User login
FDA approves adhesive treatment for superficial varicose veins
The VenaSeal closure system, which uses an adhesive directly injected into the vein, has been approved as a permanent treatment for symptomatic, superficial varicose veins, the Food and Drug Administration announced on Feb. 20.
“This new system is the first to permanently treat varicose veins by sealing them with an adhesive,” Dr. William Maisel, acting director of the Office of Device Evaluation in the FDA’s Center for Devices and Radiological Health, said in the FDA’s statement. Because the system “does not incorporate heat application or cutting, the in-office procedure can allow patients to quickly return to their normal activities, with less bruising,” he added.
The VenaSeal system differs from other procedures used to treat varicose veins, which use drugs, lasers, radiofrequency, or incisions, the FDA statement points out. The complete sterile kit includes the adhesive (n-butyl-2-cyanoacrylate), which solidifies when injected directly into the target vein via a catheter, under ultrasound guidance. The additional system components include the catheter, the adhesive, a guidewire, dispenser gun, dispenser tips, and syringes.
Approval was based on data from three clinical trials sponsored by the manufacturer. In the U.S. study that compared results in 108 patients treated with the VenaSeal system and 114 patients treated with radiofrequency ablation therapy, the device was shown “to be safe and effective for vein closure for the treatment of symptomatic superficial varicose veins of the legs,” according to the FDA. In the study, adverse events associated with the VenaSeal treatment included phlebitis and paresthesias in the treated areas, which are “generally associated with treatments of this condition,” the FDA statement noted.
The agency reviewed the VenaSeal System as a class III medical device, considered the highest risk type of medical devices that are subjected to the highest level of regulatory control, and which must be approved before marketing.
VenaSeal is manufactured by Covidien, which acquired Sapheon, the company that developed VenaSeal, in 2014. The system has also been approved in Canada, Europe, and Hong Kong, according to a Covidien statement issued last year.
The VenaSeal closure system, which uses an adhesive directly injected into the vein, has been approved as a permanent treatment for symptomatic, superficial varicose veins, the Food and Drug Administration announced on Feb. 20.
“This new system is the first to permanently treat varicose veins by sealing them with an adhesive,” Dr. William Maisel, acting director of the Office of Device Evaluation in the FDA’s Center for Devices and Radiological Health, said in the FDA’s statement. Because the system “does not incorporate heat application or cutting, the in-office procedure can allow patients to quickly return to their normal activities, with less bruising,” he added.
The VenaSeal system differs from other procedures used to treat varicose veins, which use drugs, lasers, radiofrequency, or incisions, the FDA statement points out. The complete sterile kit includes the adhesive (n-butyl-2-cyanoacrylate), which solidifies when injected directly into the target vein via a catheter, under ultrasound guidance. The additional system components include the catheter, the adhesive, a guidewire, dispenser gun, dispenser tips, and syringes.
Approval was based on data from three clinical trials sponsored by the manufacturer. In the U.S. study that compared results in 108 patients treated with the VenaSeal system and 114 patients treated with radiofrequency ablation therapy, the device was shown “to be safe and effective for vein closure for the treatment of symptomatic superficial varicose veins of the legs,” according to the FDA. In the study, adverse events associated with the VenaSeal treatment included phlebitis and paresthesias in the treated areas, which are “generally associated with treatments of this condition,” the FDA statement noted.
The agency reviewed the VenaSeal System as a class III medical device, considered the highest risk type of medical devices that are subjected to the highest level of regulatory control, and which must be approved before marketing.
VenaSeal is manufactured by Covidien, which acquired Sapheon, the company that developed VenaSeal, in 2014. The system has also been approved in Canada, Europe, and Hong Kong, according to a Covidien statement issued last year.
The VenaSeal closure system, which uses an adhesive directly injected into the vein, has been approved as a permanent treatment for symptomatic, superficial varicose veins, the Food and Drug Administration announced on Feb. 20.
“This new system is the first to permanently treat varicose veins by sealing them with an adhesive,” Dr. William Maisel, acting director of the Office of Device Evaluation in the FDA’s Center for Devices and Radiological Health, said in the FDA’s statement. Because the system “does not incorporate heat application or cutting, the in-office procedure can allow patients to quickly return to their normal activities, with less bruising,” he added.
The VenaSeal system differs from other procedures used to treat varicose veins, which use drugs, lasers, radiofrequency, or incisions, the FDA statement points out. The complete sterile kit includes the adhesive (n-butyl-2-cyanoacrylate), which solidifies when injected directly into the target vein via a catheter, under ultrasound guidance. The additional system components include the catheter, the adhesive, a guidewire, dispenser gun, dispenser tips, and syringes.
Approval was based on data from three clinical trials sponsored by the manufacturer. In the U.S. study that compared results in 108 patients treated with the VenaSeal system and 114 patients treated with radiofrequency ablation therapy, the device was shown “to be safe and effective for vein closure for the treatment of symptomatic superficial varicose veins of the legs,” according to the FDA. In the study, adverse events associated with the VenaSeal treatment included phlebitis and paresthesias in the treated areas, which are “generally associated with treatments of this condition,” the FDA statement noted.
The agency reviewed the VenaSeal System as a class III medical device, considered the highest risk type of medical devices that are subjected to the highest level of regulatory control, and which must be approved before marketing.
VenaSeal is manufactured by Covidien, which acquired Sapheon, the company that developed VenaSeal, in 2014. The system has also been approved in Canada, Europe, and Hong Kong, according to a Covidien statement issued last year.
Abnormal calcium level in a psychiatric presentation? Rule out parathyroid disease
In some patients, symptoms of depression, psychosis, delirium, or dementia exist concomitantly with, or as a result of, an abnormal (elevated or low) serum calcium concentration that has been precipitated by an unrecognized endocrinopathy. The apparent psychiatric presentations of such patients might reflect parathyroid pathology—not psychopathology.
Hypercalcemia and hypocalcemia often are related to a distinct spectrum of conditions, such as diseases of the parathyroid glands, kidneys, and various neoplasms including malignancies. Be alert to the possibility of parathyroid disease in patients whose presentation suggests mental illness concurrent with, or as a direct consequence of, an abnormal calcium level, and investigate appropriately.
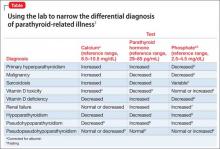
The Table1-9 illustrates how 3 clinical laboratory tests—serum calcium, serum parathyroid hormone (PTH), and phosphate—can narrow the differential diagnosis when the clinical impression is parathyroid-related illness. Seek endocrinology consultation whenever a parathyroid-associated ailment is discovered or suspected. Serum calcium is routinely assayed in hospitalized patients; when managing a patient with treatment-refractory psychiatric illness, (1) always check the reported result of that test and (2) consider measuring PTH.
Case reports1
Case 1: Woman with chronic depression. The patient was hospitalized while suicidal. Serial serum calcium levels were 12.5 mg/dL and 15.8 mg/dL (reference range, 8.2–10.2 mg/dL). The PTH level was elevated at 287 pg/mL (reference range, 10–65 pg/mL).
After thyroid imaging, surgery revealed a parathyroid mass, which was resected. Histologic examination confirmed an adenoma.
The calcium concentration declined to 8.6 mg/dL postoperatively and stabilized at 9.2 mg/dL. Psychiatric symptoms resolved fully; she experienced a complete recovery.
Case 2: Man on long-term lithium maintenance. The patient was admitted in a delusional psychotic state. The serum calcium level was 14.3 mg/dL initially, decreasing to 11.5 mg/dL after lithium was discontinued. The PTH level was elevated at 97 pg/mL at admission, consistent with hyperparathyroidism.
A parathyroid adenoma was resected. Serum calcium level normalized at 10.7 mg/dL; psychosis resolved with striking, sustained improvement in mental status.
Full return to mental, physical health
The diagnosis of parathyroid adenoma in these 2 patients, which began with a psychiatric presentation, was properly made after an abnormal serum calcium level was documented. Surgical treatment of the endocrinopathy produced full remission and a return to normal mental and physical health.
Although psychiatric manifestations are associated with an abnormal serum calcium concentration, the severity of those presentations does not correlate with the degree of abnormality of the calcium level.10
Disclosures
The authors report no financial relationships with any company whose products are mentioned in this article or with manufacturers of competing products.
1. Velasco PJ, Manshadi M, Breen K, et al. Psychiatric aspects of parathyroid disease. Psychosomatics. 1999;40(6):486-490.
2. Harrop JS, Bailey JE, Woodhead JS. Incidence of hypercalcaemia and primary hyperparathyroidism in relation to the biochemical profile. J Clin Pathol. 1982; 35(4):395-400.
3. Assadi F. Hypophosphatemia: an evidence-based problem-solving approach to clinical cases. Iran J Kidney Dis. 2010;4(3):195-201.
4. Ozkhan B, Hatun S, Bereket A. Vitamin D intoxication. Turk J Pediatr. 2012;54(2):93-98.
5. Studdy PR, Bird R, Neville E, et al. Biochemical findings in sarcoidosis. J Clin Pathol. 1980;33(6):528-533.
6. Geller JL, Adam JS. Vitamin D therapy. Curr Osteoporos Rep. 2008;6(1):5-11.
7. Albaaj F, Hutchison A. Hyperphosphatemia in renal failure: causes, consequences and current management. Drugs. 2003;63(6):577-596.
8. Al-Azem H, Khan AA. Hypoparathyroidism. Best Pract Res Clin Endocrinol Metab. 2012;26(4):517-522.
9. Brown H, Englert E, Wallach S. The syndrome of pseudo-pseudohypoparathyroidism. AMA Arch Intern Med. 1956;98(4):517-524.
10. Pfitzenmeyer P, Besancenot JF, Verges B, et al. Primary hyperparathyroidism in very old patients. Eur J Med. 1993;2(8):453-456.
In some patients, symptoms of depression, psychosis, delirium, or dementia exist concomitantly with, or as a result of, an abnormal (elevated or low) serum calcium concentration that has been precipitated by an unrecognized endocrinopathy. The apparent psychiatric presentations of such patients might reflect parathyroid pathology—not psychopathology.
Hypercalcemia and hypocalcemia often are related to a distinct spectrum of conditions, such as diseases of the parathyroid glands, kidneys, and various neoplasms including malignancies. Be alert to the possibility of parathyroid disease in patients whose presentation suggests mental illness concurrent with, or as a direct consequence of, an abnormal calcium level, and investigate appropriately.
The Table1-9 illustrates how 3 clinical laboratory tests—serum calcium, serum parathyroid hormone (PTH), and phosphate—can narrow the differential diagnosis when the clinical impression is parathyroid-related illness. Seek endocrinology consultation whenever a parathyroid-associated ailment is discovered or suspected. Serum calcium is routinely assayed in hospitalized patients; when managing a patient with treatment-refractory psychiatric illness, (1) always check the reported result of that test and (2) consider measuring PTH.
Case reports1
Case 1: Woman with chronic depression. The patient was hospitalized while suicidal. Serial serum calcium levels were 12.5 mg/dL and 15.8 mg/dL (reference range, 8.2–10.2 mg/dL). The PTH level was elevated at 287 pg/mL (reference range, 10–65 pg/mL).
After thyroid imaging, surgery revealed a parathyroid mass, which was resected. Histologic examination confirmed an adenoma.
The calcium concentration declined to 8.6 mg/dL postoperatively and stabilized at 9.2 mg/dL. Psychiatric symptoms resolved fully; she experienced a complete recovery.
Case 2: Man on long-term lithium maintenance. The patient was admitted in a delusional psychotic state. The serum calcium level was 14.3 mg/dL initially, decreasing to 11.5 mg/dL after lithium was discontinued. The PTH level was elevated at 97 pg/mL at admission, consistent with hyperparathyroidism.
A parathyroid adenoma was resected. Serum calcium level normalized at 10.7 mg/dL; psychosis resolved with striking, sustained improvement in mental status.
Full return to mental, physical health
The diagnosis of parathyroid adenoma in these 2 patients, which began with a psychiatric presentation, was properly made after an abnormal serum calcium level was documented. Surgical treatment of the endocrinopathy produced full remission and a return to normal mental and physical health.
Although psychiatric manifestations are associated with an abnormal serum calcium concentration, the severity of those presentations does not correlate with the degree of abnormality of the calcium level.10
Disclosures
The authors report no financial relationships with any company whose products are mentioned in this article or with manufacturers of competing products.
In some patients, symptoms of depression, psychosis, delirium, or dementia exist concomitantly with, or as a result of, an abnormal (elevated or low) serum calcium concentration that has been precipitated by an unrecognized endocrinopathy. The apparent psychiatric presentations of such patients might reflect parathyroid pathology—not psychopathology.
Hypercalcemia and hypocalcemia often are related to a distinct spectrum of conditions, such as diseases of the parathyroid glands, kidneys, and various neoplasms including malignancies. Be alert to the possibility of parathyroid disease in patients whose presentation suggests mental illness concurrent with, or as a direct consequence of, an abnormal calcium level, and investigate appropriately.
The Table1-9 illustrates how 3 clinical laboratory tests—serum calcium, serum parathyroid hormone (PTH), and phosphate—can narrow the differential diagnosis when the clinical impression is parathyroid-related illness. Seek endocrinology consultation whenever a parathyroid-associated ailment is discovered or suspected. Serum calcium is routinely assayed in hospitalized patients; when managing a patient with treatment-refractory psychiatric illness, (1) always check the reported result of that test and (2) consider measuring PTH.
Case reports1
Case 1: Woman with chronic depression. The patient was hospitalized while suicidal. Serial serum calcium levels were 12.5 mg/dL and 15.8 mg/dL (reference range, 8.2–10.2 mg/dL). The PTH level was elevated at 287 pg/mL (reference range, 10–65 pg/mL).
After thyroid imaging, surgery revealed a parathyroid mass, which was resected. Histologic examination confirmed an adenoma.
The calcium concentration declined to 8.6 mg/dL postoperatively and stabilized at 9.2 mg/dL. Psychiatric symptoms resolved fully; she experienced a complete recovery.
Case 2: Man on long-term lithium maintenance. The patient was admitted in a delusional psychotic state. The serum calcium level was 14.3 mg/dL initially, decreasing to 11.5 mg/dL after lithium was discontinued. The PTH level was elevated at 97 pg/mL at admission, consistent with hyperparathyroidism.
A parathyroid adenoma was resected. Serum calcium level normalized at 10.7 mg/dL; psychosis resolved with striking, sustained improvement in mental status.
Full return to mental, physical health
The diagnosis of parathyroid adenoma in these 2 patients, which began with a psychiatric presentation, was properly made after an abnormal serum calcium level was documented. Surgical treatment of the endocrinopathy produced full remission and a return to normal mental and physical health.
Although psychiatric manifestations are associated with an abnormal serum calcium concentration, the severity of those presentations does not correlate with the degree of abnormality of the calcium level.10
Disclosures
The authors report no financial relationships with any company whose products are mentioned in this article or with manufacturers of competing products.
1. Velasco PJ, Manshadi M, Breen K, et al. Psychiatric aspects of parathyroid disease. Psychosomatics. 1999;40(6):486-490.
2. Harrop JS, Bailey JE, Woodhead JS. Incidence of hypercalcaemia and primary hyperparathyroidism in relation to the biochemical profile. J Clin Pathol. 1982; 35(4):395-400.
3. Assadi F. Hypophosphatemia: an evidence-based problem-solving approach to clinical cases. Iran J Kidney Dis. 2010;4(3):195-201.
4. Ozkhan B, Hatun S, Bereket A. Vitamin D intoxication. Turk J Pediatr. 2012;54(2):93-98.
5. Studdy PR, Bird R, Neville E, et al. Biochemical findings in sarcoidosis. J Clin Pathol. 1980;33(6):528-533.
6. Geller JL, Adam JS. Vitamin D therapy. Curr Osteoporos Rep. 2008;6(1):5-11.
7. Albaaj F, Hutchison A. Hyperphosphatemia in renal failure: causes, consequences and current management. Drugs. 2003;63(6):577-596.
8. Al-Azem H, Khan AA. Hypoparathyroidism. Best Pract Res Clin Endocrinol Metab. 2012;26(4):517-522.
9. Brown H, Englert E, Wallach S. The syndrome of pseudo-pseudohypoparathyroidism. AMA Arch Intern Med. 1956;98(4):517-524.
10. Pfitzenmeyer P, Besancenot JF, Verges B, et al. Primary hyperparathyroidism in very old patients. Eur J Med. 1993;2(8):453-456.
1. Velasco PJ, Manshadi M, Breen K, et al. Psychiatric aspects of parathyroid disease. Psychosomatics. 1999;40(6):486-490.
2. Harrop JS, Bailey JE, Woodhead JS. Incidence of hypercalcaemia and primary hyperparathyroidism in relation to the biochemical profile. J Clin Pathol. 1982; 35(4):395-400.
