User login
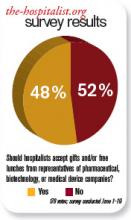
Should hospitalists accept gifts from pharmaceutical, medical device, and biotech companies?
The pharmaceutical industry is big business, and its goal is to make money. If the industry can convince physicians to prescribe its medicines, then it makes more money.
Although pharmaceutical representatives brief physicians on new medications in an effort to encourage the use of their brand-name products, they also provide substantive information on the drugs that serves an educational purpose.
In the past, pharmaceutical companies—along with the medical device and biotechnology industries—showered physicians with expensive gifts, raising ethical questions about physicians’ obligation to the drug companies. Fair enough. These excessive practices were identified and curtailed—to my knowledge—some years ago.
Watchdog groups, however, have continued to call into question every suggestion of “being in the pay” of big pharma. Everything from a plastic pen to a piece of pizza is suspect. There is considerable concern that practicing clinicians are influenced by the smallest gesture, while many large medical institutions continue to accept pharmaceutical-company-funded research grants. If big-pharma investment in research does not corrupt institutions, why is it assumed that carrying a pharmaceutical pen has such a pernicious effect on clinicians?
As a corollary to this question, does anyone really want to discontinue these important research studies just because they are funded by industry dollars?
Listening to drug representatives—even being seen in the vicinity—raises the eyebrows of purists. Do we really want physicians completely divorced from all pharmaceutical company education and communication? Do we feel there is zero benefit to hearing about new medications from the company’s viewpoint?
If physicians completely shut out the representatives, it would be expected that pharmaceutical companies would direct their efforts elsewhere—most likely, to consumers. Is that a better and healthier scenario?
Clearly, there is potential for abuse in pharmaceutical gifts to physicians. The practice should be controlled and monitored. The suspicions raised by purist groups that physicians’ prescribing habits are unalterably biased after a five-minute pharmaceutical representative detail and a chicken sandwich is hyperbole. The voice of reason is silenced in the midst of the inquisition.
In the academic setting, fear of being accused of “bought bias” has physicians clearing their pockets of tainted pens and checking their desks for corrupting paraphernalia. The positive aspects of pharma-sponsored programs and medical lectures are lost for fear of appearing to be complicit with drug companies.
The Aristotelian Golden Mean is superior to extreme positions, and I submit that the best road is the center. Listen to what the drug company representatives have to say, just like you listen to a car salesman: You can learn from both—as long as you research the data and form your own opinion. TH
Dr. Brezina is a hospitalist at Durham Regional Hospital in North Carolina.
The pharmaceutical industry is big business, and its goal is to make money. If the industry can convince physicians to prescribe its medicines, then it makes more money.
Although pharmaceutical representatives brief physicians on new medications in an effort to encourage the use of their brand-name products, they also provide substantive information on the drugs that serves an educational purpose.
In the past, pharmaceutical companies—along with the medical device and biotechnology industries—showered physicians with expensive gifts, raising ethical questions about physicians’ obligation to the drug companies. Fair enough. These excessive practices were identified and curtailed—to my knowledge—some years ago.
Watchdog groups, however, have continued to call into question every suggestion of “being in the pay” of big pharma. Everything from a plastic pen to a piece of pizza is suspect. There is considerable concern that practicing clinicians are influenced by the smallest gesture, while many large medical institutions continue to accept pharmaceutical-company-funded research grants. If big-pharma investment in research does not corrupt institutions, why is it assumed that carrying a pharmaceutical pen has such a pernicious effect on clinicians?
As a corollary to this question, does anyone really want to discontinue these important research studies just because they are funded by industry dollars?
Listening to drug representatives—even being seen in the vicinity—raises the eyebrows of purists. Do we really want physicians completely divorced from all pharmaceutical company education and communication? Do we feel there is zero benefit to hearing about new medications from the company’s viewpoint?
If physicians completely shut out the representatives, it would be expected that pharmaceutical companies would direct their efforts elsewhere—most likely, to consumers. Is that a better and healthier scenario?
Clearly, there is potential for abuse in pharmaceutical gifts to physicians. The practice should be controlled and monitored. The suspicions raised by purist groups that physicians’ prescribing habits are unalterably biased after a five-minute pharmaceutical representative detail and a chicken sandwich is hyperbole. The voice of reason is silenced in the midst of the inquisition.
In the academic setting, fear of being accused of “bought bias” has physicians clearing their pockets of tainted pens and checking their desks for corrupting paraphernalia. The positive aspects of pharma-sponsored programs and medical lectures are lost for fear of appearing to be complicit with drug companies.
The Aristotelian Golden Mean is superior to extreme positions, and I submit that the best road is the center. Listen to what the drug company representatives have to say, just like you listen to a car salesman: You can learn from both—as long as you research the data and form your own opinion. TH
Dr. Brezina is a hospitalist at Durham Regional Hospital in North Carolina.
The pharmaceutical industry is big business, and its goal is to make money. If the industry can convince physicians to prescribe its medicines, then it makes more money.
Although pharmaceutical representatives brief physicians on new medications in an effort to encourage the use of their brand-name products, they also provide substantive information on the drugs that serves an educational purpose.
In the past, pharmaceutical companies—along with the medical device and biotechnology industries—showered physicians with expensive gifts, raising ethical questions about physicians’ obligation to the drug companies. Fair enough. These excessive practices were identified and curtailed—to my knowledge—some years ago.
Watchdog groups, however, have continued to call into question every suggestion of “being in the pay” of big pharma. Everything from a plastic pen to a piece of pizza is suspect. There is considerable concern that practicing clinicians are influenced by the smallest gesture, while many large medical institutions continue to accept pharmaceutical-company-funded research grants. If big-pharma investment in research does not corrupt institutions, why is it assumed that carrying a pharmaceutical pen has such a pernicious effect on clinicians?
As a corollary to this question, does anyone really want to discontinue these important research studies just because they are funded by industry dollars?
Listening to drug representatives—even being seen in the vicinity—raises the eyebrows of purists. Do we really want physicians completely divorced from all pharmaceutical company education and communication? Do we feel there is zero benefit to hearing about new medications from the company’s viewpoint?
If physicians completely shut out the representatives, it would be expected that pharmaceutical companies would direct their efforts elsewhere—most likely, to consumers. Is that a better and healthier scenario?
Clearly, there is potential for abuse in pharmaceutical gifts to physicians. The practice should be controlled and monitored. The suspicions raised by purist groups that physicians’ prescribing habits are unalterably biased after a five-minute pharmaceutical representative detail and a chicken sandwich is hyperbole. The voice of reason is silenced in the midst of the inquisition.
In the academic setting, fear of being accused of “bought bias” has physicians clearing their pockets of tainted pens and checking their desks for corrupting paraphernalia. The positive aspects of pharma-sponsored programs and medical lectures are lost for fear of appearing to be complicit with drug companies.
The Aristotelian Golden Mean is superior to extreme positions, and I submit that the best road is the center. Listen to what the drug company representatives have to say, just like you listen to a car salesman: You can learn from both—as long as you research the data and form your own opinion. TH
Dr. Brezina is a hospitalist at Durham Regional Hospital in North Carolina.
To Err is Human
The challenges facing SHM are very different than they were 10 years ago. In the 1990s, the focus was on building a society that would represent the needs of the practicing hospitalist. Converting NAIP, with its 200 members, to SHM, with its now 10,000 members, was certainly no easy task, but the society then enjoyed some luxuries no longer afforded to an organization the size of the modern-day SHM. Early on, SHM was far from the public eye, escaping public scrutiny for each of its actions. With only a few hundred members, the society was intimate: Almost every member knew of every action before it happened. And the agenda, compared with today’s standards, was reasonably focused.
But times are different now. The organization is much larger and complex, and the challenges we now face are collectively a product of our success. SHM is squarely in the spotlight; every decision is closely monitored by the public eye. We now have a voice such that when we speak, people listen. But with greatness comes responsibility, and because we are in the spotlight, we must be especially careful in how we speak, lest the message be misunderstood. Further, with more than 10,000 members, 50 full-time staff, 44 committees, and nearly 500 physician volunteers, the organization no longer has the luxury of every action being known by every member prior to its enactment.
More challenging still is our agenda, which has grown to be a diverse and far-reaching strategy. While impressive and admirable, the size of this “footprint” creates new challenges in balancing the need to be “nimble” (i.e., being able to act quickly enough to be timely and effective) versus being “thorough” (i.e., ensuring that each action is appropriately vetted prior to execution).
I suspect that there are few practicing hospitalists who have not read To Err is Human or Crossing the Quality Chasm.1,2 Both make this essential point about quality: In complex systems, mistakes are bound to happen. And when errors do occur, each member of the team must be ready to take responsibility for the mistake, and immediately begin seeking systematic solutions to ensure that it does not happen again. SHM’s focus is to advance quality for all hospitalized patients. But an organization can only be effective if it emulates the principles that it hopes its members will individually espouse.
So let me start with this: There have been mistakes along the way.
That’s the hard truth. I believe that none of the mistakes have been intentional; rather, these missteps have been a product of an organization that has grown so fast, and whose success has gained so much public attention, that its infrastructure has struggled to keep pace with its growth. Any hospitalist who has seen his or her service size double in the span of a year or two knows of what I speak: As growth occurs, the approach to dealing with daily business has to evolve to meet new demands. If it does not, errors result.
One of the areas in which SHM’s growth has outpaced its policies and procedures regards SHM’s relationship with industry. I will say from the outset that having relationships with industry is not in and of itself a mistake. The reality is that without such relationships, in the setting of a landscape where governmental and philanthropic funding is disproportionately in deficit to the need, it would be almost impossible to advance the quality initiatives that have defined SHM’s success. SHM has, and will likely continue to have, relationships with industry. But requisite for having these relationships, especially for an organization that is a national leader, is going above and beyond the minimum standards to ensure transparency and ethics.
Two years ago, SHM began the arduous process of reviewing its partnerships and how it interacts with industry. I am pleased to announce that this has culminated in the Council of Medical Specialty Societies (CMSS; www.cmss.org) asking SHM to apply to become an affiliate member. Acceptance of SHM into CMSS is evidence of SHM’s demonstrated compliance with CMSS’s requirements, with respect to industry relationships, disclosure of conflicts of interest, and other measures of organizational transparency, all of which can be found at www.hospitalmedicine.org/industry.
But meeting the minimum standards has never been sufficient for SHM. The cost of greatness is responsibility, and as a national leader, SHM has a responsibility to ensure that its approaches to potential conflicts of interest and external relationships are above reproach.
COI Disclosure
The conflict of interest statements for each board member have long been posted on the SHM website. In an effort to go above and beyond the minimum standards, the format of the disclosure form has been revised, making it the most compete and detailed COI disclosure form of any physician organization in the country. In the coming months, SHM will make even tighter restrictions regarding disclosing potential conflicts of interest. While board members are required to report any and all financial receipts, the amended version will require board members who receive any contribution in excess of $5,000 to provide a detailed narrative as to what was required in service for the receipt of those funds. Further, to ensure collective accountability, any board member may call upon any other board member to provide a similarly detailed description of any item on his or her COI disclosure form.
Recognizing that other leaders in the organization might also have influence over important decisions, thereby being at risk for a conflict of interest, SHM is one of the first physician organizations to require public reporting of COI disclosures for all editors, course directors, and senior staff.
Next year, all committee chairs and quality-improvement (QI) project leaders will be required to submit similar COI disclosures.
But reporting potential conflicts is one thing; ensuring that those with significant conflicts are not put in a position of inescapable conflict of interest by virtue of their appointments is another. To be proactive, the executive committee has a designated meeting each year to individually review each nominee being considered for election to the board, committee chairs, editors, and course directors prior to their appointment.
The society will enforce CMSS Standard 1.4, which prohibits key society leaders (president, past-president, president-elect, CEO, editors, course directors) from having direct financial relationships with companies during his or her term of service. All people seeking such positions will be required to attest, at the time of the nomination, to cease all direct financial relationships prior to seeking office; failure to do so will negate their candidacy for the position they seek.
External Communications Regarding Industry
It is one thing to have potential conflicts disclosed on a website; it is quite another to ensure, with 100% confidence, that all recipients of all communications from SHM are aware of this website. Reminding all representatives of SHM to alert communication recipients to our potential conflicts of interest is a good start, but in quality parlance, this is tantamount to “telling people to try harder,” which is rarely an effective strategy to ensure 100% compliance. In response, SHM has designed a fail-safe systems solution to ensure that every communication alerts the recipient to SHM’s potential conflicts of interest. Beginning this year, SHM letterheads and e-mail, used for all written communications with external parties, will carry the following statement on the bottom of each page: “To Learn More About SHM’s Relationship with Industry Partners, Visit www.hospitalmedicine.org/industry.”
One of SHM’s missteps over the years has been the failure to distinguish external communications regarding pharmaceuticals/devices as being different from the organization’s other nonpharmaceutical communications. This unintentional oversight has been a product of the exponential increase in the society’s external communications during the past 10 years. But nonetheless, the distinction between these types of communications is important, especially for a society that receives industry support for its quality initiatives.
At the August board meeting in Chicago, a special ad hoc committee was appointed to develop specific policies regarding SHM’s communication strategy. This committee will bring to the board in November the following policy for approval: “Before SHM makes a specific comment, writes a letter, or posts an official statement on the SHM website about a pharmaceutical agent, a medical device, a specific disease state, or any medical IT services or products, the communication must be approved by the Executive Committee and reflected in the minutes of the Executive Committee. At the President’s discretion, the proposed communication will be brought to the entire Board for discussion and approval.”
As noted below, all agendas and decisions by the executive committee are communicated to the board, further ensuring accountability and oversight for any such decision.
Choices and Definitions
In the early years, all external relationships were initiated by SHM. Because SHM was a relative unknown on the national scene, if a relationship was to be entertained, it was based on SHM’s initiative to do so. Naturally, the smaller number of relationships, and the fact that the choice and nature of the relationship were initiated by SHM, made it easier to define the scope of such relationships. But now things are different: SHM’s agenda now encompasses a vast set of domains, and SHM is regularly on the receiving end of invitations to establish relationships with other organizations. Once again, as a leader of medical specialty organizations, SHM’s policies and procedures have to adapt to fit the needs of a larger and more diverse organization.
An intense amount of work has been devoted to evolving the mechanism by which SHM chooses and defines its relationships. An ad hoc committee from the board of directors has defined the 10 principles of SHM’s business relationships (see “10 Principles of SHM Business Relationships,” p. 42). In November, the board will adopt policies and procedures that will ensure that SHM will continue to only enter into relationships with external organizations with which it shares common interests or goals for advancing the quality and safety of patient care. SHM will continue to avoid influence from external organizations with respect to the policies, conduct, actions, and priorities of SHM.
Further, by policy, SHM will continue to reserve absolute control over all content and speakers at its educational conferences; content will continue to not be influenced by brand or product consideration during development or revisions. All potential partners will be informed from the outset that a partnership with SHM does not imply that SHM endorses the policies, values, and missions of the partner organization; any significant deviation from the values and mission of SHM will dissolve the partnership. SHM will establish from the outset that a partnership does not imply SHM’s support or endorsement of any products from a partner. As noted above, transparency of these relationships will be of paramount importance: All relationships, including the dollar amounts received as a product of those relationships, will be posted on SHM’s website.
Transparency in Decision-Making
As noted from the outset, the benefit of small organizations with limited agendas is that every member knows every decision. With limited decisions to be made, the vetting and review process is virtually assured. As organizations grow, as agendas expand exponentially, and as the pace quickens, the multiperson review of each decision becomes difficult to assure. The result is that errors start to appear—not due to intentional wrongdoing, but because the luxury of intense oversight is lost as the organization expands. For an organization to grow and still maintain oversight of its decision-making process, it is vital that the organization evolve to develop new methods of accountability and transparency.
To meet this need, SHM has enacted a change in its communication infrastructure to ensure “double-checks” for all of the important organizational decisions. An SHM leadership and staff “wiki” has been developed to promote and ensure transparency of all organizational decisions. Because it is accessible only to the SHM staff, board, and committee chairs, the wiki is invisible to the SHM membership. Nonetheless, you should know of this important innovation.
The wiki requires that all committee chairs post the results of their committee activities. This ensures that staff and committee leadership are on the same page, ensures that other committees are not duplicating work, augments collaboration across committees, and, most importantly, ensures collective accountability for each decision made.
Technology-based innovations have been enacted to improve the transparency of the executive leadership of the organization. The board of directors meets four times a year; the purpose of the board is to ensure oversight for all SHM decisions. Because the board comprises exclusively volunteer members meeting only four times a year, it is practically impossible for the board to approve every decision made by an organization as large as SHM. To ensure the necessary efficiency and effectiveness (i.e., being sufficiently “nimble” to act on important issues in between scheduled board meetings), the executive committee (EC) was established years ago. The EC, comprised of the president, the past-president, the president-elect, and the CEO, meets every two weeks via teleconference to review and approve all essential SHM decisions.
As an innovation to augment accountability and transparency, the agenda and minutes of the EC are now posted on the SHM board portal, allowing all board members to review and comment upon the decisions made by the president, the CEO, or the EC as a whole. Any board member, at any time, can request that the full board be convened to review an agenda item or decision.
And underlying all of these initiatives to improve an already exceptional organization are the tireless efforts of the SHM staff. Though there are nearly 50 staff members now, each continues to do the work of multiple people. SHM is arguably the fastest-growing organization in history, and advancing the organization to level after level has been an exceptional challenge. But regardless of the challenge, SHM leadership and staff has come through. I have no doubt that during this next chapter in SHM’s history, the result will be the same.
SHM is committed to advancing quality. Intrinsic to the “culture of quality” is the commitment to honesty, transparency, and ethics. Any permutation of the society that does not fully exemplify these standards will be ineffective in accomplishing our wished-for goal. In short, the actions of the society must model those that we wish to inspire in the day-to-day practice of our members. Although the unprecedented growth of the society is responsible for errors in the past, the importance of admitting our shortcomings is no less significant. We’ve had some missteps along the way, and while these mistakes are a product of events preceding my tenure, it does not matter. As president of the organization, I am taking responsibility for them, with a pledge to devote all time and energy, with all due speed, to finding systematic solutions that will prevent these errors from happening again.
And let me be even more honest. As we go forward, there are probably going to be more missteps; such is the nature of a growing and active organization. I cannot promise an error-free organization, but I can promise that if and when errors are made in the future, the same intensity will be applied to seek systematic solutions to ensure that we continue to evolve in becoming an organization that is emblematic of quality. Such is the promise of SHM; such is the promise of the hospitalist. TH
Dr. Wiese is president of SHM.
References
- Kohn LT, Corrigan JM, Donaldson MS, et al. To Err Is Human: Building a Safer Health System. Washington, D.C.: National Academies Press; 2000.
- Institute of Medicine Committee on Quality Health Care in America. Crossing the Quality Chasm: A New Health System for the 21st Century. Washington, D.C.: National Academies Press; 2001.
The challenges facing SHM are very different than they were 10 years ago. In the 1990s, the focus was on building a society that would represent the needs of the practicing hospitalist. Converting NAIP, with its 200 members, to SHM, with its now 10,000 members, was certainly no easy task, but the society then enjoyed some luxuries no longer afforded to an organization the size of the modern-day SHM. Early on, SHM was far from the public eye, escaping public scrutiny for each of its actions. With only a few hundred members, the society was intimate: Almost every member knew of every action before it happened. And the agenda, compared with today’s standards, was reasonably focused.
But times are different now. The organization is much larger and complex, and the challenges we now face are collectively a product of our success. SHM is squarely in the spotlight; every decision is closely monitored by the public eye. We now have a voice such that when we speak, people listen. But with greatness comes responsibility, and because we are in the spotlight, we must be especially careful in how we speak, lest the message be misunderstood. Further, with more than 10,000 members, 50 full-time staff, 44 committees, and nearly 500 physician volunteers, the organization no longer has the luxury of every action being known by every member prior to its enactment.
More challenging still is our agenda, which has grown to be a diverse and far-reaching strategy. While impressive and admirable, the size of this “footprint” creates new challenges in balancing the need to be “nimble” (i.e., being able to act quickly enough to be timely and effective) versus being “thorough” (i.e., ensuring that each action is appropriately vetted prior to execution).
I suspect that there are few practicing hospitalists who have not read To Err is Human or Crossing the Quality Chasm.1,2 Both make this essential point about quality: In complex systems, mistakes are bound to happen. And when errors do occur, each member of the team must be ready to take responsibility for the mistake, and immediately begin seeking systematic solutions to ensure that it does not happen again. SHM’s focus is to advance quality for all hospitalized patients. But an organization can only be effective if it emulates the principles that it hopes its members will individually espouse.
So let me start with this: There have been mistakes along the way.
That’s the hard truth. I believe that none of the mistakes have been intentional; rather, these missteps have been a product of an organization that has grown so fast, and whose success has gained so much public attention, that its infrastructure has struggled to keep pace with its growth. Any hospitalist who has seen his or her service size double in the span of a year or two knows of what I speak: As growth occurs, the approach to dealing with daily business has to evolve to meet new demands. If it does not, errors result.
One of the areas in which SHM’s growth has outpaced its policies and procedures regards SHM’s relationship with industry. I will say from the outset that having relationships with industry is not in and of itself a mistake. The reality is that without such relationships, in the setting of a landscape where governmental and philanthropic funding is disproportionately in deficit to the need, it would be almost impossible to advance the quality initiatives that have defined SHM’s success. SHM has, and will likely continue to have, relationships with industry. But requisite for having these relationships, especially for an organization that is a national leader, is going above and beyond the minimum standards to ensure transparency and ethics.
Two years ago, SHM began the arduous process of reviewing its partnerships and how it interacts with industry. I am pleased to announce that this has culminated in the Council of Medical Specialty Societies (CMSS; www.cmss.org) asking SHM to apply to become an affiliate member. Acceptance of SHM into CMSS is evidence of SHM’s demonstrated compliance with CMSS’s requirements, with respect to industry relationships, disclosure of conflicts of interest, and other measures of organizational transparency, all of which can be found at www.hospitalmedicine.org/industry.
But meeting the minimum standards has never been sufficient for SHM. The cost of greatness is responsibility, and as a national leader, SHM has a responsibility to ensure that its approaches to potential conflicts of interest and external relationships are above reproach.
COI Disclosure
The conflict of interest statements for each board member have long been posted on the SHM website. In an effort to go above and beyond the minimum standards, the format of the disclosure form has been revised, making it the most compete and detailed COI disclosure form of any physician organization in the country. In the coming months, SHM will make even tighter restrictions regarding disclosing potential conflicts of interest. While board members are required to report any and all financial receipts, the amended version will require board members who receive any contribution in excess of $5,000 to provide a detailed narrative as to what was required in service for the receipt of those funds. Further, to ensure collective accountability, any board member may call upon any other board member to provide a similarly detailed description of any item on his or her COI disclosure form.
Recognizing that other leaders in the organization might also have influence over important decisions, thereby being at risk for a conflict of interest, SHM is one of the first physician organizations to require public reporting of COI disclosures for all editors, course directors, and senior staff.
Next year, all committee chairs and quality-improvement (QI) project leaders will be required to submit similar COI disclosures.
But reporting potential conflicts is one thing; ensuring that those with significant conflicts are not put in a position of inescapable conflict of interest by virtue of their appointments is another. To be proactive, the executive committee has a designated meeting each year to individually review each nominee being considered for election to the board, committee chairs, editors, and course directors prior to their appointment.
The society will enforce CMSS Standard 1.4, which prohibits key society leaders (president, past-president, president-elect, CEO, editors, course directors) from having direct financial relationships with companies during his or her term of service. All people seeking such positions will be required to attest, at the time of the nomination, to cease all direct financial relationships prior to seeking office; failure to do so will negate their candidacy for the position they seek.
External Communications Regarding Industry
It is one thing to have potential conflicts disclosed on a website; it is quite another to ensure, with 100% confidence, that all recipients of all communications from SHM are aware of this website. Reminding all representatives of SHM to alert communication recipients to our potential conflicts of interest is a good start, but in quality parlance, this is tantamount to “telling people to try harder,” which is rarely an effective strategy to ensure 100% compliance. In response, SHM has designed a fail-safe systems solution to ensure that every communication alerts the recipient to SHM’s potential conflicts of interest. Beginning this year, SHM letterheads and e-mail, used for all written communications with external parties, will carry the following statement on the bottom of each page: “To Learn More About SHM’s Relationship with Industry Partners, Visit www.hospitalmedicine.org/industry.”
One of SHM’s missteps over the years has been the failure to distinguish external communications regarding pharmaceuticals/devices as being different from the organization’s other nonpharmaceutical communications. This unintentional oversight has been a product of the exponential increase in the society’s external communications during the past 10 years. But nonetheless, the distinction between these types of communications is important, especially for a society that receives industry support for its quality initiatives.
At the August board meeting in Chicago, a special ad hoc committee was appointed to develop specific policies regarding SHM’s communication strategy. This committee will bring to the board in November the following policy for approval: “Before SHM makes a specific comment, writes a letter, or posts an official statement on the SHM website about a pharmaceutical agent, a medical device, a specific disease state, or any medical IT services or products, the communication must be approved by the Executive Committee and reflected in the minutes of the Executive Committee. At the President’s discretion, the proposed communication will be brought to the entire Board for discussion and approval.”
As noted below, all agendas and decisions by the executive committee are communicated to the board, further ensuring accountability and oversight for any such decision.
Choices and Definitions
In the early years, all external relationships were initiated by SHM. Because SHM was a relative unknown on the national scene, if a relationship was to be entertained, it was based on SHM’s initiative to do so. Naturally, the smaller number of relationships, and the fact that the choice and nature of the relationship were initiated by SHM, made it easier to define the scope of such relationships. But now things are different: SHM’s agenda now encompasses a vast set of domains, and SHM is regularly on the receiving end of invitations to establish relationships with other organizations. Once again, as a leader of medical specialty organizations, SHM’s policies and procedures have to adapt to fit the needs of a larger and more diverse organization.
An intense amount of work has been devoted to evolving the mechanism by which SHM chooses and defines its relationships. An ad hoc committee from the board of directors has defined the 10 principles of SHM’s business relationships (see “10 Principles of SHM Business Relationships,” p. 42). In November, the board will adopt policies and procedures that will ensure that SHM will continue to only enter into relationships with external organizations with which it shares common interests or goals for advancing the quality and safety of patient care. SHM will continue to avoid influence from external organizations with respect to the policies, conduct, actions, and priorities of SHM.
Further, by policy, SHM will continue to reserve absolute control over all content and speakers at its educational conferences; content will continue to not be influenced by brand or product consideration during development or revisions. All potential partners will be informed from the outset that a partnership with SHM does not imply that SHM endorses the policies, values, and missions of the partner organization; any significant deviation from the values and mission of SHM will dissolve the partnership. SHM will establish from the outset that a partnership does not imply SHM’s support or endorsement of any products from a partner. As noted above, transparency of these relationships will be of paramount importance: All relationships, including the dollar amounts received as a product of those relationships, will be posted on SHM’s website.
Transparency in Decision-Making
As noted from the outset, the benefit of small organizations with limited agendas is that every member knows every decision. With limited decisions to be made, the vetting and review process is virtually assured. As organizations grow, as agendas expand exponentially, and as the pace quickens, the multiperson review of each decision becomes difficult to assure. The result is that errors start to appear—not due to intentional wrongdoing, but because the luxury of intense oversight is lost as the organization expands. For an organization to grow and still maintain oversight of its decision-making process, it is vital that the organization evolve to develop new methods of accountability and transparency.
To meet this need, SHM has enacted a change in its communication infrastructure to ensure “double-checks” for all of the important organizational decisions. An SHM leadership and staff “wiki” has been developed to promote and ensure transparency of all organizational decisions. Because it is accessible only to the SHM staff, board, and committee chairs, the wiki is invisible to the SHM membership. Nonetheless, you should know of this important innovation.
The wiki requires that all committee chairs post the results of their committee activities. This ensures that staff and committee leadership are on the same page, ensures that other committees are not duplicating work, augments collaboration across committees, and, most importantly, ensures collective accountability for each decision made.
Technology-based innovations have been enacted to improve the transparency of the executive leadership of the organization. The board of directors meets four times a year; the purpose of the board is to ensure oversight for all SHM decisions. Because the board comprises exclusively volunteer members meeting only four times a year, it is practically impossible for the board to approve every decision made by an organization as large as SHM. To ensure the necessary efficiency and effectiveness (i.e., being sufficiently “nimble” to act on important issues in between scheduled board meetings), the executive committee (EC) was established years ago. The EC, comprised of the president, the past-president, the president-elect, and the CEO, meets every two weeks via teleconference to review and approve all essential SHM decisions.
As an innovation to augment accountability and transparency, the agenda and minutes of the EC are now posted on the SHM board portal, allowing all board members to review and comment upon the decisions made by the president, the CEO, or the EC as a whole. Any board member, at any time, can request that the full board be convened to review an agenda item or decision.
And underlying all of these initiatives to improve an already exceptional organization are the tireless efforts of the SHM staff. Though there are nearly 50 staff members now, each continues to do the work of multiple people. SHM is arguably the fastest-growing organization in history, and advancing the organization to level after level has been an exceptional challenge. But regardless of the challenge, SHM leadership and staff has come through. I have no doubt that during this next chapter in SHM’s history, the result will be the same.
SHM is committed to advancing quality. Intrinsic to the “culture of quality” is the commitment to honesty, transparency, and ethics. Any permutation of the society that does not fully exemplify these standards will be ineffective in accomplishing our wished-for goal. In short, the actions of the society must model those that we wish to inspire in the day-to-day practice of our members. Although the unprecedented growth of the society is responsible for errors in the past, the importance of admitting our shortcomings is no less significant. We’ve had some missteps along the way, and while these mistakes are a product of events preceding my tenure, it does not matter. As president of the organization, I am taking responsibility for them, with a pledge to devote all time and energy, with all due speed, to finding systematic solutions that will prevent these errors from happening again.
And let me be even more honest. As we go forward, there are probably going to be more missteps; such is the nature of a growing and active organization. I cannot promise an error-free organization, but I can promise that if and when errors are made in the future, the same intensity will be applied to seek systematic solutions to ensure that we continue to evolve in becoming an organization that is emblematic of quality. Such is the promise of SHM; such is the promise of the hospitalist. TH
Dr. Wiese is president of SHM.
References
- Kohn LT, Corrigan JM, Donaldson MS, et al. To Err Is Human: Building a Safer Health System. Washington, D.C.: National Academies Press; 2000.
- Institute of Medicine Committee on Quality Health Care in America. Crossing the Quality Chasm: A New Health System for the 21st Century. Washington, D.C.: National Academies Press; 2001.
The challenges facing SHM are very different than they were 10 years ago. In the 1990s, the focus was on building a society that would represent the needs of the practicing hospitalist. Converting NAIP, with its 200 members, to SHM, with its now 10,000 members, was certainly no easy task, but the society then enjoyed some luxuries no longer afforded to an organization the size of the modern-day SHM. Early on, SHM was far from the public eye, escaping public scrutiny for each of its actions. With only a few hundred members, the society was intimate: Almost every member knew of every action before it happened. And the agenda, compared with today’s standards, was reasonably focused.
But times are different now. The organization is much larger and complex, and the challenges we now face are collectively a product of our success. SHM is squarely in the spotlight; every decision is closely monitored by the public eye. We now have a voice such that when we speak, people listen. But with greatness comes responsibility, and because we are in the spotlight, we must be especially careful in how we speak, lest the message be misunderstood. Further, with more than 10,000 members, 50 full-time staff, 44 committees, and nearly 500 physician volunteers, the organization no longer has the luxury of every action being known by every member prior to its enactment.
More challenging still is our agenda, which has grown to be a diverse and far-reaching strategy. While impressive and admirable, the size of this “footprint” creates new challenges in balancing the need to be “nimble” (i.e., being able to act quickly enough to be timely and effective) versus being “thorough” (i.e., ensuring that each action is appropriately vetted prior to execution).
I suspect that there are few practicing hospitalists who have not read To Err is Human or Crossing the Quality Chasm.1,2 Both make this essential point about quality: In complex systems, mistakes are bound to happen. And when errors do occur, each member of the team must be ready to take responsibility for the mistake, and immediately begin seeking systematic solutions to ensure that it does not happen again. SHM’s focus is to advance quality for all hospitalized patients. But an organization can only be effective if it emulates the principles that it hopes its members will individually espouse.
So let me start with this: There have been mistakes along the way.
That’s the hard truth. I believe that none of the mistakes have been intentional; rather, these missteps have been a product of an organization that has grown so fast, and whose success has gained so much public attention, that its infrastructure has struggled to keep pace with its growth. Any hospitalist who has seen his or her service size double in the span of a year or two knows of what I speak: As growth occurs, the approach to dealing with daily business has to evolve to meet new demands. If it does not, errors result.
One of the areas in which SHM’s growth has outpaced its policies and procedures regards SHM’s relationship with industry. I will say from the outset that having relationships with industry is not in and of itself a mistake. The reality is that without such relationships, in the setting of a landscape where governmental and philanthropic funding is disproportionately in deficit to the need, it would be almost impossible to advance the quality initiatives that have defined SHM’s success. SHM has, and will likely continue to have, relationships with industry. But requisite for having these relationships, especially for an organization that is a national leader, is going above and beyond the minimum standards to ensure transparency and ethics.
Two years ago, SHM began the arduous process of reviewing its partnerships and how it interacts with industry. I am pleased to announce that this has culminated in the Council of Medical Specialty Societies (CMSS; www.cmss.org) asking SHM to apply to become an affiliate member. Acceptance of SHM into CMSS is evidence of SHM’s demonstrated compliance with CMSS’s requirements, with respect to industry relationships, disclosure of conflicts of interest, and other measures of organizational transparency, all of which can be found at www.hospitalmedicine.org/industry.
But meeting the minimum standards has never been sufficient for SHM. The cost of greatness is responsibility, and as a national leader, SHM has a responsibility to ensure that its approaches to potential conflicts of interest and external relationships are above reproach.
COI Disclosure
The conflict of interest statements for each board member have long been posted on the SHM website. In an effort to go above and beyond the minimum standards, the format of the disclosure form has been revised, making it the most compete and detailed COI disclosure form of any physician organization in the country. In the coming months, SHM will make even tighter restrictions regarding disclosing potential conflicts of interest. While board members are required to report any and all financial receipts, the amended version will require board members who receive any contribution in excess of $5,000 to provide a detailed narrative as to what was required in service for the receipt of those funds. Further, to ensure collective accountability, any board member may call upon any other board member to provide a similarly detailed description of any item on his or her COI disclosure form.
Recognizing that other leaders in the organization might also have influence over important decisions, thereby being at risk for a conflict of interest, SHM is one of the first physician organizations to require public reporting of COI disclosures for all editors, course directors, and senior staff.
Next year, all committee chairs and quality-improvement (QI) project leaders will be required to submit similar COI disclosures.
But reporting potential conflicts is one thing; ensuring that those with significant conflicts are not put in a position of inescapable conflict of interest by virtue of their appointments is another. To be proactive, the executive committee has a designated meeting each year to individually review each nominee being considered for election to the board, committee chairs, editors, and course directors prior to their appointment.
The society will enforce CMSS Standard 1.4, which prohibits key society leaders (president, past-president, president-elect, CEO, editors, course directors) from having direct financial relationships with companies during his or her term of service. All people seeking such positions will be required to attest, at the time of the nomination, to cease all direct financial relationships prior to seeking office; failure to do so will negate their candidacy for the position they seek.
External Communications Regarding Industry
It is one thing to have potential conflicts disclosed on a website; it is quite another to ensure, with 100% confidence, that all recipients of all communications from SHM are aware of this website. Reminding all representatives of SHM to alert communication recipients to our potential conflicts of interest is a good start, but in quality parlance, this is tantamount to “telling people to try harder,” which is rarely an effective strategy to ensure 100% compliance. In response, SHM has designed a fail-safe systems solution to ensure that every communication alerts the recipient to SHM’s potential conflicts of interest. Beginning this year, SHM letterheads and e-mail, used for all written communications with external parties, will carry the following statement on the bottom of each page: “To Learn More About SHM’s Relationship with Industry Partners, Visit www.hospitalmedicine.org/industry.”
One of SHM’s missteps over the years has been the failure to distinguish external communications regarding pharmaceuticals/devices as being different from the organization’s other nonpharmaceutical communications. This unintentional oversight has been a product of the exponential increase in the society’s external communications during the past 10 years. But nonetheless, the distinction between these types of communications is important, especially for a society that receives industry support for its quality initiatives.
At the August board meeting in Chicago, a special ad hoc committee was appointed to develop specific policies regarding SHM’s communication strategy. This committee will bring to the board in November the following policy for approval: “Before SHM makes a specific comment, writes a letter, or posts an official statement on the SHM website about a pharmaceutical agent, a medical device, a specific disease state, or any medical IT services or products, the communication must be approved by the Executive Committee and reflected in the minutes of the Executive Committee. At the President’s discretion, the proposed communication will be brought to the entire Board for discussion and approval.”
As noted below, all agendas and decisions by the executive committee are communicated to the board, further ensuring accountability and oversight for any such decision.
Choices and Definitions
In the early years, all external relationships were initiated by SHM. Because SHM was a relative unknown on the national scene, if a relationship was to be entertained, it was based on SHM’s initiative to do so. Naturally, the smaller number of relationships, and the fact that the choice and nature of the relationship were initiated by SHM, made it easier to define the scope of such relationships. But now things are different: SHM’s agenda now encompasses a vast set of domains, and SHM is regularly on the receiving end of invitations to establish relationships with other organizations. Once again, as a leader of medical specialty organizations, SHM’s policies and procedures have to adapt to fit the needs of a larger and more diverse organization.
An intense amount of work has been devoted to evolving the mechanism by which SHM chooses and defines its relationships. An ad hoc committee from the board of directors has defined the 10 principles of SHM’s business relationships (see “10 Principles of SHM Business Relationships,” p. 42). In November, the board will adopt policies and procedures that will ensure that SHM will continue to only enter into relationships with external organizations with which it shares common interests or goals for advancing the quality and safety of patient care. SHM will continue to avoid influence from external organizations with respect to the policies, conduct, actions, and priorities of SHM.
Further, by policy, SHM will continue to reserve absolute control over all content and speakers at its educational conferences; content will continue to not be influenced by brand or product consideration during development or revisions. All potential partners will be informed from the outset that a partnership with SHM does not imply that SHM endorses the policies, values, and missions of the partner organization; any significant deviation from the values and mission of SHM will dissolve the partnership. SHM will establish from the outset that a partnership does not imply SHM’s support or endorsement of any products from a partner. As noted above, transparency of these relationships will be of paramount importance: All relationships, including the dollar amounts received as a product of those relationships, will be posted on SHM’s website.
Transparency in Decision-Making
As noted from the outset, the benefit of small organizations with limited agendas is that every member knows every decision. With limited decisions to be made, the vetting and review process is virtually assured. As organizations grow, as agendas expand exponentially, and as the pace quickens, the multiperson review of each decision becomes difficult to assure. The result is that errors start to appear—not due to intentional wrongdoing, but because the luxury of intense oversight is lost as the organization expands. For an organization to grow and still maintain oversight of its decision-making process, it is vital that the organization evolve to develop new methods of accountability and transparency.
To meet this need, SHM has enacted a change in its communication infrastructure to ensure “double-checks” for all of the important organizational decisions. An SHM leadership and staff “wiki” has been developed to promote and ensure transparency of all organizational decisions. Because it is accessible only to the SHM staff, board, and committee chairs, the wiki is invisible to the SHM membership. Nonetheless, you should know of this important innovation.
The wiki requires that all committee chairs post the results of their committee activities. This ensures that staff and committee leadership are on the same page, ensures that other committees are not duplicating work, augments collaboration across committees, and, most importantly, ensures collective accountability for each decision made.
Technology-based innovations have been enacted to improve the transparency of the executive leadership of the organization. The board of directors meets four times a year; the purpose of the board is to ensure oversight for all SHM decisions. Because the board comprises exclusively volunteer members meeting only four times a year, it is practically impossible for the board to approve every decision made by an organization as large as SHM. To ensure the necessary efficiency and effectiveness (i.e., being sufficiently “nimble” to act on important issues in between scheduled board meetings), the executive committee (EC) was established years ago. The EC, comprised of the president, the past-president, the president-elect, and the CEO, meets every two weeks via teleconference to review and approve all essential SHM decisions.
As an innovation to augment accountability and transparency, the agenda and minutes of the EC are now posted on the SHM board portal, allowing all board members to review and comment upon the decisions made by the president, the CEO, or the EC as a whole. Any board member, at any time, can request that the full board be convened to review an agenda item or decision.
