User login
Multi-Site Hospitalist Leaders: HM15 Session Summary
Session: Multi-site Hospitalist Leaders: Unique Challenges/What You Should Know
HM15 Presenter/Moderator: Scott Rissmiller, MD
Summation: This standing-room-only session was the result of a popular HMX e-community, which has become an active discussion board. As hospitals and health systems continue to consolidate across the country, there has been a rapid growth of multi-hospital systems. The role of the “Chief Hospitalist,” whose job is to lead multiple hospitalist groups within these systems, is evolving. These “Chief Hospitalists” are growing in number and they, as well as their followers, face unique challenges.
These points regarding organization structure were discussed, and as you look at your own organizational structure, these questions deserve your attention:
- Purpose of your structure?
- Is your structure centralized or decentralized?
- How does your organizational structure support decision-making?
- How does the structure ensure proper communication?
- How are resources shared across geography?
- What is your administrative support structure?
- How is administrative time allocated for physician leaders?
- How do you ensure engagement from all providers?
- How does your organization structure create alignment with the healthcare system?
The following compensation issues were discussed, and can be used as a discussion outline for most groups:
- How does your compensation (comp) plan align with the goals and values of the system?
- How does your comp plan account for regional variances?
- How does the comp plan encourage teamwork and sharing of resources?
- How does comp plan account for differences in acuity, hospital size, night frequency, etc.?
- Are goals and incentives group based, site based, or individual based?
- How does the comp plan fairly reward “non-RVU” work? (teaching, committee service, etc.)
- Should all site leaders receive the same comp regardless of group size?
- Does the comp plan incorporate “minimum work standards”/social compact?
Key Points/HM Takeaways:
- Panel discussion was valuable and reassured attendees that there are multiple ways to make groups successful. One common variable of successful groups is open lines of communication at all levels.
- Physician on-boarding is critical and should be utilized to set clear expectations.
- HM Goals/expectations must be aligned with those of the hospital and health system.
- When multiple hospitals are part of a larger system, it is desirable for goals to be aligned across the health system.
- Two-way open communication is necessary for success.
- Try to take a walk in your colleague’s/stakeholder’s shoes:
- How does my hospital administrative partner see this issue?
- How does my regional director/system lead see this issue?
- How does my bedside hospitalist physician/provider see this issue?
- How would my patients view this issue?
- Issues facing different types of groups, academic vs. community and for profit vs. not for profit, are somewhat variable.
- The leadership Dyad consisting of a physician and practice management professional in partnership is an effective and well-proven management model.
Many thanks to Drs. T.J. Richardson and Dan Duzan for their input and assistance with this session summary. Dr. Richardson is a Regional Medical Director and Dr. Duzan is a Facility Medical Director, both work for TeamHealth.
Julianna Lindsey is a hospitalist and physician leader based in the Dallas-Fort Worth Metroplex. Her focus is patient safety/quality and physician leadership. She is a member of TeamHospitalist.
Session: Multi-site Hospitalist Leaders: Unique Challenges/What You Should Know
HM15 Presenter/Moderator: Scott Rissmiller, MD
Summation: This standing-room-only session was the result of a popular HMX e-community, which has become an active discussion board. As hospitals and health systems continue to consolidate across the country, there has been a rapid growth of multi-hospital systems. The role of the “Chief Hospitalist,” whose job is to lead multiple hospitalist groups within these systems, is evolving. These “Chief Hospitalists” are growing in number and they, as well as their followers, face unique challenges.
These points regarding organization structure were discussed, and as you look at your own organizational structure, these questions deserve your attention:
- Purpose of your structure?
- Is your structure centralized or decentralized?
- How does your organizational structure support decision-making?
- How does the structure ensure proper communication?
- How are resources shared across geography?
- What is your administrative support structure?
- How is administrative time allocated for physician leaders?
- How do you ensure engagement from all providers?
- How does your organization structure create alignment with the healthcare system?
The following compensation issues were discussed, and can be used as a discussion outline for most groups:
- How does your compensation (comp) plan align with the goals and values of the system?
- How does your comp plan account for regional variances?
- How does the comp plan encourage teamwork and sharing of resources?
- How does comp plan account for differences in acuity, hospital size, night frequency, etc.?
- Are goals and incentives group based, site based, or individual based?
- How does the comp plan fairly reward “non-RVU” work? (teaching, committee service, etc.)
- Should all site leaders receive the same comp regardless of group size?
- Does the comp plan incorporate “minimum work standards”/social compact?
Key Points/HM Takeaways:
- Panel discussion was valuable and reassured attendees that there are multiple ways to make groups successful. One common variable of successful groups is open lines of communication at all levels.
- Physician on-boarding is critical and should be utilized to set clear expectations.
- HM Goals/expectations must be aligned with those of the hospital and health system.
- When multiple hospitals are part of a larger system, it is desirable for goals to be aligned across the health system.
- Two-way open communication is necessary for success.
- Try to take a walk in your colleague’s/stakeholder’s shoes:
- How does my hospital administrative partner see this issue?
- How does my regional director/system lead see this issue?
- How does my bedside hospitalist physician/provider see this issue?
- How would my patients view this issue?
- Issues facing different types of groups, academic vs. community and for profit vs. not for profit, are somewhat variable.
- The leadership Dyad consisting of a physician and practice management professional in partnership is an effective and well-proven management model.
Many thanks to Drs. T.J. Richardson and Dan Duzan for their input and assistance with this session summary. Dr. Richardson is a Regional Medical Director and Dr. Duzan is a Facility Medical Director, both work for TeamHealth.
Julianna Lindsey is a hospitalist and physician leader based in the Dallas-Fort Worth Metroplex. Her focus is patient safety/quality and physician leadership. She is a member of TeamHospitalist.
Session: Multi-site Hospitalist Leaders: Unique Challenges/What You Should Know
HM15 Presenter/Moderator: Scott Rissmiller, MD
Summation: This standing-room-only session was the result of a popular HMX e-community, which has become an active discussion board. As hospitals and health systems continue to consolidate across the country, there has been a rapid growth of multi-hospital systems. The role of the “Chief Hospitalist,” whose job is to lead multiple hospitalist groups within these systems, is evolving. These “Chief Hospitalists” are growing in number and they, as well as their followers, face unique challenges.
These points regarding organization structure were discussed, and as you look at your own organizational structure, these questions deserve your attention:
- Purpose of your structure?
- Is your structure centralized or decentralized?
- How does your organizational structure support decision-making?
- How does the structure ensure proper communication?
- How are resources shared across geography?
- What is your administrative support structure?
- How is administrative time allocated for physician leaders?
- How do you ensure engagement from all providers?
- How does your organization structure create alignment with the healthcare system?
The following compensation issues were discussed, and can be used as a discussion outline for most groups:
- How does your compensation (comp) plan align with the goals and values of the system?
- How does your comp plan account for regional variances?
- How does the comp plan encourage teamwork and sharing of resources?
- How does comp plan account for differences in acuity, hospital size, night frequency, etc.?
- Are goals and incentives group based, site based, or individual based?
- How does the comp plan fairly reward “non-RVU” work? (teaching, committee service, etc.)
- Should all site leaders receive the same comp regardless of group size?
- Does the comp plan incorporate “minimum work standards”/social compact?
Key Points/HM Takeaways:
- Panel discussion was valuable and reassured attendees that there are multiple ways to make groups successful. One common variable of successful groups is open lines of communication at all levels.
- Physician on-boarding is critical and should be utilized to set clear expectations.
- HM Goals/expectations must be aligned with those of the hospital and health system.
- When multiple hospitals are part of a larger system, it is desirable for goals to be aligned across the health system.
- Two-way open communication is necessary for success.
- Try to take a walk in your colleague’s/stakeholder’s shoes:
- How does my hospital administrative partner see this issue?
- How does my regional director/system lead see this issue?
- How does my bedside hospitalist physician/provider see this issue?
- How would my patients view this issue?
- Issues facing different types of groups, academic vs. community and for profit vs. not for profit, are somewhat variable.
- The leadership Dyad consisting of a physician and practice management professional in partnership is an effective and well-proven management model.
Many thanks to Drs. T.J. Richardson and Dan Duzan for their input and assistance with this session summary. Dr. Richardson is a Regional Medical Director and Dr. Duzan is a Facility Medical Director, both work for TeamHealth.
Julianna Lindsey is a hospitalist and physician leader based in the Dallas-Fort Worth Metroplex. Her focus is patient safety/quality and physician leadership. She is a member of TeamHospitalist.
Use of Smartphones and Mobile Devices
Over 90% of Americans own mobile phones, and their use for internet access is rising rapidly (31% in 2009, 63% in 2013).[1] This has prompted growth in mobile health (mHealth) programs for outpatient settings,[2] and similar growth is anticipated for inpatient settings.[3] Hospitals and the healthcare systems they operate within are increasingly tied to patient experience scores (eg, Hospital Consumer Assessment of Healthcare Providers and Systems, Press Ganey) for both reputation and reimbursement.[4, 5] As a result, hospitals will need to invest future resources in a consumer‐facing digital experience. Despite these trends, basic information on mobile device ownership and usage by hospitalized patients is limited. This knowledge is needed to guide successful mHealth approaches to engage patients in acute care settings.
METHODS
We administered a 27‐question survey about mobile device use to all adult inpatients at a large urban California teaching hospital over 2 dates (October 27, 2013 and November 11, 2013) to create a cross‐sectional view of mobile device use at a hospital that offers free wireless Internet (WiFi) and personal health records (Internet‐accessible individualized medical records). Average census was 447, and we excluded patients for: age under 18 years (98), admission for neurological problems (75), altered mental status (35), nonEnglish speaking (30), or unavailable if patients were not in their room after 2 attempts spaced 30 to 60 minutes apart (36), leaving 173 eligible. We performed descriptive statistics and unadjusted associations ([2] test) to explore patterns of mobile device use.
RESULTS
We enrolled 152 patients (88% response rate): 77 (51%) male, average age 53 years (1992 years), 84 (56%) white, 115 (75%) with Medicare or commercial insurance. We found 85 (56%) patients brought a smartphone, and 82/85 (95%) used it during their hospital stay. Additionally, 41 (27%) patients brought a tablet, and 29 (19%) brought a laptop; usage was 37/41 (90%) for tablets and 24/29 (83%) for laptops. One hundred three (68%) patients brought at least 1 mobile computing device (smartphone, tablet, laptop) during their hospital stay. Overall device usage was highest among oncology patients (85%) and lowest among medicine patients (54%) (Table 1). Device usage also varied by age (65 years old: 79% vs 65 years old: 27%), insurance status (private/Medicare: 70% vs Medicaid/other: 59%), and race/ethnicity (white: 73% vs non‐white: 62%), although only age was statistically significant (P0.01; all others >0.05).
| Total, N=152 | Medicine, n=39 | Surgery, n=47 | Oncology, n=34 | All Others, n=32* | |
|---|---|---|---|---|---|
| |||||
| Demographics | |||||
| Average age, y (range) | 53.2 (1992) | 55.7 (2092) | 51.7 (1979) | 51.2 (2377) | 53.9 (2584) |
| Medicare or commercial insurance | 75% (115) | 64% (25) | 87% (41) | 76% (26) | 72% (23) |
| Medicaid, other, or no insurance | 25% (37) | 36% (14) | 13% (6) | 24% (8) | 28% (9) |
| Non‐white race/ethnicity | 44% (68) | 56% (22) | 36% (17) | 38% (13) | 50%(16) |
| Female gender | 49% (75) | 49% (19) | 45% (21) | 47% (16) | 59% (19) |
| Device ownership/usage | |||||
| Own smartphone | 62% (94) | 54% (21) | 66% (31) | 74% (25) | 53% (17) |
| Brought smartphone | 55% (83) | 41% (16) | 60% (28) | 71% (24) | 48% (15) |
| Brought laptop | 19% (29) | 18% (7) | 11% (5) | 41% (14) | 10% (3) |
| Brought tablet | 27% (41) | 18% (7) | 26% (12) | 50% (17) | 16% (5) |
| Brought 1 above devices | 68% (103) | 54% (21) | 68% (32) | 85% (29) | 68% (21) |
| Ever used an app | 63% (95) | 51% (20) | 72% (34) | 79% (27) | 45% (14) |
| Ever used an app for health purposes | 22% (34) | 18% (7) | 21% (10) | 24% (8) | 29% (9) |
| Accessed PHR with mobile device | 31% (47) | 26% (10) | 26% (12) | 47% (16) | 29% (9) |
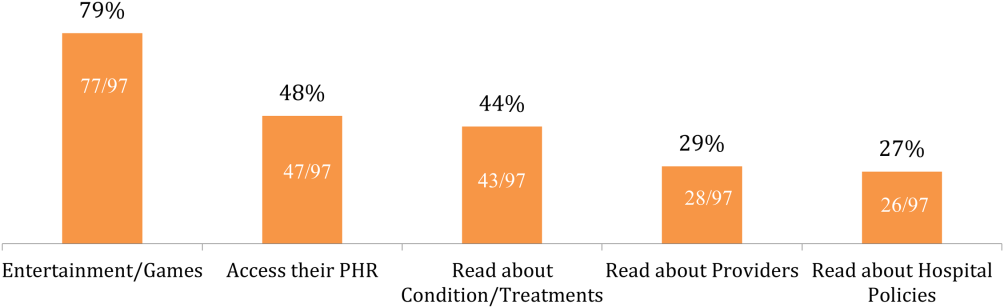
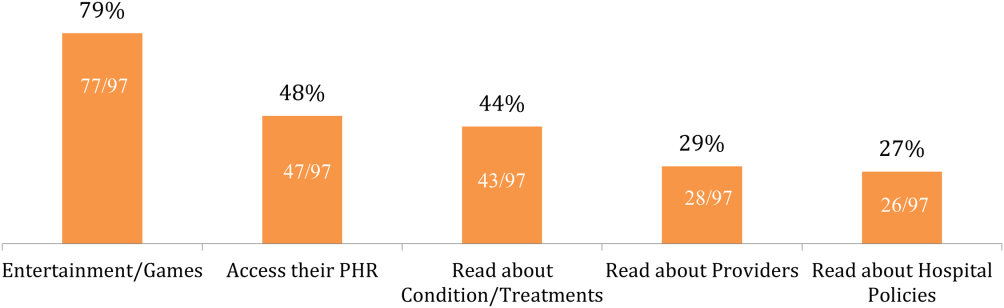
Of the patients with mobile devices (smartphone, tablet, laptop), 97/103 (94%) used them during their hospitalization and for a wide array of activities (Figure 1): 47/97 (48%) accessed their personal health record (PHR), and most of these patients (38/47, 81%) reported this improved their inpatient experience. Additionally, 43/97 (44%) patients used their mobile devices to search for information about doctors, conditions, or treatments; most of these patients (39/43, 91%) used Google to search for this information, and most 29/43 (67%) felt this information made them more confident in their care.
COMMENT
Over two‐thirds of patients in our study brought and used 1 or more mobile devices to the hospital. Despite this level of engagement with mobile devices, relatively few inpatients used their device to access their online PHR, which suggests information technology access is not the leading barrier to PHR access or mHealth engagement during hospitalization. In light of growing patient enthusiasm for PHRs,[6, 7] this represents an untapped opportunity to deliver personalized, patient‐centered care at the hospital bedside.
We also found that among the patients who did access their PHR on their mobile device, the vast majority (38/47, 81%) felt it improved their inpatient experience. Our PHR provides information such as test results and medications, but our survey suggests a number of patients look for health information, such as patient education tools, medication references, and provider information, outside of the PHR. For those patients, 29/43 (67%) felt these health‐related searches improved their experience. Although we did not ask patients why they used Web searches outside their PHR, we believe this suggests that patients desire more information than currently available via the PHR. Although this information might be difficult to incorporate into the PHR, at minimum, hospitals could develop mobile applications to provide patients with basic information about their providers and conditions. Beyond this, hospitals could develop or adopt mobile applications that align with strategic priorities such as improved physician‐provider communication, reduced hospital readmissions, and improved accuracy of medication reconciliation.
Our study has limitations. First, although we used a cross‐sectional, point‐in‐time approach to canvas the entire adult population in our hospital on 2 separate dates, our study was limited to 1 large urban hospital in California; device ownership and usage may vary in other settings. Second, although our hospital provides free WiFi, we did not assess whether patients experienced any connectivity issues that influenced their device usage patterns. Finally, we did not explore questions of access, ownership, and usage of mobile computing devices for family and friends who visited inpatients in our study. These questions are ripe for future research in this emerging area of mHeath.
In summary, our study suggests a role for hospitals to provide universal WiFi access to patients, and a role for both hospitals and healthcare providers to promote digital health programs. Our findings on mobile device use in the hospital are consistent with the growing popularity of mobile device usage nationwide. Patients are increasingly wired for new opportunities to both engage in their care and optimize their hospital experience through use of their mobile computing devices. Hospitals and providers should explore this potential for engagement, but may need to explore local trends in usage to target specific service lines and patient populations given differences in access and use.
Acknowledgements
The authors acknowledge contributions by Christina Quist, MD, and Emily Gottenborg, MD, who assisted in data collection.
Disclosures: Data from this project were presented at the 2014 Annual Scientific Meeting of the Society of Hospital Medicine, March 25, 2014 in Las Vegas, Nevada. The authors have no conflicts of interest to declare relative to this study. Dr. Ludwin, MD had full access to all data in the study and takes responsibility for the integrity of the data and the accuracy of the data analysis. This project by Drs. Ludwin and Greysen was supported by grants from the University of California, San Francisco (UCSF) Partners in Care (Ronald Rankin Award) and the UCSF Mount Zion Health Fund. Dr. Greysen is also funded by a Pilot Award for Junior Investigators in Digital Health from the UCSF Dean's Office, Research Evaluation and Allocation Committee (REAC). Additionally, Dr. Greysen receives career development support from the National Institutes of Health (NIH)National Institute of Aging (NIA) through the Claude D. Pepper Older Americans Independence Center at UCSF Division of Geriatric Medicine (#P30AG021342 NIH/NIA), a Career Development Award (1K23AG045338‐01), and the NIH‐NIA Loan Repayment Program.
- Device ownership over time. Pew Research Center. Available at: http://www.pewinternet.org/data‐trend/mobile/device‐ownership. Accessed April 3, 2014.
- , , , et al. The effectiveness of mobile‐health technologies to improve health care service delivery processes: a systematic review and meta‐analysis. PLoS Med. 2013;10(1):e1001363.
- , , . Can mobile health technologies transform health care? JAMA. 2013;310(22):2395–2396.
- Look ahead to succeed under VBP. Hosp Case Manag. 2014;22(7):92–93.
- , , , . The relationship between commercial website ratings and traditional hospital performance measures in the USA. BMJ Qual Saf. 2013;22(3):194–202.
- , , , , . Consumers' perceptions of patient‐accessible electronic medical records. J Med Internet Res. 2013;15(8):e168.
- , , , et al. Access, interest, and attitudes toward electronic communication for health care among patients in the medical safety net. J Gen Intern Med. 2013;28(7):914–920.
Over 90% of Americans own mobile phones, and their use for internet access is rising rapidly (31% in 2009, 63% in 2013).[1] This has prompted growth in mobile health (mHealth) programs for outpatient settings,[2] and similar growth is anticipated for inpatient settings.[3] Hospitals and the healthcare systems they operate within are increasingly tied to patient experience scores (eg, Hospital Consumer Assessment of Healthcare Providers and Systems, Press Ganey) for both reputation and reimbursement.[4, 5] As a result, hospitals will need to invest future resources in a consumer‐facing digital experience. Despite these trends, basic information on mobile device ownership and usage by hospitalized patients is limited. This knowledge is needed to guide successful mHealth approaches to engage patients in acute care settings.
METHODS
We administered a 27‐question survey about mobile device use to all adult inpatients at a large urban California teaching hospital over 2 dates (October 27, 2013 and November 11, 2013) to create a cross‐sectional view of mobile device use at a hospital that offers free wireless Internet (WiFi) and personal health records (Internet‐accessible individualized medical records). Average census was 447, and we excluded patients for: age under 18 years (98), admission for neurological problems (75), altered mental status (35), nonEnglish speaking (30), or unavailable if patients were not in their room after 2 attempts spaced 30 to 60 minutes apart (36), leaving 173 eligible. We performed descriptive statistics and unadjusted associations ([2] test) to explore patterns of mobile device use.
RESULTS
We enrolled 152 patients (88% response rate): 77 (51%) male, average age 53 years (1992 years), 84 (56%) white, 115 (75%) with Medicare or commercial insurance. We found 85 (56%) patients brought a smartphone, and 82/85 (95%) used it during their hospital stay. Additionally, 41 (27%) patients brought a tablet, and 29 (19%) brought a laptop; usage was 37/41 (90%) for tablets and 24/29 (83%) for laptops. One hundred three (68%) patients brought at least 1 mobile computing device (smartphone, tablet, laptop) during their hospital stay. Overall device usage was highest among oncology patients (85%) and lowest among medicine patients (54%) (Table 1). Device usage also varied by age (65 years old: 79% vs 65 years old: 27%), insurance status (private/Medicare: 70% vs Medicaid/other: 59%), and race/ethnicity (white: 73% vs non‐white: 62%), although only age was statistically significant (P0.01; all others >0.05).
| Total, N=152 | Medicine, n=39 | Surgery, n=47 | Oncology, n=34 | All Others, n=32* | |
|---|---|---|---|---|---|
| |||||
| Demographics | |||||
| Average age, y (range) | 53.2 (1992) | 55.7 (2092) | 51.7 (1979) | 51.2 (2377) | 53.9 (2584) |
| Medicare or commercial insurance | 75% (115) | 64% (25) | 87% (41) | 76% (26) | 72% (23) |
| Medicaid, other, or no insurance | 25% (37) | 36% (14) | 13% (6) | 24% (8) | 28% (9) |
| Non‐white race/ethnicity | 44% (68) | 56% (22) | 36% (17) | 38% (13) | 50%(16) |
| Female gender | 49% (75) | 49% (19) | 45% (21) | 47% (16) | 59% (19) |
| Device ownership/usage | |||||
| Own smartphone | 62% (94) | 54% (21) | 66% (31) | 74% (25) | 53% (17) |
| Brought smartphone | 55% (83) | 41% (16) | 60% (28) | 71% (24) | 48% (15) |
| Brought laptop | 19% (29) | 18% (7) | 11% (5) | 41% (14) | 10% (3) |
| Brought tablet | 27% (41) | 18% (7) | 26% (12) | 50% (17) | 16% (5) |
| Brought 1 above devices | 68% (103) | 54% (21) | 68% (32) | 85% (29) | 68% (21) |
| Ever used an app | 63% (95) | 51% (20) | 72% (34) | 79% (27) | 45% (14) |
| Ever used an app for health purposes | 22% (34) | 18% (7) | 21% (10) | 24% (8) | 29% (9) |
| Accessed PHR with mobile device | 31% (47) | 26% (10) | 26% (12) | 47% (16) | 29% (9) |
Of the patients with mobile devices (smartphone, tablet, laptop), 97/103 (94%) used them during their hospitalization and for a wide array of activities (Figure 1): 47/97 (48%) accessed their personal health record (PHR), and most of these patients (38/47, 81%) reported this improved their inpatient experience. Additionally, 43/97 (44%) patients used their mobile devices to search for information about doctors, conditions, or treatments; most of these patients (39/43, 91%) used Google to search for this information, and most 29/43 (67%) felt this information made them more confident in their care.

COMMENT
Over two‐thirds of patients in our study brought and used 1 or more mobile devices to the hospital. Despite this level of engagement with mobile devices, relatively few inpatients used their device to access their online PHR, which suggests information technology access is not the leading barrier to PHR access or mHealth engagement during hospitalization. In light of growing patient enthusiasm for PHRs,[6, 7] this represents an untapped opportunity to deliver personalized, patient‐centered care at the hospital bedside.
We also found that among the patients who did access their PHR on their mobile device, the vast majority (38/47, 81%) felt it improved their inpatient experience. Our PHR provides information such as test results and medications, but our survey suggests a number of patients look for health information, such as patient education tools, medication references, and provider information, outside of the PHR. For those patients, 29/43 (67%) felt these health‐related searches improved their experience. Although we did not ask patients why they used Web searches outside their PHR, we believe this suggests that patients desire more information than currently available via the PHR. Although this information might be difficult to incorporate into the PHR, at minimum, hospitals could develop mobile applications to provide patients with basic information about their providers and conditions. Beyond this, hospitals could develop or adopt mobile applications that align with strategic priorities such as improved physician‐provider communication, reduced hospital readmissions, and improved accuracy of medication reconciliation.
Our study has limitations. First, although we used a cross‐sectional, point‐in‐time approach to canvas the entire adult population in our hospital on 2 separate dates, our study was limited to 1 large urban hospital in California; device ownership and usage may vary in other settings. Second, although our hospital provides free WiFi, we did not assess whether patients experienced any connectivity issues that influenced their device usage patterns. Finally, we did not explore questions of access, ownership, and usage of mobile computing devices for family and friends who visited inpatients in our study. These questions are ripe for future research in this emerging area of mHeath.
In summary, our study suggests a role for hospitals to provide universal WiFi access to patients, and a role for both hospitals and healthcare providers to promote digital health programs. Our findings on mobile device use in the hospital are consistent with the growing popularity of mobile device usage nationwide. Patients are increasingly wired for new opportunities to both engage in their care and optimize their hospital experience through use of their mobile computing devices. Hospitals and providers should explore this potential for engagement, but may need to explore local trends in usage to target specific service lines and patient populations given differences in access and use.
Acknowledgements
The authors acknowledge contributions by Christina Quist, MD, and Emily Gottenborg, MD, who assisted in data collection.
Disclosures: Data from this project were presented at the 2014 Annual Scientific Meeting of the Society of Hospital Medicine, March 25, 2014 in Las Vegas, Nevada. The authors have no conflicts of interest to declare relative to this study. Dr. Ludwin, MD had full access to all data in the study and takes responsibility for the integrity of the data and the accuracy of the data analysis. This project by Drs. Ludwin and Greysen was supported by grants from the University of California, San Francisco (UCSF) Partners in Care (Ronald Rankin Award) and the UCSF Mount Zion Health Fund. Dr. Greysen is also funded by a Pilot Award for Junior Investigators in Digital Health from the UCSF Dean's Office, Research Evaluation and Allocation Committee (REAC). Additionally, Dr. Greysen receives career development support from the National Institutes of Health (NIH)National Institute of Aging (NIA) through the Claude D. Pepper Older Americans Independence Center at UCSF Division of Geriatric Medicine (#P30AG021342 NIH/NIA), a Career Development Award (1K23AG045338‐01), and the NIH‐NIA Loan Repayment Program.
Over 90% of Americans own mobile phones, and their use for internet access is rising rapidly (31% in 2009, 63% in 2013).[1] This has prompted growth in mobile health (mHealth) programs for outpatient settings,[2] and similar growth is anticipated for inpatient settings.[3] Hospitals and the healthcare systems they operate within are increasingly tied to patient experience scores (eg, Hospital Consumer Assessment of Healthcare Providers and Systems, Press Ganey) for both reputation and reimbursement.[4, 5] As a result, hospitals will need to invest future resources in a consumer‐facing digital experience. Despite these trends, basic information on mobile device ownership and usage by hospitalized patients is limited. This knowledge is needed to guide successful mHealth approaches to engage patients in acute care settings.
METHODS
We administered a 27‐question survey about mobile device use to all adult inpatients at a large urban California teaching hospital over 2 dates (October 27, 2013 and November 11, 2013) to create a cross‐sectional view of mobile device use at a hospital that offers free wireless Internet (WiFi) and personal health records (Internet‐accessible individualized medical records). Average census was 447, and we excluded patients for: age under 18 years (98), admission for neurological problems (75), altered mental status (35), nonEnglish speaking (30), or unavailable if patients were not in their room after 2 attempts spaced 30 to 60 minutes apart (36), leaving 173 eligible. We performed descriptive statistics and unadjusted associations ([2] test) to explore patterns of mobile device use.