3. Assadi F. Hypophosphatemia: an evidence-based problem-solving approach to clinical cases. Iran J Kidney Dis. 2010;4(3):195-201.
4. Ozkhan B, Hatun S, Bereket A. Vitamin D intoxication. Turk J Pediatr. 2012;54(2):93-98.
5. Studdy PR, Bird R, Neville E, et al. Biochemical findings in sarcoidosis. J Clin Pathol. 1980;33(6):528-533.
6. Geller JL, Adam JS. Vitamin D therapy. Curr Osteoporos Rep. 2008;6(1):5-11.
7. Albaaj F, Hutchison A. Hyperphosphatemia in renal failure: causes, consequences and current management. Drugs. 2003;63(6):577-596.
8. Al-Azem H, Khan AA. Hypoparathyroidism. Best Pract Res Clin Endocrinol Metab. 2012;26(4):517-522.
9. Brown H, Englert E, Wallach S. The syndrome of pseudo-pseudohypoparathyroidism. AMA Arch Intern Med. 1956;98(4):517-524.
10. Pfitzenmeyer P, Besancenot JF, Verges B, et al. Primary hyperparathyroidism in very old patients. Eur J Med. 1993;2(8):453-456.
Can Vitamin D Supplements Help With Hypertension?
Q) One of my patients came in and said he had read that vitamin D supplementation will help with hypertension. Now he wants to quit his blood pressure meds and use vitamin D instead. Do you have any background on this?
Vitamin D is critical for utilization of calcium, a vital nutrient for multiple metabolic and cellular processes; deficiency is associated with worsening of autoimmune disorders, osteoporosis, and certain cardiovascular conditions, among others.7 An association between vitamin D level and blood pressure has been recognized for some time, but the pathophysiology is not well understood.
A literature review of studies from 1988 to 2013 found contradictory results regarding vitamin D deficiency and concurrent elevated blood pressure (systolic and/or diastolic), as well as the impact on blood pressure with restoration of vitamin D levels. The findings were limited by several factors, including differences in study design, variables evaluated, and type of vitamin D compound used. The results suggested a link between the renin-angiotensin-aldosterone system, fibroblast growth factor 23/klotho axis, and vitamin D level.8
A study of 158 subjects (98 with newly diagnosed essential hypertension, 60 with normal blood pressure) found significantly lower 25(OH)D3 serum levels in hypertensive patients. Furthermore, the 25(OH)D3 level was significantly correlated with both systolic (r = –0.33) and diastolic blood pressure (r = –0.26). Using multiple regression analysis, after adjustment for age, smoking status, and BMI, the impact of 25(OH)D3 level accounted for 10% of the variation in systolic blood pressure.9
In a mendelian randomization study of 108,173 subjects from 35 studies, an inverse association between vitamin D level and systolic blood pressure (P = .0003) was found. A reduced risk for essential hypertension with increased vitamin D level (P = .0003) was also noted. However, no association was found between increasing vitamin D level and a reduction in diastolic blood pressure
(P = .37).10
With the ever-increasing access to health information from sources such as “Doctor Google,” it can be difficult for a non–health care professional to separate hype from evidence-based recommendations. While current evidence suggests optimal vitamin D levels may be beneficial for improving blood pressure control and may be a useful adjunctive therapy, there is no evidence to support discontinuing antihypertensive therapy and replacing it with vitamin D therapy.
Cynthia A. Smith, DNP, APRN, FNP-BC
Renal Consultants, South Charleston, West Virginia
REFERENCES
1. Monfared A, Heidarzadeh A, Ghaffari M, Akbarpour M. Effect of megestrol acetate on serum albumin level in malnourished dialysis patients. J Renal Nutr. 2009;19(2):167-171.
2. Byham-Gray L, Stover J, Wiesen K. A clinical guide to nutrition care in kidney disease. Acad Nutr Diet. 2013.
3. White JV, Guenter P, Jensen G, Malone A, Schofield M; Academy of Nutrition and Dietetics Malnutrition Work Group; ASPEN Malnutrition Task Force; ASPEN Board of Directors. Consensus statement of the Academy of Nutrition and Dietetics/American Society for Parenteral and Enteral Nutrition: characteristics recommended for the identification and documentation of adult malnutrition (undernutrition) [erratum appears in J Acad Nutr Diet. 2012 Nov;112(11):1899].
J Acad Nutr Diet. 2012;112(5):730-738.
4. Rammohan M, Kalantar-Zedeh K, Liang A, Ghossein C. Megestrol acetate in a moderate dose for the treatment of malnutrition-inflammation complex in maintenance dialysis patients. J Ren Nutr. 2005;15(3):345-355.
5. Yeh S, Marandi M, Thode H Jr, et al. Report of a pilot, double blind, placebo-controlled study of megestrol acetate in elderly dialysis patients with cachexia. J Ren Nutr. 2010; 20(1):52-62.
6. Golebiewska JE, Lichodziejewska-Niemierko M, Aleksandrowicz-Wrona E, et al. Megestrol acetate use in hypoalbuminemic dialysis patients [comment]. J Ren Nutr. 2011;21(2): 200-202.
7. Bendik I, Friedel A, Roos FF, et al. Vitamin D: a critical and necessary micronutrient for human health. Front Physiol. 2014;5:248.
8. Cabone F, Mach F, Vuilleumier N, Montecucco F. Potential pathophysiological role for the vitamin D deficiency in essential hypertension. World J Cardiol. 2014;6(5):260-276.
9. Sypniewska G, Pollak J, Strozecki P, et al. 25-hydroxyvitamin D, biomarkers of endothelial dysfunction and subclinical organ damage in adults with hypertension. Am J Hypertens. 2014;27(1):114-121.
10. Vimaleswaran KS, Cavadino A, Berry DJ, et al. Association of vitamin D status with arterial blood pressure and hypertension risk: a mendelian randomisation study. Lancet Diabetes Endocrinol. 2014;2(9):719-729.
Q) One of my patients came in and said he had read that vitamin D supplementation will help with hypertension. Now he wants to quit his blood pressure meds and use vitamin D instead. Do you have any background on this?
Vitamin D is critical for utilization of calcium, a vital nutrient for multiple metabolic and cellular processes; deficiency is associated with worsening of autoimmune disorders, osteoporosis, and certain cardiovascular conditions, among others.7 An association between vitamin D level and blood pressure has been recognized for some time, but the pathophysiology is not well understood.
A literature review of studies from 1988 to 2013 found contradictory results regarding vitamin D deficiency and concurrent elevated blood pressure (systolic and/or diastolic), as well as the impact on blood pressure with restoration of vitamin D levels. The findings were limited by several factors, including differences in study design, variables evaluated, and type of vitamin D compound used. The results suggested a link between the renin-angiotensin-aldosterone system, fibroblast growth factor 23/klotho axis, and vitamin D level.8
A study of 158 subjects (98 with newly diagnosed essential hypertension, 60 with normal blood pressure) found significantly lower 25(OH)D3 serum levels in hypertensive patients. Furthermore, the 25(OH)D3 level was significantly correlated with both systolic (r = –0.33) and diastolic blood pressure (r = –0.26). Using multiple regression analysis, after adjustment for age, smoking status, and BMI, the impact of 25(OH)D3 level accounted for 10% of the variation in systolic blood pressure.9
In a mendelian randomization study of 108,173 subjects from 35 studies, an inverse association between vitamin D level and systolic blood pressure (P = .0003) was found. A reduced risk for essential hypertension with increased vitamin D level (P = .0003) was also noted. However, no association was found between increasing vitamin D level and a reduction in diastolic blood pressure
(P = .37).10
With the ever-increasing access to health information from sources such as “Doctor Google,” it can be difficult for a non–health care professional to separate hype from evidence-based recommendations. While current evidence suggests optimal vitamin D levels may be beneficial for improving blood pressure control and may be a useful adjunctive therapy, there is no evidence to support discontinuing antihypertensive therapy and replacing it with vitamin D therapy.
Cynthia A. Smith, DNP, APRN, FNP-BC
Renal Consultants, South Charleston, West Virginia
REFERENCES
1. Monfared A, Heidarzadeh A, Ghaffari M, Akbarpour M. Effect of megestrol acetate on serum albumin level in malnourished dialysis patients. J Renal Nutr. 2009;19(2):167-171.
2. Byham-Gray L, Stover J, Wiesen K. A clinical guide to nutrition care in kidney disease. Acad Nutr Diet. 2013.
3. White JV, Guenter P, Jensen G, Malone A, Schofield M; Academy of Nutrition and Dietetics Malnutrition Work Group; ASPEN Malnutrition Task Force; ASPEN Board of Directors. Consensus statement of the Academy of Nutrition and Dietetics/American Society for Parenteral and Enteral Nutrition: characteristics recommended for the identification and documentation of adult malnutrition (undernutrition) [erratum appears in J Acad Nutr Diet. 2012 Nov;112(11):1899].
J Acad Nutr Diet. 2012;112(5):730-738.
4. Rammohan M, Kalantar-Zedeh K, Liang A, Ghossein C. Megestrol acetate in a moderate dose for the treatment of malnutrition-inflammation complex in maintenance dialysis patients. J Ren Nutr. 2005;15(3):345-355.
5. Yeh S, Marandi M, Thode H Jr, et al. Report of a pilot, double blind, placebo-controlled study of megestrol acetate in elderly dialysis patients with cachexia. J Ren Nutr. 2010; 20(1):52-62.
6. Golebiewska JE, Lichodziejewska-Niemierko M, Aleksandrowicz-Wrona E, et al. Megestrol acetate use in hypoalbuminemic dialysis patients [comment]. J Ren Nutr. 2011;21(2): 200-202.
7. Bendik I, Friedel A, Roos FF, et al. Vitamin D: a critical and necessary micronutrient for human health. Front Physiol. 2014;5:248.
8. Cabone F, Mach F, Vuilleumier N, Montecucco F. Potential pathophysiological role for the vitamin D deficiency in essential hypertension. World J Cardiol. 2014;6(5):260-276.
9. Sypniewska G, Pollak J, Strozecki P, et al. 25-hydroxyvitamin D, biomarkers of endothelial dysfunction and subclinical organ damage in adults with hypertension. Am J Hypertens. 2014;27(1):114-121.
10. Vimaleswaran KS, Cavadino A, Berry DJ, et al. Association of vitamin D status with arterial blood pressure and hypertension risk: a mendelian randomisation study. Lancet Diabetes Endocrinol. 2014;2(9):719-729.
Q) One of my patients came in and said he had read that vitamin D supplementation will help with hypertension. Now he wants to quit his blood pressure meds and use vitamin D instead. Do you have any background on this?
Vitamin D is critical for utilization of calcium, a vital nutrient for multiple metabolic and cellular processes; deficiency is associated with worsening of autoimmune disorders, osteoporosis, and certain cardiovascular conditions, among others.7 An association between vitamin D level and blood pressure has been recognized for some time, but the pathophysiology is not well understood.
A literature review of studies from 1988 to 2013 found contradictory results regarding vitamin D deficiency and concurrent elevated blood pressure (systolic and/or diastolic), as well as the impact on blood pressure with restoration of vitamin D levels. The findings were limited by several factors, including differences in study design, variables evaluated, and type of vitamin D compound used. The results suggested a link between the renin-angiotensin-aldosterone system, fibroblast growth factor 23/klotho axis, and vitamin D level.8
A study of 158 subjects (98 with newly diagnosed essential hypertension, 60 with normal blood pressure) found significantly lower 25(OH)D3 serum levels in hypertensive patients. Furthermore, the 25(OH)D3 level was significantly correlated with both systolic (r = –0.33) and diastolic blood pressure (r = –0.26). Using multiple regression analysis, after adjustment for age, smoking status, and BMI, the impact of 25(OH)D3 level accounted for 10% of the variation in systolic blood pressure.9
In a mendelian randomization study of 108,173 subjects from 35 studies, an inverse association between vitamin D level and systolic blood pressure (P = .0003) was found. A reduced risk for essential hypertension with increased vitamin D level (P = .0003) was also noted. However, no association was found between increasing vitamin D level and a reduction in diastolic blood pressure
(P = .37).10
With the ever-increasing access to health information from sources such as “Doctor Google,” it can be difficult for a non–health care professional to separate hype from evidence-based recommendations. While current evidence suggests optimal vitamin D levels may be beneficial for improving blood pressure control and may be a useful adjunctive therapy, there is no evidence to support discontinuing antihypertensive therapy and replacing it with vitamin D therapy.
Cynthia A. Smith, DNP, APRN, FNP-BC
Renal Consultants, South Charleston, West Virginia
REFERENCES
1. Monfared A, Heidarzadeh A, Ghaffari M, Akbarpour M. Effect of megestrol acetate on serum albumin level in malnourished dialysis patients. J Renal Nutr. 2009;19(2):167-171.
2. Byham-Gray L, Stover J, Wiesen K. A clinical guide to nutrition care in kidney disease. Acad Nutr Diet. 2013.
3. White JV, Guenter P, Jensen G, Malone A, Schofield M; Academy of Nutrition and Dietetics Malnutrition Work Group; ASPEN Malnutrition Task Force; ASPEN Board of Directors. Consensus statement of the Academy of Nutrition and Dietetics/American Society for Parenteral and Enteral Nutrition: characteristics recommended for the identification and documentation of adult malnutrition (undernutrition) [erratum appears in J Acad Nutr Diet. 2012 Nov;112(11):1899].
J Acad Nutr Diet. 2012;112(5):730-738.
4. Rammohan M, Kalantar-Zedeh K, Liang A, Ghossein C. Megestrol acetate in a moderate dose for the treatment of malnutrition-inflammation complex in maintenance dialysis patients. J Ren Nutr. 2005;15(3):345-355.
5. Yeh S, Marandi M, Thode H Jr, et al. Report of a pilot, double blind, placebo-controlled study of megestrol acetate in elderly dialysis patients with cachexia. J Ren Nutr. 2010; 20(1):52-62.
6. Golebiewska JE, Lichodziejewska-Niemierko M, Aleksandrowicz-Wrona E, et al. Megestrol acetate use in hypoalbuminemic dialysis patients [comment]. J Ren Nutr. 2011;21(2): 200-202.
7. Bendik I, Friedel A, Roos FF, et al. Vitamin D: a critical and necessary micronutrient for human health. Front Physiol. 2014;5:248.
8. Cabone F, Mach F, Vuilleumier N, Montecucco F. Potential pathophysiological role for the vitamin D deficiency in essential hypertension. World J Cardiol. 2014;6(5):260-276.
9. Sypniewska G, Pollak J, Strozecki P, et al. 25-hydroxyvitamin D, biomarkers of endothelial dysfunction and subclinical organ damage in adults with hypertension. Am J Hypertens. 2014;27(1):114-121.
10. Vimaleswaran KS, Cavadino A, Berry DJ, et al. Association of vitamin D status with arterial blood pressure and hypertension risk: a mendelian randomisation study. Lancet Diabetes Endocrinol. 2014;2(9):719-729.
How to write a suicide risk assessment that’s clinically sound and legally defensible
Suicidologists and legal experts implore clinicians to document their suicide risk assessments (SRAs) thoroughly. It’s difficult, however, to find practical guidance on how to write a clinically sound, legally defensible SRA.
The crux of every SRA is written justification of suicide risk. That justification should reveal your thinking and present a well-reasoned basis for your decision.
Reasoned vs right
It’s more important to provide a justification of suicide risk that’s well-reasoned rather than one that’s right. Suicide is impossible to predict. Instead of prediction, legally we are asked to reasonably anticipate suicide based on clinical facts. In hindsight, especially in the context of a courtroom, decisions might look ill-considered. You need to craft a logical argument, be clear, and avoid jargon.
Convey thoroughness by covering each component of an SRA. Use the mnemonic device CAIPS to help the reader (and you) understand how a conclusion was reached based on the facts of the case.
Chronic and Acute factors. Address the chronic and acute factors that weigh heaviest in your mind. Chronic factors are conditions, past events, and demographics that generally do not change. Acute factors are recent events or conditions that potentially are modifiable. Pay attention to combinations of factors that dramatically elevate risk (eg, previous attempts in the context of acute depression). Avoid repeating every factor, especially when these are documented elsewhere, such as on a checklist.
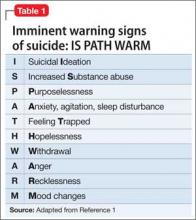
Imminent warning signs for suicide. Address warning signs (Table 1),1 the nature of current suicidal thoughts (Table 2), and other aspects of mental status (eg, future orientation) that influenced your decision. Use words like “moreover,” “however,” and “in addition” to draw the reader’s attention to the building blocks of your argument.
Protective factors. Discuss the protective factors last; they deserve the least weight because none has been shown to immunize people against suicide. Don’t solely rely on your judgment of what is protective (eg, children in the home). Instead, elicit the patient’s reasons for living and dying. Be concerned if he (she) reports more of the latter.
