User login
Anterior vaginal wall prolapse: The challenge of cystocele repair
- At this time, the traditional anterior colporrhaphy with attention to apical suspension remains the gold standard.
- If only some defects of the anterior wall are addressed at the time of reconstructive surgery, failure may be more likely.
- Women with grade 3 or 4 cystoceles often have evidence of bladder outlet obstruction on urodynamic testing.
- In 52% of cases, cystoceles coexist with detrusor instability and evidence of impaired detrusor contractility.
- A thorough preoperative evaluation includes assessing the apex, having the patient strain to maximize the defect, looking for paravaginal detachments, and making every effort to “unmask” occult stress urinary incontinence.
Ask a pelvic reconstructive surgeon to name the most difficult challenge, and the answer is likely to be anterior vaginal wall prolapse. The reason: The anterior wall usually is the leading edge of prolapse and the most common site of relaxation or failure following reconstructive surgery. This appears to hold true regardless of surgical route or technique.
Short-term success rates of anterior wall repairs appear promising, but long-term outcomes are not as encouraging. Success usually is claimed as long as the anterior wall is kept above the hymen, since the patient rarely reports symptoms in these cases.
Another challenge involves the use of allografts or xenografts, which have not undergone sufficient study to determine their long-term benefit or risks in comparison with traditional repairs.
This article reviews anatomy of the anterior vaginal wall and its supports, as well as surgical technique and outcomes.
Why the anterior wall is more susceptible to prolapse
One theory is that, in comparison with the posterior compartment, the anterior wall is not as well supported by the levator plate, which counters the effects of gravity and abdominal pressure. Normally, the anterior wall rests horizontally on the posterior wall, which in turn rests on the levator plate. When the levator muscles weaken, the anterior wall is the first to fall as increasing force is placed on the connective tissue supports.
Other possibilities: The anterior compartment’s attachments to the pelvic sidewall or apex may be weaker, the anterior wall may be more elastic or less dense than the posterior wall, and the anterior wall may be more susceptible to damage during childbirth or to the effects of age and loss of estrogen.
If only some defects are addressed at surgery, failure may be more likely. Some experts believe pelvic surgeons have focused too much attention on the urethrovesical junction in patients with concomitant urinary incontinence and not enough attention on suspending the anterior wall at the apex.
For most women, it is probably a combination of many of these factors that renders the anterior compartment so vulnerable.
Anatomy of the pelvic floor
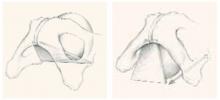
The anterior vaginal wall resembles a trapezoidal plane due to ventral and more medial attachments near the pubic symphysis, and dorsal and more lateral attachments near the ischial spine (FIGURE 1).1 This helps explain the many appearances of the cystocele. The type of cystocele is defined by the location of the break in the fascial attachments.
Paravaginal defects. The trapezoidal anterior wall is suspended on both sides from the parietal fascia overlying the levator ani muscles at the arcus tendineus fascia pelvis (ATFP). Prolapse can occur when there is loss of attachment to the pelvic sidewall at any point between the pubis and ischial spine.
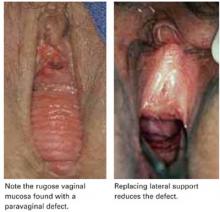
First described by White2 and characterized later by Richardson et al,3 this loss of lateral attachment is called a paravaginal defect or displacement cystocele (FIGURE 2). The goal of paravaginal repair is to reattach the lateral vaginal walls to the ATFP, either abdominally, laparoscopically, or vaginally.
Central defects, the rarest type of anterior wall prolapse, involve a loss of support near the pubis and tend to be smaller. The most common manifestation is urethral hypermobility.
Transverse defects occur when the top of the pubocervical fascia detaches from the cervix or vaginal apex, both of which are suspended from the uterosacral-cardinal ligament complex. A transverse cystocele is evidenced by loss of the anterior fornix. The anterior wall appears to be attenuated in the midline, and the vaginal mucosa is pale, thin, and smooth (FIGURE 3).
Goals of traditional repair. The traditional anterior colporrhaphy aims to excise or reinforce the attenuated transverse defect with plication of the “endopelvic fascia” in the midline of the anterior vaginal wall. The endopelvic fascia is not true fascia but the muscularis of the vaginal wall. It is comprised of smooth muscle and elastin along with the collagenous adventitia layer.4
The importance of restoring apical wall support becomes apparent when one considers the trapezoidal anatomy. The most common sites of defects or detachments of the anterior wall are near the ischial spines laterally. In an operative case series of paravaginal defects, DeLancey1 found the site of defect to be near the ischial spine in 96% of cases. The reattachment of the apex near the level of the spine becomes the highest point of support for the anterior vaginal wall.
This cephalad apical attachment can be accomplished in a variety of ways, by suspending the vaginal apex from the uterosacral ligaments, from the sacrospinous ligament, or via abdominal sacrocolpopexy.
FIGURE 1 Anatomy of the anterior wall
The anterior vaginal wall resembles a trapezoidal plane, with ventral and more medial attachments near the pubic symphysis, and dorsal and more lateral attachments to the ischial spine. Detachment from the pelvic sidewall and ischial spine results in anterior wall prolapse (right).
FIGURE 2 Paravaginal defect
FIGURE 3 Transverse defect
A transverse defect with loss of the anterior fornix. The loss of cephalad apical attachment at the level of the ischial spine leads to anterior wall prolapse. Suspending the upper vagina from shortened cardinal/uterosacral ligaments, the sacrospinous ligament, or via abdominal sacrocolpopexy is as important as plication.
Symptoms of anterior wall prolapse
As with other forms of pelvic organ prolapse, many patients complain of a bulge or feeling of pelvic pressure when the anterior vaginal wall has come through the introitus. However, some symptoms of anterior wall prolapse are unique.
Incontinence is not universal. A common misperception is that most patients with cystocele also experience stress urinary incontinence (SUI), which can develop when there is loss of urethral support and descent of the lower vaginal wall along with urethral hypermobility. However, there is no defining degree of hypermobility that links anterior wall prolapse with SUI. That is because the continence mechanism relies not only on urethral position and lateral attachments, but also on the neuromuscular function of the pelvis and lower urinary tract.
In fact, descent of the midvagina under the bladder base may actually reduce the chance of SUI. The reason: As a woman strains, the increased abdominal pressure pushes the cystocele farther and farther out. As the cystocele enlarges, it creates a functional outlet obstruction by kinking the urethra shut. When this is the case, patients may complain of prolonged voiding, an intermittent urine stream, and/or urinary retention. The woman may have to elevate the vaginal wall to empty her bladder. Patients with chronic urinary retention are at risk of developing recurrent urinary tract infections.
Bladder outlet obstruction and detrusor dysfunction. Women with grade 3 or 4 cystoceles often have evidence of bladder outlet obstruction on urodynamic testing, according to a study that found such evidence in 57% of subjects.5 After reduction of the prolapse with a pessary, obstructed flow reverted to normal in 94% of these women.
A large proportion (52%) of women with cystoceles also have detrusor instability, as well as evidence of impaired detrusor contractility. Many complain of urinary frequency and urgency and difficulty emptying the bladder.5
Again, this phenomenon is complex, related not only to anatomy but to altered neuromuscular function of the lower urinary tract. Incomplete emptying, frequency, and urgency may arise from stretching of the bladder base as it prolapses through the vaginal introitus, resulting in urinary retention. These symptoms often are less pronounced at night when the patient is supine.
We reviewed 35 cases of anterior wall prolapse greater than 1 cm outside the hymen, with elevated postvoid residuals exceeding 100 cc on 2 separate occasions.6 Thirty-one (89%) had normal postvoid residuals following reconstructive surgery and correction of their anterior wall prolapse.
Preoperative assessment
A careful physical exam is a prerequisite for all surgical repairs of pelvic organ prolapse. During this exam, identify the sites of defects and detachments.
Maximize the defect. Have the patient perform the Valsalva maneuver, cough, and/or strain while sitting upright or standing. As she is performing these maneuvers, ask her if this feels like her maximum prolapse. A split speculum often aids in visualizing the anterior and posterior compartments without pressure from the opposite vaginal wall.
Assess the apex. Place a large swab in the vagina, hold it gently against the apex, and ask the patient to strain. If the swab is pushed out, the apex needs suspension.
This technique can help identify apical relaxation that may be masked by a large anterior or posterior wall defect. A standardized staging system, such as the Pelvic Organ Prolapse Quantitative Examination (POP-Q) or Baden-Walker, aids in communicating and documenting the prolapse. In addition, it allows the surgeon to track anatomical outcomes after surgery.
Look for paravaginal defects by supporting the lateral anterior walls with a ring forceps at the level of the ATFP. Barber et al7 found this maneuver to be highly sensitive (90–94%): If no paravaginal defect was suspected clinically, none was found intraoperatively. However, the positive predictive value was poor (57%), in that defects suspected preoperatively were confirmed during surgery in less than two thirds of patients.
These findings point to the importance of careful intraoperative assessment, both before and during the repair procedure.
Limited utility of imaging studies. The use of radiologic studies such as defecography or dynamic magnetic resonance imaging of the pelvis may aid in the evaluation of defecatory disorders or suspected sigmoidocele or rectal prolapse, but have not been studied sufficiently to determine the impact on surgical outcome.
Unmasking SUI
As mentioned above, women with anterior wall prolapse do not always complain of stress incontinence. However, correction of the cystocele can relieve their obstructive voiding and unmask “occult” SUI. Various techniques have been described to elevate the anterior wall with pessaries, swabs, etc, during urodynamic testing to predict which women should have an incontinence procedure performed at the time of reconstructive surgery.
Conflicting rates of occult SUI have been reported, with estimates ranging from 36% to 80%.8 Although preoperative urodynamic testing indicates a high rate of occult stress incontinence, a study by Borstad et al9 suggests that the rate of de novo incontinence may be lower and that preoperative urodynamic findings are not predictive of postoperative continence status. In that study, 16 of 73 women (22%) developed stress incontinence following surgery for prolapse when no incontinence procedure was performed. Advanced age increased the risk of incontinence after surgery.
Contrast these findings with those of Chaikin and colleagues,10 who prospectively followed 24 patients with grade 3 or 4 cystoceles. Preoperative urodynamics showed a 58% rate of occult stress incontinence. All these patients were also defined as having intrinsic sphincter deficiency with leak point pressures below 60 cm water. The incontinent group underwent anterior colporrhaphy and concomitant pubovaginal sling, compared with anterior colporrhaphy alone for those without incontinence. Postoperatively, 2 patients who had the pubovaginal sling procedure reported continued stress incontinence (14%). No new symptoms of incontinence were reported in the patients without leakage on preoperative urodynamics. Thus, preoperative urodynamics were 100% accurate in determining which women did not need additional surgery for SUI.
Implications of a negative stress test. Our experience has shown that, despite our best attempts, a negative stress test with the prolapse reduced prior to surgery is less than 100% predictive. Occasionally, new SUI occurs after reconstructive surgery. It is unclear whether this incontinence is caused by straightening the urethra and reducing the bulge or secondary to the dissection of surgery.
