User login
Three trials cement embolectomy for acute ischemic stroke
NASHVILLE, TENN. – Treatment of selected patients with acute ischemic stroke underwent a dramatic, sudden shift with reports from three randomized, controlled trials that showed substantial added benefit and no incremental risk with the use of catheter-based embolic retrieval to open blocked intracerebral arteries when performed on top of standard thrombolytic therapy.
The three studies, each run independently and based in different countries, supported the results first reported last October and published online in December (N. Engl. J. Med. 2015;372:11-20) from the MR CLEAN (Multicenter Randomized Clinical Trial of Endovascular Treatment for Acute Ischemic Stroke in the Netherlands) study. These were the first contemporary trial results to show a jump in functional outcomes with use of a stent retriever catheter to pluck out the occluding embolus from an artery in the stroke patient’s brain to restore normal blood flow.
All three of the newly-reported studies stopped before reaching their prespecified enrollment levels because of overwhelming evidence for embolectomy’s incremental efficacy.
With four reports from prospective, randomized trials showing similar benefits and no added harm to patients, experts at the International Stroke Conference uniformly anointed catheter-based embolectomy the new standard of care for the small percentage of acute, ischemic-stroke patients who present with proximal, large-artery obstructions and also match the other strict clinical and imaging inclusion and exclusion criteria used in the studies.
“Starting now, in patients with an acute ischemic stroke due to proximal vessel occlusion, rapid endovascular treatment using a retrieval stent is the standard of care,” Dr. Mayank Goyal declared from the plenary-session podium. He is a professor of diagnostic imaging at the University of Calgary (Canada) and an investigator in two of the three trials presented at the conference, which was sponsored by the American Heart Association.
“Today the world changed. We are now in a new era, the era of highly-effective intravascular recanalization therapy,” said Dr. Jeffrey L. Saver, professor of neurology and director of the Stroke Center at the University of California, Los Angeles, and lead investigator for one of the new studies.
In three of the four studies, the researchers did not report specific numbers on how selective they were in focusing in on the ischemic stroke patients most likely to benefit from this treatment, but the one study that did, EXTEND-IA (Extending the Time for Thrombolysis in Emergency Neurological Deficits – Intra-Arterial), run at nine Australian centers and one in New Zealand, showed the extensive winnowing that occurred. Of 7,796 patients with an acute ischemic stroke who initially presented, 1,044 (13%) were eligible to receive thrombolytic therapy (alteplase in this study). And from among these 1,044 patients, a mere 70 – less than 1% of the initial group – were deemed eligible for randomization into the embolectomy trial. The top three reasons for exclusion of patients who qualified for thrombolytic treatment from the trial was an absence of a major-vessel occlusion (45% of the excluded patients), presentation outside of the times when enrollment personnel were available (22%), and poor premorbid function (16%).
But subgroup analyses in three of the four studies (EXTEND-IA with a total of 70 patients was too small for subgroup analyses) showed no subgroup of patients who failed to benefit from embolectomy, including elderly patients who in some cases were nonagenarians.
The unusual confluence of having four major trials showing remarkably consistent results meant that the stroke experts gathered at the meeting focused their attention not on whether stent retrievers should now be widely and routinely used in appropriate patients but instead on how this technology will roll out worldwide.
“From here on out we are obligated to treat patients with this technology at centers that can do this, and we are obligated to have more centers that can provide it,” said Dr. Kyra J. Becker, professor of neurology and neurological surgery and codirector of the Stroke Center at the University of Washington, Seattle. Dr. Becker had no involvement in any of the stent retriever trials. “I had been a doubter of this technology,” primarily because results reported at the International Stroke Conference a couple of years ago failed to prove the efficacy of clot retrieval in ischemic stroke patients, she noted. “Our ability to select appropriate patients and do it in a timely fashion hadn’t gotten to where it had to be until now,” Dr. Becker said in an interview.
“We only enrolled patients with blockages, we treated them quickly, and we used much better devices to open their arteries,” Dr. Saver added, explaining why the new studies succeeded when earlier studies had not.
The trial led by Dr. Saver, SWIFT-PRIME (SOLITAIRE™ FR With the Intention for Thrombectomy as Primary Endovascular Treatment for Acute Ischemic Stroke), enrolled 195 patients at 39 sites in the United States and in Europe. At 90 days after treatment, 59 patients (60%) among those treated with thrombolysis plus embolectomy had a modified Rankin Scale score of 0-2, compared with 33 patients (36%) among those treated only with thrombolysis (in this trial intravenous treatment with tissue plasminogen activator), a highly significant difference for the study’s primary endpoint.
“For every two and half patients treated, one more patient had a better disability outcome, and for every four patients treated, one more patient was independent at long-term follow-up,” Dr. Saver said. Safety measures were similar among patients in the study’s two arms.
The EXTEND-IA results showed a 90-day modified Rankin Scale score of 0-2 in 52% of the embolectomy patients, compared with 28% of those treated only with thrombolysis. The study’s co–primary endpoints were median level of reperfusion at 24 hours after treatment, 100% with embolectomy and 37% with thrombolysis only, and early neurologic recovery, defined as at least an 8-point drop from the baseline in the National Institutes of Health Stroke Scale score or a score of 0 or 1 when assessed 3 days after treatment. Patients met this second endpoint at an 80% rate with embolectomy and a 37% rate with thrombolysis only. Results of EXTEND-IA appeared in an article published online concurrently with the meeting report (N. Engl J. Med. 2015 Feb. 11 [doi:10.1056/NEJMoa1414792]).
The third, and largest, of the three studies presented at the conference, ESCAPE (Endovascular Treatment for Small Core and Anterior Circulation Proximal Occlusion with Emphasis on Minimizing CT to Recanalization Times), enrolled 316 patients at 11 centers in Canada, 6 in the United States, 3 in South Korea, and 1 in Ireland. After 90 days, 53% of patients in the embolectomy arm had achieved a modified Rankin Scale score of 0-2, this study’s primary endpoint, compared with 29% of patients in the thrombolysis-only arm (treatment with alteplase). These results also appeared in an article published online concurrently with the conference report (N. Engl. J. Med. 2015 Feb. 11 [doi:10.1056/NEJMoa1414905]).
SWIFT PRIME was sponsored by Covidien, which markets the stent retriever used in the study. Dr. Saver and Dr. Goyal are consultants to Covidien. EXTEND-IA used stent retrievers provided by Covidien. ESCAPE received a grant from Covidien. Dr. Becker had no relevant disclosures.
On Twitter @mitchelzoler
Many U.S. centers have interventionalists who already perform endovascular treatments within intracerebral arteries, but the issue is can they do this form of embolectomy in the high-quality, highly-reliable, rapid way that it was done in these trials? Stent-retriever catheters are relatively straightforward to use by operators who are experienced doing vascular procedures in the brain, but they don’t deliver this treatment by themselves. You need a team that is focused on doing it quickly, and that will be the kind of training we’ll need to roll out this treatment broadly. We achieved it for stroke thrombolytic treatment through the Target Stroke program (JAMA 2014;311:1632-40), so we know that we can achieve this sort of goal. Delivering embolectomy requires more people and more technology than thrombolysis, but it is not rocket science; it just needs a system.

|
| Dr. Lee H. Schwamm |
Embolectomy will not replace routine thrombolysis treatment; it will piggyback on top of it. The percentage of patients with a proximal occlusion in a large artery is relatively small. The results we have seen suggest that using embolectomy plus thrombolysis has no adverse-effect downside, compared with thrombolysis alone. Once routine use of embolectomy becomes established, we can directly compare catheter treatment only against combined embolectomy and thrombolysis. My impression today is that what we’d compare is transporting stroke patients directly to a center that can perform embolectomy against taking patients to the closest center that can treat them with thrombolysis and then transporting them to the center that performs embolectomy.
The results of these three new studies plus the previously-reported results from MR CLEAN are not exactly a game changer, because many centers were already performing embolectomy but in a limited way. Now we have the data to give us confidence to do it routinely and to know which patients to select for embolectomy. Because many centers are already doing this, it will not take 5 years to diffuse the technology.
Embolectomy is already a treatment cited in the guidelines, but now it will be a level 1A recommendation.
The significance of the new reports is that they will have a dramatic impact on public health systems and in the triage of patients with stroke. It will affect how patients get triaged, and will allow us to identify which patients should go to which centers. I believe we will soon develop clinical examination tools that will allow prehospital providers to discern patients with mild strokes who can go to the nearest center that can administer thrombolysis and which patients need to go to comprehensive centers that can perform embolectomy. We now need to do what we did for thrombolysis, and help centers develop the expertise to do embolectomy as a team and to shave minutes off the delivery at every step of the process. It’s clear that it is the time from stroke onset to getting the artery open that is the key to improved patient outcomes.
If I have my way, we will launch later this year a big effort to focus on improving embolectomy delivery. Now that we know for certain that it works we need to turn the crank and make sure that as many patients as possible who qualify get this treatment.
Dr. Lee H. Schwamm is professor of neurology at Harvard Medical School, and director of acute stroke services at Massachusetts General Hospital, both in Boston. He is a consultant to Penumbra and has received research support from Genentech. He made these comments in an interview.
Many U.S. centers have interventionalists who already perform endovascular treatments within intracerebral arteries, but the issue is can they do this form of embolectomy in the high-quality, highly-reliable, rapid way that it was done in these trials? Stent-retriever catheters are relatively straightforward to use by operators who are experienced doing vascular procedures in the brain, but they don’t deliver this treatment by themselves. You need a team that is focused on doing it quickly, and that will be the kind of training we’ll need to roll out this treatment broadly. We achieved it for stroke thrombolytic treatment through the Target Stroke program (JAMA 2014;311:1632-40), so we know that we can achieve this sort of goal. Delivering embolectomy requires more people and more technology than thrombolysis, but it is not rocket science; it just needs a system.

|
| Dr. Lee H. Schwamm |
Embolectomy will not replace routine thrombolysis treatment; it will piggyback on top of it. The percentage of patients with a proximal occlusion in a large artery is relatively small. The results we have seen suggest that using embolectomy plus thrombolysis has no adverse-effect downside, compared with thrombolysis alone. Once routine use of embolectomy becomes established, we can directly compare catheter treatment only against combined embolectomy and thrombolysis. My impression today is that what we’d compare is transporting stroke patients directly to a center that can perform embolectomy against taking patients to the closest center that can treat them with thrombolysis and then transporting them to the center that performs embolectomy.
The results of these three new studies plus the previously-reported results from MR CLEAN are not exactly a game changer, because many centers were already performing embolectomy but in a limited way. Now we have the data to give us confidence to do it routinely and to know which patients to select for embolectomy. Because many centers are already doing this, it will not take 5 years to diffuse the technology.
Embolectomy is already a treatment cited in the guidelines, but now it will be a level 1A recommendation.
The significance of the new reports is that they will have a dramatic impact on public health systems and in the triage of patients with stroke. It will affect how patients get triaged, and will allow us to identify which patients should go to which centers. I believe we will soon develop clinical examination tools that will allow prehospital providers to discern patients with mild strokes who can go to the nearest center that can administer thrombolysis and which patients need to go to comprehensive centers that can perform embolectomy. We now need to do what we did for thrombolysis, and help centers develop the expertise to do embolectomy as a team and to shave minutes off the delivery at every step of the process. It’s clear that it is the time from stroke onset to getting the artery open that is the key to improved patient outcomes.
If I have my way, we will launch later this year a big effort to focus on improving embolectomy delivery. Now that we know for certain that it works we need to turn the crank and make sure that as many patients as possible who qualify get this treatment.
Dr. Lee H. Schwamm is professor of neurology at Harvard Medical School, and director of acute stroke services at Massachusetts General Hospital, both in Boston. He is a consultant to Penumbra and has received research support from Genentech. He made these comments in an interview.
Many U.S. centers have interventionalists who already perform endovascular treatments within intracerebral arteries, but the issue is can they do this form of embolectomy in the high-quality, highly-reliable, rapid way that it was done in these trials? Stent-retriever catheters are relatively straightforward to use by operators who are experienced doing vascular procedures in the brain, but they don’t deliver this treatment by themselves. You need a team that is focused on doing it quickly, and that will be the kind of training we’ll need to roll out this treatment broadly. We achieved it for stroke thrombolytic treatment through the Target Stroke program (JAMA 2014;311:1632-40), so we know that we can achieve this sort of goal. Delivering embolectomy requires more people and more technology than thrombolysis, but it is not rocket science; it just needs a system.

|
| Dr. Lee H. Schwamm |
Embolectomy will not replace routine thrombolysis treatment; it will piggyback on top of it. The percentage of patients with a proximal occlusion in a large artery is relatively small. The results we have seen suggest that using embolectomy plus thrombolysis has no adverse-effect downside, compared with thrombolysis alone. Once routine use of embolectomy becomes established, we can directly compare catheter treatment only against combined embolectomy and thrombolysis. My impression today is that what we’d compare is transporting stroke patients directly to a center that can perform embolectomy against taking patients to the closest center that can treat them with thrombolysis and then transporting them to the center that performs embolectomy.
The results of these three new studies plus the previously-reported results from MR CLEAN are not exactly a game changer, because many centers were already performing embolectomy but in a limited way. Now we have the data to give us confidence to do it routinely and to know which patients to select for embolectomy. Because many centers are already doing this, it will not take 5 years to diffuse the technology.
Embolectomy is already a treatment cited in the guidelines, but now it will be a level 1A recommendation.
The significance of the new reports is that they will have a dramatic impact on public health systems and in the triage of patients with stroke. It will affect how patients get triaged, and will allow us to identify which patients should go to which centers. I believe we will soon develop clinical examination tools that will allow prehospital providers to discern patients with mild strokes who can go to the nearest center that can administer thrombolysis and which patients need to go to comprehensive centers that can perform embolectomy. We now need to do what we did for thrombolysis, and help centers develop the expertise to do embolectomy as a team and to shave minutes off the delivery at every step of the process. It’s clear that it is the time from stroke onset to getting the artery open that is the key to improved patient outcomes.
If I have my way, we will launch later this year a big effort to focus on improving embolectomy delivery. Now that we know for certain that it works we need to turn the crank and make sure that as many patients as possible who qualify get this treatment.
Dr. Lee H. Schwamm is professor of neurology at Harvard Medical School, and director of acute stroke services at Massachusetts General Hospital, both in Boston. He is a consultant to Penumbra and has received research support from Genentech. He made these comments in an interview.
NASHVILLE, TENN. – Treatment of selected patients with acute ischemic stroke underwent a dramatic, sudden shift with reports from three randomized, controlled trials that showed substantial added benefit and no incremental risk with the use of catheter-based embolic retrieval to open blocked intracerebral arteries when performed on top of standard thrombolytic therapy.
