User login
Charcot neuroarthropathy: An often overlooked complication of diabetes
Several weeks before coming to our orthopedic surgery clinic, a 53-year-old man presented to an emergency department because of pain, swelling, and redness in his right foot, which began 3 days before. He recalled no overt trauma, but he was jogging when he first noticed the pain, which he described as a constant aching and rated as high as 8 on a scale of 10.
At that time, he had no fever, chills, or night sweats, no cough, and no shortness of breath. About 10 years ago he was diagnosed with diabetes mellitus, for which he currently takes rosiglitazone (Avandia) 2 mg/day and metformin (Glucophage XR) 500 mg four tablets daily. He also takes ramipril (Altace) 10 mg/day for hypertension, as well as a daily multivitamin. He has a history of hyperlipidemia and a family history of diabetes mellitus and Parkinson disease. He has never been hospitalized and has never undergone surgery.
His blood glucose level was 239 mg/dL (normal 70–110), hemoglobin A1c 9.7% (normal 4%–6%), and white blood cell count 13.41 × 109/L (normal 4.5–11.0).
Based on that evaluation, the patient was admitted to the hospital with a diagnosis of cellulitis. He received intravenous antibiotics for 3 days and then was discharged with a prescription for oral antibiotics. He visited his primary care physician several times over the next 2 to 4 weeks and then was referred to our orthopedic surgery clinic for further evaluation. A neurologic evaluation in our clinic revealed a loss of protective sensation, contraction of the toes, and dryness, consistent with peripheral neuropathy. Given what we know so far, which is the most likely diagnosis?
DIFFERENTIAL DIAGNOSIS
While cellulitis may seem to be the likely diagnosis, if a patient with long-standing diabetes, a history of poor glycemic control, and peripheral neuropathy presents with a red, hot, swollen foot with no history of open ulceration, then Charcot neuroarthropathy should be at the top of the list in the differential diagnosis. Other possibilities include osteomyelitis, acute gout, cellulitis, abscess, neuropathic fracture, and deep venous thrombosis. However, if the patient has no open ulceration or history of an open wound, infection is probably not the culprit. Most diabetic foot infections begin with a direct inoculation through an opening in the skin, such as a diabetic neuropathic foot ulcer.
Further, in the case of cellulitis or deep venous thrombosis, the predominating feature would be asymmetric edema of the leg. Also, the location of the edema and ecchymosis in our patient—namely, the midfoot—leads to suspicion of an acute musculoskeletal injury, particularly Charcot neuroarthropathy of the midfoot and neuropathic fractures in the region of the ecchymotic second and third digits. Acute gout could be discounted because gout pain is severe, with rapid onset, and slowly improves even without treatment.
A COMPLICATION OF DIABETES
Charcot neuroarthropathy presents as a warm, swollen, erythematous foot and ankle, a picture that may be indistinguishable from that of infection. Most patients are in their 50s or 60s, and most present on an emergency basis; they often present late in the process, ie, 2 to 3 months after the initial symptoms, because the symptoms often are not painful.
This condition has been reported to occur with leprosy, syringomyelia, toxic exposure, poliomyelitis, rheumatoid arthritis, multiple sclerosis, congenital neuropathy, traumatic injury, and tertiary syphilis.1–4 Other conditions that reportedly trigger it include cellulitis, osteomyelitis, synovitis, surgery of the foot, and renal transplant surgery.5–7 However, today, the most common cause is diabetes mellitus.4,8
Other names for this condition are diabetic neuropathic osteoarthropathy and neuropathic arthropathy.
Current estimates of its prevalence range from 0.08% in the general diabetic population to 13% in high-risk diabetic patients.9
CHARCOT NEUROARTHROPATHY BEGINS WITH PERIPHERAL NEUROPATHY
The pathophysiologic mechanism of Charcot neuroarthropathy is not completely known, but it is thought to begin with peripheral neuropathy. Being insensitive to pain, patients may subject the joints of the foot (most commonly in the midfoot) to stress injuries that lead to the active Charcot process.10–12 About half of Charcot patients present with pain, as did our patient.
Although our patient remembered no trauma, he was physically active at the time he first noticed the symptoms.
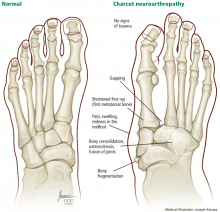
Four stages of Charcot neuroarthropathy are recognized11–15:
Stage 0 (inflammation), also called Charcot in situ or pre-stage 1, is characterized by erythema, edema, and heat but no structural changes.11,12,14,15
Stage 1 (development) is characterized by bone resorption, bone fragmentation, and joint dislocation. The swelling, warmth, and redness persist, but there are also radiographic changes such as evidence of debris formation at the articular margins, osseous fragmentation, and joint disruption.
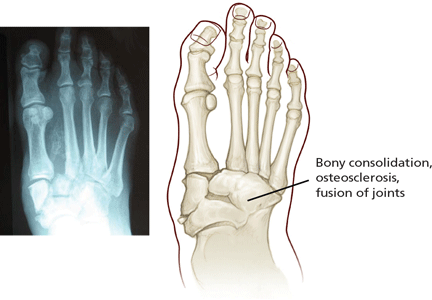
Stage 2 (coalescence) involves bony consolidation, osteosclerosis, and fusion after bony destruction. Absorption of small bone fragments, fusion of joints, and sclerosis of the bone are noticeable.
