User login
Use of Cross-Leg Flap for Wound Complications Resulting From Open Pilon Fracture
Soft-tissue complications are a known problem in the treatment of pilon fractures of the distal end of the tibia. These fractures typically occur as the result of a high-energy mechanism, and axial load and shear forces often lead to a severe soft-tissue injury. In many cases, these injuries may require additional procedures to provide adequate soft-tissue coverage. These procedures can include use of either a rotational muscle flap or a free flap transfer. In some cases, however, these flaps are not possible secondary to vascular compromise.
In this article, we report the case of a pilon fracture combined with severe soft-tissue injury and vascular compromise of the leg. A cross-leg fasciocutaneous flap was performed as a salvage procedure for coverage of the soft-tissue defect. The patient provided written informed consent for print and electronic publication of this case report.
Case Report
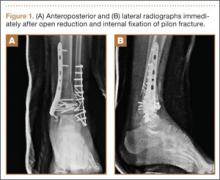
A 23-year-old man sustained a left grade III open pilon fracture after a fall off a cherry picker. He was initially treated with irrigation and débridement of the open anteromedial wound, wound closure, application of external fixation, and open reduction and internal fixation (ORIF) of the concomitant comminuted fibular fracture. Operative fixation of the pilon was performed 3 weeks after injury, once skin and soft tissues were in acceptable condition (Figure 1). Skin closure was performed with 2-0 Vicryl sutures (Ethicon, Inc, Somerville, New Jersey) followed by 3-0 nylon skin sutures and No. 2 nylon retention sutures to reduce tension at the incision.
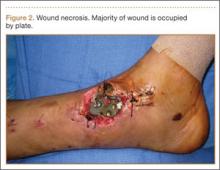
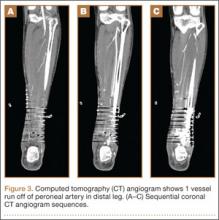
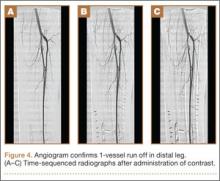
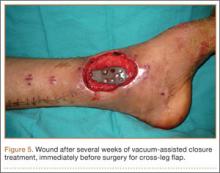
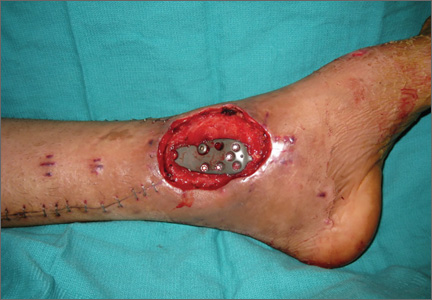
On postoperative day 17, the patient was found to have skin necrosis with exposed hardware over the medial laceration that had resulted from the open fracture (Figure 2). The wound measured 7×6 cm. The plastic surgery team was consulted, and a soft-tissue flap was recommended. Preoperative computed tomography angiogram (Figure 3) revealed 1 vessel runoff in the leg, constituting the peroneal artery, and a conventional angiogram confirmed this finding (Figure 4). Despite these findings, the patient was taken to the operating room 4 weeks after initial injury to try to find a vessel compatible with anastomosis. Intraoperative wound exploration confirmed no patent blood supply for local soft-tissue flap coverage. Therefore, the wound was irrigated and débrided, and a vacuum-assisted closure (VAC) dressing was applied despite exposed hardware and bone. A decision was then made to attempt a cross-leg flap as a salvage procedure, and VAC dressing therapy was continued for several weeks to prepare the recipient site (Figure 5).
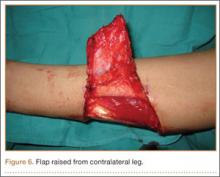
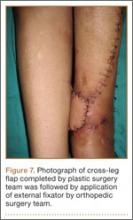
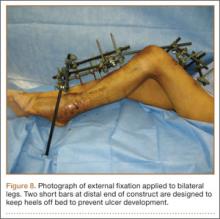
Seven weeks after injury, the patient was taken to the operating room by the orthopedic surgery and plastic surgery teams. After débridement, a fasciocutaneous flap was raised from the middle third of the contralateral leg (Figure 6) based on a posterior tibial artery perforator. The flap, which measured 7×7 cm (sufficient to cover the defect), was raised from lateral to medial from the posterior aspect of the leg with the pedicle located on the medial aspect of the right leg. Flap placement was facilitated by flexing the left knee to 80°. The flap was sutured into place with 4-0 Vicryl deep sutures followed by 4-0 nylon and superficial sutures in an interrupted fashion (Figure 7). Rigid external fixation was then applied to both extremities, bridging them together in optimal position (Figure 8). This construct included 2 short bars that would elevate the patient’s heels off the bed to reduce the chance of heel decubiti. Although including the feet in the external fixator construct may help prevent equinus contracture, we splinted the ankles in neutral position immediately after surgery so that we could begin early range-of-motion (ROM) exercises of the ankles to prevent stiffness. Ankle ROM exercises were started once the flap incorporated, 3 weeks after placement of the external fixator. Lacking medical insurance coverage, the patient could not be admitted to a rehabilitation facility or receive home care. He lived independently and had no help at home, so he had to remain hospitalized after placement of the external fixator. While hospitalized, the surgical site was treated with frequent dressing changes, including use of bacitracin and nonadherent dressing.
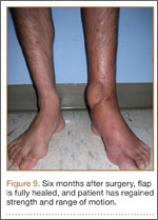
After flap coverage and 4 weeks of bed rest, a base clamping test confirmed the flap was incorporated into the recipient bed. The patient was then returned to the operating room for removal of the external fixator and skin grafting of the donor site. After surgery, he was started on physical therapy, including exercises for bilateral hip, knee, and ankle ROM and strengthening of the lower extremities. Four months after initial injury, the fracture was healed, based on bone consolidation, seen on radiographs, that is consistent with other pilon fractures treated at our institution. Six months after external fixator removal, the patient was able to ambulate independently with minimal discomfort (Figure 9). Passive and active ankle ROM was 20° of dorsiflexion and 25° of plantarflexion, compared with 25° of dorsiflexion and 45° of plantarflexion on the contralateral extremity. Subtalar motion had some stiffness with a 10° arc, compared with a 25° arc on the contralateral extremity. On simple manual testing, the patient had 5/5 motor strength with dorsiflexion, plantarflexion, inversion, and eversion. He returned to full duty as a landscaper about 1 year after initial injury and had no recurrence of wound complications or infection.
Discussion
Fractures of the distal tibia are commonly known as pilon or plafond fractures. They represent up to 10% of all tibial fractures. The injury consists of an intra-articular fracture of the tibiotalar joint with varying degrees of proximal extension into the tibial metaphysis. The etiology is an axial load on the tibia with or without a rotational force.1 Treatment is challenging. The literature includes many reports of wound and soft-tissue complications after ORIF. In 1969, Rüedi and Allgöwer2 published recommendations that have become the standard for treatment of pilon fractures. Twelve percent of the 84 fractures included in their study were associated with wound complications. In 2004, Sirkin and colleagues3 suggested that wound problems associated with ORIF of pilon fractures may be caused by attempts at immediate fixation through swollen soft tissue. They postulated that staging the procedure and waiting for decreased soft-tissue swelling may reduce the incidence of wound complications. In their series, only 2.9% of closed pilon fractures and only 9.1% of open fractures had any wound complications, and none of their patients required skin grafts, rotation flaps, or free tissue transfers.
However, soft-tissue complications still remain a significant threat in the treatment of pilon fracture, and cases that require additional procedures for soft-tissue coverage are common. In some cases, wound necrosis may lead to below-knee amputation.4 There are several coverage options, including local rotational flaps using the soleus muscle5,6 as well as free flaps using the latissimus dorsi, gracilis, or rectus abdominis muscles.7 These options require a sufficient blood supply to the region.
Many high-energy pilon fractures may be associated with vascular injury, and therefore flap survival may be compromised. We have reported such a case in the present article. Our patient’s preoperative angiogram indicated he had 1-vessel runoff to the distal leg—a situation incompatible with free tissue transfer. It is not clear whether this finding is secondary to trauma to the leg or is caused by an anatomical anomaly. Nevertheless, the poor vascularity posed a challenge to providing soft-tissue coverage. Cross-finger8 and cross-foot9 flaps have been described in upper and lower extremity injuries. In 2006, Zhao and colleagues10 reported on 5 patients with tibia and/or hardware exposure after operative fixation of tibia fractures. These patients had poor local soft tissue around the wound and therefore underwent cross-leg flap for coverage. It is not clear where the soft-tissue defects were located and whether any studies were performed to assess the local blood flow.
From our patient’s case, we learned that multiple factors should be considered when assessing such high-energy injuries. First, respecting the soft tissues is of paramount importance. Our initial management on presentation consisted of irrigation and débridement of the wound, fixation of the fibula, and application of an external fixator to allow for soft-tissue healing before definitive fixation of the pilon. Although ultimately the patient required soft-tissue coverage, soft-tissue healing and viability are important in preventing unnecessary soft-tissue procedures, and therefore we would not have handled our initial treatment differently.
Patient selection is also important. The ideal candidate for a cross-leg flap is a young, healthy person who is compliant and has a strong support system to help with activities of daily living. Unfortunately, because of financial issues and lack of home support, our patient remained hospitalized during his treatment course. For a patient who has support, it is possible to be discharged either home or to a rehabilitation facility once flap viability has been confirmed after surgery.
Another consideration is type of immobilization. Immobilization options include casting, use of Kirschner wires (K-wires), and use of rigid external fixation. For cross-leg flaps, external fixation is superior to casting and K-wires, as it provides a more rigid construct and easier access to the flap for serial evaluation. Further, it is easier for the patient to maintain personal hygiene, and it can provide heel rises to avoid pressure ulcers.
Conclusion
To our knowledge, there have been no reports of using a cross-leg flap for wound complications in high-energy pilon fractures. As already mentioned, many of these fractures may be associated with severe soft-tissue injury and may need flap coverage. A cross-leg flap with external fixation of both legs provides a limb salvage option with satisfactory patient outcomes.
1. McCann PA, Jackson M, Mitchell ST, Atkins RM. Complications of definitive open reduction and internal fixation of pilon fractures of the distal tibia. Int Orthop. 2011;35(3):413-418.
2. Rüedi TP, Allgöwer M. Fractures of the lower end of the tibia into the ankle joint. Injury. 1969;1:92-99.
3. Sirkin M, Sanders R, DiPasquale T, Herscovici D Jr. A staged protocol for soft tissue management in the treatment of complex pilon fractures. J Orthop Trauma. 2004;18(8 suppl):S32-S38.
4. Boraiah S, Kemp TJ, Erwteman A, Lucas PA, Asprinio DE. Outcome following open reduction and internal fixation of open pilon fractures. J Bone Joint Surg Am. 2010;92(2):346-352.
5. Cheng C, Li X, Abudu S. Repairing postoperative soft tissue defects of tibia and ankle open fractures with muscle flap pedicled with medial half of soleus [in Chinese]. Zhongguo Xiu Fu Chong Jian Wai Ke Za Zhi. 2009;23(12):1440-1442.
6. Yunus A, Yusuf A, Chen G. Repair of soft tissue defect by reverse soleus muscle flap after pilon fracture fixation [in Chinese]. Zhongguo Xiu Fu Chong Jian Wai Ke Za Zhi. 2007;21(9):925-927.
7. Conroy J, Agarwal M, Giannoudis PV, Matthews SJ. Early internal fixation and soft tissue cover of severe open tibial pilon fractures. Int Orthop. 2003;27(6):343-347.
8. Megerle K, Palm-Bröking K, Germann G. The cross-finger flap [in German]. Oper Orthop Traumatol. 2008;20(2):97-102.
9. Largey A, Faline A, Hebrard W, Hamoui M, Canovas F. Management of massive traumatic compound defects of the foot. Orthop Traumatol Surg Res. 2009;95(4):301-304.
10. Zhao L, Wan L, Wang S. Clinical studies on maintenance of cross-leg position through internal fixation with Kirschner wire after cross-leg flap procedure. Zhongguo Xiu Fu Chong Jian Wai Ke Za Zhi. 2006;20(12):1211-1213.
Soft-tissue complications are a known problem in the treatment of pilon fractures of the distal end of the tibia. These fractures typically occur as the result of a high-energy mechanism, and axial load and shear forces often lead to a severe soft-tissue injury. In many cases, these injuries may require additional procedures to provide adequate soft-tissue coverage. These procedures can include use of either a rotational muscle flap or a free flap transfer. In some cases, however, these flaps are not possible secondary to vascular compromise.
In this article, we report the case of a pilon fracture combined with severe soft-tissue injury and vascular compromise of the leg. A cross-leg fasciocutaneous flap was performed as a salvage procedure for coverage of the soft-tissue defect. The patient provided written informed consent for print and electronic publication of this case report.
Case Report
A 23-year-old man sustained a left grade III open pilon fracture after a fall off a cherry picker. He was initially treated with irrigation and débridement of the open anteromedial wound, wound closure, application of external fixation, and open reduction and internal fixation (ORIF) of the concomitant comminuted fibular fracture. Operative fixation of the pilon was performed 3 weeks after injury, once skin and soft tissues were in acceptable condition (Figure 1). Skin closure was performed with 2-0 Vicryl sutures (Ethicon, Inc, Somerville, New Jersey) followed by 3-0 nylon skin sutures and No. 2 nylon retention sutures to reduce tension at the incision.
On postoperative day 17, the patient was found to have skin necrosis with exposed hardware over the medial laceration that had resulted from the open fracture (Figure 2). The wound measured 7×6 cm. The plastic surgery team was consulted, and a soft-tissue flap was recommended. Preoperative computed tomography angiogram (Figure 3) revealed 1 vessel runoff in the leg, constituting the peroneal artery, and a conventional angiogram confirmed this finding (Figure 4). Despite these findings, the patient was taken to the operating room 4 weeks after initial injury to try to find a vessel compatible with anastomosis. Intraoperative wound exploration confirmed no patent blood supply for local soft-tissue flap coverage. Therefore, the wound was irrigated and débrided, and a vacuum-assisted closure (VAC) dressing was applied despite exposed hardware and bone. A decision was then made to attempt a cross-leg flap as a salvage procedure, and VAC dressing therapy was continued for several weeks to prepare the recipient site (Figure 5).
Seven weeks after injury, the patient was taken to the operating room by the orthopedic surgery and plastic surgery teams. After débridement, a fasciocutaneous flap was raised from the middle third of the contralateral leg (Figure 6) based on a posterior tibial artery perforator. The flap, which measured 7×7 cm (sufficient to cover the defect), was raised from lateral to medial from the posterior aspect of the leg with the pedicle located on the medial aspect of the right leg. Flap placement was facilitated by flexing the left knee to 80°. The flap was sutured into place with 4-0 Vicryl deep sutures followed by 4-0 nylon and superficial sutures in an interrupted fashion (Figure 7). Rigid external fixation was then applied to both extremities, bridging them together in optimal position (Figure 8). This construct included 2 short bars that would elevate the patient’s heels off the bed to reduce the chance of heel decubiti. Although including the feet in the external fixator construct may help prevent equinus contracture, we splinted the ankles in neutral position immediately after surgery so that we could begin early range-of-motion (ROM) exercises of the ankles to prevent stiffness. Ankle ROM exercises were started once the flap incorporated, 3 weeks after placement of the external fixator. Lacking medical insurance coverage, the patient could not be admitted to a rehabilitation facility or receive home care. He lived independently and had no help at home, so he had to remain hospitalized after placement of the external fixator. While hospitalized, the surgical site was treated with frequent dressing changes, including use of bacitracin and nonadherent dressing.
After flap coverage and 4 weeks of bed rest, a base clamping test confirmed the flap was incorporated into the recipient bed. The patient was then returned to the operating room for removal of the external fixator and skin grafting of the donor site. After surgery, he was started on physical therapy, including exercises for bilateral hip, knee, and ankle ROM and strengthening of the lower extremities. Four months after initial injury, the fracture was healed, based on bone consolidation, seen on radiographs, that is consistent with other pilon fractures treated at our institution. Six months after external fixator removal, the patient was able to ambulate independently with minimal discomfort (Figure 9). Passive and active ankle ROM was 20° of dorsiflexion and 25° of plantarflexion, compared with 25° of dorsiflexion and 45° of plantarflexion on the contralateral extremity. Subtalar motion had some stiffness with a 10° arc, compared with a 25° arc on the contralateral extremity. On simple manual testing, the patient had 5/5 motor strength with dorsiflexion, plantarflexion, inversion, and eversion. He returned to full duty as a landscaper about 1 year after initial injury and had no recurrence of wound complications or infection.
Discussion
Fractures of the distal tibia are commonly known as pilon or plafond fractures. They represent up to 10% of all tibial fractures. The injury consists of an intra-articular fracture of the tibiotalar joint with varying degrees of proximal extension into the tibial metaphysis. The etiology is an axial load on the tibia with or without a rotational force.1 Treatment is challenging. The literature includes many reports of wound and soft-tissue complications after ORIF. In 1969, Rüedi and Allgöwer2 published recommendations that have become the standard for treatment of pilon fractures. Twelve percent of the 84 fractures included in their study were associated with wound complications. In 2004, Sirkin and colleagues3 suggested that wound problems associated with ORIF of pilon fractures may be caused by attempts at immediate fixation through swollen soft tissue. They postulated that staging the procedure and waiting for decreased soft-tissue swelling may reduce the incidence of wound complications. In their series, only 2.9% of closed pilon fractures and only 9.1% of open fractures had any wound complications, and none of their patients required skin grafts, rotation flaps, or free tissue transfers.
However, soft-tissue complications still remain a significant threat in the treatment of pilon fracture, and cases that require additional procedures for soft-tissue coverage are common. In some cases, wound necrosis may lead to below-knee amputation.4 There are several coverage options, including local rotational flaps using the soleus muscle5,6 as well as free flaps using the latissimus dorsi, gracilis, or rectus abdominis muscles.7 These options require a sufficient blood supply to the region.
Many high-energy pilon fractures may be associated with vascular injury, and therefore flap survival may be compromised. We have reported such a case in the present article. Our patient’s preoperative angiogram indicated he had 1-vessel runoff to the distal leg—a situation incompatible with free tissue transfer. It is not clear whether this finding is secondary to trauma to the leg or is caused by an anatomical anomaly. Nevertheless, the poor vascularity posed a challenge to providing soft-tissue coverage. Cross-finger8 and cross-foot9 flaps have been described in upper and lower extremity injuries. In 2006, Zhao and colleagues10 reported on 5 patients with tibia and/or hardware exposure after operative fixation of tibia fractures. These patients had poor local soft tissue around the wound and therefore underwent cross-leg flap for coverage. It is not clear where the soft-tissue defects were located and whether any studies were performed to assess the local blood flow.
From our patient’s case, we learned that multiple factors should be considered when assessing such high-energy injuries. First, respecting the soft tissues is of paramount importance. Our initial management on presentation consisted of irrigation and débridement of the wound, fixation of the fibula, and application of an external fixator to allow for soft-tissue healing before definitive fixation of the pilon. Although ultimately the patient required soft-tissue coverage, soft-tissue healing and viability are important in preventing unnecessary soft-tissue procedures, and therefore we would not have handled our initial treatment differently.
Patient selection is also important. The ideal candidate for a cross-leg flap is a young, healthy person who is compliant and has a strong support system to help with activities of daily living. Unfortunately, because of financial issues and lack of home support, our patient remained hospitalized during his treatment course. For a patient who has support, it is possible to be discharged either home or to a rehabilitation facility once flap viability has been confirmed after surgery.
Another consideration is type of immobilization. Immobilization options include casting, use of Kirschner wires (K-wires), and use of rigid external fixation. For cross-leg flaps, external fixation is superior to casting and K-wires, as it provides a more rigid construct and easier access to the flap for serial evaluation. Further, it is easier for the patient to maintain personal hygiene, and it can provide heel rises to avoid pressure ulcers.
Conclusion
To our knowledge, there have been no reports of using a cross-leg flap for wound complications in high-energy pilon fractures. As already mentioned, many of these fractures may be associated with severe soft-tissue injury and may need flap coverage. A cross-leg flap with external fixation of both legs provides a limb salvage option with satisfactory patient outcomes.
Soft-tissue complications are a known problem in the treatment of pilon fractures of the distal end of the tibia. These fractures typically occur as the result of a high-energy mechanism, and axial load and shear forces often lead to a severe soft-tissue injury. In many cases, these injuries may require additional procedures to provide adequate soft-tissue coverage. These procedures can include use of either a rotational muscle flap or a free flap transfer. In some cases, however, these flaps are not possible secondary to vascular compromise.
In this article, we report the case of a pilon fracture combined with severe soft-tissue injury and vascular compromise of the leg. A cross-leg fasciocutaneous flap was performed as a salvage procedure for coverage of the soft-tissue defect. The patient provided written informed consent for print and electronic publication of this case report.
Case Report
A 23-year-old man sustained a left grade III open pilon fracture after a fall off a cherry picker. He was initially treated with irrigation and débridement of the open anteromedial wound, wound closure, application of external fixation, and open reduction and internal fixation (ORIF) of the concomitant comminuted fibular fracture. Operative fixation of the pilon was performed 3 weeks after injury, once skin and soft tissues were in acceptable condition (Figure 1). Skin closure was performed with 2-0 Vicryl sutures (Ethicon, Inc, Somerville, New Jersey) followed by 3-0 nylon skin sutures and No. 2 nylon retention sutures to reduce tension at the incision.
