User login
Stroke thrombolysis achieved inside an hour works best
NASHVILLE, TENN. – Fast thrombolytic treatment of acute ischemic stroke clearly helps patients, but being really, really fast is even better.
Patients treated with intravenous tissue plasminogen activator (tPA) within the first 60 minutes of their stroke onset, the putative “golden hour,” had significantly better outcomes at hospital discharge, compared with patients treated just an hour later, based on U.S. data from more than 65,000 acute ischemic stroke patients.
“In national U.S. clinical practice, treatment with intravenous tPA in the golden hour, compared with later, is associated with more frequent independent ambulation at discharge, discharge to home, and freedom from disability or dependence at discharge,” compared with patients treated at 61-180 minutes or later, Dr. Jeffrey L. Saver said at the International Stroke Conference.
“These findings support intensive efforts to accelerate patient presentation and treatment initiation, such as Target: Stroke Phase II and mobile CT ambulances, to maximize benefit of thrombolytic therapy for acute ischemic stroke,” said Dr. Saver, professor of neurology and director of the stroke center at the University of California, Los Angeles.
His study used data collected from 65,348 patients with acute ischemic stroke treated at any of 1,456 U.S. hospitals participating in the Get With the Guidelines–Stroke program during 2009-2013. Of those patients, 878 (1.3%) received tPA within the first hour following onset of their stroke, 10% within 61-90 minutes, 71% within 91-180 minutes, and 18% within 181-270 minutes.
Although the 878 patients treated within an hour of symptom onset constituted little more than 1% of all patients, the series was 10-fold larger than any prior report, which allowed a venture into “terra incognita” for insight into thrombolysis efficacy when used so early during a stroke, Dr. Saver noted. “Innovations in prehospital and emergency department systems increasingly enable intravenous tPA delivery in the first 60 minutes,” he said at the meeting, which was sponsored by the American Heart Association.
In a multivariate analysis that adjusted for many potential confounders, treatment in the first 60 minutes linked with statistically significant improvements, compared with patients treated at 1-4.5 hours, for several outcome measures at hospital discharge, including a 72% relative increase in being nondisabled – a modified Rankin score of 0 – and a 58% relative increase in being independent – a modified Rankin scale score of 0-2.
Dr. Saver highlighted how even a 30-minute drop in the time to treatment produced substantively better outcomes. The percentage of patients with a modified Rankin score of 0-1 at discharge was 38% in the 0- to 60-minute patients, 33% in those treated after 91-120 minutes had elapsed, and 28% in those treated 121-180 minutes after symptom onset.
Dr. Saver has been a consultant to BrainGate, Covidien, Grifols, and Stryker and has received research support from Covidien, Lundbeck, and Stryker.
On Twitter @mitchelzoler
NASHVILLE, TENN. – Fast thrombolytic treatment of acute ischemic stroke clearly helps patients, but being really, really fast is even better.
Patients treated with intravenous tissue plasminogen activator (tPA) within the first 60 minutes of their stroke onset, the putative “golden hour,” had significantly better outcomes at hospital discharge, compared with patients treated just an hour later, based on U.S. data from more than 65,000 acute ischemic stroke patients.
“In national U.S. clinical practice, treatment with intravenous tPA in the golden hour, compared with later, is associated with more frequent independent ambulation at discharge, discharge to home, and freedom from disability or dependence at discharge,” compared with patients treated at 61-180 minutes or later, Dr. Jeffrey L. Saver said at the International Stroke Conference.
“These findings support intensive efforts to accelerate patient presentation and treatment initiation, such as Target: Stroke Phase II and mobile CT ambulances, to maximize benefit of thrombolytic therapy for acute ischemic stroke,” said Dr. Saver, professor of neurology and director of the stroke center at the University of California, Los Angeles.
His study used data collected from 65,348 patients with acute ischemic stroke treated at any of 1,456 U.S. hospitals participating in the Get With the Guidelines–Stroke program during 2009-2013. Of those patients, 878 (1.3%) received tPA within the first hour following onset of their stroke, 10% within 61-90 minutes, 71% within 91-180 minutes, and 18% within 181-270 minutes.
Although the 878 patients treated within an hour of symptom onset constituted little more than 1% of all patients, the series was 10-fold larger than any prior report, which allowed a venture into “terra incognita” for insight into thrombolysis efficacy when used so early during a stroke, Dr. Saver noted. “Innovations in prehospital and emergency department systems increasingly enable intravenous tPA delivery in the first 60 minutes,” he said at the meeting, which was sponsored by the American Heart Association.
In a multivariate analysis that adjusted for many potential confounders, treatment in the first 60 minutes linked with statistically significant improvements, compared with patients treated at 1-4.5 hours, for several outcome measures at hospital discharge, including a 72% relative increase in being nondisabled – a modified Rankin score of 0 – and a 58% relative increase in being independent – a modified Rankin scale score of 0-2.
Dr. Saver highlighted how even a 30-minute drop in the time to treatment produced substantively better outcomes. The percentage of patients with a modified Rankin score of 0-1 at discharge was 38% in the 0- to 60-minute patients, 33% in those treated after 91-120 minutes had elapsed, and 28% in those treated 121-180 minutes after symptom onset.
Dr. Saver has been a consultant to BrainGate, Covidien, Grifols, and Stryker and has received research support from Covidien, Lundbeck, and Stryker.
On Twitter @mitchelzoler
NASHVILLE, TENN. – Fast thrombolytic treatment of acute ischemic stroke clearly helps patients, but being really, really fast is even better.
Patients treated with intravenous tissue plasminogen activator (tPA) within the first 60 minutes of their stroke onset, the putative “golden hour,” had significantly better outcomes at hospital discharge, compared with patients treated just an hour later, based on U.S. data from more than 65,000 acute ischemic stroke patients.
“In national U.S. clinical practice, treatment with intravenous tPA in the golden hour, compared with later, is associated with more frequent independent ambulation at discharge, discharge to home, and freedom from disability or dependence at discharge,” compared with patients treated at 61-180 minutes or later, Dr. Jeffrey L. Saver said at the International Stroke Conference.
“These findings support intensive efforts to accelerate patient presentation and treatment initiation, such as Target: Stroke Phase II and mobile CT ambulances, to maximize benefit of thrombolytic therapy for acute ischemic stroke,” said Dr. Saver, professor of neurology and director of the stroke center at the University of California, Los Angeles.
His study used data collected from 65,348 patients with acute ischemic stroke treated at any of 1,456 U.S. hospitals participating in the Get With the Guidelines–Stroke program during 2009-2013. Of those patients, 878 (1.3%) received tPA within the first hour following onset of their stroke, 10% within 61-90 minutes, 71% within 91-180 minutes, and 18% within 181-270 minutes.
Although the 878 patients treated within an hour of symptom onset constituted little more than 1% of all patients, the series was 10-fold larger than any prior report, which allowed a venture into “terra incognita” for insight into thrombolysis efficacy when used so early during a stroke, Dr. Saver noted. “Innovations in prehospital and emergency department systems increasingly enable intravenous tPA delivery in the first 60 minutes,” he said at the meeting, which was sponsored by the American Heart Association.
In a multivariate analysis that adjusted for many potential confounders, treatment in the first 60 minutes linked with statistically significant improvements, compared with patients treated at 1-4.5 hours, for several outcome measures at hospital discharge, including a 72% relative increase in being nondisabled – a modified Rankin score of 0 – and a 58% relative increase in being independent – a modified Rankin scale score of 0-2.
Dr. Saver highlighted how even a 30-minute drop in the time to treatment produced substantively better outcomes. The percentage of patients with a modified Rankin score of 0-1 at discharge was 38% in the 0- to 60-minute patients, 33% in those treated after 91-120 minutes had elapsed, and 28% in those treated 121-180 minutes after symptom onset.
Dr. Saver has been a consultant to BrainGate, Covidien, Grifols, and Stryker and has received research support from Covidien, Lundbeck, and Stryker.
On Twitter @mitchelzoler
AT THE INTERNATIONAL STROKE CONFERENCE
Key clinical point: Acute ischemic stroke patients who started intravenous tPA within an hour of onset had the best outcomes.
Major finding: Stroke thrombolysis within an hour produced 72% more nondisabled patients at hospital discharge, compared with treatment after 1-4.5 hours.
Data source: Review of 65,348 U.S. acute ischemic patients who arrived at hospitals participating in Get With the Guidelines during 2009-2013.
Disclosures: Dr. Saver has been a consultant to BrainGate, Covidien, Grifols, and Stryker and has received research support from Covidien, Lundbeck, and Stryker.
Aneurysmal Bone Cyst Involving the Metacarpal Bone in a Child
Less than 5% of aneurysmal bone cysts (ABCs) are located in the hand,1 and only a few cases have been reported in the literature.2-7 Unfortunately, it is impossible to predict when an ABC will exhibit aggressive behavior.4,8 Aneurysmal bone cysts and giant cell bone tumors have been considered benign9 lesions that can behave in a locally aggressive fashion.1 Optimal treatment has not been established because treatment is variable depending on the condition of the lesion. Several authors have recommended more radical treatment modalities, such as en bloc resection or excision diaphysectomy followed by strut bone grafting, which had a relatively low rate of recurrence. A relatively low rate of recurrence and other complications indicate that those techniques would serve as a good strategy for patients with expansile hand ABCs in terms of safety, simplicity, and reduced number of reoperations.3,7,10
This article reports a case of an ABC of the second metacarpal bone of the right hand in a 12-year-old boy treated with curettage and autologous morselized iliac bone grafting. The patient’s guardian provided written informed consent for print and electronic publication of this case report.
Case Report
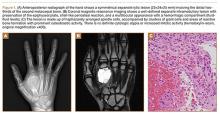
The patient was a right hand–dominant 12-year-old-boy, who noticed the development of a lump in the dorsum of his right hand. On examination, we found a large, firm swelling of the dorsum of his right hand over the second metacarpal. Radiographic examination showed a symmetrical expansile lytic lesion (22×24×25 mm) involving the entire second metacarpal bone (Figure 1A). Magnetic resonance imaging (MRI) showed a well-defined expansile intramedullary lesion with preservation of the epiphyseal plate, shell-like periosteal reaction, and a multilocular appearance with a hemorrhagic compartment (fluid-fluid levels) (Figure 1B).
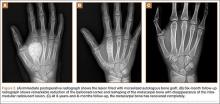
At surgery, we found a blood-filled cyst, and the cortex was very thin. The lesion extended to the distal two-thirds of the bone to the level of the physeal plate. We had considered using allograft or other bone substitutes. However, we did not have confidence in the bone-induction potential and power of osteogenesis of bone substitutes or allograft compared with autologous bone graft. Consequently, we performed autologous bone grafting, despite its being an invasive procedure, on the immature iliac crest. We performed thorough curettage of the intramedullary material without damaging the physeal plate, followed by impact morselized autologous bone grafting. Histologic examination confirmed that the final diagnosis was identical to the provisional diagnosis shown on MRI (Figure 1C). A thumb spica cast was applied for 4 weeks after surgery, and regular follow-up radiographs were taken for 3 years and 6 months until confirmation of complete normalization of the lesion without recurrence (Figures 2A-2C).
Discussion
Primary ABCs in the small tubular bones of the hands are rare. Less than 5% of aneurysmal cysts are located in the hand.1 Only a few small cases of this condition have been reported in the literature.2-7 Radiographic examination showed that, in all cases, the lesion was both expansile and completely lucent.7 Although radiographic finding of ABC in short tubular bone characteristically shows central symmetry with expansion into the diaphysis and subarticular bone, the appearance of an ABC on radiographs and angiograms is usually not diagnostic.8 Even though fluid-fluid levels are highly suggestive of ABC, only pathologic study confirms the diagnosis. MRI may be a good tool for postsurgery follow-up. On the basis of these ideas, we performed histological examination and confirmed the diagnosis of ABC of the metacarpus by radiograph and MRI.
The goals in the treatment of primary ABCs are preservation of function and avoidance of recurrence. Unfortunately, it is impossible to predict the possible aggressive behavior in ABCs. Active or aggressive character in certain localizations of ABC in children requires either curettage, which has a considerable recurrence rate, or radical segmental excision, which raises complex reconstructive challenges. Frassica and colleagues7 reported no recurrences in 3 patients treated by complete excision and bone grafting. Curettage and bone grafting in 7 cases were associated with 4 recurrences.7
Because optimal treatment has not been established,3 current recommendations vary, depending on the condition of the lesion. Several authors recommend more radical treatment modalities, such as en bloc resection, excision diaphysectomy, cryotherapy, and strut bone grafting, and a relatively low rate of recurrence and other complications indicates that those techniques would serve as a good strategy for patients with expansile ABCs in the hand.3,7,10 On the other hand, successful results with less aggressive procedures, such as curettage and autologous bone grafting, have been reported.4,5,8
In pediatric patients, surgery to preserve the growth plate is recommended.5 Ropars and colleagues4 suggested that aggressive treatment approaches, such as cryotherapy and resection with reconstruction, should be used only in cases when the articular surface is involved, when full-bone invasion of the phalanx or metacarpal has occurred, or in cases of more than 1 recurrence.
In conclusion, despite the high risk of recurrence of ABC treated with curettage with bone grafting, the findings of the present case show that ABC of the metacarpal bone in children can be treated successfully with curettage followed by morselized autologous bone grafting without recurrence.
1. Athanasian EA. Aneurysmal bone cyst and giant cell tumor of bone of the hand and distal radius. Hand Clin. 2004;20(3):269-281, vi.
2. Tarazona-Velutini P, Romo-Rodriguez R, Saleme-Cruz J. Aneurysmatic bone cyst in the proximal phalanx of a finger. Case report and literature review. Acta Ortop Mex. 2012;26(4):245-249.
3. Jafari D, Jamshidi K, Najdmazhar F, Shariatzade H, Liaghat O. Expansile aneurysmal bone cyst in the tubular bones of the hand treated with en bloc excision and autograft reconstruction: a report of 12 cases. J Hand Surg Eur Vol. 2011;36(8):648-655.
4. Ropars M, Kaila R, Briggs T, Cannon S. Aneurysmal bone cysts of the metacarpals and phalanges of the hand. A 6 case series and literature review. Chir Main. 2007;26(4-5):214-217.
5. Sproule JA, Salmo E, Mortimer G, O’Sullivan M. Aneursymal bone cyst of the proximal phalanx of the thumb in a child. Hand Surg. 2002;7(1):147-150.
6. Schwartz GB, Hammerman MZ. Aneurysmal bone cyst of the fifth metacarpal. Orthop Rev. 1989;18(12):1309-1314.
7. Frassica FJ, Amadio PC, Wold LE, Beabout JW. Aneurysmal bone cyst: clinicopathologic features and treatment of ten cases involving the hand. J Hand Surg Am. 1988;13(5):676-683.
8. Louahem D, Kouyoumdjian P, Ghanem I, et al. Active aneurysmal bone cysts in children: possible evolution after biopsy. J Child Orthop. 2012;6(4):333-338.
9. Lindfors NC. Treatment of a recurrent aneurysmal bone cyst with bioactive glass in a child allows for good bone remodelling and growth. Bone. 2009;45(2):398-400.
10. Salon A, Rémi J, Brunelle F, Drapé JL, Glorion Ch. Total replacement of a middle phalanx by free non-vascularized chondral graft, after failure of sclerotherapy for treatment of an aneurysmal bone cyst. Chir Main. 2005;24(3-4):187-192.
Less than 5% of aneurysmal bone cysts (ABCs) are located in the hand,1 and only a few cases have been reported in the literature.2-7 Unfortunately, it is impossible to predict when an ABC will exhibit aggressive behavior.4,8 Aneurysmal bone cysts and giant cell bone tumors have been considered benign9 lesions that can behave in a locally aggressive fashion.1 Optimal treatment has not been established because treatment is variable depending on the condition of the lesion. Several authors have recommended more radical treatment modalities, such as en bloc resection or excision diaphysectomy followed by strut bone grafting, which had a relatively low rate of recurrence. A relatively low rate of recurrence and other complications indicate that those techniques would serve as a good strategy for patients with expansile hand ABCs in terms of safety, simplicity, and reduced number of reoperations.3,7,10
This article reports a case of an ABC of the second metacarpal bone of the right hand in a 12-year-old boy treated with curettage and autologous morselized iliac bone grafting. The patient’s guardian provided written informed consent for print and electronic publication of this case report.
Case Report
The patient was a right hand–dominant 12-year-old-boy, who noticed the development of a lump in the dorsum of his right hand. On examination, we found a large, firm swelling of the dorsum of his right hand over the second metacarpal. Radiographic examination showed a symmetrical expansile lytic lesion (22×24×25 mm) involving the entire second metacarpal bone (Figure 1A). Magnetic resonance imaging (MRI) showed a well-defined expansile intramedullary lesion with preservation of the epiphyseal plate, shell-like periosteal reaction, and a multilocular appearance with a hemorrhagic compartment (fluid-fluid levels) (Figure 1B).
At surgery, we found a blood-filled cyst, and the cortex was very thin. The lesion extended to the distal two-thirds of the bone to the level of the physeal plate. We had considered using allograft or other bone substitutes. However, we did not have confidence in the bone-induction potential and power of osteogenesis of bone substitutes or allograft compared with autologous bone graft. Consequently, we performed autologous bone grafting, despite its being an invasive procedure, on the immature iliac crest. We performed thorough curettage of the intramedullary material without damaging the physeal plate, followed by impact morselized autologous bone grafting. Histologic examination confirmed that the final diagnosis was identical to the provisional diagnosis shown on MRI (Figure 1C). A thumb spica cast was applied for 4 weeks after surgery, and regular follow-up radiographs were taken for 3 years and 6 months until confirmation of complete normalization of the lesion without recurrence (Figures 2A-2C).
Discussion
Primary ABCs in the small tubular bones of the hands are rare. Less than 5% of aneurysmal cysts are located in the hand.1 Only a few small cases of this condition have been reported in the literature.2-7 Radiographic examination showed that, in all cases, the lesion was both expansile and completely lucent.7 Although radiographic finding of ABC in short tubular bone characteristically shows central symmetry with expansion into the diaphysis and subarticular bone, the appearance of an ABC on radiographs and angiograms is usually not diagnostic.8 Even though fluid-fluid levels are highly suggestive of ABC, only pathologic study confirms the diagnosis. MRI may be a good tool for postsurgery follow-up. On the basis of these ideas, we performed histological examination and confirmed the diagnosis of ABC of the metacarpus by radiograph and MRI.
