User login
- In appropriately selected patients, the ability to easily and skillfully remove tissue during laparoscopy facilitates patient recovery and healing and limits hospitalization time.
- Even dense tissues such as partially calcified leiomyomata are readily removed with automatic morcellators, and the size of masses is less significant than with “manual” approaches.
Novel surgical devices and techniques have transformed operative laparoscopy by improving the inefficiencies in tissue extraction that limited early acceptance.
In the beginning, it was relatively easy to isolate a myoma and dissect it from the underlying myometrium, but it took hours to extract the tissue using a hand-held morcellator. This article focuses on the 4 techniques commonly used today, as well as the products that make them possible.
In appropriately selected patients, the ability to remove tissue through any of these methods facilitates patient recovery and healing and limits hospitalization time.
Posterior colpotomy
In the 1980s and early 1990s, it was common for tissue to be extracted through a posterior colpotomy. This is not surprising given that gynecologists were trained to perform transvaginal tubal ligation and to use colpotomies when incising and draining tubo-ovarian abscesses—not to mention our ease in entering the posterior cul-de-sac during vaginal surgery.
The elasticity of the vagina facilitates removal of fairly sizeable masses. Large ovarian cysts or masses can be brought to the cul-de-sac and incised and drained in a manner that markedly reduces the risk of intraperitoneal spillage.
There are disadvantages, however. For example, if the surgeon wants to maintain laparoscopic visualization once the colpotomy has been made, the tissue to be removed must be grasped and brought toward the opening to plug the defect and maintain pneumoperitoneum.
This may not be particularly problematic if there is only 1 mass to be removed, but it can be troublesome if there are several. An option is to place the masses in the posterior cul-de-sac so they can be readily grasped once the posterior colpotomy has been made.
One conceptual concern is the issue of subsequent adhesion formation, especially in patients desiring fertility. Unfortunately, no substantive trials exist to better answer this question.
Removal through the trocar or trocar site
Although some physicians still remove tissue through a posterior colpotomy, most have abandoned that approach in favor of extraction through a primary or lateral laparoscopic port. Indeed, this is the simplest technique for extracting tissue. I often change from a 10-mm laparoscope to a 5-mm instrument, placing the smaller endoscope in one of the lower ports and removing tissue under direct visualization through the 10- or 11-mm infraumbilical port.
If a cystic mass placed in a laparoscopic bag is too large to be removed, carefully aspirate it with a large-gauge needle.
Trapped tissue. One potential problem is the trapping of tissue in trocars that contain a flap valve. If this occurs, remove the trocar, clear the tissue, and replace the trocar in the original site using a blunt instrument such as the 10-mm laparoscope. Do not use the sharp inner blade to replace this port, as it is unduly risky.
For large masses, remove the port to create extra space. It also may be necessary to enlarge the skin or the fascial incision using a blunt instrument such as forceps.
Before the widespread availability of laparoscopic bags, tissue extraction was generally performed in this manner.
Risks include spillage of cyst contents during extraction and development of a hernia secondary to the wider disruption of fascia. This risk is particularly high in the infraumbilical area, which is inherently weak to begin with. It is thus critical—in any methodology—that the fascia be appropriately closed.
Laparoscopic bags
Many of the laparoscopic bags now widely available are easily opened once they have been placed in the abdomen, though some must be opened with graspers after the bag is positioned in the peritoneal cavity. Laparoscopic bags have greater utility when the extracted tissue is soft, such as with a dermoid cyst or ovary. Dense tissue is more difficult to manage.
Some surgeons fashion their own bags using sterile gloves or baggies.
Durability. The bags vary in their ability to withstand manipulation and puncture. For example, one type of nylon bag has a polyurethane inner coating and drawstring closure, making it quite durable. It also comes in a range of sizes, allowing the surgeon to choose the bag most suitable for the mass being removed.
To use a laparoscopic bag, insert it through the infraumbilical trocar and place the mass inside it. Then remove the trocar to provide maximal room for the mass to be extracted.
If the mass is cystic and too large to be removed, carefully aspirate it with a largegauge needle, taking care not to puncture the bag. Otherwise, morcellate the mass in the sac and remove it piecemeal, allowing no spillage of contents.
This may be performed under laparoscopic visualization through the lower ancillary trocars or trocar site. If a larger port has been placed—or there is a clinical need for one—tissue extraction also could be performed through the lower port.
