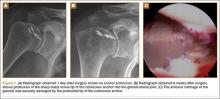
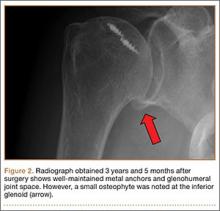
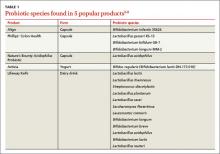
User login
Headway being made in developing biomarkers for PTSD
HUNTINGTON BEACH, CALIF. – Researchers are making significant headway in developing objective, reliable, and valid biomarkers to discriminate individuals with warzone post traumatic stress disorder from healthy controls, according to Dr. Charles R. Marmar.
“It’s clear that over the next four or five years we will identify very clear biological, psychological, and other behavioral risk and resilience profiles,” Dr. Marmar told attendees at the annual meeting of the American College of Psychiatrists.
Currently, clinicians largely rely on patient self-reports and clinical observations to diagnose PTSD in military personnel, said Dr. Marmar, professor and chair of the department of psychiatry at NYU Langone Medical Center and director of NYU’s Steven and Alexandra Cohen Veterans Center.
“The problem from the military and law enforcement perspective is that the majority of war fighters experience tremendous stigma in acknowledging their symptoms, particularly active duty military personnel,” he said. “A minority will exaggerate to avoid service or for compensation. Given that we’ve had nearly three million men and women serve in Iraq and Afghanistan, and the fact that we have no objective way yet of determining which ones continue to be fit for redeployment, which ones are in urgent need of help, and which ones deserve compensation, we need to develop better ways to determine if treatments are effective, to inform new treatment selection, and to define new targets for treatment.”
The scope of the problem is underscored in an analysis of data from 289,328 veterans entering VA Healthcare for the first time beginning on April 1, 2002 through March 31, 2006 (Am J. Pub. Health 2009;99[9]:1651-8). Prior to the invasion of Iraq, the distribution of mental health problems was very similar among veterans as in the general population: depression being most common, and low rates of PTSD and alcohol and drug abuse. However, “with each quarter since the invasion of Iraq, there’s been an incubative growth in the prevalence of PTSD, which has now eclipsed depression,” Dr. Marmar said. “We have a toll, a generational effect which looks similar in magnitude with the Vietnam War, both in the number of men and women who serve and in the prevalence of PTSD, depression and alcohol- and drug-related disorders.”
In the general population, risk factors include female sex, child abuse, genetics, which in twin studies account for 30-40% of the risk, lower IQ and lower educational attainment, stressful life events in the prior and following year, and panic reaction at the time of event, such as racing heart, shaking, and sweating.
According to findings from the National Vietnam Veterans Readjustment Study, risk factors for chronic warzone PTSD include high school dropout rate, history of child abuse, high warzone exposure, serious warzone injury, killing combatants, prisoners, and civilians, peritraumatic dissociation, hostile homecoming, post-discharge trauma, and genetics. “These are the risk profiles, and they should give us some clues about where to look for biological factors,” Dr. Marmar said.
The risks of service are not limited to stress, anxiety, depression, alcohol and drug abuse, or traumatic brain injury (TBI). “If you compare men and women returning from Iraq and Afghanistan with no mental health issues to those who have a diagnosis of either PTSD, depression, or the combination, the [diagnosed] cases have 2.5 times the risk of tobacco use, hypertension, dyslipidemia, obesity, and type 2 diabetes,” he said. “These are people in their late 20s and early 30s. So the costs of warzone-related stress and depression are enormous on general health.”
Dr. Marmar presented preliminary findings from the ongoing PTSD Systems Biology Consortium, an effort by researchers at seven universities to establish biomarkers for PTSD. Funded by the Department of Defense, the National Institutes of Health, and other sources, the consortium is comprised of integrated cores including neurocognition, genetics, structural and functional brain imaging, endocrinology, metabolism, genomics, proteomics, metabolomics, and bioinformatics.
To date, the researchers have screened 2,215 veterans from service in Iraq and Afghanistan, all of whom have been deployed to war at least once. Cases were PTSD positive and had a CAPS (Clinician-Administered PTSD scale) score of 20 or greater. Controls were PTSD negative and had a CAPS score of less than 20. They excluded subjects with lifetime psychosis, bipolar disorder, or OCD, as well as alcohol dependence in the past eight months, and drug abuse in the past year. They also excluded veterans with TBI “because we’re trying to be very careful to see if we can get a biological signal comparing combat PTSD cases with controls,” Dr. Marmar noted.
Dr. Marmar presented preliminary findings from 52 PTSD cases and 52 controls that were matched for sex, ethnicity, and age. The sample was entirely men, their mean age was 34 years, and they had a mean of 14.8 years of education. The researchers covaried for depression and other known confounders. “It’s very difficult to disentangle the effects of PTSD and depression because 50% of the cases of warzone PTSD also meet criteria for current major depression, and over 80% meet criteria for lifetime depression,” he said.
In results from the clinical diagnostic evaluation, PTSD cases, compared with controls, were significantly more likely to have current anxiety (7% vs. 0%, respectively; P = .041); lifetime anxiety (9.6% vs. 0%; P = .022); current major depressive disorder (51.5% vs. 1.9%; P<.001); lifetime MDD (84.6% vs. 23.1%; P<.001); and lifetime alcohol abuse dependence (63.5% vs. 25%; P = .001). There was also a non-signficant trend toward lifetime substance abuse/dependence (13.5% vs. 3.9%; P = .081).
Results from the neurocognitive assessments revealed that PTSD positive men had a significantly lower estimated IQ, compared with their PTSD negative counterparts (a mean of 99.3 vs. 107.9, respectively; P = .031). Other significant differences between the two groups were observed in tests of auditory and working memory, specifically digit span (8.67 vs. 10.04; P = .02), and the visual memory sum (9.1 vs. 10.67; P = .01).
One of the consortium collaborators developed a test to compare reward and punishment learning. For the test, “the subject is required to understand what the meaning of a symbol is in a task, and they have no prior knowledge [of the meaning],” Dr. Marmar explained. “They’re either rewarded for guessing correctly or punished for guessing incorrectly.” So far, the healthy controls “are performing much better in identifying the symbols when they’re rewarded, compared with the PTSD cases, and there’s no difference in punishment,” he said. “So there’s impaired reward learning and intact punishment learning in PTSD cases compared to controls, which likely reflects underlying disturbances in dopamine reward circuitry.”
Investigators in the neurogenetics core hypothesized that DNA variants in stress-response genes identified from previous medical studies will be associated with PTSD. These included FKBP5, COMT, APOE, BDNF, PACAP/PAC1R, and OPRL1. Initial analysis revealed that there were a greater number of BDNF allele frequencies among cases, compared with controls (P = .008). “It would appear that BDNF variants confer resilience for combat-related PTSD,” Dr. Marmar said.
The researchers also found a single nucleotide polymorphism never previously described on Chromosome 4. “It’s in a region between genes, probably a micro-RNA regulatory gene on the 4th chromosome,” he said. “That gene in our sample was associated with higher levels of PTSD. In addition, fMRI studies found that carrying this allele was associated with weaker activation of prefrontal cortical areas in the brain to empirical faces tasks.”
The endocrine core found that PTSD cases had lower ambient cortisol levels, compared with controls (P = .051). They also had significantly greater cortisol suppression following dexamethasone administration, compared with controls (P = .013). “This is evidence that there is increased glucocorticoid receptor sensitivity in PTSD expressing as elevated cortisol suppression,” Dr. Marmar said.
Investigators from the structural imaging core found no significant differences in overall hippocampal volume or in the five major hippocampal subfields between PTSD cases and controls, nor in difference in the volume of other brain structures previously implicated in PTSD, such as the amygdala and the thalamus. However, the researchers are finding some differences between cases and controls on functional imaging, including increased spontaneous activity in the amygdala and the insula, and decreased spontaneous activity in the precuneus. “The overall findings on fMRI are that there’s increased activity in the regions [of the brain] associated with fear and decreased connectivity between the frontal cortex and the amygdala,” he said. “This is consistent with the model of dysregulated fear activity in PTSD.”
Researchers have also observed that many markers of metabolic syndrome are significantly elevated between PTSD cases and controls, including fasting glucose (P = .001), weight (P = .03), and resting pulse (P = .003). “When you covary for depression, these findings remain,” Dr. Marmar said. “It’s important to note that these are men mostly in their early 30s recently returned from war and recently in military training, physically fit to be deployed to war.”
He closed his presentation by noting that mounting evidence from animal and human studies suggests evidence of mitochondrial dysfunction in PTSD. In the current analysis, researchers observed a reduced abundance of citrate and other mitochondrial metabolites in PTSD cases compared with controls, as well as an increased abundance of “premitochondrial” metabolites such as pyruvate and lactate. “These findings stand when you covary for depression and for metabolic syndrome,” Dr. Marmar said.
“We believe that these may be very important potential future candidate biomarkers to differentiate PTSD cases from controls.”
The next step in this effort, he added, is to replicate the consortium’s overall findings in a cross-validation sample of 50 male cases and 50 male controls. “We also have a sample of 40 female cases and 40 controls to see if the markers are the same or different,” he said. The researchers are also conducting a prospective study of 1,200 active duty military personnel, who will be evaluated before and after deployment.
For now, some clinicians wonder what should be done for men and women who carry the PTSD risk alleles, or carry the endocrine or metabolism vulnerability to develop complications from combat exposure. “That’s a very sensitive national question,” Dr. Marmar said. “People want to serve their country. The answer may be to allow service but to have a more nuanced approach to what people’s roles should be within the military, to match individuals’ stress resilience with the responsibilities they have.”
Dr. Marmar reported that he had no relevant financial conflicts.
On Twitter @dougbrunk
HUNTINGTON BEACH, CALIF. – Researchers are making significant headway in developing objective, reliable, and valid biomarkers to discriminate individuals with warzone post traumatic stress disorder from healthy controls, according to Dr. Charles R. Marmar.
“It’s clear that over the next four or five years we will identify very clear biological, psychological, and other behavioral risk and resilience profiles,” Dr. Marmar told attendees at the annual meeting of the American College of Psychiatrists.
Currently, clinicians largely rely on patient self-reports and clinical observations to diagnose PTSD in military personnel, said Dr. Marmar, professor and chair of the department of psychiatry at NYU Langone Medical Center and director of NYU’s Steven and Alexandra Cohen Veterans Center.
“The problem from the military and law enforcement perspective is that the majority of war fighters experience tremendous stigma in acknowledging their symptoms, particularly active duty military personnel,” he said. “A minority will exaggerate to avoid service or for compensation. Given that we’ve had nearly three million men and women serve in Iraq and Afghanistan, and the fact that we have no objective way yet of determining which ones continue to be fit for redeployment, which ones are in urgent need of help, and which ones deserve compensation, we need to develop better ways to determine if treatments are effective, to inform new treatment selection, and to define new targets for treatment.”
The scope of the problem is underscored in an analysis of data from 289,328 veterans entering VA Healthcare for the first time beginning on April 1, 2002 through March 31, 2006 (Am J. Pub. Health 2009;99[9]:1651-8). Prior to the invasion of Iraq, the distribution of mental health problems was very similar among veterans as in the general population: depression being most common, and low rates of PTSD and alcohol and drug abuse. However, “with each quarter since the invasion of Iraq, there’s been an incubative growth in the prevalence of PTSD, which has now eclipsed depression,” Dr. Marmar said. “We have a toll, a generational effect which looks similar in magnitude with the Vietnam War, both in the number of men and women who serve and in the prevalence of PTSD, depression and alcohol- and drug-related disorders.”
In the general population, risk factors include female sex, child abuse, genetics, which in twin studies account for 30-40% of the risk, lower IQ and lower educational attainment, stressful life events in the prior and following year, and panic reaction at the time of event, such as racing heart, shaking, and sweating.
According to findings from the National Vietnam Veterans Readjustment Study, risk factors for chronic warzone PTSD include high school dropout rate, history of child abuse, high warzone exposure, serious warzone injury, killing combatants, prisoners, and civilians, peritraumatic dissociation, hostile homecoming, post-discharge trauma, and genetics. “These are the risk profiles, and they should give us some clues about where to look for biological factors,” Dr. Marmar said.
The risks of service are not limited to stress, anxiety, depression, alcohol and drug abuse, or traumatic brain injury (TBI). “If you compare men and women returning from Iraq and Afghanistan with no mental health issues to those who have a diagnosis of either PTSD, depression, or the combination, the [diagnosed] cases have 2.5 times the risk of tobacco use, hypertension, dyslipidemia, obesity, and type 2 diabetes,” he said. “These are people in their late 20s and early 30s. So the costs of warzone-related stress and depression are enormous on general health.”
Dr. Marmar presented preliminary findings from the ongoing PTSD Systems Biology Consortium, an effort by researchers at seven universities to establish biomarkers for PTSD. Funded by the Department of Defense, the National Institutes of Health, and other sources, the consortium is comprised of integrated cores including neurocognition, genetics, structural and functional brain imaging, endocrinology, metabolism, genomics, proteomics, metabolomics, and bioinformatics.
To date, the researchers have screened 2,215 veterans from service in Iraq and Afghanistan, all of whom have been deployed to war at least once. Cases were PTSD positive and had a CAPS (Clinician-Administered PTSD scale) score of 20 or greater. Controls were PTSD negative and had a CAPS score of less than 20. They excluded subjects with lifetime psychosis, bipolar disorder, or OCD, as well as alcohol dependence in the past eight months, and drug abuse in the past year. They also excluded veterans with TBI “because we’re trying to be very careful to see if we can get a biological signal comparing combat PTSD cases with controls,” Dr. Marmar noted.
Dr. Marmar presented preliminary findings from 52 PTSD cases and 52 controls that were matched for sex, ethnicity, and age. The sample was entirely men, their mean age was 34 years, and they had a mean of 14.8 years of education. The researchers covaried for depression and other known confounders. “It’s very difficult to disentangle the effects of PTSD and depression because 50% of the cases of warzone PTSD also meet criteria for current major depression, and over 80% meet criteria for lifetime depression,” he said.
In results from the clinical diagnostic evaluation, PTSD cases, compared with controls, were significantly more likely to have current anxiety (7% vs. 0%, respectively; P = .041); lifetime anxiety (9.6% vs. 0%; P = .022); current major depressive disorder (51.5% vs. 1.9%; P<.001); lifetime MDD (84.6% vs. 23.1%; P<.001); and lifetime alcohol abuse dependence (63.5% vs. 25%; P = .001). There was also a non-signficant trend toward lifetime substance abuse/dependence (13.5% vs. 3.9%; P = .081).
Results from the neurocognitive assessments revealed that PTSD positive men had a significantly lower estimated IQ, compared with their PTSD negative counterparts (a mean of 99.3 vs. 107.9, respectively; P = .031). Other significant differences between the two groups were observed in tests of auditory and working memory, specifically digit span (8.67 vs. 10.04; P = .02), and the visual memory sum (9.1 vs. 10.67; P = .01).
One of the consortium collaborators developed a test to compare reward and punishment learning. For the test, “the subject is required to understand what the meaning of a symbol is in a task, and they have no prior knowledge [of the meaning],” Dr. Marmar explained. “They’re either rewarded for guessing correctly or punished for guessing incorrectly.” So far, the healthy controls “are performing much better in identifying the symbols when they’re rewarded, compared with the PTSD cases, and there’s no difference in punishment,” he said. “So there’s impaired reward learning and intact punishment learning in PTSD cases compared to controls, which likely reflects underlying disturbances in dopamine reward circuitry.”
Investigators in the neurogenetics core hypothesized that DNA variants in stress-response genes identified from previous medical studies will be associated with PTSD. These included FKBP5, COMT, APOE, BDNF, PACAP/PAC1R, and OPRL1. Initial analysis revealed that there were a greater number of BDNF allele frequencies among cases, compared with controls (P = .008). “It would appear that BDNF variants confer resilience for combat-related PTSD,” Dr. Marmar said.
The researchers also found a single nucleotide polymorphism never previously described on Chromosome 4. “It’s in a region between genes, probably a micro-RNA regulatory gene on the 4th chromosome,” he said. “That gene in our sample was associated with higher levels of PTSD. In addition, fMRI studies found that carrying this allele was associated with weaker activation of prefrontal cortical areas in the brain to empirical faces tasks.”
The endocrine core found that PTSD cases had lower ambient cortisol levels, compared with controls (P = .051). They also had significantly greater cortisol suppression following dexamethasone administration, compared with controls (P = .013). “This is evidence that there is increased glucocorticoid receptor sensitivity in PTSD expressing as elevated cortisol suppression,” Dr. Marmar said.
Investigators from the structural imaging core found no significant differences in overall hippocampal volume or in the five major hippocampal subfields between PTSD cases and controls, nor in difference in the volume of other brain structures previously implicated in PTSD, such as the amygdala and the thalamus. However, the researchers are finding some differences between cases and controls on functional imaging, including increased spontaneous activity in the amygdala and the insula, and decreased spontaneous activity in the precuneus. “The overall findings on fMRI are that there’s increased activity in the regions [of the brain] associated with fear and decreased connectivity between the frontal cortex and the amygdala,” he said. “This is consistent with the model of dysregulated fear activity in PTSD.”
Researchers have also observed that many markers of metabolic syndrome are significantly elevated between PTSD cases and controls, including fasting glucose (P = .001), weight (P = .03), and resting pulse (P = .003). “When you covary for depression, these findings remain,” Dr. Marmar said. “It’s important to note that these are men mostly in their early 30s recently returned from war and recently in military training, physically fit to be deployed to war.”
He closed his presentation by noting that mounting evidence from animal and human studies suggests evidence of mitochondrial dysfunction in PTSD. In the current analysis, researchers observed a reduced abundance of citrate and other mitochondrial metabolites in PTSD cases compared with controls, as well as an increased abundance of “premitochondrial” metabolites such as pyruvate and lactate. “These findings stand when you covary for depression and for metabolic syndrome,” Dr. Marmar said.
“We believe that these may be very important potential future candidate biomarkers to differentiate PTSD cases from controls.”
The next step in this effort, he added, is to replicate the consortium’s overall findings in a cross-validation sample of 50 male cases and 50 male controls. “We also have a sample of 40 female cases and 40 controls to see if the markers are the same or different,” he said. The researchers are also conducting a prospective study of 1,200 active duty military personnel, who will be evaluated before and after deployment.
For now, some clinicians wonder what should be done for men and women who carry the PTSD risk alleles, or carry the endocrine or metabolism vulnerability to develop complications from combat exposure. “That’s a very sensitive national question,” Dr. Marmar said. “People want to serve their country. The answer may be to allow service but to have a more nuanced approach to what people’s roles should be within the military, to match individuals’ stress resilience with the responsibilities they have.”
Dr. Marmar reported that he had no relevant financial conflicts.
On Twitter @dougbrunk
HUNTINGTON BEACH, CALIF. – Researchers are making significant headway in developing objective, reliable, and valid biomarkers to discriminate individuals with warzone post traumatic stress disorder from healthy controls, according to Dr. Charles R. Marmar.
“It’s clear that over the next four or five years we will identify very clear biological, psychological, and other behavioral risk and resilience profiles,” Dr. Marmar told attendees at the annual meeting of the American College of Psychiatrists.
Currently, clinicians largely rely on patient self-reports and clinical observations to diagnose PTSD in military personnel, said Dr. Marmar, professor and chair of the department of psychiatry at NYU Langone Medical Center and director of NYU’s Steven and Alexandra Cohen Veterans Center.
“The problem from the military and law enforcement perspective is that the majority of war fighters experience tremendous stigma in acknowledging their symptoms, particularly active duty military personnel,” he said. “A minority will exaggerate to avoid service or for compensation. Given that we’ve had nearly three million men and women serve in Iraq and Afghanistan, and the fact that we have no objective way yet of determining which ones continue to be fit for redeployment, which ones are in urgent need of help, and which ones deserve compensation, we need to develop better ways to determine if treatments are effective, to inform new treatment selection, and to define new targets for treatment.”
The scope of the problem is underscored in an analysis of data from 289,328 veterans entering VA Healthcare for the first time beginning on April 1, 2002 through March 31, 2006 (Am J. Pub. Health 2009;99[9]:1651-8). Prior to the invasion of Iraq, the distribution of mental health problems was very similar among veterans as in the general population: depression being most common, and low rates of PTSD and alcohol and drug abuse. However, “with each quarter since the invasion of Iraq, there’s been an incubative growth in the prevalence of PTSD, which has now eclipsed depression,” Dr. Marmar said. “We have a toll, a generational effect which looks similar in magnitude with the Vietnam War, both in the number of men and women who serve and in the prevalence of PTSD, depression and alcohol- and drug-related disorders.”
In the general population, risk factors include female sex, child abuse, genetics, which in twin studies account for 30-40% of the risk, lower IQ and lower educational attainment, stressful life events in the prior and following year, and panic reaction at the time of event, such as racing heart, shaking, and sweating.
According to findings from the National Vietnam Veterans Readjustment Study, risk factors for chronic warzone PTSD include high school dropout rate, history of child abuse, high warzone exposure, serious warzone injury, killing combatants, prisoners, and civilians, peritraumatic dissociation, hostile homecoming, post-discharge trauma, and genetics. “These are the risk profiles, and they should give us some clues about where to look for biological factors,” Dr. Marmar said.
The risks of service are not limited to stress, anxiety, depression, alcohol and drug abuse, or traumatic brain injury (TBI). “If you compare men and women returning from Iraq and Afghanistan with no mental health issues to those who have a diagnosis of either PTSD, depression, or the combination, the [diagnosed] cases have 2.5 times the risk of tobacco use, hypertension, dyslipidemia, obesity, and type 2 diabetes,” he said. “These are people in their late 20s and early 30s. So the costs of warzone-related stress and depression are enormous on general health.”
Dr. Marmar presented preliminary findings from the ongoing PTSD Systems Biology Consortium, an effort by researchers at seven universities to establish biomarkers for PTSD. Funded by the Department of Defense, the National Institutes of Health, and other sources, the consortium is comprised of integrated cores including neurocognition, genetics, structural and functional brain imaging, endocrinology, metabolism, genomics, proteomics, metabolomics, and bioinformatics.
To date, the researchers have screened 2,215 veterans from service in Iraq and Afghanistan, all of whom have been deployed to war at least once. Cases were PTSD positive and had a CAPS (Clinician-Administered PTSD scale) score of 20 or greater. Controls were PTSD negative and had a CAPS score of less than 20. They excluded subjects with lifetime psychosis, bipolar disorder, or OCD, as well as alcohol dependence in the past eight months, and drug abuse in the past year. They also excluded veterans with TBI “because we’re trying to be very careful to see if we can get a biological signal comparing combat PTSD cases with controls,” Dr. Marmar noted.
Dr. Marmar presented preliminary findings from 52 PTSD cases and 52 controls that were matched for sex, ethnicity, and age. The sample was entirely men, their mean age was 34 years, and they had a mean of 14.8 years of education. The researchers covaried for depression and other known confounders. “It’s very difficult to disentangle the effects of PTSD and depression because 50% of the cases of warzone PTSD also meet criteria for current major depression, and over 80% meet criteria for lifetime depression,” he said.
In results from the clinical diagnostic evaluation, PTSD cases, compared with controls, were significantly more likely to have current anxiety (7% vs. 0%, respectively; P = .041); lifetime anxiety (9.6% vs. 0%; P = .022); current major depressive disorder (51.5% vs. 1.9%; P<.001); lifetime MDD (84.6% vs. 23.1%; P<.001); and lifetime alcohol abuse dependence (63.5% vs. 25%; P = .001). There was also a non-signficant trend toward lifetime substance abuse/dependence (13.5% vs. 3.9%; P = .081).
Results from the neurocognitive assessments revealed that PTSD positive men had a significantly lower estimated IQ, compared with their PTSD negative counterparts (a mean of 99.3 vs. 107.9, respectively; P = .031). Other significant differences between the two groups were observed in tests of auditory and working memory, specifically digit span (8.67 vs. 10.04; P = .02), and the visual memory sum (9.1 vs. 10.67; P = .01).
One of the consortium collaborators developed a test to compare reward and punishment learning. For the test, “the subject is required to understand what the meaning of a symbol is in a task, and they have no prior knowledge [of the meaning],” Dr. Marmar explained. “They’re either rewarded for guessing correctly or punished for guessing incorrectly.” So far, the healthy controls “are performing much better in identifying the symbols when they’re rewarded, compared with the PTSD cases, and there’s no difference in punishment,” he said. “So there’s impaired reward learning and intact punishment learning in PTSD cases compared to controls, which likely reflects underlying disturbances in dopamine reward circuitry.”
Investigators in the neurogenetics core hypothesized that DNA variants in stress-response genes identified from previous medical studies will be associated with PTSD. These included FKBP5, COMT, APOE, BDNF, PACAP/PAC1R, and OPRL1. Initial analysis revealed that there were a greater number of BDNF allele frequencies among cases, compared with controls (P = .008). “It would appear that BDNF variants confer resilience for combat-related PTSD,” Dr. Marmar said.
The researchers also found a single nucleotide polymorphism never previously described on Chromosome 4. “It’s in a region between genes, probably a micro-RNA regulatory gene on the 4th chromosome,” he said. “That gene in our sample was associated with higher levels of PTSD. In addition, fMRI studies found that carrying this allele was associated with weaker activation of prefrontal cortical areas in the brain to empirical faces tasks.”
The endocrine core found that PTSD cases had lower ambient cortisol levels, compared with controls (P = .051). They also had significantly greater cortisol suppression following dexamethasone administration, compared with controls (P = .013). “This is evidence that there is increased glucocorticoid receptor sensitivity in PTSD expressing as elevated cortisol suppression,” Dr. Marmar said.
Investigators from the structural imaging core found no significant differences in overall hippocampal volume or in the five major hippocampal subfields between PTSD cases and controls, nor in difference in the volume of other brain structures previously implicated in PTSD, such as the amygdala and the thalamus. However, the researchers are finding some differences between cases and controls on functional imaging, including increased spontaneous activity in the amygdala and the insula, and decreased spontaneous activity in the precuneus. “The overall findings on fMRI are that there’s increased activity in the regions [of the brain] associated with fear and decreased connectivity between the frontal cortex and the amygdala,” he said. “This is consistent with the model of dysregulated fear activity in PTSD.”
Researchers have also observed that many markers of metabolic syndrome are significantly elevated between PTSD cases and controls, including fasting glucose (P = .001), weight (P = .03), and resting pulse (P = .003). “When you covary for depression, these findings remain,” Dr. Marmar said. “It’s important to note that these are men mostly in their early 30s recently returned from war and recently in military training, physically fit to be deployed to war.”
He closed his presentation by noting that mounting evidence from animal and human studies suggests evidence of mitochondrial dysfunction in PTSD. In the current analysis, researchers observed a reduced abundance of citrate and other mitochondrial metabolites in PTSD cases compared with controls, as well as an increased abundance of “premitochondrial” metabolites such as pyruvate and lactate. “These findings stand when you covary for depression and for metabolic syndrome,” Dr. Marmar said.
“We believe that these may be very important potential future candidate biomarkers to differentiate PTSD cases from controls.”
The next step in this effort, he added, is to replicate the consortium’s overall findings in a cross-validation sample of 50 male cases and 50 male controls. “We also have a sample of 40 female cases and 40 controls to see if the markers are the same or different,” he said. The researchers are also conducting a prospective study of 1,200 active duty military personnel, who will be evaluated before and after deployment.
For now, some clinicians wonder what should be done for men and women who carry the PTSD risk alleles, or carry the endocrine or metabolism vulnerability to develop complications from combat exposure. “That’s a very sensitive national question,” Dr. Marmar said. “People want to serve their country. The answer may be to allow service but to have a more nuanced approach to what people’s roles should be within the military, to match individuals’ stress resilience with the responsibilities they have.”
Dr. Marmar reported that he had no relevant financial conflicts.
On Twitter @dougbrunk
EXPERT ANALYSIS AT THE ANNUAL MEETING OF THE AMERICAN COLLEGE OF PSYCHIATRISTS
Telltale sonographic features of simple and hemorrhagic cysts
Pelvic ultrasonography remains the preferred imaging method to evaluate most adnexal cysts, given its ability to accurately characterize their various aspects:
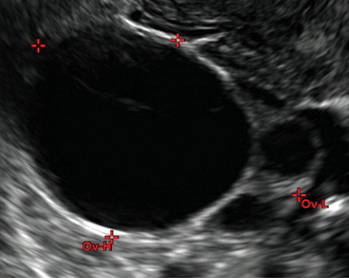
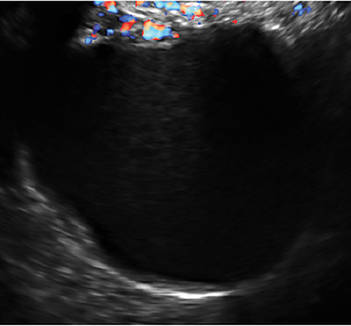
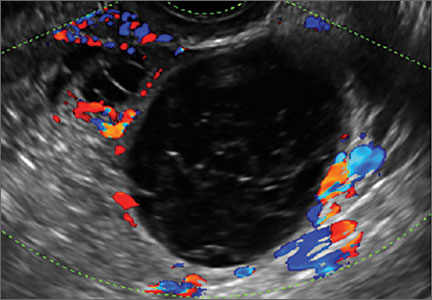
- Simple cysts are uniformly hypoechoic, with thin walls and no blood flow on color Doppler (FIGURE 1).
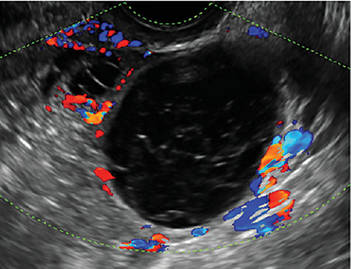
- Hemorrhagic cysts produce lacy/reticular echoes and clot with concave margins
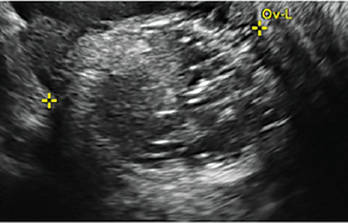
(FIGURE 2). - Mature cystic teratomas produce hyperechoic lines and dots, sometimes known as “dermoid mesh,” acoustic shadowing, and a hyperechoic nodule (FIGURE 3).
- Endometriomas produce diffuse, low-level internal echoes and a “ground glass” appearance (FIGURE 4).
In the first of this 4-part series on the sonographic features of cystic adnexal pathology, we focus on simple and hemorrhagic cysts. In the following parts we will highlight:
- mature cystic teratomas and endometriomas (Part 2)
- hydrosalpinx and pelvic inclusion cysts (Part 3)
- cystadenoma and ovarian neoplasia (Part 4).
An earlier installment of this series entitled “Hemorrhagic ovarian cysts: one entity with many appearances” (May 2014) also focused on cystic pathology.
Figure 1: Simple cyst
A simple cyst in a 32-year-old patient. Figure 2: Hemorrhagic cyst
Note the lacy/reticular internal echoes and lack of internal blood flow on color Doppler. Figure 3: Cystic teratoma
This cyst exhibits the “dermoid mesh” and hyperechoic lines that correspond with hair. Figure 4: Endometrioma
Note diffuse low-level internal echoes (“ground glass”) and no “ring of fire” on color Doppler. |
Characteristics of simple cysts
A simple cyst typically is round or oval, anechoic, and has smooth, thin walls. It contains no solid component or septation (with rare exceptions), and no internal flow is visible on color Doppler imaging.
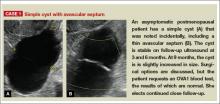
Levine and colleagues observed that simple adnexal cysts as large as 10 cm carry a risk of malignancy of less than 1%, regardless of the age of the patient. In its 2010 Consensus Conference Statement,1 the Society of Radiologists in Ultrasound recommended the following management strategies for women with simple cysts:
Reproductive-aged women
- Cyst <3 cm: No action necessary; the cyst is a normal physiologic finding and should be referred to as a follicle.
- 3–5 cm: No follow-up necessary; the cyst is almost certainly benign.
- 5–7 cm: Yearly imaging; the cyst is highly likely to be benign.
- >7 cm: Additional imaging is recommended.
Postmenopausal women
- <1 cm: No follow-up necessary; the cyst is almost certainly benign.
- 1–7 cm: Yearly imaging; the cyst is likely to be benign.
- >7 cm: Additional imaging is recommended.
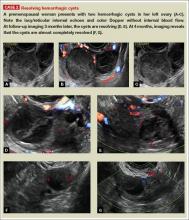
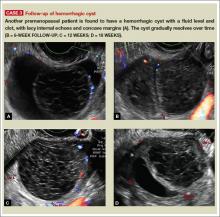
Characteristics of hemorrhagic cysts
These cysts can be quite variable in appearance. Among their sonographic features:
- reticular (lacy, cobweb, or fishnet) internal echoes, due to fibrin strands
- solid-appearing areas with concave margins
- on color Doppler, there may be circumferential peripheral flow (“ring of fire”) and no internal flow.
In its 2010 Consensus Conference Statement, the Society of Radiologists in Ultrasound recommended the following management strategies1:
Premenopausal women
- ≤5 cm: No follow-up imaging unless the diagnosis is uncertain.
- >5 cm: Short-interval follow-up ultrasound (6–12 weeks).
Recently menopausal women
- Any size: Follow-up ultrasound in 6–12 weeks to ensure resolution.
Later postmenopausal women
- Any size: Consider surgical removal, as the cyst may be neoplastic.
Share your thoughts on this article! Send your Letter to the Editor to [email protected]. Please include your name and the city and state in which you practice.
Reference
1. Levine D, Brown DL, Andreotti RF, et al. Management of asymptomatic ovarian and other adnexal cysts imaged at US: Society of Radiologists in Ultrasound Consensus Conference Statement. Radiology. 2010;256(3):943–954.
