User login
Crisugabalin Alleviates Postherpetic Neuralgia Symptoms in Phase 3 Study
TOPLINE:
METHODOLOGY:
- Researchers conducted a phase 3 multicenter, double-blind study involving 366 patients in China (median age, 63 years; 52.7% men) with PHN with an average daily pain score (ADPS) of 4 or greater on the numeric pain rating scale who were randomly assigned to receive either crisugabalin 40 mg/d (n = 121), 80 mg/d (n = 121), or placebo (n = 124) for 12 weeks.
- Patients who did not experience any serious toxic effects in these 12 weeks entered a 14-week open-label extension phase and received crisugabalin 40 mg twice daily.
- The primary efficacy endpoint was the change in ADPS from baseline at week 12.
- Secondary efficacy endpoints included the proportion of patients achieving at least 30% and 50% reduction in ADPS at week 12; changes in the Short-Form McGill Pain Questionnaire (SF-MPQ), Visual Analog Scale, and Average Daily Sleep Interference Scale scores at week 12; and change in the SF-MPQ Present Pain Intensity scores at weeks 12 and 26.
TAKEAWAY:
- At week 12, among those on crisugabalin 40 mg/d and 80 mg/d, there were significant reductions in ADPS compared with placebo (least squares mean [LSM] change from baseline, −2.2 and −2.6 vs −1.1, respectively; P < .001).
- A greater proportion of patients on crisugabalin 40 mg/d (61.2%) and 80 mg/d (54.5%) achieved 30% or greater reduction in ADPS (P < .001) than patients who received placebo (35.5%). Similarly, a 50% or greater reduction in ADPS was achieved by 37.2% of patients on crisugabalin 40 mg/d (P = .002) and 38% on 80 mg/d (P < .001), compared with 20.2% for placebo.
- Crisugabalin 40 mg/d and crisugabalin 80 mg/d were associated with greater reductions in the pain intensity at week 12 than placebo (LSM, −1.0 and −1.2 vs −0.5, respectively; P < .001). Similar patterns were noted for other pain-related measures at weeks 12 and 26.
- Serious treatment-emergent adverse events occurred in four patients in each group; only 2.4% of those on 40 mg/d and 1.6% on 80 mg/d discontinued treatment because of side effects.
IN PRACTICE:
“Crisugabalin 40 mg/d or crisugabalin 80 mg/d was well-tolerated and significantly improved ADPS compared to placebo,” the authors wrote, adding that “crisugabalin can be flexibly selected depending on individual patient response and tolerability at 40 mg/d or 80 mg/d.”
SOURCE:
The study was led by Daying Zhang, PhD, of the Department of Pain Medicine at The First Affiliated Hospital of Nanchang University, Nanchang, China. It was published online in JAMA Dermatology.
LIMITATIONS:
The findings may not be generalizable to the global population as the study population was limited to Chinese patients. The study only provided short-term efficacy and safety data on crisugabalin, lacked an active comparator, and did not reflect the standard of care observed in the United States or Europe, where oral tricyclic antidepressants, pregabalin, and the lidocaine patch are recommended as first-line therapies.
DISCLOSURES:
The study was sponsored and funded by Haisco Pharmaceutical. Dr. Zhang and another author reported receiving support from Haisco. Two authors are company employees.
This article was created using several editorial tools, including AI, as part of the process. Human editors reviewed this content before publication. A version of this article first appeared on Medscape.com.
TOPLINE:
METHODOLOGY:
- Researchers conducted a phase 3 multicenter, double-blind study involving 366 patients in China (median age, 63 years; 52.7% men) with PHN with an average daily pain score (ADPS) of 4 or greater on the numeric pain rating scale who were randomly assigned to receive either crisugabalin 40 mg/d (n = 121), 80 mg/d (n = 121), or placebo (n = 124) for 12 weeks.
- Patients who did not experience any serious toxic effects in these 12 weeks entered a 14-week open-label extension phase and received crisugabalin 40 mg twice daily.
- The primary efficacy endpoint was the change in ADPS from baseline at week 12.
- Secondary efficacy endpoints included the proportion of patients achieving at least 30% and 50% reduction in ADPS at week 12; changes in the Short-Form McGill Pain Questionnaire (SF-MPQ), Visual Analog Scale, and Average Daily Sleep Interference Scale scores at week 12; and change in the SF-MPQ Present Pain Intensity scores at weeks 12 and 26.
TAKEAWAY:
- At week 12, among those on crisugabalin 40 mg/d and 80 mg/d, there were significant reductions in ADPS compared with placebo (least squares mean [LSM] change from baseline, −2.2 and −2.6 vs −1.1, respectively; P < .001).
- A greater proportion of patients on crisugabalin 40 mg/d (61.2%) and 80 mg/d (54.5%) achieved 30% or greater reduction in ADPS (P < .001) than patients who received placebo (35.5%). Similarly, a 50% or greater reduction in ADPS was achieved by 37.2% of patients on crisugabalin 40 mg/d (P = .002) and 38% on 80 mg/d (P < .001), compared with 20.2% for placebo.
- Crisugabalin 40 mg/d and crisugabalin 80 mg/d were associated with greater reductions in the pain intensity at week 12 than placebo (LSM, −1.0 and −1.2 vs −0.5, respectively; P < .001). Similar patterns were noted for other pain-related measures at weeks 12 and 26.
- Serious treatment-emergent adverse events occurred in four patients in each group; only 2.4% of those on 40 mg/d and 1.6% on 80 mg/d discontinued treatment because of side effects.
IN PRACTICE:
“Crisugabalin 40 mg/d or crisugabalin 80 mg/d was well-tolerated and significantly improved ADPS compared to placebo,” the authors wrote, adding that “crisugabalin can be flexibly selected depending on individual patient response and tolerability at 40 mg/d or 80 mg/d.”
SOURCE:
The study was led by Daying Zhang, PhD, of the Department of Pain Medicine at The First Affiliated Hospital of Nanchang University, Nanchang, China. It was published online in JAMA Dermatology.
LIMITATIONS:
The findings may not be generalizable to the global population as the study population was limited to Chinese patients. The study only provided short-term efficacy and safety data on crisugabalin, lacked an active comparator, and did not reflect the standard of care observed in the United States or Europe, where oral tricyclic antidepressants, pregabalin, and the lidocaine patch are recommended as first-line therapies.
DISCLOSURES:
The study was sponsored and funded by Haisco Pharmaceutical. Dr. Zhang and another author reported receiving support from Haisco. Two authors are company employees.
This article was created using several editorial tools, including AI, as part of the process. Human editors reviewed this content before publication. A version of this article first appeared on Medscape.com.
TOPLINE:
METHODOLOGY:
- Researchers conducted a phase 3 multicenter, double-blind study involving 366 patients in China (median age, 63 years; 52.7% men) with PHN with an average daily pain score (ADPS) of 4 or greater on the numeric pain rating scale who were randomly assigned to receive either crisugabalin 40 mg/d (n = 121), 80 mg/d (n = 121), or placebo (n = 124) for 12 weeks.
- Patients who did not experience any serious toxic effects in these 12 weeks entered a 14-week open-label extension phase and received crisugabalin 40 mg twice daily.
- The primary efficacy endpoint was the change in ADPS from baseline at week 12.
- Secondary efficacy endpoints included the proportion of patients achieving at least 30% and 50% reduction in ADPS at week 12; changes in the Short-Form McGill Pain Questionnaire (SF-MPQ), Visual Analog Scale, and Average Daily Sleep Interference Scale scores at week 12; and change in the SF-MPQ Present Pain Intensity scores at weeks 12 and 26.
TAKEAWAY:
- At week 12, among those on crisugabalin 40 mg/d and 80 mg/d, there were significant reductions in ADPS compared with placebo (least squares mean [LSM] change from baseline, −2.2 and −2.6 vs −1.1, respectively; P < .001).
- A greater proportion of patients on crisugabalin 40 mg/d (61.2%) and 80 mg/d (54.5%) achieved 30% or greater reduction in ADPS (P < .001) than patients who received placebo (35.5%). Similarly, a 50% or greater reduction in ADPS was achieved by 37.2% of patients on crisugabalin 40 mg/d (P = .002) and 38% on 80 mg/d (P < .001), compared with 20.2% for placebo.
- Crisugabalin 40 mg/d and crisugabalin 80 mg/d were associated with greater reductions in the pain intensity at week 12 than placebo (LSM, −1.0 and −1.2 vs −0.5, respectively; P < .001). Similar patterns were noted for other pain-related measures at weeks 12 and 26.
- Serious treatment-emergent adverse events occurred in four patients in each group; only 2.4% of those on 40 mg/d and 1.6% on 80 mg/d discontinued treatment because of side effects.
IN PRACTICE:
“Crisugabalin 40 mg/d or crisugabalin 80 mg/d was well-tolerated and significantly improved ADPS compared to placebo,” the authors wrote, adding that “crisugabalin can be flexibly selected depending on individual patient response and tolerability at 40 mg/d or 80 mg/d.”
SOURCE:
The study was led by Daying Zhang, PhD, of the Department of Pain Medicine at The First Affiliated Hospital of Nanchang University, Nanchang, China. It was published online in JAMA Dermatology.
LIMITATIONS:
The findings may not be generalizable to the global population as the study population was limited to Chinese patients. The study only provided short-term efficacy and safety data on crisugabalin, lacked an active comparator, and did not reflect the standard of care observed in the United States or Europe, where oral tricyclic antidepressants, pregabalin, and the lidocaine patch are recommended as first-line therapies.
DISCLOSURES:
The study was sponsored and funded by Haisco Pharmaceutical. Dr. Zhang and another author reported receiving support from Haisco. Two authors are company employees.
This article was created using several editorial tools, including AI, as part of the process. Human editors reviewed this content before publication. A version of this article first appeared on Medscape.com.
Nonalcoholic Beer and Underage Drinking
Several months ago in a letter about healthcare providers and the decision to use alcohol and other mind-altering substances on the job, I waxed enthusiastically about the new wave of no alcohol (NA) and zero (00) alcohol beers that have come on the market. In the last 2 years our local grocery store’s cooler space for nonalcoholic beer has grown from less than 24 inches to something approaching the height of the average sixth grader.
In a bold act of chivalry at the beginning of the pandemic I accepted the mantle of designated grocery shopper and over the last 3 years have become uncommonly proud of my ability to bring home the groceries efficiently and cost effectively, without catching COVID in the process. I have developed a sixth sense of choosing which human checker/bagger combination is fastest or whether the self-checkout is the way to go.
For obvious reasons the human checkers don’t ask for my ID when I am buying adult beverages. However, the self-check register freezes up instantly when I scan my 12-pack of Run Wild nonalcoholic. This necessitates a search for the MIA store person assigned to patrol the self-check corral, ever on the lookout for shoplifters, underage drinkers, and other generally shifty looking characters.
When I find one of the grocery store detectives (who is likely to have been a former patient), I say: “You know, this doesn’t have any alcohol in it.” They invariably reply with a shrug. “I know. But, the rules are the rules.” Occasionally, they may add: “It doesn’t make sense, does it?”
At first blush checking IDs for a nonalcoholic beverage may sound dumb, certainly to someone who is just a few years on either side of the legal drinking age. Why are we trying to protect some crazy teenager from the futility of getting high on a six-pack of something that at worst will make him spend most of the next couple of hours peeing?
But, there is concern in some corners that nonalcoholic drinks pose a significant threat to teenagers. Two PhDs at Stanford University have recently published a paper in which they worry that the dramatic rise in US sales of nonalcoholic drinks from 15% to 30% since 2018 may be socializing “users of alcohol drinking experiences by exposing them to the taste, look, and even brands of alcoholic beverages”.
Is there evidence to support their concern? I could only find one brief report in the Japanese literature that states that among young people “who experienced the nonalcoholic beverage intake, interest in or motivation for drinking alcoholic beverages, and/or smoking is higher than [among] those who did not.” The study didn’t appear to clearly separate the exposure in a family setting from the actual intake.
Beer is an acquired taste. If someone offered you your first taste of beer after a hot-weather set of tennis most of you would reject it and ask for water or lemonade. I can recall my first taste of beer. For some reason my father thought at age 11 or 12 I might like to try some from his glass. I’m not sure of his motivation, but he tried the same thing with oysters. I didn’t drink beer again until I was 16, motivated at that time by a group dynamic. The oyster trial, however, backfired on him and from then on he had to share his coveted dozen with me. Alcohol, unless heavily disguised by a mixer, is also not a taste that most young people find appealing.
It is unlikely that the average thrill-seeking teenager is going to ask his older-appearing buddy with a fake ID to buy him some nonalcoholic beer. Nor would he go to the effort or risk of acquiring his own fake ID just to see how it tastes. It just doesn’t compute, especially to a self-check corral patroller.
I guess one could envision a scenario in which a teenager wanting to fit in with the fast crowd would ask a trusted adult (or clueless parent) to buy him some nonalcoholic beer to bring to a party. He is running a serious risk of being laughed at by his friends if they find he’s drinking the fake stuff. It also seems unlikely that a parent would buy nonalcoholic beer to introduce his teenager to the taste of beer.
So,
Although it runs counter to my usual commitment to evidence-based decisions, making it difficult for adolescents to buy nonalcoholic beverages feels like the right think to do. As long as alcoholic and nonalcoholic beverages share the same display space and are packaged in nearly identical containers, there is ample opportunity for confusion. Recent evidence suggesting that even small amounts of alcohol increases some health risks should strengthen our resolve to minimize that confusion.
Dr. Wilkoff practiced primary care pediatrics in Brunswick, Maine, for nearly 40 years. He has authored several books on behavioral pediatrics, including “How to Say No to Your Toddler.” Other than a Littman stethoscope he accepted as a first-year medical student in 1966, Dr. Wilkoff reports having nothing to disclose. Email him at [email protected].
Several months ago in a letter about healthcare providers and the decision to use alcohol and other mind-altering substances on the job, I waxed enthusiastically about the new wave of no alcohol (NA) and zero (00) alcohol beers that have come on the market. In the last 2 years our local grocery store’s cooler space for nonalcoholic beer has grown from less than 24 inches to something approaching the height of the average sixth grader.
In a bold act of chivalry at the beginning of the pandemic I accepted the mantle of designated grocery shopper and over the last 3 years have become uncommonly proud of my ability to bring home the groceries efficiently and cost effectively, without catching COVID in the process. I have developed a sixth sense of choosing which human checker/bagger combination is fastest or whether the self-checkout is the way to go.
For obvious reasons the human checkers don’t ask for my ID when I am buying adult beverages. However, the self-check register freezes up instantly when I scan my 12-pack of Run Wild nonalcoholic. This necessitates a search for the MIA store person assigned to patrol the self-check corral, ever on the lookout for shoplifters, underage drinkers, and other generally shifty looking characters.
When I find one of the grocery store detectives (who is likely to have been a former patient), I say: “You know, this doesn’t have any alcohol in it.” They invariably reply with a shrug. “I know. But, the rules are the rules.” Occasionally, they may add: “It doesn’t make sense, does it?”
At first blush checking IDs for a nonalcoholic beverage may sound dumb, certainly to someone who is just a few years on either side of the legal drinking age. Why are we trying to protect some crazy teenager from the futility of getting high on a six-pack of something that at worst will make him spend most of the next couple of hours peeing?
But, there is concern in some corners that nonalcoholic drinks pose a significant threat to teenagers. Two PhDs at Stanford University have recently published a paper in which they worry that the dramatic rise in US sales of nonalcoholic drinks from 15% to 30% since 2018 may be socializing “users of alcohol drinking experiences by exposing them to the taste, look, and even brands of alcoholic beverages”.
Is there evidence to support their concern? I could only find one brief report in the Japanese literature that states that among young people “who experienced the nonalcoholic beverage intake, interest in or motivation for drinking alcoholic beverages, and/or smoking is higher than [among] those who did not.” The study didn’t appear to clearly separate the exposure in a family setting from the actual intake.
Beer is an acquired taste. If someone offered you your first taste of beer after a hot-weather set of tennis most of you would reject it and ask for water or lemonade. I can recall my first taste of beer. For some reason my father thought at age 11 or 12 I might like to try some from his glass. I’m not sure of his motivation, but he tried the same thing with oysters. I didn’t drink beer again until I was 16, motivated at that time by a group dynamic. The oyster trial, however, backfired on him and from then on he had to share his coveted dozen with me. Alcohol, unless heavily disguised by a mixer, is also not a taste that most young people find appealing.
It is unlikely that the average thrill-seeking teenager is going to ask his older-appearing buddy with a fake ID to buy him some nonalcoholic beer. Nor would he go to the effort or risk of acquiring his own fake ID just to see how it tastes. It just doesn’t compute, especially to a self-check corral patroller.
I guess one could envision a scenario in which a teenager wanting to fit in with the fast crowd would ask a trusted adult (or clueless parent) to buy him some nonalcoholic beer to bring to a party. He is running a serious risk of being laughed at by his friends if they find he’s drinking the fake stuff. It also seems unlikely that a parent would buy nonalcoholic beer to introduce his teenager to the taste of beer.
So,
Although it runs counter to my usual commitment to evidence-based decisions, making it difficult for adolescents to buy nonalcoholic beverages feels like the right think to do. As long as alcoholic and nonalcoholic beverages share the same display space and are packaged in nearly identical containers, there is ample opportunity for confusion. Recent evidence suggesting that even small amounts of alcohol increases some health risks should strengthen our resolve to minimize that confusion.
Dr. Wilkoff practiced primary care pediatrics in Brunswick, Maine, for nearly 40 years. He has authored several books on behavioral pediatrics, including “How to Say No to Your Toddler.” Other than a Littman stethoscope he accepted as a first-year medical student in 1966, Dr. Wilkoff reports having nothing to disclose. Email him at [email protected].
Several months ago in a letter about healthcare providers and the decision to use alcohol and other mind-altering substances on the job, I waxed enthusiastically about the new wave of no alcohol (NA) and zero (00) alcohol beers that have come on the market. In the last 2 years our local grocery store’s cooler space for nonalcoholic beer has grown from less than 24 inches to something approaching the height of the average sixth grader.
In a bold act of chivalry at the beginning of the pandemic I accepted the mantle of designated grocery shopper and over the last 3 years have become uncommonly proud of my ability to bring home the groceries efficiently and cost effectively, without catching COVID in the process. I have developed a sixth sense of choosing which human checker/bagger combination is fastest or whether the self-checkout is the way to go.
For obvious reasons the human checkers don’t ask for my ID when I am buying adult beverages. However, the self-check register freezes up instantly when I scan my 12-pack of Run Wild nonalcoholic. This necessitates a search for the MIA store person assigned to patrol the self-check corral, ever on the lookout for shoplifters, underage drinkers, and other generally shifty looking characters.
When I find one of the grocery store detectives (who is likely to have been a former patient), I say: “You know, this doesn’t have any alcohol in it.” They invariably reply with a shrug. “I know. But, the rules are the rules.” Occasionally, they may add: “It doesn’t make sense, does it?”
At first blush checking IDs for a nonalcoholic beverage may sound dumb, certainly to someone who is just a few years on either side of the legal drinking age. Why are we trying to protect some crazy teenager from the futility of getting high on a six-pack of something that at worst will make him spend most of the next couple of hours peeing?
But, there is concern in some corners that nonalcoholic drinks pose a significant threat to teenagers. Two PhDs at Stanford University have recently published a paper in which they worry that the dramatic rise in US sales of nonalcoholic drinks from 15% to 30% since 2018 may be socializing “users of alcohol drinking experiences by exposing them to the taste, look, and even brands of alcoholic beverages”.
Is there evidence to support their concern? I could only find one brief report in the Japanese literature that states that among young people “who experienced the nonalcoholic beverage intake, interest in or motivation for drinking alcoholic beverages, and/or smoking is higher than [among] those who did not.” The study didn’t appear to clearly separate the exposure in a family setting from the actual intake.
Beer is an acquired taste. If someone offered you your first taste of beer after a hot-weather set of tennis most of you would reject it and ask for water or lemonade. I can recall my first taste of beer. For some reason my father thought at age 11 or 12 I might like to try some from his glass. I’m not sure of his motivation, but he tried the same thing with oysters. I didn’t drink beer again until I was 16, motivated at that time by a group dynamic. The oyster trial, however, backfired on him and from then on he had to share his coveted dozen with me. Alcohol, unless heavily disguised by a mixer, is also not a taste that most young people find appealing.