The three studies, each run independently and based in different countries, supported the results first reported last October and published online in December (N. Engl. J. Med. 2015;372:11-20) from the MR CLEAN (Multicenter Randomized Clinical Trial of Endovascular Treatment for Acute Ischemic Stroke in the Netherlands) study. These were the first contemporary trial results to show a jump in functional outcomes with use of a stent retriever catheter to pluck out the occluding embolus from an artery in the stroke patient’s brain to restore normal blood flow.
All three of the newly-reported studies stopped before reaching their prespecified enrollment levels because of overwhelming evidence for embolectomy’s incremental efficacy.
With four reports from prospective, randomized trials showing similar benefits and no added harm to patients, experts at the International Stroke Conference uniformly anointed catheter-based embolectomy the new standard of care for the small percentage of acute, ischemic-stroke patients who present with proximal, large-artery obstructions and also match the other strict clinical and imaging inclusion and exclusion criteria used in the studies.
“Starting now, in patients with an acute ischemic stroke due to proximal vessel occlusion, rapid endovascular treatment using a retrieval stent is the standard of care,” Dr. Mayank Goyal declared from the plenary-session podium. He is a professor of diagnostic imaging at the University of Calgary (Canada) and an investigator in two of the three trials presented at the conference, which was sponsored by the American Heart Association.
“Today the world changed. We are now in a new era, the era of highly-effective intravascular recanalization therapy,” said Dr. Jeffrey L. Saver, professor of neurology and director of the Stroke Center at the University of California, Los Angeles, and lead investigator for one of the new studies.
In three of the four studies, the researchers did not report specific numbers on how selective they were in focusing in on the ischemic stroke patients most likely to benefit from this treatment, but the one study that did, EXTEND-IA (Extending the Time for Thrombolysis in Emergency Neurological Deficits – Intra-Arterial), run at nine Australian centers and one in New Zealand, showed the extensive winnowing that occurred. Of 7,796 patients with an acute ischemic stroke who initially presented, 1,044 (13%) were eligible to receive thrombolytic therapy (alteplase in this study). And from among these 1,044 patients, a mere 70 – less than 1% of the initial group – were deemed eligible for randomization into the embolectomy trial. The top three reasons for exclusion of patients who qualified for thrombolytic treatment from the trial was an absence of a major-vessel occlusion (45% of the excluded patients), presentation outside of the times when enrollment personnel were available (22%), and poor premorbid function (16%).
But subgroup analyses in three of the four studies (EXTEND-IA with a total of 70 patients was too small for subgroup analyses) showed no subgroup of patients who failed to benefit from embolectomy, including elderly patients who in some cases were nonagenarians.
The unusual confluence of having four major trials showing remarkably consistent results meant that the stroke experts gathered at the meeting focused their attention not on whether stent retrievers should now be widely and routinely used in appropriate patients but instead on how this technology will roll out worldwide.
“From here on out we are obligated to treat patients with this technology at centers that can do this, and we are obligated to have more centers that can provide it,” said Dr. Kyra J. Becker, professor of neurology and neurological surgery and codirector of the Stroke Center at the University of Washington, Seattle. Dr. Becker had no involvement in any of the stent retriever trials. “I had been a doubter of this technology,” primarily because results reported at the International Stroke Conference a couple of years ago failed to prove the efficacy of clot retrieval in ischemic stroke patients, she noted. “Our ability to select appropriate patients and do it in a timely fashion hadn’t gotten to where it had to be until now,” Dr. Becker said in an interview.
“We only enrolled patients with blockages, we treated them quickly, and we used much better devices to open their arteries,” Dr. Saver added, explaining why the new studies succeeded when earlier studies had not.
The trial led by Dr. Saver, SWIFT-PRIME (SOLITAIRE™ FR With the Intention for Thrombectomy as Primary Endovascular Treatment for Acute Ischemic Stroke), enrolled 195 patients at 39 sites in the United States and in Europe. At 90 days after treatment, 59 patients (60%) among those treated with thrombolysis plus embolectomy had a modified Rankin Scale score of 0-2, compared with 33 patients (36%) among those treated only with thrombolysis (in this trial intravenous treatment with tissue plasminogen activator), a highly significant difference for the study’s primary endpoint.
“For every two and half patients treated, one more patient had a better disability outcome, and for every four patients treated, one more patient was independent at long-term follow-up,” Dr. Saver said. Safety measures were similar among patients in the study’s two arms.
The EXTEND-IA results showed a 90-day modified Rankin Scale score of 0-2 in 52% of the embolectomy patients, compared with 28% of those treated only with thrombolysis. The study’s co–primary endpoints were median level of reperfusion at 24 hours after treatment, 100% with embolectomy and 37% with thrombolysis only, and early neurologic recovery, defined as at least an 8-point drop from the baseline in the National Institutes of Health Stroke Scale score or a score of 0 or 1 when assessed 3 days after treatment. Patients met this second endpoint at an 80% rate with embolectomy and a 37% rate with thrombolysis only. Results of EXTEND-IA appeared in an article published online concurrently with the meeting report (N. Engl J. Med. 2015 Feb. 11 [doi:10.1056/NEJMoa1414792]).
The third, and largest, of the three studies presented at the conference, ESCAPE (Endovascular Treatment for Small Core and Anterior Circulation Proximal Occlusion with Emphasis on Minimizing CT to Recanalization Times), enrolled 316 patients at 11 centers in Canada, 6 in the United States, 3 in South Korea, and 1 in Ireland. After 90 days, 53% of patients in the embolectomy arm had achieved a modified Rankin Scale score of 0-2, this study’s primary endpoint, compared with 29% of patients in the thrombolysis-only arm (treatment with alteplase). These results also appeared in an article published online concurrently with the conference report (N. Engl. J. Med. 2015 Feb. 11 [doi:10.1056/NEJMoa1414905]).
SWIFT PRIME was sponsored by Covidien, which markets the stent retriever used in the study. Dr. Saver and Dr. Goyal are consultants to Covidien. EXTEND-IA used stent retrievers provided by Covidien. ESCAPE received a grant from Covidien. Dr. Becker had no relevant disclosures.
On Twitter @mitchelzoler
NASHVILLE, TENN. – Treatment of selected patients with acute ischemic stroke underwent a dramatic, sudden shift with reports from three randomized, controlled trials that showed substantial added benefit and no incremental risk with the use of catheter-based embolic retrieval to open blocked intracerebral arteries when performed on top of standard thrombolytic therapy.
The three studies, each run independently and based in different countries, supported the results first reported last October and published online in December (N. Engl. J. Med. 2015;372:11-20) from the MR CLEAN (Multicenter Randomized Clinical Trial of Endovascular Treatment for Acute Ischemic Stroke in the Netherlands) study. These were the first contemporary trial results to show a jump in functional outcomes with use of a stent retriever catheter to pluck out the occluding embolus from an artery in the stroke patient’s brain to restore normal blood flow.
All three of the newly-reported studies stopped before reaching their prespecified enrollment levels because of overwhelming evidence for embolectomy’s incremental efficacy.
With four reports from prospective, randomized trials showing similar benefits and no added harm to patients, experts at the International Stroke Conference uniformly anointed catheter-based embolectomy the new standard of care for the small percentage of acute, ischemic-stroke patients who present with proximal, large-artery obstructions and also match the other strict clinical and imaging inclusion and exclusion criteria used in the studies.
“Starting now, in patients with an acute ischemic stroke due to proximal vessel occlusion, rapid endovascular treatment using a retrieval stent is the standard of care,” Dr. Mayank Goyal declared from the plenary-session podium. He is a professor of diagnostic imaging at the University of Calgary (Canada) and an investigator in two of the three trials presented at the conference, which was sponsored by the American Heart Association.
“Today the world changed. We are now in a new era, the era of highly-effective intravascular recanalization therapy,” said Dr. Jeffrey L. Saver, professor of neurology and director of the Stroke Center at the University of California, Los Angeles, and lead investigator for one of the new studies.
In three of the four studies, the researchers did not report specific numbers on how selective they were in focusing in on the ischemic stroke patients most likely to benefit from this treatment, but the one study that did, EXTEND-IA (Extending the Time for Thrombolysis in Emergency Neurological Deficits – Intra-Arterial), run at nine Australian centers and one in New Zealand, showed the extensive winnowing that occurred. Of 7,796 patients with an acute ischemic stroke who initially presented, 1,044 (13%) were eligible to receive thrombolytic therapy (alteplase in this study). And from among these 1,044 patients, a mere 70 – less than 1% of the initial group – were deemed eligible for randomization into the embolectomy trial. The top three reasons for exclusion of patients who qualified for thrombolytic treatment from the trial was an absence of a major-vessel occlusion (45% of the excluded patients), presentation outside of the times when enrollment personnel were available (22%), and poor premorbid function (16%).
But subgroup analyses in three of the four studies (EXTEND-IA with a total of 70 patients was too small for subgroup analyses) showed no subgroup of patients who failed to benefit from embolectomy, including elderly patients who in some cases were nonagenarians.
The unusual confluence of having four major trials showing remarkably consistent results meant that the stroke experts gathered at the meeting focused their attention not on whether stent retrievers should now be widely and routinely used in appropriate patients but instead on how this technology will roll out worldwide.
“From here on out we are obligated to treat patients with this technology at centers that can do this, and we are obligated to have more centers that can provide it,” said Dr. Kyra J. Becker, professor of neurology and neurological surgery and codirector of the Stroke Center at the University of Washington, Seattle. Dr. Becker had no involvement in any of the stent retriever trials. “I had been a doubter of this technology,” primarily because results reported at the International Stroke Conference a couple of years ago failed to prove the efficacy of clot retrieval in ischemic stroke patients, she noted. “Our ability to select appropriate patients and do it in a timely fashion hadn’t gotten to where it had to be until now,” Dr. Becker said in an interview.
“We only enrolled patients with blockages, we treated them quickly, and we used much better devices to open their arteries,” Dr. Saver added, explaining why the new studies succeeded when earlier studies had not.
The trial led by Dr. Saver, SWIFT-PRIME (SOLITAIRE™ FR With the Intention for Thrombectomy as Primary Endovascular Treatment for Acute Ischemic Stroke), enrolled 195 patients at 39 sites in the United States and in Europe. At 90 days after treatment, 59 patients (60%) among those treated with thrombolysis plus embolectomy had a modified Rankin Scale score of 0-2, compared with 33 patients (36%) among those treated only with thrombolysis (in this trial intravenous treatment with tissue plasminogen activator), a highly significant difference for the study’s primary endpoint.
“For every two and half patients treated, one more patient had a better disability outcome, and for every four patients treated, one more patient was independent at long-term follow-up,” Dr. Saver said. Safety measures were similar among patients in the study’s two arms.
The EXTEND-IA results showed a 90-day modified Rankin Scale score of 0-2 in 52% of the embolectomy patients, compared with 28% of those treated only with thrombolysis. The study’s co–primary endpoints were median level of reperfusion at 24 hours after treatment, 100% with embolectomy and 37% with thrombolysis only, and early neurologic recovery, defined as at least an 8-point drop from the baseline in the National Institutes of Health Stroke Scale score or a score of 0 or 1 when assessed 3 days after treatment. Patients met this second endpoint at an 80% rate with embolectomy and a 37% rate with thrombolysis only. Results of EXTEND-IA appeared in an article published online concurrently with the meeting report (N. Engl J. Med. 2015 Feb. 11 [doi:10.1056/NEJMoa1414792]).
The third, and largest, of the three studies presented at the conference, ESCAPE (Endovascular Treatment for Small Core and Anterior Circulation Proximal Occlusion with Emphasis on Minimizing CT to Recanalization Times), enrolled 316 patients at 11 centers in Canada, 6 in the United States, 3 in South Korea, and 1 in Ireland. After 90 days, 53% of patients in the embolectomy arm had achieved a modified Rankin Scale score of 0-2, this study’s primary endpoint, compared with 29% of patients in the thrombolysis-only arm (treatment with alteplase). These results also appeared in an article published online concurrently with the conference report (N. Engl. J. Med. 2015 Feb. 11 [doi:10.1056/NEJMoa1414905]).
SWIFT PRIME was sponsored by Covidien, which markets the stent retriever used in the study. Dr. Saver and Dr. Goyal are consultants to Covidien. EXTEND-IA used stent retrievers provided by Covidien. ESCAPE received a grant from Covidien. Dr. Becker had no relevant disclosures.
On Twitter @mitchelzoler
AT THE INTERNATIONAL STROKE CONFERENCE
Key clinical point: Results from three randomized, controlled trials confirmed the safety and dramatic efficacy of endovascular embolectomy for selected patients with acute, ischemic stroke.
Major finding: In SWIFT PRIME, a 90-day modified Rankin Scale score of 0-2 occurred in 60% of patients treated with thrombolysis plus embolectomy and 36% of patients treated with thrombolysis only.
Data source: SWIFT PRIME, a prospective, multicenter randomized trial that enrolled 195 patients at 39 centers in the United States and Europe.
Disclosures: SWIFT PRIME was sponsored by Covidien, which markets the stent retriever used in the study. Dr. Saver and Dr. Goyal are consultants to Covidien. EXTEND-IA used stent retrievers provided by Covidien. ESCAPE received a grant from Covidien. Dr. Becker had no relevant disclosures.
The tipping point for value-based pay?
Over the last several years, doctors and other health care professionals – no doubt including many readers of this column – have worked to develop the accountable care organization model from an academic idea into a meaningful presence in the health care marketplace.
In January, the federal government threw its considerable weight squarely behind that effort, for the first time setting clear goals for ramping up the use of ACOs and other alternative payment models in Medicare.
In an editorial in the New England Journal of Medicine, Department of Health and Human Services Secretary Sylvia M. Burwell announced that by the end of 2016, her agency plans to have 30% of all Medicare payments “tied to quality through alternative payment models,” including ACOs, patient-centered medical homes, and bundled payments – and to have 50% of Medicare payments made under alternative payment models by the end of 2018.
Furthermore, even among the payments that remain under the fee-for-service model, the vast majority will be linked to quality and value in some way – 85% by 2016, and 90% by 2018.
Right now, only about 20% of Medicare payments are made through alternative payment models, meaning that HHS’ new goals entail a 50% increase in the quantity of Medicare dollars going to alternative payment models by the end of next year, and a 150% increase by the end of 2018. In 2014, Medicare made $362 billion in fee-for-service payments – a huge number, much of which increasingly will be directed toward ACOs.
“We believe these goals can drive transformative change, help us manage and track progress, and create accountability for measurable improvement,” Secretary Burwell said in a press release accompanying the announcement.
