User login
What you can do to optimize blood conservation in ObGyn practice
The authors report no financial relationships relevant to this article.
Obstetric hemorrhage is responsible for approximately 17% to 25% of all pregnancy-related deaths.1 Excessive blood loss also is a risk during gynecologic surgery. Iron-deficiency anemia increases the risk of complication and the need for transfusion in both settings. By identifying and treating anemia before childbirth and elective surgery, you can optimize the patient’s condition and usually avert the need for emergency transfusion.
The Geisinger Health System has developed a unique Blood Conservation Program that focuses on the prevention of major blood loss by identifying and treating anemia in antepartum, postpartum, and gynecologic patients. The program’s protocols for treating anemia in antepartum and surgical patients are illustrated in FIGURES 1 AND 2. Geisinger practitioners have found that adherence to these protocols reduces the need for transfusion in many patients and improves their quality of life.
Here, we 1) look at the key data that will help you identify and then treat anemia in gynecologic, obstetric, and postpartum patients and 2) describe a variety of treatment options.
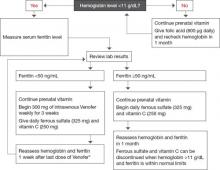
FIGURE 1 The gynecologic surgical patient: Preop diagnosis and treatment of anemia
* If anemia is refractory to iron therapy, consider erythropoietin therapy if benefits outweigh risks.
FIGURE 2 For the antepartum patient: Diagnosis and treatment of anemia
* If anemia is refractory to iron therapy, consider erythropoietin therapy if benefits outweigh risks.
Focus on the baseline hemoglobin level
The key to prevention of emergency transfusion—as well as postpartum anemia—is optimization of the patient’s hemoglobin level before delivery. It also is prudent when elective surgery is planned. In our institution, clinicians whose patients are at risk for hemorrhage or significant blood loss have the option of consulting with the Blood Conservation Program.
When the program began in November 2004, its primary purpose was to reduce the need for blood transfusion in elective surgery, including gynecologic procedures. It later expanded to include obstetric patients who have a hemoglobin level below 11 g/dL and patients who are considered to be at risk of major blood loss.
In addition to obstetric and surgical patients, the Geisinger Blood Conservation Program provides support for patients who will not accept blood or blood products for religious or personal reasons, even in life-threatening situations. The program has provided more than 8,000 consultations to date.
When to evaluate patients for anemia
Anemia in women is most often defined as a hemoglobin level below 12 g/dL or a hematocrit below 36%. In pregnant patients, the cutoff points are lower: 11 g/dL and 33%, respectively. During pregnancy, hemoglobin and hematocrit levels reach their nadir during the second trimester and then begin to rise until term.
Symptoms of anemia include fatigue, depression, shortness of breath, hypotension, and heart palpitations. However, some patients at risk of major blood loss during delivery or surgery do not display typical symptoms associated with anemia, and the condition can be confirmed only by laboratory testing.
At Geisinger, we recommend consultation with the Blood Conservation Program for any patient who exhibits symptoms of anemia or who is at risk of major blood loss. For example, the risk of blood loss during childbirth varies with the method of delivery.1 On average, obstetric patients lose 500 mL of blood during vaginal delivery; 1,000 mL during cesarean delivery; and 1,500 mL when cesarean delivery is followed by hysterectomy.1,2 Hemorrhage is classified as follows:
- Class 1 – Blood loss as high as 750 mL, or 15% of blood volume
- Class 2 – 750 to 1,500 mL, or 15% to 30% of blood volume
- Class 3 – 1,500 to 2,400 mL, or 30% to 40% of blood volume
- Class 4 – more than 2,400 mL, or more than 40% of blood volume.1
Abnormal placentation, such as placenta accreta, percreta, increta, and previa, which can often be diagnosed antepartum, may lead to significant blood loss during and after delivery. Obstetric emergencies, including abruption, trauma, and uterine rupture, may also be associated with major blood loss.
Iron deficiency lies at the root of most cases of anemia
Iron deficiency affects an estimated 2.15 billion people globally, with a prevalence of 12% to 43% worldwide.3,4 The daily iron requirement is 1 mg of elemental iron for nonobstetric patients, 2 mg for pregnant and lactating women. Latent iron deficiency is common in women who have had multiple pregnancies. These and other important facts about iron are described in the Box “Essential facts about iron”.
In iron-deficiency anemia, the following serum levels are reduced:
- Iron. A normal reading is 60 to 170 μg/dL.
- Hemoglobin, a measure of the production and turnover of red blood cells. A normal level is ≥12 g/dL (≥11 g/dL in pregnancy).
- Serum ferritin (a protein that stores iron). A normal reading is 12 to 150 ng/mL.
- Transferrin saturation. Transferrin is a transporting protein that shuttles iron to the bone marrow. The normal transferrin saturation level ranges from 20% to 50%.
Ferritin and hemoglobin levels tend to be the most efficient indicators of iron status.5
Some clinicians may also use:
- Mean corpuscular volume (MCV). Normal is 80 to 96 fL.
- Random distribution of red blood cell weight (RDW). A normal value is 11.5% to 15.5%.
- Reticulocyte count. Normal is 0.4% to 2.3%.
Laboratory tests for iron deficiency
When the Blood Conservation Program is initially consulted, the laboratory studies we recommend are based on the clinical presentation and condition of the patient. During pregnancy, we try to take account of the normal hemodynamic changes that occur during gestation. Therefore, we recommend:
- assessment of the serum ferritin level
- complete blood count (CBC) with differential. (If the hemoglobin/hematocrit is low, a peripheral smear is recommended to further evaluate microcytic anemia.)
Transferrin saturation and serum iron levels have not been shown to be useful markers in pregnant women because they are not specific for iron-restricted erythropoiesis and can be abnormally low during pregnancy.6
In nonpregnant patients, we recommend initial evaluation of:
- serum iron level
- total iron-binding capacity (TIBC). Normal levels are 240 to 450 μg/dL
- transferrin saturation.
A caveat about the ferritin level
Ferritin is both an iron-storage indicator and an acute-phase protein, so the clinician must be careful to exclude inflammatory processes that can elevate the ferritin level, giving a false indicator of iron stability in the maternal system. These inflammatory processes can include preeclampsia and neoplastic or infectious conditions.7 Transferrin saturation, however, is not affected by inflammatory processes and can be used as a confirmatory test for iron deficiency.4
Try oral iron supplementation first
When laboratory testing confirms the presence of iron-deficiency anemia, initial management is oral iron supplementation for 2 weeks, followed by repeat laboratory evaluation.
For patients scheduled for surgery, oral therapy includes a daily dosage of:
- 325 mg of ferrous sulfate
- 250 mg of vitamin C
- 800 μg of folic acid
- a multivitamin.
For perinatal patients, the daily oral regimen is:
- 325 mg of ferrous sulfate
- 250 mg of vitamin C
- a prenatal vitamin.
These medications are the least expensive alternatives for treating anemia.
Advise patients who are taking iron supplements not to ingest the medication with dairy products, coffee, tea, or foods that have a high content of phytic acid (e.g., grains, seeds, and legumes). Foods and prescription drugs that interact with iron supplements are listed in TABLE 1, along with recommendations on optimal timing of iron supplementation and other medications.
When you prescribe oral iron supplementation, bear in mind that some patients experience gastrointestinal side effects—constipation, nausea, diarrhea—so unpleasant that they stop taking their medication. In that scenario, you will need to find alternative formulations or delivery routes. One alternative you can suggest is a daily helping of blackstrap molasses, which supplies 27 mg of elemental iron per tablespoon.
Oral therapy should be continued even after hemoglobin and ferritin levels normalize. If laboratory values remain low after 2 weeks of oral therapy, parenteral therapy can be added to the oral regimen.
Therapy may be discontinued 2 months after delivery of the infant or surgery as long as the cause of the blood loss has been remedied. If the mother is breastfeeding, she should continue taking a prenatal vitamin until nursing has stopped.
TABLE 1
Some foods and drugs don’t mix well with iron
| Food or drug | Interaction | Recommendations |
|---|---|---|
| Foods high in phytic acid (grains, seeds, legumes) | Decreased absorption of iron | Do not take iron within 2 hours of eating foods high in phytic acid |
| Dairy products | Decreased bioavailability of iron | Do not take iron supplements within 1 hour of consuming dairy products, which can significantly decrease iron absorption |
| Levothyroxine | Iron reduces levothyroxine serum levels and efficacy | Take levothyroxine and iron at least 4 hours apart |
| Methyldopa | Oral iron reduces the efficacy of methyldopa | Consider IV iron or take oral iron and methyldopa as far apart as possible |
| Proton pump inhibitors (PPIs) | Absorption of oral iron is enhanced by gastric acid. PPIs decrease gastric acid production, thereby reducing the bioavailability of iron | Consider IV iron preparations |
| Ofloxacin | Iron reduces efficacy of ofloxacin | Administer ofloxacin and iron 2 hours apart |
| Cholestyramine | Decreased efficacy of iron | Administer iron and cholestyramine at least 4 hours apart |
| Calcium, aluminum, magnesium | Decreased absorption of iron | Iron should be taken at least 1 hour before or 2 hours after products that contain calcium, aluminum, or magnesium |
| Note: This table is not a comprehensive summary of all medications used in practice, but a list of those used commonly in obstetric and gynecologic populations | ||
IV iron isn’t as risky as you think
Historically, clinicians have avoided using parenteral iron sucrose (Venofer) because they have been taught that it can cause an anaphylactic reaction. In fact, although anaphylaxis may have been associated with older intravenous (IV) iron preparations, clinical trials have demonstrated the safety of IV iron sucrose and low-molecular-weight iron dextran. In a study involving 800 patients, Breymann and colleagues demonstrated that parenteral iron preparations containing dextran or iron dextrin could be safely given to pregnant women.4 Only 1.5% of the patients in the study experienced side effects from the therapy, and no anaphylactic reactions were observed.
In another study, 25 pregnant patients were given IV iron sucrose, and the only adverse reaction reported was a “not-unpleasant taste” during the injection.8
In an additional study, Breymann and colleagues found no adverse outcomes in 20 postpartum patients who received IV iron sucrose in addition to erythropoietin therapy.9
- The daily iron requirement is 2 mg of elemental iron in pregnancy and lactation, 1 mg at all other times
- The typical US diet contains about 18 mg of iron a day, of which only about 1 mg is absorbed
- Iron absorption occurs primarily in the second portion of the duodenum
- Iron absorption increases with iron deficiency
- One unit of blood contains 250 mg of iron
- Total body iron store is between 1,000 and 3,000 mg, depending on body size
- Each pregnancy depletes maternal iron stores by about 750 mg
- Latent iron deficiency is common in women who have had many pregnancies and in women who have menorrhagia.
Our preference for parenteral therapy is iron sucrose, classified by the Food and Drug Administration (FDA) as Pregnancy Category B. Iron sucrose is contraindicated in patients who have iron overload, hypersensitivity to inactive components of iron sucrose, or anemia that is not caused by iron deficiency. Adverse reactions to iron sucrose include, but are not limited to, anaphylaxis, hypotension, cramping, nausea, headache, vomiting, diarrhea, and chest pain. Adverse reactions are very rare, occurring in fewer than 1% of patients.
