User login
Tradition is yielding to new technology’s advantages, time-tested though they are not—yet
Even as we scramble to gather definitive evidence on the immediate and long-term benefits of new technologies, they are supplanting tradition in the surgical treatment of incontinence and prolapse. Surgeons have been swift to adopt synthetic mesh and the new generation of needle suspension procedures, which offer the double advantage of a shorter operative time and shorter postoperative recovery. Yet, we lack well-designed randomized prospective clinical studies on whether outcomes and complication rates are better than traditional therapies such as vaginal colporrhaphy and paravaginal repair.
There hasn’t been time.
These innovations came onto the market in rapid succession, accompanied by aggressive corporate promotion, physician interest, and, in turn, pressure from patients. Improved reimbursement for quicker, easier procedures also entices many physicians to become “early adopters.” (Recent addition of the CPT code for mesh/graft use in prolapse surgery [CPT 57267], increases reimbursement over traditional procedures.)
It is important to keep a cautious but open mind. Given the blind needle techniques and use of biomaterial grafts and synthetic meshes, these procedures may not be for every surgeon or every patient. As always, astute clinical judgment and critical analysis of the data and anecdotal experience are recommended.
Transobturator sling
The needle-guided synthetic mesh midurethral sling was rapidly adopted as the treatment of choice for stress urinary incontinence due to urethral hypermobility and intrinsic sphincter deficiency, soon after it was described in 1995.1
With the transvaginal tape (TVT) procedure, the learning curve was shorter and so were hospital stays and recovery, compared with abdominal Burch colposuspension and traditional bladder neck slings. Furthermore, cost efficiency improved,2 and the persistent cure rate was 85% from 2 to 8 years.3
However, needle passage through the retropubic space can cause vascular, bowel, or bladder injury, even in the hands of experienced surgeons. An August 2005 French survey4 of 92 surgeons who performed 12,280 TVT procedures reported these complications: perioperative bladder injuries, 901 (7.34%); cases of complete postoperative urinary retention requiring catheterization, 809 (6.59%); vaginal mesh exposure, 26 (0.21%); retropubic or vulvovaginal hematoma, 39 (0.32%); and major organ injuries, 10 (0.08%).
The transobturator (TOT) approach, introduced in 2003,5 is simpler, with fewer complications. The sling is placed in a similar manner in the midurethral position, but the insertion points overlie the obturator space in the genitofemoral crease lateral to the vagina. A needle passing through the obturator membrane exits the vaginal incision without entering the retropubic space, theoretically averting risk of bowel, bladder, and major blood vessel injury.
Although the TOT is thought to be safer in this regard, complications including urinary retention, obturator hematoma and nerve injury, and urethral injury/erosion have been reported.6
A variety of TOT sling kits are available, none with proven superiority.
In a recent randomized, prospective trial in which 61 women had TVT or TOT, there were no bladder injuries in the TOT group, and 9.7% (n=3) in the TVT group (P>.05). The postoperative urinary retention rate was 25.8% (n=8) in the TVT group and 13.3% (n=4) in the TOT group (P>.05). Cure rates (83.9% vs 90%), improvement (9.7% vs 3.3%), and failure (6.5% vs 6.7%) were similar.7
The transobturator suburethral sling is encouraging, although it is unclear whether it is effective in patients with intrinsic sphincter deficiency, especially with a fixed or lead-pipe urethra. We need studies to determine how to match the right procedure to the right patient.
Which sling for which patients?
My indications for TOT vs. TVT, which are based on personal experience and available data, may change as data accumulate (TABLE). Indications are often surgeon-specific, depending on clinical experience.
In our review of 210 TOT slings over a 16-month period at 2 centers, we found a cure rate of 88% and an improvement rate of 1.9%. The complication rate was 24%; intraoperative and postoperative complications were all minor and mostly self-limited8: 1 cystotomy, 1 urethral injury, 2 hematomas, 1 erosion, 16 complaints of transient groin pain, 5 cases of urinary retention requiring reoperation, and 23 cases of de novo urge incontinence.
TABLE
Transvaginal vs transobturator sling
| INDICATIONS | ADVANTAGES |
|---|---|
| Transvaginal (retropubic) | |
| Physically active patient | Avoids groin discomfort with activity |
| Thin, young patient | Long term data available |
| Limited urethral hypermobility/internal sphincter dysfunction | Data supports use/dynamic backboard |
| Transobturator | |
| Elderly patient | Less postop voiding dysfunction |
| Significant overactive bladder/urge incontinence | Less urethral obstruction |
| Previous retropubic surgery | Less risk of retropubic complication |
| Obesity | Less risk of needle-passage complication |
| Inexperience with TVT | Less risk of periop complications |
Adjustable suburethral sling
One of the challenges in placing a suburethral sling is adjustment for efficacy without overcorrection and resultant bladder neck obstruction, urinary retention, or persistent and refractory overactive bladder symptoms. An adjustable transvaginal midurethral synthetic sling procedure was recently introduced in the United States: the Remeex Tensionfree Readjustable Tape, (Neomedic International, Spain). A retropubic minimally invasive midurethral sling is attached to sutures that are taken through a tensioning device placed above the fascia in the suprapubic region. The tensioning device has a small adjustment kit similar to a screwdriver, which is left in place at the time of surgery. The sling is intentionally left loose for postoperative adjustment. Following surgery, a filling cystometrogram confirms stress incontinence. The sling is then progressively tightened until the leaking ceases. This technology is designed to prevent or correct overtightening, and avert bladder outlet obstruction. The sling can be adjusted via a small suprapubic incision, even years later; adjustment has been reported up to 7 years later.
In a recent study of 62 patients with stress urinary incontinence, 58 patients (94%) were completely dry and cured, and 4 patients (6%) reported occasional slight urine leakage. Operative time was 20 to 40 minutes (only stress urinary incontinence and cystocele). Six patients required long-term readjustment (5 to increase tension and 1 to reduce tension). No major intra-operative complications occurred. Late complications included suprapubic wound pain (12 transitional and relieved with analgesics), 3 urinary tract infections, 2 wound seromas, 1 case requiring prosthesis removal due to infection, and 3 cases of hyperactivity de novo, which required anticholinergic treatment.9
Although postoperative urinary retention or postoperative failure is relatively uncommon in transvaginal or transobturator suburethral sling procedures performed by experienced surgeons, the adjustable sling may be especially useful in patients with increased risk of postoperative voiding dysfunction, as well as limited urethral hypermobility/fixed urethra, because the sling can be adjusted long after the operation. Risks include infection due to foreign body (indwelling placement of the tensioning device) as well as palpation and incisional discomfort in very thin patients. Further clinical experience is needed, but the concept of a sling that can be adjusted immediately or even years later is appealing.
Graft/mesh augmentation for prolapse repair
Augmentation of pelvic prolapse repair using mesh and graft materials is used increasingly in an effort to improve long-term outcomes, although we lack randomized prospective data and long-term outcome studies. Synthetic materials offer ready availability, consistent tissue properties, cost effectiveness, and permanent placement, although there are risks: infection, dyspareunia, and erosion or exposure. Success and complications may depend on surgical technique, choice of material, patient selection, postoperative management, or other factors.
The overall success rate was 94% at a mean of 17 months after operation, in a study of 63 women in whom polypropylene mesh was used for augmentation of cystocele and rectocele. However, the authors recommended abandonment of the procedure due to an unacceptably high rate of complications.10 In the 32 women undergoing anterior repair, sexual activity rate did not alter, but dyspareunia increased in 20%. Urge and stress incontinence did not change, but urgency improved in 10%; 13% had vaginal erosion of the mesh. Of the 31 patients undergoing posterior repair, sexual activity decreased by 12% and dyspareunia increased in 63%. Constipation improved in 15% and anal incontinence in 4%; 6.5% had vaginal erosion of mesh and 1 required mesh removal for abscess.
In another study, results were improved and complications were fewer. After 2 years, 24 of 26 women who had posterior repair with polypropylene mesh were cured (92.3%) and 1 had asymptomatic stage 2 rectocele. All but 1 reported improved symptoms and quality of life. No postop infection or rectovaginal fistula was reported; there were 3 vaginal erosions (12%), and 2 patients had de novo dyspareunia (7.7%).11
To make graft/mesh augmentation easier and faster, needle-suspension techniques were recently introduced. Needles are inserted either through the transobturator space (anterior mesh placement) or ischiorectal fascia (posterior placement) and exit through the pelvic sidewall in proximity to the ischial spine. A multi-arm mesh is then attached to the needles, which are withdrawn. Tension secures the mesh and provides “tension-free” anterior or posterior wall support. Colporrhaphy can be performed prior to mesh placement at the surgeon’s discretion.
Because we have few data on patient selection or long-term safety and efficacy (most of it presented at recent meetings12,13 ), these techniques call for caution. Blind needle passage can be associated with complications such as rectal injury and rectovaginal fistula.14 But complication rates may reflect early evolution and may improve with time and experience.
The author receives grant/research support and/or serves as a consultant for American Medical Systems, Boston Scientific, CR Bard, Mentor, Novartis, and Pfizer.
1. Ulmsten U, Petros P. Intravaginal slingplasty (IVS): an ambulatory surgical procedure for treatment of female urinary incontinence. Scand J Urol Nephrol. 1995;29:75-82.
2. Kilonzo M, Vale L, Stearns SC, et al. Cost effectiveness of tension-free vaginal tape for the surgical management of female stress incontinence. Int J Technol Assess Health Care. 2004;20:455-463.
3. Holmgren C, Nilsson S, Lanner L, Hellberg D. Long-term results with tension-free vaginal tape on mixed and stress urinary incontinence. Obstet Gynecol. 2005;106:38-43.
4. Agostini A, Bretelle F, Franchi F, Roger V, Cravello L, Blanc B. Immediate complications of tension-free vaginal tape (TVT): results of a French survey. Eur J Obstet Gynecol Reprod Biol. 2005 Aug 8; [Epub ahead of print].
5. Delorme E, Droupy S, de Tayrac R, Delmas V. Transobturator tape (Uratape). A new minimally invasive method in the treatment of urinary incontinence in women. Prog Urol. 2003;13:656-659.
6. Game X, et al. Obturator infected hematoma and urethral erosion following transobturator tape implantation. J Urol. 2004;171:1629.-
7. deTayrac R, Deffieux X, Droupy S, et al. A prospective randomized trial comparing tension-free vaginal tape and transobturator suburethral tape for surgical treatment of stress urinary incontinence [retracted in: Am J Obstet Gynecol. 2005;192:339]. Am J Obstet Gynecol. 2004;190:602-608.
8. Rajan S, Diwadkar G, Hurwitz S, Kohli N, Roberts L, Moore R, Miklos J. Transobturator (TOT) suburethral sling: early US data on safety and efficacy. Abstract presented at: 2005 Meeting of the International Urogynecology Association; August 9–12, 2005; Copenhagen, Denmark. Poster 52, abstract ID 982.
9. Cabrera Pérez J, Bravo Fernández I, Pérez G, González Enguita C, Vela Navarrete R. Analysis of the suburethral sling TRT (tension free readjustable tape) results in female SUI treatment. Abstract presented at: LXX Congreso Nacional de Urología; June 4–7, 2005; San Sebastián, Spain.
10. Milani R, Salvatore S, Soligo M, Pifarotti P, Meschia M, Cortese M. Functional and anatomical outcome of anterior and posterior vaginal prolapse repair with Prolene mesh. BJOG. 2005;112:107-111.
11. de Tayrac R, Picone O, Chauveaud-Lambling A, Fernandez H. A 2-year anatomical and functional assessment of transvaginal rectocele repair using a polypropylene mesh. Int Urogynecol J Pelvic Floor Dysfunct. 2005 May 21; [Epub ahead of print].
12. Rane AM, Naidu AK, Barry CL, Nyok LY, Corstiaans AC. A novel transobturator system for the repair of anterior vaginal wall prolapse: a pilot study. Abstract presented at: 2005 Meeting of the International Urogynecology Association; August 9–12, 2005; Copenhagen, Denmark.
13. Cosson M, Caquent F, Collinet P, Rosenthal C, Clave H, Debodinance P, Garbin O, Berrocal J, Villet R, Jacquetin B. Prolift mesh for pelvic organ prolapse surgical treatment using the TVM group technique: a retrospective study of 687 patients. Abstract presented at: 2005 Meeting of the International Urogynecology Association; August 9–12, 2005; Copenhagen, Denmark.
14. Hilger WS, Cornella JL. Rectovaginal fistula after posterior intravaginal slingplasty and polypropylene mesh augmented rectocele repair. Int Urogynecol J Pelvic Floor Dysfunct. 2005 Jul 29; [Epub ahead of print].
Even as we scramble to gather definitive evidence on the immediate and long-term benefits of new technologies, they are supplanting tradition in the surgical treatment of incontinence and prolapse. Surgeons have been swift to adopt synthetic mesh and the new generation of needle suspension procedures, which offer the double advantage of a shorter operative time and shorter postoperative recovery. Yet, we lack well-designed randomized prospective clinical studies on whether outcomes and complication rates are better than traditional therapies such as vaginal colporrhaphy and paravaginal repair.
There hasn’t been time.
These innovations came onto the market in rapid succession, accompanied by aggressive corporate promotion, physician interest, and, in turn, pressure from patients. Improved reimbursement for quicker, easier procedures also entices many physicians to become “early adopters.” (Recent addition of the CPT code for mesh/graft use in prolapse surgery [CPT 57267], increases reimbursement over traditional procedures.)
It is important to keep a cautious but open mind. Given the blind needle techniques and use of biomaterial grafts and synthetic meshes, these procedures may not be for every surgeon or every patient. As always, astute clinical judgment and critical analysis of the data and anecdotal experience are recommended.
Transobturator sling
The needle-guided synthetic mesh midurethral sling was rapidly adopted as the treatment of choice for stress urinary incontinence due to urethral hypermobility and intrinsic sphincter deficiency, soon after it was described in 1995.1
With the transvaginal tape (TVT) procedure, the learning curve was shorter and so were hospital stays and recovery, compared with abdominal Burch colposuspension and traditional bladder neck slings. Furthermore, cost efficiency improved,2 and the persistent cure rate was 85% from 2 to 8 years.3
However, needle passage through the retropubic space can cause vascular, bowel, or bladder injury, even in the hands of experienced surgeons. An August 2005 French survey4 of 92 surgeons who performed 12,280 TVT procedures reported these complications: perioperative bladder injuries, 901 (7.34%); cases of complete postoperative urinary retention requiring catheterization, 809 (6.59%); vaginal mesh exposure, 26 (0.21%); retropubic or vulvovaginal hematoma, 39 (0.32%); and major organ injuries, 10 (0.08%).
The transobturator (TOT) approach, introduced in 2003,5 is simpler, with fewer complications. The sling is placed in a similar manner in the midurethral position, but the insertion points overlie the obturator space in the genitofemoral crease lateral to the vagina. A needle passing through the obturator membrane exits the vaginal incision without entering the retropubic space, theoretically averting risk of bowel, bladder, and major blood vessel injury.
Although the TOT is thought to be safer in this regard, complications including urinary retention, obturator hematoma and nerve injury, and urethral injury/erosion have been reported.6
A variety of TOT sling kits are available, none with proven superiority.
In a recent randomized, prospective trial in which 61 women had TVT or TOT, there were no bladder injuries in the TOT group, and 9.7% (n=3) in the TVT group (P>.05). The postoperative urinary retention rate was 25.8% (n=8) in the TVT group and 13.3% (n=4) in the TOT group (P>.05). Cure rates (83.9% vs 90%), improvement (9.7% vs 3.3%), and failure (6.5% vs 6.7%) were similar.7
The transobturator suburethral sling is encouraging, although it is unclear whether it is effective in patients with intrinsic sphincter deficiency, especially with a fixed or lead-pipe urethra. We need studies to determine how to match the right procedure to the right patient.
Which sling for which patients?
My indications for TOT vs. TVT, which are based on personal experience and available data, may change as data accumulate (TABLE). Indications are often surgeon-specific, depending on clinical experience.
In our review of 210 TOT slings over a 16-month period at 2 centers, we found a cure rate of 88% and an improvement rate of 1.9%. The complication rate was 24%; intraoperative and postoperative complications were all minor and mostly self-limited8: 1 cystotomy, 1 urethral injury, 2 hematomas, 1 erosion, 16 complaints of transient groin pain, 5 cases of urinary retention requiring reoperation, and 23 cases of de novo urge incontinence.