“Ultimately, this is about improving the health of each person by making the best use of our resources for patient good,” Dr. Douglas E. Henley, CEO of the American Academy of Family Physicians, noted in the same press release. “We’re on board, and we’re committed to changing how we pay for and deliver care to achieve better health.”
Of course, setting ambitious goals is not the same thing as meeting them, and many details have yet to be ironed out. Will the administration focus on ACOs or on other alternative payment models such as bundled payments? How will it measure quality? And Medicare, though massive, is only one part of the health industry. To what extent will the rest of the industry join in the federal government’s push toward accountable care?
To help answer these questions, HHS also announced that it is creating the Health Care Payment Learning and Action Network, which “will accelerate the transition to more advanced payment models by fostering collaboration between HHS, private payers, large employers, providers, consumers, and state and federal partners.”
January’s announcement is the strongest signal yet that the federal government has bought into the idea of paying for value, not volume, and that it is willing to invest substantially in the emerging accountable care model.
Mr. Bobbitt is a senior partner and head of the health law group at the Smith Anderson law firm in Raleigh, N.C. Mr. Wilson is an associate at Smith Anderson. This article is meant to be educational and does not constitute legal advice. For additional information, readers may contact the authors at [email protected] or [email protected], or by phone at 919-821-6612.
Over the last several years, doctors and other health care professionals – no doubt including many readers of this column – have worked to develop the accountable care organization model from an academic idea into a meaningful presence in the health care marketplace.
In January, the federal government threw its considerable weight squarely behind that effort, for the first time setting clear goals for ramping up the use of ACOs and other alternative payment models in Medicare.
In an editorial in the New England Journal of Medicine, Department of Health and Human Services Secretary Sylvia M. Burwell announced that by the end of 2016, her agency plans to have 30% of all Medicare payments “tied to quality through alternative payment models,” including ACOs, patient-centered medical homes, and bundled payments – and to have 50% of Medicare payments made under alternative payment models by the end of 2018.
Furthermore, even among the payments that remain under the fee-for-service model, the vast majority will be linked to quality and value in some way – 85% by 2016, and 90% by 2018.
Right now, only about 20% of Medicare payments are made through alternative payment models, meaning that HHS’ new goals entail a 50% increase in the quantity of Medicare dollars going to alternative payment models by the end of next year, and a 150% increase by the end of 2018. In 2014, Medicare made $362 billion in fee-for-service payments – a huge number, much of which increasingly will be directed toward ACOs.
“We believe these goals can drive transformative change, help us manage and track progress, and create accountability for measurable improvement,” Secretary Burwell said in a press release accompanying the announcement.
“Ultimately, this is about improving the health of each person by making the best use of our resources for patient good,” Dr. Douglas E. Henley, CEO of the American Academy of Family Physicians, noted in the same press release. “We’re on board, and we’re committed to changing how we pay for and deliver care to achieve better health.”
Of course, setting ambitious goals is not the same thing as meeting them, and many details have yet to be ironed out. Will the administration focus on ACOs or on other alternative payment models such as bundled payments? How will it measure quality? And Medicare, though massive, is only one part of the health industry. To what extent will the rest of the industry join in the federal government’s push toward accountable care?
To help answer these questions, HHS also announced that it is creating the Health Care Payment Learning and Action Network, which “will accelerate the transition to more advanced payment models by fostering collaboration between HHS, private payers, large employers, providers, consumers, and state and federal partners.”
January’s announcement is the strongest signal yet that the federal government has bought into the idea of paying for value, not volume, and that it is willing to invest substantially in the emerging accountable care model.
Mr. Bobbitt is a senior partner and head of the health law group at the Smith Anderson law firm in Raleigh, N.C. Mr. Wilson is an associate at Smith Anderson. This article is meant to be educational and does not constitute legal advice. For additional information, readers may contact the authors at [email protected] or [email protected], or by phone at 919-821-6612.
Over the last several years, doctors and other health care professionals – no doubt including many readers of this column – have worked to develop the accountable care organization model from an academic idea into a meaningful presence in the health care marketplace.
In January, the federal government threw its considerable weight squarely behind that effort, for the first time setting clear goals for ramping up the use of ACOs and other alternative payment models in Medicare.
In an editorial in the New England Journal of Medicine, Department of Health and Human Services Secretary Sylvia M. Burwell announced that by the end of 2016, her agency plans to have 30% of all Medicare payments “tied to quality through alternative payment models,” including ACOs, patient-centered medical homes, and bundled payments – and to have 50% of Medicare payments made under alternative payment models by the end of 2018.
Furthermore, even among the payments that remain under the fee-for-service model, the vast majority will be linked to quality and value in some way – 85% by 2016, and 90% by 2018.
Right now, only about 20% of Medicare payments are made through alternative payment models, meaning that HHS’ new goals entail a 50% increase in the quantity of Medicare dollars going to alternative payment models by the end of next year, and a 150% increase by the end of 2018. In 2014, Medicare made $362 billion in fee-for-service payments – a huge number, much of which increasingly will be directed toward ACOs.
“We believe these goals can drive transformative change, help us manage and track progress, and create accountability for measurable improvement,” Secretary Burwell said in a press release accompanying the announcement.
“Ultimately, this is about improving the health of each person by making the best use of our resources for patient good,” Dr. Douglas E. Henley, CEO of the American Academy of Family Physicians, noted in the same press release. “We’re on board, and we’re committed to changing how we pay for and deliver care to achieve better health.”
Of course, setting ambitious goals is not the same thing as meeting them, and many details have yet to be ironed out. Will the administration focus on ACOs or on other alternative payment models such as bundled payments? How will it measure quality? And Medicare, though massive, is only one part of the health industry. To what extent will the rest of the industry join in the federal government’s push toward accountable care?
To help answer these questions, HHS also announced that it is creating the Health Care Payment Learning and Action Network, which “will accelerate the transition to more advanced payment models by fostering collaboration between HHS, private payers, large employers, providers, consumers, and state and federal partners.”
January’s announcement is the strongest signal yet that the federal government has bought into the idea of paying for value, not volume, and that it is willing to invest substantially in the emerging accountable care model.
Mr. Bobbitt is a senior partner and head of the health law group at the Smith Anderson law firm in Raleigh, N.C. Mr. Wilson is an associate at Smith Anderson. This article is meant to be educational and does not constitute legal advice. For additional information, readers may contact the authors at [email protected] or [email protected], or by phone at 919-821-6612.
Visit your office
Every year around now, as spring begins to revive the landscape, I like to take a tour of my office from the perspective of a patient visiting our facility for the first time, because more often than not, the internal environment could use a bit of a revival as well.
We tend not to notice gradual deterioration in the workplace we inhabit every day: Carpets fade and dull with constant traffic and cleaning; wallpaper and paint accumulate dirt, stains, and damage; furniture gets dirty and dented, fabric rips, hardware goes missing.
When did you last take a good look at your waiting room? Have your patients been snacking and spilling drinks in there, despite the signs begging them not to? Is the wallpaper smudged on the walls behind chairs, where they rest their heads? How are the carpeting and upholstery holding up?
Even if you don’t find anything obvious, it’s wise to check periodically for subtle evidence of age: Find some patches of protected carpeting and flooring – under desks, for example – and compare them with exposed floors.
And look at the decor itself; is it dated or just plain old looking? Any interior designer will tell you he or she can determine quite accurately when a space was last decorated, simply by the color and style of the materials used. If your office is stuck in the ’90s, it’s probably time for a change.
If you’re planning a vacation this summer (and I hope you are), that would be the perfect time for a redo. Your patients will be spared the dust and turmoil, tradespeople won’t have to work around your office hours, and you won’t have to cancel any hours that weren’t already canceled. Best of all, you’ll come back to a clean, fresh environment.
Start by reviewing your color scheme. If it’s hopelessly out of date and style, or if you are just tired of it, change it. Wallpaper and carpeting should be long-wearing industrial quality, paint should be high-quality eggshell finish to facilitate cleaning, and everything should be professionally applied. (This is neither the time nor place for do-it-yourself experiments.) And get your building’s maintenance crew to fix any nagging plumbing, electrical, or heating/air conditioning problems while pipes, ducts, and wires are more readily accessible.
If your wall decorations are dated and unattractive, now would be a good time to replace at least some of them. This need not be an expensive proposition. I recently redecorated my exam room walls with framed photos from my travel adventures, to very positive responses from patients and staff alike. If you’re not an artist or photographer, invite family members, local artists, or talented patients to display some of their creations on your walls.
Plants are great accents and excellent stress reducers for apprehensive patients, yet many offices have little or no plant life. If you are hesitant to take on the extra work of plant upkeep, consider using one of the many corporate plant services that rent you the plants, keep them healthy, and replace them as necessary.
Furniture is another important element in keeping your office environment fresh and inviting. You may be able to resurface and reupholster what you have now, but if not, shop carefully. Beware of nonmedical products promoted specifically to physicians, as they tend to be overpriced. If you shop online, remember to factor in shipping costs, which can be considerable for furniture. Don’t be afraid to ask for discounts; you won’t get them if you don’t ask.
This is also a good time to clear out old textbooks, magazines, and files that you will never open again – not in this digital age.
Finally, spruce-up time is an excellent opportunity to inventory your medical equipment. We’ve all seen vintage offices full of gadgets that were state-of-the-art decades ago. Nostalgia is nice, but would you want to be treated by a physician whose office could be a Smithsonian exhibit titled, “Doctor’s Office Circa 1975?” Neither would your patients, for the most part. In fact, many of them – particularly younger ones – assume that doctors who don’t keep up with technologic innovations don’t keep up with anything else, either.
Dr. Eastern practices dermatology and dermatologic surgery in Belleville, N.J. He is the author of numerous articles and textbook chapters, and is a longtime monthly columnist for Dermatology News.
Every year around now, as spring begins to revive the landscape, I like to take a tour of my office from the perspective of a patient visiting our facility for the first time, because more often than not, the internal environment could use a bit of a revival as well.
We tend not to notice gradual deterioration in the workplace we inhabit every day: Carpets fade and dull with constant traffic and cleaning; wallpaper and paint accumulate dirt, stains, and damage; furniture gets dirty and dented, fabric rips, hardware goes missing.
When did you last take a good look at your waiting room? Have your patients been snacking and spilling drinks in there, despite the signs begging them not to? Is the wallpaper smudged on the walls behind chairs, where they rest their heads? How are the carpeting and upholstery holding up?
Even if you don’t find anything obvious, it’s wise to check periodically for subtle evidence of age: Find some patches of protected carpeting and flooring – under desks, for example – and compare them with exposed floors.
And look at the decor itself; is it dated or just plain old looking? Any interior designer will tell you he or she can determine quite accurately when a space was last decorated, simply by the color and style of the materials used. If your office is stuck in the ’90s, it’s probably time for a change.
If you’re planning a vacation this summer (and I hope you are), that would be the perfect time for a redo. Your patients will be spared the dust and turmoil, tradespeople won’t have to work around your office hours, and you won’t have to cancel any hours that weren’t already canceled. Best of all, you’ll come back to a clean, fresh environment.
Start by reviewing your color scheme. If it’s hopelessly out of date and style, or if you are just tired of it, change it. Wallpaper and carpeting should be long-wearing industrial quality, paint should be high-quality eggshell finish to facilitate cleaning, and everything should be professionally applied. (This is neither the time nor place for do-it-yourself experiments.) And get your building’s maintenance crew to fix any nagging plumbing, electrical, or heating/air conditioning problems while pipes, ducts, and wires are more readily accessible.
If your wall decorations are dated and unattractive, now would be a good time to replace at least some of them. This need not be an expensive proposition. I recently redecorated my exam room walls with framed photos from my travel adventures, to very positive responses from patients and staff alike. If you’re not an artist or photographer, invite family members, local artists, or talented patients to display some of their creations on your walls.
Plants are great accents and excellent stress reducers for apprehensive patients, yet many offices have little or no plant life. If you are hesitant to take on the extra work of plant upkeep, consider using one of the many corporate plant services that rent you the plants, keep them healthy, and replace them as necessary.
Furniture is another important element in keeping your office environment fresh and inviting. You may be able to resurface and reupholster what you have now, but if not, shop carefully. Beware of nonmedical products promoted specifically to physicians, as they tend to be overpriced. If you shop online, remember to factor in shipping costs, which can be considerable for furniture. Don’t be afraid to ask for discounts; you won’t get them if you don’t ask.
This is also a good time to clear out old textbooks, magazines, and files that you will never open again – not in this digital age.
Finally, spruce-up time is an excellent opportunity to inventory your medical equipment. We’ve all seen vintage offices full of gadgets that were state-of-the-art decades ago. Nostalgia is nice, but would you want to be treated by a physician whose office could be a Smithsonian exhibit titled, “Doctor’s Office Circa 1975?” Neither would your patients, for the most part. In fact, many of them – particularly younger ones – assume that doctors who don’t keep up with technologic innovations don’t keep up with anything else, either.
Dr. Eastern practices dermatology and dermatologic surgery in Belleville, N.J. He is the author of numerous articles and textbook chapters, and is a longtime monthly columnist for Dermatology News.
Every year around now, as spring begins to revive the landscape, I like to take a tour of my office from the perspective of a patient visiting our facility for the first time, because more often than not, the internal environment could use a bit of a revival as well.
We tend not to notice gradual deterioration in the workplace we inhabit every day: Carpets fade and dull with constant traffic and cleaning; wallpaper and paint accumulate dirt, stains, and damage; furniture gets dirty and dented, fabric rips, hardware goes missing.
When did you last take a good look at your waiting room? Have your patients been snacking and spilling drinks in there, despite the signs begging them not to? Is the wallpaper smudged on the walls behind chairs, where they rest their heads? How are the carpeting and upholstery holding up?
Even if you don’t find anything obvious, it’s wise to check periodically for subtle evidence of age: Find some patches of protected carpeting and flooring – under desks, for example – and compare them with exposed floors.
And look at the decor itself; is it dated or just plain old looking? Any interior designer will tell you he or she can determine quite accurately when a space was last decorated, simply by the color and style of the materials used. If your office is stuck in the ’90s, it’s probably time for a change.
If you’re planning a vacation this summer (and I hope you are), that would be the perfect time for a redo. Your patients will be spared the dust and turmoil, tradespeople won’t have to work around your office hours, and you won’t have to cancel any hours that weren’t already canceled. Best of all, you’ll come back to a clean, fresh environment.
Start by reviewing your color scheme. If it’s hopelessly out of date and style, or if you are just tired of it, change it. Wallpaper and carpeting should be long-wearing industrial quality, paint should be high-quality eggshell finish to facilitate cleaning, and everything should be professionally applied. (This is neither the time nor place for do-it-yourself experiments.) And get your building’s maintenance crew to fix any nagging plumbing, electrical, or heating/air conditioning problems while pipes, ducts, and wires are more readily accessible.
