User login
FDA grants drug orphan status to treat PKD

Image courtesy of NHLBI
The US Food and Drug Administration (FDA) has granted orphan drug designation to AG-348 for the treatment of pyruvate kinase deficiency (PKD), a rare form of hemolytic anemia.
AG-348 is a small molecule allosteric activator of pyruvate kinase-R enzymes that directly targets the underlying metabolic defect in PKD.
The orphan designation will provide Agios Pharmaceuticals, the company developing AG-348, with certain benefits. These include market exclusivity upon regulatory approval and exemption from FDA application fees and tax credits for qualified clinical trials.
According to Agios, AG-348 exhibited favorable safety and pharmacokinetic profiles in a pair of phase 1 studies conducted in healthy volunteers.
Study investigators also observed dose-dependent changes in adenosine triphosphate (ATP) and 2,3-DPG blood levels, which are consistent with increased activity of the glycolytic pathway, the expected pharmacodynamic effect of AG-348.
Results from both studies were presented in a poster at the 2014 ASH Annual Meeting (abstract 4007*). One of the studies was a single ascending dose (SAD) study, and the other was a multiple ascending dose (MAD) study.
SAD study
In this study, healthy volunteers were randomized to receive AG-348 (n=36) or placebo (n=12). Patients were divided into 6 dosing cohorts: 30 mg, 120 mg, 360 mg, 700 mg, 1400 mg, and 2500 mg.
The maximum-tolerated dose of AG-348 was not reached, and there were no serious adverse events (AEs) or early withdrawals among AG-348-treated subjects. Overall, the rate of AEs was 33.3% in the placebo arm and 44.4% in the AG-348 arm.
The rate of AEs that were considered possibly treatment-related was 16.7% in the placebo arm and 30.6% in the AG-348 arm. The most common treatment-related AEs were headache (occurring in 16.7% and 11.1% of patients, respectively), nausea (0% and 13.9%, respectively), and vomiting (0% and 5.6%, respectively).
Exposure to AG-348, as measured by area under the concentration × time curve (AUC), increased in a dose-proportional manner after a single dose. And absorption was rapid (median Tmax ranged from 0.77 to 4.07 hours), although Tmax increased and there was a less-than-proportional increase in Cmax at higher doses.
When AG-348 was administered from 30 mg to 360 mg, there was a dose-dependent decrease in blood 2,3-DPG levels over 24 hours—up to a 49% mean decrease. Increasing the dose beyond 360 mg did not result in additional decreases in 2,3-DPG levels. And levels returned to placebo levels after about 72 hours.
There were minimal increases in blood ATP levels after AG-348 treatment at any dose.
MAD study
At the time of the presentation, 2 cohorts of 8 subjects each (6 receiving AG-348 and 2 receiving placebo) had completed treatment in the MAD study. One cohort received drug or placebo at 120 mg BID, and the other received 360 mg BID.
The pharmacokinetic results for day 1 of this study were consistent with those of the SAD study. However, the Cmax and AUC0-Ʈ were lower on day 14 than day 1. Investigators said this suggests that multiple doses of AG-348 increase the rate of its own metabolism.
They also said the decrease in exposure observed on day 14 is consistent with preclinical data that suggest AG-348 is a moderate inducer of CYP3A4, which is the major route of the oxidative metabolism of AG-348.
As in the SAD study, the investigators observed decreases in 2,3-DPG blood levels after the first dose in cohorts 1 and 2—up to a 48% mean decrease from baseline for both doses. Concentrations returned to placebo levels between 48 and 72 hours after the last dose.
Unlike in the SAD study, patients in this study had increases in ATP—up to a 52% mean increase from baseline in both dosing cohorts. ATP levels remained elevated through 72 hours after the last dose.
The investigators said the results of these 2 studies have informed dose selection for the planned phase 2 study of AG-348 in PKD patients, which is expected to begin soon. ![]()
*Information in the abstract differs from that presented at the meeting.

Image courtesy of NHLBI
The US Food and Drug Administration (FDA) has granted orphan drug designation to AG-348 for the treatment of pyruvate kinase deficiency (PKD), a rare form of hemolytic anemia.
AG-348 is a small molecule allosteric activator of pyruvate kinase-R enzymes that directly targets the underlying metabolic defect in PKD.
The orphan designation will provide Agios Pharmaceuticals, the company developing AG-348, with certain benefits. These include market exclusivity upon regulatory approval and exemption from FDA application fees and tax credits for qualified clinical trials.
According to Agios, AG-348 exhibited favorable safety and pharmacokinetic profiles in a pair of phase 1 studies conducted in healthy volunteers.
Study investigators also observed dose-dependent changes in adenosine triphosphate (ATP) and 2,3-DPG blood levels, which are consistent with increased activity of the glycolytic pathway, the expected pharmacodynamic effect of AG-348.
Results from both studies were presented in a poster at the 2014 ASH Annual Meeting (abstract 4007*). One of the studies was a single ascending dose (SAD) study, and the other was a multiple ascending dose (MAD) study.
SAD study
In this study, healthy volunteers were randomized to receive AG-348 (n=36) or placebo (n=12). Patients were divided into 6 dosing cohorts: 30 mg, 120 mg, 360 mg, 700 mg, 1400 mg, and 2500 mg.
The maximum-tolerated dose of AG-348 was not reached, and there were no serious adverse events (AEs) or early withdrawals among AG-348-treated subjects. Overall, the rate of AEs was 33.3% in the placebo arm and 44.4% in the AG-348 arm.
The rate of AEs that were considered possibly treatment-related was 16.7% in the placebo arm and 30.6% in the AG-348 arm. The most common treatment-related AEs were headache (occurring in 16.7% and 11.1% of patients, respectively), nausea (0% and 13.9%, respectively), and vomiting (0% and 5.6%, respectively).
Exposure to AG-348, as measured by area under the concentration × time curve (AUC), increased in a dose-proportional manner after a single dose. And absorption was rapid (median Tmax ranged from 0.77 to 4.07 hours), although Tmax increased and there was a less-than-proportional increase in Cmax at higher doses.
When AG-348 was administered from 30 mg to 360 mg, there was a dose-dependent decrease in blood 2,3-DPG levels over 24 hours—up to a 49% mean decrease. Increasing the dose beyond 360 mg did not result in additional decreases in 2,3-DPG levels. And levels returned to placebo levels after about 72 hours.
There were minimal increases in blood ATP levels after AG-348 treatment at any dose.
MAD study
At the time of the presentation, 2 cohorts of 8 subjects each (6 receiving AG-348 and 2 receiving placebo) had completed treatment in the MAD study. One cohort received drug or placebo at 120 mg BID, and the other received 360 mg BID.
The pharmacokinetic results for day 1 of this study were consistent with those of the SAD study. However, the Cmax and AUC0-Ʈ were lower on day 14 than day 1. Investigators said this suggests that multiple doses of AG-348 increase the rate of its own metabolism.
They also said the decrease in exposure observed on day 14 is consistent with preclinical data that suggest AG-348 is a moderate inducer of CYP3A4, which is the major route of the oxidative metabolism of AG-348.
As in the SAD study, the investigators observed decreases in 2,3-DPG blood levels after the first dose in cohorts 1 and 2—up to a 48% mean decrease from baseline for both doses. Concentrations returned to placebo levels between 48 and 72 hours after the last dose.
Unlike in the SAD study, patients in this study had increases in ATP—up to a 52% mean increase from baseline in both dosing cohorts. ATP levels remained elevated through 72 hours after the last dose.
The investigators said the results of these 2 studies have informed dose selection for the planned phase 2 study of AG-348 in PKD patients, which is expected to begin soon. ![]()
*Information in the abstract differs from that presented at the meeting.

