User login
Semaglutide a Potential Treatment Option for Opioid Use Disorder?
Semaglutide (Ozempic, Novo Nordisk) is associated with a significantly lower risk for overdose in individuals with opioid use disorder (OUD), new research shows.
The findings suggest that the drug may be a promising treatment option for OUD, adding to the growing evidence of the potential psychiatric benefits of glucagon-like peptide 1 (GLP-1) inhibitors.
“Our study provided real-world evidence suggesting that semaglutide could have benefits in preventing opioid overdose and treating opioid use disorder,” co–lead author Rong Xu, PhD, director of the Center for Artificial Intelligence in Drug Discovery at Case Western Reserve University School of Medicine, Cleveland, Ohio, said in an interview.
However, Xu cautioned that this evidence is preliminary and randomized clinical trials are required to confirm these findings.
The study published online in a research letter on September 25 in JAMA Network Open.
New Addiction Meds an Urgent Priority
Investigators analyzed electronic medical records from 33,006 patients with type 2 diabetes and OUD who were prescribed one of eight antidiabetic medications between 2017 and 2023.
Drugs included in the study were semaglutide, insulin, metformin, albiglutide, dulaglutide, exenatide, liraglutide, lixisenatide, dipeptidyl peptidase–4 inhibitors, sodium-glucose cotransporter-2 inhibitors, sulfonylureas, and thiazolidinediones.
Participants in the semaglutide and each comparison group were matched for certain covariates at baseline, such as socioeconomic status and OUD medications.
After 1 year, semaglutide was associated with a 42%-68% lower risk for opioid overdose than other antidiabetic medications, including other GLP-1s (range of hazard ratio [HR]: HR, 0.32; 95% CI, 0.12-0.89; to HR, 0.58; 95%CI, 0.38-0.87).
Xu noted a number of study limitations including the effect of possible confounders and sole reliance on prescription data.
However, the findings are in line with those of prior studies showing that semaglutide may be associated with lower rates of alcohol and nicotine use, she said.
Earlier this year, Xu, along with National Institute on Drug Abuse Director Nora Volkow, MD, and colleagues, published a retrospective cohort study of nearly 84,000 patients with obesity. That analysis showed that semaglutide was associated with a significantly lower risk of new alcohol use disorder diagnoses.
In a previous editorial by Xu and Volkow that summarized the research to-date on GLP-1s for nicotine, alcohol, and substance use disorders, they note that “closing the addiction treatment gap and discovering new, more effective addiction medications are urgent priorities. In this regard, investigating the potential of GLP-1 analogue medications to treat substance use disorder deserves fast and rigorous testing.”
Caution Warranted
Commenting on the study, Riccardo De Giorgi, MD, PhD, department of psychiatry, University of Oxford in England, said at this point, “we have to be very careful about how we interpret these data.”
In August, De Giorgi published a study showing that semaglutide was associated with reduced risk for several neurologic and psychiatric outcomes including dementia and nicotine misuse.
While there is enough observational evidence linking GLP-1 medications with reduced SUD risk, he noted that “now is the time to move on and conduct some randomized clinical trials, specifically testing our hypothesis in people who have psychiatric disorders.”
De Giorgi also called for mechanistic studies of semaglutide and other so that researchers could learn more about how it works to reduce cravings. “Instead of going from bench to bed, we need to go back to the bench,” he said.
As previously reported, De Giorgi recently called on experts in the field to actively explore the potential of GLP-1 inhibitors for mental illness.
The study was funded by National Institute on Alcohol Abuse and Alcoholism, National Institute on Aging, the National Center for Advancing Translational Sciences, and the Intramural Research Program of the National Institutes of Health. Xu reported no relevant financial relationships. De Giorgi reported receiving funding from the National Institute for Health Research Oxford Health Biomedical Research Centre.
A version of this article first appeared on Medscape.com.
Semaglutide (Ozempic, Novo Nordisk) is associated with a significantly lower risk for overdose in individuals with opioid use disorder (OUD), new research shows.
The findings suggest that the drug may be a promising treatment option for OUD, adding to the growing evidence of the potential psychiatric benefits of glucagon-like peptide 1 (GLP-1) inhibitors.
“Our study provided real-world evidence suggesting that semaglutide could have benefits in preventing opioid overdose and treating opioid use disorder,” co–lead author Rong Xu, PhD, director of the Center for Artificial Intelligence in Drug Discovery at Case Western Reserve University School of Medicine, Cleveland, Ohio, said in an interview.
However, Xu cautioned that this evidence is preliminary and randomized clinical trials are required to confirm these findings.
The study published online in a research letter on September 25 in JAMA Network Open.
New Addiction Meds an Urgent Priority
Investigators analyzed electronic medical records from 33,006 patients with type 2 diabetes and OUD who were prescribed one of eight antidiabetic medications between 2017 and 2023.
Drugs included in the study were semaglutide, insulin, metformin, albiglutide, dulaglutide, exenatide, liraglutide, lixisenatide, dipeptidyl peptidase–4 inhibitors, sodium-glucose cotransporter-2 inhibitors, sulfonylureas, and thiazolidinediones.
Participants in the semaglutide and each comparison group were matched for certain covariates at baseline, such as socioeconomic status and OUD medications.
After 1 year, semaglutide was associated with a 42%-68% lower risk for opioid overdose than other antidiabetic medications, including other GLP-1s (range of hazard ratio [HR]: HR, 0.32; 95% CI, 0.12-0.89; to HR, 0.58; 95%CI, 0.38-0.87).
Xu noted a number of study limitations including the effect of possible confounders and sole reliance on prescription data.
However, the findings are in line with those of prior studies showing that semaglutide may be associated with lower rates of alcohol and nicotine use, she said.
Earlier this year, Xu, along with National Institute on Drug Abuse Director Nora Volkow, MD, and colleagues, published a retrospective cohort study of nearly 84,000 patients with obesity. That analysis showed that semaglutide was associated with a significantly lower risk of new alcohol use disorder diagnoses.
In a previous editorial by Xu and Volkow that summarized the research to-date on GLP-1s for nicotine, alcohol, and substance use disorders, they note that “closing the addiction treatment gap and discovering new, more effective addiction medications are urgent priorities. In this regard, investigating the potential of GLP-1 analogue medications to treat substance use disorder deserves fast and rigorous testing.”
Caution Warranted
Commenting on the study, Riccardo De Giorgi, MD, PhD, department of psychiatry, University of Oxford in England, said at this point, “we have to be very careful about how we interpret these data.”
In August, De Giorgi published a study showing that semaglutide was associated with reduced risk for several neurologic and psychiatric outcomes including dementia and nicotine misuse.
While there is enough observational evidence linking GLP-1 medications with reduced SUD risk, he noted that “now is the time to move on and conduct some randomized clinical trials, specifically testing our hypothesis in people who have psychiatric disorders.”
De Giorgi also called for mechanistic studies of semaglutide and other so that researchers could learn more about how it works to reduce cravings. “Instead of going from bench to bed, we need to go back to the bench,” he said.
As previously reported, De Giorgi recently called on experts in the field to actively explore the potential of GLP-1 inhibitors for mental illness.
The study was funded by National Institute on Alcohol Abuse and Alcoholism, National Institute on Aging, the National Center for Advancing Translational Sciences, and the Intramural Research Program of the National Institutes of Health. Xu reported no relevant financial relationships. De Giorgi reported receiving funding from the National Institute for Health Research Oxford Health Biomedical Research Centre.
A version of this article first appeared on Medscape.com.
Semaglutide (Ozempic, Novo Nordisk) is associated with a significantly lower risk for overdose in individuals with opioid use disorder (OUD), new research shows.
The findings suggest that the drug may be a promising treatment option for OUD, adding to the growing evidence of the potential psychiatric benefits of glucagon-like peptide 1 (GLP-1) inhibitors.
“Our study provided real-world evidence suggesting that semaglutide could have benefits in preventing opioid overdose and treating opioid use disorder,” co–lead author Rong Xu, PhD, director of the Center for Artificial Intelligence in Drug Discovery at Case Western Reserve University School of Medicine, Cleveland, Ohio, said in an interview.
However, Xu cautioned that this evidence is preliminary and randomized clinical trials are required to confirm these findings.
The study published online in a research letter on September 25 in JAMA Network Open.
New Addiction Meds an Urgent Priority
Investigators analyzed electronic medical records from 33,006 patients with type 2 diabetes and OUD who were prescribed one of eight antidiabetic medications between 2017 and 2023.
Drugs included in the study were semaglutide, insulin, metformin, albiglutide, dulaglutide, exenatide, liraglutide, lixisenatide, dipeptidyl peptidase–4 inhibitors, sodium-glucose cotransporter-2 inhibitors, sulfonylureas, and thiazolidinediones.
Participants in the semaglutide and each comparison group were matched for certain covariates at baseline, such as socioeconomic status and OUD medications.
After 1 year, semaglutide was associated with a 42%-68% lower risk for opioid overdose than other antidiabetic medications, including other GLP-1s (range of hazard ratio [HR]: HR, 0.32; 95% CI, 0.12-0.89; to HR, 0.58; 95%CI, 0.38-0.87).
Xu noted a number of study limitations including the effect of possible confounders and sole reliance on prescription data.
However, the findings are in line with those of prior studies showing that semaglutide may be associated with lower rates of alcohol and nicotine use, she said.
Earlier this year, Xu, along with National Institute on Drug Abuse Director Nora Volkow, MD, and colleagues, published a retrospective cohort study of nearly 84,000 patients with obesity. That analysis showed that semaglutide was associated with a significantly lower risk of new alcohol use disorder diagnoses.
In a previous editorial by Xu and Volkow that summarized the research to-date on GLP-1s for nicotine, alcohol, and substance use disorders, they note that “closing the addiction treatment gap and discovering new, more effective addiction medications are urgent priorities. In this regard, investigating the potential of GLP-1 analogue medications to treat substance use disorder deserves fast and rigorous testing.”
Caution Warranted
Commenting on the study, Riccardo De Giorgi, MD, PhD, department of psychiatry, University of Oxford in England, said at this point, “we have to be very careful about how we interpret these data.”
In August, De Giorgi published a study showing that semaglutide was associated with reduced risk for several neurologic and psychiatric outcomes including dementia and nicotine misuse.
While there is enough observational evidence linking GLP-1 medications with reduced SUD risk, he noted that “now is the time to move on and conduct some randomized clinical trials, specifically testing our hypothesis in people who have psychiatric disorders.”
De Giorgi also called for mechanistic studies of semaglutide and other so that researchers could learn more about how it works to reduce cravings. “Instead of going from bench to bed, we need to go back to the bench,” he said.
As previously reported, De Giorgi recently called on experts in the field to actively explore the potential of GLP-1 inhibitors for mental illness.
The study was funded by National Institute on Alcohol Abuse and Alcoholism, National Institute on Aging, the National Center for Advancing Translational Sciences, and the Intramural Research Program of the National Institutes of Health. Xu reported no relevant financial relationships. De Giorgi reported receiving funding from the National Institute for Health Research Oxford Health Biomedical Research Centre.
A version of this article first appeared on Medscape.com.
FROM JAMA NETWORK OPEN
Our Biggest Turnout Ever for Advocacy Day!
That’s why we gathered our leaders from across the United States in Washington, DC, to meet with congressional offices during our annual Advocacy Day.
GIs from California to Massachusetts and many states in between met with House and Senate offices to educate members of Congress and their staff about the most critical policy issues impacting you and your patients. In total, 28 states were represented and we attended more than 100 meetings in 64 different districts, which was a mix of both Republican and Democratic offices.
For the second year in a row, we were fortunate to be joined by GI patient advocates as well, who shared personal stories about the challenges they encountered in the health care system, and the negative effects to their well-being and quality of life because of red tape caused by prior authorization and step therapy.
The in-person advocacy of our members and patient advocates makes a difference. In one of AGA President Dr. Maria Abreu’s meetings, the congressional staffer remembered that he met with her, Dr. Mel Wilcox, and a patient advocate during 2023’s Advocacy Day and recounted the impact of their conversation about delays to timely access to care for inflammatory bowel disease medication.
Numerous GIs had similar experiences on Advocacy Day and recounted the benefits of being able to walk into House and Senate offices and educate congressional staff on the issues they’re experiencing in their clinic or lab.
Being able to start these conversations about health care and GI and build these relationships showcases the value of Advocacy Day, and demonstrates how AGA works with members to make it easy to advocate for the issues important to them. We were able to have a full day of constructive meetings with lawmakers and their staff thanks to members and patient advocates. Thank you for being engaged and using your voices to protect GI patient care!
That’s why we gathered our leaders from across the United States in Washington, DC, to meet with congressional offices during our annual Advocacy Day.
GIs from California to Massachusetts and many states in between met with House and Senate offices to educate members of Congress and their staff about the most critical policy issues impacting you and your patients. In total, 28 states were represented and we attended more than 100 meetings in 64 different districts, which was a mix of both Republican and Democratic offices.
For the second year in a row, we were fortunate to be joined by GI patient advocates as well, who shared personal stories about the challenges they encountered in the health care system, and the negative effects to their well-being and quality of life because of red tape caused by prior authorization and step therapy.
The in-person advocacy of our members and patient advocates makes a difference. In one of AGA President Dr. Maria Abreu’s meetings, the congressional staffer remembered that he met with her, Dr. Mel Wilcox, and a patient advocate during 2023’s Advocacy Day and recounted the impact of their conversation about delays to timely access to care for inflammatory bowel disease medication.
Numerous GIs had similar experiences on Advocacy Day and recounted the benefits of being able to walk into House and Senate offices and educate congressional staff on the issues they’re experiencing in their clinic or lab.
Being able to start these conversations about health care and GI and build these relationships showcases the value of Advocacy Day, and demonstrates how AGA works with members to make it easy to advocate for the issues important to them. We were able to have a full day of constructive meetings with lawmakers and their staff thanks to members and patient advocates. Thank you for being engaged and using your voices to protect GI patient care!
That’s why we gathered our leaders from across the United States in Washington, DC, to meet with congressional offices during our annual Advocacy Day.
GIs from California to Massachusetts and many states in between met with House and Senate offices to educate members of Congress and their staff about the most critical policy issues impacting you and your patients. In total, 28 states were represented and we attended more than 100 meetings in 64 different districts, which was a mix of both Republican and Democratic offices.
For the second year in a row, we were fortunate to be joined by GI patient advocates as well, who shared personal stories about the challenges they encountered in the health care system, and the negative effects to their well-being and quality of life because of red tape caused by prior authorization and step therapy.
The in-person advocacy of our members and patient advocates makes a difference. In one of AGA President Dr. Maria Abreu’s meetings, the congressional staffer remembered that he met with her, Dr. Mel Wilcox, and a patient advocate during 2023’s Advocacy Day and recounted the impact of their conversation about delays to timely access to care for inflammatory bowel disease medication.
Numerous GIs had similar experiences on Advocacy Day and recounted the benefits of being able to walk into House and Senate offices and educate congressional staff on the issues they’re experiencing in their clinic or lab.
Being able to start these conversations about health care and GI and build these relationships showcases the value of Advocacy Day, and demonstrates how AGA works with members to make it easy to advocate for the issues important to them. We were able to have a full day of constructive meetings with lawmakers and their staff thanks to members and patient advocates. Thank you for being engaged and using your voices to protect GI patient care!
An Investment in the Future of GI: The AGA Research Foundation
What will the practice of gastroenterology look like in 20 years? It is our hope that physicians have an abundance of new tools and treatments to care for their patients suffering from digestive disorders.
How will we get there? New treatments and devices are the result of years of research.
To help make this dream a reality, AGA — through the AGA Research Foundation — has made a commitment to support investigators in GI and hepatology with its Research Awards Program.
These diverse researchers range from young investigators to more seasoned leaders in GI, all embarking on novel research projects that will advance our understanding of digestive conditions and pave the way for future discoveries in the field.
To our AGA Research Foundation donors, we sincerely thank you for your gifts.
We invite the GI community to join others in supporting and helping spark the scientific breakthroughs of today so clinicians will have the tools to improve care tomorrow.
Make your tax-deductible gift today at www.gastro.org/donateonline.
What will the practice of gastroenterology look like in 20 years? It is our hope that physicians have an abundance of new tools and treatments to care for their patients suffering from digestive disorders.
How will we get there? New treatments and devices are the result of years of research.
To help make this dream a reality, AGA — through the AGA Research Foundation — has made a commitment to support investigators in GI and hepatology with its Research Awards Program.
These diverse researchers range from young investigators to more seasoned leaders in GI, all embarking on novel research projects that will advance our understanding of digestive conditions and pave the way for future discoveries in the field.
To our AGA Research Foundation donors, we sincerely thank you for your gifts.
We invite the GI community to join others in supporting and helping spark the scientific breakthroughs of today so clinicians will have the tools to improve care tomorrow.
Make your tax-deductible gift today at www.gastro.org/donateonline.
What will the practice of gastroenterology look like in 20 years? It is our hope that physicians have an abundance of new tools and treatments to care for their patients suffering from digestive disorders.
How will we get there? New treatments and devices are the result of years of research.
To help make this dream a reality, AGA — through the AGA Research Foundation — has made a commitment to support investigators in GI and hepatology with its Research Awards Program.
These diverse researchers range from young investigators to more seasoned leaders in GI, all embarking on novel research projects that will advance our understanding of digestive conditions and pave the way for future discoveries in the field.
To our AGA Research Foundation donors, we sincerely thank you for your gifts.
We invite the GI community to join others in supporting and helping spark the scientific breakthroughs of today so clinicians will have the tools to improve care tomorrow.
Make your tax-deductible gift today at www.gastro.org/donateonline.
Gastro Journal Club: Proximal Cancers in FIT-Positive Patients
For our next installment of the Gastro Journal Club, They are joined by fellows from the Icahn School of Medicine at Mount Sinai in New York City for a discussion of the article “Risk of Cancers Proximal to the Colon in Fecal Immunochemical Test Positive Screenees in a Colorectal Cancer Screening Program,” published in the September 2024 issue of Gastroenterology .
Visit our YouTube Channel (youtube.com/@AmerGastroAssn) to watch the session.
The Gastro Journal Club is by and for fellows and residents. During these sessions, fellows and residents have the opportunity to ask authors questions about their recently published work in Gastroenterology. If you are interested in arranging a Gastro Journal Club session at your institution, please contact [email protected].
For our next installment of the Gastro Journal Club, They are joined by fellows from the Icahn School of Medicine at Mount Sinai in New York City for a discussion of the article “Risk of Cancers Proximal to the Colon in Fecal Immunochemical Test Positive Screenees in a Colorectal Cancer Screening Program,” published in the September 2024 issue of Gastroenterology .
Visit our YouTube Channel (youtube.com/@AmerGastroAssn) to watch the session.
The Gastro Journal Club is by and for fellows and residents. During these sessions, fellows and residents have the opportunity to ask authors questions about their recently published work in Gastroenterology. If you are interested in arranging a Gastro Journal Club session at your institution, please contact [email protected].
For our next installment of the Gastro Journal Club, They are joined by fellows from the Icahn School of Medicine at Mount Sinai in New York City for a discussion of the article “Risk of Cancers Proximal to the Colon in Fecal Immunochemical Test Positive Screenees in a Colorectal Cancer Screening Program,” published in the September 2024 issue of Gastroenterology .
Visit our YouTube Channel (youtube.com/@AmerGastroAssn) to watch the session.
The Gastro Journal Club is by and for fellows and residents. During these sessions, fellows and residents have the opportunity to ask authors questions about their recently published work in Gastroenterology. If you are interested in arranging a Gastro Journal Club session at your institution, please contact [email protected].
Artificial Intelligence Helps Diagnose Lung Disease in Infants and Outperforms Trainee Doctors
VIENNA — Artificial Intelligence (AI) can assist doctors in assessing and diagnosing respiratory illnesses in infants and children, according to two new studies presented at the European Respiratory Society (ERS) 2024 Congress.
Researchers can train artificial neural networks (ANNs) to detect lung disease in premature babies by analyzing their breathing patterns while they sleep. “Our noninvasive test is less distressing for the baby and their parents, meaning they can access treatment more quickly, and may also be relevant for their long-term prognosis,” said Edgar Delgado-Eckert, PhD, adjunct professor in the Department of Biomedical Engineering at The University of Basel, Switzerland, and a research group leader at the University Children’s Hospital, Switzerland.
Manjith Narayanan, MD, a consultant in pediatric pulmonology at the Royal Hospital for Children and Young People, Edinburgh, and honorary senior clinical lecturer at The University of Edinburgh, United Kingdom, said chatbots such as ChatGPT, Bard, and Bing can perform as well as or better than trainee doctors when assessing children with respiratory issues. He said chatbots could triage patients more quickly and ease pressure on health services.
