User login
After several decades of slow progress, the field of urogynecology is experiencing dynamic change, including:
- new minimally invasive, tension-free, midurethral sling procedures, especially the transobturator approach,
- correction of size-specific defects to repair prolapse, and
- use of mesh/graft augmentation in pro lapse repair.
These developments are some of the most important since Kelly and Dunn first described suburethral fascial plication for stress incontinence and cystocele in 1914.1 They have come about through increased understanding of the pathophysiology of incontinence and prolapse, innovative technology and techniques, and improved communication and coordination among physicians worldwide.
New sling procedures, promising outcomes
The minimally invasive midurethral sling procedure spawned notable new approaches and is a mainstay of surgical treatment for stress urinary incontinence.
First described in Sweden by Ulmsten in 1995,2 the tension-free vaginal tape procedure is a revolutionary change in the suburethral sling procedure and is now the most widely performed surgery for stress incontinence worldwide.
In it, a tension-free vaginal tape (TVT) (Gynecare, Somerville, NJ) of synthetic polypropylene mesh is attached to 2 needles and passed through a vaginal incision and the retropubic space, exiting to small incisions in the suprapubic region to create a suburethral sling or hammock and provide urethral support during increased abdominal pressure. The sling remains fixed by friction and subsequent adhesions.
Although the traditional suburethral sling was less invasive than other abdominal incontinence procedures, it was associated with a steep learning curve and a high incidence of postoperative irritative bladder symptoms and voiding dysfunction.3
Tension-free vaginal tape: Excellent long-term cure
It can be performed routinely in under 30 minutes using local anesthesia, with minimal postoperative complications. Five-year cure rates approach 95%,4 and data presented at the 2003 International Urogynecology Association clinical meeting describe an objective 7-year cure rate of 82%.5
The incidence and severity of postoperative voiding dysfunction following the TVT procedure is significantly lower than that reported after traditional suburethral sling (2%–40%) or transvaginal needle suspension (2%–50%) procedures.6-9 Although bladder perforation has occurred in up to 10% of patients, reports of complications, including major hemorrhage, tape erosion, and bowel and nerve injury, are rare.10,11
Modifications
These products have a modified approach, materials, or refinements in technique to address various needs. For example, American Medical Systems (Minnetonka, Minn) introduced the SPARC procedure, which allows abdominal placement of the midurethral sling, similar to a needle suspension technique.
CR Bard (Murray Hill, NJ) introduced a midurethral sling of porcine dermis (Pelvicol) to address concerns physicians may have about using synthetic materials.
Minimally invasive midurethral slings include the Advantage (Boston Scientific, Natick, Mass), Centrasorb (Caldera Medical, Thousand Oaks, Calif), Stratasis TF (Cook, West Lafayette, Ind), and Uretex (CR Bard).
Newest approach: Transobturator sling
In this technique, the sling is placed in the midurethral position, but the insertion points are in the genital area lateral to the vagina, and the needle passes through the obturator membrane and paraurethral space. Because it avoids passage of the needles through the retropubic space, the transobturator approach theoretically should reduce the risk of bowel, bladder, and major blood vessel injury.
The procedure was initially described in Europe and introduced in the United States in 2003. Current product offerings include the outside-in approach of ObTape (Mentor, Santa Barbara, Calif), Monarc (American Medical Systems), and Uretex (CR Bard). Gynecare offers a variation of its TVT—the TVT-Obturator—which involves an inside-out approach to further minimize risk of vascular injury.
Shortage of long-term data, but good early results
In a 1-year follow-up of patients undergoing a sling procedure with the UraTape transobturator sling (Mentor), Delorme and colleagues12 reported that 29 of 32 patients (90.6%) were cured and 3 (9.4%) improved. De Leval13 described his inside-out approach with equally good results: no bladder or urethral injuries and no vascular (hematoma or bleeding) or neurological complications. A transobturator technique using porcine dermis has also been described.
Tension-free vaginal tape versus transobturator sling
In 2004, a small randomized, prospective trial of TVT (n = 29) versus transobturator tape (n = 27) with 1-year follow-up found the transobturator approach to be safer and easier to place with equivalent short-term results.14 Mean operative time was significantly shorter in the transobturator group (15±4 minutes versus 27±8 minutes, P <.001).
No bladder injury occurred in the transobturator group versus 9.7% (n = 3) in the TVT group (P >.05). The rate of postoperative urinary retention was 25.8% (n = 8) in the TVT group versus 13.3% (n = 4) in the transobturator group (P >.05). Cure rates were similar for the TVT and transobturator groups: 83.9% versus 90%, respectively), improvement (9.7% versus 3.3%), and failure (6.5% versus 6.7%). No vaginal erosion occurred in either group.
Additional investigations of the transobturator tape procedure are underway.
Correcting site-specific defects in prolapse repair
This repair rationale should become the standard, although it has yet to be widely adopted and procedural refinement and research are continuing.
As early as 1908, site-specific defects in the endopelvic fascia were identified as the likely cause of anterior vaginal segment prolapse. Like hernia repair, which requires closure of the fascial defect, the “cystocele hernia” repair advocated by George White involved reattaching the endopelvic fascia to the arcus tendineus fascia pelvis using a series of interrupted sutures through an abdominal retropubic approach.
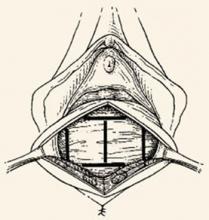
Although this view was later abandoned, it resurfaced in the 1980s, when Cullen Richardson described midline, lateral, and transverse defects (FIGURE 1) in the endopelvic fascia as the cause of cystocele and rectocele.15 Richardson advocated diagnosis that identified these fascial defects, along with treatment with site-specific repair.
(In the intervening decades, prolapse was thought to result from generalized weakening or attenuation of the endopelvic fascia that supports the bladder, rectum, or vagina, leading to cystocele, rectocele, or uterine prolapse, respectively. Traditional repairs, still widely performed by most gynecologists, consist of the anterior and posterior colporrhaphy, which involve midline plication of the endopelvic fascia to reduce the prolapse and recreate support by strengthening the weakened fascial layer.)
FIGURE 1 Identify the site of defect
Whether the defect that causes a rectocele or cystocele is transverse, central, or lateral determines the type of repair to be performed. Reprinted with permission from Richardson AC. The rectovaginal septum revisited: its relationship to rectocele and its importance in rectocele repair. Clin Obstet Gynecol.1993;36(4):976–983.
Excellent cure rates with fewer complications
Site-specific repair (FIGURE 2) has been adopted by many pelvic surgeons, who report excellent cure rates with fewer complications such as dyspareunia, vaginal narrowing, and increased blood loss—all of which are more common with traditional anterior and posterior colporrhaphy.