On postoperative day 17, the patient was found to have skin necrosis with exposed hardware over the medial laceration that had resulted from the open fracture (Figure 2). The wound measured 7×6 cm. The plastic surgery team was consulted, and a soft-tissue flap was recommended. Preoperative computed tomography angiogram (Figure 3) revealed 1 vessel runoff in the leg, constituting the peroneal artery, and a conventional angiogram confirmed this finding (Figure 4). Despite these findings, the patient was taken to the operating room 4 weeks after initial injury to try to find a vessel compatible with anastomosis. Intraoperative wound exploration confirmed no patent blood supply for local soft-tissue flap coverage. Therefore, the wound was irrigated and débrided, and a vacuum-assisted closure (VAC) dressing was applied despite exposed hardware and bone. A decision was then made to attempt a cross-leg flap as a salvage procedure, and VAC dressing therapy was continued for several weeks to prepare the recipient site (Figure 5).
Seven weeks after injury, the patient was taken to the operating room by the orthopedic surgery and plastic surgery teams. After débridement, a fasciocutaneous flap was raised from the middle third of the contralateral leg (Figure 6) based on a posterior tibial artery perforator. The flap, which measured 7×7 cm (sufficient to cover the defect), was raised from lateral to medial from the posterior aspect of the leg with the pedicle located on the medial aspect of the right leg. Flap placement was facilitated by flexing the left knee to 80°. The flap was sutured into place with 4-0 Vicryl deep sutures followed by 4-0 nylon and superficial sutures in an interrupted fashion (Figure 7). Rigid external fixation was then applied to both extremities, bridging them together in optimal position (Figure 8). This construct included 2 short bars that would elevate the patient’s heels off the bed to reduce the chance of heel decubiti. Although including the feet in the external fixator construct may help prevent equinus contracture, we splinted the ankles in neutral position immediately after surgery so that we could begin early range-of-motion (ROM) exercises of the ankles to prevent stiffness. Ankle ROM exercises were started once the flap incorporated, 3 weeks after placement of the external fixator. Lacking medical insurance coverage, the patient could not be admitted to a rehabilitation facility or receive home care. He lived independently and had no help at home, so he had to remain hospitalized after placement of the external fixator. While hospitalized, the surgical site was treated with frequent dressing changes, including use of bacitracin and nonadherent dressing.
After flap coverage and 4 weeks of bed rest, a base clamping test confirmed the flap was incorporated into the recipient bed. The patient was then returned to the operating room for removal of the external fixator and skin grafting of the donor site. After surgery, he was started on physical therapy, including exercises for bilateral hip, knee, and ankle ROM and strengthening of the lower extremities. Four months after initial injury, the fracture was healed, based on bone consolidation, seen on radiographs, that is consistent with other pilon fractures treated at our institution. Six months after external fixator removal, the patient was able to ambulate independently with minimal discomfort (Figure 9). Passive and active ankle ROM was 20° of dorsiflexion and 25° of plantarflexion, compared with 25° of dorsiflexion and 45° of plantarflexion on the contralateral extremity. Subtalar motion had some stiffness with a 10° arc, compared with a 25° arc on the contralateral extremity. On simple manual testing, the patient had 5/5 motor strength with dorsiflexion, plantarflexion, inversion, and eversion. He returned to full duty as a landscaper about 1 year after initial injury and had no recurrence of wound complications or infection.
Discussion
Fractures of the distal tibia are commonly known as pilon or plafond fractures. They represent up to 10% of all tibial fractures. The injury consists of an intra-articular fracture of the tibiotalar joint with varying degrees of proximal extension into the tibial metaphysis. The etiology is an axial load on the tibia with or without a rotational force.1 Treatment is challenging. The literature includes many reports of wound and soft-tissue complications after ORIF. In 1969, Rüedi and Allgöwer2 published recommendations that have become the standard for treatment of pilon fractures. Twelve percent of the 84 fractures included in their study were associated with wound complications. In 2004, Sirkin and colleagues3 suggested that wound problems associated with ORIF of pilon fractures may be caused by attempts at immediate fixation through swollen soft tissue. They postulated that staging the procedure and waiting for decreased soft-tissue swelling may reduce the incidence of wound complications. In their series, only 2.9% of closed pilon fractures and only 9.1% of open fractures had any wound complications, and none of their patients required skin grafts, rotation flaps, or free tissue transfers.
However, soft-tissue complications still remain a significant threat in the treatment of pilon fracture, and cases that require additional procedures for soft-tissue coverage are common. In some cases, wound necrosis may lead to below-knee amputation.4 There are several coverage options, including local rotational flaps using the soleus muscle5,6 as well as free flaps using the latissimus dorsi, gracilis, or rectus abdominis muscles.7 These options require a sufficient blood supply to the region.
Many high-energy pilon fractures may be associated with vascular injury, and therefore flap survival may be compromised. We have reported such a case in the present article. Our patient’s preoperative angiogram indicated he had 1-vessel runoff to the distal leg—a situation incompatible with free tissue transfer. It is not clear whether this finding is secondary to trauma to the leg or is caused by an anatomical anomaly. Nevertheless, the poor vascularity posed a challenge to providing soft-tissue coverage. Cross-finger8 and cross-foot9 flaps have been described in upper and lower extremity injuries. In 2006, Zhao and colleagues10 reported on 5 patients with tibia and/or hardware exposure after operative fixation of tibia fractures. These patients had poor local soft tissue around the wound and therefore underwent cross-leg flap for coverage. It is not clear where the soft-tissue defects were located and whether any studies were performed to assess the local blood flow.
From our patient’s case, we learned that multiple factors should be considered when assessing such high-energy injuries. First, respecting the soft tissues is of paramount importance. Our initial management on presentation consisted of irrigation and débridement of the wound, fixation of the fibula, and application of an external fixator to allow for soft-tissue healing before definitive fixation of the pilon. Although ultimately the patient required soft-tissue coverage, soft-tissue healing and viability are important in preventing unnecessary soft-tissue procedures, and therefore we would not have handled our initial treatment differently.
Patient selection is also important. The ideal candidate for a cross-leg flap is a young, healthy person who is compliant and has a strong support system to help with activities of daily living. Unfortunately, because of financial issues and lack of home support, our patient remained hospitalized during his treatment course. For a patient who has support, it is possible to be discharged either home or to a rehabilitation facility once flap viability has been confirmed after surgery.
Another consideration is type of immobilization. Immobilization options include casting, use of Kirschner wires (K-wires), and use of rigid external fixation. For cross-leg flaps, external fixation is superior to casting and K-wires, as it provides a more rigid construct and easier access to the flap for serial evaluation. Further, it is easier for the patient to maintain personal hygiene, and it can provide heel rises to avoid pressure ulcers.
Conclusion
To our knowledge, there have been no reports of using a cross-leg flap for wound complications in high-energy pilon fractures. As already mentioned, many of these fractures may be associated with severe soft-tissue injury and may need flap coverage. A cross-leg flap with external fixation of both legs provides a limb salvage option with satisfactory patient outcomes.
1. McCann PA, Jackson M, Mitchell ST, Atkins RM. Complications of definitive open reduction and internal fixation of pilon fractures of the distal tibia. Int Orthop. 2011;35(3):413-418.
2. Rüedi TP, Allgöwer M. Fractures of the lower end of the tibia into the ankle joint. Injury. 1969;1:92-99.
3. Sirkin M, Sanders R, DiPasquale T, Herscovici D Jr. A staged protocol for soft tissue management in the treatment of complex pilon fractures. J Orthop Trauma. 2004;18(8 suppl):S32-S38.
4. Boraiah S, Kemp TJ, Erwteman A, Lucas PA, Asprinio DE. Outcome following open reduction and internal fixation of open pilon fractures. J Bone Joint Surg Am. 2010;92(2):346-352.
5. Cheng C, Li X, Abudu S. Repairing postoperative soft tissue defects of tibia and ankle open fractures with muscle flap pedicled with medial half of soleus [in Chinese]. Zhongguo Xiu Fu Chong Jian Wai Ke Za Zhi. 2009;23(12):1440-1442.
6. Yunus A, Yusuf A, Chen G. Repair of soft tissue defect by reverse soleus muscle flap after pilon fracture fixation [in Chinese]. Zhongguo Xiu Fu Chong Jian Wai Ke Za Zhi. 2007;21(9):925-927.
7. Conroy J, Agarwal M, Giannoudis PV, Matthews SJ. Early internal fixation and soft tissue cover of severe open tibial pilon fractures. Int Orthop. 2003;27(6):343-347.
8. Megerle K, Palm-Bröking K, Germann G. The cross-finger flap [in German]. Oper Orthop Traumatol. 2008;20(2):97-102.
9. Largey A, Faline A, Hebrard W, Hamoui M, Canovas F. Management of massive traumatic compound defects of the foot. Orthop Traumatol Surg Res. 2009;95(4):301-304.
10. Zhao L, Wan L, Wang S. Clinical studies on maintenance of cross-leg position through internal fixation with Kirschner wire after cross-leg flap procedure. Zhongguo Xiu Fu Chong Jian Wai Ke Za Zhi. 2006;20(12):1211-1213.
1. McCann PA, Jackson M, Mitchell ST, Atkins RM. Complications of definitive open reduction and internal fixation of pilon fractures of the distal tibia. Int Orthop. 2011;35(3):413-418.
2. Rüedi TP, Allgöwer M. Fractures of the lower end of the tibia into the ankle joint. Injury. 1969;1:92-99.
3. Sirkin M, Sanders R, DiPasquale T, Herscovici D Jr. A staged protocol for soft tissue management in the treatment of complex pilon fractures. J Orthop Trauma. 2004;18(8 suppl):S32-S38.
4. Boraiah S, Kemp TJ, Erwteman A, Lucas PA, Asprinio DE. Outcome following open reduction and internal fixation of open pilon fractures. J Bone Joint Surg Am. 2010;92(2):346-352.
5. Cheng C, Li X, Abudu S. Repairing postoperative soft tissue defects of tibia and ankle open fractures with muscle flap pedicled with medial half of soleus [in Chinese]. Zhongguo Xiu Fu Chong Jian Wai Ke Za Zhi. 2009;23(12):1440-1442.
6. Yunus A, Yusuf A, Chen G. Repair of soft tissue defect by reverse soleus muscle flap after pilon fracture fixation [in Chinese]. Zhongguo Xiu Fu Chong Jian Wai Ke Za Zhi. 2007;21(9):925-927.
7. Conroy J, Agarwal M, Giannoudis PV, Matthews SJ. Early internal fixation and soft tissue cover of severe open tibial pilon fractures. Int Orthop. 2003;27(6):343-347.
8. Megerle K, Palm-Bröking K, Germann G. The cross-finger flap [in German]. Oper Orthop Traumatol. 2008;20(2):97-102.
9. Largey A, Faline A, Hebrard W, Hamoui M, Canovas F. Management of massive traumatic compound defects of the foot. Orthop Traumatol Surg Res. 2009;95(4):301-304.
10. Zhao L, Wan L, Wang S. Clinical studies on maintenance of cross-leg position through internal fixation with Kirschner wire after cross-leg flap procedure. Zhongguo Xiu Fu Chong Jian Wai Ke Za Zhi. 2006;20(12):1211-1213.
Complications of Open Reduction and Internal Fixation of Ankle Fractures in Patients With Positive Urine Drug Screen
Open treatment of ankle fractures is one of the most common procedures performed by orthopedic surgeons.1 Among the younger patient population, ankle fractures represent a significant proportion of orthopedic injuries.2 The reported incidence of illicit drug and alcohol use in the urban trauma population ranges from 36% to 86%,2 and medical and anesthetic complications associated with illicit drug use have been well documented in surgical patients.2 However, patients with a recent history of drug abuse may be subject to a separate but related set of complications of open treatment of ankle fractures.
The perioperative complications associated with open treatment of ankle fractures in patients with diabetes mellitus have been well described.3-6 Similarly, previous studies have suggested that peripheral vascular disease, complicated diabetes, and smoking are risk factors for poor outcomes in patients who require open reduction and internal fixation (ORIF) in lower extremity trauma.7-9 However, there are few data on the complications specifically associated with illicit drug use and orthopedic surgery. Properly identifying these high-risk groups and being cognizant of commonly associated complications are likely important in ensuring proper perioperative care and may alter follow-up protocols in these patients.
We conducted a study to identify the complications associated with open treatment of ankle fractures in patients who tested positive for illicit drugs on urine drug screen (UDS). We hypothesized that patients who had a history of positive UDS and underwent ORIF of an ankle fracture would have a higher incidence of major and minor complications.
Materials and Methods
After obtaining institutional review board approval, we retrospectively reviewed the cases of 142 patients who underwent open treatment of an ankle fracture between 2006 and 2010. Data sources included patient demographic information, radiographs, preoperative UDS, attending surgeons’ clinical office notes, and clinical laboratory data. Our institution’s standard protocol for ankle fractures was followed for all patients in the study. All patients were evaluated by an orthopedic physician, in either the emergency department or the office, during application of a well-padded Jones splint before surgery. Oral narcotic pain medication was routinely prescribed. All patients were seen, within 10 days of injury, for surgery planning. A board-certified orthopedic surgeon surgically stabilized the ankle fractures. The postoperative treatment regimen, per protocol, included non-weight-bearing in a padded Jones splint dressing; oral narcotic pain medication; physical therapy; and routine scheduled follow-up. In open fracture cases, patients were taken urgently to the operating room for irrigation and débridement with stabilization. Which treatment would be initially used—external fixation or ORIF—was determined on a case-by-case basis.
The sample consisted of adults (age, >18 years) who had undergone definitive ORIF of a lateral malleolar, bimalleolar, or trimalleolar ankle fracture during the study period. Polytrauma patients, patients with external fixation as definitive treatment, and patients with nonoperative treatment were excluded. Before surgical management, all patients were tested for recent illicit drug use by UDS (standard protocol at our institution). UDS, measured for cocaine, marijuana, PCP (phencyclidine), opiates, and barbiturates, was obtained in the office setting or emergency department or on day of surgery. The patients were divided into 2 groups, positive and negative UDS. Patients with documented receipt of narcotic pain medication before UDS were excluded.
The outcomes identified as dependent variables included nonunion, malunion, superficial or deep infection, amputation, delay in treatment, days to healing, repeat surgery, long-term bracing, and loss to follow-up. A nonunion was defined as lasting longer than 9 months and not showing radiographic signs of progression toward healing for 3 consecutive months. These complications were identified with use of attending surgeon clinical progress notes, laboratory values, radiographic parameters, and inpatient readmissions/surgeries associated with these outcomes. Nonunion, malunion, superficial or deep infection, and amputation were then grouped as major complications and analyzed as pooled major complications.
The Fisher exact test was used to analyze categorical variables with respect to UDS. The Wilcoxon rank sum test was used to determine statistical significance for continuous variables. Univariate logistic regression examined both continuous and categorical variables to evaluate predictors for a selected outcome. Statistical significance was set a priori at P ≤ .05, with significant factors indicating an increase (or decrease) in the outcome variable being tested.
Results
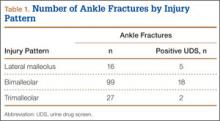
We retrospectively reviewed the cases of 142 patients. Table 1 lists the number of cases by fracture type. Bimalleolar fractures were most common, accounting for 99 (69.8%) of the 142 cases. Isolated lateral malleolar fractures accounted for 16 cases (11.2%), and trimalleolar fractures accounted for 27 cases (19%).
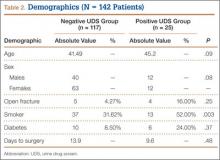
Twenty-five (18%) of the 142 patients tested positive for illicit drugs. Mean age was 45.2 years for positive UDS patients and 41.5 years for negative UDS patients. Open fracture cases represented 4.3% of negative UDS patients and 16% of positive UDS patients. Fifty-two percent of positive UDS patients and 32% of negative UDS patients were also tobacco users. These data were statistically significant (P = .003) There were no significant differences in age, sex, incidence of diabetes, incidence of open fracture, or time to surgery between the groups (Table 2).
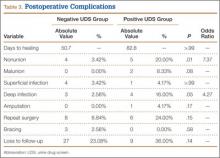
Incidence of nonunion was higher in positive UDS patients (n = 5; P = .01), as was incidence of deep infection (n = 4; P = .05) (Table 3).
Mean time to radiographic healing was 50.7 days in negative UDS patients and 82.8 days in positive UDS patients (P > .99). Incidence of nonunion was 3.5% in negative UDS patients and 20% in positive UDS patients (P = .01). There were no malunions in negative UDS patients and 2 malunions in positive UDS patients. Incidence of deep infections was 2.5% in negative UDS patients and 16% in positive UDS patients (P = .04). No significant differences were found in incidence of malunions, superficial infections, amputations, need for repeat surgery, continued bracing, or loss to follow-up.
Major complications were defined as superficial or deep infections, amputations, malunions, and nonunions. The rate of major complication was significantly (P = .03) higher in positive UDS patients (24.24%) than in negative UDS patients (7.69%) (Table 4).
Discussion
In the present study, we retrospectively reviewed the cases of patients treated with ORIF for varying types of ankle fractures. Important major and minor complications were analyzed. The overall incidence of major complications in negative UDS patients was only 7.69%, consistent with previously reported results in patients with ankle fractures.6,10 However, a statistically significant (P = .03) increased incidence of major complications—an alarmingly high rate of almost 1 in 4—was found in positive UDS patients. Our results also demonstrated a significantly higher rate of nonunion and deep infection in positive UDS patients. Calculated odds ratios were 7.37 and 4.27 for nonunion and deep infection, respectively—arguably 2 of the most devastating postoperative complications in positive UDS patients.
Previous studies have found that open fractures, age, and medical comorbidities are significant predictors of short-term complications, such as wound healing, infection, persistent pain, and delayed union.3-6 Levy and colleagues11 examined the incidence of orthopedic trauma in positive UDS patients. These patients had orthopedic injuries that were more severe and required longer hospitalization. However, the study did not address patients with ankle fractures or the incidence of major complications. Diabetes and peripheral vascular disease are significant risk factors for many surgical procedures in orthopedic surgery.3,7-9,12,13 Tight glycemic control and optimization of medical comorbidities decrease postoperative complications.12,13 SooHoo and colleagues6 found that history of diabetes and history of peripheral vascular disease were significant predictors of short-term complications of mortality, infection, reoperation, and amputation. The rate of infection in the complicated diabetes group was statistically higher as well. The effect of illicit drug use was not analyzed in that study. We think the findings of the present study highlight the importance of screening for high-risk populations (eg, patients with diabetes, patients with peripheral vascular disease, drug abusers) before orthopedic surgery, especially during definitive treatment of ankle fracture.
Recently, Nåsell and colleagues10 found that a well-implemented smoking cessation program was associated with a statistically significant reduction in complications 6 and 12 weeks after surgery. The target treatment groups were patients who underwent major lower extremity and upper extremity orthopedic surgery. The most common surgery performed in the study was ORIF of ankle fractures. The authors concluded that a smoking cessation intervention program during the first 6 weeks after acute fracture surgery decreases the risk for postoperative complications. However, no recommendations were made for treating patients with other addictions, such as alcohol and illicit drug addictions.
To our knowledge, our study is the first to critically examine postoperative complications in ankle fracture patients with a history of illicit drug abuse as determined by preoperative positive UDS. These data suggest the importance of critically evaluating this patient population. The rates of deep infection, nonunion, and pooled major complications were all notable. Furthermore, compared with negative UDS patients, positive UDS patients were more than 7 times likely to develop a nonunion and more than 4 times likely to develop a deep infection. The reasons are likely multifactorial but may involve factors such as injury severity, poor nutrition, suboptimal living conditions, difficulty complying with weight-bearing restrictions, and, possibly, poor compliance with wound-care recommendations. Determining the influence of each factor was beyond the scope of this study. However, further investigation is warranted.
The difference in incidence of smoking between the 2 groups was statistically significant. As smoking has been well documented as contributing to poor wound and bone healing,14-16 it is likely to have been a contributory factor. However, nicotine levels are not routinely part of UDS, and people who quit smoking typically take 7 to 10 days to demonstrate a measurable drop in cotinine levels. On the other hand, screening for drugs takes only a few minutes and can provide useful information during the preoperative period. It was suggested that positive UDS patients were significantly likely to be tobacco users as well.
The 2 groups were not significantly different with respect to mean follow-up time or loss to follow-up. Although mean follow-up was longer in negative UDS patients, the standard deviation was large in both groups. Given the positive UDS patients’ higher incidence of deep infection and nonunion, both of which typically prolong the course of treatment, the results were likely deceptive. Patients with a history of illicit drug use have confounding variables (eg, psychiatric disorders, financial strife) that make treatment compliance and follow-up difficult.17
Some of the weaknesses of this study are inherent to its retrospective design and limited sample size. Furthermore, patient satisfaction scores and ankle-specific outcome measures, such as AOFAS (American Orthopaedic Foot and Ankle Society) scores, were not considered. Prospective collection of data that include patient satisfaction scores and ankle-specific outcome measures would be optimal. Our current recommendation is to obtain preoperative UDS and illicit drug use history for all trauma patients. In addition, operating surgeons should exercise caution when caring for patients who test positive for illicit drugs.
Conclusion
We evaluated the incidence of complications experienced by positive UDS patients undergoing surgical treatment of ankle fractures. It is well documented that illicit drug users who receive general anesthesia have complications. However, little is known about the untoward effects of illicit drugs on postoperative complications. Furthermore, the efficacy of drug cessation programs in minimizing these complications has not been fully explored.