RESULTS
We enrolled 152 patients (88% response rate): 77 (51%) male, average age 53 years (1992 years), 84 (56%) white, 115 (75%) with Medicare or commercial insurance. We found 85 (56%) patients brought a smartphone, and 82/85 (95%) used it during their hospital stay. Additionally, 41 (27%) patients brought a tablet, and 29 (19%) brought a laptop; usage was 37/41 (90%) for tablets and 24/29 (83%) for laptops. One hundred three (68%) patients brought at least 1 mobile computing device (smartphone, tablet, laptop) during their hospital stay. Overall device usage was highest among oncology patients (85%) and lowest among medicine patients (54%) (Table 1). Device usage also varied by age (65 years old: 79% vs 65 years old: 27%), insurance status (private/Medicare: 70% vs Medicaid/other: 59%), and race/ethnicity (white: 73% vs non‐white: 62%), although only age was statistically significant (P0.01; all others >0.05).
| Total, N=152 | Medicine, n=39 | Surgery, n=47 | Oncology, n=34 | All Others, n=32* | |
|---|---|---|---|---|---|
| |||||
| Demographics | |||||
| Average age, y (range) | 53.2 (1992) | 55.7 (2092) | 51.7 (1979) | 51.2 (2377) | 53.9 (2584) |
| Medicare or commercial insurance | 75% (115) | 64% (25) | 87% (41) | 76% (26) | 72% (23) |
| Medicaid, other, or no insurance | 25% (37) | 36% (14) | 13% (6) | 24% (8) | 28% (9) |
| Non‐white race/ethnicity | 44% (68) | 56% (22) | 36% (17) | 38% (13) | 50%(16) |
| Female gender | 49% (75) | 49% (19) | 45% (21) | 47% (16) | 59% (19) |
| Device ownership/usage | |||||
| Own smartphone | 62% (94) | 54% (21) | 66% (31) | 74% (25) | 53% (17) |
| Brought smartphone | 55% (83) | 41% (16) | 60% (28) | 71% (24) | 48% (15) |
| Brought laptop | 19% (29) | 18% (7) | 11% (5) | 41% (14) | 10% (3) |
| Brought tablet | 27% (41) | 18% (7) | 26% (12) | 50% (17) | 16% (5) |
| Brought 1 above devices | 68% (103) | 54% (21) | 68% (32) | 85% (29) | 68% (21) |
| Ever used an app | 63% (95) | 51% (20) | 72% (34) | 79% (27) | 45% (14) |
| Ever used an app for health purposes | 22% (34) | 18% (7) | 21% (10) | 24% (8) | 29% (9) |
| Accessed PHR with mobile device | 31% (47) | 26% (10) | 26% (12) | 47% (16) | 29% (9) |
Of the patients with mobile devices (smartphone, tablet, laptop), 97/103 (94%) used them during their hospitalization and for a wide array of activities (Figure 1): 47/97 (48%) accessed their personal health record (PHR), and most of these patients (38/47, 81%) reported this improved their inpatient experience. Additionally, 43/97 (44%) patients used their mobile devices to search for information about doctors, conditions, or treatments; most of these patients (39/43, 91%) used Google to search for this information, and most 29/43 (67%) felt this information made them more confident in their care.

COMMENT
Over two‐thirds of patients in our study brought and used 1 or more mobile devices to the hospital. Despite this level of engagement with mobile devices, relatively few inpatients used their device to access their online PHR, which suggests information technology access is not the leading barrier to PHR access or mHealth engagement during hospitalization. In light of growing patient enthusiasm for PHRs,[6, 7] this represents an untapped opportunity to deliver personalized, patient‐centered care at the hospital bedside.
We also found that among the patients who did access their PHR on their mobile device, the vast majority (38/47, 81%) felt it improved their inpatient experience. Our PHR provides information such as test results and medications, but our survey suggests a number of patients look for health information, such as patient education tools, medication references, and provider information, outside of the PHR. For those patients, 29/43 (67%) felt these health‐related searches improved their experience. Although we did not ask patients why they used Web searches outside their PHR, we believe this suggests that patients desire more information than currently available via the PHR. Although this information might be difficult to incorporate into the PHR, at minimum, hospitals could develop mobile applications to provide patients with basic information about their providers and conditions. Beyond this, hospitals could develop or adopt mobile applications that align with strategic priorities such as improved physician‐provider communication, reduced hospital readmissions, and improved accuracy of medication reconciliation.
Our study has limitations. First, although we used a cross‐sectional, point‐in‐time approach to canvas the entire adult population in our hospital on 2 separate dates, our study was limited to 1 large urban hospital in California; device ownership and usage may vary in other settings. Second, although our hospital provides free WiFi, we did not assess whether patients experienced any connectivity issues that influenced their device usage patterns. Finally, we did not explore questions of access, ownership, and usage of mobile computing devices for family and friends who visited inpatients in our study. These questions are ripe for future research in this emerging area of mHeath.
In summary, our study suggests a role for hospitals to provide universal WiFi access to patients, and a role for both hospitals and healthcare providers to promote digital health programs. Our findings on mobile device use in the hospital are consistent with the growing popularity of mobile device usage nationwide. Patients are increasingly wired for new opportunities to both engage in their care and optimize their hospital experience through use of their mobile computing devices. Hospitals and providers should explore this potential for engagement, but may need to explore local trends in usage to target specific service lines and patient populations given differences in access and use.
Acknowledgements
The authors acknowledge contributions by Christina Quist, MD, and Emily Gottenborg, MD, who assisted in data collection.
Disclosures: Data from this project were presented at the 2014 Annual Scientific Meeting of the Society of Hospital Medicine, March 25, 2014 in Las Vegas, Nevada. The authors have no conflicts of interest to declare relative to this study. Dr. Ludwin, MD had full access to all data in the study and takes responsibility for the integrity of the data and the accuracy of the data analysis. This project by Drs. Ludwin and Greysen was supported by grants from the University of California, San Francisco (UCSF) Partners in Care (Ronald Rankin Award) and the UCSF Mount Zion Health Fund. Dr. Greysen is also funded by a Pilot Award for Junior Investigators in Digital Health from the UCSF Dean's Office, Research Evaluation and Allocation Committee (REAC). Additionally, Dr. Greysen receives career development support from the National Institutes of Health (NIH)National Institute of Aging (NIA) through the Claude D. Pepper Older Americans Independence Center at UCSF Division of Geriatric Medicine (#P30AG021342 NIH/NIA), a Career Development Award (1K23AG045338‐01), and the NIH‐NIA Loan Repayment Program.
- Device ownership over time. Pew Research Center. Available at: http://www.pewinternet.org/data‐trend/mobile/device‐ownership. Accessed April 3, 2014.
- , , , et al. The effectiveness of mobile‐health technologies to improve health care service delivery processes: a systematic review and meta‐analysis. PLoS Med. 2013;10(1):e1001363.
- , , . Can mobile health technologies transform health care? JAMA. 2013;310(22):2395–2396.
- Look ahead to succeed under VBP. Hosp Case Manag. 2014;22(7):92–93.
- , , , . The relationship between commercial website ratings and traditional hospital performance measures in the USA. BMJ Qual Saf. 2013;22(3):194–202.
- , , , , . Consumers' perceptions of patient‐accessible electronic medical records. J Med Internet Res. 2013;15(8):e168.
- , , , et al. Access, interest, and attitudes toward electronic communication for health care among patients in the medical safety net. J Gen Intern Med. 2013;28(7):914–920.
- Device ownership over time. Pew Research Center. Available at: http://www.pewinternet.org/data‐trend/mobile/device‐ownership. Accessed April 3, 2014.
- , , , et al. The effectiveness of mobile‐health technologies to improve health care service delivery processes: a systematic review and meta‐analysis. PLoS Med. 2013;10(1):e1001363.
- , , . Can mobile health technologies transform health care? JAMA. 2013;310(22):2395–2396.
- Look ahead to succeed under VBP. Hosp Case Manag. 2014;22(7):92–93.
- , , , . The relationship between commercial website ratings and traditional hospital performance measures in the USA. BMJ Qual Saf. 2013;22(3):194–202.
- , , , , . Consumers' perceptions of patient‐accessible electronic medical records. J Med Internet Res. 2013;15(8):e168.
- , , , et al. Access, interest, and attitudes toward electronic communication for health care among patients in the medical safety net. J Gen Intern Med. 2013;28(7):914–920.
Memory and Sleep in Hospital Patients
Hospitalization is often utilized as a teachable moment, as patients are provided with education about treatment and disease management, particularly at discharge.[1, 2, 3] However, memory impairment among hospitalized patients may undermine the utility of the teachable moment. In one study of community‐dwelling seniors admitted to the hospital, one‐third had previously unrecognized poor memory at discharge.[4]
Sleep loss may be an underappreciated contributor to short‐term memory deficits in inpatients, particularly in seniors, who have baseline higher rates of sleep disruptions and sleep disorders.[5] Patients often receive 2 hours less sleep than at home and experience poor quality sleep due to disruptions.[6, 7] Robust studies of healthy subjects in laboratory settings demonstrate that sleep loss leads to decreased attention and worse recall, and that more sleep is associated with better memory performance.[8, 9]
Very few studies have examined memory in hospitalized patients. Although word‐list tasks are often used to assess memory because they are quick and easy to administer, these tasks may not accurately reflect memory for a set of instructions provided at patient discharge. Finally, no studies have examined the association between inpatient sleep loss and memory. Thus, our primary aim in this study was to examine memory performance in older, hospitalized patients using a word listbased memory task and a more complex medical vignette task. Our second aim was to investigate the relationship between in‐hospital sleep and memory.
METHODS
Study Design
We conducted a prospective cohort study with subjects enrolled in an ongoing sleep study at the University of Chicago Medical Center.[10] Eligible subjects were on the general medicine or hematology/oncology service, at least 50 years old, community dwelling, ambulatory, and without detectable cognitive impairment on the Mini Mental State Exam[11] or Short Portable Mental Status Questionnaire.[12, 13] Patients were excluded if they had a documented sleep disorder (ie, obstructive sleep apnea), were transferred from an intensive care unit or were in droplet or airborne isolation, had a bedrest order, or had already spent over 72 hours in the hospital prior to enrollment. These criteria were used to select a population appropriate for wristwatch actigraphy and with low likelihood of baseline memory impairment. The University of Chicago Institutional Review Board approved this study, and participants provided written consent.
Data Collection
Memory Testing
Memory was evaluated using the University of Southern California Repeatable Episodic Memory Test (USC‐REMT), a validated verbal memory test in which subjects listen to a list of 15 words and then complete free‐recall and recognition of the list.[14, 15] Free‐recall tests subjects' ability to procure information without cues. In contrast, recognition requires subjects to pick out the words they just heard from distractors, an easier task. The USC‐REMT contains multiple functionally equivalent different word lists, and may be administered more than once to the same subject without learning effects.[15] Immediate and delayed memory were tested by asking the subject to complete the tasks immediately after listening to the word list and 24‐hours after listening to the list, respectively.
Immediate Recall and Recognition
Recall and recognition following a night of sleep in the hospital was the primary outcome for this study. After 1 night of actigraphy recorded sleep, subjects listened as a 15‐item word list (word list A) was read aloud. For the free‐recall task, subjects were asked to repeat back all the words they could remember immediately after hearing the list. For the recognition task, subjects were read a new list of 15 words, including a mix of words from the previous list and new distractor words. They answered yes if they thought the word had previously been read to them and no if they thought the word was new.
Delayed Recall and Delayed Recognition
At the conclusion of study enrollment on day 1 prior to the night of actigraphy, subjects were shown a laminated paper with a printed word list (word list B) from the USC‐REMT. They were given 2 minutes to study the sheet and were informed they would be asked to remember the words the following day. One day later, after the night of actigraphy recorded sleep, subjects completed the free recall and yes/no recognition task based on what they remembered from word list B. This established delayed recall and recognition scores.
Medical Vignette
Because it is unclear how word recall and recognition tasks approximate remembering discharge instructions, we developed a 5‐sentence vignette about an outpatient medical encounter, based on the logical memory component of the Wechsler Memory Scale IV, a commonly used, validated test of memory assessment.[16, 17] After the USC‐REMT was administered following a night of sleep in the hospital, patients listened to a story and were immediately asked to repeat back in free form as much information as possible from the story. Responses were recorded by trained research assistants. The story is comprised of short sentences with simple ideas and vocabulary (see Supporting Information, Appendix 1, in the online version of this article).
Sleep: Wrist Actigraphy and Karolinska Sleep Log
Patient sleep was measured by actigraphy following the protocol described previously by our group.[7] Patients wore a wrist actigraphy monitor (Actiwatch 2; Philips Respironics, Inc., Murrysville, PA) to collect data on sleep duration and quality. The monitor detects wrist movement by measuring acceleration.[18] Actigraphy has been validated against polysomnography, demonstrating a correlation in sleep duration of 0.82 in insomniacs and 0.97 in healthy subjects.[19] Sleep duration and sleep efficiency overnight were calculated from the actigraphy data using Actiware 5 software.[20] Sleep duration was defined by the software based on low levels of recorded movement. Sleep efficiency was calculated as the percentage of time asleep out of the subjects' self‐reported time in bed, which was obtained using the Karolinska Sleep Log.[21]
The Karolinska Sleep Log questionnaire also asks patients to rate their sleep quality, restlessness during sleep, ease of falling asleep and the ability to sleep through the night on a 5‐point scale. The Karolinska Sleep Quality Index (KSQI) is calculated by averaging the latter 4 items.[22] A score of 3 or less classifies the subject in an insomniac range.[7, 21]
Demographic Information
Demographic information, including age, race, and gender were obtained by chart audit.
Data Analysis
Data were entered into REDCap, a secure online tool for managing survey data.[23]
Memory Scoring
For immediate and delayed recall scores, subjects received 1 point for every word they remembered correctly, with a maximum score of 15 words. We defined poor memory on the immediate recall test as a score of 3 or lower, based on a score utilized by Lindquist et al.[4] in a similar task. This score was less than half of the mean score of 6.63 obtained by Parker et al. for a sample of healthy 60 to 79 year olds in a sensitivity study of the USC‐REMT.[14] For immediate and delayed recognition, subjects received 1 point for correctly identifying whether a word had been on the word list they heard or whether it was a distractor, with a maximum score of 15.
A key was created to standardize scoring of the medical vignette by assigning 1 point to specific correctly remembered items from the story (see Supporting Information, Appendix 2A, in the online version of this article). These points were added to obtain a total score for correctly remembered vignette items. It was also noted when a vignette item was remembered incorrectly, for example, when the patient remembered left foot instead of right foot. Each incorrectly remembered item received 1 point, and these were summed to create the total score for incorrectly remembered vignette items (see Supporting Information, Appendix 2A, in the online version of this article for the scoring guide). Forgotten items were assigned 0 points. Two independent raters scored each subject's responses, and their scores were averaged for each item. Inter‐rater reliability was calculated as percentage of agreement across responses.
Statistical Analysis
Descriptive statistics were performed on the memory task data. Tests for skew and curtosis were performed for recall and recognition task data. The mean and standard deviation (SD) were calculated for normally distributed data, and the median and interquartile range (IQR) were obtained for data that showed significant skew. Mean and SD were also calculated for sleep duration and sleep efficiency measured by actigraphy.
Two‐tailed t tests were used to examine the association between memory and gender and African American race. Cuzick's nonparametric test of trend was used to test the association between age quartile and recall and recognition scores.[24] Mean and standard deviation for the correct total score and incorrect total score for the medical vignette were calculated. Pearson's correlation coefficient was used to examine the association between USC‐REMT memory measures and medical vignette score.
Pearson's correlation coefficient was calculated to test the associations between sleep duration and memory scores (immediate and delayed recall, immediate and delayed recognition, medical vignette task). This test was repeated to examine the relationship between sleep efficiency and the above memory scores. Linear regression models were used to characterize the relationship between inpatient sleep duration and efficiency and memory task performance. Two‐tailed t tests were used to compare sleep metrics (duration and efficiency) between high‐ and low‐memory groups, with low memory defined as immediate recall of 3 words.
All statistical tests were conducted using Stata 12.0 software (StataCorp, College Station, TX). Statistical significance was defined as P<0.05.
RESULTS
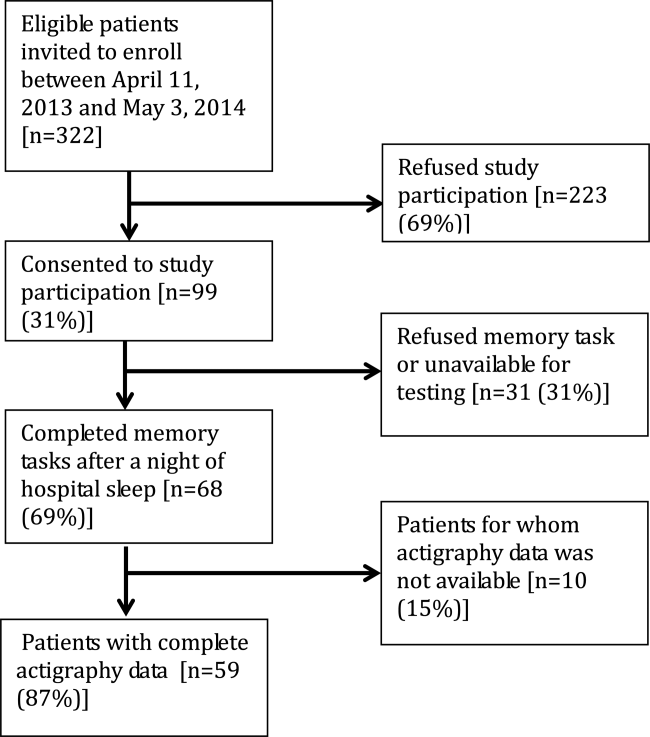
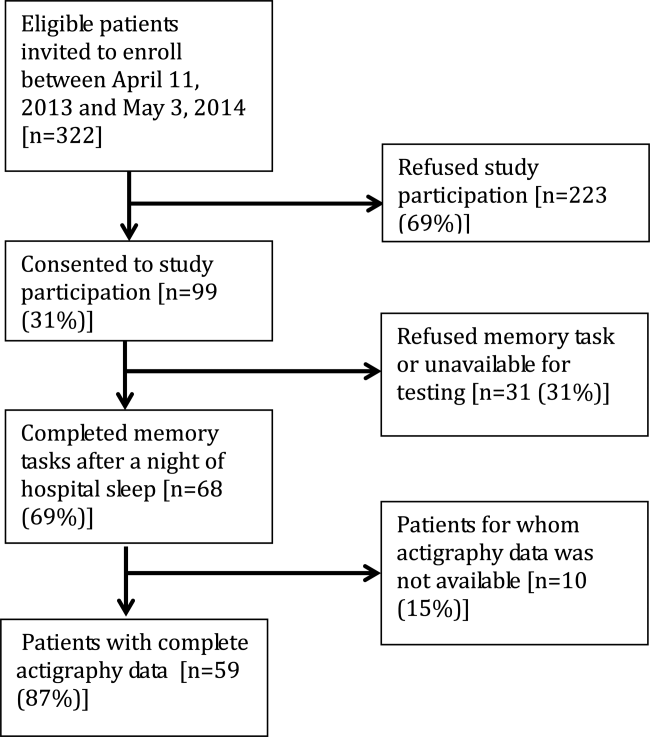
From April 11, 2013 to May 3, 2014, 322 patients were eligible for our study. Of these, 99 patients were enrolled in the study. We were able to collect sleep actigraphy data and immediate memory scores from 59 on day 2 of the study (Figure 1).
The study population had a mean age of 61.6 years (SD=9.3 years). Demographic information is presented in Table 1. Average nightly sleep in the hospital was 5.44 hours (326.4 minutes, SD=134.5 minutes), whereas mean sleep efficiency was 70.9 (SD=17.1), which is below the normal threshold of 85%.[25, 26] Forty‐four percent had a KSQI score of 3, representing in‐hospital sleep quality in the insomniac range.
| Value | |
|---|---|
| |
| Patient characteristics | |
| Age, y, mean (SD) | 61.6 (9.3) |
| Female, n (%) | 36 (61.0%) |
| BMI, n (%) | |
| Underweight (<18.5) | 3 (5.1%) |
| Normal weight (18.524.9) | 16 (27.1%) |
| Overweight (25.029.9) | 14 (23.7%) |
| Obese (30.0) | 26 (44.1%) |
| African American, n (%) | 43 (72.9%) |
| Non‐Hispanic, n (%) | 57 (96.6%) |
| Education, n (%) | |
| Did not finish high school | 13 (23.2%) |
| High school graduate | 13 (23.2%) |
| Some college or junior college | 16 (28.6%) |
| College graduate or postgraduate degree | 13 (23.2%) |
| Discharge diagnosis (ICD‐9‐CM classification), n (%) | |
| Circulatory system disease | 5 (8.5%) |
| Digestive system disease | 9 (15.3%) |
| Genitourinary system disease | 4 (6.8%) |
| Musculoskeletal system disease | 3 (5.1%) |
| Respiratory system disease | 5 (8.5%) |
| Sensory organ disease | 1 (1.7%) |
| Skin and subcutaneous tissue disease | 3 (5.1%) |
| Endocrine, nutritional, and metabolic disease | 7 (11.9%) |
| Infection and parasitic disease | 6 (10.2%) |
| Injury and poisoning | 4 (6.8%) |
| Mental disorders | 2 (3.4%) |
| Neoplasm | 5 (8.5%) |
| Symptoms, signs, and ill‐defined conditions | 5 (8.5%) |
| Comorbidities by self‐report, n=57, n (%) | |
| Cancer | 6 (10.5%) |
| Depression | 15 (26.3%) |
| Diabetes | 15 (26.3%) |
| Heart trouble | 16 (28.1%) |
| HIV/AIDS | 2 (3.5%) |
| Kidney disease | 10 (17.5%) |
| Liver disease | 9 (15.8%) |
| Stroke | 4 (7.0%) |
| Subject on the hematology and oncology service, n (%) | 6 (10.2%) |
| Sleep characteristics | |
| Nights in hospital prior to enrollment, n (%) | |
| 0 nights | 12 (20.3%) |
| 1 night | 24 (40.7%) |
| 2 nights | 17 (28.8%) |
| 3 nights | 6 (10.1%) |
| Received pharmacologic sleep aids, n (%) | 10 (17.0%) |
| Karolinska Sleep Quality Index scores, score 3, n (%) | 26 (44.1%) |
| Sleep duration, min, mean (SD) | 326.4 (134.5) |
| Sleep efficiency, %, mean (SD) | 70.9 (17.1) |
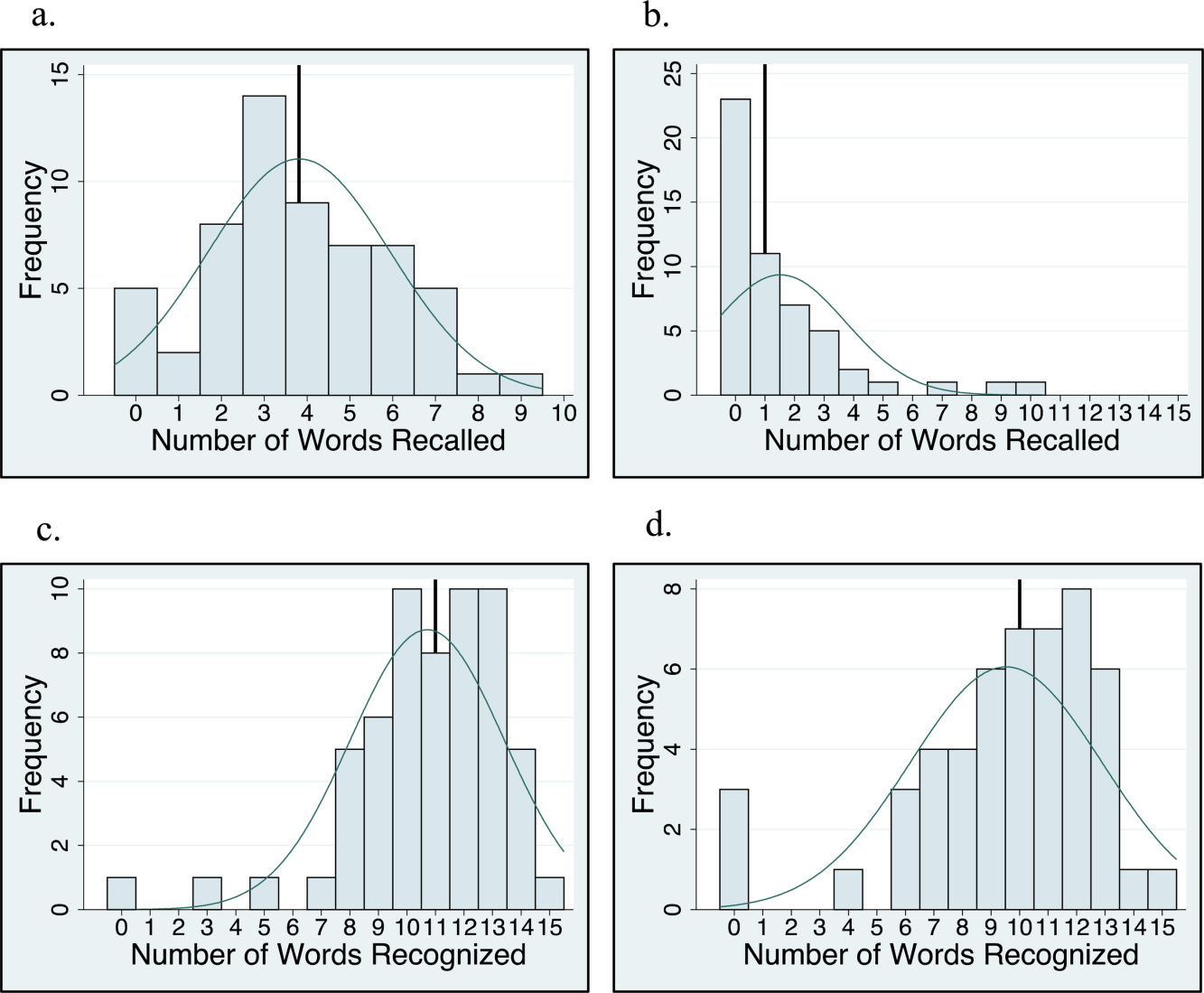
Memory test scores are presented in Figure 2. Nearly half (49%) of patients had poor memory, defined by a score of 3 words (Figure 2). Immediate recall scores varied significantly with age quartile, with older subjects recalling fewer words (Q1 [age 50.453.6 years] mean=4.9 words; Q2 [age 54.059.2 years] mean=4.1 words; Q3 [age 59.466.9 years] mean=3.7 words; Q4 [age 68.285.0 years] mean=2.5 words; P=0.001). Immediate recognition scores did not vary significantly by age quartile (Q1 [age 50.453.6 years] mean=10.3 words; Q2 [age 54.059.2 years] mean =10.3 words; Q3 [age 59.466.9 years)] mean=11.8 words; Q4 [age 68.285.0 years] mean=10.4 words; P=0.992). Fifty‐two subjects completed the delayed memory tasks. The median delayed recall score was low, at 1 word (IQR=02), with 44% of subjects remembering 0 items. Delayed memory scores were not associated with age quartile. There was no association between any memory scores and gender or African American race.
For 35 subjects in this study, we piloted the use of the medical vignette memory task. Two raters scored subject responses. Of the 525 total items, there was 98.1% agreement between the 2 raters, and only 7 out of 35 subjects' total scores differed between the 2 raters (see Supporting Information, Appendix 2B, in the online version of this article for detailed results). Median number of items remembered correctly was 4 out of 15 (IQR=26). Median number of incorrectly remembered items was 0.5 (IQR=01). Up to 57% (20 subjects) incorrectly remembered at least 1 item. The medical vignette memory score was significantly correlated with immediate recall score (r=0.49, P<0.01), but not immediate recognition score (r=0.24, P=0.16), delayed recall (r=0.13, P=0.47), or delayed recognition (r=0.01, P=0.96). There was a negative relationship between the number of items correctly recalled by a subject and the number of incorrectly recalled items on the medical vignette memory task that did not reach statistical significance (r=0.32, P=0.06).
