User login
Preventing BRCA-related cancers: The case for oophorectomy
- Mutations in BRCA1 and BRCA2 may be responsible for more than 90% of inherited predisposition to ovarian cancer.
- BRCA1 and BRCA2 mutations are associated with a lifetime risk of breast cancer of up to 85% and a 15% to 45% lifetime risk of ovarian cancer.
- The only prospective trial to date found risk-reducing salpingo-oophorectomy (RRSO) was associated with an 85% reduction in ovarian cancer and a 68% reduction in breast cancer.
- Because microscopic cancer may be found in 2% to 4% of RRSO specimens upon careful pathologic review, the ovaries and fallopian tubes should be sectioned in their entirety and examined by an experienced gynecologic pathologist.
When A.M. Liber encountered a family of 5 sisters and their mother with histologically confirmed papillary adenocarcinoma of the ovary, he recommended frequent gynecologic cancer screening for all family members and suggested prophylactic oophorectomy as an option.1 The year was 1950.
Flash forward half a century or so, and prophylactic oophorectomy has gained wider acceptance for the prevention of hereditary ovarian and breast cancer, with the only prospective trial to date confirming its overall efficacy for women with BRCA1 and BRCA2 mutations. These mutations are related to the vast majority of inherited ovarian cancers.
Using the evidence published thus far, including the recently published prospective trial, we discuss surgical technique, post-oophorectomy estrogens, psychosocial impact, insurance reimbursement, and other issues.
Three hereditary syndromes
The single biggest risk factor for ovarian cancer is a family history, although only about 10% of cases are believed to be due to an inherited predisposition. Three syndromes are associated with such a predisposition:
- Hereditary breast-ovarian cancer syndrome, caused by mutations in BRCA1 and BRCA2, is thought to be responsible for more than 90% of inherited predisposition to ovarian cancer.
- Hereditary nonpolyposis colon cancer (HNPCC) syndrome is associated with mutations in the mismatch repair genes and a greatly increased risk of cancers of the colon, endometrium, ovaries, and urinary tract. HNPCC accounts for about 2% of inherited ovarian cancers.
- A syndrome of site-specific ovarian cancer also has been proposed, though we lack conclusive evidence that it exists as a separate entity at the genetic level.
How BRCA mutations lead to cancer
BRCA1 and BRCA2 are tumor suppressor genes that play a role in genomic stability and double-stranded DNA break repair. BRCA1 is located on chromosome 17; BRCA2 on chromosome 13. Both genes function as classic tumor suppressors, as described by Knudson.2 Only a single working copy of each gene is needed for the genes to effectively suppress tumors.
In patients with no inherited mutation in these genes, carcinogenesis caused by dysfunction of this pathway can occur only if both working copies of the gene are lost. In contrast, women with an inherited mutation in BRCA1 or BRCA2 start out with only a single working copy of the gene. If any cell loses this single copy, DNA repair cannot occur via this pathway, and cancer can develop.
These repair pathways seem to be particularly important in dividing breast and ovarian cells. This explains why women with inherited mutations in these genes develop cancers more frequently and at an earlier age.
Quantifying the risk
Specific risks associated with BRCA1 and BRCA2 mutations include:
- a lifetime risk of breast cancer of up to 85%, with half of these cancers occurring prior to age 50
- a 15% to 45% lifetime risk of ovarian cancer3,4
Mutations in these genes can be inherited from a mother or father. In the general population, between 1 in 385 and 1 in 800 individuals carry a deleterious mutation in either BRCA1 or BRCA2.
In certain populations, such as Icelandic, French Canadian, or Eastern European Jewish populations, founder effects can contribute to a greatly increased frequency of mutation. For example, the Eastern European Jewish population, from which approximately 90% of North American Jews are descended, has one of the highest known frequencies of BRCA1 and BRCA2 mutation: 1 in 40 individuals carries a deleterious mutation in 1 of these 2 genes.5,6
Most evidence is historical or retrospective
Liber was not the first to suggest oophorectomy to impact the risk of breast or ovarian cancer: The procedure was initially proposed by Schinziner in 1889 as a treatment for breast cancer.7 However, the earliest evidence that oophorectomy was performed as adjuvant therapy did not appear until 7 years later, in 1896 (reviewed by Love and Philips).8
In 1968, Feinleib9 reported that premenopausal oophorectomy decreased the rate of subsequent breast cancer. Twenty years later, Brinton suggested that prophylactic oophorectomy might reduce breast cancer risk in women with a family history of the disease.10
In the sole prospective trial, salpingo-oophorectomy was associated with a 75% reduction in breast and gynecologic cancer.
Post-oophorectomy cancers identified. Possible limitations to the strategy became apparent in the early 1980s, when Tobacman and colleagues11 reported adenocarcinoma histologically indistinguishable from ovarian cancer after oophorectomy in a series of women with a strong family history.
In 1993, Piver et al12 reported a series of 6 cases of primary peritoneal cancer after prophylactic oophorectomy in 324 women from hereditary ovarian cancer families.
In 1997, the Cancer Genetics Studies Consortium reviewed all available data and concluded: “There is insufficient evidence to recommend for or against prophylactic oophorectomy as a measure for reducing ovarian cancer risk. Women with BRCA1 mutations should be counseled that this is an option available to them. Those considering prophylactic oophorectomy should be counseled that cancer has been documented to occur after the procedure.”13
Although the Cancer Genetics Studies Consortium did not specifically comment on prophylactic oophorectomy in carriers of BRCA2 mutations, most authorities interpreted these recommendations to apply to these women as well.
Predicting life expectancy. After these findings, several groups undertook decision analyses to evaluate the effect of prophylactic oophorectomy on life expectancy in women with BRCA mutations. Schrag et al14 reported that prophylactic oophorectomy in a 30-year-old with a BRCA mutation increased life expectancy by 0.3 to 1.7 years. This compares to 0.9 years for adjuvant chemotherapy in node-negative breast cancer.
A subsequent report by Grann and colleagues15 also suggested that prophylactic oophorectomy was associated with an increased life expectancy of 0.4 to 2.6 years. However, surgery was not cost-effective for quality-adjusted life-years saved.
Investigators cite need for prospective studies. In 1999, Rebbeck and colleagues16 conducted a retrospective case-control study of 43 women with BRCA1 mutations who underwent oophorectomy and 79 age-matched women with BRCA1 mutations who had ovaries in situ. In this series, oophorectomy was associated with a 47% decreased risk of subsequent breast cancer (hazard ratio 0.53). However, several investigators cited the need for prospective studies before incorporating oophorectomy into routine clinical practice for the prevention of cancer.17
The first prospective look at risk-reducing surgery
It was in this setting that our group launched a prospective trial to determine whether salpingo-oophorectomy offers any benefit over surveillance in preventing breast and gynecologic (ovarian, fallopian tube, and peritoneal) cancers in women with BRCA mutations.18
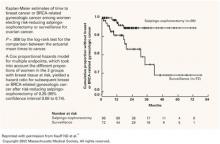
Proportional hazard analysis demonstrated that salpingo-oophorectomy was associated with a 75% reduction in subsequent breast and gynecologic cancer incidence in women with BRCA mutations (hazard ratio 0.25, 95% confidence interval 0.08 to 0.74). When the individual endpoints of breast and gynecologic cancer were observed, risk-reducing salpingo-oophorectomy (RRSO) was associated with an 85% reduction in subsequent ovarian cancer and a 68% reduction in subsequent breast cancer.
Methods. From June 1995 through May 2001, we enrolled 265 women with documented BRCA1 or BRCA2 mutations. Patients were followed by annual questionnaire, telephone contact, and medical-record review. Pathology reports were obtained for all new cancers diagnosed during follow-up.
After excluding women who underwent bilateral salpingo-oophorectomy before genetic testing, who were younger than 35 years at the time of testing, or who did not provide any follow-up information, 173 women with ovaries at risk and a documented BRCA mutation remained. These women participated in formal pre- and post-test genetic counseling and received uniform recommendations for cancer risk reduction, as detailed in the TABLE.
During follow-up, we calculated the incidence of new breast and gynecologic cancers diagnosed in the cohort who elected RRSO and compared it with the incidence of these cancers in women who chose surveillance.
Salpingo-oophorectomy was elected by 101 of the 173 women.
Findings. In 3 of these women, early-stage ovarian or fallopian-tube cancer that had not been detected during preoperative evaluation was found at the time of surgery. In the remaining 98 patients who underwent RRSO, 1 peritoneal cancer and 3 breast cancers were diagnosed during a mean 23 months of follow-up. In the 72 women who chose surveillance, 5 ovarian or peritoneal cancers and 8 breast cancers were diagnosed in a mean 25 months of follow-up.
Kaplan-Meier analysis of time to breast or BRCA-related gynecologic cancer is illustrated in FIGURE 1.