To determine whether the patient has an allergy to iron sucrose, give a test dosage of 25 mg via slow IV push and wait 20 minutes. If a reaction occurs, hold the remainder of the dose and consider alternative therapies. If no allergic reaction occurs, administer the remaining 275 mg in 50 mL to 100 mL of saline.
You may need to add erythropoietin to the regimen
Erythropoietin is a hormone made by the kidneys to promote formation of red blood cells in the bone marrow. A deficiency in this hormone causes anemia in patients who have renal disease, and nephrologists use a synthetic form of epoetin alfa (Epogen) to increase the hemoglobin level in dialysis patients.10 Epoetin alfa falls into FDA Pregnancy Category C.
In rare instances, erythropoietin-stimulating agents (ESAs), such as epoetin alfa, in addition to both IV and oral iron supplementation, are needed to increase the patient’s hemoglobin level and hematocrit before delivery or surgery. Before beginning ESA therapy, the patient’s platelet count and activity level need to be considered. ESAs have been linked to thrombolytic events and, therefore, should not be used in patients who have an elevated platelet count. The risk of thrombolytic events is a particular danger for antepartum patients on bed rest, and ESAs may be contraindicated for that reason.
Obstetric and surgical patients whose anemia has proven refractory to iron therapy may be considered for an ESA, as long as the benefits of this choice outweigh the risks. At an approximate cost of $400 for every 40,000 U, ESA therapy is by far the most expensive alternative to blood transfusion for patients who have iron-deficiency anemia. The patient typically receives one to two doses of an ESA.
Cost comparisons for alternative treatment modalities in iron-deficiency anemia can be found in TABLE 2.
TABLE 2
Estimated cost of treatment of anemia*
| Therapy | Dosage | Cost per dose |
|---|---|---|
| ORAL THERAPY | ||
| Ferrous sulfate | 325 mg | $0.05–$0.09 |
| Vitamin C | 500 mg | $0.04 |
| Vitron C | 1 tablet | $0.20 |
| Folic acid | 800 μg | $0.02 |
| INTRAVENOUS THERAPY | ||
| Iron sucrose | 100 mg | $80.00 |
| OTHER INTERVENTIONS | ||
| Transfusion | 1 U | $500.00–$600.00 |
| Erythropoietin | 40,000 U | $400.00 |
| * Local averages in central Pennsylvania | ||
TABLE 3
How safe are iron compounds in pregnancy and lactation?
| Compound | FDA pregnancy category | World Health Organization lactation recommendation | Thompson lactation rating |
|---|---|---|---|
| Parenteral iron dextran | C | Compatible with breastfeeding | Risk to infant cannot be ruled out |
| Parenteral iron sucrose | B | ||
| Oral iron | A | Unavailable | |
| Oral sodium ferric gluconate | A | Compatible with breastfeeding |
Erythropoietin-stimulating agents carry serious risks
The FDA issued the first of a series of letters to health-care professionals warning of adverse events associated with the use of ESAs in March 2007, after several randomized, controlled trials found an increased risk of stroke, blood clots, myocardial infarction, and death with high dosages. In November 2008, the FDA approved a black-box warning for the labels of Procrit and Aranesp, the two ESAs in general use in the United States. The new labels advise clinicians to modify dosages for patients who are in renal failure to maintain a target hemoglobin level between 11 and 12 g/dL, rather than the higher targets that had been in use.11,12
Transfusion is the last resort
Blood transfusion must also be considered as prophylaxis for blood loss in patients who have critically low hemoglobin levels, with due consideration of the procedure’s risks and benefits. Because the definition of “critically low” varies from patient to patient, other variables should be taken into consideration, including blood pressure; heart rate; urine output; tolerance for performing activities of daily living without dizziness, chest discomfort, or shortness of breath; and medical history. Potential drawbacks are considerable.
The multiple risks associated with transfusion include:
- immunosuppression
- fever
- chills
- urticaria
- hemolytic transfusion reaction
- septic transfusion reaction
- bacterial contamination
- anaphylaxis
- graft-versus-host reactions
- transfer of viral diseases, including hepatitis B and C and human immunodeficiency virus (HIV).
The risk of immunosuppression, in particular, should be weighed heavily for pregnant patients and those who are planning an elective surgical procedure. The possibility of viral transmission is also a deterrent. According to the Red Cross, the transmission rate is one in every 205,000 transfusions for hepatitis B, one in 2 million for hepatitis C, and one in 2,135,000 for HIV. These considerations, as well as the blood shortages that sometimes occur in practice, are sufficient reason to seek safer alternatives, when possible.
When a patient refuses transfusion
Caring for a patient who has an elevated risk of major blood loss can be particularly difficult when she is a member of a religious group such as Jehovah’s Witnesses. These patients generally decline the transfusion of plasma, packed red blood cells, white blood cells, platelets, and whole blood products.
In the Geisinger Health System, consultation with the Blood Conservation Program has been particularly helpful in these circumstances, offering clinicians alternative ways to correct anemia and prepare for the possibility of major blood loss. Patients who will not allow blood transfusion are often willing to accept plasma volume expanders that are not derived from blood, such as perfluorocarbon solutions, hydroxyethyl starch, crystalloid, or dextran.13 ESA therapy may be acceptable to some patients who refuse transfusion. Most are willing to go along with oral or IV iron supplementation to reduce their need for transfusion.
Postpartum patients may need special consideration
Iron supplementation is safe for breastfeeding mothers
Anemia in a breastfeeding woman is not uncommon and should be identified and treated. Iron supplementation with oral or IV compounds is considered safe for pregnant and breastfeeding women.
ESA therapy is a riskier strategy, whose benefits must clearly outweigh risks for all patients.
Anemia and postpartum depression
Studies have demonstrated a correlation between anemia and postpartum depression. Beard and colleagues showed a 25% improvement in cognition and improved scores on stress and depression scales in postpartum women who had iron-deficiency anemia when they were treated with daily iron and vitamin C.14 Other studies have addressed an increased risk of anemia in low-income postpartum women and the deleterious impact of iron-deficiency anemia on the quality of mother–child interactions and subsequent child development. Correcting maternal iron deficiency could prevent adverse outcomes in these mothers and their offspring.15,16
1. Gabbe SG, Niebyl JR, Simpson JL. Obstetrics: Normal and Problem Pregnancies. 5th ed. Philadelphia, Pa: Churchill Livingstone; 2007.
2. Creasy RK, Resnik R, Iams JD. Creasy and Resnik’s Maternal–Fetal Medicine: Principles and Practice. 6th ed. Philadelphia, Pa: Saunders; 2009.
3. Khusun H, Yip R, Schultink W, Dillon DHS. World Health Organization hemoglobin cutoff points for the detection of anemia are valid for an Indonesian population. J Nutr. 1999;129:1669-1674.
4. Breymann C. Intravenous iron in surgery and obstetrics. Transfus Altern Transfus Med. 2002;4(Suppl 2):22-23.
5. Mei Z, Cogswell ME, Parvanta I, et al. Hemoglobin and ferritin are currently the most efficient indicators of population response to iron interventions: an analysis of nine randomized controlled trials. J Nutr. 2005;135:1974-1980.
6. Gronowski AM, ed. Current Clinical Pathology: Handbook of Clinical Laboratory Testing During Pregnancy. Totowa, NJ: Humana Press; 2004:200.
7. Mani S, Duffy TP. Anemia of pregnancy. Clin Perinatol. 1995;22:593-607.
8. Bayoumeu F, Subiran-Buisset C, Baka NE, Legagneur H, Monnier-Barbarino P, Laxenaire MC. Iron therapy in iron deficiency anemia in pregnancy: intravenous route versus oral route. Am J Obstet Gynecol. 2002;186:518-522.
9. Breymann C, Richter C, Hüttner C, Huch R, Huch A. Effectiveness of recombinant erythropoietin and iron sucrose vs. iron therapy only, in patients with postpartum anaemia and blunted erythropoiesis. Eur J Clin Invest. 2000;30:154-161.
10. Bieber E. Erythropoietin, the biology of erythropoiesis and epoetin alfa. An overview. J Reprod Med. 2001;46(5 Suppl):521-530.
11. Patient Information for Procrit. Available at: www.procrit.com. Accessed December 1, 2009.
12. Patient Information for Aranesp. Available at: www.aranesp.com. Accessed December 1, 2009.
13. Baker BW. Blood conservation, obstetrics, and Jehovah’s Witnesses. Anesthesiol Clin North America. 1998;16:375-384.
14. Beard JL, Hendricks MK, Perez EM, et al. Maternal iron deficiency anemia affects postpartum emotions and cognition. J Nutr. 2005;135:267-272.
15. Bodnar LM, Cogswell ME, Scanlon KS. Low income postpartum women are at risk of iron deficiency. J Nutr. 2002;132:2298-2302.
16. Perez EM, Hendricks MK, Beard JL, et al. Mother–infant interactions and infant development are altered by maternal iron deficiency anemia. J Nutr. 2005;135:850-855.
The authors report no financial relationships relevant to this article.
Obstetric hemorrhage is responsible for approximately 17% to 25% of all pregnancy-related deaths.1 Excessive blood loss also is a risk during gynecologic surgery. Iron-deficiency anemia increases the risk of complication and the need for transfusion in both settings. By identifying and treating anemia before childbirth and elective surgery, you can optimize the patient’s condition and usually avert the need for emergency transfusion.
The Geisinger Health System has developed a unique Blood Conservation Program that focuses on the prevention of major blood loss by identifying and treating anemia in antepartum, postpartum, and gynecologic patients. The program’s protocols for treating anemia in antepartum and surgical patients are illustrated in FIGURES 1 AND 2. Geisinger practitioners have found that adherence to these protocols reduces the need for transfusion in many patients and improves their quality of life.
Here, we 1) look at the key data that will help you identify and then treat anemia in gynecologic, obstetric, and postpartum patients and 2) describe a variety of treatment options.
FIGURE 1 The gynecologic surgical patient: Preop diagnosis and treatment of anemia
* If anemia is refractory to iron therapy, consider erythropoietin therapy if benefits outweigh risks.
FIGURE 2 For the antepartum patient: Diagnosis and treatment of anemia
* If anemia is refractory to iron therapy, consider erythropoietin therapy if benefits outweigh risks.
Focus on the baseline hemoglobin level
The key to prevention of emergency transfusion—as well as postpartum anemia—is optimization of the patient’s hemoglobin level before delivery. It also is prudent when elective surgery is planned. In our institution, clinicians whose patients are at risk for hemorrhage or significant blood loss have the option of consulting with the Blood Conservation Program.
When the program began in November 2004, its primary purpose was to reduce the need for blood transfusion in elective surgery, including gynecologic procedures. It later expanded to include obstetric patients who have a hemoglobin level below 11 g/dL and patients who are considered to be at risk of major blood loss.
In addition to obstetric and surgical patients, the Geisinger Blood Conservation Program provides support for patients who will not accept blood or blood products for religious or personal reasons, even in life-threatening situations. The program has provided more than 8,000 consultations to date.
When to evaluate patients for anemia
Anemia in women is most often defined as a hemoglobin level below 12 g/dL or a hematocrit below 36%. In pregnant patients, the cutoff points are lower: 11 g/dL and 33%, respectively. During pregnancy, hemoglobin and hematocrit levels reach their nadir during the second trimester and then begin to rise until term.