Pelvic ultrasonography remains the preferred imaging method to evaluate most adnexal cysts, given its ability to accurately characterize their various aspects:
- Simple cysts are uniformly hypoechoic, with thin walls and no blood flow on color Doppler (FIGURE 1).
- Hemorrhagic cysts produce lacy/reticular echoes and clot with concave margins
(FIGURE 2). - Mature cystic teratomas produce hyperechoic lines and dots, sometimes known as “dermoid mesh,” acoustic shadowing, and a hyperechoic nodule (FIGURE 3).
- Endometriomas produce diffuse, low-level internal echoes and a “ground glass” appearance (FIGURE 4).
In the first of this 4-part series on the sonographic features of cystic adnexal pathology, we focus on simple and hemorrhagic cysts. In the following parts we will highlight:
- mature cystic teratomas and endometriomas (Part 2)
- hydrosalpinx and pelvic inclusion cysts (Part 3)
- cystadenoma and ovarian neoplasia (Part 4).
An earlier installment of this series entitled “Hemorrhagic ovarian cysts: one entity with many appearances” (May 2014) also focused on cystic pathology.
Figure 1: Simple cyst
A simple cyst in a 32-year-old patient. Figure 2: Hemorrhagic cyst
Note the lacy/reticular internal echoes and lack of internal blood flow on color Doppler. Figure 3: Cystic teratoma
This cyst exhibits the “dermoid mesh” and hyperechoic lines that correspond with hair. Figure 4: Endometrioma
Note diffuse low-level internal echoes (“ground glass”) and no “ring of fire” on color Doppler. |
Characteristics of simple cysts
A simple cyst typically is round or oval, anechoic, and has smooth, thin walls. It contains no solid component or septation (with rare exceptions), and no internal flow is visible on color Doppler imaging.
Levine and colleagues observed that simple adnexal cysts as large as 10 cm carry a risk of malignancy of less than 1%, regardless of the age of the patient. In its 2010 Consensus Conference Statement,1 the Society of Radiologists in Ultrasound recommended the following management strategies for women with simple cysts:
Reproductive-aged women
- Cyst <3 cm: No action necessary; the cyst is a normal physiologic finding and should be referred to as a follicle.
- 3–5 cm: No follow-up necessary; the cyst is almost certainly benign.
- 5–7 cm: Yearly imaging; the cyst is highly likely to be benign.
- >7 cm: Additional imaging is recommended.
Postmenopausal women
- <1 cm: No follow-up necessary; the cyst is almost certainly benign.
- 1–7 cm: Yearly imaging; the cyst is likely to be benign.
- >7 cm: Additional imaging is recommended.
Characteristics of hemorrhagic cysts
These cysts can be quite variable in appearance. Among their sonographic features:
- reticular (lacy, cobweb, or fishnet) internal echoes, due to fibrin strands
- solid-appearing areas with concave margins
- on color Doppler, there may be circumferential peripheral flow (“ring of fire”) and no internal flow.
In its 2010 Consensus Conference Statement, the Society of Radiologists in Ultrasound recommended the following management strategies1:
Premenopausal women
- ≤5 cm: No follow-up imaging unless the diagnosis is uncertain.
- >5 cm: Short-interval follow-up ultrasound (6–12 weeks).
Recently menopausal women
- Any size: Follow-up ultrasound in 6–12 weeks to ensure resolution.
Later postmenopausal women
- Any size: Consider surgical removal, as the cyst may be neoplastic.
Share your thoughts on this article! Send your Letter to the Editor to [email protected]. Please include your name and the city and state in which you practice.
Pelvic ultrasonography remains the preferred imaging method to evaluate most adnexal cysts, given its ability to accurately characterize their various aspects:
- Simple cysts are uniformly hypoechoic, with thin walls and no blood flow on color Doppler (FIGURE 1).
- Hemorrhagic cysts produce lacy/reticular echoes and clot with concave margins
(FIGURE 2). - Mature cystic teratomas produce hyperechoic lines and dots, sometimes known as “dermoid mesh,” acoustic shadowing, and a hyperechoic nodule (FIGURE 3).
- Endometriomas produce diffuse, low-level internal echoes and a “ground glass” appearance (FIGURE 4).
In the first of this 4-part series on the sonographic features of cystic adnexal pathology, we focus on simple and hemorrhagic cysts. In the following parts we will highlight:
- mature cystic teratomas and endometriomas (Part 2)
- hydrosalpinx and pelvic inclusion cysts (Part 3)
- cystadenoma and ovarian neoplasia (Part 4).
An earlier installment of this series entitled “Hemorrhagic ovarian cysts: one entity with many appearances” (May 2014) also focused on cystic pathology.
Figure 1: Simple cyst
A simple cyst in a 32-year-old patient. Figure 2: Hemorrhagic cyst
Note the lacy/reticular internal echoes and lack of internal blood flow on color Doppler. Figure 3: Cystic teratoma
This cyst exhibits the “dermoid mesh” and hyperechoic lines that correspond with hair. Figure 4: Endometrioma
Note diffuse low-level internal echoes (“ground glass”) and no “ring of fire” on color Doppler. |
Characteristics of simple cysts
A simple cyst typically is round or oval, anechoic, and has smooth, thin walls. It contains no solid component or septation (with rare exceptions), and no internal flow is visible on color Doppler imaging.
Levine and colleagues observed that simple adnexal cysts as large as 10 cm carry a risk of malignancy of less than 1%, regardless of the age of the patient. In its 2010 Consensus Conference Statement,1 the Society of Radiologists in Ultrasound recommended the following management strategies for women with simple cysts:
Reproductive-aged women
- Cyst <3 cm: No action necessary; the cyst is a normal physiologic finding and should be referred to as a follicle.
- 3–5 cm: No follow-up necessary; the cyst is almost certainly benign.
- 5–7 cm: Yearly imaging; the cyst is highly likely to be benign.
- >7 cm: Additional imaging is recommended.
Postmenopausal women
- <1 cm: No follow-up necessary; the cyst is almost certainly benign.
- 1–7 cm: Yearly imaging; the cyst is likely to be benign.
- >7 cm: Additional imaging is recommended.
Characteristics of hemorrhagic cysts
These cysts can be quite variable in appearance. Among their sonographic features:
- reticular (lacy, cobweb, or fishnet) internal echoes, due to fibrin strands
- solid-appearing areas with concave margins
- on color Doppler, there may be circumferential peripheral flow (“ring of fire”) and no internal flow.
In its 2010 Consensus Conference Statement, the Society of Radiologists in Ultrasound recommended the following management strategies1:
Premenopausal women
- ≤5 cm: No follow-up imaging unless the diagnosis is uncertain.
- >5 cm: Short-interval follow-up ultrasound (6–12 weeks).
Recently menopausal women
- Any size: Follow-up ultrasound in 6–12 weeks to ensure resolution.
Later postmenopausal women
- Any size: Consider surgical removal, as the cyst may be neoplastic.
Share your thoughts on this article! Send your Letter to the Editor to [email protected]. Please include your name and the city and state in which you practice.
Reference
1. Levine D, Brown DL, Andreotti RF, et al. Management of asymptomatic ovarian and other adnexal cysts imaged at US: Society of Radiologists in Ultrasound Consensus Conference Statement. Radiology. 2010;256(3):943–954.
Reference
1. Levine D, Brown DL, Andreotti RF, et al. Management of asymptomatic ovarian and other adnexal cysts imaged at US: Society of Radiologists in Ultrasound Consensus Conference Statement. Radiology. 2010;256(3):943–954.
Product Update: InTone, E-Sacs, traxi, Saliva Fertility Monitor
FEMALE URINARY INCONTINENCE DEVICE
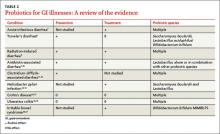
The InTone® system from InControl Medical™ is a noninvasive home-based pelvic-floor rehabilitation program to treat female urinary incontinence. InControl offers two customizable probes. Apex, for women with mild to moderate stress urinary incontinence, delivers electro-stimulation to strengthen pelvic floor muscles and eliminate leakage associated with coughing, laughing, or exercise. ApexM, for women with urgency and mixed urinary incontinence, provides alternating-frequency stimulation to strengthen the pelvic floor muscles and calm detrusor muscle spasms.
The InTone system for moderate to severe incontinence combines muscle stimulation with pelvic-floor exercises. A hand-held control unit allows the patient to listen to prerecorded exercises at home. The control unit also offers visual biofeedback to ensure that the exercises are being completed properly. The patient can present the data from her workouts, stored by the handheld control unit, to her physician, who can then individualize the exercise plan. InTone is indicated for use in 12-minute sessions, six times a week, for 90 days to treat stress urinary incontinence, and for 180 days for urgency or mixed incontinence.
FOR MORE INFORMATION, VISIT www.incontrolmedical.com
TISSUE RETRIEVAL SACS
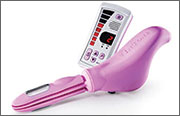
Espiner Medical offers E-Sacs, a variety of tissue retrieval sacs for minimally invasive surgery. E-Sacs are manufactured from Superamine66™ fabric, a lightweight, ripstop nylon coated with polyurethane designed to make them strong, rupture proof, leak resistant, easy to deploy, and x-ray opaque. Standard E-Sacs, made of one-piece construction with an integral tail that can be deployed using 5-mm instruments, do not require claw forceps to extract. Super E-Sacs are ideal for larger specimens and have colored tabs to facilitate placement. Master E-Sacs open automatically, have stronger arms to reinforce the mouth opening, a drawstring closure, and can accept tissue sizes up to a large spleen, ovary, or kidney. EcoSacs, for bottom-first abdominal entry, have a large open mouth to facilitate tissue capture with a drawstring closure.
FOR MORE INFORMATION, VISIT www.espinermedical.com
PANNICULUS RETRACTOR
traxi can be applied and used as a sterile or nonsterile product in conjunction with both external and internal retractors. The manufacturer explains that traxi works by anchoring at the xiphoid and distributing the patient’s weight rather than applying it fully on the sternum, which can make it difficult for the patient to breathe. traxi should not be left on the patient for longer than 24 hours. traxi is labeled with HOLD and PULL HERE tabs, as well as A, B, and C tabs to guide the clinician through the retractor application process.
FOR MORE INFORMATION, VISIT www.clinicalinnovations.com
SALIVA FERTILITY MONITOR
The KNOWHEN® Saliva Fertility Monitor is a handheld mini-microscope that monitors a woman’s ovulation using just a single drop of saliva. A woman’s daily test results can be tracked on an ovulation mobile app provided with the product, allowing her to better plan her sexual activity to achieve fertility goals.
First thing each morning, before eating, drinking, smoking, or brushing teeth, a woman applies a thick drop of saliva to the glass surface and waits for it to dry (5–15 minutes). Then she compares the image she sees through the lens to the chart supplied with the kit and inputs her results on the KNOWHEN app. If she is not ovulating, dots and circles may appear. When she sees a few fernlike crystals, it means she is starting to ovulate, or has just finished ovulating. Consistent fern-like crystals in the viewer indicate that she is ovulating. During ovulation, high estrogen levels raise the percentage of salt in a woman’s body. In saliva, these salts take the shape of ferns under the 60X magnification of the KNOWHEN microscope. The kit includes the app and educational material, including an instructional video.
FOR MORE INFORMATION, VISIT www.knowhen.com
FEMALE URINARY INCONTINENCE DEVICE

The InTone® system from InControl Medical™ is a noninvasive home-based pelvic-floor rehabilitation program to treat female urinary incontinence. InControl offers two customizable probes. Apex, for women with mild to moderate stress urinary incontinence, delivers electro-stimulation to strengthen pelvic floor muscles and eliminate leakage associated with coughing, laughing, or exercise. ApexM, for women with urgency and mixed urinary incontinence, provides alternating-frequency stimulation to strengthen the pelvic floor muscles and calm detrusor muscle spasms.
The InTone system for moderate to severe incontinence combines muscle stimulation with pelvic-floor exercises. A hand-held control unit allows the patient to listen to prerecorded exercises at home. The control unit also offers visual biofeedback to ensure that the exercises are being completed properly. The patient can present the data from her workouts, stored by the handheld control unit, to her physician, who can then individualize the exercise plan. InTone is indicated for use in 12-minute sessions, six times a week, for 90 days to treat stress urinary incontinence, and for 180 days for urgency or mixed incontinence.
FOR MORE INFORMATION, VISIT www.incontrolmedical.com
TISSUE RETRIEVAL SACS

Espiner Medical offers E-Sacs, a variety of tissue retrieval sacs for minimally invasive surgery. E-Sacs are manufactured from Superamine66™ fabric, a lightweight, ripstop nylon coated with polyurethane designed to make them strong, rupture proof, leak resistant, easy to deploy, and x-ray opaque. Standard E-Sacs, made of one-piece construction with an integral tail that can be deployed using 5-mm instruments, do not require claw forceps to extract. Super E-Sacs are ideal for larger specimens and have colored tabs to facilitate placement. Master E-Sacs open automatically, have stronger arms to reinforce the mouth opening, a drawstring closure, and can accept tissue sizes up to a large spleen, ovary, or kidney. EcoSacs, for bottom-first abdominal entry, have a large open mouth to facilitate tissue capture with a drawstring closure.
FOR MORE INFORMATION, VISIT www.espinermedical.com
PANNICULUS RETRACTOR

traxi can be applied and used as a sterile or nonsterile product in conjunction with both external and internal retractors. The manufacturer explains that traxi works by anchoring at the xiphoid and distributing the patient’s weight rather than applying it fully on the sternum, which can make it difficult for the patient to breathe. traxi should not be left on the patient for longer than 24 hours. traxi is labeled with HOLD and PULL HERE tabs, as well as A, B, and C tabs to guide the clinician through the retractor application process.
FOR MORE INFORMATION, VISIT www.clinicalinnovations.com
SALIVA FERTILITY MONITOR

The KNOWHEN® Saliva Fertility Monitor is a handheld mini-microscope that monitors a woman’s ovulation using just a single drop of saliva. A woman’s daily test results can be tracked on an ovulation mobile app provided with the product, allowing her to better plan her sexual activity to achieve fertility goals.
First thing each morning, before eating, drinking, smoking, or brushing teeth, a woman applies a thick drop of saliva to the glass surface and waits for it to dry (5–15 minutes). Then she compares the image she sees through the lens to the chart supplied with the kit and inputs her results on the KNOWHEN app. If she is not ovulating, dots and circles may appear. When she sees a few fernlike crystals, it means she is starting to ovulate, or has just finished ovulating. Consistent fern-like crystals in the viewer indicate that she is ovulating. During ovulation, high estrogen levels raise the percentage of salt in a woman’s body. In saliva, these salts take the shape of ferns under the 60X magnification of the KNOWHEN microscope. The kit includes the app and educational material, including an instructional video.
FOR MORE INFORMATION, VISIT www.knowhen.com
FEMALE URINARY INCONTINENCE DEVICE

The InTone® system from InControl Medical™ is a noninvasive home-based pelvic-floor rehabilitation program to treat female urinary incontinence. InControl offers two customizable probes. Apex, for women with mild to moderate stress urinary incontinence, delivers electro-stimulation to strengthen pelvic floor muscles and eliminate leakage associated with coughing, laughing, or exercise. ApexM, for women with urgency and mixed urinary incontinence, provides alternating-frequency stimulation to strengthen the pelvic floor muscles and calm detrusor muscle spasms.
The InTone system for moderate to severe incontinence combines muscle stimulation with pelvic-floor exercises. A hand-held control unit allows the patient to listen to prerecorded exercises at home. The control unit also offers visual biofeedback to ensure that the exercises are being completed properly. The patient can present the data from her workouts, stored by the handheld control unit, to her physician, who can then individualize the exercise plan. InTone is indicated for use in 12-minute sessions, six times a week, for 90 days to treat stress urinary incontinence, and for 180 days for urgency or mixed incontinence.
FOR MORE INFORMATION, VISIT www.incontrolmedical.com
TISSUE RETRIEVAL SACS

Espiner Medical offers E-Sacs, a variety of tissue retrieval sacs for minimally invasive surgery. E-Sacs are manufactured from Superamine66™ fabric, a lightweight, ripstop nylon coated with polyurethane designed to make them strong, rupture proof, leak resistant, easy to deploy, and x-ray opaque. Standard E-Sacs, made of one-piece construction with an integral tail that can be deployed using 5-mm instruments, do not require claw forceps to extract. Super E-Sacs are ideal for larger specimens and have colored tabs to facilitate placement. Master E-Sacs open automatically, have stronger arms to reinforce the mouth opening, a drawstring closure, and can accept tissue sizes up to a large spleen, ovary, or kidney. EcoSacs, for bottom-first abdominal entry, have a large open mouth to facilitate tissue capture with a drawstring closure.
FOR MORE INFORMATION, VISIT www.espinermedical.com
PANNICULUS RETRACTOR

traxi can be applied and used as a sterile or nonsterile product in conjunction with both external and internal retractors. The manufacturer explains that traxi works by anchoring at the xiphoid and distributing the patient’s weight rather than applying it fully on the sternum, which can make it difficult for the patient to breathe. traxi should not be left on the patient for longer than 24 hours. traxi is labeled with HOLD and PULL HERE tabs, as well as A, B, and C tabs to guide the clinician through the retractor application process.
FOR MORE INFORMATION, VISIT www.clinicalinnovations.com
SALIVA FERTILITY MONITOR

The KNOWHEN® Saliva Fertility Monitor is a handheld mini-microscope that monitors a woman’s ovulation using just a single drop of saliva. A woman’s daily test results can be tracked on an ovulation mobile app provided with the product, allowing her to better plan her sexual activity to achieve fertility goals.
First thing each morning, before eating, drinking, smoking, or brushing teeth, a woman applies a thick drop of saliva to the glass surface and waits for it to dry (5–15 minutes). Then she compares the image she sees through the lens to the chart supplied with the kit and inputs her results on the KNOWHEN app. If she is not ovulating, dots and circles may appear. When she sees a few fernlike crystals, it means she is starting to ovulate, or has just finished ovulating. Consistent fern-like crystals in the viewer indicate that she is ovulating. During ovulation, high estrogen levels raise the percentage of salt in a woman’s body. In saliva, these salts take the shape of ferns under the 60X magnification of the KNOWHEN microscope. The kit includes the app and educational material, including an instructional video.
FOR MORE INFORMATION, VISIT www.knowhen.com
David Henry's JCSO podcast, February 2015
The line-up for David Henry’s monthly podcast for The Journal of Community and Supportive Oncology includes an Original Report in which investigators report complete response rates (no emesis, no rescue medication) APF530, a sustained-release granisetron, during the acute and delayed phases of chemotherapy-induced nausea and vomiting over multiple cycles of the therapy. He also discusses the findings from a study on joint breast and colorectal cancer screenings in medically underserved women, and another in women with self-reported lower limb lymphedema after treatment for gynecological cancers and their use of services. A fourth Original Report examines perceptions about participation in cancer clinical trials in New York state and how increased outreach and a team approach to educating and enrolling patients in trials are important to increase participation and ensure a diverse sample of participants. Finally, a feature article on new therapies looks at the significant challenges – and recent advances – in the treatment of head and neck cancers.
The line-up for David Henry’s monthly podcast for The Journal of Community and Supportive Oncology includes an Original Report in which investigators report complete response rates (no emesis, no rescue medication) APF530, a sustained-release granisetron, during the acute and delayed phases of chemotherapy-induced nausea and vomiting over multiple cycles of the therapy. He also discusses the findings from a study on joint breast and colorectal cancer screenings in medically underserved women, and another in women with self-reported lower limb lymphedema after treatment for gynecological cancers and their use of services. A fourth Original Report examines perceptions about participation in cancer clinical trials in New York state and how increased outreach and a team approach to educating and enrolling patients in trials are important to increase participation and ensure a diverse sample of participants. Finally, a feature article on new therapies looks at the significant challenges – and recent advances – in the treatment of head and neck cancers.
The line-up for David Henry’s monthly podcast for The Journal of Community and Supportive Oncology includes an Original Report in which investigators report complete response rates (no emesis, no rescue medication) APF530, a sustained-release granisetron, during the acute and delayed phases of chemotherapy-induced nausea and vomiting over multiple cycles of the therapy. He also discusses the findings from a study on joint breast and colorectal cancer screenings in medically underserved women, and another in women with self-reported lower limb lymphedema after treatment for gynecological cancers and their use of services. A fourth Original Report examines perceptions about participation in cancer clinical trials in New York state and how increased outreach and a team approach to educating and enrolling patients in trials are important to increase participation and ensure a diverse sample of participants. Finally, a feature article on new therapies looks at the significant challenges – and recent advances – in the treatment of head and neck cancers.
Factor Xa antidote gets orphan designation

Photo by Piotr Bodzek
The US Food and Drug Administration (FDA) has granted orphan designation to andexanet alfa for reversing the anticoagulant effect of factor Xa inhibitors in patients experiencing a serious, uncontrolled bleeding event and those who require urgent or emergent surgery.
At present, there is no approved antidote for these patients.
Andexanet alfa is the only compound being studied as a reversal agent for factor Xa inhibitors that directly and specifically corrects anti-factor Xa activity.
The drug is a modified human factor Xa molecule that acts as a decoy to target and sequester both oral and injectable factor Xa inhibitors in the blood. Once bound, the inhibitors are unable to bind to and inhibit native factor Xa, thus allowing for the restoration of normal hemostatic processes.
Andexanet alfa has the potential to address several clinical scenarios where a factor Xa antidote is needed by allowing for flexible and controlled reversal. This can be short-acting, through the administration of an intravenous (IV) bolus, or longer-acting with the addition of an extended infusion.
The FDA previously granted andexanet alfa breakthrough therapy designation, which is intended to expedite the development and review of a drug candidate intended to treat a serious or life-threatening condition.
“Orphan drug designation for andexanet alfa recognizes its potential to address a significant unmet medical need and to advance the field by helping patients who currently have no treatment options,” said Bill Lis, chief executive officer of Portola Pharmaceuticals, the company developing andexanet alfa.
The FDA’s orphan drug designation program provides orphan status to drugs and biologics that are intended for the treatment, diagnosis, or prevention of rare diseases/disorders that currently affect fewer than 200,000 people in the US.
Orphan designation qualifies a company for certain benefits, including an accelerated approval process, 7 years of market exclusivity following the drug’s approval, tax credits on US clinical trials, eligibility for orphan drug grants, and a waiver of certain administrative fees.
Clinical development of andexanet alfa
Researchers are currently evaluating andexanet alfa in 2 randomized, placebo-controlled, phase 3 trials—ANNEXA-A and ANNEXA-R.
They previously reported promising results from the first part of the ANNEXA-A study, a test of andexanet alfa’s ability to reverse the effects of apixaban in healthy subjects when the antidote was given as a single IV bolus.
Researchers also reported favorable results from the first part of the ANNEXA-R study, in which they evaluated andexanet alfa’s ability to reverse the effects of rivaroxaban in healthy subjects when the antidote was given as a single IV bolus.
The second parts of the ANNEXA-A and ANNEXA-R studies are ongoing. The researchers are evaluating the use of andexanet alfa given as a bolus and a continuous infusion.
ANNEXA-4, a phase 4, single-arm, confirmatory study is also ongoing. Researchers are evaluating the drug in patients receiving apixaban, rivaroxaban, edoxaban, or enoxaparin who present with an acute major bleed. ![]()

Photo by Piotr Bodzek
The US Food and Drug Administration (FDA) has granted orphan designation to andexanet alfa for reversing the anticoagulant effect of factor Xa inhibitors in patients experiencing a serious, uncontrolled bleeding event and those who require urgent or emergent surgery.
At present, there is no approved antidote for these patients.
Andexanet alfa is the only compound being studied as a reversal agent for factor Xa inhibitors that directly and specifically corrects anti-factor Xa activity.
The drug is a modified human factor Xa molecule that acts as a decoy to target and sequester both oral and injectable factor Xa inhibitors in the blood. Once bound, the inhibitors are unable to bind to and inhibit native factor Xa, thus allowing for the restoration of normal hemostatic processes.
Andexanet alfa has the potential to address several clinical scenarios where a factor Xa antidote is needed by allowing for flexible and controlled reversal. This can be short-acting, through the administration of an intravenous (IV) bolus, or longer-acting with the addition of an extended infusion.
The FDA previously granted andexanet alfa breakthrough therapy designation, which is intended to expedite the development and review of a drug candidate intended to treat a serious or life-threatening condition.
“Orphan drug designation for andexanet alfa recognizes its potential to address a significant unmet medical need and to advance the field by helping patients who currently have no treatment options,” said Bill Lis, chief executive officer of Portola Pharmaceuticals, the company developing andexanet alfa.
The FDA’s orphan drug designation program provides orphan status to drugs and biologics that are intended for the treatment, diagnosis, or prevention of rare diseases/disorders that currently affect fewer than 200,000 people in the US.
Orphan designation qualifies a company for certain benefits, including an accelerated approval process, 7 years of market exclusivity following the drug’s approval, tax credits on US clinical trials, eligibility for orphan drug grants, and a waiver of certain administrative fees.
Clinical development of andexanet alfa
Researchers are currently evaluating andexanet alfa in 2 randomized, placebo-controlled, phase 3 trials—ANNEXA-A and ANNEXA-R.
They previously reported promising results from the first part of the ANNEXA-A study, a test of andexanet alfa’s ability to reverse the effects of apixaban in healthy subjects when the antidote was given as a single IV bolus.
Researchers also reported favorable results from the first part of the ANNEXA-R study, in which they evaluated andexanet alfa’s ability to reverse the effects of rivaroxaban in healthy subjects when the antidote was given as a single IV bolus.
The second parts of the ANNEXA-A and ANNEXA-R studies are ongoing. The researchers are evaluating the use of andexanet alfa given as a bolus and a continuous infusion.
ANNEXA-4, a phase 4, single-arm, confirmatory study is also ongoing. Researchers are evaluating the drug in patients receiving apixaban, rivaroxaban, edoxaban, or enoxaparin who present with an acute major bleed. ![]()

Photo by Piotr Bodzek
The US Food and Drug Administration (FDA) has granted orphan designation to andexanet alfa for reversing the anticoagulant effect of factor Xa inhibitors in patients experiencing a serious, uncontrolled bleeding event and those who require urgent or emergent surgery.
At present, there is no approved antidote for these patients.
Andexanet alfa is the only compound being studied as a reversal agent for factor Xa inhibitors that directly and specifically corrects anti-factor Xa activity.
The drug is a modified human factor Xa molecule that acts as a decoy to target and sequester both oral and injectable factor Xa inhibitors in the blood. Once bound, the inhibitors are unable to bind to and inhibit native factor Xa, thus allowing for the restoration of normal hemostatic processes.
Andexanet alfa has the potential to address several clinical scenarios where a factor Xa antidote is needed by allowing for flexible and controlled reversal. This can be short-acting, through the administration of an intravenous (IV) bolus, or longer-acting with the addition of an extended infusion.
The FDA previously granted andexanet alfa breakthrough therapy designation, which is intended to expedite the development and review of a drug candidate intended to treat a serious or life-threatening condition.
“Orphan drug designation for andexanet alfa recognizes its potential to address a significant unmet medical need and to advance the field by helping patients who currently have no treatment options,” said Bill Lis, chief executive officer of Portola Pharmaceuticals, the company developing andexanet alfa.
The FDA’s orphan drug designation program provides orphan status to drugs and biologics that are intended for the treatment, diagnosis, or prevention of rare diseases/disorders that currently affect fewer than 200,000 people in the US.
Orphan designation qualifies a company for certain benefits, including an accelerated approval process, 7 years of market exclusivity following the drug’s approval, tax credits on US clinical trials, eligibility for orphan drug grants, and a waiver of certain administrative fees.
Clinical development of andexanet alfa
Researchers are currently evaluating andexanet alfa in 2 randomized, placebo-controlled, phase 3 trials—ANNEXA-A and ANNEXA-R.
They previously reported promising results from the first part of the ANNEXA-A study, a test of andexanet alfa’s ability to reverse the effects of apixaban in healthy subjects when the antidote was given as a single IV bolus.
Researchers also reported favorable results from the first part of the ANNEXA-R study, in which they evaluated andexanet alfa’s ability to reverse the effects of rivaroxaban in healthy subjects when the antidote was given as a single IV bolus.
The second parts of the ANNEXA-A and ANNEXA-R studies are ongoing. The researchers are evaluating the use of andexanet alfa given as a bolus and a continuous infusion.
ANNEXA-4, a phase 4, single-arm, confirmatory study is also ongoing. Researchers are evaluating the drug in patients receiving apixaban, rivaroxaban, edoxaban, or enoxaparin who present with an acute major bleed. ![]()
Nanotechnology: Why Should We Care?
The orthopedic community is increasingly deluged with advancements in the basic sciences. With each step, we must evaluate the necessity of new information and the relevance of these topics for clinical practice. Since the late 1990s, the promise of nanotechnology to effect significant changes in the medical field has been heralded. However, in this coming decade, we as a profession will see unprecedented advances in the movement of this technology “from the bench to the bedside.” Not unlike many other basic science advancements in our field, nanotechnology is poorly understood among clinicians and residents. As the use of biologics and drug delivery systems expands in orthopedics, nanoparticle-based devices will become more prevalent and have a momentous impact on the way we treat and diagnose orthopedic patients.
A nanoparticle is generally defined as a particle in which at least 1 dimension is between 1 to 100 nanometers and has material properties consistent with quantum mechanics.1 Nanomaterials can be composed of organic and inorganic chemical elements that enable basic chemical processes to create more complex systems. Individual nanoparticle units can be synthesized to form nanostructures, including nanotubes, nanoscaffolds, nanofibers, and even nanodiamonds.2-4 Nanoparticles at this scale display unique optical, chemical, and physical properties that can be manipulated to create specific end-use applications. Such uses may include glass fabrication, optical probes, television screens, drug delivery, gene delivery, and multiplex diagnostic assays.5-7 By crossing disciplines of physics, engineering, and medical sciences, we can create novel technology that includes nanomanufacturing, targeted drug delivery, nanorobotics in conjunction with artificial intelligence, and point-of-care diagnostics.7-9
The field of orthopedics has benefited from nanotechnologic advances, such as new therapeutics and implant-related technology. Nanotubes are hollow nanosized cylinders that are commonly created from titania, silica, or carbon-based substrates. They have garnered significant interest for their high tensile and shear strength, favorable microstructure for bony ingrowth, and their capacity to hold antibiotics or growth factors, such as bone morphogenic proteins (BMPs).10 The current local delivery limitations of BMPs via a collagen sponge have the potential to be maximized and better controlled with a nanotechnology-based approach. The size, internal structure, and shape of the nanoparticle can be manipulated to control the release of these growth factors, and certain nanoparticles can be dual-layered, allowing for release of multiple growth factors at once or in succession.11,12 A more powerful and targeted delivery system of these types of growth factors may result in improved or more robust outcomes, and further research is warranted.
It is possible that carbon-based nanotubes can be categorized as a biomedical implant secondary to their mechanical properties.13 Their strength and ability to be augmented with osteogenic materials has made them an attractive area of research as alternative implant surfaces and stand-alone implants. Nanotubes are capable of acting as a scaffold for antibiotic-loaded, carbon-based nanodiamonds for localized treatment of periprosthetic infection, and research has been directed toward controlled release of the nanodiamond-antibiotic construct from these scaffolds or hydrogels.4,14 Technologies like this may allow the clinician to treat periprosthetic infections locally and minimize the use of systemic antibiotics. The perfection of this type of delivery system may augment the role of antibiotic-laden cement and improve our treatment success rates, even in traditionally hard-to-treat organisms.
Nanoscaffolds and nanofibers are created from nanosized polymers and rendered into a 3-dimensional structure that can be loaded with biologic particles or acting as a scaffold/template for tissue or bone ingrowth. Nanofibers created using biodegradable substrates such as poly(lactic-co-glycolic acid) (PLGA) and chitosan have been extensively studied for their delayed-release properties and biocompatibility.15 These scaffolds are often soaked or loaded with chondrogenic, osteogenic, or antibacterial agents, and have been evaluated in both in vitro and in vivo studies with promising results.15,16 They have been an exciting area of research in tissue engineering, and have been accepted as an adjunct in tendon-repair treatments and local bone regeneration.3,17 As this technology is perfected, the potential to treat more effectively massive rotator cuff tears or tears with poor tissue integrity will dramatically improve and expand the indications for rotator cuff repair.
Augmentation of implant surfaces with nanomaterials that improve osseointegration, or that act as antimicrobial agents have also been a focus of research in hopes of decreasing the rates of aseptic failure and periprosthetic infection in arthroplasty procedures. Nanocrystalline surfaces made of hydroxyapatite and cobalt chromium have been evaluated for their enhanced osteoconductive properties, and may replace standard surfaces.18-20 Recent work evaluating nanoparticle-antibiotic constructs that have been covalently bound to implant surfaces for delayed release of antibiotics during the perioperative period has shown promise, and may allow a more targeted and localized treatment strategy for periprosthetic infection.21,22
Major limitations regarding successful clinical implementation of nanotechnology include both cost and regulatory processes. Currently, pharmaceutical companies estimate that, on average, successful clinical trials from phase 1 to completion for new drugs can cost hundreds of millions of dollars.23 Such high costs result partially from the laborious and capital-intensive process of conducting clinical trials that meet US Food and Drug Administration (FDA) requirements. These regulations would apply to both surface-coated implants and nanoparticle-based drug delivery systems. These types of implants would not be expedited into the market secondary to their drug delivery component and would likely require lengthy clinical studies. Implant companies may be reluctant to invest millions of dollars in multiple FDA trials when they have lucrative implants on the market.