Tips on technique
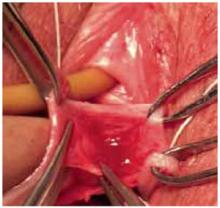
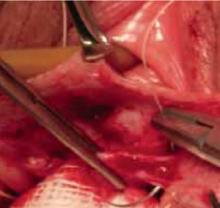
Anterior colporrhaphy traditionally is performed with plication of the “endopelvic fascia” or fibromuscular layer at the bladder neck with a Kelly plication stitch. Using “3-point” traction aids in dissecting the muscularis (FIGURE 4). Repair the remainder of the cystocele using vertical mattress stitches (1 or 2 layers) from the bladder neck to the apex.
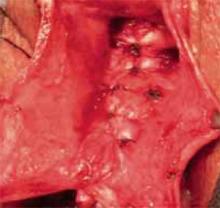
Avoid creating weak areas. Using this technique, the repair frequently stops short of the apex, leaving a “gap” or weak area. One way to avoid this is to begin plication at the apex instead of the bladder neck (FIGURE 5).
Next, excise the excess vaginal tissue and close with interrupted fine absorbable sutures (FIGURE 6).
Recreate apical support. Another problem with traditional repairs is that they do not reestablish apical support. In many patients with anterior wall prolapse, reattachment of the apex reduces the cystocele. Therefore, it often is necessary to combine anterior colporrhaphy with an apical repair procedure such as uterosacral ligament suspension or sacrospinous ligament suspension.
Sutures for the apical repair should be placed and held prior to initiating the anterior colporrhaphy. At the end of the anterior repair, incorporate the apical sutures into the vaginal cuff.
Careful attention to the integrity and strength of the tissue is crucial. Regardless of the type of transvaginal suspension, we advocate bringing 1 arm of the suspension suture through the anterior wall of the cuff. Then place the other suture arm through the posterior cuff so that, when tied, anterior and posterior walls are brought together and suspended.
Using prolonged-delayed absorbable suture allows for a full-thickness bite, ensuring scarring to the suspensory ligament. If permanent suture is used for the uterosacral suspension, place the stitches along the inside surface of the anterior wall with a strong, broad bite that incorporates the muscularis or “endopelvic fascia.”
The occasional enterocele. When a transverse cystocele occurs following hysterectomy, the surgeon should be on the lookout for an enterocele, which sometimes accompanies anterior wall prolapse. The enterocele should be corrected at the time of surgery by closing the defect and suspending the cuff.
FIGURE 4 Three-point traction
Three-point traction using Allis clamps. The assistant retracts with DeBackey forceps to allow dissection of the muscularis. An index finger placed firmly against the vaginal mucosa enables the surgeon to judge depth of dissection.
FIGURE 5 Begin plication at the apex
Plication begins at the apex with vertical mattress stitches. Use 3-0 prolonged delayed absorbable or permanent suture in the anterior wall.
FIGURE 6 The reduced cystocele
The cystocele reduced following midline plication of the vaginal muscularis. The excess vagina is then trimmed and closed with interrupted 3-0 absorbable suture.
Functional surgical outcomes
Because of the long association between anterior wall prolapse and SUI, most surgeons evaluate patients preoperatively to determine the need for concomitant incontinence procedures. As a result, the literature reporting surgical cystocele repair via anterior colporrhaphy frequently uses continence of urine as the functional outcome. This is not surprising considering that anterior repair and Kelly plication, as reported by Howard Kelly more than 75 years ago, have been the gold standard for surgical correction of anterior wall prolapse and SUI.
In a series of 194 SUI patients who underwent anterior colporrhaphy, Beck11 found that adding a modified Kelly plication, including a vaginal retropubic urethropexy, increased the cure rate for SUI from 75% to 94%. Unfortunately, he did not report the anatomic success of the anterior colporrhaphy.
Kohli et al12 also retrospectively examined patients who had undergone anterior colporrhaphy with and without needle bladder-neck suspension. Although the cure rate for SUI was not reported, patients who underwent concomitant needle suspension had a higher rate of recurrent cystocele: 33% (n = 40) versus 7% (n = 27). Investigators theorized that retropubic dissection at the time of transvaginal needle suspension resulted in an iatrogenic paravaginal defect and denervation of the anterior vaginal wall.
The risks of needle suspension. A randomized controlled trial by Bump et al8 also suggests that needle suspension should be avoided. In that trial, 29 patients with stage 3 and 4 prolapse were randomized to needle colposuspension or endopelvic fascia plication. They, too, found that needle colposuspension carried a higher rate of recurrent anterior prolapse. Further, it did not reduce the rates of SUI compared with fascia plication.
Although incontinence surgery performed at the time of cystocele repair will reduce the rates of de novo incontinence, the higher rates of cystocele recurrence associated with some procedures warrants judicious preoperative planning. Clearly, needle suspension should not be performed as an incontinence procedure or repair of anterior wall prolapse. Whether other vaginal incontinence procedures, eg, midurethral slings, lead to recurrence of anterior wall prolapse deserves further investigation.
Anatomic outcomes
Midline colporrhaphy. Because anterior colporrhaphy is rarely performed alone, few series describe patients having undergone simply an anterior repair. Stanton et al13 followed 54 women for up to 2 years after they underwent traditional midline plication with vaginal hysterectomy for prolapse. Eight (15%) of the women had recurrent anterior wall prolapse.
Colombo et al14 randomized 71 women with clinical SUI and stage 2 or 3 prolapse to Burch colposuspension or anterior colporrhaphy with Kelly plication. All women were followed for at least 8 years. The cure rate for SUI was 86% for the Burch procedure, compared with 52% for anterior repair and Kelly plication. However, 12 (34%) women treated with Burch colposuspension and 1 (3%) treated with anterior colporrhaphy had recurrent cystocele of grade 2 or 3 with or without prolapse at other vaginal sites.
Abdominal paravaginal repair. Shull15 followed 149 women for 6 months to 4 years after they underwent abdominal paravaginal repair with the urethrovesical stitch brought through Cooper’s ligament for treatment of SUI and paravaginal cystocele. He reported a 5% recurrence of anterior wall prolapse.
In another series, Shull16 reported on 62 women who were followed for a mean of 18 months after abdominal paravaginal repair. Four of 57 (7%) had recurrent vaginal prolapse to the hymen.
In a cohort of 102 patients evaluated with the Pelvic Organ Prolapse Quantitative Examination (POP-Q) at our institution, the maximal point of prolapse was the anterior wall in 60% of cases; the apex and posterior wall each accounted for roughly half the remaining cases (unpublished data).
Ellerkmann et al30 reported on 237 consecutive patients who presented with symptoms of pelvic organ prolapse. In 77 women (33%), anterior compartment pelvic organ prolapse predominated; 46 patients (19%) had posterior compartment prolapse; and 22 patients (11%) had apical prolapse.
Hendrix et al31 analyzed patients from the Women’s Health Initiative (WHI) and found that the anterior compartment predominated over the posterior compartment. In a follow-up study from the WHI, Handa32 reported on 412 women followed for 2 to 8 years. Among those who entered the WHI protocol without cystocele, 1 in 4 was diagnosed with it at some point in the study. This compares to 1 in 6 for rectocele and 1 in 100 for uterine prolapse. The majority of all defects were grade 1 or relaxation above the hymen.
Vaginal paravaginal repair. Mallipeddi et al17 reported on 45 patients undergoing vaginal paravaginal repair over 2 years, with 35 women followed for a mean of 1.6 years. Incontinent patients had a Kelly plication performed at the time of vaginal paravaginal repair. Recurrence rates were 3% for cystocele, 14% for rectocele, and 20% for enterocele.
Young et al18 followed 100 women for as long as 36 months after bilateral paravaginal repair using 1 to 6 expanded polytetrafluoroethylene (Gore-Tex) CV-0 sutures and midline colporrhaphy. Two patients had grade 1 or 2 failure at the lateral fixation points, but 21 patients had recurrent midline defects, all but 1 inside the hymen. Several patients had bloody discharge from the permanent sutures.
Sacrocolpopexy. Brubaker19 retrospectively reviewed 65 women who underwent sacrocolpopexy for apical prolapse. Three months postoperatively 19 patients (29%) had persistent anterior wall defects.
Uterosacral suspension. Shull et al20 also found the anterior segment to have the most recurrent defects. In that study, which had an average follow-up of a little over a year, 289 patients underwent vaginal uterosacral ligament repair of the apex, and 264 had an anterior wall defect preoperatively. At the time of furthest follow-up, 26 patients (9%) had failure at this site. This study confirmed that the anterior compartment is the most likely site to fail, and also that it fails the quickest.
Sacrospinous ligament suspension (SSLS). Morley and DeLancey21 found a 22% cystocele recurrence rate in 71 women 1 year after SSLS, with most of them asymptomatic. Shull22 reported a 30% incidence of cystoceles after SSLS. Paraiso and colleagues23 reported on 243 women undergoing SSLS and pelvic reconstructive surgery. Of these, 217 patients underwent concomitant anterior colporrhaphy. Follow-up at 74 months found 37% with symptomatic recurrence at the anterior wall, 13% at the posterior wall, and 8% at the apex.
Vaginal versus abdominal repair
Few studies have compared vaginal and abdominal repair of pelvic organ prolapse, including anterior wall prolapse.
In a trial by Benson et al,24 women with prolapse to or beyond the hymen were randomized to bilateral sacrospinous vault suspension and vaginal paravaginal repair (n = 48) or abdominal sacrocolpopexy with abdominal paravaginal repair (n = 40). One third of patients in each group also underwent anterior colporrhaphy. After a mean follow-up of 2.5 years, 16 of 20 women required reoperation for recurrent cystocele—12 (29%) from the vaginal group and 4 (10.5%) from the abdominal group. Vaginal vault eversion recurred in 5 women from the vaginal group and 1 from the abdominal group.
Investigators concluded that the anterior wall was the most likely site of failure because of the posterior placement of the vaginal apex with SSLS, predisposing the anterior wall to greater pressures and to neuropathy caused by lateral dissection of the anterior wall. Earlier studies have demonstrated that neuropathy may occur after extensive dissection of the vaginal wall and may affect the strength and integrity of the muscular support tissues.25,26
Allografts and xenografts
The difficulty of repairing anterior wall prolapse has led some pelvic surgeons to use mesh for cystocele repair. When Julian27 randomized 24 patients with recurrent cystocele to transvaginal repair with and without polypropylene (Marlex) mesh, 4 patients in the control group and no patients in the mesh group had recurrences (P <.05). However, 3 patients (25%) had mesh-related complications.
Weber et al28 randomized patients to standard midline plication, plication of the paravaginal tissue more laterally, or standard plication plus polyglactin 910 (Vicryl) mesh. Among 83 patients who returned for follow-up, there were no differences in anatomic outcome. Weber and colleagues concluded that there is little benefit to using mesh to correct cystoceles.
Still, although the overall cure rate was low (30–46%), most patients had cystocele to the hymen and not beyond, with significant improvement of symptoms. Although this cannot be defined as an anatomic cure, it is encouraging that the majority of patients appear to have benefited from surgery.