Other studies confirm findings. A second retrospective study by Rebbeck et al19 was released simultaneously with our findings and showed similar benefits. They found a 53% reduction in subsequent breast cancer risk and a 96% reduction in subsequent ovarian cancer risk. In the summer of 2003, a study from Israel by Rutter et al provided further confirmation of the substantially decreased incidence of cancer following risk-reducing surgery.20
TABLE
Breast and ovarian cancer risk-reduction strategies for women with BRCA1 or BRCA2 mutations
| TYPE OF CANCER | STRATEGY | ALSO CONSIDER … |
|---|---|---|
| Breast | Monthly self-examination beginning at age 18 | Imaging Breast ultrasound or magnetic resonance imaging |
| 2-4 physician examinations per year, starting at age 25 | Risk–reducing surgery Mastectomy, no earlier than mid-20s | |
| Annual mammography beginning at age 25 | Salpingo-oophorectomy, after age 35 and completion of childbearing | |
| Chemoprevention Tamoxifen. Need to discuss conflicting reports on efficacy | ||
| Ovarian | CA 125 and ultrasound twice yearly, starting at age 35 | Salpingo-oophorectomy After age 35 and the completion of childbearing |
| Chemoprevention Oral contraceptives, though they may be associated with an increased risk of breast cancer | ||
| Source: Adapted from Scheuer et al28 | ||
FIGURE 1 Reduction in cancer cases associated with salpingo-oophorectomy
Reprinted with permission from Kauff ND et al.18
Copyright 2002 Massachusetts Medical Society. All rights reserved.
Good technique and pathologic review may prevent post-oophorectomy cancer
There are 3 theories about the origin of primary peritoneal cancer after oophorectomy:
- The cancer represents undetected occult cancer present at the time of risk-reducing surgery.
- It represents cancer arising in an ovarian remnant left behind after risk-reducing surgery.
- The peritoneal cancer arises de novo from the peritoneal surface epithelium.
Reasonable evidence supports each of these theories; thus, each may play some role in the incidence of “peritoneal” cancer after risk-reducing surgery.21-23
While surgical technique and detailed pathologic review are unlikely to decrease the incidence of de novo peritoneal cancer, they may play a substantial role in reducing ovarian and related cancers after risk-reducing surgery.
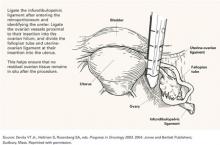
Surgical requirements. Obviously, if a surgery is to be risk-reducing, as much as possible of the tissue at risk should be removed. To do so effectively, the surgeon should be comfortable operating in the retroperitoneum so that the infundibulopelvic ligament can be ligated sufficiently proximal from the ovarian hilum to minimize the possibility of an ovarian remnant. Similarly, if a salpingo-oophorectomy without hysterectomy is to be performed, the fallopian tube should be amputated as close as possible to the uterine cornua (FIGURE 2).
Laparoscopy versus open surgery. RRSO can be performed using either a laparoscopic or open approach. The appropriate choice is best determined by the patient’s history, associated comorbid conditions, need for additional procedures, and experience of the surgeon. At our institution, in the absence of contraindications, we generally offer a laparoscopic approach due to its decreased morbidity.
Concomitant hysterectomy? An area of substantial controversy is whether the uterus should be removed at the time of RRSO. In most studies exploring this issue, hereditary breast-ovarian cancer syndrome does not appear to be associated with an increased risk of uterine cancer.24 However, there is concern that the portion of interstitial fallopian tube left behind after salpingo-oophorectomy may be at risk for malignant transformation.25,26
In our series, almost 90% of risk-reducing procedures were salpingo-oophorectomies without hysterectomy. If there is an additional benefit to concomitant hysterectomy, it has yet to be demonstrated by clinical trials.
In several studies, a patient’s level of anxiety was more important than objective cancer risk in the choice of RRSO.
Close pathologic scrutiny advised. Microscopic cancer may be found in 2% to 4% of RRSO specimens upon careful pathologic review.21,27,28 Thus, it is essential that the ovaries and fallopian tubes are sectioned in their entirety and examined by an experienced gynecologic pathologist to minimize the chance that microscopic cancer goes undetected.
It is not clear whether cytology should be routinely done at the time of risk-reducing surgery. A single report documents malignant cells in a woman with a BRCA1 mutation and no obvious foci of malignancy despite hysterectomy with bilateral salpingo-oophorectomy and staging.29 Pending further studies, we routinely send cytology for review.
FIGURE 2 Careful surgical ligation and division to eliminate residual tissue
Source: Devita VT Jr., Hellman S, Rosenberg SA, eds. Progress in Oncology 2003. 2004: Jones and Bartlett Publishers; Sudbury, Mass. Reprinted with permission.
When no BRCA mutation is present
Most of the data cited thus far apply to women with documented BRCA mutations. There is much less information about the relative risks and benefits of RRSO in women with a personal or family history of breast or ovarian cancer who lack a documented BRCA mutation.
Although RRSO may be appropriate for some of these women, in 2004 it is not the standard of care to recommend RRSO to all individuals with a personal or family history suggestive of an inherited predisposition to ovarian cancer. These patients are best managed by an interdisciplinary team of gynecologists, gynecologic oncologists, and clinical geneticists, all with experience caring for women who may have an inherited predisposition.
Is anxiety a factor?
We have limited information about the psychosocial impact of RRSO. Several studies have found that a patient’s level of anxiety is a more important factor than objective cancer risk in the decision to undergo RRSO.30,31 Unfortunately, we do not yet know whether the surgery successfully reduces these patients’ subjective concerns.
A recent study showed that risk-reducing surgery did not impair women’s overall health or psychological well-being.32 However, 20.7% of the women reported substantial cancer-related anxiety despite the risk-reducing surgery. This issue requires further investigation.
Is estrogen the best option for surgical menopause?
The role of hormone replacement after RRSO is unclear. The issue is important because many women considering salpingo-oophorectomy are in their late 30s or early 40s, when premature surgical menopause is a predictable result. Consequences can include considerable vasomotor and pelvic symptoms.
Preliminary data suggest that a woman’s satisfaction with RRSO depends in large part on its impact on sexual functioning.32 Urogenital symptoms that adversely affected sexual function, such as vaginal dryness and dyspareunia, were the most significant predictors of dissatisfaction with surgery.
Premature surgical menopause also has a substantial impact on osteoporosis risk, while its effect on heart disease remains uncertain.
While nonhormonal therapies can address each of these issues, we need more data on their long-term use. We counsel women considering RRSO that hormone replacement may be an option. We believe it is unlikely to reduce the efficacy of RRSO in preventing ovarian cancer, but it may reduce the protective effect of RRSO against subsequent breast cancer. Until further studies are available, we recommend that decisions regarding hormone replacement be individualized to the patient’s specific symptoms and personal history.
Not all insurers cover RRSO
One study explored insurance carriers’ policies about reimbursing risk-reducing surgical procedures and found that 10% to 11% of private insurers and 48% to 50% of governmental carriers had policies specifically denying coverage for such operations.
An additional 40% to 64% of insurers had no identifiable policy regarding these procedures in women with BRCA mutations.33 The authors speculated that, without identifiable policies, this critical health-care decision may be subject to arbitrary criteria that result in substantial variation.
When we recently investigated the reimbursement experience of women with BRCA mutations undergoing RRSO at our institution, we found that 97% of the procedures were reimbursed in full, less any applicable coinsurance and deductibles.34 Two important limitations of our study: It was conducted at a tertiary cancer center and was retrospective. It is not known if the findings reflect the experience of women with BRCA mutation who have risk-reducing surgery in other settings.
Unresolved issues
RRSO clearly has a role in preventing breast and ovarian cancer in women at inherited risk. However, several questions remain unanswered:
- Who is the best candidate?
- What is optimal timing of the procedure?
- What, if any, concomitant procedures should be performed?
- What is the role of hormone replacement after the surgery?
These issues will be best addressed through multicenter prospective trials, such as the one now being conducted by the Gynecologic Oncology Group.
Hope also remains that further research will improve serum and radiological detection of early ovarian cancer, and that basic research on the molecular etiology and progression of these cancers will ultimately render it unnecessary to remove organs at risk.
The authors report no financial relationships relevant to this article.
1. Liber AM. Ovarian cancer in a mother and five daughters. Arch Pathol. 1950;49:280-290.
2. Knudson AG, Jr. Mutation and cancer: statistical study of retinoblastoma. Proc Natl Acad Sci USA. 1971;68:820-823.
3. Ford D, Easton DF, Stratton M, et al. Genetic heterogeneity and penetrance analysis of the BRCA1 and BRCA2 genes in breast cancer families. The Breast Cancer Linkage Consortium. Am J Hum Genet. 1998;62:676-689.
4. Risch HA, McLaughlin JR, Cole DE, et al. Prevalence and penetrance of germline BRCA1 and BRCA2 mutations in a population series of 649 women with ovarian cancer. Am J Hum Genet. 2001;68:700-710.
5. Oddoux C, Struewing JP, Clayton CM, et al. The carrier frequency of the BRCA2 6174delT mutation among Ashkenazi Jewish individuals is approximately 1%. Nat Genet. 1996;14:188-190.
6. Tonin P, Weber B, Offit K, et al. Frequency of recurrent BRCA1 and BRCA2 mutations in Ashkenazi Jewish breast cancer families. Nat Med. 1996;2:1179-1183.
