User login
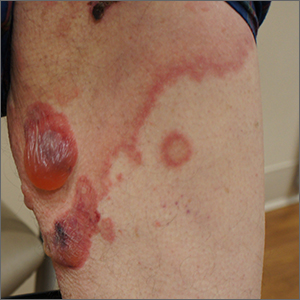
Blisters on arms and legs
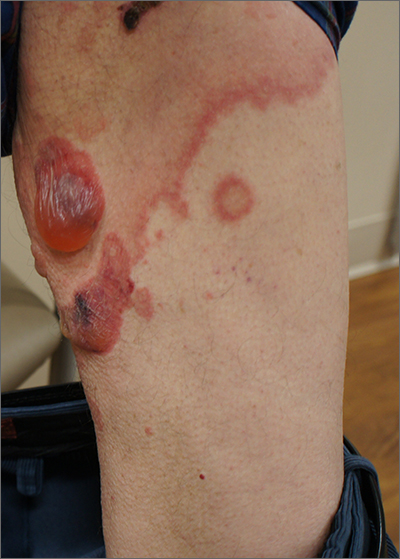
This patient was given a diagnosis of bullous pemphigoid. Although there were a number of clues that pointed to this diagnosis, confirming that this was the case required 2 biopsies and a blood draw. (More on this in a bit.)
Although rare and potentially lethal, bullous pemphigoid is the most common autoimmune blistering disease in the elderly. Patients present with tense bullae over limited or widespread areas of the skin. The pathogenesis includes development of autoimmune antibodies that target important proteins (BP180 and BP230) that bind basal epidermal keratinocytes to the dermis. When weakened by inflammation at these sites, the skin delaminates at the dermal-epidermal junction, while the cells of the epidermis continue to bind to each other. This leads to itching, hive-like wheals, and tense fluid-filled bullae.
The differential diagnosis of an acute or semi-acute bullous disease includes bullous pemphigoid, IgA pemphigoid, linear IgA bullous dermatosis, epidermolysis bullosa acquisita, and Senear-Usher syndrome. In this case, the large tense bullae suggested bullous pemphigoid over the other diagnoses.
Initial diagnosis requires 2 biopsies be performed: One at the edge of a bulla for a standard pathologic exam to identify the skin level at which the bulla is forming, and another biopsy of skin near the site of inflammation (5-10 mm away) to be sent for direct immunofluorescence (DIF) in Michel’s medium or Zeus medium. In bullous pemphigoid, the separation is at the dermal-epidermal junction, and IgG and C3 are found in the DIF in the same location. There are a couple ways to differentiate this disorder from epidermolysis bullosa acquisita—a similar blistering disorder in which autoantibodies attack collagen at the dermal-epidermal junction. A common approach is to send a patient’s serum for indirect immunofluorescence. This is done because it is impossible to distinguish between the 2 clinically.
While bullous pemphigoid has historically been treated with high-dose prednisone, it is more common now to treat with whole-body topical clobetasol and oral doxycycline 100 mg twice a day to avoid the adverse effects of the prednisone. Other immunosuppressive options, such as mycophenolate mofetil and cyclosporine, can provide the potency of prednisone with a more favorable long-term safety profile. Rituximab infusions are another very powerful and durable option in refractory or severe cases.1
This patient was treated with topical clobetasol and doxycycline 100 mg twice a day, but he had incomplete clearance after 2 to 3 weeks. At that point, mycophenolate mofetil was added to the regimen and was titrated up to 1000 mg twice daily. When clearance occurred, the clobetasol was discontinued and the mycophenolate mofetil was titrated down to 250 mg/d; the patient continues to maintain clearance at this dose. He continues on doxycycline 100 mg bid.
Photos and text for Photo Rounds Friday courtesy of Jonathan Karnes, MD (copyright retained). Dr. Karnes is the medical director of MDFMR Dermatology Services, Augusta, ME.
1. Ruggiero A, Megna M, Villani A, et al. Strategies to improve outcomes of bullous pemphigoid: a comprehensive review of clinical presentations, diagnosis, and patients' assessment. Clin Cosmet Investig Dermatol. 2022;15:661-673. doi:10.2147/CCID.S267573

This patient was given a diagnosis of bullous pemphigoid. Although there were a number of clues that pointed to this diagnosis, confirming that this was the case required 2 biopsies and a blood draw. (More on this in a bit.)
Although rare and potentially lethal, bullous pemphigoid is the most common autoimmune blistering disease in the elderly. Patients present with tense bullae over limited or widespread areas of the skin. The pathogenesis includes development of autoimmune antibodies that target important proteins (BP180 and BP230) that bind basal epidermal keratinocytes to the dermis. When weakened by inflammation at these sites, the skin delaminates at the dermal-epidermal junction, while the cells of the epidermis continue to bind to each other. This leads to itching, hive-like wheals, and tense fluid-filled bullae.
The differential diagnosis of an acute or semi-acute bullous disease includes bullous pemphigoid, IgA pemphigoid, linear IgA bullous dermatosis, epidermolysis bullosa acquisita, and Senear-Usher syndrome. In this case, the large tense bullae suggested bullous pemphigoid over the other diagnoses.
Initial diagnosis requires 2 biopsies be performed: One at the edge of a bulla for a standard pathologic exam to identify the skin level at which the bulla is forming, and another biopsy of skin near the site of inflammation (5-10 mm away) to be sent for direct immunofluorescence (DIF) in Michel’s medium or Zeus medium. In bullous pemphigoid, the separation is at the dermal-epidermal junction, and IgG and C3 are found in the DIF in the same location. There are a couple ways to differentiate this disorder from epidermolysis bullosa acquisita—a similar blistering disorder in which autoantibodies attack collagen at the dermal-epidermal junction. A common approach is to send a patient’s serum for indirect immunofluorescence. This is done because it is impossible to distinguish between the 2 clinically.
While bullous pemphigoid has historically been treated with high-dose prednisone, it is more common now to treat with whole-body topical clobetasol and oral doxycycline 100 mg twice a day to avoid the adverse effects of the prednisone. Other immunosuppressive options, such as mycophenolate mofetil and cyclosporine, can provide the potency of prednisone with a more favorable long-term safety profile. Rituximab infusions are another very powerful and durable option in refractory or severe cases.1
This patient was treated with topical clobetasol and doxycycline 100 mg twice a day, but he had incomplete clearance after 2 to 3 weeks. At that point, mycophenolate mofetil was added to the regimen and was titrated up to 1000 mg twice daily. When clearance occurred, the clobetasol was discontinued and the mycophenolate mofetil was titrated down to 250 mg/d; the patient continues to maintain clearance at this dose. He continues on doxycycline 100 mg bid.
Photos and text for Photo Rounds Friday courtesy of Jonathan Karnes, MD (copyright retained). Dr. Karnes is the medical director of MDFMR Dermatology Services, Augusta, ME.

This patient was given a diagnosis of bullous pemphigoid. Although there were a number of clues that pointed to this diagnosis, confirming that this was the case required 2 biopsies and a blood draw. (More on this in a bit.)
Although rare and potentially lethal, bullous pemphigoid is the most common autoimmune blistering disease in the elderly. Patients present with tense bullae over limited or widespread areas of the skin. The pathogenesis includes development of autoimmune antibodies that target important proteins (BP180 and BP230) that bind basal epidermal keratinocytes to the dermis. When weakened by inflammation at these sites, the skin delaminates at the dermal-epidermal junction, while the cells of the epidermis continue to bind to each other. This leads to itching, hive-like wheals, and tense fluid-filled bullae.
The differential diagnosis of an acute or semi-acute bullous disease includes bullous pemphigoid, IgA pemphigoid, linear IgA bullous dermatosis, epidermolysis bullosa acquisita, and Senear-Usher syndrome. In this case, the large tense bullae suggested bullous pemphigoid over the other diagnoses.
Initial diagnosis requires 2 biopsies be performed: One at the edge of a bulla for a standard pathologic exam to identify the skin level at which the bulla is forming, and another biopsy of skin near the site of inflammation (5-10 mm away) to be sent for direct immunofluorescence (DIF) in Michel’s medium or Zeus medium. In bullous pemphigoid, the separation is at the dermal-epidermal junction, and IgG and C3 are found in the DIF in the same location. There are a couple ways to differentiate this disorder from epidermolysis bullosa acquisita—a similar blistering disorder in which autoantibodies attack collagen at the dermal-epidermal junction. A common approach is to send a patient’s serum for indirect immunofluorescence. This is done because it is impossible to distinguish between the 2 clinically.
While bullous pemphigoid has historically been treated with high-dose prednisone, it is more common now to treat with whole-body topical clobetasol and oral doxycycline 100 mg twice a day to avoid the adverse effects of the prednisone. Other immunosuppressive options, such as mycophenolate mofetil and cyclosporine, can provide the potency of prednisone with a more favorable long-term safety profile. Rituximab infusions are another very powerful and durable option in refractory or severe cases.1
This patient was treated with topical clobetasol and doxycycline 100 mg twice a day, but he had incomplete clearance after 2 to 3 weeks. At that point, mycophenolate mofetil was added to the regimen and was titrated up to 1000 mg twice daily. When clearance occurred, the clobetasol was discontinued and the mycophenolate mofetil was titrated down to 250 mg/d; the patient continues to maintain clearance at this dose. He continues on doxycycline 100 mg bid.
Photos and text for Photo Rounds Friday courtesy of Jonathan Karnes, MD (copyright retained). Dr. Karnes is the medical director of MDFMR Dermatology Services, Augusta, ME.
1. Ruggiero A, Megna M, Villani A, et al. Strategies to improve outcomes of bullous pemphigoid: a comprehensive review of clinical presentations, diagnosis, and patients' assessment. Clin Cosmet Investig Dermatol. 2022;15:661-673. doi:10.2147/CCID.S267573
1. Ruggiero A, Megna M, Villani A, et al. Strategies to improve outcomes of bullous pemphigoid: a comprehensive review of clinical presentations, diagnosis, and patients' assessment. Clin Cosmet Investig Dermatol. 2022;15:661-673. doi:10.2147/CCID.S267573
COVID can mimic prostate cancer symptoms
This patient has a strong likelihood of aggressive prostate cancer, right? If that same patient also presents with severe, burning bone pain with no precipitating trauma to the area and rest and over-the-counter painkillers are not helping, you’d think, “check for metastases,” right?
That patient was me in late January 2023.
As a research scientist member of the American Urological Association, I knew enough to know I had to consult my urologist ASAP.
With the above symptoms, I’ll admit I was scared. Fortunately, if that’s the right word, I was no stranger to a rapid, dramatic spike in PSA. In 2021 I was temporarily living in a new city, and I wanted to form a relationship with a good local urologist. The urologist that I was referred to gave me a thorough consultation, including a vigorous digital rectal exam (DRE) and sent me across the street for a blood draw.
To my shock, my PSA had spiked over 2 points, to 9.9 from 7.8 a few months earlier. I freaked. Had my 3-cm tumor burst out into an aggressive cancer? Research on PubMed provided an array of studies showing what could cause PSA to suddenly rise, including a DRE performed 72 hours before the blood draw.1 A week later, my PSA was back down to its normal 7.6.
But in January 2023, I had none of those previously reported experiences that could suddenly trigger a spike in PSA, like a DRE or riding on a thin bicycle seat for a few hours before the lab visit.
The COVID effect
I went back to PubMed and found a new circumstance that could cause a surge in PSA: COVID-19. A recent study2 of 91 men with benign prostatic hypertrophy by researchers in Turkey found that PSA spiked from 0 to 5 points during the COVID infection period and up to 2 points higher 3 months after the infection had cleared. I had tested positive for COVID-19 in mid-December 2022, 4 weeks before my 9.9 PSA reading.
Using Google translate, I communicated with the team in Turkey and found out that the PSA spike can last up to 6 months.
That study helps explain why my PSA dropped over 1.5 points to 8.5 just 2 weeks after the 9.9 reading, with the expectation that it would return to its previous normal of 7.8 within 6 months of infection with SARS-CoV-2. To be safe, my urologist scheduled another PSA test in May, along with an updated multiparametric MRI, which may be followed by an in-bore MRI-guided biopsy of the 3-cm tumor if the mass has enlarged.
COVID-19 pain
What about my burning bone pain in my upper right humerus and right rotator cuff that was not precipitated by trauma or strain? A radiograph found no evidence of metastasis, thank goodness. And my research showed that several studies3 have found that COVID-19 can cause burning musculoskeletal pain, including enthesopathy, which is what I had per the radiology report. So my PSA spike and searing pain were likely consequences of the infection.
To avoid the risk for a gross misdiagnosis after a radical spike in PSA, the informed urologist should ask the patient if he has had COVID-19 in the previous 6 months. Overlooking that question could lead to the wrong diagnostic decisions about a rapid jump in PSA or unexplained bone pain.
References
1. Bossens MM et al. Eur J Cancer. 1995;31A:682-5.
2. Cinislioglu AE et al. Urology. 2022;159:16-21.
3. Ciaffi J et al. Joint Bone Spine. 2021;88:105158.
Dr. Keller is founder of the Keller Research Institute, Jacksonville, Fla. He reported serving as a research scientist for the American Urological Association, serving on the advisory board of Active Surveillance Patient’s International, and serving on the boards of numerous nonprofit organizations.
A version of this article first appeared on Medscape.com.
This patient has a strong likelihood of aggressive prostate cancer, right? If that same patient also presents with severe, burning bone pain with no precipitating trauma to the area and rest and over-the-counter painkillers are not helping, you’d think, “check for metastases,” right?
That patient was me in late January 2023.
As a research scientist member of the American Urological Association, I knew enough to know I had to consult my urologist ASAP.
With the above symptoms, I’ll admit I was scared. Fortunately, if that’s the right word, I was no stranger to a rapid, dramatic spike in PSA. In 2021 I was temporarily living in a new city, and I wanted to form a relationship with a good local urologist. The urologist that I was referred to gave me a thorough consultation, including a vigorous digital rectal exam (DRE) and sent me across the street for a blood draw.
To my shock, my PSA had spiked over 2 points, to 9.9 from 7.8 a few months earlier. I freaked. Had my 3-cm tumor burst out into an aggressive cancer? Research on PubMed provided an array of studies showing what could cause PSA to suddenly rise, including a DRE performed 72 hours before the blood draw.1 A week later, my PSA was back down to its normal 7.6.
But in January 2023, I had none of those previously reported experiences that could suddenly trigger a spike in PSA, like a DRE or riding on a thin bicycle seat for a few hours before the lab visit.
The COVID effect
I went back to PubMed and found a new circumstance that could cause a surge in PSA: COVID-19. A recent study2 of 91 men with benign prostatic hypertrophy by researchers in Turkey found that PSA spiked from 0 to 5 points during the COVID infection period and up to 2 points higher 3 months after the infection had cleared. I had tested positive for COVID-19 in mid-December 2022, 4 weeks before my 9.9 PSA reading.
Using Google translate, I communicated with the team in Turkey and found out that the PSA spike can last up to 6 months.
That study helps explain why my PSA dropped over 1.5 points to 8.5 just 2 weeks after the 9.9 reading, with the expectation that it would return to its previous normal of 7.8 within 6 months of infection with SARS-CoV-2. To be safe, my urologist scheduled another PSA test in May, along with an updated multiparametric MRI, which may be followed by an in-bore MRI-guided biopsy of the 3-cm tumor if the mass has enlarged.
COVID-19 pain
What about my burning bone pain in my upper right humerus and right rotator cuff that was not precipitated by trauma or strain? A radiograph found no evidence of metastasis, thank goodness. And my research showed that several studies3 have found that COVID-19 can cause burning musculoskeletal pain, including enthesopathy, which is what I had per the radiology report. So my PSA spike and searing pain were likely consequences of the infection.
To avoid the risk for a gross misdiagnosis after a radical spike in PSA, the informed urologist should ask the patient if he has had COVID-19 in the previous 6 months. Overlooking that question could lead to the wrong diagnostic decisions about a rapid jump in PSA or unexplained bone pain.
References
1. Bossens MM et al. Eur J Cancer. 1995;31A:682-5.
2. Cinislioglu AE et al. Urology. 2022;159:16-21.
3. Ciaffi J et al. Joint Bone Spine. 2021;88:105158.
Dr. Keller is founder of the Keller Research Institute, Jacksonville, Fla. He reported serving as a research scientist for the American Urological Association, serving on the advisory board of Active Surveillance Patient’s International, and serving on the boards of numerous nonprofit organizations.
A version of this article first appeared on Medscape.com.
This patient has a strong likelihood of aggressive prostate cancer, right? If that same patient also presents with severe, burning bone pain with no precipitating trauma to the area and rest and over-the-counter painkillers are not helping, you’d think, “check for metastases,” right?
That patient was me in late January 2023.
As a research scientist member of the American Urological Association, I knew enough to know I had to consult my urologist ASAP.
With the above symptoms, I’ll admit I was scared. Fortunately, if that’s the right word, I was no stranger to a rapid, dramatic spike in PSA. In 2021 I was temporarily living in a new city, and I wanted to form a relationship with a good local urologist. The urologist that I was referred to gave me a thorough consultation, including a vigorous digital rectal exam (DRE) and sent me across the street for a blood draw.
To my shock, my PSA had spiked over 2 points, to 9.9 from 7.8 a few months earlier. I freaked. Had my 3-cm tumor burst out into an aggressive cancer? Research on PubMed provided an array of studies showing what could cause PSA to suddenly rise, including a DRE performed 72 hours before the blood draw.1 A week later, my PSA was back down to its normal 7.6.
But in January 2023, I had none of those previously reported experiences that could suddenly trigger a spike in PSA, like a DRE or riding on a thin bicycle seat for a few hours before the lab visit.
The COVID effect
I went back to PubMed and found a new circumstance that could cause a surge in PSA: COVID-19. A recent study2 of 91 men with benign prostatic hypertrophy by researchers in Turkey found that PSA spiked from 0 to 5 points during the COVID infection period and up to 2 points higher 3 months after the infection had cleared. I had tested positive for COVID-19 in mid-December 2022, 4 weeks before my 9.9 PSA reading.
Using Google translate, I communicated with the team in Turkey and found out that the PSA spike can last up to 6 months.
That study helps explain why my PSA dropped over 1.5 points to 8.5 just 2 weeks after the 9.9 reading, with the expectation that it would return to its previous normal of 7.8 within 6 months of infection with SARS-CoV-2. To be safe, my urologist scheduled another PSA test in May, along with an updated multiparametric MRI, which may be followed by an in-bore MRI-guided biopsy of the 3-cm tumor if the mass has enlarged.
COVID-19 pain
What about my burning bone pain in my upper right humerus and right rotator cuff that was not precipitated by trauma or strain? A radiograph found no evidence of metastasis, thank goodness. And my research showed that several studies3 have found that COVID-19 can cause burning musculoskeletal pain, including enthesopathy, which is what I had per the radiology report. So my PSA spike and searing pain were likely consequences of the infection.
To avoid the risk for a gross misdiagnosis after a radical spike in PSA, the informed urologist should ask the patient if he has had COVID-19 in the previous 6 months. Overlooking that question could lead to the wrong diagnostic decisions about a rapid jump in PSA or unexplained bone pain.
References
1. Bossens MM et al. Eur J Cancer. 1995;31A:682-5.
2. Cinislioglu AE et al. Urology. 2022;159:16-21.
3. Ciaffi J et al. Joint Bone Spine. 2021;88:105158.
Dr. Keller is founder of the Keller Research Institute, Jacksonville, Fla. He reported serving as a research scientist for the American Urological Association, serving on the advisory board of Active Surveillance Patient’s International, and serving on the boards of numerous nonprofit organizations.
A version of this article first appeared on Medscape.com.
Like mother, like daughter? Moms pass obesity risk to girls
Girls between 4 and 9 years old were more likely to have high fat mass and a high body mass index if their mothers had excess adiposity – but this relationship was not seen between mothers and sons, or between fathers and sons or daughters, in a new study.
The researchers measured fat mass, lean mass, and BMI in the sons and daughters when they were age 4 (before a phenomenon known as “adiposity rebound”), ages 6-7 (around the adiposity rebound), and ages 8-9 (before or at the onset of puberty).
They also obtained measurements from the mothers and fathers when the offspring were ages 8-9.
The group found “a strong association between the fat mass of mothers and their daughters but not their sons,” Rebecca J. Moon, BM, PhD, and colleagues report.
“It would be important to establish persistence through puberty,” according to the researchers, “but nonetheless, these findings are clinically important, highlighting girls who are born to mothers with high BMI and excess adiposity are at high risk of themselves of becoming overweight/obese or having unfavorable body composition early in childhood.”
The mother-daughter relationship for fat mass appears to be established by age 4 years, note Dr. Moon, of the MRC Lifecourse Epidemiology Centre, University of Southampton (England), and colleagues.
Therefore, “early awareness and intervention is needed in mothers with excess adiposity, and potentially beginning even in the periconception and in utero period.”
Because 97% of the mothers and fathers were White, the findings may not be generalizable to other populations, they caution.
The results, from the Southampton Women’s Survey prospective cohort study, were published online in the Journal of Clinical Endocrinology & Metabolism.
One of the first studies to look at fat mass, not just BMI
Children with overweight or obesity are more likely to have excess weight in adulthood that puts them at risk of developing type 2 diabetes, cardiovascular disease, cancer, and osteoarthritis. Previous research has reported that children with overweight or obesity were more likely to have mothers with adiposity.
However, most prior studies have looked at BMI alone and did not measure fat mass, and it was not known how a father’s obesity might affect offspring or how risk may differ in boy versus girl children.
Researchers analyzed data from a subset of participants in the Southampton Women’s Survey of 3,158 women who were aged 20-34 in 1998-2002 and delivered a liveborn infant.
The current study included 240 mother-father-offspring trios who had data for BMI and dual-energy X-ray absorptiometry (DXA) scans (whole body less head).
Mothers were a mean age of 31 years at delivery and had a median pre-pregnancy BMI of 23.7 kg/m2.
The offspring were 129 boys (54%) and 111 girls.
The offspring had DXA scans at ages 4, 6-7, and 8-9 years, and the mothers and fathers had a DXA scan at the last time point.
At ages 6-7 and ages 8-9, BMI and fat mass of the girls reflected that of their mothers (a significant association).
At age 4, BMI and fat mass of the daughters tended to be associated with that of their mothers, but the 95% confidence interval crossed zero.
There were no significant mother-son, father-son, or father-daughter associations for BMI or fat mass at each of the three studied ages.
The study received funding from the Medical Research Council, the British Heart Foundation, the National Institute for Health and Care Research Southampton Biomedical Research Centre, the NIHR Oxford Biomedical Research Centre, the Seventh Framework Program, the Biotechnology and Biological Sciences Research Council, the Horizon 2020 Framework Program, and the National Institute on Aging. Dr. Moon has reported receiving travel bursaries from Kyowa Kirin unrelated to the current study. Disclosures for the other authors are listed with the article.
A version of this article originally appeared on Medscape.com.
Girls between 4 and 9 years old were more likely to have high fat mass and a high body mass index if their mothers had excess adiposity – but this relationship was not seen between mothers and sons, or between fathers and sons or daughters, in a new study.
The researchers measured fat mass, lean mass, and BMI in the sons and daughters when they were age 4 (before a phenomenon known as “adiposity rebound”), ages 6-7 (around the adiposity rebound), and ages 8-9 (before or at the onset of puberty).
They also obtained measurements from the mothers and fathers when the offspring were ages 8-9.
The group found “a strong association between the fat mass of mothers and their daughters but not their sons,” Rebecca J. Moon, BM, PhD, and colleagues report.
“It would be important to establish persistence through puberty,” according to the researchers, “but nonetheless, these findings are clinically important, highlighting girls who are born to mothers with high BMI and excess adiposity are at high risk of themselves of becoming overweight/obese or having unfavorable body composition early in childhood.”
The mother-daughter relationship for fat mass appears to be established by age 4 years, note Dr. Moon, of the MRC Lifecourse Epidemiology Centre, University of Southampton (England), and colleagues.
Therefore, “early awareness and intervention is needed in mothers with excess adiposity, and potentially beginning even in the periconception and in utero period.”
Because 97% of the mothers and fathers were White, the findings may not be generalizable to other populations, they caution.
The results, from the Southampton Women’s Survey prospective cohort study, were published online in the Journal of Clinical Endocrinology & Metabolism.
One of the first studies to look at fat mass, not just BMI
Children with overweight or obesity are more likely to have excess weight in adulthood that puts them at risk of developing type 2 diabetes, cardiovascular disease, cancer, and osteoarthritis. Previous research has reported that children with overweight or obesity were more likely to have mothers with adiposity.
However, most prior studies have looked at BMI alone and did not measure fat mass, and it was not known how a father’s obesity might affect offspring or how risk may differ in boy versus girl children.
Researchers analyzed data from a subset of participants in the Southampton Women’s Survey of 3,158 women who were aged 20-34 in 1998-2002 and delivered a liveborn infant.
The current study included 240 mother-father-offspring trios who had data for BMI and dual-energy X-ray absorptiometry (DXA) scans (whole body less head).
Mothers were a mean age of 31 years at delivery and had a median pre-pregnancy BMI of 23.7 kg/m2.
The offspring were 129 boys (54%) and 111 girls.
The offspring had DXA scans at ages 4, 6-7, and 8-9 years, and the mothers and fathers had a DXA scan at the last time point.
At ages 6-7 and ages 8-9, BMI and fat mass of the girls reflected that of their mothers (a significant association).
At age 4, BMI and fat mass of the daughters tended to be associated with that of their mothers, but the 95% confidence interval crossed zero.
There were no significant mother-son, father-son, or father-daughter associations for BMI or fat mass at each of the three studied ages.
The study received funding from the Medical Research Council, the British Heart Foundation, the National Institute for Health and Care Research Southampton Biomedical Research Centre, the NIHR Oxford Biomedical Research Centre, the Seventh Framework Program, the Biotechnology and Biological Sciences Research Council, the Horizon 2020 Framework Program, and the National Institute on Aging. Dr. Moon has reported receiving travel bursaries from Kyowa Kirin unrelated to the current study. Disclosures for the other authors are listed with the article.
A version of this article originally appeared on Medscape.com.
Girls between 4 and 9 years old were more likely to have high fat mass and a high body mass index if their mothers had excess adiposity – but this relationship was not seen between mothers and sons, or between fathers and sons or daughters, in a new study.
The researchers measured fat mass, lean mass, and BMI in the sons and daughters when they were age 4 (before a phenomenon known as “adiposity rebound”), ages 6-7 (around the adiposity rebound), and ages 8-9 (before or at the onset of puberty).
They also obtained measurements from the mothers and fathers when the offspring were ages 8-9.
The group found “a strong association between the fat mass of mothers and their daughters but not their sons,” Rebecca J. Moon, BM, PhD, and colleagues report.
“It would be important to establish persistence through puberty,” according to the researchers, “but nonetheless, these findings are clinically important, highlighting girls who are born to mothers with high BMI and excess adiposity are at high risk of themselves of becoming overweight/obese or having unfavorable body composition early in childhood.”
The mother-daughter relationship for fat mass appears to be established by age 4 years, note Dr. Moon, of the MRC Lifecourse Epidemiology Centre, University of Southampton (England), and colleagues.
Therefore, “early awareness and intervention is needed in mothers with excess adiposity, and potentially beginning even in the periconception and in utero period.”
Because 97% of the mothers and fathers were White, the findings may not be generalizable to other populations, they caution.
The results, from the Southampton Women’s Survey prospective cohort study, were published online in the Journal of Clinical Endocrinology & Metabolism.
One of the first studies to look at fat mass, not just BMI
Children with overweight or obesity are more likely to have excess weight in adulthood that puts them at risk of developing type 2 diabetes, cardiovascular disease, cancer, and osteoarthritis. Previous research has reported that children with overweight or obesity were more likely to have mothers with adiposity.
However, most prior studies have looked at BMI alone and did not measure fat mass, and it was not known how a father’s obesity might affect offspring or how risk may differ in boy versus girl children.
Researchers analyzed data from a subset of participants in the Southampton Women’s Survey of 3,158 women who were aged 20-34 in 1998-2002 and delivered a liveborn infant.
The current study included 240 mother-father-offspring trios who had data for BMI and dual-energy X-ray absorptiometry (DXA) scans (whole body less head).
Mothers were a mean age of 31 years at delivery and had a median pre-pregnancy BMI of 23.7 kg/m2.
The offspring were 129 boys (54%) and 111 girls.
The offspring had DXA scans at ages 4, 6-7, and 8-9 years, and the mothers and fathers had a DXA scan at the last time point.
At ages 6-7 and ages 8-9, BMI and fat mass of the girls reflected that of their mothers (a significant association).
At age 4, BMI and fat mass of the daughters tended to be associated with that of their mothers, but the 95% confidence interval crossed zero.
There were no significant mother-son, father-son, or father-daughter associations for BMI or fat mass at each of the three studied ages.
The study received funding from the Medical Research Council, the British Heart Foundation, the National Institute for Health and Care Research Southampton Biomedical Research Centre, the NIHR Oxford Biomedical Research Centre, the Seventh Framework Program, the Biotechnology and Biological Sciences Research Council, the Horizon 2020 Framework Program, and the National Institute on Aging. Dr. Moon has reported receiving travel bursaries from Kyowa Kirin unrelated to the current study. Disclosures for the other authors are listed with the article.
A version of this article originally appeared on Medscape.com.
SGS 2023 Meeting: Daily Reporting from Tucson
Wednesday, March 22. Day 4 of SGS.
Day 4, and the final day of the 49th SGS conference started with a sunrise run up and down the hills surrounding the JW Marriott Starr Resort. After breakfast, I entered the Tucson Ballroom to attend the last 2 scientific sessions of the conference.

