User login
Q Which is better at stopping acute uterine bleeding—oral MPA or combination OCs?
For the purposes of this study, acute uterine bleeding is excessive or prolonged bleeding that necessitates urgent or emergent intervention. As Munro and colleagues point out, it is a “substantial drain on health-care resources” because so many women with this complaint require hospitalization for surgical intervention. Among the options are dilation and curettage, endometrial ablation, uterine artery embolization, and hysterectomy, the definitive “cure.”
Expert Commentary
In the United States, acute menorrhagia affects at least 10% of the female population and requires immediate attention. Surgical management is generally reserved for the hemodynamically unstable patient, for those who fail medical management, and for those in whom medical management is contraindicated. The gold standard is high-dose, intravenous estrogen, which halts bleeding in 72% of women within 5 hours.
Although combination oral contraceptives (OCs) are frequently used for acute uterine bleeding, a 2000 Cochrane Review1 found only 1 randomized controlled trial comparing OCs with other medical therapies—and none with comparison with placebo. The Cochrane Review concluded there is not enough evidence to draw any conclusions about the efficacy of combined OCs for menorrhagia.
Until now, support for the use of combination OCs for this indication has been based primarily on textbooks and expert opinion, and we have very little information on the degree of patient satisfaction with the method.
Progesterone-dominant regimens are not as effective as estrogen
Munro and colleagues aimed to correct the paucity of data by treating women with acute menorrhagia with either combined high-dose OCs or high-dose medroxyprogesterone acetate (MPA). Unfortunately, both therapeutic regimens are progesterone-dominant. Estrogen is the gold standard because it stabilizes the endometrial lining by promoting rapid regrowth. Progesterone impedes the action of estrogen, making it less likely to be effective.
A placebo group was believed to be potentially unethical due to the outpatient nature of the study, but a better comparison could have been achieved with an estrogen-only arm.
Sample size fell far below initial projections
Another difficulty with this study is the level of enrollment (n=40), which was far below the number needed (n=400), based on the initial power analysis. Reasons given for the small sample include bias of the referring clinician and patient, and the refusal of many women to submit to randomization. It is difficult to draw significant conclusions based on such a small sample.
What this study reveals
Despite its shortcomings, this study does offer some new information. In the primary outcome of the study—avoidance of emergent surgery—both therapies appeared to be effective, with only 1 patient requiring an unscheduled surgical procedure during the 4 weeks of follow-up. At 2 weeks of follow-up, bleeding had stopped in 76% and 88% of the MPA- and OC-treated patients, respectively. Side effects were minimal in both groups.
Intravenous estrogen is still the gold standard
Although Munro and colleagues add to our understanding of treatments for acute uterine bleeding, estrogen remains the gold standard. Intravenous estrogen is indicated in the inpatient setting for up to 24 hours, followed by tapering to an oral regimen. High-dose oral estrogen is used in the outpatient setting until a significant reduction or cessation of bleeding occurs.
Progesterone therapy should be started in close sequence with estrogen to minimize the likelihood of heavy withdrawal bleeding.
REFERENCE
1. Iyer V, Farquhar C, Jepson R. Oral contraceptive pills for heavy menstrual bleeding. Cochrane Database Syst Rev. 2000;(2):CD000154.-
For the purposes of this study, acute uterine bleeding is excessive or prolonged bleeding that necessitates urgent or emergent intervention. As Munro and colleagues point out, it is a “substantial drain on health-care resources” because so many women with this complaint require hospitalization for surgical intervention. Among the options are dilation and curettage, endometrial ablation, uterine artery embolization, and hysterectomy, the definitive “cure.”
Expert Commentary
In the United States, acute menorrhagia affects at least 10% of the female population and requires immediate attention. Surgical management is generally reserved for the hemodynamically unstable patient, for those who fail medical management, and for those in whom medical management is contraindicated. The gold standard is high-dose, intravenous estrogen, which halts bleeding in 72% of women within 5 hours.
Although combination oral contraceptives (OCs) are frequently used for acute uterine bleeding, a 2000 Cochrane Review1 found only 1 randomized controlled trial comparing OCs with other medical therapies—and none with comparison with placebo. The Cochrane Review concluded there is not enough evidence to draw any conclusions about the efficacy of combined OCs for menorrhagia.
Until now, support for the use of combination OCs for this indication has been based primarily on textbooks and expert opinion, and we have very little information on the degree of patient satisfaction with the method.
Progesterone-dominant regimens are not as effective as estrogen
Munro and colleagues aimed to correct the paucity of data by treating women with acute menorrhagia with either combined high-dose OCs or high-dose medroxyprogesterone acetate (MPA). Unfortunately, both therapeutic regimens are progesterone-dominant. Estrogen is the gold standard because it stabilizes the endometrial lining by promoting rapid regrowth. Progesterone impedes the action of estrogen, making it less likely to be effective.
A placebo group was believed to be potentially unethical due to the outpatient nature of the study, but a better comparison could have been achieved with an estrogen-only arm.
Sample size fell far below initial projections
Another difficulty with this study is the level of enrollment (n=40), which was far below the number needed (n=400), based on the initial power analysis. Reasons given for the small sample include bias of the referring clinician and patient, and the refusal of many women to submit to randomization. It is difficult to draw significant conclusions based on such a small sample.
What this study reveals
Despite its shortcomings, this study does offer some new information. In the primary outcome of the study—avoidance of emergent surgery—both therapies appeared to be effective, with only 1 patient requiring an unscheduled surgical procedure during the 4 weeks of follow-up. At 2 weeks of follow-up, bleeding had stopped in 76% and 88% of the MPA- and OC-treated patients, respectively. Side effects were minimal in both groups.
Intravenous estrogen is still the gold standard
Although Munro and colleagues add to our understanding of treatments for acute uterine bleeding, estrogen remains the gold standard. Intravenous estrogen is indicated in the inpatient setting for up to 24 hours, followed by tapering to an oral regimen. High-dose oral estrogen is used in the outpatient setting until a significant reduction or cessation of bleeding occurs.
Progesterone therapy should be started in close sequence with estrogen to minimize the likelihood of heavy withdrawal bleeding.
For the purposes of this study, acute uterine bleeding is excessive or prolonged bleeding that necessitates urgent or emergent intervention. As Munro and colleagues point out, it is a “substantial drain on health-care resources” because so many women with this complaint require hospitalization for surgical intervention. Among the options are dilation and curettage, endometrial ablation, uterine artery embolization, and hysterectomy, the definitive “cure.”
Expert Commentary
In the United States, acute menorrhagia affects at least 10% of the female population and requires immediate attention. Surgical management is generally reserved for the hemodynamically unstable patient, for those who fail medical management, and for those in whom medical management is contraindicated. The gold standard is high-dose, intravenous estrogen, which halts bleeding in 72% of women within 5 hours.
Although combination oral contraceptives (OCs) are frequently used for acute uterine bleeding, a 2000 Cochrane Review1 found only 1 randomized controlled trial comparing OCs with other medical therapies—and none with comparison with placebo. The Cochrane Review concluded there is not enough evidence to draw any conclusions about the efficacy of combined OCs for menorrhagia.
Until now, support for the use of combination OCs for this indication has been based primarily on textbooks and expert opinion, and we have very little information on the degree of patient satisfaction with the method.
Progesterone-dominant regimens are not as effective as estrogen
Munro and colleagues aimed to correct the paucity of data by treating women with acute menorrhagia with either combined high-dose OCs or high-dose medroxyprogesterone acetate (MPA). Unfortunately, both therapeutic regimens are progesterone-dominant. Estrogen is the gold standard because it stabilizes the endometrial lining by promoting rapid regrowth. Progesterone impedes the action of estrogen, making it less likely to be effective.
A placebo group was believed to be potentially unethical due to the outpatient nature of the study, but a better comparison could have been achieved with an estrogen-only arm.
Sample size fell far below initial projections
Another difficulty with this study is the level of enrollment (n=40), which was far below the number needed (n=400), based on the initial power analysis. Reasons given for the small sample include bias of the referring clinician and patient, and the refusal of many women to submit to randomization. It is difficult to draw significant conclusions based on such a small sample.
What this study reveals
Despite its shortcomings, this study does offer some new information. In the primary outcome of the study—avoidance of emergent surgery—both therapies appeared to be effective, with only 1 patient requiring an unscheduled surgical procedure during the 4 weeks of follow-up. At 2 weeks of follow-up, bleeding had stopped in 76% and 88% of the MPA- and OC-treated patients, respectively. Side effects were minimal in both groups.
Intravenous estrogen is still the gold standard
Although Munro and colleagues add to our understanding of treatments for acute uterine bleeding, estrogen remains the gold standard. Intravenous estrogen is indicated in the inpatient setting for up to 24 hours, followed by tapering to an oral regimen. High-dose oral estrogen is used in the outpatient setting until a significant reduction or cessation of bleeding occurs.
Progesterone therapy should be started in close sequence with estrogen to minimize the likelihood of heavy withdrawal bleeding.
REFERENCE
1. Iyer V, Farquhar C, Jepson R. Oral contraceptive pills for heavy menstrual bleeding. Cochrane Database Syst Rev. 2000;(2):CD000154.-
REFERENCE
1. Iyer V, Farquhar C, Jepson R. Oral contraceptive pills for heavy menstrual bleeding. Cochrane Database Syst Rev. 2000;(2):CD000154.-
Hysterectomy for AUB: Better short-term outcomes than medical therapy
To compare the effect of hysterectomy versus expanded medical treatment on health-related quality of life in premenopausal women with abnormal uterine bleeding (AUB).
Results
In this multicenter, randomized, controlled trial involving 63 women 30 to 50 years of age in whom medical therapy with medroxyprogesterone acetate had failed, those randomized to hysterectomy had greater improvement at 6 months in Mental Component Survey scores (8 vs 2; P = .04). They also had greater improvement in symptom resolution (75 vs 29; P .001 symptom satisfaction vs>P .001 interference with sex vs>P = .003), sexual desire (21 vs 3; P = .01), health distress (33 vs 13; P = .009), sleep problems (13 vs 1; P =. 03), overall health (12 vs 2; P = .006), and satisfaction with health (31 vs 14; P = .01).
Expert commentary
This study is one of the first randomized studies to compare the effects of surgery versus medical management on quality of life. Unfortunately, participants were included only after 1 course of medical therapy had failed. Thus, although investigators avoided potential skewing of the results by excluding patients who responded easily to treatment, they also caused selection bias.
It also is likely that some of the patients randomized to medical therapy were frustrated after failing 1 course of therapy, which caused a large crossover group: 17 of 32 women in the medical-therapy group eventually underwent hysterectomy. A more accurate outcome may have resulted if all women initially presenting with AUB had been included.
Shortcomings
Sample size was small, and did not reach the projected numbers even after the target sample size was officially decreased. Again, if investigators had randomized any patient initially presenting with AUB, more women might have been willing to participate. If the reason for the low numbers is that more women wanted to undergo surgical treatment after failing therapy with medroxy-progesterone acetate, researchers could have implemented a 2:1 randomization scheme to encourage higher enrollment in the study.
Although performing surgery without attempting medical therapy does not reflect typical management, it might have provided insight into whether medical management is worthwhile and yielded information on which option truly does lead to better quality of life.
Further, researchers compared each patient’s quality-of-life scores to her baseline, which, in the medicine group, was after a mean of 4 years of treatment. Therefore, one would expect less of a difference between scores in the medicine group, since all women were already refractory to medroxyprogesterone acetate at the beginning of the study.
The large crossover from the medical management group makes it difficult to decipher what the outcome of the study really is, despite the intragroup analysis. This leads me to conclude that, at some point, most patients get tired of trying medications and want a guaranteed fast cure.
No data on effects of hysterectomy route
One area that should have been addressed and subanalyzed: whether the type of hysterectomy (36% abdominal versus 64% vaginal) had any effect on short- or longterm outcomes.