Image courtesy of NHLBI
The US Food and Drug Administration (FDA) has granted orphan drug designation to AG-348 for the treatment of pyruvate kinase deficiency (PKD), a rare form of hemolytic anemia.
AG-348 is a small molecule allosteric activator of pyruvate kinase-R enzymes that directly targets the underlying metabolic defect in PKD.
The orphan designation will provide Agios Pharmaceuticals, the company developing AG-348, with certain benefits. These include market exclusivity upon regulatory approval and exemption from FDA application fees and tax credits for qualified clinical trials.
According to Agios, AG-348 exhibited favorable safety and pharmacokinetic profiles in a pair of phase 1 studies conducted in healthy volunteers.
Study investigators also observed dose-dependent changes in adenosine triphosphate (ATP) and 2,3-DPG blood levels, which are consistent with increased activity of the glycolytic pathway, the expected pharmacodynamic effect of AG-348.
Results from both studies were presented in a poster at the 2014 ASH Annual Meeting (abstract 4007*). One of the studies was a single ascending dose (SAD) study, and the other was a multiple ascending dose (MAD) study.
SAD study
In this study, healthy volunteers were randomized to receive AG-348 (n=36) or placebo (n=12). Patients were divided into 6 dosing cohorts: 30 mg, 120 mg, 360 mg, 700 mg, 1400 mg, and 2500 mg.
The maximum-tolerated dose of AG-348 was not reached, and there were no serious adverse events (AEs) or early withdrawals among AG-348-treated subjects. Overall, the rate of AEs was 33.3% in the placebo arm and 44.4% in the AG-348 arm.
The rate of AEs that were considered possibly treatment-related was 16.7% in the placebo arm and 30.6% in the AG-348 arm. The most common treatment-related AEs were headache (occurring in 16.7% and 11.1% of patients, respectively), nausea (0% and 13.9%, respectively), and vomiting (0% and 5.6%, respectively).
Exposure to AG-348, as measured by area under the concentration × time curve (AUC), increased in a dose-proportional manner after a single dose. And absorption was rapid (median Tmax ranged from 0.77 to 4.07 hours), although Tmax increased and there was a less-than-proportional increase in Cmax at higher doses.
When AG-348 was administered from 30 mg to 360 mg, there was a dose-dependent decrease in blood 2,3-DPG levels over 24 hours—up to a 49% mean decrease. Increasing the dose beyond 360 mg did not result in additional decreases in 2,3-DPG levels. And levels returned to placebo levels after about 72 hours.
There were minimal increases in blood ATP levels after AG-348 treatment at any dose.
MAD study
At the time of the presentation, 2 cohorts of 8 subjects each (6 receiving AG-348 and 2 receiving placebo) had completed treatment in the MAD study. One cohort received drug or placebo at 120 mg BID, and the other received 360 mg BID.
The pharmacokinetic results for day 1 of this study were consistent with those of the SAD study. However, the Cmax and AUC0-Ʈ were lower on day 14 than day 1. Investigators said this suggests that multiple doses of AG-348 increase the rate of its own metabolism.
They also said the decrease in exposure observed on day 14 is consistent with preclinical data that suggest AG-348 is a moderate inducer of CYP3A4, which is the major route of the oxidative metabolism of AG-348.
As in the SAD study, the investigators observed decreases in 2,3-DPG blood levels after the first dose in cohorts 1 and 2—up to a 48% mean decrease from baseline for both doses. Concentrations returned to placebo levels between 48 and 72 hours after the last dose.
Unlike in the SAD study, patients in this study had increases in ATP—up to a 52% mean increase from baseline in both dosing cohorts. ATP levels remained elevated through 72 hours after the last dose.
The investigators said the results of these 2 studies have informed dose selection for the planned phase 2 study of AG-348 in PKD patients, which is expected to begin soon. ![]()
*Information in the abstract differs from that presented at the meeting.
Manufacturer releases new reprocessing instructions for TJF-Q180V duodenoscope
Olympus, the manufacturer of the TJF-Q180V duodenoscope, has issued new, validated instructions for reprocessing this particular model, as part of the response to recent reports of a possible association between multidrug-resistant bacterial infections and improperly processed duodenoscopes, according to the Food and Drug Administration.
The new instructions, which replace the manual reprocessing instructions included in the original labeling, and validation data have been reviewed by the FDA as part of its ongoing review of the device. The agency “recommends that any facilities that are using Olympus’ TJF-Q180V duodenoscope train staff on the new instructions and implement them as soon as possible,” according to an FDA statement. The instructions are provided in letters sent by Olympus to health care and other facilities that use this particular model.
“Key changes” have been made to the procedures for precleaning, manual cleaning, and manual high-level disinfection reprocessing procedures, the FDA said.
The TJF-Q180 V duodenoscope was the model used in four patients who had undergone an endoscopic retrograde cholangiopancreatography (ERCP) procedure between August 2014 and January 2015 with the same duodenoscope at Cedars-Sinai Medical Center in Los Angeles and had been infected with carbapenem-resistant Enterobacteriaceae (CRE). This outbreak was announced by the medical center in early March in a statement that said the infections occurred “despite the fact that Cedars-Sinai meticulously followed the disinfection procedure for duodenoscopes” recommended in instructions provided by Olympus and the FDA.
In February, the FDA first announced that the agency had received reports of multidrug-resistant bacterial infections in patients who had undergone ERCP procedures with duodenoscopes, despite proper cleaning and disinfection of the devices and that the “complex design of ERCP endoscopes (also called duodenoscopes) may impede effective reprocessing.”
In the latest statement, the FDA said it “is closely monitoring the possible association between reprocessed duodenoscopes and the transmission of infectious agents,” including multidrug-resistant bacterial infections caused by CRE. If they are not properly reprocessed, the statement adds, “residual body fluids and organic debris may remain in microscopic crevices of the device following an attempted cleaning and high-level disinfection. If these residual fluids contain microbial contamination, subsequent patients may be exposed to serious infections.”
Adverse events associated with duodenoscopes should be reported to the FDA’s MedWatch Program at 800-332-1088 or www.accessdata.fda.gov/scripts/medwatch.
AGA Resource
Read more about AGA’s efforts and recommendations to stop duodenoscope infections here.
Olympus, the manufacturer of the TJF-Q180V duodenoscope, has issued new, validated instructions for reprocessing this particular model, as part of the response to recent reports of a possible association between multidrug-resistant bacterial infections and improperly processed duodenoscopes, according to the Food and Drug Administration.
The new instructions, which replace the manual reprocessing instructions included in the original labeling, and validation data have been reviewed by the FDA as part of its ongoing review of the device. The agency “recommends that any facilities that are using Olympus’ TJF-Q180V duodenoscope train staff on the new instructions and implement them as soon as possible,” according to an FDA statement. The instructions are provided in letters sent by Olympus to health care and other facilities that use this particular model.
“Key changes” have been made to the procedures for precleaning, manual cleaning, and manual high-level disinfection reprocessing procedures, the FDA said.
The TJF-Q180 V duodenoscope was the model used in four patients who had undergone an endoscopic retrograde cholangiopancreatography (ERCP) procedure between August 2014 and January 2015 with the same duodenoscope at Cedars-Sinai Medical Center in Los Angeles and had been infected with carbapenem-resistant Enterobacteriaceae (CRE). This outbreak was announced by the medical center in early March in a statement that said the infections occurred “despite the fact that Cedars-Sinai meticulously followed the disinfection procedure for duodenoscopes” recommended in instructions provided by Olympus and the FDA.
In February, the FDA first announced that the agency had received reports of multidrug-resistant bacterial infections in patients who had undergone ERCP procedures with duodenoscopes, despite proper cleaning and disinfection of the devices and that the “complex design of ERCP endoscopes (also called duodenoscopes) may impede effective reprocessing.”
