User login
Surgery for early breast cancer can worsen frailty in older women
according to a new study.
About 1 in 5 experienced clinically significant deterioration in frailty status after treatment, the study team found. Women at highest risk for declines in frailty following treatment had “robust” baseline frailty status at diagnosis and underwent more invasive mastectomy compared with lumpectomy.
The fact that “robust” older women were more likely to become frail after locoregional therapy suggests that “thoughtful treatment decisions should be undertaken in all older women, not simply those who have frailty at diagnosis,” said the investigators, led by Christina Minami, MD, of Dana-Farber/Brigham and Women’s Cancer Center in Boston.
The study findings emphasize that there is no one-size-fits-all approach to breast cancer treatment in the elderly, said Sarah P. Cate, MD, director, Breast Surgery Quality Program, Mount Sinai Health System, New York, who wasn’t involved in the research. “Some patients will sail through a surgery, and others are severely affected by it.”
The study was published online in JAMA Surgery.
Given the growing number of older adults with breast cancer, understanding how age-related syndromes, such as frailty, may alter cancer outcomes and how cancer treatments change aging trajectories remains important.
To investigate, Dr. Minami and colleagues used Surveillance, Epidemiology, and End Results Medicare data to identify 31,084 women (mean age, 73) who had been diagnosed with ductal carcinoma in situ (DCIS) or stage I HR-positive, ERBB2-positive breast cancer and who underwent surgery (23% mastectomy, 77% lumpectomy) and radiation therapy.
Worsening frailty status was defined as a decline of 0.03 or greater in a validated frailty index from the time of diagnosis to 1 year. This level of change has been linked to greater mortality risk and greater cost of care.
Frailty status at diagnosis was “robust” in 56% of the women, prefrail in 40%, mildly frail in 4%, and moderately to severely frail in 0.3%.
According to the researchers, 21.4% of the women experienced clinically significant declines in their frailty status after treatment. These declines occurred in 25% of women who underwent mastectomy and 20% of those who underwent lumpectomy.
After adjusting for covariates, there was a higher likelihood of worsening frailty among women who were robustly frail at baseline, in comparison with those who were moderately to severely frail at baseline (odds ratio, 6.12), and in those who underwent mastectomy vs. lumpectomy (OR, 1.31).
Older age and race were also linked to worsening frailty status following treatment. Compared with younger women (aged 65-74 years), older women were more likely to experience worsening frailty (OR, 1.21 for women aged 75-79; OR, 1.53 for those aged 80-84; OR, 1.94 for those aged 85 and older). In addition, Black women were more likely than non-Hispanic White women to experience worsening frailty after treatment (OR, 1.12).
“Previous studies have documented lasting declines in functional status after surgery in older patients with breast cancer, but breast cancer treatment has not been implicated in worsening frailty to date,” Dr. Minami and colleagues explain. But “given the substantial proportion of women experiencing worsening frailty and the significant difference by breast surgery type, frailty status as a cancer therapy outcome should be further explored.” In addition, “tailoring locoregional therapy intensity in this population is important,” they write.
Dr. Cate explained that randomized clinical trials such as COMET and LORIS, which explore the monitoring of patients with DCIS in lieu of active treatment, “will likely make a big impact on this population, as we currently do not have randomized controlled data for observation of breast cancer.”
Dr. Cate added as well that assessing a patient’s ECOG [Eastern Cooperative Oncology Group] performance status is vital “to determine who can really tolerate a breast cancer surgery” and that opting for antiestrogens, such as aromatase inhibitors, which can keep cancer at bay for years, “may be preferable for many older patients.”
The study was funded by Brigham and Women’s Hospital’s Department of Surgery’s Beal Fellowship. Dr. Minami and Dr. Cate have disclosed no relevant financial relationships.
A version of this article first appeared on Medscape.com.
according to a new study.
About 1 in 5 experienced clinically significant deterioration in frailty status after treatment, the study team found. Women at highest risk for declines in frailty following treatment had “robust” baseline frailty status at diagnosis and underwent more invasive mastectomy compared with lumpectomy.
The fact that “robust” older women were more likely to become frail after locoregional therapy suggests that “thoughtful treatment decisions should be undertaken in all older women, not simply those who have frailty at diagnosis,” said the investigators, led by Christina Minami, MD, of Dana-Farber/Brigham and Women’s Cancer Center in Boston.
The study findings emphasize that there is no one-size-fits-all approach to breast cancer treatment in the elderly, said Sarah P. Cate, MD, director, Breast Surgery Quality Program, Mount Sinai Health System, New York, who wasn’t involved in the research. “Some patients will sail through a surgery, and others are severely affected by it.”
The study was published online in JAMA Surgery.
Given the growing number of older adults with breast cancer, understanding how age-related syndromes, such as frailty, may alter cancer outcomes and how cancer treatments change aging trajectories remains important.
To investigate, Dr. Minami and colleagues used Surveillance, Epidemiology, and End Results Medicare data to identify 31,084 women (mean age, 73) who had been diagnosed with ductal carcinoma in situ (DCIS) or stage I HR-positive, ERBB2-positive breast cancer and who underwent surgery (23% mastectomy, 77% lumpectomy) and radiation therapy.
Worsening frailty status was defined as a decline of 0.03 or greater in a validated frailty index from the time of diagnosis to 1 year. This level of change has been linked to greater mortality risk and greater cost of care.
Frailty status at diagnosis was “robust” in 56% of the women, prefrail in 40%, mildly frail in 4%, and moderately to severely frail in 0.3%.
According to the researchers, 21.4% of the women experienced clinically significant declines in their frailty status after treatment. These declines occurred in 25% of women who underwent mastectomy and 20% of those who underwent lumpectomy.
After adjusting for covariates, there was a higher likelihood of worsening frailty among women who were robustly frail at baseline, in comparison with those who were moderately to severely frail at baseline (odds ratio, 6.12), and in those who underwent mastectomy vs. lumpectomy (OR, 1.31).
Older age and race were also linked to worsening frailty status following treatment. Compared with younger women (aged 65-74 years), older women were more likely to experience worsening frailty (OR, 1.21 for women aged 75-79; OR, 1.53 for those aged 80-84; OR, 1.94 for those aged 85 and older). In addition, Black women were more likely than non-Hispanic White women to experience worsening frailty after treatment (OR, 1.12).
“Previous studies have documented lasting declines in functional status after surgery in older patients with breast cancer, but breast cancer treatment has not been implicated in worsening frailty to date,” Dr. Minami and colleagues explain. But “given the substantial proportion of women experiencing worsening frailty and the significant difference by breast surgery type, frailty status as a cancer therapy outcome should be further explored.” In addition, “tailoring locoregional therapy intensity in this population is important,” they write.
Dr. Cate explained that randomized clinical trials such as COMET and LORIS, which explore the monitoring of patients with DCIS in lieu of active treatment, “will likely make a big impact on this population, as we currently do not have randomized controlled data for observation of breast cancer.”
Dr. Cate added as well that assessing a patient’s ECOG [Eastern Cooperative Oncology Group] performance status is vital “to determine who can really tolerate a breast cancer surgery” and that opting for antiestrogens, such as aromatase inhibitors, which can keep cancer at bay for years, “may be preferable for many older patients.”
The study was funded by Brigham and Women’s Hospital’s Department of Surgery’s Beal Fellowship. Dr. Minami and Dr. Cate have disclosed no relevant financial relationships.
A version of this article first appeared on Medscape.com.
according to a new study.
About 1 in 5 experienced clinically significant deterioration in frailty status after treatment, the study team found. Women at highest risk for declines in frailty following treatment had “robust” baseline frailty status at diagnosis and underwent more invasive mastectomy compared with lumpectomy.
The fact that “robust” older women were more likely to become frail after locoregional therapy suggests that “thoughtful treatment decisions should be undertaken in all older women, not simply those who have frailty at diagnosis,” said the investigators, led by Christina Minami, MD, of Dana-Farber/Brigham and Women’s Cancer Center in Boston.
The study findings emphasize that there is no one-size-fits-all approach to breast cancer treatment in the elderly, said Sarah P. Cate, MD, director, Breast Surgery Quality Program, Mount Sinai Health System, New York, who wasn’t involved in the research. “Some patients will sail through a surgery, and others are severely affected by it.”
The study was published online in JAMA Surgery.
Given the growing number of older adults with breast cancer, understanding how age-related syndromes, such as frailty, may alter cancer outcomes and how cancer treatments change aging trajectories remains important.
To investigate, Dr. Minami and colleagues used Surveillance, Epidemiology, and End Results Medicare data to identify 31,084 women (mean age, 73) who had been diagnosed with ductal carcinoma in situ (DCIS) or stage I HR-positive, ERBB2-positive breast cancer and who underwent surgery (23% mastectomy, 77% lumpectomy) and radiation therapy.
Worsening frailty status was defined as a decline of 0.03 or greater in a validated frailty index from the time of diagnosis to 1 year. This level of change has been linked to greater mortality risk and greater cost of care.
Frailty status at diagnosis was “robust” in 56% of the women, prefrail in 40%, mildly frail in 4%, and moderately to severely frail in 0.3%.
According to the researchers, 21.4% of the women experienced clinically significant declines in their frailty status after treatment. These declines occurred in 25% of women who underwent mastectomy and 20% of those who underwent lumpectomy.
After adjusting for covariates, there was a higher likelihood of worsening frailty among women who were robustly frail at baseline, in comparison with those who were moderately to severely frail at baseline (odds ratio, 6.12), and in those who underwent mastectomy vs. lumpectomy (OR, 1.31).
Older age and race were also linked to worsening frailty status following treatment. Compared with younger women (aged 65-74 years), older women were more likely to experience worsening frailty (OR, 1.21 for women aged 75-79; OR, 1.53 for those aged 80-84; OR, 1.94 for those aged 85 and older). In addition, Black women were more likely than non-Hispanic White women to experience worsening frailty after treatment (OR, 1.12).
“Previous studies have documented lasting declines in functional status after surgery in older patients with breast cancer, but breast cancer treatment has not been implicated in worsening frailty to date,” Dr. Minami and colleagues explain. But “given the substantial proportion of women experiencing worsening frailty and the significant difference by breast surgery type, frailty status as a cancer therapy outcome should be further explored.” In addition, “tailoring locoregional therapy intensity in this population is important,” they write.
Dr. Cate explained that randomized clinical trials such as COMET and LORIS, which explore the monitoring of patients with DCIS in lieu of active treatment, “will likely make a big impact on this population, as we currently do not have randomized controlled data for observation of breast cancer.”
Dr. Cate added as well that assessing a patient’s ECOG [Eastern Cooperative Oncology Group] performance status is vital “to determine who can really tolerate a breast cancer surgery” and that opting for antiestrogens, such as aromatase inhibitors, which can keep cancer at bay for years, “may be preferable for many older patients.”
The study was funded by Brigham and Women’s Hospital’s Department of Surgery’s Beal Fellowship. Dr. Minami and Dr. Cate have disclosed no relevant financial relationships.
A version of this article first appeared on Medscape.com.
FROM JAMA SURGERY
Prostate cancer drug shortage leaves some with uncertainty
according to the Food and Drug Administration.
The therapy lutetium Lu 177 vipivotide tetraxetan (Pluvicto), approved in March 2022, will remain in limited supply until the drug’s manufacturer, Novartis, can ramp up production of the drug over the next 12 months.
In a letter in February, Novartis said it is giving priority to patients who have already started the regimen so they can “appropriately complete their course of therapy.” The manufacturer will not be taking any orders for new patients over the next 4-6 months, as they work to increase supply.
“We are operating our production site at full capacity to treat as many patients as possible, as quickly as possible,” Novartis said. “However, with a nuclear medicine like Pluvicto, there is no backup supply that we can draw from when we experience a delay.”
Pluvicto is currently made in small batches in the company’s manufacturing facility in Italy. The drug only has a 5-day window to reach its intended patient, after which time it cannot be used. Any disruption in the production or shipping process can create a delay.
Novartis said the facility in Italy is currently operating at full capacity and the company is “working to increase production capacity and supply” of the drug over the next 12 months at two new manufacturing sites in the United States.
The company also encountered supply problems with Pluvicto in 2022 after quality issues were discovered in the manufacturing process.
Currently, patients who are waiting for their first dose of Pluvicto will need to be rescheduled. The manufacturer will be reaching out to health care professionals with options for rescheduling.
Jonathan McConathy, MD, PhD, told The Wall Street Journal that “people will die from this shortage, for sure.”
Dr. McConathy, a radiologist at the University of Alabama at Birmingham who has consulted for Novartis, explained that some patients who would have benefited from the drug likely won’t receive it in time.
A version of this article first appeared on Medscape.com.
according to the Food and Drug Administration.
The therapy lutetium Lu 177 vipivotide tetraxetan (Pluvicto), approved in March 2022, will remain in limited supply until the drug’s manufacturer, Novartis, can ramp up production of the drug over the next 12 months.
In a letter in February, Novartis said it is giving priority to patients who have already started the regimen so they can “appropriately complete their course of therapy.” The manufacturer will not be taking any orders for new patients over the next 4-6 months, as they work to increase supply.
“We are operating our production site at full capacity to treat as many patients as possible, as quickly as possible,” Novartis said. “However, with a nuclear medicine like Pluvicto, there is no backup supply that we can draw from when we experience a delay.”
Pluvicto is currently made in small batches in the company’s manufacturing facility in Italy. The drug only has a 5-day window to reach its intended patient, after which time it cannot be used. Any disruption in the production or shipping process can create a delay.
Novartis said the facility in Italy is currently operating at full capacity and the company is “working to increase production capacity and supply” of the drug over the next 12 months at two new manufacturing sites in the United States.
The company also encountered supply problems with Pluvicto in 2022 after quality issues were discovered in the manufacturing process.
Currently, patients who are waiting for their first dose of Pluvicto will need to be rescheduled. The manufacturer will be reaching out to health care professionals with options for rescheduling.
Jonathan McConathy, MD, PhD, told The Wall Street Journal that “people will die from this shortage, for sure.”
Dr. McConathy, a radiologist at the University of Alabama at Birmingham who has consulted for Novartis, explained that some patients who would have benefited from the drug likely won’t receive it in time.
A version of this article first appeared on Medscape.com.
according to the Food and Drug Administration.
The therapy lutetium Lu 177 vipivotide tetraxetan (Pluvicto), approved in March 2022, will remain in limited supply until the drug’s manufacturer, Novartis, can ramp up production of the drug over the next 12 months.
In a letter in February, Novartis said it is giving priority to patients who have already started the regimen so they can “appropriately complete their course of therapy.” The manufacturer will not be taking any orders for new patients over the next 4-6 months, as they work to increase supply.
“We are operating our production site at full capacity to treat as many patients as possible, as quickly as possible,” Novartis said. “However, with a nuclear medicine like Pluvicto, there is no backup supply that we can draw from when we experience a delay.”
Pluvicto is currently made in small batches in the company’s manufacturing facility in Italy. The drug only has a 5-day window to reach its intended patient, after which time it cannot be used. Any disruption in the production or shipping process can create a delay.
Novartis said the facility in Italy is currently operating at full capacity and the company is “working to increase production capacity and supply” of the drug over the next 12 months at two new manufacturing sites in the United States.
The company also encountered supply problems with Pluvicto in 2022 after quality issues were discovered in the manufacturing process.
Currently, patients who are waiting for their first dose of Pluvicto will need to be rescheduled. The manufacturer will be reaching out to health care professionals with options for rescheduling.
Jonathan McConathy, MD, PhD, told The Wall Street Journal that “people will die from this shortage, for sure.”
Dr. McConathy, a radiologist at the University of Alabama at Birmingham who has consulted for Novartis, explained that some patients who would have benefited from the drug likely won’t receive it in time.
A version of this article first appeared on Medscape.com.
Restless legs a new modifiable risk factor for dementia?
suggesting the disorder may be a risk factor for dementia or a very early noncognitive sign of dementia, researchers say.
In a large population-based cohort study, adults with RLS were significantly more likely to develop dementia over more than a decade than were their peers without RLS.
If confirmed in future studies, “regular check-ups for cognitive decline in older patients with RLS may facilitate earlier detection and intervention for those with dementia risk,” wrote investigators led by Eosu Kim, MD, PhD, with Yonsei University, Seoul, Republic of Korea.
The study was published online in Alzheimer’s Research and Therapy.
Sleep disorders and dementia
RLS is associated with poor sleep, depression/anxiety, poor diet, microvasculopathy, and hypoxia – all of which are known risk factors for dementia. However, the relationship between RLS and incident dementia has been unclear.
The researchers compared risk for all-cause dementia, Alzheimer’s disease (AD), and vascular dementia (VaD) among 2,501 adults with newly diagnosed RLS and 9,977 matched control persons participating in the Korean National Health Insurance Service–Elderly Cohort, a nationwide population-based cohort of adults aged 60 and older.
The mean age of the cohort was 73 years; most of the participants were women (65%). Among all 12,478 participants, 874 (7%) developed all-cause dementia during follow-up – 475 (54%) developed AD, and 194 (22%) developed VaD.
The incidence of all-cause dementia was significantly higher among the RLS group than among the control group (10.4% vs. 6.2%). Incidence rates of AD and VaD (5.6% and 2.6%, respectively) were also higher in the RLS group than in the control group (3.4% and 1.3%, respectively).
In Cox regression analysis, RLS was significantly associated with an increased risk of all-cause dementia (adjusted hazard ratio [aHR], 1.46; 95% confidence interval [CI], 1.24-1.72), AD (aHR 1.38; 95% CI, 1.11-1.72) and VaD (aHR, 1.81; 95% CI, 1.30-2.53).
The researchers noted that RLS may precede deterioration of cognitive function, leading to dementia, and they suggest that RLS could be regarded as a “newly identified” risk factor or prodromal sign of dementia.
Modifiable risk factor
Reached for comment, Thanh Dang-Vu, MD, PhD, professor and research chair in sleep, neuroimaging, and cognitive health at Concordia University in Montreal, said there is now “increasing literature that shows sleep as a modifiable risk factor for cognitive decline.
“Previous evidence indicates that both sleep apnea and insomnia disorder increase the risk for cognitive decline and possibly dementia. Here the study adds to this body of evidence linking sleep disorders to dementia, suggesting that RLS should also be considered as a sleep-related risk factor,” Dr. Dang-Vu told this news organization.
“More evidence is needed, though, as here, all diagnoses were based on national health insurance diagnostic codes, and it is likely there were missed diagnoses for RLS but also for other sleep disorders, as there was no systematic screening for them,” Dr. Dang-Vu cautioned.
Support for the study was provided by the Ministry of Health and Welfare, the Korean government, and Yonsei University. Dr. Kim and Dr. Dang-Vu reported no relevant financial relationships.
A version of this article first appeared on Medscape.com.
suggesting the disorder may be a risk factor for dementia or a very early noncognitive sign of dementia, researchers say.
In a large population-based cohort study, adults with RLS were significantly more likely to develop dementia over more than a decade than were their peers without RLS.
If confirmed in future studies, “regular check-ups for cognitive decline in older patients with RLS may facilitate earlier detection and intervention for those with dementia risk,” wrote investigators led by Eosu Kim, MD, PhD, with Yonsei University, Seoul, Republic of Korea.
The study was published online in Alzheimer’s Research and Therapy.
Sleep disorders and dementia
RLS is associated with poor sleep, depression/anxiety, poor diet, microvasculopathy, and hypoxia – all of which are known risk factors for dementia. However, the relationship between RLS and incident dementia has been unclear.