Sand et al29 randomized 161 women with the anterior wall to or beyond the hymen to traditional anterior colporrhaphy with or without Vicryl. The 2-inch square mesh was not placed over the repair as described above, but was folded into the anterior colporrhaphy stitches. At 1 year, 16 (22%) of 73 women with mesh and 28 (40%) of the 88 women without mesh had recurrent central cystoceles beyond the midvagina (P = .02). No women had cystoceles beyond the hymen or vaginal erosions.
Difficulty of interpreting the evidence
Because of the broad range of study designs, small number of patients per series, variety of concomitant procedures, and wide range of variables used to describe recurrence and success, it is difficult to draw conclusions from the literature. The evidence does suggest that the risks of wide vaginal dissection required for vaginal paravaginal repair outweigh the benefits. As a result, we have abandoned this technique. As mentioned above, it remains unclear whether graft materials will prove to be of long-term benefit for either midline plication or paravaginal repair.
The gold standard, for now
Prolapse of the anterior vaginal wall remains a challenge for the gynecologic surgeon. Careful preoperative and intraoperative evaluation and identification of support defects should guide repairs.
Randomized, controlled trials of midline versus paravaginal repair, as well as use of various graft materials, are greatly needed. These studies should not only address recurrence of prolapse symptoms, but the impact of surgery on sexual and lower urinary tract function.
At this time, the traditional anterior colporrhaphy with attention to apical suspension remains the gold standard.
The authors report no financial relationships relevant to this article.
1. DeLancey JOL. Fascial and muscular abnormalities in women with urethral hypermobility and anterior vaginal wall prolapse. Am J Obstet Gynecol. 2002;187:93-98.
2. White GR. Cystocele: a radical cure by suturing lateral sulci of vagina to white line of pelvic fascia. JAMA. 1909;21:1707-1710.
3. Richardson AC, Lyon JB, Williams NL. A new look at pelvic relaxation. Am J Obstet Gynecol. 1976;126:568-571.
4. Weber AM, Walters MD. Anterior vaginal prolapse: review of anatomy and techniques of surgical repair. Obstet Gynecol. 1997;89:311-318.
5. Romanzi LJ, Chaikin DC, Blaivas JG. The effect of genital prolapse on voiding. J Urol. 1999;161:581-586.
6. FitzGerald MP, Kulkarni N, Fenner D. Postoperative resolution of urinary retention in patients with advanced pelvic organ prolapse. Am J Obstet Gynecol. 2000;183:1361-1364.
7. Barber MD, Cundiff GW, Weidner AC, Cotes KW, Bump RC, Addison WA. Accuracy of clinical assessment of paravaginal defects in women with anterior vaginal wall proloapse. Am J Obstet Gynecol. 1999;181:1-7.
8. Bump RC, Hurt WG, Theofrastous JP, et al. Randomized prospective comparison of needle colposuspension versus endopelvic fascia plication for potential stress incontinence prophylaxis in women undergoing vaginal reconstruction for stage III or IV pelvic organ prolapse. The Continence Program for Women Research Group. Am J Obstet Gynecol. 1996;175:326-333.
9. Borstad E, Rud T. The risk of developing urinary stress incontinence after vaginal repair in continent women. Acta Obstet Gynecol Scand. 1989;68:545-549.
10. Chaikin DC, Groutz A, Blaivas JG. Predicting the need for anti-incontinence surgery in continent women undergoing repair of severe urogenital prolapse. J Urol. 2000;163:531-534.
11. Beck RP, McCormick S, Nordstrom L. A 25-year experience with 519 anterior colporrhaphy procedures. Obstet Gynecol. 1991;78:1011-1018.
12. Kohli N, Sze EHM, Roat TW, Karram M. Incidence of recurrent cystocele after anterior colporrhaphy with and without concomitant transvaginal needle suspension. Am J Obstet Gynecol. 1996;175:1476-1482.
13. Stanton SL, Hilton P, Norton C, Cardozo L. Clinical and urodynamics effect of anterior colporrhaphy and vaginal hysterectomy for prolapse with and without incontinence. Br J Obstet Gynecol. 1982;89:459-463.
14. Colombo M, Vitobello D, Proiette F, Milani R. Randomized comparison of Burch colposuspension versus anterior colporrhaphy in women with stress urinary incontinence and anterior vaginal wall prolapse. BJOG. 2000;107:544-551.
15. Shull BL, Baden WF. A six-year experience with paravaginal defect repair for stress urinary incontinence. Am J Obstet Gynecol. 1989;160:1432-1440.
16. Shull BL, Benn SJ, Kuehl TJ. Surgical management of prolapse of the anterior vaginal segment: an analysis of support defects, operative morbidity, and anatomic outcome. Am J Obstet Gynecol. 1994;171:1429-1439.
17. Mallipeddi PK, Steele AC, Kohli N, Karram MM. Anatomic and functional outcome of vaginal paravaginal repair in the correction of anterior wall prolapse. Int Urogynecol J. 2001;12:83-88.
18. Young SB, Daman JJ, Bony LG. Vaginal paravaginal repair: one-year outcomes. Am J Obstet Gynecol. 2001;185:1360-1367.
19. Brubaker L. Sacrocolpopexy and the anterior compartment: support and function. Am J Obstet Gynecol. 1995;173:1690-1694.
20. Shull BL, Bachofen C, Coates KW, Kuehl TJ. A transvaginal approach to repair of apical and other associated sites of pelvic organ prolapse with uterosacral ligaments. Am J Obstet Gynecol. 2000;183:1365-1374.
21. Morley GW, DeLancey JOL. Sacrospinous ligament fixation for eversion of the vagina. Am J Obstet Gynecol. 1988;158:872-881.
22. Shull BL, Capen CV, Riggs MW, Kuehl TJ. Preoperative and postoperative analysis of site-specific pelvic support defects in 81 women treated with sacrospinous ligament suspension and pelvic reconstruction. Am J Obstet Gynecol. 1992;166:1764-1771.
23. Paraiso MFR, Ballard LA, Walter MD, et al. Pelvic support defects and visceral and sexual function in women treated with sacrospinous ligament suspension and pelvic reconstruction. Am J Obstet Gynecol. 1996;175:1423-1431.
24. Benson JT, Lucente V, McClellan E. Vaginal versus abdominal reconstructive surgery for the treatment of pelvic support defects: a prospective randomized study with long-term outcome evaluation. Am J Obstet Gynecol. 1996;175:1418-1421.
25. Benson JT, McClellan E. The effect of vaginal dissection of the pudendal nerve. Obstet Gynecol. 1993;82:387-389.
26. Zivkovic F, Tamussino K, Ralph G, et al. Long-term effects of vaginal dissection on the innervation of the striated urethral sphincter. Obstet Gynecol. 1996;87:257-260.
27. Julian T. The efficacy of Marlex mesh in the repair of severe, recurrent vaginal prolapse of the anterior midvaginal wall. Am J Obstet Gynecol. 1996;175:1472-1475.
28. Weber AM, Walters MD, Piedmonte MR, et al. Anterior colporrhaphy: a randomized trial of three surgical techniques. Am J Obstet Gynecol. 2001;185:1299-1306.
29. Sand PK, et al. Prospective randomized trial of polyglactin 910 mesh to prevent recurrence of cystocele and rectocele. Am J Obstet Gynecol. 2001;184:1357-1364.
30. Ellerkmann RM, Cundiff GW, Melick CF, Nihira MA, Leffler K, Bent AE. Correlation of symptoms with location and severity of pelvic organ prolapse. Am J Obstet Gynecol. 2001;185:1332-1337.
31. Hendrix SL, Clark A, Nygaard I, Aragaki A, Barnabei V, McTiernan A. Pelvic organ prolapse in the Women’s Health Initiative: gravity and gravidity. Am J Obstet Gynecol. 2002;186:1160-1166.
32. Handa VL, Garrett E, Hendrix S, Gold E, Robbins J. Progression and remission of pelvic organ prolapse: a longitudinal study of menopausal women. Am J Obstet Gynecol. 2004;190:27-32.
- At this time, the traditional anterior colporrhaphy with attention to apical suspension remains the gold standard.
- If only some defects of the anterior wall are addressed at the time of reconstructive surgery, failure may be more likely.
- Women with grade 3 or 4 cystoceles often have evidence of bladder outlet obstruction on urodynamic testing.
- In 52% of cases, cystoceles coexist with detrusor instability and evidence of impaired detrusor contractility.
- A thorough preoperative evaluation includes assessing the apex, having the patient strain to maximize the defect, looking for paravaginal detachments, and making every effort to “unmask” occult stress urinary incontinence.
Ask a pelvic reconstructive surgeon to name the most difficult challenge, and the answer is likely to be anterior vaginal wall prolapse. The reason: The anterior wall usually is the leading edge of prolapse and the most common site of relaxation or failure following reconstructive surgery. This appears to hold true regardless of surgical route or technique.
Short-term success rates of anterior wall repairs appear promising, but long-term outcomes are not as encouraging. Success usually is claimed as long as the anterior wall is kept above the hymen, since the patient rarely reports symptoms in these cases.
Another challenge involves the use of allografts or xenografts, which have not undergone sufficient study to determine their long-term benefit or risks in comparison with traditional repairs.
This article reviews anatomy of the anterior vaginal wall and its supports, as well as surgical technique and outcomes.
Why the anterior wall is more susceptible to prolapse
One theory is that, in comparison with the posterior compartment, the anterior wall is not as well supported by the levator plate, which counters the effects of gravity and abdominal pressure. Normally, the anterior wall rests horizontally on the posterior wall, which in turn rests on the levator plate. When the levator muscles weaken, the anterior wall is the first to fall as increasing force is placed on the connective tissue supports.
Other possibilities: The anterior compartment’s attachments to the pelvic sidewall or apex may be weaker, the anterior wall may be more elastic or less dense than the posterior wall, and the anterior wall may be more susceptible to damage during childbirth or to the effects of age and loss of estrogen.
If only some defects are addressed at surgery, failure may be more likely. Some experts believe pelvic surgeons have focused too much attention on the urethrovesical junction in patients with concomitant urinary incontinence and not enough attention on suspending the anterior wall at the apex.
For most women, it is probably a combination of many of these factors that renders the anterior compartment so vulnerable.
Anatomy of the pelvic floor
The anterior vaginal wall resembles a trapezoidal plane due to ventral and more medial attachments near the pubic symphysis, and dorsal and more lateral attachments near the ischial spine (FIGURE 1).1 This helps explain the many appearances of the cystocele. The type of cystocele is defined by the location of the break in the fascial attachments.
Paravaginal defects. The trapezoidal anterior wall is suspended on both sides from the parietal fascia overlying the levator ani muscles at the arcus tendineus fascia pelvis (ATFP). Prolapse can occur when there is loss of attachment to the pelvic sidewall at any point between the pubis and ischial spine.
First described by White2 and characterized later by Richardson et al,3 this loss of lateral attachment is called a paravaginal defect or displacement cystocele (FIGURE 2). The goal of paravaginal repair is to reattach the lateral vaginal walls to the ATFP, either abdominally, laparoscopically, or vaginally.