In the latest statement, the FDA said it “is closely monitoring the possible association between reprocessed duodenoscopes and the transmission of infectious agents,” including multidrug-resistant bacterial infections caused by CRE. If they are not properly reprocessed, the statement adds, “residual body fluids and organic debris may remain in microscopic crevices of the device following an attempted cleaning and high-level disinfection. If these residual fluids contain microbial contamination, subsequent patients may be exposed to serious infections.”
Adverse events associated with duodenoscopes should be reported to the FDA’s MedWatch Program at 800-332-1088 or www.accessdata.fda.gov/scripts/medwatch.
AGA Resource
Read more about AGA’s efforts and recommendations to stop duodenoscope infections here.
Olympus, the manufacturer of the TJF-Q180V duodenoscope, has issued new, validated instructions for reprocessing this particular model, as part of the response to recent reports of a possible association between multidrug-resistant bacterial infections and improperly processed duodenoscopes, according to the Food and Drug Administration.
The new instructions, which replace the manual reprocessing instructions included in the original labeling, and validation data have been reviewed by the FDA as part of its ongoing review of the device. The agency “recommends that any facilities that are using Olympus’ TJF-Q180V duodenoscope train staff on the new instructions and implement them as soon as possible,” according to an FDA statement. The instructions are provided in letters sent by Olympus to health care and other facilities that use this particular model.
“Key changes” have been made to the procedures for precleaning, manual cleaning, and manual high-level disinfection reprocessing procedures, the FDA said.
The TJF-Q180 V duodenoscope was the model used in four patients who had undergone an endoscopic retrograde cholangiopancreatography (ERCP) procedure between August 2014 and January 2015 with the same duodenoscope at Cedars-Sinai Medical Center in Los Angeles and had been infected with carbapenem-resistant Enterobacteriaceae (CRE). This outbreak was announced by the medical center in early March in a statement that said the infections occurred “despite the fact that Cedars-Sinai meticulously followed the disinfection procedure for duodenoscopes” recommended in instructions provided by Olympus and the FDA.
In February, the FDA first announced that the agency had received reports of multidrug-resistant bacterial infections in patients who had undergone ERCP procedures with duodenoscopes, despite proper cleaning and disinfection of the devices and that the “complex design of ERCP endoscopes (also called duodenoscopes) may impede effective reprocessing.”
In the latest statement, the FDA said it “is closely monitoring the possible association between reprocessed duodenoscopes and the transmission of infectious agents,” including multidrug-resistant bacterial infections caused by CRE. If they are not properly reprocessed, the statement adds, “residual body fluids and organic debris may remain in microscopic crevices of the device following an attempted cleaning and high-level disinfection. If these residual fluids contain microbial contamination, subsequent patients may be exposed to serious infections.”
Adverse events associated with duodenoscopes should be reported to the FDA’s MedWatch Program at 800-332-1088 or www.accessdata.fda.gov/scripts/medwatch.
AGA Resource
Read more about AGA’s efforts and recommendations to stop duodenoscope infections here.
Hip replacements not just for the elderly anymore
Hip replacement is becoming more common among middle-aged Americans at the same time as the number of surgeons who perform the procedure is declining, Dr. Alexander S. McLawhorn said at the annual meeting of the American Academy of Orthopaedic Surgeons in Las Vegas.
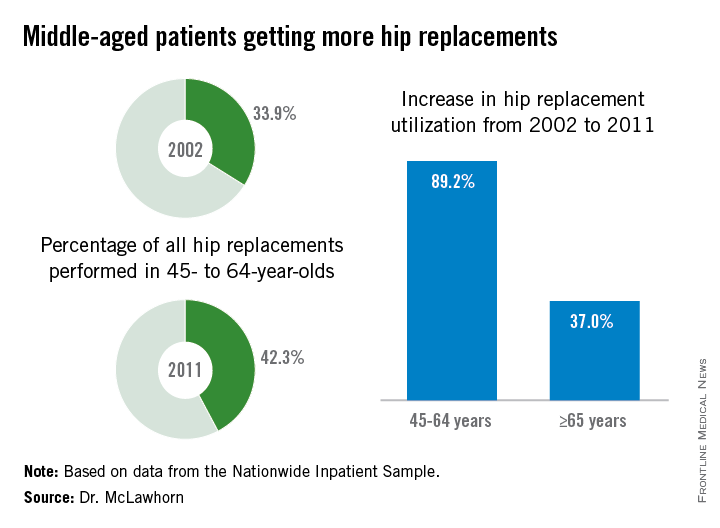
In 2011, patients aged 45-64 years underwent 42.3% of the hip replacements performed, compared with 33.9% in 2002. The number of replacements performed rose from approximately 68,000 in 2002 to 128,000 in 2011, an increase of 89.2%, compared with an increase of 37.0% among those aged 65 years and older, according to data from the Nationwide Inpatient Sample.
This “observed growth was best explained by an expansion of the middle-aged population in the United States. This particular age group is projected to continue expanding, and as such the demand for [hip replacement] in this active group of patients will likely continue to rise as well,” Dr. McLawhorn of the Hospital for Special Surgery, New York, said in a written statement.
According to membership data from the AAOS, however, the number of physicians performing hip replacements declined by almost 29% from 2002 to 2011, which will “increase the future revision burden” on those surgeons who are still doing the procedure, the investigators said.
Dr. McLawhorn had no conflicts to report, but one of his associates disclosed relationships with Ethicon, the Knee Society, Medtronic, Mekanika, and Zimmer.
Hip replacement is becoming more common among middle-aged Americans at the same time as the number of surgeons who perform the procedure is declining, Dr. Alexander S. McLawhorn said at the annual meeting of the American Academy of Orthopaedic Surgeons in Las Vegas.
In 2011, patients aged 45-64 years underwent 42.3% of the hip replacements performed, compared with 33.9% in 2002. The number of replacements performed rose from approximately 68,000 in 2002 to 128,000 in 2011, an increase of 89.2%, compared with an increase of 37.0% among those aged 65 years and older, according to data from the Nationwide Inpatient Sample.
This “observed growth was best explained by an expansion of the middle-aged population in the United States. This particular age group is projected to continue expanding, and as such the demand for [hip replacement] in this active group of patients will likely continue to rise as well,” Dr. McLawhorn of the Hospital for Special Surgery, New York, said in a written statement.
According to membership data from the AAOS, however, the number of physicians performing hip replacements declined by almost 29% from 2002 to 2011, which will “increase the future revision burden” on those surgeons who are still doing the procedure, the investigators said.
Dr. McLawhorn had no conflicts to report, but one of his associates disclosed relationships with Ethicon, the Knee Society, Medtronic, Mekanika, and Zimmer.

Hip replacement is becoming more common among middle-aged Americans at the same time as the number of surgeons who perform the procedure is declining, Dr. Alexander S. McLawhorn said at the annual meeting of the American Academy of Orthopaedic Surgeons in Las Vegas.
In 2011, patients aged 45-64 years underwent 42.3% of the hip replacements performed, compared with 33.9% in 2002. The number of replacements performed rose from approximately 68,000 in 2002 to 128,000 in 2011, an increase of 89.2%, compared with an increase of 37.0% among those aged 65 years and older, according to data from the Nationwide Inpatient Sample.
This “observed growth was best explained by an expansion of the middle-aged population in the United States. This particular age group is projected to continue expanding, and as such the demand for [hip replacement] in this active group of patients will likely continue to rise as well,” Dr. McLawhorn of the Hospital for Special Surgery, New York, said in a written statement.
According to membership data from the AAOS, however, the number of physicians performing hip replacements declined by almost 29% from 2002 to 2011, which will “increase the future revision burden” on those surgeons who are still doing the procedure, the investigators said.