TABLE
Transvaginal vs transobturator sling
| INDICATIONS | ADVANTAGES |
|---|---|
| Transvaginal (retropubic) | |
| Physically active patient | Avoids groin discomfort with activity |
| Thin, young patient | Long term data available |
| Limited urethral hypermobility/internal sphincter dysfunction | Data supports use/dynamic backboard |
| Transobturator | |
| Elderly patient | Less postop voiding dysfunction |
| Significant overactive bladder/urge incontinence | Less urethral obstruction |
| Previous retropubic surgery | Less risk of retropubic complication |
| Obesity | Less risk of needle-passage complication |
| Inexperience with TVT | Less risk of periop complications |
Adjustable suburethral sling
One of the challenges in placing a suburethral sling is adjustment for efficacy without overcorrection and resultant bladder neck obstruction, urinary retention, or persistent and refractory overactive bladder symptoms. An adjustable transvaginal midurethral synthetic sling procedure was recently introduced in the United States: the Remeex Tensionfree Readjustable Tape, (Neomedic International, Spain). A retropubic minimally invasive midurethral sling is attached to sutures that are taken through a tensioning device placed above the fascia in the suprapubic region. The tensioning device has a small adjustment kit similar to a screwdriver, which is left in place at the time of surgery. The sling is intentionally left loose for postoperative adjustment. Following surgery, a filling cystometrogram confirms stress incontinence. The sling is then progressively tightened until the leaking ceases. This technology is designed to prevent or correct overtightening, and avert bladder outlet obstruction. The sling can be adjusted via a small suprapubic incision, even years later; adjustment has been reported up to 7 years later.
In a recent study of 62 patients with stress urinary incontinence, 58 patients (94%) were completely dry and cured, and 4 patients (6%) reported occasional slight urine leakage. Operative time was 20 to 40 minutes (only stress urinary incontinence and cystocele). Six patients required long-term readjustment (5 to increase tension and 1 to reduce tension). No major intra-operative complications occurred. Late complications included suprapubic wound pain (12 transitional and relieved with analgesics), 3 urinary tract infections, 2 wound seromas, 1 case requiring prosthesis removal due to infection, and 3 cases of hyperactivity de novo, which required anticholinergic treatment.9
Although postoperative urinary retention or postoperative failure is relatively uncommon in transvaginal or transobturator suburethral sling procedures performed by experienced surgeons, the adjustable sling may be especially useful in patients with increased risk of postoperative voiding dysfunction, as well as limited urethral hypermobility/fixed urethra, because the sling can be adjusted long after the operation. Risks include infection due to foreign body (indwelling placement of the tensioning device) as well as palpation and incisional discomfort in very thin patients. Further clinical experience is needed, but the concept of a sling that can be adjusted immediately or even years later is appealing.
Graft/mesh augmentation for prolapse repair
Augmentation of pelvic prolapse repair using mesh and graft materials is used increasingly in an effort to improve long-term outcomes, although we lack randomized prospective data and long-term outcome studies. Synthetic materials offer ready availability, consistent tissue properties, cost effectiveness, and permanent placement, although there are risks: infection, dyspareunia, and erosion or exposure. Success and complications may depend on surgical technique, choice of material, patient selection, postoperative management, or other factors.
The overall success rate was 94% at a mean of 17 months after operation, in a study of 63 women in whom polypropylene mesh was used for augmentation of cystocele and rectocele. However, the authors recommended abandonment of the procedure due to an unacceptably high rate of complications.10 In the 32 women undergoing anterior repair, sexual activity rate did not alter, but dyspareunia increased in 20%. Urge and stress incontinence did not change, but urgency improved in 10%; 13% had vaginal erosion of the mesh. Of the 31 patients undergoing posterior repair, sexual activity decreased by 12% and dyspareunia increased in 63%. Constipation improved in 15% and anal incontinence in 4%; 6.5% had vaginal erosion of mesh and 1 required mesh removal for abscess.
In another study, results were improved and complications were fewer. After 2 years, 24 of 26 women who had posterior repair with polypropylene mesh were cured (92.3%) and 1 had asymptomatic stage 2 rectocele. All but 1 reported improved symptoms and quality of life. No postop infection or rectovaginal fistula was reported; there were 3 vaginal erosions (12%), and 2 patients had de novo dyspareunia (7.7%).11
To make graft/mesh augmentation easier and faster, needle-suspension techniques were recently introduced. Needles are inserted either through the transobturator space (anterior mesh placement) or ischiorectal fascia (posterior placement) and exit through the pelvic sidewall in proximity to the ischial spine. A multi-arm mesh is then attached to the needles, which are withdrawn. Tension secures the mesh and provides “tension-free” anterior or posterior wall support. Colporrhaphy can be performed prior to mesh placement at the surgeon’s discretion.
Because we have few data on patient selection or long-term safety and efficacy (most of it presented at recent meetings12,13 ), these techniques call for caution. Blind needle passage can be associated with complications such as rectal injury and rectovaginal fistula.14 But complication rates may reflect early evolution and may improve with time and experience.
The author receives grant/research support and/or serves as a consultant for American Medical Systems, Boston Scientific, CR Bard, Mentor, Novartis, and Pfizer.
Even as we scramble to gather definitive evidence on the immediate and long-term benefits of new technologies, they are supplanting tradition in the surgical treatment of incontinence and prolapse. Surgeons have been swift to adopt synthetic mesh and the new generation of needle suspension procedures, which offer the double advantage of a shorter operative time and shorter postoperative recovery. Yet, we lack well-designed randomized prospective clinical studies on whether outcomes and complication rates are better than traditional therapies such as vaginal colporrhaphy and paravaginal repair.
There hasn’t been time.
These innovations came onto the market in rapid succession, accompanied by aggressive corporate promotion, physician interest, and, in turn, pressure from patients. Improved reimbursement for quicker, easier procedures also entices many physicians to become “early adopters.” (Recent addition of the CPT code for mesh/graft use in prolapse surgery [CPT 57267], increases reimbursement over traditional procedures.)
It is important to keep a cautious but open mind. Given the blind needle techniques and use of biomaterial grafts and synthetic meshes, these procedures may not be for every surgeon or every patient. As always, astute clinical judgment and critical analysis of the data and anecdotal experience are recommended.
Transobturator sling
The needle-guided synthetic mesh midurethral sling was rapidly adopted as the treatment of choice for stress urinary incontinence due to urethral hypermobility and intrinsic sphincter deficiency, soon after it was described in 1995.1
With the transvaginal tape (TVT) procedure, the learning curve was shorter and so were hospital stays and recovery, compared with abdominal Burch colposuspension and traditional bladder neck slings. Furthermore, cost efficiency improved,2 and the persistent cure rate was 85% from 2 to 8 years.3
However, needle passage through the retropubic space can cause vascular, bowel, or bladder injury, even in the hands of experienced surgeons. An August 2005 French survey4 of 92 surgeons who performed 12,280 TVT procedures reported these complications: perioperative bladder injuries, 901 (7.34%); cases of complete postoperative urinary retention requiring catheterization, 809 (6.59%); vaginal mesh exposure, 26 (0.21%); retropubic or vulvovaginal hematoma, 39 (0.32%); and major organ injuries, 10 (0.08%).
The transobturator (TOT) approach, introduced in 2003,5 is simpler, with fewer complications. The sling is placed in a similar manner in the midurethral position, but the insertion points overlie the obturator space in the genitofemoral crease lateral to the vagina. A needle passing through the obturator membrane exits the vaginal incision without entering the retropubic space, theoretically averting risk of bowel, bladder, and major blood vessel injury.
Although the TOT is thought to be safer in this regard, complications including urinary retention, obturator hematoma and nerve injury, and urethral injury/erosion have been reported.6
A variety of TOT sling kits are available, none with proven superiority.
In a recent randomized, prospective trial in which 61 women had TVT or TOT, there were no bladder injuries in the TOT group, and 9.7% (n=3) in the TVT group (P>.05). The postoperative urinary retention rate was 25.8% (n=8) in the TVT group and 13.3% (n=4) in the TOT group (P>.05). Cure rates (83.9% vs 90%), improvement (9.7% vs 3.3%), and failure (6.5% vs 6.7%) were similar.7
The transobturator suburethral sling is encouraging, although it is unclear whether it is effective in patients with intrinsic sphincter deficiency, especially with a fixed or lead-pipe urethra. We need studies to determine how to match the right procedure to the right patient.
Which sling for which patients?
My indications for TOT vs. TVT, which are based on personal experience and available data, may change as data accumulate (TABLE). Indications are often surgeon-specific, depending on clinical experience.
In our review of 210 TOT slings over a 16-month period at 2 centers, we found a cure rate of 88% and an improvement rate of 1.9%. The complication rate was 24%; intraoperative and postoperative complications were all minor and mostly self-limited8: 1 cystotomy, 1 urethral injury, 2 hematomas, 1 erosion, 16 complaints of transient groin pain, 5 cases of urinary retention requiring reoperation, and 23 cases of de novo urge incontinence.
TABLE
Transvaginal vs transobturator sling
| INDICATIONS | ADVANTAGES |
|---|---|
| Transvaginal (retropubic) | |
| Physically active patient | Avoids groin discomfort with activity |
| Thin, young patient | Long term data available |
| Limited urethral hypermobility/internal sphincter dysfunction | Data supports use/dynamic backboard |
| Transobturator | |
| Elderly patient | Less postop voiding dysfunction |
| Significant overactive bladder/urge incontinence | Less urethral obstruction |
| Previous retropubic surgery | Less risk of retropubic complication |
| Obesity | Less risk of needle-passage complication |
| Inexperience with TVT | Less risk of periop complications |
Adjustable suburethral sling
One of the challenges in placing a suburethral sling is adjustment for efficacy without overcorrection and resultant bladder neck obstruction, urinary retention, or persistent and refractory overactive bladder symptoms. An adjustable transvaginal midurethral synthetic sling procedure was recently introduced in the United States: the Remeex Tensionfree Readjustable Tape, (Neomedic International, Spain). A retropubic minimally invasive midurethral sling is attached to sutures that are taken through a tensioning device placed above the fascia in the suprapubic region. The tensioning device has a small adjustment kit similar to a screwdriver, which is left in place at the time of surgery. The sling is intentionally left loose for postoperative adjustment. Following surgery, a filling cystometrogram confirms stress incontinence. The sling is then progressively tightened until the leaking ceases. This technology is designed to prevent or correct overtightening, and avert bladder outlet obstruction. The sling can be adjusted via a small suprapubic incision, even years later; adjustment has been reported up to 7 years later.
In a recent study of 62 patients with stress urinary incontinence, 58 patients (94%) were completely dry and cured, and 4 patients (6%) reported occasional slight urine leakage. Operative time was 20 to 40 minutes (only stress urinary incontinence and cystocele). Six patients required long-term readjustment (5 to increase tension and 1 to reduce tension). No major intra-operative complications occurred. Late complications included suprapubic wound pain (12 transitional and relieved with analgesics), 3 urinary tract infections, 2 wound seromas, 1 case requiring prosthesis removal due to infection, and 3 cases of hyperactivity de novo, which required anticholinergic treatment.9
Although postoperative urinary retention or postoperative failure is relatively uncommon in transvaginal or transobturator suburethral sling procedures performed by experienced surgeons, the adjustable sling may be especially useful in patients with increased risk of postoperative voiding dysfunction, as well as limited urethral hypermobility/fixed urethra, because the sling can be adjusted long after the operation. Risks include infection due to foreign body (indwelling placement of the tensioning device) as well as palpation and incisional discomfort in very thin patients. Further clinical experience is needed, but the concept of a sling that can be adjusted immediately or even years later is appealing.
Graft/mesh augmentation for prolapse repair
Augmentation of pelvic prolapse repair using mesh and graft materials is used increasingly in an effort to improve long-term outcomes, although we lack randomized prospective data and long-term outcome studies. Synthetic materials offer ready availability, consistent tissue properties, cost effectiveness, and permanent placement, although there are risks: infection, dyspareunia, and erosion or exposure. Success and complications may depend on surgical technique, choice of material, patient selection, postoperative management, or other factors.
The overall success rate was 94% at a mean of 17 months after operation, in a study of 63 women in whom polypropylene mesh was used for augmentation of cystocele and rectocele. However, the authors recommended abandonment of the procedure due to an unacceptably high rate of complications.10 In the 32 women undergoing anterior repair, sexual activity rate did not alter, but dyspareunia increased in 20%. Urge and stress incontinence did not change, but urgency improved in 10%; 13% had vaginal erosion of the mesh. Of the 31 patients undergoing posterior repair, sexual activity decreased by 12% and dyspareunia increased in 63%. Constipation improved in 15% and anal incontinence in 4%; 6.5% had vaginal erosion of mesh and 1 required mesh removal for abscess.
In another study, results were improved and complications were fewer. After 2 years, 24 of 26 women who had posterior repair with polypropylene mesh were cured (92.3%) and 1 had asymptomatic stage 2 rectocele. All but 1 reported improved symptoms and quality of life. No postop infection or rectovaginal fistula was reported; there were 3 vaginal erosions (12%), and 2 patients had de novo dyspareunia (7.7%).11
To make graft/mesh augmentation easier and faster, needle-suspension techniques were recently introduced. Needles are inserted either through the transobturator space (anterior mesh placement) or ischiorectal fascia (posterior placement) and exit through the pelvic sidewall in proximity to the ischial spine. A multi-arm mesh is then attached to the needles, which are withdrawn. Tension secures the mesh and provides “tension-free” anterior or posterior wall support. Colporrhaphy can be performed prior to mesh placement at the surgeon’s discretion.
Because we have few data on patient selection or long-term safety and efficacy (most of it presented at recent meetings12,13 ), these techniques call for caution. Blind needle passage can be associated with complications such as rectal injury and rectovaginal fistula.14 But complication rates may reflect early evolution and may improve with time and experience.
The author receives grant/research support and/or serves as a consultant for American Medical Systems, Boston Scientific, CR Bard, Mentor, Novartis, and Pfizer.
1. Ulmsten U, Petros P. Intravaginal slingplasty (IVS): an ambulatory surgical procedure for treatment of female urinary incontinence. Scand J Urol Nephrol. 1995;29:75-82.
2. Kilonzo M, Vale L, Stearns SC, et al. Cost effectiveness of tension-free vaginal tape for the surgical management of female stress incontinence. Int J Technol Assess Health Care. 2004;20:455-463.
3. Holmgren C, Nilsson S, Lanner L, Hellberg D. Long-term results with tension-free vaginal tape on mixed and stress urinary incontinence. Obstet Gynecol. 2005;106:38-43.
4. Agostini A, Bretelle F, Franchi F, Roger V, Cravello L, Blanc B. Immediate complications of tension-free vaginal tape (TVT): results of a French survey. Eur J Obstet Gynecol Reprod Biol. 2005 Aug 8; [Epub ahead of print].
5. Delorme E, Droupy S, de Tayrac R, Delmas V. Transobturator tape (Uratape). A new minimally invasive method in the treatment of urinary incontinence in women. Prog Urol. 2003;13:656-659.
6. Game X, et al. Obturator infected hematoma and urethral erosion following transobturator tape implantation. J Urol. 2004;171:1629.-
7. deTayrac R, Deffieux X, Droupy S, et al. A prospective randomized trial comparing tension-free vaginal tape and transobturator suburethral tape for surgical treatment of stress urinary incontinence [retracted in: Am J Obstet Gynecol. 2005;192:339]. Am J Obstet Gynecol. 2004;190:602-608.
8. Rajan S, Diwadkar G, Hurwitz S, Kohli N, Roberts L, Moore R, Miklos J. Transobturator (TOT) suburethral sling: early US data on safety and efficacy. Abstract presented at: 2005 Meeting of the International Urogynecology Association; August 9–12, 2005; Copenhagen, Denmark. Poster 52, abstract ID 982.
9. Cabrera Pérez J, Bravo Fernández I, Pérez G, González Enguita C, Vela Navarrete R. Analysis of the suburethral sling TRT (tension free readjustable tape) results in female SUI treatment. Abstract presented at: LXX Congreso Nacional de Urología; June 4–7, 2005; San Sebastián, Spain.
10. Milani R, Salvatore S, Soligo M, Pifarotti P, Meschia M, Cortese M. Functional and anatomical outcome of anterior and posterior vaginal prolapse repair with Prolene mesh. BJOG. 2005;112:107-111.
11. de Tayrac R, Picone O, Chauveaud-Lambling A, Fernandez H. A 2-year anatomical and functional assessment of transvaginal rectocele repair using a polypropylene mesh. Int Urogynecol J Pelvic Floor Dysfunct. 2005 May 21; [Epub ahead of print].
12. Rane AM, Naidu AK, Barry CL, Nyok LY, Corstiaans AC. A novel transobturator system for the repair of anterior vaginal wall prolapse: a pilot study. Abstract presented at: 2005 Meeting of the International Urogynecology Association; August 9–12, 2005; Copenhagen, Denmark.
13. Cosson M, Caquent F, Collinet P, Rosenthal C, Clave H, Debodinance P, Garbin O, Berrocal J, Villet R, Jacquetin B. Prolift mesh for pelvic organ prolapse surgical treatment using the TVM group technique: a retrospective study of 687 patients. Abstract presented at: 2005 Meeting of the International Urogynecology Association; August 9–12, 2005; Copenhagen, Denmark.
14. Hilger WS, Cornella JL. Rectovaginal fistula after posterior intravaginal slingplasty and polypropylene mesh augmented rectocele repair. Int Urogynecol J Pelvic Floor Dysfunct. 2005 Jul 29; [Epub ahead of print].
1. Ulmsten U, Petros P. Intravaginal slingplasty (IVS): an ambulatory surgical procedure for treatment of female urinary incontinence. Scand J Urol Nephrol. 1995;29:75-82.