Highlights from the first session included a look at postoperative outcomes and complication rates between gynecologic surgeons and general surgeons using the National Surgical Quality Improvement Program (NSQIP) database by Dr. Douglas Luchristt, who showed no difference between the 2 surgical specialties (and even better outcomes by gynecologists in certain operative measures), as well as the work of Dr. Christopher Hong who used 2 separate surgical databases (NSQIP and Michigan Surgical Quality Collaborative) to show that rates of vaginal hysterectomy have been decreasing from 2017 to 2020, even amongst patients who are likely good candidates for a vaginal route of hysterectomy. Dr. Jocelyn Fitzgerald presented her unique mixed methods research on how to better design the gynecologic office to improve the patient experience, using 3,000 Twitter responses to a question on this topic. Lastly, Dr. Emily Aldrich shared her work on better understanding the patient perception of same day discharge after major vaginal reconstructive surgery. An interesting finding of Dr. Aldrich’s study was that the most common response to her question about the “worst part of the surgical experience” was going home with a postoperative catheter, which surgeons often consider a small and temporary discomfort. The first session ended with the passing of the gavel from current SGS president Dr. Cheryl Iglesia, to the incoming SGS president Dr. Rosanne Kho, with much applause and excitement for what Dr. Kho will bring to the table in her new role.
The research presented at the final scientific session of the conference did not disappoint. A retrospective study on the influence of body mass index (BMI) on the time to surgical diagnosis of endometriosis by Dr. Melissa Markowitz found that obesity was associated with a delay of over 1 year in surgical diagnosis of endometriosis compared with normal and underweight patients. Dr. David (Ike) Rahn presented additional findings on his randomized, double-blinded, multicenter trial on perioperative use of vaginal estrogen cream in postmenopausal patients with prolapse. He found that 5 weeks of estrogen cream use was not associated with any improvement in urinary incontinence or sexual function.
Dr. Stephanie Glass Clark used the Premier Healthcare Database to show that that there was no difference in postoperative mesh exposure in patients who underwent a total hysterectomy compared with supracervical hysterectomy at the time of sacrocolpopexy. Dr. Kavita Mishra presented results from the FLOWER trial, which found no difference in postoperative outcomes for transgender women undergoing vaginoplasty for gender affirmation who did and did not undergo preoperative pelvic floor physical therapy. Finally, Dr. Carly Crowder shared her video of anatomy for sacral neuromodulation with some excellent cadaveric dissections to exhibit the peri-sacral and gluteal anatomy.

As the conference ended, raindrops pounded the sandy grounds of the resort as I waited in the lobby for my Uber to the airport. Dr. Rosanne Kho happened to walk by and stopped to speak with me and one of my attendings. She smiled as she asked about our experience at the conference and to wish us safe travels. To me, this moment embodies the spirit of mentorship and connection that is so unique to the SGS conference. I feel incredibly lucky to have met some of the physician leaders of our field, who genuinely want to get to know and help the next generation. This year’s meeting was attended by ObGyn generalists and surgeons of all gynecologic subspecialties and certainly met its goal in addressing topics with an “Impact Factor.” I am inspired by all the work that is happening across the country to move the needle and better our field. This was my first SGS experience, but it certainly won’t be the last. I hope you too will consider attending in the future!
Tuesday, March 21. Day 3 of SGS.
It’s Day 3 of the SGS conference! In addition to the academic roundtables, conference attendees had the option of doing early-morning yoga with Dr. Mireille Truong. Yoga sounded nice, but I spent the morning in bed, catching up on sleep. (My own version of wellness!) The scientific sessions of the day started at 7:30 am, and I especially want to highlight the work of Dr. Amy Askew who performed a randomized controlled trial comparing patient removal of urinary catheters placed for postoperative urinary retention to office removal. She found that patient urinary catheter removal was a feasible and safe option with excellent patient satisfaction and a reduction of in-person postoperative office visits. At the end of the session, Dr. Cheryl Iglesia gave her presidential address, where she shared her journey to becoming the physician, educator, researcher, and leader she is today. She emphasized the importance of being a continual learner and to give back by mentoring and educating the next generation. “Learn it, earn it, and return it.”

This was followed by the Te Linde lecture, given by Dr. Pamela Moalli. An exceptional surgeon-scientist, Dr. Moalli shared about her work on the impact of mesh on tissue, as well as alternative biologic options being developed, such as 3D printed membranes, extracellular matrix scaffolds, and living tissue grafts to create new ligamentous supports for the vagina. She discussed novel research using stem cell transplantation to harness the power of regeneration in the urethra or vagina following injury. I think it is safe to say that the entire room was in awe of the work she has done, and what she continues to do to find better therapeutic options for girls and women with pelvic floor disorders. Her talk ended in a standing ovation. Afterwards, all the University of Pittsburgh Medical Center-Magee Womens Hospital trainees, faculty, and several alumni took a picture with Dr. Moalli (fifth from the right in the picture).
Lunch followed, which included a brief walk around the industry exhibition hall. I then returned back to the Tucson Ballroom to listen in on the next scientific session on surgical ergonomics. Organized by Dr. Amy Park who herself suffered from work-related musculoskeletal injuries, the session was composed of an excellent video by Dr. Abby Stork on stretches to prevent and reduce the risk of surgeon-associated musculoskeletal injuries, especially in vaginal surgeons. There was then a panel of 3 experts, Dr. Noor Abu-Alnadi, Dr. Ladin Yurteri-Kaplan, and Dr. Susan Hallbeck (PhD ergonomics expert), moderated by Dr. Amanda Fader and Dr. Kimberly Kho. In particular, Dr. Hallbeck developed a timer app as a reminder for surgeons to stop every 40 minutes to stretch for 1.5 minutes (orstretch.mayoclinic.org). This has been studied and found to reduce musculoskeletal pain after surgery and improve physical performance without increasing total operating time. If you would like to see some of these between- and in-OR stretches yourself, an informative handout can be accessed at mcforms.mayo.edu.
Tuesday afternoon was left open. I joined Dr. Veronica Lerner, Dr. Kelly Wright, and Dr. Louise Perkins King on a 7.5-mile hike into the surrounding desert hills. We marveled at the many Saguaro cacti, some over 100 years old and towering many feet high, as well as the beautiful yellow, purple, and magenta flowers that were scattered among the desert brush. Several rabbits and deer wandered by us during our hike. On one of the trails, the stone skeleton of an old house stood, once a home to the Bowen family who had moved to Arizona for health reasons. I could see why they would want to move here—I felt such a peace looking at the gorgeous view from what was once their doorway.

After a shower and a lot of stretching, I got ready for the evening event, A Taste and Toast with SGS: Under the Arizona Skies. The food and drink were delicious, and I got to spend the evening catching up with a good friend. We watched as conference attendees assigned to different color teams (red, green, blue, and yellow), fought for the hallowed Golden Uterus Trophy in several competitive gynecology-themed games (eg, throwing sacral neuromodulation needle “darts” at balloons and removing small pom poms from a water bottle with a disposable operative hysteroscope). As the evening progressed, the DJ turned up the music and people made their way to the dance floor. The event served as a fundraiser for the SGS Pelvic Anatomy Group and successfully raised $35,000.
Monday, March 20. Day 2 of SGS.
Day 2 of the SGS meeting started off with a gentle sunrise over the cacti-covered hills surrounding the JW Marriot Starr Pass Hotel, the venue for the 49th SGS annual scientific meeting. The first official event of the day after some engaging academic round tables was the recognition of the new SGS members. Much celebration was had over the 18 gynecologic surgeons who were inducted.