Traditional colporrhaphy does correct the underlying fascial defect when the “hernia” is in the midline. However, for lateral and transverse defects, traditional colporrhaphy leaves the defect uncorrected and may even create additional tension, resulting in recurrence.
Site-specific repair also is useful in the treatment of enterocele, which has been described as a herniation of the peritoneum through a defect of the anterior and posterior fascial planes at the vaginal apex.16
Widening but not broad acceptance
Although cure rates for site-specific prolapse repair range from 75% to 85%,17,18 the concept has yet to be widely adopted and continues to undergo procedural refinement and research, as well as physician education. With increasing experience and clinical data, it may become the standard for pelvic prolapse repair.
FIGURE 2 Repair the defect to correct the rectocele
Surgical technique for a transverse tear in the rectovaginal fascia: After identifying the defect, place a series of interrupted sutures to reapproximate the fascial edges (copyright Miklos/Kohli).
Mesh augmentation: Useful in selected patients
Although augmentation with mesh or biomaterials is easy to perform, it remains unclear which technique and materials are optimal.
The use of mesh and biomaterials to augment repair of a cystocele, rectocele, or enterocele is slowly increasing, although general surgeons have been utilizing these materials for many years in hernia repair.
Augmentation has been advocated for “pelvic hernias” because of poor long-term cure rates for traditional prolapse surgeries (which range from 40% to 80%—well behind rates of 90% or more for incontinence procedures).
A recent informal survey of the members of the American Urogynecologic Society and International Urogynecologic Association revealed that most pelvic surgeons are using some type of graft or mesh to augment repairs in selected patients.
Synthetic mesh versus autologous and heterologous grafts
Synthetic materials have the advantage of being readily available, cost-effective, and consistent in quality, but they may cause significant complications, including infection, stricture, and erosion. This is especially important in the vagina, which needs to stretch during intercourse and which alters in thickness and other properties during a woman’s lifetime.
In contrast, autologous and heterologous donor grafts provide naturally occurring biomaterials capable of remodeling. Unfortunately, the in vivo tissue response is not yet fully understood. Other disadvantages: Biomaterials may lack consistent tissue properties and can be expensive.
For these reasons, graft materials remain in an early period of evaluation, although their use is expected to rise steadily with increasing experience and new product development.
Limited data on safety and efficacy
Although many pelvic surgeons use graft/mesh materials in prolapse repair, data on their safety and efficacy are limited, partly due to the variety of surgical techniques and materials available. Another factor is the difficulty of obtaining good long-term data with large patient numbers. Most of the literature is comprised of case reports, with few prospective, randomized trials.
That said, initial data suggest a significant improvement in cure rates, compared with traditional techniques, with minimal short-term complications. Long-term results and complication rates are not yet available.
What existing studies show
A variety of synthetic materials have been used in surgical correction of cystocele. In the largest series to date, Flood and colleagues19 reported their 12-year experience with 142 women undergoing a modified anterior colporrhaphy reinforced with Marlex (Davol, Cranston, RI) mesh: 100% success in correcting cystocele and a 74% success rate for urinary stress incontinence, with a mean follow-up of 3.2 years and no significant intraoperative complications.
In a prospective randomized trial of 125 patients utilizing absorbable polyglactin 910 mesh (Vicryl) (Ethicon, Somerville, NJ) to augment standard anterior colporrhaphy, Koduri et al20 reported a failure rate of 13% in the colporrhaphy-alone group compared to 1% in the colporrhaphy-mesh group at the 1-year follow-up. Subjectively, both groups improved equally.
Clemons et al21 used a human dermis graft to treat advanced recurrent cystocele in 33 women, with a follow-up of 18 months. They noted 13 (41%) objective failures and 1 (3%) subjective failure. Complications included 1 case of febrile morbidity, 1 cystotomy, and 1 anterior wall breakdown secondary to hematoma formation caused by heparin therapy. No other erosions or rejections were seen.
Most effective applications
Graft/mesh augmentation may be most effective in posterior vaginal segment reconstruction and rectocele repair, as it obviates the need for levatorplasty in patients with poor rectovaginal fascia. A variety of synthetic materials have been used for posterior wall reconstruction in small series.
Mersilene mesh
Mersilene mesh (Ethicon) was used by Fox and Stanton22 to augment traditional rectocele repair in 29 women followed for 14 months. Most of the women had undergone previous rectocele repair with recurrence of their prolapse. All women with stage II and stage III vault prolapse were corrected, with an increase in stage I prolapse from 20% to 27%. All women with stage II and stage III rectocele were corrected, with a decrease in stage I prolapse from 36% to 7%. The only significant intraoperative complication was a cystotomy. One mesh became infected postoperatively, requiring removal.
Cadaveric dermal graft
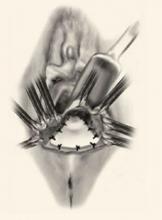
Recently, Kohli and Miklos23 reported their experience with 57 patients undergoing augmented rectocele repair using a cadaveric dermal graft (FIGURE 3) over a 2-year period. Average follow-up was 11 months. Average patient age in the follow-up group was 63.6±10.9 years (range: 33–79 years) and average parity was 2.8±1.5 (range: 0–7). No major intraoperative complications (hollow viscous injury, blood loss greater than 500 cc, or transfusion) or post-operative complications (infection, abscess, or hematoma) were noted. No graft-related complications such as rejection, erosion, infection, and fistula formation were noted during the follow-up period.
Using previously accepted Pelvic Organ Prolapse Quantification (POP-Q) parameters for success, Kohli and Miklos found 54 of 57 women (95%) to have surgical cure at follow-up. These authors have also described the use of a dermal graft in the repair of a complicated rectovaginal fistula.24
FIGURE 3 Placement of mesh augmentation
Augment enterocele/rectocele repair by attaching the mesh apically, laterally, and distally (copyright Miklos/Kohli).
Future outlook
Further data on the efficacy and safety of graft/mesh augmentation—including identification of the optimal technique and ideal material—are necessary before this approach can be widely adopted. However, in selected patients, it represents a significant advance in the surgical treatment of pelvic prolapse.
The author is a preceptor for American Medical Systems, Gynecare, CR Bard, and Mentor and a consultant for Boston Scientific.
1. Kelly HA, Dunn WM. Urinary incontinence in women without manifest injury to the bladder. Surg Gynecol Obstet. 1914;18:444.-
2. Ulmsten U, Petros P. Intravaginal slingplasty (IVS): an ambulatory surgical procedure for treatment of female urinary incontinence. Scand J Urol Nephrol. 1995;29:75-82.
3. Weinberger MW, Ostergard DR. Postoperative catheterization, urinary retention, and permanent voiding dysfunction after polytetrafluroethylene suburethral sling placement. Obstet Gynecol. 1996;87:50-54.