Symptoms of anemia include fatigue, depression, shortness of breath, hypotension, and heart palpitations. However, some patients at risk of major blood loss during delivery or surgery do not display typical symptoms associated with anemia, and the condition can be confirmed only by laboratory testing.
At Geisinger, we recommend consultation with the Blood Conservation Program for any patient who exhibits symptoms of anemia or who is at risk of major blood loss. For example, the risk of blood loss during childbirth varies with the method of delivery.1 On average, obstetric patients lose 500 mL of blood during vaginal delivery; 1,000 mL during cesarean delivery; and 1,500 mL when cesarean delivery is followed by hysterectomy.1,2 Hemorrhage is classified as follows:
- Class 1 – Blood loss as high as 750 mL, or 15% of blood volume
- Class 2 – 750 to 1,500 mL, or 15% to 30% of blood volume
- Class 3 – 1,500 to 2,400 mL, or 30% to 40% of blood volume
- Class 4 – more than 2,400 mL, or more than 40% of blood volume.1
Abnormal placentation, such as placenta accreta, percreta, increta, and previa, which can often be diagnosed antepartum, may lead to significant blood loss during and after delivery. Obstetric emergencies, including abruption, trauma, and uterine rupture, may also be associated with major blood loss.
Iron deficiency lies at the root of most cases of anemia
Iron deficiency affects an estimated 2.15 billion people globally, with a prevalence of 12% to 43% worldwide.3,4 The daily iron requirement is 1 mg of elemental iron for nonobstetric patients, 2 mg for pregnant and lactating women. Latent iron deficiency is common in women who have had multiple pregnancies. These and other important facts about iron are described in the Box “Essential facts about iron”.
In iron-deficiency anemia, the following serum levels are reduced:
- Iron. A normal reading is 60 to 170 μg/dL.
- Hemoglobin, a measure of the production and turnover of red blood cells. A normal level is ≥12 g/dL (≥11 g/dL in pregnancy).
- Serum ferritin (a protein that stores iron). A normal reading is 12 to 150 ng/mL.
- Transferrin saturation. Transferrin is a transporting protein that shuttles iron to the bone marrow. The normal transferrin saturation level ranges from 20% to 50%.
Ferritin and hemoglobin levels tend to be the most efficient indicators of iron status.5
Some clinicians may also use:
- Mean corpuscular volume (MCV). Normal is 80 to 96 fL.
- Random distribution of red blood cell weight (RDW). A normal value is 11.5% to 15.5%.
- Reticulocyte count. Normal is 0.4% to 2.3%.
Laboratory tests for iron deficiency
When the Blood Conservation Program is initially consulted, the laboratory studies we recommend are based on the clinical presentation and condition of the patient. During pregnancy, we try to take account of the normal hemodynamic changes that occur during gestation. Therefore, we recommend:
- assessment of the serum ferritin level
- complete blood count (CBC) with differential. (If the hemoglobin/hematocrit is low, a peripheral smear is recommended to further evaluate microcytic anemia.)
Transferrin saturation and serum iron levels have not been shown to be useful markers in pregnant women because they are not specific for iron-restricted erythropoiesis and can be abnormally low during pregnancy.6
In nonpregnant patients, we recommend initial evaluation of:
- serum iron level
- total iron-binding capacity (TIBC). Normal levels are 240 to 450 μg/dL
- transferrin saturation.
A caveat about the ferritin level
Ferritin is both an iron-storage indicator and an acute-phase protein, so the clinician must be careful to exclude inflammatory processes that can elevate the ferritin level, giving a false indicator of iron stability in the maternal system. These inflammatory processes can include preeclampsia and neoplastic or infectious conditions.7 Transferrin saturation, however, is not affected by inflammatory processes and can be used as a confirmatory test for iron deficiency.4
Try oral iron supplementation first
When laboratory testing confirms the presence of iron-deficiency anemia, initial management is oral iron supplementation for 2 weeks, followed by repeat laboratory evaluation.
For patients scheduled for surgery, oral therapy includes a daily dosage of:
- 325 mg of ferrous sulfate
- 250 mg of vitamin C
- 800 μg of folic acid
- a multivitamin.
For perinatal patients, the daily oral regimen is:
- 325 mg of ferrous sulfate
- 250 mg of vitamin C
- a prenatal vitamin.
These medications are the least expensive alternatives for treating anemia.
Advise patients who are taking iron supplements not to ingest the medication with dairy products, coffee, tea, or foods that have a high content of phytic acid (e.g., grains, seeds, and legumes). Foods and prescription drugs that interact with iron supplements are listed in TABLE 1, along with recommendations on optimal timing of iron supplementation and other medications.
When you prescribe oral iron supplementation, bear in mind that some patients experience gastrointestinal side effects—constipation, nausea, diarrhea—so unpleasant that they stop taking their medication. In that scenario, you will need to find alternative formulations or delivery routes. One alternative you can suggest is a daily helping of blackstrap molasses, which supplies 27 mg of elemental iron per tablespoon.
Oral therapy should be continued even after hemoglobin and ferritin levels normalize. If laboratory values remain low after 2 weeks of oral therapy, parenteral therapy can be added to the oral regimen.
Therapy may be discontinued 2 months after delivery of the infant or surgery as long as the cause of the blood loss has been remedied. If the mother is breastfeeding, she should continue taking a prenatal vitamin until nursing has stopped.
TABLE 1
Some foods and drugs don’t mix well with iron
| Food or drug | Interaction | Recommendations |
|---|---|---|
| Foods high in phytic acid (grains, seeds, legumes) | Decreased absorption of iron | Do not take iron within 2 hours of eating foods high in phytic acid |
| Dairy products | Decreased bioavailability of iron | Do not take iron supplements within 1 hour of consuming dairy products, which can significantly decrease iron absorption |
| Levothyroxine | Iron reduces levothyroxine serum levels and efficacy | Take levothyroxine and iron at least 4 hours apart |
| Methyldopa | Oral iron reduces the efficacy of methyldopa | Consider IV iron or take oral iron and methyldopa as far apart as possible |
| Proton pump inhibitors (PPIs) | Absorption of oral iron is enhanced by gastric acid. PPIs decrease gastric acid production, thereby reducing the bioavailability of iron | Consider IV iron preparations |
| Ofloxacin | Iron reduces efficacy of ofloxacin | Administer ofloxacin and iron 2 hours apart |
| Cholestyramine | Decreased efficacy of iron | Administer iron and cholestyramine at least 4 hours apart |
| Calcium, aluminum, magnesium | Decreased absorption of iron | Iron should be taken at least 1 hour before or 2 hours after products that contain calcium, aluminum, or magnesium |
| Note: This table is not a comprehensive summary of all medications used in practice, but a list of those used commonly in obstetric and gynecologic populations | ||
IV iron isn’t as risky as you think
Historically, clinicians have avoided using parenteral iron sucrose (Venofer) because they have been taught that it can cause an anaphylactic reaction. In fact, although anaphylaxis may have been associated with older intravenous (IV) iron preparations, clinical trials have demonstrated the safety of IV iron sucrose and low-molecular-weight iron dextran. In a study involving 800 patients, Breymann and colleagues demonstrated that parenteral iron preparations containing dextran or iron dextrin could be safely given to pregnant women.4 Only 1.5% of the patients in the study experienced side effects from the therapy, and no anaphylactic reactions were observed.
In another study, 25 pregnant patients were given IV iron sucrose, and the only adverse reaction reported was a “not-unpleasant taste” during the injection.8
In an additional study, Breymann and colleagues found no adverse outcomes in 20 postpartum patients who received IV iron sucrose in addition to erythropoietin therapy.9
- The daily iron requirement is 2 mg of elemental iron in pregnancy and lactation, 1 mg at all other times
- The typical US diet contains about 18 mg of iron a day, of which only about 1 mg is absorbed
- Iron absorption occurs primarily in the second portion of the duodenum
- Iron absorption increases with iron deficiency
- One unit of blood contains 250 mg of iron
- Total body iron store is between 1,000 and 3,000 mg, depending on body size
- Each pregnancy depletes maternal iron stores by about 750 mg
- Latent iron deficiency is common in women who have had many pregnancies and in women who have menorrhagia.
Our preference for parenteral therapy is iron sucrose, classified by the Food and Drug Administration (FDA) as Pregnancy Category B. Iron sucrose is contraindicated in patients who have iron overload, hypersensitivity to inactive components of iron sucrose, or anemia that is not caused by iron deficiency. Adverse reactions to iron sucrose include, but are not limited to, anaphylaxis, hypotension, cramping, nausea, headache, vomiting, diarrhea, and chest pain. Adverse reactions are very rare, occurring in fewer than 1% of patients.
To determine whether the patient has an allergy to iron sucrose, give a test dosage of 25 mg via slow IV push and wait 20 minutes. If a reaction occurs, hold the remainder of the dose and consider alternative therapies. If no allergic reaction occurs, administer the remaining 275 mg in 50 mL to 100 mL of saline.
You may need to add erythropoietin to the regimen
Erythropoietin is a hormone made by the kidneys to promote formation of red blood cells in the bone marrow. A deficiency in this hormone causes anemia in patients who have renal disease, and nephrologists use a synthetic form of epoetin alfa (Epogen) to increase the hemoglobin level in dialysis patients.10 Epoetin alfa falls into FDA Pregnancy Category C.
In rare instances, erythropoietin-stimulating agents (ESAs), such as epoetin alfa, in addition to both IV and oral iron supplementation, are needed to increase the patient’s hemoglobin level and hematocrit before delivery or surgery. Before beginning ESA therapy, the patient’s platelet count and activity level need to be considered. ESAs have been linked to thrombolytic events and, therefore, should not be used in patients who have an elevated platelet count. The risk of thrombolytic events is a particular danger for antepartum patients on bed rest, and ESAs may be contraindicated for that reason.
Obstetric and surgical patients whose anemia has proven refractory to iron therapy may be considered for an ESA, as long as the benefits of this choice outweigh the risks. At an approximate cost of $400 for every 40,000 U, ESA therapy is by far the most expensive alternative to blood transfusion for patients who have iron-deficiency anemia. The patient typically receives one to two doses of an ESA.
Cost comparisons for alternative treatment modalities in iron-deficiency anemia can be found in TABLE 2.