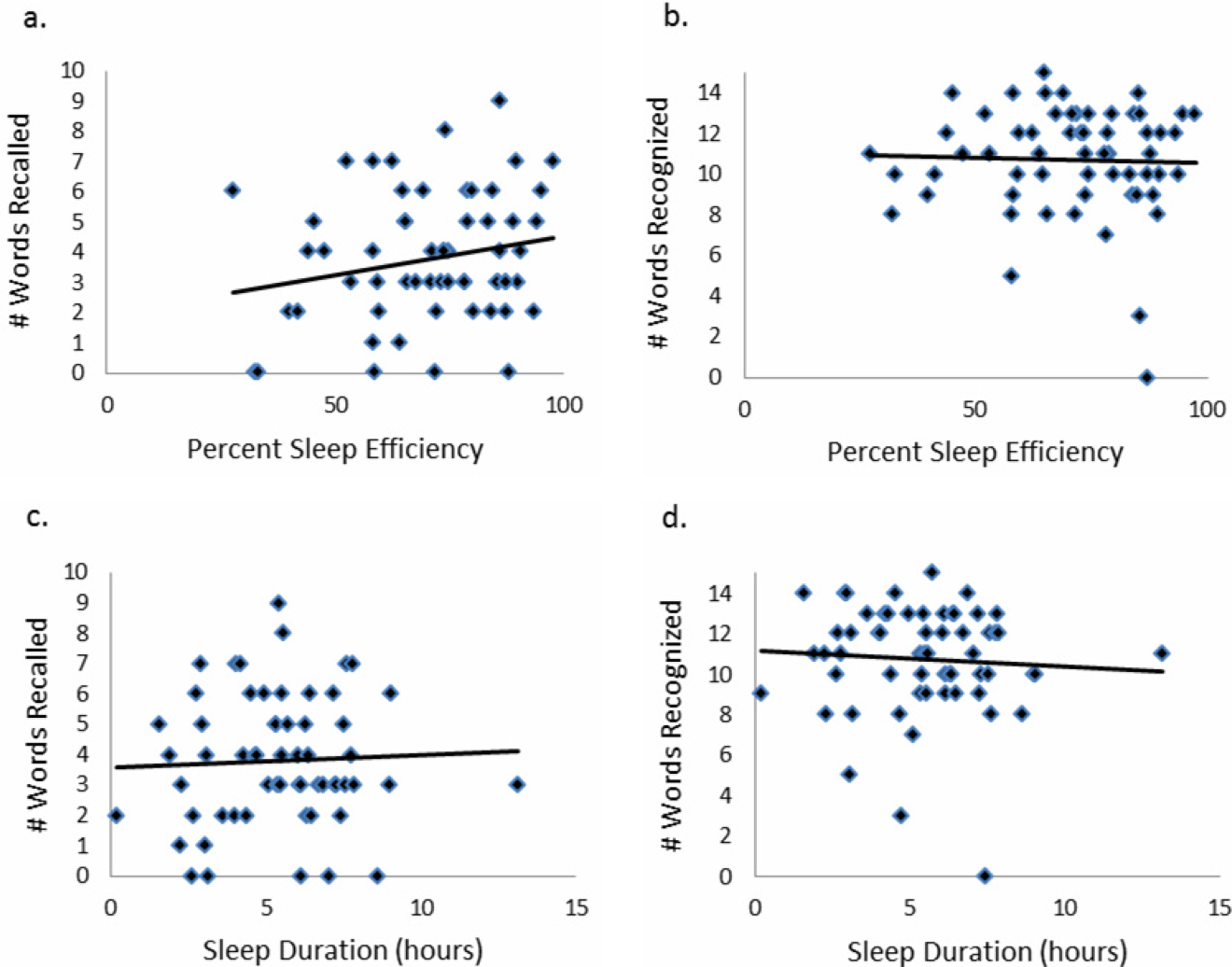
There was no association between sleep duration, sleep efficiency, and KSQI with memory scores (immediate and delayed recall, immediate and delayed recognition, medical vignette task) (Table 2.) The relationship between objective sleep measures and immediate memory are plotted in Figure 3. Finally, there was no significant difference in sleep duration or efficiency between groups with high memory (immediate recall of >3 words) and low memory (immediate recall of 3 words).
| Independent Variables | ||||
|---|---|---|---|---|
| Sleep Duration, h | Sleep Efficiency, % | Karolinska Sleep Quality Index | ||
| Immediate recall (n=59) | Pearson's r | 0.044 | 0.2 | 0.18 |
| coefficient | 0.042 | 0.025 | 0.27 | |
| P value | 0.74 | 0.12 | 0.16 | |
| Immediate recognition (n=59) | Pearson's r | 0.066 | 0.037 | 0.13 |
| coefficient | 0.080 | 0.0058 | 0.25 | |
| P value | 0.62 | 0.78 | 0.31 | |
| Delayed recall (n=52) | Pearson's r | 0.028 | 0.0020 | 0.0081 |
| coefficient | 0.027 | 0.00025 | 0.012 | |
| P value | 0.85 | 0.99 | 0.96 | |
| Delayed recognition (n=52) | Pearson's r | 0.21 | 0.12 | 0.15 |
| coefficient | 0.31 | 0.024 | 0.35 | |
| P value | 0.13 | 0.39 | 0.29 | |
CONCLUSIONS/DISCUSSION
This study demonstrated that roughly half of hospitalized older adults without diagnosed memory or cognitive impairment had poor memory using an immediate word recall task. Although performance on an immediate word recall task may not be considered a good approximation for remembering discharge instructions, immediate recall did correlate with performance on a more complex medical vignette memory task. Though our subjects had low sleep efficiency and duration while in the hospital, memory performance was not significantly associated with inpatient sleep.
Perhaps the most concerning finding in this study was the substantial number of subjects who had poor memory. In addition to scoring approximately 1 SD lower than the community sample of healthy older adults tested in the sensitivity study of USC‐REMT,[14] our subjects also scored lower on immediate recall when compared to another hospitalized patient study.[4] In the study by Lindquist et al. that utilized a similar 15‐item word recall task in hospitalized patients, 29% of subjects were found to have poor memory (recall score of 3 words), compared to 49% in our study. In our 24‐hour delayed recall task we found that 44% of our patients could not recall a single word, with 65% remembering 1 word or fewer. In their study, Lindquist et al. similarly found that greater than 50% of subjects qualified as poor memory by recalling 1 or fewer words after merely an 8‐minute delay. Given these findings, hospitalization may not be the optimal teachable moment that it is often suggested to be. Use of transition coaches, memory aids like written instructions and reminders, and involvement of caregivers are likely critical to ensuring inpatients retain instructions and knowledge. More focus also needs to be given to older patients, who often have the worst memory. Technology tools, such as the Vocera Good To Go app, could allow medical professionals to make audio recordings of discharge instructions that patients may access at any time on a mobile device.
This study also has implications for how to measure memory in inpatients. For example, a vignette‐based memory test may be appropriate for assessing inpatient memory for discharge instructions. Our task was easy to administer and correlated with immediate recall scores. Furthermore, the story‐based task helps us to establish a sense of how much information from a paragraph is truly remembered. Our data show that only 4 items of 15 were remembered, and the majority of subjects actually misremembered 1 item. This latter measure sheds light on the rate of inaccuracy of patient recall. It is worth noting also that word recognition showed a ceiling effect in our sample, suggesting the task was too easy. In contrast, delayed recall was too difficult, as scores showed a floor effect, with over half of our sample unable to recall a single word after a 24‐hour delay.
This is the first study to assess the relationship between sleep loss and memory in hospitalized patients. We found that memory scores were not significantly associated with sleep duration, sleep efficiency, or with the self‐reported KSQI. Memory during hospitalization may be affected by factors other than sleep, like cognition, obscuring the relationship between sleep and memory. It is also possible that we were unable to see a significant association between sleep and memory because of universally low sleep duration and efficiency scores in the hospital.
Our study has several limitations. Most importantly, this study includes a small number of subjects who were hospitalized on a general medicine service at a single institution, limiting generalizability. Also importantly, our data capture only 1 night of sleep, and this may limit our study's ability to detect an association between hospital sleep and memory. More longitudinal data measuring sleep and memory across a longer period of time may reveal the distinct contribution of in‐hospital sleep. We also excluded patients with known cognitive impairment from enrollment, limiting our patient population to those with only high cognitive reserve. We hypothesize that patients with dementia experience both increased sleep disturbance and greater decline in memory during hospitalization. In addition, we are unable to test causal associations in this observational study. Furthermore, we applied a standardized memory test, the USC‐REMT, in a hospital setting, where noise and other disruptions at the time of test administration cannot be completely controlled. This makes it difficult to compare our results with those of community‐dwelling members taking the test under optimal conditions. Finally, because we created our own medical vignette task, future testing to validate this method against other memory testing is warranted.
In conclusion, our results show that memory in older hospitalized inpatients is often impaired, despite patients' appearing cognitively intact. These deficits in memory are revealed by a word recall task and also by a medical vignette task that more closely approximates memory for complex discharge instructions.
Disclosure
This work was funded by the National Institute on Aging Short‐Term Aging‐Related Research Program (5T35AG029795),the National Institute on Aging Career Development Award (K23AG033763), and the National Heart Lung and Blood Institute (R25 HL116372).
- . Importance of in‐hospital initiation of evidence‐based medical therapies for heart failure: taking advantage of the teachable moment. Congest Heart Fail. 2005;11(3):153–154.
- , , , , . Smoking cessation in hospitalized patients: results of a randomized trial. Arch Intern Med. 1997;157(4):409–415.
- , , . Smoking cessation interventions for hospitalized smokers: a systematic review. Arch Intern Med. 2008;168(18):1950–1960.
- , , , , . Improvements in cognition following hospital discharge of community dwelling seniors. J Gen Intern Med. 2011;26(7):765–770.
- , , , . Sleep and aging: 1. sleep disorders commonly found in older people. Can Med Assoc J. 2007;176(9):1299–1304.
- . Noise and sleep among adult medical inpatients: far from a quiet night. Arch Intern Med. 2012;172(1):68–70.
- , , , , , . Perceived control and sleep in hospitalized older adults: a sound hypothesis? J Hosp Med. 2013;8(4):184–190.
- , . A meta‐analysis of the impact of short‐term sleep deprivation on cognitive variables. Psychol Bull. 2010;136(3):375–389.
- , . Sleep deprivation: Impact on cognitive performance. Neuropsychiatr Dis Treat. 2007;3(5):553–567.
- , , , et al. Effects of physician experience on costs and outcomes on an academic general medicine service: results of a trial of hospitalists. Ann Intern Med. 2002;137(11):866–874.
- , , . “Mini‐mental state”: a practical method for grading the cognitive state of patients for the clinician. J Psychiatr Res. 1975;12(3):189–198.
- . A short portable mental status questionnaire for the assessment of organic brain deficit in elderly patients. J Am Geriatr Soc. 1975;10:433–441.
- , , , . Reliability and validity of the Short Portable Mental Status Questionnaire administered by telephone. J Geriatr Psychiatry Neurol. 1994;7(1):33–38.
- , , , . Aging, recall and recognition: a study on the sensitivity of the University of Southern California Repeatable Episodic Memory Test (USC‐REMT). J Clin Exp Neuropsychol. 2004;26(3):428–440.
- , , , , . University of southern california repeatable episodic memory test. J Clin Exp Neuropsychol. 1995;17(6):926–936.
- , , . Development of alternate paragraphs for the logical memory subtest of the Wechsler Memory Scale‐Revised. Clin Neuropsychol. 1997;11(4):370–374.
- , , . A Compendium of Neuropsychological Tests: Administration, Norms, and Commentary. 3rd ed. New York, NY: Oxford University Press; 2009.
- . Review of physical activity measurement using accelerometers in older adults: considerations for research design and conduct. Prev Med. 2009;48(2):108–114.
- , , , , , . The actigraph data analysis software: I. A novel approach to scoring and interpreting sleep‐wake activity. Percept Mot Skills. 1997;85(1):207–216.
- , , , et al. Evaluation of immobility time for sleep latency in actigraphy. Sleep Med. 2009;10(6):621–625.
- , , , , . The subjective meaning of sleep quality: a comparison of individuals with and without insomnia. Sleep. 2008;31(3):383–393.
- , . Objective components of individual differences in subjective sleep quality. J Sleep Res. 1997;6(4):217–220.
- , , , , , . Research electronic data capture (REDCap)—a metadata‐driven methodology and workflow process for providing translational research informatics support. J Biomed Inform. 2009;42(2):377–381.
- . A Wilcoxon‐type test for trend. Stat Med. 1985;4(1):87–90.
- , , , et al. Derivation of research diagnostic criteria for insomnia: report of an American Academy of Sleep Medicine Work Group. Sleep. 2004;27(8):1567–1596.
- , , , , . Quantitative criteria for insomnia. Behav Res Ther. 2003;41(4):427–445.
Hospitalization is often utilized as a teachable moment, as patients are provided with education about treatment and disease management, particularly at discharge.[1, 2, 3] However, memory impairment among hospitalized patients may undermine the utility of the teachable moment. In one study of community‐dwelling seniors admitted to the hospital, one‐third had previously unrecognized poor memory at discharge.[4]
Sleep loss may be an underappreciated contributor to short‐term memory deficits in inpatients, particularly in seniors, who have baseline higher rates of sleep disruptions and sleep disorders.[5] Patients often receive 2 hours less sleep than at home and experience poor quality sleep due to disruptions.[6, 7] Robust studies of healthy subjects in laboratory settings demonstrate that sleep loss leads to decreased attention and worse recall, and that more sleep is associated with better memory performance.[8, 9]
Very few studies have examined memory in hospitalized patients. Although word‐list tasks are often used to assess memory because they are quick and easy to administer, these tasks may not accurately reflect memory for a set of instructions provided at patient discharge. Finally, no studies have examined the association between inpatient sleep loss and memory. Thus, our primary aim in this study was to examine memory performance in older, hospitalized patients using a word listbased memory task and a more complex medical vignette task. Our second aim was to investigate the relationship between in‐hospital sleep and memory.
METHODS
Study Design
We conducted a prospective cohort study with subjects enrolled in an ongoing sleep study at the University of Chicago Medical Center.[10] Eligible subjects were on the general medicine or hematology/oncology service, at least 50 years old, community dwelling, ambulatory, and without detectable cognitive impairment on the Mini Mental State Exam[11] or Short Portable Mental Status Questionnaire.[12, 13] Patients were excluded if they had a documented sleep disorder (ie, obstructive sleep apnea), were transferred from an intensive care unit or were in droplet or airborne isolation, had a bedrest order, or had already spent over 72 hours in the hospital prior to enrollment. These criteria were used to select a population appropriate for wristwatch actigraphy and with low likelihood of baseline memory impairment. The University of Chicago Institutional Review Board approved this study, and participants provided written consent.
Data Collection
Memory Testing
Memory was evaluated using the University of Southern California Repeatable Episodic Memory Test (USC‐REMT), a validated verbal memory test in which subjects listen to a list of 15 words and then complete free‐recall and recognition of the list.[14, 15] Free‐recall tests subjects' ability to procure information without cues. In contrast, recognition requires subjects to pick out the words they just heard from distractors, an easier task. The USC‐REMT contains multiple functionally equivalent different word lists, and may be administered more than once to the same subject without learning effects.[15] Immediate and delayed memory were tested by asking the subject to complete the tasks immediately after listening to the word list and 24‐hours after listening to the list, respectively.
Immediate Recall and Recognition
Recall and recognition following a night of sleep in the hospital was the primary outcome for this study. After 1 night of actigraphy recorded sleep, subjects listened as a 15‐item word list (word list A) was read aloud. For the free‐recall task, subjects were asked to repeat back all the words they could remember immediately after hearing the list. For the recognition task, subjects were read a new list of 15 words, including a mix of words from the previous list and new distractor words. They answered yes if they thought the word had previously been read to them and no if they thought the word was new.
Delayed Recall and Delayed Recognition
At the conclusion of study enrollment on day 1 prior to the night of actigraphy, subjects were shown a laminated paper with a printed word list (word list B) from the USC‐REMT. They were given 2 minutes to study the sheet and were informed they would be asked to remember the words the following day. One day later, after the night of actigraphy recorded sleep, subjects completed the free recall and yes/no recognition task based on what they remembered from word list B. This established delayed recall and recognition scores.
Medical Vignette
Because it is unclear how word recall and recognition tasks approximate remembering discharge instructions, we developed a 5‐sentence vignette about an outpatient medical encounter, based on the logical memory component of the Wechsler Memory Scale IV, a commonly used, validated test of memory assessment.[16, 17] After the USC‐REMT was administered following a night of sleep in the hospital, patients listened to a story and were immediately asked to repeat back in free form as much information as possible from the story. Responses were recorded by trained research assistants. The story is comprised of short sentences with simple ideas and vocabulary (see Supporting Information, Appendix 1, in the online version of this article).
Sleep: Wrist Actigraphy and Karolinska Sleep Log
Patient sleep was measured by actigraphy following the protocol described previously by our group.[7] Patients wore a wrist actigraphy monitor (Actiwatch 2; Philips Respironics, Inc., Murrysville, PA) to collect data on sleep duration and quality. The monitor detects wrist movement by measuring acceleration.[18] Actigraphy has been validated against polysomnography, demonstrating a correlation in sleep duration of 0.82 in insomniacs and 0.97 in healthy subjects.[19] Sleep duration and sleep efficiency overnight were calculated from the actigraphy data using Actiware 5 software.[20] Sleep duration was defined by the software based on low levels of recorded movement. Sleep efficiency was calculated as the percentage of time asleep out of the subjects' self‐reported time in bed, which was obtained using the Karolinska Sleep Log.[21]
The Karolinska Sleep Log questionnaire also asks patients to rate their sleep quality, restlessness during sleep, ease of falling asleep and the ability to sleep through the night on a 5‐point scale. The Karolinska Sleep Quality Index (KSQI) is calculated by averaging the latter 4 items.[22] A score of 3 or less classifies the subject in an insomniac range.[7, 21]
Demographic Information
Demographic information, including age, race, and gender were obtained by chart audit.
Data Analysis
Data were entered into REDCap, a secure online tool for managing survey data.[23]
Memory Scoring
For immediate and delayed recall scores, subjects received 1 point for every word they remembered correctly, with a maximum score of 15 words. We defined poor memory on the immediate recall test as a score of 3 or lower, based on a score utilized by Lindquist et al.[4] in a similar task. This score was less than half of the mean score of 6.63 obtained by Parker et al. for a sample of healthy 60 to 79 year olds in a sensitivity study of the USC‐REMT.[14] For immediate and delayed recognition, subjects received 1 point for correctly identifying whether a word had been on the word list they heard or whether it was a distractor, with a maximum score of 15.
A key was created to standardize scoring of the medical vignette by assigning 1 point to specific correctly remembered items from the story (see Supporting Information, Appendix 2A, in the online version of this article). These points were added to obtain a total score for correctly remembered vignette items. It was also noted when a vignette item was remembered incorrectly, for example, when the patient remembered left foot instead of right foot. Each incorrectly remembered item received 1 point, and these were summed to create the total score for incorrectly remembered vignette items (see Supporting Information, Appendix 2A, in the online version of this article for the scoring guide). Forgotten items were assigned 0 points. Two independent raters scored each subject's responses, and their scores were averaged for each item. Inter‐rater reliability was calculated as percentage of agreement across responses.
Statistical Analysis
Descriptive statistics were performed on the memory task data. Tests for skew and curtosis were performed for recall and recognition task data. The mean and standard deviation (SD) were calculated for normally distributed data, and the median and interquartile range (IQR) were obtained for data that showed significant skew. Mean and SD were also calculated for sleep duration and sleep efficiency measured by actigraphy.
Two‐tailed t tests were used to examine the association between memory and gender and African American race. Cuzick's nonparametric test of trend was used to test the association between age quartile and recall and recognition scores.[24] Mean and standard deviation for the correct total score and incorrect total score for the medical vignette were calculated. Pearson's correlation coefficient was used to examine the association between USC‐REMT memory measures and medical vignette score.
Pearson's correlation coefficient was calculated to test the associations between sleep duration and memory scores (immediate and delayed recall, immediate and delayed recognition, medical vignette task). This test was repeated to examine the relationship between sleep efficiency and the above memory scores. Linear regression models were used to characterize the relationship between inpatient sleep duration and efficiency and memory task performance. Two‐tailed t tests were used to compare sleep metrics (duration and efficiency) between high‐ and low‐memory groups, with low memory defined as immediate recall of 3 words.
All statistical tests were conducted using Stata 12.0 software (StataCorp, College Station, TX). Statistical significance was defined as P<0.05.
RESULTS
From April 11, 2013 to May 3, 2014, 322 patients were eligible for our study. Of these, 99 patients were enrolled in the study. We were able to collect sleep actigraphy data and immediate memory scores from 59 on day 2 of the study (Figure 1).

The study population had a mean age of 61.6 years (SD=9.3 years). Demographic information is presented in Table 1. Average nightly sleep in the hospital was 5.44 hours (326.4 minutes, SD=134.5 minutes), whereas mean sleep efficiency was 70.9 (SD=17.1), which is below the normal threshold of 85%.[25, 26] Forty‐four percent had a KSQI score of 3, representing in‐hospital sleep quality in the insomniac range.
| Value | |
|---|---|
| |
| Patient characteristics | |
| Age, y, mean (SD) | 61.6 (9.3) |
| Female, n (%) | 36 (61.0%) |
| BMI, n (%) | |
| Underweight (<18.5) | 3 (5.1%) |
| Normal weight (18.524.9) | 16 (27.1%) |
| Overweight (25.029.9) | 14 (23.7%) |
| Obese (30.0) | 26 (44.1%) |
| African American, n (%) | 43 (72.9%) |
| Non‐Hispanic, n (%) | 57 (96.6%) |
| Education, n (%) | |
| Did not finish high school | 13 (23.2%) |
| High school graduate | 13 (23.2%) |
| Some college or junior college | 16 (28.6%) |
| College graduate or postgraduate degree | 13 (23.2%) |
| Discharge diagnosis (ICD‐9‐CM classification), n (%) | |
| Circulatory system disease | 5 (8.5%) |
| Digestive system disease | 9 (15.3%) |
| Genitourinary system disease | 4 (6.8%) |
| Musculoskeletal system disease | 3 (5.1%) |
| Respiratory system disease | 5 (8.5%) |
| Sensory organ disease | 1 (1.7%) |
| Skin and subcutaneous tissue disease | 3 (5.1%) |
| Endocrine, nutritional, and metabolic disease | 7 (11.9%) |
| Infection and parasitic disease | 6 (10.2%) |
| Injury and poisoning | 4 (6.8%) |
| Mental disorders | 2 (3.4%) |
| Neoplasm | 5 (8.5%) |
| Symptoms, signs, and ill‐defined conditions | 5 (8.5%) |
| Comorbidities by self‐report, n=57, n (%) | |
| Cancer | 6 (10.5%) |
| Depression | 15 (26.3%) |
| Diabetes | 15 (26.3%) |
| Heart trouble | 16 (28.1%) |
| HIV/AIDS | 2 (3.5%) |
| Kidney disease | 10 (17.5%) |
| Liver disease | 9 (15.8%) |
| Stroke | 4 (7.0%) |
| Subject on the hematology and oncology service, n (%) | 6 (10.2%) |
| Sleep characteristics | |
| Nights in hospital prior to enrollment, n (%) | |
| 0 nights | 12 (20.3%) |
| 1 night | 24 (40.7%) |
| 2 nights | 17 (28.8%) |
| 3 nights | 6 (10.1%) |
| Received pharmacologic sleep aids, n (%) | 10 (17.0%) |
| Karolinska Sleep Quality Index scores, score 3, n (%) | 26 (44.1%) |
| Sleep duration, min, mean (SD) | 326.4 (134.5) |
| Sleep efficiency, %, mean (SD) | 70.9 (17.1) |
Memory test scores are presented in Figure 2. Nearly half (49%) of patients had poor memory, defined by a score of 3 words (Figure 2). Immediate recall scores varied significantly with age quartile, with older subjects recalling fewer words (Q1 [age 50.453.6 years] mean=4.9 words; Q2 [age 54.059.2 years] mean=4.1 words; Q3 [age 59.466.9 years] mean=3.7 words; Q4 [age 68.285.0 years] mean=2.5 words; P=0.001). Immediate recognition scores did not vary significantly by age quartile (Q1 [age 50.453.6 years] mean=10.3 words; Q2 [age 54.059.2 years] mean =10.3 words; Q3 [age 59.466.9 years)] mean=11.8 words; Q4 [age 68.285.0 years] mean=10.4 words; P=0.992). Fifty‐two subjects completed the delayed memory tasks. The median delayed recall score was low, at 1 word (IQR=02), with 44% of subjects remembering 0 items. Delayed memory scores were not associated with age quartile. There was no association between any memory scores and gender or African American race.

For 35 subjects in this study, we piloted the use of the medical vignette memory task. Two raters scored subject responses. Of the 525 total items, there was 98.1% agreement between the 2 raters, and only 7 out of 35 subjects' total scores differed between the 2 raters (see Supporting Information, Appendix 2B, in the online version of this article for detailed results). Median number of items remembered correctly was 4 out of 15 (IQR=26). Median number of incorrectly remembered items was 0.5 (IQR=01). Up to 57% (20 subjects) incorrectly remembered at least 1 item. The medical vignette memory score was significantly correlated with immediate recall score (r=0.49, P<0.01), but not immediate recognition score (r=0.24, P=0.16), delayed recall (r=0.13, P=0.47), or delayed recognition (r=0.01, P=0.96). There was a negative relationship between the number of items correctly recalled by a subject and the number of incorrectly recalled items on the medical vignette memory task that did not reach statistical significance (r=0.32, P=0.06).
There was no association between sleep duration, sleep efficiency, and KSQI with memory scores (immediate and delayed recall, immediate and delayed recognition, medical vignette task) (Table 2.) The relationship between objective sleep measures and immediate memory are plotted in Figure 3. Finally, there was no significant difference in sleep duration or efficiency between groups with high memory (immediate recall of >3 words) and low memory (immediate recall of 3 words).
| Independent Variables | ||||
|---|---|---|---|---|
| Sleep Duration, h | Sleep Efficiency, % | Karolinska Sleep Quality Index | ||
| Immediate recall (n=59) | Pearson's r | 0.044 | 0.2 | 0.18 |
| coefficient | 0.042 | 0.025 | 0.27 | |
| P value | 0.74 | 0.12 | 0.16 | |
| Immediate recognition (n=59) | Pearson's r | 0.066 | 0.037 | 0.13 |
| coefficient | 0.080 | 0.0058 | 0.25 | |
| P value | 0.62 | 0.78 | 0.31 | |
| Delayed recall (n=52) | Pearson's r | 0.028 | 0.0020 | 0.0081 |
| coefficient | 0.027 | 0.00025 | 0.012 | |
| P value | 0.85 | 0.99 | 0.96 | |
| Delayed recognition (n=52) | Pearson's r | 0.21 | 0.12 | 0.15 |
| coefficient | 0.31 | 0.024 | 0.35 | |
| P value | 0.13 | 0.39 | 0.29 | |

CONCLUSIONS/DISCUSSION
This study demonstrated that roughly half of hospitalized older adults without diagnosed memory or cognitive impairment had poor memory using an immediate word recall task. Although performance on an immediate word recall task may not be considered a good approximation for remembering discharge instructions, immediate recall did correlate with performance on a more complex medical vignette memory task. Though our subjects had low sleep efficiency and duration while in the hospital, memory performance was not significantly associated with inpatient sleep.
Perhaps the most concerning finding in this study was the substantial number of subjects who had poor memory. In addition to scoring approximately 1 SD lower than the community sample of healthy older adults tested in the sensitivity study of USC‐REMT,[14] our subjects also scored lower on immediate recall when compared to another hospitalized patient study.[4] In the study by Lindquist et al. that utilized a similar 15‐item word recall task in hospitalized patients, 29% of subjects were found to have poor memory (recall score of 3 words), compared to 49% in our study. In our 24‐hour delayed recall task we found that 44% of our patients could not recall a single word, with 65% remembering 1 word or fewer. In their study, Lindquist et al. similarly found that greater than 50% of subjects qualified as poor memory by recalling 1 or fewer words after merely an 8‐minute delay. Given these findings, hospitalization may not be the optimal teachable moment that it is often suggested to be. Use of transition coaches, memory aids like written instructions and reminders, and involvement of caregivers are likely critical to ensuring inpatients retain instructions and knowledge. More focus also needs to be given to older patients, who often have the worst memory. Technology tools, such as the Vocera Good To Go app, could allow medical professionals to make audio recordings of discharge instructions that patients may access at any time on a mobile device.
This study also has implications for how to measure memory in inpatients. For example, a vignette‐based memory test may be appropriate for assessing inpatient memory for discharge instructions. Our task was easy to administer and correlated with immediate recall scores. Furthermore, the story‐based task helps us to establish a sense of how much information from a paragraph is truly remembered. Our data show that only 4 items of 15 were remembered, and the majority of subjects actually misremembered 1 item. This latter measure sheds light on the rate of inaccuracy of patient recall. It is worth noting also that word recognition showed a ceiling effect in our sample, suggesting the task was too easy. In contrast, delayed recall was too difficult, as scores showed a floor effect, with over half of our sample unable to recall a single word after a 24‐hour delay.
This is the first study to assess the relationship between sleep loss and memory in hospitalized patients. We found that memory scores were not significantly associated with sleep duration, sleep efficiency, or with the self‐reported KSQI. Memory during hospitalization may be affected by factors other than sleep, like cognition, obscuring the relationship between sleep and memory. It is also possible that we were unable to see a significant association between sleep and memory because of universally low sleep duration and efficiency scores in the hospital.