If your wall decorations are dated and unattractive, now would be a good time to replace at least some of them. This need not be an expensive proposition. I recently redecorated my exam room walls with framed photos from my travel adventures, to very positive responses from patients and staff alike. If you’re not an artist or photographer, invite family members, local artists, or talented patients to display some of their creations on your walls.
Plants are great accents and excellent stress reducers for apprehensive patients, yet many offices have little or no plant life. If you are hesitant to take on the extra work of plant upkeep, consider using one of the many corporate plant services that rent you the plants, keep them healthy, and replace them as necessary.
Furniture is another important element in keeping your office environment fresh and inviting. You may be able to resurface and reupholster what you have now, but if not, shop carefully. Beware of nonmedical products promoted specifically to physicians, as they tend to be overpriced. If you shop online, remember to factor in shipping costs, which can be considerable for furniture. Don’t be afraid to ask for discounts; you won’t get them if you don’t ask.
This is also a good time to clear out old textbooks, magazines, and files that you will never open again – not in this digital age.
Finally, spruce-up time is an excellent opportunity to inventory your medical equipment. We’ve all seen vintage offices full of gadgets that were state-of-the-art decades ago. Nostalgia is nice, but would you want to be treated by a physician whose office could be a Smithsonian exhibit titled, “Doctor’s Office Circa 1975?” Neither would your patients, for the most part. In fact, many of them – particularly younger ones – assume that doctors who don’t keep up with technologic innovations don’t keep up with anything else, either.
Dr. Eastern practices dermatology and dermatologic surgery in Belleville, N.J. He is the author of numerous articles and textbook chapters, and is a longtime monthly columnist for Dermatology News.
Dermatologic Emergencies
Dermatologic emergency may sound like an oxymoron, but there are many emergencies that dermatology residents may encounter in their careers. In some instances the skin is the primary organ that is affected, while in others cutaneous symptoms and life-threatening signs are important diagnostic clues for what may lie beneath the skin.
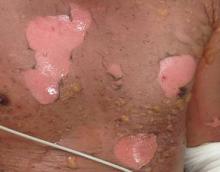
As residents who are occasionally on call or on consultation services, it is important for us to recognize dermatologic emergencies quickly because some of these conditions can acutely evolve and become lethal if a diagnosis is not made early in the disease course with the appropriate treatment administered. Dermatologic emergencies can range from severe drug reactions, infections, autoimmune exacerbations, and inflammatory conditions (eg, erythroderma) to environmental insults such as burns (Figure 1) and child abuse.1
Critical Infections
Some dermatologic emergencies are infectious in origin, and although these infections are most commonly bacterial (eg, necrotizing fasciitis), they also can range from viral to fungal (eg, mucormycosis) in nature. Some areas with large populations of immunocompromised patients (eg, human immunodeficiency virus–positive patients, organ transplant recipients) may warrant a high index of suspicion for possible zebras (rare conditions) and opportunistic infections that may quickly escalate to life-threatening situations.
Although few cutaneous manifestations in emergent infections are pathognomonic, they sometimes can be categorized according to the appearance of the primary lesion: erythrodermic (eg, staphylococcal scalded skin syndrome), maculopapular (eg, Lyme disease), purpuric/petechial (eg, Rocky Mountain spotted fever), pustular (eg, disseminated candidiasis), or vesicular (eg, neonatal herpes simplex virus)(Table). On consultations, dermatology residents frequently get called to evaluate hemorrhagic and ischemic lesions in inpatients (Figure 2). Aside from infectious causes, the differential diagnosis may include coagulation abnormalities (eg, concurrent anticoagulant therapies), vasculitides, poisoning, vascular disease, or Stevens-Johnson syndrome and toxic epidermal necrolysis, which can occasionally present with hemorrhagic lesions.1,2
Necrotizing Fasciitis
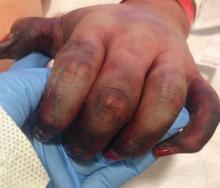
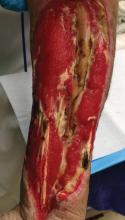
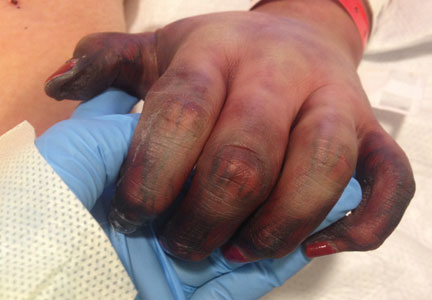
Dermatology residents may frequently encounter necrotizing fasciitis, either in clinic or on the wards (Figure 3). Recognition of the skin signs in this condition is essential to patient survival. As an intern, I once had an attending teach me that patients with necrotizing fasciitis only have a couple of hours to live. The rapid unfolding of this flesh-eating disease and its high morbidity and mortality has led to recent attention in the press and media.
Although necrotizing fasciitis may be caused by several different bacterial organisms (eg, gram positive, gram negative, polymicrobial), it usually is rapidly progressive, destroying muscle and subcutaneous tissues in a matter of hours.3 Bacteria usually enter through a traumatic or present wound and quickly move along fascial planes, destroying blood vessels and whatever subcutaneous tissues happen to be in the way. Within the first few hours, the involved area that was initially erythematous becomes indurated, woody, extremely painful, and dusky, indicating a lack of circulation to the area. Extensive debridement is required until reaching noninfected tissue that is no longer purulent, necrotic, or woody to the touch. If necrotizing fasciitis is not diagnosed and treated early, patients may lose one or several limbs and death may occur.
Key findings of necrotizing fasciitis include systemic toxicity, localized painful induration, well-defined dusky blue discoloration, and a lack of bleeding or purulent discharge on incision and squeezing of the affected tissue. Crepitation or a crackling sensation can occasionally be felt when palpating the area secondary to gas formation in the tissue, though it is not always present. Patients with necrotizing fasciitis often initially present to dermatology clinics because the first manifestation happens to be in the skin. The role of dermatologists in treating this critical condition may prompt recognition and collaboration with other specialists to reach a viable outcome for the patient.3
Drug Reactions
Cutaneous drug eruptions usually are relatively benign, consisting of a morbilliform eruption often without any other accompanying symptoms. However, sometimes these reactions can present as exfoliative dermatitis or red man syndrome in which patients can develop total body erythema with diffuse scaling and pruritus.4 Aside from drug reactions, other causes of exfoliative dermatitis such as psoriasis, atopic and seborrheic dermatitis, mycosis fungoides, and lymphoma should be ruled out. Other drug eruptions that can be classified as dermatologic emergencies include leukocytoclastic vasculitis, severe urticaria or angioedema, erythema multiforme, or Stevens-Johnson syndrome and toxic epidermal necrolysis.
Severe Acne
If not treated promptly, serious cases of acne can lead to severe scarring and psychologic problems. Acne fulminans is characterized by a rapid eruption of suppurative and large, highly inflamed nodules, plaques, and cysts that result in ragged ulcerations and cicatrization of the chest, back, and occasionally the face. Systemic symptoms of fever, arthralgia, leukocytosis, and myalgia suggest an upregulation of the immune system in affected patients.
Final Comment
In summary, dermatologic emergencies do exist and some may present with characteristic skin findings. In almost all cases, collaboration with other departments such as trauma, burn, internal medicine, rheumatology, and infectious diseases is extremely helpful in diagnosing and treating these medical emergencies. Collaboration can provide insight into how brainstorming through different approaches can lead to a better outcome whether it be solving the cause of a puzzling rash in a patient with multiple comorbidities or surgically removing a bullet from a trauma patient (Figure 4). Recognition of specific cutaneous manifestations and early diagnosis of dermatologic emergencies can be lifesaving.
1. McQueen A, Martin SA, Lio PA. Derm emergencies: detecting early signs of trouble. J Fam Pract. 2012;61:71-78.
2. Bennion S. Dermatologic emergencies. In: Fitzpatrick J, Morelli J, eds. Dermatology Secrets Plus. 4th ed. Philadelphia, PA: Mosby; 2011:442-452.
3. Sarani B, Strong M, Pascual J, et al. Necrotizing fasciitis: current concepts and review of the literature. J Am Coll Surg. 2009;208:279-288.
4. Wolf R, Orion E, Marcos B, et al. Life-threatening acute adverse cutaneous drug reactions. Clin Dermatol. 2005;23:171-181.
Dermatologic emergency may sound like an oxymoron, but there are many emergencies that dermatology residents may encounter in their careers. In some instances the skin is the primary organ that is affected, while in others cutaneous symptoms and life-threatening signs are important diagnostic clues for what may lie beneath the skin.
As residents who are occasionally on call or on consultation services, it is important for us to recognize dermatologic emergencies quickly because some of these conditions can acutely evolve and become lethal if a diagnosis is not made early in the disease course with the appropriate treatment administered. Dermatologic emergencies can range from severe drug reactions, infections, autoimmune exacerbations, and inflammatory conditions (eg, erythroderma) to environmental insults such as burns (Figure 1) and child abuse.1
Critical Infections
Some dermatologic emergencies are infectious in origin, and although these infections are most commonly bacterial (eg, necrotizing fasciitis), they also can range from viral to fungal (eg, mucormycosis) in nature. Some areas with large populations of immunocompromised patients (eg, human immunodeficiency virus–positive patients, organ transplant recipients) may warrant a high index of suspicion for possible zebras (rare conditions) and opportunistic infections that may quickly escalate to life-threatening situations.
Although few cutaneous manifestations in emergent infections are pathognomonic, they sometimes can be categorized according to the appearance of the primary lesion: erythrodermic (eg, staphylococcal scalded skin syndrome), maculopapular (eg, Lyme disease), purpuric/petechial (eg, Rocky Mountain spotted fever), pustular (eg, disseminated candidiasis), or vesicular (eg, neonatal herpes simplex virus)(Table). On consultations, dermatology residents frequently get called to evaluate hemorrhagic and ischemic lesions in inpatients (Figure 2). Aside from infectious causes, the differential diagnosis may include coagulation abnormalities (eg, concurrent anticoagulant therapies), vasculitides, poisoning, vascular disease, or Stevens-Johnson syndrome and toxic epidermal necrolysis, which can occasionally present with hemorrhagic lesions.1,2
Necrotizing Fasciitis
Dermatology residents may frequently encounter necrotizing fasciitis, either in clinic or on the wards (Figure 3). Recognition of the skin signs in this condition is essential to patient survival. As an intern, I once had an attending teach me that patients with necrotizing fasciitis only have a couple of hours to live. The rapid unfolding of this flesh-eating disease and its high morbidity and mortality has led to recent attention in the press and media.
Although necrotizing fasciitis may be caused by several different bacterial organisms (eg, gram positive, gram negative, polymicrobial), it usually is rapidly progressive, destroying muscle and subcutaneous tissues in a matter of hours.3 Bacteria usually enter through a traumatic or present wound and quickly move along fascial planes, destroying blood vessels and whatever subcutaneous tissues happen to be in the way. Within the first few hours, the involved area that was initially erythematous becomes indurated, woody, extremely painful, and dusky, indicating a lack of circulation to the area. Extensive debridement is required until reaching noninfected tissue that is no longer purulent, necrotic, or woody to the touch. If necrotizing fasciitis is not diagnosed and treated early, patients may lose one or several limbs and death may occur.
Key findings of necrotizing fasciitis include systemic toxicity, localized painful induration, well-defined dusky blue discoloration, and a lack of bleeding or purulent discharge on incision and squeezing of the affected tissue. Crepitation or a crackling sensation can occasionally be felt when palpating the area secondary to gas formation in the tissue, though it is not always present. Patients with necrotizing fasciitis often initially present to dermatology clinics because the first manifestation happens to be in the skin. The role of dermatologists in treating this critical condition may prompt recognition and collaboration with other specialists to reach a viable outcome for the patient.3
Drug Reactions
Cutaneous drug eruptions usually are relatively benign, consisting of a morbilliform eruption often without any other accompanying symptoms. However, sometimes these reactions can present as exfoliative dermatitis or red man syndrome in which patients can develop total body erythema with diffuse scaling and pruritus.4 Aside from drug reactions, other causes of exfoliative dermatitis such as psoriasis, atopic and seborrheic dermatitis, mycosis fungoides, and lymphoma should be ruled out. Other drug eruptions that can be classified as dermatologic emergencies include leukocytoclastic vasculitis, severe urticaria or angioedema, erythema multiforme, or Stevens-Johnson syndrome and toxic epidermal necrolysis.
Severe Acne
If not treated promptly, serious cases of acne can lead to severe scarring and psychologic problems. Acne fulminans is characterized by a rapid eruption of suppurative and large, highly inflamed nodules, plaques, and cysts that result in ragged ulcerations and cicatrization of the chest, back, and occasionally the face. Systemic symptoms of fever, arthralgia, leukocytosis, and myalgia suggest an upregulation of the immune system in affected patients.
Final Comment
In summary, dermatologic emergencies do exist and some may present with characteristic skin findings. In almost all cases, collaboration with other departments such as trauma, burn, internal medicine, rheumatology, and infectious diseases is extremely helpful in diagnosing and treating these medical emergencies. Collaboration can provide insight into how brainstorming through different approaches can lead to a better outcome whether it be solving the cause of a puzzling rash in a patient with multiple comorbidities or surgically removing a bullet from a trauma patient (Figure 4). Recognition of specific cutaneous manifestations and early diagnosis of dermatologic emergencies can be lifesaving.
Dermatologic emergency may sound like an oxymoron, but there are many emergencies that dermatology residents may encounter in their careers. In some instances the skin is the primary organ that is affected, while in others cutaneous symptoms and life-threatening signs are important diagnostic clues for what may lie beneath the skin.
As residents who are occasionally on call or on consultation services, it is important for us to recognize dermatologic emergencies quickly because some of these conditions can acutely evolve and become lethal if a diagnosis is not made early in the disease course with the appropriate treatment administered. Dermatologic emergencies can range from severe drug reactions, infections, autoimmune exacerbations, and inflammatory conditions (eg, erythroderma) to environmental insults such as burns (Figure 1) and child abuse.1
Critical Infections
Some dermatologic emergencies are infectious in origin, and although these infections are most commonly bacterial (eg, necrotizing fasciitis), they also can range from viral to fungal (eg, mucormycosis) in nature. Some areas with large populations of immunocompromised patients (eg, human immunodeficiency virus–positive patients, organ transplant recipients) may warrant a high index of suspicion for possible zebras (rare conditions) and opportunistic infections that may quickly escalate to life-threatening situations.