Risks include bag breakage and potential spillage. In addition, it sometimes is necessary to change to a larger bag.
Morcellators
Early morcellators were hand-held, requiring the operator to continuously bite into the tissue and remove the small fragments. While this approach was effective for soft tissues and small myomas, it was ineffective for larger or more solid masses.
“Orange peel” technique. Scissors have been used to achieve the same effect as the handheld morcellator. Harrith Hasson described the “orange peel” technique, in which the surgeon uses scissors to peel away the tissue as one would peel an orange.1 The long, thin strips of tissue then can be extracted through the trocar. Unfortunately, laparoscopic scissors are often too small or dull to adequately incise larger fibroids.
Automatic morcellators have markedly enhanced our ability to perform laparoscopic myomectomy and similar procedures. They also have had a strong impact on nongynecologic procedures such as splenectomy or nephrectomy, in which large amounts of tissue must be removed. Although these devices are costly, the time savings associated with their use are significant. Current devices range from disposable to semidisposable and are available in a wide variety of sizes.
Even dense tissues such as partially calcified leiomyomata are readily removed with automatic morcellators.
Hasson’s orange-peel technique also can be employed with automatic morcellators. This allows long, thin strips of tissue to be removed while facilitating constant visualization of anatomy surrounding the tissue being extracted.
An alternative is making multiple “through-passes” into the myoma using the morcellator. In this method, the strips of tissue obtained will be smaller and the myoma will develop a Swiss-cheese appearance. Note that this approach takes longer and may increase the number of myoma fragments that fall into the pelvis and need to be removed.
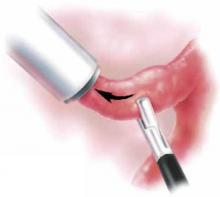
Effective for a range of masses. Not surprisingly, even dense tissues such as partially calcified leiomyomata are readily removed with automatic morcellators, and the size of masses is less significant than with “manual” approaches. Nevertheless, it is critical that the surgeon maintain constant visualization and that tissue be brought toward the morcellator and away from underlying structures (FIGURE).
Do not move the morcellator toward the tissue. Because of its sharpness, the automatic morcellator will cut through vital structures as easily as it penetrates fibroids.
FIGURE Automatic morcellation: Move excised tissue toward device
To prevent injury, tissue should be brought toward the morcellator and away from underlying structures. Do not move the morcellator toward the tissue or vital structures may be cut.
Spillage
An early and continuing concern regarding ovarian cystectomy or oophorectomy is spillage of the mass’s contents into the peritoneal cavity. This is more of an issue in the case of borderline or malignant ovarian lesions or mucinous or dermoid ovarian cysts. In fact, nowhere is there more contention than over the clinical ramifications of spillage in the case of malignancy.
Mixed data on impact of spillage. Clinical data suggest that the impact of spillage is inconsequential, whereas other evidence suggests a worsening prognosis.2-5 In the event of spillage, most gynecologic oncologists would convert an ovarian cancer patient with a 1A or 1B staged lesion to stage 1C and would likely administer chemotherapy.
Concern about spillage of a mucinous or dermoid cyst centers on the theoretical risk of pseudomyxoma peritonei or, in the case of a teratoma, chemical peritonitis. Some surgeons routinely enter dermoids and intentionally spill the contents.6,7 Of note, we lack significant case series of ensuing infections or problems with this technique. Still, removing an intact cyst negates this issue and expedites surgery, eliminating the need to irrigate the abdomen and pelvis with large quantities of fluid.
Ectopic pregnancy has also been a concern, as there have been reports of chorionic tissue being disseminated in the abdomen and pelvis during laparoscopic procedures.8
Nowhere is there more contention than over the clinical ramifications of spillage in the case of malignancy.
Patient selection
Preoperative evaluation is a critical component of patient selection. A thorough ultrasound examination can help determine who is and who is not an appropriate candidate for laparoscopic management.
Cases that suggest a high risk of ovarian malignancy may be best managed in the traditional manner, as may patients with a large number of myomas or other compounding factors.
Dr. Bieber reports no financial relationships relevant to this article.
1. Hasson HM, Rotman C, Rana N, Sistos F, Dmowski WP. Laparoscopic myomectomy. Obstet Gynecol. 1992;80:884-888.
2. Mayer C, Miller DM, Ehlen TG. Peritoneal implantation of squamous cell carcinoma following rupture of a dermoid cyst during laparoscopic removal. Gynecol Oncol. 2002;84:180-183.
