User login
- Surgical risk factors include prior pelvic surgery, history of pelvic inflammatory disease, pelvic malignancy, endometriosis, infection, diabetes, and anatomic distortion.
- Conservative therapy should be reserved for simple fistulae that are less than 1 cm in size, diagnosed within 7 days of the index surgery, lacking associated carcinoma or radiation, and subject to at least 4 weeks of constant bladder drainage.
- In surgical repair, the Latzko partial colpocleisis or fistulectomy with flap-splitting closure is preferred.
Recent advances have improved the success of vesicovaginal fistula (VVF) repair—a challenge that can test even the most experienced gynecologic surgeon. For example, it now is apparent that some small uncomplicated fistulae respond to conservative treatment. Further, in selected cases, laparoscopic repair can eliminate the need for complicated laparotomy.
In addition, timing of fistula repair no longer requires long periods of observation, and good surgical technique for identifying and repairing bladder injuries at the time of the index surgery can often prevent the development or reduce the severity of VVF.
Vesicovaginal fistula is the most common type of urogenital fistula. Presentation and prognosis vary, depending on location and size of the defect, as well as coexisting factors such as tissue devascularization and previous radiation. However, surgical repair is associated with a high cure rate if it is performed by an experienced surgeon.
Most US cases follow gynecologic surgery
Vesicovaginal fistula was first documented in the mummified remains of Egyptian Queen Henhenit (11th Dynasty, 2050 BC), which were examined in 1923 by Derry.1 Although the exact incidence of VVF in the United States is unknown, the primary cause is gynecologic surgery, especially hysterectomy. The defect is estimated to occur in 0.01% to 0.04% of gynecologic procedures.
A study of 303 women with genitourinary fistula found that the defect was related to gynecologic surgery in 82% of cases, obstetric events in 8%, radiation therapy in 6%, and trauma or fulguration in 4%.2 Rare causes of VVF include lymphogranuloma venereum, tuberculosis, syphilis, bladder stones, and a retained foreign body in the vagina. In rare instances, spontaneous vesicouterine fistulae were reported following uncomplicated vaginal birth after cesarean section.3
Gynecologic surgery may lead to VVF due to extensive dissection between the bladder and the uterus, unrecognized bladder laceration, inappropriate stitch placement, and/or devascularization injury to the tissue planes. Concurrent ureteric involvement has been reported in as many as 10% to 15% of vesicovaginal fistula cases.
In developing countries, vesicovaginal fistulae are far more common and generally related to obstetric factors such as obstructed labor (due to unattended deliveries), small pelvic dimensions, malpresentation, poor uterine contractions, and introital stenosis.
Risk factors. Conditions that may predispose patients to VVF include prior pelvic surgery, a history of pelvic inflammatory disease, pelvic malignancy, endometriosis, infection, diabetes, and anatomic distortion. If these risk factors are present, the patient should be counseled accordingly prior to gynecologic surgery.
Correct classification crucial to surgical success
Proper classification of VVF can help the gynecologic surgeon plan operative intervention. Obstetric vesicovaginal fistulae usually are categorized according to their cause, complexity, and site of obstruction. In contrast, gynecologic fistulae are generally classified as simple or complicated (TABLE).
These levels may have important implications for the surgical approach and prognosis.4 For example, simple vesicovaginal fistulae are usually uncomplicated surgical cases with good prognosis. Complicated vesicovaginal fistulae, on the other hand, can challenge even highly practiced and skilled gynecologic surgeons and are associated with a high rate of recurrence.
Women typically present within specific intervals after the various antecedent events (pelvic surgery, childbirth, radiation therapy) with a primary complaint of constant, painless urinary incontinence. If the fistula is related to traumatic childbirth, most patients experience urine leakage within the first 24 to 48 hours. Following pelvic surgery, symptoms usually occur within the first 30 days. In contrast, radiation-induced fistulae develop over a much longer interval secondary to progressive devascularization necrosis, and may present 30 days to 30 years after the antecedent event.
Some patients report exacerbation during physical activities, which can sometimes lead to erroneous diagnosis of uncomplicated stress incontinence. If the fistula is small, intermittent leakage with increased bladder distention or physical activity may be noted.
Other patients may complain of vaginal discharge or hematuria.
If there is concurrent ureteric involvement, the patient may experience constitutional symptoms (such as fever, chills, and flank pain) or even gastrointestinal symptoms.
Physical findings. Any pooling of fluid in the vagina that is noted should be sent for analysis if the diagnosis is unclear. Next, perform a careful speculum exam that allows visualization of the entire anterior vaginal wall to identify the fistula tract (FIGURE 1). In many cases, the fistula is grossly visible.
Determine the location of the fistula in relation to the vaginal apex and bladder trigone and assess the quality of surrounding tissue (eg, presence of inflammation, edema, or infection). Fistulas near the vaginal apex may require a more complicated abdominal approach, and those close to the trigone may be associated with increased risk of ureteral injury during repair.
If the fistula is particularly small, no tract may be apparent. In such cases, bimanual exam with careful palpation of the anterior wall may help isolate the fistula (eg, when there is a surrounding zone of induration).
Office tests. If no fistula is noted despite highly suspicious signs and symptoms and careful examination, a simple office test can be performed. Using a catheter, fill the bladder with a dyed solution such as normal saline with indigo carmine and repeat the pelvic exam with a half-speculum to visualize the anterior wall. Ask the patient to cough and bear down, and identify the fistula by visualizing urine leakage.
If this test fails to locate the fistula, insert a tampon and ask the patient to perform 10 to 15 minutes of exertional maneuvers, including stair climbing and jumping in place. Then remove the tampon. Visualization of dye beyond the most distal edge of the tampon confirms the presence of a fistula.
A variation of this technique is the double-dye test: Give the patient oral phenazopyridine (Pyridium), fill the bladder with the blue-tinted solution, and insert a tampon. The presence of blue staining suggests vesicovaginal or urethrovaginal fistula, while red staining (Pyridium) suggests ureterovaginal fistula.