TABLE 2
Estimated cost of treatment of anemia*
| Therapy | Dosage | Cost per dose |
|---|---|---|
| ORAL THERAPY | ||
| Ferrous sulfate | 325 mg | $0.05–$0.09 |
| Vitamin C | 500 mg | $0.04 |
| Vitron C | 1 tablet | $0.20 |
| Folic acid | 800 μg | $0.02 |
| INTRAVENOUS THERAPY | ||
| Iron sucrose | 100 mg | $80.00 |
| OTHER INTERVENTIONS | ||
| Transfusion | 1 U | $500.00–$600.00 |
| Erythropoietin | 40,000 U | $400.00 |
| * Local averages in central Pennsylvania | ||
TABLE 3
How safe are iron compounds in pregnancy and lactation?
| Compound | FDA pregnancy category | World Health Organization lactation recommendation | Thompson lactation rating |
|---|---|---|---|
| Parenteral iron dextran | C | Compatible with breastfeeding | Risk to infant cannot be ruled out |
| Parenteral iron sucrose | B | ||
| Oral iron | A | Unavailable | |
| Oral sodium ferric gluconate | A | Compatible with breastfeeding |
Erythropoietin-stimulating agents carry serious risks
The FDA issued the first of a series of letters to health-care professionals warning of adverse events associated with the use of ESAs in March 2007, after several randomized, controlled trials found an increased risk of stroke, blood clots, myocardial infarction, and death with high dosages. In November 2008, the FDA approved a black-box warning for the labels of Procrit and Aranesp, the two ESAs in general use in the United States. The new labels advise clinicians to modify dosages for patients who are in renal failure to maintain a target hemoglobin level between 11 and 12 g/dL, rather than the higher targets that had been in use.11,12
Transfusion is the last resort
Blood transfusion must also be considered as prophylaxis for blood loss in patients who have critically low hemoglobin levels, with due consideration of the procedure’s risks and benefits. Because the definition of “critically low” varies from patient to patient, other variables should be taken into consideration, including blood pressure; heart rate; urine output; tolerance for performing activities of daily living without dizziness, chest discomfort, or shortness of breath; and medical history. Potential drawbacks are considerable.
The multiple risks associated with transfusion include:
- immunosuppression
- fever
- chills
- urticaria
- hemolytic transfusion reaction
- septic transfusion reaction
- bacterial contamination
- anaphylaxis
- graft-versus-host reactions
- transfer of viral diseases, including hepatitis B and C and human immunodeficiency virus (HIV).
The risk of immunosuppression, in particular, should be weighed heavily for pregnant patients and those who are planning an elective surgical procedure. The possibility of viral transmission is also a deterrent. According to the Red Cross, the transmission rate is one in every 205,000 transfusions for hepatitis B, one in 2 million for hepatitis C, and one in 2,135,000 for HIV. These considerations, as well as the blood shortages that sometimes occur in practice, are sufficient reason to seek safer alternatives, when possible.
When a patient refuses transfusion
Caring for a patient who has an elevated risk of major blood loss can be particularly difficult when she is a member of a religious group such as Jehovah’s Witnesses. These patients generally decline the transfusion of plasma, packed red blood cells, white blood cells, platelets, and whole blood products.
In the Geisinger Health System, consultation with the Blood Conservation Program has been particularly helpful in these circumstances, offering clinicians alternative ways to correct anemia and prepare for the possibility of major blood loss. Patients who will not allow blood transfusion are often willing to accept plasma volume expanders that are not derived from blood, such as perfluorocarbon solutions, hydroxyethyl starch, crystalloid, or dextran.13 ESA therapy may be acceptable to some patients who refuse transfusion. Most are willing to go along with oral or IV iron supplementation to reduce their need for transfusion.
Postpartum patients may need special consideration
Iron supplementation is safe for breastfeeding mothers
Anemia in a breastfeeding woman is not uncommon and should be identified and treated. Iron supplementation with oral or IV compounds is considered safe for pregnant and breastfeeding women.
ESA therapy is a riskier strategy, whose benefits must clearly outweigh risks for all patients.
Anemia and postpartum depression
Studies have demonstrated a correlation between anemia and postpartum depression. Beard and colleagues showed a 25% improvement in cognition and improved scores on stress and depression scales in postpartum women who had iron-deficiency anemia when they were treated with daily iron and vitamin C.14 Other studies have addressed an increased risk of anemia in low-income postpartum women and the deleterious impact of iron-deficiency anemia on the quality of mother–child interactions and subsequent child development. Correcting maternal iron deficiency could prevent adverse outcomes in these mothers and their offspring.15,16
The authors report no financial relationships relevant to this article.
Obstetric hemorrhage is responsible for approximately 17% to 25% of all pregnancy-related deaths.1 Excessive blood loss also is a risk during gynecologic surgery. Iron-deficiency anemia increases the risk of complication and the need for transfusion in both settings. By identifying and treating anemia before childbirth and elective surgery, you can optimize the patient’s condition and usually avert the need for emergency transfusion.
The Geisinger Health System has developed a unique Blood Conservation Program that focuses on the prevention of major blood loss by identifying and treating anemia in antepartum, postpartum, and gynecologic patients. The program’s protocols for treating anemia in antepartum and surgical patients are illustrated in FIGURES 1 AND 2. Geisinger practitioners have found that adherence to these protocols reduces the need for transfusion in many patients and improves their quality of life.
Here, we 1) look at the key data that will help you identify and then treat anemia in gynecologic, obstetric, and postpartum patients and 2) describe a variety of treatment options.
FIGURE 1 The gynecologic surgical patient: Preop diagnosis and treatment of anemia
* If anemia is refractory to iron therapy, consider erythropoietin therapy if benefits outweigh risks.
FIGURE 2 For the antepartum patient: Diagnosis and treatment of anemia
* If anemia is refractory to iron therapy, consider erythropoietin therapy if benefits outweigh risks.
Focus on the baseline hemoglobin level
The key to prevention of emergency transfusion—as well as postpartum anemia—is optimization of the patient’s hemoglobin level before delivery. It also is prudent when elective surgery is planned. In our institution, clinicians whose patients are at risk for hemorrhage or significant blood loss have the option of consulting with the Blood Conservation Program.
When the program began in November 2004, its primary purpose was to reduce the need for blood transfusion in elective surgery, including gynecologic procedures. It later expanded to include obstetric patients who have a hemoglobin level below 11 g/dL and patients who are considered to be at risk of major blood loss.
In addition to obstetric and surgical patients, the Geisinger Blood Conservation Program provides support for patients who will not accept blood or blood products for religious or personal reasons, even in life-threatening situations. The program has provided more than 8,000 consultations to date.
When to evaluate patients for anemia
Anemia in women is most often defined as a hemoglobin level below 12 g/dL or a hematocrit below 36%. In pregnant patients, the cutoff points are lower: 11 g/dL and 33%, respectively. During pregnancy, hemoglobin and hematocrit levels reach their nadir during the second trimester and then begin to rise until term.
Symptoms of anemia include fatigue, depression, shortness of breath, hypotension, and heart palpitations. However, some patients at risk of major blood loss during delivery or surgery do not display typical symptoms associated with anemia, and the condition can be confirmed only by laboratory testing.
At Geisinger, we recommend consultation with the Blood Conservation Program for any patient who exhibits symptoms of anemia or who is at risk of major blood loss. For example, the risk of blood loss during childbirth varies with the method of delivery.1 On average, obstetric patients lose 500 mL of blood during vaginal delivery; 1,000 mL during cesarean delivery; and 1,500 mL when cesarean delivery is followed by hysterectomy.1,2 Hemorrhage is classified as follows:
- Class 1 – Blood loss as high as 750 mL, or 15% of blood volume
- Class 2 – 750 to 1,500 mL, or 15% to 30% of blood volume
- Class 3 – 1,500 to 2,400 mL, or 30% to 40% of blood volume
- Class 4 – more than 2,400 mL, or more than 40% of blood volume.1
Abnormal placentation, such as placenta accreta, percreta, increta, and previa, which can often be diagnosed antepartum, may lead to significant blood loss during and after delivery. Obstetric emergencies, including abruption, trauma, and uterine rupture, may also be associated with major blood loss.
Iron deficiency lies at the root of most cases of anemia
Iron deficiency affects an estimated 2.15 billion people globally, with a prevalence of 12% to 43% worldwide.3,4 The daily iron requirement is 1 mg of elemental iron for nonobstetric patients, 2 mg for pregnant and lactating women. Latent iron deficiency is common in women who have had multiple pregnancies. These and other important facts about iron are described in the Box “Essential facts about iron”.
In iron-deficiency anemia, the following serum levels are reduced:
- Iron. A normal reading is 60 to 170 μg/dL.
- Hemoglobin, a measure of the production and turnover of red blood cells. A normal level is ≥12 g/dL (≥11 g/dL in pregnancy).
- Serum ferritin (a protein that stores iron). A normal reading is 12 to 150 ng/mL.
- Transferrin saturation. Transferrin is a transporting protein that shuttles iron to the bone marrow. The normal transferrin saturation level ranges from 20% to 50%.
Ferritin and hemoglobin levels tend to be the most efficient indicators of iron status.5
Some clinicians may also use:
- Mean corpuscular volume (MCV). Normal is 80 to 96 fL.
- Random distribution of red blood cell weight (RDW). A normal value is 11.5% to 15.5%.
- Reticulocyte count. Normal is 0.4% to 2.3%.
Laboratory tests for iron deficiency
When the Blood Conservation Program is initially consulted, the laboratory studies we recommend are based on the clinical presentation and condition of the patient. During pregnancy, we try to take account of the normal hemodynamic changes that occur during gestation. Therefore, we recommend:
- assessment of the serum ferritin level
- complete blood count (CBC) with differential. (If the hemoglobin/hematocrit is low, a peripheral smear is recommended to further evaluate microcytic anemia.)
Transferrin saturation and serum iron levels have not been shown to be useful markers in pregnant women because they are not specific for iron-restricted erythropoiesis and can be abnormally low during pregnancy.6
In nonpregnant patients, we recommend initial evaluation of:
- serum iron level
- total iron-binding capacity (TIBC). Normal levels are 240 to 450 μg/dL
- transferrin saturation.
A caveat about the ferritin level
Ferritin is both an iron-storage indicator and an acute-phase protein, so the clinician must be careful to exclude inflammatory processes that can elevate the ferritin level, giving a false indicator of iron stability in the maternal system. These inflammatory processes can include preeclampsia and neoplastic or infectious conditions.7 Transferrin saturation, however, is not affected by inflammatory processes and can be used as a confirmatory test for iron deficiency.4
Try oral iron supplementation first
When laboratory testing confirms the presence of iron-deficiency anemia, initial management is oral iron supplementation for 2 weeks, followed by repeat laboratory evaluation.
For patients scheduled for surgery, oral therapy includes a daily dosage of:
- 325 mg of ferrous sulfate
- 250 mg of vitamin C
- 800 μg of folic acid
- a multivitamin.
For perinatal patients, the daily oral regimen is:
- 325 mg of ferrous sulfate
- 250 mg of vitamin C
- a prenatal vitamin.
These medications are the least expensive alternatives for treating anemia.
Advise patients who are taking iron supplements not to ingest the medication with dairy products, coffee, tea, or foods that have a high content of phytic acid (e.g., grains, seeds, and legumes). Foods and prescription drugs that interact with iron supplements are listed in TABLE 1, along with recommendations on optimal timing of iron supplementation and other medications.
When you prescribe oral iron supplementation, bear in mind that some patients experience gastrointestinal side effects—constipation, nausea, diarrhea—so unpleasant that they stop taking their medication. In that scenario, you will need to find alternative formulations or delivery routes. One alternative you can suggest is a daily helping of blackstrap molasses, which supplies 27 mg of elemental iron per tablespoon.
Oral therapy should be continued even after hemoglobin and ferritin levels normalize. If laboratory values remain low after 2 weeks of oral therapy, parenteral therapy can be added to the oral regimen.