Chatbots Show Promise in Triage of Pediatric Respiratory Illnesses
Researchers at The University of Edinburgh provided 10 trainee doctors with less than 4 months of clinical experience in pediatrics with clinical scenarios that covered topics such as cystic fibrosis, asthma, sleep-disordered breathing, breathlessness, chest infections, or no obvious diagnosis.
The trainee doctors had 1 hour to use the internet, although they were not allowed to use chatbots to solve each scenario with a descriptive answer.
Each scenario was also presented to the three large language models (LLMs): OpenAI’s ChatGPT, Google’s Bard, and Microsoft’s Bing.
Six pediatric respiratory experts assessed all responses, scoring correctness, comprehensiveness, usefulness, plausibility, and coherence on a scale of 0-9. They were also asked to say whether they thought a human or a chatbot generated each response.
ChatGPT scored an average of 7 out of 9 overall and was believed to be more human-like than responses from the other chatbots. Bard scored an average of 6 out of 9 and was more “coherent” than trainee doctors, but in other respects, it was no better or worse than trainee doctors. Bing and trainee doctors scored an average of 4 out of 9.
“Our study is the first, to our knowledge, to test LLMs against trainee doctors in situations that reflect real-life clinical practice,” Narayanan said. “We did this by allowing the trainee doctors to have full access to resources available on the internet, as they would in real life. This moves the focus away from testing memory, where LLMs have a clear advantage.”
Narayanan said that these models could help nurses, trainee doctors, and primary care physicians triage patients quickly and assist medical professionals in their studies by summarizing their thought processes. “The key word, though, is “assist.” They cannot replace conventional medical training yet,” he told Medscape Medical News.
The researchers found no obvious hallucinations — seemingly made-up information — with any of the three LLMs. Still, Narayanan said, “We need to be aware of this possibility and build mitigations.”
Hilary Pinnock, ERS education council chair and professor of primary care respiratory medicine at The University of Edinburgh who was not involved in the research, said seeing how widely available AI tools can provide solutions to complex cases of respiratory illness in children is exciting and worrying at the same time. “It certainly points the way to a brave new world of AI-supported care.”
“However, before we start to use AI in routine clinical practice, we need to be confident that it will not create errors either through ‘hallucinating’ fake information or because it has been trained on data that does not equitably represent the population we serve,” she said.
AI Predicts Lung Disease in Premature Babies
Identifying bronchopulmonary dysplasia (BPD) in premature babies remains a challenge. Lung function tests usually require blowing out on request, which is a task babies cannot perform. Current techniques require sophisticated equipment to measure an infant’s lung ventilation characteristics, so doctors usually diagnose BPD by the presence of its leading causes, prematurity and the need for respiratory support.
Researchers at the University of Basel in Switzerland trained an ANN model to predict BPD in premature babies.
The team studied a group of 139 full-term and 190 premature infants who had been assessed for BPD, recording their breathing for 10 minutes while they slept. For each baby, 100 consecutive regular breaths, carefully inspected to exclude sighs or other artifacts, were used to train, validate, and test an ANN called a Long Short-Term Memory model (LSTM), which is particularly effective at classifying sequential data such as tidal breathing.
Researchers used 60% of the data to teach the network how to recognize BPD, 20% to validate the model, and then fed the remaining 20% of the data to the model to see if it could correctly identify those babies with BPD.
The LSTM model classified a series of flow values in the unseen test data set as belonging to a patient diagnosed with BPD or not with 96% accuracy.
“Until recently, this need for large amounts of data has hindered efforts to create accurate models for lung disease in infants because it is so difficult to assess their lung function,” Delgado-Eckert said. “Our research delivers, for the first time, a comprehensive way of analyzing infants’ breathing and allows us to detect which babies have BPD as early as 1 month of corrected age.”
The study presented by Delgado-Eckert received funding from the Swiss National Science Foundation. Narayanan and Pinnock reported no relevant financial relationships.
A version of this article appeared on Medscape.com.
VIENNA — Artificial Intelligence (AI) can assist doctors in assessing and diagnosing respiratory illnesses in infants and children, according to two new studies presented at the European Respiratory Society (ERS) 2024 Congress.
Researchers can train artificial neural networks (ANNs) to detect lung disease in premature babies by analyzing their breathing patterns while they sleep. “Our noninvasive test is less distressing for the baby and their parents, meaning they can access treatment more quickly, and may also be relevant for their long-term prognosis,” said Edgar Delgado-Eckert, PhD, adjunct professor in the Department of Biomedical Engineering at The University of Basel, Switzerland, and a research group leader at the University Children’s Hospital, Switzerland.
Manjith Narayanan, MD, a consultant in pediatric pulmonology at the Royal Hospital for Children and Young People, Edinburgh, and honorary senior clinical lecturer at The University of Edinburgh, United Kingdom, said chatbots such as ChatGPT, Bard, and Bing can perform as well as or better than trainee doctors when assessing children with respiratory issues. He said chatbots could triage patients more quickly and ease pressure on health services.
Chatbots Show Promise in Triage of Pediatric Respiratory Illnesses
Researchers at The University of Edinburgh provided 10 trainee doctors with less than 4 months of clinical experience in pediatrics with clinical scenarios that covered topics such as cystic fibrosis, asthma, sleep-disordered breathing, breathlessness, chest infections, or no obvious diagnosis.
The trainee doctors had 1 hour to use the internet, although they were not allowed to use chatbots to solve each scenario with a descriptive answer.
Each scenario was also presented to the three large language models (LLMs): OpenAI’s ChatGPT, Google’s Bard, and Microsoft’s Bing.
Six pediatric respiratory experts assessed all responses, scoring correctness, comprehensiveness, usefulness, plausibility, and coherence on a scale of 0-9. They were also asked to say whether they thought a human or a chatbot generated each response.
ChatGPT scored an average of 7 out of 9 overall and was believed to be more human-like than responses from the other chatbots. Bard scored an average of 6 out of 9 and was more “coherent” than trainee doctors, but in other respects, it was no better or worse than trainee doctors. Bing and trainee doctors scored an average of 4 out of 9.
“Our study is the first, to our knowledge, to test LLMs against trainee doctors in situations that reflect real-life clinical practice,” Narayanan said. “We did this by allowing the trainee doctors to have full access to resources available on the internet, as they would in real life. This moves the focus away from testing memory, where LLMs have a clear advantage.”
Narayanan said that these models could help nurses, trainee doctors, and primary care physicians triage patients quickly and assist medical professionals in their studies by summarizing their thought processes. “The key word, though, is “assist.” They cannot replace conventional medical training yet,” he told Medscape Medical News.
The researchers found no obvious hallucinations — seemingly made-up information — with any of the three LLMs. Still, Narayanan said, “We need to be aware of this possibility and build mitigations.”
Hilary Pinnock, ERS education council chair and professor of primary care respiratory medicine at The University of Edinburgh who was not involved in the research, said seeing how widely available AI tools can provide solutions to complex cases of respiratory illness in children is exciting and worrying at the same time. “It certainly points the way to a brave new world of AI-supported care.”
“However, before we start to use AI in routine clinical practice, we need to be confident that it will not create errors either through ‘hallucinating’ fake information or because it has been trained on data that does not equitably represent the population we serve,” she said.
AI Predicts Lung Disease in Premature Babies
Identifying bronchopulmonary dysplasia (BPD) in premature babies remains a challenge. Lung function tests usually require blowing out on request, which is a task babies cannot perform. Current techniques require sophisticated equipment to measure an infant’s lung ventilation characteristics, so doctors usually diagnose BPD by the presence of its leading causes, prematurity and the need for respiratory support.
Researchers at the University of Basel in Switzerland trained an ANN model to predict BPD in premature babies.
The team studied a group of 139 full-term and 190 premature infants who had been assessed for BPD, recording their breathing for 10 minutes while they slept. For each baby, 100 consecutive regular breaths, carefully inspected to exclude sighs or other artifacts, were used to train, validate, and test an ANN called a Long Short-Term Memory model (LSTM), which is particularly effective at classifying sequential data such as tidal breathing.
Researchers used 60% of the data to teach the network how to recognize BPD, 20% to validate the model, and then fed the remaining 20% of the data to the model to see if it could correctly identify those babies with BPD.
The LSTM model classified a series of flow values in the unseen test data set as belonging to a patient diagnosed with BPD or not with 96% accuracy.
“Until recently, this need for large amounts of data has hindered efforts to create accurate models for lung disease in infants because it is so difficult to assess their lung function,” Delgado-Eckert said. “Our research delivers, for the first time, a comprehensive way of analyzing infants’ breathing and allows us to detect which babies have BPD as early as 1 month of corrected age.”
The study presented by Delgado-Eckert received funding from the Swiss National Science Foundation. Narayanan and Pinnock reported no relevant financial relationships.
A version of this article appeared on Medscape.com.
VIENNA — Artificial Intelligence (AI) can assist doctors in assessing and diagnosing respiratory illnesses in infants and children, according to two new studies presented at the European Respiratory Society (ERS) 2024 Congress.
Researchers can train artificial neural networks (ANNs) to detect lung disease in premature babies by analyzing their breathing patterns while they sleep. “Our noninvasive test is less distressing for the baby and their parents, meaning they can access treatment more quickly, and may also be relevant for their long-term prognosis,” said Edgar Delgado-Eckert, PhD, adjunct professor in the Department of Biomedical Engineering at The University of Basel, Switzerland, and a research group leader at the University Children’s Hospital, Switzerland.
Manjith Narayanan, MD, a consultant in pediatric pulmonology at the Royal Hospital for Children and Young People, Edinburgh, and honorary senior clinical lecturer at The University of Edinburgh, United Kingdom, said chatbots such as ChatGPT, Bard, and Bing can perform as well as or better than trainee doctors when assessing children with respiratory issues. He said chatbots could triage patients more quickly and ease pressure on health services.
Chatbots Show Promise in Triage of Pediatric Respiratory Illnesses
Researchers at The University of Edinburgh provided 10 trainee doctors with less than 4 months of clinical experience in pediatrics with clinical scenarios that covered topics such as cystic fibrosis, asthma, sleep-disordered breathing, breathlessness, chest infections, or no obvious diagnosis.
The trainee doctors had 1 hour to use the internet, although they were not allowed to use chatbots to solve each scenario with a descriptive answer.
Each scenario was also presented to the three large language models (LLMs): OpenAI’s ChatGPT, Google’s Bard, and Microsoft’s Bing.
Six pediatric respiratory experts assessed all responses, scoring correctness, comprehensiveness, usefulness, plausibility, and coherence on a scale of 0-9. They were also asked to say whether they thought a human or a chatbot generated each response.
ChatGPT scored an average of 7 out of 9 overall and was believed to be more human-like than responses from the other chatbots. Bard scored an average of 6 out of 9 and was more “coherent” than trainee doctors, but in other respects, it was no better or worse than trainee doctors. Bing and trainee doctors scored an average of 4 out of 9.
“Our study is the first, to our knowledge, to test LLMs against trainee doctors in situations that reflect real-life clinical practice,” Narayanan said. “We did this by allowing the trainee doctors to have full access to resources available on the internet, as they would in real life. This moves the focus away from testing memory, where LLMs have a clear advantage.”
Narayanan said that these models could help nurses, trainee doctors, and primary care physicians triage patients quickly and assist medical professionals in their studies by summarizing their thought processes. “The key word, though, is “assist.” They cannot replace conventional medical training yet,” he told Medscape Medical News.
The researchers found no obvious hallucinations — seemingly made-up information — with any of the three LLMs. Still, Narayanan said, “We need to be aware of this possibility and build mitigations.”
Hilary Pinnock, ERS education council chair and professor of primary care respiratory medicine at The University of Edinburgh who was not involved in the research, said seeing how widely available AI tools can provide solutions to complex cases of respiratory illness in children is exciting and worrying at the same time. “It certainly points the way to a brave new world of AI-supported care.”
“However, before we start to use AI in routine clinical practice, we need to be confident that it will not create errors either through ‘hallucinating’ fake information or because it has been trained on data that does not equitably represent the population we serve,” she said.
AI Predicts Lung Disease in Premature Babies
Identifying bronchopulmonary dysplasia (BPD) in premature babies remains a challenge. Lung function tests usually require blowing out on request, which is a task babies cannot perform. Current techniques require sophisticated equipment to measure an infant’s lung ventilation characteristics, so doctors usually diagnose BPD by the presence of its leading causes, prematurity and the need for respiratory support.
Researchers at the University of Basel in Switzerland trained an ANN model to predict BPD in premature babies.
The team studied a group of 139 full-term and 190 premature infants who had been assessed for BPD, recording their breathing for 10 minutes while they slept. For each baby, 100 consecutive regular breaths, carefully inspected to exclude sighs or other artifacts, were used to train, validate, and test an ANN called a Long Short-Term Memory model (LSTM), which is particularly effective at classifying sequential data such as tidal breathing.
Researchers used 60% of the data to teach the network how to recognize BPD, 20% to validate the model, and then fed the remaining 20% of the data to the model to see if it could correctly identify those babies with BPD.
The LSTM model classified a series of flow values in the unseen test data set as belonging to a patient diagnosed with BPD or not with 96% accuracy.
“Until recently, this need for large amounts of data has hindered efforts to create accurate models for lung disease in infants because it is so difficult to assess their lung function,” Delgado-Eckert said. “Our research delivers, for the first time, a comprehensive way of analyzing infants’ breathing and allows us to detect which babies have BPD as early as 1 month of corrected age.”
The study presented by Delgado-Eckert received funding from the Swiss National Science Foundation. Narayanan and Pinnock reported no relevant financial relationships.
A version of this article appeared on Medscape.com.
FROM ERS 2024
Pediatricians Must Prepare for Impact on Allergies and Asthma From Climate Change
ORLANDO — It’s important for pediatricians not only to understand the causes and effects of climate change but also to know how to discuss this issue with families and make risk-based adjustments to their clinical practice based on the individual health and circumstances of each patient. That’s one of the key messages delivered at the annual meeting of the American Academy of Pediatrics (AAP) by Elizabeth C. Matsui, MD, MHS, professor of population health and pediatrics and director of the Center for Health and Environment Education and Research at the University of Texas at Austin Dell Medical School.
“Even though climate change has been here and has been affecting health already for a while, it’s just really impossible to ignore right now,” she told attendees in a session focused on climate change impacts on allergies and asthma. “The challenge is connecting the dots between something that is much larger, or feels much larger, than the patient and the family that’s in front of you.”
The reality, however, is that climate change is now impacting patients’ health on an individual level, and pediatricians have a responsibility to understand how that’s happening and to help their families prepare for it.
“From the perspective of someone who went into medicine to practice and take care of the individual patient, I think it has been more difficult to connect those dots, and for the people in this room, it’s our job to connect those dots,” Matsui said. She also acknowledged that many of the solutions are frustratingly limited to the policy level and challenging to implement, “but it doesn’t mean that we can’t make a difference for the patients who are in front of us.”
Charles Moon, MD, a pediatrician and Pediatric Environmental Health Fellow at the Children’s Environmental Health Center, Icahn School of Medicine at Mount Sinai, New York City, found the talk particularly helpful in providing information about both the broader issue and what it means on a local practice level.
“The biggest takeaway is that more people and more pediatricians are tuning in to this issue and realizing the dangers,” Moon said. “It’s clear that a larger community is forming around this, and I think we are at the cusp where more and more people will be coming in. We are really focusing on taking all the data and trying to figure out solutions. I think the solutions orientation is the most important part.”
Understanding the Big Picture
Matsui opened with a general discussion of the human causes of climate change and the effects on a global scale presently and in the future. For example, over the past 800,000 years, carbon dioxide levels have never been above 300 ppm, but they surpassed that threshold in 1911 and have reached 420 ppm today. The trapping of heat in Earth’s atmosphere caused by the increase in carbon dioxide and other greenhouse gases is leading to multiple phenomena that impact health, such as longer growing seasons; increased droughts, heat waves, and wildfire seasons; and higher temperatures. These changes, in turn, affect allergens and asthma.
, Matsui said. Childhood is a critical period for lung and immune development, and the Environmental Protection Agency’s 2023 Climate Change and Children’s Health and Well-Being report projects that an increase of 2° C in global warming will result in an additional 34,500 pediatric asthma cases and 228,000 allergic rhinitis cases per year, driven largely by predicted increases in ozone and 2.5-µm particulate matter. The report also forecasts an increase in 6240 asthma emergency department visits and 332 additional respiratory hospitalizations per year.
“We know that these associations that we see between climate change exposures and poor respiratory health outcomes in kids are biologically plausible,” Matsui said. “They’re not just correlation without causation. A lot of the mechanisms for how air pollution, allergies, and other factors directly affect the lungs of the airway epithelium have been worked out.”
An Increase in Allergens and Viral Infections
Pediatricians should prepare for anticipated growth in allergens and viral infections. The longer growing seasons mean that pollen seasons will also lengthen. Meanwhile, higher concentrations of carbon dioxide cause individual plants to produce more pollen.
“As the winters get warmer, mice that might not be able to survive during the winter are surviving, and mice reproduce at a very rapid rate,” she said. “The increase in moisture means that dust mites, which absorb their water — they drink by absorbing humidity that’s in the air — will be present in higher concentrations, and their range will expand.”
Fungal and mold exposures are also increasing, not just outdoors but also indoors, “and there are all sorts of allergic and respiratory health consequences of fungal exposure,” Matsui said. As hurricanes and flooding increase, storm damage can also make indoor environments more conducive to fungal and mold growth.
Extreme weather from climate change also affects infrastructure. “When there’s healthcare infrastructure disruption and other infrastructure disruption, it adds to the challenge,” she said. “It compounds all the other threat to health from climate change, so this overall problem of climate change and health is multidimensional and very complicated.”
Then there’s the impact of climate change on respiratory viruses, which are a major driver of asthma exacerbations, Matsui said. The greater variability in daytime temperatures affects environmental reservoirs, transmission patterns, geographical ranges, and seasonality of various respiratory pathogens. The prevalence of respiratory syncytial virus infections, for example, increases during humid periods.
“This is coupled with the fact that the projected increases in air pollution increase susceptibility to respiratory virus infections,” Matsui said. “In fact, climate change and air pollution are inextricably linked.”
Climate Change and Air Pollution
Climate disruption creates extreme weather patterns that then lead to worsening air quality due to high temperatures; heavier precipitation; and more forest fires, droughts, dust storms, thunderstorms, hurricanes, stagnation events, and other extreme weather. Matsui shared a map showing the substantial increase in days with stagnant air since 1973. During stagnation events, air pollution builds up in the atmosphere because of a stable air mass that remains over a region for several days, with low-level winds and no precipitation.
The pollutants can then contribute to rising temperatures. Black carbon particulate matter released from the burning of forests and other biomass absorbs more solar radiation, further contributing to temperature increases. Data from the National Bureau of Economic Research has shown that the US made big strides in reducing air pollution from 2009 through 2016, but it began to reverse in 2016 as severe weather events picked up.
Pediatricians need to be cognizant of the synergistic effect of these different impacts as well. “We oftentimes talk about these problems in a silo, so we may talk about air pollution and health effects, or allergens and health effects, or heat and health effects, but all of these interact with each other and further compound the health effects,” compared to just one of them in isolation, Matsui said.
For example, air pollution increases sensitivity to allergen exposure and increases reaction severity, which disrupts the immune tolerance to allergens. “Heat and air pollution also interact, and the combination of the two is more deadly than either one alone,” she said.
Air pollution from wildfire smoke is also more toxic to the lungs than air pollution from other sources, so if there’s wildfire-based air pollution, the impact on respiratory hospitalizations is significantly greater. Even in places that would not otherwise be at risk for wildfires, the threat remains of air pollution from more distant fires, as New York City experienced from Canadian wildfires last year.
“This is a problem that is not just isolated to the parts of the world where the wildfires are located,” Matsui said.
Moon, who practices in New York City, said he really appreciated Matsui’s perspectives and nuanced advice as a subspecialist “because it’s obvious that the way we deliver healthcare is going to have to change based on climate change.” He hopes to see more subspecialists from other pediatric areas getting involved in looking at climate impacts and providing nuanced advice about changing clinical care similar to the examples Matsui provided.
Air pollution can also be deadly, as a landmark case in the United Kingdom revealed a few years ago when the court ruled that a child’s death from an asthma attack was directly due to air pollution. In addition to causing worse asthma symptoms and exacerbations, air pollution also adds to the risk of developing asthma and impedes lung growth, all of which disproportionately affects disadvantaged and minoritized communities, she said.
Greater Impact on Disadvantaged Populations
Matsui called attention to the equity implications of climate change impacts on health.
“If you have a community that does not have the infrastructure and access to resources, and that same community has a prevalence of asthma that is double that of their more advantaged and white counterparts, then the impacts of climate change are going to be amplified even more,” she said.