It is unlikely that the average thrill-seeking teenager is going to ask his older-appearing buddy with a fake ID to buy him some nonalcoholic beer. Nor would he go to the effort or risk of acquiring his own fake ID just to see how it tastes. It just doesn’t compute, especially to a self-check corral patroller.
I guess one could envision a scenario in which a teenager wanting to fit in with the fast crowd would ask a trusted adult (or clueless parent) to buy him some nonalcoholic beer to bring to a party. He is running a serious risk of being laughed at by his friends if they find he’s drinking the fake stuff. It also seems unlikely that a parent would buy nonalcoholic beer to introduce his teenager to the taste of beer.
So,
Although it runs counter to my usual commitment to evidence-based decisions, making it difficult for adolescents to buy nonalcoholic beverages feels like the right think to do. As long as alcoholic and nonalcoholic beverages share the same display space and are packaged in nearly identical containers, there is ample opportunity for confusion. Recent evidence suggesting that even small amounts of alcohol increases some health risks should strengthen our resolve to minimize that confusion.
Dr. Wilkoff practiced primary care pediatrics in Brunswick, Maine, for nearly 40 years. He has authored several books on behavioral pediatrics, including “How to Say No to Your Toddler.” Other than a Littman stethoscope he accepted as a first-year medical student in 1966, Dr. Wilkoff reports having nothing to disclose. Email him at [email protected].
Age-Friendly Health Systems Transformation: A Whole Person Approach to Support the Well-Being of Older Adults
The COVID-19 pandemic established a new normal for health care delivery, with leaders rethinking core practices to survive and thrive in a changing environment and improve the health and well-being of patients. The Veterans Health Administration (VHA) is embracing a shift in focus from “what is the matter” to “what really matters” to address pre- and postpandemic challenges through a whole health approach.1 Initially conceptualized by the VHA in 2011, whole health “is an approach to health care that empowers and equips people to take charge of their health and well-being so that they can live their life to the fullest.”1 Whole health integrates evidence-based complementary and integrative health (CIH) therapies to manage pain; this includes acupuncture, meditation, tai chi, yoga, massage therapy, guided imagery, biofeedback, and clinical hypnosis.1 The VHA now recognizes well-being as a core value, helping clinicians respond to emerging challenges related to the social determinants of health (eg, access to health care, physical activity, and healthy foods) and guiding health care decision making.1,2
Well-being through empowerment—elements of whole health and Age-Friendly Health Systems (AFHS)—encourages health care institutions to work with employees, patients, and other stakeholders to address global challenges, clinician burnout, and social issues faced by their communities. This approach focuses on life’s purpose and meaning for individuals and inspires leaders to engage with patients, staff, and communities in new, impactful ways by focusing on wellbeing and wholeness rather than illness and disease. Having a higher sense of purpose is associated with lower all-cause mortality, reduced risk of specific diseases, better health behaviors, greater use of preventive services, and fewer hospital days of care.3
This article describes how AFHS supports the well-being of older adults and aligns with the whole health model of care. It also outlines the VHA investment to transform health care to be more person-centered by documenting what matters in the electronic health record (EHR).
AGE-FRIENDLY CARE
Given that nearly half of veterans enrolled in the VHA are aged ≥ 65 years, there is an increased need to identify models of care to support this aging population.4 This is especially critical because older veterans often have multiple chronic conditions and complex care needs that benefit from a whole person approach. The AFHS movement aims to provide evidence-based care aligned with what matters to older adults and provides a mechanism for transforming care to meet the needs of older veterans. This includes addressing age-related health concerns while promoting optimal health outcomes and quality of life. AFHS follows the 4Ms framework: what matters, medication, mentation, and mobility.5 The 4Ms serve as a guide for the health care of older adults in any setting, where each “M” is assessed and acted on to support what matters.5 Since 2020, > 390 teams have developed a plan to implement the 4Ms at 156 VHA facilities, demonstrating the VHA commitment to transforming health care for veterans.6
When VHA teams join the AFHS movement, they may also engage older veterans in a whole health system (WHS) (Figure). While AFHS is designed to improve care for patients aged ≥ 65 years, it also complements whole health, a person-centered approach available to all veterans enrolled in the VHA. Through the WHS and AFHS, veterans are empowered and equipped to take charge of their health and well-being through conversations about their unique goals, preferences, and health priorities.4 Clinicians are challenged to assess what matters by asking questions like, “What brings you joy?” and, “How can we help you meet your health goals?”1,5 These questions shift the conversation from disease-based treatment and enable clinicians to better understand the veteran as a person.1,5
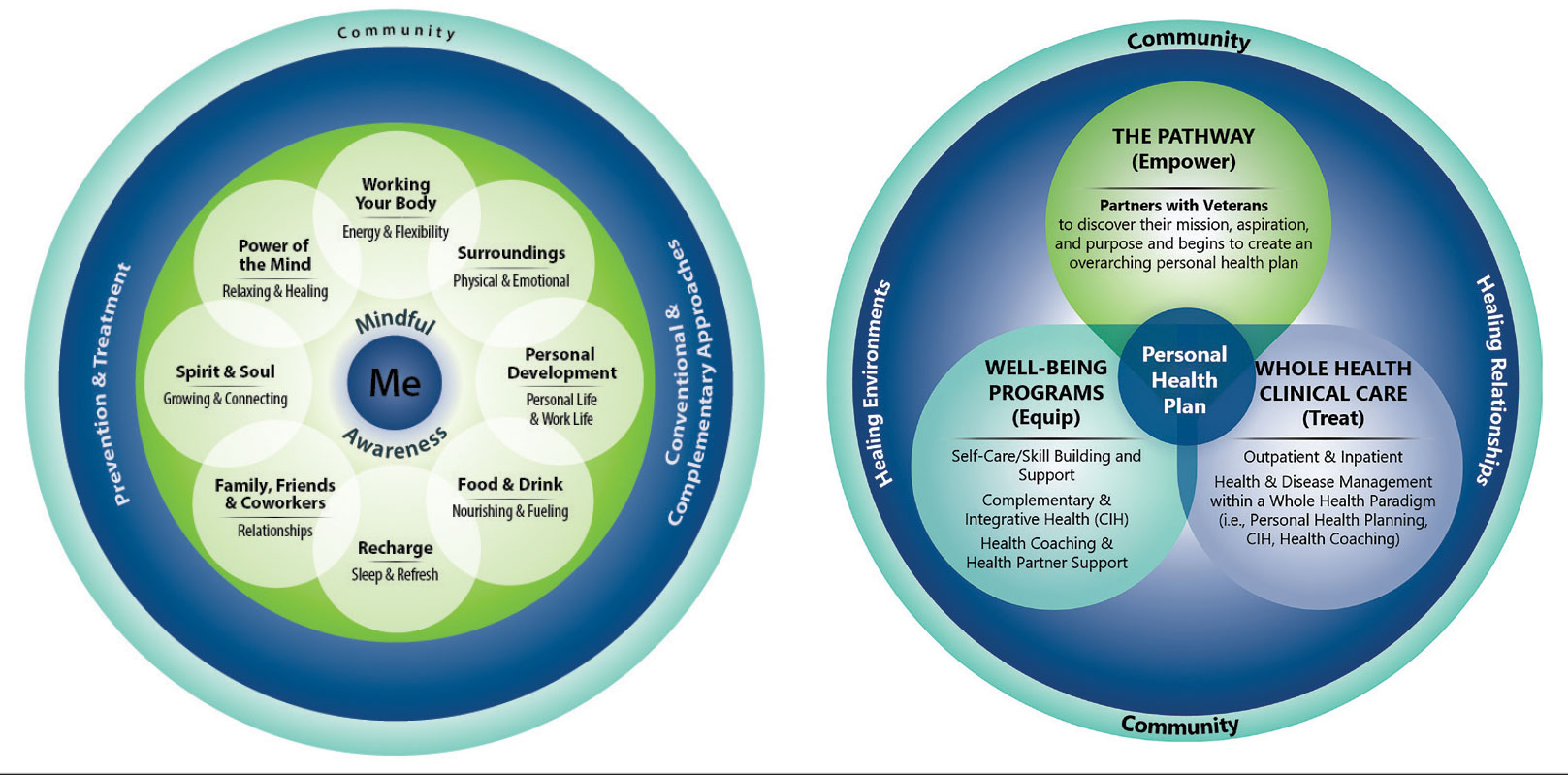
For whole health and AFHS, conversations about what matters are anchored in the veteran’s goals and preferences, especially those facing a significant health change (ie, a new diagnosis or treatment decision).5,7 Together, the veteran’s goals and priorities serve as the foundation for developing person-centered care plans that often go beyond conventional medical treatments to address the physical, mental, emotional, and social aspects of health.
SYSTEM-WIDE DIRECTIVE
The WHS enhances AFHS discussions about what matters to veterans by adding a system-level lens for conceptualizing health care delivery by leveraging the 3 components of WHS: the “pathway,” well-being programs, and whole health clinical care.
The Pathway
Discovering what matters, or the veteran’s “mission, aspiration, and purpose,” begins with the WHS pathway. When stepping into the pathway, veterans begin completing a personal health inventory, or “walking the circle of health,” which encourages self-reflection that focuses on components of their life that can influence health and well-being.1,8 The circle of health offers a visual representation of the 4 most important aspects of health and well-being: First, “Me” at the center as an individual who is the expert on their life, values, goals, and priorities. Only the individual can know what really matters through mindful awareness and what works for their life. Second, self-care consists of 8 areas that impact health and wellbeing: working your body; surroundings; personal development; food and drink; recharge; family, friends, and coworkers; spirit and soul; and power of the mind. Third, professional care consists of prevention, conventional care, and complementary care. Finally, the community that supports the individual.
Well-Being Programs
VHA provides WHS programs that support veterans in building self-care skills and improving their quality of life, often through integrative care clinics that offer coaching and CIH therapies. For example, a veteran who prioritizes mobility when seeking care at an integrative care clinic will not only receive conventional medical treatment for their physical symptoms but may also be offered CIH therapies depending on their goals. The veteran may set a daily mobility goal with their care team that supports what matters, incorporating CIH approaches, such as yoga and tai chi into the care plan.5 These holistic approaches for moving the body can help alleviate physical symptoms, reduce stress, improve mindful awareness, and provide opportunities for self-discovery and growth, thus promote overall well-being
Whole Health Clinical Care
AFHS and the 4Ms embody the clinical care component of the WHS. Because what matters is the driver of the 4Ms, every action taken by the care team supports wellbeing and quality of life by promoting independence, connection, and support, and addressing external factors, such as social determinants of health. At a minimum, well-being includes “functioning well: the experience of positive emotions such as happiness and contentment as well as the development of one’s potential, having some control over one’s life, having a sense of purpose, and experiencing positive relationships.”9 From a system perspective, the VHA has begun to normalize focusing on what matters to veterans, using an interprofessional approach, one of the first steps to implementing AFHS.
As the programs expand, AFHS teams can learn from whole health well-being programs and increase the capacity for self-care in older veterans. Learning about the key elements included in the circle of health helps clinicians understand each veteran’s perceived strengths and weaknesses to support their self-care. From there, teams can act on the 4Ms and connect older veterans with the most appropriate programs and services at their facility, ensuring continuum of care.
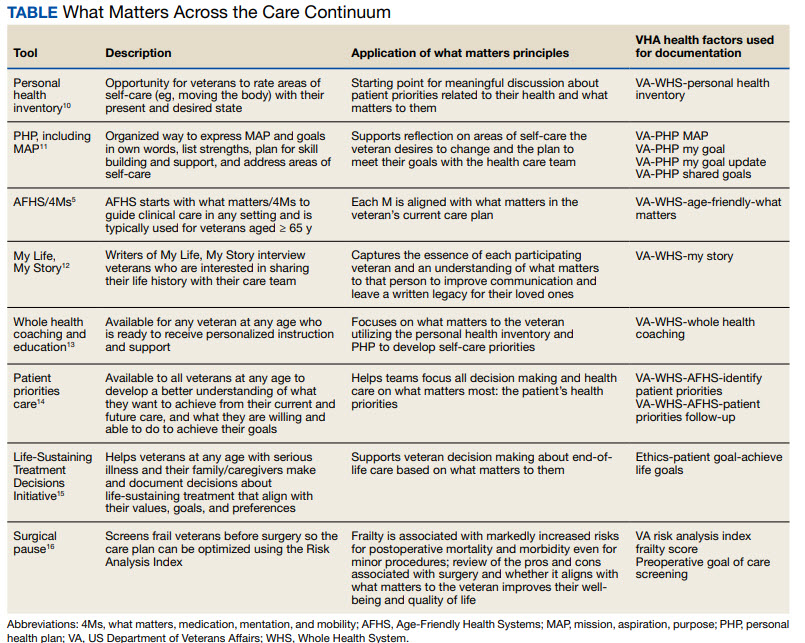
DOCUMENTATION
The VHA leverages several tools and evidence-based practices to assess and act on what matters for veterans of all ages (Table).5,10-16 The VHA EHR and associated dashboards contain a wealth of information about whole health and AFHS implementation, scale up, and spread. A national AFHS 4Ms note template contains standardized data elements called health factors, which provide a mechanism for monitoring 4Ms care via its related dashboard. This template was developed by an interprofessional workgroup of VHA staff and underwent a thorough human factors engineering review and testing process prior to its release. Although teams continue to personalize care based on what matters to the veteran, data from the standardized 4Ms note template and dashboard provide a way to establish consistent, equitable care across multiple care settings.17
Between January 2022 and December 2023, > 612,000 participants aged ≥ 65 years identified what matters to them through 1.35 million assessments. During that period, > 36,000 veterans aged ≥ 65 years participated in AFHS and had what matters conversations documented. A personalized health plan was completed by 585,270 veterans for a total of 1.1 million assessments.11 Whole health coaching has been documented for > 57,000 veterans with > 200,000 assessments completed.13 In fiscal year 2023, a total of 1,802,131 veterans participated in whole health.
When teams share information about what matters to the veteran in a clinicianfacing format in the EHR, this helps ensure that the VHA honors veteran preferences throughout transitions of care and across all phases of health care. Although the EHR captures data on what matters, measurement of the overall impact on veteran and health system outcomes is essential. Further evaluation and ongoing education are needed to ensure clinicians are accurately and efficiently capturing the care provided by completing the appropriate EHR. Additional challenges include identifying ways to balance the documentation burden, while ensuring notes include valuable patient-centered information to guide care. EHR tools and templates have helped to unlock important insights on health care delivery in the VHA; however, health systems must consider how these clinical practices support the overall well-being of patients. How leaders empower frontline clinicians in any care setting to use these data to drive meaningful change is also important.
TRANSFORMING VHA CARE DELIVERY
In Achieving Whole Health: A New Approach for Veterans and the Nation, the National Academy of Science proposes a framework for the transformation of health care institutions to provide better whole health to veterans.3 Transformation requires change in entire systems and leaders who mobilize people “for participation in the process of change, encouraging a sense of collective identity and collective efficacy, which in turn brings stronger feelings of self-worth and self-efficacy,” and an enhanced sense of meaningfulness in their work and lives.18
Shifting health care approaches to equipping and empowering veterans and employees with whole health and AFHS resources is transformational and requires radically different assumptions and approaches that cannot be realized through traditional approaches. This change requires robust and multifaceted cultural transformation spanning all levels of the organization. Whole health and AFHS are facilitating this transformation by supporting documentation and data needs, tracking outcomes across settings, and accelerating spread to new facilities and care settings nationwide to support older veterans in improving their health and well-being.
Whole health and AFHS are complementary approaches to care that can work to empower veterans (as well as caregivers and clinicians) to align services with what matters most to veterans. Lessons such as standardizing person-centered assessments of what matters, creating supportive structures to better align care with veterans’ priorities, and identifying meaningful veteran and system-level outcomes to help sustain transformational change can be applied from whole health to AFHS. Together these programs have the potential to enhance overall health outcomes and quality of life for veterans.
- Kligler B, Hyde J, Gantt C, Bokhour B. The Whole Health transformation at the Veterans Health Administration: moving from “what’s the matter with you?” to “what matters to you?” Med Care. 2022;60(5):387-391. doi:10.1097/MLR.0000000000001706
- Centers for Disease Control and Prevention. Social determinants of health (SDOH) at CDC. January 17, 2024. Accessed September 12, 2024. https://www.cdc.gov/public-health-gateway/php/about/social-determinants-of-health.html
- National Academies of Sciences, Engineering, and Medicine. Achieving Whole Health: A New Approach for Veterans and the Nation. The National Academies Press; 2023. Accessed September 9, 2024. doi:10.17226/26854
- Church K, Munro S, Shaughnessy M, Clancy C. Age-friendly health systems: improving care for older adults in the Veterans Health Administration. Health Serv Res. 2023;58 Suppl 1(Suppl 1):5-8. doi:10.1111/1475-6773.14110
- Laderman M, Jackson C, Little K, Duong T, Pelton L. “What Matters” to older adults? A toolkit for health systems to design better care with older adults. Institute for Healthcare Improvement; 2019. Accessed September 9, 2024. https://www.ihi.org/Engage/Initiatives/Age-Friendly-Health-Systems/Documents/IHI_Age_Friendly_What_Matters_to_Older_Adults_Toolkit.pdf
- U.S. Department of Veterans Affairs. Age-Friendly Health Systems. Updated September 4, 2024. Accessed September 9, 2024. https://marketplace.va.gov/innovations/age-friendly-health-systems
- Brown TT, Hurley VB, Rodriguez HP, et al. Shared dec i s i o n - m a k i n g l o w e r s m e d i c a l e x p e n d i t u re s a n d the effect is amplified in racially-ethnically concordant relationships. Med Care. 2023;61(8):528-535. doi:10.1097/MLR.0000000000001881
- Kligler B. Whole Health in the Veterans Health Administration. Glob Adv Health Med. 2022;11:2164957X221077214.
- Ruggeri K, Garcia-Garzon E, Maguire Á, Matz S, Huppert FA. Well-being is more than happiness and life satisfaction: a multidimensional analysis of 21 countries. Health Qual Life Outcomes. 2020;18(1):192. doi:10.1186/s12955-020-01423-y
- U.S. Department of Veterans Affairs. Personal Health Inventory. Updated May 2022. Accessed September 9, 2024. https://www.va.gov/WHOLEHEALTH/docs/PHI-long-May22-fillable-508.pdf doi:10.1177/2164957X221077214
- Veterans Health Administration. Personal Health Plan. Updated March 2019. Accessed September 9, 2024. https:// www.va.gov/WHOLEHEALTH/docs/PersonalHealthPlan_508_03-2019.pdf
- Veterans Health Administration. Whole Health: My Life, My Story. Updated March 20, 2024. Accessed September 9, 2024. https://www.va.gov/WHOLEHEALTH/mylifemystory/index.asp
- U.S. Department of Veterans Affairs. Whole Health Library: Whole Health for Skill Building. Updated April 17, 2024. Accessed September 9, 2024. https://www.va.gov/WHOLEHEALTHLIBRARY/courses/whole-health-skill-building.asp
- U.S. Department of Veterans Affairs. Making Decisions: Current Care Planning. Updated May 21, 2024. Accessed September 9, 2024. https://www.va.gov/geriatrics/pages/making_decisions.asp
- U.S. Department of Veterans Affairs. Life-Sustaining Treatment Decisions Initiative (LSTDI). Updated March 2024. Accessed September 12, 2024. https://marketplace.va.gov/innovations/life-sustaining-treatment-decisions-initiative
- U.S. Department of Veterans Affairs. Center for Health Equity Research and Promotion: Surgical Pause Saving Veterans Lives. Updated September 22, 2021. Accessed September 9, 2024. https://www.cherp.research.va.gov/features/Surgical_Pause_Saving_Veterans_Lives.asp
- Munro S, Church K, Berner C, et al. Implementation of an agefriendly template in the Veterans Health Administration electronic health record. J Inform Nurs. 2023;8(3):6-11.
- Burns JM. Transforming Leadership: A New Pursuit of Happiness. Grove Press; 2003.