And underlying all of these initiatives to improve an already exceptional organization are the tireless efforts of the SHM staff. Though there are nearly 50 staff members now, each continues to do the work of multiple people. SHM is arguably the fastest-growing organization in history, and advancing the organization to level after level has been an exceptional challenge. But regardless of the challenge, SHM leadership and staff has come through. I have no doubt that during this next chapter in SHM’s history, the result will be the same.
SHM is committed to advancing quality. Intrinsic to the “culture of quality” is the commitment to honesty, transparency, and ethics. Any permutation of the society that does not fully exemplify these standards will be ineffective in accomplishing our wished-for goal. In short, the actions of the society must model those that we wish to inspire in the day-to-day practice of our members. Although the unprecedented growth of the society is responsible for errors in the past, the importance of admitting our shortcomings is no less significant. We’ve had some missteps along the way, and while these mistakes are a product of events preceding my tenure, it does not matter. As president of the organization, I am taking responsibility for them, with a pledge to devote all time and energy, with all due speed, to finding systematic solutions that will prevent these errors from happening again.
And let me be even more honest. As we go forward, there are probably going to be more missteps; such is the nature of a growing and active organization. I cannot promise an error-free organization, but I can promise that if and when errors are made in the future, the same intensity will be applied to seek systematic solutions to ensure that we continue to evolve in becoming an organization that is emblematic of quality. Such is the promise of SHM; such is the promise of the hospitalist. TH
Dr. Wiese is president of SHM.
References
- Kohn LT, Corrigan JM, Donaldson MS, et al. To Err Is Human: Building a Safer Health System. Washington, D.C.: National Academies Press; 2000.
- Institute of Medicine Committee on Quality Health Care in America. Crossing the Quality Chasm: A New Health System for the 21st Century. Washington, D.C.: National Academies Press; 2001.
28,999 and Me
How many people have to die before you’ll pay attention? Like many of you, I read the article but it didn’t really stick. Rather, I filed it in the “interesting tidbits” folder on my brain’s hard drive. Somehow 29,000 people with cancer just didn’t register as a big number.
Until I thought I could be one of them.
The Number
I was harried, running late for a meeting, questioning my decision to try to shoehorn a PCP appointment into my lunch break. Then again, this was a routine follow-up of some labs and I, of course, am the picture of health. Well, I am if you exclude my LDL. It turns out that on a check 12 months earlier, my LDL was found to be running a few heart attacks higher than normal. I took this as a sign, combined with my ballooning waist, middle-ish age, and nagging wife, that I needed to do something.
Still, I wasn’t ready for “something” to include an anticholesterol medication. Instead, I chose the masochistic route and hit the treadmill. And the bike. And a little less of the dinner plate. As a result, I had lost 30 pounds, a handful of pant sizes and, while I wasn’t exactly “in shape,” I did find myself shaped a little less like the Michelin Man.
Triumphantly, I was returning to vanquish my tormentor—the PCP who foolhardily recommended I start a medication.
Sitting in the office awaiting the news of my post-weight-loss cholesterol, my grin was wide and smug—and apparently still overflowing with LDL. I was devastated. 259? I lose weight and my LDL actually goes up!?! I could feel the foam cells in my coronary plaques twitch with delight as they mockingly gorged on chylomicrons.
Undeterred, I inquired what my options were, secretly hoping the answer would be more red wine. Emboldened by my supersaturated serum, my PCP declared it was time for a statin. Alternatively, he noted that I could get a CT angiogram of my coronaries and, if they were clean, I potentially could bypass drug therapy. Thoughts of avoided myalgias happily flittered across my mind until they stumbled onto the number 29,000. It was then that I recalled the recent Archives of Internal Medicine paper.1
The Study
Using risk models based on the known biological effects of radiation, researchers estimated that approximately 29,000 people would develop cancer from the radiation associated with CT scans in 2007 alone. To arrive at this number, the authors used data showing that 1.5% to 2% of all U.S. cancers could be traced to the radiation from CT scans.
Not surprisingly, the most commonly utilized CT scans—namely, abdominal (14,000 a year), chest (4,100 a year), and head (4,000 a year)—accounted for the most morbidity. However, CT angiography, with its super-high dose of radiation, was projected to contribute 2,700 cancers a year. Apparently, my PCP didn’t read this article.
In terms of types of malignancy, lung cancer leads the list with 6,200 projected CT-induced cancers per year, followed by colon cancer (3,500 a year) and leukemia (2,800 a year).
The Names
If the numbers from this study hold, then about 1 in every 2,000 CT scans results in a new cancer. That would mean that I’ve dished out several cancers during my practice. In fact, I’ve ordered many thousand CT scans over my career—give or take a cancer. So my pen has, statistically, caused approximately three cancers.
I wondered which three patients it was. Was it Mr. Reynolds, who would’ve very likely died had we not diagnosed his post-operative abdominal abscess? Perhaps it was Mr. Jenson, who surely would have fared poorly if his pulmonary embolism had not been diagnosed and treated. Maybe it was Mrs. Hernandez, who wouldn’t have received thrombolytics for her stroke without a head CT.
Yes, I might have played a role in causing cancer in these three patients, but I did so knowing that I also saved, or at least improved, their lives. Most patients would accept that calculus.
But what if it were a different three? What if my cancer was that head CT I ordered for Mr. Davidson’s confusion, even though I know that head scans are rarely helpful in the evaluation of delirium? Perhaps my cancer-causer was that abdominal CT scan for Mrs. Ramirez’s chronic pain, which was clearly referable to her irritable bowel syndrome. Maybe it will be that CT scan I ordered last week because the patient insisted it be done, even though I strongly suspected, correctly, that it wouldn’t alter my management.
Which three would it be?
The Questions
This triggered more questions. How many of the 70 million-plus CT scans we order every year really are necessary? How many could be avoided by a robust physical examination, crisper clinical reasoning, or an alternate test? Do our patients really know the risk of these “innocuous” tests? Do we?
And, more personally, what if my PCP was still sitting on two? Would I be his number three?
Moving forward, I vow to remember 29,000. It will remain in the forefront of my mind, constantly badgering me about the next CT scan I order. To be sure, I will continue to order CTs—a lot of CTs. However, I will do so through the prism of the following query. If a patient developed a cancer from the CT scan I was about to order, could I sincerely look them in the eye and tell them I would do the test again?
And I’m agitated by one final question. How is that it took my own carcinogenic brush with CT scans for me to realize the gravity of 29,000? It’s not that 29,000 is not a big number. In fact, it’s precisely because it is a big number that we miss its importance. It’s too easy to hide behind the anonymity of the number. Because in the end, numbers don’t have names until the name is yours. TH
Dr. Glasheen is associate professor of medicine at the University of Colorado Denver, where he serves as director of the Hospital Medicine Program and the Hospitalist Training Program, and as associate program director of the Internal Medicine Residency Program.
Reference
- Berrington de González A, Mahesh M, Kim KP, et al. Projected cancer risks from computed tomographic scans performed in the United States in 2007. Arch Intern Med. 2009;169(22):2071-2077.
How many people have to die before you’ll pay attention? Like many of you, I read the article but it didn’t really stick. Rather, I filed it in the “interesting tidbits” folder on my brain’s hard drive. Somehow 29,000 people with cancer just didn’t register as a big number.
Until I thought I could be one of them.
The Number
I was harried, running late for a meeting, questioning my decision to try to shoehorn a PCP appointment into my lunch break. Then again, this was a routine follow-up of some labs and I, of course, am the picture of health. Well, I am if you exclude my LDL. It turns out that on a check 12 months earlier, my LDL was found to be running a few heart attacks higher than normal. I took this as a sign, combined with my ballooning waist, middle-ish age, and nagging wife, that I needed to do something.
Still, I wasn’t ready for “something” to include an anticholesterol medication. Instead, I chose the masochistic route and hit the treadmill. And the bike. And a little less of the dinner plate. As a result, I had lost 30 pounds, a handful of pant sizes and, while I wasn’t exactly “in shape,” I did find myself shaped a little less like the Michelin Man.
Triumphantly, I was returning to vanquish my tormentor—the PCP who foolhardily recommended I start a medication.
Sitting in the office awaiting the news of my post-weight-loss cholesterol, my grin was wide and smug—and apparently still overflowing with LDL. I was devastated. 259? I lose weight and my LDL actually goes up!?! I could feel the foam cells in my coronary plaques twitch with delight as they mockingly gorged on chylomicrons.
Undeterred, I inquired what my options were, secretly hoping the answer would be more red wine. Emboldened by my supersaturated serum, my PCP declared it was time for a statin. Alternatively, he noted that I could get a CT angiogram of my coronaries and, if they were clean, I potentially could bypass drug therapy. Thoughts of avoided myalgias happily flittered across my mind until they stumbled onto the number 29,000. It was then that I recalled the recent Archives of Internal Medicine paper.1
The Study
Using risk models based on the known biological effects of radiation, researchers estimated that approximately 29,000 people would develop cancer from the radiation associated with CT scans in 2007 alone. To arrive at this number, the authors used data showing that 1.5% to 2% of all U.S. cancers could be traced to the radiation from CT scans.
Not surprisingly, the most commonly utilized CT scans—namely, abdominal (14,000 a year), chest (4,100 a year), and head (4,000 a year)—accounted for the most morbidity. However, CT angiography, with its super-high dose of radiation, was projected to contribute 2,700 cancers a year. Apparently, my PCP didn’t read this article.
In terms of types of malignancy, lung cancer leads the list with 6,200 projected CT-induced cancers per year, followed by colon cancer (3,500 a year) and leukemia (2,800 a year).
The Names
If the numbers from this study hold, then about 1 in every 2,000 CT scans results in a new cancer. That would mean that I’ve dished out several cancers during my practice. In fact, I’ve ordered many thousand CT scans over my career—give or take a cancer. So my pen has, statistically, caused approximately three cancers.
I wondered which three patients it was. Was it Mr. Reynolds, who would’ve very likely died had we not diagnosed his post-operative abdominal abscess? Perhaps it was Mr. Jenson, who surely would have fared poorly if his pulmonary embolism had not been diagnosed and treated. Maybe it was Mrs. Hernandez, who wouldn’t have received thrombolytics for her stroke without a head CT.
Yes, I might have played a role in causing cancer in these three patients, but I did so knowing that I also saved, or at least improved, their lives. Most patients would accept that calculus.
But what if it were a different three? What if my cancer was that head CT I ordered for Mr. Davidson’s confusion, even though I know that head scans are rarely helpful in the evaluation of delirium? Perhaps my cancer-causer was that abdominal CT scan for Mrs. Ramirez’s chronic pain, which was clearly referable to her irritable bowel syndrome. Maybe it will be that CT scan I ordered last week because the patient insisted it be done, even though I strongly suspected, correctly, that it wouldn’t alter my management.
Which three would it be?
The Questions
This triggered more questions. How many of the 70 million-plus CT scans we order every year really are necessary? How many could be avoided by a robust physical examination, crisper clinical reasoning, or an alternate test? Do our patients really know the risk of these “innocuous” tests? Do we?
And, more personally, what if my PCP was still sitting on two? Would I be his number three?
Moving forward, I vow to remember 29,000. It will remain in the forefront of my mind, constantly badgering me about the next CT scan I order. To be sure, I will continue to order CTs—a lot of CTs. However, I will do so through the prism of the following query. If a patient developed a cancer from the CT scan I was about to order, could I sincerely look them in the eye and tell them I would do the test again?
And I’m agitated by one final question. How is that it took my own carcinogenic brush with CT scans for me to realize the gravity of 29,000? It’s not that 29,000 is not a big number. In fact, it’s precisely because it is a big number that we miss its importance. It’s too easy to hide behind the anonymity of the number. Because in the end, numbers don’t have names until the name is yours. TH
Dr. Glasheen is associate professor of medicine at the University of Colorado Denver, where he serves as director of the Hospital Medicine Program and the Hospitalist Training Program, and as associate program director of the Internal Medicine Residency Program.
Reference
- Berrington de González A, Mahesh M, Kim KP, et al. Projected cancer risks from computed tomographic scans performed in the United States in 2007. Arch Intern Med. 2009;169(22):2071-2077.
How many people have to die before you’ll pay attention? Like many of you, I read the article but it didn’t really stick. Rather, I filed it in the “interesting tidbits” folder on my brain’s hard drive. Somehow 29,000 people with cancer just didn’t register as a big number.
Until I thought I could be one of them.
The Number
I was harried, running late for a meeting, questioning my decision to try to shoehorn a PCP appointment into my lunch break. Then again, this was a routine follow-up of some labs and I, of course, am the picture of health. Well, I am if you exclude my LDL. It turns out that on a check 12 months earlier, my LDL was found to be running a few heart attacks higher than normal. I took this as a sign, combined with my ballooning waist, middle-ish age, and nagging wife, that I needed to do something.
Still, I wasn’t ready for “something” to include an anticholesterol medication. Instead, I chose the masochistic route and hit the treadmill. And the bike. And a little less of the dinner plate. As a result, I had lost 30 pounds, a handful of pant sizes and, while I wasn’t exactly “in shape,” I did find myself shaped a little less like the Michelin Man.
Triumphantly, I was returning to vanquish my tormentor—the PCP who foolhardily recommended I start a medication.
Sitting in the office awaiting the news of my post-weight-loss cholesterol, my grin was wide and smug—and apparently still overflowing with LDL. I was devastated. 259? I lose weight and my LDL actually goes up!?! I could feel the foam cells in my coronary plaques twitch with delight as they mockingly gorged on chylomicrons.
Undeterred, I inquired what my options were, secretly hoping the answer would be more red wine. Emboldened by my supersaturated serum, my PCP declared it was time for a statin. Alternatively, he noted that I could get a CT angiogram of my coronaries and, if they were clean, I potentially could bypass drug therapy. Thoughts of avoided myalgias happily flittered across my mind until they stumbled onto the number 29,000. It was then that I recalled the recent Archives of Internal Medicine paper.1
The Study
Using risk models based on the known biological effects of radiation, researchers estimated that approximately 29,000 people would develop cancer from the radiation associated with CT scans in 2007 alone. To arrive at this number, the authors used data showing that 1.5% to 2% of all U.S. cancers could be traced to the radiation from CT scans.
Not surprisingly, the most commonly utilized CT scans—namely, abdominal (14,000 a year), chest (4,100 a year), and head (4,000 a year)—accounted for the most morbidity. However, CT angiography, with its super-high dose of radiation, was projected to contribute 2,700 cancers a year. Apparently, my PCP didn’t read this article.
In terms of types of malignancy, lung cancer leads the list with 6,200 projected CT-induced cancers per year, followed by colon cancer (3,500 a year) and leukemia (2,800 a year).
The Names
If the numbers from this study hold, then about 1 in every 2,000 CT scans results in a new cancer. That would mean that I’ve dished out several cancers during my practice. In fact, I’ve ordered many thousand CT scans over my career—give or take a cancer. So my pen has, statistically, caused approximately three cancers.
I wondered which three patients it was. Was it Mr. Reynolds, who would’ve very likely died had we not diagnosed his post-operative abdominal abscess? Perhaps it was Mr. Jenson, who surely would have fared poorly if his pulmonary embolism had not been diagnosed and treated. Maybe it was Mrs. Hernandez, who wouldn’t have received thrombolytics for her stroke without a head CT.
Yes, I might have played a role in causing cancer in these three patients, but I did so knowing that I also saved, or at least improved, their lives. Most patients would accept that calculus.
But what if it were a different three? What if my cancer was that head CT I ordered for Mr. Davidson’s confusion, even though I know that head scans are rarely helpful in the evaluation of delirium? Perhaps my cancer-causer was that abdominal CT scan for Mrs. Ramirez’s chronic pain, which was clearly referable to her irritable bowel syndrome. Maybe it will be that CT scan I ordered last week because the patient insisted it be done, even though I strongly suspected, correctly, that it wouldn’t alter my management.
Which three would it be?
The Questions
This triggered more questions. How many of the 70 million-plus CT scans we order every year really are necessary? How many could be avoided by a robust physical examination, crisper clinical reasoning, or an alternate test? Do our patients really know the risk of these “innocuous” tests? Do we?
And, more personally, what if my PCP was still sitting on two? Would I be his number three?
Moving forward, I vow to remember 29,000. It will remain in the forefront of my mind, constantly badgering me about the next CT scan I order. To be sure, I will continue to order CTs—a lot of CTs. However, I will do so through the prism of the following query. If a patient developed a cancer from the CT scan I was about to order, could I sincerely look them in the eye and tell them I would do the test again?
And I’m agitated by one final question. How is that it took my own carcinogenic brush with CT scans for me to realize the gravity of 29,000? It’s not that 29,000 is not a big number. In fact, it’s precisely because it is a big number that we miss its importance. It’s too easy to hide behind the anonymity of the number. Because in the end, numbers don’t have names until the name is yours. TH
Dr. Glasheen is associate professor of medicine at the University of Colorado Denver, where he serves as director of the Hospital Medicine Program and the Hospitalist Training Program, and as associate program director of the Internal Medicine Residency Program.
Reference
- Berrington de González A, Mahesh M, Kim KP, et al. Projected cancer risks from computed tomographic scans performed in the United States in 2007. Arch Intern Med. 2009;169(22):2071-2077.
Volume Control, Part II
Last month I began looking at ways hospitalist practices can manage unpredictable increases in patient volume, also known as surge staffing. I provided my view of a “jeopardy” system and a patient volume cap for hospitalists. While both are potentially very effective, they have a high cost and in my view are imperfect solutions. This month I’ll examine some less common strategies to provide surge staffing. Although less popular, I think these options are more valuable.
Schedule More Providers
I’ve worked with a lot of practices and am struck by how patient volume for nearly all of them falls within a reasonably predictable range. While no one can predict with certainty which days will be unusually busy or slow, nearly all practices have a range of daily encounters that is roughly half to 1 1/2 of the mean. For example, if a practice has a mean of 60 billable encounters per day, it probably ranges from about 30 to 90 encounters on any given day. (The larger the practice, the more likely they are to conform to this range. Small practices, with average daily encounters fewer than 20, have a much wider range of daily volumes as a percent of the mean.)
Despite knowing that volumes will vary unpredictably, most practices provide the same fixed “dose” of provider staffing every day—that is, the single most common model for staffing and scheduling is to provide a fixed number of day-shift doctors (“rounders”) who work a fixed number of hours. For example, with an average of 60 billable encounters a day, a hospitalist group might decide to staff with four day-shift rounders working 12-hour shifts. This equates to a fixed 48 hours of daytime staffing. This is reasonable until the busy days arrive. Those four doctors will be much busier than average when there are 90 patients to see in a day, and will probably have a hard time seeing 22 or 23 patients each during their 12-hour shift. If such a busy day occurs more than a couple of times annually, then the practice should probably make some changes.
One approach to solving this type of staffing predicament is to add a fifth day-shift rounder. In other words, when making staffing decisions, consider giving more weight to the busiest days than the average day. This sounds fine until thinking about the practice budget. It will be pretty expensive to add doctors every day just so there are enough on duty when things get really busy. But if the hospitalists are willing to accept reduced compensation, then it might be financially reasonable to go ahead and add staff. This is easiest to do when the hospitalists are paid a significant (e.g. ≥50%) portion of their income based on their productivity, which will enable the hospitalists themselves to have a lot of say about when it is time to add staff. (Being paid on a nearly fixed annual salary means that it is the finance person who usually has the say about when it is time to add staff. And you can bet he’ll be making staffing decisions based on the average daily encounters, rather than the busy days.)
My own preference would be to do just that: Accept a reduction in compensation in return for protection against really busy and stressful days. I’m not suggesting others should agree with me, and in my experience, most don’t. (My own practice partners don’t agree with me on this one.) So I’m not really recommending it as a best practice, but I want to ensure that you don’t forget it is an option. And keep in mind you could adjust staffing by degrees; some settings might add a half-time physician or a nonphysician provider to try to find the sweet spot between having enough staff on duty every day to handle surges in volume and the cost of that staffing to the employer—or the hospitalists themselves.
Of course, if I were willing to reduce my compensation and average daily workload, then I would expect to be freed from the expectation that all rounding doctors work 12-hour shifts. Let’s turn our attention to the interplay between fixed day-shift durations and surge staffing.
Fixed-Shift Schedules Inhibit Surge Capacity
I think it usually is best to avoid fixed durations for day shifts. It might be necessary to require at least one daytime rounder to stay at least until a specified time (e.g. the arrival of the night-shift doctor), but in most cases it is reasonable for some rounders to leave when their work is done. They might need to continue responding to pages until the start of the night shift, but it usually isn’t necessary to have all rounders in the hospital until a predetermined end of the shift.
The problem is that when shifts have a fixed duration, the providers will focus on the start and stop time of their shift and might be unwilling to work beyond it. If instead there are no clearly fixed start and stop times for each day shift, then the hospitalists are likely to be willing to simply work longer on busy days, as long as they can work shorter on slow days. This is probably the most effective method of surge capacity, and it fits well with staffing each day with more providers than are required for the average patient volume.
Simply having the rounding doctors work longer on busy days must be done within reason. And there is a really wide range of opinion about what is reasonable. I think it is reasonable if a hospitalist works two or three hours longer than usual for three or four consecutive busy days, as long as the hospitalist is allowed to work less on days that are not very busy. But just what is a reasonable maximum daily amount of work for even one day is a topic that can lead to passionate debate. You’ll have to decide the details of what is and isn’t acceptable in your group.
Unit-Based Assignments
Aside from fixed-duration day shifts, unit-based assignment of hospitalists is the most common practice inhibiting surge capacity. Not long ago I worked with a practice that followed very strict unit-based assignments, which significantly inhibited “load-leveling,” and thus surge capacity. On any given day the patient volume for the whole practice might be very reasonable, but because it was never distributed evenly among the rounders, there was a very good chance that at least one doctor was drowning in work. And because of the strict approach, the other doctors didn’t come to the rescue.
I think the only reasonable approach is to deviate from such a strict unit-based assignment, at least a little. One rounder could be a utility doctor who doesn’t have her own unit and instead roams throughout the hospital, having been assigned patients based on the workload of each of her unit-based colleagues. TH
Dr. Nelson has been a practicing hospitalist since 1988 and is cofounder and past president of SHM. He is a principal in Nelson Flores Hospital Medicine Consultants (www.nelsonflores.com) and codirector and faculty for SHM’s “Best Practices in Managing a Hospital Medicine Program” course. This column represents his views and is not intended to reflect an official position of SHM.
Last month I began looking at ways hospitalist practices can manage unpredictable increases in patient volume, also known as surge staffing. I provided my view of a “jeopardy” system and a patient volume cap for hospitalists. While both are potentially very effective, they have a high cost and in my view are imperfect solutions. This month I’ll examine some less common strategies to provide surge staffing. Although less popular, I think these options are more valuable.
Schedule More Providers
I’ve worked with a lot of practices and am struck by how patient volume for nearly all of them falls within a reasonably predictable range. While no one can predict with certainty which days will be unusually busy or slow, nearly all practices have a range of daily encounters that is roughly half to 1 1/2 of the mean. For example, if a practice has a mean of 60 billable encounters per day, it probably ranges from about 30 to 90 encounters on any given day. (The larger the practice, the more likely they are to conform to this range. Small practices, with average daily encounters fewer than 20, have a much wider range of daily volumes as a percent of the mean.)
Despite knowing that volumes will vary unpredictably, most practices provide the same fixed “dose” of provider staffing every day—that is, the single most common model for staffing and scheduling is to provide a fixed number of day-shift doctors (“rounders”) who work a fixed number of hours. For example, with an average of 60 billable encounters a day, a hospitalist group might decide to staff with four day-shift rounders working 12-hour shifts. This equates to a fixed 48 hours of daytime staffing. This is reasonable until the busy days arrive. Those four doctors will be much busier than average when there are 90 patients to see in a day, and will probably have a hard time seeing 22 or 23 patients each during their 12-hour shift. If such a busy day occurs more than a couple of times annually, then the practice should probably make some changes.
One approach to solving this type of staffing predicament is to add a fifth day-shift rounder. In other words, when making staffing decisions, consider giving more weight to the busiest days than the average day. This sounds fine until thinking about the practice budget. It will be pretty expensive to add doctors every day just so there are enough on duty when things get really busy. But if the hospitalists are willing to accept reduced compensation, then it might be financially reasonable to go ahead and add staff. This is easiest to do when the hospitalists are paid a significant (e.g. ≥50%) portion of their income based on their productivity, which will enable the hospitalists themselves to have a lot of say about when it is time to add staff. (Being paid on a nearly fixed annual salary means that it is the finance person who usually has the say about when it is time to add staff. And you can bet he’ll be making staffing decisions based on the average daily encounters, rather than the busy days.)
My own preference would be to do just that: Accept a reduction in compensation in return for protection against really busy and stressful days. I’m not suggesting others should agree with me, and in my experience, most don’t. (My own practice partners don’t agree with me on this one.) So I’m not really recommending it as a best practice, but I want to ensure that you don’t forget it is an option. And keep in mind you could adjust staffing by degrees; some settings might add a half-time physician or a nonphysician provider to try to find the sweet spot between having enough staff on duty every day to handle surges in volume and the cost of that staffing to the employer—or the hospitalists themselves.
Of course, if I were willing to reduce my compensation and average daily workload, then I would expect to be freed from the expectation that all rounding doctors work 12-hour shifts. Let’s turn our attention to the interplay between fixed day-shift durations and surge staffing.
Fixed-Shift Schedules Inhibit Surge Capacity
I think it usually is best to avoid fixed durations for day shifts. It might be necessary to require at least one daytime rounder to stay at least until a specified time (e.g. the arrival of the night-shift doctor), but in most cases it is reasonable for some rounders to leave when their work is done. They might need to continue responding to pages until the start of the night shift, but it usually isn’t necessary to have all rounders in the hospital until a predetermined end of the shift.
The problem is that when shifts have a fixed duration, the providers will focus on the start and stop time of their shift and might be unwilling to work beyond it. If instead there are no clearly fixed start and stop times for each day shift, then the hospitalists are likely to be willing to simply work longer on busy days, as long as they can work shorter on slow days. This is probably the most effective method of surge capacity, and it fits well with staffing each day with more providers than are required for the average patient volume.
Simply having the rounding doctors work longer on busy days must be done within reason. And there is a really wide range of opinion about what is reasonable. I think it is reasonable if a hospitalist works two or three hours longer than usual for three or four consecutive busy days, as long as the hospitalist is allowed to work less on days that are not very busy. But just what is a reasonable maximum daily amount of work for even one day is a topic that can lead to passionate debate. You’ll have to decide the details of what is and isn’t acceptable in your group.
Unit-Based Assignments
Aside from fixed-duration day shifts, unit-based assignment of hospitalists is the most common practice inhibiting surge capacity. Not long ago I worked with a practice that followed very strict unit-based assignments, which significantly inhibited “load-leveling,” and thus surge capacity. On any given day the patient volume for the whole practice might be very reasonable, but because it was never distributed evenly among the rounders, there was a very good chance that at least one doctor was drowning in work. And because of the strict approach, the other doctors didn’t come to the rescue.
I think the only reasonable approach is to deviate from such a strict unit-based assignment, at least a little. One rounder could be a utility doctor who doesn’t have her own unit and instead roams throughout the hospital, having been assigned patients based on the workload of each of her unit-based colleagues. TH
Dr. Nelson has been a practicing hospitalist since 1988 and is cofounder and past president of SHM. He is a principal in Nelson Flores Hospital Medicine Consultants (www.nelsonflores.com) and codirector and faculty for SHM’s “Best Practices in Managing a Hospital Medicine Program” course. This column represents his views and is not intended to reflect an official position of SHM.
Last month I began looking at ways hospitalist practices can manage unpredictable increases in patient volume, also known as surge staffing. I provided my view of a “jeopardy” system and a patient volume cap for hospitalists. While both are potentially very effective, they have a high cost and in my view are imperfect solutions. This month I’ll examine some less common strategies to provide surge staffing. Although less popular, I think these options are more valuable.
Schedule More Providers
I’ve worked with a lot of practices and am struck by how patient volume for nearly all of them falls within a reasonably predictable range. While no one can predict with certainty which days will be unusually busy or slow, nearly all practices have a range of daily encounters that is roughly half to 1 1/2 of the mean. For example, if a practice has a mean of 60 billable encounters per day, it probably ranges from about 30 to 90 encounters on any given day. (The larger the practice, the more likely they are to conform to this range. Small practices, with average daily encounters fewer than 20, have a much wider range of daily volumes as a percent of the mean.)
Despite knowing that volumes will vary unpredictably, most practices provide the same fixed “dose” of provider staffing every day—that is, the single most common model for staffing and scheduling is to provide a fixed number of day-shift doctors (“rounders”) who work a fixed number of hours. For example, with an average of 60 billable encounters a day, a hospitalist group might decide to staff with four day-shift rounders working 12-hour shifts. This equates to a fixed 48 hours of daytime staffing. This is reasonable until the busy days arrive. Those four doctors will be much busier than average when there are 90 patients to see in a day, and will probably have a hard time seeing 22 or 23 patients each during their 12-hour shift. If such a busy day occurs more than a couple of times annually, then the practice should probably make some changes.
One approach to solving this type of staffing predicament is to add a fifth day-shift rounder. In other words, when making staffing decisions, consider giving more weight to the busiest days than the average day. This sounds fine until thinking about the practice budget. It will be pretty expensive to add doctors every day just so there are enough on duty when things get really busy. But if the hospitalists are willing to accept reduced compensation, then it might be financially reasonable to go ahead and add staff. This is easiest to do when the hospitalists are paid a significant (e.g. ≥50%) portion of their income based on their productivity, which will enable the hospitalists themselves to have a lot of say about when it is time to add staff. (Being paid on a nearly fixed annual salary means that it is the finance person who usually has the say about when it is time to add staff. And you can bet he’ll be making staffing decisions based on the average daily encounters, rather than the busy days.)
My own preference would be to do just that: Accept a reduction in compensation in return for protection against really busy and stressful days. I’m not suggesting others should agree with me, and in my experience, most don’t. (My own practice partners don’t agree with me on this one.) So I’m not really recommending it as a best practice, but I want to ensure that you don’t forget it is an option. And keep in mind you could adjust staffing by degrees; some settings might add a half-time physician or a nonphysician provider to try to find the sweet spot between having enough staff on duty every day to handle surges in volume and the cost of that staffing to the employer—or the hospitalists themselves.
Of course, if I were willing to reduce my compensation and average daily workload, then I would expect to be freed from the expectation that all rounding doctors work 12-hour shifts. Let’s turn our attention to the interplay between fixed day-shift durations and surge staffing.
Fixed-Shift Schedules Inhibit Surge Capacity
I think it usually is best to avoid fixed durations for day shifts. It might be necessary to require at least one daytime rounder to stay at least until a specified time (e.g. the arrival of the night-shift doctor), but in most cases it is reasonable for some rounders to leave when their work is done. They might need to continue responding to pages until the start of the night shift, but it usually isn’t necessary to have all rounders in the hospital until a predetermined end of the shift.
The problem is that when shifts have a fixed duration, the providers will focus on the start and stop time of their shift and might be unwilling to work beyond it. If instead there are no clearly fixed start and stop times for each day shift, then the hospitalists are likely to be willing to simply work longer on busy days, as long as they can work shorter on slow days. This is probably the most effective method of surge capacity, and it fits well with staffing each day with more providers than are required for the average patient volume.
Simply having the rounding doctors work longer on busy days must be done within reason. And there is a really wide range of opinion about what is reasonable. I think it is reasonable if a hospitalist works two or three hours longer than usual for three or four consecutive busy days, as long as the hospitalist is allowed to work less on days that are not very busy. But just what is a reasonable maximum daily amount of work for even one day is a topic that can lead to passionate debate. You’ll have to decide the details of what is and isn’t acceptable in your group.
Unit-Based Assignments
Aside from fixed-duration day shifts, unit-based assignment of hospitalists is the most common practice inhibiting surge capacity. Not long ago I worked with a practice that followed very strict unit-based assignments, which significantly inhibited “load-leveling,” and thus surge capacity. On any given day the patient volume for the whole practice might be very reasonable, but because it was never distributed evenly among the rounders, there was a very good chance that at least one doctor was drowning in work. And because of the strict approach, the other doctors didn’t come to the rescue.
I think the only reasonable approach is to deviate from such a strict unit-based assignment, at least a little. One rounder could be a utility doctor who doesn’t have her own unit and instead roams throughout the hospital, having been assigned patients based on the workload of each of her unit-based colleagues. TH
Dr. Nelson has been a practicing hospitalist since 1988 and is cofounder and past president of SHM. He is a principal in Nelson Flores Hospital Medicine Consultants (www.nelsonflores.com) and codirector and faculty for SHM’s “Best Practices in Managing a Hospital Medicine Program” course. This column represents his views and is not intended to reflect an official position of SHM.
Should HM Redefine Its Role as Provider and Adjust Expectations for Inpatient Care?
Did you happen to read a recent New York Times article (www.nytimes.com/2010/05/27/us/27hosp.html) about hospitalists? I thought the article was great, but I was surprised by some of the negative reader feedback. What did you think?
George Eppley, MD
Reed, Ga.
Dr. Hospitalist responds: I read the NYT article by Jane Gross, “New Breed of Specialist Steps in for Family Doctor,” which was published May 26. The accompanying reader comment section is available at http://newoldage.blogs.nytimes.com/2010/05/26/in-hospitals-new-fingers -on-the-pulse/?ref=us.
The article provides the statistics that all of us in HM have come to know: HM is the fastest-growing medical specialty in the U.S. and, over the past decade, the number of hospitalists in the U.S. has grown from hundreds to 30,000. Gross talks about the care a hospitalist at the Hospital of the University of Pennsylvania provides for her patient. She highlights the challenges of transitions of care and references the work being done by hospitalists and SHM to make sure patients are making safe transitions. While the article was largely supportive of HM, she does provide a shade of balance when she mentions the risks to the patient when hospitalists fail to do their job when it comes to communication.
As a practicing hospitalist, I kind of wished I had stopped reading at the end of the article. I honestly did not like most of what I read in the reader comment section. Although the article was a feel-good story, I think it is fair to say the reader comments were largely negative. I understand that readers with negative experiences with hospitalists might be more likely to post a comment; nevertheless, some of the comments are hard to ignore—mainly because I suspect some of it is true.
One reader from Raleigh, N.C., wrote, “Hospitalists proved inept at contacting the patients’ existing doctors or even talking to the patients. Then, on discharge to nursing homes for further recovery, the ball was dropped further, with very poor communication of medication dosages, etc.” Yikes! What happened to the communication and the medication reconciliation process?
A reader from Massachusetts wrote about “the hospitalist … (who) ordered five blood draws in the space of several hours to replicate tests that had already been taken by the primary-care physician before admission.” So, not only are hospitalists poor communicators and do not do a good job with transitions of care, but their care is also driving up the cost of healthcare needlessly?
The most positive comments seem to come from outpatient providers and, quite honestly, I found them lukewarm at best. A PCP from Charleston, S.C., wrote, “I no longer have to cancel the appointments of the patients at the last minute in order to attend to an emergency occurring outside my office. It is a very efficient system.” Glad to hear the hospitalist relationship is working out for you, Dr. PCP, but as a patient in the hospital, I am worried more about the competency of this doctor, whom I have never met before this hospitalization, as opposed to how this doctor is going to make you more “efficient” in your office practice.
I came away with several thoughts after reading the article and the comments.
First, we need to set the right expectations. Is this the equivalent of the star athlete who makes a brash statement followed shortly thereafter by the statement, “I was misquoted”? Well … maybe. Are we who we say we are? The headline is “New Breed of Specialist Steps in for Family Doctor.”
As a practicing hospitalist, I never describe myself as replacing the family doctor, because this is the worst position I could put myself in. A patient might have a relationship with a family doctor for three or four decades. This family doctor might not only care for this patient, but also his children and grandchildren. The patient visits the family doctor at least once a year for a checkup. But when the patient is as sick as they have ever been in their life and needs their family doctor whom they trust, I am supposed to “step in” for this family doctor? Good luck trying to meet that standard. It’s like putting me next to Justin Timberlake on stage at a teenybopper concert. Who do you think is going to look better in that sort of comparison?
We, as hospitalists, should never allow anyone to think we are replacing their family doctor. We are here to work with the family doctor to provide the best care possible. Do surgeons, medical subspecialists, or ED doctors “replace” the family doctor? No way! They are working with the family doctor. Perhaps the problem here is that we have not set the appropriate expectations for our patients.
Next, we need to be clear in saying what we say we do or doing we what we say we do. A line in this article bothers me more than any of the reader comments: “The most compelling argument in favor of hospitalists, who are now in 5,000 institutions, from academic giants like the Hospital of the University of Pennsylvania to small community hospitals to innovators like the Mayo and Cleveland Clinics—is that they are there all the time.”
Why does it bother me so much? It is troubling because it is misleading and might simply be untrue. Many hospitalists are not there “all the time.” While many of our hospitalist programs have providers in the hospital 24 hours a day, many do not. I know a number of hospitalists who make rounds at multiple hospitals throughout the day. Are they really hospitalists or are they inpatient rounders?
Hospitalists are physicians defined by their location, not unlike ED physicians. Do we have ED doctors going from hospital to hospital, leaving nurses alone to care for patients when they are at another hospital? So what do we expect from our hospitalists? Should they be in the hospital 24/7? That would seem to be more consistent with the thought that “they are there all the time.” Remember, Gross did not say hospitalists are “reachable” all the time. She did say hospitalists are “on top of everything that happens to a patient—from entry through treatment and discharge.” It is time that we, as hospitalists, uniformly meet those expectations. Patients all over the country are figuring out that not all hospitalists are doing what they are supposed to do when it comes to communications and establishing safe transitions of care. Remember the adage: It does not take many rotten apples to spoil the barrel.
Last, let us talk more about how hospitalists can provide patient-centric care, as opposed to cost savings and carrying out President Obama’s marching orders. The article describes how a study published in the Journal of the American Medical Association found that patients have a reduced length of stay in the hospital when cared for by hospitalists; how hospitalists are being viewed as leaders in healthcare reform; and how the hospitalist spends her nonclinical time “design(ing) computer programs to contain costs.” Do not get me wrong. I am supportive as anyone of the notion that hospitalists should provide cost-effective care. But the reality is that our patients’ No. 1 priority is to believe that their doctor is providing the best care possible. They do not want to feel someone is short-changing them.
Talk all you want to insurers and hospitals about cost savings, but when speaking with patients, I think it makes more sense to discuss the quality as opposed to cost of care. Ask your next patient whether they give a hoot what you do when you are not caring for them. TH
Did you happen to read a recent New York Times article (www.nytimes.com/2010/05/27/us/27hosp.html) about hospitalists? I thought the article was great, but I was surprised by some of the negative reader feedback. What did you think?
George Eppley, MD
Reed, Ga.
Dr. Hospitalist responds: I read the NYT article by Jane Gross, “New Breed of Specialist Steps in for Family Doctor,” which was published May 26. The accompanying reader comment section is available at http://newoldage.blogs.nytimes.com/2010/05/26/in-hospitals-new-fingers -on-the-pulse/?ref=us.
The article provides the statistics that all of us in HM have come to know: HM is the fastest-growing medical specialty in the U.S. and, over the past decade, the number of hospitalists in the U.S. has grown from hundreds to 30,000. Gross talks about the care a hospitalist at the Hospital of the University of Pennsylvania provides for her patient. She highlights the challenges of transitions of care and references the work being done by hospitalists and SHM to make sure patients are making safe transitions. While the article was largely supportive of HM, she does provide a shade of balance when she mentions the risks to the patient when hospitalists fail to do their job when it comes to communication.
As a practicing hospitalist, I kind of wished I had stopped reading at the end of the article. I honestly did not like most of what I read in the reader comment section. Although the article was a feel-good story, I think it is fair to say the reader comments were largely negative. I understand that readers with negative experiences with hospitalists might be more likely to post a comment; nevertheless, some of the comments are hard to ignore—mainly because I suspect some of it is true.
One reader from Raleigh, N.C., wrote, “Hospitalists proved inept at contacting the patients’ existing doctors or even talking to the patients. Then, on discharge to nursing homes for further recovery, the ball was dropped further, with very poor communication of medication dosages, etc.” Yikes! What happened to the communication and the medication reconciliation process?
A reader from Massachusetts wrote about “the hospitalist … (who) ordered five blood draws in the space of several hours to replicate tests that had already been taken by the primary-care physician before admission.” So, not only are hospitalists poor communicators and do not do a good job with transitions of care, but their care is also driving up the cost of healthcare needlessly?
The most positive comments seem to come from outpatient providers and, quite honestly, I found them lukewarm at best. A PCP from Charleston, S.C., wrote, “I no longer have to cancel the appointments of the patients at the last minute in order to attend to an emergency occurring outside my office. It is a very efficient system.” Glad to hear the hospitalist relationship is working out for you, Dr. PCP, but as a patient in the hospital, I am worried more about the competency of this doctor, whom I have never met before this hospitalization, as opposed to how this doctor is going to make you more “efficient” in your office practice.