For example, a 2019 study found that the biggest predictor of the location of ragweed plants has to do with vacant lots and demolition of housing. Ragweed plants being more common in neighborhoods with vacant lots will disproportionately affect disadvantaged neighborhoods, she said. Another study found in Baltimore that mouse allergens — specifically urine — were a bigger cause of asthma in low-income children than were cockroach allergens.
“It’s important to consider context,” including age, gender and social and behavioral context, she said. “We as pediatricians know that children are particularly vulnerable, and what happens to them has an effect across the lifespan.”
Furthermore, pediatricians are aware that disadvantaged and minoritized communities lack infrastructure; often live in areas with greater air pollution; often have heat islands in their communities without protection, such as tree canopy; and may be at greater flooding risk. “Poverty is also associated with increased vulnerability” because of poorer housing and infrastructure, less education, less access to care, more preexisting health conditions and greater discrimination, she said.
Three Cornerstone Interventions
Interventions fall into three main buckets, Matsui said: mitigation, adaption, and resilience.
“Mitigation means reducing greenhouse gas and air pollution production and trying to enhance sinks for greenhouse gases,” she said. Mitigation strategies primarily occur at the policy level, with improved regulation, treaties, and market-based approaches, such as carbon tax and cap and trade.
Adaptation includes actions that lessen the impact on health and environment, such as infrastructure changes and implementation of air conditioning. Examples of climate change adaptation strategies also mostly come from policy but largely at state and local levels, where individual pediatricians have a greater voice and influence. These can include changes in urban planning to address heat islands, flooding risk, and public transportation’s contribution to air pollution and climate change. It can also include changes in housing regulation and policy and investments in healthcare, such as expanded Medicaid and health insurance and investing in disaster planning and readiness.
“Resilience is a more holistic concept,” Matsui said, “which advocates for system-wide, multilevel changes and involves a range of strategies to enhance social, human, natural, physical, and financial capacities.”
What Pediatricians Can Do
Pediatricians have an important role to play when it comes to climate change and health impacts.
“The first step is sort of understanding the complexity of climate change in terms of its potential health effects, but also being prepared to talk with our patients and their families about it,” Matsui said. “The second step is advocacy.” She drew attention to the February policy statement in Pediatrics that discusses precisely the ways in which pediatricians can leverage their expertise and credibility.
“Pediatricians are ideal advocates with whom to partner and uplift youth and community voices working to advance zero-carbon energy policy and climate justice,” she said. “There are many opportunities to advocate for climate solution policies at the local, state, national, and even international level.”
These roles can include educating elected officials and health insurance entities about the risks that climate change poses to allergies, asthma, and child health more broadly, as well as the benefits of local solutions, including improved air quality, tree canopy, and green space. “There are lots of opportunities to engage with the community, including speaking at public hearings, serving as an expert testimony, and writing letters to the editor,” she said.
The impact of these efforts can be further maximized by working with other healthcare professionals. Lori Byron, MD, a pediatrician from Red Lodge, Montana, who heads the AAP Chapter Climate Advocates program, noted during Q&A that every AAP chapter in the country has climate advocates. She added that the AAP is the first medical board to have climate modules in their maintenance of certification specifically designed to incorporate climate change education into well visits.
Adjusting Clinical Care
Meanwhile, in patient care, Matsui acknowledged it can be frustrating to think about what a massive impact climate has and simultaneously challenging to engage families in discussions about it. However, a wide range of resources are available that can be provided to patients.
“For a patient in front of you, being informed and prepared to talk about it is the first step to being able to assess their climate change risk and provide tailored guidance,” she said. Tailored guidance takes into account the child’s specific health situation and the risks they’re most likely to encounter, such as wildfire smoke, air pollution, longer pollen seasons, environmental allergens, or disruption of infrastructure.
“If I am seeing a patient with asthma who is allergic to a particular pollen, I can anticipate that pollen may be present in higher levels of the future, and that the season for that pollen may be longer,” Matsui said. “So if I’m thinking about allergen immunotherapy for that patient, future risk may be something that would push the conversation and the shared decision-making” from possible consideration to more serious consideration, depending on the child’s age.
“Another example is a patient with asthma, thinking about wildfire risk and having them prepared, because we know from data that wildfire air pollution is going to be worse for that child than pollution from other sources, and there are ways for them to be prepared,” Matsui said. For instance, having an HVAC system with a high-grade air filter (at least a MERV 13) will filter the air better if a wildfire causes smoke to descend over an area. Portable, less expensive HEPA filters are also an option if a family cannot upgrade their system, and wearing an N95 or N95-equivalent mask can also reduce the impact of high air pollution levels.
An example of thinking about the impact of potential infrastructure disruption could be ensuring patients have enough of all their medications if they’re close to running out. “It’s important for them to always have think about their medications and get those refills ahead of a storm,” she said.
Additional Resources
Understanding that pediatricians may not have time to discuss all these issues or have broader conversations about climate change during visits, Matsui highlighted the AAP website of resources on climate change. In addition to resources for pediatricians, such as a basic fact sheet about climate change impacts on children’s health and the technical report that informed the policy statement, the site has multiple resources for families:
- Climate Change Impact: Safeguarding Your Family’s Health and Well-being (video), How to Talk With Children About Climate Change, Climate Change & Children’s Health: AAP Policy Explained, Climate Checkup for Children’s Health: Little Changes With Big Impact, How Climate Change Can Make Children Sick: What Parents Need to Know, Climate Change & Wildfires: Why Kids Are Most at Risk, Climate Change, Extreme Weather & Children: What Families Need to Know, Extreme Heat & Air Pollution: Health Effects on Babies & Pregnant People, and
The following resources can also be helpful to pediatricians and/or families:
- Ready.gov, AirNow, Patient Exposure and the Air Quality Index, Protecting Vulnerable Patient Populations from Climate Hazards: A Referral Guide for Health Professionals from the US Department of Health and Human Services, Low Income Home Energy Assistance Program (LIHEAP), Weatherization Assistance Program, and the Disaster Supplemental Nutrition Assistance Program (D-SNAP)
In some states, Medicaid will provide or cover the cost of air conditioning and/or air filters.
The presentation did not involve external funding. Drs. Matsui and Moon had no disclosures.
A version of this article first appeared on Medscape.com.
ORLANDO — It’s important for pediatricians not only to understand the causes and effects of climate change but also to know how to discuss this issue with families and make risk-based adjustments to their clinical practice based on the individual health and circumstances of each patient. That’s one of the key messages delivered at the annual meeting of the American Academy of Pediatrics (AAP) by Elizabeth C. Matsui, MD, MHS, professor of population health and pediatrics and director of the Center for Health and Environment Education and Research at the University of Texas at Austin Dell Medical School.
“Even though climate change has been here and has been affecting health already for a while, it’s just really impossible to ignore right now,” she told attendees in a session focused on climate change impacts on allergies and asthma. “The challenge is connecting the dots between something that is much larger, or feels much larger, than the patient and the family that’s in front of you.”
The reality, however, is that climate change is now impacting patients’ health on an individual level, and pediatricians have a responsibility to understand how that’s happening and to help their families prepare for it.
“From the perspective of someone who went into medicine to practice and take care of the individual patient, I think it has been more difficult to connect those dots, and for the people in this room, it’s our job to connect those dots,” Matsui said. She also acknowledged that many of the solutions are frustratingly limited to the policy level and challenging to implement, “but it doesn’t mean that we can’t make a difference for the patients who are in front of us.”
Charles Moon, MD, a pediatrician and Pediatric Environmental Health Fellow at the Children’s Environmental Health Center, Icahn School of Medicine at Mount Sinai, New York City, found the talk particularly helpful in providing information about both the broader issue and what it means on a local practice level.
“The biggest takeaway is that more people and more pediatricians are tuning in to this issue and realizing the dangers,” Moon said. “It’s clear that a larger community is forming around this, and I think we are at the cusp where more and more people will be coming in. We are really focusing on taking all the data and trying to figure out solutions. I think the solutions orientation is the most important part.”
Understanding the Big Picture
Matsui opened with a general discussion of the human causes of climate change and the effects on a global scale presently and in the future. For example, over the past 800,000 years, carbon dioxide levels have never been above 300 ppm, but they surpassed that threshold in 1911 and have reached 420 ppm today. The trapping of heat in Earth’s atmosphere caused by the increase in carbon dioxide and other greenhouse gases is leading to multiple phenomena that impact health, such as longer growing seasons; increased droughts, heat waves, and wildfire seasons; and higher temperatures. These changes, in turn, affect allergens and asthma.
, Matsui said. Childhood is a critical period for lung and immune development, and the Environmental Protection Agency’s 2023 Climate Change and Children’s Health and Well-Being report projects that an increase of 2° C in global warming will result in an additional 34,500 pediatric asthma cases and 228,000 allergic rhinitis cases per year, driven largely by predicted increases in ozone and 2.5-µm particulate matter. The report also forecasts an increase in 6240 asthma emergency department visits and 332 additional respiratory hospitalizations per year.
“We know that these associations that we see between climate change exposures and poor respiratory health outcomes in kids are biologically plausible,” Matsui said. “They’re not just correlation without causation. A lot of the mechanisms for how air pollution, allergies, and other factors directly affect the lungs of the airway epithelium have been worked out.”
An Increase in Allergens and Viral Infections
Pediatricians should prepare for anticipated growth in allergens and viral infections. The longer growing seasons mean that pollen seasons will also lengthen. Meanwhile, higher concentrations of carbon dioxide cause individual plants to produce more pollen.
“As the winters get warmer, mice that might not be able to survive during the winter are surviving, and mice reproduce at a very rapid rate,” she said. “The increase in moisture means that dust mites, which absorb their water — they drink by absorbing humidity that’s in the air — will be present in higher concentrations, and their range will expand.”
Fungal and mold exposures are also increasing, not just outdoors but also indoors, “and there are all sorts of allergic and respiratory health consequences of fungal exposure,” Matsui said. As hurricanes and flooding increase, storm damage can also make indoor environments more conducive to fungal and mold growth.
Extreme weather from climate change also affects infrastructure. “When there’s healthcare infrastructure disruption and other infrastructure disruption, it adds to the challenge,” she said. “It compounds all the other threat to health from climate change, so this overall problem of climate change and health is multidimensional and very complicated.”
Then there’s the impact of climate change on respiratory viruses, which are a major driver of asthma exacerbations, Matsui said. The greater variability in daytime temperatures affects environmental reservoirs, transmission patterns, geographical ranges, and seasonality of various respiratory pathogens. The prevalence of respiratory syncytial virus infections, for example, increases during humid periods.
“This is coupled with the fact that the projected increases in air pollution increase susceptibility to respiratory virus infections,” Matsui said. “In fact, climate change and air pollution are inextricably linked.”
Climate Change and Air Pollution
Climate disruption creates extreme weather patterns that then lead to worsening air quality due to high temperatures; heavier precipitation; and more forest fires, droughts, dust storms, thunderstorms, hurricanes, stagnation events, and other extreme weather. Matsui shared a map showing the substantial increase in days with stagnant air since 1973. During stagnation events, air pollution builds up in the atmosphere because of a stable air mass that remains over a region for several days, with low-level winds and no precipitation.
The pollutants can then contribute to rising temperatures. Black carbon particulate matter released from the burning of forests and other biomass absorbs more solar radiation, further contributing to temperature increases. Data from the National Bureau of Economic Research has shown that the US made big strides in reducing air pollution from 2009 through 2016, but it began to reverse in 2016 as severe weather events picked up.
Pediatricians need to be cognizant of the synergistic effect of these different impacts as well. “We oftentimes talk about these problems in a silo, so we may talk about air pollution and health effects, or allergens and health effects, or heat and health effects, but all of these interact with each other and further compound the health effects,” compared to just one of them in isolation, Matsui said.
For example, air pollution increases sensitivity to allergen exposure and increases reaction severity, which disrupts the immune tolerance to allergens. “Heat and air pollution also interact, and the combination of the two is more deadly than either one alone,” she said.
Air pollution from wildfire smoke is also more toxic to the lungs than air pollution from other sources, so if there’s wildfire-based air pollution, the impact on respiratory hospitalizations is significantly greater. Even in places that would not otherwise be at risk for wildfires, the threat remains of air pollution from more distant fires, as New York City experienced from Canadian wildfires last year.
“This is a problem that is not just isolated to the parts of the world where the wildfires are located,” Matsui said.
Moon, who practices in New York City, said he really appreciated Matsui’s perspectives and nuanced advice as a subspecialist “because it’s obvious that the way we deliver healthcare is going to have to change based on climate change.” He hopes to see more subspecialists from other pediatric areas getting involved in looking at climate impacts and providing nuanced advice about changing clinical care similar to the examples Matsui provided.
Air pollution can also be deadly, as a landmark case in the United Kingdom revealed a few years ago when the court ruled that a child’s death from an asthma attack was directly due to air pollution. In addition to causing worse asthma symptoms and exacerbations, air pollution also adds to the risk of developing asthma and impedes lung growth, all of which disproportionately affects disadvantaged and minoritized communities, she said.
Greater Impact on Disadvantaged Populations
Matsui called attention to the equity implications of climate change impacts on health.
“If you have a community that does not have the infrastructure and access to resources, and that same community has a prevalence of asthma that is double that of their more advantaged and white counterparts, then the impacts of climate change are going to be amplified even more,” she said.
For example, a 2019 study found that the biggest predictor of the location of ragweed plants has to do with vacant lots and demolition of housing. Ragweed plants being more common in neighborhoods with vacant lots will disproportionately affect disadvantaged neighborhoods, she said. Another study found in Baltimore that mouse allergens — specifically urine — were a bigger cause of asthma in low-income children than were cockroach allergens.
“It’s important to consider context,” including age, gender and social and behavioral context, she said. “We as pediatricians know that children are particularly vulnerable, and what happens to them has an effect across the lifespan.”
Furthermore, pediatricians are aware that disadvantaged and minoritized communities lack infrastructure; often live in areas with greater air pollution; often have heat islands in their communities without protection, such as tree canopy; and may be at greater flooding risk. “Poverty is also associated with increased vulnerability” because of poorer housing and infrastructure, less education, less access to care, more preexisting health conditions and greater discrimination, she said.
Three Cornerstone Interventions
Interventions fall into three main buckets, Matsui said: mitigation, adaption, and resilience.
“Mitigation means reducing greenhouse gas and air pollution production and trying to enhance sinks for greenhouse gases,” she said. Mitigation strategies primarily occur at the policy level, with improved regulation, treaties, and market-based approaches, such as carbon tax and cap and trade.
Adaptation includes actions that lessen the impact on health and environment, such as infrastructure changes and implementation of air conditioning. Examples of climate change adaptation strategies also mostly come from policy but largely at state and local levels, where individual pediatricians have a greater voice and influence. These can include changes in urban planning to address heat islands, flooding risk, and public transportation’s contribution to air pollution and climate change. It can also include changes in housing regulation and policy and investments in healthcare, such as expanded Medicaid and health insurance and investing in disaster planning and readiness.
“Resilience is a more holistic concept,” Matsui said, “which advocates for system-wide, multilevel changes and involves a range of strategies to enhance social, human, natural, physical, and financial capacities.”
What Pediatricians Can Do
Pediatricians have an important role to play when it comes to climate change and health impacts.
“The first step is sort of understanding the complexity of climate change in terms of its potential health effects, but also being prepared to talk with our patients and their families about it,” Matsui said. “The second step is advocacy.” She drew attention to the February policy statement in Pediatrics that discusses precisely the ways in which pediatricians can leverage their expertise and credibility.
“Pediatricians are ideal advocates with whom to partner and uplift youth and community voices working to advance zero-carbon energy policy and climate justice,” she said. “There are many opportunities to advocate for climate solution policies at the local, state, national, and even international level.”
These roles can include educating elected officials and health insurance entities about the risks that climate change poses to allergies, asthma, and child health more broadly, as well as the benefits of local solutions, including improved air quality, tree canopy, and green space. “There are lots of opportunities to engage with the community, including speaking at public hearings, serving as an expert testimony, and writing letters to the editor,” she said.
The impact of these efforts can be further maximized by working with other healthcare professionals. Lori Byron, MD, a pediatrician from Red Lodge, Montana, who heads the AAP Chapter Climate Advocates program, noted during Q&A that every AAP chapter in the country has climate advocates. She added that the AAP is the first medical board to have climate modules in their maintenance of certification specifically designed to incorporate climate change education into well visits.
Adjusting Clinical Care
Meanwhile, in patient care, Matsui acknowledged it can be frustrating to think about what a massive impact climate has and simultaneously challenging to engage families in discussions about it. However, a wide range of resources are available that can be provided to patients.
“For a patient in front of you, being informed and prepared to talk about it is the first step to being able to assess their climate change risk and provide tailored guidance,” she said. Tailored guidance takes into account the child’s specific health situation and the risks they’re most likely to encounter, such as wildfire smoke, air pollution, longer pollen seasons, environmental allergens, or disruption of infrastructure.
“If I am seeing a patient with asthma who is allergic to a particular pollen, I can anticipate that pollen may be present in higher levels of the future, and that the season for that pollen may be longer,” Matsui said. “So if I’m thinking about allergen immunotherapy for that patient, future risk may be something that would push the conversation and the shared decision-making” from possible consideration to more serious consideration, depending on the child’s age.
“Another example is a patient with asthma, thinking about wildfire risk and having them prepared, because we know from data that wildfire air pollution is going to be worse for that child than pollution from other sources, and there are ways for them to be prepared,” Matsui said. For instance, having an HVAC system with a high-grade air filter (at least a MERV 13) will filter the air better if a wildfire causes smoke to descend over an area. Portable, less expensive HEPA filters are also an option if a family cannot upgrade their system, and wearing an N95 or N95-equivalent mask can also reduce the impact of high air pollution levels.
An example of thinking about the impact of potential infrastructure disruption could be ensuring patients have enough of all their medications if they’re close to running out. “It’s important for them to always have think about their medications and get those refills ahead of a storm,” she said.
Additional Resources
Understanding that pediatricians may not have time to discuss all these issues or have broader conversations about climate change during visits, Matsui highlighted the AAP website of resources on climate change. In addition to resources for pediatricians, such as a basic fact sheet about climate change impacts on children’s health and the technical report that informed the policy statement, the site has multiple resources for families:
- Climate Change Impact: Safeguarding Your Family’s Health and Well-being (video), How to Talk With Children About Climate Change, Climate Change & Children’s Health: AAP Policy Explained, Climate Checkup for Children’s Health: Little Changes With Big Impact, How Climate Change Can Make Children Sick: What Parents Need to Know, Climate Change & Wildfires: Why Kids Are Most at Risk, Climate Change, Extreme Weather & Children: What Families Need to Know, Extreme Heat & Air Pollution: Health Effects on Babies & Pregnant People, and
The following resources can also be helpful to pediatricians and/or families:
- Ready.gov, AirNow, Patient Exposure and the Air Quality Index, Protecting Vulnerable Patient Populations from Climate Hazards: A Referral Guide for Health Professionals from the US Department of Health and Human Services, Low Income Home Energy Assistance Program (LIHEAP), Weatherization Assistance Program, and the Disaster Supplemental Nutrition Assistance Program (D-SNAP)
In some states, Medicaid will provide or cover the cost of air conditioning and/or air filters.
The presentation did not involve external funding. Drs. Matsui and Moon had no disclosures.
A version of this article first appeared on Medscape.com.
ORLANDO — It’s important for pediatricians not only to understand the causes and effects of climate change but also to know how to discuss this issue with families and make risk-based adjustments to their clinical practice based on the individual health and circumstances of each patient. That’s one of the key messages delivered at the annual meeting of the American Academy of Pediatrics (AAP) by Elizabeth C. Matsui, MD, MHS, professor of population health and pediatrics and director of the Center for Health and Environment Education and Research at the University of Texas at Austin Dell Medical School.
“Even though climate change has been here and has been affecting health already for a while, it’s just really impossible to ignore right now,” she told attendees in a session focused on climate change impacts on allergies and asthma. “The challenge is connecting the dots between something that is much larger, or feels much larger, than the patient and the family that’s in front of you.”
The reality, however, is that climate change is now impacting patients’ health on an individual level, and pediatricians have a responsibility to understand how that’s happening and to help their families prepare for it.
“From the perspective of someone who went into medicine to practice and take care of the individual patient, I think it has been more difficult to connect those dots, and for the people in this room, it’s our job to connect those dots,” Matsui said. She also acknowledged that many of the solutions are frustratingly limited to the policy level and challenging to implement, “but it doesn’t mean that we can’t make a difference for the patients who are in front of us.”
Charles Moon, MD, a pediatrician and Pediatric Environmental Health Fellow at the Children’s Environmental Health Center, Icahn School of Medicine at Mount Sinai, New York City, found the talk particularly helpful in providing information about both the broader issue and what it means on a local practice level.
“The biggest takeaway is that more people and more pediatricians are tuning in to this issue and realizing the dangers,” Moon said. “It’s clear that a larger community is forming around this, and I think we are at the cusp where more and more people will be coming in. We are really focusing on taking all the data and trying to figure out solutions. I think the solutions orientation is the most important part.”