Summary statement. Make an explicit statement about risk, focusing on imminent risk (ie, the next few hours and days). Avoid a “plot twist,” which is a risk level inconsistent with the preceding evidence, because it suggests an error in judgment. The Box gives an example of a justification that follows the CAIPS method.
Additional tips
Consider these strategies:
• Bolster your argument by explicitly addressing hopelessness (the strongest psychological correlate of suicide); use quotes from the patient that support your decision; refer to consultation with family members and colleagues; and include pertinent negatives to show completeness2 (ie, “denied suicide plans”).
• Critically resolve discrepancies between what the patient says and behavior that suggests suicidal intent (eg, a patient who minimizes suicidal intent but shopped for a gun yesterday).
• Last, while reviewing your justification, imagine that your patient completed suicide after leaving your office and that you are in court for negligence. In our experience, this exercise reveals dangerous errors of judgment. A clear and reasoned justification will reduce the risk of litigation and help you make prudent treatment plans.
Disclosures
The authors report no financial relationships with any companies whose products are mentioned in this article or with manufacturers of competing products.
1. American Association of Suicidology. Know the warning signs of suicide. http://www.suicidology.org/resources/ warning-signs. Accessed February 9, 2014.
2. Ballas C. How to write a suicide note: practical tips for documenting the evaluation of a suicidal patient. Psychiatric Times. http://www.psychiatrictimes.com/articles/how-write-suicide-note-practical-tips-documenting-evaluation-suicidal-patient. Published May 1, 2007. Accessed July 29, 2013.
Suicidologists and legal experts implore clinicians to document their suicide risk assessments (SRAs) thoroughly. It’s difficult, however, to find practical guidance on how to write a clinically sound, legally defensible SRA.
The crux of every SRA is written justification of suicide risk. That justification should reveal your thinking and present a well-reasoned basis for your decision.
Reasoned vs right
It’s more important to provide a justification of suicide risk that’s well-reasoned rather than one that’s right. Suicide is impossible to predict. Instead of prediction, legally we are asked to reasonably anticipate suicide based on clinical facts. In hindsight, especially in the context of a courtroom, decisions might look ill-considered. You need to craft a logical argument, be clear, and avoid jargon.
Convey thoroughness by covering each component of an SRA. Use the mnemonic device CAIPS to help the reader (and you) understand how a conclusion was reached based on the facts of the case.
Chronic and Acute factors. Address the chronic and acute factors that weigh heaviest in your mind. Chronic factors are conditions, past events, and demographics that generally do not change. Acute factors are recent events or conditions that potentially are modifiable. Pay attention to combinations of factors that dramatically elevate risk (eg, previous attempts in the context of acute depression). Avoid repeating every factor, especially when these are documented elsewhere, such as on a checklist.
Imminent warning signs for suicide. Address warning signs (Table 1),1 the nature of current suicidal thoughts (Table 2), and other aspects of mental status (eg, future orientation) that influenced your decision. Use words like “moreover,” “however,” and “in addition” to draw the reader’s attention to the building blocks of your argument.
Protective factors. Discuss the protective factors last; they deserve the least weight because none has been shown to immunize people against suicide. Don’t solely rely on your judgment of what is protective (eg, children in the home). Instead, elicit the patient’s reasons for living and dying. Be concerned if he (she) reports more of the latter.
Summary statement. Make an explicit statement about risk, focusing on imminent risk (ie, the next few hours and days). Avoid a “plot twist,” which is a risk level inconsistent with the preceding evidence, because it suggests an error in judgment. The Box gives an example of a justification that follows the CAIPS method.
Additional tips
Consider these strategies:
• Bolster your argument by explicitly addressing hopelessness (the strongest psychological correlate of suicide); use quotes from the patient that support your decision; refer to consultation with family members and colleagues; and include pertinent negatives to show completeness2 (ie, “denied suicide plans”).
• Critically resolve discrepancies between what the patient says and behavior that suggests suicidal intent (eg, a patient who minimizes suicidal intent but shopped for a gun yesterday).
• Last, while reviewing your justification, imagine that your patient completed suicide after leaving your office and that you are in court for negligence. In our experience, this exercise reveals dangerous errors of judgment. A clear and reasoned justification will reduce the risk of litigation and help you make prudent treatment plans.
Disclosures
The authors report no financial relationships with any companies whose products are mentioned in this article or with manufacturers of competing products.
Suicidologists and legal experts implore clinicians to document their suicide risk assessments (SRAs) thoroughly. It’s difficult, however, to find practical guidance on how to write a clinically sound, legally defensible SRA.
The crux of every SRA is written justification of suicide risk. That justification should reveal your thinking and present a well-reasoned basis for your decision.
Reasoned vs right
It’s more important to provide a justification of suicide risk that’s well-reasoned rather than one that’s right. Suicide is impossible to predict. Instead of prediction, legally we are asked to reasonably anticipate suicide based on clinical facts. In hindsight, especially in the context of a courtroom, decisions might look ill-considered. You need to craft a logical argument, be clear, and avoid jargon.
Convey thoroughness by covering each component of an SRA. Use the mnemonic device CAIPS to help the reader (and you) understand how a conclusion was reached based on the facts of the case.
Chronic and Acute factors. Address the chronic and acute factors that weigh heaviest in your mind. Chronic factors are conditions, past events, and demographics that generally do not change. Acute factors are recent events or conditions that potentially are modifiable. Pay attention to combinations of factors that dramatically elevate risk (eg, previous attempts in the context of acute depression). Avoid repeating every factor, especially when these are documented elsewhere, such as on a checklist.
Imminent warning signs for suicide. Address warning signs (Table 1),1 the nature of current suicidal thoughts (Table 2), and other aspects of mental status (eg, future orientation) that influenced your decision. Use words like “moreover,” “however,” and “in addition” to draw the reader’s attention to the building blocks of your argument.
Protective factors. Discuss the protective factors last; they deserve the least weight because none has been shown to immunize people against suicide. Don’t solely rely on your judgment of what is protective (eg, children in the home). Instead, elicit the patient’s reasons for living and dying. Be concerned if he (she) reports more of the latter.
Summary statement. Make an explicit statement about risk, focusing on imminent risk (ie, the next few hours and days). Avoid a “plot twist,” which is a risk level inconsistent with the preceding evidence, because it suggests an error in judgment. The Box gives an example of a justification that follows the CAIPS method.
Additional tips
Consider these strategies:
• Bolster your argument by explicitly addressing hopelessness (the strongest psychological correlate of suicide); use quotes from the patient that support your decision; refer to consultation with family members and colleagues; and include pertinent negatives to show completeness2 (ie, “denied suicide plans”).
• Critically resolve discrepancies between what the patient says and behavior that suggests suicidal intent (eg, a patient who minimizes suicidal intent but shopped for a gun yesterday).
• Last, while reviewing your justification, imagine that your patient completed suicide after leaving your office and that you are in court for negligence. In our experience, this exercise reveals dangerous errors of judgment. A clear and reasoned justification will reduce the risk of litigation and help you make prudent treatment plans.
Disclosures
The authors report no financial relationships with any companies whose products are mentioned in this article or with manufacturers of competing products.
1. American Association of Suicidology. Know the warning signs of suicide. http://www.suicidology.org/resources/ warning-signs. Accessed February 9, 2014.
2. Ballas C. How to write a suicide note: practical tips for documenting the evaluation of a suicidal patient. Psychiatric Times. http://www.psychiatrictimes.com/articles/how-write-suicide-note-practical-tips-documenting-evaluation-suicidal-patient. Published May 1, 2007. Accessed July 29, 2013.
1. American Association of Suicidology. Know the warning signs of suicide. http://www.suicidology.org/resources/ warning-signs. Accessed February 9, 2014.
2. Ballas C. How to write a suicide note: practical tips for documenting the evaluation of a suicidal patient. Psychiatric Times. http://www.psychiatrictimes.com/articles/how-write-suicide-note-practical-tips-documenting-evaluation-suicidal-patient. Published May 1, 2007. Accessed July 29, 2013.
When your diagnosis is questioned
When patients question your diagnosis, how do you react?
As physicians, we take great pride in our ability to diagnose and treat disease, and as hospitalists, our patients are sicker, so we need to make the right diagnosis and make it fast. A diagnostic delay of even a few days can sometimes cost a patient his life.
So when the patient or a family member disagrees with your diagnosis – especially when they have no remote understanding of the condition – it can be easy to dismiss their concerns. And then there are the times when you’ve missed something and they are right.
I will never forget a 60-year-old male patient I encountered early in my career as a hospitalist. He had presented with diffuse abdominal pain which later localized to both lower quadrants, diarrhea, and CT scan evidence of gastroenteritis. Multiple doctors who saw the patient before me all had the same diagnosis, a simple case of gastroenteritis. By day 2, he was afebrile, had a normal white blood cell count, was eating, and was ambulating down the hallway with his large family, seemingly in no distress.
He related that he still had abdominal pain, but felt comfortable with his diagnosis and was amenable to being discharged to follow-up with the gastroenterologist who had consulted on him during his stay in the hospital. His niece, on the other hand, was not happy with the diagnosis. The look on her face was intense, not disrespectful, as she related her conviction that her uncle had something more going on than a bout of gastroenteritis. She knew her uncle far better than I did, and his pain was concerning to her.
So I went back to the drawing board to make sure nothing had been missed, and there, hidden in plain sight, was a vital piece of information that we had all overlooked. The CT scan report that showed signs consistent with gastroenteritis made no mention whatsoever of his appendix.
Not satisfied with simply having another radiologist read the film, I insisted that a surgeon see the patient. To the surgeon’s great surprise, and mine, he found evidence of appendicitis. By 10 a.m. the next morning, the patient was in the OR having a now-perforated appendix removed. After numerous apologies to the family and patient, he was discharged home on postop day 2, doing well.
That very scary near miss taught me a valuable lesson: Sometimes the gut instinct of patients and their family members is just as accurate as the gut instinct of a physician, and we need to fully respect their input, whether or not we agree with them.
Dr. Hester is a hospitalist at Baltimore-Washington Medical Center in Glen Burnie, Md. She is the creator of the Patient Whiz, a patient-engagement app for iOS. Reach her at [email protected].
When patients question your diagnosis, how do you react?
As physicians, we take great pride in our ability to diagnose and treat disease, and as hospitalists, our patients are sicker, so we need to make the right diagnosis and make it fast. A diagnostic delay of even a few days can sometimes cost a patient his life.
So when the patient or a family member disagrees with your diagnosis – especially when they have no remote understanding of the condition – it can be easy to dismiss their concerns. And then there are the times when you’ve missed something and they are right.
I will never forget a 60-year-old male patient I encountered early in my career as a hospitalist. He had presented with diffuse abdominal pain which later localized to both lower quadrants, diarrhea, and CT scan evidence of gastroenteritis. Multiple doctors who saw the patient before me all had the same diagnosis, a simple case of gastroenteritis. By day 2, he was afebrile, had a normal white blood cell count, was eating, and was ambulating down the hallway with his large family, seemingly in no distress.
He related that he still had abdominal pain, but felt comfortable with his diagnosis and was amenable to being discharged to follow-up with the gastroenterologist who had consulted on him during his stay in the hospital. His niece, on the other hand, was not happy with the diagnosis. The look on her face was intense, not disrespectful, as she related her conviction that her uncle had something more going on than a bout of gastroenteritis. She knew her uncle far better than I did, and his pain was concerning to her.
So I went back to the drawing board to make sure nothing had been missed, and there, hidden in plain sight, was a vital piece of information that we had all overlooked. The CT scan report that showed signs consistent with gastroenteritis made no mention whatsoever of his appendix.
Not satisfied with simply having another radiologist read the film, I insisted that a surgeon see the patient. To the surgeon’s great surprise, and mine, he found evidence of appendicitis. By 10 a.m. the next morning, the patient was in the OR having a now-perforated appendix removed. After numerous apologies to the family and patient, he was discharged home on postop day 2, doing well.
That very scary near miss taught me a valuable lesson: Sometimes the gut instinct of patients and their family members is just as accurate as the gut instinct of a physician, and we need to fully respect their input, whether or not we agree with them.
Dr. Hester is a hospitalist at Baltimore-Washington Medical Center in Glen Burnie, Md. She is the creator of the Patient Whiz, a patient-engagement app for iOS. Reach her at [email protected].
When patients question your diagnosis, how do you react?
As physicians, we take great pride in our ability to diagnose and treat disease, and as hospitalists, our patients are sicker, so we need to make the right diagnosis and make it fast. A diagnostic delay of even a few days can sometimes cost a patient his life.
So when the patient or a family member disagrees with your diagnosis – especially when they have no remote understanding of the condition – it can be easy to dismiss their concerns. And then there are the times when you’ve missed something and they are right.
I will never forget a 60-year-old male patient I encountered early in my career as a hospitalist. He had presented with diffuse abdominal pain which later localized to both lower quadrants, diarrhea, and CT scan evidence of gastroenteritis. Multiple doctors who saw the patient before me all had the same diagnosis, a simple case of gastroenteritis. By day 2, he was afebrile, had a normal white blood cell count, was eating, and was ambulating down the hallway with his large family, seemingly in no distress.
He related that he still had abdominal pain, but felt comfortable with his diagnosis and was amenable to being discharged to follow-up with the gastroenterologist who had consulted on him during his stay in the hospital. His niece, on the other hand, was not happy with the diagnosis. The look on her face was intense, not disrespectful, as she related her conviction that her uncle had something more going on than a bout of gastroenteritis. She knew her uncle far better than I did, and his pain was concerning to her.
So I went back to the drawing board to make sure nothing had been missed, and there, hidden in plain sight, was a vital piece of information that we had all overlooked. The CT scan report that showed signs consistent with gastroenteritis made no mention whatsoever of his appendix.
Not satisfied with simply having another radiologist read the film, I insisted that a surgeon see the patient. To the surgeon’s great surprise, and mine, he found evidence of appendicitis. By 10 a.m. the next morning, the patient was in the OR having a now-perforated appendix removed. After numerous apologies to the family and patient, he was discharged home on postop day 2, doing well.
That very scary near miss taught me a valuable lesson: Sometimes the gut instinct of patients and their family members is just as accurate as the gut instinct of a physician, and we need to fully respect their input, whether or not we agree with them.
Dr. Hester is a hospitalist at Baltimore-Washington Medical Center in Glen Burnie, Md. She is the creator of the Patient Whiz, a patient-engagement app for iOS. Reach her at [email protected].
Maternal age, cardioseptal defects are major risk factors for peripartum thrombosis
NASHVILLE, TENN. – The risk of a peripartum thrombotic event is rare, but significantly increased for women who have a cardioseptal defect. In a large national sample, the rate of thrombotic events was seven times higher among women with an atrial or ventral septal defect.
Advanced maternal age also was a significant independent predictor of this complication; among more than 7,000 women who developed a thrombotic complication, 81% were older than 45 years.
Dr. Ali Razmara of the University of Southern California, Los Angeles, mined the National Inpatient Sample for data linking peripartum thrombotic events to patient demographics and medical comorbidities. His cohort comprised 4.3 million normal vaginal and cesarean deliveries from 2000 to 2010. Events of interest included transient ischemic attack, ischemic stroke, hemorrhagic stroke, acute MI, and venous thromboembolism.
There were 7,242 peripartum thrombotic events (0.17%).The majority occurred in women who were older than 45 years (81%); white (58%); and admitted through the emergency department (67%). Women with thrombotic events were more likely to have hypertension (52% vs. 2%), dyslipidemia (26% vs. 0.52%), diabetes (20% vs. 2%), atrial fibrillation (10% vs. 0.23%), and heart failure (10% vs. 0.26%), he said at the International Stroke Conference.
A multivariate regression model controlled for patient demographics and comorbidities, including, among others, preeclampsia, hypercoagulable states, chorioamnionitis, renal and liver disease, hypertension, diabetes, and cardiovascular disorders including atrial fibrillation, heart failure, and atrial/ventral septal defects.
In a multivariate regression analysis, maternal age shook out as the most powerful independent risk factor; the rate of thrombosis was 91 times greater among women older than age 45 years.
Other significant independent predictors included emergency vs. routine admission (RR 3.3), cardiac septal defect (RR 7), preeclampsia (RR 3.3), and hypercoagulability (RR 3).
Dyslipidemia and hypertension doubled the rate of a thrombotic event. Hypertension, migraine, renal disease, heart disease, atrial fibrillation, and heart failure were also significant factors, increasing the rate of thrombosis by 40%-50%, Dr. Razmara said at the meeting, which was sponsored by the American Heart Association.
“Our goal is development of targeted interventions for screening, prevention, and treatment of thrombosis related to pregnancy.”
Dr. Razmara had no relevant financial disclosures.