2. Kilonzo M, Vale L, Stearns SC, et al. Cost effectiveness of tension-free vaginal tape for the surgical management of female stress incontinence. Int J Technol Assess Health Care. 2004;20:455-463.
3. Holmgren C, Nilsson S, Lanner L, Hellberg D. Long-term results with tension-free vaginal tape on mixed and stress urinary incontinence. Obstet Gynecol. 2005;106:38-43.
4. Agostini A, Bretelle F, Franchi F, Roger V, Cravello L, Blanc B. Immediate complications of tension-free vaginal tape (TVT): results of a French survey. Eur J Obstet Gynecol Reprod Biol. 2005 Aug 8; [Epub ahead of print].
5. Delorme E, Droupy S, de Tayrac R, Delmas V. Transobturator tape (Uratape). A new minimally invasive method in the treatment of urinary incontinence in women. Prog Urol. 2003;13:656-659.
6. Game X, et al. Obturator infected hematoma and urethral erosion following transobturator tape implantation. J Urol. 2004;171:1629.-
7. deTayrac R, Deffieux X, Droupy S, et al. A prospective randomized trial comparing tension-free vaginal tape and transobturator suburethral tape for surgical treatment of stress urinary incontinence [retracted in: Am J Obstet Gynecol. 2005;192:339]. Am J Obstet Gynecol. 2004;190:602-608.
8. Rajan S, Diwadkar G, Hurwitz S, Kohli N, Roberts L, Moore R, Miklos J. Transobturator (TOT) suburethral sling: early US data on safety and efficacy. Abstract presented at: 2005 Meeting of the International Urogynecology Association; August 9–12, 2005; Copenhagen, Denmark. Poster 52, abstract ID 982.
9. Cabrera Pérez J, Bravo Fernández I, Pérez G, González Enguita C, Vela Navarrete R. Analysis of the suburethral sling TRT (tension free readjustable tape) results in female SUI treatment. Abstract presented at: LXX Congreso Nacional de Urología; June 4–7, 2005; San Sebastián, Spain.
10. Milani R, Salvatore S, Soligo M, Pifarotti P, Meschia M, Cortese M. Functional and anatomical outcome of anterior and posterior vaginal prolapse repair with Prolene mesh. BJOG. 2005;112:107-111.
11. de Tayrac R, Picone O, Chauveaud-Lambling A, Fernandez H. A 2-year anatomical and functional assessment of transvaginal rectocele repair using a polypropylene mesh. Int Urogynecol J Pelvic Floor Dysfunct. 2005 May 21; [Epub ahead of print].
12. Rane AM, Naidu AK, Barry CL, Nyok LY, Corstiaans AC. A novel transobturator system for the repair of anterior vaginal wall prolapse: a pilot study. Abstract presented at: 2005 Meeting of the International Urogynecology Association; August 9–12, 2005; Copenhagen, Denmark.
13. Cosson M, Caquent F, Collinet P, Rosenthal C, Clave H, Debodinance P, Garbin O, Berrocal J, Villet R, Jacquetin B. Prolift mesh for pelvic organ prolapse surgical treatment using the TVM group technique: a retrospective study of 687 patients. Abstract presented at: 2005 Meeting of the International Urogynecology Association; August 9–12, 2005; Copenhagen, Denmark.
14. Hilger WS, Cornella JL. Rectovaginal fistula after posterior intravaginal slingplasty and polypropylene mesh augmented rectocele repair. Int Urogynecol J Pelvic Floor Dysfunct. 2005 Jul 29; [Epub ahead of print].
• New sling procedures • Correcting site-specific defects • Mesh augmentation
After several decades of slow progress, the field of urogynecology is experiencing dynamic change, including:
- new minimally invasive, tension-free, midurethral sling procedures, especially the transobturator approach,
- correction of size-specific defects to repair prolapse, and
- use of mesh/graft augmentation in pro lapse repair.
These developments are some of the most important since Kelly and Dunn first described suburethral fascial plication for stress incontinence and cystocele in 1914.1 They have come about through increased understanding of the pathophysiology of incontinence and prolapse, innovative technology and techniques, and improved communication and coordination among physicians worldwide.
New sling procedures, promising outcomes
The minimally invasive midurethral sling procedure spawned notable new approaches and is a mainstay of surgical treatment for stress urinary incontinence.
First described in Sweden by Ulmsten in 1995,2 the tension-free vaginal tape procedure is a revolutionary change in the suburethral sling procedure and is now the most widely performed surgery for stress incontinence worldwide.
In it, a tension-free vaginal tape (TVT) (Gynecare, Somerville, NJ) of synthetic polypropylene mesh is attached to 2 needles and passed through a vaginal incision and the retropubic space, exiting to small incisions in the suprapubic region to create a suburethral sling or hammock and provide urethral support during increased abdominal pressure. The sling remains fixed by friction and subsequent adhesions.
Although the traditional suburethral sling was less invasive than other abdominal incontinence procedures, it was associated with a steep learning curve and a high incidence of postoperative irritative bladder symptoms and voiding dysfunction.3
Tension-free vaginal tape: Excellent long-term cure
It can be performed routinely in under 30 minutes using local anesthesia, with minimal postoperative complications. Five-year cure rates approach 95%,4 and data presented at the 2003 International Urogynecology Association clinical meeting describe an objective 7-year cure rate of 82%.5
The incidence and severity of postoperative voiding dysfunction following the TVT procedure is significantly lower than that reported after traditional suburethral sling (2%–40%) or transvaginal needle suspension (2%–50%) procedures.6-9 Although bladder perforation has occurred in up to 10% of patients, reports of complications, including major hemorrhage, tape erosion, and bowel and nerve injury, are rare.10,11
Modifications
These products have a modified approach, materials, or refinements in technique to address various needs. For example, American Medical Systems (Minnetonka, Minn) introduced the SPARC procedure, which allows abdominal placement of the midurethral sling, similar to a needle suspension technique.
CR Bard (Murray Hill, NJ) introduced a midurethral sling of porcine dermis (Pelvicol) to address concerns physicians may have about using synthetic materials.
Minimally invasive midurethral slings include the Advantage (Boston Scientific, Natick, Mass), Centrasorb (Caldera Medical, Thousand Oaks, Calif), Stratasis TF (Cook, West Lafayette, Ind), and Uretex (CR Bard).
Newest approach: Transobturator sling
In this technique, the sling is placed in the midurethral position, but the insertion points are in the genital area lateral to the vagina, and the needle passes through the obturator membrane and paraurethral space. Because it avoids passage of the needles through the retropubic space, the transobturator approach theoretically should reduce the risk of bowel, bladder, and major blood vessel injury.
The procedure was initially described in Europe and introduced in the United States in 2003. Current product offerings include the outside-in approach of ObTape (Mentor, Santa Barbara, Calif), Monarc (American Medical Systems), and Uretex (CR Bard). Gynecare offers a variation of its TVT—the TVT-Obturator—which involves an inside-out approach to further minimize risk of vascular injury.
Shortage of long-term data, but good early results
In a 1-year follow-up of patients undergoing a sling procedure with the UraTape transobturator sling (Mentor), Delorme and colleagues12 reported that 29 of 32 patients (90.6%) were cured and 3 (9.4%) improved. De Leval13 described his inside-out approach with equally good results: no bladder or urethral injuries and no vascular (hematoma or bleeding) or neurological complications. A transobturator technique using porcine dermis has also been described.
Tension-free vaginal tape versus transobturator sling
In 2004, a small randomized, prospective trial of TVT (n = 29) versus transobturator tape (n = 27) with 1-year follow-up found the transobturator approach to be safer and easier to place with equivalent short-term results.14 Mean operative time was significantly shorter in the transobturator group (15±4 minutes versus 27±8 minutes, P <.001).
No bladder injury occurred in the transobturator group versus 9.7% (n = 3) in the TVT group (P >.05). The rate of postoperative urinary retention was 25.8% (n = 8) in the TVT group versus 13.3% (n = 4) in the transobturator group (P >.05). Cure rates were similar for the TVT and transobturator groups: 83.9% versus 90%, respectively), improvement (9.7% versus 3.3%), and failure (6.5% versus 6.7%). No vaginal erosion occurred in either group.
Additional investigations of the transobturator tape procedure are underway.
Correcting site-specific defects in prolapse repair
This repair rationale should become the standard, although it has yet to be widely adopted and procedural refinement and research are continuing.
As early as 1908, site-specific defects in the endopelvic fascia were identified as the likely cause of anterior vaginal segment prolapse. Like hernia repair, which requires closure of the fascial defect, the “cystocele hernia” repair advocated by George White involved reattaching the endopelvic fascia to the arcus tendineus fascia pelvis using a series of interrupted sutures through an abdominal retropubic approach.
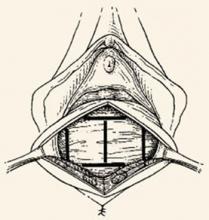
Although this view was later abandoned, it resurfaced in the 1980s, when Cullen Richardson described midline, lateral, and transverse defects (FIGURE 1) in the endopelvic fascia as the cause of cystocele and rectocele.15 Richardson advocated diagnosis that identified these fascial defects, along with treatment with site-specific repair.
(In the intervening decades, prolapse was thought to result from generalized weakening or attenuation of the endopelvic fascia that supports the bladder, rectum, or vagina, leading to cystocele, rectocele, or uterine prolapse, respectively. Traditional repairs, still widely performed by most gynecologists, consist of the anterior and posterior colporrhaphy, which involve midline plication of the endopelvic fascia to reduce the prolapse and recreate support by strengthening the weakened fascial layer.)
FIGURE 1 Identify the site of defect
Whether the defect that causes a rectocele or cystocele is transverse, central, or lateral determines the type of repair to be performed. Reprinted with permission from Richardson AC. The rectovaginal septum revisited: its relationship to rectocele and its importance in rectocele repair. Clin Obstet Gynecol.1993;36(4):976–983.
Excellent cure rates with fewer complications
Site-specific repair (FIGURE 2) has been adopted by many pelvic surgeons, who report excellent cure rates with fewer complications such as dyspareunia, vaginal narrowing, and increased blood loss—all of which are more common with traditional anterior and posterior colporrhaphy.
Traditional colporrhaphy does correct the underlying fascial defect when the “hernia” is in the midline. However, for lateral and transverse defects, traditional colporrhaphy leaves the defect uncorrected and may even create additional tension, resulting in recurrence.
Site-specific repair also is useful in the treatment of enterocele, which has been described as a herniation of the peritoneum through a defect of the anterior and posterior fascial planes at the vaginal apex.16
Widening but not broad acceptance
Although cure rates for site-specific prolapse repair range from 75% to 85%,17,18 the concept has yet to be widely adopted and continues to undergo procedural refinement and research, as well as physician education. With increasing experience and clinical data, it may become the standard for pelvic prolapse repair.
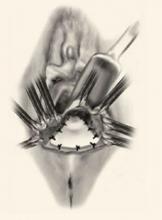
FIGURE 2 Repair the defect to correct the rectocele
Surgical technique for a transverse tear in the rectovaginal fascia: After identifying the defect, place a series of interrupted sutures to reapproximate the fascial edges (copyright Miklos/Kohli).
Mesh augmentation: Useful in selected patients
Although augmentation with mesh or biomaterials is easy to perform, it remains unclear which technique and materials are optimal.
The use of mesh and biomaterials to augment repair of a cystocele, rectocele, or enterocele is slowly increasing, although general surgeons have been utilizing these materials for many years in hernia repair.
Augmentation has been advocated for “pelvic hernias” because of poor long-term cure rates for traditional prolapse surgeries (which range from 40% to 80%—well behind rates of 90% or more for incontinence procedures).
A recent informal survey of the members of the American Urogynecologic Society and International Urogynecologic Association revealed that most pelvic surgeons are using some type of graft or mesh to augment repairs in selected patients.
Synthetic mesh versus autologous and heterologous grafts
Synthetic materials have the advantage of being readily available, cost-effective, and consistent in quality, but they may cause significant complications, including infection, stricture, and erosion. This is especially important in the vagina, which needs to stretch during intercourse and which alters in thickness and other properties during a woman’s lifetime.
In contrast, autologous and heterologous donor grafts provide naturally occurring biomaterials capable of remodeling. Unfortunately, the in vivo tissue response is not yet fully understood. Other disadvantages: Biomaterials may lack consistent tissue properties and can be expensive.
For these reasons, graft materials remain in an early period of evaluation, although their use is expected to rise steadily with increasing experience and new product development.
Limited data on safety and efficacy
Although many pelvic surgeons use graft/mesh materials in prolapse repair, data on their safety and efficacy are limited, partly due to the variety of surgical techniques and materials available. Another factor is the difficulty of obtaining good long-term data with large patient numbers. Most of the literature is comprised of case reports, with few prospective, randomized trials.
That said, initial data suggest a significant improvement in cure rates, compared with traditional techniques, with minimal short-term complications. Long-term results and complication rates are not yet available.
What existing studies show
A variety of synthetic materials have been used in surgical correction of cystocele. In the largest series to date, Flood and colleagues19 reported their 12-year experience with 142 women undergoing a modified anterior colporrhaphy reinforced with Marlex (Davol, Cranston, RI) mesh: 100% success in correcting cystocele and a 74% success rate for urinary stress incontinence, with a mean follow-up of 3.2 years and no significant intraoperative complications.
In a prospective randomized trial of 125 patients utilizing absorbable polyglactin 910 mesh (Vicryl) (Ethicon, Somerville, NJ) to augment standard anterior colporrhaphy, Koduri et al20 reported a failure rate of 13% in the colporrhaphy-alone group compared to 1% in the colporrhaphy-mesh group at the 1-year follow-up. Subjectively, both groups improved equally.
Clemons et al21 used a human dermis graft to treat advanced recurrent cystocele in 33 women, with a follow-up of 18 months. They noted 13 (41%) objective failures and 1 (3%) subjective failure. Complications included 1 case of febrile morbidity, 1 cystotomy, and 1 anterior wall breakdown secondary to hematoma formation caused by heparin therapy. No other erosions or rejections were seen.
Most effective applications
Graft/mesh augmentation may be most effective in posterior vaginal segment reconstruction and rectocele repair, as it obviates the need for levatorplasty in patients with poor rectovaginal fascia. A variety of synthetic materials have been used for posterior wall reconstruction in small series.
Mersilene mesh
Mersilene mesh (Ethicon) was used by Fox and Stanton22 to augment traditional rectocele repair in 29 women followed for 14 months. Most of the women had undergone previous rectocele repair with recurrence of their prolapse. All women with stage II and stage III vault prolapse were corrected, with an increase in stage I prolapse from 20% to 27%. All women with stage II and stage III rectocele were corrected, with a decrease in stage I prolapse from 36% to 7%. The only significant intraoperative complication was a cystotomy. One mesh became infected postoperatively, requiring removal.
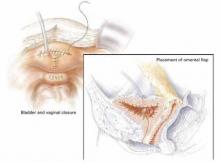
Cadaveric dermal graft
Recently, Kohli and Miklos23 reported their experience with 57 patients undergoing augmented rectocele repair using a cadaveric dermal graft (FIGURE 3) over a 2-year period. Average follow-up was 11 months. Average patient age in the follow-up group was 63.6±10.9 years (range: 33–79 years) and average parity was 2.8±1.5 (range: 0–7). No major intraoperative complications (hollow viscous injury, blood loss greater than 500 cc, or transfusion) or post-operative complications (infection, abscess, or hematoma) were noted. No graft-related complications such as rejection, erosion, infection, and fistula formation were noted during the follow-up period.
Using previously accepted Pelvic Organ Prolapse Quantification (POP-Q) parameters for success, Kohli and Miklos found 54 of 57 women (95%) to have surgical cure at follow-up. These authors have also described the use of a dermal graft in the repair of a complicated rectovaginal fistula.24
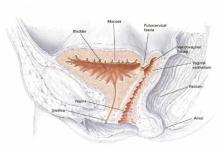
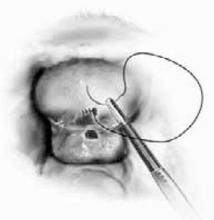
FIGURE 3 Placement of mesh augmentation
Augment enterocele/rectocele repair by attaching the mesh apically, laterally, and distally (copyright Miklos/Kohli).
Future outlook
Further data on the efficacy and safety of graft/mesh augmentation—including identification of the optimal technique and ideal material—are necessary before this approach can be widely adopted. However, in selected patients, it represents a significant advance in the surgical treatment of pelvic prolapse.
The author is a preceptor for American Medical Systems, Gynecare, CR Bard, and Mentor and a consultant for Boston Scientific.
1. Kelly HA, Dunn WM. Urinary incontinence in women without manifest injury to the bladder. Surg Gynecol Obstet. 1914;18:444.-
2. Ulmsten U, Petros P. Intravaginal slingplasty (IVS): an ambulatory surgical procedure for treatment of female urinary incontinence. Scand J Urol Nephrol. 1995;29:75-82.