Other testing. Further assessment is recommended to rule out concurrent pathology and formulate an appropriate treatment plan. Routine testing should include a urinalysis and culture to exclude coexisting urinary tract infection, an electrolyte panel to evaluate renal function, and a complete blood cell count to rule out systemic infection.
Cystoscopy should be performed to visualize the fistulous tract, assess its location in relation to the ureters and trigone, assure bilateral ureteral patency, and exclude the presence of a foreign body or suture in the bladder.
In patients with a history of urogenital malignancy, biopsy of the fistula tract and urine cytology is warranted.
Comparable success rates have been reported for early and late repair of surgery-induced fistulae.
Radiologic studies are recommended prior to surgical repair of a vesicovaginal fistula to fully assess the defect and exclude the presence of multiple fistulae. An intravenous pyelogram is helpful to exclude concurrent ureterovaginal fistulae or ureteral obstruction. A targeted fistulogram may be indicated if conservative therapy is planned, including expectant management, continuous bladder drainage, fulguration, or fibrin occlusion.
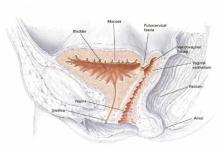
FIGURE 1 Vesicovaginal fistula
Pelvic cross section depicting high vesicovaginal fistula.TABLE
Classification of vesicovaginal fistulae17
| CLASSIFICATION | DESCRIPTION |
|---|---|
| Simple |
|
| Complicated |
|
Indications for conservative management
Because spontaneous closure is uncommon, symptomatic VVF merits treatment. Appropriate therapy depends on various factors, including fistula size and location, timing from the antecedent event, severity of symptoms, quality of surrounding tissue, and clinician experience and surgical skills.
Occasionally, a fistula heals following prolonged bladder drainage through a transurethral or suprapubic catheter—provided it is diagnosed within a few days of surgery. Zimmern5 recommends a conservative approach to small fistulae if the patient’s complaints of urinary incontinence are resolved with insertion of a Foley catheter. In this case, bladder drainage should be continued for 3 weeks, followed by reevaluation of the fistula. If the fistula has diminished in size, an additional 3 weeks of catheter drainage is associated with a high rate of spontaneous closure; if there is no change, the fistula is unlikely to resolve spontaneously.5
Varying success rates have been reported for conservative management, ranging from 2% to 80%.4,6 The chances of success are apparently greater if the fistula is:
- diagnosed within 7 days of the index surgery,
- less than 1 cm in size,
- simple, without associated carcinoma or radiation, and
- subject to at least 4 weeks of constant bladder drainage.
Persistent, large, or complex fistulae are best treated surgically.
Indications for surgical management
The basic principles of fistula closure apply. They are:
- adequate exposure,
- good hemostasis,
- wide mobilization of the bladder and vagina,
- resection of devascularized tissue,
- removal of any foreign bodies,
- tension-free closure,
- nonopposition of suture lines,
- confirmation of a water-tight seal on bladder closure, and
- bladder drainage for 10 to 14 days following the repair.
Timing of the repair has been the subject of controversy and can pose a dilemma to physician and patient alike. Traditionally, an interval of 3 months was recommended between the index surgery and fistula repair, with a delay of up to 1 year when the fistula was radiation-induced. However, little data support these recommendations.
Today most experts recommend an individualized approach, delaying the surgery until inflammation and infection of the surrounding tissue have resolved. The use of estrogen, antibiotics, or steroids to facilitate healing during this period also has been recommended.7 Comparable success rates have been reported for early and late repair of surgery-induced fistulae based on these principles.8-10
Vaginal approach. Most vesicovaginal fistulae can be surgically corrected using a vaginal approach. Traditionally, a Latzko partial colpocleisis or fistulectomy with flap-splitting closure has been advocated.
Debate continues about whether resection of the fistulous tract is necessary. Some experts believe that wide resection increases the size of the fistula and, therefore, the risk of recurrence. They also maintain that the fibrous tissue surrounding the fistula helps to reinforce the surgical repair. Proponents of fistulectomy counter that resection of the fistula and exposure of healthy tissue optimizes wound healing and improves surgical success rates. Comparable success has been reported for both techniques.11,12
We prefer an individualized approach, with minimal resection of the fistulous tract to simplify the procedure and minimize associated complications, including recurrence.
- Latzko partial colpocleisis. This technique, first reported in 1942, remains a common procedure, with success rates of 90% to 100%.13 Advantages include a short operative time, low intraoperative and postoperative morbidity, and low risk of ureteral injury.
- Fistulectomy technique. Alternatively, to perform fistulectomy with a flap-splitting closure, begin by resecting the fistulous tract to expose healthy tissue at the wound margins. Then close the defect in a multilayer fashion, beginning with the bladder mucosa, bladder serosa, pubocervical fascia, and vaginal mucosa. Be careful to avoid tension on suture lines. In addition, create a fascial flap to prevent opposition of the incision planes and reduce the risk of recurrence.
- Grafts. In cases with a high risk of recurrence, such as complex or large fistulae, a Martius fat-pad graft should be interposed between the closure layers to promote vascularization and reduce the risk of recurrence.14 Placement of a cadaveric biomaterial graft also has been reported, reducing the need for complicated flap procedures.15
Abdominal approach. Although most vesicovaginal fistulae can be surgically corrected via the vaginal approach, the abdominal route may be preferred when the fistula is high and inaccessible, large and complex, multiple in number, or when there is concurrent uterine or bowel involvement or a need for ureteral reimplantation. The abdominal approach may be facilitated by cystoscopically guided placement of a catheter through the fistulous tract to assist in subsequent identification and dissection.
To begin, make a vertical skin incision to optimize visualization and allow mobilization of an omental flap, if necessary. Expose the bladder and perform a high extraperitoneal cystotomy to visualize the fistulous tract. Place ureteral stents if the fistula is in close proximity to the ureteral orifice.