Stage 3 (reconstruction) is characterized by osteogenesis, decreased osteosclerosis, and progressive fusion.13 Healing and new bone formation occur. Decreased sclerosis and bony remodeling signify that the deformity (for example, subluxation, incongruity, and dislocation) is permanent.4
MISDIAGNOSIS IS COMMON
Charcot neuroarthropathy is an often overlooked complication in diabetic patients with peripheral neuropathy. A group of experts reported that 25% of patients referred to their facility who had Charcot neuroarthropathy had not received a correct diagnosis at the referring institution.16 The incorrect diagnoses included infection, gout, arthritis, fracture, venous insufficiency, and tumor.
Laboratory tests can narrow the differential diagnosis
There are no laboratory criteria for the diagnosis of Charcot neuroarthropathy and no hematologic markers, but laboratory testing can help narrow the differential diagnosis. Leukocytosis, an elevated C-reactive protein and erythrocyte sedimentation rate, and recent unexplained hyperglycemia suggest infection.17 However, unremarkable results on clinical tests in this population may not comprehensively exclude infection.
Our patient’s elevated white blood cell count confused the diagnosis. Further, when he was treated with antibiotics, he reported having less pain, although the edema and erythema continued.
Imaging studies
Although advanced imaging may help confirm the diagnosis of Charcot neuropathy in some patients, it is not always necessary.
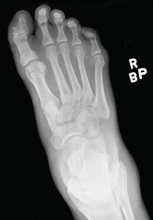
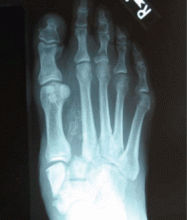
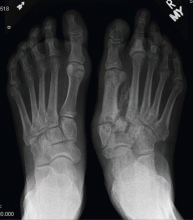
Radiography. Radiographic findings are important in diagnosing Charcot neuroarthropathy, although they are less helpful in patients with stage 0 disease, such as our patient, in whom the condition has not yet progressed to fracture or dislocation. All foot and ankle radiographs should be taken in the weight-bearing position. Subtle changes may be missed if non-weight-bearing images are taken.
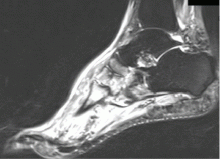
Magnetic resonance imaging (MRI) can show changes in stage 0, thus enabling treatment to be started sooner,18 and it is increasingly being recommended for diagnosing Charcot neuroarthropathy, especially in the early stages.3 Although bone scintigraphy and white blood cell scans have been traditionally advocated, MRI offers the highest diagnostic accuracy.19 Signs on MRI consistent with Charcot neuroarthropathy include ligamentous disruption, concomitant joint deformity, and the center of signal enhancement within joints and subchondral bone.20
MRI can also differentiate Charcot neuroarthropathy from transient regional osteoporosis. The latter has a different anatomic location and does not cause fractures and dislocations, and patients do not have a clinical history of pain.
Another condition MRI can identify is complex regional pain syndrome. In this condition, patients have no radiographic abnormalities except for periarticular osteopenia, but they may have severe pain out of proportion with the clinical appearance, and they may develop soft-tissue deformity in the late stages, which is not seen in Charcot neuroarthropathy.
Positron emission tomography (PET) with fluorine-18 fluorodeoxyglucose is also gaining support,21 especially when combined with computed tomography (CT). This PET-CT hybrid has better anatomic localization than PET alone.
PET-CT is very reliable for differentiating Charcot neuroarthropathy from osteomyelitis, a distinction that can be difficult to make when Charcot neuroarthropathy is complicated by adjacent loss of skin integrity. The sensitivity of PET-CT in this situation has been reported as 100%, and its sensitivity 93.8%.22
Patients with Charcot neuroarthropathy demonstrate a low-intensity diffuse uptake that is easily distinguishable from normal joints on visual examination of the images. In addition, the maximum standardized uptake value, a quantitative measurement, is low to intermediate in Charcot neuroarthropathy but significantly higher in osteomyelitis. In one study,22 the mean standardized uptake values were 0.42 in normal feet, 1.3 in Charcot neuroarthropathy, and 4.38 in osteomyelitis.
TREATMENT: IMMOBILIZATION, BISPHOSPHONATES, SURGERY
The goals of treatment for acute or quiescent Charcot neuroarthropathy should be to maintain or achieve structural stability of the foot and ankle, to prevent skin ulceration, and to preserve the plantigrade shape of the foot so that prescription footwear can be used.
Patient and family education is important for compliance with the regimen, particularly because patients with diabetic neuropathy lack the protective pain response.
Immobilization. A total-contact cast is worn until the redness, swelling, and heat subside, generally 8 to 12 weeks, after which the patient should use removable braces or a Charcot restraint orthotic walker for a total of 4 to 6 months of treatment.23 The cast is typically changed every 1 to 2 weeks as the swelling subsides to minimize irritation to the insensate limb.
Many physicians also recommend elastic stockings (eg, Stockinette) or an elastic tubular bandage (eg, Tubigrip) to reduce edema under the cast.
Bisphosphonates. Some clinicians also prescribe bisphosphonates in the early stages of treatment, as the bone mineral density of the affected foot is low.24 Unfortunately, while these drugs can significantly reduce the levels of bone turnover markers, temperature, and pain, evidence of clinical benefit such as an earlier return to ambulation or radiographic improvement is weak at best.
Surgery is reserved for severe ankle and midfoot deformities that are susceptible to skin ulcerations and that make braces and orthotic devices difficult to use.
TREATMENT OUTCOME
The patient’s condition resolved, with eventual multiplanar deformity and with widening of the midfoot and increased pressure points, particularly to the first ray. He is able to wear an extra-depth shoe, with a custom totalcontact inlay. He continues his profession as an attorney and goes about his normal daily activities; however, he is no longer able to golf and must limit his walking. He subsequently developed ulcerations to both feet, but they resolved with conservative wound care and surgical care. He is seen in the diabetic foot clinic every 6 to 8 weeks.