The researchers compared risk for all-cause dementia, Alzheimer’s disease (AD), and vascular dementia (VaD) among 2,501 adults with newly diagnosed RLS and 9,977 matched control persons participating in the Korean National Health Insurance Service–Elderly Cohort, a nationwide population-based cohort of adults aged 60 and older.
The mean age of the cohort was 73 years; most of the participants were women (65%). Among all 12,478 participants, 874 (7%) developed all-cause dementia during follow-up – 475 (54%) developed AD, and 194 (22%) developed VaD.
The incidence of all-cause dementia was significantly higher among the RLS group than among the control group (10.4% vs. 6.2%). Incidence rates of AD and VaD (5.6% and 2.6%, respectively) were also higher in the RLS group than in the control group (3.4% and 1.3%, respectively).
In Cox regression analysis, RLS was significantly associated with an increased risk of all-cause dementia (adjusted hazard ratio [aHR], 1.46; 95% confidence interval [CI], 1.24-1.72), AD (aHR 1.38; 95% CI, 1.11-1.72) and VaD (aHR, 1.81; 95% CI, 1.30-2.53).
The researchers noted that RLS may precede deterioration of cognitive function, leading to dementia, and they suggest that RLS could be regarded as a “newly identified” risk factor or prodromal sign of dementia.
Modifiable risk factor
Reached for comment, Thanh Dang-Vu, MD, PhD, professor and research chair in sleep, neuroimaging, and cognitive health at Concordia University in Montreal, said there is now “increasing literature that shows sleep as a modifiable risk factor for cognitive decline.
“Previous evidence indicates that both sleep apnea and insomnia disorder increase the risk for cognitive decline and possibly dementia. Here the study adds to this body of evidence linking sleep disorders to dementia, suggesting that RLS should also be considered as a sleep-related risk factor,” Dr. Dang-Vu told this news organization.
“More evidence is needed, though, as here, all diagnoses were based on national health insurance diagnostic codes, and it is likely there were missed diagnoses for RLS but also for other sleep disorders, as there was no systematic screening for them,” Dr. Dang-Vu cautioned.
Support for the study was provided by the Ministry of Health and Welfare, the Korean government, and Yonsei University. Dr. Kim and Dr. Dang-Vu reported no relevant financial relationships.
A version of this article first appeared on Medscape.com.
suggesting the disorder may be a risk factor for dementia or a very early noncognitive sign of dementia, researchers say.
In a large population-based cohort study, adults with RLS were significantly more likely to develop dementia over more than a decade than were their peers without RLS.
If confirmed in future studies, “regular check-ups for cognitive decline in older patients with RLS may facilitate earlier detection and intervention for those with dementia risk,” wrote investigators led by Eosu Kim, MD, PhD, with Yonsei University, Seoul, Republic of Korea.
The study was published online in Alzheimer’s Research and Therapy.
Sleep disorders and dementia
RLS is associated with poor sleep, depression/anxiety, poor diet, microvasculopathy, and hypoxia – all of which are known risk factors for dementia. However, the relationship between RLS and incident dementia has been unclear.
The researchers compared risk for all-cause dementia, Alzheimer’s disease (AD), and vascular dementia (VaD) among 2,501 adults with newly diagnosed RLS and 9,977 matched control persons participating in the Korean National Health Insurance Service–Elderly Cohort, a nationwide population-based cohort of adults aged 60 and older.
The mean age of the cohort was 73 years; most of the participants were women (65%). Among all 12,478 participants, 874 (7%) developed all-cause dementia during follow-up – 475 (54%) developed AD, and 194 (22%) developed VaD.
The incidence of all-cause dementia was significantly higher among the RLS group than among the control group (10.4% vs. 6.2%). Incidence rates of AD and VaD (5.6% and 2.6%, respectively) were also higher in the RLS group than in the control group (3.4% and 1.3%, respectively).
In Cox regression analysis, RLS was significantly associated with an increased risk of all-cause dementia (adjusted hazard ratio [aHR], 1.46; 95% confidence interval [CI], 1.24-1.72), AD (aHR 1.38; 95% CI, 1.11-1.72) and VaD (aHR, 1.81; 95% CI, 1.30-2.53).
The researchers noted that RLS may precede deterioration of cognitive function, leading to dementia, and they suggest that RLS could be regarded as a “newly identified” risk factor or prodromal sign of dementia.
Modifiable risk factor
Reached for comment, Thanh Dang-Vu, MD, PhD, professor and research chair in sleep, neuroimaging, and cognitive health at Concordia University in Montreal, said there is now “increasing literature that shows sleep as a modifiable risk factor for cognitive decline.
“Previous evidence indicates that both sleep apnea and insomnia disorder increase the risk for cognitive decline and possibly dementia. Here the study adds to this body of evidence linking sleep disorders to dementia, suggesting that RLS should also be considered as a sleep-related risk factor,” Dr. Dang-Vu told this news organization.
“More evidence is needed, though, as here, all diagnoses were based on national health insurance diagnostic codes, and it is likely there were missed diagnoses for RLS but also for other sleep disorders, as there was no systematic screening for them,” Dr. Dang-Vu cautioned.
Support for the study was provided by the Ministry of Health and Welfare, the Korean government, and Yonsei University. Dr. Kim and Dr. Dang-Vu reported no relevant financial relationships.
A version of this article first appeared on Medscape.com.
FROM ALZHEIMER’S RESEARCH AND THERAPY
Cases of potentially deadly fungus jump 200%: CDC
prompting the Centers for Disease Control and Prevention to issue a warning to health care facilities about the rising threat.
C. auris is a yeast that spreads easily from touching it on a surface like a countertop. It can also spread from person to person. It isn’t a threat to healthy people, but people in hospitals and nursing homes are at a heightened risk because they might have weakened immune systems or be using invasive medical devices that can introduce the fungus inside their bodies. When C. auris progresses to causing an infection that reaches the brain, blood, or lungs, more than one in three people die.
The worrying increase was detailed in the journal Annals of Internal Medicine. In 2021, cases reached a count of 3,270 with an active infection, and 7,413 cases showed the fungus was present but hadn’t caused an infection. Infection counts were up 95% over the previous year, and the fungus showed up on screenings three times as often. The number of cases resistant to medication also tripled.
The CDC called the figures “alarming,” noting that the fungus was only detected in the United States in 2016.
“The timing of this increase and findings from public health investigations suggest C. auris spread may have worsened due to strain on health care and public health systems during the COVID-19 pandemic,” the CDC explained in a news release.
Another potential reason for the jump could be that screening for C. auris has simply increased and it’s being found more often because it’s being looked for more often. But researchers believe that, even with the increase in testing, the reported counts are underestimated. That’s because even though screening has increased, health care providers still aren’t looking for the presence of the fungus as often as the CDC would like.
“The rapid rise and geographic spread of cases is concerning and emphasizes the need for continued surveillance, expanded lab capacity, quicker diagnostic tests, and adherence to proven infection prevention and control,” said study author Meghan Lyman, MD, a CDC epidemiologist in Atlanta, in a statement.
Cases of C. auris continued to rise in 2022, the CDC said. A map on the agency’s website of reported cases from 2022 shows it was found in more than half of U.S. states, with the highest counts occurring in California, Florida, Illinois, Nevada, New York, and Texas. The fungus is a problem worldwide and is listed among the most threatening treatment-resistant fungi by the World Health Organization.
The study authors concluded that screening capacity for the fungus needs to be expanded nationwide so that when C. auris is detected, measures can be taken to prevent its spread.
A version of this article originally appeared on WebMD.com.
prompting the Centers for Disease Control and Prevention to issue a warning to health care facilities about the rising threat.
C. auris is a yeast that spreads easily from touching it on a surface like a countertop. It can also spread from person to person. It isn’t a threat to healthy people, but people in hospitals and nursing homes are at a heightened risk because they might have weakened immune systems or be using invasive medical devices that can introduce the fungus inside their bodies. When C. auris progresses to causing an infection that reaches the brain, blood, or lungs, more than one in three people die.
The worrying increase was detailed in the journal Annals of Internal Medicine. In 2021, cases reached a count of 3,270 with an active infection, and 7,413 cases showed the fungus was present but hadn’t caused an infection. Infection counts were up 95% over the previous year, and the fungus showed up on screenings three times as often. The number of cases resistant to medication also tripled.
The CDC called the figures “alarming,” noting that the fungus was only detected in the United States in 2016.
“The timing of this increase and findings from public health investigations suggest C. auris spread may have worsened due to strain on health care and public health systems during the COVID-19 pandemic,” the CDC explained in a news release.
Another potential reason for the jump could be that screening for C. auris has simply increased and it’s being found more often because it’s being looked for more often. But researchers believe that, even with the increase in testing, the reported counts are underestimated. That’s because even though screening has increased, health care providers still aren’t looking for the presence of the fungus as often as the CDC would like.
“The rapid rise and geographic spread of cases is concerning and emphasizes the need for continued surveillance, expanded lab capacity, quicker diagnostic tests, and adherence to proven infection prevention and control,” said study author Meghan Lyman, MD, a CDC epidemiologist in Atlanta, in a statement.
Cases of C. auris continued to rise in 2022, the CDC said. A map on the agency’s website of reported cases from 2022 shows it was found in more than half of U.S. states, with the highest counts occurring in California, Florida, Illinois, Nevada, New York, and Texas. The fungus is a problem worldwide and is listed among the most threatening treatment-resistant fungi by the World Health Organization.
The study authors concluded that screening capacity for the fungus needs to be expanded nationwide so that when C. auris is detected, measures can be taken to prevent its spread.
A version of this article originally appeared on WebMD.com.
prompting the Centers for Disease Control and Prevention to issue a warning to health care facilities about the rising threat.
C. auris is a yeast that spreads easily from touching it on a surface like a countertop. It can also spread from person to person. It isn’t a threat to healthy people, but people in hospitals and nursing homes are at a heightened risk because they might have weakened immune systems or be using invasive medical devices that can introduce the fungus inside their bodies. When C. auris progresses to causing an infection that reaches the brain, blood, or lungs, more than one in three people die.
The worrying increase was detailed in the journal Annals of Internal Medicine. In 2021, cases reached a count of 3,270 with an active infection, and 7,413 cases showed the fungus was present but hadn’t caused an infection. Infection counts were up 95% over the previous year, and the fungus showed up on screenings three times as often. The number of cases resistant to medication also tripled.
The CDC called the figures “alarming,” noting that the fungus was only detected in the United States in 2016.
“The timing of this increase and findings from public health investigations suggest C. auris spread may have worsened due to strain on health care and public health systems during the COVID-19 pandemic,” the CDC explained in a news release.
Another potential reason for the jump could be that screening for C. auris has simply increased and it’s being found more often because it’s being looked for more often. But researchers believe that, even with the increase in testing, the reported counts are underestimated. That’s because even though screening has increased, health care providers still aren’t looking for the presence of the fungus as often as the CDC would like.
“The rapid rise and geographic spread of cases is concerning and emphasizes the need for continued surveillance, expanded lab capacity, quicker diagnostic tests, and adherence to proven infection prevention and control,” said study author Meghan Lyman, MD, a CDC epidemiologist in Atlanta, in a statement.
Cases of C. auris continued to rise in 2022, the CDC said. A map on the agency’s website of reported cases from 2022 shows it was found in more than half of U.S. states, with the highest counts occurring in California, Florida, Illinois, Nevada, New York, and Texas. The fungus is a problem worldwide and is listed among the most threatening treatment-resistant fungi by the World Health Organization.
The study authors concluded that screening capacity for the fungus needs to be expanded nationwide so that when C. auris is detected, measures can be taken to prevent its spread.
A version of this article originally appeared on WebMD.com.
After the Match: Next steps for new residents, unmatched
Medical school graduates around the US took to social media after last week's Match Day to share their joy ― or explore their options if they did not match.
Take this post March 19 on Twitter: “I went unmatched this year; looking for research position at any institute for internal medicine.”
including an international medical graduate who matched into his chosen specialty after multiple disappointments.
“I’ve waited for this email for 8 years,” Sahil Bawa, MD, posted on Twitter on March 13. A few days later, when he learned about his residency position, he posted: “I’m beyond grateful. Will be moving to Alabama soon #familymedicine.”
Dr. Bawa, who matched into UAB Medicine Selma (Ala.), graduated from medical school in India in 2014. He said in an interview that he has visited the United States periodically since then to pass medical tests, obtain letters of recommendation, and participate in research.
Over the years he watched his Indian colleagues give up on becoming American doctors, find alternative careers, or resolve to practice in their native country. But he held onto the few success stories he saw on social media. “There were always one to two every year. It kept me going. If they can do it, I can do it.”
International medical graduates (IMGs) like Dr. Bawa applied in record numbers to Match2023, according to the National Resident Matching Program (NRMP), which announced the results on March 13 of its main residency match and the Supplemental Offer and Acceptance Program (SOAP) for unfilled positions or unmatched applicants.
Overall, 48,156 total applicants registered for the match, which was driven by the increase of non-U.S. IMG applicants and U.S. DO seniors over the past year, NRMP stated in its release. U.S. MD seniors had a match rate of nearly 94%, and U.S. DO seniors, nearly 92%. U.S. IMGs had a match rate of nearly 68%, an “all-time high,” and non-U.S. IMGs, nearly 60%, NRMP stated.
Three specialties that filled all of their 30 or more available positions were orthopedic surgery, plastic surgery (integrated), radiology – diagnostic, and thoracic surgery. Specialties with 30 or more positions that filled with the highest percentage of U.S. MD and DO seniors were plastic surgery (integrated), internal medicine-pediatrics, ob.gyn., and orthopedic surgery.
The number of available primary care positions increased slightly, NRMP reported. Considering “a serious and growing shortage of primary care physicians across the U.S.,” there were 571 more primary care positions than 2022. That’s an increase of about 3% over last year and 17% over the past 5 years. Primary care positions filled at a rate of 94%, which remained steady from 2022.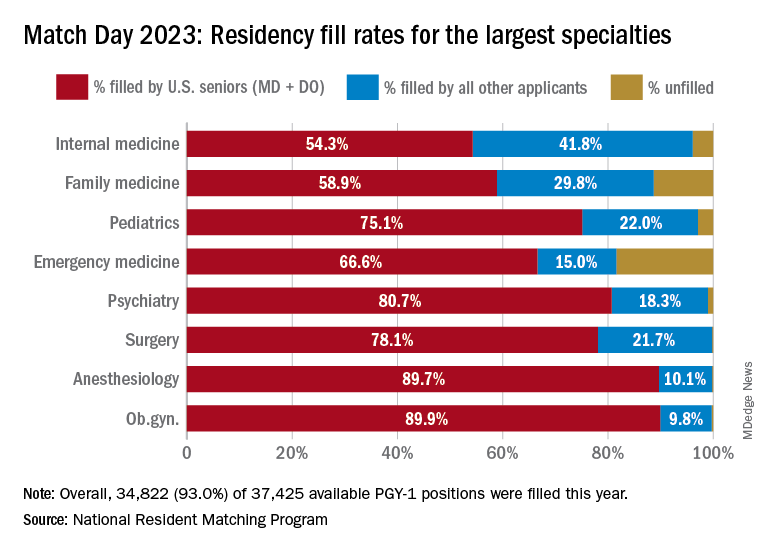
NRMP also pointed out specialties with increases in the number of positions filled by U.S. MD seniors of more than 10% and 10 positions in the past 5 years: anesthesiology, child neurology, interventional radiology, neurology, pathology, physical medicine and rehabilitation, plastic surgery (integrated), psychiatry, radiology-diagnostic, transitional year, and vascular surgery.
Bryan Carmody, MD, MPH, a pediatric nephrologist known for his medical school commentaries, said in an interview that the most competitive specialties he noted in 2023 were radiology, pathology, and neurology.
“The surgical specialties are always competitive, so it wasn’t a surprise that orthopedics, plastic surgery, and thoracic surgery filled all of their positions. But I was surprised to see diagnostic radiology fill every single one of their positions in the match. And although pathology and neurology aren’t typically considered extremely competitive specialties, they filled over 99% of their positions in the Match this year.”
On Dr. Carmody’s blog about the winners and losers of Match Day, he said that despite the record number of primary care positions offered, family medicine programs suffered. “Only 89% of family medicine programs filled in the Match, and graduating U.S. MD and DO students only filled a little more than half of all the available positions,” he wrote.
For a record number of applicants that match each year, and “the most favorable ratio in the past 2 decades” of applicants-to-positions in 2023, there are still a lot unmatched, Dr. Carmody said. “It’s a tough thing to talk about. The reality is the number of residency positions should be determined by the number of physicians needed.”
One student, Asim Ansari, didn’t match into a traditional residency or through SOAP. It was his fifth attempt. He was serving a transitional-year residency at Merit Health Wesley in Hattiesburg, Miss., and when he didn’t match, he accepted a child and adolescent psychiatry fellowship at the University of Kansas Medical Center, Kansas City.
He said he was “relieved and excited” to have found a program in his chosen specialty. Still, in 2 years, Mr. Ansari must again try to match into a traditional psychiatry residency.
Meanwhile, Dr. Bawa will prepare for his 3-year residency in Alabama after completing his interim research year in the surgery department at Wayne State University, Detroit, in May.
Despite his years in limbo, Dr. Bawa said, “I have no regrets, no complaints. I am still very happy.”
A version of this article originally appeared on Medscape.com.
Medical school graduates around the US took to social media after last week's Match Day to share their joy ― or explore their options if they did not match.
Take this post March 19 on Twitter: “I went unmatched this year; looking for research position at any institute for internal medicine.”
including an international medical graduate who matched into his chosen specialty after multiple disappointments.
“I’ve waited for this email for 8 years,” Sahil Bawa, MD, posted on Twitter on March 13. A few days later, when he learned about his residency position, he posted: “I’m beyond grateful. Will be moving to Alabama soon #familymedicine.”
Dr. Bawa, who matched into UAB Medicine Selma (Ala.), graduated from medical school in India in 2014. He said in an interview that he has visited the United States periodically since then to pass medical tests, obtain letters of recommendation, and participate in research.
Over the years he watched his Indian colleagues give up on becoming American doctors, find alternative careers, or resolve to practice in their native country. But he held onto the few success stories he saw on social media. “There were always one to two every year. It kept me going. If they can do it, I can do it.”
International medical graduates (IMGs) like Dr. Bawa applied in record numbers to Match2023, according to the National Resident Matching Program (NRMP), which announced the results on March 13 of its main residency match and the Supplemental Offer and Acceptance Program (SOAP) for unfilled positions or unmatched applicants.
Overall, 48,156 total applicants registered for the match, which was driven by the increase of non-U.S. IMG applicants and U.S. DO seniors over the past year, NRMP stated in its release. U.S. MD seniors had a match rate of nearly 94%, and U.S. DO seniors, nearly 92%. U.S. IMGs had a match rate of nearly 68%, an “all-time high,” and non-U.S. IMGs, nearly 60%, NRMP stated.
Three specialties that filled all of their 30 or more available positions were orthopedic surgery, plastic surgery (integrated), radiology – diagnostic, and thoracic surgery. Specialties with 30 or more positions that filled with the highest percentage of U.S. MD and DO seniors were plastic surgery (integrated), internal medicine-pediatrics, ob.gyn., and orthopedic surgery.