Central defects, the rarest type of anterior wall prolapse, involve a loss of support near the pubis and tend to be smaller. The most common manifestation is urethral hypermobility.
Transverse defects occur when the top of the pubocervical fascia detaches from the cervix or vaginal apex, both of which are suspended from the uterosacral-cardinal ligament complex. A transverse cystocele is evidenced by loss of the anterior fornix. The anterior wall appears to be attenuated in the midline, and the vaginal mucosa is pale, thin, and smooth (FIGURE 3).
Goals of traditional repair. The traditional anterior colporrhaphy aims to excise or reinforce the attenuated transverse defect with plication of the “endopelvic fascia” in the midline of the anterior vaginal wall. The endopelvic fascia is not true fascia but the muscularis of the vaginal wall. It is comprised of smooth muscle and elastin along with the collagenous adventitia layer.4
The importance of restoring apical wall support becomes apparent when one considers the trapezoidal anatomy. The most common sites of defects or detachments of the anterior wall are near the ischial spines laterally. In an operative case series of paravaginal defects, DeLancey1 found the site of defect to be near the ischial spine in 96% of cases. The reattachment of the apex near the level of the spine becomes the highest point of support for the anterior vaginal wall.
This cephalad apical attachment can be accomplished in a variety of ways, by suspending the vaginal apex from the uterosacral ligaments, from the sacrospinous ligament, or via abdominal sacrocolpopexy.
FIGURE 1 Anatomy of the anterior wall
The anterior vaginal wall resembles a trapezoidal plane, with ventral and more medial attachments near the pubic symphysis, and dorsal and more lateral attachments to the ischial spine. Detachment from the pelvic sidewall and ischial spine results in anterior wall prolapse (right).
FIGURE 2 Paravaginal defect
FIGURE 3 Transverse defect
A transverse defect with loss of the anterior fornix. The loss of cephalad apical attachment at the level of the ischial spine leads to anterior wall prolapse. Suspending the upper vagina from shortened cardinal/uterosacral ligaments, the sacrospinous ligament, or via abdominal sacrocolpopexy is as important as plication.
Symptoms of anterior wall prolapse
As with other forms of pelvic organ prolapse, many patients complain of a bulge or feeling of pelvic pressure when the anterior vaginal wall has come through the introitus. However, some symptoms of anterior wall prolapse are unique.
Incontinence is not universal. A common misperception is that most patients with cystocele also experience stress urinary incontinence (SUI), which can develop when there is loss of urethral support and descent of the lower vaginal wall along with urethral hypermobility. However, there is no defining degree of hypermobility that links anterior wall prolapse with SUI. That is because the continence mechanism relies not only on urethral position and lateral attachments, but also on the neuromuscular function of the pelvis and lower urinary tract.
In fact, descent of the midvagina under the bladder base may actually reduce the chance of SUI. The reason: As a woman strains, the increased abdominal pressure pushes the cystocele farther and farther out. As the cystocele enlarges, it creates a functional outlet obstruction by kinking the urethra shut. When this is the case, patients may complain of prolonged voiding, an intermittent urine stream, and/or urinary retention. The woman may have to elevate the vaginal wall to empty her bladder. Patients with chronic urinary retention are at risk of developing recurrent urinary tract infections.
Bladder outlet obstruction and detrusor dysfunction. Women with grade 3 or 4 cystoceles often have evidence of bladder outlet obstruction on urodynamic testing, according to a study that found such evidence in 57% of subjects.5 After reduction of the prolapse with a pessary, obstructed flow reverted to normal in 94% of these women.
A large proportion (52%) of women with cystoceles also have detrusor instability, as well as evidence of impaired detrusor contractility. Many complain of urinary frequency and urgency and difficulty emptying the bladder.5
Again, this phenomenon is complex, related not only to anatomy but to altered neuromuscular function of the lower urinary tract. Incomplete emptying, frequency, and urgency may arise from stretching of the bladder base as it prolapses through the vaginal introitus, resulting in urinary retention. These symptoms often are less pronounced at night when the patient is supine.
We reviewed 35 cases of anterior wall prolapse greater than 1 cm outside the hymen, with elevated postvoid residuals exceeding 100 cc on 2 separate occasions.6 Thirty-one (89%) had normal postvoid residuals following reconstructive surgery and correction of their anterior wall prolapse.
Preoperative assessment
A careful physical exam is a prerequisite for all surgical repairs of pelvic organ prolapse. During this exam, identify the sites of defects and detachments.
Maximize the defect. Have the patient perform the Valsalva maneuver, cough, and/or strain while sitting upright or standing. As she is performing these maneuvers, ask her if this feels like her maximum prolapse. A split speculum often aids in visualizing the anterior and posterior compartments without pressure from the opposite vaginal wall.
Assess the apex. Place a large swab in the vagina, hold it gently against the apex, and ask the patient to strain. If the swab is pushed out, the apex needs suspension.
This technique can help identify apical relaxation that may be masked by a large anterior or posterior wall defect. A standardized staging system, such as the Pelvic Organ Prolapse Quantitative Examination (POP-Q) or Baden-Walker, aids in communicating and documenting the prolapse. In addition, it allows the surgeon to track anatomical outcomes after surgery.
Look for paravaginal defects by supporting the lateral anterior walls with a ring forceps at the level of the ATFP. Barber et al7 found this maneuver to be highly sensitive (90–94%): If no paravaginal defect was suspected clinically, none was found intraoperatively. However, the positive predictive value was poor (57%), in that defects suspected preoperatively were confirmed during surgery in less than two thirds of patients.
These findings point to the importance of careful intraoperative assessment, both before and during the repair procedure.
Limited utility of imaging studies. The use of radiologic studies such as defecography or dynamic magnetic resonance imaging of the pelvis may aid in the evaluation of defecatory disorders or suspected sigmoidocele or rectal prolapse, but have not been studied sufficiently to determine the impact on surgical outcome.
Unmasking SUI
As mentioned above, women with anterior wall prolapse do not always complain of stress incontinence. However, correction of the cystocele can relieve their obstructive voiding and unmask “occult” SUI. Various techniques have been described to elevate the anterior wall with pessaries, swabs, etc, during urodynamic testing to predict which women should have an incontinence procedure performed at the time of reconstructive surgery.
Conflicting rates of occult SUI have been reported, with estimates ranging from 36% to 80%.8 Although preoperative urodynamic testing indicates a high rate of occult stress incontinence, a study by Borstad et al9 suggests that the rate of de novo incontinence may be lower and that preoperative urodynamic findings are not predictive of postoperative continence status. In that study, 16 of 73 women (22%) developed stress incontinence following surgery for prolapse when no incontinence procedure was performed. Advanced age increased the risk of incontinence after surgery.
Contrast these findings with those of Chaikin and colleagues,10 who prospectively followed 24 patients with grade 3 or 4 cystoceles. Preoperative urodynamics showed a 58% rate of occult stress incontinence. All these patients were also defined as having intrinsic sphincter deficiency with leak point pressures below 60 cm water. The incontinent group underwent anterior colporrhaphy and concomitant pubovaginal sling, compared with anterior colporrhaphy alone for those without incontinence. Postoperatively, 2 patients who had the pubovaginal sling procedure reported continued stress incontinence (14%). No new symptoms of incontinence were reported in the patients without leakage on preoperative urodynamics. Thus, preoperative urodynamics were 100% accurate in determining which women did not need additional surgery for SUI.
Implications of a negative stress test. Our experience has shown that, despite our best attempts, a negative stress test with the prolapse reduced prior to surgery is less than 100% predictive. Occasionally, new SUI occurs after reconstructive surgery. It is unclear whether this incontinence is caused by straightening the urethra and reducing the bulge or secondary to the dissection of surgery.
Tips on technique
Anterior colporrhaphy traditionally is performed with plication of the “endopelvic fascia” or fibromuscular layer at the bladder neck with a Kelly plication stitch. Using “3-point” traction aids in dissecting the muscularis (FIGURE 4). Repair the remainder of the cystocele using vertical mattress stitches (1 or 2 layers) from the bladder neck to the apex.
Avoid creating weak areas. Using this technique, the repair frequently stops short of the apex, leaving a “gap” or weak area. One way to avoid this is to begin plication at the apex instead of the bladder neck (FIGURE 5).
Next, excise the excess vaginal tissue and close with interrupted fine absorbable sutures (FIGURE 6).
Recreate apical support. Another problem with traditional repairs is that they do not reestablish apical support. In many patients with anterior wall prolapse, reattachment of the apex reduces the cystocele. Therefore, it often is necessary to combine anterior colporrhaphy with an apical repair procedure such as uterosacral ligament suspension or sacrospinous ligament suspension.
Sutures for the apical repair should be placed and held prior to initiating the anterior colporrhaphy. At the end of the anterior repair, incorporate the apical sutures into the vaginal cuff.
Careful attention to the integrity and strength of the tissue is crucial. Regardless of the type of transvaginal suspension, we advocate bringing 1 arm of the suspension suture through the anterior wall of the cuff. Then place the other suture arm through the posterior cuff so that, when tied, anterior and posterior walls are brought together and suspended.
Using prolonged-delayed absorbable suture allows for a full-thickness bite, ensuring scarring to the suspensory ligament. If permanent suture is used for the uterosacral suspension, place the stitches along the inside surface of the anterior wall with a strong, broad bite that incorporates the muscularis or “endopelvic fascia.”
The occasional enterocele. When a transverse cystocele occurs following hysterectomy, the surgeon should be on the lookout for an enterocele, which sometimes accompanies anterior wall prolapse. The enterocele should be corrected at the time of surgery by closing the defect and suspending the cuff.
FIGURE 4 Three-point traction
Three-point traction using Allis clamps. The assistant retracts with DeBackey forceps to allow dissection of the muscularis. An index finger placed firmly against the vaginal mucosa enables the surgeon to judge depth of dissection.
FIGURE 5 Begin plication at the apex
Plication begins at the apex with vertical mattress stitches. Use 3-0 prolonged delayed absorbable or permanent suture in the anterior wall.
FIGURE 6 The reduced cystocele
The cystocele reduced following midline plication of the vaginal muscularis. The excess vagina is then trimmed and closed with interrupted 3-0 absorbable suture.
Functional surgical outcomes
Because of the long association between anterior wall prolapse and SUI, most surgeons evaluate patients preoperatively to determine the need for concomitant incontinence procedures. As a result, the literature reporting surgical cystocele repair via anterior colporrhaphy frequently uses continence of urine as the functional outcome. This is not surprising considering that anterior repair and Kelly plication, as reported by Howard Kelly more than 75 years ago, have been the gold standard for surgical correction of anterior wall prolapse and SUI.
In a series of 194 SUI patients who underwent anterior colporrhaphy, Beck11 found that adding a modified Kelly plication, including a vaginal retropubic urethropexy, increased the cure rate for SUI from 75% to 94%. Unfortunately, he did not report the anatomic success of the anterior colporrhaphy.
Kohli et al12 also retrospectively examined patients who had undergone anterior colporrhaphy with and without needle bladder-neck suspension. Although the cure rate for SUI was not reported, patients who underwent concomitant needle suspension had a higher rate of recurrent cystocele: 33% (n = 40) versus 7% (n = 27). Investigators theorized that retropubic dissection at the time of transvaginal needle suspension resulted in an iatrogenic paravaginal defect and denervation of the anterior vaginal wall.