Ablation option not included
There is also another treatment option that was not addressed: endometrial ablation. This surgery typically has a quicker recovery than hysterectomy and should correct the bleeding faster than medical management. It would be interesting to see the differences in quality-of-life and sexual-function outcomes with this option, compared with the others.
Bottom line
Until these issues have been thoroughly evaluated, women with AUB should initially be treated with medical therapy. Based on the preliminary screening study for this trial, medroxyprogesterone acetate appears to effectively control most patients’ symptoms. However, when medical therapy fails, the physician should explain to the patient that the improved short-term outcome seen with hysterectomy does not necessarily translate into significant long-term quality-of-life outcomes, as this study points out. The final decision between medical management and hysterectomy thus should fall to the individual patient.
To compare the effect of hysterectomy versus expanded medical treatment on health-related quality of life in premenopausal women with abnormal uterine bleeding (AUB).
Results
In this multicenter, randomized, controlled trial involving 63 women 30 to 50 years of age in whom medical therapy with medroxyprogesterone acetate had failed, those randomized to hysterectomy had greater improvement at 6 months in Mental Component Survey scores (8 vs 2; P = .04). They also had greater improvement in symptom resolution (75 vs 29; P .001 symptom satisfaction vs>P .001 interference with sex vs>P = .003), sexual desire (21 vs 3; P = .01), health distress (33 vs 13; P = .009), sleep problems (13 vs 1; P =. 03), overall health (12 vs 2; P = .006), and satisfaction with health (31 vs 14; P = .01).
Expert commentary
This study is one of the first randomized studies to compare the effects of surgery versus medical management on quality of life. Unfortunately, participants were included only after 1 course of medical therapy had failed. Thus, although investigators avoided potential skewing of the results by excluding patients who responded easily to treatment, they also caused selection bias.
It also is likely that some of the patients randomized to medical therapy were frustrated after failing 1 course of therapy, which caused a large crossover group: 17 of 32 women in the medical-therapy group eventually underwent hysterectomy. A more accurate outcome may have resulted if all women initially presenting with AUB had been included.
Shortcomings
Sample size was small, and did not reach the projected numbers even after the target sample size was officially decreased. Again, if investigators had randomized any patient initially presenting with AUB, more women might have been willing to participate. If the reason for the low numbers is that more women wanted to undergo surgical treatment after failing therapy with medroxy-progesterone acetate, researchers could have implemented a 2:1 randomization scheme to encourage higher enrollment in the study.
Although performing surgery without attempting medical therapy does not reflect typical management, it might have provided insight into whether medical management is worthwhile and yielded information on which option truly does lead to better quality of life.
Further, researchers compared each patient’s quality-of-life scores to her baseline, which, in the medicine group, was after a mean of 4 years of treatment. Therefore, one would expect less of a difference between scores in the medicine group, since all women were already refractory to medroxyprogesterone acetate at the beginning of the study.
The large crossover from the medical management group makes it difficult to decipher what the outcome of the study really is, despite the intragroup analysis. This leads me to conclude that, at some point, most patients get tired of trying medications and want a guaranteed fast cure.
No data on effects of hysterectomy route
One area that should have been addressed and subanalyzed: whether the type of hysterectomy (36% abdominal versus 64% vaginal) had any effect on short- or longterm outcomes.
Ablation option not included
There is also another treatment option that was not addressed: endometrial ablation. This surgery typically has a quicker recovery than hysterectomy and should correct the bleeding faster than medical management. It would be interesting to see the differences in quality-of-life and sexual-function outcomes with this option, compared with the others.
Bottom line
Until these issues have been thoroughly evaluated, women with AUB should initially be treated with medical therapy. Based on the preliminary screening study for this trial, medroxyprogesterone acetate appears to effectively control most patients’ symptoms. However, when medical therapy fails, the physician should explain to the patient that the improved short-term outcome seen with hysterectomy does not necessarily translate into significant long-term quality-of-life outcomes, as this study points out. The final decision between medical management and hysterectomy thus should fall to the individual patient.
To compare the effect of hysterectomy versus expanded medical treatment on health-related quality of life in premenopausal women with abnormal uterine bleeding (AUB).
Results
In this multicenter, randomized, controlled trial involving 63 women 30 to 50 years of age in whom medical therapy with medroxyprogesterone acetate had failed, those randomized to hysterectomy had greater improvement at 6 months in Mental Component Survey scores (8 vs 2; P = .04). They also had greater improvement in symptom resolution (75 vs 29; P .001 symptom satisfaction vs>P .001 interference with sex vs>P = .003), sexual desire (21 vs 3; P = .01), health distress (33 vs 13; P = .009), sleep problems (13 vs 1; P =. 03), overall health (12 vs 2; P = .006), and satisfaction with health (31 vs 14; P = .01).
Expert commentary
This study is one of the first randomized studies to compare the effects of surgery versus medical management on quality of life. Unfortunately, participants were included only after 1 course of medical therapy had failed. Thus, although investigators avoided potential skewing of the results by excluding patients who responded easily to treatment, they also caused selection bias.
It also is likely that some of the patients randomized to medical therapy were frustrated after failing 1 course of therapy, which caused a large crossover group: 17 of 32 women in the medical-therapy group eventually underwent hysterectomy. A more accurate outcome may have resulted if all women initially presenting with AUB had been included.
Shortcomings
Sample size was small, and did not reach the projected numbers even after the target sample size was officially decreased. Again, if investigators had randomized any patient initially presenting with AUB, more women might have been willing to participate. If the reason for the low numbers is that more women wanted to undergo surgical treatment after failing therapy with medroxy-progesterone acetate, researchers could have implemented a 2:1 randomization scheme to encourage higher enrollment in the study.
Although performing surgery without attempting medical therapy does not reflect typical management, it might have provided insight into whether medical management is worthwhile and yielded information on which option truly does lead to better quality of life.
Further, researchers compared each patient’s quality-of-life scores to her baseline, which, in the medicine group, was after a mean of 4 years of treatment. Therefore, one would expect less of a difference between scores in the medicine group, since all women were already refractory to medroxyprogesterone acetate at the beginning of the study.
The large crossover from the medical management group makes it difficult to decipher what the outcome of the study really is, despite the intragroup analysis. This leads me to conclude that, at some point, most patients get tired of trying medications and want a guaranteed fast cure.
No data on effects of hysterectomy route
One area that should have been addressed and subanalyzed: whether the type of hysterectomy (36% abdominal versus 64% vaginal) had any effect on short- or longterm outcomes.
Ablation option not included
There is also another treatment option that was not addressed: endometrial ablation. This surgery typically has a quicker recovery than hysterectomy and should correct the bleeding faster than medical management. It would be interesting to see the differences in quality-of-life and sexual-function outcomes with this option, compared with the others.
Bottom line
Until these issues have been thoroughly evaluated, women with AUB should initially be treated with medical therapy. Based on the preliminary screening study for this trial, medroxyprogesterone acetate appears to effectively control most patients’ symptoms. However, when medical therapy fails, the physician should explain to the patient that the improved short-term outcome seen with hysterectomy does not necessarily translate into significant long-term quality-of-life outcomes, as this study points out. The final decision between medical management and hysterectomy thus should fall to the individual patient.
Preventing adhesions after abdominal myomectomy: Tools and techniques
- To reduce the incidence of postoperative adhesions, follow basic principles of microsurgery: Minimize the number and extent of incisions, handle all tissue gently, strive for absolute hemostasis, and use small, nonreactive suture.
- Despite limited data from prospective, randomized studies, both fluid and barrier adjuvants have proved effective in reducing the incidence and extent of adhesions after abdominal myomectomy.
Abdominal myomectomy is the preferred treatment in women with large or numerous intramural myomas, especially in the setting of infertility, recurrent pregnancy loss, and preservation of future fertility.1,2 However, postoperative adhesions are distressingly common following this procedure, resulting in significant potential morbidity. Fortunately, a number of products can reduce their occurrence. Proper surgical techniques and a thorough knowledge of these products are invaluable in helping reduce the incidence of adhesions.
The association between adhesions and diminished fertility is well-established,3,4 particularly when peritubal involvement is present (FIGURES 1-3). Abdominopelvic adhesions also contribute to significant chronic pelvic pain, bowel obstruction, and technical difficulty in subsequent surgical or assisted-reproduction procedures.5 Unfortunately, most attempts at adhesiolysis meet with less than complete success, since adhesions recur in 55% to 100% of patients (FIGURE 4).6 Thus, preventing adhesions in the first place would seem to be key to successful outcomes in abdominal myomectomy.
This article reviews the evidence on various approaches and products. While the number of studies examining each adjuvant in the setting of abdominal myomectomy is limited, the overall evidence supports the safety and efficacy of both liquid and barrier adjuvants.
Adhesions present a significant clinical dilemma after abdominal myomectomy, occurring in 50% to 90% of patients.5,7 In 1 prospective series of women undergoing second-look laparoscopy (SLL) after myomectomy, adnexal adhesions were noted in 94% of patients with posterior uterine incisions and in 56% of patients with only anterior or fundal incisions; adhesions between the uterus and omentum or bowel occurred in 88% of all patients.5
In another study of early SLL following abdominal myomectomy, 83% of patients had adhesions between the surgical site and the bowel or omentum, and 65% had adhesions involving the adnexae.2 Removal of large, bulky fibroids (with uterine mass exceeding 13 weeks’ gestational size) resulted in higher adhesion scores than did small myomas. Again, the incidence and severity of adhesions also correlated with location of the uterine incision: Adnexal adhesions were more common after posterior uterine incisions (76%) than after anterior or fundal entries (45%).2
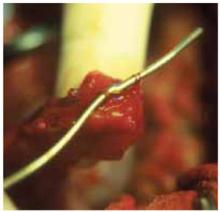
FIGURE 1 Peritubal adhesion
Adhesion at distal end of the fallopian tube. Peritubal involvement often leads to diminished fertility.
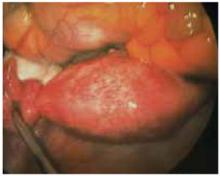
FIGURE 2 Adherent structures
Postoperative adhesion between fallopian tube and the uterus.
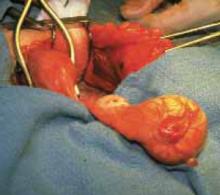
FIGURE 3 Cyst dissection
Adhesions can follow common surgeries such as paratubal cyst dissection.
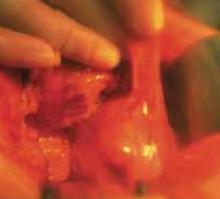
FIGURE 4 Recurrent adhesions
Adhesions may recur following adhesiolysis.
Causes of pelvic adhesions
Adhesion prevention requires an understanding of risk factors and maneuvers that increase the likelihood of injury (see “Pathophysiology of adhesion formation”). A number of causes have been proposed, most of them centering on tissue and peritoneal trauma (TABLE 1).
Injury can arise from excessive or rough manipulation of tissue and peritoneal surfaces or from common effects such as cutting, abrasion, and denudation (FIGURE 5). Tissue desiccation or manual blotting may lead to peritoneal desquamation and fibrin deposition.
Exposure of surfaces to intraperitoneal blood in the setting of tissue hypoxia—virtually unavoidable during abdominal myomectomy—disrupts normal fibrinolytic activity, resulting in stimulation of angiogenesis.8
Introduction of reactive foreign bodies such as talc powder, residual suture material, and even lint from laparotomy pads can favor adhesion formation. These serve as substrates or niduses of fibrin deposition.
FIGURE 5 Conducive conditions
Raw surface area can become a potent substrate for adhesions.TABLE 1
Proposed causes of adhesion formation
| Tissue hypoxia or ischemia |
| Tissue desiccation |
| Intra-abdominal infection |
| Introduction of reactive foreign body |
| Presence of intraperitoneal blood |
| Dissection of adhesions |
Techniques that may help prevent adhesions
Based on findings from studies of second-look procedures, most physicians advocate avoiding posterior uterine incisions, as well as minimizing the number and extent of incisions, to help reduce the likelihood of adhesion formation. Further, many Ob/Gyns favor removing myomas through as few uterine incisions as possible.9 We select anterior hysterotomy sites that enable removal of multiple fibroids, avoiding posterior incisions and the uterotubal junction whenever possible.