4. Nilsson CG, Kuuva N, Falconer C, Rezapour M, Ulmsten U. Long-term results of the tension-free vaginal tape (TVT) procedure for surgical treatment of female stress urinary incontinence. Int Urogynecol J Pelvic Floor Dysfunct. 2001;12(Suppl 2):S5-8.
5. Nilsson CG, Rezapour M, Falconer C. Seven years follow-up of the tension-free vaginal tape (TVT) procedure. Presented at the 2003 International Urogynecology Association Annual Meeting.
6. Horbach N. Suburethral sling procedures. In: Ostergard DR, Bent AE, eds. Urogynecology and Urodynamics: Theory and Practice. 3rd ed. Baltimore: Williams & Wilkins; 1991;449-458.
7. Stanton SL, Reynolds SF, Creighton SM. The modified Pereyra (Raz) procedure for genuine stress incontinence—a useful option in the elderly or frail patient? Int Urogynecol J. 1995;6:22-25.
8. Nygaard IE, Kreder KJ. Complications of incontinence surgery. Int Urogynecol J. 1994;5:353-360.
9. Lockhart vJL, Tirado A, Morillo G, Politano VA. Vesicourethral dysfunction following cystourethropexy. J Urol. 1982;128:943-945.
10. Leboeuf L, Tellez CA, Ead D, Gousse AE. Complication of bowel perforation during insertion of tension-free vaginal tape. J Urol. 2003;170(4 Pt 1):1310.-
11. Abouassaly R, Steinberg JR, Lemieux M, et al. Complications of tension-free vaginal tape surgery: a multi-institutional review. BJU Int. 2004;94:110-113.
12. Delorme E, Droupy S, de Tayrac R, Delmas V. [Transobturator tape (UraTape). A new minimally invasive method in the treatment of urinary incontinence in women.] Prog Urol. 2003;13:656-659.
13. de Leval J. Novel surgical technique for the treatment of female stress urinary incontinence: transobturator vaginal tape inside-out. Eur Urol. 2003;44:724-730.
14. De Tayrac R, Deffieux X, Droupy S, ChauveaudLambling A, Calvanese-Benamour L, Fernandez H. A prospective randomized trial comparing tensionfree vaginal tape and transobturator suburethral tape for surgical treatment of stress urinary incontinence. Am J Obstet Gynecol. 2004;190:602-608.
15. Richardson AC. The rectovaginal septum revisited: its relationship to rectocele and its importance in rectocele repair. Clin Obstet Gynecol. 1993;36:976-983.
16. Miklos JR, Kohli N, Lucente V, Saye WB. Site-specific fascial defects in the diagnosis and surgical management of enterocele. Am J Obstet Gynecol. 1998;179 (6 Pt 1):1418-1422; discussion 1422-823.
17. Porter WE, Steele A, Walsh P, Kohli N, Karram MM. The anatomic and functional outcomes of defectspecific rectocele repairs. Am J Obstet Gynecol. 1999;181:1353-1358; discussion 1358-1359.
18. Kenton K, Shott S, Brubaker L. Outcome after rectovaginal fascia reattachment for rectocele repair. Am J Obstet Gynecol. 1999;181:1360-1363; discussion 1363-1364.
19. Flood CG, Drutz HP, Waja L. Anterior colporrhaphy reinforced with Marlex mesh for the treatment of cystoceles. Int Urogynecol J Pelvic Floor Dysfunct. 1998;9:200-204.
20. Koduri S, Lobel RW, Winkler HA, Tomezsko J, Culligan PJ, Sand PK. Prospective randomized trial of polyglactin 910 mesh to prevent recurrence of cystoceles and rectoceles. Proceedings of the 25th Annual Meeting of the International Urogynecological Association, 2000. Int Urogynecol J Pelvic Floor Dysfunct. 2000;11:S80.-
21. Clemons JL, Myers DL, Aguilar VC, Arya LA. Vaginal paravaginal repair with an AlloDerm graft. Am J Obstet Gynecol. 2003;189:1612-1618; discussion 1618-1619.
22. Fox SD, Stanton SL. Vault prolapse and rectocele: assessment of repair using sacrocolpopexy with mesh interposition. BJOG. 2000;107:1371-1375.
23. Kohli N, Miklos JR. Site specific rectocele repair augmented with a cadaveric dermal graft. Proceedings of the 21st Annual Scientific Meeting of the American Urogynecologic Society, 2000. Int Urogynecol J Pelvic Floor Dysfunct. 2001;12:S9.-
24. Miklos JR, Kohli N. Rectovaginal fistula repair utilizing a cadaveric dermal allograft. Int Urogynecol J Pelvic Floor Dysfunct. 1999;10:405-406.
After several decades of slow progress, the field of urogynecology is experiencing dynamic change, including:
- new minimally invasive, tension-free, midurethral sling procedures, especially the transobturator approach,
- correction of size-specific defects to repair prolapse, and
- use of mesh/graft augmentation in pro lapse repair.
These developments are some of the most important since Kelly and Dunn first described suburethral fascial plication for stress incontinence and cystocele in 1914.1 They have come about through increased understanding of the pathophysiology of incontinence and prolapse, innovative technology and techniques, and improved communication and coordination among physicians worldwide.
New sling procedures, promising outcomes
The minimally invasive midurethral sling procedure spawned notable new approaches and is a mainstay of surgical treatment for stress urinary incontinence.
First described in Sweden by Ulmsten in 1995,2 the tension-free vaginal tape procedure is a revolutionary change in the suburethral sling procedure and is now the most widely performed surgery for stress incontinence worldwide.
In it, a tension-free vaginal tape (TVT) (Gynecare, Somerville, NJ) of synthetic polypropylene mesh is attached to 2 needles and passed through a vaginal incision and the retropubic space, exiting to small incisions in the suprapubic region to create a suburethral sling or hammock and provide urethral support during increased abdominal pressure. The sling remains fixed by friction and subsequent adhesions.
Although the traditional suburethral sling was less invasive than other abdominal incontinence procedures, it was associated with a steep learning curve and a high incidence of postoperative irritative bladder symptoms and voiding dysfunction.3
Tension-free vaginal tape: Excellent long-term cure
It can be performed routinely in under 30 minutes using local anesthesia, with minimal postoperative complications. Five-year cure rates approach 95%,4 and data presented at the 2003 International Urogynecology Association clinical meeting describe an objective 7-year cure rate of 82%.5
The incidence and severity of postoperative voiding dysfunction following the TVT procedure is significantly lower than that reported after traditional suburethral sling (2%–40%) or transvaginal needle suspension (2%–50%) procedures.6-9 Although bladder perforation has occurred in up to 10% of patients, reports of complications, including major hemorrhage, tape erosion, and bowel and nerve injury, are rare.10,11
Modifications
These products have a modified approach, materials, or refinements in technique to address various needs. For example, American Medical Systems (Minnetonka, Minn) introduced the SPARC procedure, which allows abdominal placement of the midurethral sling, similar to a needle suspension technique.