The number of available primary care positions increased slightly, NRMP reported. Considering “a serious and growing shortage of primary care physicians across the U.S.,” there were 571 more primary care positions than 2022. That’s an increase of about 3% over last year and 17% over the past 5 years. Primary care positions filled at a rate of 94%, which remained steady from 2022.
NRMP also pointed out specialties with increases in the number of positions filled by U.S. MD seniors of more than 10% and 10 positions in the past 5 years: anesthesiology, child neurology, interventional radiology, neurology, pathology, physical medicine and rehabilitation, plastic surgery (integrated), psychiatry, radiology-diagnostic, transitional year, and vascular surgery.
Bryan Carmody, MD, MPH, a pediatric nephrologist known for his medical school commentaries, said in an interview that the most competitive specialties he noted in 2023 were radiology, pathology, and neurology.
“The surgical specialties are always competitive, so it wasn’t a surprise that orthopedics, plastic surgery, and thoracic surgery filled all of their positions. But I was surprised to see diagnostic radiology fill every single one of their positions in the match. And although pathology and neurology aren’t typically considered extremely competitive specialties, they filled over 99% of their positions in the Match this year.”
On Dr. Carmody’s blog about the winners and losers of Match Day, he said that despite the record number of primary care positions offered, family medicine programs suffered. “Only 89% of family medicine programs filled in the Match, and graduating U.S. MD and DO students only filled a little more than half of all the available positions,” he wrote.
For a record number of applicants that match each year, and “the most favorable ratio in the past 2 decades” of applicants-to-positions in 2023, there are still a lot unmatched, Dr. Carmody said. “It’s a tough thing to talk about. The reality is the number of residency positions should be determined by the number of physicians needed.”
One student, Asim Ansari, didn’t match into a traditional residency or through SOAP. It was his fifth attempt. He was serving a transitional-year residency at Merit Health Wesley in Hattiesburg, Miss., and when he didn’t match, he accepted a child and adolescent psychiatry fellowship at the University of Kansas Medical Center, Kansas City.
He said he was “relieved and excited” to have found a program in his chosen specialty. Still, in 2 years, Mr. Ansari must again try to match into a traditional psychiatry residency.
Meanwhile, Dr. Bawa will prepare for his 3-year residency in Alabama after completing his interim research year in the surgery department at Wayne State University, Detroit, in May.
Despite his years in limbo, Dr. Bawa said, “I have no regrets, no complaints. I am still very happy.”
A version of this article originally appeared on Medscape.com.
Medical school graduates around the US took to social media after last week's Match Day to share their joy ― or explore their options if they did not match.
Take this post March 19 on Twitter: “I went unmatched this year; looking for research position at any institute for internal medicine.”
including an international medical graduate who matched into his chosen specialty after multiple disappointments.
“I’ve waited for this email for 8 years,” Sahil Bawa, MD, posted on Twitter on March 13. A few days later, when he learned about his residency position, he posted: “I’m beyond grateful. Will be moving to Alabama soon #familymedicine.”
Dr. Bawa, who matched into UAB Medicine Selma (Ala.), graduated from medical school in India in 2014. He said in an interview that he has visited the United States periodically since then to pass medical tests, obtain letters of recommendation, and participate in research.
Over the years he watched his Indian colleagues give up on becoming American doctors, find alternative careers, or resolve to practice in their native country. But he held onto the few success stories he saw on social media. “There were always one to two every year. It kept me going. If they can do it, I can do it.”
International medical graduates (IMGs) like Dr. Bawa applied in record numbers to Match2023, according to the National Resident Matching Program (NRMP), which announced the results on March 13 of its main residency match and the Supplemental Offer and Acceptance Program (SOAP) for unfilled positions or unmatched applicants.
Overall, 48,156 total applicants registered for the match, which was driven by the increase of non-U.S. IMG applicants and U.S. DO seniors over the past year, NRMP stated in its release. U.S. MD seniors had a match rate of nearly 94%, and U.S. DO seniors, nearly 92%. U.S. IMGs had a match rate of nearly 68%, an “all-time high,” and non-U.S. IMGs, nearly 60%, NRMP stated.
Three specialties that filled all of their 30 or more available positions were orthopedic surgery, plastic surgery (integrated), radiology – diagnostic, and thoracic surgery. Specialties with 30 or more positions that filled with the highest percentage of U.S. MD and DO seniors were plastic surgery (integrated), internal medicine-pediatrics, ob.gyn., and orthopedic surgery.
The number of available primary care positions increased slightly, NRMP reported. Considering “a serious and growing shortage of primary care physicians across the U.S.,” there were 571 more primary care positions than 2022. That’s an increase of about 3% over last year and 17% over the past 5 years. Primary care positions filled at a rate of 94%, which remained steady from 2022.
NRMP also pointed out specialties with increases in the number of positions filled by U.S. MD seniors of more than 10% and 10 positions in the past 5 years: anesthesiology, child neurology, interventional radiology, neurology, pathology, physical medicine and rehabilitation, plastic surgery (integrated), psychiatry, radiology-diagnostic, transitional year, and vascular surgery.
Bryan Carmody, MD, MPH, a pediatric nephrologist known for his medical school commentaries, said in an interview that the most competitive specialties he noted in 2023 were radiology, pathology, and neurology.
“The surgical specialties are always competitive, so it wasn’t a surprise that orthopedics, plastic surgery, and thoracic surgery filled all of their positions. But I was surprised to see diagnostic radiology fill every single one of their positions in the match. And although pathology and neurology aren’t typically considered extremely competitive specialties, they filled over 99% of their positions in the Match this year.”
On Dr. Carmody’s blog about the winners and losers of Match Day, he said that despite the record number of primary care positions offered, family medicine programs suffered. “Only 89% of family medicine programs filled in the Match, and graduating U.S. MD and DO students only filled a little more than half of all the available positions,” he wrote.
For a record number of applicants that match each year, and “the most favorable ratio in the past 2 decades” of applicants-to-positions in 2023, there are still a lot unmatched, Dr. Carmody said. “It’s a tough thing to talk about. The reality is the number of residency positions should be determined by the number of physicians needed.”
One student, Asim Ansari, didn’t match into a traditional residency or through SOAP. It was his fifth attempt. He was serving a transitional-year residency at Merit Health Wesley in Hattiesburg, Miss., and when he didn’t match, he accepted a child and adolescent psychiatry fellowship at the University of Kansas Medical Center, Kansas City.
He said he was “relieved and excited” to have found a program in his chosen specialty. Still, in 2 years, Mr. Ansari must again try to match into a traditional psychiatry residency.
Meanwhile, Dr. Bawa will prepare for his 3-year residency in Alabama after completing his interim research year in the surgery department at Wayne State University, Detroit, in May.
Despite his years in limbo, Dr. Bawa said, “I have no regrets, no complaints. I am still very happy.”
A version of this article originally appeared on Medscape.com.
Old-school printer helps scientists quickly spot bacteria in blood
When a bacterial infection reaches the bloodstream, every second is critical. The person’s life is on the line. Yet blood tests to identify bacteria take hours to days. While waiting, doctors often prescribe broad-spectrum antibiotics in hopes of killing whatever bug may be at fault.
Someday soon, that wait time could shrink significantly, allowing health care providers to more quickly zero in on the best antibiotic for each infection – thanks to an innovation from Stanford (Calif.) University that identifies bacteria in seconds.
The cutting-edge method relies on old-school tech: an inkjet printer similar the kind you might have at home – except this one has been modified to print blood instead of ink.
The very small sample size – each drop is two trillionths of a liter, or about a billion times smaller than a raindrop – make spotting bacteria easier. Smaller samples mean fewer cells, so lab techs can more swiftly separate the bacterial spectra from other components, like red blood cells and white blood cells.
To boost efficiency even more, the researchers added gold nanoparticles, which attach to the bacteria, serving like antennas to focus the light. Machine learning – a type of artificial intelligence – helps interpret the spectrum of light and identify which fingerprint goes with which bacteria.
“It kind of wound up being this really interesting historical period where we could put the pieces together from different technologies, including nanophotonics, printing, and artificial intelligence, to help accelerate identification of bacteria in these complex samples,” says study author Jennifer Dionne, PhD, associate professor of materials science and engineering at Stanford.
Compare that to blood culture testing in hospitals, where it takes days for bacterial cells to grow and multiply inside a large machine that looks like a refrigerator. For some bacteria, like the kinds that cause tuberculosis, cultures take weeks.
Then further testing is needed to identify which antibiotics will quell the infection. The new technology from Stanford could accelerate this process, too.
“The promise of our technique is that you don’t need to have a culture of cells to put the antibiotic on top,” says Dr. Dionne. “What we’re finding is that from the Raman scattering, we can use that to identify – even without incubating with antibiotics – which drug the bacteria would respond to, and that’s really exciting.”
If patients can receive the antibiotic best suited for their infection, they will likely have better outcomes.
“Blood cultures can typically take 48-72 hours to come back, and then you base your clinical decisions and adjusting antibiotics based on those blood cultures,” says Richard Watkins, MD, an infectious disease physician and professor of medicine at the Northeastern Ohio Universities, Rootstown. Dr. Watkins was not involved in the study.
“Sometimes, despite your best guess, you’re wrong,” Dr. Watkins says, “and obviously, the patient could have an adverse outcome. So, if you can diagnose the pathogen sooner, that is ideal. Whatever technology enables clinicians to do that is definitely progress and a step forward.”
On a global scale, this technology could help reduce the overuse of broad-spectrum antibiotics, which contributes to antimicrobial resistance, an emerging health threat, says Dr. Dionne.
The team is working to develop the technology further into an instrument the size of a shoebox and, with further testing, commercialize the product. That could take a few years.
This technology has potential beyond bloodstream infections, too. It could be used to identify bacteria in other fluids, such as in wastewater or contaminated food.
A version of this article originally appeared on WebMD.com.
When a bacterial infection reaches the bloodstream, every second is critical. The person’s life is on the line. Yet blood tests to identify bacteria take hours to days. While waiting, doctors often prescribe broad-spectrum antibiotics in hopes of killing whatever bug may be at fault.
Someday soon, that wait time could shrink significantly, allowing health care providers to more quickly zero in on the best antibiotic for each infection – thanks to an innovation from Stanford (Calif.) University that identifies bacteria in seconds.
The cutting-edge method relies on old-school tech: an inkjet printer similar the kind you might have at home – except this one has been modified to print blood instead of ink.
The very small sample size – each drop is two trillionths of a liter, or about a billion times smaller than a raindrop – make spotting bacteria easier. Smaller samples mean fewer cells, so lab techs can more swiftly separate the bacterial spectra from other components, like red blood cells and white blood cells.
To boost efficiency even more, the researchers added gold nanoparticles, which attach to the bacteria, serving like antennas to focus the light. Machine learning – a type of artificial intelligence – helps interpret the spectrum of light and identify which fingerprint goes with which bacteria.
“It kind of wound up being this really interesting historical period where we could put the pieces together from different technologies, including nanophotonics, printing, and artificial intelligence, to help accelerate identification of bacteria in these complex samples,” says study author Jennifer Dionne, PhD, associate professor of materials science and engineering at Stanford.
Compare that to blood culture testing in hospitals, where it takes days for bacterial cells to grow and multiply inside a large machine that looks like a refrigerator. For some bacteria, like the kinds that cause tuberculosis, cultures take weeks.
Then further testing is needed to identify which antibiotics will quell the infection. The new technology from Stanford could accelerate this process, too.
“The promise of our technique is that you don’t need to have a culture of cells to put the antibiotic on top,” says Dr. Dionne. “What we’re finding is that from the Raman scattering, we can use that to identify – even without incubating with antibiotics – which drug the bacteria would respond to, and that’s really exciting.”
If patients can receive the antibiotic best suited for their infection, they will likely have better outcomes.
“Blood cultures can typically take 48-72 hours to come back, and then you base your clinical decisions and adjusting antibiotics based on those blood cultures,” says Richard Watkins, MD, an infectious disease physician and professor of medicine at the Northeastern Ohio Universities, Rootstown. Dr. Watkins was not involved in the study.
“Sometimes, despite your best guess, you’re wrong,” Dr. Watkins says, “and obviously, the patient could have an adverse outcome. So, if you can diagnose the pathogen sooner, that is ideal. Whatever technology enables clinicians to do that is definitely progress and a step forward.”
On a global scale, this technology could help reduce the overuse of broad-spectrum antibiotics, which contributes to antimicrobial resistance, an emerging health threat, says Dr. Dionne.
The team is working to develop the technology further into an instrument the size of a shoebox and, with further testing, commercialize the product. That could take a few years.
This technology has potential beyond bloodstream infections, too. It could be used to identify bacteria in other fluids, such as in wastewater or contaminated food.
A version of this article originally appeared on WebMD.com.
When a bacterial infection reaches the bloodstream, every second is critical. The person’s life is on the line. Yet blood tests to identify bacteria take hours to days. While waiting, doctors often prescribe broad-spectrum antibiotics in hopes of killing whatever bug may be at fault.
Someday soon, that wait time could shrink significantly, allowing health care providers to more quickly zero in on the best antibiotic for each infection – thanks to an innovation from Stanford (Calif.) University that identifies bacteria in seconds.
The cutting-edge method relies on old-school tech: an inkjet printer similar the kind you might have at home – except this one has been modified to print blood instead of ink.
The very small sample size – each drop is two trillionths of a liter, or about a billion times smaller than a raindrop – make spotting bacteria easier. Smaller samples mean fewer cells, so lab techs can more swiftly separate the bacterial spectra from other components, like red blood cells and white blood cells.
To boost efficiency even more, the researchers added gold nanoparticles, which attach to the bacteria, serving like antennas to focus the light. Machine learning – a type of artificial intelligence – helps interpret the spectrum of light and identify which fingerprint goes with which bacteria.
“It kind of wound up being this really interesting historical period where we could put the pieces together from different technologies, including nanophotonics, printing, and artificial intelligence, to help accelerate identification of bacteria in these complex samples,” says study author Jennifer Dionne, PhD, associate professor of materials science and engineering at Stanford.
Compare that to blood culture testing in hospitals, where it takes days for bacterial cells to grow and multiply inside a large machine that looks like a refrigerator. For some bacteria, like the kinds that cause tuberculosis, cultures take weeks.
Then further testing is needed to identify which antibiotics will quell the infection. The new technology from Stanford could accelerate this process, too.
“The promise of our technique is that you don’t need to have a culture of cells to put the antibiotic on top,” says Dr. Dionne. “What we’re finding is that from the Raman scattering, we can use that to identify – even without incubating with antibiotics – which drug the bacteria would respond to, and that’s really exciting.”
If patients can receive the antibiotic best suited for their infection, they will likely have better outcomes.
“Blood cultures can typically take 48-72 hours to come back, and then you base your clinical decisions and adjusting antibiotics based on those blood cultures,” says Richard Watkins, MD, an infectious disease physician and professor of medicine at the Northeastern Ohio Universities, Rootstown. Dr. Watkins was not involved in the study.
“Sometimes, despite your best guess, you’re wrong,” Dr. Watkins says, “and obviously, the patient could have an adverse outcome. So, if you can diagnose the pathogen sooner, that is ideal. Whatever technology enables clinicians to do that is definitely progress and a step forward.”
On a global scale, this technology could help reduce the overuse of broad-spectrum antibiotics, which contributes to antimicrobial resistance, an emerging health threat, says Dr. Dionne.
The team is working to develop the technology further into an instrument the size of a shoebox and, with further testing, commercialize the product. That could take a few years.
This technology has potential beyond bloodstream infections, too. It could be used to identify bacteria in other fluids, such as in wastewater or contaminated food.
A version of this article originally appeared on WebMD.com.
Ectopic pregnancy risk and levonorgestrel-releasing IUDs
Researchers report that use of any levonorgestrel-releasing intrauterine system was associated with a significantly increased risk of ectopic pregnancy, compared with other hormonal contraceptives, in a study published in JAMA.
A national health database analysis headed by Amani Meaidi, MD, PhD, of the Danish Cancer Society Research Center, Cancer Surveillance and Pharmacoepidemiology, in Copenhagen, compared the 13.5-mg with the 19.5-mg and 52-mg dosages of levonorgestrel-releasing intrauterine systems (IUSs).
The hormone content in levonorgestrel-releasing IUSs must be high enough to maintain optimal contraceptive effect but sufficiently low to minimize progestin-related adverse events, Dr. Meaidi and colleagues noted; they advised using the middle dosage of 19.5 mg. All dosages are recommended for contraception, with the highest dosage also recommended for heavy menstrual bleeding.
“If 10,000 women using the hormonal IUD for 1 year were given the 19.5-mg hormonal IUD instead of the 13.5-mg hormonal IUD, around nine ectopic pregnancies would be avoided,” Dr. Meaidi said in an interview.
“Ectopic pregnancy is an acknowledged adverse event of hormonal IUD use. Although a rare event, it is a serious one, and a difference in ectopic pregnancy safety between the two low-dose hormonal IUDs would impact my recommendations to women.”
The study
Dr. Meaidi’s group followed 963,964 women for 7.8 million person-years. For users of levonorgestrel IUS dosages 52 mg, 19.5 mg, and 13.5 mg, and other hormonal contraceptives, the median ages were 24, 22, 22, and 21 years, respectively.
Eligible women were nulliparous with no previous ectopic pregnancy, abdominal or pelvic surgery, infertility treatment, endometriosis, or use of a levonorgestrel IUS. They were followed from Jan. 1, 2001, or their 15th birthday, until July 1, 2021, age 35, pregnancy, death, emigration, or the occurrence of any exclusion criterion.
During the study period, the cohort registered 2,925 ectopic pregnancies, including 35 at 52 mg, 32 at 19.5 mg, and 80 at 13.5 mg of levonorgestrel. For all other types of hormonal contraception, there were 763 ectopic pregnancies.
In terms of adjusted absolute rates of ectopic pregnancy per 10,000 person-years, compared with other hormonal contraceptives (rate = 2.4), these were 7.7 with 52 mg levonorgestrel IUS, 7.1 with 19.5 mg, and 15.7 with 13.5 mg. They translated to comparative differences of 5.3 (95% confidence interval, 1.9-8.7), 4.8 (95% CI, 1.5-8.0), and 13.4 (95% CI, 8.8-18.1), respectively.
Corresponding adjusted relative rate ratios were 3.4, 4.1, and 7.9. For each levonorgestrel IUS dosage; the ectopic pregnancy rate increased with duration of use.
The adjusted ectopic pregnancy rate difference per 10,000 person-years between the 19.5-mg and 52-mg levonorgestrel dosages was −0.6 , and between the 13.5-mg and 52-mg doses, 8.0, with a rate ratio of 2.3. The rate difference between the 13.5-mg and 19.5-mg levonorgestrel IUS was 8.6, with a rate ratio of 1.9.
An outsider’s perspective
Offering an outsider’s perspective on the study, Eran Bornstein, MD, vice-chair of obstetrics and gynecology at Lenox Hill Hospital in New York, said these data should spark further evaluation of risk of ectopic pregnancy with levonorgestrel-releasing IUDs. “The best advice for clinicians is to individualize the choice of which contraceptive to use, and when levonorgestrel IUD is selected, to individualize the appropriate dose and timing of placement,” he said in an interview.
Several additional factors may determine the best choice, Dr. Bornstein added, including medical conditions that contraindicate other contraceptives and those conditions that justify avoidance of pregnancy, as well as uterine myomas or malformation, the ability of the patient to comply with other options, and informed patient choice. “It is important to remember the potential risk for expulsion and ectopic pregnancy, maintain alertness, and use ultrasound to exclude these potential complications if suspected,” he said.
