses surgical or chemical means to obliterate the endometrial surface. Newer devices (FDA approved since 1997) allow office-based or same-day surgery; recovery time is shorter, and complication rates are lower than for hysterectomy.
This approach has gained popularity as instrumentation has improved; yet, because the endometrial surface is so resilient, success rates fall well shy of 100%. This article summaries the data on efficacy, and describes the indications, preoperative evaluation, and technique for 4 ablation options:
- thermal balloon ablation
- thermal fluid ablation
- cryotherapy
- impedance-controlled ablation
Other modalities include microwave, laser, and a progestin-releasing intrauterine contraception system.
Each uses a different energy-transfer technique to destroy the endometrium ( TABLE).
TABLE
4 global ablation devices at a glance
| THERMAL BALLOON (THERMACHOICE) | THERMAL FLUID (HYDROTHERMABLATOR) | CRYOTHERAPY (HER OPTION) | IMPEDANCE-CONTROLLED (NOVASURE) | |
|---|---|---|---|---|
| Pretreatment | Yes | Yes | Yes | No |
| Time of energy delivery | 8 minutes | 10 minutes | 10–12 minutes | 90–120 seconds |
| Cornual ablation | No | Yes | User-dependent | Yes |
| Principle | Balloon filled with fluid (5% dextrose in water) at 87°C | Hydrothermal circulation of saline at 90°C | Probe with transfer media creates ice ball at –100 to –120°C | Bipolar, radiofrequency ablation at 100°C |
| Direct visualization | None | Hysteroscopy | Ultrasound guidance | None |
| Safety features | Pressure and temperature-sensing cutoffs | Fluid loss detection system | Ultrasound guidance | Uterine cavity integrity assessment system |
Indications
The typical candidate for endometrial ablation has heavy menses requiring excessive sanitary protection (eg, tampon and pad simultaneously); her daily activity frequently is limited. The patient may have tried such management as nonsteroidal anti-inflammatory agents, oral contraceptives, or surgical dilatation and curettage (D&C) without success.
Excessive or abnormal uterine bleeding is defined as blood loss exceeding 80 mL per menses or a menstrual flow longer than 7 days. Abnormal uterine bleeding affects 22% of women of reproductive age.1 Each year in the United States, approximately 180,000 women undergo hysterectomy for this indication.2
The optimal patient for endometrial ablation has a history of regular menses without excessive dysmenorrhea, which could suggest an underlying diagnosis of adenomyosis. (Findings suggestive of this difficult-to-diagnose condition include a tender, soft, boggy uterus at the time of menses.) Many women with adenomyosis fail to achieve adequate pain relief with endometrial ablation alone and eventually require a hysterectomy.
The patient should have completed childbearing and have a permanent method of contraception in place—endometrial ablation only reduces fertility, it does not eliminate it.
Preoperative evaluation
Laboratory studies include a complete blood count and urine human chorionic gonadotropin level, as well as screening for bleeding disorders when indicated.
A bleeding diary helps quantify symptoms. Its use should be encouraged.
Other tests and examinations. Also recommended are endometrial biopsy, a Pap test, and assessment of the endometrial cavity via hysteroscopy or sonohysterography.
Biopsy should reflect histologically normal tissue. The patient should have:
- regular menstrual cycles lasting 25 to 34 days
- no uterine anomaly or potential myometrial wall defect from a previous classical cesarean or transmural myomectomy
Contraindications
Anovulatory patients may not be good candidates because islands of endometrial tissue can remain after ablation. These tissue “nests” may spontaneously change to hyperplasia or endometrial carcinoma (due to unopposed estrogen). Further, uterine bleeding may not always occur when hyperplasia is present after endometrial ablation, delaying this serious diagnosis.
Relative contraindications include intramural or submucosal uterine myomas.
Earlier techniques: Hysteroscopic ablation
Techniques developed earlier require expert operative hysteroscopic skills and should be performed by experienced gynecologic surgeons to minimize complications.
For example, the first treatment of menorrhagia using a hysteroscopic approach with a Nd:YAG laser power source, reported in 1981 by Goldrath et al,3 utilized 55 watts of power with a 600-micron fiber dragged across the endometrium. Later, other surgeons used a rollerball unipolar electrode that coagulated the surface with continuous contact.4,5
Modifications of this technique included a loop electrode that used monopolar electrical energy to “shave” the thicker portions of endometrium. In some reports, the rollerball electrode was used to reach the uterine cornu and endocoagulate the lower uterine segments. The most successful reports of this approach used a loop electrode to shave the endometrium followed by rollerball coagulation of the shaved areas. Amenorrhea rates with these techniques approached 60%.
Thermal balloon ablation
ThermaChoice (Gynecare, Somerville, NJ), the first global-ablation device to be marketed, was FDA-approved in 1997.6,7 It is a single-use balloon that is filled with fluid (5% dextrose and water) and inflated to a pressure of 180 mm Hg.
Technique. After general or regional anesthesia and prior to balloon insertion, remove the superficial endometrium by suction curettage.
The balloon contains a central heating element that warms the fluid to 87°C for 8 minutes via electronic control. Pressure within the balloon must be stabilized within the uterine cavity.
Safety features include a pressure shut-off device that activates at 210 mm Hg or higher and 45 mm Hg or below. The procedure is terminated if the temperature exceeds 95°C or falls below 75°C.
Caveats. The device may not function optimally if the cavity is irregular. In addition, it may not destroy residual and endometrial tissue in cornual regions of the uterus.
Postoperative response. Patients have reported increased uterine pain secondary to release of prostaglandins and other tissue factors that may increase uterine contractility.
What the data show. In a series of 296 patients followed for 1 year, 88% reported decreased flow and 14% achieved amenorrhea.6 Meyer et al7 compared thermal balloon ablation with the rollerball technique and found an amenorrhea rate of 27% with rollerball and 15% with the balloon. Patient satisfaction remained high in both groups: 87% for rollerball versus 86% with the balloon.
More recently, 5- and 7-year follow-up studies have been published. At 5-year follow-up, Loffer and Grainger8 concluded that thermal balloon ablation therapy was an effective treatment of menorrhagia in premenopausal women, with clinical outcomes similar to rollerball ablation. Patient satisfaction was noted in 93% of women treated with thermal balloon ablation and 100% of those treated with rollerball ablation. A 7-year multicenter follow-up study of thermal balloon therapy defined avoidance of hysterectomy as the primary outcome.9 Overall, the probability of avoiding any surgery was 75% at 6.5 or 7 years.
Thermal fluid ablation
The HydroThermAblator (Boston Scientific, Natick, Mass) is similar to the balloon. It delivers heated saline at 90°C directly to the uterine cavity under hysteroscopic guidance.10 This solution is circulated at gravity pressure so that it remains in the uterine cavity and does not flow out the fallopian tubes into the peritoneal cavity. Approximately 10 minutes is required for the procedure.
Preparation is via GnRH-agonist hormonal suppression or a suction D&C.
Technique. Following regional or general anesthesia, the uterus is sounded and the endocervical canal dilated sufficiently to insert the operative hysteroscope. After inspection of the uterine cavity by direct visualization via the hysteroscope/TV monitor, the tubing that delivers the heated saline is connected to the operative hysteroscope to perform the ablation. The procedure takes place under direct visualization.
Safety features include automatic shutdown if there is a 10-mL fluid loss or an increase in fluid accumulation in excess of 20 mL.
What the data show. At 12 months, 1 trial reported an amenorrhea rate of 50%, hypomenorrhea of 39%, and eumenorrhea of 5.5%.11
Cryotherapy
The Her Option cryoablation system (American Medical Systems, Minnetonka, Minn) involves insertion of a cryoprobe into the uterine cavity, cooling it to –100 to –120°C to form an ice ball, and destroying adjacent endometrium.
Preparation is via preoperative hormonal suppression with a GnRH agonist.
Abdominal ultrasound monitoring is necessary for insertion of the cryoprobe and ice ball formation.
Technique. In some patients, multiple ice balls may be needed to thoroughly ablate the endometrial cavity, which can prolong the procedure.
What the data show. A multicenter randomized trial comparing durability of treatment effects after endometrial cryoablation versus rollerball electroablation for abnormal uterine bleeding found 94% of patients (n = 94) free of abnormal uterine bleeding at 24 months of follow-up, compared to 93% of rollerball electroablation patients (n = 43).12
Impedance-controlled endometrial ablation
The NovaSure device (Novacept, Palo Alto, Calif) consists of a hand-held, disposable, 3-dimensional ablation wand that functions as a bipolar electrode. It is constructed of gold-plated fabric mesh mounted on a metal wire frame.
Treatment time. The procedure can be completed in less than 120 seconds. Because it is so quick, this technique can be accomplished with paracervical blockade and conscious sedation in suitable patients.
Pretreatment is not necessary. The procedure can be performed any time during the menstrual cycle.
Technique. After measuring the uterine cavity with a sound, insert and deploy the wand. Because it is flexible, it will make contact with and conform to the shape of the uterine cavity. Ablation depth is controlled by tissue impedance (electrical resistance).
As the wand makes contact with the endometrial surface, tissue is vaporized, and vapors are evacuated from the uterine cavity by continuous suction—which also brings additional endometrial tissue layers into contact with the bipolar electrode. As the device reaches myometrial tissue, resistance increases to a preset threshold and the device automatically shuts down.
The ablation electrode is configured so that the ablation zone in the lower uterine segment and corneal region will not exceed 2 mm; in the miduterine cavity, meanwhile, it reaches a depth of 5 to 7 mm.
Safety features. If inadvertent perforation occurs before the treatment cycle begins, the device will not activate.
What the data show. In a large multicenter clinical trial of 265 patients followed for 12 months, 41% reported amenorrhea and 88% eumenorrhea or hypomenorrhea.13
Other techniques
Microwave. Novel endometrial ablation techniques include use of microwave energies delivered to the uterine cavity via an 8-mm probe (Microsoulis, Waterloo, UK).14
Laser. A procedure known as endometrial laser intrauterine thermal therapy, or ELITT,15 delivers laser energy via a tri-fibershaped intrauterine device.
Progestin-releasing intrauterine system. Recently, the medical treatment of excessive uterine bleeding has been advanced by the levonorgestrel-releasing intrauterine device, approved by the FDA in 2000 for intrauterine contraception. The Mirena device (Berlex, Montville, NJ) has a Pearl index of 0.11 and is more reliable than tubal ligation. It can induce endometrial thinning and reduce menstrual blood loss by as much as 90%. When Mirena was compared with rollerball endometrial ablation, it was more effective in reducing menstrual blood loss and had similar satisfaction rates.16 No doubt future trials will compare Mirena with the newer ablation devices.
Complications and long-term considerations
Short-term complications, which are rare, include uterine perforation, low-grade endometritis, cervical stenosis, hematometra, and pelvic infection. These problems can be minimized by giving preoperative antibiotics and reducing tissue destruction in the lower uterine segment and cervix.
Long-term complications. Development of occult endometrial carcinoma in islands of endometrial tissue is a remote possibility. The likelihood of this rare occurrence remains low if the patient is ovulatory. Once a woman transitions into menopause and desires hormone therapy, a progestin should be included in treatment to reduce the risk of endometrial hyperplasia.
Pregnancy after endometrial ablation has been reported even in the absence of significant amounts of normal endometrial tissue.17 Thus, it is vital that the patient have a reliable and permanent form of contraception, such as tubal ligation or vasectomy.
Failure rates. Long-term failure rates in women undergoing ablation are not known, but clinical trials exploring the issue are under way.18
Dr. Brzozowski is a speaker for Novasure. Dr. Liu reports no financial relationships relevant to this article.
1. Shah A, Grainger D. Contemporary concepts in managing menorrhagia. Medscape General Medicine. December 24, 1996. Available at http://www.medscape.com/Medscape/viewarticle/408831. Accessed June 16, 2004.
2. Sculpher M, Bryan S, Dwyer N, Hutton J, Stirrat G. An economic evaluation of transcervical endometrial resection versus abdominal hysterectomy for the treatment of menorrhagia. Br J Obstet Gynecol. 1993;100:244-252.
3. Goldrath MH, Fuller TA, Segal S. Laser photo vaporization of the endometrium for the treatment of menorrhagia. Am J Obstet Gynecol. 1981;140:14-19.
4. Vancaillie TG. Electrocoagulation of the endometrium with the ball-end resectoscope. Obstet Gynecol. 1989;74:425-427.
5. Townsend DE, Richart RM, Paskowitz RA, Woolfork RE. “Rollerball” coagulation of the endometrium. Obstet Gynecol. 1990;76:310-313.
6. Amso NN, Stabinsky SA, McFaul P, Blanc B, Pendley L, Neuwirth R. Uterine thermal balloon therapy for the treatment of menorrhagia: the first 300 patients from a multi-centered study. Br J Obstet Gynaecol. 1998;105:517-523.
7. Meyer WR, Walsh BW, Grainger DA, Peacock LM, Loffer FD, Steege JF. Thermal balloon and rollerball to treat menorrhagia: a multicenter comparison. Obstet Gynecol. 1998;92:98-103.
8. Loffer FD, Grainger D. Five-year follow-up of patients participating in a randomized trial of uterine balloon therapy versus rollerball ablation for treatment of menorrhagia. J Am Assoc Gynecol Laparosc. 2002;9:429-435.
9. Amso NN, Fernandez H, Vilos G, et al. Uterine endometrial thermal balloon therapy for the treatment of menorrhagia: long-term multicenter follow-up study. Hum Reprod. 2003;18:1082-1087.
10. Corson S. A multicenter evaluation of endometrial ablation by HydroThermAblator and rollerball for treatment of menorrhagia. J Am Assoc Gynecol Laparosc. 2001;8:359-367.
11. Romer T, Muller J. A simple method of coagulating endometrium in patients with therapy-resistant recurring hypermenorrhea. J Am Assoc Gynecol Laparosc. 1999;6:265-268.
12. Townsend DE, Duleba AJ, Wilkes MM. Durability of treatment effects after endometrial cryoablation versus rollerball electroablation for abnormal uterine bleeding: two-year results of a multicenter randomized trial. Am J Obstet Gynecol. 2003;188:699-701.
13. Cooper J, Gimpelson R, Laberge P, et al. A randomized, multicenter trial of safety and efficacy of the NovaSure System in the treatment of menorrhagia. J Am Assoc Gynecol Laparosc. 2002;9:418-428.
14. Bain C, Cooper K, Parkin D. Microwave endometrial ablation versus endometrial resection: a randomized controlled trial. Obstet Gynecol. 2002;99:983-987.
15. Donnez J, Polet R, Rabinovitz R, Ak M, Squiffler J, Nisolle M. Endometrial laser intrauterine thermotherapy: the first series of 100 patients observed for 1 year. Fertil Steril. 2000;74:791-796.
16. Lethaby A, Cooke I, Rees M. Progesterone/progestogen releasing intrauterine systems versus either placebo or any other medications for heavy menstrual bleeding. Cochrane Database System Rev. 2000;CD002126.-
17. Cook JR, Seman EI. Pregnancy following endometrial ablation: case history and literature review. Obstet Gynecol Survey. 2003;58:551-556.
18. Dickersin K, Munro M, Langenberg P, et al. Surgical treatments outcomes project for dysfunctional uterine bleeding (STOP-DUB): design and methods. Clinical Trials. 2003;24:591-609.
1. Shah A, Grainger D. Contemporary concepts in managing menorrhagia. Medscape General Medicine. December 24, 1996. Available at http://www.medscape.com/Medscape/viewarticle/408831. Accessed June 16, 2004.
2. Sculpher M, Bryan S, Dwyer N, Hutton J, Stirrat G. An economic evaluation of transcervical endometrial resection versus abdominal hysterectomy for the treatment of menorrhagia. Br J Obstet Gynecol. 1993;100:244-252.
3. Goldrath MH, Fuller TA, Segal S. Laser photo vaporization of the endometrium for the treatment of menorrhagia. Am J Obstet Gynecol. 1981;140:14-19.
4. Vancaillie TG. Electrocoagulation of the endometrium with the ball-end resectoscope. Obstet Gynecol. 1989;74:425-427.
5. Townsend DE, Richart RM, Paskowitz RA, Woolfork RE. “Rollerball” coagulation of the endometrium. Obstet Gynecol. 1990;76:310-313.
6. Amso NN, Stabinsky SA, McFaul P, Blanc B, Pendley L, Neuwirth R. Uterine thermal balloon therapy for the treatment of menorrhagia: the first 300 patients from a multi-centered study. Br J Obstet Gynaecol. 1998;105:517-523.
7. Meyer WR, Walsh BW, Grainger DA, Peacock LM, Loffer FD, Steege JF. Thermal balloon and rollerball to treat menorrhagia: a multicenter comparison. Obstet Gynecol. 1998;92:98-103.
8. Loffer FD, Grainger D. Five-year follow-up of patients participating in a randomized trial of uterine balloon therapy versus rollerball ablation for treatment of menorrhagia. J Am Assoc Gynecol Laparosc. 2002;9:429-435.
9. Amso NN, Fernandez H, Vilos G, et al. Uterine endometrial thermal balloon therapy for the treatment of menorrhagia: long-term multicenter follow-up study. Hum Reprod. 2003;18:1082-1087.
10. Corson S. A multicenter evaluation of endometrial ablation by HydroThermAblator and rollerball for treatment of menorrhagia. J Am Assoc Gynecol Laparosc. 2001;8:359-367.
11. Romer T, Muller J. A simple method of coagulating endometrium in patients with therapy-resistant recurring hypermenorrhea. J Am Assoc Gynecol Laparosc. 1999;6:265-268.
12. Townsend DE, Duleba AJ, Wilkes MM. Durability of treatment effects after endometrial cryoablation versus rollerball electroablation for abnormal uterine bleeding: two-year results of a multicenter randomized trial. Am J Obstet Gynecol. 2003;188:699-701.
13. Cooper J, Gimpelson R, Laberge P, et al. A randomized, multicenter trial of safety and efficacy of the NovaSure System in the treatment of menorrhagia. J Am Assoc Gynecol Laparosc. 2002;9:418-428.
14. Bain C, Cooper K, Parkin D. Microwave endometrial ablation versus endometrial resection: a randomized controlled trial. Obstet Gynecol. 2002;99:983-987.
15. Donnez J, Polet R, Rabinovitz R, Ak M, Squiffler J, Nisolle M. Endometrial laser intrauterine thermotherapy: the first series of 100 patients observed for 1 year. Fertil Steril. 2000;74:791-796.
16. Lethaby A, Cooke I, Rees M. Progesterone/progestogen releasing intrauterine systems versus either placebo or any other medications for heavy menstrual bleeding. Cochrane Database System Rev. 2000;CD002126.-
17. Cook JR, Seman EI. Pregnancy following endometrial ablation: case history and literature review. Obstet Gynecol Survey. 2003;58:551-556.
18. Dickersin K, Munro M, Langenberg P, et al. Surgical treatments outcomes project for dysfunctional uterine bleeding (STOP-DUB): design and methods. Clinical Trials. 2003;24:591-609.
Minimally invasive cesarean: Improving an innovative technique
- A simplified abdominal incision makes the traditional extensive dissection associated with the Pfannenstiel incision unnecessary.
- A soft, self-retaining abdominal retractor offers increased exposure, atraumatic retraction, incision protection, and adjustable height while facilitating delivery of the fetal head by creating a rigid border around the abdominal incision.
- Bladder-flap omission has been associated with reduced operative time and incision-delivery interval, decreased blood loss, and less need for postoperative analgesics.
Is the extensive dissection of the Pfannenstiel incision necessary in cesarean delivery? Is bladder dissection essential? Must the visceral and parietal peritoneum be closed?
The success of our minimally invasive cesarean technique suggests the answer is “no.”
The approach described here features a short operative time; minimal instrumentation; reduced surgical dissection; decreased postoperative pain; and reduced risk of blood loss, infection, and wound complications. It is easily learned and cost-effective, with a brief postoperative recovery period.
Among the updates made from the technique’s initial publication in the mid-1990s1,2:
- addition of routine perioperative oxygen (80%), to reduce the risk of surgical wound infection (see “Perioperative considerations”)
- regular use of forced warm air covers applied to the anterior skin surface, to help patients maintain normothermia
- addition of a soft, self-retaining abdominal retractor—which creates an atraumatic circle of exposure up to a calculated 177 cm2 for a 15-cm incision (versus 113 cm2 calculated for traditional retraction)
- vertical, rather than lateral, digital extension of the initial transverse uterine incision
- identification of a subgroup in whom peritoneal closure is strongly recommended
- new data on the procedure’s effectiveness.3
Routinely use prophylactic antibiotics. Several recent studies have concluded that perioperative antibiotics reduce the incidence of endometritis and wound infection following elective and nonelective cesarean section.37 Administer ampicillin or a first-generation cephalosporin at umbilical cord clamping. For patients allergic to penicillin and cephalosporins, choose an alternative, such as clindamycin.
Give antacids 30 minutes before anesthesia (general or regional) to prevent pneumonitis from inhalation of gastric contents.
Clip pubic hair, rather than shave, to reduce the risk of wound infection.
Insert a Foley catheter, empty the bladder, and keep the catheter in place.
Position the patient in a 10°left lateral tilt, to avoid hypotension associated with aortocaval occlusion.
Routinely administer supplemental perioperative oxygen (80%), with either general or regional anesthesia; this activates alveolar immune defenses and halves the risk of surgical wound infections.
Neutrophil oxidative killing and phagocytosis—the most important defenses against surgical pathogens—depend on the partial pressure of oxygen in contaminated tissue. Giving supplemental oxygen during and for the first 2 hours after the procedure (by mask) is a practical, inexpensive way to reduce the incidence of surgical wound infection.38,39
Using 80% oxygen during and, for a short period, after surgery does not cause pulmonary toxicity such as atelectasis or impaired pulmonary function.40
Ensure normal body heat during and after cesarean to reduce the risk of postoperative surgical infection.40,41 Forced warm air covers applied to the anterior skin surface are the most effective way for warming surgical patients.9 IV fluid warming, though appropriate when large volumes are to be administered, is unnecessary for smaller operations.41
Modified abdominal incision reduces dissection
Make a straight low transverse incision with a scalpel, at a point approximately 3 to 4 cm above the symphysis pubis (FIGURE 1A).
The length of the incision is individualized (13 to 15 cm), though difficult fetal extraction is more likely if the abdominal incision is less than 15 cm.4,5
Divide the subcutaneous tissue transversely with an electrocautery knife. In a cutting and coagulation blend mode, the knife divides the fat while achieving hemostasis. To improve hemostasis, coagulate the blood vessels that cross the subcutaneous fat layer in a brushing manner before dividing them. To prevent unnecessary dead space, avoid filleting the fat and separating adherent subcutaneous fat from the anterior rectus fascia beyond what is needed to expose the fascia.
Open the fascia transversely with the electrocautery knife to the same length as the skin incision. Coagulate the blood vessels that cross the fascia before dividing them. Identify the median raphe by pulling up the superior edge of the abdominal incision.
Separate the rectus muscles in the midline by vertical blunt finger dissection (FIGURE 1B). If digital dissection is inefficient due to a dense, thick, or scarred median raphe, use an electrocautery knife, a scalpel, or scissors.
Open the peritoneum. This is facilitated by upward traction and elevation of the superior edge of the abdominal incision that lifts the peritoneum, allowing easy digital perforation using the index or middle finger (FIGURE 1C).
If this maneuver is not feasible, open the peritoneum in the traditional fashion.
Stretch the full thickness of the abdominal wall to full size of the skin incision, using 1 or 2 fingers of each hand (FIGURE 1D). Incorporate the skin, subcutaneous tissue, fascia layer, rectus muscles, and peritoneum. An assistant’s hands may be required. When needed, extend the peritoneal opening transversely on either side, to the midline and away from the bladder.
Digitally stretching the full-thickness abdominal incision is easily achieved due to the mechanical stretching of the anterior abdominal wall, edema, and increased vascularization that occur during pregnancy.
FIGURE 1A Create a modified abdominal incision
FIGURE 1B Create a modified abdominal incision
FIGURE 1C Create a modified abdominal incision
FIGURE 1D Create a modified abdominal incision
Retractor facilitates exposure
Our positive experience with the soft, self-retaining abdominal retractor for minilaparotomy and laparotomy6-8 compelled us to incorporate its use for cesarean 3 years ago.
The device consists of a flexible plastic inner ring and a firmer outer ring connected by a soft plastic sleeve. We use the large size of either of the 2 models currently available: the Mobius (Apple Medical Corporation, Marlboro, Miss) and the Protractor (Weck Closure Systems, Research Triangle Park, NC).
Introduce a hand through the laparotomy incision and evaluate the pelvis to ensure that no significant adhesions are present that may interfere with swift placement of the inner ring. If significant adhesions are found, use traditional metal retractors instead.
Squeeze the inner ring and insert it into the abdominal cavity toward the patient’s head, allowing the device to spring open against the parietal peritoneum (FIGURE 2A). Apply upper traction and elevate the superior edge of the abdominal incision to facilitate placement. Perform a digital check to ensure no tissue is trapped between the inner ring and the abdominal wall.
Hold up the outer ring, then roll the plastic sleeve until the ring completely inverts (FIGURE 2B). Repeat the process until the top ring is snug against the patient’s skin.
Advantages of the soft, self-retaining abdominal retractor include atraumatic retraction; incision protection; and adjustable height, making it ideal for obese patients.
FIGURE 2A Place the abdominal retractor
FIGURE 2B Place the abdominal retractor
Transverse hysterotomy in lower uterine segment
Make a transverse 2-cm uterine incision with a scalpel, approximately 1 cm above the vesicouterine peritoneal fold (identify this using gentle digital pressure to elevate the uterus) (FIGURE 3A).
Traditional bladder dissection is eliminated. We have found that when an adequate transverse hysterotomy is performed, a bladder flap is not required.1-3 In a recent randomized trial, Hohlagschwandtner et al9 confirmed our findings that bladder-flap omission was associated with reduced operative time and incision-delivery interval, decreased blood loss, and less need for postoperative analgesics.
An additional advantage of this omission: We can avoid making the uterine incision too low—especially when the cervix is fully dilated. Further, making the hysterotomy (uterine serosa and myometrium together) slightly above the vesicouterine peritoneal fold without bladder flap dissection frees the loose connective tissue between the uterus and the urinary bladder, allowing the spontaneous descent of the bladder.
Digitally extend the transverse uterine incision. We extend the initial incision not laterally but, instead, vertically (FIGURE 3B). The vertical digital traction on the initial transverse uterine incision creates a transverse dissociation of the horizontal myometrium fibers; this results in a transverse extension of the original incision.
This modification prevents unintended and uncontrolled lateral extensions of the incision that may lacerate the uterine vessels.10 It also prevents the uterine incision from becoming an inverted “U” and the undesirable accumulation of myometrium fibers at the ends of the incision. Such incisions usually do not reapproximate well during hysterotomy closure and may lead to sacculation-type defects.11
FIGURE 3A Use a standard hysterotomy in the lower uterine segment
FIGURE 3B Use a standard hysterotomy in the lower uterine segment
Delivering the fetus
For vertex presentations, place your hand into the uterine cavity between the lower edge of the hysterotomy and fetal head. While applying transabdominal fundal pressure, lift the head with your fingers and deliver it through the incision (FIGURE 4A).
The self-retaining retractor facilitates delivery of the fetal head by creating a rigid border around the abdominal incision. The back of the surgeon’s hand that is in the uterine cavity achieves better leverage, as it now rests on a rigid plane on the inferior part of the incision rather than on the back hand and the softer and pliable wound edge of the standard abdominal incision.
Vacuum-assistance. When delivery of the fetal head proves difficult, we use the soft vacuum cup, which avoids unnecessary intrauterine manipulation that may result in fetal or maternal trauma (FIGURE 4B).12
Breech extraction or transverse lie delivery is performed using standard extraction maneuvers (FIGURE 4C).13 We have found no need to perform T or J vertical extensions of the low transverse uterine incision, even in cases of a poorly developed lower uterine segment, abnormal presentation, or prematurity.
FIGURE 4A Deliver the fetal head
FIGURE 4B Deliver the fetal head
FIGURE 4C Deliver the fetal head
Uterine incision: 1-layer, in situ close
Remove the placenta only after it separates spontaneously—this greatly reduces blood loss compared with immediate manual placental delivery or umbilical-cord traction. Manually deliver the placenta only if it has not separated spontaneously after 5 minutes. Avoid routine manual clean-out of the uterine cavity if the placenta is completely delivered.1-3,14
Dilate the cervix to facilitate lochial discharge, when necessary.
Decrease uterine bleeding by massaging the uterus and administering diluted oxytocin.
Close the uterine incision in situ. Disadvantages of routine uterine exteriorization include discomfort and vomiting resulting from traction, exposure of the fallopian tubes to unnecessary trauma, increased risk of infection, possible rupture of the uteroovarian veins, and pulmonary embolism.
Exteriorization may also increase the risk of adhesion formation by exposing the uterine serosa to the abrasive effects of drying or microscopic abrasion if sponges are used to hold the uterus in place.1,14-17
Use a single-layer closure with a running suture of polyglycolic acid to close the uterine incision (FIGURE 5A). If needed, use atraumatic clamps to grasp the edges of the hysterotomy to facilitate visualization and closure.
Begin suturing at a point just beyond one end of the incision, continuing to the opposite side. Be sure to penetrate the full thickness of the myometrium and avoid incorporating the decidua in the suture line. After the uterine closure, use individual figure-of-8 sutures to control areas of persistent bleeding.
Compared with 2-layer closures, single-layer closure of a low transverse incision is associated with reduced operating time, improved hemostasis, and less tissue disruption; introduces less foreign material into the surgical site; reduces the need for postoperative analgesia; and potentially reduces infectious morbidity. A trial of labor after a cesarean section with 1-layer closure appears safe.18-22
We do not close the vesicouterine fold and parietal peritoneum (FIGURE 5B) or reapproximate the rectus muscles. It has been shown that a new peritoneal layer is formed within days of the original incision closure.
Leaving the peritoneum open leads to no increased postoperative complications, nor obvious differences in wound healing, wound dehiscence, or incidence of postoperative adhesions. Compared to women without peritoneal closure, those with peritoneal closure require a longer operative time, greater amounts of post-operative narcotics for postoperative pain, a greater need for bowel stimulants, and a longer postoperative hospitalization.23
In 1995, we performed laparoscopy for gynecologic conditions in 23 patients and repeat cesarean in 10 patients who had undergone our technique (with visceral and parietal peritoneum left open).2 We found no adhesions and a normal peritoneal lining in all cases.
In 1 subgroup, peritoneal closure is strongly recommended: cases in which 1 or both rectus muscles have been transected to increase surgical exposure. Extremely thick fibromuscular adhesions between the low anterior surface of the uterus and the under-surface of the rectus musculature may develop if the parietal peritoneum is not closed.
FIGURE 5A Close the incisions
FIGURE 5B Close the incisions
Abdominal closure
Close the rectus fascia with a continuous nonlocking 0 suture after the rectus muscles fall into place. Place entry and exit sites at 1-cm intervals, 1.5 cm beyond the wound edges—this minimizes the risk of sutures pulling through fascia. Interrupted sutures offer no greater tensile strength than continuous suturing.24 Tight, continuous suturing can lead to tissue ischemia, which may weaken the sutured fascia, and thus should be avoided.
The subcutaneous layer is not closed separately. One exception is obese patients with at least 2 cm of thick subcutaneous tissue. Thickness of subcutaneous tissue appears to be a significant risk factor for wound infection after cesarean section.25
In patients with thick subcutaneous tissue, you can reduce tension on the skin edges and the risk of subcutaneous infection, seroma formation, and wound disruption by placing interrupted 3-0 absorbable synthetic sutures to eliminate dead space.26-28 We have found no need to place Penrose drains or closed drainage systems in the subcutaneous layer.29-31
Close the skin using a subcuticular continuous absorbable 4-0 suture or, alternatively, metal staples, which are removed 4 days after surgery (FIGURE 5C).
FIGURE 5C Close the incisions
Prompt postoperative recovery
The patient may drink fluids in the recovery room, can resume a regular solid food diet within 4 hours after surgery, and may resume mobility as soon as anesthesia wears off.
Early breast-feeding is also allowed in the recovery room. In our experience, this has not been associated with complications, has been highly appreciated by the patients, and has led to earlier hospital discharge.
Control pain with meperidine (50–75 mg) or morphine (10 mg) parenterally every 3 to 4 hours. Other options include oxycodone and acetaminophen, oxycodone and aspirin, and ibuprofen.
Patients are usually discharged 48 to 72 hours following surgery.
Our experience: The numbers
Using this updated system, we have successfully completed 300 consecutive cesarean sections (210 primary, 90 repeat).
The average operating time was 19 minutes; average blood loss, 405 mL.
There was no postoperative febrile morbidity, wound infection, wound disruption, or wound hematoma.Only 3 patients developed superficial wound seromas, which were easily resolved. There were no intraoperative or postoperative complications.
We attribute the absence of wound infection to routine prophylactic antibiotics; supplemental perioperative oxygen; maintenance of normothermia; use of an electrocautery knife to create rapid hemostatic division of subcutaneous fat and anterior rectus fascia; a simplified abdominal wall opening; and placement of a self-retaining atraumatic retractor.10,32-36
Additional surgical procedures (tubal sterilization, myomectomy, appendectomy, and adhesiolysis) did not alter the recovery period, and all patients were able to get out of bed, shower, breast-feed, and care for their infants within 8 hours of surgery.
No infant complications related to the cesarean procedure occurred.
Patients were discharged from the hospital within 72 hours.
A prospective comparison study demonstrated that the original Pelosi-type of cesarean delivery was faster to perform, more cost-effective, and resulted in less maternal morbidity than the traditional cesarean technique.3
Dr. Pelosi II is a consultant for Apple Medical Corporation. Dr. Pelosi III reports no financial relationships relevant to this article.