Other limitations include the particles’ complex 3-dimensional structure, which can present challenges for mass production. Producing large quantities of nanoparticles at a consistent quality may be a major limitation to the more unique and target-based nanotherapies. Recent concerns with the toxicity profile of nanotechnology-based medicines have resulted in more intense scrutiny of the nanotechnology safety profile.24,25 Currently, nanoparticle technology is evaluated case by case with each technology requiring its own toxicology and safety profile testing if it is intended for human use. These tests can be cost-prohibitive and require extensive private and government capital for successful market entry. Despite these limitations, nanotechnology will impact the next generation of orthopedic surgeons. Current estimates project the nanomedicine market to be worth $177.6 billion by 2019.26
Advances in nanobased orthopedic technologies have expanded dramatically in the past decade, and we, as the treating physicians, must make educated decisions on how and when to use nanoparticle-based therapies and treatment options. Nanotechnology’s basic science is confusing and often burdensome, but contemporary review articles may be helpful in keeping the orthopedic resident and clinician current with advancements.10,27,28 The more we educate ourselves about evolving nanotechnologies, the less reluctance we will have when evaluating new diagnostic and therapeutic treatment modalities.
1. Hewakuruppu YL, Dombrovsky LA, Chen C, et al. Plasmonic “pump-probe” method to study semi-transparent nanofluids. Appl Opt. 2013;52(24):6041-6050.
2. Balasundaram G, Webster TJ. An overview of nano-polymers for orthopedic applications. Macromol Biosci. 2007;7(5):635-642.
3. Zhang Z, Hu J, Ma PX. Nanofiber-based delivery of bioactive agents and stem cells to bone sites. Adv Drug Deliv Rev. 2012;64(12):1129-1141.
4. Mochalin VN, Shenderova O, Ho D, Gogotsi Y. The properties and applications of nanodiamonds. Nat Nanotechnol. 2012;7(1):11-23.
5. Kneipp J, Kneipp H, Rice WL, Kneipp K. Optical probes for biological applications based on surface-enhanced Raman scattering from indocyanine green on gold nanoparticles. Anal Chem. 2005;77(8):2381-2385.
6. Wang L, O’Donoghue MB, Tan W. Nanoparticles for multiplex diagnostics and imaging. Nanomedicine (Lond). 2006;1(4):413-426.
7. Krebs MD, Salter E, Chen E, Sutter KA, Alsberg E. Calcium phosphate-DNA nanoparticle gene delivery from alginate hydrogels induces in vivo osteogenesis. J Biomed Mater Res A. 2010;92(3):1131-1138.
8. Myers FB, Lee LP. Innovations in optical microfluidic technologies for point-of-care diagnostics. Lab Chip. 2008;8(12):2015-2031.
9. Sacha GM, Varona P. Artificial intelligence in nanotechnology. Nanotechnology. 2013;24(45):452002.
10. Ganguly DY, Shahbazian R, Shokuhfar T. Recent advances in nanotubes for orthopedic implants. J Nanotech Smart Mater. 2014;1:1-10.
11. Srivastava S, Kotov NA. Composite Layer-by-Layer (LBL) assembly with inorganic nanoparticles and nanowires. Acc Chem Res. 2008;41(12):1831-1841.
12. Panda HS, Srivastava R, Bahadur D. Shape and size control of nano dispersed Mg/Al layered double hydroxide. J Nanosci Nanotechnol. 2008;8(8):4218-4223.
13. Wang X, Li Q, Xie J, et al. Fabrication of ultralong and electrically uniform single-walled carbon nanotubes on clean substrates. Nano Lett. 2009;9(9):3137-3141.
14. Zhu Y, Li J, Li W, et al. The biocompatibility of nanodiamonds and their application in drug delivery systems. Theranostics. 2012;2(3):302-312.
15. Wu L, Ding J. In vitro degradation of three-dimensional porous poly(D,L-lactide-co-glycolide) scaffolds for tissue engineering. Biomaterials. 2004;25(2):5821-5830.
16. Wu X, Rabkin-Aikawa E, Guleserian KJ, et al. Tissue-engineered microvessels on three-dimensional biodegradable scaffolds using human endothelial progenitor cells. Am J Physiol Heart Circ Physiol. 2004;287(2):H480-H487.
17. Xia W, Liu W, Cui L, et al. Tissue engineering of cartilage with the use of chitosan-gelatin complex scaffolds. J Biomed Mater Res B Appl Biomater. 2004;71(2):373-380.
18. Laurencin CT, Kumbar SG, Nukavarapu SP. Nanotechnology and orthopedics: a personal perspective. Wiley Interdiscip Rev Nanomed Nanobiotechnol. 2009;1(1):6-10.
19. Webster TJ, Ejiofor JU. Increased osteoblast adhesion on nanophase metals: Ti, Ti6Al4V, and CoCrMo. Biomaterials. 2004;25(19):4731-4739.
20. Webster TJ, Ergun C, Doremus RH, Siegel RW, Bizios R. Enhanced functions of osteoblasts on nanophase ceramics. Biomaterials. 2000;21(17):1803-1810.
21. Stewart S, Barr S, Engiles J, et al. Vancomycin-modified implant surface inhibits biofilm formation and supports bone-healing in an infected osteotomy model in sheep: a proof-of-concept study. J Bone Joint Surg Am. 2012;94(15):1406-1415.
22. Hickok NJ, Shapiro IM. Immobilized antibiotics to prevent orthopaedic implant infections. Adv Drug Deliv Rev. 2012;64(12):1165-1176.
23. DiMasi JA, Hansen RW, Grabowski HG. The price of innovation: new estimates of drug development costs. J Health Econ. 2003;22(2):151-185.
24. Vines T, Faunce T. Assessing the safety and cost-effectiveness of early nanodrugs. J Law Med. 2009;16(5):822-845.
25. Nel A, Xia T, Mädler L, Li N. Toxic potential of materials at the nanolevel. Science. 2006;311(5761):622-627.
26. Nanomedicine Market (Neurology, Cardiovascular, Anti-Inflammatory, Anti-Infective, and Oncology Applications): Global Industry Analysis, Size, Share, Growth, Trends and Forecast, 2013-2019. Transparency Market Research website. http://www.transparencymarketresearch.com/nanomedicine-market.html. Published August 1, 2014. Accessed January 20, 2015.
27. Sullivan MP, McHale KJ, Parvizi J, Mehta S. Nanotechnology: current concepts in orthopaedic surgery and future directions. Bone Joint J. 2014;96-B(5):569-573.
28. Pleshko N, Grande DA, Myers KR. Nanotechnology in orthopaedics. J Am Acad Orthop Surg. 2012;20(1):60-62.
The orthopedic community is increasingly deluged with advancements in the basic sciences. With each step, we must evaluate the necessity of new information and the relevance of these topics for clinical practice. Since the late 1990s, the promise of nanotechnology to effect significant changes in the medical field has been heralded. However, in this coming decade, we as a profession will see unprecedented advances in the movement of this technology “from the bench to the bedside.” Not unlike many other basic science advancements in our field, nanotechnology is poorly understood among clinicians and residents. As the use of biologics and drug delivery systems expands in orthopedics, nanoparticle-based devices will become more prevalent and have a momentous impact on the way we treat and diagnose orthopedic patients.
A nanoparticle is generally defined as a particle in which at least 1 dimension is between 1 to 100 nanometers and has material properties consistent with quantum mechanics.1 Nanomaterials can be composed of organic and inorganic chemical elements that enable basic chemical processes to create more complex systems. Individual nanoparticle units can be synthesized to form nanostructures, including nanotubes, nanoscaffolds, nanofibers, and even nanodiamonds.2-4 Nanoparticles at this scale display unique optical, chemical, and physical properties that can be manipulated to create specific end-use applications. Such uses may include glass fabrication, optical probes, television screens, drug delivery, gene delivery, and multiplex diagnostic assays.5-7 By crossing disciplines of physics, engineering, and medical sciences, we can create novel technology that includes nanomanufacturing, targeted drug delivery, nanorobotics in conjunction with artificial intelligence, and point-of-care diagnostics.7-9
The field of orthopedics has benefited from nanotechnologic advances, such as new therapeutics and implant-related technology. Nanotubes are hollow nanosized cylinders that are commonly created from titania, silica, or carbon-based substrates. They have garnered significant interest for their high tensile and shear strength, favorable microstructure for bony ingrowth, and their capacity to hold antibiotics or growth factors, such as bone morphogenic proteins (BMPs).10 The current local delivery limitations of BMPs via a collagen sponge have the potential to be maximized and better controlled with a nanotechnology-based approach. The size, internal structure, and shape of the nanoparticle can be manipulated to control the release of these growth factors, and certain nanoparticles can be dual-layered, allowing for release of multiple growth factors at once or in succession.11,12 A more powerful and targeted delivery system of these types of growth factors may result in improved or more robust outcomes, and further research is warranted.
It is possible that carbon-based nanotubes can be categorized as a biomedical implant secondary to their mechanical properties.13 Their strength and ability to be augmented with osteogenic materials has made them an attractive area of research as alternative implant surfaces and stand-alone implants. Nanotubes are capable of acting as a scaffold for antibiotic-loaded, carbon-based nanodiamonds for localized treatment of periprosthetic infection, and research has been directed toward controlled release of the nanodiamond-antibiotic construct from these scaffolds or hydrogels.4,14 Technologies like this may allow the clinician to treat periprosthetic infections locally and minimize the use of systemic antibiotics. The perfection of this type of delivery system may augment the role of antibiotic-laden cement and improve our treatment success rates, even in traditionally hard-to-treat organisms.
Nanoscaffolds and nanofibers are created from nanosized polymers and rendered into a 3-dimensional structure that can be loaded with biologic particles or acting as a scaffold/template for tissue or bone ingrowth. Nanofibers created using biodegradable substrates such as poly(lactic-co-glycolic acid) (PLGA) and chitosan have been extensively studied for their delayed-release properties and biocompatibility.15 These scaffolds are often soaked or loaded with chondrogenic, osteogenic, or antibacterial agents, and have been evaluated in both in vitro and in vivo studies with promising results.15,16 They have been an exciting area of research in tissue engineering, and have been accepted as an adjunct in tendon-repair treatments and local bone regeneration.3,17 As this technology is perfected, the potential to treat more effectively massive rotator cuff tears or tears with poor tissue integrity will dramatically improve and expand the indications for rotator cuff repair.
Augmentation of implant surfaces with nanomaterials that improve osseointegration, or that act as antimicrobial agents have also been a focus of research in hopes of decreasing the rates of aseptic failure and periprosthetic infection in arthroplasty procedures. Nanocrystalline surfaces made of hydroxyapatite and cobalt chromium have been evaluated for their enhanced osteoconductive properties, and may replace standard surfaces.18-20 Recent work evaluating nanoparticle-antibiotic constructs that have been covalently bound to implant surfaces for delayed release of antibiotics during the perioperative period has shown promise, and may allow a more targeted and localized treatment strategy for periprosthetic infection.21,22
Major limitations regarding successful clinical implementation of nanotechnology include both cost and regulatory processes. Currently, pharmaceutical companies estimate that, on average, successful clinical trials from phase 1 to completion for new drugs can cost hundreds of millions of dollars.23 Such high costs result partially from the laborious and capital-intensive process of conducting clinical trials that meet US Food and Drug Administration (FDA) requirements. These regulations would apply to both surface-coated implants and nanoparticle-based drug delivery systems. These types of implants would not be expedited into the market secondary to their drug delivery component and would likely require lengthy clinical studies. Implant companies may be reluctant to invest millions of dollars in multiple FDA trials when they have lucrative implants on the market.
Other limitations include the particles’ complex 3-dimensional structure, which can present challenges for mass production. Producing large quantities of nanoparticles at a consistent quality may be a major limitation to the more unique and target-based nanotherapies. Recent concerns with the toxicity profile of nanotechnology-based medicines have resulted in more intense scrutiny of the nanotechnology safety profile.24,25 Currently, nanoparticle technology is evaluated case by case with each technology requiring its own toxicology and safety profile testing if it is intended for human use. These tests can be cost-prohibitive and require extensive private and government capital for successful market entry. Despite these limitations, nanotechnology will impact the next generation of orthopedic surgeons. Current estimates project the nanomedicine market to be worth $177.6 billion by 2019.26
Advances in nanobased orthopedic technologies have expanded dramatically in the past decade, and we, as the treating physicians, must make educated decisions on how and when to use nanoparticle-based therapies and treatment options. Nanotechnology’s basic science is confusing and often burdensome, but contemporary review articles may be helpful in keeping the orthopedic resident and clinician current with advancements.10,27,28 The more we educate ourselves about evolving nanotechnologies, the less reluctance we will have when evaluating new diagnostic and therapeutic treatment modalities.
The orthopedic community is increasingly deluged with advancements in the basic sciences. With each step, we must evaluate the necessity of new information and the relevance of these topics for clinical practice. Since the late 1990s, the promise of nanotechnology to effect significant changes in the medical field has been heralded. However, in this coming decade, we as a profession will see unprecedented advances in the movement of this technology “from the bench to the bedside.” Not unlike many other basic science advancements in our field, nanotechnology is poorly understood among clinicians and residents. As the use of biologics and drug delivery systems expands in orthopedics, nanoparticle-based devices will become more prevalent and have a momentous impact on the way we treat and diagnose orthopedic patients.
A nanoparticle is generally defined as a particle in which at least 1 dimension is between 1 to 100 nanometers and has material properties consistent with quantum mechanics.1 Nanomaterials can be composed of organic and inorganic chemical elements that enable basic chemical processes to create more complex systems. Individual nanoparticle units can be synthesized to form nanostructures, including nanotubes, nanoscaffolds, nanofibers, and even nanodiamonds.2-4 Nanoparticles at this scale display unique optical, chemical, and physical properties that can be manipulated to create specific end-use applications. Such uses may include glass fabrication, optical probes, television screens, drug delivery, gene delivery, and multiplex diagnostic assays.5-7 By crossing disciplines of physics, engineering, and medical sciences, we can create novel technology that includes nanomanufacturing, targeted drug delivery, nanorobotics in conjunction with artificial intelligence, and point-of-care diagnostics.7-9
The field of orthopedics has benefited from nanotechnologic advances, such as new therapeutics and implant-related technology. Nanotubes are hollow nanosized cylinders that are commonly created from titania, silica, or carbon-based substrates. They have garnered significant interest for their high tensile and shear strength, favorable microstructure for bony ingrowth, and their capacity to hold antibiotics or growth factors, such as bone morphogenic proteins (BMPs).10 The current local delivery limitations of BMPs via a collagen sponge have the potential to be maximized and better controlled with a nanotechnology-based approach. The size, internal structure, and shape of the nanoparticle can be manipulated to control the release of these growth factors, and certain nanoparticles can be dual-layered, allowing for release of multiple growth factors at once or in succession.11,12 A more powerful and targeted delivery system of these types of growth factors may result in improved or more robust outcomes, and further research is warranted.
It is possible that carbon-based nanotubes can be categorized as a biomedical implant secondary to their mechanical properties.13 Their strength and ability to be augmented with osteogenic materials has made them an attractive area of research as alternative implant surfaces and stand-alone implants. Nanotubes are capable of acting as a scaffold for antibiotic-loaded, carbon-based nanodiamonds for localized treatment of periprosthetic infection, and research has been directed toward controlled release of the nanodiamond-antibiotic construct from these scaffolds or hydrogels.4,14 Technologies like this may allow the clinician to treat periprosthetic infections locally and minimize the use of systemic antibiotics. The perfection of this type of delivery system may augment the role of antibiotic-laden cement and improve our treatment success rates, even in traditionally hard-to-treat organisms.
Nanoscaffolds and nanofibers are created from nanosized polymers and rendered into a 3-dimensional structure that can be loaded with biologic particles or acting as a scaffold/template for tissue or bone ingrowth. Nanofibers created using biodegradable substrates such as poly(lactic-co-glycolic acid) (PLGA) and chitosan have been extensively studied for their delayed-release properties and biocompatibility.15 These scaffolds are often soaked or loaded with chondrogenic, osteogenic, or antibacterial agents, and have been evaluated in both in vitro and in vivo studies with promising results.15,16 They have been an exciting area of research in tissue engineering, and have been accepted as an adjunct in tendon-repair treatments and local bone regeneration.3,17 As this technology is perfected, the potential to treat more effectively massive rotator cuff tears or tears with poor tissue integrity will dramatically improve and expand the indications for rotator cuff repair.
Augmentation of implant surfaces with nanomaterials that improve osseointegration, or that act as antimicrobial agents have also been a focus of research in hopes of decreasing the rates of aseptic failure and periprosthetic infection in arthroplasty procedures. Nanocrystalline surfaces made of hydroxyapatite and cobalt chromium have been evaluated for their enhanced osteoconductive properties, and may replace standard surfaces.18-20 Recent work evaluating nanoparticle-antibiotic constructs that have been covalently bound to implant surfaces for delayed release of antibiotics during the perioperative period has shown promise, and may allow a more targeted and localized treatment strategy for periprosthetic infection.21,22
Major limitations regarding successful clinical implementation of nanotechnology include both cost and regulatory processes. Currently, pharmaceutical companies estimate that, on average, successful clinical trials from phase 1 to completion for new drugs can cost hundreds of millions of dollars.23 Such high costs result partially from the laborious and capital-intensive process of conducting clinical trials that meet US Food and Drug Administration (FDA) requirements. These regulations would apply to both surface-coated implants and nanoparticle-based drug delivery systems. These types of implants would not be expedited into the market secondary to their drug delivery component and would likely require lengthy clinical studies. Implant companies may be reluctant to invest millions of dollars in multiple FDA trials when they have lucrative implants on the market.
Other limitations include the particles’ complex 3-dimensional structure, which can present challenges for mass production. Producing large quantities of nanoparticles at a consistent quality may be a major limitation to the more unique and target-based nanotherapies. Recent concerns with the toxicity profile of nanotechnology-based medicines have resulted in more intense scrutiny of the nanotechnology safety profile.24,25 Currently, nanoparticle technology is evaluated case by case with each technology requiring its own toxicology and safety profile testing if it is intended for human use. These tests can be cost-prohibitive and require extensive private and government capital for successful market entry. Despite these limitations, nanotechnology will impact the next generation of orthopedic surgeons. Current estimates project the nanomedicine market to be worth $177.6 billion by 2019.26
Advances in nanobased orthopedic technologies have expanded dramatically in the past decade, and we, as the treating physicians, must make educated decisions on how and when to use nanoparticle-based therapies and treatment options. Nanotechnology’s basic science is confusing and often burdensome, but contemporary review articles may be helpful in keeping the orthopedic resident and clinician current with advancements.10,27,28 The more we educate ourselves about evolving nanotechnologies, the less reluctance we will have when evaluating new diagnostic and therapeutic treatment modalities.
1. Hewakuruppu YL, Dombrovsky LA, Chen C, et al. Plasmonic “pump-probe” method to study semi-transparent nanofluids. Appl Opt. 2013;52(24):6041-6050.
2. Balasundaram G, Webster TJ. An overview of nano-polymers for orthopedic applications. Macromol Biosci. 2007;7(5):635-642.
3. Zhang Z, Hu J, Ma PX. Nanofiber-based delivery of bioactive agents and stem cells to bone sites. Adv Drug Deliv Rev. 2012;64(12):1129-1141.
4. Mochalin VN, Shenderova O, Ho D, Gogotsi Y. The properties and applications of nanodiamonds. Nat Nanotechnol. 2012;7(1):11-23.
5. Kneipp J, Kneipp H, Rice WL, Kneipp K. Optical probes for biological applications based on surface-enhanced Raman scattering from indocyanine green on gold nanoparticles. Anal Chem. 2005;77(8):2381-2385.
6. Wang L, O’Donoghue MB, Tan W. Nanoparticles for multiplex diagnostics and imaging. Nanomedicine (Lond). 2006;1(4):413-426.
7. Krebs MD, Salter E, Chen E, Sutter KA, Alsberg E. Calcium phosphate-DNA nanoparticle gene delivery from alginate hydrogels induces in vivo osteogenesis. J Biomed Mater Res A. 2010;92(3):1131-1138.
8. Myers FB, Lee LP. Innovations in optical microfluidic technologies for point-of-care diagnostics. Lab Chip. 2008;8(12):2015-2031.
9. Sacha GM, Varona P. Artificial intelligence in nanotechnology. Nanotechnology. 2013;24(45):452002.
10. Ganguly DY, Shahbazian R, Shokuhfar T. Recent advances in nanotubes for orthopedic implants. J Nanotech Smart Mater. 2014;1:1-10.
11. Srivastava S, Kotov NA. Composite Layer-by-Layer (LBL) assembly with inorganic nanoparticles and nanowires. Acc Chem Res. 2008;41(12):1831-1841.
12. Panda HS, Srivastava R, Bahadur D. Shape and size control of nano dispersed Mg/Al layered double hydroxide. J Nanosci Nanotechnol. 2008;8(8):4218-4223.
13. Wang X, Li Q, Xie J, et al. Fabrication of ultralong and electrically uniform single-walled carbon nanotubes on clean substrates. Nano Lett. 2009;9(9):3137-3141.
14. Zhu Y, Li J, Li W, et al. The biocompatibility of nanodiamonds and their application in drug delivery systems. Theranostics. 2012;2(3):302-312.
15. Wu L, Ding J. In vitro degradation of three-dimensional porous poly(D,L-lactide-co-glycolide) scaffolds for tissue engineering. Biomaterials. 2004;25(2):5821-5830.
16. Wu X, Rabkin-Aikawa E, Guleserian KJ, et al. Tissue-engineered microvessels on three-dimensional biodegradable scaffolds using human endothelial progenitor cells. Am J Physiol Heart Circ Physiol. 2004;287(2):H480-H487.
17. Xia W, Liu W, Cui L, et al. Tissue engineering of cartilage with the use of chitosan-gelatin complex scaffolds. J Biomed Mater Res B Appl Biomater. 2004;71(2):373-380.
18. Laurencin CT, Kumbar SG, Nukavarapu SP. Nanotechnology and orthopedics: a personal perspective. Wiley Interdiscip Rev Nanomed Nanobiotechnol. 2009;1(1):6-10.
19. Webster TJ, Ejiofor JU. Increased osteoblast adhesion on nanophase metals: Ti, Ti6Al4V, and CoCrMo. Biomaterials. 2004;25(19):4731-4739.
20. Webster TJ, Ergun C, Doremus RH, Siegel RW, Bizios R. Enhanced functions of osteoblasts on nanophase ceramics. Biomaterials. 2000;21(17):1803-1810.
21. Stewart S, Barr S, Engiles J, et al. Vancomycin-modified implant surface inhibits biofilm formation and supports bone-healing in an infected osteotomy model in sheep: a proof-of-concept study. J Bone Joint Surg Am. 2012;94(15):1406-1415.
22. Hickok NJ, Shapiro IM. Immobilized antibiotics to prevent orthopaedic implant infections. Adv Drug Deliv Rev. 2012;64(12):1165-1176.
23. DiMasi JA, Hansen RW, Grabowski HG. The price of innovation: new estimates of drug development costs. J Health Econ. 2003;22(2):151-185.
24. Vines T, Faunce T. Assessing the safety and cost-effectiveness of early nanodrugs. J Law Med. 2009;16(5):822-845.
25. Nel A, Xia T, Mädler L, Li N. Toxic potential of materials at the nanolevel. Science. 2006;311(5761):622-627.
26. Nanomedicine Market (Neurology, Cardiovascular, Anti-Inflammatory, Anti-Infective, and Oncology Applications): Global Industry Analysis, Size, Share, Growth, Trends and Forecast, 2013-2019. Transparency Market Research website. http://www.transparencymarketresearch.com/nanomedicine-market.html. Published August 1, 2014. Accessed January 20, 2015.
27. Sullivan MP, McHale KJ, Parvizi J, Mehta S. Nanotechnology: current concepts in orthopaedic surgery and future directions. Bone Joint J. 2014;96-B(5):569-573.
28. Pleshko N, Grande DA, Myers KR. Nanotechnology in orthopaedics. J Am Acad Orthop Surg. 2012;20(1):60-62.
1. Hewakuruppu YL, Dombrovsky LA, Chen C, et al. Plasmonic “pump-probe” method to study semi-transparent nanofluids. Appl Opt. 2013;52(24):6041-6050.
2. Balasundaram G, Webster TJ. An overview of nano-polymers for orthopedic applications. Macromol Biosci. 2007;7(5):635-642.
3. Zhang Z, Hu J, Ma PX. Nanofiber-based delivery of bioactive agents and stem cells to bone sites. Adv Drug Deliv Rev. 2012;64(12):1129-1141.
4. Mochalin VN, Shenderova O, Ho D, Gogotsi Y. The properties and applications of nanodiamonds. Nat Nanotechnol. 2012;7(1):11-23.
5. Kneipp J, Kneipp H, Rice WL, Kneipp K. Optical probes for biological applications based on surface-enhanced Raman scattering from indocyanine green on gold nanoparticles. Anal Chem. 2005;77(8):2381-2385.
6. Wang L, O’Donoghue MB, Tan W. Nanoparticles for multiplex diagnostics and imaging. Nanomedicine (Lond). 2006;1(4):413-426.
7. Krebs MD, Salter E, Chen E, Sutter KA, Alsberg E. Calcium phosphate-DNA nanoparticle gene delivery from alginate hydrogels induces in vivo osteogenesis. J Biomed Mater Res A. 2010;92(3):1131-1138.
8. Myers FB, Lee LP. Innovations in optical microfluidic technologies for point-of-care diagnostics. Lab Chip. 2008;8(12):2015-2031.
9. Sacha GM, Varona P. Artificial intelligence in nanotechnology. Nanotechnology. 2013;24(45):452002.
10. Ganguly DY, Shahbazian R, Shokuhfar T. Recent advances in nanotubes for orthopedic implants. J Nanotech Smart Mater. 2014;1:1-10.
11. Srivastava S, Kotov NA. Composite Layer-by-Layer (LBL) assembly with inorganic nanoparticles and nanowires. Acc Chem Res. 2008;41(12):1831-1841.
12. Panda HS, Srivastava R, Bahadur D. Shape and size control of nano dispersed Mg/Al layered double hydroxide. J Nanosci Nanotechnol. 2008;8(8):4218-4223.
13. Wang X, Li Q, Xie J, et al. Fabrication of ultralong and electrically uniform single-walled carbon nanotubes on clean substrates. Nano Lett. 2009;9(9):3137-3141.
14. Zhu Y, Li J, Li W, et al. The biocompatibility of nanodiamonds and their application in drug delivery systems. Theranostics. 2012;2(3):302-312.
15. Wu L, Ding J. In vitro degradation of three-dimensional porous poly(D,L-lactide-co-glycolide) scaffolds for tissue engineering. Biomaterials. 2004;25(2):5821-5830.
16. Wu X, Rabkin-Aikawa E, Guleserian KJ, et al. Tissue-engineered microvessels on three-dimensional biodegradable scaffolds using human endothelial progenitor cells. Am J Physiol Heart Circ Physiol. 2004;287(2):H480-H487.
17. Xia W, Liu W, Cui L, et al. Tissue engineering of cartilage with the use of chitosan-gelatin complex scaffolds. J Biomed Mater Res B Appl Biomater. 2004;71(2):373-380.
18. Laurencin CT, Kumbar SG, Nukavarapu SP. Nanotechnology and orthopedics: a personal perspective. Wiley Interdiscip Rev Nanomed Nanobiotechnol. 2009;1(1):6-10.
19. Webster TJ, Ejiofor JU. Increased osteoblast adhesion on nanophase metals: Ti, Ti6Al4V, and CoCrMo. Biomaterials. 2004;25(19):4731-4739.
20. Webster TJ, Ergun C, Doremus RH, Siegel RW, Bizios R. Enhanced functions of osteoblasts on nanophase ceramics. Biomaterials. 2000;21(17):1803-1810.
21. Stewart S, Barr S, Engiles J, et al. Vancomycin-modified implant surface inhibits biofilm formation and supports bone-healing in an infected osteotomy model in sheep: a proof-of-concept study. J Bone Joint Surg Am. 2012;94(15):1406-1415.
22. Hickok NJ, Shapiro IM. Immobilized antibiotics to prevent orthopaedic implant infections. Adv Drug Deliv Rev. 2012;64(12):1165-1176.
23. DiMasi JA, Hansen RW, Grabowski HG. The price of innovation: new estimates of drug development costs. J Health Econ. 2003;22(2):151-185.
24. Vines T, Faunce T. Assessing the safety and cost-effectiveness of early nanodrugs. J Law Med. 2009;16(5):822-845.
25. Nel A, Xia T, Mädler L, Li N. Toxic potential of materials at the nanolevel. Science. 2006;311(5761):622-627.
26. Nanomedicine Market (Neurology, Cardiovascular, Anti-Inflammatory, Anti-Infective, and Oncology Applications): Global Industry Analysis, Size, Share, Growth, Trends and Forecast, 2013-2019. Transparency Market Research website. http://www.transparencymarketresearch.com/nanomedicine-market.html. Published August 1, 2014. Accessed January 20, 2015.
27. Sullivan MP, McHale KJ, Parvizi J, Mehta S. Nanotechnology: current concepts in orthopaedic surgery and future directions. Bone Joint J. 2014;96-B(5):569-573.
28. Pleshko N, Grande DA, Myers KR. Nanotechnology in orthopaedics. J Am Acad Orthop Surg. 2012;20(1):60-62.
Intragrade Intramedullary Nailing of an Open Tibial Shaft Fracture in a Patient With Concomitant Ipsilateral Total Knee Arthroplasty
Fracture of the tibial shaft below an ipsilateral total knee arthroplasty (TKA) is an infrequently occurring injury pattern that presents a unique treatment scenario. The high predilection for open wounds associated with these diaphyseal fractures further complicates the treatment algorithm.1,2 The standard principles of treatment for open tibial shaft fractures entail open fracture débridement followed by adequate fracture reduction and stable skeletal fixation in a manner that limits adverse complications of this injury, which include nonunion, malunion, infection, soft-tissue compromise, and reoperation.3,4
Antegrade intramedullary (IM) tibial nailing has become standard treatment for tibial shaft fractures.5-7 This minimally invasive method of fixation limits damage to the soft-tissue envelope, provides superior neutralization of the mechanical forces to provide a template for biologic fracture healing, and allows the best options for revision procedures in the event of inadequate healing. This case report examines treatment options for an open tibial shaft fracture of an ipsilateral TKA, complicating the standard treatment of antegrade tibial nailing. The patient provided written informed consent for print and electronic publication of this case report.
Case Report
A 66-year-old woman became light-headed and fell down a flight of stairs at her home. She was taken to the local emergency room where she presented with left leg pain, deformity, and a skin wound. The wound was dressed with sterile gauze and the extremity immobilized in a temporary plaster splint after which the patient was transferred to our level I trauma center. The accident occurred shortly after dawn, and she received definitive evaluation at the level I trauma center before noon the same day, making the time from injury to evaluation less than 6 hours.
The patient’s medical history was significant for depressive and anxiety disorders, fibromyalgia, hypertension, peripheral vascular disease, and lymphedema. Her surgical history was significant for a remote left TKA and remote open reduction with internal fixation of a left lateral malleolus fracture. She was prescribed antidepressant and anti-anxiolytic medications, narcotic medication, and antihypertensive therapy. She smoked 1 pack of cigarettes per day for approximately 20 years and denied alcohol consumption or illicit drug use. Her body mass index was 37.5, and she ambulated independently in the community.
Upon presentation at our hospital, the patient was hemodynamically stable with no discernable systemic compromise from the extremity injury. An examination of the left lower extremity showed a large longitudinal skin wound over the anteromedial surface of the lower leg measuring roughly 10 cm in length with obvious periosteal stripping and protrusion of the proximal fracture segment. Neurologic motor and sensory function was intact in the lower extremities and pulses were strong. Lower leg compartments were soft. Radiographic imaging confirmed a short oblique fracture of the distal third of the tibial diaphysis. The left TKA was intact with no signs of component loosening or periprosthetic fracture (Figures 1A, 1B).
The patient urgently received broad-spectrum antibiotics with intravenous (IV) cefazolin and IV gentamicin as well as tetanus vaccination. Her fracture was temporarily stabilized in a long-leg splint before she was transported to the operating room. Based upon the characteristics of the patient and the open fracture, we had an extensive discussion with the patient regarding the severity of her injury and treatment options, including nonoperative treatment, operative irrigation and débridement with skeletal stabilization, or below-knee amputation. The patient was adamant that limb salvage be attempted despite adequate understanding that she was exposing herself to risk of multiple reoperations from potential complications, as well as systemic medical compromise. Thus, we considered possible techniques for internal fixation of the tibial shaft fracture and treatment of the open wound.
Two primary technical concerns were addressed in the preoperative planning phase: the first was the need for primary closure of the open wound. This patient had a large wound over the anteromedial surface of the distal third of the tibia with scant soft-tissue coverage. Consequently, skin graft alone would not be adequate. While a muscle flap is another option, it would be prone to failure because of the patient’s age and comorbidities, including hypertension, peripheral vascular disease, lymphedema, and tobacco use. Therefore, we hoped to achieve primary closure. Our second major concern was that the method of fixation must be biomechanically sound without impeding our first goal of primary wound closure. In the setting of an ipsilateral TKA, standard antegrade IM nail fixation would not be possible. While we considered plate fixation, it is biomechanically less stable than an IM nail, and we had great concerns about wound complications. External fixation—uniplanar and mutliplanar (eg, Ilizarov)—was limited by issues of long-term fracture stability and risk of pin-site infection. Both methods appeared less desirable compared with IM nail fixation. Thus, we devised an innovative technique to implant an IM nail into the tibial canal.