Dr. Meaidi said the mechanism of ectopic pregnancy with hormonal IUDs is unclear, but in vitro and animal studies have observed that levonorgestrel reduces the ciliary beat frequency in the fallopian tubes. “Thus, it could be hypothesized that if a woman was unfortunate enough to become pregnant using a hormonal IUD, the hormone could inhibit or slow down the movement of the zygote into the uterus for rightful intrauterine implantation and thereby increase the risk of ectopic pregnancy.”
Two coauthors of the study reported financial support from private-sector companies. Dr. Meaidi had no conflicts of interest. Dr. Bornstein disclosed no competing interests.
Researchers report that use of any levonorgestrel-releasing intrauterine system was associated with a significantly increased risk of ectopic pregnancy, compared with other hormonal contraceptives, in a study published in JAMA.
A national health database analysis headed by Amani Meaidi, MD, PhD, of the Danish Cancer Society Research Center, Cancer Surveillance and Pharmacoepidemiology, in Copenhagen, compared the 13.5-mg with the 19.5-mg and 52-mg dosages of levonorgestrel-releasing intrauterine systems (IUSs).
The hormone content in levonorgestrel-releasing IUSs must be high enough to maintain optimal contraceptive effect but sufficiently low to minimize progestin-related adverse events, Dr. Meaidi and colleagues noted; they advised using the middle dosage of 19.5 mg. All dosages are recommended for contraception, with the highest dosage also recommended for heavy menstrual bleeding.
“If 10,000 women using the hormonal IUD for 1 year were given the 19.5-mg hormonal IUD instead of the 13.5-mg hormonal IUD, around nine ectopic pregnancies would be avoided,” Dr. Meaidi said in an interview.
“Ectopic pregnancy is an acknowledged adverse event of hormonal IUD use. Although a rare event, it is a serious one, and a difference in ectopic pregnancy safety between the two low-dose hormonal IUDs would impact my recommendations to women.”
The study
Dr. Meaidi’s group followed 963,964 women for 7.8 million person-years. For users of levonorgestrel IUS dosages 52 mg, 19.5 mg, and 13.5 mg, and other hormonal contraceptives, the median ages were 24, 22, 22, and 21 years, respectively.
Eligible women were nulliparous with no previous ectopic pregnancy, abdominal or pelvic surgery, infertility treatment, endometriosis, or use of a levonorgestrel IUS. They were followed from Jan. 1, 2001, or their 15th birthday, until July 1, 2021, age 35, pregnancy, death, emigration, or the occurrence of any exclusion criterion.
During the study period, the cohort registered 2,925 ectopic pregnancies, including 35 at 52 mg, 32 at 19.5 mg, and 80 at 13.5 mg of levonorgestrel. For all other types of hormonal contraception, there were 763 ectopic pregnancies.
In terms of adjusted absolute rates of ectopic pregnancy per 10,000 person-years, compared with other hormonal contraceptives (rate = 2.4), these were 7.7 with 52 mg levonorgestrel IUS, 7.1 with 19.5 mg, and 15.7 with 13.5 mg. They translated to comparative differences of 5.3 (95% confidence interval, 1.9-8.7), 4.8 (95% CI, 1.5-8.0), and 13.4 (95% CI, 8.8-18.1), respectively.
Corresponding adjusted relative rate ratios were 3.4, 4.1, and 7.9. For each levonorgestrel IUS dosage; the ectopic pregnancy rate increased with duration of use.
The adjusted ectopic pregnancy rate difference per 10,000 person-years between the 19.5-mg and 52-mg levonorgestrel dosages was −0.6 , and between the 13.5-mg and 52-mg doses, 8.0, with a rate ratio of 2.3. The rate difference between the 13.5-mg and 19.5-mg levonorgestrel IUS was 8.6, with a rate ratio of 1.9.
An outsider’s perspective
Offering an outsider’s perspective on the study, Eran Bornstein, MD, vice-chair of obstetrics and gynecology at Lenox Hill Hospital in New York, said these data should spark further evaluation of risk of ectopic pregnancy with levonorgestrel-releasing IUDs. “The best advice for clinicians is to individualize the choice of which contraceptive to use, and when levonorgestrel IUD is selected, to individualize the appropriate dose and timing of placement,” he said in an interview.
Several additional factors may determine the best choice, Dr. Bornstein added, including medical conditions that contraindicate other contraceptives and those conditions that justify avoidance of pregnancy, as well as uterine myomas or malformation, the ability of the patient to comply with other options, and informed patient choice. “It is important to remember the potential risk for expulsion and ectopic pregnancy, maintain alertness, and use ultrasound to exclude these potential complications if suspected,” he said.
Dr. Meaidi said the mechanism of ectopic pregnancy with hormonal IUDs is unclear, but in vitro and animal studies have observed that levonorgestrel reduces the ciliary beat frequency in the fallopian tubes. “Thus, it could be hypothesized that if a woman was unfortunate enough to become pregnant using a hormonal IUD, the hormone could inhibit or slow down the movement of the zygote into the uterus for rightful intrauterine implantation and thereby increase the risk of ectopic pregnancy.”
Two coauthors of the study reported financial support from private-sector companies. Dr. Meaidi had no conflicts of interest. Dr. Bornstein disclosed no competing interests.
Researchers report that use of any levonorgestrel-releasing intrauterine system was associated with a significantly increased risk of ectopic pregnancy, compared with other hormonal contraceptives, in a study published in JAMA.
A national health database analysis headed by Amani Meaidi, MD, PhD, of the Danish Cancer Society Research Center, Cancer Surveillance and Pharmacoepidemiology, in Copenhagen, compared the 13.5-mg with the 19.5-mg and 52-mg dosages of levonorgestrel-releasing intrauterine systems (IUSs).
The hormone content in levonorgestrel-releasing IUSs must be high enough to maintain optimal contraceptive effect but sufficiently low to minimize progestin-related adverse events, Dr. Meaidi and colleagues noted; they advised using the middle dosage of 19.5 mg. All dosages are recommended for contraception, with the highest dosage also recommended for heavy menstrual bleeding.
“If 10,000 women using the hormonal IUD for 1 year were given the 19.5-mg hormonal IUD instead of the 13.5-mg hormonal IUD, around nine ectopic pregnancies would be avoided,” Dr. Meaidi said in an interview.
“Ectopic pregnancy is an acknowledged adverse event of hormonal IUD use. Although a rare event, it is a serious one, and a difference in ectopic pregnancy safety between the two low-dose hormonal IUDs would impact my recommendations to women.”
The study
Dr. Meaidi’s group followed 963,964 women for 7.8 million person-years. For users of levonorgestrel IUS dosages 52 mg, 19.5 mg, and 13.5 mg, and other hormonal contraceptives, the median ages were 24, 22, 22, and 21 years, respectively.
Eligible women were nulliparous with no previous ectopic pregnancy, abdominal or pelvic surgery, infertility treatment, endometriosis, or use of a levonorgestrel IUS. They were followed from Jan. 1, 2001, or their 15th birthday, until July 1, 2021, age 35, pregnancy, death, emigration, or the occurrence of any exclusion criterion.
During the study period, the cohort registered 2,925 ectopic pregnancies, including 35 at 52 mg, 32 at 19.5 mg, and 80 at 13.5 mg of levonorgestrel. For all other types of hormonal contraception, there were 763 ectopic pregnancies.
In terms of adjusted absolute rates of ectopic pregnancy per 10,000 person-years, compared with other hormonal contraceptives (rate = 2.4), these were 7.7 with 52 mg levonorgestrel IUS, 7.1 with 19.5 mg, and 15.7 with 13.5 mg. They translated to comparative differences of 5.3 (95% confidence interval, 1.9-8.7), 4.8 (95% CI, 1.5-8.0), and 13.4 (95% CI, 8.8-18.1), respectively.
Corresponding adjusted relative rate ratios were 3.4, 4.1, and 7.9. For each levonorgestrel IUS dosage; the ectopic pregnancy rate increased with duration of use.
The adjusted ectopic pregnancy rate difference per 10,000 person-years between the 19.5-mg and 52-mg levonorgestrel dosages was −0.6 , and between the 13.5-mg and 52-mg doses, 8.0, with a rate ratio of 2.3. The rate difference between the 13.5-mg and 19.5-mg levonorgestrel IUS was 8.6, with a rate ratio of 1.9.
An outsider’s perspective
Offering an outsider’s perspective on the study, Eran Bornstein, MD, vice-chair of obstetrics and gynecology at Lenox Hill Hospital in New York, said these data should spark further evaluation of risk of ectopic pregnancy with levonorgestrel-releasing IUDs. “The best advice for clinicians is to individualize the choice of which contraceptive to use, and when levonorgestrel IUD is selected, to individualize the appropriate dose and timing of placement,” he said in an interview.
Several additional factors may determine the best choice, Dr. Bornstein added, including medical conditions that contraindicate other contraceptives and those conditions that justify avoidance of pregnancy, as well as uterine myomas or malformation, the ability of the patient to comply with other options, and informed patient choice. “It is important to remember the potential risk for expulsion and ectopic pregnancy, maintain alertness, and use ultrasound to exclude these potential complications if suspected,” he said.
Dr. Meaidi said the mechanism of ectopic pregnancy with hormonal IUDs is unclear, but in vitro and animal studies have observed that levonorgestrel reduces the ciliary beat frequency in the fallopian tubes. “Thus, it could be hypothesized that if a woman was unfortunate enough to become pregnant using a hormonal IUD, the hormone could inhibit or slow down the movement of the zygote into the uterus for rightful intrauterine implantation and thereby increase the risk of ectopic pregnancy.”
Two coauthors of the study reported financial support from private-sector companies. Dr. Meaidi had no conflicts of interest. Dr. Bornstein disclosed no competing interests.
FROM JAMA
Phase 3 prurigo nodularis trial shows positive results for nemolizumab
NEW ORLEANS – demonstrated.
Nemolizumab is a first-in-class investigational monoclonal antibody directed against the interleukin-31 receptor alpha that blocks signaling from IL-31. “From prior studies we know that it modulates pruritus, but also alters keratinocyte differentiation, inflammation, and fibrosis,” one of the investigators, Shawn G. Kwatra, MD, of the department of dermatology, Johns Hopkins University, Baltimore, said during a late-breaking research session at the annual meeting of the American Academy of Dermatology.
OLYMPIA 2 was a phase 3, multicenter, double-blind study in adults with PN presenting with 20 or more nodules, and Investigator’s Global Assessment (IGA) score of 3 or more, and the Peak Pruritus Numerical Rating Scale (PP-NRS) score of 7 or more. Exclusion criteria included chronic pruritus resulting from an active condition other than PN, such as neuropathic and psychogenic pruritus and active atopic dermatitis. In addition, the use of topical steroids, considered a rescue therapy, was not allowed in the trial, Dr. Kwatra said.
After an initial screening period, 274 patients at 73 sites in nine countries were randomized 2:1 either to the nemolizumab monotherapy or placebo. Following an initial 60-mg subcutaneous dose, patients received 30 mg or 60 mg (depending on their baseline weight) every 4 weeks for 16 weeks. The primary endpoint was the proportion of patients with a 4-point or greater improvement in the PP-NRS from baseline at week 16 and the proportion of patients with IGA success at week 16.
Selected key secondary endpoints included the proportion of patients with a 4 point or greater improvement from baseline in the PP-NRS at week 4, the Sleep Disturbance Numerical Rating Scale at week 4, and the SD-NRS at week 16. Safety endpoints included the incidence and severity of all adverse events.
Of the 274 patients randomized, 183 received nemolizumab and 91 received placebo. A total of 174 patients in the nemolizumab group completed the study, compared with 88 in the placebo group. The mean age of study participants was 53 years, 61% were women, 79% were White, 14% were Asian, and the rest were from other racial groups. More than half (57%) had IGA category 3 disease (moderate) and the remainder had IGA category 4 disease (severe); 63% had 20-100 lesions, and the remainder had more than 100. About one-third of study enrollees (32%) had a history of atopy.
Primary, secondary endpoint results
Dr. Kwatra reported that 56.3% of the patients in the nemolizumab group achieved a 4-point or greater improvement in the PP-NRS at week 16, compared with 20.9% of those in the placebo group (P < .0001), while 37.7% of those in the nemolizumab group achieved IGA success at week 16, compared with 11% of those in the placebo group (P < .0001).
As for secondary endpoints, 41% of patients in the nemolizumab group achieved a 4-point or greater improvement in PP-NRS at week 4, compared with 7.7% of those in the placebo group (P < .0001); and 37.2% of patients in the nemolizumab group achieved a 4-point or greater improvement in SD-NRS at week 4, compared with 9.9% of those in the placebo group (P < .0001). Almost 52% of patients in the nemolizumab group achieved a 4-point or greater improvement in SD-NRS at week 16, compared with 20.9% of those in the placebo group (P < .0001); and 9.8% of those in the nemolizumab group achieved IGA success at week 4, compared with 1.1% of those in the placebo group (P < .0074).
Adverse events
Treatment-emergent adverse events occurred in 61.2% of subjects in the nemolizumab group, compared with 52.7% of those in the placebo group. “There were no imbalances overall, [including] no injection-related reactions in either group,” Dr. Kwatra said. There was one case of newly diagnosed asthma in the placebo arm, and none in the treatment arm.
The researchers observed a slightly increased onset of atopic dermatitis in the treatment arm, compared with the placebo arm (5.5% vs. 0%). “Seven out of those 10 patients actually had a history of atopic dermatitis or high IgE [levels] and they were mostly managed with topical steroids without study drug discontinuation,” Dr. Kwatra added. Neurodermatitis, or worsening of PN, occurred in 3.8% of patients in the nemolizumab group, compared with 11% of those in the placebo group.
“The results of this study extend the efficacy and safety findings from the phase 2 study of nemolizumab in patients with PN,” Dr. Kwatra concluded. “I think they also help to usher in a new era of PN [treatment] in prime time.”
Kenneth B. Gordon, MD, who chairs the department of dermatology at the Medical College of Wisconsin, Milwaukee, and was asked to comment on the study, was impressed with nemolizumab’s propensity for blocking IL-31. “To be able to treat PN effectively by simply blocking the itch and not having a significant inflammatory function is really interesting,” he said in an interview at the meeting. If approved, nemolizumab “gives us another treatment option for a disease that is really debilitating. It’s very promising and we hope [the drug] will be available to us in the near future.”
Nemolizumab is being developed by Galderma. According to a press release from the company, nemolizumab was granted Breakthrough Therapy designation by the Food and Drug Administration in December 2019 for the treatment of pruritus associated with PN, a status that was reconfirmed in February 2023.
Dr. Kwatra disclosed that he is an advisory board member/consultant for Galderma, AbbVie, Amgen, Arcutis, ASLAN Pharmaceuticals, Cara Therapeutics, Castle Biosciences, Celldex, Incyte, Johnson and Johnson, Leo Pharma, Novartis, Pfizer, Regeneron, and Sanofi. Dr. Gordon disclosed that he is a consultant to, an investigator for, and/or a member of the advisory board for several pharmaceutical companies, but not Galderma.
NEW ORLEANS – demonstrated.
Nemolizumab is a first-in-class investigational monoclonal antibody directed against the interleukin-31 receptor alpha that blocks signaling from IL-31. “From prior studies we know that it modulates pruritus, but also alters keratinocyte differentiation, inflammation, and fibrosis,” one of the investigators, Shawn G. Kwatra, MD, of the department of dermatology, Johns Hopkins University, Baltimore, said during a late-breaking research session at the annual meeting of the American Academy of Dermatology.
OLYMPIA 2 was a phase 3, multicenter, double-blind study in adults with PN presenting with 20 or more nodules, and Investigator’s Global Assessment (IGA) score of 3 or more, and the Peak Pruritus Numerical Rating Scale (PP-NRS) score of 7 or more. Exclusion criteria included chronic pruritus resulting from an active condition other than PN, such as neuropathic and psychogenic pruritus and active atopic dermatitis. In addition, the use of topical steroids, considered a rescue therapy, was not allowed in the trial, Dr. Kwatra said.
After an initial screening period, 274 patients at 73 sites in nine countries were randomized 2:1 either to the nemolizumab monotherapy or placebo. Following an initial 60-mg subcutaneous dose, patients received 30 mg or 60 mg (depending on their baseline weight) every 4 weeks for 16 weeks. The primary endpoint was the proportion of patients with a 4-point or greater improvement in the PP-NRS from baseline at week 16 and the proportion of patients with IGA success at week 16.
Selected key secondary endpoints included the proportion of patients with a 4 point or greater improvement from baseline in the PP-NRS at week 4, the Sleep Disturbance Numerical Rating Scale at week 4, and the SD-NRS at week 16. Safety endpoints included the incidence and severity of all adverse events.
Of the 274 patients randomized, 183 received nemolizumab and 91 received placebo. A total of 174 patients in the nemolizumab group completed the study, compared with 88 in the placebo group. The mean age of study participants was 53 years, 61% were women, 79% were White, 14% were Asian, and the rest were from other racial groups. More than half (57%) had IGA category 3 disease (moderate) and the remainder had IGA category 4 disease (severe); 63% had 20-100 lesions, and the remainder had more than 100. About one-third of study enrollees (32%) had a history of atopy.
Primary, secondary endpoint results
Dr. Kwatra reported that 56.3% of the patients in the nemolizumab group achieved a 4-point or greater improvement in the PP-NRS at week 16, compared with 20.9% of those in the placebo group (P < .0001), while 37.7% of those in the nemolizumab group achieved IGA success at week 16, compared with 11% of those in the placebo group (P < .0001).
As for secondary endpoints, 41% of patients in the nemolizumab group achieved a 4-point or greater improvement in PP-NRS at week 4, compared with 7.7% of those in the placebo group (P < .0001); and 37.2% of patients in the nemolizumab group achieved a 4-point or greater improvement in SD-NRS at week 4, compared with 9.9% of those in the placebo group (P < .0001). Almost 52% of patients in the nemolizumab group achieved a 4-point or greater improvement in SD-NRS at week 16, compared with 20.9% of those in the placebo group (P < .0001); and 9.8% of those in the nemolizumab group achieved IGA success at week 4, compared with 1.1% of those in the placebo group (P < .0074).
Adverse events
Treatment-emergent adverse events occurred in 61.2% of subjects in the nemolizumab group, compared with 52.7% of those in the placebo group. “There were no imbalances overall, [including] no injection-related reactions in either group,” Dr. Kwatra said. There was one case of newly diagnosed asthma in the placebo arm, and none in the treatment arm.
The researchers observed a slightly increased onset of atopic dermatitis in the treatment arm, compared with the placebo arm (5.5% vs. 0%). “Seven out of those 10 patients actually had a history of atopic dermatitis or high IgE [levels] and they were mostly managed with topical steroids without study drug discontinuation,” Dr. Kwatra added. Neurodermatitis, or worsening of PN, occurred in 3.8% of patients in the nemolizumab group, compared with 11% of those in the placebo group.
“The results of this study extend the efficacy and safety findings from the phase 2 study of nemolizumab in patients with PN,” Dr. Kwatra concluded. “I think they also help to usher in a new era of PN [treatment] in prime time.”