Therapy may be discontinued 2 months after delivery of the infant or surgery as long as the cause of the blood loss has been remedied. If the mother is breastfeeding, she should continue taking a prenatal vitamin until nursing has stopped.
TABLE 1
Some foods and drugs don’t mix well with iron
| Food or drug | Interaction | Recommendations |
|---|---|---|
| Foods high in phytic acid (grains, seeds, legumes) | Decreased absorption of iron | Do not take iron within 2 hours of eating foods high in phytic acid |
| Dairy products | Decreased bioavailability of iron | Do not take iron supplements within 1 hour of consuming dairy products, which can significantly decrease iron absorption |
| Levothyroxine | Iron reduces levothyroxine serum levels and efficacy | Take levothyroxine and iron at least 4 hours apart |
| Methyldopa | Oral iron reduces the efficacy of methyldopa | Consider IV iron or take oral iron and methyldopa as far apart as possible |
| Proton pump inhibitors (PPIs) | Absorption of oral iron is enhanced by gastric acid. PPIs decrease gastric acid production, thereby reducing the bioavailability of iron | Consider IV iron preparations |
| Ofloxacin | Iron reduces efficacy of ofloxacin | Administer ofloxacin and iron 2 hours apart |
| Cholestyramine | Decreased efficacy of iron | Administer iron and cholestyramine at least 4 hours apart |
| Calcium, aluminum, magnesium | Decreased absorption of iron | Iron should be taken at least 1 hour before or 2 hours after products that contain calcium, aluminum, or magnesium |
| Note: This table is not a comprehensive summary of all medications used in practice, but a list of those used commonly in obstetric and gynecologic populations | ||
IV iron isn’t as risky as you think
Historically, clinicians have avoided using parenteral iron sucrose (Venofer) because they have been taught that it can cause an anaphylactic reaction. In fact, although anaphylaxis may have been associated with older intravenous (IV) iron preparations, clinical trials have demonstrated the safety of IV iron sucrose and low-molecular-weight iron dextran. In a study involving 800 patients, Breymann and colleagues demonstrated that parenteral iron preparations containing dextran or iron dextrin could be safely given to pregnant women.4 Only 1.5% of the patients in the study experienced side effects from the therapy, and no anaphylactic reactions were observed.
In another study, 25 pregnant patients were given IV iron sucrose, and the only adverse reaction reported was a “not-unpleasant taste” during the injection.8
In an additional study, Breymann and colleagues found no adverse outcomes in 20 postpartum patients who received IV iron sucrose in addition to erythropoietin therapy.9
- The daily iron requirement is 2 mg of elemental iron in pregnancy and lactation, 1 mg at all other times
- The typical US diet contains about 18 mg of iron a day, of which only about 1 mg is absorbed
- Iron absorption occurs primarily in the second portion of the duodenum
- Iron absorption increases with iron deficiency
- One unit of blood contains 250 mg of iron
- Total body iron store is between 1,000 and 3,000 mg, depending on body size
- Each pregnancy depletes maternal iron stores by about 750 mg
- Latent iron deficiency is common in women who have had many pregnancies and in women who have menorrhagia.
Our preference for parenteral therapy is iron sucrose, classified by the Food and Drug Administration (FDA) as Pregnancy Category B. Iron sucrose is contraindicated in patients who have iron overload, hypersensitivity to inactive components of iron sucrose, or anemia that is not caused by iron deficiency. Adverse reactions to iron sucrose include, but are not limited to, anaphylaxis, hypotension, cramping, nausea, headache, vomiting, diarrhea, and chest pain. Adverse reactions are very rare, occurring in fewer than 1% of patients.
To determine whether the patient has an allergy to iron sucrose, give a test dosage of 25 mg via slow IV push and wait 20 minutes. If a reaction occurs, hold the remainder of the dose and consider alternative therapies. If no allergic reaction occurs, administer the remaining 275 mg in 50 mL to 100 mL of saline.
You may need to add erythropoietin to the regimen
Erythropoietin is a hormone made by the kidneys to promote formation of red blood cells in the bone marrow. A deficiency in this hormone causes anemia in patients who have renal disease, and nephrologists use a synthetic form of epoetin alfa (Epogen) to increase the hemoglobin level in dialysis patients.10 Epoetin alfa falls into FDA Pregnancy Category C.
In rare instances, erythropoietin-stimulating agents (ESAs), such as epoetin alfa, in addition to both IV and oral iron supplementation, are needed to increase the patient’s hemoglobin level and hematocrit before delivery or surgery. Before beginning ESA therapy, the patient’s platelet count and activity level need to be considered. ESAs have been linked to thrombolytic events and, therefore, should not be used in patients who have an elevated platelet count. The risk of thrombolytic events is a particular danger for antepartum patients on bed rest, and ESAs may be contraindicated for that reason.
Obstetric and surgical patients whose anemia has proven refractory to iron therapy may be considered for an ESA, as long as the benefits of this choice outweigh the risks. At an approximate cost of $400 for every 40,000 U, ESA therapy is by far the most expensive alternative to blood transfusion for patients who have iron-deficiency anemia. The patient typically receives one to two doses of an ESA.
Cost comparisons for alternative treatment modalities in iron-deficiency anemia can be found in TABLE 2.
TABLE 2
Estimated cost of treatment of anemia*
| Therapy | Dosage | Cost per dose |
|---|---|---|
| ORAL THERAPY | ||
| Ferrous sulfate | 325 mg | $0.05–$0.09 |
| Vitamin C | 500 mg | $0.04 |
| Vitron C | 1 tablet | $0.20 |
| Folic acid | 800 μg | $0.02 |
| INTRAVENOUS THERAPY | ||
| Iron sucrose | 100 mg | $80.00 |
| OTHER INTERVENTIONS | ||
| Transfusion | 1 U | $500.00–$600.00 |
| Erythropoietin | 40,000 U | $400.00 |
| * Local averages in central Pennsylvania | ||
TABLE 3
How safe are iron compounds in pregnancy and lactation?
| Compound | FDA pregnancy category | World Health Organization lactation recommendation | Thompson lactation rating |
|---|---|---|---|
| Parenteral iron dextran | C | Compatible with breastfeeding | Risk to infant cannot be ruled out |
| Parenteral iron sucrose | B | ||
| Oral iron | A | Unavailable | |
| Oral sodium ferric gluconate | A | Compatible with breastfeeding |
Erythropoietin-stimulating agents carry serious risks
The FDA issued the first of a series of letters to health-care professionals warning of adverse events associated with the use of ESAs in March 2007, after several randomized, controlled trials found an increased risk of stroke, blood clots, myocardial infarction, and death with high dosages. In November 2008, the FDA approved a black-box warning for the labels of Procrit and Aranesp, the two ESAs in general use in the United States. The new labels advise clinicians to modify dosages for patients who are in renal failure to maintain a target hemoglobin level between 11 and 12 g/dL, rather than the higher targets that had been in use.11,12
Transfusion is the last resort
Blood transfusion must also be considered as prophylaxis for blood loss in patients who have critically low hemoglobin levels, with due consideration of the procedure’s risks and benefits. Because the definition of “critically low” varies from patient to patient, other variables should be taken into consideration, including blood pressure; heart rate; urine output; tolerance for performing activities of daily living without dizziness, chest discomfort, or shortness of breath; and medical history. Potential drawbacks are considerable.
The multiple risks associated with transfusion include:
- immunosuppression
- fever
- chills
- urticaria
- hemolytic transfusion reaction
- septic transfusion reaction
- bacterial contamination
- anaphylaxis
- graft-versus-host reactions
- transfer of viral diseases, including hepatitis B and C and human immunodeficiency virus (HIV).
The risk of immunosuppression, in particular, should be weighed heavily for pregnant patients and those who are planning an elective surgical procedure. The possibility of viral transmission is also a deterrent. According to the Red Cross, the transmission rate is one in every 205,000 transfusions for hepatitis B, one in 2 million for hepatitis C, and one in 2,135,000 for HIV. These considerations, as well as the blood shortages that sometimes occur in practice, are sufficient reason to seek safer alternatives, when possible.
When a patient refuses transfusion
Caring for a patient who has an elevated risk of major blood loss can be particularly difficult when she is a member of a religious group such as Jehovah’s Witnesses. These patients generally decline the transfusion of plasma, packed red blood cells, white blood cells, platelets, and whole blood products.
In the Geisinger Health System, consultation with the Blood Conservation Program has been particularly helpful in these circumstances, offering clinicians alternative ways to correct anemia and prepare for the possibility of major blood loss. Patients who will not allow blood transfusion are often willing to accept plasma volume expanders that are not derived from blood, such as perfluorocarbon solutions, hydroxyethyl starch, crystalloid, or dextran.13 ESA therapy may be acceptable to some patients who refuse transfusion. Most are willing to go along with oral or IV iron supplementation to reduce their need for transfusion.
Postpartum patients may need special consideration
Iron supplementation is safe for breastfeeding mothers
Anemia in a breastfeeding woman is not uncommon and should be identified and treated. Iron supplementation with oral or IV compounds is considered safe for pregnant and breastfeeding women.
ESA therapy is a riskier strategy, whose benefits must clearly outweigh risks for all patients.
Anemia and postpartum depression
Studies have demonstrated a correlation between anemia and postpartum depression. Beard and colleagues showed a 25% improvement in cognition and improved scores on stress and depression scales in postpartum women who had iron-deficiency anemia when they were treated with daily iron and vitamin C.14 Other studies have addressed an increased risk of anemia in low-income postpartum women and the deleterious impact of iron-deficiency anemia on the quality of mother–child interactions and subsequent child development. Correcting maternal iron deficiency could prevent adverse outcomes in these mothers and their offspring.15,16
1. Gabbe SG, Niebyl JR, Simpson JL. Obstetrics: Normal and Problem Pregnancies. 5th ed. Philadelphia, Pa: Churchill Livingstone; 2007.
2. Creasy RK, Resnik R, Iams JD. Creasy and Resnik’s Maternal–Fetal Medicine: Principles and Practice. 6th ed. Philadelphia, Pa: Saunders; 2009.
3. Khusun H, Yip R, Schultink W, Dillon DHS. World Health Organization hemoglobin cutoff points for the detection of anemia are valid for an Indonesian population. J Nutr. 1999;129:1669-1674.
4. Breymann C. Intravenous iron in surgery and obstetrics. Transfus Altern Transfus Med. 2002;4(Suppl 2):22-23.
5. Mei Z, Cogswell ME, Parvanta I, et al. Hemoglobin and ferritin are currently the most efficient indicators of population response to iron interventions: an analysis of nine randomized controlled trials. J Nutr. 2005;135:1974-1980.
6. Gronowski AM, ed. Current Clinical Pathology: Handbook of Clinical Laboratory Testing During Pregnancy. Totowa, NJ: Humana Press; 2004:200.
7. Mani S, Duffy TP. Anemia of pregnancy. Clin Perinatol. 1995;22:593-607.
8. Bayoumeu F, Subiran-Buisset C, Baka NE, Legagneur H, Monnier-Barbarino P, Laxenaire MC. Iron therapy in iron deficiency anemia in pregnancy: intravenous route versus oral route. Am J Obstet Gynecol. 2002;186:518-522.