The operative procedure first entailed standard open fracture care comprising débridement of nonviable soft tissue from the traumatic anteromedial tibial wound, curettage of the fractured bone ends, and irrigation with pulse-jet lavage. Then, we turned to reduction and internal fixation of the bony injury. The large traumatic wound was not extended and was used as the primary surgical approach to permit introduction of the IM nail into the canal. Through the traumatic wound, we performed limited reaming of the proximal and distal fracture segments. Using a cannulated technique over guide wires, we reamed to 11 mm (Figure 2). The tourniquet was not used during the IM reaming. We determined the maximum nail length (approximately 22 cm) by measuring the distance from the fracture to the bone interface with the tibial component. We used a 10×200-mm femoral retrograde Synthes nail (Synthes, Inc, West Chester, Pennsylvania) for the procedure, although we considered an IM humerus nail. Through the traumatic wound, the nail was advanced in its entirety into the proximal tibial segment (Figure 3). The fracture was reduced anatomically and held with a bone tenaculum (Figures 4A, 4B). A medial cortical window proximal to the proximal extent of the IM nail was created through which the Synthes IM reduction tool (aluminum femoral finger) was advanced to impact the IM nail antegrade through the fracture site into the distal segment (Figure 5). After placement of the nail was complete, the excised fragment of bone was reinserted into the cortical window. The Synthes IM reduction tool was chosen for its diameter, length, and, most important, its relative flexibility. While maintaining reduction of the fracture, cross-locking of the nail was performed at the distal and proximal ends with perfect circle technique through stab incisions. Length, alignment, and rotation of the affected tibia were deemed symmetric to the contralateral side based on preoperative clinical measurements. Final fluoroscopic images showed appropriate alignment and proper implant placement.
Following open reduction and internal fixation of the fracture, the traumatic and surgical wounds were closed in a layered fashion. A subcutaneous drain and an incisional vacuum-assisted closure (VAC) device were applied to the closed traumatic wound, and a second subcutaneous drain was placed at the site of the cortical window. The patient tolerated the procedure well without perioperative complications.
In the acute period after surgery, the patient’s neurologic and vascular status remained stable. Her muscular compartments remained soft and compressible on physical examination, and her pain was well controlled. The incisional VAC and the 2 Hemovac drains were removed within a few days of the operation. Intravenous cefazolin was continued through her hospital stay and she was transitioned to oral cephalexin at discharge as recommended by our infectious disease colleagues to complete a 10-day course of antibiotic therapy.
At the time of discharge—within 1 week of her initial injury—the patient’s wounds were dry and she was ambulatory with a walker. She was instructed to remain non-weight-bearing and to keep her wounds clean and dry with follow-up in 2 weeks. Over 6 to 8 weeks after surgery, the patient’s weight-bearing status was gradually advanced to full weight-bearing, and she achieved union of the fracture and uneventful healing of the traumatic wound (Figures 6A, 6B, 7).
Discussion
We have presented a case of an open distal-third tibial shaft fracture in a 66-year-old obese woman with an ipsilateral TKA. Open fracture of the tibial shaft is potentially limb-threatening because of the challenging management of the bone and soft-tissue injury. The presence of an ipsilateral TKA adds a degree of complexity. From a biomechanical standpoint, the lower interdigitation of cortical bone, coupled with weight-bearing of the lower extremity, subjects the tibia diaphysis to issues of rotation, length, and angular control.8 Due to the diaphyseal nature of the fracture, consisting of cortical bone with comparably lower vascularity and a small soft-tissue envelope, these fractures heal very slowly and often take as many as 6 to 9 months to achieve union.9,10 Furthermore, as was the case here, short oblique fractures of the tibial shaft often occur under bending stresses that also cause significant damage to the tibial soft-tissue envelope and periosteum, as indicated by the open wound. This disruption deprives the fracture and soft tissues of important vascular supply that is critical to healing and to avoiding infection and soft-tissue necrosis.11-13 The effects of treatment may magnify these biomechanical and biologic consequences. Ideal fixation serves to minimize potential complications by neutralizing the biomechanical forces to permit fracture healing while also limiting the amount of soft-tissue trauma and tension. Because the challenges associated with treatment of open tibial shaft fractures make it a limb-threatening injury in a patient with poor peripheral circulation, it is appropriate to consider primary amputation.14
If circumstances warrant an attempt at limb salvage, IM nailing with static interlocking screws would typically be the standard of care for treatment of an open fracture of the tibia shaft. This provides stable internal fixation that controls tibial alignment in 6° of freedom and neutralizes bending forces with less strain on the implant because of the IM position.15,16 In addition to superior neutralization of the biomechanical forces, IM nailing is also a minimally invasive approach that limits further trauma to the periosteum and soft-tissue envelope surrounding the fracture site. This optimizes biologic fracture healing and minimizes complications of malunion, infection, and nonunion.17-19 Moreover, by limiting further damage to the surrounding soft tissue, there is a diminished need for a plastic surgery procedure to reestablish soft-tissue integrity overlying the fracture site. This is particularly advantageous in patients with medical comorbidities that make skin grafts and muscle flaps less likely to succeed. For these reasons, IM nailing was our preferred method of fixation in our patient; however, the presence of an ipsilateral TKA made this standard treatment through an antegrade approach impossible.
Consequently, we considered other methods of fixation, including internal fixation with plate application or external fixation with a multiplanar construct, such as an Ilizarov frame. Some orthopedists consider plate application a superior technique for achieving fracture union because it results in interfragmentary compression, which promotes primary healing. Interestingly, some would argue that the absolute stability provided by the plate may be too rigid a construct to enable optimal fracture healing biology if compression is not achieved.20 However, to allow primary healing to complete fracture union, absolute stability with rigid and strong fixation must be provided. In the tibial shaft, with large bending forces and rotational moments, this is difficult to achieve with plate fixation alone.8 Furthermore, plate application often requires relatively extensive soft-tissue dissection and may impede biologic factors in healing of the bone and soft tissue, increasing the likelihood of infection.21 Finally, adequate plate fixation would significantly increase the soft-tissue volume at this location, further compromising the soft tissues and impeding our goal of primary wound closure.
A uniplanar or mutliplanar external fixator would be an appealing option for definitive fixation because of minimal additional soft-tissue damage that is created during its application. However, it is difficult to achieve adequate stability to encourage either primary, or more commonly, secondary healing in the adult or elderly population.22 An Ilizarov frame is a multiplanar external construct, which allows reconstructive applications because of multiple points of fixation in bone.23 However, the multiple fixation points result in burdensome size of the implant for the patient and requires patient compliance to minimize risk of pin-site infection, which is magnified in a patient with multiple medical comorbid conditions. Furthermore, when comparing treatment options that aim to minimize additional soft-tissue trauma at the site of injury, there is little evidence to show a lower risk of infection at the open fracture site compared with IM nailing.24,25 Thus, in our patient, customary treatment of an open tibial shaft fracture using antegrade IM nailing was not possible, while plate application and external fixation, though potential treatment options, would be relatively contraindicated due to a higher likelihood of failure.
Consequently, primary amputation may be the most appropriate treatment option in a patient with multiple comorbid medical conditions, including peripheral vascular disease. Primary amputation prevents morbidity and mortality associated with complications related to the aforementioned treatment options, as well as limiting risks associated with multiple reoperations.14,25 Studies illustrate that patient functional outcomes after primary amputation are equal to and, in some cases, superior to those patients undergoing limb salvage procedures for open tibial shaft fractures.26-28
Despite the appropriateness of primary amputation in this case, the patient requested limb salvage. Therefore, other innovative treatment options were explored to achieve our goals of primary wound closure and stable internal fixation. Previous case reports have examined retrograde IM nailing as a means of rigidly fixing tibial shaft fractures in the setting of poor soft tissues or ipsilateral knee arthroplasty.29-31 However, the retrograde approach to IM nailing requires passage of reamers through the subtalar and ankle joints, leading to associated arthritis in these joints or, more commonly, rigidity because the final nail position often crosses these joints in addition to the fracture site. Therefore, a novel approach for IM nailing was performed using the large open-fracture wound. Through the traumatic wound, open-fracture débridement was first performed, followed by placement of a nail into the medullary canal with little additional disruption of the surrounding periosteum or soft tissue.
Possible complications of this novel method for IM nail passage warrant discussion. First, potentially unfavorable aspects associated with IM reaming include impairment of endosteal blood circulation in the subacute postoperative period.32-34 If the patient develops complications, such as deep infection, nonunion, hardware failure, or periprosthetic fracture, treatment options that require removal of the nail would be very difficult to execute because this nail was passed “intragrade,” or through the fracture site, not from the knee or the calcaneus. However, unique to this case of intragrade nailing, complications associated with the proximal cortical window may occur. In particular, unintended cortical fracture may happen during impaction of the nail into the distal segment of the fracture after reduction. However, this complication may be avoided with the use of a 1-cm wide and 2-cm long window and the use of the malleable aluminum femoral finger (Synthes). Furthermore, use of a femoral nail is recommended because the Herzog curve of a tibial nail cannot be inserted in the proximal tibial segment using an “intragrade” nailing technique. However, fracture may occur intraoperatively or during rehabilitation after surgery because the cortical window creates a region of high stress distal to the tibial arthroplasty component. Likewise, the area of bone between the proximal extent of the IM nail and tibial component of the TKA represents an area of high stress susceptible to periprosthetic fracture.
Conclusion
We have presented a case of a high-energy open distal tibial diaphyseal fracture in a 66-year-old woman with medical comorbidities and treatment complicated by the presence of an ipsilateral TKA. Intramedullary nailing has become the standard of care for open fractures of the tibial diaphysis because of the high rate of union with little additional soft-tissue damage at the fracture site. Despite these advantages, the ipsilateral TKA complicated the placement of an antegrade tibial nail. An alternative treatment, such as an external fixation using an Ilizarov frame, would present equally challenging treatment aspects, including patient compliance, with little proven benefit over an IM nail. Application of a plate would be less desirable because of increased risk of infection at the fracture site, soft-tissue and periosteum disruption, and muscle necrosis compared with other treatment options. Primary amputation was an appropriate consideration for this patient given her comorbid medical circumstances, but the patient refused this treatment option. Therefore, we created a novel approach to place an IM nail, using the traumatic wound to achieve access to the medullary canal proximally and distally.
1. Patzakis MJ, Wilkins J. Factors influencing infection rate in open fracture wounds. Clin Orthop. 1989;243:36-40.
2. Court-Brown CM, McBirnie J. The epidemiology of tibial fractures. J Bone Joint Surg Br. 1995;77(3):417-421.
3. Puno RM, Teynor JT, Nagano J, Gustilo RB. Critical analysis of results of treatment of 201 tibial shaft fractures. Clin Orthop. 1986;212:113-121.
4. Melvin JS, Dombroski DG, Torbert JT, Kovach SJ, Esterhal JL, Mehta S. Open tibial shaft fractures: I. Evaluation and initial wound management. J Am Acad Orthop Surg. 2010;18(1):10-19.
5. Bhandari M, Guyatt GH, Swiontkowski MF, Schemitsch EH. Treatment of open fractures of the shaft of the tibia. J Bone Joint Surg Br. 2001;83(1):62-68.
6. SPRINT Investigators, Bhandari M, Guyatt G, Tornetta P 3rd, et al. Study to prospectively evaluate reamed intramedually nails in patients with tibial fractures (S.P.R.I.N.T.): study rationale and design. BMC Musculoskelet Disord. 2008;9:91.
7. Study to Prospectively Evaluate Reamed Intramedullary Nails in Patients with Tibial Fractures Investigators, Bhandari M, Guyatt G, Tornetta P 3rd, et al. Randomized trial of reamed and unreamed intramedullary nailing of tibial shaft fractures. J Bone Joint Surg Am. 2008;90(12):2567-2578.
8. Burr DB, Milgrom C, Fyhrie D, et al. In vivo measurement of human tibial strains during vigorous activity. Bone. 1996;18(5):405-410.
9. Edwards P. Fracture of the shaft of the tibia: 492 consecutive cases in adults: Importance of soft tissue injury. Acta Orthop Scand (Suppl). 1965;76(suppl 76):1-82.
10. Papakostidis C, Kanakaris NK, Pretel J, Faour O, Morell DJ, Giannoudis PV. Prevalence of complications of open tibial shaft fractures stratified as per the Gustilo–Anderson classification. Injury. 2011;42(12):1408-1415.
11. Gustilo RB, Mendoza RM, Williams DN. Problems in the management of type III (severe) open fractures: a new classification of type III open fractures. J Trauma. 1984;24(8):742-746.
12. DeLong WG Jr, Born CT, Wei SY, Petrik ME, Ponzio R, Schwab CW. Aggressive treatment of 119 open fracture wounds. J Trauma. 1999;46(6):1049-1054.
13. Tielinen L, Lindahl JE, Tukiainen EJ. Acute unreamed intramedullary nailing and soft tissue reconstruction with muscle flaps for the treatment of severe open tibial shaft fractures. Injury. 2007;38(8):906-912.
14. Georgiadis GM, Behrens FF, Joyce MJ, Earle AS, Simmons AL. Open tibial fractures with severe soft-tissue loss. Limb salvage compared with below-the-knee amputation. J Bone Joint Surg Am. 1993;75(10):1431-1441.
15. Hansen M, Mehler D, Hessmann MH, Blum J, Rommens PM. Intramedullary stabilization of extraarticular proximal tibial fractures: a biomechanical comparison of intramedullary and extramedullary implants including a new proximal tibia nail (PTN). J Orthop Trauma. 2007;21(10):701-709.
16. Hoegel FW, Hoffmann S, Weninger P, Bühren V, Augat P. Biomechanical comparison of locked plate osteosynthesis, reamed and unreamed nailing in conventional interlocking technique, and unreamed angle stable nailing in distal tibia fractures. J Trauma Acute Care Surg. 2012;73(4):933-938.
17. Brumback RJ, Reilly JP, Poka A, Lakatos RP, Bathon GH, Burgess AR. Intramedullary nailing of femoral shaft fractures. Part 1: Decision-making errors with interlocking fixation. J Bone Joint Surg Am. 1988;70(10):1441-1452.
18. Hooper GJ, Keddell RG, Penny ID. Conservative management or closed nailing for tibial shaft fractures. A randomised prospective trial. J Bone Joint Surg Br. 1991;73(1):83-85.
19. Karladani AH, Granhed H, Edshage B, Jerre R, Styf J. Displaced tibial shaft fractures: a prospective randomized study of closed intramedullary nailing versus cast treatment in 53 patients. Acta Orthop Scand. 2000;71(12):160-167.
20. Kenwright J, Richardson JB, Goodship AE, et al. Effect of controlled axial micromovement on healing of tibial fractures. Lancet. 1986;22(8517):1185-1187.
21. Im GI, Tae SK. Distal metaphyseal fractures of tibia: a prospective randomized trial of closed reduction and intramedullary nail versus open reduction and plate and screws fixation. J Trauma. 2005;59(5):1219-1223.
22. Henley MB, Chapman JR, Agel J, Harvey EJ, Whorton AM, Swiontkowski MF. Treatment of type II, IIIA, and IIIB open fractures of the tibial shaft: a prospective comparison of unreamed interlocking intramedullary nails and half-pin external fixators. J Orthop Trauma. 1998;12(1):1-7.
23. Ramos T, Ekholm C, Eriksson BI, Karlsson J, Nistor L. The Ilizarov external fixator - a useful alternative for the treatment of proximal tibial fractures. A prospective observational study of 30 consecutive patients. BMC Musculoskelet Disord. 2013;14:11.
24. Bhandari M, Guyatt GH, Swiontkowski MF, Schemitsch EH. Treatment of open fractures of the shaft of the tibia. J Bone Joint Surg Br. 2001;83(1):62-68.
25. Webb LX, Bosse MJ, Castillo RC, MacKenzie EJ; LEAP Study Group. Analysis of surgeon-controlled variables in the treatment of limb-threatening type-III open tibial diaphyseal fractures. J Bone Joint Surg Am. 2007;89(5):923-928.
26. Bondurant FJ, Cotler HB, Buckle R, Miller-Crotchett P, Browner BD. The medical and economic impact of severely injured lower extremities. J Trauma. 1988;28(8):1270-1273.
27. Bosse MJ, MacKenzie EJ, Kellam JF, et al. An analysis of outcomes of reconstruction or amputation of leg-threatening injuries. N Engl J Med. 2002;347(24):1924-1931.
28. MacKenzie EJ, Bosse MJ, Pollak AN, et al. Long-term persistence of disability following severe lower-limb trauma. Results of a seven-year follow-up. J Bone Joint Surg Am. 2005;87(8):1801-1809.
29. Doulens KM, Joshi AB, Wagner RA. Tibial fracture after total knee arthroplasty treated with retrograde intramedullary fixation. Am J Orthop. 2007;36(7):E111-E113.
30. Zafra-Jiménez JA, Pretell-Mazzini J, Resines-Erasun C. Distal tibial fracture below a total knee arthroplasty: retrograde intramedullary nailing as an alternative method of treatment: a case report. J Orthop Trauma. 2011;25(7):e74-e76.
31. Loosen S, Preuss S, Zelle BA, Pape HC, Tarken IS. Multimorbid patients with poor soft tissue conditions: Treatment of distal tibia fractures with retrograde intramedullary nailing. Unfallchirurg. 2012;116(6):553-558.
32. Kessler SB, Hallfeldt KJ, Perren SM, Schweiberer L. The effects of reaming and intramedullary nailing on fracture healing. Clin Orthop. 1986;212:18-25.
33. Klein MP, Rahn BA, Frigg R, Kessler S, Perren SM. Reaming versus non-reaming in medullary nailing: interference with cortical circulation of the canine tibia. Arch Orthop Trauma Surg. 1990;109(6):314-316.
34. Reichert IL, McCarthy ID, Hughes SP. The acute vascular response to intramedullary reaming. Microsphere estimation of blood flow in the intact ovine tibia. J Bone Joint Surg Br. 1995;77(3):490-493.
Fracture of the tibial shaft below an ipsilateral total knee arthroplasty (TKA) is an infrequently occurring injury pattern that presents a unique treatment scenario. The high predilection for open wounds associated with these diaphyseal fractures further complicates the treatment algorithm.1,2 The standard principles of treatment for open tibial shaft fractures entail open fracture débridement followed by adequate fracture reduction and stable skeletal fixation in a manner that limits adverse complications of this injury, which include nonunion, malunion, infection, soft-tissue compromise, and reoperation.3,4
Antegrade intramedullary (IM) tibial nailing has become standard treatment for tibial shaft fractures.5-7 This minimally invasive method of fixation limits damage to the soft-tissue envelope, provides superior neutralization of the mechanical forces to provide a template for biologic fracture healing, and allows the best options for revision procedures in the event of inadequate healing. This case report examines treatment options for an open tibial shaft fracture of an ipsilateral TKA, complicating the standard treatment of antegrade tibial nailing. The patient provided written informed consent for print and electronic publication of this case report.
Case Report
A 66-year-old woman became light-headed and fell down a flight of stairs at her home. She was taken to the local emergency room where she presented with left leg pain, deformity, and a skin wound. The wound was dressed with sterile gauze and the extremity immobilized in a temporary plaster splint after which the patient was transferred to our level I trauma center. The accident occurred shortly after dawn, and she received definitive evaluation at the level I trauma center before noon the same day, making the time from injury to evaluation less than 6 hours.
The patient’s medical history was significant for depressive and anxiety disorders, fibromyalgia, hypertension, peripheral vascular disease, and lymphedema. Her surgical history was significant for a remote left TKA and remote open reduction with internal fixation of a left lateral malleolus fracture. She was prescribed antidepressant and anti-anxiolytic medications, narcotic medication, and antihypertensive therapy. She smoked 1 pack of cigarettes per day for approximately 20 years and denied alcohol consumption or illicit drug use. Her body mass index was 37.5, and she ambulated independently in the community.
Upon presentation at our hospital, the patient was hemodynamically stable with no discernable systemic compromise from the extremity injury. An examination of the left lower extremity showed a large longitudinal skin wound over the anteromedial surface of the lower leg measuring roughly 10 cm in length with obvious periosteal stripping and protrusion of the proximal fracture segment. Neurologic motor and sensory function was intact in the lower extremities and pulses were strong. Lower leg compartments were soft. Radiographic imaging confirmed a short oblique fracture of the distal third of the tibial diaphysis. The left TKA was intact with no signs of component loosening or periprosthetic fracture (Figures 1A, 1B).
The patient urgently received broad-spectrum antibiotics with intravenous (IV) cefazolin and IV gentamicin as well as tetanus vaccination. Her fracture was temporarily stabilized in a long-leg splint before she was transported to the operating room. Based upon the characteristics of the patient and the open fracture, we had an extensive discussion with the patient regarding the severity of her injury and treatment options, including nonoperative treatment, operative irrigation and débridement with skeletal stabilization, or below-knee amputation. The patient was adamant that limb salvage be attempted despite adequate understanding that she was exposing herself to risk of multiple reoperations from potential complications, as well as systemic medical compromise. Thus, we considered possible techniques for internal fixation of the tibial shaft fracture and treatment of the open wound.
Two primary technical concerns were addressed in the preoperative planning phase: the first was the need for primary closure of the open wound. This patient had a large wound over the anteromedial surface of the distal third of the tibia with scant soft-tissue coverage. Consequently, skin graft alone would not be adequate. While a muscle flap is another option, it would be prone to failure because of the patient’s age and comorbidities, including hypertension, peripheral vascular disease, lymphedema, and tobacco use. Therefore, we hoped to achieve primary closure. Our second major concern was that the method of fixation must be biomechanically sound without impeding our first goal of primary wound closure. In the setting of an ipsilateral TKA, standard antegrade IM nail fixation would not be possible. While we considered plate fixation, it is biomechanically less stable than an IM nail, and we had great concerns about wound complications. External fixation—uniplanar and mutliplanar (eg, Ilizarov)—was limited by issues of long-term fracture stability and risk of pin-site infection. Both methods appeared less desirable compared with IM nail fixation. Thus, we devised an innovative technique to implant an IM nail into the tibial canal.
The operative procedure first entailed standard open fracture care comprising débridement of nonviable soft tissue from the traumatic anteromedial tibial wound, curettage of the fractured bone ends, and irrigation with pulse-jet lavage. Then, we turned to reduction and internal fixation of the bony injury. The large traumatic wound was not extended and was used as the primary surgical approach to permit introduction of the IM nail into the canal. Through the traumatic wound, we performed limited reaming of the proximal and distal fracture segments. Using a cannulated technique over guide wires, we reamed to 11 mm (Figure 2). The tourniquet was not used during the IM reaming. We determined the maximum nail length (approximately 22 cm) by measuring the distance from the fracture to the bone interface with the tibial component. We used a 10×200-mm femoral retrograde Synthes nail (Synthes, Inc, West Chester, Pennsylvania) for the procedure, although we considered an IM humerus nail. Through the traumatic wound, the nail was advanced in its entirety into the proximal tibial segment (Figure 3). The fracture was reduced anatomically and held with a bone tenaculum (Figures 4A, 4B). A medial cortical window proximal to the proximal extent of the IM nail was created through which the Synthes IM reduction tool (aluminum femoral finger) was advanced to impact the IM nail antegrade through the fracture site into the distal segment (Figure 5). After placement of the nail was complete, the excised fragment of bone was reinserted into the cortical window. The Synthes IM reduction tool was chosen for its diameter, length, and, most important, its relative flexibility. While maintaining reduction of the fracture, cross-locking of the nail was performed at the distal and proximal ends with perfect circle technique through stab incisions. Length, alignment, and rotation of the affected tibia were deemed symmetric to the contralateral side based on preoperative clinical measurements. Final fluoroscopic images showed appropriate alignment and proper implant placement.
Following open reduction and internal fixation of the fracture, the traumatic and surgical wounds were closed in a layered fashion. A subcutaneous drain and an incisional vacuum-assisted closure (VAC) device were applied to the closed traumatic wound, and a second subcutaneous drain was placed at the site of the cortical window. The patient tolerated the procedure well without perioperative complications.
In the acute period after surgery, the patient’s neurologic and vascular status remained stable. Her muscular compartments remained soft and compressible on physical examination, and her pain was well controlled. The incisional VAC and the 2 Hemovac drains were removed within a few days of the operation. Intravenous cefazolin was continued through her hospital stay and she was transitioned to oral cephalexin at discharge as recommended by our infectious disease colleagues to complete a 10-day course of antibiotic therapy.
At the time of discharge—within 1 week of her initial injury—the patient’s wounds were dry and she was ambulatory with a walker. She was instructed to remain non-weight-bearing and to keep her wounds clean and dry with follow-up in 2 weeks. Over 6 to 8 weeks after surgery, the patient’s weight-bearing status was gradually advanced to full weight-bearing, and she achieved union of the fracture and uneventful healing of the traumatic wound (Figures 6A, 6B, 7).
Discussion
We have presented a case of an open distal-third tibial shaft fracture in a 66-year-old obese woman with an ipsilateral TKA. Open fracture of the tibial shaft is potentially limb-threatening because of the challenging management of the bone and soft-tissue injury. The presence of an ipsilateral TKA adds a degree of complexity. From a biomechanical standpoint, the lower interdigitation of cortical bone, coupled with weight-bearing of the lower extremity, subjects the tibia diaphysis to issues of rotation, length, and angular control.8 Due to the diaphyseal nature of the fracture, consisting of cortical bone with comparably lower vascularity and a small soft-tissue envelope, these fractures heal very slowly and often take as many as 6 to 9 months to achieve union.9,10 Furthermore, as was the case here, short oblique fractures of the tibial shaft often occur under bending stresses that also cause significant damage to the tibial soft-tissue envelope and periosteum, as indicated by the open wound. This disruption deprives the fracture and soft tissues of important vascular supply that is critical to healing and to avoiding infection and soft-tissue necrosis.11-13 The effects of treatment may magnify these biomechanical and biologic consequences. Ideal fixation serves to minimize potential complications by neutralizing the biomechanical forces to permit fracture healing while also limiting the amount of soft-tissue trauma and tension. Because the challenges associated with treatment of open tibial shaft fractures make it a limb-threatening injury in a patient with poor peripheral circulation, it is appropriate to consider primary amputation.14
If circumstances warrant an attempt at limb salvage, IM nailing with static interlocking screws would typically be the standard of care for treatment of an open fracture of the tibia shaft. This provides stable internal fixation that controls tibial alignment in 6° of freedom and neutralizes bending forces with less strain on the implant because of the IM position.15,16 In addition to superior neutralization of the biomechanical forces, IM nailing is also a minimally invasive approach that limits further trauma to the periosteum and soft-tissue envelope surrounding the fracture site. This optimizes biologic fracture healing and minimizes complications of malunion, infection, and nonunion.17-19 Moreover, by limiting further damage to the surrounding soft tissue, there is a diminished need for a plastic surgery procedure to reestablish soft-tissue integrity overlying the fracture site. This is particularly advantageous in patients with medical comorbidities that make skin grafts and muscle flaps less likely to succeed. For these reasons, IM nailing was our preferred method of fixation in our patient; however, the presence of an ipsilateral TKA made this standard treatment through an antegrade approach impossible.
Consequently, we considered other methods of fixation, including internal fixation with plate application or external fixation with a multiplanar construct, such as an Ilizarov frame. Some orthopedists consider plate application a superior technique for achieving fracture union because it results in interfragmentary compression, which promotes primary healing. Interestingly, some would argue that the absolute stability provided by the plate may be too rigid a construct to enable optimal fracture healing biology if compression is not achieved.20 However, to allow primary healing to complete fracture union, absolute stability with rigid and strong fixation must be provided. In the tibial shaft, with large bending forces and rotational moments, this is difficult to achieve with plate fixation alone.8 Furthermore, plate application often requires relatively extensive soft-tissue dissection and may impede biologic factors in healing of the bone and soft tissue, increasing the likelihood of infection.21 Finally, adequate plate fixation would significantly increase the soft-tissue volume at this location, further compromising the soft tissues and impeding our goal of primary wound closure.
A uniplanar or mutliplanar external fixator would be an appealing option for definitive fixation because of minimal additional soft-tissue damage that is created during its application. However, it is difficult to achieve adequate stability to encourage either primary, or more commonly, secondary healing in the adult or elderly population.22 An Ilizarov frame is a multiplanar external construct, which allows reconstructive applications because of multiple points of fixation in bone.23 However, the multiple fixation points result in burdensome size of the implant for the patient and requires patient compliance to minimize risk of pin-site infection, which is magnified in a patient with multiple medical comorbid conditions. Furthermore, when comparing treatment options that aim to minimize additional soft-tissue trauma at the site of injury, there is little evidence to show a lower risk of infection at the open fracture site compared with IM nailing.24,25 Thus, in our patient, customary treatment of an open tibial shaft fracture using antegrade IM nailing was not possible, while plate application and external fixation, though potential treatment options, would be relatively contraindicated due to a higher likelihood of failure.
Consequently, primary amputation may be the most appropriate treatment option in a patient with multiple comorbid medical conditions, including peripheral vascular disease. Primary amputation prevents morbidity and mortality associated with complications related to the aforementioned treatment options, as well as limiting risks associated with multiple reoperations.14,25 Studies illustrate that patient functional outcomes after primary amputation are equal to and, in some cases, superior to those patients undergoing limb salvage procedures for open tibial shaft fractures.26-28
Despite the appropriateness of primary amputation in this case, the patient requested limb salvage. Therefore, other innovative treatment options were explored to achieve our goals of primary wound closure and stable internal fixation. Previous case reports have examined retrograde IM nailing as a means of rigidly fixing tibial shaft fractures in the setting of poor soft tissues or ipsilateral knee arthroplasty.29-31 However, the retrograde approach to IM nailing requires passage of reamers through the subtalar and ankle joints, leading to associated arthritis in these joints or, more commonly, rigidity because the final nail position often crosses these joints in addition to the fracture site. Therefore, a novel approach for IM nailing was performed using the large open-fracture wound. Through the traumatic wound, open-fracture débridement was first performed, followed by placement of a nail into the medullary canal with little additional disruption of the surrounding periosteum or soft tissue.
Possible complications of this novel method for IM nail passage warrant discussion. First, potentially unfavorable aspects associated with IM reaming include impairment of endosteal blood circulation in the subacute postoperative period.32-34 If the patient develops complications, such as deep infection, nonunion, hardware failure, or periprosthetic fracture, treatment options that require removal of the nail would be very difficult to execute because this nail was passed “intragrade,” or through the fracture site, not from the knee or the calcaneus. However, unique to this case of intragrade nailing, complications associated with the proximal cortical window may occur. In particular, unintended cortical fracture may happen during impaction of the nail into the distal segment of the fracture after reduction. However, this complication may be avoided with the use of a 1-cm wide and 2-cm long window and the use of the malleable aluminum femoral finger (Synthes). Furthermore, use of a femoral nail is recommended because the Herzog curve of a tibial nail cannot be inserted in the proximal tibial segment using an “intragrade” nailing technique. However, fracture may occur intraoperatively or during rehabilitation after surgery because the cortical window creates a region of high stress distal to the tibial arthroplasty component. Likewise, the area of bone between the proximal extent of the IM nail and tibial component of the TKA represents an area of high stress susceptible to periprosthetic fracture.
Conclusion
We have presented a case of a high-energy open distal tibial diaphyseal fracture in a 66-year-old woman with medical comorbidities and treatment complicated by the presence of an ipsilateral TKA. Intramedullary nailing has become the standard of care for open fractures of the tibial diaphysis because of the high rate of union with little additional soft-tissue damage at the fracture site. Despite these advantages, the ipsilateral TKA complicated the placement of an antegrade tibial nail. An alternative treatment, such as an external fixation using an Ilizarov frame, would present equally challenging treatment aspects, including patient compliance, with little proven benefit over an IM nail. Application of a plate would be less desirable because of increased risk of infection at the fracture site, soft-tissue and periosteum disruption, and muscle necrosis compared with other treatment options. Primary amputation was an appropriate consideration for this patient given her comorbid medical circumstances, but the patient refused this treatment option. Therefore, we created a novel approach to place an IM nail, using the traumatic wound to achieve access to the medullary canal proximally and distally.
Fracture of the tibial shaft below an ipsilateral total knee arthroplasty (TKA) is an infrequently occurring injury pattern that presents a unique treatment scenario. The high predilection for open wounds associated with these diaphyseal fractures further complicates the treatment algorithm.1,2 The standard principles of treatment for open tibial shaft fractures entail open fracture débridement followed by adequate fracture reduction and stable skeletal fixation in a manner that limits adverse complications of this injury, which include nonunion, malunion, infection, soft-tissue compromise, and reoperation.3,4
Antegrade intramedullary (IM) tibial nailing has become standard treatment for tibial shaft fractures.5-7 This minimally invasive method of fixation limits damage to the soft-tissue envelope, provides superior neutralization of the mechanical forces to provide a template for biologic fracture healing, and allows the best options for revision procedures in the event of inadequate healing. This case report examines treatment options for an open tibial shaft fracture of an ipsilateral TKA, complicating the standard treatment of antegrade tibial nailing. The patient provided written informed consent for print and electronic publication of this case report.
Case Report
A 66-year-old woman became light-headed and fell down a flight of stairs at her home. She was taken to the local emergency room where she presented with left leg pain, deformity, and a skin wound. The wound was dressed with sterile gauze and the extremity immobilized in a temporary plaster splint after which the patient was transferred to our level I trauma center. The accident occurred shortly after dawn, and she received definitive evaluation at the level I trauma center before noon the same day, making the time from injury to evaluation less than 6 hours.
The patient’s medical history was significant for depressive and anxiety disorders, fibromyalgia, hypertension, peripheral vascular disease, and lymphedema. Her surgical history was significant for a remote left TKA and remote open reduction with internal fixation of a left lateral malleolus fracture. She was prescribed antidepressant and anti-anxiolytic medications, narcotic medication, and antihypertensive therapy. She smoked 1 pack of cigarettes per day for approximately 20 years and denied alcohol consumption or illicit drug use. Her body mass index was 37.5, and she ambulated independently in the community.