Kenneth B. Gordon, MD, who chairs the department of dermatology at the Medical College of Wisconsin, Milwaukee, and was asked to comment on the study, was impressed with nemolizumab’s propensity for blocking IL-31. “To be able to treat PN effectively by simply blocking the itch and not having a significant inflammatory function is really interesting,” he said in an interview at the meeting. If approved, nemolizumab “gives us another treatment option for a disease that is really debilitating. It’s very promising and we hope [the drug] will be available to us in the near future.”
Nemolizumab is being developed by Galderma. According to a press release from the company, nemolizumab was granted Breakthrough Therapy designation by the Food and Drug Administration in December 2019 for the treatment of pruritus associated with PN, a status that was reconfirmed in February 2023.
Dr. Kwatra disclosed that he is an advisory board member/consultant for Galderma, AbbVie, Amgen, Arcutis, ASLAN Pharmaceuticals, Cara Therapeutics, Castle Biosciences, Celldex, Incyte, Johnson and Johnson, Leo Pharma, Novartis, Pfizer, Regeneron, and Sanofi. Dr. Gordon disclosed that he is a consultant to, an investigator for, and/or a member of the advisory board for several pharmaceutical companies, but not Galderma.
NEW ORLEANS – demonstrated.
Nemolizumab is a first-in-class investigational monoclonal antibody directed against the interleukin-31 receptor alpha that blocks signaling from IL-31. “From prior studies we know that it modulates pruritus, but also alters keratinocyte differentiation, inflammation, and fibrosis,” one of the investigators, Shawn G. Kwatra, MD, of the department of dermatology, Johns Hopkins University, Baltimore, said during a late-breaking research session at the annual meeting of the American Academy of Dermatology.
OLYMPIA 2 was a phase 3, multicenter, double-blind study in adults with PN presenting with 20 or more nodules, and Investigator’s Global Assessment (IGA) score of 3 or more, and the Peak Pruritus Numerical Rating Scale (PP-NRS) score of 7 or more. Exclusion criteria included chronic pruritus resulting from an active condition other than PN, such as neuropathic and psychogenic pruritus and active atopic dermatitis. In addition, the use of topical steroids, considered a rescue therapy, was not allowed in the trial, Dr. Kwatra said.
After an initial screening period, 274 patients at 73 sites in nine countries were randomized 2:1 either to the nemolizumab monotherapy or placebo. Following an initial 60-mg subcutaneous dose, patients received 30 mg or 60 mg (depending on their baseline weight) every 4 weeks for 16 weeks. The primary endpoint was the proportion of patients with a 4-point or greater improvement in the PP-NRS from baseline at week 16 and the proportion of patients with IGA success at week 16.
Selected key secondary endpoints included the proportion of patients with a 4 point or greater improvement from baseline in the PP-NRS at week 4, the Sleep Disturbance Numerical Rating Scale at week 4, and the SD-NRS at week 16. Safety endpoints included the incidence and severity of all adverse events.
Of the 274 patients randomized, 183 received nemolizumab and 91 received placebo. A total of 174 patients in the nemolizumab group completed the study, compared with 88 in the placebo group. The mean age of study participants was 53 years, 61% were women, 79% were White, 14% were Asian, and the rest were from other racial groups. More than half (57%) had IGA category 3 disease (moderate) and the remainder had IGA category 4 disease (severe); 63% had 20-100 lesions, and the remainder had more than 100. About one-third of study enrollees (32%) had a history of atopy.
Primary, secondary endpoint results
Dr. Kwatra reported that 56.3% of the patients in the nemolizumab group achieved a 4-point or greater improvement in the PP-NRS at week 16, compared with 20.9% of those in the placebo group (P < .0001), while 37.7% of those in the nemolizumab group achieved IGA success at week 16, compared with 11% of those in the placebo group (P < .0001).
As for secondary endpoints, 41% of patients in the nemolizumab group achieved a 4-point or greater improvement in PP-NRS at week 4, compared with 7.7% of those in the placebo group (P < .0001); and 37.2% of patients in the nemolizumab group achieved a 4-point or greater improvement in SD-NRS at week 4, compared with 9.9% of those in the placebo group (P < .0001). Almost 52% of patients in the nemolizumab group achieved a 4-point or greater improvement in SD-NRS at week 16, compared with 20.9% of those in the placebo group (P < .0001); and 9.8% of those in the nemolizumab group achieved IGA success at week 4, compared with 1.1% of those in the placebo group (P < .0074).
Adverse events
Treatment-emergent adverse events occurred in 61.2% of subjects in the nemolizumab group, compared with 52.7% of those in the placebo group. “There were no imbalances overall, [including] no injection-related reactions in either group,” Dr. Kwatra said. There was one case of newly diagnosed asthma in the placebo arm, and none in the treatment arm.
The researchers observed a slightly increased onset of atopic dermatitis in the treatment arm, compared with the placebo arm (5.5% vs. 0%). “Seven out of those 10 patients actually had a history of atopic dermatitis or high IgE [levels] and they were mostly managed with topical steroids without study drug discontinuation,” Dr. Kwatra added. Neurodermatitis, or worsening of PN, occurred in 3.8% of patients in the nemolizumab group, compared with 11% of those in the placebo group.
“The results of this study extend the efficacy and safety findings from the phase 2 study of nemolizumab in patients with PN,” Dr. Kwatra concluded. “I think they also help to usher in a new era of PN [treatment] in prime time.”
Kenneth B. Gordon, MD, who chairs the department of dermatology at the Medical College of Wisconsin, Milwaukee, and was asked to comment on the study, was impressed with nemolizumab’s propensity for blocking IL-31. “To be able to treat PN effectively by simply blocking the itch and not having a significant inflammatory function is really interesting,” he said in an interview at the meeting. If approved, nemolizumab “gives us another treatment option for a disease that is really debilitating. It’s very promising and we hope [the drug] will be available to us in the near future.”
Nemolizumab is being developed by Galderma. According to a press release from the company, nemolizumab was granted Breakthrough Therapy designation by the Food and Drug Administration in December 2019 for the treatment of pruritus associated with PN, a status that was reconfirmed in February 2023.
Dr. Kwatra disclosed that he is an advisory board member/consultant for Galderma, AbbVie, Amgen, Arcutis, ASLAN Pharmaceuticals, Cara Therapeutics, Castle Biosciences, Celldex, Incyte, Johnson and Johnson, Leo Pharma, Novartis, Pfizer, Regeneron, and Sanofi. Dr. Gordon disclosed that he is a consultant to, an investigator for, and/or a member of the advisory board for several pharmaceutical companies, but not Galderma.
AT AAD 2023
Current approaches and challenges to cervical cancer prevention in the United States
CASE Intervention approaches for decreasing the risk of cervical cancer
A 25-year-old woman presents to your practice for routine examination. She has never undergone cervical cancer screening or received the human papillomavirus (HPV) vaccine series. The patient has had 3 lifetime sexual partners and currently uses condoms as contraception. What interventions are appropriate to offer this patient to decrease her risk of cervical cancer? Choose as many that may apply:
1. cervical cytology with reflex HPV testing
2. cervical cytology with HPV cotesting
3. primary HPV testing
4. HPV vaccine series (3 doses)
5. all of the above
The answer is number 5, all of the above.
Choices 1, 2, and 3 are acceptable methods of cervical cancer screening for this patient. Catch-up HPV vaccination should be offered as well.
Equitable preventive care is needed
Cervical cancer is a unique cancer because it has a known preventative strategy. HPV vaccination, paired with cervical screening and management of abnormal results, has contributed to decreased rates of cervical cancer in the United States, from 13,914 cases in 1999 to 12,795 cases in 2019.1 In less-developed countries, however, cervical cancer continues to be a leading cause of mortality, with 90% of cervical cancer deaths in 2020 occurring in low- and middle-income countries.2
Disparate outcomes in cervical cancer are often a reflection of disparities in health access. Within the United States, Black women have a higher incidence of cervical cancer, advanced-stage disease, and mortality from cervical cancer than White women.3,4 Furthermore, the incidence of cervical cancer increased among American Indian and Alaska Native people between 2000 and 2019.5 The rate for patients who are overdue for cervical cancer screening is higher among Asian and Hispanic patients compared with non-Hispanic White patients (31.4% vs 20.1%; P=.01) and among patients who identify as LGBTQ+ compared with patients who identify as heterosexual (32.0% vs 22.2%; P<.001).6 Younger patients have a significantly higher rate for overdue screening compared with their older counterparts (29.1% vs 21.1%; P<.001), as do uninsured patients compared with those who are privately insured (41.7% vs 18.1%; P<.001). Overall, the proportion of women without up-to-date screening increased significantly from 2005 to 2019 (14.4% vs 23.0%; P<.001).6
Unfortunately, despite a known strategy to eliminate cervical cancer, we are not accomplishing equitable preventative care. Barriers to care can include patient-centered issues, such as fear of cancer or of painful evaluations, lack of trust in the health care system, and inadequate understanding of the benefits of cancer prevention, in addition to systemic and structural barriers. As we assess new technologies, one of our most important goals is to consider how such innovations can increase health access—whether through increasing ease and acceptability of testing or by creating more effective screening tests.
Updates to cervical screening guidance
In 2020, the American Cancer Society (ACS) updated its cervical screening guidelines to start screening at age 25 years with the “preferred” strategy of HPV primary testing every 5 years.7 By contrast, the US Preventive Services Task Force (USPSTF) continues to recommend 1 of 3 methods: cytology alone every 3 years; cytology alone every 3 years between ages 21 and 29 followed by cytology and HPV cotesting every 5 years at age 30 or older; or high-risk HPV testing alone every 5 years (TABLE).8
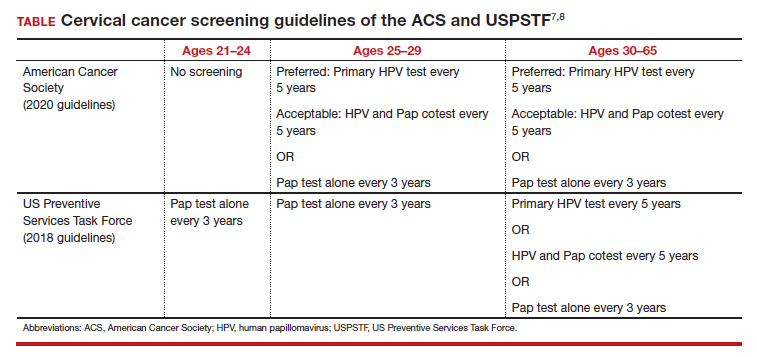
To successfully prevent cervical cancer, abnormal results are managed by performing either colposcopy with biopsy, immediate treatment, or close surveillance based on the risk of developing cervical intraepithelial neoplasia (CIN) 3 or worse. A patient’s risk is determined based on both current and prior test results. The ASCCP (American Society for Colposcopy and Cervical Pathology) transitioned to risk-based management guidelines in 2019 and has both an app and a web-based risk assessment tool available for clinicians (https://www.asccp.org).9
All organizations recommend stopping screening after age 65 provided there has been a history of adequate screening in the prior 10 years (defined as 2 normal cotests or 3 normal cytology tests, with the most recent test within 5 years) and no history of CIN 2 or worse within the prior 25 years.10,11 Recent studies that examined the rate of cervical cancer diagnosed in patients older than 65 years have questioned whether patients should continue screening beyond 65.10 In the United States, 20% of cervical cancer still occurs in women older than age 65.11 One reason may be that many women have not met the requirement for adequate and normal prior screening and may still need ongoing testing.12
Continue to: Primary HPV screening...
Primary HPV screening
Primary HPV testing means that an HPV test is performed first, and if it is positive for high-risk HPV, further testing is performed to determine next steps. This contrasts with the currently used method of obtaining cytology (Pap) first with either concurrent HPV testing or reflex HPV testing. The first HPV primary screening test was approved by the US Food and Drug Administration (FDA) in 2014.13
Multiple randomized controlled trials in Europe have demonstrated the accuracy of HPV-based screening compared with cytology in the detection of cervical cancer and its precursors.14-17 The HPV FOCAL trial demonstrated increased efficacy of primary HPV screening in the detection of CIN 2+ lesions.18 This trial recruited a total of 19,000 women, ages 25 to 65, in Canada and randomly assigned them to receive primary HPV testing or liquid-based cytology. If primary HPV testing was negative, participants would return in 48 months for cytology and HPV cotesting. If primary liquid-based cytology testing was negative, participants would return at 24 months for cytology testing alone and at 48 months for cytology and HPV cotesting. Both groups had similar incidences of CIN 2+ over the study period. HPV testing was shown to detect CIN 2+ at higher rates at the time of initial screen (risk ratio [RR], 1.61; 95% confidence interval [CI], 1.24–2.09) and then significantly lower rates at the time of exit screening at 48 months (RR, 0.36; 95% CI, 0.24–0.54).18 These results demonstrated that primary HPV testing detects CIN 2+ earlier than cytology alone. In follow-up analyses, primary HPV screening missed fewer CIN 2+ diagnoses than cytology screening.19
While not as many studies have compared primary HPV testing to cytology with an HPV cotest, the current most common practice in the United States, one study performed in the United States found that a negative cytology result did not further decrease the risk of CIN 3 for HPV-negative patients (risk of CIN 3+ at 5 years: 0.16% vs 0.17%; P=0.8) and concluded that a negative HPV test was enough reassurance for a low risk of CIN 3+.20
Another study, the ATHENA trial, evaluated more than 42,000 women who were 25 years and older over a 3-year period.21 Patients underwent either primary HPV testing or combination cytology and reflex HPV (if ages 25–29) or HPV cotesting (if age 30 or older). Primary HPV testing was found to have a sensitivity and specificity of 76.1% and 93.5%, respectively, compared with 61.7% and 94.6% for cytology with HPV cotesting, but it also increased the total number of colposcopies performed.21
Subsequent management of a primary HPV-positive result can be triaged using genotyping, cytology, or a combination of both. FDA-approved HPV screening tests provide genotyping and current management guidelines use genotyping to triage positive HPV results into HPV 16, 18, or 1 of 12 other high-risk HPV genotypes.
In the ATHENA trial, the 3-year incidence of CIN 3+ for HPV 16/18-positive results was 21.16% (95% CI, 18.39%–24.01%) compared with 5.4% (95% CI, 4.5%–6.4%) among patients with an HPV test positive for 1 of the other HPV genotypes.21 While a patient with an HPV result positive for HPV 16/18 should directly undergo colposcopy, clinical guidance for an HPV-positive result for one of the other genotypes suggests using reflex cytology to triage patients. The ASCCP recommended management of primary HPV testing is included in the FIGURE.22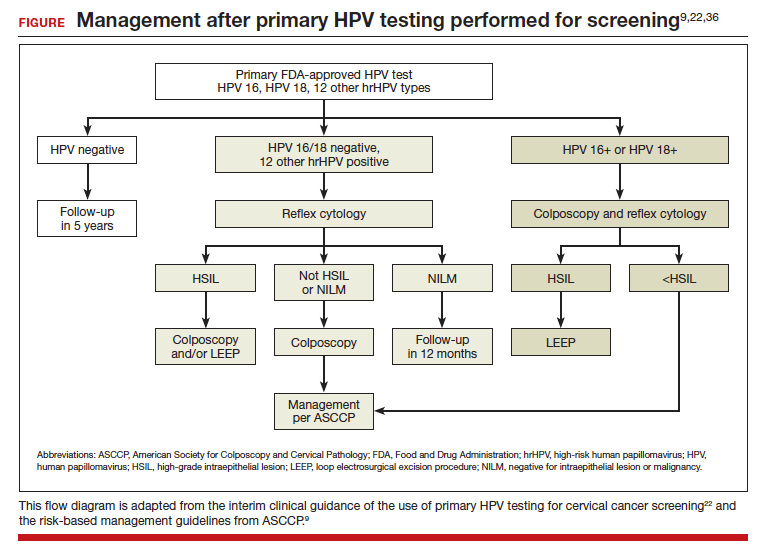
Many barriers remain to transitioning to primary HPV testing, including laboratory test availability as well as patient and provider acceptance. At present, 2 FDA-approved primary HPV screening tests are available: the Cobas HPV test (Roche Molecular Systems, Inc) and the BD Onclarity HPV assay (Becton, Dickinson and Company). Changes to screening recommendations need to be accompanied by patient and provider outreach and education.
In a survey of more than 500 US women in 2015 after guidelines allowed for increased screening intervals after negative results, a majority of women (55.6%; 95% CI, 51.4%–59.8%) were aware that screening recommendations had changed; however, 74.1% (95% CI, 70.3%–77.7%) still believed that women should be screened annually.23 By contrast, participants in the HPV FOCAL trial, who were able to learn more about HPV-based screening, were surveyed about their willingness to undergo primary HPV testing rather than Pap testing at the conclusion of the trial.24 Of the participants, 63% were comfortable with primary HPV testing, and 54% were accepting of an extended screening interval of 4 to 5 years.24
Continue to: p16/Ki-67 dual-stain cytology...
p16/Ki-67 dual-stain cytology
An additional tool for triaging HPV-positive patients is the p16/Ki-67 dual stain test (CINtec Plus Cytology; Roche), which was FDA approved in March 2020. A tumor suppressor protein, p16 is found to be overexpressed by HPV oncogenic activity, and Ki-67 is a marker of cellular proliferation. Coexpression of p16 and Ki-67 indicates a loss of cell cycle regulation and is a hallmark of neoplastic transformation. When positive, this test is supportive of active HPV infection and of a high-grade lesion. While the dual stain test is not yet formally incorporated into triage algorithms by national guidelines, it has demonstrated efficacy in detecting CIN 3+
In the IMPACT trial, nearly 5,000 HPV-positive patients underwent p16/Ki-67 dual stain testing compared with cytology and HPV genotyping.25 The sensitivity of dual stain for CIN 3+ was 91.9% (95% CI, 86.1%–95.4%) in HPV 16/18–positive and 86.0% (95% CI, 77.5%–91.6%) in the 12 other genotypes. Using dual stain testing alone to triage HPV-positive results showed significantly higher sensitivity but lower specificity than using cytology alone to triage HPV-positive results. Importantly, triage with dual stain testing alone would have referred significantly fewer women to colposcopy than HPV 16/18 genotyping with cytology triage for the 12 other genotypes (48.6% vs 56.0%; P< .0001).
Self-sampling methods: An approach for potentially improving access to screening
One technology that may help bridge gaps in access to cervical cancer screening is self-collected HPV testing, which would preclude the need for a clinician-performed pelvic exam. At present, no self-sampling method is approved by the FDA. However, many studies have examined the efficacy and safety of various self-sampling kits.26
One randomized controlled trial in the Netherlands compared sensitivity and specificity of CIN 2+ detection in patient-collected versus clinician-collected swabs.27 After a median follow-up of 20 months, the sensitivity and specificity of HPV testing did not differ between the patient-collected and the clinician-collected groups (specificity 100%; 95% CI, 0.91–1.08; sensitivity 96%; 95% CI, 0.90–1.03).27 This analysis did not include patients who did not return their self-collected sample, which leaves the question of whether self-sampling may exacerbate issues with patients who are lost to follow-up.
In a study performed in the United States, 16,590 patients who were overdue for cervical cancer screening were randomly assigned to usual care reminders (annual mailed reminders and phone calls from clinics) or to the addition of a mailed HPV self-sampling test kit.28 While the study did not demonstrate significant difference in the detection of overall CIN 2+ between the 2 groups, screening uptake was higher in the self-sampling kit group than in the usual care reminders group (RR, 1.51; 95% CI, 1.43–1.60), and the number of abnormal screens that warranted colposcopy referral was similar between the 2 groups (36.4% vs 36.8%).28 In qualitative interviews of the participants of this trial, patients who were sent at-home self-sampling kits found that the convenience of at-home testing lowered barriers to scheduling an in-office appointment.29 The hope is that self-sampling methods will expand access of cervical cancer screening to vulnerable populations that face significant barriers to having an in-office pelvic exam.