The risks of needle suspension. A randomized controlled trial by Bump et al8 also suggests that needle suspension should be avoided. In that trial, 29 patients with stage 3 and 4 prolapse were randomized to needle colposuspension or endopelvic fascia plication. They, too, found that needle colposuspension carried a higher rate of recurrent anterior prolapse. Further, it did not reduce the rates of SUI compared with fascia plication.
Although incontinence surgery performed at the time of cystocele repair will reduce the rates of de novo incontinence, the higher rates of cystocele recurrence associated with some procedures warrants judicious preoperative planning. Clearly, needle suspension should not be performed as an incontinence procedure or repair of anterior wall prolapse. Whether other vaginal incontinence procedures, eg, midurethral slings, lead to recurrence of anterior wall prolapse deserves further investigation.
Anatomic outcomes
Midline colporrhaphy. Because anterior colporrhaphy is rarely performed alone, few series describe patients having undergone simply an anterior repair. Stanton et al13 followed 54 women for up to 2 years after they underwent traditional midline plication with vaginal hysterectomy for prolapse. Eight (15%) of the women had recurrent anterior wall prolapse.
Colombo et al14 randomized 71 women with clinical SUI and stage 2 or 3 prolapse to Burch colposuspension or anterior colporrhaphy with Kelly plication. All women were followed for at least 8 years. The cure rate for SUI was 86% for the Burch procedure, compared with 52% for anterior repair and Kelly plication. However, 12 (34%) women treated with Burch colposuspension and 1 (3%) treated with anterior colporrhaphy had recurrent cystocele of grade 2 or 3 with or without prolapse at other vaginal sites.
Abdominal paravaginal repair. Shull15 followed 149 women for 6 months to 4 years after they underwent abdominal paravaginal repair with the urethrovesical stitch brought through Cooper’s ligament for treatment of SUI and paravaginal cystocele. He reported a 5% recurrence of anterior wall prolapse.
In another series, Shull16 reported on 62 women who were followed for a mean of 18 months after abdominal paravaginal repair. Four of 57 (7%) had recurrent vaginal prolapse to the hymen.
In a cohort of 102 patients evaluated with the Pelvic Organ Prolapse Quantitative Examination (POP-Q) at our institution, the maximal point of prolapse was the anterior wall in 60% of cases; the apex and posterior wall each accounted for roughly half the remaining cases (unpublished data).
Ellerkmann et al30 reported on 237 consecutive patients who presented with symptoms of pelvic organ prolapse. In 77 women (33%), anterior compartment pelvic organ prolapse predominated; 46 patients (19%) had posterior compartment prolapse; and 22 patients (11%) had apical prolapse.
Hendrix et al31 analyzed patients from the Women’s Health Initiative (WHI) and found that the anterior compartment predominated over the posterior compartment. In a follow-up study from the WHI, Handa32 reported on 412 women followed for 2 to 8 years. Among those who entered the WHI protocol without cystocele, 1 in 4 was diagnosed with it at some point in the study. This compares to 1 in 6 for rectocele and 1 in 100 for uterine prolapse. The majority of all defects were grade 1 or relaxation above the hymen.
Vaginal paravaginal repair. Mallipeddi et al17 reported on 45 patients undergoing vaginal paravaginal repair over 2 years, with 35 women followed for a mean of 1.6 years. Incontinent patients had a Kelly plication performed at the time of vaginal paravaginal repair. Recurrence rates were 3% for cystocele, 14% for rectocele, and 20% for enterocele.
Young et al18 followed 100 women for as long as 36 months after bilateral paravaginal repair using 1 to 6 expanded polytetrafluoroethylene (Gore-Tex) CV-0 sutures and midline colporrhaphy. Two patients had grade 1 or 2 failure at the lateral fixation points, but 21 patients had recurrent midline defects, all but 1 inside the hymen. Several patients had bloody discharge from the permanent sutures.
Sacrocolpopexy. Brubaker19 retrospectively reviewed 65 women who underwent sacrocolpopexy for apical prolapse. Three months postoperatively 19 patients (29%) had persistent anterior wall defects.
Uterosacral suspension. Shull et al20 also found the anterior segment to have the most recurrent defects. In that study, which had an average follow-up of a little over a year, 289 patients underwent vaginal uterosacral ligament repair of the apex, and 264 had an anterior wall defect preoperatively. At the time of furthest follow-up, 26 patients (9%) had failure at this site. This study confirmed that the anterior compartment is the most likely site to fail, and also that it fails the quickest.
Sacrospinous ligament suspension (SSLS). Morley and DeLancey21 found a 22% cystocele recurrence rate in 71 women 1 year after SSLS, with most of them asymptomatic. Shull22 reported a 30% incidence of cystoceles after SSLS. Paraiso and colleagues23 reported on 243 women undergoing SSLS and pelvic reconstructive surgery. Of these, 217 patients underwent concomitant anterior colporrhaphy. Follow-up at 74 months found 37% with symptomatic recurrence at the anterior wall, 13% at the posterior wall, and 8% at the apex.
Vaginal versus abdominal repair
Few studies have compared vaginal and abdominal repair of pelvic organ prolapse, including anterior wall prolapse.
In a trial by Benson et al,24 women with prolapse to or beyond the hymen were randomized to bilateral sacrospinous vault suspension and vaginal paravaginal repair (n = 48) or abdominal sacrocolpopexy with abdominal paravaginal repair (n = 40). One third of patients in each group also underwent anterior colporrhaphy. After a mean follow-up of 2.5 years, 16 of 20 women required reoperation for recurrent cystocele—12 (29%) from the vaginal group and 4 (10.5%) from the abdominal group. Vaginal vault eversion recurred in 5 women from the vaginal group and 1 from the abdominal group.
Investigators concluded that the anterior wall was the most likely site of failure because of the posterior placement of the vaginal apex with SSLS, predisposing the anterior wall to greater pressures and to neuropathy caused by lateral dissection of the anterior wall. Earlier studies have demonstrated that neuropathy may occur after extensive dissection of the vaginal wall and may affect the strength and integrity of the muscular support tissues.25,26
Allografts and xenografts
The difficulty of repairing anterior wall prolapse has led some pelvic surgeons to use mesh for cystocele repair. When Julian27 randomized 24 patients with recurrent cystocele to transvaginal repair with and without polypropylene (Marlex) mesh, 4 patients in the control group and no patients in the mesh group had recurrences (P <.05). However, 3 patients (25%) had mesh-related complications.
Weber et al28 randomized patients to standard midline plication, plication of the paravaginal tissue more laterally, or standard plication plus polyglactin 910 (Vicryl) mesh. Among 83 patients who returned for follow-up, there were no differences in anatomic outcome. Weber and colleagues concluded that there is little benefit to using mesh to correct cystoceles.
Still, although the overall cure rate was low (30–46%), most patients had cystocele to the hymen and not beyond, with significant improvement of symptoms. Although this cannot be defined as an anatomic cure, it is encouraging that the majority of patients appear to have benefited from surgery.
Sand et al29 randomized 161 women with the anterior wall to or beyond the hymen to traditional anterior colporrhaphy with or without Vicryl. The 2-inch square mesh was not placed over the repair as described above, but was folded into the anterior colporrhaphy stitches. At 1 year, 16 (22%) of 73 women with mesh and 28 (40%) of the 88 women without mesh had recurrent central cystoceles beyond the midvagina (P = .02). No women had cystoceles beyond the hymen or vaginal erosions.
Difficulty of interpreting the evidence
Because of the broad range of study designs, small number of patients per series, variety of concomitant procedures, and wide range of variables used to describe recurrence and success, it is difficult to draw conclusions from the literature. The evidence does suggest that the risks of wide vaginal dissection required for vaginal paravaginal repair outweigh the benefits. As a result, we have abandoned this technique. As mentioned above, it remains unclear whether graft materials will prove to be of long-term benefit for either midline plication or paravaginal repair.
The gold standard, for now
Prolapse of the anterior vaginal wall remains a challenge for the gynecologic surgeon. Careful preoperative and intraoperative evaluation and identification of support defects should guide repairs.
Randomized, controlled trials of midline versus paravaginal repair, as well as use of various graft materials, are greatly needed. These studies should not only address recurrence of prolapse symptoms, but the impact of surgery on sexual and lower urinary tract function.
At this time, the traditional anterior colporrhaphy with attention to apical suspension remains the gold standard.
The authors report no financial relationships relevant to this article.
- At this time, the traditional anterior colporrhaphy with attention to apical suspension remains the gold standard.
- If only some defects of the anterior wall are addressed at the time of reconstructive surgery, failure may be more likely.
- Women with grade 3 or 4 cystoceles often have evidence of bladder outlet obstruction on urodynamic testing.
- In 52% of cases, cystoceles coexist with detrusor instability and evidence of impaired detrusor contractility.
- A thorough preoperative evaluation includes assessing the apex, having the patient strain to maximize the defect, looking for paravaginal detachments, and making every effort to “unmask” occult stress urinary incontinence.
Ask a pelvic reconstructive surgeon to name the most difficult challenge, and the answer is likely to be anterior vaginal wall prolapse. The reason: The anterior wall usually is the leading edge of prolapse and the most common site of relaxation or failure following reconstructive surgery. This appears to hold true regardless of surgical route or technique.
Short-term success rates of anterior wall repairs appear promising, but long-term outcomes are not as encouraging. Success usually is claimed as long as the anterior wall is kept above the hymen, since the patient rarely reports symptoms in these cases.
Another challenge involves the use of allografts or xenografts, which have not undergone sufficient study to determine their long-term benefit or risks in comparison with traditional repairs.
This article reviews anatomy of the anterior vaginal wall and its supports, as well as surgical technique and outcomes.
Why the anterior wall is more susceptible to prolapse
One theory is that, in comparison with the posterior compartment, the anterior wall is not as well supported by the levator plate, which counters the effects of gravity and abdominal pressure. Normally, the anterior wall rests horizontally on the posterior wall, which in turn rests on the levator plate. When the levator muscles weaken, the anterior wall is the first to fall as increasing force is placed on the connective tissue supports.
Other possibilities: The anterior compartment’s attachments to the pelvic sidewall or apex may be weaker, the anterior wall may be more elastic or less dense than the posterior wall, and the anterior wall may be more susceptible to damage during childbirth or to the effects of age and loss of estrogen.
If only some defects are addressed at surgery, failure may be more likely. Some experts believe pelvic surgeons have focused too much attention on the urethrovesical junction in patients with concomitant urinary incontinence and not enough attention on suspending the anterior wall at the apex.
For most women, it is probably a combination of many of these factors that renders the anterior compartment so vulnerable.
Anatomy of the pelvic floor
The anterior vaginal wall resembles a trapezoidal plane due to ventral and more medial attachments near the pubic symphysis, and dorsal and more lateral attachments near the ischial spine (FIGURE 1).1 This helps explain the many appearances of the cystocele. The type of cystocele is defined by the location of the break in the fascial attachments.