Interestingly, reapproximation of peritoneal defects after reproductive surgery (and, probably, myomectomy) does not appear to help prevent adhesions. Tulandi and colleagues10 examined the clinical and SLL outcomes of peritoneal closure in patients undergoing Pfannenstiel incisions with or without peritoneal closure at the end of the procedure. There was no difference in postoperative complications or wound healing in the 2 groups. At the time of SLL, there was no significant difference in the incidence of adhesion formation at the anterior abdominal wall.
Principles of microsurgery have been adopted by reproductive surgeons to minimize the likelihood of adhesions after myomectomy and gynecologic surgery in general. The basic techniques reflect respect for tissue integrity:
- Gentle handling of tissue, with minimal manipulation of all peritoneal surfaces.
- Meticulous hemostasis. Examine all myomectomy sites to ensure adequate hemostasis prior to closure.
- Continuous irrigation to prevent tissue desiccation. We favor continuous saline irrigation throughout the procedure. We also use only moistened laparotomy sponges and pads, and avoid applying dry gauze to any tissue surface.
- Avoidance of foreign-body introduction. We use only talc-free gloves and remove all residual suture fragments and tissue debris before closure. We also perform copious saline suction-irrigation at the end of the procedure to remove as much residue as possible.
- Use of fine, nonreactive suture. We favor fine, resorbable sutures that incite as little tissue reactivity as possible. For closure of large myomectomy defects, we use braided multifilaments such as Vicryl (polyglactine 910) (Ethicon, Somerville, NJ) or Dexon (polyglycolic acid) (Davis and Geck, Danbury, Conn) for strength and ease of handling. These sutures are absorbed through simple hydrolysis and stimulate less tissue reactivity than do chromic or catgut sutures.
The idea is appealing, but a randomized, blinded comparison of “good” and “bad” microsurgical technique is unlikely, since no one would wish to perform “bad” technique.
With barrier adjuvants, optimal benefit is obtained when the physician can predict potential sites of adhesions.
Wide range of prevention tools has been studied
Many types of agents have been studied in an attempt to reduce postsurgical adhesions after gynecologic surgery. Although most offer little or no benefit (TABLE 2), a few have potential in myomectomy procedures.
Barriers that form mechanical separation (TABLE 3) theoretically physically separate damaged tissues during early peritoneal wound healing, when adhesions form.11 The original adhesion barriers consisted of omental and peritoneal grafts that were placed over surgical sites. However, studies demonstrated that devitalized tissue positioned on damaged peritoneum serves as a potent substrate—not inhibitor—for adhesions.12 More recent trials have examined the adhesion-prevention benefit of other types of absorbable and nonabsorbable barriers.
TABLE 2
Pharmacologic agents studied for adhesion prevention in reproductive surgery
| AGENT | THEORETICAL ACTION | EXAMPLES | MODE OF USE/APPLICATION | RISKS/PROBLEMS |
|---|---|---|---|---|
| Antibiotics | Prevent infection or inflammation | Cephalosporins Tetracyclines | Intraperitoneal irrigation with antibiotic fluid Hydrotubation fluid with antibiotic | Theoretical reaction to antibiotic |
| Anticoagulants | Clot prevention Fibrin prevention | Heparin | In conjunction with Interceed | Risk of postoperative bleeding |
| Anti-inflammatory agents | Decrease permeability and histamine release | Nonsteroidal anti-inflammatory drugs Corticosteroids | Awaiting further investigation | Theoretical reaction to agent |
| Crystalloid solutions | Hydroflotation effect, decrease surface contact between pelvic organs | Normal saline Ringer’s lactate | Intra-abdominal instillation | Possible volume overload from intravascular absorption |
| Fibrinolytic agents | Fibrinolysis Plasminogen activation | Streptokinase Trypsin Fibrinolysin | Awaiting further investigation | Theoretical risk of postoperative bleeding |
| Steroids | Decrease inflammatory response | Dexamethasone | Systemic and/or intraperitoneal | Possible suppression of hypothalamic-pituitary axis |
| Polysaccharide polymer | "Siliconizing" effect to coat raw surfaces | Dextran 70 (Hyskon) | 200 mL placed in posterior cul-de-sac or coating surgical site surfaces | Abdominal bloating, anaphylaxis, pleural effusion, liver function abnormalities, wound separation, rare diffuse intravascular coagulation |
| Other fluid and barrier agents* | Peritoneal surface separation Hydroflotation | Absorbable and nonabsorbable barriers (see Table 3) | See Table 3 | See Table 3 |
| *Adjuvants studied in the setting of abdominal myomectomy | ||||
TABLE 3
Fluid and barrier adjuvants studied for adhesion reduction after myomectomy
| AGENT | THEORETICAL ACTION | MODE OF USE/APPLICATION | PROBLEMS | EVIDENCE FOR ADHESION REDUCTION |
|---|---|---|---|---|
| ABSORBABLE (BARRIER) | ||||
| Oxidized regenerated cellulose (Interceed) | Protective layer over surgical sites to prevent surface contact | Direct placement onto surface of uterus; no suturing required | Requires hemostasis | Prospective studies and meta-analysis support benefit in reproductive surgery including abdominal myomectomy |
| Hyaluronatecarboxymethycellulose derivative film (Seprafilm) | Protective layer over surgical sites to prevent surface contact | Direct placement around entire uterine surface; no suturing required | Requires hemostasis | Multicenter prospective, randomized study supports benefit in reducing adhesions after abdominal myomectomy |
| NONABSORBABLE (BARRIER) | ||||
| Expanded polytetrafluoroethylene (GoreTex) | Prevent contact between surgical surfaces | Patch sutured onto surface of uterus | Usually must be removed Report of fistula formation when left in situ | Multicenter prospective studies support adhesion-preventive benefit after abdominal myomectomy |
| Pericardial patch (Shelhigh No-React) | Prevent contact between surgical surfaces | Patch sutured onto surface of uterus | Early clinical use in myomectomy Proven safety as pericardial patch in humans | Preliminary study (case series data only) shows potential benefit |
| FLUID | ||||
| Hyaluronic acid-coat (Sepracoat) | Diffuse coating on surgical sites and potential sites of contact | 100-mL to 250-mL aliquots injected into peritoneal cavity | Limited data on efficacy in abdominal myomectomy | Small studies (multicenter, prospective, randomized, controlled trials) demonstrated reduced postoperative adhesions after reproductive surgery via laparotomy, including myomectomy |
| Hyaluronate-carboxymethycellulose derivative gel (Intergel) | Diffuse coating on surgical sites and potential sites of contact | 300-mL aliquot into peritoneal cavity | Withdrawn from market for reports of postoperative pain, complications | Reduced adhesion formation in animal studies and in preliminary human studies |
Adhesions are fibrous or fibrovascular bands that connect tissue surfaces in abnormal locations.1,2 Their development likely results from an imbalance in inflammatory mediators or fibrin degradation during peritoneal wound healing.
Peritoneal injury initiates the release of histamine and vasoactive kinins that mediate increased capillary permeability and outpouring of serosanguineous fluid.3 This proteinaceous exudate coagulates, depositing fibrinous bands between areas of denuded tissue.
Under normal circumstances, the fibrinolytic system is activated to lyse these bands within 72 hours. Peritoneal healing occurs when mesothelial cells migrate from the underlying mesenchyme to reepithelialize the injured site.4 Disequilibrium of the fibrin deposition-fibrinolysis system results in a persistent band that will eventually undergo fibroblast and vascular invasion.
REFERENCES
1. Diamond MP, DeCherney AH. Pathogenesis of adhesion formation/reformation: application to reproductive pelvic surgery. Microsurgery. 1987;8:103-107.
2. Diamond MP, Freeman ML. Clinical implications of postsurgical adhesions. Hum Reprod. 2001;7:567-576.
3. Diamond MP, El-Mowafi DM. Pelvic adhesions. Surg Technol Int. 1998;VII:273-283.
4. Farquhar C, Vandekerckhove P, Watson A, Vail A, Wiseman D. Barrier agents for preventing adhesions after surgery for subfertility. Cochrane Database Systematic Rev. 2002;(4):1-34.
Absorbable barriers
These are largely derivatives of organic materials. Their application to myomectomy may be limited by the requirement for absolute hemostasis at the site of application.
Interceed (Gynecare, a division of Ethicon), which is derived from oxidized regenerated cellulose, is one of the first and most extensively evaluated barriers. A mesh synthetic designed to be placed over injured tissue, it is a derivative of the hemostatic agent Surgicell (Johnson & Johnson, New Brunswick, NJ), with modifications in weave and pore size.
Interceed offers ease of application: It can be cut to the size or shape necessary and requires no suturing. It forms a gelatinous protective layer within 8 hours of placement, and is degraded into monosaccharides and absorbed within 2 weeks.13
The use of Interceed has been shown to reduce adhesions following adhesiolysis and ovarian surgery. In a large, multicenter, prospective, randomized trial, it significantly decreased the incidence of adhesion reformation after adnexal adhesiolysis in infertility patients with bilateral tubal disease.14
In a retrospective series of 38 infertility patients, including 19 patients after myomectomy (13 with the barrier, 6 without), reproductive outcomes were significantly better in the Interceed group, and adhesion development was reduced.15 Pregnancy rates in the 2 years following surgery were 78% in the Interceed group, compared with 47% in controls. In addition, among 23 patients who had second-look procedures, postoperative adhesions were noted in 38% of the Interceed group, compared with 86% in controls.15
Seprafilm (hyaluronic acid-film) (Genzyme Corp, Cambridge, Mass) is a bioresorbable membrane derived from sodium hyaluronate and carboxymethylcellulose. It is absorbed from the peritoneal cavity within 1 week and is completely excreted within 1 month.
Its potential for adhesion prevention after abdominal myomectomy was examined by SLL in a multicenter, prospective, randomized, blinded study11 in which 127 women undergoing abdominal myomectomy at 19 institutions were randomized to either Seprafilm or no barrier. In the Seprafilm group, the barrier was wrapped circumferentially around the uterus, covering all uterine defects, at the time of abdominal closure. Clinical outcomes—including vital signs, adverse events (pain, fever, nausea), and abdominal wound complications—were similar in the control and Seprafilm groups.
In this study, the incidence (mean number of sites), severity, and extent (mean area) of uterine adhesions were significantly lower in the Seprafilm-treated patients. The proportion of patients undergoing anterior hysterotomies who were found to have no anterior uterine adhesions was 39% in the Seprafilm group, compared with 6% in the control group. The percentage of patients with at least 1 adnexa totally free of adhesions to the posterior uterus also was higher in the Seprafilm group—48% versus 31%.11
Nonabsorbable barriers
GoreTex Surgical Membrane (W.L. Gore and Associates, Newark, Del) is an inert expanded polytetrafluoroethylene (PTFE) derivative that must be sutured in place. It is of potential utility in myomectomy because its application does not require absolute hemostasis. However, its usefulness and application are limited by the need for later removal.
In a multicenter, randomized, controlled trial exploring its adhesion-preventive properties after myomectomy, the GoreTex membrane significantly outperformed the barrierfree group.16
In a separate multicenter, randomized clinical trial, the GoreTex membrane was more effective than Interceed in preventing adhesions after pelvic/tubal reconstructive surgery.17
Yet another multicenter, randomized trial—this one involving 27 women undergoing abdominal myomectomy—used SLL to determine the extent of adhesions and to remove the barrier. The percentage of adhesion-free surgical sites was significantly higher in the GoreTex group: 56% compared with 7% in no-barrier controls.16
The adhesion scores, determined by the extent (area of involvement), nature (filmy versus opaque), and tenacity (ease of lysis or dissection) of the adhesions, were significantly lower in the GoreTex group.16
Despite the few prospective, randomized trials, the evidence supports the efficacy of various fluid and barrier adjuvants.