Extend the bladder incision to the fistulous tract and completely excise it following mobilization of the vagina. Then close the vagina and bladder with interrupted, delayed absorbable suture in a double layer.
Transpose an omental flap between the vaginal and bladder incisions to promote vascularization, minimize opposition of suture lines, and reduce the risk of recurrence (FIGURE 2).
Laparoscopic approach. A similar laparoscopic repair has been reported with comparable results, but requires advanced skills with endoscopic suturing and knot tying (FIGURE 3).16
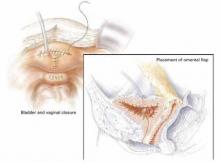
FIGURE 2 Abdominal repair
Abdominal repair of vesicovaginal fistula, with closure of bladder defect and posterior cystotomy and separate closure of vaginal defect. Note the omental flap pictured in the insert.
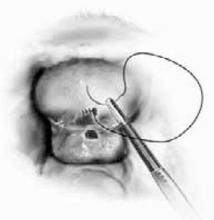
FIGURE 3 Laparoscopic repair
Laparoscopic repair of vesicovaginal fistula.The authors report no financial relationship with any companies whose products are mentioned in this article.
1. Kremling H. Labor-induced bladder injuries: historical observations. Gynakol Geburtshilfliche Rundsch. 1996;36:197-200.
2. Lee RA, Symmonds RE, Williams TJ. Current state of genitourinary fistula. Obstet Gynecol. 1998;72:313-315.
3. Miklos JR, Sze EHM, Parobeck D, et al. Vesicouterine fistula: a rare complication of vaginal birth after cesarean. Obstet Gynecol. 1995;86:638-639.
4. Elkins TE, Thompson JR. Lower urinary tract fistulas. In: Walters M, Karram MM, eds. Urogynecology and Reconstructive Pelvic Surgery. 2nd ed. St. Louis, Mo: Mosby; 1999;355-365.
5. Zimmern PE, Hadley HR, Staskin D. Genitourinary fistulas: vaginal approach for repair of vesicovaginal fistulas. Clin Obstet Gynaecol. 1985;12:403-413.
6. Davits RJ, Miranda SI. Conservative treatment of vesicovaginal fistulas by bladder drainage alone. Br J Urol. 1991;68:155-156.
7. Margolis T, Mercer LJ. Vesicovaginal fistula. Obstet Gynecol Surv. 1995;49:840-847.
8. Blaivas JG, Heritz DM, Romanzi LJ. Early versus late repair of vesicovaginal fistulas: vaginal and abdominal approaches. J Urol. 1995;153:1110-1112.
9. Blandy JP, Badenoch DF, Fowler CG. Early repair of iatrogenic injury to the ureter or bladder after gynecological surgery. J Urol. 1991;146:761-765.
10. Cruikshank SH. Early closure of posthysterectomy vesicovaginal fistulas. South Med J. 1988;81:1525-1528.
11. Raz S, Bregg KJ, Nitti VW. Transvaginal repair of vesicovaginal fistula using a peritoneal flap. J Urol. 1993;150:56-59.
12. Iselin CE, Aslan P, Webster GD. Transvaginal repair of vesicovaginal fistulas after hysterectomy by vaginal cuff excision. J Urol. 1998;160(3 Pt 1):728-730.
13. Latzko W. Postoperative vesicovaginal fistulas: genesis and therapy. Am J Surg. 1942;58:211-218.
14. Punekar SV, Buch DN, Soni AB. Martius’ labial fat pad interposition and its modification in complex lower urinary fistulae. J Postgrad Med. 1999;10:405-406.
15. Miklos JR, Kohli N. Rectovaginal fistula repair utilizing a cadaveric dermal allograft. Int Urogynecol J Pelvic Floor Dysfunct. 1999;10:405-406.
16. Nezhat CH, Nezhat F, Nezhat C. Laparoscopic repair of a vesicovaginal fistula: a case report. Obstet Gynecol. 1994;83(5 Pt 2):899-901.
17. Walters MD, Karram MM, eds. Urogynecology and Reconstructive Pelvic Surgery. 2nd ed. St. Louis, Mo: Mosby; 1999.
- Surgical risk factors include prior pelvic surgery, history of pelvic inflammatory disease, pelvic malignancy, endometriosis, infection, diabetes, and anatomic distortion.
- Conservative therapy should be reserved for simple fistulae that are less than 1 cm in size, diagnosed within 7 days of the index surgery, lacking associated carcinoma or radiation, and subject to at least 4 weeks of constant bladder drainage.
- In surgical repair, the Latzko partial colpocleisis or fistulectomy with flap-splitting closure is preferred.
Recent advances have improved the success of vesicovaginal fistula (VVF) repair—a challenge that can test even the most experienced gynecologic surgeon. For example, it now is apparent that some small uncomplicated fistulae respond to conservative treatment. Further, in selected cases, laparoscopic repair can eliminate the need for complicated laparotomy.
In addition, timing of fistula repair no longer requires long periods of observation, and good surgical technique for identifying and repairing bladder injuries at the time of the index surgery can often prevent the development or reduce the severity of VVF.
Vesicovaginal fistula is the most common type of urogenital fistula. Presentation and prognosis vary, depending on location and size of the defect, as well as coexisting factors such as tissue devascularization and previous radiation. However, surgical repair is associated with a high cure rate if it is performed by an experienced surgeon.
Most US cases follow gynecologic surgery
Vesicovaginal fistula was first documented in the mummified remains of Egyptian Queen Henhenit (11th Dynasty, 2050 BC), which were examined in 1923 by Derry.1 Although the exact incidence of VVF in the United States is unknown, the primary cause is gynecologic surgery, especially hysterectomy. The defect is estimated to occur in 0.01% to 0.04% of gynecologic procedures.