7. Schinzinger A. Ueber Carcinoma Mammae. Verhandlungen der Deutschen Gesellschaft fur Chirurgie. 18th Kongress, Berlin, Apr 24–27, 1889. Berlin, Germany: Hirschwald; 1889:28[abstract].
8. Love RR, Philips J. Oophorectomy for breast cancer: history revisited. J Natl Cancer Inst. 2002;94:1433-1434.
9. Feinleib M. Breast cancer and artificial menopause: a cohort study. J Natl Cancer Inst. 1968;41:315-329.
10. Brinton LA, Schairer C, Hoover RN, Fraumeni JF, Jr. Menstrual factors and risk of breast cancer. Cancer Invest. 1988;6:245-254.
11. Tobacman JK, Greene MH, Tucker MA, Costa J, Kase R, Fraumeni JF, Jr. Intraabdominal carcinomatosis after prophylactic oophorectomy in ovarian-cancer-prone families. Lancet. 1982;2:795-797.
12. Piver MS, Jishi MF, Tsukada Y, Nava G. Primary peritoneal carcinoma after prophylactic oophorectomy in women with a family history of ovarian cancer. A report of the Gilda Radner Familial Ovarian Cancer Registry. Cancer. 1993;71:2751-2755.
13. Burke W, Daly M, Garber J, et al. Recommendations for follow-up care of individuals with an inherited predisposition to cancer. II. BRCA1 and BRCA2. Cancer Genetics Studies Consortium. JAMA. 1997;277:997-1003.
14. Schrag D, Kuntz KM, Garber JE, Weeks JC. Decision analysis—effects of prophylactic mastectomy and oophorectomy on life expectancy among women with BRCA1 or BRCA2 mutations. N Engl J Med. 1997;336:1465-1471.
15. Grann VR, Panageas KS, Whang W, Antman KH, Neugut AI. Decision analysis of prophylactic mastectomy and oophorectomy in BRCA1-positive or BRCA2-positive patients. J Clin Oncol. 1998;16:979-985.
16. Rebbeck TR, Levin AM, Eisen A, et al. Breast cancer risk after bilateral prophylactic oophorectomy in BRCA1 mutation carriers. J Natl Cancer Inst. 1999;91:1475-1479.
17. Eisen A, Rebbeck TR, Wood WC, Weber BL. Prophylactic surgery in women with a hereditary predisposition to breast and ovarian cancer. J Clin Oncol. 2000;18:1980-1995.
18. Kauff ND, Satagopan JM, Robson ME, et al. Risk-reducing salpingo-oophorectomy in women with a BRCA1 or BRCA2 mutation. N Engl J Med. 2002;346:1609-1615.
19. Rebbeck TR, Lynch HT, Neuhausen SL, et al. Prophylactic oophorectomy in carriers of BRCA1 or BRCA2 mutations. N Engl J Med. 2002;346:1616-1622.
20. Rutter JL, Wacholder S, Chetrit A, et al. Gynecologic surgeries and risk of ovarian cancer in women with BRCA1 and BRCA2 Ashkenazi founder mutations: an Israeli population-based case-control study. J Natl Cancer Inst. 2003;95:1072-1078.
21. Lu KH, Garber JE, Cramer DW, et al. Occult ovarian tumors in women with BRCA1 or BRCA2 mutations undergoing prophylactic oophorectomy. J Clin Oncol. 2000;18:2728-2732.
22. Lafferty HW, Angioli R, Rudolph J, Penalver MA. Ovarian remnant syndrome: experience at Jackson Memorial Hospital, University of Miami, 1985 through 1993. Am J Obstet Gynecol. 1996;174:641-645.
23. Schorge JO, Muto MG, Welch WR, et al. Molecular evidence for multifocal papillary serous carcinoma of the peritoneum in patients with germline BRCA1 mutations. J Natl Cancer Inst. 1998;90:841-845.
24. Levine DA, Lin O, Barakat RR, et al. Risk of endometrial carcinoma associated with BRCA mutation. Gynecol Oncol. 2001;80:395-398.
25. Paley PJ, Swisher EM, Garcia RL, et al. Occult cancer of the fallopian tube in BRCA-1 germline mutation carriers at prophylactic oophorectomy: a case for recommending hysterectomy at surgical prophylaxis. Gynecol Oncol. 2001;80:176-180.
26. Aziz S, Kuperstein G, Rosen B, et al. A genetic epidemiological study of carcinoma of the fallopian tube. Gynecol Oncol. 2001;80:341-345.
27. Colgan TJ, Murphy J, Cole DE, Narod S, Rosen B. Occult carcinoma in prophylactic oophorectomy specimens: prevalence and association with BRCA germline mutation status. Am J Surg Pathol. 2001;25:1283-1289.
28. Scheuer L, Kauff N, Robson M, et al. Outcome of preventive surgery and screening for breast and ovarian cancer in BRCA mutation carriers. J Clin Oncol. 2002;20:1260-1268.
29. Colgan TJ, Boerner SL, Murphy J, Cole DE, Narod S, Rosen B. Peritoneal lavage cytology: an assessment of its value during prophylactic oophorectomy. Gynecol Oncol. 2002;85:397-403.
30. Meiser B, Butow P, Barratt A, et al. Attitudes toward prophylactic oophorectomy and screening utilization in women at increased risk of developing hereditary breast/ovarian cancer. Gynecol Oncol. 1999;75:122-129.
31. Hurley KE, Miller SM, Costalas JW, Gillespie D, Daly MB. Anxiety/uncertainty reduction as a motivation for interest in prophylactic oophorectomy in women with a family history of ovarian cancer. J Womens Health Gend Based Med. 2001;10:189-199.
32. Robson M, Hensley M, Barakat R, et al. Quality of life in women at risk for ovarian cancer who have undergone risk-reducing oophorectomy. Gynecol Oncol. 2003;89:281-287.
33. Kuerer HM, Hwang ES, Anthony JP, et al. Current national health insurance coverage policies for breast and ovarian cancer prophylactic surgery. Ann Surg Oncol. 2000;7:325-332.
34. Kauff ND, Scheuer L, Robson ME, et al. Insurance reimbursement for risk-reducing mastectomy and oophorectomy in women with BRCA1 or BRCA2 mutations. Genet Med. 2001;3:422-425.
- Mutations in BRCA1 and BRCA2 may be responsible for more than 90% of inherited predisposition to ovarian cancer.
- BRCA1 and BRCA2 mutations are associated with a lifetime risk of breast cancer of up to 85% and a 15% to 45% lifetime risk of ovarian cancer.
- The only prospective trial to date found risk-reducing salpingo-oophorectomy (RRSO) was associated with an 85% reduction in ovarian cancer and a 68% reduction in breast cancer.
- Because microscopic cancer may be found in 2% to 4% of RRSO specimens upon careful pathologic review, the ovaries and fallopian tubes should be sectioned in their entirety and examined by an experienced gynecologic pathologist.
When A.M. Liber encountered a family of 5 sisters and their mother with histologically confirmed papillary adenocarcinoma of the ovary, he recommended frequent gynecologic cancer screening for all family members and suggested prophylactic oophorectomy as an option.1 The year was 1950.
Flash forward half a century or so, and prophylactic oophorectomy has gained wider acceptance for the prevention of hereditary ovarian and breast cancer, with the only prospective trial to date confirming its overall efficacy for women with BRCA1 and BRCA2 mutations. These mutations are related to the vast majority of inherited ovarian cancers.
Using the evidence published thus far, including the recently published prospective trial, we discuss surgical technique, post-oophorectomy estrogens, psychosocial impact, insurance reimbursement, and other issues.
Three hereditary syndromes
The single biggest risk factor for ovarian cancer is a family history, although only about 10% of cases are believed to be due to an inherited predisposition. Three syndromes are associated with such a predisposition:
- Hereditary breast-ovarian cancer syndrome, caused by mutations in BRCA1 and BRCA2, is thought to be responsible for more than 90% of inherited predisposition to ovarian cancer.
- Hereditary nonpolyposis colon cancer (HNPCC) syndrome is associated with mutations in the mismatch repair genes and a greatly increased risk of cancers of the colon, endometrium, ovaries, and urinary tract. HNPCC accounts for about 2% of inherited ovarian cancers.
- A syndrome of site-specific ovarian cancer also has been proposed, though we lack conclusive evidence that it exists as a separate entity at the genetic level.
How BRCA mutations lead to cancer
BRCA1 and BRCA2 are tumor suppressor genes that play a role in genomic stability and double-stranded DNA break repair. BRCA1 is located on chromosome 17; BRCA2 on chromosome 13. Both genes function as classic tumor suppressors, as described by Knudson.2 Only a single working copy of each gene is needed for the genes to effectively suppress tumors.
In patients with no inherited mutation in these genes, carcinogenesis caused by dysfunction of this pathway can occur only if both working copies of the gene are lost. In contrast, women with an inherited mutation in BRCA1 or BRCA2 start out with only a single working copy of the gene. If any cell loses this single copy, DNA repair cannot occur via this pathway, and cancer can develop.