CR Bard (Murray Hill, NJ) introduced a midurethral sling of porcine dermis (Pelvicol) to address concerns physicians may have about using synthetic materials.
Minimally invasive midurethral slings include the Advantage (Boston Scientific, Natick, Mass), Centrasorb (Caldera Medical, Thousand Oaks, Calif), Stratasis TF (Cook, West Lafayette, Ind), and Uretex (CR Bard).
Newest approach: Transobturator sling
In this technique, the sling is placed in the midurethral position, but the insertion points are in the genital area lateral to the vagina, and the needle passes through the obturator membrane and paraurethral space. Because it avoids passage of the needles through the retropubic space, the transobturator approach theoretically should reduce the risk of bowel, bladder, and major blood vessel injury.
The procedure was initially described in Europe and introduced in the United States in 2003. Current product offerings include the outside-in approach of ObTape (Mentor, Santa Barbara, Calif), Monarc (American Medical Systems), and Uretex (CR Bard). Gynecare offers a variation of its TVT—the TVT-Obturator—which involves an inside-out approach to further minimize risk of vascular injury.
Shortage of long-term data, but good early results
In a 1-year follow-up of patients undergoing a sling procedure with the UraTape transobturator sling (Mentor), Delorme and colleagues12 reported that 29 of 32 patients (90.6%) were cured and 3 (9.4%) improved. De Leval13 described his inside-out approach with equally good results: no bladder or urethral injuries and no vascular (hematoma or bleeding) or neurological complications. A transobturator technique using porcine dermis has also been described.
Tension-free vaginal tape versus transobturator sling
In 2004, a small randomized, prospective trial of TVT (n = 29) versus transobturator tape (n = 27) with 1-year follow-up found the transobturator approach to be safer and easier to place with equivalent short-term results.14 Mean operative time was significantly shorter in the transobturator group (15±4 minutes versus 27±8 minutes, P <.001).
No bladder injury occurred in the transobturator group versus 9.7% (n = 3) in the TVT group (P >.05). The rate of postoperative urinary retention was 25.8% (n = 8) in the TVT group versus 13.3% (n = 4) in the transobturator group (P >.05). Cure rates were similar for the TVT and transobturator groups: 83.9% versus 90%, respectively), improvement (9.7% versus 3.3%), and failure (6.5% versus 6.7%). No vaginal erosion occurred in either group.
Additional investigations of the transobturator tape procedure are underway.
Correcting site-specific defects in prolapse repair
This repair rationale should become the standard, although it has yet to be widely adopted and procedural refinement and research are continuing.
As early as 1908, site-specific defects in the endopelvic fascia were identified as the likely cause of anterior vaginal segment prolapse. Like hernia repair, which requires closure of the fascial defect, the “cystocele hernia” repair advocated by George White involved reattaching the endopelvic fascia to the arcus tendineus fascia pelvis using a series of interrupted sutures through an abdominal retropubic approach.
Although this view was later abandoned, it resurfaced in the 1980s, when Cullen Richardson described midline, lateral, and transverse defects (FIGURE 1) in the endopelvic fascia as the cause of cystocele and rectocele.15 Richardson advocated diagnosis that identified these fascial defects, along with treatment with site-specific repair.
(In the intervening decades, prolapse was thought to result from generalized weakening or attenuation of the endopelvic fascia that supports the bladder, rectum, or vagina, leading to cystocele, rectocele, or uterine prolapse, respectively. Traditional repairs, still widely performed by most gynecologists, consist of the anterior and posterior colporrhaphy, which involve midline plication of the endopelvic fascia to reduce the prolapse and recreate support by strengthening the weakened fascial layer.)
FIGURE 1 Identify the site of defect
Whether the defect that causes a rectocele or cystocele is transverse, central, or lateral determines the type of repair to be performed. Reprinted with permission from Richardson AC. The rectovaginal septum revisited: its relationship to rectocele and its importance in rectocele repair. Clin Obstet Gynecol.1993;36(4):976–983.
Excellent cure rates with fewer complications
Site-specific repair (FIGURE 2) has been adopted by many pelvic surgeons, who report excellent cure rates with fewer complications such as dyspareunia, vaginal narrowing, and increased blood loss—all of which are more common with traditional anterior and posterior colporrhaphy.
Traditional colporrhaphy does correct the underlying fascial defect when the “hernia” is in the midline. However, for lateral and transverse defects, traditional colporrhaphy leaves the defect uncorrected and may even create additional tension, resulting in recurrence.
Site-specific repair also is useful in the treatment of enterocele, which has been described as a herniation of the peritoneum through a defect of the anterior and posterior fascial planes at the vaginal apex.16
Widening but not broad acceptance
Although cure rates for site-specific prolapse repair range from 75% to 85%,17,18 the concept has yet to be widely adopted and continues to undergo procedural refinement and research, as well as physician education. With increasing experience and clinical data, it may become the standard for pelvic prolapse repair.
FIGURE 2 Repair the defect to correct the rectocele
Surgical technique for a transverse tear in the rectovaginal fascia: After identifying the defect, place a series of interrupted sutures to reapproximate the fascial edges (copyright Miklos/Kohli).
Mesh augmentation: Useful in selected patients
Although augmentation with mesh or biomaterials is easy to perform, it remains unclear which technique and materials are optimal.
The use of mesh and biomaterials to augment repair of a cystocele, rectocele, or enterocele is slowly increasing, although general surgeons have been utilizing these materials for many years in hernia repair.
Augmentation has been advocated for “pelvic hernias” because of poor long-term cure rates for traditional prolapse surgeries (which range from 40% to 80%—well behind rates of 90% or more for incontinence procedures).
A recent informal survey of the members of the American Urogynecologic Society and International Urogynecologic Association revealed that most pelvic surgeons are using some type of graft or mesh to augment repairs in selected patients.
Synthetic mesh versus autologous and heterologous grafts
Synthetic materials have the advantage of being readily available, cost-effective, and consistent in quality, but they may cause significant complications, including infection, stricture, and erosion. This is especially important in the vagina, which needs to stretch during intercourse and which alters in thickness and other properties during a woman’s lifetime.
In contrast, autologous and heterologous donor grafts provide naturally occurring biomaterials capable of remodeling. Unfortunately, the in vivo tissue response is not yet fully understood. Other disadvantages: Biomaterials may lack consistent tissue properties and can be expensive.
For these reasons, graft materials remain in an early period of evaluation, although their use is expected to rise steadily with increasing experience and new product development.