Our study has several limitations. Most importantly, this study includes a small number of subjects who were hospitalized on a general medicine service at a single institution, limiting generalizability. Also importantly, our data capture only 1 night of sleep, and this may limit our study's ability to detect an association between hospital sleep and memory. More longitudinal data measuring sleep and memory across a longer period of time may reveal the distinct contribution of in‐hospital sleep. We also excluded patients with known cognitive impairment from enrollment, limiting our patient population to those with only high cognitive reserve. We hypothesize that patients with dementia experience both increased sleep disturbance and greater decline in memory during hospitalization. In addition, we are unable to test causal associations in this observational study. Furthermore, we applied a standardized memory test, the USC‐REMT, in a hospital setting, where noise and other disruptions at the time of test administration cannot be completely controlled. This makes it difficult to compare our results with those of community‐dwelling members taking the test under optimal conditions. Finally, because we created our own medical vignette task, future testing to validate this method against other memory testing is warranted.
In conclusion, our results show that memory in older hospitalized inpatients is often impaired, despite patients' appearing cognitively intact. These deficits in memory are revealed by a word recall task and also by a medical vignette task that more closely approximates memory for complex discharge instructions.
Disclosure
This work was funded by the National Institute on Aging Short‐Term Aging‐Related Research Program (5T35AG029795),the National Institute on Aging Career Development Award (K23AG033763), and the National Heart Lung and Blood Institute (R25 HL116372).
Hospitalization is often utilized as a teachable moment, as patients are provided with education about treatment and disease management, particularly at discharge.[1, 2, 3] However, memory impairment among hospitalized patients may undermine the utility of the teachable moment. In one study of community‐dwelling seniors admitted to the hospital, one‐third had previously unrecognized poor memory at discharge.[4]
Sleep loss may be an underappreciated contributor to short‐term memory deficits in inpatients, particularly in seniors, who have baseline higher rates of sleep disruptions and sleep disorders.[5] Patients often receive 2 hours less sleep than at home and experience poor quality sleep due to disruptions.[6, 7] Robust studies of healthy subjects in laboratory settings demonstrate that sleep loss leads to decreased attention and worse recall, and that more sleep is associated with better memory performance.[8, 9]
Very few studies have examined memory in hospitalized patients. Although word‐list tasks are often used to assess memory because they are quick and easy to administer, these tasks may not accurately reflect memory for a set of instructions provided at patient discharge. Finally, no studies have examined the association between inpatient sleep loss and memory. Thus, our primary aim in this study was to examine memory performance in older, hospitalized patients using a word listbased memory task and a more complex medical vignette task. Our second aim was to investigate the relationship between in‐hospital sleep and memory.
METHODS
Study Design
We conducted a prospective cohort study with subjects enrolled in an ongoing sleep study at the University of Chicago Medical Center.[10] Eligible subjects were on the general medicine or hematology/oncology service, at least 50 years old, community dwelling, ambulatory, and without detectable cognitive impairment on the Mini Mental State Exam[11] or Short Portable Mental Status Questionnaire.[12, 13] Patients were excluded if they had a documented sleep disorder (ie, obstructive sleep apnea), were transferred from an intensive care unit or were in droplet or airborne isolation, had a bedrest order, or had already spent over 72 hours in the hospital prior to enrollment. These criteria were used to select a population appropriate for wristwatch actigraphy and with low likelihood of baseline memory impairment. The University of Chicago Institutional Review Board approved this study, and participants provided written consent.
Data Collection
Memory Testing
Memory was evaluated using the University of Southern California Repeatable Episodic Memory Test (USC‐REMT), a validated verbal memory test in which subjects listen to a list of 15 words and then complete free‐recall and recognition of the list.[14, 15] Free‐recall tests subjects' ability to procure information without cues. In contrast, recognition requires subjects to pick out the words they just heard from distractors, an easier task. The USC‐REMT contains multiple functionally equivalent different word lists, and may be administered more than once to the same subject without learning effects.[15] Immediate and delayed memory were tested by asking the subject to complete the tasks immediately after listening to the word list and 24‐hours after listening to the list, respectively.
Immediate Recall and Recognition
Recall and recognition following a night of sleep in the hospital was the primary outcome for this study. After 1 night of actigraphy recorded sleep, subjects listened as a 15‐item word list (word list A) was read aloud. For the free‐recall task, subjects were asked to repeat back all the words they could remember immediately after hearing the list. For the recognition task, subjects were read a new list of 15 words, including a mix of words from the previous list and new distractor words. They answered yes if they thought the word had previously been read to them and no if they thought the word was new.
Delayed Recall and Delayed Recognition
At the conclusion of study enrollment on day 1 prior to the night of actigraphy, subjects were shown a laminated paper with a printed word list (word list B) from the USC‐REMT. They were given 2 minutes to study the sheet and were informed they would be asked to remember the words the following day. One day later, after the night of actigraphy recorded sleep, subjects completed the free recall and yes/no recognition task based on what they remembered from word list B. This established delayed recall and recognition scores.
Medical Vignette
Because it is unclear how word recall and recognition tasks approximate remembering discharge instructions, we developed a 5‐sentence vignette about an outpatient medical encounter, based on the logical memory component of the Wechsler Memory Scale IV, a commonly used, validated test of memory assessment.[16, 17] After the USC‐REMT was administered following a night of sleep in the hospital, patients listened to a story and were immediately asked to repeat back in free form as much information as possible from the story. Responses were recorded by trained research assistants. The story is comprised of short sentences with simple ideas and vocabulary (see Supporting Information, Appendix 1, in the online version of this article).
Sleep: Wrist Actigraphy and Karolinska Sleep Log
Patient sleep was measured by actigraphy following the protocol described previously by our group.[7] Patients wore a wrist actigraphy monitor (Actiwatch 2; Philips Respironics, Inc., Murrysville, PA) to collect data on sleep duration and quality. The monitor detects wrist movement by measuring acceleration.[18] Actigraphy has been validated against polysomnography, demonstrating a correlation in sleep duration of 0.82 in insomniacs and 0.97 in healthy subjects.[19] Sleep duration and sleep efficiency overnight were calculated from the actigraphy data using Actiware 5 software.[20] Sleep duration was defined by the software based on low levels of recorded movement. Sleep efficiency was calculated as the percentage of time asleep out of the subjects' self‐reported time in bed, which was obtained using the Karolinska Sleep Log.[21]
The Karolinska Sleep Log questionnaire also asks patients to rate their sleep quality, restlessness during sleep, ease of falling asleep and the ability to sleep through the night on a 5‐point scale. The Karolinska Sleep Quality Index (KSQI) is calculated by averaging the latter 4 items.[22] A score of 3 or less classifies the subject in an insomniac range.[7, 21]
Demographic Information
Demographic information, including age, race, and gender were obtained by chart audit.
Data Analysis
Data were entered into REDCap, a secure online tool for managing survey data.[23]
Memory Scoring
For immediate and delayed recall scores, subjects received 1 point for every word they remembered correctly, with a maximum score of 15 words. We defined poor memory on the immediate recall test as a score of 3 or lower, based on a score utilized by Lindquist et al.[4] in a similar task. This score was less than half of the mean score of 6.63 obtained by Parker et al. for a sample of healthy 60 to 79 year olds in a sensitivity study of the USC‐REMT.[14] For immediate and delayed recognition, subjects received 1 point for correctly identifying whether a word had been on the word list they heard or whether it was a distractor, with a maximum score of 15.
A key was created to standardize scoring of the medical vignette by assigning 1 point to specific correctly remembered items from the story (see Supporting Information, Appendix 2A, in the online version of this article). These points were added to obtain a total score for correctly remembered vignette items. It was also noted when a vignette item was remembered incorrectly, for example, when the patient remembered left foot instead of right foot. Each incorrectly remembered item received 1 point, and these were summed to create the total score for incorrectly remembered vignette items (see Supporting Information, Appendix 2A, in the online version of this article for the scoring guide). Forgotten items were assigned 0 points. Two independent raters scored each subject's responses, and their scores were averaged for each item. Inter‐rater reliability was calculated as percentage of agreement across responses.
Statistical Analysis
Descriptive statistics were performed on the memory task data. Tests for skew and curtosis were performed for recall and recognition task data. The mean and standard deviation (SD) were calculated for normally distributed data, and the median and interquartile range (IQR) were obtained for data that showed significant skew. Mean and SD were also calculated for sleep duration and sleep efficiency measured by actigraphy.
Two‐tailed t tests were used to examine the association between memory and gender and African American race. Cuzick's nonparametric test of trend was used to test the association between age quartile and recall and recognition scores.[24] Mean and standard deviation for the correct total score and incorrect total score for the medical vignette were calculated. Pearson's correlation coefficient was used to examine the association between USC‐REMT memory measures and medical vignette score.
Pearson's correlation coefficient was calculated to test the associations between sleep duration and memory scores (immediate and delayed recall, immediate and delayed recognition, medical vignette task). This test was repeated to examine the relationship between sleep efficiency and the above memory scores. Linear regression models were used to characterize the relationship between inpatient sleep duration and efficiency and memory task performance. Two‐tailed t tests were used to compare sleep metrics (duration and efficiency) between high‐ and low‐memory groups, with low memory defined as immediate recall of 3 words.
All statistical tests were conducted using Stata 12.0 software (StataCorp, College Station, TX). Statistical significance was defined as P<0.05.
RESULTS
From April 11, 2013 to May 3, 2014, 322 patients were eligible for our study. Of these, 99 patients were enrolled in the study. We were able to collect sleep actigraphy data and immediate memory scores from 59 on day 2 of the study (Figure 1).

The study population had a mean age of 61.6 years (SD=9.3 years). Demographic information is presented in Table 1. Average nightly sleep in the hospital was 5.44 hours (326.4 minutes, SD=134.5 minutes), whereas mean sleep efficiency was 70.9 (SD=17.1), which is below the normal threshold of 85%.[25, 26] Forty‐four percent had a KSQI score of 3, representing in‐hospital sleep quality in the insomniac range.
| Value | |
|---|---|
| |
| Patient characteristics | |
| Age, y, mean (SD) | 61.6 (9.3) |
| Female, n (%) | 36 (61.0%) |
| BMI, n (%) | |
| Underweight (<18.5) | 3 (5.1%) |
| Normal weight (18.524.9) | 16 (27.1%) |
| Overweight (25.029.9) | 14 (23.7%) |
| Obese (30.0) | 26 (44.1%) |
| African American, n (%) | 43 (72.9%) |
| Non‐Hispanic, n (%) | 57 (96.6%) |
| Education, n (%) | |
| Did not finish high school | 13 (23.2%) |
| High school graduate | 13 (23.2%) |
| Some college or junior college | 16 (28.6%) |
| College graduate or postgraduate degree | 13 (23.2%) |
| Discharge diagnosis (ICD‐9‐CM classification), n (%) | |
| Circulatory system disease | 5 (8.5%) |
| Digestive system disease | 9 (15.3%) |
| Genitourinary system disease | 4 (6.8%) |
| Musculoskeletal system disease | 3 (5.1%) |
| Respiratory system disease | 5 (8.5%) |
| Sensory organ disease | 1 (1.7%) |
| Skin and subcutaneous tissue disease | 3 (5.1%) |
| Endocrine, nutritional, and metabolic disease | 7 (11.9%) |
| Infection and parasitic disease | 6 (10.2%) |
| Injury and poisoning | 4 (6.8%) |
| Mental disorders | 2 (3.4%) |
| Neoplasm | 5 (8.5%) |
| Symptoms, signs, and ill‐defined conditions | 5 (8.5%) |
| Comorbidities by self‐report, n=57, n (%) | |
| Cancer | 6 (10.5%) |
| Depression | 15 (26.3%) |
| Diabetes | 15 (26.3%) |
| Heart trouble | 16 (28.1%) |
| HIV/AIDS | 2 (3.5%) |
| Kidney disease | 10 (17.5%) |
| Liver disease | 9 (15.8%) |
| Stroke | 4 (7.0%) |
| Subject on the hematology and oncology service, n (%) | 6 (10.2%) |
| Sleep characteristics | |
| Nights in hospital prior to enrollment, n (%) | |
| 0 nights | 12 (20.3%) |
| 1 night | 24 (40.7%) |
| 2 nights | 17 (28.8%) |
| 3 nights | 6 (10.1%) |
| Received pharmacologic sleep aids, n (%) | 10 (17.0%) |
| Karolinska Sleep Quality Index scores, score 3, n (%) | 26 (44.1%) |
| Sleep duration, min, mean (SD) | 326.4 (134.5) |
| Sleep efficiency, %, mean (SD) | 70.9 (17.1) |
Memory test scores are presented in Figure 2. Nearly half (49%) of patients had poor memory, defined by a score of 3 words (Figure 2). Immediate recall scores varied significantly with age quartile, with older subjects recalling fewer words (Q1 [age 50.453.6 years] mean=4.9 words; Q2 [age 54.059.2 years] mean=4.1 words; Q3 [age 59.466.9 years] mean=3.7 words; Q4 [age 68.285.0 years] mean=2.5 words; P=0.001). Immediate recognition scores did not vary significantly by age quartile (Q1 [age 50.453.6 years] mean=10.3 words; Q2 [age 54.059.2 years] mean =10.3 words; Q3 [age 59.466.9 years)] mean=11.8 words; Q4 [age 68.285.0 years] mean=10.4 words; P=0.992). Fifty‐two subjects completed the delayed memory tasks. The median delayed recall score was low, at 1 word (IQR=02), with 44% of subjects remembering 0 items. Delayed memory scores were not associated with age quartile. There was no association between any memory scores and gender or African American race.

For 35 subjects in this study, we piloted the use of the medical vignette memory task. Two raters scored subject responses. Of the 525 total items, there was 98.1% agreement between the 2 raters, and only 7 out of 35 subjects' total scores differed between the 2 raters (see Supporting Information, Appendix 2B, in the online version of this article for detailed results). Median number of items remembered correctly was 4 out of 15 (IQR=26). Median number of incorrectly remembered items was 0.5 (IQR=01). Up to 57% (20 subjects) incorrectly remembered at least 1 item. The medical vignette memory score was significantly correlated with immediate recall score (r=0.49, P<0.01), but not immediate recognition score (r=0.24, P=0.16), delayed recall (r=0.13, P=0.47), or delayed recognition (r=0.01, P=0.96). There was a negative relationship between the number of items correctly recalled by a subject and the number of incorrectly recalled items on the medical vignette memory task that did not reach statistical significance (r=0.32, P=0.06).
There was no association between sleep duration, sleep efficiency, and KSQI with memory scores (immediate and delayed recall, immediate and delayed recognition, medical vignette task) (Table 2.) The relationship between objective sleep measures and immediate memory are plotted in Figure 3. Finally, there was no significant difference in sleep duration or efficiency between groups with high memory (immediate recall of >3 words) and low memory (immediate recall of 3 words).
| Independent Variables | ||||
|---|---|---|---|---|
| Sleep Duration, h | Sleep Efficiency, % | Karolinska Sleep Quality Index | ||
| Immediate recall (n=59) | Pearson's r | 0.044 | 0.2 | 0.18 |
| coefficient | 0.042 | 0.025 | 0.27 | |
| P value | 0.74 | 0.12 | 0.16 | |
| Immediate recognition (n=59) | Pearson's r | 0.066 | 0.037 | 0.13 |
| coefficient | 0.080 | 0.0058 | 0.25 | |
| P value | 0.62 | 0.78 | 0.31 | |
| Delayed recall (n=52) | Pearson's r | 0.028 | 0.0020 | 0.0081 |
| coefficient | 0.027 | 0.00025 | 0.012 | |
| P value | 0.85 | 0.99 | 0.96 | |
| Delayed recognition (n=52) | Pearson's r | 0.21 | 0.12 | 0.15 |
| coefficient | 0.31 | 0.024 | 0.35 | |
| P value | 0.13 | 0.39 | 0.29 | |

CONCLUSIONS/DISCUSSION
This study demonstrated that roughly half of hospitalized older adults without diagnosed memory or cognitive impairment had poor memory using an immediate word recall task. Although performance on an immediate word recall task may not be considered a good approximation for remembering discharge instructions, immediate recall did correlate with performance on a more complex medical vignette memory task. Though our subjects had low sleep efficiency and duration while in the hospital, memory performance was not significantly associated with inpatient sleep.
Perhaps the most concerning finding in this study was the substantial number of subjects who had poor memory. In addition to scoring approximately 1 SD lower than the community sample of healthy older adults tested in the sensitivity study of USC‐REMT,[14] our subjects also scored lower on immediate recall when compared to another hospitalized patient study.[4] In the study by Lindquist et al. that utilized a similar 15‐item word recall task in hospitalized patients, 29% of subjects were found to have poor memory (recall score of 3 words), compared to 49% in our study. In our 24‐hour delayed recall task we found that 44% of our patients could not recall a single word, with 65% remembering 1 word or fewer. In their study, Lindquist et al. similarly found that greater than 50% of subjects qualified as poor memory by recalling 1 or fewer words after merely an 8‐minute delay. Given these findings, hospitalization may not be the optimal teachable moment that it is often suggested to be. Use of transition coaches, memory aids like written instructions and reminders, and involvement of caregivers are likely critical to ensuring inpatients retain instructions and knowledge. More focus also needs to be given to older patients, who often have the worst memory. Technology tools, such as the Vocera Good To Go app, could allow medical professionals to make audio recordings of discharge instructions that patients may access at any time on a mobile device.
This study also has implications for how to measure memory in inpatients. For example, a vignette‐based memory test may be appropriate for assessing inpatient memory for discharge instructions. Our task was easy to administer and correlated with immediate recall scores. Furthermore, the story‐based task helps us to establish a sense of how much information from a paragraph is truly remembered. Our data show that only 4 items of 15 were remembered, and the majority of subjects actually misremembered 1 item. This latter measure sheds light on the rate of inaccuracy of patient recall. It is worth noting also that word recognition showed a ceiling effect in our sample, suggesting the task was too easy. In contrast, delayed recall was too difficult, as scores showed a floor effect, with over half of our sample unable to recall a single word after a 24‐hour delay.
This is the first study to assess the relationship between sleep loss and memory in hospitalized patients. We found that memory scores were not significantly associated with sleep duration, sleep efficiency, or with the self‐reported KSQI. Memory during hospitalization may be affected by factors other than sleep, like cognition, obscuring the relationship between sleep and memory. It is also possible that we were unable to see a significant association between sleep and memory because of universally low sleep duration and efficiency scores in the hospital.
Our study has several limitations. Most importantly, this study includes a small number of subjects who were hospitalized on a general medicine service at a single institution, limiting generalizability. Also importantly, our data capture only 1 night of sleep, and this may limit our study's ability to detect an association between hospital sleep and memory. More longitudinal data measuring sleep and memory across a longer period of time may reveal the distinct contribution of in‐hospital sleep. We also excluded patients with known cognitive impairment from enrollment, limiting our patient population to those with only high cognitive reserve. We hypothesize that patients with dementia experience both increased sleep disturbance and greater decline in memory during hospitalization. In addition, we are unable to test causal associations in this observational study. Furthermore, we applied a standardized memory test, the USC‐REMT, in a hospital setting, where noise and other disruptions at the time of test administration cannot be completely controlled. This makes it difficult to compare our results with those of community‐dwelling members taking the test under optimal conditions. Finally, because we created our own medical vignette task, future testing to validate this method against other memory testing is warranted.
In conclusion, our results show that memory in older hospitalized inpatients is often impaired, despite patients' appearing cognitively intact. These deficits in memory are revealed by a word recall task and also by a medical vignette task that more closely approximates memory for complex discharge instructions.
Disclosure
This work was funded by the National Institute on Aging Short‐Term Aging‐Related Research Program (5T35AG029795),the National Institute on Aging Career Development Award (K23AG033763), and the National Heart Lung and Blood Institute (R25 HL116372).
- . Importance of in‐hospital initiation of evidence‐based medical therapies for heart failure: taking advantage of the teachable moment. Congest Heart Fail. 2005;11(3):153–154.
- , , , , . Smoking cessation in hospitalized patients: results of a randomized trial. Arch Intern Med. 1997;157(4):409–415.
- , , . Smoking cessation interventions for hospitalized smokers: a systematic review. Arch Intern Med. 2008;168(18):1950–1960.
- , , , , . Improvements in cognition following hospital discharge of community dwelling seniors. J Gen Intern Med. 2011;26(7):765–770.
- , , , . Sleep and aging: 1. sleep disorders commonly found in older people. Can Med Assoc J. 2007;176(9):1299–1304.
- . Noise and sleep among adult medical inpatients: far from a quiet night. Arch Intern Med. 2012;172(1):68–70.
- , , , , , . Perceived control and sleep in hospitalized older adults: a sound hypothesis? J Hosp Med. 2013;8(4):184–190.
- , . A meta‐analysis of the impact of short‐term sleep deprivation on cognitive variables. Psychol Bull. 2010;136(3):375–389.
- , . Sleep deprivation: Impact on cognitive performance. Neuropsychiatr Dis Treat. 2007;3(5):553–567.
- , , , et al. Effects of physician experience on costs and outcomes on an academic general medicine service: results of a trial of hospitalists. Ann Intern Med. 2002;137(11):866–874.
- , , . “Mini‐mental state”: a practical method for grading the cognitive state of patients for the clinician. J Psychiatr Res. 1975;12(3):189–198.
- . A short portable mental status questionnaire for the assessment of organic brain deficit in elderly patients. J Am Geriatr Soc. 1975;10:433–441.
- , , , . Reliability and validity of the Short Portable Mental Status Questionnaire administered by telephone. J Geriatr Psychiatry Neurol. 1994;7(1):33–38.
- , , , . Aging, recall and recognition: a study on the sensitivity of the University of Southern California Repeatable Episodic Memory Test (USC‐REMT). J Clin Exp Neuropsychol. 2004;26(3):428–440.
- , , , , . University of southern california repeatable episodic memory test. J Clin Exp Neuropsychol. 1995;17(6):926–936.
- , , . Development of alternate paragraphs for the logical memory subtest of the Wechsler Memory Scale‐Revised. Clin Neuropsychol. 1997;11(4):370–374.
- , , . A Compendium of Neuropsychological Tests: Administration, Norms, and Commentary. 3rd ed. New York, NY: Oxford University Press; 2009.
- . Review of physical activity measurement using accelerometers in older adults: considerations for research design and conduct. Prev Med. 2009;48(2):108–114.
- , , , , , . The actigraph data analysis software: I. A novel approach to scoring and interpreting sleep‐wake activity. Percept Mot Skills. 1997;85(1):207–216.
- , , , et al. Evaluation of immobility time for sleep latency in actigraphy. Sleep Med. 2009;10(6):621–625.
- , , , , . The subjective meaning of sleep quality: a comparison of individuals with and without insomnia. Sleep. 2008;31(3):383–393.
- , . Objective components of individual differences in subjective sleep quality. J Sleep Res. 1997;6(4):217–220.
- , , , , , . Research electronic data capture (REDCap)—a metadata‐driven methodology and workflow process for providing translational research informatics support. J Biomed Inform. 2009;42(2):377–381.
- . A Wilcoxon‐type test for trend. Stat Med. 1985;4(1):87–90.
- , , , et al. Derivation of research diagnostic criteria for insomnia: report of an American Academy of Sleep Medicine Work Group. Sleep. 2004;27(8):1567–1596.
- , , , , . Quantitative criteria for insomnia. Behav Res Ther. 2003;41(4):427–445.
- . Importance of in‐hospital initiation of evidence‐based medical therapies for heart failure: taking advantage of the teachable moment. Congest Heart Fail. 2005;11(3):153–154.
- , , , , . Smoking cessation in hospitalized patients: results of a randomized trial. Arch Intern Med. 1997;157(4):409–415.
- , , . Smoking cessation interventions for hospitalized smokers: a systematic review. Arch Intern Med. 2008;168(18):1950–1960.
- , , , , . Improvements in cognition following hospital discharge of community dwelling seniors. J Gen Intern Med. 2011;26(7):765–770.
- , , , . Sleep and aging: 1. sleep disorders commonly found in older people. Can Med Assoc J. 2007;176(9):1299–1304.
- . Noise and sleep among adult medical inpatients: far from a quiet night. Arch Intern Med. 2012;172(1):68–70.
- , , , , , . Perceived control and sleep in hospitalized older adults: a sound hypothesis? J Hosp Med. 2013;8(4):184–190.
- , . A meta‐analysis of the impact of short‐term sleep deprivation on cognitive variables. Psychol Bull. 2010;136(3):375–389.
- , . Sleep deprivation: Impact on cognitive performance. Neuropsychiatr Dis Treat. 2007;3(5):553–567.
- , , , et al. Effects of physician experience on costs and outcomes on an academic general medicine service: results of a trial of hospitalists. Ann Intern Med. 2002;137(11):866–874.
- , , . “Mini‐mental state”: a practical method for grading the cognitive state of patients for the clinician. J Psychiatr Res. 1975;12(3):189–198.
- . A short portable mental status questionnaire for the assessment of organic brain deficit in elderly patients. J Am Geriatr Soc. 1975;10:433–441.
- , , , . Reliability and validity of the Short Portable Mental Status Questionnaire administered by telephone. J Geriatr Psychiatry Neurol. 1994;7(1):33–38.
- , , , . Aging, recall and recognition: a study on the sensitivity of the University of Southern California Repeatable Episodic Memory Test (USC‐REMT). J Clin Exp Neuropsychol. 2004;26(3):428–440.
- , , , , . University of southern california repeatable episodic memory test. J Clin Exp Neuropsychol. 1995;17(6):926–936.
- , , . Development of alternate paragraphs for the logical memory subtest of the Wechsler Memory Scale‐Revised. Clin Neuropsychol. 1997;11(4):370–374.
- , , . A Compendium of Neuropsychological Tests: Administration, Norms, and Commentary. 3rd ed. New York, NY: Oxford University Press; 2009.
- . Review of physical activity measurement using accelerometers in older adults: considerations for research design and conduct. Prev Med. 2009;48(2):108–114.
- , , , , , . The actigraph data analysis software: I. A novel approach to scoring and interpreting sleep‐wake activity. Percept Mot Skills. 1997;85(1):207–216.
- , , , et al. Evaluation of immobility time for sleep latency in actigraphy. Sleep Med. 2009;10(6):621–625.
- , , , , . The subjective meaning of sleep quality: a comparison of individuals with and without insomnia. Sleep. 2008;31(3):383–393.
- , . Objective components of individual differences in subjective sleep quality. J Sleep Res. 1997;6(4):217–220.
- , , , , , . Research electronic data capture (REDCap)—a metadata‐driven methodology and workflow process for providing translational research informatics support. J Biomed Inform. 2009;42(2):377–381.
- . A Wilcoxon‐type test for trend. Stat Med. 1985;4(1):87–90.
- , , , et al. Derivation of research diagnostic criteria for insomnia: report of an American Academy of Sleep Medicine Work Group. Sleep. 2004;27(8):1567–1596.
- , , , , . Quantitative criteria for insomnia. Behav Res Ther. 2003;41(4):427–445.
© 2015 Society of Hospital Medicine
Managing Superutilizers
We have known for years that the distribution of healthcare expenditures in the United States is skewed, with a small portion of the population consuming a disproportionately high share of resources. In 2010, 1% of the population accounted for 21.4% of the $1.3 trillion spent on healthcare.[1] Growing evidence documents that most of these high‐cost patients are not receiving coordinated care, preventive care, or care in the most appropriate settings.[2] The term superutilizer describes individuals with complex physical, behavioral, and social needs who have frequent emergency department (ED) visits and multiple costly hospital admissions.[3] Not surprisingly, multiple superutilizer programs and new funding opportunities target this population attempting to reduce their healthcare costs while improving their care, as public and private insurers shift to value‐based care.[4]
Beginning in 2006, the Robert Wood Johnson Foundation supported the Camden Coalition[5] with 3 grants to develop a community‐based approach to identify high‐utilizer patients and provide them with coordinated medical and social services.[6] These programs include community‐based teams that focus on the highest utilizers in a specific geographic area and provide intensive outpatient case management. Building on these efforts, the Center for Medicare and Medicaid Innovation (CMMI) awarded 2 Health Care Innovation Awards totaling $17.2 million to target Medicaid superutilizers.[7] Through its State Innovation Models initiative, CMMI also encourages states to pilot superutilizer programs to increase care coordination and support of persons with certain risk factors such as homelessness or mental illness.[8] Additionally, the National Governors Association developed a 1‐year, multistate policy academy to develop state‐level capacity and state action plans that guide how to improve the delivery and financing of care for superutilizers.[9]
With all these ongoing activities in the setting of a paucity of research identifying the most cost‐efficient practices to manage super‐utilizers, we are glad to see the Journal of Hospital Medicine publish an evaluation of a quality‐improvement project targeting superutilizers.[10] Mercer and colleagues at Duke University Hospital show that developing an individualized care plan and integrating it into their electronic health record (EHR) reduced hospital admissions, but not ED visits. Although we applaud the reportedly individualized patient approach and recognize the effort required to refer patients to a more appropriate care setting, we believe the researchers neglected 3 important components for the intervention: (1) patient engagement in developing individualized care plans, (2) care coordination integrated with community collaboration, and (3) feedback on continuum of care relayed back to providers. The managing strategies mentioned in the article seem to have evolved exclusively from the provider's perspective, a common mistake that the Patient‐Centered Outcomes Research Institute emphasizes must be avoided. We are concerned about the lack of clarity regarding the set of management strategies focused on providing high‐quality care while limiting unnecessary admissions reported by them. We fear this strategy was imposed on patients and not developed collaboratively with them. Effective interventions for superutilizers should do more than just guide providers actions, but also connect services to the patient's needs. There should be coordination and continuous improvement of these efforts, which requires engagement of the patient and their community with feedback to the system.