The goals in the treatment of primary ABCs are preservation of function and avoidance of recurrence. Unfortunately, it is impossible to predict the possible aggressive behavior in ABCs. Active or aggressive character in certain localizations of ABC in children requires either curettage, which has a considerable recurrence rate, or radical segmental excision, which raises complex reconstructive challenges. Frassica and colleagues7 reported no recurrences in 3 patients treated by complete excision and bone grafting. Curettage and bone grafting in 7 cases were associated with 4 recurrences.7
Because optimal treatment has not been established,3 current recommendations vary, depending on the condition of the lesion. Several authors recommend more radical treatment modalities, such as en bloc resection, excision diaphysectomy, cryotherapy, and strut bone grafting, and a relatively low rate of recurrence and other complications indicates that those techniques would serve as a good strategy for patients with expansile ABCs in the hand.3,7,10 On the other hand, successful results with less aggressive procedures, such as curettage and autologous bone grafting, have been reported.4,5,8
In pediatric patients, surgery to preserve the growth plate is recommended.5 Ropars and colleagues4 suggested that aggressive treatment approaches, such as cryotherapy and resection with reconstruction, should be used only in cases when the articular surface is involved, when full-bone invasion of the phalanx or metacarpal has occurred, or in cases of more than 1 recurrence.
In conclusion, despite the high risk of recurrence of ABC treated with curettage with bone grafting, the findings of the present case show that ABC of the metacarpal bone in children can be treated successfully with curettage followed by morselized autologous bone grafting without recurrence.
Less than 5% of aneurysmal bone cysts (ABCs) are located in the hand,1 and only a few cases have been reported in the literature.2-7 Unfortunately, it is impossible to predict when an ABC will exhibit aggressive behavior.4,8 Aneurysmal bone cysts and giant cell bone tumors have been considered benign9 lesions that can behave in a locally aggressive fashion.1 Optimal treatment has not been established because treatment is variable depending on the condition of the lesion. Several authors have recommended more radical treatment modalities, such as en bloc resection or excision diaphysectomy followed by strut bone grafting, which had a relatively low rate of recurrence. A relatively low rate of recurrence and other complications indicate that those techniques would serve as a good strategy for patients with expansile hand ABCs in terms of safety, simplicity, and reduced number of reoperations.3,7,10
This article reports a case of an ABC of the second metacarpal bone of the right hand in a 12-year-old boy treated with curettage and autologous morselized iliac bone grafting. The patient’s guardian provided written informed consent for print and electronic publication of this case report.
Case Report
The patient was a right hand–dominant 12-year-old-boy, who noticed the development of a lump in the dorsum of his right hand. On examination, we found a large, firm swelling of the dorsum of his right hand over the second metacarpal. Radiographic examination showed a symmetrical expansile lytic lesion (22×24×25 mm) involving the entire second metacarpal bone (Figure 1A). Magnetic resonance imaging (MRI) showed a well-defined expansile intramedullary lesion with preservation of the epiphyseal plate, shell-like periosteal reaction, and a multilocular appearance with a hemorrhagic compartment (fluid-fluid levels) (Figure 1B).
At surgery, we found a blood-filled cyst, and the cortex was very thin. The lesion extended to the distal two-thirds of the bone to the level of the physeal plate. We had considered using allograft or other bone substitutes. However, we did not have confidence in the bone-induction potential and power of osteogenesis of bone substitutes or allograft compared with autologous bone graft. Consequently, we performed autologous bone grafting, despite its being an invasive procedure, on the immature iliac crest. We performed thorough curettage of the intramedullary material without damaging the physeal plate, followed by impact morselized autologous bone grafting. Histologic examination confirmed that the final diagnosis was identical to the provisional diagnosis shown on MRI (Figure 1C). A thumb spica cast was applied for 4 weeks after surgery, and regular follow-up radiographs were taken for 3 years and 6 months until confirmation of complete normalization of the lesion without recurrence (Figures 2A-2C).
Discussion
Primary ABCs in the small tubular bones of the hands are rare. Less than 5% of aneurysmal cysts are located in the hand.1 Only a few small cases of this condition have been reported in the literature.2-7 Radiographic examination showed that, in all cases, the lesion was both expansile and completely lucent.7 Although radiographic finding of ABC in short tubular bone characteristically shows central symmetry with expansion into the diaphysis and subarticular bone, the appearance of an ABC on radiographs and angiograms is usually not diagnostic.8 Even though fluid-fluid levels are highly suggestive of ABC, only pathologic study confirms the diagnosis. MRI may be a good tool for postsurgery follow-up. On the basis of these ideas, we performed histological examination and confirmed the diagnosis of ABC of the metacarpus by radiograph and MRI.
The goals in the treatment of primary ABCs are preservation of function and avoidance of recurrence. Unfortunately, it is impossible to predict the possible aggressive behavior in ABCs. Active or aggressive character in certain localizations of ABC in children requires either curettage, which has a considerable recurrence rate, or radical segmental excision, which raises complex reconstructive challenges. Frassica and colleagues7 reported no recurrences in 3 patients treated by complete excision and bone grafting. Curettage and bone grafting in 7 cases were associated with 4 recurrences.7
Because optimal treatment has not been established,3 current recommendations vary, depending on the condition of the lesion. Several authors recommend more radical treatment modalities, such as en bloc resection, excision diaphysectomy, cryotherapy, and strut bone grafting, and a relatively low rate of recurrence and other complications indicates that those techniques would serve as a good strategy for patients with expansile ABCs in the hand.3,7,10 On the other hand, successful results with less aggressive procedures, such as curettage and autologous bone grafting, have been reported.4,5,8
In pediatric patients, surgery to preserve the growth plate is recommended.5 Ropars and colleagues4 suggested that aggressive treatment approaches, such as cryotherapy and resection with reconstruction, should be used only in cases when the articular surface is involved, when full-bone invasion of the phalanx or metacarpal has occurred, or in cases of more than 1 recurrence.
In conclusion, despite the high risk of recurrence of ABC treated with curettage with bone grafting, the findings of the present case show that ABC of the metacarpal bone in children can be treated successfully with curettage followed by morselized autologous bone grafting without recurrence.
1. Athanasian EA. Aneurysmal bone cyst and giant cell tumor of bone of the hand and distal radius. Hand Clin. 2004;20(3):269-281, vi.
2. Tarazona-Velutini P, Romo-Rodriguez R, Saleme-Cruz J. Aneurysmatic bone cyst in the proximal phalanx of a finger. Case report and literature review. Acta Ortop Mex. 2012;26(4):245-249.
3. Jafari D, Jamshidi K, Najdmazhar F, Shariatzade H, Liaghat O. Expansile aneurysmal bone cyst in the tubular bones of the hand treated with en bloc excision and autograft reconstruction: a report of 12 cases. J Hand Surg Eur Vol. 2011;36(8):648-655.
4. Ropars M, Kaila R, Briggs T, Cannon S. Aneurysmal bone cysts of the metacarpals and phalanges of the hand. A 6 case series and literature review. Chir Main. 2007;26(4-5):214-217.
5. Sproule JA, Salmo E, Mortimer G, O’Sullivan M. Aneursymal bone cyst of the proximal phalanx of the thumb in a child. Hand Surg. 2002;7(1):147-150.
6. Schwartz GB, Hammerman MZ. Aneurysmal bone cyst of the fifth metacarpal. Orthop Rev. 1989;18(12):1309-1314.
7. Frassica FJ, Amadio PC, Wold LE, Beabout JW. Aneurysmal bone cyst: clinicopathologic features and treatment of ten cases involving the hand. J Hand Surg Am. 1988;13(5):676-683.
8. Louahem D, Kouyoumdjian P, Ghanem I, et al. Active aneurysmal bone cysts in children: possible evolution after biopsy. J Child Orthop. 2012;6(4):333-338.
9. Lindfors NC. Treatment of a recurrent aneurysmal bone cyst with bioactive glass in a child allows for good bone remodelling and growth. Bone. 2009;45(2):398-400.
10. Salon A, Rémi J, Brunelle F, Drapé JL, Glorion Ch. Total replacement of a middle phalanx by free non-vascularized chondral graft, after failure of sclerotherapy for treatment of an aneurysmal bone cyst. Chir Main. 2005;24(3-4):187-192.
1. Athanasian EA. Aneurysmal bone cyst and giant cell tumor of bone of the hand and distal radius. Hand Clin. 2004;20(3):269-281, vi.
2. Tarazona-Velutini P, Romo-Rodriguez R, Saleme-Cruz J. Aneurysmatic bone cyst in the proximal phalanx of a finger. Case report and literature review. Acta Ortop Mex. 2012;26(4):245-249.
3. Jafari D, Jamshidi K, Najdmazhar F, Shariatzade H, Liaghat O. Expansile aneurysmal bone cyst in the tubular bones of the hand treated with en bloc excision and autograft reconstruction: a report of 12 cases. J Hand Surg Eur Vol. 2011;36(8):648-655.
4. Ropars M, Kaila R, Briggs T, Cannon S. Aneurysmal bone cysts of the metacarpals and phalanges of the hand. A 6 case series and literature review. Chir Main. 2007;26(4-5):214-217.
5. Sproule JA, Salmo E, Mortimer G, O’Sullivan M. Aneursymal bone cyst of the proximal phalanx of the thumb in a child. Hand Surg. 2002;7(1):147-150.
6. Schwartz GB, Hammerman MZ. Aneurysmal bone cyst of the fifth metacarpal. Orthop Rev. 1989;18(12):1309-1314.
7. Frassica FJ, Amadio PC, Wold LE, Beabout JW. Aneurysmal bone cyst: clinicopathologic features and treatment of ten cases involving the hand. J Hand Surg Am. 1988;13(5):676-683.
8. Louahem D, Kouyoumdjian P, Ghanem I, et al. Active aneurysmal bone cysts in children: possible evolution after biopsy. J Child Orthop. 2012;6(4):333-338.
9. Lindfors NC. Treatment of a recurrent aneurysmal bone cyst with bioactive glass in a child allows for good bone remodelling and growth. Bone. 2009;45(2):398-400.
10. Salon A, Rémi J, Brunelle F, Drapé JL, Glorion Ch. Total replacement of a middle phalanx by free non-vascularized chondral graft, after failure of sclerotherapy for treatment of an aneurysmal bone cyst. Chir Main. 2005;24(3-4):187-192.
Team-based care: Worth a second look
Team care is not a new idea. For many years, our office teams have included physicians, nurse practitioners, physician assistants, nurses, medical assistants, front office staff, and administrative staff who functioned quite well in caring for our patients.
But primary care changed drastically after the publication of 2 landmark Institute of Medicine reports: To Err is Human: Building a Safer Health System1 (in 1999) and Crossing the Quality Chasm: A New Health System for the 21st Century2 (in 2001). These scathing reports told us we were providing inadequate care to our patients, and they contained plenty of truth. What followed is that expectations increased exponentially, and we found our offices were not prepared to deal with the new mandates for computerized medical records, high performance on quality and patient satisfaction measures, and population management.
Addressing these expanded expectations requires redefining roles and adding new players to our office teams, including nurse care coordinators, “navigators,” clinical pharmacists, psychologists, information technologists, and who knows what else. One innovative role that has seen limited testing is what some call practice facilitators.3 These are trained agents who do some of the heavy lifting required to change things like office systems and work flow.
I think that expanding the role of nurses and medical assistants is one of best ways to ensure that all of our patients get the care they deserve. Each office is unique, however, and physicians need to do the hard work of selecting the best team configuration to care for their patients. One of the more successful team-based practices is the Nuka System of Care in Alaska, which was crafted in collaboration with the tribal council. Read this fascinating story at http://www.ncbi.nlm.nih.gov/pmc/articles/PMC3752290 and then create your own story of a successful, high-quality primary care office.
1. Kohn LT, Corrigan JM, Donaldson MS (eds); Committee on Quality of Health Care in America, Institute of Medicine. To Err is Human: Building a Safer Health System. Washington, DC: National Academy Press; 1999.
2. Committee on Quality of Health Care in America; Institute of Medicine. Crossing the Quality Chasm: A New Health System for the 21st Century. Washington, DC: National Academy Press; 2001.
3. Nagykaldi Z, Mold JW, Aspy CB. Practice facilitators: a review of the literature. Fam Med. 2005;37:581-588.
Team care is not a new idea. For many years, our office teams have included physicians, nurse practitioners, physician assistants, nurses, medical assistants, front office staff, and administrative staff who functioned quite well in caring for our patients.
But primary care changed drastically after the publication of 2 landmark Institute of Medicine reports: To Err is Human: Building a Safer Health System1 (in 1999) and Crossing the Quality Chasm: A New Health System for the 21st Century2 (in 2001). These scathing reports told us we were providing inadequate care to our patients, and they contained plenty of truth. What followed is that expectations increased exponentially, and we found our offices were not prepared to deal with the new mandates for computerized medical records, high performance on quality and patient satisfaction measures, and population management.
Addressing these expanded expectations requires redefining roles and adding new players to our office teams, including nurse care coordinators, “navigators,” clinical pharmacists, psychologists, information technologists, and who knows what else. One innovative role that has seen limited testing is what some call practice facilitators.3 These are trained agents who do some of the heavy lifting required to change things like office systems and work flow.
I think that expanding the role of nurses and medical assistants is one of best ways to ensure that all of our patients get the care they deserve. Each office is unique, however, and physicians need to do the hard work of selecting the best team configuration to care for their patients. One of the more successful team-based practices is the Nuka System of Care in Alaska, which was crafted in collaboration with the tribal council. Read this fascinating story at http://www.ncbi.nlm.nih.gov/pmc/articles/PMC3752290 and then create your own story of a successful, high-quality primary care office.
Team care is not a new idea. For many years, our office teams have included physicians, nurse practitioners, physician assistants, nurses, medical assistants, front office staff, and administrative staff who functioned quite well in caring for our patients.
But primary care changed drastically after the publication of 2 landmark Institute of Medicine reports: To Err is Human: Building a Safer Health System1 (in 1999) and Crossing the Quality Chasm: A New Health System for the 21st Century2 (in 2001). These scathing reports told us we were providing inadequate care to our patients, and they contained plenty of truth. What followed is that expectations increased exponentially, and we found our offices were not prepared to deal with the new mandates for computerized medical records, high performance on quality and patient satisfaction measures, and population management.
Addressing these expanded expectations requires redefining roles and adding new players to our office teams, including nurse care coordinators, “navigators,” clinical pharmacists, psychologists, information technologists, and who knows what else. One innovative role that has seen limited testing is what some call practice facilitators.3 These are trained agents who do some of the heavy lifting required to change things like office systems and work flow.
I think that expanding the role of nurses and medical assistants is one of best ways to ensure that all of our patients get the care they deserve. Each office is unique, however, and physicians need to do the hard work of selecting the best team configuration to care for their patients. One of the more successful team-based practices is the Nuka System of Care in Alaska, which was crafted in collaboration with the tribal council. Read this fascinating story at http://www.ncbi.nlm.nih.gov/pmc/articles/PMC3752290 and then create your own story of a successful, high-quality primary care office.
1. Kohn LT, Corrigan JM, Donaldson MS (eds); Committee on Quality of Health Care in America, Institute of Medicine. To Err is Human: Building a Safer Health System. Washington, DC: National Academy Press; 1999.
2. Committee on Quality of Health Care in America; Institute of Medicine. Crossing the Quality Chasm: A New Health System for the 21st Century. Washington, DC: National Academy Press; 2001.
3. Nagykaldi Z, Mold JW, Aspy CB. Practice facilitators: a review of the literature. Fam Med. 2005;37:581-588.
1. Kohn LT, Corrigan JM, Donaldson MS (eds); Committee on Quality of Health Care in America, Institute of Medicine. To Err is Human: Building a Safer Health System. Washington, DC: National Academy Press; 1999.
2. Committee on Quality of Health Care in America; Institute of Medicine. Crossing the Quality Chasm: A New Health System for the 21st Century. Washington, DC: National Academy Press; 2001.
3. Nagykaldi Z, Mold JW, Aspy CB. Practice facilitators: a review of the literature. Fam Med. 2005;37:581-588.
Vasomotor symptoms of menopause often persist longer than 7 years
Frequent menopausal vasomotor symptoms (VMS), including hot flashes and night sweats, lasted longer than 7 years during the transition to menopause for more than 50% of women in the Study of Women’s Health Across the Nation (SWAN).1 Among the factors related to a longer duration of VMS:
- younger age
- African American heritage
- lower educational level
- greater perceived stress and symptom sensitivity
- higher depressive symptoms and anxiety at the first report of VMS.
Details of the study
Avis and colleagues analyzed data from SWAN, a multiracial/multiethnic study of women transitioning to menopause that was conducted from February 1996 through April 2013. The analyses included 1,449 women with frequent VMS (ie, occurring at least 6 days in the previous 2 weeks).
Baseline eligibility was age between 42 and 52 years, an intact uterus and at least one ovary, report of a menstrual cycle in the 3 months before screening, absence of pregnancy and lactation, and no use of oral contraceptives or hormone therapy (HT). Women were assessed in person at baseline and approximately annually over the course of the study (mean and maximum follow-up durations were 12.7 and 17.2 years, respectively).
The main outcomes were total VMS duration (in years) and persistence of VMS (in years) beyond the final menstrual period (FMP).
Among the findings:
- The unadjusted median total VMS duration was 7.4 years
- Women who were premenopausal or early perimenopausal when they first reported frequent VMS had the longest total duration of VMS (median, >11.8 years) and longest persistence of VMS beyond the FMP (median, 9.4 years)
- Women who were postmenopausal at the onset of VMS had the shortest total VMS duration after the FMP (median, 3.4 years; P<.001).
- The median total VMS duration varied significantly by race, with African American women reporting the longest total VMS duration (median, 10.1 years) and Japanese and Chinese women reporting the shortest total duration (median, 4.8 and 5.4 years, respectively). Non-Hispanic white women had a median total VMS duration of 6.5 years; among Hispanic women, the median was 8.9 years.
Key takeaway
“These findings can help health-care professionals counsel patients about expectations regarding VMS and assist women in making treatment decisions based on the probability of their VMS persisting,” Avis and colleagues concluded. “In addition, the median total duration of 7.4 years highlights the limitations of guidance recommending short-term HT use and emphasizes the need to identify safe long-term therapies for the treatment of VMS.”
SWAN is the largest and longest longitudinal study to date to report on total duration of VMS and their persistence beyond the FMP.
“More than 50% of midlife women experience frequent VMS, yet clinical guidelines typically underestimate their true duration,” Avis and colleagues observed.
Reference
- Avis NE, Crawford SL, Greendale G, et al; Study of Women’s Health Across the Nation (SWAN). Duration of menopausal symptoms over the menopause transition [published online ahead of print February 16, 2015]. JAMA Intern Med. doi:10.1001/jamainternmed.2014.8063.