These repair pathways seem to be particularly important in dividing breast and ovarian cells. This explains why women with inherited mutations in these genes develop cancers more frequently and at an earlier age.
Quantifying the risk
Specific risks associated with BRCA1 and BRCA2 mutations include:
- a lifetime risk of breast cancer of up to 85%, with half of these cancers occurring prior to age 50
- a 15% to 45% lifetime risk of ovarian cancer3,4
Mutations in these genes can be inherited from a mother or father. In the general population, between 1 in 385 and 1 in 800 individuals carry a deleterious mutation in either BRCA1 or BRCA2.
In certain populations, such as Icelandic, French Canadian, or Eastern European Jewish populations, founder effects can contribute to a greatly increased frequency of mutation. For example, the Eastern European Jewish population, from which approximately 90% of North American Jews are descended, has one of the highest known frequencies of BRCA1 and BRCA2 mutation: 1 in 40 individuals carries a deleterious mutation in 1 of these 2 genes.5,6
Most evidence is historical or retrospective
Liber was not the first to suggest oophorectomy to impact the risk of breast or ovarian cancer: The procedure was initially proposed by Schinziner in 1889 as a treatment for breast cancer.7 However, the earliest evidence that oophorectomy was performed as adjuvant therapy did not appear until 7 years later, in 1896 (reviewed by Love and Philips).8
In 1968, Feinleib9 reported that premenopausal oophorectomy decreased the rate of subsequent breast cancer. Twenty years later, Brinton suggested that prophylactic oophorectomy might reduce breast cancer risk in women with a family history of the disease.10
In the sole prospective trial, salpingo-oophorectomy was associated with a 75% reduction in breast and gynecologic cancer.
Post-oophorectomy cancers identified. Possible limitations to the strategy became apparent in the early 1980s, when Tobacman and colleagues11 reported adenocarcinoma histologically indistinguishable from ovarian cancer after oophorectomy in a series of women with a strong family history.
In 1993, Piver et al12 reported a series of 6 cases of primary peritoneal cancer after prophylactic oophorectomy in 324 women from hereditary ovarian cancer families.
In 1997, the Cancer Genetics Studies Consortium reviewed all available data and concluded: “There is insufficient evidence to recommend for or against prophylactic oophorectomy as a measure for reducing ovarian cancer risk. Women with BRCA1 mutations should be counseled that this is an option available to them. Those considering prophylactic oophorectomy should be counseled that cancer has been documented to occur after the procedure.”13
Although the Cancer Genetics Studies Consortium did not specifically comment on prophylactic oophorectomy in carriers of BRCA2 mutations, most authorities interpreted these recommendations to apply to these women as well.
Predicting life expectancy. After these findings, several groups undertook decision analyses to evaluate the effect of prophylactic oophorectomy on life expectancy in women with BRCA mutations. Schrag et al14 reported that prophylactic oophorectomy in a 30-year-old with a BRCA mutation increased life expectancy by 0.3 to 1.7 years. This compares to 0.9 years for adjuvant chemotherapy in node-negative breast cancer.
A subsequent report by Grann and colleagues15 also suggested that prophylactic oophorectomy was associated with an increased life expectancy of 0.4 to 2.6 years. However, surgery was not cost-effective for quality-adjusted life-years saved.
Investigators cite need for prospective studies. In 1999, Rebbeck and colleagues16 conducted a retrospective case-control study of 43 women with BRCA1 mutations who underwent oophorectomy and 79 age-matched women with BRCA1 mutations who had ovaries in situ. In this series, oophorectomy was associated with a 47% decreased risk of subsequent breast cancer (hazard ratio 0.53). However, several investigators cited the need for prospective studies before incorporating oophorectomy into routine clinical practice for the prevention of cancer.17
The first prospective look at risk-reducing surgery
It was in this setting that our group launched a prospective trial to determine whether salpingo-oophorectomy offers any benefit over surveillance in preventing breast and gynecologic (ovarian, fallopian tube, and peritoneal) cancers in women with BRCA mutations.18
Proportional hazard analysis demonstrated that salpingo-oophorectomy was associated with a 75% reduction in subsequent breast and gynecologic cancer incidence in women with BRCA mutations (hazard ratio 0.25, 95% confidence interval 0.08 to 0.74). When the individual endpoints of breast and gynecologic cancer were observed, risk-reducing salpingo-oophorectomy (RRSO) was associated with an 85% reduction in subsequent ovarian cancer and a 68% reduction in subsequent breast cancer.
Methods. From June 1995 through May 2001, we enrolled 265 women with documented BRCA1 or BRCA2 mutations. Patients were followed by annual questionnaire, telephone contact, and medical-record review. Pathology reports were obtained for all new cancers diagnosed during follow-up.
After excluding women who underwent bilateral salpingo-oophorectomy before genetic testing, who were younger than 35 years at the time of testing, or who did not provide any follow-up information, 173 women with ovaries at risk and a documented BRCA mutation remained. These women participated in formal pre- and post-test genetic counseling and received uniform recommendations for cancer risk reduction, as detailed in the TABLE.
During follow-up, we calculated the incidence of new breast and gynecologic cancers diagnosed in the cohort who elected RRSO and compared it with the incidence of these cancers in women who chose surveillance.
Salpingo-oophorectomy was elected by 101 of the 173 women.
Findings. In 3 of these women, early-stage ovarian or fallopian-tube cancer that had not been detected during preoperative evaluation was found at the time of surgery. In the remaining 98 patients who underwent RRSO, 1 peritoneal cancer and 3 breast cancers were diagnosed during a mean 23 months of follow-up. In the 72 women who chose surveillance, 5 ovarian or peritoneal cancers and 8 breast cancers were diagnosed in a mean 25 months of follow-up.
Kaplan-Meier analysis of time to breast or BRCA-related gynecologic cancer is illustrated in FIGURE 1.
Other studies confirm findings. A second retrospective study by Rebbeck et al19 was released simultaneously with our findings and showed similar benefits. They found a 53% reduction in subsequent breast cancer risk and a 96% reduction in subsequent ovarian cancer risk. In the summer of 2003, a study from Israel by Rutter et al provided further confirmation of the substantially decreased incidence of cancer following risk-reducing surgery.20
TABLE
Breast and ovarian cancer risk-reduction strategies for women with BRCA1 or BRCA2 mutations
| TYPE OF CANCER | STRATEGY | ALSO CONSIDER … |
|---|---|---|
| Breast | Monthly self-examination beginning at age 18 | Imaging Breast ultrasound or magnetic resonance imaging |
| 2-4 physician examinations per year, starting at age 25 | Risk–reducing surgery Mastectomy, no earlier than mid-20s | |
| Annual mammography beginning at age 25 | Salpingo-oophorectomy, after age 35 and completion of childbearing | |
| Chemoprevention Tamoxifen. Need to discuss conflicting reports on efficacy | ||
| Ovarian | CA 125 and ultrasound twice yearly, starting at age 35 | Salpingo-oophorectomy After age 35 and the completion of childbearing |
| Chemoprevention Oral contraceptives, though they may be associated with an increased risk of breast cancer | ||
| Source: Adapted from Scheuer et al28 | ||
FIGURE 1 Reduction in cancer cases associated with salpingo-oophorectomy
Reprinted with permission from Kauff ND et al.18
Copyright 2002 Massachusetts Medical Society. All rights reserved.
Good technique and pathologic review may prevent post-oophorectomy cancer
There are 3 theories about the origin of primary peritoneal cancer after oophorectomy:
- The cancer represents undetected occult cancer present at the time of risk-reducing surgery.
- It represents cancer arising in an ovarian remnant left behind after risk-reducing surgery.
- The peritoneal cancer arises de novo from the peritoneal surface epithelium.
Reasonable evidence supports each of these theories; thus, each may play some role in the incidence of “peritoneal” cancer after risk-reducing surgery.21-23
While surgical technique and detailed pathologic review are unlikely to decrease the incidence of de novo peritoneal cancer, they may play a substantial role in reducing ovarian and related cancers after risk-reducing surgery.
Surgical requirements. Obviously, if a surgery is to be risk-reducing, as much as possible of the tissue at risk should be removed. To do so effectively, the surgeon should be comfortable operating in the retroperitoneum so that the infundibulopelvic ligament can be ligated sufficiently proximal from the ovarian hilum to minimize the possibility of an ovarian remnant. Similarly, if a salpingo-oophorectomy without hysterectomy is to be performed, the fallopian tube should be amputated as close as possible to the uterine cornua (FIGURE 2).
Laparoscopy versus open surgery. RRSO can be performed using either a laparoscopic or open approach. The appropriate choice is best determined by the patient’s history, associated comorbid conditions, need for additional procedures, and experience of the surgeon. At our institution, in the absence of contraindications, we generally offer a laparoscopic approach due to its decreased morbidity.