Possibly most important, an individualized approach to superutilizers needs to be patient‐centeredprioritizing patient goals and preferences, selecting interventions and services guided by the needs of the individual, and emphasizing modifiable outcomes that matter to the patient. Such a patient‐centered approach goes beyond the individual patient to incorporate information about social support and family dynamics, highlighting the role of caregivers. Patients and their caregivers must be engaged or activated to ensure adherence to appropriate care and behaviors in any superutilizer programs. Additionally, individualized patient‐centered care plans should be dynamic and bidirectional to accommodate changes in health priorities that may occur over time. Such lack of patient and community engagement may explain why ED‐visit frequency was unchanged in their study.
The approach of having a Complex Care Plan Committee deserves attention as it appropriately included the right people at the academic medical center. However, why is it voluntary? Should not an important, or even essential, committee such as this be supported by the health system? Moreover, although the care plan developed by members of the committee possesses understandable aspects to be considered in a patient's care, why is this not shown to the patient for their input? Instead of being done to the patient, we recommend including patients in this process, believing such patient engagement would improve care further and likely yield sustained changes. We suggest the researchers remember the maxim nothing about me, without me.
Patients who use the most healthcare services typically have complicated social situations that directly impact their ability to improve their health and stay well.[2, 11] Addressing the social determinants of health is not a new concept; however, creating healthy communities as a core responsibility of the healthcare industry is. Contributing to the dizzying state of change in US healthcare are efforts to shift to value‐based purchasing and population health management.[12] This transformation from a fee‐for‐service hospital‐centric industry into one focused on the continuum of care requires outreach into communities where superutilizers live. Ultimately, all healthcare is local, as this is where patients receive the vast majority of their care. Improving quality and reducing costs requires healthcare providers to work together on a collaborative mission that focuses on the needs of patients and community, not just efforts to reduce utilization. Even hospitalists must forge collaborative relationships with skilled nursing facilities and patient‐centered medical homes.
Given the successes of some superutilizer programs,[3] a key issue is how to scale or disseminate such labor‐intensive highly individualized programs. Each patient has very complex and specific medical, behavioral, and social needs that require creativity and flexibility to adequately address these needs. Without question, patients and/or their caregivers should be members of the care team aiming to optimize their care. Unfortunately, our current healthcare system is not designed to address the complexity and uniqueness of each superutilizer. Nonetheless, summarizing patients history into the EHR and integrating recommendations offers an opportunity to share information as originally hoped by the transition from paper‐based records. It additionally offers an opportunity to learn from use of this information as academic medical centers aim to become learning health systems.[13] Future implementation science research in this area should assess how to scale patient‐centered approaches to care, particularly for those with chronic illness and other vulnerabilities. We must eschew efforts that solely focus on reducing utilization by patients without involving them; after all, they are the focus of healthcare.
Disclosure
Nothing to report.
- , . Differentials in the concentration in the level of health expenditures across population subgroups in the U.S, 2010. Statistical brief #421. Rockville, MD: Agency for Healthcare Research and Quality; 2013.
- , , . Preventing avoidable rehospitalizations by understanding the characteristics of “frequent fliers.” J Nurs Care Qual. 2012;27(1):77–82.
- Robert Wood Johnson Foundation. Super‐utilizer summit: common themes from innovative complex care management programs. Available at: http://www.rwjf.org/en/library/research/2013/10/super‐utilizer‐summit.html. Published October 2013; accessed March 22, 2015.
- . Setting value‐based payment goals—HHS efforts to improve U.S. health care. N Engl J Med. 2015;372(10):897–899.
- . Medical Report: The hot spotters—can we lower medical costs by giving the neediest patients better care? Available at: http://www.newyorker.com/magazine/2011/01/24/the-hot-spotters. Published January 24, 2011; accessed March 22, 2015.
- Robert Wood Johnson Foundation. A coalition creates a citywide care management system. Available at: http://www.rwjf.org/content/dam/farm/reports/program_results_reports/2014/rwjf69151. Published January 13, 2011; revised June 13, 2014; accessed March 22, 2015.
- Centers for Medicare 10(XX):XXX–XXX.
- , , , . The faces of Medicaid II: recognizing the care needs of people with multiple chronic conditions. Center for Health Care Strategies, Inc. Available at: http://www.chcs.org/resource/the-faces-of-medicaid-ii-recognizing-the-care-needs-of-people-with-multiple-chronic-conditions. Published October 2007; accessed March 22, 2015.
- . Accountable care organizations—the risk of failure and the risks of success. N Engl J Med. 2014;371(18):1750–1751.
- , , . Implementing the learning health system: from concept to action. Ann Intern Med. 2012;157(3):207–210.
We have known for years that the distribution of healthcare expenditures in the United States is skewed, with a small portion of the population consuming a disproportionately high share of resources. In 2010, 1% of the population accounted for 21.4% of the $1.3 trillion spent on healthcare.[1] Growing evidence documents that most of these high‐cost patients are not receiving coordinated care, preventive care, or care in the most appropriate settings.[2] The term superutilizer describes individuals with complex physical, behavioral, and social needs who have frequent emergency department (ED) visits and multiple costly hospital admissions.[3] Not surprisingly, multiple superutilizer programs and new funding opportunities target this population attempting to reduce their healthcare costs while improving their care, as public and private insurers shift to value‐based care.[4]
Beginning in 2006, the Robert Wood Johnson Foundation supported the Camden Coalition[5] with 3 grants to develop a community‐based approach to identify high‐utilizer patients and provide them with coordinated medical and social services.[6] These programs include community‐based teams that focus on the highest utilizers in a specific geographic area and provide intensive outpatient case management. Building on these efforts, the Center for Medicare and Medicaid Innovation (CMMI) awarded 2 Health Care Innovation Awards totaling $17.2 million to target Medicaid superutilizers.[7] Through its State Innovation Models initiative, CMMI also encourages states to pilot superutilizer programs to increase care coordination and support of persons with certain risk factors such as homelessness or mental illness.[8] Additionally, the National Governors Association developed a 1‐year, multistate policy academy to develop state‐level capacity and state action plans that guide how to improve the delivery and financing of care for superutilizers.[9]
With all these ongoing activities in the setting of a paucity of research identifying the most cost‐efficient practices to manage super‐utilizers, we are glad to see the Journal of Hospital Medicine publish an evaluation of a quality‐improvement project targeting superutilizers.[10] Mercer and colleagues at Duke University Hospital show that developing an individualized care plan and integrating it into their electronic health record (EHR) reduced hospital admissions, but not ED visits. Although we applaud the reportedly individualized patient approach and recognize the effort required to refer patients to a more appropriate care setting, we believe the researchers neglected 3 important components for the intervention: (1) patient engagement in developing individualized care plans, (2) care coordination integrated with community collaboration, and (3) feedback on continuum of care relayed back to providers. The managing strategies mentioned in the article seem to have evolved exclusively from the provider's perspective, a common mistake that the Patient‐Centered Outcomes Research Institute emphasizes must be avoided. We are concerned about the lack of clarity regarding the set of management strategies focused on providing high‐quality care while limiting unnecessary admissions reported by them. We fear this strategy was imposed on patients and not developed collaboratively with them. Effective interventions for superutilizers should do more than just guide providers actions, but also connect services to the patient's needs. There should be coordination and continuous improvement of these efforts, which requires engagement of the patient and their community with feedback to the system.
Possibly most important, an individualized approach to superutilizers needs to be patient‐centeredprioritizing patient goals and preferences, selecting interventions and services guided by the needs of the individual, and emphasizing modifiable outcomes that matter to the patient. Such a patient‐centered approach goes beyond the individual patient to incorporate information about social support and family dynamics, highlighting the role of caregivers. Patients and their caregivers must be engaged or activated to ensure adherence to appropriate care and behaviors in any superutilizer programs. Additionally, individualized patient‐centered care plans should be dynamic and bidirectional to accommodate changes in health priorities that may occur over time. Such lack of patient and community engagement may explain why ED‐visit frequency was unchanged in their study.
The approach of having a Complex Care Plan Committee deserves attention as it appropriately included the right people at the academic medical center. However, why is it voluntary? Should not an important, or even essential, committee such as this be supported by the health system? Moreover, although the care plan developed by members of the committee possesses understandable aspects to be considered in a patient's care, why is this not shown to the patient for their input? Instead of being done to the patient, we recommend including patients in this process, believing such patient engagement would improve care further and likely yield sustained changes. We suggest the researchers remember the maxim nothing about me, without me.
Patients who use the most healthcare services typically have complicated social situations that directly impact their ability to improve their health and stay well.[2, 11] Addressing the social determinants of health is not a new concept; however, creating healthy communities as a core responsibility of the healthcare industry is. Contributing to the dizzying state of change in US healthcare are efforts to shift to value‐based purchasing and population health management.[12] This transformation from a fee‐for‐service hospital‐centric industry into one focused on the continuum of care requires outreach into communities where superutilizers live. Ultimately, all healthcare is local, as this is where patients receive the vast majority of their care. Improving quality and reducing costs requires healthcare providers to work together on a collaborative mission that focuses on the needs of patients and community, not just efforts to reduce utilization. Even hospitalists must forge collaborative relationships with skilled nursing facilities and patient‐centered medical homes.
Given the successes of some superutilizer programs,[3] a key issue is how to scale or disseminate such labor‐intensive highly individualized programs. Each patient has very complex and specific medical, behavioral, and social needs that require creativity and flexibility to adequately address these needs. Without question, patients and/or their caregivers should be members of the care team aiming to optimize their care. Unfortunately, our current healthcare system is not designed to address the complexity and uniqueness of each superutilizer. Nonetheless, summarizing patients history into the EHR and integrating recommendations offers an opportunity to share information as originally hoped by the transition from paper‐based records. It additionally offers an opportunity to learn from use of this information as academic medical centers aim to become learning health systems.[13] Future implementation science research in this area should assess how to scale patient‐centered approaches to care, particularly for those with chronic illness and other vulnerabilities. We must eschew efforts that solely focus on reducing utilization by patients without involving them; after all, they are the focus of healthcare.
Disclosure
Nothing to report.
We have known for years that the distribution of healthcare expenditures in the United States is skewed, with a small portion of the population consuming a disproportionately high share of resources. In 2010, 1% of the population accounted for 21.4% of the $1.3 trillion spent on healthcare.[1] Growing evidence documents that most of these high‐cost patients are not receiving coordinated care, preventive care, or care in the most appropriate settings.[2] The term superutilizer describes individuals with complex physical, behavioral, and social needs who have frequent emergency department (ED) visits and multiple costly hospital admissions.[3] Not surprisingly, multiple superutilizer programs and new funding opportunities target this population attempting to reduce their healthcare costs while improving their care, as public and private insurers shift to value‐based care.[4]
Beginning in 2006, the Robert Wood Johnson Foundation supported the Camden Coalition[5] with 3 grants to develop a community‐based approach to identify high‐utilizer patients and provide them with coordinated medical and social services.[6] These programs include community‐based teams that focus on the highest utilizers in a specific geographic area and provide intensive outpatient case management. Building on these efforts, the Center for Medicare and Medicaid Innovation (CMMI) awarded 2 Health Care Innovation Awards totaling $17.2 million to target Medicaid superutilizers.[7] Through its State Innovation Models initiative, CMMI also encourages states to pilot superutilizer programs to increase care coordination and support of persons with certain risk factors such as homelessness or mental illness.[8] Additionally, the National Governors Association developed a 1‐year, multistate policy academy to develop state‐level capacity and state action plans that guide how to improve the delivery and financing of care for superutilizers.[9]
With all these ongoing activities in the setting of a paucity of research identifying the most cost‐efficient practices to manage super‐utilizers, we are glad to see the Journal of Hospital Medicine publish an evaluation of a quality‐improvement project targeting superutilizers.[10] Mercer and colleagues at Duke University Hospital show that developing an individualized care plan and integrating it into their electronic health record (EHR) reduced hospital admissions, but not ED visits. Although we applaud the reportedly individualized patient approach and recognize the effort required to refer patients to a more appropriate care setting, we believe the researchers neglected 3 important components for the intervention: (1) patient engagement in developing individualized care plans, (2) care coordination integrated with community collaboration, and (3) feedback on continuum of care relayed back to providers. The managing strategies mentioned in the article seem to have evolved exclusively from the provider's perspective, a common mistake that the Patient‐Centered Outcomes Research Institute emphasizes must be avoided. We are concerned about the lack of clarity regarding the set of management strategies focused on providing high‐quality care while limiting unnecessary admissions reported by them. We fear this strategy was imposed on patients and not developed collaboratively with them. Effective interventions for superutilizers should do more than just guide providers actions, but also connect services to the patient's needs. There should be coordination and continuous improvement of these efforts, which requires engagement of the patient and their community with feedback to the system.
Possibly most important, an individualized approach to superutilizers needs to be patient‐centeredprioritizing patient goals and preferences, selecting interventions and services guided by the needs of the individual, and emphasizing modifiable outcomes that matter to the patient. Such a patient‐centered approach goes beyond the individual patient to incorporate information about social support and family dynamics, highlighting the role of caregivers. Patients and their caregivers must be engaged or activated to ensure adherence to appropriate care and behaviors in any superutilizer programs. Additionally, individualized patient‐centered care plans should be dynamic and bidirectional to accommodate changes in health priorities that may occur over time. Such lack of patient and community engagement may explain why ED‐visit frequency was unchanged in their study.
The approach of having a Complex Care Plan Committee deserves attention as it appropriately included the right people at the academic medical center. However, why is it voluntary? Should not an important, or even essential, committee such as this be supported by the health system? Moreover, although the care plan developed by members of the committee possesses understandable aspects to be considered in a patient's care, why is this not shown to the patient for their input? Instead of being done to the patient, we recommend including patients in this process, believing such patient engagement would improve care further and likely yield sustained changes. We suggest the researchers remember the maxim nothing about me, without me.
Patients who use the most healthcare services typically have complicated social situations that directly impact their ability to improve their health and stay well.[2, 11] Addressing the social determinants of health is not a new concept; however, creating healthy communities as a core responsibility of the healthcare industry is. Contributing to the dizzying state of change in US healthcare are efforts to shift to value‐based purchasing and population health management.[12] This transformation from a fee‐for‐service hospital‐centric industry into one focused on the continuum of care requires outreach into communities where superutilizers live. Ultimately, all healthcare is local, as this is where patients receive the vast majority of their care. Improving quality and reducing costs requires healthcare providers to work together on a collaborative mission that focuses on the needs of patients and community, not just efforts to reduce utilization. Even hospitalists must forge collaborative relationships with skilled nursing facilities and patient‐centered medical homes.
Given the successes of some superutilizer programs,[3] a key issue is how to scale or disseminate such labor‐intensive highly individualized programs. Each patient has very complex and specific medical, behavioral, and social needs that require creativity and flexibility to adequately address these needs. Without question, patients and/or their caregivers should be members of the care team aiming to optimize their care. Unfortunately, our current healthcare system is not designed to address the complexity and uniqueness of each superutilizer. Nonetheless, summarizing patients history into the EHR and integrating recommendations offers an opportunity to share information as originally hoped by the transition from paper‐based records. It additionally offers an opportunity to learn from use of this information as academic medical centers aim to become learning health systems.[13] Future implementation science research in this area should assess how to scale patient‐centered approaches to care, particularly for those with chronic illness and other vulnerabilities. We must eschew efforts that solely focus on reducing utilization by patients without involving them; after all, they are the focus of healthcare.
Disclosure
Nothing to report.
- , . Differentials in the concentration in the level of health expenditures across population subgroups in the U.S, 2010. Statistical brief #421. Rockville, MD: Agency for Healthcare Research and Quality; 2013.
- , , . Preventing avoidable rehospitalizations by understanding the characteristics of “frequent fliers.” J Nurs Care Qual. 2012;27(1):77–82.
- Robert Wood Johnson Foundation. Super‐utilizer summit: common themes from innovative complex care management programs. Available at: http://www.rwjf.org/en/library/research/2013/10/super‐utilizer‐summit.html. Published October 2013; accessed March 22, 2015.
- . Setting value‐based payment goals—HHS efforts to improve U.S. health care. N Engl J Med. 2015;372(10):897–899.
- . Medical Report: The hot spotters—can we lower medical costs by giving the neediest patients better care? Available at: http://www.newyorker.com/magazine/2011/01/24/the-hot-spotters. Published January 24, 2011; accessed March 22, 2015.
- Robert Wood Johnson Foundation. A coalition creates a citywide care management system. Available at: http://www.rwjf.org/content/dam/farm/reports/program_results_reports/2014/rwjf69151. Published January 13, 2011; revised June 13, 2014; accessed March 22, 2015.
- Centers for Medicare 10(XX):XXX–XXX.
- , , , . The faces of Medicaid II: recognizing the care needs of people with multiple chronic conditions. Center for Health Care Strategies, Inc. Available at: http://www.chcs.org/resource/the-faces-of-medicaid-ii-recognizing-the-care-needs-of-people-with-multiple-chronic-conditions. Published October 2007; accessed March 22, 2015.
- . Accountable care organizations—the risk of failure and the risks of success. N Engl J Med. 2014;371(18):1750–1751.
- , , . Implementing the learning health system: from concept to action. Ann Intern Med. 2012;157(3):207–210.
- , . Differentials in the concentration in the level of health expenditures across population subgroups in the U.S, 2010. Statistical brief #421. Rockville, MD: Agency for Healthcare Research and Quality; 2013.
- , , . Preventing avoidable rehospitalizations by understanding the characteristics of “frequent fliers.” J Nurs Care Qual. 2012;27(1):77–82.
- Robert Wood Johnson Foundation. Super‐utilizer summit: common themes from innovative complex care management programs. Available at: http://www.rwjf.org/en/library/research/2013/10/super‐utilizer‐summit.html. Published October 2013; accessed March 22, 2015.
- . Setting value‐based payment goals—HHS efforts to improve U.S. health care. N Engl J Med. 2015;372(10):897–899.
- . Medical Report: The hot spotters—can we lower medical costs by giving the neediest patients better care? Available at: http://www.newyorker.com/magazine/2011/01/24/the-hot-spotters. Published January 24, 2011; accessed March 22, 2015.
- Robert Wood Johnson Foundation. A coalition creates a citywide care management system. Available at: http://www.rwjf.org/content/dam/farm/reports/program_results_reports/2014/rwjf69151. Published January 13, 2011; revised June 13, 2014; accessed March 22, 2015.
- Centers for Medicare 10(XX):XXX–XXX.
- , , , . The faces of Medicaid II: recognizing the care needs of people with multiple chronic conditions. Center for Health Care Strategies, Inc. Available at: http://www.chcs.org/resource/the-faces-of-medicaid-ii-recognizing-the-care-needs-of-people-with-multiple-chronic-conditions. Published October 2007; accessed March 22, 2015.
- . Accountable care organizations—the risk of failure and the risks of success. N Engl J Med. 2014;371(18):1750–1751.
- , , . Implementing the learning health system: from concept to action. Ann Intern Med. 2012;157(3):207–210.
Patient Complexities and Antibiotics
Clinical management of patients with medical and social comorbidities has become increasingly complex.[1, 2, 3, 4] This complexity stems from lack of data for these groups of patients who are often excluded from clinical trials.[5] There are data demonstrating that older patients and patients with multiple comorbidities including diabetes, renal disease, obesity, limited mobility, and poor access to healthcare have worse outcomes for specific conditions compared to otherwise equal counterparts, and that the cost of care for these patients is more expensive.[3, 4, 6] Moreover, traditional risk assessment of disease severity and outcomes are not accurate when applied to medically and socially complex patients.[7, 8]
Treatment decisions regarding antibiotic use add additional complexity. Specifically, physicians antibiotic prescribing decisions can promote the emergence of multidrug resistant pathogens in a hospital or population.[9, 10] Multidrug resistant organisms (MDROs) are particularly problematic, as their prevalence is increasing while the development of new antimicrobial agents is declining.[11] Infections caused by antibiotic‐resistant pathogens are associated with increased morbidity and mortality and healthcare costs.[12, 13, 14] Antibiotic use, although potentially lifesaving, can also result in severe complications such as Clostridium difficile‐associated diarrhea, acute kidney injury, and anaphylaxis, among other adverse events, particularly in older patients with medical comorbidities.[15, 16, 17, 18] Judicious antibiotic use is critical to halt the epidemic of MDROs and to minimize antibiotic‐associated adverse effects.[19, 20, 21]
Evidence‐based guidelines have the potential to assist physicians in choosing the antibiotic that achieves the best clinical outcome for a specific infection or situation.[11] This includes using the narrowest spectrum agent to minimize selection pressure on microorganisms and avoiding unneeded drugs to minimize adverse drug effects.[9, 11] Importantly, guideline adherence regarding antibiotic selection has been shown to be associated with increased clinical success and decreased mortality.[22, 23] Unfortunately, 30% to 50% of antibiotic use in hospitalized patients is inconsistent with national guidelines.[24, 25, 26] Reasons for physicians ordering of tests and treatments inconsistent with guidelines are not fully understood, and potentially include patient and physician factors, and the cultural and social context of the healthcare system.[27]
To optimize the use of antibiotics, it is important to understand how medical complexities (defined as demographic, comorbid, and limited healthcare access characteristics that are associated with suboptimal patient care and outcomes) influence physicians antibiotic prescribing practices.[28] We created 3 clinical vignettes for common diagnoses (dyspnea with initial concern for pneumonia, skin and soft tissue infection, and asymptomatic bacteriuria) among hospitalized patients. We selected these conditions because of their high prevalence, frequent management by hospitalists, generalist physicians, and noninfectious disease specialists, and because well‐documented evidence suggests either no antibiotics or narrower spectrum antibiotics are usually the treatments of choice. Using the Infectious Diseases Society of America (IDSA) guidelines relevant to each clinical vignette,[29, 30, 31] we assessed physicians recommendations for guideline‐appropriate antibiotic management for patients without and with medical complexities using an electronic multiple‐choice survey.
METHODS
Survey Participants
We surveyed internal medicine generalist and subspecialty inpatient physicians from 3 academic medical centers in the metropolitan Los Angeles, California area. Potential participants included attending and housestaff physicians in the departments of internal medicine and family medicine at the 3 medical centers associated with the University of California Los Angeles (UCLA) Clinical and Translational Science Institute: (1) Ronald ReaganUCLA Medical Center, a tertiary care academic medical center; (2) Harbor UCLA Medical Center, a county (public) medical center; and (3) CedarsSinai Medical Center, a tertiary care medical center. Each center was affiliated with a residency training program, although not all attending physicians were associated with the training programs. Physicians were eligible to perform the survey if they attended 2 weeks per year in the inpatient setting. We collected physician‐level information including level of training (resident/fellow vs attending), specialization or not, proportion of time spent working in the hospital, and proportion of time spent providing direct clinical care (compared to activities such as administration and research). All eligible participants were emailed a brief study description with a hyperlink to the electronic survey created in REDCap (Research Electronic Data Capture version 5.6.0, 2013). Administrative staff provided email lists for potential participants at 2 of the hospitals. Per hospital policy, an email list was not provided by the third hospital, and potential participants were emailed the survey link directly by the hospital administrative staff. We incentivized study participation by entering participants who completed the survey into a raffle to win either a $100 gift card or a computer tablet. Physicians had 3 months to complete the survey and were sent up to 5 emails encouraging them to complete it.
Survey
The survey consisted of 3 clinical vignettes describing common hospital‐based situations that required decision making about antibiotic use. The 3 clinical vignettes described: (1) a patient with dyspnea and no infiltrate on chest radiograph who is initially treated empirically with antibiotics for pneumonia but is ultimately diagnosed with a congestive heart failure exacerbation, (2) a patient admitted with a skin infection that grows methicillin‐sensitive Staphylococcus aureus, and (3) a patient with a urinary catheter who develops asymptomatic bacteriuria. The first vignette was chosen because congestive heart failure and pneumonia are among the most common reasons for hospitalization in the United States, and their overlapping syndromes can make the diagnosis challenging.[32, 33, 34, 35] The second vignette was chosen because skin infections are some of the most common infectious diseases, with an incidence that is twice that of urinary tract infections and 10 times that of pneumonia, and can lead to serious complications among hospitalized patients.[30, 36] The third vignette was chosen because the prevalence of asymptomatic bacteriuria approaches 100% among catheterized patients, and rates of unnecessary treatment for this condition are as high as 80%.[31, 37]
Each clinical vignette was then modified to include 1 of the 4 studied patient complexities (Table 1). Medical comorbidities were represented by modifying the baseline vignette to describe patients with poorly controlled diabetes, morbid obesity, chronic kidney disease, and/or heavy tobacco use (Table 2). Patients with poor functional status were described in the vignettes as having difficulty with ambulation, requiring a mobility device, or needing assistance with self‐care (Table 2). Clinical vignettes varied the age of the patient from 47 years in the baseline case to 86 years (Table 2). Patients expected to have limited postdischarge follow‐up were described as being uninsured, with the first available follow‐up occurring in a public clinic no sooner than 2 weeks after their hospital discharge (Table 2). These complexities were chosen because they are common among hospitalized patients and have been shown to be associated with worse outcomes in a variety of conditions.[38, 39, 40, 41, 42] The asymptomatic bacteriuria vignette did not have a question about limited postdischarge follow‐up, because the clinical decision making in this question only pertained to the initial diagnosis and management. All physicians were queried about antibiotic use in each of the 3 baseline vignettes and in the subsequent 4 modified vignettes for each baseline scenario, with each physician making antibiotic management decisions for 15 vignettes in total. Physicians responses were recorded using categorical responses describing treatment options.
|
| Dyspnea case (baseline scenario) |
| A 47‐year‐old male with a history of stage III congestive heart failure is hospitalized after presenting with shortness of breath and a nonproductive cough. In the ED, he had a temp 99.6F, a HR of 120, and an RR 30. Exam was notable for bilateral crackles. CXR on admission was interpreted as having cardiomegaly, bilateral base atelectasis versus infiltrate and prominent pulmonary arteries with cephalization consistent with cardiogenic pulmonary edema. Admission laboratories were notable for an elevated BNP (950 pg/mL) and WBC (11.5 cells 10*9/L) with 75% neutrophils. Troponin and CK‐MB were not elevated. The patient was treated with diuretics, ceftriaxone, and azithromycin, and over the course of the next 48 hours has improved shortness of breath and no fevers. Because of his improvement he is prepared for hospital discharge. A repeat chest x‐ray shows cardiomegaly and bibasilar atelectasis. Blood cultures have been negative. He is insured and can follow with his primary physician within 3 days of discharge. Upon discharge you: |
| A. Discharge on his usual cardiac medications. |
| B. Discharge on his cardiac medications plus azithromycin to complete a 5‐day course of antibiotics. |
| C. Discharge on his cardiac medications plus azithromycin to complete a 7‐day course of antibiotics. |
| D. Discharge on usual cardiac medications plus levofloxacin to complete a 7‐day course of antibiotics. |
| Skin infection case (baseline scenario) |
| A 47‐year‐old obese female (BMI=32.9) is hospitalized after presenting with leg erythema, pain, and an 8 8‐cm fluctuant mass on her thigh. Admission temperature was 101.4F. In the ED, she was given vancomycin, and piperacillin/tazobactam and underwent incision and drainage of a thigh abscess, which drained a large amount of pus. The wound was packed. On day 3 she is afebrile, and the pain has improved. Cultures from the wound grow methicillin‐susceptible Staphylococcus aureus. Blood cultures are negative to date. She is ready for discharge. She has no allergies. She is insured and can be followed up with her primary physician within a few days of discharge. You: |
| A. Discharge on vancomycin and piperacillin/tazobactam through a PICC line to complete a 10‐day course. |
| B. Discharge on amoxicillin/clavulanate orally to complete a 10‐day course. |
| C. Discharge on cephalexin to complete a 10‐day course. |
| Asymptomatic bacteriuria case (baseline scenario) |
| A generally healthy 47‐year‐old female is admitted to the hospital for a femoral head fracture and undergoes total hip replacement. On post operative day 2, the nurse sends a urinalysis and culture from the patient's Foley catheter because the patient's urine is cloudy. Labs from that day show a WBC of 8900 and a urinalysis with 12 WBCs/HPF and 2+ bacteria. She has surgical site discomfort and no dysuria or other lower urinary tract symptoms. She has not had a fever during her hospital stay. Two days later her urine culture grows 100,000 CFU/mL of Escherichia coli susceptible to ciprofloxacin but resistant to all other oral antibiotics. That day, the patient is still afebrile and has no dysuria or other lower urinary tract symptoms. Her Foley is changed and a repeat urinalysis shows that the urine has persistent leukocytes and bacteria. You: |
| A. Initiate intravenous ciprofloxacin. |
| B. Initiate oral ciprofloxacin. |
| C. Give no antibiotics. |
| |
| Dyspnea case | |
| Comorbidities | A 47‐year‐old male with a history of stage III congestive heart failure, moderate to heavy tobacco use, poorly controlled type 2 diabetes (last HbA1C=10.9), chronic renal insufficiency (baseline creatinine of 1.3), diabetic retinopathy, and diabetic neuropathy is hospitalized after presenting with shortness of breath. |
| Poor functional status | A 47‐year‐old male with a history of stage III congestive heart failure, and poor functional status with difficulty ambulating and with self‐care is hospitalized after presenting with shortness of breath. |
| Older age | An 86‐year‐old male with a history of stage III congestive heart failure is hospitalized after presenting with shortness of breath. |
| Limited follow‐up | A 47‐year‐old male with a history of stage III congestive heart failure is hospitalized after presenting with shortness of breath.Blood cultures have been negative. He is uninsured and will be referred for follow‐up to a public clinic, which has a 2‐week wait for the next available clinic appointment. |
| Skin infection case | |
| Comorbidities | A 47‐year‐old morbidly obese female (BMI=32.9) with type 2 diabetes is hospitalized after presenting with leg erythema, pain, and an 8 8‐cm fluctuant mass on her thigh. |
| Poor functional status | A 47‐year‐old obese female (BMI=32.9) and poor functional status due to her obesity (poor mobility, uses either a walker or an electric scooter at all times to move around) is hospitalized after presenting with leg erythema, pain, and an 8 8‐cm fluctuant mass on her thigh. |
| Older age | An 86‐year‐old obese female (BMI=32.9) is hospitalized after presenting with leg erythema, pain, and an 8 8‐cm fluctuant mass on her thigh. |
| Limited follow‐up | A 47‐year‐old obese female (BMI=32.9) is hospitalized after presenting with leg erythema, pain, and an 8 8‐cm fluctuant mass on her thigh. She is uninsured and will be referred for follow‐up in a public clinic, which has a 2‐week wait for the next available clinic appointment. |
| Asymptomatic bacteriuria case | |
| Comorbidities | A 47‐year‐old female with a history type 2 diabetes with diabetic nephropathy and retinopathy is admitted to the hospital for a femoral head fracture and undergoes total hip replacement. On postoperative day 2, the nurse sends a urinalysis and culture from the patient's Foley catheter because the patient's urine is cloudy. |
| Poor functional status | A 47‐year‐old female with a history of poor functional status (needs assistance with activities of daily living) due to her obesity and musculoskeletal comorbidities, such as osteoarthritis, is admitted to the hospital for a femoral head fracture and undergoes total hip replacement. On postoperative day 2, the nurse sends a urinalysis and culture from the patient's Foley catheter because the patient's urine is cloudy. |
| Older age | A generally healthy 86‐year‐old female is admitted to the hospital for a femoral head fracture and undergoes total hip replacement. On post operative day 2, the nurse sends a urinalysis and culture from the patient's Foley catheter because the patient's urine is cloudy. |
The institutional review boards at all 3 medical centers approved the study.