A study of 303 women with genitourinary fistula found that the defect was related to gynecologic surgery in 82% of cases, obstetric events in 8%, radiation therapy in 6%, and trauma or fulguration in 4%.2 Rare causes of VVF include lymphogranuloma venereum, tuberculosis, syphilis, bladder stones, and a retained foreign body in the vagina. In rare instances, spontaneous vesicouterine fistulae were reported following uncomplicated vaginal birth after cesarean section.3
Gynecologic surgery may lead to VVF due to extensive dissection between the bladder and the uterus, unrecognized bladder laceration, inappropriate stitch placement, and/or devascularization injury to the tissue planes. Concurrent ureteric involvement has been reported in as many as 10% to 15% of vesicovaginal fistula cases.
In developing countries, vesicovaginal fistulae are far more common and generally related to obstetric factors such as obstructed labor (due to unattended deliveries), small pelvic dimensions, malpresentation, poor uterine contractions, and introital stenosis.
Risk factors. Conditions that may predispose patients to VVF include prior pelvic surgery, a history of pelvic inflammatory disease, pelvic malignancy, endometriosis, infection, diabetes, and anatomic distortion. If these risk factors are present, the patient should be counseled accordingly prior to gynecologic surgery.
Correct classification crucial to surgical success
Proper classification of VVF can help the gynecologic surgeon plan operative intervention. Obstetric vesicovaginal fistulae usually are categorized according to their cause, complexity, and site of obstruction. In contrast, gynecologic fistulae are generally classified as simple or complicated (TABLE).
These levels may have important implications for the surgical approach and prognosis.4 For example, simple vesicovaginal fistulae are usually uncomplicated surgical cases with good prognosis. Complicated vesicovaginal fistulae, on the other hand, can challenge even highly practiced and skilled gynecologic surgeons and are associated with a high rate of recurrence.
Women typically present within specific intervals after the various antecedent events (pelvic surgery, childbirth, radiation therapy) with a primary complaint of constant, painless urinary incontinence. If the fistula is related to traumatic childbirth, most patients experience urine leakage within the first 24 to 48 hours. Following pelvic surgery, symptoms usually occur within the first 30 days. In contrast, radiation-induced fistulae develop over a much longer interval secondary to progressive devascularization necrosis, and may present 30 days to 30 years after the antecedent event.
Some patients report exacerbation during physical activities, which can sometimes lead to erroneous diagnosis of uncomplicated stress incontinence. If the fistula is small, intermittent leakage with increased bladder distention or physical activity may be noted.
Other patients may complain of vaginal discharge or hematuria.
If there is concurrent ureteric involvement, the patient may experience constitutional symptoms (such as fever, chills, and flank pain) or even gastrointestinal symptoms.
Physical findings. Any pooling of fluid in the vagina that is noted should be sent for analysis if the diagnosis is unclear. Next, perform a careful speculum exam that allows visualization of the entire anterior vaginal wall to identify the fistula tract (FIGURE 1). In many cases, the fistula is grossly visible.
Determine the location of the fistula in relation to the vaginal apex and bladder trigone and assess the quality of surrounding tissue (eg, presence of inflammation, edema, or infection). Fistulas near the vaginal apex may require a more complicated abdominal approach, and those close to the trigone may be associated with increased risk of ureteral injury during repair.
If the fistula is particularly small, no tract may be apparent. In such cases, bimanual exam with careful palpation of the anterior wall may help isolate the fistula (eg, when there is a surrounding zone of induration).
Office tests. If no fistula is noted despite highly suspicious signs and symptoms and careful examination, a simple office test can be performed. Using a catheter, fill the bladder with a dyed solution such as normal saline with indigo carmine and repeat the pelvic exam with a half-speculum to visualize the anterior wall. Ask the patient to cough and bear down, and identify the fistula by visualizing urine leakage.
If this test fails to locate the fistula, insert a tampon and ask the patient to perform 10 to 15 minutes of exertional maneuvers, including stair climbing and jumping in place. Then remove the tampon. Visualization of dye beyond the most distal edge of the tampon confirms the presence of a fistula.
A variation of this technique is the double-dye test: Give the patient oral phenazopyridine (Pyridium), fill the bladder with the blue-tinted solution, and insert a tampon. The presence of blue staining suggests vesicovaginal or urethrovaginal fistula, while red staining (Pyridium) suggests ureterovaginal fistula.
Other testing. Further assessment is recommended to rule out concurrent pathology and formulate an appropriate treatment plan. Routine testing should include a urinalysis and culture to exclude coexisting urinary tract infection, an electrolyte panel to evaluate renal function, and a complete blood cell count to rule out systemic infection.
Cystoscopy should be performed to visualize the fistulous tract, assess its location in relation to the ureters and trigone, assure bilateral ureteral patency, and exclude the presence of a foreign body or suture in the bladder.
In patients with a history of urogenital malignancy, biopsy of the fistula tract and urine cytology is warranted.
Comparable success rates have been reported for early and late repair of surgery-induced fistulae.
Radiologic studies are recommended prior to surgical repair of a vesicovaginal fistula to fully assess the defect and exclude the presence of multiple fistulae. An intravenous pyelogram is helpful to exclude concurrent ureterovaginal fistulae or ureteral obstruction. A targeted fistulogram may be indicated if conservative therapy is planned, including expectant management, continuous bladder drainage, fulguration, or fibrin occlusion.
FIGURE 1 Vesicovaginal fistula
Pelvic cross section depicting high vesicovaginal fistula.TABLE
Classification of vesicovaginal fistulae17
| CLASSIFICATION | DESCRIPTION |
|---|---|
| Simple |
|
| Complicated |
|
Indications for conservative management
Because spontaneous closure is uncommon, symptomatic VVF merits treatment. Appropriate therapy depends on various factors, including fistula size and location, timing from the antecedent event, severity of symptoms, quality of surrounding tissue, and clinician experience and surgical skills.