Frequent menopausal vasomotor symptoms (VMS), including hot flashes and night sweats, lasted longer than 7 years during the transition to menopause for more than 50% of women in the Study of Women’s Health Across the Nation (SWAN).1 Among the factors related to a longer duration of VMS:
- younger age
- African American heritage
- lower educational level
- greater perceived stress and symptom sensitivity
- higher depressive symptoms and anxiety at the first report of VMS.
Details of the study
Avis and colleagues analyzed data from SWAN, a multiracial/multiethnic study of women transitioning to menopause that was conducted from February 1996 through April 2013. The analyses included 1,449 women with frequent VMS (ie, occurring at least 6 days in the previous 2 weeks).
Baseline eligibility was age between 42 and 52 years, an intact uterus and at least one ovary, report of a menstrual cycle in the 3 months before screening, absence of pregnancy and lactation, and no use of oral contraceptives or hormone therapy (HT). Women were assessed in person at baseline and approximately annually over the course of the study (mean and maximum follow-up durations were 12.7 and 17.2 years, respectively).
The main outcomes were total VMS duration (in years) and persistence of VMS (in years) beyond the final menstrual period (FMP).
Among the findings:
- The unadjusted median total VMS duration was 7.4 years
- Women who were premenopausal or early perimenopausal when they first reported frequent VMS had the longest total duration of VMS (median, >11.8 years) and longest persistence of VMS beyond the FMP (median, 9.4 years)
- Women who were postmenopausal at the onset of VMS had the shortest total VMS duration after the FMP (median, 3.4 years; P<.001).
- The median total VMS duration varied significantly by race, with African American women reporting the longest total VMS duration (median, 10.1 years) and Japanese and Chinese women reporting the shortest total duration (median, 4.8 and 5.4 years, respectively). Non-Hispanic white women had a median total VMS duration of 6.5 years; among Hispanic women, the median was 8.9 years.
Key takeaway
“These findings can help health-care professionals counsel patients about expectations regarding VMS and assist women in making treatment decisions based on the probability of their VMS persisting,” Avis and colleagues concluded. “In addition, the median total duration of 7.4 years highlights the limitations of guidance recommending short-term HT use and emphasizes the need to identify safe long-term therapies for the treatment of VMS.”
SWAN is the largest and longest longitudinal study to date to report on total duration of VMS and their persistence beyond the FMP.
“More than 50% of midlife women experience frequent VMS, yet clinical guidelines typically underestimate their true duration,” Avis and colleagues observed.
Frequent menopausal vasomotor symptoms (VMS), including hot flashes and night sweats, lasted longer than 7 years during the transition to menopause for more than 50% of women in the Study of Women’s Health Across the Nation (SWAN).1 Among the factors related to a longer duration of VMS:
- younger age
- African American heritage
- lower educational level
- greater perceived stress and symptom sensitivity
- higher depressive symptoms and anxiety at the first report of VMS.
Details of the study
Avis and colleagues analyzed data from SWAN, a multiracial/multiethnic study of women transitioning to menopause that was conducted from February 1996 through April 2013. The analyses included 1,449 women with frequent VMS (ie, occurring at least 6 days in the previous 2 weeks).
Baseline eligibility was age between 42 and 52 years, an intact uterus and at least one ovary, report of a menstrual cycle in the 3 months before screening, absence of pregnancy and lactation, and no use of oral contraceptives or hormone therapy (HT). Women were assessed in person at baseline and approximately annually over the course of the study (mean and maximum follow-up durations were 12.7 and 17.2 years, respectively).
The main outcomes were total VMS duration (in years) and persistence of VMS (in years) beyond the final menstrual period (FMP).
Among the findings:
- The unadjusted median total VMS duration was 7.4 years
- Women who were premenopausal or early perimenopausal when they first reported frequent VMS had the longest total duration of VMS (median, >11.8 years) and longest persistence of VMS beyond the FMP (median, 9.4 years)
- Women who were postmenopausal at the onset of VMS had the shortest total VMS duration after the FMP (median, 3.4 years; P<.001).
- The median total VMS duration varied significantly by race, with African American women reporting the longest total VMS duration (median, 10.1 years) and Japanese and Chinese women reporting the shortest total duration (median, 4.8 and 5.4 years, respectively). Non-Hispanic white women had a median total VMS duration of 6.5 years; among Hispanic women, the median was 8.9 years.
Key takeaway
“These findings can help health-care professionals counsel patients about expectations regarding VMS and assist women in making treatment decisions based on the probability of their VMS persisting,” Avis and colleagues concluded. “In addition, the median total duration of 7.4 years highlights the limitations of guidance recommending short-term HT use and emphasizes the need to identify safe long-term therapies for the treatment of VMS.”
SWAN is the largest and longest longitudinal study to date to report on total duration of VMS and their persistence beyond the FMP.
“More than 50% of midlife women experience frequent VMS, yet clinical guidelines typically underestimate their true duration,” Avis and colleagues observed.
Reference
- Avis NE, Crawford SL, Greendale G, et al; Study of Women’s Health Across the Nation (SWAN). Duration of menopausal symptoms over the menopause transition [published online ahead of print February 16, 2015]. JAMA Intern Med. doi:10.1001/jamainternmed.2014.8063.
Reference
- Avis NE, Crawford SL, Greendale G, et al; Study of Women’s Health Across the Nation (SWAN). Duration of menopausal symptoms over the menopause transition [published online ahead of print February 16, 2015]. JAMA Intern Med. doi:10.1001/jamainternmed.2014.8063.
Turning team-based care into a winning proposition
› Explore the potential benefits of team-based care by conducting a full assessment of your practice, including patient panels, payer mix, current finances, regional pay-for-performance programs, leadership support, and your staff’s training and talents. A
› Consider partnering with a local pharmacist or with insurers to use their community health workers, nurse case managers, and other self-management support tools. B
Strength of recommendation (SOR)
A Good-quality patient-oriented evidence
B Inconsistent or limited-quality patient-oriented evidence
C Consensus, usual practice, opinion, disease-oriented evidence, case series
The Institute for Healthcare Improvement’s “Triple Aim” approach to optimizing the delivery of health care in the United States calls for improving the patient’s experience of care, including both quality and satisfaction; improving the health of populations; and reducing the per-capita cost of health care.1 Unfortunately, achieving these goals is being made more challenging by a perfect storm of conditions: The age of the population and the number of people accessing the systems are increasing, while the number of providers available to care for these patients is decreasing. The number of annual office visits to family physicians (FPs) in the United States is projected to increase from 462 million in 2008 to 565 million in 2025, which will require an estimated 51,880 additional FPs.2
One of the health care delivery models that has recently gained traction to help address this is team-based care. By practicing in a team-based care model, physicians and other clinicians can care for more patients, better manage those with high-risk and high-cost needs, and improve overall quality of care and satisfaction for all involved. Here we review the evidence for team-based care and its use for chronic disease management, and offer suggestions for its implementation.
The many providers who comprise the team
There is little consistency in the definition, composition, training, or maintenance of health care teams. Naylor et al3 defined team-based care as “the provision of health services to individuals, families, and/or their communities by at least two health providers who work collaboratively with patients and their caregivers—to the extent preferred by each patient—to accomplish shared goals within and across settings to achieve coordinated, high-quality care.”
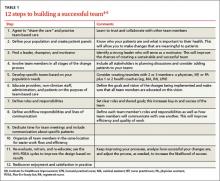
While the team construct will vary based on the needs of your practice and your patients, developing a high-functioning team is essential to achieving success. Our 12-step checklist for building a successful team is a good starting point (TABLE 1).4-6 Many other resources are available to help with each step of this process (TABLE 2).
Teams should be led by a primary care provider—a physician, nurse practitioner (NP), or physician assistant (PA)—and consist of other members that complement the other’s expertise and roles, such as nurse case managers, clinical pharmacists, social workers, and behavioral health experts. Some practices have large teams with interdisciplinary members, including pharmacists, PAs, and NPs (the “expanded staffing” model), while others form smaller “teamlets” consisting of a physician and a registered nurse (RN) who serves as a health coach.
In the expanded staffing model, RNs and clinical pharmacists assume greater care management, while medical assistants (MAs) and licensed practice nurses (LPNs) are responsible for pre-visit, outreach, and follow-up activities.7 Redefining roles can spread the work among all team members, which allows each member to work to their level of training and licensure and permits the MD/NP/PA to focus on more complex tasks.
The teamlet model has 2 main features: 1) Patient encounters involve a clinician (MD, NP, PA) and a health coach (MA, RN, LPN); and 2) Care is expanded beyond the usual 15-minute visit to include pre-visit, visit, post-visit, and between-visit care.8 Incorporating a health coach puts an increased focus on the patient and self-management support, with the goals of increasing satisfaction for both the patient and the health care team, improving outcomes, and lowering cost due to fewer emergency department (ED) visits and hospital admissions/readmissions.
Smaller teams seem to be more effective and more manageable.8,9 In one example of well-functioning teamlet composed of RNs and MAs, an MA is responsible for patients coming in for timely chronic and preventive care needs, while the RNs focus their efforts on tasks that require their expertise, including health coaching, self-management support, and patient education.9 Although smaller offices may not have the resources of a large academic practice, this model of maximizing the role of the MAs is reasonable and achievable.
Another example of a successful teamlet model is a clinical microsystem, in which a small group of clinicians and support staff work together to provide care to a discrete group of patients.10,11 (For more information on clinical microsystems, go to the Dartmouth Institute Microsystem Academy at https://clinicalmicrosystem.org.)
What are the barriers to creating team-based care?
Many providers and administrators are concerned about the costs of creating a team-based model of care. These include the cost of hiring new staff, retraining current staff, and educating team members and patients, as well as the cost of developing and maintaining the necessary information technology.
There is, of course, always the concern about physicians relinquishing patient care tasks to other team members. The flip side of that is that staff members may not be eager to increase their roles and responsibilities. In addition, developing a high-functioning team requires ongoing efforts to train and retrain, as well as dedicated leadership and an ongoing commitment to team building.12
Team-based care can work well for managing chronic diseases
Despite the challenges of developing and maintaining this approach to care, the evidence suggests that implementing a team-based model can be especially useful for patients with chronic diseases, because it can improve patient outcomes and access to care, decrease costs, and improve clinician satisfaction—as detailed below.
Improved patient outcomes. Initial evidence suggests that implementing a team-based model can improve patients’ health and experience of care.13,14 The most positive findings have been observed for team-based efforts at managing specific diseases, such as diabetes and congestive heart failure (CHF), or specific populations, such as older patients with chronic illness. Studies have shown that using a team approach results in improved metrics, including HbA1c, low-density lipoprotein cholesterol, blood pressure (BP), and body mass index.7,15-20 Team-based models that pair physicians and other primary care providers with a clinical pharmacist have increased patients’ medication adherence and provider adherence to recommended prescribing habits.15,21-23
One small clinical microsystem that focused on self-management support with health coaching increased patients’ ratings of their confidence in self-management from 40% to 60% at baseline to 80% to 90% after one year. This program also increased the proportion of patients in whom BP was controlled by 10% to 15%.10
Despite these successes, some team-based models may not always be “doable” because of the costs of adding an advanced practice clinician to the staff, or the challenges of recruiting the right person for the job. (How to adapt team-based care for smaller practices is discussed below.)
Improved access to care. A preponderance of data shows that team-based care increases the volume of patient visits, thereby improving access to care.7,21,24-28 The critical elements to successfully achieving this are effective training and delegation. In private practice, using well-trained clinical assistants to create a physician-driven team can increase patient visit volume by an estimated 30% (using 1 assistant) to 60% (using 2 assistants).24
Similar increases in visit volume are seen in larger patient-centered medical home (PCMH) models that consist of physicians, PAs or NPs, MAs, LPNs, RNs, and clinical pharmacists.7,25 Teams with defined ratios of assistants to physicians/NPs/PAs see the most patients per day compared to care coordinator models (ie, 1 assistant for multiple physicians) or enhanced traditional models.21 When focusing on disease-specific care, the impact on access can be even greater. A diabetes-specific team-based care program resulted in a >50% increase in daily patient encounters and 4-fold increase in annual office visits.28
In addition to increasing visits, team-based care also increases access to care by decreasing wait times for an appointment and increasing the use of secure messaging and telephone visits.7,25 In a prospective cohort pilot study of more than 2000 patients enrolled in a team-based care model, the average scheduling time for a face-to-face visit for nonurgent care decreased from a mean of 26.5 days to 14 days, compared to a mean of 31.5 days to 17.8 days for controls.25 (The decrease in the control group was likely due to implementation of an electronic medical record in the practice.) Furthermore, a non-controlled evaluation of health plan-based practice groups with very large patient populations (ie, >300,000 patients) reported up to a 3-fold decrease in appointment waiting time when using a team-based model.29
Some studies have found a decrease in office visits after implementing team-based care.7 However, these reports also found a corresponding increase (by as much as 80%) in the use of secure messaging and telephone encounters, which translated to an overall enhanced communication with patients and ultimately increased access to care.7
Decreased costs. Several controlled trials have looked at the financial impact of using team-based care to manage chronic conditions such as asthma, CHF, and diabetes. Rich et al30 found a nurse-directed program of patient self-management support via telephone and home visit follow-up was associated with a 56% reduction in hospital readmissions, which translated to a $460 decrease in cost per patient over a 3-month period compared to a control group. In a study by Domurat,31 hospital stays were 50% shorter for high-risk diabetes patients who were managed by a team that offered planned visits, telephone contact, and group visits; this resulted in a lower cost of care. Katon et al32 found that when a nurse manager was added to a primary care team to enhance self-management support, intensify treatment, and coordinate continuity of care for patients with multiple chronic conditions, outpatient health costs were decreased by $594 per patient over 24 months.
Liu et al33 randomly assigned 354 patients in a VA primary care clinic who met criteria for major depression or dysthymia to usual care or a collaborative care model. The collaborative care model included a mental health care team that provided telephone contact to encourage medication adherence and reviewed and suggested modifications to the treatment plan. After an initial expenditure of $519 per patient, a savings of approximately $33 per patient for total outpatient costs was realized.
A team-based coordinated care program for patients with multiple chronic conditions reduced patient visits to specialists by 24%, ED visits by 13%, and hospitalizations by 39%.34 An internal evaluation found that the program saved money by reducing admissions, including intensive care unit stays and “observational” stays for Medicare fee-for-service patients.35
What about reimbursement? Most studies that have evaluated the financial aspects of implementing team-based care have calculated the cost savings for the health system—rather than for an individual practice—through decreased hospital admissions, readmissions, and ED visits. Efficient, high-quality teams will require a substantial initial investment of time and hiring and training of staff before savings can be realized.
Team-based care may not be financially sustainable unless current reimbursement models are changed. The current US system bases payment on quantity of care instead of quality of care, reimburses only for clinician services, and does not compensate teams.36 The Centers for Medicare and Medicaid Services (CMS) has begun to recognize the need to reimburse for services that are not delivered in face-to-face patient encounters. For example, the agency established a new G-code that can be used for non-face-to-face care management services for Medicare patients with 2 or more significant chronic conditions; this code took effect on January 1, 2015.37
Some insurers are reimbursing practices for obtaining designation as a PCMH. This type of reimbursement could be expanded to include other types of team-based efforts—such as self-management support and health coaching.
Improved team satisfaction. While many primary care providers are experiencing fatigue and burnout,38 support staff in many practices also experience job dissatisfaction, which leads to increased absenteeism and high turnover. Several studies indicate that involving all levels of staff in the improvement process and empowering them to work to their full potential by enhancing their roles and realigning responsibilities can increase satisfaction.7,11,21,38,39 This in turn can lead to increased loyalty, commitment, and productivity, with decreased burnout and turnover.
TABLE 2
| Team-based care: Additional resources | |
| Resource | Comments |
The Dartmouth Institute Microsystem Academy | This site includes assessment tools and strategies for implementing clinical microsystems into practices |
Improving Chronic Illness Care | This site provides information about the chronic care model, care coordination, and patient-centered medical homes |
TeamSTEPPS | TeamSTEPPS is an evidence-based teamwork system to improve communication and teamwork skills among health care professionals. All resources, including training materials, are free and downloadable |
Godfrey MM, Melin CN, Muething SE, et al. Clinical microsystems, Part 3. Transformation of two hospitals using microsystem, mesosystem, and macrosystem strategies. Jt Comm J Qual Patient Saf. 2008;34:591-603. | This article provides resources and strategies to engage all levels of the health system in team-based care |
McKinley KE, Berry SA, Laam LA, et al. Clinical microsystems, Part 4. Building innovative population-specific mesosystems. Jt Comm J Qual Patient Saf. 2008;34:655-663. | This article describes how to engage leadership at the health systems level |
Adapting team-based care for smaller practices
Physicians who practice alone or in small groups may have limited capacity to employ allied health professionals. However, your “team” doesn’t need to be housed only in your office. One innovative approach is the community-based medical home, where physicians with medical homes and/or care teams in their offices refer to, and collaborate with, a network of community-based professionals and agencies for clinical and social service support for their patients.22 Some options are to partner with a local pharmacist or with insurers to use their community health workers, nurse case managers, and other self-management support tools.
While having team-based care strategies is necessary to achieve a PCMH designation, you do not need to seek such designation in order to practice team-based care. Start by conducting a full assessment of your practice, including patient panels, payer mix, current finances, regional pay-for-performance programs, leadership support, and your staff’s training and talents. In addition, determine what you value for your practice and what outcomes you hope for, along with a clear plan of how to measure these outcomes. This will allow you to determine if the estimated cost of the proposed strategy is “worth it” in terms of your individual situation and goals.
CORRESPONDENCE
Michele Q. Zawora, MD, Thomas Jefferson University, 1015 Walnut Street, Suite 401, Philadelphia, Pa 19107; [email protected]
1. Institute of Healthcare Improvement. The IHI Triple Aim. Institute for Healthcare Improvement Web site. Available at http://www.ihi.org/offerings/Initiatives/TripleAim/Pages/default.aspx. Accessed February 5, 2015.
2.Petterson SM, Liaw WR, Phillips RL Jr, et al. Projecting US primary care physician workforce needs: 2010-2025. Ann Fam Med. 2012;10:503-509.
3. Naylor MD, Coburn KD, Kurtzman ET, et al. Inter-professional team-based primary care for chronically ill adults: State of the science. White paper presented at: the ABIM Foundation meeting to Advance Team-Based Care for the Chronically Ill in Ambulatory Settings; March 24-25, 2010; Philadelphia, PA.
4.Boult C, Green AF, Boult LB, et al. Successful models of comprehensive care for older adults with chronic conditions: Evidence for the Institute of Medicine’s Retooling for an Aging America report. J Am Geriatr Soc. 2009;57:2328-2337.
5.Kuzel AJ. Keys to high-functioning office teams. Family Practice Management. 2011;18:15-18.