Limited data on safety and efficacy
Although many pelvic surgeons use graft/mesh materials in prolapse repair, data on their safety and efficacy are limited, partly due to the variety of surgical techniques and materials available. Another factor is the difficulty of obtaining good long-term data with large patient numbers. Most of the literature is comprised of case reports, with few prospective, randomized trials.
That said, initial data suggest a significant improvement in cure rates, compared with traditional techniques, with minimal short-term complications. Long-term results and complication rates are not yet available.
What existing studies show
A variety of synthetic materials have been used in surgical correction of cystocele. In the largest series to date, Flood and colleagues19 reported their 12-year experience with 142 women undergoing a modified anterior colporrhaphy reinforced with Marlex (Davol, Cranston, RI) mesh: 100% success in correcting cystocele and a 74% success rate for urinary stress incontinence, with a mean follow-up of 3.2 years and no significant intraoperative complications.
In a prospective randomized trial of 125 patients utilizing absorbable polyglactin 910 mesh (Vicryl) (Ethicon, Somerville, NJ) to augment standard anterior colporrhaphy, Koduri et al20 reported a failure rate of 13% in the colporrhaphy-alone group compared to 1% in the colporrhaphy-mesh group at the 1-year follow-up. Subjectively, both groups improved equally.
Clemons et al21 used a human dermis graft to treat advanced recurrent cystocele in 33 women, with a follow-up of 18 months. They noted 13 (41%) objective failures and 1 (3%) subjective failure. Complications included 1 case of febrile morbidity, 1 cystotomy, and 1 anterior wall breakdown secondary to hematoma formation caused by heparin therapy. No other erosions or rejections were seen.
Most effective applications
Graft/mesh augmentation may be most effective in posterior vaginal segment reconstruction and rectocele repair, as it obviates the need for levatorplasty in patients with poor rectovaginal fascia. A variety of synthetic materials have been used for posterior wall reconstruction in small series.
Mersilene mesh
Mersilene mesh (Ethicon) was used by Fox and Stanton22 to augment traditional rectocele repair in 29 women followed for 14 months. Most of the women had undergone previous rectocele repair with recurrence of their prolapse. All women with stage II and stage III vault prolapse were corrected, with an increase in stage I prolapse from 20% to 27%. All women with stage II and stage III rectocele were corrected, with a decrease in stage I prolapse from 36% to 7%. The only significant intraoperative complication was a cystotomy. One mesh became infected postoperatively, requiring removal.
Cadaveric dermal graft
Recently, Kohli and Miklos23 reported their experience with 57 patients undergoing augmented rectocele repair using a cadaveric dermal graft (FIGURE 3) over a 2-year period. Average follow-up was 11 months. Average patient age in the follow-up group was 63.6±10.9 years (range: 33–79 years) and average parity was 2.8±1.5 (range: 0–7). No major intraoperative complications (hollow viscous injury, blood loss greater than 500 cc, or transfusion) or post-operative complications (infection, abscess, or hematoma) were noted. No graft-related complications such as rejection, erosion, infection, and fistula formation were noted during the follow-up period.
Using previously accepted Pelvic Organ Prolapse Quantification (POP-Q) parameters for success, Kohli and Miklos found 54 of 57 women (95%) to have surgical cure at follow-up. These authors have also described the use of a dermal graft in the repair of a complicated rectovaginal fistula.24
FIGURE 3 Placement of mesh augmentation
Augment enterocele/rectocele repair by attaching the mesh apically, laterally, and distally (copyright Miklos/Kohli).
Future outlook
Further data on the efficacy and safety of graft/mesh augmentation—including identification of the optimal technique and ideal material—are necessary before this approach can be widely adopted. However, in selected patients, it represents a significant advance in the surgical treatment of pelvic prolapse.
The author is a preceptor for American Medical Systems, Gynecare, CR Bard, and Mentor and a consultant for Boston Scientific.
After several decades of slow progress, the field of urogynecology is experiencing dynamic change, including:
- new minimally invasive, tension-free, midurethral sling procedures, especially the transobturator approach,
- correction of size-specific defects to repair prolapse, and
- use of mesh/graft augmentation in pro lapse repair.
These developments are some of the most important since Kelly and Dunn first described suburethral fascial plication for stress incontinence and cystocele in 1914.1 They have come about through increased understanding of the pathophysiology of incontinence and prolapse, innovative technology and techniques, and improved communication and coordination among physicians worldwide.
New sling procedures, promising outcomes
The minimally invasive midurethral sling procedure spawned notable new approaches and is a mainstay of surgical treatment for stress urinary incontinence.
First described in Sweden by Ulmsten in 1995,2 the tension-free vaginal tape procedure is a revolutionary change in the suburethral sling procedure and is now the most widely performed surgery for stress incontinence worldwide.
In it, a tension-free vaginal tape (TVT) (Gynecare, Somerville, NJ) of synthetic polypropylene mesh is attached to 2 needles and passed through a vaginal incision and the retropubic space, exiting to small incisions in the suprapubic region to create a suburethral sling or hammock and provide urethral support during increased abdominal pressure. The sling remains fixed by friction and subsequent adhesions.
Although the traditional suburethral sling was less invasive than other abdominal incontinence procedures, it was associated with a steep learning curve and a high incidence of postoperative irritative bladder symptoms and voiding dysfunction.3
Tension-free vaginal tape: Excellent long-term cure
It can be performed routinely in under 30 minutes using local anesthesia, with minimal postoperative complications. Five-year cure rates approach 95%,4 and data presented at the 2003 International Urogynecology Association clinical meeting describe an objective 7-year cure rate of 82%.5
The incidence and severity of postoperative voiding dysfunction following the TVT procedure is significantly lower than that reported after traditional suburethral sling (2%–40%) or transvaginal needle suspension (2%–50%) procedures.6-9 Although bladder perforation has occurred in up to 10% of patients, reports of complications, including major hemorrhage, tape erosion, and bowel and nerve injury, are rare.10,11
Modifications
These products have a modified approach, materials, or refinements in technique to address various needs. For example, American Medical Systems (Minnetonka, Minn) introduced the SPARC procedure, which allows abdominal placement of the midurethral sling, similar to a needle suspension technique.
CR Bard (Murray Hill, NJ) introduced a midurethral sling of porcine dermis (Pelvicol) to address concerns physicians may have about using synthetic materials.
Minimally invasive midurethral slings include the Advantage (Boston Scientific, Natick, Mass), Centrasorb (Caldera Medical, Thousand Oaks, Calif), Stratasis TF (Cook, West Lafayette, Ind), and Uretex (CR Bard).