3. Kodama S, Tanaka K, Tokunaga A, et al. Multivariate analysis of prognostic fators in patients with ovarian cancer stage I and II. Int J Gynaecol Obstet. 1997;56:147-153.
4. Mizuno M, Kikkawa F, Shibata K, et al. Long-term prognosis of stage I ovarian carcinoma. Prognostic importance of intraoperative rupture. Oncology. 2003;65:29-36.
5. Vergote I, De Brabanter J, Fyles A, et al. Prognostic importance of degree of differentiation and cyst rupture in stage I invasive epithelial ovarian carcinoma. Lancet. 2001;357:176-182.
6. Zanetta G, Ferrari L, Mignini-Renzini M, Vignali M, Fadini R. Laparoscopic excision of ovarian dermoid cysts with controlled intraoperative spillage. Safety andeffectiveness. J Reprod Med. 1999;44:815-820.
7. Mecke H, Sawas V. Laparoscopic surgery of dermoid cysts—intraoperative spillage and complications. Eur J Obstet Gynecol Reprod Biol. 2001;96:80-84.
8. Billieux MH, Petignat P, Anguenot JL, Campana A, Bischof P. Early and late halflife of human chorionic gonadotropin as a predictor of persistent trophoblast after laparoscopic conservative surgery for tubal pregnancy. Acta Obstet Gynecol Scand. 2003;82:550-555.
- In appropriately selected patients, the ability to easily and skillfully remove tissue during laparoscopy facilitates patient recovery and healing and limits hospitalization time.
- Even dense tissues such as partially calcified leiomyomata are readily removed with automatic morcellators, and the size of masses is less significant than with “manual” approaches.
Novel surgical devices and techniques have transformed operative laparoscopy by improving the inefficiencies in tissue extraction that limited early acceptance.
In the beginning, it was relatively easy to isolate a myoma and dissect it from the underlying myometrium, but it took hours to extract the tissue using a hand-held morcellator. This article focuses on the 4 techniques commonly used today, as well as the products that make them possible.
In appropriately selected patients, the ability to remove tissue through any of these methods facilitates patient recovery and healing and limits hospitalization time.
Posterior colpotomy
In the 1980s and early 1990s, it was common for tissue to be extracted through a posterior colpotomy. This is not surprising given that gynecologists were trained to perform transvaginal tubal ligation and to use colpotomies when incising and draining tubo-ovarian abscesses—not to mention our ease in entering the posterior cul-de-sac during vaginal surgery.
The elasticity of the vagina facilitates removal of fairly sizeable masses. Large ovarian cysts or masses can be brought to the cul-de-sac and incised and drained in a manner that markedly reduces the risk of intraperitoneal spillage.
There are disadvantages, however. For example, if the surgeon wants to maintain laparoscopic visualization once the colpotomy has been made, the tissue to be removed must be grasped and brought toward the opening to plug the defect and maintain pneumoperitoneum.
This may not be particularly problematic if there is only 1 mass to be removed, but it can be troublesome if there are several. An option is to place the masses in the posterior cul-de-sac so they can be readily grasped once the posterior colpotomy has been made.
One conceptual concern is the issue of subsequent adhesion formation, especially in patients desiring fertility. Unfortunately, no substantive trials exist to better answer this question.
Removal through the trocar or trocar site
Although some physicians still remove tissue through a posterior colpotomy, most have abandoned that approach in favor of extraction through a primary or lateral laparoscopic port. Indeed, this is the simplest technique for extracting tissue. I often change from a 10-mm laparoscope to a 5-mm instrument, placing the smaller endoscope in one of the lower ports and removing tissue under direct visualization through the 10- or 11-mm infraumbilical port.
If a cystic mass placed in a laparoscopic bag is too large to be removed, carefully aspirate it with a large-gauge needle.
Trapped tissue. One potential problem is the trapping of tissue in trocars that contain a flap valve. If this occurs, remove the trocar, clear the tissue, and replace the trocar in the original site using a blunt instrument such as the 10-mm laparoscope. Do not use the sharp inner blade to replace this port, as it is unduly risky.