Understanding the Big Picture
Matsui opened with a general discussion of the human causes of climate change and the effects on a global scale presently and in the future. For example, over the past 800,000 years, carbon dioxide levels have never been above 300 ppm, but they surpassed that threshold in 1911 and have reached 420 ppm today. The trapping of heat in Earth’s atmosphere caused by the increase in carbon dioxide and other greenhouse gases is leading to multiple phenomena that impact health, such as longer growing seasons; increased droughts, heat waves, and wildfire seasons; and higher temperatures. These changes, in turn, affect allergens and asthma.
, Matsui said. Childhood is a critical period for lung and immune development, and the Environmental Protection Agency’s 2023 Climate Change and Children’s Health and Well-Being report projects that an increase of 2° C in global warming will result in an additional 34,500 pediatric asthma cases and 228,000 allergic rhinitis cases per year, driven largely by predicted increases in ozone and 2.5-µm particulate matter. The report also forecasts an increase in 6240 asthma emergency department visits and 332 additional respiratory hospitalizations per year.
“We know that these associations that we see between climate change exposures and poor respiratory health outcomes in kids are biologically plausible,” Matsui said. “They’re not just correlation without causation. A lot of the mechanisms for how air pollution, allergies, and other factors directly affect the lungs of the airway epithelium have been worked out.”
An Increase in Allergens and Viral Infections
Pediatricians should prepare for anticipated growth in allergens and viral infections. The longer growing seasons mean that pollen seasons will also lengthen. Meanwhile, higher concentrations of carbon dioxide cause individual plants to produce more pollen.
“As the winters get warmer, mice that might not be able to survive during the winter are surviving, and mice reproduce at a very rapid rate,” she said. “The increase in moisture means that dust mites, which absorb their water — they drink by absorbing humidity that’s in the air — will be present in higher concentrations, and their range will expand.”
Fungal and mold exposures are also increasing, not just outdoors but also indoors, “and there are all sorts of allergic and respiratory health consequences of fungal exposure,” Matsui said. As hurricanes and flooding increase, storm damage can also make indoor environments more conducive to fungal and mold growth.
Extreme weather from climate change also affects infrastructure. “When there’s healthcare infrastructure disruption and other infrastructure disruption, it adds to the challenge,” she said. “It compounds all the other threat to health from climate change, so this overall problem of climate change and health is multidimensional and very complicated.”
Then there’s the impact of climate change on respiratory viruses, which are a major driver of asthma exacerbations, Matsui said. The greater variability in daytime temperatures affects environmental reservoirs, transmission patterns, geographical ranges, and seasonality of various respiratory pathogens. The prevalence of respiratory syncytial virus infections, for example, increases during humid periods.
“This is coupled with the fact that the projected increases in air pollution increase susceptibility to respiratory virus infections,” Matsui said. “In fact, climate change and air pollution are inextricably linked.”
Climate Change and Air Pollution
Climate disruption creates extreme weather patterns that then lead to worsening air quality due to high temperatures; heavier precipitation; and more forest fires, droughts, dust storms, thunderstorms, hurricanes, stagnation events, and other extreme weather. Matsui shared a map showing the substantial increase in days with stagnant air since 1973. During stagnation events, air pollution builds up in the atmosphere because of a stable air mass that remains over a region for several days, with low-level winds and no precipitation.
The pollutants can then contribute to rising temperatures. Black carbon particulate matter released from the burning of forests and other biomass absorbs more solar radiation, further contributing to temperature increases. Data from the National Bureau of Economic Research has shown that the US made big strides in reducing air pollution from 2009 through 2016, but it began to reverse in 2016 as severe weather events picked up.
Pediatricians need to be cognizant of the synergistic effect of these different impacts as well. “We oftentimes talk about these problems in a silo, so we may talk about air pollution and health effects, or allergens and health effects, or heat and health effects, but all of these interact with each other and further compound the health effects,” compared to just one of them in isolation, Matsui said.
For example, air pollution increases sensitivity to allergen exposure and increases reaction severity, which disrupts the immune tolerance to allergens. “Heat and air pollution also interact, and the combination of the two is more deadly than either one alone,” she said.
Air pollution from wildfire smoke is also more toxic to the lungs than air pollution from other sources, so if there’s wildfire-based air pollution, the impact on respiratory hospitalizations is significantly greater. Even in places that would not otherwise be at risk for wildfires, the threat remains of air pollution from more distant fires, as New York City experienced from Canadian wildfires last year.
“This is a problem that is not just isolated to the parts of the world where the wildfires are located,” Matsui said.
Moon, who practices in New York City, said he really appreciated Matsui’s perspectives and nuanced advice as a subspecialist “because it’s obvious that the way we deliver healthcare is going to have to change based on climate change.” He hopes to see more subspecialists from other pediatric areas getting involved in looking at climate impacts and providing nuanced advice about changing clinical care similar to the examples Matsui provided.
Air pollution can also be deadly, as a landmark case in the United Kingdom revealed a few years ago when the court ruled that a child’s death from an asthma attack was directly due to air pollution. In addition to causing worse asthma symptoms and exacerbations, air pollution also adds to the risk of developing asthma and impedes lung growth, all of which disproportionately affects disadvantaged and minoritized communities, she said.
Greater Impact on Disadvantaged Populations
Matsui called attention to the equity implications of climate change impacts on health.
“If you have a community that does not have the infrastructure and access to resources, and that same community has a prevalence of asthma that is double that of their more advantaged and white counterparts, then the impacts of climate change are going to be amplified even more,” she said.
For example, a 2019 study found that the biggest predictor of the location of ragweed plants has to do with vacant lots and demolition of housing. Ragweed plants being more common in neighborhoods with vacant lots will disproportionately affect disadvantaged neighborhoods, she said. Another study found in Baltimore that mouse allergens — specifically urine — were a bigger cause of asthma in low-income children than were cockroach allergens.
“It’s important to consider context,” including age, gender and social and behavioral context, she said. “We as pediatricians know that children are particularly vulnerable, and what happens to them has an effect across the lifespan.”
Furthermore, pediatricians are aware that disadvantaged and minoritized communities lack infrastructure; often live in areas with greater air pollution; often have heat islands in their communities without protection, such as tree canopy; and may be at greater flooding risk. “Poverty is also associated with increased vulnerability” because of poorer housing and infrastructure, less education, less access to care, more preexisting health conditions and greater discrimination, she said.
Three Cornerstone Interventions
Interventions fall into three main buckets, Matsui said: mitigation, adaption, and resilience.
“Mitigation means reducing greenhouse gas and air pollution production and trying to enhance sinks for greenhouse gases,” she said. Mitigation strategies primarily occur at the policy level, with improved regulation, treaties, and market-based approaches, such as carbon tax and cap and trade.
Adaptation includes actions that lessen the impact on health and environment, such as infrastructure changes and implementation of air conditioning. Examples of climate change adaptation strategies also mostly come from policy but largely at state and local levels, where individual pediatricians have a greater voice and influence. These can include changes in urban planning to address heat islands, flooding risk, and public transportation’s contribution to air pollution and climate change. It can also include changes in housing regulation and policy and investments in healthcare, such as expanded Medicaid and health insurance and investing in disaster planning and readiness.
“Resilience is a more holistic concept,” Matsui said, “which advocates for system-wide, multilevel changes and involves a range of strategies to enhance social, human, natural, physical, and financial capacities.”
What Pediatricians Can Do
Pediatricians have an important role to play when it comes to climate change and health impacts.
“The first step is sort of understanding the complexity of climate change in terms of its potential health effects, but also being prepared to talk with our patients and their families about it,” Matsui said. “The second step is advocacy.” She drew attention to the February policy statement in Pediatrics that discusses precisely the ways in which pediatricians can leverage their expertise and credibility.
“Pediatricians are ideal advocates with whom to partner and uplift youth and community voices working to advance zero-carbon energy policy and climate justice,” she said. “There are many opportunities to advocate for climate solution policies at the local, state, national, and even international level.”
These roles can include educating elected officials and health insurance entities about the risks that climate change poses to allergies, asthma, and child health more broadly, as well as the benefits of local solutions, including improved air quality, tree canopy, and green space. “There are lots of opportunities to engage with the community, including speaking at public hearings, serving as an expert testimony, and writing letters to the editor,” she said.
The impact of these efforts can be further maximized by working with other healthcare professionals. Lori Byron, MD, a pediatrician from Red Lodge, Montana, who heads the AAP Chapter Climate Advocates program, noted during Q&A that every AAP chapter in the country has climate advocates. She added that the AAP is the first medical board to have climate modules in their maintenance of certification specifically designed to incorporate climate change education into well visits.
Adjusting Clinical Care
Meanwhile, in patient care, Matsui acknowledged it can be frustrating to think about what a massive impact climate has and simultaneously challenging to engage families in discussions about it. However, a wide range of resources are available that can be provided to patients.
“For a patient in front of you, being informed and prepared to talk about it is the first step to being able to assess their climate change risk and provide tailored guidance,” she said. Tailored guidance takes into account the child’s specific health situation and the risks they’re most likely to encounter, such as wildfire smoke, air pollution, longer pollen seasons, environmental allergens, or disruption of infrastructure.
“If I am seeing a patient with asthma who is allergic to a particular pollen, I can anticipate that pollen may be present in higher levels of the future, and that the season for that pollen may be longer,” Matsui said. “So if I’m thinking about allergen immunotherapy for that patient, future risk may be something that would push the conversation and the shared decision-making” from possible consideration to more serious consideration, depending on the child’s age.
“Another example is a patient with asthma, thinking about wildfire risk and having them prepared, because we know from data that wildfire air pollution is going to be worse for that child than pollution from other sources, and there are ways for them to be prepared,” Matsui said. For instance, having an HVAC system with a high-grade air filter (at least a MERV 13) will filter the air better if a wildfire causes smoke to descend over an area. Portable, less expensive HEPA filters are also an option if a family cannot upgrade their system, and wearing an N95 or N95-equivalent mask can also reduce the impact of high air pollution levels.
An example of thinking about the impact of potential infrastructure disruption could be ensuring patients have enough of all their medications if they’re close to running out. “It’s important for them to always have think about their medications and get those refills ahead of a storm,” she said.
Additional Resources
Understanding that pediatricians may not have time to discuss all these issues or have broader conversations about climate change during visits, Matsui highlighted the AAP website of resources on climate change. In addition to resources for pediatricians, such as a basic fact sheet about climate change impacts on children’s health and the technical report that informed the policy statement, the site has multiple resources for families:
- Climate Change Impact: Safeguarding Your Family’s Health and Well-being (video), How to Talk With Children About Climate Change, Climate Change & Children’s Health: AAP Policy Explained, Climate Checkup for Children’s Health: Little Changes With Big Impact, How Climate Change Can Make Children Sick: What Parents Need to Know, Climate Change & Wildfires: Why Kids Are Most at Risk, Climate Change, Extreme Weather & Children: What Families Need to Know, Extreme Heat & Air Pollution: Health Effects on Babies & Pregnant People, and
The following resources can also be helpful to pediatricians and/or families:
- Ready.gov, AirNow, Patient Exposure and the Air Quality Index, Protecting Vulnerable Patient Populations from Climate Hazards: A Referral Guide for Health Professionals from the US Department of Health and Human Services, Low Income Home Energy Assistance Program (LIHEAP), Weatherization Assistance Program, and the Disaster Supplemental Nutrition Assistance Program (D-SNAP)
In some states, Medicaid will provide or cover the cost of air conditioning and/or air filters.
The presentation did not involve external funding. Drs. Matsui and Moon had no disclosures.
A version of this article first appeared on Medscape.com.
FROM AAP 2024
High Levels of Indoor Pollutants Promote Wheezing in Preschoolers
“There is an increasing concern about of the role of Indoor Air Quality (IAQ) in development of respiratory disorders like asthma, especially in children whose immune system is under development, and they are more vulnerable to the effects of poor air quality,” lead author Ioannis Sakellaris, PhD, of Université Paris-Saclay, Villejuif, France, said in an interview. However, the effects of specific pollutants on the health of young children in daycare settings has not been examined, he said.
In a presentation at the European Respiratory Society Congress, Sakellaris reviewed data from the French CRESPI cohort study, an epidemiological study of the impact of exposures to disinfectants and cleaning products on workers and children in daycare centers in France.
The study population included 532 children (47.4% girls) with a mean age of 22.3 months (aged 3 months to 4 years) in 106 daycare centers. A total of 171 children reportedly experienced at least one episode of wheezing since birth.
A total of 67 VOCs were measured during one day, and concentrations were studied in four categories based on quartiles. The researchers evaluated three child wheezing outcomes based on parental questionnaires: Ever wheeze since birth, recurrent wheeze (≥ 3 times since birth), and ever wheeze with inhaled corticosteroid use. The researchers adjusted for factors including child age and parental smoking status and education level.
Overall, ever wheezing was significantly associated with higher concentrations of 1,2,4-trimethylbenzene (odds ratio [OR] for Q4 vs Q1, 1.56; P = .08 for trend), 1-methoxy-2-propylacetate (OR, 1.62; P = .01), decamethylcyclopentasiloxane (OR, 2.12; P = .004), and methylisobutylcetone (OR, 1.85; P < .001).
The results emphasize the significant role of IAQ in respiratory health, said Sakellaris. “Further efforts to reduce pollutant concentrations and limit sources are needed,” he said. In addition, more studies on the combined effect of multiple VOCs are necessary for a deeper understanding of the complex relations between IAQ and children’s respiratory health, he said.
Pay Attention to Indoor Pollutants
“Since the COVID-19 pandemic, the use of cleaning products and disinfectants has exploded,” Alexander S. Rabin, MD, of the University of Michigan, Ann Arbor, Michigan, said in an interview. Although many of these cleaning agents contain chemicals, including VOCs, that are known respiratory irritants, little is known about the relationship between VOCs and children’s respiratory outcomes in daycare settings, said Rabin, who was not involved in the study.
“I was struck by the wide array of VOCs detected in daycare settings,” Rabin said. However, the relationship to childhood wheeze was not entirely surprising as the VOCs included the known irritants benzene and toluene, he added.
The results suggest that exposure to VOCs, not only in cleaning agents but also building materials and other consumer products in daycare settings, may be associated with an increased risk for wheeze in children, said Rabin.
However, “it is important to know more about confounding variables, including concurrent rates of respiratory infection that are common among children,” said Rabin. “As the authors highlight, further work on the compound effects of multiple pollutants would be of interest. Lastly, it would be helpful to clearly identify the most common sources of VOCs that place children at greatest risk for wheeze, so that appropriate steps can be taken to mitigate risk,” he said.
The original CRESPI cohort study was supported by ANSES, ADEME, Fondation de France, and ARS Ile-de-France. Sakellaris and Rabin had no financial conflicts to disclose.
A version of this article appeared on Medscape.com.
“There is an increasing concern about of the role of Indoor Air Quality (IAQ) in development of respiratory disorders like asthma, especially in children whose immune system is under development, and they are more vulnerable to the effects of poor air quality,” lead author Ioannis Sakellaris, PhD, of Université Paris-Saclay, Villejuif, France, said in an interview. However, the effects of specific pollutants on the health of young children in daycare settings has not been examined, he said.
In a presentation at the European Respiratory Society Congress, Sakellaris reviewed data from the French CRESPI cohort study, an epidemiological study of the impact of exposures to disinfectants and cleaning products on workers and children in daycare centers in France.
The study population included 532 children (47.4% girls) with a mean age of 22.3 months (aged 3 months to 4 years) in 106 daycare centers. A total of 171 children reportedly experienced at least one episode of wheezing since birth.
A total of 67 VOCs were measured during one day, and concentrations were studied in four categories based on quartiles. The researchers evaluated three child wheezing outcomes based on parental questionnaires: Ever wheeze since birth, recurrent wheeze (≥ 3 times since birth), and ever wheeze with inhaled corticosteroid use. The researchers adjusted for factors including child age and parental smoking status and education level.
Overall, ever wheezing was significantly associated with higher concentrations of 1,2,4-trimethylbenzene (odds ratio [OR] for Q4 vs Q1, 1.56; P = .08 for trend), 1-methoxy-2-propylacetate (OR, 1.62; P = .01), decamethylcyclopentasiloxane (OR, 2.12; P = .004), and methylisobutylcetone (OR, 1.85; P < .001).
The results emphasize the significant role of IAQ in respiratory health, said Sakellaris. “Further efforts to reduce pollutant concentrations and limit sources are needed,” he said. In addition, more studies on the combined effect of multiple VOCs are necessary for a deeper understanding of the complex relations between IAQ and children’s respiratory health, he said.
Pay Attention to Indoor Pollutants
“Since the COVID-19 pandemic, the use of cleaning products and disinfectants has exploded,” Alexander S. Rabin, MD, of the University of Michigan, Ann Arbor, Michigan, said in an interview. Although many of these cleaning agents contain chemicals, including VOCs, that are known respiratory irritants, little is known about the relationship between VOCs and children’s respiratory outcomes in daycare settings, said Rabin, who was not involved in the study.
“I was struck by the wide array of VOCs detected in daycare settings,” Rabin said. However, the relationship to childhood wheeze was not entirely surprising as the VOCs included the known irritants benzene and toluene, he added.
The results suggest that exposure to VOCs, not only in cleaning agents but also building materials and other consumer products in daycare settings, may be associated with an increased risk for wheeze in children, said Rabin.
However, “it is important to know more about confounding variables, including concurrent rates of respiratory infection that are common among children,” said Rabin. “As the authors highlight, further work on the compound effects of multiple pollutants would be of interest. Lastly, it would be helpful to clearly identify the most common sources of VOCs that place children at greatest risk for wheeze, so that appropriate steps can be taken to mitigate risk,” he said.
The original CRESPI cohort study was supported by ANSES, ADEME, Fondation de France, and ARS Ile-de-France. Sakellaris and Rabin had no financial conflicts to disclose.
A version of this article appeared on Medscape.com.
“There is an increasing concern about of the role of Indoor Air Quality (IAQ) in development of respiratory disorders like asthma, especially in children whose immune system is under development, and they are more vulnerable to the effects of poor air quality,” lead author Ioannis Sakellaris, PhD, of Université Paris-Saclay, Villejuif, France, said in an interview. However, the effects of specific pollutants on the health of young children in daycare settings has not been examined, he said.
In a presentation at the European Respiratory Society Congress, Sakellaris reviewed data from the French CRESPI cohort study, an epidemiological study of the impact of exposures to disinfectants and cleaning products on workers and children in daycare centers in France.
The study population included 532 children (47.4% girls) with a mean age of 22.3 months (aged 3 months to 4 years) in 106 daycare centers. A total of 171 children reportedly experienced at least one episode of wheezing since birth.
A total of 67 VOCs were measured during one day, and concentrations were studied in four categories based on quartiles. The researchers evaluated three child wheezing outcomes based on parental questionnaires: Ever wheeze since birth, recurrent wheeze (≥ 3 times since birth), and ever wheeze with inhaled corticosteroid use. The researchers adjusted for factors including child age and parental smoking status and education level.
Overall, ever wheezing was significantly associated with higher concentrations of 1,2,4-trimethylbenzene (odds ratio [OR] for Q4 vs Q1, 1.56; P = .08 for trend), 1-methoxy-2-propylacetate (OR, 1.62; P = .01), decamethylcyclopentasiloxane (OR, 2.12; P = .004), and methylisobutylcetone (OR, 1.85; P < .001).
The results emphasize the significant role of IAQ in respiratory health, said Sakellaris. “Further efforts to reduce pollutant concentrations and limit sources are needed,” he said. In addition, more studies on the combined effect of multiple VOCs are necessary for a deeper understanding of the complex relations between IAQ and children’s respiratory health, he said.
Pay Attention to Indoor Pollutants
“Since the COVID-19 pandemic, the use of cleaning products and disinfectants has exploded,” Alexander S. Rabin, MD, of the University of Michigan, Ann Arbor, Michigan, said in an interview. Although many of these cleaning agents contain chemicals, including VOCs, that are known respiratory irritants, little is known about the relationship between VOCs and children’s respiratory outcomes in daycare settings, said Rabin, who was not involved in the study.
“I was struck by the wide array of VOCs detected in daycare settings,” Rabin said. However, the relationship to childhood wheeze was not entirely surprising as the VOCs included the known irritants benzene and toluene, he added.
The results suggest that exposure to VOCs, not only in cleaning agents but also building materials and other consumer products in daycare settings, may be associated with an increased risk for wheeze in children, said Rabin.
However, “it is important to know more about confounding variables, including concurrent rates of respiratory infection that are common among children,” said Rabin. “As the authors highlight, further work on the compound effects of multiple pollutants would be of interest. Lastly, it would be helpful to clearly identify the most common sources of VOCs that place children at greatest risk for wheeze, so that appropriate steps can be taken to mitigate risk,” he said.
