User login
Thiamine Prescribing Practices
Thiamine pyrophosphate, the biologically active form of thiamine (vitamin B1), is an essential cofactor for the aerobic breakdown of glucose, with daily requirements related to total caloric intake and the proportion of calories provided as carbohydrates. Patients presenting to the hospital are at risk of thiamine deficiency due to a preponderance of factors including baseline poor nutritional status,[1, 2, 3] diminished intake (associated with illness prodrome), increased metabolic demand (eg, occurring with sepsis, malignancy, surgery, pregnancy), and resuscitation with intravenous glucose‐containing fluids. Without thiamine pyrophosphate, glucose is metabolized through less‐efficient anaerobic pathways, producing lactic acid as a by‐product. The brain uses glucose as its main source of energy and is susceptible to injury due to acute thiamine deficiency. Petechial hemorrhage and demyelination within periventricular structures (thalami, mammillary bodies, ocular motor nuclei, and cerebellar vermis) account for the classical triad of Wernicke's encephalopathy (WE), including confusion/encephalopathy, ophthalmoplegia, and ataxia.[4, 5, 6, 7] Additional symptoms and signs are reported in WE, implicating more widespread consequences of thiamine deficiency.[6, 7, 8, 9]
Wernicke's encephalopathy remains an historically well‐documented illness,[4, 5] of continued relevance to hospitalist practice. Pathologic changes diagnostic of WE are reported in 1.9% to 2.8% of all patients at autopsy,[6, 10] and in as many as 20% of hospitalized patients following unexplained death.[11, 12] In cases of alcohol‐related death, the incidence may be as high as 59%.[13] Most troubling is the observation that the diagnosis is frequently missed in clinical settings,[14, 15, 16, 17, 18, 19] with devastating results. Case series report mortality in upward of 20% of patients with untreated or undertreated WE, with 85% of survivors developing Korsakoff syndromethe chronic form of WE.[8, 20, 21] The clinical diagnosis may be missed in as many as 94% of nonalcoholic patients, including patients admitted to surgical (ie, postgastrointestinal surgery), obstetrical (ie, hyperemesis gravidarum), psychiatric (ie, eating disorders), general internal medicine (ie, cancer‐associated cachexia and related complications), and subspecialty services (ie, dialysis and renal diseases).[15]
Recognition of WE is critical, as effective intervention and treatment is possible. For hospitalized patients, intervention should prioritize parenteral administration of thiamine[22, 23] to circumvent problems with oral absorption common in the medically ill, and to maximize serum thiamine concentrations, promoting passive (concentration‐dependent) movement of thiamine across the blood brain barrier. In lieu of evidence from randomized controlled trials supporting specific doses and schedules for thiamine administration,[24, 25] this recommendation relies on ample experiential evidence emphasizing the importance of higher doses of parenteral thiamine (in excess of 200 mg provided 3 times daily) for the prevention/treatment of WE.[12, 15, 20, 26, 27]
It remains to be determined whether the evidence emphasizing parenteral prescribing has affected current practice within inpatient populations. To address this need, we sought to quantify thiamine‐prescribing practices within Canadian university‐affiliated hospitals across a 2‐year period. We hypothesized that thiamine would be prescribed more frequently via the parenteral route, in line with published guidelines promoting parenteral administration of thiamine.[15, 20]
METHODS
Study Design and Recruitment
A retrospective observational study was used to evaluate thiamine prescribing within Canadian academic hospitals between January 2010 and December 2011. University‐affiliated institutions with English‐speaking postgraduate (ie, residency) adult medicine programs were eligible to participate. Participating centers all utilized computerized pharmacy information systems, allowing anonymized retrospective data to be reported for participants. Study objectives, methods, and procedures were approved by institutional research ethics boards at participating centers.
Data were extracted for the study period from computerized pharmacy information systems recording prescriptions processed by centralized hospital pharmacies. Thiamine prescribed as part of total parenteral nutrition was excluded from analysis, as prescribing was automated in most centers. Participants were assigned a randomized study number linked to prescription information specifying the prescribed dose of thiamine, route of administration (oral: per os, nasogastric tube, orogastric tube, gastric tube; versus parenteral: intravenous, intramuscular), frequency of dosing (eg, daily, twice daily, 3 times daily), and start/end dates. Complete data were available from 12 hospitals (12/14, 85.7%). One hospital was missing data concerning the frequency of prescribing. One hospital provided only summary data, specifying the number of prescriptions issued by route and dose. Information concerning the prescribing service was captured within records from 7 hospitals (7/14, 50.0%), allowing prescriptions to be stratified by prescribing services. Subspecialty designations were simplified to emergency department, intensive care unit (ICU) (including medical, surgical, and trauma ICUs), medical subspecialty (ie, cardiology, endocrinology, gastroenterology, medical oncology, rheumatology), general internal medicine, neurology, psychiatry, and surgical (ie, general surgery, cardiac surgery, neurosurgery, orthopedics, gynecology) services.
Statistical Analysis
Prescriptions for thiamine were summarized across each center specifying the route of administration (all prescriptions and initial prescription). Prescribing behaviors across centers were summarized using descriptive statistics. Differences in parenteral versus oral prescribing were evaluated using the z test for the difference between weighted averages, assuming a null hypothesis of equal prescribing (ie, 50% parenteral and 50% oral) within categories (i.e., all prescriptions, initial prescriptions, total doses).
Factors that may affect rates of parenteral prescribing were considered across centers. Hospitals were stratified based upon the presence of protocols promoting parenteral thiamine prescribing for the treatment of patients at risk of deficiency. The effect of protocols on prescribing behaviors was evaluated by comparing prescribing practices in centers with and without protocols using the z test for the difference between proportions. Forward linear regression was used to evaluate the correlation between the number of prescriptions and/or doses prescribed within centers, and the rates of parenteral prescribing. Rates of parenteral prescribing across hospital services were compared using pairwise comparisons.
Statistical analyses were completed using IBM SPSS Statistics 20 (IBM Corp., Armonk, NY). Significance was defined as P<0.05, using P values corrected for the total number of comparisons (Bonferroni correction), equivalent to a single‐comparison P value<0.001.
RESULTS
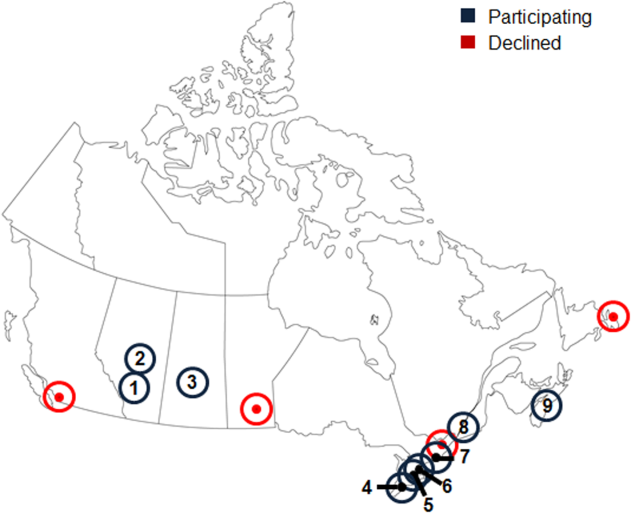
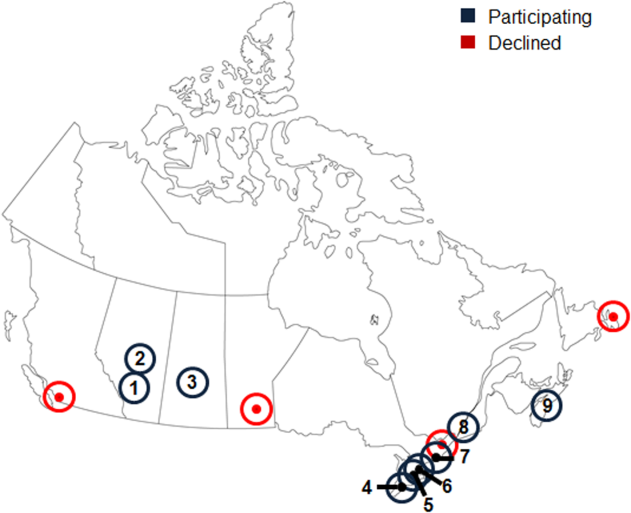
Thirteen university‐affiliated academic centers met inclusion criterion and were invited to participate. Data were obtained from 9 organizations (9/13, 69%), encompassing 14 geographically distributed academic hospitals (Figure 1). Centers that declined to participate cited a lack of comprehensive electronic databases tracking prescriptions as the main barrier. One of the 14 participating hospitals represented a network of affiliated health centers (site 2), rendering it an outlier in terms of total number of doses prescribed (>3 standard deviations above the mean). This affiliated network was unable to provide prescribing data separated by hospital and was excluded from analyses.
In total, data were collected corresponding to 48,806 prescriptions for 209,762 doses of thiamine, provided to 32,213 patients (Table 1). Prescriptions were divided by route of administration (parenteral vs oral) and prescribing practices summarized across centers. Rates of parenteral prescribing varied widely between centers (maximum=82.0%, minimum=33.9%). Overall, however, parenteral thiamine was prescribed more frequently than oral thiamine, accounting for 57.6% of all prescriptions (z=33.59, P<0.001) and 59.2% of initial prescriptions issued to patients (z=168.93, P<0.001). Oral thiamine constituted a significant majority of the total doses prescribed (68.4%, z=168.9; P<0.001).
| All Prescriptions | First Prescriptions | Doses | ||||||||
|---|---|---|---|---|---|---|---|---|---|---|
| Hospital | Total | Parenteral % | Oral % | Total | Parenteral % | Oral % | Total | Parenteral % | Oral % | |
| ||||||||||
| 1A* | 7,499 | 66.42 | 33.58 | 6,189 | 66.41 | 33.59 | 36,032 | 26.60 | 73.40 | |
| 1B* | 4,372 | 70.38 | 29.62 | 2,314 | 78.69 | 21.31 | 17,133 | 27.61 | 72.39 | |
| 1C* | 5,805 | 57.64 | 42.36 | 4,742 | 58.10 | 41.90 | 30,910 | 17.12 | 82.88 | |
| 3* | 3,519 | 45.27 | 54.73 | 2,236 | 59.57 | 40.43 | 8,037 | 52.37 | 47.63 | |
| 4* | 5,322 | 60.50 | 39.50 | 2,900 | 57.83 | 42.17 | 22,159 | 44.49 | 55.51 | |
| 5 | 3,857 | 46.85 | 53.15 | 2,858 | 56.37 | 43.63 | 16,035 | 33.51 | 66.49 | |
| 6A* | 6,105 | 55.46 | 44.54 | 2,907 | 55.04 | 44.96 | 15,947 | 44.22 | 55.78 | |
| 6B | 3,127 | 55.39 | 44.61 | 1,710 | 64.62 | 35.38 | 9,048 | 45.11 | 54.89 | |
| 6C* | 2,134 | 55.62 | 44.38 | 1,499 | 56.24 | 43.76 | 10,327 | 32.33 | 67.67 | |
| 6D | 617 | 35.82 | 64.18 | 505 | 34.65 | 65.35 | 2,647 | 35.70 | 64.30 | |
| 7* | 2,467 | 82.00 | 18.00 | 1,373 | 87.40 | 12.60 | 9,924 | 59.06 | 40.94 | |
| 8 | 1,122 | 50.98 | 49.02 | 694 | 58.79 | 41.21 | 3,048 | 64.73 | 35.27 | |
| 9 | 2,860 | 33.85 | 66.15 | 2,286 | 19.34 | 80.66 | 28,515 | 14.73 | 85.27 | |
| Total | 48,806 | 57.60 | 42.40 | 32,213 | 59.23 | 40.77 | 209,762 | 31.56 | 68.44 | |
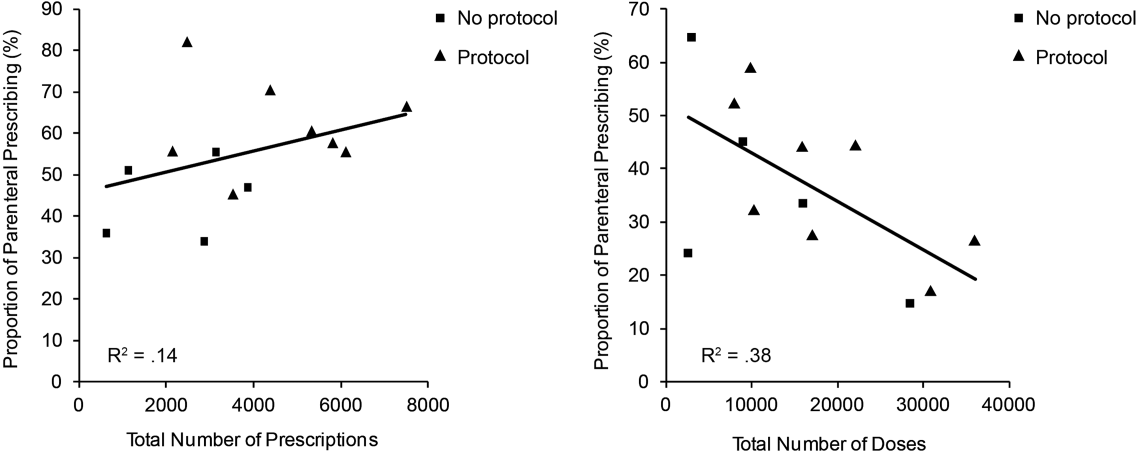
The factors associated with higher rates of parenteral prescribing were further considered. Rates of parenteral prescribing were compared between centers with and without published guidelines governing thiamine usage. Eight of 13 hospitals (61.5%) had hospital‐wide protocols that promoted initial administration of thiamine via the parenteral route in patients at risk of deficiency. The presence of a protocol was associated with significantly higher overall rates of parenteral prescribing (61.3% with protocol, 45.7% without protocol; z=29.5; P<0.001). Linear regression revealed no predictive relationship between the number of prescriptions issued and the proportion of parenteral thiamine prescribed across centers (total prescriptions, standard 0.38, P=0.20; Figure 2). A negative correlation was observed between the proportion of doses prescribed via the parenteral route and the number of total doses prescribed (standard =0.61, P=0.03), suggesting that centers prescribing the greatest numbers of doses were less likely to prescribe parenteral thiamine.