3. Weinberger MW, Ostergard DR. Postoperative catheterization, urinary retention, and permanent voiding dysfunction after polytetrafluroethylene suburethral sling placement. Obstet Gynecol. 1996;87:50-54.
4. Nilsson CG, Kuuva N, Falconer C, Rezapour M, Ulmsten U. Long-term results of the tension-free vaginal tape (TVT) procedure for surgical treatment of female stress urinary incontinence. Int Urogynecol J Pelvic Floor Dysfunct. 2001;12(Suppl 2):S5-8.
5. Nilsson CG, Rezapour M, Falconer C. Seven years follow-up of the tension-free vaginal tape (TVT) procedure. Presented at the 2003 International Urogynecology Association Annual Meeting.
6. Horbach N. Suburethral sling procedures. In: Ostergard DR, Bent AE, eds. Urogynecology and Urodynamics: Theory and Practice. 3rd ed. Baltimore: Williams & Wilkins; 1991;449-458.
7. Stanton SL, Reynolds SF, Creighton SM. The modified Pereyra (Raz) procedure for genuine stress incontinence—a useful option in the elderly or frail patient? Int Urogynecol J. 1995;6:22-25.
8. Nygaard IE, Kreder KJ. Complications of incontinence surgery. Int Urogynecol J. 1994;5:353-360.
9. Lockhart vJL, Tirado A, Morillo G, Politano VA. Vesicourethral dysfunction following cystourethropexy. J Urol. 1982;128:943-945.
10. Leboeuf L, Tellez CA, Ead D, Gousse AE. Complication of bowel perforation during insertion of tension-free vaginal tape. J Urol. 2003;170(4 Pt 1):1310.-
11. Abouassaly R, Steinberg JR, Lemieux M, et al. Complications of tension-free vaginal tape surgery: a multi-institutional review. BJU Int. 2004;94:110-113.
12. Delorme E, Droupy S, de Tayrac R, Delmas V. [Transobturator tape (UraTape). A new minimally invasive method in the treatment of urinary incontinence in women.] Prog Urol. 2003;13:656-659.
13. de Leval J. Novel surgical technique for the treatment of female stress urinary incontinence: transobturator vaginal tape inside-out. Eur Urol. 2003;44:724-730.
14. De Tayrac R, Deffieux X, Droupy S, ChauveaudLambling A, Calvanese-Benamour L, Fernandez H. A prospective randomized trial comparing tensionfree vaginal tape and transobturator suburethral tape for surgical treatment of stress urinary incontinence. Am J Obstet Gynecol. 2004;190:602-608.
15. Richardson AC. The rectovaginal septum revisited: its relationship to rectocele and its importance in rectocele repair. Clin Obstet Gynecol. 1993;36:976-983.
16. Miklos JR, Kohli N, Lucente V, Saye WB. Site-specific fascial defects in the diagnosis and surgical management of enterocele. Am J Obstet Gynecol. 1998;179 (6 Pt 1):1418-1422; discussion 1422-823.
17. Porter WE, Steele A, Walsh P, Kohli N, Karram MM. The anatomic and functional outcomes of defectspecific rectocele repairs. Am J Obstet Gynecol. 1999;181:1353-1358; discussion 1358-1359.
18. Kenton K, Shott S, Brubaker L. Outcome after rectovaginal fascia reattachment for rectocele repair. Am J Obstet Gynecol. 1999;181:1360-1363; discussion 1363-1364.
19. Flood CG, Drutz HP, Waja L. Anterior colporrhaphy reinforced with Marlex mesh for the treatment of cystoceles. Int Urogynecol J Pelvic Floor Dysfunct. 1998;9:200-204.
20. Koduri S, Lobel RW, Winkler HA, Tomezsko J, Culligan PJ, Sand PK. Prospective randomized trial of polyglactin 910 mesh to prevent recurrence of cystoceles and rectoceles. Proceedings of the 25th Annual Meeting of the International Urogynecological Association, 2000. Int Urogynecol J Pelvic Floor Dysfunct. 2000;11:S80.-
21. Clemons JL, Myers DL, Aguilar VC, Arya LA. Vaginal paravaginal repair with an AlloDerm graft. Am J Obstet Gynecol. 2003;189:1612-1618; discussion 1618-1619.
22. Fox SD, Stanton SL. Vault prolapse and rectocele: assessment of repair using sacrocolpopexy with mesh interposition. BJOG. 2000;107:1371-1375.
23. Kohli N, Miklos JR. Site specific rectocele repair augmented with a cadaveric dermal graft. Proceedings of the 21st Annual Scientific Meeting of the American Urogynecologic Society, 2000. Int Urogynecol J Pelvic Floor Dysfunct. 2001;12:S9.-
24. Miklos JR, Kohli N. Rectovaginal fistula repair utilizing a cadaveric dermal allograft. Int Urogynecol J Pelvic Floor Dysfunct. 1999;10:405-406.
After several decades of slow progress, the field of urogynecology is experiencing dynamic change, including:
- new minimally invasive, tension-free, midurethral sling procedures, especially the transobturator approach,
- correction of size-specific defects to repair prolapse, and
- use of mesh/graft augmentation in pro lapse repair.
These developments are some of the most important since Kelly and Dunn first described suburethral fascial plication for stress incontinence and cystocele in 1914.1 They have come about through increased understanding of the pathophysiology of incontinence and prolapse, innovative technology and techniques, and improved communication and coordination among physicians worldwide.
New sling procedures, promising outcomes
The minimally invasive midurethral sling procedure spawned notable new approaches and is a mainstay of surgical treatment for stress urinary incontinence.
First described in Sweden by Ulmsten in 1995,2 the tension-free vaginal tape procedure is a revolutionary change in the suburethral sling procedure and is now the most widely performed surgery for stress incontinence worldwide.
In it, a tension-free vaginal tape (TVT) (Gynecare, Somerville, NJ) of synthetic polypropylene mesh is attached to 2 needles and passed through a vaginal incision and the retropubic space, exiting to small incisions in the suprapubic region to create a suburethral sling or hammock and provide urethral support during increased abdominal pressure. The sling remains fixed by friction and subsequent adhesions.
Although the traditional suburethral sling was less invasive than other abdominal incontinence procedures, it was associated with a steep learning curve and a high incidence of postoperative irritative bladder symptoms and voiding dysfunction.3
Tension-free vaginal tape: Excellent long-term cure
It can be performed routinely in under 30 minutes using local anesthesia, with minimal postoperative complications. Five-year cure rates approach 95%,4 and data presented at the 2003 International Urogynecology Association clinical meeting describe an objective 7-year cure rate of 82%.5
The incidence and severity of postoperative voiding dysfunction following the TVT procedure is significantly lower than that reported after traditional suburethral sling (2%–40%) or transvaginal needle suspension (2%–50%) procedures.6-9 Although bladder perforation has occurred in up to 10% of patients, reports of complications, including major hemorrhage, tape erosion, and bowel and nerve injury, are rare.10,11
Modifications
These products have a modified approach, materials, or refinements in technique to address various needs. For example, American Medical Systems (Minnetonka, Minn) introduced the SPARC procedure, which allows abdominal placement of the midurethral sling, similar to a needle suspension technique.
CR Bard (Murray Hill, NJ) introduced a midurethral sling of porcine dermis (Pelvicol) to address concerns physicians may have about using synthetic materials.
Minimally invasive midurethral slings include the Advantage (Boston Scientific, Natick, Mass), Centrasorb (Caldera Medical, Thousand Oaks, Calif), Stratasis TF (Cook, West Lafayette, Ind), and Uretex (CR Bard).
Newest approach: Transobturator sling
In this technique, the sling is placed in the midurethral position, but the insertion points are in the genital area lateral to the vagina, and the needle passes through the obturator membrane and paraurethral space. Because it avoids passage of the needles through the retropubic space, the transobturator approach theoretically should reduce the risk of bowel, bladder, and major blood vessel injury.
The procedure was initially described in Europe and introduced in the United States in 2003. Current product offerings include the outside-in approach of ObTape (Mentor, Santa Barbara, Calif), Monarc (American Medical Systems), and Uretex (CR Bard). Gynecare offers a variation of its TVT—the TVT-Obturator—which involves an inside-out approach to further minimize risk of vascular injury.
Shortage of long-term data, but good early results
In a 1-year follow-up of patients undergoing a sling procedure with the UraTape transobturator sling (Mentor), Delorme and colleagues12 reported that 29 of 32 patients (90.6%) were cured and 3 (9.4%) improved. De Leval13 described his inside-out approach with equally good results: no bladder or urethral injuries and no vascular (hematoma or bleeding) or neurological complications. A transobturator technique using porcine dermis has also been described.
Tension-free vaginal tape versus transobturator sling
In 2004, a small randomized, prospective trial of TVT (n = 29) versus transobturator tape (n = 27) with 1-year follow-up found the transobturator approach to be safer and easier to place with equivalent short-term results.14 Mean operative time was significantly shorter in the transobturator group (15±4 minutes versus 27±8 minutes, P <.001).
No bladder injury occurred in the transobturator group versus 9.7% (n = 3) in the TVT group (P >.05). The rate of postoperative urinary retention was 25.8% (n = 8) in the TVT group versus 13.3% (n = 4) in the transobturator group (P >.05). Cure rates were similar for the TVT and transobturator groups: 83.9% versus 90%, respectively), improvement (9.7% versus 3.3%), and failure (6.5% versus 6.7%). No vaginal erosion occurred in either group.
Additional investigations of the transobturator tape procedure are underway.
Correcting site-specific defects in prolapse repair
This repair rationale should become the standard, although it has yet to be widely adopted and procedural refinement and research are continuing.
As early as 1908, site-specific defects in the endopelvic fascia were identified as the likely cause of anterior vaginal segment prolapse. Like hernia repair, which requires closure of the fascial defect, the “cystocele hernia” repair advocated by George White involved reattaching the endopelvic fascia to the arcus tendineus fascia pelvis using a series of interrupted sutures through an abdominal retropubic approach.
Although this view was later abandoned, it resurfaced in the 1980s, when Cullen Richardson described midline, lateral, and transverse defects (FIGURE 1) in the endopelvic fascia as the cause of cystocele and rectocele.15 Richardson advocated diagnosis that identified these fascial defects, along with treatment with site-specific repair.
(In the intervening decades, prolapse was thought to result from generalized weakening or attenuation of the endopelvic fascia that supports the bladder, rectum, or vagina, leading to cystocele, rectocele, or uterine prolapse, respectively. Traditional repairs, still widely performed by most gynecologists, consist of the anterior and posterior colporrhaphy, which involve midline plication of the endopelvic fascia to reduce the prolapse and recreate support by strengthening the weakened fascial layer.)
FIGURE 1 Identify the site of defect
Whether the defect that causes a rectocele or cystocele is transverse, central, or lateral determines the type of repair to be performed. Reprinted with permission from Richardson AC. The rectovaginal septum revisited: its relationship to rectocele and its importance in rectocele repair. Clin Obstet Gynecol.1993;36(4):976–983.
Excellent cure rates with fewer complications
Site-specific repair (FIGURE 2) has been adopted by many pelvic surgeons, who report excellent cure rates with fewer complications such as dyspareunia, vaginal narrowing, and increased blood loss—all of which are more common with traditional anterior and posterior colporrhaphy.
Traditional colporrhaphy does correct the underlying fascial defect when the “hernia” is in the midline. However, for lateral and transverse defects, traditional colporrhaphy leaves the defect uncorrected and may even create additional tension, resulting in recurrence.
Site-specific repair also is useful in the treatment of enterocele, which has been described as a herniation of the peritoneum through a defect of the anterior and posterior fascial planes at the vaginal apex.16
Widening but not broad acceptance
Although cure rates for site-specific prolapse repair range from 75% to 85%,17,18 the concept has yet to be widely adopted and continues to undergo procedural refinement and research, as well as physician education. With increasing experience and clinical data, it may become the standard for pelvic prolapse repair.
FIGURE 2 Repair the defect to correct the rectocele
Surgical technique for a transverse tear in the rectovaginal fascia: After identifying the defect, place a series of interrupted sutures to reapproximate the fascial edges (copyright Miklos/Kohli).
Mesh augmentation: Useful in selected patients
Although augmentation with mesh or biomaterials is easy to perform, it remains unclear which technique and materials are optimal.
The use of mesh and biomaterials to augment repair of a cystocele, rectocele, or enterocele is slowly increasing, although general surgeons have been utilizing these materials for many years in hernia repair.
Augmentation has been advocated for “pelvic hernias” because of poor long-term cure rates for traditional prolapse surgeries (which range from 40% to 80%—well behind rates of 90% or more for incontinence procedures).
A recent informal survey of the members of the American Urogynecologic Society and International Urogynecologic Association revealed that most pelvic surgeons are using some type of graft or mesh to augment repairs in selected patients.
Synthetic mesh versus autologous and heterologous grafts
Synthetic materials have the advantage of being readily available, cost-effective, and consistent in quality, but they may cause significant complications, including infection, stricture, and erosion. This is especially important in the vagina, which needs to stretch during intercourse and which alters in thickness and other properties during a woman’s lifetime.
In contrast, autologous and heterologous donor grafts provide naturally occurring biomaterials capable of remodeling. Unfortunately, the in vivo tissue response is not yet fully understood. Other disadvantages: Biomaterials may lack consistent tissue properties and can be expensive.
For these reasons, graft materials remain in an early period of evaluation, although their use is expected to rise steadily with increasing experience and new product development.
Limited data on safety and efficacy
Although many pelvic surgeons use graft/mesh materials in prolapse repair, data on their safety and efficacy are limited, partly due to the variety of surgical techniques and materials available. Another factor is the difficulty of obtaining good long-term data with large patient numbers. Most of the literature is comprised of case reports, with few prospective, randomized trials.
That said, initial data suggest a significant improvement in cure rates, compared with traditional techniques, with minimal short-term complications. Long-term results and complication rates are not yet available.
What existing studies show
A variety of synthetic materials have been used in surgical correction of cystocele. In the largest series to date, Flood and colleagues19 reported their 12-year experience with 142 women undergoing a modified anterior colporrhaphy reinforced with Marlex (Davol, Cranston, RI) mesh: 100% success in correcting cystocele and a 74% success rate for urinary stress incontinence, with a mean follow-up of 3.2 years and no significant intraoperative complications.
In a prospective randomized trial of 125 patients utilizing absorbable polyglactin 910 mesh (Vicryl) (Ethicon, Somerville, NJ) to augment standard anterior colporrhaphy, Koduri et al20 reported a failure rate of 13% in the colporrhaphy-alone group compared to 1% in the colporrhaphy-mesh group at the 1-year follow-up. Subjectively, both groups improved equally.
Clemons et al21 used a human dermis graft to treat advanced recurrent cystocele in 33 women, with a follow-up of 18 months. They noted 13 (41%) objective failures and 1 (3%) subjective failure. Complications included 1 case of febrile morbidity, 1 cystotomy, and 1 anterior wall breakdown secondary to hematoma formation caused by heparin therapy. No other erosions or rejections were seen.
Most effective applications
Graft/mesh augmentation may be most effective in posterior vaginal segment reconstruction and rectocele repair, as it obviates the need for levatorplasty in patients with poor rectovaginal fascia. A variety of synthetic materials have been used for posterior wall reconstruction in small series.
Mersilene mesh
Mersilene mesh (Ethicon) was used by Fox and Stanton22 to augment traditional rectocele repair in 29 women followed for 14 months. Most of the women had undergone previous rectocele repair with recurrence of their prolapse. All women with stage II and stage III vault prolapse were corrected, with an increase in stage I prolapse from 20% to 27%. All women with stage II and stage III rectocele were corrected, with a decrease in stage I prolapse from 36% to 7%. The only significant intraoperative complication was a cystotomy. One mesh became infected postoperatively, requiring removal.
Cadaveric dermal graft
Recently, Kohli and Miklos23 reported their experience with 57 patients undergoing augmented rectocele repair using a cadaveric dermal graft (FIGURE 3) over a 2-year period. Average follow-up was 11 months. Average patient age in the follow-up group was 63.6±10.9 years (range: 33–79 years) and average parity was 2.8±1.5 (range: 0–7). No major intraoperative complications (hollow viscous injury, blood loss greater than 500 cc, or transfusion) or post-operative complications (infection, abscess, or hematoma) were noted. No graft-related complications such as rejection, erosion, infection, and fistula formation were noted during the follow-up period.
Using previously accepted Pelvic Organ Prolapse Quantification (POP-Q) parameters for success, Kohli and Miklos found 54 of 57 women (95%) to have surgical cure at follow-up. These authors have also described the use of a dermal graft in the repair of a complicated rectovaginal fistula.24
FIGURE 3 Placement of mesh augmentation
Augment enterocele/rectocele repair by attaching the mesh apically, laterally, and distally (copyright Miklos/Kohli).
Future outlook
Further data on the efficacy and safety of graft/mesh augmentation—including identification of the optimal technique and ideal material—are necessary before this approach can be widely adopted. However, in selected patients, it represents a significant advance in the surgical treatment of pelvic prolapse.
The author is a preceptor for American Medical Systems, Gynecare, CR Bard, and Mentor and a consultant for Boston Scientific.