- US Department of Veterans Affairs, Veterans Health Administration. Whole Health: Circle of Health Overview. Updated May 20, 2024. Accessed September 12, 2024. https://www.va.gov/WHOLEHEALTH/circle-of-health/index.asp
The COVID-19 pandemic established a new normal for health care delivery, with leaders rethinking core practices to survive and thrive in a changing environment and improve the health and well-being of patients. The Veterans Health Administration (VHA) is embracing a shift in focus from “what is the matter” to “what really matters” to address pre- and postpandemic challenges through a whole health approach.1 Initially conceptualized by the VHA in 2011, whole health “is an approach to health care that empowers and equips people to take charge of their health and well-being so that they can live their life to the fullest.”1 Whole health integrates evidence-based complementary and integrative health (CIH) therapies to manage pain; this includes acupuncture, meditation, tai chi, yoga, massage therapy, guided imagery, biofeedback, and clinical hypnosis.1 The VHA now recognizes well-being as a core value, helping clinicians respond to emerging challenges related to the social determinants of health (eg, access to health care, physical activity, and healthy foods) and guiding health care decision making.1,2
Well-being through empowerment—elements of whole health and Age-Friendly Health Systems (AFHS)—encourages health care institutions to work with employees, patients, and other stakeholders to address global challenges, clinician burnout, and social issues faced by their communities. This approach focuses on life’s purpose and meaning for individuals and inspires leaders to engage with patients, staff, and communities in new, impactful ways by focusing on wellbeing and wholeness rather than illness and disease. Having a higher sense of purpose is associated with lower all-cause mortality, reduced risk of specific diseases, better health behaviors, greater use of preventive services, and fewer hospital days of care.3
This article describes how AFHS supports the well-being of older adults and aligns with the whole health model of care. It also outlines the VHA investment to transform health care to be more person-centered by documenting what matters in the electronic health record (EHR).
AGE-FRIENDLY CARE
Given that nearly half of veterans enrolled in the VHA are aged ≥ 65 years, there is an increased need to identify models of care to support this aging population.4 This is especially critical because older veterans often have multiple chronic conditions and complex care needs that benefit from a whole person approach. The AFHS movement aims to provide evidence-based care aligned with what matters to older adults and provides a mechanism for transforming care to meet the needs of older veterans. This includes addressing age-related health concerns while promoting optimal health outcomes and quality of life. AFHS follows the 4Ms framework: what matters, medication, mentation, and mobility.5 The 4Ms serve as a guide for the health care of older adults in any setting, where each “M” is assessed and acted on to support what matters.5 Since 2020, > 390 teams have developed a plan to implement the 4Ms at 156 VHA facilities, demonstrating the VHA commitment to transforming health care for veterans.6
When VHA teams join the AFHS movement, they may also engage older veterans in a whole health system (WHS) (Figure). While AFHS is designed to improve care for patients aged ≥ 65 years, it also complements whole health, a person-centered approach available to all veterans enrolled in the VHA. Through the WHS and AFHS, veterans are empowered and equipped to take charge of their health and well-being through conversations about their unique goals, preferences, and health priorities.4 Clinicians are challenged to assess what matters by asking questions like, “What brings you joy?” and, “How can we help you meet your health goals?”1,5 These questions shift the conversation from disease-based treatment and enable clinicians to better understand the veteran as a person.1,5

For whole health and AFHS, conversations about what matters are anchored in the veteran’s goals and preferences, especially those facing a significant health change (ie, a new diagnosis or treatment decision).5,7 Together, the veteran’s goals and priorities serve as the foundation for developing person-centered care plans that often go beyond conventional medical treatments to address the physical, mental, emotional, and social aspects of health.
SYSTEM-WIDE DIRECTIVE
The WHS enhances AFHS discussions about what matters to veterans by adding a system-level lens for conceptualizing health care delivery by leveraging the 3 components of WHS: the “pathway,” well-being programs, and whole health clinical care.
The Pathway
Discovering what matters, or the veteran’s “mission, aspiration, and purpose,” begins with the WHS pathway. When stepping into the pathway, veterans begin completing a personal health inventory, or “walking the circle of health,” which encourages self-reflection that focuses on components of their life that can influence health and well-being.1,8 The circle of health offers a visual representation of the 4 most important aspects of health and well-being: First, “Me” at the center as an individual who is the expert on their life, values, goals, and priorities. Only the individual can know what really matters through mindful awareness and what works for their life. Second, self-care consists of 8 areas that impact health and wellbeing: working your body; surroundings; personal development; food and drink; recharge; family, friends, and coworkers; spirit and soul; and power of the mind. Third, professional care consists of prevention, conventional care, and complementary care. Finally, the community that supports the individual.
Well-Being Programs
VHA provides WHS programs that support veterans in building self-care skills and improving their quality of life, often through integrative care clinics that offer coaching and CIH therapies. For example, a veteran who prioritizes mobility when seeking care at an integrative care clinic will not only receive conventional medical treatment for their physical symptoms but may also be offered CIH therapies depending on their goals. The veteran may set a daily mobility goal with their care team that supports what matters, incorporating CIH approaches, such as yoga and tai chi into the care plan.5 These holistic approaches for moving the body can help alleviate physical symptoms, reduce stress, improve mindful awareness, and provide opportunities for self-discovery and growth, thus promote overall well-being
Whole Health Clinical Care
AFHS and the 4Ms embody the clinical care component of the WHS. Because what matters is the driver of the 4Ms, every action taken by the care team supports wellbeing and quality of life by promoting independence, connection, and support, and addressing external factors, such as social determinants of health. At a minimum, well-being includes “functioning well: the experience of positive emotions such as happiness and contentment as well as the development of one’s potential, having some control over one’s life, having a sense of purpose, and experiencing positive relationships.”9 From a system perspective, the VHA has begun to normalize focusing on what matters to veterans, using an interprofessional approach, one of the first steps to implementing AFHS.
As the programs expand, AFHS teams can learn from whole health well-being programs and increase the capacity for self-care in older veterans. Learning about the key elements included in the circle of health helps clinicians understand each veteran’s perceived strengths and weaknesses to support their self-care. From there, teams can act on the 4Ms and connect older veterans with the most appropriate programs and services at their facility, ensuring continuum of care.
DOCUMENTATION
The VHA leverages several tools and evidence-based practices to assess and act on what matters for veterans of all ages (Table).5,10-16 The VHA EHR and associated dashboards contain a wealth of information about whole health and AFHS implementation, scale up, and spread. A national AFHS 4Ms note template contains standardized data elements called health factors, which provide a mechanism for monitoring 4Ms care via its related dashboard. This template was developed by an interprofessional workgroup of VHA staff and underwent a thorough human factors engineering review and testing process prior to its release. Although teams continue to personalize care based on what matters to the veteran, data from the standardized 4Ms note template and dashboard provide a way to establish consistent, equitable care across multiple care settings.17

Between January 2022 and December 2023, > 612,000 participants aged ≥ 65 years identified what matters to them through 1.35 million assessments. During that period, > 36,000 veterans aged ≥ 65 years participated in AFHS and had what matters conversations documented. A personalized health plan was completed by 585,270 veterans for a total of 1.1 million assessments.11 Whole health coaching has been documented for > 57,000 veterans with > 200,000 assessments completed.13 In fiscal year 2023, a total of 1,802,131 veterans participated in whole health.
When teams share information about what matters to the veteran in a clinicianfacing format in the EHR, this helps ensure that the VHA honors veteran preferences throughout transitions of care and across all phases of health care. Although the EHR captures data on what matters, measurement of the overall impact on veteran and health system outcomes is essential. Further evaluation and ongoing education are needed to ensure clinicians are accurately and efficiently capturing the care provided by completing the appropriate EHR. Additional challenges include identifying ways to balance the documentation burden, while ensuring notes include valuable patient-centered information to guide care. EHR tools and templates have helped to unlock important insights on health care delivery in the VHA; however, health systems must consider how these clinical practices support the overall well-being of patients. How leaders empower frontline clinicians in any care setting to use these data to drive meaningful change is also important.
TRANSFORMING VHA CARE DELIVERY
In Achieving Whole Health: A New Approach for Veterans and the Nation, the National Academy of Science proposes a framework for the transformation of health care institutions to provide better whole health to veterans.3 Transformation requires change in entire systems and leaders who mobilize people “for participation in the process of change, encouraging a sense of collective identity and collective efficacy, which in turn brings stronger feelings of self-worth and self-efficacy,” and an enhanced sense of meaningfulness in their work and lives.18
Shifting health care approaches to equipping and empowering veterans and employees with whole health and AFHS resources is transformational and requires radically different assumptions and approaches that cannot be realized through traditional approaches. This change requires robust and multifaceted cultural transformation spanning all levels of the organization. Whole health and AFHS are facilitating this transformation by supporting documentation and data needs, tracking outcomes across settings, and accelerating spread to new facilities and care settings nationwide to support older veterans in improving their health and well-being.
Whole health and AFHS are complementary approaches to care that can work to empower veterans (as well as caregivers and clinicians) to align services with what matters most to veterans. Lessons such as standardizing person-centered assessments of what matters, creating supportive structures to better align care with veterans’ priorities, and identifying meaningful veteran and system-level outcomes to help sustain transformational change can be applied from whole health to AFHS. Together these programs have the potential to enhance overall health outcomes and quality of life for veterans.
The COVID-19 pandemic established a new normal for health care delivery, with leaders rethinking core practices to survive and thrive in a changing environment and improve the health and well-being of patients. The Veterans Health Administration (VHA) is embracing a shift in focus from “what is the matter” to “what really matters” to address pre- and postpandemic challenges through a whole health approach.1 Initially conceptualized by the VHA in 2011, whole health “is an approach to health care that empowers and equips people to take charge of their health and well-being so that they can live their life to the fullest.”1 Whole health integrates evidence-based complementary and integrative health (CIH) therapies to manage pain; this includes acupuncture, meditation, tai chi, yoga, massage therapy, guided imagery, biofeedback, and clinical hypnosis.1 The VHA now recognizes well-being as a core value, helping clinicians respond to emerging challenges related to the social determinants of health (eg, access to health care, physical activity, and healthy foods) and guiding health care decision making.1,2
Well-being through empowerment—elements of whole health and Age-Friendly Health Systems (AFHS)—encourages health care institutions to work with employees, patients, and other stakeholders to address global challenges, clinician burnout, and social issues faced by their communities. This approach focuses on life’s purpose and meaning for individuals and inspires leaders to engage with patients, staff, and communities in new, impactful ways by focusing on wellbeing and wholeness rather than illness and disease. Having a higher sense of purpose is associated with lower all-cause mortality, reduced risk of specific diseases, better health behaviors, greater use of preventive services, and fewer hospital days of care.3
This article describes how AFHS supports the well-being of older adults and aligns with the whole health model of care. It also outlines the VHA investment to transform health care to be more person-centered by documenting what matters in the electronic health record (EHR).
AGE-FRIENDLY CARE
Given that nearly half of veterans enrolled in the VHA are aged ≥ 65 years, there is an increased need to identify models of care to support this aging population.4 This is especially critical because older veterans often have multiple chronic conditions and complex care needs that benefit from a whole person approach. The AFHS movement aims to provide evidence-based care aligned with what matters to older adults and provides a mechanism for transforming care to meet the needs of older veterans. This includes addressing age-related health concerns while promoting optimal health outcomes and quality of life. AFHS follows the 4Ms framework: what matters, medication, mentation, and mobility.5 The 4Ms serve as a guide for the health care of older adults in any setting, where each “M” is assessed and acted on to support what matters.5 Since 2020, > 390 teams have developed a plan to implement the 4Ms at 156 VHA facilities, demonstrating the VHA commitment to transforming health care for veterans.6
When VHA teams join the AFHS movement, they may also engage older veterans in a whole health system (WHS) (Figure). While AFHS is designed to improve care for patients aged ≥ 65 years, it also complements whole health, a person-centered approach available to all veterans enrolled in the VHA. Through the WHS and AFHS, veterans are empowered and equipped to take charge of their health and well-being through conversations about their unique goals, preferences, and health priorities.4 Clinicians are challenged to assess what matters by asking questions like, “What brings you joy?” and, “How can we help you meet your health goals?”1,5 These questions shift the conversation from disease-based treatment and enable clinicians to better understand the veteran as a person.1,5

For whole health and AFHS, conversations about what matters are anchored in the veteran’s goals and preferences, especially those facing a significant health change (ie, a new diagnosis or treatment decision).5,7 Together, the veteran’s goals and priorities serve as the foundation for developing person-centered care plans that often go beyond conventional medical treatments to address the physical, mental, emotional, and social aspects of health.
SYSTEM-WIDE DIRECTIVE
The WHS enhances AFHS discussions about what matters to veterans by adding a system-level lens for conceptualizing health care delivery by leveraging the 3 components of WHS: the “pathway,” well-being programs, and whole health clinical care.
The Pathway
Discovering what matters, or the veteran’s “mission, aspiration, and purpose,” begins with the WHS pathway. When stepping into the pathway, veterans begin completing a personal health inventory, or “walking the circle of health,” which encourages self-reflection that focuses on components of their life that can influence health and well-being.1,8 The circle of health offers a visual representation of the 4 most important aspects of health and well-being: First, “Me” at the center as an individual who is the expert on their life, values, goals, and priorities. Only the individual can know what really matters through mindful awareness and what works for their life. Second, self-care consists of 8 areas that impact health and wellbeing: working your body; surroundings; personal development; food and drink; recharge; family, friends, and coworkers; spirit and soul; and power of the mind. Third, professional care consists of prevention, conventional care, and complementary care. Finally, the community that supports the individual.
Well-Being Programs
VHA provides WHS programs that support veterans in building self-care skills and improving their quality of life, often through integrative care clinics that offer coaching and CIH therapies. For example, a veteran who prioritizes mobility when seeking care at an integrative care clinic will not only receive conventional medical treatment for their physical symptoms but may also be offered CIH therapies depending on their goals. The veteran may set a daily mobility goal with their care team that supports what matters, incorporating CIH approaches, such as yoga and tai chi into the care plan.5 These holistic approaches for moving the body can help alleviate physical symptoms, reduce stress, improve mindful awareness, and provide opportunities for self-discovery and growth, thus promote overall well-being
Whole Health Clinical Care
AFHS and the 4Ms embody the clinical care component of the WHS. Because what matters is the driver of the 4Ms, every action taken by the care team supports wellbeing and quality of life by promoting independence, connection, and support, and addressing external factors, such as social determinants of health. At a minimum, well-being includes “functioning well: the experience of positive emotions such as happiness and contentment as well as the development of one’s potential, having some control over one’s life, having a sense of purpose, and experiencing positive relationships.”9 From a system perspective, the VHA has begun to normalize focusing on what matters to veterans, using an interprofessional approach, one of the first steps to implementing AFHS.
As the programs expand, AFHS teams can learn from whole health well-being programs and increase the capacity for self-care in older veterans. Learning about the key elements included in the circle of health helps clinicians understand each veteran’s perceived strengths and weaknesses to support their self-care. From there, teams can act on the 4Ms and connect older veterans with the most appropriate programs and services at their facility, ensuring continuum of care.
DOCUMENTATION
The VHA leverages several tools and evidence-based practices to assess and act on what matters for veterans of all ages (Table).5,10-16 The VHA EHR and associated dashboards contain a wealth of information about whole health and AFHS implementation, scale up, and spread. A national AFHS 4Ms note template contains standardized data elements called health factors, which provide a mechanism for monitoring 4Ms care via its related dashboard. This template was developed by an interprofessional workgroup of VHA staff and underwent a thorough human factors engineering review and testing process prior to its release. Although teams continue to personalize care based on what matters to the veteran, data from the standardized 4Ms note template and dashboard provide a way to establish consistent, equitable care across multiple care settings.17

Between January 2022 and December 2023, > 612,000 participants aged ≥ 65 years identified what matters to them through 1.35 million assessments. During that period, > 36,000 veterans aged ≥ 65 years participated in AFHS and had what matters conversations documented. A personalized health plan was completed by 585,270 veterans for a total of 1.1 million assessments.11 Whole health coaching has been documented for > 57,000 veterans with > 200,000 assessments completed.13 In fiscal year 2023, a total of 1,802,131 veterans participated in whole health.
When teams share information about what matters to the veteran in a clinicianfacing format in the EHR, this helps ensure that the VHA honors veteran preferences throughout transitions of care and across all phases of health care. Although the EHR captures data on what matters, measurement of the overall impact on veteran and health system outcomes is essential. Further evaluation and ongoing education are needed to ensure clinicians are accurately and efficiently capturing the care provided by completing the appropriate EHR. Additional challenges include identifying ways to balance the documentation burden, while ensuring notes include valuable patient-centered information to guide care. EHR tools and templates have helped to unlock important insights on health care delivery in the VHA; however, health systems must consider how these clinical practices support the overall well-being of patients. How leaders empower frontline clinicians in any care setting to use these data to drive meaningful change is also important.
TRANSFORMING VHA CARE DELIVERY
In Achieving Whole Health: A New Approach for Veterans and the Nation, the National Academy of Science proposes a framework for the transformation of health care institutions to provide better whole health to veterans.3 Transformation requires change in entire systems and leaders who mobilize people “for participation in the process of change, encouraging a sense of collective identity and collective efficacy, which in turn brings stronger feelings of self-worth and self-efficacy,” and an enhanced sense of meaningfulness in their work and lives.18
Shifting health care approaches to equipping and empowering veterans and employees with whole health and AFHS resources is transformational and requires radically different assumptions and approaches that cannot be realized through traditional approaches. This change requires robust and multifaceted cultural transformation spanning all levels of the organization. Whole health and AFHS are facilitating this transformation by supporting documentation and data needs, tracking outcomes across settings, and accelerating spread to new facilities and care settings nationwide to support older veterans in improving their health and well-being.
Whole health and AFHS are complementary approaches to care that can work to empower veterans (as well as caregivers and clinicians) to align services with what matters most to veterans. Lessons such as standardizing person-centered assessments of what matters, creating supportive structures to better align care with veterans’ priorities, and identifying meaningful veteran and system-level outcomes to help sustain transformational change can be applied from whole health to AFHS. Together these programs have the potential to enhance overall health outcomes and quality of life for veterans.
- Kligler B, Hyde J, Gantt C, Bokhour B. The Whole Health transformation at the Veterans Health Administration: moving from “what’s the matter with you?” to “what matters to you?” Med Care. 2022;60(5):387-391. doi:10.1097/MLR.0000000000001706
- Centers for Disease Control and Prevention. Social determinants of health (SDOH) at CDC. January 17, 2024. Accessed September 12, 2024. https://www.cdc.gov/public-health-gateway/php/about/social-determinants-of-health.html
- National Academies of Sciences, Engineering, and Medicine. Achieving Whole Health: A New Approach for Veterans and the Nation. The National Academies Press; 2023. Accessed September 9, 2024. doi:10.17226/26854
- Church K, Munro S, Shaughnessy M, Clancy C. Age-friendly health systems: improving care for older adults in the Veterans Health Administration. Health Serv Res. 2023;58 Suppl 1(Suppl 1):5-8. doi:10.1111/1475-6773.14110
- Laderman M, Jackson C, Little K, Duong T, Pelton L. “What Matters” to older adults? A toolkit for health systems to design better care with older adults. Institute for Healthcare Improvement; 2019. Accessed September 9, 2024. https://www.ihi.org/Engage/Initiatives/Age-Friendly-Health-Systems/Documents/IHI_Age_Friendly_What_Matters_to_Older_Adults_Toolkit.pdf
- U.S. Department of Veterans Affairs. Age-Friendly Health Systems. Updated September 4, 2024. Accessed September 9, 2024. https://marketplace.va.gov/innovations/age-friendly-health-systems
- Brown TT, Hurley VB, Rodriguez HP, et al. Shared dec i s i o n - m a k i n g l o w e r s m e d i c a l e x p e n d i t u re s a n d the effect is amplified in racially-ethnically concordant relationships. Med Care. 2023;61(8):528-535. doi:10.1097/MLR.0000000000001881
- Kligler B. Whole Health in the Veterans Health Administration. Glob Adv Health Med. 2022;11:2164957X221077214.