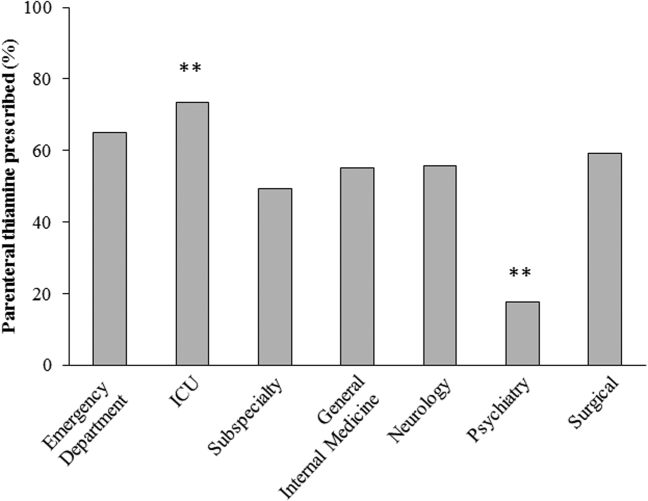
The effect of the inpatient practice environment on prescribing behavior was considered across the 7 centers for which service‐specific prescribing data were provided (Figure 3). Patients receiving care within emergency departments or intensive care, general medical or surgical units were more likely to be prescribed parenteral thiamine (z>3.0, P<0.001), whereas patients admitted to psychiatry units were more likely to be prescribed thiamine via the oral route (z=23.7, P<0.001). No differences were observed between rates of parenteral and oral prescribing for patients admitted under medical subspecialty (z=0.6, P=not significant) and neurology services (z=3.1, P=not significant). Pair‐wise comparisons for means confirmed that patients admitted to the ICU were significantly more likely to be prescribed parenteral thiamine than patients admitted to any other service; psychiatry inpatients were the least likely to be prescribed parenteral thiamine (P<0.001).
A post hoc analysis was used to determine the service‐specific effect of hospital‐wide protocols promoting parenteral prescribing. Protocols were associated with significantly higher rates of parenteral prescribing for patients receiving care under all services (ICU, z=3.76; medical subspecialties, z=16.07; general medicine, z=15.40; neurology, z=7.02; surgery, z=13.19; P<0.001), except psychiatry (z=2.0, P=not significant) and those within emergency departments (z=2.05, P=not significant).
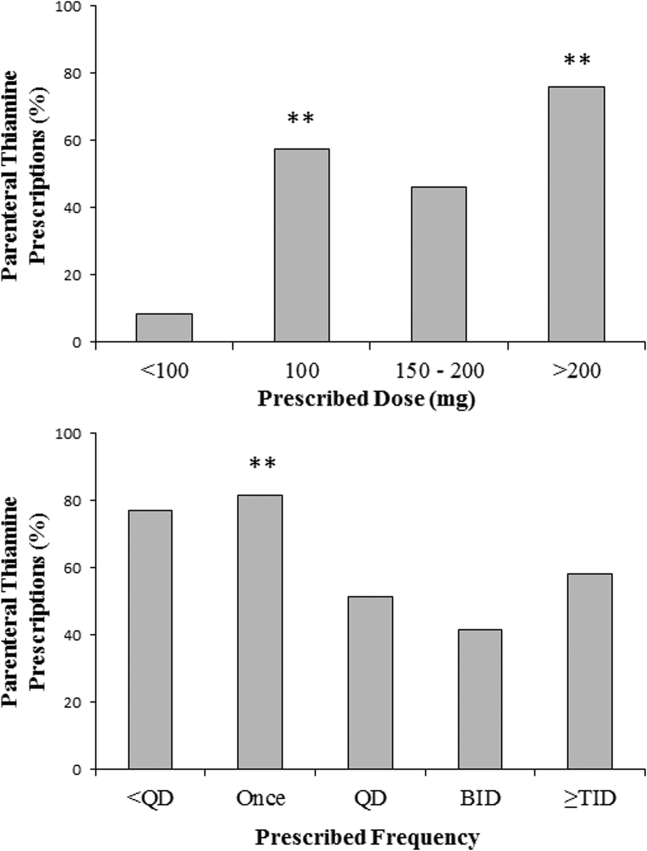
In contrast to the differences in the rates of parenteral prescribing across centers, quantitative review of the doses and schedule of thiamine administration revealed a near‐universal approach to prescribing. Overall, 92.7% (45,266/48,806) of prescriptions were for 100 mg of thiamine (z=188.8, P<0.001); 74.6% (33,551/44,948) of prescriptions were ordered once daily (z=104.5, P<0.001). Thiamine was more likely to be prescribed via the parenteral route when prescribed in doses of 100 mg (57.6%, z=32.5, P<0.001) or >200 mg (76.1%, z=25.51, P<0.001), or when ordered as single doses (81.5%, z=64.86, P<0.001) (Figure 4).
DISCUSSION
The causal relationship between thiamine deficiency and WE has been recognized since 1941,[28] with the importance of parenteral thiamine replacement in vulnerable populations emphasized in numerous case series,[29, 30] population‐based studies,[14, 31, 32] and consensus guidelines.[15, 20] Consistent with guideline recommendations, thiamine was significantly more likely to be prescribed via the parenteral route across a large network of geographically distributed Canadian academic healthcare centers. Somewhat surprisingly, however, oral thiamine accounted for 42.4% of all prescriptions, and a significant majority of doses prescribed to the over 30,000 patients studied. These findings confirm that oral thiamine continues to be prescribed to inpatients within Canadian academic hospitals.
The critical importance of parenteral thiamine administration for the treatment and prevention of WE is supported by an understanding of the pathophysiology of this disease. Wernicke's encephalopathy results from brain‐thiamine deficiency, leading to a cellular energy deficit, focal acidosis, regional increases in glutamate, and cell death.[20, 33, 34] Serum thiamine crosses the blood brain barrier through active (transporter‐mediated) and passive (concentration‐dependent) means.[20] It is therefore possible to drive thiamine into the central nervous system by establishing high serum thiamine levels. Interestingly, forward linear regression suggested that the centers prescribing the largest number of doses were less likely to prescribe parenteral thiamine. This may reflect a misguided preference for the use of prolonged/frequent courses of oral thiamine for the prevention and/or treatment of thiamine deficiency in hospitalized patients. Oral absorption of thiamine occurs within the duodenum by a rate‐limited process, with maximum absorption of 4.5 mg per dose.[20] This rate may be higher in healthy individuals, arguing for passive and active transport across enterocytes.[35] In sick individuals, however, oral absorption cannot be relied upon to attain the high serum thiamine levels necessary to reverse the effects of deficiency, exemplifying the importance of parenteral administration in hospitalized patients.
Protocols promoting parenteral administration of thiamine were associated with higher rates of parenteral prescribing across centers, and may represent an effective and convenient means of effecting prescriber behaviors. Efforts must be made, however, to identify additional barriers limiting parenteral thiamine prescribing within hospitals. One such barrier relates to the identification of at‐risk individuals. Despite advances in biochemical measures quantifying thiamine deficiency[36] and neuroimaging studies confirming changes within the brains of affected patients,[37] WE remains a clinical diagnosis. As such, clinical criteria have been proposed to identify those at risk of deficiency, with an emphasis on detection of WE in patients with alcohol‐use disorders. Specifically, guidelines from the Royal College of Physicians advocate that WE be considered in patients with evidence of alcohol misuse, and 1 of the following: (1) acute confusion, (2) decreased consciousness, (3) ataxia, (4) ophthalmoplegia, (5) memory disturbances, and (6) hypothermia with hypotension.[20] The European Federation of Neurological Sciences broadens the clinical criteria to include patients with and without alcohol‐use disorders, encouraging diagnosis and treatment in individuals with any 2 of the following: (1) dietary deficiencies, (2) oculomotor abnormalities, (3) cerebellar dysfunction, and (4) altered mental status or mild memory impairment.[15] Once identified, it remains imperative that patients receive appropriate therapies to reverse thiamine deficiency. To this end, the results of the present study may be used to identify potential inpatient populations at risk of undertreatment.
Psychiatric patients were the least likely to be prescribed parenteral thiamine, regardless of whether protocols promoting parenteral prescribing were in place within the study hospital. This observation is concerning, as psychiatric inpatients may be at risk of thiamine deficiency due to a confluence of factors related to mental illness (ie, malnutrition associated with eating disorders, substance abuse)[21, 38] and increased rates of comorbid physical illnesses.[39] Low rates of parenteral prescribing may reflect a number of service‐specific (ie, decreased ease of administration of parenteral medications) and patient‐specific factors (ie, challenges of maintaining intravenous catheter access in acutely ill psychiatric patients) that are not adequately addressed by hospital‐wide protocols. Alternatively, lower rates of parenteral prescribing may reflect a systematic preference for the use of oral thiamine in a patient population perceived to be at lower risk of thiamine deficiency. Although oral thiamine has been shown to be effective in correcting thiamine deficiency in a group of community‐dwelling elderly patients without clinical symptoms or signs suggesting WE (ie, subclinical thiamine deficiency),[40] it remains to be determined whether a similar treatment strategy can be endorsed in select inpatients with subclinical deficiency, in whom oral absorption and compliance can be reasonably assured.
Randomized control trial evidence supporting specific doses and schedules for administration of parenteral thiamine is not available.[24, 25] Accordingly, uncertainty exists concerning the doses and frequency of administration of thiamine required to prevent or reverse suspected WE. Despite this uncertainty, the dose and schedule of thiamine prescribed in our study population was remarkably uniform, with thiamine most commonly prescribed in 100‐mg doses once daily. Similar findings were reported in a retrospective study considering thiamine prescribing to 217 patients with alcohol‐use disorders admitted to an urban US teaching hospital: 76.9% of inpatients were prescribed 100‐mg daily doses of thiamine.[19] Interestingly, no differences in prescribing behaviors were noted when high‐risk patients presenting with alcohol intoxication, withdrawal, or delirium tremens were considered separately, suggesting that patient‐specific factors had little impact on the dosing strategy endorsed by clinicians.[19]
Although pervasive, the provision of 100 mg of thiamine daily is not supported by biochemical or clinical studies.[27] On the contrary, clinical‐pathological studies suggest that doses of thiamine between 50 and 100 mg per day may not be sufficient to reverse clinical signs or prevent death in patients with WE, whereas doses up to 250 mg may not reverse the biochemical abnormalities associated with clinically significant deficiency.[12, 41, 42] Such rationale is cited in support of consensus recommendations promoting administration of high doses of parenteral thiamine for the treatment of WE (200 or 500 mg, provided 3 times daily).[15, 20] As this project illustrates, however, rational, well‐justified guidelines are not enough to transform clinical practice.