- Gupta R. A short history of neuropathic arthropathy. Clin Orthop Relat Res 1993; 296:43–49.
- Johnson JT. Neuropathic fractures and joint injuries. Pathogenesis and rationale of prevention and treatment. J Bone Joint Surg Am 1967; 49:1–30.
- Sanders LJ, Frykberg RG. The Charcot Foot (Pied de Charcot). In:Bowker JH, Pfeifer MA, editors. Levin and O’Neal’s The Diabetic Foot. 7th ed. Philadelphia, PA: Mosby Elsevier; 2008:257–283.
- Wukich DK, Sung W. Charcot arthropathy of the foot and ankle: modern concepts and management review. J Diabetes Complications 2009; 23:409–426.
- Armstrong DG, Todd WF, Lavery LA, Harkless LB, Bushman TR. The natural history of acute Charcot’s arthropathy in a diabetic foot specialty clinic. J Am Podiatr Med Assoc 1997; 87:272–278.
- Jeffcoate WJ. Theories concerning the pathogenesis of the acute Charcot foot suggest future therapy. Curr Diab Rep 2005; 5:430–435.
- Matricali GA, Bammens B, Kuypers D, Flour M, Mathieu C. High rate of Charcot foot attacks early after simultaneous pancreas-kidney transplantation. Transplantation 2007; 83:245–246.
- Miller DS, Lichtman WF. Diabetic neuropathic arthropathy of feet; summary report of seventeen cases. AMA Arch Surg 1955; 70:513–518.
- Frykberg RG, Belczyk R. Epidemiology of the Charcot foot. Clin Podiatr Med Surg 2008; 25:17–28,
- Chantelau E. The perils of procrastination: effects of early vs delayed detection and treatment of incipient Charcot fracture. Diabet Med 2005; 22:1707–1712.
- Schon LC, Marks RM. The management of neuroarthropathic fracture-dislocations in the diabetic patient. Orthop Clin North Am 1995; 26:375–392.
- Sella EJ, Barrette C. Staging of Charcot neuroarthropathy along the medial column of the foot in the diabetic patient. J Foot Ankle Surg 1999; 38:34–40.
- Eichenholtz SN. Charcot Joints. Springfield, IL: CC Thomas; 1966.
- Shibata T, Tada K, Hashizume C. The results of arthrodesis of the ankle for leprotic neuroarthropathy. J Bone Joint Surg Am 1990; 72:749–756.
- Yu GV, Hudson JR. Evaluation and treatment of stage 0 Charcot’s neuroarthropathy of the foot and ankle. J Am Podiatr Med Assoc 2002; 92:210–220.
- Myerson MS, Henderson MR, Saxby T, Short KW. Management of midfoot diabetic neuroarthropathy. Foot Ankle Int 1994; 15:233–241.
- Judge MS. Using serologic screening to Identify and monitor at-risk Charcot patients. Podiatry Today Magazine 2004; 17:75–82.
- Chantelau E, Poll LW. Evaluation of the diabetic Charcot foot by MR imaging or plain radiography—an observational study. Exp Clin Endocrinol Diabetes 2006; 114:428–431.
- Tan PL, Teh J. MRI of the diabetic foot: differentiation of infection from neuropathic change. Br J Radiol 2007; 80:939–948.
- Ledermann HP, Morrison WB. Differential diagnosis of pedal osteomyelitis and diabetic neuroarthropathy: MR Imaging. Semin Musculoskelet Radiol 2005; 9:272–283.
- Höpfner S, Krolak C, Kessler S, et al. Preoperative imaging of Charcot neuroarthropathy in diabetic patients: comparison of ring PET, hybrid PET, and magnetic resonance imaging. Foot Ankle Int 2004; 25:890–895.
- Basu S, Chryssikos T, Houseni M, et al. Potential role of FDG PET in the setting of diabetic neuro-osteoarthropathy: can it differentiate uncomplicated Charcot’s neuroarthropathy from osteomyelitis and soft-tissue infection? Nucl Med Commun 2007; 28:465–472.
- Frykberg RG, Zgonis T, Armstrong DG, et al; American College of Foot and Ankle Surgeons. Diabetic foot disorders. A clinical practice guideline (2006 revision). J Foot Ankle Surg 2006; 45(suppl 5):S1–S66.
- Young MJ, Marshall A, Adams JE, Selby PL, Boulton AJ. Osteopenia, neurological dysfunction, and the development of Charcot neuroarthropathy. Diabetes Care 1995; 18:34–38.
Several weeks before coming to our orthopedic surgery clinic, a 53-year-old man presented to an emergency department because of pain, swelling, and redness in his right foot, which began 3 days before. He recalled no overt trauma, but he was jogging when he first noticed the pain, which he described as a constant aching and rated as high as 8 on a scale of 10.
At that time, he had no fever, chills, or night sweats, no cough, and no shortness of breath. About 10 years ago he was diagnosed with diabetes mellitus, for which he currently takes rosiglitazone (Avandia) 2 mg/day and metformin (Glucophage XR) 500 mg four tablets daily. He also takes ramipril (Altace) 10 mg/day for hypertension, as well as a daily multivitamin. He has a history of hyperlipidemia and a family history of diabetes mellitus and Parkinson disease. He has never been hospitalized and has never undergone surgery.
His blood glucose level was 239 mg/dL (normal 70–110), hemoglobin A1c 9.7% (normal 4%–6%), and white blood cell count 13.41 × 109/L (normal 4.5–11.0).