The second day included the first 3 scientific sessions of the conference. Some highlights include the work of Dr. Shawn Menefee on a randomized trial of sacral colpopexy, transvaginal mesh, and native tissue apical repair for posthysterectomy vault prolapse; a video by Dr. Matthew Fallon on a robotic-assisted laparoscopic approach to repairing a chronic uterine inversion; and the impact of age on regret following hysterectomy by Dr. Nathan King. Dr. Candace Parker-Autry also presented her work on the impact of perineorrhaphy on both female and male sexual function, and Dr. Cassie (Clarissa) Niino spoke elegantly on the “red bag problem” that exists in all of our operating rooms, which increases pollution and cost unnecessarily.
There were also several excellent talks given. Dr. Jason Wright spoke about the importance of surgical volume on gynecologic surgery. In particular, he noted that surgical volume needs to be considered not only at the surgeon level but also at the hospital level. Higher-volume hospitals will provide better care, in the same way that general, high-volume surgeons have less complications and better long-term outcomes. Of note, volume is not the whole picture. We need to also consider measurements of surgery and hospital quality and surgeon skill in addition to volume, as Dr. Shawn Menefee insightfully commented.
Dr. Beri Ridgeway gave the Mark D. Walters Lecture about surgeons in the c-suite and the importance of having a seat at the leadership table as surgeons and medical providers. In her words: “If we aren’t at the table, then we are on the menu.” Overworked and underpaid, burned out doctors feel powerless because they are managed by leaders with a business and not a medical background, and we need to have physicians in leadership who understand how medicine is practiced and to ensure equitable care
Dr. Kelly Wright gave a talk on the environmental impact of gynecologic care—from OR to clinic. She gave examples of how metal, reusable speculums become more cost-effective and produce less waste after only 2-3 uses and how there is no evidence that bouffants reduce surgical site infections (and a reusable scrub cap could work just as well without creating waste). Finally, Dr. Ebony Carter gave an impassioned talk on the need for equity in publication and grant funding in our field. She shared about her initiative through the Green Journal (Obstetrics and Gynecology) to create an issue focused on furthering equity and dispelling racism in medical research.
Later in the afternoon, I attended the Fellows’ Pelvic Research Network (FPRN) meeting, which includes AUGS-SGS (urogynecology fellows) and FMIGS-SGS (fellows of all other gynecologic subspecialities, including minimally invasive gynecologic surgery, family planning, reproductive endocrinology and infertility, and pediatric and adolescent gynecology). Dr. John Gebhart gave an excellent lecture with some impressive photos and videos on how to manage mesh exposure and erosion.
Afterwards, updates were given on the current FPRN projects, and 4 new projects were proposed and underwent audience feedback for improvement. It was exciting to see the multicenter collaborations fostered through the FPRN, and I look forward to seeing which projects will get funded for this upcoming year!

The evening ended with the President's Award Ceremony led by Dr. Cheryl Iglesia, the 49th SGS President, as well as the President's Reception. I also wantd to highlight the winner of the Distinguished Surgeon Award: Dr. Dee Fenner. The remaining awardees are listed on the SGS website (https://sgsonline.org).
Sunday, March 19. Day 1 of SGS.
Last night around midnight, bleary-eyed from the long flight from Pittsburgh, I walked out into the dimly lit, mild air of Tucson, Arizona. The Saguaro cacti that lined the entrance to the airport stood tall and tree-like, with welcoming green arms. It was as if they too knew that the next 4 days would be filled with the building of new relationships and the strengthening of old ones, as well as with education, innovation, and the sharing of research. That spirit of collegiality, approachability, and connection in an intimate and vibrant meeting is what the Society of Gynecologic Surgeons (SGS) meeting has been known for and why it draws people to come back, year after year.
The first day of the conference was fantastic. As a first-time attendant at SGS, I was excited to have the opportunity to meet and rub elbows with mentors and role models from across the country. My day started off with the SGS Fellows and Young Attendings Course, moderated by 3 incredible faculty: Dr. Matthew Barker, Dr. Sadikah Behbehani, and Dr. Traci Ito. Some high-yield topics such as contract negotiation, developing a urogynecology- or MIGS-based practice, billing, academic promotion, and taking advantage of relationships with industry were discussed at length, and the session ended with a roundtable, where the experts had time to answer questions in smaller groups. One of the quotes that will ring true for many fellows about to embark on the job search was from Dr. Amanda Ecker: “Up until now, you were told where to go and what your schedule is. This is the first time you have flexibility and power to decide for yourself.” Therefore, it is important to reflect on what you really desire and/or prioritize in a job, whether it is location, compensation, protected time, or opportunities for advancement.

In the afternoon, I attended a postgraduate course led by Dr. Veronica Lerner and Dr. Mireille Truong called “The Sim Factor: Making an Impact in Surgical Education.” Several other excellent postgraduate courses were available, including “Advanced Endometriosis Surgery and Pelvic Pain Patient-Centered Approach,” “Social Media Workshop- #Gynfluencing: Using Social Media to Find Your Digital Voice,” and “Urologic Surgery for the Gynecologic Surgeon: GU Injury, Ureteral Stents, Complex Fistula and More.” I was grateful for the hands-on and tangible tools that Drs. Lerner and Truong left the group with—including ideas such as Zoom-based virtual coaching for trainees learning fundamentals of laparoscopic surgery types of tasks, table-top simulation for high-stakes scenarios (eg, operative vascular injury), and the importance of grounding educational activity in objectives and evaluation. I even got to make and take home my own myomectomy model. (Fun fact: The myoma is actually a stress ball wrapped in an Ace bandage and then Glad Press n’ Seal!

The late afternoon transitioned to an opportunity for trainees to interact with senior SGS members and a welcome reception. The indoor and outdoor spaces were filled with laughing and talking as people connected over drinks and snacks. Finally, the evening ended with a session presented by the SGS Equity Council, “What your Patients REALLY Want to Know.” Patty Brisben, of the Patty Brisben Foundation and founder of the company Pure Romance, was interviewed by Dr. Christine Vaccaro. It was heartwarming to hear how Patty took the stories of women suffering from sexual pain and dissatisfaction and chose to make it her life’s mission to improve women’s sexual health.
Wednesday, March 22. Day 4 of SGS.
Day 4, and the final day of the 49th SGS conference started with a sunrise run up and down the hills surrounding the JW Marriott Starr Resort. After breakfast, I entered the Tucson Ballroom to attend the last 2 scientific sessions of the conference.

Highlights from the first session included a look at postoperative outcomes and complication rates between gynecologic surgeons and general surgeons using the National Surgical Quality Improvement Program (NSQIP) database by Dr. Douglas Luchristt, who showed no difference between the 2 surgical specialties (and even better outcomes by gynecologists in certain operative measures), as well as the work of Dr. Christopher Hong who used 2 separate surgical databases (NSQIP and Michigan Surgical Quality Collaborative) to show that rates of vaginal hysterectomy have been decreasing from 2017 to 2020, even amongst patients who are likely good candidates for a vaginal route of hysterectomy. Dr. Jocelyn Fitzgerald presented her unique mixed methods research on how to better design the gynecologic office to improve the patient experience, using 3,000 Twitter responses to a question on this topic. Lastly, Dr. Emily Aldrich shared her work on better understanding the patient perception of same day discharge after major vaginal reconstructive surgery. An interesting finding of Dr. Aldrich’s study was that the most common response to her question about the “worst part of the surgical experience” was going home with a postoperative catheter, which surgeons often consider a small and temporary discomfort. The first session ended with the passing of the gavel from current SGS president Dr. Cheryl Iglesia, to the incoming SGS president Dr. Rosanne Kho, with much applause and excitement for what Dr. Kho will bring to the table in her new role.
The research presented at the final scientific session of the conference did not disappoint. A retrospective study on the influence of body mass index (BMI) on the time to surgical diagnosis of endometriosis by Dr. Melissa Markowitz found that obesity was associated with a delay of over 1 year in surgical diagnosis of endometriosis compared with normal and underweight patients. Dr. David (Ike) Rahn presented additional findings on his randomized, double-blinded, multicenter trial on perioperative use of vaginal estrogen cream in postmenopausal patients with prolapse. He found that 5 weeks of estrogen cream use was not associated with any improvement in urinary incontinence or sexual function.
Dr. Stephanie Glass Clark used the Premier Healthcare Database to show that that there was no difference in postoperative mesh exposure in patients who underwent a total hysterectomy compared with supracervical hysterectomy at the time of sacrocolpopexy. Dr. Kavita Mishra presented results from the FLOWER trial, which found no difference in postoperative outcomes for transgender women undergoing vaginoplasty for gender affirmation who did and did not undergo preoperative pelvic floor physical therapy. Finally, Dr. Carly Crowder shared her video of anatomy for sacral neuromodulation with some excellent cadaveric dissections to exhibit the peri-sacral and gluteal anatomy.

As the conference ended, raindrops pounded the sandy grounds of the resort as I waited in the lobby for my Uber to the airport. Dr. Rosanne Kho happened to walk by and stopped to speak with me and one of my attendings. She smiled as she asked about our experience at the conference and to wish us safe travels. To me, this moment embodies the spirit of mentorship and connection that is so unique to the SGS conference. I feel incredibly lucky to have met some of the physician leaders of our field, who genuinely want to get to know and help the next generation. This year’s meeting was attended by ObGyn generalists and surgeons of all gynecologic subspecialties and certainly met its goal in addressing topics with an “Impact Factor.” I am inspired by all the work that is happening across the country to move the needle and better our field. This was my first SGS experience, but it certainly won’t be the last. I hope you too will consider attending in the future!
Tuesday, March 21. Day 3 of SGS.
It’s Day 3 of the SGS conference! In addition to the academic roundtables, conference attendees had the option of doing early-morning yoga with Dr. Mireille Truong. Yoga sounded nice, but I spent the morning in bed, catching up on sleep. (My own version of wellness!) The scientific sessions of the day started at 7:30 am, and I especially want to highlight the work of Dr. Amy Askew who performed a randomized controlled trial comparing patient removal of urinary catheters placed for postoperative urinary retention to office removal. She found that patient urinary catheter removal was a feasible and safe option with excellent patient satisfaction and a reduction of in-person postoperative office visits. At the end of the session, Dr. Cheryl Iglesia gave her presidential address, where she shared her journey to becoming the physician, educator, researcher, and leader she is today. She emphasized the importance of being a continual learner and to give back by mentoring and educating the next generation. “Learn it, earn it, and return it.”

This was followed by the Te Linde lecture, given by Dr. Pamela Moalli. An exceptional surgeon-scientist, Dr. Moalli shared about her work on the impact of mesh on tissue, as well as alternative biologic options being developed, such as 3D printed membranes, extracellular matrix scaffolds, and living tissue grafts to create new ligamentous supports for the vagina. She discussed novel research using stem cell transplantation to harness the power of regeneration in the urethra or vagina following injury. I think it is safe to say that the entire room was in awe of the work she has done, and what she continues to do to find better therapeutic options for girls and women with pelvic floor disorders. Her talk ended in a standing ovation. Afterwards, all the University of Pittsburgh Medical Center-Magee Womens Hospital trainees, faculty, and several alumni took a picture with Dr. Moalli (fifth from the right in the picture).
Lunch followed, which included a brief walk around the industry exhibition hall. I then returned back to the Tucson Ballroom to listen in on the next scientific session on surgical ergonomics. Organized by Dr. Amy Park who herself suffered from work-related musculoskeletal injuries, the session was composed of an excellent video by Dr. Abby Stork on stretches to prevent and reduce the risk of surgeon-associated musculoskeletal injuries, especially in vaginal surgeons. There was then a panel of 3 experts, Dr. Noor Abu-Alnadi, Dr. Ladin Yurteri-Kaplan, and Dr. Susan Hallbeck (PhD ergonomics expert), moderated by Dr. Amanda Fader and Dr. Kimberly Kho. In particular, Dr. Hallbeck developed a timer app as a reminder for surgeons to stop every 40 minutes to stretch for 1.5 minutes (orstretch.mayoclinic.org). This has been studied and found to reduce musculoskeletal pain after surgery and improve physical performance without increasing total operating time. If you would like to see some of these between- and in-OR stretches yourself, an informative handout can be accessed at mcforms.mayo.edu.
Tuesday afternoon was left open. I joined Dr. Veronica Lerner, Dr. Kelly Wright, and Dr. Louise Perkins King on a 7.5-mile hike into the surrounding desert hills. We marveled at the many Saguaro cacti, some over 100 years old and towering many feet high, as well as the beautiful yellow, purple, and magenta flowers that were scattered among the desert brush. Several rabbits and deer wandered by us during our hike. On one of the trails, the stone skeleton of an old house stood, once a home to the Bowen family who had moved to Arizona for health reasons. I could see why they would want to move here—I felt such a peace looking at the gorgeous view from what was once their doorway.

After a shower and a lot of stretching, I got ready for the evening event, A Taste and Toast with SGS: Under the Arizona Skies. The food and drink were delicious, and I got to spend the evening catching up with a good friend. We watched as conference attendees assigned to different color teams (red, green, blue, and yellow), fought for the hallowed Golden Uterus Trophy in several competitive gynecology-themed games (eg, throwing sacral neuromodulation needle “darts” at balloons and removing small pom poms from a water bottle with a disposable operative hysteroscope). As the evening progressed, the DJ turned up the music and people made their way to the dance floor. The event served as a fundraiser for the SGS Pelvic Anatomy Group and successfully raised $35,000.
Monday, March 20. Day 2 of SGS.
Day 2 of the SGS meeting started off with a gentle sunrise over the cacti-covered hills surrounding the JW Marriot Starr Pass Hotel, the venue for the 49th SGS annual scientific meeting. The first official event of the day after some engaging academic round tables was the recognition of the new SGS members. Much celebration was had over the 18 gynecologic surgeons who were inducted.

The second day included the first 3 scientific sessions of the conference. Some highlights include the work of Dr. Shawn Menefee on a randomized trial of sacral colpopexy, transvaginal mesh, and native tissue apical repair for posthysterectomy vault prolapse; a video by Dr. Matthew Fallon on a robotic-assisted laparoscopic approach to repairing a chronic uterine inversion; and the impact of age on regret following hysterectomy by Dr. Nathan King. Dr. Candace Parker-Autry also presented her work on the impact of perineorrhaphy on both female and male sexual function, and Dr. Cassie (Clarissa) Niino spoke elegantly on the “red bag problem” that exists in all of our operating rooms, which increases pollution and cost unnecessarily.
There were also several excellent talks given. Dr. Jason Wright spoke about the importance of surgical volume on gynecologic surgery. In particular, he noted that surgical volume needs to be considered not only at the surgeon level but also at the hospital level. Higher-volume hospitals will provide better care, in the same way that general, high-volume surgeons have less complications and better long-term outcomes. Of note, volume is not the whole picture. We need to also consider measurements of surgery and hospital quality and surgeon skill in addition to volume, as Dr. Shawn Menefee insightfully commented.
Dr. Beri Ridgeway gave the Mark D. Walters Lecture about surgeons in the c-suite and the importance of having a seat at the leadership table as surgeons and medical providers. In her words: “If we aren’t at the table, then we are on the menu.” Overworked and underpaid, burned out doctors feel powerless because they are managed by leaders with a business and not a medical background, and we need to have physicians in leadership who understand how medicine is practiced and to ensure equitable care
Dr. Kelly Wright gave a talk on the environmental impact of gynecologic care—from OR to clinic. She gave examples of how metal, reusable speculums become more cost-effective and produce less waste after only 2-3 uses and how there is no evidence that bouffants reduce surgical site infections (and a reusable scrub cap could work just as well without creating waste). Finally, Dr. Ebony Carter gave an impassioned talk on the need for equity in publication and grant funding in our field. She shared about her initiative through the Green Journal (Obstetrics and Gynecology) to create an issue focused on furthering equity and dispelling racism in medical research.
Later in the afternoon, I attended the Fellows’ Pelvic Research Network (FPRN) meeting, which includes AUGS-SGS (urogynecology fellows) and FMIGS-SGS (fellows of all other gynecologic subspecialities, including minimally invasive gynecologic surgery, family planning, reproductive endocrinology and infertility, and pediatric and adolescent gynecology). Dr. John Gebhart gave an excellent lecture with some impressive photos and videos on how to manage mesh exposure and erosion.
Afterwards, updates were given on the current FPRN projects, and 4 new projects were proposed and underwent audience feedback for improvement. It was exciting to see the multicenter collaborations fostered through the FPRN, and I look forward to seeing which projects will get funded for this upcoming year!

The evening ended with the President's Award Ceremony led by Dr. Cheryl Iglesia, the 49th SGS President, as well as the President's Reception. I also wantd to highlight the winner of the Distinguished Surgeon Award: Dr. Dee Fenner. The remaining awardees are listed on the SGS website (https://sgsonline.org).
Sunday, March 19. Day 1 of SGS.
Last night around midnight, bleary-eyed from the long flight from Pittsburgh, I walked out into the dimly lit, mild air of Tucson, Arizona. The Saguaro cacti that lined the entrance to the airport stood tall and tree-like, with welcoming green arms. It was as if they too knew that the next 4 days would be filled with the building of new relationships and the strengthening of old ones, as well as with education, innovation, and the sharing of research. That spirit of collegiality, approachability, and connection in an intimate and vibrant meeting is what the Society of Gynecologic Surgeons (SGS) meeting has been known for and why it draws people to come back, year after year.
The first day of the conference was fantastic. As a first-time attendant at SGS, I was excited to have the opportunity to meet and rub elbows with mentors and role models from across the country. My day started off with the SGS Fellows and Young Attendings Course, moderated by 3 incredible faculty: Dr. Matthew Barker, Dr. Sadikah Behbehani, and Dr. Traci Ito. Some high-yield topics such as contract negotiation, developing a urogynecology- or MIGS-based practice, billing, academic promotion, and taking advantage of relationships with industry were discussed at length, and the session ended with a roundtable, where the experts had time to answer questions in smaller groups. One of the quotes that will ring true for many fellows about to embark on the job search was from Dr. Amanda Ecker: “Up until now, you were told where to go and what your schedule is. This is the first time you have flexibility and power to decide for yourself.” Therefore, it is important to reflect on what you really desire and/or prioritize in a job, whether it is location, compensation, protected time, or opportunities for advancement.