6.Sinsky CA, Willard-Grace R, Schutzbank AM, et al. In search of joy in practice: a report of 23 high-functioning primary care practices. Ann Fam Med. 2013;11:272-278.
7. Reid RJ, Coleman K, Johnson EA, et al. The Group Health medical home at year two: cost savings, higher patient satisfaction, and less burnout for providers. Health Aff (Millwood). 2010;29:835-843.
8. Bodenheimer T, Laing BY. The teamlet model of primary care. Ann Fam Med. 2007;5:457-461.
9. Chen EH, Thom DH, Hessler DM, et al. Using the teamlet model to improve chronic care in an academic primary care practice. J Gen Intern Med. 2010;25(suppl 4):S610-S614.
10. Wasson JH, Anders SG, Moore LG, et al. Clinical microsystems, part 2. Learning from micro practices about providing patients the care they want and need. Jt Comm J Qual Patient Saf. 2008;34:445-452.
11. Williams I, Dickinson H, Robinson S. Clinical microsystems: An evaluation. Health Services Management Centre, School of Public Policy, University of Birmingham, England; 2007. Available at: http://chain.ulcc.ac.uk/chain/documents/hsmc_evaluation_report_CMS2007final.pdf. Accessed February 20, 2015.
12. Willard R, Bodenheimer T. The building blocks of high-performing primary care: Lessons from the field. April 2012. Available at: http://www.chcf.org/~/media/MEDIA%20LIBRARY%20Files/PDF/B/PDF%20BuildingBlocksPrimaryCare.pdf. Accessed February 10, 2015.
13. Wagner EH. The role of patient care teams in chronic disease management. BMJ. 2000;320:569-572.
14. Boult C, Karm L, Groves C. Improving chronic care: the “guided care” model. Perm J. 2008;12:50-54.
15. Smith SM, Soubhi H, Fortin M, et al. Interventions for improving outcomes in patients with multimorbidity in primary care and community settings. Cochrane Database Syst Rev. 2012;4:CD006560.
16. Bloom FJ, Graf TR, Steele GD. Improved patient outcomes in 3 years with a system of care for diabetes. October 2012. Available at: http://iom.edu/~/media/Files/Perspectives-Files/2012/Commentaries/VSRT-Improved-Patient-Outcomes.pdf. Accessed February 10, 2015.
17. Bloom FJ Jr, Yan X, Stewart WF, et al. Primary care diabetes bundle management: 3-year outcomes for microvascular and macrovascular events. Am J Manag Care. 2014;20:e175-e182.
18. Pape GA, Hunt JS, Butler KL, et al. Team-based care approach to cholesterol management in diabetes mellitus: two-year cluster randomized controlled trial. Arch Intern Med. 2011;171:1480-1486.
19. Scanlon DP, Hollenbeak CS, Beich J, et al. Financial and clinical impact of team-based treatment for medicaid enrollees with diabetes in a federally qualified health center. Diabetes Care. 2008;31:2160-2165.
20. Renders CM, Valk GD, Griffin S, et al. Interventions to improve the management of diabetes mellitus in primary care, outpatient and community settings. Cochrane Database Syst Rev. 2001;(1):CD001481.
21. Goldberg GD, Beeson T, Kuzel AJ, et al. Team-based care: a critical element of primary care practice transformation. Popul Health Manag. 2013;16:150-156.
22. Lipton HL. Home is where the health is: advancing team-based care in chronic disease management. Arch Intern Med. 2009;169:1945-1948.
23. Farris KB, Côté I, Feeny D, et al. Enhancing primary care for complex patients. Demonstration project using multidisciplinary teams. Can Fam Physician. 2004;50:998-1003.
24. Anderson P, Halley MD. A new approach to making your doctor-nurse team more productive. Fam Pract Manag. 2008;15:35-40.
25. Grumbach K, Bodenheimer T, Grundy P. The outcomes of implementing patient-centered medical home interventions: A review of the evidence on quality, access and costs from recent prospective evaluation studies, August 2009. Washington, DC; Patient-Centered Primary Care Collaborative; 2009.
26. Smith M, Giuliano MR, Starkowski MP. In Connecticut: improving patient medication management in primary care. Health Aff (Millwood). 2011;30:646-654.
27. Kaboli PJ, Hoth AB, McClimon BJ, et al. Clinical pharmacists and inpatient medical care: a systematic review. Arch Intern Med. 2006;16:955-964.
28. Bray P, Roupe M, Young S, et al. Feasibility and effectiveness of system redesign for diabetes care management in rural areas: the eastern North Carolina experience. Diabetes Educ. 2005;31:712-718.
29. Institute for Healthcare Improvement. Health Partners uses “BestCare” practices to improve care and outcomes, reduce costs. Institute for Healthcare Improvement Web site. Available at http://www.ihi.org/engage/initiatives/TripleAim/Documents/IHITripleAimHealthPartnersSummaryofSuccessJul09v2.pdf. Accessed February 5, 2015.
30. Rich MW, Beckman V, Wittenberg C, et al. A multidisciplinary intervention to prevent the readmission of elderly patients with congestive heart failure. N Engl J Med. 1995;333:1190-1195.
31. Domurat ES. Diabetes managed care and clinical outcomes: the Harbor City, California Kaiser Permanente diabetes care system. Am J Manag Care. 1999;5:1299-1307.
32. Katon W, Russo J, Lin EH, et al. Cost-effectiveness of a multicondition collaborative care intervention: a randomized controlled trial. Arch Gen Psychiatry. 2012;69:506-514.
33. Liu CF, Hendrick SC, Chaney EF, et al. Cost-effectiveness of collaborative care for depression in a primary care veteran population. Psychiatr Serv. 2003;54:698-704.
34. Berenson RA; The Urban Institute. Challenging the status quo in chronic disease care: seven case studies. California Healthcare Foundation: 2006. California HealthCare Foundation Web site. Available at: http://www.chcf.org/~/media/MEDIA%20LIBRARY%20Files/PDF/C/PDF%20ChallengingStatusQuoCaseStudies.pdf. Accessed February 5, 2015.
35. Coleman EA, Smith JD, Frank JC, et al. Preparing patients and caregivers to participate in care delivered across settings: the Care Transitions Intervention. J Am Geriatr Soc. 2004;52:1817-1825.
36. Schneider ME. Medicare finalizes plan for non-face-to-face payments. Family Practice News Web site. Available at: http://www.familypracticenews.com/?id=2633&tx_ttnews%5Btt_news%5D=226457&cHash=2aeafe0585c7156dcf23891d010cd12f. Accessed December 2, 2013.
37. Centers for Medicare & Medicaid Services. Fact sheets: Policy and payment changes to the Medicare Physician Fee Schedule for 2015. Centers for Medicare & Medicaid Services Web site. Available at: http://www.cms.gov/newsroom/mediareleasedatabase/fact-sheets/2014-Fact-sheets-items/2014-10-31-7.html. Accessed February 13, 2015.
38. Berry LL, Dunham J. Redefining the patient experience with collaborative care. Harvard Business Review Blog Network. Harvard Business Review Web site. Available at: https://hbr.org/2013/09/redefining-the-patient-experience-with-collaborative-care/. Accessed February 5, 2015.
39. Lyon RK, Slawson J. An organized approach to chronic disease care. Fam Pract Manag. 2011;18:27-31.
› Explore the potential benefits of team-based care by conducting a full assessment of your practice, including patient panels, payer mix, current finances, regional pay-for-performance programs, leadership support, and your staff’s training and talents. A
› Consider partnering with a local pharmacist or with insurers to use their community health workers, nurse case managers, and other self-management support tools. B
Strength of recommendation (SOR)
A Good-quality patient-oriented evidence
B Inconsistent or limited-quality patient-oriented evidence
C Consensus, usual practice, opinion, disease-oriented evidence, case series
The Institute for Healthcare Improvement’s “Triple Aim” approach to optimizing the delivery of health care in the United States calls for improving the patient’s experience of care, including both quality and satisfaction; improving the health of populations; and reducing the per-capita cost of health care.1 Unfortunately, achieving these goals is being made more challenging by a perfect storm of conditions: The age of the population and the number of people accessing the systems are increasing, while the number of providers available to care for these patients is decreasing. The number of annual office visits to family physicians (FPs) in the United States is projected to increase from 462 million in 2008 to 565 million in 2025, which will require an estimated 51,880 additional FPs.2
One of the health care delivery models that has recently gained traction to help address this is team-based care. By practicing in a team-based care model, physicians and other clinicians can care for more patients, better manage those with high-risk and high-cost needs, and improve overall quality of care and satisfaction for all involved. Here we review the evidence for team-based care and its use for chronic disease management, and offer suggestions for its implementation.
The many providers who comprise the team
There is little consistency in the definition, composition, training, or maintenance of health care teams. Naylor et al3 defined team-based care as “the provision of health services to individuals, families, and/or their communities by at least two health providers who work collaboratively with patients and their caregivers—to the extent preferred by each patient—to accomplish shared goals within and across settings to achieve coordinated, high-quality care.”
While the team construct will vary based on the needs of your practice and your patients, developing a high-functioning team is essential to achieving success. Our 12-step checklist for building a successful team is a good starting point (TABLE 1).4-6 Many other resources are available to help with each step of this process (TABLE 2).
Teams should be led by a primary care provider—a physician, nurse practitioner (NP), or physician assistant (PA)—and consist of other members that complement the other’s expertise and roles, such as nurse case managers, clinical pharmacists, social workers, and behavioral health experts. Some practices have large teams with interdisciplinary members, including pharmacists, PAs, and NPs (the “expanded staffing” model), while others form smaller “teamlets” consisting of a physician and a registered nurse (RN) who serves as a health coach.
In the expanded staffing model, RNs and clinical pharmacists assume greater care management, while medical assistants (MAs) and licensed practice nurses (LPNs) are responsible for pre-visit, outreach, and follow-up activities.7 Redefining roles can spread the work among all team members, which allows each member to work to their level of training and licensure and permits the MD/NP/PA to focus on more complex tasks.
The teamlet model has 2 main features: 1) Patient encounters involve a clinician (MD, NP, PA) and a health coach (MA, RN, LPN); and 2) Care is expanded beyond the usual 15-minute visit to include pre-visit, visit, post-visit, and between-visit care.8 Incorporating a health coach puts an increased focus on the patient and self-management support, with the goals of increasing satisfaction for both the patient and the health care team, improving outcomes, and lowering cost due to fewer emergency department (ED) visits and hospital admissions/readmissions.
Smaller teams seem to be more effective and more manageable.8,9 In one example of well-functioning teamlet composed of RNs and MAs, an MA is responsible for patients coming in for timely chronic and preventive care needs, while the RNs focus their efforts on tasks that require their expertise, including health coaching, self-management support, and patient education.9 Although smaller offices may not have the resources of a large academic practice, this model of maximizing the role of the MAs is reasonable and achievable.
Another example of a successful teamlet model is a clinical microsystem, in which a small group of clinicians and support staff work together to provide care to a discrete group of patients.10,11 (For more information on clinical microsystems, go to the Dartmouth Institute Microsystem Academy at https://clinicalmicrosystem.org.)
What are the barriers to creating team-based care?
Many providers and administrators are concerned about the costs of creating a team-based model of care. These include the cost of hiring new staff, retraining current staff, and educating team members and patients, as well as the cost of developing and maintaining the necessary information technology.
There is, of course, always the concern about physicians relinquishing patient care tasks to other team members. The flip side of that is that staff members may not be eager to increase their roles and responsibilities. In addition, developing a high-functioning team requires ongoing efforts to train and retrain, as well as dedicated leadership and an ongoing commitment to team building.12
Team-based care can work well for managing chronic diseases
Despite the challenges of developing and maintaining this approach to care, the evidence suggests that implementing a team-based model can be especially useful for patients with chronic diseases, because it can improve patient outcomes and access to care, decrease costs, and improve clinician satisfaction—as detailed below.
Improved patient outcomes. Initial evidence suggests that implementing a team-based model can improve patients’ health and experience of care.13,14 The most positive findings have been observed for team-based efforts at managing specific diseases, such as diabetes and congestive heart failure (CHF), or specific populations, such as older patients with chronic illness. Studies have shown that using a team approach results in improved metrics, including HbA1c, low-density lipoprotein cholesterol, blood pressure (BP), and body mass index.7,15-20 Team-based models that pair physicians and other primary care providers with a clinical pharmacist have increased patients’ medication adherence and provider adherence to recommended prescribing habits.15,21-23
One small clinical microsystem that focused on self-management support with health coaching increased patients’ ratings of their confidence in self-management from 40% to 60% at baseline to 80% to 90% after one year. This program also increased the proportion of patients in whom BP was controlled by 10% to 15%.10
Despite these successes, some team-based models may not always be “doable” because of the costs of adding an advanced practice clinician to the staff, or the challenges of recruiting the right person for the job. (How to adapt team-based care for smaller practices is discussed below.)
Improved access to care. A preponderance of data shows that team-based care increases the volume of patient visits, thereby improving access to care.7,21,24-28 The critical elements to successfully achieving this are effective training and delegation. In private practice, using well-trained clinical assistants to create a physician-driven team can increase patient visit volume by an estimated 30% (using 1 assistant) to 60% (using 2 assistants).24
Similar increases in visit volume are seen in larger patient-centered medical home (PCMH) models that consist of physicians, PAs or NPs, MAs, LPNs, RNs, and clinical pharmacists.7,25 Teams with defined ratios of assistants to physicians/NPs/PAs see the most patients per day compared to care coordinator models (ie, 1 assistant for multiple physicians) or enhanced traditional models.21 When focusing on disease-specific care, the impact on access can be even greater. A diabetes-specific team-based care program resulted in a >50% increase in daily patient encounters and 4-fold increase in annual office visits.28
In addition to increasing visits, team-based care also increases access to care by decreasing wait times for an appointment and increasing the use of secure messaging and telephone visits.7,25 In a prospective cohort pilot study of more than 2000 patients enrolled in a team-based care model, the average scheduling time for a face-to-face visit for nonurgent care decreased from a mean of 26.5 days to 14 days, compared to a mean of 31.5 days to 17.8 days for controls.25 (The decrease in the control group was likely due to implementation of an electronic medical record in the practice.) Furthermore, a non-controlled evaluation of health plan-based practice groups with very large patient populations (ie, >300,000 patients) reported up to a 3-fold decrease in appointment waiting time when using a team-based model.29
Some studies have found a decrease in office visits after implementing team-based care.7 However, these reports also found a corresponding increase (by as much as 80%) in the use of secure messaging and telephone encounters, which translated to an overall enhanced communication with patients and ultimately increased access to care.7
Decreased costs. Several controlled trials have looked at the financial impact of using team-based care to manage chronic conditions such as asthma, CHF, and diabetes. Rich et al30 found a nurse-directed program of patient self-management support via telephone and home visit follow-up was associated with a 56% reduction in hospital readmissions, which translated to a $460 decrease in cost per patient over a 3-month period compared to a control group. In a study by Domurat,31 hospital stays were 50% shorter for high-risk diabetes patients who were managed by a team that offered planned visits, telephone contact, and group visits; this resulted in a lower cost of care. Katon et al32 found that when a nurse manager was added to a primary care team to enhance self-management support, intensify treatment, and coordinate continuity of care for patients with multiple chronic conditions, outpatient health costs were decreased by $594 per patient over 24 months.
Liu et al33 randomly assigned 354 patients in a VA primary care clinic who met criteria for major depression or dysthymia to usual care or a collaborative care model. The collaborative care model included a mental health care team that provided telephone contact to encourage medication adherence and reviewed and suggested modifications to the treatment plan. After an initial expenditure of $519 per patient, a savings of approximately $33 per patient for total outpatient costs was realized.
A team-based coordinated care program for patients with multiple chronic conditions reduced patient visits to specialists by 24%, ED visits by 13%, and hospitalizations by 39%.34 An internal evaluation found that the program saved money by reducing admissions, including intensive care unit stays and “observational” stays for Medicare fee-for-service patients.35
What about reimbursement? Most studies that have evaluated the financial aspects of implementing team-based care have calculated the cost savings for the health system—rather than for an individual practice—through decreased hospital admissions, readmissions, and ED visits. Efficient, high-quality teams will require a substantial initial investment of time and hiring and training of staff before savings can be realized.
Team-based care may not be financially sustainable unless current reimbursement models are changed. The current US system bases payment on quantity of care instead of quality of care, reimburses only for clinician services, and does not compensate teams.36 The Centers for Medicare and Medicaid Services (CMS) has begun to recognize the need to reimburse for services that are not delivered in face-to-face patient encounters. For example, the agency established a new G-code that can be used for non-face-to-face care management services for Medicare patients with 2 or more significant chronic conditions; this code took effect on January 1, 2015.37
Some insurers are reimbursing practices for obtaining designation as a PCMH. This type of reimbursement could be expanded to include other types of team-based efforts—such as self-management support and health coaching.
Improved team satisfaction. While many primary care providers are experiencing fatigue and burnout,38 support staff in many practices also experience job dissatisfaction, which leads to increased absenteeism and high turnover. Several studies indicate that involving all levels of staff in the improvement process and empowering them to work to their full potential by enhancing their roles and realigning responsibilities can increase satisfaction.7,11,21,38,39 This in turn can lead to increased loyalty, commitment, and productivity, with decreased burnout and turnover.
TABLE 2
| Team-based care: Additional resources | |
| Resource | Comments |
The Dartmouth Institute Microsystem Academy | This site includes assessment tools and strategies for implementing clinical microsystems into practices |
Improving Chronic Illness Care | This site provides information about the chronic care model, care coordination, and patient-centered medical homes |
TeamSTEPPS | TeamSTEPPS is an evidence-based teamwork system to improve communication and teamwork skills among health care professionals. All resources, including training materials, are free and downloadable |
Godfrey MM, Melin CN, Muething SE, et al. Clinical microsystems, Part 3. Transformation of two hospitals using microsystem, mesosystem, and macrosystem strategies. Jt Comm J Qual Patient Saf. 2008;34:591-603. | This article provides resources and strategies to engage all levels of the health system in team-based care |
McKinley KE, Berry SA, Laam LA, et al. Clinical microsystems, Part 4. Building innovative population-specific mesosystems. Jt Comm J Qual Patient Saf. 2008;34:655-663. | This article describes how to engage leadership at the health systems level |
Adapting team-based care for smaller practices
Physicians who practice alone or in small groups may have limited capacity to employ allied health professionals. However, your “team” doesn’t need to be housed only in your office. One innovative approach is the community-based medical home, where physicians with medical homes and/or care teams in their offices refer to, and collaborate with, a network of community-based professionals and agencies for clinical and social service support for their patients.22 Some options are to partner with a local pharmacist or with insurers to use their community health workers, nurse case managers, and other self-management support tools.