It is important to note that self-collection and self-sample testing requires multidisciplinary systems for processing results and assuring necessary patient follow-up. Implementing and disseminating such a program has been well tested only in developed countries27,30 with universal health care systems or within an integrated care delivery system. Bringing such technology broadly to the United States and less developed countries will require continued commitment to increasing laboratory capacity, a central electronic health record or system for monitoring results, educational materials for clinicians and patients, and expanding insurance reimbursement for such testing.
HPV vaccination rates must increase
While we continue to investigate which screening methods will most improve our secondary prevention of cervical cancer, our path to increasing primary prevention of cervical cancer is clear: We must increase rates of HPV vaccination. The 9-valent HPV vaccine is FDA approved for use in all patients aged 9 to 45 years.
The American College of Obstetricians and Gynecologists and other organizations recommend HPV vaccination between the ages of 9 and 13, and a “catch-up period” from ages 13 to 26 in which patients previously not vaccinated should receive the vaccine.31 Initiation of the vaccine course earlier (ages 9–10) compared with later (ages 11–12) is correlated with higher overall completion rates by age 15 and has been suggested to be associated with a stronger immune response.32
A study from Sweden found that HPV vaccination before age 17 was most strongly correlated with the lowest rates of cervical cancer, although vaccination between ages 17 and 30 still significantly decreased the risk of cervical cancer compared with those who were unvaccinated.33
Overall HPV vaccination rates in the United States continue to improve, with 58.6%34 of US adolescents having completed vaccination in 2020. However, these rates still are significantly lower than those in many other developed countries, including Australia, which had a complete vaccination rate of 80.5% in 2020.35 Continued disparities in vaccination rates could be contributing to the rise in cervical cancer among certain groups, such as American Indian and Alaska Native populations.5
Work—and innovations—must continue
In conclusion, the incidence of cervical cancer in the United States continues to decrease, although at disparate rates among marginalized populations. To ensure that we are working toward eliminating cervical cancer for all patients, we must continue efforts to eliminate disparities in health access. Continued innovations, including primary HPV testing and self-collection samples, may contribute to lowering barriers to all patients being able to access the preventative care they need. ●
- Centers for Disease Control and Prevention. United States Cancer Statistics: data visualizations. Trends: changes over time: cervix. Accessed January 8, 2023. https://gis.cdc.gov /Cancer/USCS/#/Trends/
- Sung H, Ferlay J, Siegel RL, et al. Global Cancer Statistics 2020: GLOBOCAN estimates of incidence and mortality worldwide for 36 cancers in 185 countries. CA Cancer J Clin. 2021;71:209-249. doi:10.3322/caac.21660.
- Francoeur AA, Liao CI, Casear MA, et al. The increasing incidence of stage IV cervical cancer in the USA: what factors are related? Int J Gynecol Cancer. 2022;32:ijgc-2022-003728. doi:10.1136/ijgc-2022-003728.
- Abdalla E, Habtemariam T, Fall S, et al. A comparative study of health disparities in cervical cancer mortality rates through time between Black and Caucasian women in Alabama and the US. Int J Stud Nurs. 2021;6:9-23. doi:10.20849/ijsn. v6i1.864.
- Bruegl AS, Emerson J, Tirumala K. Persistent disparities of cervical cancer among American Indians/Alaska natives: are we maximizing prevention tools? Gynecol Oncol. 2023;168:5661. doi:10.1016/j.ygyno.2022.11.007.
- Suk R, Hong YR, Rajan SS, et al. Assessment of US Preventive Services Task Force Guideline–Concordant cervical cancer screening rates and reasons for underscreening by age, race and ethnicity, sexual orientation, rurality, and insurance, 2005 to 2019. JAMA Netw Open. 2022;5:e2143582. doi:10.1001/ jamanetworkopen.2021.43582.
- Fontham ETH, Wolf AMD, Church TR, et al. Cervical cancer screening for individuals at average risk: 2020 guideline update from the American Cancer Society. CA Cancer J Clin. 2020;70:321-346. doi:10.3322/caac.21628.
- US Preventive Services Task Force; Curry SJ, Krist AH, Owens DK, et al. Screening for cervical cancer: US Preventive Services Task Force Recommendation statement. JAMA. 2018;320:674-686. doi:10.1001/jama.2018.10897.
- Nayar R, Chhieng DC, Crothers B, et al. Moving forward—the 2019 ASCCP risk-based management consensus guidelines for abnormal cervical cancer screening tests and cancer precursors and beyond: implications and suggestions for laboratories. J Am Soc Cytopathol. 2020;9:291-303. doi:10.1016/j.jasc.2020.05.002.
- Cooley JJP, Maguire FB, Morris CR, et al. Cervical cancer stage at diagnosis and survival among women ≥65 years in California. Cancer Epidemiol Biomarkers Prev. 2023;32:91-97. doi:10.1158/1055-9965.EPI-22-0793.
- National Cancer Institute. Surveillance, Epidemiology, and End Results Program. Cancer Stat Facts: Cervical Cancer. Accessed February 21, 2023. https://seer.cancer.gov /statfacts/html/cervix.html
- Feldman S. Screening options for preventing cervical cancer. JAMA Intern Med. 2019;179:879-880. doi:10.1001/ jamainternmed.2019.0298.
- ASCO Post Staff. FDA approves first HPV test for primary cervical cancer screening. ASCO Post. May 15, 2014. Accessed January 8, 2023. https://ascopost.com/issues/may-15-2014 /fda-approves-first-hpv-test-for-primary-cervical-cancer -screening/
- Rijkaart DC, Berkhof J, Rozendaal L, et al. Human papillomavirus testing for the detection of high-grade cervical intraepithelial neoplasia and cancer: final results of the POBASCAM randomised controlled trial. Lancet Oncol. 2012;13:78-88. doi:10.1016/S1470-2045(11)70296-0.
- Ronco G, Giorgi-Rossi P, Carozzi F, et al; New Technologies for Cervical Cancer Screening (NTCC) Working Group. Efficacy of human papillomavirus testing for the detection of invasive cervical cancers and cervical intraepithelial neoplasia: a randomised controlled trial. Lancet Oncol. 2010;11:249-257. doi:10.1016/S1470-2045(09)70360-2.
- Kitchener HC, Almonte M, Thomson C, et al. HPV testing in combination with liquid-based cytology in primary cervical screening (ARTISTIC): a randomised controlled trial. Lancet Oncol. 2009;10:672-682. doi:10.1016/S1470-2045(09)70156-1.
- Bulkmans NWJ, Berkhof J, Rozendaal L, et al. Human papillomavirus DNA testing for the detection of cervical intraepithelial neoplasia grade 3 and cancer: 5-year followup of a randomised controlled implementation trial. Lancet. 2007;370:1764-1772. doi:10.1016/S0140-6736(07)61450-0.
- Ogilvie GS, Van Niekerk D, Krajden M, et al. Effect of screening with primary cervical HPV testing vs cytology testing on high-grade cervical intraepithelial neoplasia at 48 months: the HPV FOCAL randomized clinical trial. JAMA. 2018;320:43-52. doi:10.1001/jama.2018.7464.
- Gottschlich A, Gondara L, Smith LW, et al. Human papillomavirus‐based screening at extended intervals missed fewer cervical precancers than cytology in the HPV For Cervical Cancer (HPV FOCAL) trial. Int J Cancer. 2022;151:897-905. doi:10.1002/ijc.34039.
- Katki HA, Kinney WK, Fetterman B, et al. Cervical cancer risk for women undergoing concurrent testing for human papillomavirus and cervical cytology: a population-based study in routine clinical practice. Lancet Oncol. 2011;12:663672. doi:10.1016/S1470-2045(11)70145-0.
- Wright TC, Stoler MH, Behrens CM, et al. Primary cervical cancer screening with human papillomavirus: end of study results from the ATHENA study using HPV as the first-line screening test. Gynecol Oncol. 2015;136:189-197. doi:10.1016/j.ygyno.2014.11.076
- Huh WK, Ault KA, Chelmow D, et al. Use of primary high-risk human papillomavirus testing for cervical cancer screening: interim clinical guidance. Obstet Gynecol. 2015;125:330-337. doi:10.1097/AOG.0000000000000669.
- Silver MI, Rositch AF, Burke AE, et al. Patient concerns about human papillomavirus testing and 5-year intervals in routine cervical cancer screening. Obstet Gynecol. 2015;125:317-329. doi:10.1097/AOG.0000000000000638.
- Smith LW, Racey CS, Gondara L, et al. Women’s acceptability of and experience with primary human papillomavirus testing for cervical screening: HPV FOCAL trial cross-sectional online survey results. BMJ Open. 2021;11:e052084. doi:10.1136/bmjopen-2021-052084.
- Wright TC, Stoler MH, Ranger-Moore J, et al. Clinical validation of p16/Ki-67 dual-stained cytology triage of HPV-positive women: results from the IMPACT trial. Int J Cancer. 2022;150:461-471. doi:10.1002/ijc.33812.
- Yeh PT, Kennedy CE, De Vuyst H, et al. Self-sampling for human papillomavirus (HPV) testing: a systematic review and meta-analysis. BMJ Global Health. 2019;4:e001351. doi:10.1136/bmjgh-2018-001351.
- Polman NJ, Ebisch RMF, Heideman DAM, et al. Performance of human papillomavirus testing on self-collected versus clinician-collected samples for the detection of cervical intraepithelial neoplasia of grade 2 or worse: a randomised, paired screen-positive, non-inferiority trial. Lancet Oncol. 2019;20:229-238. doi:10.1016/S1470-2045(18)30763-0.
- Winer RL, Lin J, Tiro JA, et al. Effect of mailed human papillomavirus test kits vs usual care reminders on cervical cancer screening uptake, precancer detection, and treatment: a randomized clinical trial. JAMA Netw Open. 2019;2:e1914729. doi:10.1001/jamanetworkopen.2019.14729.
- Tiro JA, Betts AC, Kimbel K, et al. Understanding patients’ perspectives and information needs following a positive home human papillomavirus self-sampling kit result. J Womens Health (Larchmt). 2019;28:384-392. doi:10.1089/ jwh.2018.7070.
- Knauss T, Hansen BT, Pedersen K, et al. The cost-effectiveness of opt-in and send-to-all HPV self-sampling among long-term non-attenders to cervical cancer screening in Norway: the Equalscreen randomized controlled trial. Gynecol Oncol. 2023;168:39-47. doi:10.1016/j.ygyno.2022.10.027.
- ACOG committee opinion no. 809. Human papillomavirus vaccination: correction. Obstet Gynecol. 2022;139:345. doi:10.1097/AOG.0000000000004680.
- St Sauver JL, Finney Rutten LJF, Ebbert JO, et al. Younger age at initiation of the human papillomavirus (HPV) vaccination series is associated with higher rates of on-time completion. Prev Med. 2016;89:327-333. doi:10.1016/j.ypmed.2016.02.039.
- Lei J, Ploner A, Elfström KM, et al. HPV vaccination and the risk of invasive cervical cancer. N Engl J Med. 2020;383:13401348. doi:10.1056/NEJMoa1917338.
- Pingali C, Yankey D, Elam-Evans LD, et al. National, regional, state, and selected local area vaccination coverage among adolescents aged 13–17 years — United States, 2020. MMWR Morb Mortal Wkly Rep. 2021;70:1183-1190. doi:10.15585/ mmwr.mm7035a1.
- National Centre for Immunisation Research and Surveillance Australia. Annual Immunisation Coverage Report 2020. November 29, 2021. Accessed March 1, 2023. https://ncirs .org.au/sites/default/files/2021-11/NCIRS%20Annual%20 Immunisation%20Coverage%20Report%202020_FINAL.pdf
- Leung SOA, Feldman S. 2022 Update on cervical disease. OBG Manag. 2022;34(5):16-17, 22-24, 26, 28. doi:10.12788/ obgm.0197.
CASE Intervention approaches for decreasing the risk of cervical cancer
A 25-year-old woman presents to your practice for routine examination. She has never undergone cervical cancer screening or received the human papillomavirus (HPV) vaccine series. The patient has had 3 lifetime sexual partners and currently uses condoms as contraception. What interventions are appropriate to offer this patient to decrease her risk of cervical cancer? Choose as many that may apply:
1. cervical cytology with reflex HPV testing
2. cervical cytology with HPV cotesting
3. primary HPV testing
4. HPV vaccine series (3 doses)
5. all of the above
The answer is number 5, all of the above.
Choices 1, 2, and 3 are acceptable methods of cervical cancer screening for this patient. Catch-up HPV vaccination should be offered as well.
Equitable preventive care is needed
Cervical cancer is a unique cancer because it has a known preventative strategy. HPV vaccination, paired with cervical screening and management of abnormal results, has contributed to decreased rates of cervical cancer in the United States, from 13,914 cases in 1999 to 12,795 cases in 2019.1 In less-developed countries, however, cervical cancer continues to be a leading cause of mortality, with 90% of cervical cancer deaths in 2020 occurring in low- and middle-income countries.2
Disparate outcomes in cervical cancer are often a reflection of disparities in health access. Within the United States, Black women have a higher incidence of cervical cancer, advanced-stage disease, and mortality from cervical cancer than White women.3,4 Furthermore, the incidence of cervical cancer increased among American Indian and Alaska Native people between 2000 and 2019.5 The rate for patients who are overdue for cervical cancer screening is higher among Asian and Hispanic patients compared with non-Hispanic White patients (31.4% vs 20.1%; P=.01) and among patients who identify as LGBTQ+ compared with patients who identify as heterosexual (32.0% vs 22.2%; P<.001).6 Younger patients have a significantly higher rate for overdue screening compared with their older counterparts (29.1% vs 21.1%; P<.001), as do uninsured patients compared with those who are privately insured (41.7% vs 18.1%; P<.001). Overall, the proportion of women without up-to-date screening increased significantly from 2005 to 2019 (14.4% vs 23.0%; P<.001).6
Unfortunately, despite a known strategy to eliminate cervical cancer, we are not accomplishing equitable preventative care. Barriers to care can include patient-centered issues, such as fear of cancer or of painful evaluations, lack of trust in the health care system, and inadequate understanding of the benefits of cancer prevention, in addition to systemic and structural barriers. As we assess new technologies, one of our most important goals is to consider how such innovations can increase health access—whether through increasing ease and acceptability of testing or by creating more effective screening tests.
Updates to cervical screening guidance
In 2020, the American Cancer Society (ACS) updated its cervical screening guidelines to start screening at age 25 years with the “preferred” strategy of HPV primary testing every 5 years.7 By contrast, the US Preventive Services Task Force (USPSTF) continues to recommend 1 of 3 methods: cytology alone every 3 years; cytology alone every 3 years between ages 21 and 29 followed by cytology and HPV cotesting every 5 years at age 30 or older; or high-risk HPV testing alone every 5 years (TABLE).8

To successfully prevent cervical cancer, abnormal results are managed by performing either colposcopy with biopsy, immediate treatment, or close surveillance based on the risk of developing cervical intraepithelial neoplasia (CIN) 3 or worse. A patient’s risk is determined based on both current and prior test results. The ASCCP (American Society for Colposcopy and Cervical Pathology) transitioned to risk-based management guidelines in 2019 and has both an app and a web-based risk assessment tool available for clinicians (https://www.asccp.org).9
All organizations recommend stopping screening after age 65 provided there has been a history of adequate screening in the prior 10 years (defined as 2 normal cotests or 3 normal cytology tests, with the most recent test within 5 years) and no history of CIN 2 or worse within the prior 25 years.10,11 Recent studies that examined the rate of cervical cancer diagnosed in patients older than 65 years have questioned whether patients should continue screening beyond 65.10 In the United States, 20% of cervical cancer still occurs in women older than age 65.11 One reason may be that many women have not met the requirement for adequate and normal prior screening and may still need ongoing testing.12

Continue to: Primary HPV screening...
Primary HPV screening
Primary HPV testing means that an HPV test is performed first, and if it is positive for high-risk HPV, further testing is performed to determine next steps. This contrasts with the currently used method of obtaining cytology (Pap) first with either concurrent HPV testing or reflex HPV testing. The first HPV primary screening test was approved by the US Food and Drug Administration (FDA) in 2014.13
Multiple randomized controlled trials in Europe have demonstrated the accuracy of HPV-based screening compared with cytology in the detection of cervical cancer and its precursors.14-17 The HPV FOCAL trial demonstrated increased efficacy of primary HPV screening in the detection of CIN 2+ lesions.18 This trial recruited a total of 19,000 women, ages 25 to 65, in Canada and randomly assigned them to receive primary HPV testing or liquid-based cytology. If primary HPV testing was negative, participants would return in 48 months for cytology and HPV cotesting. If primary liquid-based cytology testing was negative, participants would return at 24 months for cytology testing alone and at 48 months for cytology and HPV cotesting. Both groups had similar incidences of CIN 2+ over the study period. HPV testing was shown to detect CIN 2+ at higher rates at the time of initial screen (risk ratio [RR], 1.61; 95% confidence interval [CI], 1.24–2.09) and then significantly lower rates at the time of exit screening at 48 months (RR, 0.36; 95% CI, 0.24–0.54).18 These results demonstrated that primary HPV testing detects CIN 2+ earlier than cytology alone. In follow-up analyses, primary HPV screening missed fewer CIN 2+ diagnoses than cytology screening.19
While not as many studies have compared primary HPV testing to cytology with an HPV cotest, the current most common practice in the United States, one study performed in the United States found that a negative cytology result did not further decrease the risk of CIN 3 for HPV-negative patients (risk of CIN 3+ at 5 years: 0.16% vs 0.17%; P=0.8) and concluded that a negative HPV test was enough reassurance for a low risk of CIN 3+.20
Another study, the ATHENA trial, evaluated more than 42,000 women who were 25 years and older over a 3-year period.21 Patients underwent either primary HPV testing or combination cytology and reflex HPV (if ages 25–29) or HPV cotesting (if age 30 or older). Primary HPV testing was found to have a sensitivity and specificity of 76.1% and 93.5%, respectively, compared with 61.7% and 94.6% for cytology with HPV cotesting, but it also increased the total number of colposcopies performed.21
Subsequent management of a primary HPV-positive result can be triaged using genotyping, cytology, or a combination of both. FDA-approved HPV screening tests provide genotyping and current management guidelines use genotyping to triage positive HPV results into HPV 16, 18, or 1 of 12 other high-risk HPV genotypes.
In the ATHENA trial, the 3-year incidence of CIN 3+ for HPV 16/18-positive results was 21.16% (95% CI, 18.39%–24.01%) compared with 5.4% (95% CI, 4.5%–6.4%) among patients with an HPV test positive for 1 of the other HPV genotypes.21 While a patient with an HPV result positive for HPV 16/18 should directly undergo colposcopy, clinical guidance for an HPV-positive result for one of the other genotypes suggests using reflex cytology to triage patients. The ASCCP recommended management of primary HPV testing is included in the FIGURE.22
Many barriers remain to transitioning to primary HPV testing, including laboratory test availability as well as patient and provider acceptance. At present, 2 FDA-approved primary HPV screening tests are available: the Cobas HPV test (Roche Molecular Systems, Inc) and the BD Onclarity HPV assay (Becton, Dickinson and Company). Changes to screening recommendations need to be accompanied by patient and provider outreach and education.