Based on that evaluation, the patient was admitted to the hospital with a diagnosis of cellulitis. He received intravenous antibiotics for 3 days and then was discharged with a prescription for oral antibiotics. He visited his primary care physician several times over the next 2 to 4 weeks and then was referred to our orthopedic surgery clinic for further evaluation. A neurologic evaluation in our clinic revealed a loss of protective sensation, contraction of the toes, and dryness, consistent with peripheral neuropathy. Given what we know so far, which is the most likely diagnosis?
DIFFERENTIAL DIAGNOSIS
While cellulitis may seem to be the likely diagnosis, if a patient with long-standing diabetes, a history of poor glycemic control, and peripheral neuropathy presents with a red, hot, swollen foot with no history of open ulceration, then Charcot neuroarthropathy should be at the top of the list in the differential diagnosis. Other possibilities include osteomyelitis, acute gout, cellulitis, abscess, neuropathic fracture, and deep venous thrombosis. However, if the patient has no open ulceration or history of an open wound, infection is probably not the culprit. Most diabetic foot infections begin with a direct inoculation through an opening in the skin, such as a diabetic neuropathic foot ulcer.
Further, in the case of cellulitis or deep venous thrombosis, the predominating feature would be asymmetric edema of the leg. Also, the location of the edema and ecchymosis in our patient—namely, the midfoot—leads to suspicion of an acute musculoskeletal injury, particularly Charcot neuroarthropathy of the midfoot and neuropathic fractures in the region of the ecchymotic second and third digits. Acute gout could be discounted because gout pain is severe, with rapid onset, and slowly improves even without treatment.
A COMPLICATION OF DIABETES
Charcot neuroarthropathy presents as a warm, swollen, erythematous foot and ankle, a picture that may be indistinguishable from that of infection. Most patients are in their 50s or 60s, and most present on an emergency basis; they often present late in the process, ie, 2 to 3 months after the initial symptoms, because the symptoms often are not painful.
This condition has been reported to occur with leprosy, syringomyelia, toxic exposure, poliomyelitis, rheumatoid arthritis, multiple sclerosis, congenital neuropathy, traumatic injury, and tertiary syphilis.1–4 Other conditions that reportedly trigger it include cellulitis, osteomyelitis, synovitis, surgery of the foot, and renal transplant surgery.5–7 However, today, the most common cause is diabetes mellitus.4,8
Other names for this condition are diabetic neuropathic osteoarthropathy and neuropathic arthropathy.
Current estimates of its prevalence range from 0.08% in the general diabetic population to 13% in high-risk diabetic patients.9
CHARCOT NEUROARTHROPATHY BEGINS WITH PERIPHERAL NEUROPATHY
The pathophysiologic mechanism of Charcot neuroarthropathy is not completely known, but it is thought to begin with peripheral neuropathy. Being insensitive to pain, patients may subject the joints of the foot (most commonly in the midfoot) to stress injuries that lead to the active Charcot process.10–12 About half of Charcot patients present with pain, as did our patient.
Although our patient remembered no trauma, he was physically active at the time he first noticed the symptoms.
Four stages of Charcot neuroarthropathy are recognized11–15:
Stage 0 (inflammation), also called Charcot in situ or pre-stage 1, is characterized by erythema, edema, and heat but no structural changes.11,12,14,15
Stage 1 (development) is characterized by bone resorption, bone fragmentation, and joint dislocation. The swelling, warmth, and redness persist, but there are also radiographic changes such as evidence of debris formation at the articular margins, osseous fragmentation, and joint disruption.
Stage 2 (coalescence) involves bony consolidation, osteosclerosis, and fusion after bony destruction. Absorption of small bone fragments, fusion of joints, and sclerosis of the bone are noticeable.
Stage 3 (reconstruction) is characterized by osteogenesis, decreased osteosclerosis, and progressive fusion.13 Healing and new bone formation occur. Decreased sclerosis and bony remodeling signify that the deformity (for example, subluxation, incongruity, and dislocation) is permanent.4
MISDIAGNOSIS IS COMMON
Charcot neuroarthropathy is an often overlooked complication in diabetic patients with peripheral neuropathy. A group of experts reported that 25% of patients referred to their facility who had Charcot neuroarthropathy had not received a correct diagnosis at the referring institution.16 The incorrect diagnoses included infection, gout, arthritis, fracture, venous insufficiency, and tumor.
Laboratory tests can narrow the differential diagnosis
There are no laboratory criteria for the diagnosis of Charcot neuroarthropathy and no hematologic markers, but laboratory testing can help narrow the differential diagnosis. Leukocytosis, an elevated C-reactive protein and erythrocyte sedimentation rate, and recent unexplained hyperglycemia suggest infection.17 However, unremarkable results on clinical tests in this population may not comprehensively exclude infection.
Our patient’s elevated white blood cell count confused the diagnosis. Further, when he was treated with antibiotics, he reported having less pain, although the edema and erythema continued.
Imaging studies
Although advanced imaging may help confirm the diagnosis of Charcot neuropathy in some patients, it is not always necessary.
Radiography. Radiographic findings are important in diagnosing Charcot neuroarthropathy, although they are less helpful in patients with stage 0 disease, such as our patient, in whom the condition has not yet progressed to fracture or dislocation. All foot and ankle radiographs should be taken in the weight-bearing position. Subtle changes may be missed if non-weight-bearing images are taken.
Magnetic resonance imaging (MRI) can show changes in stage 0, thus enabling treatment to be started sooner,18 and it is increasingly being recommended for diagnosing Charcot neuroarthropathy, especially in the early stages.3 Although bone scintigraphy and white blood cell scans have been traditionally advocated, MRI offers the highest diagnostic accuracy.19 Signs on MRI consistent with Charcot neuroarthropathy include ligamentous disruption, concomitant joint deformity, and the center of signal enhancement within joints and subchondral bone.20
MRI can also differentiate Charcot neuroarthropathy from transient regional osteoporosis. The latter has a different anatomic location and does not cause fractures and dislocations, and patients do not have a clinical history of pain.