In conclusion, similar to patients with diabetes, patients with a history of recent illicit drug use, as evidenced by preoperative positive UDS, are at increased risk for complications during treatment for ankle fracture. These data suggest that practicing orthopedists should be more vigilant when caring for ankle fracture patients with preoperative positive UDS.
1. Michelson JD. Fractures about the ankle. J Bone Joint Surg Am. 1995;77(1):142-152.
2. Culver JL, Walker JR. Anesthetic implications of illicit drug use. J Perianesth Nurs. 1999;14(2):82-90.
3. Bibbo C, Lin SS, Beam HA, Behrens FF. Complications of ankle fractures in diabetic patients. Orthop Clin North Am. 2001;32(1):113-133.
4. Leininger RE, Knox CL, Comstock RD. Epidemiology of 1.6 million pediatric soccer-related injuries presenting to US emergency departments from 1990 to 2003. Am J Sports Med. 2007;35(2);288-293.
5. Clark RF, Harchelroad F. Toxicology screening of the trauma patient: a changing profile. Ann Emerg Med. 1991;20(2):151-153.
6. SooHoo NF, Krenek L, Eagan MJ, Gurbani B, Ko CY, Zingmond DS. Complication rates following open reduction and internal fixation of ankle fractures. J Bone Joint Surg Am. 2009;91(5):1042-1049.
7. Wukich DK, Kline AJ. The management of ankle fractures in patients with diabetes. J Bone Joint Surg Am. 2008;90(7):1570-1578.
8. Egol KA, Tejwani NC, Walsh MG, Capla EL, Koval KJ. Predictors of short-term functional outcome following ankle fracture surgery. J Bone Joint Surg Am. 2006;88(5):974-979.
9. Jones KB, Maiers-Yelden KA, Marsh JL, Zimmerman MB, Estin M, Saltzman CL. Ankle fractures in patients with diabetes mellitus J Bone Joint Surg Br. 2005;87(4):489-495.
10. Nåsell H, Adami J, Samnegård E, Tønnesen H, Ponzer S. Effect of smoking cessation intervention on results of acute fracture surgery: a randomized controlled trial. J Bone Joint Surg Am. 2010;92(6):1335-1342.
11. Levy RS, Hebert CK, Munn BG, Barrack RL. Drug and alcohol use in orthopedic trauma patients: a prospective study. J Orthop Trauma. 1996;10(1):21-27.
12. Flynn JM, Rodriguez-del Rio F, Pizá PA. Closed ankle fractures in the diabetic patient. Foot Ankle Int. 2000;21(4):311-319.
13. Dronge AS, Perkal MF, Kancir S, Concato J, Aslan M, Rosenthal RA. Long-term glycemic control and postoperative infectious complications. Arch Surg. 2006;141(4):375-380.
14. Sorensen LT, Karlsmark T, Gottrup F. Abstinence from smoking reduces incisional wound infection: a randomized controlled trial. Ann Surg. 2003;238(1):1-5.
15. Møller AM, Pedersen T, Villebro N, Munksgaard A. Effect of smoking on early complications after elective orthopaedic surgery. J Bone Joint Surg Br. 2003;85(2):178-181.
16. Castillo RC, Bosse MJ, MacKenzie EJ, Patterson BM; LEAP Study Group. Impact of smoking on fracture healing and risk of complications in limb-threatening open tibia fractures. J Orthop Trauma. 2005;19(3):151-157.
17. Torrens M, Gilchrist G, Domingo-Salvany A; PsyCoBarcelona Group. Psychiatric comorbidity in illicit drug users: substance-induced versus independent disorders. Drug Alcohol Depend. 2010;113(2-3):147-156.
Open treatment of ankle fractures is one of the most common procedures performed by orthopedic surgeons.1 Among the younger patient population, ankle fractures represent a significant proportion of orthopedic injuries.2 The reported incidence of illicit drug and alcohol use in the urban trauma population ranges from 36% to 86%,2 and medical and anesthetic complications associated with illicit drug use have been well documented in surgical patients.2 However, patients with a recent history of drug abuse may be subject to a separate but related set of complications of open treatment of ankle fractures.
The perioperative complications associated with open treatment of ankle fractures in patients with diabetes mellitus have been well described.3-6 Similarly, previous studies have suggested that peripheral vascular disease, complicated diabetes, and smoking are risk factors for poor outcomes in patients who require open reduction and internal fixation (ORIF) in lower extremity trauma.7-9 However, there are few data on the complications specifically associated with illicit drug use and orthopedic surgery. Properly identifying these high-risk groups and being cognizant of commonly associated complications are likely important in ensuring proper perioperative care and may alter follow-up protocols in these patients.
We conducted a study to identify the complications associated with open treatment of ankle fractures in patients who tested positive for illicit drugs on urine drug screen (UDS). We hypothesized that patients who had a history of positive UDS and underwent ORIF of an ankle fracture would have a higher incidence of major and minor complications.
Materials and Methods
After obtaining institutional review board approval, we retrospectively reviewed the cases of 142 patients who underwent open treatment of an ankle fracture between 2006 and 2010. Data sources included patient demographic information, radiographs, preoperative UDS, attending surgeons’ clinical office notes, and clinical laboratory data. Our institution’s standard protocol for ankle fractures was followed for all patients in the study. All patients were evaluated by an orthopedic physician, in either the emergency department or the office, during application of a well-padded Jones splint before surgery. Oral narcotic pain medication was routinely prescribed. All patients were seen, within 10 days of injury, for surgery planning. A board-certified orthopedic surgeon surgically stabilized the ankle fractures. The postoperative treatment regimen, per protocol, included non-weight-bearing in a padded Jones splint dressing; oral narcotic pain medication; physical therapy; and routine scheduled follow-up. In open fracture cases, patients were taken urgently to the operating room for irrigation and débridement with stabilization. Which treatment would be initially used—external fixation or ORIF—was determined on a case-by-case basis.
The sample consisted of adults (age, >18 years) who had undergone definitive ORIF of a lateral malleolar, bimalleolar, or trimalleolar ankle fracture during the study period. Polytrauma patients, patients with external fixation as definitive treatment, and patients with nonoperative treatment were excluded. Before surgical management, all patients were tested for recent illicit drug use by UDS (standard protocol at our institution). UDS, measured for cocaine, marijuana, PCP (phencyclidine), opiates, and barbiturates, was obtained in the office setting or emergency department or on day of surgery. The patients were divided into 2 groups, positive and negative UDS. Patients with documented receipt of narcotic pain medication before UDS were excluded.
The outcomes identified as dependent variables included nonunion, malunion, superficial or deep infection, amputation, delay in treatment, days to healing, repeat surgery, long-term bracing, and loss to follow-up. A nonunion was defined as lasting longer than 9 months and not showing radiographic signs of progression toward healing for 3 consecutive months. These complications were identified with use of attending surgeon clinical progress notes, laboratory values, radiographic parameters, and inpatient readmissions/surgeries associated with these outcomes. Nonunion, malunion, superficial or deep infection, and amputation were then grouped as major complications and analyzed as pooled major complications.
The Fisher exact test was used to analyze categorical variables with respect to UDS. The Wilcoxon rank sum test was used to determine statistical significance for continuous variables. Univariate logistic regression examined both continuous and categorical variables to evaluate predictors for a selected outcome. Statistical significance was set a priori at P ≤ .05, with significant factors indicating an increase (or decrease) in the outcome variable being tested.
Results
We retrospectively reviewed the cases of 142 patients. Table 1 lists the number of cases by fracture type. Bimalleolar fractures were most common, accounting for 99 (69.8%) of the 142 cases. Isolated lateral malleolar fractures accounted for 16 cases (11.2%), and trimalleolar fractures accounted for 27 cases (19%).
Twenty-five (18%) of the 142 patients tested positive for illicit drugs. Mean age was 45.2 years for positive UDS patients and 41.5 years for negative UDS patients. Open fracture cases represented 4.3% of negative UDS patients and 16% of positive UDS patients. Fifty-two percent of positive UDS patients and 32% of negative UDS patients were also tobacco users. These data were statistically significant (P = .003) There were no significant differences in age, sex, incidence of diabetes, incidence of open fracture, or time to surgery between the groups (Table 2).
Incidence of nonunion was higher in positive UDS patients (n = 5; P = .01), as was incidence of deep infection (n = 4; P = .05) (Table 3).
Mean time to radiographic healing was 50.7 days in negative UDS patients and 82.8 days in positive UDS patients (P > .99). Incidence of nonunion was 3.5% in negative UDS patients and 20% in positive UDS patients (P = .01). There were no malunions in negative UDS patients and 2 malunions in positive UDS patients. Incidence of deep infections was 2.5% in negative UDS patients and 16% in positive UDS patients (P = .04). No significant differences were found in incidence of malunions, superficial infections, amputations, need for repeat surgery, continued bracing, or loss to follow-up.
Major complications were defined as superficial or deep infections, amputations, malunions, and nonunions. The rate of major complication was significantly (P = .03) higher in positive UDS patients (24.24%) than in negative UDS patients (7.69%) (Table 4).
Discussion
In the present study, we retrospectively reviewed the cases of patients treated with ORIF for varying types of ankle fractures. Important major and minor complications were analyzed. The overall incidence of major complications in negative UDS patients was only 7.69%, consistent with previously reported results in patients with ankle fractures.6,10 However, a statistically significant (P = .03) increased incidence of major complications—an alarmingly high rate of almost 1 in 4—was found in positive UDS patients. Our results also demonstrated a significantly higher rate of nonunion and deep infection in positive UDS patients. Calculated odds ratios were 7.37 and 4.27 for nonunion and deep infection, respectively—arguably 2 of the most devastating postoperative complications in positive UDS patients.
Previous studies have found that open fractures, age, and medical comorbidities are significant predictors of short-term complications, such as wound healing, infection, persistent pain, and delayed union.3-6 Levy and colleagues11 examined the incidence of orthopedic trauma in positive UDS patients. These patients had orthopedic injuries that were more severe and required longer hospitalization. However, the study did not address patients with ankle fractures or the incidence of major complications. Diabetes and peripheral vascular disease are significant risk factors for many surgical procedures in orthopedic surgery.3,7-9,12,13 Tight glycemic control and optimization of medical comorbidities decrease postoperative complications.12,13 SooHoo and colleagues6 found that history of diabetes and history of peripheral vascular disease were significant predictors of short-term complications of mortality, infection, reoperation, and amputation. The rate of infection in the complicated diabetes group was statistically higher as well. The effect of illicit drug use was not analyzed in that study. We think the findings of the present study highlight the importance of screening for high-risk populations (eg, patients with diabetes, patients with peripheral vascular disease, drug abusers) before orthopedic surgery, especially during definitive treatment of ankle fracture.
Recently, Nåsell and colleagues10 found that a well-implemented smoking cessation program was associated with a statistically significant reduction in complications 6 and 12 weeks after surgery. The target treatment groups were patients who underwent major lower extremity and upper extremity orthopedic surgery. The most common surgery performed in the study was ORIF of ankle fractures. The authors concluded that a smoking cessation intervention program during the first 6 weeks after acute fracture surgery decreases the risk for postoperative complications. However, no recommendations were made for treating patients with other addictions, such as alcohol and illicit drug addictions.
To our knowledge, our study is the first to critically examine postoperative complications in ankle fracture patients with a history of illicit drug abuse as determined by preoperative positive UDS. These data suggest the importance of critically evaluating this patient population. The rates of deep infection, nonunion, and pooled major complications were all notable. Furthermore, compared with negative UDS patients, positive UDS patients were more than 7 times likely to develop a nonunion and more than 4 times likely to develop a deep infection. The reasons are likely multifactorial but may involve factors such as injury severity, poor nutrition, suboptimal living conditions, difficulty complying with weight-bearing restrictions, and, possibly, poor compliance with wound-care recommendations. Determining the influence of each factor was beyond the scope of this study. However, further investigation is warranted.
The difference in incidence of smoking between the 2 groups was statistically significant. As smoking has been well documented as contributing to poor wound and bone healing,14-16 it is likely to have been a contributory factor. However, nicotine levels are not routinely part of UDS, and people who quit smoking typically take 7 to 10 days to demonstrate a measurable drop in cotinine levels. On the other hand, screening for drugs takes only a few minutes and can provide useful information during the preoperative period. It was suggested that positive UDS patients were significantly likely to be tobacco users as well.
The 2 groups were not significantly different with respect to mean follow-up time or loss to follow-up. Although mean follow-up was longer in negative UDS patients, the standard deviation was large in both groups. Given the positive UDS patients’ higher incidence of deep infection and nonunion, both of which typically prolong the course of treatment, the results were likely deceptive. Patients with a history of illicit drug use have confounding variables (eg, psychiatric disorders, financial strife) that make treatment compliance and follow-up difficult.17
Some of the weaknesses of this study are inherent to its retrospective design and limited sample size. Furthermore, patient satisfaction scores and ankle-specific outcome measures, such as AOFAS (American Orthopaedic Foot and Ankle Society) scores, were not considered. Prospective collection of data that include patient satisfaction scores and ankle-specific outcome measures would be optimal. Our current recommendation is to obtain preoperative UDS and illicit drug use history for all trauma patients. In addition, operating surgeons should exercise caution when caring for patients who test positive for illicit drugs.
Conclusion
We evaluated the incidence of complications experienced by positive UDS patients undergoing surgical treatment of ankle fractures. It is well documented that illicit drug users who receive general anesthesia have complications. However, little is known about the untoward effects of illicit drugs on postoperative complications. Furthermore, the efficacy of drug cessation programs in minimizing these complications has not been fully explored.
In conclusion, similar to patients with diabetes, patients with a history of recent illicit drug use, as evidenced by preoperative positive UDS, are at increased risk for complications during treatment for ankle fracture. These data suggest that practicing orthopedists should be more vigilant when caring for ankle fracture patients with preoperative positive UDS.
Open treatment of ankle fractures is one of the most common procedures performed by orthopedic surgeons.1 Among the younger patient population, ankle fractures represent a significant proportion of orthopedic injuries.2 The reported incidence of illicit drug and alcohol use in the urban trauma population ranges from 36% to 86%,2 and medical and anesthetic complications associated with illicit drug use have been well documented in surgical patients.2 However, patients with a recent history of drug abuse may be subject to a separate but related set of complications of open treatment of ankle fractures.
The perioperative complications associated with open treatment of ankle fractures in patients with diabetes mellitus have been well described.3-6 Similarly, previous studies have suggested that peripheral vascular disease, complicated diabetes, and smoking are risk factors for poor outcomes in patients who require open reduction and internal fixation (ORIF) in lower extremity trauma.7-9 However, there are few data on the complications specifically associated with illicit drug use and orthopedic surgery. Properly identifying these high-risk groups and being cognizant of commonly associated complications are likely important in ensuring proper perioperative care and may alter follow-up protocols in these patients.
We conducted a study to identify the complications associated with open treatment of ankle fractures in patients who tested positive for illicit drugs on urine drug screen (UDS). We hypothesized that patients who had a history of positive UDS and underwent ORIF of an ankle fracture would have a higher incidence of major and minor complications.
Materials and Methods
After obtaining institutional review board approval, we retrospectively reviewed the cases of 142 patients who underwent open treatment of an ankle fracture between 2006 and 2010. Data sources included patient demographic information, radiographs, preoperative UDS, attending surgeons’ clinical office notes, and clinical laboratory data. Our institution’s standard protocol for ankle fractures was followed for all patients in the study. All patients were evaluated by an orthopedic physician, in either the emergency department or the office, during application of a well-padded Jones splint before surgery. Oral narcotic pain medication was routinely prescribed. All patients were seen, within 10 days of injury, for surgery planning. A board-certified orthopedic surgeon surgically stabilized the ankle fractures. The postoperative treatment regimen, per protocol, included non-weight-bearing in a padded Jones splint dressing; oral narcotic pain medication; physical therapy; and routine scheduled follow-up. In open fracture cases, patients were taken urgently to the operating room for irrigation and débridement with stabilization. Which treatment would be initially used—external fixation or ORIF—was determined on a case-by-case basis.
The sample consisted of adults (age, >18 years) who had undergone definitive ORIF of a lateral malleolar, bimalleolar, or trimalleolar ankle fracture during the study period. Polytrauma patients, patients with external fixation as definitive treatment, and patients with nonoperative treatment were excluded. Before surgical management, all patients were tested for recent illicit drug use by UDS (standard protocol at our institution). UDS, measured for cocaine, marijuana, PCP (phencyclidine), opiates, and barbiturates, was obtained in the office setting or emergency department or on day of surgery. The patients were divided into 2 groups, positive and negative UDS. Patients with documented receipt of narcotic pain medication before UDS were excluded.
The outcomes identified as dependent variables included nonunion, malunion, superficial or deep infection, amputation, delay in treatment, days to healing, repeat surgery, long-term bracing, and loss to follow-up. A nonunion was defined as lasting longer than 9 months and not showing radiographic signs of progression toward healing for 3 consecutive months. These complications were identified with use of attending surgeon clinical progress notes, laboratory values, radiographic parameters, and inpatient readmissions/surgeries associated with these outcomes. Nonunion, malunion, superficial or deep infection, and amputation were then grouped as major complications and analyzed as pooled major complications.
The Fisher exact test was used to analyze categorical variables with respect to UDS. The Wilcoxon rank sum test was used to determine statistical significance for continuous variables. Univariate logistic regression examined both continuous and categorical variables to evaluate predictors for a selected outcome. Statistical significance was set a priori at P ≤ .05, with significant factors indicating an increase (or decrease) in the outcome variable being tested.
Results
We retrospectively reviewed the cases of 142 patients. Table 1 lists the number of cases by fracture type. Bimalleolar fractures were most common, accounting for 99 (69.8%) of the 142 cases. Isolated lateral malleolar fractures accounted for 16 cases (11.2%), and trimalleolar fractures accounted for 27 cases (19%).
Twenty-five (18%) of the 142 patients tested positive for illicit drugs. Mean age was 45.2 years for positive UDS patients and 41.5 years for negative UDS patients. Open fracture cases represented 4.3% of negative UDS patients and 16% of positive UDS patients. Fifty-two percent of positive UDS patients and 32% of negative UDS patients were also tobacco users. These data were statistically significant (P = .003) There were no significant differences in age, sex, incidence of diabetes, incidence of open fracture, or time to surgery between the groups (Table 2).
Incidence of nonunion was higher in positive UDS patients (n = 5; P = .01), as was incidence of deep infection (n = 4; P = .05) (Table 3).
Mean time to radiographic healing was 50.7 days in negative UDS patients and 82.8 days in positive UDS patients (P > .99). Incidence of nonunion was 3.5% in negative UDS patients and 20% in positive UDS patients (P = .01). There were no malunions in negative UDS patients and 2 malunions in positive UDS patients. Incidence of deep infections was 2.5% in negative UDS patients and 16% in positive UDS patients (P = .04). No significant differences were found in incidence of malunions, superficial infections, amputations, need for repeat surgery, continued bracing, or loss to follow-up.
Major complications were defined as superficial or deep infections, amputations, malunions, and nonunions. The rate of major complication was significantly (P = .03) higher in positive UDS patients (24.24%) than in negative UDS patients (7.69%) (Table 4).
Discussion
In the present study, we retrospectively reviewed the cases of patients treated with ORIF for varying types of ankle fractures. Important major and minor complications were analyzed. The overall incidence of major complications in negative UDS patients was only 7.69%, consistent with previously reported results in patients with ankle fractures.6,10 However, a statistically significant (P = .03) increased incidence of major complications—an alarmingly high rate of almost 1 in 4—was found in positive UDS patients. Our results also demonstrated a significantly higher rate of nonunion and deep infection in positive UDS patients. Calculated odds ratios were 7.37 and 4.27 for nonunion and deep infection, respectively—arguably 2 of the most devastating postoperative complications in positive UDS patients.
Previous studies have found that open fractures, age, and medical comorbidities are significant predictors of short-term complications, such as wound healing, infection, persistent pain, and delayed union.3-6 Levy and colleagues11 examined the incidence of orthopedic trauma in positive UDS patients. These patients had orthopedic injuries that were more severe and required longer hospitalization. However, the study did not address patients with ankle fractures or the incidence of major complications. Diabetes and peripheral vascular disease are significant risk factors for many surgical procedures in orthopedic surgery.3,7-9,12,13 Tight glycemic control and optimization of medical comorbidities decrease postoperative complications.12,13 SooHoo and colleagues6 found that history of diabetes and history of peripheral vascular disease were significant predictors of short-term complications of mortality, infection, reoperation, and amputation. The rate of infection in the complicated diabetes group was statistically higher as well. The effect of illicit drug use was not analyzed in that study. We think the findings of the present study highlight the importance of screening for high-risk populations (eg, patients with diabetes, patients with peripheral vascular disease, drug abusers) before orthopedic surgery, especially during definitive treatment of ankle fracture.
Recently, Nåsell and colleagues10 found that a well-implemented smoking cessation program was associated with a statistically significant reduction in complications 6 and 12 weeks after surgery. The target treatment groups were patients who underwent major lower extremity and upper extremity orthopedic surgery. The most common surgery performed in the study was ORIF of ankle fractures. The authors concluded that a smoking cessation intervention program during the first 6 weeks after acute fracture surgery decreases the risk for postoperative complications. However, no recommendations were made for treating patients with other addictions, such as alcohol and illicit drug addictions.