Statistical Analysis
We used physician self‐report of recommended antibiotic use stratified by baseline and modified clinical vignettes to calculate the proportion of physicians recommending antibiotic use. We examined how physicians antibiotic use varied according to both patient complexity (medical comorbidity, poor functional status, older age, and limited follow‐up after hospital discharge), and provider characteristics (level of training, degree of specialization, time spent working in the hospital, and time spent providing direct patient care).
Data were analyzed using SAS (version 9.1.3; SAS Institute, Cary, NC). Antibiotic use inconsistent with IDSA guideline recommendations was considered the outcome of interest for the data analysis. Statistical analyses were performed using [2] or Fischer exact test, Student t test, and analysis of variance, as appropriate.
RESULTS
Physician Survey
Of the 874 invited physicians, 255 (29%) responded to the survey. Of these, 8/255 (3.1%) responded that they did not spend 2 or more weeks in the inpatient setting and were thus ineligible for the survey. We analyzed data from the remaining 247 physician respondents. Most respondents (217/233, 93%) reported their primary role was direct clinical care (Table 3). Most (185/241, 77%) reported at least half of their clinical work occurred in the hospital. Approximately three‐quarters (183/241, 76%) of the respondents were residents; the remaining respondents were attending physicians (57/241, 24%). Almost half of attending physicians (46%) were internal medicine subspecialists.
| Physician Characteristic | No. (%) Completing the Survey | % of Physicians Not Adhering to Guidelines in Baseline Scenarios | P Value |
|---|---|---|---|
| |||
| Affiliated medical center, n =241 | |||
| Ronald Reagan UCLA | 47 (20%) | 37% | 0.37 |
| Harbor‐UCLA | 106 (44%) | 41% | |
| Cedars‐Sinai | 86 (35%) | 43% | |
| Primary professional activity, n=233 | |||
| Direct clinical care/teaching | 217 (93%) | 42% | 0.90 |
| Research/administration | 16 (7%) | 27% | |
| Percent of clinical duties in the hospital, n=241 | |||
| 1%25% | 57 (23%) | 41% | 0.71 |
| 51%75% | 93 (39%) | 42% | |
| 76%100% | 92 (38%) | 41% | |
| Level of training and subspecialization, n=241 | |||
| Resident/fellow | 183 (76%) | 43% | 0.05 |
| Attending | 58 (24%) | 34% | |
| Subspecialist | 27 (47%) | 34% | 0.90 |
| Hospitalist | 28 (48%) | 33% | |
Physician recommendation for the use of antibiotics inconsistent with IDSA guidelines was prevalent in the baseline vignettes: 42% (303/729) overall, and 49% (120/246) for the dyspnea, 28% (68/242) for the skin infection, and 48% (115/241) for the asymptomatic bacteriuria cases. When the vignettes were modified to include patient complexities, the proportion of physicians recommending antibiotics increased significantly compared to the baseline vignette (63% (459/728), 54% (393/728), 51% (371/728), and 48% (232/487) for medical comorbidities, poor functional status, older age, and limited follow‐up respectively, P<0.001 for all comparisons) (Figure 1). The increase in the proportion of physicians recommending antibiotics inconsistent with guidelines for patients with medical complexities was the same when stratified by case (data not shown).

We found no association between provider characteristics (medical center, degree of attending physician specialization, percentage of clinical time spent practicing hospital‐based medicine, or percentage of time providing direct clinical care) and prescribing antibiotics in the baseline vignettes (Table 3). However, resident physicians (n=183) were more likely than attending physicians (n=57) to have recommended antibiotics in the baseline vignettes (43% vs 34%, P<0.05) (Table 3) and in all 4 vignettes with patient complexities (data not shown).
DISCUSSION
In our survey, almost half of the physician respondents recommended antibiotics that were inconsistent with national guidelines in the baseline vignettes. One explanation for this finding is that physicians may underestimate the risk associated with antibiotic use, such as the emergence of antimicrobial resistant pathogens and drug‐associated adverse effects. Although physicians generally agree that antibiotic resistance is an important problem, many believe that it is not a prominent issue in their practice or through their antibiotic prescribing practices.[43, 44] Others have shown that physicians underestimate the risk and severity of antibiotic‐associated complications such as C difficile.[45, 46] An accurate assessment and heightened awareness of the risks associated with antibiotics is important in clinical decision making, and should potentially be included not only in antibiotic stewardship educational efforts, but in national guideline recommendations as well.
Our survey also demonstrated that the tendency of physician respondents to recommend antibiotics was amplified for patients with medical complexities. This suggests that patient characteristics related to medical and social complexities play an important role in physicians clinical decision making about prescribing antibiotics. Previous investigations have shown that when physicians are deciding whether or not to prescribe antibiotics, they tend to deprioritize guideline recommendations and give greater weight to the risk of disease progression and complications that might occur if antibiotics are withheld.[47, 48, 49] Physicians are believed to prescribe antibiotics for complex patients more often, in part, because complex patients are more likely to suffer bad outcomes if undertreated.[50, 51] Axiomatically, patients with medical complexities are also at higher risk for antibiotic‐associated adverse effects including polypharmacy, drug‐drug interactions, and more severe side effects.[52, 53, 54]
An additional factor contributing to the overuse of antibiotics in our survey could be the lack of clear guideline recommendations for antibiotic management, especially among patients with complexities. We reviewed 20 national guidelines that addressed the medical decision making relevant to the survey's 3 clinical vignettes (see Supporting Information, Appendix 1, in the online version of this article). Fifteen of the guidelines provided recommendations for antibiotic management in the baseline vignettes, though most of the recommendations were not explicit about stopping or de‐escalating antibiotics. Furthermore, when antibiotic recommendations were present, they often lacked supporting data for the recommendation. Guidelines were even less complete for patients with medical complexities. For the asymptomatic bacteriuria vignette, 4 of 6 guidelines provided recommendations for patients with medical comorbidities described in our survey's modified vignettes.[31, 55, 56, 57] None of the guidelines related to the dyspnea or skin infection vignette provided specific antibiotic recommendations for complex patients with medical comorbidities, poor functional status, older age, or limited follow‐up (see Supporting Information, Appendix 1, in the online version of this article). Given these findings, along with evidence that medically complex patients are more likely to receive antibiotics compared to their less complex counterparts, there is a need for subsequent guidelines to more explicitly recommend best antibiotic practices for patients with medical and social complexities.
We also found that resident physicians were significantly more likely to recommend antibiotics inconsistent with guidelines compared to attending physicians. Although the underlying explanations for this finding were not explored in this study, possibilities include a lack of familiarity with guideline recommendations, less comfort in discontinuing antibiotics in the setting of clinical uncertainty, and/or a preference to accept the risks of overtreatment over the risks of undertreatment.
There are limitations to our study. First, although previous investigations have shown a high degree of correlation between actual antibiotic prescribing practices and antibiotic prescribing decisions self‐reported in clinical vignettes, physician responses in our survey may not reflect actual practices.[58] Second, we were not able to directly measure how physicians knowledge and interpretation of national guidelines influenced their antibiotic management decisions in the survey. Third, while our study was multisite including physicians from 3 different centers, not all invited physicians responded. Because of the confidentiality procedures surrounding the email distribution lists of potential participants, we were unable to obtain additional details about the non‐responders. Finally, because the large majority of respondents were trainees (76%), the generalizability of our findings to attending physicians may be limited. Nevertheless, because residents soon become staff physicians, resident‐reported data supplemented by that from attending physicians seems relevant to identifying opportunities for improving medical care.
In conclusion, we found that a large proportion of physicians recommended antibiotics that were not indicated based on IDSA guidelines for 3 vignettes depicting common hospital‐based clinical scenarios. This pattern of physicians recommending antibiotics inconsistent with guidelines was accentuated with significantly higher reported use for patients with medical comorbidities, poor functional status, older age, and limited healthcare access. Although good clinical judgment requires increased monitoring of patients with medical complexities, it is important for clinicians not to conflate the need for increased patient monitoring with the need for increased antibiotic use. Additional studies and corresponding guideline recommendations for frail, complex patients could be instrumental in reducing frequent use of antibiotics and the resultant cost, adverse effects, and emergence of antibiotic resistant pathogens. Educational efforts, particularly among trainees, regarding appropriate antibiotic use for clinical indications among patients without and with medical complexities would also likely contribute to these aims. Treatment guidelines should consider explicitly addressing medically complex patients in the context of management of infectious syndromes.
Disclosure
Nothing to report.
- , , , , , . Heart failure and chronic obstructive pulmonary disease multimorbidity at hospital discharge transition: a study of patient and carer experience [published online ahead of print May 16. 2014]. Health Expect. doi: 10.1111/hex.12208.
- , , , , , . Development of clinical practice guidelines for patients with comorbidity and multiple diseases [in Spanish]. Aten Primaria. 2014;46(7):385–392.
- , , , et al. Cardiac complications in patients with community‐acquired pneumonia: a systematic review and meta‐analysis of observational studies. PLoS Med. 2011;8(6):e1001048.
- , , , , , . Cost and incidence of social comorbidities in low‐risk patients with community‐acquired pneumonia admitted to a public hospital. Chest. 2003;124(6):2148–2155.
- , , , et al. Why do GPs exclude patients from participating in research? An exploration of adherence to and divergence from trial criteria. Fam Pract. 2014;31(3):364–370.
- , , , , . The impact of pre‐existing heart failure on pneumonia prognosis: population‐based cohort study. J Gen Intern Med. 2008;23(9):1407–1413.
- , , , et al. Severe community‐acquired pneumonia: use of intensive care services and evaluation of American and British Thoracic Society Diagnostic criteria. Am J Respir Crit Care Med. 2002;166(5):717–723.
- . Decisions about treating community‐acquired pneumonia. Ann Intern Med. 2005;142(3):215–216.
- , , , et al. The epidemic of antibiotic‐resistant infections: a call to action for the medical community from the Infectious Diseases Society of America. Clin Infect Dis. 2008;46(2):155–164.
- Will antibiotic misuse now stop? Nat Rev Microbiol. 2003;1(2):85.
- , , , et al. Infectious Diseases Society of America and the Society for Healthcare Epidemiology of America guidelines for developing an institutional program to enhance antimicrobial stewardship. Clin Infect Dis. 2007;44(2):159–177.
- . Economic impact of antimicrobial resistance. Emerg Infect Dis. 2001;7(2):286–292.
- , , , , . Guideline‐concordant therapy and reduced mortality and length of stay in adults with community‐acquired pneumonia: playing by the rules. Arch Intern Med. 2009;169(16):1525–1531.
- , , , et al. Adherence to guidelines' empirical antibiotic recommendations and community‐acquired pneumonia outcome. Eur Respir J. 2008;32(4):892–901.
- , , , et al. Community‐associated Clostridium difficile infection and antibiotics: a meta‐analysis. J Antimicrob Chemother. 2013;68(9):1951–1961.
- , , , . Vancomycin‐induced nephrotoxicity: mechanism, incidence, risk factors and special populations. A literature review. Eur J Clin Pharmacol. 2012;68(9):1243–1255.
- , . Diagnosis and management of immediate hypersensitivity reactions to cephalosporins. Clin Rev Allergy Immunol. 2013;45(1):131–142.
- , . Neurotoxic effects associated with antibiotic use: management considerations. Br J Clin Pharmacol. 2011;72(3):381–393.
- , , , et al. A clinician's guide to the appropriate and accurate use of antibiotics: the Council for Appropriate and Rational Antibiotic Therapy (CARAT) criteria. Am J Med. 2005;118(suppl 7A):1S–6S.
- , , , , . Evaluation of rational antibiotic use. Int J Antimicrob Agents. 2000;15(2):131–135.
- , . Rational antibiotic prescribing. Challenges and successes [in German]. Bundesgesundheitsblatt Gesundheitsforschung Gesundheitsschutz. 2012;55(11–12):1418–1426.
- , , , et al. Antibiotic prescription for community‐acquired pneumonia in the intensive care unit: impact of adherence to Infectious Diseases Society of America guidelines on survival. Clin Infect Dis. 2005;41(12):1709–1716.
- , , . Guideline‐adherent initial intravenous antibiotic therapy for hospital‐acquired/ventilator‐associated pneumonia is clinically superior, saves lives and is cheaper than non guideline adherent therapy. Eur J Med Res. 2011;16(7):315–323.
- , , , , , . Antibiotic misuse: a prospective clinical audit in a French university hospital. Eur J Clin Microbiol Infect Dis. 2007;26(4):277–280.
- , , , , . Unnecessary use of antimicrobials in hospitalized patients: current patterns of misuse with an emphasis on the antianaerobic spectrum of activity. Arch Intern Med. 2003;163(8):972–978.
- , , , . A systematic review and meta‐analysis of misuse of antibiotic therapies in the community. Int J Antimicrob Agents. 2005;26(2):106–113.
- , . From best evidence to best practice: effective implementation of change in patients' care. Lancet. 2003;362(9391):1225–1230.
- , , , et al. The complexity of care for patients with rheumatoid arthritis: metrics for better understanding chronic disease care. Med Care. 2007;45(1):55–65.
- , , , et al. Infectious Diseases Society of America/American Thoracic Society consensus guidelines on the management of community‐acquired pneumonia in adults. Clin Infect Dis. 2007;44(suppl 2):S27–S72.
- , , , et al. Practice guidelines for the diagnosis and management of skin and soft tissue infections: 2014 update by the Infectious Diseases Society of America. Clin Infect Dis. 2014;59(2):e10–e52.
- , , , , , . Infectious Diseases Society of America guidelines for the diagnosis and treatment of asymptomatic bacteriuria in adults. Clin Infect Dis. 2005;40(5):643–654.
- , , , et al. Heart disease and stroke statistics—2010 update: a report from the American Heart Association. Circulation. 2010;121(7):e46–e215.
- , , , et al. ESC Guidelines for the diagnosis and treatment of acute and chronic heart failure 2008: the Task Force for the Diagnosis and Treatment of Acute and Chronic Heart Failure 2008 of the European Society of Cardiology. Developed in collaboration with the Heart Failure Association of the ESC (HFA) and endorsed by the European Society of Intensive Care Medicine (ESICM). Eur Heart J. 2008;29(19):2388–2442.
- , , , . Prevalence of negative chest radiography results in the emergency department patient with decompensated heart failure. Ann Emerg Med. 2006;47(1):13–18.
- , , , . Patients admitted to hospital with suspected pneumonia and normal chest radiographs: epidemiology, microbiology, and outcomes. Am J Med. 2004;117(5):305–311.
- , , , et al. Incidence of skin and soft tissue infections in ambulatory and inpatient settings, 2005–2010. BMC Infect Dis. In press.
- . Asymptomatic bacteriuria: when the treatment is worse than the disease. Nat Rev Urol. 2012;9(2):85–93.
- , , , et al. Prevalence and incidence trends for diagnosed diabetes among adults aged 20 to 79 years, United States, 1980–2012. JAMA. 2014;312(12):1218–1226.
- , , , . Prevalence of childhood and adult obesity in the United States, 2011–2012. JAMA. 2014;311(8):806–814.
- , , , , . Frailty in elderly people. Lancet. 2013;381(9868):752–762.
- , , , , , . Risk factors for death in homeless adults in Boston. Arch Intern Med. 1998;158(13):1454–1460.
- . Taking it to the streets: homelessness, health, and health care in the United States. J Gen Intern Med. 2003;18(11):964–965.
- , , , , , . Antibiotic resistance: a survey of physician perceptions. Arch Intern Med. 2002;162(19):2210–2216.
- , , , et al. Primary care clinicians' perceptions of antibiotic resistance: a multi‐country qualitative interview study. J Antimicrob Chemother. 2013;68(1):237–243.
- , , , , , . Underestimation of Clostridium difficile infection among clinicians: an international survey. Eur J Clin Microbiol Infect Dis. 2012;31(9):2439–2444.
- , , , , , . Unnecessary antimicrobial use in patients with current or recent Clostridium difficile infection. Infect Control Hosp Epidemiol. 2013;34(2):109–116.
- , , , , . Attitudes of primary care physicians to the prescribing of antibiotics and antimicrobial resistance: a qualitative study from Spain. Fam Pract. 2012;29(3):352–360.
- , , , , . Understanding physician antibiotic prescribing behaviour: a systematic review of qualitative studies. Int J Antimicrob Agents. 2013;41(3):203–212.
- , , , . Guidelines on uncomplicated urinary tract infections are difficult to follow: perceived barriers and suggested interventions. BMC Fam Pract. 2010;11:51.
- , , , . Why don't physicians adhere to guideline recommendations in practice? An analysis of barriers among Dutch general practitioners. Implement Sci. 2009;4:54.
- , , , , . Perceived barriers to guideline adherence: a survey among general practitioners. BMC Fam Pract. 2011;12:98.
- , , , . Clostridium difficile in acute and long‐stay elderly patients. Age Ageing. 1988;17(5):333–336.
- , , , , , . Pharmacodynamics of vancomycin in elderly patients aged 75 years or older with methicillin‐resistant Staphylococcus aureus hospital‐acquired pneumonia. Clin Interv Aging. 2013;8:1015–1021.
- , , , et al. Empiric guideline‐recommended weight‐based vancomycin dosing and nephrotoxicity rates in patients with methicillin‐resistant Staphylococcus aureus bacteremia: a retrospective cohort study. BMC Pharmacol Toxicol. 2013;14:12.
- , , , , , , , , . Guidelines on urological infections. Arnhem, The Netherlands: European Association of Urology (EAU); 2011. p. 15–27.
- Scottish Intercollegiate Guidelines Network. Management of suspected bacterial urinary tract infection in adults. Available at: http://www.sign.ac.uk/guidelines/fulltext/88/. Accessed on July 25, 2014.
- , , , et al. [Optimisation of the antibiotic policy in the Netherlands. X. The SWAB guideline for antimicrobial treatment of complicated urinary tract infections]. Ned Tijdschr Geneeskd 2006;150(43):2370–2376.
- , , , et al. Do case vignettes accurately reflect antibiotic prescription? Infect Control Hosp Epidemiol. 2011;32(10):1003–1009.
- The committee for The Japanese Respiratory Society guidelines in management of respiratory infections. Principles for the development of the guidelines. Respirology 2004;9(suppl 1):S1–S2.
Clinical management of patients with medical and social comorbidities has become increasingly complex.[1, 2, 3, 4] This complexity stems from lack of data for these groups of patients who are often excluded from clinical trials.[5] There are data demonstrating that older patients and patients with multiple comorbidities including diabetes, renal disease, obesity, limited mobility, and poor access to healthcare have worse outcomes for specific conditions compared to otherwise equal counterparts, and that the cost of care for these patients is more expensive.[3, 4, 6] Moreover, traditional risk assessment of disease severity and outcomes are not accurate when applied to medically and socially complex patients.[7, 8]
Treatment decisions regarding antibiotic use add additional complexity. Specifically, physicians antibiotic prescribing decisions can promote the emergence of multidrug resistant pathogens in a hospital or population.[9, 10] Multidrug resistant organisms (MDROs) are particularly problematic, as their prevalence is increasing while the development of new antimicrobial agents is declining.[11] Infections caused by antibiotic‐resistant pathogens are associated with increased morbidity and mortality and healthcare costs.[12, 13, 14] Antibiotic use, although potentially lifesaving, can also result in severe complications such as Clostridium difficile‐associated diarrhea, acute kidney injury, and anaphylaxis, among other adverse events, particularly in older patients with medical comorbidities.[15, 16, 17, 18] Judicious antibiotic use is critical to halt the epidemic of MDROs and to minimize antibiotic‐associated adverse effects.[19, 20, 21]
Evidence‐based guidelines have the potential to assist physicians in choosing the antibiotic that achieves the best clinical outcome for a specific infection or situation.[11] This includes using the narrowest spectrum agent to minimize selection pressure on microorganisms and avoiding unneeded drugs to minimize adverse drug effects.[9, 11] Importantly, guideline adherence regarding antibiotic selection has been shown to be associated with increased clinical success and decreased mortality.[22, 23] Unfortunately, 30% to 50% of antibiotic use in hospitalized patients is inconsistent with national guidelines.[24, 25, 26] Reasons for physicians ordering of tests and treatments inconsistent with guidelines are not fully understood, and potentially include patient and physician factors, and the cultural and social context of the healthcare system.[27]
To optimize the use of antibiotics, it is important to understand how medical complexities (defined as demographic, comorbid, and limited healthcare access characteristics that are associated with suboptimal patient care and outcomes) influence physicians antibiotic prescribing practices.[28] We created 3 clinical vignettes for common diagnoses (dyspnea with initial concern for pneumonia, skin and soft tissue infection, and asymptomatic bacteriuria) among hospitalized patients. We selected these conditions because of their high prevalence, frequent management by hospitalists, generalist physicians, and noninfectious disease specialists, and because well‐documented evidence suggests either no antibiotics or narrower spectrum antibiotics are usually the treatments of choice. Using the Infectious Diseases Society of America (IDSA) guidelines relevant to each clinical vignette,[29, 30, 31] we assessed physicians recommendations for guideline‐appropriate antibiotic management for patients without and with medical complexities using an electronic multiple‐choice survey.
METHODS
Survey Participants
We surveyed internal medicine generalist and subspecialty inpatient physicians from 3 academic medical centers in the metropolitan Los Angeles, California area. Potential participants included attending and housestaff physicians in the departments of internal medicine and family medicine at the 3 medical centers associated with the University of California Los Angeles (UCLA) Clinical and Translational Science Institute: (1) Ronald ReaganUCLA Medical Center, a tertiary care academic medical center; (2) Harbor UCLA Medical Center, a county (public) medical center; and (3) CedarsSinai Medical Center, a tertiary care medical center. Each center was affiliated with a residency training program, although not all attending physicians were associated with the training programs. Physicians were eligible to perform the survey if they attended 2 weeks per year in the inpatient setting. We collected physician‐level information including level of training (resident/fellow vs attending), specialization or not, proportion of time spent working in the hospital, and proportion of time spent providing direct clinical care (compared to activities such as administration and research). All eligible participants were emailed a brief study description with a hyperlink to the electronic survey created in REDCap (Research Electronic Data Capture version 5.6.0, 2013). Administrative staff provided email lists for potential participants at 2 of the hospitals. Per hospital policy, an email list was not provided by the third hospital, and potential participants were emailed the survey link directly by the hospital administrative staff. We incentivized study participation by entering participants who completed the survey into a raffle to win either a $100 gift card or a computer tablet. Physicians had 3 months to complete the survey and were sent up to 5 emails encouraging them to complete it.