The original CRESPI cohort study was supported by ANSES, ADEME, Fondation de France, and ARS Ile-de-France. Sakellaris and Rabin had no financial conflicts to disclose.
A version of this article appeared on Medscape.com.
FROM ERS 2024
Maternal Immunization to Prevent Serious Respiratory Illness
Editor’s Note: Sadly, this is the last column in the Master Class Obstetrics series. This award-winning column has been part of Ob.Gyn. News for 20 years. The deep discussion of cutting-edge topics in obstetrics by specialists and researchers will be missed as will the leadership and curation of topics by Dr. E. Albert Reece.
Introduction: The Need for Increased Vigilance About Maternal Immunization
Viruses are becoming increasingly prevalent in our world and the consequences of viral infections are implicated in a growing number of disease states. It is well established that certain cancers are caused by viruses and it is increasingly evident that viral infections can trigger the development of chronic illness. In pregnant women, viruses such as cytomegalovirus can cause infection in utero and lead to long-term impairments for the baby.
Likewise, it appears that the virulence of viruses is increasing, whether it be the respiratory syncytial virus (RSV) in children or the severe acute respiratory syndrome (SARS) coronaviruses in adults. Clearly, our environment is changing, with increases in population growth and urbanization, for instance, and an intensification of climate change and its effects. Viruses are part of this changing background.
Vaccines are our most powerful tool to protect people of all ages against viral threats, and fortunately, we benefit from increasing expertise in vaccinology. Since 1974, the University of Maryland School of Medicine has a Center for Vaccine Development and Global Health that has conducted research on vaccines to defend against the Zika virus, H1N1, Ebola, and SARS-CoV-2.
We’re not alone. Other vaccinology centers across the country — as well as the National Institutes of Health at the national level, through its National Institute of Allergy and Infectious Diseases — are doing research and developing vaccines to combat viral diseases.
In this column, we are focused on viral diseases in pregnancy and the role that vaccines can play in preventing serious respiratory illness in mothers and their newborns. I have invited Laura E. Riley, MD, the Given Foundation Professor and Chair of Obstetrics and Gynecology at Weill Cornell Medicine, to address the importance of maternal immunization and how we can best counsel our patients and improve immunization rates.
As Dr. Riley explains, we are in a new era, and it behooves us all to be more vigilant about recommending vaccines, combating misperceptions, addressing patients’ knowledge gaps, and administering vaccines whenever possible.
Dr. Reece is the former Dean of Medicine & University Executive VP, and The Distinguished University and Endowed Professor & Director of the Center for Advanced Research Training and Innovation (CARTI) at the University of Maryland School of Medicine, as well as senior scientist at the Center for Birth Defects Research.
The alarming decline in maternal immunization rates that occurred in the wake of the COVID-19 pandemic means that, now more than ever, we must fully embrace our responsibility to recommend immunizations in pregnancy and to communicate what is known about their efficacy and safety. Data show that vaccination rates drop when we do not offer vaccines in our offices, so whenever possible, we should administer them as well.
The ob.gyn. is the patient’s most trusted person in pregnancy. When patients decline or express hesitancy about vaccines, it is incumbent upon us to ask why. Oftentimes, we can identify areas in which patients lack knowledge or have misperceptions and we can successfully educate the patient or change their perspective or misunderstanding concerning the importance of vaccination for themselves and their babies. (See Table 1.) We can also successfully address concerns about safety.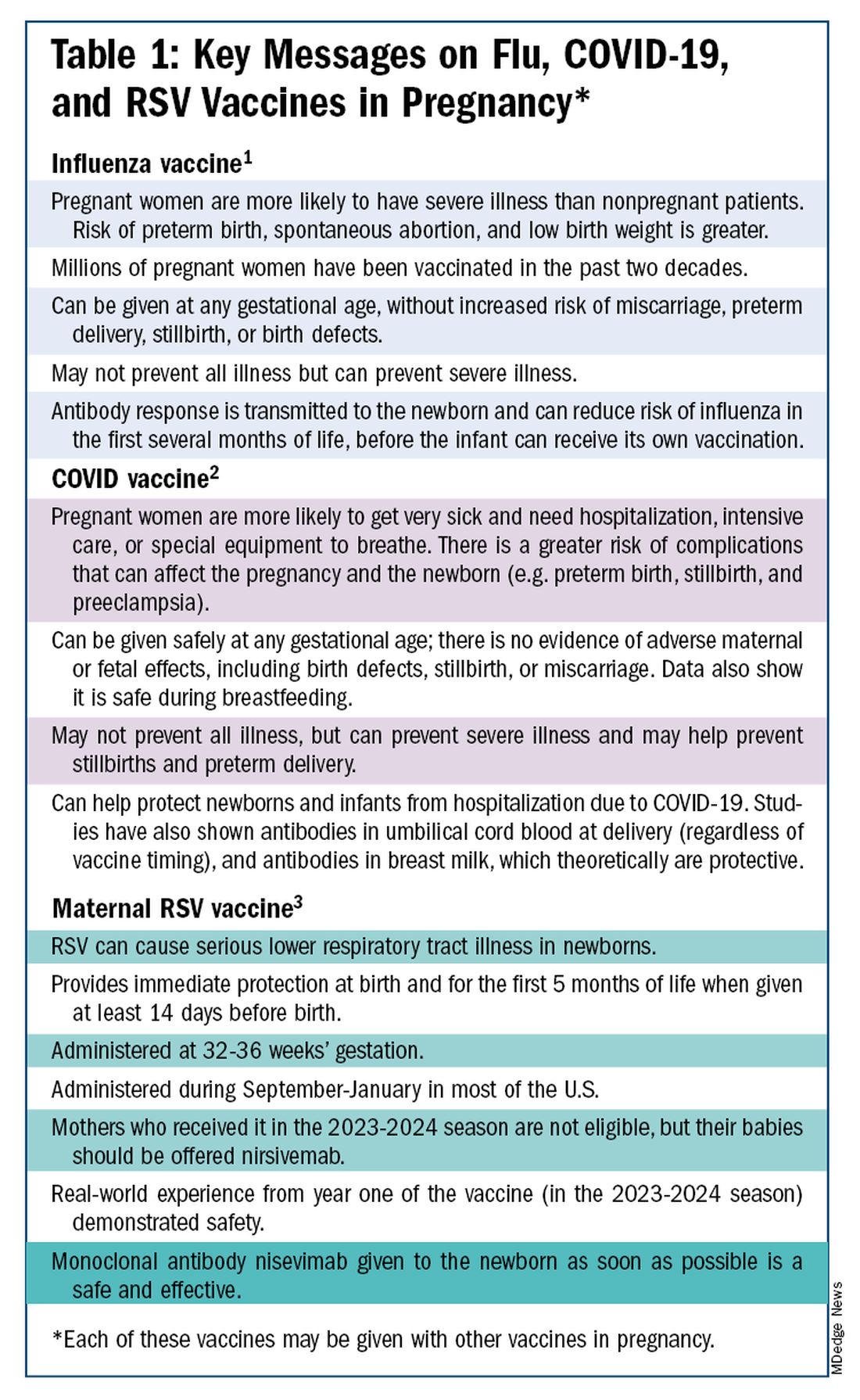
The safety of COVID-19 vaccinations in pregnancy is now backed by several years of data from multiple studies showing no increase in birth defects, preterm delivery, miscarriage, or stillbirth.
Data also show that pregnant patients are more likely than patients who are not pregnant to need hospitalization and intensive care when infected with SARS-CoV-2 and are at risk of having complications that can affect pregnancy and the newborn, including preterm birth and stillbirth. Vaccination has been shown to reduce the risk of severe illness and the risk of such adverse obstetrical outcomes, in addition to providing protection for the infant early on.
Similarly, influenza has long been more likely to be severe in pregnant patients, with an increased risk of poor obstetrical outcomes. Vaccines similarly provide “two for one protection,” protecting both mother and baby, and are, of course, backed by many years of safety and efficacy data.
With the new maternal respiratory syncytial virus (RSV) vaccine, now in its second year of availability, the goal is to protect the baby from RSV-caused serious lower respiratory tract illness. The illness has contributed to tens of thousands of annual hospitalizations and up to several hundred deaths every year in children younger than 5 years — particularly in those under age 6 months.
The RSV monoclonal antibody nirsevimab is available for the newborn as an alternative to maternal immunization but the maternal vaccine is optimal in that it will provide immediate rather than delayed protection for the newborn. The maternal vaccine is recommended during weeks 32-36 of pregnancy in mothers who were not vaccinated during last year’s RSV season. With real-world experience from year one, the available safety data are reassuring.
Counseling About Influenza and COVID-19 Vaccination
The COVID-19 pandemic took a toll on vaccination interest/receptivity broadly in pregnant and nonpregnant people. Among pregnant individuals, influenza vaccination coverage declined from 71% in the 2019-2020 influenza season to 56% in the 2021-2022 season, according to data from the Centers for Disease Control and Prevention’s Vaccine Safety Datalink.4 Coverage for the 2022-2023 and 2023-2024 influenza seasons was even worse: well under 50%.5
Fewer pregnant women have received updated COVID-19 vaccines. Only 13% of pregnant persons overall received the updated 2023-2024 COVID-19 booster vaccine (through March 30, 2024), according to the CDC.6
Maternal immunization for influenza has been recommended in the United States since 2004 (part of the recommendation that everyone over the age of 6 months receive an annual flu vaccine), and flu vaccines have been given to millions of pregnant women, but the H1N1 pandemic of 2009 reinforced its value as a priority for prenatal care. Most of the women who became severely ill from the H1N1 virus were young and healthy, without co-existing conditions known to increase risk.7
It became clearer during the H1N1 pandemic that pregnancy itself — which is associated with physiologic changes such as decreased lung capacity, increased nasal congestion and changes in the immune system – is its own significant risk factor for severe illness from the influenza virus. This increased risk applies to COVID-19 as well.
As COVID-19 has become endemic, with hospitalizations and deaths not reaching the levels of previous surges — and with mask-wearing and other preventive measures having declined — patients understandably have become more complacent. Some patients are vaccine deniers, but in my practice, these patients are a much smaller group than those who believe COVID-19 “is no big deal,” especially if they have had infections recently.
This is why it’s important to actively listen to concerns and to ask patients who decline a vaccination why they are hesitant. Blanket messages about vaccine efficacy and safety are the first step, but individualized, more pointed conversations based on the patient’s personal experiences and beliefs have become increasingly important.
I routinely tell pregnant patients about the risks of COVID-19 and I explain that it has been difficult to predict who will develop severe illness. Sometimes more conversation is needed. For those who are still hesitant or who tell me they feel protected by a recent infection, for instance, I provide more detail on the unique risks of pregnancy — the fact that “pregnancy is different” — and that natural immunity wanes while the protection afforded by immunization is believed to last longer. Many women are also concerned about the safety of the COVID-19 vaccine, so having safety data at your fingertips is helpful. (See Table 2.)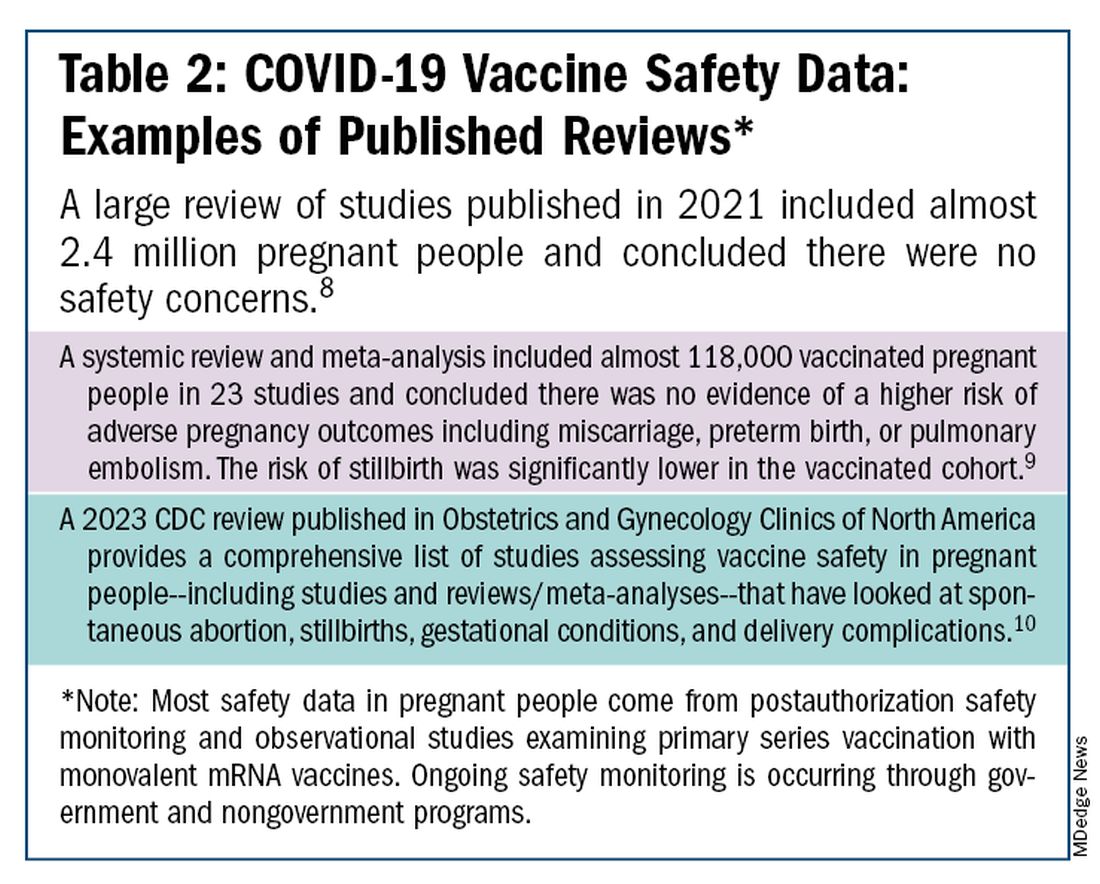
The fact that influenza and COVID-19 vaccination protect the newborn as well as the mother is something that I find is underappreciated by many patients. Explaining that infants likely benefit from the passage of antibodies across the placenta should be part of patient counseling.
Counseling About RSV Vaccination
Importantly, for the 2024-2025 RSV season, the maternal RSV vaccine (Abrysvo, Pfizer) is recommended only for pregnant women who did not receive the vaccine during the 2023-2024 season. When more research is done and more data are obtained showing how long the immune response persists post vaccination, it may be that the US Food and Drug Administration (FDA) will approve the maternal RSV vaccine for use in every pregnancy.
The later timing of the vaccination recommendation — 32-36 weeks’ gestation — reflects a conservative approach taken by the FDA in response to data from one of the pivotal trials showing a numerical trend toward more preterm deliveries among vaccinated compared with unvaccinated patients. This imbalance in the original trial, which administered the vaccine during 24-36 weeks of gestation, was seen only in low-income countries with no temporal association, however.
In our experience at two Weill Cornell Medical College–associated hospitals we did not see this trend. Our cohort study of almost 3000 pregnant patients who delivered at 32 weeks’ gestation or later found no increased risk of preterm birth among the 35% of patients who received the RSV vaccine during the 2023-2024 RSV season. We also did not see any difference in preeclampsia, in contrast with original trial data that showed a signal for increased risk.11
When fewer than 2 weeks have elapsed between maternal vaccination and delivery, the monoclonal antibody nirsevimab is recommended for the newborn — ideally before the newborn leaves the hospital. Nirsevimab is also recommended for newborns of mothers who decline vaccination or were not candidates (e.g. vaccinated in a previous pregnancy), or when there is concern about the adequacy of the maternal immune response to the vaccine (e.g. in cases of immunosuppression).
While there was a limited supply of the monoclonal antibody last year, limitations are not expected this year, especially after October.
The ultimate goal is that patients choose the vaccine or the immunoglobulin, given the severity of RSV disease. Patient preferences should be considered. However, given that it takes 2 weeks after vaccination for protection to build up, I stress to patients that if they’ve vaccinated themselves, their newborn will leave the hospital with protection. If nirsevimab is relied upon, I explain, their newborn may not be protected for some period of time.
Take-home Messages
- When patients decline or are hesitant about vaccines, ask why. Listen actively, and work to correct misperceptions and knowledge gaps.
- Whenever possible, offer vaccines in your practice. Vaccination rates drop when this does not occur.
- COVID-vaccine safety is backed by many studies showing no increase in birth defects, preterm delivery, miscarriage, or stillbirth.
- Pregnant women are more likely to have severe illness from the influenza and SARS-CoV-2 viruses. Vaccines can prevent severe illness and can protect the newborn as well as the mother.
- Recommend/administer the maternal RSV vaccine at 32-36 weeks’ gestation in women who did not receive the vaccine in the 2023-2024 season. If mothers aren’t eligible their babies should be offered nirsevimab.
Dr. Riley is the Given Foundation Professor and Chair of Obstetrics and Gynecology at Weill Cornell Medicine and the obstetrician and gynecologist-in-chief at New York Presbyterian Hospital. She disclosed that she has provided one-time consultations to Pfizer (Abrysvo RSV vaccine) and GSK (cytomegalovirus vaccine), and is providing consultant education on CMV for Moderna. She is chair of ACOG’s task force on immunization and emerging infectious diseases, serves on the medical advisory board for MAVEN, and serves as an editor or editorial board member for several medical publications.
References
1. ACOG Committee Opinion No. 741: Maternal Immunization. Obstet Gynecol. 2018;131(6):e214-e217.
2. Centers for Disease Control and Prevention. COVID-19 Vaccination for People Who are Pregnant or Breastfeeding. https://www.cdc.gov/covid/vaccines/pregnant-or-breastfeeding.html.
3. ACOG Practice Advisory on Maternal Respiratory Syncytial Virus Vaccination, September 2023. (Updated August 2024).4. Irving S et al. Open Forum Infect Dis. 2023;10(Suppl 2):ofad500.1002.
5. Flu Vaccination Dashboard, CDC, National Center for Immunization and Respiratory Diseases.
6. Weekly COVID-19 Vaccination Dashboard, CDC. https://www.cdc.gov/covidvaxview/weekly-dashboard/index.html
7. Louie JK et al. N Engl J Med. 2010;362:27-35. 8. Ciapponi A et al. Vaccine. 2021;39(40):5891-908.
9. Prasad S et al. Nature Communications. 2022;13:2414. 10. Fleming-Dutra KE et al. Obstet Gynecol Clin North Am 2023;50(2):279-97. 11. Mouen S et al. JAMA Network Open 2024;7(7):e2419268.
Editor’s Note: Sadly, this is the last column in the Master Class Obstetrics series. This award-winning column has been part of Ob.Gyn. News for 20 years. The deep discussion of cutting-edge topics in obstetrics by specialists and researchers will be missed as will the leadership and curation of topics by Dr. E. Albert Reece.
Introduction: The Need for Increased Vigilance About Maternal Immunization
Viruses are becoming increasingly prevalent in our world and the consequences of viral infections are implicated in a growing number of disease states. It is well established that certain cancers are caused by viruses and it is increasingly evident that viral infections can trigger the development of chronic illness. In pregnant women, viruses such as cytomegalovirus can cause infection in utero and lead to long-term impairments for the baby.
Likewise, it appears that the virulence of viruses is increasing, whether it be the respiratory syncytial virus (RSV) in children or the severe acute respiratory syndrome (SARS) coronaviruses in adults. Clearly, our environment is changing, with increases in population growth and urbanization, for instance, and an intensification of climate change and its effects. Viruses are part of this changing background.
Vaccines are our most powerful tool to protect people of all ages against viral threats, and fortunately, we benefit from increasing expertise in vaccinology. Since 1974, the University of Maryland School of Medicine has a Center for Vaccine Development and Global Health that has conducted research on vaccines to defend against the Zika virus, H1N1, Ebola, and SARS-CoV-2.
We’re not alone. Other vaccinology centers across the country — as well as the National Institutes of Health at the national level, through its National Institute of Allergy and Infectious Diseases — are doing research and developing vaccines to combat viral diseases.
In this column, we are focused on viral diseases in pregnancy and the role that vaccines can play in preventing serious respiratory illness in mothers and their newborns. I have invited Laura E. Riley, MD, the Given Foundation Professor and Chair of Obstetrics and Gynecology at Weill Cornell Medicine, to address the importance of maternal immunization and how we can best counsel our patients and improve immunization rates.
As Dr. Riley explains, we are in a new era, and it behooves us all to be more vigilant about recommending vaccines, combating misperceptions, addressing patients’ knowledge gaps, and administering vaccines whenever possible.
Dr. Reece is the former Dean of Medicine & University Executive VP, and The Distinguished University and Endowed Professor & Director of the Center for Advanced Research Training and Innovation (CARTI) at the University of Maryland School of Medicine, as well as senior scientist at the Center for Birth Defects Research.