Another condition MRI can identify is complex regional pain syndrome. In this condition, patients have no radiographic abnormalities except for periarticular osteopenia, but they may have severe pain out of proportion with the clinical appearance, and they may develop soft-tissue deformity in the late stages, which is not seen in Charcot neuroarthropathy.
Positron emission tomography (PET) with fluorine-18 fluorodeoxyglucose is also gaining support,21 especially when combined with computed tomography (CT). This PET-CT hybrid has better anatomic localization than PET alone.
PET-CT is very reliable for differentiating Charcot neuroarthropathy from osteomyelitis, a distinction that can be difficult to make when Charcot neuroarthropathy is complicated by adjacent loss of skin integrity. The sensitivity of PET-CT in this situation has been reported as 100%, and its sensitivity 93.8%.22
Patients with Charcot neuroarthropathy demonstrate a low-intensity diffuse uptake that is easily distinguishable from normal joints on visual examination of the images. In addition, the maximum standardized uptake value, a quantitative measurement, is low to intermediate in Charcot neuroarthropathy but significantly higher in osteomyelitis. In one study,22 the mean standardized uptake values were 0.42 in normal feet, 1.3 in Charcot neuroarthropathy, and 4.38 in osteomyelitis.
TREATMENT: IMMOBILIZATION, BISPHOSPHONATES, SURGERY
The goals of treatment for acute or quiescent Charcot neuroarthropathy should be to maintain or achieve structural stability of the foot and ankle, to prevent skin ulceration, and to preserve the plantigrade shape of the foot so that prescription footwear can be used.
Patient and family education is important for compliance with the regimen, particularly because patients with diabetic neuropathy lack the protective pain response.
Immobilization. A total-contact cast is worn until the redness, swelling, and heat subside, generally 8 to 12 weeks, after which the patient should use removable braces or a Charcot restraint orthotic walker for a total of 4 to 6 months of treatment.23 The cast is typically changed every 1 to 2 weeks as the swelling subsides to minimize irritation to the insensate limb.
Many physicians also recommend elastic stockings (eg, Stockinette) or an elastic tubular bandage (eg, Tubigrip) to reduce edema under the cast.
Bisphosphonates. Some clinicians also prescribe bisphosphonates in the early stages of treatment, as the bone mineral density of the affected foot is low.24 Unfortunately, while these drugs can significantly reduce the levels of bone turnover markers, temperature, and pain, evidence of clinical benefit such as an earlier return to ambulation or radiographic improvement is weak at best.
Surgery is reserved for severe ankle and midfoot deformities that are susceptible to skin ulcerations and that make braces and orthotic devices difficult to use.
TREATMENT OUTCOME
The patient’s condition resolved, with eventual multiplanar deformity and with widening of the midfoot and increased pressure points, particularly to the first ray. He is able to wear an extra-depth shoe, with a custom totalcontact inlay. He continues his profession as an attorney and goes about his normal daily activities; however, he is no longer able to golf and must limit his walking. He subsequently developed ulcerations to both feet, but they resolved with conservative wound care and surgical care. He is seen in the diabetic foot clinic every 6 to 8 weeks.
Several weeks before coming to our orthopedic surgery clinic, a 53-year-old man presented to an emergency department because of pain, swelling, and redness in his right foot, which began 3 days before. He recalled no overt trauma, but he was jogging when he first noticed the pain, which he described as a constant aching and rated as high as 8 on a scale of 10.
At that time, he had no fever, chills, or night sweats, no cough, and no shortness of breath. About 10 years ago he was diagnosed with diabetes mellitus, for which he currently takes rosiglitazone (Avandia) 2 mg/day and metformin (Glucophage XR) 500 mg four tablets daily. He also takes ramipril (Altace) 10 mg/day for hypertension, as well as a daily multivitamin. He has a history of hyperlipidemia and a family history of diabetes mellitus and Parkinson disease. He has never been hospitalized and has never undergone surgery.
His blood glucose level was 239 mg/dL (normal 70–110), hemoglobin A1c 9.7% (normal 4%–6%), and white blood cell count 13.41 × 109/L (normal 4.5–11.0).
Based on that evaluation, the patient was admitted to the hospital with a diagnosis of cellulitis. He received intravenous antibiotics for 3 days and then was discharged with a prescription for oral antibiotics. He visited his primary care physician several times over the next 2 to 4 weeks and then was referred to our orthopedic surgery clinic for further evaluation. A neurologic evaluation in our clinic revealed a loss of protective sensation, contraction of the toes, and dryness, consistent with peripheral neuropathy. Given what we know so far, which is the most likely diagnosis?
DIFFERENTIAL DIAGNOSIS
While cellulitis may seem to be the likely diagnosis, if a patient with long-standing diabetes, a history of poor glycemic control, and peripheral neuropathy presents with a red, hot, swollen foot with no history of open ulceration, then Charcot neuroarthropathy should be at the top of the list in the differential diagnosis. Other possibilities include osteomyelitis, acute gout, cellulitis, abscess, neuropathic fracture, and deep venous thrombosis. However, if the patient has no open ulceration or history of an open wound, infection is probably not the culprit. Most diabetic foot infections begin with a direct inoculation through an opening in the skin, such as a diabetic neuropathic foot ulcer.
Further, in the case of cellulitis or deep venous thrombosis, the predominating feature would be asymmetric edema of the leg. Also, the location of the edema and ecchymosis in our patient—namely, the midfoot—leads to suspicion of an acute musculoskeletal injury, particularly Charcot neuroarthropathy of the midfoot and neuropathic fractures in the region of the ecchymotic second and third digits. Acute gout could be discounted because gout pain is severe, with rapid onset, and slowly improves even without treatment.