1. Pelosi MA, Ortega I. Cesarean section: Pelosi simplified technique. Rev Chil Obstet Gynecol. 1994;59:372-377.
2. Pelosi MA, II, Pelosi MA, III. Simplified cesarean section. Contemp OB/GYN. 1995;40:89-100.
3. Wood RM, Simon H, Oz AU. Pelosi-type vs traditional cesarean delivery: a prospective comparison. J Reprod Med. 1999;44:788-795.
4. Finan MA, Mastrogiannis DS, Spellacy WN. The “Allis” test for easy cesarean delivery. Am J Obstet Gynecol. 1991;164:772-775.
5. Ayers IW, Morley GW. Surgical incision for cesarean section. Obstet Gynecol. 1987;70:706-712.
6. Pelosi MA, II, Pelosi MA, III. Self-retaining abdominal retractor for minilaparotomy. Obstet Gynecol. 2000;96:775-778.
7. Pelosi MA, II, Pelosi MA, III. Pelosi minilaparotomy hysterectomy: Effective alternative to laparoscopy and laparotomy. OBG Manag. April 2003;16-33.
8. Pelosi MA, II, Pelosi MA, III. A novel minilaparotomy approach for large ovarian cysts. OBG Manag. February 2004:;17-30.
9. Hohlagschwandtner M, Ruecklinger E, Husslein P, et al. Is the formation of a bladder flap at cesarean necessary? A randomized trial. Obstet Gynecol. 2001;98:1089-1092.
10. Ferrari A, Frigerio L, Origoni M, et al. Modified Stark procedure for cesarean section. J Pelv Surg. 1996;2:239-244.
11. Field CS. Surgical techniques for cesarean section. Obstet Gynecol Clin North Amer. 1988;15:657-672.
12. Pelosi MA, Apuzzio J. Use of the soft, silicone obstetric vacuum cup for delivery of the fetal head at cesarean section. J Reprod Med. 1984;29:289-292.
13. Pelosi MA, Apuzzio J, Fricchione D, et al. The intra-abdominal version technique for delivery of transverse lie by low segment cesarean section. Am J Obstet Gynecol. 1979;135:1009-1012.
14. Magann EF, Dodson MK, Albert JR, et al. Blood loss at the time of cesarean section by method of placental removal and exteriorization versus in situ repair of the uterine incision. Surg Gynecol Obstet. 1993;177:389-392.
15. Stock RJ, Shelton H. Fatal pulmonary embolism occurring 2 hours after exteriorization of the uterus for repair following cesareans section. Milit Med. 1985;150:549-550.
16. Hershey DW, Quilligan EJ. Extra-abdominal uterine exteriorization at cesarean section. Obstet Genecol. 1978;52:189-191.
17. Wahab MA, Karantzis P, Eccersley PS, et al. A randomized, controlled study of uterine exteriorization and repair at cesarean section. Br J Obstet Gynecol. 1999;106:913-916.
18. Hauth JC, Owen J, Davis RO. Transverse uterine incision closure: one versus two layers. Am J Obstet Gynecol. 1992;167:1108-1111.
19. Ohel G, Younis JS, Lang N, et al. Double-layer closure of uterine incision with visceral and parietal peritoneal closure: Are they obligatory steps of routine cesarean sections? J Matern Fetal Med. 1996;5:366-369.
20. Tucker JM, Hauth JC, Hodgkins P, et al. Trial of labor after a one- or two-layer closure of a low transverse uterine incision. Am J Obstet Gynecol. 1993;168:545-546.
21. Chapman SJ, Owen J, Hauth JC. One- versus two-layer closure of a low transverse cesarean: the next pregnancy. Obstet Gynecol. 1997;89:16-18.
22. Jelsema RD, Wittinger JA, Vander Kolk KJ. Continuous, nonlocking, single-layer repair of the low transverse uterine incision. J Reprod Med. 1993;38:393-396.
23. Tulandi T, Al-Jaroudi D. Nonclosure of peritoneum: a reappraisal. Am J Obstet Gynecol. 2003;189:609-612.
24. Fagniez PL, Hay JM, Lacaine F. Abdominal midline incision closure. Arch Surg. 1985;120:1351-1355.
25. Vermillion ST, Lamoutte C, Soper DE, et al. Wound infection after cesarean: effect of subcutaneous tissue thickness. Obstet Gynecol. 2000;95:923-926.
26. Naumann RW, Hauth JC, Owen J, et al. Subcutaneous tissue approximation in relation to wound disruption after cesarean delivery in obese women. Obstet Gynecol. 1995;85:412-416.
27. Cetin A, Cetin M. Superficial wound disruption after cesarean delivery: effect of the depth and closure of subcutaneous tissue. Int J Gynaecol Obstet. 1997;57:17-21.
28. Del Valle GO, Combs P, Qualls C, et al. Does closure of Camper fascia reduce the incidence of post-cesarean superficial wound disruption? Obstet Gynecol. 1992;80:1013-1016.
29. Loong RL, Rogers MS, Chang AM. A controlled trial of wound drainage at cesarean section. Aust NZ J Obstet Gynaecol. 1988;28:266-269.
30. Saunders NJ, Barclay C. Closed suction wound drainage and lower-segment cesarean section. Br J Obstet Gynaecol. 1988;95:1060-1062.
31. Magann EF, Chauhan SP, Rodts-Palenik S, Bufkin L, Martin JN, Jr, Morrison JC. Subcutaneous stitch closure versus subcutaneous drain to prevent wound disruption after cesarean delivery: a randomized clinical trial. Am J Obstet Gynecol. 2002;186:1119-1123.
32. Stark M, Finkel AR. Comparison between the Joel-Cohen and Pfannenstiel incisions in cesarean section. Eur J Obstet Gynecol Reprod Biol. 1994;53:121-122.
33. Joel-Cohen S. Abdominal and vaginal hysterectomy. New techniques based on time and motion studies. London, England: William Heinemann Medical Books; 1972.
34. Hemsell DL, Hemsell PG, Nobles B, et al. Abdominal wound problems after hysterectomy with electrocautery versus scalpel subcutaneous incision. Infect Dis Obstet Gynecol. 1993;1:27-30.
35. Johnson CD, Serpell JW. Wound infection after abdominal incision with scalpel or diathermy. Br J Surg. 1990;77:626-629.
36. Hussain SA, Hussain S. Incisions with knife or diathermy and postoperative pain. Br J Surg. 1988;75:1179-1182.
37. American College of Obstetricians and Gynecologist. ACOG Practice Bulletin. Clinical management guidelines for obstetricians-gynecologists. No. 43, May, 2003. Management of preterm labor. Obstet Gynecol. 2003;101:1039-1047.
38. Greif R, Akca O, Hurn E-P, et al. Supplemental perioperative oxygen to reduce the incidence of surgical wound infection. N Engl J Med. 2000;342:161-167.
39. Sorensen LT, Karlsmark T, Gottrup F. Abstinence from smoking reduces incisional wound infection: a randomized controlled trial. Ann Surg. 2003;238:1-5.
40. Sessler DI, Akca O. Preventing surgical site infections without drugs. Contemp OB/GYN. 2004;49:78-87.
41. Kurz A, Sessler DI, Lenhardt R. Perioperative normothermia to reduce the incidence of surgical wound infection and shorten hospitalization. N Engl J Med. 1996;334:1209-1215.
- A simplified abdominal incision makes the traditional extensive dissection associated with the Pfannenstiel incision unnecessary.
- A soft, self-retaining abdominal retractor offers increased exposure, atraumatic retraction, incision protection, and adjustable height while facilitating delivery of the fetal head by creating a rigid border around the abdominal incision.
- Bladder-flap omission has been associated with reduced operative time and incision-delivery interval, decreased blood loss, and less need for postoperative analgesics.
Is the extensive dissection of the Pfannenstiel incision necessary in cesarean delivery? Is bladder dissection essential? Must the visceral and parietal peritoneum be closed?
The success of our minimally invasive cesarean technique suggests the answer is “no.”
The approach described here features a short operative time; minimal instrumentation; reduced surgical dissection; decreased postoperative pain; and reduced risk of blood loss, infection, and wound complications. It is easily learned and cost-effective, with a brief postoperative recovery period.
Among the updates made from the technique’s initial publication in the mid-1990s1,2:
- addition of routine perioperative oxygen (80%), to reduce the risk of surgical wound infection (see “Perioperative considerations”)
- regular use of forced warm air covers applied to the anterior skin surface, to help patients maintain normothermia
- addition of a soft, self-retaining abdominal retractor—which creates an atraumatic circle of exposure up to a calculated 177 cm2 for a 15-cm incision (versus 113 cm2 calculated for traditional retraction)
- vertical, rather than lateral, digital extension of the initial transverse uterine incision
- identification of a subgroup in whom peritoneal closure is strongly recommended
- new data on the procedure’s effectiveness.3
Routinely use prophylactic antibiotics. Several recent studies have concluded that perioperative antibiotics reduce the incidence of endometritis and wound infection following elective and nonelective cesarean section.37 Administer ampicillin or a first-generation cephalosporin at umbilical cord clamping. For patients allergic to penicillin and cephalosporins, choose an alternative, such as clindamycin.
Give antacids 30 minutes before anesthesia (general or regional) to prevent pneumonitis from inhalation of gastric contents.
Clip pubic hair, rather than shave, to reduce the risk of wound infection.
Insert a Foley catheter, empty the bladder, and keep the catheter in place.
Position the patient in a 10°left lateral tilt, to avoid hypotension associated with aortocaval occlusion.
Routinely administer supplemental perioperative oxygen (80%), with either general or regional anesthesia; this activates alveolar immune defenses and halves the risk of surgical wound infections.
Neutrophil oxidative killing and phagocytosis—the most important defenses against surgical pathogens—depend on the partial pressure of oxygen in contaminated tissue. Giving supplemental oxygen during and for the first 2 hours after the procedure (by mask) is a practical, inexpensive way to reduce the incidence of surgical wound infection.38,39
Using 80% oxygen during and, for a short period, after surgery does not cause pulmonary toxicity such as atelectasis or impaired pulmonary function.40
Ensure normal body heat during and after cesarean to reduce the risk of postoperative surgical infection.40,41 Forced warm air covers applied to the anterior skin surface are the most effective way for warming surgical patients.9 IV fluid warming, though appropriate when large volumes are to be administered, is unnecessary for smaller operations.41
Modified abdominal incision reduces dissection
Make a straight low transverse incision with a scalpel, at a point approximately 3 to 4 cm above the symphysis pubis (FIGURE 1A).
The length of the incision is individualized (13 to 15 cm), though difficult fetal extraction is more likely if the abdominal incision is less than 15 cm.4,5
Divide the subcutaneous tissue transversely with an electrocautery knife. In a cutting and coagulation blend mode, the knife divides the fat while achieving hemostasis. To improve hemostasis, coagulate the blood vessels that cross the subcutaneous fat layer in a brushing manner before dividing them. To prevent unnecessary dead space, avoid filleting the fat and separating adherent subcutaneous fat from the anterior rectus fascia beyond what is needed to expose the fascia.
Open the fascia transversely with the electrocautery knife to the same length as the skin incision. Coagulate the blood vessels that cross the fascia before dividing them. Identify the median raphe by pulling up the superior edge of the abdominal incision.
Separate the rectus muscles in the midline by vertical blunt finger dissection (FIGURE 1B). If digital dissection is inefficient due to a dense, thick, or scarred median raphe, use an electrocautery knife, a scalpel, or scissors.
Open the peritoneum. This is facilitated by upward traction and elevation of the superior edge of the abdominal incision that lifts the peritoneum, allowing easy digital perforation using the index or middle finger (FIGURE 1C).
If this maneuver is not feasible, open the peritoneum in the traditional fashion.
Stretch the full thickness of the abdominal wall to full size of the skin incision, using 1 or 2 fingers of each hand (FIGURE 1D). Incorporate the skin, subcutaneous tissue, fascia layer, rectus muscles, and peritoneum. An assistant’s hands may be required. When needed, extend the peritoneal opening transversely on either side, to the midline and away from the bladder.
Digitally stretching the full-thickness abdominal incision is easily achieved due to the mechanical stretching of the anterior abdominal wall, edema, and increased vascularization that occur during pregnancy.
FIGURE 1A Create a modified abdominal incision
FIGURE 1B Create a modified abdominal incision
FIGURE 1C Create a modified abdominal incision
FIGURE 1D Create a modified abdominal incision
Retractor facilitates exposure
Our positive experience with the soft, self-retaining abdominal retractor for minilaparotomy and laparotomy6-8 compelled us to incorporate its use for cesarean 3 years ago.
The device consists of a flexible plastic inner ring and a firmer outer ring connected by a soft plastic sleeve. We use the large size of either of the 2 models currently available: the Mobius (Apple Medical Corporation, Marlboro, Miss) and the Protractor (Weck Closure Systems, Research Triangle Park, NC).
Introduce a hand through the laparotomy incision and evaluate the pelvis to ensure that no significant adhesions are present that may interfere with swift placement of the inner ring. If significant adhesions are found, use traditional metal retractors instead.
Squeeze the inner ring and insert it into the abdominal cavity toward the patient’s head, allowing the device to spring open against the parietal peritoneum (FIGURE 2A). Apply upper traction and elevate the superior edge of the abdominal incision to facilitate placement. Perform a digital check to ensure no tissue is trapped between the inner ring and the abdominal wall.
Hold up the outer ring, then roll the plastic sleeve until the ring completely inverts (FIGURE 2B). Repeat the process until the top ring is snug against the patient’s skin.
Advantages of the soft, self-retaining abdominal retractor include atraumatic retraction; incision protection; and adjustable height, making it ideal for obese patients.
FIGURE 2A Place the abdominal retractor
FIGURE 2B Place the abdominal retractor
Transverse hysterotomy in lower uterine segment
Make a transverse 2-cm uterine incision with a scalpel, approximately 1 cm above the vesicouterine peritoneal fold (identify this using gentle digital pressure to elevate the uterus) (FIGURE 3A).
Traditional bladder dissection is eliminated. We have found that when an adequate transverse hysterotomy is performed, a bladder flap is not required.1-3 In a recent randomized trial, Hohlagschwandtner et al9 confirmed our findings that bladder-flap omission was associated with reduced operative time and incision-delivery interval, decreased blood loss, and less need for postoperative analgesics.
An additional advantage of this omission: We can avoid making the uterine incision too low—especially when the cervix is fully dilated. Further, making the hysterotomy (uterine serosa and myometrium together) slightly above the vesicouterine peritoneal fold without bladder flap dissection frees the loose connective tissue between the uterus and the urinary bladder, allowing the spontaneous descent of the bladder.
Digitally extend the transverse uterine incision. We extend the initial incision not laterally but, instead, vertically (FIGURE 3B). The vertical digital traction on the initial transverse uterine incision creates a transverse dissociation of the horizontal myometrium fibers; this results in a transverse extension of the original incision.
This modification prevents unintended and uncontrolled lateral extensions of the incision that may lacerate the uterine vessels.10 It also prevents the uterine incision from becoming an inverted “U” and the undesirable accumulation of myometrium fibers at the ends of the incision. Such incisions usually do not reapproximate well during hysterotomy closure and may lead to sacculation-type defects.11
FIGURE 3A Use a standard hysterotomy in the lower uterine segment
FIGURE 3B Use a standard hysterotomy in the lower uterine segment
Delivering the fetus
For vertex presentations, place your hand into the uterine cavity between the lower edge of the hysterotomy and fetal head. While applying transabdominal fundal pressure, lift the head with your fingers and deliver it through the incision (FIGURE 4A).
The self-retaining retractor facilitates delivery of the fetal head by creating a rigid border around the abdominal incision. The back of the surgeon’s hand that is in the uterine cavity achieves better leverage, as it now rests on a rigid plane on the inferior part of the incision rather than on the back hand and the softer and pliable wound edge of the standard abdominal incision.
Vacuum-assistance. When delivery of the fetal head proves difficult, we use the soft vacuum cup, which avoids unnecessary intrauterine manipulation that may result in fetal or maternal trauma (FIGURE 4B).12
Breech extraction or transverse lie delivery is performed using standard extraction maneuvers (FIGURE 4C).13 We have found no need to perform T or J vertical extensions of the low transverse uterine incision, even in cases of a poorly developed lower uterine segment, abnormal presentation, or prematurity.
FIGURE 4A Deliver the fetal head
FIGURE 4B Deliver the fetal head
FIGURE 4C Deliver the fetal head
Uterine incision: 1-layer, in situ close
Remove the placenta only after it separates spontaneously—this greatly reduces blood loss compared with immediate manual placental delivery or umbilical-cord traction. Manually deliver the placenta only if it has not separated spontaneously after 5 minutes. Avoid routine manual clean-out of the uterine cavity if the placenta is completely delivered.1-3,14
Dilate the cervix to facilitate lochial discharge, when necessary.
Decrease uterine bleeding by massaging the uterus and administering diluted oxytocin.
Close the uterine incision in situ. Disadvantages of routine uterine exteriorization include discomfort and vomiting resulting from traction, exposure of the fallopian tubes to unnecessary trauma, increased risk of infection, possible rupture of the uteroovarian veins, and pulmonary embolism.
Exteriorization may also increase the risk of adhesion formation by exposing the uterine serosa to the abrasive effects of drying or microscopic abrasion if sponges are used to hold the uterus in place.1,14-17
Use a single-layer closure with a running suture of polyglycolic acid to close the uterine incision (FIGURE 5A). If needed, use atraumatic clamps to grasp the edges of the hysterotomy to facilitate visualization and closure.
Begin suturing at a point just beyond one end of the incision, continuing to the opposite side. Be sure to penetrate the full thickness of the myometrium and avoid incorporating the decidua in the suture line. After the uterine closure, use individual figure-of-8 sutures to control areas of persistent bleeding.
Compared with 2-layer closures, single-layer closure of a low transverse incision is associated with reduced operating time, improved hemostasis, and less tissue disruption; introduces less foreign material into the surgical site; reduces the need for postoperative analgesia; and potentially reduces infectious morbidity. A trial of labor after a cesarean section with 1-layer closure appears safe.18-22
We do not close the vesicouterine fold and parietal peritoneum (FIGURE 5B) or reapproximate the rectus muscles. It has been shown that a new peritoneal layer is formed within days of the original incision closure.
Leaving the peritoneum open leads to no increased postoperative complications, nor obvious differences in wound healing, wound dehiscence, or incidence of postoperative adhesions. Compared to women without peritoneal closure, those with peritoneal closure require a longer operative time, greater amounts of post-operative narcotics for postoperative pain, a greater need for bowel stimulants, and a longer postoperative hospitalization.23
In 1995, we performed laparoscopy for gynecologic conditions in 23 patients and repeat cesarean in 10 patients who had undergone our technique (with visceral and parietal peritoneum left open).2 We found no adhesions and a normal peritoneal lining in all cases.
In 1 subgroup, peritoneal closure is strongly recommended: cases in which 1 or both rectus muscles have been transected to increase surgical exposure. Extremely thick fibromuscular adhesions between the low anterior surface of the uterus and the under-surface of the rectus musculature may develop if the parietal peritoneum is not closed.
FIGURE 5A Close the incisions
FIGURE 5B Close the incisions
Abdominal closure
Close the rectus fascia with a continuous nonlocking 0 suture after the rectus muscles fall into place. Place entry and exit sites at 1-cm intervals, 1.5 cm beyond the wound edges—this minimizes the risk of sutures pulling through fascia. Interrupted sutures offer no greater tensile strength than continuous suturing.24 Tight, continuous suturing can lead to tissue ischemia, which may weaken the sutured fascia, and thus should be avoided.
The subcutaneous layer is not closed separately. One exception is obese patients with at least 2 cm of thick subcutaneous tissue. Thickness of subcutaneous tissue appears to be a significant risk factor for wound infection after cesarean section.25
In patients with thick subcutaneous tissue, you can reduce tension on the skin edges and the risk of subcutaneous infection, seroma formation, and wound disruption by placing interrupted 3-0 absorbable synthetic sutures to eliminate dead space.26-28 We have found no need to place Penrose drains or closed drainage systems in the subcutaneous layer.29-31
Close the skin using a subcuticular continuous absorbable 4-0 suture or, alternatively, metal staples, which are removed 4 days after surgery (FIGURE 5C).
FIGURE 5C Close the incisions
Prompt postoperative recovery
The patient may drink fluids in the recovery room, can resume a regular solid food diet within 4 hours after surgery, and may resume mobility as soon as anesthesia wears off.
Early breast-feeding is also allowed in the recovery room. In our experience, this has not been associated with complications, has been highly appreciated by the patients, and has led to earlier hospital discharge.
Control pain with meperidine (50–75 mg) or morphine (10 mg) parenterally every 3 to 4 hours. Other options include oxycodone and acetaminophen, oxycodone and aspirin, and ibuprofen.
Patients are usually discharged 48 to 72 hours following surgery.
Our experience: The numbers
Using this updated system, we have successfully completed 300 consecutive cesarean sections (210 primary, 90 repeat).
The average operating time was 19 minutes; average blood loss, 405 mL.
There was no postoperative febrile morbidity, wound infection, wound disruption, or wound hematoma.Only 3 patients developed superficial wound seromas, which were easily resolved. There were no intraoperative or postoperative complications.
We attribute the absence of wound infection to routine prophylactic antibiotics; supplemental perioperative oxygen; maintenance of normothermia; use of an electrocautery knife to create rapid hemostatic division of subcutaneous fat and anterior rectus fascia; a simplified abdominal wall opening; and placement of a self-retaining atraumatic retractor.10,32-36
Additional surgical procedures (tubal sterilization, myomectomy, appendectomy, and adhesiolysis) did not alter the recovery period, and all patients were able to get out of bed, shower, breast-feed, and care for their infants within 8 hours of surgery.
No infant complications related to the cesarean procedure occurred.
Patients were discharged from the hospital within 72 hours.
A prospective comparison study demonstrated that the original Pelosi-type of cesarean delivery was faster to perform, more cost-effective, and resulted in less maternal morbidity than the traditional cesarean technique.3
Dr. Pelosi II is a consultant for Apple Medical Corporation. Dr. Pelosi III reports no financial relationships relevant to this article.
- A simplified abdominal incision makes the traditional extensive dissection associated with the Pfannenstiel incision unnecessary.
- A soft, self-retaining abdominal retractor offers increased exposure, atraumatic retraction, incision protection, and adjustable height while facilitating delivery of the fetal head by creating a rigid border around the abdominal incision.
- Bladder-flap omission has been associated with reduced operative time and incision-delivery interval, decreased blood loss, and less need for postoperative analgesics.
Is the extensive dissection of the Pfannenstiel incision necessary in cesarean delivery? Is bladder dissection essential? Must the visceral and parietal peritoneum be closed?
The success of our minimally invasive cesarean technique suggests the answer is “no.”
The approach described here features a short operative time; minimal instrumentation; reduced surgical dissection; decreased postoperative pain; and reduced risk of blood loss, infection, and wound complications. It is easily learned and cost-effective, with a brief postoperative recovery period.
Among the updates made from the technique’s initial publication in the mid-1990s1,2:
- addition of routine perioperative oxygen (80%), to reduce the risk of surgical wound infection (see “Perioperative considerations”)
- regular use of forced warm air covers applied to the anterior skin surface, to help patients maintain normothermia
- addition of a soft, self-retaining abdominal retractor—which creates an atraumatic circle of exposure up to a calculated 177 cm2 for a 15-cm incision (versus 113 cm2 calculated for traditional retraction)
- vertical, rather than lateral, digital extension of the initial transverse uterine incision
- identification of a subgroup in whom peritoneal closure is strongly recommended
- new data on the procedure’s effectiveness.3
Routinely use prophylactic antibiotics. Several recent studies have concluded that perioperative antibiotics reduce the incidence of endometritis and wound infection following elective and nonelective cesarean section.37 Administer ampicillin or a first-generation cephalosporin at umbilical cord clamping. For patients allergic to penicillin and cephalosporins, choose an alternative, such as clindamycin.
Give antacids 30 minutes before anesthesia (general or regional) to prevent pneumonitis from inhalation of gastric contents.
Clip pubic hair, rather than shave, to reduce the risk of wound infection.
Insert a Foley catheter, empty the bladder, and keep the catheter in place.
Position the patient in a 10°left lateral tilt, to avoid hypotension associated with aortocaval occlusion.
Routinely administer supplemental perioperative oxygen (80%), with either general or regional anesthesia; this activates alveolar immune defenses and halves the risk of surgical wound infections.
Neutrophil oxidative killing and phagocytosis—the most important defenses against surgical pathogens—depend on the partial pressure of oxygen in contaminated tissue. Giving supplemental oxygen during and for the first 2 hours after the procedure (by mask) is a practical, inexpensive way to reduce the incidence of surgical wound infection.38,39
Using 80% oxygen during and, for a short period, after surgery does not cause pulmonary toxicity such as atelectasis or impaired pulmonary function.40
Ensure normal body heat during and after cesarean to reduce the risk of postoperative surgical infection.40,41 Forced warm air covers applied to the anterior skin surface are the most effective way for warming surgical patients.9 IV fluid warming, though appropriate when large volumes are to be administered, is unnecessary for smaller operations.41
Modified abdominal incision reduces dissection
Make a straight low transverse incision with a scalpel, at a point approximately 3 to 4 cm above the symphysis pubis (FIGURE 1A).
The length of the incision is individualized (13 to 15 cm), though difficult fetal extraction is more likely if the abdominal incision is less than 15 cm.4,5
Divide the subcutaneous tissue transversely with an electrocautery knife. In a cutting and coagulation blend mode, the knife divides the fat while achieving hemostasis. To improve hemostasis, coagulate the blood vessels that cross the subcutaneous fat layer in a brushing manner before dividing them. To prevent unnecessary dead space, avoid filleting the fat and separating adherent subcutaneous fat from the anterior rectus fascia beyond what is needed to expose the fascia.
Open the fascia transversely with the electrocautery knife to the same length as the skin incision. Coagulate the blood vessels that cross the fascia before dividing them. Identify the median raphe by pulling up the superior edge of the abdominal incision.
Separate the rectus muscles in the midline by vertical blunt finger dissection (FIGURE 1B). If digital dissection is inefficient due to a dense, thick, or scarred median raphe, use an electrocautery knife, a scalpel, or scissors.
Open the peritoneum. This is facilitated by upward traction and elevation of the superior edge of the abdominal incision that lifts the peritoneum, allowing easy digital perforation using the index or middle finger (FIGURE 1C).
If this maneuver is not feasible, open the peritoneum in the traditional fashion.
Stretch the full thickness of the abdominal wall to full size of the skin incision, using 1 or 2 fingers of each hand (FIGURE 1D). Incorporate the skin, subcutaneous tissue, fascia layer, rectus muscles, and peritoneum. An assistant’s hands may be required. When needed, extend the peritoneal opening transversely on either side, to the midline and away from the bladder.
Digitally stretching the full-thickness abdominal incision is easily achieved due to the mechanical stretching of the anterior abdominal wall, edema, and increased vascularization that occur during pregnancy.
FIGURE 1A Create a modified abdominal incision
FIGURE 1B Create a modified abdominal incision
FIGURE 1C Create a modified abdominal incision
FIGURE 1D Create a modified abdominal incision
Retractor facilitates exposure
Our positive experience with the soft, self-retaining abdominal retractor for minilaparotomy and laparotomy6-8 compelled us to incorporate its use for cesarean 3 years ago.
The device consists of a flexible plastic inner ring and a firmer outer ring connected by a soft plastic sleeve. We use the large size of either of the 2 models currently available: the Mobius (Apple Medical Corporation, Marlboro, Miss) and the Protractor (Weck Closure Systems, Research Triangle Park, NC).
Introduce a hand through the laparotomy incision and evaluate the pelvis to ensure that no significant adhesions are present that may interfere with swift placement of the inner ring. If significant adhesions are found, use traditional metal retractors instead.
Squeeze the inner ring and insert it into the abdominal cavity toward the patient’s head, allowing the device to spring open against the parietal peritoneum (FIGURE 2A). Apply upper traction and elevate the superior edge of the abdominal incision to facilitate placement. Perform a digital check to ensure no tissue is trapped between the inner ring and the abdominal wall.
Hold up the outer ring, then roll the plastic sleeve until the ring completely inverts (FIGURE 2B). Repeat the process until the top ring is snug against the patient’s skin.
Advantages of the soft, self-retaining abdominal retractor include atraumatic retraction; incision protection; and adjustable height, making it ideal for obese patients.
FIGURE 2A Place the abdominal retractor
FIGURE 2B Place the abdominal retractor
Transverse hysterotomy in lower uterine segment
Make a transverse 2-cm uterine incision with a scalpel, approximately 1 cm above the vesicouterine peritoneal fold (identify this using gentle digital pressure to elevate the uterus) (FIGURE 3A).
Traditional bladder dissection is eliminated. We have found that when an adequate transverse hysterotomy is performed, a bladder flap is not required.1-3 In a recent randomized trial, Hohlagschwandtner et al9 confirmed our findings that bladder-flap omission was associated with reduced operative time and incision-delivery interval, decreased blood loss, and less need for postoperative analgesics.
An additional advantage of this omission: We can avoid making the uterine incision too low—especially when the cervix is fully dilated. Further, making the hysterotomy (uterine serosa and myometrium together) slightly above the vesicouterine peritoneal fold without bladder flap dissection frees the loose connective tissue between the uterus and the urinary bladder, allowing the spontaneous descent of the bladder.
Digitally extend the transverse uterine incision. We extend the initial incision not laterally but, instead, vertically (FIGURE 3B). The vertical digital traction on the initial transverse uterine incision creates a transverse dissociation of the horizontal myometrium fibers; this results in a transverse extension of the original incision.
This modification prevents unintended and uncontrolled lateral extensions of the incision that may lacerate the uterine vessels.10 It also prevents the uterine incision from becoming an inverted “U” and the undesirable accumulation of myometrium fibers at the ends of the incision. Such incisions usually do not reapproximate well during hysterotomy closure and may lead to sacculation-type defects.11
FIGURE 3A Use a standard hysterotomy in the lower uterine segment
FIGURE 3B Use a standard hysterotomy in the lower uterine segment
Delivering the fetus
For vertex presentations, place your hand into the uterine cavity between the lower edge of the hysterotomy and fetal head. While applying transabdominal fundal pressure, lift the head with your fingers and deliver it through the incision (FIGURE 4A).
The self-retaining retractor facilitates delivery of the fetal head by creating a rigid border around the abdominal incision. The back of the surgeon’s hand that is in the uterine cavity achieves better leverage, as it now rests on a rigid plane on the inferior part of the incision rather than on the back hand and the softer and pliable wound edge of the standard abdominal incision.
Vacuum-assistance. When delivery of the fetal head proves difficult, we use the soft vacuum cup, which avoids unnecessary intrauterine manipulation that may result in fetal or maternal trauma (FIGURE 4B).12
Breech extraction or transverse lie delivery is performed using standard extraction maneuvers (FIGURE 4C).13 We have found no need to perform T or J vertical extensions of the low transverse uterine incision, even in cases of a poorly developed lower uterine segment, abnormal presentation, or prematurity.
FIGURE 4A Deliver the fetal head
FIGURE 4B Deliver the fetal head
FIGURE 4C Deliver the fetal head
Uterine incision: 1-layer, in situ close
Remove the placenta only after it separates spontaneously—this greatly reduces blood loss compared with immediate manual placental delivery or umbilical-cord traction. Manually deliver the placenta only if it has not separated spontaneously after 5 minutes. Avoid routine manual clean-out of the uterine cavity if the placenta is completely delivered.1-3,14
Dilate the cervix to facilitate lochial discharge, when necessary.
Decrease uterine bleeding by massaging the uterus and administering diluted oxytocin.
Close the uterine incision in situ. Disadvantages of routine uterine exteriorization include discomfort and vomiting resulting from traction, exposure of the fallopian tubes to unnecessary trauma, increased risk of infection, possible rupture of the uteroovarian veins, and pulmonary embolism.
Exteriorization may also increase the risk of adhesion formation by exposing the uterine serosa to the abrasive effects of drying or microscopic abrasion if sponges are used to hold the uterus in place.1,14-17
Use a single-layer closure with a running suture of polyglycolic acid to close the uterine incision (FIGURE 5A). If needed, use atraumatic clamps to grasp the edges of the hysterotomy to facilitate visualization and closure.
Begin suturing at a point just beyond one end of the incision, continuing to the opposite side. Be sure to penetrate the full thickness of the myometrium and avoid incorporating the decidua in the suture line. After the uterine closure, use individual figure-of-8 sutures to control areas of persistent bleeding.
Compared with 2-layer closures, single-layer closure of a low transverse incision is associated with reduced operating time, improved hemostasis, and less tissue disruption; introduces less foreign material into the surgical site; reduces the need for postoperative analgesia; and potentially reduces infectious morbidity. A trial of labor after a cesarean section with 1-layer closure appears safe.18-22
We do not close the vesicouterine fold and parietal peritoneum (FIGURE 5B) or reapproximate the rectus muscles. It has been shown that a new peritoneal layer is formed within days of the original incision closure.
Leaving the peritoneum open leads to no increased postoperative complications, nor obvious differences in wound healing, wound dehiscence, or incidence of postoperative adhesions. Compared to women without peritoneal closure, those with peritoneal closure require a longer operative time, greater amounts of post-operative narcotics for postoperative pain, a greater need for bowel stimulants, and a longer postoperative hospitalization.23
In 1995, we performed laparoscopy for gynecologic conditions in 23 patients and repeat cesarean in 10 patients who had undergone our technique (with visceral and parietal peritoneum left open).2 We found no adhesions and a normal peritoneal lining in all cases.
In 1 subgroup, peritoneal closure is strongly recommended: cases in which 1 or both rectus muscles have been transected to increase surgical exposure. Extremely thick fibromuscular adhesions between the low anterior surface of the uterus and the under-surface of the rectus musculature may develop if the parietal peritoneum is not closed.