9. Breymann C, Richter C, Hüttner C, Huch R, Huch A. Effectiveness of recombinant erythropoietin and iron sucrose vs. iron therapy only, in patients with postpartum anaemia and blunted erythropoiesis. Eur J Clin Invest. 2000;30:154-161.
10. Bieber E. Erythropoietin, the biology of erythropoiesis and epoetin alfa. An overview. J Reprod Med. 2001;46(5 Suppl):521-530.
11. Patient Information for Procrit. Available at: www.procrit.com. Accessed December 1, 2009.
12. Patient Information for Aranesp. Available at: www.aranesp.com. Accessed December 1, 2009.
13. Baker BW. Blood conservation, obstetrics, and Jehovah’s Witnesses. Anesthesiol Clin North America. 1998;16:375-384.
14. Beard JL, Hendricks MK, Perez EM, et al. Maternal iron deficiency anemia affects postpartum emotions and cognition. J Nutr. 2005;135:267-272.
15. Bodnar LM, Cogswell ME, Scanlon KS. Low income postpartum women are at risk of iron deficiency. J Nutr. 2002;132:2298-2302.
16. Perez EM, Hendricks MK, Beard JL, et al. Mother–infant interactions and infant development are altered by maternal iron deficiency anemia. J Nutr. 2005;135:850-855.
1. Gabbe SG, Niebyl JR, Simpson JL. Obstetrics: Normal and Problem Pregnancies. 5th ed. Philadelphia, Pa: Churchill Livingstone; 2007.
2. Creasy RK, Resnik R, Iams JD. Creasy and Resnik’s Maternal–Fetal Medicine: Principles and Practice. 6th ed. Philadelphia, Pa: Saunders; 2009.
3. Khusun H, Yip R, Schultink W, Dillon DHS. World Health Organization hemoglobin cutoff points for the detection of anemia are valid for an Indonesian population. J Nutr. 1999;129:1669-1674.
4. Breymann C. Intravenous iron in surgery and obstetrics. Transfus Altern Transfus Med. 2002;4(Suppl 2):22-23.
5. Mei Z, Cogswell ME, Parvanta I, et al. Hemoglobin and ferritin are currently the most efficient indicators of population response to iron interventions: an analysis of nine randomized controlled trials. J Nutr. 2005;135:1974-1980.
6. Gronowski AM, ed. Current Clinical Pathology: Handbook of Clinical Laboratory Testing During Pregnancy. Totowa, NJ: Humana Press; 2004:200.
7. Mani S, Duffy TP. Anemia of pregnancy. Clin Perinatol. 1995;22:593-607.
8. Bayoumeu F, Subiran-Buisset C, Baka NE, Legagneur H, Monnier-Barbarino P, Laxenaire MC. Iron therapy in iron deficiency anemia in pregnancy: intravenous route versus oral route. Am J Obstet Gynecol. 2002;186:518-522.
9. Breymann C, Richter C, Hüttner C, Huch R, Huch A. Effectiveness of recombinant erythropoietin and iron sucrose vs. iron therapy only, in patients with postpartum anaemia and blunted erythropoiesis. Eur J Clin Invest. 2000;30:154-161.
10. Bieber E. Erythropoietin, the biology of erythropoiesis and epoetin alfa. An overview. J Reprod Med. 2001;46(5 Suppl):521-530.
11. Patient Information for Procrit. Available at: www.procrit.com. Accessed December 1, 2009.
12. Patient Information for Aranesp. Available at: www.aranesp.com. Accessed December 1, 2009.
13. Baker BW. Blood conservation, obstetrics, and Jehovah’s Witnesses. Anesthesiol Clin North America. 1998;16:375-384.
14. Beard JL, Hendricks MK, Perez EM, et al. Maternal iron deficiency anemia affects postpartum emotions and cognition. J Nutr. 2005;135:267-272.
15. Bodnar LM, Cogswell ME, Scanlon KS. Low income postpartum women are at risk of iron deficiency. J Nutr. 2002;132:2298-2302.
16. Perez EM, Hendricks MK, Beard JL, et al. Mother–infant interactions and infant development are altered by maternal iron deficiency anemia. J Nutr. 2005;135:850-855.
Laparoscopic tissue extraction: Pros and cons of 4 techniques
- In appropriately selected patients, the ability to easily and skillfully remove tissue during laparoscopy facilitates patient recovery and healing and limits hospitalization time.
- Even dense tissues such as partially calcified leiomyomata are readily removed with automatic morcellators, and the size of masses is less significant than with “manual” approaches.
Novel surgical devices and techniques have transformed operative laparoscopy by improving the inefficiencies in tissue extraction that limited early acceptance.
In the beginning, it was relatively easy to isolate a myoma and dissect it from the underlying myometrium, but it took hours to extract the tissue using a hand-held morcellator. This article focuses on the 4 techniques commonly used today, as well as the products that make them possible.
In appropriately selected patients, the ability to remove tissue through any of these methods facilitates patient recovery and healing and limits hospitalization time.
Posterior colpotomy
In the 1980s and early 1990s, it was common for tissue to be extracted through a posterior colpotomy. This is not surprising given that gynecologists were trained to perform transvaginal tubal ligation and to use colpotomies when incising and draining tubo-ovarian abscesses—not to mention our ease in entering the posterior cul-de-sac during vaginal surgery.
The elasticity of the vagina facilitates removal of fairly sizeable masses. Large ovarian cysts or masses can be brought to the cul-de-sac and incised and drained in a manner that markedly reduces the risk of intraperitoneal spillage.
There are disadvantages, however. For example, if the surgeon wants to maintain laparoscopic visualization once the colpotomy has been made, the tissue to be removed must be grasped and brought toward the opening to plug the defect and maintain pneumoperitoneum.
This may not be particularly problematic if there is only 1 mass to be removed, but it can be troublesome if there are several. An option is to place the masses in the posterior cul-de-sac so they can be readily grasped once the posterior colpotomy has been made.
One conceptual concern is the issue of subsequent adhesion formation, especially in patients desiring fertility. Unfortunately, no substantive trials exist to better answer this question.
Removal through the trocar or trocar site
Although some physicians still remove tissue through a posterior colpotomy, most have abandoned that approach in favor of extraction through a primary or lateral laparoscopic port. Indeed, this is the simplest technique for extracting tissue. I often change from a 10-mm laparoscope to a 5-mm instrument, placing the smaller endoscope in one of the lower ports and removing tissue under direct visualization through the 10- or 11-mm infraumbilical port.
If a cystic mass placed in a laparoscopic bag is too large to be removed, carefully aspirate it with a large-gauge needle.
Trapped tissue. One potential problem is the trapping of tissue in trocars that contain a flap valve. If this occurs, remove the trocar, clear the tissue, and replace the trocar in the original site using a blunt instrument such as the 10-mm laparoscope. Do not use the sharp inner blade to replace this port, as it is unduly risky.
For large masses, remove the port to create extra space. It also may be necessary to enlarge the skin or the fascial incision using a blunt instrument such as forceps.
Before the widespread availability of laparoscopic bags, tissue extraction was generally performed in this manner.
Risks include spillage of cyst contents during extraction and development of a hernia secondary to the wider disruption of fascia. This risk is particularly high in the infraumbilical area, which is inherently weak to begin with. It is thus critical—in any methodology—that the fascia be appropriately closed.
Laparoscopic bags
Many of the laparoscopic bags now widely available are easily opened once they have been placed in the abdomen, though some must be opened with graspers after the bag is positioned in the peritoneal cavity. Laparoscopic bags have greater utility when the extracted tissue is soft, such as with a dermoid cyst or ovary. Dense tissue is more difficult to manage.
Some surgeons fashion their own bags using sterile gloves or baggies.
Durability. The bags vary in their ability to withstand manipulation and puncture. For example, one type of nylon bag has a polyurethane inner coating and drawstring closure, making it quite durable. It also comes in a range of sizes, allowing the surgeon to choose the bag most suitable for the mass being removed.
To use a laparoscopic bag, insert it through the infraumbilical trocar and place the mass inside it. Then remove the trocar to provide maximal room for the mass to be extracted.
If the mass is cystic and too large to be removed, carefully aspirate it with a largegauge needle, taking care not to puncture the bag. Otherwise, morcellate the mass in the sac and remove it piecemeal, allowing no spillage of contents.
This may be performed under laparoscopic visualization through the lower ancillary trocars or trocar site. If a larger port has been placed—or there is a clinical need for one—tissue extraction also could be performed through the lower port.
Risks include bag breakage and potential spillage. In addition, it sometimes is necessary to change to a larger bag.
Morcellators
Early morcellators were hand-held, requiring the operator to continuously bite into the tissue and remove the small fragments. While this approach was effective for soft tissues and small myomas, it was ineffective for larger or more solid masses.
“Orange peel” technique. Scissors have been used to achieve the same effect as the handheld morcellator. Harrith Hasson described the “orange peel” technique, in which the surgeon uses scissors to peel away the tissue as one would peel an orange.1 The long, thin strips of tissue then can be extracted through the trocar. Unfortunately, laparoscopic scissors are often too small or dull to adequately incise larger fibroids.
Automatic morcellators have markedly enhanced our ability to perform laparoscopic myomectomy and similar procedures. They also have had a strong impact on nongynecologic procedures such as splenectomy or nephrectomy, in which large amounts of tissue must be removed. Although these devices are costly, the time savings associated with their use are significant. Current devices range from disposable to semidisposable and are available in a wide variety of sizes.
Even dense tissues such as partially calcified leiomyomata are readily removed with automatic morcellators.
Hasson’s orange-peel technique also can be employed with automatic morcellators. This allows long, thin strips of tissue to be removed while facilitating constant visualization of anatomy surrounding the tissue being extracted.
An alternative is making multiple “through-passes” into the myoma using the morcellator. In this method, the strips of tissue obtained will be smaller and the myoma will develop a Swiss-cheese appearance. Note that this approach takes longer and may increase the number of myoma fragments that fall into the pelvis and need to be removed.
Effective for a range of masses. Not surprisingly, even dense tissues such as partially calcified leiomyomata are readily removed with automatic morcellators, and the size of masses is less significant than with “manual” approaches. Nevertheless, it is critical that the surgeon maintain constant visualization and that tissue be brought toward the morcellator and away from underlying structures (FIGURE).
Do not move the morcellator toward the tissue. Because of its sharpness, the automatic morcellator will cut through vital structures as easily as it penetrates fibroids.
FIGURE Automatic morcellation: Move excised tissue toward device
To prevent injury, tissue should be brought toward the morcellator and away from underlying structures. Do not move the morcellator toward the tissue or vital structures may be cut.
Spillage
An early and continuing concern regarding ovarian cystectomy or oophorectomy is spillage of the mass’s contents into the peritoneal cavity. This is more of an issue in the case of borderline or malignant ovarian lesions or mucinous or dermoid ovarian cysts. In fact, nowhere is there more contention than over the clinical ramifications of spillage in the case of malignancy.
Mixed data on impact of spillage. Clinical data suggest that the impact of spillage is inconsequential, whereas other evidence suggests a worsening prognosis.2-5 In the event of spillage, most gynecologic oncologists would convert an ovarian cancer patient with a 1A or 1B staged lesion to stage 1C and would likely administer chemotherapy.