Paravaginal defects. The trapezoidal anterior wall is suspended on both sides from the parietal fascia overlying the levator ani muscles at the arcus tendineus fascia pelvis (ATFP). Prolapse can occur when there is loss of attachment to the pelvic sidewall at any point between the pubis and ischial spine.
First described by White2 and characterized later by Richardson et al,3 this loss of lateral attachment is called a paravaginal defect or displacement cystocele (FIGURE 2). The goal of paravaginal repair is to reattach the lateral vaginal walls to the ATFP, either abdominally, laparoscopically, or vaginally.
Central defects, the rarest type of anterior wall prolapse, involve a loss of support near the pubis and tend to be smaller. The most common manifestation is urethral hypermobility.
Transverse defects occur when the top of the pubocervical fascia detaches from the cervix or vaginal apex, both of which are suspended from the uterosacral-cardinal ligament complex. A transverse cystocele is evidenced by loss of the anterior fornix. The anterior wall appears to be attenuated in the midline, and the vaginal mucosa is pale, thin, and smooth (FIGURE 3).
Goals of traditional repair. The traditional anterior colporrhaphy aims to excise or reinforce the attenuated transverse defect with plication of the “endopelvic fascia” in the midline of the anterior vaginal wall. The endopelvic fascia is not true fascia but the muscularis of the vaginal wall. It is comprised of smooth muscle and elastin along with the collagenous adventitia layer.4
The importance of restoring apical wall support becomes apparent when one considers the trapezoidal anatomy. The most common sites of defects or detachments of the anterior wall are near the ischial spines laterally. In an operative case series of paravaginal defects, DeLancey1 found the site of defect to be near the ischial spine in 96% of cases. The reattachment of the apex near the level of the spine becomes the highest point of support for the anterior vaginal wall.
This cephalad apical attachment can be accomplished in a variety of ways, by suspending the vaginal apex from the uterosacral ligaments, from the sacrospinous ligament, or via abdominal sacrocolpopexy.
FIGURE 1 Anatomy of the anterior wall
The anterior vaginal wall resembles a trapezoidal plane, with ventral and more medial attachments near the pubic symphysis, and dorsal and more lateral attachments to the ischial spine. Detachment from the pelvic sidewall and ischial spine results in anterior wall prolapse (right).
FIGURE 2 Paravaginal defect
FIGURE 3 Transverse defect
A transverse defect with loss of the anterior fornix. The loss of cephalad apical attachment at the level of the ischial spine leads to anterior wall prolapse. Suspending the upper vagina from shortened cardinal/uterosacral ligaments, the sacrospinous ligament, or via abdominal sacrocolpopexy is as important as plication.
Symptoms of anterior wall prolapse
As with other forms of pelvic organ prolapse, many patients complain of a bulge or feeling of pelvic pressure when the anterior vaginal wall has come through the introitus. However, some symptoms of anterior wall prolapse are unique.
Incontinence is not universal. A common misperception is that most patients with cystocele also experience stress urinary incontinence (SUI), which can develop when there is loss of urethral support and descent of the lower vaginal wall along with urethral hypermobility. However, there is no defining degree of hypermobility that links anterior wall prolapse with SUI. That is because the continence mechanism relies not only on urethral position and lateral attachments, but also on the neuromuscular function of the pelvis and lower urinary tract.
In fact, descent of the midvagina under the bladder base may actually reduce the chance of SUI. The reason: As a woman strains, the increased abdominal pressure pushes the cystocele farther and farther out. As the cystocele enlarges, it creates a functional outlet obstruction by kinking the urethra shut. When this is the case, patients may complain of prolonged voiding, an intermittent urine stream, and/or urinary retention. The woman may have to elevate the vaginal wall to empty her bladder. Patients with chronic urinary retention are at risk of developing recurrent urinary tract infections.
Bladder outlet obstruction and detrusor dysfunction. Women with grade 3 or 4 cystoceles often have evidence of bladder outlet obstruction on urodynamic testing, according to a study that found such evidence in 57% of subjects.5 After reduction of the prolapse with a pessary, obstructed flow reverted to normal in 94% of these women.
A large proportion (52%) of women with cystoceles also have detrusor instability, as well as evidence of impaired detrusor contractility. Many complain of urinary frequency and urgency and difficulty emptying the bladder.5
Again, this phenomenon is complex, related not only to anatomy but to altered neuromuscular function of the lower urinary tract. Incomplete emptying, frequency, and urgency may arise from stretching of the bladder base as it prolapses through the vaginal introitus, resulting in urinary retention. These symptoms often are less pronounced at night when the patient is supine.
We reviewed 35 cases of anterior wall prolapse greater than 1 cm outside the hymen, with elevated postvoid residuals exceeding 100 cc on 2 separate occasions.6 Thirty-one (89%) had normal postvoid residuals following reconstructive surgery and correction of their anterior wall prolapse.
Preoperative assessment
A careful physical exam is a prerequisite for all surgical repairs of pelvic organ prolapse. During this exam, identify the sites of defects and detachments.
Maximize the defect. Have the patient perform the Valsalva maneuver, cough, and/or strain while sitting upright or standing. As she is performing these maneuvers, ask her if this feels like her maximum prolapse. A split speculum often aids in visualizing the anterior and posterior compartments without pressure from the opposite vaginal wall.
Assess the apex. Place a large swab in the vagina, hold it gently against the apex, and ask the patient to strain. If the swab is pushed out, the apex needs suspension.
This technique can help identify apical relaxation that may be masked by a large anterior or posterior wall defect. A standardized staging system, such as the Pelvic Organ Prolapse Quantitative Examination (POP-Q) or Baden-Walker, aids in communicating and documenting the prolapse. In addition, it allows the surgeon to track anatomical outcomes after surgery.
Look for paravaginal defects by supporting the lateral anterior walls with a ring forceps at the level of the ATFP. Barber et al7 found this maneuver to be highly sensitive (90–94%): If no paravaginal defect was suspected clinically, none was found intraoperatively. However, the positive predictive value was poor (57%), in that defects suspected preoperatively were confirmed during surgery in less than two thirds of patients.
These findings point to the importance of careful intraoperative assessment, both before and during the repair procedure.
Limited utility of imaging studies. The use of radiologic studies such as defecography or dynamic magnetic resonance imaging of the pelvis may aid in the evaluation of defecatory disorders or suspected sigmoidocele or rectal prolapse, but have not been studied sufficiently to determine the impact on surgical outcome.
Unmasking SUI
As mentioned above, women with anterior wall prolapse do not always complain of stress incontinence. However, correction of the cystocele can relieve their obstructive voiding and unmask “occult” SUI. Various techniques have been described to elevate the anterior wall with pessaries, swabs, etc, during urodynamic testing to predict which women should have an incontinence procedure performed at the time of reconstructive surgery.
Conflicting rates of occult SUI have been reported, with estimates ranging from 36% to 80%.8 Although preoperative urodynamic testing indicates a high rate of occult stress incontinence, a study by Borstad et al9 suggests that the rate of de novo incontinence may be lower and that preoperative urodynamic findings are not predictive of postoperative continence status. In that study, 16 of 73 women (22%) developed stress incontinence following surgery for prolapse when no incontinence procedure was performed. Advanced age increased the risk of incontinence after surgery.
Contrast these findings with those of Chaikin and colleagues,10 who prospectively followed 24 patients with grade 3 or 4 cystoceles. Preoperative urodynamics showed a 58% rate of occult stress incontinence. All these patients were also defined as having intrinsic sphincter deficiency with leak point pressures below 60 cm water. The incontinent group underwent anterior colporrhaphy and concomitant pubovaginal sling, compared with anterior colporrhaphy alone for those without incontinence. Postoperatively, 2 patients who had the pubovaginal sling procedure reported continued stress incontinence (14%). No new symptoms of incontinence were reported in the patients without leakage on preoperative urodynamics. Thus, preoperative urodynamics were 100% accurate in determining which women did not need additional surgery for SUI.
Implications of a negative stress test. Our experience has shown that, despite our best attempts, a negative stress test with the prolapse reduced prior to surgery is less than 100% predictive. Occasionally, new SUI occurs after reconstructive surgery. It is unclear whether this incontinence is caused by straightening the urethra and reducing the bulge or secondary to the dissection of surgery.
Tips on technique
Anterior colporrhaphy traditionally is performed with plication of the “endopelvic fascia” or fibromuscular layer at the bladder neck with a Kelly plication stitch. Using “3-point” traction aids in dissecting the muscularis (FIGURE 4). Repair the remainder of the cystocele using vertical mattress stitches (1 or 2 layers) from the bladder neck to the apex.
Avoid creating weak areas. Using this technique, the repair frequently stops short of the apex, leaving a “gap” or weak area. One way to avoid this is to begin plication at the apex instead of the bladder neck (FIGURE 5).
Next, excise the excess vaginal tissue and close with interrupted fine absorbable sutures (FIGURE 6).
Recreate apical support. Another problem with traditional repairs is that they do not reestablish apical support. In many patients with anterior wall prolapse, reattachment of the apex reduces the cystocele. Therefore, it often is necessary to combine anterior colporrhaphy with an apical repair procedure such as uterosacral ligament suspension or sacrospinous ligament suspension.
Sutures for the apical repair should be placed and held prior to initiating the anterior colporrhaphy. At the end of the anterior repair, incorporate the apical sutures into the vaginal cuff.
Careful attention to the integrity and strength of the tissue is crucial. Regardless of the type of transvaginal suspension, we advocate bringing 1 arm of the suspension suture through the anterior wall of the cuff. Then place the other suture arm through the posterior cuff so that, when tied, anterior and posterior walls are brought together and suspended.
Using prolonged-delayed absorbable suture allows for a full-thickness bite, ensuring scarring to the suspensory ligament. If permanent suture is used for the uterosacral suspension, place the stitches along the inside surface of the anterior wall with a strong, broad bite that incorporates the muscularis or “endopelvic fascia.”
The occasional enterocele. When a transverse cystocele occurs following hysterectomy, the surgeon should be on the lookout for an enterocele, which sometimes accompanies anterior wall prolapse. The enterocele should be corrected at the time of surgery by closing the defect and suspending the cuff.
FIGURE 4 Three-point traction
Three-point traction using Allis clamps. The assistant retracts with DeBackey forceps to allow dissection of the muscularis. An index finger placed firmly against the vaginal mucosa enables the surgeon to judge depth of dissection.
FIGURE 5 Begin plication at the apex
Plication begins at the apex with vertical mattress stitches. Use 3-0 prolonged delayed absorbable or permanent suture in the anterior wall.
FIGURE 6 The reduced cystocele
The cystocele reduced following midline plication of the vaginal muscularis. The excess vagina is then trimmed and closed with interrupted 3-0 absorbable suture.
Functional surgical outcomes
Because of the long association between anterior wall prolapse and SUI, most surgeons evaluate patients preoperatively to determine the need for concomitant incontinence procedures. As a result, the literature reporting surgical cystocele repair via anterior colporrhaphy frequently uses continence of urine as the functional outcome. This is not surprising considering that anterior repair and Kelly plication, as reported by Howard Kelly more than 75 years ago, have been the gold standard for surgical correction of anterior wall prolapse and SUI.