Although few cutaneous manifestations in emergent infections are pathognomonic, they sometimes can be categorized according to the appearance of the primary lesion: erythrodermic (eg, staphylococcal scalded skin syndrome), maculopapular (eg, Lyme disease), purpuric/petechial (eg, Rocky Mountain spotted fever), pustular (eg, disseminated candidiasis), or vesicular (eg, neonatal herpes simplex virus)(Table). On consultations, dermatology residents frequently get called to evaluate hemorrhagic and ischemic lesions in inpatients (Figure 2). Aside from infectious causes, the differential diagnosis may include coagulation abnormalities (eg, concurrent anticoagulant therapies), vasculitides, poisoning, vascular disease, or Stevens-Johnson syndrome and toxic epidermal necrolysis, which can occasionally present with hemorrhagic lesions.1,2
Necrotizing Fasciitis
Dermatology residents may frequently encounter necrotizing fasciitis, either in clinic or on the wards (Figure 3). Recognition of the skin signs in this condition is essential to patient survival. As an intern, I once had an attending teach me that patients with necrotizing fasciitis only have a couple of hours to live. The rapid unfolding of this flesh-eating disease and its high morbidity and mortality has led to recent attention in the press and media.
Although necrotizing fasciitis may be caused by several different bacterial organisms (eg, gram positive, gram negative, polymicrobial), it usually is rapidly progressive, destroying muscle and subcutaneous tissues in a matter of hours.3 Bacteria usually enter through a traumatic or present wound and quickly move along fascial planes, destroying blood vessels and whatever subcutaneous tissues happen to be in the way. Within the first few hours, the involved area that was initially erythematous becomes indurated, woody, extremely painful, and dusky, indicating a lack of circulation to the area. Extensive debridement is required until reaching noninfected tissue that is no longer purulent, necrotic, or woody to the touch. If necrotizing fasciitis is not diagnosed and treated early, patients may lose one or several limbs and death may occur.
Key findings of necrotizing fasciitis include systemic toxicity, localized painful induration, well-defined dusky blue discoloration, and a lack of bleeding or purulent discharge on incision and squeezing of the affected tissue. Crepitation or a crackling sensation can occasionally be felt when palpating the area secondary to gas formation in the tissue, though it is not always present. Patients with necrotizing fasciitis often initially present to dermatology clinics because the first manifestation happens to be in the skin. The role of dermatologists in treating this critical condition may prompt recognition and collaboration with other specialists to reach a viable outcome for the patient.3
Drug Reactions
Cutaneous drug eruptions usually are relatively benign, consisting of a morbilliform eruption often without any other accompanying symptoms. However, sometimes these reactions can present as exfoliative dermatitis or red man syndrome in which patients can develop total body erythema with diffuse scaling and pruritus.4 Aside from drug reactions, other causes of exfoliative dermatitis such as psoriasis, atopic and seborrheic dermatitis, mycosis fungoides, and lymphoma should be ruled out. Other drug eruptions that can be classified as dermatologic emergencies include leukocytoclastic vasculitis, severe urticaria or angioedema, erythema multiforme, or Stevens-Johnson syndrome and toxic epidermal necrolysis.
Severe Acne
If not treated promptly, serious cases of acne can lead to severe scarring and psychologic problems. Acne fulminans is characterized by a rapid eruption of suppurative and large, highly inflamed nodules, plaques, and cysts that result in ragged ulcerations and cicatrization of the chest, back, and occasionally the face. Systemic symptoms of fever, arthralgia, leukocytosis, and myalgia suggest an upregulation of the immune system in affected patients.
Final Comment
In summary, dermatologic emergencies do exist and some may present with characteristic skin findings. In almost all cases, collaboration with other departments such as trauma, burn, internal medicine, rheumatology, and infectious diseases is extremely helpful in diagnosing and treating these medical emergencies. Collaboration can provide insight into how brainstorming through different approaches can lead to a better outcome whether it be solving the cause of a puzzling rash in a patient with multiple comorbidities or surgically removing a bullet from a trauma patient (Figure 4). Recognition of specific cutaneous manifestations and early diagnosis of dermatologic emergencies can be lifesaving.
1. McQueen A, Martin SA, Lio PA. Derm emergencies: detecting early signs of trouble. J Fam Pract. 2012;61:71-78.
2. Bennion S. Dermatologic emergencies. In: Fitzpatrick J, Morelli J, eds. Dermatology Secrets Plus. 4th ed. Philadelphia, PA: Mosby; 2011:442-452.
3. Sarani B, Strong M, Pascual J, et al. Necrotizing fasciitis: current concepts and review of the literature. J Am Coll Surg. 2009;208:279-288.
4. Wolf R, Orion E, Marcos B, et al. Life-threatening acute adverse cutaneous drug reactions. Clin Dermatol. 2005;23:171-181.
1. McQueen A, Martin SA, Lio PA. Derm emergencies: detecting early signs of trouble. J Fam Pract. 2012;61:71-78.
2. Bennion S. Dermatologic emergencies. In: Fitzpatrick J, Morelli J, eds. Dermatology Secrets Plus. 4th ed. Philadelphia, PA: Mosby; 2011:442-452.
3. Sarani B, Strong M, Pascual J, et al. Necrotizing fasciitis: current concepts and review of the literature. J Am Coll Surg. 2009;208:279-288.
4. Wolf R, Orion E, Marcos B, et al. Life-threatening acute adverse cutaneous drug reactions. Clin Dermatol. 2005;23:171-181.
Order of mutations impacts MPN behavior
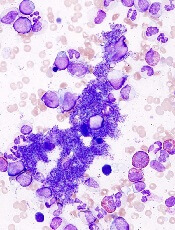
essential thrombocythemia
The order in which genetic mutations are acquired determines how myeloproliferative neoplasms (MPNs) behave, according to research published in NEJM.
Investigators found that mutation order impacts everything from the type of MPN a patient develops to how the disease responds to treatment.
“This surprising finding could help us offer more accurate prognoses to MPN patients based on their mutation order and tailor potential therapies towards them,” said study author David Kent, PhD, of the University of Cambridge in the UK.
“For example, our results predict that targeted JAK2 therapy would be more effective in patients with one mutation order but not the other.”
To uncover this finding, Dr Kent and his colleagues screened 246 MPN patients for mutations in JAK2 and TET2. By studying patients who carried both mutations, the team was able to determine which mutation came first and study the effect of mutation order on the behavior of hematopoietic stem cells.
The investigators found that patients who acquired mutations in JAK2 prior to those in TET2 displayed aberrant blood counts more than a decade earlier.
These patients were more likely to present with polycythemia vera than with essential thrombocythemia, and they were more likely to develop thromboses.
At the same time, JAK2-mutant progenitors from these patients exhibited increased sensitivity to the JAK1/2 inhibitor ruxolitinib in vitro.
“This is the first time that mutation order has been shown to affect any cancer, and it is likely that this phenomenon occurs in many types of malignancy,” said study author Tony Green, MD, PhD, of the University of Cambridge.
“These results show how the study of MPNs provides unparalleled access to the earliest stages of tumor development (inaccessible in other cancers, which usually cannot be detected until many mutations have accumulated). This should give us powerful insights into the origins of cancer.” ![]()

essential thrombocythemia
The order in which genetic mutations are acquired determines how myeloproliferative neoplasms (MPNs) behave, according to research published in NEJM.
Investigators found that mutation order impacts everything from the type of MPN a patient develops to how the disease responds to treatment.
“This surprising finding could help us offer more accurate prognoses to MPN patients based on their mutation order and tailor potential therapies towards them,” said study author David Kent, PhD, of the University of Cambridge in the UK.
“For example, our results predict that targeted JAK2 therapy would be more effective in patients with one mutation order but not the other.”
To uncover this finding, Dr Kent and his colleagues screened 246 MPN patients for mutations in JAK2 and TET2. By studying patients who carried both mutations, the team was able to determine which mutation came first and study the effect of mutation order on the behavior of hematopoietic stem cells.
The investigators found that patients who acquired mutations in JAK2 prior to those in TET2 displayed aberrant blood counts more than a decade earlier.
These patients were more likely to present with polycythemia vera than with essential thrombocythemia, and they were more likely to develop thromboses.
At the same time, JAK2-mutant progenitors from these patients exhibited increased sensitivity to the JAK1/2 inhibitor ruxolitinib in vitro.
“This is the first time that mutation order has been shown to affect any cancer, and it is likely that this phenomenon occurs in many types of malignancy,” said study author Tony Green, MD, PhD, of the University of Cambridge.
“These results show how the study of MPNs provides unparalleled access to the earliest stages of tumor development (inaccessible in other cancers, which usually cannot be detected until many mutations have accumulated). This should give us powerful insights into the origins of cancer.” ![]()

essential thrombocythemia
The order in which genetic mutations are acquired determines how myeloproliferative neoplasms (MPNs) behave, according to research published in NEJM.
Investigators found that mutation order impacts everything from the type of MPN a patient develops to how the disease responds to treatment.
“This surprising finding could help us offer more accurate prognoses to MPN patients based on their mutation order and tailor potential therapies towards them,” said study author David Kent, PhD, of the University of Cambridge in the UK.
“For example, our results predict that targeted JAK2 therapy would be more effective in patients with one mutation order but not the other.”
To uncover this finding, Dr Kent and his colleagues screened 246 MPN patients for mutations in JAK2 and TET2. By studying patients who carried both mutations, the team was able to determine which mutation came first and study the effect of mutation order on the behavior of hematopoietic stem cells.
The investigators found that patients who acquired mutations in JAK2 prior to those in TET2 displayed aberrant blood counts more than a decade earlier.
These patients were more likely to present with polycythemia vera than with essential thrombocythemia, and they were more likely to develop thromboses.
At the same time, JAK2-mutant progenitors from these patients exhibited increased sensitivity to the JAK1/2 inhibitor ruxolitinib in vitro.
“This is the first time that mutation order has been shown to affect any cancer, and it is likely that this phenomenon occurs in many types of malignancy,” said study author Tony Green, MD, PhD, of the University of Cambridge.
“These results show how the study of MPNs provides unparalleled access to the earliest stages of tumor development (inaccessible in other cancers, which usually cannot be detected until many mutations have accumulated). This should give us powerful insights into the origins of cancer.” ![]()
Robotic sock could prevent DVT, team says
the bio-inspired robotic sock
Photo courtesy of the National
University of Singapore
Researchers have invented a robotic sock that may be able to prevent deep vein thrombosis (DVT), although it has not yet been tested in clinical trials.
Equipped with soft actuators that mimic the tentacle movements of corals, the robotic sock emulates natural lower leg muscle contractions in the wearer’s leg, thereby promoting blood circulation throughout the body.
The device also allows the patient’s lower leg movements to be monitored to improve therapy outcomes.
The sock was created by Lim Jeong Hoon, MD, PhD, Raye Yeow Chen Hua, PhD, and Low Fanzhe (a PhD student), all from the National University of Singapore.
While exploring a way to prevent DVT, Dr Lim was inspired by the natural role of the human ankle muscles in facilitating venous blood flow back to the heart. He worked with Dr Yeow and Low to identify a way to perform this function for patients who are bedridden or unable to move their legs.
The team turned to nature for inspiration. They found similarities in the structural design of the coral tentacle, which can extend to grab food and contract to bring the food closer for consumption, and invented soft actuators that mimic this push-and-pull mechanism.
By integrating the actuators with a sock and the use of a programmable pneumatic pump-valve control system, the invention can create the desired robot-assisted ankle joint motions to facilitate blood flow in the leg.
“We chose to use only soft components and actuators to increase patient comfort during use, hence minimizing the risk of injury from excessive mechanical forces,” Low said. “Compression stockings are currently used in the hospital wards, so it makes sense to use a similar sock-based approach to provide comfort and minimize bulk on the ankle and foot.”
The researchers noted that the sock complements conventional ankle therapy exercises that therapists perform on patients, thereby optimizing therapy time and productivity.
In addition, the sock can be worn for prolonged periods to provide robot-assisted therapy, on top of the therapist-assisted sessions. The sock is also embedded with sensors to track the ankle joint angle, allowing the patient’s ankle motion to be monitored for better treatment.
“Given its compact size, modular design, and ease of use, the soft robotic sock can be adopted in hospital wards and rehabilitation centers for on-bed applications to prevent DVT among stroke patients or even at home for bedridden patients,” Dr Yeow said. “By reducing the risk of DVT using this device, we hope to improve survival rates of these patients.”
To investigate the effectiveness of the robotic sock, the researchers will be conducting pilot clinical trials with about 30 patients at the National University Hospital over 6 months, starting in March.
They hope the pilot trials will help them obtain patient and clinical feedback to further improve the design and capabilities of the device. The team intends to conduct trials across different local hospitals for better evaluation, and they also hope to commercialize the device in the future. ![]()

the bio-inspired robotic sock
Photo courtesy of the National
University of Singapore
Researchers have invented a robotic sock that may be able to prevent deep vein thrombosis (DVT), although it has not yet been tested in clinical trials.
Equipped with soft actuators that mimic the tentacle movements of corals, the robotic sock emulates natural lower leg muscle contractions in the wearer’s leg, thereby promoting blood circulation throughout the body.
The device also allows the patient’s lower leg movements to be monitored to improve therapy outcomes.
The sock was created by Lim Jeong Hoon, MD, PhD, Raye Yeow Chen Hua, PhD, and Low Fanzhe (a PhD student), all from the National University of Singapore.
While exploring a way to prevent DVT, Dr Lim was inspired by the natural role of the human ankle muscles in facilitating venous blood flow back to the heart. He worked with Dr Yeow and Low to identify a way to perform this function for patients who are bedridden or unable to move their legs.
The team turned to nature for inspiration. They found similarities in the structural design of the coral tentacle, which can extend to grab food and contract to bring the food closer for consumption, and invented soft actuators that mimic this push-and-pull mechanism.
By integrating the actuators with a sock and the use of a programmable pneumatic pump-valve control system, the invention can create the desired robot-assisted ankle joint motions to facilitate blood flow in the leg.
“We chose to use only soft components and actuators to increase patient comfort during use, hence minimizing the risk of injury from excessive mechanical forces,” Low said. “Compression stockings are currently used in the hospital wards, so it makes sense to use a similar sock-based approach to provide comfort and minimize bulk on the ankle and foot.”
The researchers noted that the sock complements conventional ankle therapy exercises that therapists perform on patients, thereby optimizing therapy time and productivity.
In addition, the sock can be worn for prolonged periods to provide robot-assisted therapy, on top of the therapist-assisted sessions. The sock is also embedded with sensors to track the ankle joint angle, allowing the patient’s ankle motion to be monitored for better treatment.