Histologic examination of the removed barriers demonstrated no tissue attachment to the PTFE. Despite these positive findings, however, both fistula formation and graft infection have been reported after placement of a GoreTex barrier.18
The Shelhigh Pericardial No-React Patch (Herzog Surgical, Sacramento, Calif) is a new nonabsorbable adhesion barrier that was first described in gynecologic use by Pelosi and Pelosi in a case series of 20 patients.19 Consisting of a 12-cm-diameter patch of glutaraldehyde-treated bovine/porcine pericardium, this product has an excellent safety record as a permanent pericardial substitute in the cardiovascular literature and has been shown to resist calcification and adhesions.
In the Pelosi series, the patch was placed over the uterine fundus and secured by 4 monofilament sutures at the dome of the uterus and the parietal peritoneum.19 All patients underwent SLL at 6 weeks, and 3 patients also underwent third-look laparoscopy. None of the 20 women had adhesions between the abdominal wall and the bladder, bowel, uterus, or adnexae at SLL. However, minimal adhesions of the ovaries and tubes were found in 7 patients who had undergone posterior hysterotomy. Clinical trials are likely to follow this small pilot study.
Absorbable fluid adjuvants
With barrier adjuvants, optimal benefit is obtained when the physician can predict potential sites of adhesions. This is not a strict requirement with fluid barriers, which is their chief advantage. Among the agents described below, Sepracoat (Genzyme Corp) is available in the United States, while Intergel (Lifecore Biomedical, Chaska, Minn) was withdrawn from the market in March.
Intergel, a 0.5% ferric hyaluronade formulation, is a sterile nonpyrogenic gel of highly purified sodium hyaluronate, which is ionically cross-linked with ferric ion and adjusted to isotonicity with sodium chloride.11 (Hyaluronic acid is a major component of body tissues and fluids such as peritoneal fluid, where it performs physically supportive and mechanically protective roles.)
Johns and colleagues20 studied Intergel in a randomized, multicenter, third-party– blinded, placebo-controlled study. Of the 265 patients who completed the study, 131 were given 300 mL of Intergel and 134 were given lactated Ringer’s solution (the placebo) at the time of their surgery, through the laparoscopic port. When SLL was performed 6 to 12 weeks after surgery, the mean number and severity of adhesions—overall and at the surgical site—were significantly lower in the Intergel group. Adhesions reformed in 91% of those in the control group, compared with 63% in the Intergel group.
In myomectomy patients, the modified American Fertility Society Score, which uses 24 potential adhesion sites, was reduced by 42% in the Intergel group—a statistically significant improvement.20
One major advantage of Intergel is that it reduces adhesion formation at sites distant from the area of application, secondary to its wide intra-abdominal circulation. However, as mentioned above, sales were voluntarily suspended due to post-market reports of tissue adherence, sterile foreign-body reaction, and late-onset pain that sometimes required surgical intervention. In some patients, persistent residual material was noted at the time of subsequent surgery.
Sepracoat (hyaluronic acid-coat) is a dilute solution of 0.4% hyaluronic acid in phosphate-buffered saline. It is bioresorbable, persists at the application site less than 24 hours, and is completely cleared in less than 5 days.
In a prospective, randomized, blinded, placebo-controlled, multicenter study in 1998, Diamond and colleagues compared Sepracoat with a pure phosphate-buffered saline solution in 227 women undergoing gynecologic procedures via laparotomy.21 The aim of the study was to assess the efficacy and safety of the fluid at sites without direct surgical trauma or adhesiolysis. Both solutions were warmed to room temperature and injected into the abdominal cavity before the procedure began (250 mL after skin incision). The solution was reapplied after irrigation or every 30 minutes (100 mL), and at the end of the procedure (250 mL) before closure. The maximum volume used was 1,000 mL. After application, the fluid was left 1 minute before suctioning. Patients underwent SLL 40 days later, and adhesions were identified at the initial procedure and at SLL.
In the Sepracoat group, there was a reduction in de novo adhesions at nonsurgical sites by a factor of 2.8. The proportion of sites with de novo adhesions also decreased, and 80% of Sepracoat patients had at least 1 ovary that was adhesion-free compared with 58% of placebo-treated patients. These findings occurred in areas of indirect trauma, demonstrating that Sepracoat limited trauma at tissue injury.21
Conclusion
Adhesion prevention is of utmost importance after abdominal myomectomy, especially in patients who desire future fertility. Despite the limited number of prospective, randomized studies, the literature does support the efficacy of various fluid and barrier adjuvants.
Our practice is to use an adjuvant in all abdominal myomectomies. We have long relied on Interceed, which enjoys both ease of application and an excellent safety record. However, as reviewed above, other potentially useful products are available or in development. As ever, the reproductive surgeon should adhere to principles of microsurgery, strive for meticulous hemostasis, and demonstrate respect for tissue integrity.
Dr. DeCherney reports small holdings with Lifecore Biomedical. Drs. Chang and Marin report no affiliations or financial arrangements with any of the manufacturers of products mentioned in this article.
1. Stewart EA. Treatment of uterine leiomyomas. UpToDate (Version 10.3) 2002; 1-8. Available at: www.uptodate.com.
2. Ugur M, Tura C, Mungan T, Aydogdu T, Sahin Y, Gokmen O. Laparoscopy for adhesion prevention following myomectomy. Int J Gynecol Obstet. 1996;53:145-149.
3. Berkeley AS, DeCherney AH, Plan ML. Abdominal myomectomy and subsequent fertility. Surg Gynecol Obstet. 1983;153:319-322.
4. Tulandi T, Collin JA, Burrows E, et al. Treatment-dependent and treatment-independent pregnancy among women with periadnexal adhesions. Am J Obstet Gynecol. 1990;162:354-357.
5. Tulandi T, Murria C, Guralnick M. Adhesion formation and reproductive outcome after myomectomy and second-look laparoscopy. Obstet Gynecol. 1993;82:213-215.
6. Diamond MP, Freeman ML. Clinical implications of postsurgical adhesions. Hum Reprod. 2001;7:567-576.
7. Lau S, Tulandi T. Myomectomy and adhesion formation. In: diZerega GS, ed. Peritoneal Surgery. New York: Springer-Verlag 1999;289-290.
8. Diamond MP, El-Mowafi DM. Pelvic adhesions. Surg Technol Int. 1998;VII:273-283.
9. Guarnaccia MM, Rein MS. Traditional surgical approaches to uterine fibroids: abdominal myomectomy and hysterectomy. Clin Obstet Gynecol. 2001;44:385-400.
10. Tulandi T, Hum HS, Gelfand MM. Closure of laparotomy incisions with or without peritoneal suturing and second-look laparoscopy. Am J Obstet Gynecol. 1988;158:536-537.
11. Diamond MP. Reduction of adhesions after uterine myomectomy by Seprafilm membrane (HAL-F): a blinded, prospective, randomized, multicenter clinical study. Fertil Steril. 1996;66:904-910.
12. Johns D. Reduction of postsurgical adhesions with Intergel adhesion prevention solution: a multicenter study of safety and efficacy after conservative gynecologic surgery. Fertil Steril. 2001;76:595-604.
13. Farquhar C, vandekerckhove P, Watson A, Vail A, Wiseman D. Barrier agents for preventing adhesions after surgery for subfertility. Cochrane Database Systematic Rev. 2002(4);1-34.
14. Nordic Adhesion Prevention Study Group. The efficacy of Interceed (TC7) for prevention of reformation of postoperative adhesions on ovaries, fallopian tubes, and fimbriae in microsurgical operations for fertility: a multicenter study. Fertil Steril. 1995;63:709-714.
15. Sawada T, Nishizawa H, Nishio E, Kadowaki M. Postoperative adhesion prevention with an oxidized regenerated cellulose adhesion barrier in infertile women. J Reprod Med. 2000;45:387-389.
16. The Myomectomy Adhesions Multicenter Study Group. An expanded polytetrafluoroethylene barrier (Gore-Tex Surgical Membrane) reduces post-myomectomy adhesion formation. Fertil Steril. 1995;63:491-493.
17. Haney AF, Hesla J, Hurst BS, et al. Expanded polytetrafluoroethylene (Gore-Tex Surgical Membrane) is superior to oxidized regenerated cellulose (Interceed TC7) in preventing adhesions. Fertil Steril. 1995;63:1021-1026.
18. Monteforte CA, Queirazza R, Francescetting P, Herbst TJ. Fistula formation after implanting an ePTFE membrane. A case report. J Reprod Med. 1997;42:184-187.
19. Pelosi MA, II, Pelosi MA, III. A new nonabsorbable adhesion barrier for myomectomy. Am J Surg. 2002;184:428-432.
20. Johns D. Reduction of postsurgical adhesions with Intergel adhesion prevention solution: a multicenter study of safety and efficacy after conservative gynecologic surgery. Fertil Steril. 2001;76:596-604.
21. Diamond MP and The Sepracoat Adhesion Study Group. Reduction of de novo postsurgical adhesions by intraoperative precoating with Sepracoat (HAL-C) solution: a prospective, randomized, blinded, placebo-controlled multicenter study. Fertil Steril. 1998;69:1067-1074.
- To reduce the incidence of postoperative adhesions, follow basic principles of microsurgery: Minimize the number and extent of incisions, handle all tissue gently, strive for absolute hemostasis, and use small, nonreactive suture.
- Despite limited data from prospective, randomized studies, both fluid and barrier adjuvants have proved effective in reducing the incidence and extent of adhesions after abdominal myomectomy.
Abdominal myomectomy is the preferred treatment in women with large or numerous intramural myomas, especially in the setting of infertility, recurrent pregnancy loss, and preservation of future fertility.1,2 However, postoperative adhesions are distressingly common following this procedure, resulting in significant potential morbidity. Fortunately, a number of products can reduce their occurrence. Proper surgical techniques and a thorough knowledge of these products are invaluable in helping reduce the incidence of adhesions.
The association between adhesions and diminished fertility is well-established,3,4 particularly when peritubal involvement is present (FIGURES 1-3). Abdominopelvic adhesions also contribute to significant chronic pelvic pain, bowel obstruction, and technical difficulty in subsequent surgical or assisted-reproduction procedures.5 Unfortunately, most attempts at adhesiolysis meet with less than complete success, since adhesions recur in 55% to 100% of patients (FIGURE 4).6 Thus, preventing adhesions in the first place would seem to be key to successful outcomes in abdominal myomectomy.
This article reviews the evidence on various approaches and products. While the number of studies examining each adjuvant in the setting of abdominal myomectomy is limited, the overall evidence supports the safety and efficacy of both liquid and barrier adjuvants.
Adhesions present a significant clinical dilemma after abdominal myomectomy, occurring in 50% to 90% of patients.5,7 In 1 prospective series of women undergoing second-look laparoscopy (SLL) after myomectomy, adnexal adhesions were noted in 94% of patients with posterior uterine incisions and in 56% of patients with only anterior or fundal incisions; adhesions between the uterus and omentum or bowel occurred in 88% of all patients.5
In another study of early SLL following abdominal myomectomy, 83% of patients had adhesions between the surgical site and the bowel or omentum, and 65% had adhesions involving the adnexae.2 Removal of large, bulky fibroids (with uterine mass exceeding 13 weeks’ gestational size) resulted in higher adhesion scores than did small myomas. Again, the incidence and severity of adhesions also correlated with location of the uterine incision: Adnexal adhesions were more common after posterior uterine incisions (76%) than after anterior or fundal entries (45%).2
FIGURE 1 Peritubal adhesion
Adhesion at distal end of the fallopian tube. Peritubal involvement often leads to diminished fertility.
FIGURE 2 Adherent structures
Postoperative adhesion between fallopian tube and the uterus.
FIGURE 3 Cyst dissection
Adhesions can follow common surgeries such as paratubal cyst dissection.
FIGURE 4 Recurrent adhesions
Adhesions may recur following adhesiolysis.