A COMPLICATION OF DIABETES
Charcot neuroarthropathy presents as a warm, swollen, erythematous foot and ankle, a picture that may be indistinguishable from that of infection. Most patients are in their 50s or 60s, and most present on an emergency basis; they often present late in the process, ie, 2 to 3 months after the initial symptoms, because the symptoms often are not painful.
This condition has been reported to occur with leprosy, syringomyelia, toxic exposure, poliomyelitis, rheumatoid arthritis, multiple sclerosis, congenital neuropathy, traumatic injury, and tertiary syphilis.1–4 Other conditions that reportedly trigger it include cellulitis, osteomyelitis, synovitis, surgery of the foot, and renal transplant surgery.5–7 However, today, the most common cause is diabetes mellitus.4,8
Other names for this condition are diabetic neuropathic osteoarthropathy and neuropathic arthropathy.
Current estimates of its prevalence range from 0.08% in the general diabetic population to 13% in high-risk diabetic patients.9
CHARCOT NEUROARTHROPATHY BEGINS WITH PERIPHERAL NEUROPATHY
The pathophysiologic mechanism of Charcot neuroarthropathy is not completely known, but it is thought to begin with peripheral neuropathy. Being insensitive to pain, patients may subject the joints of the foot (most commonly in the midfoot) to stress injuries that lead to the active Charcot process.10–12 About half of Charcot patients present with pain, as did our patient.
Although our patient remembered no trauma, he was physically active at the time he first noticed the symptoms.
Four stages of Charcot neuroarthropathy are recognized11–15:
Stage 0 (inflammation), also called Charcot in situ or pre-stage 1, is characterized by erythema, edema, and heat but no structural changes.11,12,14,15
Stage 1 (development) is characterized by bone resorption, bone fragmentation, and joint dislocation. The swelling, warmth, and redness persist, but there are also radiographic changes such as evidence of debris formation at the articular margins, osseous fragmentation, and joint disruption.
Stage 2 (coalescence) involves bony consolidation, osteosclerosis, and fusion after bony destruction. Absorption of small bone fragments, fusion of joints, and sclerosis of the bone are noticeable.
Stage 3 (reconstruction) is characterized by osteogenesis, decreased osteosclerosis, and progressive fusion.13 Healing and new bone formation occur. Decreased sclerosis and bony remodeling signify that the deformity (for example, subluxation, incongruity, and dislocation) is permanent.4
MISDIAGNOSIS IS COMMON
Charcot neuroarthropathy is an often overlooked complication in diabetic patients with peripheral neuropathy. A group of experts reported that 25% of patients referred to their facility who had Charcot neuroarthropathy had not received a correct diagnosis at the referring institution.16 The incorrect diagnoses included infection, gout, arthritis, fracture, venous insufficiency, and tumor.
Laboratory tests can narrow the differential diagnosis
There are no laboratory criteria for the diagnosis of Charcot neuroarthropathy and no hematologic markers, but laboratory testing can help narrow the differential diagnosis. Leukocytosis, an elevated C-reactive protein and erythrocyte sedimentation rate, and recent unexplained hyperglycemia suggest infection.17 However, unremarkable results on clinical tests in this population may not comprehensively exclude infection.
Our patient’s elevated white blood cell count confused the diagnosis. Further, when he was treated with antibiotics, he reported having less pain, although the edema and erythema continued.
Imaging studies
Although advanced imaging may help confirm the diagnosis of Charcot neuropathy in some patients, it is not always necessary.
Radiography. Radiographic findings are important in diagnosing Charcot neuroarthropathy, although they are less helpful in patients with stage 0 disease, such as our patient, in whom the condition has not yet progressed to fracture or dislocation. All foot and ankle radiographs should be taken in the weight-bearing position. Subtle changes may be missed if non-weight-bearing images are taken.
Magnetic resonance imaging (MRI) can show changes in stage 0, thus enabling treatment to be started sooner,18 and it is increasingly being recommended for diagnosing Charcot neuroarthropathy, especially in the early stages.3 Although bone scintigraphy and white blood cell scans have been traditionally advocated, MRI offers the highest diagnostic accuracy.19 Signs on MRI consistent with Charcot neuroarthropathy include ligamentous disruption, concomitant joint deformity, and the center of signal enhancement within joints and subchondral bone.20
MRI can also differentiate Charcot neuroarthropathy from transient regional osteoporosis. The latter has a different anatomic location and does not cause fractures and dislocations, and patients do not have a clinical history of pain.
Another condition MRI can identify is complex regional pain syndrome. In this condition, patients have no radiographic abnormalities except for periarticular osteopenia, but they may have severe pain out of proportion with the clinical appearance, and they may develop soft-tissue deformity in the late stages, which is not seen in Charcot neuroarthropathy.
Positron emission tomography (PET) with fluorine-18 fluorodeoxyglucose is also gaining support,21 especially when combined with computed tomography (CT). This PET-CT hybrid has better anatomic localization than PET alone.
PET-CT is very reliable for differentiating Charcot neuroarthropathy from osteomyelitis, a distinction that can be difficult to make when Charcot neuroarthropathy is complicated by adjacent loss of skin integrity. The sensitivity of PET-CT in this situation has been reported as 100%, and its sensitivity 93.8%.22
Patients with Charcot neuroarthropathy demonstrate a low-intensity diffuse uptake that is easily distinguishable from normal joints on visual examination of the images. In addition, the maximum standardized uptake value, a quantitative measurement, is low to intermediate in Charcot neuroarthropathy but significantly higher in osteomyelitis. In one study,22 the mean standardized uptake values were 0.42 in normal feet, 1.3 in Charcot neuroarthropathy, and 4.38 in osteomyelitis.