NASHVILLE, TENN. – The risk of a peripartum thrombotic event is rare, but significantly increased for women who have a cardioseptal defect. In a large national sample, the rate of thrombotic events was seven times higher among women with an atrial or ventral septal defect.
Advanced maternal age also was a significant independent predictor of this complication; among more than 7,000 women who developed a thrombotic complication, 81% were older than 45 years.
Dr. Ali Razmara of the University of Southern California, Los Angeles, mined the National Inpatient Sample for data linking peripartum thrombotic events to patient demographics and medical comorbidities. His cohort comprised 4.3 million normal vaginal and cesarean deliveries from 2000 to 2010. Events of interest included transient ischemic attack, ischemic stroke, hemorrhagic stroke, acute MI, and venous thromboembolism.
There were 7,242 peripartum thrombotic events (0.17%).The majority occurred in women who were older than 45 years (81%); white (58%); and admitted through the emergency department (67%). Women with thrombotic events were more likely to have hypertension (52% vs. 2%), dyslipidemia (26% vs. 0.52%), diabetes (20% vs. 2%), atrial fibrillation (10% vs. 0.23%), and heart failure (10% vs. 0.26%), he said at the International Stroke Conference.
A multivariate regression model controlled for patient demographics and comorbidities, including, among others, preeclampsia, hypercoagulable states, chorioamnionitis, renal and liver disease, hypertension, diabetes, and cardiovascular disorders including atrial fibrillation, heart failure, and atrial/ventral septal defects.
In a multivariate regression analysis, maternal age shook out as the most powerful independent risk factor; the rate of thrombosis was 91 times greater among women older than age 45 years.
Other significant independent predictors included emergency vs. routine admission (RR 3.3), cardiac septal defect (RR 7), preeclampsia (RR 3.3), and hypercoagulability (RR 3).
Dyslipidemia and hypertension doubled the rate of a thrombotic event. Hypertension, migraine, renal disease, heart disease, atrial fibrillation, and heart failure were also significant factors, increasing the rate of thrombosis by 40%-50%, Dr. Razmara said at the meeting, which was sponsored by the American Heart Association.
“Our goal is development of targeted interventions for screening, prevention, and treatment of thrombosis related to pregnancy.”
Dr. Razmara had no relevant financial disclosures.
NASHVILLE, TENN. – The risk of a peripartum thrombotic event is rare, but significantly increased for women who have a cardioseptal defect. In a large national sample, the rate of thrombotic events was seven times higher among women with an atrial or ventral septal defect.
Advanced maternal age also was a significant independent predictor of this complication; among more than 7,000 women who developed a thrombotic complication, 81% were older than 45 years.
Dr. Ali Razmara of the University of Southern California, Los Angeles, mined the National Inpatient Sample for data linking peripartum thrombotic events to patient demographics and medical comorbidities. His cohort comprised 4.3 million normal vaginal and cesarean deliveries from 2000 to 2010. Events of interest included transient ischemic attack, ischemic stroke, hemorrhagic stroke, acute MI, and venous thromboembolism.
There were 7,242 peripartum thrombotic events (0.17%).The majority occurred in women who were older than 45 years (81%); white (58%); and admitted through the emergency department (67%). Women with thrombotic events were more likely to have hypertension (52% vs. 2%), dyslipidemia (26% vs. 0.52%), diabetes (20% vs. 2%), atrial fibrillation (10% vs. 0.23%), and heart failure (10% vs. 0.26%), he said at the International Stroke Conference.
A multivariate regression model controlled for patient demographics and comorbidities, including, among others, preeclampsia, hypercoagulable states, chorioamnionitis, renal and liver disease, hypertension, diabetes, and cardiovascular disorders including atrial fibrillation, heart failure, and atrial/ventral septal defects.
In a multivariate regression analysis, maternal age shook out as the most powerful independent risk factor; the rate of thrombosis was 91 times greater among women older than age 45 years.
Other significant independent predictors included emergency vs. routine admission (RR 3.3), cardiac septal defect (RR 7), preeclampsia (RR 3.3), and hypercoagulability (RR 3).
Dyslipidemia and hypertension doubled the rate of a thrombotic event. Hypertension, migraine, renal disease, heart disease, atrial fibrillation, and heart failure were also significant factors, increasing the rate of thrombosis by 40%-50%, Dr. Razmara said at the meeting, which was sponsored by the American Heart Association.
“Our goal is development of targeted interventions for screening, prevention, and treatment of thrombosis related to pregnancy.”
Dr. Razmara had no relevant financial disclosures.
AT THE INTERNATIONAL STROKE CONFERENCE
Key clinical point: Advanced maternal age and a cardioseptal defect increase the risk of a peripartum thrombotic event.
Major finding: The rate of peripartum thrombotic events was 0.17%; cardioseptal defects increased the rate of a peripartum thombotic event by more than seven times.
Data source: A sample that comprised 4.5 million deliveries during 2000-2010.
Disclosures: Dr. Razmara had no relevant disclosures.
Megestrol Acetate for CKD and Dialysis Patients
Q) Some of my CKD patients are malnourished; in fact, some of those on dialysis do not eat well and have low albumin levels. Previously in this column, it was stated that higher albumin levels (> 4 g/dL) confer survival benefits to dialysis patients. Should I consider prescribing megestrol acetate to improve appetite? If I do prescribe it, what dose is safe for CKD and dialysis patients?
Malnutrition affects one-third of dialysis patients,1 and malnutrition-inflammation complex syndrome (MICS) is common in those with stage 5 CKD. Albumin is used as an indicator of MICS in dialysis patients; however, since other factors (stress, infection, inflammation, comorbidities) affect nutritional status,2 serum albumin alone may not be sufficient to assess it.
In fact, a recent consensus statement on malnutrition from the Academy of Nutrition and Dietetics and the American Society for Parenteral and Enteral Nutrition excluded serum albumin as a diagnostic characteristic; the criteria included percentage of energy requirement, percentage of weight loss and time frame, loss of body fat and muscle mass, presence of edema, and reduced grip strength.3 These may be better measures of malnutrition in dialysis patients and could be used as criteria for determining when to prescribe an appetite stimulant, such as megestrol acetate.
In recent years, megestrol acetate (an antineoplastic drug) has been used to improve appetite, weight, albumin levels, and MICS in patients receiving maintenance dialysis.1,4-6 Rammohan et al found significant increases in weight, BMI, body fat, triceps skinfold thickness, protein/energy intake, and serum albumin in 10 dialysis patients who took megestrol acetate (400 mg/d) for 16 weeks.4
Continue for megestrol acetate's effects >>
In a 20-week randomized, double-blind, placebo-controlled trial, Yeh et al found significant increases in weight, body fat, and fat-free mass in elderly hemodialysis patients receiving megestrol acetate (800 mg/d). The treatment group also demonstrated greater improvement in ability to exercise.5
Monfared and colleagues looked specifically at megestrol acetate’s effect on serum albumin levels in dialysis patients.1 Using a much lower dose (40 mg bid for two months), they found a significant increase in serum albumin in the treatment group. Although an increase in appetite was noted, the researchers did not observe any significant change in total weight following treatment.1
In a letter to the editor of the Journal of Renal Nutrition, Golebiewska et al reported their use of megestrol acetate in maintenance hemodialysis and peritoneal dialysis patients.6 Hypoalbuminemic patients were given megestrol acetate (160 mg/d). Significant increases in weight, BMI, subjective global assessment scores (a measure of nutritional status based on clinical indices such as weight, appetite, muscle, and fat mass), and serum albumin levels were seen. Only 12 of the 32 patients completed the study; the others dropped out due to adverse effects, including high intradialytic weight gain (the amount of fluid gained between dialysis sessions), dyspnea, diarrhea, and nausea.6
Currently, there is no consensus in the literature regarding the most effective dosage of megestrol acetate. Furthermore, evidence is lacking as to whether megestrol acetate–induced increases in appetite, oral intake, weight, and serum albumin level bestow any survival benefit or affect outcomes in dialysis patients.4 However, the increased sense of well-being a patient experiences when appetite returns and weight is restored may be worth the effort.
Luanne DiGuglielmo, MS, RD, CSR
DaVita Summit Renal Center
Mountainside, New Jersey
REFERENCES
1. Monfared A, Heidarzadeh A, Ghaffari M, Akbarpour M. Effect of megestrol acetate on serum albumin level in malnourished dialysis patients. J Renal Nutr. 2009;19(2):167-171.
2. Byham-Gray L, Stover J, Wiesen K. A clinical guide to nutrition care in kidney disease. Acad Nutr Diet. 2013.
3. White JV, Guenter P, Jensen G, Malone A, Schofield M; Academy of Nutrition and Dietetics Malnutrition Work Group; ASPEN Malnutrition Task Force; ASPEN Board of Directors. Consensus statement of the Academy of Nutrition and Dietetics/American Society for Parenteral and Enteral Nutrition: characteristics recommended for the identification and documentation of adult malnutrition (undernutrition) [erratum appears in J Acad Nutr Diet. 2012 Nov;112(11):1899].
J Acad Nutr Diet. 2012;112(5):730-738.
4. Rammohan M, Kalantar-Zedeh K, Liang A, Ghossein C. Megestrol acetate in a moderate dose for the treatment of malnutrition-inflammation complex in maintenance dialysis patients. J Ren Nutr. 2005;15(3):345-355.
5. Yeh S, Marandi M, Thode H Jr, et al. Report of a pilot, double blind, placebo-controlled study of megestrol acetate in elderly dialysis patients with cachexia. J Ren Nutr. 2010; 20(1):52-62.
6. Golebiewska JE, Lichodziejewska-Niemierko M, Aleksandrowicz-Wrona E, et al. Megestrol acetate use in hypoalbuminemic dialysis patients [comment]. J Ren Nutr. 2011;21(2): 200-202.
7. Bendik I, Friedel A, Roos FF, et al. Vitamin D: a critical and necessary micronutrient for human health. Front Physiol. 2014;5:248.
8. Cabone F, Mach F, Vuilleumier N, Montecucco F. Potential pathophysiological role for the vitamin D deficiency in essential hypertension. World J Cardiol. 2014;6(5):260-276.
9. Sypniewska G, Pollak J, Strozecki P, et al. 25-hydroxyvitamin D, biomarkers of endothelial dysfunction and subclinical organ damage in adults with hypertension. Am J Hypertens. 2014;27(1):114-121.
10. Vimaleswaran KS, Cavadino A, Berry DJ, et al. Association of vitamin D status with arterial blood pressure and hypertension risk: a mendelian randomisation study. Lancet Diabetes Endocrinol. 2014;2(9):719-729.
Q) Some of my CKD patients are malnourished; in fact, some of those on dialysis do not eat well and have low albumin levels. Previously in this column, it was stated that higher albumin levels (> 4 g/dL) confer survival benefits to dialysis patients. Should I consider prescribing megestrol acetate to improve appetite? If I do prescribe it, what dose is safe for CKD and dialysis patients?
Malnutrition affects one-third of dialysis patients,1 and malnutrition-inflammation complex syndrome (MICS) is common in those with stage 5 CKD. Albumin is used as an indicator of MICS in dialysis patients; however, since other factors (stress, infection, inflammation, comorbidities) affect nutritional status,2 serum albumin alone may not be sufficient to assess it.
In fact, a recent consensus statement on malnutrition from the Academy of Nutrition and Dietetics and the American Society for Parenteral and Enteral Nutrition excluded serum albumin as a diagnostic characteristic; the criteria included percentage of energy requirement, percentage of weight loss and time frame, loss of body fat and muscle mass, presence of edema, and reduced grip strength.3 These may be better measures of malnutrition in dialysis patients and could be used as criteria for determining when to prescribe an appetite stimulant, such as megestrol acetate.
In recent years, megestrol acetate (an antineoplastic drug) has been used to improve appetite, weight, albumin levels, and MICS in patients receiving maintenance dialysis.1,4-6 Rammohan et al found significant increases in weight, BMI, body fat, triceps skinfold thickness, protein/energy intake, and serum albumin in 10 dialysis patients who took megestrol acetate (400 mg/d) for 16 weeks.4
Continue for megestrol acetate's effects >>
In a 20-week randomized, double-blind, placebo-controlled trial, Yeh et al found significant increases in weight, body fat, and fat-free mass in elderly hemodialysis patients receiving megestrol acetate (800 mg/d). The treatment group also demonstrated greater improvement in ability to exercise.5
Monfared and colleagues looked specifically at megestrol acetate’s effect on serum albumin levels in dialysis patients.1 Using a much lower dose (40 mg bid for two months), they found a significant increase in serum albumin in the treatment group. Although an increase in appetite was noted, the researchers did not observe any significant change in total weight following treatment.1
In a letter to the editor of the Journal of Renal Nutrition, Golebiewska et al reported their use of megestrol acetate in maintenance hemodialysis and peritoneal dialysis patients.6 Hypoalbuminemic patients were given megestrol acetate (160 mg/d). Significant increases in weight, BMI, subjective global assessment scores (a measure of nutritional status based on clinical indices such as weight, appetite, muscle, and fat mass), and serum albumin levels were seen. Only 12 of the 32 patients completed the study; the others dropped out due to adverse effects, including high intradialytic weight gain (the amount of fluid gained between dialysis sessions), dyspnea, diarrhea, and nausea.6
Currently, there is no consensus in the literature regarding the most effective dosage of megestrol acetate. Furthermore, evidence is lacking as to whether megestrol acetate–induced increases in appetite, oral intake, weight, and serum albumin level bestow any survival benefit or affect outcomes in dialysis patients.4 However, the increased sense of well-being a patient experiences when appetite returns and weight is restored may be worth the effort.
Luanne DiGuglielmo, MS, RD, CSR
DaVita Summit Renal Center
Mountainside, New Jersey
REFERENCES
1. Monfared A, Heidarzadeh A, Ghaffari M, Akbarpour M. Effect of megestrol acetate on serum albumin level in malnourished dialysis patients. J Renal Nutr. 2009;19(2):167-171.
2. Byham-Gray L, Stover J, Wiesen K. A clinical guide to nutrition care in kidney disease. Acad Nutr Diet. 2013.
3. White JV, Guenter P, Jensen G, Malone A, Schofield M; Academy of Nutrition and Dietetics Malnutrition Work Group; ASPEN Malnutrition Task Force; ASPEN Board of Directors. Consensus statement of the Academy of Nutrition and Dietetics/American Society for Parenteral and Enteral Nutrition: characteristics recommended for the identification and documentation of adult malnutrition (undernutrition) [erratum appears in J Acad Nutr Diet. 2012 Nov;112(11):1899].
J Acad Nutr Diet. 2012;112(5):730-738.
4. Rammohan M, Kalantar-Zedeh K, Liang A, Ghossein C. Megestrol acetate in a moderate dose for the treatment of malnutrition-inflammation complex in maintenance dialysis patients. J Ren Nutr. 2005;15(3):345-355.
5. Yeh S, Marandi M, Thode H Jr, et al. Report of a pilot, double blind, placebo-controlled study of megestrol acetate in elderly dialysis patients with cachexia. J Ren Nutr. 2010; 20(1):52-62.
6. Golebiewska JE, Lichodziejewska-Niemierko M, Aleksandrowicz-Wrona E, et al. Megestrol acetate use in hypoalbuminemic dialysis patients [comment]. J Ren Nutr. 2011;21(2): 200-202.
7. Bendik I, Friedel A, Roos FF, et al. Vitamin D: a critical and necessary micronutrient for human health. Front Physiol. 2014;5:248.
8. Cabone F, Mach F, Vuilleumier N, Montecucco F. Potential pathophysiological role for the vitamin D deficiency in essential hypertension. World J Cardiol. 2014;6(5):260-276.
9. Sypniewska G, Pollak J, Strozecki P, et al. 25-hydroxyvitamin D, biomarkers of endothelial dysfunction and subclinical organ damage in adults with hypertension. Am J Hypertens. 2014;27(1):114-121.
10. Vimaleswaran KS, Cavadino A, Berry DJ, et al. Association of vitamin D status with arterial blood pressure and hypertension risk: a mendelian randomisation study. Lancet Diabetes Endocrinol. 2014;2(9):719-729.
Q) Some of my CKD patients are malnourished; in fact, some of those on dialysis do not eat well and have low albumin levels. Previously in this column, it was stated that higher albumin levels (> 4 g/dL) confer survival benefits to dialysis patients. Should I consider prescribing megestrol acetate to improve appetite? If I do prescribe it, what dose is safe for CKD and dialysis patients?
Malnutrition affects one-third of dialysis patients,1 and malnutrition-inflammation complex syndrome (MICS) is common in those with stage 5 CKD. Albumin is used as an indicator of MICS in dialysis patients; however, since other factors (stress, infection, inflammation, comorbidities) affect nutritional status,2 serum albumin alone may not be sufficient to assess it.