After several decades of slow progress, the field of urogynecology is experiencing dynamic change, including:
- new minimally invasive, tension-free, midurethral sling procedures, especially the transobturator approach,
- correction of size-specific defects to repair prolapse, and
- use of mesh/graft augmentation in pro lapse repair.
These developments are some of the most important since Kelly and Dunn first described suburethral fascial plication for stress incontinence and cystocele in 1914.1 They have come about through increased understanding of the pathophysiology of incontinence and prolapse, innovative technology and techniques, and improved communication and coordination among physicians worldwide.
New sling procedures, promising outcomes
The minimally invasive midurethral sling procedure spawned notable new approaches and is a mainstay of surgical treatment for stress urinary incontinence.
First described in Sweden by Ulmsten in 1995,2 the tension-free vaginal tape procedure is a revolutionary change in the suburethral sling procedure and is now the most widely performed surgery for stress incontinence worldwide.
In it, a tension-free vaginal tape (TVT) (Gynecare, Somerville, NJ) of synthetic polypropylene mesh is attached to 2 needles and passed through a vaginal incision and the retropubic space, exiting to small incisions in the suprapubic region to create a suburethral sling or hammock and provide urethral support during increased abdominal pressure. The sling remains fixed by friction and subsequent adhesions.
Although the traditional suburethral sling was less invasive than other abdominal incontinence procedures, it was associated with a steep learning curve and a high incidence of postoperative irritative bladder symptoms and voiding dysfunction.3
Tension-free vaginal tape: Excellent long-term cure
It can be performed routinely in under 30 minutes using local anesthesia, with minimal postoperative complications. Five-year cure rates approach 95%,4 and data presented at the 2003 International Urogynecology Association clinical meeting describe an objective 7-year cure rate of 82%.5
The incidence and severity of postoperative voiding dysfunction following the TVT procedure is significantly lower than that reported after traditional suburethral sling (2%–40%) or transvaginal needle suspension (2%–50%) procedures.6-9 Although bladder perforation has occurred in up to 10% of patients, reports of complications, including major hemorrhage, tape erosion, and bowel and nerve injury, are rare.10,11
Modifications
These products have a modified approach, materials, or refinements in technique to address various needs. For example, American Medical Systems (Minnetonka, Minn) introduced the SPARC procedure, which allows abdominal placement of the midurethral sling, similar to a needle suspension technique.
CR Bard (Murray Hill, NJ) introduced a midurethral sling of porcine dermis (Pelvicol) to address concerns physicians may have about using synthetic materials.
Minimally invasive midurethral slings include the Advantage (Boston Scientific, Natick, Mass), Centrasorb (Caldera Medical, Thousand Oaks, Calif), Stratasis TF (Cook, West Lafayette, Ind), and Uretex (CR Bard).
Newest approach: Transobturator sling
In this technique, the sling is placed in the midurethral position, but the insertion points are in the genital area lateral to the vagina, and the needle passes through the obturator membrane and paraurethral space. Because it avoids passage of the needles through the retropubic space, the transobturator approach theoretically should reduce the risk of bowel, bladder, and major blood vessel injury.
The procedure was initially described in Europe and introduced in the United States in 2003. Current product offerings include the outside-in approach of ObTape (Mentor, Santa Barbara, Calif), Monarc (American Medical Systems), and Uretex (CR Bard). Gynecare offers a variation of its TVT—the TVT-Obturator—which involves an inside-out approach to further minimize risk of vascular injury.
Shortage of long-term data, but good early results
In a 1-year follow-up of patients undergoing a sling procedure with the UraTape transobturator sling (Mentor), Delorme and colleagues12 reported that 29 of 32 patients (90.6%) were cured and 3 (9.4%) improved. De Leval13 described his inside-out approach with equally good results: no bladder or urethral injuries and no vascular (hematoma or bleeding) or neurological complications. A transobturator technique using porcine dermis has also been described.
Tension-free vaginal tape versus transobturator sling
In 2004, a small randomized, prospective trial of TVT (n = 29) versus transobturator tape (n = 27) with 1-year follow-up found the transobturator approach to be safer and easier to place with equivalent short-term results.14 Mean operative time was significantly shorter in the transobturator group (15±4 minutes versus 27±8 minutes, P <.001).
No bladder injury occurred in the transobturator group versus 9.7% (n = 3) in the TVT group (P >.05). The rate of postoperative urinary retention was 25.8% (n = 8) in the TVT group versus 13.3% (n = 4) in the transobturator group (P >.05). Cure rates were similar for the TVT and transobturator groups: 83.9% versus 90%, respectively), improvement (9.7% versus 3.3%), and failure (6.5% versus 6.7%). No vaginal erosion occurred in either group.
Additional investigations of the transobturator tape procedure are underway.
Correcting site-specific defects in prolapse repair
This repair rationale should become the standard, although it has yet to be widely adopted and procedural refinement and research are continuing.
As early as 1908, site-specific defects in the endopelvic fascia were identified as the likely cause of anterior vaginal segment prolapse. Like hernia repair, which requires closure of the fascial defect, the “cystocele hernia” repair advocated by George White involved reattaching the endopelvic fascia to the arcus tendineus fascia pelvis using a series of interrupted sutures through an abdominal retropubic approach.
Although this view was later abandoned, it resurfaced in the 1980s, when Cullen Richardson described midline, lateral, and transverse defects (FIGURE 1) in the endopelvic fascia as the cause of cystocele and rectocele.15 Richardson advocated diagnosis that identified these fascial defects, along with treatment with site-specific repair.
(In the intervening decades, prolapse was thought to result from generalized weakening or attenuation of the endopelvic fascia that supports the bladder, rectum, or vagina, leading to cystocele, rectocele, or uterine prolapse, respectively. Traditional repairs, still widely performed by most gynecologists, consist of the anterior and posterior colporrhaphy, which involve midline plication of the endopelvic fascia to reduce the prolapse and recreate support by strengthening the weakened fascial layer.)
FIGURE 1 Identify the site of defect
Whether the defect that causes a rectocele or cystocele is transverse, central, or lateral determines the type of repair to be performed. Reprinted with permission from Richardson AC. The rectovaginal septum revisited: its relationship to rectocele and its importance in rectocele repair. Clin Obstet Gynecol.1993;36(4):976–983.
Excellent cure rates with fewer complications
Site-specific repair (FIGURE 2) has been adopted by many pelvic surgeons, who report excellent cure rates with fewer complications such as dyspareunia, vaginal narrowing, and increased blood loss—all of which are more common with traditional anterior and posterior colporrhaphy.
Traditional colporrhaphy does correct the underlying fascial defect when the “hernia” is in the midline. However, for lateral and transverse defects, traditional colporrhaphy leaves the defect uncorrected and may even create additional tension, resulting in recurrence.
Site-specific repair also is useful in the treatment of enterocele, which has been described as a herniation of the peritoneum through a defect of the anterior and posterior fascial planes at the vaginal apex.16
Widening but not broad acceptance
Although cure rates for site-specific prolapse repair range from 75% to 85%,17,18 the concept has yet to be widely adopted and continues to undergo procedural refinement and research, as well as physician education. With increasing experience and clinical data, it may become the standard for pelvic prolapse repair.
FIGURE 2 Repair the defect to correct the rectocele
Surgical technique for a transverse tear in the rectovaginal fascia: After identifying the defect, place a series of interrupted sutures to reapproximate the fascial edges (copyright Miklos/Kohli).
Mesh augmentation: Useful in selected patients
Although augmentation with mesh or biomaterials is easy to perform, it remains unclear which technique and materials are optimal.
The use of mesh and biomaterials to augment repair of a cystocele, rectocele, or enterocele is slowly increasing, although general surgeons have been utilizing these materials for many years in hernia repair.
Augmentation has been advocated for “pelvic hernias” because of poor long-term cure rates for traditional prolapse surgeries (which range from 40% to 80%—well behind rates of 90% or more for incontinence procedures).
A recent informal survey of the members of the American Urogynecologic Society and International Urogynecologic Association revealed that most pelvic surgeons are using some type of graft or mesh to augment repairs in selected patients.
Synthetic mesh versus autologous and heterologous grafts
Synthetic materials have the advantage of being readily available, cost-effective, and consistent in quality, but they may cause significant complications, including infection, stricture, and erosion. This is especially important in the vagina, which needs to stretch during intercourse and which alters in thickness and other properties during a woman’s lifetime.
In contrast, autologous and heterologous donor grafts provide naturally occurring biomaterials capable of remodeling. Unfortunately, the in vivo tissue response is not yet fully understood. Other disadvantages: Biomaterials may lack consistent tissue properties and can be expensive.
For these reasons, graft materials remain in an early period of evaluation, although their use is expected to rise steadily with increasing experience and new product development.
Limited data on safety and efficacy
Although many pelvic surgeons use graft/mesh materials in prolapse repair, data on their safety and efficacy are limited, partly due to the variety of surgical techniques and materials available. Another factor is the difficulty of obtaining good long-term data with large patient numbers. Most of the literature is comprised of case reports, with few prospective, randomized trials.
That said, initial data suggest a significant improvement in cure rates, compared with traditional techniques, with minimal short-term complications. Long-term results and complication rates are not yet available.
What existing studies show
A variety of synthetic materials have been used in surgical correction of cystocele. In the largest series to date, Flood and colleagues19 reported their 12-year experience with 142 women undergoing a modified anterior colporrhaphy reinforced with Marlex (Davol, Cranston, RI) mesh: 100% success in correcting cystocele and a 74% success rate for urinary stress incontinence, with a mean follow-up of 3.2 years and no significant intraoperative complications.
In a prospective randomized trial of 125 patients utilizing absorbable polyglactin 910 mesh (Vicryl) (Ethicon, Somerville, NJ) to augment standard anterior colporrhaphy, Koduri et al20 reported a failure rate of 13% in the colporrhaphy-alone group compared to 1% in the colporrhaphy-mesh group at the 1-year follow-up. Subjectively, both groups improved equally.
Clemons et al21 used a human dermis graft to treat advanced recurrent cystocele in 33 women, with a follow-up of 18 months. They noted 13 (41%) objective failures and 1 (3%) subjective failure. Complications included 1 case of febrile morbidity, 1 cystotomy, and 1 anterior wall breakdown secondary to hematoma formation caused by heparin therapy. No other erosions or rejections were seen.
Most effective applications
Graft/mesh augmentation may be most effective in posterior vaginal segment reconstruction and rectocele repair, as it obviates the need for levatorplasty in patients with poor rectovaginal fascia. A variety of synthetic materials have been used for posterior wall reconstruction in small series.
Mersilene mesh
Mersilene mesh (Ethicon) was used by Fox and Stanton22 to augment traditional rectocele repair in 29 women followed for 14 months. Most of the women had undergone previous rectocele repair with recurrence of their prolapse. All women with stage II and stage III vault prolapse were corrected, with an increase in stage I prolapse from 20% to 27%. All women with stage II and stage III rectocele were corrected, with a decrease in stage I prolapse from 36% to 7%. The only significant intraoperative complication was a cystotomy. One mesh became infected postoperatively, requiring removal.
Cadaveric dermal graft
Recently, Kohli and Miklos23 reported their experience with 57 patients undergoing augmented rectocele repair using a cadaveric dermal graft (FIGURE 3) over a 2-year period. Average follow-up was 11 months. Average patient age in the follow-up group was 63.6±10.9 years (range: 33–79 years) and average parity was 2.8±1.5 (range: 0–7). No major intraoperative complications (hollow viscous injury, blood loss greater than 500 cc, or transfusion) or post-operative complications (infection, abscess, or hematoma) were noted. No graft-related complications such as rejection, erosion, infection, and fistula formation were noted during the follow-up period.
Using previously accepted Pelvic Organ Prolapse Quantification (POP-Q) parameters for success, Kohli and Miklos found 54 of 57 women (95%) to have surgical cure at follow-up. These authors have also described the use of a dermal graft in the repair of a complicated rectovaginal fistula.24
FIGURE 3 Placement of mesh augmentation
Augment enterocele/rectocele repair by attaching the mesh apically, laterally, and distally (copyright Miklos/Kohli).
Future outlook
Further data on the efficacy and safety of graft/mesh augmentation—including identification of the optimal technique and ideal material—are necessary before this approach can be widely adopted. However, in selected patients, it represents a significant advance in the surgical treatment of pelvic prolapse.
The author is a preceptor for American Medical Systems, Gynecare, CR Bard, and Mentor and a consultant for Boston Scientific.
1. Kelly HA, Dunn WM. Urinary incontinence in women without manifest injury to the bladder. Surg Gynecol Obstet. 1914;18:444.-
2. Ulmsten U, Petros P. Intravaginal slingplasty (IVS): an ambulatory surgical procedure for treatment of female urinary incontinence. Scand J Urol Nephrol. 1995;29:75-82.
3. Weinberger MW, Ostergard DR. Postoperative catheterization, urinary retention, and permanent voiding dysfunction after polytetrafluroethylene suburethral sling placement. Obstet Gynecol. 1996;87:50-54.
4. Nilsson CG, Kuuva N, Falconer C, Rezapour M, Ulmsten U. Long-term results of the tension-free vaginal tape (TVT) procedure for surgical treatment of female stress urinary incontinence. Int Urogynecol J Pelvic Floor Dysfunct. 2001;12(Suppl 2):S5-8.
5. Nilsson CG, Rezapour M, Falconer C. Seven years follow-up of the tension-free vaginal tape (TVT) procedure. Presented at the 2003 International Urogynecology Association Annual Meeting.
6. Horbach N. Suburethral sling procedures. In: Ostergard DR, Bent AE, eds. Urogynecology and Urodynamics: Theory and Practice. 3rd ed. Baltimore: Williams & Wilkins; 1991;449-458.
7. Stanton SL, Reynolds SF, Creighton SM. The modified Pereyra (Raz) procedure for genuine stress incontinence—a useful option in the elderly or frail patient? Int Urogynecol J. 1995;6:22-25.
8. Nygaard IE, Kreder KJ. Complications of incontinence surgery. Int Urogynecol J. 1994;5:353-360.
9. Lockhart vJL, Tirado A, Morillo G, Politano VA. Vesicourethral dysfunction following cystourethropexy. J Urol. 1982;128:943-945.
10. Leboeuf L, Tellez CA, Ead D, Gousse AE. Complication of bowel perforation during insertion of tension-free vaginal tape. J Urol. 2003;170(4 Pt 1):1310.-
11. Abouassaly R, Steinberg JR, Lemieux M, et al. Complications of tension-free vaginal tape surgery: a multi-institutional review. BJU Int. 2004;94:110-113.
12. Delorme E, Droupy S, de Tayrac R, Delmas V. [Transobturator tape (UraTape). A new minimally invasive method in the treatment of urinary incontinence in women.] Prog Urol. 2003;13:656-659.
13. de Leval J. Novel surgical technique for the treatment of female stress urinary incontinence: transobturator vaginal tape inside-out. Eur Urol. 2003;44:724-730.
14. De Tayrac R, Deffieux X, Droupy S, ChauveaudLambling A, Calvanese-Benamour L, Fernandez H. A prospective randomized trial comparing tensionfree vaginal tape and transobturator suburethral tape for surgical treatment of stress urinary incontinence. Am J Obstet Gynecol. 2004;190:602-608.
15. Richardson AC. The rectovaginal septum revisited: its relationship to rectocele and its importance in rectocele repair. Clin Obstet Gynecol. 1993;36:976-983.
16. Miklos JR, Kohli N, Lucente V, Saye WB. Site-specific fascial defects in the diagnosis and surgical management of enterocele. Am J Obstet Gynecol. 1998;179 (6 Pt 1):1418-1422; discussion 1422-823.
17. Porter WE, Steele A, Walsh P, Kohli N, Karram MM. The anatomic and functional outcomes of defectspecific rectocele repairs. Am J Obstet Gynecol. 1999;181:1353-1358; discussion 1358-1359.
18. Kenton K, Shott S, Brubaker L. Outcome after rectovaginal fascia reattachment for rectocele repair. Am J Obstet Gynecol. 1999;181:1360-1363; discussion 1363-1364.
19. Flood CG, Drutz HP, Waja L. Anterior colporrhaphy reinforced with Marlex mesh for the treatment of cystoceles. Int Urogynecol J Pelvic Floor Dysfunct. 1998;9:200-204.
20. Koduri S, Lobel RW, Winkler HA, Tomezsko J, Culligan PJ, Sand PK. Prospective randomized trial of polyglactin 910 mesh to prevent recurrence of cystoceles and rectoceles. Proceedings of the 25th Annual Meeting of the International Urogynecological Association, 2000. Int Urogynecol J Pelvic Floor Dysfunct. 2000;11:S80.-
21. Clemons JL, Myers DL, Aguilar VC, Arya LA. Vaginal paravaginal repair with an AlloDerm graft. Am J Obstet Gynecol. 2003;189:1612-1618; discussion 1618-1619.
22. Fox SD, Stanton SL. Vault prolapse and rectocele: assessment of repair using sacrocolpopexy with mesh interposition. BJOG. 2000;107:1371-1375.