FIGURE 5A Close the incisions
FIGURE 5B Close the incisions
Abdominal closure
Close the rectus fascia with a continuous nonlocking 0 suture after the rectus muscles fall into place. Place entry and exit sites at 1-cm intervals, 1.5 cm beyond the wound edges—this minimizes the risk of sutures pulling through fascia. Interrupted sutures offer no greater tensile strength than continuous suturing.24 Tight, continuous suturing can lead to tissue ischemia, which may weaken the sutured fascia, and thus should be avoided.
The subcutaneous layer is not closed separately. One exception is obese patients with at least 2 cm of thick subcutaneous tissue. Thickness of subcutaneous tissue appears to be a significant risk factor for wound infection after cesarean section.25
In patients with thick subcutaneous tissue, you can reduce tension on the skin edges and the risk of subcutaneous infection, seroma formation, and wound disruption by placing interrupted 3-0 absorbable synthetic sutures to eliminate dead space.26-28 We have found no need to place Penrose drains or closed drainage systems in the subcutaneous layer.29-31
Close the skin using a subcuticular continuous absorbable 4-0 suture or, alternatively, metal staples, which are removed 4 days after surgery (FIGURE 5C).
FIGURE 5C Close the incisions
Prompt postoperative recovery
The patient may drink fluids in the recovery room, can resume a regular solid food diet within 4 hours after surgery, and may resume mobility as soon as anesthesia wears off.
Early breast-feeding is also allowed in the recovery room. In our experience, this has not been associated with complications, has been highly appreciated by the patients, and has led to earlier hospital discharge.
Control pain with meperidine (50–75 mg) or morphine (10 mg) parenterally every 3 to 4 hours. Other options include oxycodone and acetaminophen, oxycodone and aspirin, and ibuprofen.
Patients are usually discharged 48 to 72 hours following surgery.
Our experience: The numbers
Using this updated system, we have successfully completed 300 consecutive cesarean sections (210 primary, 90 repeat).
The average operating time was 19 minutes; average blood loss, 405 mL.
There was no postoperative febrile morbidity, wound infection, wound disruption, or wound hematoma.Only 3 patients developed superficial wound seromas, which were easily resolved. There were no intraoperative or postoperative complications.
We attribute the absence of wound infection to routine prophylactic antibiotics; supplemental perioperative oxygen; maintenance of normothermia; use of an electrocautery knife to create rapid hemostatic division of subcutaneous fat and anterior rectus fascia; a simplified abdominal wall opening; and placement of a self-retaining atraumatic retractor.10,32-36
Additional surgical procedures (tubal sterilization, myomectomy, appendectomy, and adhesiolysis) did not alter the recovery period, and all patients were able to get out of bed, shower, breast-feed, and care for their infants within 8 hours of surgery.
No infant complications related to the cesarean procedure occurred.
Patients were discharged from the hospital within 72 hours.
A prospective comparison study demonstrated that the original Pelosi-type of cesarean delivery was faster to perform, more cost-effective, and resulted in less maternal morbidity than the traditional cesarean technique.3
Dr. Pelosi II is a consultant for Apple Medical Corporation. Dr. Pelosi III reports no financial relationships relevant to this article.
1. Pelosi MA, Ortega I. Cesarean section: Pelosi simplified technique. Rev Chil Obstet Gynecol. 1994;59:372-377.
2. Pelosi MA, II, Pelosi MA, III. Simplified cesarean section. Contemp OB/GYN. 1995;40:89-100.
3. Wood RM, Simon H, Oz AU. Pelosi-type vs traditional cesarean delivery: a prospective comparison. J Reprod Med. 1999;44:788-795.
4. Finan MA, Mastrogiannis DS, Spellacy WN. The “Allis” test for easy cesarean delivery. Am J Obstet Gynecol. 1991;164:772-775.
5. Ayers IW, Morley GW. Surgical incision for cesarean section. Obstet Gynecol. 1987;70:706-712.
6. Pelosi MA, II, Pelosi MA, III. Self-retaining abdominal retractor for minilaparotomy. Obstet Gynecol. 2000;96:775-778.
7. Pelosi MA, II, Pelosi MA, III. Pelosi minilaparotomy hysterectomy: Effective alternative to laparoscopy and laparotomy. OBG Manag. April 2003;16-33.
8. Pelosi MA, II, Pelosi MA, III. A novel minilaparotomy approach for large ovarian cysts. OBG Manag. February 2004:;17-30.
9. Hohlagschwandtner M, Ruecklinger E, Husslein P, et al. Is the formation of a bladder flap at cesarean necessary? A randomized trial. Obstet Gynecol. 2001;98:1089-1092.
10. Ferrari A, Frigerio L, Origoni M, et al. Modified Stark procedure for cesarean section. J Pelv Surg. 1996;2:239-244.
11. Field CS. Surgical techniques for cesarean section. Obstet Gynecol Clin North Amer. 1988;15:657-672.
12. Pelosi MA, Apuzzio J. Use of the soft, silicone obstetric vacuum cup for delivery of the fetal head at cesarean section. J Reprod Med. 1984;29:289-292.
13. Pelosi MA, Apuzzio J, Fricchione D, et al. The intra-abdominal version technique for delivery of transverse lie by low segment cesarean section. Am J Obstet Gynecol. 1979;135:1009-1012.
14. Magann EF, Dodson MK, Albert JR, et al. Blood loss at the time of cesarean section by method of placental removal and exteriorization versus in situ repair of the uterine incision. Surg Gynecol Obstet. 1993;177:389-392.
15. Stock RJ, Shelton H. Fatal pulmonary embolism occurring 2 hours after exteriorization of the uterus for repair following cesareans section. Milit Med. 1985;150:549-550.
16. Hershey DW, Quilligan EJ. Extra-abdominal uterine exteriorization at cesarean section. Obstet Genecol. 1978;52:189-191.
17. Wahab MA, Karantzis P, Eccersley PS, et al. A randomized, controlled study of uterine exteriorization and repair at cesarean section. Br J Obstet Gynecol. 1999;106:913-916.
18. Hauth JC, Owen J, Davis RO. Transverse uterine incision closure: one versus two layers. Am J Obstet Gynecol. 1992;167:1108-1111.
19. Ohel G, Younis JS, Lang N, et al. Double-layer closure of uterine incision with visceral and parietal peritoneal closure: Are they obligatory steps of routine cesarean sections? J Matern Fetal Med. 1996;5:366-369.
20. Tucker JM, Hauth JC, Hodgkins P, et al. Trial of labor after a one- or two-layer closure of a low transverse uterine incision. Am J Obstet Gynecol. 1993;168:545-546.
21. Chapman SJ, Owen J, Hauth JC. One- versus two-layer closure of a low transverse cesarean: the next pregnancy. Obstet Gynecol. 1997;89:16-18.
22. Jelsema RD, Wittinger JA, Vander Kolk KJ. Continuous, nonlocking, single-layer repair of the low transverse uterine incision. J Reprod Med. 1993;38:393-396.
23. Tulandi T, Al-Jaroudi D. Nonclosure of peritoneum: a reappraisal. Am J Obstet Gynecol. 2003;189:609-612.
24. Fagniez PL, Hay JM, Lacaine F. Abdominal midline incision closure. Arch Surg. 1985;120:1351-1355.
25. Vermillion ST, Lamoutte C, Soper DE, et al. Wound infection after cesarean: effect of subcutaneous tissue thickness. Obstet Gynecol. 2000;95:923-926.
26. Naumann RW, Hauth JC, Owen J, et al. Subcutaneous tissue approximation in relation to wound disruption after cesarean delivery in obese women. Obstet Gynecol. 1995;85:412-416.
27. Cetin A, Cetin M. Superficial wound disruption after cesarean delivery: effect of the depth and closure of subcutaneous tissue. Int J Gynaecol Obstet. 1997;57:17-21.
28. Del Valle GO, Combs P, Qualls C, et al. Does closure of Camper fascia reduce the incidence of post-cesarean superficial wound disruption? Obstet Gynecol. 1992;80:1013-1016.
29. Loong RL, Rogers MS, Chang AM. A controlled trial of wound drainage at cesarean section. Aust NZ J Obstet Gynaecol. 1988;28:266-269.
30. Saunders NJ, Barclay C. Closed suction wound drainage and lower-segment cesarean section. Br J Obstet Gynaecol. 1988;95:1060-1062.
31. Magann EF, Chauhan SP, Rodts-Palenik S, Bufkin L, Martin JN, Jr, Morrison JC. Subcutaneous stitch closure versus subcutaneous drain to prevent wound disruption after cesarean delivery: a randomized clinical trial. Am J Obstet Gynecol. 2002;186:1119-1123.
32. Stark M, Finkel AR. Comparison between the Joel-Cohen and Pfannenstiel incisions in cesarean section. Eur J Obstet Gynecol Reprod Biol. 1994;53:121-122.
33. Joel-Cohen S. Abdominal and vaginal hysterectomy. New techniques based on time and motion studies. London, England: William Heinemann Medical Books; 1972.
34. Hemsell DL, Hemsell PG, Nobles B, et al. Abdominal wound problems after hysterectomy with electrocautery versus scalpel subcutaneous incision. Infect Dis Obstet Gynecol. 1993;1:27-30.
35. Johnson CD, Serpell JW. Wound infection after abdominal incision with scalpel or diathermy. Br J Surg. 1990;77:626-629.
36. Hussain SA, Hussain S. Incisions with knife or diathermy and postoperative pain. Br J Surg. 1988;75:1179-1182.
37. American College of Obstetricians and Gynecologist. ACOG Practice Bulletin. Clinical management guidelines for obstetricians-gynecologists. No. 43, May, 2003. Management of preterm labor. Obstet Gynecol. 2003;101:1039-1047.
38. Greif R, Akca O, Hurn E-P, et al. Supplemental perioperative oxygen to reduce the incidence of surgical wound infection. N Engl J Med. 2000;342:161-167.
39. Sorensen LT, Karlsmark T, Gottrup F. Abstinence from smoking reduces incisional wound infection: a randomized controlled trial. Ann Surg. 2003;238:1-5.
40. Sessler DI, Akca O. Preventing surgical site infections without drugs. Contemp OB/GYN. 2004;49:78-87.
41. Kurz A, Sessler DI, Lenhardt R. Perioperative normothermia to reduce the incidence of surgical wound infection and shorten hospitalization. N Engl J Med. 1996;334:1209-1215.
1. Pelosi MA, Ortega I. Cesarean section: Pelosi simplified technique. Rev Chil Obstet Gynecol. 1994;59:372-377.
2. Pelosi MA, II, Pelosi MA, III. Simplified cesarean section. Contemp OB/GYN. 1995;40:89-100.
3. Wood RM, Simon H, Oz AU. Pelosi-type vs traditional cesarean delivery: a prospective comparison. J Reprod Med. 1999;44:788-795.
4. Finan MA, Mastrogiannis DS, Spellacy WN. The “Allis” test for easy cesarean delivery. Am J Obstet Gynecol. 1991;164:772-775.
5. Ayers IW, Morley GW. Surgical incision for cesarean section. Obstet Gynecol. 1987;70:706-712.
6. Pelosi MA, II, Pelosi MA, III. Self-retaining abdominal retractor for minilaparotomy. Obstet Gynecol. 2000;96:775-778.
7. Pelosi MA, II, Pelosi MA, III. Pelosi minilaparotomy hysterectomy: Effective alternative to laparoscopy and laparotomy. OBG Manag. April 2003;16-33.
8. Pelosi MA, II, Pelosi MA, III. A novel minilaparotomy approach for large ovarian cysts. OBG Manag. February 2004:;17-30.
9. Hohlagschwandtner M, Ruecklinger E, Husslein P, et al. Is the formation of a bladder flap at cesarean necessary? A randomized trial. Obstet Gynecol. 2001;98:1089-1092.
10. Ferrari A, Frigerio L, Origoni M, et al. Modified Stark procedure for cesarean section. J Pelv Surg. 1996;2:239-244.
11. Field CS. Surgical techniques for cesarean section. Obstet Gynecol Clin North Amer. 1988;15:657-672.
12. Pelosi MA, Apuzzio J. Use of the soft, silicone obstetric vacuum cup for delivery of the fetal head at cesarean section. J Reprod Med. 1984;29:289-292.
13. Pelosi MA, Apuzzio J, Fricchione D, et al. The intra-abdominal version technique for delivery of transverse lie by low segment cesarean section. Am J Obstet Gynecol. 1979;135:1009-1012.
14. Magann EF, Dodson MK, Albert JR, et al. Blood loss at the time of cesarean section by method of placental removal and exteriorization versus in situ repair of the uterine incision. Surg Gynecol Obstet. 1993;177:389-392.
15. Stock RJ, Shelton H. Fatal pulmonary embolism occurring 2 hours after exteriorization of the uterus for repair following cesareans section. Milit Med. 1985;150:549-550.
16. Hershey DW, Quilligan EJ. Extra-abdominal uterine exteriorization at cesarean section. Obstet Genecol. 1978;52:189-191.
17. Wahab MA, Karantzis P, Eccersley PS, et al. A randomized, controlled study of uterine exteriorization and repair at cesarean section. Br J Obstet Gynecol. 1999;106:913-916.
18. Hauth JC, Owen J, Davis RO. Transverse uterine incision closure: one versus two layers. Am J Obstet Gynecol. 1992;167:1108-1111.
19. Ohel G, Younis JS, Lang N, et al. Double-layer closure of uterine incision with visceral and parietal peritoneal closure: Are they obligatory steps of routine cesarean sections? J Matern Fetal Med. 1996;5:366-369.
20. Tucker JM, Hauth JC, Hodgkins P, et al. Trial of labor after a one- or two-layer closure of a low transverse uterine incision. Am J Obstet Gynecol. 1993;168:545-546.
21. Chapman SJ, Owen J, Hauth JC. One- versus two-layer closure of a low transverse cesarean: the next pregnancy. Obstet Gynecol. 1997;89:16-18.
22. Jelsema RD, Wittinger JA, Vander Kolk KJ. Continuous, nonlocking, single-layer repair of the low transverse uterine incision. J Reprod Med. 1993;38:393-396.
23. Tulandi T, Al-Jaroudi D. Nonclosure of peritoneum: a reappraisal. Am J Obstet Gynecol. 2003;189:609-612.
24. Fagniez PL, Hay JM, Lacaine F. Abdominal midline incision closure. Arch Surg. 1985;120:1351-1355.
25. Vermillion ST, Lamoutte C, Soper DE, et al. Wound infection after cesarean: effect of subcutaneous tissue thickness. Obstet Gynecol. 2000;95:923-926.
26. Naumann RW, Hauth JC, Owen J, et al. Subcutaneous tissue approximation in relation to wound disruption after cesarean delivery in obese women. Obstet Gynecol. 1995;85:412-416.
27. Cetin A, Cetin M. Superficial wound disruption after cesarean delivery: effect of the depth and closure of subcutaneous tissue. Int J Gynaecol Obstet. 1997;57:17-21.
28. Del Valle GO, Combs P, Qualls C, et al. Does closure of Camper fascia reduce the incidence of post-cesarean superficial wound disruption? Obstet Gynecol. 1992;80:1013-1016.
29. Loong RL, Rogers MS, Chang AM. A controlled trial of wound drainage at cesarean section. Aust NZ J Obstet Gynaecol. 1988;28:266-269.
30. Saunders NJ, Barclay C. Closed suction wound drainage and lower-segment cesarean section. Br J Obstet Gynaecol. 1988;95:1060-1062.
31. Magann EF, Chauhan SP, Rodts-Palenik S, Bufkin L, Martin JN, Jr, Morrison JC. Subcutaneous stitch closure versus subcutaneous drain to prevent wound disruption after cesarean delivery: a randomized clinical trial. Am J Obstet Gynecol. 2002;186:1119-1123.
32. Stark M, Finkel AR. Comparison between the Joel-Cohen and Pfannenstiel incisions in cesarean section. Eur J Obstet Gynecol Reprod Biol. 1994;53:121-122.
33. Joel-Cohen S. Abdominal and vaginal hysterectomy. New techniques based on time and motion studies. London, England: William Heinemann Medical Books; 1972.
34. Hemsell DL, Hemsell PG, Nobles B, et al. Abdominal wound problems after hysterectomy with electrocautery versus scalpel subcutaneous incision. Infect Dis Obstet Gynecol. 1993;1:27-30.
35. Johnson CD, Serpell JW. Wound infection after abdominal incision with scalpel or diathermy. Br J Surg. 1990;77:626-629.
36. Hussain SA, Hussain S. Incisions with knife or diathermy and postoperative pain. Br J Surg. 1988;75:1179-1182.
37. American College of Obstetricians and Gynecologist. ACOG Practice Bulletin. Clinical management guidelines for obstetricians-gynecologists. No. 43, May, 2003. Management of preterm labor. Obstet Gynecol. 2003;101:1039-1047.
38. Greif R, Akca O, Hurn E-P, et al. Supplemental perioperative oxygen to reduce the incidence of surgical wound infection. N Engl J Med. 2000;342:161-167.
39. Sorensen LT, Karlsmark T, Gottrup F. Abstinence from smoking reduces incisional wound infection: a randomized controlled trial. Ann Surg. 2003;238:1-5.
40. Sessler DI, Akca O. Preventing surgical site infections without drugs. Contemp OB/GYN. 2004;49:78-87.
41. Kurz A, Sessler DI, Lenhardt R. Perioperative normothermia to reduce the incidence of surgical wound infection and shorten hospitalization. N Engl J Med. 1996;334:1209-1215.
Anterior vaginal wall prolapse: The challenge of cystocele repair
- At this time, the traditional anterior colporrhaphy with attention to apical suspension remains the gold standard.
- If only some defects of the anterior wall are addressed at the time of reconstructive surgery, failure may be more likely.
- Women with grade 3 or 4 cystoceles often have evidence of bladder outlet obstruction on urodynamic testing.
- In 52% of cases, cystoceles coexist with detrusor instability and evidence of impaired detrusor contractility.
- A thorough preoperative evaluation includes assessing the apex, having the patient strain to maximize the defect, looking for paravaginal detachments, and making every effort to “unmask” occult stress urinary incontinence.
Ask a pelvic reconstructive surgeon to name the most difficult challenge, and the answer is likely to be anterior vaginal wall prolapse. The reason: The anterior wall usually is the leading edge of prolapse and the most common site of relaxation or failure following reconstructive surgery. This appears to hold true regardless of surgical route or technique.
Short-term success rates of anterior wall repairs appear promising, but long-term outcomes are not as encouraging. Success usually is claimed as long as the anterior wall is kept above the hymen, since the patient rarely reports symptoms in these cases.
Another challenge involves the use of allografts or xenografts, which have not undergone sufficient study to determine their long-term benefit or risks in comparison with traditional repairs.
This article reviews anatomy of the anterior vaginal wall and its supports, as well as surgical technique and outcomes.
Why the anterior wall is more susceptible to prolapse
One theory is that, in comparison with the posterior compartment, the anterior wall is not as well supported by the levator plate, which counters the effects of gravity and abdominal pressure. Normally, the anterior wall rests horizontally on the posterior wall, which in turn rests on the levator plate. When the levator muscles weaken, the anterior wall is the first to fall as increasing force is placed on the connective tissue supports.
Other possibilities: The anterior compartment’s attachments to the pelvic sidewall or apex may be weaker, the anterior wall may be more elastic or less dense than the posterior wall, and the anterior wall may be more susceptible to damage during childbirth or to the effects of age and loss of estrogen.
If only some defects are addressed at surgery, failure may be more likely. Some experts believe pelvic surgeons have focused too much attention on the urethrovesical junction in patients with concomitant urinary incontinence and not enough attention on suspending the anterior wall at the apex.
For most women, it is probably a combination of many of these factors that renders the anterior compartment so vulnerable.
Anatomy of the pelvic floor
The anterior vaginal wall resembles a trapezoidal plane due to ventral and more medial attachments near the pubic symphysis, and dorsal and more lateral attachments near the ischial spine (FIGURE 1).1 This helps explain the many appearances of the cystocele. The type of cystocele is defined by the location of the break in the fascial attachments.
Paravaginal defects. The trapezoidal anterior wall is suspended on both sides from the parietal fascia overlying the levator ani muscles at the arcus tendineus fascia pelvis (ATFP). Prolapse can occur when there is loss of attachment to the pelvic sidewall at any point between the pubis and ischial spine.
First described by White2 and characterized later by Richardson et al,3 this loss of lateral attachment is called a paravaginal defect or displacement cystocele (FIGURE 2). The goal of paravaginal repair is to reattach the lateral vaginal walls to the ATFP, either abdominally, laparoscopically, or vaginally.
Central defects, the rarest type of anterior wall prolapse, involve a loss of support near the pubis and tend to be smaller. The most common manifestation is urethral hypermobility.
Transverse defects occur when the top of the pubocervical fascia detaches from the cervix or vaginal apex, both of which are suspended from the uterosacral-cardinal ligament complex. A transverse cystocele is evidenced by loss of the anterior fornix. The anterior wall appears to be attenuated in the midline, and the vaginal mucosa is pale, thin, and smooth (FIGURE 3).
Goals of traditional repair. The traditional anterior colporrhaphy aims to excise or reinforce the attenuated transverse defect with plication of the “endopelvic fascia” in the midline of the anterior vaginal wall. The endopelvic fascia is not true fascia but the muscularis of the vaginal wall. It is comprised of smooth muscle and elastin along with the collagenous adventitia layer.4
The importance of restoring apical wall support becomes apparent when one considers the trapezoidal anatomy. The most common sites of defects or detachments of the anterior wall are near the ischial spines laterally. In an operative case series of paravaginal defects, DeLancey1 found the site of defect to be near the ischial spine in 96% of cases. The reattachment of the apex near the level of the spine becomes the highest point of support for the anterior vaginal wall.
This cephalad apical attachment can be accomplished in a variety of ways, by suspending the vaginal apex from the uterosacral ligaments, from the sacrospinous ligament, or via abdominal sacrocolpopexy.
FIGURE 1 Anatomy of the anterior wall
The anterior vaginal wall resembles a trapezoidal plane, with ventral and more medial attachments near the pubic symphysis, and dorsal and more lateral attachments to the ischial spine. Detachment from the pelvic sidewall and ischial spine results in anterior wall prolapse (right).
FIGURE 2 Paravaginal defect
FIGURE 3 Transverse defect
A transverse defect with loss of the anterior fornix. The loss of cephalad apical attachment at the level of the ischial spine leads to anterior wall prolapse. Suspending the upper vagina from shortened cardinal/uterosacral ligaments, the sacrospinous ligament, or via abdominal sacrocolpopexy is as important as plication.
Symptoms of anterior wall prolapse
As with other forms of pelvic organ prolapse, many patients complain of a bulge or feeling of pelvic pressure when the anterior vaginal wall has come through the introitus. However, some symptoms of anterior wall prolapse are unique.
Incontinence is not universal. A common misperception is that most patients with cystocele also experience stress urinary incontinence (SUI), which can develop when there is loss of urethral support and descent of the lower vaginal wall along with urethral hypermobility. However, there is no defining degree of hypermobility that links anterior wall prolapse with SUI. That is because the continence mechanism relies not only on urethral position and lateral attachments, but also on the neuromuscular function of the pelvis and lower urinary tract.
In fact, descent of the midvagina under the bladder base may actually reduce the chance of SUI. The reason: As a woman strains, the increased abdominal pressure pushes the cystocele farther and farther out. As the cystocele enlarges, it creates a functional outlet obstruction by kinking the urethra shut. When this is the case, patients may complain of prolonged voiding, an intermittent urine stream, and/or urinary retention. The woman may have to elevate the vaginal wall to empty her bladder. Patients with chronic urinary retention are at risk of developing recurrent urinary tract infections.
Bladder outlet obstruction and detrusor dysfunction. Women with grade 3 or 4 cystoceles often have evidence of bladder outlet obstruction on urodynamic testing, according to a study that found such evidence in 57% of subjects.5 After reduction of the prolapse with a pessary, obstructed flow reverted to normal in 94% of these women.
A large proportion (52%) of women with cystoceles also have detrusor instability, as well as evidence of impaired detrusor contractility. Many complain of urinary frequency and urgency and difficulty emptying the bladder.5
Again, this phenomenon is complex, related not only to anatomy but to altered neuromuscular function of the lower urinary tract. Incomplete emptying, frequency, and urgency may arise from stretching of the bladder base as it prolapses through the vaginal introitus, resulting in urinary retention. These symptoms often are less pronounced at night when the patient is supine.
We reviewed 35 cases of anterior wall prolapse greater than 1 cm outside the hymen, with elevated postvoid residuals exceeding 100 cc on 2 separate occasions.6 Thirty-one (89%) had normal postvoid residuals following reconstructive surgery and correction of their anterior wall prolapse.
Preoperative assessment
A careful physical exam is a prerequisite for all surgical repairs of pelvic organ prolapse. During this exam, identify the sites of defects and detachments.
Maximize the defect. Have the patient perform the Valsalva maneuver, cough, and/or strain while sitting upright or standing. As she is performing these maneuvers, ask her if this feels like her maximum prolapse. A split speculum often aids in visualizing the anterior and posterior compartments without pressure from the opposite vaginal wall.
Assess the apex. Place a large swab in the vagina, hold it gently against the apex, and ask the patient to strain. If the swab is pushed out, the apex needs suspension.
This technique can help identify apical relaxation that may be masked by a large anterior or posterior wall defect. A standardized staging system, such as the Pelvic Organ Prolapse Quantitative Examination (POP-Q) or Baden-Walker, aids in communicating and documenting the prolapse. In addition, it allows the surgeon to track anatomical outcomes after surgery.
Look for paravaginal defects by supporting the lateral anterior walls with a ring forceps at the level of the ATFP. Barber et al7 found this maneuver to be highly sensitive (90–94%): If no paravaginal defect was suspected clinically, none was found intraoperatively. However, the positive predictive value was poor (57%), in that defects suspected preoperatively were confirmed during surgery in less than two thirds of patients.
These findings point to the importance of careful intraoperative assessment, both before and during the repair procedure.
Limited utility of imaging studies. The use of radiologic studies such as defecography or dynamic magnetic resonance imaging of the pelvis may aid in the evaluation of defecatory disorders or suspected sigmoidocele or rectal prolapse, but have not been studied sufficiently to determine the impact on surgical outcome.
Unmasking SUI
As mentioned above, women with anterior wall prolapse do not always complain of stress incontinence. However, correction of the cystocele can relieve their obstructive voiding and unmask “occult” SUI. Various techniques have been described to elevate the anterior wall with pessaries, swabs, etc, during urodynamic testing to predict which women should have an incontinence procedure performed at the time of reconstructive surgery.
Conflicting rates of occult SUI have been reported, with estimates ranging from 36% to 80%.8 Although preoperative urodynamic testing indicates a high rate of occult stress incontinence, a study by Borstad et al9 suggests that the rate of de novo incontinence may be lower and that preoperative urodynamic findings are not predictive of postoperative continence status. In that study, 16 of 73 women (22%) developed stress incontinence following surgery for prolapse when no incontinence procedure was performed. Advanced age increased the risk of incontinence after surgery.
Contrast these findings with those of Chaikin and colleagues,10 who prospectively followed 24 patients with grade 3 or 4 cystoceles. Preoperative urodynamics showed a 58% rate of occult stress incontinence. All these patients were also defined as having intrinsic sphincter deficiency with leak point pressures below 60 cm water. The incontinent group underwent anterior colporrhaphy and concomitant pubovaginal sling, compared with anterior colporrhaphy alone for those without incontinence. Postoperatively, 2 patients who had the pubovaginal sling procedure reported continued stress incontinence (14%). No new symptoms of incontinence were reported in the patients without leakage on preoperative urodynamics. Thus, preoperative urodynamics were 100% accurate in determining which women did not need additional surgery for SUI.
Implications of a negative stress test. Our experience has shown that, despite our best attempts, a negative stress test with the prolapse reduced prior to surgery is less than 100% predictive. Occasionally, new SUI occurs after reconstructive surgery. It is unclear whether this incontinence is caused by straightening the urethra and reducing the bulge or secondary to the dissection of surgery.
Tips on technique
Anterior colporrhaphy traditionally is performed with plication of the “endopelvic fascia” or fibromuscular layer at the bladder neck with a Kelly plication stitch. Using “3-point” traction aids in dissecting the muscularis (FIGURE 4). Repair the remainder of the cystocele using vertical mattress stitches (1 or 2 layers) from the bladder neck to the apex.
Avoid creating weak areas. Using this technique, the repair frequently stops short of the apex, leaving a “gap” or weak area. One way to avoid this is to begin plication at the apex instead of the bladder neck (FIGURE 5).
Next, excise the excess vaginal tissue and close with interrupted fine absorbable sutures (FIGURE 6).
Recreate apical support. Another problem with traditional repairs is that they do not reestablish apical support. In many patients with anterior wall prolapse, reattachment of the apex reduces the cystocele. Therefore, it often is necessary to combine anterior colporrhaphy with an apical repair procedure such as uterosacral ligament suspension or sacrospinous ligament suspension.
Sutures for the apical repair should be placed and held prior to initiating the anterior colporrhaphy. At the end of the anterior repair, incorporate the apical sutures into the vaginal cuff.
Careful attention to the integrity and strength of the tissue is crucial. Regardless of the type of transvaginal suspension, we advocate bringing 1 arm of the suspension suture through the anterior wall of the cuff. Then place the other suture arm through the posterior cuff so that, when tied, anterior and posterior walls are brought together and suspended.
Using prolonged-delayed absorbable suture allows for a full-thickness bite, ensuring scarring to the suspensory ligament. If permanent suture is used for the uterosacral suspension, place the stitches along the inside surface of the anterior wall with a strong, broad bite that incorporates the muscularis or “endopelvic fascia.”
The occasional enterocele. When a transverse cystocele occurs following hysterectomy, the surgeon should be on the lookout for an enterocele, which sometimes accompanies anterior wall prolapse. The enterocele should be corrected at the time of surgery by closing the defect and suspending the cuff.
FIGURE 4 Three-point traction
Three-point traction using Allis clamps. The assistant retracts with DeBackey forceps to allow dissection of the muscularis. An index finger placed firmly against the vaginal mucosa enables the surgeon to judge depth of dissection.
FIGURE 5 Begin plication at the apex
Plication begins at the apex with vertical mattress stitches. Use 3-0 prolonged delayed absorbable or permanent suture in the anterior wall.
FIGURE 6 The reduced cystocele
The cystocele reduced following midline plication of the vaginal muscularis. The excess vagina is then trimmed and closed with interrupted 3-0 absorbable suture.
Functional surgical outcomes
Because of the long association between anterior wall prolapse and SUI, most surgeons evaluate patients preoperatively to determine the need for concomitant incontinence procedures. As a result, the literature reporting surgical cystocele repair via anterior colporrhaphy frequently uses continence of urine as the functional outcome. This is not surprising considering that anterior repair and Kelly plication, as reported by Howard Kelly more than 75 years ago, have been the gold standard for surgical correction of anterior wall prolapse and SUI.
In a series of 194 SUI patients who underwent anterior colporrhaphy, Beck11 found that adding a modified Kelly plication, including a vaginal retropubic urethropexy, increased the cure rate for SUI from 75% to 94%. Unfortunately, he did not report the anatomic success of the anterior colporrhaphy.
Kohli et al12 also retrospectively examined patients who had undergone anterior colporrhaphy with and without needle bladder-neck suspension. Although the cure rate for SUI was not reported, patients who underwent concomitant needle suspension had a higher rate of recurrent cystocele: 33% (n = 40) versus 7% (n = 27). Investigators theorized that retropubic dissection at the time of transvaginal needle suspension resulted in an iatrogenic paravaginal defect and denervation of the anterior vaginal wall.
The risks of needle suspension. A randomized controlled trial by Bump et al8 also suggests that needle suspension should be avoided. In that trial, 29 patients with stage 3 and 4 prolapse were randomized to needle colposuspension or endopelvic fascia plication. They, too, found that needle colposuspension carried a higher rate of recurrent anterior prolapse. Further, it did not reduce the rates of SUI compared with fascia plication.
Although incontinence surgery performed at the time of cystocele repair will reduce the rates of de novo incontinence, the higher rates of cystocele recurrence associated with some procedures warrants judicious preoperative planning. Clearly, needle suspension should not be performed as an incontinence procedure or repair of anterior wall prolapse. Whether other vaginal incontinence procedures, eg, midurethral slings, lead to recurrence of anterior wall prolapse deserves further investigation.
Anatomic outcomes
Midline colporrhaphy. Because anterior colporrhaphy is rarely performed alone, few series describe patients having undergone simply an anterior repair. Stanton et al13 followed 54 women for up to 2 years after they underwent traditional midline plication with vaginal hysterectomy for prolapse. Eight (15%) of the women had recurrent anterior wall prolapse.
Colombo et al14 randomized 71 women with clinical SUI and stage 2 or 3 prolapse to Burch colposuspension or anterior colporrhaphy with Kelly plication. All women were followed for at least 8 years. The cure rate for SUI was 86% for the Burch procedure, compared with 52% for anterior repair and Kelly plication. However, 12 (34%) women treated with Burch colposuspension and 1 (3%) treated with anterior colporrhaphy had recurrent cystocele of grade 2 or 3 with or without prolapse at other vaginal sites.
Abdominal paravaginal repair. Shull15 followed 149 women for 6 months to 4 years after they underwent abdominal paravaginal repair with the urethrovesical stitch brought through Cooper’s ligament for treatment of SUI and paravaginal cystocele. He reported a 5% recurrence of anterior wall prolapse.
In another series, Shull16 reported on 62 women who were followed for a mean of 18 months after abdominal paravaginal repair. Four of 57 (7%) had recurrent vaginal prolapse to the hymen.
In a cohort of 102 patients evaluated with the Pelvic Organ Prolapse Quantitative Examination (POP-Q) at our institution, the maximal point of prolapse was the anterior wall in 60% of cases; the apex and posterior wall each accounted for roughly half the remaining cases (unpublished data).
Ellerkmann et al30 reported on 237 consecutive patients who presented with symptoms of pelvic organ prolapse. In 77 women (33%), anterior compartment pelvic organ prolapse predominated; 46 patients (19%) had posterior compartment prolapse; and 22 patients (11%) had apical prolapse.