Causes of pelvic adhesions
Adhesion prevention requires an understanding of risk factors and maneuvers that increase the likelihood of injury (see “Pathophysiology of adhesion formation”). A number of causes have been proposed, most of them centering on tissue and peritoneal trauma (TABLE 1).
Injury can arise from excessive or rough manipulation of tissue and peritoneal surfaces or from common effects such as cutting, abrasion, and denudation (FIGURE 5). Tissue desiccation or manual blotting may lead to peritoneal desquamation and fibrin deposition.
Exposure of surfaces to intraperitoneal blood in the setting of tissue hypoxia—virtually unavoidable during abdominal myomectomy—disrupts normal fibrinolytic activity, resulting in stimulation of angiogenesis.8
Introduction of reactive foreign bodies such as talc powder, residual suture material, and even lint from laparotomy pads can favor adhesion formation. These serve as substrates or niduses of fibrin deposition.
FIGURE 5 Conducive conditions
Raw surface area can become a potent substrate for adhesions.TABLE 1
Proposed causes of adhesion formation
| Tissue hypoxia or ischemia |
| Tissue desiccation |
| Intra-abdominal infection |
| Introduction of reactive foreign body |
| Presence of intraperitoneal blood |
| Dissection of adhesions |
Techniques that may help prevent adhesions
Based on findings from studies of second-look procedures, most physicians advocate avoiding posterior uterine incisions, as well as minimizing the number and extent of incisions, to help reduce the likelihood of adhesion formation. Further, many Ob/Gyns favor removing myomas through as few uterine incisions as possible.9 We select anterior hysterotomy sites that enable removal of multiple fibroids, avoiding posterior incisions and the uterotubal junction whenever possible.
Interestingly, reapproximation of peritoneal defects after reproductive surgery (and, probably, myomectomy) does not appear to help prevent adhesions. Tulandi and colleagues10 examined the clinical and SLL outcomes of peritoneal closure in patients undergoing Pfannenstiel incisions with or without peritoneal closure at the end of the procedure. There was no difference in postoperative complications or wound healing in the 2 groups. At the time of SLL, there was no significant difference in the incidence of adhesion formation at the anterior abdominal wall.
Principles of microsurgery have been adopted by reproductive surgeons to minimize the likelihood of adhesions after myomectomy and gynecologic surgery in general. The basic techniques reflect respect for tissue integrity:
- Gentle handling of tissue, with minimal manipulation of all peritoneal surfaces.
- Meticulous hemostasis. Examine all myomectomy sites to ensure adequate hemostasis prior to closure.
- Continuous irrigation to prevent tissue desiccation. We favor continuous saline irrigation throughout the procedure. We also use only moistened laparotomy sponges and pads, and avoid applying dry gauze to any tissue surface.
- Avoidance of foreign-body introduction. We use only talc-free gloves and remove all residual suture fragments and tissue debris before closure. We also perform copious saline suction-irrigation at the end of the procedure to remove as much residue as possible.
- Use of fine, nonreactive suture. We favor fine, resorbable sutures that incite as little tissue reactivity as possible. For closure of large myomectomy defects, we use braided multifilaments such as Vicryl (polyglactine 910) (Ethicon, Somerville, NJ) or Dexon (polyglycolic acid) (Davis and Geck, Danbury, Conn) for strength and ease of handling. These sutures are absorbed through simple hydrolysis and stimulate less tissue reactivity than do chromic or catgut sutures.
The idea is appealing, but a randomized, blinded comparison of “good” and “bad” microsurgical technique is unlikely, since no one would wish to perform “bad” technique.
With barrier adjuvants, optimal benefit is obtained when the physician can predict potential sites of adhesions.
Wide range of prevention tools has been studied
Many types of agents have been studied in an attempt to reduce postsurgical adhesions after gynecologic surgery. Although most offer little or no benefit (TABLE 2), a few have potential in myomectomy procedures.
Barriers that form mechanical separation (TABLE 3) theoretically physically separate damaged tissues during early peritoneal wound healing, when adhesions form.11 The original adhesion barriers consisted of omental and peritoneal grafts that were placed over surgical sites. However, studies demonstrated that devitalized tissue positioned on damaged peritoneum serves as a potent substrate—not inhibitor—for adhesions.12 More recent trials have examined the adhesion-prevention benefit of other types of absorbable and nonabsorbable barriers.
TABLE 2
Pharmacologic agents studied for adhesion prevention in reproductive surgery
| AGENT | THEORETICAL ACTION | EXAMPLES | MODE OF USE/APPLICATION | RISKS/PROBLEMS |
|---|---|---|---|---|
| Antibiotics | Prevent infection or inflammation | Cephalosporins Tetracyclines | Intraperitoneal irrigation with antibiotic fluid Hydrotubation fluid with antibiotic | Theoretical reaction to antibiotic |
| Anticoagulants | Clot prevention Fibrin prevention | Heparin | In conjunction with Interceed | Risk of postoperative bleeding |
| Anti-inflammatory agents | Decrease permeability and histamine release | Nonsteroidal anti-inflammatory drugs Corticosteroids | Awaiting further investigation | Theoretical reaction to agent |
| Crystalloid solutions | Hydroflotation effect, decrease surface contact between pelvic organs | Normal saline Ringer’s lactate | Intra-abdominal instillation | Possible volume overload from intravascular absorption |
| Fibrinolytic agents | Fibrinolysis Plasminogen activation | Streptokinase Trypsin Fibrinolysin | Awaiting further investigation | Theoretical risk of postoperative bleeding |
| Steroids | Decrease inflammatory response | Dexamethasone | Systemic and/or intraperitoneal | Possible suppression of hypothalamic-pituitary axis |
| Polysaccharide polymer | "Siliconizing" effect to coat raw surfaces | Dextran 70 (Hyskon) | 200 mL placed in posterior cul-de-sac or coating surgical site surfaces | Abdominal bloating, anaphylaxis, pleural effusion, liver function abnormalities, wound separation, rare diffuse intravascular coagulation |
| Other fluid and barrier agents* | Peritoneal surface separation Hydroflotation | Absorbable and nonabsorbable barriers (see Table 3) | See Table 3 | See Table 3 |
| *Adjuvants studied in the setting of abdominal myomectomy | ||||
TABLE 3
Fluid and barrier adjuvants studied for adhesion reduction after myomectomy
| AGENT | THEORETICAL ACTION | MODE OF USE/APPLICATION | PROBLEMS | EVIDENCE FOR ADHESION REDUCTION |
|---|---|---|---|---|
| ABSORBABLE (BARRIER) | ||||
| Oxidized regenerated cellulose (Interceed) | Protective layer over surgical sites to prevent surface contact | Direct placement onto surface of uterus; no suturing required | Requires hemostasis | Prospective studies and meta-analysis support benefit in reproductive surgery including abdominal myomectomy |
| Hyaluronatecarboxymethycellulose derivative film (Seprafilm) | Protective layer over surgical sites to prevent surface contact | Direct placement around entire uterine surface; no suturing required | Requires hemostasis | Multicenter prospective, randomized study supports benefit in reducing adhesions after abdominal myomectomy |
| NONABSORBABLE (BARRIER) | ||||
| Expanded polytetrafluoroethylene (GoreTex) | Prevent contact between surgical surfaces | Patch sutured onto surface of uterus | Usually must be removed Report of fistula formation when left in situ | Multicenter prospective studies support adhesion-preventive benefit after abdominal myomectomy |
| Pericardial patch (Shelhigh No-React) | Prevent contact between surgical surfaces | Patch sutured onto surface of uterus | Early clinical use in myomectomy Proven safety as pericardial patch in humans | Preliminary study (case series data only) shows potential benefit |
| FLUID | ||||
| Hyaluronic acid-coat (Sepracoat) | Diffuse coating on surgical sites and potential sites of contact | 100-mL to 250-mL aliquots injected into peritoneal cavity | Limited data on efficacy in abdominal myomectomy | Small studies (multicenter, prospective, randomized, controlled trials) demonstrated reduced postoperative adhesions after reproductive surgery via laparotomy, including myomectomy |
| Hyaluronate-carboxymethycellulose derivative gel (Intergel) | Diffuse coating on surgical sites and potential sites of contact | 300-mL aliquot into peritoneal cavity | Withdrawn from market for reports of postoperative pain, complications | Reduced adhesion formation in animal studies and in preliminary human studies |
Adhesions are fibrous or fibrovascular bands that connect tissue surfaces in abnormal locations.1,2 Their development likely results from an imbalance in inflammatory mediators or fibrin degradation during peritoneal wound healing.
Peritoneal injury initiates the release of histamine and vasoactive kinins that mediate increased capillary permeability and outpouring of serosanguineous fluid.3 This proteinaceous exudate coagulates, depositing fibrinous bands between areas of denuded tissue.
Under normal circumstances, the fibrinolytic system is activated to lyse these bands within 72 hours. Peritoneal healing occurs when mesothelial cells migrate from the underlying mesenchyme to reepithelialize the injured site.4 Disequilibrium of the fibrin deposition-fibrinolysis system results in a persistent band that will eventually undergo fibroblast and vascular invasion.
REFERENCES
1. Diamond MP, DeCherney AH. Pathogenesis of adhesion formation/reformation: application to reproductive pelvic surgery. Microsurgery. 1987;8:103-107.
2. Diamond MP, Freeman ML. Clinical implications of postsurgical adhesions. Hum Reprod. 2001;7:567-576.
3. Diamond MP, El-Mowafi DM. Pelvic adhesions. Surg Technol Int. 1998;VII:273-283.
4. Farquhar C, Vandekerckhove P, Watson A, Vail A, Wiseman D. Barrier agents for preventing adhesions after surgery for subfertility. Cochrane Database Systematic Rev. 2002;(4):1-34.
Absorbable barriers
These are largely derivatives of organic materials. Their application to myomectomy may be limited by the requirement for absolute hemostasis at the site of application.
Interceed (Gynecare, a division of Ethicon), which is derived from oxidized regenerated cellulose, is one of the first and most extensively evaluated barriers. A mesh synthetic designed to be placed over injured tissue, it is a derivative of the hemostatic agent Surgicell (Johnson & Johnson, New Brunswick, NJ), with modifications in weave and pore size.
Interceed offers ease of application: It can be cut to the size or shape necessary and requires no suturing. It forms a gelatinous protective layer within 8 hours of placement, and is degraded into monosaccharides and absorbed within 2 weeks.13
The use of Interceed has been shown to reduce adhesions following adhesiolysis and ovarian surgery. In a large, multicenter, prospective, randomized trial, it significantly decreased the incidence of adhesion reformation after adnexal adhesiolysis in infertility patients with bilateral tubal disease.14
In a retrospective series of 38 infertility patients, including 19 patients after myomectomy (13 with the barrier, 6 without), reproductive outcomes were significantly better in the Interceed group, and adhesion development was reduced.15 Pregnancy rates in the 2 years following surgery were 78% in the Interceed group, compared with 47% in controls. In addition, among 23 patients who had second-look procedures, postoperative adhesions were noted in 38% of the Interceed group, compared with 86% in controls.15
Seprafilm (hyaluronic acid-film) (Genzyme Corp, Cambridge, Mass) is a bioresorbable membrane derived from sodium hyaluronate and carboxymethylcellulose. It is absorbed from the peritoneal cavity within 1 week and is completely excreted within 1 month.
Its potential for adhesion prevention after abdominal myomectomy was examined by SLL in a multicenter, prospective, randomized, blinded study11 in which 127 women undergoing abdominal myomectomy at 19 institutions were randomized to either Seprafilm or no barrier. In the Seprafilm group, the barrier was wrapped circumferentially around the uterus, covering all uterine defects, at the time of abdominal closure. Clinical outcomes—including vital signs, adverse events (pain, fever, nausea), and abdominal wound complications—were similar in the control and Seprafilm groups.