For large masses, remove the port to create extra space. It also may be necessary to enlarge the skin or the fascial incision using a blunt instrument such as forceps.
Before the widespread availability of laparoscopic bags, tissue extraction was generally performed in this manner.
Risks include spillage of cyst contents during extraction and development of a hernia secondary to the wider disruption of fascia. This risk is particularly high in the infraumbilical area, which is inherently weak to begin with. It is thus critical—in any methodology—that the fascia be appropriately closed.
Laparoscopic bags
Many of the laparoscopic bags now widely available are easily opened once they have been placed in the abdomen, though some must be opened with graspers after the bag is positioned in the peritoneal cavity. Laparoscopic bags have greater utility when the extracted tissue is soft, such as with a dermoid cyst or ovary. Dense tissue is more difficult to manage.
Some surgeons fashion their own bags using sterile gloves or baggies.
Durability. The bags vary in their ability to withstand manipulation and puncture. For example, one type of nylon bag has a polyurethane inner coating and drawstring closure, making it quite durable. It also comes in a range of sizes, allowing the surgeon to choose the bag most suitable for the mass being removed.
To use a laparoscopic bag, insert it through the infraumbilical trocar and place the mass inside it. Then remove the trocar to provide maximal room for the mass to be extracted.
If the mass is cystic and too large to be removed, carefully aspirate it with a largegauge needle, taking care not to puncture the bag. Otherwise, morcellate the mass in the sac and remove it piecemeal, allowing no spillage of contents.
This may be performed under laparoscopic visualization through the lower ancillary trocars or trocar site. If a larger port has been placed—or there is a clinical need for one—tissue extraction also could be performed through the lower port.
Risks include bag breakage and potential spillage. In addition, it sometimes is necessary to change to a larger bag.
Morcellators
Early morcellators were hand-held, requiring the operator to continuously bite into the tissue and remove the small fragments. While this approach was effective for soft tissues and small myomas, it was ineffective for larger or more solid masses.
“Orange peel” technique. Scissors have been used to achieve the same effect as the handheld morcellator. Harrith Hasson described the “orange peel” technique, in which the surgeon uses scissors to peel away the tissue as one would peel an orange.1 The long, thin strips of tissue then can be extracted through the trocar. Unfortunately, laparoscopic scissors are often too small or dull to adequately incise larger fibroids.
Automatic morcellators have markedly enhanced our ability to perform laparoscopic myomectomy and similar procedures. They also have had a strong impact on nongynecologic procedures such as splenectomy or nephrectomy, in which large amounts of tissue must be removed. Although these devices are costly, the time savings associated with their use are significant. Current devices range from disposable to semidisposable and are available in a wide variety of sizes.
Even dense tissues such as partially calcified leiomyomata are readily removed with automatic morcellators.
Hasson’s orange-peel technique also can be employed with automatic morcellators. This allows long, thin strips of tissue to be removed while facilitating constant visualization of anatomy surrounding the tissue being extracted.
An alternative is making multiple “through-passes” into the myoma using the morcellator. In this method, the strips of tissue obtained will be smaller and the myoma will develop a Swiss-cheese appearance. Note that this approach takes longer and may increase the number of myoma fragments that fall into the pelvis and need to be removed.
Effective for a range of masses. Not surprisingly, even dense tissues such as partially calcified leiomyomata are readily removed with automatic morcellators, and the size of masses is less significant than with “manual” approaches. Nevertheless, it is critical that the surgeon maintain constant visualization and that tissue be brought toward the morcellator and away from underlying structures (FIGURE).
Do not move the morcellator toward the tissue. Because of its sharpness, the automatic morcellator will cut through vital structures as easily as it penetrates fibroids.
FIGURE Automatic morcellation: Move excised tissue toward device
To prevent injury, tissue should be brought toward the morcellator and away from underlying structures. Do not move the morcellator toward the tissue or vital structures may be cut.
Spillage
An early and continuing concern regarding ovarian cystectomy or oophorectomy is spillage of the mass’s contents into the peritoneal cavity. This is more of an issue in the case of borderline or malignant ovarian lesions or mucinous or dermoid ovarian cysts. In fact, nowhere is there more contention than over the clinical ramifications of spillage in the case of malignancy.
Mixed data on impact of spillage. Clinical data suggest that the impact of spillage is inconsequential, whereas other evidence suggests a worsening prognosis.2-5 In the event of spillage, most gynecologic oncologists would convert an ovarian cancer patient with a 1A or 1B staged lesion to stage 1C and would likely administer chemotherapy.