23. Kohli N, Miklos JR. Site specific rectocele repair augmented with a cadaveric dermal graft. Proceedings of the 21st Annual Scientific Meeting of the American Urogynecologic Society, 2000. Int Urogynecol J Pelvic Floor Dysfunct. 2001;12:S9.-
24. Miklos JR, Kohli N. Rectovaginal fistula repair utilizing a cadaveric dermal allograft. Int Urogynecol J Pelvic Floor Dysfunct. 1999;10:405-406.
1. Kelly HA, Dunn WM. Urinary incontinence in women without manifest injury to the bladder. Surg Gynecol Obstet. 1914;18:444.-
2. Ulmsten U, Petros P. Intravaginal slingplasty (IVS): an ambulatory surgical procedure for treatment of female urinary incontinence. Scand J Urol Nephrol. 1995;29:75-82.
3. Weinberger MW, Ostergard DR. Postoperative catheterization, urinary retention, and permanent voiding dysfunction after polytetrafluroethylene suburethral sling placement. Obstet Gynecol. 1996;87:50-54.
4. Nilsson CG, Kuuva N, Falconer C, Rezapour M, Ulmsten U. Long-term results of the tension-free vaginal tape (TVT) procedure for surgical treatment of female stress urinary incontinence. Int Urogynecol J Pelvic Floor Dysfunct. 2001;12(Suppl 2):S5-8.
5. Nilsson CG, Rezapour M, Falconer C. Seven years follow-up of the tension-free vaginal tape (TVT) procedure. Presented at the 2003 International Urogynecology Association Annual Meeting.
6. Horbach N. Suburethral sling procedures. In: Ostergard DR, Bent AE, eds. Urogynecology and Urodynamics: Theory and Practice. 3rd ed. Baltimore: Williams & Wilkins; 1991;449-458.
7. Stanton SL, Reynolds SF, Creighton SM. The modified Pereyra (Raz) procedure for genuine stress incontinence—a useful option in the elderly or frail patient? Int Urogynecol J. 1995;6:22-25.
8. Nygaard IE, Kreder KJ. Complications of incontinence surgery. Int Urogynecol J. 1994;5:353-360.
9. Lockhart vJL, Tirado A, Morillo G, Politano VA. Vesicourethral dysfunction following cystourethropexy. J Urol. 1982;128:943-945.
10. Leboeuf L, Tellez CA, Ead D, Gousse AE. Complication of bowel perforation during insertion of tension-free vaginal tape. J Urol. 2003;170(4 Pt 1):1310.-
11. Abouassaly R, Steinberg JR, Lemieux M, et al. Complications of tension-free vaginal tape surgery: a multi-institutional review. BJU Int. 2004;94:110-113.
12. Delorme E, Droupy S, de Tayrac R, Delmas V. [Transobturator tape (UraTape). A new minimally invasive method in the treatment of urinary incontinence in women.] Prog Urol. 2003;13:656-659.
13. de Leval J. Novel surgical technique for the treatment of female stress urinary incontinence: transobturator vaginal tape inside-out. Eur Urol. 2003;44:724-730.
14. De Tayrac R, Deffieux X, Droupy S, ChauveaudLambling A, Calvanese-Benamour L, Fernandez H. A prospective randomized trial comparing tensionfree vaginal tape and transobturator suburethral tape for surgical treatment of stress urinary incontinence. Am J Obstet Gynecol. 2004;190:602-608.
15. Richardson AC. The rectovaginal septum revisited: its relationship to rectocele and its importance in rectocele repair. Clin Obstet Gynecol. 1993;36:976-983.
16. Miklos JR, Kohli N, Lucente V, Saye WB. Site-specific fascial defects in the diagnosis and surgical management of enterocele. Am J Obstet Gynecol. 1998;179 (6 Pt 1):1418-1422; discussion 1422-823.
17. Porter WE, Steele A, Walsh P, Kohli N, Karram MM. The anatomic and functional outcomes of defectspecific rectocele repairs. Am J Obstet Gynecol. 1999;181:1353-1358; discussion 1358-1359.
18. Kenton K, Shott S, Brubaker L. Outcome after rectovaginal fascia reattachment for rectocele repair. Am J Obstet Gynecol. 1999;181:1360-1363; discussion 1363-1364.
19. Flood CG, Drutz HP, Waja L. Anterior colporrhaphy reinforced with Marlex mesh for the treatment of cystoceles. Int Urogynecol J Pelvic Floor Dysfunct. 1998;9:200-204.
20. Koduri S, Lobel RW, Winkler HA, Tomezsko J, Culligan PJ, Sand PK. Prospective randomized trial of polyglactin 910 mesh to prevent recurrence of cystoceles and rectoceles. Proceedings of the 25th Annual Meeting of the International Urogynecological Association, 2000. Int Urogynecol J Pelvic Floor Dysfunct. 2000;11:S80.-
21. Clemons JL, Myers DL, Aguilar VC, Arya LA. Vaginal paravaginal repair with an AlloDerm graft. Am J Obstet Gynecol. 2003;189:1612-1618; discussion 1618-1619.
22. Fox SD, Stanton SL. Vault prolapse and rectocele: assessment of repair using sacrocolpopexy with mesh interposition. BJOG. 2000;107:1371-1375.
23. Kohli N, Miklos JR. Site specific rectocele repair augmented with a cadaveric dermal graft. Proceedings of the 21st Annual Scientific Meeting of the American Urogynecologic Society, 2000. Int Urogynecol J Pelvic Floor Dysfunct. 2001;12:S9.-
24. Miklos JR, Kohli N. Rectovaginal fistula repair utilizing a cadaveric dermal allograft. Int Urogynecol J Pelvic Floor Dysfunct. 1999;10:405-406.
Meeting the challenge of vesicovaginal fistula repair: Conservative and surgical measures
- Surgical risk factors include prior pelvic surgery, history of pelvic inflammatory disease, pelvic malignancy, endometriosis, infection, diabetes, and anatomic distortion.
- Conservative therapy should be reserved for simple fistulae that are less than 1 cm in size, diagnosed within 7 days of the index surgery, lacking associated carcinoma or radiation, and subject to at least 4 weeks of constant bladder drainage.
- In surgical repair, the Latzko partial colpocleisis or fistulectomy with flap-splitting closure is preferred.
Recent advances have improved the success of vesicovaginal fistula (VVF) repair—a challenge that can test even the most experienced gynecologic surgeon. For example, it now is apparent that some small uncomplicated fistulae respond to conservative treatment. Further, in selected cases, laparoscopic repair can eliminate the need for complicated laparotomy.
In addition, timing of fistula repair no longer requires long periods of observation, and good surgical technique for identifying and repairing bladder injuries at the time of the index surgery can often prevent the development or reduce the severity of VVF.
Vesicovaginal fistula is the most common type of urogenital fistula. Presentation and prognosis vary, depending on location and size of the defect, as well as coexisting factors such as tissue devascularization and previous radiation. However, surgical repair is associated with a high cure rate if it is performed by an experienced surgeon.
Most US cases follow gynecologic surgery
Vesicovaginal fistula was first documented in the mummified remains of Egyptian Queen Henhenit (11th Dynasty, 2050 BC), which were examined in 1923 by Derry.1 Although the exact incidence of VVF in the United States is unknown, the primary cause is gynecologic surgery, especially hysterectomy. The defect is estimated to occur in 0.01% to 0.04% of gynecologic procedures.
A study of 303 women with genitourinary fistula found that the defect was related to gynecologic surgery in 82% of cases, obstetric events in 8%, radiation therapy in 6%, and trauma or fulguration in 4%.2 Rare causes of VVF include lymphogranuloma venereum, tuberculosis, syphilis, bladder stones, and a retained foreign body in the vagina. In rare instances, spontaneous vesicouterine fistulae were reported following uncomplicated vaginal birth after cesarean section.3
Gynecologic surgery may lead to VVF due to extensive dissection between the bladder and the uterus, unrecognized bladder laceration, inappropriate stitch placement, and/or devascularization injury to the tissue planes. Concurrent ureteric involvement has been reported in as many as 10% to 15% of vesicovaginal fistula cases.
In developing countries, vesicovaginal fistulae are far more common and generally related to obstetric factors such as obstructed labor (due to unattended deliveries), small pelvic dimensions, malpresentation, poor uterine contractions, and introital stenosis.
Risk factors. Conditions that may predispose patients to VVF include prior pelvic surgery, a history of pelvic inflammatory disease, pelvic malignancy, endometriosis, infection, diabetes, and anatomic distortion. If these risk factors are present, the patient should be counseled accordingly prior to gynecologic surgery.
Correct classification crucial to surgical success
Proper classification of VVF can help the gynecologic surgeon plan operative intervention. Obstetric vesicovaginal fistulae usually are categorized according to their cause, complexity, and site of obstruction. In contrast, gynecologic fistulae are generally classified as simple or complicated (TABLE).
These levels may have important implications for the surgical approach and prognosis.4 For example, simple vesicovaginal fistulae are usually uncomplicated surgical cases with good prognosis. Complicated vesicovaginal fistulae, on the other hand, can challenge even highly practiced and skilled gynecologic surgeons and are associated with a high rate of recurrence.
Women typically present within specific intervals after the various antecedent events (pelvic surgery, childbirth, radiation therapy) with a primary complaint of constant, painless urinary incontinence. If the fistula is related to traumatic childbirth, most patients experience urine leakage within the first 24 to 48 hours. Following pelvic surgery, symptoms usually occur within the first 30 days. In contrast, radiation-induced fistulae develop over a much longer interval secondary to progressive devascularization necrosis, and may present 30 days to 30 years after the antecedent event.
Some patients report exacerbation during physical activities, which can sometimes lead to erroneous diagnosis of uncomplicated stress incontinence. If the fistula is small, intermittent leakage with increased bladder distention or physical activity may be noted.
Other patients may complain of vaginal discharge or hematuria.
If there is concurrent ureteric involvement, the patient may experience constitutional symptoms (such as fever, chills, and flank pain) or even gastrointestinal symptoms.
Physical findings. Any pooling of fluid in the vagina that is noted should be sent for analysis if the diagnosis is unclear. Next, perform a careful speculum exam that allows visualization of the entire anterior vaginal wall to identify the fistula tract (FIGURE 1). In many cases, the fistula is grossly visible.
Determine the location of the fistula in relation to the vaginal apex and bladder trigone and assess the quality of surrounding tissue (eg, presence of inflammation, edema, or infection). Fistulas near the vaginal apex may require a more complicated abdominal approach, and those close to the trigone may be associated with increased risk of ureteral injury during repair.
If the fistula is particularly small, no tract may be apparent. In such cases, bimanual exam with careful palpation of the anterior wall may help isolate the fistula (eg, when there is a surrounding zone of induration).
Office tests. If no fistula is noted despite highly suspicious signs and symptoms and careful examination, a simple office test can be performed. Using a catheter, fill the bladder with a dyed solution such as normal saline with indigo carmine and repeat the pelvic exam with a half-speculum to visualize the anterior wall. Ask the patient to cough and bear down, and identify the fistula by visualizing urine leakage.
If this test fails to locate the fistula, insert a tampon and ask the patient to perform 10 to 15 minutes of exertional maneuvers, including stair climbing and jumping in place. Then remove the tampon. Visualization of dye beyond the most distal edge of the tampon confirms the presence of a fistula.
A variation of this technique is the double-dye test: Give the patient oral phenazopyridine (Pyridium), fill the bladder with the blue-tinted solution, and insert a tampon. The presence of blue staining suggests vesicovaginal or urethrovaginal fistula, while red staining (Pyridium) suggests ureterovaginal fistula.
Other testing. Further assessment is recommended to rule out concurrent pathology and formulate an appropriate treatment plan. Routine testing should include a urinalysis and culture to exclude coexisting urinary tract infection, an electrolyte panel to evaluate renal function, and a complete blood cell count to rule out systemic infection.
Cystoscopy should be performed to visualize the fistulous tract, assess its location in relation to the ureters and trigone, assure bilateral ureteral patency, and exclude the presence of a foreign body or suture in the bladder.
In patients with a history of urogenital malignancy, biopsy of the fistula tract and urine cytology is warranted.
Comparable success rates have been reported for early and late repair of surgery-induced fistulae.
Radiologic studies are recommended prior to surgical repair of a vesicovaginal fistula to fully assess the defect and exclude the presence of multiple fistulae. An intravenous pyelogram is helpful to exclude concurrent ureterovaginal fistulae or ureteral obstruction. A targeted fistulogram may be indicated if conservative therapy is planned, including expectant management, continuous bladder drainage, fulguration, or fibrin occlusion.
FIGURE 1 Vesicovaginal fistula
Pelvic cross section depicting high vesicovaginal fistula.TABLE
Classification of vesicovaginal fistulae17
| CLASSIFICATION | DESCRIPTION |
|---|---|
| Simple |
|
| Complicated |
|
Indications for conservative management
Because spontaneous closure is uncommon, symptomatic VVF merits treatment. Appropriate therapy depends on various factors, including fistula size and location, timing from the antecedent event, severity of symptoms, quality of surrounding tissue, and clinician experience and surgical skills.
Occasionally, a fistula heals following prolonged bladder drainage through a transurethral or suprapubic catheter—provided it is diagnosed within a few days of surgery. Zimmern5 recommends a conservative approach to small fistulae if the patient’s complaints of urinary incontinence are resolved with insertion of a Foley catheter. In this case, bladder drainage should be continued for 3 weeks, followed by reevaluation of the fistula. If the fistula has diminished in size, an additional 3 weeks of catheter drainage is associated with a high rate of spontaneous closure; if there is no change, the fistula is unlikely to resolve spontaneously.5
Varying success rates have been reported for conservative management, ranging from 2% to 80%.4,6 The chances of success are apparently greater if the fistula is:
- diagnosed within 7 days of the index surgery,
- less than 1 cm in size,
- simple, without associated carcinoma or radiation, and
- subject to at least 4 weeks of constant bladder drainage.
Persistent, large, or complex fistulae are best treated surgically.
Indications for surgical management
The basic principles of fistula closure apply. They are:
- adequate exposure,
- good hemostasis,
- wide mobilization of the bladder and vagina,
- resection of devascularized tissue,
- removal of any foreign bodies,
- tension-free closure,
- nonopposition of suture lines,
- confirmation of a water-tight seal on bladder closure, and
- bladder drainage for 10 to 14 days following the repair.
Timing of the repair has been the subject of controversy and can pose a dilemma to physician and patient alike. Traditionally, an interval of 3 months was recommended between the index surgery and fistula repair, with a delay of up to 1 year when the fistula was radiation-induced. However, little data support these recommendations.
Today most experts recommend an individualized approach, delaying the surgery until inflammation and infection of the surrounding tissue have resolved. The use of estrogen, antibiotics, or steroids to facilitate healing during this period also has been recommended.7 Comparable success rates have been reported for early and late repair of surgery-induced fistulae based on these principles.8-10
Vaginal approach. Most vesicovaginal fistulae can be surgically corrected using a vaginal approach. Traditionally, a Latzko partial colpocleisis or fistulectomy with flap-splitting closure has been advocated.
Debate continues about whether resection of the fistulous tract is necessary. Some experts believe that wide resection increases the size of the fistula and, therefore, the risk of recurrence. They also maintain that the fibrous tissue surrounding the fistula helps to reinforce the surgical repair. Proponents of fistulectomy counter that resection of the fistula and exposure of healthy tissue optimizes wound healing and improves surgical success rates. Comparable success has been reported for both techniques.11,12
We prefer an individualized approach, with minimal resection of the fistulous tract to simplify the procedure and minimize associated complications, including recurrence.
- Latzko partial colpocleisis. This technique, first reported in 1942, remains a common procedure, with success rates of 90% to 100%.13 Advantages include a short operative time, low intraoperative and postoperative morbidity, and low risk of ureteral injury.
- Fistulectomy technique. Alternatively, to perform fistulectomy with a flap-splitting closure, begin by resecting the fistulous tract to expose healthy tissue at the wound margins. Then close the defect in a multilayer fashion, beginning with the bladder mucosa, bladder serosa, pubocervical fascia, and vaginal mucosa. Be careful to avoid tension on suture lines. In addition, create a fascial flap to prevent opposition of the incision planes and reduce the risk of recurrence.
- Grafts. In cases with a high risk of recurrence, such as complex or large fistulae, a Martius fat-pad graft should be interposed between the closure layers to promote vascularization and reduce the risk of recurrence.14 Placement of a cadaveric biomaterial graft also has been reported, reducing the need for complicated flap procedures.15
Abdominal approach. Although most vesicovaginal fistulae can be surgically corrected via the vaginal approach, the abdominal route may be preferred when the fistula is high and inaccessible, large and complex, multiple in number, or when there is concurrent uterine or bowel involvement or a need for ureteral reimplantation. The abdominal approach may be facilitated by cystoscopically guided placement of a catheter through the fistulous tract to assist in subsequent identification and dissection.
To begin, make a vertical skin incision to optimize visualization and allow mobilization of an omental flap, if necessary. Expose the bladder and perform a high extraperitoneal cystotomy to visualize the fistulous tract. Place ureteral stents if the fistula is in close proximity to the ureteral orifice.