In this study, the incidence (mean number of sites), severity, and extent (mean area) of uterine adhesions were significantly lower in the Seprafilm-treated patients. The proportion of patients undergoing anterior hysterotomies who were found to have no anterior uterine adhesions was 39% in the Seprafilm group, compared with 6% in the control group. The percentage of patients with at least 1 adnexa totally free of adhesions to the posterior uterus also was higher in the Seprafilm group—48% versus 31%.11
Nonabsorbable barriers
GoreTex Surgical Membrane (W.L. Gore and Associates, Newark, Del) is an inert expanded polytetrafluoroethylene (PTFE) derivative that must be sutured in place. It is of potential utility in myomectomy because its application does not require absolute hemostasis. However, its usefulness and application are limited by the need for later removal.
In a multicenter, randomized, controlled trial exploring its adhesion-preventive properties after myomectomy, the GoreTex membrane significantly outperformed the barrierfree group.16
In a separate multicenter, randomized clinical trial, the GoreTex membrane was more effective than Interceed in preventing adhesions after pelvic/tubal reconstructive surgery.17
Yet another multicenter, randomized trial—this one involving 27 women undergoing abdominal myomectomy—used SLL to determine the extent of adhesions and to remove the barrier. The percentage of adhesion-free surgical sites was significantly higher in the GoreTex group: 56% compared with 7% in no-barrier controls.16
The adhesion scores, determined by the extent (area of involvement), nature (filmy versus opaque), and tenacity (ease of lysis or dissection) of the adhesions, were significantly lower in the GoreTex group.16
Despite the few prospective, randomized trials, the evidence supports the efficacy of various fluid and barrier adjuvants.
Histologic examination of the removed barriers demonstrated no tissue attachment to the PTFE. Despite these positive findings, however, both fistula formation and graft infection have been reported after placement of a GoreTex barrier.18
The Shelhigh Pericardial No-React Patch (Herzog Surgical, Sacramento, Calif) is a new nonabsorbable adhesion barrier that was first described in gynecologic use by Pelosi and Pelosi in a case series of 20 patients.19 Consisting of a 12-cm-diameter patch of glutaraldehyde-treated bovine/porcine pericardium, this product has an excellent safety record as a permanent pericardial substitute in the cardiovascular literature and has been shown to resist calcification and adhesions.
In the Pelosi series, the patch was placed over the uterine fundus and secured by 4 monofilament sutures at the dome of the uterus and the parietal peritoneum.19 All patients underwent SLL at 6 weeks, and 3 patients also underwent third-look laparoscopy. None of the 20 women had adhesions between the abdominal wall and the bladder, bowel, uterus, or adnexae at SLL. However, minimal adhesions of the ovaries and tubes were found in 7 patients who had undergone posterior hysterotomy. Clinical trials are likely to follow this small pilot study.
Absorbable fluid adjuvants
With barrier adjuvants, optimal benefit is obtained when the physician can predict potential sites of adhesions. This is not a strict requirement with fluid barriers, which is their chief advantage. Among the agents described below, Sepracoat (Genzyme Corp) is available in the United States, while Intergel (Lifecore Biomedical, Chaska, Minn) was withdrawn from the market in March.
Intergel, a 0.5% ferric hyaluronade formulation, is a sterile nonpyrogenic gel of highly purified sodium hyaluronate, which is ionically cross-linked with ferric ion and adjusted to isotonicity with sodium chloride.11 (Hyaluronic acid is a major component of body tissues and fluids such as peritoneal fluid, where it performs physically supportive and mechanically protective roles.)
Johns and colleagues20 studied Intergel in a randomized, multicenter, third-party– blinded, placebo-controlled study. Of the 265 patients who completed the study, 131 were given 300 mL of Intergel and 134 were given lactated Ringer’s solution (the placebo) at the time of their surgery, through the laparoscopic port. When SLL was performed 6 to 12 weeks after surgery, the mean number and severity of adhesions—overall and at the surgical site—were significantly lower in the Intergel group. Adhesions reformed in 91% of those in the control group, compared with 63% in the Intergel group.
In myomectomy patients, the modified American Fertility Society Score, which uses 24 potential adhesion sites, was reduced by 42% in the Intergel group—a statistically significant improvement.20
One major advantage of Intergel is that it reduces adhesion formation at sites distant from the area of application, secondary to its wide intra-abdominal circulation. However, as mentioned above, sales were voluntarily suspended due to post-market reports of tissue adherence, sterile foreign-body reaction, and late-onset pain that sometimes required surgical intervention. In some patients, persistent residual material was noted at the time of subsequent surgery.
Sepracoat (hyaluronic acid-coat) is a dilute solution of 0.4% hyaluronic acid in phosphate-buffered saline. It is bioresorbable, persists at the application site less than 24 hours, and is completely cleared in less than 5 days.
In a prospective, randomized, blinded, placebo-controlled, multicenter study in 1998, Diamond and colleagues compared Sepracoat with a pure phosphate-buffered saline solution in 227 women undergoing gynecologic procedures via laparotomy.21 The aim of the study was to assess the efficacy and safety of the fluid at sites without direct surgical trauma or adhesiolysis. Both solutions were warmed to room temperature and injected into the abdominal cavity before the procedure began (250 mL after skin incision). The solution was reapplied after irrigation or every 30 minutes (100 mL), and at the end of the procedure (250 mL) before closure. The maximum volume used was 1,000 mL. After application, the fluid was left 1 minute before suctioning. Patients underwent SLL 40 days later, and adhesions were identified at the initial procedure and at SLL.
In the Sepracoat group, there was a reduction in de novo adhesions at nonsurgical sites by a factor of 2.8. The proportion of sites with de novo adhesions also decreased, and 80% of Sepracoat patients had at least 1 ovary that was adhesion-free compared with 58% of placebo-treated patients. These findings occurred in areas of indirect trauma, demonstrating that Sepracoat limited trauma at tissue injury.21
Conclusion
Adhesion prevention is of utmost importance after abdominal myomectomy, especially in patients who desire future fertility. Despite the limited number of prospective, randomized studies, the literature does support the efficacy of various fluid and barrier adjuvants.
Our practice is to use an adjuvant in all abdominal myomectomies. We have long relied on Interceed, which enjoys both ease of application and an excellent safety record. However, as reviewed above, other potentially useful products are available or in development. As ever, the reproductive surgeon should adhere to principles of microsurgery, strive for meticulous hemostasis, and demonstrate respect for tissue integrity.
Dr. DeCherney reports small holdings with Lifecore Biomedical. Drs. Chang and Marin report no affiliations or financial arrangements with any of the manufacturers of products mentioned in this article.
- To reduce the incidence of postoperative adhesions, follow basic principles of microsurgery: Minimize the number and extent of incisions, handle all tissue gently, strive for absolute hemostasis, and use small, nonreactive suture.
- Despite limited data from prospective, randomized studies, both fluid and barrier adjuvants have proved effective in reducing the incidence and extent of adhesions after abdominal myomectomy.
Abdominal myomectomy is the preferred treatment in women with large or numerous intramural myomas, especially in the setting of infertility, recurrent pregnancy loss, and preservation of future fertility.1,2 However, postoperative adhesions are distressingly common following this procedure, resulting in significant potential morbidity. Fortunately, a number of products can reduce their occurrence. Proper surgical techniques and a thorough knowledge of these products are invaluable in helping reduce the incidence of adhesions.
The association between adhesions and diminished fertility is well-established,3,4 particularly when peritubal involvement is present (FIGURES 1-3). Abdominopelvic adhesions also contribute to significant chronic pelvic pain, bowel obstruction, and technical difficulty in subsequent surgical or assisted-reproduction procedures.5 Unfortunately, most attempts at adhesiolysis meet with less than complete success, since adhesions recur in 55% to 100% of patients (FIGURE 4).6 Thus, preventing adhesions in the first place would seem to be key to successful outcomes in abdominal myomectomy.
This article reviews the evidence on various approaches and products. While the number of studies examining each adjuvant in the setting of abdominal myomectomy is limited, the overall evidence supports the safety and efficacy of both liquid and barrier adjuvants.
Adhesions present a significant clinical dilemma after abdominal myomectomy, occurring in 50% to 90% of patients.5,7 In 1 prospective series of women undergoing second-look laparoscopy (SLL) after myomectomy, adnexal adhesions were noted in 94% of patients with posterior uterine incisions and in 56% of patients with only anterior or fundal incisions; adhesions between the uterus and omentum or bowel occurred in 88% of all patients.5
In another study of early SLL following abdominal myomectomy, 83% of patients had adhesions between the surgical site and the bowel or omentum, and 65% had adhesions involving the adnexae.2 Removal of large, bulky fibroids (with uterine mass exceeding 13 weeks’ gestational size) resulted in higher adhesion scores than did small myomas. Again, the incidence and severity of adhesions also correlated with location of the uterine incision: Adnexal adhesions were more common after posterior uterine incisions (76%) than after anterior or fundal entries (45%).2
FIGURE 1 Peritubal adhesion
Adhesion at distal end of the fallopian tube. Peritubal involvement often leads to diminished fertility.
FIGURE 2 Adherent structures
Postoperative adhesion between fallopian tube and the uterus.
FIGURE 3 Cyst dissection
Adhesions can follow common surgeries such as paratubal cyst dissection.
FIGURE 4 Recurrent adhesions
Adhesions may recur following adhesiolysis.
Causes of pelvic adhesions
Adhesion prevention requires an understanding of risk factors and maneuvers that increase the likelihood of injury (see “Pathophysiology of adhesion formation”). A number of causes have been proposed, most of them centering on tissue and peritoneal trauma (TABLE 1).
Injury can arise from excessive or rough manipulation of tissue and peritoneal surfaces or from common effects such as cutting, abrasion, and denudation (FIGURE 5). Tissue desiccation or manual blotting may lead to peritoneal desquamation and fibrin deposition.
Exposure of surfaces to intraperitoneal blood in the setting of tissue hypoxia—virtually unavoidable during abdominal myomectomy—disrupts normal fibrinolytic activity, resulting in stimulation of angiogenesis.8
Introduction of reactive foreign bodies such as talc powder, residual suture material, and even lint from laparotomy pads can favor adhesion formation. These serve as substrates or niduses of fibrin deposition.
FIGURE 5 Conducive conditions
Raw surface area can become a potent substrate for adhesions.TABLE 1
Proposed causes of adhesion formation
| Tissue hypoxia or ischemia |
| Tissue desiccation |
| Intra-abdominal infection |
| Introduction of reactive foreign body |
| Presence of intraperitoneal blood |
| Dissection of adhesions |
Techniques that may help prevent adhesions
Based on findings from studies of second-look procedures, most physicians advocate avoiding posterior uterine incisions, as well as minimizing the number and extent of incisions, to help reduce the likelihood of adhesion formation. Further, many Ob/Gyns favor removing myomas through as few uterine incisions as possible.9 We select anterior hysterotomy sites that enable removal of multiple fibroids, avoiding posterior incisions and the uterotubal junction whenever possible.
Interestingly, reapproximation of peritoneal defects after reproductive surgery (and, probably, myomectomy) does not appear to help prevent adhesions. Tulandi and colleagues10 examined the clinical and SLL outcomes of peritoneal closure in patients undergoing Pfannenstiel incisions with or without peritoneal closure at the end of the procedure. There was no difference in postoperative complications or wound healing in the 2 groups. At the time of SLL, there was no significant difference in the incidence of adhesion formation at the anterior abdominal wall.
Principles of microsurgery have been adopted by reproductive surgeons to minimize the likelihood of adhesions after myomectomy and gynecologic surgery in general. The basic techniques reflect respect for tissue integrity:
- Gentle handling of tissue, with minimal manipulation of all peritoneal surfaces.
- Meticulous hemostasis. Examine all myomectomy sites to ensure adequate hemostasis prior to closure.
- Continuous irrigation to prevent tissue desiccation. We favor continuous saline irrigation throughout the procedure. We also use only moistened laparotomy sponges and pads, and avoid applying dry gauze to any tissue surface.