Hendrix et al31 analyzed patients from the Women’s Health Initiative (WHI) and found that the anterior compartment predominated over the posterior compartment. In a follow-up study from the WHI, Handa32 reported on 412 women followed for 2 to 8 years. Among those who entered the WHI protocol without cystocele, 1 in 4 was diagnosed with it at some point in the study. This compares to 1 in 6 for rectocele and 1 in 100 for uterine prolapse. The majority of all defects were grade 1 or relaxation above the hymen.
Vaginal paravaginal repair. Mallipeddi et al17 reported on 45 patients undergoing vaginal paravaginal repair over 2 years, with 35 women followed for a mean of 1.6 years. Incontinent patients had a Kelly plication performed at the time of vaginal paravaginal repair. Recurrence rates were 3% for cystocele, 14% for rectocele, and 20% for enterocele.
Young et al18 followed 100 women for as long as 36 months after bilateral paravaginal repair using 1 to 6 expanded polytetrafluoroethylene (Gore-Tex) CV-0 sutures and midline colporrhaphy. Two patients had grade 1 or 2 failure at the lateral fixation points, but 21 patients had recurrent midline defects, all but 1 inside the hymen. Several patients had bloody discharge from the permanent sutures.
Sacrocolpopexy. Brubaker19 retrospectively reviewed 65 women who underwent sacrocolpopexy for apical prolapse. Three months postoperatively 19 patients (29%) had persistent anterior wall defects.
Uterosacral suspension. Shull et al20 also found the anterior segment to have the most recurrent defects. In that study, which had an average follow-up of a little over a year, 289 patients underwent vaginal uterosacral ligament repair of the apex, and 264 had an anterior wall defect preoperatively. At the time of furthest follow-up, 26 patients (9%) had failure at this site. This study confirmed that the anterior compartment is the most likely site to fail, and also that it fails the quickest.
Sacrospinous ligament suspension (SSLS). Morley and DeLancey21 found a 22% cystocele recurrence rate in 71 women 1 year after SSLS, with most of them asymptomatic. Shull22 reported a 30% incidence of cystoceles after SSLS. Paraiso and colleagues23 reported on 243 women undergoing SSLS and pelvic reconstructive surgery. Of these, 217 patients underwent concomitant anterior colporrhaphy. Follow-up at 74 months found 37% with symptomatic recurrence at the anterior wall, 13% at the posterior wall, and 8% at the apex.
Vaginal versus abdominal repair
Few studies have compared vaginal and abdominal repair of pelvic organ prolapse, including anterior wall prolapse.
In a trial by Benson et al,24 women with prolapse to or beyond the hymen were randomized to bilateral sacrospinous vault suspension and vaginal paravaginal repair (n = 48) or abdominal sacrocolpopexy with abdominal paravaginal repair (n = 40). One third of patients in each group also underwent anterior colporrhaphy. After a mean follow-up of 2.5 years, 16 of 20 women required reoperation for recurrent cystocele—12 (29%) from the vaginal group and 4 (10.5%) from the abdominal group. Vaginal vault eversion recurred in 5 women from the vaginal group and 1 from the abdominal group.
Investigators concluded that the anterior wall was the most likely site of failure because of the posterior placement of the vaginal apex with SSLS, predisposing the anterior wall to greater pressures and to neuropathy caused by lateral dissection of the anterior wall. Earlier studies have demonstrated that neuropathy may occur after extensive dissection of the vaginal wall and may affect the strength and integrity of the muscular support tissues.25,26
Allografts and xenografts
The difficulty of repairing anterior wall prolapse has led some pelvic surgeons to use mesh for cystocele repair. When Julian27 randomized 24 patients with recurrent cystocele to transvaginal repair with and without polypropylene (Marlex) mesh, 4 patients in the control group and no patients in the mesh group had recurrences (P <.05). However, 3 patients (25%) had mesh-related complications.
Weber et al28 randomized patients to standard midline plication, plication of the paravaginal tissue more laterally, or standard plication plus polyglactin 910 (Vicryl) mesh. Among 83 patients who returned for follow-up, there were no differences in anatomic outcome. Weber and colleagues concluded that there is little benefit to using mesh to correct cystoceles.
Still, although the overall cure rate was low (30–46%), most patients had cystocele to the hymen and not beyond, with significant improvement of symptoms. Although this cannot be defined as an anatomic cure, it is encouraging that the majority of patients appear to have benefited from surgery.
Sand et al29 randomized 161 women with the anterior wall to or beyond the hymen to traditional anterior colporrhaphy with or without Vicryl. The 2-inch square mesh was not placed over the repair as described above, but was folded into the anterior colporrhaphy stitches. At 1 year, 16 (22%) of 73 women with mesh and 28 (40%) of the 88 women without mesh had recurrent central cystoceles beyond the midvagina (P = .02). No women had cystoceles beyond the hymen or vaginal erosions.
Difficulty of interpreting the evidence
Because of the broad range of study designs, small number of patients per series, variety of concomitant procedures, and wide range of variables used to describe recurrence and success, it is difficult to draw conclusions from the literature. The evidence does suggest that the risks of wide vaginal dissection required for vaginal paravaginal repair outweigh the benefits. As a result, we have abandoned this technique. As mentioned above, it remains unclear whether graft materials will prove to be of long-term benefit for either midline plication or paravaginal repair.
The gold standard, for now
Prolapse of the anterior vaginal wall remains a challenge for the gynecologic surgeon. Careful preoperative and intraoperative evaluation and identification of support defects should guide repairs.
Randomized, controlled trials of midline versus paravaginal repair, as well as use of various graft materials, are greatly needed. These studies should not only address recurrence of prolapse symptoms, but the impact of surgery on sexual and lower urinary tract function.
At this time, the traditional anterior colporrhaphy with attention to apical suspension remains the gold standard.
The authors report no financial relationships relevant to this article.
1. DeLancey JOL. Fascial and muscular abnormalities in women with urethral hypermobility and anterior vaginal wall prolapse. Am J Obstet Gynecol. 2002;187:93-98.
2. White GR. Cystocele: a radical cure by suturing lateral sulci of vagina to white line of pelvic fascia. JAMA. 1909;21:1707-1710.
3. Richardson AC, Lyon JB, Williams NL. A new look at pelvic relaxation. Am J Obstet Gynecol. 1976;126:568-571.
4. Weber AM, Walters MD. Anterior vaginal prolapse: review of anatomy and techniques of surgical repair. Obstet Gynecol. 1997;89:311-318.
5. Romanzi LJ, Chaikin DC, Blaivas JG. The effect of genital prolapse on voiding. J Urol. 1999;161:581-586.
6. FitzGerald MP, Kulkarni N, Fenner D. Postoperative resolution of urinary retention in patients with advanced pelvic organ prolapse. Am J Obstet Gynecol. 2000;183:1361-1364.
7. Barber MD, Cundiff GW, Weidner AC, Cotes KW, Bump RC, Addison WA. Accuracy of clinical assessment of paravaginal defects in women with anterior vaginal wall proloapse. Am J Obstet Gynecol. 1999;181:1-7.
8. Bump RC, Hurt WG, Theofrastous JP, et al. Randomized prospective comparison of needle colposuspension versus endopelvic fascia plication for potential stress incontinence prophylaxis in women undergoing vaginal reconstruction for stage III or IV pelvic organ prolapse. The Continence Program for Women Research Group. Am J Obstet Gynecol. 1996;175:326-333.
9. Borstad E, Rud T. The risk of developing urinary stress incontinence after vaginal repair in continent women. Acta Obstet Gynecol Scand. 1989;68:545-549.
10. Chaikin DC, Groutz A, Blaivas JG. Predicting the need for anti-incontinence surgery in continent women undergoing repair of severe urogenital prolapse. J Urol. 2000;163:531-534.
11. Beck RP, McCormick S, Nordstrom L. A 25-year experience with 519 anterior colporrhaphy procedures. Obstet Gynecol. 1991;78:1011-1018.
12. Kohli N, Sze EHM, Roat TW, Karram M. Incidence of recurrent cystocele after anterior colporrhaphy with and without concomitant transvaginal needle suspension. Am J Obstet Gynecol. 1996;175:1476-1482.
13. Stanton SL, Hilton P, Norton C, Cardozo L. Clinical and urodynamics effect of anterior colporrhaphy and vaginal hysterectomy for prolapse with and without incontinence. Br J Obstet Gynecol. 1982;89:459-463.
14. Colombo M, Vitobello D, Proiette F, Milani R. Randomized comparison of Burch colposuspension versus anterior colporrhaphy in women with stress urinary incontinence and anterior vaginal wall prolapse. BJOG. 2000;107:544-551.
15. Shull BL, Baden WF. A six-year experience with paravaginal defect repair for stress urinary incontinence. Am J Obstet Gynecol. 1989;160:1432-1440.
16. Shull BL, Benn SJ, Kuehl TJ. Surgical management of prolapse of the anterior vaginal segment: an analysis of support defects, operative morbidity, and anatomic outcome. Am J Obstet Gynecol. 1994;171:1429-1439.
17. Mallipeddi PK, Steele AC, Kohli N, Karram MM. Anatomic and functional outcome of vaginal paravaginal repair in the correction of anterior wall prolapse. Int Urogynecol J. 2001;12:83-88.
18. Young SB, Daman JJ, Bony LG. Vaginal paravaginal repair: one-year outcomes. Am J Obstet Gynecol. 2001;185:1360-1367.
19. Brubaker L. Sacrocolpopexy and the anterior compartment: support and function. Am J Obstet Gynecol. 1995;173:1690-1694.
20. Shull BL, Bachofen C, Coates KW, Kuehl TJ. A transvaginal approach to repair of apical and other associated sites of pelvic organ prolapse with uterosacral ligaments. Am J Obstet Gynecol. 2000;183:1365-1374.
21. Morley GW, DeLancey JOL. Sacrospinous ligament fixation for eversion of the vagina. Am J Obstet Gynecol. 1988;158:872-881.
22. Shull BL, Capen CV, Riggs MW, Kuehl TJ. Preoperative and postoperative analysis of site-specific pelvic support defects in 81 women treated with sacrospinous ligament suspension and pelvic reconstruction. Am J Obstet Gynecol. 1992;166:1764-1771.
23. Paraiso MFR, Ballard LA, Walter MD, et al. Pelvic support defects and visceral and sexual function in women treated with sacrospinous ligament suspension and pelvic reconstruction. Am J Obstet Gynecol. 1996;175:1423-1431.
24. Benson JT, Lucente V, McClellan E. Vaginal versus abdominal reconstructive surgery for the treatment of pelvic support defects: a prospective randomized study with long-term outcome evaluation. Am J Obstet Gynecol. 1996;175:1418-1421.
25. Benson JT, McClellan E. The effect of vaginal dissection of the pudendal nerve. Obstet Gynecol. 1993;82:387-389.
26. Zivkovic F, Tamussino K, Ralph G, et al. Long-term effects of vaginal dissection on the innervation of the striated urethral sphincter. Obstet Gynecol. 1996;87:257-260.
27. Julian T. The efficacy of Marlex mesh in the repair of severe, recurrent vaginal prolapse of the anterior midvaginal wall. Am J Obstet Gynecol. 1996;175:1472-1475.
28. Weber AM, Walters MD, Piedmonte MR, et al. Anterior colporrhaphy: a randomized trial of three surgical techniques. Am J Obstet Gynecol. 2001;185:1299-1306.
29. Sand PK, et al. Prospective randomized trial of polyglactin 910 mesh to prevent recurrence of cystocele and rectocele. Am J Obstet Gynecol. 2001;184:1357-1364.
30. Ellerkmann RM, Cundiff GW, Melick CF, Nihira MA, Leffler K, Bent AE. Correlation of symptoms with location and severity of pelvic organ prolapse. Am J Obstet Gynecol. 2001;185:1332-1337.
31. Hendrix SL, Clark A, Nygaard I, Aragaki A, Barnabei V, McTiernan A. Pelvic organ prolapse in the Women’s Health Initiative: gravity and gravidity. Am J Obstet Gynecol. 2002;186:1160-1166.
32. Handa VL, Garrett E, Hendrix S, Gold E, Robbins J. Progression and remission of pelvic organ prolapse: a longitudinal study of menopausal women. Am J Obstet Gynecol. 2004;190:27-32.
- At this time, the traditional anterior colporrhaphy with attention to apical suspension remains the gold standard.
- If only some defects of the anterior wall are addressed at the time of reconstructive surgery, failure may be more likely.
- Women with grade 3 or 4 cystoceles often have evidence of bladder outlet obstruction on urodynamic testing.
- In 52% of cases, cystoceles coexist with detrusor instability and evidence of impaired detrusor contractility.
- A thorough preoperative evaluation includes assessing the apex, having the patient strain to maximize the defect, looking for paravaginal detachments, and making every effort to “unmask” occult stress urinary incontinence.
Ask a pelvic reconstructive surgeon to name the most difficult challenge, and the answer is likely to be anterior vaginal wall prolapse. The reason: The anterior wall usually is the leading edge of prolapse and the most common site of relaxation or failure following reconstructive surgery. This appears to hold true regardless of surgical route or technique.
Short-term success rates of anterior wall repairs appear promising, but long-term outcomes are not as encouraging. Success usually is claimed as long as the anterior wall is kept above the hymen, since the patient rarely reports symptoms in these cases.
Another challenge involves the use of allografts or xenografts, which have not undergone sufficient study to determine their long-term benefit or risks in comparison with traditional repairs.
This article reviews anatomy of the anterior vaginal wall and its supports, as well as surgical technique and outcomes.
Why the anterior wall is more susceptible to prolapse
One theory is that, in comparison with the posterior compartment, the anterior wall is not as well supported by the levator plate, which counters the effects of gravity and abdominal pressure. Normally, the anterior wall rests horizontally on the posterior wall, which in turn rests on the levator plate. When the levator muscles weaken, the anterior wall is the first to fall as increasing force is placed on the connective tissue supports.
Other possibilities: The anterior compartment’s attachments to the pelvic sidewall or apex may be weaker, the anterior wall may be more elastic or less dense than the posterior wall, and the anterior wall may be more susceptible to damage during childbirth or to the effects of age and loss of estrogen.
If only some defects are addressed at surgery, failure may be more likely. Some experts believe pelvic surgeons have focused too much attention on the urethrovesical junction in patients with concomitant urinary incontinence and not enough attention on suspending the anterior wall at the apex.
For most women, it is probably a combination of many of these factors that renders the anterior compartment so vulnerable.
Anatomy of the pelvic floor
The anterior vaginal wall resembles a trapezoidal plane due to ventral and more medial attachments near the pubic symphysis, and dorsal and more lateral attachments near the ischial spine (FIGURE 1).1 This helps explain the many appearances of the cystocele. The type of cystocele is defined by the location of the break in the fascial attachments.
Paravaginal defects. The trapezoidal anterior wall is suspended on both sides from the parietal fascia overlying the levator ani muscles at the arcus tendineus fascia pelvis (ATFP). Prolapse can occur when there is loss of attachment to the pelvic sidewall at any point between the pubis and ischial spine.
First described by White2 and characterized later by Richardson et al,3 this loss of lateral attachment is called a paravaginal defect or displacement cystocele (FIGURE 2). The goal of paravaginal repair is to reattach the lateral vaginal walls to the ATFP, either abdominally, laparoscopically, or vaginally.
Central defects, the rarest type of anterior wall prolapse, involve a loss of support near the pubis and tend to be smaller. The most common manifestation is urethral hypermobility.
Transverse defects occur when the top of the pubocervical fascia detaches from the cervix or vaginal apex, both of which are suspended from the uterosacral-cardinal ligament complex. A transverse cystocele is evidenced by loss of the anterior fornix. The anterior wall appears to be attenuated in the midline, and the vaginal mucosa is pale, thin, and smooth (FIGURE 3).
Goals of traditional repair. The traditional anterior colporrhaphy aims to excise or reinforce the attenuated transverse defect with plication of the “endopelvic fascia” in the midline of the anterior vaginal wall. The endopelvic fascia is not true fascia but the muscularis of the vaginal wall. It is comprised of smooth muscle and elastin along with the collagenous adventitia layer.4
The importance of restoring apical wall support becomes apparent when one considers the trapezoidal anatomy. The most common sites of defects or detachments of the anterior wall are near the ischial spines laterally. In an operative case series of paravaginal defects, DeLancey1 found the site of defect to be near the ischial spine in 96% of cases. The reattachment of the apex near the level of the spine becomes the highest point of support for the anterior vaginal wall.
This cephalad apical attachment can be accomplished in a variety of ways, by suspending the vaginal apex from the uterosacral ligaments, from the sacrospinous ligament, or via abdominal sacrocolpopexy.
FIGURE 1 Anatomy of the anterior wall
The anterior vaginal wall resembles a trapezoidal plane, with ventral and more medial attachments near the pubic symphysis, and dorsal and more lateral attachments to the ischial spine. Detachment from the pelvic sidewall and ischial spine results in anterior wall prolapse (right).
FIGURE 2 Paravaginal defect
FIGURE 3 Transverse defect
A transverse defect with loss of the anterior fornix. The loss of cephalad apical attachment at the level of the ischial spine leads to anterior wall prolapse. Suspending the upper vagina from shortened cardinal/uterosacral ligaments, the sacrospinous ligament, or via abdominal sacrocolpopexy is as important as plication.
Symptoms of anterior wall prolapse
As with other forms of pelvic organ prolapse, many patients complain of a bulge or feeling of pelvic pressure when the anterior vaginal wall has come through the introitus. However, some symptoms of anterior wall prolapse are unique.
Incontinence is not universal. A common misperception is that most patients with cystocele also experience stress urinary incontinence (SUI), which can develop when there is loss of urethral support and descent of the lower vaginal wall along with urethral hypermobility. However, there is no defining degree of hypermobility that links anterior wall prolapse with SUI. That is because the continence mechanism relies not only on urethral position and lateral attachments, but also on the neuromuscular function of the pelvis and lower urinary tract.
In fact, descent of the midvagina under the bladder base may actually reduce the chance of SUI. The reason: As a woman strains, the increased abdominal pressure pushes the cystocele farther and farther out. As the cystocele enlarges, it creates a functional outlet obstruction by kinking the urethra shut. When this is the case, patients may complain of prolonged voiding, an intermittent urine stream, and/or urinary retention. The woman may have to elevate the vaginal wall to empty her bladder. Patients with chronic urinary retention are at risk of developing recurrent urinary tract infections.
Bladder outlet obstruction and detrusor dysfunction. Women with grade 3 or 4 cystoceles often have evidence of bladder outlet obstruction on urodynamic testing, according to a study that found such evidence in 57% of subjects.5 After reduction of the prolapse with a pessary, obstructed flow reverted to normal in 94% of these women.
A large proportion (52%) of women with cystoceles also have detrusor instability, as well as evidence of impaired detrusor contractility. Many complain of urinary frequency and urgency and difficulty emptying the bladder.5
Again, this phenomenon is complex, related not only to anatomy but to altered neuromuscular function of the lower urinary tract. Incomplete emptying, frequency, and urgency may arise from stretching of the bladder base as it prolapses through the vaginal introitus, resulting in urinary retention. These symptoms often are less pronounced at night when the patient is supine.
We reviewed 35 cases of anterior wall prolapse greater than 1 cm outside the hymen, with elevated postvoid residuals exceeding 100 cc on 2 separate occasions.6 Thirty-one (89%) had normal postvoid residuals following reconstructive surgery and correction of their anterior wall prolapse.
Preoperative assessment
A careful physical exam is a prerequisite for all surgical repairs of pelvic organ prolapse. During this exam, identify the sites of defects and detachments.
Maximize the defect. Have the patient perform the Valsalva maneuver, cough, and/or strain while sitting upright or standing. As she is performing these maneuvers, ask her if this feels like her maximum prolapse. A split speculum often aids in visualizing the anterior and posterior compartments without pressure from the opposite vaginal wall.
Assess the apex. Place a large swab in the vagina, hold it gently against the apex, and ask the patient to strain. If the swab is pushed out, the apex needs suspension.
This technique can help identify apical relaxation that may be masked by a large anterior or posterior wall defect. A standardized staging system, such as the Pelvic Organ Prolapse Quantitative Examination (POP-Q) or Baden-Walker, aids in communicating and documenting the prolapse. In addition, it allows the surgeon to track anatomical outcomes after surgery.
Look for paravaginal defects by supporting the lateral anterior walls with a ring forceps at the level of the ATFP. Barber et al7 found this maneuver to be highly sensitive (90–94%): If no paravaginal defect was suspected clinically, none was found intraoperatively. However, the positive predictive value was poor (57%), in that defects suspected preoperatively were confirmed during surgery in less than two thirds of patients.
These findings point to the importance of careful intraoperative assessment, both before and during the repair procedure.
Limited utility of imaging studies. The use of radiologic studies such as defecography or dynamic magnetic resonance imaging of the pelvis may aid in the evaluation of defecatory disorders or suspected sigmoidocele or rectal prolapse, but have not been studied sufficiently to determine the impact on surgical outcome.
Unmasking SUI
As mentioned above, women with anterior wall prolapse do not always complain of stress incontinence. However, correction of the cystocele can relieve their obstructive voiding and unmask “occult” SUI. Various techniques have been described to elevate the anterior wall with pessaries, swabs, etc, during urodynamic testing to predict which women should have an incontinence procedure performed at the time of reconstructive surgery.
Conflicting rates of occult SUI have been reported, with estimates ranging from 36% to 80%.8 Although preoperative urodynamic testing indicates a high rate of occult stress incontinence, a study by Borstad et al9 suggests that the rate of de novo incontinence may be lower and that preoperative urodynamic findings are not predictive of postoperative continence status. In that study, 16 of 73 women (22%) developed stress incontinence following surgery for prolapse when no incontinence procedure was performed. Advanced age increased the risk of incontinence after surgery.
Contrast these findings with those of Chaikin and colleagues,10 who prospectively followed 24 patients with grade 3 or 4 cystoceles. Preoperative urodynamics showed a 58% rate of occult stress incontinence. All these patients were also defined as having intrinsic sphincter deficiency with leak point pressures below 60 cm water. The incontinent group underwent anterior colporrhaphy and concomitant pubovaginal sling, compared with anterior colporrhaphy alone for those without incontinence. Postoperatively, 2 patients who had the pubovaginal sling procedure reported continued stress incontinence (14%). No new symptoms of incontinence were reported in the patients without leakage on preoperative urodynamics. Thus, preoperative urodynamics were 100% accurate in determining which women did not need additional surgery for SUI.
Implications of a negative stress test. Our experience has shown that, despite our best attempts, a negative stress test with the prolapse reduced prior to surgery is less than 100% predictive. Occasionally, new SUI occurs after reconstructive surgery. It is unclear whether this incontinence is caused by straightening the urethra and reducing the bulge or secondary to the dissection of surgery.
Tips on technique
Anterior colporrhaphy traditionally is performed with plication of the “endopelvic fascia” or fibromuscular layer at the bladder neck with a Kelly plication stitch. Using “3-point” traction aids in dissecting the muscularis (FIGURE 4). Repair the remainder of the cystocele using vertical mattress stitches (1 or 2 layers) from the bladder neck to the apex.
Avoid creating weak areas. Using this technique, the repair frequently stops short of the apex, leaving a “gap” or weak area. One way to avoid this is to begin plication at the apex instead of the bladder neck (FIGURE 5).
Next, excise the excess vaginal tissue and close with interrupted fine absorbable sutures (FIGURE 6).
Recreate apical support. Another problem with traditional repairs is that they do not reestablish apical support. In many patients with anterior wall prolapse, reattachment of the apex reduces the cystocele. Therefore, it often is necessary to combine anterior colporrhaphy with an apical repair procedure such as uterosacral ligament suspension or sacrospinous ligament suspension.
Sutures for the apical repair should be placed and held prior to initiating the anterior colporrhaphy. At the end of the anterior repair, incorporate the apical sutures into the vaginal cuff.
Careful attention to the integrity and strength of the tissue is crucial. Regardless of the type of transvaginal suspension, we advocate bringing 1 arm of the suspension suture through the anterior wall of the cuff. Then place the other suture arm through the posterior cuff so that, when tied, anterior and posterior walls are brought together and suspended.
Using prolonged-delayed absorbable suture allows for a full-thickness bite, ensuring scarring to the suspensory ligament. If permanent suture is used for the uterosacral suspension, place the stitches along the inside surface of the anterior wall with a strong, broad bite that incorporates the muscularis or “endopelvic fascia.”
The occasional enterocele. When a transverse cystocele occurs following hysterectomy, the surgeon should be on the lookout for an enterocele, which sometimes accompanies anterior wall prolapse. The enterocele should be corrected at the time of surgery by closing the defect and suspending the cuff.
FIGURE 4 Three-point traction
Three-point traction using Allis clamps. The assistant retracts with DeBackey forceps to allow dissection of the muscularis. An index finger placed firmly against the vaginal mucosa enables the surgeon to judge depth of dissection.
FIGURE 5 Begin plication at the apex
Plication begins at the apex with vertical mattress stitches. Use 3-0 prolonged delayed absorbable or permanent suture in the anterior wall.
FIGURE 6 The reduced cystocele
The cystocele reduced following midline plication of the vaginal muscularis. The excess vagina is then trimmed and closed with interrupted 3-0 absorbable suture.
Functional surgical outcomes
Because of the long association between anterior wall prolapse and SUI, most surgeons evaluate patients preoperatively to determine the need for concomitant incontinence procedures. As a result, the literature reporting surgical cystocele repair via anterior colporrhaphy frequently uses continence of urine as the functional outcome. This is not surprising considering that anterior repair and Kelly plication, as reported by Howard Kelly more than 75 years ago, have been the gold standard for surgical correction of anterior wall prolapse and SUI.
In a series of 194 SUI patients who underwent anterior colporrhaphy, Beck11 found that adding a modified Kelly plication, including a vaginal retropubic urethropexy, increased the cure rate for SUI from 75% to 94%. Unfortunately, he did not report the anatomic success of the anterior colporrhaphy.
Kohli et al12 also retrospectively examined patients who had undergone anterior colporrhaphy with and without needle bladder-neck suspension. Although the cure rate for SUI was not reported, patients who underwent concomitant needle suspension had a higher rate of recurrent cystocele: 33% (n = 40) versus 7% (n = 27). Investigators theorized that retropubic dissection at the time of transvaginal needle suspension resulted in an iatrogenic paravaginal defect and denervation of the anterior vaginal wall.
The risks of needle suspension. A randomized controlled trial by Bump et al8 also suggests that needle suspension should be avoided. In that trial, 29 patients with stage 3 and 4 prolapse were randomized to needle colposuspension or endopelvic fascia plication. They, too, found that needle colposuspension carried a higher rate of recurrent anterior prolapse. Further, it did not reduce the rates of SUI compared with fascia plication.
Although incontinence surgery performed at the time of cystocele repair will reduce the rates of de novo incontinence, the higher rates of cystocele recurrence associated with some procedures warrants judicious preoperative planning. Clearly, needle suspension should not be performed as an incontinence procedure or repair of anterior wall prolapse. Whether other vaginal incontinence procedures, eg, midurethral slings, lead to recurrence of anterior wall prolapse deserves further investigation.
Anatomic outcomes
Midline colporrhaphy. Because anterior colporrhaphy is rarely performed alone, few series describe patients having undergone simply an anterior repair. Stanton et al13 followed 54 women for up to 2 years after they underwent traditional midline plication with vaginal hysterectomy for prolapse. Eight (15%) of the women had recurrent anterior wall prolapse.
Colombo et al14 randomized 71 women with clinical SUI and stage 2 or 3 prolapse to Burch colposuspension or anterior colporrhaphy with Kelly plication. All women were followed for at least 8 years. The cure rate for SUI was 86% for the Burch procedure, compared with 52% for anterior repair and Kelly plication. However, 12 (34%) women treated with Burch colposuspension and 1 (3%) treated with anterior colporrhaphy had recurrent cystocele of grade 2 or 3 with or without prolapse at other vaginal sites.
Abdominal paravaginal repair. Shull15 followed 149 women for 6 months to 4 years after they underwent abdominal paravaginal repair with the urethrovesical stitch brought through Cooper’s ligament for treatment of SUI and paravaginal cystocele. He reported a 5% recurrence of anterior wall prolapse.
In another series, Shull16 reported on 62 women who were followed for a mean of 18 months after abdominal paravaginal repair. Four of 57 (7%) had recurrent vaginal prolapse to the hymen.
In a cohort of 102 patients evaluated with the Pelvic Organ Prolapse Quantitative Examination (POP-Q) at our institution, the maximal point of prolapse was the anterior wall in 60% of cases; the apex and posterior wall each accounted for roughly half the remaining cases (unpublished data).
Ellerkmann et al30 reported on 237 consecutive patients who presented with symptoms of pelvic organ prolapse. In 77 women (33%), anterior compartment pelvic organ prolapse predominated; 46 patients (19%) had posterior compartment prolapse; and 22 patients (11%) had apical prolapse.
Hendrix et al31 analyzed patients from the Women’s Health Initiative (WHI) and found that the anterior compartment predominated over the posterior compartment. In a follow-up study from the WHI, Handa32 reported on 412 women followed for 2 to 8 years. Among those who entered the WHI protocol without cystocele, 1 in 4 was diagnosed with it at some point in the study. This compares to 1 in 6 for rectocele and 1 in 100 for uterine prolapse. The majority of all defects were grade 1 or relaxation above the hymen.
Vaginal paravaginal repair. Mallipeddi et al17 reported on 45 patients undergoing vaginal paravaginal repair over 2 years, with 35 women followed for a mean of 1.6 years. Incontinent patients had a Kelly plication performed at the time of vaginal paravaginal repair. Recurrence rates were 3% for cystocele, 14% for rectocele, and 20% for enterocele.
Young et al18 followed 100 women for as long as 36 months after bilateral paravaginal repair using 1 to 6 expanded polytetrafluoroethylene (Gore-Tex) CV-0 sutures and midline colporrhaphy. Two patients had grade 1 or 2 failure at the lateral fixation points, but 21 patients had recurrent midline defects, all but 1 inside the hymen. Several patients had bloody discharge from the permanent sutures.
Sacrocolpopexy. Brubaker19 retrospectively reviewed 65 women who underwent sacrocolpopexy for apical prolapse. Three months postoperatively 19 patients (29%) had persistent anterior wall defects.
Uterosacral suspension. Shull et al20 also found the anterior segment to have the most recurrent defects. In that study, which had an average follow-up of a little over a year, 289 patients underwent vaginal uterosacral ligament repair of the apex, and 264 had an anterior wall defect preoperatively. At the time of furthest follow-up, 26 patients (9%) had failure at this site. This study confirmed that the anterior compartment is the most likely site to fail, and also that it fails the quickest.
Sacrospinous ligament suspension (SSLS). Morley and DeLancey21 found a 22% cystocele recurrence rate in 71 women 1 year after SSLS, with most of them asymptomatic. Shull22 reported a 30% incidence of cystoceles after SSLS. Paraiso and colleagues23 reported on 243 women undergoing SSLS and pelvic reconstructive surgery. Of these, 217 patients underwent concomitant anterior colporrhaphy. Follow-up at 74 months found 37% with symptomatic recurrence at the anterior wall, 13% at the posterior wall, and 8% at the apex.
Vaginal versus abdominal repair
Few studies have compared vaginal and abdominal repair of pelvic organ prolapse, including anterior wall prolapse.
In a trial by Benson et al,24 women with prolapse to or beyond the hymen were randomized to bilateral sacrospinous vault suspension and vaginal paravaginal repair (n = 48) or abdominal sacrocolpopexy with abdominal paravaginal repair (n = 40). One third of patients in each group also underwent anterior colporrhaphy. After a mean follow-up of 2.5 years, 16 of 20 women required reoperation for recurrent cystocele—12 (29%) from the vaginal group and 4 (10.5%) from the abdominal group. Vaginal vault eversion recurred in 5 women from the vaginal group and 1 from the abdominal group.
Investigators concluded that the anterior wall was the most likely site of failure because of the posterior placement of the vaginal apex with SSLS, predisposing the anterior wall to greater pressures and to neuropathy caused by lateral dissection of the anterior wall. Earlier studies have demonstrated that neuropathy may occur after extensive dissection of the vaginal wall and may affect the strength and integrity of the muscular support tissues.25,26
Allografts and xenografts
The difficulty of repairing anterior wall prolapse has led some pelvic surgeons to use mesh for cystocele repair. When Julian27 randomized 24 patients with recurrent cystocele to transvaginal repair with and without polypropylene (Marlex) mesh, 4 patients in the control group and no patients in the mesh group had recurrences (P <.05). However, 3 patients (25%) had mesh-related complications.
Weber et al28 randomized patients to standard midline plication, plication of the paravaginal tissue more laterally, or standard plication plus polyglactin 910 (Vicryl) mesh. Among 83 patients who returned for follow-up, there were no differences in anatomic outcome. Weber and colleagues concluded that there is little benefit to using mesh to correct cystoceles.
Still, although the overall cure rate was low (30–46%), most patients had cystocele to the hymen and not beyond, with significant improvement of symptoms. Although this cannot be defined as an anatomic cure, it is encouraging that the majority of patients appear to have benefited from surgery.
Sand et al29 randomized 161 women with the anterior wall to or beyond the hymen to traditional anterior colporrhaphy with or without Vicryl. The 2-inch square mesh was not placed over the repair as described above, but was folded into the anterior colporrhaphy stitches. At 1 year, 16 (22%) of 73 women with mesh and 28 (40%) of the 88 women without mesh had recurrent central cystoceles beyond the midvagina (P = .02). No women had cystoceles beyond the hymen or vaginal erosions.
Difficulty of interpreting the evidence
Because of the broad range of study designs, small number of patients per series, variety of concomitant procedures, and wide range of variables used to describe recurrence and success, it is difficult to draw conclusions from the literature. The evidence does suggest that the risks of wide vaginal dissection required for vaginal paravaginal repair outweigh the benefits. As a result, we have abandoned this technique. As mentioned above, it remains unclear whether graft materials will prove to be of long-term benefit for either midline plication or paravaginal repair.