The limitations of consensus guidelines and hospital‐specific protocols promoting thiamine prescribing have been explored in other specialty[43] and hospital environments.[44, 45] These studies offer several insights into the factors that may contribute to the disparity between recommended and real‐world practices, including continued under‐recognition of malnourished hospitalized patients at risk of thiamine deficiency,[1, 45, 46, 47] variations in consensus‐based guidelines governing thiamine prescribing,[15, 20] and challenges in communication of protocol rationales and recommendations.[44, 48] Together, these findings exemplify the need for additional strategies aimed at improving parenteral prescribing in vulnerable hospitalized populations. The proliferation of computerized physician order entry and clinical decision support systems may offer the opportunity to effect prescribing behaviors, with the possibility of specifying routes and doses of thiamine administration in accordance with guidelines,[49] without the requirement for dedicated monitoring and personnel‐driven interventions.
Limitations
By design, our study was limited to the assessment of thiamine‐prescribing data obtained directly from computerized pharmacy information systems. Consequently, only the minimum details required to safely prescribe a medication were captured. As a result, we were unable to evaluate the potential effect of patient‐specific factors (including clinical diagnosis) on prescriber behaviors. Thus, it remains possible that prescribing behaviors varied according to perceived patient risks in our study population. An additional limitation relates to the generalizability of results beyond academic hospitals in Canada. We suggest, however, that potential concerns relating to generalizability are counterbalanced by 2 advantages inherent within our study population. The first is that the majority of community‐based clinicians are trained within university‐affiliated hospitals. As a result, prescribing behaviors measured in these training centers should reflect optimal behaviors within downstream networks of community hospitals. The second is that the recruitment of hospitals funded by a universal single‐payer served to minimize variability in prescribing behaviors attributable to prescriber and patient concerns regarding reimbursement, thus providing a more accurate assessment of prescriber behaviors based on clinical evidence, independent of financial factors.
Acknowledging these limitations, we assert that parenteral administration of thiamine remains the best means of rapidly correcting thiamine deficiency, and should be considered for the treatment of clinically relevant thiamine deficiency in hospitalized patients. This recommendation effectively balances the potentially deleterious consequences of undertreatment of thiamine deficiency, with the favorable risk‐ and cost‐profile associated with the administration of parenteral thiamine.[15, 20, 23, 27, 50, 51]
CLINICAL AND RESEARCH IMPLICATIONS
In an era of overuse of vitamin supplementation,[52] it is increasingly important for healthcare providers to recognize not only when vitamin supplementation is required, but also how replacement therapies should be delivered. As shown in this study, protocols promoting the use of parenteral thiamine may improve overall compliance with recommendations. However, additional strategies are required to further improve rates of parenteral prescribing to hospitalized patients at risk of thiamine deficiency.
Acknowledgements
The authors are grateful for the contributions of support staff within local hospital pharmacy and information technology departments who made collection of these data possible. Dr. David F. Tang‐Wai reviewed an earlier draft of the manuscript and provided useful comments for which we are grateful.
Disclosures: G. S. Day developed the study concept and methods for implementation, and was primarily responsible for acquisition, analysis, and interpretation of data, as well as drafting, revision, and finalization of the manuscript. G. S. Day had full access to all study data, and takes responsibility for the integrity of the data and the accuracy of the analysis and interpretation. S. Ladak participated in the development of methods, acquisition of data, and revision and finalization of the manuscript. K. Curley participated in the development of methods, acquisition of data, and revision and finalization of the manuscript. N. A. S. Farb participated in analysis of data, and revision and finalization of the manuscript. P. Masiowski participated in acquisition of data, and revision and finalization of the manuscript. T. Pringsheim participated in acquisition of data, and revision and finalization of the manuscript. M. Ritchie participated in acquisition of data, and revision and finalization of the manuscript. A. Cheung participated in acquisition of data, and revision and finalization of the manuscript. S. Jansen participated in acquisition of data, and revision and finalization of the manuscript. L. Methot participated in acquisition of data, and revision and finalization of the manuscript. H. L. Neville participated in acquisition of data, and revision and finalization of the manuscript. D. Bates participated in acquisition of data, and revision and finalization of the manuscript. D. Lowe participated in acquisition of data, and revision and finalization of the manuscript. N. Fernandes participated in acquisition of data, and revision and finalization of the manuscript. A. Ferland participated in acquisition of data, and revision and finalization of the manuscript. C. M. del Campo acted as primary supervisor for this project, and approved study design and methods. He assisted with interpretation of data, and revision and finalization of the manuscript. Preliminary data were reported in abstract form at the 2013 Annual Meeting of the American Academy of Neurology (March 2013, San Diego, CA) and the 2014 Annual Meeting of the Canadian Neurological Sciences Foundation (June 2014, Banff, AB, Canada). No sources of funding are reported for this study. The authors report no conflicts of interest.
- , , , , . Nutrition in the hospitalized patient. J Hosp Med. 2013;8(1):52–58.
- , . The impact of malnutrition on morbidity, mortality, length of hospital stay and costs evaluated through a multivariate model analysis. Clin Nutr. 2003;22(3):235–239.
- , , , et al. Prevalence of malnutrition on admission to four hospitals in England. The Malnutrition Prevalence Group. Clin Nutr. 2000;19(3):191–195.
- . Die akute haemorrhagische polioencephalitis superior: Kassel, Germany; Fisher; 1881.
- . Diseases of the Nervous System. 5th ed. London: Oxford University Press; 1955.
- , , . Wernicke's encephalopathy. A clinical and pathological study of 28 autopsied cases. Arch Neurol. 1961;4:510–519.
- , , , . Diagnosis by treatment. J Hosp Med. 2011;6(9):546–549.
- , , . The Wernicke‐Korsakoff syndrome. A clinical and pathological study of 245 patients, 82 with post‐mortem examinations. Contemp Neurol Ser. 1971;7:1–206.
- , , , . Operational criteria for the classification of chronic alcoholics: identification of Wernicke's encephalopathy. J Neurol Neurosurg Psychiatry. 1997;62(1):51–60.
- . The incidence of Wernicke's encephalopathy in Australia–a neuropathological study of 131 cases. J Neurol Neurosurg Psychiatry. 1983;46(7):593–598.
- . Wernicke's encephalopathy: a more common disease than realised. A neuropathological study of 51 cases. J Neurol Neurosurg Psychiatry. 1979;42:226–231.
- , , . Clinical signs in the Wernicke‐Korsakoff complex: a retrospective analysis of 131 cases diagnosed at necropsy. J Neurol Neurosurg Psychiatry. 1986;49:341–345.
- , , . Autopsy prevalence of Wernicke's encephalopathy in alcohol‐related disease. S Afr Med J. 1996;86(9):1110–1112.
- , . Increasing incidence of Korsakoff's psychosis in the east end of Glasgow. Alcohol Alcohol. 1997;32(3):281–285.
- , , , et al. EFNS guidelines for diagnosis, therapy and prevention of Wernicke encephalopathy. Eur J Neurol. 2010;17(12):1408–1418.
- , , . A survey of the current clinical practice of psychiatrists and accident and emergency specialists in the United Kingdom concerning vitamin supplementation for chronic alcohol misusers. Alcohol Alcohol. 1999;34(6):862–867.
- , , . Thiamine deficiency in head injury: a missed insult? Alcohol Alcohol. 1997;32(4):493–500.
- . Thiamine (vitamin B1) deficiency and associated brain damage is still common throughout the world and prevention is simple and safe! Eur J Neurol. 2006;13(10):1078–1082.
- , , . Prescribing thiamine to inpatients with alcohol use disorders: how well are we doing? J Addict Med. 2014;8(1):1–5.
- , , , ; Royal College of Physicians, London. The Royal College of Physicians report on alcohol: guidelines for managing Wernicke's encephalopathy in the accident and Emergency Department. Alcohol Alcohol. 2002;37(6):513–521.
- , , . B Vitamin deficiency and neuropsychiatric syndromes in alcohol misuse. Alcohol Alcohol. 1998;33(4):317–336.
- , . Five things to know about Wernicke's Encephalopathy: a medical emergency. CMAJ. 2013;186(8):E295.
- , , . Wernicke‐Korsakoff‐syndrome: under‐recognized and under‐treated. Psychosomatics. 2012;53(6):507–516.
- , , , , . Thiamine for Wernicke‐Korsakoff Syndrome in people at risk from alcohol abuse. Cochrane Database Syst Rev. 2004;(1):CD004033.
- , , , , . Thiamine for prevention and treatment of Wernicke‐Korsakoff Syndrome in people who abuse alcohol. Cochrane Database Syst Rev. 2013;7:CD004033.
- , , . Thiamine Treatment and working memory function of alcohol‐dependent people: preliminary findings. Alcohol Clin Exp Res. 2001;25(1):112–116.
- , , , . Myths and misconceptions of Wernicke's encephalopathy: what every emergency physician should know. Ann Emerg Med. 2007;50(6):715–721.
- , . Wernicke's Encephalopathy: The clinical features and their probable relationship to vitamin B deficiency. Q J Med. 1941;10:(37):41–64.
- , , . Patterns of 35S‐thiamine hydrochloride absorption in the malnourished alcoholic patient. J Lab Clin Med. 1970;76(1):34–45.
- , , , . Thiamine propyl disulfide: absorption and utilization. Ann Intern Med. 1971;74(4):529–534.
- , . Parenteral thiamine and Wernicke's encephalopathy: the balance of risks and perception of concern. Alcohol Alcohol. 1997;32(3):207–209.
- , , , et al. Efficacy of vitamin supplementation in chronic alcoholics undergoing detoxification. Alcohol Alcohol Suppl. 1983;18:157–166.
- , , . Mechanisms of neuronal cell death in Wernicke's encephalopathy. Metab Brain Dis. 1998;13(2):97–122.
- , , , . Brain lactate synthesis in thiamine deficiency: a re‐evaluation using 1H‐13C nuclear magnetic resonance spectroscopy. J Neurosci Res. 2005;79(1‐2):33–41.
- , , . Pharmacokinetics of high‐dose oral thiamine hydrochloride in healthy subjects. BMC Clin Pharmacol. 2012;12(4):4.
- , , , et al. Simultaneous liquid chromatographic assessment of thiamine, thiamine monophosphate and thiamine diphosphate in human erythrocytes: a study on alcoholics. J Chromatogr B Analyt Technol Biomed Life Sci. 2003;789(2):355–363.
- , . Neuroimaging findings in acute Wernicke's encephalopathy: review of the literature. AJR Am J Roentgenol. 2009;192(2):501–508.
- , , , , . Beyond alcoholism: Wernicke‐Korsakoff syndrome in patients with psychiatric disorders. Cogn Behav Neurol. 2011;24(4):209–216.
- , , , , . Mental health status and gender as risk factors for onset of physical illness over 10 years. J Epidemiol Community Health. 2014;68(1):64–70.
- , , , , . The response to treatment of subclinical thiamine deficiency in the elderly. Am J Clin Nutr. 1997;66(4):925–928.
- , , . Thiamin and thiamin phosphate ester deficiency assessed by high performance liquid chromatography in four clinical cases of Wernicke's encephalopathy. Alcohol Clin Exp Res. 1993;17:712–716.
- . Prevention and treatment of Wernicke‐Korsakoff syndrome. Alcohol Alcohol Suppl. 2000;35(1):19–20.
- , , . Pabrinex prescribing in Scottish Emergency Departments. Poster presented at: Inaugural Scientific Conference of the College of Emergency Medicine; May 14–16, 2008; London, United Kingdom.
- , , , , , . Pharmacy‐based intervention in Wernicke's encephalopathy. Psychiatrist. 2010;34(6):234–238.
- , , . Time to act on the inadequate management of Wernicke's encephalopathy in the UK. Alcohol alcohol. Jan‐Feb 2013;48(1):4–8.
- , , , , . Malnutrition is prevalent in hospitalized medical patients: are housestaff identifying the malnourished patient? Nutrition. Apr 2006;22(4):350–354.
- , , , . Malnutrition among Hospitalized‐Patients ‐ a Problem of Physician Awareness. Archives of Internal Medicine. Aug 1987;147(8):1462–1465.
- , . BNF recommendations for the treatment of Wernicke's encephalopathy: lost in translation? Alcohol Alcohol. 2013;48(4):514–515.
- , , , , , . Reduction in medication errors in hospitals due to adoption of computerized provider order entry systems. J Am Med Inform Assoc. 2013;20(3):470–476.