While having team-based care strategies is necessary to achieve a PCMH designation, you do not need to seek such designation in order to practice team-based care. Start by conducting a full assessment of your practice, including patient panels, payer mix, current finances, regional pay-for-performance programs, leadership support, and your staff’s training and talents. In addition, determine what you value for your practice and what outcomes you hope for, along with a clear plan of how to measure these outcomes. This will allow you to determine if the estimated cost of the proposed strategy is “worth it” in terms of your individual situation and goals.
CORRESPONDENCE
Michele Q. Zawora, MD, Thomas Jefferson University, 1015 Walnut Street, Suite 401, Philadelphia, Pa 19107; [email protected]
› Explore the potential benefits of team-based care by conducting a full assessment of your practice, including patient panels, payer mix, current finances, regional pay-for-performance programs, leadership support, and your staff’s training and talents. A
› Consider partnering with a local pharmacist or with insurers to use their community health workers, nurse case managers, and other self-management support tools. B
Strength of recommendation (SOR)
A Good-quality patient-oriented evidence
B Inconsistent or limited-quality patient-oriented evidence
C Consensus, usual practice, opinion, disease-oriented evidence, case series
The Institute for Healthcare Improvement’s “Triple Aim” approach to optimizing the delivery of health care in the United States calls for improving the patient’s experience of care, including both quality and satisfaction; improving the health of populations; and reducing the per-capita cost of health care.1 Unfortunately, achieving these goals is being made more challenging by a perfect storm of conditions: The age of the population and the number of people accessing the systems are increasing, while the number of providers available to care for these patients is decreasing. The number of annual office visits to family physicians (FPs) in the United States is projected to increase from 462 million in 2008 to 565 million in 2025, which will require an estimated 51,880 additional FPs.2
One of the health care delivery models that has recently gained traction to help address this is team-based care. By practicing in a team-based care model, physicians and other clinicians can care for more patients, better manage those with high-risk and high-cost needs, and improve overall quality of care and satisfaction for all involved. Here we review the evidence for team-based care and its use for chronic disease management, and offer suggestions for its implementation.
The many providers who comprise the team
There is little consistency in the definition, composition, training, or maintenance of health care teams. Naylor et al3 defined team-based care as “the provision of health services to individuals, families, and/or their communities by at least two health providers who work collaboratively with patients and their caregivers—to the extent preferred by each patient—to accomplish shared goals within and across settings to achieve coordinated, high-quality care.”
While the team construct will vary based on the needs of your practice and your patients, developing a high-functioning team is essential to achieving success. Our 12-step checklist for building a successful team is a good starting point (TABLE 1).4-6 Many other resources are available to help with each step of this process (TABLE 2).
Teams should be led by a primary care provider—a physician, nurse practitioner (NP), or physician assistant (PA)—and consist of other members that complement the other’s expertise and roles, such as nurse case managers, clinical pharmacists, social workers, and behavioral health experts. Some practices have large teams with interdisciplinary members, including pharmacists, PAs, and NPs (the “expanded staffing” model), while others form smaller “teamlets” consisting of a physician and a registered nurse (RN) who serves as a health coach.
In the expanded staffing model, RNs and clinical pharmacists assume greater care management, while medical assistants (MAs) and licensed practice nurses (LPNs) are responsible for pre-visit, outreach, and follow-up activities.7 Redefining roles can spread the work among all team members, which allows each member to work to their level of training and licensure and permits the MD/NP/PA to focus on more complex tasks.
The teamlet model has 2 main features: 1) Patient encounters involve a clinician (MD, NP, PA) and a health coach (MA, RN, LPN); and 2) Care is expanded beyond the usual 15-minute visit to include pre-visit, visit, post-visit, and between-visit care.8 Incorporating a health coach puts an increased focus on the patient and self-management support, with the goals of increasing satisfaction for both the patient and the health care team, improving outcomes, and lowering cost due to fewer emergency department (ED) visits and hospital admissions/readmissions.
Smaller teams seem to be more effective and more manageable.8,9 In one example of well-functioning teamlet composed of RNs and MAs, an MA is responsible for patients coming in for timely chronic and preventive care needs, while the RNs focus their efforts on tasks that require their expertise, including health coaching, self-management support, and patient education.9 Although smaller offices may not have the resources of a large academic practice, this model of maximizing the role of the MAs is reasonable and achievable.
Another example of a successful teamlet model is a clinical microsystem, in which a small group of clinicians and support staff work together to provide care to a discrete group of patients.10,11 (For more information on clinical microsystems, go to the Dartmouth Institute Microsystem Academy at https://clinicalmicrosystem.org.)
What are the barriers to creating team-based care?
Many providers and administrators are concerned about the costs of creating a team-based model of care. These include the cost of hiring new staff, retraining current staff, and educating team members and patients, as well as the cost of developing and maintaining the necessary information technology.
There is, of course, always the concern about physicians relinquishing patient care tasks to other team members. The flip side of that is that staff members may not be eager to increase their roles and responsibilities. In addition, developing a high-functioning team requires ongoing efforts to train and retrain, as well as dedicated leadership and an ongoing commitment to team building.12
Team-based care can work well for managing chronic diseases
Despite the challenges of developing and maintaining this approach to care, the evidence suggests that implementing a team-based model can be especially useful for patients with chronic diseases, because it can improve patient outcomes and access to care, decrease costs, and improve clinician satisfaction—as detailed below.
Improved patient outcomes. Initial evidence suggests that implementing a team-based model can improve patients’ health and experience of care.13,14 The most positive findings have been observed for team-based efforts at managing specific diseases, such as diabetes and congestive heart failure (CHF), or specific populations, such as older patients with chronic illness. Studies have shown that using a team approach results in improved metrics, including HbA1c, low-density lipoprotein cholesterol, blood pressure (BP), and body mass index.7,15-20 Team-based models that pair physicians and other primary care providers with a clinical pharmacist have increased patients’ medication adherence and provider adherence to recommended prescribing habits.15,21-23
One small clinical microsystem that focused on self-management support with health coaching increased patients’ ratings of their confidence in self-management from 40% to 60% at baseline to 80% to 90% after one year. This program also increased the proportion of patients in whom BP was controlled by 10% to 15%.10
Despite these successes, some team-based models may not always be “doable” because of the costs of adding an advanced practice clinician to the staff, or the challenges of recruiting the right person for the job. (How to adapt team-based care for smaller practices is discussed below.)
Improved access to care. A preponderance of data shows that team-based care increases the volume of patient visits, thereby improving access to care.7,21,24-28 The critical elements to successfully achieving this are effective training and delegation. In private practice, using well-trained clinical assistants to create a physician-driven team can increase patient visit volume by an estimated 30% (using 1 assistant) to 60% (using 2 assistants).24
Similar increases in visit volume are seen in larger patient-centered medical home (PCMH) models that consist of physicians, PAs or NPs, MAs, LPNs, RNs, and clinical pharmacists.7,25 Teams with defined ratios of assistants to physicians/NPs/PAs see the most patients per day compared to care coordinator models (ie, 1 assistant for multiple physicians) or enhanced traditional models.21 When focusing on disease-specific care, the impact on access can be even greater. A diabetes-specific team-based care program resulted in a >50% increase in daily patient encounters and 4-fold increase in annual office visits.28
In addition to increasing visits, team-based care also increases access to care by decreasing wait times for an appointment and increasing the use of secure messaging and telephone visits.7,25 In a prospective cohort pilot study of more than 2000 patients enrolled in a team-based care model, the average scheduling time for a face-to-face visit for nonurgent care decreased from a mean of 26.5 days to 14 days, compared to a mean of 31.5 days to 17.8 days for controls.25 (The decrease in the control group was likely due to implementation of an electronic medical record in the practice.) Furthermore, a non-controlled evaluation of health plan-based practice groups with very large patient populations (ie, >300,000 patients) reported up to a 3-fold decrease in appointment waiting time when using a team-based model.29
Some studies have found a decrease in office visits after implementing team-based care.7 However, these reports also found a corresponding increase (by as much as 80%) in the use of secure messaging and telephone encounters, which translated to an overall enhanced communication with patients and ultimately increased access to care.7
Decreased costs. Several controlled trials have looked at the financial impact of using team-based care to manage chronic conditions such as asthma, CHF, and diabetes. Rich et al30 found a nurse-directed program of patient self-management support via telephone and home visit follow-up was associated with a 56% reduction in hospital readmissions, which translated to a $460 decrease in cost per patient over a 3-month period compared to a control group. In a study by Domurat,31 hospital stays were 50% shorter for high-risk diabetes patients who were managed by a team that offered planned visits, telephone contact, and group visits; this resulted in a lower cost of care. Katon et al32 found that when a nurse manager was added to a primary care team to enhance self-management support, intensify treatment, and coordinate continuity of care for patients with multiple chronic conditions, outpatient health costs were decreased by $594 per patient over 24 months.
Liu et al33 randomly assigned 354 patients in a VA primary care clinic who met criteria for major depression or dysthymia to usual care or a collaborative care model. The collaborative care model included a mental health care team that provided telephone contact to encourage medication adherence and reviewed and suggested modifications to the treatment plan. After an initial expenditure of $519 per patient, a savings of approximately $33 per patient for total outpatient costs was realized.
A team-based coordinated care program for patients with multiple chronic conditions reduced patient visits to specialists by 24%, ED visits by 13%, and hospitalizations by 39%.34 An internal evaluation found that the program saved money by reducing admissions, including intensive care unit stays and “observational” stays for Medicare fee-for-service patients.35
What about reimbursement? Most studies that have evaluated the financial aspects of implementing team-based care have calculated the cost savings for the health system—rather than for an individual practice—through decreased hospital admissions, readmissions, and ED visits. Efficient, high-quality teams will require a substantial initial investment of time and hiring and training of staff before savings can be realized.
Team-based care may not be financially sustainable unless current reimbursement models are changed. The current US system bases payment on quantity of care instead of quality of care, reimburses only for clinician services, and does not compensate teams.36 The Centers for Medicare and Medicaid Services (CMS) has begun to recognize the need to reimburse for services that are not delivered in face-to-face patient encounters. For example, the agency established a new G-code that can be used for non-face-to-face care management services for Medicare patients with 2 or more significant chronic conditions; this code took effect on January 1, 2015.37
Some insurers are reimbursing practices for obtaining designation as a PCMH. This type of reimbursement could be expanded to include other types of team-based efforts—such as self-management support and health coaching.
Improved team satisfaction. While many primary care providers are experiencing fatigue and burnout,38 support staff in many practices also experience job dissatisfaction, which leads to increased absenteeism and high turnover. Several studies indicate that involving all levels of staff in the improvement process and empowering them to work to their full potential by enhancing their roles and realigning responsibilities can increase satisfaction.7,11,21,38,39 This in turn can lead to increased loyalty, commitment, and productivity, with decreased burnout and turnover.
TABLE 2
| Team-based care: Additional resources | |
| Resource | Comments |
The Dartmouth Institute Microsystem Academy | This site includes assessment tools and strategies for implementing clinical microsystems into practices |
Improving Chronic Illness Care | This site provides information about the chronic care model, care coordination, and patient-centered medical homes |
TeamSTEPPS | TeamSTEPPS is an evidence-based teamwork system to improve communication and teamwork skills among health care professionals. All resources, including training materials, are free and downloadable |
Godfrey MM, Melin CN, Muething SE, et al. Clinical microsystems, Part 3. Transformation of two hospitals using microsystem, mesosystem, and macrosystem strategies. Jt Comm J Qual Patient Saf. 2008;34:591-603. | This article provides resources and strategies to engage all levels of the health system in team-based care |
McKinley KE, Berry SA, Laam LA, et al. Clinical microsystems, Part 4. Building innovative population-specific mesosystems. Jt Comm J Qual Patient Saf. 2008;34:655-663. | This article describes how to engage leadership at the health systems level |
Adapting team-based care for smaller practices
Physicians who practice alone or in small groups may have limited capacity to employ allied health professionals. However, your “team” doesn’t need to be housed only in your office. One innovative approach is the community-based medical home, where physicians with medical homes and/or care teams in their offices refer to, and collaborate with, a network of community-based professionals and agencies for clinical and social service support for their patients.22 Some options are to partner with a local pharmacist or with insurers to use their community health workers, nurse case managers, and other self-management support tools.
While having team-based care strategies is necessary to achieve a PCMH designation, you do not need to seek such designation in order to practice team-based care. Start by conducting a full assessment of your practice, including patient panels, payer mix, current finances, regional pay-for-performance programs, leadership support, and your staff’s training and talents. In addition, determine what you value for your practice and what outcomes you hope for, along with a clear plan of how to measure these outcomes. This will allow you to determine if the estimated cost of the proposed strategy is “worth it” in terms of your individual situation and goals.
CORRESPONDENCE
Michele Q. Zawora, MD, Thomas Jefferson University, 1015 Walnut Street, Suite 401, Philadelphia, Pa 19107; [email protected]
1. Institute of Healthcare Improvement. The IHI Triple Aim. Institute for Healthcare Improvement Web site. Available at http://www.ihi.org/offerings/Initiatives/TripleAim/Pages/default.aspx. Accessed February 5, 2015.
2.Petterson SM, Liaw WR, Phillips RL Jr, et al. Projecting US primary care physician workforce needs: 2010-2025. Ann Fam Med. 2012;10:503-509.
3. Naylor MD, Coburn KD, Kurtzman ET, et al. Inter-professional team-based primary care for chronically ill adults: State of the science. White paper presented at: the ABIM Foundation meeting to Advance Team-Based Care for the Chronically Ill in Ambulatory Settings; March 24-25, 2010; Philadelphia, PA.
4.Boult C, Green AF, Boult LB, et al. Successful models of comprehensive care for older adults with chronic conditions: Evidence for the Institute of Medicine’s Retooling for an Aging America report. J Am Geriatr Soc. 2009;57:2328-2337.
5.Kuzel AJ. Keys to high-functioning office teams. Family Practice Management. 2011;18:15-18.
6.Sinsky CA, Willard-Grace R, Schutzbank AM, et al. In search of joy in practice: a report of 23 high-functioning primary care practices. Ann Fam Med. 2013;11:272-278.
7. Reid RJ, Coleman K, Johnson EA, et al. The Group Health medical home at year two: cost savings, higher patient satisfaction, and less burnout for providers. Health Aff (Millwood). 2010;29:835-843.
8. Bodenheimer T, Laing BY. The teamlet model of primary care. Ann Fam Med. 2007;5:457-461.
9. Chen EH, Thom DH, Hessler DM, et al. Using the teamlet model to improve chronic care in an academic primary care practice. J Gen Intern Med. 2010;25(suppl 4):S610-S614.
10. Wasson JH, Anders SG, Moore LG, et al. Clinical microsystems, part 2. Learning from micro practices about providing patients the care they want and need. Jt Comm J Qual Patient Saf. 2008;34:445-452.
11. Williams I, Dickinson H, Robinson S. Clinical microsystems: An evaluation. Health Services Management Centre, School of Public Policy, University of Birmingham, England; 2007. Available at: http://chain.ulcc.ac.uk/chain/documents/hsmc_evaluation_report_CMS2007final.pdf. Accessed February 20, 2015.
12. Willard R, Bodenheimer T. The building blocks of high-performing primary care: Lessons from the field. April 2012. Available at: http://www.chcf.org/~/media/MEDIA%20LIBRARY%20Files/PDF/B/PDF%20BuildingBlocksPrimaryCare.pdf. Accessed February 10, 2015.
13. Wagner EH. The role of patient care teams in chronic disease management. BMJ. 2000;320:569-572.
14. Boult C, Karm L, Groves C. Improving chronic care: the “guided care” model. Perm J. 2008;12:50-54.
15. Smith SM, Soubhi H, Fortin M, et al. Interventions for improving outcomes in patients with multimorbidity in primary care and community settings. Cochrane Database Syst Rev. 2012;4:CD006560.
16. Bloom FJ, Graf TR, Steele GD. Improved patient outcomes in 3 years with a system of care for diabetes. October 2012. Available at: http://iom.edu/~/media/Files/Perspectives-Files/2012/Commentaries/VSRT-Improved-Patient-Outcomes.pdf. Accessed February 10, 2015.
17. Bloom FJ Jr, Yan X, Stewart WF, et al. Primary care diabetes bundle management: 3-year outcomes for microvascular and macrovascular events. Am J Manag Care. 2014;20:e175-e182.
18. Pape GA, Hunt JS, Butler KL, et al. Team-based care approach to cholesterol management in diabetes mellitus: two-year cluster randomized controlled trial. Arch Intern Med. 2011;171:1480-1486.
19. Scanlon DP, Hollenbeak CS, Beich J, et al. Financial and clinical impact of team-based treatment for medicaid enrollees with diabetes in a federally qualified health center. Diabetes Care. 2008;31:2160-2165.
20. Renders CM, Valk GD, Griffin S, et al. Interventions to improve the management of diabetes mellitus in primary care, outpatient and community settings. Cochrane Database Syst Rev. 2001;(1):CD001481.
21. Goldberg GD, Beeson T, Kuzel AJ, et al. Team-based care: a critical element of primary care practice transformation. Popul Health Manag. 2013;16:150-156.
22. Lipton HL. Home is where the health is: advancing team-based care in chronic disease management. Arch Intern Med. 2009;169:1945-1948.
23. Farris KB, Côté I, Feeny D, et al. Enhancing primary care for complex patients. Demonstration project using multidisciplinary teams. Can Fam Physician. 2004;50:998-1003.
24. Anderson P, Halley MD. A new approach to making your doctor-nurse team more productive. Fam Pract Manag. 2008;15:35-40.
25. Grumbach K, Bodenheimer T, Grundy P. The outcomes of implementing patient-centered medical home interventions: A review of the evidence on quality, access and costs from recent prospective evaluation studies, August 2009. Washington, DC; Patient-Centered Primary Care Collaborative; 2009.
26. Smith M, Giuliano MR, Starkowski MP. In Connecticut: improving patient medication management in primary care. Health Aff (Millwood). 2011;30:646-654.
27. Kaboli PJ, Hoth AB, McClimon BJ, et al. Clinical pharmacists and inpatient medical care: a systematic review. Arch Intern Med. 2006;16:955-964.
28. Bray P, Roupe M, Young S, et al. Feasibility and effectiveness of system redesign for diabetes care management in rural areas: the eastern North Carolina experience. Diabetes Educ. 2005;31:712-718.
29. Institute for Healthcare Improvement. Health Partners uses “BestCare” practices to improve care and outcomes, reduce costs. Institute for Healthcare Improvement Web site. Available at http://www.ihi.org/engage/initiatives/TripleAim/Documents/IHITripleAimHealthPartnersSummaryofSuccessJul09v2.pdf. Accessed February 5, 2015.