Occasionally, a fistula heals following prolonged bladder drainage through a transurethral or suprapubic catheter—provided it is diagnosed within a few days of surgery. Zimmern5 recommends a conservative approach to small fistulae if the patient’s complaints of urinary incontinence are resolved with insertion of a Foley catheter. In this case, bladder drainage should be continued for 3 weeks, followed by reevaluation of the fistula. If the fistula has diminished in size, an additional 3 weeks of catheter drainage is associated with a high rate of spontaneous closure; if there is no change, the fistula is unlikely to resolve spontaneously.5
Varying success rates have been reported for conservative management, ranging from 2% to 80%.4,6 The chances of success are apparently greater if the fistula is:
- diagnosed within 7 days of the index surgery,
- less than 1 cm in size,
- simple, without associated carcinoma or radiation, and
- subject to at least 4 weeks of constant bladder drainage.
Persistent, large, or complex fistulae are best treated surgically.
Indications for surgical management
The basic principles of fistula closure apply. They are:
- adequate exposure,
- good hemostasis,
- wide mobilization of the bladder and vagina,
- resection of devascularized tissue,
- removal of any foreign bodies,
- tension-free closure,
- nonopposition of suture lines,
- confirmation of a water-tight seal on bladder closure, and
- bladder drainage for 10 to 14 days following the repair.
Timing of the repair has been the subject of controversy and can pose a dilemma to physician and patient alike. Traditionally, an interval of 3 months was recommended between the index surgery and fistula repair, with a delay of up to 1 year when the fistula was radiation-induced. However, little data support these recommendations.
Today most experts recommend an individualized approach, delaying the surgery until inflammation and infection of the surrounding tissue have resolved. The use of estrogen, antibiotics, or steroids to facilitate healing during this period also has been recommended.7 Comparable success rates have been reported for early and late repair of surgery-induced fistulae based on these principles.8-10
Vaginal approach. Most vesicovaginal fistulae can be surgically corrected using a vaginal approach. Traditionally, a Latzko partial colpocleisis or fistulectomy with flap-splitting closure has been advocated.
Debate continues about whether resection of the fistulous tract is necessary. Some experts believe that wide resection increases the size of the fistula and, therefore, the risk of recurrence. They also maintain that the fibrous tissue surrounding the fistula helps to reinforce the surgical repair. Proponents of fistulectomy counter that resection of the fistula and exposure of healthy tissue optimizes wound healing and improves surgical success rates. Comparable success has been reported for both techniques.11,12
We prefer an individualized approach, with minimal resection of the fistulous tract to simplify the procedure and minimize associated complications, including recurrence.
- Latzko partial colpocleisis. This technique, first reported in 1942, remains a common procedure, with success rates of 90% to 100%.13 Advantages include a short operative time, low intraoperative and postoperative morbidity, and low risk of ureteral injury.
- Fistulectomy technique. Alternatively, to perform fistulectomy with a flap-splitting closure, begin by resecting the fistulous tract to expose healthy tissue at the wound margins. Then close the defect in a multilayer fashion, beginning with the bladder mucosa, bladder serosa, pubocervical fascia, and vaginal mucosa. Be careful to avoid tension on suture lines. In addition, create a fascial flap to prevent opposition of the incision planes and reduce the risk of recurrence.
- Grafts. In cases with a high risk of recurrence, such as complex or large fistulae, a Martius fat-pad graft should be interposed between the closure layers to promote vascularization and reduce the risk of recurrence.14 Placement of a cadaveric biomaterial graft also has been reported, reducing the need for complicated flap procedures.15
Abdominal approach. Although most vesicovaginal fistulae can be surgically corrected via the vaginal approach, the abdominal route may be preferred when the fistula is high and inaccessible, large and complex, multiple in number, or when there is concurrent uterine or bowel involvement or a need for ureteral reimplantation. The abdominal approach may be facilitated by cystoscopically guided placement of a catheter through the fistulous tract to assist in subsequent identification and dissection.
To begin, make a vertical skin incision to optimize visualization and allow mobilization of an omental flap, if necessary. Expose the bladder and perform a high extraperitoneal cystotomy to visualize the fistulous tract. Place ureteral stents if the fistula is in close proximity to the ureteral orifice.
Extend the bladder incision to the fistulous tract and completely excise it following mobilization of the vagina. Then close the vagina and bladder with interrupted, delayed absorbable suture in a double layer.
Transpose an omental flap between the vaginal and bladder incisions to promote vascularization, minimize opposition of suture lines, and reduce the risk of recurrence (FIGURE 2).
Laparoscopic approach. A similar laparoscopic repair has been reported with comparable results, but requires advanced skills with endoscopic suturing and knot tying (FIGURE 3).16
FIGURE 2 Abdominal repair
Abdominal repair of vesicovaginal fistula, with closure of bladder defect and posterior cystotomy and separate closure of vaginal defect. Note the omental flap pictured in the insert.
FIGURE 3 Laparoscopic repair
Laparoscopic repair of vesicovaginal fistula.The authors report no financial relationship with any companies whose products are mentioned in this article.
- Surgical risk factors include prior pelvic surgery, history of pelvic inflammatory disease, pelvic malignancy, endometriosis, infection, diabetes, and anatomic distortion.
- Conservative therapy should be reserved for simple fistulae that are less than 1 cm in size, diagnosed within 7 days of the index surgery, lacking associated carcinoma or radiation, and subject to at least 4 weeks of constant bladder drainage.
- In surgical repair, the Latzko partial colpocleisis or fistulectomy with flap-splitting closure is preferred.
Recent advances have improved the success of vesicovaginal fistula (VVF) repair—a challenge that can test even the most experienced gynecologic surgeon. For example, it now is apparent that some small uncomplicated fistulae respond to conservative treatment. Further, in selected cases, laparoscopic repair can eliminate the need for complicated laparotomy.