- , . Is intravenous thiamine safe? Am J Emerg Med. 1992;10(2):165.
- , , . A toxicity study of parenteral thiamine hydrochloride. Ann Emerg Med. 1989;18(8):867–870.
- , , , , . Enough is enough: Stop wasting money on vitamin and mineral supplements. Ann Intern Med. 2013;159:850–851.
Thiamine pyrophosphate, the biologically active form of thiamine (vitamin B1), is an essential cofactor for the aerobic breakdown of glucose, with daily requirements related to total caloric intake and the proportion of calories provided as carbohydrates. Patients presenting to the hospital are at risk of thiamine deficiency due to a preponderance of factors including baseline poor nutritional status,[1, 2, 3] diminished intake (associated with illness prodrome), increased metabolic demand (eg, occurring with sepsis, malignancy, surgery, pregnancy), and resuscitation with intravenous glucose‐containing fluids. Without thiamine pyrophosphate, glucose is metabolized through less‐efficient anaerobic pathways, producing lactic acid as a by‐product. The brain uses glucose as its main source of energy and is susceptible to injury due to acute thiamine deficiency. Petechial hemorrhage and demyelination within periventricular structures (thalami, mammillary bodies, ocular motor nuclei, and cerebellar vermis) account for the classical triad of Wernicke's encephalopathy (WE), including confusion/encephalopathy, ophthalmoplegia, and ataxia.[4, 5, 6, 7] Additional symptoms and signs are reported in WE, implicating more widespread consequences of thiamine deficiency.[6, 7, 8, 9]
Wernicke's encephalopathy remains an historically well‐documented illness,[4, 5] of continued relevance to hospitalist practice. Pathologic changes diagnostic of WE are reported in 1.9% to 2.8% of all patients at autopsy,[6, 10] and in as many as 20% of hospitalized patients following unexplained death.[11, 12] In cases of alcohol‐related death, the incidence may be as high as 59%.[13] Most troubling is the observation that the diagnosis is frequently missed in clinical settings,[14, 15, 16, 17, 18, 19] with devastating results. Case series report mortality in upward of 20% of patients with untreated or undertreated WE, with 85% of survivors developing Korsakoff syndromethe chronic form of WE.[8, 20, 21] The clinical diagnosis may be missed in as many as 94% of nonalcoholic patients, including patients admitted to surgical (ie, postgastrointestinal surgery), obstetrical (ie, hyperemesis gravidarum), psychiatric (ie, eating disorders), general internal medicine (ie, cancer‐associated cachexia and related complications), and subspecialty services (ie, dialysis and renal diseases).[15]
Recognition of WE is critical, as effective intervention and treatment is possible. For hospitalized patients, intervention should prioritize parenteral administration of thiamine[22, 23] to circumvent problems with oral absorption common in the medically ill, and to maximize serum thiamine concentrations, promoting passive (concentration‐dependent) movement of thiamine across the blood brain barrier. In lieu of evidence from randomized controlled trials supporting specific doses and schedules for thiamine administration,[24, 25] this recommendation relies on ample experiential evidence emphasizing the importance of higher doses of parenteral thiamine (in excess of 200 mg provided 3 times daily) for the prevention/treatment of WE.[12, 15, 20, 26, 27]
It remains to be determined whether the evidence emphasizing parenteral prescribing has affected current practice within inpatient populations. To address this need, we sought to quantify thiamine‐prescribing practices within Canadian university‐affiliated hospitals across a 2‐year period. We hypothesized that thiamine would be prescribed more frequently via the parenteral route, in line with published guidelines promoting parenteral administration of thiamine.[15, 20]
METHODS
Study Design and Recruitment
A retrospective observational study was used to evaluate thiamine prescribing within Canadian academic hospitals between January 2010 and December 2011. University‐affiliated institutions with English‐speaking postgraduate (ie, residency) adult medicine programs were eligible to participate. Participating centers all utilized computerized pharmacy information systems, allowing anonymized retrospective data to be reported for participants. Study objectives, methods, and procedures were approved by institutional research ethics boards at participating centers.
Data were extracted for the study period from computerized pharmacy information systems recording prescriptions processed by centralized hospital pharmacies. Thiamine prescribed as part of total parenteral nutrition was excluded from analysis, as prescribing was automated in most centers. Participants were assigned a randomized study number linked to prescription information specifying the prescribed dose of thiamine, route of administration (oral: per os, nasogastric tube, orogastric tube, gastric tube; versus parenteral: intravenous, intramuscular), frequency of dosing (eg, daily, twice daily, 3 times daily), and start/end dates. Complete data were available from 12 hospitals (12/14, 85.7%). One hospital was missing data concerning the frequency of prescribing. One hospital provided only summary data, specifying the number of prescriptions issued by route and dose. Information concerning the prescribing service was captured within records from 7 hospitals (7/14, 50.0%), allowing prescriptions to be stratified by prescribing services. Subspecialty designations were simplified to emergency department, intensive care unit (ICU) (including medical, surgical, and trauma ICUs), medical subspecialty (ie, cardiology, endocrinology, gastroenterology, medical oncology, rheumatology), general internal medicine, neurology, psychiatry, and surgical (ie, general surgery, cardiac surgery, neurosurgery, orthopedics, gynecology) services.
Statistical Analysis
Prescriptions for thiamine were summarized across each center specifying the route of administration (all prescriptions and initial prescription). Prescribing behaviors across centers were summarized using descriptive statistics. Differences in parenteral versus oral prescribing were evaluated using the z test for the difference between weighted averages, assuming a null hypothesis of equal prescribing (ie, 50% parenteral and 50% oral) within categories (i.e., all prescriptions, initial prescriptions, total doses).
Factors that may affect rates of parenteral prescribing were considered across centers. Hospitals were stratified based upon the presence of protocols promoting parenteral thiamine prescribing for the treatment of patients at risk of deficiency. The effect of protocols on prescribing behaviors was evaluated by comparing prescribing practices in centers with and without protocols using the z test for the difference between proportions. Forward linear regression was used to evaluate the correlation between the number of prescriptions and/or doses prescribed within centers, and the rates of parenteral prescribing. Rates of parenteral prescribing across hospital services were compared using pairwise comparisons.
Statistical analyses were completed using IBM SPSS Statistics 20 (IBM Corp., Armonk, NY). Significance was defined as P<0.05, using P values corrected for the total number of comparisons (Bonferroni correction), equivalent to a single‐comparison P value<0.001.
RESULTS
Thirteen university‐affiliated academic centers met inclusion criterion and were invited to participate. Data were obtained from 9 organizations (9/13, 69%), encompassing 14 geographically distributed academic hospitals (Figure 1). Centers that declined to participate cited a lack of comprehensive electronic databases tracking prescriptions as the main barrier. One of the 14 participating hospitals represented a network of affiliated health centers (site 2), rendering it an outlier in terms of total number of doses prescribed (>3 standard deviations above the mean). This affiliated network was unable to provide prescribing data separated by hospital and was excluded from analyses.

In total, data were collected corresponding to 48,806 prescriptions for 209,762 doses of thiamine, provided to 32,213 patients (Table 1). Prescriptions were divided by route of administration (parenteral vs oral) and prescribing practices summarized across centers. Rates of parenteral prescribing varied widely between centers (maximum=82.0%, minimum=33.9%). Overall, however, parenteral thiamine was prescribed more frequently than oral thiamine, accounting for 57.6% of all prescriptions (z=33.59, P<0.001) and 59.2% of initial prescriptions issued to patients (z=168.93, P<0.001). Oral thiamine constituted a significant majority of the total doses prescribed (68.4%, z=168.9; P<0.001).
| All Prescriptions | First Prescriptions | Doses | ||||||||
|---|---|---|---|---|---|---|---|---|---|---|
| Hospital | Total | Parenteral % | Oral % | Total | Parenteral % | Oral % | Total | Parenteral % | Oral % | |
| ||||||||||
| 1A* | 7,499 | 66.42 | 33.58 | 6,189 | 66.41 | 33.59 | 36,032 | 26.60 | 73.40 | |
| 1B* | 4,372 | 70.38 | 29.62 | 2,314 | 78.69 | 21.31 | 17,133 | 27.61 | 72.39 | |
| 1C* | 5,805 | 57.64 | 42.36 | 4,742 | 58.10 | 41.90 | 30,910 | 17.12 | 82.88 | |
| 3* | 3,519 | 45.27 | 54.73 | 2,236 | 59.57 | 40.43 | 8,037 | 52.37 | 47.63 | |
| 4* | 5,322 | 60.50 | 39.50 | 2,900 | 57.83 | 42.17 | 22,159 | 44.49 | 55.51 | |
| 5 | 3,857 | 46.85 | 53.15 | 2,858 | 56.37 | 43.63 | 16,035 | 33.51 | 66.49 | |
| 6A* | 6,105 | 55.46 | 44.54 | 2,907 | 55.04 | 44.96 | 15,947 | 44.22 | 55.78 | |
| 6B | 3,127 | 55.39 | 44.61 | 1,710 | 64.62 | 35.38 | 9,048 | 45.11 | 54.89 | |
| 6C* | 2,134 | 55.62 | 44.38 | 1,499 | 56.24 | 43.76 | 10,327 | 32.33 | 67.67 | |
| 6D | 617 | 35.82 | 64.18 | 505 | 34.65 | 65.35 | 2,647 | 35.70 | 64.30 | |
| 7* | 2,467 | 82.00 | 18.00 | 1,373 | 87.40 | 12.60 | 9,924 | 59.06 | 40.94 | |
| 8 | 1,122 | 50.98 | 49.02 | 694 | 58.79 | 41.21 | 3,048 | 64.73 | 35.27 | |
| 9 | 2,860 | 33.85 | 66.15 | 2,286 | 19.34 | 80.66 | 28,515 | 14.73 | 85.27 | |
| Total | 48,806 | 57.60 | 42.40 | 32,213 | 59.23 | 40.77 | 209,762 | 31.56 | 68.44 | |
The factors associated with higher rates of parenteral prescribing were further considered. Rates of parenteral prescribing were compared between centers with and without published guidelines governing thiamine usage. Eight of 13 hospitals (61.5%) had hospital‐wide protocols that promoted initial administration of thiamine via the parenteral route in patients at risk of deficiency. The presence of a protocol was associated with significantly higher overall rates of parenteral prescribing (61.3% with protocol, 45.7% without protocol; z=29.5; P<0.001). Linear regression revealed no predictive relationship between the number of prescriptions issued and the proportion of parenteral thiamine prescribed across centers (total prescriptions, standard 0.38, P=0.20; Figure 2). A negative correlation was observed between the proportion of doses prescribed via the parenteral route and the number of total doses prescribed (standard =0.61, P=0.03), suggesting that centers prescribing the greatest numbers of doses were less likely to prescribe parenteral thiamine.

The effect of the inpatient practice environment on prescribing behavior was considered across the 7 centers for which service‐specific prescribing data were provided (Figure 3). Patients receiving care within emergency departments or intensive care, general medical or surgical units were more likely to be prescribed parenteral thiamine (z>3.0, P<0.001), whereas patients admitted to psychiatry units were more likely to be prescribed thiamine via the oral route (z=23.7, P<0.001). No differences were observed between rates of parenteral and oral prescribing for patients admitted under medical subspecialty (z=0.6, P=not significant) and neurology services (z=3.1, P=not significant). Pair‐wise comparisons for means confirmed that patients admitted to the ICU were significantly more likely to be prescribed parenteral thiamine than patients admitted to any other service; psychiatry inpatients were the least likely to be prescribed parenteral thiamine (P<0.001).

A post hoc analysis was used to determine the service‐specific effect of hospital‐wide protocols promoting parenteral prescribing. Protocols were associated with significantly higher rates of parenteral prescribing for patients receiving care under all services (ICU, z=3.76; medical subspecialties, z=16.07; general medicine, z=15.40; neurology, z=7.02; surgery, z=13.19; P<0.001), except psychiatry (z=2.0, P=not significant) and those within emergency departments (z=2.05, P=not significant).
In contrast to the differences in the rates of parenteral prescribing across centers, quantitative review of the doses and schedule of thiamine administration revealed a near‐universal approach to prescribing. Overall, 92.7% (45,266/48,806) of prescriptions were for 100 mg of thiamine (z=188.8, P<0.001); 74.6% (33,551/44,948) of prescriptions were ordered once daily (z=104.5, P<0.001). Thiamine was more likely to be prescribed via the parenteral route when prescribed in doses of 100 mg (57.6%, z=32.5, P<0.001) or >200 mg (76.1%, z=25.51, P<0.001), or when ordered as single doses (81.5%, z=64.86, P<0.001) (Figure 4).