The alarming decline in maternal immunization rates that occurred in the wake of the COVID-19 pandemic means that, now more than ever, we must fully embrace our responsibility to recommend immunizations in pregnancy and to communicate what is known about their efficacy and safety. Data show that vaccination rates drop when we do not offer vaccines in our offices, so whenever possible, we should administer them as well.
The ob.gyn. is the patient’s most trusted person in pregnancy. When patients decline or express hesitancy about vaccines, it is incumbent upon us to ask why. Oftentimes, we can identify areas in which patients lack knowledge or have misperceptions and we can successfully educate the patient or change their perspective or misunderstanding concerning the importance of vaccination for themselves and their babies. (See Table 1.) We can also successfully address concerns about safety.
The safety of COVID-19 vaccinations in pregnancy is now backed by several years of data from multiple studies showing no increase in birth defects, preterm delivery, miscarriage, or stillbirth.
Data also show that pregnant patients are more likely than patients who are not pregnant to need hospitalization and intensive care when infected with SARS-CoV-2 and are at risk of having complications that can affect pregnancy and the newborn, including preterm birth and stillbirth. Vaccination has been shown to reduce the risk of severe illness and the risk of such adverse obstetrical outcomes, in addition to providing protection for the infant early on.
Similarly, influenza has long been more likely to be severe in pregnant patients, with an increased risk of poor obstetrical outcomes. Vaccines similarly provide “two for one protection,” protecting both mother and baby, and are, of course, backed by many years of safety and efficacy data.
With the new maternal respiratory syncytial virus (RSV) vaccine, now in its second year of availability, the goal is to protect the baby from RSV-caused serious lower respiratory tract illness. The illness has contributed to tens of thousands of annual hospitalizations and up to several hundred deaths every year in children younger than 5 years — particularly in those under age 6 months.
The RSV monoclonal antibody nirsevimab is available for the newborn as an alternative to maternal immunization but the maternal vaccine is optimal in that it will provide immediate rather than delayed protection for the newborn. The maternal vaccine is recommended during weeks 32-36 of pregnancy in mothers who were not vaccinated during last year’s RSV season. With real-world experience from year one, the available safety data are reassuring.
Counseling About Influenza and COVID-19 Vaccination
The COVID-19 pandemic took a toll on vaccination interest/receptivity broadly in pregnant and nonpregnant people. Among pregnant individuals, influenza vaccination coverage declined from 71% in the 2019-2020 influenza season to 56% in the 2021-2022 season, according to data from the Centers for Disease Control and Prevention’s Vaccine Safety Datalink.4 Coverage for the 2022-2023 and 2023-2024 influenza seasons was even worse: well under 50%.5
Fewer pregnant women have received updated COVID-19 vaccines. Only 13% of pregnant persons overall received the updated 2023-2024 COVID-19 booster vaccine (through March 30, 2024), according to the CDC.6
Maternal immunization for influenza has been recommended in the United States since 2004 (part of the recommendation that everyone over the age of 6 months receive an annual flu vaccine), and flu vaccines have been given to millions of pregnant women, but the H1N1 pandemic of 2009 reinforced its value as a priority for prenatal care. Most of the women who became severely ill from the H1N1 virus were young and healthy, without co-existing conditions known to increase risk.7
It became clearer during the H1N1 pandemic that pregnancy itself — which is associated with physiologic changes such as decreased lung capacity, increased nasal congestion and changes in the immune system – is its own significant risk factor for severe illness from the influenza virus. This increased risk applies to COVID-19 as well.
As COVID-19 has become endemic, with hospitalizations and deaths not reaching the levels of previous surges — and with mask-wearing and other preventive measures having declined — patients understandably have become more complacent. Some patients are vaccine deniers, but in my practice, these patients are a much smaller group than those who believe COVID-19 “is no big deal,” especially if they have had infections recently.
This is why it’s important to actively listen to concerns and to ask patients who decline a vaccination why they are hesitant. Blanket messages about vaccine efficacy and safety are the first step, but individualized, more pointed conversations based on the patient’s personal experiences and beliefs have become increasingly important.
I routinely tell pregnant patients about the risks of COVID-19 and I explain that it has been difficult to predict who will develop severe illness. Sometimes more conversation is needed. For those who are still hesitant or who tell me they feel protected by a recent infection, for instance, I provide more detail on the unique risks of pregnancy — the fact that “pregnancy is different” — and that natural immunity wanes while the protection afforded by immunization is believed to last longer. Many women are also concerned about the safety of the COVID-19 vaccine, so having safety data at your fingertips is helpful. (See Table 2.)
The fact that influenza and COVID-19 vaccination protect the newborn as well as the mother is something that I find is underappreciated by many patients. Explaining that infants likely benefit from the passage of antibodies across the placenta should be part of patient counseling.
Counseling About RSV Vaccination
Importantly, for the 2024-2025 RSV season, the maternal RSV vaccine (Abrysvo, Pfizer) is recommended only for pregnant women who did not receive the vaccine during the 2023-2024 season. When more research is done and more data are obtained showing how long the immune response persists post vaccination, it may be that the US Food and Drug Administration (FDA) will approve the maternal RSV vaccine for use in every pregnancy.
The later timing of the vaccination recommendation — 32-36 weeks’ gestation — reflects a conservative approach taken by the FDA in response to data from one of the pivotal trials showing a numerical trend toward more preterm deliveries among vaccinated compared with unvaccinated patients. This imbalance in the original trial, which administered the vaccine during 24-36 weeks of gestation, was seen only in low-income countries with no temporal association, however.
In our experience at two Weill Cornell Medical College–associated hospitals we did not see this trend. Our cohort study of almost 3000 pregnant patients who delivered at 32 weeks’ gestation or later found no increased risk of preterm birth among the 35% of patients who received the RSV vaccine during the 2023-2024 RSV season. We also did not see any difference in preeclampsia, in contrast with original trial data that showed a signal for increased risk.11
When fewer than 2 weeks have elapsed between maternal vaccination and delivery, the monoclonal antibody nirsevimab is recommended for the newborn — ideally before the newborn leaves the hospital. Nirsevimab is also recommended for newborns of mothers who decline vaccination or were not candidates (e.g. vaccinated in a previous pregnancy), or when there is concern about the adequacy of the maternal immune response to the vaccine (e.g. in cases of immunosuppression).
While there was a limited supply of the monoclonal antibody last year, limitations are not expected this year, especially after October.
The ultimate goal is that patients choose the vaccine or the immunoglobulin, given the severity of RSV disease. Patient preferences should be considered. However, given that it takes 2 weeks after vaccination for protection to build up, I stress to patients that if they’ve vaccinated themselves, their newborn will leave the hospital with protection. If nirsevimab is relied upon, I explain, their newborn may not be protected for some period of time.
Take-home Messages
- When patients decline or are hesitant about vaccines, ask why. Listen actively, and work to correct misperceptions and knowledge gaps.
- Whenever possible, offer vaccines in your practice. Vaccination rates drop when this does not occur.
- COVID-vaccine safety is backed by many studies showing no increase in birth defects, preterm delivery, miscarriage, or stillbirth.
- Pregnant women are more likely to have severe illness from the influenza and SARS-CoV-2 viruses. Vaccines can prevent severe illness and can protect the newborn as well as the mother.
- Recommend/administer the maternal RSV vaccine at 32-36 weeks’ gestation in women who did not receive the vaccine in the 2023-2024 season. If mothers aren’t eligible their babies should be offered nirsevimab.
Dr. Riley is the Given Foundation Professor and Chair of Obstetrics and Gynecology at Weill Cornell Medicine and the obstetrician and gynecologist-in-chief at New York Presbyterian Hospital. She disclosed that she has provided one-time consultations to Pfizer (Abrysvo RSV vaccine) and GSK (cytomegalovirus vaccine), and is providing consultant education on CMV for Moderna. She is chair of ACOG’s task force on immunization and emerging infectious diseases, serves on the medical advisory board for MAVEN, and serves as an editor or editorial board member for several medical publications.
References
1. ACOG Committee Opinion No. 741: Maternal Immunization. Obstet Gynecol. 2018;131(6):e214-e217.
2. Centers for Disease Control and Prevention. COVID-19 Vaccination for People Who are Pregnant or Breastfeeding. https://www.cdc.gov/covid/vaccines/pregnant-or-breastfeeding.html.
3. ACOG Practice Advisory on Maternal Respiratory Syncytial Virus Vaccination, September 2023. (Updated August 2024).4. Irving S et al. Open Forum Infect Dis. 2023;10(Suppl 2):ofad500.1002.
5. Flu Vaccination Dashboard, CDC, National Center for Immunization and Respiratory Diseases.
6. Weekly COVID-19 Vaccination Dashboard, CDC. https://www.cdc.gov/covidvaxview/weekly-dashboard/index.html
7. Louie JK et al. N Engl J Med. 2010;362:27-35. 8. Ciapponi A et al. Vaccine. 2021;39(40):5891-908.
9. Prasad S et al. Nature Communications. 2022;13:2414. 10. Fleming-Dutra KE et al. Obstet Gynecol Clin North Am 2023;50(2):279-97. 11. Mouen S et al. JAMA Network Open 2024;7(7):e2419268.
Editor’s Note: Sadly, this is the last column in the Master Class Obstetrics series. This award-winning column has been part of Ob.Gyn. News for 20 years. The deep discussion of cutting-edge topics in obstetrics by specialists and researchers will be missed as will the leadership and curation of topics by Dr. E. Albert Reece.
Introduction: The Need for Increased Vigilance About Maternal Immunization
Viruses are becoming increasingly prevalent in our world and the consequences of viral infections are implicated in a growing number of disease states. It is well established that certain cancers are caused by viruses and it is increasingly evident that viral infections can trigger the development of chronic illness. In pregnant women, viruses such as cytomegalovirus can cause infection in utero and lead to long-term impairments for the baby.
Likewise, it appears that the virulence of viruses is increasing, whether it be the respiratory syncytial virus (RSV) in children or the severe acute respiratory syndrome (SARS) coronaviruses in adults. Clearly, our environment is changing, with increases in population growth and urbanization, for instance, and an intensification of climate change and its effects. Viruses are part of this changing background.
Vaccines are our most powerful tool to protect people of all ages against viral threats, and fortunately, we benefit from increasing expertise in vaccinology. Since 1974, the University of Maryland School of Medicine has a Center for Vaccine Development and Global Health that has conducted research on vaccines to defend against the Zika virus, H1N1, Ebola, and SARS-CoV-2.
We’re not alone. Other vaccinology centers across the country — as well as the National Institutes of Health at the national level, through its National Institute of Allergy and Infectious Diseases — are doing research and developing vaccines to combat viral diseases.
In this column, we are focused on viral diseases in pregnancy and the role that vaccines can play in preventing serious respiratory illness in mothers and their newborns. I have invited Laura E. Riley, MD, the Given Foundation Professor and Chair of Obstetrics and Gynecology at Weill Cornell Medicine, to address the importance of maternal immunization and how we can best counsel our patients and improve immunization rates.
As Dr. Riley explains, we are in a new era, and it behooves us all to be more vigilant about recommending vaccines, combating misperceptions, addressing patients’ knowledge gaps, and administering vaccines whenever possible.
Dr. Reece is the former Dean of Medicine & University Executive VP, and The Distinguished University and Endowed Professor & Director of the Center for Advanced Research Training and Innovation (CARTI) at the University of Maryland School of Medicine, as well as senior scientist at the Center for Birth Defects Research.
The alarming decline in maternal immunization rates that occurred in the wake of the COVID-19 pandemic means that, now more than ever, we must fully embrace our responsibility to recommend immunizations in pregnancy and to communicate what is known about their efficacy and safety. Data show that vaccination rates drop when we do not offer vaccines in our offices, so whenever possible, we should administer them as well.
The ob.gyn. is the patient’s most trusted person in pregnancy. When patients decline or express hesitancy about vaccines, it is incumbent upon us to ask why. Oftentimes, we can identify areas in which patients lack knowledge or have misperceptions and we can successfully educate the patient or change their perspective or misunderstanding concerning the importance of vaccination for themselves and their babies. (See Table 1.) We can also successfully address concerns about safety.
The safety of COVID-19 vaccinations in pregnancy is now backed by several years of data from multiple studies showing no increase in birth defects, preterm delivery, miscarriage, or stillbirth.
Data also show that pregnant patients are more likely than patients who are not pregnant to need hospitalization and intensive care when infected with SARS-CoV-2 and are at risk of having complications that can affect pregnancy and the newborn, including preterm birth and stillbirth. Vaccination has been shown to reduce the risk of severe illness and the risk of such adverse obstetrical outcomes, in addition to providing protection for the infant early on.
Similarly, influenza has long been more likely to be severe in pregnant patients, with an increased risk of poor obstetrical outcomes. Vaccines similarly provide “two for one protection,” protecting both mother and baby, and are, of course, backed by many years of safety and efficacy data.
With the new maternal respiratory syncytial virus (RSV) vaccine, now in its second year of availability, the goal is to protect the baby from RSV-caused serious lower respiratory tract illness. The illness has contributed to tens of thousands of annual hospitalizations and up to several hundred deaths every year in children younger than 5 years — particularly in those under age 6 months.
The RSV monoclonal antibody nirsevimab is available for the newborn as an alternative to maternal immunization but the maternal vaccine is optimal in that it will provide immediate rather than delayed protection for the newborn. The maternal vaccine is recommended during weeks 32-36 of pregnancy in mothers who were not vaccinated during last year’s RSV season. With real-world experience from year one, the available safety data are reassuring.
Counseling About Influenza and COVID-19 Vaccination
The COVID-19 pandemic took a toll on vaccination interest/receptivity broadly in pregnant and nonpregnant people. Among pregnant individuals, influenza vaccination coverage declined from 71% in the 2019-2020 influenza season to 56% in the 2021-2022 season, according to data from the Centers for Disease Control and Prevention’s Vaccine Safety Datalink.4 Coverage for the 2022-2023 and 2023-2024 influenza seasons was even worse: well under 50%.5
Fewer pregnant women have received updated COVID-19 vaccines. Only 13% of pregnant persons overall received the updated 2023-2024 COVID-19 booster vaccine (through March 30, 2024), according to the CDC.6
Maternal immunization for influenza has been recommended in the United States since 2004 (part of the recommendation that everyone over the age of 6 months receive an annual flu vaccine), and flu vaccines have been given to millions of pregnant women, but the H1N1 pandemic of 2009 reinforced its value as a priority for prenatal care. Most of the women who became severely ill from the H1N1 virus were young and healthy, without co-existing conditions known to increase risk.7
It became clearer during the H1N1 pandemic that pregnancy itself — which is associated with physiologic changes such as decreased lung capacity, increased nasal congestion and changes in the immune system – is its own significant risk factor for severe illness from the influenza virus. This increased risk applies to COVID-19 as well.
As COVID-19 has become endemic, with hospitalizations and deaths not reaching the levels of previous surges — and with mask-wearing and other preventive measures having declined — patients understandably have become more complacent. Some patients are vaccine deniers, but in my practice, these patients are a much smaller group than those who believe COVID-19 “is no big deal,” especially if they have had infections recently.
This is why it’s important to actively listen to concerns and to ask patients who decline a vaccination why they are hesitant. Blanket messages about vaccine efficacy and safety are the first step, but individualized, more pointed conversations based on the patient’s personal experiences and beliefs have become increasingly important.
I routinely tell pregnant patients about the risks of COVID-19 and I explain that it has been difficult to predict who will develop severe illness. Sometimes more conversation is needed. For those who are still hesitant or who tell me they feel protected by a recent infection, for instance, I provide more detail on the unique risks of pregnancy — the fact that “pregnancy is different” — and that natural immunity wanes while the protection afforded by immunization is believed to last longer. Many women are also concerned about the safety of the COVID-19 vaccine, so having safety data at your fingertips is helpful. (See Table 2.)
The fact that influenza and COVID-19 vaccination protect the newborn as well as the mother is something that I find is underappreciated by many patients. Explaining that infants likely benefit from the passage of antibodies across the placenta should be part of patient counseling.
Counseling About RSV Vaccination
Importantly, for the 2024-2025 RSV season, the maternal RSV vaccine (Abrysvo, Pfizer) is recommended only for pregnant women who did not receive the vaccine during the 2023-2024 season. When more research is done and more data are obtained showing how long the immune response persists post vaccination, it may be that the US Food and Drug Administration (FDA) will approve the maternal RSV vaccine for use in every pregnancy.
The later timing of the vaccination recommendation — 32-36 weeks’ gestation — reflects a conservative approach taken by the FDA in response to data from one of the pivotal trials showing a numerical trend toward more preterm deliveries among vaccinated compared with unvaccinated patients. This imbalance in the original trial, which administered the vaccine during 24-36 weeks of gestation, was seen only in low-income countries with no temporal association, however.
In our experience at two Weill Cornell Medical College–associated hospitals we did not see this trend. Our cohort study of almost 3000 pregnant patients who delivered at 32 weeks’ gestation or later found no increased risk of preterm birth among the 35% of patients who received the RSV vaccine during the 2023-2024 RSV season. We also did not see any difference in preeclampsia, in contrast with original trial data that showed a signal for increased risk.11
When fewer than 2 weeks have elapsed between maternal vaccination and delivery, the monoclonal antibody nirsevimab is recommended for the newborn — ideally before the newborn leaves the hospital. Nirsevimab is also recommended for newborns of mothers who decline vaccination or were not candidates (e.g. vaccinated in a previous pregnancy), or when there is concern about the adequacy of the maternal immune response to the vaccine (e.g. in cases of immunosuppression).
While there was a limited supply of the monoclonal antibody last year, limitations are not expected this year, especially after October.
The ultimate goal is that patients choose the vaccine or the immunoglobulin, given the severity of RSV disease. Patient preferences should be considered. However, given that it takes 2 weeks after vaccination for protection to build up, I stress to patients that if they’ve vaccinated themselves, their newborn will leave the hospital with protection. If nirsevimab is relied upon, I explain, their newborn may not be protected for some period of time.
Take-home Messages
- When patients decline or are hesitant about vaccines, ask why. Listen actively, and work to correct misperceptions and knowledge gaps.
- Whenever possible, offer vaccines in your practice. Vaccination rates drop when this does not occur.
- COVID-vaccine safety is backed by many studies showing no increase in birth defects, preterm delivery, miscarriage, or stillbirth.
- Pregnant women are more likely to have severe illness from the influenza and SARS-CoV-2 viruses. Vaccines can prevent severe illness and can protect the newborn as well as the mother.
- Recommend/administer the maternal RSV vaccine at 32-36 weeks’ gestation in women who did not receive the vaccine in the 2023-2024 season. If mothers aren’t eligible their babies should be offered nirsevimab.
Dr. Riley is the Given Foundation Professor and Chair of Obstetrics and Gynecology at Weill Cornell Medicine and the obstetrician and gynecologist-in-chief at New York Presbyterian Hospital. She disclosed that she has provided one-time consultations to Pfizer (Abrysvo RSV vaccine) and GSK (cytomegalovirus vaccine), and is providing consultant education on CMV for Moderna. She is chair of ACOG’s task force on immunization and emerging infectious diseases, serves on the medical advisory board for MAVEN, and serves as an editor or editorial board member for several medical publications.
References
1. ACOG Committee Opinion No. 741: Maternal Immunization. Obstet Gynecol. 2018;131(6):e214-e217.
2. Centers for Disease Control and Prevention. COVID-19 Vaccination for People Who are Pregnant or Breastfeeding. https://www.cdc.gov/covid/vaccines/pregnant-or-breastfeeding.html.
3. ACOG Practice Advisory on Maternal Respiratory Syncytial Virus Vaccination, September 2023. (Updated August 2024).4. Irving S et al. Open Forum Infect Dis. 2023;10(Suppl 2):ofad500.1002.
5. Flu Vaccination Dashboard, CDC, National Center for Immunization and Respiratory Diseases.
6. Weekly COVID-19 Vaccination Dashboard, CDC. https://www.cdc.gov/covidvaxview/weekly-dashboard/index.html
7. Louie JK et al. N Engl J Med. 2010;362:27-35. 8. Ciapponi A et al. Vaccine. 2021;39(40):5891-908.
9. Prasad S et al. Nature Communications. 2022;13:2414. 10. Fleming-Dutra KE et al. Obstet Gynecol Clin North Am 2023;50(2):279-97. 11. Mouen S et al. JAMA Network Open 2024;7(7):e2419268.
Wide Availability of Naloxone and Education on Its Use Can Save Pediatric Lives
ORLANDO — More than half of youth improved after receiving a dose of naloxone by emergency medical services (EMS) after an emergency dispatch call, according to research presented at the American Academy of Pediatrics 2024 National Conference.