In a survey of more than 500 US women in 2015 after guidelines allowed for increased screening intervals after negative results, a majority of women (55.6%; 95% CI, 51.4%–59.8%) were aware that screening recommendations had changed; however, 74.1% (95% CI, 70.3%–77.7%) still believed that women should be screened annually.23 By contrast, participants in the HPV FOCAL trial, who were able to learn more about HPV-based screening, were surveyed about their willingness to undergo primary HPV testing rather than Pap testing at the conclusion of the trial.24 Of the participants, 63% were comfortable with primary HPV testing, and 54% were accepting of an extended screening interval of 4 to 5 years.24
Continue to: p16/Ki-67 dual-stain cytology...
p16/Ki-67 dual-stain cytology
An additional tool for triaging HPV-positive patients is the p16/Ki-67 dual stain test (CINtec Plus Cytology; Roche), which was FDA approved in March 2020. A tumor suppressor protein, p16 is found to be overexpressed by HPV oncogenic activity, and Ki-67 is a marker of cellular proliferation. Coexpression of p16 and Ki-67 indicates a loss of cell cycle regulation and is a hallmark of neoplastic transformation. When positive, this test is supportive of active HPV infection and of a high-grade lesion. While the dual stain test is not yet formally incorporated into triage algorithms by national guidelines, it has demonstrated efficacy in detecting CIN 3+
In the IMPACT trial, nearly 5,000 HPV-positive patients underwent p16/Ki-67 dual stain testing compared with cytology and HPV genotyping.25 The sensitivity of dual stain for CIN 3+ was 91.9% (95% CI, 86.1%–95.4%) in HPV 16/18–positive and 86.0% (95% CI, 77.5%–91.6%) in the 12 other genotypes. Using dual stain testing alone to triage HPV-positive results showed significantly higher sensitivity but lower specificity than using cytology alone to triage HPV-positive results. Importantly, triage with dual stain testing alone would have referred significantly fewer women to colposcopy than HPV 16/18 genotyping with cytology triage for the 12 other genotypes (48.6% vs 56.0%; P< .0001).
Self-sampling methods: An approach for potentially improving access to screening
One technology that may help bridge gaps in access to cervical cancer screening is self-collected HPV testing, which would preclude the need for a clinician-performed pelvic exam. At present, no self-sampling method is approved by the FDA. However, many studies have examined the efficacy and safety of various self-sampling kits.26
One randomized controlled trial in the Netherlands compared sensitivity and specificity of CIN 2+ detection in patient-collected versus clinician-collected swabs.27 After a median follow-up of 20 months, the sensitivity and specificity of HPV testing did not differ between the patient-collected and the clinician-collected groups (specificity 100%; 95% CI, 0.91–1.08; sensitivity 96%; 95% CI, 0.90–1.03).27 This analysis did not include patients who did not return their self-collected sample, which leaves the question of whether self-sampling may exacerbate issues with patients who are lost to follow-up.
In a study performed in the United States, 16,590 patients who were overdue for cervical cancer screening were randomly assigned to usual care reminders (annual mailed reminders and phone calls from clinics) or to the addition of a mailed HPV self-sampling test kit.28 While the study did not demonstrate significant difference in the detection of overall CIN 2+ between the 2 groups, screening uptake was higher in the self-sampling kit group than in the usual care reminders group (RR, 1.51; 95% CI, 1.43–1.60), and the number of abnormal screens that warranted colposcopy referral was similar between the 2 groups (36.4% vs 36.8%).28 In qualitative interviews of the participants of this trial, patients who were sent at-home self-sampling kits found that the convenience of at-home testing lowered barriers to scheduling an in-office appointment.29 The hope is that self-sampling methods will expand access of cervical cancer screening to vulnerable populations that face significant barriers to having an in-office pelvic exam.
It is important to note that self-collection and self-sample testing requires multidisciplinary systems for processing results and assuring necessary patient follow-up. Implementing and disseminating such a program has been well tested only in developed countries27,30 with universal health care systems or within an integrated care delivery system. Bringing such technology broadly to the United States and less developed countries will require continued commitment to increasing laboratory capacity, a central electronic health record or system for monitoring results, educational materials for clinicians and patients, and expanding insurance reimbursement for such testing.
HPV vaccination rates must increase
While we continue to investigate which screening methods will most improve our secondary prevention of cervical cancer, our path to increasing primary prevention of cervical cancer is clear: We must increase rates of HPV vaccination. The 9-valent HPV vaccine is FDA approved for use in all patients aged 9 to 45 years.
The American College of Obstetricians and Gynecologists and other organizations recommend HPV vaccination between the ages of 9 and 13, and a “catch-up period” from ages 13 to 26 in which patients previously not vaccinated should receive the vaccine.31 Initiation of the vaccine course earlier (ages 9–10) compared with later (ages 11–12) is correlated with higher overall completion rates by age 15 and has been suggested to be associated with a stronger immune response.32
A study from Sweden found that HPV vaccination before age 17 was most strongly correlated with the lowest rates of cervical cancer, although vaccination between ages 17 and 30 still significantly decreased the risk of cervical cancer compared with those who were unvaccinated.33
Overall HPV vaccination rates in the United States continue to improve, with 58.6%34 of US adolescents having completed vaccination in 2020. However, these rates still are significantly lower than those in many other developed countries, including Australia, which had a complete vaccination rate of 80.5% in 2020.35 Continued disparities in vaccination rates could be contributing to the rise in cervical cancer among certain groups, such as American Indian and Alaska Native populations.5
Work—and innovations—must continue
In conclusion, the incidence of cervical cancer in the United States continues to decrease, although at disparate rates among marginalized populations. To ensure that we are working toward eliminating cervical cancer for all patients, we must continue efforts to eliminate disparities in health access. Continued innovations, including primary HPV testing and self-collection samples, may contribute to lowering barriers to all patients being able to access the preventative care they need. ●
CASE Intervention approaches for decreasing the risk of cervical cancer
A 25-year-old woman presents to your practice for routine examination. She has never undergone cervical cancer screening or received the human papillomavirus (HPV) vaccine series. The patient has had 3 lifetime sexual partners and currently uses condoms as contraception. What interventions are appropriate to offer this patient to decrease her risk of cervical cancer? Choose as many that may apply:
1. cervical cytology with reflex HPV testing
2. cervical cytology with HPV cotesting
3. primary HPV testing
4. HPV vaccine series (3 doses)
5. all of the above
The answer is number 5, all of the above.
Choices 1, 2, and 3 are acceptable methods of cervical cancer screening for this patient. Catch-up HPV vaccination should be offered as well.
Equitable preventive care is needed
Cervical cancer is a unique cancer because it has a known preventative strategy. HPV vaccination, paired with cervical screening and management of abnormal results, has contributed to decreased rates of cervical cancer in the United States, from 13,914 cases in 1999 to 12,795 cases in 2019.1 In less-developed countries, however, cervical cancer continues to be a leading cause of mortality, with 90% of cervical cancer deaths in 2020 occurring in low- and middle-income countries.2
Disparate outcomes in cervical cancer are often a reflection of disparities in health access. Within the United States, Black women have a higher incidence of cervical cancer, advanced-stage disease, and mortality from cervical cancer than White women.3,4 Furthermore, the incidence of cervical cancer increased among American Indian and Alaska Native people between 2000 and 2019.5 The rate for patients who are overdue for cervical cancer screening is higher among Asian and Hispanic patients compared with non-Hispanic White patients (31.4% vs 20.1%; P=.01) and among patients who identify as LGBTQ+ compared with patients who identify as heterosexual (32.0% vs 22.2%; P<.001).6 Younger patients have a significantly higher rate for overdue screening compared with their older counterparts (29.1% vs 21.1%; P<.001), as do uninsured patients compared with those who are privately insured (41.7% vs 18.1%; P<.001). Overall, the proportion of women without up-to-date screening increased significantly from 2005 to 2019 (14.4% vs 23.0%; P<.001).6
Unfortunately, despite a known strategy to eliminate cervical cancer, we are not accomplishing equitable preventative care. Barriers to care can include patient-centered issues, such as fear of cancer or of painful evaluations, lack of trust in the health care system, and inadequate understanding of the benefits of cancer prevention, in addition to systemic and structural barriers. As we assess new technologies, one of our most important goals is to consider how such innovations can increase health access—whether through increasing ease and acceptability of testing or by creating more effective screening tests.
Updates to cervical screening guidance
In 2020, the American Cancer Society (ACS) updated its cervical screening guidelines to start screening at age 25 years with the “preferred” strategy of HPV primary testing every 5 years.7 By contrast, the US Preventive Services Task Force (USPSTF) continues to recommend 1 of 3 methods: cytology alone every 3 years; cytology alone every 3 years between ages 21 and 29 followed by cytology and HPV cotesting every 5 years at age 30 or older; or high-risk HPV testing alone every 5 years (TABLE).8

To successfully prevent cervical cancer, abnormal results are managed by performing either colposcopy with biopsy, immediate treatment, or close surveillance based on the risk of developing cervical intraepithelial neoplasia (CIN) 3 or worse. A patient’s risk is determined based on both current and prior test results. The ASCCP (American Society for Colposcopy and Cervical Pathology) transitioned to risk-based management guidelines in 2019 and has both an app and a web-based risk assessment tool available for clinicians (https://www.asccp.org).9
All organizations recommend stopping screening after age 65 provided there has been a history of adequate screening in the prior 10 years (defined as 2 normal cotests or 3 normal cytology tests, with the most recent test within 5 years) and no history of CIN 2 or worse within the prior 25 years.10,11 Recent studies that examined the rate of cervical cancer diagnosed in patients older than 65 years have questioned whether patients should continue screening beyond 65.10 In the United States, 20% of cervical cancer still occurs in women older than age 65.11 One reason may be that many women have not met the requirement for adequate and normal prior screening and may still need ongoing testing.12

Continue to: Primary HPV screening...
Primary HPV screening
Primary HPV testing means that an HPV test is performed first, and if it is positive for high-risk HPV, further testing is performed to determine next steps. This contrasts with the currently used method of obtaining cytology (Pap) first with either concurrent HPV testing or reflex HPV testing. The first HPV primary screening test was approved by the US Food and Drug Administration (FDA) in 2014.13
Multiple randomized controlled trials in Europe have demonstrated the accuracy of HPV-based screening compared with cytology in the detection of cervical cancer and its precursors.14-17 The HPV FOCAL trial demonstrated increased efficacy of primary HPV screening in the detection of CIN 2+ lesions.18 This trial recruited a total of 19,000 women, ages 25 to 65, in Canada and randomly assigned them to receive primary HPV testing or liquid-based cytology. If primary HPV testing was negative, participants would return in 48 months for cytology and HPV cotesting. If primary liquid-based cytology testing was negative, participants would return at 24 months for cytology testing alone and at 48 months for cytology and HPV cotesting. Both groups had similar incidences of CIN 2+ over the study period. HPV testing was shown to detect CIN 2+ at higher rates at the time of initial screen (risk ratio [RR], 1.61; 95% confidence interval [CI], 1.24–2.09) and then significantly lower rates at the time of exit screening at 48 months (RR, 0.36; 95% CI, 0.24–0.54).18 These results demonstrated that primary HPV testing detects CIN 2+ earlier than cytology alone. In follow-up analyses, primary HPV screening missed fewer CIN 2+ diagnoses than cytology screening.19
While not as many studies have compared primary HPV testing to cytology with an HPV cotest, the current most common practice in the United States, one study performed in the United States found that a negative cytology result did not further decrease the risk of CIN 3 for HPV-negative patients (risk of CIN 3+ at 5 years: 0.16% vs 0.17%; P=0.8) and concluded that a negative HPV test was enough reassurance for a low risk of CIN 3+.20
Another study, the ATHENA trial, evaluated more than 42,000 women who were 25 years and older over a 3-year period.21 Patients underwent either primary HPV testing or combination cytology and reflex HPV (if ages 25–29) or HPV cotesting (if age 30 or older). Primary HPV testing was found to have a sensitivity and specificity of 76.1% and 93.5%, respectively, compared with 61.7% and 94.6% for cytology with HPV cotesting, but it also increased the total number of colposcopies performed.21
Subsequent management of a primary HPV-positive result can be triaged using genotyping, cytology, or a combination of both. FDA-approved HPV screening tests provide genotyping and current management guidelines use genotyping to triage positive HPV results into HPV 16, 18, or 1 of 12 other high-risk HPV genotypes.
In the ATHENA trial, the 3-year incidence of CIN 3+ for HPV 16/18-positive results was 21.16% (95% CI, 18.39%–24.01%) compared with 5.4% (95% CI, 4.5%–6.4%) among patients with an HPV test positive for 1 of the other HPV genotypes.21 While a patient with an HPV result positive for HPV 16/18 should directly undergo colposcopy, clinical guidance for an HPV-positive result for one of the other genotypes suggests using reflex cytology to triage patients. The ASCCP recommended management of primary HPV testing is included in the FIGURE.22
Many barriers remain to transitioning to primary HPV testing, including laboratory test availability as well as patient and provider acceptance. At present, 2 FDA-approved primary HPV screening tests are available: the Cobas HPV test (Roche Molecular Systems, Inc) and the BD Onclarity HPV assay (Becton, Dickinson and Company). Changes to screening recommendations need to be accompanied by patient and provider outreach and education.
In a survey of more than 500 US women in 2015 after guidelines allowed for increased screening intervals after negative results, a majority of women (55.6%; 95% CI, 51.4%–59.8%) were aware that screening recommendations had changed; however, 74.1% (95% CI, 70.3%–77.7%) still believed that women should be screened annually.23 By contrast, participants in the HPV FOCAL trial, who were able to learn more about HPV-based screening, were surveyed about their willingness to undergo primary HPV testing rather than Pap testing at the conclusion of the trial.24 Of the participants, 63% were comfortable with primary HPV testing, and 54% were accepting of an extended screening interval of 4 to 5 years.24
Continue to: p16/Ki-67 dual-stain cytology...
p16/Ki-67 dual-stain cytology
An additional tool for triaging HPV-positive patients is the p16/Ki-67 dual stain test (CINtec Plus Cytology; Roche), which was FDA approved in March 2020. A tumor suppressor protein, p16 is found to be overexpressed by HPV oncogenic activity, and Ki-67 is a marker of cellular proliferation. Coexpression of p16 and Ki-67 indicates a loss of cell cycle regulation and is a hallmark of neoplastic transformation. When positive, this test is supportive of active HPV infection and of a high-grade lesion. While the dual stain test is not yet formally incorporated into triage algorithms by national guidelines, it has demonstrated efficacy in detecting CIN 3+
In the IMPACT trial, nearly 5,000 HPV-positive patients underwent p16/Ki-67 dual stain testing compared with cytology and HPV genotyping.25 The sensitivity of dual stain for CIN 3+ was 91.9% (95% CI, 86.1%–95.4%) in HPV 16/18–positive and 86.0% (95% CI, 77.5%–91.6%) in the 12 other genotypes. Using dual stain testing alone to triage HPV-positive results showed significantly higher sensitivity but lower specificity than using cytology alone to triage HPV-positive results. Importantly, triage with dual stain testing alone would have referred significantly fewer women to colposcopy than HPV 16/18 genotyping with cytology triage for the 12 other genotypes (48.6% vs 56.0%; P< .0001).
Self-sampling methods: An approach for potentially improving access to screening
One technology that may help bridge gaps in access to cervical cancer screening is self-collected HPV testing, which would preclude the need for a clinician-performed pelvic exam. At present, no self-sampling method is approved by the FDA. However, many studies have examined the efficacy and safety of various self-sampling kits.26
One randomized controlled trial in the Netherlands compared sensitivity and specificity of CIN 2+ detection in patient-collected versus clinician-collected swabs.27 After a median follow-up of 20 months, the sensitivity and specificity of HPV testing did not differ between the patient-collected and the clinician-collected groups (specificity 100%; 95% CI, 0.91–1.08; sensitivity 96%; 95% CI, 0.90–1.03).27 This analysis did not include patients who did not return their self-collected sample, which leaves the question of whether self-sampling may exacerbate issues with patients who are lost to follow-up.
In a study performed in the United States, 16,590 patients who were overdue for cervical cancer screening were randomly assigned to usual care reminders (annual mailed reminders and phone calls from clinics) or to the addition of a mailed HPV self-sampling test kit.28 While the study did not demonstrate significant difference in the detection of overall CIN 2+ between the 2 groups, screening uptake was higher in the self-sampling kit group than in the usual care reminders group (RR, 1.51; 95% CI, 1.43–1.60), and the number of abnormal screens that warranted colposcopy referral was similar between the 2 groups (36.4% vs 36.8%).28 In qualitative interviews of the participants of this trial, patients who were sent at-home self-sampling kits found that the convenience of at-home testing lowered barriers to scheduling an in-office appointment.29 The hope is that self-sampling methods will expand access of cervical cancer screening to vulnerable populations that face significant barriers to having an in-office pelvic exam.
It is important to note that self-collection and self-sample testing requires multidisciplinary systems for processing results and assuring necessary patient follow-up. Implementing and disseminating such a program has been well tested only in developed countries27,30 with universal health care systems or within an integrated care delivery system. Bringing such technology broadly to the United States and less developed countries will require continued commitment to increasing laboratory capacity, a central electronic health record or system for monitoring results, educational materials for clinicians and patients, and expanding insurance reimbursement for such testing.
HPV vaccination rates must increase
While we continue to investigate which screening methods will most improve our secondary prevention of cervical cancer, our path to increasing primary prevention of cervical cancer is clear: We must increase rates of HPV vaccination. The 9-valent HPV vaccine is FDA approved for use in all patients aged 9 to 45 years.
The American College of Obstetricians and Gynecologists and other organizations recommend HPV vaccination between the ages of 9 and 13, and a “catch-up period” from ages 13 to 26 in which patients previously not vaccinated should receive the vaccine.31 Initiation of the vaccine course earlier (ages 9–10) compared with later (ages 11–12) is correlated with higher overall completion rates by age 15 and has been suggested to be associated with a stronger immune response.32
A study from Sweden found that HPV vaccination before age 17 was most strongly correlated with the lowest rates of cervical cancer, although vaccination between ages 17 and 30 still significantly decreased the risk of cervical cancer compared with those who were unvaccinated.33
Overall HPV vaccination rates in the United States continue to improve, with 58.6%34 of US adolescents having completed vaccination in 2020. However, these rates still are significantly lower than those in many other developed countries, including Australia, which had a complete vaccination rate of 80.5% in 2020.35 Continued disparities in vaccination rates could be contributing to the rise in cervical cancer among certain groups, such as American Indian and Alaska Native populations.5
Work—and innovations—must continue
In conclusion, the incidence of cervical cancer in the United States continues to decrease, although at disparate rates among marginalized populations. To ensure that we are working toward eliminating cervical cancer for all patients, we must continue efforts to eliminate disparities in health access. Continued innovations, including primary HPV testing and self-collection samples, may contribute to lowering barriers to all patients being able to access the preventative care they need. ●
- Centers for Disease Control and Prevention. United States Cancer Statistics: data visualizations. Trends: changes over time: cervix. Accessed January 8, 2023. https://gis.cdc.gov /Cancer/USCS/#/Trends/
- Sung H, Ferlay J, Siegel RL, et al. Global Cancer Statistics 2020: GLOBOCAN estimates of incidence and mortality worldwide for 36 cancers in 185 countries. CA Cancer J Clin. 2021;71:209-249. doi:10.3322/caac.21660.