The gold standard, for now
Prolapse of the anterior vaginal wall remains a challenge for the gynecologic surgeon. Careful preoperative and intraoperative evaluation and identification of support defects should guide repairs.
Randomized, controlled trials of midline versus paravaginal repair, as well as use of various graft materials, are greatly needed. These studies should not only address recurrence of prolapse symptoms, but the impact of surgery on sexual and lower urinary tract function.
At this time, the traditional anterior colporrhaphy with attention to apical suspension remains the gold standard.
The authors report no financial relationships relevant to this article.
- At this time, the traditional anterior colporrhaphy with attention to apical suspension remains the gold standard.
- If only some defects of the anterior wall are addressed at the time of reconstructive surgery, failure may be more likely.
- Women with grade 3 or 4 cystoceles often have evidence of bladder outlet obstruction on urodynamic testing.
- In 52% of cases, cystoceles coexist with detrusor instability and evidence of impaired detrusor contractility.
- A thorough preoperative evaluation includes assessing the apex, having the patient strain to maximize the defect, looking for paravaginal detachments, and making every effort to “unmask” occult stress urinary incontinence.
Ask a pelvic reconstructive surgeon to name the most difficult challenge, and the answer is likely to be anterior vaginal wall prolapse. The reason: The anterior wall usually is the leading edge of prolapse and the most common site of relaxation or failure following reconstructive surgery. This appears to hold true regardless of surgical route or technique.
Short-term success rates of anterior wall repairs appear promising, but long-term outcomes are not as encouraging. Success usually is claimed as long as the anterior wall is kept above the hymen, since the patient rarely reports symptoms in these cases.
Another challenge involves the use of allografts or xenografts, which have not undergone sufficient study to determine their long-term benefit or risks in comparison with traditional repairs.
This article reviews anatomy of the anterior vaginal wall and its supports, as well as surgical technique and outcomes.
Why the anterior wall is more susceptible to prolapse
One theory is that, in comparison with the posterior compartment, the anterior wall is not as well supported by the levator plate, which counters the effects of gravity and abdominal pressure. Normally, the anterior wall rests horizontally on the posterior wall, which in turn rests on the levator plate. When the levator muscles weaken, the anterior wall is the first to fall as increasing force is placed on the connective tissue supports.
Other possibilities: The anterior compartment’s attachments to the pelvic sidewall or apex may be weaker, the anterior wall may be more elastic or less dense than the posterior wall, and the anterior wall may be more susceptible to damage during childbirth or to the effects of age and loss of estrogen.
If only some defects are addressed at surgery, failure may be more likely. Some experts believe pelvic surgeons have focused too much attention on the urethrovesical junction in patients with concomitant urinary incontinence and not enough attention on suspending the anterior wall at the apex.
For most women, it is probably a combination of many of these factors that renders the anterior compartment so vulnerable.
Anatomy of the pelvic floor
The anterior vaginal wall resembles a trapezoidal plane due to ventral and more medial attachments near the pubic symphysis, and dorsal and more lateral attachments near the ischial spine (FIGURE 1).1 This helps explain the many appearances of the cystocele. The type of cystocele is defined by the location of the break in the fascial attachments.
Paravaginal defects. The trapezoidal anterior wall is suspended on both sides from the parietal fascia overlying the levator ani muscles at the arcus tendineus fascia pelvis (ATFP). Prolapse can occur when there is loss of attachment to the pelvic sidewall at any point between the pubis and ischial spine.
First described by White2 and characterized later by Richardson et al,3 this loss of lateral attachment is called a paravaginal defect or displacement cystocele (FIGURE 2). The goal of paravaginal repair is to reattach the lateral vaginal walls to the ATFP, either abdominally, laparoscopically, or vaginally.
Central defects, the rarest type of anterior wall prolapse, involve a loss of support near the pubis and tend to be smaller. The most common manifestation is urethral hypermobility.
Transverse defects occur when the top of the pubocervical fascia detaches from the cervix or vaginal apex, both of which are suspended from the uterosacral-cardinal ligament complex. A transverse cystocele is evidenced by loss of the anterior fornix. The anterior wall appears to be attenuated in the midline, and the vaginal mucosa is pale, thin, and smooth (FIGURE 3).
Goals of traditional repair. The traditional anterior colporrhaphy aims to excise or reinforce the attenuated transverse defect with plication of the “endopelvic fascia” in the midline of the anterior vaginal wall. The endopelvic fascia is not true fascia but the muscularis of the vaginal wall. It is comprised of smooth muscle and elastin along with the collagenous adventitia layer.4
The importance of restoring apical wall support becomes apparent when one considers the trapezoidal anatomy. The most common sites of defects or detachments of the anterior wall are near the ischial spines laterally. In an operative case series of paravaginal defects, DeLancey1 found the site of defect to be near the ischial spine in 96% of cases. The reattachment of the apex near the level of the spine becomes the highest point of support for the anterior vaginal wall.
This cephalad apical attachment can be accomplished in a variety of ways, by suspending the vaginal apex from the uterosacral ligaments, from the sacrospinous ligament, or via abdominal sacrocolpopexy.
FIGURE 1 Anatomy of the anterior wall
The anterior vaginal wall resembles a trapezoidal plane, with ventral and more medial attachments near the pubic symphysis, and dorsal and more lateral attachments to the ischial spine. Detachment from the pelvic sidewall and ischial spine results in anterior wall prolapse (right).
FIGURE 2 Paravaginal defect
FIGURE 3 Transverse defect
A transverse defect with loss of the anterior fornix. The loss of cephalad apical attachment at the level of the ischial spine leads to anterior wall prolapse. Suspending the upper vagina from shortened cardinal/uterosacral ligaments, the sacrospinous ligament, or via abdominal sacrocolpopexy is as important as plication.
Symptoms of anterior wall prolapse
As with other forms of pelvic organ prolapse, many patients complain of a bulge or feeling of pelvic pressure when the anterior vaginal wall has come through the introitus. However, some symptoms of anterior wall prolapse are unique.
Incontinence is not universal. A common misperception is that most patients with cystocele also experience stress urinary incontinence (SUI), which can develop when there is loss of urethral support and descent of the lower vaginal wall along with urethral hypermobility. However, there is no defining degree of hypermobility that links anterior wall prolapse with SUI. That is because the continence mechanism relies not only on urethral position and lateral attachments, but also on the neuromuscular function of the pelvis and lower urinary tract.
In fact, descent of the midvagina under the bladder base may actually reduce the chance of SUI. The reason: As a woman strains, the increased abdominal pressure pushes the cystocele farther and farther out. As the cystocele enlarges, it creates a functional outlet obstruction by kinking the urethra shut. When this is the case, patients may complain of prolonged voiding, an intermittent urine stream, and/or urinary retention. The woman may have to elevate the vaginal wall to empty her bladder. Patients with chronic urinary retention are at risk of developing recurrent urinary tract infections.
Bladder outlet obstruction and detrusor dysfunction. Women with grade 3 or 4 cystoceles often have evidence of bladder outlet obstruction on urodynamic testing, according to a study that found such evidence in 57% of subjects.5 After reduction of the prolapse with a pessary, obstructed flow reverted to normal in 94% of these women.
A large proportion (52%) of women with cystoceles also have detrusor instability, as well as evidence of impaired detrusor contractility. Many complain of urinary frequency and urgency and difficulty emptying the bladder.5
Again, this phenomenon is complex, related not only to anatomy but to altered neuromuscular function of the lower urinary tract. Incomplete emptying, frequency, and urgency may arise from stretching of the bladder base as it prolapses through the vaginal introitus, resulting in urinary retention. These symptoms often are less pronounced at night when the patient is supine.
We reviewed 35 cases of anterior wall prolapse greater than 1 cm outside the hymen, with elevated postvoid residuals exceeding 100 cc on 2 separate occasions.6 Thirty-one (89%) had normal postvoid residuals following reconstructive surgery and correction of their anterior wall prolapse.
Preoperative assessment
A careful physical exam is a prerequisite for all surgical repairs of pelvic organ prolapse. During this exam, identify the sites of defects and detachments.
Maximize the defect. Have the patient perform the Valsalva maneuver, cough, and/or strain while sitting upright or standing. As she is performing these maneuvers, ask her if this feels like her maximum prolapse. A split speculum often aids in visualizing the anterior and posterior compartments without pressure from the opposite vaginal wall.
Assess the apex. Place a large swab in the vagina, hold it gently against the apex, and ask the patient to strain. If the swab is pushed out, the apex needs suspension.
This technique can help identify apical relaxation that may be masked by a large anterior or posterior wall defect. A standardized staging system, such as the Pelvic Organ Prolapse Quantitative Examination (POP-Q) or Baden-Walker, aids in communicating and documenting the prolapse. In addition, it allows the surgeon to track anatomical outcomes after surgery.
Look for paravaginal defects by supporting the lateral anterior walls with a ring forceps at the level of the ATFP. Barber et al7 found this maneuver to be highly sensitive (90–94%): If no paravaginal defect was suspected clinically, none was found intraoperatively. However, the positive predictive value was poor (57%), in that defects suspected preoperatively were confirmed during surgery in less than two thirds of patients.
These findings point to the importance of careful intraoperative assessment, both before and during the repair procedure.
Limited utility of imaging studies. The use of radiologic studies such as defecography or dynamic magnetic resonance imaging of the pelvis may aid in the evaluation of defecatory disorders or suspected sigmoidocele or rectal prolapse, but have not been studied sufficiently to determine the impact on surgical outcome.
Unmasking SUI
As mentioned above, women with anterior wall prolapse do not always complain of stress incontinence. However, correction of the cystocele can relieve their obstructive voiding and unmask “occult” SUI. Various techniques have been described to elevate the anterior wall with pessaries, swabs, etc, during urodynamic testing to predict which women should have an incontinence procedure performed at the time of reconstructive surgery.
Conflicting rates of occult SUI have been reported, with estimates ranging from 36% to 80%.8 Although preoperative urodynamic testing indicates a high rate of occult stress incontinence, a study by Borstad et al9 suggests that the rate of de novo incontinence may be lower and that preoperative urodynamic findings are not predictive of postoperative continence status. In that study, 16 of 73 women (22%) developed stress incontinence following surgery for prolapse when no incontinence procedure was performed. Advanced age increased the risk of incontinence after surgery.
Contrast these findings with those of Chaikin and colleagues,10 who prospectively followed 24 patients with grade 3 or 4 cystoceles. Preoperative urodynamics showed a 58% rate of occult stress incontinence. All these patients were also defined as having intrinsic sphincter deficiency with leak point pressures below 60 cm water. The incontinent group underwent anterior colporrhaphy and concomitant pubovaginal sling, compared with anterior colporrhaphy alone for those without incontinence. Postoperatively, 2 patients who had the pubovaginal sling procedure reported continued stress incontinence (14%). No new symptoms of incontinence were reported in the patients without leakage on preoperative urodynamics. Thus, preoperative urodynamics were 100% accurate in determining which women did not need additional surgery for SUI.
Implications of a negative stress test. Our experience has shown that, despite our best attempts, a negative stress test with the prolapse reduced prior to surgery is less than 100% predictive. Occasionally, new SUI occurs after reconstructive surgery. It is unclear whether this incontinence is caused by straightening the urethra and reducing the bulge or secondary to the dissection of surgery.
Tips on technique
Anterior colporrhaphy traditionally is performed with plication of the “endopelvic fascia” or fibromuscular layer at the bladder neck with a Kelly plication stitch. Using “3-point” traction aids in dissecting the muscularis (FIGURE 4). Repair the remainder of the cystocele using vertical mattress stitches (1 or 2 layers) from the bladder neck to the apex.
Avoid creating weak areas. Using this technique, the repair frequently stops short of the apex, leaving a “gap” or weak area. One way to avoid this is to begin plication at the apex instead of the bladder neck (FIGURE 5).
Next, excise the excess vaginal tissue and close with interrupted fine absorbable sutures (FIGURE 6).
Recreate apical support. Another problem with traditional repairs is that they do not reestablish apical support. In many patients with anterior wall prolapse, reattachment of the apex reduces the cystocele. Therefore, it often is necessary to combine anterior colporrhaphy with an apical repair procedure such as uterosacral ligament suspension or sacrospinous ligament suspension.
Sutures for the apical repair should be placed and held prior to initiating the anterior colporrhaphy. At the end of the anterior repair, incorporate the apical sutures into the vaginal cuff.
Careful attention to the integrity and strength of the tissue is crucial. Regardless of the type of transvaginal suspension, we advocate bringing 1 arm of the suspension suture through the anterior wall of the cuff. Then place the other suture arm through the posterior cuff so that, when tied, anterior and posterior walls are brought together and suspended.
Using prolonged-delayed absorbable suture allows for a full-thickness bite, ensuring scarring to the suspensory ligament. If permanent suture is used for the uterosacral suspension, place the stitches along the inside surface of the anterior wall with a strong, broad bite that incorporates the muscularis or “endopelvic fascia.”
The occasional enterocele. When a transverse cystocele occurs following hysterectomy, the surgeon should be on the lookout for an enterocele, which sometimes accompanies anterior wall prolapse. The enterocele should be corrected at the time of surgery by closing the defect and suspending the cuff.
FIGURE 4 Three-point traction
Three-point traction using Allis clamps. The assistant retracts with DeBackey forceps to allow dissection of the muscularis. An index finger placed firmly against the vaginal mucosa enables the surgeon to judge depth of dissection.
FIGURE 5 Begin plication at the apex
Plication begins at the apex with vertical mattress stitches. Use 3-0 prolonged delayed absorbable or permanent suture in the anterior wall.
FIGURE 6 The reduced cystocele
The cystocele reduced following midline plication of the vaginal muscularis. The excess vagina is then trimmed and closed with interrupted 3-0 absorbable suture.
Functional surgical outcomes
Because of the long association between anterior wall prolapse and SUI, most surgeons evaluate patients preoperatively to determine the need for concomitant incontinence procedures. As a result, the literature reporting surgical cystocele repair via anterior colporrhaphy frequently uses continence of urine as the functional outcome. This is not surprising considering that anterior repair and Kelly plication, as reported by Howard Kelly more than 75 years ago, have been the gold standard for surgical correction of anterior wall prolapse and SUI.
In a series of 194 SUI patients who underwent anterior colporrhaphy, Beck11 found that adding a modified Kelly plication, including a vaginal retropubic urethropexy, increased the cure rate for SUI from 75% to 94%. Unfortunately, he did not report the anatomic success of the anterior colporrhaphy.
Kohli et al12 also retrospectively examined patients who had undergone anterior colporrhaphy with and without needle bladder-neck suspension. Although the cure rate for SUI was not reported, patients who underwent concomitant needle suspension had a higher rate of recurrent cystocele: 33% (n = 40) versus 7% (n = 27). Investigators theorized that retropubic dissection at the time of transvaginal needle suspension resulted in an iatrogenic paravaginal defect and denervation of the anterior vaginal wall.
The risks of needle suspension. A randomized controlled trial by Bump et al8 also suggests that needle suspension should be avoided. In that trial, 29 patients with stage 3 and 4 prolapse were randomized to needle colposuspension or endopelvic fascia plication. They, too, found that needle colposuspension carried a higher rate of recurrent anterior prolapse. Further, it did not reduce the rates of SUI compared with fascia plication.
Although incontinence surgery performed at the time of cystocele repair will reduce the rates of de novo incontinence, the higher rates of cystocele recurrence associated with some procedures warrants judicious preoperative planning. Clearly, needle suspension should not be performed as an incontinence procedure or repair of anterior wall prolapse. Whether other vaginal incontinence procedures, eg, midurethral slings, lead to recurrence of anterior wall prolapse deserves further investigation.
Anatomic outcomes
Midline colporrhaphy. Because anterior colporrhaphy is rarely performed alone, few series describe patients having undergone simply an anterior repair. Stanton et al13 followed 54 women for up to 2 years after they underwent traditional midline plication with vaginal hysterectomy for prolapse. Eight (15%) of the women had recurrent anterior wall prolapse.
Colombo et al14 randomized 71 women with clinical SUI and stage 2 or 3 prolapse to Burch colposuspension or anterior colporrhaphy with Kelly plication. All women were followed for at least 8 years. The cure rate for SUI was 86% for the Burch procedure, compared with 52% for anterior repair and Kelly plication. However, 12 (34%) women treated with Burch colposuspension and 1 (3%) treated with anterior colporrhaphy had recurrent cystocele of grade 2 or 3 with or without prolapse at other vaginal sites.
Abdominal paravaginal repair. Shull15 followed 149 women for 6 months to 4 years after they underwent abdominal paravaginal repair with the urethrovesical stitch brought through Cooper’s ligament for treatment of SUI and paravaginal cystocele. He reported a 5% recurrence of anterior wall prolapse.
In another series, Shull16 reported on 62 women who were followed for a mean of 18 months after abdominal paravaginal repair. Four of 57 (7%) had recurrent vaginal prolapse to the hymen.
In a cohort of 102 patients evaluated with the Pelvic Organ Prolapse Quantitative Examination (POP-Q) at our institution, the maximal point of prolapse was the anterior wall in 60% of cases; the apex and posterior wall each accounted for roughly half the remaining cases (unpublished data).
Ellerkmann et al30 reported on 237 consecutive patients who presented with symptoms of pelvic organ prolapse. In 77 women (33%), anterior compartment pelvic organ prolapse predominated; 46 patients (19%) had posterior compartment prolapse; and 22 patients (11%) had apical prolapse.
Hendrix et al31 analyzed patients from the Women’s Health Initiative (WHI) and found that the anterior compartment predominated over the posterior compartment. In a follow-up study from the WHI, Handa32 reported on 412 women followed for 2 to 8 years. Among those who entered the WHI protocol without cystocele, 1 in 4 was diagnosed with it at some point in the study. This compares to 1 in 6 for rectocele and 1 in 100 for uterine prolapse. The majority of all defects were grade 1 or relaxation above the hymen.
Vaginal paravaginal repair. Mallipeddi et al17 reported on 45 patients undergoing vaginal paravaginal repair over 2 years, with 35 women followed for a mean of 1.6 years. Incontinent patients had a Kelly plication performed at the time of vaginal paravaginal repair. Recurrence rates were 3% for cystocele, 14% for rectocele, and 20% for enterocele.
Young et al18 followed 100 women for as long as 36 months after bilateral paravaginal repair using 1 to 6 expanded polytetrafluoroethylene (Gore-Tex) CV-0 sutures and midline colporrhaphy. Two patients had grade 1 or 2 failure at the lateral fixation points, but 21 patients had recurrent midline defects, all but 1 inside the hymen. Several patients had bloody discharge from the permanent sutures.
Sacrocolpopexy. Brubaker19 retrospectively reviewed 65 women who underwent sacrocolpopexy for apical prolapse. Three months postoperatively 19 patients (29%) had persistent anterior wall defects.
Uterosacral suspension. Shull et al20 also found the anterior segment to have the most recurrent defects. In that study, which had an average follow-up of a little over a year, 289 patients underwent vaginal uterosacral ligament repair of the apex, and 264 had an anterior wall defect preoperatively. At the time of furthest follow-up, 26 patients (9%) had failure at this site. This study confirmed that the anterior compartment is the most likely site to fail, and also that it fails the quickest.
Sacrospinous ligament suspension (SSLS). Morley and DeLancey21 found a 22% cystocele recurrence rate in 71 women 1 year after SSLS, with most of them asymptomatic. Shull22 reported a 30% incidence of cystoceles after SSLS. Paraiso and colleagues23 reported on 243 women undergoing SSLS and pelvic reconstructive surgery. Of these, 217 patients underwent concomitant anterior colporrhaphy. Follow-up at 74 months found 37% with symptomatic recurrence at the anterior wall, 13% at the posterior wall, and 8% at the apex.
Vaginal versus abdominal repair
Few studies have compared vaginal and abdominal repair of pelvic organ prolapse, including anterior wall prolapse.
In a trial by Benson et al,24 women with prolapse to or beyond the hymen were randomized to bilateral sacrospinous vault suspension and vaginal paravaginal repair (n = 48) or abdominal sacrocolpopexy with abdominal paravaginal repair (n = 40). One third of patients in each group also underwent anterior colporrhaphy. After a mean follow-up of 2.5 years, 16 of 20 women required reoperation for recurrent cystocele—12 (29%) from the vaginal group and 4 (10.5%) from the abdominal group. Vaginal vault eversion recurred in 5 women from the vaginal group and 1 from the abdominal group.
Investigators concluded that the anterior wall was the most likely site of failure because of the posterior placement of the vaginal apex with SSLS, predisposing the anterior wall to greater pressures and to neuropathy caused by lateral dissection of the anterior wall. Earlier studies have demonstrated that neuropathy may occur after extensive dissection of the vaginal wall and may affect the strength and integrity of the muscular support tissues.25,26
Allografts and xenografts
The difficulty of repairing anterior wall prolapse has led some pelvic surgeons to use mesh for cystocele repair. When Julian27 randomized 24 patients with recurrent cystocele to transvaginal repair with and without polypropylene (Marlex) mesh, 4 patients in the control group and no patients in the mesh group had recurrences (P <.05). However, 3 patients (25%) had mesh-related complications.
Weber et al28 randomized patients to standard midline plication, plication of the paravaginal tissue more laterally, or standard plication plus polyglactin 910 (Vicryl) mesh. Among 83 patients who returned for follow-up, there were no differences in anatomic outcome. Weber and colleagues concluded that there is little benefit to using mesh to correct cystoceles.
Still, although the overall cure rate was low (30–46%), most patients had cystocele to the hymen and not beyond, with significant improvement of symptoms. Although this cannot be defined as an anatomic cure, it is encouraging that the majority of patients appear to have benefited from surgery.
Sand et al29 randomized 161 women with the anterior wall to or beyond the hymen to traditional anterior colporrhaphy with or without Vicryl. The 2-inch square mesh was not placed over the repair as described above, but was folded into the anterior colporrhaphy stitches. At 1 year, 16 (22%) of 73 women with mesh and 28 (40%) of the 88 women without mesh had recurrent central cystoceles beyond the midvagina (P = .02). No women had cystoceles beyond the hymen or vaginal erosions.
Difficulty of interpreting the evidence
Because of the broad range of study designs, small number of patients per series, variety of concomitant procedures, and wide range of variables used to describe recurrence and success, it is difficult to draw conclusions from the literature. The evidence does suggest that the risks of wide vaginal dissection required for vaginal paravaginal repair outweigh the benefits. As a result, we have abandoned this technique. As mentioned above, it remains unclear whether graft materials will prove to be of long-term benefit for either midline plication or paravaginal repair.
The gold standard, for now
Prolapse of the anterior vaginal wall remains a challenge for the gynecologic surgeon. Careful preoperative and intraoperative evaluation and identification of support defects should guide repairs.
Randomized, controlled trials of midline versus paravaginal repair, as well as use of various graft materials, are greatly needed. These studies should not only address recurrence of prolapse symptoms, but the impact of surgery on sexual and lower urinary tract function.
At this time, the traditional anterior colporrhaphy with attention to apical suspension remains the gold standard.
The authors report no financial relationships relevant to this article.
1. DeLancey JOL. Fascial and muscular abnormalities in women with urethral hypermobility and anterior vaginal wall prolapse. Am J Obstet Gynecol. 2002;187:93-98.
2. White GR. Cystocele: a radical cure by suturing lateral sulci of vagina to white line of pelvic fascia. JAMA. 1909;21:1707-1710.
3. Richardson AC, Lyon JB, Williams NL. A new look at pelvic relaxation. Am J Obstet Gynecol. 1976;126:568-571.
4. Weber AM, Walters MD. Anterior vaginal prolapse: review of anatomy and techniques of surgical repair. Obstet Gynecol. 1997;89:311-318.
5. Romanzi LJ, Chaikin DC, Blaivas JG. The effect of genital prolapse on voiding. J Urol. 1999;161:581-586.
6. FitzGerald MP, Kulkarni N, Fenner D. Postoperative resolution of urinary retention in patients with advanced pelvic organ prolapse. Am J Obstet Gynecol. 2000;183:1361-1364.
7. Barber MD, Cundiff GW, Weidner AC, Cotes KW, Bump RC, Addison WA. Accuracy of clinical assessment of paravaginal defects in women with anterior vaginal wall proloapse. Am J Obstet Gynecol. 1999;181:1-7.
8. Bump RC, Hurt WG, Theofrastous JP, et al. Randomized prospective comparison of needle colposuspension versus endopelvic fascia plication for potential stress incontinence prophylaxis in women undergoing vaginal reconstruction for stage III or IV pelvic organ prolapse. The Continence Program for Women Research Group. Am J Obstet Gynecol. 1996;175:326-333.
9. Borstad E, Rud T. The risk of developing urinary stress incontinence after vaginal repair in continent women. Acta Obstet Gynecol Scand. 1989;68:545-549.
10. Chaikin DC, Groutz A, Blaivas JG. Predicting the need for anti-incontinence surgery in continent women undergoing repair of severe urogenital prolapse. J Urol. 2000;163:531-534.
11. Beck RP, McCormick S, Nordstrom L. A 25-year experience with 519 anterior colporrhaphy procedures. Obstet Gynecol. 1991;78:1011-1018.
12. Kohli N, Sze EHM, Roat TW, Karram M. Incidence of recurrent cystocele after anterior colporrhaphy with and without concomitant transvaginal needle suspension. Am J Obstet Gynecol. 1996;175:1476-1482.
13. Stanton SL, Hilton P, Norton C, Cardozo L. Clinical and urodynamics effect of anterior colporrhaphy and vaginal hysterectomy for prolapse with and without incontinence. Br J Obstet Gynecol. 1982;89:459-463.
14. Colombo M, Vitobello D, Proiette F, Milani R. Randomized comparison of Burch colposuspension versus anterior colporrhaphy in women with stress urinary incontinence and anterior vaginal wall prolapse. BJOG. 2000;107:544-551.
15. Shull BL, Baden WF. A six-year experience with paravaginal defect repair for stress urinary incontinence. Am J Obstet Gynecol. 1989;160:1432-1440.
16. Shull BL, Benn SJ, Kuehl TJ. Surgical management of prolapse of the anterior vaginal segment: an analysis of support defects, operative morbidity, and anatomic outcome. Am J Obstet Gynecol. 1994;171:1429-1439.
17. Mallipeddi PK, Steele AC, Kohli N, Karram MM. Anatomic and functional outcome of vaginal paravaginal repair in the correction of anterior wall prolapse. Int Urogynecol J. 2001;12:83-88.
18. Young SB, Daman JJ, Bony LG. Vaginal paravaginal repair: one-year outcomes. Am J Obstet Gynecol. 2001;185:1360-1367.
19. Brubaker L. Sacrocolpopexy and the anterior compartment: support and function. Am J Obstet Gynecol. 1995;173:1690-1694.
20. Shull BL, Bachofen C, Coates KW, Kuehl TJ. A transvaginal approach to repair of apical and other associated sites of pelvic organ prolapse with uterosacral ligaments. Am J Obstet Gynecol. 2000;183:1365-1374.
21. Morley GW, DeLancey JOL. Sacrospinous ligament fixation for eversion of the vagina. Am J Obstet Gynecol. 1988;158:872-881.
22. Shull BL, Capen CV, Riggs MW, Kuehl TJ. Preoperative and postoperative analysis of site-specific pelvic support defects in 81 women treated with sacrospinous ligament suspension and pelvic reconstruction. Am J Obstet Gynecol. 1992;166:1764-1771.
23. Paraiso MFR, Ballard LA, Walter MD, et al. Pelvic support defects and visceral and sexual function in women treated with sacrospinous ligament suspension and pelvic reconstruction. Am J Obstet Gynecol. 1996;175:1423-1431.
24. Benson JT, Lucente V, McClellan E. Vaginal versus abdominal reconstructive surgery for the treatment of pelvic support defects: a prospective randomized study with long-term outcome evaluation. Am J Obstet Gynecol. 1996;175:1418-1421.
25. Benson JT, McClellan E. The effect of vaginal dissection of the pudendal nerve. Obstet Gynecol. 1993;82:387-389.
26. Zivkovic F, Tamussino K, Ralph G, et al. Long-term effects of vaginal dissection on the innervation of the striated urethral sphincter. Obstet Gynecol. 1996;87:257-260.
27. Julian T. The efficacy of Marlex mesh in the repair of severe, recurrent vaginal prolapse of the anterior midvaginal wall. Am J Obstet Gynecol. 1996;175:1472-1475.
28. Weber AM, Walters MD, Piedmonte MR, et al. Anterior colporrhaphy: a randomized trial of three surgical techniques. Am J Obstet Gynecol. 2001;185:1299-1306.
29. Sand PK, et al. Prospective randomized trial of polyglactin 910 mesh to prevent recurrence of cystocele and rectocele. Am J Obstet Gynecol. 2001;184:1357-1364.
30. Ellerkmann RM, Cundiff GW, Melick CF, Nihira MA, Leffler K, Bent AE. Correlation of symptoms with location and severity of pelvic organ prolapse. Am J Obstet Gynecol. 2001;185:1332-1337.
31. Hendrix SL, Clark A, Nygaard I, Aragaki A, Barnabei V, McTiernan A. Pelvic organ prolapse in the Women’s Health Initiative: gravity and gravidity. Am J Obstet Gynecol. 2002;186:1160-1166.
32. Handa VL, Garrett E, Hendrix S, Gold E, Robbins J. Progression and remission of pelvic organ prolapse: a longitudinal study of menopausal women. Am J Obstet Gynecol. 2004;190:27-32.
1. DeLancey JOL. Fascial and muscular abnormalities in women with urethral hypermobility and anterior vaginal wall prolapse. Am J Obstet Gynecol. 2002;187:93-98.
2. White GR. Cystocele: a radical cure by suturing lateral sulci of vagina to white line of pelvic fascia. JAMA. 1909;21:1707-1710.
3. Richardson AC, Lyon JB, Williams NL. A new look at pelvic relaxation. Am J Obstet Gynecol. 1976;126:568-571.
4. Weber AM, Walters MD. Anterior vaginal prolapse: review of anatomy and techniques of surgical repair. Obstet Gynecol. 1997;89:311-318.
5. Romanzi LJ, Chaikin DC, Blaivas JG. The effect of genital prolapse on voiding. J Urol. 1999;161:581-586.
6. FitzGerald MP, Kulkarni N, Fenner D. Postoperative resolution of urinary retention in patients with advanced pelvic organ prolapse. Am J Obstet Gynecol. 2000;183:1361-1364.
7. Barber MD, Cundiff GW, Weidner AC, Cotes KW, Bump RC, Addison WA. Accuracy of clinical assessment of paravaginal defects in women with anterior vaginal wall proloapse. Am J Obstet Gynecol. 1999;181:1-7.
8. Bump RC, Hurt WG, Theofrastous JP, et al. Randomized prospective comparison of needle colposuspension versus endopelvic fascia plication for potential stress incontinence prophylaxis in women undergoing vaginal reconstruction for stage III or IV pelvic organ prolapse. The Continence Program for Women Research Group. Am J Obstet Gynecol. 1996;175:326-333.
9. Borstad E, Rud T. The risk of developing urinary stress incontinence after vaginal repair in continent women. Acta Obstet Gynecol Scand. 1989;68:545-549.
10. Chaikin DC, Groutz A, Blaivas JG. Predicting the need for anti-incontinence surgery in continent women undergoing repair of severe urogenital prolapse. J Urol. 2000;163:531-534.
11. Beck RP, McCormick S, Nordstrom L. A 25-year experience with 519 anterior colporrhaphy procedures. Obstet Gynecol. 1991;78:1011-1018.
12. Kohli N, Sze EHM, Roat TW, Karram M. Incidence of recurrent cystocele after anterior colporrhaphy with and without concomitant transvaginal needle suspension. Am J Obstet Gynecol. 1996;175:1476-1482.
13. Stanton SL, Hilton P, Norton C, Cardozo L. Clinical and urodynamics effect of anterior colporrhaphy and vaginal hysterectomy for prolapse with and without incontinence. Br J Obstet Gynecol. 1982;89:459-463.
14. Colombo M, Vitobello D, Proiette F, Milani R. Randomized comparison of Burch colposuspension versus anterior colporrhaphy in women with stress urinary incontinence and anterior vaginal wall prolapse. BJOG. 2000;107:544-551.
15. Shull BL, Baden WF. A six-year experience with paravaginal defect repair for stress urinary incontinence. Am J Obstet Gynecol. 1989;160:1432-1440.
16. Shull BL, Benn SJ, Kuehl TJ. Surgical management of prolapse of the anterior vaginal segment: an analysis of support defects, operative morbidity, and anatomic outcome. Am J Obstet Gynecol. 1994;171:1429-1439.
17. Mallipeddi PK, Steele AC, Kohli N, Karram MM. Anatomic and functional outcome of vaginal paravaginal repair in the correction of anterior wall prolapse. Int Urogynecol J. 2001;12:83-88.
18. Young SB, Daman JJ, Bony LG. Vaginal paravaginal repair: one-year outcomes. Am J Obstet Gynecol. 2001;185:1360-1367.
19. Brubaker L. Sacrocolpopexy and the anterior compartment: support and function. Am J Obstet Gynecol. 1995;173:1690-1694.
20. Shull BL, Bachofen C, Coates KW, Kuehl TJ. A transvaginal approach to repair of apical and other associated sites of pelvic organ prolapse with uterosacral ligaments. Am J Obstet Gynecol. 2000;183:1365-1374.
21. Morley GW, DeLancey JOL. Sacrospinous ligament fixation for eversion of the vagina. Am J Obstet Gynecol. 1988;158:872-881.
22. Shull BL, Capen CV, Riggs MW, Kuehl TJ. Preoperative and postoperative analysis of site-specific pelvic support defects in 81 women treated with sacrospinous ligament suspension and pelvic reconstruction. Am J Obstet Gynecol. 1992;166:1764-1771.