To our knowledge, our study is the first to critically examine postoperative complications in ankle fracture patients with a history of illicit drug abuse as determined by preoperative positive UDS. These data suggest the importance of critically evaluating this patient population. The rates of deep infection, nonunion, and pooled major complications were all notable. Furthermore, compared with negative UDS patients, positive UDS patients were more than 7 times likely to develop a nonunion and more than 4 times likely to develop a deep infection. The reasons are likely multifactorial but may involve factors such as injury severity, poor nutrition, suboptimal living conditions, difficulty complying with weight-bearing restrictions, and, possibly, poor compliance with wound-care recommendations. Determining the influence of each factor was beyond the scope of this study. However, further investigation is warranted.
The difference in incidence of smoking between the 2 groups was statistically significant. As smoking has been well documented as contributing to poor wound and bone healing,14-16 it is likely to have been a contributory factor. However, nicotine levels are not routinely part of UDS, and people who quit smoking typically take 7 to 10 days to demonstrate a measurable drop in cotinine levels. On the other hand, screening for drugs takes only a few minutes and can provide useful information during the preoperative period. It was suggested that positive UDS patients were significantly likely to be tobacco users as well.
The 2 groups were not significantly different with respect to mean follow-up time or loss to follow-up. Although mean follow-up was longer in negative UDS patients, the standard deviation was large in both groups. Given the positive UDS patients’ higher incidence of deep infection and nonunion, both of which typically prolong the course of treatment, the results were likely deceptive. Patients with a history of illicit drug use have confounding variables (eg, psychiatric disorders, financial strife) that make treatment compliance and follow-up difficult.17
Some of the weaknesses of this study are inherent to its retrospective design and limited sample size. Furthermore, patient satisfaction scores and ankle-specific outcome measures, such as AOFAS (American Orthopaedic Foot and Ankle Society) scores, were not considered. Prospective collection of data that include patient satisfaction scores and ankle-specific outcome measures would be optimal. Our current recommendation is to obtain preoperative UDS and illicit drug use history for all trauma patients. In addition, operating surgeons should exercise caution when caring for patients who test positive for illicit drugs.
Conclusion
We evaluated the incidence of complications experienced by positive UDS patients undergoing surgical treatment of ankle fractures. It is well documented that illicit drug users who receive general anesthesia have complications. However, little is known about the untoward effects of illicit drugs on postoperative complications. Furthermore, the efficacy of drug cessation programs in minimizing these complications has not been fully explored.
In conclusion, similar to patients with diabetes, patients with a history of recent illicit drug use, as evidenced by preoperative positive UDS, are at increased risk for complications during treatment for ankle fracture. These data suggest that practicing orthopedists should be more vigilant when caring for ankle fracture patients with preoperative positive UDS.
1. Michelson JD. Fractures about the ankle. J Bone Joint Surg Am. 1995;77(1):142-152.
2. Culver JL, Walker JR. Anesthetic implications of illicit drug use. J Perianesth Nurs. 1999;14(2):82-90.
3. Bibbo C, Lin SS, Beam HA, Behrens FF. Complications of ankle fractures in diabetic patients. Orthop Clin North Am. 2001;32(1):113-133.
4. Leininger RE, Knox CL, Comstock RD. Epidemiology of 1.6 million pediatric soccer-related injuries presenting to US emergency departments from 1990 to 2003. Am J Sports Med. 2007;35(2);288-293.
5. Clark RF, Harchelroad F. Toxicology screening of the trauma patient: a changing profile. Ann Emerg Med. 1991;20(2):151-153.
6. SooHoo NF, Krenek L, Eagan MJ, Gurbani B, Ko CY, Zingmond DS. Complication rates following open reduction and internal fixation of ankle fractures. J Bone Joint Surg Am. 2009;91(5):1042-1049.
7. Wukich DK, Kline AJ. The management of ankle fractures in patients with diabetes. J Bone Joint Surg Am. 2008;90(7):1570-1578.
8. Egol KA, Tejwani NC, Walsh MG, Capla EL, Koval KJ. Predictors of short-term functional outcome following ankle fracture surgery. J Bone Joint Surg Am. 2006;88(5):974-979.
9. Jones KB, Maiers-Yelden KA, Marsh JL, Zimmerman MB, Estin M, Saltzman CL. Ankle fractures in patients with diabetes mellitus J Bone Joint Surg Br. 2005;87(4):489-495.
10. Nåsell H, Adami J, Samnegård E, Tønnesen H, Ponzer S. Effect of smoking cessation intervention on results of acute fracture surgery: a randomized controlled trial. J Bone Joint Surg Am. 2010;92(6):1335-1342.
11. Levy RS, Hebert CK, Munn BG, Barrack RL. Drug and alcohol use in orthopedic trauma patients: a prospective study. J Orthop Trauma. 1996;10(1):21-27.
12. Flynn JM, Rodriguez-del Rio F, Pizá PA. Closed ankle fractures in the diabetic patient. Foot Ankle Int. 2000;21(4):311-319.
13. Dronge AS, Perkal MF, Kancir S, Concato J, Aslan M, Rosenthal RA. Long-term glycemic control and postoperative infectious complications. Arch Surg. 2006;141(4):375-380.
14. Sorensen LT, Karlsmark T, Gottrup F. Abstinence from smoking reduces incisional wound infection: a randomized controlled trial. Ann Surg. 2003;238(1):1-5.
15. Møller AM, Pedersen T, Villebro N, Munksgaard A. Effect of smoking on early complications after elective orthopaedic surgery. J Bone Joint Surg Br. 2003;85(2):178-181.
16. Castillo RC, Bosse MJ, MacKenzie EJ, Patterson BM; LEAP Study Group. Impact of smoking on fracture healing and risk of complications in limb-threatening open tibia fractures. J Orthop Trauma. 2005;19(3):151-157.
17. Torrens M, Gilchrist G, Domingo-Salvany A; PsyCoBarcelona Group. Psychiatric comorbidity in illicit drug users: substance-induced versus independent disorders. Drug Alcohol Depend. 2010;113(2-3):147-156.
1. Michelson JD. Fractures about the ankle. J Bone Joint Surg Am. 1995;77(1):142-152.
2. Culver JL, Walker JR. Anesthetic implications of illicit drug use. J Perianesth Nurs. 1999;14(2):82-90.
3. Bibbo C, Lin SS, Beam HA, Behrens FF. Complications of ankle fractures in diabetic patients. Orthop Clin North Am. 2001;32(1):113-133.
4. Leininger RE, Knox CL, Comstock RD. Epidemiology of 1.6 million pediatric soccer-related injuries presenting to US emergency departments from 1990 to 2003. Am J Sports Med. 2007;35(2);288-293.
5. Clark RF, Harchelroad F. Toxicology screening of the trauma patient: a changing profile. Ann Emerg Med. 1991;20(2):151-153.
6. SooHoo NF, Krenek L, Eagan MJ, Gurbani B, Ko CY, Zingmond DS. Complication rates following open reduction and internal fixation of ankle fractures. J Bone Joint Surg Am. 2009;91(5):1042-1049.
7. Wukich DK, Kline AJ. The management of ankle fractures in patients with diabetes. J Bone Joint Surg Am. 2008;90(7):1570-1578.
8. Egol KA, Tejwani NC, Walsh MG, Capla EL, Koval KJ. Predictors of short-term functional outcome following ankle fracture surgery. J Bone Joint Surg Am. 2006;88(5):974-979.
9. Jones KB, Maiers-Yelden KA, Marsh JL, Zimmerman MB, Estin M, Saltzman CL. Ankle fractures in patients with diabetes mellitus J Bone Joint Surg Br. 2005;87(4):489-495.
10. Nåsell H, Adami J, Samnegård E, Tønnesen H, Ponzer S. Effect of smoking cessation intervention on results of acute fracture surgery: a randomized controlled trial. J Bone Joint Surg Am. 2010;92(6):1335-1342.
11. Levy RS, Hebert CK, Munn BG, Barrack RL. Drug and alcohol use in orthopedic trauma patients: a prospective study. J Orthop Trauma. 1996;10(1):21-27.
12. Flynn JM, Rodriguez-del Rio F, Pizá PA. Closed ankle fractures in the diabetic patient. Foot Ankle Int. 2000;21(4):311-319.
13. Dronge AS, Perkal MF, Kancir S, Concato J, Aslan M, Rosenthal RA. Long-term glycemic control and postoperative infectious complications. Arch Surg. 2006;141(4):375-380.
14. Sorensen LT, Karlsmark T, Gottrup F. Abstinence from smoking reduces incisional wound infection: a randomized controlled trial. Ann Surg. 2003;238(1):1-5.
15. Møller AM, Pedersen T, Villebro N, Munksgaard A. Effect of smoking on early complications after elective orthopaedic surgery. J Bone Joint Surg Br. 2003;85(2):178-181.
16. Castillo RC, Bosse MJ, MacKenzie EJ, Patterson BM; LEAP Study Group. Impact of smoking on fracture healing and risk of complications in limb-threatening open tibia fractures. J Orthop Trauma. 2005;19(3):151-157.
17. Torrens M, Gilchrist G, Domingo-Salvany A; PsyCoBarcelona Group. Psychiatric comorbidity in illicit drug users: substance-induced versus independent disorders. Drug Alcohol Depend. 2010;113(2-3):147-156.
A Novel Treatment for Refractory Plantar Fasciitis
Chronic plantar fasciitis is a major health care problem worldwide and affects nearly 10% of the US population. Plantar fasciitis presents as heel pain in the mornings and usually gets better and then gets worse. Inflammation at the plantar fascia attachment causes acute and sometimes disabling pain. Chronic pain at the site can develop as time goes on because of long-standing inflammatory changes. Fibrotic tissues may develop at the site. On a continuum, symptoms may begin in an insidious phase and progress to chronic pain. Although most cases resolve with conservative care, the numerous treatments for refractory plantar fasciitis attest to the lack of consensus regarding these cases. The condition frustrates patient and physician alike.
Treatments for refractory plantar fasciitis include conservative measures, including rest, analgesics, walking orthosis, heel cup, night splint, walking boot, and then, in a standard and logical progression, cortisone or platelet-rich plasma injections. Improved magnetic resonance imaging and ultrasonographic imaging allow accurate localization of the pathologic process,1-3 and this localization in turn provides an opportunity to deliver a more reliable and focused intervention, as in needle-guided therapy.4 Surgical procedures for plantar fasciitis have included open or endoscopically assisted plantar fasciectomies with or without gastrocnemius recession; these procedures have had varying results. The emerging goals for this condition are a minimally invasive percutaneous intervention that is safe, effective, and well-tolerated and has minimal morbidity and a low complication rate.
We conducted a prospective study in which patients were allowed either to continue with noninvasive treatment or to undergo focal aspiration and partial fasciotomy with an ultrasonic probe. Study inclusion criteria were plantar fasciitis symptoms lasting 12 months or longer. Exclusion criteria were unwillingness to participate in the study. Prior treatments, even surgeries, were not exclusionary.
Twelve patients with refractory plantar fasciitis lasting a mean of 19 months (minimum, 12 months; range, 12-24 months) chose the procedure. They all had failed conservative care, including physical therapy, casting, shockwave therapy, and invasive procedures such as injections and endoscopic partial releases. Four of the 12 had undergone an open or endoscopic partial release at a different institution but had experienced no improvement in symptoms.
Based on the study protocol, patients continued noninvasive care (night splint, stretching exercises) for 2 to 6 weeks after the initial visit. When this conservative care failed, they were offered focal partial fasciectomy with a percutaneous ultrasonic probe. American Orthopaedic Foot and Ankle Society (AOFAS) scores were obtained before and after surgery. Follow-up consisted of clinic visits 2 weeks after surgery and monthly thereafter. I saw all 12 patients 3 months after surgery (range, 11-14 weeks), and all 12 underwent postoperative physical therapy.
Technique
The TX1 Tissue Removal System (Tenex Health, Lake Forest, California) (Figure 1) consists of an energy module, a pump/suction cassette that provides irrigation and suction through a probe, and the probe itself, the TX1, which is the size of an 18-gauge needle and delivers ultrasonic energy. The cassette is inserted into the energy module, and the ultrasonic energy probe is primed so it will deliver the irrigation fluid, normal saline. The safety features of the energy module are such that no energy is expended unless the system is properly irrigating and aspirating the diseased tissue. Ultrasonic treatment may be performed in a clinical or ambulatory surgical center. The patient is placed supine on an operating table, on a clinical examining table, or, if in a cast room, on a cart. A pillow is placed under the distal tibia so the knees can flex slightly, and the patient is positioned so the feet are free of the edge of the bed or gurney (Figure 2).
The pathology is first confirmed by ultrasonography (Figures 3–5). The first step is to identify the calcaneus with the sensor along the long axis of the foot. Then the plantar fascia is visualized and followed along its long axis to the site of attachment at the medial tubercle. As the pathologic process involves the medial site of attachment, a transverse image may also be obtained to better understand the medial/lateral extent of the disease process. The ultrasonographic image of plantar fasciitis has been well characterized.2,5 The pathology is visualized as an area of edema or of disruption of the linear appearance of the fascia as it attaches to the calcaneus. While the diagnosis is being confirmed, the optimal site for probe insertion should be considered based on the location of the pain and the localization of the pathology by the 2 orthogonal images.
The area is prepared as if for an injection and is squared off with sterile towels. Then the sensor is placed in the sterile sleeve. The area of maximum tenderness is again confirmed. Determining the location of the probe insertion site is a crucial step. We use the ultrasonic sensor in the longitudinal and transverse planes to direct the injection of a fast-acting local anesthetic to the medial aspect of the calcaneus. A skin wheal is created, and the fast-acting local anesthetic (3-4 mL) is injected into the region of the fascia pathology.
An 11-blade knife is used to create a site for the probe through the skin wheal at the medial aspect of the heel, in line with the pathology (Figure 6). The probe is then introduced through the puncture site and is identified, along with the pathology, with the sensor, which may be oriented transverse or longitudinal to the long axis of the foot.
Once the pathologic area is identified, the ultrasonic energy is delivered to the region by the probe, which is activated with a foot pedal, effectively releasing the pathologic tissue from its insertion at the medial tubercle of the calcaneus. The probe is moved in a linear fashion medially and laterally within the lesion across the site of attachment. Treatment continues until the entire soft-tissue lesion is addressed.
Postoperative Care
The wound or wounds are closed with a nylon stitch and Steri-Strip (3M, St. Paul, Minnesota) and covered with Tegaderm (3M) or similar dressing (Figure 7). A compressive dressing is applied. The dressing is removed in 2 to 3 days; the Steri-Strip and stitch are removed in 10 to 14 days. A walking boot is put on immediately after the procedure (most patients in this study already have a boot) and is worn for a few days, or until the symptoms have resolved. How long the boot is used is very much based on patient preference. Patients may continue stretching exercises at home, but there should be no high-impact activity. As-needed ice and analgesics are recommended for the first few days.
The 12 patients had a mean preoperative AOFAS score of 30 (range, 17-46) and a mean postoperative score of 88 (range, 25-92). By the 3-month postoperative visit, symptoms were resolved in 11 patients (no activity restricted by plantar fascia pain). On physical examination, 11 patients had no palpable tenderness at the site of preoperative pain. Pain relief was documented as having occurred between 5 and 13 weeks after treatment. One patient had bilateral procedures. One foot was treated, pain resolved by the 3-month postoperative visit, and the patient asked for the other foot to be treated. Three months after the second procedure, he had minimal non-activity-restricting pain. There were no postoperative infections or wound complications.
I phoned my patients during postoperative month 24. All 12 patients (13 feet) indicated they were essentially pain-free. None admitted to activity restriction or required over-the-counter pain medication. All indicated they were satisfied with the procedure and would have it again.
The refractory nature of plantar fasciitis, and the resistance to and unpredictability of current treatment options, is well known. Considerable efforts have been made to develop treatment guidelines and algorithms.6 A standard and logical treatment plan involves initial attempts with rest, analgesics, and a walking orthosis and then, if those fail, cortisone or platelet-rich plasma injections. Reluctance to perform surgery is well justified because of the unpredictability of the intervention. As might be expected, the utility of ultrasonography has been on the rise. The diagnostic value of ultrasonography, first recognized in the early 1970s, is of increasing importance.7,8 Subsequent use of ultrasonographic imaging as guidance for various treatments, including percutaneous release, has also been recognized and documented.4,9-12 The present article is the first to describe and document the outcome of using ultrasonic energy for percutaneous release of the diseased attachment of the plantar fascia.
This report is preliminary and was designed to alert the orthopedic community to a safe and promising treatment for a chronic, refractory condition. The safety and efficacy of this treatment are reflected in our experience and have been documented for tennis elbow as well.13
This study was limited by its single-surgeon and relatively small clinical experience. Nevertheless, the benefits of this novel technique—effectiveness, safety, tolerability, and rapid recovery—are encouraging enough to share at this time. Prospective randomized controlled studies are needed.
Conclusion
This is the first report of a plantar fascia partial release guided by ultrasonic energy delivered by a percutaneously inserted probe under local anesthesia. The procedure appears to be a safe, effective, well-tolerated treatment for a condition that is refractory to other options. More studies are needed to further validate the safety and efficacy of this innovative treatment modality.
1. Wall JR, Harkness MA, Crawford A. Ultrasound diagnosis of plantar fasciitis. Foot Ankle. 1993;14(8):465-470.
2. Maffulli N, Regine R, Angelillo M, Capasso G, Filice S. Ultrasound diagnosis of Achilles tendon pathology in runners. Br J Sports Med. 1987;21(4):158-162.
3. Patil P, Dasgupta B. Role of diagnostic ultrasound in the assessment of musculoskeletal diseases. Ther Adv Musculoskelet Dis. 2012;4(5):341-355.
4. Royall NA, Farrin E, Bahner DP, Stawicki SP. Ultrasound-assisted musculoskeletal procedures: a practical overview of current literature. World J Orthop. 2011;2(7):57-66.
5. Tsai WC, Chiu MF, Wang CL, Tang FT, Wong MK. Ultrasound evaluation of plantar fasciitis. Scand J Rheumatol. 2000;29(4):255-259.
6. Thomas JL, Christensen JC, Kravitz SR, et al; American College of Foot and Ankle Surgeons Heel Pain Committee. The diagnosis and treatment of heel pain: a clinical practice guideline—revision 2010. J Foot Ankle Surg. 2010;49(3 suppl):S1-S19.
7. McDonald DG, Leopold GR. Ultrasound B–scanning in the differentiation of Baker’s cyst and thrombophlebitis. Br J Radiol. 1972;45(538):729-732.
8. Blankstein A. Ultrasound in the diagnosis of clinical orthopedics: the orthopedic stethoscope. World J Orthop. 2011;2(2):13-24.
9. Rubens DJ, Fultz PJ, Gottlieb RH, Rubin SJ. Effective ultrasonographically guided intervention for diagnosis of musculoskeletal lesions. J Ultrasound Med. 1997;16(12):831-842.
10. Testa V, Capasso G, Benazzo F, Maffulli N. Management of Achilles tendinopathy by ultrasound-guided percutaneous tenotomy. Med Sci Sports Exerc. 2002;34(4):573-580.
11. Debrule MB. Ultrasound-guided Weil percutaneous plantar fasciotomy. J Am Podiatr Med Assoc. 2010;100(2):146-148.
12. Vohra PK, Japour CJ. Ultrasound-guided plantar fascia release technique: a retrospective study of 46 feet. J Am Podiatr Med Assoc. 2009;99(3):183-190.
13. Koh JS, Mohan PC, Howe TS, et al. Fasciotomy and surgical tenotomy for recalcitrant lateral elbow tendinopathy: early clinical experience with a novel device for minimally invasive percutaneous microresection. Am J Sports Med. 2013;41(3):636-644.
Chronic plantar fasciitis is a major health care problem worldwide and affects nearly 10% of the US population. Plantar fasciitis presents as heel pain in the mornings and usually gets better and then gets worse. Inflammation at the plantar fascia attachment causes acute and sometimes disabling pain. Chronic pain at the site can develop as time goes on because of long-standing inflammatory changes. Fibrotic tissues may develop at the site. On a continuum, symptoms may begin in an insidious phase and progress to chronic pain. Although most cases resolve with conservative care, the numerous treatments for refractory plantar fasciitis attest to the lack of consensus regarding these cases. The condition frustrates patient and physician alike.
Treatments for refractory plantar fasciitis include conservative measures, including rest, analgesics, walking orthosis, heel cup, night splint, walking boot, and then, in a standard and logical progression, cortisone or platelet-rich plasma injections. Improved magnetic resonance imaging and ultrasonographic imaging allow accurate localization of the pathologic process,1-3 and this localization in turn provides an opportunity to deliver a more reliable and focused intervention, as in needle-guided therapy.4 Surgical procedures for plantar fasciitis have included open or endoscopically assisted plantar fasciectomies with or without gastrocnemius recession; these procedures have had varying results. The emerging goals for this condition are a minimally invasive percutaneous intervention that is safe, effective, and well-tolerated and has minimal morbidity and a low complication rate.
We conducted a prospective study in which patients were allowed either to continue with noninvasive treatment or to undergo focal aspiration and partial fasciotomy with an ultrasonic probe. Study inclusion criteria were plantar fasciitis symptoms lasting 12 months or longer. Exclusion criteria were unwillingness to participate in the study. Prior treatments, even surgeries, were not exclusionary.
Twelve patients with refractory plantar fasciitis lasting a mean of 19 months (minimum, 12 months; range, 12-24 months) chose the procedure. They all had failed conservative care, including physical therapy, casting, shockwave therapy, and invasive procedures such as injections and endoscopic partial releases. Four of the 12 had undergone an open or endoscopic partial release at a different institution but had experienced no improvement in symptoms.