DISCUSSION
The causal relationship between thiamine deficiency and WE has been recognized since 1941,[28] with the importance of parenteral thiamine replacement in vulnerable populations emphasized in numerous case series,[29, 30] population‐based studies,[14, 31, 32] and consensus guidelines.[15, 20] Consistent with guideline recommendations, thiamine was significantly more likely to be prescribed via the parenteral route across a large network of geographically distributed Canadian academic healthcare centers. Somewhat surprisingly, however, oral thiamine accounted for 42.4% of all prescriptions, and a significant majority of doses prescribed to the over 30,000 patients studied. These findings confirm that oral thiamine continues to be prescribed to inpatients within Canadian academic hospitals.
The critical importance of parenteral thiamine administration for the treatment and prevention of WE is supported by an understanding of the pathophysiology of this disease. Wernicke's encephalopathy results from brain‐thiamine deficiency, leading to a cellular energy deficit, focal acidosis, regional increases in glutamate, and cell death.[20, 33, 34] Serum thiamine crosses the blood brain barrier through active (transporter‐mediated) and passive (concentration‐dependent) means.[20] It is therefore possible to drive thiamine into the central nervous system by establishing high serum thiamine levels. Interestingly, forward linear regression suggested that the centers prescribing the largest number of doses were less likely to prescribe parenteral thiamine. This may reflect a misguided preference for the use of prolonged/frequent courses of oral thiamine for the prevention and/or treatment of thiamine deficiency in hospitalized patients. Oral absorption of thiamine occurs within the duodenum by a rate‐limited process, with maximum absorption of 4.5 mg per dose.[20] This rate may be higher in healthy individuals, arguing for passive and active transport across enterocytes.[35] In sick individuals, however, oral absorption cannot be relied upon to attain the high serum thiamine levels necessary to reverse the effects of deficiency, exemplifying the importance of parenteral administration in hospitalized patients.
Protocols promoting parenteral administration of thiamine were associated with higher rates of parenteral prescribing across centers, and may represent an effective and convenient means of effecting prescriber behaviors. Efforts must be made, however, to identify additional barriers limiting parenteral thiamine prescribing within hospitals. One such barrier relates to the identification of at‐risk individuals. Despite advances in biochemical measures quantifying thiamine deficiency[36] and neuroimaging studies confirming changes within the brains of affected patients,[37] WE remains a clinical diagnosis. As such, clinical criteria have been proposed to identify those at risk of deficiency, with an emphasis on detection of WE in patients with alcohol‐use disorders. Specifically, guidelines from the Royal College of Physicians advocate that WE be considered in patients with evidence of alcohol misuse, and 1 of the following: (1) acute confusion, (2) decreased consciousness, (3) ataxia, (4) ophthalmoplegia, (5) memory disturbances, and (6) hypothermia with hypotension.[20] The European Federation of Neurological Sciences broadens the clinical criteria to include patients with and without alcohol‐use disorders, encouraging diagnosis and treatment in individuals with any 2 of the following: (1) dietary deficiencies, (2) oculomotor abnormalities, (3) cerebellar dysfunction, and (4) altered mental status or mild memory impairment.[15] Once identified, it remains imperative that patients receive appropriate therapies to reverse thiamine deficiency. To this end, the results of the present study may be used to identify potential inpatient populations at risk of undertreatment.
Psychiatric patients were the least likely to be prescribed parenteral thiamine, regardless of whether protocols promoting parenteral prescribing were in place within the study hospital. This observation is concerning, as psychiatric inpatients may be at risk of thiamine deficiency due to a confluence of factors related to mental illness (ie, malnutrition associated with eating disorders, substance abuse)[21, 38] and increased rates of comorbid physical illnesses.[39] Low rates of parenteral prescribing may reflect a number of service‐specific (ie, decreased ease of administration of parenteral medications) and patient‐specific factors (ie, challenges of maintaining intravenous catheter access in acutely ill psychiatric patients) that are not adequately addressed by hospital‐wide protocols. Alternatively, lower rates of parenteral prescribing may reflect a systematic preference for the use of oral thiamine in a patient population perceived to be at lower risk of thiamine deficiency. Although oral thiamine has been shown to be effective in correcting thiamine deficiency in a group of community‐dwelling elderly patients without clinical symptoms or signs suggesting WE (ie, subclinical thiamine deficiency),[40] it remains to be determined whether a similar treatment strategy can be endorsed in select inpatients with subclinical deficiency, in whom oral absorption and compliance can be reasonably assured.
Randomized control trial evidence supporting specific doses and schedules for administration of parenteral thiamine is not available.[24, 25] Accordingly, uncertainty exists concerning the doses and frequency of administration of thiamine required to prevent or reverse suspected WE. Despite this uncertainty, the dose and schedule of thiamine prescribed in our study population was remarkably uniform, with thiamine most commonly prescribed in 100‐mg doses once daily. Similar findings were reported in a retrospective study considering thiamine prescribing to 217 patients with alcohol‐use disorders admitted to an urban US teaching hospital: 76.9% of inpatients were prescribed 100‐mg daily doses of thiamine.[19] Interestingly, no differences in prescribing behaviors were noted when high‐risk patients presenting with alcohol intoxication, withdrawal, or delirium tremens were considered separately, suggesting that patient‐specific factors had little impact on the dosing strategy endorsed by clinicians.[19]
Although pervasive, the provision of 100 mg of thiamine daily is not supported by biochemical or clinical studies.[27] On the contrary, clinical‐pathological studies suggest that doses of thiamine between 50 and 100 mg per day may not be sufficient to reverse clinical signs or prevent death in patients with WE, whereas doses up to 250 mg may not reverse the biochemical abnormalities associated with clinically significant deficiency.[12, 41, 42] Such rationale is cited in support of consensus recommendations promoting administration of high doses of parenteral thiamine for the treatment of WE (200 or 500 mg, provided 3 times daily).[15, 20] As this project illustrates, however, rational, well‐justified guidelines are not enough to transform clinical practice.
The limitations of consensus guidelines and hospital‐specific protocols promoting thiamine prescribing have been explored in other specialty[43] and hospital environments.[44, 45] These studies offer several insights into the factors that may contribute to the disparity between recommended and real‐world practices, including continued under‐recognition of malnourished hospitalized patients at risk of thiamine deficiency,[1, 45, 46, 47] variations in consensus‐based guidelines governing thiamine prescribing,[15, 20] and challenges in communication of protocol rationales and recommendations.[44, 48] Together, these findings exemplify the need for additional strategies aimed at improving parenteral prescribing in vulnerable hospitalized populations. The proliferation of computerized physician order entry and clinical decision support systems may offer the opportunity to effect prescribing behaviors, with the possibility of specifying routes and doses of thiamine administration in accordance with guidelines,[49] without the requirement for dedicated monitoring and personnel‐driven interventions.
Limitations
By design, our study was limited to the assessment of thiamine‐prescribing data obtained directly from computerized pharmacy information systems. Consequently, only the minimum details required to safely prescribe a medication were captured. As a result, we were unable to evaluate the potential effect of patient‐specific factors (including clinical diagnosis) on prescriber behaviors. Thus, it remains possible that prescribing behaviors varied according to perceived patient risks in our study population. An additional limitation relates to the generalizability of results beyond academic hospitals in Canada. We suggest, however, that potential concerns relating to generalizability are counterbalanced by 2 advantages inherent within our study population. The first is that the majority of community‐based clinicians are trained within university‐affiliated hospitals. As a result, prescribing behaviors measured in these training centers should reflect optimal behaviors within downstream networks of community hospitals. The second is that the recruitment of hospitals funded by a universal single‐payer served to minimize variability in prescribing behaviors attributable to prescriber and patient concerns regarding reimbursement, thus providing a more accurate assessment of prescriber behaviors based on clinical evidence, independent of financial factors.
Acknowledging these limitations, we assert that parenteral administration of thiamine remains the best means of rapidly correcting thiamine deficiency, and should be considered for the treatment of clinically relevant thiamine deficiency in hospitalized patients. This recommendation effectively balances the potentially deleterious consequences of undertreatment of thiamine deficiency, with the favorable risk‐ and cost‐profile associated with the administration of parenteral thiamine.[15, 20, 23, 27, 50, 51]
CLINICAL AND RESEARCH IMPLICATIONS
In an era of overuse of vitamin supplementation,[52] it is increasingly important for healthcare providers to recognize not only when vitamin supplementation is required, but also how replacement therapies should be delivered. As shown in this study, protocols promoting the use of parenteral thiamine may improve overall compliance with recommendations. However, additional strategies are required to further improve rates of parenteral prescribing to hospitalized patients at risk of thiamine deficiency.
Acknowledgements
The authors are grateful for the contributions of support staff within local hospital pharmacy and information technology departments who made collection of these data possible. Dr. David F. Tang‐Wai reviewed an earlier draft of the manuscript and provided useful comments for which we are grateful.
Disclosures: G. S. Day developed the study concept and methods for implementation, and was primarily responsible for acquisition, analysis, and interpretation of data, as well as drafting, revision, and finalization of the manuscript. G. S. Day had full access to all study data, and takes responsibility for the integrity of the data and the accuracy of the analysis and interpretation. S. Ladak participated in the development of methods, acquisition of data, and revision and finalization of the manuscript. K. Curley participated in the development of methods, acquisition of data, and revision and finalization of the manuscript. N. A. S. Farb participated in analysis of data, and revision and finalization of the manuscript. P. Masiowski participated in acquisition of data, and revision and finalization of the manuscript. T. Pringsheim participated in acquisition of data, and revision and finalization of the manuscript. M. Ritchie participated in acquisition of data, and revision and finalization of the manuscript. A. Cheung participated in acquisition of data, and revision and finalization of the manuscript. S. Jansen participated in acquisition of data, and revision and finalization of the manuscript. L. Methot participated in acquisition of data, and revision and finalization of the manuscript. H. L. Neville participated in acquisition of data, and revision and finalization of the manuscript. D. Bates participated in acquisition of data, and revision and finalization of the manuscript. D. Lowe participated in acquisition of data, and revision and finalization of the manuscript. N. Fernandes participated in acquisition of data, and revision and finalization of the manuscript. A. Ferland participated in acquisition of data, and revision and finalization of the manuscript. C. M. del Campo acted as primary supervisor for this project, and approved study design and methods. He assisted with interpretation of data, and revision and finalization of the manuscript. Preliminary data were reported in abstract form at the 2013 Annual Meeting of the American Academy of Neurology (March 2013, San Diego, CA) and the 2014 Annual Meeting of the Canadian Neurological Sciences Foundation (June 2014, Banff, AB, Canada). No sources of funding are reported for this study. The authors report no conflicts of interest.