- Ruggeri K, Garcia-Garzon E, Maguire Á, Matz S, Huppert FA. Well-being is more than happiness and life satisfaction: a multidimensional analysis of 21 countries. Health Qual Life Outcomes. 2020;18(1):192. doi:10.1186/s12955-020-01423-y
- U.S. Department of Veterans Affairs. Personal Health Inventory. Updated May 2022. Accessed September 9, 2024. https://www.va.gov/WHOLEHEALTH/docs/PHI-long-May22-fillable-508.pdf doi:10.1177/2164957X221077214
- Veterans Health Administration. Personal Health Plan. Updated March 2019. Accessed September 9, 2024. https:// www.va.gov/WHOLEHEALTH/docs/PersonalHealthPlan_508_03-2019.pdf
- Veterans Health Administration. Whole Health: My Life, My Story. Updated March 20, 2024. Accessed September 9, 2024. https://www.va.gov/WHOLEHEALTH/mylifemystory/index.asp
- U.S. Department of Veterans Affairs. Whole Health Library: Whole Health for Skill Building. Updated April 17, 2024. Accessed September 9, 2024. https://www.va.gov/WHOLEHEALTHLIBRARY/courses/whole-health-skill-building.asp
- U.S. Department of Veterans Affairs. Making Decisions: Current Care Planning. Updated May 21, 2024. Accessed September 9, 2024. https://www.va.gov/geriatrics/pages/making_decisions.asp
- U.S. Department of Veterans Affairs. Life-Sustaining Treatment Decisions Initiative (LSTDI). Updated March 2024. Accessed September 12, 2024. https://marketplace.va.gov/innovations/life-sustaining-treatment-decisions-initiative
- U.S. Department of Veterans Affairs. Center for Health Equity Research and Promotion: Surgical Pause Saving Veterans Lives. Updated September 22, 2021. Accessed September 9, 2024. https://www.cherp.research.va.gov/features/Surgical_Pause_Saving_Veterans_Lives.asp
- Munro S, Church K, Berner C, et al. Implementation of an agefriendly template in the Veterans Health Administration electronic health record. J Inform Nurs. 2023;8(3):6-11.
- Burns JM. Transforming Leadership: A New Pursuit of Happiness. Grove Press; 2003.
- US Department of Veterans Affairs, Veterans Health Administration. Whole Health: Circle of Health Overview. Updated May 20, 2024. Accessed September 12, 2024. https://www.va.gov/WHOLEHEALTH/circle-of-health/index.asp
- Kligler B, Hyde J, Gantt C, Bokhour B. The Whole Health transformation at the Veterans Health Administration: moving from “what’s the matter with you?” to “what matters to you?” Med Care. 2022;60(5):387-391. doi:10.1097/MLR.0000000000001706
- Centers for Disease Control and Prevention. Social determinants of health (SDOH) at CDC. January 17, 2024. Accessed September 12, 2024. https://www.cdc.gov/public-health-gateway/php/about/social-determinants-of-health.html
- National Academies of Sciences, Engineering, and Medicine. Achieving Whole Health: A New Approach for Veterans and the Nation. The National Academies Press; 2023. Accessed September 9, 2024. doi:10.17226/26854
- Church K, Munro S, Shaughnessy M, Clancy C. Age-friendly health systems: improving care for older adults in the Veterans Health Administration. Health Serv Res. 2023;58 Suppl 1(Suppl 1):5-8. doi:10.1111/1475-6773.14110
- Laderman M, Jackson C, Little K, Duong T, Pelton L. “What Matters” to older adults? A toolkit for health systems to design better care with older adults. Institute for Healthcare Improvement; 2019. Accessed September 9, 2024. https://www.ihi.org/Engage/Initiatives/Age-Friendly-Health-Systems/Documents/IHI_Age_Friendly_What_Matters_to_Older_Adults_Toolkit.pdf
- U.S. Department of Veterans Affairs. Age-Friendly Health Systems. Updated September 4, 2024. Accessed September 9, 2024. https://marketplace.va.gov/innovations/age-friendly-health-systems
- Brown TT, Hurley VB, Rodriguez HP, et al. Shared dec i s i o n - m a k i n g l o w e r s m e d i c a l e x p e n d i t u re s a n d the effect is amplified in racially-ethnically concordant relationships. Med Care. 2023;61(8):528-535. doi:10.1097/MLR.0000000000001881
- Kligler B. Whole Health in the Veterans Health Administration. Glob Adv Health Med. 2022;11:2164957X221077214.
- Ruggeri K, Garcia-Garzon E, Maguire Á, Matz S, Huppert FA. Well-being is more than happiness and life satisfaction: a multidimensional analysis of 21 countries. Health Qual Life Outcomes. 2020;18(1):192. doi:10.1186/s12955-020-01423-y
- U.S. Department of Veterans Affairs. Personal Health Inventory. Updated May 2022. Accessed September 9, 2024. https://www.va.gov/WHOLEHEALTH/docs/PHI-long-May22-fillable-508.pdf doi:10.1177/2164957X221077214
- Veterans Health Administration. Personal Health Plan. Updated March 2019. Accessed September 9, 2024. https:// www.va.gov/WHOLEHEALTH/docs/PersonalHealthPlan_508_03-2019.pdf
- Veterans Health Administration. Whole Health: My Life, My Story. Updated March 20, 2024. Accessed September 9, 2024. https://www.va.gov/WHOLEHEALTH/mylifemystory/index.asp
- U.S. Department of Veterans Affairs. Whole Health Library: Whole Health for Skill Building. Updated April 17, 2024. Accessed September 9, 2024. https://www.va.gov/WHOLEHEALTHLIBRARY/courses/whole-health-skill-building.asp
- U.S. Department of Veterans Affairs. Making Decisions: Current Care Planning. Updated May 21, 2024. Accessed September 9, 2024. https://www.va.gov/geriatrics/pages/making_decisions.asp
- U.S. Department of Veterans Affairs. Life-Sustaining Treatment Decisions Initiative (LSTDI). Updated March 2024. Accessed September 12, 2024. https://marketplace.va.gov/innovations/life-sustaining-treatment-decisions-initiative
- U.S. Department of Veterans Affairs. Center for Health Equity Research and Promotion: Surgical Pause Saving Veterans Lives. Updated September 22, 2021. Accessed September 9, 2024. https://www.cherp.research.va.gov/features/Surgical_Pause_Saving_Veterans_Lives.asp
- Munro S, Church K, Berner C, et al. Implementation of an agefriendly template in the Veterans Health Administration electronic health record. J Inform Nurs. 2023;8(3):6-11.
- Burns JM. Transforming Leadership: A New Pursuit of Happiness. Grove Press; 2003.
- US Department of Veterans Affairs, Veterans Health Administration. Whole Health: Circle of Health Overview. Updated May 20, 2024. Accessed September 12, 2024. https://www.va.gov/WHOLEHEALTH/circle-of-health/index.asp
Isatuximab Quadruplet Approval Could Change the Landscape for Treating Myeloma
The findings, presented on September 26 at the annual meeting of the International Myeloma Society, support the four-drug combination known as Isa-VRd as a potential new standard of care (SOC) supplanting VRd alone as the SOC in this setting, according to Meletios Dimopoulos, MD, of the University of Athens, Greece.
The IMROZ findings — the first from a phase 3 study of an anti-CD38 monoclonal antibody given in combination with VRd — were also reported in May at the annual meeting of the American Society of Clinical Oncology (ASCO) and published simultaneously in The New England Journal of Medicine.
“The significant progression-free benefit observed with Sarclisa with combination therapy compared to VRd is important and encouraging for patients with newly diagnosed multiple myeloma,” first author Thierry Facon, MD, told this news organization at ASCO.
Dr. Thierry, of the University of Lille, and the French Academy of Medicine in Paris, France, added that Isa-VRd has the potential as “a first-in-class combination to address gaps in care for newly diagnosed multiple myeloma transplant-ineligible patients.”
Isatuximab in combination with VRd was subsequently approved by the US Food and Drug Administration (FDA) for this indication, as reported on September 23 by this news organization.
So, what will this quadruplet mean for the treatment of multiple myeloma? IMROZ study coauthors Meral Beksac, MD, of Istinye University, Istanbul, and Liv Hospital Ankara, Turkey, and Mohamad Mohty, MD, of Sorbonne University, Saint-Antoine Hospital, Paris, France, provided some insights in a recent interview, telling the European Medical Journal (EMJ) Hematology that Isa-VRd is a “welcome addition” to the multiple myeloma armamentarium.
Should Isa-VRd Be Considered the New First-Choice Frontline Treatment for Transplant-Ineligible Patients?
“The short answer is yes,” Dr. Mohty told EMJ. “Based on this trial, quadruplet should become the preferred regimen in the population of patients represented by these inclusion criteria.”
Dr. Beksac agreed that Isa-VRd will play a role in frontline management for transplant-ineligible patients.
However, both noted that despite having a favorable safety profile similar to VRd, Isa-VRd may not be well tolerated in elderly and frail patients. Demonstrably frail patients were excluded from IMROZ, and this is a factor that should be considered in the practice setting, they agreed.
Will Isa-VRd Change How Patients Are Evaluated for Transplant Eligibility?
“The cutoff for transplant eligibility differs from one country to another, and today, we do not have consensus around an agreed-upon age limit,” Dr. Beksac said. “We further rely on frailty and the patient’s performance status, not only at diagnosis but at later stages as well.”
She also noted that “[t]he introduction of very effective systemic regimens with similar efficacy to [hematopoietic stem cell transplant (HSCT)] has seen a shift towards non-transplant regimens, particularly in the USA.”
“In many centers in Europe, these patients [in IMROZ] would be considered transplant eligible. Hence, for this group of patients who are not too old, but not too young, and fit, IMROZ is offering a non-transplant-based treatment with similar efficacy to what can be achieved with HSCT,” Dr. Mohty added.
Patient preference and access are also important considerations, as is cost, he noted.
Younger transplant-eligible patients may prefer transplant over continuous treatment for life, whereas some might prefer long-term treatment over a stem cell protocol that will require months off of work, he and Dr. Beksac explained.
“Based on this trial, we will likely see a decline in the number of transplants,” Dr. Mohty predicted. “With the IMROZ data, we have something valid that we can offer patients without any prejudice to their outcome.”
How Will This Combination Be Integrated Into Daily Clinical Practice?
“My interpretation would be that this protocol will be conceived as an applicable protocol that can be adapted to our daily practice,” Dr. Beksac said.
Dr. Mohty added that the multiple myeloma story is changing and evolving.
“It’s not transplant versus no transplant, it’s who is going to receive quadruplet and who’s going to receive less than a quadruplet, who is fit and who is unfit,” he explained, adding that physicians will likely adapt the Isa-VRd regimen for real-world use based on clinical judgment.
For example, the quadruplet may be combined “in a kind of VRd-light version to start with, and maybe we can adapt later depending on the tolerability of the patient,” Dr. Beksac added.
“Until recently, we thought that transplant is the gold standard for everybody whenever possible. Now, we have a more nuanced answer, offering a regimen that actually is as effective, and may even be better, than transplant,” Dr. Mohty said. “So, it’s a most welcome addition to what we do.”
Both the IMROZ study and the EMJ article were funded by Sanofi.
Dr. Dimopoulos reported ties with Amgen, BeiGene, BMS, Janssen, Sanofi, and Takeda. Dr. Beksac disclosed relationships with Amgen, BMS, GSK, Janssen, Sanofi, and Takeda. Dr. Mohty reported ties with Adaptive Biotechnologies, Amgen, Astellas Pharma, BMS, GSK, Janssen-Cilag, Jazz Pharmaceuticals, and others.
A version of this article appeared on Medscape.com.
The findings, presented on September 26 at the annual meeting of the International Myeloma Society, support the four-drug combination known as Isa-VRd as a potential new standard of care (SOC) supplanting VRd alone as the SOC in this setting, according to Meletios Dimopoulos, MD, of the University of Athens, Greece.
The IMROZ findings — the first from a phase 3 study of an anti-CD38 monoclonal antibody given in combination with VRd — were also reported in May at the annual meeting of the American Society of Clinical Oncology (ASCO) and published simultaneously in The New England Journal of Medicine.
“The significant progression-free benefit observed with Sarclisa with combination therapy compared to VRd is important and encouraging for patients with newly diagnosed multiple myeloma,” first author Thierry Facon, MD, told this news organization at ASCO.
Dr. Thierry, of the University of Lille, and the French Academy of Medicine in Paris, France, added that Isa-VRd has the potential as “a first-in-class combination to address gaps in care for newly diagnosed multiple myeloma transplant-ineligible patients.”
Isatuximab in combination with VRd was subsequently approved by the US Food and Drug Administration (FDA) for this indication, as reported on September 23 by this news organization.
So, what will this quadruplet mean for the treatment of multiple myeloma? IMROZ study coauthors Meral Beksac, MD, of Istinye University, Istanbul, and Liv Hospital Ankara, Turkey, and Mohamad Mohty, MD, of Sorbonne University, Saint-Antoine Hospital, Paris, France, provided some insights in a recent interview, telling the European Medical Journal (EMJ) Hematology that Isa-VRd is a “welcome addition” to the multiple myeloma armamentarium.
Should Isa-VRd Be Considered the New First-Choice Frontline Treatment for Transplant-Ineligible Patients?
“The short answer is yes,” Dr. Mohty told EMJ. “Based on this trial, quadruplet should become the preferred regimen in the population of patients represented by these inclusion criteria.”
Dr. Beksac agreed that Isa-VRd will play a role in frontline management for transplant-ineligible patients.
However, both noted that despite having a favorable safety profile similar to VRd, Isa-VRd may not be well tolerated in elderly and frail patients. Demonstrably frail patients were excluded from IMROZ, and this is a factor that should be considered in the practice setting, they agreed.
Will Isa-VRd Change How Patients Are Evaluated for Transplant Eligibility?
“The cutoff for transplant eligibility differs from one country to another, and today, we do not have consensus around an agreed-upon age limit,” Dr. Beksac said. “We further rely on frailty and the patient’s performance status, not only at diagnosis but at later stages as well.”
She also noted that “[t]he introduction of very effective systemic regimens with similar efficacy to [hematopoietic stem cell transplant (HSCT)] has seen a shift towards non-transplant regimens, particularly in the USA.”
“In many centers in Europe, these patients [in IMROZ] would be considered transplant eligible. Hence, for this group of patients who are not too old, but not too young, and fit, IMROZ is offering a non-transplant-based treatment with similar efficacy to what can be achieved with HSCT,” Dr. Mohty added.
Patient preference and access are also important considerations, as is cost, he noted.
Younger transplant-eligible patients may prefer transplant over continuous treatment for life, whereas some might prefer long-term treatment over a stem cell protocol that will require months off of work, he and Dr. Beksac explained.
“Based on this trial, we will likely see a decline in the number of transplants,” Dr. Mohty predicted. “With the IMROZ data, we have something valid that we can offer patients without any prejudice to their outcome.”
How Will This Combination Be Integrated Into Daily Clinical Practice?
“My interpretation would be that this protocol will be conceived as an applicable protocol that can be adapted to our daily practice,” Dr. Beksac said.
Dr. Mohty added that the multiple myeloma story is changing and evolving.
“It’s not transplant versus no transplant, it’s who is going to receive quadruplet and who’s going to receive less than a quadruplet, who is fit and who is unfit,” he explained, adding that physicians will likely adapt the Isa-VRd regimen for real-world use based on clinical judgment.
For example, the quadruplet may be combined “in a kind of VRd-light version to start with, and maybe we can adapt later depending on the tolerability of the patient,” Dr. Beksac added.
“Until recently, we thought that transplant is the gold standard for everybody whenever possible. Now, we have a more nuanced answer, offering a regimen that actually is as effective, and may even be better, than transplant,” Dr. Mohty said. “So, it’s a most welcome addition to what we do.”
Both the IMROZ study and the EMJ article were funded by Sanofi.
Dr. Dimopoulos reported ties with Amgen, BeiGene, BMS, Janssen, Sanofi, and Takeda. Dr. Beksac disclosed relationships with Amgen, BMS, GSK, Janssen, Sanofi, and Takeda. Dr. Mohty reported ties with Adaptive Biotechnologies, Amgen, Astellas Pharma, BMS, GSK, Janssen-Cilag, Jazz Pharmaceuticals, and others.
A version of this article appeared on Medscape.com.
The findings, presented on September 26 at the annual meeting of the International Myeloma Society, support the four-drug combination known as Isa-VRd as a potential new standard of care (SOC) supplanting VRd alone as the SOC in this setting, according to Meletios Dimopoulos, MD, of the University of Athens, Greece.
The IMROZ findings — the first from a phase 3 study of an anti-CD38 monoclonal antibody given in combination with VRd — were also reported in May at the annual meeting of the American Society of Clinical Oncology (ASCO) and published simultaneously in The New England Journal of Medicine.
“The significant progression-free benefit observed with Sarclisa with combination therapy compared to VRd is important and encouraging for patients with newly diagnosed multiple myeloma,” first author Thierry Facon, MD, told this news organization at ASCO.
Dr. Thierry, of the University of Lille, and the French Academy of Medicine in Paris, France, added that Isa-VRd has the potential as “a first-in-class combination to address gaps in care for newly diagnosed multiple myeloma transplant-ineligible patients.”
Isatuximab in combination with VRd was subsequently approved by the US Food and Drug Administration (FDA) for this indication, as reported on September 23 by this news organization.
So, what will this quadruplet mean for the treatment of multiple myeloma? IMROZ study coauthors Meral Beksac, MD, of Istinye University, Istanbul, and Liv Hospital Ankara, Turkey, and Mohamad Mohty, MD, of Sorbonne University, Saint-Antoine Hospital, Paris, France, provided some insights in a recent interview, telling the European Medical Journal (EMJ) Hematology that Isa-VRd is a “welcome addition” to the multiple myeloma armamentarium.
Should Isa-VRd Be Considered the New First-Choice Frontline Treatment for Transplant-Ineligible Patients?
“The short answer is yes,” Dr. Mohty told EMJ. “Based on this trial, quadruplet should become the preferred regimen in the population of patients represented by these inclusion criteria.”
Dr. Beksac agreed that Isa-VRd will play a role in frontline management for transplant-ineligible patients.
However, both noted that despite having a favorable safety profile similar to VRd, Isa-VRd may not be well tolerated in elderly and frail patients. Demonstrably frail patients were excluded from IMROZ, and this is a factor that should be considered in the practice setting, they agreed.
Will Isa-VRd Change How Patients Are Evaluated for Transplant Eligibility?
“The cutoff for transplant eligibility differs from one country to another, and today, we do not have consensus around an agreed-upon age limit,” Dr. Beksac said. “We further rely on frailty and the patient’s performance status, not only at diagnosis but at later stages as well.”
She also noted that “[t]he introduction of very effective systemic regimens with similar efficacy to [hematopoietic stem cell transplant (HSCT)] has seen a shift towards non-transplant regimens, particularly in the USA.”
“In many centers in Europe, these patients [in IMROZ] would be considered transplant eligible. Hence, for this group of patients who are not too old, but not too young, and fit, IMROZ is offering a non-transplant-based treatment with similar efficacy to what can be achieved with HSCT,” Dr. Mohty added.
Patient preference and access are also important considerations, as is cost, he noted.
Younger transplant-eligible patients may prefer transplant over continuous treatment for life, whereas some might prefer long-term treatment over a stem cell protocol that will require months off of work, he and Dr. Beksac explained.
“Based on this trial, we will likely see a decline in the number of transplants,” Dr. Mohty predicted. “With the IMROZ data, we have something valid that we can offer patients without any prejudice to their outcome.”
How Will This Combination Be Integrated Into Daily Clinical Practice?
“My interpretation would be that this protocol will be conceived as an applicable protocol that can be adapted to our daily practice,” Dr. Beksac said.
Dr. Mohty added that the multiple myeloma story is changing and evolving.
“It’s not transplant versus no transplant, it’s who is going to receive quadruplet and who’s going to receive less than a quadruplet, who is fit and who is unfit,” he explained, adding that physicians will likely adapt the Isa-VRd regimen for real-world use based on clinical judgment.
For example, the quadruplet may be combined “in a kind of VRd-light version to start with, and maybe we can adapt later depending on the tolerability of the patient,” Dr. Beksac added.
“Until recently, we thought that transplant is the gold standard for everybody whenever possible. Now, we have a more nuanced answer, offering a regimen that actually is as effective, and may even be better, than transplant,” Dr. Mohty said. “So, it’s a most welcome addition to what we do.”