In fact, a recent consensus statement on malnutrition from the Academy of Nutrition and Dietetics and the American Society for Parenteral and Enteral Nutrition excluded serum albumin as a diagnostic characteristic; the criteria included percentage of energy requirement, percentage of weight loss and time frame, loss of body fat and muscle mass, presence of edema, and reduced grip strength.3 These may be better measures of malnutrition in dialysis patients and could be used as criteria for determining when to prescribe an appetite stimulant, such as megestrol acetate.
In recent years, megestrol acetate (an antineoplastic drug) has been used to improve appetite, weight, albumin levels, and MICS in patients receiving maintenance dialysis.1,4-6 Rammohan et al found significant increases in weight, BMI, body fat, triceps skinfold thickness, protein/energy intake, and serum albumin in 10 dialysis patients who took megestrol acetate (400 mg/d) for 16 weeks.4
Continue for megestrol acetate's effects >>
In a 20-week randomized, double-blind, placebo-controlled trial, Yeh et al found significant increases in weight, body fat, and fat-free mass in elderly hemodialysis patients receiving megestrol acetate (800 mg/d). The treatment group also demonstrated greater improvement in ability to exercise.5
Monfared and colleagues looked specifically at megestrol acetate’s effect on serum albumin levels in dialysis patients.1 Using a much lower dose (40 mg bid for two months), they found a significant increase in serum albumin in the treatment group. Although an increase in appetite was noted, the researchers did not observe any significant change in total weight following treatment.1
In a letter to the editor of the Journal of Renal Nutrition, Golebiewska et al reported their use of megestrol acetate in maintenance hemodialysis and peritoneal dialysis patients.6 Hypoalbuminemic patients were given megestrol acetate (160 mg/d). Significant increases in weight, BMI, subjective global assessment scores (a measure of nutritional status based on clinical indices such as weight, appetite, muscle, and fat mass), and serum albumin levels were seen. Only 12 of the 32 patients completed the study; the others dropped out due to adverse effects, including high intradialytic weight gain (the amount of fluid gained between dialysis sessions), dyspnea, diarrhea, and nausea.6
Currently, there is no consensus in the literature regarding the most effective dosage of megestrol acetate. Furthermore, evidence is lacking as to whether megestrol acetate–induced increases in appetite, oral intake, weight, and serum albumin level bestow any survival benefit or affect outcomes in dialysis patients.4 However, the increased sense of well-being a patient experiences when appetite returns and weight is restored may be worth the effort.
Luanne DiGuglielmo, MS, RD, CSR
DaVita Summit Renal Center
Mountainside, New Jersey
REFERENCES
1. Monfared A, Heidarzadeh A, Ghaffari M, Akbarpour M. Effect of megestrol acetate on serum albumin level in malnourished dialysis patients. J Renal Nutr. 2009;19(2):167-171.
2. Byham-Gray L, Stover J, Wiesen K. A clinical guide to nutrition care in kidney disease. Acad Nutr Diet. 2013.
3. White JV, Guenter P, Jensen G, Malone A, Schofield M; Academy of Nutrition and Dietetics Malnutrition Work Group; ASPEN Malnutrition Task Force; ASPEN Board of Directors. Consensus statement of the Academy of Nutrition and Dietetics/American Society for Parenteral and Enteral Nutrition: characteristics recommended for the identification and documentation of adult malnutrition (undernutrition) [erratum appears in J Acad Nutr Diet. 2012 Nov;112(11):1899].
J Acad Nutr Diet. 2012;112(5):730-738.
4. Rammohan M, Kalantar-Zedeh K, Liang A, Ghossein C. Megestrol acetate in a moderate dose for the treatment of malnutrition-inflammation complex in maintenance dialysis patients. J Ren Nutr. 2005;15(3):345-355.
5. Yeh S, Marandi M, Thode H Jr, et al. Report of a pilot, double blind, placebo-controlled study of megestrol acetate in elderly dialysis patients with cachexia. J Ren Nutr. 2010; 20(1):52-62.
6. Golebiewska JE, Lichodziejewska-Niemierko M, Aleksandrowicz-Wrona E, et al. Megestrol acetate use in hypoalbuminemic dialysis patients [comment]. J Ren Nutr. 2011;21(2): 200-202.
7. Bendik I, Friedel A, Roos FF, et al. Vitamin D: a critical and necessary micronutrient for human health. Front Physiol. 2014;5:248.
8. Cabone F, Mach F, Vuilleumier N, Montecucco F. Potential pathophysiological role for the vitamin D deficiency in essential hypertension. World J Cardiol. 2014;6(5):260-276.
9. Sypniewska G, Pollak J, Strozecki P, et al. 25-hydroxyvitamin D, biomarkers of endothelial dysfunction and subclinical organ damage in adults with hypertension. Am J Hypertens. 2014;27(1):114-121.
10. Vimaleswaran KS, Cavadino A, Berry DJ, et al. Association of vitamin D status with arterial blood pressure and hypertension risk: a mendelian randomisation study. Lancet Diabetes Endocrinol. 2014;2(9):719-729.
A teen with seizures, amnesia, and troubled family dynamics
CASE Seizures, amnesia
Ms. A, age 13, who has a history of seizures, presents to the emergency department (ED) with sudden onset of memory loss. Her family reports that she had been spending a normal evening at home with family and friends. After going to the bathroom, Ms. A became acutely confused and extremely upset, had slurred speech, and did not recognize anyone in the room except her mother.
Initial neurologic examination in the ED reports that Ms. A does not remember recent or remote past events. Her family denies any recent stressors.
Vital signs are within normal range. She has mild muscle soreness and gait instability, which is attributed to a presumed postictal phase. Her medication regimen includes: levetiracetam, 500 mg, 3 times a day; valproic acid, 1,000 mg/d; and oxcarbazepine, 2,400 mg/d, for seizure management.
Complete blood count and comprehensive metabolic panel are within normal limits. Pregnancy test is negative. Urine toxicology report is negative. Serum valproic acid level is 71 μg/mL; oxcarbazepine level, <2 μg/mL; ammonia level, 71 μg/dL (reference range, 15 to 45 μg/dL). Other than the aforementioned deficits, she is neurologically intact. The team thinks that her symptoms are part of a postictal phase of an unwitnessed seizure.
Ms. A is admitted to the inpatient medical unit for further work up. Along with the memory loss and seizures, she reports visual hallucinations.
What could be causing Ms. A’s amnesia?
a) a seizure disorder
b) malingering
c) posttraumatic stress disorder
d) traumatic brain injury
HISTORY Repeat ED visits
Ms. A’s mother reports that 3 years ago her daughter was treated for tics with quetiapine and aripiprazole, prescribed by a primary care physician. She received a short course of counseling 6 years ago after her sister was sexually abused by her grandfather. Approximately 6 months ago, Ms. A engaged in self-injurious behavior by cutting herself, and she briefly received counseling. There is no history of suicide attempts, psychiatric hospitalization, or a psychiatric diagnosis.
Medical and surgical history include viral meningitis at age 6 months. Medical records show a visit to the ED for abdominal pain after a classmate punched her in the abdomen, which resolved with supportive care. She was given a diagnosis of pediatric autoimmune neuropsychiatric disorders associated with streptococcal infections 6 years ago.
Ms. A developed multiple recurrent methicillin-resistant Staphylococcus aureus abscesses a year ago, which lasted for 4 months; it was noted that she was self-inoculating by scratching eczema. She had a possible syncopal episode 5 months ago, but the medical work-up was normal. The pediatric neurology service diagnosed and treated seizures 4 months ago.
Levetiracetam was prescribed after a possible syncopal episode followed by a tonic-clonic seizure. Because she was still having seizure-like episodes with a single antiepileptic drug (AED), oxcarbazepine, then valproic acid were added. Whether her seizures were generalized or partial was inconclusive. The seizures were followed by a postictal phase lasting 3 minutes to 1 hour. Her last generalized tonic-clonic seizure was 1 month before admission.
Ms. A had 3 MRI studies of the brain over the past 3 years, which showed consistent and unchanged multifocal punctate white matter lesions. The findings represented gliosis from an old perivascular inflammation, trauma, or ischemic damage. There is no history of traumatic brain injury.
Her perinatal history is unremarkable, with normal vaginal delivery at 36 weeks (pre-term birth). All developmental milestones were on target.
Ms. A lives at home with her mother, 6-year-old brother, and stepfather. Her parents are divorced, but her biological father has been involved in her upbringing. She is in seventh grade, but is home schooled after she withdrew from school because of multiple seizure episodes. Ms. A denied bullying at school although she had been punched by a peer. It was unclear if it was a single incident or bullying continued and she was hesitant to disclose it.
The authors’ observations
We focus on the amnesia because it has an acute onset and it seems this is the first time Ms. A presented with this symptom. There is no need to wait for neurology consultation, even though organic causes of amnesia need to be ruled out. Our plan is to develop rapport with Ms. A, and then administer a mental status examination focusing on memory assessment. We understand that, because Ms. A’s chief concern is amnesia, she might not be able to provide many details. We start the initial interview with the family in the patient’s room to understand family dynamics, and then interview Ms. A alone.
EVALUATION Memory problems
On initial psychiatric interview, Ms. A can recognize some of her family members. She is seen in clean attire, with short hair, lying in the bed with good eye contact and a calm demeanor. She seems to be difficult to engage because of her reserved nature.
Ms. A displays some psychomotor retardation. She reports her mood as tired, and her affect is flat and mood incongruent. She is alert and oriented to person only; not to place, time, or situation. She can do a simple spelling task, perform 5-minute recall of 3 words, complete serial 3 subtractions, repeat phrases, read aloud, focus on a coin task, and name simple objects. She does not compare similar objects or answer simple historical or factual questions.
Ms. A replies “I don’t know” to most historical questions, such as her birthday, favorite color, and family members; she does not answer when asked how many legs a dog has, who is the current or past president, what month the Fourth of July is in, or when Christmas is. She can complete some memory tasks on the Mini-Mental State Examination, but does not attempt many others. Ms. A says she is upset about her memory deficit, but her affect was flat. Her mood and her affect were incongruent. She describes a vision of a “girl with black holes [for eyes]” in the corner of her hospital room telling her not to believe anyone and that the interviewers are lying to her. Also, she reports that “the girl” tells her to hurt herself and others, but she is not going to act on the commands because she knows it is not the right thing to do. When we ask Ms. A about a history of substance abuse, she says she has never heard of drugs or alcohol.
Overall, she displays multiple apparent deficits in declarative memory, both episodic and semantic. Regarding non-declarative or procedural memory, she can dress herself, use the bathroom independently, order meals off the menu, and feed herself, among other routine tasks, without difficulty.
According to Ms. A’s mother, Ms. A has shown a decline in overall functioning and personality changes during the past 5 months. She started to cut herself superficially on her forearms 6 months ago and also tried to change her appearance with a new hairstyle when school started. She displayed noticeably intense and disturbing writings, artwork, and conversations with others over 3 to 4 months.
She started experiencing seizures, with 3 to 4 seizures a day; however, she could attend sleepovers seizure-free. She had prolonged periods of seizures lasting up to an hour, much longer than would be expected clinically. She also had requested to go to the cemetery for unclear reasons (because the spirit wanted her to visit), and was observed mumbling under her breath.
Six years ago, Ms. A’s 6-year-old sister tried to suffocate her infant brother. Child protective services was involved and the sister was hospitalized in a psychiatric facility, where she was given a diagnosis of bipolar disorder; she was then transferred to foster care, and later placed in residential treatment. Her mother relinquished her parental rights and gave custody of Ms. A’s sister to the state.
Ms. A’s mother has a history of depression, but her younger brother is healthy. There is no history of autism, attention problems, tics, substance abuse, brain tumor, or intellectual disabilities in the family.
Which diagnosis does Ms. A’s presentation and history suggest?
a) dissociative amnesia
b) factitious disorder imposed on self
c) conversion disorder (neurological symptom disorder)
d) psychosis not otherwise specified
e) malingering
The authors’ observations
The history of unwitnessed seizures, sudden onset of visual hallucinations, and transient amnesia points to a possible postictal cause. Selective amnesia brings up the question of whether psychological components are driving the symptoms.
Her psychotic symptoms appear to be mediated by anxiety and possibly related to the trauma of losing her only sister when her mother relinquished custody to the state; the circumstances might have aroused feelings of insecurity or fear of abandonment and raised questions about her mother’s love toward her. Her sister’s abuse by a family member might have created reticence to trust others. These background experiences could be intensely conflicting at this age when the second separation individuation process commences, especially in an emotionally immature adolescent.
OUTCOME Medication change
The neurology team recommends discontinuing levetiracetam because the visual hallucinations, mood disturbance, and personality change could be adverse effects of the drug. Because of generalized uncontrolled body movements with staring episodes and unresponsiveness, an EEG is ordered to rule out ongoing seizures.
Ms. A recognizes the psychosomatic medicine team members when they interview her again. The team employs consistent reassurance and a non-confrontational approach. She spends 3 days in the medical unit during which she reports that the frequency of visual and auditory hallucinations decreases and her memory symptoms resolve. Her 24-hour EEG is negative for seizure activity, and the 24-hour video EEG does not show any signs of epileptogenic foci. Ms. A’s family declines inpatient psychiatric hospitalization.
Because of gradual improvement in Ms. A’s symptoms and no imminent safety concerns, she is discharged home with valproic acid, 1,000 mg/d, and oxcarbazepine, 1,200 mg/d, and follow-up appointments with her primary care physician, a neurologist, and a psychiatrist.
The authors’ observations
Dissociative amnesia
Generalized dissociative amnesia is difficult to differentiate from factitious disorder or malingering. According to DSM-5, there is loss of episodic memory in dissociative amnesia, in which the person is unable to recall the stressful event after trauma (Table 1).1 Although there have been case reports of dissociative amnesia with loss of semantic and procedural memory, episodic memory is the last to return.2 In Ms. A’s case, there was no immediate basis to explain amnesia onset, although she had experienced the trauma of losing her sister. She had episodic and mostly semantic memory loss.
Although organic causes can precipitate amnesia,3 Ms. A’s EEG and MRI results did not reflect that. Patients with a dissociative disorder often report some physical, sexual, or emotional abuse.4 Although Ms. A did not report any abuse, it cannot be completely ruled out because of her sister’s history of abuse.
Suicidality or self-injurious behavior is common among adults with dissociative amnesia, although it is not well studied in children.4,5 Generally, the constellation of primary dissociative symptoms that patients develop are forgetfulness, fragmentation, and emotional numbing. Ms. A presented with some of these features; did she, in fact, have dissociative amnesia?
Factitious amnesia
Factious amnesia (Table 2)6 is a symptom of factious disorder in which amnesia appears with the motivation to assume a sick role.3 Ms. A’s amnesia garnered significant attention from her mother and other family members; this may have been related to insecurity in her family relationships because her sister was given up to the state. She also could be afraid of entering adolescence and leaving her sister behind. Did she want more time to bond with her mother? Did she experience emotional benefit from being cared for by medical professionals?7 Her affect during interviews was blunted and her attitude was nonchalant, and her multiple visits to the hospital since childhood for abdominal pain, abscesses (it isn’t clear whether the abscesses were related to self-injury and scratching), tics, seizures, and, recently, amnesia and hallucinations indicated some desire to occupy a sick role. Furthermore, the severity of her symptoms seemed to be increasing over time, from somatic to neurologic (seizure-like episodes) to significant and less frequent psychiatric symptoms (amnesia and hallucinations). One could speculate that her symptoms were escalating because she was not receiving the attention she needed.
Malingered amnesia
Although malingering is not a psychiatric diagnosis, it can be a focus of clinical attention. It is challenging to identify malingered cognitive impairments.8 Children often have difficulty malingering symptoms because they have limited understanding of the illness they are trying to simulate.9 Many malingerers do not want to participate in their medical work up and might exhibit a hostile attitude toward examiners (Table 26). Clinicians could rely on family to provide information regarding history and inconsistencies in clinical deficits.9 The clinical interview, mental status examination, and collateral information are crucial for identifying malingering.
Most of Ms. A’s seizure-like episodes happened in specific contexts, such as in school, but not at friends’ houses, raising the question of whether she is aware of her episodes. Ms. A’s grades are consistently good; because she is being home schooled, there is no secondary gain from not going to school. There is no other reason to speculate that she was malingering.
The inconsistency of Ms. A’s symptoms and her compliance with assessment and treatment did not reflect malingering. Interestingly, Ms. A’s amnesia was retrograde in nature. There have been more studies on malingered anterograde amnesia8 than on retrograde amnesia, making her presentation even more unusual.
Amnesia presenting as conversion disorder
Amnesia as a symptom of conversion disorder is referred as psychogenic amnesia; the memory loss mostly is isolated retrograde amnesia.10 Ms. A likely had unconsciously produced symptoms of non-epileptic seizures, followed by auditory and visual hallucinations not related to her seizures, and then later developed selective transient amnesia. Conversion disorder seemed to be the diagnosis most consistent with her indifference (“la belle indifference”) and the significant attention she gained from the acute memory loss (Table 3).1 It seemed that she developed multiple symptoms in progression leading toward a conversion disorder diagnosis. The question arises whether Ms. A’s presentation is a gradually increasing cry for help or reflects depressive or anxiety symptoms, which often are comorbid with conversion disorder.