In a series of 194 SUI patients who underwent anterior colporrhaphy, Beck11 found that adding a modified Kelly plication, including a vaginal retropubic urethropexy, increased the cure rate for SUI from 75% to 94%. Unfortunately, he did not report the anatomic success of the anterior colporrhaphy.
Kohli et al12 also retrospectively examined patients who had undergone anterior colporrhaphy with and without needle bladder-neck suspension. Although the cure rate for SUI was not reported, patients who underwent concomitant needle suspension had a higher rate of recurrent cystocele: 33% (n = 40) versus 7% (n = 27). Investigators theorized that retropubic dissection at the time of transvaginal needle suspension resulted in an iatrogenic paravaginal defect and denervation of the anterior vaginal wall.
The risks of needle suspension. A randomized controlled trial by Bump et al8 also suggests that needle suspension should be avoided. In that trial, 29 patients with stage 3 and 4 prolapse were randomized to needle colposuspension or endopelvic fascia plication. They, too, found that needle colposuspension carried a higher rate of recurrent anterior prolapse. Further, it did not reduce the rates of SUI compared with fascia plication.
Although incontinence surgery performed at the time of cystocele repair will reduce the rates of de novo incontinence, the higher rates of cystocele recurrence associated with some procedures warrants judicious preoperative planning. Clearly, needle suspension should not be performed as an incontinence procedure or repair of anterior wall prolapse. Whether other vaginal incontinence procedures, eg, midurethral slings, lead to recurrence of anterior wall prolapse deserves further investigation.
Anatomic outcomes
Midline colporrhaphy. Because anterior colporrhaphy is rarely performed alone, few series describe patients having undergone simply an anterior repair. Stanton et al13 followed 54 women for up to 2 years after they underwent traditional midline plication with vaginal hysterectomy for prolapse. Eight (15%) of the women had recurrent anterior wall prolapse.
Colombo et al14 randomized 71 women with clinical SUI and stage 2 or 3 prolapse to Burch colposuspension or anterior colporrhaphy with Kelly plication. All women were followed for at least 8 years. The cure rate for SUI was 86% for the Burch procedure, compared with 52% for anterior repair and Kelly plication. However, 12 (34%) women treated with Burch colposuspension and 1 (3%) treated with anterior colporrhaphy had recurrent cystocele of grade 2 or 3 with or without prolapse at other vaginal sites.
Abdominal paravaginal repair. Shull15 followed 149 women for 6 months to 4 years after they underwent abdominal paravaginal repair with the urethrovesical stitch brought through Cooper’s ligament for treatment of SUI and paravaginal cystocele. He reported a 5% recurrence of anterior wall prolapse.
In another series, Shull16 reported on 62 women who were followed for a mean of 18 months after abdominal paravaginal repair. Four of 57 (7%) had recurrent vaginal prolapse to the hymen.
In a cohort of 102 patients evaluated with the Pelvic Organ Prolapse Quantitative Examination (POP-Q) at our institution, the maximal point of prolapse was the anterior wall in 60% of cases; the apex and posterior wall each accounted for roughly half the remaining cases (unpublished data).
Ellerkmann et al30 reported on 237 consecutive patients who presented with symptoms of pelvic organ prolapse. In 77 women (33%), anterior compartment pelvic organ prolapse predominated; 46 patients (19%) had posterior compartment prolapse; and 22 patients (11%) had apical prolapse.
Hendrix et al31 analyzed patients from the Women’s Health Initiative (WHI) and found that the anterior compartment predominated over the posterior compartment. In a follow-up study from the WHI, Handa32 reported on 412 women followed for 2 to 8 years. Among those who entered the WHI protocol without cystocele, 1 in 4 was diagnosed with it at some point in the study. This compares to 1 in 6 for rectocele and 1 in 100 for uterine prolapse. The majority of all defects were grade 1 or relaxation above the hymen.
Vaginal paravaginal repair. Mallipeddi et al17 reported on 45 patients undergoing vaginal paravaginal repair over 2 years, with 35 women followed for a mean of 1.6 years. Incontinent patients had a Kelly plication performed at the time of vaginal paravaginal repair. Recurrence rates were 3% for cystocele, 14% for rectocele, and 20% for enterocele.
Young et al18 followed 100 women for as long as 36 months after bilateral paravaginal repair using 1 to 6 expanded polytetrafluoroethylene (Gore-Tex) CV-0 sutures and midline colporrhaphy. Two patients had grade 1 or 2 failure at the lateral fixation points, but 21 patients had recurrent midline defects, all but 1 inside the hymen. Several patients had bloody discharge from the permanent sutures.
Sacrocolpopexy. Brubaker19 retrospectively reviewed 65 women who underwent sacrocolpopexy for apical prolapse. Three months postoperatively 19 patients (29%) had persistent anterior wall defects.
Uterosacral suspension. Shull et al20 also found the anterior segment to have the most recurrent defects. In that study, which had an average follow-up of a little over a year, 289 patients underwent vaginal uterosacral ligament repair of the apex, and 264 had an anterior wall defect preoperatively. At the time of furthest follow-up, 26 patients (9%) had failure at this site. This study confirmed that the anterior compartment is the most likely site to fail, and also that it fails the quickest.
Sacrospinous ligament suspension (SSLS). Morley and DeLancey21 found a 22% cystocele recurrence rate in 71 women 1 year after SSLS, with most of them asymptomatic. Shull22 reported a 30% incidence of cystoceles after SSLS. Paraiso and colleagues23 reported on 243 women undergoing SSLS and pelvic reconstructive surgery. Of these, 217 patients underwent concomitant anterior colporrhaphy. Follow-up at 74 months found 37% with symptomatic recurrence at the anterior wall, 13% at the posterior wall, and 8% at the apex.
Vaginal versus abdominal repair
Few studies have compared vaginal and abdominal repair of pelvic organ prolapse, including anterior wall prolapse.
In a trial by Benson et al,24 women with prolapse to or beyond the hymen were randomized to bilateral sacrospinous vault suspension and vaginal paravaginal repair (n = 48) or abdominal sacrocolpopexy with abdominal paravaginal repair (n = 40). One third of patients in each group also underwent anterior colporrhaphy. After a mean follow-up of 2.5 years, 16 of 20 women required reoperation for recurrent cystocele—12 (29%) from the vaginal group and 4 (10.5%) from the abdominal group. Vaginal vault eversion recurred in 5 women from the vaginal group and 1 from the abdominal group.
Investigators concluded that the anterior wall was the most likely site of failure because of the posterior placement of the vaginal apex with SSLS, predisposing the anterior wall to greater pressures and to neuropathy caused by lateral dissection of the anterior wall. Earlier studies have demonstrated that neuropathy may occur after extensive dissection of the vaginal wall and may affect the strength and integrity of the muscular support tissues.25,26
Allografts and xenografts
The difficulty of repairing anterior wall prolapse has led some pelvic surgeons to use mesh for cystocele repair. When Julian27 randomized 24 patients with recurrent cystocele to transvaginal repair with and without polypropylene (Marlex) mesh, 4 patients in the control group and no patients in the mesh group had recurrences (P <.05). However, 3 patients (25%) had mesh-related complications.
Weber et al28 randomized patients to standard midline plication, plication of the paravaginal tissue more laterally, or standard plication plus polyglactin 910 (Vicryl) mesh. Among 83 patients who returned for follow-up, there were no differences in anatomic outcome. Weber and colleagues concluded that there is little benefit to using mesh to correct cystoceles.
Still, although the overall cure rate was low (30–46%), most patients had cystocele to the hymen and not beyond, with significant improvement of symptoms. Although this cannot be defined as an anatomic cure, it is encouraging that the majority of patients appear to have benefited from surgery.
Sand et al29 randomized 161 women with the anterior wall to or beyond the hymen to traditional anterior colporrhaphy with or without Vicryl. The 2-inch square mesh was not placed over the repair as described above, but was folded into the anterior colporrhaphy stitches. At 1 year, 16 (22%) of 73 women with mesh and 28 (40%) of the 88 women without mesh had recurrent central cystoceles beyond the midvagina (P = .02). No women had cystoceles beyond the hymen or vaginal erosions.
Difficulty of interpreting the evidence
Because of the broad range of study designs, small number of patients per series, variety of concomitant procedures, and wide range of variables used to describe recurrence and success, it is difficult to draw conclusions from the literature. The evidence does suggest that the risks of wide vaginal dissection required for vaginal paravaginal repair outweigh the benefits. As a result, we have abandoned this technique. As mentioned above, it remains unclear whether graft materials will prove to be of long-term benefit for either midline plication or paravaginal repair.
The gold standard, for now
Prolapse of the anterior vaginal wall remains a challenge for the gynecologic surgeon. Careful preoperative and intraoperative evaluation and identification of support defects should guide repairs.
Randomized, controlled trials of midline versus paravaginal repair, as well as use of various graft materials, are greatly needed. These studies should not only address recurrence of prolapse symptoms, but the impact of surgery on sexual and lower urinary tract function.
At this time, the traditional anterior colporrhaphy with attention to apical suspension remains the gold standard.
The authors report no financial relationships relevant to this article.
1. DeLancey JOL. Fascial and muscular abnormalities in women with urethral hypermobility and anterior vaginal wall prolapse. Am J Obstet Gynecol. 2002;187:93-98.
2. White GR. Cystocele: a radical cure by suturing lateral sulci of vagina to white line of pelvic fascia. JAMA. 1909;21:1707-1710.
3. Richardson AC, Lyon JB, Williams NL. A new look at pelvic relaxation. Am J Obstet Gynecol. 1976;126:568-571.
4. Weber AM, Walters MD. Anterior vaginal prolapse: review of anatomy and techniques of surgical repair. Obstet Gynecol. 1997;89:311-318.
5. Romanzi LJ, Chaikin DC, Blaivas JG. The effect of genital prolapse on voiding. J Urol. 1999;161:581-586.
6. FitzGerald MP, Kulkarni N, Fenner D. Postoperative resolution of urinary retention in patients with advanced pelvic organ prolapse. Am J Obstet Gynecol. 2000;183:1361-1364.
7. Barber MD, Cundiff GW, Weidner AC, Cotes KW, Bump RC, Addison WA. Accuracy of clinical assessment of paravaginal defects in women with anterior vaginal wall proloapse. Am J Obstet Gynecol. 1999;181:1-7.
8. Bump RC, Hurt WG, Theofrastous JP, et al. Randomized prospective comparison of needle colposuspension versus endopelvic fascia plication for potential stress incontinence prophylaxis in women undergoing vaginal reconstruction for stage III or IV pelvic organ prolapse. The Continence Program for Women Research Group. Am J Obstet Gynecol. 1996;175:326-333.
9. Borstad E, Rud T. The risk of developing urinary stress incontinence after vaginal repair in continent women. Acta Obstet Gynecol Scand. 1989;68:545-549.
10. Chaikin DC, Groutz A, Blaivas JG. Predicting the need for anti-incontinence surgery in continent women undergoing repair of severe urogenital prolapse. J Urol. 2000;163:531-534.