“Emergency responders or EMS are often the first to arrive to an opioid poisoning, and they’re often the first to give naloxone, a potentially lifesaving medication,” said Christopher E. Gaw, MD, MPH, MBE, assistant professor of pediatrics at The Ohio State University College of Medicine and an emergency medicine physician at Nationwide Children’s Hospital in Columbus, Ohio.
“Our study highlights and underscores its safety of use in the prehospital setting, and this is also supported by other data,” Gaw said.
Additional research at the meeting showed that teens’ knowledge, attitudes, and confidence about recognizing overdoses and assisting with naloxone administration improved following a peer-to-peer training program, suggesting that teens can play an important role in reducing youth mortality from overdoses.
An average of 22 American teens died from overdose every week in 2022, and as counterfeit pill use has increased among youth, research has found that fentanyl was detected in 93% of overdose deaths with counterfeit pills, according to Talia Puzantian, PharmD, BCPP, of the Keck Graduate Institute School of Pharmacy, Claremont, California, who led the study on peer education. Yet a recent survey had found that less than a third of teens (30%) knew what naloxone was, and only 14% knew how to administer it.
“Ensuring that adolescents have easy and confidential access to naloxone is important and can save lives,” said Taylor Nichols, MD, assistant clinical professor at the University of California San Francisco and an emergency medicine and addiction medicine–certified physician. “I have had teen patients who have told me that they have had to use naloxone obtained from our clinic on friends when they have accidentally overdosed.”
Nichols, who was not involved in either study, added that all 50 states have some version of Good Samaritan laws that offer protection to individuals who attempt to aid in emergency assistance in good faith, and all except Kansas and Wyoming have laws specifically protecting people trying to help with overdose prevention.
“I tell people that everyone should carry naloxone and have naloxone available to be able to reverse an overdose, whether they personally use opioids or know people who use opioids because if they happen to come into a situation in which someone is passed out and unresponsive, that timely administration of naloxone may save their life,” Nichols said.
He added that primary care physicians, “particularly in family medicine and pediatrics, should be asking about any opioids in the home prescribed to anyone else and ensure that those patients also are prescribed or have access to naloxone to keep at home. Just as with asking about any other potential safety hazards, making sure they have naloxone available is crucial.”
EMS Naloxone Administration to Youth
EMS clinicians are often the first healthcare providers to respond to an opioid overdose or poisoning event, and evidence-based guidelines for EMS naloxone administration were developed in 2019 to support this intervention. Gaw’s team investigated the frequency and demographics of pediatric administration of naloxone.
They analyzed data from the National Emergency Medical Services Information System on EMS activations for administration of at least one dose of naloxone during 2022 to those aged 0-17. There were 6215 EMS pediatric administrations of naloxone that year, and in the vast majority of cases (82%), the patient had not received a naloxone injection prior to EMS’s arrival.
Most patients (79%) were aged 13-17 years, but 10% were in the 6-12 age group. The remaining patients included 6% infants younger than 1 year and 4% aged 6-12 years. Just over half were for males (55%), and most were dispatched to a home or residential setting (61%). One in five incidents (22%) occurred at a non-healthcare business, 9% on a street or highway, and the rest at a healthcare facility or another location.
Most of the incidents occurred in urban areas (86%), followed by rural (7%), suburban (6%), and wilderness (1.4%). More occurred in the US South (42%) than in the West (29%), Midwest (22%), or Northeast (7.5%).
A key takeaway of those demographic findings is that ingestions and accidental poisonings with opioids can occur in children of any age, Nichols said. “Every single home that has any opioids in the home should absolutely have naloxone immediately available as well,” he said. “Every single person who is prescribed opioids should also have naloxone available and accessible and to be sure that the naloxone is not expired or otherwise tampered with and update that every few years.” He noted that Narcan expiration was recently extended from 3 years to 4 years by the US Food and Drug Administration (FDA).
“I always advise that people who have opioid medications keep them stored safely and securely,” Nichols said. “However, I also acknowledge that even perfect systems fail and that people make mistakes and may accidentally leave medication out, within reach, or otherwise unsecured. If that happens, and someone were to intentionally or unintentionally get into that medication and potentially overdose as a result, we want to have that reversal medication immediately available to reverse the overdose.”
In nearly all cases (91%), EMS provided advanced life support, with only 7.5% patients receiving basic life support and 1.5% receiving specialty critical care. Just under a third (29%) of the dispatch calls were for “overdose/poisoning/ingestion.” Other dispatch calls included “unconscious/fainting/near-fainting” (21%) or “cardiac arrest/death” (17%), but the frequency of each dispatch label varied by age groups.
For example, 38% of calls for infants were for cardiac arrest, compared with 15% of calls for older teens and 18% of calls for 6-12 year olds. An overdose/poisoning dispatch was meanwhile more common for teens (32%) than for infants (13%), younger children (23%), and older children/tweens (18%). Other dispatch complaints included “sick person/person down/unknown problem” (12%) and “breathing problem” (5%).
A possible reason for these variations is that “an overdose might be mistaken for another medical emergency, or vice versa, because opioid poisonings can be challenging to recognize, especially in young children and in the pediatric population,” Gaw said. “Both the public and emergency responders should maintain a high level of suspicion” of possible overdose for children with the signs or symptoms of it, such as low breathing, unresponsiveness, or small pupils.
In most cases (87%), the patient was not in cardiac arrest, though the patient had entered cardiac arrest before EMS’s arrival in 11.5% of cases. Two thirds of cases only involved one dose of naloxone, while the other 33% involved two doses.
Ryan Marino, MD, an addiction medicine specialist and an associate professor of emergency medicine at Case Western Reserve University School of Medicine in Cleveland, Ohio, who was not involved in the study, took note of the high proportion of cases in which two doses were administered.
“While there is, in my professional opinion, almost no downside to giving naloxone in situations like this, and everybody should have it available and know how to use it, I would caution people on the risk of anchor bias, especially when more than two doses of naloxone are given, since we know that one should be an effective amount for any known opioid overdose,” Marino said. Anchoring bias refers to the tendency for individuals to rely more heavily on the first piece of information they receive about a topic or situation.
“For first responders and healthcare professionals, the importance of additional resuscitation measures like oxygenation and ventilation are just as crucial,” Marino said. “People should not be discouraged if someone doesn’t immediately respond to naloxone as overdose physiology can cause mental status to stay impaired for other reasons beyond direct drug effect, such as hypercarbia, but continue to seek and/or provide additional emergency care in these situations.”
Patients improved after one dose in just over half the cases (54%), and their conditions were unchanged in 46% of cases. There were only 11 cases in which the patient’s condition worsened after a naloxone dose (0.2%). Most of the cases (88%) were transported by EMS, and there were 13 total deaths at the scene (0.2%).
Nichols found the low incidence of worsening clinical status particularly striking. “This is further evidence of a critically important point — naloxone is purely an opioid antagonist, and only binds to opioid receptors, such that if a person has not overdosed on opioids or does not otherwise have opioids in their system, naloxone will not have a significant effect and will not cause them harm,” Nichols said.
“The most common causes of harm are due to rapid reversal of overdose and the potential risks involved in the rapid reversal of opioid effects and potentially precipitating withdrawal, and as this paper demonstrates, these are exceedingly rare,” he said. “Given that, we should have an incredibly low barrier to administer naloxone appropriately.”
The study was limited by inability to know how many true pediatric opioid poisonings are managed by EMS, so future research could look at linking EMS and emergency room hospital databases.
Improved Self-Efficacy in Teens
Another study showed that a peer-to-peer training program increased teens’ knowledge about overdoses from 34% before training to 79% after (P < .0001), and it substantially improved their confidence in recognizing an overdose and administering naloxone.
Nichols said the study shows the importance of ensuring “that adolescents know how to keep themselves and their friends safe in the case that they or anyone they know does end up using illicit substances which either intentionally or unintentionally contain opioids.”
This study assessed a training program with 206 students in a Los Angeles County high school who were trained by their peers between November 2023 and March 2024. The training included trends in teen overdose deaths, defining what opioids and fentanyl are, recognizing an overdose, and responding to one with naloxone.
The teens were an average 16 years old, about evenly split between boys and girls, and mostly in 11th (40%) or 12th (28%) grade, though nearly a third (29%) were 9th graders.
The students’ knowledge about fentanyl’s presence in counterfeit pills increased from 21% before the training to 68% afterward, and their correct identification of an overdose increased from 47% of participants to 90%.
The students’ confidence and attitudes toward helping with an overdose also improved substantially after the training. About two thirds agreed that non-medical people should be able to carry naloxone before the training, and that rose to 88% agreeing after the training. The proportion who agreed they would be willing to assist in an overdose rose from 77% before to 89% after training.
More dramatically, the teens’ confidence after training more than doubled in recognizing an overdose (from 31% to 81%) and more than tripled in their ability to give naloxone during an overdose (from 26% to 83%).
“The critical piece to keep in mind is that the concern about opioid overdose is respiratory depression leading to a lack of oxygen getting to the brain,” Nichols explained. “In the event of an overdose, time is brain — the longer the brain is deprived of oxygen, the lower the chance of survival. There is no specific time at which naloxone would become less effective at reversing an overdose.”
Therefore, people do not need to know the exact time that someone may have overdosed or how long they have been passed out in order to administer naloxone, he said. “The sooner naloxone is administered to someone who is unresponsive and who may have overdosed on opioids, the higher the likelihood of a successful reversal of an overdose and of saving a life.”
The peer-to-peer program was sponsored by the CARLOW Center for Medical Innovation, and the EMS study used no external funding. The authors of both studies and Marino had no disclosures. Nichols has consulted or clinically advised TV shows and health tech startup companies and has no disclosures related to naloxone or the pharmaceutical industry.
A version of this article first appeared on Medscape.com.
ORLANDO — More than half of youth improved after receiving a dose of naloxone by emergency medical services (EMS) after an emergency dispatch call, according to research presented at the American Academy of Pediatrics 2024 National Conference.
“Emergency responders or EMS are often the first to arrive to an opioid poisoning, and they’re often the first to give naloxone, a potentially lifesaving medication,” said Christopher E. Gaw, MD, MPH, MBE, assistant professor of pediatrics at The Ohio State University College of Medicine and an emergency medicine physician at Nationwide Children’s Hospital in Columbus, Ohio.
“Our study highlights and underscores its safety of use in the prehospital setting, and this is also supported by other data,” Gaw said.
Additional research at the meeting showed that teens’ knowledge, attitudes, and confidence about recognizing overdoses and assisting with naloxone administration improved following a peer-to-peer training program, suggesting that teens can play an important role in reducing youth mortality from overdoses.
An average of 22 American teens died from overdose every week in 2022, and as counterfeit pill use has increased among youth, research has found that fentanyl was detected in 93% of overdose deaths with counterfeit pills, according to Talia Puzantian, PharmD, BCPP, of the Keck Graduate Institute School of Pharmacy, Claremont, California, who led the study on peer education. Yet a recent survey had found that less than a third of teens (30%) knew what naloxone was, and only 14% knew how to administer it.
“Ensuring that adolescents have easy and confidential access to naloxone is important and can save lives,” said Taylor Nichols, MD, assistant clinical professor at the University of California San Francisco and an emergency medicine and addiction medicine–certified physician. “I have had teen patients who have told me that they have had to use naloxone obtained from our clinic on friends when they have accidentally overdosed.”
Nichols, who was not involved in either study, added that all 50 states have some version of Good Samaritan laws that offer protection to individuals who attempt to aid in emergency assistance in good faith, and all except Kansas and Wyoming have laws specifically protecting people trying to help with overdose prevention.
“I tell people that everyone should carry naloxone and have naloxone available to be able to reverse an overdose, whether they personally use opioids or know people who use opioids because if they happen to come into a situation in which someone is passed out and unresponsive, that timely administration of naloxone may save their life,” Nichols said.
He added that primary care physicians, “particularly in family medicine and pediatrics, should be asking about any opioids in the home prescribed to anyone else and ensure that those patients also are prescribed or have access to naloxone to keep at home. Just as with asking about any other potential safety hazards, making sure they have naloxone available is crucial.”
EMS Naloxone Administration to Youth
EMS clinicians are often the first healthcare providers to respond to an opioid overdose or poisoning event, and evidence-based guidelines for EMS naloxone administration were developed in 2019 to support this intervention. Gaw’s team investigated the frequency and demographics of pediatric administration of naloxone.
They analyzed data from the National Emergency Medical Services Information System on EMS activations for administration of at least one dose of naloxone during 2022 to those aged 0-17. There were 6215 EMS pediatric administrations of naloxone that year, and in the vast majority of cases (82%), the patient had not received a naloxone injection prior to EMS’s arrival.
Most patients (79%) were aged 13-17 years, but 10% were in the 6-12 age group. The remaining patients included 6% infants younger than 1 year and 4% aged 6-12 years. Just over half were for males (55%), and most were dispatched to a home or residential setting (61%). One in five incidents (22%) occurred at a non-healthcare business, 9% on a street or highway, and the rest at a healthcare facility or another location.
Most of the incidents occurred in urban areas (86%), followed by rural (7%), suburban (6%), and wilderness (1.4%). More occurred in the US South (42%) than in the West (29%), Midwest (22%), or Northeast (7.5%).
A key takeaway of those demographic findings is that ingestions and accidental poisonings with opioids can occur in children of any age, Nichols said. “Every single home that has any opioids in the home should absolutely have naloxone immediately available as well,” he said. “Every single person who is prescribed opioids should also have naloxone available and accessible and to be sure that the naloxone is not expired or otherwise tampered with and update that every few years.” He noted that Narcan expiration was recently extended from 3 years to 4 years by the US Food and Drug Administration (FDA).
“I always advise that people who have opioid medications keep them stored safely and securely,” Nichols said. “However, I also acknowledge that even perfect systems fail and that people make mistakes and may accidentally leave medication out, within reach, or otherwise unsecured. If that happens, and someone were to intentionally or unintentionally get into that medication and potentially overdose as a result, we want to have that reversal medication immediately available to reverse the overdose.”
In nearly all cases (91%), EMS provided advanced life support, with only 7.5% patients receiving basic life support and 1.5% receiving specialty critical care. Just under a third (29%) of the dispatch calls were for “overdose/poisoning/ingestion.” Other dispatch calls included “unconscious/fainting/near-fainting” (21%) or “cardiac arrest/death” (17%), but the frequency of each dispatch label varied by age groups.
For example, 38% of calls for infants were for cardiac arrest, compared with 15% of calls for older teens and 18% of calls for 6-12 year olds. An overdose/poisoning dispatch was meanwhile more common for teens (32%) than for infants (13%), younger children (23%), and older children/tweens (18%). Other dispatch complaints included “sick person/person down/unknown problem” (12%) and “breathing problem” (5%).
A possible reason for these variations is that “an overdose might be mistaken for another medical emergency, or vice versa, because opioid poisonings can be challenging to recognize, especially in young children and in the pediatric population,” Gaw said. “Both the public and emergency responders should maintain a high level of suspicion” of possible overdose for children with the signs or symptoms of it, such as low breathing, unresponsiveness, or small pupils.
In most cases (87%), the patient was not in cardiac arrest, though the patient had entered cardiac arrest before EMS’s arrival in 11.5% of cases. Two thirds of cases only involved one dose of naloxone, while the other 33% involved two doses.
Ryan Marino, MD, an addiction medicine specialist and an associate professor of emergency medicine at Case Western Reserve University School of Medicine in Cleveland, Ohio, who was not involved in the study, took note of the high proportion of cases in which two doses were administered.
“While there is, in my professional opinion, almost no downside to giving naloxone in situations like this, and everybody should have it available and know how to use it, I would caution people on the risk of anchor bias, especially when more than two doses of naloxone are given, since we know that one should be an effective amount for any known opioid overdose,” Marino said. Anchoring bias refers to the tendency for individuals to rely more heavily on the first piece of information they receive about a topic or situation.
“For first responders and healthcare professionals, the importance of additional resuscitation measures like oxygenation and ventilation are just as crucial,” Marino said. “People should not be discouraged if someone doesn’t immediately respond to naloxone as overdose physiology can cause mental status to stay impaired for other reasons beyond direct drug effect, such as hypercarbia, but continue to seek and/or provide additional emergency care in these situations.”
Patients improved after one dose in just over half the cases (54%), and their conditions were unchanged in 46% of cases. There were only 11 cases in which the patient’s condition worsened after a naloxone dose (0.2%). Most of the cases (88%) were transported by EMS, and there were 13 total deaths at the scene (0.2%).
Nichols found the low incidence of worsening clinical status particularly striking. “This is further evidence of a critically important point — naloxone is purely an opioid antagonist, and only binds to opioid receptors, such that if a person has not overdosed on opioids or does not otherwise have opioids in their system, naloxone will not have a significant effect and will not cause them harm,” Nichols said.
“The most common causes of harm are due to rapid reversal of overdose and the potential risks involved in the rapid reversal of opioid effects and potentially precipitating withdrawal, and as this paper demonstrates, these are exceedingly rare,” he said. “Given that, we should have an incredibly low barrier to administer naloxone appropriately.”
The study was limited by inability to know how many true pediatric opioid poisonings are managed by EMS, so future research could look at linking EMS and emergency room hospital databases.
Improved Self-Efficacy in Teens
Another study showed that a peer-to-peer training program increased teens’ knowledge about overdoses from 34% before training to 79% after (P < .0001), and it substantially improved their confidence in recognizing an overdose and administering naloxone.
Nichols said the study shows the importance of ensuring “that adolescents know how to keep themselves and their friends safe in the case that they or anyone they know does end up using illicit substances which either intentionally or unintentionally contain opioids.”
This study assessed a training program with 206 students in a Los Angeles County high school who were trained by their peers between November 2023 and March 2024. The training included trends in teen overdose deaths, defining what opioids and fentanyl are, recognizing an overdose, and responding to one with naloxone.
The teens were an average 16 years old, about evenly split between boys and girls, and mostly in 11th (40%) or 12th (28%) grade, though nearly a third (29%) were 9th graders.
The students’ knowledge about fentanyl’s presence in counterfeit pills increased from 21% before the training to 68% afterward, and their correct identification of an overdose increased from 47% of participants to 90%.
The students’ confidence and attitudes toward helping with an overdose also improved substantially after the training. About two thirds agreed that non-medical people should be able to carry naloxone before the training, and that rose to 88% agreeing after the training. The proportion who agreed they would be willing to assist in an overdose rose from 77% before to 89% after training.
More dramatically, the teens’ confidence after training more than doubled in recognizing an overdose (from 31% to 81%) and more than tripled in their ability to give naloxone during an overdose (from 26% to 83%).
“The critical piece to keep in mind is that the concern about opioid overdose is respiratory depression leading to a lack of oxygen getting to the brain,” Nichols explained. “In the event of an overdose, time is brain — the longer the brain is deprived of oxygen, the lower the chance of survival. There is no specific time at which naloxone would become less effective at reversing an overdose.”
Therefore, people do not need to know the exact time that someone may have overdosed or how long they have been passed out in order to administer naloxone, he said. “The sooner naloxone is administered to someone who is unresponsive and who may have overdosed on opioids, the higher the likelihood of a successful reversal of an overdose and of saving a life.”
The peer-to-peer program was sponsored by the CARLOW Center for Medical Innovation, and the EMS study used no external funding. The authors of both studies and Marino had no disclosures. Nichols has consulted or clinically advised TV shows and health tech startup companies and has no disclosures related to naloxone or the pharmaceutical industry.
A version of this article first appeared on Medscape.com.
ORLANDO — More than half of youth improved after receiving a dose of naloxone by emergency medical services (EMS) after an emergency dispatch call, according to research presented at the American Academy of Pediatrics 2024 National Conference.
“Emergency responders or EMS are often the first to arrive to an opioid poisoning, and they’re often the first to give naloxone, a potentially lifesaving medication,” said Christopher E. Gaw, MD, MPH, MBE, assistant professor of pediatrics at The Ohio State University College of Medicine and an emergency medicine physician at Nationwide Children’s Hospital in Columbus, Ohio.
“Our study highlights and underscores its safety of use in the prehospital setting, and this is also supported by other data,” Gaw said.
Additional research at the meeting showed that teens’ knowledge, attitudes, and confidence about recognizing overdoses and assisting with naloxone administration improved following a peer-to-peer training program, suggesting that teens can play an important role in reducing youth mortality from overdoses.
An average of 22 American teens died from overdose every week in 2022, and as counterfeit pill use has increased among youth, research has found that fentanyl was detected in 93% of overdose deaths with counterfeit pills, according to Talia Puzantian, PharmD, BCPP, of the Keck Graduate Institute School of Pharmacy, Claremont, California, who led the study on peer education. Yet a recent survey had found that less than a third of teens (30%) knew what naloxone was, and only 14% knew how to administer it.
“Ensuring that adolescents have easy and confidential access to naloxone is important and can save lives,” said Taylor Nichols, MD, assistant clinical professor at the University of California San Francisco and an emergency medicine and addiction medicine–certified physician. “I have had teen patients who have told me that they have had to use naloxone obtained from our clinic on friends when they have accidentally overdosed.”