Both the IMROZ study and the EMJ article were funded by Sanofi.
Dr. Dimopoulos reported ties with Amgen, BeiGene, BMS, Janssen, Sanofi, and Takeda. Dr. Beksac disclosed relationships with Amgen, BMS, GSK, Janssen, Sanofi, and Takeda. Dr. Mohty reported ties with Adaptive Biotechnologies, Amgen, Astellas Pharma, BMS, GSK, Janssen-Cilag, Jazz Pharmaceuticals, and others.
A version of this article appeared on Medscape.com.
FROM IMS 2024
Clozapine and Respiratory Infection Risk: What to Know
Clozapine is considered the drug of choice for treatment-resistant schizophrenia in guidelines globally, but it remains significantly underutilized. This is largely due to its range of side effects, particularly its increased infection risk which prompted the US Food and Drug Administration (FDA) to mandate regular blood testing to monitor neutrophil counts.
The COVID-19 pandemic raised new concerns about the care of clozapine-treated patients, leading clinicians and patients to urge the FDA to relax prescription requirements for the drug under the Risk Evaluation and Mitigation Strategy (REMS) program.
As the FDA prepares for a public hearing in November on proposed adjustments to the drug’s REMS criteria, a growing body of research is challenging the previous understanding of clozapine and infection risk.
Clarifying the Risk
Research on the link between clozapine and respiratory infections has produced conflicting results. Some studies indicate little to no increased risk for mild COVID-19 and other respiratory illnesses, while others have shown a higher likelihood of severe infection.
A recent nationwide Danish registry study of respiratory infections in people with a schizophrenia spectrum disorder could bring some clarity, Maxime Taquet, MD, a clinical lecturer at the University of Oxford, Warneford Hospital, Oxford, England, told this news organization.
By tracking periods when patients were on and off clozapine and other antipsychotics, the study offers more precise risk estimates, distinguishing the risks associated with the antipsychotic from those related to underlying schizophrenia, said Dr. Taquet, who authored an accompanying editorial on the study.
“It’s very important to try to disentangle the effects of schizophrenia, its severity, from the medication,” Dr. Taquet said. “I think that the Danish study is the first to try and really do that with as much precision as possible.”
After adjusting for key confounders including economic status and COVID-19 vaccination status, the researchers found that individuals taking antipsychotics had lower odds of testing positive for SARS-CoV-2 and similar rates of filled anti-infective prescriptions as those not taking the drugs.
Although antipsychotic use was not linked to higher rates of mild infection, it was linked to an increased risk for COVID-19 hospitalization in individuals older than 70 years, as well as hospitalization and death from other respiratory infections, mainly pneumonia, in those older than 40 years.
Notably, there was no excess risk for any outcome with clozapine vs other antipsychotics.
Strong Link to Pneumonia Risk
Results from a longitudinal Finnish study, just published in The American Journal of Psychiatry, also show an increased risk for severe outcomes from ileus and pneumonia among more than 2600 patients with schizophrenia taking clozapine.
Twenty years after initiating clozapine, the cumulative incidence estimate for ileus was 5.3% — more than sixfold higher than previously reported. The incidence of pneumonia was also high, at 29.5%.
Both illnesses were significantly associated with mortality, with odds ratios of 4.5 and 2.8, respectively.
These findings align with previous pharmacovigilance studies, with reported mortality rates for gastrointestinal hypomotility and pneumonia that were 4-10 times higher than those for agranulocytosis, the researchers said.
The study “really adds to a growing body of research suggesting a connection between clozapine use and a higher risk of developing pneumonia,” Robert O. Cotes, MD, a professor of psychiatry and behavioral sciences at Emory University, Atlanta, who specializes in the use of clozapine, told this news organization.
“Additionally, when people on clozapine do contract pneumonia, there’s concern the condition may be more dangerous,” he added.
A Closer Look at Neutropenia Risk
Neutropenia receives the lion’s share of attention among clozapine’s potential side effects, but this focus may need to be re-evaluated, Dr. Cotes said.
He pointed out that recent data suggest the risk for severe neutropenia, 2-3 years after initiating clozapine, is comparable to that of other antipsychotics.
A study of 26,630 clozapine users in Australia and New Zealand showed that most cases of severe neutropenia leading to clozapine cessation peaked within 18 weeks and was negligible after 2 years. This suggests weekly hematologic monitoring could potentially be discontinued after the 2-year mark.
Another study reported earlier this year by this news organization showed a low risk for mild or moderate neutropenia and no severe cases in nearly 1000 people taking clozapine.
“I worry that we may be missing the forest for the trees by hyperfocusing on neutropenia and not considering clozapine’s other potential serious side effects like pneumonia, myocarditis, and gastrointestinal hypermotility,” Dr. Cotes said.
Importance of Vaccines
The findings of these studies highlight the importance of vaccines in this at-risk group, said Dr. Taquet, a point emphasized by investigators of the Danish study he reviewed.
“Inspired by the experience of COVID-19 vaccine prioritization in severe mental illness and based on our findings, there is momentum for preventive action,” the authors wrote. “Our findings do not suggest the avoidance of specific antipsychotics but rather a call for increased vigilance regarding this at-risk group.”
This includes recommending pneumococcal, influenza, COVID-19, and other anti-infective vaccines in those older than 40 years treated with, or due to start, an antipsychotic.
“It’s not mandatory, but we do recommend that patients on clozapine get the regular vaccines,” Dr. Taquet said.
Pointing to the recent study on pneumonia risk, Dr. Cotes said addressing underlying risk factors, such as smoking, obesity, and possibly sedation and excessive salivation caused by clozapine, is key.
“And to make sure that vaccinations are up to date, particularly heading into this fall,” he added.
Rethinking Clozapine REMS
One of the most challenging issues facing clinicians and researchers is how to help people understand the safety profile of clozapine and to use it with more confidence, Dr. Cotes said.
“A lot of people hear about clozapine and they think about neutropenia, they think about side effects, the REMS system, and all of these factors really drive down clozapine utilization,” he said.
Treatment-resistant schizophrenia affects about a quarter of those with schizophrenia, yet only 4% of these patients receive clozapine in the United States, Dr. Cotes said. That number may be even lower for its other indication of reducing suicidal behavior in patients with schizophrenia or schizoaffective disorder.
The clozapine REMS is viewed as a major barrier to utilization and requires certification of pharmacists and physicians and use of a central system to monitor absolute neutrophil counts for neutropenia in patients.
As previously reported by this news organization in November 2022, the FDA opted to temporarily exercise enforcement discretion for certain aspects of the drug safety program to ensure continuity of care for patients after concerns were raised by the American Psychiatric Association (APA) along with other professional organizations.
Even with that temporary enforcement discretion, “reports have shown that over half of those prescribed clozapine have trouble accessing the medication because of the REMS program,” a spokesperson for the APA told this news organization.
“Not only are patients having trouble accessing the medication, many have trouble finding a prescriber in their geographic locations and others because of the monitoring requirements have their treatment discontinued leading to negative outcomes,” the spokesperson said.
The FDA is currently reviewing the clozapine REMS and is holding a joint advisory committee meeting on November 19 to discuss the review and “possible changes to minimize burden on patients, pharmacies, and prescribers while maintaining safe use of clozapine.”
The APA plans to submit written and oral comments to the advisory committees.
“We are hopeful that the re-evaluation meeting in November will remove barriers and increase access to clozapine, which is currently highly underutilized, especially in marginalized communities,” the spokesperson said.
Dr. Cotes reported serving as a speaker and consultant for Saladax Biomedical and as a consultant for Syneos Health. Dr. Taquet reported having no competing interests.
A version of this article first appeared on Medscape.com.
Clozapine is considered the drug of choice for treatment-resistant schizophrenia in guidelines globally, but it remains significantly underutilized. This is largely due to its range of side effects, particularly its increased infection risk which prompted the US Food and Drug Administration (FDA) to mandate regular blood testing to monitor neutrophil counts.
The COVID-19 pandemic raised new concerns about the care of clozapine-treated patients, leading clinicians and patients to urge the FDA to relax prescription requirements for the drug under the Risk Evaluation and Mitigation Strategy (REMS) program.
As the FDA prepares for a public hearing in November on proposed adjustments to the drug’s REMS criteria, a growing body of research is challenging the previous understanding of clozapine and infection risk.
Clarifying the Risk
Research on the link between clozapine and respiratory infections has produced conflicting results. Some studies indicate little to no increased risk for mild COVID-19 and other respiratory illnesses, while others have shown a higher likelihood of severe infection.
A recent nationwide Danish registry study of respiratory infections in people with a schizophrenia spectrum disorder could bring some clarity, Maxime Taquet, MD, a clinical lecturer at the University of Oxford, Warneford Hospital, Oxford, England, told this news organization.
By tracking periods when patients were on and off clozapine and other antipsychotics, the study offers more precise risk estimates, distinguishing the risks associated with the antipsychotic from those related to underlying schizophrenia, said Dr. Taquet, who authored an accompanying editorial on the study.
“It’s very important to try to disentangle the effects of schizophrenia, its severity, from the medication,” Dr. Taquet said. “I think that the Danish study is the first to try and really do that with as much precision as possible.”
After adjusting for key confounders including economic status and COVID-19 vaccination status, the researchers found that individuals taking antipsychotics had lower odds of testing positive for SARS-CoV-2 and similar rates of filled anti-infective prescriptions as those not taking the drugs.
Although antipsychotic use was not linked to higher rates of mild infection, it was linked to an increased risk for COVID-19 hospitalization in individuals older than 70 years, as well as hospitalization and death from other respiratory infections, mainly pneumonia, in those older than 40 years.
Notably, there was no excess risk for any outcome with clozapine vs other antipsychotics.
Strong Link to Pneumonia Risk
Results from a longitudinal Finnish study, just published in The American Journal of Psychiatry, also show an increased risk for severe outcomes from ileus and pneumonia among more than 2600 patients with schizophrenia taking clozapine.
Twenty years after initiating clozapine, the cumulative incidence estimate for ileus was 5.3% — more than sixfold higher than previously reported. The incidence of pneumonia was also high, at 29.5%.
Both illnesses were significantly associated with mortality, with odds ratios of 4.5 and 2.8, respectively.
These findings align with previous pharmacovigilance studies, with reported mortality rates for gastrointestinal hypomotility and pneumonia that were 4-10 times higher than those for agranulocytosis, the researchers said.
The study “really adds to a growing body of research suggesting a connection between clozapine use and a higher risk of developing pneumonia,” Robert O. Cotes, MD, a professor of psychiatry and behavioral sciences at Emory University, Atlanta, who specializes in the use of clozapine, told this news organization.
“Additionally, when people on clozapine do contract pneumonia, there’s concern the condition may be more dangerous,” he added.
A Closer Look at Neutropenia Risk
Neutropenia receives the lion’s share of attention among clozapine’s potential side effects, but this focus may need to be re-evaluated, Dr. Cotes said.
He pointed out that recent data suggest the risk for severe neutropenia, 2-3 years after initiating clozapine, is comparable to that of other antipsychotics.
A study of 26,630 clozapine users in Australia and New Zealand showed that most cases of severe neutropenia leading to clozapine cessation peaked within 18 weeks and was negligible after 2 years. This suggests weekly hematologic monitoring could potentially be discontinued after the 2-year mark.
Another study reported earlier this year by this news organization showed a low risk for mild or moderate neutropenia and no severe cases in nearly 1000 people taking clozapine.
“I worry that we may be missing the forest for the trees by hyperfocusing on neutropenia and not considering clozapine’s other potential serious side effects like pneumonia, myocarditis, and gastrointestinal hypermotility,” Dr. Cotes said.
Importance of Vaccines
The findings of these studies highlight the importance of vaccines in this at-risk group, said Dr. Taquet, a point emphasized by investigators of the Danish study he reviewed.
“Inspired by the experience of COVID-19 vaccine prioritization in severe mental illness and based on our findings, there is momentum for preventive action,” the authors wrote. “Our findings do not suggest the avoidance of specific antipsychotics but rather a call for increased vigilance regarding this at-risk group.”
This includes recommending pneumococcal, influenza, COVID-19, and other anti-infective vaccines in those older than 40 years treated with, or due to start, an antipsychotic.
“It’s not mandatory, but we do recommend that patients on clozapine get the regular vaccines,” Dr. Taquet said.
Pointing to the recent study on pneumonia risk, Dr. Cotes said addressing underlying risk factors, such as smoking, obesity, and possibly sedation and excessive salivation caused by clozapine, is key.
“And to make sure that vaccinations are up to date, particularly heading into this fall,” he added.
Rethinking Clozapine REMS
One of the most challenging issues facing clinicians and researchers is how to help people understand the safety profile of clozapine and to use it with more confidence, Dr. Cotes said.
“A lot of people hear about clozapine and they think about neutropenia, they think about side effects, the REMS system, and all of these factors really drive down clozapine utilization,” he said.
Treatment-resistant schizophrenia affects about a quarter of those with schizophrenia, yet only 4% of these patients receive clozapine in the United States, Dr. Cotes said. That number may be even lower for its other indication of reducing suicidal behavior in patients with schizophrenia or schizoaffective disorder.
The clozapine REMS is viewed as a major barrier to utilization and requires certification of pharmacists and physicians and use of a central system to monitor absolute neutrophil counts for neutropenia in patients.
As previously reported by this news organization in November 2022, the FDA opted to temporarily exercise enforcement discretion for certain aspects of the drug safety program to ensure continuity of care for patients after concerns were raised by the American Psychiatric Association (APA) along with other professional organizations.
Even with that temporary enforcement discretion, “reports have shown that over half of those prescribed clozapine have trouble accessing the medication because of the REMS program,” a spokesperson for the APA told this news organization.
“Not only are patients having trouble accessing the medication, many have trouble finding a prescriber in their geographic locations and others because of the monitoring requirements have their treatment discontinued leading to negative outcomes,” the spokesperson said.
The FDA is currently reviewing the clozapine REMS and is holding a joint advisory committee meeting on November 19 to discuss the review and “possible changes to minimize burden on patients, pharmacies, and prescribers while maintaining safe use of clozapine.”
The APA plans to submit written and oral comments to the advisory committees.
“We are hopeful that the re-evaluation meeting in November will remove barriers and increase access to clozapine, which is currently highly underutilized, especially in marginalized communities,” the spokesperson said.
Dr. Cotes reported serving as a speaker and consultant for Saladax Biomedical and as a consultant for Syneos Health. Dr. Taquet reported having no competing interests.
A version of this article first appeared on Medscape.com.
Clozapine is considered the drug of choice for treatment-resistant schizophrenia in guidelines globally, but it remains significantly underutilized. This is largely due to its range of side effects, particularly its increased infection risk which prompted the US Food and Drug Administration (FDA) to mandate regular blood testing to monitor neutrophil counts.
The COVID-19 pandemic raised new concerns about the care of clozapine-treated patients, leading clinicians and patients to urge the FDA to relax prescription requirements for the drug under the Risk Evaluation and Mitigation Strategy (REMS) program.
As the FDA prepares for a public hearing in November on proposed adjustments to the drug’s REMS criteria, a growing body of research is challenging the previous understanding of clozapine and infection risk.
Clarifying the Risk
Research on the link between clozapine and respiratory infections has produced conflicting results. Some studies indicate little to no increased risk for mild COVID-19 and other respiratory illnesses, while others have shown a higher likelihood of severe infection.
A recent nationwide Danish registry study of respiratory infections in people with a schizophrenia spectrum disorder could bring some clarity, Maxime Taquet, MD, a clinical lecturer at the University of Oxford, Warneford Hospital, Oxford, England, told this news organization.
By tracking periods when patients were on and off clozapine and other antipsychotics, the study offers more precise risk estimates, distinguishing the risks associated with the antipsychotic from those related to underlying schizophrenia, said Dr. Taquet, who authored an accompanying editorial on the study.
“It’s very important to try to disentangle the effects of schizophrenia, its severity, from the medication,” Dr. Taquet said. “I think that the Danish study is the first to try and really do that with as much precision as possible.”
After adjusting for key confounders including economic status and COVID-19 vaccination status, the researchers found that individuals taking antipsychotics had lower odds of testing positive for SARS-CoV-2 and similar rates of filled anti-infective prescriptions as those not taking the drugs.
Although antipsychotic use was not linked to higher rates of mild infection, it was linked to an increased risk for COVID-19 hospitalization in individuals older than 70 years, as well as hospitalization and death from other respiratory infections, mainly pneumonia, in those older than 40 years.
Notably, there was no excess risk for any outcome with clozapine vs other antipsychotics.
Strong Link to Pneumonia Risk
Results from a longitudinal Finnish study, just published in The American Journal of Psychiatry, also show an increased risk for severe outcomes from ileus and pneumonia among more than 2600 patients with schizophrenia taking clozapine.
Twenty years after initiating clozapine, the cumulative incidence estimate for ileus was 5.3% — more than sixfold higher than previously reported. The incidence of pneumonia was also high, at 29.5%.
Both illnesses were significantly associated with mortality, with odds ratios of 4.5 and 2.8, respectively.
These findings align with previous pharmacovigilance studies, with reported mortality rates for gastrointestinal hypomotility and pneumonia that were 4-10 times higher than those for agranulocytosis, the researchers said.
The study “really adds to a growing body of research suggesting a connection between clozapine use and a higher risk of developing pneumonia,” Robert O. Cotes, MD, a professor of psychiatry and behavioral sciences at Emory University, Atlanta, who specializes in the use of clozapine, told this news organization.
“Additionally, when people on clozapine do contract pneumonia, there’s concern the condition may be more dangerous,” he added.
A Closer Look at Neutropenia Risk
Neutropenia receives the lion’s share of attention among clozapine’s potential side effects, but this focus may need to be re-evaluated, Dr. Cotes said.
He pointed out that recent data suggest the risk for severe neutropenia, 2-3 years after initiating clozapine, is comparable to that of other antipsychotics.
A study of 26,630 clozapine users in Australia and New Zealand showed that most cases of severe neutropenia leading to clozapine cessation peaked within 18 weeks and was negligible after 2 years. This suggests weekly hematologic monitoring could potentially be discontinued after the 2-year mark.
Another study reported earlier this year by this news organization showed a low risk for mild or moderate neutropenia and no severe cases in nearly 1000 people taking clozapine.
“I worry that we may be missing the forest for the trees by hyperfocusing on neutropenia and not considering clozapine’s other potential serious side effects like pneumonia, myocarditis, and gastrointestinal hypermotility,” Dr. Cotes said.
Importance of Vaccines
The findings of these studies highlight the importance of vaccines in this at-risk group, said Dr. Taquet, a point emphasized by investigators of the Danish study he reviewed.
“Inspired by the experience of COVID-19 vaccine prioritization in severe mental illness and based on our findings, there is momentum for preventive action,” the authors wrote. “Our findings do not suggest the avoidance of specific antipsychotics but rather a call for increased vigilance regarding this at-risk group.”
This includes recommending pneumococcal, influenza, COVID-19, and other anti-infective vaccines in those older than 40 years treated with, or due to start, an antipsychotic.
“It’s not mandatory, but we do recommend that patients on clozapine get the regular vaccines,” Dr. Taquet said.
Pointing to the recent study on pneumonia risk, Dr. Cotes said addressing underlying risk factors, such as smoking, obesity, and possibly sedation and excessive salivation caused by clozapine, is key.
“And to make sure that vaccinations are up to date, particularly heading into this fall,” he added.
Rethinking Clozapine REMS
One of the most challenging issues facing clinicians and researchers is how to help people understand the safety profile of clozapine and to use it with more confidence, Dr. Cotes said.
“A lot of people hear about clozapine and they think about neutropenia, they think about side effects, the REMS system, and all of these factors really drive down clozapine utilization,” he said.
Treatment-resistant schizophrenia affects about a quarter of those with schizophrenia, yet only 4% of these patients receive clozapine in the United States, Dr. Cotes said. That number may be even lower for its other indication of reducing suicidal behavior in patients with schizophrenia or schizoaffective disorder.
The clozapine REMS is viewed as a major barrier to utilization and requires certification of pharmacists and physicians and use of a central system to monitor absolute neutrophil counts for neutropenia in patients.