FOLLOW-UP Suicide attempt
Ms. A has frequent visits to the ED with symptoms of syncope and seizures and undergoes medical work-up and multiple EEGs. A prolonged 5-day video EEG is performed to assess seizure episodes after AEDs were withdrawn, but no seizure activity is elicited. She also has an ED visit for recurrent tic emergence.
The last visit in the ED is for a suicide attempt with overdose of an unknown quantity of unspecified pills. Ms. A talks to a social worker, who reports that Ms. A needed answers to such questions as why her grandfather abused her sister? Could she have stopped them and made a difference for the family?
The authors’ observations
Conversion disorder arises from unconscious psychological conflicts, needs, or responses to trauma. Ms. A’s consistent conflict about her sister and grandfather’s relationship was evident from occasions when she tried to confide in hospital staff. During an ED visit, she reported her sister’s abuse to a staff member. Another time, while recovering from sedation, she spontaneously spoke about her sister’s abuse. When asked again, she said she did not remember saying it.
Freud said that patients develop conversion disorder to avoid unacceptable conflicting thoughts and feelings.10 It appeared that Ms. A was struggling with these questions because she brought them up again when she visited the ED after the suicide attempt.
Dissociative symptoms arise from unstable parenting and disciplining styles with variable family dynamics. Patients show extreme detachment and emotional unresponsiveness akin to attachment disorder.11 Ms. A had inconsistent parenting because both her stepfather and biological father were involved with her care. Her mother had relinquished her parental rights to her sister, which indicated some attachment issues.
Ms. A’s idea that her mother was indifferent stemmed from her uncaring approach toward her sister and not able to understand her emotionally. Her amnesia could be thought of as “I don’t know you because I don’t remember that I am related to you.” The traumas of infancy (referred to as hidden traumas) that were a result of parent-child mismatch of needs and availability at times of distress might not be obvious to the examiner.11
Although Ms. A’s infancy was reported to be unremarkable, there always is a question, especially in a consultation-liaison setting, of whether conversion disorder might be masking an attachment problem. Perhaps with long-term psychotherapy, an attachment issue would be revealed.
Excluding an organic cause or a neurologic disorder is important when diagnosing conversion disorder10; Ms. A’s negative neurologic tests favored a diagnosis of amnesia due to conversion disorder. It appears that, although Ms. A presented with “transient amnesia,” she had underlying psychiatric symptoms, likely depression or anxiety. We were concerned about possible psychiatric comorbidity and recommended inpatient hospitalization to clarify the diagnosis and provide intensive therapy, but her family declined. She may have received outpatient services, but that was not documented.
Bottom Line
Psychogenic amnesia can be a form of conversion disorder or a symptom of
malingering; can occur in dissociative disorder; and can be factitious in nature.
Regardless of the cause, the condition requires continuous close follow up. Although organic causes of amnesia should be ruled out, mental health care can help address comorbid psychiatric symptoms and might change the course of the illness.
Related Resources
• Byatt N, Toor R. Young, pregnant, ataxic—and jilted. Current Psychiatry. 2015;14(1):44-49.
• Leipsic J. A teen who is wasting away. Current Psychiatry. 2013;12(6):40-45.
Drug Brand Names
Aripiprazole • Abilify Quetiapine • Seroquel
Levetiracetam • Keppra Valproic acid • Depakote
Oxcarbazepine • Trileptal
Disclosures
The authors report no financial relationship with any company whose products are mentioned in this article or with manufacturers of competing products.
1. Diagnostic and statistical manual of mental disorders, 5th ed. Washington, DC: American Psychiatric Association; 2013.
2. van der Hart O, Nijenhuis E. Generalized dissociative amnesia: episodic, semantic and procedural memories lost and found. Aust N Z J Psychiatry. 2001;35(5):589-560.
3. Ehrlich S, Pfeiffer E, Salbach H, et al. Factitious disorder in children and adolescents: a retrospective study. Psychosomatics. 2008;45(5):392-398.
4. Sar V, Akyüz G, Kundakçi T, et al. Childhood trauma, dissociation, and psychiatric comorbidity in patients with conversion disorder. Am J Psychiatry. 2004;161(12):2271-2276.
5. Kisiel CL, Lyons JS. Dissociation as a mediator of psychopathology among sexually abused children and adolescents. Am J Psychiatry. 2001;158(7):1034-1039.
6. Worley CB, Feldman MD, Hamilton JC. The case of factitious disorder versus malingering. http://www.psychiatrictimes. com/munchausen-syndrome/case-factitious-disorder-versus-malingering. Published October 30, 2009. Accessed January 27, 2015.
7. Hagglund LA. Challenges in the treatment of factitious disorder: a case study. Arch Psychiatr Nurs. 2009;23(1):58-64.
8. Jenkins KG, Kapur N, Kopelman MD. Retrograde amnesia and malingering. Curr Opin Neurol. 2009;22(6):601-605.
9. Walker JS. Malingering in children: fibs and faking. Child Adolesc Psychiatr Clin N Am. 2011;20(3):547-556.
10. Levenson JL. Psychiatric issues in neurology, part 4: amnestic syndromes and conversion disorder. Primary Psychiatry. http://primarypsychiatry.com/psychiatric-issues-in-neurology-part-4-amnestic-syndromes-and-conversion-disorder. Published March 1, 2008. Accessed February 3, 2015.
11. Lyons-Ruth K, Dutra L, Schuder MR, et al. From infant attachment disorganization to adult dissociation: relational adaptations or traumatic experiences? Psychiatr Clin North Am. 2006;29(1):63-86, viii.
CASE Seizures, amnesia
Ms. A, age 13, who has a history of seizures, presents to the emergency department (ED) with sudden onset of memory loss. Her family reports that she had been spending a normal evening at home with family and friends. After going to the bathroom, Ms. A became acutely confused and extremely upset, had slurred speech, and did not recognize anyone in the room except her mother.
Initial neurologic examination in the ED reports that Ms. A does not remember recent or remote past events. Her family denies any recent stressors.
Vital signs are within normal range. She has mild muscle soreness and gait instability, which is attributed to a presumed postictal phase. Her medication regimen includes: levetiracetam, 500 mg, 3 times a day; valproic acid, 1,000 mg/d; and oxcarbazepine, 2,400 mg/d, for seizure management.
Complete blood count and comprehensive metabolic panel are within normal limits. Pregnancy test is negative. Urine toxicology report is negative. Serum valproic acid level is 71 μg/mL; oxcarbazepine level, <2 μg/mL; ammonia level, 71 μg/dL (reference range, 15 to 45 μg/dL). Other than the aforementioned deficits, she is neurologically intact. The team thinks that her symptoms are part of a postictal phase of an unwitnessed seizure.
Ms. A is admitted to the inpatient medical unit for further work up. Along with the memory loss and seizures, she reports visual hallucinations.
What could be causing Ms. A’s amnesia?
a) a seizure disorder
b) malingering
c) posttraumatic stress disorder
d) traumatic brain injury
HISTORY Repeat ED visits
Ms. A’s mother reports that 3 years ago her daughter was treated for tics with quetiapine and aripiprazole, prescribed by a primary care physician. She received a short course of counseling 6 years ago after her sister was sexually abused by her grandfather. Approximately 6 months ago, Ms. A engaged in self-injurious behavior by cutting herself, and she briefly received counseling. There is no history of suicide attempts, psychiatric hospitalization, or a psychiatric diagnosis.
Medical and surgical history include viral meningitis at age 6 months. Medical records show a visit to the ED for abdominal pain after a classmate punched her in the abdomen, which resolved with supportive care. She was given a diagnosis of pediatric autoimmune neuropsychiatric disorders associated with streptococcal infections 6 years ago.
Ms. A developed multiple recurrent methicillin-resistant Staphylococcus aureus abscesses a year ago, which lasted for 4 months; it was noted that she was self-inoculating by scratching eczema. She had a possible syncopal episode 5 months ago, but the medical work-up was normal. The pediatric neurology service diagnosed and treated seizures 4 months ago.
Levetiracetam was prescribed after a possible syncopal episode followed by a tonic-clonic seizure. Because she was still having seizure-like episodes with a single antiepileptic drug (AED), oxcarbazepine, then valproic acid were added. Whether her seizures were generalized or partial was inconclusive. The seizures were followed by a postictal phase lasting 3 minutes to 1 hour. Her last generalized tonic-clonic seizure was 1 month before admission.
Ms. A had 3 MRI studies of the brain over the past 3 years, which showed consistent and unchanged multifocal punctate white matter lesions. The findings represented gliosis from an old perivascular inflammation, trauma, or ischemic damage. There is no history of traumatic brain injury.
Her perinatal history is unremarkable, with normal vaginal delivery at 36 weeks (pre-term birth). All developmental milestones were on target.
Ms. A lives at home with her mother, 6-year-old brother, and stepfather. Her parents are divorced, but her biological father has been involved in her upbringing. She is in seventh grade, but is home schooled after she withdrew from school because of multiple seizure episodes. Ms. A denied bullying at school although she had been punched by a peer. It was unclear if it was a single incident or bullying continued and she was hesitant to disclose it.
The authors’ observations
We focus on the amnesia because it has an acute onset and it seems this is the first time Ms. A presented with this symptom. There is no need to wait for neurology consultation, even though organic causes of amnesia need to be ruled out. Our plan is to develop rapport with Ms. A, and then administer a mental status examination focusing on memory assessment. We understand that, because Ms. A’s chief concern is amnesia, she might not be able to provide many details. We start the initial interview with the family in the patient’s room to understand family dynamics, and then interview Ms. A alone.
EVALUATION Memory problems
On initial psychiatric interview, Ms. A can recognize some of her family members. She is seen in clean attire, with short hair, lying in the bed with good eye contact and a calm demeanor. She seems to be difficult to engage because of her reserved nature.
Ms. A displays some psychomotor retardation. She reports her mood as tired, and her affect is flat and mood incongruent. She is alert and oriented to person only; not to place, time, or situation. She can do a simple spelling task, perform 5-minute recall of 3 words, complete serial 3 subtractions, repeat phrases, read aloud, focus on a coin task, and name simple objects. She does not compare similar objects or answer simple historical or factual questions.
Ms. A replies “I don’t know” to most historical questions, such as her birthday, favorite color, and family members; she does not answer when asked how many legs a dog has, who is the current or past president, what month the Fourth of July is in, or when Christmas is. She can complete some memory tasks on the Mini-Mental State Examination, but does not attempt many others. Ms. A says she is upset about her memory deficit, but her affect was flat. Her mood and her affect were incongruent. She describes a vision of a “girl with black holes [for eyes]” in the corner of her hospital room telling her not to believe anyone and that the interviewers are lying to her. Also, she reports that “the girl” tells her to hurt herself and others, but she is not going to act on the commands because she knows it is not the right thing to do. When we ask Ms. A about a history of substance abuse, she says she has never heard of drugs or alcohol.
Overall, she displays multiple apparent deficits in declarative memory, both episodic and semantic. Regarding non-declarative or procedural memory, she can dress herself, use the bathroom independently, order meals off the menu, and feed herself, among other routine tasks, without difficulty.
According to Ms. A’s mother, Ms. A has shown a decline in overall functioning and personality changes during the past 5 months. She started to cut herself superficially on her forearms 6 months ago and also tried to change her appearance with a new hairstyle when school started. She displayed noticeably intense and disturbing writings, artwork, and conversations with others over 3 to 4 months.
She started experiencing seizures, with 3 to 4 seizures a day; however, she could attend sleepovers seizure-free. She had prolonged periods of seizures lasting up to an hour, much longer than would be expected clinically. She also had requested to go to the cemetery for unclear reasons (because the spirit wanted her to visit), and was observed mumbling under her breath.
Six years ago, Ms. A’s 6-year-old sister tried to suffocate her infant brother. Child protective services was involved and the sister was hospitalized in a psychiatric facility, where she was given a diagnosis of bipolar disorder; she was then transferred to foster care, and later placed in residential treatment. Her mother relinquished her parental rights and gave custody of Ms. A’s sister to the state.
Ms. A’s mother has a history of depression, but her younger brother is healthy. There is no history of autism, attention problems, tics, substance abuse, brain tumor, or intellectual disabilities in the family.
Which diagnosis does Ms. A’s presentation and history suggest?
a) dissociative amnesia
b) factitious disorder imposed on self
c) conversion disorder (neurological symptom disorder)
d) psychosis not otherwise specified
e) malingering
The authors’ observations
The history of unwitnessed seizures, sudden onset of visual hallucinations, and transient amnesia points to a possible postictal cause. Selective amnesia brings up the question of whether psychological components are driving the symptoms.
Her psychotic symptoms appear to be mediated by anxiety and possibly related to the trauma of losing her only sister when her mother relinquished custody to the state; the circumstances might have aroused feelings of insecurity or fear of abandonment and raised questions about her mother’s love toward her. Her sister’s abuse by a family member might have created reticence to trust others. These background experiences could be intensely conflicting at this age when the second separation individuation process commences, especially in an emotionally immature adolescent.
OUTCOME Medication change
The neurology team recommends discontinuing levetiracetam because the visual hallucinations, mood disturbance, and personality change could be adverse effects of the drug. Because of generalized uncontrolled body movements with staring episodes and unresponsiveness, an EEG is ordered to rule out ongoing seizures.
Ms. A recognizes the psychosomatic medicine team members when they interview her again. The team employs consistent reassurance and a non-confrontational approach. She spends 3 days in the medical unit during which she reports that the frequency of visual and auditory hallucinations decreases and her memory symptoms resolve. Her 24-hour EEG is negative for seizure activity, and the 24-hour video EEG does not show any signs of epileptogenic foci. Ms. A’s family declines inpatient psychiatric hospitalization.
Because of gradual improvement in Ms. A’s symptoms and no imminent safety concerns, she is discharged home with valproic acid, 1,000 mg/d, and oxcarbazepine, 1,200 mg/d, and follow-up appointments with her primary care physician, a neurologist, and a psychiatrist.
The authors’ observations
Dissociative amnesia
Generalized dissociative amnesia is difficult to differentiate from factitious disorder or malingering. According to DSM-5, there is loss of episodic memory in dissociative amnesia, in which the person is unable to recall the stressful event after trauma (Table 1).1 Although there have been case reports of dissociative amnesia with loss of semantic and procedural memory, episodic memory is the last to return.2 In Ms. A’s case, there was no immediate basis to explain amnesia onset, although she had experienced the trauma of losing her sister. She had episodic and mostly semantic memory loss.
Although organic causes can precipitate amnesia,3 Ms. A’s EEG and MRI results did not reflect that. Patients with a dissociative disorder often report some physical, sexual, or emotional abuse.4 Although Ms. A did not report any abuse, it cannot be completely ruled out because of her sister’s history of abuse.
Suicidality or self-injurious behavior is common among adults with dissociative amnesia, although it is not well studied in children.4,5 Generally, the constellation of primary dissociative symptoms that patients develop are forgetfulness, fragmentation, and emotional numbing. Ms. A presented with some of these features; did she, in fact, have dissociative amnesia?
Factitious amnesia
Factious amnesia (Table 2)6 is a symptom of factious disorder in which amnesia appears with the motivation to assume a sick role.3 Ms. A’s amnesia garnered significant attention from her mother and other family members; this may have been related to insecurity in her family relationships because her sister was given up to the state. She also could be afraid of entering adolescence and leaving her sister behind. Did she want more time to bond with her mother? Did she experience emotional benefit from being cared for by medical professionals?7 Her affect during interviews was blunted and her attitude was nonchalant, and her multiple visits to the hospital since childhood for abdominal pain, abscesses (it isn’t clear whether the abscesses were related to self-injury and scratching), tics, seizures, and, recently, amnesia and hallucinations indicated some desire to occupy a sick role. Furthermore, the severity of her symptoms seemed to be increasing over time, from somatic to neurologic (seizure-like episodes) to significant and less frequent psychiatric symptoms (amnesia and hallucinations). One could speculate that her symptoms were escalating because she was not receiving the attention she needed.
Malingered amnesia
Although malingering is not a psychiatric diagnosis, it can be a focus of clinical attention. It is challenging to identify malingered cognitive impairments.8 Children often have difficulty malingering symptoms because they have limited understanding of the illness they are trying to simulate.9 Many malingerers do not want to participate in their medical work up and might exhibit a hostile attitude toward examiners (Table 26). Clinicians could rely on family to provide information regarding history and inconsistencies in clinical deficits.9 The clinical interview, mental status examination, and collateral information are crucial for identifying malingering.