TREATMENT: IMMOBILIZATION, BISPHOSPHONATES, SURGERY
The goals of treatment for acute or quiescent Charcot neuroarthropathy should be to maintain or achieve structural stability of the foot and ankle, to prevent skin ulceration, and to preserve the plantigrade shape of the foot so that prescription footwear can be used.
Patient and family education is important for compliance with the regimen, particularly because patients with diabetic neuropathy lack the protective pain response.
Immobilization. A total-contact cast is worn until the redness, swelling, and heat subside, generally 8 to 12 weeks, after which the patient should use removable braces or a Charcot restraint orthotic walker for a total of 4 to 6 months of treatment.23 The cast is typically changed every 1 to 2 weeks as the swelling subsides to minimize irritation to the insensate limb.
Many physicians also recommend elastic stockings (eg, Stockinette) or an elastic tubular bandage (eg, Tubigrip) to reduce edema under the cast.
Bisphosphonates. Some clinicians also prescribe bisphosphonates in the early stages of treatment, as the bone mineral density of the affected foot is low.24 Unfortunately, while these drugs can significantly reduce the levels of bone turnover markers, temperature, and pain, evidence of clinical benefit such as an earlier return to ambulation or radiographic improvement is weak at best.
Surgery is reserved for severe ankle and midfoot deformities that are susceptible to skin ulcerations and that make braces and orthotic devices difficult to use.
TREATMENT OUTCOME
The patient’s condition resolved, with eventual multiplanar deformity and with widening of the midfoot and increased pressure points, particularly to the first ray. He is able to wear an extra-depth shoe, with a custom totalcontact inlay. He continues his profession as an attorney and goes about his normal daily activities; however, he is no longer able to golf and must limit his walking. He subsequently developed ulcerations to both feet, but they resolved with conservative wound care and surgical care. He is seen in the diabetic foot clinic every 6 to 8 weeks.
- Gupta R. A short history of neuropathic arthropathy. Clin Orthop Relat Res 1993; 296:43–49.
- Johnson JT. Neuropathic fractures and joint injuries. Pathogenesis and rationale of prevention and treatment. J Bone Joint Surg Am 1967; 49:1–30.
- Sanders LJ, Frykberg RG. The Charcot Foot (Pied de Charcot). In:Bowker JH, Pfeifer MA, editors. Levin and O’Neal’s The Diabetic Foot. 7th ed. Philadelphia, PA: Mosby Elsevier; 2008:257–283.
- Wukich DK, Sung W. Charcot arthropathy of the foot and ankle: modern concepts and management review. J Diabetes Complications 2009; 23:409–426.
- Armstrong DG, Todd WF, Lavery LA, Harkless LB, Bushman TR. The natural history of acute Charcot’s arthropathy in a diabetic foot specialty clinic. J Am Podiatr Med Assoc 1997; 87:272–278.
- Jeffcoate WJ. Theories concerning the pathogenesis of the acute Charcot foot suggest future therapy. Curr Diab Rep 2005; 5:430–435.
- Matricali GA, Bammens B, Kuypers D, Flour M, Mathieu C. High rate of Charcot foot attacks early after simultaneous pancreas-kidney transplantation. Transplantation 2007; 83:245–246.
- Miller DS, Lichtman WF. Diabetic neuropathic arthropathy of feet; summary report of seventeen cases. AMA Arch Surg 1955; 70:513–518.
- Frykberg RG, Belczyk R. Epidemiology of the Charcot foot. Clin Podiatr Med Surg 2008; 25:17–28,
- Chantelau E. The perils of procrastination: effects of early vs delayed detection and treatment of incipient Charcot fracture. Diabet Med 2005; 22:1707–1712.
- Schon LC, Marks RM. The management of neuroarthropathic fracture-dislocations in the diabetic patient. Orthop Clin North Am 1995; 26:375–392.
- Sella EJ, Barrette C. Staging of Charcot neuroarthropathy along the medial column of the foot in the diabetic patient. J Foot Ankle Surg 1999; 38:34–40.
- Eichenholtz SN. Charcot Joints. Springfield, IL: CC Thomas; 1966.
- Shibata T, Tada K, Hashizume C. The results of arthrodesis of the ankle for leprotic neuroarthropathy. J Bone Joint Surg Am 1990; 72:749–756.
- Yu GV, Hudson JR. Evaluation and treatment of stage 0 Charcot’s neuroarthropathy of the foot and ankle. J Am Podiatr Med Assoc 2002; 92:210–220.
- Myerson MS, Henderson MR, Saxby T, Short KW. Management of midfoot diabetic neuroarthropathy. Foot Ankle Int 1994; 15:233–241.
- Judge MS. Using serologic screening to Identify and monitor at-risk Charcot patients. Podiatry Today Magazine 2004; 17:75–82.
- Chantelau E, Poll LW. Evaluation of the diabetic Charcot foot by MR imaging or plain radiography—an observational study. Exp Clin Endocrinol Diabetes 2006; 114:428–431.
- Tan PL, Teh J. MRI of the diabetic foot: differentiation of infection from neuropathic change. Br J Radiol 2007; 80:939–948.
- Ledermann HP, Morrison WB. Differential diagnosis of pedal osteomyelitis and diabetic neuroarthropathy: MR Imaging. Semin Musculoskelet Radiol 2005; 9:272–283.
- Höpfner S, Krolak C, Kessler S, et al. Preoperative imaging of Charcot neuroarthropathy in diabetic patients: comparison of ring PET, hybrid PET, and magnetic resonance imaging. Foot Ankle Int 2004; 25:890–895.