Upon presentation at our hospital, the patient was hemodynamically stable with no discernable systemic compromise from the extremity injury. An examination of the left lower extremity showed a large longitudinal skin wound over the anteromedial surface of the lower leg measuring roughly 10 cm in length with obvious periosteal stripping and protrusion of the proximal fracture segment. Neurologic motor and sensory function was intact in the lower extremities and pulses were strong. Lower leg compartments were soft. Radiographic imaging confirmed a short oblique fracture of the distal third of the tibial diaphysis. The left TKA was intact with no signs of component loosening or periprosthetic fracture (Figures 1A, 1B).
The patient urgently received broad-spectrum antibiotics with intravenous (IV) cefazolin and IV gentamicin as well as tetanus vaccination. Her fracture was temporarily stabilized in a long-leg splint before she was transported to the operating room. Based upon the characteristics of the patient and the open fracture, we had an extensive discussion with the patient regarding the severity of her injury and treatment options, including nonoperative treatment, operative irrigation and débridement with skeletal stabilization, or below-knee amputation. The patient was adamant that limb salvage be attempted despite adequate understanding that she was exposing herself to risk of multiple reoperations from potential complications, as well as systemic medical compromise. Thus, we considered possible techniques for internal fixation of the tibial shaft fracture and treatment of the open wound.
Two primary technical concerns were addressed in the preoperative planning phase: the first was the need for primary closure of the open wound. This patient had a large wound over the anteromedial surface of the distal third of the tibia with scant soft-tissue coverage. Consequently, skin graft alone would not be adequate. While a muscle flap is another option, it would be prone to failure because of the patient’s age and comorbidities, including hypertension, peripheral vascular disease, lymphedema, and tobacco use. Therefore, we hoped to achieve primary closure. Our second major concern was that the method of fixation must be biomechanically sound without impeding our first goal of primary wound closure. In the setting of an ipsilateral TKA, standard antegrade IM nail fixation would not be possible. While we considered plate fixation, it is biomechanically less stable than an IM nail, and we had great concerns about wound complications. External fixation—uniplanar and mutliplanar (eg, Ilizarov)—was limited by issues of long-term fracture stability and risk of pin-site infection. Both methods appeared less desirable compared with IM nail fixation. Thus, we devised an innovative technique to implant an IM nail into the tibial canal.
The operative procedure first entailed standard open fracture care comprising débridement of nonviable soft tissue from the traumatic anteromedial tibial wound, curettage of the fractured bone ends, and irrigation with pulse-jet lavage. Then, we turned to reduction and internal fixation of the bony injury. The large traumatic wound was not extended and was used as the primary surgical approach to permit introduction of the IM nail into the canal. Through the traumatic wound, we performed limited reaming of the proximal and distal fracture segments. Using a cannulated technique over guide wires, we reamed to 11 mm (Figure 2). The tourniquet was not used during the IM reaming. We determined the maximum nail length (approximately 22 cm) by measuring the distance from the fracture to the bone interface with the tibial component. We used a 10×200-mm femoral retrograde Synthes nail (Synthes, Inc, West Chester, Pennsylvania) for the procedure, although we considered an IM humerus nail. Through the traumatic wound, the nail was advanced in its entirety into the proximal tibial segment (Figure 3). The fracture was reduced anatomically and held with a bone tenaculum (Figures 4A, 4B). A medial cortical window proximal to the proximal extent of the IM nail was created through which the Synthes IM reduction tool (aluminum femoral finger) was advanced to impact the IM nail antegrade through the fracture site into the distal segment (Figure 5). After placement of the nail was complete, the excised fragment of bone was reinserted into the cortical window. The Synthes IM reduction tool was chosen for its diameter, length, and, most important, its relative flexibility. While maintaining reduction of the fracture, cross-locking of the nail was performed at the distal and proximal ends with perfect circle technique through stab incisions. Length, alignment, and rotation of the affected tibia were deemed symmetric to the contralateral side based on preoperative clinical measurements. Final fluoroscopic images showed appropriate alignment and proper implant placement.
Following open reduction and internal fixation of the fracture, the traumatic and surgical wounds were closed in a layered fashion. A subcutaneous drain and an incisional vacuum-assisted closure (VAC) device were applied to the closed traumatic wound, and a second subcutaneous drain was placed at the site of the cortical window. The patient tolerated the procedure well without perioperative complications.
In the acute period after surgery, the patient’s neurologic and vascular status remained stable. Her muscular compartments remained soft and compressible on physical examination, and her pain was well controlled. The incisional VAC and the 2 Hemovac drains were removed within a few days of the operation. Intravenous cefazolin was continued through her hospital stay and she was transitioned to oral cephalexin at discharge as recommended by our infectious disease colleagues to complete a 10-day course of antibiotic therapy.
At the time of discharge—within 1 week of her initial injury—the patient’s wounds were dry and she was ambulatory with a walker. She was instructed to remain non-weight-bearing and to keep her wounds clean and dry with follow-up in 2 weeks. Over 6 to 8 weeks after surgery, the patient’s weight-bearing status was gradually advanced to full weight-bearing, and she achieved union of the fracture and uneventful healing of the traumatic wound (Figures 6A, 6B, 7).
Discussion
We have presented a case of an open distal-third tibial shaft fracture in a 66-year-old obese woman with an ipsilateral TKA. Open fracture of the tibial shaft is potentially limb-threatening because of the challenging management of the bone and soft-tissue injury. The presence of an ipsilateral TKA adds a degree of complexity. From a biomechanical standpoint, the lower interdigitation of cortical bone, coupled with weight-bearing of the lower extremity, subjects the tibia diaphysis to issues of rotation, length, and angular control.8 Due to the diaphyseal nature of the fracture, consisting of cortical bone with comparably lower vascularity and a small soft-tissue envelope, these fractures heal very slowly and often take as many as 6 to 9 months to achieve union.9,10 Furthermore, as was the case here, short oblique fractures of the tibial shaft often occur under bending stresses that also cause significant damage to the tibial soft-tissue envelope and periosteum, as indicated by the open wound. This disruption deprives the fracture and soft tissues of important vascular supply that is critical to healing and to avoiding infection and soft-tissue necrosis.11-13 The effects of treatment may magnify these biomechanical and biologic consequences. Ideal fixation serves to minimize potential complications by neutralizing the biomechanical forces to permit fracture healing while also limiting the amount of soft-tissue trauma and tension. Because the challenges associated with treatment of open tibial shaft fractures make it a limb-threatening injury in a patient with poor peripheral circulation, it is appropriate to consider primary amputation.14
If circumstances warrant an attempt at limb salvage, IM nailing with static interlocking screws would typically be the standard of care for treatment of an open fracture of the tibia shaft. This provides stable internal fixation that controls tibial alignment in 6° of freedom and neutralizes bending forces with less strain on the implant because of the IM position.15,16 In addition to superior neutralization of the biomechanical forces, IM nailing is also a minimally invasive approach that limits further trauma to the periosteum and soft-tissue envelope surrounding the fracture site. This optimizes biologic fracture healing and minimizes complications of malunion, infection, and nonunion.17-19 Moreover, by limiting further damage to the surrounding soft tissue, there is a diminished need for a plastic surgery procedure to reestablish soft-tissue integrity overlying the fracture site. This is particularly advantageous in patients with medical comorbidities that make skin grafts and muscle flaps less likely to succeed. For these reasons, IM nailing was our preferred method of fixation in our patient; however, the presence of an ipsilateral TKA made this standard treatment through an antegrade approach impossible.
Consequently, we considered other methods of fixation, including internal fixation with plate application or external fixation with a multiplanar construct, such as an Ilizarov frame. Some orthopedists consider plate application a superior technique for achieving fracture union because it results in interfragmentary compression, which promotes primary healing. Interestingly, some would argue that the absolute stability provided by the plate may be too rigid a construct to enable optimal fracture healing biology if compression is not achieved.20 However, to allow primary healing to complete fracture union, absolute stability with rigid and strong fixation must be provided. In the tibial shaft, with large bending forces and rotational moments, this is difficult to achieve with plate fixation alone.8 Furthermore, plate application often requires relatively extensive soft-tissue dissection and may impede biologic factors in healing of the bone and soft tissue, increasing the likelihood of infection.21 Finally, adequate plate fixation would significantly increase the soft-tissue volume at this location, further compromising the soft tissues and impeding our goal of primary wound closure.
A uniplanar or mutliplanar external fixator would be an appealing option for definitive fixation because of minimal additional soft-tissue damage that is created during its application. However, it is difficult to achieve adequate stability to encourage either primary, or more commonly, secondary healing in the adult or elderly population.22 An Ilizarov frame is a multiplanar external construct, which allows reconstructive applications because of multiple points of fixation in bone.23 However, the multiple fixation points result in burdensome size of the implant for the patient and requires patient compliance to minimize risk of pin-site infection, which is magnified in a patient with multiple medical comorbid conditions. Furthermore, when comparing treatment options that aim to minimize additional soft-tissue trauma at the site of injury, there is little evidence to show a lower risk of infection at the open fracture site compared with IM nailing.24,25 Thus, in our patient, customary treatment of an open tibial shaft fracture using antegrade IM nailing was not possible, while plate application and external fixation, though potential treatment options, would be relatively contraindicated due to a higher likelihood of failure.
Consequently, primary amputation may be the most appropriate treatment option in a patient with multiple comorbid medical conditions, including peripheral vascular disease. Primary amputation prevents morbidity and mortality associated with complications related to the aforementioned treatment options, as well as limiting risks associated with multiple reoperations.14,25 Studies illustrate that patient functional outcomes after primary amputation are equal to and, in some cases, superior to those patients undergoing limb salvage procedures for open tibial shaft fractures.26-28
Despite the appropriateness of primary amputation in this case, the patient requested limb salvage. Therefore, other innovative treatment options were explored to achieve our goals of primary wound closure and stable internal fixation. Previous case reports have examined retrograde IM nailing as a means of rigidly fixing tibial shaft fractures in the setting of poor soft tissues or ipsilateral knee arthroplasty.29-31 However, the retrograde approach to IM nailing requires passage of reamers through the subtalar and ankle joints, leading to associated arthritis in these joints or, more commonly, rigidity because the final nail position often crosses these joints in addition to the fracture site. Therefore, a novel approach for IM nailing was performed using the large open-fracture wound. Through the traumatic wound, open-fracture débridement was first performed, followed by placement of a nail into the medullary canal with little additional disruption of the surrounding periosteum or soft tissue.
Possible complications of this novel method for IM nail passage warrant discussion. First, potentially unfavorable aspects associated with IM reaming include impairment of endosteal blood circulation in the subacute postoperative period.32-34 If the patient develops complications, such as deep infection, nonunion, hardware failure, or periprosthetic fracture, treatment options that require removal of the nail would be very difficult to execute because this nail was passed “intragrade,” or through the fracture site, not from the knee or the calcaneus. However, unique to this case of intragrade nailing, complications associated with the proximal cortical window may occur. In particular, unintended cortical fracture may happen during impaction of the nail into the distal segment of the fracture after reduction. However, this complication may be avoided with the use of a 1-cm wide and 2-cm long window and the use of the malleable aluminum femoral finger (Synthes). Furthermore, use of a femoral nail is recommended because the Herzog curve of a tibial nail cannot be inserted in the proximal tibial segment using an “intragrade” nailing technique. However, fracture may occur intraoperatively or during rehabilitation after surgery because the cortical window creates a region of high stress distal to the tibial arthroplasty component. Likewise, the area of bone between the proximal extent of the IM nail and tibial component of the TKA represents an area of high stress susceptible to periprosthetic fracture.
Conclusion
We have presented a case of a high-energy open distal tibial diaphyseal fracture in a 66-year-old woman with medical comorbidities and treatment complicated by the presence of an ipsilateral TKA. Intramedullary nailing has become the standard of care for open fractures of the tibial diaphysis because of the high rate of union with little additional soft-tissue damage at the fracture site. Despite these advantages, the ipsilateral TKA complicated the placement of an antegrade tibial nail. An alternative treatment, such as an external fixation using an Ilizarov frame, would present equally challenging treatment aspects, including patient compliance, with little proven benefit over an IM nail. Application of a plate would be less desirable because of increased risk of infection at the fracture site, soft-tissue and periosteum disruption, and muscle necrosis compared with other treatment options. Primary amputation was an appropriate consideration for this patient given her comorbid medical circumstances, but the patient refused this treatment option. Therefore, we created a novel approach to place an IM nail, using the traumatic wound to achieve access to the medullary canal proximally and distally.
1. Patzakis MJ, Wilkins J. Factors influencing infection rate in open fracture wounds. Clin Orthop. 1989;243:36-40.
2. Court-Brown CM, McBirnie J. The epidemiology of tibial fractures. J Bone Joint Surg Br. 1995;77(3):417-421.
3. Puno RM, Teynor JT, Nagano J, Gustilo RB. Critical analysis of results of treatment of 201 tibial shaft fractures. Clin Orthop. 1986;212:113-121.
4. Melvin JS, Dombroski DG, Torbert JT, Kovach SJ, Esterhal JL, Mehta S. Open tibial shaft fractures: I. Evaluation and initial wound management. J Am Acad Orthop Surg. 2010;18(1):10-19.
5. Bhandari M, Guyatt GH, Swiontkowski MF, Schemitsch EH. Treatment of open fractures of the shaft of the tibia. J Bone Joint Surg Br. 2001;83(1):62-68.
6. SPRINT Investigators, Bhandari M, Guyatt G, Tornetta P 3rd, et al. Study to prospectively evaluate reamed intramedually nails in patients with tibial fractures (S.P.R.I.N.T.): study rationale and design. BMC Musculoskelet Disord. 2008;9:91.
7. Study to Prospectively Evaluate Reamed Intramedullary Nails in Patients with Tibial Fractures Investigators, Bhandari M, Guyatt G, Tornetta P 3rd, et al. Randomized trial of reamed and unreamed intramedullary nailing of tibial shaft fractures. J Bone Joint Surg Am. 2008;90(12):2567-2578.
8. Burr DB, Milgrom C, Fyhrie D, et al. In vivo measurement of human tibial strains during vigorous activity. Bone. 1996;18(5):405-410.
9. Edwards P. Fracture of the shaft of the tibia: 492 consecutive cases in adults: Importance of soft tissue injury. Acta Orthop Scand (Suppl). 1965;76(suppl 76):1-82.
10. Papakostidis C, Kanakaris NK, Pretel J, Faour O, Morell DJ, Giannoudis PV. Prevalence of complications of open tibial shaft fractures stratified as per the Gustilo–Anderson classification. Injury. 2011;42(12):1408-1415.
11. Gustilo RB, Mendoza RM, Williams DN. Problems in the management of type III (severe) open fractures: a new classification of type III open fractures. J Trauma. 1984;24(8):742-746.
12. DeLong WG Jr, Born CT, Wei SY, Petrik ME, Ponzio R, Schwab CW. Aggressive treatment of 119 open fracture wounds. J Trauma. 1999;46(6):1049-1054.
13. Tielinen L, Lindahl JE, Tukiainen EJ. Acute unreamed intramedullary nailing and soft tissue reconstruction with muscle flaps for the treatment of severe open tibial shaft fractures. Injury. 2007;38(8):906-912.
14. Georgiadis GM, Behrens FF, Joyce MJ, Earle AS, Simmons AL. Open tibial fractures with severe soft-tissue loss. Limb salvage compared with below-the-knee amputation. J Bone Joint Surg Am. 1993;75(10):1431-1441.
15. Hansen M, Mehler D, Hessmann MH, Blum J, Rommens PM. Intramedullary stabilization of extraarticular proximal tibial fractures: a biomechanical comparison of intramedullary and extramedullary implants including a new proximal tibia nail (PTN). J Orthop Trauma. 2007;21(10):701-709.
16. Hoegel FW, Hoffmann S, Weninger P, Bühren V, Augat P. Biomechanical comparison of locked plate osteosynthesis, reamed and unreamed nailing in conventional interlocking technique, and unreamed angle stable nailing in distal tibia fractures. J Trauma Acute Care Surg. 2012;73(4):933-938.
17. Brumback RJ, Reilly JP, Poka A, Lakatos RP, Bathon GH, Burgess AR. Intramedullary nailing of femoral shaft fractures. Part 1: Decision-making errors with interlocking fixation. J Bone Joint Surg Am. 1988;70(10):1441-1452.
18. Hooper GJ, Keddell RG, Penny ID. Conservative management or closed nailing for tibial shaft fractures. A randomised prospective trial. J Bone Joint Surg Br. 1991;73(1):83-85.
19. Karladani AH, Granhed H, Edshage B, Jerre R, Styf J. Displaced tibial shaft fractures: a prospective randomized study of closed intramedullary nailing versus cast treatment in 53 patients. Acta Orthop Scand. 2000;71(12):160-167.
20. Kenwright J, Richardson JB, Goodship AE, et al. Effect of controlled axial micromovement on healing of tibial fractures. Lancet. 1986;22(8517):1185-1187.
21. Im GI, Tae SK. Distal metaphyseal fractures of tibia: a prospective randomized trial of closed reduction and intramedullary nail versus open reduction and plate and screws fixation. J Trauma. 2005;59(5):1219-1223.
22. Henley MB, Chapman JR, Agel J, Harvey EJ, Whorton AM, Swiontkowski MF. Treatment of type II, IIIA, and IIIB open fractures of the tibial shaft: a prospective comparison of unreamed interlocking intramedullary nails and half-pin external fixators. J Orthop Trauma. 1998;12(1):1-7.
23. Ramos T, Ekholm C, Eriksson BI, Karlsson J, Nistor L. The Ilizarov external fixator - a useful alternative for the treatment of proximal tibial fractures. A prospective observational study of 30 consecutive patients. BMC Musculoskelet Disord. 2013;14:11.
24. Bhandari M, Guyatt GH, Swiontkowski MF, Schemitsch EH. Treatment of open fractures of the shaft of the tibia. J Bone Joint Surg Br. 2001;83(1):62-68.
25. Webb LX, Bosse MJ, Castillo RC, MacKenzie EJ; LEAP Study Group. Analysis of surgeon-controlled variables in the treatment of limb-threatening type-III open tibial diaphyseal fractures. J Bone Joint Surg Am. 2007;89(5):923-928.
26. Bondurant FJ, Cotler HB, Buckle R, Miller-Crotchett P, Browner BD. The medical and economic impact of severely injured lower extremities. J Trauma. 1988;28(8):1270-1273.
27. Bosse MJ, MacKenzie EJ, Kellam JF, et al. An analysis of outcomes of reconstruction or amputation of leg-threatening injuries. N Engl J Med. 2002;347(24):1924-1931.
28. MacKenzie EJ, Bosse MJ, Pollak AN, et al. Long-term persistence of disability following severe lower-limb trauma. Results of a seven-year follow-up. J Bone Joint Surg Am. 2005;87(8):1801-1809.
29. Doulens KM, Joshi AB, Wagner RA. Tibial fracture after total knee arthroplasty treated with retrograde intramedullary fixation. Am J Orthop. 2007;36(7):E111-E113.
30. Zafra-Jiménez JA, Pretell-Mazzini J, Resines-Erasun C. Distal tibial fracture below a total knee arthroplasty: retrograde intramedullary nailing as an alternative method of treatment: a case report. J Orthop Trauma. 2011;25(7):e74-e76.
31. Loosen S, Preuss S, Zelle BA, Pape HC, Tarken IS. Multimorbid patients with poor soft tissue conditions: Treatment of distal tibia fractures with retrograde intramedullary nailing. Unfallchirurg. 2012;116(6):553-558.
32. Kessler SB, Hallfeldt KJ, Perren SM, Schweiberer L. The effects of reaming and intramedullary nailing on fracture healing. Clin Orthop. 1986;212:18-25.
33. Klein MP, Rahn BA, Frigg R, Kessler S, Perren SM. Reaming versus non-reaming in medullary nailing: interference with cortical circulation of the canine tibia. Arch Orthop Trauma Surg. 1990;109(6):314-316.
34. Reichert IL, McCarthy ID, Hughes SP. The acute vascular response to intramedullary reaming. Microsphere estimation of blood flow in the intact ovine tibia. J Bone Joint Surg Br. 1995;77(3):490-493.
1. Patzakis MJ, Wilkins J. Factors influencing infection rate in open fracture wounds. Clin Orthop. 1989;243:36-40.
2. Court-Brown CM, McBirnie J. The epidemiology of tibial fractures. J Bone Joint Surg Br. 1995;77(3):417-421.
3. Puno RM, Teynor JT, Nagano J, Gustilo RB. Critical analysis of results of treatment of 201 tibial shaft fractures. Clin Orthop. 1986;212:113-121.
4. Melvin JS, Dombroski DG, Torbert JT, Kovach SJ, Esterhal JL, Mehta S. Open tibial shaft fractures: I. Evaluation and initial wound management. J Am Acad Orthop Surg. 2010;18(1):10-19.
5. Bhandari M, Guyatt GH, Swiontkowski MF, Schemitsch EH. Treatment of open fractures of the shaft of the tibia. J Bone Joint Surg Br. 2001;83(1):62-68.
6. SPRINT Investigators, Bhandari M, Guyatt G, Tornetta P 3rd, et al. Study to prospectively evaluate reamed intramedually nails in patients with tibial fractures (S.P.R.I.N.T.): study rationale and design. BMC Musculoskelet Disord. 2008;9:91.
7. Study to Prospectively Evaluate Reamed Intramedullary Nails in Patients with Tibial Fractures Investigators, Bhandari M, Guyatt G, Tornetta P 3rd, et al. Randomized trial of reamed and unreamed intramedullary nailing of tibial shaft fractures. J Bone Joint Surg Am. 2008;90(12):2567-2578.
8. Burr DB, Milgrom C, Fyhrie D, et al. In vivo measurement of human tibial strains during vigorous activity. Bone. 1996;18(5):405-410.
9. Edwards P. Fracture of the shaft of the tibia: 492 consecutive cases in adults: Importance of soft tissue injury. Acta Orthop Scand (Suppl). 1965;76(suppl 76):1-82.
10. Papakostidis C, Kanakaris NK, Pretel J, Faour O, Morell DJ, Giannoudis PV. Prevalence of complications of open tibial shaft fractures stratified as per the Gustilo–Anderson classification. Injury. 2011;42(12):1408-1415.
11. Gustilo RB, Mendoza RM, Williams DN. Problems in the management of type III (severe) open fractures: a new classification of type III open fractures. J Trauma. 1984;24(8):742-746.
12. DeLong WG Jr, Born CT, Wei SY, Petrik ME, Ponzio R, Schwab CW. Aggressive treatment of 119 open fracture wounds. J Trauma. 1999;46(6):1049-1054.
13. Tielinen L, Lindahl JE, Tukiainen EJ. Acute unreamed intramedullary nailing and soft tissue reconstruction with muscle flaps for the treatment of severe open tibial shaft fractures. Injury. 2007;38(8):906-912.
14. Georgiadis GM, Behrens FF, Joyce MJ, Earle AS, Simmons AL. Open tibial fractures with severe soft-tissue loss. Limb salvage compared with below-the-knee amputation. J Bone Joint Surg Am. 1993;75(10):1431-1441.
15. Hansen M, Mehler D, Hessmann MH, Blum J, Rommens PM. Intramedullary stabilization of extraarticular proximal tibial fractures: a biomechanical comparison of intramedullary and extramedullary implants including a new proximal tibia nail (PTN). J Orthop Trauma. 2007;21(10):701-709.
16. Hoegel FW, Hoffmann S, Weninger P, Bühren V, Augat P. Biomechanical comparison of locked plate osteosynthesis, reamed and unreamed nailing in conventional interlocking technique, and unreamed angle stable nailing in distal tibia fractures. J Trauma Acute Care Surg. 2012;73(4):933-938.
17. Brumback RJ, Reilly JP, Poka A, Lakatos RP, Bathon GH, Burgess AR. Intramedullary nailing of femoral shaft fractures. Part 1: Decision-making errors with interlocking fixation. J Bone Joint Surg Am. 1988;70(10):1441-1452.
18. Hooper GJ, Keddell RG, Penny ID. Conservative management or closed nailing for tibial shaft fractures. A randomised prospective trial. J Bone Joint Surg Br. 1991;73(1):83-85.
19. Karladani AH, Granhed H, Edshage B, Jerre R, Styf J. Displaced tibial shaft fractures: a prospective randomized study of closed intramedullary nailing versus cast treatment in 53 patients. Acta Orthop Scand. 2000;71(12):160-167.
20. Kenwright J, Richardson JB, Goodship AE, et al. Effect of controlled axial micromovement on healing of tibial fractures. Lancet. 1986;22(8517):1185-1187.
21. Im GI, Tae SK. Distal metaphyseal fractures of tibia: a prospective randomized trial of closed reduction and intramedullary nail versus open reduction and plate and screws fixation. J Trauma. 2005;59(5):1219-1223.
22. Henley MB, Chapman JR, Agel J, Harvey EJ, Whorton AM, Swiontkowski MF. Treatment of type II, IIIA, and IIIB open fractures of the tibial shaft: a prospective comparison of unreamed interlocking intramedullary nails and half-pin external fixators. J Orthop Trauma. 1998;12(1):1-7.
23. Ramos T, Ekholm C, Eriksson BI, Karlsson J, Nistor L. The Ilizarov external fixator - a useful alternative for the treatment of proximal tibial fractures. A prospective observational study of 30 consecutive patients. BMC Musculoskelet Disord. 2013;14:11.
24. Bhandari M, Guyatt GH, Swiontkowski MF, Schemitsch EH. Treatment of open fractures of the shaft of the tibia. J Bone Joint Surg Br. 2001;83(1):62-68.
25. Webb LX, Bosse MJ, Castillo RC, MacKenzie EJ; LEAP Study Group. Analysis of surgeon-controlled variables in the treatment of limb-threatening type-III open tibial diaphyseal fractures. J Bone Joint Surg Am. 2007;89(5):923-928.
26. Bondurant FJ, Cotler HB, Buckle R, Miller-Crotchett P, Browner BD. The medical and economic impact of severely injured lower extremities. J Trauma. 1988;28(8):1270-1273.
27. Bosse MJ, MacKenzie EJ, Kellam JF, et al. An analysis of outcomes of reconstruction or amputation of leg-threatening injuries. N Engl J Med. 2002;347(24):1924-1931.
28. MacKenzie EJ, Bosse MJ, Pollak AN, et al. Long-term persistence of disability following severe lower-limb trauma. Results of a seven-year follow-up. J Bone Joint Surg Am. 2005;87(8):1801-1809.
29. Doulens KM, Joshi AB, Wagner RA. Tibial fracture after total knee arthroplasty treated with retrograde intramedullary fixation. Am J Orthop. 2007;36(7):E111-E113.
30. Zafra-Jiménez JA, Pretell-Mazzini J, Resines-Erasun C. Distal tibial fracture below a total knee arthroplasty: retrograde intramedullary nailing as an alternative method of treatment: a case report. J Orthop Trauma. 2011;25(7):e74-e76.
31. Loosen S, Preuss S, Zelle BA, Pape HC, Tarken IS. Multimorbid patients with poor soft tissue conditions: Treatment of distal tibia fractures with retrograde intramedullary nailing. Unfallchirurg. 2012;116(6):553-558.
32. Kessler SB, Hallfeldt KJ, Perren SM, Schweiberer L. The effects of reaming and intramedullary nailing on fracture healing. Clin Orthop. 1986;212:18-25.
33. Klein MP, Rahn BA, Frigg R, Kessler S, Perren SM. Reaming versus non-reaming in medullary nailing: interference with cortical circulation of the canine tibia. Arch Orthop Trauma Surg. 1990;109(6):314-316.
34. Reichert IL, McCarthy ID, Hughes SP. The acute vascular response to intramedullary reaming. Microsphere estimation of blood flow in the intact ovine tibia. J Bone Joint Surg Br. 1995;77(3):490-493.
Glenoid Damage From Articular Protrusion of Metal Suture Anchor After Arthroscopic Rotator Cuff Repair
Complications with the use of anchor screws in shoulder surgery have been well-documented1,2 and can be divided into 3 categories: insertion (eg, incomplete seating, inadequate insertion, and migration), biologic (eg, large tacks producing synovitis and bone reaction), and, less commonly, mechanical (eg, intra- and extra-articular bone pull-out with migration) complications.
Prominent hardware, including suture anchors, as a cause of arthritis and joint damage has been well-documented in shoulder surgery.3,4 For example, anchors placed on the glenoid rim have been implicated in severe cartilage loss if they protrude above the level of the glenoid rim.3 However, to the authors’ knowledge, prominent anchor placement after rotator cuff repair has not been reported as a cause of arthritis unless the anchor dislodges into the glenohumeral joint. The authors present a case in which a suture anchor used for rotator cuff repair protruded through the humeral head, resulting in glenohumeral arthritis. The patient provided written informed consent for print and electronic publication of this case report.
Case Report
A 61-year-old woman presented with complaints of persistent right shoulder pain for 5 months after a fall from a bicycle. She had taken nonsteroidal anti-inflammatory medication without pain relief. On presentation, she had no atrophy or deformity, was neurologically intact for sensation and reflexes, and had full range of motion (ROM) but a painful arc. She had tenderness over the greater tuberosity and positive Neer and Hawkins-Kennedy impingement signs. She had pain but no weakness to resisted abduction or to resisted external rotation with the arms at the sides.
Preoperative conventional radiographs of the shoulder were normal. A gadolinium-enhanced magnetic resonance arthrogram showed a high-grade articular partial tear of the supraspinatus, which was judged to be at least two-thirds of the tendon width. Because nonoperative methods had failed, the patient elected operative intervention for this tear.
Diagnostic arthroscopy (with the patient in a lateral decubitus position) showed a normal joint except for a high-grade, 8×8-mm, greater than 6 mm deep, partial tear of the articular side of the supraspinatus tendon. The subacromial space had moderate to severe bursal tissue inflammation but no full-thickness component to the rotator cuff tear. A bursectomy, coracoacromial ligament release, and partial anterolateral acromioplasty were performed.
A transtendinous technique was used to repair this high-grade tear. For an anatomically rigid repair, we used 3 suture anchors with a straight configuration because each metal anchor has only 1 suture. According to the standard arthroscopic transtendinous repair technique, the suture anchors were placed through the rotator cuff tendon (at the lateral articular margin at the medial extent of the footprint) after localization of the angle with a spinal needle. A shuttle relay was used to pass the sutures, and the knot was pulled into the subacromial space, cinching the rotator cuff on top of the suture anchors and reestablishing the contact of the tendon to the footprint. We used two 2.4-mm FASTak suture anchors (Arthrex, Naples, Florida) and one 3.5-mm Corkscrew suture anchor (Arthrex). This process was repeated for the remaining suture limbs. The placement of the suture anchors adequately reduced the articular part of the cuff to the footprint.
After surgery, the patient had no complications, and radiographs taken the next day suggested no abnormalities (Figure 1A). The shoulder was immobilized for 4 weeks after surgery, and passive, gentle ROM exercise was supervised by a physical therapist twice a week during this period. After the first 4 weeks, an active ROM program was begun. However, shortly after initiating motion in the shoulder, the patient complained of a recurrence of pain that she described as a sharp and grinding sensation.
The patient was reevaluated 8 weeks after surgery. Her pain was worsening, and she was having difficulty regaining ROM. Conventional radiographs showed the tip of the metal anchor protruding through the articular cartilage of the humeral head (Figure 1B). The patient was informed of the findings, and immediate surgery was performed to remove the anchor.
Arthroscopic examination showed extensive damage to the glenoid cartilage (Figure 1C) and an intra-articularly intact rotator cuff repair. The cartilage damage was located in the posterior and inferior half of the glenoid, which is related to the forward flexion of the arm; the depth of the cartilage defect was approximately 2 mm. Under the image intensifier, an empty suture anchor driver was inserted into the previous screw insertion hole, and the anchor was screwed back out and removed.
After surgery, the patient’s arm was placed in a sling, and an ROM program began 4 weeks later. The sensation of grinding was eliminated, and her pain gradually improved. Three years after surgery, she had no pain, no weakness, and full ROM without limitations (Figure 2).
Discussion
Protrusion and migration of suture anchors in shoulder surgery has been documented extensively.3,4 Zuckerman and Matsen4 divided these complications into 4 groups: (1) incorrect placement, (2) migration after placement, (3) loosening, and (4) device breakage. These complications may be frequently related to surgical technique, and all these studies describe backward migration of the anchor out of the drill hole. In the current case, the anchor tip penetrated the articular surface of the humeral head, not because of anchor migration but because the anchor was inserted too far. To the authors’ knowledge, there is only 1 reported case of anchor protrusion through the humeral head; it involved a different type of anchor insertion system.5 In that case, there was only mild cartilage damage to the glenoid, and the patient recovered after removal of the anchors.
Several factors contributed to the improper insertion of the anchor in the current patient. First, repairing a high-grade articular side defect or partial articular supraspinatus tendon avulsion lesion can be technically challenging because rotator cuff tissue obscures the view when inserting the anchor. Second, the anchor was inserted too medially on the greater tuberosity, which made the distance from the tuberosity to the joint shorter. Wong and colleagues5 performed an analysis of the angle of insertion that would be safe using a PEEK PushLock SP system (Arthrex), but they emphasized that the angle depends on the configuration of the particular insertion system. The current case also shows that the surgeon should be cognizant of the fact that penetration of the humeral head by the anchor can occur if the surgeon is unaware of the distance from the anchor to the laser line on the insertion device or of the distance from the tuberosity to the articular surface of the humeral head.
The current case also shows that the type of anchor and delivery system may contribute to this complication. Double-loaded suture anchors can decrease the number of anchors needed for secure fixation. Bioabsorbable anchors can be used for this purpose, but they may be technically more difficult to use for repairing partial tears of the rotator cuff. Better visualization of the laser line on the anchor may be facilitated by using a probe from an anterior portal to hold the cuff up while the anchor is inserted.