Concern about spillage of a mucinous or dermoid cyst centers on the theoretical risk of pseudomyxoma peritonei or, in the case of a teratoma, chemical peritonitis. Some surgeons routinely enter dermoids and intentionally spill the contents.6,7 Of note, we lack significant case series of ensuing infections or problems with this technique. Still, removing an intact cyst negates this issue and expedites surgery, eliminating the need to irrigate the abdomen and pelvis with large quantities of fluid.
Ectopic pregnancy has also been a concern, as there have been reports of chorionic tissue being disseminated in the abdomen and pelvis during laparoscopic procedures.8
Nowhere is there more contention than over the clinical ramifications of spillage in the case of malignancy.
Patient selection
Preoperative evaluation is a critical component of patient selection. A thorough ultrasound examination can help determine who is and who is not an appropriate candidate for laparoscopic management.
Cases that suggest a high risk of ovarian malignancy may be best managed in the traditional manner, as may patients with a large number of myomas or other compounding factors.
Dr. Bieber reports no financial relationships relevant to this article.
- In appropriately selected patients, the ability to easily and skillfully remove tissue during laparoscopy facilitates patient recovery and healing and limits hospitalization time.
- Even dense tissues such as partially calcified leiomyomata are readily removed with automatic morcellators, and the size of masses is less significant than with “manual” approaches.
Novel surgical devices and techniques have transformed operative laparoscopy by improving the inefficiencies in tissue extraction that limited early acceptance.
In the beginning, it was relatively easy to isolate a myoma and dissect it from the underlying myometrium, but it took hours to extract the tissue using a hand-held morcellator. This article focuses on the 4 techniques commonly used today, as well as the products that make them possible.
In appropriately selected patients, the ability to remove tissue through any of these methods facilitates patient recovery and healing and limits hospitalization time.
Posterior colpotomy
In the 1980s and early 1990s, it was common for tissue to be extracted through a posterior colpotomy. This is not surprising given that gynecologists were trained to perform transvaginal tubal ligation and to use colpotomies when incising and draining tubo-ovarian abscesses—not to mention our ease in entering the posterior cul-de-sac during vaginal surgery.
The elasticity of the vagina facilitates removal of fairly sizeable masses. Large ovarian cysts or masses can be brought to the cul-de-sac and incised and drained in a manner that markedly reduces the risk of intraperitoneal spillage.
There are disadvantages, however. For example, if the surgeon wants to maintain laparoscopic visualization once the colpotomy has been made, the tissue to be removed must be grasped and brought toward the opening to plug the defect and maintain pneumoperitoneum.
This may not be particularly problematic if there is only 1 mass to be removed, but it can be troublesome if there are several. An option is to place the masses in the posterior cul-de-sac so they can be readily grasped once the posterior colpotomy has been made.
One conceptual concern is the issue of subsequent adhesion formation, especially in patients desiring fertility. Unfortunately, no substantive trials exist to better answer this question.
Removal through the trocar or trocar site
Although some physicians still remove tissue through a posterior colpotomy, most have abandoned that approach in favor of extraction through a primary or lateral laparoscopic port. Indeed, this is the simplest technique for extracting tissue. I often change from a 10-mm laparoscope to a 5-mm instrument, placing the smaller endoscope in one of the lower ports and removing tissue under direct visualization through the 10- or 11-mm infraumbilical port.
If a cystic mass placed in a laparoscopic bag is too large to be removed, carefully aspirate it with a large-gauge needle.
Trapped tissue. One potential problem is the trapping of tissue in trocars that contain a flap valve. If this occurs, remove the trocar, clear the tissue, and replace the trocar in the original site using a blunt instrument such as the 10-mm laparoscope. Do not use the sharp inner blade to replace this port, as it is unduly risky.
For large masses, remove the port to create extra space. It also may be necessary to enlarge the skin or the fascial incision using a blunt instrument such as forceps.
Before the widespread availability of laparoscopic bags, tissue extraction was generally performed in this manner.
Risks include spillage of cyst contents during extraction and development of a hernia secondary to the wider disruption of fascia. This risk is particularly high in the infraumbilical area, which is inherently weak to begin with. It is thus critical—in any methodology—that the fascia be appropriately closed.