23. Paraiso MFR, Ballard LA, Walter MD, et al. Pelvic support defects and visceral and sexual function in women treated with sacrospinous ligament suspension and pelvic reconstruction. Am J Obstet Gynecol. 1996;175:1423-1431.
24. Benson JT, Lucente V, McClellan E. Vaginal versus abdominal reconstructive surgery for the treatment of pelvic support defects: a prospective randomized study with long-term outcome evaluation. Am J Obstet Gynecol. 1996;175:1418-1421.
25. Benson JT, McClellan E. The effect of vaginal dissection of the pudendal nerve. Obstet Gynecol. 1993;82:387-389.
26. Zivkovic F, Tamussino K, Ralph G, et al. Long-term effects of vaginal dissection on the innervation of the striated urethral sphincter. Obstet Gynecol. 1996;87:257-260.
27. Julian T. The efficacy of Marlex mesh in the repair of severe, recurrent vaginal prolapse of the anterior midvaginal wall. Am J Obstet Gynecol. 1996;175:1472-1475.
28. Weber AM, Walters MD, Piedmonte MR, et al. Anterior colporrhaphy: a randomized trial of three surgical techniques. Am J Obstet Gynecol. 2001;185:1299-1306.
29. Sand PK, et al. Prospective randomized trial of polyglactin 910 mesh to prevent recurrence of cystocele and rectocele. Am J Obstet Gynecol. 2001;184:1357-1364.
30. Ellerkmann RM, Cundiff GW, Melick CF, Nihira MA, Leffler K, Bent AE. Correlation of symptoms with location and severity of pelvic organ prolapse. Am J Obstet Gynecol. 2001;185:1332-1337.
31. Hendrix SL, Clark A, Nygaard I, Aragaki A, Barnabei V, McTiernan A. Pelvic organ prolapse in the Women’s Health Initiative: gravity and gravidity. Am J Obstet Gynecol. 2002;186:1160-1166.
32. Handa VL, Garrett E, Hendrix S, Gold E, Robbins J. Progression and remission of pelvic organ prolapse: a longitudinal study of menopausal women. Am J Obstet Gynecol. 2004;190:27-32.
Preventing BRCA-related cancers: The case for oophorectomy
- Mutations in BRCA1 and BRCA2 may be responsible for more than 90% of inherited predisposition to ovarian cancer.
- BRCA1 and BRCA2 mutations are associated with a lifetime risk of breast cancer of up to 85% and a 15% to 45% lifetime risk of ovarian cancer.
- The only prospective trial to date found risk-reducing salpingo-oophorectomy (RRSO) was associated with an 85% reduction in ovarian cancer and a 68% reduction in breast cancer.
- Because microscopic cancer may be found in 2% to 4% of RRSO specimens upon careful pathologic review, the ovaries and fallopian tubes should be sectioned in their entirety and examined by an experienced gynecologic pathologist.
When A.M. Liber encountered a family of 5 sisters and their mother with histologically confirmed papillary adenocarcinoma of the ovary, he recommended frequent gynecologic cancer screening for all family members and suggested prophylactic oophorectomy as an option.1 The year was 1950.
Flash forward half a century or so, and prophylactic oophorectomy has gained wider acceptance for the prevention of hereditary ovarian and breast cancer, with the only prospective trial to date confirming its overall efficacy for women with BRCA1 and BRCA2 mutations. These mutations are related to the vast majority of inherited ovarian cancers.
Using the evidence published thus far, including the recently published prospective trial, we discuss surgical technique, post-oophorectomy estrogens, psychosocial impact, insurance reimbursement, and other issues.
Three hereditary syndromes
The single biggest risk factor for ovarian cancer is a family history, although only about 10% of cases are believed to be due to an inherited predisposition. Three syndromes are associated with such a predisposition:
- Hereditary breast-ovarian cancer syndrome, caused by mutations in BRCA1 and BRCA2, is thought to be responsible for more than 90% of inherited predisposition to ovarian cancer.
- Hereditary nonpolyposis colon cancer (HNPCC) syndrome is associated with mutations in the mismatch repair genes and a greatly increased risk of cancers of the colon, endometrium, ovaries, and urinary tract. HNPCC accounts for about 2% of inherited ovarian cancers.
- A syndrome of site-specific ovarian cancer also has been proposed, though we lack conclusive evidence that it exists as a separate entity at the genetic level.
How BRCA mutations lead to cancer
BRCA1 and BRCA2 are tumor suppressor genes that play a role in genomic stability and double-stranded DNA break repair. BRCA1 is located on chromosome 17; BRCA2 on chromosome 13. Both genes function as classic tumor suppressors, as described by Knudson.2 Only a single working copy of each gene is needed for the genes to effectively suppress tumors.
In patients with no inherited mutation in these genes, carcinogenesis caused by dysfunction of this pathway can occur only if both working copies of the gene are lost. In contrast, women with an inherited mutation in BRCA1 or BRCA2 start out with only a single working copy of the gene. If any cell loses this single copy, DNA repair cannot occur via this pathway, and cancer can develop.
These repair pathways seem to be particularly important in dividing breast and ovarian cells. This explains why women with inherited mutations in these genes develop cancers more frequently and at an earlier age.
Quantifying the risk
Specific risks associated with BRCA1 and BRCA2 mutations include:
- a lifetime risk of breast cancer of up to 85%, with half of these cancers occurring prior to age 50
- a 15% to 45% lifetime risk of ovarian cancer3,4
Mutations in these genes can be inherited from a mother or father. In the general population, between 1 in 385 and 1 in 800 individuals carry a deleterious mutation in either BRCA1 or BRCA2.
In certain populations, such as Icelandic, French Canadian, or Eastern European Jewish populations, founder effects can contribute to a greatly increased frequency of mutation. For example, the Eastern European Jewish population, from which approximately 90% of North American Jews are descended, has one of the highest known frequencies of BRCA1 and BRCA2 mutation: 1 in 40 individuals carries a deleterious mutation in 1 of these 2 genes.5,6
Most evidence is historical or retrospective
Liber was not the first to suggest oophorectomy to impact the risk of breast or ovarian cancer: The procedure was initially proposed by Schinziner in 1889 as a treatment for breast cancer.7 However, the earliest evidence that oophorectomy was performed as adjuvant therapy did not appear until 7 years later, in 1896 (reviewed by Love and Philips).8
In 1968, Feinleib9 reported that premenopausal oophorectomy decreased the rate of subsequent breast cancer. Twenty years later, Brinton suggested that prophylactic oophorectomy might reduce breast cancer risk in women with a family history of the disease.10
In the sole prospective trial, salpingo-oophorectomy was associated with a 75% reduction in breast and gynecologic cancer.
Post-oophorectomy cancers identified. Possible limitations to the strategy became apparent in the early 1980s, when Tobacman and colleagues11 reported adenocarcinoma histologically indistinguishable from ovarian cancer after oophorectomy in a series of women with a strong family history.
In 1993, Piver et al12 reported a series of 6 cases of primary peritoneal cancer after prophylactic oophorectomy in 324 women from hereditary ovarian cancer families.
In 1997, the Cancer Genetics Studies Consortium reviewed all available data and concluded: “There is insufficient evidence to recommend for or against prophylactic oophorectomy as a measure for reducing ovarian cancer risk. Women with BRCA1 mutations should be counseled that this is an option available to them. Those considering prophylactic oophorectomy should be counseled that cancer has been documented to occur after the procedure.”13
Although the Cancer Genetics Studies Consortium did not specifically comment on prophylactic oophorectomy in carriers of BRCA2 mutations, most authorities interpreted these recommendations to apply to these women as well.
Predicting life expectancy. After these findings, several groups undertook decision analyses to evaluate the effect of prophylactic oophorectomy on life expectancy in women with BRCA mutations. Schrag et al14 reported that prophylactic oophorectomy in a 30-year-old with a BRCA mutation increased life expectancy by 0.3 to 1.7 years. This compares to 0.9 years for adjuvant chemotherapy in node-negative breast cancer.
A subsequent report by Grann and colleagues15 also suggested that prophylactic oophorectomy was associated with an increased life expectancy of 0.4 to 2.6 years. However, surgery was not cost-effective for quality-adjusted life-years saved.
Investigators cite need for prospective studies. In 1999, Rebbeck and colleagues16 conducted a retrospective case-control study of 43 women with BRCA1 mutations who underwent oophorectomy and 79 age-matched women with BRCA1 mutations who had ovaries in situ. In this series, oophorectomy was associated with a 47% decreased risk of subsequent breast cancer (hazard ratio 0.53). However, several investigators cited the need for prospective studies before incorporating oophorectomy into routine clinical practice for the prevention of cancer.17
The first prospective look at risk-reducing surgery
It was in this setting that our group launched a prospective trial to determine whether salpingo-oophorectomy offers any benefit over surveillance in preventing breast and gynecologic (ovarian, fallopian tube, and peritoneal) cancers in women with BRCA mutations.18
Proportional hazard analysis demonstrated that salpingo-oophorectomy was associated with a 75% reduction in subsequent breast and gynecologic cancer incidence in women with BRCA mutations (hazard ratio 0.25, 95% confidence interval 0.08 to 0.74). When the individual endpoints of breast and gynecologic cancer were observed, risk-reducing salpingo-oophorectomy (RRSO) was associated with an 85% reduction in subsequent ovarian cancer and a 68% reduction in subsequent breast cancer.
Methods. From June 1995 through May 2001, we enrolled 265 women with documented BRCA1 or BRCA2 mutations. Patients were followed by annual questionnaire, telephone contact, and medical-record review. Pathology reports were obtained for all new cancers diagnosed during follow-up.
After excluding women who underwent bilateral salpingo-oophorectomy before genetic testing, who were younger than 35 years at the time of testing, or who did not provide any follow-up information, 173 women with ovaries at risk and a documented BRCA mutation remained. These women participated in formal pre- and post-test genetic counseling and received uniform recommendations for cancer risk reduction, as detailed in the TABLE.
During follow-up, we calculated the incidence of new breast and gynecologic cancers diagnosed in the cohort who elected RRSO and compared it with the incidence of these cancers in women who chose surveillance.
Salpingo-oophorectomy was elected by 101 of the 173 women.
Findings. In 3 of these women, early-stage ovarian or fallopian-tube cancer that had not been detected during preoperative evaluation was found at the time of surgery. In the remaining 98 patients who underwent RRSO, 1 peritoneal cancer and 3 breast cancers were diagnosed during a mean 23 months of follow-up. In the 72 women who chose surveillance, 5 ovarian or peritoneal cancers and 8 breast cancers were diagnosed in a mean 25 months of follow-up.
Kaplan-Meier analysis of time to breast or BRCA-related gynecologic cancer is illustrated in FIGURE 1.
Other studies confirm findings. A second retrospective study by Rebbeck et al19 was released simultaneously with our findings and showed similar benefits. They found a 53% reduction in subsequent breast cancer risk and a 96% reduction in subsequent ovarian cancer risk. In the summer of 2003, a study from Israel by Rutter et al provided further confirmation of the substantially decreased incidence of cancer following risk-reducing surgery.20
TABLE
Breast and ovarian cancer risk-reduction strategies for women with BRCA1 or BRCA2 mutations
| TYPE OF CANCER | STRATEGY | ALSO CONSIDER … |
|---|---|---|
| Breast | Monthly self-examination beginning at age 18 | Imaging Breast ultrasound or magnetic resonance imaging |
| 2-4 physician examinations per year, starting at age 25 | Risk–reducing surgery Mastectomy, no earlier than mid-20s | |
| Annual mammography beginning at age 25 | Salpingo-oophorectomy, after age 35 and completion of childbearing | |
| Chemoprevention Tamoxifen. Need to discuss conflicting reports on efficacy | ||
| Ovarian | CA 125 and ultrasound twice yearly, starting at age 35 | Salpingo-oophorectomy After age 35 and the completion of childbearing |
| Chemoprevention Oral contraceptives, though they may be associated with an increased risk of breast cancer | ||
| Source: Adapted from Scheuer et al28 | ||
FIGURE 1 Reduction in cancer cases associated with salpingo-oophorectomy
Reprinted with permission from Kauff ND et al.18
Copyright 2002 Massachusetts Medical Society. All rights reserved.
Good technique and pathologic review may prevent post-oophorectomy cancer
There are 3 theories about the origin of primary peritoneal cancer after oophorectomy:
- The cancer represents undetected occult cancer present at the time of risk-reducing surgery.
- It represents cancer arising in an ovarian remnant left behind after risk-reducing surgery.
- The peritoneal cancer arises de novo from the peritoneal surface epithelium.
Reasonable evidence supports each of these theories; thus, each may play some role in the incidence of “peritoneal” cancer after risk-reducing surgery.21-23
While surgical technique and detailed pathologic review are unlikely to decrease the incidence of de novo peritoneal cancer, they may play a substantial role in reducing ovarian and related cancers after risk-reducing surgery.
Surgical requirements. Obviously, if a surgery is to be risk-reducing, as much as possible of the tissue at risk should be removed. To do so effectively, the surgeon should be comfortable operating in the retroperitoneum so that the infundibulopelvic ligament can be ligated sufficiently proximal from the ovarian hilum to minimize the possibility of an ovarian remnant. Similarly, if a salpingo-oophorectomy without hysterectomy is to be performed, the fallopian tube should be amputated as close as possible to the uterine cornua (FIGURE 2).
Laparoscopy versus open surgery. RRSO can be performed using either a laparoscopic or open approach. The appropriate choice is best determined by the patient’s history, associated comorbid conditions, need for additional procedures, and experience of the surgeon. At our institution, in the absence of contraindications, we generally offer a laparoscopic approach due to its decreased morbidity.
Concomitant hysterectomy? An area of substantial controversy is whether the uterus should be removed at the time of RRSO. In most studies exploring this issue, hereditary breast-ovarian cancer syndrome does not appear to be associated with an increased risk of uterine cancer.24 However, there is concern that the portion of interstitial fallopian tube left behind after salpingo-oophorectomy may be at risk for malignant transformation.25,26
In our series, almost 90% of risk-reducing procedures were salpingo-oophorectomies without hysterectomy. If there is an additional benefit to concomitant hysterectomy, it has yet to be demonstrated by clinical trials.
In several studies, a patient’s level of anxiety was more important than objective cancer risk in the choice of RRSO.
Close pathologic scrutiny advised. Microscopic cancer may be found in 2% to 4% of RRSO specimens upon careful pathologic review.21,27,28 Thus, it is essential that the ovaries and fallopian tubes are sectioned in their entirety and examined by an experienced gynecologic pathologist to minimize the chance that microscopic cancer goes undetected.
It is not clear whether cytology should be routinely done at the time of risk-reducing surgery. A single report documents malignant cells in a woman with a BRCA1 mutation and no obvious foci of malignancy despite hysterectomy with bilateral salpingo-oophorectomy and staging.29 Pending further studies, we routinely send cytology for review.
FIGURE 2 Careful surgical ligation and division to eliminate residual tissue
Source: Devita VT Jr., Hellman S, Rosenberg SA, eds. Progress in Oncology 2003. 2004: Jones and Bartlett Publishers; Sudbury, Mass. Reprinted with permission.
When no BRCA mutation is present
Most of the data cited thus far apply to women with documented BRCA mutations. There is much less information about the relative risks and benefits of RRSO in women with a personal or family history of breast or ovarian cancer who lack a documented BRCA mutation.
Although RRSO may be appropriate for some of these women, in 2004 it is not the standard of care to recommend RRSO to all individuals with a personal or family history suggestive of an inherited predisposition to ovarian cancer. These patients are best managed by an interdisciplinary team of gynecologists, gynecologic oncologists, and clinical geneticists, all with experience caring for women who may have an inherited predisposition.
Is anxiety a factor?
We have limited information about the psychosocial impact of RRSO. Several studies have found that a patient’s level of anxiety is a more important factor than objective cancer risk in the decision to undergo RRSO.30,31 Unfortunately, we do not yet know whether the surgery successfully reduces these patients’ subjective concerns.
A recent study showed that risk-reducing surgery did not impair women’s overall health or psychological well-being.32 However, 20.7% of the women reported substantial cancer-related anxiety despite the risk-reducing surgery. This issue requires further investigation.
Is estrogen the best option for surgical menopause?
The role of hormone replacement after RRSO is unclear. The issue is important because many women considering salpingo-oophorectomy are in their late 30s or early 40s, when premature surgical menopause is a predictable result. Consequences can include considerable vasomotor and pelvic symptoms.
Preliminary data suggest that a woman’s satisfaction with RRSO depends in large part on its impact on sexual functioning.32 Urogenital symptoms that adversely affected sexual function, such as vaginal dryness and dyspareunia, were the most significant predictors of dissatisfaction with surgery.
Premature surgical menopause also has a substantial impact on osteoporosis risk, while its effect on heart disease remains uncertain.
While nonhormonal therapies can address each of these issues, we need more data on their long-term use. We counsel women considering RRSO that hormone replacement may be an option. We believe it is unlikely to reduce the efficacy of RRSO in preventing ovarian cancer, but it may reduce the protective effect of RRSO against subsequent breast cancer. Until further studies are available, we recommend that decisions regarding hormone replacement be individualized to the patient’s specific symptoms and personal history.
Not all insurers cover RRSO
One study explored insurance carriers’ policies about reimbursing risk-reducing surgical procedures and found that 10% to 11% of private insurers and 48% to 50% of governmental carriers had policies specifically denying coverage for such operations.
An additional 40% to 64% of insurers had no identifiable policy regarding these procedures in women with BRCA mutations.33 The authors speculated that, without identifiable policies, this critical health-care decision may be subject to arbitrary criteria that result in substantial variation.
When we recently investigated the reimbursement experience of women with BRCA mutations undergoing RRSO at our institution, we found that 97% of the procedures were reimbursed in full, less any applicable coinsurance and deductibles.34 Two important limitations of our study: It was conducted at a tertiary cancer center and was retrospective. It is not known if the findings reflect the experience of women with BRCA mutation who have risk-reducing surgery in other settings.
Unresolved issues
RRSO clearly has a role in preventing breast and ovarian cancer in women at inherited risk. However, several questions remain unanswered:
- Who is the best candidate?
- What is optimal timing of the procedure?
- What, if any, concomitant procedures should be performed?
- What is the role of hormone replacement after the surgery?
These issues will be best addressed through multicenter prospective trials, such as the one now being conducted by the Gynecologic Oncology Group.
Hope also remains that further research will improve serum and radiological detection of early ovarian cancer, and that basic research on the molecular etiology and progression of these cancers will ultimately render it unnecessary to remove organs at risk.
The authors report no financial relationships relevant to this article.
1. Liber AM. Ovarian cancer in a mother and five daughters. Arch Pathol. 1950;49:280-290.
2. Knudson AG, Jr. Mutation and cancer: statistical study of retinoblastoma. Proc Natl Acad Sci USA. 1971;68:820-823.
3. Ford D, Easton DF, Stratton M, et al. Genetic heterogeneity and penetrance analysis of the BRCA1 and BRCA2 genes in breast cancer families. The Breast Cancer Linkage Consortium. Am J Hum Genet. 1998;62:676-689.
4. Risch HA, McLaughlin JR, Cole DE, et al. Prevalence and penetrance of germline BRCA1 and BRCA2 mutations in a population series of 649 women with ovarian cancer. Am J Hum Genet. 2001;68:700-710.
5. Oddoux C, Struewing JP, Clayton CM, et al. The carrier frequency of the BRCA2 6174delT mutation among Ashkenazi Jewish individuals is approximately 1%. Nat Genet. 1996;14:188-190.
6. Tonin P, Weber B, Offit K, et al. Frequency of recurrent BRCA1 and BRCA2 mutations in Ashkenazi Jewish breast cancer families. Nat Med. 1996;2:1179-1183.
7. Schinzinger A. Ueber Carcinoma Mammae. Verhandlungen der Deutschen Gesellschaft fur Chirurgie. 18th Kongress, Berlin, Apr 24–27, 1889. Berlin, Germany: Hirschwald; 1889:28[abstract].
8. Love RR, Philips J. Oophorectomy for breast cancer: history revisited. J Natl Cancer Inst. 2002;94:1433-1434.
9. Feinleib M. Breast cancer and artificial menopause: a cohort study. J Natl Cancer Inst. 1968;41:315-329.
10. Brinton LA, Schairer C, Hoover RN, Fraumeni JF, Jr. Menstrual factors and risk of breast cancer. Cancer Invest. 1988;6:245-254.
11. Tobacman JK, Greene MH, Tucker MA, Costa J, Kase R, Fraumeni JF, Jr. Intraabdominal carcinomatosis after prophylactic oophorectomy in ovarian-cancer-prone families. Lancet. 1982;2:795-797.
12. Piver MS, Jishi MF, Tsukada Y, Nava G. Primary peritoneal carcinoma after prophylactic oophorectomy in women with a family history of ovarian cancer. A report of the Gilda Radner Familial Ovarian Cancer Registry. Cancer. 1993;71:2751-2755.
13. Burke W, Daly M, Garber J, et al. Recommendations for follow-up care of individuals with an inherited predisposition to cancer. II. BRCA1 and BRCA2. Cancer Genetics Studies Consortium. JAMA. 1997;277:997-1003.
14. Schrag D, Kuntz KM, Garber JE, Weeks JC. Decision analysis—effects of prophylactic mastectomy and oophorectomy on life expectancy among women with BRCA1 or BRCA2 mutations. N Engl J Med. 1997;336:1465-1471.
15. Grann VR, Panageas KS, Whang W, Antman KH, Neugut AI. Decision analysis of prophylactic mastectomy and oophorectomy in BRCA1-positive or BRCA2-positive patients. J Clin Oncol. 1998;16:979-985.
16. Rebbeck TR, Levin AM, Eisen A, et al. Breast cancer risk after bilateral prophylactic oophorectomy in BRCA1 mutation carriers. J Natl Cancer Inst. 1999;91:1475-1479.
17. Eisen A, Rebbeck TR, Wood WC, Weber BL. Prophylactic surgery in women with a hereditary predisposition to breast and ovarian cancer. J Clin Oncol. 2000;18:1980-1995.
18. Kauff ND, Satagopan JM, Robson ME, et al. Risk-reducing salpingo-oophorectomy in women with a BRCA1 or BRCA2 mutation. N Engl J Med. 2002;346:1609-1615.
19. Rebbeck TR, Lynch HT, Neuhausen SL, et al. Prophylactic oophorectomy in carriers of BRCA1 or BRCA2 mutations. N Engl J Med. 2002;346:1616-1622.
20. Rutter JL, Wacholder S, Chetrit A, et al. Gynecologic surgeries and risk of ovarian cancer in women with BRCA1 and BRCA2 Ashkenazi founder mutations: an Israeli population-based case-control study. J Natl Cancer Inst. 2003;95:1072-1078.
21. Lu KH, Garber JE, Cramer DW, et al. Occult ovarian tumors in women with BRCA1 or BRCA2 mutations undergoing prophylactic oophorectomy. J Clin Oncol. 2000;18:2728-2732.
22. Lafferty HW, Angioli R, Rudolph J, Penalver MA. Ovarian remnant syndrome: experience at Jackson Memorial Hospital, University of Miami, 1985 through 1993. Am J Obstet Gynecol. 1996;174:641-645.
23. Schorge JO, Muto MG, Welch WR, et al. Molecular evidence for multifocal papillary serous carcinoma of the peritoneum in patients with germline BRCA1 mutations. J Natl Cancer Inst. 1998;90:841-845.
24. Levine DA, Lin O, Barakat RR, et al. Risk of endometrial carcinoma associated with BRCA mutation. Gynecol Oncol. 2001;80:395-398.
25. Paley PJ, Swisher EM, Garcia RL, et al. Occult cancer of the fallopian tube in BRCA-1 germline mutation carriers at prophylactic oophorectomy: a case for recommending hysterectomy at surgical prophylaxis. Gynecol Oncol. 2001;80:176-180.
26. Aziz S, Kuperstein G, Rosen B, et al. A genetic epidemiological study of carcinoma of the fallopian tube. Gynecol Oncol. 2001;80:341-345.
27. Colgan TJ, Murphy J, Cole DE, Narod S, Rosen B. Occult carcinoma in prophylactic oophorectomy specimens: prevalence and association with BRCA germline mutation status. Am J Surg Pathol. 2001;25:1283-1289.
28. Scheuer L, Kauff N, Robson M, et al. Outcome of preventive surgery and screening for breast and ovarian cancer in BRCA mutation carriers. J Clin Oncol. 2002;20:1260-1268.
29. Colgan TJ, Boerner SL, Murphy J, Cole DE, Narod S, Rosen B. Peritoneal lavage cytology: an assessment of its value during prophylactic oophorectomy. Gynecol Oncol. 2002;85:397-403.
30. Meiser B, Butow P, Barratt A, et al. Attitudes toward prophylactic oophorectomy and screening utilization in women at increased risk of developing hereditary breast/ovarian cancer. Gynecol Oncol. 1999;75:122-129.
31. Hurley KE, Miller SM, Costalas JW, Gillespie D, Daly MB. Anxiety/uncertainty reduction as a motivation for interest in prophylactic oophorectomy in women with a family history of ovarian cancer. J Womens Health Gend Based Med. 2001;10:189-199.
32. Robson M, Hensley M, Barakat R, et al. Quality of life in women at risk for ovarian cancer who have undergone risk-reducing oophorectomy. Gynecol Oncol. 2003;89:281-287.
33. Kuerer HM, Hwang ES, Anthony JP, et al. Current national health insurance coverage policies for breast and ovarian cancer prophylactic surgery. Ann Surg Oncol. 2000;7:325-332.
34. Kauff ND, Scheuer L, Robson ME, et al. Insurance reimbursement for risk-reducing mastectomy and oophorectomy in women with BRCA1 or BRCA2 mutations. Genet Med. 2001;3:422-425.
- Mutations in BRCA1 and BRCA2 may be responsible for more than 90% of inherited predisposition to ovarian cancer.
- BRCA1 and BRCA2 mutations are associated with a lifetime risk of breast cancer of up to 85% and a 15% to 45% lifetime risk of ovarian cancer.
- The only prospective trial to date found risk-reducing salpingo-oophorectomy (RRSO) was associated with an 85% reduction in ovarian cancer and a 68% reduction in breast cancer.
- Because microscopic cancer may be found in 2% to 4% of RRSO specimens upon careful pathologic review, the ovaries and fallopian tubes should be sectioned in their entirety and examined by an experienced gynecologic pathologist.
When A.M. Liber encountered a family of 5 sisters and their mother with histologically confirmed papillary adenocarcinoma of the ovary, he recommended frequent gynecologic cancer screening for all family members and suggested prophylactic oophorectomy as an option.1 The year was 1950.
Flash forward half a century or so, and prophylactic oophorectomy has gained wider acceptance for the prevention of hereditary ovarian and breast cancer, with the only prospective trial to date confirming its overall efficacy for women with BRCA1 and BRCA2 mutations. These mutations are related to the vast majority of inherited ovarian cancers.
Using the evidence published thus far, including the recently published prospective trial, we discuss surgical technique, post-oophorectomy estrogens, psychosocial impact, insurance reimbursement, and other issues.
Three hereditary syndromes
The single biggest risk factor for ovarian cancer is a family history, although only about 10% of cases are believed to be due to an inherited predisposition. Three syndromes are associated with such a predisposition:
- Hereditary breast-ovarian cancer syndrome, caused by mutations in BRCA1 and BRCA2, is thought to be responsible for more than 90% of inherited predisposition to ovarian cancer.
- Hereditary nonpolyposis colon cancer (HNPCC) syndrome is associated with mutations in the mismatch repair genes and a greatly increased risk of cancers of the colon, endometrium, ovaries, and urinary tract. HNPCC accounts for about 2% of inherited ovarian cancers.
- A syndrome of site-specific ovarian cancer also has been proposed, though we lack conclusive evidence that it exists as a separate entity at the genetic level.
How BRCA mutations lead to cancer
BRCA1 and BRCA2 are tumor suppressor genes that play a role in genomic stability and double-stranded DNA break repair. BRCA1 is located on chromosome 17; BRCA2 on chromosome 13. Both genes function as classic tumor suppressors, as described by Knudson.2 Only a single working copy of each gene is needed for the genes to effectively suppress tumors.
In patients with no inherited mutation in these genes, carcinogenesis caused by dysfunction of this pathway can occur only if both working copies of the gene are lost. In contrast, women with an inherited mutation in BRCA1 or BRCA2 start out with only a single working copy of the gene. If any cell loses this single copy, DNA repair cannot occur via this pathway, and cancer can develop.
These repair pathways seem to be particularly important in dividing breast and ovarian cells. This explains why women with inherited mutations in these genes develop cancers more frequently and at an earlier age.
Quantifying the risk
Specific risks associated with BRCA1 and BRCA2 mutations include:
- a lifetime risk of breast cancer of up to 85%, with half of these cancers occurring prior to age 50
- a 15% to 45% lifetime risk of ovarian cancer3,4
Mutations in these genes can be inherited from a mother or father. In the general population, between 1 in 385 and 1 in 800 individuals carry a deleterious mutation in either BRCA1 or BRCA2.
In certain populations, such as Icelandic, French Canadian, or Eastern European Jewish populations, founder effects can contribute to a greatly increased frequency of mutation. For example, the Eastern European Jewish population, from which approximately 90% of North American Jews are descended, has one of the highest known frequencies of BRCA1 and BRCA2 mutation: 1 in 40 individuals carries a deleterious mutation in 1 of these 2 genes.5,6
Most evidence is historical or retrospective
Liber was not the first to suggest oophorectomy to impact the risk of breast or ovarian cancer: The procedure was initially proposed by Schinziner in 1889 as a treatment for breast cancer.7 However, the earliest evidence that oophorectomy was performed as adjuvant therapy did not appear until 7 years later, in 1896 (reviewed by Love and Philips).8
In 1968, Feinleib9 reported that premenopausal oophorectomy decreased the rate of subsequent breast cancer. Twenty years later, Brinton suggested that prophylactic oophorectomy might reduce breast cancer risk in women with a family history of the disease.10
In the sole prospective trial, salpingo-oophorectomy was associated with a 75% reduction in breast and gynecologic cancer.
Post-oophorectomy cancers identified. Possible limitations to the strategy became apparent in the early 1980s, when Tobacman and colleagues11 reported adenocarcinoma histologically indistinguishable from ovarian cancer after oophorectomy in a series of women with a strong family history.
In 1993, Piver et al12 reported a series of 6 cases of primary peritoneal cancer after prophylactic oophorectomy in 324 women from hereditary ovarian cancer families.
In 1997, the Cancer Genetics Studies Consortium reviewed all available data and concluded: “There is insufficient evidence to recommend for or against prophylactic oophorectomy as a measure for reducing ovarian cancer risk. Women with BRCA1 mutations should be counseled that this is an option available to them. Those considering prophylactic oophorectomy should be counseled that cancer has been documented to occur after the procedure.”13
Although the Cancer Genetics Studies Consortium did not specifically comment on prophylactic oophorectomy in carriers of BRCA2 mutations, most authorities interpreted these recommendations to apply to these women as well.
Predicting life expectancy. After these findings, several groups undertook decision analyses to evaluate the effect of prophylactic oophorectomy on life expectancy in women with BRCA mutations. Schrag et al14 reported that prophylactic oophorectomy in a 30-year-old with a BRCA mutation increased life expectancy by 0.3 to 1.7 years. This compares to 0.9 years for adjuvant chemotherapy in node-negative breast cancer.
A subsequent report by Grann and colleagues15 also suggested that prophylactic oophorectomy was associated with an increased life expectancy of 0.4 to 2.6 years. However, surgery was not cost-effective for quality-adjusted life-years saved.
Investigators cite need for prospective studies. In 1999, Rebbeck and colleagues16 conducted a retrospective case-control study of 43 women with BRCA1 mutations who underwent oophorectomy and 79 age-matched women with BRCA1 mutations who had ovaries in situ. In this series, oophorectomy was associated with a 47% decreased risk of subsequent breast cancer (hazard ratio 0.53). However, several investigators cited the need for prospective studies before incorporating oophorectomy into routine clinical practice for the prevention of cancer.17
The first prospective look at risk-reducing surgery
It was in this setting that our group launched a prospective trial to determine whether salpingo-oophorectomy offers any benefit over surveillance in preventing breast and gynecologic (ovarian, fallopian tube, and peritoneal) cancers in women with BRCA mutations.18
Proportional hazard analysis demonstrated that salpingo-oophorectomy was associated with a 75% reduction in subsequent breast and gynecologic cancer incidence in women with BRCA mutations (hazard ratio 0.25, 95% confidence interval 0.08 to 0.74). When the individual endpoints of breast and gynecologic cancer were observed, risk-reducing salpingo-oophorectomy (RRSO) was associated with an 85% reduction in subsequent ovarian cancer and a 68% reduction in subsequent breast cancer.
Methods. From June 1995 through May 2001, we enrolled 265 women with documented BRCA1 or BRCA2 mutations. Patients were followed by annual questionnaire, telephone contact, and medical-record review. Pathology reports were obtained for all new cancers diagnosed during follow-up.
After excluding women who underwent bilateral salpingo-oophorectomy before genetic testing, who were younger than 35 years at the time of testing, or who did not provide any follow-up information, 173 women with ovaries at risk and a documented BRCA mutation remained. These women participated in formal pre- and post-test genetic counseling and received uniform recommendations for cancer risk reduction, as detailed in the TABLE.
During follow-up, we calculated the incidence of new breast and gynecologic cancers diagnosed in the cohort who elected RRSO and compared it with the incidence of these cancers in women who chose surveillance.
Salpingo-oophorectomy was elected by 101 of the 173 women.
Findings. In 3 of these women, early-stage ovarian or fallopian-tube cancer that had not been detected during preoperative evaluation was found at the time of surgery. In the remaining 98 patients who underwent RRSO, 1 peritoneal cancer and 3 breast cancers were diagnosed during a mean 23 months of follow-up. In the 72 women who chose surveillance, 5 ovarian or peritoneal cancers and 8 breast cancers were diagnosed in a mean 25 months of follow-up.