This case has shown the importance of obtaining postoperative radiographic studies in patients who have metal anchors placed during shoulder surgery, especially if they complain of continued pain, new pain, crepitus, or grinding. When conventional radiography is insufficient for locating the anchor or its proximity to the joint line, computed tomography can be helpful.1
Conclusion
Removing failed suture anchors can be challenging, especially when they protrude into the joint on the humeral side.1,6 The best way to prevent this complication is through careful technique. The anchors should not be inserted beyond the depth of the laser line on the anchors, and every attempt should be made to make sure the laser line is visible at the time of anchor insertion. Postoperative radiographs should be considered for patients with metal anchors in the shoulder, especially if the patient continues to have symptoms or develops new symptoms in the shoulder after surgery.
1. Park HB, Keyurapan E, Gill HS, Selhi HS, McFarland EG. Suture anchors and tacks for shoulder surgery. Part II: The prevention and treatment of complications. Am J Sports Med. 2006;34(1):136-144.
2. McFarland EG, Park HB, Keyurapan E, Gill HS, Selhi HS. Suture anchors and tacks for shoulder surgery. Part I: Biology and biomechanics. Am J Sports Med. 2005;33(12):1918-1923.
3. Rhee YG, Lee DH, Chun IH, Bae SC. Glenohumeral arthropathy after arthroscopic anterior shoulder stabilization. Arthroscopy. 2004;20(4):402-406.
4. Zuckerman JD, Matsen FA III. Complications about the glenohumeral joint related to the use of screws and staples. J Bone Joint Surg Am. 1984;66(2):175-180.
5. Wong AS, Kokkalis ZT, Schmidt CC. Proper insertion angle is essential to prevent intra-articular protrusion of a knotless suture anchor in shoulder rotator cuff repair. Arthroscopy. 2010;26(2):286-290.
6. Grutter PW, McFarland EG, Zikria BA, Dai Z, Petersen SA. Techniques for suture anchor removal in shoulder surgery. Am J Sports Med. 2010;38(8):1706-1710.
Complications with the use of anchor screws in shoulder surgery have been well-documented1,2 and can be divided into 3 categories: insertion (eg, incomplete seating, inadequate insertion, and migration), biologic (eg, large tacks producing synovitis and bone reaction), and, less commonly, mechanical (eg, intra- and extra-articular bone pull-out with migration) complications.
Prominent hardware, including suture anchors, as a cause of arthritis and joint damage has been well-documented in shoulder surgery.3,4 For example, anchors placed on the glenoid rim have been implicated in severe cartilage loss if they protrude above the level of the glenoid rim.3 However, to the authors’ knowledge, prominent anchor placement after rotator cuff repair has not been reported as a cause of arthritis unless the anchor dislodges into the glenohumeral joint. The authors present a case in which a suture anchor used for rotator cuff repair protruded through the humeral head, resulting in glenohumeral arthritis. The patient provided written informed consent for print and electronic publication of this case report.
Case Report
A 61-year-old woman presented with complaints of persistent right shoulder pain for 5 months after a fall from a bicycle. She had taken nonsteroidal anti-inflammatory medication without pain relief. On presentation, she had no atrophy or deformity, was neurologically intact for sensation and reflexes, and had full range of motion (ROM) but a painful arc. She had tenderness over the greater tuberosity and positive Neer and Hawkins-Kennedy impingement signs. She had pain but no weakness to resisted abduction or to resisted external rotation with the arms at the sides.
Preoperative conventional radiographs of the shoulder were normal. A gadolinium-enhanced magnetic resonance arthrogram showed a high-grade articular partial tear of the supraspinatus, which was judged to be at least two-thirds of the tendon width. Because nonoperative methods had failed, the patient elected operative intervention for this tear.
Diagnostic arthroscopy (with the patient in a lateral decubitus position) showed a normal joint except for a high-grade, 8×8-mm, greater than 6 mm deep, partial tear of the articular side of the supraspinatus tendon. The subacromial space had moderate to severe bursal tissue inflammation but no full-thickness component to the rotator cuff tear. A bursectomy, coracoacromial ligament release, and partial anterolateral acromioplasty were performed.
A transtendinous technique was used to repair this high-grade tear. For an anatomically rigid repair, we used 3 suture anchors with a straight configuration because each metal anchor has only 1 suture. According to the standard arthroscopic transtendinous repair technique, the suture anchors were placed through the rotator cuff tendon (at the lateral articular margin at the medial extent of the footprint) after localization of the angle with a spinal needle. A shuttle relay was used to pass the sutures, and the knot was pulled into the subacromial space, cinching the rotator cuff on top of the suture anchors and reestablishing the contact of the tendon to the footprint. We used two 2.4-mm FASTak suture anchors (Arthrex, Naples, Florida) and one 3.5-mm Corkscrew suture anchor (Arthrex). This process was repeated for the remaining suture limbs. The placement of the suture anchors adequately reduced the articular part of the cuff to the footprint.
After surgery, the patient had no complications, and radiographs taken the next day suggested no abnormalities (Figure 1A). The shoulder was immobilized for 4 weeks after surgery, and passive, gentle ROM exercise was supervised by a physical therapist twice a week during this period. After the first 4 weeks, an active ROM program was begun. However, shortly after initiating motion in the shoulder, the patient complained of a recurrence of pain that she described as a sharp and grinding sensation.
The patient was reevaluated 8 weeks after surgery. Her pain was worsening, and she was having difficulty regaining ROM. Conventional radiographs showed the tip of the metal anchor protruding through the articular cartilage of the humeral head (Figure 1B). The patient was informed of the findings, and immediate surgery was performed to remove the anchor.
Arthroscopic examination showed extensive damage to the glenoid cartilage (Figure 1C) and an intra-articularly intact rotator cuff repair. The cartilage damage was located in the posterior and inferior half of the glenoid, which is related to the forward flexion of the arm; the depth of the cartilage defect was approximately 2 mm. Under the image intensifier, an empty suture anchor driver was inserted into the previous screw insertion hole, and the anchor was screwed back out and removed.
After surgery, the patient’s arm was placed in a sling, and an ROM program began 4 weeks later. The sensation of grinding was eliminated, and her pain gradually improved. Three years after surgery, she had no pain, no weakness, and full ROM without limitations (Figure 2).
Discussion
Protrusion and migration of suture anchors in shoulder surgery has been documented extensively.3,4 Zuckerman and Matsen4 divided these complications into 4 groups: (1) incorrect placement, (2) migration after placement, (3) loosening, and (4) device breakage. These complications may be frequently related to surgical technique, and all these studies describe backward migration of the anchor out of the drill hole. In the current case, the anchor tip penetrated the articular surface of the humeral head, not because of anchor migration but because the anchor was inserted too far. To the authors’ knowledge, there is only 1 reported case of anchor protrusion through the humeral head; it involved a different type of anchor insertion system.5 In that case, there was only mild cartilage damage to the glenoid, and the patient recovered after removal of the anchors.
Several factors contributed to the improper insertion of the anchor in the current patient. First, repairing a high-grade articular side defect or partial articular supraspinatus tendon avulsion lesion can be technically challenging because rotator cuff tissue obscures the view when inserting the anchor. Second, the anchor was inserted too medially on the greater tuberosity, which made the distance from the tuberosity to the joint shorter. Wong and colleagues5 performed an analysis of the angle of insertion that would be safe using a PEEK PushLock SP system (Arthrex), but they emphasized that the angle depends on the configuration of the particular insertion system. The current case also shows that the surgeon should be cognizant of the fact that penetration of the humeral head by the anchor can occur if the surgeon is unaware of the distance from the anchor to the laser line on the insertion device or of the distance from the tuberosity to the articular surface of the humeral head.
The current case also shows that the type of anchor and delivery system may contribute to this complication. Double-loaded suture anchors can decrease the number of anchors needed for secure fixation. Bioabsorbable anchors can be used for this purpose, but they may be technically more difficult to use for repairing partial tears of the rotator cuff. Better visualization of the laser line on the anchor may be facilitated by using a probe from an anterior portal to hold the cuff up while the anchor is inserted.
This case has shown the importance of obtaining postoperative radiographic studies in patients who have metal anchors placed during shoulder surgery, especially if they complain of continued pain, new pain, crepitus, or grinding. When conventional radiography is insufficient for locating the anchor or its proximity to the joint line, computed tomography can be helpful.1
Conclusion
Removing failed suture anchors can be challenging, especially when they protrude into the joint on the humeral side.1,6 The best way to prevent this complication is through careful technique. The anchors should not be inserted beyond the depth of the laser line on the anchors, and every attempt should be made to make sure the laser line is visible at the time of anchor insertion. Postoperative radiographs should be considered for patients with metal anchors in the shoulder, especially if the patient continues to have symptoms or develops new symptoms in the shoulder after surgery.
Complications with the use of anchor screws in shoulder surgery have been well-documented1,2 and can be divided into 3 categories: insertion (eg, incomplete seating, inadequate insertion, and migration), biologic (eg, large tacks producing synovitis and bone reaction), and, less commonly, mechanical (eg, intra- and extra-articular bone pull-out with migration) complications.
Prominent hardware, including suture anchors, as a cause of arthritis and joint damage has been well-documented in shoulder surgery.3,4 For example, anchors placed on the glenoid rim have been implicated in severe cartilage loss if they protrude above the level of the glenoid rim.3 However, to the authors’ knowledge, prominent anchor placement after rotator cuff repair has not been reported as a cause of arthritis unless the anchor dislodges into the glenohumeral joint. The authors present a case in which a suture anchor used for rotator cuff repair protruded through the humeral head, resulting in glenohumeral arthritis. The patient provided written informed consent for print and electronic publication of this case report.
Case Report
A 61-year-old woman presented with complaints of persistent right shoulder pain for 5 months after a fall from a bicycle. She had taken nonsteroidal anti-inflammatory medication without pain relief. On presentation, she had no atrophy or deformity, was neurologically intact for sensation and reflexes, and had full range of motion (ROM) but a painful arc. She had tenderness over the greater tuberosity and positive Neer and Hawkins-Kennedy impingement signs. She had pain but no weakness to resisted abduction or to resisted external rotation with the arms at the sides.
Preoperative conventional radiographs of the shoulder were normal. A gadolinium-enhanced magnetic resonance arthrogram showed a high-grade articular partial tear of the supraspinatus, which was judged to be at least two-thirds of the tendon width. Because nonoperative methods had failed, the patient elected operative intervention for this tear.
Diagnostic arthroscopy (with the patient in a lateral decubitus position) showed a normal joint except for a high-grade, 8×8-mm, greater than 6 mm deep, partial tear of the articular side of the supraspinatus tendon. The subacromial space had moderate to severe bursal tissue inflammation but no full-thickness component to the rotator cuff tear. A bursectomy, coracoacromial ligament release, and partial anterolateral acromioplasty were performed.
A transtendinous technique was used to repair this high-grade tear. For an anatomically rigid repair, we used 3 suture anchors with a straight configuration because each metal anchor has only 1 suture. According to the standard arthroscopic transtendinous repair technique, the suture anchors were placed through the rotator cuff tendon (at the lateral articular margin at the medial extent of the footprint) after localization of the angle with a spinal needle. A shuttle relay was used to pass the sutures, and the knot was pulled into the subacromial space, cinching the rotator cuff on top of the suture anchors and reestablishing the contact of the tendon to the footprint. We used two 2.4-mm FASTak suture anchors (Arthrex, Naples, Florida) and one 3.5-mm Corkscrew suture anchor (Arthrex). This process was repeated for the remaining suture limbs. The placement of the suture anchors adequately reduced the articular part of the cuff to the footprint.
After surgery, the patient had no complications, and radiographs taken the next day suggested no abnormalities (Figure 1A). The shoulder was immobilized for 4 weeks after surgery, and passive, gentle ROM exercise was supervised by a physical therapist twice a week during this period. After the first 4 weeks, an active ROM program was begun. However, shortly after initiating motion in the shoulder, the patient complained of a recurrence of pain that she described as a sharp and grinding sensation.
The patient was reevaluated 8 weeks after surgery. Her pain was worsening, and she was having difficulty regaining ROM. Conventional radiographs showed the tip of the metal anchor protruding through the articular cartilage of the humeral head (Figure 1B). The patient was informed of the findings, and immediate surgery was performed to remove the anchor.
Arthroscopic examination showed extensive damage to the glenoid cartilage (Figure 1C) and an intra-articularly intact rotator cuff repair. The cartilage damage was located in the posterior and inferior half of the glenoid, which is related to the forward flexion of the arm; the depth of the cartilage defect was approximately 2 mm. Under the image intensifier, an empty suture anchor driver was inserted into the previous screw insertion hole, and the anchor was screwed back out and removed.
After surgery, the patient’s arm was placed in a sling, and an ROM program began 4 weeks later. The sensation of grinding was eliminated, and her pain gradually improved. Three years after surgery, she had no pain, no weakness, and full ROM without limitations (Figure 2).
Discussion
Protrusion and migration of suture anchors in shoulder surgery has been documented extensively.3,4 Zuckerman and Matsen4 divided these complications into 4 groups: (1) incorrect placement, (2) migration after placement, (3) loosening, and (4) device breakage. These complications may be frequently related to surgical technique, and all these studies describe backward migration of the anchor out of the drill hole. In the current case, the anchor tip penetrated the articular surface of the humeral head, not because of anchor migration but because the anchor was inserted too far. To the authors’ knowledge, there is only 1 reported case of anchor protrusion through the humeral head; it involved a different type of anchor insertion system.5 In that case, there was only mild cartilage damage to the glenoid, and the patient recovered after removal of the anchors.
Several factors contributed to the improper insertion of the anchor in the current patient. First, repairing a high-grade articular side defect or partial articular supraspinatus tendon avulsion lesion can be technically challenging because rotator cuff tissue obscures the view when inserting the anchor. Second, the anchor was inserted too medially on the greater tuberosity, which made the distance from the tuberosity to the joint shorter. Wong and colleagues5 performed an analysis of the angle of insertion that would be safe using a PEEK PushLock SP system (Arthrex), but they emphasized that the angle depends on the configuration of the particular insertion system. The current case also shows that the surgeon should be cognizant of the fact that penetration of the humeral head by the anchor can occur if the surgeon is unaware of the distance from the anchor to the laser line on the insertion device or of the distance from the tuberosity to the articular surface of the humeral head.
The current case also shows that the type of anchor and delivery system may contribute to this complication. Double-loaded suture anchors can decrease the number of anchors needed for secure fixation. Bioabsorbable anchors can be used for this purpose, but they may be technically more difficult to use for repairing partial tears of the rotator cuff. Better visualization of the laser line on the anchor may be facilitated by using a probe from an anterior portal to hold the cuff up while the anchor is inserted.
This case has shown the importance of obtaining postoperative radiographic studies in patients who have metal anchors placed during shoulder surgery, especially if they complain of continued pain, new pain, crepitus, or grinding. When conventional radiography is insufficient for locating the anchor or its proximity to the joint line, computed tomography can be helpful.1
Conclusion
Removing failed suture anchors can be challenging, especially when they protrude into the joint on the humeral side.1,6 The best way to prevent this complication is through careful technique. The anchors should not be inserted beyond the depth of the laser line on the anchors, and every attempt should be made to make sure the laser line is visible at the time of anchor insertion. Postoperative radiographs should be considered for patients with metal anchors in the shoulder, especially if the patient continues to have symptoms or develops new symptoms in the shoulder after surgery.
1. Park HB, Keyurapan E, Gill HS, Selhi HS, McFarland EG. Suture anchors and tacks for shoulder surgery. Part II: The prevention and treatment of complications. Am J Sports Med. 2006;34(1):136-144.
2. McFarland EG, Park HB, Keyurapan E, Gill HS, Selhi HS. Suture anchors and tacks for shoulder surgery. Part I: Biology and biomechanics. Am J Sports Med. 2005;33(12):1918-1923.
3. Rhee YG, Lee DH, Chun IH, Bae SC. Glenohumeral arthropathy after arthroscopic anterior shoulder stabilization. Arthroscopy. 2004;20(4):402-406.
4. Zuckerman JD, Matsen FA III. Complications about the glenohumeral joint related to the use of screws and staples. J Bone Joint Surg Am. 1984;66(2):175-180.
5. Wong AS, Kokkalis ZT, Schmidt CC. Proper insertion angle is essential to prevent intra-articular protrusion of a knotless suture anchor in shoulder rotator cuff repair. Arthroscopy. 2010;26(2):286-290.
6. Grutter PW, McFarland EG, Zikria BA, Dai Z, Petersen SA. Techniques for suture anchor removal in shoulder surgery. Am J Sports Med. 2010;38(8):1706-1710.
1. Park HB, Keyurapan E, Gill HS, Selhi HS, McFarland EG. Suture anchors and tacks for shoulder surgery. Part II: The prevention and treatment of complications. Am J Sports Med. 2006;34(1):136-144.
2. McFarland EG, Park HB, Keyurapan E, Gill HS, Selhi HS. Suture anchors and tacks for shoulder surgery. Part I: Biology and biomechanics. Am J Sports Med. 2005;33(12):1918-1923.
3. Rhee YG, Lee DH, Chun IH, Bae SC. Glenohumeral arthropathy after arthroscopic anterior shoulder stabilization. Arthroscopy. 2004;20(4):402-406.
4. Zuckerman JD, Matsen FA III. Complications about the glenohumeral joint related to the use of screws and staples. J Bone Joint Surg Am. 1984;66(2):175-180.
5. Wong AS, Kokkalis ZT, Schmidt CC. Proper insertion angle is essential to prevent intra-articular protrusion of a knotless suture anchor in shoulder rotator cuff repair. Arthroscopy. 2010;26(2):286-290.
6. Grutter PW, McFarland EG, Zikria BA, Dai Z, Petersen SA. Techniques for suture anchor removal in shoulder surgery. Am J Sports Med. 2010;38(8):1706-1710.
Atypical Presentation of Fat Embolism Syndrome After Gunshot Wound to the Foot
Fat embolism syndrome (FES) is a rare complication reported primarily after long bone fractures, with an incidence of 0.3% to 2.2%.1-3 It is most commonly caused by trauma and is thought to result from movement of bone fragments or to occur during intramedullary reaming.1 Both of these factors lead to a distortion of the bone marrow cavity, allowing marrow and fat to enter the circulatory system.1
Although the true pathophysiology remains poorly understood, it is possible that, once in systemic circulation, the fat particles become lodged in the vascular system, inciting an inflammatory response, leading to organ dysfunction via mechanical or biochemical processes.4 Typically, the diagnosis is made after clinical features are observed, including hypoxemia, petechial rash, and cerebral signs not related to a head injury or other conditions.5,6
Although FES is an uncommon complication after traumatic injuries, mortality after FES in a recent study was reported to be 10%.1 FES is most commonly seen after fractures of the femur and tibia, although cases have been described involving fractures of the radius, ulna, and humerus.1,3 We present an atypical case of cerebral FES after multiple fractures of the foot; to our knowledge, such a case has not been reported in the English-language literature. The patient provided written informed consent for print and electronic publication of this case report.
Case Report
A 42-year-old man was hunting with his son when he was accidentally shot in the left foot with a .270-caliber rifle bullet at close range. The patient sought care at a local hospital and, in the ensuing 3 hours, his mentation appeared normal. He reported pain and numbness distal to the injury in the tibial nerve distribution, but he remained vascularly intact, alert, and oriented. He was given 7 mg of hydromorphone hydrochloride over 2 hours for pain control and was transferred to our hospital via ambulance approximately 6 hours after injury. Upon arrival, he was noted to be extremely sedated and obtunded, responding only to pain with spontaneous eye opening. He was unable to follow commands. He was given
1.2 mg of naloxone intravenously to reverse what was presumed to be acute opioid intoxication; however, his mental status did not improve.
On examination, the patient was noted to have a small entrance wound through the Achilles tendon (Figures 1A, 1B) and an exit wound on the plantar aspect of the foot near the heads of the first and second metatarsals (Figures 1C, 1D) with minimal bleeding and no gross contamination. There was significant edema on the medial and proximal aspects of the left foot, 3+ dorsalis pedis pulse, and a capillary refill of 4 seconds. Radiographs showed traumatic fracture deformities of the calcaneus, navicular, medial cuneiform, and first and second metatarsal bases, as well as an intra-articular fracture deformity of the left talus extending to the talar dome (Figures 2A-2C). Neurologic examination could not be reliably obtained because of the patient’s mental status. He was determined to be unstable for immediate surgery, and his left leg was splinted pending neurologic evaluation.
The patient’s oxygen saturation was 94%, and his temperature was 38.2°C (100.76°F). Although his heart rate was in the 90s upon arrival, he became tachycardic over the next 4 hours, with heart rate ranging from the 110s to 130s; he remained tachycardic for approximately 72 hours. Laboratory values upon arrival showed a hemoglobin value of 12.8 g/dL and platelets of 249,000/μL. He developed anemia and thrombocytopenia within 72 hours of the injury, with a low of 6.6 g/dL and 88,000/μL, respectively, by postinjury day 4. Computed tomography of the head, electroencephalography, urine drug screen, and lumbar puncture were unremarkable. The patient never became hypoxemic. Within 14 hours after injury, he was completely comatose with extensor posturing. In the intensive care unit (ICU), the patient was intubated for airway protection.
The next day, the patient underwent magnetic resonance imaging (MRI) of the brain, which showed innumerable tiny infarcts throughout cerebral hemispheres, cerebellum, and brainstem in a characteristic “starfield” pattern on T2-weighted images (Figure 3). This was radiographically consistent with fat emboli related to the left lower extremity gunshot wound. An echocardiogram showed small right-to-left shunt and a possible intrapulmonary shunt, although this was never confirmed. The echocardiogram was technically challenging secondary to his persistent tachycardia. He also developed a subtle petechial rash (Figure 4A).
The patient underwent percutaneous gastrostomy-tube placement for nutrition on postinjury day 4 and remained intubated, unable to protect his airway, and nonresponsive with extensor posturing (Figure 4B). He was also taken to the operating room for spanning external fixator placement on postinjury day 3 to restore calcaneal height and length as well as foot stability (Figures 5A, 5B).
The patient was treated with supportive care and was discharged from the hospital in a comatose state on hospital day 17 to a rehabilitation facility. He began to emerge from the coma 6 weeks after injury, and his external fixator was removed and a cast applied to his lower extremity. His entrance and exit wounds healed as expected. Initial agitation was treated with propranolol and quetiapine. Because he continued to have difficulty with spasticity and increased tone, he was given botulinum toxin type A injections in the pectoral muscles, biceps, and forearms. He made continued and rapid improvement in response to intensive multidisciplinary therapy and returned home 4½ months after injury. Eight months after the injury, he is now walking independently with a cane and independent with his activities of daily living. Unfortunately, he has substantial pain in his foot, which appears to be a combination of both neuropathic and posttraumatic arthrosis causes. He is undergoing consultation for a possible amputation. Radiographs show consolidation of the hind and midfoot fractures with retained bullet fragments (Figures 6A-6C). He continues to receive multidisciplinary care to address cognitive limitations and is making progress.
Discussion
FES is a life-threatening disease affecting multiple organ systems.7 Classically, the pulmonary, central nervous, and dermatologic systems are affected.5,6,8 While FES is most recognizable after long bone fractures and orthopedic procedures involving the intramedullary canal, to our knowledge, FES after gunshot wound and concomitant fractures of the foot has never been reported.
The syndrome is defined by major and minor criteria as outlined by Gurd.5 Major criteria include hypoxia, deteriorating mental status, and petechiae. This case represents a somewhat atypical presentation of FES, because dermatologic manifestations and pulmonary compromise were subtle. The minor criteria consisting of tachycardia, fever, anemia, and thrombocytopenia were present in our patient, although at different phases during the progression of the syndrome. This emphasizes the difficulty in diagnosing FES because the symptoms do not occur simultaneously.
In the classic syndrome, after an initial asymptomatic interval of 12 to 72 hours, pulmonary, neurologic, and/or dermatologic changes usually ensue.9 Altered mental status, including headache, confusion, stupor, coma, rigidity, or convulsions, has been documented in 86% of patients.10 In our case, the neurologic symptoms presented earlier, at around 6 hours after injury, and respiratory symptoms, including hypoxia, tachypnea, and dyspnea, reported in 75% of cases,2,11 did not occur at all. In fact, continued intubation was only required in this case for neuromuscular airway protection. Classic dermatologic manifestations, a reddish-brown nonpalpable petechial rash diffusely covering the upper torso and extremities, normally appears within 12 to 36 hours.12,13 Nevertheless, in our case, these findings were subtle compared with others previously reported.14,15 In fact, despite being seen by numerous physicians, including neurologists and ICU intensivists, only the orthopedists’ notes made reference to this modest finding (Figure 4A).
Further complicating the diagnosis is that, during the onset of symptoms, patients are typically victims of polytrauma and/or routinely given narcotics to help with significant pain. Therefore, it is appropriate to rule out opioid overdose and other metabolic sources of mental-status change. This can be done fairly expeditiously with laboratory testing and narcotic reversal. After these have been eliminated, FES should be considered in a patient with rapid neurologic deterioration, because a delay in treatment can affect outcomes.2,4,16
Because continuous showering of emboli to the brain and other organs occurs without fracture stabilization, rapid diagnosis with high clinical suspicion of FES is essential and can be aided immensely with MRI. In fact, MRI is the most sensitive test for this diagnosis and correlates with clinical severity of brain injury.17 T2-weighted images show regions of high-signal intensity and “starfield” pattern, which are sensitive markers for FES (Figure 3).18 These tests can be done concomitantly with a well-splinted extremity, and definitive stabilization should be carried out promptly because early splinting and fixation of orthopedic fractures improves outcomes.17
Perhaps the most important reason to make an expeditious diagnosis is to help counsel families, who are undoubtedly in shock and disbelief. Recovery times can vary widely, with the patient often continuing to regain cognitive and motor function over the course of months to years.2 Without knowledge of signs of improvement in neurologic outcome, families cannot be accurately counseled regarding potential for recovery. The practicing orthopedist should be aware of this disorder, because initial neurologic deterioration may seem hopeless. Furthermore, supportive care should be initiated early with multidisciplinary teams and extensive rehabilitation because these offer the best outcomes in patients with FES.4,18 Although our patient continues to have cognitive impairment, his recovery in the preceding 8 months has been aided by rapid diagnosis and multidisciplinary care and should offer hope to other patients faced with this situation.
1. Akhtar S. Fat embolism. Anesthesiol Clin. 2009;27(3):533-550.
2. Müller C, Rahn BA, Pfister U, Meinig RP. The incidence, pathogenesis, diagnosis, and treatment of fat embolism. Orthop Rev. 1994;23(2):107-117.
3. Stein PD, Yaekoub AY, Matta F, Kleerekoper M. Fat embolism syndrome. Am J Med Sci. 2008;336(6):472-477.
4. Parisi DM, Koval K, Egol K. Fat embolism syndrome. Am J Orthop. 2002;31(9):507-512.
5. Gurd AR. Fat embolism: an aid to diagnosis. J Bone Joint Surb Br. 1970;52(4):732-737.
6. Lee SC, Yoon JY, Nam CH, Kim TK, Jung KA, Lee DW. Cerebral fat embolism syndrome after simultaneous bilateral total knee arthroplasty: a case series. J Arthroplasty. 2012;27(3):409-414.
7. Gurd AR, Wilson RI. Fat-embolism syndrome. Lancet. 1972;2(7770):
231-232.
8. Habashi NM, Andrews PL, Scalea TM. Therapeutic aspects of fat embolism syndrome. Injury. 2006;37(Suppl 4):S68-S73.
9. Weiss W, Bardana D, Yen D. Delayed presentation of fat embolism syndrome after intramedullary nailing of a fractured femur: a case report. J Trauma. 2009;66(3):E42-E45.
10. Byrick RJ. Fat embolism and postoperative coagulopathy. Can J Anaesth. 2001;48(7):618-621.
11. Gurd AR, Wilson RI. The fat embolism syndrome. J Bone Joint Surg Br. 1974;56(3):408-416.
12. Burgher LW. Fat embolism syndrome. Chest. 1981;79(2):131-132.
13. Burgher LW, Dines DE, Linscheid RL, Didier EP. Fat embolism and the adult respiratory distress syndrome. Mayo Clin Proc. 1974;49(2):107-109.
14. Liu DD, Hsieh NK, Chen HI. Histopathological and biochemical changes following fat embolism with administration of corn oil micelles: a new animal model for fat embolism syndrome. J Bone Joint Surg Br. 2008;90(11):
1517-1521.
15. Liu HK, Chen WC. Images in clinical medicine. Fat embolism syndrome. N Engl J Med. 2011;364(18):1761.
16. Pinney SJ, Keating JF, Meek RN. Fat embolism syndrome in isolated femoral fractures: does timing of nailing influence incidence? Injury. 1998;29(2):
131-133.
17. Takahashi M, Suzuki R, Osakabe Y, et al. Magnetic resonance imaging findings in cerebral fat embolism: correlation with clinical manifestations. J Trauma. 1999;46(2):324-327.
18. Parizel PM, Demey HE, Veeckmans G, et al. Early diagnosis of cerebral fat embolism syndrome by diffusion-weighted MRI (starfield pattern). Stroke. 2001;32(12):2942-2944.
Fat embolism syndrome (FES) is a rare complication reported primarily after long bone fractures, with an incidence of 0.3% to 2.2%.1-3 It is most commonly caused by trauma and is thought to result from movement of bone fragments or to occur during intramedullary reaming.1 Both of these factors lead to a distortion of the bone marrow cavity, allowing marrow and fat to enter the circulatory system.1
Although the true pathophysiology remains poorly understood, it is possible that, once in systemic circulation, the fat particles become lodged in the vascular system, inciting an inflammatory response, leading to organ dysfunction via mechanical or biochemical processes.4 Typically, the diagnosis is made after clinical features are observed, including hypoxemia, petechial rash, and cerebral signs not related to a head injury or other conditions.5,6
Although FES is an uncommon complication after traumatic injuries, mortality after FES in a recent study was reported to be 10%.1 FES is most commonly seen after fractures of the femur and tibia, although cases have been described involving fractures of the radius, ulna, and humerus.1,3 We present an atypical case of cerebral FES after multiple fractures of the foot; to our knowledge, such a case has not been reported in the English-language literature. The patient provided written informed consent for print and electronic publication of this case report.
Case Report
A 42-year-old man was hunting with his son when he was accidentally shot in the left foot with a .270-caliber rifle bullet at close range. The patient sought care at a local hospital and, in the ensuing 3 hours, his mentation appeared normal. He reported pain and numbness distal to the injury in the tibial nerve distribution, but he remained vascularly intact, alert, and oriented. He was given 7 mg of hydromorphone hydrochloride over 2 hours for pain control and was transferred to our hospital via ambulance approximately 6 hours after injury. Upon arrival, he was noted to be extremely sedated and obtunded, responding only to pain with spontaneous eye opening. He was unable to follow commands. He was given
1.2 mg of naloxone intravenously to reverse what was presumed to be acute opioid intoxication; however, his mental status did not improve.
On examination, the patient was noted to have a small entrance wound through the Achilles tendon (Figures 1A, 1B) and an exit wound on the plantar aspect of the foot near the heads of the first and second metatarsals (Figures 1C, 1D) with minimal bleeding and no gross contamination. There was significant edema on the medial and proximal aspects of the left foot, 3+ dorsalis pedis pulse, and a capillary refill of 4 seconds. Radiographs showed traumatic fracture deformities of the calcaneus, navicular, medial cuneiform, and first and second metatarsal bases, as well as an intra-articular fracture deformity of the left talus extending to the talar dome (Figures 2A-2C). Neurologic examination could not be reliably obtained because of the patient’s mental status. He was determined to be unstable for immediate surgery, and his left leg was splinted pending neurologic evaluation.
The patient’s oxygen saturation was 94%, and his temperature was 38.2°C (100.76°F). Although his heart rate was in the 90s upon arrival, he became tachycardic over the next 4 hours, with heart rate ranging from the 110s to 130s; he remained tachycardic for approximately 72 hours. Laboratory values upon arrival showed a hemoglobin value of 12.8 g/dL and platelets of 249,000/μL. He developed anemia and thrombocytopenia within 72 hours of the injury, with a low of 6.6 g/dL and 88,000/μL, respectively, by postinjury day 4. Computed tomography of the head, electroencephalography, urine drug screen, and lumbar puncture were unremarkable. The patient never became hypoxemic. Within 14 hours after injury, he was completely comatose with extensor posturing. In the intensive care unit (ICU), the patient was intubated for airway protection.
The next day, the patient underwent magnetic resonance imaging (MRI) of the brain, which showed innumerable tiny infarcts throughout cerebral hemispheres, cerebellum, and brainstem in a characteristic “starfield” pattern on T2-weighted images (Figure 3). This was radiographically consistent with fat emboli related to the left lower extremity gunshot wound. An echocardiogram showed small right-to-left shunt and a possible intrapulmonary shunt, although this was never confirmed. The echocardiogram was technically challenging secondary to his persistent tachycardia. He also developed a subtle petechial rash (Figure 4A).
The patient underwent percutaneous gastrostomy-tube placement for nutrition on postinjury day 4 and remained intubated, unable to protect his airway, and nonresponsive with extensor posturing (Figure 4B). He was also taken to the operating room for spanning external fixator placement on postinjury day 3 to restore calcaneal height and length as well as foot stability (Figures 5A, 5B).
The patient was treated with supportive care and was discharged from the hospital in a comatose state on hospital day 17 to a rehabilitation facility. He began to emerge from the coma 6 weeks after injury, and his external fixator was removed and a cast applied to his lower extremity. His entrance and exit wounds healed as expected. Initial agitation was treated with propranolol and quetiapine. Because he continued to have difficulty with spasticity and increased tone, he was given botulinum toxin type A injections in the pectoral muscles, biceps, and forearms. He made continued and rapid improvement in response to intensive multidisciplinary therapy and returned home 4½ months after injury. Eight months after the injury, he is now walking independently with a cane and independent with his activities of daily living. Unfortunately, he has substantial pain in his foot, which appears to be a combination of both neuropathic and posttraumatic arthrosis causes. He is undergoing consultation for a possible amputation. Radiographs show consolidation of the hind and midfoot fractures with retained bullet fragments (Figures 6A-6C). He continues to receive multidisciplinary care to address cognitive limitations and is making progress.