Laparoscopic bags
Many of the laparoscopic bags now widely available are easily opened once they have been placed in the abdomen, though some must be opened with graspers after the bag is positioned in the peritoneal cavity. Laparoscopic bags have greater utility when the extracted tissue is soft, such as with a dermoid cyst or ovary. Dense tissue is more difficult to manage.
Some surgeons fashion their own bags using sterile gloves or baggies.
Durability. The bags vary in their ability to withstand manipulation and puncture. For example, one type of nylon bag has a polyurethane inner coating and drawstring closure, making it quite durable. It also comes in a range of sizes, allowing the surgeon to choose the bag most suitable for the mass being removed.
To use a laparoscopic bag, insert it through the infraumbilical trocar and place the mass inside it. Then remove the trocar to provide maximal room for the mass to be extracted.
If the mass is cystic and too large to be removed, carefully aspirate it with a largegauge needle, taking care not to puncture the bag. Otherwise, morcellate the mass in the sac and remove it piecemeal, allowing no spillage of contents.
This may be performed under laparoscopic visualization through the lower ancillary trocars or trocar site. If a larger port has been placed—or there is a clinical need for one—tissue extraction also could be performed through the lower port.
Risks include bag breakage and potential spillage. In addition, it sometimes is necessary to change to a larger bag.
Morcellators
Early morcellators were hand-held, requiring the operator to continuously bite into the tissue and remove the small fragments. While this approach was effective for soft tissues and small myomas, it was ineffective for larger or more solid masses.
“Orange peel” technique. Scissors have been used to achieve the same effect as the handheld morcellator. Harrith Hasson described the “orange peel” technique, in which the surgeon uses scissors to peel away the tissue as one would peel an orange.1 The long, thin strips of tissue then can be extracted through the trocar. Unfortunately, laparoscopic scissors are often too small or dull to adequately incise larger fibroids.
Automatic morcellators have markedly enhanced our ability to perform laparoscopic myomectomy and similar procedures. They also have had a strong impact on nongynecologic procedures such as splenectomy or nephrectomy, in which large amounts of tissue must be removed. Although these devices are costly, the time savings associated with their use are significant. Current devices range from disposable to semidisposable and are available in a wide variety of sizes.
Even dense tissues such as partially calcified leiomyomata are readily removed with automatic morcellators.
Hasson’s orange-peel technique also can be employed with automatic morcellators. This allows long, thin strips of tissue to be removed while facilitating constant visualization of anatomy surrounding the tissue being extracted.
An alternative is making multiple “through-passes” into the myoma using the morcellator. In this method, the strips of tissue obtained will be smaller and the myoma will develop a Swiss-cheese appearance. Note that this approach takes longer and may increase the number of myoma fragments that fall into the pelvis and need to be removed.
Effective for a range of masses. Not surprisingly, even dense tissues such as partially calcified leiomyomata are readily removed with automatic morcellators, and the size of masses is less significant than with “manual” approaches. Nevertheless, it is critical that the surgeon maintain constant visualization and that tissue be brought toward the morcellator and away from underlying structures (FIGURE).
Do not move the morcellator toward the tissue. Because of its sharpness, the automatic morcellator will cut through vital structures as easily as it penetrates fibroids.
FIGURE Automatic morcellation: Move excised tissue toward device
To prevent injury, tissue should be brought toward the morcellator and away from underlying structures. Do not move the morcellator toward the tissue or vital structures may be cut.
Spillage
An early and continuing concern regarding ovarian cystectomy or oophorectomy is spillage of the mass’s contents into the peritoneal cavity. This is more of an issue in the case of borderline or malignant ovarian lesions or mucinous or dermoid ovarian cysts. In fact, nowhere is there more contention than over the clinical ramifications of spillage in the case of malignancy.
Mixed data on impact of spillage. Clinical data suggest that the impact of spillage is inconsequential, whereas other evidence suggests a worsening prognosis.2-5 In the event of spillage, most gynecologic oncologists would convert an ovarian cancer patient with a 1A or 1B staged lesion to stage 1C and would likely administer chemotherapy.
Concern about spillage of a mucinous or dermoid cyst centers on the theoretical risk of pseudomyxoma peritonei or, in the case of a teratoma, chemical peritonitis. Some surgeons routinely enter dermoids and intentionally spill the contents.6,7 Of note, we lack significant case series of ensuing infections or problems with this technique. Still, removing an intact cyst negates this issue and expedites surgery, eliminating the need to irrigate the abdomen and pelvis with large quantities of fluid.