Based on the study protocol, patients continued noninvasive care (night splint, stretching exercises) for 2 to 6 weeks after the initial visit. When this conservative care failed, they were offered focal partial fasciectomy with a percutaneous ultrasonic probe. American Orthopaedic Foot and Ankle Society (AOFAS) scores were obtained before and after surgery. Follow-up consisted of clinic visits 2 weeks after surgery and monthly thereafter. I saw all 12 patients 3 months after surgery (range, 11-14 weeks), and all 12 underwent postoperative physical therapy.
Technique
The TX1 Tissue Removal System (Tenex Health, Lake Forest, California) (Figure 1) consists of an energy module, a pump/suction cassette that provides irrigation and suction through a probe, and the probe itself, the TX1, which is the size of an 18-gauge needle and delivers ultrasonic energy. The cassette is inserted into the energy module, and the ultrasonic energy probe is primed so it will deliver the irrigation fluid, normal saline. The safety features of the energy module are such that no energy is expended unless the system is properly irrigating and aspirating the diseased tissue. Ultrasonic treatment may be performed in a clinical or ambulatory surgical center. The patient is placed supine on an operating table, on a clinical examining table, or, if in a cast room, on a cart. A pillow is placed under the distal tibia so the knees can flex slightly, and the patient is positioned so the feet are free of the edge of the bed or gurney (Figure 2).
The pathology is first confirmed by ultrasonography (Figures 3–5). The first step is to identify the calcaneus with the sensor along the long axis of the foot. Then the plantar fascia is visualized and followed along its long axis to the site of attachment at the medial tubercle. As the pathologic process involves the medial site of attachment, a transverse image may also be obtained to better understand the medial/lateral extent of the disease process. The ultrasonographic image of plantar fasciitis has been well characterized.2,5 The pathology is visualized as an area of edema or of disruption of the linear appearance of the fascia as it attaches to the calcaneus. While the diagnosis is being confirmed, the optimal site for probe insertion should be considered based on the location of the pain and the localization of the pathology by the 2 orthogonal images.
The area is prepared as if for an injection and is squared off with sterile towels. Then the sensor is placed in the sterile sleeve. The area of maximum tenderness is again confirmed. Determining the location of the probe insertion site is a crucial step. We use the ultrasonic sensor in the longitudinal and transverse planes to direct the injection of a fast-acting local anesthetic to the medial aspect of the calcaneus. A skin wheal is created, and the fast-acting local anesthetic (3-4 mL) is injected into the region of the fascia pathology.
An 11-blade knife is used to create a site for the probe through the skin wheal at the medial aspect of the heel, in line with the pathology (Figure 6). The probe is then introduced through the puncture site and is identified, along with the pathology, with the sensor, which may be oriented transverse or longitudinal to the long axis of the foot.
Once the pathologic area is identified, the ultrasonic energy is delivered to the region by the probe, which is activated with a foot pedal, effectively releasing the pathologic tissue from its insertion at the medial tubercle of the calcaneus. The probe is moved in a linear fashion medially and laterally within the lesion across the site of attachment. Treatment continues until the entire soft-tissue lesion is addressed.
Postoperative Care
The wound or wounds are closed with a nylon stitch and Steri-Strip (3M, St. Paul, Minnesota) and covered with Tegaderm (3M) or similar dressing (Figure 7). A compressive dressing is applied. The dressing is removed in 2 to 3 days; the Steri-Strip and stitch are removed in 10 to 14 days. A walking boot is put on immediately after the procedure (most patients in this study already have a boot) and is worn for a few days, or until the symptoms have resolved. How long the boot is used is very much based on patient preference. Patients may continue stretching exercises at home, but there should be no high-impact activity. As-needed ice and analgesics are recommended for the first few days.
The 12 patients had a mean preoperative AOFAS score of 30 (range, 17-46) and a mean postoperative score of 88 (range, 25-92). By the 3-month postoperative visit, symptoms were resolved in 11 patients (no activity restricted by plantar fascia pain). On physical examination, 11 patients had no palpable tenderness at the site of preoperative pain. Pain relief was documented as having occurred between 5 and 13 weeks after treatment. One patient had bilateral procedures. One foot was treated, pain resolved by the 3-month postoperative visit, and the patient asked for the other foot to be treated. Three months after the second procedure, he had minimal non-activity-restricting pain. There were no postoperative infections or wound complications.
I phoned my patients during postoperative month 24. All 12 patients (13 feet) indicated they were essentially pain-free. None admitted to activity restriction or required over-the-counter pain medication. All indicated they were satisfied with the procedure and would have it again.
The refractory nature of plantar fasciitis, and the resistance to and unpredictability of current treatment options, is well known. Considerable efforts have been made to develop treatment guidelines and algorithms.6 A standard and logical treatment plan involves initial attempts with rest, analgesics, and a walking orthosis and then, if those fail, cortisone or platelet-rich plasma injections. Reluctance to perform surgery is well justified because of the unpredictability of the intervention. As might be expected, the utility of ultrasonography has been on the rise. The diagnostic value of ultrasonography, first recognized in the early 1970s, is of increasing importance.7,8 Subsequent use of ultrasonographic imaging as guidance for various treatments, including percutaneous release, has also been recognized and documented.4,9-12 The present article is the first to describe and document the outcome of using ultrasonic energy for percutaneous release of the diseased attachment of the plantar fascia.
This report is preliminary and was designed to alert the orthopedic community to a safe and promising treatment for a chronic, refractory condition. The safety and efficacy of this treatment are reflected in our experience and have been documented for tennis elbow as well.13
This study was limited by its single-surgeon and relatively small clinical experience. Nevertheless, the benefits of this novel technique—effectiveness, safety, tolerability, and rapid recovery—are encouraging enough to share at this time. Prospective randomized controlled studies are needed.
Conclusion
This is the first report of a plantar fascia partial release guided by ultrasonic energy delivered by a percutaneously inserted probe under local anesthesia. The procedure appears to be a safe, effective, well-tolerated treatment for a condition that is refractory to other options. More studies are needed to further validate the safety and efficacy of this innovative treatment modality.
Chronic plantar fasciitis is a major health care problem worldwide and affects nearly 10% of the US population. Plantar fasciitis presents as heel pain in the mornings and usually gets better and then gets worse. Inflammation at the plantar fascia attachment causes acute and sometimes disabling pain. Chronic pain at the site can develop as time goes on because of long-standing inflammatory changes. Fibrotic tissues may develop at the site. On a continuum, symptoms may begin in an insidious phase and progress to chronic pain. Although most cases resolve with conservative care, the numerous treatments for refractory plantar fasciitis attest to the lack of consensus regarding these cases. The condition frustrates patient and physician alike.
Treatments for refractory plantar fasciitis include conservative measures, including rest, analgesics, walking orthosis, heel cup, night splint, walking boot, and then, in a standard and logical progression, cortisone or platelet-rich plasma injections. Improved magnetic resonance imaging and ultrasonographic imaging allow accurate localization of the pathologic process,1-3 and this localization in turn provides an opportunity to deliver a more reliable and focused intervention, as in needle-guided therapy.4 Surgical procedures for plantar fasciitis have included open or endoscopically assisted plantar fasciectomies with or without gastrocnemius recession; these procedures have had varying results. The emerging goals for this condition are a minimally invasive percutaneous intervention that is safe, effective, and well-tolerated and has minimal morbidity and a low complication rate.
We conducted a prospective study in which patients were allowed either to continue with noninvasive treatment or to undergo focal aspiration and partial fasciotomy with an ultrasonic probe. Study inclusion criteria were plantar fasciitis symptoms lasting 12 months or longer. Exclusion criteria were unwillingness to participate in the study. Prior treatments, even surgeries, were not exclusionary.
Twelve patients with refractory plantar fasciitis lasting a mean of 19 months (minimum, 12 months; range, 12-24 months) chose the procedure. They all had failed conservative care, including physical therapy, casting, shockwave therapy, and invasive procedures such as injections and endoscopic partial releases. Four of the 12 had undergone an open or endoscopic partial release at a different institution but had experienced no improvement in symptoms.
Based on the study protocol, patients continued noninvasive care (night splint, stretching exercises) for 2 to 6 weeks after the initial visit. When this conservative care failed, they were offered focal partial fasciectomy with a percutaneous ultrasonic probe. American Orthopaedic Foot and Ankle Society (AOFAS) scores were obtained before and after surgery. Follow-up consisted of clinic visits 2 weeks after surgery and monthly thereafter. I saw all 12 patients 3 months after surgery (range, 11-14 weeks), and all 12 underwent postoperative physical therapy.
Technique
The TX1 Tissue Removal System (Tenex Health, Lake Forest, California) (Figure 1) consists of an energy module, a pump/suction cassette that provides irrigation and suction through a probe, and the probe itself, the TX1, which is the size of an 18-gauge needle and delivers ultrasonic energy. The cassette is inserted into the energy module, and the ultrasonic energy probe is primed so it will deliver the irrigation fluid, normal saline. The safety features of the energy module are such that no energy is expended unless the system is properly irrigating and aspirating the diseased tissue. Ultrasonic treatment may be performed in a clinical or ambulatory surgical center. The patient is placed supine on an operating table, on a clinical examining table, or, if in a cast room, on a cart. A pillow is placed under the distal tibia so the knees can flex slightly, and the patient is positioned so the feet are free of the edge of the bed or gurney (Figure 2).
The pathology is first confirmed by ultrasonography (Figures 3–5). The first step is to identify the calcaneus with the sensor along the long axis of the foot. Then the plantar fascia is visualized and followed along its long axis to the site of attachment at the medial tubercle. As the pathologic process involves the medial site of attachment, a transverse image may also be obtained to better understand the medial/lateral extent of the disease process. The ultrasonographic image of plantar fasciitis has been well characterized.2,5 The pathology is visualized as an area of edema or of disruption of the linear appearance of the fascia as it attaches to the calcaneus. While the diagnosis is being confirmed, the optimal site for probe insertion should be considered based on the location of the pain and the localization of the pathology by the 2 orthogonal images.
The area is prepared as if for an injection and is squared off with sterile towels. Then the sensor is placed in the sterile sleeve. The area of maximum tenderness is again confirmed. Determining the location of the probe insertion site is a crucial step. We use the ultrasonic sensor in the longitudinal and transverse planes to direct the injection of a fast-acting local anesthetic to the medial aspect of the calcaneus. A skin wheal is created, and the fast-acting local anesthetic (3-4 mL) is injected into the region of the fascia pathology.
An 11-blade knife is used to create a site for the probe through the skin wheal at the medial aspect of the heel, in line with the pathology (Figure 6). The probe is then introduced through the puncture site and is identified, along with the pathology, with the sensor, which may be oriented transverse or longitudinal to the long axis of the foot.
Once the pathologic area is identified, the ultrasonic energy is delivered to the region by the probe, which is activated with a foot pedal, effectively releasing the pathologic tissue from its insertion at the medial tubercle of the calcaneus. The probe is moved in a linear fashion medially and laterally within the lesion across the site of attachment. Treatment continues until the entire soft-tissue lesion is addressed.
Postoperative Care
The wound or wounds are closed with a nylon stitch and Steri-Strip (3M, St. Paul, Minnesota) and covered with Tegaderm (3M) or similar dressing (Figure 7). A compressive dressing is applied. The dressing is removed in 2 to 3 days; the Steri-Strip and stitch are removed in 10 to 14 days. A walking boot is put on immediately after the procedure (most patients in this study already have a boot) and is worn for a few days, or until the symptoms have resolved. How long the boot is used is very much based on patient preference. Patients may continue stretching exercises at home, but there should be no high-impact activity. As-needed ice and analgesics are recommended for the first few days.
The 12 patients had a mean preoperative AOFAS score of 30 (range, 17-46) and a mean postoperative score of 88 (range, 25-92). By the 3-month postoperative visit, symptoms were resolved in 11 patients (no activity restricted by plantar fascia pain). On physical examination, 11 patients had no palpable tenderness at the site of preoperative pain. Pain relief was documented as having occurred between 5 and 13 weeks after treatment. One patient had bilateral procedures. One foot was treated, pain resolved by the 3-month postoperative visit, and the patient asked for the other foot to be treated. Three months after the second procedure, he had minimal non-activity-restricting pain. There were no postoperative infections or wound complications.
I phoned my patients during postoperative month 24. All 12 patients (13 feet) indicated they were essentially pain-free. None admitted to activity restriction or required over-the-counter pain medication. All indicated they were satisfied with the procedure and would have it again.
The refractory nature of plantar fasciitis, and the resistance to and unpredictability of current treatment options, is well known. Considerable efforts have been made to develop treatment guidelines and algorithms.6 A standard and logical treatment plan involves initial attempts with rest, analgesics, and a walking orthosis and then, if those fail, cortisone or platelet-rich plasma injections. Reluctance to perform surgery is well justified because of the unpredictability of the intervention. As might be expected, the utility of ultrasonography has been on the rise. The diagnostic value of ultrasonography, first recognized in the early 1970s, is of increasing importance.7,8 Subsequent use of ultrasonographic imaging as guidance for various treatments, including percutaneous release, has also been recognized and documented.4,9-12 The present article is the first to describe and document the outcome of using ultrasonic energy for percutaneous release of the diseased attachment of the plantar fascia.
This report is preliminary and was designed to alert the orthopedic community to a safe and promising treatment for a chronic, refractory condition. The safety and efficacy of this treatment are reflected in our experience and have been documented for tennis elbow as well.13
This study was limited by its single-surgeon and relatively small clinical experience. Nevertheless, the benefits of this novel technique—effectiveness, safety, tolerability, and rapid recovery—are encouraging enough to share at this time. Prospective randomized controlled studies are needed.
Conclusion
This is the first report of a plantar fascia partial release guided by ultrasonic energy delivered by a percutaneously inserted probe under local anesthesia. The procedure appears to be a safe, effective, well-tolerated treatment for a condition that is refractory to other options. More studies are needed to further validate the safety and efficacy of this innovative treatment modality.
1. Wall JR, Harkness MA, Crawford A. Ultrasound diagnosis of plantar fasciitis. Foot Ankle. 1993;14(8):465-470.
2. Maffulli N, Regine R, Angelillo M, Capasso G, Filice S. Ultrasound diagnosis of Achilles tendon pathology in runners. Br J Sports Med. 1987;21(4):158-162.
3. Patil P, Dasgupta B. Role of diagnostic ultrasound in the assessment of musculoskeletal diseases. Ther Adv Musculoskelet Dis. 2012;4(5):341-355.
4. Royall NA, Farrin E, Bahner DP, Stawicki SP. Ultrasound-assisted musculoskeletal procedures: a practical overview of current literature. World J Orthop. 2011;2(7):57-66.
5. Tsai WC, Chiu MF, Wang CL, Tang FT, Wong MK. Ultrasound evaluation of plantar fasciitis. Scand J Rheumatol. 2000;29(4):255-259.
6. Thomas JL, Christensen JC, Kravitz SR, et al; American College of Foot and Ankle Surgeons Heel Pain Committee. The diagnosis and treatment of heel pain: a clinical practice guideline—revision 2010. J Foot Ankle Surg. 2010;49(3 suppl):S1-S19.
7. McDonald DG, Leopold GR. Ultrasound B–scanning in the differentiation of Baker’s cyst and thrombophlebitis. Br J Radiol. 1972;45(538):729-732.
8. Blankstein A. Ultrasound in the diagnosis of clinical orthopedics: the orthopedic stethoscope. World J Orthop. 2011;2(2):13-24.
9. Rubens DJ, Fultz PJ, Gottlieb RH, Rubin SJ. Effective ultrasonographically guided intervention for diagnosis of musculoskeletal lesions. J Ultrasound Med. 1997;16(12):831-842.
10. Testa V, Capasso G, Benazzo F, Maffulli N. Management of Achilles tendinopathy by ultrasound-guided percutaneous tenotomy. Med Sci Sports Exerc. 2002;34(4):573-580.
11. Debrule MB. Ultrasound-guided Weil percutaneous plantar fasciotomy. J Am Podiatr Med Assoc. 2010;100(2):146-148.
12. Vohra PK, Japour CJ. Ultrasound-guided plantar fascia release technique: a retrospective study of 46 feet. J Am Podiatr Med Assoc. 2009;99(3):183-190.
13. Koh JS, Mohan PC, Howe TS, et al. Fasciotomy and surgical tenotomy for recalcitrant lateral elbow tendinopathy: early clinical experience with a novel device for minimally invasive percutaneous microresection. Am J Sports Med. 2013;41(3):636-644.
1. Wall JR, Harkness MA, Crawford A. Ultrasound diagnosis of plantar fasciitis. Foot Ankle. 1993;14(8):465-470.
2. Maffulli N, Regine R, Angelillo M, Capasso G, Filice S. Ultrasound diagnosis of Achilles tendon pathology in runners. Br J Sports Med. 1987;21(4):158-162.
3. Patil P, Dasgupta B. Role of diagnostic ultrasound in the assessment of musculoskeletal diseases. Ther Adv Musculoskelet Dis. 2012;4(5):341-355.
4. Royall NA, Farrin E, Bahner DP, Stawicki SP. Ultrasound-assisted musculoskeletal procedures: a practical overview of current literature. World J Orthop. 2011;2(7):57-66.
5. Tsai WC, Chiu MF, Wang CL, Tang FT, Wong MK. Ultrasound evaluation of plantar fasciitis. Scand J Rheumatol. 2000;29(4):255-259.
6. Thomas JL, Christensen JC, Kravitz SR, et al; American College of Foot and Ankle Surgeons Heel Pain Committee. The diagnosis and treatment of heel pain: a clinical practice guideline—revision 2010. J Foot Ankle Surg. 2010;49(3 suppl):S1-S19.
7. McDonald DG, Leopold GR. Ultrasound B–scanning in the differentiation of Baker’s cyst and thrombophlebitis. Br J Radiol. 1972;45(538):729-732.
8. Blankstein A. Ultrasound in the diagnosis of clinical orthopedics: the orthopedic stethoscope. World J Orthop. 2011;2(2):13-24.
9. Rubens DJ, Fultz PJ, Gottlieb RH, Rubin SJ. Effective ultrasonographically guided intervention for diagnosis of musculoskeletal lesions. J Ultrasound Med. 1997;16(12):831-842.
10. Testa V, Capasso G, Benazzo F, Maffulli N. Management of Achilles tendinopathy by ultrasound-guided percutaneous tenotomy. Med Sci Sports Exerc. 2002;34(4):573-580.
11. Debrule MB. Ultrasound-guided Weil percutaneous plantar fasciotomy. J Am Podiatr Med Assoc. 2010;100(2):146-148.
12. Vohra PK, Japour CJ. Ultrasound-guided plantar fascia release technique: a retrospective study of 46 feet. J Am Podiatr Med Assoc. 2009;99(3):183-190.
13. Koh JS, Mohan PC, Howe TS, et al. Fasciotomy and surgical tenotomy for recalcitrant lateral elbow tendinopathy: early clinical experience with a novel device for minimally invasive percutaneous microresection. Am J Sports Med. 2013;41(3):636-644.
AGA guideline addresses asymptomatic neoplastic pancreatic cysts
For asymptomatic neoplastic pancreatic cysts discovered incidentally on abdominal imaging, surgery is warranted only if both a solid component and a dilated pancreatic duct are shown and/or if endoscopic* ultrasound with or without fine-needle aspiration has detected “concerning features,” according to a clinical practice guideline published in the April issue of Gastroenterology (doi:10.1053/j.gastro.2015.01.015).
Even then, patients should be referred for the procedure only to centers that perform high volumes of pancreatic surgery, so as to minimize the relatively high rates of morbidity and mortality associated with these invasive, expensive, and potentially harmful surgeries.
These are 2 of the 10 recommendations and “suggestions” in the American Gastroenterological Association guideline, which is the first such guideline to be based on a systematic evaluation of the available evidence, said Dr. Santhi Swaroop Vege of the division of gastroenterology and hepatology, Mayo Clinic, Rochester, Minn., and his associates.
Incidental discovery of asymptomatic pancreatic cysts is common with the increasing use of sophisticated abdominal imaging techniques. For example, approximately 15% of patients undergoing abdominal MRI for other indications are found to have them. Clinical management is very difficult because only a small fraction of these lesions prove to be malignant, and the data to guide diagnostic and treatment decisions are sparse and of very low quality, based almost entirely on retrospective case series. Nevertheless, Dr. Vege and his associates developed the guideline from the limited evidence that is available, because of the seriousness of the outcomes for that minority of cancers and the complexity of management strategies.
“These recommendations may result in significant controversy, as they advocate less frequent follow-up and a higher threshold before offering endoscopic ultrasound and/or surgery. However, consistent utilization should decrease inadvertent harm to patients and reduce the costs of health care delivery,” they noted.
After reviewing the literature, the investigators estimated that an asymptomatic cyst found incidentally on MRI has only a 10 in 100,000 chance of being a mucinous invasive malignancy and a 17 in 100,000 chance of being a ductal cancer. The guideline therefore suggests that surveillance is sufficient for asymptomatic pancreatic cysts smaller than 3 cm that don’t have a solid component or a dilated pancreatic duct. The preferred imaging modality is MRI, and the preferred surveillance interval is at 1 year after discovery. If no change is noted, surveillance every 2 years for a total of 5 years should be sufficient.
The risk of malignant transformation is estimated to be only 0.24% per year, and is even lower among cysts that show no changes over time. “The small risk of malignant progression in stable cysts is likely outweighed by the costs of surveillance and the risks of surgery,” so the guideline suggests that surveillance can be discontinued if no change has occurred after 5 years or if the patient is no longer a candidate for surgery. However, some patients, such as those with a family history of pancreatic cancer, may opt to continue surveillance.