Dr. McLawhorn had no conflicts to report, but one of his associates disclosed relationships with Ethicon, the Knee Society, Medtronic, Mekanika, and Zimmer.

FROM AAOS 2015
Women Fare Better Than Men Following Total Knee, Hip Replacement
LAS VEGAS—While women may have their first total joint replacement (TJR) at an older age, they are less likely to have complications related to their surgery or require revision surgery, according to a study presented at the 2015 Annual Meeting of the American Academy of Orthopaedic Surgeons (AAOS). The findings contradict the theory that TJR is underutilized in female patients because they have worse outcomes than men.
Researchers reviewed patient databases from an Ontario hospital for first-time primary total hip replacement (THR) and total knee replacement (TKR) patients between 2002 and 2009. There were 37,881 THR surgeries (53.8% female) and 59,564 TKR surgeries (60.5% female). Women who underwent THR were significantly older than males (70 years vs. 65 years); however, there was no difference in age between male and female patients undergoing TKR (median age 68 years for both). A greater proportion of female patients undergoing TJR were defined as frail (6.6% vs. 3.5% for THR; and, 6.7% vs. 4% for TKR).
Following surgery, men were:
• 15% more likely to return to the emergency department within 30 days of hospital discharge following either THR or TKR.
• 60% and 70% more likely to have an acute myocardial infarction within 3 months following THR and TKR, respectively.
• 50% more likely to require a revision arthroplasty within 2 years of TKR.
• 25% more likely to be readmitted to the hospital and 70% more likely to experience an infection or revision surgery within 2 years of TKR, compared to women.
“Despite the fact that women have a higher prevalence of advanced hip and knee arthritis, prior research indicates that North American women with arthritis are less likely to receive joint replacement than men,” said lead study author Bheeshma Ravi, MD, PhD, an orthopedic surgery resident at the University of Toronto. “One possible explanation is that women are less often offered or accept surgery because their risk of serious complications following surgery is greater than that of men.
“In this study, we found that while overall rates of serious complications were low for both groups, they were lower for women than for men for both hip and knee replacement, particularly the latter” said Dr. Ravi. “Thus, the previously documented sex difference utilization of TJR cannot be explained by differential risks of complications following surgery.”
LAS VEGAS—While women may have their first total joint replacement (TJR) at an older age, they are less likely to have complications related to their surgery or require revision surgery, according to a study presented at the 2015 Annual Meeting of the American Academy of Orthopaedic Surgeons (AAOS). The findings contradict the theory that TJR is underutilized in female patients because they have worse outcomes than men.
Researchers reviewed patient databases from an Ontario hospital for first-time primary total hip replacement (THR) and total knee replacement (TKR) patients between 2002 and 2009. There were 37,881 THR surgeries (53.8% female) and 59,564 TKR surgeries (60.5% female). Women who underwent THR were significantly older than males (70 years vs. 65 years); however, there was no difference in age between male and female patients undergoing TKR (median age 68 years for both). A greater proportion of female patients undergoing TJR were defined as frail (6.6% vs. 3.5% for THR; and, 6.7% vs. 4% for TKR).
Following surgery, men were:
• 15% more likely to return to the emergency department within 30 days of hospital discharge following either THR or TKR.
• 60% and 70% more likely to have an acute myocardial infarction within 3 months following THR and TKR, respectively.
• 50% more likely to require a revision arthroplasty within 2 years of TKR.
• 25% more likely to be readmitted to the hospital and 70% more likely to experience an infection or revision surgery within 2 years of TKR, compared to women.
“Despite the fact that women have a higher prevalence of advanced hip and knee arthritis, prior research indicates that North American women with arthritis are less likely to receive joint replacement than men,” said lead study author Bheeshma Ravi, MD, PhD, an orthopedic surgery resident at the University of Toronto. “One possible explanation is that women are less often offered or accept surgery because their risk of serious complications following surgery is greater than that of men.
“In this study, we found that while overall rates of serious complications were low for both groups, they were lower for women than for men for both hip and knee replacement, particularly the latter” said Dr. Ravi. “Thus, the previously documented sex difference utilization of TJR cannot be explained by differential risks of complications following surgery.”
LAS VEGAS—While women may have their first total joint replacement (TJR) at an older age, they are less likely to have complications related to their surgery or require revision surgery, according to a study presented at the 2015 Annual Meeting of the American Academy of Orthopaedic Surgeons (AAOS). The findings contradict the theory that TJR is underutilized in female patients because they have worse outcomes than men.
Researchers reviewed patient databases from an Ontario hospital for first-time primary total hip replacement (THR) and total knee replacement (TKR) patients between 2002 and 2009. There were 37,881 THR surgeries (53.8% female) and 59,564 TKR surgeries (60.5% female). Women who underwent THR were significantly older than males (70 years vs. 65 years); however, there was no difference in age between male and female patients undergoing TKR (median age 68 years for both). A greater proportion of female patients undergoing TJR were defined as frail (6.6% vs. 3.5% for THR; and, 6.7% vs. 4% for TKR).
Following surgery, men were:
• 15% more likely to return to the emergency department within 30 days of hospital discharge following either THR or TKR.
• 60% and 70% more likely to have an acute myocardial infarction within 3 months following THR and TKR, respectively.
• 50% more likely to require a revision arthroplasty within 2 years of TKR.
• 25% more likely to be readmitted to the hospital and 70% more likely to experience an infection or revision surgery within 2 years of TKR, compared to women.
“Despite the fact that women have a higher prevalence of advanced hip and knee arthritis, prior research indicates that North American women with arthritis are less likely to receive joint replacement than men,” said lead study author Bheeshma Ravi, MD, PhD, an orthopedic surgery resident at the University of Toronto. “One possible explanation is that women are less often offered or accept surgery because their risk of serious complications following surgery is greater than that of men.
“In this study, we found that while overall rates of serious complications were low for both groups, they were lower for women than for men for both hip and knee replacement, particularly the latter” said Dr. Ravi. “Thus, the previously documented sex difference utilization of TJR cannot be explained by differential risks of complications following surgery.”
Black, Hispanic Patients More Likely to Be Readmitted to the Hospital Within 30 Days Following Hip or Knee Replacement Surgery
LAS VEGAS—Black and Hispanic patients were 62% and 50%, respectively, more likely to be readmitted to the hospital within 30 days after total joint replacement (TJR) surgery compared to white patients, according to a study presented at the 2015 Annual Meeting of the American Academy of Orthopaedic Surgeons (AAOS). In addition, Medicaid patients were 40% more likely to be readmitted to the hospital than patients with private insurance. Poorer outcomes, due in part to patient comorbidities, may reflect limited access to primary care, insufficient patient-doctor communication, researchers suggest.
Disparities in the provision of health care services have long been documented, including that black patients utilize hip and total knee replacement at rates nearly 40% less than white patients, despite having comparable or higher rates of osteoarthritis.
In this study, researchers analyzed 5 years of data—demographic (including race/ethnicity), clinical, and billing—on nearly 53,000 patients admitted to Connecticut hospitals for TJR from 2008 to 2012. The average patient age was 67 years, and the vast majority of patients were white (87%), covered by Medicare (56.7%), and female (61%).
The overall 30-day readmission rate for patients was 5.2%. The most common reasons for readmission were postoperative infection (8%), infection and inflammatory reaction due to internal joint prosthesis (6%), hematoma complications during a procedure (3%), and dislocation of a prosthetic joint (3%). Among the other study findings:
• Readmission rates were 83.5 per thousand for black patients, 78.9 for Hispanic patients, and 53.3 for white patients.
• Longer length of hospital stay was significantly associated with increased odds of readmission.