Kaplan-Meier analysis of time to breast or BRCA-related gynecologic cancer is illustrated in FIGURE 1.
Other studies confirm findings. A second retrospective study by Rebbeck et al19 was released simultaneously with our findings and showed similar benefits. They found a 53% reduction in subsequent breast cancer risk and a 96% reduction in subsequent ovarian cancer risk. In the summer of 2003, a study from Israel by Rutter et al provided further confirmation of the substantially decreased incidence of cancer following risk-reducing surgery.20
TABLE
Breast and ovarian cancer risk-reduction strategies for women with BRCA1 or BRCA2 mutations
| TYPE OF CANCER | STRATEGY | ALSO CONSIDER … |
|---|---|---|
| Breast | Monthly self-examination beginning at age 18 | Imaging Breast ultrasound or magnetic resonance imaging |
| 2-4 physician examinations per year, starting at age 25 | Risk–reducing surgery Mastectomy, no earlier than mid-20s | |
| Annual mammography beginning at age 25 | Salpingo-oophorectomy, after age 35 and completion of childbearing | |
| Chemoprevention Tamoxifen. Need to discuss conflicting reports on efficacy | ||
| Ovarian | CA 125 and ultrasound twice yearly, starting at age 35 | Salpingo-oophorectomy After age 35 and the completion of childbearing |
| Chemoprevention Oral contraceptives, though they may be associated with an increased risk of breast cancer | ||
| Source: Adapted from Scheuer et al28 | ||
FIGURE 1 Reduction in cancer cases associated with salpingo-oophorectomy
Reprinted with permission from Kauff ND et al.18
Copyright 2002 Massachusetts Medical Society. All rights reserved.
Good technique and pathologic review may prevent post-oophorectomy cancer
There are 3 theories about the origin of primary peritoneal cancer after oophorectomy:
- The cancer represents undetected occult cancer present at the time of risk-reducing surgery.
- It represents cancer arising in an ovarian remnant left behind after risk-reducing surgery.
- The peritoneal cancer arises de novo from the peritoneal surface epithelium.
Reasonable evidence supports each of these theories; thus, each may play some role in the incidence of “peritoneal” cancer after risk-reducing surgery.21-23
While surgical technique and detailed pathologic review are unlikely to decrease the incidence of de novo peritoneal cancer, they may play a substantial role in reducing ovarian and related cancers after risk-reducing surgery.
Surgical requirements. Obviously, if a surgery is to be risk-reducing, as much as possible of the tissue at risk should be removed. To do so effectively, the surgeon should be comfortable operating in the retroperitoneum so that the infundibulopelvic ligament can be ligated sufficiently proximal from the ovarian hilum to minimize the possibility of an ovarian remnant. Similarly, if a salpingo-oophorectomy without hysterectomy is to be performed, the fallopian tube should be amputated as close as possible to the uterine cornua (FIGURE 2).
Laparoscopy versus open surgery. RRSO can be performed using either a laparoscopic or open approach. The appropriate choice is best determined by the patient’s history, associated comorbid conditions, need for additional procedures, and experience of the surgeon. At our institution, in the absence of contraindications, we generally offer a laparoscopic approach due to its decreased morbidity.
Concomitant hysterectomy? An area of substantial controversy is whether the uterus should be removed at the time of RRSO. In most studies exploring this issue, hereditary breast-ovarian cancer syndrome does not appear to be associated with an increased risk of uterine cancer.24 However, there is concern that the portion of interstitial fallopian tube left behind after salpingo-oophorectomy may be at risk for malignant transformation.25,26
In our series, almost 90% of risk-reducing procedures were salpingo-oophorectomies without hysterectomy. If there is an additional benefit to concomitant hysterectomy, it has yet to be demonstrated by clinical trials.
In several studies, a patient’s level of anxiety was more important than objective cancer risk in the choice of RRSO.
Close pathologic scrutiny advised. Microscopic cancer may be found in 2% to 4% of RRSO specimens upon careful pathologic review.21,27,28 Thus, it is essential that the ovaries and fallopian tubes are sectioned in their entirety and examined by an experienced gynecologic pathologist to minimize the chance that microscopic cancer goes undetected.
It is not clear whether cytology should be routinely done at the time of risk-reducing surgery. A single report documents malignant cells in a woman with a BRCA1 mutation and no obvious foci of malignancy despite hysterectomy with bilateral salpingo-oophorectomy and staging.29 Pending further studies, we routinely send cytology for review.
FIGURE 2 Careful surgical ligation and division to eliminate residual tissue
Source: Devita VT Jr., Hellman S, Rosenberg SA, eds. Progress in Oncology 2003. 2004: Jones and Bartlett Publishers; Sudbury, Mass. Reprinted with permission.
When no BRCA mutation is present
Most of the data cited thus far apply to women with documented BRCA mutations. There is much less information about the relative risks and benefits of RRSO in women with a personal or family history of breast or ovarian cancer who lack a documented BRCA mutation.
Although RRSO may be appropriate for some of these women, in 2004 it is not the standard of care to recommend RRSO to all individuals with a personal or family history suggestive of an inherited predisposition to ovarian cancer. These patients are best managed by an interdisciplinary team of gynecologists, gynecologic oncologists, and clinical geneticists, all with experience caring for women who may have an inherited predisposition.
Is anxiety a factor?
We have limited information about the psychosocial impact of RRSO. Several studies have found that a patient’s level of anxiety is a more important factor than objective cancer risk in the decision to undergo RRSO.30,31 Unfortunately, we do not yet know whether the surgery successfully reduces these patients’ subjective concerns.
A recent study showed that risk-reducing surgery did not impair women’s overall health or psychological well-being.32 However, 20.7% of the women reported substantial cancer-related anxiety despite the risk-reducing surgery. This issue requires further investigation.
Is estrogen the best option for surgical menopause?
The role of hormone replacement after RRSO is unclear. The issue is important because many women considering salpingo-oophorectomy are in their late 30s or early 40s, when premature surgical menopause is a predictable result. Consequences can include considerable vasomotor and pelvic symptoms.
Preliminary data suggest that a woman’s satisfaction with RRSO depends in large part on its impact on sexual functioning.32 Urogenital symptoms that adversely affected sexual function, such as vaginal dryness and dyspareunia, were the most significant predictors of dissatisfaction with surgery.
Premature surgical menopause also has a substantial impact on osteoporosis risk, while its effect on heart disease remains uncertain.
While nonhormonal therapies can address each of these issues, we need more data on their long-term use. We counsel women considering RRSO that hormone replacement may be an option. We believe it is unlikely to reduce the efficacy of RRSO in preventing ovarian cancer, but it may reduce the protective effect of RRSO against subsequent breast cancer. Until further studies are available, we recommend that decisions regarding hormone replacement be individualized to the patient’s specific symptoms and personal history.
Not all insurers cover RRSO
One study explored insurance carriers’ policies about reimbursing risk-reducing surgical procedures and found that 10% to 11% of private insurers and 48% to 50% of governmental carriers had policies specifically denying coverage for such operations.
An additional 40% to 64% of insurers had no identifiable policy regarding these procedures in women with BRCA mutations.33 The authors speculated that, without identifiable policies, this critical health-care decision may be subject to arbitrary criteria that result in substantial variation.
When we recently investigated the reimbursement experience of women with BRCA mutations undergoing RRSO at our institution, we found that 97% of the procedures were reimbursed in full, less any applicable coinsurance and deductibles.34 Two important limitations of our study: It was conducted at a tertiary cancer center and was retrospective. It is not known if the findings reflect the experience of women with BRCA mutation who have risk-reducing surgery in other settings.
Unresolved issues
RRSO clearly has a role in preventing breast and ovarian cancer in women at inherited risk. However, several questions remain unanswered:
- Who is the best candidate?
- What is optimal timing of the procedure?
- What, if any, concomitant procedures should be performed?
- What is the role of hormone replacement after the surgery?
These issues will be best addressed through multicenter prospective trials, such as the one now being conducted by the Gynecologic Oncology Group.
Hope also remains that further research will improve serum and radiological detection of early ovarian cancer, and that basic research on the molecular etiology and progression of these cancers will ultimately render it unnecessary to remove organs at risk.
The authors report no financial relationships relevant to this article.
- Mutations in BRCA1 and BRCA2 may be responsible for more than 90% of inherited predisposition to ovarian cancer.
- BRCA1 and BRCA2 mutations are associated with a lifetime risk of breast cancer of up to 85% and a 15% to 45% lifetime risk of ovarian cancer.
- The only prospective trial to date found risk-reducing salpingo-oophorectomy (RRSO) was associated with an 85% reduction in ovarian cancer and a 68% reduction in breast cancer.
- Because microscopic cancer may be found in 2% to 4% of RRSO specimens upon careful pathologic review, the ovaries and fallopian tubes should be sectioned in their entirety and examined by an experienced gynecologic pathologist.
When A.M. Liber encountered a family of 5 sisters and their mother with histologically confirmed papillary adenocarcinoma of the ovary, he recommended frequent gynecologic cancer screening for all family members and suggested prophylactic oophorectomy as an option.1 The year was 1950.
Flash forward half a century or so, and prophylactic oophorectomy has gained wider acceptance for the prevention of hereditary ovarian and breast cancer, with the only prospective trial to date confirming its overall efficacy for women with BRCA1 and BRCA2 mutations. These mutations are related to the vast majority of inherited ovarian cancers.
Using the evidence published thus far, including the recently published prospective trial, we discuss surgical technique, post-oophorectomy estrogens, psychosocial impact, insurance reimbursement, and other issues.
Three hereditary syndromes
The single biggest risk factor for ovarian cancer is a family history, although only about 10% of cases are believed to be due to an inherited predisposition. Three syndromes are associated with such a predisposition:
- Hereditary breast-ovarian cancer syndrome, caused by mutations in BRCA1 and BRCA2, is thought to be responsible for more than 90% of inherited predisposition to ovarian cancer.
- Hereditary nonpolyposis colon cancer (HNPCC) syndrome is associated with mutations in the mismatch repair genes and a greatly increased risk of cancers of the colon, endometrium, ovaries, and urinary tract. HNPCC accounts for about 2% of inherited ovarian cancers.
- A syndrome of site-specific ovarian cancer also has been proposed, though we lack conclusive evidence that it exists as a separate entity at the genetic level.
How BRCA mutations lead to cancer
BRCA1 and BRCA2 are tumor suppressor genes that play a role in genomic stability and double-stranded DNA break repair. BRCA1 is located on chromosome 17; BRCA2 on chromosome 13. Both genes function as classic tumor suppressors, as described by Knudson.2 Only a single working copy of each gene is needed for the genes to effectively suppress tumors.
In patients with no inherited mutation in these genes, carcinogenesis caused by dysfunction of this pathway can occur only if both working copies of the gene are lost. In contrast, women with an inherited mutation in BRCA1 or BRCA2 start out with only a single working copy of the gene. If any cell loses this single copy, DNA repair cannot occur via this pathway, and cancer can develop.
These repair pathways seem to be particularly important in dividing breast and ovarian cells. This explains why women with inherited mutations in these genes develop cancers more frequently and at an earlier age.
Quantifying the risk
Specific risks associated with BRCA1 and BRCA2 mutations include:
- a lifetime risk of breast cancer of up to 85%, with half of these cancers occurring prior to age 50
- a 15% to 45% lifetime risk of ovarian cancer3,4
Mutations in these genes can be inherited from a mother or father. In the general population, between 1 in 385 and 1 in 800 individuals carry a deleterious mutation in either BRCA1 or BRCA2.
In certain populations, such as Icelandic, French Canadian, or Eastern European Jewish populations, founder effects can contribute to a greatly increased frequency of mutation. For example, the Eastern European Jewish population, from which approximately 90% of North American Jews are descended, has one of the highest known frequencies of BRCA1 and BRCA2 mutation: 1 in 40 individuals carries a deleterious mutation in 1 of these 2 genes.5,6
Most evidence is historical or retrospective
Liber was not the first to suggest oophorectomy to impact the risk of breast or ovarian cancer: The procedure was initially proposed by Schinziner in 1889 as a treatment for breast cancer.7 However, the earliest evidence that oophorectomy was performed as adjuvant therapy did not appear until 7 years later, in 1896 (reviewed by Love and Philips).8
In 1968, Feinleib9 reported that premenopausal oophorectomy decreased the rate of subsequent breast cancer. Twenty years later, Brinton suggested that prophylactic oophorectomy might reduce breast cancer risk in women with a family history of the disease.10
In the sole prospective trial, salpingo-oophorectomy was associated with a 75% reduction in breast and gynecologic cancer.
Post-oophorectomy cancers identified. Possible limitations to the strategy became apparent in the early 1980s, when Tobacman and colleagues11 reported adenocarcinoma histologically indistinguishable from ovarian cancer after oophorectomy in a series of women with a strong family history.
In 1993, Piver et al12 reported a series of 6 cases of primary peritoneal cancer after prophylactic oophorectomy in 324 women from hereditary ovarian cancer families.
In 1997, the Cancer Genetics Studies Consortium reviewed all available data and concluded: “There is insufficient evidence to recommend for or against prophylactic oophorectomy as a measure for reducing ovarian cancer risk. Women with BRCA1 mutations should be counseled that this is an option available to them. Those considering prophylactic oophorectomy should be counseled that cancer has been documented to occur after the procedure.”13
Although the Cancer Genetics Studies Consortium did not specifically comment on prophylactic oophorectomy in carriers of BRCA2 mutations, most authorities interpreted these recommendations to apply to these women as well.
Predicting life expectancy. After these findings, several groups undertook decision analyses to evaluate the effect of prophylactic oophorectomy on life expectancy in women with BRCA mutations. Schrag et al14 reported that prophylactic oophorectomy in a 30-year-old with a BRCA mutation increased life expectancy by 0.3 to 1.7 years. This compares to 0.9 years for adjuvant chemotherapy in node-negative breast cancer.
A subsequent report by Grann and colleagues15 also suggested that prophylactic oophorectomy was associated with an increased life expectancy of 0.4 to 2.6 years. However, surgery was not cost-effective for quality-adjusted life-years saved.
Investigators cite need for prospective studies. In 1999, Rebbeck and colleagues16 conducted a retrospective case-control study of 43 women with BRCA1 mutations who underwent oophorectomy and 79 age-matched women with BRCA1 mutations who had ovaries in situ. In this series, oophorectomy was associated with a 47% decreased risk of subsequent breast cancer (hazard ratio 0.53). However, several investigators cited the need for prospective studies before incorporating oophorectomy into routine clinical practice for the prevention of cancer.17
The first prospective look at risk-reducing surgery
It was in this setting that our group launched a prospective trial to determine whether salpingo-oophorectomy offers any benefit over surveillance in preventing breast and gynecologic (ovarian, fallopian tube, and peritoneal) cancers in women with BRCA mutations.18
Proportional hazard analysis demonstrated that salpingo-oophorectomy was associated with a 75% reduction in subsequent breast and gynecologic cancer incidence in women with BRCA mutations (hazard ratio 0.25, 95% confidence interval 0.08 to 0.74). When the individual endpoints of breast and gynecologic cancer were observed, risk-reducing salpingo-oophorectomy (RRSO) was associated with an 85% reduction in subsequent ovarian cancer and a 68% reduction in subsequent breast cancer.
Methods. From June 1995 through May 2001, we enrolled 265 women with documented BRCA1 or BRCA2 mutations. Patients were followed by annual questionnaire, telephone contact, and medical-record review. Pathology reports were obtained for all new cancers diagnosed during follow-up.
After excluding women who underwent bilateral salpingo-oophorectomy before genetic testing, who were younger than 35 years at the time of testing, or who did not provide any follow-up information, 173 women with ovaries at risk and a documented BRCA mutation remained. These women participated in formal pre- and post-test genetic counseling and received uniform recommendations for cancer risk reduction, as detailed in the TABLE.
During follow-up, we calculated the incidence of new breast and gynecologic cancers diagnosed in the cohort who elected RRSO and compared it with the incidence of these cancers in women who chose surveillance.
Salpingo-oophorectomy was elected by 101 of the 173 women.
Findings. In 3 of these women, early-stage ovarian or fallopian-tube cancer that had not been detected during preoperative evaluation was found at the time of surgery. In the remaining 98 patients who underwent RRSO, 1 peritoneal cancer and 3 breast cancers were diagnosed during a mean 23 months of follow-up. In the 72 women who chose surveillance, 5 ovarian or peritoneal cancers and 8 breast cancers were diagnosed in a mean 25 months of follow-up.
Kaplan-Meier analysis of time to breast or BRCA-related gynecologic cancer is illustrated in FIGURE 1.
Other studies confirm findings. A second retrospective study by Rebbeck et al19 was released simultaneously with our findings and showed similar benefits. They found a 53% reduction in subsequent breast cancer risk and a 96% reduction in subsequent ovarian cancer risk. In the summer of 2003, a study from Israel by Rutter et al provided further confirmation of the substantially decreased incidence of cancer following risk-reducing surgery.20
TABLE
Breast and ovarian cancer risk-reduction strategies for women with BRCA1 or BRCA2 mutations
| TYPE OF CANCER | STRATEGY | ALSO CONSIDER … |
|---|---|---|
| Breast | Monthly self-examination beginning at age 18 | Imaging Breast ultrasound or magnetic resonance imaging |
| 2-4 physician examinations per year, starting at age 25 | Risk–reducing surgery Mastectomy, no earlier than mid-20s | |
| Annual mammography beginning at age 25 | Salpingo-oophorectomy, after age 35 and completion of childbearing | |
| Chemoprevention Tamoxifen. Need to discuss conflicting reports on efficacy | ||
| Ovarian | CA 125 and ultrasound twice yearly, starting at age 35 | Salpingo-oophorectomy After age 35 and the completion of childbearing |
| Chemoprevention Oral contraceptives, though they may be associated with an increased risk of breast cancer | ||
| Source: Adapted from Scheuer et al28 | ||
FIGURE 1 Reduction in cancer cases associated with salpingo-oophorectomy
Reprinted with permission from Kauff ND et al.18
Copyright 2002 Massachusetts Medical Society. All rights reserved.
Good technique and pathologic review may prevent post-oophorectomy cancer
There are 3 theories about the origin of primary peritoneal cancer after oophorectomy:
- The cancer represents undetected occult cancer present at the time of risk-reducing surgery.
- It represents cancer arising in an ovarian remnant left behind after risk-reducing surgery.
- The peritoneal cancer arises de novo from the peritoneal surface epithelium.
Reasonable evidence supports each of these theories; thus, each may play some role in the incidence of “peritoneal” cancer after risk-reducing surgery.21-23
While surgical technique and detailed pathologic review are unlikely to decrease the incidence of de novo peritoneal cancer, they may play a substantial role in reducing ovarian and related cancers after risk-reducing surgery.
Surgical requirements. Obviously, if a surgery is to be risk-reducing, as much as possible of the tissue at risk should be removed. To do so effectively, the surgeon should be comfortable operating in the retroperitoneum so that the infundibulopelvic ligament can be ligated sufficiently proximal from the ovarian hilum to minimize the possibility of an ovarian remnant. Similarly, if a salpingo-oophorectomy without hysterectomy is to be performed, the fallopian tube should be amputated as close as possible to the uterine cornua (FIGURE 2).
Laparoscopy versus open surgery. RRSO can be performed using either a laparoscopic or open approach. The appropriate choice is best determined by the patient’s history, associated comorbid conditions, need for additional procedures, and experience of the surgeon. At our institution, in the absence of contraindications, we generally offer a laparoscopic approach due to its decreased morbidity.
Concomitant hysterectomy? An area of substantial controversy is whether the uterus should be removed at the time of RRSO. In most studies exploring this issue, hereditary breast-ovarian cancer syndrome does not appear to be associated with an increased risk of uterine cancer.24 However, there is concern that the portion of interstitial fallopian tube left behind after salpingo-oophorectomy may be at risk for malignant transformation.25,26
In our series, almost 90% of risk-reducing procedures were salpingo-oophorectomies without hysterectomy. If there is an additional benefit to concomitant hysterectomy, it has yet to be demonstrated by clinical trials.
In several studies, a patient’s level of anxiety was more important than objective cancer risk in the choice of RRSO.
Close pathologic scrutiny advised. Microscopic cancer may be found in 2% to 4% of RRSO specimens upon careful pathologic review.21,27,28 Thus, it is essential that the ovaries and fallopian tubes are sectioned in their entirety and examined by an experienced gynecologic pathologist to minimize the chance that microscopic cancer goes undetected.
It is not clear whether cytology should be routinely done at the time of risk-reducing surgery. A single report documents malignant cells in a woman with a BRCA1 mutation and no obvious foci of malignancy despite hysterectomy with bilateral salpingo-oophorectomy and staging.29 Pending further studies, we routinely send cytology for review.
FIGURE 2 Careful surgical ligation and division to eliminate residual tissue
Source: Devita VT Jr., Hellman S, Rosenberg SA, eds. Progress in Oncology 2003. 2004: Jones and Bartlett Publishers; Sudbury, Mass. Reprinted with permission.
When no BRCA mutation is present
Most of the data cited thus far apply to women with documented BRCA mutations. There is much less information about the relative risks and benefits of RRSO in women with a personal or family history of breast or ovarian cancer who lack a documented BRCA mutation.
Although RRSO may be appropriate for some of these women, in 2004 it is not the standard of care to recommend RRSO to all individuals with a personal or family history suggestive of an inherited predisposition to ovarian cancer. These patients are best managed by an interdisciplinary team of gynecologists, gynecologic oncologists, and clinical geneticists, all with experience caring for women who may have an inherited predisposition.
Is anxiety a factor?
We have limited information about the psychosocial impact of RRSO. Several studies have found that a patient’s level of anxiety is a more important factor than objective cancer risk in the decision to undergo RRSO.30,31 Unfortunately, we do not yet know whether the surgery successfully reduces these patients’ subjective concerns.
A recent study showed that risk-reducing surgery did not impair women’s overall health or psychological well-being.32 However, 20.7% of the women reported substantial cancer-related anxiety despite the risk-reducing surgery. This issue requires further investigation.
Is estrogen the best option for surgical menopause?
The role of hormone replacement after RRSO is unclear. The issue is important because many women considering salpingo-oophorectomy are in their late 30s or early 40s, when premature surgical menopause is a predictable result. Consequences can include considerable vasomotor and pelvic symptoms.
Preliminary data suggest that a woman’s satisfaction with RRSO depends in large part on its impact on sexual functioning.32 Urogenital symptoms that adversely affected sexual function, such as vaginal dryness and dyspareunia, were the most significant predictors of dissatisfaction with surgery.
Premature surgical menopause also has a substantial impact on osteoporosis risk, while its effect on heart disease remains uncertain.
While nonhormonal therapies can address each of these issues, we need more data on their long-term use. We counsel women considering RRSO that hormone replacement may be an option. We believe it is unlikely to reduce the efficacy of RRSO in preventing ovarian cancer, but it may reduce the protective effect of RRSO against subsequent breast cancer. Until further studies are available, we recommend that decisions regarding hormone replacement be individualized to the patient’s specific symptoms and personal history.
Not all insurers cover RRSO
One study explored insurance carriers’ policies about reimbursing risk-reducing surgical procedures and found that 10% to 11% of private insurers and 48% to 50% of governmental carriers had policies specifically denying coverage for such operations.
An additional 40% to 64% of insurers had no identifiable policy regarding these procedures in women with BRCA mutations.33 The authors speculated that, without identifiable policies, this critical health-care decision may be subject to arbitrary criteria that result in substantial variation.
When we recently investigated the reimbursement experience of women with BRCA mutations undergoing RRSO at our institution, we found that 97% of the procedures were reimbursed in full, less any applicable coinsurance and deductibles.34 Two important limitations of our study: It was conducted at a tertiary cancer center and was retrospective. It is not known if the findings reflect the experience of women with BRCA mutation who have risk-reducing surgery in other settings.
Unresolved issues
RRSO clearly has a role in preventing breast and ovarian cancer in women at inherited risk. However, several questions remain unanswered:
- Who is the best candidate?
- What is optimal timing of the procedure?
- What, if any, concomitant procedures should be performed?
- What is the role of hormone replacement after the surgery?
These issues will be best addressed through multicenter prospective trials, such as the one now being conducted by the Gynecologic Oncology Group.
Hope also remains that further research will improve serum and radiological detection of early ovarian cancer, and that basic research on the molecular etiology and progression of these cancers will ultimately render it unnecessary to remove organs at risk.
The authors report no financial relationships relevant to this article.
1. Liber AM. Ovarian cancer in a mother and five daughters. Arch Pathol. 1950;49:280-290.
2. Knudson AG, Jr. Mutation and cancer: statistical study of retinoblastoma. Proc Natl Acad Sci USA. 1971;68:820-823.
3. Ford D, Easton DF, Stratton M, et al. Genetic heterogeneity and penetrance analysis of the BRCA1 and BRCA2 genes in breast cancer families. The Breast Cancer Linkage Consortium. Am J Hum Genet. 1998;62:676-689.
4. Risch HA, McLaughlin JR, Cole DE, et al. Prevalence and penetrance of germline BRCA1 and BRCA2 mutations in a population series of 649 women with ovarian cancer. Am J Hum Genet. 2001;68:700-710.
5. Oddoux C, Struewing JP, Clayton CM, et al. The carrier frequency of the BRCA2 6174delT mutation among Ashkenazi Jewish individuals is approximately 1%. Nat Genet. 1996;14:188-190.
6. Tonin P, Weber B, Offit K, et al. Frequency of recurrent BRCA1 and BRCA2 mutations in Ashkenazi Jewish breast cancer families. Nat Med. 1996;2:1179-1183.
7. Schinzinger A. Ueber Carcinoma Mammae. Verhandlungen der Deutschen Gesellschaft fur Chirurgie. 18th Kongress, Berlin, Apr 24–27, 1889. Berlin, Germany: Hirschwald; 1889:28[abstract].
8. Love RR, Philips J. Oophorectomy for breast cancer: history revisited. J Natl Cancer Inst. 2002;94:1433-1434.
9. Feinleib M. Breast cancer and artificial menopause: a cohort study. J Natl Cancer Inst. 1968;41:315-329.
10. Brinton LA, Schairer C, Hoover RN, Fraumeni JF, Jr. Menstrual factors and risk of breast cancer. Cancer Invest. 1988;6:245-254.
11. Tobacman JK, Greene MH, Tucker MA, Costa J, Kase R, Fraumeni JF, Jr. Intraabdominal carcinomatosis after prophylactic oophorectomy in ovarian-cancer-prone families. Lancet. 1982;2:795-797.
12. Piver MS, Jishi MF, Tsukada Y, Nava G. Primary peritoneal carcinoma after prophylactic oophorectomy in women with a family history of ovarian cancer. A report of the Gilda Radner Familial Ovarian Cancer Registry. Cancer. 1993;71:2751-2755.
13. Burke W, Daly M, Garber J, et al. Recommendations for follow-up care of individuals with an inherited predisposition to cancer. II. BRCA1 and BRCA2. Cancer Genetics Studies Consortium. JAMA. 1997;277:997-1003.
14. Schrag D, Kuntz KM, Garber JE, Weeks JC. Decision analysis—effects of prophylactic mastectomy and oophorectomy on life expectancy among women with BRCA1 or BRCA2 mutations. N Engl J Med. 1997;336:1465-1471.
15. Grann VR, Panageas KS, Whang W, Antman KH, Neugut AI. Decision analysis of prophylactic mastectomy and oophorectomy in BRCA1-positive or BRCA2-positive patients. J Clin Oncol. 1998;16:979-985.
16. Rebbeck TR, Levin AM, Eisen A, et al. Breast cancer risk after bilateral prophylactic oophorectomy in BRCA1 mutation carriers. J Natl Cancer Inst. 1999;91:1475-1479.
17. Eisen A, Rebbeck TR, Wood WC, Weber BL. Prophylactic surgery in women with a hereditary predisposition to breast and ovarian cancer. J Clin Oncol. 2000;18:1980-1995.
18. Kauff ND, Satagopan JM, Robson ME, et al. Risk-reducing salpingo-oophorectomy in women with a BRCA1 or BRCA2 mutation. N Engl J Med. 2002;346:1609-1615.
19. Rebbeck TR, Lynch HT, Neuhausen SL, et al. Prophylactic oophorectomy in carriers of BRCA1 or BRCA2 mutations. N Engl J Med. 2002;346:1616-1622.
20. Rutter JL, Wacholder S, Chetrit A, et al. Gynecologic surgeries and risk of ovarian cancer in women with BRCA1 and BRCA2 Ashkenazi founder mutations: an Israeli population-based case-control study. J Natl Cancer Inst. 2003;95:1072-1078.
21. Lu KH, Garber JE, Cramer DW, et al. Occult ovarian tumors in women with BRCA1 or BRCA2 mutations undergoing prophylactic oophorectomy. J Clin Oncol. 2000;18:2728-2732.
22. Lafferty HW, Angioli R, Rudolph J, Penalver MA. Ovarian remnant syndrome: experience at Jackson Memorial Hospital, University of Miami, 1985 through 1993. Am J Obstet Gynecol. 1996;174:641-645.
23. Schorge JO, Muto MG, Welch WR, et al. Molecular evidence for multifocal papillary serous carcinoma of the peritoneum in patients with germline BRCA1 mutations. J Natl Cancer Inst. 1998;90:841-845.
24. Levine DA, Lin O, Barakat RR, et al. Risk of endometrial carcinoma associated with BRCA mutation. Gynecol Oncol. 2001;80:395-398.
25. Paley PJ, Swisher EM, Garcia RL, et al. Occult cancer of the fallopian tube in BRCA-1 germline mutation carriers at prophylactic oophorectomy: a case for recommending hysterectomy at surgical prophylaxis. Gynecol Oncol. 2001;80:176-180.
26. Aziz S, Kuperstein G, Rosen B, et al. A genetic epidemiological study of carcinoma of the fallopian tube. Gynecol Oncol. 2001;80:341-345.
27. Colgan TJ, Murphy J, Cole DE, Narod S, Rosen B. Occult carcinoma in prophylactic oophorectomy specimens: prevalence and association with BRCA germline mutation status. Am J Surg Pathol. 2001;25:1283-1289.
28. Scheuer L, Kauff N, Robson M, et al. Outcome of preventive surgery and screening for breast and ovarian cancer in BRCA mutation carriers. J Clin Oncol. 2002;20:1260-1268.
29. Colgan TJ, Boerner SL, Murphy J, Cole DE, Narod S, Rosen B. Peritoneal lavage cytology: an assessment of its value during prophylactic oophorectomy. Gynecol Oncol. 2002;85:397-403.
30. Meiser B, Butow P, Barratt A, et al. Attitudes toward prophylactic oophorectomy and screening utilization in women at increased risk of developing hereditary breast/ovarian cancer. Gynecol Oncol. 1999;75:122-129.
31. Hurley KE, Miller SM, Costalas JW, Gillespie D, Daly MB. Anxiety/uncertainty reduction as a motivation for interest in prophylactic oophorectomy in women with a family history of ovarian cancer. J Womens Health Gend Based Med. 2001;10:189-199.
32. Robson M, Hensley M, Barakat R, et al. Quality of life in women at risk for ovarian cancer who have undergone risk-reducing oophorectomy. Gynecol Oncol. 2003;89:281-287.
33. Kuerer HM, Hwang ES, Anthony JP, et al. Current national health insurance coverage policies for breast and ovarian cancer prophylactic surgery. Ann Surg Oncol. 2000;7:325-332.
34. Kauff ND, Scheuer L, Robson ME, et al. Insurance reimbursement for risk-reducing mastectomy and oophorectomy in women with BRCA1 or BRCA2 mutations. Genet Med. 2001;3:422-425.
1. Liber AM. Ovarian cancer in a mother and five daughters. Arch Pathol. 1950;49:280-290.
2. Knudson AG, Jr. Mutation and cancer: statistical study of retinoblastoma. Proc Natl Acad Sci USA. 1971;68:820-823.
3. Ford D, Easton DF, Stratton M, et al. Genetic heterogeneity and penetrance analysis of the BRCA1 and BRCA2 genes in breast cancer families. The Breast Cancer Linkage Consortium. Am J Hum Genet. 1998;62:676-689.
4. Risch HA, McLaughlin JR, Cole DE, et al. Prevalence and penetrance of germline BRCA1 and BRCA2 mutations in a population series of 649 women with ovarian cancer. Am J Hum Genet. 2001;68:700-710.
5. Oddoux C, Struewing JP, Clayton CM, et al. The carrier frequency of the BRCA2 6174delT mutation among Ashkenazi Jewish individuals is approximately 1%. Nat Genet. 1996;14:188-190.
6. Tonin P, Weber B, Offit K, et al. Frequency of recurrent BRCA1 and BRCA2 mutations in Ashkenazi Jewish breast cancer families. Nat Med. 1996;2:1179-1183.
7. Schinzinger A. Ueber Carcinoma Mammae. Verhandlungen der Deutschen Gesellschaft fur Chirurgie. 18th Kongress, Berlin, Apr 24–27, 1889. Berlin, Germany: Hirschwald; 1889:28[abstract].
8. Love RR, Philips J. Oophorectomy for breast cancer: history revisited. J Natl Cancer Inst. 2002;94:1433-1434.
9. Feinleib M. Breast cancer and artificial menopause: a cohort study. J Natl Cancer Inst. 1968;41:315-329.
10. Brinton LA, Schairer C, Hoover RN, Fraumeni JF, Jr. Menstrual factors and risk of breast cancer. Cancer Invest. 1988;6:245-254.
11. Tobacman JK, Greene MH, Tucker MA, Costa J, Kase R, Fraumeni JF, Jr. Intraabdominal carcinomatosis after prophylactic oophorectomy in ovarian-cancer-prone families. Lancet. 1982;2:795-797.
12. Piver MS, Jishi MF, Tsukada Y, Nava G. Primary peritoneal carcinoma after prophylactic oophorectomy in women with a family history of ovarian cancer. A report of the Gilda Radner Familial Ovarian Cancer Registry. Cancer. 1993;71:2751-2755.