I came away with several thoughts after reading the article and the comments.
First, we need to set the right expectations. Is this the equivalent of the star athlete who makes a brash statement followed shortly thereafter by the statement, “I was misquoted”? Well … maybe. Are we who we say we are? The headline is “New Breed of Specialist Steps in for Family Doctor.”
As a practicing hospitalist, I never describe myself as replacing the family doctor, because this is the worst position I could put myself in. A patient might have a relationship with a family doctor for three or four decades. This family doctor might not only care for this patient, but also his children and grandchildren. The patient visits the family doctor at least once a year for a checkup. But when the patient is as sick as they have ever been in their life and needs their family doctor whom they trust, I am supposed to “step in” for this family doctor? Good luck trying to meet that standard. It’s like putting me next to Justin Timberlake on stage at a teenybopper concert. Who do you think is going to look better in that sort of comparison?
We, as hospitalists, should never allow anyone to think we are replacing their family doctor. We are here to work with the family doctor to provide the best care possible. Do surgeons, medical subspecialists, or ED doctors “replace” the family doctor? No way! They are working with the family doctor. Perhaps the problem here is that we have not set the appropriate expectations for our patients.
Next, we need to be clear in saying what we say we do or doing we what we say we do. A line in this article bothers me more than any of the reader comments: “The most compelling argument in favor of hospitalists, who are now in 5,000 institutions, from academic giants like the Hospital of the University of Pennsylvania to small community hospitals to innovators like the Mayo and Cleveland Clinics—is that they are there all the time.”
Why does it bother me so much? It is troubling because it is misleading and might simply be untrue. Many hospitalists are not there “all the time.” While many of our hospitalist programs have providers in the hospital 24 hours a day, many do not. I know a number of hospitalists who make rounds at multiple hospitals throughout the day. Are they really hospitalists or are they inpatient rounders?
Hospitalists are physicians defined by their location, not unlike ED physicians. Do we have ED doctors going from hospital to hospital, leaving nurses alone to care for patients when they are at another hospital? So what do we expect from our hospitalists? Should they be in the hospital 24/7? That would seem to be more consistent with the thought that “they are there all the time.” Remember, Gross did not say hospitalists are “reachable” all the time. She did say hospitalists are “on top of everything that happens to a patient—from entry through treatment and discharge.” It is time that we, as hospitalists, uniformly meet those expectations. Patients all over the country are figuring out that not all hospitalists are doing what they are supposed to do when it comes to communications and establishing safe transitions of care. Remember the adage: It does not take many rotten apples to spoil the barrel.
Last, let us talk more about how hospitalists can provide patient-centric care, as opposed to cost savings and carrying out President Obama’s marching orders. The article describes how a study published in the Journal of the American Medical Association found that patients have a reduced length of stay in the hospital when cared for by hospitalists; how hospitalists are being viewed as leaders in healthcare reform; and how the hospitalist spends her nonclinical time “design(ing) computer programs to contain costs.” Do not get me wrong. I am supportive as anyone of the notion that hospitalists should provide cost-effective care. But the reality is that our patients’ No. 1 priority is to believe that their doctor is providing the best care possible. They do not want to feel someone is short-changing them.
Talk all you want to insurers and hospitals about cost savings, but when speaking with patients, I think it makes more sense to discuss the quality as opposed to cost of care. Ask your next patient whether they give a hoot what you do when you are not caring for them. TH
Did you happen to read a recent New York Times article (www.nytimes.com/2010/05/27/us/27hosp.html) about hospitalists? I thought the article was great, but I was surprised by some of the negative reader feedback. What did you think?
George Eppley, MD
Reed, Ga.
Dr. Hospitalist responds: I read the NYT article by Jane Gross, “New Breed of Specialist Steps in for Family Doctor,” which was published May 26. The accompanying reader comment section is available at http://newoldage.blogs.nytimes.com/2010/05/26/in-hospitals-new-fingers -on-the-pulse/?ref=us.
The article provides the statistics that all of us in HM have come to know: HM is the fastest-growing medical specialty in the U.S. and, over the past decade, the number of hospitalists in the U.S. has grown from hundreds to 30,000. Gross talks about the care a hospitalist at the Hospital of the University of Pennsylvania provides for her patient. She highlights the challenges of transitions of care and references the work being done by hospitalists and SHM to make sure patients are making safe transitions. While the article was largely supportive of HM, she does provide a shade of balance when she mentions the risks to the patient when hospitalists fail to do their job when it comes to communication.
As a practicing hospitalist, I kind of wished I had stopped reading at the end of the article. I honestly did not like most of what I read in the reader comment section. Although the article was a feel-good story, I think it is fair to say the reader comments were largely negative. I understand that readers with negative experiences with hospitalists might be more likely to post a comment; nevertheless, some of the comments are hard to ignore—mainly because I suspect some of it is true.
One reader from Raleigh, N.C., wrote, “Hospitalists proved inept at contacting the patients’ existing doctors or even talking to the patients. Then, on discharge to nursing homes for further recovery, the ball was dropped further, with very poor communication of medication dosages, etc.” Yikes! What happened to the communication and the medication reconciliation process?
A reader from Massachusetts wrote about “the hospitalist … (who) ordered five blood draws in the space of several hours to replicate tests that had already been taken by the primary-care physician before admission.” So, not only are hospitalists poor communicators and do not do a good job with transitions of care, but their care is also driving up the cost of healthcare needlessly?
The most positive comments seem to come from outpatient providers and, quite honestly, I found them lukewarm at best. A PCP from Charleston, S.C., wrote, “I no longer have to cancel the appointments of the patients at the last minute in order to attend to an emergency occurring outside my office. It is a very efficient system.” Glad to hear the hospitalist relationship is working out for you, Dr. PCP, but as a patient in the hospital, I am worried more about the competency of this doctor, whom I have never met before this hospitalization, as opposed to how this doctor is going to make you more “efficient” in your office practice.
I came away with several thoughts after reading the article and the comments.
First, we need to set the right expectations. Is this the equivalent of the star athlete who makes a brash statement followed shortly thereafter by the statement, “I was misquoted”? Well … maybe. Are we who we say we are? The headline is “New Breed of Specialist Steps in for Family Doctor.”
As a practicing hospitalist, I never describe myself as replacing the family doctor, because this is the worst position I could put myself in. A patient might have a relationship with a family doctor for three or four decades. This family doctor might not only care for this patient, but also his children and grandchildren. The patient visits the family doctor at least once a year for a checkup. But when the patient is as sick as they have ever been in their life and needs their family doctor whom they trust, I am supposed to “step in” for this family doctor? Good luck trying to meet that standard. It’s like putting me next to Justin Timberlake on stage at a teenybopper concert. Who do you think is going to look better in that sort of comparison?
We, as hospitalists, should never allow anyone to think we are replacing their family doctor. We are here to work with the family doctor to provide the best care possible. Do surgeons, medical subspecialists, or ED doctors “replace” the family doctor? No way! They are working with the family doctor. Perhaps the problem here is that we have not set the appropriate expectations for our patients.
Next, we need to be clear in saying what we say we do or doing we what we say we do. A line in this article bothers me more than any of the reader comments: “The most compelling argument in favor of hospitalists, who are now in 5,000 institutions, from academic giants like the Hospital of the University of Pennsylvania to small community hospitals to innovators like the Mayo and Cleveland Clinics—is that they are there all the time.”
Why does it bother me so much? It is troubling because it is misleading and might simply be untrue. Many hospitalists are not there “all the time.” While many of our hospitalist programs have providers in the hospital 24 hours a day, many do not. I know a number of hospitalists who make rounds at multiple hospitals throughout the day. Are they really hospitalists or are they inpatient rounders?
Hospitalists are physicians defined by their location, not unlike ED physicians. Do we have ED doctors going from hospital to hospital, leaving nurses alone to care for patients when they are at another hospital? So what do we expect from our hospitalists? Should they be in the hospital 24/7? That would seem to be more consistent with the thought that “they are there all the time.” Remember, Gross did not say hospitalists are “reachable” all the time. She did say hospitalists are “on top of everything that happens to a patient—from entry through treatment and discharge.” It is time that we, as hospitalists, uniformly meet those expectations. Patients all over the country are figuring out that not all hospitalists are doing what they are supposed to do when it comes to communications and establishing safe transitions of care. Remember the adage: It does not take many rotten apples to spoil the barrel.
Last, let us talk more about how hospitalists can provide patient-centric care, as opposed to cost savings and carrying out President Obama’s marching orders. The article describes how a study published in the Journal of the American Medical Association found that patients have a reduced length of stay in the hospital when cared for by hospitalists; how hospitalists are being viewed as leaders in healthcare reform; and how the hospitalist spends her nonclinical time “design(ing) computer programs to contain costs.” Do not get me wrong. I am supportive as anyone of the notion that hospitalists should provide cost-effective care. But the reality is that our patients’ No. 1 priority is to believe that their doctor is providing the best care possible. They do not want to feel someone is short-changing them.
Talk all you want to insurers and hospitals about cost savings, but when speaking with patients, I think it makes more sense to discuss the quality as opposed to cost of care. Ask your next patient whether they give a hoot what you do when you are not caring for them. TH
When CAM might be appropriate for your patient with anxiety
Palmoplantar eruption
A 38-year-old woman presents with recurrent asymptomatic lesions on the palms and soles and on the sides of both feet. The lesions have been developing for 2 months, unaccompanied by fever or other systemic symptoms.
Laboratory tests of C-reactive protein, erythrocyte sedimentation rate, viral serologies, and antinuclear antibodies are normal. A pustule culture is negative, and a cutaneous biopsy shows parakeratosis and elongation of rete ridges, neutrophils migrating from papillary capillaries to the epidermis, and spongiform Kogoj pustule.
Q: Which is the most likely diagnosis?
- Pustular psoriasis
- Impetigo contagiosa
- Syndrome of synovitis, acne, pustulosis, hyperostosis, osteitis (SAPHO)
- Dyshidrotic eczema
- Acute exanthematous pustulosis (drug eruption)
A: SAPHO syndrome is the most likely diagnosis. The presence of pustules and aseptic osteitis of the anterior chest wall is compatible with SAPHO syndrome. Treatment with topical clobetasol propionate (Temovate) for pustules and NSAIDs for osteitis brought a good response.
Pustular psoriasis is an uncommon type of psoriasis characterized by erythema and pustules involving the flexural and anogenital areas. Cutaneous lesions of psoriasis vulgaris may be present before an acute pustular episode. Withdrawal of systemic corticosteroids in a patient with psoriasis has been reported as a precipitating factor.
Impetigo contagiosa is a superficial cutaneous infection characterized by an erythematous macule that evolves into a vesicle or pustule. These lesions are more common in children. Culture of the fluid usually reveals Staphylococcus aureus or S pyogenes.
Dyshidrotic eczema is a pruritic vesicular eruption of unknown cause on the palm and soles (bilateral and symmetric). The typical histologic findings are spongiotic and intraepidermal vesicles.
Acute exanthematous pustulosis is a drug-induced reaction characterized by confluent erythema, blisters, and pustules, mucous membrane erosions with fever, and lymphadenopathy. Cultures of the pustules are negative, and biopsy can help confirm the diagnosis of drug eruption.
SAPHO SYNDROME
SAPHO syndrome is a rare condition of unknown pathogenesis originally described by Chamot et al1 in 1987. The onset is usually in young adulthood, and is similar in men and women. It is characterized by synovitis, acne, pustulosis, hyperostosis, and osteitis. Of paramount importance is the finding of a non-infectious inflammatory osteitis in a patient with skin lesions.
Clinical findings: Pustules plus rheumatic pain
SAPHO syndrome must be suspected when a patient is affected by a pustular skin disease associated with rheumatic pain. If examination shows that the pain is caused by a sterile inflammation of bone or joints, the diagnosis tends to be confirmed.2
Osteoarticular involvement tends to be limited to the anterior chest wall. It may include aseptic osteitis, hyperostosis, and symmetrical arthritis. Peripheral and axial osteitis is one of the main characteristics of the syndrome and is found in around 90% of cases.
Cutaneous manifestations are present in two-thirds of patients and consist chiefly of severe acne (acne fulminans, acne conglobata, and hidradenitis suppurativa), pustular psoriasis, and palmoplantar pustulosis. Neutrophilic dermatoses associated with this syndrome include Sweet syndrome and pyoderma gangrenosum. Acne lesions are usually seen in men, whereas palmoplantar pustulosis is seen in women,3 often accompanying osteoarticular manifestations.
Radiologic findings in the spine are spondylodiskitis, osteosclerosis, sacroiliac joint involvement, and paravertebral ossification. In anterior chest wall hyperostosis, common findings are bone hypertrophy and sclerosis with a soft-tissue component. Laboratory test results are uncharacteristic, with variable signs of inflammation, and the C-reactive protein and sedimentation rate are usually elevated in the absence of leukocytosis.
Pathogenesis remains elusive
The pathogenesis of this syndrome remains elusive. Since SAPHO syndrome usually involves the axial skeleton, some investigators have suggested a possible link between the SAPHO syndrome and the seronegative spondyloarthopathies.3 It has also been related to an infection by Propionibacterium acnes and Corynebacterium species,4 which have been isolated in cultures of bone and skin lesions. However, the fact that these bacteria are contaminant agents makes their involvement in the pathogenesis of this syndrome unlikely. More recently, high concentrations of tumor necrosis factor alpha in bone specimens of patients with SAPHO syndrome have been reported, thus highlighting the central role of this cytokine in maintaining inflammation.
Treatment is to relieve symptoms
Since understanding of the pathogenesis of SAPHO syndrome is limited, a wide range of therapies has been used,5 mostly to relieve symptoms. These include NSAIDs; steroids; antibiotics; bisphosphonates such as pamidronate (Aredia) and zoledronic acid (Reclast); and immunosuppressors and immunomodulators such as methotrexate (Trexall), leflunomide (Arava), sulfasalazine (Azulfidine), cyclosporine (Sandimmune). The results with these therapies have been quite varied. Good response has been reported with tumor necrosis factor alpha blockers—infliximab (Remicade), etanercept (Enbrel), and adalimumab (Humira).6
- Chamot AM, Benhamou CL, Kahn MF, Beraneck L, Kaplan G, Prost A. Acne-pustulosis-hyperostosis-osteitis syndrome. Results of a national survey. 85 cases [in French]. Rev Rhum Mal Osteoartic 1987; 54:187–196.
- Benhamou CL, Chamot AM, Kahn MF. Synovitis-acnepustulosis hyperostosis-osteomyelitis syndrome (SAPHO). A new syndrome among the spondyloarthropathies? Clin Exp Rheumatol 1988; 6:109–112.
- Hayem G, Bouchaud-Chabot A, Benali K, et al. SAPHO syndrome: a long-term follow-up study of 120 cases. Semin Arthritis Rheum 1999; 29:159–171.
- Moll C, Hernández MV, Cañete JD, et al. Ilium osteitis as the main manifestation of the SAPHO syndrome: response to infliximab therapy and review of the literature. Semin Arthritis Rheum 2008; 37:299–306.
- Olivieri I, Padula A, Palazzi C. Pharmacological management of SAPHO syndrome. Expert Opin Investig Drugs 2006; 15:1229–1233.
- Arias-Santiago S, Sanchez-Cano D, Callejas-Rubio JL, Fernández-Pugnaire MA, Ortego-Centeno N. Adalimumab treatment for SAPHO syndrome. Acta Derm Venereol 2010; 90:301–302.
A 38-year-old woman presents with recurrent asymptomatic lesions on the palms and soles and on the sides of both feet. The lesions have been developing for 2 months, unaccompanied by fever or other systemic symptoms.
Laboratory tests of C-reactive protein, erythrocyte sedimentation rate, viral serologies, and antinuclear antibodies are normal. A pustule culture is negative, and a cutaneous biopsy shows parakeratosis and elongation of rete ridges, neutrophils migrating from papillary capillaries to the epidermis, and spongiform Kogoj pustule.
Q: Which is the most likely diagnosis?
- Pustular psoriasis
- Impetigo contagiosa
- Syndrome of synovitis, acne, pustulosis, hyperostosis, osteitis (SAPHO)
- Dyshidrotic eczema
- Acute exanthematous pustulosis (drug eruption)
A: SAPHO syndrome is the most likely diagnosis. The presence of pustules and aseptic osteitis of the anterior chest wall is compatible with SAPHO syndrome. Treatment with topical clobetasol propionate (Temovate) for pustules and NSAIDs for osteitis brought a good response.
Pustular psoriasis is an uncommon type of psoriasis characterized by erythema and pustules involving the flexural and anogenital areas. Cutaneous lesions of psoriasis vulgaris may be present before an acute pustular episode. Withdrawal of systemic corticosteroids in a patient with psoriasis has been reported as a precipitating factor.
Impetigo contagiosa is a superficial cutaneous infection characterized by an erythematous macule that evolves into a vesicle or pustule. These lesions are more common in children. Culture of the fluid usually reveals Staphylococcus aureus or S pyogenes.
Dyshidrotic eczema is a pruritic vesicular eruption of unknown cause on the palm and soles (bilateral and symmetric). The typical histologic findings are spongiotic and intraepidermal vesicles.
Acute exanthematous pustulosis is a drug-induced reaction characterized by confluent erythema, blisters, and pustules, mucous membrane erosions with fever, and lymphadenopathy. Cultures of the pustules are negative, and biopsy can help confirm the diagnosis of drug eruption.
SAPHO SYNDROME
SAPHO syndrome is a rare condition of unknown pathogenesis originally described by Chamot et al1 in 1987. The onset is usually in young adulthood, and is similar in men and women. It is characterized by synovitis, acne, pustulosis, hyperostosis, and osteitis. Of paramount importance is the finding of a non-infectious inflammatory osteitis in a patient with skin lesions.
Clinical findings: Pustules plus rheumatic pain
SAPHO syndrome must be suspected when a patient is affected by a pustular skin disease associated with rheumatic pain. If examination shows that the pain is caused by a sterile inflammation of bone or joints, the diagnosis tends to be confirmed.2
Osteoarticular involvement tends to be limited to the anterior chest wall. It may include aseptic osteitis, hyperostosis, and symmetrical arthritis. Peripheral and axial osteitis is one of the main characteristics of the syndrome and is found in around 90% of cases.
Cutaneous manifestations are present in two-thirds of patients and consist chiefly of severe acne (acne fulminans, acne conglobata, and hidradenitis suppurativa), pustular psoriasis, and palmoplantar pustulosis. Neutrophilic dermatoses associated with this syndrome include Sweet syndrome and pyoderma gangrenosum. Acne lesions are usually seen in men, whereas palmoplantar pustulosis is seen in women,3 often accompanying osteoarticular manifestations.
Radiologic findings in the spine are spondylodiskitis, osteosclerosis, sacroiliac joint involvement, and paravertebral ossification. In anterior chest wall hyperostosis, common findings are bone hypertrophy and sclerosis with a soft-tissue component. Laboratory test results are uncharacteristic, with variable signs of inflammation, and the C-reactive protein and sedimentation rate are usually elevated in the absence of leukocytosis.
Pathogenesis remains elusive
The pathogenesis of this syndrome remains elusive. Since SAPHO syndrome usually involves the axial skeleton, some investigators have suggested a possible link between the SAPHO syndrome and the seronegative spondyloarthopathies.3 It has also been related to an infection by Propionibacterium acnes and Corynebacterium species,4 which have been isolated in cultures of bone and skin lesions. However, the fact that these bacteria are contaminant agents makes their involvement in the pathogenesis of this syndrome unlikely. More recently, high concentrations of tumor necrosis factor alpha in bone specimens of patients with SAPHO syndrome have been reported, thus highlighting the central role of this cytokine in maintaining inflammation.
Treatment is to relieve symptoms
Since understanding of the pathogenesis of SAPHO syndrome is limited, a wide range of therapies has been used,5 mostly to relieve symptoms. These include NSAIDs; steroids; antibiotics; bisphosphonates such as pamidronate (Aredia) and zoledronic acid (Reclast); and immunosuppressors and immunomodulators such as methotrexate (Trexall), leflunomide (Arava), sulfasalazine (Azulfidine), cyclosporine (Sandimmune). The results with these therapies have been quite varied. Good response has been reported with tumor necrosis factor alpha blockers—infliximab (Remicade), etanercept (Enbrel), and adalimumab (Humira).6
A 38-year-old woman presents with recurrent asymptomatic lesions on the palms and soles and on the sides of both feet. The lesions have been developing for 2 months, unaccompanied by fever or other systemic symptoms.
Laboratory tests of C-reactive protein, erythrocyte sedimentation rate, viral serologies, and antinuclear antibodies are normal. A pustule culture is negative, and a cutaneous biopsy shows parakeratosis and elongation of rete ridges, neutrophils migrating from papillary capillaries to the epidermis, and spongiform Kogoj pustule.
Q: Which is the most likely diagnosis?
- Pustular psoriasis
- Impetigo contagiosa
- Syndrome of synovitis, acne, pustulosis, hyperostosis, osteitis (SAPHO)
- Dyshidrotic eczema
- Acute exanthematous pustulosis (drug eruption)
A: SAPHO syndrome is the most likely diagnosis. The presence of pustules and aseptic osteitis of the anterior chest wall is compatible with SAPHO syndrome. Treatment with topical clobetasol propionate (Temovate) for pustules and NSAIDs for osteitis brought a good response.
Pustular psoriasis is an uncommon type of psoriasis characterized by erythema and pustules involving the flexural and anogenital areas. Cutaneous lesions of psoriasis vulgaris may be present before an acute pustular episode. Withdrawal of systemic corticosteroids in a patient with psoriasis has been reported as a precipitating factor.
Impetigo contagiosa is a superficial cutaneous infection characterized by an erythematous macule that evolves into a vesicle or pustule. These lesions are more common in children. Culture of the fluid usually reveals Staphylococcus aureus or S pyogenes.
Dyshidrotic eczema is a pruritic vesicular eruption of unknown cause on the palm and soles (bilateral and symmetric). The typical histologic findings are spongiotic and intraepidermal vesicles.
Acute exanthematous pustulosis is a drug-induced reaction characterized by confluent erythema, blisters, and pustules, mucous membrane erosions with fever, and lymphadenopathy. Cultures of the pustules are negative, and biopsy can help confirm the diagnosis of drug eruption.
SAPHO SYNDROME
SAPHO syndrome is a rare condition of unknown pathogenesis originally described by Chamot et al1 in 1987. The onset is usually in young adulthood, and is similar in men and women. It is characterized by synovitis, acne, pustulosis, hyperostosis, and osteitis. Of paramount importance is the finding of a non-infectious inflammatory osteitis in a patient with skin lesions.
Clinical findings: Pustules plus rheumatic pain
SAPHO syndrome must be suspected when a patient is affected by a pustular skin disease associated with rheumatic pain. If examination shows that the pain is caused by a sterile inflammation of bone or joints, the diagnosis tends to be confirmed.2
Osteoarticular involvement tends to be limited to the anterior chest wall. It may include aseptic osteitis, hyperostosis, and symmetrical arthritis. Peripheral and axial osteitis is one of the main characteristics of the syndrome and is found in around 90% of cases.
Cutaneous manifestations are present in two-thirds of patients and consist chiefly of severe acne (acne fulminans, acne conglobata, and hidradenitis suppurativa), pustular psoriasis, and palmoplantar pustulosis. Neutrophilic dermatoses associated with this syndrome include Sweet syndrome and pyoderma gangrenosum. Acne lesions are usually seen in men, whereas palmoplantar pustulosis is seen in women,3 often accompanying osteoarticular manifestations.
Radiologic findings in the spine are spondylodiskitis, osteosclerosis, sacroiliac joint involvement, and paravertebral ossification. In anterior chest wall hyperostosis, common findings are bone hypertrophy and sclerosis with a soft-tissue component. Laboratory test results are uncharacteristic, with variable signs of inflammation, and the C-reactive protein and sedimentation rate are usually elevated in the absence of leukocytosis.
Pathogenesis remains elusive
The pathogenesis of this syndrome remains elusive. Since SAPHO syndrome usually involves the axial skeleton, some investigators have suggested a possible link between the SAPHO syndrome and the seronegative spondyloarthopathies.3 It has also been related to an infection by Propionibacterium acnes and Corynebacterium species,4 which have been isolated in cultures of bone and skin lesions. However, the fact that these bacteria are contaminant agents makes their involvement in the pathogenesis of this syndrome unlikely. More recently, high concentrations of tumor necrosis factor alpha in bone specimens of patients with SAPHO syndrome have been reported, thus highlighting the central role of this cytokine in maintaining inflammation.
Treatment is to relieve symptoms
Since understanding of the pathogenesis of SAPHO syndrome is limited, a wide range of therapies has been used,5 mostly to relieve symptoms. These include NSAIDs; steroids; antibiotics; bisphosphonates such as pamidronate (Aredia) and zoledronic acid (Reclast); and immunosuppressors and immunomodulators such as methotrexate (Trexall), leflunomide (Arava), sulfasalazine (Azulfidine), cyclosporine (Sandimmune). The results with these therapies have been quite varied. Good response has been reported with tumor necrosis factor alpha blockers—infliximab (Remicade), etanercept (Enbrel), and adalimumab (Humira).6
- Chamot AM, Benhamou CL, Kahn MF, Beraneck L, Kaplan G, Prost A. Acne-pustulosis-hyperostosis-osteitis syndrome. Results of a national survey. 85 cases [in French]. Rev Rhum Mal Osteoartic 1987; 54:187–196.
- Benhamou CL, Chamot AM, Kahn MF. Synovitis-acnepustulosis hyperostosis-osteomyelitis syndrome (SAPHO). A new syndrome among the spondyloarthropathies? Clin Exp Rheumatol 1988; 6:109–112.
- Hayem G, Bouchaud-Chabot A, Benali K, et al. SAPHO syndrome: a long-term follow-up study of 120 cases. Semin Arthritis Rheum 1999; 29:159–171.
- Moll C, Hernández MV, Cañete JD, et al. Ilium osteitis as the main manifestation of the SAPHO syndrome: response to infliximab therapy and review of the literature. Semin Arthritis Rheum 2008; 37:299–306.
- Olivieri I, Padula A, Palazzi C. Pharmacological management of SAPHO syndrome. Expert Opin Investig Drugs 2006; 15:1229–1233.
- Arias-Santiago S, Sanchez-Cano D, Callejas-Rubio JL, Fernández-Pugnaire MA, Ortego-Centeno N. Adalimumab treatment for SAPHO syndrome. Acta Derm Venereol 2010; 90:301–302.
- Chamot AM, Benhamou CL, Kahn MF, Beraneck L, Kaplan G, Prost A. Acne-pustulosis-hyperostosis-osteitis syndrome. Results of a national survey. 85 cases [in French]. Rev Rhum Mal Osteoartic 1987; 54:187–196.
- Benhamou CL, Chamot AM, Kahn MF. Synovitis-acnepustulosis hyperostosis-osteomyelitis syndrome (SAPHO). A new syndrome among the spondyloarthropathies? Clin Exp Rheumatol 1988; 6:109–112.
- Hayem G, Bouchaud-Chabot A, Benali K, et al. SAPHO syndrome: a long-term follow-up study of 120 cases. Semin Arthritis Rheum 1999; 29:159–171.
- Moll C, Hernández MV, Cañete JD, et al. Ilium osteitis as the main manifestation of the SAPHO syndrome: response to infliximab therapy and review of the literature. Semin Arthritis Rheum 2008; 37:299–306.
- Olivieri I, Padula A, Palazzi C. Pharmacological management of SAPHO syndrome. Expert Opin Investig Drugs 2006; 15:1229–1233.
- Arias-Santiago S, Sanchez-Cano D, Callejas-Rubio JL, Fernández-Pugnaire MA, Ortego-Centeno N. Adalimumab treatment for SAPHO syndrome. Acta Derm Venereol 2010; 90:301–302.
Noninvasive positive pressure ventilation for stable outpatients: CPAP and beyond
Noninvasive positive pressure ventilation (NIPPV) is any form of positive ventilatory support applied without an endotracheal tube, including continuous positive airway pressure (CPAP).1 The role of NIPPV in acute care has been discussed in an earlier review in the Cleveland Clinic Journal of Medicine.2
NIPPV is also used at night in outpatients with stable chronic conditions, first used in the 1980s in the treatment of obstructive sleep apnea3 and neuromuscular diseases,4 and since then in several other conditions including sleep disorders associated with congestive heart failure (including sleep apnea and the Cheyne-Stokes respiration-central sleep apnea syndrome), chronic obstructive pulmonary disease (COPD), and the obesity-hypoventilation syndrome.
In this review, we discuss the different types of NIPPV, the specific conditions in which they can be used, and the indications, recommendations, and evidence supporting the efficacy of NIPPV in outpatients.
THE TYPES OF NIPPV AND THEIR USES
Although the conditions for which different types of NIPPV can be used overlap significantly, each type has general indications and different goals of treatment. This section begins with types of NIPPV that are predominantly used to treat sleep-disordered breathing, and then proceeds to those predominantly used for conditions associated with hypoventilation and hypercapnia.
Continuous positive airway pressure
CPAP, currently the most widely used form of NIPPV, applies a constant level of positive pressure at the airway opening during spontaneous breathing.
CPAP is commonly used to treat obstructive sleep-disordered breathing, with the goals of improving daytime sleepiness and reducing cardiovascular risk. It has also been used to treat sleep-disordered breathing associated with congestive heart failure.
CPAP’s role in the support of ventilation is limited and indirect. For instance, it has been used in the obesity-hypoventilation syndrome and in the “overlap” syndrome (in which both sleep apnea and COPD coexist). However, its benefits in those conditions are probably derived in large part from correction of underlying obstructive sleep apnea.
The mechanisms of action of CPAP include:
- Preventing intermittent narrowing and collapse of the airway in patients with obstructive sleep apnea-hypopnea syndrome by acting as a pneumatic splint during sleep3,5,6
- Counteracting auto-positive end-expiratory pressure, thereby reducing respiratory muscle load, reducing the work of breathing, and lowering daytime Paco2 in patients with coexistent COPD and obstructive sleep apnea-hypopnea syndrome (the overlap syndrome)7–9
- Improving lung function (particularly the functional residual capacity) and daytime gas exchange in obstructive sleep apnea-hypopnea syndrome 10
- Improving left ventricular systolic function in patients with heart failure coexisting with obstructive sleep apnea-hypopnea syndrome.11,12
Auto-CPAP is delivered via a self-titrating CPAP device, which uses algorithms to detect variations in the degree of obstruction and consequently adjusts the pressure level to restore normal breathing. Auto-CPAP therefore compensates for factors that modify the upper airway collapsibility, such as body posture during sleep, stage of sleep, use of alcohol, and drugs that affect upper airway muscle tone.13
Although one of the premises of using auto-CPAP is that it improves the patient’s satisfaction and compliance, several studies found it to be no more effective than fixed CPAP for treating obstructive sleep apnea-hypopnea syndrome.14–16 Current guidelines of the American Academy of Sleep Medicine do not recommend self-titrating CPAP devices to diagnose obstructive sleep apnea or to treat patients with cardiopulmonary disorders or other conditions in which nocturnal desaturation may be unrelated to obstructive events.17
Adaptive servo-ventilation
Adaptive servo-ventilation was developed for Cheyne-Stokes respiration-central sleep apnea syndrome in patients with congestive heart failure, who may have periods of crescendo-decrescendo change in tidal volume (Cheyne-Stokes respiration) with possible intercalated episodes of central apnea or hypopnea. It is also applied in patients with the complex sleep apnea syndrome.
Adaptive servo-ventilation devices are usually set at an expiratory positive airway pressure (EPAP) level sufficient to control obstructive sleep apnea. The device then automatically adjusts the pressure support for each inspiration, within a prespecified range, to maintain a moving-target ventilation set at 90% of the patient’s recent average ventilation. The aim is to stabilize breathing and reduce respiratory alkalosis, which can trigger apnea reentry cycles.18
Bilevel positive airway pressure
Bilevel positive airway pressure (bilevel PAP) can be of use in sleep-disordered breathing (including cases associated with congestive heart failure), but it is predominantly applied in conditions associated with hypoventilation.
Bilevel PAP devices deliver a higher pressure during inspiration (inspiratory positive airway pressure, or IPAP) and a lower pressure during expiration (EPAP). The gradient between IPAP and EPAP is of key importance in maintaining alveolar ventilation and reducing Paco2. The IPAP acts as pressure support to augment the patient’s effort, maintain adequate alveolar ventilation, unload respiratory muscles, decrease the work of breathing, and control obstructive hypopnea, whereas EPAP is set to maintain upper airway patency, control obstructive apnea, improve functional residual capacity, and prevent microatelectasis.
Although there is no evidence that bilevel PAP is better adhered to or more effective than CPAP, current guidelines propose it as an option for patients who require high pressures to control obstructive sleep apnea-hypopnea syndrome or for those who cannot tolerate exhaling against a high fixed CPAP pressure.19
Other, more-common uses of bilevel PAP are to treat coexisting central sleep apnea or hypoventilation,19 the obesity-hypoventilation syndrome with residual alveolar hypoventilation despite CPAP and control of concomitant obstructive sleep apnea-hypopnea syndrome,5,20 severe stable COPD with significant nocturnal hypoventilation and daytime hypercarbia,21 and restrictive pulmonary diseases.21
Average volume-assured pressure support
CONDITIONS IN WHICH NOCTURNAL NIPPV IS USED IN OUT PATIENTS
Obstructive sleep apnea-hypopnea syndrome
Obstructive sleep apnea-hypopnea syndrome is estimated to affect 2% of women and 4% of men.25 It is characterized by recurrent episodes of partial (hypopnea) or complete (apnea) upper airway obstruction during sleep despite ongoing inspiratory efforts, with associated episodes of arousal or desaturation or both. Corresponding symptoms include excessive daytime sleepiness, choking and gasping during sleep, recurrent awakenings from sleep, unrefreshing sleep, daytime fatigue, and impaired concentration that is not explained by other factors.26
Current understanding of the pathophysiology of obstructive sleep apnea implicates an impairment in the balance between factors that promote collapsibility of the airway (including obesity and anatomic issues such as the volume of the soft-tissue structures surrounding the upper airway) and the compensatory neuromuscular response.27,28
Long-standing obstructive sleep apnea-hypopnea syndrome has been linked in prospective studies to the development of hypertension,29–31 coronary artery disease,32,33 increased coagulation,34,35 and stroke or death from any cause.36,37 It is also associated with a greater rate and severity of motor vehicular accidents,38 greater health care utilization, impaired work performance, and occupational injuries.39
Strong evidence exists that NIPPV (most commonly CPAP) is beneficial in obstructive sleep apnea-hypopnea syndrome, improving sleep quality, sleepiness, cognitive impairment, and quality of life,40,41 decreasing motor vehicle accidents,42 lowering blood pressure, 43,44 and decreasing the rates of myocardial infarction,32 stroke,32 and death.45
The American Academy of Sleep Medicine recommends CPAP as an optional adjunctive therapy to lower blood pressure in patients with obstructive sleep apnea-hypopnea syndrome with concomitant hypertension.19 This is supported by a recent study that suggested that CPAP may have additional benefits on blood pressure in a subgroup of patients with uncontrolled hypertension while on antihypertensive medications.46 Indications with Medicare guidelines for reimbursement of CPAP devices are summarized in Table 2.
Complex sleep apnea syndrome
The complex sleep apnea syndrome is characterized by the emergence of significant central sleep apnea or Cheyne-Stokes respiration after obstructive events have been brought under control with NIPPV in patients who initially appear to have obstructive sleep apnea-hypopnea syndrome. A retrospective study of patients with the complex sleep apnea syndrome who continued their NIPPV use until a subsequent polysomnographic study and NIPPV titration showed that the syndrome may resolve spontaneously, but that 46% of patients had a persistently elevated apnea-hypopnea index with central apnea activity.47
Restoration of upper airway patency by CPAP and dysregulation or delayed adaptation of chemosensitive ventilatory control to a changing Paco2 level may be a key pathophysiologic mechanism of the complex sleep apnea syndrome.48 In this mechanism, increased ventilation from restored airway patency and from an increase in the slope of the ventilatory response may intermittently draw the Paco2 to below the hypocapnic apneic threshold and trigger episodes of central apnea.48
The ideal NIPPV device for use in the complex sleep apnea syndrome should be able to provide enough pressure to resolve the obstructive sleep apnea-hypopnea syndrome while maintaining proper ventilatory support during central apnea episodes without fluctuations of Paco2, which could further worsen the dysregulated ventilatory control. Currently, adaptive servo-ventilation appears to be superior to bilevel PAP and CPAP in the management of the complex sleep apnea syndrome.49,50
Sleep disturbances associated with cardiac dysfunction
There are specific indications for NIPPV modes in the setting of respiratory sleep disturbances associated with heart failure.
Obstructive sleep apnea in congestive heart failure. The prevalence of obstructive sleep apnea in patients with impaired left ventricular ejection fraction is 11% to 53%.51 Obstructive sleep apnea-hypopnea syndrome can worsen congestive heart failure by causing a periodic increase in negative intrathoracic pressure from breathing against an occluded airway, by raising arterial blood pressure, and causing tachycardia from sympathetic nervous system stimulation from hypoxia, hypercarbia, and arousals.52,53 Both heart failure and sleep apnea contribute in an additive manner to the increased sympathetic nervous activity.54
Fortunately, treatment with CPAP has been found to reduce systolic blood pressure and improve left ventricular systolic function in medically treated patients with heart failure and coexisting obstructive sleep apnea.11,12 Furthermore, in a randomized trial in patients with stable congestive heart failure and newly diagnosed obstructive sleep apnea-hypopnea syndrome, a greater improvement in cardiac function was observed in patients on bilevel PAP than in those on CPAP.55 The authors speculated that bilevel PAP might provide more unloading of the respiratory muscles, reduce the work of breathing more, and result in less positive intrathoracic pressure than with CPAP, and that the higher intrathoracic pressure with CPAP could reduce the left ventricular ejection fraction in patients with low filling pressures (pulmonary capillary wedge pressure < 12 mm Hg) and low baseline left ventricular ejection fractions (< 30%).55
Whether these interventions reduce the mortality rate is uncertain. In a prospective nonrandomized study, 9 (24%) of 37 patients who had heart failure with untreated obstructive sleep apnea died, compared with no deaths in 14 treated patients (P = .07).56
Cheyne-Stokes respiration with central sleep apnea in congestive heart failure. A related but different situation is central apnea associated with congestive heart failure.
There are several pathophysiologic mechanisms of Cheyne-Stokes respiration with central sleep apnea. Specifically, the elevation of left ventricular filling pressures, end-diastolic volumes, and pulmonary congestion generate hyperventilation, chronic hypocapnia, and increased chemoreceptor responsiveness, which contribute to the development of central apnea by promoting a decrease in the Paco2 during sleep to below the hypocapnic apneic threshold.57,58 Additionally, an increase in circulation time may result in periodicity of breathing and hyperpnea.59 Obstructive events can then occur at the end of the central events corresponding with the nadir of the inspiratory drive.60,61
The Canadian Continuous Positive Airway Pressure for Patients With Central Sleep Apnea and Heart Failure (CANPAP) trial, a randomized trial of CPAP in this clinical setting, showed that compared with optimal medical therapy alone, CPAP plus optimal medical therapy improved the ejection fraction, reduced central sleep apnea, improved nocturnal oxygenation, and improved the 6-minute walking distance, but without a survival benefit.62 The disappointing survival results from CANPAP have to be interpreted in the context that CPAP may have failed to control the central apnea in some patients, such that the mean apnea-hypopnea index in treated patients (19 events/hour) remained above the entry criterion for recruitment (15 events/hour). In a post hoc analysis of this study, the heart-transplantation-free survival rate was significantly greater in the subgroup of patients in whom CPAP effectively suppressed central sleep apnea (< 15 events/hour).63
Other NIPPV modes such as bilevel PAP with backup rate and adaptive servo-ventilation have been shown in some studies to be superior to CPAP in controlling respiratory events, with adaptive servo-ventilation being the most effective in controlling central, mixed, and complex sleep apnea in this setting. 49,50 Whether more effective resolution of obstructive and central events with these treatment modes translates into improved mortality rates and transplantation-free survival rates remains to be determined.
Obesity-hypoventilation syndrome
Obesity-hypoventilation syndrome refers to daytime hypercapnia (Paco2 > 45 mm Hg) in obese people when no other cause of hypoventilation is present.