“Given its compact size, modular design, and ease of use, the soft robotic sock can be adopted in hospital wards and rehabilitation centers for on-bed applications to prevent DVT among stroke patients or even at home for bedridden patients,” Dr Yeow said. “By reducing the risk of DVT using this device, we hope to improve survival rates of these patients.”
To investigate the effectiveness of the robotic sock, the researchers will be conducting pilot clinical trials with about 30 patients at the National University Hospital over 6 months, starting in March.
They hope the pilot trials will help them obtain patient and clinical feedback to further improve the design and capabilities of the device. The team intends to conduct trials across different local hospitals for better evaluation, and they also hope to commercialize the device in the future. ![]()

the bio-inspired robotic sock
Photo courtesy of the National
University of Singapore
Researchers have invented a robotic sock that may be able to prevent deep vein thrombosis (DVT), although it has not yet been tested in clinical trials.
Equipped with soft actuators that mimic the tentacle movements of corals, the robotic sock emulates natural lower leg muscle contractions in the wearer’s leg, thereby promoting blood circulation throughout the body.
The device also allows the patient’s lower leg movements to be monitored to improve therapy outcomes.
The sock was created by Lim Jeong Hoon, MD, PhD, Raye Yeow Chen Hua, PhD, and Low Fanzhe (a PhD student), all from the National University of Singapore.
While exploring a way to prevent DVT, Dr Lim was inspired by the natural role of the human ankle muscles in facilitating venous blood flow back to the heart. He worked with Dr Yeow and Low to identify a way to perform this function for patients who are bedridden or unable to move their legs.
The team turned to nature for inspiration. They found similarities in the structural design of the coral tentacle, which can extend to grab food and contract to bring the food closer for consumption, and invented soft actuators that mimic this push-and-pull mechanism.
By integrating the actuators with a sock and the use of a programmable pneumatic pump-valve control system, the invention can create the desired robot-assisted ankle joint motions to facilitate blood flow in the leg.
“We chose to use only soft components and actuators to increase patient comfort during use, hence minimizing the risk of injury from excessive mechanical forces,” Low said. “Compression stockings are currently used in the hospital wards, so it makes sense to use a similar sock-based approach to provide comfort and minimize bulk on the ankle and foot.”
The researchers noted that the sock complements conventional ankle therapy exercises that therapists perform on patients, thereby optimizing therapy time and productivity.
In addition, the sock can be worn for prolonged periods to provide robot-assisted therapy, on top of the therapist-assisted sessions. The sock is also embedded with sensors to track the ankle joint angle, allowing the patient’s ankle motion to be monitored for better treatment.
“Given its compact size, modular design, and ease of use, the soft robotic sock can be adopted in hospital wards and rehabilitation centers for on-bed applications to prevent DVT among stroke patients or even at home for bedridden patients,” Dr Yeow said. “By reducing the risk of DVT using this device, we hope to improve survival rates of these patients.”
To investigate the effectiveness of the robotic sock, the researchers will be conducting pilot clinical trials with about 30 patients at the National University Hospital over 6 months, starting in March.
They hope the pilot trials will help them obtain patient and clinical feedback to further improve the design and capabilities of the device. The team intends to conduct trials across different local hospitals for better evaluation, and they also hope to commercialize the device in the future. ![]()
Gene variations tied to drug-related hearing loss

Photo by Peter Barta
New research has revealed inherited genetic variations associated with rapid hearing loss in young cancer patients who receive cisplatin.
The drug is used to treat a range of cancers and is known to pose a risk of severe hearing loss, but the risk factors involved are not completely understood.
Now, researchers have found that variations in the gene ACYP2 are associated with an increased risk of cisplatin-related hearing loss.
Jun J. Yang, PhD, of St Jude Children’s Research Hospital in Memphis, Tennessee, and his colleagues reported this discovery in Nature Genetics.
The researchers checked the DNA of 238 young patients with brain tumors for more than 1.7 million common genetic variations. The team found that variations in ACYP2 were associated with as much as a 4-fold greater risk of cisplatin-related hearing loss.
The screening is among the first to survey the genetic landscape for clues to help explain why the risk of cisplatin-related hearing loss varies so widely among patients.
“This is an important first step in being able to pinpoint patients who are at higher risk of developing cisplatin toxicity and to learn how to better manage that risk,” said study author Clinton Stewart, PharmD, also of St Jude.
The researchers confirmed the association between the high-risk ACYP2 variants and cisplatin-related hearing loss in a separate group of 68 brain tumor patients. The association was independent of other risk factors for cisplatin-related hearing loss, including patient age and receipt of radiation therapy.
Twenty-four of the 306 patients in this study had at least one copy of the high-risk ACYP2 variant. All 24 patients had measurable hearing loss that occurred as early as weeks after beginning cisplatin therapy.
Overall, however, the ACYP2 variant explained a relatively small proportion of hearing damage. Just 12.4% of the 194 patients in this study with cisplatin-related hearing loss carried the ACYP2 variant.
“This suggests that other genes also contribute to the risk of hearing loss and are yet to be identified,” Dr Yang said. “Further research is needed to understand how the ACYP2 variations modify the risk . . . of cisplatin toxicity.”
Such studies could potentially lead to new medications to protect high-risk patients from cisplatin-related toxicity or help identify candidates for intensive monitoring of their hearing, Dr Stewart said. Early intervention could then be offered if problems are identified.
This study included patients enrolled in 1 of 3 trials designed by St Jude investigators for newly diagnosed pediatric brain tumors. The protocols involved similar treatment, including surgery to remove as much of the tumor as possible, followed by radiation, which was modified based on patient age and other risk factors.
The patients were scheduled to receive 4 rounds of cisplatin therapy. Patients’ hearing was tested before treatment began, after radiation therapy, after each round of chemotherapy, and then at regular standardized intervals. Analysis of the resulting data led to identification of ACYP2 and other variants.
“Our primary goal is to cure children with brain tumors, but we also have a duty to help patients survive with a high quality of life,” said Giles Robinson, MD, also of St Jude.
“Hearing loss can have a significant impact on a child’s quality of life, language development, and academic performance. There is no easy fix, but the more we know about the risk factors, the better we will understand how to use cisplatin.” ![]()

Photo by Peter Barta
New research has revealed inherited genetic variations associated with rapid hearing loss in young cancer patients who receive cisplatin.
The drug is used to treat a range of cancers and is known to pose a risk of severe hearing loss, but the risk factors involved are not completely understood.
Now, researchers have found that variations in the gene ACYP2 are associated with an increased risk of cisplatin-related hearing loss.
Jun J. Yang, PhD, of St Jude Children’s Research Hospital in Memphis, Tennessee, and his colleagues reported this discovery in Nature Genetics.
The researchers checked the DNA of 238 young patients with brain tumors for more than 1.7 million common genetic variations. The team found that variations in ACYP2 were associated with as much as a 4-fold greater risk of cisplatin-related hearing loss.
The screening is among the first to survey the genetic landscape for clues to help explain why the risk of cisplatin-related hearing loss varies so widely among patients.
“This is an important first step in being able to pinpoint patients who are at higher risk of developing cisplatin toxicity and to learn how to better manage that risk,” said study author Clinton Stewart, PharmD, also of St Jude.
The researchers confirmed the association between the high-risk ACYP2 variants and cisplatin-related hearing loss in a separate group of 68 brain tumor patients. The association was independent of other risk factors for cisplatin-related hearing loss, including patient age and receipt of radiation therapy.
Twenty-four of the 306 patients in this study had at least one copy of the high-risk ACYP2 variant. All 24 patients had measurable hearing loss that occurred as early as weeks after beginning cisplatin therapy.
Overall, however, the ACYP2 variant explained a relatively small proportion of hearing damage. Just 12.4% of the 194 patients in this study with cisplatin-related hearing loss carried the ACYP2 variant.
“This suggests that other genes also contribute to the risk of hearing loss and are yet to be identified,” Dr Yang said. “Further research is needed to understand how the ACYP2 variations modify the risk . . . of cisplatin toxicity.”
Such studies could potentially lead to new medications to protect high-risk patients from cisplatin-related toxicity or help identify candidates for intensive monitoring of their hearing, Dr Stewart said. Early intervention could then be offered if problems are identified.
This study included patients enrolled in 1 of 3 trials designed by St Jude investigators for newly diagnosed pediatric brain tumors. The protocols involved similar treatment, including surgery to remove as much of the tumor as possible, followed by radiation, which was modified based on patient age and other risk factors.
The patients were scheduled to receive 4 rounds of cisplatin therapy. Patients’ hearing was tested before treatment began, after radiation therapy, after each round of chemotherapy, and then at regular standardized intervals. Analysis of the resulting data led to identification of ACYP2 and other variants.
“Our primary goal is to cure children with brain tumors, but we also have a duty to help patients survive with a high quality of life,” said Giles Robinson, MD, also of St Jude.
“Hearing loss can have a significant impact on a child’s quality of life, language development, and academic performance. There is no easy fix, but the more we know about the risk factors, the better we will understand how to use cisplatin.” ![]()

Photo by Peter Barta
New research has revealed inherited genetic variations associated with rapid hearing loss in young cancer patients who receive cisplatin.
The drug is used to treat a range of cancers and is known to pose a risk of severe hearing loss, but the risk factors involved are not completely understood.
Now, researchers have found that variations in the gene ACYP2 are associated with an increased risk of cisplatin-related hearing loss.
Jun J. Yang, PhD, of St Jude Children’s Research Hospital in Memphis, Tennessee, and his colleagues reported this discovery in Nature Genetics.
The researchers checked the DNA of 238 young patients with brain tumors for more than 1.7 million common genetic variations. The team found that variations in ACYP2 were associated with as much as a 4-fold greater risk of cisplatin-related hearing loss.
The screening is among the first to survey the genetic landscape for clues to help explain why the risk of cisplatin-related hearing loss varies so widely among patients.
“This is an important first step in being able to pinpoint patients who are at higher risk of developing cisplatin toxicity and to learn how to better manage that risk,” said study author Clinton Stewart, PharmD, also of St Jude.
The researchers confirmed the association between the high-risk ACYP2 variants and cisplatin-related hearing loss in a separate group of 68 brain tumor patients. The association was independent of other risk factors for cisplatin-related hearing loss, including patient age and receipt of radiation therapy.
Twenty-four of the 306 patients in this study had at least one copy of the high-risk ACYP2 variant. All 24 patients had measurable hearing loss that occurred as early as weeks after beginning cisplatin therapy.
Overall, however, the ACYP2 variant explained a relatively small proportion of hearing damage. Just 12.4% of the 194 patients in this study with cisplatin-related hearing loss carried the ACYP2 variant.
“This suggests that other genes also contribute to the risk of hearing loss and are yet to be identified,” Dr Yang said. “Further research is needed to understand how the ACYP2 variations modify the risk . . . of cisplatin toxicity.”
Such studies could potentially lead to new medications to protect high-risk patients from cisplatin-related toxicity or help identify candidates for intensive monitoring of their hearing, Dr Stewart said. Early intervention could then be offered if problems are identified.
This study included patients enrolled in 1 of 3 trials designed by St Jude investigators for newly diagnosed pediatric brain tumors. The protocols involved similar treatment, including surgery to remove as much of the tumor as possible, followed by radiation, which was modified based on patient age and other risk factors.
The patients were scheduled to receive 4 rounds of cisplatin therapy. Patients’ hearing was tested before treatment began, after radiation therapy, after each round of chemotherapy, and then at regular standardized intervals. Analysis of the resulting data led to identification of ACYP2 and other variants.
“Our primary goal is to cure children with brain tumors, but we also have a duty to help patients survive with a high quality of life,” said Giles Robinson, MD, also of St Jude.
“Hearing loss can have a significant impact on a child’s quality of life, language development, and academic performance. There is no easy fix, but the more we know about the risk factors, the better we will understand how to use cisplatin.” ![]()
Signs may predict death in cancer patients

Researchers have identified 8 highly specific physical and cognitive signs that seem to be associated with imminent death in cancer patients.
The findings, published in Cancer, could offer clinicians the ability to better communicate with patients and their families.
The research might also help guide the medical team and caregivers when it comes to complex decision making, such as discontinuing tests and therapy, plans for hospital discharge, and hospice referral.
Previous studies in end-of-life care have focused on physicians prognosticating better. However, research on how to tell if a patient has entered the final days of life has been minimal, according to David Hui, MD, of the University of Texas MD Anderson Cancer Center in Houston.
“In the past, studies trying to understand the signs associated with impending death were conducted in people who were recognized as dying, so there’s a potential bias built into this model,” Dr Hui said.
“With our study, we observed a list of signs in patients from the time they were admitted to the palliative care unit. They were observed systematically, twice a day, without knowing if the patient would die or be discharged.”
Dr Hui and his colleagues observed 357 cancer patients, 57% of whom ultimately died. The researchers observed 52 physical and cognitive signs—identified by Dr Hui and his colleagues in previous research—twice a day from the patient’s admission to discharge or death.
Of those 52 signs, the 8 most highly associated with impending death within 3 days were:
- Nonreactive pupils
- Decreased response to verbal stimuli
- Decreased response to visual stimuli
- Inability to close eyelids
- Drooping of the nasolabial fold
- Neck hyperextension
- Grunting of vocal cords
- Upper gastrointestinal bleeding.
“When cancer patients reach the last days of life, this is an extremely emotional time for families; their stress levels cannot be understated,” Dr Hui said.
“Knowing when death is imminent would provide more information so caregivers can plan appropriately. For clinicians, having this information could help reassure families that we are providing the best care possible.”
Dr Hui stressed that this research is not yet practice-changing, but is an important step in understanding these 8 signs and their relation to impending death. In addition, the findings are only representative of imminent cancer death and should not be generalized to other causes of death.
Follow-up studies in different settings are planned. Dr Hui and his colleagues plan to look at the reliability of the identified signs, as well as evaluate this research in other countries and in the hospice setting. ![]()

Researchers have identified 8 highly specific physical and cognitive signs that seem to be associated with imminent death in cancer patients.
The findings, published in Cancer, could offer clinicians the ability to better communicate with patients and their families.
The research might also help guide the medical team and caregivers when it comes to complex decision making, such as discontinuing tests and therapy, plans for hospital discharge, and hospice referral.
Previous studies in end-of-life care have focused on physicians prognosticating better. However, research on how to tell if a patient has entered the final days of life has been minimal, according to David Hui, MD, of the University of Texas MD Anderson Cancer Center in Houston.
“In the past, studies trying to understand the signs associated with impending death were conducted in people who were recognized as dying, so there’s a potential bias built into this model,” Dr Hui said.
“With our study, we observed a list of signs in patients from the time they were admitted to the palliative care unit. They were observed systematically, twice a day, without knowing if the patient would die or be discharged.”