30. Rich MW, Beckman V, Wittenberg C, et al. A multidisciplinary intervention to prevent the readmission of elderly patients with congestive heart failure. N Engl J Med. 1995;333:1190-1195.
31. Domurat ES. Diabetes managed care and clinical outcomes: the Harbor City, California Kaiser Permanente diabetes care system. Am J Manag Care. 1999;5:1299-1307.
32. Katon W, Russo J, Lin EH, et al. Cost-effectiveness of a multicondition collaborative care intervention: a randomized controlled trial. Arch Gen Psychiatry. 2012;69:506-514.
33. Liu CF, Hendrick SC, Chaney EF, et al. Cost-effectiveness of collaborative care for depression in a primary care veteran population. Psychiatr Serv. 2003;54:698-704.
34. Berenson RA; The Urban Institute. Challenging the status quo in chronic disease care: seven case studies. California Healthcare Foundation: 2006. California HealthCare Foundation Web site. Available at: http://www.chcf.org/~/media/MEDIA%20LIBRARY%20Files/PDF/C/PDF%20ChallengingStatusQuoCaseStudies.pdf. Accessed February 5, 2015.
35. Coleman EA, Smith JD, Frank JC, et al. Preparing patients and caregivers to participate in care delivered across settings: the Care Transitions Intervention. J Am Geriatr Soc. 2004;52:1817-1825.
36. Schneider ME. Medicare finalizes plan for non-face-to-face payments. Family Practice News Web site. Available at: http://www.familypracticenews.com/?id=2633&tx_ttnews%5Btt_news%5D=226457&cHash=2aeafe0585c7156dcf23891d010cd12f. Accessed December 2, 2013.
37. Centers for Medicare & Medicaid Services. Fact sheets: Policy and payment changes to the Medicare Physician Fee Schedule for 2015. Centers for Medicare & Medicaid Services Web site. Available at: http://www.cms.gov/newsroom/mediareleasedatabase/fact-sheets/2014-Fact-sheets-items/2014-10-31-7.html. Accessed February 13, 2015.
38. Berry LL, Dunham J. Redefining the patient experience with collaborative care. Harvard Business Review Blog Network. Harvard Business Review Web site. Available at: https://hbr.org/2013/09/redefining-the-patient-experience-with-collaborative-care/. Accessed February 5, 2015.
39. Lyon RK, Slawson J. An organized approach to chronic disease care. Fam Pract Manag. 2011;18:27-31.
1. Institute of Healthcare Improvement. The IHI Triple Aim. Institute for Healthcare Improvement Web site. Available at http://www.ihi.org/offerings/Initiatives/TripleAim/Pages/default.aspx. Accessed February 5, 2015.
2.Petterson SM, Liaw WR, Phillips RL Jr, et al. Projecting US primary care physician workforce needs: 2010-2025. Ann Fam Med. 2012;10:503-509.
3. Naylor MD, Coburn KD, Kurtzman ET, et al. Inter-professional team-based primary care for chronically ill adults: State of the science. White paper presented at: the ABIM Foundation meeting to Advance Team-Based Care for the Chronically Ill in Ambulatory Settings; March 24-25, 2010; Philadelphia, PA.
4.Boult C, Green AF, Boult LB, et al. Successful models of comprehensive care for older adults with chronic conditions: Evidence for the Institute of Medicine’s Retooling for an Aging America report. J Am Geriatr Soc. 2009;57:2328-2337.
5.Kuzel AJ. Keys to high-functioning office teams. Family Practice Management. 2011;18:15-18.
6.Sinsky CA, Willard-Grace R, Schutzbank AM, et al. In search of joy in practice: a report of 23 high-functioning primary care practices. Ann Fam Med. 2013;11:272-278.
7. Reid RJ, Coleman K, Johnson EA, et al. The Group Health medical home at year two: cost savings, higher patient satisfaction, and less burnout for providers. Health Aff (Millwood). 2010;29:835-843.
8. Bodenheimer T, Laing BY. The teamlet model of primary care. Ann Fam Med. 2007;5:457-461.
9. Chen EH, Thom DH, Hessler DM, et al. Using the teamlet model to improve chronic care in an academic primary care practice. J Gen Intern Med. 2010;25(suppl 4):S610-S614.
10. Wasson JH, Anders SG, Moore LG, et al. Clinical microsystems, part 2. Learning from micro practices about providing patients the care they want and need. Jt Comm J Qual Patient Saf. 2008;34:445-452.
11. Williams I, Dickinson H, Robinson S. Clinical microsystems: An evaluation. Health Services Management Centre, School of Public Policy, University of Birmingham, England; 2007. Available at: http://chain.ulcc.ac.uk/chain/documents/hsmc_evaluation_report_CMS2007final.pdf. Accessed February 20, 2015.
12. Willard R, Bodenheimer T. The building blocks of high-performing primary care: Lessons from the field. April 2012. Available at: http://www.chcf.org/~/media/MEDIA%20LIBRARY%20Files/PDF/B/PDF%20BuildingBlocksPrimaryCare.pdf. Accessed February 10, 2015.
13. Wagner EH. The role of patient care teams in chronic disease management. BMJ. 2000;320:569-572.
14. Boult C, Karm L, Groves C. Improving chronic care: the “guided care” model. Perm J. 2008;12:50-54.
15. Smith SM, Soubhi H, Fortin M, et al. Interventions for improving outcomes in patients with multimorbidity in primary care and community settings. Cochrane Database Syst Rev. 2012;4:CD006560.
16. Bloom FJ, Graf TR, Steele GD. Improved patient outcomes in 3 years with a system of care for diabetes. October 2012. Available at: http://iom.edu/~/media/Files/Perspectives-Files/2012/Commentaries/VSRT-Improved-Patient-Outcomes.pdf. Accessed February 10, 2015.
17. Bloom FJ Jr, Yan X, Stewart WF, et al. Primary care diabetes bundle management: 3-year outcomes for microvascular and macrovascular events. Am J Manag Care. 2014;20:e175-e182.
18. Pape GA, Hunt JS, Butler KL, et al. Team-based care approach to cholesterol management in diabetes mellitus: two-year cluster randomized controlled trial. Arch Intern Med. 2011;171:1480-1486.
19. Scanlon DP, Hollenbeak CS, Beich J, et al. Financial and clinical impact of team-based treatment for medicaid enrollees with diabetes in a federally qualified health center. Diabetes Care. 2008;31:2160-2165.
20. Renders CM, Valk GD, Griffin S, et al. Interventions to improve the management of diabetes mellitus in primary care, outpatient and community settings. Cochrane Database Syst Rev. 2001;(1):CD001481.
21. Goldberg GD, Beeson T, Kuzel AJ, et al. Team-based care: a critical element of primary care practice transformation. Popul Health Manag. 2013;16:150-156.
22. Lipton HL. Home is where the health is: advancing team-based care in chronic disease management. Arch Intern Med. 2009;169:1945-1948.
23. Farris KB, Côté I, Feeny D, et al. Enhancing primary care for complex patients. Demonstration project using multidisciplinary teams. Can Fam Physician. 2004;50:998-1003.
24. Anderson P, Halley MD. A new approach to making your doctor-nurse team more productive. Fam Pract Manag. 2008;15:35-40.
25. Grumbach K, Bodenheimer T, Grundy P. The outcomes of implementing patient-centered medical home interventions: A review of the evidence on quality, access and costs from recent prospective evaluation studies, August 2009. Washington, DC; Patient-Centered Primary Care Collaborative; 2009.
26. Smith M, Giuliano MR, Starkowski MP. In Connecticut: improving patient medication management in primary care. Health Aff (Millwood). 2011;30:646-654.
27. Kaboli PJ, Hoth AB, McClimon BJ, et al. Clinical pharmacists and inpatient medical care: a systematic review. Arch Intern Med. 2006;16:955-964.
28. Bray P, Roupe M, Young S, et al. Feasibility and effectiveness of system redesign for diabetes care management in rural areas: the eastern North Carolina experience. Diabetes Educ. 2005;31:712-718.
29. Institute for Healthcare Improvement. Health Partners uses “BestCare” practices to improve care and outcomes, reduce costs. Institute for Healthcare Improvement Web site. Available at http://www.ihi.org/engage/initiatives/TripleAim/Documents/IHITripleAimHealthPartnersSummaryofSuccessJul09v2.pdf. Accessed February 5, 2015.
30. Rich MW, Beckman V, Wittenberg C, et al. A multidisciplinary intervention to prevent the readmission of elderly patients with congestive heart failure. N Engl J Med. 1995;333:1190-1195.
31. Domurat ES. Diabetes managed care and clinical outcomes: the Harbor City, California Kaiser Permanente diabetes care system. Am J Manag Care. 1999;5:1299-1307.
32. Katon W, Russo J, Lin EH, et al. Cost-effectiveness of a multicondition collaborative care intervention: a randomized controlled trial. Arch Gen Psychiatry. 2012;69:506-514.
33. Liu CF, Hendrick SC, Chaney EF, et al. Cost-effectiveness of collaborative care for depression in a primary care veteran population. Psychiatr Serv. 2003;54:698-704.
34. Berenson RA; The Urban Institute. Challenging the status quo in chronic disease care: seven case studies. California Healthcare Foundation: 2006. California HealthCare Foundation Web site. Available at: http://www.chcf.org/~/media/MEDIA%20LIBRARY%20Files/PDF/C/PDF%20ChallengingStatusQuoCaseStudies.pdf. Accessed February 5, 2015.
35. Coleman EA, Smith JD, Frank JC, et al. Preparing patients and caregivers to participate in care delivered across settings: the Care Transitions Intervention. J Am Geriatr Soc. 2004;52:1817-1825.
36. Schneider ME. Medicare finalizes plan for non-face-to-face payments. Family Practice News Web site. Available at: http://www.familypracticenews.com/?id=2633&tx_ttnews%5Btt_news%5D=226457&cHash=2aeafe0585c7156dcf23891d010cd12f. Accessed December 2, 2013.
37. Centers for Medicare & Medicaid Services. Fact sheets: Policy and payment changes to the Medicare Physician Fee Schedule for 2015. Centers for Medicare & Medicaid Services Web site. Available at: http://www.cms.gov/newsroom/mediareleasedatabase/fact-sheets/2014-Fact-sheets-items/2014-10-31-7.html. Accessed February 13, 2015.
38. Berry LL, Dunham J. Redefining the patient experience with collaborative care. Harvard Business Review Blog Network. Harvard Business Review Web site. Available at: https://hbr.org/2013/09/redefining-the-patient-experience-with-collaborative-care/. Accessed February 5, 2015.
39. Lyon RK, Slawson J. An organized approach to chronic disease care. Fam Pract Manag. 2011;18:27-31.
A new adjunctive Tx option for venous stasis ulcers
Consider adding simvastatin 40 mg/d to standard wound care and compression for patients with venous stasis ulcers.1
Strength of recommendation
B: Based on a high-quality randomized controlled trial (RCT).
Evangelista MT, Casintahan MF, Villafuerte LL. Simvastatin as a novel therapeutic agent for venous ulcers: a randomized, double-blind, placebo-controlled trial. Br J Dermatol. 2014;170:1151-1157.
Illustrative case
A 74-year-old woman with chronic lower extremity edema seeks treatment for a nonhealing venous stasis ulcer. For the past 9 months, she’s been wearing compression stockings and receiving intermittent home-based wound care, but nothing seems to help. She asks if there’s anything else she can try.
Venous stasis ulcers affect 1% of US adults and lead to substantial morbidity and more than $2 billion in annual health care expenditures.1,2 Edema management—generally limb elevation and compression therapy—has been the mainstay of therapy. Treatment can be lengthy, and ulcer recurrences are common.2,3
Statins have been found to help wound healing through their diverse physiologic (pleiotropic) effects. Evidence shows they can be beneficial for treating diabetic foot ulcers,4 pressure ulcers,5 and ulcerations associated with systemic sclerosis and Raynaud’s phenomenon.6 Evangelista et al1 investigated whether adding a statin to standard wound care and compression could improve venous stasis ulcer healing.
STUDY SUMMARY: Ulcers are more likely to close when a statin is added to standard care
This randomized, double-blind, placebo-controlled trial was performed at a large medical center in the Philippines. It was designed to assess the efficacy and safety of simvastatin 40 mg/d for venous ulcer healing when combined with standard treatment (compression therapy, limb elevation, and standard wound care).1
Researchers randomized 66 patients ages 41 to 71 who’d had one or more venous ulcers for at least 3 months to receive either simvastatin 40 mg/d (N=32) or an identical appearing placebo (N=34). Patients were excluded if they were pregnant, had an ulcer that was infected or >10 cm in diameter, or were taking any medication that could interact with a statin. Patients were stratified according to ulcer diameter (≤5 cm and >5 cm). There was no statistically significant difference between the 2 groups in the duration of venous ulceration (3.80 years in the placebo group vs 3.93 years in the simvastatin group) or incidence of diabetes (5% in the placebo group vs 3% in the simvastatin group).
The primary outcome was the proportion of patients whose ulcers completely healed at 10 weeks. Secondary outcomes were measures of the total surface area healed and healing time, and Dermatology Life Quality Index (DLQI) scores. Baseline ulcer diameter and surface area and DLQI scores were obtained prior to therapy. The same dermatologist, who was blinded to the patients’ assigned group, evaluated all patients every 2 weeks until wound closure or for a maximum of 10 weeks.
Overall, 90% of the patients who received simvastatin had complete ulcer closure at 10 weeks, compared with 34% of patients in the control group (relative risk [RR]=0.16; 95% confidence interval [CI], 0.05-0.47; number needed to treat [NNT]=2).
Among patients with ulcers ≤5 cm, 100% of the ulcers healed in the simvastatin group, compared to 50% in the control group (RR=0.10; 95% CI, 0.01-0.71; NNT=2). Perhaps more importantly, in patients with ulcers >5 cm, 67% of the ulcers in the simvastatin group had closure with a mean healing time of 9 weeks, whereas none of the ulcers of this size closed in the control group (RR=0.33; 95% CI, 0.12-0.84; NNT=1.5), and the mean healed area was significantly larger in patients who received simvastatin (28.9 cm2 vs 19.6 cm2; P=.03).
In addition, in the simvastatin group, healing times were significantly reduced (7.53±1.34 weeks vs 8.55±1.13 weeks) and quality of life (as evaluated by DLQI scoring) significantly improved compared to the control group.
Study dropouts (8%; 2 in the placebo group and 3 in the intervention group) were minimal. Using intention-to-treat analysis and worst-case scenarios for dropouts did not affect the primary outcome. There were no withdrawals for adverse reactions.
WHAT’S NEW: Statins offer significant benefits for treating venous stasis ulcers
This is the first human study to investigate the use of a statin in venous stasis ulcer healing. This intervention demonstrated significant improvements in healing rate and time, a very small NNT for benefit, and improved patient quality of life compared to placebo.
CAVEATS: Results were found in a carefully selected group of patients
Many wounds will heal with compression therapy alone, as occurred in this study, where 50% of ulcers ≤5 cm treated with standard therapy healed, albeit at a somewhat slower rate. Adding another medication to the regimen when these patients generally have multiple comorbidities should always prompt caution.
The study by Evangelista et al1 was performed in a select population, and the exclusion criteria included the use of some commonly prescribed medications, such as angiotensin-converting enzyme inhibitors. No data were collected on patient body mass index, which is a risk factor for delayed healing. The prevalence of obesity is lower in the Philippines than in the United States, and it is uncertain what role this difference would have in the statin’s effectiveness. Further studies, especially those conducted with a less selective population, would better clarify the generalizability of this intervention.
We found the results of this study impressive. The methods reported are rigorous and consistent with standard RCT methodologies. This is the only study of a statin in human venous stasis disease, but studies in animals—and studies of statins for other types of ulcers in humans—have consistently suggested benefit. It seems hard to argue against adding this low-cost, low-risk intervention.
CHALLENGES TO IMPLEMENTATION
There are no known barriers to implementing this practice.
ACKNOWLEDGEMENT
The PURLs Surveillance System was supported in part by Grant Number UL1RR024999 from the National Center For Research Resources, a Clinical Translational Science Award to the University of Chicago. The content is solely the responsibility of the authors and does not necessarily represent the official views of the National Center For Research Resources or the National Institutes of Health.
1. Evangelista MT, Casintahan MF, Villafuerte LL. Simvastatin as a novel therapeutic agent for venous ulcers: a randomized, double-blind, placebo-controlled trial. Br J Dermatol. 2014;170:1151-1157.
2. Collins L, Seraj S. Diagnosis and treatment of venous ulcers. Am Fam Physician. 2010;81:989-996.
3. The Australian Wound Management Association Inc, New Zealand Wound Care Society Inc. Australian and New Zealand clinical practice guideline for prevention and management of venous leg ulcers. 2011. Australian Government National Health and Medical Research Council Web site. Available at: http://www.nhmrc.gov.au/_files_nhmrc/publications/attachments/ext003_venous_leg_ulcers_aust_nz_0.pdf. Accessed February 13, 2015.
4. Johansen OE, Birkeland KI, Jørgensen AP, et al. Diabetic foot ulcer burden may be modified by high-dose atorvastatin: A 6-month randomized controlled pilot trial. J Diabetes. 2009;1:182-187.
5. Farsaei S, Khalili H, Farboud ES, et al. Efficacy of topical atorvastatin for the treatment of pressure ulcers: a randomized clinical trial. Pharmacotherapy. 2014;34:19-27.
6. Abou-Raya A, Abou-Raya S, Helmii M. Statins: potentially useful in therapy of systemic sclerosis-related Raynaud’s phenomenon and digital ulcers. J Rheumatol. 2008;35:1801-1808.
Consider adding simvastatin 40 mg/d to standard wound care and compression for patients with venous stasis ulcers.1
Strength of recommendation
B: Based on a high-quality randomized controlled trial (RCT).
Evangelista MT, Casintahan MF, Villafuerte LL. Simvastatin as a novel therapeutic agent for venous ulcers: a randomized, double-blind, placebo-controlled trial. Br J Dermatol. 2014;170:1151-1157.
Illustrative case
A 74-year-old woman with chronic lower extremity edema seeks treatment for a nonhealing venous stasis ulcer. For the past 9 months, she’s been wearing compression stockings and receiving intermittent home-based wound care, but nothing seems to help. She asks if there’s anything else she can try.
Venous stasis ulcers affect 1% of US adults and lead to substantial morbidity and more than $2 billion in annual health care expenditures.1,2 Edema management—generally limb elevation and compression therapy—has been the mainstay of therapy. Treatment can be lengthy, and ulcer recurrences are common.2,3
Statins have been found to help wound healing through their diverse physiologic (pleiotropic) effects. Evidence shows they can be beneficial for treating diabetic foot ulcers,4 pressure ulcers,5 and ulcerations associated with systemic sclerosis and Raynaud’s phenomenon.6 Evangelista et al1 investigated whether adding a statin to standard wound care and compression could improve venous stasis ulcer healing.