13. Burke W, Daly M, Garber J, et al. Recommendations for follow-up care of individuals with an inherited predisposition to cancer. II. BRCA1 and BRCA2. Cancer Genetics Studies Consortium. JAMA. 1997;277:997-1003.
14. Schrag D, Kuntz KM, Garber JE, Weeks JC. Decision analysis—effects of prophylactic mastectomy and oophorectomy on life expectancy among women with BRCA1 or BRCA2 mutations. N Engl J Med. 1997;336:1465-1471.
15. Grann VR, Panageas KS, Whang W, Antman KH, Neugut AI. Decision analysis of prophylactic mastectomy and oophorectomy in BRCA1-positive or BRCA2-positive patients. J Clin Oncol. 1998;16:979-985.
16. Rebbeck TR, Levin AM, Eisen A, et al. Breast cancer risk after bilateral prophylactic oophorectomy in BRCA1 mutation carriers. J Natl Cancer Inst. 1999;91:1475-1479.
17. Eisen A, Rebbeck TR, Wood WC, Weber BL. Prophylactic surgery in women with a hereditary predisposition to breast and ovarian cancer. J Clin Oncol. 2000;18:1980-1995.
18. Kauff ND, Satagopan JM, Robson ME, et al. Risk-reducing salpingo-oophorectomy in women with a BRCA1 or BRCA2 mutation. N Engl J Med. 2002;346:1609-1615.
19. Rebbeck TR, Lynch HT, Neuhausen SL, et al. Prophylactic oophorectomy in carriers of BRCA1 or BRCA2 mutations. N Engl J Med. 2002;346:1616-1622.
20. Rutter JL, Wacholder S, Chetrit A, et al. Gynecologic surgeries and risk of ovarian cancer in women with BRCA1 and BRCA2 Ashkenazi founder mutations: an Israeli population-based case-control study. J Natl Cancer Inst. 2003;95:1072-1078.
21. Lu KH, Garber JE, Cramer DW, et al. Occult ovarian tumors in women with BRCA1 or BRCA2 mutations undergoing prophylactic oophorectomy. J Clin Oncol. 2000;18:2728-2732.
22. Lafferty HW, Angioli R, Rudolph J, Penalver MA. Ovarian remnant syndrome: experience at Jackson Memorial Hospital, University of Miami, 1985 through 1993. Am J Obstet Gynecol. 1996;174:641-645.
23. Schorge JO, Muto MG, Welch WR, et al. Molecular evidence for multifocal papillary serous carcinoma of the peritoneum in patients with germline BRCA1 mutations. J Natl Cancer Inst. 1998;90:841-845.
24. Levine DA, Lin O, Barakat RR, et al. Risk of endometrial carcinoma associated with BRCA mutation. Gynecol Oncol. 2001;80:395-398.
25. Paley PJ, Swisher EM, Garcia RL, et al. Occult cancer of the fallopian tube in BRCA-1 germline mutation carriers at prophylactic oophorectomy: a case for recommending hysterectomy at surgical prophylaxis. Gynecol Oncol. 2001;80:176-180.
26. Aziz S, Kuperstein G, Rosen B, et al. A genetic epidemiological study of carcinoma of the fallopian tube. Gynecol Oncol. 2001;80:341-345.
27. Colgan TJ, Murphy J, Cole DE, Narod S, Rosen B. Occult carcinoma in prophylactic oophorectomy specimens: prevalence and association with BRCA germline mutation status. Am J Surg Pathol. 2001;25:1283-1289.
28. Scheuer L, Kauff N, Robson M, et al. Outcome of preventive surgery and screening for breast and ovarian cancer in BRCA mutation carriers. J Clin Oncol. 2002;20:1260-1268.
29. Colgan TJ, Boerner SL, Murphy J, Cole DE, Narod S, Rosen B. Peritoneal lavage cytology: an assessment of its value during prophylactic oophorectomy. Gynecol Oncol. 2002;85:397-403.
30. Meiser B, Butow P, Barratt A, et al. Attitudes toward prophylactic oophorectomy and screening utilization in women at increased risk of developing hereditary breast/ovarian cancer. Gynecol Oncol. 1999;75:122-129.
31. Hurley KE, Miller SM, Costalas JW, Gillespie D, Daly MB. Anxiety/uncertainty reduction as a motivation for interest in prophylactic oophorectomy in women with a family history of ovarian cancer. J Womens Health Gend Based Med. 2001;10:189-199.
32. Robson M, Hensley M, Barakat R, et al. Quality of life in women at risk for ovarian cancer who have undergone risk-reducing oophorectomy. Gynecol Oncol. 2003;89:281-287.
33. Kuerer HM, Hwang ES, Anthony JP, et al. Current national health insurance coverage policies for breast and ovarian cancer prophylactic surgery. Ann Surg Oncol. 2000;7:325-332.
34. Kauff ND, Scheuer L, Robson ME, et al. Insurance reimbursement for risk-reducing mastectomy and oophorectomy in women with BRCA1 or BRCA2 mutations. Genet Med. 2001;3:422-425.
Laparoscopic tissue extraction: Pros and cons of 4 techniques
- In appropriately selected patients, the ability to easily and skillfully remove tissue during laparoscopy facilitates patient recovery and healing and limits hospitalization time.
- Even dense tissues such as partially calcified leiomyomata are readily removed with automatic morcellators, and the size of masses is less significant than with “manual” approaches.
Novel surgical devices and techniques have transformed operative laparoscopy by improving the inefficiencies in tissue extraction that limited early acceptance.
In the beginning, it was relatively easy to isolate a myoma and dissect it from the underlying myometrium, but it took hours to extract the tissue using a hand-held morcellator. This article focuses on the 4 techniques commonly used today, as well as the products that make them possible.
In appropriately selected patients, the ability to remove tissue through any of these methods facilitates patient recovery and healing and limits hospitalization time.
Posterior colpotomy
In the 1980s and early 1990s, it was common for tissue to be extracted through a posterior colpotomy. This is not surprising given that gynecologists were trained to perform transvaginal tubal ligation and to use colpotomies when incising and draining tubo-ovarian abscesses—not to mention our ease in entering the posterior cul-de-sac during vaginal surgery.
The elasticity of the vagina facilitates removal of fairly sizeable masses. Large ovarian cysts or masses can be brought to the cul-de-sac and incised and drained in a manner that markedly reduces the risk of intraperitoneal spillage.
There are disadvantages, however. For example, if the surgeon wants to maintain laparoscopic visualization once the colpotomy has been made, the tissue to be removed must be grasped and brought toward the opening to plug the defect and maintain pneumoperitoneum.
This may not be particularly problematic if there is only 1 mass to be removed, but it can be troublesome if there are several. An option is to place the masses in the posterior cul-de-sac so they can be readily grasped once the posterior colpotomy has been made.
One conceptual concern is the issue of subsequent adhesion formation, especially in patients desiring fertility. Unfortunately, no substantive trials exist to better answer this question.
Removal through the trocar or trocar site
Although some physicians still remove tissue through a posterior colpotomy, most have abandoned that approach in favor of extraction through a primary or lateral laparoscopic port. Indeed, this is the simplest technique for extracting tissue. I often change from a 10-mm laparoscope to a 5-mm instrument, placing the smaller endoscope in one of the lower ports and removing tissue under direct visualization through the 10- or 11-mm infraumbilical port.
If a cystic mass placed in a laparoscopic bag is too large to be removed, carefully aspirate it with a large-gauge needle.
Trapped tissue. One potential problem is the trapping of tissue in trocars that contain a flap valve. If this occurs, remove the trocar, clear the tissue, and replace the trocar in the original site using a blunt instrument such as the 10-mm laparoscope. Do not use the sharp inner blade to replace this port, as it is unduly risky.
For large masses, remove the port to create extra space. It also may be necessary to enlarge the skin or the fascial incision using a blunt instrument such as forceps.
Before the widespread availability of laparoscopic bags, tissue extraction was generally performed in this manner.
Risks include spillage of cyst contents during extraction and development of a hernia secondary to the wider disruption of fascia. This risk is particularly high in the infraumbilical area, which is inherently weak to begin with. It is thus critical—in any methodology—that the fascia be appropriately closed.
Laparoscopic bags
Many of the laparoscopic bags now widely available are easily opened once they have been placed in the abdomen, though some must be opened with graspers after the bag is positioned in the peritoneal cavity. Laparoscopic bags have greater utility when the extracted tissue is soft, such as with a dermoid cyst or ovary. Dense tissue is more difficult to manage.
Some surgeons fashion their own bags using sterile gloves or baggies.
Durability. The bags vary in their ability to withstand manipulation and puncture. For example, one type of nylon bag has a polyurethane inner coating and drawstring closure, making it quite durable. It also comes in a range of sizes, allowing the surgeon to choose the bag most suitable for the mass being removed.
To use a laparoscopic bag, insert it through the infraumbilical trocar and place the mass inside it. Then remove the trocar to provide maximal room for the mass to be extracted.
If the mass is cystic and too large to be removed, carefully aspirate it with a largegauge needle, taking care not to puncture the bag. Otherwise, morcellate the mass in the sac and remove it piecemeal, allowing no spillage of contents.
This may be performed under laparoscopic visualization through the lower ancillary trocars or trocar site. If a larger port has been placed—or there is a clinical need for one—tissue extraction also could be performed through the lower port.
Risks include bag breakage and potential spillage. In addition, it sometimes is necessary to change to a larger bag.
Morcellators
Early morcellators were hand-held, requiring the operator to continuously bite into the tissue and remove the small fragments. While this approach was effective for soft tissues and small myomas, it was ineffective for larger or more solid masses.
“Orange peel” technique. Scissors have been used to achieve the same effect as the handheld morcellator. Harrith Hasson described the “orange peel” technique, in which the surgeon uses scissors to peel away the tissue as one would peel an orange.1 The long, thin strips of tissue then can be extracted through the trocar. Unfortunately, laparoscopic scissors are often too small or dull to adequately incise larger fibroids.
Automatic morcellators have markedly enhanced our ability to perform laparoscopic myomectomy and similar procedures. They also have had a strong impact on nongynecologic procedures such as splenectomy or nephrectomy, in which large amounts of tissue must be removed. Although these devices are costly, the time savings associated with their use are significant. Current devices range from disposable to semidisposable and are available in a wide variety of sizes.
Even dense tissues such as partially calcified leiomyomata are readily removed with automatic morcellators.
Hasson’s orange-peel technique also can be employed with automatic morcellators. This allows long, thin strips of tissue to be removed while facilitating constant visualization of anatomy surrounding the tissue being extracted.
An alternative is making multiple “through-passes” into the myoma using the morcellator. In this method, the strips of tissue obtained will be smaller and the myoma will develop a Swiss-cheese appearance. Note that this approach takes longer and may increase the number of myoma fragments that fall into the pelvis and need to be removed.
Effective for a range of masses. Not surprisingly, even dense tissues such as partially calcified leiomyomata are readily removed with automatic morcellators, and the size of masses is less significant than with “manual” approaches. Nevertheless, it is critical that the surgeon maintain constant visualization and that tissue be brought toward the morcellator and away from underlying structures (FIGURE).
Do not move the morcellator toward the tissue. Because of its sharpness, the automatic morcellator will cut through vital structures as easily as it penetrates fibroids.
FIGURE Automatic morcellation: Move excised tissue toward device
To prevent injury, tissue should be brought toward the morcellator and away from underlying structures. Do not move the morcellator toward the tissue or vital structures may be cut.
Spillage
An early and continuing concern regarding ovarian cystectomy or oophorectomy is spillage of the mass’s contents into the peritoneal cavity. This is more of an issue in the case of borderline or malignant ovarian lesions or mucinous or dermoid ovarian cysts. In fact, nowhere is there more contention than over the clinical ramifications of spillage in the case of malignancy.
Mixed data on impact of spillage. Clinical data suggest that the impact of spillage is inconsequential, whereas other evidence suggests a worsening prognosis.2-5 In the event of spillage, most gynecologic oncologists would convert an ovarian cancer patient with a 1A or 1B staged lesion to stage 1C and would likely administer chemotherapy.
Concern about spillage of a mucinous or dermoid cyst centers on the theoretical risk of pseudomyxoma peritonei or, in the case of a teratoma, chemical peritonitis. Some surgeons routinely enter dermoids and intentionally spill the contents.6,7 Of note, we lack significant case series of ensuing infections or problems with this technique. Still, removing an intact cyst negates this issue and expedites surgery, eliminating the need to irrigate the abdomen and pelvis with large quantities of fluid.
Ectopic pregnancy has also been a concern, as there have been reports of chorionic tissue being disseminated in the abdomen and pelvis during laparoscopic procedures.8
Nowhere is there more contention than over the clinical ramifications of spillage in the case of malignancy.
Patient selection
Preoperative evaluation is a critical component of patient selection. A thorough ultrasound examination can help determine who is and who is not an appropriate candidate for laparoscopic management.
Cases that suggest a high risk of ovarian malignancy may be best managed in the traditional manner, as may patients with a large number of myomas or other compounding factors.
Dr. Bieber reports no financial relationships relevant to this article.
1. Hasson HM, Rotman C, Rana N, Sistos F, Dmowski WP. Laparoscopic myomectomy. Obstet Gynecol. 1992;80:884-888.
2. Mayer C, Miller DM, Ehlen TG. Peritoneal implantation of squamous cell carcinoma following rupture of a dermoid cyst during laparoscopic removal. Gynecol Oncol. 2002;84:180-183.
3. Kodama S, Tanaka K, Tokunaga A, et al. Multivariate analysis of prognostic fators in patients with ovarian cancer stage I and II. Int J Gynaecol Obstet. 1997;56:147-153.
4. Mizuno M, Kikkawa F, Shibata K, et al. Long-term prognosis of stage I ovarian carcinoma. Prognostic importance of intraoperative rupture. Oncology. 2003;65:29-36.
5. Vergote I, De Brabanter J, Fyles A, et al. Prognostic importance of degree of differentiation and cyst rupture in stage I invasive epithelial ovarian carcinoma. Lancet. 2001;357:176-182.
6. Zanetta G, Ferrari L, Mignini-Renzini M, Vignali M, Fadini R. Laparoscopic excision of ovarian dermoid cysts with controlled intraoperative spillage. Safety andeffectiveness. J Reprod Med. 1999;44:815-820.
7. Mecke H, Sawas V. Laparoscopic surgery of dermoid cysts—intraoperative spillage and complications. Eur J Obstet Gynecol Reprod Biol. 2001;96:80-84.
8. Billieux MH, Petignat P, Anguenot JL, Campana A, Bischof P. Early and late halflife of human chorionic gonadotropin as a predictor of persistent trophoblast after laparoscopic conservative surgery for tubal pregnancy. Acta Obstet Gynecol Scand. 2003;82:550-555.
- In appropriately selected patients, the ability to easily and skillfully remove tissue during laparoscopy facilitates patient recovery and healing and limits hospitalization time.
- Even dense tissues such as partially calcified leiomyomata are readily removed with automatic morcellators, and the size of masses is less significant than with “manual” approaches.
Novel surgical devices and techniques have transformed operative laparoscopy by improving the inefficiencies in tissue extraction that limited early acceptance.
In the beginning, it was relatively easy to isolate a myoma and dissect it from the underlying myometrium, but it took hours to extract the tissue using a hand-held morcellator. This article focuses on the 4 techniques commonly used today, as well as the products that make them possible.
In appropriately selected patients, the ability to remove tissue through any of these methods facilitates patient recovery and healing and limits hospitalization time.
Posterior colpotomy
In the 1980s and early 1990s, it was common for tissue to be extracted through a posterior colpotomy. This is not surprising given that gynecologists were trained to perform transvaginal tubal ligation and to use colpotomies when incising and draining tubo-ovarian abscesses—not to mention our ease in entering the posterior cul-de-sac during vaginal surgery.
The elasticity of the vagina facilitates removal of fairly sizeable masses. Large ovarian cysts or masses can be brought to the cul-de-sac and incised and drained in a manner that markedly reduces the risk of intraperitoneal spillage.
There are disadvantages, however. For example, if the surgeon wants to maintain laparoscopic visualization once the colpotomy has been made, the tissue to be removed must be grasped and brought toward the opening to plug the defect and maintain pneumoperitoneum.
This may not be particularly problematic if there is only 1 mass to be removed, but it can be troublesome if there are several. An option is to place the masses in the posterior cul-de-sac so they can be readily grasped once the posterior colpotomy has been made.
One conceptual concern is the issue of subsequent adhesion formation, especially in patients desiring fertility. Unfortunately, no substantive trials exist to better answer this question.
Removal through the trocar or trocar site
Although some physicians still remove tissue through a posterior colpotomy, most have abandoned that approach in favor of extraction through a primary or lateral laparoscopic port. Indeed, this is the simplest technique for extracting tissue. I often change from a 10-mm laparoscope to a 5-mm instrument, placing the smaller endoscope in one of the lower ports and removing tissue under direct visualization through the 10- or 11-mm infraumbilical port.
If a cystic mass placed in a laparoscopic bag is too large to be removed, carefully aspirate it with a large-gauge needle.
Trapped tissue. One potential problem is the trapping of tissue in trocars that contain a flap valve. If this occurs, remove the trocar, clear the tissue, and replace the trocar in the original site using a blunt instrument such as the 10-mm laparoscope. Do not use the sharp inner blade to replace this port, as it is unduly risky.
For large masses, remove the port to create extra space. It also may be necessary to enlarge the skin or the fascial incision using a blunt instrument such as forceps.
Before the widespread availability of laparoscopic bags, tissue extraction was generally performed in this manner.
Risks include spillage of cyst contents during extraction and development of a hernia secondary to the wider disruption of fascia. This risk is particularly high in the infraumbilical area, which is inherently weak to begin with. It is thus critical—in any methodology—that the fascia be appropriately closed.
Laparoscopic bags
Many of the laparoscopic bags now widely available are easily opened once they have been placed in the abdomen, though some must be opened with graspers after the bag is positioned in the peritoneal cavity. Laparoscopic bags have greater utility when the extracted tissue is soft, such as with a dermoid cyst or ovary. Dense tissue is more difficult to manage.
Some surgeons fashion their own bags using sterile gloves or baggies.
Durability. The bags vary in their ability to withstand manipulation and puncture. For example, one type of nylon bag has a polyurethane inner coating and drawstring closure, making it quite durable. It also comes in a range of sizes, allowing the surgeon to choose the bag most suitable for the mass being removed.
To use a laparoscopic bag, insert it through the infraumbilical trocar and place the mass inside it. Then remove the trocar to provide maximal room for the mass to be extracted.
If the mass is cystic and too large to be removed, carefully aspirate it with a largegauge needle, taking care not to puncture the bag. Otherwise, morcellate the mass in the sac and remove it piecemeal, allowing no spillage of contents.
This may be performed under laparoscopic visualization through the lower ancillary trocars or trocar site. If a larger port has been placed—or there is a clinical need for one—tissue extraction also could be performed through the lower port.
Risks include bag breakage and potential spillage. In addition, it sometimes is necessary to change to a larger bag.
Morcellators
Early morcellators were hand-held, requiring the operator to continuously bite into the tissue and remove the small fragments. While this approach was effective for soft tissues and small myomas, it was ineffective for larger or more solid masses.
“Orange peel” technique. Scissors have been used to achieve the same effect as the handheld morcellator. Harrith Hasson described the “orange peel” technique, in which the surgeon uses scissors to peel away the tissue as one would peel an orange.1 The long, thin strips of tissue then can be extracted through the trocar. Unfortunately, laparoscopic scissors are often too small or dull to adequately incise larger fibroids.
Automatic morcellators have markedly enhanced our ability to perform laparoscopic myomectomy and similar procedures. They also have had a strong impact on nongynecologic procedures such as splenectomy or nephrectomy, in which large amounts of tissue must be removed. Although these devices are costly, the time savings associated with their use are significant. Current devices range from disposable to semidisposable and are available in a wide variety of sizes.
Even dense tissues such as partially calcified leiomyomata are readily removed with automatic morcellators.
Hasson’s orange-peel technique also can be employed with automatic morcellators. This allows long, thin strips of tissue to be removed while facilitating constant visualization of anatomy surrounding the tissue being extracted.
An alternative is making multiple “through-passes” into the myoma using the morcellator. In this method, the strips of tissue obtained will be smaller and the myoma will develop a Swiss-cheese appearance. Note that this approach takes longer and may increase the number of myoma fragments that fall into the pelvis and need to be removed.
Effective for a range of masses. Not surprisingly, even dense tissues such as partially calcified leiomyomata are readily removed with automatic morcellators, and the size of masses is less significant than with “manual” approaches. Nevertheless, it is critical that the surgeon maintain constant visualization and that tissue be brought toward the morcellator and away from underlying structures (FIGURE).
Do not move the morcellator toward the tissue. Because of its sharpness, the automatic morcellator will cut through vital structures as easily as it penetrates fibroids.
FIGURE Automatic morcellation: Move excised tissue toward device
To prevent injury, tissue should be brought toward the morcellator and away from underlying structures. Do not move the morcellator toward the tissue or vital structures may be cut.
Spillage
An early and continuing concern regarding ovarian cystectomy or oophorectomy is spillage of the mass’s contents into the peritoneal cavity. This is more of an issue in the case of borderline or malignant ovarian lesions or mucinous or dermoid ovarian cysts. In fact, nowhere is there more contention than over the clinical ramifications of spillage in the case of malignancy.
Mixed data on impact of spillage. Clinical data suggest that the impact of spillage is inconsequential, whereas other evidence suggests a worsening prognosis.2-5 In the event of spillage, most gynecologic oncologists would convert an ovarian cancer patient with a 1A or 1B staged lesion to stage 1C and would likely administer chemotherapy.
Concern about spillage of a mucinous or dermoid cyst centers on the theoretical risk of pseudomyxoma peritonei or, in the case of a teratoma, chemical peritonitis. Some surgeons routinely enter dermoids and intentionally spill the contents.6,7 Of note, we lack significant case series of ensuing infections or problems with this technique. Still, removing an intact cyst negates this issue and expedites surgery, eliminating the need to irrigate the abdomen and pelvis with large quantities of fluid.
Ectopic pregnancy has also been a concern, as there have been reports of chorionic tissue being disseminated in the abdomen and pelvis during laparoscopic procedures.8
Nowhere is there more contention than over the clinical ramifications of spillage in the case of malignancy.
Patient selection
Preoperative evaluation is a critical component of patient selection. A thorough ultrasound examination can help determine who is and who is not an appropriate candidate for laparoscopic management.
Cases that suggest a high risk of ovarian malignancy may be best managed in the traditional manner, as may patients with a large number of myomas or other compounding factors.
Dr. Bieber reports no financial relationships relevant to this article.
- In appropriately selected patients, the ability to easily and skillfully remove tissue during laparoscopy facilitates patient recovery and healing and limits hospitalization time.
- Even dense tissues such as partially calcified leiomyomata are readily removed with automatic morcellators, and the size of masses is less significant than with “manual” approaches.
Novel surgical devices and techniques have transformed operative laparoscopy by improving the inefficiencies in tissue extraction that limited early acceptance.
In the beginning, it was relatively easy to isolate a myoma and dissect it from the underlying myometrium, but it took hours to extract the tissue using a hand-held morcellator. This article focuses on the 4 techniques commonly used today, as well as the products that make them possible.
In appropriately selected patients, the ability to remove tissue through any of these methods facilitates patient recovery and healing and limits hospitalization time.
Posterior colpotomy
In the 1980s and early 1990s, it was common for tissue to be extracted through a posterior colpotomy. This is not surprising given that gynecologists were trained to perform transvaginal tubal ligation and to use colpotomies when incising and draining tubo-ovarian abscesses—not to mention our ease in entering the posterior cul-de-sac during vaginal surgery.
The elasticity of the vagina facilitates removal of fairly sizeable masses. Large ovarian cysts or masses can be brought to the cul-de-sac and incised and drained in a manner that markedly reduces the risk of intraperitoneal spillage.
There are disadvantages, however. For example, if the surgeon wants to maintain laparoscopic visualization once the colpotomy has been made, the tissue to be removed must be grasped and brought toward the opening to plug the defect and maintain pneumoperitoneum.
This may not be particularly problematic if there is only 1 mass to be removed, but it can be troublesome if there are several. An option is to place the masses in the posterior cul-de-sac so they can be readily grasped once the posterior colpotomy has been made.
One conceptual concern is the issue of subsequent adhesion formation, especially in patients desiring fertility. Unfortunately, no substantive trials exist to better answer this question.
Removal through the trocar or trocar site
Although some physicians still remove tissue through a posterior colpotomy, most have abandoned that approach in favor of extraction through a primary or lateral laparoscopic port. Indeed, this is the simplest technique for extracting tissue. I often change from a 10-mm laparoscope to a 5-mm instrument, placing the smaller endoscope in one of the lower ports and removing tissue under direct visualization through the 10- or 11-mm infraumbilical port.
If a cystic mass placed in a laparoscopic bag is too large to be removed, carefully aspirate it with a large-gauge needle.
Trapped tissue. One potential problem is the trapping of tissue in trocars that contain a flap valve. If this occurs, remove the trocar, clear the tissue, and replace the trocar in the original site using a blunt instrument such as the 10-mm laparoscope. Do not use the sharp inner blade to replace this port, as it is unduly risky.
For large masses, remove the port to create extra space. It also may be necessary to enlarge the skin or the fascial incision using a blunt instrument such as forceps.
Before the widespread availability of laparoscopic bags, tissue extraction was generally performed in this manner.
Risks include spillage of cyst contents during extraction and development of a hernia secondary to the wider disruption of fascia. This risk is particularly high in the infraumbilical area, which is inherently weak to begin with. It is thus critical—in any methodology—that the fascia be appropriately closed.
Laparoscopic bags
Many of the laparoscopic bags now widely available are easily opened once they have been placed in the abdomen, though some must be opened with graspers after the bag is positioned in the peritoneal cavity. Laparoscopic bags have greater utility when the extracted tissue is soft, such as with a dermoid cyst or ovary. Dense tissue is more difficult to manage.
Some surgeons fashion their own bags using sterile gloves or baggies.
Durability. The bags vary in their ability to withstand manipulation and puncture. For example, one type of nylon bag has a polyurethane inner coating and drawstring closure, making it quite durable. It also comes in a range of sizes, allowing the surgeon to choose the bag most suitable for the mass being removed.
To use a laparoscopic bag, insert it through the infraumbilical trocar and place the mass inside it. Then remove the trocar to provide maximal room for the mass to be extracted.
If the mass is cystic and too large to be removed, carefully aspirate it with a largegauge needle, taking care not to puncture the bag. Otherwise, morcellate the mass in the sac and remove it piecemeal, allowing no spillage of contents.
This may be performed under laparoscopic visualization through the lower ancillary trocars or trocar site. If a larger port has been placed—or there is a clinical need for one—tissue extraction also could be performed through the lower port.
Risks include bag breakage and potential spillage. In addition, it sometimes is necessary to change to a larger bag.
Morcellators
Early morcellators were hand-held, requiring the operator to continuously bite into the tissue and remove the small fragments. While this approach was effective for soft tissues and small myomas, it was ineffective for larger or more solid masses.
“Orange peel” technique. Scissors have been used to achieve the same effect as the handheld morcellator. Harrith Hasson described the “orange peel” technique, in which the surgeon uses scissors to peel away the tissue as one would peel an orange.1 The long, thin strips of tissue then can be extracted through the trocar. Unfortunately, laparoscopic scissors are often too small or dull to adequately incise larger fibroids.
Automatic morcellators have markedly enhanced our ability to perform laparoscopic myomectomy and similar procedures. They also have had a strong impact on nongynecologic procedures such as splenectomy or nephrectomy, in which large amounts of tissue must be removed. Although these devices are costly, the time savings associated with their use are significant. Current devices range from disposable to semidisposable and are available in a wide variety of sizes.
Even dense tissues such as partially calcified leiomyomata are readily removed with automatic morcellators.
Hasson’s orange-peel technique also can be employed with automatic morcellators. This allows long, thin strips of tissue to be removed while facilitating constant visualization of anatomy surrounding the tissue being extracted.
An alternative is making multiple “through-passes” into the myoma using the morcellator. In this method, the strips of tissue obtained will be smaller and the myoma will develop a Swiss-cheese appearance. Note that this approach takes longer and may increase the number of myoma fragments that fall into the pelvis and need to be removed.
Effective for a range of masses. Not surprisingly, even dense tissues such as partially calcified leiomyomata are readily removed with automatic morcellators, and the size of masses is less significant than with “manual” approaches. Nevertheless, it is critical that the surgeon maintain constant visualization and that tissue be brought toward the morcellator and away from underlying structures (FIGURE).
Do not move the morcellator toward the tissue. Because of its sharpness, the automatic morcellator will cut through vital structures as easily as it penetrates fibroids.
FIGURE Automatic morcellation: Move excised tissue toward device
To prevent injury, tissue should be brought toward the morcellator and away from underlying structures. Do not move the morcellator toward the tissue or vital structures may be cut.
Spillage
An early and continuing concern regarding ovarian cystectomy or oophorectomy is spillage of the mass’s contents into the peritoneal cavity. This is more of an issue in the case of borderline or malignant ovarian lesions or mucinous or dermoid ovarian cysts. In fact, nowhere is there more contention than over the clinical ramifications of spillage in the case of malignancy.
Mixed data on impact of spillage. Clinical data suggest that the impact of spillage is inconsequential, whereas other evidence suggests a worsening prognosis.2-5 In the event of spillage, most gynecologic oncologists would convert an ovarian cancer patient with a 1A or 1B staged lesion to stage 1C and would likely administer chemotherapy.
Concern about spillage of a mucinous or dermoid cyst centers on the theoretical risk of pseudomyxoma peritonei or, in the case of a teratoma, chemical peritonitis. Some surgeons routinely enter dermoids and intentionally spill the contents.6,7 Of note, we lack significant case series of ensuing infections or problems with this technique. Still, removing an intact cyst negates this issue and expedites surgery, eliminating the need to irrigate the abdomen and pelvis with large quantities of fluid.
Ectopic pregnancy has also been a concern, as there have been reports of chorionic tissue being disseminated in the abdomen and pelvis during laparoscopic procedures.8
Nowhere is there more contention than over the clinical ramifications of spillage in the case of malignancy.
Patient selection
Preoperative evaluation is a critical component of patient selection. A thorough ultrasound examination can help determine who is and who is not an appropriate candidate for laparoscopic management.
Cases that suggest a high risk of ovarian malignancy may be best managed in the traditional manner, as may patients with a large number of myomas or other compounding factors.
Dr. Bieber reports no financial relationships relevant to this article.
1. Hasson HM, Rotman C, Rana N, Sistos F, Dmowski WP. Laparoscopic myomectomy. Obstet Gynecol. 1992;80:884-888.
2. Mayer C, Miller DM, Ehlen TG. Peritoneal implantation of squamous cell carcinoma following rupture of a dermoid cyst during laparoscopic removal. Gynecol Oncol. 2002;84:180-183.
3. Kodama S, Tanaka K, Tokunaga A, et al. Multivariate analysis of prognostic fators in patients with ovarian cancer stage I and II. Int J Gynaecol Obstet. 1997;56:147-153.
4. Mizuno M, Kikkawa F, Shibata K, et al. Long-term prognosis of stage I ovarian carcinoma. Prognostic importance of intraoperative rupture. Oncology. 2003;65:29-36.
5. Vergote I, De Brabanter J, Fyles A, et al. Prognostic importance of degree of differentiation and cyst rupture in stage I invasive epithelial ovarian carcinoma. Lancet. 2001;357:176-182.
6. Zanetta G, Ferrari L, Mignini-Renzini M, Vignali M, Fadini R. Laparoscopic excision of ovarian dermoid cysts with controlled intraoperative spillage. Safety andeffectiveness. J Reprod Med. 1999;44:815-820.
7. Mecke H, Sawas V. Laparoscopic surgery of dermoid cysts—intraoperative spillage and complications. Eur J Obstet Gynecol Reprod Biol. 2001;96:80-84.
8. Billieux MH, Petignat P, Anguenot JL, Campana A, Bischof P. Early and late halflife of human chorionic gonadotropin as a predictor of persistent trophoblast after laparoscopic conservative surgery for tubal pregnancy. Acta Obstet Gynecol Scand. 2003;82:550-555.
1. Hasson HM, Rotman C, Rana N, Sistos F, Dmowski WP. Laparoscopic myomectomy. Obstet Gynecol. 1992;80:884-888.
2. Mayer C, Miller DM, Ehlen TG. Peritoneal implantation of squamous cell carcinoma following rupture of a dermoid cyst during laparoscopic removal. Gynecol Oncol. 2002;84:180-183.
3. Kodama S, Tanaka K, Tokunaga A, et al. Multivariate analysis of prognostic fators in patients with ovarian cancer stage I and II. Int J Gynaecol Obstet. 1997;56:147-153.
4. Mizuno M, Kikkawa F, Shibata K, et al. Long-term prognosis of stage I ovarian carcinoma. Prognostic importance of intraoperative rupture. Oncology. 2003;65:29-36.
5. Vergote I, De Brabanter J, Fyles A, et al. Prognostic importance of degree of differentiation and cyst rupture in stage I invasive epithelial ovarian carcinoma. Lancet. 2001;357:176-182.
6. Zanetta G, Ferrari L, Mignini-Renzini M, Vignali M, Fadini R. Laparoscopic excision of ovarian dermoid cysts with controlled intraoperative spillage. Safety andeffectiveness. J Reprod Med. 1999;44:815-820.
7. Mecke H, Sawas V. Laparoscopic surgery of dermoid cysts—intraoperative spillage and complications. Eur J Obstet Gynecol Reprod Biol. 2001;96:80-84.
8. Billieux MH, Petignat P, Anguenot JL, Campana A, Bischof P. Early and late halflife of human chorionic gonadotropin as a predictor of persistent trophoblast after laparoscopic conservative surgery for tubal pregnancy. Acta Obstet Gynecol Scand. 2003;82:550-555.