In the afternoon, I attended a postgraduate course led by Dr. Veronica Lerner and Dr. Mireille Truong called “The Sim Factor: Making an Impact in Surgical Education.” Several other excellent postgraduate courses were available, including “Advanced Endometriosis Surgery and Pelvic Pain Patient-Centered Approach,” “Social Media Workshop- #Gynfluencing: Using Social Media to Find Your Digital Voice,” and “Urologic Surgery for the Gynecologic Surgeon: GU Injury, Ureteral Stents, Complex Fistula and More.” I was grateful for the hands-on and tangible tools that Drs. Lerner and Truong left the group with—including ideas such as Zoom-based virtual coaching for trainees learning fundamentals of laparoscopic surgery types of tasks, table-top simulation for high-stakes scenarios (eg, operative vascular injury), and the importance of grounding educational activity in objectives and evaluation. I even got to make and take home my own myomectomy model. (Fun fact: The myoma is actually a stress ball wrapped in an Ace bandage and then Glad Press n’ Seal!

The late afternoon transitioned to an opportunity for trainees to interact with senior SGS members and a welcome reception. The indoor and outdoor spaces were filled with laughing and talking as people connected over drinks and snacks. Finally, the evening ended with a session presented by the SGS Equity Council, “What your Patients REALLY Want to Know.” Patty Brisben, of the Patty Brisben Foundation and founder of the company Pure Romance, was interviewed by Dr. Christine Vaccaro. It was heartwarming to hear how Patty took the stories of women suffering from sexual pain and dissatisfaction and chose to make it her life’s mission to improve women’s sexual health.
Wednesday, March 22. Day 4 of SGS.
Day 4, and the final day of the 49th SGS conference started with a sunrise run up and down the hills surrounding the JW Marriott Starr Resort. After breakfast, I entered the Tucson Ballroom to attend the last 2 scientific sessions of the conference.

Highlights from the first session included a look at postoperative outcomes and complication rates between gynecologic surgeons and general surgeons using the National Surgical Quality Improvement Program (NSQIP) database by Dr. Douglas Luchristt, who showed no difference between the 2 surgical specialties (and even better outcomes by gynecologists in certain operative measures), as well as the work of Dr. Christopher Hong who used 2 separate surgical databases (NSQIP and Michigan Surgical Quality Collaborative) to show that rates of vaginal hysterectomy have been decreasing from 2017 to 2020, even amongst patients who are likely good candidates for a vaginal route of hysterectomy. Dr. Jocelyn Fitzgerald presented her unique mixed methods research on how to better design the gynecologic office to improve the patient experience, using 3,000 Twitter responses to a question on this topic. Lastly, Dr. Emily Aldrich shared her work on better understanding the patient perception of same day discharge after major vaginal reconstructive surgery. An interesting finding of Dr. Aldrich’s study was that the most common response to her question about the “worst part of the surgical experience” was going home with a postoperative catheter, which surgeons often consider a small and temporary discomfort. The first session ended with the passing of the gavel from current SGS president Dr. Cheryl Iglesia, to the incoming SGS president Dr. Rosanne Kho, with much applause and excitement for what Dr. Kho will bring to the table in her new role.
The research presented at the final scientific session of the conference did not disappoint. A retrospective study on the influence of body mass index (BMI) on the time to surgical diagnosis of endometriosis by Dr. Melissa Markowitz found that obesity was associated with a delay of over 1 year in surgical diagnosis of endometriosis compared with normal and underweight patients. Dr. David (Ike) Rahn presented additional findings on his randomized, double-blinded, multicenter trial on perioperative use of vaginal estrogen cream in postmenopausal patients with prolapse. He found that 5 weeks of estrogen cream use was not associated with any improvement in urinary incontinence or sexual function.
Dr. Stephanie Glass Clark used the Premier Healthcare Database to show that that there was no difference in postoperative mesh exposure in patients who underwent a total hysterectomy compared with supracervical hysterectomy at the time of sacrocolpopexy. Dr. Kavita Mishra presented results from the FLOWER trial, which found no difference in postoperative outcomes for transgender women undergoing vaginoplasty for gender affirmation who did and did not undergo preoperative pelvic floor physical therapy. Finally, Dr. Carly Crowder shared her video of anatomy for sacral neuromodulation with some excellent cadaveric dissections to exhibit the peri-sacral and gluteal anatomy.

As the conference ended, raindrops pounded the sandy grounds of the resort as I waited in the lobby for my Uber to the airport. Dr. Rosanne Kho happened to walk by and stopped to speak with me and one of my attendings. She smiled as she asked about our experience at the conference and to wish us safe travels. To me, this moment embodies the spirit of mentorship and connection that is so unique to the SGS conference. I feel incredibly lucky to have met some of the physician leaders of our field, who genuinely want to get to know and help the next generation. This year’s meeting was attended by ObGyn generalists and surgeons of all gynecologic subspecialties and certainly met its goal in addressing topics with an “Impact Factor.” I am inspired by all the work that is happening across the country to move the needle and better our field. This was my first SGS experience, but it certainly won’t be the last. I hope you too will consider attending in the future!
Tuesday, March 21. Day 3 of SGS.
It’s Day 3 of the SGS conference! In addition to the academic roundtables, conference attendees had the option of doing early-morning yoga with Dr. Mireille Truong. Yoga sounded nice, but I spent the morning in bed, catching up on sleep. (My own version of wellness!) The scientific sessions of the day started at 7:30 am, and I especially want to highlight the work of Dr. Amy Askew who performed a randomized controlled trial comparing patient removal of urinary catheters placed for postoperative urinary retention to office removal. She found that patient urinary catheter removal was a feasible and safe option with excellent patient satisfaction and a reduction of in-person postoperative office visits. At the end of the session, Dr. Cheryl Iglesia gave her presidential address, where she shared her journey to becoming the physician, educator, researcher, and leader she is today. She emphasized the importance of being a continual learner and to give back by mentoring and educating the next generation. “Learn it, earn it, and return it.”

This was followed by the Te Linde lecture, given by Dr. Pamela Moalli. An exceptional surgeon-scientist, Dr. Moalli shared about her work on the impact of mesh on tissue, as well as alternative biologic options being developed, such as 3D printed membranes, extracellular matrix scaffolds, and living tissue grafts to create new ligamentous supports for the vagina. She discussed novel research using stem cell transplantation to harness the power of regeneration in the urethra or vagina following injury. I think it is safe to say that the entire room was in awe of the work she has done, and what she continues to do to find better therapeutic options for girls and women with pelvic floor disorders. Her talk ended in a standing ovation. Afterwards, all the University of Pittsburgh Medical Center-Magee Womens Hospital trainees, faculty, and several alumni took a picture with Dr. Moalli (fifth from the right in the picture).
Lunch followed, which included a brief walk around the industry exhibition hall. I then returned back to the Tucson Ballroom to listen in on the next scientific session on surgical ergonomics. Organized by Dr. Amy Park who herself suffered from work-related musculoskeletal injuries, the session was composed of an excellent video by Dr. Abby Stork on stretches to prevent and reduce the risk of surgeon-associated musculoskeletal injuries, especially in vaginal surgeons. There was then a panel of 3 experts, Dr. Noor Abu-Alnadi, Dr. Ladin Yurteri-Kaplan, and Dr. Susan Hallbeck (PhD ergonomics expert), moderated by Dr. Amanda Fader and Dr. Kimberly Kho. In particular, Dr. Hallbeck developed a timer app as a reminder for surgeons to stop every 40 minutes to stretch for 1.5 minutes (orstretch.mayoclinic.org). This has been studied and found to reduce musculoskeletal pain after surgery and improve physical performance without increasing total operating time. If you would like to see some of these between- and in-OR stretches yourself, an informative handout can be accessed at mcforms.mayo.edu.
Tuesday afternoon was left open. I joined Dr. Veronica Lerner, Dr. Kelly Wright, and Dr. Louise Perkins King on a 7.5-mile hike into the surrounding desert hills. We marveled at the many Saguaro cacti, some over 100 years old and towering many feet high, as well as the beautiful yellow, purple, and magenta flowers that were scattered among the desert brush. Several rabbits and deer wandered by us during our hike. On one of the trails, the stone skeleton of an old house stood, once a home to the Bowen family who had moved to Arizona for health reasons. I could see why they would want to move here—I felt such a peace looking at the gorgeous view from what was once their doorway.

After a shower and a lot of stretching, I got ready for the evening event, A Taste and Toast with SGS: Under the Arizona Skies. The food and drink were delicious, and I got to spend the evening catching up with a good friend. We watched as conference attendees assigned to different color teams (red, green, blue, and yellow), fought for the hallowed Golden Uterus Trophy in several competitive gynecology-themed games (eg, throwing sacral neuromodulation needle “darts” at balloons and removing small pom poms from a water bottle with a disposable operative hysteroscope). As the evening progressed, the DJ turned up the music and people made their way to the dance floor. The event served as a fundraiser for the SGS Pelvic Anatomy Group and successfully raised $35,000.
Monday, March 20. Day 2 of SGS.
Day 2 of the SGS meeting started off with a gentle sunrise over the cacti-covered hills surrounding the JW Marriot Starr Pass Hotel, the venue for the 49th SGS annual scientific meeting. The first official event of the day after some engaging academic round tables was the recognition of the new SGS members. Much celebration was had over the 18 gynecologic surgeons who were inducted.

The second day included the first 3 scientific sessions of the conference. Some highlights include the work of Dr. Shawn Menefee on a randomized trial of sacral colpopexy, transvaginal mesh, and native tissue apical repair for posthysterectomy vault prolapse; a video by Dr. Matthew Fallon on a robotic-assisted laparoscopic approach to repairing a chronic uterine inversion; and the impact of age on regret following hysterectomy by Dr. Nathan King. Dr. Candace Parker-Autry also presented her work on the impact of perineorrhaphy on both female and male sexual function, and Dr. Cassie (Clarissa) Niino spoke elegantly on the “red bag problem” that exists in all of our operating rooms, which increases pollution and cost unnecessarily.
There were also several excellent talks given. Dr. Jason Wright spoke about the importance of surgical volume on gynecologic surgery. In particular, he noted that surgical volume needs to be considered not only at the surgeon level but also at the hospital level. Higher-volume hospitals will provide better care, in the same way that general, high-volume surgeons have less complications and better long-term outcomes. Of note, volume is not the whole picture. We need to also consider measurements of surgery and hospital quality and surgeon skill in addition to volume, as Dr. Shawn Menefee insightfully commented.
Dr. Beri Ridgeway gave the Mark D. Walters Lecture about surgeons in the c-suite and the importance of having a seat at the leadership table as surgeons and medical providers. In her words: “If we aren’t at the table, then we are on the menu.” Overworked and underpaid, burned out doctors feel powerless because they are managed by leaders with a business and not a medical background, and we need to have physicians in leadership who understand how medicine is practiced and to ensure equitable care
Dr. Kelly Wright gave a talk on the environmental impact of gynecologic care—from OR to clinic. She gave examples of how metal, reusable speculums become more cost-effective and produce less waste after only 2-3 uses and how there is no evidence that bouffants reduce surgical site infections (and a reusable scrub cap could work just as well without creating waste). Finally, Dr. Ebony Carter gave an impassioned talk on the need for equity in publication and grant funding in our field. She shared about her initiative through the Green Journal (Obstetrics and Gynecology) to create an issue focused on furthering equity and dispelling racism in medical research.
Later in the afternoon, I attended the Fellows’ Pelvic Research Network (FPRN) meeting, which includes AUGS-SGS (urogynecology fellows) and FMIGS-SGS (fellows of all other gynecologic subspecialities, including minimally invasive gynecologic surgery, family planning, reproductive endocrinology and infertility, and pediatric and adolescent gynecology). Dr. John Gebhart gave an excellent lecture with some impressive photos and videos on how to manage mesh exposure and erosion.
Afterwards, updates were given on the current FPRN projects, and 4 new projects were proposed and underwent audience feedback for improvement. It was exciting to see the multicenter collaborations fostered through the FPRN, and I look forward to seeing which projects will get funded for this upcoming year!

The evening ended with the President's Award Ceremony led by Dr. Cheryl Iglesia, the 49th SGS President, as well as the President's Reception. I also wantd to highlight the winner of the Distinguished Surgeon Award: Dr. Dee Fenner. The remaining awardees are listed on the SGS website (https://sgsonline.org).
Sunday, March 19. Day 1 of SGS.
Last night around midnight, bleary-eyed from the long flight from Pittsburgh, I walked out into the dimly lit, mild air of Tucson, Arizona. The Saguaro cacti that lined the entrance to the airport stood tall and tree-like, with welcoming green arms. It was as if they too knew that the next 4 days would be filled with the building of new relationships and the strengthening of old ones, as well as with education, innovation, and the sharing of research. That spirit of collegiality, approachability, and connection in an intimate and vibrant meeting is what the Society of Gynecologic Surgeons (SGS) meeting has been known for and why it draws people to come back, year after year.
The first day of the conference was fantastic. As a first-time attendant at SGS, I was excited to have the opportunity to meet and rub elbows with mentors and role models from across the country. My day started off with the SGS Fellows and Young Attendings Course, moderated by 3 incredible faculty: Dr. Matthew Barker, Dr. Sadikah Behbehani, and Dr. Traci Ito. Some high-yield topics such as contract negotiation, developing a urogynecology- or MIGS-based practice, billing, academic promotion, and taking advantage of relationships with industry were discussed at length, and the session ended with a roundtable, where the experts had time to answer questions in smaller groups. One of the quotes that will ring true for many fellows about to embark on the job search was from Dr. Amanda Ecker: “Up until now, you were told where to go and what your schedule is. This is the first time you have flexibility and power to decide for yourself.” Therefore, it is important to reflect on what you really desire and/or prioritize in a job, whether it is location, compensation, protected time, or opportunities for advancement.

In the afternoon, I attended a postgraduate course led by Dr. Veronica Lerner and Dr. Mireille Truong called “The Sim Factor: Making an Impact in Surgical Education.” Several other excellent postgraduate courses were available, including “Advanced Endometriosis Surgery and Pelvic Pain Patient-Centered Approach,” “Social Media Workshop- #Gynfluencing: Using Social Media to Find Your Digital Voice,” and “Urologic Surgery for the Gynecologic Surgeon: GU Injury, Ureteral Stents, Complex Fistula and More.” I was grateful for the hands-on and tangible tools that Drs. Lerner and Truong left the group with—including ideas such as Zoom-based virtual coaching for trainees learning fundamentals of laparoscopic surgery types of tasks, table-top simulation for high-stakes scenarios (eg, operative vascular injury), and the importance of grounding educational activity in objectives and evaluation. I even got to make and take home my own myomectomy model. (Fun fact: The myoma is actually a stress ball wrapped in an Ace bandage and then Glad Press n’ Seal!