Extend the bladder incision to the fistulous tract and completely excise it following mobilization of the vagina. Then close the vagina and bladder with interrupted, delayed absorbable suture in a double layer.
Transpose an omental flap between the vaginal and bladder incisions to promote vascularization, minimize opposition of suture lines, and reduce the risk of recurrence (FIGURE 2).
Laparoscopic approach. A similar laparoscopic repair has been reported with comparable results, but requires advanced skills with endoscopic suturing and knot tying (FIGURE 3).16
FIGURE 2 Abdominal repair
Abdominal repair of vesicovaginal fistula, with closure of bladder defect and posterior cystotomy and separate closure of vaginal defect. Note the omental flap pictured in the insert.
FIGURE 3 Laparoscopic repair
Laparoscopic repair of vesicovaginal fistula.The authors report no financial relationship with any companies whose products are mentioned in this article.
1. Kremling H. Labor-induced bladder injuries: historical observations. Gynakol Geburtshilfliche Rundsch. 1996;36:197-200.
2. Lee RA, Symmonds RE, Williams TJ. Current state of genitourinary fistula. Obstet Gynecol. 1998;72:313-315.
3. Miklos JR, Sze EHM, Parobeck D, et al. Vesicouterine fistula: a rare complication of vaginal birth after cesarean. Obstet Gynecol. 1995;86:638-639.
4. Elkins TE, Thompson JR. Lower urinary tract fistulas. In: Walters M, Karram MM, eds. Urogynecology and Reconstructive Pelvic Surgery. 2nd ed. St. Louis, Mo: Mosby; 1999;355-365.
5. Zimmern PE, Hadley HR, Staskin D. Genitourinary fistulas: vaginal approach for repair of vesicovaginal fistulas. Clin Obstet Gynaecol. 1985;12:403-413.
6. Davits RJ, Miranda SI. Conservative treatment of vesicovaginal fistulas by bladder drainage alone. Br J Urol. 1991;68:155-156.
7. Margolis T, Mercer LJ. Vesicovaginal fistula. Obstet Gynecol Surv. 1995;49:840-847.
8. Blaivas JG, Heritz DM, Romanzi LJ. Early versus late repair of vesicovaginal fistulas: vaginal and abdominal approaches. J Urol. 1995;153:1110-1112.
9. Blandy JP, Badenoch DF, Fowler CG. Early repair of iatrogenic injury to the ureter or bladder after gynecological surgery. J Urol. 1991;146:761-765.
10. Cruikshank SH. Early closure of posthysterectomy vesicovaginal fistulas. South Med J. 1988;81:1525-1528.
11. Raz S, Bregg KJ, Nitti VW. Transvaginal repair of vesicovaginal fistula using a peritoneal flap. J Urol. 1993;150:56-59.
12. Iselin CE, Aslan P, Webster GD. Transvaginal repair of vesicovaginal fistulas after hysterectomy by vaginal cuff excision. J Urol. 1998;160(3 Pt 1):728-730.
13. Latzko W. Postoperative vesicovaginal fistulas: genesis and therapy. Am J Surg. 1942;58:211-218.
14. Punekar SV, Buch DN, Soni AB. Martius’ labial fat pad interposition and its modification in complex lower urinary fistulae. J Postgrad Med. 1999;10:405-406.
15. Miklos JR, Kohli N. Rectovaginal fistula repair utilizing a cadaveric dermal allograft. Int Urogynecol J Pelvic Floor Dysfunct. 1999;10:405-406.
16. Nezhat CH, Nezhat F, Nezhat C. Laparoscopic repair of a vesicovaginal fistula: a case report. Obstet Gynecol. 1994;83(5 Pt 2):899-901.
17. Walters MD, Karram MM, eds. Urogynecology and Reconstructive Pelvic Surgery. 2nd ed. St. Louis, Mo: Mosby; 1999.
- Surgical risk factors include prior pelvic surgery, history of pelvic inflammatory disease, pelvic malignancy, endometriosis, infection, diabetes, and anatomic distortion.
- Conservative therapy should be reserved for simple fistulae that are less than 1 cm in size, diagnosed within 7 days of the index surgery, lacking associated carcinoma or radiation, and subject to at least 4 weeks of constant bladder drainage.
- In surgical repair, the Latzko partial colpocleisis or fistulectomy with flap-splitting closure is preferred.
Recent advances have improved the success of vesicovaginal fistula (VVF) repair—a challenge that can test even the most experienced gynecologic surgeon. For example, it now is apparent that some small uncomplicated fistulae respond to conservative treatment. Further, in selected cases, laparoscopic repair can eliminate the need for complicated laparotomy.
In addition, timing of fistula repair no longer requires long periods of observation, and good surgical technique for identifying and repairing bladder injuries at the time of the index surgery can often prevent the development or reduce the severity of VVF.
Vesicovaginal fistula is the most common type of urogenital fistula. Presentation and prognosis vary, depending on location and size of the defect, as well as coexisting factors such as tissue devascularization and previous radiation. However, surgical repair is associated with a high cure rate if it is performed by an experienced surgeon.
Most US cases follow gynecologic surgery
Vesicovaginal fistula was first documented in the mummified remains of Egyptian Queen Henhenit (11th Dynasty, 2050 BC), which were examined in 1923 by Derry.1 Although the exact incidence of VVF in the United States is unknown, the primary cause is gynecologic surgery, especially hysterectomy. The defect is estimated to occur in 0.01% to 0.04% of gynecologic procedures.
A study of 303 women with genitourinary fistula found that the defect was related to gynecologic surgery in 82% of cases, obstetric events in 8%, radiation therapy in 6%, and trauma or fulguration in 4%.2 Rare causes of VVF include lymphogranuloma venereum, tuberculosis, syphilis, bladder stones, and a retained foreign body in the vagina. In rare instances, spontaneous vesicouterine fistulae were reported following uncomplicated vaginal birth after cesarean section.3
Gynecologic surgery may lead to VVF due to extensive dissection between the bladder and the uterus, unrecognized bladder laceration, inappropriate stitch placement, and/or devascularization injury to the tissue planes. Concurrent ureteric involvement has been reported in as many as 10% to 15% of vesicovaginal fistula cases.
In developing countries, vesicovaginal fistulae are far more common and generally related to obstetric factors such as obstructed labor (due to unattended deliveries), small pelvic dimensions, malpresentation, poor uterine contractions, and introital stenosis.
Risk factors. Conditions that may predispose patients to VVF include prior pelvic surgery, a history of pelvic inflammatory disease, pelvic malignancy, endometriosis, infection, diabetes, and anatomic distortion. If these risk factors are present, the patient should be counseled accordingly prior to gynecologic surgery.
Correct classification crucial to surgical success
Proper classification of VVF can help the gynecologic surgeon plan operative intervention. Obstetric vesicovaginal fistulae usually are categorized according to their cause, complexity, and site of obstruction. In contrast, gynecologic fistulae are generally classified as simple or complicated (TABLE).
These levels may have important implications for the surgical approach and prognosis.4 For example, simple vesicovaginal fistulae are usually uncomplicated surgical cases with good prognosis. Complicated vesicovaginal fistulae, on the other hand, can challenge even highly practiced and skilled gynecologic surgeons and are associated with a high rate of recurrence.
Women typically present within specific intervals after the various antecedent events (pelvic surgery, childbirth, radiation therapy) with a primary complaint of constant, painless urinary incontinence. If the fistula is related to traumatic childbirth, most patients experience urine leakage within the first 24 to 48 hours. Following pelvic surgery, symptoms usually occur within the first 30 days. In contrast, radiation-induced fistulae develop over a much longer interval secondary to progressive devascularization necrosis, and may present 30 days to 30 years after the antecedent event.
Some patients report exacerbation during physical activities, which can sometimes lead to erroneous diagnosis of uncomplicated stress incontinence. If the fistula is small, intermittent leakage with increased bladder distention or physical activity may be noted.
Other patients may complain of vaginal discharge or hematuria.
If there is concurrent ureteric involvement, the patient may experience constitutional symptoms (such as fever, chills, and flank pain) or even gastrointestinal symptoms.
Physical findings. Any pooling of fluid in the vagina that is noted should be sent for analysis if the diagnosis is unclear. Next, perform a careful speculum exam that allows visualization of the entire anterior vaginal wall to identify the fistula tract (FIGURE 1). In many cases, the fistula is grossly visible.
Determine the location of the fistula in relation to the vaginal apex and bladder trigone and assess the quality of surrounding tissue (eg, presence of inflammation, edema, or infection). Fistulas near the vaginal apex may require a more complicated abdominal approach, and those close to the trigone may be associated with increased risk of ureteral injury during repair.
If the fistula is particularly small, no tract may be apparent. In such cases, bimanual exam with careful palpation of the anterior wall may help isolate the fistula (eg, when there is a surrounding zone of induration).
Office tests. If no fistula is noted despite highly suspicious signs and symptoms and careful examination, a simple office test can be performed. Using a catheter, fill the bladder with a dyed solution such as normal saline with indigo carmine and repeat the pelvic exam with a half-speculum to visualize the anterior wall. Ask the patient to cough and bear down, and identify the fistula by visualizing urine leakage.
If this test fails to locate the fistula, insert a tampon and ask the patient to perform 10 to 15 minutes of exertional maneuvers, including stair climbing and jumping in place. Then remove the tampon. Visualization of dye beyond the most distal edge of the tampon confirms the presence of a fistula.
A variation of this technique is the double-dye test: Give the patient oral phenazopyridine (Pyridium), fill the bladder with the blue-tinted solution, and insert a tampon. The presence of blue staining suggests vesicovaginal or urethrovaginal fistula, while red staining (Pyridium) suggests ureterovaginal fistula.
Other testing. Further assessment is recommended to rule out concurrent pathology and formulate an appropriate treatment plan. Routine testing should include a urinalysis and culture to exclude coexisting urinary tract infection, an electrolyte panel to evaluate renal function, and a complete blood cell count to rule out systemic infection.
Cystoscopy should be performed to visualize the fistulous tract, assess its location in relation to the ureters and trigone, assure bilateral ureteral patency, and exclude the presence of a foreign body or suture in the bladder.
In patients with a history of urogenital malignancy, biopsy of the fistula tract and urine cytology is warranted.
Comparable success rates have been reported for early and late repair of surgery-induced fistulae.
Radiologic studies are recommended prior to surgical repair of a vesicovaginal fistula to fully assess the defect and exclude the presence of multiple fistulae. An intravenous pyelogram is helpful to exclude concurrent ureterovaginal fistulae or ureteral obstruction. A targeted fistulogram may be indicated if conservative therapy is planned, including expectant management, continuous bladder drainage, fulguration, or fibrin occlusion.
FIGURE 1 Vesicovaginal fistula
Pelvic cross section depicting high vesicovaginal fistula.TABLE
Classification of vesicovaginal fistulae17
| CLASSIFICATION | DESCRIPTION |
|---|---|
| Simple |
|
| Complicated |
|
Indications for conservative management
Because spontaneous closure is uncommon, symptomatic VVF merits treatment. Appropriate therapy depends on various factors, including fistula size and location, timing from the antecedent event, severity of symptoms, quality of surrounding tissue, and clinician experience and surgical skills.
Occasionally, a fistula heals following prolonged bladder drainage through a transurethral or suprapubic catheter—provided it is diagnosed within a few days of surgery. Zimmern5 recommends a conservative approach to small fistulae if the patient’s complaints of urinary incontinence are resolved with insertion of a Foley catheter. In this case, bladder drainage should be continued for 3 weeks, followed by reevaluation of the fistula. If the fistula has diminished in size, an additional 3 weeks of catheter drainage is associated with a high rate of spontaneous closure; if there is no change, the fistula is unlikely to resolve spontaneously.5
Varying success rates have been reported for conservative management, ranging from 2% to 80%.4,6 The chances of success are apparently greater if the fistula is:
- diagnosed within 7 days of the index surgery,
- less than 1 cm in size,
- simple, without associated carcinoma or radiation, and
- subject to at least 4 weeks of constant bladder drainage.
Persistent, large, or complex fistulae are best treated surgically.
Indications for surgical management
The basic principles of fistula closure apply. They are:
- adequate exposure,
- good hemostasis,
- wide mobilization of the bladder and vagina,
- resection of devascularized tissue,
- removal of any foreign bodies,
- tension-free closure,
- nonopposition of suture lines,
- confirmation of a water-tight seal on bladder closure, and
- bladder drainage for 10 to 14 days following the repair.
Timing of the repair has been the subject of controversy and can pose a dilemma to physician and patient alike. Traditionally, an interval of 3 months was recommended between the index surgery and fistula repair, with a delay of up to 1 year when the fistula was radiation-induced. However, little data support these recommendations.
Today most experts recommend an individualized approach, delaying the surgery until inflammation and infection of the surrounding tissue have resolved. The use of estrogen, antibiotics, or steroids to facilitate healing during this period also has been recommended.7 Comparable success rates have been reported for early and late repair of surgery-induced fistulae based on these principles.8-10
Vaginal approach. Most vesicovaginal fistulae can be surgically corrected using a vaginal approach. Traditionally, a Latzko partial colpocleisis or fistulectomy with flap-splitting closure has been advocated.
Debate continues about whether resection of the fistulous tract is necessary. Some experts believe that wide resection increases the size of the fistula and, therefore, the risk of recurrence. They also maintain that the fibrous tissue surrounding the fistula helps to reinforce the surgical repair. Proponents of fistulectomy counter that resection of the fistula and exposure of healthy tissue optimizes wound healing and improves surgical success rates. Comparable success has been reported for both techniques.11,12
We prefer an individualized approach, with minimal resection of the fistulous tract to simplify the procedure and minimize associated complications, including recurrence.
- Latzko partial colpocleisis. This technique, first reported in 1942, remains a common procedure, with success rates of 90% to 100%.13 Advantages include a short operative time, low intraoperative and postoperative morbidity, and low risk of ureteral injury.
- Fistulectomy technique. Alternatively, to perform fistulectomy with a flap-splitting closure, begin by resecting the fistulous tract to expose healthy tissue at the wound margins. Then close the defect in a multilayer fashion, beginning with the bladder mucosa, bladder serosa, pubocervical fascia, and vaginal mucosa. Be careful to avoid tension on suture lines. In addition, create a fascial flap to prevent opposition of the incision planes and reduce the risk of recurrence.
- Grafts. In cases with a high risk of recurrence, such as complex or large fistulae, a Martius fat-pad graft should be interposed between the closure layers to promote vascularization and reduce the risk of recurrence.14 Placement of a cadaveric biomaterial graft also has been reported, reducing the need for complicated flap procedures.15
Abdominal approach. Although most vesicovaginal fistulae can be surgically corrected via the vaginal approach, the abdominal route may be preferred when the fistula is high and inaccessible, large and complex, multiple in number, or when there is concurrent uterine or bowel involvement or a need for ureteral reimplantation. The abdominal approach may be facilitated by cystoscopically guided placement of a catheter through the fistulous tract to assist in subsequent identification and dissection.
To begin, make a vertical skin incision to optimize visualization and allow mobilization of an omental flap, if necessary. Expose the bladder and perform a high extraperitoneal cystotomy to visualize the fistulous tract. Place ureteral stents if the fistula is in close proximity to the ureteral orifice.
Extend the bladder incision to the fistulous tract and completely excise it following mobilization of the vagina. Then close the vagina and bladder with interrupted, delayed absorbable suture in a double layer.
Transpose an omental flap between the vaginal and bladder incisions to promote vascularization, minimize opposition of suture lines, and reduce the risk of recurrence (FIGURE 2).
Laparoscopic approach. A similar laparoscopic repair has been reported with comparable results, but requires advanced skills with endoscopic suturing and knot tying (FIGURE 3).16
FIGURE 2 Abdominal repair
Abdominal repair of vesicovaginal fistula, with closure of bladder defect and posterior cystotomy and separate closure of vaginal defect. Note the omental flap pictured in the insert.
FIGURE 3 Laparoscopic repair
Laparoscopic repair of vesicovaginal fistula.The authors report no financial relationship with any companies whose products are mentioned in this article.
- Surgical risk factors include prior pelvic surgery, history of pelvic inflammatory disease, pelvic malignancy, endometriosis, infection, diabetes, and anatomic distortion.
- Conservative therapy should be reserved for simple fistulae that are less than 1 cm in size, diagnosed within 7 days of the index surgery, lacking associated carcinoma or radiation, and subject to at least 4 weeks of constant bladder drainage.
- In surgical repair, the Latzko partial colpocleisis or fistulectomy with flap-splitting closure is preferred.
Recent advances have improved the success of vesicovaginal fistula (VVF) repair—a challenge that can test even the most experienced gynecologic surgeon. For example, it now is apparent that some small uncomplicated fistulae respond to conservative treatment. Further, in selected cases, laparoscopic repair can eliminate the need for complicated laparotomy.
In addition, timing of fistula repair no longer requires long periods of observation, and good surgical technique for identifying and repairing bladder injuries at the time of the index surgery can often prevent the development or reduce the severity of VVF.