Dr Hui and his colleagues observed 357 cancer patients, 57% of whom ultimately died. The researchers observed 52 physical and cognitive signs—identified by Dr Hui and his colleagues in previous research—twice a day from the patient’s admission to discharge or death.
Of those 52 signs, the 8 most highly associated with impending death within 3 days were:
- Nonreactive pupils
- Decreased response to verbal stimuli
- Decreased response to visual stimuli
- Inability to close eyelids
- Drooping of the nasolabial fold
- Neck hyperextension
- Grunting of vocal cords
- Upper gastrointestinal bleeding.
“When cancer patients reach the last days of life, this is an extremely emotional time for families; their stress levels cannot be understated,” Dr Hui said.
“Knowing when death is imminent would provide more information so caregivers can plan appropriately. For clinicians, having this information could help reassure families that we are providing the best care possible.”
Dr Hui stressed that this research is not yet practice-changing, but is an important step in understanding these 8 signs and their relation to impending death. In addition, the findings are only representative of imminent cancer death and should not be generalized to other causes of death.
Follow-up studies in different settings are planned. Dr Hui and his colleagues plan to look at the reliability of the identified signs, as well as evaluate this research in other countries and in the hospice setting. ![]()

Researchers have identified 8 highly specific physical and cognitive signs that seem to be associated with imminent death in cancer patients.
The findings, published in Cancer, could offer clinicians the ability to better communicate with patients and their families.
The research might also help guide the medical team and caregivers when it comes to complex decision making, such as discontinuing tests and therapy, plans for hospital discharge, and hospice referral.
Previous studies in end-of-life care have focused on physicians prognosticating better. However, research on how to tell if a patient has entered the final days of life has been minimal, according to David Hui, MD, of the University of Texas MD Anderson Cancer Center in Houston.
“In the past, studies trying to understand the signs associated with impending death were conducted in people who were recognized as dying, so there’s a potential bias built into this model,” Dr Hui said.
“With our study, we observed a list of signs in patients from the time they were admitted to the palliative care unit. They were observed systematically, twice a day, without knowing if the patient would die or be discharged.”
Dr Hui and his colleagues observed 357 cancer patients, 57% of whom ultimately died. The researchers observed 52 physical and cognitive signs—identified by Dr Hui and his colleagues in previous research—twice a day from the patient’s admission to discharge or death.
Of those 52 signs, the 8 most highly associated with impending death within 3 days were:
- Nonreactive pupils
- Decreased response to verbal stimuli
- Decreased response to visual stimuli
- Inability to close eyelids
- Drooping of the nasolabial fold
- Neck hyperextension
- Grunting of vocal cords
- Upper gastrointestinal bleeding.
“When cancer patients reach the last days of life, this is an extremely emotional time for families; their stress levels cannot be understated,” Dr Hui said.
“Knowing when death is imminent would provide more information so caregivers can plan appropriately. For clinicians, having this information could help reassure families that we are providing the best care possible.”
Dr Hui stressed that this research is not yet practice-changing, but is an important step in understanding these 8 signs and their relation to impending death. In addition, the findings are only representative of imminent cancer death and should not be generalized to other causes of death.
Follow-up studies in different settings are planned. Dr Hui and his colleagues plan to look at the reliability of the identified signs, as well as evaluate this research in other countries and in the hospice setting. ![]()
“Drip-and-ship” thrombolysis remains common for ischemic stroke
NASHVILLE, TENN. – About 25% of patients with ischemic stroke who receive thrombolytic therapy get it in the field before hospital transfer with the “drip-and-ship” paradigm.
While there were only modest differences in clinical outcomes between these patients and those treated when admitted to an emergency department, drip-and-ship may actually increase the overall use of tissue plasminogen activator (TPA), Dr. Kevin N. Sheth said at the International Stroke Conference, sponsored by the American Heart Association.
The retrospective analysis, which was simultaneously published in Stroke (2015 Feb. 11 [doi:10.1161/STROKEAHA.114.007506]), plumbed the Get With the Guidelines registry for data to describe trends in the use of TPA and drip-and-ship administration across the United States over time. The study involved 1,440 hospitals and 44,667 patients who had an ischemic stroke during 2003-2010 and received TPA. Of these, 10,475 (23.5%) received it in the field before optional admission and within 3 hours of symptom onset.
Baseline characteristics were similar between the treatment groups. The patients’ mean age was 70 years, and the sex distribution was evenly split. More than 75% of each group was white.
The National Institutes of Health Stroke Scale (NIHSS) score was significantly higher among those who presented for hospital treatment (12.9 vs. 11). However, these patients were seen before TPA administration, while the drip-and-ship group had already been treated, a temporal difference that could have accounted for the score finding, cautioned Dr. Sheth, director of the neuroscience ICU and chief of clinical research at Yale University, New Haven, Conn.
In hospitals that employed drip-and-ship, there were significantly higher rates of stroke patients treated each year as well as more beds. Those hospitals also were more often teaching facilities and were designated as a primary stroke center.
Drip-and-ship frequency remained fairly steady over the study period – about 25% of all eligible patients had it in both 2003 and 2010. Among those treated at the hospital, the frequency of TPA administration within 3 hours of stroke onset rose sharply over the study period, from about 11% in 2003 to 25% in 2010. In contrast, the percentage of timely thrombolysis in drip-and-ship patients moved very little, from about 5% to 9% over the same period.
Overall inpatient mortality was 10%, but was slightly higher among drip-and-ship patients (10.93% vs. 9.67). Symptomatic intracranial hemorrhage occurred in 5.79% of those treated via drip-and-ship and 5.22% of those treated in the hospital. Nearly the same percentage of patients were discharged walking independently (38.4% vs. 38.8%) and discharged home (40.3% vs. 40.6%).
Among the hospital-treated patients, fewer than 4% (1,200) underwent endovascular therapy; this occurred in 707 (7%) of drip-and-ship patients. Those who got endovascular treatment had higher median NIHSS scores at TPA administration than did those who did not (17 vs. 12, respectively). Endovascular treatment was significantly associated with higher mortality (20% vs. 10%) and intracranial hemorrhage (11% vs. 5%).
In a multivariate analysis that adjusted for NIHSS score, in-hospital mortality was significantly more likely in drip-and-ship patients (odds ratio, 1.23). Those patients also were significantly less likely to be independently walking at discharge (OR, 0.66) or discharge to home (OR, 0.66). Intracranial hemorrhage was significantly more likely in drip-and-ship patients (OR, 1.4), as was a hospital stay of longer than 4 days (OR, 1.20).
“These are very modest differences clinically,” Dr. Sheth said, adding that selection bias or unmeasured confounding could have influenced the findings.
Dr. Sheth is a member of the Get with the Guidelines (GWTG) Stroke Clinical Workgroup, and he is a coinvestigator and executive committee member for Glyburide Advantage in Malignant Edema and Stroke-Remedy Pharmaceuticals (GAMES-RP), a phase II trial to prevent swelling in patients with large stroke, funded by Remedy Pharmaceuticals.
On Twitter @alz_gal
NASHVILLE, TENN. – About 25% of patients with ischemic stroke who receive thrombolytic therapy get it in the field before hospital transfer with the “drip-and-ship” paradigm.
While there were only modest differences in clinical outcomes between these patients and those treated when admitted to an emergency department, drip-and-ship may actually increase the overall use of tissue plasminogen activator (TPA), Dr. Kevin N. Sheth said at the International Stroke Conference, sponsored by the American Heart Association.
The retrospective analysis, which was simultaneously published in Stroke (2015 Feb. 11 [doi:10.1161/STROKEAHA.114.007506]), plumbed the Get With the Guidelines registry for data to describe trends in the use of TPA and drip-and-ship administration across the United States over time. The study involved 1,440 hospitals and 44,667 patients who had an ischemic stroke during 2003-2010 and received TPA. Of these, 10,475 (23.5%) received it in the field before optional admission and within 3 hours of symptom onset.
Baseline characteristics were similar between the treatment groups. The patients’ mean age was 70 years, and the sex distribution was evenly split. More than 75% of each group was white.
The National Institutes of Health Stroke Scale (NIHSS) score was significantly higher among those who presented for hospital treatment (12.9 vs. 11). However, these patients were seen before TPA administration, while the drip-and-ship group had already been treated, a temporal difference that could have accounted for the score finding, cautioned Dr. Sheth, director of the neuroscience ICU and chief of clinical research at Yale University, New Haven, Conn.
In hospitals that employed drip-and-ship, there were significantly higher rates of stroke patients treated each year as well as more beds. Those hospitals also were more often teaching facilities and were designated as a primary stroke center.
Drip-and-ship frequency remained fairly steady over the study period – about 25% of all eligible patients had it in both 2003 and 2010. Among those treated at the hospital, the frequency of TPA administration within 3 hours of stroke onset rose sharply over the study period, from about 11% in 2003 to 25% in 2010. In contrast, the percentage of timely thrombolysis in drip-and-ship patients moved very little, from about 5% to 9% over the same period.
Overall inpatient mortality was 10%, but was slightly higher among drip-and-ship patients (10.93% vs. 9.67). Symptomatic intracranial hemorrhage occurred in 5.79% of those treated via drip-and-ship and 5.22% of those treated in the hospital. Nearly the same percentage of patients were discharged walking independently (38.4% vs. 38.8%) and discharged home (40.3% vs. 40.6%).
Among the hospital-treated patients, fewer than 4% (1,200) underwent endovascular therapy; this occurred in 707 (7%) of drip-and-ship patients. Those who got endovascular treatment had higher median NIHSS scores at TPA administration than did those who did not (17 vs. 12, respectively). Endovascular treatment was significantly associated with higher mortality (20% vs. 10%) and intracranial hemorrhage (11% vs. 5%).
In a multivariate analysis that adjusted for NIHSS score, in-hospital mortality was significantly more likely in drip-and-ship patients (odds ratio, 1.23). Those patients also were significantly less likely to be independently walking at discharge (OR, 0.66) or discharge to home (OR, 0.66). Intracranial hemorrhage was significantly more likely in drip-and-ship patients (OR, 1.4), as was a hospital stay of longer than 4 days (OR, 1.20).
“These are very modest differences clinically,” Dr. Sheth said, adding that selection bias or unmeasured confounding could have influenced the findings.
Dr. Sheth is a member of the Get with the Guidelines (GWTG) Stroke Clinical Workgroup, and he is a coinvestigator and executive committee member for Glyburide Advantage in Malignant Edema and Stroke-Remedy Pharmaceuticals (GAMES-RP), a phase II trial to prevent swelling in patients with large stroke, funded by Remedy Pharmaceuticals.
On Twitter @alz_gal
NASHVILLE, TENN. – About 25% of patients with ischemic stroke who receive thrombolytic therapy get it in the field before hospital transfer with the “drip-and-ship” paradigm.
While there were only modest differences in clinical outcomes between these patients and those treated when admitted to an emergency department, drip-and-ship may actually increase the overall use of tissue plasminogen activator (TPA), Dr. Kevin N. Sheth said at the International Stroke Conference, sponsored by the American Heart Association.
The retrospective analysis, which was simultaneously published in Stroke (2015 Feb. 11 [doi:10.1161/STROKEAHA.114.007506]), plumbed the Get With the Guidelines registry for data to describe trends in the use of TPA and drip-and-ship administration across the United States over time. The study involved 1,440 hospitals and 44,667 patients who had an ischemic stroke during 2003-2010 and received TPA. Of these, 10,475 (23.5%) received it in the field before optional admission and within 3 hours of symptom onset.
Baseline characteristics were similar between the treatment groups. The patients’ mean age was 70 years, and the sex distribution was evenly split. More than 75% of each group was white.
The National Institutes of Health Stroke Scale (NIHSS) score was significantly higher among those who presented for hospital treatment (12.9 vs. 11). However, these patients were seen before TPA administration, while the drip-and-ship group had already been treated, a temporal difference that could have accounted for the score finding, cautioned Dr. Sheth, director of the neuroscience ICU and chief of clinical research at Yale University, New Haven, Conn.
In hospitals that employed drip-and-ship, there were significantly higher rates of stroke patients treated each year as well as more beds. Those hospitals also were more often teaching facilities and were designated as a primary stroke center.
Drip-and-ship frequency remained fairly steady over the study period – about 25% of all eligible patients had it in both 2003 and 2010. Among those treated at the hospital, the frequency of TPA administration within 3 hours of stroke onset rose sharply over the study period, from about 11% in 2003 to 25% in 2010. In contrast, the percentage of timely thrombolysis in drip-and-ship patients moved very little, from about 5% to 9% over the same period.
Overall inpatient mortality was 10%, but was slightly higher among drip-and-ship patients (10.93% vs. 9.67). Symptomatic intracranial hemorrhage occurred in 5.79% of those treated via drip-and-ship and 5.22% of those treated in the hospital. Nearly the same percentage of patients were discharged walking independently (38.4% vs. 38.8%) and discharged home (40.3% vs. 40.6%).
Among the hospital-treated patients, fewer than 4% (1,200) underwent endovascular therapy; this occurred in 707 (7%) of drip-and-ship patients. Those who got endovascular treatment had higher median NIHSS scores at TPA administration than did those who did not (17 vs. 12, respectively). Endovascular treatment was significantly associated with higher mortality (20% vs. 10%) and intracranial hemorrhage (11% vs. 5%).
In a multivariate analysis that adjusted for NIHSS score, in-hospital mortality was significantly more likely in drip-and-ship patients (odds ratio, 1.23). Those patients also were significantly less likely to be independently walking at discharge (OR, 0.66) or discharge to home (OR, 0.66). Intracranial hemorrhage was significantly more likely in drip-and-ship patients (OR, 1.4), as was a hospital stay of longer than 4 days (OR, 1.20).
“These are very modest differences clinically,” Dr. Sheth said, adding that selection bias or unmeasured confounding could have influenced the findings.
Dr. Sheth is a member of the Get with the Guidelines (GWTG) Stroke Clinical Workgroup, and he is a coinvestigator and executive committee member for Glyburide Advantage in Malignant Edema and Stroke-Remedy Pharmaceuticals (GAMES-RP), a phase II trial to prevent swelling in patients with large stroke, funded by Remedy Pharmaceuticals.
On Twitter @alz_gal
AT THE INTERNATIONAL STROKE CONFERENCE
Key clinical point: The rate of thrombolysis for ischemic stroke via drip-and-ship has remained steady over the past 12 years.
Major finding: About a quarter of ischemic stroke patients eligible for TPA are getting it in the field, via the “drip-and-ship” paradigm.
Data source: A retrospective study comprising 44,667 patients with ischemic stroke.