In contrast, asymptomatic pancreatic cysts that have at least two high-risk features should be assessed using endoscopic ultrasound, with or without fine-needle aspiration. If these procedures reveal “concerning features,” the benefits of surgery probably outweigh the risks, and surgical excision/resection is conditionally recommended. However, even in these “suspect” lesions only an estimated 17% are found to harbor high-grade dysplasia. Any benefit ascribed to surgery must be balanced against “an overall postoperative mortality of 2% and major morbidity of 30% from our review of the literature,” Dr. Vege and his associates said.
In contrast to its suggestions and conditional recommendations, the AGA guideline strongly recommends that if surgery is being considered, patients be referred to “a center with demonstrated expertise in pancreatic surgery.” Their investigation showed that in the U.S. overall, all pancreatic surgeries carry a postoperative mortality of 6.6%, while in centers of excellence, the postoperative mortality is only 2%.
The guideline conditionally suggests that patients found to have invasive cancer or dysplasia in a resected cyst can undergo MRI surveillance of any remaining pancreas every 2 years, for as long as the patient remains a good candidate for further surgery.
Another recommendation is that patients be given a clear understanding of the benefits and risks of any surveillance program, because surveillance may not be appropriate for some. Certain patients have a high tolerance for risk and may decide against surveillance once the small risk of malignancy is explained to them. Others have a limited life expectancy and are unlikely to benefit from surveillance or surgery, and still others who are poor surgical candidates because of age or comorbidities shouldn’t be subjected to surveillance.
Finally, this AGA guideline pertains only to asymptomatic neoplastic pancreatic cysts. It doesn’t address lesions such as solid papillary neoplasms, cystic degeneration of adenocarcinomas, neuroendocrine tumors, or main duct intraductal papillary mucinous neoplasms without side-branch involvement, because identification of these lesions is more straightforward and the accepted management approach is surgical resection, Dr. Vege and his associates added.
*A correction was made on April 29, 2015.
For asymptomatic neoplastic pancreatic cysts discovered incidentally on abdominal imaging, surgery is warranted only if both a solid component and a dilated pancreatic duct are shown and/or if endoscopic* ultrasound with or without fine-needle aspiration has detected “concerning features,” according to a clinical practice guideline published in the April issue of Gastroenterology (doi:10.1053/j.gastro.2015.01.015).
Even then, patients should be referred for the procedure only to centers that perform high volumes of pancreatic surgery, so as to minimize the relatively high rates of morbidity and mortality associated with these invasive, expensive, and potentially harmful surgeries.
These are 2 of the 10 recommendations and “suggestions” in the American Gastroenterological Association guideline, which is the first such guideline to be based on a systematic evaluation of the available evidence, said Dr. Santhi Swaroop Vege of the division of gastroenterology and hepatology, Mayo Clinic, Rochester, Minn., and his associates.
Incidental discovery of asymptomatic pancreatic cysts is common with the increasing use of sophisticated abdominal imaging techniques. For example, approximately 15% of patients undergoing abdominal MRI for other indications are found to have them. Clinical management is very difficult because only a small fraction of these lesions prove to be malignant, and the data to guide diagnostic and treatment decisions are sparse and of very low quality, based almost entirely on retrospective case series. Nevertheless, Dr. Vege and his associates developed the guideline from the limited evidence that is available, because of the seriousness of the outcomes for that minority of cancers and the complexity of management strategies.
“These recommendations may result in significant controversy, as they advocate less frequent follow-up and a higher threshold before offering endoscopic ultrasound and/or surgery. However, consistent utilization should decrease inadvertent harm to patients and reduce the costs of health care delivery,” they noted.
After reviewing the literature, the investigators estimated that an asymptomatic cyst found incidentally on MRI has only a 10 in 100,000 chance of being a mucinous invasive malignancy and a 17 in 100,000 chance of being a ductal cancer. The guideline therefore suggests that surveillance is sufficient for asymptomatic pancreatic cysts smaller than 3 cm that don’t have a solid component or a dilated pancreatic duct. The preferred imaging modality is MRI, and the preferred surveillance interval is at 1 year after discovery. If no change is noted, surveillance every 2 years for a total of 5 years should be sufficient.
The risk of malignant transformation is estimated to be only 0.24% per year, and is even lower among cysts that show no changes over time. “The small risk of malignant progression in stable cysts is likely outweighed by the costs of surveillance and the risks of surgery,” so the guideline suggests that surveillance can be discontinued if no change has occurred after 5 years or if the patient is no longer a candidate for surgery. However, some patients, such as those with a family history of pancreatic cancer, may opt to continue surveillance.
In contrast, asymptomatic pancreatic cysts that have at least two high-risk features should be assessed using endoscopic ultrasound, with or without fine-needle aspiration. If these procedures reveal “concerning features,” the benefits of surgery probably outweigh the risks, and surgical excision/resection is conditionally recommended. However, even in these “suspect” lesions only an estimated 17% are found to harbor high-grade dysplasia. Any benefit ascribed to surgery must be balanced against “an overall postoperative mortality of 2% and major morbidity of 30% from our review of the literature,” Dr. Vege and his associates said.
In contrast to its suggestions and conditional recommendations, the AGA guideline strongly recommends that if surgery is being considered, patients be referred to “a center with demonstrated expertise in pancreatic surgery.” Their investigation showed that in the U.S. overall, all pancreatic surgeries carry a postoperative mortality of 6.6%, while in centers of excellence, the postoperative mortality is only 2%.
The guideline conditionally suggests that patients found to have invasive cancer or dysplasia in a resected cyst can undergo MRI surveillance of any remaining pancreas every 2 years, for as long as the patient remains a good candidate for further surgery.
Another recommendation is that patients be given a clear understanding of the benefits and risks of any surveillance program, because surveillance may not be appropriate for some. Certain patients have a high tolerance for risk and may decide against surveillance once the small risk of malignancy is explained to them. Others have a limited life expectancy and are unlikely to benefit from surveillance or surgery, and still others who are poor surgical candidates because of age or comorbidities shouldn’t be subjected to surveillance.
Finally, this AGA guideline pertains only to asymptomatic neoplastic pancreatic cysts. It doesn’t address lesions such as solid papillary neoplasms, cystic degeneration of adenocarcinomas, neuroendocrine tumors, or main duct intraductal papillary mucinous neoplasms without side-branch involvement, because identification of these lesions is more straightforward and the accepted management approach is surgical resection, Dr. Vege and his associates added.
*A correction was made on April 29, 2015.
For asymptomatic neoplastic pancreatic cysts discovered incidentally on abdominal imaging, surgery is warranted only if both a solid component and a dilated pancreatic duct are shown and/or if endoscopic* ultrasound with or without fine-needle aspiration has detected “concerning features,” according to a clinical practice guideline published in the April issue of Gastroenterology (doi:10.1053/j.gastro.2015.01.015).
Even then, patients should be referred for the procedure only to centers that perform high volumes of pancreatic surgery, so as to minimize the relatively high rates of morbidity and mortality associated with these invasive, expensive, and potentially harmful surgeries.
These are 2 of the 10 recommendations and “suggestions” in the American Gastroenterological Association guideline, which is the first such guideline to be based on a systematic evaluation of the available evidence, said Dr. Santhi Swaroop Vege of the division of gastroenterology and hepatology, Mayo Clinic, Rochester, Minn., and his associates.
Incidental discovery of asymptomatic pancreatic cysts is common with the increasing use of sophisticated abdominal imaging techniques. For example, approximately 15% of patients undergoing abdominal MRI for other indications are found to have them. Clinical management is very difficult because only a small fraction of these lesions prove to be malignant, and the data to guide diagnostic and treatment decisions are sparse and of very low quality, based almost entirely on retrospective case series. Nevertheless, Dr. Vege and his associates developed the guideline from the limited evidence that is available, because of the seriousness of the outcomes for that minority of cancers and the complexity of management strategies.
“These recommendations may result in significant controversy, as they advocate less frequent follow-up and a higher threshold before offering endoscopic ultrasound and/or surgery. However, consistent utilization should decrease inadvertent harm to patients and reduce the costs of health care delivery,” they noted.
After reviewing the literature, the investigators estimated that an asymptomatic cyst found incidentally on MRI has only a 10 in 100,000 chance of being a mucinous invasive malignancy and a 17 in 100,000 chance of being a ductal cancer. The guideline therefore suggests that surveillance is sufficient for asymptomatic pancreatic cysts smaller than 3 cm that don’t have a solid component or a dilated pancreatic duct. The preferred imaging modality is MRI, and the preferred surveillance interval is at 1 year after discovery. If no change is noted, surveillance every 2 years for a total of 5 years should be sufficient.
The risk of malignant transformation is estimated to be only 0.24% per year, and is even lower among cysts that show no changes over time. “The small risk of malignant progression in stable cysts is likely outweighed by the costs of surveillance and the risks of surgery,” so the guideline suggests that surveillance can be discontinued if no change has occurred after 5 years or if the patient is no longer a candidate for surgery. However, some patients, such as those with a family history of pancreatic cancer, may opt to continue surveillance.
In contrast, asymptomatic pancreatic cysts that have at least two high-risk features should be assessed using endoscopic ultrasound, with or without fine-needle aspiration. If these procedures reveal “concerning features,” the benefits of surgery probably outweigh the risks, and surgical excision/resection is conditionally recommended. However, even in these “suspect” lesions only an estimated 17% are found to harbor high-grade dysplasia. Any benefit ascribed to surgery must be balanced against “an overall postoperative mortality of 2% and major morbidity of 30% from our review of the literature,” Dr. Vege and his associates said.
In contrast to its suggestions and conditional recommendations, the AGA guideline strongly recommends that if surgery is being considered, patients be referred to “a center with demonstrated expertise in pancreatic surgery.” Their investigation showed that in the U.S. overall, all pancreatic surgeries carry a postoperative mortality of 6.6%, while in centers of excellence, the postoperative mortality is only 2%.
The guideline conditionally suggests that patients found to have invasive cancer or dysplasia in a resected cyst can undergo MRI surveillance of any remaining pancreas every 2 years, for as long as the patient remains a good candidate for further surgery.
Another recommendation is that patients be given a clear understanding of the benefits and risks of any surveillance program, because surveillance may not be appropriate for some. Certain patients have a high tolerance for risk and may decide against surveillance once the small risk of malignancy is explained to them. Others have a limited life expectancy and are unlikely to benefit from surveillance or surgery, and still others who are poor surgical candidates because of age or comorbidities shouldn’t be subjected to surveillance.
Finally, this AGA guideline pertains only to asymptomatic neoplastic pancreatic cysts. It doesn’t address lesions such as solid papillary neoplasms, cystic degeneration of adenocarcinomas, neuroendocrine tumors, or main duct intraductal papillary mucinous neoplasms without side-branch involvement, because identification of these lesions is more straightforward and the accepted management approach is surgical resection, Dr. Vege and his associates added.
*A correction was made on April 29, 2015.
Key clinical point: A new AGA clinical practice guideline suggests surgery is warranted only if asymptomatic neoplastic pancreatic cysts have both a solid component and a dilated pancreatic duct and/or concerning features on endoscopic ultrasound with or without fine-needle aspiration.
Major finding: An asymptomatic pancreatic cyst found incidentally on MRI is estimated to have only a 10 in 100,000 chance of being a mucinous invasive malignancy and a 17 in 100,000 chance of being a ductal cancer.
Data source: A review and summary of the available evidence regarding management of asymptomatic neoplastic pancreatic cysts, and a compilation of recommendations for clinicians.
Disclosures: Dr. Vege and his associates’ disclosures are available at the American Gastroenterological Association, Bethesda, Md.
Memory disorders
The video associated with this article is no longer available on this site. Please view all of our videos on the MDedge YouTube channel
The video associated with this article is no longer available on this site. Please view all of our videos on the MDedge YouTube channel
The video associated with this article is no longer available on this site. Please view all of our videos on the MDedge YouTube channel
Quick antibiotic delivery reduces intensive care needs

Photo by Logan Tuttle
Time is of the essence when delivering antibiotics to pediatric cancer patients who present with fever and neutropenia, a new study suggests.
Patients who received antibiotics within 60 minutes of hospital admission were significantly less likely to require intensive care than patients who received antibiotics outside of an hour.
Children who received antibiotics faster also had a lower mortality rate, but the difference between the 2 groups was not statistically significant.
Joanne Hilden, MD, of Children’s Hospital Colorado in Aurora, and her colleagues detailed these results in Pediatric Blood & Cancer.
Dr Hilden noted that administering antibiotics within 60 minutes of a patient’s admission can be difficult, but she and her colleagues were able to adopt policies that sped up the process at their institution.
“We’re talking about kids who have gone home after chemotherapy and then a parent calls the hospital reporting a fever,” Dr Hilden said. “The question is, can we get the patient back to the hospital, then get a white cell count, and get antibiotics on board when needed all within an hour of their arrival?”
“It’s a huge challenge. This study shows that it’s important we make it happen. There’s less intensive care and fewer fatalities for kids who get antibiotics sooner.”
To determine the impact of timely antibiotic administration, Dr Hilden and her colleagues initially analyzed 116 children with hematologic and solid tumor malignancies who developed fever and neutropenia.
But the team found no significant differences in outcomes whether patients received antibiotics within or outside of the 60-minute window.
So the researchers extended the time period of their study and expanded the cohort to 220 patients.
This time, only the need for intensive care unit (ICU)-level care was significantly different between the 2 groups, with 12.6% of patients who received antibiotics within 60 minutes requiring ICU-level care, compared to 29.9% of patients who received antibiotics outside of an hour (P=0.003).
The researchers also found differences between the 2 groups with regard to the mean length of hospital stay (6.9 days vs 5.7 days), the mean duration of fever (3 days vs 2 days), the need for imaging workup (5.2% vs 9.1%), the incidence of bacteremia (13% vs 15.4%), and mortality rate (3.9% vs 0.7%). But none of these differences were statistically significant.
Still, Dr Hilden and her colleagues said it was important to reduce the time to antibiotic delivery at their institution, which took an average of 150 minutes when this study began. By instituting new policies, the team found they could deliver antibiotics in less than 60 minutes nearly 100% of the time.
To do this, hospital staff began prescribing antibiotics upon a pediatric cancer patient’s arrival, holding that order, and then allowing antibiotics to be delivered immediately after learning the results of neutrophil count testing. This eliminated the need to find a prescriber once the patient’s white blood cell count was known.
The researchers also found they could cut the time needed to determine a patient’s neutrophil count. Traditionally, determining neutropenia requires a full white blood cell count, followed by a differential by a human technician. But human verification reverses the machine results in less than 0.5% of cases.
The team discovered that the benefit of speed obtained by eliminating human verification outweighed the risk of administering unneeded antibiotics in very few cases. Depending on preliminary rather than technician-verified results of white cell counts reduced the time of testing from 45 minutes to 20.
The researchers also instituted changes to clinic flow procedures, such as notifying the full care team as soon as a family was advised to come into the hospital.
“Another thing we show is that just increasing the awareness of how important it is to get antibiotics on board quickly in these cases speeds delivery,” Dr Hilden said.
This knowledge and the aforementioned interventions allowed the researchers to reduce the time to antibiotic delivery to a median of 46 minutes.
“Only 11% of pediatric cancer patients with fever and neutropenia have serious complications,” Dr Hilden noted. “That’s low, but we can make it 0%, and this study shows that getting antibiotics onboard quickly goes a long way toward that goal.” ![]()

Photo by Logan Tuttle
Time is of the essence when delivering antibiotics to pediatric cancer patients who present with fever and neutropenia, a new study suggests.
Patients who received antibiotics within 60 minutes of hospital admission were significantly less likely to require intensive care than patients who received antibiotics outside of an hour.
Children who received antibiotics faster also had a lower mortality rate, but the difference between the 2 groups was not statistically significant.
Joanne Hilden, MD, of Children’s Hospital Colorado in Aurora, and her colleagues detailed these results in Pediatric Blood & Cancer.
Dr Hilden noted that administering antibiotics within 60 minutes of a patient’s admission can be difficult, but she and her colleagues were able to adopt policies that sped up the process at their institution.
“We’re talking about kids who have gone home after chemotherapy and then a parent calls the hospital reporting a fever,” Dr Hilden said. “The question is, can we get the patient back to the hospital, then get a white cell count, and get antibiotics on board when needed all within an hour of their arrival?”
“It’s a huge challenge. This study shows that it’s important we make it happen. There’s less intensive care and fewer fatalities for kids who get antibiotics sooner.”
To determine the impact of timely antibiotic administration, Dr Hilden and her colleagues initially analyzed 116 children with hematologic and solid tumor malignancies who developed fever and neutropenia.
But the team found no significant differences in outcomes whether patients received antibiotics within or outside of the 60-minute window.
So the researchers extended the time period of their study and expanded the cohort to 220 patients.
This time, only the need for intensive care unit (ICU)-level care was significantly different between the 2 groups, with 12.6% of patients who received antibiotics within 60 minutes requiring ICU-level care, compared to 29.9% of patients who received antibiotics outside of an hour (P=0.003).
The researchers also found differences between the 2 groups with regard to the mean length of hospital stay (6.9 days vs 5.7 days), the mean duration of fever (3 days vs 2 days), the need for imaging workup (5.2% vs 9.1%), the incidence of bacteremia (13% vs 15.4%), and mortality rate (3.9% vs 0.7%). But none of these differences were statistically significant.
Still, Dr Hilden and her colleagues said it was important to reduce the time to antibiotic delivery at their institution, which took an average of 150 minutes when this study began. By instituting new policies, the team found they could deliver antibiotics in less than 60 minutes nearly 100% of the time.
To do this, hospital staff began prescribing antibiotics upon a pediatric cancer patient’s arrival, holding that order, and then allowing antibiotics to be delivered immediately after learning the results of neutrophil count testing. This eliminated the need to find a prescriber once the patient’s white blood cell count was known.
The researchers also found they could cut the time needed to determine a patient’s neutrophil count. Traditionally, determining neutropenia requires a full white blood cell count, followed by a differential by a human technician. But human verification reverses the machine results in less than 0.5% of cases.
The team discovered that the benefit of speed obtained by eliminating human verification outweighed the risk of administering unneeded antibiotics in very few cases. Depending on preliminary rather than technician-verified results of white cell counts reduced the time of testing from 45 minutes to 20.
The researchers also instituted changes to clinic flow procedures, such as notifying the full care team as soon as a family was advised to come into the hospital.
“Another thing we show is that just increasing the awareness of how important it is to get antibiotics on board quickly in these cases speeds delivery,” Dr Hilden said.
This knowledge and the aforementioned interventions allowed the researchers to reduce the time to antibiotic delivery to a median of 46 minutes.
“Only 11% of pediatric cancer patients with fever and neutropenia have serious complications,” Dr Hilden noted. “That’s low, but we can make it 0%, and this study shows that getting antibiotics onboard quickly goes a long way toward that goal.” ![]()

Photo by Logan Tuttle
Time is of the essence when delivering antibiotics to pediatric cancer patients who present with fever and neutropenia, a new study suggests.
Patients who received antibiotics within 60 minutes of hospital admission were significantly less likely to require intensive care than patients who received antibiotics outside of an hour.
Children who received antibiotics faster also had a lower mortality rate, but the difference between the 2 groups was not statistically significant.
Joanne Hilden, MD, of Children’s Hospital Colorado in Aurora, and her colleagues detailed these results in Pediatric Blood & Cancer.
Dr Hilden noted that administering antibiotics within 60 minutes of a patient’s admission can be difficult, but she and her colleagues were able to adopt policies that sped up the process at their institution.
“We’re talking about kids who have gone home after chemotherapy and then a parent calls the hospital reporting a fever,” Dr Hilden said. “The question is, can we get the patient back to the hospital, then get a white cell count, and get antibiotics on board when needed all within an hour of their arrival?”
“It’s a huge challenge. This study shows that it’s important we make it happen. There’s less intensive care and fewer fatalities for kids who get antibiotics sooner.”
To determine the impact of timely antibiotic administration, Dr Hilden and her colleagues initially analyzed 116 children with hematologic and solid tumor malignancies who developed fever and neutropenia.
But the team found no significant differences in outcomes whether patients received antibiotics within or outside of the 60-minute window.
So the researchers extended the time period of their study and expanded the cohort to 220 patients.
This time, only the need for intensive care unit (ICU)-level care was significantly different between the 2 groups, with 12.6% of patients who received antibiotics within 60 minutes requiring ICU-level care, compared to 29.9% of patients who received antibiotics outside of an hour (P=0.003).
The researchers also found differences between the 2 groups with regard to the mean length of hospital stay (6.9 days vs 5.7 days), the mean duration of fever (3 days vs 2 days), the need for imaging workup (5.2% vs 9.1%), the incidence of bacteremia (13% vs 15.4%), and mortality rate (3.9% vs 0.7%). But none of these differences were statistically significant.
Still, Dr Hilden and her colleagues said it was important to reduce the time to antibiotic delivery at their institution, which took an average of 150 minutes when this study began. By instituting new policies, the team found they could deliver antibiotics in less than 60 minutes nearly 100% of the time.
To do this, hospital staff began prescribing antibiotics upon a pediatric cancer patient’s arrival, holding that order, and then allowing antibiotics to be delivered immediately after learning the results of neutrophil count testing. This eliminated the need to find a prescriber once the patient’s white blood cell count was known.