Most of Ms. A’s seizure-like episodes happened in specific contexts, such as in school, but not at friends’ houses, raising the question of whether she is aware of her episodes. Ms. A’s grades are consistently good; because she is being home schooled, there is no secondary gain from not going to school. There is no other reason to speculate that she was malingering.
The inconsistency of Ms. A’s symptoms and her compliance with assessment and treatment did not reflect malingering. Interestingly, Ms. A’s amnesia was retrograde in nature. There have been more studies on malingered anterograde amnesia8 than on retrograde amnesia, making her presentation even more unusual.
Amnesia presenting as conversion disorder
Amnesia as a symptom of conversion disorder is referred as psychogenic amnesia; the memory loss mostly is isolated retrograde amnesia.10 Ms. A likely had unconsciously produced symptoms of non-epileptic seizures, followed by auditory and visual hallucinations not related to her seizures, and then later developed selective transient amnesia. Conversion disorder seemed to be the diagnosis most consistent with her indifference (“la belle indifference”) and the significant attention she gained from the acute memory loss (Table 3).1 It seemed that she developed multiple symptoms in progression leading toward a conversion disorder diagnosis. The question arises whether Ms. A’s presentation is a gradually increasing cry for help or reflects depressive or anxiety symptoms, which often are comorbid with conversion disorder.
FOLLOW-UP Suicide attempt
Ms. A has frequent visits to the ED with symptoms of syncope and seizures and undergoes medical work-up and multiple EEGs. A prolonged 5-day video EEG is performed to assess seizure episodes after AEDs were withdrawn, but no seizure activity is elicited. She also has an ED visit for recurrent tic emergence.
The last visit in the ED is for a suicide attempt with overdose of an unknown quantity of unspecified pills. Ms. A talks to a social worker, who reports that Ms. A needed answers to such questions as why her grandfather abused her sister? Could she have stopped them and made a difference for the family?
The authors’ observations
Conversion disorder arises from unconscious psychological conflicts, needs, or responses to trauma. Ms. A’s consistent conflict about her sister and grandfather’s relationship was evident from occasions when she tried to confide in hospital staff. During an ED visit, she reported her sister’s abuse to a staff member. Another time, while recovering from sedation, she spontaneously spoke about her sister’s abuse. When asked again, she said she did not remember saying it.
Freud said that patients develop conversion disorder to avoid unacceptable conflicting thoughts and feelings.10 It appeared that Ms. A was struggling with these questions because she brought them up again when she visited the ED after the suicide attempt.
Dissociative symptoms arise from unstable parenting and disciplining styles with variable family dynamics. Patients show extreme detachment and emotional unresponsiveness akin to attachment disorder.11 Ms. A had inconsistent parenting because both her stepfather and biological father were involved with her care. Her mother had relinquished her parental rights to her sister, which indicated some attachment issues.
Ms. A’s idea that her mother was indifferent stemmed from her uncaring approach toward her sister and not able to understand her emotionally. Her amnesia could be thought of as “I don’t know you because I don’t remember that I am related to you.” The traumas of infancy (referred to as hidden traumas) that were a result of parent-child mismatch of needs and availability at times of distress might not be obvious to the examiner.11
Although Ms. A’s infancy was reported to be unremarkable, there always is a question, especially in a consultation-liaison setting, of whether conversion disorder might be masking an attachment problem. Perhaps with long-term psychotherapy, an attachment issue would be revealed.
Excluding an organic cause or a neurologic disorder is important when diagnosing conversion disorder10; Ms. A’s negative neurologic tests favored a diagnosis of amnesia due to conversion disorder. It appears that, although Ms. A presented with “transient amnesia,” she had underlying psychiatric symptoms, likely depression or anxiety. We were concerned about possible psychiatric comorbidity and recommended inpatient hospitalization to clarify the diagnosis and provide intensive therapy, but her family declined. She may have received outpatient services, but that was not documented.
Bottom Line
Psychogenic amnesia can be a form of conversion disorder or a symptom of
malingering; can occur in dissociative disorder; and can be factitious in nature.
Regardless of the cause, the condition requires continuous close follow up. Although organic causes of amnesia should be ruled out, mental health care can help address comorbid psychiatric symptoms and might change the course of the illness.
Related Resources
• Byatt N, Toor R. Young, pregnant, ataxic—and jilted. Current Psychiatry. 2015;14(1):44-49.
• Leipsic J. A teen who is wasting away. Current Psychiatry. 2013;12(6):40-45.
Drug Brand Names
Aripiprazole • Abilify Quetiapine • Seroquel
Levetiracetam • Keppra Valproic acid • Depakote
Oxcarbazepine • Trileptal
Disclosures
The authors report no financial relationship with any company whose products are mentioned in this article or with manufacturers of competing products.
CASE Seizures, amnesia
Ms. A, age 13, who has a history of seizures, presents to the emergency department (ED) with sudden onset of memory loss. Her family reports that she had been spending a normal evening at home with family and friends. After going to the bathroom, Ms. A became acutely confused and extremely upset, had slurred speech, and did not recognize anyone in the room except her mother.
Initial neurologic examination in the ED reports that Ms. A does not remember recent or remote past events. Her family denies any recent stressors.
Vital signs are within normal range. She has mild muscle soreness and gait instability, which is attributed to a presumed postictal phase. Her medication regimen includes: levetiracetam, 500 mg, 3 times a day; valproic acid, 1,000 mg/d; and oxcarbazepine, 2,400 mg/d, for seizure management.
Complete blood count and comprehensive metabolic panel are within normal limits. Pregnancy test is negative. Urine toxicology report is negative. Serum valproic acid level is 71 μg/mL; oxcarbazepine level, <2 μg/mL; ammonia level, 71 μg/dL (reference range, 15 to 45 μg/dL). Other than the aforementioned deficits, she is neurologically intact. The team thinks that her symptoms are part of a postictal phase of an unwitnessed seizure.
Ms. A is admitted to the inpatient medical unit for further work up. Along with the memory loss and seizures, she reports visual hallucinations.
What could be causing Ms. A’s amnesia?
a) a seizure disorder
b) malingering
c) posttraumatic stress disorder
d) traumatic brain injury
HISTORY Repeat ED visits
Ms. A’s mother reports that 3 years ago her daughter was treated for tics with quetiapine and aripiprazole, prescribed by a primary care physician. She received a short course of counseling 6 years ago after her sister was sexually abused by her grandfather. Approximately 6 months ago, Ms. A engaged in self-injurious behavior by cutting herself, and she briefly received counseling. There is no history of suicide attempts, psychiatric hospitalization, or a psychiatric diagnosis.
Medical and surgical history include viral meningitis at age 6 months. Medical records show a visit to the ED for abdominal pain after a classmate punched her in the abdomen, which resolved with supportive care. She was given a diagnosis of pediatric autoimmune neuropsychiatric disorders associated with streptococcal infections 6 years ago.
Ms. A developed multiple recurrent methicillin-resistant Staphylococcus aureus abscesses a year ago, which lasted for 4 months; it was noted that she was self-inoculating by scratching eczema. She had a possible syncopal episode 5 months ago, but the medical work-up was normal. The pediatric neurology service diagnosed and treated seizures 4 months ago.
Levetiracetam was prescribed after a possible syncopal episode followed by a tonic-clonic seizure. Because she was still having seizure-like episodes with a single antiepileptic drug (AED), oxcarbazepine, then valproic acid were added. Whether her seizures were generalized or partial was inconclusive. The seizures were followed by a postictal phase lasting 3 minutes to 1 hour. Her last generalized tonic-clonic seizure was 1 month before admission.
Ms. A had 3 MRI studies of the brain over the past 3 years, which showed consistent and unchanged multifocal punctate white matter lesions. The findings represented gliosis from an old perivascular inflammation, trauma, or ischemic damage. There is no history of traumatic brain injury.
Her perinatal history is unremarkable, with normal vaginal delivery at 36 weeks (pre-term birth). All developmental milestones were on target.
Ms. A lives at home with her mother, 6-year-old brother, and stepfather. Her parents are divorced, but her biological father has been involved in her upbringing. She is in seventh grade, but is home schooled after she withdrew from school because of multiple seizure episodes. Ms. A denied bullying at school although she had been punched by a peer. It was unclear if it was a single incident or bullying continued and she was hesitant to disclose it.
The authors’ observations
We focus on the amnesia because it has an acute onset and it seems this is the first time Ms. A presented with this symptom. There is no need to wait for neurology consultation, even though organic causes of amnesia need to be ruled out. Our plan is to develop rapport with Ms. A, and then administer a mental status examination focusing on memory assessment. We understand that, because Ms. A’s chief concern is amnesia, she might not be able to provide many details. We start the initial interview with the family in the patient’s room to understand family dynamics, and then interview Ms. A alone.
EVALUATION Memory problems
On initial psychiatric interview, Ms. A can recognize some of her family members. She is seen in clean attire, with short hair, lying in the bed with good eye contact and a calm demeanor. She seems to be difficult to engage because of her reserved nature.
Ms. A displays some psychomotor retardation. She reports her mood as tired, and her affect is flat and mood incongruent. She is alert and oriented to person only; not to place, time, or situation. She can do a simple spelling task, perform 5-minute recall of 3 words, complete serial 3 subtractions, repeat phrases, read aloud, focus on a coin task, and name simple objects. She does not compare similar objects or answer simple historical or factual questions.
Ms. A replies “I don’t know” to most historical questions, such as her birthday, favorite color, and family members; she does not answer when asked how many legs a dog has, who is the current or past president, what month the Fourth of July is in, or when Christmas is. She can complete some memory tasks on the Mini-Mental State Examination, but does not attempt many others. Ms. A says she is upset about her memory deficit, but her affect was flat. Her mood and her affect were incongruent. She describes a vision of a “girl with black holes [for eyes]” in the corner of her hospital room telling her not to believe anyone and that the interviewers are lying to her. Also, she reports that “the girl” tells her to hurt herself and others, but she is not going to act on the commands because she knows it is not the right thing to do. When we ask Ms. A about a history of substance abuse, she says she has never heard of drugs or alcohol.
Overall, she displays multiple apparent deficits in declarative memory, both episodic and semantic. Regarding non-declarative or procedural memory, she can dress herself, use the bathroom independently, order meals off the menu, and feed herself, among other routine tasks, without difficulty.
According to Ms. A’s mother, Ms. A has shown a decline in overall functioning and personality changes during the past 5 months. She started to cut herself superficially on her forearms 6 months ago and also tried to change her appearance with a new hairstyle when school started. She displayed noticeably intense and disturbing writings, artwork, and conversations with others over 3 to 4 months.
She started experiencing seizures, with 3 to 4 seizures a day; however, she could attend sleepovers seizure-free. She had prolonged periods of seizures lasting up to an hour, much longer than would be expected clinically. She also had requested to go to the cemetery for unclear reasons (because the spirit wanted her to visit), and was observed mumbling under her breath.
Six years ago, Ms. A’s 6-year-old sister tried to suffocate her infant brother. Child protective services was involved and the sister was hospitalized in a psychiatric facility, where she was given a diagnosis of bipolar disorder; she was then transferred to foster care, and later placed in residential treatment. Her mother relinquished her parental rights and gave custody of Ms. A’s sister to the state.
Ms. A’s mother has a history of depression, but her younger brother is healthy. There is no history of autism, attention problems, tics, substance abuse, brain tumor, or intellectual disabilities in the family.
Which diagnosis does Ms. A’s presentation and history suggest?
a) dissociative amnesia
b) factitious disorder imposed on self
c) conversion disorder (neurological symptom disorder)
d) psychosis not otherwise specified
e) malingering
The authors’ observations
The history of unwitnessed seizures, sudden onset of visual hallucinations, and transient amnesia points to a possible postictal cause. Selective amnesia brings up the question of whether psychological components are driving the symptoms.
Her psychotic symptoms appear to be mediated by anxiety and possibly related to the trauma of losing her only sister when her mother relinquished custody to the state; the circumstances might have aroused feelings of insecurity or fear of abandonment and raised questions about her mother’s love toward her. Her sister’s abuse by a family member might have created reticence to trust others. These background experiences could be intensely conflicting at this age when the second separation individuation process commences, especially in an emotionally immature adolescent.
OUTCOME Medication change
The neurology team recommends discontinuing levetiracetam because the visual hallucinations, mood disturbance, and personality change could be adverse effects of the drug. Because of generalized uncontrolled body movements with staring episodes and unresponsiveness, an EEG is ordered to rule out ongoing seizures.
Ms. A recognizes the psychosomatic medicine team members when they interview her again. The team employs consistent reassurance and a non-confrontational approach. She spends 3 days in the medical unit during which she reports that the frequency of visual and auditory hallucinations decreases and her memory symptoms resolve. Her 24-hour EEG is negative for seizure activity, and the 24-hour video EEG does not show any signs of epileptogenic foci. Ms. A’s family declines inpatient psychiatric hospitalization.
Because of gradual improvement in Ms. A’s symptoms and no imminent safety concerns, she is discharged home with valproic acid, 1,000 mg/d, and oxcarbazepine, 1,200 mg/d, and follow-up appointments with her primary care physician, a neurologist, and a psychiatrist.
The authors’ observations
Dissociative amnesia
Generalized dissociative amnesia is difficult to differentiate from factitious disorder or malingering. According to DSM-5, there is loss of episodic memory in dissociative amnesia, in which the person is unable to recall the stressful event after trauma (Table 1).1 Although there have been case reports of dissociative amnesia with loss of semantic and procedural memory, episodic memory is the last to return.2 In Ms. A’s case, there was no immediate basis to explain amnesia onset, although she had experienced the trauma of losing her sister. She had episodic and mostly semantic memory loss.
Although organic causes can precipitate amnesia,3 Ms. A’s EEG and MRI results did not reflect that. Patients with a dissociative disorder often report some physical, sexual, or emotional abuse.4 Although Ms. A did not report any abuse, it cannot be completely ruled out because of her sister’s history of abuse.
Suicidality or self-injurious behavior is common among adults with dissociative amnesia, although it is not well studied in children.4,5 Generally, the constellation of primary dissociative symptoms that patients develop are forgetfulness, fragmentation, and emotional numbing. Ms. A presented with some of these features; did she, in fact, have dissociative amnesia?
Factitious amnesia
Factious amnesia (Table 2)6 is a symptom of factious disorder in which amnesia appears with the motivation to assume a sick role.3 Ms. A’s amnesia garnered significant attention from her mother and other family members; this may have been related to insecurity in her family relationships because her sister was given up to the state. She also could be afraid of entering adolescence and leaving her sister behind. Did she want more time to bond with her mother? Did she experience emotional benefit from being cared for by medical professionals?7 Her affect during interviews was blunted and her attitude was nonchalant, and her multiple visits to the hospital since childhood for abdominal pain, abscesses (it isn’t clear whether the abscesses were related to self-injury and scratching), tics, seizures, and, recently, amnesia and hallucinations indicated some desire to occupy a sick role. Furthermore, the severity of her symptoms seemed to be increasing over time, from somatic to neurologic (seizure-like episodes) to significant and less frequent psychiatric symptoms (amnesia and hallucinations). One could speculate that her symptoms were escalating because she was not receiving the attention she needed.
Malingered amnesia
Although malingering is not a psychiatric diagnosis, it can be a focus of clinical attention. It is challenging to identify malingered cognitive impairments.8 Children often have difficulty malingering symptoms because they have limited understanding of the illness they are trying to simulate.9 Many malingerers do not want to participate in their medical work up and might exhibit a hostile attitude toward examiners (Table 26). Clinicians could rely on family to provide information regarding history and inconsistencies in clinical deficits.9 The clinical interview, mental status examination, and collateral information are crucial for identifying malingering.
Most of Ms. A’s seizure-like episodes happened in specific contexts, such as in school, but not at friends’ houses, raising the question of whether she is aware of her episodes. Ms. A’s grades are consistently good; because she is being home schooled, there is no secondary gain from not going to school. There is no other reason to speculate that she was malingering.
The inconsistency of Ms. A’s symptoms and her compliance with assessment and treatment did not reflect malingering. Interestingly, Ms. A’s amnesia was retrograde in nature. There have been more studies on malingered anterograde amnesia8 than on retrograde amnesia, making her presentation even more unusual.
Amnesia presenting as conversion disorder
Amnesia as a symptom of conversion disorder is referred as psychogenic amnesia; the memory loss mostly is isolated retrograde amnesia.10 Ms. A likely had unconsciously produced symptoms of non-epileptic seizures, followed by auditory and visual hallucinations not related to her seizures, and then later developed selective transient amnesia. Conversion disorder seemed to be the diagnosis most consistent with her indifference (“la belle indifference”) and the significant attention she gained from the acute memory loss (Table 3).1 It seemed that she developed multiple symptoms in progression leading toward a conversion disorder diagnosis. The question arises whether Ms. A’s presentation is a gradually increasing cry for help or reflects depressive or anxiety symptoms, which often are comorbid with conversion disorder.