Concomitant hysterectomy? An area of substantial controversy is whether the uterus should be removed at the time of RRSO. In most studies exploring this issue, hereditary breast-ovarian cancer syndrome does not appear to be associated with an increased risk of uterine cancer.24 However, there is concern that the portion of interstitial fallopian tube left behind after salpingo-oophorectomy may be at risk for malignant transformation.25,26
In our series, almost 90% of risk-reducing procedures were salpingo-oophorectomies without hysterectomy. If there is an additional benefit to concomitant hysterectomy, it has yet to be demonstrated by clinical trials.
In several studies, a patient’s level of anxiety was more important than objective cancer risk in the choice of RRSO.
Close pathologic scrutiny advised. Microscopic cancer may be found in 2% to 4% of RRSO specimens upon careful pathologic review.21,27,28 Thus, it is essential that the ovaries and fallopian tubes are sectioned in their entirety and examined by an experienced gynecologic pathologist to minimize the chance that microscopic cancer goes undetected.
It is not clear whether cytology should be routinely done at the time of risk-reducing surgery. A single report documents malignant cells in a woman with a BRCA1 mutation and no obvious foci of malignancy despite hysterectomy with bilateral salpingo-oophorectomy and staging.29 Pending further studies, we routinely send cytology for review.
FIGURE 2 Careful surgical ligation and division to eliminate residual tissue
Source: Devita VT Jr., Hellman S, Rosenberg SA, eds. Progress in Oncology 2003. 2004: Jones and Bartlett Publishers; Sudbury, Mass. Reprinted with permission.
When no BRCA mutation is present
Most of the data cited thus far apply to women with documented BRCA mutations. There is much less information about the relative risks and benefits of RRSO in women with a personal or family history of breast or ovarian cancer who lack a documented BRCA mutation.
Although RRSO may be appropriate for some of these women, in 2004 it is not the standard of care to recommend RRSO to all individuals with a personal or family history suggestive of an inherited predisposition to ovarian cancer. These patients are best managed by an interdisciplinary team of gynecologists, gynecologic oncologists, and clinical geneticists, all with experience caring for women who may have an inherited predisposition.
Is anxiety a factor?
We have limited information about the psychosocial impact of RRSO. Several studies have found that a patient’s level of anxiety is a more important factor than objective cancer risk in the decision to undergo RRSO.30,31 Unfortunately, we do not yet know whether the surgery successfully reduces these patients’ subjective concerns.
A recent study showed that risk-reducing surgery did not impair women’s overall health or psychological well-being.32 However, 20.7% of the women reported substantial cancer-related anxiety despite the risk-reducing surgery. This issue requires further investigation.
Is estrogen the best option for surgical menopause?
The role of hormone replacement after RRSO is unclear. The issue is important because many women considering salpingo-oophorectomy are in their late 30s or early 40s, when premature surgical menopause is a predictable result. Consequences can include considerable vasomotor and pelvic symptoms.
Preliminary data suggest that a woman’s satisfaction with RRSO depends in large part on its impact on sexual functioning.32 Urogenital symptoms that adversely affected sexual function, such as vaginal dryness and dyspareunia, were the most significant predictors of dissatisfaction with surgery.
Premature surgical menopause also has a substantial impact on osteoporosis risk, while its effect on heart disease remains uncertain.
While nonhormonal therapies can address each of these issues, we need more data on their long-term use. We counsel women considering RRSO that hormone replacement may be an option. We believe it is unlikely to reduce the efficacy of RRSO in preventing ovarian cancer, but it may reduce the protective effect of RRSO against subsequent breast cancer. Until further studies are available, we recommend that decisions regarding hormone replacement be individualized to the patient’s specific symptoms and personal history.
Not all insurers cover RRSO
One study explored insurance carriers’ policies about reimbursing risk-reducing surgical procedures and found that 10% to 11% of private insurers and 48% to 50% of governmental carriers had policies specifically denying coverage for such operations.
An additional 40% to 64% of insurers had no identifiable policy regarding these procedures in women with BRCA mutations.33 The authors speculated that, without identifiable policies, this critical health-care decision may be subject to arbitrary criteria that result in substantial variation.
When we recently investigated the reimbursement experience of women with BRCA mutations undergoing RRSO at our institution, we found that 97% of the procedures were reimbursed in full, less any applicable coinsurance and deductibles.34 Two important limitations of our study: It was conducted at a tertiary cancer center and was retrospective. It is not known if the findings reflect the experience of women with BRCA mutation who have risk-reducing surgery in other settings.
Unresolved issues
RRSO clearly has a role in preventing breast and ovarian cancer in women at inherited risk. However, several questions remain unanswered:
- Who is the best candidate?
- What is optimal timing of the procedure?
- What, if any, concomitant procedures should be performed?
- What is the role of hormone replacement after the surgery?
These issues will be best addressed through multicenter prospective trials, such as the one now being conducted by the Gynecologic Oncology Group.
Hope also remains that further research will improve serum and radiological detection of early ovarian cancer, and that basic research on the molecular etiology and progression of these cancers will ultimately render it unnecessary to remove organs at risk.
The authors report no financial relationships relevant to this article.
- Mutations in BRCA1 and BRCA2 may be responsible for more than 90% of inherited predisposition to ovarian cancer.
- BRCA1 and BRCA2 mutations are associated with a lifetime risk of breast cancer of up to 85% and a 15% to 45% lifetime risk of ovarian cancer.
- The only prospective trial to date found risk-reducing salpingo-oophorectomy (RRSO) was associated with an 85% reduction in ovarian cancer and a 68% reduction in breast cancer.
- Because microscopic cancer may be found in 2% to 4% of RRSO specimens upon careful pathologic review, the ovaries and fallopian tubes should be sectioned in their entirety and examined by an experienced gynecologic pathologist.
When A.M. Liber encountered a family of 5 sisters and their mother with histologically confirmed papillary adenocarcinoma of the ovary, he recommended frequent gynecologic cancer screening for all family members and suggested prophylactic oophorectomy as an option.1 The year was 1950.
Flash forward half a century or so, and prophylactic oophorectomy has gained wider acceptance for the prevention of hereditary ovarian and breast cancer, with the only prospective trial to date confirming its overall efficacy for women with BRCA1 and BRCA2 mutations. These mutations are related to the vast majority of inherited ovarian cancers.
Using the evidence published thus far, including the recently published prospective trial, we discuss surgical technique, post-oophorectomy estrogens, psychosocial impact, insurance reimbursement, and other issues.
Three hereditary syndromes
The single biggest risk factor for ovarian cancer is a family history, although only about 10% of cases are believed to be due to an inherited predisposition. Three syndromes are associated with such a predisposition:
- Hereditary breast-ovarian cancer syndrome, caused by mutations in BRCA1 and BRCA2, is thought to be responsible for more than 90% of inherited predisposition to ovarian cancer.
- Hereditary nonpolyposis colon cancer (HNPCC) syndrome is associated with mutations in the mismatch repair genes and a greatly increased risk of cancers of the colon, endometrium, ovaries, and urinary tract. HNPCC accounts for about 2% of inherited ovarian cancers.
- A syndrome of site-specific ovarian cancer also has been proposed, though we lack conclusive evidence that it exists as a separate entity at the genetic level.
How BRCA mutations lead to cancer
BRCA1 and BRCA2 are tumor suppressor genes that play a role in genomic stability and double-stranded DNA break repair. BRCA1 is located on chromosome 17; BRCA2 on chromosome 13. Both genes function as classic tumor suppressors, as described by Knudson.2 Only a single working copy of each gene is needed for the genes to effectively suppress tumors.
In patients with no inherited mutation in these genes, carcinogenesis caused by dysfunction of this pathway can occur only if both working copies of the gene are lost. In contrast, women with an inherited mutation in BRCA1 or BRCA2 start out with only a single working copy of the gene. If any cell loses this single copy, DNA repair cannot occur via this pathway, and cancer can develop.
These repair pathways seem to be particularly important in dividing breast and ovarian cells. This explains why women with inherited mutations in these genes develop cancers more frequently and at an earlier age.
Quantifying the risk
Specific risks associated with BRCA1 and BRCA2 mutations include:
- a lifetime risk of breast cancer of up to 85%, with half of these cancers occurring prior to age 50
- a 15% to 45% lifetime risk of ovarian cancer3,4
Mutations in these genes can be inherited from a mother or father. In the general population, between 1 in 385 and 1 in 800 individuals carry a deleterious mutation in either BRCA1 or BRCA2.
In certain populations, such as Icelandic, French Canadian, or Eastern European Jewish populations, founder effects can contribute to a greatly increased frequency of mutation. For example, the Eastern European Jewish population, from which approximately 90% of North American Jews are descended, has one of the highest known frequencies of BRCA1 and BRCA2 mutation: 1 in 40 individuals carries a deleterious mutation in 1 of these 2 genes.5,6
Most evidence is historical or retrospective
Liber was not the first to suggest oophorectomy to impact the risk of breast or ovarian cancer: The procedure was initially proposed by Schinziner in 1889 as a treatment for breast cancer.7 However, the earliest evidence that oophorectomy was performed as adjuvant therapy did not appear until 7 years later, in 1896 (reviewed by Love and Philips).8
In 1968, Feinleib9 reported that premenopausal oophorectomy decreased the rate of subsequent breast cancer. Twenty years later, Brinton suggested that prophylactic oophorectomy might reduce breast cancer risk in women with a family history of the disease.10
In the sole prospective trial, salpingo-oophorectomy was associated with a 75% reduction in breast and gynecologic cancer.