The researchers also found they could cut the time needed to determine a patient’s neutrophil count. Traditionally, determining neutropenia requires a full white blood cell count, followed by a differential by a human technician. But human verification reverses the machine results in less than 0.5% of cases.
The team discovered that the benefit of speed obtained by eliminating human verification outweighed the risk of administering unneeded antibiotics in very few cases. Depending on preliminary rather than technician-verified results of white cell counts reduced the time of testing from 45 minutes to 20.
The researchers also instituted changes to clinic flow procedures, such as notifying the full care team as soon as a family was advised to come into the hospital.
“Another thing we show is that just increasing the awareness of how important it is to get antibiotics on board quickly in these cases speeds delivery,” Dr Hilden said.
This knowledge and the aforementioned interventions allowed the researchers to reduce the time to antibiotic delivery to a median of 46 minutes.
“Only 11% of pediatric cancer patients with fever and neutropenia have serious complications,” Dr Hilden noted. “That’s low, but we can make it 0%, and this study shows that getting antibiotics onboard quickly goes a long way toward that goal.” ![]()
Managing anticoagulant-related bleeding in the brain
Photo courtesy of NIH
A retrospective study has revealed insights that may help physicians manage patients with anticoagulant-associated intracerebral hemorrhage.
Attaining an international normalized ratio (INR) below 1.3 and systolic blood pressure below 160 mm Hg, both within 4 hours of hospital admission, were associated with lower rates of hematoma enlargement.
And resuming anticoagulant therapy conferred a lower risk of ischemic events without increasing bleeding complications.
Hagen B. Huttner, MD, of the University of Erlangen-Nuremberg in Erlangen, Germany, and his colleagues published these findings in JAMA.
The investigators noted that, among all types of stroke, there is a substantial lack of data about how to manage oral-anticoagulant-related intracranial hemorrhage. Two of the most pressing questions are how to prevent hematoma enlargement and how to manage anticoagulation in the long-term.
There is a consensus that elevated INR levels should be reversed to minimize hematoma enlargement, but the mode of reversal, timing, and extent of INR reversal are unclear. And valid data on the safety and clinical benefit of resuming oral anticoagulant use have not been established.
So Dr Huttner and his colleagues conducted their study to gain some insight. They looked at patients treated at 19 German tertiary care centers from 2006 to 2012, assessing long-term functional outcomes in 1176 patients, hematoma enlargement in 853 patients, and anticoagulant resumption in 719 patients.
Thirty-six percent of patients experienced hematoma enlargement. Patients were less likely to experience enlargement if they had achieved INR levels below 1.3 within 4 hours of hospital admission. Enlargement occurred in 19.8% of these patients, compared to 41.5% of other patients (P<0.001).
Patients were also less likely to have hematoma enlargement if their systolic blood pressure was lower than 160 mm Hg at 4 hours after admission. Enlargement occurred in 33.1% of these patients and 52.4% of other patients (P<0.001).
Patients who had both of these favorable factors had even lower rates of hematoma enlargement than patients with higher INR and blood pressure levels—18.1% and 44.2%, respectively (P<0.001). And having both favorable factors conferred a lower rate of in-hospital mortality as well—13.5% and 20.7%, respectively (P=0.03).
About 24% of patients resumed oral anticoagulant therapy. Those who did had fewer ischemic complications than their peers—5.2% and 15%, respectively (P<0.001). But there was no significant difference between the groups with regard to hemorrhagic complications—8.1% and 6.6%, respectively (P=0.48).
The investigators concluded that, although this study has revealed clinically valuable associations, the results must be replicated in prospective studies. ![]()

Photo courtesy of NIH
A retrospective study has revealed insights that may help physicians manage patients with anticoagulant-associated intracerebral hemorrhage.
Attaining an international normalized ratio (INR) below 1.3 and systolic blood pressure below 160 mm Hg, both within 4 hours of hospital admission, were associated with lower rates of hematoma enlargement.
And resuming anticoagulant therapy conferred a lower risk of ischemic events without increasing bleeding complications.
Hagen B. Huttner, MD, of the University of Erlangen-Nuremberg in Erlangen, Germany, and his colleagues published these findings in JAMA.
The investigators noted that, among all types of stroke, there is a substantial lack of data about how to manage oral-anticoagulant-related intracranial hemorrhage. Two of the most pressing questions are how to prevent hematoma enlargement and how to manage anticoagulation in the long-term.
There is a consensus that elevated INR levels should be reversed to minimize hematoma enlargement, but the mode of reversal, timing, and extent of INR reversal are unclear. And valid data on the safety and clinical benefit of resuming oral anticoagulant use have not been established.
So Dr Huttner and his colleagues conducted their study to gain some insight. They looked at patients treated at 19 German tertiary care centers from 2006 to 2012, assessing long-term functional outcomes in 1176 patients, hematoma enlargement in 853 patients, and anticoagulant resumption in 719 patients.
Thirty-six percent of patients experienced hematoma enlargement. Patients were less likely to experience enlargement if they had achieved INR levels below 1.3 within 4 hours of hospital admission. Enlargement occurred in 19.8% of these patients, compared to 41.5% of other patients (P<0.001).
Patients were also less likely to have hematoma enlargement if their systolic blood pressure was lower than 160 mm Hg at 4 hours after admission. Enlargement occurred in 33.1% of these patients and 52.4% of other patients (P<0.001).
Patients who had both of these favorable factors had even lower rates of hematoma enlargement than patients with higher INR and blood pressure levels—18.1% and 44.2%, respectively (P<0.001). And having both favorable factors conferred a lower rate of in-hospital mortality as well—13.5% and 20.7%, respectively (P=0.03).
About 24% of patients resumed oral anticoagulant therapy. Those who did had fewer ischemic complications than their peers—5.2% and 15%, respectively (P<0.001). But there was no significant difference between the groups with regard to hemorrhagic complications—8.1% and 6.6%, respectively (P=0.48).
The investigators concluded that, although this study has revealed clinically valuable associations, the results must be replicated in prospective studies. ![]()

Photo courtesy of NIH
A retrospective study has revealed insights that may help physicians manage patients with anticoagulant-associated intracerebral hemorrhage.
Attaining an international normalized ratio (INR) below 1.3 and systolic blood pressure below 160 mm Hg, both within 4 hours of hospital admission, were associated with lower rates of hematoma enlargement.
And resuming anticoagulant therapy conferred a lower risk of ischemic events without increasing bleeding complications.
Hagen B. Huttner, MD, of the University of Erlangen-Nuremberg in Erlangen, Germany, and his colleagues published these findings in JAMA.
The investigators noted that, among all types of stroke, there is a substantial lack of data about how to manage oral-anticoagulant-related intracranial hemorrhage. Two of the most pressing questions are how to prevent hematoma enlargement and how to manage anticoagulation in the long-term.
There is a consensus that elevated INR levels should be reversed to minimize hematoma enlargement, but the mode of reversal, timing, and extent of INR reversal are unclear. And valid data on the safety and clinical benefit of resuming oral anticoagulant use have not been established.
So Dr Huttner and his colleagues conducted their study to gain some insight. They looked at patients treated at 19 German tertiary care centers from 2006 to 2012, assessing long-term functional outcomes in 1176 patients, hematoma enlargement in 853 patients, and anticoagulant resumption in 719 patients.
Thirty-six percent of patients experienced hematoma enlargement. Patients were less likely to experience enlargement if they had achieved INR levels below 1.3 within 4 hours of hospital admission. Enlargement occurred in 19.8% of these patients, compared to 41.5% of other patients (P<0.001).
Patients were also less likely to have hematoma enlargement if their systolic blood pressure was lower than 160 mm Hg at 4 hours after admission. Enlargement occurred in 33.1% of these patients and 52.4% of other patients (P<0.001).
Patients who had both of these favorable factors had even lower rates of hematoma enlargement than patients with higher INR and blood pressure levels—18.1% and 44.2%, respectively (P<0.001). And having both favorable factors conferred a lower rate of in-hospital mortality as well—13.5% and 20.7%, respectively (P=0.03).
About 24% of patients resumed oral anticoagulant therapy. Those who did had fewer ischemic complications than their peers—5.2% and 15%, respectively (P<0.001). But there was no significant difference between the groups with regard to hemorrhagic complications—8.1% and 6.6%, respectively (P=0.48).
The investigators concluded that, although this study has revealed clinically valuable associations, the results must be replicated in prospective studies. ![]()
Team uses 3D printing to create drug carrier

Photo by Aaron Logan
Researchers have used a 3D printer to create a carrier that allows for local and sustained delivery of the immunosuppressive drug cyclosporine A (CsA) after cell transplantation.
The carrier is a combination of microspheres and hydrogel. In murine experiments, it delivered a local, sustained load of CsA in an amount that eliminated the need for additional drugs to treat immune rejection.
The researchers described these results in Cell Transplantation.
“Our objective was to show the feasibility of using a subcutaneous, 3D-printed drug delivery system to achieve local and sustained CsA release and to investigate the local immunosuppressive effects of the CsA after cell transplantation,” said study author Dong-Woo Cho, PhD, of the Pohang University of Science and Technology in Korea.
“The improved load-bearing capacity of the combined microsphere and hydrogel system, and its ability to maintain its integrity and shape during the implantation period, helped to deliver a sustained CsA release, preventing the acceleration of the secretion of cytokines related to immune rejection.”
The researchers noted that CsA improves the success rate of transplants, but systemic administration requires high doses that can have severe side effects. The benefit of a carrier is that it provides local drug delivery.
Other research groups have attempted CsA delivery via either microspheres or hydrogels, but most encountered serious problems, such as embolisms or organ damage due to migration of the microspheres from the injection site.
In addition, weak mechanical properties in some delivery systems caused premature dissolution and placed limitations on drug load quantity.
However, Dr Cho’s group said their carrier’s improved structure and load-bearing capacity allowed for sustained release of CsA at the desired site.
Their carrier is a hybrid of a CsA-poly (lactic-co-glycolic) acid microsphere-loaded hydrogel and a polymeric framework, which ensures the carrier can endure external force under physiological conditions.
In in vitro experiments with the carrier, the researchers observed decreased expression of cytokines, which are secreted by spleen cells activated by Concanavalin A and are related to immune rejection.
The team also implanted in mice drug carriers seeded with xenogeneic cells, and they observed significant suppression of T-cell-mediated rejection for 4 weeks.
The researchers believe this study could help overcome existing cell transplantation limitations caused by systemic immunosuppression. They said their carrier could be a promising solution for treating a range of diseases that require cell-based therapy. ![]()

Photo by Aaron Logan
Researchers have used a 3D printer to create a carrier that allows for local and sustained delivery of the immunosuppressive drug cyclosporine A (CsA) after cell transplantation.
The carrier is a combination of microspheres and hydrogel. In murine experiments, it delivered a local, sustained load of CsA in an amount that eliminated the need for additional drugs to treat immune rejection.
The researchers described these results in Cell Transplantation.
“Our objective was to show the feasibility of using a subcutaneous, 3D-printed drug delivery system to achieve local and sustained CsA release and to investigate the local immunosuppressive effects of the CsA after cell transplantation,” said study author Dong-Woo Cho, PhD, of the Pohang University of Science and Technology in Korea.
“The improved load-bearing capacity of the combined microsphere and hydrogel system, and its ability to maintain its integrity and shape during the implantation period, helped to deliver a sustained CsA release, preventing the acceleration of the secretion of cytokines related to immune rejection.”
The researchers noted that CsA improves the success rate of transplants, but systemic administration requires high doses that can have severe side effects. The benefit of a carrier is that it provides local drug delivery.
Other research groups have attempted CsA delivery via either microspheres or hydrogels, but most encountered serious problems, such as embolisms or organ damage due to migration of the microspheres from the injection site.
In addition, weak mechanical properties in some delivery systems caused premature dissolution and placed limitations on drug load quantity.
However, Dr Cho’s group said their carrier’s improved structure and load-bearing capacity allowed for sustained release of CsA at the desired site.
Their carrier is a hybrid of a CsA-poly (lactic-co-glycolic) acid microsphere-loaded hydrogel and a polymeric framework, which ensures the carrier can endure external force under physiological conditions.
In in vitro experiments with the carrier, the researchers observed decreased expression of cytokines, which are secreted by spleen cells activated by Concanavalin A and are related to immune rejection.
The team also implanted in mice drug carriers seeded with xenogeneic cells, and they observed significant suppression of T-cell-mediated rejection for 4 weeks.
The researchers believe this study could help overcome existing cell transplantation limitations caused by systemic immunosuppression. They said their carrier could be a promising solution for treating a range of diseases that require cell-based therapy. ![]()

Photo by Aaron Logan
Researchers have used a 3D printer to create a carrier that allows for local and sustained delivery of the immunosuppressive drug cyclosporine A (CsA) after cell transplantation.
The carrier is a combination of microspheres and hydrogel. In murine experiments, it delivered a local, sustained load of CsA in an amount that eliminated the need for additional drugs to treat immune rejection.
The researchers described these results in Cell Transplantation.
“Our objective was to show the feasibility of using a subcutaneous, 3D-printed drug delivery system to achieve local and sustained CsA release and to investigate the local immunosuppressive effects of the CsA after cell transplantation,” said study author Dong-Woo Cho, PhD, of the Pohang University of Science and Technology in Korea.
“The improved load-bearing capacity of the combined microsphere and hydrogel system, and its ability to maintain its integrity and shape during the implantation period, helped to deliver a sustained CsA release, preventing the acceleration of the secretion of cytokines related to immune rejection.”
The researchers noted that CsA improves the success rate of transplants, but systemic administration requires high doses that can have severe side effects. The benefit of a carrier is that it provides local drug delivery.
Other research groups have attempted CsA delivery via either microspheres or hydrogels, but most encountered serious problems, such as embolisms or organ damage due to migration of the microspheres from the injection site.
In addition, weak mechanical properties in some delivery systems caused premature dissolution and placed limitations on drug load quantity.
However, Dr Cho’s group said their carrier’s improved structure and load-bearing capacity allowed for sustained release of CsA at the desired site.
Their carrier is a hybrid of a CsA-poly (lactic-co-glycolic) acid microsphere-loaded hydrogel and a polymeric framework, which ensures the carrier can endure external force under physiological conditions.
In in vitro experiments with the carrier, the researchers observed decreased expression of cytokines, which are secreted by spleen cells activated by Concanavalin A and are related to immune rejection.
The team also implanted in mice drug carriers seeded with xenogeneic cells, and they observed significant suppression of T-cell-mediated rejection for 4 weeks.
The researchers believe this study could help overcome existing cell transplantation limitations caused by systemic immunosuppression. They said their carrier could be a promising solution for treating a range of diseases that require cell-based therapy. ![]()
Skiing accident claims life of leukemia expert

Photo courtesy of RPCI
Meir Wetzler, MD, Chief of the Leukemia Section at the Roswell Park Cancer Institute (RPCI) in Buffalo, New York, has died at the age of 60.
Dr Wetzler passed away on February 23, in a Denver, Colorado, hospital a little more than 2 weeks after a skiing accident.
He was nationally prominent in his field and served on the Chronic Myelogenous Leukemia (CML) Treatment Committee of the National Comprehensive Cancer Network, helping set the standard of care for CML patients.
Originally from Israel, Dr Wetzler earned his medical degree at Hebrew University’s Hadassah Medical School in Jerusalem and did his residency in internal medicine at Kaplan Hospital in Rehovot before coming to the US.
From 1988 to 1992, he served 2 fellowships—in clinical immunology/biologic therapy and medical oncology—at MD Anderson Cancer Center in Houston, Texas. He joined the Leukemia Division of RPCI in 1994.
Dr Wetzler’s colleagues said he worked tirelessly with cooperative groups and pharmaceutical companies to attract new trials to RPCI for the benefit of his patients.
“He gave a piece of himself in everything he did, from his research to his care for patients to his interactions with his team of colleagues,” said Kara Eaton-Weaver, RPCI’s Executive Director of the Patient and Family Experience. “Meir was a transformational leader who built a culture of empathy, compassion, integrity, and innovation.”
“He was like a father,” said Linda Lutgen-Dunckley, a pathology resource technician at RPCI. “Everybody was part of a team, and nobody was less important than he was. He felt everybody played their part on the team.”
Dr Wetzler is survived by his wife, Chana, and their 4 children: Mor, Shira, Adam, and Modi.
The Dr Meir Wetzler Memorial Fund for Leukemia Research has been established to benefit leukemia research. A portion of the donations will be used to plant a tree in his memory in RPCI’s Kaminski Park & Gardens. To donate directly, visit giving.roswellpark.org/wetzler.
To send a personal message to Dr Wetzler’s family, direct it to Jamie Genovese at Roswell Park Cancer Institute, Department of Medicine, Elm and Carlton Streets, Buffalo, NY 14263. Messages can also be dropped off at RPCI’s Leukemia Center. ![]()

Photo courtesy of RPCI
Meir Wetzler, MD, Chief of the Leukemia Section at the Roswell Park Cancer Institute (RPCI) in Buffalo, New York, has died at the age of 60.
Dr Wetzler passed away on February 23, in a Denver, Colorado, hospital a little more than 2 weeks after a skiing accident.
He was nationally prominent in his field and served on the Chronic Myelogenous Leukemia (CML) Treatment Committee of the National Comprehensive Cancer Network, helping set the standard of care for CML patients.
Originally from Israel, Dr Wetzler earned his medical degree at Hebrew University’s Hadassah Medical School in Jerusalem and did his residency in internal medicine at Kaplan Hospital in Rehovot before coming to the US.
From 1988 to 1992, he served 2 fellowships—in clinical immunology/biologic therapy and medical oncology—at MD Anderson Cancer Center in Houston, Texas. He joined the Leukemia Division of RPCI in 1994.
Dr Wetzler’s colleagues said he worked tirelessly with cooperative groups and pharmaceutical companies to attract new trials to RPCI for the benefit of his patients.
“He gave a piece of himself in everything he did, from his research to his care for patients to his interactions with his team of colleagues,” said Kara Eaton-Weaver, RPCI’s Executive Director of the Patient and Family Experience. “Meir was a transformational leader who built a culture of empathy, compassion, integrity, and innovation.”
“He was like a father,” said Linda Lutgen-Dunckley, a pathology resource technician at RPCI. “Everybody was part of a team, and nobody was less important than he was. He felt everybody played their part on the team.”
Dr Wetzler is survived by his wife, Chana, and their 4 children: Mor, Shira, Adam, and Modi.
The Dr Meir Wetzler Memorial Fund for Leukemia Research has been established to benefit leukemia research. A portion of the donations will be used to plant a tree in his memory in RPCI’s Kaminski Park & Gardens. To donate directly, visit giving.roswellpark.org/wetzler.
To send a personal message to Dr Wetzler’s family, direct it to Jamie Genovese at Roswell Park Cancer Institute, Department of Medicine, Elm and Carlton Streets, Buffalo, NY 14263. Messages can also be dropped off at RPCI’s Leukemia Center. ![]()

Photo courtesy of RPCI
Meir Wetzler, MD, Chief of the Leukemia Section at the Roswell Park Cancer Institute (RPCI) in Buffalo, New York, has died at the age of 60.
Dr Wetzler passed away on February 23, in a Denver, Colorado, hospital a little more than 2 weeks after a skiing accident.
He was nationally prominent in his field and served on the Chronic Myelogenous Leukemia (CML) Treatment Committee of the National Comprehensive Cancer Network, helping set the standard of care for CML patients.
Originally from Israel, Dr Wetzler earned his medical degree at Hebrew University’s Hadassah Medical School in Jerusalem and did his residency in internal medicine at Kaplan Hospital in Rehovot before coming to the US.
From 1988 to 1992, he served 2 fellowships—in clinical immunology/biologic therapy and medical oncology—at MD Anderson Cancer Center in Houston, Texas. He joined the Leukemia Division of RPCI in 1994.
Dr Wetzler’s colleagues said he worked tirelessly with cooperative groups and pharmaceutical companies to attract new trials to RPCI for the benefit of his patients.
“He gave a piece of himself in everything he did, from his research to his care for patients to his interactions with his team of colleagues,” said Kara Eaton-Weaver, RPCI’s Executive Director of the Patient and Family Experience. “Meir was a transformational leader who built a culture of empathy, compassion, integrity, and innovation.”
“He was like a father,” said Linda Lutgen-Dunckley, a pathology resource technician at RPCI. “Everybody was part of a team, and nobody was less important than he was. He felt everybody played their part on the team.”
Dr Wetzler is survived by his wife, Chana, and their 4 children: Mor, Shira, Adam, and Modi.
The Dr Meir Wetzler Memorial Fund for Leukemia Research has been established to benefit leukemia research. A portion of the donations will be used to plant a tree in his memory in RPCI’s Kaminski Park & Gardens. To donate directly, visit giving.roswellpark.org/wetzler.
To send a personal message to Dr Wetzler’s family, direct it to Jamie Genovese at Roswell Park Cancer Institute, Department of Medicine, Elm and Carlton Streets, Buffalo, NY 14263. Messages can also be dropped off at RPCI’s Leukemia Center. ![]()
Ob.gyns. can help end the HIV epidemic
Despite staggering scientific and medical advances, the HIV epidemic in the United States has not changed significantly over the past decade. The estimated incidence of HIV infection has remained stable overall, with between 45,000 and 55,000 new HIV infections diagnosed per year.
This is disheartening because, even without a vaccine, I believe we have the tools today to drive the epidemic down to zero. First of all, we know how to effectively diagnose and treat the infection, and we have evidence that antiretroviral treatment is an effective prevention tool. Secondly, advances in chemoprophylaxis have made pre-exposure prophylaxis a reality.