Newest approach: Transobturator sling
In this technique, the sling is placed in the midurethral position, but the insertion points are in the genital area lateral to the vagina, and the needle passes through the obturator membrane and paraurethral space. Because it avoids passage of the needles through the retropubic space, the transobturator approach theoretically should reduce the risk of bowel, bladder, and major blood vessel injury.
The procedure was initially described in Europe and introduced in the United States in 2003. Current product offerings include the outside-in approach of ObTape (Mentor, Santa Barbara, Calif), Monarc (American Medical Systems), and Uretex (CR Bard). Gynecare offers a variation of its TVT—the TVT-Obturator—which involves an inside-out approach to further minimize risk of vascular injury.
Shortage of long-term data, but good early results
In a 1-year follow-up of patients undergoing a sling procedure with the UraTape transobturator sling (Mentor), Delorme and colleagues12 reported that 29 of 32 patients (90.6%) were cured and 3 (9.4%) improved. De Leval13 described his inside-out approach with equally good results: no bladder or urethral injuries and no vascular (hematoma or bleeding) or neurological complications. A transobturator technique using porcine dermis has also been described.
Tension-free vaginal tape versus transobturator sling
In 2004, a small randomized, prospective trial of TVT (n = 29) versus transobturator tape (n = 27) with 1-year follow-up found the transobturator approach to be safer and easier to place with equivalent short-term results.14 Mean operative time was significantly shorter in the transobturator group (15±4 minutes versus 27±8 minutes, P <.001).
No bladder injury occurred in the transobturator group versus 9.7% (n = 3) in the TVT group (P >.05). The rate of postoperative urinary retention was 25.8% (n = 8) in the TVT group versus 13.3% (n = 4) in the transobturator group (P >.05). Cure rates were similar for the TVT and transobturator groups: 83.9% versus 90%, respectively), improvement (9.7% versus 3.3%), and failure (6.5% versus 6.7%). No vaginal erosion occurred in either group.
Additional investigations of the transobturator tape procedure are underway.
Correcting site-specific defects in prolapse repair
This repair rationale should become the standard, although it has yet to be widely adopted and procedural refinement and research are continuing.
As early as 1908, site-specific defects in the endopelvic fascia were identified as the likely cause of anterior vaginal segment prolapse. Like hernia repair, which requires closure of the fascial defect, the “cystocele hernia” repair advocated by George White involved reattaching the endopelvic fascia to the arcus tendineus fascia pelvis using a series of interrupted sutures through an abdominal retropubic approach.
Although this view was later abandoned, it resurfaced in the 1980s, when Cullen Richardson described midline, lateral, and transverse defects (FIGURE 1) in the endopelvic fascia as the cause of cystocele and rectocele.15 Richardson advocated diagnosis that identified these fascial defects, along with treatment with site-specific repair.
(In the intervening decades, prolapse was thought to result from generalized weakening or attenuation of the endopelvic fascia that supports the bladder, rectum, or vagina, leading to cystocele, rectocele, or uterine prolapse, respectively. Traditional repairs, still widely performed by most gynecologists, consist of the anterior and posterior colporrhaphy, which involve midline plication of the endopelvic fascia to reduce the prolapse and recreate support by strengthening the weakened fascial layer.)
FIGURE 1 Identify the site of defect
Whether the defect that causes a rectocele or cystocele is transverse, central, or lateral determines the type of repair to be performed. Reprinted with permission from Richardson AC. The rectovaginal septum revisited: its relationship to rectocele and its importance in rectocele repair. Clin Obstet Gynecol.1993;36(4):976–983.
Excellent cure rates with fewer complications
Site-specific repair (FIGURE 2) has been adopted by many pelvic surgeons, who report excellent cure rates with fewer complications such as dyspareunia, vaginal narrowing, and increased blood loss—all of which are more common with traditional anterior and posterior colporrhaphy.
Traditional colporrhaphy does correct the underlying fascial defect when the “hernia” is in the midline. However, for lateral and transverse defects, traditional colporrhaphy leaves the defect uncorrected and may even create additional tension, resulting in recurrence.
Site-specific repair also is useful in the treatment of enterocele, which has been described as a herniation of the peritoneum through a defect of the anterior and posterior fascial planes at the vaginal apex.16
Widening but not broad acceptance
Although cure rates for site-specific prolapse repair range from 75% to 85%,17,18 the concept has yet to be widely adopted and continues to undergo procedural refinement and research, as well as physician education. With increasing experience and clinical data, it may become the standard for pelvic prolapse repair.
FIGURE 2 Repair the defect to correct the rectocele
Surgical technique for a transverse tear in the rectovaginal fascia: After identifying the defect, place a series of interrupted sutures to reapproximate the fascial edges (copyright Miklos/Kohli).
Mesh augmentation: Useful in selected patients
Although augmentation with mesh or biomaterials is easy to perform, it remains unclear which technique and materials are optimal.
The use of mesh and biomaterials to augment repair of a cystocele, rectocele, or enterocele is slowly increasing, although general surgeons have been utilizing these materials for many years in hernia repair.
Augmentation has been advocated for “pelvic hernias” because of poor long-term cure rates for traditional prolapse surgeries (which range from 40% to 80%—well behind rates of 90% or more for incontinence procedures).
A recent informal survey of the members of the American Urogynecologic Society and International Urogynecologic Association revealed that most pelvic surgeons are using some type of graft or mesh to augment repairs in selected patients.
Synthetic mesh versus autologous and heterologous grafts
Synthetic materials have the advantage of being readily available, cost-effective, and consistent in quality, but they may cause significant complications, including infection, stricture, and erosion. This is especially important in the vagina, which needs to stretch during intercourse and which alters in thickness and other properties during a woman’s lifetime.
In contrast, autologous and heterologous donor grafts provide naturally occurring biomaterials capable of remodeling. Unfortunately, the in vivo tissue response is not yet fully understood. Other disadvantages: Biomaterials may lack consistent tissue properties and can be expensive.
For these reasons, graft materials remain in an early period of evaluation, although their use is expected to rise steadily with increasing experience and new product development.
Limited data on safety and efficacy
Although many pelvic surgeons use graft/mesh materials in prolapse repair, data on their safety and efficacy are limited, partly due to the variety of surgical techniques and materials available. Another factor is the difficulty of obtaining good long-term data with large patient numbers. Most of the literature is comprised of case reports, with few prospective, randomized trials.
That said, initial data suggest a significant improvement in cure rates, compared with traditional techniques, with minimal short-term complications. Long-term results and complication rates are not yet available.
What existing studies show
A variety of synthetic materials have been used in surgical correction of cystocele. In the largest series to date, Flood and colleagues19 reported their 12-year experience with 142 women undergoing a modified anterior colporrhaphy reinforced with Marlex (Davol, Cranston, RI) mesh: 100% success in correcting cystocele and a 74% success rate for urinary stress incontinence, with a mean follow-up of 3.2 years and no significant intraoperative complications.