Concern about spillage of a mucinous or dermoid cyst centers on the theoretical risk of pseudomyxoma peritonei or, in the case of a teratoma, chemical peritonitis. Some surgeons routinely enter dermoids and intentionally spill the contents.6,7 Of note, we lack significant case series of ensuing infections or problems with this technique. Still, removing an intact cyst negates this issue and expedites surgery, eliminating the need to irrigate the abdomen and pelvis with large quantities of fluid.
Ectopic pregnancy has also been a concern, as there have been reports of chorionic tissue being disseminated in the abdomen and pelvis during laparoscopic procedures.8
Nowhere is there more contention than over the clinical ramifications of spillage in the case of malignancy.
Patient selection
Preoperative evaluation is a critical component of patient selection. A thorough ultrasound examination can help determine who is and who is not an appropriate candidate for laparoscopic management.
Cases that suggest a high risk of ovarian malignancy may be best managed in the traditional manner, as may patients with a large number of myomas or other compounding factors.
Dr. Bieber reports no financial relationships relevant to this article.
1. Hasson HM, Rotman C, Rana N, Sistos F, Dmowski WP. Laparoscopic myomectomy. Obstet Gynecol. 1992;80:884-888.
2. Mayer C, Miller DM, Ehlen TG. Peritoneal implantation of squamous cell carcinoma following rupture of a dermoid cyst during laparoscopic removal. Gynecol Oncol. 2002;84:180-183.
3. Kodama S, Tanaka K, Tokunaga A, et al. Multivariate analysis of prognostic fators in patients with ovarian cancer stage I and II. Int J Gynaecol Obstet. 1997;56:147-153.
4. Mizuno M, Kikkawa F, Shibata K, et al. Long-term prognosis of stage I ovarian carcinoma. Prognostic importance of intraoperative rupture. Oncology. 2003;65:29-36.
5. Vergote I, De Brabanter J, Fyles A, et al. Prognostic importance of degree of differentiation and cyst rupture in stage I invasive epithelial ovarian carcinoma. Lancet. 2001;357:176-182.
6. Zanetta G, Ferrari L, Mignini-Renzini M, Vignali M, Fadini R. Laparoscopic excision of ovarian dermoid cysts with controlled intraoperative spillage. Safety andeffectiveness. J Reprod Med. 1999;44:815-820.
7. Mecke H, Sawas V. Laparoscopic surgery of dermoid cysts—intraoperative spillage and complications. Eur J Obstet Gynecol Reprod Biol. 2001;96:80-84.
8. Billieux MH, Petignat P, Anguenot JL, Campana A, Bischof P. Early and late halflife of human chorionic gonadotropin as a predictor of persistent trophoblast after laparoscopic conservative surgery for tubal pregnancy. Acta Obstet Gynecol Scand. 2003;82:550-555.
- In appropriately selected patients, the ability to easily and skillfully remove tissue during laparoscopy facilitates patient recovery and healing and limits hospitalization time.
- Even dense tissues such as partially calcified leiomyomata are readily removed with automatic morcellators, and the size of masses is less significant than with “manual” approaches.
Novel surgical devices and techniques have transformed operative laparoscopy by improving the inefficiencies in tissue extraction that limited early acceptance.
In the beginning, it was relatively easy to isolate a myoma and dissect it from the underlying myometrium, but it took hours to extract the tissue using a hand-held morcellator. This article focuses on the 4 techniques commonly used today, as well as the products that make them possible.
In appropriately selected patients, the ability to remove tissue through any of these methods facilitates patient recovery and healing and limits hospitalization time.
Posterior colpotomy
In the 1980s and early 1990s, it was common for tissue to be extracted through a posterior colpotomy. This is not surprising given that gynecologists were trained to perform transvaginal tubal ligation and to use colpotomies when incising and draining tubo-ovarian abscesses—not to mention our ease in entering the posterior cul-de-sac during vaginal surgery.
The elasticity of the vagina facilitates removal of fairly sizeable masses. Large ovarian cysts or masses can be brought to the cul-de-sac and incised and drained in a manner that markedly reduces the risk of intraperitoneal spillage.
There are disadvantages, however. For example, if the surgeon wants to maintain laparoscopic visualization once the colpotomy has been made, the tissue to be removed must be grasped and brought toward the opening to plug the defect and maintain pneumoperitoneum.
This may not be particularly problematic if there is only 1 mass to be removed, but it can be troublesome if there are several. An option is to place the masses in the posterior cul-de-sac so they can be readily grasped once the posterior colpotomy has been made.
One conceptual concern is the issue of subsequent adhesion formation, especially in patients desiring fertility. Unfortunately, no substantive trials exist to better answer this question.
Removal through the trocar or trocar site
Although some physicians still remove tissue through a posterior colpotomy, most have abandoned that approach in favor of extraction through a primary or lateral laparoscopic port. Indeed, this is the simplest technique for extracting tissue. I often change from a 10-mm laparoscope to a 5-mm instrument, placing the smaller endoscope in one of the lower ports and removing tissue under direct visualization through the 10- or 11-mm infraumbilical port.
If a cystic mass placed in a laparoscopic bag is too large to be removed, carefully aspirate it with a large-gauge needle.
Trapped tissue. One potential problem is the trapping of tissue in trocars that contain a flap valve. If this occurs, remove the trocar, clear the tissue, and replace the trocar in the original site using a blunt instrument such as the 10-mm laparoscope. Do not use the sharp inner blade to replace this port, as it is unduly risky.
For large masses, remove the port to create extra space. It also may be necessary to enlarge the skin or the fascial incision using a blunt instrument such as forceps.
Before the widespread availability of laparoscopic bags, tissue extraction was generally performed in this manner.
Risks include spillage of cyst contents during extraction and development of a hernia secondary to the wider disruption of fascia. This risk is particularly high in the infraumbilical area, which is inherently weak to begin with. It is thus critical—in any methodology—that the fascia be appropriately closed.
Laparoscopic bags
Many of the laparoscopic bags now widely available are easily opened once they have been placed in the abdomen, though some must be opened with graspers after the bag is positioned in the peritoneal cavity. Laparoscopic bags have greater utility when the extracted tissue is soft, such as with a dermoid cyst or ovary. Dense tissue is more difficult to manage.
Some surgeons fashion their own bags using sterile gloves or baggies.
Durability. The bags vary in their ability to withstand manipulation and puncture. For example, one type of nylon bag has a polyurethane inner coating and drawstring closure, making it quite durable. It also comes in a range of sizes, allowing the surgeon to choose the bag most suitable for the mass being removed.
To use a laparoscopic bag, insert it through the infraumbilical trocar and place the mass inside it. Then remove the trocar to provide maximal room for the mass to be extracted.
If the mass is cystic and too large to be removed, carefully aspirate it with a largegauge needle, taking care not to puncture the bag. Otherwise, morcellate the mass in the sac and remove it piecemeal, allowing no spillage of contents.
This may be performed under laparoscopic visualization through the lower ancillary trocars or trocar site. If a larger port has been placed—or there is a clinical need for one—tissue extraction also could be performed through the lower port.
Risks include bag breakage and potential spillage. In addition, it sometimes is necessary to change to a larger bag.
Morcellators
Early morcellators were hand-held, requiring the operator to continuously bite into the tissue and remove the small fragments. While this approach was effective for soft tissues and small myomas, it was ineffective for larger or more solid masses.
“Orange peel” technique. Scissors have been used to achieve the same effect as the handheld morcellator. Harrith Hasson described the “orange peel” technique, in which the surgeon uses scissors to peel away the tissue as one would peel an orange.1 The long, thin strips of tissue then can be extracted through the trocar. Unfortunately, laparoscopic scissors are often too small or dull to adequately incise larger fibroids.
Automatic morcellators have markedly enhanced our ability to perform laparoscopic myomectomy and similar procedures. They also have had a strong impact on nongynecologic procedures such as splenectomy or nephrectomy, in which large amounts of tissue must be removed. Although these devices are costly, the time savings associated with their use are significant. Current devices range from disposable to semidisposable and are available in a wide variety of sizes.
Even dense tissues such as partially calcified leiomyomata are readily removed with automatic morcellators.
Hasson’s orange-peel technique also can be employed with automatic morcellators. This allows long, thin strips of tissue to be removed while facilitating constant visualization of anatomy surrounding the tissue being extracted.
An alternative is making multiple “through-passes” into the myoma using the morcellator. In this method, the strips of tissue obtained will be smaller and the myoma will develop a Swiss-cheese appearance. Note that this approach takes longer and may increase the number of myoma fragments that fall into the pelvis and need to be removed.
Effective for a range of masses. Not surprisingly, even dense tissues such as partially calcified leiomyomata are readily removed with automatic morcellators, and the size of masses is less significant than with “manual” approaches. Nevertheless, it is critical that the surgeon maintain constant visualization and that tissue be brought toward the morcellator and away from underlying structures (FIGURE).
Do not move the morcellator toward the tissue. Because of its sharpness, the automatic morcellator will cut through vital structures as easily as it penetrates fibroids.
FIGURE Automatic morcellation: Move excised tissue toward device
To prevent injury, tissue should be brought toward the morcellator and away from underlying structures. Do not move the morcellator toward the tissue or vital structures may be cut.
Spillage
An early and continuing concern regarding ovarian cystectomy or oophorectomy is spillage of the mass’s contents into the peritoneal cavity. This is more of an issue in the case of borderline or malignant ovarian lesions or mucinous or dermoid ovarian cysts. In fact, nowhere is there more contention than over the clinical ramifications of spillage in the case of malignancy.
Mixed data on impact of spillage. Clinical data suggest that the impact of spillage is inconsequential, whereas other evidence suggests a worsening prognosis.2-5 In the event of spillage, most gynecologic oncologists would convert an ovarian cancer patient with a 1A or 1B staged lesion to stage 1C and would likely administer chemotherapy.
Concern about spillage of a mucinous or dermoid cyst centers on the theoretical risk of pseudomyxoma peritonei or, in the case of a teratoma, chemical peritonitis. Some surgeons routinely enter dermoids and intentionally spill the contents.6,7 Of note, we lack significant case series of ensuing infections or problems with this technique. Still, removing an intact cyst negates this issue and expedites surgery, eliminating the need to irrigate the abdomen and pelvis with large quantities of fluid.
Ectopic pregnancy has also been a concern, as there have been reports of chorionic tissue being disseminated in the abdomen and pelvis during laparoscopic procedures.8
Nowhere is there more contention than over the clinical ramifications of spillage in the case of malignancy.
Patient selection
Preoperative evaluation is a critical component of patient selection. A thorough ultrasound examination can help determine who is and who is not an appropriate candidate for laparoscopic management.
Cases that suggest a high risk of ovarian malignancy may be best managed in the traditional manner, as may patients with a large number of myomas or other compounding factors.
Dr. Bieber reports no financial relationships relevant to this article.
- In appropriately selected patients, the ability to easily and skillfully remove tissue during laparoscopy facilitates patient recovery and healing and limits hospitalization time.
- Even dense tissues such as partially calcified leiomyomata are readily removed with automatic morcellators, and the size of masses is less significant than with “manual” approaches.
Novel surgical devices and techniques have transformed operative laparoscopy by improving the inefficiencies in tissue extraction that limited early acceptance.