Thiamine pyrophosphate, the biologically active form of thiamine (vitamin B1), is an essential cofactor for the aerobic breakdown of glucose, with daily requirements related to total caloric intake and the proportion of calories provided as carbohydrates. Patients presenting to the hospital are at risk of thiamine deficiency due to a preponderance of factors including baseline poor nutritional status,[1, 2, 3] diminished intake (associated with illness prodrome), increased metabolic demand (eg, occurring with sepsis, malignancy, surgery, pregnancy), and resuscitation with intravenous glucose‐containing fluids. Without thiamine pyrophosphate, glucose is metabolized through less‐efficient anaerobic pathways, producing lactic acid as a by‐product. The brain uses glucose as its main source of energy and is susceptible to injury due to acute thiamine deficiency. Petechial hemorrhage and demyelination within periventricular structures (thalami, mammillary bodies, ocular motor nuclei, and cerebellar vermis) account for the classical triad of Wernicke's encephalopathy (WE), including confusion/encephalopathy, ophthalmoplegia, and ataxia.[4, 5, 6, 7] Additional symptoms and signs are reported in WE, implicating more widespread consequences of thiamine deficiency.[6, 7, 8, 9]
Wernicke's encephalopathy remains an historically well‐documented illness,[4, 5] of continued relevance to hospitalist practice. Pathologic changes diagnostic of WE are reported in 1.9% to 2.8% of all patients at autopsy,[6, 10] and in as many as 20% of hospitalized patients following unexplained death.[11, 12] In cases of alcohol‐related death, the incidence may be as high as 59%.[13] Most troubling is the observation that the diagnosis is frequently missed in clinical settings,[14, 15, 16, 17, 18, 19] with devastating results. Case series report mortality in upward of 20% of patients with untreated or undertreated WE, with 85% of survivors developing Korsakoff syndromethe chronic form of WE.[8, 20, 21] The clinical diagnosis may be missed in as many as 94% of nonalcoholic patients, including patients admitted to surgical (ie, postgastrointestinal surgery), obstetrical (ie, hyperemesis gravidarum), psychiatric (ie, eating disorders), general internal medicine (ie, cancer‐associated cachexia and related complications), and subspecialty services (ie, dialysis and renal diseases).[15]
Recognition of WE is critical, as effective intervention and treatment is possible. For hospitalized patients, intervention should prioritize parenteral administration of thiamine[22, 23] to circumvent problems with oral absorption common in the medically ill, and to maximize serum thiamine concentrations, promoting passive (concentration‐dependent) movement of thiamine across the blood brain barrier. In lieu of evidence from randomized controlled trials supporting specific doses and schedules for thiamine administration,[24, 25] this recommendation relies on ample experiential evidence emphasizing the importance of higher doses of parenteral thiamine (in excess of 200 mg provided 3 times daily) for the prevention/treatment of WE.[12, 15, 20, 26, 27]
It remains to be determined whether the evidence emphasizing parenteral prescribing has affected current practice within inpatient populations. To address this need, we sought to quantify thiamine‐prescribing practices within Canadian university‐affiliated hospitals across a 2‐year period. We hypothesized that thiamine would be prescribed more frequently via the parenteral route, in line with published guidelines promoting parenteral administration of thiamine.[15, 20]
METHODS
Study Design and Recruitment
A retrospective observational study was used to evaluate thiamine prescribing within Canadian academic hospitals between January 2010 and December 2011. University‐affiliated institutions with English‐speaking postgraduate (ie, residency) adult medicine programs were eligible to participate. Participating centers all utilized computerized pharmacy information systems, allowing anonymized retrospective data to be reported for participants. Study objectives, methods, and procedures were approved by institutional research ethics boards at participating centers.
Data were extracted for the study period from computerized pharmacy information systems recording prescriptions processed by centralized hospital pharmacies. Thiamine prescribed as part of total parenteral nutrition was excluded from analysis, as prescribing was automated in most centers. Participants were assigned a randomized study number linked to prescription information specifying the prescribed dose of thiamine, route of administration (oral: per os, nasogastric tube, orogastric tube, gastric tube; versus parenteral: intravenous, intramuscular), frequency of dosing (eg, daily, twice daily, 3 times daily), and start/end dates. Complete data were available from 12 hospitals (12/14, 85.7%). One hospital was missing data concerning the frequency of prescribing. One hospital provided only summary data, specifying the number of prescriptions issued by route and dose. Information concerning the prescribing service was captured within records from 7 hospitals (7/14, 50.0%), allowing prescriptions to be stratified by prescribing services. Subspecialty designations were simplified to emergency department, intensive care unit (ICU) (including medical, surgical, and trauma ICUs), medical subspecialty (ie, cardiology, endocrinology, gastroenterology, medical oncology, rheumatology), general internal medicine, neurology, psychiatry, and surgical (ie, general surgery, cardiac surgery, neurosurgery, orthopedics, gynecology) services.
Statistical Analysis
Prescriptions for thiamine were summarized across each center specifying the route of administration (all prescriptions and initial prescription). Prescribing behaviors across centers were summarized using descriptive statistics. Differences in parenteral versus oral prescribing were evaluated using the z test for the difference between weighted averages, assuming a null hypothesis of equal prescribing (ie, 50% parenteral and 50% oral) within categories (i.e., all prescriptions, initial prescriptions, total doses).
Factors that may affect rates of parenteral prescribing were considered across centers. Hospitals were stratified based upon the presence of protocols promoting parenteral thiamine prescribing for the treatment of patients at risk of deficiency. The effect of protocols on prescribing behaviors was evaluated by comparing prescribing practices in centers with and without protocols using the z test for the difference between proportions. Forward linear regression was used to evaluate the correlation between the number of prescriptions and/or doses prescribed within centers, and the rates of parenteral prescribing. Rates of parenteral prescribing across hospital services were compared using pairwise comparisons.
Statistical analyses were completed using IBM SPSS Statistics 20 (IBM Corp., Armonk, NY). Significance was defined as P<0.05, using P values corrected for the total number of comparisons (Bonferroni correction), equivalent to a single‐comparison P value<0.001.
RESULTS
Thirteen university‐affiliated academic centers met inclusion criterion and were invited to participate. Data were obtained from 9 organizations (9/13, 69%), encompassing 14 geographically distributed academic hospitals (Figure 1). Centers that declined to participate cited a lack of comprehensive electronic databases tracking prescriptions as the main barrier. One of the 14 participating hospitals represented a network of affiliated health centers (site 2), rendering it an outlier in terms of total number of doses prescribed (>3 standard deviations above the mean). This affiliated network was unable to provide prescribing data separated by hospital and was excluded from analyses.

In total, data were collected corresponding to 48,806 prescriptions for 209,762 doses of thiamine, provided to 32,213 patients (Table 1). Prescriptions were divided by route of administration (parenteral vs oral) and prescribing practices summarized across centers. Rates of parenteral prescribing varied widely between centers (maximum=82.0%, minimum=33.9%). Overall, however, parenteral thiamine was prescribed more frequently than oral thiamine, accounting for 57.6% of all prescriptions (z=33.59, P<0.001) and 59.2% of initial prescriptions issued to patients (z=168.93, P<0.001). Oral thiamine constituted a significant majority of the total doses prescribed (68.4%, z=168.9; P<0.001).
| All Prescriptions | First Prescriptions | Doses | ||||||||
|---|---|---|---|---|---|---|---|---|---|---|
| Hospital | Total | Parenteral % | Oral % | Total | Parenteral % | Oral % | Total | Parenteral % | Oral % | |
| ||||||||||
| 1A* | 7,499 | 66.42 | 33.58 | 6,189 | 66.41 | 33.59 | 36,032 | 26.60 | 73.40 | |
| 1B* | 4,372 | 70.38 | 29.62 | 2,314 | 78.69 | 21.31 | 17,133 | 27.61 | 72.39 | |
| 1C* | 5,805 | 57.64 | 42.36 | 4,742 | 58.10 | 41.90 | 30,910 | 17.12 | 82.88 | |
| 3* | 3,519 | 45.27 | 54.73 | 2,236 | 59.57 | 40.43 | 8,037 | 52.37 | 47.63 | |
| 4* | 5,322 | 60.50 | 39.50 | 2,900 | 57.83 | 42.17 | 22,159 | 44.49 | 55.51 | |
| 5 | 3,857 | 46.85 | 53.15 | 2,858 | 56.37 | 43.63 | 16,035 | 33.51 | 66.49 | |
| 6A* | 6,105 | 55.46 | 44.54 | 2,907 | 55.04 | 44.96 | 15,947 | 44.22 | 55.78 | |
| 6B | 3,127 | 55.39 | 44.61 | 1,710 | 64.62 | 35.38 | 9,048 | 45.11 | 54.89 | |
| 6C* | 2,134 | 55.62 | 44.38 | 1,499 | 56.24 | 43.76 | 10,327 | 32.33 | 67.67 | |
| 6D | 617 | 35.82 | 64.18 | 505 | 34.65 | 65.35 | 2,647 | 35.70 | 64.30 | |
| 7* | 2,467 | 82.00 | 18.00 | 1,373 | 87.40 | 12.60 | 9,924 | 59.06 | 40.94 | |
| 8 | 1,122 | 50.98 | 49.02 | 694 | 58.79 | 41.21 | 3,048 | 64.73 | 35.27 | |
| 9 | 2,860 | 33.85 | 66.15 | 2,286 | 19.34 | 80.66 | 28,515 | 14.73 | 85.27 | |
| Total | 48,806 | 57.60 | 42.40 | 32,213 | 59.23 | 40.77 | 209,762 | 31.56 | 68.44 | |
The factors associated with higher rates of parenteral prescribing were further considered. Rates of parenteral prescribing were compared between centers with and without published guidelines governing thiamine usage. Eight of 13 hospitals (61.5%) had hospital‐wide protocols that promoted initial administration of thiamine via the parenteral route in patients at risk of deficiency. The presence of a protocol was associated with significantly higher overall rates of parenteral prescribing (61.3% with protocol, 45.7% without protocol; z=29.5; P<0.001). Linear regression revealed no predictive relationship between the number of prescriptions issued and the proportion of parenteral thiamine prescribed across centers (total prescriptions, standard 0.38, P=0.20; Figure 2). A negative correlation was observed between the proportion of doses prescribed via the parenteral route and the number of total doses prescribed (standard =0.61, P=0.03), suggesting that centers prescribing the greatest numbers of doses were less likely to prescribe parenteral thiamine.

The effect of the inpatient practice environment on prescribing behavior was considered across the 7 centers for which service‐specific prescribing data were provided (Figure 3). Patients receiving care within emergency departments or intensive care, general medical or surgical units were more likely to be prescribed parenteral thiamine (z>3.0, P<0.001), whereas patients admitted to psychiatry units were more likely to be prescribed thiamine via the oral route (z=23.7, P<0.001). No differences were observed between rates of parenteral and oral prescribing for patients admitted under medical subspecialty (z=0.6, P=not significant) and neurology services (z=3.1, P=not significant). Pair‐wise comparisons for means confirmed that patients admitted to the ICU were significantly more likely to be prescribed parenteral thiamine than patients admitted to any other service; psychiatry inpatients were the least likely to be prescribed parenteral thiamine (P<0.001).

A post hoc analysis was used to determine the service‐specific effect of hospital‐wide protocols promoting parenteral prescribing. Protocols were associated with significantly higher rates of parenteral prescribing for patients receiving care under all services (ICU, z=3.76; medical subspecialties, z=16.07; general medicine, z=15.40; neurology, z=7.02; surgery, z=13.19; P<0.001), except psychiatry (z=2.0, P=not significant) and those within emergency departments (z=2.05, P=not significant).
In contrast to the differences in the rates of parenteral prescribing across centers, quantitative review of the doses and schedule of thiamine administration revealed a near‐universal approach to prescribing. Overall, 92.7% (45,266/48,806) of prescriptions were for 100 mg of thiamine (z=188.8, P<0.001); 74.6% (33,551/44,948) of prescriptions were ordered once daily (z=104.5, P<0.001). Thiamine was more likely to be prescribed via the parenteral route when prescribed in doses of 100 mg (57.6%, z=32.5, P<0.001) or >200 mg (76.1%, z=25.51, P<0.001), or when ordered as single doses (81.5%, z=64.86, P<0.001) (Figure 4).

DISCUSSION
The causal relationship between thiamine deficiency and WE has been recognized since 1941,[28] with the importance of parenteral thiamine replacement in vulnerable populations emphasized in numerous case series,[29, 30] population‐based studies,[14, 31, 32] and consensus guidelines.[15, 20] Consistent with guideline recommendations, thiamine was significantly more likely to be prescribed via the parenteral route across a large network of geographically distributed Canadian academic healthcare centers. Somewhat surprisingly, however, oral thiamine accounted for 42.4% of all prescriptions, and a significant majority of doses prescribed to the over 30,000 patients studied. These findings confirm that oral thiamine continues to be prescribed to inpatients within Canadian academic hospitals.
The critical importance of parenteral thiamine administration for the treatment and prevention of WE is supported by an understanding of the pathophysiology of this disease. Wernicke's encephalopathy results from brain‐thiamine deficiency, leading to a cellular energy deficit, focal acidosis, regional increases in glutamate, and cell death.[20, 33, 34] Serum thiamine crosses the blood brain barrier through active (transporter‐mediated) and passive (concentration‐dependent) means.[20] It is therefore possible to drive thiamine into the central nervous system by establishing high serum thiamine levels. Interestingly, forward linear regression suggested that the centers prescribing the largest number of doses were less likely to prescribe parenteral thiamine. This may reflect a misguided preference for the use of prolonged/frequent courses of oral thiamine for the prevention and/or treatment of thiamine deficiency in hospitalized patients. Oral absorption of thiamine occurs within the duodenum by a rate‐limited process, with maximum absorption of 4.5 mg per dose.[20] This rate may be higher in healthy individuals, arguing for passive and active transport across enterocytes.[35] In sick individuals, however, oral absorption cannot be relied upon to attain the high serum thiamine levels necessary to reverse the effects of deficiency, exemplifying the importance of parenteral administration in hospitalized patients.