11. Beck RP, McCormick S, Nordstrom L. A 25-year experience with 519 anterior colporrhaphy procedures. Obstet Gynecol. 1991;78:1011-1018.
12. Kohli N, Sze EHM, Roat TW, Karram M. Incidence of recurrent cystocele after anterior colporrhaphy with and without concomitant transvaginal needle suspension. Am J Obstet Gynecol. 1996;175:1476-1482.
13. Stanton SL, Hilton P, Norton C, Cardozo L. Clinical and urodynamics effect of anterior colporrhaphy and vaginal hysterectomy for prolapse with and without incontinence. Br J Obstet Gynecol. 1982;89:459-463.
14. Colombo M, Vitobello D, Proiette F, Milani R. Randomized comparison of Burch colposuspension versus anterior colporrhaphy in women with stress urinary incontinence and anterior vaginal wall prolapse. BJOG. 2000;107:544-551.
15. Shull BL, Baden WF. A six-year experience with paravaginal defect repair for stress urinary incontinence. Am J Obstet Gynecol. 1989;160:1432-1440.
16. Shull BL, Benn SJ, Kuehl TJ. Surgical management of prolapse of the anterior vaginal segment: an analysis of support defects, operative morbidity, and anatomic outcome. Am J Obstet Gynecol. 1994;171:1429-1439.
17. Mallipeddi PK, Steele AC, Kohli N, Karram MM. Anatomic and functional outcome of vaginal paravaginal repair in the correction of anterior wall prolapse. Int Urogynecol J. 2001;12:83-88.
18. Young SB, Daman JJ, Bony LG. Vaginal paravaginal repair: one-year outcomes. Am J Obstet Gynecol. 2001;185:1360-1367.
19. Brubaker L. Sacrocolpopexy and the anterior compartment: support and function. Am J Obstet Gynecol. 1995;173:1690-1694.
20. Shull BL, Bachofen C, Coates KW, Kuehl TJ. A transvaginal approach to repair of apical and other associated sites of pelvic organ prolapse with uterosacral ligaments. Am J Obstet Gynecol. 2000;183:1365-1374.
21. Morley GW, DeLancey JOL. Sacrospinous ligament fixation for eversion of the vagina. Am J Obstet Gynecol. 1988;158:872-881.
22. Shull BL, Capen CV, Riggs MW, Kuehl TJ. Preoperative and postoperative analysis of site-specific pelvic support defects in 81 women treated with sacrospinous ligament suspension and pelvic reconstruction. Am J Obstet Gynecol. 1992;166:1764-1771.
23. Paraiso MFR, Ballard LA, Walter MD, et al. Pelvic support defects and visceral and sexual function in women treated with sacrospinous ligament suspension and pelvic reconstruction. Am J Obstet Gynecol. 1996;175:1423-1431.
24. Benson JT, Lucente V, McClellan E. Vaginal versus abdominal reconstructive surgery for the treatment of pelvic support defects: a prospective randomized study with long-term outcome evaluation. Am J Obstet Gynecol. 1996;175:1418-1421.
25. Benson JT, McClellan E. The effect of vaginal dissection of the pudendal nerve. Obstet Gynecol. 1993;82:387-389.
26. Zivkovic F, Tamussino K, Ralph G, et al. Long-term effects of vaginal dissection on the innervation of the striated urethral sphincter. Obstet Gynecol. 1996;87:257-260.
27. Julian T. The efficacy of Marlex mesh in the repair of severe, recurrent vaginal prolapse of the anterior midvaginal wall. Am J Obstet Gynecol. 1996;175:1472-1475.
28. Weber AM, Walters MD, Piedmonte MR, et al. Anterior colporrhaphy: a randomized trial of three surgical techniques. Am J Obstet Gynecol. 2001;185:1299-1306.
29. Sand PK, et al. Prospective randomized trial of polyglactin 910 mesh to prevent recurrence of cystocele and rectocele. Am J Obstet Gynecol. 2001;184:1357-1364.
30. Ellerkmann RM, Cundiff GW, Melick CF, Nihira MA, Leffler K, Bent AE. Correlation of symptoms with location and severity of pelvic organ prolapse. Am J Obstet Gynecol. 2001;185:1332-1337.
31. Hendrix SL, Clark A, Nygaard I, Aragaki A, Barnabei V, McTiernan A. Pelvic organ prolapse in the Women’s Health Initiative: gravity and gravidity. Am J Obstet Gynecol. 2002;186:1160-1166.
32. Handa VL, Garrett E, Hendrix S, Gold E, Robbins J. Progression and remission of pelvic organ prolapse: a longitudinal study of menopausal women. Am J Obstet Gynecol. 2004;190:27-32.
1. DeLancey JOL. Fascial and muscular abnormalities in women with urethral hypermobility and anterior vaginal wall prolapse. Am J Obstet Gynecol. 2002;187:93-98.
2. White GR. Cystocele: a radical cure by suturing lateral sulci of vagina to white line of pelvic fascia. JAMA. 1909;21:1707-1710.
3. Richardson AC, Lyon JB, Williams NL. A new look at pelvic relaxation. Am J Obstet Gynecol. 1976;126:568-571.
4. Weber AM, Walters MD. Anterior vaginal prolapse: review of anatomy and techniques of surgical repair. Obstet Gynecol. 1997;89:311-318.
5. Romanzi LJ, Chaikin DC, Blaivas JG. The effect of genital prolapse on voiding. J Urol. 1999;161:581-586.
6. FitzGerald MP, Kulkarni N, Fenner D. Postoperative resolution of urinary retention in patients with advanced pelvic organ prolapse. Am J Obstet Gynecol. 2000;183:1361-1364.
7. Barber MD, Cundiff GW, Weidner AC, Cotes KW, Bump RC, Addison WA. Accuracy of clinical assessment of paravaginal defects in women with anterior vaginal wall proloapse. Am J Obstet Gynecol. 1999;181:1-7.
8. Bump RC, Hurt WG, Theofrastous JP, et al. Randomized prospective comparison of needle colposuspension versus endopelvic fascia plication for potential stress incontinence prophylaxis in women undergoing vaginal reconstruction for stage III or IV pelvic organ prolapse. The Continence Program for Women Research Group. Am J Obstet Gynecol. 1996;175:326-333.
9. Borstad E, Rud T. The risk of developing urinary stress incontinence after vaginal repair in continent women. Acta Obstet Gynecol Scand. 1989;68:545-549.
10. Chaikin DC, Groutz A, Blaivas JG. Predicting the need for anti-incontinence surgery in continent women undergoing repair of severe urogenital prolapse. J Urol. 2000;163:531-534.
11. Beck RP, McCormick S, Nordstrom L. A 25-year experience with 519 anterior colporrhaphy procedures. Obstet Gynecol. 1991;78:1011-1018.
12. Kohli N, Sze EHM, Roat TW, Karram M. Incidence of recurrent cystocele after anterior colporrhaphy with and without concomitant transvaginal needle suspension. Am J Obstet Gynecol. 1996;175:1476-1482.
13. Stanton SL, Hilton P, Norton C, Cardozo L. Clinical and urodynamics effect of anterior colporrhaphy and vaginal hysterectomy for prolapse with and without incontinence. Br J Obstet Gynecol. 1982;89:459-463.
14. Colombo M, Vitobello D, Proiette F, Milani R. Randomized comparison of Burch colposuspension versus anterior colporrhaphy in women with stress urinary incontinence and anterior vaginal wall prolapse. BJOG. 2000;107:544-551.
15. Shull BL, Baden WF. A six-year experience with paravaginal defect repair for stress urinary incontinence. Am J Obstet Gynecol. 1989;160:1432-1440.
16. Shull BL, Benn SJ, Kuehl TJ. Surgical management of prolapse of the anterior vaginal segment: an analysis of support defects, operative morbidity, and anatomic outcome. Am J Obstet Gynecol. 1994;171:1429-1439.
17. Mallipeddi PK, Steele AC, Kohli N, Karram MM. Anatomic and functional outcome of vaginal paravaginal repair in the correction of anterior wall prolapse. Int Urogynecol J. 2001;12:83-88.
18. Young SB, Daman JJ, Bony LG. Vaginal paravaginal repair: one-year outcomes. Am J Obstet Gynecol. 2001;185:1360-1367.
19. Brubaker L. Sacrocolpopexy and the anterior compartment: support and function. Am J Obstet Gynecol. 1995;173:1690-1694.
20. Shull BL, Bachofen C, Coates KW, Kuehl TJ. A transvaginal approach to repair of apical and other associated sites of pelvic organ prolapse with uterosacral ligaments. Am J Obstet Gynecol. 2000;183:1365-1374.
21. Morley GW, DeLancey JOL. Sacrospinous ligament fixation for eversion of the vagina. Am J Obstet Gynecol. 1988;158:872-881.
22. Shull BL, Capen CV, Riggs MW, Kuehl TJ. Preoperative and postoperative analysis of site-specific pelvic support defects in 81 women treated with sacrospinous ligament suspension and pelvic reconstruction. Am J Obstet Gynecol. 1992;166:1764-1771.
23. Paraiso MFR, Ballard LA, Walter MD, et al. Pelvic support defects and visceral and sexual function in women treated with sacrospinous ligament suspension and pelvic reconstruction. Am J Obstet Gynecol. 1996;175:1423-1431.
24. Benson JT, Lucente V, McClellan E. Vaginal versus abdominal reconstructive surgery for the treatment of pelvic support defects: a prospective randomized study with long-term outcome evaluation. Am J Obstet Gynecol. 1996;175:1418-1421.
25. Benson JT, McClellan E. The effect of vaginal dissection of the pudendal nerve. Obstet Gynecol. 1993;82:387-389.
26. Zivkovic F, Tamussino K, Ralph G, et al. Long-term effects of vaginal dissection on the innervation of the striated urethral sphincter. Obstet Gynecol. 1996;87:257-260.
27. Julian T. The efficacy of Marlex mesh in the repair of severe, recurrent vaginal prolapse of the anterior midvaginal wall. Am J Obstet Gynecol. 1996;175:1472-1475.
28. Weber AM, Walters MD, Piedmonte MR, et al. Anterior colporrhaphy: a randomized trial of three surgical techniques. Am J Obstet Gynecol. 2001;185:1299-1306.
29. Sand PK, et al. Prospective randomized trial of polyglactin 910 mesh to prevent recurrence of cystocele and rectocele. Am J Obstet Gynecol. 2001;184:1357-1364.
30. Ellerkmann RM, Cundiff GW, Melick CF, Nihira MA, Leffler K, Bent AE. Correlation of symptoms with location and severity of pelvic organ prolapse. Am J Obstet Gynecol. 2001;185:1332-1337.
31. Hendrix SL, Clark A, Nygaard I, Aragaki A, Barnabei V, McTiernan A. Pelvic organ prolapse in the Women’s Health Initiative: gravity and gravidity. Am J Obstet Gynecol. 2002;186:1160-1166.
32. Handa VL, Garrett E, Hendrix S, Gold E, Robbins J. Progression and remission of pelvic organ prolapse: a longitudinal study of menopausal women. Am J Obstet Gynecol. 2004;190:27-32.