Disclosures: Dr. Sheth is a member of the Get with the Guidelines (GWTG) Stroke Clinical Workgroup, and he is a coinvestigator and executive committee member for Glyburide Advantage in Malignant Edema and Stroke-Remedy Pharmaceuticals (GAMES-RP), a phase II trial to prevent swelling in patients with large stroke, funded by Remedy Pharmaceuticals.
Upper airway stimulation an option in some patients
CORONADO, CALIF. – Some patients with treatment-refractory obstructive sleep apnea may be candidates for upper airway stimulation, though those with complete concentric palatal collapse may not respond, according to Dr. Marion Boyd Gillespie.
“There’s been sound research showing that patients with obstructive sleep apnea have reduced neural tone, so it may not be due to fat in the tongue; some of it may have to do with reduced neural tone,” Dr. Gillespie, who directs the snoring clinics at the Medical University of South Carolina, Charleston, said at the Triological Society’s Combined Sections Meeting. “During these apneic events, there’s a reduction in the neural tone of the genioglossus muscle, which is the main dilator of the upper airway. With upper airway stimulation, we’re trying to account for that loss of neural tone by providing more neural impulse to these muscle groups that perform the dilator functions.”
In 2014 the Food and Drug Administration cleared an upper airway stimulation system manufactured by Inspire Medical Systems, a pacemakerlike device that’s implanted in the subclavicular space. The system features a stimulator lead that attaches to the right hypoglossal nerve and a sensing lead that goes between the external and internal intercostal muscles to detect breathing. “That allows the device to know when in the phase of respiration to fire,” said Dr. Gillespie, professor of otolaryngology–head and neck surgery at the university. “The sensing lead detects the respiratory wave, and the stimulatory lead starts stimulation at the end of expiration, because that’s when the airway is in its most collapsible state. It continues about two-thirds of the way through the inspiratory cycle to keep the airway open.”
Titration of the device is very similar to continuous positive airway pressure, he continued. Once implanted, the patient “will go back to the sleep lab where a tech who’s trained in the device will ramp up stimulation until observed apneas and hypopneas are adequately reduced. You would think that isolated stimulation of the hypoglossal nerve would only open up the airway at the level of the tongue. However, our initial investigation showed that there is dilation at the velopharynx as well,” Dr. Gillespie said. By moving the tongue out of the posterior airway, “you’re moving the dorsum of the tongue away from the velopharynx. You’re also getting active traction on the palatoglossal fold,” he added.
Results of the initial trial of the system in 126 patients with a mean body mass index of 28.4 kg/m2 were published last year (N. Engl. J. Med. 2014;370:139-49). At 12 months of follow-up, patients experienced a 68% overall reduction in their apnea-hypopnea index (AHI) score, from a preoperative mean of 29 to a postoperative mean of 9. In addition, patients had a 70% overall reduction in their oxygen desaturation index (ODI). The researchers also observed normalization of patient-based outcomes, with improvement in the Functional Outcomes of Sleep Questionnaire score and reduction of the Epworth Sleepiness Scale score to a level of 10 on average. “We also saw a reduction of snoring,” said Dr. Gillespie, who was a member of the research team. “Snoring went from 72% of patients having severe, annoying snoring to the point where a bed partner leaves the room, to 15% postoperatively.” Even so, 96% of patients who had a previous history of uvulopalatopharyngoplasty (UPPP) or laser-assisted uvulopalatoplasty (LAUP) still had tongue-based collapse after 12 months of follow-up. “But we found that their response to this therapy was just as good as people who had never had a UPPP or LAUP,” Dr. Gillespie said at the meeting, jointly sponsored by the Triological Society and the American College of Surgeons. “So it seems like patients who have failed UPPP are still good candidates for upper airway stimulation therapy.”
Dr. Gillespie noted that selection criteria for the trial were limited to patients with a BMI of less than 32 kg/m2 and to those who did not have complete circumferential collapse at the level of the soft palate on preoperative drug-induced endoscopy. These criteria were based on an earlier pilot study that showed that patients with complete circumferential collapse at the level of the soft palate did not respond to upper airway stimulation (J. Clin. Sleep Med. 2013;9:433-8).
Dr. Gillespie disclosed that he has received research support from Inspire Medical Systems, Olympus, and Surgical Specialties. He is also a consultant for those companies as well as for Medtronic.
On Twitter @dougbrunk
Dr. David Schulman, FCCP, comments: The data presented by Dr. Gillespie add to the growing body of literature showing the benefits of stimulation of the upper airway muscles during sleep in a selected subgroup of obstructive sleep apnea (OSA) patients, demonstrating improvements in both physiologic and functional parameters. Given the well-described issues with continuous positive airway pressure (CPAP) adherence and the lesser efficacy of currently available CPAP alternatives, patients with obstructive sleep apnea and their providers have long awaited access to hypoglossal nerve stimulators to add to the armamentarium of options for management of the disorder.
While early data continue to show promise for this treatment, a number of physiologic and anatomic characteristics serve as relative contraindications, limiting the generalizability of study results to some patient populations (such as those with body mass index greater than 32 kg/m2 or those with concentric collapse of the soft palate). While upper airway stimulation is not likely to be the first-line OSA treatment for the majority of patients, it is an important step forward for those unwilling or unable to use CPAP.
Dr. David Schulman, FCCP, comments: The data presented by Dr. Gillespie add to the growing body of literature showing the benefits of stimulation of the upper airway muscles during sleep in a selected subgroup of obstructive sleep apnea (OSA) patients, demonstrating improvements in both physiologic and functional parameters. Given the well-described issues with continuous positive airway pressure (CPAP) adherence and the lesser efficacy of currently available CPAP alternatives, patients with obstructive sleep apnea and their providers have long awaited access to hypoglossal nerve stimulators to add to the armamentarium of options for management of the disorder.
While early data continue to show promise for this treatment, a number of physiologic and anatomic characteristics serve as relative contraindications, limiting the generalizability of study results to some patient populations (such as those with body mass index greater than 32 kg/m2 or those with concentric collapse of the soft palate). While upper airway stimulation is not likely to be the first-line OSA treatment for the majority of patients, it is an important step forward for those unwilling or unable to use CPAP.
Dr. David Schulman, FCCP, comments: The data presented by Dr. Gillespie add to the growing body of literature showing the benefits of stimulation of the upper airway muscles during sleep in a selected subgroup of obstructive sleep apnea (OSA) patients, demonstrating improvements in both physiologic and functional parameters. Given the well-described issues with continuous positive airway pressure (CPAP) adherence and the lesser efficacy of currently available CPAP alternatives, patients with obstructive sleep apnea and their providers have long awaited access to hypoglossal nerve stimulators to add to the armamentarium of options for management of the disorder.
While early data continue to show promise for this treatment, a number of physiologic and anatomic characteristics serve as relative contraindications, limiting the generalizability of study results to some patient populations (such as those with body mass index greater than 32 kg/m2 or those with concentric collapse of the soft palate). While upper airway stimulation is not likely to be the first-line OSA treatment for the majority of patients, it is an important step forward for those unwilling or unable to use CPAP.
CORONADO, CALIF. – Some patients with treatment-refractory obstructive sleep apnea may be candidates for upper airway stimulation, though those with complete concentric palatal collapse may not respond, according to Dr. Marion Boyd Gillespie.
“There’s been sound research showing that patients with obstructive sleep apnea have reduced neural tone, so it may not be due to fat in the tongue; some of it may have to do with reduced neural tone,” Dr. Gillespie, who directs the snoring clinics at the Medical University of South Carolina, Charleston, said at the Triological Society’s Combined Sections Meeting. “During these apneic events, there’s a reduction in the neural tone of the genioglossus muscle, which is the main dilator of the upper airway. With upper airway stimulation, we’re trying to account for that loss of neural tone by providing more neural impulse to these muscle groups that perform the dilator functions.”
In 2014 the Food and Drug Administration cleared an upper airway stimulation system manufactured by Inspire Medical Systems, a pacemakerlike device that’s implanted in the subclavicular space. The system features a stimulator lead that attaches to the right hypoglossal nerve and a sensing lead that goes between the external and internal intercostal muscles to detect breathing. “That allows the device to know when in the phase of respiration to fire,” said Dr. Gillespie, professor of otolaryngology–head and neck surgery at the university. “The sensing lead detects the respiratory wave, and the stimulatory lead starts stimulation at the end of expiration, because that’s when the airway is in its most collapsible state. It continues about two-thirds of the way through the inspiratory cycle to keep the airway open.”
Titration of the device is very similar to continuous positive airway pressure, he continued. Once implanted, the patient “will go back to the sleep lab where a tech who’s trained in the device will ramp up stimulation until observed apneas and hypopneas are adequately reduced. You would think that isolated stimulation of the hypoglossal nerve would only open up the airway at the level of the tongue. However, our initial investigation showed that there is dilation at the velopharynx as well,” Dr. Gillespie said. By moving the tongue out of the posterior airway, “you’re moving the dorsum of the tongue away from the velopharynx. You’re also getting active traction on the palatoglossal fold,” he added.
Results of the initial trial of the system in 126 patients with a mean body mass index of 28.4 kg/m2 were published last year (N. Engl. J. Med. 2014;370:139-49). At 12 months of follow-up, patients experienced a 68% overall reduction in their apnea-hypopnea index (AHI) score, from a preoperative mean of 29 to a postoperative mean of 9. In addition, patients had a 70% overall reduction in their oxygen desaturation index (ODI). The researchers also observed normalization of patient-based outcomes, with improvement in the Functional Outcomes of Sleep Questionnaire score and reduction of the Epworth Sleepiness Scale score to a level of 10 on average. “We also saw a reduction of snoring,” said Dr. Gillespie, who was a member of the research team. “Snoring went from 72% of patients having severe, annoying snoring to the point where a bed partner leaves the room, to 15% postoperatively.” Even so, 96% of patients who had a previous history of uvulopalatopharyngoplasty (UPPP) or laser-assisted uvulopalatoplasty (LAUP) still had tongue-based collapse after 12 months of follow-up. “But we found that their response to this therapy was just as good as people who had never had a UPPP or LAUP,” Dr. Gillespie said at the meeting, jointly sponsored by the Triological Society and the American College of Surgeons. “So it seems like patients who have failed UPPP are still good candidates for upper airway stimulation therapy.”
Dr. Gillespie noted that selection criteria for the trial were limited to patients with a BMI of less than 32 kg/m2 and to those who did not have complete circumferential collapse at the level of the soft palate on preoperative drug-induced endoscopy. These criteria were based on an earlier pilot study that showed that patients with complete circumferential collapse at the level of the soft palate did not respond to upper airway stimulation (J. Clin. Sleep Med. 2013;9:433-8).
Dr. Gillespie disclosed that he has received research support from Inspire Medical Systems, Olympus, and Surgical Specialties. He is also a consultant for those companies as well as for Medtronic.
On Twitter @dougbrunk
CORONADO, CALIF. – Some patients with treatment-refractory obstructive sleep apnea may be candidates for upper airway stimulation, though those with complete concentric palatal collapse may not respond, according to Dr. Marion Boyd Gillespie.
“There’s been sound research showing that patients with obstructive sleep apnea have reduced neural tone, so it may not be due to fat in the tongue; some of it may have to do with reduced neural tone,” Dr. Gillespie, who directs the snoring clinics at the Medical University of South Carolina, Charleston, said at the Triological Society’s Combined Sections Meeting. “During these apneic events, there’s a reduction in the neural tone of the genioglossus muscle, which is the main dilator of the upper airway. With upper airway stimulation, we’re trying to account for that loss of neural tone by providing more neural impulse to these muscle groups that perform the dilator functions.”
In 2014 the Food and Drug Administration cleared an upper airway stimulation system manufactured by Inspire Medical Systems, a pacemakerlike device that’s implanted in the subclavicular space. The system features a stimulator lead that attaches to the right hypoglossal nerve and a sensing lead that goes between the external and internal intercostal muscles to detect breathing. “That allows the device to know when in the phase of respiration to fire,” said Dr. Gillespie, professor of otolaryngology–head and neck surgery at the university. “The sensing lead detects the respiratory wave, and the stimulatory lead starts stimulation at the end of expiration, because that’s when the airway is in its most collapsible state. It continues about two-thirds of the way through the inspiratory cycle to keep the airway open.”
Titration of the device is very similar to continuous positive airway pressure, he continued. Once implanted, the patient “will go back to the sleep lab where a tech who’s trained in the device will ramp up stimulation until observed apneas and hypopneas are adequately reduced. You would think that isolated stimulation of the hypoglossal nerve would only open up the airway at the level of the tongue. However, our initial investigation showed that there is dilation at the velopharynx as well,” Dr. Gillespie said. By moving the tongue out of the posterior airway, “you’re moving the dorsum of the tongue away from the velopharynx. You’re also getting active traction on the palatoglossal fold,” he added.
Results of the initial trial of the system in 126 patients with a mean body mass index of 28.4 kg/m2 were published last year (N. Engl. J. Med. 2014;370:139-49). At 12 months of follow-up, patients experienced a 68% overall reduction in their apnea-hypopnea index (AHI) score, from a preoperative mean of 29 to a postoperative mean of 9. In addition, patients had a 70% overall reduction in their oxygen desaturation index (ODI). The researchers also observed normalization of patient-based outcomes, with improvement in the Functional Outcomes of Sleep Questionnaire score and reduction of the Epworth Sleepiness Scale score to a level of 10 on average. “We also saw a reduction of snoring,” said Dr. Gillespie, who was a member of the research team. “Snoring went from 72% of patients having severe, annoying snoring to the point where a bed partner leaves the room, to 15% postoperatively.” Even so, 96% of patients who had a previous history of uvulopalatopharyngoplasty (UPPP) or laser-assisted uvulopalatoplasty (LAUP) still had tongue-based collapse after 12 months of follow-up. “But we found that their response to this therapy was just as good as people who had never had a UPPP or LAUP,” Dr. Gillespie said at the meeting, jointly sponsored by the Triological Society and the American College of Surgeons. “So it seems like patients who have failed UPPP are still good candidates for upper airway stimulation therapy.”
Dr. Gillespie noted that selection criteria for the trial were limited to patients with a BMI of less than 32 kg/m2 and to those who did not have complete circumferential collapse at the level of the soft palate on preoperative drug-induced endoscopy. These criteria were based on an earlier pilot study that showed that patients with complete circumferential collapse at the level of the soft palate did not respond to upper airway stimulation (J. Clin. Sleep Med. 2013;9:433-8).
Dr. Gillespie disclosed that he has received research support from Inspire Medical Systems, Olympus, and Surgical Specialties. He is also a consultant for those companies as well as for Medtronic.
On Twitter @dougbrunk
EXPERT ANALYSIS AT THE COMBINED SECTIONS WINTER MEETING