As previously reported by this news organization in November 2022, the FDA opted to temporarily exercise enforcement discretion for certain aspects of the drug safety program to ensure continuity of care for patients after concerns were raised by the American Psychiatric Association (APA) along with other professional organizations.
Even with that temporary enforcement discretion, “reports have shown that over half of those prescribed clozapine have trouble accessing the medication because of the REMS program,” a spokesperson for the APA told this news organization.
“Not only are patients having trouble accessing the medication, many have trouble finding a prescriber in their geographic locations and others because of the monitoring requirements have their treatment discontinued leading to negative outcomes,” the spokesperson said.
The FDA is currently reviewing the clozapine REMS and is holding a joint advisory committee meeting on November 19 to discuss the review and “possible changes to minimize burden on patients, pharmacies, and prescribers while maintaining safe use of clozapine.”
The APA plans to submit written and oral comments to the advisory committees.
“We are hopeful that the re-evaluation meeting in November will remove barriers and increase access to clozapine, which is currently highly underutilized, especially in marginalized communities,” the spokesperson said.
Dr. Cotes reported serving as a speaker and consultant for Saladax Biomedical and as a consultant for Syneos Health. Dr. Taquet reported having no competing interests.
A version of this article first appeared on Medscape.com.
Anticipated Effects of Pneumococcal Vaccines on Otitis
Acute otitis media (AOM) is caused by Streptococcus pneumoniae, Haemophilus influenzae, and Moraxella catarrhalis. Since the introduction of pneumococcal conjugate vaccines (PCVs) shifts in the proportion of these three bacteria as causes of AOM and their antibiotic susceptibility profiles and strain diversity have occurred due to multiple factors including the PCVs and antibiotic selection pressure.
The 7-valent PCV (PCV7) was introduced in 2000 and was proven to be efficacious in preventing AOM, but no subsequent PCV has received an indication for prevention of AOM because the FDA required a tympanocentesis study to prove efficacy and that approval was not achieved for PCV13, PCV15, or PCV20. This is a little known fact. After introduction of PCV7, replacement pneumococcal strains expressing serotypes not in PCV7 emerged and antibiotic non-susceptible strains became predominant causes of AOM, especially antibiotic-resistant serotype 19A. To address the phenomena of pneumococcal serotype replacement, PCV13 was introduced in 2010. But serotype replacement continued to occur under PCV13 pressure, replacement serotypes increasingly caused AOM, and antibiotic-resistant serotype 35B emerged. Now we have two new higher valency PCVs: PCV15 (Merck) where serotypes 22F and 33F were added to the PCV13 serotypes and PCV20 (Pfizer) where 22F, 33F, 8, 10A, 11A, 12F, 15B were added to PCV13. Note that neither PCV15 nor PCV20 includes the most common serotype causing AOM – serotype 35B.1
While PCV15 and PCV20 should provide protection against more pneumococcal serotypes, increasing serotypes in both vaccines decreased immunogenicity of certain shared serotypes, more so with the addition of seven more in PCV20 than two more in PCV15, compared with PCV13. Whether lower antibody concentrations will make a difference clinically in terms of vaccine failure to prevent nasopharyngeal colonization, AOM, and/or invasive pneumococcal infections is currently unknown.
Our group from greater Rochester, New York, is the only one in the United States performing tympanocentesis to determine the etiology of AOM infections. Children between ages 6 and 36 months are studied. We recently reported our results for the time span September 2021 to September 2023, the immediate 2 years prior to recommendations for use of PCV15 and PCV20 in young children.2 Tympanocentesis was performed in 139 (78%) of 179 episodes of AOM, yielding 216 middle ear fluid samples (the higher number of middle ear fluids was due to bilateral tympanocentesis in some children). H. influenzae (40%) was the most common bacterial isolate, followed by S. pneumonia (19%) and M. catarrhalis (17%), with the remainder no growth. Polymerase chain reactions (PCR) was positive in many of those culture negative samples, suggesting prior use of antibiotics before tympanocentesis was performed. Among the pneumococcal isolates, 46% were oxacillin non-susceptible. Among the H. influenzae isolates, 27% were beta-lactamase producing and all M. catarrhalis were beta-lactamase-producing.
As we previously reported,1 we once again found that serotype 35B was the most frequent non-PCV15, non-PCV20, serotype. Other frequently detected non-PCV20 pneumococcal serotypes were 23A, 23B, 35D, 35F and 15C.2
Projected Pneumococcal Serotype Coverage by PCV15 and PCV20
PCV13 serotypes were identified in 9% of middle ear fluids, consistent with vaccine failure. Assuming 100% vaccine-type effectiveness, PCV15 will provide about 11% coverage of pneumococci causing AOM, the same PCV13 and PCV20 will provide 30% coverage, leaving 70% of pneumococci causing AOM in young children uncovered (Figure).
Thus, the high proportion of pneumococcal serotype 35B and other non-PCV15 or non-PCV20 serotypes will result in a relatively small incremental benefit over PCV13 in young children for AOM.
AOM is the most common cause of pediatric outpatient visits and antibiotic prescriptions in the United States that contributes to selection of antibiotic-resistant microbes.3 The economic burden of AOM is high, estimated at about $3 billion annually in the United States, when direct and indirect costs are calculated,4 thereby making AOM a major factor in calculations of cost effectiveness analyses of PCV immunizations in children.
While PCV15 and PCV20 include common serotypes associated with invasive pneumococcal diseases, their effectiveness in preventing AOM, acute sinusitis, and non-bacteremic community-acquired pneumonia is currently unknown because these vaccines were licensed based on safety and immunogenicity data, not proven efficacy.
The data on antibiotic susceptibility of pneumococci and H. influenza and M. catarrhalis isolated in the late post PCV13 era from young children in a pediatric primary-care setting raise a question about empiric antibiotic choice for AOM today. For penicillin non-susceptible pneumococcal strains, higher dosages of amoxicillin can improve eradication. However, higher dosages of amoxicillin cannot overcome beta-lactamase production by H. influenza and M. catarrhalis. Based on the mix of pathogens causing AOM and the antibiotic susceptibility of those bacteria, high-dose amoxicillin/clavulanate or alternative cephalosporin drugs active against pneumococci and beta-lactamase producing H. influenza and M. catarrhalis would be a better empiric choice over high-dose amoxicillin.
Limitations of our study include that it occurred in one center in New York, although we have previously shown results of tympanocentesis at our center are similar to those in Virginia and Pennsylvania5 and our study population was composed of children living in urban, suburban, and rural households of all economic levels. Because this study was conducted during a relatively short time frame (2021-2023), the numbers of subjects and samples were sometimes insufficient to identify statistically significant differences in some comparisons. Some children were lost to follow-up, and not every participant was consented for tympanocentesis. Some participants received antibiotics prior to middle ear fluid specimen collection.
Dr. Pichichero is a specialist in pediatric infectious diseases, Center for Infectious Diseases and Immunology, and director of the Research Institute, at Rochester (N.Y.) General Hospital. He has no conflicts of interest to declare.
References
1. Kaur R et al. Dynamic Changes in Otopathogens Colonizing the Nasopharynx and Causing Acute Otitis Media in Children After 13-Valent (PCV13) Pneumococcal Conjugate Vaccination During 2015-2019. Eur J Clin Microbiol Infect Dis. 2022 Jan;41(1):37-44. doi: 10.1007/s10096-021-04324-0.
2. Kaur R et al. Anticipated Effects of Higher-valency Pneumococcal Conjugate Vaccines on Colonization and Acute Otitis Media. Pediatr Infect Dis J. 2024 Oct 1;43(10):1004-1010. doi: 10.1097/INF.0000000000004413.
3. King LM et al. Pediatric Outpatient Visits and Antibiotic Use Attributable to Higher Valency Pneumococcal Conjugate Vaccine Serotypes. medRxiv [Preprint]. 2023 Aug 25:2023.08.24.23294570. doi: 10.1101/2023.08.24.23294570.
4. Ahmed S et al. Incremental Health Care Utilization and Costs for Acute Otitis Media in Children. Laryngoscope. 2014 Jan;124(1):301-5. doi: 10.1002/lary.24190.
5. Pichichero ME et al. Pathogens Causing Recurrent and Difficult-to-Treat Acute Otitis Media, 2003-2006. Clin Pediatr (Phila). 2008 Nov;47(9):901-6. doi: 10.1177/0009922808319966.
Acute otitis media (AOM) is caused by Streptococcus pneumoniae, Haemophilus influenzae, and Moraxella catarrhalis. Since the introduction of pneumococcal conjugate vaccines (PCVs) shifts in the proportion of these three bacteria as causes of AOM and their antibiotic susceptibility profiles and strain diversity have occurred due to multiple factors including the PCVs and antibiotic selection pressure.
The 7-valent PCV (PCV7) was introduced in 2000 and was proven to be efficacious in preventing AOM, but no subsequent PCV has received an indication for prevention of AOM because the FDA required a tympanocentesis study to prove efficacy and that approval was not achieved for PCV13, PCV15, or PCV20. This is a little known fact. After introduction of PCV7, replacement pneumococcal strains expressing serotypes not in PCV7 emerged and antibiotic non-susceptible strains became predominant causes of AOM, especially antibiotic-resistant serotype 19A. To address the phenomena of pneumococcal serotype replacement, PCV13 was introduced in 2010. But serotype replacement continued to occur under PCV13 pressure, replacement serotypes increasingly caused AOM, and antibiotic-resistant serotype 35B emerged. Now we have two new higher valency PCVs: PCV15 (Merck) where serotypes 22F and 33F were added to the PCV13 serotypes and PCV20 (Pfizer) where 22F, 33F, 8, 10A, 11A, 12F, 15B were added to PCV13. Note that neither PCV15 nor PCV20 includes the most common serotype causing AOM – serotype 35B.1
While PCV15 and PCV20 should provide protection against more pneumococcal serotypes, increasing serotypes in both vaccines decreased immunogenicity of certain shared serotypes, more so with the addition of seven more in PCV20 than two more in PCV15, compared with PCV13. Whether lower antibody concentrations will make a difference clinically in terms of vaccine failure to prevent nasopharyngeal colonization, AOM, and/or invasive pneumococcal infections is currently unknown.
Our group from greater Rochester, New York, is the only one in the United States performing tympanocentesis to determine the etiology of AOM infections. Children between ages 6 and 36 months are studied. We recently reported our results for the time span September 2021 to September 2023, the immediate 2 years prior to recommendations for use of PCV15 and PCV20 in young children.2 Tympanocentesis was performed in 139 (78%) of 179 episodes of AOM, yielding 216 middle ear fluid samples (the higher number of middle ear fluids was due to bilateral tympanocentesis in some children). H. influenzae (40%) was the most common bacterial isolate, followed by S. pneumonia (19%) and M. catarrhalis (17%), with the remainder no growth. Polymerase chain reactions (PCR) was positive in many of those culture negative samples, suggesting prior use of antibiotics before tympanocentesis was performed. Among the pneumococcal isolates, 46% were oxacillin non-susceptible. Among the H. influenzae isolates, 27% were beta-lactamase producing and all M. catarrhalis were beta-lactamase-producing.
As we previously reported,1 we once again found that serotype 35B was the most frequent non-PCV15, non-PCV20, serotype. Other frequently detected non-PCV20 pneumococcal serotypes were 23A, 23B, 35D, 35F and 15C.2
Projected Pneumococcal Serotype Coverage by PCV15 and PCV20
PCV13 serotypes were identified in 9% of middle ear fluids, consistent with vaccine failure. Assuming 100% vaccine-type effectiveness, PCV15 will provide about 11% coverage of pneumococci causing AOM, the same PCV13 and PCV20 will provide 30% coverage, leaving 70% of pneumococci causing AOM in young children uncovered (Figure).

Thus, the high proportion of pneumococcal serotype 35B and other non-PCV15 or non-PCV20 serotypes will result in a relatively small incremental benefit over PCV13 in young children for AOM.
AOM is the most common cause of pediatric outpatient visits and antibiotic prescriptions in the United States that contributes to selection of antibiotic-resistant microbes.3 The economic burden of AOM is high, estimated at about $3 billion annually in the United States, when direct and indirect costs are calculated,4 thereby making AOM a major factor in calculations of cost effectiveness analyses of PCV immunizations in children.
While PCV15 and PCV20 include common serotypes associated with invasive pneumococcal diseases, their effectiveness in preventing AOM, acute sinusitis, and non-bacteremic community-acquired pneumonia is currently unknown because these vaccines were licensed based on safety and immunogenicity data, not proven efficacy.
The data on antibiotic susceptibility of pneumococci and H. influenza and M. catarrhalis isolated in the late post PCV13 era from young children in a pediatric primary-care setting raise a question about empiric antibiotic choice for AOM today. For penicillin non-susceptible pneumococcal strains, higher dosages of amoxicillin can improve eradication. However, higher dosages of amoxicillin cannot overcome beta-lactamase production by H. influenza and M. catarrhalis. Based on the mix of pathogens causing AOM and the antibiotic susceptibility of those bacteria, high-dose amoxicillin/clavulanate or alternative cephalosporin drugs active against pneumococci and beta-lactamase producing H. influenza and M. catarrhalis would be a better empiric choice over high-dose amoxicillin.
Limitations of our study include that it occurred in one center in New York, although we have previously shown results of tympanocentesis at our center are similar to those in Virginia and Pennsylvania5 and our study population was composed of children living in urban, suburban, and rural households of all economic levels. Because this study was conducted during a relatively short time frame (2021-2023), the numbers of subjects and samples were sometimes insufficient to identify statistically significant differences in some comparisons. Some children were lost to follow-up, and not every participant was consented for tympanocentesis. Some participants received antibiotics prior to middle ear fluid specimen collection.
Dr. Pichichero is a specialist in pediatric infectious diseases, Center for Infectious Diseases and Immunology, and director of the Research Institute, at Rochester (N.Y.) General Hospital. He has no conflicts of interest to declare.
References
1. Kaur R et al. Dynamic Changes in Otopathogens Colonizing the Nasopharynx and Causing Acute Otitis Media in Children After 13-Valent (PCV13) Pneumococcal Conjugate Vaccination During 2015-2019. Eur J Clin Microbiol Infect Dis. 2022 Jan;41(1):37-44. doi: 10.1007/s10096-021-04324-0.
2. Kaur R et al. Anticipated Effects of Higher-valency Pneumococcal Conjugate Vaccines on Colonization and Acute Otitis Media. Pediatr Infect Dis J. 2024 Oct 1;43(10):1004-1010. doi: 10.1097/INF.0000000000004413.
3. King LM et al. Pediatric Outpatient Visits and Antibiotic Use Attributable to Higher Valency Pneumococcal Conjugate Vaccine Serotypes. medRxiv [Preprint]. 2023 Aug 25:2023.08.24.23294570. doi: 10.1101/2023.08.24.23294570.
4. Ahmed S et al. Incremental Health Care Utilization and Costs for Acute Otitis Media in Children. Laryngoscope. 2014 Jan;124(1):301-5. doi: 10.1002/lary.24190.
5. Pichichero ME et al. Pathogens Causing Recurrent and Difficult-to-Treat Acute Otitis Media, 2003-2006. Clin Pediatr (Phila). 2008 Nov;47(9):901-6. doi: 10.1177/0009922808319966.
Acute otitis media (AOM) is caused by Streptococcus pneumoniae, Haemophilus influenzae, and Moraxella catarrhalis. Since the introduction of pneumococcal conjugate vaccines (PCVs) shifts in the proportion of these three bacteria as causes of AOM and their antibiotic susceptibility profiles and strain diversity have occurred due to multiple factors including the PCVs and antibiotic selection pressure.
The 7-valent PCV (PCV7) was introduced in 2000 and was proven to be efficacious in preventing AOM, but no subsequent PCV has received an indication for prevention of AOM because the FDA required a tympanocentesis study to prove efficacy and that approval was not achieved for PCV13, PCV15, or PCV20. This is a little known fact. After introduction of PCV7, replacement pneumococcal strains expressing serotypes not in PCV7 emerged and antibiotic non-susceptible strains became predominant causes of AOM, especially antibiotic-resistant serotype 19A. To address the phenomena of pneumococcal serotype replacement, PCV13 was introduced in 2010. But serotype replacement continued to occur under PCV13 pressure, replacement serotypes increasingly caused AOM, and antibiotic-resistant serotype 35B emerged. Now we have two new higher valency PCVs: PCV15 (Merck) where serotypes 22F and 33F were added to the PCV13 serotypes and PCV20 (Pfizer) where 22F, 33F, 8, 10A, 11A, 12F, 15B were added to PCV13. Note that neither PCV15 nor PCV20 includes the most common serotype causing AOM – serotype 35B.1
While PCV15 and PCV20 should provide protection against more pneumococcal serotypes, increasing serotypes in both vaccines decreased immunogenicity of certain shared serotypes, more so with the addition of seven more in PCV20 than two more in PCV15, compared with PCV13. Whether lower antibody concentrations will make a difference clinically in terms of vaccine failure to prevent nasopharyngeal colonization, AOM, and/or invasive pneumococcal infections is currently unknown.
Our group from greater Rochester, New York, is the only one in the United States performing tympanocentesis to determine the etiology of AOM infections. Children between ages 6 and 36 months are studied. We recently reported our results for the time span September 2021 to September 2023, the immediate 2 years prior to recommendations for use of PCV15 and PCV20 in young children.2 Tympanocentesis was performed in 139 (78%) of 179 episodes of AOM, yielding 216 middle ear fluid samples (the higher number of middle ear fluids was due to bilateral tympanocentesis in some children). H. influenzae (40%) was the most common bacterial isolate, followed by S. pneumonia (19%) and M. catarrhalis (17%), with the remainder no growth. Polymerase chain reactions (PCR) was positive in many of those culture negative samples, suggesting prior use of antibiotics before tympanocentesis was performed. Among the pneumococcal isolates, 46% were oxacillin non-susceptible. Among the H. influenzae isolates, 27% were beta-lactamase producing and all M. catarrhalis were beta-lactamase-producing.
As we previously reported,1 we once again found that serotype 35B was the most frequent non-PCV15, non-PCV20, serotype. Other frequently detected non-PCV20 pneumococcal serotypes were 23A, 23B, 35D, 35F and 15C.2
Projected Pneumococcal Serotype Coverage by PCV15 and PCV20
PCV13 serotypes were identified in 9% of middle ear fluids, consistent with vaccine failure. Assuming 100% vaccine-type effectiveness, PCV15 will provide about 11% coverage of pneumococci causing AOM, the same PCV13 and PCV20 will provide 30% coverage, leaving 70% of pneumococci causing AOM in young children uncovered (Figure).

Thus, the high proportion of pneumococcal serotype 35B and other non-PCV15 or non-PCV20 serotypes will result in a relatively small incremental benefit over PCV13 in young children for AOM.
AOM is the most common cause of pediatric outpatient visits and antibiotic prescriptions in the United States that contributes to selection of antibiotic-resistant microbes.3 The economic burden of AOM is high, estimated at about $3 billion annually in the United States, when direct and indirect costs are calculated,4 thereby making AOM a major factor in calculations of cost effectiveness analyses of PCV immunizations in children.
While PCV15 and PCV20 include common serotypes associated with invasive pneumococcal diseases, their effectiveness in preventing AOM, acute sinusitis, and non-bacteremic community-acquired pneumonia is currently unknown because these vaccines were licensed based on safety and immunogenicity data, not proven efficacy.
The data on antibiotic susceptibility of pneumococci and H. influenza and M. catarrhalis isolated in the late post PCV13 era from young children in a pediatric primary-care setting raise a question about empiric antibiotic choice for AOM today. For penicillin non-susceptible pneumococcal strains, higher dosages of amoxicillin can improve eradication. However, higher dosages of amoxicillin cannot overcome beta-lactamase production by H. influenza and M. catarrhalis. Based on the mix of pathogens causing AOM and the antibiotic susceptibility of those bacteria, high-dose amoxicillin/clavulanate or alternative cephalosporin drugs active against pneumococci and beta-lactamase producing H. influenza and M. catarrhalis would be a better empiric choice over high-dose amoxicillin.