The late afternoon transitioned to an opportunity for trainees to interact with senior SGS members and a welcome reception. The indoor and outdoor spaces were filled with laughing and talking as people connected over drinks and snacks. Finally, the evening ended with a session presented by the SGS Equity Council, “What your Patients REALLY Want to Know.” Patty Brisben, of the Patty Brisben Foundation and founder of the company Pure Romance, was interviewed by Dr. Christine Vaccaro. It was heartwarming to hear how Patty took the stories of women suffering from sexual pain and dissatisfaction and chose to make it her life’s mission to improve women’s sexual health.
Depression tied to inflammation and survival in lung cancer
suggests a new study.
The findings underscore the importance of assessing and treating depression in patients with cancer, particularly given the high rate of depression among those with lung cancer versus other types of cancer, the investigators said.
The study involved 186 patients with newly diagnosed stage IV non–small cell lung cancer (NSCLC), of whom 35% had self-reported moderate to severe depressive symptoms.
Depression was reliably associated with lung-relevant systemic inflammation responses (SIRs), which included neutrophil-to-lymphocyte ratio (NLR), platelet-to-lymphocyte ratio (PLR), and Advanced Lung Cancer Inflammation Index (ALI) score.
These SIRs were prognostic for 2-year OS.
Overall mortality at 2 years was 61%. Higher NLRs and PLRs and lower ALI scores all predicted worse OS (hazard ratio, 1.91, 2.08, and 0.53, respectively).
The findings were published online in PLoS ONE (2023 Feb 24.
“These patients with high levels of depression are at much higher risk for poor outcomes,” but the key finding was that patients with the highest depression levels were driving the relationship, lead author Barbara Andersen, PhD, professor of psychology at Ohio State University, Columbus, stated in a press release.
“It was patients with high depression levels who had strikingly higher inflammation levels, and that is what really drove the correlation we saw,” she explained.
For example, 56% of patients with no depression symptoms or only mild depression symptoms had a PLR above the cutoff for dangerous levels of inflammation, compared with 42% whose PLR was below the cutoff. However, among those with high depression levels, 77% and 23% had a PLR above and below the cutoff, respectively.
“These highly depressed patients were 1.3-3 times more likely to have high inflammation levels, even after controlling for other factors related to inflammation biomarker levels, including demographics and smoking status,” Dr. Andersen noted.
“Depression levels may be as important or even more important than other factors that have been associated with how people fare with lung cancer,” she suggested.
In a previous study, the team controlled for baseline depression and found that “the trajectory of depression from diagnosis through 2 years (18 assessments) predicted NSCLC patients’ survival (HR, 1.09), above and beyond baseline depression, sociodemographics, smoking status, cell type, and receipt of targeted treatments and immunotherapies.”
“Taken together, data support psychological, behavioral, and biologic toxicities of depression capable of influencing treatment response and/or survival,” they wrote.
“The results may help explain why a substantial portion of lung cancer patients fail to respond to new immunotherapy and targeted treatments that have led to significantly longer survival for many people with the disease,” Dr. Andersen said.
The investigators concluded that “intensive study of depression among patients with NSCLC, combined with measures of cell biology, inflammation, and immunity, is needed to extend these findings and discover their mechanisms, with the long-term aim to improve patients’ quality of life, treatment responses, and longevity.”
This study was funded by the Ohio State University Comprehensive Cancer Center and Pelotonia through grants to individual authors. Dr. Andersen reported having no relevant disclosures.
A version of this article first appeared on Medscape.com.
suggests a new study.
The findings underscore the importance of assessing and treating depression in patients with cancer, particularly given the high rate of depression among those with lung cancer versus other types of cancer, the investigators said.
The study involved 186 patients with newly diagnosed stage IV non–small cell lung cancer (NSCLC), of whom 35% had self-reported moderate to severe depressive symptoms.
Depression was reliably associated with lung-relevant systemic inflammation responses (SIRs), which included neutrophil-to-lymphocyte ratio (NLR), platelet-to-lymphocyte ratio (PLR), and Advanced Lung Cancer Inflammation Index (ALI) score.
These SIRs were prognostic for 2-year OS.
Overall mortality at 2 years was 61%. Higher NLRs and PLRs and lower ALI scores all predicted worse OS (hazard ratio, 1.91, 2.08, and 0.53, respectively).
The findings were published online in PLoS ONE (2023 Feb 24.
“These patients with high levels of depression are at much higher risk for poor outcomes,” but the key finding was that patients with the highest depression levels were driving the relationship, lead author Barbara Andersen, PhD, professor of psychology at Ohio State University, Columbus, stated in a press release.
“It was patients with high depression levels who had strikingly higher inflammation levels, and that is what really drove the correlation we saw,” she explained.
For example, 56% of patients with no depression symptoms or only mild depression symptoms had a PLR above the cutoff for dangerous levels of inflammation, compared with 42% whose PLR was below the cutoff. However, among those with high depression levels, 77% and 23% had a PLR above and below the cutoff, respectively.
“These highly depressed patients were 1.3-3 times more likely to have high inflammation levels, even after controlling for other factors related to inflammation biomarker levels, including demographics and smoking status,” Dr. Andersen noted.
“Depression levels may be as important or even more important than other factors that have been associated with how people fare with lung cancer,” she suggested.
In a previous study, the team controlled for baseline depression and found that “the trajectory of depression from diagnosis through 2 years (18 assessments) predicted NSCLC patients’ survival (HR, 1.09), above and beyond baseline depression, sociodemographics, smoking status, cell type, and receipt of targeted treatments and immunotherapies.”
“Taken together, data support psychological, behavioral, and biologic toxicities of depression capable of influencing treatment response and/or survival,” they wrote.
“The results may help explain why a substantial portion of lung cancer patients fail to respond to new immunotherapy and targeted treatments that have led to significantly longer survival for many people with the disease,” Dr. Andersen said.
The investigators concluded that “intensive study of depression among patients with NSCLC, combined with measures of cell biology, inflammation, and immunity, is needed to extend these findings and discover their mechanisms, with the long-term aim to improve patients’ quality of life, treatment responses, and longevity.”
This study was funded by the Ohio State University Comprehensive Cancer Center and Pelotonia through grants to individual authors. Dr. Andersen reported having no relevant disclosures.
A version of this article first appeared on Medscape.com.
suggests a new study.
The findings underscore the importance of assessing and treating depression in patients with cancer, particularly given the high rate of depression among those with lung cancer versus other types of cancer, the investigators said.
The study involved 186 patients with newly diagnosed stage IV non–small cell lung cancer (NSCLC), of whom 35% had self-reported moderate to severe depressive symptoms.
Depression was reliably associated with lung-relevant systemic inflammation responses (SIRs), which included neutrophil-to-lymphocyte ratio (NLR), platelet-to-lymphocyte ratio (PLR), and Advanced Lung Cancer Inflammation Index (ALI) score.
These SIRs were prognostic for 2-year OS.
Overall mortality at 2 years was 61%. Higher NLRs and PLRs and lower ALI scores all predicted worse OS (hazard ratio, 1.91, 2.08, and 0.53, respectively).
The findings were published online in PLoS ONE (2023 Feb 24.
“These patients with high levels of depression are at much higher risk for poor outcomes,” but the key finding was that patients with the highest depression levels were driving the relationship, lead author Barbara Andersen, PhD, professor of psychology at Ohio State University, Columbus, stated in a press release.
“It was patients with high depression levels who had strikingly higher inflammation levels, and that is what really drove the correlation we saw,” she explained.
For example, 56% of patients with no depression symptoms or only mild depression symptoms had a PLR above the cutoff for dangerous levels of inflammation, compared with 42% whose PLR was below the cutoff. However, among those with high depression levels, 77% and 23% had a PLR above and below the cutoff, respectively.
“These highly depressed patients were 1.3-3 times more likely to have high inflammation levels, even after controlling for other factors related to inflammation biomarker levels, including demographics and smoking status,” Dr. Andersen noted.
“Depression levels may be as important or even more important than other factors that have been associated with how people fare with lung cancer,” she suggested.
In a previous study, the team controlled for baseline depression and found that “the trajectory of depression from diagnosis through 2 years (18 assessments) predicted NSCLC patients’ survival (HR, 1.09), above and beyond baseline depression, sociodemographics, smoking status, cell type, and receipt of targeted treatments and immunotherapies.”
“Taken together, data support psychological, behavioral, and biologic toxicities of depression capable of influencing treatment response and/or survival,” they wrote.
“The results may help explain why a substantial portion of lung cancer patients fail to respond to new immunotherapy and targeted treatments that have led to significantly longer survival for many people with the disease,” Dr. Andersen said.
The investigators concluded that “intensive study of depression among patients with NSCLC, combined with measures of cell biology, inflammation, and immunity, is needed to extend these findings and discover their mechanisms, with the long-term aim to improve patients’ quality of life, treatment responses, and longevity.”
This study was funded by the Ohio State University Comprehensive Cancer Center and Pelotonia through grants to individual authors. Dr. Andersen reported having no relevant disclosures.
A version of this article first appeared on Medscape.com.
FROM PLOS ONE
Living kidney donors should receive money for their costs of donating
This transcript has been edited for clarity.
Hi. I’m Art Caplan. I’m at the division of medical ethics at New York University’s Grossman School of Medicine in New York City.
We try very hard to get organ donation from those who die. That’s a commendable thing to do. I think doctors should always be discussing the opportunity to donate organs upon death, even in primary care settings.
It’s good to find out what people’s attitudes are. Let them learn about organ donation as something they can think about. Let them talk about it with family and friends and partners so that they know their wishes.
However, despite these efforts to encourage organ donation, we still have far fewer organs than we could use to transplant people, many people die on waiting lists because there are no organs to give them, and we’re in a situation where demand for organ transplant is actually increasing.
There is more capacity to do transplants both in the United States and elsewhere, and more people are living longer, so organ failure starts to become more common before, let’s say, terminal illness is really there. Now, we have more people who might benefit from organ transplant in an aging population.
One place to turn to help reduce the shortage of organs is to living donation. At least insofar as kidneys go, kidney donation from living persons has become a prominent source of organs for those who need kidneys – most of whom are surviving on dialysis, by the way, at a very high cost and often with a quality of life that they don’t find particularly easy to accept.
Transplant is far preferred, even though they have to take immunosuppression to keep those organ transplants going, and that has its own risks and side effects. They still get more mobility. They still are able to have a broader diet. They enjoy life far more than they do having to show up for dialysis three times a week for a couple of hours, every week, for every week that they live.
There is an interest in living kidney donation. One battle has been that, well, maybe we could get more kidneys if we just paid people to sell us their kidneys. That has been resisted, and I’ve been resistant to that idea, too, because I worry that it leads to exploitation.
The people who sell their kidneys are poor. They’re often in debt. They feel coerced by their circumstances, so they make a kidney sale. This happens in countries like India, where there are markets underground, and you see that it’s the poorest of the poor who do this, and they don’t really work their way out of debt. They just wind up without a kidney, help relieve their debt a little bit, and pretty soon, because they don’t have a job or an income except that sale of a kidney, they’re not much better off than they were before they started.
Also, people who sell kidneys for money are more likely not to admit to their own health problems, raising risks about the quality of organs. Then, of course, it puts doctors in a position to take out an organ for pay, even though it doesn’t benefit you, so that you can sell it. This raises some questions about whether that’s consistent with medical ethics.
A different idea has emerged. New York State Governor Kathy Hochul just signed legislation that allows living donors to be compensated for legitimate costs. That’s a little different matter. You’re not buying the organ, but you’re saying that if you experience health care problems due to complications from a donation, if you need money for transportation, if you lost money because you did this altruistically and you had to take time off from work and had expenses for a babysitter, restaurants, or other things, the state is going to try to create funds that will compensate you.
That, I think we should agree, is not a bad idea. You’re in a situation there where you don’t want to make people who are heroic, altruistic, and trying to help others by donating a kidney end up financially worse off.
I think there’s a difference between making someone financially whole after the decision to make a kidney available and creating a market where the poorest of the poor come forward to just sell because they see no other choice in terms of how to get rid of debts. I see these situations as not ethically equivalent, so I support efforts to try to compensate people who are our heroes. I don’t think we should ask them to financially suffer.
We’ll watch to see what happens as the New York state law comes into effect. By the way, New York is one of the states that really lags in the supply of organs for transplant, so this measure is particularly important for that state. Many other states should be considering this legislation as well.
It’s one thing to reward, if you will, donors by making sure they don’t suffer financial loss. It’s a very different thing to say, let’s have a free market and we’ll pay whoever it is that’s willing to sell us a kidney to do so. The former seems to me to be humane and just, whereas the latter risks exploitation.
A version of this article first appeared on Medscape.com.
This transcript has been edited for clarity.
Hi. I’m Art Caplan. I’m at the division of medical ethics at New York University’s Grossman School of Medicine in New York City.
We try very hard to get organ donation from those who die. That’s a commendable thing to do. I think doctors should always be discussing the opportunity to donate organs upon death, even in primary care settings.
It’s good to find out what people’s attitudes are. Let them learn about organ donation as something they can think about. Let them talk about it with family and friends and partners so that they know their wishes.
However, despite these efforts to encourage organ donation, we still have far fewer organs than we could use to transplant people, many people die on waiting lists because there are no organs to give them, and we’re in a situation where demand for organ transplant is actually increasing.
There is more capacity to do transplants both in the United States and elsewhere, and more people are living longer, so organ failure starts to become more common before, let’s say, terminal illness is really there. Now, we have more people who might benefit from organ transplant in an aging population.
One place to turn to help reduce the shortage of organs is to living donation. At least insofar as kidneys go, kidney donation from living persons has become a prominent source of organs for those who need kidneys – most of whom are surviving on dialysis, by the way, at a very high cost and often with a quality of life that they don’t find particularly easy to accept.
Transplant is far preferred, even though they have to take immunosuppression to keep those organ transplants going, and that has its own risks and side effects. They still get more mobility. They still are able to have a broader diet. They enjoy life far more than they do having to show up for dialysis three times a week for a couple of hours, every week, for every week that they live.
There is an interest in living kidney donation. One battle has been that, well, maybe we could get more kidneys if we just paid people to sell us their kidneys. That has been resisted, and I’ve been resistant to that idea, too, because I worry that it leads to exploitation.
The people who sell their kidneys are poor. They’re often in debt. They feel coerced by their circumstances, so they make a kidney sale. This happens in countries like India, where there are markets underground, and you see that it’s the poorest of the poor who do this, and they don’t really work their way out of debt. They just wind up without a kidney, help relieve their debt a little bit, and pretty soon, because they don’t have a job or an income except that sale of a kidney, they’re not much better off than they were before they started.
Also, people who sell kidneys for money are more likely not to admit to their own health problems, raising risks about the quality of organs. Then, of course, it puts doctors in a position to take out an organ for pay, even though it doesn’t benefit you, so that you can sell it. This raises some questions about whether that’s consistent with medical ethics.
A different idea has emerged. New York State Governor Kathy Hochul just signed legislation that allows living donors to be compensated for legitimate costs. That’s a little different matter. You’re not buying the organ, but you’re saying that if you experience health care problems due to complications from a donation, if you need money for transportation, if you lost money because you did this altruistically and you had to take time off from work and had expenses for a babysitter, restaurants, or other things, the state is going to try to create funds that will compensate you.
That, I think we should agree, is not a bad idea. You’re in a situation there where you don’t want to make people who are heroic, altruistic, and trying to help others by donating a kidney end up financially worse off.
I think there’s a difference between making someone financially whole after the decision to make a kidney available and creating a market where the poorest of the poor come forward to just sell because they see no other choice in terms of how to get rid of debts. I see these situations as not ethically equivalent, so I support efforts to try to compensate people who are our heroes. I don’t think we should ask them to financially suffer.
We’ll watch to see what happens as the New York state law comes into effect. By the way, New York is one of the states that really lags in the supply of organs for transplant, so this measure is particularly important for that state. Many other states should be considering this legislation as well.
It’s one thing to reward, if you will, donors by making sure they don’t suffer financial loss. It’s a very different thing to say, let’s have a free market and we’ll pay whoever it is that’s willing to sell us a kidney to do so. The former seems to me to be humane and just, whereas the latter risks exploitation.
A version of this article first appeared on Medscape.com.
This transcript has been edited for clarity.
Hi. I’m Art Caplan. I’m at the division of medical ethics at New York University’s Grossman School of Medicine in New York City.
We try very hard to get organ donation from those who die. That’s a commendable thing to do. I think doctors should always be discussing the opportunity to donate organs upon death, even in primary care settings.
It’s good to find out what people’s attitudes are. Let them learn about organ donation as something they can think about. Let them talk about it with family and friends and partners so that they know their wishes.
However, despite these efforts to encourage organ donation, we still have far fewer organs than we could use to transplant people, many people die on waiting lists because there are no organs to give them, and we’re in a situation where demand for organ transplant is actually increasing.
There is more capacity to do transplants both in the United States and elsewhere, and more people are living longer, so organ failure starts to become more common before, let’s say, terminal illness is really there. Now, we have more people who might benefit from organ transplant in an aging population.
One place to turn to help reduce the shortage of organs is to living donation. At least insofar as kidneys go, kidney donation from living persons has become a prominent source of organs for those who need kidneys – most of whom are surviving on dialysis, by the way, at a very high cost and often with a quality of life that they don’t find particularly easy to accept.
Transplant is far preferred, even though they have to take immunosuppression to keep those organ transplants going, and that has its own risks and side effects. They still get more mobility. They still are able to have a broader diet. They enjoy life far more than they do having to show up for dialysis three times a week for a couple of hours, every week, for every week that they live.
There is an interest in living kidney donation. One battle has been that, well, maybe we could get more kidneys if we just paid people to sell us their kidneys. That has been resisted, and I’ve been resistant to that idea, too, because I worry that it leads to exploitation.
The people who sell their kidneys are poor. They’re often in debt. They feel coerced by their circumstances, so they make a kidney sale. This happens in countries like India, where there are markets underground, and you see that it’s the poorest of the poor who do this, and they don’t really work their way out of debt. They just wind up without a kidney, help relieve their debt a little bit, and pretty soon, because they don’t have a job or an income except that sale of a kidney, they’re not much better off than they were before they started.
Also, people who sell kidneys for money are more likely not to admit to their own health problems, raising risks about the quality of organs. Then, of course, it puts doctors in a position to take out an organ for pay, even though it doesn’t benefit you, so that you can sell it. This raises some questions about whether that’s consistent with medical ethics.
A different idea has emerged. New York State Governor Kathy Hochul just signed legislation that allows living donors to be compensated for legitimate costs. That’s a little different matter. You’re not buying the organ, but you’re saying that if you experience health care problems due to complications from a donation, if you need money for transportation, if you lost money because you did this altruistically and you had to take time off from work and had expenses for a babysitter, restaurants, or other things, the state is going to try to create funds that will compensate you.