Post-oophorectomy cancers identified. Possible limitations to the strategy became apparent in the early 1980s, when Tobacman and colleagues11 reported adenocarcinoma histologically indistinguishable from ovarian cancer after oophorectomy in a series of women with a strong family history.
In 1993, Piver et al12 reported a series of 6 cases of primary peritoneal cancer after prophylactic oophorectomy in 324 women from hereditary ovarian cancer families.
In 1997, the Cancer Genetics Studies Consortium reviewed all available data and concluded: “There is insufficient evidence to recommend for or against prophylactic oophorectomy as a measure for reducing ovarian cancer risk. Women with BRCA1 mutations should be counseled that this is an option available to them. Those considering prophylactic oophorectomy should be counseled that cancer has been documented to occur after the procedure.”13
Although the Cancer Genetics Studies Consortium did not specifically comment on prophylactic oophorectomy in carriers of BRCA2 mutations, most authorities interpreted these recommendations to apply to these women as well.
Predicting life expectancy. After these findings, several groups undertook decision analyses to evaluate the effect of prophylactic oophorectomy on life expectancy in women with BRCA mutations. Schrag et al14 reported that prophylactic oophorectomy in a 30-year-old with a BRCA mutation increased life expectancy by 0.3 to 1.7 years. This compares to 0.9 years for adjuvant chemotherapy in node-negative breast cancer.
A subsequent report by Grann and colleagues15 also suggested that prophylactic oophorectomy was associated with an increased life expectancy of 0.4 to 2.6 years. However, surgery was not cost-effective for quality-adjusted life-years saved.
Investigators cite need for prospective studies. In 1999, Rebbeck and colleagues16 conducted a retrospective case-control study of 43 women with BRCA1 mutations who underwent oophorectomy and 79 age-matched women with BRCA1 mutations who had ovaries in situ. In this series, oophorectomy was associated with a 47% decreased risk of subsequent breast cancer (hazard ratio 0.53). However, several investigators cited the need for prospective studies before incorporating oophorectomy into routine clinical practice for the prevention of cancer.17
The first prospective look at risk-reducing surgery
It was in this setting that our group launched a prospective trial to determine whether salpingo-oophorectomy offers any benefit over surveillance in preventing breast and gynecologic (ovarian, fallopian tube, and peritoneal) cancers in women with BRCA mutations.18
Proportional hazard analysis demonstrated that salpingo-oophorectomy was associated with a 75% reduction in subsequent breast and gynecologic cancer incidence in women with BRCA mutations (hazard ratio 0.25, 95% confidence interval 0.08 to 0.74). When the individual endpoints of breast and gynecologic cancer were observed, risk-reducing salpingo-oophorectomy (RRSO) was associated with an 85% reduction in subsequent ovarian cancer and a 68% reduction in subsequent breast cancer.
Methods. From June 1995 through May 2001, we enrolled 265 women with documented BRCA1 or BRCA2 mutations. Patients were followed by annual questionnaire, telephone contact, and medical-record review. Pathology reports were obtained for all new cancers diagnosed during follow-up.
After excluding women who underwent bilateral salpingo-oophorectomy before genetic testing, who were younger than 35 years at the time of testing, or who did not provide any follow-up information, 173 women with ovaries at risk and a documented BRCA mutation remained. These women participated in formal pre- and post-test genetic counseling and received uniform recommendations for cancer risk reduction, as detailed in the TABLE.
During follow-up, we calculated the incidence of new breast and gynecologic cancers diagnosed in the cohort who elected RRSO and compared it with the incidence of these cancers in women who chose surveillance.
Salpingo-oophorectomy was elected by 101 of the 173 women.
Findings. In 3 of these women, early-stage ovarian or fallopian-tube cancer that had not been detected during preoperative evaluation was found at the time of surgery. In the remaining 98 patients who underwent RRSO, 1 peritoneal cancer and 3 breast cancers were diagnosed during a mean 23 months of follow-up. In the 72 women who chose surveillance, 5 ovarian or peritoneal cancers and 8 breast cancers were diagnosed in a mean 25 months of follow-up.
Kaplan-Meier analysis of time to breast or BRCA-related gynecologic cancer is illustrated in FIGURE 1.
Other studies confirm findings. A second retrospective study by Rebbeck et al19 was released simultaneously with our findings and showed similar benefits. They found a 53% reduction in subsequent breast cancer risk and a 96% reduction in subsequent ovarian cancer risk. In the summer of 2003, a study from Israel by Rutter et al provided further confirmation of the substantially decreased incidence of cancer following risk-reducing surgery.20
TABLE
Breast and ovarian cancer risk-reduction strategies for women with BRCA1 or BRCA2 mutations
| TYPE OF CANCER | STRATEGY | ALSO CONSIDER … |
|---|---|---|
| Breast | Monthly self-examination beginning at age 18 | Imaging Breast ultrasound or magnetic resonance imaging |
| 2-4 physician examinations per year, starting at age 25 | Risk–reducing surgery Mastectomy, no earlier than mid-20s | |
| Annual mammography beginning at age 25 | Salpingo-oophorectomy, after age 35 and completion of childbearing | |
| Chemoprevention Tamoxifen. Need to discuss conflicting reports on efficacy | ||
| Ovarian | CA 125 and ultrasound twice yearly, starting at age 35 | Salpingo-oophorectomy After age 35 and the completion of childbearing |
| Chemoprevention Oral contraceptives, though they may be associated with an increased risk of breast cancer | ||
| Source: Adapted from Scheuer et al28 | ||
FIGURE 1 Reduction in cancer cases associated with salpingo-oophorectomy
Reprinted with permission from Kauff ND et al.18
Copyright 2002 Massachusetts Medical Society. All rights reserved.
Good technique and pathologic review may prevent post-oophorectomy cancer
There are 3 theories about the origin of primary peritoneal cancer after oophorectomy:
- The cancer represents undetected occult cancer present at the time of risk-reducing surgery.
- It represents cancer arising in an ovarian remnant left behind after risk-reducing surgery.
- The peritoneal cancer arises de novo from the peritoneal surface epithelium.
Reasonable evidence supports each of these theories; thus, each may play some role in the incidence of “peritoneal” cancer after risk-reducing surgery.21-23
While surgical technique and detailed pathologic review are unlikely to decrease the incidence of de novo peritoneal cancer, they may play a substantial role in reducing ovarian and related cancers after risk-reducing surgery.
Surgical requirements. Obviously, if a surgery is to be risk-reducing, as much as possible of the tissue at risk should be removed. To do so effectively, the surgeon should be comfortable operating in the retroperitoneum so that the infundibulopelvic ligament can be ligated sufficiently proximal from the ovarian hilum to minimize the possibility of an ovarian remnant. Similarly, if a salpingo-oophorectomy without hysterectomy is to be performed, the fallopian tube should be amputated as close as possible to the uterine cornua (FIGURE 2).
Laparoscopy versus open surgery. RRSO can be performed using either a laparoscopic or open approach. The appropriate choice is best determined by the patient’s history, associated comorbid conditions, need for additional procedures, and experience of the surgeon. At our institution, in the absence of contraindications, we generally offer a laparoscopic approach due to its decreased morbidity.
Concomitant hysterectomy? An area of substantial controversy is whether the uterus should be removed at the time of RRSO. In most studies exploring this issue, hereditary breast-ovarian cancer syndrome does not appear to be associated with an increased risk of uterine cancer.24 However, there is concern that the portion of interstitial fallopian tube left behind after salpingo-oophorectomy may be at risk for malignant transformation.25,26
In our series, almost 90% of risk-reducing procedures were salpingo-oophorectomies without hysterectomy. If there is an additional benefit to concomitant hysterectomy, it has yet to be demonstrated by clinical trials.
In several studies, a patient’s level of anxiety was more important than objective cancer risk in the choice of RRSO.
Close pathologic scrutiny advised. Microscopic cancer may be found in 2% to 4% of RRSO specimens upon careful pathologic review.21,27,28 Thus, it is essential that the ovaries and fallopian tubes are sectioned in their entirety and examined by an experienced gynecologic pathologist to minimize the chance that microscopic cancer goes undetected.
It is not clear whether cytology should be routinely done at the time of risk-reducing surgery. A single report documents malignant cells in a woman with a BRCA1 mutation and no obvious foci of malignancy despite hysterectomy with bilateral salpingo-oophorectomy and staging.29 Pending further studies, we routinely send cytology for review.
FIGURE 2 Careful surgical ligation and division to eliminate residual tissue
Source: Devita VT Jr., Hellman S, Rosenberg SA, eds. Progress in Oncology 2003. 2004: Jones and Bartlett Publishers; Sudbury, Mass. Reprinted with permission.
When no BRCA mutation is present
Most of the data cited thus far apply to women with documented BRCA mutations. There is much less information about the relative risks and benefits of RRSO in women with a personal or family history of breast or ovarian cancer who lack a documented BRCA mutation.