- Avoidance of foreign-body introduction. We use only talc-free gloves and remove all residual suture fragments and tissue debris before closure. We also perform copious saline suction-irrigation at the end of the procedure to remove as much residue as possible.
- Use of fine, nonreactive suture. We favor fine, resorbable sutures that incite as little tissue reactivity as possible. For closure of large myomectomy defects, we use braided multifilaments such as Vicryl (polyglactine 910) (Ethicon, Somerville, NJ) or Dexon (polyglycolic acid) (Davis and Geck, Danbury, Conn) for strength and ease of handling. These sutures are absorbed through simple hydrolysis and stimulate less tissue reactivity than do chromic or catgut sutures.
The idea is appealing, but a randomized, blinded comparison of “good” and “bad” microsurgical technique is unlikely, since no one would wish to perform “bad” technique.
With barrier adjuvants, optimal benefit is obtained when the physician can predict potential sites of adhesions.
Wide range of prevention tools has been studied
Many types of agents have been studied in an attempt to reduce postsurgical adhesions after gynecologic surgery. Although most offer little or no benefit (TABLE 2), a few have potential in myomectomy procedures.
Barriers that form mechanical separation (TABLE 3) theoretically physically separate damaged tissues during early peritoneal wound healing, when adhesions form.11 The original adhesion barriers consisted of omental and peritoneal grafts that were placed over surgical sites. However, studies demonstrated that devitalized tissue positioned on damaged peritoneum serves as a potent substrate—not inhibitor—for adhesions.12 More recent trials have examined the adhesion-prevention benefit of other types of absorbable and nonabsorbable barriers.
TABLE 2
Pharmacologic agents studied for adhesion prevention in reproductive surgery
| AGENT | THEORETICAL ACTION | EXAMPLES | MODE OF USE/APPLICATION | RISKS/PROBLEMS |
|---|---|---|---|---|
| Antibiotics | Prevent infection or inflammation | Cephalosporins Tetracyclines | Intraperitoneal irrigation with antibiotic fluid Hydrotubation fluid with antibiotic | Theoretical reaction to antibiotic |
| Anticoagulants | Clot prevention Fibrin prevention | Heparin | In conjunction with Interceed | Risk of postoperative bleeding |
| Anti-inflammatory agents | Decrease permeability and histamine release | Nonsteroidal anti-inflammatory drugs Corticosteroids | Awaiting further investigation | Theoretical reaction to agent |
| Crystalloid solutions | Hydroflotation effect, decrease surface contact between pelvic organs | Normal saline Ringer’s lactate | Intra-abdominal instillation | Possible volume overload from intravascular absorption |
| Fibrinolytic agents | Fibrinolysis Plasminogen activation | Streptokinase Trypsin Fibrinolysin | Awaiting further investigation | Theoretical risk of postoperative bleeding |
| Steroids | Decrease inflammatory response | Dexamethasone | Systemic and/or intraperitoneal | Possible suppression of hypothalamic-pituitary axis |
| Polysaccharide polymer | "Siliconizing" effect to coat raw surfaces | Dextran 70 (Hyskon) | 200 mL placed in posterior cul-de-sac or coating surgical site surfaces | Abdominal bloating, anaphylaxis, pleural effusion, liver function abnormalities, wound separation, rare diffuse intravascular coagulation |
| Other fluid and barrier agents* | Peritoneal surface separation Hydroflotation | Absorbable and nonabsorbable barriers (see Table 3) | See Table 3 | See Table 3 |
| *Adjuvants studied in the setting of abdominal myomectomy | ||||
TABLE 3
Fluid and barrier adjuvants studied for adhesion reduction after myomectomy
| AGENT | THEORETICAL ACTION | MODE OF USE/APPLICATION | PROBLEMS | EVIDENCE FOR ADHESION REDUCTION |
|---|---|---|---|---|
| ABSORBABLE (BARRIER) | ||||
| Oxidized regenerated cellulose (Interceed) | Protective layer over surgical sites to prevent surface contact | Direct placement onto surface of uterus; no suturing required | Requires hemostasis | Prospective studies and meta-analysis support benefit in reproductive surgery including abdominal myomectomy |
| Hyaluronatecarboxymethycellulose derivative film (Seprafilm) | Protective layer over surgical sites to prevent surface contact | Direct placement around entire uterine surface; no suturing required | Requires hemostasis | Multicenter prospective, randomized study supports benefit in reducing adhesions after abdominal myomectomy |
| NONABSORBABLE (BARRIER) | ||||
| Expanded polytetrafluoroethylene (GoreTex) | Prevent contact between surgical surfaces | Patch sutured onto surface of uterus | Usually must be removed Report of fistula formation when left in situ | Multicenter prospective studies support adhesion-preventive benefit after abdominal myomectomy |
| Pericardial patch (Shelhigh No-React) | Prevent contact between surgical surfaces | Patch sutured onto surface of uterus | Early clinical use in myomectomy Proven safety as pericardial patch in humans | Preliminary study (case series data only) shows potential benefit |
| FLUID | ||||
| Hyaluronic acid-coat (Sepracoat) | Diffuse coating on surgical sites and potential sites of contact | 100-mL to 250-mL aliquots injected into peritoneal cavity | Limited data on efficacy in abdominal myomectomy | Small studies (multicenter, prospective, randomized, controlled trials) demonstrated reduced postoperative adhesions after reproductive surgery via laparotomy, including myomectomy |
| Hyaluronate-carboxymethycellulose derivative gel (Intergel) | Diffuse coating on surgical sites and potential sites of contact | 300-mL aliquot into peritoneal cavity | Withdrawn from market for reports of postoperative pain, complications | Reduced adhesion formation in animal studies and in preliminary human studies |
Adhesions are fibrous or fibrovascular bands that connect tissue surfaces in abnormal locations.1,2 Their development likely results from an imbalance in inflammatory mediators or fibrin degradation during peritoneal wound healing.
Peritoneal injury initiates the release of histamine and vasoactive kinins that mediate increased capillary permeability and outpouring of serosanguineous fluid.3 This proteinaceous exudate coagulates, depositing fibrinous bands between areas of denuded tissue.
Under normal circumstances, the fibrinolytic system is activated to lyse these bands within 72 hours. Peritoneal healing occurs when mesothelial cells migrate from the underlying mesenchyme to reepithelialize the injured site.4 Disequilibrium of the fibrin deposition-fibrinolysis system results in a persistent band that will eventually undergo fibroblast and vascular invasion.
REFERENCES
1. Diamond MP, DeCherney AH. Pathogenesis of adhesion formation/reformation: application to reproductive pelvic surgery. Microsurgery. 1987;8:103-107.
2. Diamond MP, Freeman ML. Clinical implications of postsurgical adhesions. Hum Reprod. 2001;7:567-576.
3. Diamond MP, El-Mowafi DM. Pelvic adhesions. Surg Technol Int. 1998;VII:273-283.
4. Farquhar C, Vandekerckhove P, Watson A, Vail A, Wiseman D. Barrier agents for preventing adhesions after surgery for subfertility. Cochrane Database Systematic Rev. 2002;(4):1-34.
Absorbable barriers
These are largely derivatives of organic materials. Their application to myomectomy may be limited by the requirement for absolute hemostasis at the site of application.
Interceed (Gynecare, a division of Ethicon), which is derived from oxidized regenerated cellulose, is one of the first and most extensively evaluated barriers. A mesh synthetic designed to be placed over injured tissue, it is a derivative of the hemostatic agent Surgicell (Johnson & Johnson, New Brunswick, NJ), with modifications in weave and pore size.
Interceed offers ease of application: It can be cut to the size or shape necessary and requires no suturing. It forms a gelatinous protective layer within 8 hours of placement, and is degraded into monosaccharides and absorbed within 2 weeks.13
The use of Interceed has been shown to reduce adhesions following adhesiolysis and ovarian surgery. In a large, multicenter, prospective, randomized trial, it significantly decreased the incidence of adhesion reformation after adnexal adhesiolysis in infertility patients with bilateral tubal disease.14
In a retrospective series of 38 infertility patients, including 19 patients after myomectomy (13 with the barrier, 6 without), reproductive outcomes were significantly better in the Interceed group, and adhesion development was reduced.15 Pregnancy rates in the 2 years following surgery were 78% in the Interceed group, compared with 47% in controls. In addition, among 23 patients who had second-look procedures, postoperative adhesions were noted in 38% of the Interceed group, compared with 86% in controls.15
Seprafilm (hyaluronic acid-film) (Genzyme Corp, Cambridge, Mass) is a bioresorbable membrane derived from sodium hyaluronate and carboxymethylcellulose. It is absorbed from the peritoneal cavity within 1 week and is completely excreted within 1 month.
Its potential for adhesion prevention after abdominal myomectomy was examined by SLL in a multicenter, prospective, randomized, blinded study11 in which 127 women undergoing abdominal myomectomy at 19 institutions were randomized to either Seprafilm or no barrier. In the Seprafilm group, the barrier was wrapped circumferentially around the uterus, covering all uterine defects, at the time of abdominal closure. Clinical outcomes—including vital signs, adverse events (pain, fever, nausea), and abdominal wound complications—were similar in the control and Seprafilm groups.
In this study, the incidence (mean number of sites), severity, and extent (mean area) of uterine adhesions were significantly lower in the Seprafilm-treated patients. The proportion of patients undergoing anterior hysterotomies who were found to have no anterior uterine adhesions was 39% in the Seprafilm group, compared with 6% in the control group. The percentage of patients with at least 1 adnexa totally free of adhesions to the posterior uterus also was higher in the Seprafilm group—48% versus 31%.11
Nonabsorbable barriers
GoreTex Surgical Membrane (W.L. Gore and Associates, Newark, Del) is an inert expanded polytetrafluoroethylene (PTFE) derivative that must be sutured in place. It is of potential utility in myomectomy because its application does not require absolute hemostasis. However, its usefulness and application are limited by the need for later removal.
In a multicenter, randomized, controlled trial exploring its adhesion-preventive properties after myomectomy, the GoreTex membrane significantly outperformed the barrierfree group.16
In a separate multicenter, randomized clinical trial, the GoreTex membrane was more effective than Interceed in preventing adhesions after pelvic/tubal reconstructive surgery.17
Yet another multicenter, randomized trial—this one involving 27 women undergoing abdominal myomectomy—used SLL to determine the extent of adhesions and to remove the barrier. The percentage of adhesion-free surgical sites was significantly higher in the GoreTex group: 56% compared with 7% in no-barrier controls.16
The adhesion scores, determined by the extent (area of involvement), nature (filmy versus opaque), and tenacity (ease of lysis or dissection) of the adhesions, were significantly lower in the GoreTex group.16
Despite the few prospective, randomized trials, the evidence supports the efficacy of various fluid and barrier adjuvants.
Histologic examination of the removed barriers demonstrated no tissue attachment to the PTFE. Despite these positive findings, however, both fistula formation and graft infection have been reported after placement of a GoreTex barrier.18
The Shelhigh Pericardial No-React Patch (Herzog Surgical, Sacramento, Calif) is a new nonabsorbable adhesion barrier that was first described in gynecologic use by Pelosi and Pelosi in a case series of 20 patients.19 Consisting of a 12-cm-diameter patch of glutaraldehyde-treated bovine/porcine pericardium, this product has an excellent safety record as a permanent pericardial substitute in the cardiovascular literature and has been shown to resist calcification and adhesions.
In the Pelosi series, the patch was placed over the uterine fundus and secured by 4 monofilament sutures at the dome of the uterus and the parietal peritoneum.19 All patients underwent SLL at 6 weeks, and 3 patients also underwent third-look laparoscopy. None of the 20 women had adhesions between the abdominal wall and the bladder, bowel, uterus, or adnexae at SLL. However, minimal adhesions of the ovaries and tubes were found in 7 patients who had undergone posterior hysterotomy. Clinical trials are likely to follow this small pilot study.