STUDY SUMMARY: Ulcers are more likely to close when a statin is added to standard care
This randomized, double-blind, placebo-controlled trial was performed at a large medical center in the Philippines. It was designed to assess the efficacy and safety of simvastatin 40 mg/d for venous ulcer healing when combined with standard treatment (compression therapy, limb elevation, and standard wound care).1
Researchers randomized 66 patients ages 41 to 71 who’d had one or more venous ulcers for at least 3 months to receive either simvastatin 40 mg/d (N=32) or an identical appearing placebo (N=34). Patients were excluded if they were pregnant, had an ulcer that was infected or >10 cm in diameter, or were taking any medication that could interact with a statin. Patients were stratified according to ulcer diameter (≤5 cm and >5 cm). There was no statistically significant difference between the 2 groups in the duration of venous ulceration (3.80 years in the placebo group vs 3.93 years in the simvastatin group) or incidence of diabetes (5% in the placebo group vs 3% in the simvastatin group).
The primary outcome was the proportion of patients whose ulcers completely healed at 10 weeks. Secondary outcomes were measures of the total surface area healed and healing time, and Dermatology Life Quality Index (DLQI) scores. Baseline ulcer diameter and surface area and DLQI scores were obtained prior to therapy. The same dermatologist, who was blinded to the patients’ assigned group, evaluated all patients every 2 weeks until wound closure or for a maximum of 10 weeks.
Overall, 90% of the patients who received simvastatin had complete ulcer closure at 10 weeks, compared with 34% of patients in the control group (relative risk [RR]=0.16; 95% confidence interval [CI], 0.05-0.47; number needed to treat [NNT]=2).
Among patients with ulcers ≤5 cm, 100% of the ulcers healed in the simvastatin group, compared to 50% in the control group (RR=0.10; 95% CI, 0.01-0.71; NNT=2). Perhaps more importantly, in patients with ulcers >5 cm, 67% of the ulcers in the simvastatin group had closure with a mean healing time of 9 weeks, whereas none of the ulcers of this size closed in the control group (RR=0.33; 95% CI, 0.12-0.84; NNT=1.5), and the mean healed area was significantly larger in patients who received simvastatin (28.9 cm2 vs 19.6 cm2; P=.03).
In addition, in the simvastatin group, healing times were significantly reduced (7.53±1.34 weeks vs 8.55±1.13 weeks) and quality of life (as evaluated by DLQI scoring) significantly improved compared to the control group.
Study dropouts (8%; 2 in the placebo group and 3 in the intervention group) were minimal. Using intention-to-treat analysis and worst-case scenarios for dropouts did not affect the primary outcome. There were no withdrawals for adverse reactions.
WHAT’S NEW: Statins offer significant benefits for treating venous stasis ulcers
This is the first human study to investigate the use of a statin in venous stasis ulcer healing. This intervention demonstrated significant improvements in healing rate and time, a very small NNT for benefit, and improved patient quality of life compared to placebo.
CAVEATS: Results were found in a carefully selected group of patients
Many wounds will heal with compression therapy alone, as occurred in this study, where 50% of ulcers ≤5 cm treated with standard therapy healed, albeit at a somewhat slower rate. Adding another medication to the regimen when these patients generally have multiple comorbidities should always prompt caution.
The study by Evangelista et al1 was performed in a select population, and the exclusion criteria included the use of some commonly prescribed medications, such as angiotensin-converting enzyme inhibitors. No data were collected on patient body mass index, which is a risk factor for delayed healing. The prevalence of obesity is lower in the Philippines than in the United States, and it is uncertain what role this difference would have in the statin’s effectiveness. Further studies, especially those conducted with a less selective population, would better clarify the generalizability of this intervention.
We found the results of this study impressive. The methods reported are rigorous and consistent with standard RCT methodologies. This is the only study of a statin in human venous stasis disease, but studies in animals—and studies of statins for other types of ulcers in humans—have consistently suggested benefit. It seems hard to argue against adding this low-cost, low-risk intervention.
CHALLENGES TO IMPLEMENTATION
There are no known barriers to implementing this practice.
ACKNOWLEDGEMENT
The PURLs Surveillance System was supported in part by Grant Number UL1RR024999 from the National Center For Research Resources, a Clinical Translational Science Award to the University of Chicago. The content is solely the responsibility of the authors and does not necessarily represent the official views of the National Center For Research Resources or the National Institutes of Health.
Consider adding simvastatin 40 mg/d to standard wound care and compression for patients with venous stasis ulcers.1
Strength of recommendation
B: Based on a high-quality randomized controlled trial (RCT).
Evangelista MT, Casintahan MF, Villafuerte LL. Simvastatin as a novel therapeutic agent for venous ulcers: a randomized, double-blind, placebo-controlled trial. Br J Dermatol. 2014;170:1151-1157.
Illustrative case
A 74-year-old woman with chronic lower extremity edema seeks treatment for a nonhealing venous stasis ulcer. For the past 9 months, she’s been wearing compression stockings and receiving intermittent home-based wound care, but nothing seems to help. She asks if there’s anything else she can try.
Venous stasis ulcers affect 1% of US adults and lead to substantial morbidity and more than $2 billion in annual health care expenditures.1,2 Edema management—generally limb elevation and compression therapy—has been the mainstay of therapy. Treatment can be lengthy, and ulcer recurrences are common.2,3
Statins have been found to help wound healing through their diverse physiologic (pleiotropic) effects. Evidence shows they can be beneficial for treating diabetic foot ulcers,4 pressure ulcers,5 and ulcerations associated with systemic sclerosis and Raynaud’s phenomenon.6 Evangelista et al1 investigated whether adding a statin to standard wound care and compression could improve venous stasis ulcer healing.
STUDY SUMMARY: Ulcers are more likely to close when a statin is added to standard care
This randomized, double-blind, placebo-controlled trial was performed at a large medical center in the Philippines. It was designed to assess the efficacy and safety of simvastatin 40 mg/d for venous ulcer healing when combined with standard treatment (compression therapy, limb elevation, and standard wound care).1
Researchers randomized 66 patients ages 41 to 71 who’d had one or more venous ulcers for at least 3 months to receive either simvastatin 40 mg/d (N=32) or an identical appearing placebo (N=34). Patients were excluded if they were pregnant, had an ulcer that was infected or >10 cm in diameter, or were taking any medication that could interact with a statin. Patients were stratified according to ulcer diameter (≤5 cm and >5 cm). There was no statistically significant difference between the 2 groups in the duration of venous ulceration (3.80 years in the placebo group vs 3.93 years in the simvastatin group) or incidence of diabetes (5% in the placebo group vs 3% in the simvastatin group).
The primary outcome was the proportion of patients whose ulcers completely healed at 10 weeks. Secondary outcomes were measures of the total surface area healed and healing time, and Dermatology Life Quality Index (DLQI) scores. Baseline ulcer diameter and surface area and DLQI scores were obtained prior to therapy. The same dermatologist, who was blinded to the patients’ assigned group, evaluated all patients every 2 weeks until wound closure or for a maximum of 10 weeks.
Overall, 90% of the patients who received simvastatin had complete ulcer closure at 10 weeks, compared with 34% of patients in the control group (relative risk [RR]=0.16; 95% confidence interval [CI], 0.05-0.47; number needed to treat [NNT]=2).
Among patients with ulcers ≤5 cm, 100% of the ulcers healed in the simvastatin group, compared to 50% in the control group (RR=0.10; 95% CI, 0.01-0.71; NNT=2). Perhaps more importantly, in patients with ulcers >5 cm, 67% of the ulcers in the simvastatin group had closure with a mean healing time of 9 weeks, whereas none of the ulcers of this size closed in the control group (RR=0.33; 95% CI, 0.12-0.84; NNT=1.5), and the mean healed area was significantly larger in patients who received simvastatin (28.9 cm2 vs 19.6 cm2; P=.03).
In addition, in the simvastatin group, healing times were significantly reduced (7.53±1.34 weeks vs 8.55±1.13 weeks) and quality of life (as evaluated by DLQI scoring) significantly improved compared to the control group.
Study dropouts (8%; 2 in the placebo group and 3 in the intervention group) were minimal. Using intention-to-treat analysis and worst-case scenarios for dropouts did not affect the primary outcome. There were no withdrawals for adverse reactions.
WHAT’S NEW: Statins offer significant benefits for treating venous stasis ulcers
This is the first human study to investigate the use of a statin in venous stasis ulcer healing. This intervention demonstrated significant improvements in healing rate and time, a very small NNT for benefit, and improved patient quality of life compared to placebo.
CAVEATS: Results were found in a carefully selected group of patients
Many wounds will heal with compression therapy alone, as occurred in this study, where 50% of ulcers ≤5 cm treated with standard therapy healed, albeit at a somewhat slower rate. Adding another medication to the regimen when these patients generally have multiple comorbidities should always prompt caution.
The study by Evangelista et al1 was performed in a select population, and the exclusion criteria included the use of some commonly prescribed medications, such as angiotensin-converting enzyme inhibitors. No data were collected on patient body mass index, which is a risk factor for delayed healing. The prevalence of obesity is lower in the Philippines than in the United States, and it is uncertain what role this difference would have in the statin’s effectiveness. Further studies, especially those conducted with a less selective population, would better clarify the generalizability of this intervention.
We found the results of this study impressive. The methods reported are rigorous and consistent with standard RCT methodologies. This is the only study of a statin in human venous stasis disease, but studies in animals—and studies of statins for other types of ulcers in humans—have consistently suggested benefit. It seems hard to argue against adding this low-cost, low-risk intervention.
CHALLENGES TO IMPLEMENTATION
There are no known barriers to implementing this practice.
ACKNOWLEDGEMENT
The PURLs Surveillance System was supported in part by Grant Number UL1RR024999 from the National Center For Research Resources, a Clinical Translational Science Award to the University of Chicago. The content is solely the responsibility of the authors and does not necessarily represent the official views of the National Center For Research Resources or the National Institutes of Health.
1. Evangelista MT, Casintahan MF, Villafuerte LL. Simvastatin as a novel therapeutic agent for venous ulcers: a randomized, double-blind, placebo-controlled trial. Br J Dermatol. 2014;170:1151-1157.
2. Collins L, Seraj S. Diagnosis and treatment of venous ulcers. Am Fam Physician. 2010;81:989-996.
3. The Australian Wound Management Association Inc, New Zealand Wound Care Society Inc. Australian and New Zealand clinical practice guideline for prevention and management of venous leg ulcers. 2011. Australian Government National Health and Medical Research Council Web site. Available at: http://www.nhmrc.gov.au/_files_nhmrc/publications/attachments/ext003_venous_leg_ulcers_aust_nz_0.pdf. Accessed February 13, 2015.
4. Johansen OE, Birkeland KI, Jørgensen AP, et al. Diabetic foot ulcer burden may be modified by high-dose atorvastatin: A 6-month randomized controlled pilot trial. J Diabetes. 2009;1:182-187.
5. Farsaei S, Khalili H, Farboud ES, et al. Efficacy of topical atorvastatin for the treatment of pressure ulcers: a randomized clinical trial. Pharmacotherapy. 2014;34:19-27.
6. Abou-Raya A, Abou-Raya S, Helmii M. Statins: potentially useful in therapy of systemic sclerosis-related Raynaud’s phenomenon and digital ulcers. J Rheumatol. 2008;35:1801-1808.
1. Evangelista MT, Casintahan MF, Villafuerte LL. Simvastatin as a novel therapeutic agent for venous ulcers: a randomized, double-blind, placebo-controlled trial. Br J Dermatol. 2014;170:1151-1157.
2. Collins L, Seraj S. Diagnosis and treatment of venous ulcers. Am Fam Physician. 2010;81:989-996.
3. The Australian Wound Management Association Inc, New Zealand Wound Care Society Inc. Australian and New Zealand clinical practice guideline for prevention and management of venous leg ulcers. 2011. Australian Government National Health and Medical Research Council Web site. Available at: http://www.nhmrc.gov.au/_files_nhmrc/publications/attachments/ext003_venous_leg_ulcers_aust_nz_0.pdf. Accessed February 13, 2015.
4. Johansen OE, Birkeland KI, Jørgensen AP, et al. Diabetic foot ulcer burden may be modified by high-dose atorvastatin: A 6-month randomized controlled pilot trial. J Diabetes. 2009;1:182-187.
5. Farsaei S, Khalili H, Farboud ES, et al. Efficacy of topical atorvastatin for the treatment of pressure ulcers: a randomized clinical trial. Pharmacotherapy. 2014;34:19-27.
6. Abou-Raya A, Abou-Raya S, Helmii M. Statins: potentially useful in therapy of systemic sclerosis-related Raynaud’s phenomenon and digital ulcers. J Rheumatol. 2008;35:1801-1808.
Copyright © 2015 Family Physicians Inquiries Network. All rights reserved.
Study reveals underuse of HSCT

Photo by Chad McNeeley
A retrospective study has shown that, since the 1980s, the world has seen a substantial increase in the use of hematopoietic stem cell transplants (HSCTs) and a significant rise in the number of donor registries.
Still, it seems many patients who would benefit from HSCT do not undergo the procedure due to a chronic shortage of resources and donors.
Dietger Niederwieser, MD, of the University Hospital Leipzig in Germany, and his colleagues recounted these findings in The Lancet Haematology.
Using data collected by the Worldwide Network for Blood and Marrow Transplantation (WBMT), the researchers systematically analyzed the growth of HSCT and changes in its use in 194 WHO member countries since the first HSCT was performed in 1957.
The team also examined the link between macroeconomic factors (eg, gross national income and healthcare expenditure) and transplant frequencies per 10 million inhabitants in each country.
The data showed that about 10,000 HSCTs had been performed by 1985, but that number rose to around 100,000 by 1995. By December 2012, nearly 1 million HSCTs—42% allogeneic and 58% autologous—had been performed at 1516 transplant centers across 75 countries.
Transplants were more common in countries with greater financial resources, more transplant teams, and an unrelated donor infrastructure. Most transplants were performed in Europe (53%), followed by the Americas (31%), South East Asia and the Western Pacific (15%), and the Eastern Mediterranean and Africa (2%).
The use of allogeneic HSCT has grown rapidly in all regions, without any signs of saturation. This likely reflects substantial underuse of this therapy, according to the researchers. And it suggests more patients would have received allogeneic transplants if they were accessible or suitable donors were available.
On the other hand, the researchers found that the number of countries with donor registries increased from 2 in 1987 to 57 in 2012. And the number of volunteer donors rose from 3072 in 1987 to over 22 million in 2012.
The international exchange of stem cell products also increased to more than 10,000 a year between 2006 and 2012, with substantial differences between countries in the amount of stem cells they import or export.
Despite these increases, the researchers noted that there are still too many patients who are unable to find a suitable donor. At any time, more than 37,000 people worldwide are waiting for an HSC donation.
“Patients, many of them children, are facing a life-and-death situation,” Dr Niederwieser said. “Ultimately, they will die if they cannot get the treatment they need. All countries need to provide adequate infrastructure for patients and donors to make sure that everyone who needs a transplant gets one, rather than the present situation in which access remains restricted to countries and people with sufficient resources.” ![]()

Photo by Chad McNeeley
A retrospective study has shown that, since the 1980s, the world has seen a substantial increase in the use of hematopoietic stem cell transplants (HSCTs) and a significant rise in the number of donor registries.
Still, it seems many patients who would benefit from HSCT do not undergo the procedure due to a chronic shortage of resources and donors.
Dietger Niederwieser, MD, of the University Hospital Leipzig in Germany, and his colleagues recounted these findings in The Lancet Haematology.
Using data collected by the Worldwide Network for Blood and Marrow Transplantation (WBMT), the researchers systematically analyzed the growth of HSCT and changes in its use in 194 WHO member countries since the first HSCT was performed in 1957.
The team also examined the link between macroeconomic factors (eg, gross national income and healthcare expenditure) and transplant frequencies per 10 million inhabitants in each country.
The data showed that about 10,000 HSCTs had been performed by 1985, but that number rose to around 100,000 by 1995. By December 2012, nearly 1 million HSCTs—42% allogeneic and 58% autologous—had been performed at 1516 transplant centers across 75 countries.
Transplants were more common in countries with greater financial resources, more transplant teams, and an unrelated donor infrastructure. Most transplants were performed in Europe (53%), followed by the Americas (31%), South East Asia and the Western Pacific (15%), and the Eastern Mediterranean and Africa (2%).
The use of allogeneic HSCT has grown rapidly in all regions, without any signs of saturation. This likely reflects substantial underuse of this therapy, according to the researchers. And it suggests more patients would have received allogeneic transplants if they were accessible or suitable donors were available.
On the other hand, the researchers found that the number of countries with donor registries increased from 2 in 1987 to 57 in 2012. And the number of volunteer donors rose from 3072 in 1987 to over 22 million in 2012.
The international exchange of stem cell products also increased to more than 10,000 a year between 2006 and 2012, with substantial differences between countries in the amount of stem cells they import or export.
Despite these increases, the researchers noted that there are still too many patients who are unable to find a suitable donor. At any time, more than 37,000 people worldwide are waiting for an HSC donation.
“Patients, many of them children, are facing a life-and-death situation,” Dr Niederwieser said. “Ultimately, they will die if they cannot get the treatment they need. All countries need to provide adequate infrastructure for patients and donors to make sure that everyone who needs a transplant gets one, rather than the present situation in which access remains restricted to countries and people with sufficient resources.” ![]()

Photo by Chad McNeeley
A retrospective study has shown that, since the 1980s, the world has seen a substantial increase in the use of hematopoietic stem cell transplants (HSCTs) and a significant rise in the number of donor registries.
Still, it seems many patients who would benefit from HSCT do not undergo the procedure due to a chronic shortage of resources and donors.
Dietger Niederwieser, MD, of the University Hospital Leipzig in Germany, and his colleagues recounted these findings in The Lancet Haematology.
Using data collected by the Worldwide Network for Blood and Marrow Transplantation (WBMT), the researchers systematically analyzed the growth of HSCT and changes in its use in 194 WHO member countries since the first HSCT was performed in 1957.
The team also examined the link between macroeconomic factors (eg, gross national income and healthcare expenditure) and transplant frequencies per 10 million inhabitants in each country.
The data showed that about 10,000 HSCTs had been performed by 1985, but that number rose to around 100,000 by 1995. By December 2012, nearly 1 million HSCTs—42% allogeneic and 58% autologous—had been performed at 1516 transplant centers across 75 countries.