That, I think we should agree, is not a bad idea. You’re in a situation there where you don’t want to make people who are heroic, altruistic, and trying to help others by donating a kidney end up financially worse off.
I think there’s a difference between making someone financially whole after the decision to make a kidney available and creating a market where the poorest of the poor come forward to just sell because they see no other choice in terms of how to get rid of debts. I see these situations as not ethically equivalent, so I support efforts to try to compensate people who are our heroes. I don’t think we should ask them to financially suffer.
We’ll watch to see what happens as the New York state law comes into effect. By the way, New York is one of the states that really lags in the supply of organs for transplant, so this measure is particularly important for that state. Many other states should be considering this legislation as well.
It’s one thing to reward, if you will, donors by making sure they don’t suffer financial loss. It’s a very different thing to say, let’s have a free market and we’ll pay whoever it is that’s willing to sell us a kidney to do so. The former seems to me to be humane and just, whereas the latter risks exploitation.
A version of this article first appeared on Medscape.com.
State medical board chair steps down amid Medicaid fraud accusations
He has stepped down as board chair, and state officials have suspended all Medicaid payments to Dr. Hyatt and his practice, Pinnacle Premier Psychiatry in Rogers, Arkansas.
Dr. Hyatt billed 99.95% of the claims for his patients’ hospital care to Medicaid at the highest severity level, according to an affidavit filed by an investigator with the Medicaid Fraud Control Unit, Arkansas Attorney General’s Office. Other Arkansas psychiatrists billed that same level in only about 39% of claims, the affidavit states.
The possible upcoding alleged in the affidavit was a red flag that prompted the state to temporarily suspend Dr. Hyatt’s Medicaid payments.
Dr. Hyatt has until this Friday to file an appeal. He did not respond to requests from this news organization for comment.
The affidavit pointed to other concerns. For example, a whistleblower who worked at the Northwest Medical Center where Dr. Hyatt admitted patients claimed that Dr. Hyatt was only on the floor a few minutes a day and that he had no contact with patients. A review of hundreds of hours of video by state investigators revealed that Dr. Hyatt did not enter patients’ rooms, nor did he have any contact with patients, according to the affidavit. Dr. Hyatt served as the hospital’s behavioral unit director from 2018 until his contract was abruptly terminated in May 2022, according to the affidavit.
However, Dr. Hyatt claimed to have conducted daily face-to-face evaluation and management with patients, according to the affidavit. In addition, the whistleblower claimed that Dr. Hyatt did not want patients to know his name and instructed staff to cover up his name on patient armbands.
Detaining patients
Dr. Hyatt also faces accusations that he held patients against their will, according to civil lawsuits filed in Washington County, Ark., reports the Arkansas Advocate.
Karla Adrian-Caceres filed suit on Jan. 17. Ms. Adrian-Caceres also named Brooke Green, Northwest Arkansas Hospitals, and 25 unidentified hospital employees as defendants.
According to the complaint, Ms. Adrian-Caceres, an engineering student at the University of Arkansas, arrived at the Northwest Medical Emergency Department after accidentally taking too many Tylenol on Jan. 18, 2022. She was then taken by ambulance to a Northwest psychiatric facility in Springdale, court records show.
According to the complaint, Ms. Adrian-Caceres said that she was given a sedative and asked to sign consent for admission while on the way to Northwest. She said that she “signed some documents without being able to read or understand them at the time.”
When she asked when she could go home, Ms. Adrian-Caceres said, “more than one employee told her there was a minimum stay and that if she asked to leave, they would take her to court where a judge would give her a longer stay because the judge always sides with Dr. Hyatt and Northwest,” according to court documents. Northwest employees stripped Ms. Adrian-Caceres, searched her body, took all of her possessions from her and issued underwear and a uniform, according to the lawsuit.
Ms. Adrian-Caceres’ mother, Katty Caceres, claimed in the lawsuit that she was prohibited from seeing her daughter. Ms. Caceres spoke with five different employees, four of whom had only their first names on their badges. Each of them reportedly said that they could not help, or that the plaintiff “would be in there for some time” and that it was Dr. Hyatt’s decision regarding how long that would be, according to court documents.
Katty Caceres hired a local attorney named Aaron Cash to represent her daughter. On Jan. 20, 2022, Mr. Cash faxed a letter to the hospital demanding her release. When Ms. Caceres arrived to pick up her daughter, she claimed that staff members indicated that the daughter was there voluntarily and refused to release her “at the direction of Dr Hyatt.” During a phone call later that day, the plaintiff told her mother that her status was being changed to an involuntary hold, court documents show.
“At one point she was threatened with the longer time in there if she kept asking to leave,” Mr. Cash told this news organization. In addition, staff members reportedly told Ms. Adrian-Caceres that the “judge always sided with Dr Hyatt” and she “would get way longer there, 30-45 days if [she] went before the judge,” according to Mr. Cash.
Mr. Cash said nine other patients have contacted his firm with similar allegations against Dr. Hyatt.
“We’ve talked to many people that have experienced the same threats,” Mr. Cash said. “When they’re asking to leave, they get these threats, they get coerced … and they’re never taken to court. They’re never given opportunity to talk to a judge or to have a public defender appointed.”
A version of this article first appeared on Medscape.com.
He has stepped down as board chair, and state officials have suspended all Medicaid payments to Dr. Hyatt and his practice, Pinnacle Premier Psychiatry in Rogers, Arkansas.
Dr. Hyatt billed 99.95% of the claims for his patients’ hospital care to Medicaid at the highest severity level, according to an affidavit filed by an investigator with the Medicaid Fraud Control Unit, Arkansas Attorney General’s Office. Other Arkansas psychiatrists billed that same level in only about 39% of claims, the affidavit states.
The possible upcoding alleged in the affidavit was a red flag that prompted the state to temporarily suspend Dr. Hyatt’s Medicaid payments.
Dr. Hyatt has until this Friday to file an appeal. He did not respond to requests from this news organization for comment.
The affidavit pointed to other concerns. For example, a whistleblower who worked at the Northwest Medical Center where Dr. Hyatt admitted patients claimed that Dr. Hyatt was only on the floor a few minutes a day and that he had no contact with patients. A review of hundreds of hours of video by state investigators revealed that Dr. Hyatt did not enter patients’ rooms, nor did he have any contact with patients, according to the affidavit. Dr. Hyatt served as the hospital’s behavioral unit director from 2018 until his contract was abruptly terminated in May 2022, according to the affidavit.
However, Dr. Hyatt claimed to have conducted daily face-to-face evaluation and management with patients, according to the affidavit. In addition, the whistleblower claimed that Dr. Hyatt did not want patients to know his name and instructed staff to cover up his name on patient armbands.
Detaining patients
Dr. Hyatt also faces accusations that he held patients against their will, according to civil lawsuits filed in Washington County, Ark., reports the Arkansas Advocate.
Karla Adrian-Caceres filed suit on Jan. 17. Ms. Adrian-Caceres also named Brooke Green, Northwest Arkansas Hospitals, and 25 unidentified hospital employees as defendants.
According to the complaint, Ms. Adrian-Caceres, an engineering student at the University of Arkansas, arrived at the Northwest Medical Emergency Department after accidentally taking too many Tylenol on Jan. 18, 2022. She was then taken by ambulance to a Northwest psychiatric facility in Springdale, court records show.
According to the complaint, Ms. Adrian-Caceres said that she was given a sedative and asked to sign consent for admission while on the way to Northwest. She said that she “signed some documents without being able to read or understand them at the time.”
When she asked when she could go home, Ms. Adrian-Caceres said, “more than one employee told her there was a minimum stay and that if she asked to leave, they would take her to court where a judge would give her a longer stay because the judge always sides with Dr. Hyatt and Northwest,” according to court documents. Northwest employees stripped Ms. Adrian-Caceres, searched her body, took all of her possessions from her and issued underwear and a uniform, according to the lawsuit.
Ms. Adrian-Caceres’ mother, Katty Caceres, claimed in the lawsuit that she was prohibited from seeing her daughter. Ms. Caceres spoke with five different employees, four of whom had only their first names on their badges. Each of them reportedly said that they could not help, or that the plaintiff “would be in there for some time” and that it was Dr. Hyatt’s decision regarding how long that would be, according to court documents.
Katty Caceres hired a local attorney named Aaron Cash to represent her daughter. On Jan. 20, 2022, Mr. Cash faxed a letter to the hospital demanding her release. When Ms. Caceres arrived to pick up her daughter, she claimed that staff members indicated that the daughter was there voluntarily and refused to release her “at the direction of Dr Hyatt.” During a phone call later that day, the plaintiff told her mother that her status was being changed to an involuntary hold, court documents show.
“At one point she was threatened with the longer time in there if she kept asking to leave,” Mr. Cash told this news organization. In addition, staff members reportedly told Ms. Adrian-Caceres that the “judge always sided with Dr Hyatt” and she “would get way longer there, 30-45 days if [she] went before the judge,” according to Mr. Cash.
Mr. Cash said nine other patients have contacted his firm with similar allegations against Dr. Hyatt.
“We’ve talked to many people that have experienced the same threats,” Mr. Cash said. “When they’re asking to leave, they get these threats, they get coerced … and they’re never taken to court. They’re never given opportunity to talk to a judge or to have a public defender appointed.”
A version of this article first appeared on Medscape.com.
He has stepped down as board chair, and state officials have suspended all Medicaid payments to Dr. Hyatt and his practice, Pinnacle Premier Psychiatry in Rogers, Arkansas.
Dr. Hyatt billed 99.95% of the claims for his patients’ hospital care to Medicaid at the highest severity level, according to an affidavit filed by an investigator with the Medicaid Fraud Control Unit, Arkansas Attorney General’s Office. Other Arkansas psychiatrists billed that same level in only about 39% of claims, the affidavit states.
The possible upcoding alleged in the affidavit was a red flag that prompted the state to temporarily suspend Dr. Hyatt’s Medicaid payments.
Dr. Hyatt has until this Friday to file an appeal. He did not respond to requests from this news organization for comment.
The affidavit pointed to other concerns. For example, a whistleblower who worked at the Northwest Medical Center where Dr. Hyatt admitted patients claimed that Dr. Hyatt was only on the floor a few minutes a day and that he had no contact with patients. A review of hundreds of hours of video by state investigators revealed that Dr. Hyatt did not enter patients’ rooms, nor did he have any contact with patients, according to the affidavit. Dr. Hyatt served as the hospital’s behavioral unit director from 2018 until his contract was abruptly terminated in May 2022, according to the affidavit.
However, Dr. Hyatt claimed to have conducted daily face-to-face evaluation and management with patients, according to the affidavit. In addition, the whistleblower claimed that Dr. Hyatt did not want patients to know his name and instructed staff to cover up his name on patient armbands.
Detaining patients
Dr. Hyatt also faces accusations that he held patients against their will, according to civil lawsuits filed in Washington County, Ark., reports the Arkansas Advocate.
Karla Adrian-Caceres filed suit on Jan. 17. Ms. Adrian-Caceres also named Brooke Green, Northwest Arkansas Hospitals, and 25 unidentified hospital employees as defendants.
According to the complaint, Ms. Adrian-Caceres, an engineering student at the University of Arkansas, arrived at the Northwest Medical Emergency Department after accidentally taking too many Tylenol on Jan. 18, 2022. She was then taken by ambulance to a Northwest psychiatric facility in Springdale, court records show.
According to the complaint, Ms. Adrian-Caceres said that she was given a sedative and asked to sign consent for admission while on the way to Northwest. She said that she “signed some documents without being able to read or understand them at the time.”
When she asked when she could go home, Ms. Adrian-Caceres said, “more than one employee told her there was a minimum stay and that if she asked to leave, they would take her to court where a judge would give her a longer stay because the judge always sides with Dr. Hyatt and Northwest,” according to court documents. Northwest employees stripped Ms. Adrian-Caceres, searched her body, took all of her possessions from her and issued underwear and a uniform, according to the lawsuit.
Ms. Adrian-Caceres’ mother, Katty Caceres, claimed in the lawsuit that she was prohibited from seeing her daughter. Ms. Caceres spoke with five different employees, four of whom had only their first names on their badges. Each of them reportedly said that they could not help, or that the plaintiff “would be in there for some time” and that it was Dr. Hyatt’s decision regarding how long that would be, according to court documents.
Katty Caceres hired a local attorney named Aaron Cash to represent her daughter. On Jan. 20, 2022, Mr. Cash faxed a letter to the hospital demanding her release. When Ms. Caceres arrived to pick up her daughter, she claimed that staff members indicated that the daughter was there voluntarily and refused to release her “at the direction of Dr Hyatt.” During a phone call later that day, the plaintiff told her mother that her status was being changed to an involuntary hold, court documents show.
“At one point she was threatened with the longer time in there if she kept asking to leave,” Mr. Cash told this news organization. In addition, staff members reportedly told Ms. Adrian-Caceres that the “judge always sided with Dr Hyatt” and she “would get way longer there, 30-45 days if [she] went before the judge,” according to Mr. Cash.
Mr. Cash said nine other patients have contacted his firm with similar allegations against Dr. Hyatt.
“We’ve talked to many people that have experienced the same threats,” Mr. Cash said. “When they’re asking to leave, they get these threats, they get coerced … and they’re never taken to court. They’re never given opportunity to talk to a judge or to have a public defender appointed.”
A version of this article first appeared on Medscape.com.
Bruce Willis’ frontotemporal dementia is not your grandpa’s dementia
What is remarkable about the swamp that we call FTD is that it’s a somewhat rare and unusual type of dementia. We tend to characterize dementia as the erosion of memory, but FTD is more characterized by the loss of control over emotions and other cognitive functions. What›s especially tragic for performers like Mr. Willis is the loss of the verbal fluency required for delivering one’s lines.
Frontotemporal dementia
To this casual observer, Bruce Willis was an almost invincible force, vigorous, vital, one of the “immortals.” Alas, with his FTD diagnosis, we know that even a die-hard like Mr. Willis, now only 67 years of age, may have to endure years of progressive decline. If the disease follows its typical path, that will probably include slowly disconnecting and progressively losing emotional judgment and control as well as losing a reasonable understanding of what or why any of it is happening. He may also experience a progressive deterioration of the control of bodily functions and general health.
Most people with dementia lose their neurocognitive abilities through a number of different pathways, all of which result in brain shrinkage, disconnection, evident neuropathology, neurobehavioral expressions of loss, and forms of befuddlement. Alzheimer’s disease leads the list as the most common form of dementia, but vascular dementias; dementia with Lewy bodies; “mixed” dementias; dementias associated with Parkinson’s, Huntington’s, or other diseases; dementia rising from alcoholic or other brain poisoning, HIV, Lyme disease, or a host of other brain infections; or from traumatic encephalopathy (chronic or more current) may present at any active neurology clinic. These are what you might think of as your “grandpa’s dementia” – the common types often associated with old age.
FTD is a particularly interesting variant for several reasons. First, it usually arises in relatively young individuals, with initial symptoms emerging in one’s 50s or 60s. In most cases, there is no genetic and, with rare exception, any other explanation of origin – except that old medical standby, bad luck.
Second, FTD has little initial impact on a patient’s broader memory and associated cognitive abilities. The patient will stumble to come up with that next word and ultimately slow down their speech as their brain struggles with verbal fluency; they will struggle with translating their feelings and emotions into fast and appropriate actions expressed in their mind and their physical body while their memory will appear intact.
In all other dementias, cognitive losses can be profound, whereas social and emotional control and voluble speech production are generally better sustained. Imagine the impact that these struggles in verbal fluency and in emotional calibration and response must have for an established actor. By all reports, Mr. Willis vigorously pursued the work that he loved right up until the time of his dementia diagnosis, even as his colleagues would almost certainly have seen that he was struggling. Sadly, a lack of that type of self-awareness is an expected consequence of FTD.
The salience network and von Economo neurons
Third and most intriguing to a neuroscientific nerd like me is that patients with FTD experience an initial loss of a special population of cortical neurons located within the salience network in our brains, called the von Economo neurons. That salience network is designed to quickly read and evaluate our complex thoughts and emotions and via those Economo neurons, initiate appropriate neurologic and physical responses.
We share this special von Economo machinery with great apes, whales, elephants, and a handful of other especially social mammalian species.
When we see or hear or otherwise sense something that induces fear, alarm, or a potential reward, the salience network in our brain acts as a kind of gatekeeper. First, it assesses the emergent or changing situation, then it rapidly initiates an emotional and physical response. As I sit with a patient in obvious distress in my office, my salience network turns on an empathetic alarm. My brain and body immediately adjust to initiate appropriately sympathetic reactions. The von Economo neurons – those very neurons that have substantially died off in a brain with FTD – are the linchpins in this fast-response emotion and complex body signal-informed system.
Controlled emotional response is at the heart of our humanity. It’s a sad day when we lose it.
In other neurologic clinical conditions marked by the loss of specific brain cells, different forms of “disuse atrophy” are partly the cause. We don’t know whether that’s the case for FTD. Scientists have shown that specific forms of computerized brain exercises can sharply increase activity levels in the salience network which is linked to improvements in the regulatory control of the autonomic nervous system – one of the key response-mediating targets of the network’s von Economo neurons.
Interestingly, superagers who sustain body and brain health into their 90s (and beyond) die with a full complement of von Economo neurons operating happily in a still-vigorous salience network.
This neuroscientist can foresee a day when we routinely assess the integrity of this important brain system and more reliably maintain its good health. Keeping those very special neurons alive would have probably allowed Mr. Willis to sustain himself on the soundstage and on the grander stage of life for a long time to come. Alas, like so many things in medicine, there is promise. But at this moment for this famous patient, our current medical science appears to be a day late, and a dollar short.
Dr. Merzenichis is professor emeritus at the University of California, San Francisco, and a Kavli Laureate in Neuroscience. He reported conflicts of interest with the National Institutes of Health, Stronger Brains, and Posit Science.
A version of this article first appeared on Medscape.com.
What is remarkable about the swamp that we call FTD is that it’s a somewhat rare and unusual type of dementia. We tend to characterize dementia as the erosion of memory, but FTD is more characterized by the loss of control over emotions and other cognitive functions. What›s especially tragic for performers like Mr. Willis is the loss of the verbal fluency required for delivering one’s lines.
Frontotemporal dementia
To this casual observer, Bruce Willis was an almost invincible force, vigorous, vital, one of the “immortals.” Alas, with his FTD diagnosis, we know that even a die-hard like Mr. Willis, now only 67 years of age, may have to endure years of progressive decline. If the disease follows its typical path, that will probably include slowly disconnecting and progressively losing emotional judgment and control as well as losing a reasonable understanding of what or why any of it is happening. He may also experience a progressive deterioration of the control of bodily functions and general health.
Most people with dementia lose their neurocognitive abilities through a number of different pathways, all of which result in brain shrinkage, disconnection, evident neuropathology, neurobehavioral expressions of loss, and forms of befuddlement. Alzheimer’s disease leads the list as the most common form of dementia, but vascular dementias; dementia with Lewy bodies; “mixed” dementias; dementias associated with Parkinson’s, Huntington’s, or other diseases; dementia rising from alcoholic or other brain poisoning, HIV, Lyme disease, or a host of other brain infections; or from traumatic encephalopathy (chronic or more current) may present at any active neurology clinic. These are what you might think of as your “grandpa’s dementia” – the common types often associated with old age.
FTD is a particularly interesting variant for several reasons. First, it usually arises in relatively young individuals, with initial symptoms emerging in one’s 50s or 60s. In most cases, there is no genetic and, with rare exception, any other explanation of origin – except that old medical standby, bad luck.
Second, FTD has little initial impact on a patient’s broader memory and associated cognitive abilities. The patient will stumble to come up with that next word and ultimately slow down their speech as their brain struggles with verbal fluency; they will struggle with translating their feelings and emotions into fast and appropriate actions expressed in their mind and their physical body while their memory will appear intact.
In all other dementias, cognitive losses can be profound, whereas social and emotional control and voluble speech production are generally better sustained. Imagine the impact that these struggles in verbal fluency and in emotional calibration and response must have for an established actor. By all reports, Mr. Willis vigorously pursued the work that he loved right up until the time of his dementia diagnosis, even as his colleagues would almost certainly have seen that he was struggling. Sadly, a lack of that type of self-awareness is an expected consequence of FTD.