Ob.gyns. played a central role in one of the greatest successes of the use of antiretroviral drugs: the virtual elimination of mother-to-child transmission of HIV in the United States. Now, by fully utilizing the tools available today, ob.gyns. can play a critical role in ending the epidemic in the United States and beyond.
Tools for diagnosis and treatment
We have so many missed opportunities in fighting the HIV epidemic.
This is evident in data compiled for a model called the “HIV Care Continuum,” or HIV “Cascade of Care.” The model captures the sequential stages of HIV care from diagnosis to suppression of the virus. It was developed in 2011 by Dr. Edward Gardner, an infectious disease/HIV expert at Denver Public Health, and has since been used at the federal, state, and local levels to help identify gaps in HIV services.
Not too long ago, diagnosis was the biggest problem in reducing the public health burden of HIV. Today, the biggest problem is linking and keeping individuals in care. According to the latest analysis by the U.S. Centers for Disease Control and Prevention of the HIV Care Continuum, of the 1.2 million people estimated to be living with HIV in America in 2011, approximately 86% were diagnosed, but only 40% were linked to and stayed in care, 37% were prescribed antiretroviral therapy (ART), and 30% had achieved viral suppression.
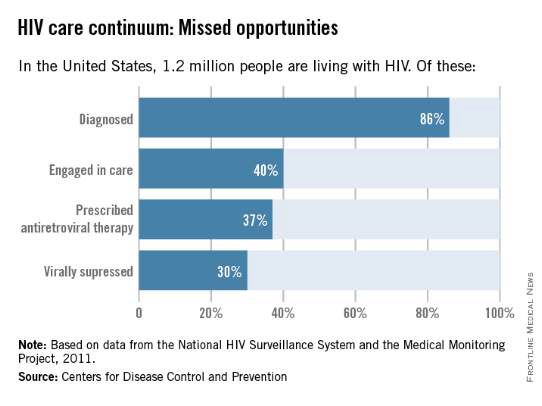
Only 30% of Americans living with HIV infection today are effectively treated, according to these data, even though we have the drugs and drug regimens available to treat everyone effectively.
Other analyses have included an additional stage of being initially linked to care (rather than being linked to care and retained in care). This presentation of the cascade, or continuum, further illuminates the progressive drop-off and that shows why an effective, sustained linkage to care is a critical component to ending the HIV epidemic.
One of these studies – an analysis published in 2013 – showed that approximately 82% of people were diagnosed, 66% were linked to care, 37% were retained in care, 33% were prescribed antiretroviral therapy, and 25% had a suppressed viral load of 200 copies/mL or less (JAMA. Intern. Med. 2013;173:1337-44).
With regard to women specifically, the CDC estimates that one in four people living with HIV infection are women, and that only about half of the women who are diagnosed with the infection are staying in care. Even fewer – 4 in 10 – have viral suppression, according to the CDC.
Expanding the management of HIV in the primary care setting could move us closer to ensuring that everyone in the United States who is infected with HIV is aware of the infection, is committed to treatment, and is virologically suppressed.
Like other primary care physicians, ob.gyns often have some degree of long-term continuity with patients – or the ability to create such continuity – that can be helpful for ensuring treatment compliance.
Ob.gyns also have valuable contact with adolescents, who fare worse throughout the cascade and are significantly more likely than older individuals to have unknown infections. An analysis published in 2014 of data for youth ages 13-29 shows that only 40% of HIV-infected youth were aware of their diagnosis and that an estimated 6% or less of HIV-infected youth were virally suppressed (AIDS. Patient. Care. STDS. 2014;28:128-135).
HIV testing should occur much more frequently than a decade ago, given the move in 2006 by the CDC from targeted risk-based testing to routine opt-out testing for all patients aged 13-64.
Treatment, moreover, has become much simpler in many respects. We have available to us more than 30 different drugs for individualizing therapy and providing treatment that allows patients to live a natural lifetime.
While such a large array of options may require those ob.gyns. who see only a few HIV-infected patients a year to work in consultation with an expert, many of the regimens require only a single, once-a-day pill. And while there was much debate as recently as five years ago about when to start treatment, there now is consensus that treatment should be started immediately after diagnosis (even in pregnant women), rather than waiting for the immune system to show signs of decline.
In fact, there is growing evidence that early treatment is key for both the infected individual and for individuals at risk. In the HIV Prevention Trials Network 052 study of discordant couples, for instance, early antiretroviral therapy in an infected partner not only reduced the number of clinical events; it almost completely blocked sexual transmission of the virus to an HIV-negative partner (N. Engl. J. Med. 2011;365:493-505).
The 052 study was a landmark “treatment as prevention” study. Other research has similarly shown that when the viral load of HIV-infected individuals is significantly reduced, their infectivity is reduced. And on a larger scale, research has shown that when we do this on a population basis, achieving widespread and continual treatment success, we can significantly impact the epidemic. This has been the case with the population of intravenous drug users in Vancouver, where the community viral load was significantly reduced by successful treatment that prevented new infections in this once-high-risk population.
Emerging data suggests that early diagnosis and treatment will likely also impact the likelihood of infected individuals achieving “functional cure.” The issue of functional cure – of achieving viral loads that are so low that drug therapy is no longer needed – has been receiving increasing attention in recent years, with the most promising findings reported thus far involving early treatment.
Tools for preexposure prophylaxis
For many years, we fit HIV care neatly into either the treatment or prevention category. More recently, we have come to appreciate that treatment is prevention, that a comprehensive prevention strategy must include treatment of infected individuals.
On the purely prevention side, it is important to continue educating women about safe sex behaviors. Most new HIV infections in women (84%) result from heterosexual contact, according to the CDC. For those who remain at risk of acquiring HIV despite education and counseling (eg., individuals who continue to engage in high-risk behaviors, or who have an HIV-positive partner), pre-exposure prophylaxis (PrEP) is now a safe and effective tool for preventing transmission. Patients deemed to be at high risk of acquiring HIV need to be made aware of this option.
PrEP originally was recommended only for gay or bisexual men, but in May 2014, the CDC recommended it for all individuals at risk and released the first comprehensive clinical practice guidelines for the prevention tool (www.cdc.gov/hiv/pdf/guidelines/PrEPguidelines2014.pdf).
The PrEP medication, Truvada, is a combination of two drugs (tenovovir and emtricitabine) that, when taken daily on a consistent basis, significantly reduces the risk of getting HIV infection. Several large national and international studies have documented risk reductions of 73% to 92% when the medication was taken every day or almost every day. It is clearly within the purview of any ob.gyn to prescribe, monitor, and manage such prevention therapy.
The availability and relative ease of such a tool, along with advances in treatment and knowledge gained from the HIV Care Continuum, should re-energize ob.gyns. to up the ante in efforts to end the epidemic.
Experience in our clinical program that provides care and treatment to patients in the Baltimore-Washington area has taught us that we do much better when we integrate HIV care within primary care. It’s much more likely that patients will “stay close” with their ob.gyn than to another specialist.
Certainly, HIV infection has its “hot spots” and areas of much lower prevalence, but regardless of where we reside, we must continue to appreciate that the epidemic has had a significant impact on women and that this will persist unless we can all better utilize our available tools, such as early diagnosis and effective treatment that are linked long-term with other primary care physicians.
For women, ob.gyns represent a great resource for our nation to make progress toward President Obama’s National HIV Strategy.
Dr. Redfield reported that he has no disclosures relevant to this Master Class.
Despite staggering scientific and medical advances, the HIV epidemic in the United States has not changed significantly over the past decade. The estimated incidence of HIV infection has remained stable overall, with between 45,000 and 55,000 new HIV infections diagnosed per year.
This is disheartening because, even without a vaccine, I believe we have the tools today to drive the epidemic down to zero. First of all, we know how to effectively diagnose and treat the infection, and we have evidence that antiretroviral treatment is an effective prevention tool. Secondly, advances in chemoprophylaxis have made pre-exposure prophylaxis a reality.
Ob.gyns. played a central role in one of the greatest successes of the use of antiretroviral drugs: the virtual elimination of mother-to-child transmission of HIV in the United States. Now, by fully utilizing the tools available today, ob.gyns. can play a critical role in ending the epidemic in the United States and beyond.
Tools for diagnosis and treatment
We have so many missed opportunities in fighting the HIV epidemic.
This is evident in data compiled for a model called the “HIV Care Continuum,” or HIV “Cascade of Care.” The model captures the sequential stages of HIV care from diagnosis to suppression of the virus. It was developed in 2011 by Dr. Edward Gardner, an infectious disease/HIV expert at Denver Public Health, and has since been used at the federal, state, and local levels to help identify gaps in HIV services.
Not too long ago, diagnosis was the biggest problem in reducing the public health burden of HIV. Today, the biggest problem is linking and keeping individuals in care. According to the latest analysis by the U.S. Centers for Disease Control and Prevention of the HIV Care Continuum, of the 1.2 million people estimated to be living with HIV in America in 2011, approximately 86% were diagnosed, but only 40% were linked to and stayed in care, 37% were prescribed antiretroviral therapy (ART), and 30% had achieved viral suppression.

Only 30% of Americans living with HIV infection today are effectively treated, according to these data, even though we have the drugs and drug regimens available to treat everyone effectively.
Other analyses have included an additional stage of being initially linked to care (rather than being linked to care and retained in care). This presentation of the cascade, or continuum, further illuminates the progressive drop-off and that shows why an effective, sustained linkage to care is a critical component to ending the HIV epidemic.
One of these studies – an analysis published in 2013 – showed that approximately 82% of people were diagnosed, 66% were linked to care, 37% were retained in care, 33% were prescribed antiretroviral therapy, and 25% had a suppressed viral load of 200 copies/mL or less (JAMA. Intern. Med. 2013;173:1337-44).
With regard to women specifically, the CDC estimates that one in four people living with HIV infection are women, and that only about half of the women who are diagnosed with the infection are staying in care. Even fewer – 4 in 10 – have viral suppression, according to the CDC.
Expanding the management of HIV in the primary care setting could move us closer to ensuring that everyone in the United States who is infected with HIV is aware of the infection, is committed to treatment, and is virologically suppressed.
Like other primary care physicians, ob.gyns often have some degree of long-term continuity with patients – or the ability to create such continuity – that can be helpful for ensuring treatment compliance.
Ob.gyns also have valuable contact with adolescents, who fare worse throughout the cascade and are significantly more likely than older individuals to have unknown infections. An analysis published in 2014 of data for youth ages 13-29 shows that only 40% of HIV-infected youth were aware of their diagnosis and that an estimated 6% or less of HIV-infected youth were virally suppressed (AIDS. Patient. Care. STDS. 2014;28:128-135).
HIV testing should occur much more frequently than a decade ago, given the move in 2006 by the CDC from targeted risk-based testing to routine opt-out testing for all patients aged 13-64.
Treatment, moreover, has become much simpler in many respects. We have available to us more than 30 different drugs for individualizing therapy and providing treatment that allows patients to live a natural lifetime.
While such a large array of options may require those ob.gyns. who see only a few HIV-infected patients a year to work in consultation with an expert, many of the regimens require only a single, once-a-day pill. And while there was much debate as recently as five years ago about when to start treatment, there now is consensus that treatment should be started immediately after diagnosis (even in pregnant women), rather than waiting for the immune system to show signs of decline.
In fact, there is growing evidence that early treatment is key for both the infected individual and for individuals at risk. In the HIV Prevention Trials Network 052 study of discordant couples, for instance, early antiretroviral therapy in an infected partner not only reduced the number of clinical events; it almost completely blocked sexual transmission of the virus to an HIV-negative partner (N. Engl. J. Med. 2011;365:493-505).
The 052 study was a landmark “treatment as prevention” study. Other research has similarly shown that when the viral load of HIV-infected individuals is significantly reduced, their infectivity is reduced. And on a larger scale, research has shown that when we do this on a population basis, achieving widespread and continual treatment success, we can significantly impact the epidemic. This has been the case with the population of intravenous drug users in Vancouver, where the community viral load was significantly reduced by successful treatment that prevented new infections in this once-high-risk population.
Emerging data suggests that early diagnosis and treatment will likely also impact the likelihood of infected individuals achieving “functional cure.” The issue of functional cure – of achieving viral loads that are so low that drug therapy is no longer needed – has been receiving increasing attention in recent years, with the most promising findings reported thus far involving early treatment.
Tools for preexposure prophylaxis
For many years, we fit HIV care neatly into either the treatment or prevention category. More recently, we have come to appreciate that treatment is prevention, that a comprehensive prevention strategy must include treatment of infected individuals.
On the purely prevention side, it is important to continue educating women about safe sex behaviors. Most new HIV infections in women (84%) result from heterosexual contact, according to the CDC. For those who remain at risk of acquiring HIV despite education and counseling (eg., individuals who continue to engage in high-risk behaviors, or who have an HIV-positive partner), pre-exposure prophylaxis (PrEP) is now a safe and effective tool for preventing transmission. Patients deemed to be at high risk of acquiring HIV need to be made aware of this option.
PrEP originally was recommended only for gay or bisexual men, but in May 2014, the CDC recommended it for all individuals at risk and released the first comprehensive clinical practice guidelines for the prevention tool (www.cdc.gov/hiv/pdf/guidelines/PrEPguidelines2014.pdf).
The PrEP medication, Truvada, is a combination of two drugs (tenovovir and emtricitabine) that, when taken daily on a consistent basis, significantly reduces the risk of getting HIV infection. Several large national and international studies have documented risk reductions of 73% to 92% when the medication was taken every day or almost every day. It is clearly within the purview of any ob.gyn to prescribe, monitor, and manage such prevention therapy.
The availability and relative ease of such a tool, along with advances in treatment and knowledge gained from the HIV Care Continuum, should re-energize ob.gyns. to up the ante in efforts to end the epidemic.
Experience in our clinical program that provides care and treatment to patients in the Baltimore-Washington area has taught us that we do much better when we integrate HIV care within primary care. It’s much more likely that patients will “stay close” with their ob.gyn than to another specialist.
Certainly, HIV infection has its “hot spots” and areas of much lower prevalence, but regardless of where we reside, we must continue to appreciate that the epidemic has had a significant impact on women and that this will persist unless we can all better utilize our available tools, such as early diagnosis and effective treatment that are linked long-term with other primary care physicians.
For women, ob.gyns represent a great resource for our nation to make progress toward President Obama’s National HIV Strategy.
Dr. Redfield reported that he has no disclosures relevant to this Master Class.
Despite staggering scientific and medical advances, the HIV epidemic in the United States has not changed significantly over the past decade. The estimated incidence of HIV infection has remained stable overall, with between 45,000 and 55,000 new HIV infections diagnosed per year.
This is disheartening because, even without a vaccine, I believe we have the tools today to drive the epidemic down to zero. First of all, we know how to effectively diagnose and treat the infection, and we have evidence that antiretroviral treatment is an effective prevention tool. Secondly, advances in chemoprophylaxis have made pre-exposure prophylaxis a reality.
Ob.gyns. played a central role in one of the greatest successes of the use of antiretroviral drugs: the virtual elimination of mother-to-child transmission of HIV in the United States. Now, by fully utilizing the tools available today, ob.gyns. can play a critical role in ending the epidemic in the United States and beyond.
Tools for diagnosis and treatment
We have so many missed opportunities in fighting the HIV epidemic.
This is evident in data compiled for a model called the “HIV Care Continuum,” or HIV “Cascade of Care.” The model captures the sequential stages of HIV care from diagnosis to suppression of the virus. It was developed in 2011 by Dr. Edward Gardner, an infectious disease/HIV expert at Denver Public Health, and has since been used at the federal, state, and local levels to help identify gaps in HIV services.
Not too long ago, diagnosis was the biggest problem in reducing the public health burden of HIV. Today, the biggest problem is linking and keeping individuals in care. According to the latest analysis by the U.S. Centers for Disease Control and Prevention of the HIV Care Continuum, of the 1.2 million people estimated to be living with HIV in America in 2011, approximately 86% were diagnosed, but only 40% were linked to and stayed in care, 37% were prescribed antiretroviral therapy (ART), and 30% had achieved viral suppression.

Only 30% of Americans living with HIV infection today are effectively treated, according to these data, even though we have the drugs and drug regimens available to treat everyone effectively.
Other analyses have included an additional stage of being initially linked to care (rather than being linked to care and retained in care). This presentation of the cascade, or continuum, further illuminates the progressive drop-off and that shows why an effective, sustained linkage to care is a critical component to ending the HIV epidemic.
One of these studies – an analysis published in 2013 – showed that approximately 82% of people were diagnosed, 66% were linked to care, 37% were retained in care, 33% were prescribed antiretroviral therapy, and 25% had a suppressed viral load of 200 copies/mL or less (JAMA. Intern. Med. 2013;173:1337-44).
With regard to women specifically, the CDC estimates that one in four people living with HIV infection are women, and that only about half of the women who are diagnosed with the infection are staying in care. Even fewer – 4 in 10 – have viral suppression, according to the CDC.
Expanding the management of HIV in the primary care setting could move us closer to ensuring that everyone in the United States who is infected with HIV is aware of the infection, is committed to treatment, and is virologically suppressed.
Like other primary care physicians, ob.gyns often have some degree of long-term continuity with patients – or the ability to create such continuity – that can be helpful for ensuring treatment compliance.
Ob.gyns also have valuable contact with adolescents, who fare worse throughout the cascade and are significantly more likely than older individuals to have unknown infections. An analysis published in 2014 of data for youth ages 13-29 shows that only 40% of HIV-infected youth were aware of their diagnosis and that an estimated 6% or less of HIV-infected youth were virally suppressed (AIDS. Patient. Care. STDS. 2014;28:128-135).
HIV testing should occur much more frequently than a decade ago, given the move in 2006 by the CDC from targeted risk-based testing to routine opt-out testing for all patients aged 13-64.
Treatment, moreover, has become much simpler in many respects. We have available to us more than 30 different drugs for individualizing therapy and providing treatment that allows patients to live a natural lifetime.
While such a large array of options may require those ob.gyns. who see only a few HIV-infected patients a year to work in consultation with an expert, many of the regimens require only a single, once-a-day pill. And while there was much debate as recently as five years ago about when to start treatment, there now is consensus that treatment should be started immediately after diagnosis (even in pregnant women), rather than waiting for the immune system to show signs of decline.
In fact, there is growing evidence that early treatment is key for both the infected individual and for individuals at risk. In the HIV Prevention Trials Network 052 study of discordant couples, for instance, early antiretroviral therapy in an infected partner not only reduced the number of clinical events; it almost completely blocked sexual transmission of the virus to an HIV-negative partner (N. Engl. J. Med. 2011;365:493-505).
The 052 study was a landmark “treatment as prevention” study. Other research has similarly shown that when the viral load of HIV-infected individuals is significantly reduced, their infectivity is reduced. And on a larger scale, research has shown that when we do this on a population basis, achieving widespread and continual treatment success, we can significantly impact the epidemic. This has been the case with the population of intravenous drug users in Vancouver, where the community viral load was significantly reduced by successful treatment that prevented new infections in this once-high-risk population.
Emerging data suggests that early diagnosis and treatment will likely also impact the likelihood of infected individuals achieving “functional cure.” The issue of functional cure – of achieving viral loads that are so low that drug therapy is no longer needed – has been receiving increasing attention in recent years, with the most promising findings reported thus far involving early treatment.
Tools for preexposure prophylaxis
For many years, we fit HIV care neatly into either the treatment or prevention category. More recently, we have come to appreciate that treatment is prevention, that a comprehensive prevention strategy must include treatment of infected individuals.
On the purely prevention side, it is important to continue educating women about safe sex behaviors. Most new HIV infections in women (84%) result from heterosexual contact, according to the CDC. For those who remain at risk of acquiring HIV despite education and counseling (eg., individuals who continue to engage in high-risk behaviors, or who have an HIV-positive partner), pre-exposure prophylaxis (PrEP) is now a safe and effective tool for preventing transmission. Patients deemed to be at high risk of acquiring HIV need to be made aware of this option.
PrEP originally was recommended only for gay or bisexual men, but in May 2014, the CDC recommended it for all individuals at risk and released the first comprehensive clinical practice guidelines for the prevention tool (www.cdc.gov/hiv/pdf/guidelines/PrEPguidelines2014.pdf).
The PrEP medication, Truvada, is a combination of two drugs (tenovovir and emtricitabine) that, when taken daily on a consistent basis, significantly reduces the risk of getting HIV infection. Several large national and international studies have documented risk reductions of 73% to 92% when the medication was taken every day or almost every day. It is clearly within the purview of any ob.gyn to prescribe, monitor, and manage such prevention therapy.
The availability and relative ease of such a tool, along with advances in treatment and knowledge gained from the HIV Care Continuum, should re-energize ob.gyns. to up the ante in efforts to end the epidemic.
Experience in our clinical program that provides care and treatment to patients in the Baltimore-Washington area has taught us that we do much better when we integrate HIV care within primary care. It’s much more likely that patients will “stay close” with their ob.gyn than to another specialist.
Certainly, HIV infection has its “hot spots” and areas of much lower prevalence, but regardless of where we reside, we must continue to appreciate that the epidemic has had a significant impact on women and that this will persist unless we can all better utilize our available tools, such as early diagnosis and effective treatment that are linked long-term with other primary care physicians.
For women, ob.gyns represent a great resource for our nation to make progress toward President Obama’s National HIV Strategy.
Dr. Redfield reported that he has no disclosures relevant to this Master Class.