In a prospective randomized trial of 125 patients utilizing absorbable polyglactin 910 mesh (Vicryl) (Ethicon, Somerville, NJ) to augment standard anterior colporrhaphy, Koduri et al20 reported a failure rate of 13% in the colporrhaphy-alone group compared to 1% in the colporrhaphy-mesh group at the 1-year follow-up. Subjectively, both groups improved equally.
Clemons et al21 used a human dermis graft to treat advanced recurrent cystocele in 33 women, with a follow-up of 18 months. They noted 13 (41%) objective failures and 1 (3%) subjective failure. Complications included 1 case of febrile morbidity, 1 cystotomy, and 1 anterior wall breakdown secondary to hematoma formation caused by heparin therapy. No other erosions or rejections were seen.
Most effective applications
Graft/mesh augmentation may be most effective in posterior vaginal segment reconstruction and rectocele repair, as it obviates the need for levatorplasty in patients with poor rectovaginal fascia. A variety of synthetic materials have been used for posterior wall reconstruction in small series.
Mersilene mesh
Mersilene mesh (Ethicon) was used by Fox and Stanton22 to augment traditional rectocele repair in 29 women followed for 14 months. Most of the women had undergone previous rectocele repair with recurrence of their prolapse. All women with stage II and stage III vault prolapse were corrected, with an increase in stage I prolapse from 20% to 27%. All women with stage II and stage III rectocele were corrected, with a decrease in stage I prolapse from 36% to 7%. The only significant intraoperative complication was a cystotomy. One mesh became infected postoperatively, requiring removal.
Cadaveric dermal graft
Recently, Kohli and Miklos23 reported their experience with 57 patients undergoing augmented rectocele repair using a cadaveric dermal graft (FIGURE 3) over a 2-year period. Average follow-up was 11 months. Average patient age in the follow-up group was 63.6±10.9 years (range: 33–79 years) and average parity was 2.8±1.5 (range: 0–7). No major intraoperative complications (hollow viscous injury, blood loss greater than 500 cc, or transfusion) or post-operative complications (infection, abscess, or hematoma) were noted. No graft-related complications such as rejection, erosion, infection, and fistula formation were noted during the follow-up period.
Using previously accepted Pelvic Organ Prolapse Quantification (POP-Q) parameters for success, Kohli and Miklos found 54 of 57 women (95%) to have surgical cure at follow-up. These authors have also described the use of a dermal graft in the repair of a complicated rectovaginal fistula.24
FIGURE 3 Placement of mesh augmentation
Augment enterocele/rectocele repair by attaching the mesh apically, laterally, and distally (copyright Miklos/Kohli).
Future outlook
Further data on the efficacy and safety of graft/mesh augmentation—including identification of the optimal technique and ideal material—are necessary before this approach can be widely adopted. However, in selected patients, it represents a significant advance in the surgical treatment of pelvic prolapse.
The author is a preceptor for American Medical Systems, Gynecare, CR Bard, and Mentor and a consultant for Boston Scientific.
1. Kelly HA, Dunn WM. Urinary incontinence in women without manifest injury to the bladder. Surg Gynecol Obstet. 1914;18:444.-
2. Ulmsten U, Petros P. Intravaginal slingplasty (IVS): an ambulatory surgical procedure for treatment of female urinary incontinence. Scand J Urol Nephrol. 1995;29:75-82.
3. Weinberger MW, Ostergard DR. Postoperative catheterization, urinary retention, and permanent voiding dysfunction after polytetrafluroethylene suburethral sling placement. Obstet Gynecol. 1996;87:50-54.
4. Nilsson CG, Kuuva N, Falconer C, Rezapour M, Ulmsten U. Long-term results of the tension-free vaginal tape (TVT) procedure for surgical treatment of female stress urinary incontinence. Int Urogynecol J Pelvic Floor Dysfunct. 2001;12(Suppl 2):S5-8.
5. Nilsson CG, Rezapour M, Falconer C. Seven years follow-up of the tension-free vaginal tape (TVT) procedure. Presented at the 2003 International Urogynecology Association Annual Meeting.
6. Horbach N. Suburethral sling procedures. In: Ostergard DR, Bent AE, eds. Urogynecology and Urodynamics: Theory and Practice. 3rd ed. Baltimore: Williams & Wilkins; 1991;449-458.
7. Stanton SL, Reynolds SF, Creighton SM. The modified Pereyra (Raz) procedure for genuine stress incontinence—a useful option in the elderly or frail patient? Int Urogynecol J. 1995;6:22-25.
8. Nygaard IE, Kreder KJ. Complications of incontinence surgery. Int Urogynecol J. 1994;5:353-360.
9. Lockhart vJL, Tirado A, Morillo G, Politano VA. Vesicourethral dysfunction following cystourethropexy. J Urol. 1982;128:943-945.
10. Leboeuf L, Tellez CA, Ead D, Gousse AE. Complication of bowel perforation during insertion of tension-free vaginal tape. J Urol. 2003;170(4 Pt 1):1310.-
11. Abouassaly R, Steinberg JR, Lemieux M, et al. Complications of tension-free vaginal tape surgery: a multi-institutional review. BJU Int. 2004;94:110-113.
12. Delorme E, Droupy S, de Tayrac R, Delmas V. [Transobturator tape (UraTape). A new minimally invasive method in the treatment of urinary incontinence in women.] Prog Urol. 2003;13:656-659.
13. de Leval J. Novel surgical technique for the treatment of female stress urinary incontinence: transobturator vaginal tape inside-out. Eur Urol. 2003;44:724-730.
14. De Tayrac R, Deffieux X, Droupy S, ChauveaudLambling A, Calvanese-Benamour L, Fernandez H. A prospective randomized trial comparing tensionfree vaginal tape and transobturator suburethral tape for surgical treatment of stress urinary incontinence. Am J Obstet Gynecol. 2004;190:602-608.
15. Richardson AC. The rectovaginal septum revisited: its relationship to rectocele and its importance in rectocele repair. Clin Obstet Gynecol. 1993;36:976-983.
16. Miklos JR, Kohli N, Lucente V, Saye WB. Site-specific fascial defects in the diagnosis and surgical management of enterocele. Am J Obstet Gynecol. 1998;179 (6 Pt 1):1418-1422; discussion 1422-823.
17. Porter WE, Steele A, Walsh P, Kohli N, Karram MM. The anatomic and functional outcomes of defectspecific rectocele repairs. Am J Obstet Gynecol. 1999;181:1353-1358; discussion 1358-1359.
18. Kenton K, Shott S, Brubaker L. Outcome after rectovaginal fascia reattachment for rectocele repair. Am J Obstet Gynecol. 1999;181:1360-1363; discussion 1363-1364.