Survey
The survey consisted of 3 clinical vignettes describing common hospital‐based situations that required decision making about antibiotic use. The 3 clinical vignettes described: (1) a patient with dyspnea and no infiltrate on chest radiograph who is initially treated empirically with antibiotics for pneumonia but is ultimately diagnosed with a congestive heart failure exacerbation, (2) a patient admitted with a skin infection that grows methicillin‐sensitive Staphylococcus aureus, and (3) a patient with a urinary catheter who develops asymptomatic bacteriuria. The first vignette was chosen because congestive heart failure and pneumonia are among the most common reasons for hospitalization in the United States, and their overlapping syndromes can make the diagnosis challenging.[32, 33, 34, 35] The second vignette was chosen because skin infections are some of the most common infectious diseases, with an incidence that is twice that of urinary tract infections and 10 times that of pneumonia, and can lead to serious complications among hospitalized patients.[30, 36] The third vignette was chosen because the prevalence of asymptomatic bacteriuria approaches 100% among catheterized patients, and rates of unnecessary treatment for this condition are as high as 80%.[31, 37]
Each clinical vignette was then modified to include 1 of the 4 studied patient complexities (Table 1). Medical comorbidities were represented by modifying the baseline vignette to describe patients with poorly controlled diabetes, morbid obesity, chronic kidney disease, and/or heavy tobacco use (Table 2). Patients with poor functional status were described in the vignettes as having difficulty with ambulation, requiring a mobility device, or needing assistance with self‐care (Table 2). Clinical vignettes varied the age of the patient from 47 years in the baseline case to 86 years (Table 2). Patients expected to have limited postdischarge follow‐up were described as being uninsured, with the first available follow‐up occurring in a public clinic no sooner than 2 weeks after their hospital discharge (Table 2). These complexities were chosen because they are common among hospitalized patients and have been shown to be associated with worse outcomes in a variety of conditions.[38, 39, 40, 41, 42] The asymptomatic bacteriuria vignette did not have a question about limited postdischarge follow‐up, because the clinical decision making in this question only pertained to the initial diagnosis and management. All physicians were queried about antibiotic use in each of the 3 baseline vignettes and in the subsequent 4 modified vignettes for each baseline scenario, with each physician making antibiotic management decisions for 15 vignettes in total. Physicians responses were recorded using categorical responses describing treatment options.
|
| Dyspnea case (baseline scenario) |
| A 47‐year‐old male with a history of stage III congestive heart failure is hospitalized after presenting with shortness of breath and a nonproductive cough. In the ED, he had a temp 99.6F, a HR of 120, and an RR 30. Exam was notable for bilateral crackles. CXR on admission was interpreted as having cardiomegaly, bilateral base atelectasis versus infiltrate and prominent pulmonary arteries with cephalization consistent with cardiogenic pulmonary edema. Admission laboratories were notable for an elevated BNP (950 pg/mL) and WBC (11.5 cells 10*9/L) with 75% neutrophils. Troponin and CK‐MB were not elevated. The patient was treated with diuretics, ceftriaxone, and azithromycin, and over the course of the next 48 hours has improved shortness of breath and no fevers. Because of his improvement he is prepared for hospital discharge. A repeat chest x‐ray shows cardiomegaly and bibasilar atelectasis. Blood cultures have been negative. He is insured and can follow with his primary physician within 3 days of discharge. Upon discharge you: |
| A. Discharge on his usual cardiac medications. |
| B. Discharge on his cardiac medications plus azithromycin to complete a 5‐day course of antibiotics. |
| C. Discharge on his cardiac medications plus azithromycin to complete a 7‐day course of antibiotics. |
| D. Discharge on usual cardiac medications plus levofloxacin to complete a 7‐day course of antibiotics. |
| Skin infection case (baseline scenario) |
| A 47‐year‐old obese female (BMI=32.9) is hospitalized after presenting with leg erythema, pain, and an 8 8‐cm fluctuant mass on her thigh. Admission temperature was 101.4F. In the ED, she was given vancomycin, and piperacillin/tazobactam and underwent incision and drainage of a thigh abscess, which drained a large amount of pus. The wound was packed. On day 3 she is afebrile, and the pain has improved. Cultures from the wound grow methicillin‐susceptible Staphylococcus aureus. Blood cultures are negative to date. She is ready for discharge. She has no allergies. She is insured and can be followed up with her primary physician within a few days of discharge. You: |
| A. Discharge on vancomycin and piperacillin/tazobactam through a PICC line to complete a 10‐day course. |
| B. Discharge on amoxicillin/clavulanate orally to complete a 10‐day course. |
| C. Discharge on cephalexin to complete a 10‐day course. |
| Asymptomatic bacteriuria case (baseline scenario) |
| A generally healthy 47‐year‐old female is admitted to the hospital for a femoral head fracture and undergoes total hip replacement. On post operative day 2, the nurse sends a urinalysis and culture from the patient's Foley catheter because the patient's urine is cloudy. Labs from that day show a WBC of 8900 and a urinalysis with 12 WBCs/HPF and 2+ bacteria. She has surgical site discomfort and no dysuria or other lower urinary tract symptoms. She has not had a fever during her hospital stay. Two days later her urine culture grows 100,000 CFU/mL of Escherichia coli susceptible to ciprofloxacin but resistant to all other oral antibiotics. That day, the patient is still afebrile and has no dysuria or other lower urinary tract symptoms. Her Foley is changed and a repeat urinalysis shows that the urine has persistent leukocytes and bacteria. You: |
| A. Initiate intravenous ciprofloxacin. |
| B. Initiate oral ciprofloxacin. |
| C. Give no antibiotics. |
| |
| Dyspnea case | |
| Comorbidities | A 47‐year‐old male with a history of stage III congestive heart failure, moderate to heavy tobacco use, poorly controlled type 2 diabetes (last HbA1C=10.9), chronic renal insufficiency (baseline creatinine of 1.3), diabetic retinopathy, and diabetic neuropathy is hospitalized after presenting with shortness of breath. |
| Poor functional status | A 47‐year‐old male with a history of stage III congestive heart failure, and poor functional status with difficulty ambulating and with self‐care is hospitalized after presenting with shortness of breath. |
| Older age | An 86‐year‐old male with a history of stage III congestive heart failure is hospitalized after presenting with shortness of breath. |
| Limited follow‐up | A 47‐year‐old male with a history of stage III congestive heart failure is hospitalized after presenting with shortness of breath.Blood cultures have been negative. He is uninsured and will be referred for follow‐up to a public clinic, which has a 2‐week wait for the next available clinic appointment. |
| Skin infection case | |
| Comorbidities | A 47‐year‐old morbidly obese female (BMI=32.9) with type 2 diabetes is hospitalized after presenting with leg erythema, pain, and an 8 8‐cm fluctuant mass on her thigh. |
| Poor functional status | A 47‐year‐old obese female (BMI=32.9) and poor functional status due to her obesity (poor mobility, uses either a walker or an electric scooter at all times to move around) is hospitalized after presenting with leg erythema, pain, and an 8 8‐cm fluctuant mass on her thigh. |
| Older age | An 86‐year‐old obese female (BMI=32.9) is hospitalized after presenting with leg erythema, pain, and an 8 8‐cm fluctuant mass on her thigh. |
| Limited follow‐up | A 47‐year‐old obese female (BMI=32.9) is hospitalized after presenting with leg erythema, pain, and an 8 8‐cm fluctuant mass on her thigh. She is uninsured and will be referred for follow‐up in a public clinic, which has a 2‐week wait for the next available clinic appointment. |
| Asymptomatic bacteriuria case | |
| Comorbidities | A 47‐year‐old female with a history type 2 diabetes with diabetic nephropathy and retinopathy is admitted to the hospital for a femoral head fracture and undergoes total hip replacement. On postoperative day 2, the nurse sends a urinalysis and culture from the patient's Foley catheter because the patient's urine is cloudy. |
| Poor functional status | A 47‐year‐old female with a history of poor functional status (needs assistance with activities of daily living) due to her obesity and musculoskeletal comorbidities, such as osteoarthritis, is admitted to the hospital for a femoral head fracture and undergoes total hip replacement. On postoperative day 2, the nurse sends a urinalysis and culture from the patient's Foley catheter because the patient's urine is cloudy. |
| Older age | A generally healthy 86‐year‐old female is admitted to the hospital for a femoral head fracture and undergoes total hip replacement. On post operative day 2, the nurse sends a urinalysis and culture from the patient's Foley catheter because the patient's urine is cloudy. |
The institutional review boards at all 3 medical centers approved the study.
Statistical Analysis
We used physician self‐report of recommended antibiotic use stratified by baseline and modified clinical vignettes to calculate the proportion of physicians recommending antibiotic use. We examined how physicians antibiotic use varied according to both patient complexity (medical comorbidity, poor functional status, older age, and limited follow‐up after hospital discharge), and provider characteristics (level of training, degree of specialization, time spent working in the hospital, and time spent providing direct patient care).
Data were analyzed using SAS (version 9.1.3; SAS Institute, Cary, NC). Antibiotic use inconsistent with IDSA guideline recommendations was considered the outcome of interest for the data analysis. Statistical analyses were performed using [2] or Fischer exact test, Student t test, and analysis of variance, as appropriate.
RESULTS
Physician Survey
Of the 874 invited physicians, 255 (29%) responded to the survey. Of these, 8/255 (3.1%) responded that they did not spend 2 or more weeks in the inpatient setting and were thus ineligible for the survey. We analyzed data from the remaining 247 physician respondents. Most respondents (217/233, 93%) reported their primary role was direct clinical care (Table 3). Most (185/241, 77%) reported at least half of their clinical work occurred in the hospital. Approximately three‐quarters (183/241, 76%) of the respondents were residents; the remaining respondents were attending physicians (57/241, 24%). Almost half of attending physicians (46%) were internal medicine subspecialists.
| Physician Characteristic | No. (%) Completing the Survey | % of Physicians Not Adhering to Guidelines in Baseline Scenarios | P Value |
|---|---|---|---|
| |||
| Affiliated medical center, n =241 | |||
| Ronald Reagan UCLA | 47 (20%) | 37% | 0.37 |
| Harbor‐UCLA | 106 (44%) | 41% | |
| Cedars‐Sinai | 86 (35%) | 43% | |
| Primary professional activity, n=233 | |||
| Direct clinical care/teaching | 217 (93%) | 42% | 0.90 |
| Research/administration | 16 (7%) | 27% | |
| Percent of clinical duties in the hospital, n=241 | |||
| 1%25% | 57 (23%) | 41% | 0.71 |
| 51%75% | 93 (39%) | 42% | |
| 76%100% | 92 (38%) | 41% | |
| Level of training and subspecialization, n=241 | |||
| Resident/fellow | 183 (76%) | 43% | 0.05 |
| Attending | 58 (24%) | 34% | |
| Subspecialist | 27 (47%) | 34% | 0.90 |
| Hospitalist | 28 (48%) | 33% | |
Physician recommendation for the use of antibiotics inconsistent with IDSA guidelines was prevalent in the baseline vignettes: 42% (303/729) overall, and 49% (120/246) for the dyspnea, 28% (68/242) for the skin infection, and 48% (115/241) for the asymptomatic bacteriuria cases. When the vignettes were modified to include patient complexities, the proportion of physicians recommending antibiotics increased significantly compared to the baseline vignette (63% (459/728), 54% (393/728), 51% (371/728), and 48% (232/487) for medical comorbidities, poor functional status, older age, and limited follow‐up respectively, P<0.001 for all comparisons) (Figure 1). The increase in the proportion of physicians recommending antibiotics inconsistent with guidelines for patients with medical complexities was the same when stratified by case (data not shown).

We found no association between provider characteristics (medical center, degree of attending physician specialization, percentage of clinical time spent practicing hospital‐based medicine, or percentage of time providing direct clinical care) and prescribing antibiotics in the baseline vignettes (Table 3). However, resident physicians (n=183) were more likely than attending physicians (n=57) to have recommended antibiotics in the baseline vignettes (43% vs 34%, P<0.05) (Table 3) and in all 4 vignettes with patient complexities (data not shown).
DISCUSSION
In our survey, almost half of the physician respondents recommended antibiotics that were inconsistent with national guidelines in the baseline vignettes. One explanation for this finding is that physicians may underestimate the risk associated with antibiotic use, such as the emergence of antimicrobial resistant pathogens and drug‐associated adverse effects. Although physicians generally agree that antibiotic resistance is an important problem, many believe that it is not a prominent issue in their practice or through their antibiotic prescribing practices.[43, 44] Others have shown that physicians underestimate the risk and severity of antibiotic‐associated complications such as C difficile.[45, 46] An accurate assessment and heightened awareness of the risks associated with antibiotics is important in clinical decision making, and should potentially be included not only in antibiotic stewardship educational efforts, but in national guideline recommendations as well.
Our survey also demonstrated that the tendency of physician respondents to recommend antibiotics was amplified for patients with medical complexities. This suggests that patient characteristics related to medical and social complexities play an important role in physicians clinical decision making about prescribing antibiotics. Previous investigations have shown that when physicians are deciding whether or not to prescribe antibiotics, they tend to deprioritize guideline recommendations and give greater weight to the risk of disease progression and complications that might occur if antibiotics are withheld.[47, 48, 49] Physicians are believed to prescribe antibiotics for complex patients more often, in part, because complex patients are more likely to suffer bad outcomes if undertreated.[50, 51] Axiomatically, patients with medical complexities are also at higher risk for antibiotic‐associated adverse effects including polypharmacy, drug‐drug interactions, and more severe side effects.[52, 53, 54]
An additional factor contributing to the overuse of antibiotics in our survey could be the lack of clear guideline recommendations for antibiotic management, especially among patients with complexities. We reviewed 20 national guidelines that addressed the medical decision making relevant to the survey's 3 clinical vignettes (see Supporting Information, Appendix 1, in the online version of this article). Fifteen of the guidelines provided recommendations for antibiotic management in the baseline vignettes, though most of the recommendations were not explicit about stopping or de‐escalating antibiotics. Furthermore, when antibiotic recommendations were present, they often lacked supporting data for the recommendation. Guidelines were even less complete for patients with medical complexities. For the asymptomatic bacteriuria vignette, 4 of 6 guidelines provided recommendations for patients with medical comorbidities described in our survey's modified vignettes.[31, 55, 56, 57] None of the guidelines related to the dyspnea or skin infection vignette provided specific antibiotic recommendations for complex patients with medical comorbidities, poor functional status, older age, or limited follow‐up (see Supporting Information, Appendix 1, in the online version of this article). Given these findings, along with evidence that medically complex patients are more likely to receive antibiotics compared to their less complex counterparts, there is a need for subsequent guidelines to more explicitly recommend best antibiotic practices for patients with medical and social complexities.
We also found that resident physicians were significantly more likely to recommend antibiotics inconsistent with guidelines compared to attending physicians. Although the underlying explanations for this finding were not explored in this study, possibilities include a lack of familiarity with guideline recommendations, less comfort in discontinuing antibiotics in the setting of clinical uncertainty, and/or a preference to accept the risks of overtreatment over the risks of undertreatment.
There are limitations to our study. First, although previous investigations have shown a high degree of correlation between actual antibiotic prescribing practices and antibiotic prescribing decisions self‐reported in clinical vignettes, physician responses in our survey may not reflect actual practices.[58] Second, we were not able to directly measure how physicians knowledge and interpretation of national guidelines influenced their antibiotic management decisions in the survey. Third, while our study was multisite including physicians from 3 different centers, not all invited physicians responded. Because of the confidentiality procedures surrounding the email distribution lists of potential participants, we were unable to obtain additional details about the non‐responders. Finally, because the large majority of respondents were trainees (76%), the generalizability of our findings to attending physicians may be limited. Nevertheless, because residents soon become staff physicians, resident‐reported data supplemented by that from attending physicians seems relevant to identifying opportunities for improving medical care.
In conclusion, we found that a large proportion of physicians recommended antibiotics that were not indicated based on IDSA guidelines for 3 vignettes depicting common hospital‐based clinical scenarios. This pattern of physicians recommending antibiotics inconsistent with guidelines was accentuated with significantly higher reported use for patients with medical comorbidities, poor functional status, older age, and limited healthcare access. Although good clinical judgment requires increased monitoring of patients with medical complexities, it is important for clinicians not to conflate the need for increased patient monitoring with the need for increased antibiotic use. Additional studies and corresponding guideline recommendations for frail, complex patients could be instrumental in reducing frequent use of antibiotics and the resultant cost, adverse effects, and emergence of antibiotic resistant pathogens. Educational efforts, particularly among trainees, regarding appropriate antibiotic use for clinical indications among patients without and with medical complexities would also likely contribute to these aims. Treatment guidelines should consider explicitly addressing medically complex patients in the context of management of infectious syndromes.
Disclosure
Nothing to report.
Clinical management of patients with medical and social comorbidities has become increasingly complex.[1, 2, 3, 4] This complexity stems from lack of data for these groups of patients who are often excluded from clinical trials.[5] There are data demonstrating that older patients and patients with multiple comorbidities including diabetes, renal disease, obesity, limited mobility, and poor access to healthcare have worse outcomes for specific conditions compared to otherwise equal counterparts, and that the cost of care for these patients is more expensive.[3, 4, 6] Moreover, traditional risk assessment of disease severity and outcomes are not accurate when applied to medically and socially complex patients.[7, 8]
Treatment decisions regarding antibiotic use add additional complexity. Specifically, physicians antibiotic prescribing decisions can promote the emergence of multidrug resistant pathogens in a hospital or population.[9, 10] Multidrug resistant organisms (MDROs) are particularly problematic, as their prevalence is increasing while the development of new antimicrobial agents is declining.[11] Infections caused by antibiotic‐resistant pathogens are associated with increased morbidity and mortality and healthcare costs.[12, 13, 14] Antibiotic use, although potentially lifesaving, can also result in severe complications such as Clostridium difficile‐associated diarrhea, acute kidney injury, and anaphylaxis, among other adverse events, particularly in older patients with medical comorbidities.[15, 16, 17, 18] Judicious antibiotic use is critical to halt the epidemic of MDROs and to minimize antibiotic‐associated adverse effects.[19, 20, 21]
Evidence‐based guidelines have the potential to assist physicians in choosing the antibiotic that achieves the best clinical outcome for a specific infection or situation.[11] This includes using the narrowest spectrum agent to minimize selection pressure on microorganisms and avoiding unneeded drugs to minimize adverse drug effects.[9, 11] Importantly, guideline adherence regarding antibiotic selection has been shown to be associated with increased clinical success and decreased mortality.[22, 23] Unfortunately, 30% to 50% of antibiotic use in hospitalized patients is inconsistent with national guidelines.[24, 25, 26] Reasons for physicians ordering of tests and treatments inconsistent with guidelines are not fully understood, and potentially include patient and physician factors, and the cultural and social context of the healthcare system.[27]
To optimize the use of antibiotics, it is important to understand how medical complexities (defined as demographic, comorbid, and limited healthcare access characteristics that are associated with suboptimal patient care and outcomes) influence physicians antibiotic prescribing practices.[28] We created 3 clinical vignettes for common diagnoses (dyspnea with initial concern for pneumonia, skin and soft tissue infection, and asymptomatic bacteriuria) among hospitalized patients. We selected these conditions because of their high prevalence, frequent management by hospitalists, generalist physicians, and noninfectious disease specialists, and because well‐documented evidence suggests either no antibiotics or narrower spectrum antibiotics are usually the treatments of choice. Using the Infectious Diseases Society of America (IDSA) guidelines relevant to each clinical vignette,[29, 30, 31] we assessed physicians recommendations for guideline‐appropriate antibiotic management for patients without and with medical complexities using an electronic multiple‐choice survey.
METHODS
Survey Participants
We surveyed internal medicine generalist and subspecialty inpatient physicians from 3 academic medical centers in the metropolitan Los Angeles, California area. Potential participants included attending and housestaff physicians in the departments of internal medicine and family medicine at the 3 medical centers associated with the University of California Los Angeles (UCLA) Clinical and Translational Science Institute: (1) Ronald ReaganUCLA Medical Center, a tertiary care academic medical center; (2) Harbor UCLA Medical Center, a county (public) medical center; and (3) CedarsSinai Medical Center, a tertiary care medical center. Each center was affiliated with a residency training program, although not all attending physicians were associated with the training programs. Physicians were eligible to perform the survey if they attended 2 weeks per year in the inpatient setting. We collected physician‐level information including level of training (resident/fellow vs attending), specialization or not, proportion of time spent working in the hospital, and proportion of time spent providing direct clinical care (compared to activities such as administration and research). All eligible participants were emailed a brief study description with a hyperlink to the electronic survey created in REDCap (Research Electronic Data Capture version 5.6.0, 2013). Administrative staff provided email lists for potential participants at 2 of the hospitals. Per hospital policy, an email list was not provided by the third hospital, and potential participants were emailed the survey link directly by the hospital administrative staff. We incentivized study participation by entering participants who completed the survey into a raffle to win either a $100 gift card or a computer tablet. Physicians had 3 months to complete the survey and were sent up to 5 emails encouraging them to complete it.
Survey
The survey consisted of 3 clinical vignettes describing common hospital‐based situations that required decision making about antibiotic use. The 3 clinical vignettes described: (1) a patient with dyspnea and no infiltrate on chest radiograph who is initially treated empirically with antibiotics for pneumonia but is ultimately diagnosed with a congestive heart failure exacerbation, (2) a patient admitted with a skin infection that grows methicillin‐sensitive Staphylococcus aureus, and (3) a patient with a urinary catheter who develops asymptomatic bacteriuria. The first vignette was chosen because congestive heart failure and pneumonia are among the most common reasons for hospitalization in the United States, and their overlapping syndromes can make the diagnosis challenging.[32, 33, 34, 35] The second vignette was chosen because skin infections are some of the most common infectious diseases, with an incidence that is twice that of urinary tract infections and 10 times that of pneumonia, and can lead to serious complications among hospitalized patients.[30, 36] The third vignette was chosen because the prevalence of asymptomatic bacteriuria approaches 100% among catheterized patients, and rates of unnecessary treatment for this condition are as high as 80%.[31, 37]
Each clinical vignette was then modified to include 1 of the 4 studied patient complexities (Table 1). Medical comorbidities were represented by modifying the baseline vignette to describe patients with poorly controlled diabetes, morbid obesity, chronic kidney disease, and/or heavy tobacco use (Table 2). Patients with poor functional status were described in the vignettes as having difficulty with ambulation, requiring a mobility device, or needing assistance with self‐care (Table 2). Clinical vignettes varied the age of the patient from 47 years in the baseline case to 86 years (Table 2). Patients expected to have limited postdischarge follow‐up were described as being uninsured, with the first available follow‐up occurring in a public clinic no sooner than 2 weeks after their hospital discharge (Table 2). These complexities were chosen because they are common among hospitalized patients and have been shown to be associated with worse outcomes in a variety of conditions.[38, 39, 40, 41, 42] The asymptomatic bacteriuria vignette did not have a question about limited postdischarge follow‐up, because the clinical decision making in this question only pertained to the initial diagnosis and management. All physicians were queried about antibiotic use in each of the 3 baseline vignettes and in the subsequent 4 modified vignettes for each baseline scenario, with each physician making antibiotic management decisions for 15 vignettes in total. Physicians responses were recorded using categorical responses describing treatment options.
|
| Dyspnea case (baseline scenario) |
| A 47‐year‐old male with a history of stage III congestive heart failure is hospitalized after presenting with shortness of breath and a nonproductive cough. In the ED, he had a temp 99.6F, a HR of 120, and an RR 30. Exam was notable for bilateral crackles. CXR on admission was interpreted as having cardiomegaly, bilateral base atelectasis versus infiltrate and prominent pulmonary arteries with cephalization consistent with cardiogenic pulmonary edema. Admission laboratories were notable for an elevated BNP (950 pg/mL) and WBC (11.5 cells 10*9/L) with 75% neutrophils. Troponin and CK‐MB were not elevated. The patient was treated with diuretics, ceftriaxone, and azithromycin, and over the course of the next 48 hours has improved shortness of breath and no fevers. Because of his improvement he is prepared for hospital discharge. A repeat chest x‐ray shows cardiomegaly and bibasilar atelectasis. Blood cultures have been negative. He is insured and can follow with his primary physician within 3 days of discharge. Upon discharge you: |
| A. Discharge on his usual cardiac medications. |
| B. Discharge on his cardiac medications plus azithromycin to complete a 5‐day course of antibiotics. |
| C. Discharge on his cardiac medications plus azithromycin to complete a 7‐day course of antibiotics. |
| D. Discharge on usual cardiac medications plus levofloxacin to complete a 7‐day course of antibiotics. |
| Skin infection case (baseline scenario) |
| A 47‐year‐old obese female (BMI=32.9) is hospitalized after presenting with leg erythema, pain, and an 8 8‐cm fluctuant mass on her thigh. Admission temperature was 101.4F. In the ED, she was given vancomycin, and piperacillin/tazobactam and underwent incision and drainage of a thigh abscess, which drained a large amount of pus. The wound was packed. On day 3 she is afebrile, and the pain has improved. Cultures from the wound grow methicillin‐susceptible Staphylococcus aureus. Blood cultures are negative to date. She is ready for discharge. She has no allergies. She is insured and can be followed up with her primary physician within a few days of discharge. You: |
| A. Discharge on vancomycin and piperacillin/tazobactam through a PICC line to complete a 10‐day course. |
| B. Discharge on amoxicillin/clavulanate orally to complete a 10‐day course. |
| C. Discharge on cephalexin to complete a 10‐day course. |
| Asymptomatic bacteriuria case (baseline scenario) |
| A generally healthy 47‐year‐old female is admitted to the hospital for a femoral head fracture and undergoes total hip replacement. On post operative day 2, the nurse sends a urinalysis and culture from the patient's Foley catheter because the patient's urine is cloudy. Labs from that day show a WBC of 8900 and a urinalysis with 12 WBCs/HPF and 2+ bacteria. She has surgical site discomfort and no dysuria or other lower urinary tract symptoms. She has not had a fever during her hospital stay. Two days later her urine culture grows 100,000 CFU/mL of Escherichia coli susceptible to ciprofloxacin but resistant to all other oral antibiotics. That day, the patient is still afebrile and has no dysuria or other lower urinary tract symptoms. Her Foley is changed and a repeat urinalysis shows that the urine has persistent leukocytes and bacteria. You: |
| A. Initiate intravenous ciprofloxacin. |
| B. Initiate oral ciprofloxacin. |
| C. Give no antibiotics. |
| |
| Dyspnea case | |
| Comorbidities | A 47‐year‐old male with a history of stage III congestive heart failure, moderate to heavy tobacco use, poorly controlled type 2 diabetes (last HbA1C=10.9), chronic renal insufficiency (baseline creatinine of 1.3), diabetic retinopathy, and diabetic neuropathy is hospitalized after presenting with shortness of breath. |
| Poor functional status | A 47‐year‐old male with a history of stage III congestive heart failure, and poor functional status with difficulty ambulating and with self‐care is hospitalized after presenting with shortness of breath. |
| Older age | An 86‐year‐old male with a history of stage III congestive heart failure is hospitalized after presenting with shortness of breath. |
| Limited follow‐up | A 47‐year‐old male with a history of stage III congestive heart failure is hospitalized after presenting with shortness of breath.Blood cultures have been negative. He is uninsured and will be referred for follow‐up to a public clinic, which has a 2‐week wait for the next available clinic appointment. |
| Skin infection case | |
| Comorbidities | A 47‐year‐old morbidly obese female (BMI=32.9) with type 2 diabetes is hospitalized after presenting with leg erythema, pain, and an 8 8‐cm fluctuant mass on her thigh. |
| Poor functional status | A 47‐year‐old obese female (BMI=32.9) and poor functional status due to her obesity (poor mobility, uses either a walker or an electric scooter at all times to move around) is hospitalized after presenting with leg erythema, pain, and an 8 8‐cm fluctuant mass on her thigh. |
| Older age | An 86‐year‐old obese female (BMI=32.9) is hospitalized after presenting with leg erythema, pain, and an 8 8‐cm fluctuant mass on her thigh. |
| Limited follow‐up | A 47‐year‐old obese female (BMI=32.9) is hospitalized after presenting with leg erythema, pain, and an 8 8‐cm fluctuant mass on her thigh. She is uninsured and will be referred for follow‐up in a public clinic, which has a 2‐week wait for the next available clinic appointment. |
| Asymptomatic bacteriuria case | |
| Comorbidities | A 47‐year‐old female with a history type 2 diabetes with diabetic nephropathy and retinopathy is admitted to the hospital for a femoral head fracture and undergoes total hip replacement. On postoperative day 2, the nurse sends a urinalysis and culture from the patient's Foley catheter because the patient's urine is cloudy. |
| Poor functional status | A 47‐year‐old female with a history of poor functional status (needs assistance with activities of daily living) due to her obesity and musculoskeletal comorbidities, such as osteoarthritis, is admitted to the hospital for a femoral head fracture and undergoes total hip replacement. On postoperative day 2, the nurse sends a urinalysis and culture from the patient's Foley catheter because the patient's urine is cloudy. |
| Older age | A generally healthy 86‐year‐old female is admitted to the hospital for a femoral head fracture and undergoes total hip replacement. On post operative day 2, the nurse sends a urinalysis and culture from the patient's Foley catheter because the patient's urine is cloudy. |
The institutional review boards at all 3 medical centers approved the study.
Statistical Analysis
We used physician self‐report of recommended antibiotic use stratified by baseline and modified clinical vignettes to calculate the proportion of physicians recommending antibiotic use. We examined how physicians antibiotic use varied according to both patient complexity (medical comorbidity, poor functional status, older age, and limited follow‐up after hospital discharge), and provider characteristics (level of training, degree of specialization, time spent working in the hospital, and time spent providing direct patient care).
Data were analyzed using SAS (version 9.1.3; SAS Institute, Cary, NC). Antibiotic use inconsistent with IDSA guideline recommendations was considered the outcome of interest for the data analysis. Statistical analyses were performed using [2] or Fischer exact test, Student t test, and analysis of variance, as appropriate.
RESULTS
Physician Survey
Of the 874 invited physicians, 255 (29%) responded to the survey. Of these, 8/255 (3.1%) responded that they did not spend 2 or more weeks in the inpatient setting and were thus ineligible for the survey. We analyzed data from the remaining 247 physician respondents. Most respondents (217/233, 93%) reported their primary role was direct clinical care (Table 3). Most (185/241, 77%) reported at least half of their clinical work occurred in the hospital. Approximately three‐quarters (183/241, 76%) of the respondents were residents; the remaining respondents were attending physicians (57/241, 24%). Almost half of attending physicians (46%) were internal medicine subspecialists.
| Physician Characteristic | No. (%) Completing the Survey | % of Physicians Not Adhering to Guidelines in Baseline Scenarios | P Value |
|---|---|---|---|
| |||
| Affiliated medical center, n =241 | |||
| Ronald Reagan UCLA | 47 (20%) | 37% | 0.37 |
| Harbor‐UCLA | 106 (44%) | 41% | |
| Cedars‐Sinai | 86 (35%) | 43% | |
| Primary professional activity, n=233 | |||
| Direct clinical care/teaching | 217 (93%) | 42% | 0.90 |
| Research/administration | 16 (7%) | 27% | |
| Percent of clinical duties in the hospital, n=241 | |||
| 1%25% | 57 (23%) | 41% | 0.71 |
| 51%75% | 93 (39%) | 42% | |
| 76%100% | 92 (38%) | 41% | |
| Level of training and subspecialization, n=241 | |||
| Resident/fellow | 183 (76%) | 43% | 0.05 |
| Attending | 58 (24%) | 34% | |
| Subspecialist | 27 (47%) | 34% | 0.90 |
| Hospitalist | 28 (48%) | 33% | |
Physician recommendation for the use of antibiotics inconsistent with IDSA guidelines was prevalent in the baseline vignettes: 42% (303/729) overall, and 49% (120/246) for the dyspnea, 28% (68/242) for the skin infection, and 48% (115/241) for the asymptomatic bacteriuria cases. When the vignettes were modified to include patient complexities, the proportion of physicians recommending antibiotics increased significantly compared to the baseline vignette (63% (459/728), 54% (393/728), 51% (371/728), and 48% (232/487) for medical comorbidities, poor functional status, older age, and limited follow‐up respectively, P<0.001 for all comparisons) (Figure 1). The increase in the proportion of physicians recommending antibiotics inconsistent with guidelines for patients with medical complexities was the same when stratified by case (data not shown).