• When controlling for comorbidities and type of insurance coverage, the readmission rate for Hispanic patients dropped 44%, and for black patients, 38%. Black patients remained significantly more likely than white patients to be readmitted following surgery, after controlling for comorbidities.
• Patients covered by Medicare were 30% more likely to be readmitted within 30 days following discharge compared to patients covered by private insurance, and Medicaid patients were 40% more likely.
Recent research using national data on Medicare suggests that community-based factors, such as availability of general practitioners in the area, may be as or more important than hospital factors in determining readmission rates, and that patients may have few options other than hospital care for both urgent and non-urgent conditions related to their surgery or other conditions.
“Using an all-payer database, our study shows that black patients who undergo total knee replacement may have poorer outcomes,” said lead study author and orthopedic surgeon Courtland Lewis, MD. “After controlling for two key variables implicated in race and ethnic disparities in hospital readmission—preoperative comorbidities and type of insurance coverage—black patients still have a 35% higher likelihood of all-cause, 30-day readmission compared to white patients.
“Our ongoing research in this area is focused on other factors, such as the patient’s connection to primary care and patient-provider communication, that may explain this troubling finding,” said Dr. Lewis.
LAS VEGAS—Black and Hispanic patients were 62% and 50%, respectively, more likely to be readmitted to the hospital within 30 days after total joint replacement (TJR) surgery compared to white patients, according to a study presented at the 2015 Annual Meeting of the American Academy of Orthopaedic Surgeons (AAOS). In addition, Medicaid patients were 40% more likely to be readmitted to the hospital than patients with private insurance. Poorer outcomes, due in part to patient comorbidities, may reflect limited access to primary care, insufficient patient-doctor communication, researchers suggest.
Disparities in the provision of health care services have long been documented, including that black patients utilize hip and total knee replacement at rates nearly 40% less than white patients, despite having comparable or higher rates of osteoarthritis.
In this study, researchers analyzed 5 years of data—demographic (including race/ethnicity), clinical, and billing—on nearly 53,000 patients admitted to Connecticut hospitals for TJR from 2008 to 2012. The average patient age was 67 years, and the vast majority of patients were white (87%), covered by Medicare (56.7%), and female (61%).
The overall 30-day readmission rate for patients was 5.2%. The most common reasons for readmission were postoperative infection (8%), infection and inflammatory reaction due to internal joint prosthesis (6%), hematoma complications during a procedure (3%), and dislocation of a prosthetic joint (3%). Among the other study findings:
• Readmission rates were 83.5 per thousand for black patients, 78.9 for Hispanic patients, and 53.3 for white patients.
• Longer length of hospital stay was significantly associated with increased odds of readmission.
• When controlling for comorbidities and type of insurance coverage, the readmission rate for Hispanic patients dropped 44%, and for black patients, 38%. Black patients remained significantly more likely than white patients to be readmitted following surgery, after controlling for comorbidities.
• Patients covered by Medicare were 30% more likely to be readmitted within 30 days following discharge compared to patients covered by private insurance, and Medicaid patients were 40% more likely.
Recent research using national data on Medicare suggests that community-based factors, such as availability of general practitioners in the area, may be as or more important than hospital factors in determining readmission rates, and that patients may have few options other than hospital care for both urgent and non-urgent conditions related to their surgery or other conditions.
“Using an all-payer database, our study shows that black patients who undergo total knee replacement may have poorer outcomes,” said lead study author and orthopedic surgeon Courtland Lewis, MD. “After controlling for two key variables implicated in race and ethnic disparities in hospital readmission—preoperative comorbidities and type of insurance coverage—black patients still have a 35% higher likelihood of all-cause, 30-day readmission compared to white patients.
“Our ongoing research in this area is focused on other factors, such as the patient’s connection to primary care and patient-provider communication, that may explain this troubling finding,” said Dr. Lewis.
LAS VEGAS—Black and Hispanic patients were 62% and 50%, respectively, more likely to be readmitted to the hospital within 30 days after total joint replacement (TJR) surgery compared to white patients, according to a study presented at the 2015 Annual Meeting of the American Academy of Orthopaedic Surgeons (AAOS). In addition, Medicaid patients were 40% more likely to be readmitted to the hospital than patients with private insurance. Poorer outcomes, due in part to patient comorbidities, may reflect limited access to primary care, insufficient patient-doctor communication, researchers suggest.
Disparities in the provision of health care services have long been documented, including that black patients utilize hip and total knee replacement at rates nearly 40% less than white patients, despite having comparable or higher rates of osteoarthritis.
In this study, researchers analyzed 5 years of data—demographic (including race/ethnicity), clinical, and billing—on nearly 53,000 patients admitted to Connecticut hospitals for TJR from 2008 to 2012. The average patient age was 67 years, and the vast majority of patients were white (87%), covered by Medicare (56.7%), and female (61%).
The overall 30-day readmission rate for patients was 5.2%. The most common reasons for readmission were postoperative infection (8%), infection and inflammatory reaction due to internal joint prosthesis (6%), hematoma complications during a procedure (3%), and dislocation of a prosthetic joint (3%). Among the other study findings:
• Readmission rates were 83.5 per thousand for black patients, 78.9 for Hispanic patients, and 53.3 for white patients.
• Longer length of hospital stay was significantly associated with increased odds of readmission.
• When controlling for comorbidities and type of insurance coverage, the readmission rate for Hispanic patients dropped 44%, and for black patients, 38%. Black patients remained significantly more likely than white patients to be readmitted following surgery, after controlling for comorbidities.
• Patients covered by Medicare were 30% more likely to be readmitted within 30 days following discharge compared to patients covered by private insurance, and Medicaid patients were 40% more likely.
Recent research using national data on Medicare suggests that community-based factors, such as availability of general practitioners in the area, may be as or more important than hospital factors in determining readmission rates, and that patients may have few options other than hospital care for both urgent and non-urgent conditions related to their surgery or other conditions.
“Using an all-payer database, our study shows that black patients who undergo total knee replacement may have poorer outcomes,” said lead study author and orthopedic surgeon Courtland Lewis, MD. “After controlling for two key variables implicated in race and ethnic disparities in hospital readmission—preoperative comorbidities and type of insurance coverage—black patients still have a 35% higher likelihood of all-cause, 30-day readmission compared to white patients.
“Our ongoing research in this area is focused on other factors, such as the patient’s connection to primary care and patient-provider communication, that may explain this troubling finding,” said Dr. Lewis.
Hip Replacements in Middle-Age Nearly Double From 2002-2011, Outpacing Growth in Elderly Population
LAS VEGAS—The number of total hip replacements (THRs) nearly doubled among middle-age patients between 2002 and 2011, primarily due to the expansion of the middle-age population in the United States, according to a study presented at the 2015 Annual Meeting of the American Academy of Orthopaedic Surgeons (AAOS). Continued growth in utilization of hip replacement surgery in patients ages 45 to 64 years, an increase in revision surgeries for this population as they age, and a nearly 30% decline in the number of surgeons who perform THR could have significant implications for future health care costs, THR demand, and access, researchers said.
The researchers used the Nationwide Inpatient Sample (NIS) to identify primary THRs performed between 2002 and 2011 in patients ages 45 to 64 years, as well as related hospital charges. Population data and projections were obtained from the US Census Bureau and surgeon workforce estimates from the AAOS.
In 2011, 42.3% of THRs were performed in patients ages 45 to 64 years compared to 33.9% in 2002. Utilization of THR in this age group increased 89.2% from 2002 to 2011, from approximately 68,000 THRs in 2002 to 128,000 THRs in 2011. The overall population increased 21.3%. In addition, the authors found that:
• Growth of THR utilization in the 45- to 64-year-old age group grew 2.4 times faster than it did in the Medicare-aged population (age > 65 years).