In the beginning, it was relatively easy to isolate a myoma and dissect it from the underlying myometrium, but it took hours to extract the tissue using a hand-held morcellator. This article focuses on the 4 techniques commonly used today, as well as the products that make them possible.
In appropriately selected patients, the ability to remove tissue through any of these methods facilitates patient recovery and healing and limits hospitalization time.
Posterior colpotomy
In the 1980s and early 1990s, it was common for tissue to be extracted through a posterior colpotomy. This is not surprising given that gynecologists were trained to perform transvaginal tubal ligation and to use colpotomies when incising and draining tubo-ovarian abscesses—not to mention our ease in entering the posterior cul-de-sac during vaginal surgery.
The elasticity of the vagina facilitates removal of fairly sizeable masses. Large ovarian cysts or masses can be brought to the cul-de-sac and incised and drained in a manner that markedly reduces the risk of intraperitoneal spillage.
There are disadvantages, however. For example, if the surgeon wants to maintain laparoscopic visualization once the colpotomy has been made, the tissue to be removed must be grasped and brought toward the opening to plug the defect and maintain pneumoperitoneum.
This may not be particularly problematic if there is only 1 mass to be removed, but it can be troublesome if there are several. An option is to place the masses in the posterior cul-de-sac so they can be readily grasped once the posterior colpotomy has been made.
One conceptual concern is the issue of subsequent adhesion formation, especially in patients desiring fertility. Unfortunately, no substantive trials exist to better answer this question.
Removal through the trocar or trocar site
Although some physicians still remove tissue through a posterior colpotomy, most have abandoned that approach in favor of extraction through a primary or lateral laparoscopic port. Indeed, this is the simplest technique for extracting tissue. I often change from a 10-mm laparoscope to a 5-mm instrument, placing the smaller endoscope in one of the lower ports and removing tissue under direct visualization through the 10- or 11-mm infraumbilical port.
If a cystic mass placed in a laparoscopic bag is too large to be removed, carefully aspirate it with a large-gauge needle.
Trapped tissue. One potential problem is the trapping of tissue in trocars that contain a flap valve. If this occurs, remove the trocar, clear the tissue, and replace the trocar in the original site using a blunt instrument such as the 10-mm laparoscope. Do not use the sharp inner blade to replace this port, as it is unduly risky.
For large masses, remove the port to create extra space. It also may be necessary to enlarge the skin or the fascial incision using a blunt instrument such as forceps.
Before the widespread availability of laparoscopic bags, tissue extraction was generally performed in this manner.
Risks include spillage of cyst contents during extraction and development of a hernia secondary to the wider disruption of fascia. This risk is particularly high in the infraumbilical area, which is inherently weak to begin with. It is thus critical—in any methodology—that the fascia be appropriately closed.
Laparoscopic bags
Many of the laparoscopic bags now widely available are easily opened once they have been placed in the abdomen, though some must be opened with graspers after the bag is positioned in the peritoneal cavity. Laparoscopic bags have greater utility when the extracted tissue is soft, such as with a dermoid cyst or ovary. Dense tissue is more difficult to manage.
Some surgeons fashion their own bags using sterile gloves or baggies.
Durability. The bags vary in their ability to withstand manipulation and puncture. For example, one type of nylon bag has a polyurethane inner coating and drawstring closure, making it quite durable. It also comes in a range of sizes, allowing the surgeon to choose the bag most suitable for the mass being removed.
To use a laparoscopic bag, insert it through the infraumbilical trocar and place the mass inside it. Then remove the trocar to provide maximal room for the mass to be extracted.
If the mass is cystic and too large to be removed, carefully aspirate it with a largegauge needle, taking care not to puncture the bag. Otherwise, morcellate the mass in the sac and remove it piecemeal, allowing no spillage of contents.
This may be performed under laparoscopic visualization through the lower ancillary trocars or trocar site. If a larger port has been placed—or there is a clinical need for one—tissue extraction also could be performed through the lower port.
Risks include bag breakage and potential spillage. In addition, it sometimes is necessary to change to a larger bag.
Morcellators
Early morcellators were hand-held, requiring the operator to continuously bite into the tissue and remove the small fragments. While this approach was effective for soft tissues and small myomas, it was ineffective for larger or more solid masses.
“Orange peel” technique. Scissors have been used to achieve the same effect as the handheld morcellator. Harrith Hasson described the “orange peel” technique, in which the surgeon uses scissors to peel away the tissue as one would peel an orange.1 The long, thin strips of tissue then can be extracted through the trocar. Unfortunately, laparoscopic scissors are often too small or dull to adequately incise larger fibroids.
Automatic morcellators have markedly enhanced our ability to perform laparoscopic myomectomy and similar procedures. They also have had a strong impact on nongynecologic procedures such as splenectomy or nephrectomy, in which large amounts of tissue must be removed. Although these devices are costly, the time savings associated with their use are significant. Current devices range from disposable to semidisposable and are available in a wide variety of sizes.
Even dense tissues such as partially calcified leiomyomata are readily removed with automatic morcellators.
Hasson’s orange-peel technique also can be employed with automatic morcellators. This allows long, thin strips of tissue to be removed while facilitating constant visualization of anatomy surrounding the tissue being extracted.
An alternative is making multiple “through-passes” into the myoma using the morcellator. In this method, the strips of tissue obtained will be smaller and the myoma will develop a Swiss-cheese appearance. Note that this approach takes longer and may increase the number of myoma fragments that fall into the pelvis and need to be removed.
Effective for a range of masses. Not surprisingly, even dense tissues such as partially calcified leiomyomata are readily removed with automatic morcellators, and the size of masses is less significant than with “manual” approaches. Nevertheless, it is critical that the surgeon maintain constant visualization and that tissue be brought toward the morcellator and away from underlying structures (FIGURE).
Do not move the morcellator toward the tissue. Because of its sharpness, the automatic morcellator will cut through vital structures as easily as it penetrates fibroids.
FIGURE Automatic morcellation: Move excised tissue toward device
To prevent injury, tissue should be brought toward the morcellator and away from underlying structures. Do not move the morcellator toward the tissue or vital structures may be cut.
Spillage
An early and continuing concern regarding ovarian cystectomy or oophorectomy is spillage of the mass’s contents into the peritoneal cavity. This is more of an issue in the case of borderline or malignant ovarian lesions or mucinous or dermoid ovarian cysts. In fact, nowhere is there more contention than over the clinical ramifications of spillage in the case of malignancy.
Mixed data on impact of spillage. Clinical data suggest that the impact of spillage is inconsequential, whereas other evidence suggests a worsening prognosis.2-5 In the event of spillage, most gynecologic oncologists would convert an ovarian cancer patient with a 1A or 1B staged lesion to stage 1C and would likely administer chemotherapy.
Concern about spillage of a mucinous or dermoid cyst centers on the theoretical risk of pseudomyxoma peritonei or, in the case of a teratoma, chemical peritonitis. Some surgeons routinely enter dermoids and intentionally spill the contents.6,7 Of note, we lack significant case series of ensuing infections or problems with this technique. Still, removing an intact cyst negates this issue and expedites surgery, eliminating the need to irrigate the abdomen and pelvis with large quantities of fluid.
Ectopic pregnancy has also been a concern, as there have been reports of chorionic tissue being disseminated in the abdomen and pelvis during laparoscopic procedures.8
Nowhere is there more contention than over the clinical ramifications of spillage in the case of malignancy.
Patient selection
Preoperative evaluation is a critical component of patient selection. A thorough ultrasound examination can help determine who is and who is not an appropriate candidate for laparoscopic management.
Cases that suggest a high risk of ovarian malignancy may be best managed in the traditional manner, as may patients with a large number of myomas or other compounding factors.
Dr. Bieber reports no financial relationships relevant to this article.
1. Hasson HM, Rotman C, Rana N, Sistos F, Dmowski WP. Laparoscopic myomectomy. Obstet Gynecol. 1992;80:884-888.
2. Mayer C, Miller DM, Ehlen TG. Peritoneal implantation of squamous cell carcinoma following rupture of a dermoid cyst during laparoscopic removal. Gynecol Oncol. 2002;84:180-183.
3. Kodama S, Tanaka K, Tokunaga A, et al. Multivariate analysis of prognostic fators in patients with ovarian cancer stage I and II. Int J Gynaecol Obstet. 1997;56:147-153.
4. Mizuno M, Kikkawa F, Shibata K, et al. Long-term prognosis of stage I ovarian carcinoma. Prognostic importance of intraoperative rupture. Oncology. 2003;65:29-36.
5. Vergote I, De Brabanter J, Fyles A, et al. Prognostic importance of degree of differentiation and cyst rupture in stage I invasive epithelial ovarian carcinoma. Lancet. 2001;357:176-182.
6. Zanetta G, Ferrari L, Mignini-Renzini M, Vignali M, Fadini R. Laparoscopic excision of ovarian dermoid cysts with controlled intraoperative spillage. Safety andeffectiveness. J Reprod Med. 1999;44:815-820.
7. Mecke H, Sawas V. Laparoscopic surgery of dermoid cysts—intraoperative spillage and complications. Eur J Obstet Gynecol Reprod Biol. 2001;96:80-84.
8. Billieux MH, Petignat P, Anguenot JL, Campana A, Bischof P. Early and late halflife of human chorionic gonadotropin as a predictor of persistent trophoblast after laparoscopic conservative surgery for tubal pregnancy. Acta Obstet Gynecol Scand. 2003;82:550-555.
1. Hasson HM, Rotman C, Rana N, Sistos F, Dmowski WP. Laparoscopic myomectomy. Obstet Gynecol. 1992;80:884-888.
2. Mayer C, Miller DM, Ehlen TG. Peritoneal implantation of squamous cell carcinoma following rupture of a dermoid cyst during laparoscopic removal. Gynecol Oncol. 2002;84:180-183.
3. Kodama S, Tanaka K, Tokunaga A, et al. Multivariate analysis of prognostic fators in patients with ovarian cancer stage I and II. Int J Gynaecol Obstet. 1997;56:147-153.
4. Mizuno M, Kikkawa F, Shibata K, et al. Long-term prognosis of stage I ovarian carcinoma. Prognostic importance of intraoperative rupture. Oncology. 2003;65:29-36.
5. Vergote I, De Brabanter J, Fyles A, et al. Prognostic importance of degree of differentiation and cyst rupture in stage I invasive epithelial ovarian carcinoma. Lancet. 2001;357:176-182.
6. Zanetta G, Ferrari L, Mignini-Renzini M, Vignali M, Fadini R. Laparoscopic excision of ovarian dermoid cysts with controlled intraoperative spillage. Safety andeffectiveness. J Reprod Med. 1999;44:815-820.
7. Mecke H, Sawas V. Laparoscopic surgery of dermoid cysts—intraoperative spillage and complications. Eur J Obstet Gynecol Reprod Biol. 2001;96:80-84.
8. Billieux MH, Petignat P, Anguenot JL, Campana A, Bischof P. Early and late halflife of human chorionic gonadotropin as a predictor of persistent trophoblast after laparoscopic conservative surgery for tubal pregnancy. Acta Obstet Gynecol Scand. 2003;82:550-555.