The salience network and von Economo neurons
Third and most intriguing to a neuroscientific nerd like me is that patients with FTD experience an initial loss of a special population of cortical neurons located within the salience network in our brains, called the von Economo neurons. That salience network is designed to quickly read and evaluate our complex thoughts and emotions and via those Economo neurons, initiate appropriate neurologic and physical responses.
We share this special von Economo machinery with great apes, whales, elephants, and a handful of other especially social mammalian species.
When we see or hear or otherwise sense something that induces fear, alarm, or a potential reward, the salience network in our brain acts as a kind of gatekeeper. First, it assesses the emergent or changing situation, then it rapidly initiates an emotional and physical response. As I sit with a patient in obvious distress in my office, my salience network turns on an empathetic alarm. My brain and body immediately adjust to initiate appropriately sympathetic reactions. The von Economo neurons – those very neurons that have substantially died off in a brain with FTD – are the linchpins in this fast-response emotion and complex body signal-informed system.
Controlled emotional response is at the heart of our humanity. It’s a sad day when we lose it.
In other neurologic clinical conditions marked by the loss of specific brain cells, different forms of “disuse atrophy” are partly the cause. We don’t know whether that’s the case for FTD. Scientists have shown that specific forms of computerized brain exercises can sharply increase activity levels in the salience network which is linked to improvements in the regulatory control of the autonomic nervous system – one of the key response-mediating targets of the network’s von Economo neurons.
Interestingly, superagers who sustain body and brain health into their 90s (and beyond) die with a full complement of von Economo neurons operating happily in a still-vigorous salience network.
This neuroscientist can foresee a day when we routinely assess the integrity of this important brain system and more reliably maintain its good health. Keeping those very special neurons alive would have probably allowed Mr. Willis to sustain himself on the soundstage and on the grander stage of life for a long time to come. Alas, like so many things in medicine, there is promise. But at this moment for this famous patient, our current medical science appears to be a day late, and a dollar short.
Dr. Merzenichis is professor emeritus at the University of California, San Francisco, and a Kavli Laureate in Neuroscience. He reported conflicts of interest with the National Institutes of Health, Stronger Brains, and Posit Science.
A version of this article first appeared on Medscape.com.
What is remarkable about the swamp that we call FTD is that it’s a somewhat rare and unusual type of dementia. We tend to characterize dementia as the erosion of memory, but FTD is more characterized by the loss of control over emotions and other cognitive functions. What›s especially tragic for performers like Mr. Willis is the loss of the verbal fluency required for delivering one’s lines.
Frontotemporal dementia
To this casual observer, Bruce Willis was an almost invincible force, vigorous, vital, one of the “immortals.” Alas, with his FTD diagnosis, we know that even a die-hard like Mr. Willis, now only 67 years of age, may have to endure years of progressive decline. If the disease follows its typical path, that will probably include slowly disconnecting and progressively losing emotional judgment and control as well as losing a reasonable understanding of what or why any of it is happening. He may also experience a progressive deterioration of the control of bodily functions and general health.
Most people with dementia lose their neurocognitive abilities through a number of different pathways, all of which result in brain shrinkage, disconnection, evident neuropathology, neurobehavioral expressions of loss, and forms of befuddlement. Alzheimer’s disease leads the list as the most common form of dementia, but vascular dementias; dementia with Lewy bodies; “mixed” dementias; dementias associated with Parkinson’s, Huntington’s, or other diseases; dementia rising from alcoholic or other brain poisoning, HIV, Lyme disease, or a host of other brain infections; or from traumatic encephalopathy (chronic or more current) may present at any active neurology clinic. These are what you might think of as your “grandpa’s dementia” – the common types often associated with old age.
FTD is a particularly interesting variant for several reasons. First, it usually arises in relatively young individuals, with initial symptoms emerging in one’s 50s or 60s. In most cases, there is no genetic and, with rare exception, any other explanation of origin – except that old medical standby, bad luck.
Second, FTD has little initial impact on a patient’s broader memory and associated cognitive abilities. The patient will stumble to come up with that next word and ultimately slow down their speech as their brain struggles with verbal fluency; they will struggle with translating their feelings and emotions into fast and appropriate actions expressed in their mind and their physical body while their memory will appear intact.
In all other dementias, cognitive losses can be profound, whereas social and emotional control and voluble speech production are generally better sustained. Imagine the impact that these struggles in verbal fluency and in emotional calibration and response must have for an established actor. By all reports, Mr. Willis vigorously pursued the work that he loved right up until the time of his dementia diagnosis, even as his colleagues would almost certainly have seen that he was struggling. Sadly, a lack of that type of self-awareness is an expected consequence of FTD.
The salience network and von Economo neurons
Third and most intriguing to a neuroscientific nerd like me is that patients with FTD experience an initial loss of a special population of cortical neurons located within the salience network in our brains, called the von Economo neurons. That salience network is designed to quickly read and evaluate our complex thoughts and emotions and via those Economo neurons, initiate appropriate neurologic and physical responses.
We share this special von Economo machinery with great apes, whales, elephants, and a handful of other especially social mammalian species.
When we see or hear or otherwise sense something that induces fear, alarm, or a potential reward, the salience network in our brain acts as a kind of gatekeeper. First, it assesses the emergent or changing situation, then it rapidly initiates an emotional and physical response. As I sit with a patient in obvious distress in my office, my salience network turns on an empathetic alarm. My brain and body immediately adjust to initiate appropriately sympathetic reactions. The von Economo neurons – those very neurons that have substantially died off in a brain with FTD – are the linchpins in this fast-response emotion and complex body signal-informed system.
Controlled emotional response is at the heart of our humanity. It’s a sad day when we lose it.
In other neurologic clinical conditions marked by the loss of specific brain cells, different forms of “disuse atrophy” are partly the cause. We don’t know whether that’s the case for FTD. Scientists have shown that specific forms of computerized brain exercises can sharply increase activity levels in the salience network which is linked to improvements in the regulatory control of the autonomic nervous system – one of the key response-mediating targets of the network’s von Economo neurons.
Interestingly, superagers who sustain body and brain health into their 90s (and beyond) die with a full complement of von Economo neurons operating happily in a still-vigorous salience network.
This neuroscientist can foresee a day when we routinely assess the integrity of this important brain system and more reliably maintain its good health. Keeping those very special neurons alive would have probably allowed Mr. Willis to sustain himself on the soundstage and on the grander stage of life for a long time to come. Alas, like so many things in medicine, there is promise. But at this moment for this famous patient, our current medical science appears to be a day late, and a dollar short.
Dr. Merzenichis is professor emeritus at the University of California, San Francisco, and a Kavli Laureate in Neuroscience. He reported conflicts of interest with the National Institutes of Health, Stronger Brains, and Posit Science.
A version of this article first appeared on Medscape.com.
What’s the ‘secret sauce’ to help patients move more?
“Just Do It” is a cute marketing slogan. But let’s face it: Clinically, it doesn’t work well. Most people just don’t exercise. according to recent data from the Centers for Disease Control and Prevention.
Furthermore, when surveyed about aerobic exercise and strength training, only 24.6% meet these weekly recommendations. These low rates of physical activity are alarming, given the immense benefits of exercise in improving mental and physical health and well-being.
Many people know that exercise is good for them but struggle to go workout consistently. I know firsthand how challenging this can be. In addition to being an integrative obesity specialist, I have gone from 0 minutes of physical activity in 2014 to becoming a fitness enthusiast who’s run more than 5,300 miles over 8 years. I know that as doctors and clinicians, we can profoundly influence our patients’ exercise journey.
Here are five tips to help motivate your patients make the change from “I Won’t Do It” to “I’m Doing It.”
Tip 1: ‘[Clinician], heal thyself’
Data don’t lie. Doctors who move more are more likely to counsel patients on exercise. I’ve been the doctor on both sides of the exercise spectrum. At my heaviest weight and lowest physical activity level, I felt hypocritical counseling patients on exercise.
If and when I counseled my patients on exercise, it was very directive and impersonal. When I started running consistently, I went to the opposite end of the spectrum. In my running zeal, it took a while for me to understand that not everyone wants to run dozens of miles a week. Shocking! Some people can’t handle intense workouts. The “I did it so you can too” perspective wasn’t helpful for long-term change in most patients.
What has been beneficial is recalling the obstacles and emotions I had (and still have) with staying consistent with physical activity. When physicians and clinicians move regularly, we’re more equipped to give our patients genuine counseling based on practicality rather than theory.
Now that self-reflection has been addressed, let’s get to patient counseling.
Tip 2: Motivate, don’t berate
Lectures on why patients should exercise are less helpful than asking, “Why aren›t you able to exercise more often?”
Asking open-ended questions is essential in motivational interviewing. Motivational interviewing promotes behavioral change through collaborative conversation.
Instead of telling the patient what to do, motivational interviewing seeks to establish a person’s why and create an effective plan based on their motivation. Asking open-ended questions is also helpful in determining any challenges to regular exercise, rather than calling these challenges “excuses,” which can be counterproductive.
I encourage patients to embrace challenges as opportunities for improvement. If they say: “I can’t find time to work out,” I suggest that they create time to work out by walking 10-15 minutes during lunch or after dinner. The information gleaned from open-ended questions helps set practical SMARTER goals, which we will discuss next.
Tip 3: Set SMARTER goals
After assessing the patient’s motivation and barriers, use this information to transform their desire to change into an actionable plan through a SMARTER goal. SMARTER stands for Specific, Measurable, Attainable, Relevant, Time-Sensitive, Enjoyable, and Rewarding. Practical goals have each of these components. That’s why “Just Do It” or even “Exercise 150 minutes a week” isn’t a clear path for actionable change. SMARTER goals go beyond what to do and help people personalize how to change.
For example, the SMARTER version of “exercise 150 minutes a week” for a busy person who works 50 hours a week may look like this: “My goal is to incorporate 150 minutes of physical activity through 60 minutes of aerobic exercise Monday through Friday (20-minute lunch walks) and 90 minutes of combination resistance training on the weekend (two 45-minute sessions) while listening to my favorite music. To meet my goal, I will reward myself by calling a friend to catch up or buy myself a new workout outfit.”
Exercise prescriptions are another helpful way to empower patients with a realistic exercise strategy. In my practice, I developed my own exercise prescription which focuses on overcoming time barriers to exercise and finding personally enjoyable exercises. To enhance self-directed physical activity, I›ve found it useful to have patients complete part of the “exercise prescription” on their own before or after their visit.
Tip 4: Use accountability tools
Making a SMARTER goal is one thing, but sticking with it takes regular reinforcement. Even with the best plan, once patients leave the office, there are many distractions from their goals. Accountability is the secret sauce to cultivating consistency. Fitness trackers are an affordable form of accountability. Studies show that wearing a fitness tracker can help people get up to 40 minutes of extra walking, compared with people who don’t wear trackers.
Additionally, clinicians can use different ways to offer exercise accountability. For example, more frequent check-ins, individually or in groups, can be helpful. The increase in telehealth has made interval visits easier. Reimbursement and time can limit clinician-level accountability, however. Other options are referring patients to online support groups or programs sponsored by the government or organizations. For years, I coled a Walk With a Doc chapter in Richmond, Va. There are chapters throughout the country.
Tip 5: Prepare and PLAN for setbacks
Breaking news: Most plans don’t go quite as envisioned. Accounting for the potential of setbacks early on helps patients set realistic expectations. As physicians and clinicians, we can help our patients anticipate a few likely obstacles. This may lessen the impact when a setback occurs. Also, it’s helpful to have the patient prepare for a setback with a PLAN for recovering quickly. PLAN stands for Ponder what happened; Learn from it; Adjust the original goal; Now get back on track. Getting back on track as soon as possible is important to keep patients motivated and prevent muscle deconditioning.
Exercise is medicine. Physical inactivity is a leading contributor to many preventable diseases. Although the physical activity statistics are disappointing, improvement is possible. Many systemic changes are needed to increase physical activity on a population level.
While waiting for more extensive changes, we have the power to equip patients with personalized, actionable tools for improving and maintaining physical activity.
We can transform one person at a time through our clinical encounters. Let’s use effective tools to help patients shift from “I Won’t Do It” to “I’m Doing It.”
Sylvia Gonsahn-Bollie, MD, DipABOM, is an integrative obesity specialist focused on individualized solutions for emotional and biological overeating. Her bestselling book, “Embrace You: Your Guide to Transforming Weight Loss Misconceptions Into Lifelong Wellness,” was Healthline.com’s Best Overall Weight Loss Book of 2022 and one of Livestrong.com’s 8 Best Weight-Loss Books to Read in 2022. She reported no conflicts of interest.
A version of this article first appeared on Medscape.com.
“Just Do It” is a cute marketing slogan. But let’s face it: Clinically, it doesn’t work well. Most people just don’t exercise. according to recent data from the Centers for Disease Control and Prevention.
Furthermore, when surveyed about aerobic exercise and strength training, only 24.6% meet these weekly recommendations. These low rates of physical activity are alarming, given the immense benefits of exercise in improving mental and physical health and well-being.
Many people know that exercise is good for them but struggle to go workout consistently. I know firsthand how challenging this can be. In addition to being an integrative obesity specialist, I have gone from 0 minutes of physical activity in 2014 to becoming a fitness enthusiast who’s run more than 5,300 miles over 8 years. I know that as doctors and clinicians, we can profoundly influence our patients’ exercise journey.
Here are five tips to help motivate your patients make the change from “I Won’t Do It” to “I’m Doing It.”
Tip 1: ‘[Clinician], heal thyself’
Data don’t lie. Doctors who move more are more likely to counsel patients on exercise. I’ve been the doctor on both sides of the exercise spectrum. At my heaviest weight and lowest physical activity level, I felt hypocritical counseling patients on exercise.
If and when I counseled my patients on exercise, it was very directive and impersonal. When I started running consistently, I went to the opposite end of the spectrum. In my running zeal, it took a while for me to understand that not everyone wants to run dozens of miles a week. Shocking! Some people can’t handle intense workouts. The “I did it so you can too” perspective wasn’t helpful for long-term change in most patients.
What has been beneficial is recalling the obstacles and emotions I had (and still have) with staying consistent with physical activity. When physicians and clinicians move regularly, we’re more equipped to give our patients genuine counseling based on practicality rather than theory.
Now that self-reflection has been addressed, let’s get to patient counseling.
Tip 2: Motivate, don’t berate
Lectures on why patients should exercise are less helpful than asking, “Why aren›t you able to exercise more often?”
Asking open-ended questions is essential in motivational interviewing. Motivational interviewing promotes behavioral change through collaborative conversation.
Instead of telling the patient what to do, motivational interviewing seeks to establish a person’s why and create an effective plan based on their motivation. Asking open-ended questions is also helpful in determining any challenges to regular exercise, rather than calling these challenges “excuses,” which can be counterproductive.
I encourage patients to embrace challenges as opportunities for improvement. If they say: “I can’t find time to work out,” I suggest that they create time to work out by walking 10-15 minutes during lunch or after dinner. The information gleaned from open-ended questions helps set practical SMARTER goals, which we will discuss next.
Tip 3: Set SMARTER goals
After assessing the patient’s motivation and barriers, use this information to transform their desire to change into an actionable plan through a SMARTER goal. SMARTER stands for Specific, Measurable, Attainable, Relevant, Time-Sensitive, Enjoyable, and Rewarding. Practical goals have each of these components. That’s why “Just Do It” or even “Exercise 150 minutes a week” isn’t a clear path for actionable change. SMARTER goals go beyond what to do and help people personalize how to change.
For example, the SMARTER version of “exercise 150 minutes a week” for a busy person who works 50 hours a week may look like this: “My goal is to incorporate 150 minutes of physical activity through 60 minutes of aerobic exercise Monday through Friday (20-minute lunch walks) and 90 minutes of combination resistance training on the weekend (two 45-minute sessions) while listening to my favorite music. To meet my goal, I will reward myself by calling a friend to catch up or buy myself a new workout outfit.”
Exercise prescriptions are another helpful way to empower patients with a realistic exercise strategy. In my practice, I developed my own exercise prescription which focuses on overcoming time barriers to exercise and finding personally enjoyable exercises. To enhance self-directed physical activity, I›ve found it useful to have patients complete part of the “exercise prescription” on their own before or after their visit.
Tip 4: Use accountability tools
Making a SMARTER goal is one thing, but sticking with it takes regular reinforcement. Even with the best plan, once patients leave the office, there are many distractions from their goals. Accountability is the secret sauce to cultivating consistency. Fitness trackers are an affordable form of accountability. Studies show that wearing a fitness tracker can help people get up to 40 minutes of extra walking, compared with people who don’t wear trackers.
Additionally, clinicians can use different ways to offer exercise accountability. For example, more frequent check-ins, individually or in groups, can be helpful. The increase in telehealth has made interval visits easier. Reimbursement and time can limit clinician-level accountability, however. Other options are referring patients to online support groups or programs sponsored by the government or organizations. For years, I coled a Walk With a Doc chapter in Richmond, Va. There are chapters throughout the country.
Tip 5: Prepare and PLAN for setbacks
Breaking news: Most plans don’t go quite as envisioned. Accounting for the potential of setbacks early on helps patients set realistic expectations. As physicians and clinicians, we can help our patients anticipate a few likely obstacles. This may lessen the impact when a setback occurs. Also, it’s helpful to have the patient prepare for a setback with a PLAN for recovering quickly. PLAN stands for Ponder what happened; Learn from it; Adjust the original goal; Now get back on track. Getting back on track as soon as possible is important to keep patients motivated and prevent muscle deconditioning.
Exercise is medicine. Physical inactivity is a leading contributor to many preventable diseases. Although the physical activity statistics are disappointing, improvement is possible. Many systemic changes are needed to increase physical activity on a population level.
While waiting for more extensive changes, we have the power to equip patients with personalized, actionable tools for improving and maintaining physical activity.
We can transform one person at a time through our clinical encounters. Let’s use effective tools to help patients shift from “I Won’t Do It” to “I’m Doing It.”
Sylvia Gonsahn-Bollie, MD, DipABOM, is an integrative obesity specialist focused on individualized solutions for emotional and biological overeating. Her bestselling book, “Embrace You: Your Guide to Transforming Weight Loss Misconceptions Into Lifelong Wellness,” was Healthline.com’s Best Overall Weight Loss Book of 2022 and one of Livestrong.com’s 8 Best Weight-Loss Books to Read in 2022. She reported no conflicts of interest.
A version of this article first appeared on Medscape.com.
“Just Do It” is a cute marketing slogan. But let’s face it: Clinically, it doesn’t work well. Most people just don’t exercise. according to recent data from the Centers for Disease Control and Prevention.
Furthermore, when surveyed about aerobic exercise and strength training, only 24.6% meet these weekly recommendations. These low rates of physical activity are alarming, given the immense benefits of exercise in improving mental and physical health and well-being.
Many people know that exercise is good for them but struggle to go workout consistently. I know firsthand how challenging this can be. In addition to being an integrative obesity specialist, I have gone from 0 minutes of physical activity in 2014 to becoming a fitness enthusiast who’s run more than 5,300 miles over 8 years. I know that as doctors and clinicians, we can profoundly influence our patients’ exercise journey.
Here are five tips to help motivate your patients make the change from “I Won’t Do It” to “I’m Doing It.”
Tip 1: ‘[Clinician], heal thyself’
Data don’t lie. Doctors who move more are more likely to counsel patients on exercise. I’ve been the doctor on both sides of the exercise spectrum. At my heaviest weight and lowest physical activity level, I felt hypocritical counseling patients on exercise.