The prevalence of obesity-hypoventilation syndrome among patients with obstructive sleep apnea-hypopnea syndrome is 20% to 30% and is greater in extremely obese patients (body mass index > 40 kg/m2).64 However, about 10% of patients with obesity-hypoventilation syndrome do not have obstructive sleep apnea-hypopnea syndrome.64 Additionally, nocturnal hypoxemia and diurnal hypercapnia persist in about 40% of patients with obesity-hypoventilation syndrome after CPAP eliminates their sleep apnea.65 Therefore, factors other than sleep apnea contribute to the development of obesity-hypoventilation syndrome, and in a meta-analysis, factors associated with daytime hypercapnia included, in addition to body mass index and the apnea-hypopnea index, mean overnight oxygen saturation and severity of restrictive pulmonary function.66 Predictors of success with CPAP include better spirometric findings, a higher apnea-hypopnea index, and adequate oxygenation.67,68
Bilevel PAP therapy can be tried in patients in whom CPAP by itself fails. In a study of patients with obesity-hypoventilation syndrome in whom initial CPAP treatment failed, average volume-assured pressure support lowered Paco2 compared to bilevel PAP alone, but did not further improve oxygenation, sleep quality, or quality of life.69
Restrictive pulmonary diseases
Neuromuscular diseases and thoracic cage abnormalities. Noninvasive ventilation has been used in patients with progressive neuromuscular disorders or severe thoracic cage abnormalities, with recognized benefits including an improved survival rate and improved quality of life.70,71 However, NIPPV is used in only 9% of patients with amyotrophic lateral sclerosis when clearly indicated.72 The indications and Medicare guidelines for reimbursement of NIPPV (with or without a backup rate) in this setting are shown in Table 2.
Potential contraindications to starting NIPPV in this population include upper airway obstruction, failure to clear secretions despite optimal noninvasive support, inability to achieve a mask fit, and intolerance of the intervention.73,74
The mechanisms of benefit of NIPPV in these settings include improvements in daytime blood gas levels (including hypercapnia75), a reduction in the oxygen cost of breathing,76 an increase in the ventilatory response to carbon dioxide,75 and improved lung compliance.77
Chronic hypercapnic failure due to severe COPD
The use of NIPPV in chronic COPD is less well established than in patients with exacerbations of COPD,78 and limitations in its use are reflected in the more stringent Medicare indications for NIPPV in this setting (Table 2).
A particular subset of patients with stable COPD who may benefit from NIPPV includes those with daytime hypercapnia and super-imposed nocturnal hypoventilation.78 The potential benefits of NIPPV in these patients include improved daytime and nocturnal gas exchange, increased sleep duration, and improved quality of life.78 Additionally, a recent randomized controlled trial of NIPPV plus long-term oxygen therapy compared with oxygen therapy alone in patients with severe COPD and a Paco2 greater than 46 mm Hg demonstrated a survival benefit in favor of adding NIPPV (hazard ratio 0.6).79
However, that study also found no reduction in hospitalization rates, an apparent worsening in general and mental health (as reflected on the 36-Item Short Form Health Survey or SF-36, a quality-of-life questionnaire), as well as increased confusion and bewilderment (reflected on the Profile of Mood States scale).79 These potentially deleterious effects may explain why adherence to NIPPV is low in patients with stable COPD: only 37% to 57% of patients continued to use it in several reported studies.79–81
A level of inspiratory pressure support that is insufficient to reduce hypercapnia may account for the low adherence rate and worsened quality of life in such patients. For instance, in a randomized trial,82 compared with low-intensity NIPPV (mean IPAP 14 cm H2O, backup rate 8 per minute), settings that aimed to maximally reduce Paco2 (mean IPAP 29 cm H2O with a backup rate of 17.5 per minute) increased the daily use of NIPPV by 3.6 hours/day and improved exercise-related dyspnea, daytime Paco2, forced expiratory volume in 1 second (FEV1), vital capacity, and health-related quality of life.
The overlap syndrome was first described by Flenley in 1985 as a combination of chronic respiratory disease (more generally limited to COPD) and obstructive sleep apnea-hypopnea syndrome.83 Epidemiologic studies do not consistently show a higher incidence of obstructive sleep apnea-hypopnea syndrome in patients with COPD, but the exaggerated oxygen desaturation during sleep in patients with this combination increases the risk of hypoxemia, hypercapnia, and pulmonary hypertension. 84 In addition, there was evidence of higher risks of death and of hospitalization for COPD in patients with the overlap syndrome. 85 NIPPV is the main treatment for obstructive sleep apnea-hypopnea syndrome with or without COPD.
A recent study by Marin et al85 showed that CPAP was associated with improved survival and decreased hospitalization in patients with the overlap syndrome. However, polysomnography or nocturnal oximetry while on NIPPV alone must be done, as additional nocturnal oxygen therapy may be warranted when significant chronic respiratory illness coexists with sleep apnea.
Acknowledgment: The authors gratefully acknowledge the contribution of Scott Marlow, RRT, to Table 2 of this review.
- Organized jointly by the American Thoracic Society, the European Respiratory Society, the European Society of Intensive Care Medicine, and the Société de Réanimation de Langue Française, and approved by ATS Board of Directors, December 2000. International Consensus Conferences in Intensive Care Medicine: noninvasive positive pressure ventilation in acute respiratory failure. Am J Respir Crit Care Med 2001; 163:283–291.
- Aboussouan LS, Ricaurte B. Noninvasive positive pressure ventilation: increasing use in acute care. Cleve Clin J Med 2010; 77:307–316.
- Sullivan CE, Berthon-Jones M, Issa FG. Remission of severe obesity-hypoventilation syndrome after short-term treatment during sleep with nasal continuous positive airway pressure. Am Rev Respir Dis 1983; 128:177–181.
- Ellis ER, Bye PT, Bruderer JW, Sullivan CE. Treatment of respiratory failure during sleep in patients with neuromuscular disease. Positive-pressure ventilation through a nose mask. Am Rev Respir Dis 1987; 135:148–152.
- Berger KI, Ayappa I, Chatr-Amontri B, et al. Obesity hypoventilation syndrome as a spectrum of respiratory disturbances during sleep. Chest 2001; 120:1231–1238.
- Olson AL, Zwillich C. The obesity hypoventilation syndrome. Am J Med 2005; 118:948–956.
- de Miguel J, Cabello J, Sánchez-Alarcos JM, Alvarez-Sala R, Espinós D, Alvarez-Sala JL. Long-term effects of treatment with nasal continuous positive airway pressure on lung function in patients with overlap syndrome. Sleep Breath 2002; 6:3–10.
- Mezzanotte WS, Tangel DJ, Fox AM, Ballard RD, White DP. Nocturnal nasal continuous positive airway pressure in patients with chronic obstructive pulmonary disease. Influence on waking respiratory muscle function. Chest 1994; 106:1100–1108.
- Petrof BJ, Legaré M, Goldberg P, Milic-Emili J, Gottfried SB. Continuous positive airway pressure reduces work of breathing and dyspnea during weaning from mechanical ventilation in severe chronic obstructive pulmonary disease. Am Rev Respir Dis 1990; 141:281–289.
- Verbraecken J, Willemen M, De Cock W, Van de Heyning P, De Backer WA. Continuous positive airway pressure and lung inflation in sleep apnea patients. Respiration 2001; 68:357–364.
- Kaneko Y, Floras JS, Usui K, et al. Cardiovascular effects of continuous positive airway pressure in patients with heart failure and obstructive sleep apnea. N Engl J Med 2003; 348:1233–1241.
- Mansfield DR, Gollogly NC, Kaye DM, Richardson M, Bergin P, Naughton MT. Controlled trial of continuous positive airway pressure in obstructive sleep apnea and heart failure. Am J Respir Crit Care Med 2004; 169:361–366.
- Malhotra A, Trinder J, Fogel R, et al. Postural effects on pharyngeal protective reflex mechanisms. Sleep 2004; 27:1105–1112.
- Ayas NT, Patel SR, Malhotra A, et al. Auto-titrating versus standard continuous positive airway pressure for the treatment of obstructive sleep apnea: results of a meta-analysis. Sleep 2004; 27:249–253.
- Nolan GM, Ryan S, O’Connor TM, McNicholas WT. Comparison of three auto-adjusting positive pressure devices in patients with sleep apnoea. Eur Respir J 2006; 28:159–164.
- Meurice JC, Cornette A, Philip-Joet F, et al; ANTADIR “PPC” Working Group. Evaluation of autoCPAP devices in home treatment of sleep apnea/hypopnea syndrome. Sleep Med 2007; 8:695–703.
- Morgenthaler TI, Aurora RN, Brown T, et al; Standards of Practice Committee of the AASM; American Academy of Sleep Medicine. Practice parameters for the use of autotitrating continuous positive airway pressure devices for titrating pressures and treating adult patients with obstructive sleep apnea syndrome: an update for 2007. An American Academy of Sleep Medicine report. Sleep 2008; 31:141–147.
- Teschler H, Döhring J, Wang YM, Berthon-Jones M. Adaptive pressure support servo-ventilation: a novel treatment for Cheyne-Stokes respiration in heart failure. Am J Respir Crit Care Med 2001; 164:614–619.
- Kushida CA, Littner MR, Hirshkowitz M, et al; American Academy of Sleep Medicine. Practice parameters for the use of continuous and bilevel positive airway pressure devices to treat adult patients with sleep-related breathing disorders. Sleep 2006; 29:375–380.
- Schäfer H, Ewig S, Hasper E, Lüderitz B. Failure of CPAP therapy in obstructive sleep apnoea syndrome: predictive factors and treatment with bilevel-positive airway pressure. Respir Med 1998; 92:208–215.
- Clinical indications for noninvasive positive pressure ventilation in chronic respiratory failure due to restrictive lung disease, COPD, and nocturnal hypoventilation—a consensus conference report. Chest 1999; 116:521–534.
- Aboussouan LS, Khan SU, Meeker DP, Stelmach K, Mitsumoto H. Effect of noninvasive positive-pressure ventilation on survival in amyotrophic lateral sclerosis. Ann Intern Med 1997; 127:450–453.
- Köhnlein T, Welte T, Tan LB, Elliott MW. Assisted ventilation for heart failure patients with Cheyne-Stokes respiration. Eur Respir J 2002; 20:934–941.
- Johnson KG, Johnson DC. Bilevel positive airway pressure worsens central apneas during sleep. Chest 2005; 128:2141–2150.
- Young T, Palta M, Dempsey J, Skatrud J, Weber S, Badr S. The occurrence of sleep-disordered breathing among middle-aged adults. N Engl J Med 1993; 328:1230–1235.
- Sleep-related breathing disorders in adults: recommendations for syndrome definition and measurement techniques in clinical research. The Report of an American Academy of Sleep Medicine Task Force. Sleep 1999; 22:667–689.
- Patil SP, Schneider H, Schwartz AR, Smith PL. Adult obstructive sleep apnea: pathophysiology and diagnosis. Chest 2007; 132:325–337.
- Schwab RJ, Pasirstein M, Pierson R, et al. Identification of upper airway anatomic risk factors for obstructive sleep apnea with volumetric magnetic resonance imaging. Am J Respir Crit Care Med 2003; 168:522–530.
- Hedner J, Bengtsson-Boström K, Peker Y, Grote L, Råstam L, Lindblad U. Hypertension prevalence in obstructive sleep apnoea and sex: a population-based case-control study. Eur Respir J 2006; 27:564–570.
- Nieto FJ, Young TB, Lind BK, et al. Association of sleep-disordered breathing, sleep apnea, and hypertension in a large community-based study. Sleep Heart Health Study. JAMA 2000; 283:1829–1836.
- Peppard PE, Young T, Palta M, Skatrud J. Prospective study of the association between sleep-disordered breathing and hypertension. N Engl J Med 2000; 342:1378–1384.
- Marin JM, Carrizo SJ, Vicente E, Agusti AG. Long-term cardiovascular outcomes in men with obstructive sleep apnoea-hypopnoea with or without treatment with continuous positive airway pressure: an observational study. Lancet 2005; 365:1046–1053.
- Peker Y, Carlson J, Hedner J. Increased incidence of coronary artery disease in sleep apnoea: a long-term follow-up. Eur Respir J 2006; 28:596–602.
- Guardiola JJ, Matheson PJ, Clavijo LC, Wilson MA, Fletcher EC. Hypercoagulability in patients with obstructive sleep apnea. Sleep Med 2001; 2:517–523.
- von Känel R, Loredo JS, Ancoli-Israel S, Mills PJ, Natarajan L, Dimsdale JE. Association between polysomnographic measures of disrupted sleep and prothrombotic factors. Chest 2007; 131:733–739.
- Redline S, Yenokyan G, Gottlieb DJ, et al. Obstructive sleep apnea-hypopnea and incident stroke: The Sleep Heart Health Study. Am J Respir Crit Care Med. 2010; Epub ahead of print.
- Yaggi HK, Concato J, Kernan WN, Lichtman JH, Brass LM, Mohsenin V. Obstructive sleep apnea as a risk factor for stroke and death. N Engl J Med 2005; 353:2034–2041.
- Mulgrew AT, Nasvadi G, Butt A, et al. Risk and severity of motor vehicle crashes in patients with obstructive sleep apnoea/hypopnoea. Thorax 2008; 63:536–541.
- AlGhanim N, Comondore VR, Fleetham J, Marra CA, Ayas NT. The economic impact of obstructive sleep apnea. Lung 2008; 186:7–12.
- Giles TL, Lasserson TJ, Smith BH, White J, Wright J, Cates CJ. Continuous positive airways pressure for obstructive sleep apnoea in adults. Cochrane Database Syst Rev 2006; 3:CD001106.
- Patel SR, White DP, Malhotra A, Stanchina ML, Ayas NT. Continuous positive airway pressure therapy for treating sleepiness in a diverse population with obstructive sleep apnea: results of a meta-analysis. Arch Intern Med 2003; 163:565–571.
- George CF. Reduction in motor vehicle collisions following treatment of sleep apnoea with nasal CPAP. Thorax 2001; 56:508–512.
- Becker HF, Jerrentrup A, Ploch T, et al. Effect of nasal continuous positive airway pressure treatment on blood pressure in patients with obstructive sleep apnea. Circulation 2003; 107:68–73.
- Faccenda JF, Mackay TW, Boon NA, Douglas NJ. Randomized placebo-controlled trial of continuous positive airway pressure on blood pressure in the sleep apnea-hypopnea syndrome. Am J Respir Crit Care Med 2001; 163:344–348.
- Campos-Rodriguez F, Peña-Griñan N, Reyes-Nuñez N, et al. Mortality in obstructive sleep apnea-hypopnea patients treated with positive airway pressure. Chest 2005; 128:624–633.
- Pepin JL, Tamisier R, Barone-Rochette G, Launois SH, Levy P, Baguet JP. Comparison of continuous positive airway pressure and valsartan in hypertensive sleep apnea patients. Am J Respir Crit Care Med 2010; Epub ahead of print.
- Kuzniar TJ, Pusalavidyasagar S, Gay PC, Morgenthaler TI. Natural course of complex sleep apnea—a retrospective study. Sleep Breath 2008; 12:135–139.
- Morgenthaler TI, Kagramanov V, Hanak V, Decker PA. Complex sleep apnea syndrome: is it a unique clinical syndrome? Sleep 2006; 29:1203–1209.
- Allam JS, Olson EJ, Gay PC, Morgenthaler TI. Efficacy of adaptive servoventilation in treatment of complex and central sleep apnea syndromes. Chest 2007; 132:1839–1846.
- Morgenthaler TI, Gay PC, Gordon N, Brown LK. Adaptive servoventilation versus noninvasive positive pressure ventilation for central, mixed, and complex sleep apnea syndromes. Sleep 2007; 30:468–475.
- Bordier P. Sleep apnoea in patients with heart failure. Part I: diagnosis, definitions, prevalence, pathophysiology and haemodynamic consequences. Arch Cardiovasc Dis 2009; 102:651–661.
- Romero-Corral A, Somers VK, Pellikka PA, et al. Decreased right and left ventricular myocardial performance in obstructive sleep apnea. Chest 2007; 132:1863–1870.
- Solin P, Kaye DM, Little PJ, Bergin P, Richardson M, Naughton MT. Impact of sleep apnea on sympathetic nervous system activity in heart failure. Chest 2003; 123:1119–1126.
- Floras JS. Should sleep apnoea be a specific target of therapy in chronic heart failure? Heart 2009; 95:1041–1046.
- Khayat RN, Abraham WT, Patt B, Roy M, Hua K, Jarjoura D. Cardiac effects of continuous and bilevel positive airway pressure for patients with heart failure and obstructive sleep apnea: a pilot study. Chest 2008; 134:1162–1168.
- Wang H, Parker JD, Newton GE, et al. Influence of obstructive sleep apnea on mortality in patients with heart failure. J Am Coll Cardiol 2007; 49:1625–1631.
- Javaheri S. A mechanism of central sleep apnea in patients with heart failure. N Engl J Med 1999; 341:949–954.
- Tkacova R, Hall MJ, Liu PP, Fitzgerald FS, Bradley TD. Left ventricular volume in patients with heart failure and Cheyne-Stokes respiration during sleep. Am J Respir Crit Care Med 1997; 156:1549–1555.
- Lorenzi-Filho G, Rankin F, Bies I, Douglas Bradley T. Effects of inhaled carbon dioxide and oxygen on Cheyne-Stokes respiration in patients with heart failure. Am J Respir Crit Care Med 1999; 159:1490–1498.
- Badr MS, Toiber F, Skatrud JB, Dempsey J. Pharyngeal narrowing/occlusion during central sleep apnea. J Appl Physiol 1995; 78:1806–1815.
- Onal E, Burrows DL, Hart RH, Lopata M. Induction of periodic breathing during sleep causes upper airway obstruction in humans. J Appl Physiol 1986; 61:1438–1443.
- Bradley TD, Logan AG, Kimoff RJ, et al; CANPAP Investigators. Continuous positive airway pressure for central sleep apnea and heart failure. N Engl J Med 2005; 353:2025–2033.
- Arzt M, Floras JS, Logan AG, et al; CANPAP Investigators. Suppression of central sleep apnea by continuous positive airway pressure and transplant-free survival in heart failure: a post hoc analysis of the Canadian Continuous Positive Airway Pressure for Patients with Central Sleep Apnea and Heart Failure Trial (CANPAP). Circulation 2007; 115:3173–3180.
- Mokhlesi B, Tulaimat A, Faibussowitsch I, Wang Y, Evans AT. Obesity hypoventilation syndrome: prevalence and predictors in patients with obstructive sleep apnea. Sleep Breath 2007; 11:117–124.
- Banerjee D, Yee BJ, Piper AJ, Zwillich CW, Grunstein RR. Obesity hypoventilation syndrome: hypoxemia during continuous positive airway pressure. Chest 2007; 131:1678–1684.
- Kaw R, Hernandez AV, Walker E, Aboussouan L, Mokhlesi B. Determinants of hypercapnia in obese patients with obstructive sleep apnea: a systematic review and metaanalysis of cohort studies. Chest 2009; 136:787–796.
- Pérez de Llano LA, Golpe R, Piquer MO, et al. Clinical heterogeneity among patients with obesity hypoventilation syndrome: therapeutic implications. Respiration 2008; 75:34–39.
- Piper AJ, Wang D, Yee BJ, Barnes DJ, Grunstein RR. Randomised trial of CPAP vs bilevel support in the treatment of obesity hypoventilation syndrome without severe nocturnal desaturation. Thorax 2008; 63:395–401.
- Storre JH, Seuthe B, Fiechter R, et al. Average volume-assured pressure support in obesity hypoventilation: a randomized crossover trial. Chest 2006; 130:815–821.
- Aboussouan LS, Khan SU, Banerjee M, Arroliga AC, Mitsumoto H. Objective measures of the efficacy of noninvasive positive-pressure ventilation in amyotrophic lateral sclerosis. Muscle Nerve 2001; 24:403–409.
- Bourke SC, Tomlinson M, Williams TL, Bullock RE, Shaw PJ, Gibson GJ. Effects of non-invasive ventilation on survival and quality of life in patients with amyotrophic lateral sclerosis: a randomised controlled trial. Lancet Neurol 2006; 5:140–147.
- Miller RG, Anderson F, Brooks BR, Mitsumoto H, Bradley WG, Ringel SP; ALS CARE Study Group. Outcomes research in amyotrophic lateral sclerosis: lessons learned from the amyotrophic lateral sclerosis clinical assessment, research, and education database. Ann Neurol 2009; 65(suppl 1):S24–S28.
- Bach JR. Amyotrophic lateral sclerosis: prolongation of life by noninvasive respiratory AIDS. Chest 2002; 122:92–98.
- Perrin C, Unterborn JN, Ambrosio CD, Hill NS. Pulmonary complications of chronic neuromuscular diseases and their management. Muscle Nerve 2004; 29:5–27.
- Nickol AH, Hart N, Hopkinson NS, Moxham J, Simonds A, Polkey MI. Mechanisms of improvement of respiratory failure in patients with restrictive thoracic disease treated with non-invasive ventilation. Thorax 2005; 60:754–760.
- Barle H, Söderberg P, Haegerstrand C, Markström A. Bi-level positive airway pressure ventilation reduces the oxygen cost of breathing in long-standing post-polio patients on invasive home mechanical ventilation. Acta Anaesthesiol Scand 2005; 49:197–202.
- Lechtzin N, Shade D, Clawson L, Wiener CM. Supramaximal inflation improves lung compliance in subjects with amyotrophic lateral sclerosis. Chest 2006; 129:1322–1329.
- Hill NS. Noninvasive ventilation for chronic obstructive pulmonary disease. Respir Care 2004; 49:72–87;
- McEvoy RD, Pierce RJ, Hillman D, et al; Australian trial of noninvasive Ventilation in Chronic Airflow Limitation (AVCAL) Study Group. Nocturnal non-invasive nasal ventilation in stable hypercapnic COPD: a randomised controlled trial. Thorax 2009; 64:561–566.
- Criner GJ, Brennan K, Travaline JM, Kreimer D. Efficacy and compliance with noninvasive positive pressure ventilation in patients with chronic respiratory failure. Chest 1999; 116:667–675.
- Strumpf DA, Millman RP, Carlisle CC, et al. Nocturnal positive-pressure ventilation via nasal mask in patients with severe chronic obstructive pulmonary disease. Am Rev Respir Dis 1991; 144:1234–1239.
- Dreher M, Storre JH, Schmoor C, Windisch W. High-intensity versus low-intensity non-invasive ventilation in patients with stable hypercapnic COPD: a randomised crossover trial. Thorax 2010; 65:303–308.
- Flenley DC. Sleep in chronic obstructive lung disease. Clin Chest Med 1985; 6:651–661.
- Weitzenblum E, Chaouat A, Kessler R, Canuet M. Overlap syndrome: obstructive sleep apnea in patients with chronic obstructive pulmonary disease. Proc Am Thorac Soc 2008; 5:237–241.
- Marin JM, Soriano JB, Carrizo SJ, Boldova A, Celli BR. Outcomes in patients with chronic obstructive pulmonary disease and obstructive sleep apnea. The overlap syndrome. Am J Respir Crit Care Med 2010; Epub ahead of print.
Noninvasive positive pressure ventilation (NIPPV) is any form of positive ventilatory support applied without an endotracheal tube, including continuous positive airway pressure (CPAP).1 The role of NIPPV in acute care has been discussed in an earlier review in the Cleveland Clinic Journal of Medicine.2
NIPPV is also used at night in outpatients with stable chronic conditions, first used in the 1980s in the treatment of obstructive sleep apnea3 and neuromuscular diseases,4 and since then in several other conditions including sleep disorders associated with congestive heart failure (including sleep apnea and the Cheyne-Stokes respiration-central sleep apnea syndrome), chronic obstructive pulmonary disease (COPD), and the obesity-hypoventilation syndrome.
In this review, we discuss the different types of NIPPV, the specific conditions in which they can be used, and the indications, recommendations, and evidence supporting the efficacy of NIPPV in outpatients.
THE TYPES OF NIPPV AND THEIR USES
Although the conditions for which different types of NIPPV can be used overlap significantly, each type has general indications and different goals of treatment. This section begins with types of NIPPV that are predominantly used to treat sleep-disordered breathing, and then proceeds to those predominantly used for conditions associated with hypoventilation and hypercapnia.
Continuous positive airway pressure
CPAP, currently the most widely used form of NIPPV, applies a constant level of positive pressure at the airway opening during spontaneous breathing.
CPAP is commonly used to treat obstructive sleep-disordered breathing, with the goals of improving daytime sleepiness and reducing cardiovascular risk. It has also been used to treat sleep-disordered breathing associated with congestive heart failure.
CPAP’s role in the support of ventilation is limited and indirect. For instance, it has been used in the obesity-hypoventilation syndrome and in the “overlap” syndrome (in which both sleep apnea and COPD coexist). However, its benefits in those conditions are probably derived in large part from correction of underlying obstructive sleep apnea.
The mechanisms of action of CPAP include:
- Preventing intermittent narrowing and collapse of the airway in patients with obstructive sleep apnea-hypopnea syndrome by acting as a pneumatic splint during sleep3,5,6
- Counteracting auto-positive end-expiratory pressure, thereby reducing respiratory muscle load, reducing the work of breathing, and lowering daytime Paco2 in patients with coexistent COPD and obstructive sleep apnea-hypopnea syndrome (the overlap syndrome)7–9
- Improving lung function (particularly the functional residual capacity) and daytime gas exchange in obstructive sleep apnea-hypopnea syndrome 10
- Improving left ventricular systolic function in patients with heart failure coexisting with obstructive sleep apnea-hypopnea syndrome.11,12
Auto-CPAP is delivered via a self-titrating CPAP device, which uses algorithms to detect variations in the degree of obstruction and consequently adjusts the pressure level to restore normal breathing. Auto-CPAP therefore compensates for factors that modify the upper airway collapsibility, such as body posture during sleep, stage of sleep, use of alcohol, and drugs that affect upper airway muscle tone.13
Although one of the premises of using auto-CPAP is that it improves the patient’s satisfaction and compliance, several studies found it to be no more effective than fixed CPAP for treating obstructive sleep apnea-hypopnea syndrome.14–16 Current guidelines of the American Academy of Sleep Medicine do not recommend self-titrating CPAP devices to diagnose obstructive sleep apnea or to treat patients with cardiopulmonary disorders or other conditions in which nocturnal desaturation may be unrelated to obstructive events.17
Adaptive servo-ventilation
Adaptive servo-ventilation was developed for Cheyne-Stokes respiration-central sleep apnea syndrome in patients with congestive heart failure, who may have periods of crescendo-decrescendo change in tidal volume (Cheyne-Stokes respiration) with possible intercalated episodes of central apnea or hypopnea. It is also applied in patients with the complex sleep apnea syndrome.
Adaptive servo-ventilation devices are usually set at an expiratory positive airway pressure (EPAP) level sufficient to control obstructive sleep apnea. The device then automatically adjusts the pressure support for each inspiration, within a prespecified range, to maintain a moving-target ventilation set at 90% of the patient’s recent average ventilation. The aim is to stabilize breathing and reduce respiratory alkalosis, which can trigger apnea reentry cycles.18
Bilevel positive airway pressure
Bilevel positive airway pressure (bilevel PAP) can be of use in sleep-disordered breathing (including cases associated with congestive heart failure), but it is predominantly applied in conditions associated with hypoventilation.
Bilevel PAP devices deliver a higher pressure during inspiration (inspiratory positive airway pressure, or IPAP) and a lower pressure during expiration (EPAP). The gradient between IPAP and EPAP is of key importance in maintaining alveolar ventilation and reducing Paco2. The IPAP acts as pressure support to augment the patient’s effort, maintain adequate alveolar ventilation, unload respiratory muscles, decrease the work of breathing, and control obstructive hypopnea, whereas EPAP is set to maintain upper airway patency, control obstructive apnea, improve functional residual capacity, and prevent microatelectasis.
Although there is no evidence that bilevel PAP is better adhered to or more effective than CPAP, current guidelines propose it as an option for patients who require high pressures to control obstructive sleep apnea-hypopnea syndrome or for those who cannot tolerate exhaling against a high fixed CPAP pressure.19
Other, more-common uses of bilevel PAP are to treat coexisting central sleep apnea or hypoventilation,19 the obesity-hypoventilation syndrome with residual alveolar hypoventilation despite CPAP and control of concomitant obstructive sleep apnea-hypopnea syndrome,5,20 severe stable COPD with significant nocturnal hypoventilation and daytime hypercarbia,21 and restrictive pulmonary diseases.21
Average volume-assured pressure support
CONDITIONS IN WHICH NOCTURNAL NIPPV IS USED IN OUT PATIENTS
Obstructive sleep apnea-hypopnea syndrome
Obstructive sleep apnea-hypopnea syndrome is estimated to affect 2% of women and 4% of men.25 It is characterized by recurrent episodes of partial (hypopnea) or complete (apnea) upper airway obstruction during sleep despite ongoing inspiratory efforts, with associated episodes of arousal or desaturation or both. Corresponding symptoms include excessive daytime sleepiness, choking and gasping during sleep, recurrent awakenings from sleep, unrefreshing sleep, daytime fatigue, and impaired concentration that is not explained by other factors.26
Current understanding of the pathophysiology of obstructive sleep apnea implicates an impairment in the balance between factors that promote collapsibility of the airway (including obesity and anatomic issues such as the volume of the soft-tissue structures surrounding the upper airway) and the compensatory neuromuscular response.27,28
Long-standing obstructive sleep apnea-hypopnea syndrome has been linked in prospective studies to the development of hypertension,29–31 coronary artery disease,32,33 increased coagulation,34,35 and stroke or death from any cause.36,37 It is also associated with a greater rate and severity of motor vehicular accidents,38 greater health care utilization, impaired work performance, and occupational injuries.39
Strong evidence exists that NIPPV (most commonly CPAP) is beneficial in obstructive sleep apnea-hypopnea syndrome, improving sleep quality, sleepiness, cognitive impairment, and quality of life,40,41 decreasing motor vehicle accidents,42 lowering blood pressure, 43,44 and decreasing the rates of myocardial infarction,32 stroke,32 and death.45
The American Academy of Sleep Medicine recommends CPAP as an optional adjunctive therapy to lower blood pressure in patients with obstructive sleep apnea-hypopnea syndrome with concomitant hypertension.19 This is supported by a recent study that suggested that CPAP may have additional benefits on blood pressure in a subgroup of patients with uncontrolled hypertension while on antihypertensive medications.46 Indications with Medicare guidelines for reimbursement of CPAP devices are summarized in Table 2.
Complex sleep apnea syndrome
The complex sleep apnea syndrome is characterized by the emergence of significant central sleep apnea or Cheyne-Stokes respiration after obstructive events have been brought under control with NIPPV in patients who initially appear to have obstructive sleep apnea-hypopnea syndrome. A retrospective study of patients with the complex sleep apnea syndrome who continued their NIPPV use until a subsequent polysomnographic study and NIPPV titration showed that the syndrome may resolve spontaneously, but that 46% of patients had a persistently elevated apnea-hypopnea index with central apnea activity.47
Restoration of upper airway patency by CPAP and dysregulation or delayed adaptation of chemosensitive ventilatory control to a changing Paco2 level may be a key pathophysiologic mechanism of the complex sleep apnea syndrome.48 In this mechanism, increased ventilation from restored airway patency and from an increase in the slope of the ventilatory response may intermittently draw the Paco2 to below the hypocapnic apneic threshold and trigger episodes of central apnea.48
The ideal NIPPV device for use in the complex sleep apnea syndrome should be able to provide enough pressure to resolve the obstructive sleep apnea-hypopnea syndrome while maintaining proper ventilatory support during central apnea episodes without fluctuations of Paco2, which could further worsen the dysregulated ventilatory control. Currently, adaptive servo-ventilation appears to be superior to bilevel PAP and CPAP in the management of the complex sleep apnea syndrome.49,50
Sleep disturbances associated with cardiac dysfunction
There are specific indications for NIPPV modes in the setting of respiratory sleep disturbances associated with heart failure.
Obstructive sleep apnea in congestive heart failure. The prevalence of obstructive sleep apnea in patients with impaired left ventricular ejection fraction is 11% to 53%.51 Obstructive sleep apnea-hypopnea syndrome can worsen congestive heart failure by causing a periodic increase in negative intrathoracic pressure from breathing against an occluded airway, by raising arterial blood pressure, and causing tachycardia from sympathetic nervous system stimulation from hypoxia, hypercarbia, and arousals.52,53 Both heart failure and sleep apnea contribute in an additive manner to the increased sympathetic nervous activity.54
Fortunately, treatment with CPAP has been found to reduce systolic blood pressure and improve left ventricular systolic function in medically treated patients with heart failure and coexisting obstructive sleep apnea.11,12 Furthermore, in a randomized trial in patients with stable congestive heart failure and newly diagnosed obstructive sleep apnea-hypopnea syndrome, a greater improvement in cardiac function was observed in patients on bilevel PAP than in those on CPAP.55 The authors speculated that bilevel PAP might provide more unloading of the respiratory muscles, reduce the work of breathing more, and result in less positive intrathoracic pressure than with CPAP, and that the higher intrathoracic pressure with CPAP could reduce the left ventricular ejection fraction in patients with low filling pressures (pulmonary capillary wedge pressure < 12 mm Hg) and low baseline left ventricular ejection fractions (< 30%).55
Whether these interventions reduce the mortality rate is uncertain. In a prospective nonrandomized study, 9 (24%) of 37 patients who had heart failure with untreated obstructive sleep apnea died, compared with no deaths in 14 treated patients (P = .07).56
Cheyne-Stokes respiration with central sleep apnea in congestive heart failure. A related but different situation is central apnea associated with congestive heart failure.
There are several pathophysiologic mechanisms of Cheyne-Stokes respiration with central sleep apnea. Specifically, the elevation of left ventricular filling pressures, end-diastolic volumes, and pulmonary congestion generate hyperventilation, chronic hypocapnia, and increased chemoreceptor responsiveness, which contribute to the development of central apnea by promoting a decrease in the Paco2 during sleep to below the hypocapnic apneic threshold.57,58 Additionally, an increase in circulation time may result in periodicity of breathing and hyperpnea.59 Obstructive events can then occur at the end of the central events corresponding with the nadir of the inspiratory drive.60,61
The Canadian Continuous Positive Airway Pressure for Patients With Central Sleep Apnea and Heart Failure (CANPAP) trial, a randomized trial of CPAP in this clinical setting, showed that compared with optimal medical therapy alone, CPAP plus optimal medical therapy improved the ejection fraction, reduced central sleep apnea, improved nocturnal oxygenation, and improved the 6-minute walking distance, but without a survival benefit.62 The disappointing survival results from CANPAP have to be interpreted in the context that CPAP may have failed to control the central apnea in some patients, such that the mean apnea-hypopnea index in treated patients (19 events/hour) remained above the entry criterion for recruitment (15 events/hour). In a post hoc analysis of this study, the heart-transplantation-free survival rate was significantly greater in the subgroup of patients in whom CPAP effectively suppressed central sleep apnea (< 15 events/hour).63
Other NIPPV modes such as bilevel PAP with backup rate and adaptive servo-ventilation have been shown in some studies to be superior to CPAP in controlling respiratory events, with adaptive servo-ventilation being the most effective in controlling central, mixed, and complex sleep apnea in this setting. 49,50 Whether more effective resolution of obstructive and central events with these treatment modes translates into improved mortality rates and transplantation-free survival rates remains to be determined.
Obesity-hypoventilation syndrome
Obesity-hypoventilation syndrome refers to daytime hypercapnia (Paco2 > 45 mm Hg) in obese people when no other cause of hypoventilation is present.
The prevalence of obesity-hypoventilation syndrome among patients with obstructive sleep apnea-hypopnea syndrome is 20% to 30% and is greater in extremely obese patients (body mass index > 40 kg/m2).64 However, about 10% of patients with obesity-hypoventilation syndrome do not have obstructive sleep apnea-hypopnea syndrome.64 Additionally, nocturnal hypoxemia and diurnal hypercapnia persist in about 40% of patients with obesity-hypoventilation syndrome after CPAP eliminates their sleep apnea.65 Therefore, factors other than sleep apnea contribute to the development of obesity-hypoventilation syndrome, and in a meta-analysis, factors associated with daytime hypercapnia included, in addition to body mass index and the apnea-hypopnea index, mean overnight oxygen saturation and severity of restrictive pulmonary function.66 Predictors of success with CPAP include better spirometric findings, a higher apnea-hypopnea index, and adequate oxygenation.67,68
Bilevel PAP therapy can be tried in patients in whom CPAP by itself fails. In a study of patients with obesity-hypoventilation syndrome in whom initial CPAP treatment failed, average volume-assured pressure support lowered Paco2 compared to bilevel PAP alone, but did not further improve oxygenation, sleep quality, or quality of life.69
Restrictive pulmonary diseases
Neuromuscular diseases and thoracic cage abnormalities. Noninvasive ventilation has been used in patients with progressive neuromuscular disorders or severe thoracic cage abnormalities, with recognized benefits including an improved survival rate and improved quality of life.70,71 However, NIPPV is used in only 9% of patients with amyotrophic lateral sclerosis when clearly indicated.72 The indications and Medicare guidelines for reimbursement of NIPPV (with or without a backup rate) in this setting are shown in Table 2.
Potential contraindications to starting NIPPV in this population include upper airway obstruction, failure to clear secretions despite optimal noninvasive support, inability to achieve a mask fit, and intolerance of the intervention.73,74
The mechanisms of benefit of NIPPV in these settings include improvements in daytime blood gas levels (including hypercapnia75), a reduction in the oxygen cost of breathing,76 an increase in the ventilatory response to carbon dioxide,75 and improved lung compliance.77
Chronic hypercapnic failure due to severe COPD
The use of NIPPV in chronic COPD is less well established than in patients with exacerbations of COPD,78 and limitations in its use are reflected in the more stringent Medicare indications for NIPPV in this setting (Table 2).
A particular subset of patients with stable COPD who may benefit from NIPPV includes those with daytime hypercapnia and super-imposed nocturnal hypoventilation.78 The potential benefits of NIPPV in these patients include improved daytime and nocturnal gas exchange, increased sleep duration, and improved quality of life.78 Additionally, a recent randomized controlled trial of NIPPV plus long-term oxygen therapy compared with oxygen therapy alone in patients with severe COPD and a Paco2 greater than 46 mm Hg demonstrated a survival benefit in favor of adding NIPPV (hazard ratio 0.6).79
However, that study also found no reduction in hospitalization rates, an apparent worsening in general and mental health (as reflected on the 36-Item Short Form Health Survey or SF-36, a quality-of-life questionnaire), as well as increased confusion and bewilderment (reflected on the Profile of Mood States scale).79 These potentially deleterious effects may explain why adherence to NIPPV is low in patients with stable COPD: only 37% to 57% of patients continued to use it in several reported studies.79–81
A level of inspiratory pressure support that is insufficient to reduce hypercapnia may account for the low adherence rate and worsened quality of life in such patients. For instance, in a randomized trial,82 compared with low-intensity NIPPV (mean IPAP 14 cm H2O, backup rate 8 per minute), settings that aimed to maximally reduce Paco2 (mean IPAP 29 cm H2O with a backup rate of 17.5 per minute) increased the daily use of NIPPV by 3.6 hours/day and improved exercise-related dyspnea, daytime Paco2, forced expiratory volume in 1 second (FEV1), vital capacity, and health-related quality of life.
The overlap syndrome was first described by Flenley in 1985 as a combination of chronic respiratory disease (more generally limited to COPD) and obstructive sleep apnea-hypopnea syndrome.83 Epidemiologic studies do not consistently show a higher incidence of obstructive sleep apnea-hypopnea syndrome in patients with COPD, but the exaggerated oxygen desaturation during sleep in patients with this combination increases the risk of hypoxemia, hypercapnia, and pulmonary hypertension. 84 In addition, there was evidence of higher risks of death and of hospitalization for COPD in patients with the overlap syndrome. 85 NIPPV is the main treatment for obstructive sleep apnea-hypopnea syndrome with or without COPD.
A recent study by Marin et al85 showed that CPAP was associated with improved survival and decreased hospitalization in patients with the overlap syndrome. However, polysomnography or nocturnal oximetry while on NIPPV alone must be done, as additional nocturnal oxygen therapy may be warranted when significant chronic respiratory illness coexists with sleep apnea.
Acknowledgment: The authors gratefully acknowledge the contribution of Scott Marlow, RRT, to Table 2 of this review.
Noninvasive positive pressure ventilation (NIPPV) is any form of positive ventilatory support applied without an endotracheal tube, including continuous positive airway pressure (CPAP).1 The role of NIPPV in acute care has been discussed in an earlier review in the Cleveland Clinic Journal of Medicine.2
NIPPV is also used at night in outpatients with stable chronic conditions, first used in the 1980s in the treatment of obstructive sleep apnea3 and neuromuscular diseases,4 and since then in several other conditions including sleep disorders associated with congestive heart failure (including sleep apnea and the Cheyne-Stokes respiration-central sleep apnea syndrome), chronic obstructive pulmonary disease (COPD), and the obesity-hypoventilation syndrome.