Nichols, who was not involved in either study, added that all 50 states have some version of Good Samaritan laws that offer protection to individuals who attempt to aid in emergency assistance in good faith, and all except Kansas and Wyoming have laws specifically protecting people trying to help with overdose prevention.
“I tell people that everyone should carry naloxone and have naloxone available to be able to reverse an overdose, whether they personally use opioids or know people who use opioids because if they happen to come into a situation in which someone is passed out and unresponsive, that timely administration of naloxone may save their life,” Nichols said.
He added that primary care physicians, “particularly in family medicine and pediatrics, should be asking about any opioids in the home prescribed to anyone else and ensure that those patients also are prescribed or have access to naloxone to keep at home. Just as with asking about any other potential safety hazards, making sure they have naloxone available is crucial.”
EMS Naloxone Administration to Youth
EMS clinicians are often the first healthcare providers to respond to an opioid overdose or poisoning event, and evidence-based guidelines for EMS naloxone administration were developed in 2019 to support this intervention. Gaw’s team investigated the frequency and demographics of pediatric administration of naloxone.
They analyzed data from the National Emergency Medical Services Information System on EMS activations for administration of at least one dose of naloxone during 2022 to those aged 0-17. There were 6215 EMS pediatric administrations of naloxone that year, and in the vast majority of cases (82%), the patient had not received a naloxone injection prior to EMS’s arrival.
Most patients (79%) were aged 13-17 years, but 10% were in the 6-12 age group. The remaining patients included 6% infants younger than 1 year and 4% aged 6-12 years. Just over half were for males (55%), and most were dispatched to a home or residential setting (61%). One in five incidents (22%) occurred at a non-healthcare business, 9% on a street or highway, and the rest at a healthcare facility or another location.
Most of the incidents occurred in urban areas (86%), followed by rural (7%), suburban (6%), and wilderness (1.4%). More occurred in the US South (42%) than in the West (29%), Midwest (22%), or Northeast (7.5%).
A key takeaway of those demographic findings is that ingestions and accidental poisonings with opioids can occur in children of any age, Nichols said. “Every single home that has any opioids in the home should absolutely have naloxone immediately available as well,” he said. “Every single person who is prescribed opioids should also have naloxone available and accessible and to be sure that the naloxone is not expired or otherwise tampered with and update that every few years.” He noted that Narcan expiration was recently extended from 3 years to 4 years by the US Food and Drug Administration (FDA).
“I always advise that people who have opioid medications keep them stored safely and securely,” Nichols said. “However, I also acknowledge that even perfect systems fail and that people make mistakes and may accidentally leave medication out, within reach, or otherwise unsecured. If that happens, and someone were to intentionally or unintentionally get into that medication and potentially overdose as a result, we want to have that reversal medication immediately available to reverse the overdose.”
In nearly all cases (91%), EMS provided advanced life support, with only 7.5% patients receiving basic life support and 1.5% receiving specialty critical care. Just under a third (29%) of the dispatch calls were for “overdose/poisoning/ingestion.” Other dispatch calls included “unconscious/fainting/near-fainting” (21%) or “cardiac arrest/death” (17%), but the frequency of each dispatch label varied by age groups.
For example, 38% of calls for infants were for cardiac arrest, compared with 15% of calls for older teens and 18% of calls for 6-12 year olds. An overdose/poisoning dispatch was meanwhile more common for teens (32%) than for infants (13%), younger children (23%), and older children/tweens (18%). Other dispatch complaints included “sick person/person down/unknown problem” (12%) and “breathing problem” (5%).
A possible reason for these variations is that “an overdose might be mistaken for another medical emergency, or vice versa, because opioid poisonings can be challenging to recognize, especially in young children and in the pediatric population,” Gaw said. “Both the public and emergency responders should maintain a high level of suspicion” of possible overdose for children with the signs or symptoms of it, such as low breathing, unresponsiveness, or small pupils.
In most cases (87%), the patient was not in cardiac arrest, though the patient had entered cardiac arrest before EMS’s arrival in 11.5% of cases. Two thirds of cases only involved one dose of naloxone, while the other 33% involved two doses.
Ryan Marino, MD, an addiction medicine specialist and an associate professor of emergency medicine at Case Western Reserve University School of Medicine in Cleveland, Ohio, who was not involved in the study, took note of the high proportion of cases in which two doses were administered.
“While there is, in my professional opinion, almost no downside to giving naloxone in situations like this, and everybody should have it available and know how to use it, I would caution people on the risk of anchor bias, especially when more than two doses of naloxone are given, since we know that one should be an effective amount for any known opioid overdose,” Marino said. Anchoring bias refers to the tendency for individuals to rely more heavily on the first piece of information they receive about a topic or situation.
“For first responders and healthcare professionals, the importance of additional resuscitation measures like oxygenation and ventilation are just as crucial,” Marino said. “People should not be discouraged if someone doesn’t immediately respond to naloxone as overdose physiology can cause mental status to stay impaired for other reasons beyond direct drug effect, such as hypercarbia, but continue to seek and/or provide additional emergency care in these situations.”
Patients improved after one dose in just over half the cases (54%), and their conditions were unchanged in 46% of cases. There were only 11 cases in which the patient’s condition worsened after a naloxone dose (0.2%). Most of the cases (88%) were transported by EMS, and there were 13 total deaths at the scene (0.2%).
Nichols found the low incidence of worsening clinical status particularly striking. “This is further evidence of a critically important point — naloxone is purely an opioid antagonist, and only binds to opioid receptors, such that if a person has not overdosed on opioids or does not otherwise have opioids in their system, naloxone will not have a significant effect and will not cause them harm,” Nichols said.
“The most common causes of harm are due to rapid reversal of overdose and the potential risks involved in the rapid reversal of opioid effects and potentially precipitating withdrawal, and as this paper demonstrates, these are exceedingly rare,” he said. “Given that, we should have an incredibly low barrier to administer naloxone appropriately.”
The study was limited by inability to know how many true pediatric opioid poisonings are managed by EMS, so future research could look at linking EMS and emergency room hospital databases.
Improved Self-Efficacy in Teens
Another study showed that a peer-to-peer training program increased teens’ knowledge about overdoses from 34% before training to 79% after (P < .0001), and it substantially improved their confidence in recognizing an overdose and administering naloxone.
Nichols said the study shows the importance of ensuring “that adolescents know how to keep themselves and their friends safe in the case that they or anyone they know does end up using illicit substances which either intentionally or unintentionally contain opioids.”
This study assessed a training program with 206 students in a Los Angeles County high school who were trained by their peers between November 2023 and March 2024. The training included trends in teen overdose deaths, defining what opioids and fentanyl are, recognizing an overdose, and responding to one with naloxone.
The teens were an average 16 years old, about evenly split between boys and girls, and mostly in 11th (40%) or 12th (28%) grade, though nearly a third (29%) were 9th graders.
The students’ knowledge about fentanyl’s presence in counterfeit pills increased from 21% before the training to 68% afterward, and their correct identification of an overdose increased from 47% of participants to 90%.
The students’ confidence and attitudes toward helping with an overdose also improved substantially after the training. About two thirds agreed that non-medical people should be able to carry naloxone before the training, and that rose to 88% agreeing after the training. The proportion who agreed they would be willing to assist in an overdose rose from 77% before to 89% after training.
More dramatically, the teens’ confidence after training more than doubled in recognizing an overdose (from 31% to 81%) and more than tripled in their ability to give naloxone during an overdose (from 26% to 83%).
“The critical piece to keep in mind is that the concern about opioid overdose is respiratory depression leading to a lack of oxygen getting to the brain,” Nichols explained. “In the event of an overdose, time is brain — the longer the brain is deprived of oxygen, the lower the chance of survival. There is no specific time at which naloxone would become less effective at reversing an overdose.”
Therefore, people do not need to know the exact time that someone may have overdosed or how long they have been passed out in order to administer naloxone, he said. “The sooner naloxone is administered to someone who is unresponsive and who may have overdosed on opioids, the higher the likelihood of a successful reversal of an overdose and of saving a life.”
The peer-to-peer program was sponsored by the CARLOW Center for Medical Innovation, and the EMS study used no external funding. The authors of both studies and Marino had no disclosures. Nichols has consulted or clinically advised TV shows and health tech startup companies and has no disclosures related to naloxone or the pharmaceutical industry.
A version of this article first appeared on Medscape.com.
FROM AAP 2024
Celiac Screening in Kids Appears Cost-Effective
If these screening strategies are deemed feasible by clinicians and patients, then implementation in routine care is needed, lead author Jan Heijdra Suasnabar, MSc, of Leiden University Medical Centre in the Netherlands, and colleagues reported.
“Cohort studies have shown that CD likely develops early in life and can be easily diagnosed by detection of CD-specific antibodies against the enzyme tissue transglutaminase type 2 (IgA-TG2),” the investigators wrote in Gastroenterology.
Despite the ease of diagnosis, as few as one in five cases of CD are detected using current clinical strategies, meaning many cases are diagnosed years after symptom onset.
“Such high rates of missed/delayed diagnoses have been attributed to CD’s varied and nonspecific symptoms, lack of awareness, and the resource-intensive process necessary to establish the diagnosis,” Heijdra Suasnabar and colleagues wrote. “From an economic perspective, the burden of CD translates into substantial excess healthcare and societal costs.”
These practice gaps prompted the present study, which explored the long-term cost effectiveness of mass CD screening and active case finding among pediatric patients.
The investigators employed a model-based cost-effectiveness analysis with a hypothetical cohort representing all children with CD in the Netherlands. Iterations of this model evaluated long-term costs as these children moved through the healthcare system along various CD detection strategies.
The first strategy was based on the current Dutch approach, which is the same as that in the United States: Patients are only evaluated for CD if they present with symptoms that prompt suspicion of disease. Based on data from population-based studies, the model assumed that approximately one in three cases would be detected using this strategy.
The second strategy involved mass screening using IgA-TG2 point-of-care testing (sensitivity, 0.94; specificity, 0.944) via youth health care clinics, regardless of symptoms.
The third strategy, called “active case finding,” represented something of an intermediate approach, in which children with at least 1 CD-related symptom underwent point-of-care antibody testing.
For both mass screening and active case finding strategies, a positive antibody test was followed with confirmatory diagnostic testing.
Compared with current clinical approach, mass screening added 7.46 more quality-adjusted life-years (QALYs) per CD patient with an increased cost of €28,635 per CD patient. Active case finding gained 4.33 QALYs per CD patient while incurring an additional cost of €15,585 per CD patient.
Based on a willingness-to-pay threshold of €20,000 per QALY, the investigators deemed both strategies “highly cost effective,” compared with current standard of care. Some of these costs were offset by “substantial” reductions in productivity losses, they noted, including CD-related absences from work and school.
“Our results illustrate how an earlier detection of CD through screening or case finding, although more costly, leads to improved health outcomes and a reduction in disease burden, compared with current care,” Heijdra Suasnabar and colleagues wrote.
Their concluding remarks highlighted the conservative scenarios built into their model, and suggested that their findings offer solid evidence for implementing new CD-testing strategies.
“If found to be feasible and acceptable by clinicians and patients, these strategies should be implemented in the Netherlands,” they wrote.This study was supported by the Netherlands Organization for Health Research and Development. The investigators disclosed no conflicts of interest.
Celiac disease (CD) is common, affecting about 1% of the population, but it remains underdiagnosed because of its heterogeneous presentation and limited provider awareness. Most cases are detected only after patients develop gastrointestinal symptoms or laboratory abnormalities.
In this cost-effectiveness analysis, Suasnabar and colleagues demonstrate that screening children for celiac disease would be highly cost-effective relative to the current practice of clinical detection. They modeled point-of-care-testing using tissue transglutaminase IgA in all 3-year-old children in the Netherlands. While both mass screening and case-finding (via a standardized questionnaire) would increase healthcare costs relative to current care, both strategies would improve quality of life (QoL), reduce long-term complications (such as osteoporosis and non-Hodgkin lymphoma), and minimize productivity losses in individuals with CD. In sensitivity analyses accounting for uncertainty in QoL inputs and in the utility of diagnosing and treating asymptomatic CD, each screening strategy remained well below accepted willingness-to-pay thresholds.
John B. Doyle, MD, is a gastroenterology fellow in the Division of Digestive and Liver Diseases at Columbia University Medical Center, New York City. Benjamin Lebwohl, MD, MS, AGAF, is professor of medicine and epidemiology at Columbia University Medical Center and director of clinical research at The Celiac Disease Center at Columbia. They have no conflicts of interest to declare.
Celiac disease (CD) is common, affecting about 1% of the population, but it remains underdiagnosed because of its heterogeneous presentation and limited provider awareness. Most cases are detected only after patients develop gastrointestinal symptoms or laboratory abnormalities.
In this cost-effectiveness analysis, Suasnabar and colleagues demonstrate that screening children for celiac disease would be highly cost-effective relative to the current practice of clinical detection. They modeled point-of-care-testing using tissue transglutaminase IgA in all 3-year-old children in the Netherlands. While both mass screening and case-finding (via a standardized questionnaire) would increase healthcare costs relative to current care, both strategies would improve quality of life (QoL), reduce long-term complications (such as osteoporosis and non-Hodgkin lymphoma), and minimize productivity losses in individuals with CD. In sensitivity analyses accounting for uncertainty in QoL inputs and in the utility of diagnosing and treating asymptomatic CD, each screening strategy remained well below accepted willingness-to-pay thresholds.
John B. Doyle, MD, is a gastroenterology fellow in the Division of Digestive and Liver Diseases at Columbia University Medical Center, New York City. Benjamin Lebwohl, MD, MS, AGAF, is professor of medicine and epidemiology at Columbia University Medical Center and director of clinical research at The Celiac Disease Center at Columbia. They have no conflicts of interest to declare.
Celiac disease (CD) is common, affecting about 1% of the population, but it remains underdiagnosed because of its heterogeneous presentation and limited provider awareness. Most cases are detected only after patients develop gastrointestinal symptoms or laboratory abnormalities.
In this cost-effectiveness analysis, Suasnabar and colleagues demonstrate that screening children for celiac disease would be highly cost-effective relative to the current practice of clinical detection. They modeled point-of-care-testing using tissue transglutaminase IgA in all 3-year-old children in the Netherlands. While both mass screening and case-finding (via a standardized questionnaire) would increase healthcare costs relative to current care, both strategies would improve quality of life (QoL), reduce long-term complications (such as osteoporosis and non-Hodgkin lymphoma), and minimize productivity losses in individuals with CD. In sensitivity analyses accounting for uncertainty in QoL inputs and in the utility of diagnosing and treating asymptomatic CD, each screening strategy remained well below accepted willingness-to-pay thresholds.
John B. Doyle, MD, is a gastroenterology fellow in the Division of Digestive and Liver Diseases at Columbia University Medical Center, New York City. Benjamin Lebwohl, MD, MS, AGAF, is professor of medicine and epidemiology at Columbia University Medical Center and director of clinical research at The Celiac Disease Center at Columbia. They have no conflicts of interest to declare.
If these screening strategies are deemed feasible by clinicians and patients, then implementation in routine care is needed, lead author Jan Heijdra Suasnabar, MSc, of Leiden University Medical Centre in the Netherlands, and colleagues reported.
“Cohort studies have shown that CD likely develops early in life and can be easily diagnosed by detection of CD-specific antibodies against the enzyme tissue transglutaminase type 2 (IgA-TG2),” the investigators wrote in Gastroenterology.
Despite the ease of diagnosis, as few as one in five cases of CD are detected using current clinical strategies, meaning many cases are diagnosed years after symptom onset.
“Such high rates of missed/delayed diagnoses have been attributed to CD’s varied and nonspecific symptoms, lack of awareness, and the resource-intensive process necessary to establish the diagnosis,” Heijdra Suasnabar and colleagues wrote. “From an economic perspective, the burden of CD translates into substantial excess healthcare and societal costs.”
These practice gaps prompted the present study, which explored the long-term cost effectiveness of mass CD screening and active case finding among pediatric patients.
The investigators employed a model-based cost-effectiveness analysis with a hypothetical cohort representing all children with CD in the Netherlands. Iterations of this model evaluated long-term costs as these children moved through the healthcare system along various CD detection strategies.
The first strategy was based on the current Dutch approach, which is the same as that in the United States: Patients are only evaluated for CD if they present with symptoms that prompt suspicion of disease. Based on data from population-based studies, the model assumed that approximately one in three cases would be detected using this strategy.
The second strategy involved mass screening using IgA-TG2 point-of-care testing (sensitivity, 0.94; specificity, 0.944) via youth health care clinics, regardless of symptoms.
The third strategy, called “active case finding,” represented something of an intermediate approach, in which children with at least 1 CD-related symptom underwent point-of-care antibody testing.
For both mass screening and active case finding strategies, a positive antibody test was followed with confirmatory diagnostic testing.
Compared with current clinical approach, mass screening added 7.46 more quality-adjusted life-years (QALYs) per CD patient with an increased cost of €28,635 per CD patient. Active case finding gained 4.33 QALYs per CD patient while incurring an additional cost of €15,585 per CD patient.
Based on a willingness-to-pay threshold of €20,000 per QALY, the investigators deemed both strategies “highly cost effective,” compared with current standard of care. Some of these costs were offset by “substantial” reductions in productivity losses, they noted, including CD-related absences from work and school.
“Our results illustrate how an earlier detection of CD through screening or case finding, although more costly, leads to improved health outcomes and a reduction in disease burden, compared with current care,” Heijdra Suasnabar and colleagues wrote.
Their concluding remarks highlighted the conservative scenarios built into their model, and suggested that their findings offer solid evidence for implementing new CD-testing strategies.
“If found to be feasible and acceptable by clinicians and patients, these strategies should be implemented in the Netherlands,” they wrote.This study was supported by the Netherlands Organization for Health Research and Development. The investigators disclosed no conflicts of interest.
If these screening strategies are deemed feasible by clinicians and patients, then implementation in routine care is needed, lead author Jan Heijdra Suasnabar, MSc, of Leiden University Medical Centre in the Netherlands, and colleagues reported.
“Cohort studies have shown that CD likely develops early in life and can be easily diagnosed by detection of CD-specific antibodies against the enzyme tissue transglutaminase type 2 (IgA-TG2),” the investigators wrote in Gastroenterology.
Despite the ease of diagnosis, as few as one in five cases of CD are detected using current clinical strategies, meaning many cases are diagnosed years after symptom onset.
“Such high rates of missed/delayed diagnoses have been attributed to CD’s varied and nonspecific symptoms, lack of awareness, and the resource-intensive process necessary to establish the diagnosis,” Heijdra Suasnabar and colleagues wrote. “From an economic perspective, the burden of CD translates into substantial excess healthcare and societal costs.”
These practice gaps prompted the present study, which explored the long-term cost effectiveness of mass CD screening and active case finding among pediatric patients.
The investigators employed a model-based cost-effectiveness analysis with a hypothetical cohort representing all children with CD in the Netherlands. Iterations of this model evaluated long-term costs as these children moved through the healthcare system along various CD detection strategies.
The first strategy was based on the current Dutch approach, which is the same as that in the United States: Patients are only evaluated for CD if they present with symptoms that prompt suspicion of disease. Based on data from population-based studies, the model assumed that approximately one in three cases would be detected using this strategy.
The second strategy involved mass screening using IgA-TG2 point-of-care testing (sensitivity, 0.94; specificity, 0.944) via youth health care clinics, regardless of symptoms.
The third strategy, called “active case finding,” represented something of an intermediate approach, in which children with at least 1 CD-related symptom underwent point-of-care antibody testing.
For both mass screening and active case finding strategies, a positive antibody test was followed with confirmatory diagnostic testing.
Compared with current clinical approach, mass screening added 7.46 more quality-adjusted life-years (QALYs) per CD patient with an increased cost of €28,635 per CD patient. Active case finding gained 4.33 QALYs per CD patient while incurring an additional cost of €15,585 per CD patient.
Based on a willingness-to-pay threshold of €20,000 per QALY, the investigators deemed both strategies “highly cost effective,” compared with current standard of care. Some of these costs were offset by “substantial” reductions in productivity losses, they noted, including CD-related absences from work and school.
“Our results illustrate how an earlier detection of CD through screening or case finding, although more costly, leads to improved health outcomes and a reduction in disease burden, compared with current care,” Heijdra Suasnabar and colleagues wrote.
Their concluding remarks highlighted the conservative scenarios built into their model, and suggested that their findings offer solid evidence for implementing new CD-testing strategies.
“If found to be feasible and acceptable by clinicians and patients, these strategies should be implemented in the Netherlands,” they wrote.This study was supported by the Netherlands Organization for Health Research and Development. The investigators disclosed no conflicts of interest.
FROM GASTROENTEROLOGY