• A rise in the prevalence of obesity, a known risk factor for hip osteoarthritis, among middle-age Americans was not significantly associated with increased THR utilization.
• Mean hospital charges in the THR 45- to 64-year-old age group declined 5.7% from 2002 to 2011, and declined 2.5% in the Medicare population (age > 65 years).
• Mean physician reimbursement per THR, in 2011 US dollars, declined 26.2% over the same period.
• Concurrently, the number of physicians reporting that they performed THR surgeries declined 28.2%.
“The purpose of this study was to identify potential drivers of THR utilization in the middle-age patient segment,” said lead study author Alexander S. McLawhorn, MD, MBA, an orthopedic surgery resident at the Hospital for Special Surgery in New York City. “Our multivariable statistical model suggested that the observed growth was best explained by an expansion of the middle-age population in the US. This particular age group is projected to continue expanding, and as such the demand for THR in this active group of patients will likely continue to rise as well. Our results underscore concerns about consumption of premium-priced implants in younger patients and the future revision burden this trend implies in the face of a dwindling number of physicians who specialize in hip arthroplasty surgery.”
LAS VEGAS—The number of total hip replacements (THRs) nearly doubled among middle-age patients between 2002 and 2011, primarily due to the expansion of the middle-age population in the United States, according to a study presented at the 2015 Annual Meeting of the American Academy of Orthopaedic Surgeons (AAOS). Continued growth in utilization of hip replacement surgery in patients ages 45 to 64 years, an increase in revision surgeries for this population as they age, and a nearly 30% decline in the number of surgeons who perform THR could have significant implications for future health care costs, THR demand, and access, researchers said.
The researchers used the Nationwide Inpatient Sample (NIS) to identify primary THRs performed between 2002 and 2011 in patients ages 45 to 64 years, as well as related hospital charges. Population data and projections were obtained from the US Census Bureau and surgeon workforce estimates from the AAOS.
In 2011, 42.3% of THRs were performed in patients ages 45 to 64 years compared to 33.9% in 2002. Utilization of THR in this age group increased 89.2% from 2002 to 2011, from approximately 68,000 THRs in 2002 to 128,000 THRs in 2011. The overall population increased 21.3%. In addition, the authors found that:
• Growth of THR utilization in the 45- to 64-year-old age group grew 2.4 times faster than it did in the Medicare-aged population (age > 65 years).
• A rise in the prevalence of obesity, a known risk factor for hip osteoarthritis, among middle-age Americans was not significantly associated with increased THR utilization.
• Mean hospital charges in the THR 45- to 64-year-old age group declined 5.7% from 2002 to 2011, and declined 2.5% in the Medicare population (age > 65 years).
• Mean physician reimbursement per THR, in 2011 US dollars, declined 26.2% over the same period.
• Concurrently, the number of physicians reporting that they performed THR surgeries declined 28.2%.
“The purpose of this study was to identify potential drivers of THR utilization in the middle-age patient segment,” said lead study author Alexander S. McLawhorn, MD, MBA, an orthopedic surgery resident at the Hospital for Special Surgery in New York City. “Our multivariable statistical model suggested that the observed growth was best explained by an expansion of the middle-age population in the US. This particular age group is projected to continue expanding, and as such the demand for THR in this active group of patients will likely continue to rise as well. Our results underscore concerns about consumption of premium-priced implants in younger patients and the future revision burden this trend implies in the face of a dwindling number of physicians who specialize in hip arthroplasty surgery.”
LAS VEGAS—The number of total hip replacements (THRs) nearly doubled among middle-age patients between 2002 and 2011, primarily due to the expansion of the middle-age population in the United States, according to a study presented at the 2015 Annual Meeting of the American Academy of Orthopaedic Surgeons (AAOS). Continued growth in utilization of hip replacement surgery in patients ages 45 to 64 years, an increase in revision surgeries for this population as they age, and a nearly 30% decline in the number of surgeons who perform THR could have significant implications for future health care costs, THR demand, and access, researchers said.
The researchers used the Nationwide Inpatient Sample (NIS) to identify primary THRs performed between 2002 and 2011 in patients ages 45 to 64 years, as well as related hospital charges. Population data and projections were obtained from the US Census Bureau and surgeon workforce estimates from the AAOS.
In 2011, 42.3% of THRs were performed in patients ages 45 to 64 years compared to 33.9% in 2002. Utilization of THR in this age group increased 89.2% from 2002 to 2011, from approximately 68,000 THRs in 2002 to 128,000 THRs in 2011. The overall population increased 21.3%. In addition, the authors found that:
• Growth of THR utilization in the 45- to 64-year-old age group grew 2.4 times faster than it did in the Medicare-aged population (age > 65 years).
• A rise in the prevalence of obesity, a known risk factor for hip osteoarthritis, among middle-age Americans was not significantly associated with increased THR utilization.
• Mean hospital charges in the THR 45- to 64-year-old age group declined 5.7% from 2002 to 2011, and declined 2.5% in the Medicare population (age > 65 years).
• Mean physician reimbursement per THR, in 2011 US dollars, declined 26.2% over the same period.
• Concurrently, the number of physicians reporting that they performed THR surgeries declined 28.2%.
“The purpose of this study was to identify potential drivers of THR utilization in the middle-age patient segment,” said lead study author Alexander S. McLawhorn, MD, MBA, an orthopedic surgery resident at the Hospital for Special Surgery in New York City. “Our multivariable statistical model suggested that the observed growth was best explained by an expansion of the middle-age population in the US. This particular age group is projected to continue expanding, and as such the demand for THR in this active group of patients will likely continue to rise as well. Our results underscore concerns about consumption of premium-priced implants in younger patients and the future revision burden this trend implies in the face of a dwindling number of physicians who specialize in hip arthroplasty surgery.”
ObGyns, and US women, are embracing LARCs
Use of long-acting reversible contraception (LARC) has increased nearly 5-fold in the last decade, reported the Centers for Disease Control and Prevention (CDC) in a National Center for Health Statistics (NCHS) Data Brief on the trends in LARC use among US women aged 15 to 44.1
Data from the National Survey of Family Growth indicate that LARCs, which include intrauterine devices (IUDs) and subdermal hormonal implants, are gaining popularity because of their high efficacy in preventing unintended pregnancies. LARCs have demonstrated greater efficacy in preventing unintended pregnancy among all women compared with other contraceptive methods, including the oral contraceptive pill and the transdermal patch.
Age-related trends
For women aged 15 to 44, LARC use doubled between 2002 (1.5%) and the period 2006–2010 (3.8%) and then nearly doubled again for 2011–2013 (7.2%). IUD use increased 83% from the 2006–2010 period (3.5%) to the 2011–2013 period (6.4%). Implant use tripled from 2002 (0.3%) to the 2011–2013 period (0.8%).
LARC use was higher among women aged 25 to 34 than among women aged 15 to 24. The difference in LARC use was not statistically significant between women aged 25 to 34 and women aged 35 to 44.
- LARC use increased nearly 4-fold for women aged 15 to 24 between 2002 (0.6%) and 2006–2010 (2.3%), and doubled again for 2011–2013 (5.0%).
- LARC use almost doubled among women aged 25 to 34 from 2006–2010 to 2011–2013 (5.3% to 11.1%).
- LARC use tripled between 2002 (1.1%) and 2006–2010 (3.8%) for women aged 35 to 44, and increased to 5.3% in 2011–2013.
Patterns of use by race
Although LARC use tripled for non-Hispanic white women and increased 4-fold for non-Hispanic black women between 2002 and 2006–2010, use among Hispanic women declined 10% during this period. LARC use increased by 129% among Hispanic women and by 128% among non-Hispanic white women from 2006–2010 to 2011–2013. Use of LARCs in non-Hispanic black women increased by 30% during this same period.