Vesicovaginal fistula is the most common type of urogenital fistula. Presentation and prognosis vary, depending on location and size of the defect, as well as coexisting factors such as tissue devascularization and previous radiation. However, surgical repair is associated with a high cure rate if it is performed by an experienced surgeon.
Most US cases follow gynecologic surgery
Vesicovaginal fistula was first documented in the mummified remains of Egyptian Queen Henhenit (11th Dynasty, 2050 BC), which were examined in 1923 by Derry.1 Although the exact incidence of VVF in the United States is unknown, the primary cause is gynecologic surgery, especially hysterectomy. The defect is estimated to occur in 0.01% to 0.04% of gynecologic procedures.
A study of 303 women with genitourinary fistula found that the defect was related to gynecologic surgery in 82% of cases, obstetric events in 8%, radiation therapy in 6%, and trauma or fulguration in 4%.2 Rare causes of VVF include lymphogranuloma venereum, tuberculosis, syphilis, bladder stones, and a retained foreign body in the vagina. In rare instances, spontaneous vesicouterine fistulae were reported following uncomplicated vaginal birth after cesarean section.3
Gynecologic surgery may lead to VVF due to extensive dissection between the bladder and the uterus, unrecognized bladder laceration, inappropriate stitch placement, and/or devascularization injury to the tissue planes. Concurrent ureteric involvement has been reported in as many as 10% to 15% of vesicovaginal fistula cases.
In developing countries, vesicovaginal fistulae are far more common and generally related to obstetric factors such as obstructed labor (due to unattended deliveries), small pelvic dimensions, malpresentation, poor uterine contractions, and introital stenosis.
Risk factors. Conditions that may predispose patients to VVF include prior pelvic surgery, a history of pelvic inflammatory disease, pelvic malignancy, endometriosis, infection, diabetes, and anatomic distortion. If these risk factors are present, the patient should be counseled accordingly prior to gynecologic surgery.
Correct classification crucial to surgical success
Proper classification of VVF can help the gynecologic surgeon plan operative intervention. Obstetric vesicovaginal fistulae usually are categorized according to their cause, complexity, and site of obstruction. In contrast, gynecologic fistulae are generally classified as simple or complicated (TABLE).
These levels may have important implications for the surgical approach and prognosis.4 For example, simple vesicovaginal fistulae are usually uncomplicated surgical cases with good prognosis. Complicated vesicovaginal fistulae, on the other hand, can challenge even highly practiced and skilled gynecologic surgeons and are associated with a high rate of recurrence.
Women typically present within specific intervals after the various antecedent events (pelvic surgery, childbirth, radiation therapy) with a primary complaint of constant, painless urinary incontinence. If the fistula is related to traumatic childbirth, most patients experience urine leakage within the first 24 to 48 hours. Following pelvic surgery, symptoms usually occur within the first 30 days. In contrast, radiation-induced fistulae develop over a much longer interval secondary to progressive devascularization necrosis, and may present 30 days to 30 years after the antecedent event.
Some patients report exacerbation during physical activities, which can sometimes lead to erroneous diagnosis of uncomplicated stress incontinence. If the fistula is small, intermittent leakage with increased bladder distention or physical activity may be noted.
Other patients may complain of vaginal discharge or hematuria.
If there is concurrent ureteric involvement, the patient may experience constitutional symptoms (such as fever, chills, and flank pain) or even gastrointestinal symptoms.
Physical findings. Any pooling of fluid in the vagina that is noted should be sent for analysis if the diagnosis is unclear. Next, perform a careful speculum exam that allows visualization of the entire anterior vaginal wall to identify the fistula tract (FIGURE 1). In many cases, the fistula is grossly visible.
Determine the location of the fistula in relation to the vaginal apex and bladder trigone and assess the quality of surrounding tissue (eg, presence of inflammation, edema, or infection). Fistulas near the vaginal apex may require a more complicated abdominal approach, and those close to the trigone may be associated with increased risk of ureteral injury during repair.
If the fistula is particularly small, no tract may be apparent. In such cases, bimanual exam with careful palpation of the anterior wall may help isolate the fistula (eg, when there is a surrounding zone of induration).
Office tests. If no fistula is noted despite highly suspicious signs and symptoms and careful examination, a simple office test can be performed. Using a catheter, fill the bladder with a dyed solution such as normal saline with indigo carmine and repeat the pelvic exam with a half-speculum to visualize the anterior wall. Ask the patient to cough and bear down, and identify the fistula by visualizing urine leakage.
If this test fails to locate the fistula, insert a tampon and ask the patient to perform 10 to 15 minutes of exertional maneuvers, including stair climbing and jumping in place. Then remove the tampon. Visualization of dye beyond the most distal edge of the tampon confirms the presence of a fistula.
A variation of this technique is the double-dye test: Give the patient oral phenazopyridine (Pyridium), fill the bladder with the blue-tinted solution, and insert a tampon. The presence of blue staining suggests vesicovaginal or urethrovaginal fistula, while red staining (Pyridium) suggests ureterovaginal fistula.
Other testing. Further assessment is recommended to rule out concurrent pathology and formulate an appropriate treatment plan. Routine testing should include a urinalysis and culture to exclude coexisting urinary tract infection, an electrolyte panel to evaluate renal function, and a complete blood cell count to rule out systemic infection.
Cystoscopy should be performed to visualize the fistulous tract, assess its location in relation to the ureters and trigone, assure bilateral ureteral patency, and exclude the presence of a foreign body or suture in the bladder.
In patients with a history of urogenital malignancy, biopsy of the fistula tract and urine cytology is warranted.
Comparable success rates have been reported for early and late repair of surgery-induced fistulae.
Radiologic studies are recommended prior to surgical repair of a vesicovaginal fistula to fully assess the defect and exclude the presence of multiple fistulae. An intravenous pyelogram is helpful to exclude concurrent ureterovaginal fistulae or ureteral obstruction. A targeted fistulogram may be indicated if conservative therapy is planned, including expectant management, continuous bladder drainage, fulguration, or fibrin occlusion.
FIGURE 1 Vesicovaginal fistula
Pelvic cross section depicting high vesicovaginal fistula.TABLE
Classification of vesicovaginal fistulae17
| CLASSIFICATION | DESCRIPTION |
|---|---|
| Simple |
|
| Complicated |
|
Indications for conservative management
Because spontaneous closure is uncommon, symptomatic VVF merits treatment. Appropriate therapy depends on various factors, including fistula size and location, timing from the antecedent event, severity of symptoms, quality of surrounding tissue, and clinician experience and surgical skills.
Occasionally, a fistula heals following prolonged bladder drainage through a transurethral or suprapubic catheter—provided it is diagnosed within a few days of surgery. Zimmern5 recommends a conservative approach to small fistulae if the patient’s complaints of urinary incontinence are resolved with insertion of a Foley catheter. In this case, bladder drainage should be continued for 3 weeks, followed by reevaluation of the fistula. If the fistula has diminished in size, an additional 3 weeks of catheter drainage is associated with a high rate of spontaneous closure; if there is no change, the fistula is unlikely to resolve spontaneously.5
Varying success rates have been reported for conservative management, ranging from 2% to 80%.4,6 The chances of success are apparently greater if the fistula is:
- diagnosed within 7 days of the index surgery,
- less than 1 cm in size,
- simple, without associated carcinoma or radiation, and
- subject to at least 4 weeks of constant bladder drainage.
Persistent, large, or complex fistulae are best treated surgically.
Indications for surgical management
The basic principles of fistula closure apply. They are:
- adequate exposure,
- good hemostasis,
- wide mobilization of the bladder and vagina,
- resection of devascularized tissue,
- removal of any foreign bodies,
- tension-free closure,
- nonopposition of suture lines,
- confirmation of a water-tight seal on bladder closure, and
- bladder drainage for 10 to 14 days following the repair.
Timing of the repair has been the subject of controversy and can pose a dilemma to physician and patient alike. Traditionally, an interval of 3 months was recommended between the index surgery and fistula repair, with a delay of up to 1 year when the fistula was radiation-induced. However, little data support these recommendations.
Today most experts recommend an individualized approach, delaying the surgery until inflammation and infection of the surrounding tissue have resolved. The use of estrogen, antibiotics, or steroids to facilitate healing during this period also has been recommended.7 Comparable success rates have been reported for early and late repair of surgery-induced fistulae based on these principles.8-10
Vaginal approach. Most vesicovaginal fistulae can be surgically corrected using a vaginal approach. Traditionally, a Latzko partial colpocleisis or fistulectomy with flap-splitting closure has been advocated.
Debate continues about whether resection of the fistulous tract is necessary. Some experts believe that wide resection increases the size of the fistula and, therefore, the risk of recurrence. They also maintain that the fibrous tissue surrounding the fistula helps to reinforce the surgical repair. Proponents of fistulectomy counter that resection of the fistula and exposure of healthy tissue optimizes wound healing and improves surgical success rates. Comparable success has been reported for both techniques.11,12
We prefer an individualized approach, with minimal resection of the fistulous tract to simplify the procedure and minimize associated complications, including recurrence.
- Latzko partial colpocleisis. This technique, first reported in 1942, remains a common procedure, with success rates of 90% to 100%.13 Advantages include a short operative time, low intraoperative and postoperative morbidity, and low risk of ureteral injury.
- Fistulectomy technique. Alternatively, to perform fistulectomy with a flap-splitting closure, begin by resecting the fistulous tract to expose healthy tissue at the wound margins. Then close the defect in a multilayer fashion, beginning with the bladder mucosa, bladder serosa, pubocervical fascia, and vaginal mucosa. Be careful to avoid tension on suture lines. In addition, create a fascial flap to prevent opposition of the incision planes and reduce the risk of recurrence.
- Grafts. In cases with a high risk of recurrence, such as complex or large fistulae, a Martius fat-pad graft should be interposed between the closure layers to promote vascularization and reduce the risk of recurrence.14 Placement of a cadaveric biomaterial graft also has been reported, reducing the need for complicated flap procedures.15
Abdominal approach. Although most vesicovaginal fistulae can be surgically corrected via the vaginal approach, the abdominal route may be preferred when the fistula is high and inaccessible, large and complex, multiple in number, or when there is concurrent uterine or bowel involvement or a need for ureteral reimplantation. The abdominal approach may be facilitated by cystoscopically guided placement of a catheter through the fistulous tract to assist in subsequent identification and dissection.
To begin, make a vertical skin incision to optimize visualization and allow mobilization of an omental flap, if necessary. Expose the bladder and perform a high extraperitoneal cystotomy to visualize the fistulous tract. Place ureteral stents if the fistula is in close proximity to the ureteral orifice.
Extend the bladder incision to the fistulous tract and completely excise it following mobilization of the vagina. Then close the vagina and bladder with interrupted, delayed absorbable suture in a double layer.
Transpose an omental flap between the vaginal and bladder incisions to promote vascularization, minimize opposition of suture lines, and reduce the risk of recurrence (FIGURE 2).
Laparoscopic approach. A similar laparoscopic repair has been reported with comparable results, but requires advanced skills with endoscopic suturing and knot tying (FIGURE 3).16
FIGURE 2 Abdominal repair
Abdominal repair of vesicovaginal fistula, with closure of bladder defect and posterior cystotomy and separate closure of vaginal defect. Note the omental flap pictured in the insert.
FIGURE 3 Laparoscopic repair
Laparoscopic repair of vesicovaginal fistula.The authors report no financial relationship with any companies whose products are mentioned in this article.
1. Kremling H. Labor-induced bladder injuries: historical observations. Gynakol Geburtshilfliche Rundsch. 1996;36:197-200.
2. Lee RA, Symmonds RE, Williams TJ. Current state of genitourinary fistula. Obstet Gynecol. 1998;72:313-315.
3. Miklos JR, Sze EHM, Parobeck D, et al. Vesicouterine fistula: a rare complication of vaginal birth after cesarean. Obstet Gynecol. 1995;86:638-639.
4. Elkins TE, Thompson JR. Lower urinary tract fistulas. In: Walters M, Karram MM, eds. Urogynecology and Reconstructive Pelvic Surgery. 2nd ed. St. Louis, Mo: Mosby; 1999;355-365.
5. Zimmern PE, Hadley HR, Staskin D. Genitourinary fistulas: vaginal approach for repair of vesicovaginal fistulas. Clin Obstet Gynaecol. 1985;12:403-413.
6. Davits RJ, Miranda SI. Conservative treatment of vesicovaginal fistulas by bladder drainage alone. Br J Urol. 1991;68:155-156.
7. Margolis T, Mercer LJ. Vesicovaginal fistula. Obstet Gynecol Surv. 1995;49:840-847.
8. Blaivas JG, Heritz DM, Romanzi LJ. Early versus late repair of vesicovaginal fistulas: vaginal and abdominal approaches. J Urol. 1995;153:1110-1112.
9. Blandy JP, Badenoch DF, Fowler CG. Early repair of iatrogenic injury to the ureter or bladder after gynecological surgery. J Urol. 1991;146:761-765.
10. Cruikshank SH. Early closure of posthysterectomy vesicovaginal fistulas. South Med J. 1988;81:1525-1528.
11. Raz S, Bregg KJ, Nitti VW. Transvaginal repair of vesicovaginal fistula using a peritoneal flap. J Urol. 1993;150:56-59.
12. Iselin CE, Aslan P, Webster GD. Transvaginal repair of vesicovaginal fistulas after hysterectomy by vaginal cuff excision. J Urol. 1998;160(3 Pt 1):728-730.
13. Latzko W. Postoperative vesicovaginal fistulas: genesis and therapy. Am J Surg. 1942;58:211-218.
14. Punekar SV, Buch DN, Soni AB. Martius’ labial fat pad interposition and its modification in complex lower urinary fistulae. J Postgrad Med. 1999;10:405-406.
15. Miklos JR, Kohli N. Rectovaginal fistula repair utilizing a cadaveric dermal allograft. Int Urogynecol J Pelvic Floor Dysfunct. 1999;10:405-406.
16. Nezhat CH, Nezhat F, Nezhat C. Laparoscopic repair of a vesicovaginal fistula: a case report. Obstet Gynecol. 1994;83(5 Pt 2):899-901.
17. Walters MD, Karram MM, eds. Urogynecology and Reconstructive Pelvic Surgery. 2nd ed. St. Louis, Mo: Mosby; 1999.
1. Kremling H. Labor-induced bladder injuries: historical observations. Gynakol Geburtshilfliche Rundsch. 1996;36:197-200.
2. Lee RA, Symmonds RE, Williams TJ. Current state of genitourinary fistula. Obstet Gynecol. 1998;72:313-315.
3. Miklos JR, Sze EHM, Parobeck D, et al. Vesicouterine fistula: a rare complication of vaginal birth after cesarean. Obstet Gynecol. 1995;86:638-639.
4. Elkins TE, Thompson JR. Lower urinary tract fistulas. In: Walters M, Karram MM, eds. Urogynecology and Reconstructive Pelvic Surgery. 2nd ed. St. Louis, Mo: Mosby; 1999;355-365.
5. Zimmern PE, Hadley HR, Staskin D. Genitourinary fistulas: vaginal approach for repair of vesicovaginal fistulas. Clin Obstet Gynaecol. 1985;12:403-413.
6. Davits RJ, Miranda SI. Conservative treatment of vesicovaginal fistulas by bladder drainage alone. Br J Urol. 1991;68:155-156.
7. Margolis T, Mercer LJ. Vesicovaginal fistula. Obstet Gynecol Surv. 1995;49:840-847.
8. Blaivas JG, Heritz DM, Romanzi LJ. Early versus late repair of vesicovaginal fistulas: vaginal and abdominal approaches. J Urol. 1995;153:1110-1112.
9. Blandy JP, Badenoch DF, Fowler CG. Early repair of iatrogenic injury to the ureter or bladder after gynecological surgery. J Urol. 1991;146:761-765.
10. Cruikshank SH. Early closure of posthysterectomy vesicovaginal fistulas. South Med J. 1988;81:1525-1528.
11. Raz S, Bregg KJ, Nitti VW. Transvaginal repair of vesicovaginal fistula using a peritoneal flap. J Urol. 1993;150:56-59.
12. Iselin CE, Aslan P, Webster GD. Transvaginal repair of vesicovaginal fistulas after hysterectomy by vaginal cuff excision. J Urol. 1998;160(3 Pt 1):728-730.
13. Latzko W. Postoperative vesicovaginal fistulas: genesis and therapy. Am J Surg. 1942;58:211-218.
14. Punekar SV, Buch DN, Soni AB. Martius’ labial fat pad interposition and its modification in complex lower urinary fistulae. J Postgrad Med. 1999;10:405-406.
15. Miklos JR, Kohli N. Rectovaginal fistula repair utilizing a cadaveric dermal allograft. Int Urogynecol J Pelvic Floor Dysfunct. 1999;10:405-406.
16. Nezhat CH, Nezhat F, Nezhat C. Laparoscopic repair of a vesicovaginal fistula: a case report. Obstet Gynecol. 1994;83(5 Pt 2):899-901.
17. Walters MD, Karram MM, eds. Urogynecology and Reconstructive Pelvic Surgery. 2nd ed. St. Louis, Mo: Mosby; 1999.