Ectopic pregnancy has also been a concern, as there have been reports of chorionic tissue being disseminated in the abdomen and pelvis during laparoscopic procedures.8
Nowhere is there more contention than over the clinical ramifications of spillage in the case of malignancy.
Patient selection
Preoperative evaluation is a critical component of patient selection. A thorough ultrasound examination can help determine who is and who is not an appropriate candidate for laparoscopic management.
Cases that suggest a high risk of ovarian malignancy may be best managed in the traditional manner, as may patients with a large number of myomas or other compounding factors.
Dr. Bieber reports no financial relationships relevant to this article.
1. Hasson HM, Rotman C, Rana N, Sistos F, Dmowski WP. Laparoscopic myomectomy. Obstet Gynecol. 1992;80:884-888.
2. Mayer C, Miller DM, Ehlen TG. Peritoneal implantation of squamous cell carcinoma following rupture of a dermoid cyst during laparoscopic removal. Gynecol Oncol. 2002;84:180-183.
3. Kodama S, Tanaka K, Tokunaga A, et al. Multivariate analysis of prognostic fators in patients with ovarian cancer stage I and II. Int J Gynaecol Obstet. 1997;56:147-153.
4. Mizuno M, Kikkawa F, Shibata K, et al. Long-term prognosis of stage I ovarian carcinoma. Prognostic importance of intraoperative rupture. Oncology. 2003;65:29-36.
5. Vergote I, De Brabanter J, Fyles A, et al. Prognostic importance of degree of differentiation and cyst rupture in stage I invasive epithelial ovarian carcinoma. Lancet. 2001;357:176-182.
6. Zanetta G, Ferrari L, Mignini-Renzini M, Vignali M, Fadini R. Laparoscopic excision of ovarian dermoid cysts with controlled intraoperative spillage. Safety andeffectiveness. J Reprod Med. 1999;44:815-820.
7. Mecke H, Sawas V. Laparoscopic surgery of dermoid cysts—intraoperative spillage and complications. Eur J Obstet Gynecol Reprod Biol. 2001;96:80-84.
8. Billieux MH, Petignat P, Anguenot JL, Campana A, Bischof P. Early and late halflife of human chorionic gonadotropin as a predictor of persistent trophoblast after laparoscopic conservative surgery for tubal pregnancy. Acta Obstet Gynecol Scand. 2003;82:550-555.
1. Hasson HM, Rotman C, Rana N, Sistos F, Dmowski WP. Laparoscopic myomectomy. Obstet Gynecol. 1992;80:884-888.
2. Mayer C, Miller DM, Ehlen TG. Peritoneal implantation of squamous cell carcinoma following rupture of a dermoid cyst during laparoscopic removal. Gynecol Oncol. 2002;84:180-183.
3. Kodama S, Tanaka K, Tokunaga A, et al. Multivariate analysis of prognostic fators in patients with ovarian cancer stage I and II. Int J Gynaecol Obstet. 1997;56:147-153.
4. Mizuno M, Kikkawa F, Shibata K, et al. Long-term prognosis of stage I ovarian carcinoma. Prognostic importance of intraoperative rupture. Oncology. 2003;65:29-36.
5. Vergote I, De Brabanter J, Fyles A, et al. Prognostic importance of degree of differentiation and cyst rupture in stage I invasive epithelial ovarian carcinoma. Lancet. 2001;357:176-182.
6. Zanetta G, Ferrari L, Mignini-Renzini M, Vignali M, Fadini R. Laparoscopic excision of ovarian dermoid cysts with controlled intraoperative spillage. Safety andeffectiveness. J Reprod Med. 1999;44:815-820.
7. Mecke H, Sawas V. Laparoscopic surgery of dermoid cysts—intraoperative spillage and complications. Eur J Obstet Gynecol Reprod Biol. 2001;96:80-84.
8. Billieux MH, Petignat P, Anguenot JL, Campana A, Bischof P. Early and late halflife of human chorionic gonadotropin as a predictor of persistent trophoblast after laparoscopic conservative surgery for tubal pregnancy. Acta Obstet Gynecol Scand. 2003;82:550-555.