In this review, we discuss the different types of NIPPV, the specific conditions in which they can be used, and the indications, recommendations, and evidence supporting the efficacy of NIPPV in outpatients.
THE TYPES OF NIPPV AND THEIR USES
Although the conditions for which different types of NIPPV can be used overlap significantly, each type has general indications and different goals of treatment. This section begins with types of NIPPV that are predominantly used to treat sleep-disordered breathing, and then proceeds to those predominantly used for conditions associated with hypoventilation and hypercapnia.
Continuous positive airway pressure
CPAP, currently the most widely used form of NIPPV, applies a constant level of positive pressure at the airway opening during spontaneous breathing.
CPAP is commonly used to treat obstructive sleep-disordered breathing, with the goals of improving daytime sleepiness and reducing cardiovascular risk. It has also been used to treat sleep-disordered breathing associated with congestive heart failure.
CPAP’s role in the support of ventilation is limited and indirect. For instance, it has been used in the obesity-hypoventilation syndrome and in the “overlap” syndrome (in which both sleep apnea and COPD coexist). However, its benefits in those conditions are probably derived in large part from correction of underlying obstructive sleep apnea.
The mechanisms of action of CPAP include:
- Preventing intermittent narrowing and collapse of the airway in patients with obstructive sleep apnea-hypopnea syndrome by acting as a pneumatic splint during sleep3,5,6
- Counteracting auto-positive end-expiratory pressure, thereby reducing respiratory muscle load, reducing the work of breathing, and lowering daytime Paco2 in patients with coexistent COPD and obstructive sleep apnea-hypopnea syndrome (the overlap syndrome)7–9
- Improving lung function (particularly the functional residual capacity) and daytime gas exchange in obstructive sleep apnea-hypopnea syndrome 10
- Improving left ventricular systolic function in patients with heart failure coexisting with obstructive sleep apnea-hypopnea syndrome.11,12
Auto-CPAP is delivered via a self-titrating CPAP device, which uses algorithms to detect variations in the degree of obstruction and consequently adjusts the pressure level to restore normal breathing. Auto-CPAP therefore compensates for factors that modify the upper airway collapsibility, such as body posture during sleep, stage of sleep, use of alcohol, and drugs that affect upper airway muscle tone.13
Although one of the premises of using auto-CPAP is that it improves the patient’s satisfaction and compliance, several studies found it to be no more effective than fixed CPAP for treating obstructive sleep apnea-hypopnea syndrome.14–16 Current guidelines of the American Academy of Sleep Medicine do not recommend self-titrating CPAP devices to diagnose obstructive sleep apnea or to treat patients with cardiopulmonary disorders or other conditions in which nocturnal desaturation may be unrelated to obstructive events.17
Adaptive servo-ventilation
Adaptive servo-ventilation was developed for Cheyne-Stokes respiration-central sleep apnea syndrome in patients with congestive heart failure, who may have periods of crescendo-decrescendo change in tidal volume (Cheyne-Stokes respiration) with possible intercalated episodes of central apnea or hypopnea. It is also applied in patients with the complex sleep apnea syndrome.
Adaptive servo-ventilation devices are usually set at an expiratory positive airway pressure (EPAP) level sufficient to control obstructive sleep apnea. The device then automatically adjusts the pressure support for each inspiration, within a prespecified range, to maintain a moving-target ventilation set at 90% of the patient’s recent average ventilation. The aim is to stabilize breathing and reduce respiratory alkalosis, which can trigger apnea reentry cycles.18
Bilevel positive airway pressure
Bilevel positive airway pressure (bilevel PAP) can be of use in sleep-disordered breathing (including cases associated with congestive heart failure), but it is predominantly applied in conditions associated with hypoventilation.
Bilevel PAP devices deliver a higher pressure during inspiration (inspiratory positive airway pressure, or IPAP) and a lower pressure during expiration (EPAP). The gradient between IPAP and EPAP is of key importance in maintaining alveolar ventilation and reducing Paco2. The IPAP acts as pressure support to augment the patient’s effort, maintain adequate alveolar ventilation, unload respiratory muscles, decrease the work of breathing, and control obstructive hypopnea, whereas EPAP is set to maintain upper airway patency, control obstructive apnea, improve functional residual capacity, and prevent microatelectasis.
Although there is no evidence that bilevel PAP is better adhered to or more effective than CPAP, current guidelines propose it as an option for patients who require high pressures to control obstructive sleep apnea-hypopnea syndrome or for those who cannot tolerate exhaling against a high fixed CPAP pressure.19
Other, more-common uses of bilevel PAP are to treat coexisting central sleep apnea or hypoventilation,19 the obesity-hypoventilation syndrome with residual alveolar hypoventilation despite CPAP and control of concomitant obstructive sleep apnea-hypopnea syndrome,5,20 severe stable COPD with significant nocturnal hypoventilation and daytime hypercarbia,21 and restrictive pulmonary diseases.21
Average volume-assured pressure support
CONDITIONS IN WHICH NOCTURNAL NIPPV IS USED IN OUT PATIENTS
Obstructive sleep apnea-hypopnea syndrome
Obstructive sleep apnea-hypopnea syndrome is estimated to affect 2% of women and 4% of men.25 It is characterized by recurrent episodes of partial (hypopnea) or complete (apnea) upper airway obstruction during sleep despite ongoing inspiratory efforts, with associated episodes of arousal or desaturation or both. Corresponding symptoms include excessive daytime sleepiness, choking and gasping during sleep, recurrent awakenings from sleep, unrefreshing sleep, daytime fatigue, and impaired concentration that is not explained by other factors.26
Current understanding of the pathophysiology of obstructive sleep apnea implicates an impairment in the balance between factors that promote collapsibility of the airway (including obesity and anatomic issues such as the volume of the soft-tissue structures surrounding the upper airway) and the compensatory neuromuscular response.27,28
Long-standing obstructive sleep apnea-hypopnea syndrome has been linked in prospective studies to the development of hypertension,29–31 coronary artery disease,32,33 increased coagulation,34,35 and stroke or death from any cause.36,37 It is also associated with a greater rate and severity of motor vehicular accidents,38 greater health care utilization, impaired work performance, and occupational injuries.39
Strong evidence exists that NIPPV (most commonly CPAP) is beneficial in obstructive sleep apnea-hypopnea syndrome, improving sleep quality, sleepiness, cognitive impairment, and quality of life,40,41 decreasing motor vehicle accidents,42 lowering blood pressure, 43,44 and decreasing the rates of myocardial infarction,32 stroke,32 and death.45
The American Academy of Sleep Medicine recommends CPAP as an optional adjunctive therapy to lower blood pressure in patients with obstructive sleep apnea-hypopnea syndrome with concomitant hypertension.19 This is supported by a recent study that suggested that CPAP may have additional benefits on blood pressure in a subgroup of patients with uncontrolled hypertension while on antihypertensive medications.46 Indications with Medicare guidelines for reimbursement of CPAP devices are summarized in Table 2.
Complex sleep apnea syndrome
The complex sleep apnea syndrome is characterized by the emergence of significant central sleep apnea or Cheyne-Stokes respiration after obstructive events have been brought under control with NIPPV in patients who initially appear to have obstructive sleep apnea-hypopnea syndrome. A retrospective study of patients with the complex sleep apnea syndrome who continued their NIPPV use until a subsequent polysomnographic study and NIPPV titration showed that the syndrome may resolve spontaneously, but that 46% of patients had a persistently elevated apnea-hypopnea index with central apnea activity.47
Restoration of upper airway patency by CPAP and dysregulation or delayed adaptation of chemosensitive ventilatory control to a changing Paco2 level may be a key pathophysiologic mechanism of the complex sleep apnea syndrome.48 In this mechanism, increased ventilation from restored airway patency and from an increase in the slope of the ventilatory response may intermittently draw the Paco2 to below the hypocapnic apneic threshold and trigger episodes of central apnea.48
The ideal NIPPV device for use in the complex sleep apnea syndrome should be able to provide enough pressure to resolve the obstructive sleep apnea-hypopnea syndrome while maintaining proper ventilatory support during central apnea episodes without fluctuations of Paco2, which could further worsen the dysregulated ventilatory control. Currently, adaptive servo-ventilation appears to be superior to bilevel PAP and CPAP in the management of the complex sleep apnea syndrome.49,50
Sleep disturbances associated with cardiac dysfunction
There are specific indications for NIPPV modes in the setting of respiratory sleep disturbances associated with heart failure.
Obstructive sleep apnea in congestive heart failure. The prevalence of obstructive sleep apnea in patients with impaired left ventricular ejection fraction is 11% to 53%.51 Obstructive sleep apnea-hypopnea syndrome can worsen congestive heart failure by causing a periodic increase in negative intrathoracic pressure from breathing against an occluded airway, by raising arterial blood pressure, and causing tachycardia from sympathetic nervous system stimulation from hypoxia, hypercarbia, and arousals.52,53 Both heart failure and sleep apnea contribute in an additive manner to the increased sympathetic nervous activity.54
Fortunately, treatment with CPAP has been found to reduce systolic blood pressure and improve left ventricular systolic function in medically treated patients with heart failure and coexisting obstructive sleep apnea.11,12 Furthermore, in a randomized trial in patients with stable congestive heart failure and newly diagnosed obstructive sleep apnea-hypopnea syndrome, a greater improvement in cardiac function was observed in patients on bilevel PAP than in those on CPAP.55 The authors speculated that bilevel PAP might provide more unloading of the respiratory muscles, reduce the work of breathing more, and result in less positive intrathoracic pressure than with CPAP, and that the higher intrathoracic pressure with CPAP could reduce the left ventricular ejection fraction in patients with low filling pressures (pulmonary capillary wedge pressure < 12 mm Hg) and low baseline left ventricular ejection fractions (< 30%).55
Whether these interventions reduce the mortality rate is uncertain. In a prospective nonrandomized study, 9 (24%) of 37 patients who had heart failure with untreated obstructive sleep apnea died, compared with no deaths in 14 treated patients (P = .07).56
Cheyne-Stokes respiration with central sleep apnea in congestive heart failure. A related but different situation is central apnea associated with congestive heart failure.
There are several pathophysiologic mechanisms of Cheyne-Stokes respiration with central sleep apnea. Specifically, the elevation of left ventricular filling pressures, end-diastolic volumes, and pulmonary congestion generate hyperventilation, chronic hypocapnia, and increased chemoreceptor responsiveness, which contribute to the development of central apnea by promoting a decrease in the Paco2 during sleep to below the hypocapnic apneic threshold.57,58 Additionally, an increase in circulation time may result in periodicity of breathing and hyperpnea.59 Obstructive events can then occur at the end of the central events corresponding with the nadir of the inspiratory drive.60,61
The Canadian Continuous Positive Airway Pressure for Patients With Central Sleep Apnea and Heart Failure (CANPAP) trial, a randomized trial of CPAP in this clinical setting, showed that compared with optimal medical therapy alone, CPAP plus optimal medical therapy improved the ejection fraction, reduced central sleep apnea, improved nocturnal oxygenation, and improved the 6-minute walking distance, but without a survival benefit.62 The disappointing survival results from CANPAP have to be interpreted in the context that CPAP may have failed to control the central apnea in some patients, such that the mean apnea-hypopnea index in treated patients (19 events/hour) remained above the entry criterion for recruitment (15 events/hour). In a post hoc analysis of this study, the heart-transplantation-free survival rate was significantly greater in the subgroup of patients in whom CPAP effectively suppressed central sleep apnea (< 15 events/hour).63
Other NIPPV modes such as bilevel PAP with backup rate and adaptive servo-ventilation have been shown in some studies to be superior to CPAP in controlling respiratory events, with adaptive servo-ventilation being the most effective in controlling central, mixed, and complex sleep apnea in this setting. 49,50 Whether more effective resolution of obstructive and central events with these treatment modes translates into improved mortality rates and transplantation-free survival rates remains to be determined.
Obesity-hypoventilation syndrome
Obesity-hypoventilation syndrome refers to daytime hypercapnia (Paco2 > 45 mm Hg) in obese people when no other cause of hypoventilation is present.
The prevalence of obesity-hypoventilation syndrome among patients with obstructive sleep apnea-hypopnea syndrome is 20% to 30% and is greater in extremely obese patients (body mass index > 40 kg/m2).64 However, about 10% of patients with obesity-hypoventilation syndrome do not have obstructive sleep apnea-hypopnea syndrome.64 Additionally, nocturnal hypoxemia and diurnal hypercapnia persist in about 40% of patients with obesity-hypoventilation syndrome after CPAP eliminates their sleep apnea.65 Therefore, factors other than sleep apnea contribute to the development of obesity-hypoventilation syndrome, and in a meta-analysis, factors associated with daytime hypercapnia included, in addition to body mass index and the apnea-hypopnea index, mean overnight oxygen saturation and severity of restrictive pulmonary function.66 Predictors of success with CPAP include better spirometric findings, a higher apnea-hypopnea index, and adequate oxygenation.67,68
Bilevel PAP therapy can be tried in patients in whom CPAP by itself fails. In a study of patients with obesity-hypoventilation syndrome in whom initial CPAP treatment failed, average volume-assured pressure support lowered Paco2 compared to bilevel PAP alone, but did not further improve oxygenation, sleep quality, or quality of life.69
Restrictive pulmonary diseases
Neuromuscular diseases and thoracic cage abnormalities. Noninvasive ventilation has been used in patients with progressive neuromuscular disorders or severe thoracic cage abnormalities, with recognized benefits including an improved survival rate and improved quality of life.70,71 However, NIPPV is used in only 9% of patients with amyotrophic lateral sclerosis when clearly indicated.72 The indications and Medicare guidelines for reimbursement of NIPPV (with or without a backup rate) in this setting are shown in Table 2.
Potential contraindications to starting NIPPV in this population include upper airway obstruction, failure to clear secretions despite optimal noninvasive support, inability to achieve a mask fit, and intolerance of the intervention.73,74
The mechanisms of benefit of NIPPV in these settings include improvements in daytime blood gas levels (including hypercapnia75), a reduction in the oxygen cost of breathing,76 an increase in the ventilatory response to carbon dioxide,75 and improved lung compliance.77
Chronic hypercapnic failure due to severe COPD
The use of NIPPV in chronic COPD is less well established than in patients with exacerbations of COPD,78 and limitations in its use are reflected in the more stringent Medicare indications for NIPPV in this setting (Table 2).
A particular subset of patients with stable COPD who may benefit from NIPPV includes those with daytime hypercapnia and super-imposed nocturnal hypoventilation.78 The potential benefits of NIPPV in these patients include improved daytime and nocturnal gas exchange, increased sleep duration, and improved quality of life.78 Additionally, a recent randomized controlled trial of NIPPV plus long-term oxygen therapy compared with oxygen therapy alone in patients with severe COPD and a Paco2 greater than 46 mm Hg demonstrated a survival benefit in favor of adding NIPPV (hazard ratio 0.6).79
However, that study also found no reduction in hospitalization rates, an apparent worsening in general and mental health (as reflected on the 36-Item Short Form Health Survey or SF-36, a quality-of-life questionnaire), as well as increased confusion and bewilderment (reflected on the Profile of Mood States scale).79 These potentially deleterious effects may explain why adherence to NIPPV is low in patients with stable COPD: only 37% to 57% of patients continued to use it in several reported studies.79–81
A level of inspiratory pressure support that is insufficient to reduce hypercapnia may account for the low adherence rate and worsened quality of life in such patients. For instance, in a randomized trial,82 compared with low-intensity NIPPV (mean IPAP 14 cm H2O, backup rate 8 per minute), settings that aimed to maximally reduce Paco2 (mean IPAP 29 cm H2O with a backup rate of 17.5 per minute) increased the daily use of NIPPV by 3.6 hours/day and improved exercise-related dyspnea, daytime Paco2, forced expiratory volume in 1 second (FEV1), vital capacity, and health-related quality of life.
The overlap syndrome was first described by Flenley in 1985 as a combination of chronic respiratory disease (more generally limited to COPD) and obstructive sleep apnea-hypopnea syndrome.83 Epidemiologic studies do not consistently show a higher incidence of obstructive sleep apnea-hypopnea syndrome in patients with COPD, but the exaggerated oxygen desaturation during sleep in patients with this combination increases the risk of hypoxemia, hypercapnia, and pulmonary hypertension. 84 In addition, there was evidence of higher risks of death and of hospitalization for COPD in patients with the overlap syndrome. 85 NIPPV is the main treatment for obstructive sleep apnea-hypopnea syndrome with or without COPD.
A recent study by Marin et al85 showed that CPAP was associated with improved survival and decreased hospitalization in patients with the overlap syndrome. However, polysomnography or nocturnal oximetry while on NIPPV alone must be done, as additional nocturnal oxygen therapy may be warranted when significant chronic respiratory illness coexists with sleep apnea.
Acknowledgment: The authors gratefully acknowledge the contribution of Scott Marlow, RRT, to Table 2 of this review.
- Organized jointly by the American Thoracic Society, the European Respiratory Society, the European Society of Intensive Care Medicine, and the Société de Réanimation de Langue Française, and approved by ATS Board of Directors, December 2000. International Consensus Conferences in Intensive Care Medicine: noninvasive positive pressure ventilation in acute respiratory failure. Am J Respir Crit Care Med 2001; 163:283–291.
- Aboussouan LS, Ricaurte B. Noninvasive positive pressure ventilation: increasing use in acute care. Cleve Clin J Med 2010; 77:307–316.
- Sullivan CE, Berthon-Jones M, Issa FG. Remission of severe obesity-hypoventilation syndrome after short-term treatment during sleep with nasal continuous positive airway pressure. Am Rev Respir Dis 1983; 128:177–181.
- Ellis ER, Bye PT, Bruderer JW, Sullivan CE. Treatment of respiratory failure during sleep in patients with neuromuscular disease. Positive-pressure ventilation through a nose mask. Am Rev Respir Dis 1987; 135:148–152.
- Berger KI, Ayappa I, Chatr-Amontri B, et al. Obesity hypoventilation syndrome as a spectrum of respiratory disturbances during sleep. Chest 2001; 120:1231–1238.
- Olson AL, Zwillich C. The obesity hypoventilation syndrome. Am J Med 2005; 118:948–956.
- de Miguel J, Cabello J, Sánchez-Alarcos JM, Alvarez-Sala R, Espinós D, Alvarez-Sala JL. Long-term effects of treatment with nasal continuous positive airway pressure on lung function in patients with overlap syndrome. Sleep Breath 2002; 6:3–10.
- Mezzanotte WS, Tangel DJ, Fox AM, Ballard RD, White DP. Nocturnal nasal continuous positive airway pressure in patients with chronic obstructive pulmonary disease. Influence on waking respiratory muscle function. Chest 1994; 106:1100–1108.
- Petrof BJ, Legaré M, Goldberg P, Milic-Emili J, Gottfried SB. Continuous positive airway pressure reduces work of breathing and dyspnea during weaning from mechanical ventilation in severe chronic obstructive pulmonary disease. Am Rev Respir Dis 1990; 141:281–289.
- Verbraecken J, Willemen M, De Cock W, Van de Heyning P, De Backer WA. Continuous positive airway pressure and lung inflation in sleep apnea patients. Respiration 2001; 68:357–364.
- Kaneko Y, Floras JS, Usui K, et al. Cardiovascular effects of continuous positive airway pressure in patients with heart failure and obstructive sleep apnea. N Engl J Med 2003; 348:1233–1241.
- Mansfield DR, Gollogly NC, Kaye DM, Richardson M, Bergin P, Naughton MT. Controlled trial of continuous positive airway pressure in obstructive sleep apnea and heart failure. Am J Respir Crit Care Med 2004; 169:361–366.
- Malhotra A, Trinder J, Fogel R, et al. Postural effects on pharyngeal protective reflex mechanisms. Sleep 2004; 27:1105–1112.
- Ayas NT, Patel SR, Malhotra A, et al. Auto-titrating versus standard continuous positive airway pressure for the treatment of obstructive sleep apnea: results of a meta-analysis. Sleep 2004; 27:249–253.
- Nolan GM, Ryan S, O’Connor TM, McNicholas WT. Comparison of three auto-adjusting positive pressure devices in patients with sleep apnoea. Eur Respir J 2006; 28:159–164.
- Meurice JC, Cornette A, Philip-Joet F, et al; ANTADIR “PPC” Working Group. Evaluation of autoCPAP devices in home treatment of sleep apnea/hypopnea syndrome. Sleep Med 2007; 8:695–703.
- Morgenthaler TI, Aurora RN, Brown T, et al; Standards of Practice Committee of the AASM; American Academy of Sleep Medicine. Practice parameters for the use of autotitrating continuous positive airway pressure devices for titrating pressures and treating adult patients with obstructive sleep apnea syndrome: an update for 2007. An American Academy of Sleep Medicine report. Sleep 2008; 31:141–147.
- Teschler H, Döhring J, Wang YM, Berthon-Jones M. Adaptive pressure support servo-ventilation: a novel treatment for Cheyne-Stokes respiration in heart failure. Am J Respir Crit Care Med 2001; 164:614–619.
- Kushida CA, Littner MR, Hirshkowitz M, et al; American Academy of Sleep Medicine. Practice parameters for the use of continuous and bilevel positive airway pressure devices to treat adult patients with sleep-related breathing disorders. Sleep 2006; 29:375–380.
- Schäfer H, Ewig S, Hasper E, Lüderitz B. Failure of CPAP therapy in obstructive sleep apnoea syndrome: predictive factors and treatment with bilevel-positive airway pressure. Respir Med 1998; 92:208–215.
- Clinical indications for noninvasive positive pressure ventilation in chronic respiratory failure due to restrictive lung disease, COPD, and nocturnal hypoventilation—a consensus conference report. Chest 1999; 116:521–534.
- Aboussouan LS, Khan SU, Meeker DP, Stelmach K, Mitsumoto H. Effect of noninvasive positive-pressure ventilation on survival in amyotrophic lateral sclerosis. Ann Intern Med 1997; 127:450–453.
- Köhnlein T, Welte T, Tan LB, Elliott MW. Assisted ventilation for heart failure patients with Cheyne-Stokes respiration. Eur Respir J 2002; 20:934–941.
- Johnson KG, Johnson DC. Bilevel positive airway pressure worsens central apneas during sleep. Chest 2005; 128:2141–2150.
- Young T, Palta M, Dempsey J, Skatrud J, Weber S, Badr S. The occurrence of sleep-disordered breathing among middle-aged adults. N Engl J Med 1993; 328:1230–1235.
- Sleep-related breathing disorders in adults: recommendations for syndrome definition and measurement techniques in clinical research. The Report of an American Academy of Sleep Medicine Task Force. Sleep 1999; 22:667–689.
- Patil SP, Schneider H, Schwartz AR, Smith PL. Adult obstructive sleep apnea: pathophysiology and diagnosis. Chest 2007; 132:325–337.
- Schwab RJ, Pasirstein M, Pierson R, et al. Identification of upper airway anatomic risk factors for obstructive sleep apnea with volumetric magnetic resonance imaging. Am J Respir Crit Care Med 2003; 168:522–530.
- Hedner J, Bengtsson-Boström K, Peker Y, Grote L, Råstam L, Lindblad U. Hypertension prevalence in obstructive sleep apnoea and sex: a population-based case-control study. Eur Respir J 2006; 27:564–570.
- Nieto FJ, Young TB, Lind BK, et al. Association of sleep-disordered breathing, sleep apnea, and hypertension in a large community-based study. Sleep Heart Health Study. JAMA 2000; 283:1829–1836.
- Peppard PE, Young T, Palta M, Skatrud J. Prospective study of the association between sleep-disordered breathing and hypertension. N Engl J Med 2000; 342:1378–1384.
- Marin JM, Carrizo SJ, Vicente E, Agusti AG. Long-term cardiovascular outcomes in men with obstructive sleep apnoea-hypopnoea with or without treatment with continuous positive airway pressure: an observational study. Lancet 2005; 365:1046–1053.
- Peker Y, Carlson J, Hedner J. Increased incidence of coronary artery disease in sleep apnoea: a long-term follow-up. Eur Respir J 2006; 28:596–602.
- Guardiola JJ, Matheson PJ, Clavijo LC, Wilson MA, Fletcher EC. Hypercoagulability in patients with obstructive sleep apnea. Sleep Med 2001; 2:517–523.
- von Känel R, Loredo JS, Ancoli-Israel S, Mills PJ, Natarajan L, Dimsdale JE. Association between polysomnographic measures of disrupted sleep and prothrombotic factors. Chest 2007; 131:733–739.
- Redline S, Yenokyan G, Gottlieb DJ, et al. Obstructive sleep apnea-hypopnea and incident stroke: The Sleep Heart Health Study. Am J Respir Crit Care Med. 2010; Epub ahead of print.
- Yaggi HK, Concato J, Kernan WN, Lichtman JH, Brass LM, Mohsenin V. Obstructive sleep apnea as a risk factor for stroke and death. N Engl J Med 2005; 353:2034–2041.
- Mulgrew AT, Nasvadi G, Butt A, et al. Risk and severity of motor vehicle crashes in patients with obstructive sleep apnoea/hypopnoea. Thorax 2008; 63:536–541.
- AlGhanim N, Comondore VR, Fleetham J, Marra CA, Ayas NT. The economic impact of obstructive sleep apnea. Lung 2008; 186:7–12.
- Giles TL, Lasserson TJ, Smith BH, White J, Wright J, Cates CJ. Continuous positive airways pressure for obstructive sleep apnoea in adults. Cochrane Database Syst Rev 2006; 3:CD001106.
- Patel SR, White DP, Malhotra A, Stanchina ML, Ayas NT. Continuous positive airway pressure therapy for treating sleepiness in a diverse population with obstructive sleep apnea: results of a meta-analysis. Arch Intern Med 2003; 163:565–571.
- George CF. Reduction in motor vehicle collisions following treatment of sleep apnoea with nasal CPAP. Thorax 2001; 56:508–512.
- Becker HF, Jerrentrup A, Ploch T, et al. Effect of nasal continuous positive airway pressure treatment on blood pressure in patients with obstructive sleep apnea. Circulation 2003; 107:68–73.
- Faccenda JF, Mackay TW, Boon NA, Douglas NJ. Randomized placebo-controlled trial of continuous positive airway pressure on blood pressure in the sleep apnea-hypopnea syndrome. Am J Respir Crit Care Med 2001; 163:344–348.
- Campos-Rodriguez F, Peña-Griñan N, Reyes-Nuñez N, et al. Mortality in obstructive sleep apnea-hypopnea patients treated with positive airway pressure. Chest 2005; 128:624–633.
- Pepin JL, Tamisier R, Barone-Rochette G, Launois SH, Levy P, Baguet JP. Comparison of continuous positive airway pressure and valsartan in hypertensive sleep apnea patients. Am J Respir Crit Care Med 2010; Epub ahead of print.
- Kuzniar TJ, Pusalavidyasagar S, Gay PC, Morgenthaler TI. Natural course of complex sleep apnea—a retrospective study. Sleep Breath 2008; 12:135–139.
- Morgenthaler TI, Kagramanov V, Hanak V, Decker PA. Complex sleep apnea syndrome: is it a unique clinical syndrome? Sleep 2006; 29:1203–1209.
- Allam JS, Olson EJ, Gay PC, Morgenthaler TI. Efficacy of adaptive servoventilation in treatment of complex and central sleep apnea syndromes. Chest 2007; 132:1839–1846.
- Morgenthaler TI, Gay PC, Gordon N, Brown LK. Adaptive servoventilation versus noninvasive positive pressure ventilation for central, mixed, and complex sleep apnea syndromes. Sleep 2007; 30:468–475.
- Bordier P. Sleep apnoea in patients with heart failure. Part I: diagnosis, definitions, prevalence, pathophysiology and haemodynamic consequences. Arch Cardiovasc Dis 2009; 102:651–661.
- Romero-Corral A, Somers VK, Pellikka PA, et al. Decreased right and left ventricular myocardial performance in obstructive sleep apnea. Chest 2007; 132:1863–1870.
- Solin P, Kaye DM, Little PJ, Bergin P, Richardson M, Naughton MT. Impact of sleep apnea on sympathetic nervous system activity in heart failure. Chest 2003; 123:1119–1126.
- Floras JS. Should sleep apnoea be a specific target of therapy in chronic heart failure? Heart 2009; 95:1041–1046.
- Khayat RN, Abraham WT, Patt B, Roy M, Hua K, Jarjoura D. Cardiac effects of continuous and bilevel positive airway pressure for patients with heart failure and obstructive sleep apnea: a pilot study. Chest 2008; 134:1162–1168.
- Wang H, Parker JD, Newton GE, et al. Influence of obstructive sleep apnea on mortality in patients with heart failure. J Am Coll Cardiol 2007; 49:1625–1631.
- Javaheri S. A mechanism of central sleep apnea in patients with heart failure. N Engl J Med 1999; 341:949–954.
- Tkacova R, Hall MJ, Liu PP, Fitzgerald FS, Bradley TD. Left ventricular volume in patients with heart failure and Cheyne-Stokes respiration during sleep. Am J Respir Crit Care Med 1997; 156:1549–1555.
- Lorenzi-Filho G, Rankin F, Bies I, Douglas Bradley T. Effects of inhaled carbon dioxide and oxygen on Cheyne-Stokes respiration in patients with heart failure. Am J Respir Crit Care Med 1999; 159:1490–1498.
- Badr MS, Toiber F, Skatrud JB, Dempsey J. Pharyngeal narrowing/occlusion during central sleep apnea. J Appl Physiol 1995; 78:1806–1815.
- Onal E, Burrows DL, Hart RH, Lopata M. Induction of periodic breathing during sleep causes upper airway obstruction in humans. J Appl Physiol 1986; 61:1438–1443.
- Bradley TD, Logan AG, Kimoff RJ, et al; CANPAP Investigators. Continuous positive airway pressure for central sleep apnea and heart failure. N Engl J Med 2005; 353:2025–2033.
- Arzt M, Floras JS, Logan AG, et al; CANPAP Investigators. Suppression of central sleep apnea by continuous positive airway pressure and transplant-free survival in heart failure: a post hoc analysis of the Canadian Continuous Positive Airway Pressure for Patients with Central Sleep Apnea and Heart Failure Trial (CANPAP). Circulation 2007; 115:3173–3180.
- Mokhlesi B, Tulaimat A, Faibussowitsch I, Wang Y, Evans AT. Obesity hypoventilation syndrome: prevalence and predictors in patients with obstructive sleep apnea. Sleep Breath 2007; 11:117–124.
- Banerjee D, Yee BJ, Piper AJ, Zwillich CW, Grunstein RR. Obesity hypoventilation syndrome: hypoxemia during continuous positive airway pressure. Chest 2007; 131:1678–1684.
- Kaw R, Hernandez AV, Walker E, Aboussouan L, Mokhlesi B. Determinants of hypercapnia in obese patients with obstructive sleep apnea: a systematic review and metaanalysis of cohort studies. Chest 2009; 136:787–796.
- Pérez de Llano LA, Golpe R, Piquer MO, et al. Clinical heterogeneity among patients with obesity hypoventilation syndrome: therapeutic implications. Respiration 2008; 75:34–39.
- Piper AJ, Wang D, Yee BJ, Barnes DJ, Grunstein RR. Randomised trial of CPAP vs bilevel support in the treatment of obesity hypoventilation syndrome without severe nocturnal desaturation. Thorax 2008; 63:395–401.
- Storre JH, Seuthe B, Fiechter R, et al. Average volume-assured pressure support in obesity hypoventilation: a randomized crossover trial. Chest 2006; 130:815–821.
- Aboussouan LS, Khan SU, Banerjee M, Arroliga AC, Mitsumoto H. Objective measures of the efficacy of noninvasive positive-pressure ventilation in amyotrophic lateral sclerosis. Muscle Nerve 2001; 24:403–409.
- Bourke SC, Tomlinson M, Williams TL, Bullock RE, Shaw PJ, Gibson GJ. Effects of non-invasive ventilation on survival and quality of life in patients with amyotrophic lateral sclerosis: a randomised controlled trial. Lancet Neurol 2006; 5:140–147.
- Miller RG, Anderson F, Brooks BR, Mitsumoto H, Bradley WG, Ringel SP; ALS CARE Study Group. Outcomes research in amyotrophic lateral sclerosis: lessons learned from the amyotrophic lateral sclerosis clinical assessment, research, and education database. Ann Neurol 2009; 65(suppl 1):S24–S28.
- Bach JR. Amyotrophic lateral sclerosis: prolongation of life by noninvasive respiratory AIDS. Chest 2002; 122:92–98.
- Perrin C, Unterborn JN, Ambrosio CD, Hill NS. Pulmonary complications of chronic neuromuscular diseases and their management. Muscle Nerve 2004; 29:5–27.
- Nickol AH, Hart N, Hopkinson NS, Moxham J, Simonds A, Polkey MI. Mechanisms of improvement of respiratory failure in patients with restrictive thoracic disease treated with non-invasive ventilation. Thorax 2005; 60:754–760.
- Barle H, Söderberg P, Haegerstrand C, Markström A. Bi-level positive airway pressure ventilation reduces the oxygen cost of breathing in long-standing post-polio patients on invasive home mechanical ventilation. Acta Anaesthesiol Scand 2005; 49:197–202.
- Lechtzin N, Shade D, Clawson L, Wiener CM. Supramaximal inflation improves lung compliance in subjects with amyotrophic lateral sclerosis. Chest 2006; 129:1322–1329.
- Hill NS. Noninvasive ventilation for chronic obstructive pulmonary disease. Respir Care 2004; 49:72–87;
- McEvoy RD, Pierce RJ, Hillman D, et al; Australian trial of noninvasive Ventilation in Chronic Airflow Limitation (AVCAL) Study Group. Nocturnal non-invasive nasal ventilation in stable hypercapnic COPD: a randomised controlled trial. Thorax 2009; 64:561–566.
- Criner GJ, Brennan K, Travaline JM, Kreimer D. Efficacy and compliance with noninvasive positive pressure ventilation in patients with chronic respiratory failure. Chest 1999; 116:667–675.
- Strumpf DA, Millman RP, Carlisle CC, et al. Nocturnal positive-pressure ventilation via nasal mask in patients with severe chronic obstructive pulmonary disease. Am Rev Respir Dis 1991; 144:1234–1239.
- Dreher M, Storre JH, Schmoor C, Windisch W. High-intensity versus low-intensity non-invasive ventilation in patients with stable hypercapnic COPD: a randomised crossover trial. Thorax 2010; 65:303–308.
- Flenley DC. Sleep in chronic obstructive lung disease. Clin Chest Med 1985; 6:651–661.
- Weitzenblum E, Chaouat A, Kessler R, Canuet M. Overlap syndrome: obstructive sleep apnea in patients with chronic obstructive pulmonary disease. Proc Am Thorac Soc 2008; 5:237–241.
- Marin JM, Soriano JB, Carrizo SJ, Boldova A, Celli BR. Outcomes in patients with chronic obstructive pulmonary disease and obstructive sleep apnea. The overlap syndrome. Am J Respir Crit Care Med 2010; Epub ahead of print.
- Organized jointly by the American Thoracic Society, the European Respiratory Society, the European Society of Intensive Care Medicine, and the Société de Réanimation de Langue Française, and approved by ATS Board of Directors, December 2000. International Consensus Conferences in Intensive Care Medicine: noninvasive positive pressure ventilation in acute respiratory failure. Am J Respir Crit Care Med 2001; 163:283–291.
- Aboussouan LS, Ricaurte B. Noninvasive positive pressure ventilation: increasing use in acute care. Cleve Clin J Med 2010; 77:307–316.
- Sullivan CE, Berthon-Jones M, Issa FG. Remission of severe obesity-hypoventilation syndrome after short-term treatment during sleep with nasal continuous positive airway pressure. Am Rev Respir Dis 1983; 128:177–181.
- Ellis ER, Bye PT, Bruderer JW, Sullivan CE. Treatment of respiratory failure during sleep in patients with neuromuscular disease. Positive-pressure ventilation through a nose mask. Am Rev Respir Dis 1987; 135:148–152.
- Berger KI, Ayappa I, Chatr-Amontri B, et al. Obesity hypoventilation syndrome as a spectrum of respiratory disturbances during sleep. Chest 2001; 120:1231–1238.
- Olson AL, Zwillich C. The obesity hypoventilation syndrome. Am J Med 2005; 118:948–956.
- de Miguel J, Cabello J, Sánchez-Alarcos JM, Alvarez-Sala R, Espinós D, Alvarez-Sala JL. Long-term effects of treatment with nasal continuous positive airway pressure on lung function in patients with overlap syndrome. Sleep Breath 2002; 6:3–10.
- Mezzanotte WS, Tangel DJ, Fox AM, Ballard RD, White DP. Nocturnal nasal continuous positive airway pressure in patients with chronic obstructive pulmonary disease. Influence on waking respiratory muscle function. Chest 1994; 106:1100–1108.
- Petrof BJ, Legaré M, Goldberg P, Milic-Emili J, Gottfried SB. Continuous positive airway pressure reduces work of breathing and dyspnea during weaning from mechanical ventilation in severe chronic obstructive pulmonary disease. Am Rev Respir Dis 1990; 141:281–289.
- Verbraecken J, Willemen M, De Cock W, Van de Heyning P, De Backer WA. Continuous positive airway pressure and lung inflation in sleep apnea patients. Respiration 2001; 68:357–364.
- Kaneko Y, Floras JS, Usui K, et al. Cardiovascular effects of continuous positive airway pressure in patients with heart failure and obstructive sleep apnea. N Engl J Med 2003; 348:1233–1241.
- Mansfield DR, Gollogly NC, Kaye DM, Richardson M, Bergin P, Naughton MT. Controlled trial of continuous positive airway pressure in obstructive sleep apnea and heart failure. Am J Respir Crit Care Med 2004; 169:361–366.
- Malhotra A, Trinder J, Fogel R, et al. Postural effects on pharyngeal protective reflex mechanisms. Sleep 2004; 27:1105–1112.
- Ayas NT, Patel SR, Malhotra A, et al. Auto-titrating versus standard continuous positive airway pressure for the treatment of obstructive sleep apnea: results of a meta-analysis. Sleep 2004; 27:249–253.
- Nolan GM, Ryan S, O’Connor TM, McNicholas WT. Comparison of three auto-adjusting positive pressure devices in patients with sleep apnoea. Eur Respir J 2006; 28:159–164.
- Meurice JC, Cornette A, Philip-Joet F, et al; ANTADIR “PPC” Working Group. Evaluation of autoCPAP devices in home treatment of sleep apnea/hypopnea syndrome. Sleep Med 2007; 8:695–703.
- Morgenthaler TI, Aurora RN, Brown T, et al; Standards of Practice Committee of the AASM; American Academy of Sleep Medicine. Practice parameters for the use of autotitrating continuous positive airway pressure devices for titrating pressures and treating adult patients with obstructive sleep apnea syndrome: an update for 2007. An American Academy of Sleep Medicine report. Sleep 2008; 31:141–147.
- Teschler H, Döhring J, Wang YM, Berthon-Jones M. Adaptive pressure support servo-ventilation: a novel treatment for Cheyne-Stokes respiration in heart failure. Am J Respir Crit Care Med 2001; 164:614–619.
- Kushida CA, Littner MR, Hirshkowitz M, et al; American Academy of Sleep Medicine. Practice parameters for the use of continuous and bilevel positive airway pressure devices to treat adult patients with sleep-related breathing disorders. Sleep 2006; 29:375–380.
- Schäfer H, Ewig S, Hasper E, Lüderitz B. Failure of CPAP therapy in obstructive sleep apnoea syndrome: predictive factors and treatment with bilevel-positive airway pressure. Respir Med 1998; 92:208–215.
- Clinical indications for noninvasive positive pressure ventilation in chronic respiratory failure due to restrictive lung disease, COPD, and nocturnal hypoventilation—a consensus conference report. Chest 1999; 116:521–534.
- Aboussouan LS, Khan SU, Meeker DP, Stelmach K, Mitsumoto H. Effect of noninvasive positive-pressure ventilation on survival in amyotrophic lateral sclerosis. Ann Intern Med 1997; 127:450–453.
- Köhnlein T, Welte T, Tan LB, Elliott MW. Assisted ventilation for heart failure patients with Cheyne-Stokes respiration. Eur Respir J 2002; 20:934–941.
- Johnson KG, Johnson DC. Bilevel positive airway pressure worsens central apneas during sleep. Chest 2005; 128:2141–2150.
- Young T, Palta M, Dempsey J, Skatrud J, Weber S, Badr S. The occurrence of sleep-disordered breathing among middle-aged adults. N Engl J Med 1993; 328:1230–1235.