Although RRSO may be appropriate for some of these women, in 2004 it is not the standard of care to recommend RRSO to all individuals with a personal or family history suggestive of an inherited predisposition to ovarian cancer. These patients are best managed by an interdisciplinary team of gynecologists, gynecologic oncologists, and clinical geneticists, all with experience caring for women who may have an inherited predisposition.
Is anxiety a factor?
We have limited information about the psychosocial impact of RRSO. Several studies have found that a patient’s level of anxiety is a more important factor than objective cancer risk in the decision to undergo RRSO.30,31 Unfortunately, we do not yet know whether the surgery successfully reduces these patients’ subjective concerns.
A recent study showed that risk-reducing surgery did not impair women’s overall health or psychological well-being.32 However, 20.7% of the women reported substantial cancer-related anxiety despite the risk-reducing surgery. This issue requires further investigation.
Is estrogen the best option for surgical menopause?
The role of hormone replacement after RRSO is unclear. The issue is important because many women considering salpingo-oophorectomy are in their late 30s or early 40s, when premature surgical menopause is a predictable result. Consequences can include considerable vasomotor and pelvic symptoms.
Preliminary data suggest that a woman’s satisfaction with RRSO depends in large part on its impact on sexual functioning.32 Urogenital symptoms that adversely affected sexual function, such as vaginal dryness and dyspareunia, were the most significant predictors of dissatisfaction with surgery.
Premature surgical menopause also has a substantial impact on osteoporosis risk, while its effect on heart disease remains uncertain.
While nonhormonal therapies can address each of these issues, we need more data on their long-term use. We counsel women considering RRSO that hormone replacement may be an option. We believe it is unlikely to reduce the efficacy of RRSO in preventing ovarian cancer, but it may reduce the protective effect of RRSO against subsequent breast cancer. Until further studies are available, we recommend that decisions regarding hormone replacement be individualized to the patient’s specific symptoms and personal history.
Not all insurers cover RRSO
One study explored insurance carriers’ policies about reimbursing risk-reducing surgical procedures and found that 10% to 11% of private insurers and 48% to 50% of governmental carriers had policies specifically denying coverage for such operations.
An additional 40% to 64% of insurers had no identifiable policy regarding these procedures in women with BRCA mutations.33 The authors speculated that, without identifiable policies, this critical health-care decision may be subject to arbitrary criteria that result in substantial variation.
When we recently investigated the reimbursement experience of women with BRCA mutations undergoing RRSO at our institution, we found that 97% of the procedures were reimbursed in full, less any applicable coinsurance and deductibles.34 Two important limitations of our study: It was conducted at a tertiary cancer center and was retrospective. It is not known if the findings reflect the experience of women with BRCA mutation who have risk-reducing surgery in other settings.
Unresolved issues
RRSO clearly has a role in preventing breast and ovarian cancer in women at inherited risk. However, several questions remain unanswered:
- Who is the best candidate?
- What is optimal timing of the procedure?
- What, if any, concomitant procedures should be performed?
- What is the role of hormone replacement after the surgery?
These issues will be best addressed through multicenter prospective trials, such as the one now being conducted by the Gynecologic Oncology Group.
Hope also remains that further research will improve serum and radiological detection of early ovarian cancer, and that basic research on the molecular etiology and progression of these cancers will ultimately render it unnecessary to remove organs at risk.
The authors report no financial relationships relevant to this article.
1. Liber AM. Ovarian cancer in a mother and five daughters. Arch Pathol. 1950;49:280-290.
2. Knudson AG, Jr. Mutation and cancer: statistical study of retinoblastoma. Proc Natl Acad Sci USA. 1971;68:820-823.
3. Ford D, Easton DF, Stratton M, et al. Genetic heterogeneity and penetrance analysis of the BRCA1 and BRCA2 genes in breast cancer families. The Breast Cancer Linkage Consortium. Am J Hum Genet. 1998;62:676-689.
4. Risch HA, McLaughlin JR, Cole DE, et al. Prevalence and penetrance of germline BRCA1 and BRCA2 mutations in a population series of 649 women with ovarian cancer. Am J Hum Genet. 2001;68:700-710.
5. Oddoux C, Struewing JP, Clayton CM, et al. The carrier frequency of the BRCA2 6174delT mutation among Ashkenazi Jewish individuals is approximately 1%. Nat Genet. 1996;14:188-190.
6. Tonin P, Weber B, Offit K, et al. Frequency of recurrent BRCA1 and BRCA2 mutations in Ashkenazi Jewish breast cancer families. Nat Med. 1996;2:1179-1183.
7. Schinzinger A. Ueber Carcinoma Mammae. Verhandlungen der Deutschen Gesellschaft fur Chirurgie. 18th Kongress, Berlin, Apr 24–27, 1889. Berlin, Germany: Hirschwald; 1889:28[abstract].
8. Love RR, Philips J. Oophorectomy for breast cancer: history revisited. J Natl Cancer Inst. 2002;94:1433-1434.
9. Feinleib M. Breast cancer and artificial menopause: a cohort study. J Natl Cancer Inst. 1968;41:315-329.
10. Brinton LA, Schairer C, Hoover RN, Fraumeni JF, Jr. Menstrual factors and risk of breast cancer. Cancer Invest. 1988;6:245-254.
11. Tobacman JK, Greene MH, Tucker MA, Costa J, Kase R, Fraumeni JF, Jr. Intraabdominal carcinomatosis after prophylactic oophorectomy in ovarian-cancer-prone families. Lancet. 1982;2:795-797.
12. Piver MS, Jishi MF, Tsukada Y, Nava G. Primary peritoneal carcinoma after prophylactic oophorectomy in women with a family history of ovarian cancer. A report of the Gilda Radner Familial Ovarian Cancer Registry. Cancer. 1993;71:2751-2755.
13. Burke W, Daly M, Garber J, et al. Recommendations for follow-up care of individuals with an inherited predisposition to cancer. II. BRCA1 and BRCA2. Cancer Genetics Studies Consortium. JAMA. 1997;277:997-1003.
14. Schrag D, Kuntz KM, Garber JE, Weeks JC. Decision analysis—effects of prophylactic mastectomy and oophorectomy on life expectancy among women with BRCA1 or BRCA2 mutations. N Engl J Med. 1997;336:1465-1471.
15. Grann VR, Panageas KS, Whang W, Antman KH, Neugut AI. Decision analysis of prophylactic mastectomy and oophorectomy in BRCA1-positive or BRCA2-positive patients. J Clin Oncol. 1998;16:979-985.
16. Rebbeck TR, Levin AM, Eisen A, et al. Breast cancer risk after bilateral prophylactic oophorectomy in BRCA1 mutation carriers. J Natl Cancer Inst. 1999;91:1475-1479.
17. Eisen A, Rebbeck TR, Wood WC, Weber BL. Prophylactic surgery in women with a hereditary predisposition to breast and ovarian cancer. J Clin Oncol. 2000;18:1980-1995.
18. Kauff ND, Satagopan JM, Robson ME, et al. Risk-reducing salpingo-oophorectomy in women with a BRCA1 or BRCA2 mutation. N Engl J Med. 2002;346:1609-1615.
19. Rebbeck TR, Lynch HT, Neuhausen SL, et al. Prophylactic oophorectomy in carriers of BRCA1 or BRCA2 mutations. N Engl J Med. 2002;346:1616-1622.
20. Rutter JL, Wacholder S, Chetrit A, et al. Gynecologic surgeries and risk of ovarian cancer in women with BRCA1 and BRCA2 Ashkenazi founder mutations: an Israeli population-based case-control study. J Natl Cancer Inst. 2003;95:1072-1078.
21. Lu KH, Garber JE, Cramer DW, et al. Occult ovarian tumors in women with BRCA1 or BRCA2 mutations undergoing prophylactic oophorectomy. J Clin Oncol. 2000;18:2728-2732.
22. Lafferty HW, Angioli R, Rudolph J, Penalver MA. Ovarian remnant syndrome: experience at Jackson Memorial Hospital, University of Miami, 1985 through 1993. Am J Obstet Gynecol. 1996;174:641-645.
23. Schorge JO, Muto MG, Welch WR, et al. Molecular evidence for multifocal papillary serous carcinoma of the peritoneum in patients with germline BRCA1 mutations. J Natl Cancer Inst. 1998;90:841-845.
24. Levine DA, Lin O, Barakat RR, et al. Risk of endometrial carcinoma associated with BRCA mutation. Gynecol Oncol. 2001;80:395-398.
25. Paley PJ, Swisher EM, Garcia RL, et al. Occult cancer of the fallopian tube in BRCA-1 germline mutation carriers at prophylactic oophorectomy: a case for recommending hysterectomy at surgical prophylaxis. Gynecol Oncol. 2001;80:176-180.
26. Aziz S, Kuperstein G, Rosen B, et al. A genetic epidemiological study of carcinoma of the fallopian tube. Gynecol Oncol. 2001;80:341-345.