Parous vs nulliparous women
Women who have had at least one birth use LARC at a higher rate than women who have had no previous births. During the period 2011–2013, rate of use was 3 times greater among parous (11.0%) women compared with nulliparous (2.8%) women.
- Among parous women, LARC use increased from 2.4% in 2002 to 6.3% in 2006–2010, and to 10.6% in 2011–2013.
- In nulliparous women, LARC use increased 10-fold between 2006–2010 and 2011–2013.
For additional information, visit the NCHS Data Brief at http://www.cdc.gov/nchs/data/databriefs/db188.htm
Share your thoughts on this article! Send your Letter to the Editor to [email protected]. Please include your name and the city and state in which you practice.
Reference
- Branum AM, Jones J. Trends in long-acting reversible contraception use among U.S. women aged 15–44. NCHS data brief, no 188. Hyattsville, MD: National Center for Health Statistics. 2015. http://www.cdc.gov/nchs/data/databriefs/db188.htm. Updated February 24, 2015. Accessed March 25, 2015.
Use of long-acting reversible contraception (LARC) has increased nearly 5-fold in the last decade, reported the Centers for Disease Control and Prevention (CDC) in a National Center for Health Statistics (NCHS) Data Brief on the trends in LARC use among US women aged 15 to 44.1
Data from the National Survey of Family Growth indicate that LARCs, which include intrauterine devices (IUDs) and subdermal hormonal implants, are gaining popularity because of their high efficacy in preventing unintended pregnancies. LARCs have demonstrated greater efficacy in preventing unintended pregnancy among all women compared with other contraceptive methods, including the oral contraceptive pill and the transdermal patch.
Age-related trends
For women aged 15 to 44, LARC use doubled between 2002 (1.5%) and the period 2006–2010 (3.8%) and then nearly doubled again for 2011–2013 (7.2%). IUD use increased 83% from the 2006–2010 period (3.5%) to the 2011–2013 period (6.4%). Implant use tripled from 2002 (0.3%) to the 2011–2013 period (0.8%).
LARC use was higher among women aged 25 to 34 than among women aged 15 to 24. The difference in LARC use was not statistically significant between women aged 25 to 34 and women aged 35 to 44.
- LARC use increased nearly 4-fold for women aged 15 to 24 between 2002 (0.6%) and 2006–2010 (2.3%), and doubled again for 2011–2013 (5.0%).
- LARC use almost doubled among women aged 25 to 34 from 2006–2010 to 2011–2013 (5.3% to 11.1%).
- LARC use tripled between 2002 (1.1%) and 2006–2010 (3.8%) for women aged 35 to 44, and increased to 5.3% in 2011–2013.
Patterns of use by race
Although LARC use tripled for non-Hispanic white women and increased 4-fold for non-Hispanic black women between 2002 and 2006–2010, use among Hispanic women declined 10% during this period. LARC use increased by 129% among Hispanic women and by 128% among non-Hispanic white women from 2006–2010 to 2011–2013. Use of LARCs in non-Hispanic black women increased by 30% during this same period.
Parous vs nulliparous women
Women who have had at least one birth use LARC at a higher rate than women who have had no previous births. During the period 2011–2013, rate of use was 3 times greater among parous (11.0%) women compared with nulliparous (2.8%) women.
- Among parous women, LARC use increased from 2.4% in 2002 to 6.3% in 2006–2010, and to 10.6% in 2011–2013.
- In nulliparous women, LARC use increased 10-fold between 2006–2010 and 2011–2013.
For additional information, visit the NCHS Data Brief at http://www.cdc.gov/nchs/data/databriefs/db188.htm
Share your thoughts on this article! Send your Letter to the Editor to [email protected]. Please include your name and the city and state in which you practice.
Use of long-acting reversible contraception (LARC) has increased nearly 5-fold in the last decade, reported the Centers for Disease Control and Prevention (CDC) in a National Center for Health Statistics (NCHS) Data Brief on the trends in LARC use among US women aged 15 to 44.1
Data from the National Survey of Family Growth indicate that LARCs, which include intrauterine devices (IUDs) and subdermal hormonal implants, are gaining popularity because of their high efficacy in preventing unintended pregnancies. LARCs have demonstrated greater efficacy in preventing unintended pregnancy among all women compared with other contraceptive methods, including the oral contraceptive pill and the transdermal patch.
Age-related trends
For women aged 15 to 44, LARC use doubled between 2002 (1.5%) and the period 2006–2010 (3.8%) and then nearly doubled again for 2011–2013 (7.2%). IUD use increased 83% from the 2006–2010 period (3.5%) to the 2011–2013 period (6.4%). Implant use tripled from 2002 (0.3%) to the 2011–2013 period (0.8%).
LARC use was higher among women aged 25 to 34 than among women aged 15 to 24. The difference in LARC use was not statistically significant between women aged 25 to 34 and women aged 35 to 44.
- LARC use increased nearly 4-fold for women aged 15 to 24 between 2002 (0.6%) and 2006–2010 (2.3%), and doubled again for 2011–2013 (5.0%).
- LARC use almost doubled among women aged 25 to 34 from 2006–2010 to 2011–2013 (5.3% to 11.1%).
- LARC use tripled between 2002 (1.1%) and 2006–2010 (3.8%) for women aged 35 to 44, and increased to 5.3% in 2011–2013.
Patterns of use by race
Although LARC use tripled for non-Hispanic white women and increased 4-fold for non-Hispanic black women between 2002 and 2006–2010, use among Hispanic women declined 10% during this period. LARC use increased by 129% among Hispanic women and by 128% among non-Hispanic white women from 2006–2010 to 2011–2013. Use of LARCs in non-Hispanic black women increased by 30% during this same period.
Parous vs nulliparous women
Women who have had at least one birth use LARC at a higher rate than women who have had no previous births. During the period 2011–2013, rate of use was 3 times greater among parous (11.0%) women compared with nulliparous (2.8%) women.
- Among parous women, LARC use increased from 2.4% in 2002 to 6.3% in 2006–2010, and to 10.6% in 2011–2013.
- In nulliparous women, LARC use increased 10-fold between 2006–2010 and 2011–2013.
For additional information, visit the NCHS Data Brief at http://www.cdc.gov/nchs/data/databriefs/db188.htm
Share your thoughts on this article! Send your Letter to the Editor to [email protected]. Please include your name and the city and state in which you practice.
Reference
- Branum AM, Jones J. Trends in long-acting reversible contraception use among U.S. women aged 15–44. NCHS data brief, no 188. Hyattsville, MD: National Center for Health Statistics. 2015. http://www.cdc.gov/nchs/data/databriefs/db188.htm. Updated February 24, 2015. Accessed March 25, 2015.
Reference
- Branum AM, Jones J. Trends in long-acting reversible contraception use among U.S. women aged 15–44. NCHS data brief, no 188. Hyattsville, MD: National Center for Health Statistics. 2015. http://www.cdc.gov/nchs/data/databriefs/db188.htm. Updated February 24, 2015. Accessed March 25, 2015.
Teach patients about the health benefits of sleep
The National Heart, Lung, and Blood Institute offers an online resource you can use to reinforce what you’ve taught your patients about the need for adequate sleep. “Your Guide to Healthy Sleep” can be downloaded from http://www.nhlbi.nih.gov/files/docs/public/sleep/healthy_sleep.pdf. It describes the beneficial effects of sleep on an individual’s mood, memory, hormones, and heart; explains common sleep disorders and where to find treatment for them; and dispels the Top 10 Sleep Myths.