- Sleep-related breathing disorders in adults: recommendations for syndrome definition and measurement techniques in clinical research. The Report of an American Academy of Sleep Medicine Task Force. Sleep 1999; 22:667–689.
- Patil SP, Schneider H, Schwartz AR, Smith PL. Adult obstructive sleep apnea: pathophysiology and diagnosis. Chest 2007; 132:325–337.
- Schwab RJ, Pasirstein M, Pierson R, et al. Identification of upper airway anatomic risk factors for obstructive sleep apnea with volumetric magnetic resonance imaging. Am J Respir Crit Care Med 2003; 168:522–530.
- Hedner J, Bengtsson-Boström K, Peker Y, Grote L, Råstam L, Lindblad U. Hypertension prevalence in obstructive sleep apnoea and sex: a population-based case-control study. Eur Respir J 2006; 27:564–570.
- Nieto FJ, Young TB, Lind BK, et al. Association of sleep-disordered breathing, sleep apnea, and hypertension in a large community-based study. Sleep Heart Health Study. JAMA 2000; 283:1829–1836.
- Peppard PE, Young T, Palta M, Skatrud J. Prospective study of the association between sleep-disordered breathing and hypertension. N Engl J Med 2000; 342:1378–1384.
- Marin JM, Carrizo SJ, Vicente E, Agusti AG. Long-term cardiovascular outcomes in men with obstructive sleep apnoea-hypopnoea with or without treatment with continuous positive airway pressure: an observational study. Lancet 2005; 365:1046–1053.
- Peker Y, Carlson J, Hedner J. Increased incidence of coronary artery disease in sleep apnoea: a long-term follow-up. Eur Respir J 2006; 28:596–602.
- Guardiola JJ, Matheson PJ, Clavijo LC, Wilson MA, Fletcher EC. Hypercoagulability in patients with obstructive sleep apnea. Sleep Med 2001; 2:517–523.
- von Känel R, Loredo JS, Ancoli-Israel S, Mills PJ, Natarajan L, Dimsdale JE. Association between polysomnographic measures of disrupted sleep and prothrombotic factors. Chest 2007; 131:733–739.
- Redline S, Yenokyan G, Gottlieb DJ, et al. Obstructive sleep apnea-hypopnea and incident stroke: The Sleep Heart Health Study. Am J Respir Crit Care Med. 2010; Epub ahead of print.
- Yaggi HK, Concato J, Kernan WN, Lichtman JH, Brass LM, Mohsenin V. Obstructive sleep apnea as a risk factor for stroke and death. N Engl J Med 2005; 353:2034–2041.
- Mulgrew AT, Nasvadi G, Butt A, et al. Risk and severity of motor vehicle crashes in patients with obstructive sleep apnoea/hypopnoea. Thorax 2008; 63:536–541.
- AlGhanim N, Comondore VR, Fleetham J, Marra CA, Ayas NT. The economic impact of obstructive sleep apnea. Lung 2008; 186:7–12.
- Giles TL, Lasserson TJ, Smith BH, White J, Wright J, Cates CJ. Continuous positive airways pressure for obstructive sleep apnoea in adults. Cochrane Database Syst Rev 2006; 3:CD001106.
- Patel SR, White DP, Malhotra A, Stanchina ML, Ayas NT. Continuous positive airway pressure therapy for treating sleepiness in a diverse population with obstructive sleep apnea: results of a meta-analysis. Arch Intern Med 2003; 163:565–571.
- George CF. Reduction in motor vehicle collisions following treatment of sleep apnoea with nasal CPAP. Thorax 2001; 56:508–512.
- Becker HF, Jerrentrup A, Ploch T, et al. Effect of nasal continuous positive airway pressure treatment on blood pressure in patients with obstructive sleep apnea. Circulation 2003; 107:68–73.
- Faccenda JF, Mackay TW, Boon NA, Douglas NJ. Randomized placebo-controlled trial of continuous positive airway pressure on blood pressure in the sleep apnea-hypopnea syndrome. Am J Respir Crit Care Med 2001; 163:344–348.
- Campos-Rodriguez F, Peña-Griñan N, Reyes-Nuñez N, et al. Mortality in obstructive sleep apnea-hypopnea patients treated with positive airway pressure. Chest 2005; 128:624–633.
- Pepin JL, Tamisier R, Barone-Rochette G, Launois SH, Levy P, Baguet JP. Comparison of continuous positive airway pressure and valsartan in hypertensive sleep apnea patients. Am J Respir Crit Care Med 2010; Epub ahead of print.
- Kuzniar TJ, Pusalavidyasagar S, Gay PC, Morgenthaler TI. Natural course of complex sleep apnea—a retrospective study. Sleep Breath 2008; 12:135–139.
- Morgenthaler TI, Kagramanov V, Hanak V, Decker PA. Complex sleep apnea syndrome: is it a unique clinical syndrome? Sleep 2006; 29:1203–1209.
- Allam JS, Olson EJ, Gay PC, Morgenthaler TI. Efficacy of adaptive servoventilation in treatment of complex and central sleep apnea syndromes. Chest 2007; 132:1839–1846.
- Morgenthaler TI, Gay PC, Gordon N, Brown LK. Adaptive servoventilation versus noninvasive positive pressure ventilation for central, mixed, and complex sleep apnea syndromes. Sleep 2007; 30:468–475.
- Bordier P. Sleep apnoea in patients with heart failure. Part I: diagnosis, definitions, prevalence, pathophysiology and haemodynamic consequences. Arch Cardiovasc Dis 2009; 102:651–661.
- Romero-Corral A, Somers VK, Pellikka PA, et al. Decreased right and left ventricular myocardial performance in obstructive sleep apnea. Chest 2007; 132:1863–1870.
- Solin P, Kaye DM, Little PJ, Bergin P, Richardson M, Naughton MT. Impact of sleep apnea on sympathetic nervous system activity in heart failure. Chest 2003; 123:1119–1126.
- Floras JS. Should sleep apnoea be a specific target of therapy in chronic heart failure? Heart 2009; 95:1041–1046.
- Khayat RN, Abraham WT, Patt B, Roy M, Hua K, Jarjoura D. Cardiac effects of continuous and bilevel positive airway pressure for patients with heart failure and obstructive sleep apnea: a pilot study. Chest 2008; 134:1162–1168.
- Wang H, Parker JD, Newton GE, et al. Influence of obstructive sleep apnea on mortality in patients with heart failure. J Am Coll Cardiol 2007; 49:1625–1631.
- Javaheri S. A mechanism of central sleep apnea in patients with heart failure. N Engl J Med 1999; 341:949–954.
- Tkacova R, Hall MJ, Liu PP, Fitzgerald FS, Bradley TD. Left ventricular volume in patients with heart failure and Cheyne-Stokes respiration during sleep. Am J Respir Crit Care Med 1997; 156:1549–1555.
- Lorenzi-Filho G, Rankin F, Bies I, Douglas Bradley T. Effects of inhaled carbon dioxide and oxygen on Cheyne-Stokes respiration in patients with heart failure. Am J Respir Crit Care Med 1999; 159:1490–1498.
- Badr MS, Toiber F, Skatrud JB, Dempsey J. Pharyngeal narrowing/occlusion during central sleep apnea. J Appl Physiol 1995; 78:1806–1815.
- Onal E, Burrows DL, Hart RH, Lopata M. Induction of periodic breathing during sleep causes upper airway obstruction in humans. J Appl Physiol 1986; 61:1438–1443.
- Bradley TD, Logan AG, Kimoff RJ, et al; CANPAP Investigators. Continuous positive airway pressure for central sleep apnea and heart failure. N Engl J Med 2005; 353:2025–2033.
- Arzt M, Floras JS, Logan AG, et al; CANPAP Investigators. Suppression of central sleep apnea by continuous positive airway pressure and transplant-free survival in heart failure: a post hoc analysis of the Canadian Continuous Positive Airway Pressure for Patients with Central Sleep Apnea and Heart Failure Trial (CANPAP). Circulation 2007; 115:3173–3180.
- Mokhlesi B, Tulaimat A, Faibussowitsch I, Wang Y, Evans AT. Obesity hypoventilation syndrome: prevalence and predictors in patients with obstructive sleep apnea. Sleep Breath 2007; 11:117–124.
- Banerjee D, Yee BJ, Piper AJ, Zwillich CW, Grunstein RR. Obesity hypoventilation syndrome: hypoxemia during continuous positive airway pressure. Chest 2007; 131:1678–1684.
- Kaw R, Hernandez AV, Walker E, Aboussouan L, Mokhlesi B. Determinants of hypercapnia in obese patients with obstructive sleep apnea: a systematic review and metaanalysis of cohort studies. Chest 2009; 136:787–796.
- Pérez de Llano LA, Golpe R, Piquer MO, et al. Clinical heterogeneity among patients with obesity hypoventilation syndrome: therapeutic implications. Respiration 2008; 75:34–39.
- Piper AJ, Wang D, Yee BJ, Barnes DJ, Grunstein RR. Randomised trial of CPAP vs bilevel support in the treatment of obesity hypoventilation syndrome without severe nocturnal desaturation. Thorax 2008; 63:395–401.
- Storre JH, Seuthe B, Fiechter R, et al. Average volume-assured pressure support in obesity hypoventilation: a randomized crossover trial. Chest 2006; 130:815–821.
- Aboussouan LS, Khan SU, Banerjee M, Arroliga AC, Mitsumoto H. Objective measures of the efficacy of noninvasive positive-pressure ventilation in amyotrophic lateral sclerosis. Muscle Nerve 2001; 24:403–409.
- Bourke SC, Tomlinson M, Williams TL, Bullock RE, Shaw PJ, Gibson GJ. Effects of non-invasive ventilation on survival and quality of life in patients with amyotrophic lateral sclerosis: a randomised controlled trial. Lancet Neurol 2006; 5:140–147.
- Miller RG, Anderson F, Brooks BR, Mitsumoto H, Bradley WG, Ringel SP; ALS CARE Study Group. Outcomes research in amyotrophic lateral sclerosis: lessons learned from the amyotrophic lateral sclerosis clinical assessment, research, and education database. Ann Neurol 2009; 65(suppl 1):S24–S28.
- Bach JR. Amyotrophic lateral sclerosis: prolongation of life by noninvasive respiratory AIDS. Chest 2002; 122:92–98.
- Perrin C, Unterborn JN, Ambrosio CD, Hill NS. Pulmonary complications of chronic neuromuscular diseases and their management. Muscle Nerve 2004; 29:5–27.
- Nickol AH, Hart N, Hopkinson NS, Moxham J, Simonds A, Polkey MI. Mechanisms of improvement of respiratory failure in patients with restrictive thoracic disease treated with non-invasive ventilation. Thorax 2005; 60:754–760.
- Barle H, Söderberg P, Haegerstrand C, Markström A. Bi-level positive airway pressure ventilation reduces the oxygen cost of breathing in long-standing post-polio patients on invasive home mechanical ventilation. Acta Anaesthesiol Scand 2005; 49:197–202.
- Lechtzin N, Shade D, Clawson L, Wiener CM. Supramaximal inflation improves lung compliance in subjects with amyotrophic lateral sclerosis. Chest 2006; 129:1322–1329.
- Hill NS. Noninvasive ventilation for chronic obstructive pulmonary disease. Respir Care 2004; 49:72–87;
- McEvoy RD, Pierce RJ, Hillman D, et al; Australian trial of noninvasive Ventilation in Chronic Airflow Limitation (AVCAL) Study Group. Nocturnal non-invasive nasal ventilation in stable hypercapnic COPD: a randomised controlled trial. Thorax 2009; 64:561–566.
- Criner GJ, Brennan K, Travaline JM, Kreimer D. Efficacy and compliance with noninvasive positive pressure ventilation in patients with chronic respiratory failure. Chest 1999; 116:667–675.
- Strumpf DA, Millman RP, Carlisle CC, et al. Nocturnal positive-pressure ventilation via nasal mask in patients with severe chronic obstructive pulmonary disease. Am Rev Respir Dis 1991; 144:1234–1239.
- Dreher M, Storre JH, Schmoor C, Windisch W. High-intensity versus low-intensity non-invasive ventilation in patients with stable hypercapnic COPD: a randomised crossover trial. Thorax 2010; 65:303–308.
- Flenley DC. Sleep in chronic obstructive lung disease. Clin Chest Med 1985; 6:651–661.
- Weitzenblum E, Chaouat A, Kessler R, Canuet M. Overlap syndrome: obstructive sleep apnea in patients with chronic obstructive pulmonary disease. Proc Am Thorac Soc 2008; 5:237–241.
- Marin JM, Soriano JB, Carrizo SJ, Boldova A, Celli BR. Outcomes in patients with chronic obstructive pulmonary disease and obstructive sleep apnea. The overlap syndrome. Am J Respir Crit Care Med 2010; Epub ahead of print.
KEY POINTS
- In sleep apnea, NIPPV has both short-term benefits such as improved daytime alertness and reduced fatigue, and long-term benefits such as a reduced cardiovascular risk.
- The potential development of complex sleep apnea with NIPPV may be managed by using lower pressures, by continued treatment (more than half of cases improve over time), and by advanced options such as adaptive servo-ventilation.
- In patients with concomitant obstructive sleep apnea and congestive heart failure, NIPPV, particularly bilevel positive airway pressure, improves blood pressure and left ventricular function, though it is not clear whether it has a survival benefit.
Taking blood pressure: Too important to trust to humans?
The reality of blood pressure measurement is that human beings do not do it very well. The time has come to delegate this job to machines that can do it better.
Several automatic devices are available. Used in physicians’ offices and in patients’ homes, they can eliminate some types of observer error as well as the “white-coat effect,” ie, the tendency of some patients to have higher blood pressure when medical personnel are present than in their natural environment. Since a difference of only a few millimeters of mercury can affect the physician’s decision to start or to modify treatment, measurements that more accurately reflect a person’s average blood pressure are to be desired.
In the following pages, we review the problems that plague blood pressure measurement by human observers, and we describe the advantages of automatic devices.
SHORTCOMINGS OF OFFICE BLOOD PRESSURE MEASUREMENT
For decades, large surveys have provided invaluable information on the prevalence of hypertension, its relationship to cardiovascular disease, and the benefits of treating it.1–3 Unfortunately, the percentage of hypertensive patients whose blood pressure is under control has remained low despite our increased knowledge about hypertension’s diagnosis and therapy.4
In these surveys, blood pressure was measured by auscultation by human observers using mercury or aneroid sphygmomanometers, and most physicians still use this method in clinical practice. But in spite of multiple guidelines for accurate measurement of blood pressure in the office, the overall accuracy and reproducibility of office blood pressure measurements remain poor.5–7
The accuracy of blood pressure measurement with aneroid and mercury manometers is affected by a number of observer errors and patient factors.8,9
Observer errors
Failure to prepare the patient. National guidelines5 state that before having their blood pressure taken, patients should be allowed to sit quietly for at least 5 minutes, which often does not happen. Another error is that clinicians rarely discourage patients from smoking cigarettes or drinking coffee in the 30 minutes prior to measurement.
Equipment and layout problems. Equipment should be properly calibrated and validated. 5 However, even if the sphygmomanometer is periodically calibrated, too often it is mounted on the wall adjacent to the examination table in the examination room, making it difficult to provide a comfortable seat with back and arm support during the reading. The measurement should be done with the patient sitting in a chair (not on an examination table), with feet on the floor and the arm supported at the level of the heart. If the forearm is not supported in the horizontal position and with the cuff at heart level, the blood pressure and heart rate tend to be higher.10 Further, the diastolic blood pressure and heart rate may be misleadingly low with the patient supine rather than seated,11,12 so readings should be taken with the patient sitting.
Miscuffing, ie, the use of a blood pressure cuff that is too large or, more often, too small for the patient’s arm, is a common source of error. The cuff bladder should encircle at least 80% of the arm.5 However, some offices do not have a large blood pressure cuff for overweight patients or a pediatric cuff for children or adults with arms of small circumference. It is recommended that a large blood pressure cuff be used routinely in adults, since a smaller cuff gives falsely high readings in people with large upper arms (circumference > 29 cm).13,14
Digit preference. Many physicians round off the blood pressure to the nearest 5 or 10 mm Hg. This problem may go along with:
Deflating the cuff too rapidly.
Talking to the patient while taking the blood pressure can contribute to higher readings.9
Not taking enough readings. Ideally, at the initial visit, blood pressure should be measured in both arms with the patient seated, and another reading should be taken with the patient standing. The arm with the higher pressure should be used for subsequent readings. Physicians should not make any treatment decisions based on blood pressure during an initial clinic visit, and at least two readings should be taken even on subsequent visits. However, owing to time constraints in busy clinical practices, treatment decisions are often based on single readings or on multiple readings on a single visit.
Discrepancies between observers. The blood pressure readings obtained by the nurse or medical assistant may differ significantly from those obtained by the physician. These differences can be large enough to affect treatment decisions,15,16 and they can be partially corrected by adequate training of all medical personnel who take blood pressure, doctors as well as nurses.
Given that time is tight in busy clinical practices and a trained blood pressure nurse or technician is usually not available, we will probably not see any significant improvement in the accuracy of blood pressure measurement using older technology and current physician practices.
The white-coat effect
Most patients have a higher level of anxiety, and therefore higher blood pressure, in the physician’s office or clinic than in their normal environment (as revealed by ambulatory monitoring or home blood pressure measurements), a phenomenon commonly called the white-coat effect.
Several factors can increase this effect, such as observer-patient interaction during the measurement. The effect tends to be greatest in the initial measurement, but can persist through multiple readings by the doctor or nurse during the same visit.
Whether the white-coat effect is due purely to patient anxiety about an office visit or to a conditioned response has been a point of interest in clinical studies. Regardless, it may result in the misdiagnosis of hypertension or in overestimation of the severity of hypertension and may lead to overly aggressive therapy. Antihypertensive treatment may be unnecessary in the absence of concurrent cardiovascular risk factors.17
“White-coat hypertension” or “isolated office hypertension” is the condition in which a patient who is not on antihypertensive drug therapy has persistently elevated blood pressure in the clinic or office (> 140/90 mm Hg) but normal daytime ambulatory blood pressure (< 135/85 mm Hg).18 Since patients may have an elevated reading when seen for a first office visit, at least several visits are required to establish the diagnosis. Multiple studies have suggested that white-coat hypertension may account for 20% to 25% of the hypertensive population, particularly in older patients, mainly women.19,20
Both white-coat hypertension and the white-coat effect can be avoided by using an automatic and programmable device that can take multiple readings after the clinician leaves the examination room (more about this below).21
MEASURING BLOOD PRESSURE OUTSIDE THE OFFICE
Recent studies have reported that the information obtained by 24-hour ambulatory blood pressure monitoring and by self-measurement of blood pressure in the home more accurately reflects the patient’s risk of future cardiovascular events than do conventional blood pressure measurements taken in the physician’s office. 22–24 Current national guidelines recognize this pattern and the frequent measurement inaccuracies observed in clinical practice, and they are recommending including out-of-office measurements in the diagnosis of hypertension. 25,26
Ambulatory monitoring provides the most accurate measurement of out-of-office blood pressure. With ambulatory monitoring, the normal mean daytime pressure is considered to be lower than 135/85 mm Hg, in contrast to the 140/90 mm Hg cutoff used in the physician’s office with standard aneroid or mercury devices.
Self-monitoring of blood pressure at home has now become widely available with single-measurement oscillometric devices. (Oscillometric means that these devices measure the blood pressure by sensing the oscillations in pressure in the cuff induced by the pulsation of the brachial artery, as opposed to auscultating the Korotkoff sounds.) Blood pressures lower than 135/85 mm Hg outside the clinician’s office are considered normal with these devices.
However, despite its proven value, ambulatory monitoring is neither widely available nor cost-effective for the long-term management of hypertension. Furthermore, few physicians recommend that patients take their blood pressure at home, although the information obtained can be of significant value in the patient’s long-term management.
AUTOMATED MEASUREMENT IN THE OFFICE
In recent years, several automated oscillometric sphygmomanometers have been developed for measuring blood pressure in the office, and more are on the way. These devices can be programmed to take multiple readings without a clinician observer in the examination room, thus reducing the white-coat response.
Omron (Kyoto, Japan) makes several devices, including the HEM-907 and the HEM-705, that have been used in the clinical setting. 21,27–29 They can be programmed to take two or three readings at intervals of 1 to 2 minutes, with up to 5 minutes before the first reading. Unfortunately, data were not recorded with the patient alone in the room in many studies of the Omron devices, even though the devices meet national and international standards for accuracy.
The Microlife Watch BP Office (Microlife, Widnau, Switzerland) is currently undergoing development.30
The BpTRU (BpTRU Medical Devices, Coquitlam, BC, Canada) has enjoyed greater clinical acceptance, since it can take up to five blood pressure readings at intervals of 1 to 5 minutes, and calculates the mean of all five readings, taken with the patient resting comfortably in a quiet room without a clinician present.
The accuracy and durability of the device has been well established. Since the BpTRU self-calibrates between every blood pressure measurement, periodic calibration has not been required. The device can be placed on a table, mounted on the wall, or mounted on a cart if used in several locations in the office.
At Cleveland Clinic, several departments are using the BpTRU on a daily basis. Soon, we will be able to transfer data directly from the BpTRU to our electronic medical record system.
Studies of the BpTRU device
To date, most of the studies of automated office blood pressure measurement have used the BpTRU with the recording interval set at 1 to 2 minutes.
Myers31 used the BpTRU device in 50 hypertensive patients. The physician took the patient’s blood pressure with a mercury sphygmomanometer while the BpTRU device made the first reading, and then he left the room. The next five readings were taken at 2-minute intervals with the patient alone in the room. The mean initial reading by the machine was 162/85 mm Hg; the reading by the physician was 163/86 mm Hg. The third automatic reading was the lowest (averaging 140/84 mm Hg), and the mean of the five automated readings was 142/80 mm Hg, which was significantly lower than the initial reading obtained by the physician (P < .001).
In another study, Myers et al32 compared the measurements obtained by 24-hour ambulatory monitoring and by the BpTRU device (the mean of five readings obtained at 1-minute or 2-minute intervals) in 309 hypertensive patients. The mean blood pressure with the Bp-TRU was 132/75 mm Hg, which correlated well with the mean awake ambulatory blood pressure (134/77 mm Hg; r = 0.62 for the systolic pressure and 0.72 for the diastolic pressure).
We recently reviewed the records of 278 patients seen in our preventive medicine clinic (D.G. Vidt, MD, unpublished data, November 2009). The group included patients with and without established hypertension, and among the hypertensive group, both treated and untreated individuals. We had initially set the device to take readings at 3-minute intervals following the initial nurse-initiated reading. But in view of the recent data on the Bp-TRU using shorter intervals, we also obtained readings in 51 patients with the device set to record at 2-minute intervals, and then in 72 additional patients at 1-minute intervals. In all three groups, blood pressure had stabilized by the third reading after the clinician had left the room. These observations support those reported by Myers et al.31,32 Of particular importance is the observation that the white-coat effect dissipates within 2 to 3 minutes after the clinician leaves the room.33
The shorter measurement intervals can add up in a busy office practice, in which the time relegated to taking blood pressure is often limited.
In fact, waiting 5 minutes between measurements may allow the patient to become too relaxed and the blood pressure to drop too low vis-a-vis the gold standard, ambulatory monitoring. Culleton and colleagues34 compared the blood pressure in 107 hypertensive patients as measured four ways: by the referring physician, by a nurse who was trained to adhere to the protocol of the Canadian Hypertension Education Program, by 24-hour ambulatory monitoring, and by the BpTRU (the mean of five readings obtained at 5-minute intervals). The mean measured values were:
- 150/90 mm Hg by the referring physician
- 139/86 mm Hg by the nurse
- 142/85 mm Hg by ambulatory monitoring
- 132/82 mm Hg by the BpTRU device.
Although the BpTRU reduced the white-coat effect and white-coat hypertension, it underestimated the blood pressure, leading to misclassification of hypertension. Using 140/90 mm Hg as the cutoff for whether the patient was hypertensive and using ambulatory monitoring as the gold standard, the BpTRU misclassified more than half of the patients, agreeing with the classification of hypertensive or not hypertensive by ambulatory monitoring in only 48%. The authors recommended that the BpTRU not be set at 5-minute measurement intervals.34
WHAT ROLE FOR AUTOMATED READINGS IN THE OFFICE?
Although automatic devices, by enabling the physician to leave the room, can eliminate the white-coat effect and white-coat hypertension, physicians must continue to take care to avoid the other potential errors of office blood pressure measurement addressed earlier in this review, for example, by positioning the patient correctly and using a cuff that is large enough. These issues can take on more importance as the clinician leaves the patient alone for brief periods during measurements.
In view of its perennial inaccuracies, some experts have suggested that we abandon routine office measurement of blood pressure.35,36 In its place, ambulatory monitoring would be used for diagnosis and for periodic follow-up. In addition, patients would regularly take their pressure at home with approved, single-measurement oscillometric devices. Unfortunately, in our health care system, periodic ambulatory monitoring for hypertension management would impose a significant financial burden on patients at this time.37
Of particular importance is the observation that the mean of five readings with the BpTRU device, obtained at 1- or 2-minute intervals, closely approximates the mean awake blood pressure obtained in the same patient with an ambulatory monitor.32,38 The ability to obtain readings that correlate exceptionally well with mean daytime ambulatory pressure suggests that this device could well reduce the need for ambulatory monitoring, with its associated cost. The ability to negate the white-coat effect with the use of the BpTRU in the office setting also has particular importance, not only for patient office readings, but for the diagnosis and subsequent treatment of hypertension in individual patients.
Most clinical decisions about the treatment of hypertension are still made on the basis of office determinations of blood pressure. Most office practices still rely on the aneroid manometer or, decreasingly, mercury sphygmomanometers. As noted earlier, although auscultatory blood pressure measurement appears to be simple, it is fraught with a host of observer- or patient-induced errors that not only lead to inaccurate diagnoses, but may also result in the mismanagement of hypertension. Even single-measurement oscillometric devices, now used in a minority of clinical practices, are associated with many of the same measurement issues that lead to overestimation of blood pressure.
We believe the time has come for broader use of oscillometric devices in the outpatient setting. While many available oscillometric devices for use in the home could also be used in the physician’s office, they carry the similar disadvantage of providing only a single measurement. The major disadvantage of all single-measurement devices is the continued presence of the clinician during the reading and the associated white-coat effect observed in most patients.
It is highly likely that the next Joint National Committee Report on Hypertension will further emphasize the role of automated blood pressure devices in the outpatient setting.
Acknowledgment: The authors wish to acknowledge the contributions of Deborah McCoy, RN, and Maria Eckhouse, RN.
- Burt VL, Whelton P, Roccella EJ, et al. Prevalence of hypertension in the US adult population. Results from the Third National Health and Nutrition Examination Survey, 1988–1991. Hypertension 1995; 25:305–313.
- Neaton JD, Wentworth D. Serum cholesterol, blood pressure, cigarette smoking, and death from coronary heart disease. Overall findings and differences by age for 316,099 white men. Multiple Risk Factor Intervention Trial Research Group. Arch Intern Med 1992; 152:56–64.
- Lewington S, Clarke R, Qizilbash N, Peto R, Collins R; Prospective Studies Collaboration. Age-specific relevance of usual blood pressure to vascular mortality: a meta-analysis of individual data for one million adults in 61 prospective studies. Lancet 2002; 360:1903–1913.
- Lloyd-Jones D, Adams R, Carnethon M, et al; American Heart Association Statistics Committee and Stroke Statistics Subcommittee. Heart disease and stroke statistics—2009 update: a report from the American Heart Association Statistics Committee and Stroke Statistics Subcommittee. Circulation 2009; 119:e21–e181.
- Chobanian AV, Bakris GL, Black HR, et al; National Heart, Lung, and Blood Institute Joint National Committee on Prevention, Detection, Evaluation, and Treatment of High Blood Pressure; National High Blood Pressure Education Program Coordinating Committee. The Seventh Report of the Joint National Committee on Prevention, Detection, Evaluation, and Treatment of High Blood Pressure: the JNC 7 report. JAMA 2003; 289:2560–2572.
- Grim CM, Grim CE. A curriculum for the training and certification of blood pressure measurement for health care providers. Can J Cardiol 1995; 11(suppl H):38H–42H.
- Pickering TG, Hall JE, Appel LJ, et al; Subcommittee of Professional and Public Education of the American Heart Association Council on High Blood Pressure Research. Recommendations for blood pressure measurement in humans and experimental animals: part 1: blood pressure measurement in humans: a statement for professionals from the Subcommittee of Professional and Public Education of the American Heart Association Council on High Blood Pressure Research. Hypertension 2005; 45:142–161.
- Langlois S. Measuring blood pressure: how competent are we? Perspect Cardiol 1999; 15:29–39.
- Le Pailleur C, Helft G, Landais P, et al. The effects of talking, reading, and silence on the “white coat” phenomenon in hypertensive patients. Am J Hypertens 1998; 11:203–207.
- Webster J, Newnham D, Petrie JC, Lovell HG. Influence of arm position on measurement of blood pressure. Br Med J (Clin Res Ed) 1984; 288:1574–1575.
- Netea RT, Smits P, Lenders JW, Thien T. Does it matter whether blood pressure measurements are taken with subjects sitting or supine? J Hypertens 1998; 16:263–268.
- Silverberg DS, Shemesh E, Iaina A. The unsupported arm: a cause of falsely raised blood pressure readings. Br Med J 1977; 2:1331.
- Manning DM, Kuchirka C, Kaminski J. Miscuffing: inappropriate blood pressure cuff application. Circulation 1983; 68:763–766.
- Iyriboz Y, Hearon CM, Edwards K. Agreement between large and small cuffs in sphygmomanometry: a quantitative assessment. J Clin Monit 1994; 10:127–133.
- Scherwitz LW, Evans LA, Hennrikus DJ, Vallbona C. Procedures and discrepancies of blood pressure measurements in two community health centers. Med Care 1982; 20:727–738.
- La Batide-Alanore A, Chatellier G, Bobrie G, Fofol I, Plouin PF. Comparison of nurse- and physician-determined clinic blood pressure levels in patients referred to a hypertension clinic: implications for subsequent management. J Hypertens 2000; 18:391–398.
- Verdecchia P. Prognostic value of ambulatory blood pressure: current evidence and clinical implications. Hypertension 2000; 35:844–851.
- Ogedegbe G, Pickering TG, Clemow L, et al. The misdiagnosis of hypertension: the role of patient anxiety. Arch Intern Med 2008; 168:2459–2465.
- Pickering TG. Stress, white coat hypertension and masked hypertension. In:Izzo JL, Sica DA, Black HR, editors. Hypertension Primer: The Essentials of High Blood Pressure: Basic Science, Population Science, and Clinical Management. 4th ed. Philadelphia, PA: Lippincott Williams & Wilkins; 2008:289–291.
- Pickering TG, Coats A, Mallion JM, Mancia G, Verdecchia P. Blood Pressure Monitoring. Task force V: White-coat hypertension. Blood Press Monit 1999; 4:333–341.
- Myers MG, Valdivieso MA. Use of an automated blood pressure recording device, the BpTRU, to reduce the “white coat effect” in routine practice. Am J Hypertens 2003; 16:494–497.
- Redon J, Campos C, Narciso ML, Rodicio JL, Pascual JM, Ruilope LM. Prognostic value of ambulatory blood pressure monitoring in refractory hypertension: a prospective study. Hypertension 1998; 31:712–718.
- Ohkubo T, Imai Y, Tsuji I, et al. Prediction of mortality by ambulatory blood pressure monitoring versus screening blood pressure measurements: a pilot study in Ohasama. J Hypertens 1997; 15:357–364.
- Verdecchia P, Reboldi G, Porcellati C, et al. Risk of cardiovascular disease in relation to achieved office and ambulatory blood pressure control in treated hypertensive subjects. J Am Coll Cardiol 2002; 39:878–885.
- Hemmelgarn BR, McAllister FA, Myers MG, et al; Canadian Hypertension Education Program. The 2005 Canadian Hypertension Education Program recommendations for the management of hypertension: part 1 - blood pressure measurement, diagnosis and assessment of risk. Can J Cardiol 2005; 21:645–656.
- Pickering TG. JNC 7.5. J Clin Hypertens (Greenwich) 2007; 9:901–904.
- White WB, Anwar YA. Evaluation of the overall efficacy of the Omron office digital blood pressure HEM-907 monitor in adults. Blood Press Monit 2001; 6:107–110.
- Myers MG, Meglis G, Polemidiotis G. The impact of physician vs automated blood pressure readings on office-induced hypertension. J Hum Hypertens 1997; 11:491–493.
- Myers MG, Godwin M, Dawes M, Kiss A, Tobe SW, Kaczorowski J. Measurement of blood pressure in the office: recognizing the problem and proposing the solution. Hypertension 2010; 55:195–200.
- Stergiou GS, Tzamouranis D, Protogerou A, Nasothimiou E, Kapralos C. Validation of the Microlife Watch BP Office professional device for office blood pressure measurement according to the International protocol. Blood Press Monit 2008; 13:299–303.
- Myers MG. Automated blood pressure measurement in routine clinical practice. Blood Press Monit 2006; 11:59–62.
- Myers MG, Valdivieso M, Kiss A. Optimum frequency of office blood pressure measurement using an automated sphygmomanometer. Blood Press Monit 2008; 13:333–338.
- Myers MG, Valdivieso M, Kiss A. Use of automated office blood pressure measurement to reduce the white coat response. J Hypertens 2009; 27:280–286.
- Culleton BF, McKay DW, Campbell NR. Performance of the automated BpTRU measurement device in the assessment of white-coat hypertension and white-coat effect. Blood Press Monit 2006; 11:37–42.
- Pickering TG, Miller NH, Ogedegbe G, Krakoff LR, Artinian NT, Goff D; American Heart Association. Call to action on use and reimbursement for home blood pressure monitoring: executive summary: a joint scientific statement from the American Heart Association, American Society Of Hypertension, and Preventive Cardiovascular Nurses Association. Hypertension 2008; 52:1–9.
- Parati G, Stergiou GS, Asmar R, et al; ESH Working Group on Blood Pressure Monitoring. European Society of Hypertension guidelines for blood pressure monitoring at home: a summary report of the Second International Consensus Conference on Home Blood Pressure Monitoring. J Hypertens 2008; 26:1505–1526.
- O’Brien E. Ambulatory blood pressure measurement: the case for implementation in primary care. Hypertension 2008; 51:1435–1441.
- Beckett L, Godwin M. The BpTRU automatic blood pressure monitor compared to 24 hour ambulatory blood pressure monitoring in the assessment of blood pressure in patients with hypertension. BMC Cardiovasc Disord 2005; 5:18.
The reality of blood pressure measurement is that human beings do not do it very well. The time has come to delegate this job to machines that can do it better.
Several automatic devices are available. Used in physicians’ offices and in patients’ homes, they can eliminate some types of observer error as well as the “white-coat effect,” ie, the tendency of some patients to have higher blood pressure when medical personnel are present than in their natural environment. Since a difference of only a few millimeters of mercury can affect the physician’s decision to start or to modify treatment, measurements that more accurately reflect a person’s average blood pressure are to be desired.
In the following pages, we review the problems that plague blood pressure measurement by human observers, and we describe the advantages of automatic devices.
SHORTCOMINGS OF OFFICE BLOOD PRESSURE MEASUREMENT
For decades, large surveys have provided invaluable information on the prevalence of hypertension, its relationship to cardiovascular disease, and the benefits of treating it.1–3 Unfortunately, the percentage of hypertensive patients whose blood pressure is under control has remained low despite our increased knowledge about hypertension’s diagnosis and therapy.4
In these surveys, blood pressure was measured by auscultation by human observers using mercury or aneroid sphygmomanometers, and most physicians still use this method in clinical practice. But in spite of multiple guidelines for accurate measurement of blood pressure in the office, the overall accuracy and reproducibility of office blood pressure measurements remain poor.5–7
The accuracy of blood pressure measurement with aneroid and mercury manometers is affected by a number of observer errors and patient factors.8,9
Observer errors
Failure to prepare the patient. National guidelines5 state that before having their blood pressure taken, patients should be allowed to sit quietly for at least 5 minutes, which often does not happen. Another error is that clinicians rarely discourage patients from smoking cigarettes or drinking coffee in the 30 minutes prior to measurement.
Equipment and layout problems. Equipment should be properly calibrated and validated. 5 However, even if the sphygmomanometer is periodically calibrated, too often it is mounted on the wall adjacent to the examination table in the examination room, making it difficult to provide a comfortable seat with back and arm support during the reading. The measurement should be done with the patient sitting in a chair (not on an examination table), with feet on the floor and the arm supported at the level of the heart. If the forearm is not supported in the horizontal position and with the cuff at heart level, the blood pressure and heart rate tend to be higher.10 Further, the diastolic blood pressure and heart rate may be misleadingly low with the patient supine rather than seated,11,12 so readings should be taken with the patient sitting.
Miscuffing, ie, the use of a blood pressure cuff that is too large or, more often, too small for the patient’s arm, is a common source of error. The cuff bladder should encircle at least 80% of the arm.5 However, some offices do not have a large blood pressure cuff for overweight patients or a pediatric cuff for children or adults with arms of small circumference. It is recommended that a large blood pressure cuff be used routinely in adults, since a smaller cuff gives falsely high readings in people with large upper arms (circumference > 29 cm).13,14
Digit preference. Many physicians round off the blood pressure to the nearest 5 or 10 mm Hg. This problem may go along with:
Deflating the cuff too rapidly.
Talking to the patient while taking the blood pressure can contribute to higher readings.9
Not taking enough readings. Ideally, at the initial visit, blood pressure should be measured in both arms with the patient seated, and another reading should be taken with the patient standing. The arm with the higher pressure should be used for subsequent readings. Physicians should not make any treatment decisions based on blood pressure during an initial clinic visit, and at least two readings should be taken even on subsequent visits. However, owing to time constraints in busy clinical practices, treatment decisions are often based on single readings or on multiple readings on a single visit.
Discrepancies between observers. The blood pressure readings obtained by the nurse or medical assistant may differ significantly from those obtained by the physician. These differences can be large enough to affect treatment decisions,15,16 and they can be partially corrected by adequate training of all medical personnel who take blood pressure, doctors as well as nurses.
Given that time is tight in busy clinical practices and a trained blood pressure nurse or technician is usually not available, we will probably not see any significant improvement in the accuracy of blood pressure measurement using older technology and current physician practices.
The white-coat effect
Most patients have a higher level of anxiety, and therefore higher blood pressure, in the physician’s office or clinic than in their normal environment (as revealed by ambulatory monitoring or home blood pressure measurements), a phenomenon commonly called the white-coat effect.
Several factors can increase this effect, such as observer-patient interaction during the measurement. The effect tends to be greatest in the initial measurement, but can persist through multiple readings by the doctor or nurse during the same visit.
Whether the white-coat effect is due purely to patient anxiety about an office visit or to a conditioned response has been a point of interest in clinical studies. Regardless, it may result in the misdiagnosis of hypertension or in overestimation of the severity of hypertension and may lead to overly aggressive therapy. Antihypertensive treatment may be unnecessary in the absence of concurrent cardiovascular risk factors.17
“White-coat hypertension” or “isolated office hypertension” is the condition in which a patient who is not on antihypertensive drug therapy has persistently elevated blood pressure in the clinic or office (> 140/90 mm Hg) but normal daytime ambulatory blood pressure (< 135/85 mm Hg).18 Since patients may have an elevated reading when seen for a first office visit, at least several visits are required to establish the diagnosis. Multiple studies have suggested that white-coat hypertension may account for 20% to 25% of the hypertensive population, particularly in older patients, mainly women.19,20
Both white-coat hypertension and the white-coat effect can be avoided by using an automatic and programmable device that can take multiple readings after the clinician leaves the examination room (more about this below).21
MEASURING BLOOD PRESSURE OUTSIDE THE OFFICE
Recent studies have reported that the information obtained by 24-hour ambulatory blood pressure monitoring and by self-measurement of blood pressure in the home more accurately reflects the patient’s risk of future cardiovascular events than do conventional blood pressure measurements taken in the physician’s office. 22–24 Current national guidelines recognize this pattern and the frequent measurement inaccuracies observed in clinical practice, and they are recommending including out-of-office measurements in the diagnosis of hypertension. 25,26
Ambulatory monitoring provides the most accurate measurement of out-of-office blood pressure. With ambulatory monitoring, the normal mean daytime pressure is considered to be lower than 135/85 mm Hg, in contrast to the 140/90 mm Hg cutoff used in the physician’s office with standard aneroid or mercury devices.