27. Colgan TJ, Murphy J, Cole DE, Narod S, Rosen B. Occult carcinoma in prophylactic oophorectomy specimens: prevalence and association with BRCA germline mutation status. Am J Surg Pathol. 2001;25:1283-1289.
28. Scheuer L, Kauff N, Robson M, et al. Outcome of preventive surgery and screening for breast and ovarian cancer in BRCA mutation carriers. J Clin Oncol. 2002;20:1260-1268.
29. Colgan TJ, Boerner SL, Murphy J, Cole DE, Narod S, Rosen B. Peritoneal lavage cytology: an assessment of its value during prophylactic oophorectomy. Gynecol Oncol. 2002;85:397-403.
30. Meiser B, Butow P, Barratt A, et al. Attitudes toward prophylactic oophorectomy and screening utilization in women at increased risk of developing hereditary breast/ovarian cancer. Gynecol Oncol. 1999;75:122-129.
31. Hurley KE, Miller SM, Costalas JW, Gillespie D, Daly MB. Anxiety/uncertainty reduction as a motivation for interest in prophylactic oophorectomy in women with a family history of ovarian cancer. J Womens Health Gend Based Med. 2001;10:189-199.
32. Robson M, Hensley M, Barakat R, et al. Quality of life in women at risk for ovarian cancer who have undergone risk-reducing oophorectomy. Gynecol Oncol. 2003;89:281-287.
33. Kuerer HM, Hwang ES, Anthony JP, et al. Current national health insurance coverage policies for breast and ovarian cancer prophylactic surgery. Ann Surg Oncol. 2000;7:325-332.
34. Kauff ND, Scheuer L, Robson ME, et al. Insurance reimbursement for risk-reducing mastectomy and oophorectomy in women with BRCA1 or BRCA2 mutations. Genet Med. 2001;3:422-425.
1. Liber AM. Ovarian cancer in a mother and five daughters. Arch Pathol. 1950;49:280-290.
2. Knudson AG, Jr. Mutation and cancer: statistical study of retinoblastoma. Proc Natl Acad Sci USA. 1971;68:820-823.
3. Ford D, Easton DF, Stratton M, et al. Genetic heterogeneity and penetrance analysis of the BRCA1 and BRCA2 genes in breast cancer families. The Breast Cancer Linkage Consortium. Am J Hum Genet. 1998;62:676-689.
4. Risch HA, McLaughlin JR, Cole DE, et al. Prevalence and penetrance of germline BRCA1 and BRCA2 mutations in a population series of 649 women with ovarian cancer. Am J Hum Genet. 2001;68:700-710.
5. Oddoux C, Struewing JP, Clayton CM, et al. The carrier frequency of the BRCA2 6174delT mutation among Ashkenazi Jewish individuals is approximately 1%. Nat Genet. 1996;14:188-190.
6. Tonin P, Weber B, Offit K, et al. Frequency of recurrent BRCA1 and BRCA2 mutations in Ashkenazi Jewish breast cancer families. Nat Med. 1996;2:1179-1183.
7. Schinzinger A. Ueber Carcinoma Mammae. Verhandlungen der Deutschen Gesellschaft fur Chirurgie. 18th Kongress, Berlin, Apr 24–27, 1889. Berlin, Germany: Hirschwald; 1889:28[abstract].
8. Love RR, Philips J. Oophorectomy for breast cancer: history revisited. J Natl Cancer Inst. 2002;94:1433-1434.
9. Feinleib M. Breast cancer and artificial menopause: a cohort study. J Natl Cancer Inst. 1968;41:315-329.
10. Brinton LA, Schairer C, Hoover RN, Fraumeni JF, Jr. Menstrual factors and risk of breast cancer. Cancer Invest. 1988;6:245-254.
11. Tobacman JK, Greene MH, Tucker MA, Costa J, Kase R, Fraumeni JF, Jr. Intraabdominal carcinomatosis after prophylactic oophorectomy in ovarian-cancer-prone families. Lancet. 1982;2:795-797.
12. Piver MS, Jishi MF, Tsukada Y, Nava G. Primary peritoneal carcinoma after prophylactic oophorectomy in women with a family history of ovarian cancer. A report of the Gilda Radner Familial Ovarian Cancer Registry. Cancer. 1993;71:2751-2755.
13. Burke W, Daly M, Garber J, et al. Recommendations for follow-up care of individuals with an inherited predisposition to cancer. II. BRCA1 and BRCA2. Cancer Genetics Studies Consortium. JAMA. 1997;277:997-1003.
14. Schrag D, Kuntz KM, Garber JE, Weeks JC. Decision analysis—effects of prophylactic mastectomy and oophorectomy on life expectancy among women with BRCA1 or BRCA2 mutations. N Engl J Med. 1997;336:1465-1471.
15. Grann VR, Panageas KS, Whang W, Antman KH, Neugut AI. Decision analysis of prophylactic mastectomy and oophorectomy in BRCA1-positive or BRCA2-positive patients. J Clin Oncol. 1998;16:979-985.
16. Rebbeck TR, Levin AM, Eisen A, et al. Breast cancer risk after bilateral prophylactic oophorectomy in BRCA1 mutation carriers. J Natl Cancer Inst. 1999;91:1475-1479.
17. Eisen A, Rebbeck TR, Wood WC, Weber BL. Prophylactic surgery in women with a hereditary predisposition to breast and ovarian cancer. J Clin Oncol. 2000;18:1980-1995.
18. Kauff ND, Satagopan JM, Robson ME, et al. Risk-reducing salpingo-oophorectomy in women with a BRCA1 or BRCA2 mutation. N Engl J Med. 2002;346:1609-1615.
19. Rebbeck TR, Lynch HT, Neuhausen SL, et al. Prophylactic oophorectomy in carriers of BRCA1 or BRCA2 mutations. N Engl J Med. 2002;346:1616-1622.
20. Rutter JL, Wacholder S, Chetrit A, et al. Gynecologic surgeries and risk of ovarian cancer in women with BRCA1 and BRCA2 Ashkenazi founder mutations: an Israeli population-based case-control study. J Natl Cancer Inst. 2003;95:1072-1078.
21. Lu KH, Garber JE, Cramer DW, et al. Occult ovarian tumors in women with BRCA1 or BRCA2 mutations undergoing prophylactic oophorectomy. J Clin Oncol. 2000;18:2728-2732.
22. Lafferty HW, Angioli R, Rudolph J, Penalver MA. Ovarian remnant syndrome: experience at Jackson Memorial Hospital, University of Miami, 1985 through 1993. Am J Obstet Gynecol. 1996;174:641-645.
23. Schorge JO, Muto MG, Welch WR, et al. Molecular evidence for multifocal papillary serous carcinoma of the peritoneum in patients with germline BRCA1 mutations. J Natl Cancer Inst. 1998;90:841-845.
24. Levine DA, Lin O, Barakat RR, et al. Risk of endometrial carcinoma associated with BRCA mutation. Gynecol Oncol. 2001;80:395-398.
25. Paley PJ, Swisher EM, Garcia RL, et al. Occult cancer of the fallopian tube in BRCA-1 germline mutation carriers at prophylactic oophorectomy: a case for recommending hysterectomy at surgical prophylaxis. Gynecol Oncol. 2001;80:176-180.
26. Aziz S, Kuperstein G, Rosen B, et al. A genetic epidemiological study of carcinoma of the fallopian tube. Gynecol Oncol. 2001;80:341-345.
27. Colgan TJ, Murphy J, Cole DE, Narod S, Rosen B. Occult carcinoma in prophylactic oophorectomy specimens: prevalence and association with BRCA germline mutation status. Am J Surg Pathol. 2001;25:1283-1289.
28. Scheuer L, Kauff N, Robson M, et al. Outcome of preventive surgery and screening for breast and ovarian cancer in BRCA mutation carriers. J Clin Oncol. 2002;20:1260-1268.
29. Colgan TJ, Boerner SL, Murphy J, Cole DE, Narod S, Rosen B. Peritoneal lavage cytology: an assessment of its value during prophylactic oophorectomy. Gynecol Oncol. 2002;85:397-403.
30. Meiser B, Butow P, Barratt A, et al. Attitudes toward prophylactic oophorectomy and screening utilization in women at increased risk of developing hereditary breast/ovarian cancer. Gynecol Oncol. 1999;75:122-129.
31. Hurley KE, Miller SM, Costalas JW, Gillespie D, Daly MB. Anxiety/uncertainty reduction as a motivation for interest in prophylactic oophorectomy in women with a family history of ovarian cancer. J Womens Health Gend Based Med. 2001;10:189-199.
32. Robson M, Hensley M, Barakat R, et al. Quality of life in women at risk for ovarian cancer who have undergone risk-reducing oophorectomy. Gynecol Oncol. 2003;89:281-287.
33. Kuerer HM, Hwang ES, Anthony JP, et al. Current national health insurance coverage policies for breast and ovarian cancer prophylactic surgery. Ann Surg Oncol. 2000;7:325-332.
34. Kauff ND, Scheuer L, Robson ME, et al. Insurance reimbursement for risk-reducing mastectomy and oophorectomy in women with BRCA1 or BRCA2 mutations. Genet Med. 2001;3:422-425.