If and when I counseled my patients on exercise, it was very directive and impersonal. When I started running consistently, I went to the opposite end of the spectrum. In my running zeal, it took a while for me to understand that not everyone wants to run dozens of miles a week. Shocking! Some people can’t handle intense workouts. The “I did it so you can too” perspective wasn’t helpful for long-term change in most patients.
What has been beneficial is recalling the obstacles and emotions I had (and still have) with staying consistent with physical activity. When physicians and clinicians move regularly, we’re more equipped to give our patients genuine counseling based on practicality rather than theory.
Now that self-reflection has been addressed, let’s get to patient counseling.
Tip 2: Motivate, don’t berate
Lectures on why patients should exercise are less helpful than asking, “Why aren›t you able to exercise more often?”
Asking open-ended questions is essential in motivational interviewing. Motivational interviewing promotes behavioral change through collaborative conversation.
Instead of telling the patient what to do, motivational interviewing seeks to establish a person’s why and create an effective plan based on their motivation. Asking open-ended questions is also helpful in determining any challenges to regular exercise, rather than calling these challenges “excuses,” which can be counterproductive.
I encourage patients to embrace challenges as opportunities for improvement. If they say: “I can’t find time to work out,” I suggest that they create time to work out by walking 10-15 minutes during lunch or after dinner. The information gleaned from open-ended questions helps set practical SMARTER goals, which we will discuss next.
Tip 3: Set SMARTER goals
After assessing the patient’s motivation and barriers, use this information to transform their desire to change into an actionable plan through a SMARTER goal. SMARTER stands for Specific, Measurable, Attainable, Relevant, Time-Sensitive, Enjoyable, and Rewarding. Practical goals have each of these components. That’s why “Just Do It” or even “Exercise 150 minutes a week” isn’t a clear path for actionable change. SMARTER goals go beyond what to do and help people personalize how to change.
For example, the SMARTER version of “exercise 150 minutes a week” for a busy person who works 50 hours a week may look like this: “My goal is to incorporate 150 minutes of physical activity through 60 minutes of aerobic exercise Monday through Friday (20-minute lunch walks) and 90 minutes of combination resistance training on the weekend (two 45-minute sessions) while listening to my favorite music. To meet my goal, I will reward myself by calling a friend to catch up or buy myself a new workout outfit.”
Exercise prescriptions are another helpful way to empower patients with a realistic exercise strategy. In my practice, I developed my own exercise prescription which focuses on overcoming time barriers to exercise and finding personally enjoyable exercises. To enhance self-directed physical activity, I›ve found it useful to have patients complete part of the “exercise prescription” on their own before or after their visit.
Tip 4: Use accountability tools
Making a SMARTER goal is one thing, but sticking with it takes regular reinforcement. Even with the best plan, once patients leave the office, there are many distractions from their goals. Accountability is the secret sauce to cultivating consistency. Fitness trackers are an affordable form of accountability. Studies show that wearing a fitness tracker can help people get up to 40 minutes of extra walking, compared with people who don’t wear trackers.
Additionally, clinicians can use different ways to offer exercise accountability. For example, more frequent check-ins, individually or in groups, can be helpful. The increase in telehealth has made interval visits easier. Reimbursement and time can limit clinician-level accountability, however. Other options are referring patients to online support groups or programs sponsored by the government or organizations. For years, I coled a Walk With a Doc chapter in Richmond, Va. There are chapters throughout the country.
Tip 5: Prepare and PLAN for setbacks
Breaking news: Most plans don’t go quite as envisioned. Accounting for the potential of setbacks early on helps patients set realistic expectations. As physicians and clinicians, we can help our patients anticipate a few likely obstacles. This may lessen the impact when a setback occurs. Also, it’s helpful to have the patient prepare for a setback with a PLAN for recovering quickly. PLAN stands for Ponder what happened; Learn from it; Adjust the original goal; Now get back on track. Getting back on track as soon as possible is important to keep patients motivated and prevent muscle deconditioning.
Exercise is medicine. Physical inactivity is a leading contributor to many preventable diseases. Although the physical activity statistics are disappointing, improvement is possible. Many systemic changes are needed to increase physical activity on a population level.
While waiting for more extensive changes, we have the power to equip patients with personalized, actionable tools for improving and maintaining physical activity.
We can transform one person at a time through our clinical encounters. Let’s use effective tools to help patients shift from “I Won’t Do It” to “I’m Doing It.”
Sylvia Gonsahn-Bollie, MD, DipABOM, is an integrative obesity specialist focused on individualized solutions for emotional and biological overeating. Her bestselling book, “Embrace You: Your Guide to Transforming Weight Loss Misconceptions Into Lifelong Wellness,” was Healthline.com’s Best Overall Weight Loss Book of 2022 and one of Livestrong.com’s 8 Best Weight-Loss Books to Read in 2022. She reported no conflicts of interest.
A version of this article first appeared on Medscape.com.
Presurgical expectations may influence patients’ attitudes, experiences after knee replacement
DENVER – People with lower expectations of how they would be able to use their knees during work activities after a total knee arthroplasty were more dissatisfied with their knee abilities 6 months after their surgery, according to a study presented at the OARSI 2023 World Congress.
Two out of 10 patients are dissatisfied after total knee arthroplasty, which is increasingly performed in younger and working patients who may have higher demands, presenter Yvonne van Zaanen, a physiotherapist in occupational health and ergonomics and a PhD candidate at Amsterdam University Medical Center, told attendees.
The findings suggest a correlation between patients’ low presurgical expectations of their ability to use their knees and having more difficulty with their knees postoperatively, she said. “We should take better care of working patients with low expectations by managing their preoperative expectations and improving their ability to perform work-related knee-straining activities in rehabilitation,” Ms. van Zaanen told attendees.
The researchers conducted a multicenter, prospective cohort study involving seven hospitals. They surveyed 175 employed individuals aged 18-65 years who were scheduled for a total knee arthroplasty and intended to return to work after their surgery. The first survey occurred before the operation, and the follow-up occurred 6 months after the surgery.
Just over half the participants were women (53%), and the average participant age was 59. Respondents had a mean body mass index (BMI) of 29 kg/m2, and had a Knee injury and Osteoarthritis Outcome Score (KOOS) pain score of 42 (on a 0-to-100 scale in which lower scores are worse). About half the respondents (51%) had a job that involved knee-straining activities.
The researchers assessed participants’ ability to perform work-related, knee-straining activities using the Work, Osteoarthritis, or joint-Replacement Questionnaire (WORQ) tool, which considers the following activities: kneeling, crouching, clambering, taking the stairs, walking on rough terrain, working with hands below knee height, standing, lifting or carrying, pushing or pulling, walking on ground level, operating a vehicle, operating foot pedals, and sitting. The 0-to-100 scale rates the difficulty of using knees for each particular activity, with higher scores indicating greater ease and less pain in doing that activity.
Among the 107 patients who expected to be satisfied after their surgery, half (n = 53) were satisfied, compared with 12% (n = 13) who were unsatisfied; the remaining participants (n = 41, 38%) were neither satisfied nor dissatisfied. Among the 24 patients who expected to be dissatisfied after their surgery, one-third (n = 8) were satisfied and 42% (n = 10) were dissatisfied. The remaining 44 patients didn’t expect to be satisfied or dissatisfied before their surgery, and 41% of them were satisfied while 23% were dissatisfied.
The researchers found that patients’ expectation of their satisfaction level going into the surgery was the only preoperative factor to be prognostic for dissatisfaction 6 months after surgery, based on their WORQ score. That is, patients who expected to be dissatisfied before their surgery had approximately five times greater odds of being dissatisfied after their surgery than did those who expected to be satisfied with their ability to do knee-straining activities at work (odds ratio, 5.1; 95% confidence interval, 1.7-15.5). Among those with a WORQ score of 40, indicating a greater expectation of difficulty using their knees postoperatively, 55% were dissatisfied after their surgery, compared with 19% of those with a WORQ score of 85, who expected greater knee ability after their surgery.
The other factors that the researchers examined, which had no effect on WORQ scores, included age, sex, BMI, education, comorbidities, KOOS pain subscale, having a knee-straining job, having needed surgery because of work, or having preoperative sick leave.
One discussion prompted by the presentation focused specifically on individuals’ ability to kneel without much difficulty after their surgery, an activity that’s not typically considered likely, Ms. van Zaanen noted. One audience member, Gillian Hawker, MD, MSc, a professor of medicine in the division of rheumatology at the University of Toronto, questioned whether the field should accept that current reality from surgical intervention. Dr. Hawker described a cohort she had analyzed in which two-thirds of the participants had expected they would be able to kneel after their surgery, regardless of whether it was related to work or other activities.
“Kneeling is important, not just for work; it’s important for culture and religion and lots of other things,” Dr. Hawker said. “How will you help these people to kneel after knee replacement when the surgery isn’t really performed to enable people to do that?” In response, Ms. van Zaanen noted it might not be achievable, as the research literature demonstrates, but Dr. Hawker suggested that is itself problematic.
“I guess what I’m asking is, why are we settling for that? If it’s important to so many people, and an expectation of so many people, why don’t we technologically improve such that, post arthroplasty, people can kneel?”
Another commenter suggested that the study’s findings may not indicate a need to manage patients’ expectations prior to surgery so much as showing that some patients simply have realistic expectations of what they will and will not be able to do after knee replacement.
“Is it possible that people who had low expectations – those who expected to be dissatisfied afterwards – were appropriately understanding that they were likely to be dissatisfied afterwards, in which case, managing their expectations might do nothing for their dissatisfaction afterwards?” the commenter asked. It is likely necessary to conduct additional research about expectations before surgery and experiences after surgery to address that question, Ms. van Zaanen suggested.
Ms. van Zaanen and Dr. Hawker reported having no relevant financial relationships. The presentation did not note any external funding. The Congress was sponsored by the Osteoarthritis Research Society International.
DENVER – People with lower expectations of how they would be able to use their knees during work activities after a total knee arthroplasty were more dissatisfied with their knee abilities 6 months after their surgery, according to a study presented at the OARSI 2023 World Congress.
Two out of 10 patients are dissatisfied after total knee arthroplasty, which is increasingly performed in younger and working patients who may have higher demands, presenter Yvonne van Zaanen, a physiotherapist in occupational health and ergonomics and a PhD candidate at Amsterdam University Medical Center, told attendees.
The findings suggest a correlation between patients’ low presurgical expectations of their ability to use their knees and having more difficulty with their knees postoperatively, she said. “We should take better care of working patients with low expectations by managing their preoperative expectations and improving their ability to perform work-related knee-straining activities in rehabilitation,” Ms. van Zaanen told attendees.
The researchers conducted a multicenter, prospective cohort study involving seven hospitals. They surveyed 175 employed individuals aged 18-65 years who were scheduled for a total knee arthroplasty and intended to return to work after their surgery. The first survey occurred before the operation, and the follow-up occurred 6 months after the surgery.
Just over half the participants were women (53%), and the average participant age was 59. Respondents had a mean body mass index (BMI) of 29 kg/m2, and had a Knee injury and Osteoarthritis Outcome Score (KOOS) pain score of 42 (on a 0-to-100 scale in which lower scores are worse). About half the respondents (51%) had a job that involved knee-straining activities.
The researchers assessed participants’ ability to perform work-related, knee-straining activities using the Work, Osteoarthritis, or joint-Replacement Questionnaire (WORQ) tool, which considers the following activities: kneeling, crouching, clambering, taking the stairs, walking on rough terrain, working with hands below knee height, standing, lifting or carrying, pushing or pulling, walking on ground level, operating a vehicle, operating foot pedals, and sitting. The 0-to-100 scale rates the difficulty of using knees for each particular activity, with higher scores indicating greater ease and less pain in doing that activity.
Among the 107 patients who expected to be satisfied after their surgery, half (n = 53) were satisfied, compared with 12% (n = 13) who were unsatisfied; the remaining participants (n = 41, 38%) were neither satisfied nor dissatisfied. Among the 24 patients who expected to be dissatisfied after their surgery, one-third (n = 8) were satisfied and 42% (n = 10) were dissatisfied. The remaining 44 patients didn’t expect to be satisfied or dissatisfied before their surgery, and 41% of them were satisfied while 23% were dissatisfied.
The researchers found that patients’ expectation of their satisfaction level going into the surgery was the only preoperative factor to be prognostic for dissatisfaction 6 months after surgery, based on their WORQ score. That is, patients who expected to be dissatisfied before their surgery had approximately five times greater odds of being dissatisfied after their surgery than did those who expected to be satisfied with their ability to do knee-straining activities at work (odds ratio, 5.1; 95% confidence interval, 1.7-15.5). Among those with a WORQ score of 40, indicating a greater expectation of difficulty using their knees postoperatively, 55% were dissatisfied after their surgery, compared with 19% of those with a WORQ score of 85, who expected greater knee ability after their surgery.
The other factors that the researchers examined, which had no effect on WORQ scores, included age, sex, BMI, education, comorbidities, KOOS pain subscale, having a knee-straining job, having needed surgery because of work, or having preoperative sick leave.
One discussion prompted by the presentation focused specifically on individuals’ ability to kneel without much difficulty after their surgery, an activity that’s not typically considered likely, Ms. van Zaanen noted. One audience member, Gillian Hawker, MD, MSc, a professor of medicine in the division of rheumatology at the University of Toronto, questioned whether the field should accept that current reality from surgical intervention. Dr. Hawker described a cohort she had analyzed in which two-thirds of the participants had expected they would be able to kneel after their surgery, regardless of whether it was related to work or other activities.
“Kneeling is important, not just for work; it’s important for culture and religion and lots of other things,” Dr. Hawker said. “How will you help these people to kneel after knee replacement when the surgery isn’t really performed to enable people to do that?” In response, Ms. van Zaanen noted it might not be achievable, as the research literature demonstrates, but Dr. Hawker suggested that is itself problematic.
“I guess what I’m asking is, why are we settling for that? If it’s important to so many people, and an expectation of so many people, why don’t we technologically improve such that, post arthroplasty, people can kneel?”
Another commenter suggested that the study’s findings may not indicate a need to manage patients’ expectations prior to surgery so much as showing that some patients simply have realistic expectations of what they will and will not be able to do after knee replacement.
“Is it possible that people who had low expectations – those who expected to be dissatisfied afterwards – were appropriately understanding that they were likely to be dissatisfied afterwards, in which case, managing their expectations might do nothing for their dissatisfaction afterwards?” the commenter asked. It is likely necessary to conduct additional research about expectations before surgery and experiences after surgery to address that question, Ms. van Zaanen suggested.
Ms. van Zaanen and Dr. Hawker reported having no relevant financial relationships. The presentation did not note any external funding. The Congress was sponsored by the Osteoarthritis Research Society International.
DENVER – People with lower expectations of how they would be able to use their knees during work activities after a total knee arthroplasty were more dissatisfied with their knee abilities 6 months after their surgery, according to a study presented at the OARSI 2023 World Congress.
Two out of 10 patients are dissatisfied after total knee arthroplasty, which is increasingly performed in younger and working patients who may have higher demands, presenter Yvonne van Zaanen, a physiotherapist in occupational health and ergonomics and a PhD candidate at Amsterdam University Medical Center, told attendees.
The findings suggest a correlation between patients’ low presurgical expectations of their ability to use their knees and having more difficulty with their knees postoperatively, she said. “We should take better care of working patients with low expectations by managing their preoperative expectations and improving their ability to perform work-related knee-straining activities in rehabilitation,” Ms. van Zaanen told attendees.
The researchers conducted a multicenter, prospective cohort study involving seven hospitals. They surveyed 175 employed individuals aged 18-65 years who were scheduled for a total knee arthroplasty and intended to return to work after their surgery. The first survey occurred before the operation, and the follow-up occurred 6 months after the surgery.
Just over half the participants were women (53%), and the average participant age was 59. Respondents had a mean body mass index (BMI) of 29 kg/m2, and had a Knee injury and Osteoarthritis Outcome Score (KOOS) pain score of 42 (on a 0-to-100 scale in which lower scores are worse). About half the respondents (51%) had a job that involved knee-straining activities.
The researchers assessed participants’ ability to perform work-related, knee-straining activities using the Work, Osteoarthritis, or joint-Replacement Questionnaire (WORQ) tool, which considers the following activities: kneeling, crouching, clambering, taking the stairs, walking on rough terrain, working with hands below knee height, standing, lifting or carrying, pushing or pulling, walking on ground level, operating a vehicle, operating foot pedals, and sitting. The 0-to-100 scale rates the difficulty of using knees for each particular activity, with higher scores indicating greater ease and less pain in doing that activity.
Among the 107 patients who expected to be satisfied after their surgery, half (n = 53) were satisfied, compared with 12% (n = 13) who were unsatisfied; the remaining participants (n = 41, 38%) were neither satisfied nor dissatisfied. Among the 24 patients who expected to be dissatisfied after their surgery, one-third (n = 8) were satisfied and 42% (n = 10) were dissatisfied. The remaining 44 patients didn’t expect to be satisfied or dissatisfied before their surgery, and 41% of them were satisfied while 23% were dissatisfied.
The researchers found that patients’ expectation of their satisfaction level going into the surgery was the only preoperative factor to be prognostic for dissatisfaction 6 months after surgery, based on their WORQ score. That is, patients who expected to be dissatisfied before their surgery had approximately five times greater odds of being dissatisfied after their surgery than did those who expected to be satisfied with their ability to do knee-straining activities at work (odds ratio, 5.1; 95% confidence interval, 1.7-15.5). Among those with a WORQ score of 40, indicating a greater expectation of difficulty using their knees postoperatively, 55% were dissatisfied after their surgery, compared with 19% of those with a WORQ score of 85, who expected greater knee ability after their surgery.
The other factors that the researchers examined, which had no effect on WORQ scores, included age, sex, BMI, education, comorbidities, KOOS pain subscale, having a knee-straining job, having needed surgery because of work, or having preoperative sick leave.
One discussion prompted by the presentation focused specifically on individuals’ ability to kneel without much difficulty after their surgery, an activity that’s not typically considered likely, Ms. van Zaanen noted. One audience member, Gillian Hawker, MD, MSc, a professor of medicine in the division of rheumatology at the University of Toronto, questioned whether the field should accept that current reality from surgical intervention. Dr. Hawker described a cohort she had analyzed in which two-thirds of the participants had expected they would be able to kneel after their surgery, regardless of whether it was related to work or other activities.
“Kneeling is important, not just for work; it’s important for culture and religion and lots of other things,” Dr. Hawker said. “How will you help these people to kneel after knee replacement when the surgery isn’t really performed to enable people to do that?” In response, Ms. van Zaanen noted it might not be achievable, as the research literature demonstrates, but Dr. Hawker suggested that is itself problematic.
“I guess what I’m asking is, why are we settling for that? If it’s important to so many people, and an expectation of so many people, why don’t we technologically improve such that, post arthroplasty, people can kneel?”
Another commenter suggested that the study’s findings may not indicate a need to manage patients’ expectations prior to surgery so much as showing that some patients simply have realistic expectations of what they will and will not be able to do after knee replacement.
“Is it possible that people who had low expectations – those who expected to be dissatisfied afterwards – were appropriately understanding that they were likely to be dissatisfied afterwards, in which case, managing their expectations might do nothing for their dissatisfaction afterwards?” the commenter asked. It is likely necessary to conduct additional research about expectations before surgery and experiences after surgery to address that question, Ms. van Zaanen suggested.
Ms. van Zaanen and Dr. Hawker reported having no relevant financial relationships. The presentation did not note any external funding. The Congress was sponsored by the Osteoarthritis Research Society International.
AT OARSI 2023