Limitations of our study include that it occurred in one center in New York, although we have previously shown results of tympanocentesis at our center are similar to those in Virginia and Pennsylvania5 and our study population was composed of children living in urban, suburban, and rural households of all economic levels. Because this study was conducted during a relatively short time frame (2021-2023), the numbers of subjects and samples were sometimes insufficient to identify statistically significant differences in some comparisons. Some children were lost to follow-up, and not every participant was consented for tympanocentesis. Some participants received antibiotics prior to middle ear fluid specimen collection.
Dr. Pichichero is a specialist in pediatric infectious diseases, Center for Infectious Diseases and Immunology, and director of the Research Institute, at Rochester (N.Y.) General Hospital. He has no conflicts of interest to declare.
References
1. Kaur R et al. Dynamic Changes in Otopathogens Colonizing the Nasopharynx and Causing Acute Otitis Media in Children After 13-Valent (PCV13) Pneumococcal Conjugate Vaccination During 2015-2019. Eur J Clin Microbiol Infect Dis. 2022 Jan;41(1):37-44. doi: 10.1007/s10096-021-04324-0.
2. Kaur R et al. Anticipated Effects of Higher-valency Pneumococcal Conjugate Vaccines on Colonization and Acute Otitis Media. Pediatr Infect Dis J. 2024 Oct 1;43(10):1004-1010. doi: 10.1097/INF.0000000000004413.
3. King LM et al. Pediatric Outpatient Visits and Antibiotic Use Attributable to Higher Valency Pneumococcal Conjugate Vaccine Serotypes. medRxiv [Preprint]. 2023 Aug 25:2023.08.24.23294570. doi: 10.1101/2023.08.24.23294570.
4. Ahmed S et al. Incremental Health Care Utilization and Costs for Acute Otitis Media in Children. Laryngoscope. 2014 Jan;124(1):301-5. doi: 10.1002/lary.24190.
5. Pichichero ME et al. Pathogens Causing Recurrent and Difficult-to-Treat Acute Otitis Media, 2003-2006. Clin Pediatr (Phila). 2008 Nov;47(9):901-6. doi: 10.1177/0009922808319966.
Tirzepatide Shortage Resolved? FDA Says Yes, Compounders No
The agency wrote in a clarification aimed at compounders that Lilly said it can meet the “present and projected national demand” and that compounders are restricted from making the products.
Nevertheless, patients and prescribers may still see “intermittent localized supply disruptions as the products move through the supply chain,” the FDA noted.
The Alliance for Pharmacy Compounding (APC) responded swiftly, alerting its members and the public to the resolved shortage and stating that compounders “must immediately cease preparing and dispensing compounded copies” of the two drugs.
However, APC CEO Scott Brunner added it often takes a long time for FDA-approved versions of the drug to become widely available to wholesalers, hospitals, and clinics. Even after Lilly announced greater availability for the drugs, including in a new vial format for low doses, “for most pharmacies, they’re lucky to get two or three boxes of Zepbound a day from their wholesaler — for a patient waiting list that can number in the hundreds.”
“We have already heard this morning from APC members that they are unable to fill orders for their patients,” he said.
Furthermore, he contended, “I suspect plenty of patients taking compounded tirzepatide are going to be caught flat-footed by this. They are being cut off cold turkey, their prescription no longer fillable. They’ll need to get in to see their provider to get a new prescription, and that will take some time. It’s possible that so many patients presently taking compounded GLP-1s [glucagon-like peptide 1] will be eventually switched to the FDA-approved versions — if they can afford them, of course — that it will push tirzepatide injection back into shortage.”
Commenting on the shortage resolution, endocrinologist Beverly Tchang, MD, DABOM, an assistant professor of clinical medicine at Weill Cornell Medicine in New York City told this news organization, “we are not yet experiencing relief from the shortages, but I hope this resolves at least one barrier to access for our patients.”
“I don’t think it will create confusion,” she said. “Fortunately or unfortunately, patients and clinicians are adept by now with therapeutic transitions because we’ve been forced to do so whenever insurance withdraws coverage or a shortage recurs or a coupon expires. It’s obviously not ideal but patients are motivated and clinicians don’t give up.”
This news organization has previously reported on the impact of the shortages and how endocrinologists and obesity medicine specialists were handling them, in light of concerns about compounding pharmacies that may or may not be well founded.
Dr. Tchang declared that she is an adviser to Novo Nordisk.
A version of this article appeared on Medscape.com.
The agency wrote in a clarification aimed at compounders that Lilly said it can meet the “present and projected national demand” and that compounders are restricted from making the products.
Nevertheless, patients and prescribers may still see “intermittent localized supply disruptions as the products move through the supply chain,” the FDA noted.
The Alliance for Pharmacy Compounding (APC) responded swiftly, alerting its members and the public to the resolved shortage and stating that compounders “must immediately cease preparing and dispensing compounded copies” of the two drugs.
However, APC CEO Scott Brunner added it often takes a long time for FDA-approved versions of the drug to become widely available to wholesalers, hospitals, and clinics. Even after Lilly announced greater availability for the drugs, including in a new vial format for low doses, “for most pharmacies, they’re lucky to get two or three boxes of Zepbound a day from their wholesaler — for a patient waiting list that can number in the hundreds.”
“We have already heard this morning from APC members that they are unable to fill orders for their patients,” he said.
Furthermore, he contended, “I suspect plenty of patients taking compounded tirzepatide are going to be caught flat-footed by this. They are being cut off cold turkey, their prescription no longer fillable. They’ll need to get in to see their provider to get a new prescription, and that will take some time. It’s possible that so many patients presently taking compounded GLP-1s [glucagon-like peptide 1] will be eventually switched to the FDA-approved versions — if they can afford them, of course — that it will push tirzepatide injection back into shortage.”
Commenting on the shortage resolution, endocrinologist Beverly Tchang, MD, DABOM, an assistant professor of clinical medicine at Weill Cornell Medicine in New York City told this news organization, “we are not yet experiencing relief from the shortages, but I hope this resolves at least one barrier to access for our patients.”
“I don’t think it will create confusion,” she said. “Fortunately or unfortunately, patients and clinicians are adept by now with therapeutic transitions because we’ve been forced to do so whenever insurance withdraws coverage or a shortage recurs or a coupon expires. It’s obviously not ideal but patients are motivated and clinicians don’t give up.”
This news organization has previously reported on the impact of the shortages and how endocrinologists and obesity medicine specialists were handling them, in light of concerns about compounding pharmacies that may or may not be well founded.
Dr. Tchang declared that she is an adviser to Novo Nordisk.
A version of this article appeared on Medscape.com.
The agency wrote in a clarification aimed at compounders that Lilly said it can meet the “present and projected national demand” and that compounders are restricted from making the products.
Nevertheless, patients and prescribers may still see “intermittent localized supply disruptions as the products move through the supply chain,” the FDA noted.
The Alliance for Pharmacy Compounding (APC) responded swiftly, alerting its members and the public to the resolved shortage and stating that compounders “must immediately cease preparing and dispensing compounded copies” of the two drugs.
However, APC CEO Scott Brunner added it often takes a long time for FDA-approved versions of the drug to become widely available to wholesalers, hospitals, and clinics. Even after Lilly announced greater availability for the drugs, including in a new vial format for low doses, “for most pharmacies, they’re lucky to get two or three boxes of Zepbound a day from their wholesaler — for a patient waiting list that can number in the hundreds.”
“We have already heard this morning from APC members that they are unable to fill orders for their patients,” he said.
Furthermore, he contended, “I suspect plenty of patients taking compounded tirzepatide are going to be caught flat-footed by this. They are being cut off cold turkey, their prescription no longer fillable. They’ll need to get in to see their provider to get a new prescription, and that will take some time. It’s possible that so many patients presently taking compounded GLP-1s [glucagon-like peptide 1] will be eventually switched to the FDA-approved versions — if they can afford them, of course — that it will push tirzepatide injection back into shortage.”
Commenting on the shortage resolution, endocrinologist Beverly Tchang, MD, DABOM, an assistant professor of clinical medicine at Weill Cornell Medicine in New York City told this news organization, “we are not yet experiencing relief from the shortages, but I hope this resolves at least one barrier to access for our patients.”
“I don’t think it will create confusion,” she said. “Fortunately or unfortunately, patients and clinicians are adept by now with therapeutic transitions because we’ve been forced to do so whenever insurance withdraws coverage or a shortage recurs or a coupon expires. It’s obviously not ideal but patients are motivated and clinicians don’t give up.”
This news organization has previously reported on the impact of the shortages and how endocrinologists and obesity medicine specialists were handling them, in light of concerns about compounding pharmacies that may or may not be well founded.
Dr. Tchang declared that she is an adviser to Novo Nordisk.
A version of this article appeared on Medscape.com.
Weight Loss After Anti-Obesity Medications Linked to Reduced Gout Risk
TOPLINE:
A higher rate of weight loss within 1 year of initiating orlistat is associated with lower risks for incident gout and recurrent gout flares in individuals with body mass index (BMI) > 25, particularly if they have obesity or high baseline serum urate levels.
METHODOLOGY:
- Researchers conducted a population-based cohort study using data from The Health Improvement Network in the United Kingdom to examine the association between weight loss rates after the initiation of anti-obesity medication (orlistat) and the risk for incident gout and recurrent gout flares in patients with overweight or obesity.
- The risk for incident gout was analyzed in 131,000 patients with overweight or obesity (mean age, 45 years; 77.3% women; mean BMI, 37.2) who did not have gout before initiating orlistat.
- The risk for recurrent gout flares was evaluated in 3847 individuals with overweight or obesity (mean age, 56.6 years; 29.4% women; mean BMI, 38.5), who had gout before initiating orlistat.
- Participants were divided into four groups based on their rate of weight loss during the first year of orlistat use: Weight gain or stable (< 2%), slow (2% to < 5%), moderate (5% to < 10%), and fast (≥ 10%).
- The primary outcome was incident gout, and the secondary outcome was the rate of recurrent gout flares during the 5-year follow-up period after initiating orlistat.
TAKEAWAY:
- The 5-year risk for incident gout was the lowest among patients in the fast weight loss group (1.2%) and highest among those in the weight gain or stable weight group (1.6%).
- The risk for incident gout was lower in the fast (hazard ratio [HR], 0.73; 95% CI, 0.62-0.86) and moderate (HR, 0.82; 95% CI, 0.72-0.92) weight loss groups than in the weight gain or stable weight group.
- Similarly, faster weight loss rates were linked to lower rates of recurrent gout flares, with risk ratios of 0.71 (95% CI, 0.60-0.84) and 0.83 (95% CI, 0.71-0.96) in the fast and moderate weight loss groups, respectively.
- This study found that .
IN PRACTICE:
“Pharmacologic treatments, such as orlistat, present an alternative strategy for managing overweight and obesity. Our study provides empirical evidence of a dose-response effect of weight loss after initiating orlistat within 1 year lowers the risk of incident gout and recurrent gout flares,” the authors wrote.
SOURCE:
This study was led by Jie Wei, PhD, Department of Orthopaedics, Xiangya Hospital, Central South University, Changsha, China, and was published online on September 19, 2024, in Arthritis & Rheumatology.
LIMITATIONS:
Despite adjustment for many variables, factors such as disease severity, exercise levels, and diet were not fully captured, which might have influenced the results. The lack of hospitalization data could have resulted in recurrent gout flares being underreported. The current study may have been subjected to bias due to potential exposure misclassification resulting from the timing of weight measurements and missing updated weight data.
DISCLOSURES:
This study was supported by the National Key Research and Development Plan, the National Natural Science Foundation of China, the Project Program of National Clinical Research Center for Geriatric Disorders, and other sources. No disclosures of interest were reported by the authors.
This article was created using several editorial tools, including AI, as part of the process. Human editors reviewed this content before publication. A version of this article first appeared on Medscape.com.
TOPLINE:
A higher rate of weight loss within 1 year of initiating orlistat is associated with lower risks for incident gout and recurrent gout flares in individuals with body mass index (BMI) > 25, particularly if they have obesity or high baseline serum urate levels.
METHODOLOGY:
- Researchers conducted a population-based cohort study using data from The Health Improvement Network in the United Kingdom to examine the association between weight loss rates after the initiation of anti-obesity medication (orlistat) and the risk for incident gout and recurrent gout flares in patients with overweight or obesity.
- The risk for incident gout was analyzed in 131,000 patients with overweight or obesity (mean age, 45 years; 77.3% women; mean BMI, 37.2) who did not have gout before initiating orlistat.
- The risk for recurrent gout flares was evaluated in 3847 individuals with overweight or obesity (mean age, 56.6 years; 29.4% women; mean BMI, 38.5), who had gout before initiating orlistat.
- Participants were divided into four groups based on their rate of weight loss during the first year of orlistat use: Weight gain or stable (< 2%), slow (2% to < 5%), moderate (5% to < 10%), and fast (≥ 10%).
- The primary outcome was incident gout, and the secondary outcome was the rate of recurrent gout flares during the 5-year follow-up period after initiating orlistat.
TAKEAWAY:
- The 5-year risk for incident gout was the lowest among patients in the fast weight loss group (1.2%) and highest among those in the weight gain or stable weight group (1.6%).
- The risk for incident gout was lower in the fast (hazard ratio [HR], 0.73; 95% CI, 0.62-0.86) and moderate (HR, 0.82; 95% CI, 0.72-0.92) weight loss groups than in the weight gain or stable weight group.
- Similarly, faster weight loss rates were linked to lower rates of recurrent gout flares, with risk ratios of 0.71 (95% CI, 0.60-0.84) and 0.83 (95% CI, 0.71-0.96) in the fast and moderate weight loss groups, respectively.
- This study found that .
IN PRACTICE:
“Pharmacologic treatments, such as orlistat, present an alternative strategy for managing overweight and obesity. Our study provides empirical evidence of a dose-response effect of weight loss after initiating orlistat within 1 year lowers the risk of incident gout and recurrent gout flares,” the authors wrote.
SOURCE:
This study was led by Jie Wei, PhD, Department of Orthopaedics, Xiangya Hospital, Central South University, Changsha, China, and was published online on September 19, 2024, in Arthritis & Rheumatology.
LIMITATIONS:
Despite adjustment for many variables, factors such as disease severity, exercise levels, and diet were not fully captured, which might have influenced the results. The lack of hospitalization data could have resulted in recurrent gout flares being underreported. The current study may have been subjected to bias due to potential exposure misclassification resulting from the timing of weight measurements and missing updated weight data.
DISCLOSURES:
This study was supported by the National Key Research and Development Plan, the National Natural Science Foundation of China, the Project Program of National Clinical Research Center for Geriatric Disorders, and other sources. No disclosures of interest were reported by the authors.
This article was created using several editorial tools, including AI, as part of the process. Human editors reviewed this content before publication. A version of this article first appeared on Medscape.com.
TOPLINE:
A higher rate of weight loss within 1 year of initiating orlistat is associated with lower risks for incident gout and recurrent gout flares in individuals with body mass index (BMI) > 25, particularly if they have obesity or high baseline serum urate levels.
METHODOLOGY:
- Researchers conducted a population-based cohort study using data from The Health Improvement Network in the United Kingdom to examine the association between weight loss rates after the initiation of anti-obesity medication (orlistat) and the risk for incident gout and recurrent gout flares in patients with overweight or obesity.
- The risk for incident gout was analyzed in 131,000 patients with overweight or obesity (mean age, 45 years; 77.3% women; mean BMI, 37.2) who did not have gout before initiating orlistat.
- The risk for recurrent gout flares was evaluated in 3847 individuals with overweight or obesity (mean age, 56.6 years; 29.4% women; mean BMI, 38.5), who had gout before initiating orlistat.
- Participants were divided into four groups based on their rate of weight loss during the first year of orlistat use: Weight gain or stable (< 2%), slow (2% to < 5%), moderate (5% to < 10%), and fast (≥ 10%).
- The primary outcome was incident gout, and the secondary outcome was the rate of recurrent gout flares during the 5-year follow-up period after initiating orlistat.
TAKEAWAY:
- The 5-year risk for incident gout was the lowest among patients in the fast weight loss group (1.2%) and highest among those in the weight gain or stable weight group (1.6%).
- The risk for incident gout was lower in the fast (hazard ratio [HR], 0.73; 95% CI, 0.62-0.86) and moderate (HR, 0.82; 95% CI, 0.72-0.92) weight loss groups than in the weight gain or stable weight group.
- Similarly, faster weight loss rates were linked to lower rates of recurrent gout flares, with risk ratios of 0.71 (95% CI, 0.60-0.84) and 0.83 (95% CI, 0.71-0.96) in the fast and moderate weight loss groups, respectively.
- This study found that .
IN PRACTICE:
“Pharmacologic treatments, such as orlistat, present an alternative strategy for managing overweight and obesity. Our study provides empirical evidence of a dose-response effect of weight loss after initiating orlistat within 1 year lowers the risk of incident gout and recurrent gout flares,” the authors wrote.
SOURCE:
This study was led by Jie Wei, PhD, Department of Orthopaedics, Xiangya Hospital, Central South University, Changsha, China, and was published online on September 19, 2024, in Arthritis & Rheumatology.
LIMITATIONS:
Despite adjustment for many variables, factors such as disease severity, exercise levels, and diet were not fully captured, which might have influenced the results. The lack of hospitalization data could have resulted in recurrent gout flares being underreported. The current study may have been subjected to bias due to potential exposure misclassification resulting from the timing of weight measurements and missing updated weight data.
DISCLOSURES:
This study was supported by the National Key Research and Development Plan, the National Natural Science Foundation of China, the Project Program of National Clinical Research Center for Geriatric Disorders, and other sources. No disclosures of interest were reported by the authors.
This article was created using several editorial tools, including AI, as part of the process. Human editors reviewed this content before publication. A version of this article first appeared on Medscape.com.
Surgical Center Wins $421 Million Verdict Against Blue Cross
Insurance specialists told this news organization that the September 20 verdict is unusual. If upheld on appeal, one said, it could give out-of-network providers more power to decide how much insurers must pay them.
The case, which the St. Charles Surgical Hospital and Center for Restorative Breast Surgery first filed in 2017 in Louisiana state court, will be appealed and could ultimately land in federal court. The center has seen mixed results from a similar case it filed in federal court, legal documents show. Physicians from the center declined comment.
At issue: Did Blue Cross fail to fully pay the surgery center for about 7000 out-of-network procedures that it authorized?
The lawsuit claimed that the insurer’s online system confirmed that claims would be paid and noted the percentage of patient bills that would be reimbursed.
However, “Blue Cross and Blue Shield of Louisiana either slow-paid, low-paid, or no-paid all their bills over an eight-year period, hoping to pressure the doctors and hospital to either come into the network or fail and close down,” the surgery center’s attorney, James Williams, said in a statement.
Blue Cross denied that it acted fraudulently, “arguing that because the hospital is not a member of its provider network, it had no contractual obligation to pay anything,” the Times-Picayune newspaper reported. Authorization of a procedure doesn’t guarantee payment, the insurer argued in court.
In a statement to the media, Blue Cross said it disagrees with the verdict and will appeal.
Out-of-Network Free For All
Paul B. Ginsburg, PhD, professor of the Practice of Health Policy at the Price School of Public Policy, University of Southern California, Los Angeles, said out-of-network care doesn’t come with a contractual relationship.
Without a contract, he said, “providers can charge whatever they want, and the insurers will pay them whatever they want, and then it’s up to the provider to see how much additional balance bill they can collect from the patients.” (Some states and the federal government have laws partly protecting patients from balance billing when doctors and insurers conflict over payment.)
He added that “if insurance companies were on the hook to pay whatever any provider charges, nobody would ever belong to a network, and rates would be sky high. Many fewer people would buy insurance. Providers would [then] charge as much as they think they can get from the patients.”
What about the insurer’s apparent authorization of the out-of-network procedures? “They’re authorizing them because they believe the procedures are medically warranted,” Dr. Ginsburg said. “That’s totally separate from how much they’ll pay.”
Dr. Ginsburg added that juries in the South are known for imposing high penalties against companies. “They often come up with crazy verdicts.”
Mark V. Pauly, PhD, MA, professor emeritus of health care management at the Wharton School of the University of Pennsylvania, Philadelphia, questioned why the clinic kept accepting Blue Cross patients.
“Once it became apparent that Blue Cross wasn’t going to pay them well or would give them a lot of grief,” Dr. Pauly said, “the simplest thing would have been to tell patients that we’re going to go back to the old-fashioned way of doing things: You pay us up front, or assure us that you’re going to pay.”