- Basu S, Chryssikos T, Houseni M, et al. Potential role of FDG PET in the setting of diabetic neuro-osteoarthropathy: can it differentiate uncomplicated Charcot’s neuroarthropathy from osteomyelitis and soft-tissue infection? Nucl Med Commun 2007; 28:465–472.
- Frykberg RG, Zgonis T, Armstrong DG, et al; American College of Foot and Ankle Surgeons. Diabetic foot disorders. A clinical practice guideline (2006 revision). J Foot Ankle Surg 2006; 45(suppl 5):S1–S66.
- Young MJ, Marshall A, Adams JE, Selby PL, Boulton AJ. Osteopenia, neurological dysfunction, and the development of Charcot neuroarthropathy. Diabetes Care 1995; 18:34–38.
- Gupta R. A short history of neuropathic arthropathy. Clin Orthop Relat Res 1993; 296:43–49.
- Johnson JT. Neuropathic fractures and joint injuries. Pathogenesis and rationale of prevention and treatment. J Bone Joint Surg Am 1967; 49:1–30.
- Sanders LJ, Frykberg RG. The Charcot Foot (Pied de Charcot). In:Bowker JH, Pfeifer MA, editors. Levin and O’Neal’s The Diabetic Foot. 7th ed. Philadelphia, PA: Mosby Elsevier; 2008:257–283.
- Wukich DK, Sung W. Charcot arthropathy of the foot and ankle: modern concepts and management review. J Diabetes Complications 2009; 23:409–426.
- Armstrong DG, Todd WF, Lavery LA, Harkless LB, Bushman TR. The natural history of acute Charcot’s arthropathy in a diabetic foot specialty clinic. J Am Podiatr Med Assoc 1997; 87:272–278.
- Jeffcoate WJ. Theories concerning the pathogenesis of the acute Charcot foot suggest future therapy. Curr Diab Rep 2005; 5:430–435.
- Matricali GA, Bammens B, Kuypers D, Flour M, Mathieu C. High rate of Charcot foot attacks early after simultaneous pancreas-kidney transplantation. Transplantation 2007; 83:245–246.
- Miller DS, Lichtman WF. Diabetic neuropathic arthropathy of feet; summary report of seventeen cases. AMA Arch Surg 1955; 70:513–518.
- Frykberg RG, Belczyk R. Epidemiology of the Charcot foot. Clin Podiatr Med Surg 2008; 25:17–28,
- Chantelau E. The perils of procrastination: effects of early vs delayed detection and treatment of incipient Charcot fracture. Diabet Med 2005; 22:1707–1712.
- Schon LC, Marks RM. The management of neuroarthropathic fracture-dislocations in the diabetic patient. Orthop Clin North Am 1995; 26:375–392.
- Sella EJ, Barrette C. Staging of Charcot neuroarthropathy along the medial column of the foot in the diabetic patient. J Foot Ankle Surg 1999; 38:34–40.
- Eichenholtz SN. Charcot Joints. Springfield, IL: CC Thomas; 1966.
- Shibata T, Tada K, Hashizume C. The results of arthrodesis of the ankle for leprotic neuroarthropathy. J Bone Joint Surg Am 1990; 72:749–756.
- Yu GV, Hudson JR. Evaluation and treatment of stage 0 Charcot’s neuroarthropathy of the foot and ankle. J Am Podiatr Med Assoc 2002; 92:210–220.
- Myerson MS, Henderson MR, Saxby T, Short KW. Management of midfoot diabetic neuroarthropathy. Foot Ankle Int 1994; 15:233–241.
- Judge MS. Using serologic screening to Identify and monitor at-risk Charcot patients. Podiatry Today Magazine 2004; 17:75–82.
- Chantelau E, Poll LW. Evaluation of the diabetic Charcot foot by MR imaging or plain radiography—an observational study. Exp Clin Endocrinol Diabetes 2006; 114:428–431.
- Tan PL, Teh J. MRI of the diabetic foot: differentiation of infection from neuropathic change. Br J Radiol 2007; 80:939–948.
- Ledermann HP, Morrison WB. Differential diagnosis of pedal osteomyelitis and diabetic neuroarthropathy: MR Imaging. Semin Musculoskelet Radiol 2005; 9:272–283.
- Höpfner S, Krolak C, Kessler S, et al. Preoperative imaging of Charcot neuroarthropathy in diabetic patients: comparison of ring PET, hybrid PET, and magnetic resonance imaging. Foot Ankle Int 2004; 25:890–895.
- Basu S, Chryssikos T, Houseni M, et al. Potential role of FDG PET in the setting of diabetic neuro-osteoarthropathy: can it differentiate uncomplicated Charcot’s neuroarthropathy from osteomyelitis and soft-tissue infection? Nucl Med Commun 2007; 28:465–472.
- Frykberg RG, Zgonis T, Armstrong DG, et al; American College of Foot and Ankle Surgeons. Diabetic foot disorders. A clinical practice guideline (2006 revision). J Foot Ankle Surg 2006; 45(suppl 5):S1–S66.
- Young MJ, Marshall A, Adams JE, Selby PL, Boulton AJ. Osteopenia, neurological dysfunction, and the development of Charcot neuroarthropathy. Diabetes Care 1995; 18:34–38.
KEY POINTS
- One must pay particular attention to the history in diabetic patients and assess the risk of diabetic foot complications.
- Without the presence or history of an open ulceration, infection is rare.
- Paramount to the treatment of this condition are the avoidance of weight-bearing and the immediate referral to a foot and ankle specialist. Prevention, suspicion, early diagnosis, and protection of the involved foot preserve the ability to walk and quality of life.