The National Heart, Lung, and Blood Institute offers an online resource you can use to reinforce what you’ve taught your patients about the need for adequate sleep. “Your Guide to Healthy Sleep” can be downloaded from http://www.nhlbi.nih.gov/files/docs/public/sleep/healthy_sleep.pdf. It describes the beneficial effects of sleep on an individual’s mood, memory, hormones, and heart; explains common sleep disorders and where to find treatment for them; and dispels the Top 10 Sleep Myths.
The National Heart, Lung, and Blood Institute offers an online resource you can use to reinforce what you’ve taught your patients about the need for adequate sleep. “Your Guide to Healthy Sleep” can be downloaded from http://www.nhlbi.nih.gov/files/docs/public/sleep/healthy_sleep.pdf. It describes the beneficial effects of sleep on an individual’s mood, memory, hormones, and heart; explains common sleep disorders and where to find treatment for them; and dispels the Top 10 Sleep Myths.
Give patients the facts about hepatitis C
The Centers for Disease Control and Prevention has updated its online hepatitis C fact sheet. “Hepatitis C: General Information” is available at http://www.cdc.gov/hepatitis/HCV/PDFs/HepCGeneralFactSheet.pdf. It explains what hepatitis C is, how it’s spread, who should get tested, and how it can be prevented.
The Centers for Disease Control and Prevention has updated its online hepatitis C fact sheet. “Hepatitis C: General Information” is available at http://www.cdc.gov/hepatitis/HCV/PDFs/HepCGeneralFactSheet.pdf. It explains what hepatitis C is, how it’s spread, who should get tested, and how it can be prevented.
The Centers for Disease Control and Prevention has updated its online hepatitis C fact sheet. “Hepatitis C: General Information” is available at http://www.cdc.gov/hepatitis/HCV/PDFs/HepCGeneralFactSheet.pdf. It explains what hepatitis C is, how it’s spread, who should get tested, and how it can be prevented.
Too little time and too many worries for social media
I’m not on Facebook, either professionally or personally.
My office doesn’t have a Twitter account.
In fact, my only nod to social media at all is a rarely updated LinkedIn page, which is really just a public CV.
Why, in this age of connectedness, do I hide from these things? One reason is time. There isn’t much of it in the course of a day. Between my practice (patients, dictations, forms, returning calls, reviewing tests, rinse, wash, repeat), my family (wife, kids, dogs, house), and all the other things that make up a day (driving, finances, bathing, sleep), I don’t have much extra time. I really have no desire to see what others had for breakfast, look at pictures of a distant cousin’s kids, or have an online political argument with in-laws.
Another reason is privacy. Most patients are good people, but there are scary ones, too. I don’t want them knowing my kids’ names, or what school they go to, or seeing their pictures. In this age trying to have a degree of personal privacy is hard enough. I don’t want to make it any easier for someone looking to cause trouble.
I have nothing against my patients. I like the majority of them. But I don’t want to be online friends with them, either. Practicing objective medicine requires a degree of emotional distance, and I don’t want to do anything to shorten that. Social media connections with someone may also clue you into their personal and political beliefs, and, as I’ve said before, I think knowing those about patients (and them knowing mine) can only make the relationship difficult.
And the last is from a medical-legal view. The definition of what constitutes medical advice seems to be quite vague, and I worry anything I innocuously post or tweet could be taken to mean that I had an established treating medical relationship with someone or that my malpractice carrier could raise my rates by saying I was doing online medicine.
There’s also the simple fact that anything can be interpreted in any way. I worry that something I might put up could be used against me in court. Let’s say a patient dies while I’m on vacation, and the family decides to sue. Pictures of me relaxing with my kids on the trip could be used to make me look like an uncaring, callous doctor, even if I had no idea what was going on back home.
I’ll keep my somewhat under-the-radar personal existence as it is. Others may feel I’m missing out on the wonders of the social age, but I’m happy with keeping my home life just that – at home.
Dr. Block has a solo neurology practice in Scottsdale, Ariz.
I’m not on Facebook, either professionally or personally.
My office doesn’t have a Twitter account.
In fact, my only nod to social media at all is a rarely updated LinkedIn page, which is really just a public CV.
Why, in this age of connectedness, do I hide from these things? One reason is time. There isn’t much of it in the course of a day. Between my practice (patients, dictations, forms, returning calls, reviewing tests, rinse, wash, repeat), my family (wife, kids, dogs, house), and all the other things that make up a day (driving, finances, bathing, sleep), I don’t have much extra time. I really have no desire to see what others had for breakfast, look at pictures of a distant cousin’s kids, or have an online political argument with in-laws.
Another reason is privacy. Most patients are good people, but there are scary ones, too. I don’t want them knowing my kids’ names, or what school they go to, or seeing their pictures. In this age trying to have a degree of personal privacy is hard enough. I don’t want to make it any easier for someone looking to cause trouble.
I have nothing against my patients. I like the majority of them. But I don’t want to be online friends with them, either. Practicing objective medicine requires a degree of emotional distance, and I don’t want to do anything to shorten that. Social media connections with someone may also clue you into their personal and political beliefs, and, as I’ve said before, I think knowing those about patients (and them knowing mine) can only make the relationship difficult.
And the last is from a medical-legal view. The definition of what constitutes medical advice seems to be quite vague, and I worry anything I innocuously post or tweet could be taken to mean that I had an established treating medical relationship with someone or that my malpractice carrier could raise my rates by saying I was doing online medicine.
There’s also the simple fact that anything can be interpreted in any way. I worry that something I might put up could be used against me in court. Let’s say a patient dies while I’m on vacation, and the family decides to sue. Pictures of me relaxing with my kids on the trip could be used to make me look like an uncaring, callous doctor, even if I had no idea what was going on back home.
I’ll keep my somewhat under-the-radar personal existence as it is. Others may feel I’m missing out on the wonders of the social age, but I’m happy with keeping my home life just that – at home.
Dr. Block has a solo neurology practice in Scottsdale, Ariz.
I’m not on Facebook, either professionally or personally.
My office doesn’t have a Twitter account.
In fact, my only nod to social media at all is a rarely updated LinkedIn page, which is really just a public CV.
Why, in this age of connectedness, do I hide from these things? One reason is time. There isn’t much of it in the course of a day. Between my practice (patients, dictations, forms, returning calls, reviewing tests, rinse, wash, repeat), my family (wife, kids, dogs, house), and all the other things that make up a day (driving, finances, bathing, sleep), I don’t have much extra time. I really have no desire to see what others had for breakfast, look at pictures of a distant cousin’s kids, or have an online political argument with in-laws.
Another reason is privacy. Most patients are good people, but there are scary ones, too. I don’t want them knowing my kids’ names, or what school they go to, or seeing their pictures. In this age trying to have a degree of personal privacy is hard enough. I don’t want to make it any easier for someone looking to cause trouble.
I have nothing against my patients. I like the majority of them. But I don’t want to be online friends with them, either. Practicing objective medicine requires a degree of emotional distance, and I don’t want to do anything to shorten that. Social media connections with someone may also clue you into their personal and political beliefs, and, as I’ve said before, I think knowing those about patients (and them knowing mine) can only make the relationship difficult.
And the last is from a medical-legal view. The definition of what constitutes medical advice seems to be quite vague, and I worry anything I innocuously post or tweet could be taken to mean that I had an established treating medical relationship with someone or that my malpractice carrier could raise my rates by saying I was doing online medicine.
There’s also the simple fact that anything can be interpreted in any way. I worry that something I might put up could be used against me in court. Let’s say a patient dies while I’m on vacation, and the family decides to sue. Pictures of me relaxing with my kids on the trip could be used to make me look like an uncaring, callous doctor, even if I had no idea what was going on back home.
I’ll keep my somewhat under-the-radar personal existence as it is. Others may feel I’m missing out on the wonders of the social age, but I’m happy with keeping my home life just that – at home.
Dr. Block has a solo neurology practice in Scottsdale, Ariz.