Discussion
FES is a life-threatening disease affecting multiple organ systems.7 Classically, the pulmonary, central nervous, and dermatologic systems are affected.5,6,8 While FES is most recognizable after long bone fractures and orthopedic procedures involving the intramedullary canal, to our knowledge, FES after gunshot wound and concomitant fractures of the foot has never been reported.
The syndrome is defined by major and minor criteria as outlined by Gurd.5 Major criteria include hypoxia, deteriorating mental status, and petechiae. This case represents a somewhat atypical presentation of FES, because dermatologic manifestations and pulmonary compromise were subtle. The minor criteria consisting of tachycardia, fever, anemia, and thrombocytopenia were present in our patient, although at different phases during the progression of the syndrome. This emphasizes the difficulty in diagnosing FES because the symptoms do not occur simultaneously.
In the classic syndrome, after an initial asymptomatic interval of 12 to 72 hours, pulmonary, neurologic, and/or dermatologic changes usually ensue.9 Altered mental status, including headache, confusion, stupor, coma, rigidity, or convulsions, has been documented in 86% of patients.10 In our case, the neurologic symptoms presented earlier, at around 6 hours after injury, and respiratory symptoms, including hypoxia, tachypnea, and dyspnea, reported in 75% of cases,2,11 did not occur at all. In fact, continued intubation was only required in this case for neuromuscular airway protection. Classic dermatologic manifestations, a reddish-brown nonpalpable petechial rash diffusely covering the upper torso and extremities, normally appears within 12 to 36 hours.12,13 Nevertheless, in our case, these findings were subtle compared with others previously reported.14,15 In fact, despite being seen by numerous physicians, including neurologists and ICU intensivists, only the orthopedists’ notes made reference to this modest finding (Figure 4A).
Further complicating the diagnosis is that, during the onset of symptoms, patients are typically victims of polytrauma and/or routinely given narcotics to help with significant pain. Therefore, it is appropriate to rule out opioid overdose and other metabolic sources of mental-status change. This can be done fairly expeditiously with laboratory testing and narcotic reversal. After these have been eliminated, FES should be considered in a patient with rapid neurologic deterioration, because a delay in treatment can affect outcomes.2,4,16
Because continuous showering of emboli to the brain and other organs occurs without fracture stabilization, rapid diagnosis with high clinical suspicion of FES is essential and can be aided immensely with MRI. In fact, MRI is the most sensitive test for this diagnosis and correlates with clinical severity of brain injury.17 T2-weighted images show regions of high-signal intensity and “starfield” pattern, which are sensitive markers for FES (Figure 3).18 These tests can be done concomitantly with a well-splinted extremity, and definitive stabilization should be carried out promptly because early splinting and fixation of orthopedic fractures improves outcomes.17
Perhaps the most important reason to make an expeditious diagnosis is to help counsel families, who are undoubtedly in shock and disbelief. Recovery times can vary widely, with the patient often continuing to regain cognitive and motor function over the course of months to years.2 Without knowledge of signs of improvement in neurologic outcome, families cannot be accurately counseled regarding potential for recovery. The practicing orthopedist should be aware of this disorder, because initial neurologic deterioration may seem hopeless. Furthermore, supportive care should be initiated early with multidisciplinary teams and extensive rehabilitation because these offer the best outcomes in patients with FES.4,18 Although our patient continues to have cognitive impairment, his recovery in the preceding 8 months has been aided by rapid diagnosis and multidisciplinary care and should offer hope to other patients faced with this situation.
Fat embolism syndrome (FES) is a rare complication reported primarily after long bone fractures, with an incidence of 0.3% to 2.2%.1-3 It is most commonly caused by trauma and is thought to result from movement of bone fragments or to occur during intramedullary reaming.1 Both of these factors lead to a distortion of the bone marrow cavity, allowing marrow and fat to enter the circulatory system.1
Although the true pathophysiology remains poorly understood, it is possible that, once in systemic circulation, the fat particles become lodged in the vascular system, inciting an inflammatory response, leading to organ dysfunction via mechanical or biochemical processes.4 Typically, the diagnosis is made after clinical features are observed, including hypoxemia, petechial rash, and cerebral signs not related to a head injury or other conditions.5,6
Although FES is an uncommon complication after traumatic injuries, mortality after FES in a recent study was reported to be 10%.1 FES is most commonly seen after fractures of the femur and tibia, although cases have been described involving fractures of the radius, ulna, and humerus.1,3 We present an atypical case of cerebral FES after multiple fractures of the foot; to our knowledge, such a case has not been reported in the English-language literature. The patient provided written informed consent for print and electronic publication of this case report.
Case Report
A 42-year-old man was hunting with his son when he was accidentally shot in the left foot with a .270-caliber rifle bullet at close range. The patient sought care at a local hospital and, in the ensuing 3 hours, his mentation appeared normal. He reported pain and numbness distal to the injury in the tibial nerve distribution, but he remained vascularly intact, alert, and oriented. He was given 7 mg of hydromorphone hydrochloride over 2 hours for pain control and was transferred to our hospital via ambulance approximately 6 hours after injury. Upon arrival, he was noted to be extremely sedated and obtunded, responding only to pain with spontaneous eye opening. He was unable to follow commands. He was given
1.2 mg of naloxone intravenously to reverse what was presumed to be acute opioid intoxication; however, his mental status did not improve.
On examination, the patient was noted to have a small entrance wound through the Achilles tendon (Figures 1A, 1B) and an exit wound on the plantar aspect of the foot near the heads of the first and second metatarsals (Figures 1C, 1D) with minimal bleeding and no gross contamination. There was significant edema on the medial and proximal aspects of the left foot, 3+ dorsalis pedis pulse, and a capillary refill of 4 seconds. Radiographs showed traumatic fracture deformities of the calcaneus, navicular, medial cuneiform, and first and second metatarsal bases, as well as an intra-articular fracture deformity of the left talus extending to the talar dome (Figures 2A-2C). Neurologic examination could not be reliably obtained because of the patient’s mental status. He was determined to be unstable for immediate surgery, and his left leg was splinted pending neurologic evaluation.
The patient’s oxygen saturation was 94%, and his temperature was 38.2°C (100.76°F). Although his heart rate was in the 90s upon arrival, he became tachycardic over the next 4 hours, with heart rate ranging from the 110s to 130s; he remained tachycardic for approximately 72 hours. Laboratory values upon arrival showed a hemoglobin value of 12.8 g/dL and platelets of 249,000/μL. He developed anemia and thrombocytopenia within 72 hours of the injury, with a low of 6.6 g/dL and 88,000/μL, respectively, by postinjury day 4. Computed tomography of the head, electroencephalography, urine drug screen, and lumbar puncture were unremarkable. The patient never became hypoxemic. Within 14 hours after injury, he was completely comatose with extensor posturing. In the intensive care unit (ICU), the patient was intubated for airway protection.
The next day, the patient underwent magnetic resonance imaging (MRI) of the brain, which showed innumerable tiny infarcts throughout cerebral hemispheres, cerebellum, and brainstem in a characteristic “starfield” pattern on T2-weighted images (Figure 3). This was radiographically consistent with fat emboli related to the left lower extremity gunshot wound. An echocardiogram showed small right-to-left shunt and a possible intrapulmonary shunt, although this was never confirmed. The echocardiogram was technically challenging secondary to his persistent tachycardia. He also developed a subtle petechial rash (Figure 4A).
The patient underwent percutaneous gastrostomy-tube placement for nutrition on postinjury day 4 and remained intubated, unable to protect his airway, and nonresponsive with extensor posturing (Figure 4B). He was also taken to the operating room for spanning external fixator placement on postinjury day 3 to restore calcaneal height and length as well as foot stability (Figures 5A, 5B).
The patient was treated with supportive care and was discharged from the hospital in a comatose state on hospital day 17 to a rehabilitation facility. He began to emerge from the coma 6 weeks after injury, and his external fixator was removed and a cast applied to his lower extremity. His entrance and exit wounds healed as expected. Initial agitation was treated with propranolol and quetiapine. Because he continued to have difficulty with spasticity and increased tone, he was given botulinum toxin type A injections in the pectoral muscles, biceps, and forearms. He made continued and rapid improvement in response to intensive multidisciplinary therapy and returned home 4½ months after injury. Eight months after the injury, he is now walking independently with a cane and independent with his activities of daily living. Unfortunately, he has substantial pain in his foot, which appears to be a combination of both neuropathic and posttraumatic arthrosis causes. He is undergoing consultation for a possible amputation. Radiographs show consolidation of the hind and midfoot fractures with retained bullet fragments (Figures 6A-6C). He continues to receive multidisciplinary care to address cognitive limitations and is making progress.
Discussion
FES is a life-threatening disease affecting multiple organ systems.7 Classically, the pulmonary, central nervous, and dermatologic systems are affected.5,6,8 While FES is most recognizable after long bone fractures and orthopedic procedures involving the intramedullary canal, to our knowledge, FES after gunshot wound and concomitant fractures of the foot has never been reported.
The syndrome is defined by major and minor criteria as outlined by Gurd.5 Major criteria include hypoxia, deteriorating mental status, and petechiae. This case represents a somewhat atypical presentation of FES, because dermatologic manifestations and pulmonary compromise were subtle. The minor criteria consisting of tachycardia, fever, anemia, and thrombocytopenia were present in our patient, although at different phases during the progression of the syndrome. This emphasizes the difficulty in diagnosing FES because the symptoms do not occur simultaneously.
In the classic syndrome, after an initial asymptomatic interval of 12 to 72 hours, pulmonary, neurologic, and/or dermatologic changes usually ensue.9 Altered mental status, including headache, confusion, stupor, coma, rigidity, or convulsions, has been documented in 86% of patients.10 In our case, the neurologic symptoms presented earlier, at around 6 hours after injury, and respiratory symptoms, including hypoxia, tachypnea, and dyspnea, reported in 75% of cases,2,11 did not occur at all. In fact, continued intubation was only required in this case for neuromuscular airway protection. Classic dermatologic manifestations, a reddish-brown nonpalpable petechial rash diffusely covering the upper torso and extremities, normally appears within 12 to 36 hours.12,13 Nevertheless, in our case, these findings were subtle compared with others previously reported.14,15 In fact, despite being seen by numerous physicians, including neurologists and ICU intensivists, only the orthopedists’ notes made reference to this modest finding (Figure 4A).
Further complicating the diagnosis is that, during the onset of symptoms, patients are typically victims of polytrauma and/or routinely given narcotics to help with significant pain. Therefore, it is appropriate to rule out opioid overdose and other metabolic sources of mental-status change. This can be done fairly expeditiously with laboratory testing and narcotic reversal. After these have been eliminated, FES should be considered in a patient with rapid neurologic deterioration, because a delay in treatment can affect outcomes.2,4,16
Because continuous showering of emboli to the brain and other organs occurs without fracture stabilization, rapid diagnosis with high clinical suspicion of FES is essential and can be aided immensely with MRI. In fact, MRI is the most sensitive test for this diagnosis and correlates with clinical severity of brain injury.17 T2-weighted images show regions of high-signal intensity and “starfield” pattern, which are sensitive markers for FES (Figure 3).18 These tests can be done concomitantly with a well-splinted extremity, and definitive stabilization should be carried out promptly because early splinting and fixation of orthopedic fractures improves outcomes.17
Perhaps the most important reason to make an expeditious diagnosis is to help counsel families, who are undoubtedly in shock and disbelief. Recovery times can vary widely, with the patient often continuing to regain cognitive and motor function over the course of months to years.2 Without knowledge of signs of improvement in neurologic outcome, families cannot be accurately counseled regarding potential for recovery. The practicing orthopedist should be aware of this disorder, because initial neurologic deterioration may seem hopeless. Furthermore, supportive care should be initiated early with multidisciplinary teams and extensive rehabilitation because these offer the best outcomes in patients with FES.4,18 Although our patient continues to have cognitive impairment, his recovery in the preceding 8 months has been aided by rapid diagnosis and multidisciplinary care and should offer hope to other patients faced with this situation.
1. Akhtar S. Fat embolism. Anesthesiol Clin. 2009;27(3):533-550.
2. Müller C, Rahn BA, Pfister U, Meinig RP. The incidence, pathogenesis, diagnosis, and treatment of fat embolism. Orthop Rev. 1994;23(2):107-117.
3. Stein PD, Yaekoub AY, Matta F, Kleerekoper M. Fat embolism syndrome. Am J Med Sci. 2008;336(6):472-477.
4. Parisi DM, Koval K, Egol K. Fat embolism syndrome. Am J Orthop. 2002;31(9):507-512.
5. Gurd AR. Fat embolism: an aid to diagnosis. J Bone Joint Surb Br. 1970;52(4):732-737.
6. Lee SC, Yoon JY, Nam CH, Kim TK, Jung KA, Lee DW. Cerebral fat embolism syndrome after simultaneous bilateral total knee arthroplasty: a case series. J Arthroplasty. 2012;27(3):409-414.
7. Gurd AR, Wilson RI. Fat-embolism syndrome. Lancet. 1972;2(7770):
231-232.
8. Habashi NM, Andrews PL, Scalea TM. Therapeutic aspects of fat embolism syndrome. Injury. 2006;37(Suppl 4):S68-S73.
9. Weiss W, Bardana D, Yen D. Delayed presentation of fat embolism syndrome after intramedullary nailing of a fractured femur: a case report. J Trauma. 2009;66(3):E42-E45.
10. Byrick RJ. Fat embolism and postoperative coagulopathy. Can J Anaesth. 2001;48(7):618-621.
11. Gurd AR, Wilson RI. The fat embolism syndrome. J Bone Joint Surg Br. 1974;56(3):408-416.
12. Burgher LW. Fat embolism syndrome. Chest. 1981;79(2):131-132.
13. Burgher LW, Dines DE, Linscheid RL, Didier EP. Fat embolism and the adult respiratory distress syndrome. Mayo Clin Proc. 1974;49(2):107-109.
14. Liu DD, Hsieh NK, Chen HI. Histopathological and biochemical changes following fat embolism with administration of corn oil micelles: a new animal model for fat embolism syndrome. J Bone Joint Surg Br. 2008;90(11):
1517-1521.
15. Liu HK, Chen WC. Images in clinical medicine. Fat embolism syndrome. N Engl J Med. 2011;364(18):1761.
16. Pinney SJ, Keating JF, Meek RN. Fat embolism syndrome in isolated femoral fractures: does timing of nailing influence incidence? Injury. 1998;29(2):
131-133.
17. Takahashi M, Suzuki R, Osakabe Y, et al. Magnetic resonance imaging findings in cerebral fat embolism: correlation with clinical manifestations. J Trauma. 1999;46(2):324-327.
18. Parizel PM, Demey HE, Veeckmans G, et al. Early diagnosis of cerebral fat embolism syndrome by diffusion-weighted MRI (starfield pattern). Stroke. 2001;32(12):2942-2944.
1. Akhtar S. Fat embolism. Anesthesiol Clin. 2009;27(3):533-550.
2. Müller C, Rahn BA, Pfister U, Meinig RP. The incidence, pathogenesis, diagnosis, and treatment of fat embolism. Orthop Rev. 1994;23(2):107-117.
3. Stein PD, Yaekoub AY, Matta F, Kleerekoper M. Fat embolism syndrome. Am J Med Sci. 2008;336(6):472-477.
4. Parisi DM, Koval K, Egol K. Fat embolism syndrome. Am J Orthop. 2002;31(9):507-512.
5. Gurd AR. Fat embolism: an aid to diagnosis. J Bone Joint Surb Br. 1970;52(4):732-737.
6. Lee SC, Yoon JY, Nam CH, Kim TK, Jung KA, Lee DW. Cerebral fat embolism syndrome after simultaneous bilateral total knee arthroplasty: a case series. J Arthroplasty. 2012;27(3):409-414.
7. Gurd AR, Wilson RI. Fat-embolism syndrome. Lancet. 1972;2(7770):
231-232.
8. Habashi NM, Andrews PL, Scalea TM. Therapeutic aspects of fat embolism syndrome. Injury. 2006;37(Suppl 4):S68-S73.
9. Weiss W, Bardana D, Yen D. Delayed presentation of fat embolism syndrome after intramedullary nailing of a fractured femur: a case report. J Trauma. 2009;66(3):E42-E45.
10. Byrick RJ. Fat embolism and postoperative coagulopathy. Can J Anaesth. 2001;48(7):618-621.
11. Gurd AR, Wilson RI. The fat embolism syndrome. J Bone Joint Surg Br. 1974;56(3):408-416.
12. Burgher LW. Fat embolism syndrome. Chest. 1981;79(2):131-132.
13. Burgher LW, Dines DE, Linscheid RL, Didier EP. Fat embolism and the adult respiratory distress syndrome. Mayo Clin Proc. 1974;49(2):107-109.
14. Liu DD, Hsieh NK, Chen HI. Histopathological and biochemical changes following fat embolism with administration of corn oil micelles: a new animal model for fat embolism syndrome. J Bone Joint Surg Br. 2008;90(11):
1517-1521.
15. Liu HK, Chen WC. Images in clinical medicine. Fat embolism syndrome. N Engl J Med. 2011;364(18):1761.
16. Pinney SJ, Keating JF, Meek RN. Fat embolism syndrome in isolated femoral fractures: does timing of nailing influence incidence? Injury. 1998;29(2):
131-133.
17. Takahashi M, Suzuki R, Osakabe Y, et al. Magnetic resonance imaging findings in cerebral fat embolism: correlation with clinical manifestations. J Trauma. 1999;46(2):324-327.
18. Parizel PM, Demey HE, Veeckmans G, et al. Early diagnosis of cerebral fat embolism syndrome by diffusion-weighted MRI (starfield pattern). Stroke. 2001;32(12):2942-2944.
What you must know before you recommend a probiotic
› Consider probiotics for patients with acute infectious diarrhea, antibiotic-associated diarrhea, or Clostridium difficile-associated diarrhea. A
› Do not recommend probiotics for preventing or treating Crohn’s disease or ulcerative colitis. B
› Consider the probiotic Bifidobacterium bifidum MIMBb75 for patients with irritable bowel syndrome. B
Strength of recommendation (SOR)
A Good-quality patient-oriented evidence
B Inconsistent or limited-quality patient-oriented evidence
C Consensus, usual practice, opinion, disease-oriented evidence, case series
Probiotics—live micoorganisms that are consumed as supplements or food for purported health benefits—are a popular over-the-counter remedy for various gastrointestinal (GI) ailments and other conditions, but the evidence supporting their use is mixed. Probiotics interact with the normal flora of the human body. They are believed to act by multiple mechanisms to deliver beneficial effects, including providing a protective barrier, altering intestinal pH to favor the growth of nonpathogenic bacteria, enhancing the host’s immunologic response, producing antimicrobial substances, and directly competing with pathogenic bacteria for receptors in the GI tract.1 (See “The normal human intestinal flora.”)
In the United States, Lactobacillus and Bifidobacterium are the probiotic genera that are most commonly used. (For a list of the specific probiotic species found in 5 popular products, see TABLE 1.2-6) The review that follows examines the evidence for using probiotics for select GI ailments, including several types of diarrheal illnesses, inflammatory bowel disease (Crohn’s disease and ulcerative colitis), and irritable bowel syndrome (IBS). These findings are summarized in TABLE 2.1,7-21
The human body contains approximately 1014 prokaryotic organisms, with a biomass of >1 kg. Most of these organisms are indigenous and stable, although transient members such as enteric pathogens can be found.
The gastrointestinal tract is sterile at birth but is colonized immediately, and each individual has marked variations in microbial composition. The complex symbiotic relationship between the normal intestinal flora and the human host is beneficial to both. These microbes utilize complex carbohydrates undigested by the host as energy. Fermentation results in the formation of short-chain fatty acids, which can provide up to 15% of human energy requirements.
In addition to these metabolic benefits, microbial flora dampen the human inflammatory response, induce immunosuppressive T cells (Tregs), and competitively exclude pathogens.
Colonic epithelium is nourished and proliferates in the presence of normal intestinal flora. Disruption of the normal flora can cause disease.
SOURCE: Neish AS. Microbes in gastrointestinal health and disease. Gastroenterology. 2009;136:65-80.
Probiotics may help with some types of diarrhea
Acute infectious diarrhea. Viruses, bacteria, and parasites cause acute infectious diarrhea, and probiotics are thought to act against these pathogens by competing for available nutrients and pattern recognition receptors in the GI endothelium, acidifying the local environment, and increasing immune responses within the GI tract. In a meta-analysis of 63 studies (N=8014) that used multiple strains and dosages of probiotics, investigators found probiotics shortened the duration of acute infectious diarrhea by approximately 24 hours (95% confidence interval [CI], 15.9-33.6 hours).7 Probiotics also reduced both the risk of diarrhea lasting longer than 4 days (relative risk [RR]=0.41; 95% CI, 0.32-0.53) and stool frequency on Day 2 of illness (mean difference of 0.80 stools; 95% CI, 0.45-1.14).
Traveler’s diarrhea. The incidence of traveler’s diarrhea is >50% when traveling to high-risk areas such as the Middle East, North Africa, Latin America, and Southeast Asia, and 5% to 10% when traveling to areas such as North America, Northern Europe, the United Kingdom, Australia, and New Zealand.8 Traveler’s diarrhea may be caused by ingesting food and liquids contaminated with fecal material. Symptoms include diarrhea, cramps, and nausea that if untreated typically last from 2 to 6 days but can last for as long as a month.8
In a meta-analysis of 12 studies (N=5171) that evaluated various probiotic strains, researchers found probiotics effectively prevented traveler’s diarrhea in US and European travelers who visited a variety of vacation spots (pooled RR=0.85; 95% CI, 0.79-0.91).8 No serious adverse events were reported.
Radiation-induced diarrhea. Radiation treatments to the abdomen and pelvis can damage the lower GI tract and cause diarrhea. The pooled results from a meta-analysis that included 6 studies (N=1449) significantly favored the use of probiotics over placebo for decreasing the incidence of radiation-induced diarrhea (odds ratio [OR]=0.44; 95% CI, 0.21-0.92).9 Probiotics use also was associated with decreased loperamide use (OR=0.29; 95% CI, 0.01-6.80) and decreased incidence of watery stools (OR=0.36; 95% CI, 0.05-2.81), but these outcomes did not reach statistical significance.
Antibiotic-associated diarrhea. Antibiotic use has long been associated with the development of diarrheal illness, sometimes due to the acceleration of GI motility (eg, erythromycin) or by causing osmotic diarrhea by decreasing GI bacteria that assist in carbohydrate breakdown.11 A meta-analysis that evaluated 63 randomized controlled trials (RCTs) (N=11,811) showed that probiotics are effective for treating and preventing antibiotic-associated diarrhea (AAD).1 There was a statistically significant reduction in AAD among patients who received probiotics (RR=0.58; 95% CI, 0.50-0.68; number needed to treat [NNT]=13). Most of the studies in this meta-analysis used a Lactobacillus probiotic alone or in combination with another probiotic. Researchers did not analyze whether the efficacy varied by patient population, probiotic used, causative antibiotic, or duration of treatment.1
Another meta-analysis of 34 studies (N=4138) also found probiotic therapy can prevent AAD.10 The pooled RR for AAD was 0.53 (95% CI, 0.44-0.63) for patients treated with probiotics compared to placebo, with an NNT of 8 (95% CI, 7-11). The effects remained significant when results were grouped by probiotic species, patient age, and duration of antibiotic treatment. Among a subgroup of patients in this meta-analysis who were being treated for Helicobacter pylori, the pooled RR of AAD was 0.37 (95% CI, 0.20-0.69) and the NNT was 5 (95% CI, 4-10).10 However, the 2013 PLACIDE trial (N=17,420) found no significant decrease in AAD rates in hospitalized patients over age 65 years being treated with antibiotics who received probiotics (RR=1.04; 95% CI, 0.84-1.28).22
Clostridium difficile-associated diarrhea. As we know, antibiotics can disrupt the normal GI flora and permit overgrow of Clostridium difficile, which can result in C. difficile-associated diarrhea (CDAD).12 This can occur with oral, parenteral, and even topical antibiotics.11 Researchers have investigated whether probiotics can prevent this opportunistic C. difficile overgrowth.
A 2012 meta-analysis of 20 trials (N=38,180) found probiotic prophylaxis prevented CDAD in both inpatients and outpatients while not increasing the incidence of significant adverse effects.12 Probiotics decreased the incidence of CDAD by 66% (pooled RR=0.34, 95% CI, 0.24-0.49).12 Adverse events occurred in 9.3% of patients taking probiotics, compared with 12.6% of controls (RR=0.82, 95% CI, 0.65-1.05).12
Conversely, a 2008 review of 4 studies (N=336) concluded there is insufficient evidence for using probiotics to treat CDAD, either as monotherapy or adjunct therapy.11 One trial in this meta-analysis (N=124) found patients who received the probiotic Saccharomyces boulardii in addition to antibiotic therapy were significantly less likely to experience CDAD recurrence than those who received placebo (RR=0.59; 95% CI, 0.35-0.98).11 However, this benefit was not found in the other trials in this meta-analysis.11
The PLACIDE trial found probiotics did not prevent CDAD in hospitalized patients over age 65 years; 0.8% of patients who received probiotics developed CDAD, compared to 1.2% in the placebo group (RR=0.71, 95% CI, 0.34-1.47).22
Helicobacter pylori infection. The triple therapy regimen of a proton pump inhibitor plus the antibiotics clarithromycin and amoxicillin is the recommended treatment for H. pylori infection.13 Problems with this treatment include adverse effects such as diarrhea and decreased eradication rates, in part due to antibiotic resistance. Certain Lactobacillus species have been shown to inhibit or kill H. pylori in vitro,13 and evidence from several meta-analyses suggests probiotics should be an adjunct therapy when treating H. pylori.
In a meta-analysis of 10 RCTs (N=963), fermented milk-based probiotics improved H. pylori eradication rates by 5% to 15%.14 In another meta-analysis that evaluated 5 RCTs (N=1307), S. boulardii significantly increased the H. pylori eradication rate when used as an adjunct to triple therapy (RR=1.13; 95% CI, 1.05-1.21) and reduced the rate of treatment-related adverse effects (RR=0.46; 95% CI, 0.3-0.7).13 In a third meta-analysis of 10 trials (N=1469), Lactobacillus supplementation increased H. pylori eradication rates (OR=2.1; 95% CI, 1.4-3.1) while decreasing the overall incidence of adverse effects (OR=0.3; 0.1-0.8).15
For inflammatory bowel disease, probiotics are unlikely to help
Current therapies for Crohn’s disease and ulcerative colitis, such as corticosteroids and other immunosuppressive agents, are effective but have significant adverse events.18 Researchers explored whether probiotics might help treat these diseases by improving immune response, the balance of microbes in the GI tract, and the intestinal barrier.18
Crohn’s disease. In a meta-analysis that was able to identify only one small RCT (N=11), 80% of patients receiving probiotic treatment went into remission, compared to 83% in the placebo group (OR=0.80; 95% CI, 0.04–17.20).16 Researchers concluded there was insufficient evidence for the use of probiotics for inducing remission in Crohn’s disease.
Another meta-analysis of 7 small studies (N=160) found no significant evidence supporting probiotic use for maintaining remission in Crohn’s disease compared with aminosalicylates or azathioprine.17 One small study in this review found there was a benefit to combining S. boulardii with a reduced level of standard maintenance therapy when compared to standard therapy alone, but this difference was not statistically significant.17
Ulcerative colitis. A systematic review of 4 RCTs (N=244) that compared conventional treatment alone to conventional treatment plus probiotics for remission or clinical improvement in patients with active ulcerative colitis found no significant differences between groups.18 Another meta-analysis of 4 studies (N=587) found that compared to placebo or treatment with mesalazine, probiotics had no benefit for maintaining remission in ulcerative colitis.19 The rate of relapse was 40.1% in the probiotics group compared to 34.1% in the mesalazine group. The number of adverse effects was similar in both groups.
Most evidence suggests probiotics are useful for IBS
Research suggests that imbalances in GI flora, along with subsequent dysfunction in intestinal barriers and translocation of intestinal flora, may play a role in symptoms associated with IBS, such as abdominal pain, bloating, and diarrhea/constipation.20 There are few effective therapeutic options for patients suffering with IBS.
In a systematic review of 19 RCTs (N=1650), probiotics were significantly more effective than placebo for patients with IBS, with an NNT of 4 (95% CI, 3-12.5).21 This review did not evaluate the difference between various probiotic species and strains.
In an RCT (N=122), the probiotic strain Bifidobacterium bifidum MIMBb75 was found to be safe and beneficial for treating IBS symptoms and improving patients’ quality of life.20 On a 7-point scale of global assessment of IBS symptoms, the score was reduced by 0.88 points (95% CI, 0.69-1.07) in the group that received B. bifidum MIMBb75 and 0.16 points (95% CI, -0.32-0.00) in the placebo group (P<0.0001). Almost half (47%) of the patients who received B. bifidum MIMBb75 reported adequate relief, compared to 11% in the placebo group (P<.0001).
An RCT (N=179) that compared yogurt that contained probiotics to non-probiotic yogurt found the probiotic yogurt had no benefits for treating IBS symptoms.23 After 4 weeks, 57% of patients who ate the probiotic yogurt reported adequate relief, compared to 53% of those who ate non-probiotic yogurt (P=0.71). After 8 weeks, those numbers were 47% and 68%, respectively.23
CORRESPONDENCE
Erik R. Clauson, DO, Nellis Family Medicine Residency, 99 MDOS/SGOF, 4700 Las Vegas Boulevard North, Nellis Air Force Base, NV 89191; [email protected]
1. Hempel S, Newberry SJ, Maher AR, et al. Probiotics for the prevention and treatment of antibiotic-associated diarrhea: a systematic review and meta-analysis. JAMA. 2012;307:1959-1969.
2. Procter & Gamble. Align product information. Procter & Gamble Align Web site. Available at: http://www.aligngi.com/information-on-Align-probiotic-supplement. Accessed February 13, 2015.
3. Bayer HealthCare. Phillip’s Colon Health product information. Bayer HealthCare Phillip’s Colon Health Web site. Available at: http://phillipspro.com/en/home/product-information/index.php. Accessed February 13, 2015.
4. Nature’s Bounty. Nature’s Bounty Acidophilus Probiotic product label. Nature’s Bounty Web site. Available at: http://images.vitaminimages.com/cdn/sd/pdf/L002610-NB.PDF. Accessed February 13, 2015.
5. Dannon. Activia. Dannon Activia Web site. Available at: http://activia.us.com/probiotic-yogurt/activia. Accessed February 13, 2015.
6. Lifeway. Lifeway Kefir frequently asked questions. Lifeway Kefir Web site. Available at: http://lifewaykefir.com/faq/. Accessed February 13, 2015.
7. Allen SJ, Martinez EG, Gregorio GV, et al. Probiotics for treating acute infectious diarrhoea. Cochrane Database Syst Rev. 2010;(11):CD003048.
8. McFarland LV. Meta-analysis of probiotics for the prevention of traveler’s diarrhea. Travel Med Infect Dis. 2007;5:97-105.
9. Hamad A, Fragkos KC, Forbes A. A systemic review and meta-analysis of probiotics for the management of radiation induced bowel disease. Clin Nutr. 2013;32:353-360.
10. Videlock EJ, Cremonini F. Meta-analysis: probiotics in antibiotic-associated diarrhoea. Aliment Pharmacol Ther. 2012;35:1355-1369.
11. Pillai A, Nelson RL. Probiotics for treatment of Clostridium difficile-associated colitis in adults. Cochrane Database Syst Rev. 2008;(1):CD004611.
12. Johnston BC, Ma SY, Goldenberg JZ, et al. Probiotics for the prevention of Clostridium difficile-associated diarrhea: a systematic review and meta-analysis. Ann Intern Med. 2012;157:878-888.
13. Szajewska H, Horvath A, Piwowarczyk A. Meta-analysis: the effects of Saccharomyces boulardii supplementation on Helicobacter pylori eradication rates and side effects during treatment. Aliment Pharmacol Ther. 2010;32:1069-1079.
14. Sachdeva A, Nagpal J. Effect of fermented milk-based probiotic preparations on Helicobacter pylori eradication: a systematic review and meta-analysis of randomized-controlled trials. Eur J Gastroenterol Hepatol. 2009;21:45-53.
15. Wang ZH, Gao QY, Fang JY. Meta-analysis of the efficacy and safety of Lactobacillus-containing and Bifidobacterium-containing probiotic compound preparation in Helicobacter pylori eradication therapy. J Clin Gastroenterol. 2013;47:25-32.
16. Butterworth AD, Thomas AG, Akobeng AK. Probiotics for induction of remission in Crohn’s disease. Cochrane Database Syst Rev. 2008;16:CD006634.
17. Rolfe VE, Fortun PJ, Hawkey CJ, et al. Probiotics for maintenance of remission in Crohn’s disease. Cochrane Database Syst Rev. 2006;(4):CD004826.
18. Mallon P, McKay D, Kirk SJ, et al. Probiotics for induction of remission in ulcerative colitis. Cochrane Database Syst Rev. 2007;(4):CD005573.
19. Naidoo K, Gordon M, Fagbemi AO, et al. Probiotics for maintenance of remission in ulcerative colitis. Cochrane Database Syst Rev. 2011;(12):CD007443.
20. Guglielmetti S, Mora D, Gschwender M, et al. Randomised clinical trial: Bifidobacterium bifidum MIMBb75 significantly alleviates irritable bowel syndrome and improves quality of life–– a double-blind, placebo-controlled study. Aliment Pharmacol Ther. 2011;33:1123-1132.