19. Flood CG, Drutz HP, Waja L. Anterior colporrhaphy reinforced with Marlex mesh for the treatment of cystoceles. Int Urogynecol J Pelvic Floor Dysfunct. 1998;9:200-204.
20. Koduri S, Lobel RW, Winkler HA, Tomezsko J, Culligan PJ, Sand PK. Prospective randomized trial of polyglactin 910 mesh to prevent recurrence of cystoceles and rectoceles. Proceedings of the 25th Annual Meeting of the International Urogynecological Association, 2000. Int Urogynecol J Pelvic Floor Dysfunct. 2000;11:S80.-
21. Clemons JL, Myers DL, Aguilar VC, Arya LA. Vaginal paravaginal repair with an AlloDerm graft. Am J Obstet Gynecol. 2003;189:1612-1618; discussion 1618-1619.
22. Fox SD, Stanton SL. Vault prolapse and rectocele: assessment of repair using sacrocolpopexy with mesh interposition. BJOG. 2000;107:1371-1375.
23. Kohli N, Miklos JR. Site specific rectocele repair augmented with a cadaveric dermal graft. Proceedings of the 21st Annual Scientific Meeting of the American Urogynecologic Society, 2000. Int Urogynecol J Pelvic Floor Dysfunct. 2001;12:S9.-
24. Miklos JR, Kohli N. Rectovaginal fistula repair utilizing a cadaveric dermal allograft. Int Urogynecol J Pelvic Floor Dysfunct. 1999;10:405-406.
1. Kelly HA, Dunn WM. Urinary incontinence in women without manifest injury to the bladder. Surg Gynecol Obstet. 1914;18:444.-
2. Ulmsten U, Petros P. Intravaginal slingplasty (IVS): an ambulatory surgical procedure for treatment of female urinary incontinence. Scand J Urol Nephrol. 1995;29:75-82.
3. Weinberger MW, Ostergard DR. Postoperative catheterization, urinary retention, and permanent voiding dysfunction after polytetrafluroethylene suburethral sling placement. Obstet Gynecol. 1996;87:50-54.
4. Nilsson CG, Kuuva N, Falconer C, Rezapour M, Ulmsten U. Long-term results of the tension-free vaginal tape (TVT) procedure for surgical treatment of female stress urinary incontinence. Int Urogynecol J Pelvic Floor Dysfunct. 2001;12(Suppl 2):S5-8.
5. Nilsson CG, Rezapour M, Falconer C. Seven years follow-up of the tension-free vaginal tape (TVT) procedure. Presented at the 2003 International Urogynecology Association Annual Meeting.
6. Horbach N. Suburethral sling procedures. In: Ostergard DR, Bent AE, eds. Urogynecology and Urodynamics: Theory and Practice. 3rd ed. Baltimore: Williams & Wilkins; 1991;449-458.
7. Stanton SL, Reynolds SF, Creighton SM. The modified Pereyra (Raz) procedure for genuine stress incontinence—a useful option in the elderly or frail patient? Int Urogynecol J. 1995;6:22-25.
8. Nygaard IE, Kreder KJ. Complications of incontinence surgery. Int Urogynecol J. 1994;5:353-360.
9. Lockhart vJL, Tirado A, Morillo G, Politano VA. Vesicourethral dysfunction following cystourethropexy. J Urol. 1982;128:943-945.
10. Leboeuf L, Tellez CA, Ead D, Gousse AE. Complication of bowel perforation during insertion of tension-free vaginal tape. J Urol. 2003;170(4 Pt 1):1310.-
11. Abouassaly R, Steinberg JR, Lemieux M, et al. Complications of tension-free vaginal tape surgery: a multi-institutional review. BJU Int. 2004;94:110-113.
12. Delorme E, Droupy S, de Tayrac R, Delmas V. [Transobturator tape (UraTape). A new minimally invasive method in the treatment of urinary incontinence in women.] Prog Urol. 2003;13:656-659.
13. de Leval J. Novel surgical technique for the treatment of female stress urinary incontinence: transobturator vaginal tape inside-out. Eur Urol. 2003;44:724-730.
14. De Tayrac R, Deffieux X, Droupy S, ChauveaudLambling A, Calvanese-Benamour L, Fernandez H. A prospective randomized trial comparing tensionfree vaginal tape and transobturator suburethral tape for surgical treatment of stress urinary incontinence. Am J Obstet Gynecol. 2004;190:602-608.
15. Richardson AC. The rectovaginal septum revisited: its relationship to rectocele and its importance in rectocele repair. Clin Obstet Gynecol. 1993;36:976-983.
16. Miklos JR, Kohli N, Lucente V, Saye WB. Site-specific fascial defects in the diagnosis and surgical management of enterocele. Am J Obstet Gynecol. 1998;179 (6 Pt 1):1418-1422; discussion 1422-823.
17. Porter WE, Steele A, Walsh P, Kohli N, Karram MM. The anatomic and functional outcomes of defectspecific rectocele repairs. Am J Obstet Gynecol. 1999;181:1353-1358; discussion 1358-1359.
18. Kenton K, Shott S, Brubaker L. Outcome after rectovaginal fascia reattachment for rectocele repair. Am J Obstet Gynecol. 1999;181:1360-1363; discussion 1363-1364.
19. Flood CG, Drutz HP, Waja L. Anterior colporrhaphy reinforced with Marlex mesh for the treatment of cystoceles. Int Urogynecol J Pelvic Floor Dysfunct. 1998;9:200-204.
20. Koduri S, Lobel RW, Winkler HA, Tomezsko J, Culligan PJ, Sand PK. Prospective randomized trial of polyglactin 910 mesh to prevent recurrence of cystoceles and rectoceles. Proceedings of the 25th Annual Meeting of the International Urogynecological Association, 2000. Int Urogynecol J Pelvic Floor Dysfunct. 2000;11:S80.-
21. Clemons JL, Myers DL, Aguilar VC, Arya LA. Vaginal paravaginal repair with an AlloDerm graft. Am J Obstet Gynecol. 2003;189:1612-1618; discussion 1618-1619.
22. Fox SD, Stanton SL. Vault prolapse and rectocele: assessment of repair using sacrocolpopexy with mesh interposition. BJOG. 2000;107:1371-1375.
23. Kohli N, Miklos JR. Site specific rectocele repair augmented with a cadaveric dermal graft. Proceedings of the 21st Annual Scientific Meeting of the American Urogynecologic Society, 2000. Int Urogynecol J Pelvic Floor Dysfunct. 2001;12:S9.-
24. Miklos JR, Kohli N. Rectovaginal fistula repair utilizing a cadaveric dermal allograft. Int Urogynecol J Pelvic Floor Dysfunct. 1999;10:405-406.