Absorbable fluid adjuvants
With barrier adjuvants, optimal benefit is obtained when the physician can predict potential sites of adhesions. This is not a strict requirement with fluid barriers, which is their chief advantage. Among the agents described below, Sepracoat (Genzyme Corp) is available in the United States, while Intergel (Lifecore Biomedical, Chaska, Minn) was withdrawn from the market in March.
Intergel, a 0.5% ferric hyaluronade formulation, is a sterile nonpyrogenic gel of highly purified sodium hyaluronate, which is ionically cross-linked with ferric ion and adjusted to isotonicity with sodium chloride.11 (Hyaluronic acid is a major component of body tissues and fluids such as peritoneal fluid, where it performs physically supportive and mechanically protective roles.)
Johns and colleagues20 studied Intergel in a randomized, multicenter, third-party– blinded, placebo-controlled study. Of the 265 patients who completed the study, 131 were given 300 mL of Intergel and 134 were given lactated Ringer’s solution (the placebo) at the time of their surgery, through the laparoscopic port. When SLL was performed 6 to 12 weeks after surgery, the mean number and severity of adhesions—overall and at the surgical site—were significantly lower in the Intergel group. Adhesions reformed in 91% of those in the control group, compared with 63% in the Intergel group.
In myomectomy patients, the modified American Fertility Society Score, which uses 24 potential adhesion sites, was reduced by 42% in the Intergel group—a statistically significant improvement.20
One major advantage of Intergel is that it reduces adhesion formation at sites distant from the area of application, secondary to its wide intra-abdominal circulation. However, as mentioned above, sales were voluntarily suspended due to post-market reports of tissue adherence, sterile foreign-body reaction, and late-onset pain that sometimes required surgical intervention. In some patients, persistent residual material was noted at the time of subsequent surgery.
Sepracoat (hyaluronic acid-coat) is a dilute solution of 0.4% hyaluronic acid in phosphate-buffered saline. It is bioresorbable, persists at the application site less than 24 hours, and is completely cleared in less than 5 days.
In a prospective, randomized, blinded, placebo-controlled, multicenter study in 1998, Diamond and colleagues compared Sepracoat with a pure phosphate-buffered saline solution in 227 women undergoing gynecologic procedures via laparotomy.21 The aim of the study was to assess the efficacy and safety of the fluid at sites without direct surgical trauma or adhesiolysis. Both solutions were warmed to room temperature and injected into the abdominal cavity before the procedure began (250 mL after skin incision). The solution was reapplied after irrigation or every 30 minutes (100 mL), and at the end of the procedure (250 mL) before closure. The maximum volume used was 1,000 mL. After application, the fluid was left 1 minute before suctioning. Patients underwent SLL 40 days later, and adhesions were identified at the initial procedure and at SLL.
In the Sepracoat group, there was a reduction in de novo adhesions at nonsurgical sites by a factor of 2.8. The proportion of sites with de novo adhesions also decreased, and 80% of Sepracoat patients had at least 1 ovary that was adhesion-free compared with 58% of placebo-treated patients. These findings occurred in areas of indirect trauma, demonstrating that Sepracoat limited trauma at tissue injury.21
Conclusion
Adhesion prevention is of utmost importance after abdominal myomectomy, especially in patients who desire future fertility. Despite the limited number of prospective, randomized studies, the literature does support the efficacy of various fluid and barrier adjuvants.
Our practice is to use an adjuvant in all abdominal myomectomies. We have long relied on Interceed, which enjoys both ease of application and an excellent safety record. However, as reviewed above, other potentially useful products are available or in development. As ever, the reproductive surgeon should adhere to principles of microsurgery, strive for meticulous hemostasis, and demonstrate respect for tissue integrity.
Dr. DeCherney reports small holdings with Lifecore Biomedical. Drs. Chang and Marin report no affiliations or financial arrangements with any of the manufacturers of products mentioned in this article.
1. Stewart EA. Treatment of uterine leiomyomas. UpToDate (Version 10.3) 2002; 1-8. Available at: www.uptodate.com.
2. Ugur M, Tura C, Mungan T, Aydogdu T, Sahin Y, Gokmen O. Laparoscopy for adhesion prevention following myomectomy. Int J Gynecol Obstet. 1996;53:145-149.
3. Berkeley AS, DeCherney AH, Plan ML. Abdominal myomectomy and subsequent fertility. Surg Gynecol Obstet. 1983;153:319-322.
4. Tulandi T, Collin JA, Burrows E, et al. Treatment-dependent and treatment-independent pregnancy among women with periadnexal adhesions. Am J Obstet Gynecol. 1990;162:354-357.
5. Tulandi T, Murria C, Guralnick M. Adhesion formation and reproductive outcome after myomectomy and second-look laparoscopy. Obstet Gynecol. 1993;82:213-215.
6. Diamond MP, Freeman ML. Clinical implications of postsurgical adhesions. Hum Reprod. 2001;7:567-576.
7. Lau S, Tulandi T. Myomectomy and adhesion formation. In: diZerega GS, ed. Peritoneal Surgery. New York: Springer-Verlag 1999;289-290.
8. Diamond MP, El-Mowafi DM. Pelvic adhesions. Surg Technol Int. 1998;VII:273-283.
9. Guarnaccia MM, Rein MS. Traditional surgical approaches to uterine fibroids: abdominal myomectomy and hysterectomy. Clin Obstet Gynecol. 2001;44:385-400.
10. Tulandi T, Hum HS, Gelfand MM. Closure of laparotomy incisions with or without peritoneal suturing and second-look laparoscopy. Am J Obstet Gynecol. 1988;158:536-537.
11. Diamond MP. Reduction of adhesions after uterine myomectomy by Seprafilm membrane (HAL-F): a blinded, prospective, randomized, multicenter clinical study. Fertil Steril. 1996;66:904-910.
12. Johns D. Reduction of postsurgical adhesions with Intergel adhesion prevention solution: a multicenter study of safety and efficacy after conservative gynecologic surgery. Fertil Steril. 2001;76:595-604.
13. Farquhar C, vandekerckhove P, Watson A, Vail A, Wiseman D. Barrier agents for preventing adhesions after surgery for subfertility. Cochrane Database Systematic Rev. 2002(4);1-34.
14. Nordic Adhesion Prevention Study Group. The efficacy of Interceed (TC7) for prevention of reformation of postoperative adhesions on ovaries, fallopian tubes, and fimbriae in microsurgical operations for fertility: a multicenter study. Fertil Steril. 1995;63:709-714.
15. Sawada T, Nishizawa H, Nishio E, Kadowaki M. Postoperative adhesion prevention with an oxidized regenerated cellulose adhesion barrier in infertile women. J Reprod Med. 2000;45:387-389.
16. The Myomectomy Adhesions Multicenter Study Group. An expanded polytetrafluoroethylene barrier (Gore-Tex Surgical Membrane) reduces post-myomectomy adhesion formation. Fertil Steril. 1995;63:491-493.
17. Haney AF, Hesla J, Hurst BS, et al. Expanded polytetrafluoroethylene (Gore-Tex Surgical Membrane) is superior to oxidized regenerated cellulose (Interceed TC7) in preventing adhesions. Fertil Steril. 1995;63:1021-1026.
18. Monteforte CA, Queirazza R, Francescetting P, Herbst TJ. Fistula formation after implanting an ePTFE membrane. A case report. J Reprod Med. 1997;42:184-187.
19. Pelosi MA, II, Pelosi MA, III. A new nonabsorbable adhesion barrier for myomectomy. Am J Surg. 2002;184:428-432.
20. Johns D. Reduction of postsurgical adhesions with Intergel adhesion prevention solution: a multicenter study of safety and efficacy after conservative gynecologic surgery. Fertil Steril. 2001;76:596-604.
21. Diamond MP and The Sepracoat Adhesion Study Group. Reduction of de novo postsurgical adhesions by intraoperative precoating with Sepracoat (HAL-C) solution: a prospective, randomized, blinded, placebo-controlled multicenter study. Fertil Steril. 1998;69:1067-1074.
1. Stewart EA. Treatment of uterine leiomyomas. UpToDate (Version 10.3) 2002; 1-8. Available at: www.uptodate.com.
2. Ugur M, Tura C, Mungan T, Aydogdu T, Sahin Y, Gokmen O. Laparoscopy for adhesion prevention following myomectomy. Int J Gynecol Obstet. 1996;53:145-149.
3. Berkeley AS, DeCherney AH, Plan ML. Abdominal myomectomy and subsequent fertility. Surg Gynecol Obstet. 1983;153:319-322.
4. Tulandi T, Collin JA, Burrows E, et al. Treatment-dependent and treatment-independent pregnancy among women with periadnexal adhesions. Am J Obstet Gynecol. 1990;162:354-357.
5. Tulandi T, Murria C, Guralnick M. Adhesion formation and reproductive outcome after myomectomy and second-look laparoscopy. Obstet Gynecol. 1993;82:213-215.
6. Diamond MP, Freeman ML. Clinical implications of postsurgical adhesions. Hum Reprod. 2001;7:567-576.
7. Lau S, Tulandi T. Myomectomy and adhesion formation. In: diZerega GS, ed. Peritoneal Surgery. New York: Springer-Verlag 1999;289-290.
8. Diamond MP, El-Mowafi DM. Pelvic adhesions. Surg Technol Int. 1998;VII:273-283.
9. Guarnaccia MM, Rein MS. Traditional surgical approaches to uterine fibroids: abdominal myomectomy and hysterectomy. Clin Obstet Gynecol. 2001;44:385-400.
10. Tulandi T, Hum HS, Gelfand MM. Closure of laparotomy incisions with or without peritoneal suturing and second-look laparoscopy. Am J Obstet Gynecol. 1988;158:536-537.
11. Diamond MP. Reduction of adhesions after uterine myomectomy by Seprafilm membrane (HAL-F): a blinded, prospective, randomized, multicenter clinical study. Fertil Steril. 1996;66:904-910.
12. Johns D. Reduction of postsurgical adhesions with Intergel adhesion prevention solution: a multicenter study of safety and efficacy after conservative gynecologic surgery. Fertil Steril. 2001;76:595-604.
13. Farquhar C, vandekerckhove P, Watson A, Vail A, Wiseman D. Barrier agents for preventing adhesions after surgery for subfertility. Cochrane Database Systematic Rev. 2002(4);1-34.
14. Nordic Adhesion Prevention Study Group. The efficacy of Interceed (TC7) for prevention of reformation of postoperative adhesions on ovaries, fallopian tubes, and fimbriae in microsurgical operations for fertility: a multicenter study. Fertil Steril. 1995;63:709-714.
15. Sawada T, Nishizawa H, Nishio E, Kadowaki M. Postoperative adhesion prevention with an oxidized regenerated cellulose adhesion barrier in infertile women. J Reprod Med. 2000;45:387-389.
16. The Myomectomy Adhesions Multicenter Study Group. An expanded polytetrafluoroethylene barrier (Gore-Tex Surgical Membrane) reduces post-myomectomy adhesion formation. Fertil Steril. 1995;63:491-493.
17. Haney AF, Hesla J, Hurst BS, et al. Expanded polytetrafluoroethylene (Gore-Tex Surgical Membrane) is superior to oxidized regenerated cellulose (Interceed TC7) in preventing adhesions. Fertil Steril. 1995;63:1021-1026.
18. Monteforte CA, Queirazza R, Francescetting P, Herbst TJ. Fistula formation after implanting an ePTFE membrane. A case report. J Reprod Med. 1997;42:184-187.
19. Pelosi MA, II, Pelosi MA, III. A new nonabsorbable adhesion barrier for myomectomy. Am J Surg. 2002;184:428-432.
20. Johns D. Reduction of postsurgical adhesions with Intergel adhesion prevention solution: a multicenter study of safety and efficacy after conservative gynecologic surgery. Fertil Steril. 2001;76:596-604.
21. Diamond MP and The Sepracoat Adhesion Study Group. Reduction of de novo postsurgical adhesions by intraoperative precoating with Sepracoat (HAL-C) solution: a prospective, randomized, blinded, placebo-controlled multicenter study. Fertil Steril. 1998;69:1067-1074.