21. Moayyedi P, Ford AC, Talley NJ, et al. The efficacy of probiotics in the treatment of irritable bowel syndrome: a systematic review. Gut. 2010;59:325-332.
22. Allen SJ, Wareham K, Wang D, et al. Lactobacilli and bifidobacteria in the prevention of antibiotic-associated diarrhoea and Clostridium difficile diarrhoea in older inpatients (PLACIDE): a randomised, double-blind, placebo-controlled, multicentre trial. Lancet. 2013;382:1249-1257.
23. Roberts LM, McCahon D, Holder R, et al. A randomised controlled trial of a probiotic ‘functional food’ in the management of irritable bowel syndrome. BMC Gastroenterol. 2013;13:45.
› Consider probiotics for patients with acute infectious diarrhea, antibiotic-associated diarrhea, or Clostridium difficile-associated diarrhea. A
› Do not recommend probiotics for preventing or treating Crohn’s disease or ulcerative colitis. B
› Consider the probiotic Bifidobacterium bifidum MIMBb75 for patients with irritable bowel syndrome. B
Strength of recommendation (SOR)
A Good-quality patient-oriented evidence
B Inconsistent or limited-quality patient-oriented evidence
C Consensus, usual practice, opinion, disease-oriented evidence, case series
Probiotics—live micoorganisms that are consumed as supplements or food for purported health benefits—are a popular over-the-counter remedy for various gastrointestinal (GI) ailments and other conditions, but the evidence supporting their use is mixed. Probiotics interact with the normal flora of the human body. They are believed to act by multiple mechanisms to deliver beneficial effects, including providing a protective barrier, altering intestinal pH to favor the growth of nonpathogenic bacteria, enhancing the host’s immunologic response, producing antimicrobial substances, and directly competing with pathogenic bacteria for receptors in the GI tract.1 (See “The normal human intestinal flora.”)
In the United States, Lactobacillus and Bifidobacterium are the probiotic genera that are most commonly used. (For a list of the specific probiotic species found in 5 popular products, see TABLE 1.2-6) The review that follows examines the evidence for using probiotics for select GI ailments, including several types of diarrheal illnesses, inflammatory bowel disease (Crohn’s disease and ulcerative colitis), and irritable bowel syndrome (IBS). These findings are summarized in TABLE 2.1,7-21
The human body contains approximately 1014 prokaryotic organisms, with a biomass of >1 kg. Most of these organisms are indigenous and stable, although transient members such as enteric pathogens can be found.
The gastrointestinal tract is sterile at birth but is colonized immediately, and each individual has marked variations in microbial composition. The complex symbiotic relationship between the normal intestinal flora and the human host is beneficial to both. These microbes utilize complex carbohydrates undigested by the host as energy. Fermentation results in the formation of short-chain fatty acids, which can provide up to 15% of human energy requirements.
In addition to these metabolic benefits, microbial flora dampen the human inflammatory response, induce immunosuppressive T cells (Tregs), and competitively exclude pathogens.
Colonic epithelium is nourished and proliferates in the presence of normal intestinal flora. Disruption of the normal flora can cause disease.
SOURCE: Neish AS. Microbes in gastrointestinal health and disease. Gastroenterology. 2009;136:65-80.
Probiotics may help with some types of diarrhea
Acute infectious diarrhea. Viruses, bacteria, and parasites cause acute infectious diarrhea, and probiotics are thought to act against these pathogens by competing for available nutrients and pattern recognition receptors in the GI endothelium, acidifying the local environment, and increasing immune responses within the GI tract. In a meta-analysis of 63 studies (N=8014) that used multiple strains and dosages of probiotics, investigators found probiotics shortened the duration of acute infectious diarrhea by approximately 24 hours (95% confidence interval [CI], 15.9-33.6 hours).7 Probiotics also reduced both the risk of diarrhea lasting longer than 4 days (relative risk [RR]=0.41; 95% CI, 0.32-0.53) and stool frequency on Day 2 of illness (mean difference of 0.80 stools; 95% CI, 0.45-1.14).
Traveler’s diarrhea. The incidence of traveler’s diarrhea is >50% when traveling to high-risk areas such as the Middle East, North Africa, Latin America, and Southeast Asia, and 5% to 10% when traveling to areas such as North America, Northern Europe, the United Kingdom, Australia, and New Zealand.8 Traveler’s diarrhea may be caused by ingesting food and liquids contaminated with fecal material. Symptoms include diarrhea, cramps, and nausea that if untreated typically last from 2 to 6 days but can last for as long as a month.8
In a meta-analysis of 12 studies (N=5171) that evaluated various probiotic strains, researchers found probiotics effectively prevented traveler’s diarrhea in US and European travelers who visited a variety of vacation spots (pooled RR=0.85; 95% CI, 0.79-0.91).8 No serious adverse events were reported.
Radiation-induced diarrhea. Radiation treatments to the abdomen and pelvis can damage the lower GI tract and cause diarrhea. The pooled results from a meta-analysis that included 6 studies (N=1449) significantly favored the use of probiotics over placebo for decreasing the incidence of radiation-induced diarrhea (odds ratio [OR]=0.44; 95% CI, 0.21-0.92).9 Probiotics use also was associated with decreased loperamide use (OR=0.29; 95% CI, 0.01-6.80) and decreased incidence of watery stools (OR=0.36; 95% CI, 0.05-2.81), but these outcomes did not reach statistical significance.
Antibiotic-associated diarrhea. Antibiotic use has long been associated with the development of diarrheal illness, sometimes due to the acceleration of GI motility (eg, erythromycin) or by causing osmotic diarrhea by decreasing GI bacteria that assist in carbohydrate breakdown.11 A meta-analysis that evaluated 63 randomized controlled trials (RCTs) (N=11,811) showed that probiotics are effective for treating and preventing antibiotic-associated diarrhea (AAD).1 There was a statistically significant reduction in AAD among patients who received probiotics (RR=0.58; 95% CI, 0.50-0.68; number needed to treat [NNT]=13). Most of the studies in this meta-analysis used a Lactobacillus probiotic alone or in combination with another probiotic. Researchers did not analyze whether the efficacy varied by patient population, probiotic used, causative antibiotic, or duration of treatment.1
Another meta-analysis of 34 studies (N=4138) also found probiotic therapy can prevent AAD.10 The pooled RR for AAD was 0.53 (95% CI, 0.44-0.63) for patients treated with probiotics compared to placebo, with an NNT of 8 (95% CI, 7-11). The effects remained significant when results were grouped by probiotic species, patient age, and duration of antibiotic treatment. Among a subgroup of patients in this meta-analysis who were being treated for Helicobacter pylori, the pooled RR of AAD was 0.37 (95% CI, 0.20-0.69) and the NNT was 5 (95% CI, 4-10).10 However, the 2013 PLACIDE trial (N=17,420) found no significant decrease in AAD rates in hospitalized patients over age 65 years being treated with antibiotics who received probiotics (RR=1.04; 95% CI, 0.84-1.28).22
Clostridium difficile-associated diarrhea. As we know, antibiotics can disrupt the normal GI flora and permit overgrow of Clostridium difficile, which can result in C. difficile-associated diarrhea (CDAD).12 This can occur with oral, parenteral, and even topical antibiotics.11 Researchers have investigated whether probiotics can prevent this opportunistic C. difficile overgrowth.
A 2012 meta-analysis of 20 trials (N=38,180) found probiotic prophylaxis prevented CDAD in both inpatients and outpatients while not increasing the incidence of significant adverse effects.12 Probiotics decreased the incidence of CDAD by 66% (pooled RR=0.34, 95% CI, 0.24-0.49).12 Adverse events occurred in 9.3% of patients taking probiotics, compared with 12.6% of controls (RR=0.82, 95% CI, 0.65-1.05).12
Conversely, a 2008 review of 4 studies (N=336) concluded there is insufficient evidence for using probiotics to treat CDAD, either as monotherapy or adjunct therapy.11 One trial in this meta-analysis (N=124) found patients who received the probiotic Saccharomyces boulardii in addition to antibiotic therapy were significantly less likely to experience CDAD recurrence than those who received placebo (RR=0.59; 95% CI, 0.35-0.98).11 However, this benefit was not found in the other trials in this meta-analysis.11
The PLACIDE trial found probiotics did not prevent CDAD in hospitalized patients over age 65 years; 0.8% of patients who received probiotics developed CDAD, compared to 1.2% in the placebo group (RR=0.71, 95% CI, 0.34-1.47).22
Helicobacter pylori infection. The triple therapy regimen of a proton pump inhibitor plus the antibiotics clarithromycin and amoxicillin is the recommended treatment for H. pylori infection.13 Problems with this treatment include adverse effects such as diarrhea and decreased eradication rates, in part due to antibiotic resistance. Certain Lactobacillus species have been shown to inhibit or kill H. pylori in vitro,13 and evidence from several meta-analyses suggests probiotics should be an adjunct therapy when treating H. pylori.
In a meta-analysis of 10 RCTs (N=963), fermented milk-based probiotics improved H. pylori eradication rates by 5% to 15%.14 In another meta-analysis that evaluated 5 RCTs (N=1307), S. boulardii significantly increased the H. pylori eradication rate when used as an adjunct to triple therapy (RR=1.13; 95% CI, 1.05-1.21) and reduced the rate of treatment-related adverse effects (RR=0.46; 95% CI, 0.3-0.7).13 In a third meta-analysis of 10 trials (N=1469), Lactobacillus supplementation increased H. pylori eradication rates (OR=2.1; 95% CI, 1.4-3.1) while decreasing the overall incidence of adverse effects (OR=0.3; 0.1-0.8).15
For inflammatory bowel disease, probiotics are unlikely to help
Current therapies for Crohn’s disease and ulcerative colitis, such as corticosteroids and other immunosuppressive agents, are effective but have significant adverse events.18 Researchers explored whether probiotics might help treat these diseases by improving immune response, the balance of microbes in the GI tract, and the intestinal barrier.18
Crohn’s disease. In a meta-analysis that was able to identify only one small RCT (N=11), 80% of patients receiving probiotic treatment went into remission, compared to 83% in the placebo group (OR=0.80; 95% CI, 0.04–17.20).16 Researchers concluded there was insufficient evidence for the use of probiotics for inducing remission in Crohn’s disease.
Another meta-analysis of 7 small studies (N=160) found no significant evidence supporting probiotic use for maintaining remission in Crohn’s disease compared with aminosalicylates or azathioprine.17 One small study in this review found there was a benefit to combining S. boulardii with a reduced level of standard maintenance therapy when compared to standard therapy alone, but this difference was not statistically significant.17
Ulcerative colitis. A systematic review of 4 RCTs (N=244) that compared conventional treatment alone to conventional treatment plus probiotics for remission or clinical improvement in patients with active ulcerative colitis found no significant differences between groups.18 Another meta-analysis of 4 studies (N=587) found that compared to placebo or treatment with mesalazine, probiotics had no benefit for maintaining remission in ulcerative colitis.19 The rate of relapse was 40.1% in the probiotics group compared to 34.1% in the mesalazine group. The number of adverse effects was similar in both groups.
Most evidence suggests probiotics are useful for IBS
Research suggests that imbalances in GI flora, along with subsequent dysfunction in intestinal barriers and translocation of intestinal flora, may play a role in symptoms associated with IBS, such as abdominal pain, bloating, and diarrhea/constipation.20 There are few effective therapeutic options for patients suffering with IBS.
In a systematic review of 19 RCTs (N=1650), probiotics were significantly more effective than placebo for patients with IBS, with an NNT of 4 (95% CI, 3-12.5).21 This review did not evaluate the difference between various probiotic species and strains.
In an RCT (N=122), the probiotic strain Bifidobacterium bifidum MIMBb75 was found to be safe and beneficial for treating IBS symptoms and improving patients’ quality of life.20 On a 7-point scale of global assessment of IBS symptoms, the score was reduced by 0.88 points (95% CI, 0.69-1.07) in the group that received B. bifidum MIMBb75 and 0.16 points (95% CI, -0.32-0.00) in the placebo group (P<0.0001). Almost half (47%) of the patients who received B. bifidum MIMBb75 reported adequate relief, compared to 11% in the placebo group (P<.0001).
An RCT (N=179) that compared yogurt that contained probiotics to non-probiotic yogurt found the probiotic yogurt had no benefits for treating IBS symptoms.23 After 4 weeks, 57% of patients who ate the probiotic yogurt reported adequate relief, compared to 53% of those who ate non-probiotic yogurt (P=0.71). After 8 weeks, those numbers were 47% and 68%, respectively.23
CORRESPONDENCE
Erik R. Clauson, DO, Nellis Family Medicine Residency, 99 MDOS/SGOF, 4700 Las Vegas Boulevard North, Nellis Air Force Base, NV 89191; [email protected]
› Consider probiotics for patients with acute infectious diarrhea, antibiotic-associated diarrhea, or Clostridium difficile-associated diarrhea. A
› Do not recommend probiotics for preventing or treating Crohn’s disease or ulcerative colitis. B
› Consider the probiotic Bifidobacterium bifidum MIMBb75 for patients with irritable bowel syndrome. B
Strength of recommendation (SOR)
A Good-quality patient-oriented evidence
B Inconsistent or limited-quality patient-oriented evidence
C Consensus, usual practice, opinion, disease-oriented evidence, case series
Probiotics—live micoorganisms that are consumed as supplements or food for purported health benefits—are a popular over-the-counter remedy for various gastrointestinal (GI) ailments and other conditions, but the evidence supporting their use is mixed. Probiotics interact with the normal flora of the human body. They are believed to act by multiple mechanisms to deliver beneficial effects, including providing a protective barrier, altering intestinal pH to favor the growth of nonpathogenic bacteria, enhancing the host’s immunologic response, producing antimicrobial substances, and directly competing with pathogenic bacteria for receptors in the GI tract.1 (See “The normal human intestinal flora.”)
In the United States, Lactobacillus and Bifidobacterium are the probiotic genera that are most commonly used. (For a list of the specific probiotic species found in 5 popular products, see TABLE 1.2-6) The review that follows examines the evidence for using probiotics for select GI ailments, including several types of diarrheal illnesses, inflammatory bowel disease (Crohn’s disease and ulcerative colitis), and irritable bowel syndrome (IBS). These findings are summarized in TABLE 2.1,7-21
The human body contains approximately 1014 prokaryotic organisms, with a biomass of >1 kg. Most of these organisms are indigenous and stable, although transient members such as enteric pathogens can be found.
The gastrointestinal tract is sterile at birth but is colonized immediately, and each individual has marked variations in microbial composition. The complex symbiotic relationship between the normal intestinal flora and the human host is beneficial to both. These microbes utilize complex carbohydrates undigested by the host as energy. Fermentation results in the formation of short-chain fatty acids, which can provide up to 15% of human energy requirements.
In addition to these metabolic benefits, microbial flora dampen the human inflammatory response, induce immunosuppressive T cells (Tregs), and competitively exclude pathogens.
Colonic epithelium is nourished and proliferates in the presence of normal intestinal flora. Disruption of the normal flora can cause disease.
SOURCE: Neish AS. Microbes in gastrointestinal health and disease. Gastroenterology. 2009;136:65-80.
Probiotics may help with some types of diarrhea
Acute infectious diarrhea. Viruses, bacteria, and parasites cause acute infectious diarrhea, and probiotics are thought to act against these pathogens by competing for available nutrients and pattern recognition receptors in the GI endothelium, acidifying the local environment, and increasing immune responses within the GI tract. In a meta-analysis of 63 studies (N=8014) that used multiple strains and dosages of probiotics, investigators found probiotics shortened the duration of acute infectious diarrhea by approximately 24 hours (95% confidence interval [CI], 15.9-33.6 hours).7 Probiotics also reduced both the risk of diarrhea lasting longer than 4 days (relative risk [RR]=0.41; 95% CI, 0.32-0.53) and stool frequency on Day 2 of illness (mean difference of 0.80 stools; 95% CI, 0.45-1.14).
Traveler’s diarrhea. The incidence of traveler’s diarrhea is >50% when traveling to high-risk areas such as the Middle East, North Africa, Latin America, and Southeast Asia, and 5% to 10% when traveling to areas such as North America, Northern Europe, the United Kingdom, Australia, and New Zealand.8 Traveler’s diarrhea may be caused by ingesting food and liquids contaminated with fecal material. Symptoms include diarrhea, cramps, and nausea that if untreated typically last from 2 to 6 days but can last for as long as a month.8
In a meta-analysis of 12 studies (N=5171) that evaluated various probiotic strains, researchers found probiotics effectively prevented traveler’s diarrhea in US and European travelers who visited a variety of vacation spots (pooled RR=0.85; 95% CI, 0.79-0.91).8 No serious adverse events were reported.
Radiation-induced diarrhea. Radiation treatments to the abdomen and pelvis can damage the lower GI tract and cause diarrhea. The pooled results from a meta-analysis that included 6 studies (N=1449) significantly favored the use of probiotics over placebo for decreasing the incidence of radiation-induced diarrhea (odds ratio [OR]=0.44; 95% CI, 0.21-0.92).9 Probiotics use also was associated with decreased loperamide use (OR=0.29; 95% CI, 0.01-6.80) and decreased incidence of watery stools (OR=0.36; 95% CI, 0.05-2.81), but these outcomes did not reach statistical significance.
Antibiotic-associated diarrhea. Antibiotic use has long been associated with the development of diarrheal illness, sometimes due to the acceleration of GI motility (eg, erythromycin) or by causing osmotic diarrhea by decreasing GI bacteria that assist in carbohydrate breakdown.11 A meta-analysis that evaluated 63 randomized controlled trials (RCTs) (N=11,811) showed that probiotics are effective for treating and preventing antibiotic-associated diarrhea (AAD).1 There was a statistically significant reduction in AAD among patients who received probiotics (RR=0.58; 95% CI, 0.50-0.68; number needed to treat [NNT]=13). Most of the studies in this meta-analysis used a Lactobacillus probiotic alone or in combination with another probiotic. Researchers did not analyze whether the efficacy varied by patient population, probiotic used, causative antibiotic, or duration of treatment.1
Another meta-analysis of 34 studies (N=4138) also found probiotic therapy can prevent AAD.10 The pooled RR for AAD was 0.53 (95% CI, 0.44-0.63) for patients treated with probiotics compared to placebo, with an NNT of 8 (95% CI, 7-11). The effects remained significant when results were grouped by probiotic species, patient age, and duration of antibiotic treatment. Among a subgroup of patients in this meta-analysis who were being treated for Helicobacter pylori, the pooled RR of AAD was 0.37 (95% CI, 0.20-0.69) and the NNT was 5 (95% CI, 4-10).10 However, the 2013 PLACIDE trial (N=17,420) found no significant decrease in AAD rates in hospitalized patients over age 65 years being treated with antibiotics who received probiotics (RR=1.04; 95% CI, 0.84-1.28).22
Clostridium difficile-associated diarrhea. As we know, antibiotics can disrupt the normal GI flora and permit overgrow of Clostridium difficile, which can result in C. difficile-associated diarrhea (CDAD).12 This can occur with oral, parenteral, and even topical antibiotics.11 Researchers have investigated whether probiotics can prevent this opportunistic C. difficile overgrowth.
A 2012 meta-analysis of 20 trials (N=38,180) found probiotic prophylaxis prevented CDAD in both inpatients and outpatients while not increasing the incidence of significant adverse effects.12 Probiotics decreased the incidence of CDAD by 66% (pooled RR=0.34, 95% CI, 0.24-0.49).12 Adverse events occurred in 9.3% of patients taking probiotics, compared with 12.6% of controls (RR=0.82, 95% CI, 0.65-1.05).12
Conversely, a 2008 review of 4 studies (N=336) concluded there is insufficient evidence for using probiotics to treat CDAD, either as monotherapy or adjunct therapy.11 One trial in this meta-analysis (N=124) found patients who received the probiotic Saccharomyces boulardii in addition to antibiotic therapy were significantly less likely to experience CDAD recurrence than those who received placebo (RR=0.59; 95% CI, 0.35-0.98).11 However, this benefit was not found in the other trials in this meta-analysis.11
The PLACIDE trial found probiotics did not prevent CDAD in hospitalized patients over age 65 years; 0.8% of patients who received probiotics developed CDAD, compared to 1.2% in the placebo group (RR=0.71, 95% CI, 0.34-1.47).22
Helicobacter pylori infection. The triple therapy regimen of a proton pump inhibitor plus the antibiotics clarithromycin and amoxicillin is the recommended treatment for H. pylori infection.13 Problems with this treatment include adverse effects such as diarrhea and decreased eradication rates, in part due to antibiotic resistance. Certain Lactobacillus species have been shown to inhibit or kill H. pylori in vitro,13 and evidence from several meta-analyses suggests probiotics should be an adjunct therapy when treating H. pylori.
In a meta-analysis of 10 RCTs (N=963), fermented milk-based probiotics improved H. pylori eradication rates by 5% to 15%.14 In another meta-analysis that evaluated 5 RCTs (N=1307), S. boulardii significantly increased the H. pylori eradication rate when used as an adjunct to triple therapy (RR=1.13; 95% CI, 1.05-1.21) and reduced the rate of treatment-related adverse effects (RR=0.46; 95% CI, 0.3-0.7).13 In a third meta-analysis of 10 trials (N=1469), Lactobacillus supplementation increased H. pylori eradication rates (OR=2.1; 95% CI, 1.4-3.1) while decreasing the overall incidence of adverse effects (OR=0.3; 0.1-0.8).15
For inflammatory bowel disease, probiotics are unlikely to help
Current therapies for Crohn’s disease and ulcerative colitis, such as corticosteroids and other immunosuppressive agents, are effective but have significant adverse events.18 Researchers explored whether probiotics might help treat these diseases by improving immune response, the balance of microbes in the GI tract, and the intestinal barrier.18
Crohn’s disease. In a meta-analysis that was able to identify only one small RCT (N=11), 80% of patients receiving probiotic treatment went into remission, compared to 83% in the placebo group (OR=0.80; 95% CI, 0.04–17.20).16 Researchers concluded there was insufficient evidence for the use of probiotics for inducing remission in Crohn’s disease.
Another meta-analysis of 7 small studies (N=160) found no significant evidence supporting probiotic use for maintaining remission in Crohn’s disease compared with aminosalicylates or azathioprine.17 One small study in this review found there was a benefit to combining S. boulardii with a reduced level of standard maintenance therapy when compared to standard therapy alone, but this difference was not statistically significant.17
Ulcerative colitis. A systematic review of 4 RCTs (N=244) that compared conventional treatment alone to conventional treatment plus probiotics for remission or clinical improvement in patients with active ulcerative colitis found no significant differences between groups.18 Another meta-analysis of 4 studies (N=587) found that compared to placebo or treatment with mesalazine, probiotics had no benefit for maintaining remission in ulcerative colitis.19 The rate of relapse was 40.1% in the probiotics group compared to 34.1% in the mesalazine group. The number of adverse effects was similar in both groups.
Most evidence suggests probiotics are useful for IBS
Research suggests that imbalances in GI flora, along with subsequent dysfunction in intestinal barriers and translocation of intestinal flora, may play a role in symptoms associated with IBS, such as abdominal pain, bloating, and diarrhea/constipation.20 There are few effective therapeutic options for patients suffering with IBS.
In a systematic review of 19 RCTs (N=1650), probiotics were significantly more effective than placebo for patients with IBS, with an NNT of 4 (95% CI, 3-12.5).21 This review did not evaluate the difference between various probiotic species and strains.
In an RCT (N=122), the probiotic strain Bifidobacterium bifidum MIMBb75 was found to be safe and beneficial for treating IBS symptoms and improving patients’ quality of life.20 On a 7-point scale of global assessment of IBS symptoms, the score was reduced by 0.88 points (95% CI, 0.69-1.07) in the group that received B. bifidum MIMBb75 and 0.16 points (95% CI, -0.32-0.00) in the placebo group (P<0.0001). Almost half (47%) of the patients who received B. bifidum MIMBb75 reported adequate relief, compared to 11% in the placebo group (P<.0001).
An RCT (N=179) that compared yogurt that contained probiotics to non-probiotic yogurt found the probiotic yogurt had no benefits for treating IBS symptoms.23 After 4 weeks, 57% of patients who ate the probiotic yogurt reported adequate relief, compared to 53% of those who ate non-probiotic yogurt (P=0.71). After 8 weeks, those numbers were 47% and 68%, respectively.23
CORRESPONDENCE
Erik R. Clauson, DO, Nellis Family Medicine Residency, 99 MDOS/SGOF, 4700 Las Vegas Boulevard North, Nellis Air Force Base, NV 89191; [email protected]
1. Hempel S, Newberry SJ, Maher AR, et al. Probiotics for the prevention and treatment of antibiotic-associated diarrhea: a systematic review and meta-analysis. JAMA. 2012;307:1959-1969.
2. Procter & Gamble. Align product information. Procter & Gamble Align Web site. Available at: http://www.aligngi.com/information-on-Align-probiotic-supplement. Accessed February 13, 2015.
3. Bayer HealthCare. Phillip’s Colon Health product information. Bayer HealthCare Phillip’s Colon Health Web site. Available at: http://phillipspro.com/en/home/product-information/index.php. Accessed February 13, 2015.
4. Nature’s Bounty. Nature’s Bounty Acidophilus Probiotic product label. Nature’s Bounty Web site. Available at: http://images.vitaminimages.com/cdn/sd/pdf/L002610-NB.PDF. Accessed February 13, 2015.
5. Dannon. Activia. Dannon Activia Web site. Available at: http://activia.us.com/probiotic-yogurt/activia. Accessed February 13, 2015.
6. Lifeway. Lifeway Kefir frequently asked questions. Lifeway Kefir Web site. Available at: http://lifewaykefir.com/faq/. Accessed February 13, 2015.
7. Allen SJ, Martinez EG, Gregorio GV, et al. Probiotics for treating acute infectious diarrhoea. Cochrane Database Syst Rev. 2010;(11):CD003048.
8. McFarland LV. Meta-analysis of probiotics for the prevention of traveler’s diarrhea. Travel Med Infect Dis. 2007;5:97-105.
9. Hamad A, Fragkos KC, Forbes A. A systemic review and meta-analysis of probiotics for the management of radiation induced bowel disease. Clin Nutr. 2013;32:353-360.
10. Videlock EJ, Cremonini F. Meta-analysis: probiotics in antibiotic-associated diarrhoea. Aliment Pharmacol Ther. 2012;35:1355-1369.
11. Pillai A, Nelson RL. Probiotics for treatment of Clostridium difficile-associated colitis in adults. Cochrane Database Syst Rev. 2008;(1):CD004611.
12. Johnston BC, Ma SY, Goldenberg JZ, et al. Probiotics for the prevention of Clostridium difficile-associated diarrhea: a systematic review and meta-analysis. Ann Intern Med. 2012;157:878-888.
13. Szajewska H, Horvath A, Piwowarczyk A. Meta-analysis: the effects of Saccharomyces boulardii supplementation on Helicobacter pylori eradication rates and side effects during treatment. Aliment Pharmacol Ther. 2010;32:1069-1079.
14. Sachdeva A, Nagpal J. Effect of fermented milk-based probiotic preparations on Helicobacter pylori eradication: a systematic review and meta-analysis of randomized-controlled trials. Eur J Gastroenterol Hepatol. 2009;21:45-53.
15. Wang ZH, Gao QY, Fang JY. Meta-analysis of the efficacy and safety of Lactobacillus-containing and Bifidobacterium-containing probiotic compound preparation in Helicobacter pylori eradication therapy. J Clin Gastroenterol. 2013;47:25-32.
16. Butterworth AD, Thomas AG, Akobeng AK. Probiotics for induction of remission in Crohn’s disease. Cochrane Database Syst Rev. 2008;16:CD006634.
17. Rolfe VE, Fortun PJ, Hawkey CJ, et al. Probiotics for maintenance of remission in Crohn’s disease. Cochrane Database Syst Rev. 2006;(4):CD004826.
18. Mallon P, McKay D, Kirk SJ, et al. Probiotics for induction of remission in ulcerative colitis. Cochrane Database Syst Rev. 2007;(4):CD005573.
19. Naidoo K, Gordon M, Fagbemi AO, et al. Probiotics for maintenance of remission in ulcerative colitis. Cochrane Database Syst Rev. 2011;(12):CD007443.
20. Guglielmetti S, Mora D, Gschwender M, et al. Randomised clinical trial: Bifidobacterium bifidum MIMBb75 significantly alleviates irritable bowel syndrome and improves quality of life–– a double-blind, placebo-controlled study. Aliment Pharmacol Ther. 2011;33:1123-1132.
21. Moayyedi P, Ford AC, Talley NJ, et al. The efficacy of probiotics in the treatment of irritable bowel syndrome: a systematic review. Gut. 2010;59:325-332.
22. Allen SJ, Wareham K, Wang D, et al. Lactobacilli and bifidobacteria in the prevention of antibiotic-associated diarrhoea and Clostridium difficile diarrhoea in older inpatients (PLACIDE): a randomised, double-blind, placebo-controlled, multicentre trial. Lancet. 2013;382:1249-1257.
23. Roberts LM, McCahon D, Holder R, et al. A randomised controlled trial of a probiotic ‘functional food’ in the management of irritable bowel syndrome. BMC Gastroenterol. 2013;13:45.
1. Hempel S, Newberry SJ, Maher AR, et al. Probiotics for the prevention and treatment of antibiotic-associated diarrhea: a systematic review and meta-analysis. JAMA. 2012;307:1959-1969.
2. Procter & Gamble. Align product information. Procter & Gamble Align Web site. Available at: http://www.aligngi.com/information-on-Align-probiotic-supplement. Accessed February 13, 2015.
3. Bayer HealthCare. Phillip’s Colon Health product information. Bayer HealthCare Phillip’s Colon Health Web site. Available at: http://phillipspro.com/en/home/product-information/index.php. Accessed February 13, 2015.
4. Nature’s Bounty. Nature’s Bounty Acidophilus Probiotic product label. Nature’s Bounty Web site. Available at: http://images.vitaminimages.com/cdn/sd/pdf/L002610-NB.PDF. Accessed February 13, 2015.
5. Dannon. Activia. Dannon Activia Web site. Available at: http://activia.us.com/probiotic-yogurt/activia. Accessed February 13, 2015.
6. Lifeway. Lifeway Kefir frequently asked questions. Lifeway Kefir Web site. Available at: http://lifewaykefir.com/faq/. Accessed February 13, 2015.
7. Allen SJ, Martinez EG, Gregorio GV, et al. Probiotics for treating acute infectious diarrhoea. Cochrane Database Syst Rev. 2010;(11):CD003048.
8. McFarland LV. Meta-analysis of probiotics for the prevention of traveler’s diarrhea. Travel Med Infect Dis. 2007;5:97-105.
9. Hamad A, Fragkos KC, Forbes A. A systemic review and meta-analysis of probiotics for the management of radiation induced bowel disease. Clin Nutr. 2013;32:353-360.
10. Videlock EJ, Cremonini F. Meta-analysis: probiotics in antibiotic-associated diarrhoea. Aliment Pharmacol Ther. 2012;35:1355-1369.
11. Pillai A, Nelson RL. Probiotics for treatment of Clostridium difficile-associated colitis in adults. Cochrane Database Syst Rev. 2008;(1):CD004611.
12. Johnston BC, Ma SY, Goldenberg JZ, et al. Probiotics for the prevention of Clostridium difficile-associated diarrhea: a systematic review and meta-analysis. Ann Intern Med. 2012;157:878-888.
13. Szajewska H, Horvath A, Piwowarczyk A. Meta-analysis: the effects of Saccharomyces boulardii supplementation on Helicobacter pylori eradication rates and side effects during treatment. Aliment Pharmacol Ther. 2010;32:1069-1079.
14. Sachdeva A, Nagpal J. Effect of fermented milk-based probiotic preparations on Helicobacter pylori eradication: a systematic review and meta-analysis of randomized-controlled trials. Eur J Gastroenterol Hepatol. 2009;21:45-53.
15. Wang ZH, Gao QY, Fang JY. Meta-analysis of the efficacy and safety of Lactobacillus-containing and Bifidobacterium-containing probiotic compound preparation in Helicobacter pylori eradication therapy. J Clin Gastroenterol. 2013;47:25-32.
16. Butterworth AD, Thomas AG, Akobeng AK. Probiotics for induction of remission in Crohn’s disease. Cochrane Database Syst Rev. 2008;16:CD006634.
17. Rolfe VE, Fortun PJ, Hawkey CJ, et al. Probiotics for maintenance of remission in Crohn’s disease. Cochrane Database Syst Rev. 2006;(4):CD004826.
18. Mallon P, McKay D, Kirk SJ, et al. Probiotics for induction of remission in ulcerative colitis. Cochrane Database Syst Rev. 2007;(4):CD005573.
19. Naidoo K, Gordon M, Fagbemi AO, et al. Probiotics for maintenance of remission in ulcerative colitis. Cochrane Database Syst Rev. 2011;(12):CD007443.
20. Guglielmetti S, Mora D, Gschwender M, et al. Randomised clinical trial: Bifidobacterium bifidum MIMBb75 significantly alleviates irritable bowel syndrome and improves quality of life–– a double-blind, placebo-controlled study. Aliment Pharmacol Ther. 2011;33:1123-1132.
21. Moayyedi P, Ford AC, Talley NJ, et al. The efficacy of probiotics in the treatment of irritable bowel syndrome: a systematic review. Gut. 2010;59:325-332.
22. Allen SJ, Wareham K, Wang D, et al. Lactobacilli and bifidobacteria in the prevention of antibiotic-associated diarrhoea and Clostridium difficile diarrhoea in older inpatients (PLACIDE): a randomised, double-blind, placebo-controlled, multicentre trial. Lancet. 2013;382:1249-1257.
23. Roberts LM, McCahon D, Holder R, et al. A randomised controlled trial of a probiotic ‘functional food’ in the management of irritable bowel syndrome. BMC Gastroenterol. 2013;13:45.