In addition, timing of fistula repair no longer requires long periods of observation, and good surgical technique for identifying and repairing bladder injuries at the time of the index surgery can often prevent the development or reduce the severity of VVF.
Vesicovaginal fistula is the most common type of urogenital fistula. Presentation and prognosis vary, depending on location and size of the defect, as well as coexisting factors such as tissue devascularization and previous radiation. However, surgical repair is associated with a high cure rate if it is performed by an experienced surgeon.
Most US cases follow gynecologic surgery
Vesicovaginal fistula was first documented in the mummified remains of Egyptian Queen Henhenit (11th Dynasty, 2050 BC), which were examined in 1923 by Derry.1 Although the exact incidence of VVF in the United States is unknown, the primary cause is gynecologic surgery, especially hysterectomy. The defect is estimated to occur in 0.01% to 0.04% of gynecologic procedures.
A study of 303 women with genitourinary fistula found that the defect was related to gynecologic surgery in 82% of cases, obstetric events in 8%, radiation therapy in 6%, and trauma or fulguration in 4%.2 Rare causes of VVF include lymphogranuloma venereum, tuberculosis, syphilis, bladder stones, and a retained foreign body in the vagina. In rare instances, spontaneous vesicouterine fistulae were reported following uncomplicated vaginal birth after cesarean section.3
Gynecologic surgery may lead to VVF due to extensive dissection between the bladder and the uterus, unrecognized bladder laceration, inappropriate stitch placement, and/or devascularization injury to the tissue planes. Concurrent ureteric involvement has been reported in as many as 10% to 15% of vesicovaginal fistula cases.
In developing countries, vesicovaginal fistulae are far more common and generally related to obstetric factors such as obstructed labor (due to unattended deliveries), small pelvic dimensions, malpresentation, poor uterine contractions, and introital stenosis.
Risk factors. Conditions that may predispose patients to VVF include prior pelvic surgery, a history of pelvic inflammatory disease, pelvic malignancy, endometriosis, infection, diabetes, and anatomic distortion. If these risk factors are present, the patient should be counseled accordingly prior to gynecologic surgery.
Correct classification crucial to surgical success
Proper classification of VVF can help the gynecologic surgeon plan operative intervention. Obstetric vesicovaginal fistulae usually are categorized according to their cause, complexity, and site of obstruction. In contrast, gynecologic fistulae are generally classified as simple or complicated (TABLE).
These levels may have important implications for the surgical approach and prognosis.4 For example, simple vesicovaginal fistulae are usually uncomplicated surgical cases with good prognosis. Complicated vesicovaginal fistulae, on the other hand, can challenge even highly practiced and skilled gynecologic surgeons and are associated with a high rate of recurrence.
Women typically present within specific intervals after the various antecedent events (pelvic surgery, childbirth, radiation therapy) with a primary complaint of constant, painless urinary incontinence. If the fistula is related to traumatic childbirth, most patients experience urine leakage within the first 24 to 48 hours. Following pelvic surgery, symptoms usually occur within the first 30 days. In contrast, radiation-induced fistulae develop over a much longer interval secondary to progressive devascularization necrosis, and may present 30 days to 30 years after the antecedent event.
Some patients report exacerbation during physical activities, which can sometimes lead to erroneous diagnosis of uncomplicated stress incontinence. If the fistula is small, intermittent leakage with increased bladder distention or physical activity may be noted.
Other patients may complain of vaginal discharge or hematuria.
If there is concurrent ureteric involvement, the patient may experience constitutional symptoms (such as fever, chills, and flank pain) or even gastrointestinal symptoms.
Physical findings. Any pooling of fluid in the vagina that is noted should be sent for analysis if the diagnosis is unclear. Next, perform a careful speculum exam that allows visualization of the entire anterior vaginal wall to identify the fistula tract (FIGURE 1). In many cases, the fistula is grossly visible.
Determine the location of the fistula in relation to the vaginal apex and bladder trigone and assess the quality of surrounding tissue (eg, presence of inflammation, edema, or infection). Fistulas near the vaginal apex may require a more complicated abdominal approach, and those close to the trigone may be associated with increased risk of ureteral injury during repair.
If the fistula is particularly small, no tract may be apparent. In such cases, bimanual exam with careful palpation of the anterior wall may help isolate the fistula (eg, when there is a surrounding zone of induration).
Office tests. If no fistula is noted despite highly suspicious signs and symptoms and careful examination, a simple office test can be performed. Using a catheter, fill the bladder with a dyed solution such as normal saline with indigo carmine and repeat the pelvic exam with a half-speculum to visualize the anterior wall. Ask the patient to cough and bear down, and identify the fistula by visualizing urine leakage.
If this test fails to locate the fistula, insert a tampon and ask the patient to perform 10 to 15 minutes of exertional maneuvers, including stair climbing and jumping in place. Then remove the tampon. Visualization of dye beyond the most distal edge of the tampon confirms the presence of a fistula.
A variation of this technique is the double-dye test: Give the patient oral phenazopyridine (Pyridium), fill the bladder with the blue-tinted solution, and insert a tampon. The presence of blue staining suggests vesicovaginal or urethrovaginal fistula, while red staining (Pyridium) suggests ureterovaginal fistula.
Other testing. Further assessment is recommended to rule out concurrent pathology and formulate an appropriate treatment plan. Routine testing should include a urinalysis and culture to exclude coexisting urinary tract infection, an electrolyte panel to evaluate renal function, and a complete blood cell count to rule out systemic infection.
Cystoscopy should be performed to visualize the fistulous tract, assess its location in relation to the ureters and trigone, assure bilateral ureteral patency, and exclude the presence of a foreign body or suture in the bladder.
In patients with a history of urogenital malignancy, biopsy of the fistula tract and urine cytology is warranted.
Comparable success rates have been reported for early and late repair of surgery-induced fistulae.