Self-monitoring of blood pressure at home has now become widely available with single-measurement oscillometric devices. (Oscillometric means that these devices measure the blood pressure by sensing the oscillations in pressure in the cuff induced by the pulsation of the brachial artery, as opposed to auscultating the Korotkoff sounds.) Blood pressures lower than 135/85 mm Hg outside the clinician’s office are considered normal with these devices.
However, despite its proven value, ambulatory monitoring is neither widely available nor cost-effective for the long-term management of hypertension. Furthermore, few physicians recommend that patients take their blood pressure at home, although the information obtained can be of significant value in the patient’s long-term management.
AUTOMATED MEASUREMENT IN THE OFFICE
In recent years, several automated oscillometric sphygmomanometers have been developed for measuring blood pressure in the office, and more are on the way. These devices can be programmed to take multiple readings without a clinician observer in the examination room, thus reducing the white-coat response.
Omron (Kyoto, Japan) makes several devices, including the HEM-907 and the HEM-705, that have been used in the clinical setting. 21,27–29 They can be programmed to take two or three readings at intervals of 1 to 2 minutes, with up to 5 minutes before the first reading. Unfortunately, data were not recorded with the patient alone in the room in many studies of the Omron devices, even though the devices meet national and international standards for accuracy.
The Microlife Watch BP Office (Microlife, Widnau, Switzerland) is currently undergoing development.30
The BpTRU (BpTRU Medical Devices, Coquitlam, BC, Canada) has enjoyed greater clinical acceptance, since it can take up to five blood pressure readings at intervals of 1 to 5 minutes, and calculates the mean of all five readings, taken with the patient resting comfortably in a quiet room without a clinician present.
The accuracy and durability of the device has been well established. Since the BpTRU self-calibrates between every blood pressure measurement, periodic calibration has not been required. The device can be placed on a table, mounted on the wall, or mounted on a cart if used in several locations in the office.
At Cleveland Clinic, several departments are using the BpTRU on a daily basis. Soon, we will be able to transfer data directly from the BpTRU to our electronic medical record system.
Studies of the BpTRU device
To date, most of the studies of automated office blood pressure measurement have used the BpTRU with the recording interval set at 1 to 2 minutes.
Myers31 used the BpTRU device in 50 hypertensive patients. The physician took the patient’s blood pressure with a mercury sphygmomanometer while the BpTRU device made the first reading, and then he left the room. The next five readings were taken at 2-minute intervals with the patient alone in the room. The mean initial reading by the machine was 162/85 mm Hg; the reading by the physician was 163/86 mm Hg. The third automatic reading was the lowest (averaging 140/84 mm Hg), and the mean of the five automated readings was 142/80 mm Hg, which was significantly lower than the initial reading obtained by the physician (P < .001).
In another study, Myers et al32 compared the measurements obtained by 24-hour ambulatory monitoring and by the BpTRU device (the mean of five readings obtained at 1-minute or 2-minute intervals) in 309 hypertensive patients. The mean blood pressure with the Bp-TRU was 132/75 mm Hg, which correlated well with the mean awake ambulatory blood pressure (134/77 mm Hg; r = 0.62 for the systolic pressure and 0.72 for the diastolic pressure).
We recently reviewed the records of 278 patients seen in our preventive medicine clinic (D.G. Vidt, MD, unpublished data, November 2009). The group included patients with and without established hypertension, and among the hypertensive group, both treated and untreated individuals. We had initially set the device to take readings at 3-minute intervals following the initial nurse-initiated reading. But in view of the recent data on the Bp-TRU using shorter intervals, we also obtained readings in 51 patients with the device set to record at 2-minute intervals, and then in 72 additional patients at 1-minute intervals. In all three groups, blood pressure had stabilized by the third reading after the clinician had left the room. These observations support those reported by Myers et al.31,32 Of particular importance is the observation that the white-coat effect dissipates within 2 to 3 minutes after the clinician leaves the room.33
The shorter measurement intervals can add up in a busy office practice, in which the time relegated to taking blood pressure is often limited.
In fact, waiting 5 minutes between measurements may allow the patient to become too relaxed and the blood pressure to drop too low vis-a-vis the gold standard, ambulatory monitoring. Culleton and colleagues34 compared the blood pressure in 107 hypertensive patients as measured four ways: by the referring physician, by a nurse who was trained to adhere to the protocol of the Canadian Hypertension Education Program, by 24-hour ambulatory monitoring, and by the BpTRU (the mean of five readings obtained at 5-minute intervals). The mean measured values were:
- 150/90 mm Hg by the referring physician
- 139/86 mm Hg by the nurse
- 142/85 mm Hg by ambulatory monitoring
- 132/82 mm Hg by the BpTRU device.
Although the BpTRU reduced the white-coat effect and white-coat hypertension, it underestimated the blood pressure, leading to misclassification of hypertension. Using 140/90 mm Hg as the cutoff for whether the patient was hypertensive and using ambulatory monitoring as the gold standard, the BpTRU misclassified more than half of the patients, agreeing with the classification of hypertensive or not hypertensive by ambulatory monitoring in only 48%. The authors recommended that the BpTRU not be set at 5-minute measurement intervals.34
WHAT ROLE FOR AUTOMATED READINGS IN THE OFFICE?
Although automatic devices, by enabling the physician to leave the room, can eliminate the white-coat effect and white-coat hypertension, physicians must continue to take care to avoid the other potential errors of office blood pressure measurement addressed earlier in this review, for example, by positioning the patient correctly and using a cuff that is large enough. These issues can take on more importance as the clinician leaves the patient alone for brief periods during measurements.
In view of its perennial inaccuracies, some experts have suggested that we abandon routine office measurement of blood pressure.35,36 In its place, ambulatory monitoring would be used for diagnosis and for periodic follow-up. In addition, patients would regularly take their pressure at home with approved, single-measurement oscillometric devices. Unfortunately, in our health care system, periodic ambulatory monitoring for hypertension management would impose a significant financial burden on patients at this time.37
Of particular importance is the observation that the mean of five readings with the BpTRU device, obtained at 1- or 2-minute intervals, closely approximates the mean awake blood pressure obtained in the same patient with an ambulatory monitor.32,38 The ability to obtain readings that correlate exceptionally well with mean daytime ambulatory pressure suggests that this device could well reduce the need for ambulatory monitoring, with its associated cost. The ability to negate the white-coat effect with the use of the BpTRU in the office setting also has particular importance, not only for patient office readings, but for the diagnosis and subsequent treatment of hypertension in individual patients.
Most clinical decisions about the treatment of hypertension are still made on the basis of office determinations of blood pressure. Most office practices still rely on the aneroid manometer or, decreasingly, mercury sphygmomanometers. As noted earlier, although auscultatory blood pressure measurement appears to be simple, it is fraught with a host of observer- or patient-induced errors that not only lead to inaccurate diagnoses, but may also result in the mismanagement of hypertension. Even single-measurement oscillometric devices, now used in a minority of clinical practices, are associated with many of the same measurement issues that lead to overestimation of blood pressure.
We believe the time has come for broader use of oscillometric devices in the outpatient setting. While many available oscillometric devices for use in the home could also be used in the physician’s office, they carry the similar disadvantage of providing only a single measurement. The major disadvantage of all single-measurement devices is the continued presence of the clinician during the reading and the associated white-coat effect observed in most patients.
It is highly likely that the next Joint National Committee Report on Hypertension will further emphasize the role of automated blood pressure devices in the outpatient setting.
Acknowledgment: The authors wish to acknowledge the contributions of Deborah McCoy, RN, and Maria Eckhouse, RN.
The reality of blood pressure measurement is that human beings do not do it very well. The time has come to delegate this job to machines that can do it better.
Several automatic devices are available. Used in physicians’ offices and in patients’ homes, they can eliminate some types of observer error as well as the “white-coat effect,” ie, the tendency of some patients to have higher blood pressure when medical personnel are present than in their natural environment. Since a difference of only a few millimeters of mercury can affect the physician’s decision to start or to modify treatment, measurements that more accurately reflect a person’s average blood pressure are to be desired.
In the following pages, we review the problems that plague blood pressure measurement by human observers, and we describe the advantages of automatic devices.
SHORTCOMINGS OF OFFICE BLOOD PRESSURE MEASUREMENT
For decades, large surveys have provided invaluable information on the prevalence of hypertension, its relationship to cardiovascular disease, and the benefits of treating it.1–3 Unfortunately, the percentage of hypertensive patients whose blood pressure is under control has remained low despite our increased knowledge about hypertension’s diagnosis and therapy.4
In these surveys, blood pressure was measured by auscultation by human observers using mercury or aneroid sphygmomanometers, and most physicians still use this method in clinical practice. But in spite of multiple guidelines for accurate measurement of blood pressure in the office, the overall accuracy and reproducibility of office blood pressure measurements remain poor.5–7
The accuracy of blood pressure measurement with aneroid and mercury manometers is affected by a number of observer errors and patient factors.8,9
Observer errors
Failure to prepare the patient. National guidelines5 state that before having their blood pressure taken, patients should be allowed to sit quietly for at least 5 minutes, which often does not happen. Another error is that clinicians rarely discourage patients from smoking cigarettes or drinking coffee in the 30 minutes prior to measurement.
Equipment and layout problems. Equipment should be properly calibrated and validated. 5 However, even if the sphygmomanometer is periodically calibrated, too often it is mounted on the wall adjacent to the examination table in the examination room, making it difficult to provide a comfortable seat with back and arm support during the reading. The measurement should be done with the patient sitting in a chair (not on an examination table), with feet on the floor and the arm supported at the level of the heart. If the forearm is not supported in the horizontal position and with the cuff at heart level, the blood pressure and heart rate tend to be higher.10 Further, the diastolic blood pressure and heart rate may be misleadingly low with the patient supine rather than seated,11,12 so readings should be taken with the patient sitting.
Miscuffing, ie, the use of a blood pressure cuff that is too large or, more often, too small for the patient’s arm, is a common source of error. The cuff bladder should encircle at least 80% of the arm.5 However, some offices do not have a large blood pressure cuff for overweight patients or a pediatric cuff for children or adults with arms of small circumference. It is recommended that a large blood pressure cuff be used routinely in adults, since a smaller cuff gives falsely high readings in people with large upper arms (circumference > 29 cm).13,14
Digit preference. Many physicians round off the blood pressure to the nearest 5 or 10 mm Hg. This problem may go along with:
Deflating the cuff too rapidly.
Talking to the patient while taking the blood pressure can contribute to higher readings.9
Not taking enough readings. Ideally, at the initial visit, blood pressure should be measured in both arms with the patient seated, and another reading should be taken with the patient standing. The arm with the higher pressure should be used for subsequent readings. Physicians should not make any treatment decisions based on blood pressure during an initial clinic visit, and at least two readings should be taken even on subsequent visits. However, owing to time constraints in busy clinical practices, treatment decisions are often based on single readings or on multiple readings on a single visit.
Discrepancies between observers. The blood pressure readings obtained by the nurse or medical assistant may differ significantly from those obtained by the physician. These differences can be large enough to affect treatment decisions,15,16 and they can be partially corrected by adequate training of all medical personnel who take blood pressure, doctors as well as nurses.
Given that time is tight in busy clinical practices and a trained blood pressure nurse or technician is usually not available, we will probably not see any significant improvement in the accuracy of blood pressure measurement using older technology and current physician practices.
The white-coat effect
Most patients have a higher level of anxiety, and therefore higher blood pressure, in the physician’s office or clinic than in their normal environment (as revealed by ambulatory monitoring or home blood pressure measurements), a phenomenon commonly called the white-coat effect.
Several factors can increase this effect, such as observer-patient interaction during the measurement. The effect tends to be greatest in the initial measurement, but can persist through multiple readings by the doctor or nurse during the same visit.
Whether the white-coat effect is due purely to patient anxiety about an office visit or to a conditioned response has been a point of interest in clinical studies. Regardless, it may result in the misdiagnosis of hypertension or in overestimation of the severity of hypertension and may lead to overly aggressive therapy. Antihypertensive treatment may be unnecessary in the absence of concurrent cardiovascular risk factors.17
“White-coat hypertension” or “isolated office hypertension” is the condition in which a patient who is not on antihypertensive drug therapy has persistently elevated blood pressure in the clinic or office (> 140/90 mm Hg) but normal daytime ambulatory blood pressure (< 135/85 mm Hg).18 Since patients may have an elevated reading when seen for a first office visit, at least several visits are required to establish the diagnosis. Multiple studies have suggested that white-coat hypertension may account for 20% to 25% of the hypertensive population, particularly in older patients, mainly women.19,20
Both white-coat hypertension and the white-coat effect can be avoided by using an automatic and programmable device that can take multiple readings after the clinician leaves the examination room (more about this below).21
MEASURING BLOOD PRESSURE OUTSIDE THE OFFICE
Recent studies have reported that the information obtained by 24-hour ambulatory blood pressure monitoring and by self-measurement of blood pressure in the home more accurately reflects the patient’s risk of future cardiovascular events than do conventional blood pressure measurements taken in the physician’s office. 22–24 Current national guidelines recognize this pattern and the frequent measurement inaccuracies observed in clinical practice, and they are recommending including out-of-office measurements in the diagnosis of hypertension. 25,26
Ambulatory monitoring provides the most accurate measurement of out-of-office blood pressure. With ambulatory monitoring, the normal mean daytime pressure is considered to be lower than 135/85 mm Hg, in contrast to the 140/90 mm Hg cutoff used in the physician’s office with standard aneroid or mercury devices.
Self-monitoring of blood pressure at home has now become widely available with single-measurement oscillometric devices. (Oscillometric means that these devices measure the blood pressure by sensing the oscillations in pressure in the cuff induced by the pulsation of the brachial artery, as opposed to auscultating the Korotkoff sounds.) Blood pressures lower than 135/85 mm Hg outside the clinician’s office are considered normal with these devices.
However, despite its proven value, ambulatory monitoring is neither widely available nor cost-effective for the long-term management of hypertension. Furthermore, few physicians recommend that patients take their blood pressure at home, although the information obtained can be of significant value in the patient’s long-term management.
AUTOMATED MEASUREMENT IN THE OFFICE
In recent years, several automated oscillometric sphygmomanometers have been developed for measuring blood pressure in the office, and more are on the way. These devices can be programmed to take multiple readings without a clinician observer in the examination room, thus reducing the white-coat response.
Omron (Kyoto, Japan) makes several devices, including the HEM-907 and the HEM-705, that have been used in the clinical setting. 21,27–29 They can be programmed to take two or three readings at intervals of 1 to 2 minutes, with up to 5 minutes before the first reading. Unfortunately, data were not recorded with the patient alone in the room in many studies of the Omron devices, even though the devices meet national and international standards for accuracy.
The Microlife Watch BP Office (Microlife, Widnau, Switzerland) is currently undergoing development.30
The BpTRU (BpTRU Medical Devices, Coquitlam, BC, Canada) has enjoyed greater clinical acceptance, since it can take up to five blood pressure readings at intervals of 1 to 5 minutes, and calculates the mean of all five readings, taken with the patient resting comfortably in a quiet room without a clinician present.
The accuracy and durability of the device has been well established. Since the BpTRU self-calibrates between every blood pressure measurement, periodic calibration has not been required. The device can be placed on a table, mounted on the wall, or mounted on a cart if used in several locations in the office.
At Cleveland Clinic, several departments are using the BpTRU on a daily basis. Soon, we will be able to transfer data directly from the BpTRU to our electronic medical record system.
Studies of the BpTRU device
To date, most of the studies of automated office blood pressure measurement have used the BpTRU with the recording interval set at 1 to 2 minutes.
Myers31 used the BpTRU device in 50 hypertensive patients. The physician took the patient’s blood pressure with a mercury sphygmomanometer while the BpTRU device made the first reading, and then he left the room. The next five readings were taken at 2-minute intervals with the patient alone in the room. The mean initial reading by the machine was 162/85 mm Hg; the reading by the physician was 163/86 mm Hg. The third automatic reading was the lowest (averaging 140/84 mm Hg), and the mean of the five automated readings was 142/80 mm Hg, which was significantly lower than the initial reading obtained by the physician (P < .001).
In another study, Myers et al32 compared the measurements obtained by 24-hour ambulatory monitoring and by the BpTRU device (the mean of five readings obtained at 1-minute or 2-minute intervals) in 309 hypertensive patients. The mean blood pressure with the Bp-TRU was 132/75 mm Hg, which correlated well with the mean awake ambulatory blood pressure (134/77 mm Hg; r = 0.62 for the systolic pressure and 0.72 for the diastolic pressure).
We recently reviewed the records of 278 patients seen in our preventive medicine clinic (D.G. Vidt, MD, unpublished data, November 2009). The group included patients with and without established hypertension, and among the hypertensive group, both treated and untreated individuals. We had initially set the device to take readings at 3-minute intervals following the initial nurse-initiated reading. But in view of the recent data on the Bp-TRU using shorter intervals, we also obtained readings in 51 patients with the device set to record at 2-minute intervals, and then in 72 additional patients at 1-minute intervals. In all three groups, blood pressure had stabilized by the third reading after the clinician had left the room. These observations support those reported by Myers et al.31,32 Of particular importance is the observation that the white-coat effect dissipates within 2 to 3 minutes after the clinician leaves the room.33
The shorter measurement intervals can add up in a busy office practice, in which the time relegated to taking blood pressure is often limited.
In fact, waiting 5 minutes between measurements may allow the patient to become too relaxed and the blood pressure to drop too low vis-a-vis the gold standard, ambulatory monitoring. Culleton and colleagues34 compared the blood pressure in 107 hypertensive patients as measured four ways: by the referring physician, by a nurse who was trained to adhere to the protocol of the Canadian Hypertension Education Program, by 24-hour ambulatory monitoring, and by the BpTRU (the mean of five readings obtained at 5-minute intervals). The mean measured values were:
- 150/90 mm Hg by the referring physician
- 139/86 mm Hg by the nurse
- 142/85 mm Hg by ambulatory monitoring
- 132/82 mm Hg by the BpTRU device.
Although the BpTRU reduced the white-coat effect and white-coat hypertension, it underestimated the blood pressure, leading to misclassification of hypertension. Using 140/90 mm Hg as the cutoff for whether the patient was hypertensive and using ambulatory monitoring as the gold standard, the BpTRU misclassified more than half of the patients, agreeing with the classification of hypertensive or not hypertensive by ambulatory monitoring in only 48%. The authors recommended that the BpTRU not be set at 5-minute measurement intervals.34
WHAT ROLE FOR AUTOMATED READINGS IN THE OFFICE?
Although automatic devices, by enabling the physician to leave the room, can eliminate the white-coat effect and white-coat hypertension, physicians must continue to take care to avoid the other potential errors of office blood pressure measurement addressed earlier in this review, for example, by positioning the patient correctly and using a cuff that is large enough. These issues can take on more importance as the clinician leaves the patient alone for brief periods during measurements.
In view of its perennial inaccuracies, some experts have suggested that we abandon routine office measurement of blood pressure.35,36 In its place, ambulatory monitoring would be used for diagnosis and for periodic follow-up. In addition, patients would regularly take their pressure at home with approved, single-measurement oscillometric devices. Unfortunately, in our health care system, periodic ambulatory monitoring for hypertension management would impose a significant financial burden on patients at this time.37
Of particular importance is the observation that the mean of five readings with the BpTRU device, obtained at 1- or 2-minute intervals, closely approximates the mean awake blood pressure obtained in the same patient with an ambulatory monitor.32,38 The ability to obtain readings that correlate exceptionally well with mean daytime ambulatory pressure suggests that this device could well reduce the need for ambulatory monitoring, with its associated cost. The ability to negate the white-coat effect with the use of the BpTRU in the office setting also has particular importance, not only for patient office readings, but for the diagnosis and subsequent treatment of hypertension in individual patients.
Most clinical decisions about the treatment of hypertension are still made on the basis of office determinations of blood pressure. Most office practices still rely on the aneroid manometer or, decreasingly, mercury sphygmomanometers. As noted earlier, although auscultatory blood pressure measurement appears to be simple, it is fraught with a host of observer- or patient-induced errors that not only lead to inaccurate diagnoses, but may also result in the mismanagement of hypertension. Even single-measurement oscillometric devices, now used in a minority of clinical practices, are associated with many of the same measurement issues that lead to overestimation of blood pressure.
We believe the time has come for broader use of oscillometric devices in the outpatient setting. While many available oscillometric devices for use in the home could also be used in the physician’s office, they carry the similar disadvantage of providing only a single measurement. The major disadvantage of all single-measurement devices is the continued presence of the clinician during the reading and the associated white-coat effect observed in most patients.
It is highly likely that the next Joint National Committee Report on Hypertension will further emphasize the role of automated blood pressure devices in the outpatient setting.
Acknowledgment: The authors wish to acknowledge the contributions of Deborah McCoy, RN, and Maria Eckhouse, RN.
- Burt VL, Whelton P, Roccella EJ, et al. Prevalence of hypertension in the US adult population. Results from the Third National Health and Nutrition Examination Survey, 1988–1991. Hypertension 1995; 25:305–313.
- Neaton JD, Wentworth D. Serum cholesterol, blood pressure, cigarette smoking, and death from coronary heart disease. Overall findings and differences by age for 316,099 white men. Multiple Risk Factor Intervention Trial Research Group. Arch Intern Med 1992; 152:56–64.
- Lewington S, Clarke R, Qizilbash N, Peto R, Collins R; Prospective Studies Collaboration. Age-specific relevance of usual blood pressure to vascular mortality: a meta-analysis of individual data for one million adults in 61 prospective studies. Lancet 2002; 360:1903–1913.
- Lloyd-Jones D, Adams R, Carnethon M, et al; American Heart Association Statistics Committee and Stroke Statistics Subcommittee. Heart disease and stroke statistics—2009 update: a report from the American Heart Association Statistics Committee and Stroke Statistics Subcommittee. Circulation 2009; 119:e21–e181.
- Chobanian AV, Bakris GL, Black HR, et al; National Heart, Lung, and Blood Institute Joint National Committee on Prevention, Detection, Evaluation, and Treatment of High Blood Pressure; National High Blood Pressure Education Program Coordinating Committee. The Seventh Report of the Joint National Committee on Prevention, Detection, Evaluation, and Treatment of High Blood Pressure: the JNC 7 report. JAMA 2003; 289:2560–2572.
- Grim CM, Grim CE. A curriculum for the training and certification of blood pressure measurement for health care providers. Can J Cardiol 1995; 11(suppl H):38H–42H.
- Pickering TG, Hall JE, Appel LJ, et al; Subcommittee of Professional and Public Education of the American Heart Association Council on High Blood Pressure Research. Recommendations for blood pressure measurement in humans and experimental animals: part 1: blood pressure measurement in humans: a statement for professionals from the Subcommittee of Professional and Public Education of the American Heart Association Council on High Blood Pressure Research. Hypertension 2005; 45:142–161.
- Langlois S. Measuring blood pressure: how competent are we? Perspect Cardiol 1999; 15:29–39.
- Le Pailleur C, Helft G, Landais P, et al. The effects of talking, reading, and silence on the “white coat” phenomenon in hypertensive patients. Am J Hypertens 1998; 11:203–207.
- Webster J, Newnham D, Petrie JC, Lovell HG. Influence of arm position on measurement of blood pressure. Br Med J (Clin Res Ed) 1984; 288:1574–1575.
- Netea RT, Smits P, Lenders JW, Thien T. Does it matter whether blood pressure measurements are taken with subjects sitting or supine? J Hypertens 1998; 16:263–268.
- Silverberg DS, Shemesh E, Iaina A. The unsupported arm: a cause of falsely raised blood pressure readings. Br Med J 1977; 2:1331.
- Manning DM, Kuchirka C, Kaminski J. Miscuffing: inappropriate blood pressure cuff application. Circulation 1983; 68:763–766.
- Iyriboz Y, Hearon CM, Edwards K. Agreement between large and small cuffs in sphygmomanometry: a quantitative assessment. J Clin Monit 1994; 10:127–133.
- Scherwitz LW, Evans LA, Hennrikus DJ, Vallbona C. Procedures and discrepancies of blood pressure measurements in two community health centers. Med Care 1982; 20:727–738.
- La Batide-Alanore A, Chatellier G, Bobrie G, Fofol I, Plouin PF. Comparison of nurse- and physician-determined clinic blood pressure levels in patients referred to a hypertension clinic: implications for subsequent management. J Hypertens 2000; 18:391–398.
- Verdecchia P. Prognostic value of ambulatory blood pressure: current evidence and clinical implications. Hypertension 2000; 35:844–851.
- Ogedegbe G, Pickering TG, Clemow L, et al. The misdiagnosis of hypertension: the role of patient anxiety. Arch Intern Med 2008; 168:2459–2465.
- Pickering TG. Stress, white coat hypertension and masked hypertension. In:Izzo JL, Sica DA, Black HR, editors. Hypertension Primer: The Essentials of High Blood Pressure: Basic Science, Population Science, and Clinical Management. 4th ed. Philadelphia, PA: Lippincott Williams & Wilkins; 2008:289–291.
- Pickering TG, Coats A, Mallion JM, Mancia G, Verdecchia P. Blood Pressure Monitoring. Task force V: White-coat hypertension. Blood Press Monit 1999; 4:333–341.
- Myers MG, Valdivieso MA. Use of an automated blood pressure recording device, the BpTRU, to reduce the “white coat effect” in routine practice. Am J Hypertens 2003; 16:494–497.
- Redon J, Campos C, Narciso ML, Rodicio JL, Pascual JM, Ruilope LM. Prognostic value of ambulatory blood pressure monitoring in refractory hypertension: a prospective study. Hypertension 1998; 31:712–718.
- Ohkubo T, Imai Y, Tsuji I, et al. Prediction of mortality by ambulatory blood pressure monitoring versus screening blood pressure measurements: a pilot study in Ohasama. J Hypertens 1997; 15:357–364.
- Verdecchia P, Reboldi G, Porcellati C, et al. Risk of cardiovascular disease in relation to achieved office and ambulatory blood pressure control in treated hypertensive subjects. J Am Coll Cardiol 2002; 39:878–885.
- Hemmelgarn BR, McAllister FA, Myers MG, et al; Canadian Hypertension Education Program. The 2005 Canadian Hypertension Education Program recommendations for the management of hypertension: part 1 - blood pressure measurement, diagnosis and assessment of risk. Can J Cardiol 2005; 21:645–656.
- Pickering TG. JNC 7.5. J Clin Hypertens (Greenwich) 2007; 9:901–904.
- White WB, Anwar YA. Evaluation of the overall efficacy of the Omron office digital blood pressure HEM-907 monitor in adults. Blood Press Monit 2001; 6:107–110.
- Myers MG, Meglis G, Polemidiotis G. The impact of physician vs automated blood pressure readings on office-induced hypertension. J Hum Hypertens 1997; 11:491–493.
- Myers MG, Godwin M, Dawes M, Kiss A, Tobe SW, Kaczorowski J. Measurement of blood pressure in the office: recognizing the problem and proposing the solution. Hypertension 2010; 55:195–200.
- Stergiou GS, Tzamouranis D, Protogerou A, Nasothimiou E, Kapralos C. Validation of the Microlife Watch BP Office professional device for office blood pressure measurement according to the International protocol. Blood Press Monit 2008; 13:299–303.
- Myers MG. Automated blood pressure measurement in routine clinical practice. Blood Press Monit 2006; 11:59–62.
- Myers MG, Valdivieso M, Kiss A. Optimum frequency of office blood pressure measurement using an automated sphygmomanometer. Blood Press Monit 2008; 13:333–338.
- Myers MG, Valdivieso M, Kiss A. Use of automated office blood pressure measurement to reduce the white coat response. J Hypertens 2009; 27:280–286.
- Culleton BF, McKay DW, Campbell NR. Performance of the automated BpTRU measurement device in the assessment of white-coat hypertension and white-coat effect. Blood Press Monit 2006; 11:37–42.
- Pickering TG, Miller NH, Ogedegbe G, Krakoff LR, Artinian NT, Goff D; American Heart Association. Call to action on use and reimbursement for home blood pressure monitoring: executive summary: a joint scientific statement from the American Heart Association, American Society Of Hypertension, and Preventive Cardiovascular Nurses Association. Hypertension 2008; 52:1–9.
- Parati G, Stergiou GS, Asmar R, et al; ESH Working Group on Blood Pressure Monitoring. European Society of Hypertension guidelines for blood pressure monitoring at home: a summary report of the Second International Consensus Conference on Home Blood Pressure Monitoring. J Hypertens 2008; 26:1505–1526.
- O’Brien E. Ambulatory blood pressure measurement: the case for implementation in primary care. Hypertension 2008; 51:1435–1441.
- Beckett L, Godwin M. The BpTRU automatic blood pressure monitor compared to 24 hour ambulatory blood pressure monitoring in the assessment of blood pressure in patients with hypertension. BMC Cardiovasc Disord 2005; 5:18.
- Burt VL, Whelton P, Roccella EJ, et al. Prevalence of hypertension in the US adult population. Results from the Third National Health and Nutrition Examination Survey, 1988–1991. Hypertension 1995; 25:305–313.
- Neaton JD, Wentworth D. Serum cholesterol, blood pressure, cigarette smoking, and death from coronary heart disease. Overall findings and differences by age for 316,099 white men. Multiple Risk Factor Intervention Trial Research Group. Arch Intern Med 1992; 152:56–64.
- Lewington S, Clarke R, Qizilbash N, Peto R, Collins R; Prospective Studies Collaboration. Age-specific relevance of usual blood pressure to vascular mortality: a meta-analysis of individual data for one million adults in 61 prospective studies. Lancet 2002; 360:1903–1913.
- Lloyd-Jones D, Adams R, Carnethon M, et al; American Heart Association Statistics Committee and Stroke Statistics Subcommittee. Heart disease and stroke statistics—2009 update: a report from the American Heart Association Statistics Committee and Stroke Statistics Subcommittee. Circulation 2009; 119:e21–e181.
- Chobanian AV, Bakris GL, Black HR, et al; National Heart, Lung, and Blood Institute Joint National Committee on Prevention, Detection, Evaluation, and Treatment of High Blood Pressure; National High Blood Pressure Education Program Coordinating Committee. The Seventh Report of the Joint National Committee on Prevention, Detection, Evaluation, and Treatment of High Blood Pressure: the JNC 7 report. JAMA 2003; 289:2560–2572.
- Grim CM, Grim CE. A curriculum for the training and certification of blood pressure measurement for health care providers. Can J Cardiol 1995; 11(suppl H):38H–42H.
- Pickering TG, Hall JE, Appel LJ, et al; Subcommittee of Professional and Public Education of the American Heart Association Council on High Blood Pressure Research. Recommendations for blood pressure measurement in humans and experimental animals: part 1: blood pressure measurement in humans: a statement for professionals from the Subcommittee of Professional and Public Education of the American Heart Association Council on High Blood Pressure Research. Hypertension 2005; 45:142–161.
- Langlois S. Measuring blood pressure: how competent are we? Perspect Cardiol 1999; 15:29–39.
- Le Pailleur C, Helft G, Landais P, et al. The effects of talking, reading, and silence on the “white coat” phenomenon in hypertensive patients. Am J Hypertens 1998; 11:203–207.
- Webster J, Newnham D, Petrie JC, Lovell HG. Influence of arm position on measurement of blood pressure. Br Med J (Clin Res Ed) 1984; 288:1574–1575.
- Netea RT, Smits P, Lenders JW, Thien T. Does it matter whether blood pressure measurements are taken with subjects sitting or supine? J Hypertens 1998; 16:263–268.
- Silverberg DS, Shemesh E, Iaina A. The unsupported arm: a cause of falsely raised blood pressure readings. Br Med J 1977; 2:1331.
- Manning DM, Kuchirka C, Kaminski J. Miscuffing: inappropriate blood pressure cuff application. Circulation 1983; 68:763–766.
- Iyriboz Y, Hearon CM, Edwards K. Agreement between large and small cuffs in sphygmomanometry: a quantitative assessment. J Clin Monit 1994; 10:127–133.
- Scherwitz LW, Evans LA, Hennrikus DJ, Vallbona C. Procedures and discrepancies of blood pressure measurements in two community health centers. Med Care 1982; 20:727–738.
- La Batide-Alanore A, Chatellier G, Bobrie G, Fofol I, Plouin PF. Comparison of nurse- and physician-determined clinic blood pressure levels in patients referred to a hypertension clinic: implications for subsequent management. J Hypertens 2000; 18:391–398.
- Verdecchia P. Prognostic value of ambulatory blood pressure: current evidence and clinical implications. Hypertension 2000; 35:844–851.
- Ogedegbe G, Pickering TG, Clemow L, et al. The misdiagnosis of hypertension: the role of patient anxiety. Arch Intern Med 2008; 168:2459–2465.
- Pickering TG. Stress, white coat hypertension and masked hypertension. In:Izzo JL, Sica DA, Black HR, editors. Hypertension Primer: The Essentials of High Blood Pressure: Basic Science, Population Science, and Clinical Management. 4th ed. Philadelphia, PA: Lippincott Williams & Wilkins; 2008:289–291.
- Pickering TG, Coats A, Mallion JM, Mancia G, Verdecchia P. Blood Pressure Monitoring. Task force V: White-coat hypertension. Blood Press Monit 1999; 4:333–341.
- Myers MG, Valdivieso MA. Use of an automated blood pressure recording device, the BpTRU, to reduce the “white coat effect” in routine practice. Am J Hypertens 2003; 16:494–497.
- Redon J, Campos C, Narciso ML, Rodicio JL, Pascual JM, Ruilope LM. Prognostic value of ambulatory blood pressure monitoring in refractory hypertension: a prospective study. Hypertension 1998; 31:712–718.
- Ohkubo T, Imai Y, Tsuji I, et al. Prediction of mortality by ambulatory blood pressure monitoring versus screening blood pressure measurements: a pilot study in Ohasama. J Hypertens 1997; 15:357–364.
- Verdecchia P, Reboldi G, Porcellati C, et al. Risk of cardiovascular disease in relation to achieved office and ambulatory blood pressure control in treated hypertensive subjects. J Am Coll Cardiol 2002; 39:878–885.
- Hemmelgarn BR, McAllister FA, Myers MG, et al; Canadian Hypertension Education Program. The 2005 Canadian Hypertension Education Program recommendations for the management of hypertension: part 1 - blood pressure measurement, diagnosis and assessment of risk. Can J Cardiol 2005; 21:645–656.
- Pickering TG. JNC 7.5. J Clin Hypertens (Greenwich) 2007; 9:901–904.
- White WB, Anwar YA. Evaluation of the overall efficacy of the Omron office digital blood pressure HEM-907 monitor in adults. Blood Press Monit 2001; 6:107–110.
- Myers MG, Meglis G, Polemidiotis G. The impact of physician vs automated blood pressure readings on office-induced hypertension. J Hum Hypertens 1997; 11:491–493.
- Myers MG, Godwin M, Dawes M, Kiss A, Tobe SW, Kaczorowski J. Measurement of blood pressure in the office: recognizing the problem and proposing the solution. Hypertension 2010; 55:195–200.
- Stergiou GS, Tzamouranis D, Protogerou A, Nasothimiou E, Kapralos C. Validation of the Microlife Watch BP Office professional device for office blood pressure measurement according to the International protocol. Blood Press Monit 2008; 13:299–303.
- Myers MG. Automated blood pressure measurement in routine clinical practice. Blood Press Monit 2006; 11:59–62.
- Myers MG, Valdivieso M, Kiss A. Optimum frequency of office blood pressure measurement using an automated sphygmomanometer. Blood Press Monit 2008; 13:333–338.
- Myers MG, Valdivieso M, Kiss A. Use of automated office blood pressure measurement to reduce the white coat response. J Hypertens 2009; 27:280–286.
- Culleton BF, McKay DW, Campbell NR. Performance of the automated BpTRU measurement device in the assessment of white-coat hypertension and white-coat effect. Blood Press Monit 2006; 11:37–42.
- Pickering TG, Miller NH, Ogedegbe G, Krakoff LR, Artinian NT, Goff D; American Heart Association. Call to action on use and reimbursement for home blood pressure monitoring: executive summary: a joint scientific statement from the American Heart Association, American Society Of Hypertension, and Preventive Cardiovascular Nurses Association. Hypertension 2008; 52:1–9.
- Parati G, Stergiou GS, Asmar R, et al; ESH Working Group on Blood Pressure Monitoring. European Society of Hypertension guidelines for blood pressure monitoring at home: a summary report of the Second International Consensus Conference on Home Blood Pressure Monitoring. J Hypertens 2008; 26:1505–1526.
- O’Brien E. Ambulatory blood pressure measurement: the case for implementation in primary care. Hypertension 2008; 51:1435–1441.
- Beckett L, Godwin M. The BpTRU automatic blood pressure monitor compared to 24 hour ambulatory blood pressure monitoring in the assessment of blood pressure in patients with hypertension. BMC Cardiovasc Disord 2005; 5:18.
KEY POINTS
- The white-coat effect, ie, the tendency of many patients to have higher blood pressure in the presence of medical personnel than in their own environment, can lead to inappropriate diagnosis of hypertension and unnecessary treatment.
- Out-of-office blood pressure correlates better with cardiovascular risk than does the blood pressure in the physician’s office, but ambulatory monitoring is costly and not widely available, and few physicians recommend self-measurement at home.
- Several available devices can take a series of blood pressure measurements at preset intervals while the patient sits alone in the examination room, eliminating the white-coat effect.
- The mean of five automatic readings taken at intervals of 1 or 2 minutes correlates well with the mean value while awake on ambulatory monitoring.
Bringing home the ‘medical home’ for older adults
Mrs. Smith, age 82, has chronic heart failure. She also has difficulty walking because of arthritis in her knee and osteoporosis. Her son has taken the day off work to bring her in to see her primary care physician, Dr. Jones, because of increasing swelling of her legs and feeling tired.
Even on a good day, Mrs. Smith faces challenges getting to the doctor’s office: she has difficulty getting dressed, taking the stairs, and transporting her walker and oxygen, not to mention parking the car, getting out, getting in to the doctor’s office, and then returning home.
After a careful evaluation Dr. Jones concludes that the leg swelling and fatigue are due to an exacerbation of heart failure triggered by excess dietary sodium and uncontrolled hypertension. She decides to increase the dosages of Mrs. Smith’s diuretic and angiotensin-converting enzyme inhibitor and advises her and her son about dietary sodium restriction. She reviews with them the symptoms that should trigger a call to the office, and she says she wants to see Mrs. Smith again in 3 days.
Mrs. Smith and her son do not seem to understand the instructions, and they explain how difficult it will be to make the follow-up visit, so Dr. Jones recommends hospital admission. Mrs. Smith protests, as she has had multiple hospitalizations during the past year and she dreads the idea of returning. And her son explains, “Mom always seems worse after going to the hospital. Last winter when she was there her days and nights got mixed up, and when she called out at night they gave her some drug that knocked her out for 2 days. Doctor, isn’t there any safe way to keep her at home?”
CHRONIC ILLNESS: A CHALLENGE, AND AN OPPORTUNITY
The growing number of older adults with chronic illnesses poses a serious challenge to the US health care system, placing unprecedented pressures on the financial sustainability and overall effectiveness of the Medicare program.1,2 Of particular concern is the plight of Medicare beneficiaries like Mrs. Smith who have multiple chronic conditions and whose activity and mobility are limited. These patients account for a disproportionate share of Medicare expenses and, despite all the money spent, often struggle without optimal care that is accessible, individualized, and coordinated.
But this challenge is also an opportunity. We may be able to improve the care of these vulnerable patients—and control costs—by taking their primary care to their own homes. To these ends, the Patient Protection and Affordable Care Act (ie, the “health care reform law”) has several provisions for pilot and demonstration projects.3–5 In light of the new policies and as part of a grassroots effort to change the delivery of care for patients with chronic conditions, primary care physicians like Dr. Jones are redesigning their practices to provide a patient-centered medical home.6
As envisioned, the primary care physician’s office will be the patient’s “medical home.” The primary care physician will lead, coordinate, and oversee the efforts of a multidisciplinary team, referring patients when necessary to specialists and community resources. Primary care practices that become medical homes would potentially be paid care management fees in addition to fees for visits, but with new expectations for care coordination and integration.
The emergence of the medical home, independence-at-home, and related concepts makes it a good time for physicians to explore how they can collaborate with home health providers to better meet the needs of older patients with chronic illness (Table 1).
UNDER MEDICARE, WHO IS ELIGIBLE FOR HOME HEALTH SERVICES?
Primary care physicians who are transforming their offices into a medical home must consider how to deliver the care (it must be accessible, team-based, and aimed at the “whole person”), coordinate the care, and measure its quality.7 Many Medicare beneficiaries with serious chronic illness have limited mobility that makes it difficult to regularly travel to medical offices, and thus they need home visits or regular contact by telephone or computer.