Lawton Robert Burns, PhD, MBA, professor of health care management at the Wharton School, said the case and the verdict are unusual. He noted that insurer contracts with employers often state that out-of-network care will be covered at a specific rate, such as 70% of “reasonable charges.”
A 2020 analysis found that initial breast reconstruction surgeries in the United States cost a median of $24,600-$38,000 from 2009 to 2016. According to the Times-Picayune, the New Orleans clinic billed Blue Cross for $506.7 million, averaging more than $72,385 per procedure.
Dr. Ginsburg, Dr. Pauly, and Dr. Burns had no disclosures.
A version of this article first appeared on Medscape.com.
Insurance specialists told this news organization that the September 20 verdict is unusual. If upheld on appeal, one said, it could give out-of-network providers more power to decide how much insurers must pay them.
The case, which the St. Charles Surgical Hospital and Center for Restorative Breast Surgery first filed in 2017 in Louisiana state court, will be appealed and could ultimately land in federal court. The center has seen mixed results from a similar case it filed in federal court, legal documents show. Physicians from the center declined comment.
At issue: Did Blue Cross fail to fully pay the surgery center for about 7000 out-of-network procedures that it authorized?
The lawsuit claimed that the insurer’s online system confirmed that claims would be paid and noted the percentage of patient bills that would be reimbursed.
However, “Blue Cross and Blue Shield of Louisiana either slow-paid, low-paid, or no-paid all their bills over an eight-year period, hoping to pressure the doctors and hospital to either come into the network or fail and close down,” the surgery center’s attorney, James Williams, said in a statement.
Blue Cross denied that it acted fraudulently, “arguing that because the hospital is not a member of its provider network, it had no contractual obligation to pay anything,” the Times-Picayune newspaper reported. Authorization of a procedure doesn’t guarantee payment, the insurer argued in court.
In a statement to the media, Blue Cross said it disagrees with the verdict and will appeal.
Out-of-Network Free For All
Paul B. Ginsburg, PhD, professor of the Practice of Health Policy at the Price School of Public Policy, University of Southern California, Los Angeles, said out-of-network care doesn’t come with a contractual relationship.
Without a contract, he said, “providers can charge whatever they want, and the insurers will pay them whatever they want, and then it’s up to the provider to see how much additional balance bill they can collect from the patients.” (Some states and the federal government have laws partly protecting patients from balance billing when doctors and insurers conflict over payment.)
He added that “if insurance companies were on the hook to pay whatever any provider charges, nobody would ever belong to a network, and rates would be sky high. Many fewer people would buy insurance. Providers would [then] charge as much as they think they can get from the patients.”
What about the insurer’s apparent authorization of the out-of-network procedures? “They’re authorizing them because they believe the procedures are medically warranted,” Dr. Ginsburg said. “That’s totally separate from how much they’ll pay.”
Dr. Ginsburg added that juries in the South are known for imposing high penalties against companies. “They often come up with crazy verdicts.”
Mark V. Pauly, PhD, MA, professor emeritus of health care management at the Wharton School of the University of Pennsylvania, Philadelphia, questioned why the clinic kept accepting Blue Cross patients.
“Once it became apparent that Blue Cross wasn’t going to pay them well or would give them a lot of grief,” Dr. Pauly said, “the simplest thing would have been to tell patients that we’re going to go back to the old-fashioned way of doing things: You pay us up front, or assure us that you’re going to pay.”
Lawton Robert Burns, PhD, MBA, professor of health care management at the Wharton School, said the case and the verdict are unusual. He noted that insurer contracts with employers often state that out-of-network care will be covered at a specific rate, such as 70% of “reasonable charges.”
A 2020 analysis found that initial breast reconstruction surgeries in the United States cost a median of $24,600-$38,000 from 2009 to 2016. According to the Times-Picayune, the New Orleans clinic billed Blue Cross for $506.7 million, averaging more than $72,385 per procedure.
Dr. Ginsburg, Dr. Pauly, and Dr. Burns had no disclosures.
A version of this article first appeared on Medscape.com.
Insurance specialists told this news organization that the September 20 verdict is unusual. If upheld on appeal, one said, it could give out-of-network providers more power to decide how much insurers must pay them.
The case, which the St. Charles Surgical Hospital and Center for Restorative Breast Surgery first filed in 2017 in Louisiana state court, will be appealed and could ultimately land in federal court. The center has seen mixed results from a similar case it filed in federal court, legal documents show. Physicians from the center declined comment.
At issue: Did Blue Cross fail to fully pay the surgery center for about 7000 out-of-network procedures that it authorized?
The lawsuit claimed that the insurer’s online system confirmed that claims would be paid and noted the percentage of patient bills that would be reimbursed.
However, “Blue Cross and Blue Shield of Louisiana either slow-paid, low-paid, or no-paid all their bills over an eight-year period, hoping to pressure the doctors and hospital to either come into the network or fail and close down,” the surgery center’s attorney, James Williams, said in a statement.
Blue Cross denied that it acted fraudulently, “arguing that because the hospital is not a member of its provider network, it had no contractual obligation to pay anything,” the Times-Picayune newspaper reported. Authorization of a procedure doesn’t guarantee payment, the insurer argued in court.
In a statement to the media, Blue Cross said it disagrees with the verdict and will appeal.
Out-of-Network Free For All
Paul B. Ginsburg, PhD, professor of the Practice of Health Policy at the Price School of Public Policy, University of Southern California, Los Angeles, said out-of-network care doesn’t come with a contractual relationship.
Without a contract, he said, “providers can charge whatever they want, and the insurers will pay them whatever they want, and then it’s up to the provider to see how much additional balance bill they can collect from the patients.” (Some states and the federal government have laws partly protecting patients from balance billing when doctors and insurers conflict over payment.)
He added that “if insurance companies were on the hook to pay whatever any provider charges, nobody would ever belong to a network, and rates would be sky high. Many fewer people would buy insurance. Providers would [then] charge as much as they think they can get from the patients.”
What about the insurer’s apparent authorization of the out-of-network procedures? “They’re authorizing them because they believe the procedures are medically warranted,” Dr. Ginsburg said. “That’s totally separate from how much they’ll pay.”
Dr. Ginsburg added that juries in the South are known for imposing high penalties against companies. “They often come up with crazy verdicts.”
Mark V. Pauly, PhD, MA, professor emeritus of health care management at the Wharton School of the University of Pennsylvania, Philadelphia, questioned why the clinic kept accepting Blue Cross patients.
“Once it became apparent that Blue Cross wasn’t going to pay them well or would give them a lot of grief,” Dr. Pauly said, “the simplest thing would have been to tell patients that we’re going to go back to the old-fashioned way of doing things: You pay us up front, or assure us that you’re going to pay.”
Lawton Robert Burns, PhD, MBA, professor of health care management at the Wharton School, said the case and the verdict are unusual. He noted that insurer contracts with employers often state that out-of-network care will be covered at a specific rate, such as 70% of “reasonable charges.”
A 2020 analysis found that initial breast reconstruction surgeries in the United States cost a median of $24,600-$38,000 from 2009 to 2016. According to the Times-Picayune, the New Orleans clinic billed Blue Cross for $506.7 million, averaging more than $72,385 per procedure.
Dr. Ginsburg, Dr. Pauly, and Dr. Burns had no disclosures.
A version of this article first appeared on Medscape.com.
Lifestyle Medicine: Not Just for the Wealthy
Primary care clinicians understand that addressing lifestyle-related chronic disease health disparities in minority and lower-income communities is a significant opportunity to alleviate unnecessary suffering. Disparate health outcomes associated with underlying comorbidities during the COVID pandemic exposed the urgency of this problem.
When it comes to delivering evidence-based therapeutic lifestyle behavior interventions to these populations, however, there is a misconception that lifestyle medicine is only for the wealthy. Such a misconception needlessly widens the gap in health disparities because the truth is that everyone deserves access to lifestyle medicine. Fortunately, there are numerous successful examples of delivering these services to underresourced patients. We can all contribute to narrowing health inequities by sourcing increasingly abundant lifestyle medicine resources.
All patients’ lived experiences are unique, and there is a wide range of potential challenges to achieving lifestyle behavior change. Ignoring these obstacles is a disservice to patients and almost certainly results in treatment failure. Requirements to document SDOH have been a tremendous initial step.
The next step is to have conversations with every patient about the powerful outcomes of even small lifestyle changes. All too often, clinicians forgo conversations on lifestyle change with patients affected by adverse SDOH and assume that social obstacles automatically mean that patients are neither willing nor able to attempt behavior modification. Instead, it is an opportunity for clinicians, particularly those certified in lifestyle medicine, to meet patients where they are, work with them to identify solutions, and provide referrals to community-based organizations with resources to help.
Small Steps to Big Changes
Not all lifestyle behavior interventions need to be programmatic or time intensive. Clinicians can guide patients toward simple but specific actions that can make a difference in health outcomes over time. Small steps, like eating one can of beans or two bags of frozen leafy greens each week, are a good start toward adjusted eating patterns. The American College of Lifestyle Medicine offers a whole-food, plant-predominant meal guide to share with patients.
Individuals can increase their physical activity in their living rooms by doing sit-to-stands or balancing on one leg. Deep breathing and establishing a sleep routine are other lifestyle behavior changes without a price tag.
It is true that early adopters of lifestyle medicine often had difficulty practicing in underresourced communities. Those practitioners were forced to operate on a cash-pay basis, making access to care cost-prohibitive for many patients. However, board certification has been available since 2017, and lifestyle medicine is being integrated into medical schools and residency programs. Many such board-certified clinicians now work in large health systems and bill under the usual methods. There are also frameworks, such as the community-engaged lifestyle medicine model, showing how to treat patients affected by adverse SDOH effectively.
For example, patients at risk for malnutrition because of illnesses like chronic kidney disease, cancer, and heart failure receive medically tailored meals and access to a registered dietitian through a partnership between UC San Diego Health and Mama’s Kitchen. In Pennsylvania’s Lehigh Valley, where 1 in 10 of the approximately 700,000 residents face food insecurity, the Kellyn Foundation delivers fresh food through the Eat Real Food Mobile Market and offers whole-food, plant-predominant cooking classes, interactive elementary school programs focused on healthy lifestyle choices, and therapeutic lifestyle-change programs in community locations. Three months after launching new mobile market sites in Allentown, 1200 households were utilizing $15 weekly food vouchers through the program. Lifestyle medicine clinicians serve inner-city and rural areas in independent practices, large health systems, and community-based practice activities.
To improve access to lifestyle medicine in underresourced communities, more clinicians trained and certified in lifestyle medicine are needed. The Health Equity Achieved through Lifestyle Medicine Initiative supports a diverse lifestyle medicine workforce by offering scholarships to clinicians underrepresented in medicine and is working to train and certify at least one physician within each of the 1400 federally qualified health centers where clinicians are on the front lines of delivering care to the most underserved populations.
A meaningful first step for clinicians to address health disparities is to screen patients for and document SDOH. The American Academy of Family Physicians offers useful tools to screen patients, identify community-based resources, and help patients create action plans to overcome health risks and improve outcomes. In a promising trend to better support addressing SDOH in clinical care, the 2024 Medicare Physician Fee Schedule final rule included new codes to support this effort.
Not every patient will be ready or willing to begin a lifestyle medicine treatment plan. Still, all of them will be grateful for the opportunity to decide for themselves. If we are invested in narrowing health inequities, lifestyle medicine and behavior change must be a topic in clinical encounters with all our patients.
Dr. Collings, director of lifestyle medicine, Silicon Valley Medical Development, and past president, American College of Lifestyle Medicine, Mountain View, California, has disclosed no relevant financial relationships.
A version of this article appeared on Medscape.com.
Primary care clinicians understand that addressing lifestyle-related chronic disease health disparities in minority and lower-income communities is a significant opportunity to alleviate unnecessary suffering. Disparate health outcomes associated with underlying comorbidities during the COVID pandemic exposed the urgency of this problem.
When it comes to delivering evidence-based therapeutic lifestyle behavior interventions to these populations, however, there is a misconception that lifestyle medicine is only for the wealthy. Such a misconception needlessly widens the gap in health disparities because the truth is that everyone deserves access to lifestyle medicine. Fortunately, there are numerous successful examples of delivering these services to underresourced patients. We can all contribute to narrowing health inequities by sourcing increasingly abundant lifestyle medicine resources.
All patients’ lived experiences are unique, and there is a wide range of potential challenges to achieving lifestyle behavior change. Ignoring these obstacles is a disservice to patients and almost certainly results in treatment failure. Requirements to document SDOH have been a tremendous initial step.
The next step is to have conversations with every patient about the powerful outcomes of even small lifestyle changes. All too often, clinicians forgo conversations on lifestyle change with patients affected by adverse SDOH and assume that social obstacles automatically mean that patients are neither willing nor able to attempt behavior modification. Instead, it is an opportunity for clinicians, particularly those certified in lifestyle medicine, to meet patients where they are, work with them to identify solutions, and provide referrals to community-based organizations with resources to help.
Small Steps to Big Changes
Not all lifestyle behavior interventions need to be programmatic or time intensive. Clinicians can guide patients toward simple but specific actions that can make a difference in health outcomes over time. Small steps, like eating one can of beans or two bags of frozen leafy greens each week, are a good start toward adjusted eating patterns. The American College of Lifestyle Medicine offers a whole-food, plant-predominant meal guide to share with patients.
Individuals can increase their physical activity in their living rooms by doing sit-to-stands or balancing on one leg. Deep breathing and establishing a sleep routine are other lifestyle behavior changes without a price tag.
It is true that early adopters of lifestyle medicine often had difficulty practicing in underresourced communities. Those practitioners were forced to operate on a cash-pay basis, making access to care cost-prohibitive for many patients. However, board certification has been available since 2017, and lifestyle medicine is being integrated into medical schools and residency programs. Many such board-certified clinicians now work in large health systems and bill under the usual methods. There are also frameworks, such as the community-engaged lifestyle medicine model, showing how to treat patients affected by adverse SDOH effectively.
For example, patients at risk for malnutrition because of illnesses like chronic kidney disease, cancer, and heart failure receive medically tailored meals and access to a registered dietitian through a partnership between UC San Diego Health and Mama’s Kitchen. In Pennsylvania’s Lehigh Valley, where 1 in 10 of the approximately 700,000 residents face food insecurity, the Kellyn Foundation delivers fresh food through the Eat Real Food Mobile Market and offers whole-food, plant-predominant cooking classes, interactive elementary school programs focused on healthy lifestyle choices, and therapeutic lifestyle-change programs in community locations. Three months after launching new mobile market sites in Allentown, 1200 households were utilizing $15 weekly food vouchers through the program. Lifestyle medicine clinicians serve inner-city and rural areas in independent practices, large health systems, and community-based practice activities.
To improve access to lifestyle medicine in underresourced communities, more clinicians trained and certified in lifestyle medicine are needed. The Health Equity Achieved through Lifestyle Medicine Initiative supports a diverse lifestyle medicine workforce by offering scholarships to clinicians underrepresented in medicine and is working to train and certify at least one physician within each of the 1400 federally qualified health centers where clinicians are on the front lines of delivering care to the most underserved populations.
A meaningful first step for clinicians to address health disparities is to screen patients for and document SDOH. The American Academy of Family Physicians offers useful tools to screen patients, identify community-based resources, and help patients create action plans to overcome health risks and improve outcomes. In a promising trend to better support addressing SDOH in clinical care, the 2024 Medicare Physician Fee Schedule final rule included new codes to support this effort.
Not every patient will be ready or willing to begin a lifestyle medicine treatment plan. Still, all of them will be grateful for the opportunity to decide for themselves. If we are invested in narrowing health inequities, lifestyle medicine and behavior change must be a topic in clinical encounters with all our patients.
Dr. Collings, director of lifestyle medicine, Silicon Valley Medical Development, and past president, American College of Lifestyle Medicine, Mountain View, California, has disclosed no relevant financial relationships.
A version of this article appeared on Medscape.com.
Primary care clinicians understand that addressing lifestyle-related chronic disease health disparities in minority and lower-income communities is a significant opportunity to alleviate unnecessary suffering. Disparate health outcomes associated with underlying comorbidities during the COVID pandemic exposed the urgency of this problem.
When it comes to delivering evidence-based therapeutic lifestyle behavior interventions to these populations, however, there is a misconception that lifestyle medicine is only for the wealthy. Such a misconception needlessly widens the gap in health disparities because the truth is that everyone deserves access to lifestyle medicine. Fortunately, there are numerous successful examples of delivering these services to underresourced patients. We can all contribute to narrowing health inequities by sourcing increasingly abundant lifestyle medicine resources.
All patients’ lived experiences are unique, and there is a wide range of potential challenges to achieving lifestyle behavior change. Ignoring these obstacles is a disservice to patients and almost certainly results in treatment failure. Requirements to document SDOH have been a tremendous initial step.
The next step is to have conversations with every patient about the powerful outcomes of even small lifestyle changes. All too often, clinicians forgo conversations on lifestyle change with patients affected by adverse SDOH and assume that social obstacles automatically mean that patients are neither willing nor able to attempt behavior modification. Instead, it is an opportunity for clinicians, particularly those certified in lifestyle medicine, to meet patients where they are, work with them to identify solutions, and provide referrals to community-based organizations with resources to help.
Small Steps to Big Changes
Not all lifestyle behavior interventions need to be programmatic or time intensive. Clinicians can guide patients toward simple but specific actions that can make a difference in health outcomes over time. Small steps, like eating one can of beans or two bags of frozen leafy greens each week, are a good start toward adjusted eating patterns. The American College of Lifestyle Medicine offers a whole-food, plant-predominant meal guide to share with patients.
Individuals can increase their physical activity in their living rooms by doing sit-to-stands or balancing on one leg. Deep breathing and establishing a sleep routine are other lifestyle behavior changes without a price tag.
It is true that early adopters of lifestyle medicine often had difficulty practicing in underresourced communities. Those practitioners were forced to operate on a cash-pay basis, making access to care cost-prohibitive for many patients. However, board certification has been available since 2017, and lifestyle medicine is being integrated into medical schools and residency programs. Many such board-certified clinicians now work in large health systems and bill under the usual methods. There are also frameworks, such as the community-engaged lifestyle medicine model, showing how to treat patients affected by adverse SDOH effectively.
For example, patients at risk for malnutrition because of illnesses like chronic kidney disease, cancer, and heart failure receive medically tailored meals and access to a registered dietitian through a partnership between UC San Diego Health and Mama’s Kitchen. In Pennsylvania’s Lehigh Valley, where 1 in 10 of the approximately 700,000 residents face food insecurity, the Kellyn Foundation delivers fresh food through the Eat Real Food Mobile Market and offers whole-food, plant-predominant cooking classes, interactive elementary school programs focused on healthy lifestyle choices, and therapeutic lifestyle-change programs in community locations. Three months after launching new mobile market sites in Allentown, 1200 households were utilizing $15 weekly food vouchers through the program. Lifestyle medicine clinicians serve inner-city and rural areas in independent practices, large health systems, and community-based practice activities.
To improve access to lifestyle medicine in underresourced communities, more clinicians trained and certified in lifestyle medicine are needed. The Health Equity Achieved through Lifestyle Medicine Initiative supports a diverse lifestyle medicine workforce by offering scholarships to clinicians underrepresented in medicine and is working to train and certify at least one physician within each of the 1400 federally qualified health centers where clinicians are on the front lines of delivering care to the most underserved populations.
A meaningful first step for clinicians to address health disparities is to screen patients for and document SDOH. The American Academy of Family Physicians offers useful tools to screen patients, identify community-based resources, and help patients create action plans to overcome health risks and improve outcomes. In a promising trend to better support addressing SDOH in clinical care, the 2024 Medicare Physician Fee Schedule final rule included new codes to support this effort.
Not every patient will be ready or willing to begin a lifestyle medicine treatment plan. Still, all of them will be grateful for the opportunity to decide for themselves. If we are invested in narrowing health inequities, lifestyle medicine and behavior change must be a topic in clinical encounters with all our patients.
Dr. Collings, director of lifestyle medicine, Silicon Valley Medical Development, and past president, American College of Lifestyle Medicine, Mountain View, California, has disclosed no relevant financial relationships.
A version of this article appeared on Medscape.com.