- Francoeur AA, Liao CI, Casear MA, et al. The increasing incidence of stage IV cervical cancer in the USA: what factors are related? Int J Gynecol Cancer. 2022;32:ijgc-2022-003728. doi:10.1136/ijgc-2022-003728.
- Abdalla E, Habtemariam T, Fall S, et al. A comparative study of health disparities in cervical cancer mortality rates through time between Black and Caucasian women in Alabama and the US. Int J Stud Nurs. 2021;6:9-23. doi:10.20849/ijsn. v6i1.864.
- Bruegl AS, Emerson J, Tirumala K. Persistent disparities of cervical cancer among American Indians/Alaska natives: are we maximizing prevention tools? Gynecol Oncol. 2023;168:5661. doi:10.1016/j.ygyno.2022.11.007.
- Suk R, Hong YR, Rajan SS, et al. Assessment of US Preventive Services Task Force Guideline–Concordant cervical cancer screening rates and reasons for underscreening by age, race and ethnicity, sexual orientation, rurality, and insurance, 2005 to 2019. JAMA Netw Open. 2022;5:e2143582. doi:10.1001/ jamanetworkopen.2021.43582.
- Fontham ETH, Wolf AMD, Church TR, et al. Cervical cancer screening for individuals at average risk: 2020 guideline update from the American Cancer Society. CA Cancer J Clin. 2020;70:321-346. doi:10.3322/caac.21628.
- US Preventive Services Task Force; Curry SJ, Krist AH, Owens DK, et al. Screening for cervical cancer: US Preventive Services Task Force Recommendation statement. JAMA. 2018;320:674-686. doi:10.1001/jama.2018.10897.
- Nayar R, Chhieng DC, Crothers B, et al. Moving forward—the 2019 ASCCP risk-based management consensus guidelines for abnormal cervical cancer screening tests and cancer precursors and beyond: implications and suggestions for laboratories. J Am Soc Cytopathol. 2020;9:291-303. doi:10.1016/j.jasc.2020.05.002.
- Cooley JJP, Maguire FB, Morris CR, et al. Cervical cancer stage at diagnosis and survival among women ≥65 years in California. Cancer Epidemiol Biomarkers Prev. 2023;32:91-97. doi:10.1158/1055-9965.EPI-22-0793.
- National Cancer Institute. Surveillance, Epidemiology, and End Results Program. Cancer Stat Facts: Cervical Cancer. Accessed February 21, 2023. https://seer.cancer.gov /statfacts/html/cervix.html
- Feldman S. Screening options for preventing cervical cancer. JAMA Intern Med. 2019;179:879-880. doi:10.1001/ jamainternmed.2019.0298.
- ASCO Post Staff. FDA approves first HPV test for primary cervical cancer screening. ASCO Post. May 15, 2014. Accessed January 8, 2023. https://ascopost.com/issues/may-15-2014 /fda-approves-first-hpv-test-for-primary-cervical-cancer -screening/
- Rijkaart DC, Berkhof J, Rozendaal L, et al. Human papillomavirus testing for the detection of high-grade cervical intraepithelial neoplasia and cancer: final results of the POBASCAM randomised controlled trial. Lancet Oncol. 2012;13:78-88. doi:10.1016/S1470-2045(11)70296-0.
- Ronco G, Giorgi-Rossi P, Carozzi F, et al; New Technologies for Cervical Cancer Screening (NTCC) Working Group. Efficacy of human papillomavirus testing for the detection of invasive cervical cancers and cervical intraepithelial neoplasia: a randomised controlled trial. Lancet Oncol. 2010;11:249-257. doi:10.1016/S1470-2045(09)70360-2.
- Kitchener HC, Almonte M, Thomson C, et al. HPV testing in combination with liquid-based cytology in primary cervical screening (ARTISTIC): a randomised controlled trial. Lancet Oncol. 2009;10:672-682. doi:10.1016/S1470-2045(09)70156-1.
- Bulkmans NWJ, Berkhof J, Rozendaal L, et al. Human papillomavirus DNA testing for the detection of cervical intraepithelial neoplasia grade 3 and cancer: 5-year followup of a randomised controlled implementation trial. Lancet. 2007;370:1764-1772. doi:10.1016/S0140-6736(07)61450-0.
- Ogilvie GS, Van Niekerk D, Krajden M, et al. Effect of screening with primary cervical HPV testing vs cytology testing on high-grade cervical intraepithelial neoplasia at 48 months: the HPV FOCAL randomized clinical trial. JAMA. 2018;320:43-52. doi:10.1001/jama.2018.7464.
- Gottschlich A, Gondara L, Smith LW, et al. Human papillomavirus‐based screening at extended intervals missed fewer cervical precancers than cytology in the HPV For Cervical Cancer (HPV FOCAL) trial. Int J Cancer. 2022;151:897-905. doi:10.1002/ijc.34039.
- Katki HA, Kinney WK, Fetterman B, et al. Cervical cancer risk for women undergoing concurrent testing for human papillomavirus and cervical cytology: a population-based study in routine clinical practice. Lancet Oncol. 2011;12:663672. doi:10.1016/S1470-2045(11)70145-0.
- Wright TC, Stoler MH, Behrens CM, et al. Primary cervical cancer screening with human papillomavirus: end of study results from the ATHENA study using HPV as the first-line screening test. Gynecol Oncol. 2015;136:189-197. doi:10.1016/j.ygyno.2014.11.076
- Huh WK, Ault KA, Chelmow D, et al. Use of primary high-risk human papillomavirus testing for cervical cancer screening: interim clinical guidance. Obstet Gynecol. 2015;125:330-337. doi:10.1097/AOG.0000000000000669.
- Silver MI, Rositch AF, Burke AE, et al. Patient concerns about human papillomavirus testing and 5-year intervals in routine cervical cancer screening. Obstet Gynecol. 2015;125:317-329. doi:10.1097/AOG.0000000000000638.
- Smith LW, Racey CS, Gondara L, et al. Women’s acceptability of and experience with primary human papillomavirus testing for cervical screening: HPV FOCAL trial cross-sectional online survey results. BMJ Open. 2021;11:e052084. doi:10.1136/bmjopen-2021-052084.
- Wright TC, Stoler MH, Ranger-Moore J, et al. Clinical validation of p16/Ki-67 dual-stained cytology triage of HPV-positive women: results from the IMPACT trial. Int J Cancer. 2022;150:461-471. doi:10.1002/ijc.33812.
- Yeh PT, Kennedy CE, De Vuyst H, et al. Self-sampling for human papillomavirus (HPV) testing: a systematic review and meta-analysis. BMJ Global Health. 2019;4:e001351. doi:10.1136/bmjgh-2018-001351.
- Polman NJ, Ebisch RMF, Heideman DAM, et al. Performance of human papillomavirus testing on self-collected versus clinician-collected samples for the detection of cervical intraepithelial neoplasia of grade 2 or worse: a randomised, paired screen-positive, non-inferiority trial. Lancet Oncol. 2019;20:229-238. doi:10.1016/S1470-2045(18)30763-0.
- Winer RL, Lin J, Tiro JA, et al. Effect of mailed human papillomavirus test kits vs usual care reminders on cervical cancer screening uptake, precancer detection, and treatment: a randomized clinical trial. JAMA Netw Open. 2019;2:e1914729. doi:10.1001/jamanetworkopen.2019.14729.
- Tiro JA, Betts AC, Kimbel K, et al. Understanding patients’ perspectives and information needs following a positive home human papillomavirus self-sampling kit result. J Womens Health (Larchmt). 2019;28:384-392. doi:10.1089/ jwh.2018.7070.
- Knauss T, Hansen BT, Pedersen K, et al. The cost-effectiveness of opt-in and send-to-all HPV self-sampling among long-term non-attenders to cervical cancer screening in Norway: the Equalscreen randomized controlled trial. Gynecol Oncol. 2023;168:39-47. doi:10.1016/j.ygyno.2022.10.027.
- ACOG committee opinion no. 809. Human papillomavirus vaccination: correction. Obstet Gynecol. 2022;139:345. doi:10.1097/AOG.0000000000004680.
- St Sauver JL, Finney Rutten LJF, Ebbert JO, et al. Younger age at initiation of the human papillomavirus (HPV) vaccination series is associated with higher rates of on-time completion. Prev Med. 2016;89:327-333. doi:10.1016/j.ypmed.2016.02.039.
- Lei J, Ploner A, Elfström KM, et al. HPV vaccination and the risk of invasive cervical cancer. N Engl J Med. 2020;383:13401348. doi:10.1056/NEJMoa1917338.
- Pingali C, Yankey D, Elam-Evans LD, et al. National, regional, state, and selected local area vaccination coverage among adolescents aged 13–17 years — United States, 2020. MMWR Morb Mortal Wkly Rep. 2021;70:1183-1190. doi:10.15585/ mmwr.mm7035a1.
- National Centre for Immunisation Research and Surveillance Australia. Annual Immunisation Coverage Report 2020. November 29, 2021. Accessed March 1, 2023. https://ncirs .org.au/sites/default/files/2021-11/NCIRS%20Annual%20 Immunisation%20Coverage%20Report%202020_FINAL.pdf
- Leung SOA, Feldman S. 2022 Update on cervical disease. OBG Manag. 2022;34(5):16-17, 22-24, 26, 28. doi:10.12788/ obgm.0197.
- Centers for Disease Control and Prevention. United States Cancer Statistics: data visualizations. Trends: changes over time: cervix. Accessed January 8, 2023. https://gis.cdc.gov /Cancer/USCS/#/Trends/
- Sung H, Ferlay J, Siegel RL, et al. Global Cancer Statistics 2020: GLOBOCAN estimates of incidence and mortality worldwide for 36 cancers in 185 countries. CA Cancer J Clin. 2021;71:209-249. doi:10.3322/caac.21660.
- Francoeur AA, Liao CI, Casear MA, et al. The increasing incidence of stage IV cervical cancer in the USA: what factors are related? Int J Gynecol Cancer. 2022;32:ijgc-2022-003728. doi:10.1136/ijgc-2022-003728.
- Abdalla E, Habtemariam T, Fall S, et al. A comparative study of health disparities in cervical cancer mortality rates through time between Black and Caucasian women in Alabama and the US. Int J Stud Nurs. 2021;6:9-23. doi:10.20849/ijsn. v6i1.864.
- Bruegl AS, Emerson J, Tirumala K. Persistent disparities of cervical cancer among American Indians/Alaska natives: are we maximizing prevention tools? Gynecol Oncol. 2023;168:5661. doi:10.1016/j.ygyno.2022.11.007.
- Suk R, Hong YR, Rajan SS, et al. Assessment of US Preventive Services Task Force Guideline–Concordant cervical cancer screening rates and reasons for underscreening by age, race and ethnicity, sexual orientation, rurality, and insurance, 2005 to 2019. JAMA Netw Open. 2022;5:e2143582. doi:10.1001/ jamanetworkopen.2021.43582.
- Fontham ETH, Wolf AMD, Church TR, et al. Cervical cancer screening for individuals at average risk: 2020 guideline update from the American Cancer Society. CA Cancer J Clin. 2020;70:321-346. doi:10.3322/caac.21628.
- US Preventive Services Task Force; Curry SJ, Krist AH, Owens DK, et al. Screening for cervical cancer: US Preventive Services Task Force Recommendation statement. JAMA. 2018;320:674-686. doi:10.1001/jama.2018.10897.
- Nayar R, Chhieng DC, Crothers B, et al. Moving forward—the 2019 ASCCP risk-based management consensus guidelines for abnormal cervical cancer screening tests and cancer precursors and beyond: implications and suggestions for laboratories. J Am Soc Cytopathol. 2020;9:291-303. doi:10.1016/j.jasc.2020.05.002.
- Cooley JJP, Maguire FB, Morris CR, et al. Cervical cancer stage at diagnosis and survival among women ≥65 years in California. Cancer Epidemiol Biomarkers Prev. 2023;32:91-97. doi:10.1158/1055-9965.EPI-22-0793.
- National Cancer Institute. Surveillance, Epidemiology, and End Results Program. Cancer Stat Facts: Cervical Cancer. Accessed February 21, 2023. https://seer.cancer.gov /statfacts/html/cervix.html
- Feldman S. Screening options for preventing cervical cancer. JAMA Intern Med. 2019;179:879-880. doi:10.1001/ jamainternmed.2019.0298.
- ASCO Post Staff. FDA approves first HPV test for primary cervical cancer screening. ASCO Post. May 15, 2014. Accessed January 8, 2023. https://ascopost.com/issues/may-15-2014 /fda-approves-first-hpv-test-for-primary-cervical-cancer -screening/
- Rijkaart DC, Berkhof J, Rozendaal L, et al. Human papillomavirus testing for the detection of high-grade cervical intraepithelial neoplasia and cancer: final results of the POBASCAM randomised controlled trial. Lancet Oncol. 2012;13:78-88. doi:10.1016/S1470-2045(11)70296-0.
- Ronco G, Giorgi-Rossi P, Carozzi F, et al; New Technologies for Cervical Cancer Screening (NTCC) Working Group. Efficacy of human papillomavirus testing for the detection of invasive cervical cancers and cervical intraepithelial neoplasia: a randomised controlled trial. Lancet Oncol. 2010;11:249-257. doi:10.1016/S1470-2045(09)70360-2.
- Kitchener HC, Almonte M, Thomson C, et al. HPV testing in combination with liquid-based cytology in primary cervical screening (ARTISTIC): a randomised controlled trial. Lancet Oncol. 2009;10:672-682. doi:10.1016/S1470-2045(09)70156-1.
- Bulkmans NWJ, Berkhof J, Rozendaal L, et al. Human papillomavirus DNA testing for the detection of cervical intraepithelial neoplasia grade 3 and cancer: 5-year followup of a randomised controlled implementation trial. Lancet. 2007;370:1764-1772. doi:10.1016/S0140-6736(07)61450-0.
- Ogilvie GS, Van Niekerk D, Krajden M, et al. Effect of screening with primary cervical HPV testing vs cytology testing on high-grade cervical intraepithelial neoplasia at 48 months: the HPV FOCAL randomized clinical trial. JAMA. 2018;320:43-52. doi:10.1001/jama.2018.7464.
- Gottschlich A, Gondara L, Smith LW, et al. Human papillomavirus‐based screening at extended intervals missed fewer cervical precancers than cytology in the HPV For Cervical Cancer (HPV FOCAL) trial. Int J Cancer. 2022;151:897-905. doi:10.1002/ijc.34039.
- Katki HA, Kinney WK, Fetterman B, et al. Cervical cancer risk for women undergoing concurrent testing for human papillomavirus and cervical cytology: a population-based study in routine clinical practice. Lancet Oncol. 2011;12:663672. doi:10.1016/S1470-2045(11)70145-0.
- Wright TC, Stoler MH, Behrens CM, et al. Primary cervical cancer screening with human papillomavirus: end of study results from the ATHENA study using HPV as the first-line screening test. Gynecol Oncol. 2015;136:189-197. doi:10.1016/j.ygyno.2014.11.076
- Huh WK, Ault KA, Chelmow D, et al. Use of primary high-risk human papillomavirus testing for cervical cancer screening: interim clinical guidance. Obstet Gynecol. 2015;125:330-337. doi:10.1097/AOG.0000000000000669.
- Silver MI, Rositch AF, Burke AE, et al. Patient concerns about human papillomavirus testing and 5-year intervals in routine cervical cancer screening. Obstet Gynecol. 2015;125:317-329. doi:10.1097/AOG.0000000000000638.
- Smith LW, Racey CS, Gondara L, et al. Women’s acceptability of and experience with primary human papillomavirus testing for cervical screening: HPV FOCAL trial cross-sectional online survey results. BMJ Open. 2021;11:e052084. doi:10.1136/bmjopen-2021-052084.
- Wright TC, Stoler MH, Ranger-Moore J, et al. Clinical validation of p16/Ki-67 dual-stained cytology triage of HPV-positive women: results from the IMPACT trial. Int J Cancer. 2022;150:461-471. doi:10.1002/ijc.33812.
- Yeh PT, Kennedy CE, De Vuyst H, et al. Self-sampling for human papillomavirus (HPV) testing: a systematic review and meta-analysis. BMJ Global Health. 2019;4:e001351. doi:10.1136/bmjgh-2018-001351.
- Polman NJ, Ebisch RMF, Heideman DAM, et al. Performance of human papillomavirus testing on self-collected versus clinician-collected samples for the detection of cervical intraepithelial neoplasia of grade 2 or worse: a randomised, paired screen-positive, non-inferiority trial. Lancet Oncol. 2019;20:229-238. doi:10.1016/S1470-2045(18)30763-0.
- Winer RL, Lin J, Tiro JA, et al. Effect of mailed human papillomavirus test kits vs usual care reminders on cervical cancer screening uptake, precancer detection, and treatment: a randomized clinical trial. JAMA Netw Open. 2019;2:e1914729. doi:10.1001/jamanetworkopen.2019.14729.
- Tiro JA, Betts AC, Kimbel K, et al. Understanding patients’ perspectives and information needs following a positive home human papillomavirus self-sampling kit result. J Womens Health (Larchmt). 2019;28:384-392. doi:10.1089/ jwh.2018.7070.
- Knauss T, Hansen BT, Pedersen K, et al. The cost-effectiveness of opt-in and send-to-all HPV self-sampling among long-term non-attenders to cervical cancer screening in Norway: the Equalscreen randomized controlled trial. Gynecol Oncol. 2023;168:39-47. doi:10.1016/j.ygyno.2022.10.027.
- ACOG committee opinion no. 809. Human papillomavirus vaccination: correction. Obstet Gynecol. 2022;139:345. doi:10.1097/AOG.0000000000004680.
- St Sauver JL, Finney Rutten LJF, Ebbert JO, et al. Younger age at initiation of the human papillomavirus (HPV) vaccination series is associated with higher rates of on-time completion. Prev Med. 2016;89:327-333. doi:10.1016/j.ypmed.2016.02.039.
- Lei J, Ploner A, Elfström KM, et al. HPV vaccination and the risk of invasive cervical cancer. N Engl J Med. 2020;383:13401348. doi:10.1056/NEJMoa1917338.
- Pingali C, Yankey D, Elam-Evans LD, et al. National, regional, state, and selected local area vaccination coverage among adolescents aged 13–17 years — United States, 2020. MMWR Morb Mortal Wkly Rep. 2021;70:1183-1190. doi:10.15585/ mmwr.mm7035a1.
- National Centre for Immunisation Research and Surveillance Australia. Annual Immunisation Coverage Report 2020. November 29, 2021. Accessed March 1, 2023. https://ncirs .org.au/sites/default/files/2021-11/NCIRS%20Annual%20 Immunisation%20Coverage%20Report%202020_FINAL.pdf
- Leung SOA, Feldman S. 2022 Update on cervical disease. OBG Manag. 2022;34(5):16-17, 22-24, 26, 28. doi:10.12788/ obgm.0197.
When having discussions with your patients about recommended cancer screenings, have you been asked to answer questions related to liquid biopsy technology?
[polldaddy:11991465]
[polldaddy:11991465]
[polldaddy:11991465]