Transplants were more common in countries with greater financial resources, more transplant teams, and an unrelated donor infrastructure. Most transplants were performed in Europe (53%), followed by the Americas (31%), South East Asia and the Western Pacific (15%), and the Eastern Mediterranean and Africa (2%).
The use of allogeneic HSCT has grown rapidly in all regions, without any signs of saturation. This likely reflects substantial underuse of this therapy, according to the researchers. And it suggests more patients would have received allogeneic transplants if they were accessible or suitable donors were available.
On the other hand, the researchers found that the number of countries with donor registries increased from 2 in 1987 to 57 in 2012. And the number of volunteer donors rose from 3072 in 1987 to over 22 million in 2012.
The international exchange of stem cell products also increased to more than 10,000 a year between 2006 and 2012, with substantial differences between countries in the amount of stem cells they import or export.
Despite these increases, the researchers noted that there are still too many patients who are unable to find a suitable donor. At any time, more than 37,000 people worldwide are waiting for an HSC donation.
“Patients, many of them children, are facing a life-and-death situation,” Dr Niederwieser said. “Ultimately, they will die if they cannot get the treatment they need. All countries need to provide adequate infrastructure for patients and donors to make sure that everyone who needs a transplant gets one, rather than the present situation in which access remains restricted to countries and people with sufficient resources.” ![]()
Clonal hematopoiesis is more common than we thought, group says

in the bone marrow
The incidence of clonal hematopoiesis in the general population may be higher than we thought and appears to increase dramatically with age, according to research published in Cell Reports.
Investigators used ultra-deep sequencing to search for evidence of clonal hematopoiesis driven by 15 recurrent leukemogenic mutations.
They sequenced DNA from more than 4000 subjects who had no evidence of hematologic malignancy.
And the incidence of clonal hematopoiesis was higher than has been observed in previous, exome-sequencing studies—0.8% in subjects under 60, rising to 19.5% in subjects ages 90 to 98.
“Ultra-deep sequencing has allowed us to see the very beginnings of cancer,” said study author George Vassiliou, MD, PhD, of the Wellcome Trust Sanger Institute in Cambridge, UK.
“These mutations will be harmless for the majority of people, but, for a few unlucky carriers, they will take the body on a journey towards leukemia. We are now beginning to understand the major landmarks on that journey.”
The investigators found that mutations affecting 2 genes, SF3B1 and SRSF2, appeared exclusively in 70-year-olds.
This suggests these mutations only confer a growth benefit for cells later in life, when there is less competition. And it explains why myelodysplastic syndromes, which are associated with these genes, appear almost exclusively in the elderly.
None of the 4219 subjects studied had mutations in the NPM1 gene, which is mutated in up to 40% of leukemia cases.
This unexpected result suggests that mutations in NPM1 behave as gatekeepers for leukemia, the investigators said. Once a mutation in this gene occurs in a cell with particular, previously accumulated, pre-leukemic mutations, leukemia will develop.
“The significance of mutations in this gene is astonishingly clear from these results: it simply doesn’t exist where there is no leukemia,” said study author Naomi Park, PhD, of the Wellcome Trust Sanger Institute.
“When it is mutated in the appropriate cell, the floodgates open, and leukemia is then very likely to develop. This fits with studies we’ve conducted in the past in which we found that the gene primes blood stem cells for leukemic transformation.” ![]()

in the bone marrow
The incidence of clonal hematopoiesis in the general population may be higher than we thought and appears to increase dramatically with age, according to research published in Cell Reports.
Investigators used ultra-deep sequencing to search for evidence of clonal hematopoiesis driven by 15 recurrent leukemogenic mutations.
They sequenced DNA from more than 4000 subjects who had no evidence of hematologic malignancy.
And the incidence of clonal hematopoiesis was higher than has been observed in previous, exome-sequencing studies—0.8% in subjects under 60, rising to 19.5% in subjects ages 90 to 98.
“Ultra-deep sequencing has allowed us to see the very beginnings of cancer,” said study author George Vassiliou, MD, PhD, of the Wellcome Trust Sanger Institute in Cambridge, UK.
“These mutations will be harmless for the majority of people, but, for a few unlucky carriers, they will take the body on a journey towards leukemia. We are now beginning to understand the major landmarks on that journey.”
The investigators found that mutations affecting 2 genes, SF3B1 and SRSF2, appeared exclusively in 70-year-olds.
This suggests these mutations only confer a growth benefit for cells later in life, when there is less competition. And it explains why myelodysplastic syndromes, which are associated with these genes, appear almost exclusively in the elderly.
None of the 4219 subjects studied had mutations in the NPM1 gene, which is mutated in up to 40% of leukemia cases.
This unexpected result suggests that mutations in NPM1 behave as gatekeepers for leukemia, the investigators said. Once a mutation in this gene occurs in a cell with particular, previously accumulated, pre-leukemic mutations, leukemia will develop.
“The significance of mutations in this gene is astonishingly clear from these results: it simply doesn’t exist where there is no leukemia,” said study author Naomi Park, PhD, of the Wellcome Trust Sanger Institute.
“When it is mutated in the appropriate cell, the floodgates open, and leukemia is then very likely to develop. This fits with studies we’ve conducted in the past in which we found that the gene primes blood stem cells for leukemic transformation.” ![]()

in the bone marrow
The incidence of clonal hematopoiesis in the general population may be higher than we thought and appears to increase dramatically with age, according to research published in Cell Reports.
Investigators used ultra-deep sequencing to search for evidence of clonal hematopoiesis driven by 15 recurrent leukemogenic mutations.
They sequenced DNA from more than 4000 subjects who had no evidence of hematologic malignancy.
And the incidence of clonal hematopoiesis was higher than has been observed in previous, exome-sequencing studies—0.8% in subjects under 60, rising to 19.5% in subjects ages 90 to 98.
“Ultra-deep sequencing has allowed us to see the very beginnings of cancer,” said study author George Vassiliou, MD, PhD, of the Wellcome Trust Sanger Institute in Cambridge, UK.
“These mutations will be harmless for the majority of people, but, for a few unlucky carriers, they will take the body on a journey towards leukemia. We are now beginning to understand the major landmarks on that journey.”
The investigators found that mutations affecting 2 genes, SF3B1 and SRSF2, appeared exclusively in 70-year-olds.
This suggests these mutations only confer a growth benefit for cells later in life, when there is less competition. And it explains why myelodysplastic syndromes, which are associated with these genes, appear almost exclusively in the elderly.
None of the 4219 subjects studied had mutations in the NPM1 gene, which is mutated in up to 40% of leukemia cases.
This unexpected result suggests that mutations in NPM1 behave as gatekeepers for leukemia, the investigators said. Once a mutation in this gene occurs in a cell with particular, previously accumulated, pre-leukemic mutations, leukemia will develop.
“The significance of mutations in this gene is astonishingly clear from these results: it simply doesn’t exist where there is no leukemia,” said study author Naomi Park, PhD, of the Wellcome Trust Sanger Institute.
“When it is mutated in the appropriate cell, the floodgates open, and leukemia is then very likely to develop. This fits with studies we’ve conducted in the past in which we found that the gene primes blood stem cells for leukemic transformation.” ![]()
Use of animals in research on the rise in the US

The use of animals in experimental research conducted at leading US laboratories has risen by more than 70% in recent years, according to a study published in the Journal of Medical Ethics.
The data contradict industry claims of reduced animal use and are at odds with government policies designed to curb and replace the use of animals in experiments, according to researchers.
The US is the world’s largest user of animals in experiments. And government data show declines in the use of cats, dogs, primates, rabbits, hamsters, and other larger mammals.
But the exclusion of the species most commonly used in laboratory research—mice, rats, birds, and all cold-blooded animals—from federal regulations has resulted in an absence of published data on how many of these animals are used in experiments.
To fill this gap, researchers at People for the Ethical Treatment of Animals (PETA) analyzed previously unpublished data collected by the National Institutes of Health (NIH) on the use of all vertebrate species at the top 25 institutions in receipt of NIH grants.
This includes such institutions as Harvard University, Yale University, Columbia University, the University of California-San Francisco, the University of Wisconsin-Madison, and Johns Hopkins University. Together, these institutions account for 27% of all NIH grants disbursed.
PETA’s analysis showed that the use of animals in laboratory research at these facilities rose by 72.7% between 1997 and 2012. This increase was largely driven by an increase in the use of mice. The use of other species remained mostly unchanged.
This study is the first ever to document figures on the use of mice, rats, birds, fish, reptiles, and amphibians in US laboratories. These species are not protected under the federal law governing the treatment of animals used in experiments—the Animal Welfare Act—and therefore are excluded from the law’s reporting requirements.
PETA’s study showed that these unregulated species comprise 98.8% of animals in laboratories.
The researchers said possible explanations for these results include personal and legal biases toward certain animal species. But the figures highlight a need for greater efforts to curb the use of animals in scientific research and more transparency in reporting on whether such efforts are succeeding.
A linked viewpoint article acknowledges the ongoing tensions between scientists and animal rights advocates but suggests that people on both sides of the divide do want to better understand one another.
It recommends that institutional policies be updated to better inform the public about the use of animals in scientific research, as well as opening up dialogue between a broad base of players to replace the often poorly informed and emotionally charged debate. ![]()

The use of animals in experimental research conducted at leading US laboratories has risen by more than 70% in recent years, according to a study published in the Journal of Medical Ethics.
The data contradict industry claims of reduced animal use and are at odds with government policies designed to curb and replace the use of animals in experiments, according to researchers.
The US is the world’s largest user of animals in experiments. And government data show declines in the use of cats, dogs, primates, rabbits, hamsters, and other larger mammals.
But the exclusion of the species most commonly used in laboratory research—mice, rats, birds, and all cold-blooded animals—from federal regulations has resulted in an absence of published data on how many of these animals are used in experiments.
To fill this gap, researchers at People for the Ethical Treatment of Animals (PETA) analyzed previously unpublished data collected by the National Institutes of Health (NIH) on the use of all vertebrate species at the top 25 institutions in receipt of NIH grants.
This includes such institutions as Harvard University, Yale University, Columbia University, the University of California-San Francisco, the University of Wisconsin-Madison, and Johns Hopkins University. Together, these institutions account for 27% of all NIH grants disbursed.
PETA’s analysis showed that the use of animals in laboratory research at these facilities rose by 72.7% between 1997 and 2012. This increase was largely driven by an increase in the use of mice. The use of other species remained mostly unchanged.
This study is the first ever to document figures on the use of mice, rats, birds, fish, reptiles, and amphibians in US laboratories. These species are not protected under the federal law governing the treatment of animals used in experiments—the Animal Welfare Act—and therefore are excluded from the law’s reporting requirements.
PETA’s study showed that these unregulated species comprise 98.8% of animals in laboratories.
The researchers said possible explanations for these results include personal and legal biases toward certain animal species. But the figures highlight a need for greater efforts to curb the use of animals in scientific research and more transparency in reporting on whether such efforts are succeeding.
A linked viewpoint article acknowledges the ongoing tensions between scientists and animal rights advocates but suggests that people on both sides of the divide do want to better understand one another.
It recommends that institutional policies be updated to better inform the public about the use of animals in scientific research, as well as opening up dialogue between a broad base of players to replace the often poorly informed and emotionally charged debate. ![]()

The use of animals in experimental research conducted at leading US laboratories has risen by more than 70% in recent years, according to a study published in the Journal of Medical Ethics.
The data contradict industry claims of reduced animal use and are at odds with government policies designed to curb and replace the use of animals in experiments, according to researchers.
The US is the world’s largest user of animals in experiments. And government data show declines in the use of cats, dogs, primates, rabbits, hamsters, and other larger mammals.
But the exclusion of the species most commonly used in laboratory research—mice, rats, birds, and all cold-blooded animals—from federal regulations has resulted in an absence of published data on how many of these animals are used in experiments.
To fill this gap, researchers at People for the Ethical Treatment of Animals (PETA) analyzed previously unpublished data collected by the National Institutes of Health (NIH) on the use of all vertebrate species at the top 25 institutions in receipt of NIH grants.
This includes such institutions as Harvard University, Yale University, Columbia University, the University of California-San Francisco, the University of Wisconsin-Madison, and Johns Hopkins University. Together, these institutions account for 27% of all NIH grants disbursed.
PETA’s analysis showed that the use of animals in laboratory research at these facilities rose by 72.7% between 1997 and 2012. This increase was largely driven by an increase in the use of mice. The use of other species remained mostly unchanged.
This study is the first ever to document figures on the use of mice, rats, birds, fish, reptiles, and amphibians in US laboratories. These species are not protected under the federal law governing the treatment of animals used in experiments—the Animal Welfare Act—and therefore are excluded from the law’s reporting requirements.
PETA’s study showed that these unregulated species comprise 98.8% of animals in laboratories.
The researchers said possible explanations for these results include personal and legal biases toward certain animal species. But the figures highlight a need for greater efforts to curb the use of animals in scientific research and more transparency in reporting on whether such efforts are succeeding.
A linked viewpoint article acknowledges the ongoing tensions between scientists and animal rights advocates but suggests that people on both sides of the divide do want to better understand one another.
It recommends that institutional policies be updated to better inform the public about the use of animals in scientific research, as well as opening up dialogue between a broad base of players to replace the often poorly informed and emotionally charged debate. ![]()
Why CLL patients stop taking ibrutinib

Photo courtesy of CDC
In reviewing 4 clinical trials of ibrutinib, researchers found that a quarter of chronic lymphocytic leukemia (CLL) patients discontinued treatment with the Bruton tyrosine kinase (BTK) inhibitor.
Ten percent of patients stopped taking the drug due to disease progression, and 15% stopped because of adverse and medical events.
Sequencing data indicated that mutations in BTK and PLCG2 were associated with CLL progression but not Richter’s transformation (RT).
Jennifer A. Woyach, MD, of Ohio State University in Columbus, and her colleagues detailed these discoveries in JAMA Oncology.
The researchers evaluated 308 patients participating in 4 trials conducted at a single institution. The team described the characteristics and outcomes of the patients who discontinued treatment with ibrutinib.
At a median follow-up of 20 months, 232 of the patients studied (75%) remained on therapy, including 7 patients who went off study to undergo stem cell transplant or receive ibrutinib commercially at another center.
Forty-five patients (15%) discontinued treatment following adverse or medical events—28 due to infection, 8 for other adverse events, and 9 due to other medical events. This included 2 patients who needed anticoagulation, 2 with comorbid medical conditions, 1 case of progressive multifocal leukoencephalopathy in a patient who had received rituximab, 1 case of noncompliance, 1 failure to thrive, 1 sudden cardiac death, and 1 cerebrovascular event.
Thirty-one patients (10%) discontinued treatment due to disease progression. Progression included RT or progressive CLL. RT appeared to occur early and CLL progression later. The median survival was 3.5 months after RT and 17.6 months after CLL progression.
Deep sequencing performed on 11 patients with CLL progression revealed BTK or PLCG2 mutations in all of them. But sequencing data on peripheral blood from 8 patients with RT showed that only 2 patients had mutations in BTK, and a lymph node sample showed no mutations in BTK or PLCG2. ![]()

Photo courtesy of CDC
In reviewing 4 clinical trials of ibrutinib, researchers found that a quarter of chronic lymphocytic leukemia (CLL) patients discontinued treatment with the Bruton tyrosine kinase (BTK) inhibitor.
Ten percent of patients stopped taking the drug due to disease progression, and 15% stopped because of adverse and medical events.
Sequencing data indicated that mutations in BTK and PLCG2 were associated with CLL progression but not Richter’s transformation (RT).
Jennifer A. Woyach, MD, of Ohio State University in Columbus, and her colleagues detailed these discoveries in JAMA Oncology.
The researchers evaluated 308 patients participating in 4 trials conducted at a single institution. The team described the characteristics and outcomes of the patients who discontinued treatment with ibrutinib.
At a median follow-up of 20 months, 232 of the patients studied (75%) remained on therapy, including 7 patients who went off study to undergo stem cell transplant or receive ibrutinib commercially at another center.
Forty-five patients (15%) discontinued treatment following adverse or medical events—28 due to infection, 8 for other adverse events, and 9 due to other medical events. This included 2 patients who needed anticoagulation, 2 with comorbid medical conditions, 1 case of progressive multifocal leukoencephalopathy in a patient who had received rituximab, 1 case of noncompliance, 1 failure to thrive, 1 sudden cardiac death, and 1 cerebrovascular event.
Thirty-one patients (10%) discontinued treatment due to disease progression. Progression included RT or progressive CLL. RT appeared to occur early and CLL progression later. The median survival was 3.5 months after RT and 17.6 months after CLL progression.
Deep sequencing performed on 11 patients with CLL progression revealed BTK or PLCG2 mutations in all of them. But sequencing data on peripheral blood from 8 patients with RT showed that only 2 patients had mutations in BTK, and a lymph node sample showed no mutations in BTK or PLCG2. ![]()

Photo courtesy of CDC
In reviewing 4 clinical trials of ibrutinib, researchers found that a quarter of chronic lymphocytic leukemia (CLL) patients discontinued treatment with the Bruton tyrosine kinase (BTK) inhibitor.
Ten percent of patients stopped taking the drug due to disease progression, and 15% stopped because of adverse and medical events.
Sequencing data indicated that mutations in BTK and PLCG2 were associated with CLL progression but not Richter’s transformation (RT).
Jennifer A. Woyach, MD, of Ohio State University in Columbus, and her colleagues detailed these discoveries in JAMA Oncology.
The researchers evaluated 308 patients participating in 4 trials conducted at a single institution. The team described the characteristics and outcomes of the patients who discontinued treatment with ibrutinib.
At a median follow-up of 20 months, 232 of the patients studied (75%) remained on therapy, including 7 patients who went off study to undergo stem cell transplant or receive ibrutinib commercially at another center.
Forty-five patients (15%) discontinued treatment following adverse or medical events—28 due to infection, 8 for other adverse events, and 9 due to other medical events. This included 2 patients who needed anticoagulation, 2 with comorbid medical conditions, 1 case of progressive multifocal leukoencephalopathy in a patient who had received rituximab, 1 case of noncompliance, 1 failure to thrive, 1 sudden cardiac death, and 1 cerebrovascular event.
Thirty-one patients (10%) discontinued treatment due to disease progression. Progression included RT or progressive CLL. RT appeared to occur early and CLL progression later. The median survival was 3.5 months after RT and 17.6 months after CLL progression.
Deep sequencing performed on 11 patients with CLL progression revealed BTK or PLCG2 mutations in all of them. But sequencing data on peripheral blood from 8 patients with RT showed that only 2 patients had mutations in BTK, and a lymph node sample showed no mutations in BTK or PLCG2. ![]()