Radiologic studies are recommended prior to surgical repair of a vesicovaginal fistula to fully assess the defect and exclude the presence of multiple fistulae. An intravenous pyelogram is helpful to exclude concurrent ureterovaginal fistulae or ureteral obstruction. A targeted fistulogram may be indicated if conservative therapy is planned, including expectant management, continuous bladder drainage, fulguration, or fibrin occlusion.
FIGURE 1 Vesicovaginal fistula
Pelvic cross section depicting high vesicovaginal fistula.TABLE
Classification of vesicovaginal fistulae17
| CLASSIFICATION | DESCRIPTION |
|---|---|
| Simple |
|
| Complicated |
|
Indications for conservative management
Because spontaneous closure is uncommon, symptomatic VVF merits treatment. Appropriate therapy depends on various factors, including fistula size and location, timing from the antecedent event, severity of symptoms, quality of surrounding tissue, and clinician experience and surgical skills.
Occasionally, a fistula heals following prolonged bladder drainage through a transurethral or suprapubic catheter—provided it is diagnosed within a few days of surgery. Zimmern5 recommends a conservative approach to small fistulae if the patient’s complaints of urinary incontinence are resolved with insertion of a Foley catheter. In this case, bladder drainage should be continued for 3 weeks, followed by reevaluation of the fistula. If the fistula has diminished in size, an additional 3 weeks of catheter drainage is associated with a high rate of spontaneous closure; if there is no change, the fistula is unlikely to resolve spontaneously.5
Varying success rates have been reported for conservative management, ranging from 2% to 80%.4,6 The chances of success are apparently greater if the fistula is:
- diagnosed within 7 days of the index surgery,
- less than 1 cm in size,
- simple, without associated carcinoma or radiation, and
- subject to at least 4 weeks of constant bladder drainage.
Persistent, large, or complex fistulae are best treated surgically.
Indications for surgical management
The basic principles of fistula closure apply. They are:
- adequate exposure,
- good hemostasis,
- wide mobilization of the bladder and vagina,
- resection of devascularized tissue,
- removal of any foreign bodies,
- tension-free closure,
- nonopposition of suture lines,
- confirmation of a water-tight seal on bladder closure, and
- bladder drainage for 10 to 14 days following the repair.
Timing of the repair has been the subject of controversy and can pose a dilemma to physician and patient alike. Traditionally, an interval of 3 months was recommended between the index surgery and fistula repair, with a delay of up to 1 year when the fistula was radiation-induced. However, little data support these recommendations.
Today most experts recommend an individualized approach, delaying the surgery until inflammation and infection of the surrounding tissue have resolved. The use of estrogen, antibiotics, or steroids to facilitate healing during this period also has been recommended.7 Comparable success rates have been reported for early and late repair of surgery-induced fistulae based on these principles.8-10
Vaginal approach. Most vesicovaginal fistulae can be surgically corrected using a vaginal approach. Traditionally, a Latzko partial colpocleisis or fistulectomy with flap-splitting closure has been advocated.
Debate continues about whether resection of the fistulous tract is necessary. Some experts believe that wide resection increases the size of the fistula and, therefore, the risk of recurrence. They also maintain that the fibrous tissue surrounding the fistula helps to reinforce the surgical repair. Proponents of fistulectomy counter that resection of the fistula and exposure of healthy tissue optimizes wound healing and improves surgical success rates. Comparable success has been reported for both techniques.11,12
We prefer an individualized approach, with minimal resection of the fistulous tract to simplify the procedure and minimize associated complications, including recurrence.
- Latzko partial colpocleisis. This technique, first reported in 1942, remains a common procedure, with success rates of 90% to 100%.13 Advantages include a short operative time, low intraoperative and postoperative morbidity, and low risk of ureteral injury.
- Fistulectomy technique. Alternatively, to perform fistulectomy with a flap-splitting closure, begin by resecting the fistulous tract to expose healthy tissue at the wound margins. Then close the defect in a multilayer fashion, beginning with the bladder mucosa, bladder serosa, pubocervical fascia, and vaginal mucosa. Be careful to avoid tension on suture lines. In addition, create a fascial flap to prevent opposition of the incision planes and reduce the risk of recurrence.
- Grafts. In cases with a high risk of recurrence, such as complex or large fistulae, a Martius fat-pad graft should be interposed between the closure layers to promote vascularization and reduce the risk of recurrence.14 Placement of a cadaveric biomaterial graft also has been reported, reducing the need for complicated flap procedures.15
Abdominal approach. Although most vesicovaginal fistulae can be surgically corrected via the vaginal approach, the abdominal route may be preferred when the fistula is high and inaccessible, large and complex, multiple in number, or when there is concurrent uterine or bowel involvement or a need for ureteral reimplantation. The abdominal approach may be facilitated by cystoscopically guided placement of a catheter through the fistulous tract to assist in subsequent identification and dissection.
To begin, make a vertical skin incision to optimize visualization and allow mobilization of an omental flap, if necessary. Expose the bladder and perform a high extraperitoneal cystotomy to visualize the fistulous tract. Place ureteral stents if the fistula is in close proximity to the ureteral orifice.
Extend the bladder incision to the fistulous tract and completely excise it following mobilization of the vagina. Then close the vagina and bladder with interrupted, delayed absorbable suture in a double layer.
Transpose an omental flap between the vaginal and bladder incisions to promote vascularization, minimize opposition of suture lines, and reduce the risk of recurrence (FIGURE 2).
Laparoscopic approach. A similar laparoscopic repair has been reported with comparable results, but requires advanced skills with endoscopic suturing and knot tying (FIGURE 3).16
FIGURE 2 Abdominal repair
Abdominal repair of vesicovaginal fistula, with closure of bladder defect and posterior cystotomy and separate closure of vaginal defect. Note the omental flap pictured in the insert.
FIGURE 3 Laparoscopic repair
Laparoscopic repair of vesicovaginal fistula.The authors report no financial relationship with any companies whose products are mentioned in this article.