Many home health agencies are using new conceptual models, programs, technologies, and services so they can play a supportive role.8 These agencies employ nurses, therapists, social workers, personal caregivers, and nutritionists. In many instances these people can become the physician-directed team responsible for key aspects of caring for patients with chronic illness in their homes, coordinating and integrating the care, and measuring its quality. Additionally, in-home assessment provides a holistic view of patients that potentially promotes patient- and family-centered care options.
To be eligible for home health services, a beneficiary must be “homebound,” must need intermittent skilled nursing care or skilled therapy, and must be under the care of a physician. The health reform law has also mandated that patients have a face-to-face visit with their physician or with certain nonphysician practitioners in order to certify the home health care plan.
Even though the homebound requirement limits the number of people eligible, many older adults like Mrs. Smith who have chronic illness meet this criterion. Others may only be homebound during an exacerbation of a chronic illness that temporarily limits their mobility. However, patients can still be considered homebound for the Medicare benefit even if they leave their home (infrequently) for medical care, religious services, family events, adult day programs, and other reasons.9
The Medicare Home Health benefit covers several services that are especially important for patients with chronic illness. These include nursing visits for observation and assessment, evaluation and management of a care plan, and teaching and training.
How this applies to Mrs. Smith
In the case of Mrs. Smith, Dr. Jones could order home nursing care to make sure she is taking her medications as directed, to teach her about self-management and nutrition, and to assess the impact of medication changes—both the intended effects and adverse effects such as hypotension.
Other team members bring other skills. For example, home health social workers may be able to address complex psychosocial needs that can affect adherence.
The time Dr. Jones spends developing this care plan and reviewing the patient’s condition with home health field staff by telephone or other communication methods is reimbursable under Medicare as “care plan oversight”10 and can substitute for the revenue lost due to less-frequent office visits.10 In the new practice models, a medical home or independence-at-home care-management fee or anticipated revenues from “gain-sharing” could cover nonvisit supervision of in-home services.
Oversight in the computer age
Dr. Jones may be reluctant to rely on a home health agency because she cannot directly oversee what they are doing and may in fact be uncertain as to what they are doing. Home care may seem like a “black box” to physicians, but it shouldn’t in this era of electronic health records and advanced electronic information systems. Seamless communication is possible without playing “telephone tag” and sending multiple faxes. Physicians may prefer to work only with home care providers who use electronic information systems and who can interface their systems with the physician’s electronic systems, or at least offer shared viewing through Web access. Of course, such arrangements must be initiated with respect for the patient’s preference for a home care agency.
Home health providers are also well positioned to help measure and monitor the quality of care. Medicare requires that home health providers track a comprehensive set of quality outcomes, adjusted for risk, and ranging from improvement in function to acute hospitalization rates.11,12 Given that most home care providers are swimming in data about their patients, it would be reasonable for home care agencies to provide physician partners with more nuanced reports for specific subpopulations, such as those from a particular physician practice, or for patients with a particular disease.
NEW CONCEPTS, PROCESSES, AND TECHNOLOGIES
To care for a patient like Mrs. Smith, the home health team must embrace new, chronic-care-oriented concepts, processes, and technologies. Many agencies now have nurses and therapists skilled in chronic illness care, self-management support, and health coaching. Ancillary staff collaborate with the physician by assuming time-consuming but necessary tasks such as patient education, care coordination and integration, and quality measurement and improvement initiatives.
Several groups and authors have proposed a “home-based chronic care model,” built upon the well-studied “chronic care model,” 13–16 as a framework to help home care providers change their approach to patients with chronic illness. This model offers a standardized curriculum and certification program, as well as practice guidelines, which standardize best-practice care delivery from agency to agency.
A core tenet of this model is a strong focus on teaching clinicians how to teach their patients to care for themselves, since bad outcomes are often due to patients not following physicians' recommendations. Since successful chronic care management requires adherence to specific self-care behaviors, the focus on behavior change must not be neglected if positive outcomes are to be realized.
New technologies are also emerging. Some home health providers are using in-home telemetry with remote call centers to track the patient’s health status on a daily basis. Physicians and patients can follow the data, allowing for quick intervention, if necessary, and reinforcement of self-management learning.17–20 Some home care agencies could monitor, via telemetry, Mrs. Smith’s weight, blood pressure, oxygen saturation, heart rate, and dyspnea symptoms. This information could be fed back to call-center clinicians who have predetermined parameters for titrating the diuretic dose and for notifying the physician.
Some monitoring technology allows for interactive assessment and teaching via live videoconferencing. Some home health agencies also use telephone-based health coaching.21 Information system interfaces between the home health agency and the medical home coordinator could make the content of this in-home monitoring and care management visible in the physician’s record.
TOWARD ONGOING CARE MANAGEMENT
In spite of these opportunities, the Medicare home health benefit rarely permits uninterrupted ongoing home care. Thus, the home health collaboration developed around Mrs. Smith’s heart failure exacerbation is likely to be temporary, and when her condition stabilizes she may no longer meet the criteria for home health services.
This episodic-payment model contrasts with the ongoing needs of the typical high-risk older patient with chronic illness. Changing the home health benefit to allow for ongoing home health care for beneficiaries like Mrs. Smith may be an opportunity for patient-centered reform. Although ongoing home health care for a given patient may not be possible, the medical home model offers the opportunity for ongoing physician-home health collaboration because at any time a physician’s practice is likely to have patients requiring these services. The independence-at-home model does provide for uninterrupted ongoing in-home physician and mid-level care for some patients, but it may require changing primary care physicians, and this may be undesirable to some patients. If a viable financing model is established for medical homes and independence-at-home practices, they may choose to contract with home health agencies to provide ongoing telephone or telemetric care management between (or outside of) episodes of eligibility for traditional home health care. All of these potential arrangements would need legal review and would need to be structured to avoid violation of the letter and spirit of laws prohibiting self-referrals and kickbacks.
PHYSICIAN HOME VISITS
In the case of Mrs. Smith, Dr. Jones has the option of making a follow-up home visit, or even ongoing home visits.
Granted, home visits may be impractical due to the time involved and the impact of that downtime on the physician’s medical practice and responsibilities to other patients. However, larger practices may employ a specific physician, nurse practitioner, or physician’s assistant to provide in-home care to patients in need.
Some communities have house-call practices to which Dr. Jones could refer Mrs. Smith for in-home physician care, and, where available, this may be a preferred care model— somewhat analogous to how a primary care physician might collaborate with a hospitalist for inpatient care of a specific patient.22 These homecare physician practices will likely become more prevalent if the independence-at-home Medicare demonstration project is successful.
In the future, even if Mrs. Smith needed more intensive inpatient care, an emerging concept called “hospital at home” may be able to provide this acute care in her home.23,24 These in-home physician services are increasingly supported by new mobile diagnostic technologies.25
However, adding or changing physicians may not be possible or desirable for Mrs. Smith and could lead to further fragmentation of care. In the future, teleconferencing may provide options for “virtual visits” that would partially solve this problem.
Whether the physician care is provided in the office, in the home, or as a virtual visit, much of the care Mrs. Smith needs can and should be done by nonphysician home health care providers in partnership with informal caregivers.
MRS. SMITH GETS BETTER AT HOME
Dr. Jones decided to refer Mrs. Smith for home health nursing and maintained close telephone contact with her and the home health nurse during the first 2 weeks. Mrs. Smith responded well to the changes in medication and diet, her leg swelling decreased, and she was feeling more like her usual self. At a follow-up office visit 3 months later, Mrs. Smith hugged Dr. Jones and thanked her profusely for helping her get better at home.
- Hackbarth GM. Medicare Payment Advisory Commission. June 2008 Report to the Congress: Reforming the Delivery System. http://www.medpac.gov/documents/Jun08_Entirereport.pdf. Accessed September 9, 2010.
- Congressional Budget Office. Accounting for Sources of Projected Growth in Federal Spending on Medicare and Medicaid. http://www.cbo.gov/ftpdocs/93xx/doc9316/HealthCostGrowth.shtml. Accessed September 9, 2010.
- Landers SH. The other Medical Home. JAMA 2009; 301:97–99.
- The Library of Congress: Thomas. The RE-Aligning Care Act. http://frwebgate.access.gpo.gov/cgi-bin/getdoc.cgi?dbname=111_cong_bills&docid=f:s1004is.txt.pdf. Accessed September 9, 2010.
- The Library of Congress: Thomas. Independence at Home Act of 2009. http://frwebgate.access.gpo.gov/cgi-bin/getdoc.cgi?dbname=111_cong_bills&docid=f:h2560ih.txt.pdf. Accessed August 12, 2010.
- TransforMED. http://www.transformed.com. Accessed September 9, 2010.
- Kellerman R, Kirk L. Principles of the patient-centered medical home. Am Fam Physician 2007; 76:774–775.
- Fisher ES. Building a medical neighborhood for the medical home. N Engl J Med 2008; 359:1202–1205.
- Medicare Benefit Policy Manual: Chapter 7 - Home Health Services. http://www.cms.hhs.gov/manuals/Downloads/bp102c07.pdf. Accessed September 9, 2010.
- Bluestein HM. Care plan oversight and home care/hospice revenue for telephone management. Compr Ther 2006; 32:226–229.
- Madigan EA, Fortinsky RH. Interrater reliability of the outcomes and assessment information set: results from the field. Gerontologist 2004; 44:689–692.
- Madigan EA, Tullai-McGuinness S, Fortinsky RH. Accuracy in the Outcomes and Assessment Information Set (OASIS): results of a video simulation. Res Nurs Health 2003; 26:273–283.
- Bodenheimer T, Wagner EH, Grumbach K. Improving primary care for patients with chronic illness: the chronic care model, Part 2. JAMA 2002; 288:1909–1914.
- Martin JC, Avant RF, Bowman MA, et al; Future of Family Medicine Project Leadership Committee. The Future of Family Medicine: a collaborative project of the family medicine community. Ann Fam Med 2004; 2(suppl 1):S3–S32.
- Hennessey B, Suter P. The home-based chronic care model. Caring 2009; 28:12–16.
- Suter P, Hennessey B, Harrison G, Fagan M, Norman B, Suter WN. Home-based chronic care. An expanded integrative model for home health professionals. Home Healthc Nurse 2008; 26:222–229.
- Browning SV, Tullai-McGuinness S, Madigan E, Struk C. Telehealth: is your staff ready to implement? A descriptive exploratory study of readiness for this technology in home health care. Home Healthc Nurse 2009; 27:242–248.
- Fazzi R, Ashe T, Doak L. Telehealth, disease management, home care and the future—part 2. Caring 2008; 27:36–8,40–1,3.
- Kelly K, Christians J. Best practices in implementing a telehealth program. Caring 2008; 27:44–47.
- Whitten P, Bergman A, Meese MA, Bridwell K, Jule K. St. Vincent’s Home telehealth for congestive heart failure patients. Telemed J E Health 2009; 15:148–153.
- A medisys Home Health Services. Comprehensive, continuous chronic care management in the home. http://www.amedisys.com/pdf/Whitepaper_C4M.pdf. Accessed September 9, 2010.
- Okie S. Home delivery—bringing primary care to the housebound elderly. N Engl J Med 2008; 359:2409–2412.
- Leff B, Burton JR. Acute medical care in the home. J Am Geriatr Soc 1996; 44:603–605.
- Leff B, Burton L, Mader SL, et al. Hospital at home: feasibility and outcomes of a program to provide hospital-level care at home for acutely ill older patients. Ann Intern Med 2005; 143:798–808.
- Bayne CG, Boling PA. New diagnostic and information technology for mobile medical care. Clin Geriatr Med 2009; 25:93–107.
Mrs. Smith, age 82, has chronic heart failure. She also has difficulty walking because of arthritis in her knee and osteoporosis. Her son has taken the day off work to bring her in to see her primary care physician, Dr. Jones, because of increasing swelling of her legs and feeling tired.
Even on a good day, Mrs. Smith faces challenges getting to the doctor’s office: she has difficulty getting dressed, taking the stairs, and transporting her walker and oxygen, not to mention parking the car, getting out, getting in to the doctor’s office, and then returning home.
After a careful evaluation Dr. Jones concludes that the leg swelling and fatigue are due to an exacerbation of heart failure triggered by excess dietary sodium and uncontrolled hypertension. She decides to increase the dosages of Mrs. Smith’s diuretic and angiotensin-converting enzyme inhibitor and advises her and her son about dietary sodium restriction. She reviews with them the symptoms that should trigger a call to the office, and she says she wants to see Mrs. Smith again in 3 days.
Mrs. Smith and her son do not seem to understand the instructions, and they explain how difficult it will be to make the follow-up visit, so Dr. Jones recommends hospital admission. Mrs. Smith protests, as she has had multiple hospitalizations during the past year and she dreads the idea of returning. And her son explains, “Mom always seems worse after going to the hospital. Last winter when she was there her days and nights got mixed up, and when she called out at night they gave her some drug that knocked her out for 2 days. Doctor, isn’t there any safe way to keep her at home?”
CHRONIC ILLNESS: A CHALLENGE, AND AN OPPORTUNITY
The growing number of older adults with chronic illnesses poses a serious challenge to the US health care system, placing unprecedented pressures on the financial sustainability and overall effectiveness of the Medicare program.1,2 Of particular concern is the plight of Medicare beneficiaries like Mrs. Smith who have multiple chronic conditions and whose activity and mobility are limited. These patients account for a disproportionate share of Medicare expenses and, despite all the money spent, often struggle without optimal care that is accessible, individualized, and coordinated.
But this challenge is also an opportunity. We may be able to improve the care of these vulnerable patients—and control costs—by taking their primary care to their own homes. To these ends, the Patient Protection and Affordable Care Act (ie, the “health care reform law”) has several provisions for pilot and demonstration projects.3–5 In light of the new policies and as part of a grassroots effort to change the delivery of care for patients with chronic conditions, primary care physicians like Dr. Jones are redesigning their practices to provide a patient-centered medical home.6
As envisioned, the primary care physician’s office will be the patient’s “medical home.” The primary care physician will lead, coordinate, and oversee the efforts of a multidisciplinary team, referring patients when necessary to specialists and community resources. Primary care practices that become medical homes would potentially be paid care management fees in addition to fees for visits, but with new expectations for care coordination and integration.
The emergence of the medical home, independence-at-home, and related concepts makes it a good time for physicians to explore how they can collaborate with home health providers to better meet the needs of older patients with chronic illness (Table 1).
UNDER MEDICARE, WHO IS ELIGIBLE FOR HOME HEALTH SERVICES?
Primary care physicians who are transforming their offices into a medical home must consider how to deliver the care (it must be accessible, team-based, and aimed at the “whole person”), coordinate the care, and measure its quality.7 Many Medicare beneficiaries with serious chronic illness have limited mobility that makes it difficult to regularly travel to medical offices, and thus they need home visits or regular contact by telephone or computer.
Many home health agencies are using new conceptual models, programs, technologies, and services so they can play a supportive role.8 These agencies employ nurses, therapists, social workers, personal caregivers, and nutritionists. In many instances these people can become the physician-directed team responsible for key aspects of caring for patients with chronic illness in their homes, coordinating and integrating the care, and measuring its quality. Additionally, in-home assessment provides a holistic view of patients that potentially promotes patient- and family-centered care options.
To be eligible for home health services, a beneficiary must be “homebound,” must need intermittent skilled nursing care or skilled therapy, and must be under the care of a physician. The health reform law has also mandated that patients have a face-to-face visit with their physician or with certain nonphysician practitioners in order to certify the home health care plan.
Even though the homebound requirement limits the number of people eligible, many older adults like Mrs. Smith who have chronic illness meet this criterion. Others may only be homebound during an exacerbation of a chronic illness that temporarily limits their mobility. However, patients can still be considered homebound for the Medicare benefit even if they leave their home (infrequently) for medical care, religious services, family events, adult day programs, and other reasons.9
The Medicare Home Health benefit covers several services that are especially important for patients with chronic illness. These include nursing visits for observation and assessment, evaluation and management of a care plan, and teaching and training.
How this applies to Mrs. Smith
In the case of Mrs. Smith, Dr. Jones could order home nursing care to make sure she is taking her medications as directed, to teach her about self-management and nutrition, and to assess the impact of medication changes—both the intended effects and adverse effects such as hypotension.
Other team members bring other skills. For example, home health social workers may be able to address complex psychosocial needs that can affect adherence.
The time Dr. Jones spends developing this care plan and reviewing the patient’s condition with home health field staff by telephone or other communication methods is reimbursable under Medicare as “care plan oversight”10 and can substitute for the revenue lost due to less-frequent office visits.10 In the new practice models, a medical home or independence-at-home care-management fee or anticipated revenues from “gain-sharing” could cover nonvisit supervision of in-home services.
Oversight in the computer age
Dr. Jones may be reluctant to rely on a home health agency because she cannot directly oversee what they are doing and may in fact be uncertain as to what they are doing. Home care may seem like a “black box” to physicians, but it shouldn’t in this era of electronic health records and advanced electronic information systems. Seamless communication is possible without playing “telephone tag” and sending multiple faxes. Physicians may prefer to work only with home care providers who use electronic information systems and who can interface their systems with the physician’s electronic systems, or at least offer shared viewing through Web access. Of course, such arrangements must be initiated with respect for the patient’s preference for a home care agency.
Home health providers are also well positioned to help measure and monitor the quality of care. Medicare requires that home health providers track a comprehensive set of quality outcomes, adjusted for risk, and ranging from improvement in function to acute hospitalization rates.11,12 Given that most home care providers are swimming in data about their patients, it would be reasonable for home care agencies to provide physician partners with more nuanced reports for specific subpopulations, such as those from a particular physician practice, or for patients with a particular disease.
NEW CONCEPTS, PROCESSES, AND TECHNOLOGIES
To care for a patient like Mrs. Smith, the home health team must embrace new, chronic-care-oriented concepts, processes, and technologies. Many agencies now have nurses and therapists skilled in chronic illness care, self-management support, and health coaching. Ancillary staff collaborate with the physician by assuming time-consuming but necessary tasks such as patient education, care coordination and integration, and quality measurement and improvement initiatives.
Several groups and authors have proposed a “home-based chronic care model,” built upon the well-studied “chronic care model,” 13–16 as a framework to help home care providers change their approach to patients with chronic illness. This model offers a standardized curriculum and certification program, as well as practice guidelines, which standardize best-practice care delivery from agency to agency.
A core tenet of this model is a strong focus on teaching clinicians how to teach their patients to care for themselves, since bad outcomes are often due to patients not following physicians' recommendations. Since successful chronic care management requires adherence to specific self-care behaviors, the focus on behavior change must not be neglected if positive outcomes are to be realized.
New technologies are also emerging. Some home health providers are using in-home telemetry with remote call centers to track the patient’s health status on a daily basis. Physicians and patients can follow the data, allowing for quick intervention, if necessary, and reinforcement of self-management learning.17–20 Some home care agencies could monitor, via telemetry, Mrs. Smith’s weight, blood pressure, oxygen saturation, heart rate, and dyspnea symptoms. This information could be fed back to call-center clinicians who have predetermined parameters for titrating the diuretic dose and for notifying the physician.
Some monitoring technology allows for interactive assessment and teaching via live videoconferencing. Some home health agencies also use telephone-based health coaching.21 Information system interfaces between the home health agency and the medical home coordinator could make the content of this in-home monitoring and care management visible in the physician’s record.
TOWARD ONGOING CARE MANAGEMENT
In spite of these opportunities, the Medicare home health benefit rarely permits uninterrupted ongoing home care. Thus, the home health collaboration developed around Mrs. Smith’s heart failure exacerbation is likely to be temporary, and when her condition stabilizes she may no longer meet the criteria for home health services.
This episodic-payment model contrasts with the ongoing needs of the typical high-risk older patient with chronic illness. Changing the home health benefit to allow for ongoing home health care for beneficiaries like Mrs. Smith may be an opportunity for patient-centered reform. Although ongoing home health care for a given patient may not be possible, the medical home model offers the opportunity for ongoing physician-home health collaboration because at any time a physician’s practice is likely to have patients requiring these services. The independence-at-home model does provide for uninterrupted ongoing in-home physician and mid-level care for some patients, but it may require changing primary care physicians, and this may be undesirable to some patients. If a viable financing model is established for medical homes and independence-at-home practices, they may choose to contract with home health agencies to provide ongoing telephone or telemetric care management between (or outside of) episodes of eligibility for traditional home health care. All of these potential arrangements would need legal review and would need to be structured to avoid violation of the letter and spirit of laws prohibiting self-referrals and kickbacks.
PHYSICIAN HOME VISITS
In the case of Mrs. Smith, Dr. Jones has the option of making a follow-up home visit, or even ongoing home visits.
Granted, home visits may be impractical due to the time involved and the impact of that downtime on the physician’s medical practice and responsibilities to other patients. However, larger practices may employ a specific physician, nurse practitioner, or physician’s assistant to provide in-home care to patients in need.
Some communities have house-call practices to which Dr. Jones could refer Mrs. Smith for in-home physician care, and, where available, this may be a preferred care model— somewhat analogous to how a primary care physician might collaborate with a hospitalist for inpatient care of a specific patient.22 These homecare physician practices will likely become more prevalent if the independence-at-home Medicare demonstration project is successful.
In the future, even if Mrs. Smith needed more intensive inpatient care, an emerging concept called “hospital at home” may be able to provide this acute care in her home.23,24 These in-home physician services are increasingly supported by new mobile diagnostic technologies.25
However, adding or changing physicians may not be possible or desirable for Mrs. Smith and could lead to further fragmentation of care. In the future, teleconferencing may provide options for “virtual visits” that would partially solve this problem.
Whether the physician care is provided in the office, in the home, or as a virtual visit, much of the care Mrs. Smith needs can and should be done by nonphysician home health care providers in partnership with informal caregivers.
MRS. SMITH GETS BETTER AT HOME
Dr. Jones decided to refer Mrs. Smith for home health nursing and maintained close telephone contact with her and the home health nurse during the first 2 weeks. Mrs. Smith responded well to the changes in medication and diet, her leg swelling decreased, and she was feeling more like her usual self. At a follow-up office visit 3 months later, Mrs. Smith hugged Dr. Jones and thanked her profusely for helping her get better at home.
Mrs. Smith, age 82, has chronic heart failure. She also has difficulty walking because of arthritis in her knee and osteoporosis. Her son has taken the day off work to bring her in to see her primary care physician, Dr. Jones, because of increasing swelling of her legs and feeling tired.
Even on a good day, Mrs. Smith faces challenges getting to the doctor’s office: she has difficulty getting dressed, taking the stairs, and transporting her walker and oxygen, not to mention parking the car, getting out, getting in to the doctor’s office, and then returning home.
After a careful evaluation Dr. Jones concludes that the leg swelling and fatigue are due to an exacerbation of heart failure triggered by excess dietary sodium and uncontrolled hypertension. She decides to increase the dosages of Mrs. Smith’s diuretic and angiotensin-converting enzyme inhibitor and advises her and her son about dietary sodium restriction. She reviews with them the symptoms that should trigger a call to the office, and she says she wants to see Mrs. Smith again in 3 days.
Mrs. Smith and her son do not seem to understand the instructions, and they explain how difficult it will be to make the follow-up visit, so Dr. Jones recommends hospital admission. Mrs. Smith protests, as she has had multiple hospitalizations during the past year and she dreads the idea of returning. And her son explains, “Mom always seems worse after going to the hospital. Last winter when she was there her days and nights got mixed up, and when she called out at night they gave her some drug that knocked her out for 2 days. Doctor, isn’t there any safe way to keep her at home?”
CHRONIC ILLNESS: A CHALLENGE, AND AN OPPORTUNITY
The growing number of older adults with chronic illnesses poses a serious challenge to the US health care system, placing unprecedented pressures on the financial sustainability and overall effectiveness of the Medicare program.1,2 Of particular concern is the plight of Medicare beneficiaries like Mrs. Smith who have multiple chronic conditions and whose activity and mobility are limited. These patients account for a disproportionate share of Medicare expenses and, despite all the money spent, often struggle without optimal care that is accessible, individualized, and coordinated.
But this challenge is also an opportunity. We may be able to improve the care of these vulnerable patients—and control costs—by taking their primary care to their own homes. To these ends, the Patient Protection and Affordable Care Act (ie, the “health care reform law”) has several provisions for pilot and demonstration projects.3–5 In light of the new policies and as part of a grassroots effort to change the delivery of care for patients with chronic conditions, primary care physicians like Dr. Jones are redesigning their practices to provide a patient-centered medical home.6
As envisioned, the primary care physician’s office will be the patient’s “medical home.” The primary care physician will lead, coordinate, and oversee the efforts of a multidisciplinary team, referring patients when necessary to specialists and community resources. Primary care practices that become medical homes would potentially be paid care management fees in addition to fees for visits, but with new expectations for care coordination and integration.
The emergence of the medical home, independence-at-home, and related concepts makes it a good time for physicians to explore how they can collaborate with home health providers to better meet the needs of older patients with chronic illness (Table 1).
UNDER MEDICARE, WHO IS ELIGIBLE FOR HOME HEALTH SERVICES?
Primary care physicians who are transforming their offices into a medical home must consider how to deliver the care (it must be accessible, team-based, and aimed at the “whole person”), coordinate the care, and measure its quality.7 Many Medicare beneficiaries with serious chronic illness have limited mobility that makes it difficult to regularly travel to medical offices, and thus they need home visits or regular contact by telephone or computer.
Many home health agencies are using new conceptual models, programs, technologies, and services so they can play a supportive role.8 These agencies employ nurses, therapists, social workers, personal caregivers, and nutritionists. In many instances these people can become the physician-directed team responsible for key aspects of caring for patients with chronic illness in their homes, coordinating and integrating the care, and measuring its quality. Additionally, in-home assessment provides a holistic view of patients that potentially promotes patient- and family-centered care options.
To be eligible for home health services, a beneficiary must be “homebound,” must need intermittent skilled nursing care or skilled therapy, and must be under the care of a physician. The health reform law has also mandated that patients have a face-to-face visit with their physician or with certain nonphysician practitioners in order to certify the home health care plan.
Even though the homebound requirement limits the number of people eligible, many older adults like Mrs. Smith who have chronic illness meet this criterion. Others may only be homebound during an exacerbation of a chronic illness that temporarily limits their mobility. However, patients can still be considered homebound for the Medicare benefit even if they leave their home (infrequently) for medical care, religious services, family events, adult day programs, and other reasons.9
The Medicare Home Health benefit covers several services that are especially important for patients with chronic illness. These include nursing visits for observation and assessment, evaluation and management of a care plan, and teaching and training.
How this applies to Mrs. Smith
In the case of Mrs. Smith, Dr. Jones could order home nursing care to make sure she is taking her medications as directed, to teach her about self-management and nutrition, and to assess the impact of medication changes—both the intended effects and adverse effects such as hypotension.
Other team members bring other skills. For example, home health social workers may be able to address complex psychosocial needs that can affect adherence.
The time Dr. Jones spends developing this care plan and reviewing the patient’s condition with home health field staff by telephone or other communication methods is reimbursable under Medicare as “care plan oversight”10 and can substitute for the revenue lost due to less-frequent office visits.10 In the new practice models, a medical home or independence-at-home care-management fee or anticipated revenues from “gain-sharing” could cover nonvisit supervision of in-home services.
Oversight in the computer age
Dr. Jones may be reluctant to rely on a home health agency because she cannot directly oversee what they are doing and may in fact be uncertain as to what they are doing. Home care may seem like a “black box” to physicians, but it shouldn’t in this era of electronic health records and advanced electronic information systems. Seamless communication is possible without playing “telephone tag” and sending multiple faxes. Physicians may prefer to work only with home care providers who use electronic information systems and who can interface their systems with the physician’s electronic systems, or at least offer shared viewing through Web access. Of course, such arrangements must be initiated with respect for the patient’s preference for a home care agency.
Home health providers are also well positioned to help measure and monitor the quality of care. Medicare requires that home health providers track a comprehensive set of quality outcomes, adjusted for risk, and ranging from improvement in function to acute hospitalization rates.11,12 Given that most home care providers are swimming in data about their patients, it would be reasonable for home care agencies to provide physician partners with more nuanced reports for specific subpopulations, such as those from a particular physician practice, or for patients with a particular disease.
NEW CONCEPTS, PROCESSES, AND TECHNOLOGIES
To care for a patient like Mrs. Smith, the home health team must embrace new, chronic-care-oriented concepts, processes, and technologies. Many agencies now have nurses and therapists skilled in chronic illness care, self-management support, and health coaching. Ancillary staff collaborate with the physician by assuming time-consuming but necessary tasks such as patient education, care coordination and integration, and quality measurement and improvement initiatives.
Several groups and authors have proposed a “home-based chronic care model,” built upon the well-studied “chronic care model,” 13–16 as a framework to help home care providers change their approach to patients with chronic illness. This model offers a standardized curriculum and certification program, as well as practice guidelines, which standardize best-practice care delivery from agency to agency.
A core tenet of this model is a strong focus on teaching clinicians how to teach their patients to care for themselves, since bad outcomes are often due to patients not following physicians' recommendations. Since successful chronic care management requires adherence to specific self-care behaviors, the focus on behavior change must not be neglected if positive outcomes are to be realized.
New technologies are also emerging. Some home health providers are using in-home telemetry with remote call centers to track the patient’s health status on a daily basis. Physicians and patients can follow the data, allowing for quick intervention, if necessary, and reinforcement of self-management learning.17–20 Some home care agencies could monitor, via telemetry, Mrs. Smith’s weight, blood pressure, oxygen saturation, heart rate, and dyspnea symptoms. This information could be fed back to call-center clinicians who have predetermined parameters for titrating the diuretic dose and for notifying the physician.
Some monitoring technology allows for interactive assessment and teaching via live videoconferencing. Some home health agencies also use telephone-based health coaching.21 Information system interfaces between the home health agency and the medical home coordinator could make the content of this in-home monitoring and care management visible in the physician’s record.
TOWARD ONGOING CARE MANAGEMENT
In spite of these opportunities, the Medicare home health benefit rarely permits uninterrupted ongoing home care. Thus, the home health collaboration developed around Mrs. Smith’s heart failure exacerbation is likely to be temporary, and when her condition stabilizes she may no longer meet the criteria for home health services.
This episodic-payment model contrasts with the ongoing needs of the typical high-risk older patient with chronic illness. Changing the home health benefit to allow for ongoing home health care for beneficiaries like Mrs. Smith may be an opportunity for patient-centered reform. Although ongoing home health care for a given patient may not be possible, the medical home model offers the opportunity for ongoing physician-home health collaboration because at any time a physician’s practice is likely to have patients requiring these services. The independence-at-home model does provide for uninterrupted ongoing in-home physician and mid-level care for some patients, but it may require changing primary care physicians, and this may be undesirable to some patients. If a viable financing model is established for medical homes and independence-at-home practices, they may choose to contract with home health agencies to provide ongoing telephone or telemetric care management between (or outside of) episodes of eligibility for traditional home health care. All of these potential arrangements would need legal review and would need to be structured to avoid violation of the letter and spirit of laws prohibiting self-referrals and kickbacks.
PHYSICIAN HOME VISITS
In the case of Mrs. Smith, Dr. Jones has the option of making a follow-up home visit, or even ongoing home visits.
Granted, home visits may be impractical due to the time involved and the impact of that downtime on the physician’s medical practice and responsibilities to other patients. However, larger practices may employ a specific physician, nurse practitioner, or physician’s assistant to provide in-home care to patients in need.
Some communities have house-call practices to which Dr. Jones could refer Mrs. Smith for in-home physician care, and, where available, this may be a preferred care model— somewhat analogous to how a primary care physician might collaborate with a hospitalist for inpatient care of a specific patient.22 These homecare physician practices will likely become more prevalent if the independence-at-home Medicare demonstration project is successful.
In the future, even if Mrs. Smith needed more intensive inpatient care, an emerging concept called “hospital at home” may be able to provide this acute care in her home.23,24 These in-home physician services are increasingly supported by new mobile diagnostic technologies.25
However, adding or changing physicians may not be possible or desirable for Mrs. Smith and could lead to further fragmentation of care. In the future, teleconferencing may provide options for “virtual visits” that would partially solve this problem.
Whether the physician care is provided in the office, in the home, or as a virtual visit, much of the care Mrs. Smith needs can and should be done by nonphysician home health care providers in partnership with informal caregivers.
MRS. SMITH GETS BETTER AT HOME
Dr. Jones decided to refer Mrs. Smith for home health nursing and maintained close telephone contact with her and the home health nurse during the first 2 weeks. Mrs. Smith responded well to the changes in medication and diet, her leg swelling decreased, and she was feeling more like her usual self. At a follow-up office visit 3 months later, Mrs. Smith hugged Dr. Jones and thanked her profusely for helping her get better at home.
- Hackbarth GM. Medicare Payment Advisory Commission. June 2008 Report to the Congress: Reforming the Delivery System. http://www.medpac.gov/documents/Jun08_Entirereport.pdf. Accessed September 9, 2010.
- Congressional Budget Office. Accounting for Sources of Projected Growth in Federal Spending on Medicare and Medicaid. http://www.cbo.gov/ftpdocs/93xx/doc9316/HealthCostGrowth.shtml. Accessed September 9, 2010.
- Landers SH. The other Medical Home. JAMA 2009; 301:97–99.
- The Library of Congress: Thomas. The RE-Aligning Care Act. http://frwebgate.access.gpo.gov/cgi-bin/getdoc.cgi?dbname=111_cong_bills&docid=f:s1004is.txt.pdf. Accessed September 9, 2010.
- The Library of Congress: Thomas. Independence at Home Act of 2009. http://frwebgate.access.gpo.gov/cgi-bin/getdoc.cgi?dbname=111_cong_bills&docid=f:h2560ih.txt.pdf. Accessed August 12, 2010.
- TransforMED. http://www.transformed.com. Accessed September 9, 2010.
- Kellerman R, Kirk L. Principles of the patient-centered medical home. Am Fam Physician 2007; 76:774–775.
- Fisher ES. Building a medical neighborhood for the medical home. N Engl J Med 2008; 359:1202–1205.
- Medicare Benefit Policy Manual: Chapter 7 - Home Health Services. http://www.cms.hhs.gov/manuals/Downloads/bp102c07.pdf. Accessed September 9, 2010.
- Bluestein HM. Care plan oversight and home care/hospice revenue for telephone management. Compr Ther 2006; 32:226–229.
- Madigan EA, Fortinsky RH. Interrater reliability of the outcomes and assessment information set: results from the field. Gerontologist 2004; 44:689–692.
- Madigan EA, Tullai-McGuinness S, Fortinsky RH. Accuracy in the Outcomes and Assessment Information Set (OASIS): results of a video simulation. Res Nurs Health 2003; 26:273–283.
- Bodenheimer T, Wagner EH, Grumbach K. Improving primary care for patients with chronic illness: the chronic care model, Part 2. JAMA 2002; 288:1909–1914.
- Martin JC, Avant RF, Bowman MA, et al; Future of Family Medicine Project Leadership Committee. The Future of Family Medicine: a collaborative project of the family medicine community. Ann Fam Med 2004; 2(suppl 1):S3–S32.
- Hennessey B, Suter P. The home-based chronic care model. Caring 2009; 28:12–16.
- Suter P, Hennessey B, Harrison G, Fagan M, Norman B, Suter WN. Home-based chronic care. An expanded integrative model for home health professionals. Home Healthc Nurse 2008; 26:222–229.
- Browning SV, Tullai-McGuinness S, Madigan E, Struk C. Telehealth: is your staff ready to implement? A descriptive exploratory study of readiness for this technology in home health care. Home Healthc Nurse 2009; 27:242–248.
- Fazzi R, Ashe T, Doak L. Telehealth, disease management, home care and the future—part 2. Caring 2008; 27:36–8,40–1,3.
- Kelly K, Christians J. Best practices in implementing a telehealth program. Caring 2008; 27:44–47.
- Whitten P, Bergman A, Meese MA, Bridwell K, Jule K. St. Vincent’s Home telehealth for congestive heart failure patients. Telemed J E Health 2009; 15:148–153.
- A medisys Home Health Services. Comprehensive, continuous chronic care management in the home. http://www.amedisys.com/pdf/Whitepaper_C4M.pdf. Accessed September 9, 2010.
- Okie S. Home delivery—bringing primary care to the housebound elderly. N Engl J Med 2008; 359:2409–2412.
- Leff B, Burton JR. Acute medical care in the home. J Am Geriatr Soc 1996; 44:603–605.
- Leff B, Burton L, Mader SL, et al. Hospital at home: feasibility and outcomes of a program to provide hospital-level care at home for acutely ill older patients. Ann Intern Med 2005; 143:798–808.
- Bayne CG, Boling PA. New diagnostic and information technology for mobile medical care. Clin Geriatr Med 2009; 25:93–107.
- Hackbarth GM. Medicare Payment Advisory Commission. June 2008 Report to the Congress: Reforming the Delivery System. http://www.medpac.gov/documents/Jun08_Entirereport.pdf. Accessed September 9, 2010.
- Congressional Budget Office. Accounting for Sources of Projected Growth in Federal Spending on Medicare and Medicaid. http://www.cbo.gov/ftpdocs/93xx/doc9316/HealthCostGrowth.shtml. Accessed September 9, 2010.
- Landers SH. The other Medical Home. JAMA 2009; 301:97–99.
- The Library of Congress: Thomas. The RE-Aligning Care Act. http://frwebgate.access.gpo.gov/cgi-bin/getdoc.cgi?dbname=111_cong_bills&docid=f:s1004is.txt.pdf. Accessed September 9, 2010.
- The Library of Congress: Thomas. Independence at Home Act of 2009. http://frwebgate.access.gpo.gov/cgi-bin/getdoc.cgi?dbname=111_cong_bills&docid=f:h2560ih.txt.pdf. Accessed August 12, 2010.
- TransforMED. http://www.transformed.com. Accessed September 9, 2010.
- Kellerman R, Kirk L. Principles of the patient-centered medical home. Am Fam Physician 2007; 76:774–775.
- Fisher ES. Building a medical neighborhood for the medical home. N Engl J Med 2008; 359:1202–1205.
- Medicare Benefit Policy Manual: Chapter 7 - Home Health Services. http://www.cms.hhs.gov/manuals/Downloads/bp102c07.pdf. Accessed September 9, 2010.
- Bluestein HM. Care plan oversight and home care/hospice revenue for telephone management. Compr Ther 2006; 32:226–229.
- Madigan EA, Fortinsky RH. Interrater reliability of the outcomes and assessment information set: results from the field. Gerontologist 2004; 44:689–692.
- Madigan EA, Tullai-McGuinness S, Fortinsky RH. Accuracy in the Outcomes and Assessment Information Set (OASIS): results of a video simulation. Res Nurs Health 2003; 26:273–283.
- Bodenheimer T, Wagner EH, Grumbach K. Improving primary care for patients with chronic illness: the chronic care model, Part 2. JAMA 2002; 288:1909–1914.
- Martin JC, Avant RF, Bowman MA, et al; Future of Family Medicine Project Leadership Committee. The Future of Family Medicine: a collaborative project of the family medicine community. Ann Fam Med 2004; 2(suppl 1):S3–S32.
- Hennessey B, Suter P. The home-based chronic care model. Caring 2009; 28:12–16.
- Suter P, Hennessey B, Harrison G, Fagan M, Norman B, Suter WN. Home-based chronic care. An expanded integrative model for home health professionals. Home Healthc Nurse 2008; 26:222–229.
- Browning SV, Tullai-McGuinness S, Madigan E, Struk C. Telehealth: is your staff ready to implement? A descriptive exploratory study of readiness for this technology in home health care. Home Healthc Nurse 2009; 27:242–248.
- Fazzi R, Ashe T, Doak L. Telehealth, disease management, home care and the future—part 2. Caring 2008; 27:36–8,40–1,3.
- Kelly K, Christians J. Best practices in implementing a telehealth program. Caring 2008; 27:44–47.
- Whitten P, Bergman A, Meese MA, Bridwell K, Jule K. St. Vincent’s Home telehealth for congestive heart failure patients. Telemed J E Health 2009; 15:148–153.
- A medisys Home Health Services. Comprehensive, continuous chronic care management in the home. http://www.amedisys.com/pdf/Whitepaper_C4M.pdf. Accessed September 9, 2010.
- Okie S. Home delivery—bringing primary care to the housebound elderly. N Engl J Med 2008; 359:2409–2412.
- Leff B, Burton JR. Acute medical care in the home. J Am Geriatr Soc 1996; 44:603–605.
- Leff B, Burton L, Mader SL, et al. Hospital at home: feasibility and outcomes of a program to provide hospital-level care at home for acutely ill older patients. Ann Intern Med 2005; 143:798–808.
- Bayne CG, Boling PA. New diagnostic and information technology for mobile medical care. Clin Geriatr Med 2009; 25:93–107.