Protocols promoting parenteral administration of thiamine were associated with higher rates of parenteral prescribing across centers, and may represent an effective and convenient means of effecting prescriber behaviors. Efforts must be made, however, to identify additional barriers limiting parenteral thiamine prescribing within hospitals. One such barrier relates to the identification of at‐risk individuals. Despite advances in biochemical measures quantifying thiamine deficiency[36] and neuroimaging studies confirming changes within the brains of affected patients,[37] WE remains a clinical diagnosis. As such, clinical criteria have been proposed to identify those at risk of deficiency, with an emphasis on detection of WE in patients with alcohol‐use disorders. Specifically, guidelines from the Royal College of Physicians advocate that WE be considered in patients with evidence of alcohol misuse, and 1 of the following: (1) acute confusion, (2) decreased consciousness, (3) ataxia, (4) ophthalmoplegia, (5) memory disturbances, and (6) hypothermia with hypotension.[20] The European Federation of Neurological Sciences broadens the clinical criteria to include patients with and without alcohol‐use disorders, encouraging diagnosis and treatment in individuals with any 2 of the following: (1) dietary deficiencies, (2) oculomotor abnormalities, (3) cerebellar dysfunction, and (4) altered mental status or mild memory impairment.[15] Once identified, it remains imperative that patients receive appropriate therapies to reverse thiamine deficiency. To this end, the results of the present study may be used to identify potential inpatient populations at risk of undertreatment.
Psychiatric patients were the least likely to be prescribed parenteral thiamine, regardless of whether protocols promoting parenteral prescribing were in place within the study hospital. This observation is concerning, as psychiatric inpatients may be at risk of thiamine deficiency due to a confluence of factors related to mental illness (ie, malnutrition associated with eating disorders, substance abuse)[21, 38] and increased rates of comorbid physical illnesses.[39] Low rates of parenteral prescribing may reflect a number of service‐specific (ie, decreased ease of administration of parenteral medications) and patient‐specific factors (ie, challenges of maintaining intravenous catheter access in acutely ill psychiatric patients) that are not adequately addressed by hospital‐wide protocols. Alternatively, lower rates of parenteral prescribing may reflect a systematic preference for the use of oral thiamine in a patient population perceived to be at lower risk of thiamine deficiency. Although oral thiamine has been shown to be effective in correcting thiamine deficiency in a group of community‐dwelling elderly patients without clinical symptoms or signs suggesting WE (ie, subclinical thiamine deficiency),[40] it remains to be determined whether a similar treatment strategy can be endorsed in select inpatients with subclinical deficiency, in whom oral absorption and compliance can be reasonably assured.
Randomized control trial evidence supporting specific doses and schedules for administration of parenteral thiamine is not available.[24, 25] Accordingly, uncertainty exists concerning the doses and frequency of administration of thiamine required to prevent or reverse suspected WE. Despite this uncertainty, the dose and schedule of thiamine prescribed in our study population was remarkably uniform, with thiamine most commonly prescribed in 100‐mg doses once daily. Similar findings were reported in a retrospective study considering thiamine prescribing to 217 patients with alcohol‐use disorders admitted to an urban US teaching hospital: 76.9% of inpatients were prescribed 100‐mg daily doses of thiamine.[19] Interestingly, no differences in prescribing behaviors were noted when high‐risk patients presenting with alcohol intoxication, withdrawal, or delirium tremens were considered separately, suggesting that patient‐specific factors had little impact on the dosing strategy endorsed by clinicians.[19]
Although pervasive, the provision of 100 mg of thiamine daily is not supported by biochemical or clinical studies.[27] On the contrary, clinical‐pathological studies suggest that doses of thiamine between 50 and 100 mg per day may not be sufficient to reverse clinical signs or prevent death in patients with WE, whereas doses up to 250 mg may not reverse the biochemical abnormalities associated with clinically significant deficiency.[12, 41, 42] Such rationale is cited in support of consensus recommendations promoting administration of high doses of parenteral thiamine for the treatment of WE (200 or 500 mg, provided 3 times daily).[15, 20] As this project illustrates, however, rational, well‐justified guidelines are not enough to transform clinical practice.
The limitations of consensus guidelines and hospital‐specific protocols promoting thiamine prescribing have been explored in other specialty[43] and hospital environments.[44, 45] These studies offer several insights into the factors that may contribute to the disparity between recommended and real‐world practices, including continued under‐recognition of malnourished hospitalized patients at risk of thiamine deficiency,[1, 45, 46, 47] variations in consensus‐based guidelines governing thiamine prescribing,[15, 20] and challenges in communication of protocol rationales and recommendations.[44, 48] Together, these findings exemplify the need for additional strategies aimed at improving parenteral prescribing in vulnerable hospitalized populations. The proliferation of computerized physician order entry and clinical decision support systems may offer the opportunity to effect prescribing behaviors, with the possibility of specifying routes and doses of thiamine administration in accordance with guidelines,[49] without the requirement for dedicated monitoring and personnel‐driven interventions.
Limitations
By design, our study was limited to the assessment of thiamine‐prescribing data obtained directly from computerized pharmacy information systems. Consequently, only the minimum details required to safely prescribe a medication were captured. As a result, we were unable to evaluate the potential effect of patient‐specific factors (including clinical diagnosis) on prescriber behaviors. Thus, it remains possible that prescribing behaviors varied according to perceived patient risks in our study population. An additional limitation relates to the generalizability of results beyond academic hospitals in Canada. We suggest, however, that potential concerns relating to generalizability are counterbalanced by 2 advantages inherent within our study population. The first is that the majority of community‐based clinicians are trained within university‐affiliated hospitals. As a result, prescribing behaviors measured in these training centers should reflect optimal behaviors within downstream networks of community hospitals. The second is that the recruitment of hospitals funded by a universal single‐payer served to minimize variability in prescribing behaviors attributable to prescriber and patient concerns regarding reimbursement, thus providing a more accurate assessment of prescriber behaviors based on clinical evidence, independent of financial factors.
Acknowledging these limitations, we assert that parenteral administration of thiamine remains the best means of rapidly correcting thiamine deficiency, and should be considered for the treatment of clinically relevant thiamine deficiency in hospitalized patients. This recommendation effectively balances the potentially deleterious consequences of undertreatment of thiamine deficiency, with the favorable risk‐ and cost‐profile associated with the administration of parenteral thiamine.[15, 20, 23, 27, 50, 51]
CLINICAL AND RESEARCH IMPLICATIONS
In an era of overuse of vitamin supplementation,[52] it is increasingly important for healthcare providers to recognize not only when vitamin supplementation is required, but also how replacement therapies should be delivered. As shown in this study, protocols promoting the use of parenteral thiamine may improve overall compliance with recommendations. However, additional strategies are required to further improve rates of parenteral prescribing to hospitalized patients at risk of thiamine deficiency.
Acknowledgements
The authors are grateful for the contributions of support staff within local hospital pharmacy and information technology departments who made collection of these data possible. Dr. David F. Tang‐Wai reviewed an earlier draft of the manuscript and provided useful comments for which we are grateful.
Disclosures: G. S. Day developed the study concept and methods for implementation, and was primarily responsible for acquisition, analysis, and interpretation of data, as well as drafting, revision, and finalization of the manuscript. G. S. Day had full access to all study data, and takes responsibility for the integrity of the data and the accuracy of the analysis and interpretation. S. Ladak participated in the development of methods, acquisition of data, and revision and finalization of the manuscript. K. Curley participated in the development of methods, acquisition of data, and revision and finalization of the manuscript. N. A. S. Farb participated in analysis of data, and revision and finalization of the manuscript. P. Masiowski participated in acquisition of data, and revision and finalization of the manuscript. T. Pringsheim participated in acquisition of data, and revision and finalization of the manuscript. M. Ritchie participated in acquisition of data, and revision and finalization of the manuscript. A. Cheung participated in acquisition of data, and revision and finalization of the manuscript. S. Jansen participated in acquisition of data, and revision and finalization of the manuscript. L. Methot participated in acquisition of data, and revision and finalization of the manuscript. H. L. Neville participated in acquisition of data, and revision and finalization of the manuscript. D. Bates participated in acquisition of data, and revision and finalization of the manuscript. D. Lowe participated in acquisition of data, and revision and finalization of the manuscript. N. Fernandes participated in acquisition of data, and revision and finalization of the manuscript. A. Ferland participated in acquisition of data, and revision and finalization of the manuscript. C. M. del Campo acted as primary supervisor for this project, and approved study design and methods. He assisted with interpretation of data, and revision and finalization of the manuscript. Preliminary data were reported in abstract form at the 2013 Annual Meeting of the American Academy of Neurology (March 2013, San Diego, CA) and the 2014 Annual Meeting of the Canadian Neurological Sciences Foundation (June 2014, Banff, AB, Canada). No sources of funding are reported for this study. The authors report no conflicts of interest.
- , , , , . Nutrition in the hospitalized patient. J Hosp Med. 2013;8(1):52–58.
- , . The impact of malnutrition on morbidity, mortality, length of hospital stay and costs evaluated through a multivariate model analysis. Clin Nutr. 2003;22(3):235–239.
- , , , et al. Prevalence of malnutrition on admission to four hospitals in England. The Malnutrition Prevalence Group. Clin Nutr. 2000;19(3):191–195.
- . Die akute haemorrhagische polioencephalitis superior: Kassel, Germany; Fisher; 1881.
- . Diseases of the Nervous System. 5th ed. London: Oxford University Press; 1955.
- , , . Wernicke's encephalopathy. A clinical and pathological study of 28 autopsied cases. Arch Neurol. 1961;4:510–519.
- , , , . Diagnosis by treatment. J Hosp Med. 2011;6(9):546–549.
- , , . The Wernicke‐Korsakoff syndrome. A clinical and pathological study of 245 patients, 82 with post‐mortem examinations. Contemp Neurol Ser. 1971;7:1–206.
- , , , . Operational criteria for the classification of chronic alcoholics: identification of Wernicke's encephalopathy. J Neurol Neurosurg Psychiatry. 1997;62(1):51–60.
- . The incidence of Wernicke's encephalopathy in Australia–a neuropathological study of 131 cases. J Neurol Neurosurg Psychiatry. 1983;46(7):593–598.
- . Wernicke's encephalopathy: a more common disease than realised. A neuropathological study of 51 cases. J Neurol Neurosurg Psychiatry. 1979;42:226–231.
- , , . Clinical signs in the Wernicke‐Korsakoff complex: a retrospective analysis of 131 cases diagnosed at necropsy. J Neurol Neurosurg Psychiatry. 1986;49:341–345.
- , , . Autopsy prevalence of Wernicke's encephalopathy in alcohol‐related disease. S Afr Med J. 1996;86(9):1110–1112.
- , . Increasing incidence of Korsakoff's psychosis in the east end of Glasgow. Alcohol Alcohol. 1997;32(3):281–285.
- , , , et al. EFNS guidelines for diagnosis, therapy and prevention of Wernicke encephalopathy. Eur J Neurol. 2010;17(12):1408–1418.
- , , . A survey of the current clinical practice of psychiatrists and accident and emergency specialists in the United Kingdom concerning vitamin supplementation for chronic alcohol misusers. Alcohol Alcohol. 1999;34(6):862–867.
- , , . Thiamine deficiency in head injury: a missed insult? Alcohol Alcohol. 1997;32(4):493–500.
- . Thiamine (vitamin B1) deficiency and associated brain damage is still common throughout the world and prevention is simple and safe! Eur J Neurol. 2006;13(10):1078–1082.
- , , . Prescribing thiamine to inpatients with alcohol use disorders: how well are we doing? J Addict Med. 2014;8(1):1–5.
- , , , ; Royal College of Physicians, London. The Royal College of Physicians report on alcohol: guidelines for managing Wernicke's encephalopathy in the accident and Emergency Department. Alcohol Alcohol. 2002;37(6):513–521.
- , , . B Vitamin deficiency and neuropsychiatric syndromes in alcohol misuse. Alcohol Alcohol. 1998;33(4):317–336.
- , . Five things to know about Wernicke's Encephalopathy: a medical emergency. CMAJ. 2013;186(8):E295.
- , , . Wernicke‐Korsakoff‐syndrome: under‐recognized and under‐treated. Psychosomatics. 2012;53(6):507–516.
- , , , , . Thiamine for Wernicke‐Korsakoff Syndrome in people at risk from alcohol abuse. Cochrane Database Syst Rev. 2004;(1):CD004033.