1. Kremling H. Labor-induced bladder injuries: historical observations. Gynakol Geburtshilfliche Rundsch. 1996;36:197-200.
2. Lee RA, Symmonds RE, Williams TJ. Current state of genitourinary fistula. Obstet Gynecol. 1998;72:313-315.
3. Miklos JR, Sze EHM, Parobeck D, et al. Vesicouterine fistula: a rare complication of vaginal birth after cesarean. Obstet Gynecol. 1995;86:638-639.
4. Elkins TE, Thompson JR. Lower urinary tract fistulas. In: Walters M, Karram MM, eds. Urogynecology and Reconstructive Pelvic Surgery. 2nd ed. St. Louis, Mo: Mosby; 1999;355-365.
5. Zimmern PE, Hadley HR, Staskin D. Genitourinary fistulas: vaginal approach for repair of vesicovaginal fistulas. Clin Obstet Gynaecol. 1985;12:403-413.
6. Davits RJ, Miranda SI. Conservative treatment of vesicovaginal fistulas by bladder drainage alone. Br J Urol. 1991;68:155-156.
7. Margolis T, Mercer LJ. Vesicovaginal fistula. Obstet Gynecol Surv. 1995;49:840-847.
8. Blaivas JG, Heritz DM, Romanzi LJ. Early versus late repair of vesicovaginal fistulas: vaginal and abdominal approaches. J Urol. 1995;153:1110-1112.
9. Blandy JP, Badenoch DF, Fowler CG. Early repair of iatrogenic injury to the ureter or bladder after gynecological surgery. J Urol. 1991;146:761-765.
10. Cruikshank SH. Early closure of posthysterectomy vesicovaginal fistulas. South Med J. 1988;81:1525-1528.
11. Raz S, Bregg KJ, Nitti VW. Transvaginal repair of vesicovaginal fistula using a peritoneal flap. J Urol. 1993;150:56-59.
12. Iselin CE, Aslan P, Webster GD. Transvaginal repair of vesicovaginal fistulas after hysterectomy by vaginal cuff excision. J Urol. 1998;160(3 Pt 1):728-730.
13. Latzko W. Postoperative vesicovaginal fistulas: genesis and therapy. Am J Surg. 1942;58:211-218.
14. Punekar SV, Buch DN, Soni AB. Martius’ labial fat pad interposition and its modification in complex lower urinary fistulae. J Postgrad Med. 1999;10:405-406.
15. Miklos JR, Kohli N. Rectovaginal fistula repair utilizing a cadaveric dermal allograft. Int Urogynecol J Pelvic Floor Dysfunct. 1999;10:405-406.
16. Nezhat CH, Nezhat F, Nezhat C. Laparoscopic repair of a vesicovaginal fistula: a case report. Obstet Gynecol. 1994;83(5 Pt 2):899-901.
17. Walters MD, Karram MM, eds. Urogynecology and Reconstructive Pelvic Surgery. 2nd ed. St. Louis, Mo: Mosby; 1999.
1. Kremling H. Labor-induced bladder injuries: historical observations. Gynakol Geburtshilfliche Rundsch. 1996;36:197-200.
2. Lee RA, Symmonds RE, Williams TJ. Current state of genitourinary fistula. Obstet Gynecol. 1998;72:313-315.
3. Miklos JR, Sze EHM, Parobeck D, et al. Vesicouterine fistula: a rare complication of vaginal birth after cesarean. Obstet Gynecol. 1995;86:638-639.
4. Elkins TE, Thompson JR. Lower urinary tract fistulas. In: Walters M, Karram MM, eds. Urogynecology and Reconstructive Pelvic Surgery. 2nd ed. St. Louis, Mo: Mosby; 1999;355-365.
5. Zimmern PE, Hadley HR, Staskin D. Genitourinary fistulas: vaginal approach for repair of vesicovaginal fistulas. Clin Obstet Gynaecol. 1985;12:403-413.
6. Davits RJ, Miranda SI. Conservative treatment of vesicovaginal fistulas by bladder drainage alone. Br J Urol. 1991;68:155-156.
7. Margolis T, Mercer LJ. Vesicovaginal fistula. Obstet Gynecol Surv. 1995;49:840-847.
8. Blaivas JG, Heritz DM, Romanzi LJ. Early versus late repair of vesicovaginal fistulas: vaginal and abdominal approaches. J Urol. 1995;153:1110-1112.
9. Blandy JP, Badenoch DF, Fowler CG. Early repair of iatrogenic injury to the ureter or bladder after gynecological surgery. J Urol. 1991;146:761-765.
10. Cruikshank SH. Early closure of posthysterectomy vesicovaginal fistulas. South Med J. 1988;81:1525-1528.
11. Raz S, Bregg KJ, Nitti VW. Transvaginal repair of vesicovaginal fistula using a peritoneal flap. J Urol. 1993;150:56-59.
12. Iselin CE, Aslan P, Webster GD. Transvaginal repair of vesicovaginal fistulas after hysterectomy by vaginal cuff excision. J Urol. 1998;160(3 Pt 1):728-730.
13. Latzko W. Postoperative vesicovaginal fistulas: genesis and therapy. Am J Surg. 1942;58:211-218.
14. Punekar SV, Buch DN, Soni AB. Martius’ labial fat pad interposition and its modification in complex lower urinary fistulae. J Postgrad Med. 1999;10:405-406.
15. Miklos JR, Kohli N. Rectovaginal fistula repair utilizing a cadaveric dermal allograft. Int Urogynecol J Pelvic Floor Dysfunct. 1999;10:405-406.
16. Nezhat CH, Nezhat F, Nezhat C. Laparoscopic repair of a vesicovaginal fistula: a case report. Obstet Gynecol. 1994;83(5 Pt 2):899-901.
17. Walters MD, Karram MM, eds. Urogynecology and Reconstructive Pelvic Surgery. 2nd ed. St. Louis, Mo: Mosby; 1999.