FOLLOW-UP Suicide attempt
Ms. A has frequent visits to the ED with symptoms of syncope and seizures and undergoes medical work-up and multiple EEGs. A prolonged 5-day video EEG is performed to assess seizure episodes after AEDs were withdrawn, but no seizure activity is elicited. She also has an ED visit for recurrent tic emergence.
The last visit in the ED is for a suicide attempt with overdose of an unknown quantity of unspecified pills. Ms. A talks to a social worker, who reports that Ms. A needed answers to such questions as why her grandfather abused her sister? Could she have stopped them and made a difference for the family?
The authors’ observations
Conversion disorder arises from unconscious psychological conflicts, needs, or responses to trauma. Ms. A’s consistent conflict about her sister and grandfather’s relationship was evident from occasions when she tried to confide in hospital staff. During an ED visit, she reported her sister’s abuse to a staff member. Another time, while recovering from sedation, she spontaneously spoke about her sister’s abuse. When asked again, she said she did not remember saying it.
Freud said that patients develop conversion disorder to avoid unacceptable conflicting thoughts and feelings.10 It appeared that Ms. A was struggling with these questions because she brought them up again when she visited the ED after the suicide attempt.
Dissociative symptoms arise from unstable parenting and disciplining styles with variable family dynamics. Patients show extreme detachment and emotional unresponsiveness akin to attachment disorder.11 Ms. A had inconsistent parenting because both her stepfather and biological father were involved with her care. Her mother had relinquished her parental rights to her sister, which indicated some attachment issues.
Ms. A’s idea that her mother was indifferent stemmed from her uncaring approach toward her sister and not able to understand her emotionally. Her amnesia could be thought of as “I don’t know you because I don’t remember that I am related to you.” The traumas of infancy (referred to as hidden traumas) that were a result of parent-child mismatch of needs and availability at times of distress might not be obvious to the examiner.11
Although Ms. A’s infancy was reported to be unremarkable, there always is a question, especially in a consultation-liaison setting, of whether conversion disorder might be masking an attachment problem. Perhaps with long-term psychotherapy, an attachment issue would be revealed.
Excluding an organic cause or a neurologic disorder is important when diagnosing conversion disorder10; Ms. A’s negative neurologic tests favored a diagnosis of amnesia due to conversion disorder. It appears that, although Ms. A presented with “transient amnesia,” she had underlying psychiatric symptoms, likely depression or anxiety. We were concerned about possible psychiatric comorbidity and recommended inpatient hospitalization to clarify the diagnosis and provide intensive therapy, but her family declined. She may have received outpatient services, but that was not documented.
Bottom Line
Psychogenic amnesia can be a form of conversion disorder or a symptom of
malingering; can occur in dissociative disorder; and can be factitious in nature.
Regardless of the cause, the condition requires continuous close follow up. Although organic causes of amnesia should be ruled out, mental health care can help address comorbid psychiatric symptoms and might change the course of the illness.
Related Resources
• Byatt N, Toor R. Young, pregnant, ataxic—and jilted. Current Psychiatry. 2015;14(1):44-49.
• Leipsic J. A teen who is wasting away. Current Psychiatry. 2013;12(6):40-45.
Drug Brand Names
Aripiprazole • Abilify Quetiapine • Seroquel
Levetiracetam • Keppra Valproic acid • Depakote
Oxcarbazepine • Trileptal
Disclosures
The authors report no financial relationship with any company whose products are mentioned in this article or with manufacturers of competing products.
1. Diagnostic and statistical manual of mental disorders, 5th ed. Washington, DC: American Psychiatric Association; 2013.
2. van der Hart O, Nijenhuis E. Generalized dissociative amnesia: episodic, semantic and procedural memories lost and found. Aust N Z J Psychiatry. 2001;35(5):589-560.
3. Ehrlich S, Pfeiffer E, Salbach H, et al. Factitious disorder in children and adolescents: a retrospective study. Psychosomatics. 2008;45(5):392-398.
4. Sar V, Akyüz G, Kundakçi T, et al. Childhood trauma, dissociation, and psychiatric comorbidity in patients with conversion disorder. Am J Psychiatry. 2004;161(12):2271-2276.
5. Kisiel CL, Lyons JS. Dissociation as a mediator of psychopathology among sexually abused children and adolescents. Am J Psychiatry. 2001;158(7):1034-1039.
6. Worley CB, Feldman MD, Hamilton JC. The case of factitious disorder versus malingering. http://www.psychiatrictimes. com/munchausen-syndrome/case-factitious-disorder-versus-malingering. Published October 30, 2009. Accessed January 27, 2015.
7. Hagglund LA. Challenges in the treatment of factitious disorder: a case study. Arch Psychiatr Nurs. 2009;23(1):58-64.
8. Jenkins KG, Kapur N, Kopelman MD. Retrograde amnesia and malingering. Curr Opin Neurol. 2009;22(6):601-605.
9. Walker JS. Malingering in children: fibs and faking. Child Adolesc Psychiatr Clin N Am. 2011;20(3):547-556.
10. Levenson JL. Psychiatric issues in neurology, part 4: amnestic syndromes and conversion disorder. Primary Psychiatry. http://primarypsychiatry.com/psychiatric-issues-in-neurology-part-4-amnestic-syndromes-and-conversion-disorder. Published March 1, 2008. Accessed February 3, 2015.
11. Lyons-Ruth K, Dutra L, Schuder MR, et al. From infant attachment disorganization to adult dissociation: relational adaptations or traumatic experiences? Psychiatr Clin North Am. 2006;29(1):63-86, viii.
1. Diagnostic and statistical manual of mental disorders, 5th ed. Washington, DC: American Psychiatric Association; 2013.
2. van der Hart O, Nijenhuis E. Generalized dissociative amnesia: episodic, semantic and procedural memories lost and found. Aust N Z J Psychiatry. 2001;35(5):589-560.
3. Ehrlich S, Pfeiffer E, Salbach H, et al. Factitious disorder in children and adolescents: a retrospective study. Psychosomatics. 2008;45(5):392-398.
4. Sar V, Akyüz G, Kundakçi T, et al. Childhood trauma, dissociation, and psychiatric comorbidity in patients with conversion disorder. Am J Psychiatry. 2004;161(12):2271-2276.
5. Kisiel CL, Lyons JS. Dissociation as a mediator of psychopathology among sexually abused children and adolescents. Am J Psychiatry. 2001;158(7):1034-1039.
6. Worley CB, Feldman MD, Hamilton JC. The case of factitious disorder versus malingering. http://www.psychiatrictimes. com/munchausen-syndrome/case-factitious-disorder-versus-malingering. Published October 30, 2009. Accessed January 27, 2015.
7. Hagglund LA. Challenges in the treatment of factitious disorder: a case study. Arch Psychiatr Nurs. 2009;23(1):58-64.
8. Jenkins KG, Kapur N, Kopelman MD. Retrograde amnesia and malingering. Curr Opin Neurol. 2009;22(6):601-605.
9. Walker JS. Malingering in children: fibs and faking. Child Adolesc Psychiatr Clin N Am. 2011;20(3):547-556.
10. Levenson JL. Psychiatric issues in neurology, part 4: amnestic syndromes and conversion disorder. Primary Psychiatry. http://primarypsychiatry.com/psychiatric-issues-in-neurology-part-4-amnestic-syndromes-and-conversion-disorder. Published March 1, 2008. Accessed February 3, 2015.
11. Lyons-Ruth K, Dutra L, Schuder MR, et al. From infant attachment disorganization to adult dissociation: relational adaptations or traumatic experiences? Psychiatr Clin North Am. 2006;29(1):63-86, viii.
Lisdexamfetamine for binge eating disorder: New indication
Lisdexamfetamine, approved by the FDA in 2007 for attention-deficit/hyperactivity disorder (ADHD), has a new indication: binge eating disorder (BED) (Table 1). BED is characterized by recurrent episodes of consuming a large amount of food in a short time. A prodrug of amphetamine, lisdexamfetamine is a Schedule-II controlled substance, with a high potential for abuse and the risk of severe psychological or physical dependence.
Lisdexamfetamine is not indicated for weight loss or obesity.
Dosage
For BED, the initial dosage of lisdexamfetamine is 30 mg/d in the morning, titrated by 20 mg/d per week to the target dosage of 50 to 70 mg/d. Maximum dosage is 70 mg/d. Morning dosing is recommended to avoid sleep disturbance.
Efficacy
The clinical efficacy of lisdexamfetamine was assessed in two 12-week parallel group, flexible-dose, placebo-controlled trials in adults with BED (age 18 to 55). Primary efficacy measure was the number of binge days per week. Both studies had a 4-week dose-optimization period and an 8-week dose-maintenance period and followed the same dosage protocol. Patients began treatment at 30 mg/d and after 1 week were titrated to 50 mg/d; increases to 70 mg/d were made if clinically necessary and well tolerated. Patients were maintained on the optimized dosage during the 8-week dose-maintenance period. A dosage of 30 mg/d did not produce a statistically significant effect, but 50 mg/d and 70 mg/d dosages were statistically superior to placebo. Patients taking lisdexamfetamine also had greater improvement on the Clinical Global Impression—Improvement scores, 4-week binge cessation, and greater reduction in the Yale-Brown Obsessive Compulsive Scale Modified for Binge Eating score.
The prescribing information does not state if lisdexamfetamine should be continued long-term for treating BED.
Adverse reactions
In controlled trials, 5.1% of patients receiving lisdexamfetamine for BED discontinued the drug because of an adverse event, compared with 2.4% of patients receiving placebo. The most common adverse reactions in BED studies were dry mouth (36%), insomnia (20%), decreased appetite (8%), increased heart rate (8%), constipation (6%), and feeling jittery (6%). In trials of children, adolescents, and adults with ADHD, decreased appetite was more common (39%, 34%, and 27%, respectively) than in BED trials (Table 2). Anaphylactic reactions, Stevens-Johnson syndrome, angioedema, and urticaria have been described in postmarketing reports.
The safety of lisdexamfetamine for BED has not been studied in patients age <18, but has been studied in patients with ADHD.
Contraindications
Do not give lisdexamfetamine to patients who have a known hypersensitivity to amphetamine products or other ingredients in lisdexamfetamine capsules.
Lisdexamfetamine is contraindicated in patients who are taking a monoamine oxidase inhibitor, because of a risk of hypertensive crisis.
Related Resources
• Wilens TE. Lisdexamfetamine for ADHD. Current Psychiatry. 2007;6(6):96-98,105.
• Peat CM, Brownley KA, Berkman ND, et al. Binge eating disorder: evidence-based treatments. Current Psychiatry. 2012; 11(5):32-39.
Source: Vyvanse [package insert]. Wayne, PA: Shire; 2015.
Lisdexamfetamine, approved by the FDA in 2007 for attention-deficit/hyperactivity disorder (ADHD), has a new indication: binge eating disorder (BED) (Table 1). BED is characterized by recurrent episodes of consuming a large amount of food in a short time. A prodrug of amphetamine, lisdexamfetamine is a Schedule-II controlled substance, with a high potential for abuse and the risk of severe psychological or physical dependence.
Lisdexamfetamine is not indicated for weight loss or obesity.
Dosage
For BED, the initial dosage of lisdexamfetamine is 30 mg/d in the morning, titrated by 20 mg/d per week to the target dosage of 50 to 70 mg/d. Maximum dosage is 70 mg/d. Morning dosing is recommended to avoid sleep disturbance.
Efficacy
The clinical efficacy of lisdexamfetamine was assessed in two 12-week parallel group, flexible-dose, placebo-controlled trials in adults with BED (age 18 to 55). Primary efficacy measure was the number of binge days per week. Both studies had a 4-week dose-optimization period and an 8-week dose-maintenance period and followed the same dosage protocol. Patients began treatment at 30 mg/d and after 1 week were titrated to 50 mg/d; increases to 70 mg/d were made if clinically necessary and well tolerated. Patients were maintained on the optimized dosage during the 8-week dose-maintenance period. A dosage of 30 mg/d did not produce a statistically significant effect, but 50 mg/d and 70 mg/d dosages were statistically superior to placebo. Patients taking lisdexamfetamine also had greater improvement on the Clinical Global Impression—Improvement scores, 4-week binge cessation, and greater reduction in the Yale-Brown Obsessive Compulsive Scale Modified for Binge Eating score.
The prescribing information does not state if lisdexamfetamine should be continued long-term for treating BED.
Adverse reactions
In controlled trials, 5.1% of patients receiving lisdexamfetamine for BED discontinued the drug because of an adverse event, compared with 2.4% of patients receiving placebo. The most common adverse reactions in BED studies were dry mouth (36%), insomnia (20%), decreased appetite (8%), increased heart rate (8%), constipation (6%), and feeling jittery (6%). In trials of children, adolescents, and adults with ADHD, decreased appetite was more common (39%, 34%, and 27%, respectively) than in BED trials (Table 2). Anaphylactic reactions, Stevens-Johnson syndrome, angioedema, and urticaria have been described in postmarketing reports.
The safety of lisdexamfetamine for BED has not been studied in patients age <18, but has been studied in patients with ADHD.
Contraindications
Do not give lisdexamfetamine to patients who have a known hypersensitivity to amphetamine products or other ingredients in lisdexamfetamine capsules.
Lisdexamfetamine is contraindicated in patients who are taking a monoamine oxidase inhibitor, because of a risk of hypertensive crisis.
Related Resources
• Wilens TE. Lisdexamfetamine for ADHD. Current Psychiatry. 2007;6(6):96-98,105.
• Peat CM, Brownley KA, Berkman ND, et al. Binge eating disorder: evidence-based treatments. Current Psychiatry. 2012; 11(5):32-39.
Lisdexamfetamine, approved by the FDA in 2007 for attention-deficit/hyperactivity disorder (ADHD), has a new indication: binge eating disorder (BED) (Table 1). BED is characterized by recurrent episodes of consuming a large amount of food in a short time. A prodrug of amphetamine, lisdexamfetamine is a Schedule-II controlled substance, with a high potential for abuse and the risk of severe psychological or physical dependence.
Lisdexamfetamine is not indicated for weight loss or obesity.
Dosage
For BED, the initial dosage of lisdexamfetamine is 30 mg/d in the morning, titrated by 20 mg/d per week to the target dosage of 50 to 70 mg/d. Maximum dosage is 70 mg/d. Morning dosing is recommended to avoid sleep disturbance.
Efficacy
The clinical efficacy of lisdexamfetamine was assessed in two 12-week parallel group, flexible-dose, placebo-controlled trials in adults with BED (age 18 to 55). Primary efficacy measure was the number of binge days per week. Both studies had a 4-week dose-optimization period and an 8-week dose-maintenance period and followed the same dosage protocol. Patients began treatment at 30 mg/d and after 1 week were titrated to 50 mg/d; increases to 70 mg/d were made if clinically necessary and well tolerated. Patients were maintained on the optimized dosage during the 8-week dose-maintenance period. A dosage of 30 mg/d did not produce a statistically significant effect, but 50 mg/d and 70 mg/d dosages were statistically superior to placebo. Patients taking lisdexamfetamine also had greater improvement on the Clinical Global Impression—Improvement scores, 4-week binge cessation, and greater reduction in the Yale-Brown Obsessive Compulsive Scale Modified for Binge Eating score.
The prescribing information does not state if lisdexamfetamine should be continued long-term for treating BED.
Adverse reactions
In controlled trials, 5.1% of patients receiving lisdexamfetamine for BED discontinued the drug because of an adverse event, compared with 2.4% of patients receiving placebo. The most common adverse reactions in BED studies were dry mouth (36%), insomnia (20%), decreased appetite (8%), increased heart rate (8%), constipation (6%), and feeling jittery (6%). In trials of children, adolescents, and adults with ADHD, decreased appetite was more common (39%, 34%, and 27%, respectively) than in BED trials (Table 2). Anaphylactic reactions, Stevens-Johnson syndrome, angioedema, and urticaria have been described in postmarketing reports.
The safety of lisdexamfetamine for BED has not been studied in patients age <18, but has been studied in patients with ADHD.
Contraindications
Do not give lisdexamfetamine to patients who have a known hypersensitivity to amphetamine products or other ingredients in lisdexamfetamine capsules.
Lisdexamfetamine is contraindicated in patients who are taking a monoamine oxidase inhibitor, because of a risk of hypertensive crisis.
Related Resources
• Wilens TE. Lisdexamfetamine for ADHD. Current Psychiatry. 2007;6(6):96-98,105.
• Peat CM, Brownley KA, Berkman ND, et al. Binge eating disorder: evidence-based treatments. Current Psychiatry. 2012; 11(5):32-39.
Source: Vyvanse [package insert]. Wayne, PA: Shire; 2015.
Source: Vyvanse [package insert]. Wayne, PA: Shire; 2015.