- , , , , . Thiamine for prevention and treatment of Wernicke‐Korsakoff Syndrome in people who abuse alcohol. Cochrane Database Syst Rev. 2013;7:CD004033.
- , , . Thiamine Treatment and working memory function of alcohol‐dependent people: preliminary findings. Alcohol Clin Exp Res. 2001;25(1):112–116.
- , , , . Myths and misconceptions of Wernicke's encephalopathy: what every emergency physician should know. Ann Emerg Med. 2007;50(6):715–721.
- , . Wernicke's Encephalopathy: The clinical features and their probable relationship to vitamin B deficiency. Q J Med. 1941;10:(37):41–64.
- , , . Patterns of 35S‐thiamine hydrochloride absorption in the malnourished alcoholic patient. J Lab Clin Med. 1970;76(1):34–45.
- , , , . Thiamine propyl disulfide: absorption and utilization. Ann Intern Med. 1971;74(4):529–534.
- , . Parenteral thiamine and Wernicke's encephalopathy: the balance of risks and perception of concern. Alcohol Alcohol. 1997;32(3):207–209.
- , , , et al. Efficacy of vitamin supplementation in chronic alcoholics undergoing detoxification. Alcohol Alcohol Suppl. 1983;18:157–166.
- , , . Mechanisms of neuronal cell death in Wernicke's encephalopathy. Metab Brain Dis. 1998;13(2):97–122.
- , , , . Brain lactate synthesis in thiamine deficiency: a re‐evaluation using 1H‐13C nuclear magnetic resonance spectroscopy. J Neurosci Res. 2005;79(1‐2):33–41.
- , , . Pharmacokinetics of high‐dose oral thiamine hydrochloride in healthy subjects. BMC Clin Pharmacol. 2012;12(4):4.
- , , , et al. Simultaneous liquid chromatographic assessment of thiamine, thiamine monophosphate and thiamine diphosphate in human erythrocytes: a study on alcoholics. J Chromatogr B Analyt Technol Biomed Life Sci. 2003;789(2):355–363.
- , . Neuroimaging findings in acute Wernicke's encephalopathy: review of the literature. AJR Am J Roentgenol. 2009;192(2):501–508.
- , , , , . Beyond alcoholism: Wernicke‐Korsakoff syndrome in patients with psychiatric disorders. Cogn Behav Neurol. 2011;24(4):209–216.
- , , , , . Mental health status and gender as risk factors for onset of physical illness over 10 years. J Epidemiol Community Health. 2014;68(1):64–70.
- , , , , . The response to treatment of subclinical thiamine deficiency in the elderly. Am J Clin Nutr. 1997;66(4):925–928.
- , , . Thiamin and thiamin phosphate ester deficiency assessed by high performance liquid chromatography in four clinical cases of Wernicke's encephalopathy. Alcohol Clin Exp Res. 1993;17:712–716.
- . Prevention and treatment of Wernicke‐Korsakoff syndrome. Alcohol Alcohol Suppl. 2000;35(1):19–20.
- , , . Pabrinex prescribing in Scottish Emergency Departments. Poster presented at: Inaugural Scientific Conference of the College of Emergency Medicine; May 14–16, 2008; London, United Kingdom.
- , , , , , . Pharmacy‐based intervention in Wernicke's encephalopathy. Psychiatrist. 2010;34(6):234–238.
- , , . Time to act on the inadequate management of Wernicke's encephalopathy in the UK. Alcohol alcohol. Jan‐Feb 2013;48(1):4–8.
- , , , , . Malnutrition is prevalent in hospitalized medical patients: are housestaff identifying the malnourished patient? Nutrition. Apr 2006;22(4):350–354.
- , , , . Malnutrition among Hospitalized‐Patients ‐ a Problem of Physician Awareness. Archives of Internal Medicine. Aug 1987;147(8):1462–1465.
- , . BNF recommendations for the treatment of Wernicke's encephalopathy: lost in translation? Alcohol Alcohol. 2013;48(4):514–515.
- , , , , , . Reduction in medication errors in hospitals due to adoption of computerized provider order entry systems. J Am Med Inform Assoc. 2013;20(3):470–476.
- , . Is intravenous thiamine safe? Am J Emerg Med. 1992;10(2):165.
- , , . A toxicity study of parenteral thiamine hydrochloride. Ann Emerg Med. 1989;18(8):867–870.
- , , , , . Enough is enough: Stop wasting money on vitamin and mineral supplements. Ann Intern Med. 2013;159:850–851.
- , , , , . Nutrition in the hospitalized patient. J Hosp Med. 2013;8(1):52–58.
- , . The impact of malnutrition on morbidity, mortality, length of hospital stay and costs evaluated through a multivariate model analysis. Clin Nutr. 2003;22(3):235–239.
- , , , et al. Prevalence of malnutrition on admission to four hospitals in England. The Malnutrition Prevalence Group. Clin Nutr. 2000;19(3):191–195.
- . Die akute haemorrhagische polioencephalitis superior: Kassel, Germany; Fisher; 1881.
- . Diseases of the Nervous System. 5th ed. London: Oxford University Press; 1955.
- , , . Wernicke's encephalopathy. A clinical and pathological study of 28 autopsied cases. Arch Neurol. 1961;4:510–519.
- , , , . Diagnosis by treatment. J Hosp Med. 2011;6(9):546–549.
- , , . The Wernicke‐Korsakoff syndrome. A clinical and pathological study of 245 patients, 82 with post‐mortem examinations. Contemp Neurol Ser. 1971;7:1–206.
- , , , . Operational criteria for the classification of chronic alcoholics: identification of Wernicke's encephalopathy. J Neurol Neurosurg Psychiatry. 1997;62(1):51–60.
- . The incidence of Wernicke's encephalopathy in Australia–a neuropathological study of 131 cases. J Neurol Neurosurg Psychiatry. 1983;46(7):593–598.
- . Wernicke's encephalopathy: a more common disease than realised. A neuropathological study of 51 cases. J Neurol Neurosurg Psychiatry. 1979;42:226–231.
- , , . Clinical signs in the Wernicke‐Korsakoff complex: a retrospective analysis of 131 cases diagnosed at necropsy. J Neurol Neurosurg Psychiatry. 1986;49:341–345.
- , , . Autopsy prevalence of Wernicke's encephalopathy in alcohol‐related disease. S Afr Med J. 1996;86(9):1110–1112.
- , . Increasing incidence of Korsakoff's psychosis in the east end of Glasgow. Alcohol Alcohol. 1997;32(3):281–285.
- , , , et al. EFNS guidelines for diagnosis, therapy and prevention of Wernicke encephalopathy. Eur J Neurol. 2010;17(12):1408–1418.
- , , . A survey of the current clinical practice of psychiatrists and accident and emergency specialists in the United Kingdom concerning vitamin supplementation for chronic alcohol misusers. Alcohol Alcohol. 1999;34(6):862–867.
- , , . Thiamine deficiency in head injury: a missed insult? Alcohol Alcohol. 1997;32(4):493–500.
- . Thiamine (vitamin B1) deficiency and associated brain damage is still common throughout the world and prevention is simple and safe! Eur J Neurol. 2006;13(10):1078–1082.
- , , . Prescribing thiamine to inpatients with alcohol use disorders: how well are we doing? J Addict Med. 2014;8(1):1–5.
- , , , ; Royal College of Physicians, London. The Royal College of Physicians report on alcohol: guidelines for managing Wernicke's encephalopathy in the accident and Emergency Department. Alcohol Alcohol. 2002;37(6):513–521.
- , , . B Vitamin deficiency and neuropsychiatric syndromes in alcohol misuse. Alcohol Alcohol. 1998;33(4):317–336.
- , . Five things to know about Wernicke's Encephalopathy: a medical emergency. CMAJ. 2013;186(8):E295.
- , , . Wernicke‐Korsakoff‐syndrome: under‐recognized and under‐treated. Psychosomatics. 2012;53(6):507–516.
- , , , , . Thiamine for Wernicke‐Korsakoff Syndrome in people at risk from alcohol abuse. Cochrane Database Syst Rev. 2004;(1):CD004033.
- , , , , . Thiamine for prevention and treatment of Wernicke‐Korsakoff Syndrome in people who abuse alcohol. Cochrane Database Syst Rev. 2013;7:CD004033.
- , , . Thiamine Treatment and working memory function of alcohol‐dependent people: preliminary findings. Alcohol Clin Exp Res. 2001;25(1):112–116.
- , , , . Myths and misconceptions of Wernicke's encephalopathy: what every emergency physician should know. Ann Emerg Med. 2007;50(6):715–721.
- , . Wernicke's Encephalopathy: The clinical features and their probable relationship to vitamin B deficiency. Q J Med. 1941;10:(37):41–64.
- , , . Patterns of 35S‐thiamine hydrochloride absorption in the malnourished alcoholic patient. J Lab Clin Med. 1970;76(1):34–45.
- , , , . Thiamine propyl disulfide: absorption and utilization. Ann Intern Med. 1971;74(4):529–534.
- , . Parenteral thiamine and Wernicke's encephalopathy: the balance of risks and perception of concern. Alcohol Alcohol. 1997;32(3):207–209.
- , , , et al. Efficacy of vitamin supplementation in chronic alcoholics undergoing detoxification. Alcohol Alcohol Suppl. 1983;18:157–166.
- , , . Mechanisms of neuronal cell death in Wernicke's encephalopathy. Metab Brain Dis. 1998;13(2):97–122.
- , , , . Brain lactate synthesis in thiamine deficiency: a re‐evaluation using 1H‐13C nuclear magnetic resonance spectroscopy. J Neurosci Res. 2005;79(1‐2):33–41.
- , , . Pharmacokinetics of high‐dose oral thiamine hydrochloride in healthy subjects. BMC Clin Pharmacol. 2012;12(4):4.
- , , , et al. Simultaneous liquid chromatographic assessment of thiamine, thiamine monophosphate and thiamine diphosphate in human erythrocytes: a study on alcoholics. J Chromatogr B Analyt Technol Biomed Life Sci. 2003;789(2):355–363.
- , . Neuroimaging findings in acute Wernicke's encephalopathy: review of the literature. AJR Am J Roentgenol. 2009;192(2):501–508.
- , , , , . Beyond alcoholism: Wernicke‐Korsakoff syndrome in patients with psychiatric disorders. Cogn Behav Neurol. 2011;24(4):209–216.
- , , , , . Mental health status and gender as risk factors for onset of physical illness over 10 years. J Epidemiol Community Health. 2014;68(1):64–70.
- , , , , . The response to treatment of subclinical thiamine deficiency in the elderly. Am J Clin Nutr. 1997;66(4):925–928.
- , , . Thiamin and thiamin phosphate ester deficiency assessed by high performance liquid chromatography in four clinical cases of Wernicke's encephalopathy. Alcohol Clin Exp Res. 1993;17:712–716.
- . Prevention and treatment of Wernicke‐Korsakoff syndrome. Alcohol Alcohol Suppl. 2000;35(1):19–20.
- , , . Pabrinex prescribing in Scottish Emergency Departments. Poster presented at: Inaugural Scientific Conference of the College of Emergency Medicine; May 14–16, 2008; London, United Kingdom.
- , , , , , . Pharmacy‐based intervention in Wernicke's encephalopathy. Psychiatrist. 2010;34(6):234–238.
- , , . Time to act on the inadequate management of Wernicke's encephalopathy in the UK. Alcohol alcohol. Jan‐Feb 2013;48(1):4–8.
- , , , , . Malnutrition is prevalent in hospitalized medical patients: are housestaff identifying the malnourished patient? Nutrition. Apr 2006;22(4):350–354.
- , , , . Malnutrition among Hospitalized‐Patients ‐ a Problem of Physician Awareness. Archives of Internal Medicine. Aug 1987;147(8):1462–1465.
- , . BNF recommendations for the treatment of Wernicke's encephalopathy: lost in translation? Alcohol Alcohol. 2013;48(4):514–515.
- , , , , , . Reduction in medication errors in hospitals due to adoption of computerized provider order entry systems. J Am Med Inform Assoc. 2013;20(3):470–476.
- , . Is intravenous thiamine safe? Am J Emerg Med. 1992;10(2):165.
- , , . A toxicity study of parenteral thiamine hydrochloride. Ann Emerg Med. 1989;18(8):867–870.
- , , , , . Enough is enough: Stop wasting money on vitamin and mineral supplements. Ann Intern Med. 2013;159:850–851.
© 2015 Society of Hospital Medicine