We found no association between provider characteristics (medical center, degree of attending physician specialization, percentage of clinical time spent practicing hospital‐based medicine, or percentage of time providing direct clinical care) and prescribing antibiotics in the baseline vignettes (Table 3). However, resident physicians (n=183) were more likely than attending physicians (n=57) to have recommended antibiotics in the baseline vignettes (43% vs 34%, P<0.05) (Table 3) and in all 4 vignettes with patient complexities (data not shown).
DISCUSSION
In our survey, almost half of the physician respondents recommended antibiotics that were inconsistent with national guidelines in the baseline vignettes. One explanation for this finding is that physicians may underestimate the risk associated with antibiotic use, such as the emergence of antimicrobial resistant pathogens and drug‐associated adverse effects. Although physicians generally agree that antibiotic resistance is an important problem, many believe that it is not a prominent issue in their practice or through their antibiotic prescribing practices.[43, 44] Others have shown that physicians underestimate the risk and severity of antibiotic‐associated complications such as C difficile.[45, 46] An accurate assessment and heightened awareness of the risks associated with antibiotics is important in clinical decision making, and should potentially be included not only in antibiotic stewardship educational efforts, but in national guideline recommendations as well.
Our survey also demonstrated that the tendency of physician respondents to recommend antibiotics was amplified for patients with medical complexities. This suggests that patient characteristics related to medical and social complexities play an important role in physicians clinical decision making about prescribing antibiotics. Previous investigations have shown that when physicians are deciding whether or not to prescribe antibiotics, they tend to deprioritize guideline recommendations and give greater weight to the risk of disease progression and complications that might occur if antibiotics are withheld.[47, 48, 49] Physicians are believed to prescribe antibiotics for complex patients more often, in part, because complex patients are more likely to suffer bad outcomes if undertreated.[50, 51] Axiomatically, patients with medical complexities are also at higher risk for antibiotic‐associated adverse effects including polypharmacy, drug‐drug interactions, and more severe side effects.[52, 53, 54]
An additional factor contributing to the overuse of antibiotics in our survey could be the lack of clear guideline recommendations for antibiotic management, especially among patients with complexities. We reviewed 20 national guidelines that addressed the medical decision making relevant to the survey's 3 clinical vignettes (see Supporting Information, Appendix 1, in the online version of this article). Fifteen of the guidelines provided recommendations for antibiotic management in the baseline vignettes, though most of the recommendations were not explicit about stopping or de‐escalating antibiotics. Furthermore, when antibiotic recommendations were present, they often lacked supporting data for the recommendation. Guidelines were even less complete for patients with medical complexities. For the asymptomatic bacteriuria vignette, 4 of 6 guidelines provided recommendations for patients with medical comorbidities described in our survey's modified vignettes.[31, 55, 56, 57] None of the guidelines related to the dyspnea or skin infection vignette provided specific antibiotic recommendations for complex patients with medical comorbidities, poor functional status, older age, or limited follow‐up (see Supporting Information, Appendix 1, in the online version of this article). Given these findings, along with evidence that medically complex patients are more likely to receive antibiotics compared to their less complex counterparts, there is a need for subsequent guidelines to more explicitly recommend best antibiotic practices for patients with medical and social complexities.
We also found that resident physicians were significantly more likely to recommend antibiotics inconsistent with guidelines compared to attending physicians. Although the underlying explanations for this finding were not explored in this study, possibilities include a lack of familiarity with guideline recommendations, less comfort in discontinuing antibiotics in the setting of clinical uncertainty, and/or a preference to accept the risks of overtreatment over the risks of undertreatment.
There are limitations to our study. First, although previous investigations have shown a high degree of correlation between actual antibiotic prescribing practices and antibiotic prescribing decisions self‐reported in clinical vignettes, physician responses in our survey may not reflect actual practices.[58] Second, we were not able to directly measure how physicians knowledge and interpretation of national guidelines influenced their antibiotic management decisions in the survey. Third, while our study was multisite including physicians from 3 different centers, not all invited physicians responded. Because of the confidentiality procedures surrounding the email distribution lists of potential participants, we were unable to obtain additional details about the non‐responders. Finally, because the large majority of respondents were trainees (76%), the generalizability of our findings to attending physicians may be limited. Nevertheless, because residents soon become staff physicians, resident‐reported data supplemented by that from attending physicians seems relevant to identifying opportunities for improving medical care.
In conclusion, we found that a large proportion of physicians recommended antibiotics that were not indicated based on IDSA guidelines for 3 vignettes depicting common hospital‐based clinical scenarios. This pattern of physicians recommending antibiotics inconsistent with guidelines was accentuated with significantly higher reported use for patients with medical comorbidities, poor functional status, older age, and limited healthcare access. Although good clinical judgment requires increased monitoring of patients with medical complexities, it is important for clinicians not to conflate the need for increased patient monitoring with the need for increased antibiotic use. Additional studies and corresponding guideline recommendations for frail, complex patients could be instrumental in reducing frequent use of antibiotics and the resultant cost, adverse effects, and emergence of antibiotic resistant pathogens. Educational efforts, particularly among trainees, regarding appropriate antibiotic use for clinical indications among patients without and with medical complexities would also likely contribute to these aims. Treatment guidelines should consider explicitly addressing medically complex patients in the context of management of infectious syndromes.
Disclosure
Nothing to report.
- , , , , , . Heart failure and chronic obstructive pulmonary disease multimorbidity at hospital discharge transition: a study of patient and carer experience [published online ahead of print May 16. 2014]. Health Expect. doi: 10.1111/hex.12208.
- , , , , , . Development of clinical practice guidelines for patients with comorbidity and multiple diseases [in Spanish]. Aten Primaria. 2014;46(7):385–392.
- , , , et al. Cardiac complications in patients with community‐acquired pneumonia: a systematic review and meta‐analysis of observational studies. PLoS Med. 2011;8(6):e1001048.
- , , , , , . Cost and incidence of social comorbidities in low‐risk patients with community‐acquired pneumonia admitted to a public hospital. Chest. 2003;124(6):2148–2155.
- , , , et al. Why do GPs exclude patients from participating in research? An exploration of adherence to and divergence from trial criteria. Fam Pract. 2014;31(3):364–370.
- , , , , . The impact of pre‐existing heart failure on pneumonia prognosis: population‐based cohort study. J Gen Intern Med. 2008;23(9):1407–1413.
- , , , et al. Severe community‐acquired pneumonia: use of intensive care services and evaluation of American and British Thoracic Society Diagnostic criteria. Am J Respir Crit Care Med. 2002;166(5):717–723.
- . Decisions about treating community‐acquired pneumonia. Ann Intern Med. 2005;142(3):215–216.
- , , , et al. The epidemic of antibiotic‐resistant infections: a call to action for the medical community from the Infectious Diseases Society of America. Clin Infect Dis. 2008;46(2):155–164.
- Will antibiotic misuse now stop? Nat Rev Microbiol. 2003;1(2):85.
- , , , et al. Infectious Diseases Society of America and the Society for Healthcare Epidemiology of America guidelines for developing an institutional program to enhance antimicrobial stewardship. Clin Infect Dis. 2007;44(2):159–177.
- . Economic impact of antimicrobial resistance. Emerg Infect Dis. 2001;7(2):286–292.
- , , , , . Guideline‐concordant therapy and reduced mortality and length of stay in adults with community‐acquired pneumonia: playing by the rules. Arch Intern Med. 2009;169(16):1525–1531.
- , , , et al. Adherence to guidelines' empirical antibiotic recommendations and community‐acquired pneumonia outcome. Eur Respir J. 2008;32(4):892–901.
- , , , et al. Community‐associated Clostridium difficile infection and antibiotics: a meta‐analysis. J Antimicrob Chemother. 2013;68(9):1951–1961.
- , , , . Vancomycin‐induced nephrotoxicity: mechanism, incidence, risk factors and special populations. A literature review. Eur J Clin Pharmacol. 2012;68(9):1243–1255.
- , . Diagnosis and management of immediate hypersensitivity reactions to cephalosporins. Clin Rev Allergy Immunol. 2013;45(1):131–142.
- , . Neurotoxic effects associated with antibiotic use: management considerations. Br J Clin Pharmacol. 2011;72(3):381–393.
- , , , et al. A clinician's guide to the appropriate and accurate use of antibiotics: the Council for Appropriate and Rational Antibiotic Therapy (CARAT) criteria. Am J Med. 2005;118(suppl 7A):1S–6S.
- , , , , . Evaluation of rational antibiotic use. Int J Antimicrob Agents. 2000;15(2):131–135.
- , . Rational antibiotic prescribing. Challenges and successes [in German]. Bundesgesundheitsblatt Gesundheitsforschung Gesundheitsschutz. 2012;55(11–12):1418–1426.
- , , , et al. Antibiotic prescription for community‐acquired pneumonia in the intensive care unit: impact of adherence to Infectious Diseases Society of America guidelines on survival. Clin Infect Dis. 2005;41(12):1709–1716.
- , , . Guideline‐adherent initial intravenous antibiotic therapy for hospital‐acquired/ventilator‐associated pneumonia is clinically superior, saves lives and is cheaper than non guideline adherent therapy. Eur J Med Res. 2011;16(7):315–323.
- , , , , , . Antibiotic misuse: a prospective clinical audit in a French university hospital. Eur J Clin Microbiol Infect Dis. 2007;26(4):277–280.
- , , , , . Unnecessary use of antimicrobials in hospitalized patients: current patterns of misuse with an emphasis on the antianaerobic spectrum of activity. Arch Intern Med. 2003;163(8):972–978.
- , , , . A systematic review and meta‐analysis of misuse of antibiotic therapies in the community. Int J Antimicrob Agents. 2005;26(2):106–113.
- , . From best evidence to best practice: effective implementation of change in patients' care. Lancet. 2003;362(9391):1225–1230.
- , , , et al. The complexity of care for patients with rheumatoid arthritis: metrics for better understanding chronic disease care. Med Care. 2007;45(1):55–65.
- , , , et al. Infectious Diseases Society of America/American Thoracic Society consensus guidelines on the management of community‐acquired pneumonia in adults. Clin Infect Dis. 2007;44(suppl 2):S27–S72.
- , , , et al. Practice guidelines for the diagnosis and management of skin and soft tissue infections: 2014 update by the Infectious Diseases Society of America. Clin Infect Dis. 2014;59(2):e10–e52.
- , , , , , . Infectious Diseases Society of America guidelines for the diagnosis and treatment of asymptomatic bacteriuria in adults. Clin Infect Dis. 2005;40(5):643–654.
- , , , et al. Heart disease and stroke statistics—2010 update: a report from the American Heart Association. Circulation. 2010;121(7):e46–e215.
- , , , et al. ESC Guidelines for the diagnosis and treatment of acute and chronic heart failure 2008: the Task Force for the Diagnosis and Treatment of Acute and Chronic Heart Failure 2008 of the European Society of Cardiology. Developed in collaboration with the Heart Failure Association of the ESC (HFA) and endorsed by the European Society of Intensive Care Medicine (ESICM). Eur Heart J. 2008;29(19):2388–2442.
- , , , . Prevalence of negative chest radiography results in the emergency department patient with decompensated heart failure. Ann Emerg Med. 2006;47(1):13–18.
- , , , . Patients admitted to hospital with suspected pneumonia and normal chest radiographs: epidemiology, microbiology, and outcomes. Am J Med. 2004;117(5):305–311.
- , , , et al. Incidence of skin and soft tissue infections in ambulatory and inpatient settings, 2005–2010. BMC Infect Dis. In press.
- . Asymptomatic bacteriuria: when the treatment is worse than the disease. Nat Rev Urol. 2012;9(2):85–93.
- , , , et al. Prevalence and incidence trends for diagnosed diabetes among adults aged 20 to 79 years, United States, 1980–2012. JAMA. 2014;312(12):1218–1226.
- , , , . Prevalence of childhood and adult obesity in the United States, 2011–2012. JAMA. 2014;311(8):806–814.
- , , , , . Frailty in elderly people. Lancet. 2013;381(9868):752–762.
- , , , , , . Risk factors for death in homeless adults in Boston. Arch Intern Med. 1998;158(13):1454–1460.
- . Taking it to the streets: homelessness, health, and health care in the United States. J Gen Intern Med. 2003;18(11):964–965.
- , , , , , . Antibiotic resistance: a survey of physician perceptions. Arch Intern Med. 2002;162(19):2210–2216.
- , , , et al. Primary care clinicians' perceptions of antibiotic resistance: a multi‐country qualitative interview study. J Antimicrob Chemother. 2013;68(1):237–243.
- , , , , , . Underestimation of Clostridium difficile infection among clinicians: an international survey. Eur J Clin Microbiol Infect Dis. 2012;31(9):2439–2444.
- , , , , , . Unnecessary antimicrobial use in patients with current or recent Clostridium difficile infection. Infect Control Hosp Epidemiol. 2013;34(2):109–116.
- , , , , . Attitudes of primary care physicians to the prescribing of antibiotics and antimicrobial resistance: a qualitative study from Spain. Fam Pract. 2012;29(3):352–360.
- , , , , . Understanding physician antibiotic prescribing behaviour: a systematic review of qualitative studies. Int J Antimicrob Agents. 2013;41(3):203–212.
- , , , . Guidelines on uncomplicated urinary tract infections are difficult to follow: perceived barriers and suggested interventions. BMC Fam Pract. 2010;11:51.
- , , , . Why don't physicians adhere to guideline recommendations in practice? An analysis of barriers among Dutch general practitioners. Implement Sci. 2009;4:54.
- , , , , . Perceived barriers to guideline adherence: a survey among general practitioners. BMC Fam Pract. 2011;12:98.
- , , , . Clostridium difficile in acute and long‐stay elderly patients. Age Ageing. 1988;17(5):333–336.
- , , , , , . Pharmacodynamics of vancomycin in elderly patients aged 75 years or older with methicillin‐resistant Staphylococcus aureus hospital‐acquired pneumonia. Clin Interv Aging. 2013;8:1015–1021.
- , , , et al. Empiric guideline‐recommended weight‐based vancomycin dosing and nephrotoxicity rates in patients with methicillin‐resistant Staphylococcus aureus bacteremia: a retrospective cohort study. BMC Pharmacol Toxicol. 2013;14:12.
- , , , , , , , , . Guidelines on urological infections. Arnhem, The Netherlands: European Association of Urology (EAU); 2011. p. 15–27.
- Scottish Intercollegiate Guidelines Network. Management of suspected bacterial urinary tract infection in adults. Available at: http://www.sign.ac.uk/guidelines/fulltext/88/. Accessed on July 25, 2014.
- , , , et al. [Optimisation of the antibiotic policy in the Netherlands. X. The SWAB guideline for antimicrobial treatment of complicated urinary tract infections]. Ned Tijdschr Geneeskd 2006;150(43):2370–2376.
- , , , et al. Do case vignettes accurately reflect antibiotic prescription? Infect Control Hosp Epidemiol. 2011;32(10):1003–1009.
- The committee for The Japanese Respiratory Society guidelines in management of respiratory infections. Principles for the development of the guidelines. Respirology 2004;9(suppl 1):S1–S2.
- , , , , , . Heart failure and chronic obstructive pulmonary disease multimorbidity at hospital discharge transition: a study of patient and carer experience [published online ahead of print May 16. 2014]. Health Expect. doi: 10.1111/hex.12208.
- , , , , , . Development of clinical practice guidelines for patients with comorbidity and multiple diseases [in Spanish]. Aten Primaria. 2014;46(7):385–392.
- , , , et al. Cardiac complications in patients with community‐acquired pneumonia: a systematic review and meta‐analysis of observational studies. PLoS Med. 2011;8(6):e1001048.
- , , , , , . Cost and incidence of social comorbidities in low‐risk patients with community‐acquired pneumonia admitted to a public hospital. Chest. 2003;124(6):2148–2155.
- , , , et al. Why do GPs exclude patients from participating in research? An exploration of adherence to and divergence from trial criteria. Fam Pract. 2014;31(3):364–370.
- , , , , . The impact of pre‐existing heart failure on pneumonia prognosis: population‐based cohort study. J Gen Intern Med. 2008;23(9):1407–1413.
- , , , et al. Severe community‐acquired pneumonia: use of intensive care services and evaluation of American and British Thoracic Society Diagnostic criteria. Am J Respir Crit Care Med. 2002;166(5):717–723.
- . Decisions about treating community‐acquired pneumonia. Ann Intern Med. 2005;142(3):215–216.
- , , , et al. The epidemic of antibiotic‐resistant infections: a call to action for the medical community from the Infectious Diseases Society of America. Clin Infect Dis. 2008;46(2):155–164.
- Will antibiotic misuse now stop? Nat Rev Microbiol. 2003;1(2):85.
- , , , et al. Infectious Diseases Society of America and the Society for Healthcare Epidemiology of America guidelines for developing an institutional program to enhance antimicrobial stewardship. Clin Infect Dis. 2007;44(2):159–177.
- . Economic impact of antimicrobial resistance. Emerg Infect Dis. 2001;7(2):286–292.
- , , , , . Guideline‐concordant therapy and reduced mortality and length of stay in adults with community‐acquired pneumonia: playing by the rules. Arch Intern Med. 2009;169(16):1525–1531.
- , , , et al. Adherence to guidelines' empirical antibiotic recommendations and community‐acquired pneumonia outcome. Eur Respir J. 2008;32(4):892–901.
- , , , et al. Community‐associated Clostridium difficile infection and antibiotics: a meta‐analysis. J Antimicrob Chemother. 2013;68(9):1951–1961.
- , , , . Vancomycin‐induced nephrotoxicity: mechanism, incidence, risk factors and special populations. A literature review. Eur J Clin Pharmacol. 2012;68(9):1243–1255.
- , . Diagnosis and management of immediate hypersensitivity reactions to cephalosporins. Clin Rev Allergy Immunol. 2013;45(1):131–142.
- , . Neurotoxic effects associated with antibiotic use: management considerations. Br J Clin Pharmacol. 2011;72(3):381–393.
- , , , et al. A clinician's guide to the appropriate and accurate use of antibiotics: the Council for Appropriate and Rational Antibiotic Therapy (CARAT) criteria. Am J Med. 2005;118(suppl 7A):1S–6S.
- , , , , . Evaluation of rational antibiotic use. Int J Antimicrob Agents. 2000;15(2):131–135.
- , . Rational antibiotic prescribing. Challenges and successes [in German]. Bundesgesundheitsblatt Gesundheitsforschung Gesundheitsschutz. 2012;55(11–12):1418–1426.
- , , , et al. Antibiotic prescription for community‐acquired pneumonia in the intensive care unit: impact of adherence to Infectious Diseases Society of America guidelines on survival. Clin Infect Dis. 2005;41(12):1709–1716.
- , , . Guideline‐adherent initial intravenous antibiotic therapy for hospital‐acquired/ventilator‐associated pneumonia is clinically superior, saves lives and is cheaper than non guideline adherent therapy. Eur J Med Res. 2011;16(7):315–323.
- , , , , , . Antibiotic misuse: a prospective clinical audit in a French university hospital. Eur J Clin Microbiol Infect Dis. 2007;26(4):277–280.
- , , , , . Unnecessary use of antimicrobials in hospitalized patients: current patterns of misuse with an emphasis on the antianaerobic spectrum of activity. Arch Intern Med. 2003;163(8):972–978.
- , , , . A systematic review and meta‐analysis of misuse of antibiotic therapies in the community. Int J Antimicrob Agents. 2005;26(2):106–113.
- , . From best evidence to best practice: effective implementation of change in patients' care. Lancet. 2003;362(9391):1225–1230.
- , , , et al. The complexity of care for patients with rheumatoid arthritis: metrics for better understanding chronic disease care. Med Care. 2007;45(1):55–65.
- , , , et al. Infectious Diseases Society of America/American Thoracic Society consensus guidelines on the management of community‐acquired pneumonia in adults. Clin Infect Dis. 2007;44(suppl 2):S27–S72.
- , , , et al. Practice guidelines for the diagnosis and management of skin and soft tissue infections: 2014 update by the Infectious Diseases Society of America. Clin Infect Dis. 2014;59(2):e10–e52.
- , , , , , . Infectious Diseases Society of America guidelines for the diagnosis and treatment of asymptomatic bacteriuria in adults. Clin Infect Dis. 2005;40(5):643–654.
- , , , et al. Heart disease and stroke statistics—2010 update: a report from the American Heart Association. Circulation. 2010;121(7):e46–e215.
- , , , et al. ESC Guidelines for the diagnosis and treatment of acute and chronic heart failure 2008: the Task Force for the Diagnosis and Treatment of Acute and Chronic Heart Failure 2008 of the European Society of Cardiology. Developed in collaboration with the Heart Failure Association of the ESC (HFA) and endorsed by the European Society of Intensive Care Medicine (ESICM). Eur Heart J. 2008;29(19):2388–2442.
- , , , . Prevalence of negative chest radiography results in the emergency department patient with decompensated heart failure. Ann Emerg Med. 2006;47(1):13–18.
- , , , . Patients admitted to hospital with suspected pneumonia and normal chest radiographs: epidemiology, microbiology, and outcomes. Am J Med. 2004;117(5):305–311.
- , , , et al. Incidence of skin and soft tissue infections in ambulatory and inpatient settings, 2005–2010. BMC Infect Dis. In press.
- . Asymptomatic bacteriuria: when the treatment is worse than the disease. Nat Rev Urol. 2012;9(2):85–93.
- , , , et al. Prevalence and incidence trends for diagnosed diabetes among adults aged 20 to 79 years, United States, 1980–2012. JAMA. 2014;312(12):1218–1226.
- , , , . Prevalence of childhood and adult obesity in the United States, 2011–2012. JAMA. 2014;311(8):806–814.
- , , , , . Frailty in elderly people. Lancet. 2013;381(9868):752–762.
- , , , , , . Risk factors for death in homeless adults in Boston. Arch Intern Med. 1998;158(13):1454–1460.
- . Taking it to the streets: homelessness, health, and health care in the United States. J Gen Intern Med. 2003;18(11):964–965.
- , , , , , . Antibiotic resistance: a survey of physician perceptions. Arch Intern Med. 2002;162(19):2210–2216.
- , , , et al. Primary care clinicians' perceptions of antibiotic resistance: a multi‐country qualitative interview study. J Antimicrob Chemother. 2013;68(1):237–243.
- , , , , , . Underestimation of Clostridium difficile infection among clinicians: an international survey. Eur J Clin Microbiol Infect Dis. 2012;31(9):2439–2444.
- , , , , , . Unnecessary antimicrobial use in patients with current or recent Clostridium difficile infection. Infect Control Hosp Epidemiol. 2013;34(2):109–116.
- , , , , . Attitudes of primary care physicians to the prescribing of antibiotics and antimicrobial resistance: a qualitative study from Spain. Fam Pract. 2012;29(3):352–360.
- , , , , . Understanding physician antibiotic prescribing behaviour: a systematic review of qualitative studies. Int J Antimicrob Agents. 2013;41(3):203–212.
- , , , . Guidelines on uncomplicated urinary tract infections are difficult to follow: perceived barriers and suggested interventions. BMC Fam Pract. 2010;11:51.
- , , , . Why don't physicians adhere to guideline recommendations in practice? An analysis of barriers among Dutch general practitioners. Implement Sci. 2009;4:54.
- , , , , . Perceived barriers to guideline adherence: a survey among general practitioners. BMC Fam Pract. 2011;12:98.
- , , , . Clostridium difficile in acute and long‐stay elderly patients. Age Ageing. 1988;17(5):333–336.
- , , , , , . Pharmacodynamics of vancomycin in elderly patients aged 75 years or older with methicillin‐resistant Staphylococcus aureus hospital‐acquired pneumonia. Clin Interv Aging. 2013;8:1015–1021.
- , , , et al. Empiric guideline‐recommended weight‐based vancomycin dosing and nephrotoxicity rates in patients with methicillin‐resistant Staphylococcus aureus bacteremia: a retrospective cohort study. BMC Pharmacol Toxicol. 2013;14:12.
- , , , , , , , , . Guidelines on urological infections. Arnhem, The Netherlands: European Association of Urology (EAU); 2011. p. 15–27.
- Scottish Intercollegiate Guidelines Network. Management of suspected bacterial urinary tract infection in adults. Available at: http://www.sign.ac.uk/guidelines/fulltext/88/. Accessed on July 25, 2014.
- , , , et al. [Optimisation of the antibiotic policy in the Netherlands. X. The SWAB guideline for antimicrobial treatment of complicated urinary tract infections]. Ned Tijdschr Geneeskd 2006;150(43):2370–2376.
- , , , et al. Do case vignettes accurately reflect antibiotic prescription? Infect Control Hosp Epidemiol. 2011;32(10):1003–1009.
- The committee for The Japanese Respiratory Society guidelines in management of respiratory infections. Principles for the development of the guidelines. Respirology 2004;9(suppl 1):S1–S2.
© 2015 Society of Hospital Medicine
False Alarms and Patient Safety
Despite 15 years of national and local investment in improving the safety of hospital care, patient safety remains a leading problem in both adult and pediatric hospitals. A 2010 study found that 180,000 Medicare beneficiaries likely die each year due to harm suffered as a result of medical care,[1] a death toll surpassed only by deaths due to cardiovascular disease and cancer. Even though initial efforts in the field have shown great promise for stemming the tide of healthcare‐associated infections,[2] surgical errors,[3] handoff failures,[4] and errors in the care of adults hospitalized for myocardial infarction and congestive heart failure,[5] much work remains to be done.[6] The root causes of many adverse events are poorly understood and unaddressed. Resultant tragedies remain all too common.
In the current issue of the Journal of Hospital Medicine, Bonafide and colleagues report the results of an innovative observational pilot study designed to assess the role of an inadequately addressed root cause of serious errors: alarm fatigue.[7] Alarm fatigue is the phenomenon of desensitization to alarms, particularly in the context of excessive false alarms. In a videotaped observational assessment of nurse response times to 5070 alarms on a pediatric ward and intensive care unit (ICU), the authors found that nurses responded significantly more slowly as the number of nonactionable alarms in the preceding 2 hours increased. Although a substantial majority of these alarms were technically valid (ie, representing true deviations of vital signs outside of the normal range rather than sensor or equipment problems), the vast majority required no action to be takenapproximately 7 out of 8 in the ICU and an astonishing 99 out of 100 on the ward.
As any hospitalist, intensivist, or nurse knows well, alarms are rampant throughout hospitals. It is impossible to walk down any hallway on a busy hospital wardnever mind an ICUwithout seeing a flashing light or 2 above a doorway, and hearing the incessant beeping of oxygen saturation and cardiovascular/respiratory monitors, a thousand bits of technology forever crying wolf. The problem, of course, is that sometimes there really is a wolf, but it is hard to take the risk seriously when the false alarms happen not just twice before a true threat materializes, as in Aesop's fable, but 7 times in the ICU, or worse, 99 times in the setting where most hospitalists practice. Moreover, even when the threat is real, in most cases it is caught in time one way or another, and no lasting harm results.
So why not simply shut off the unremitting noise? In 1987, outside of Baltimore, Amtrak experienced what at the time was the deadliest rail crash in its history after 1 of its passenger trains collided with a Conrail freight train. A major root cause of the crash was that the crew on the freight train had placed duct tape over an annoying automated signal alarm.[8, 9] Tragically, on this particular day, the suppressed alarm was all too relevant. Identifying the real alarm, however, can be nearly impossible when it sounds the same as 100 irritating sounds constantly emanating from the environment. It is the challenge of identifying the needle in the haystack, after you have developed an allergy to the hay.
What then to do? More research like that conducted by Bonafide and colleagues is needed to better understand how healthcare providers respond to the onslaught of alarms they encounter, and to inform refinement of these systems. Understanding how alarm fatigue plays out in the context of different clinical settings, with different workloads, varying levels of distraction, and different rates of true and false‐positive alarms will be critical. Furthermore, understanding how individuals' physiologic fatigue, circadian misalignment, mood, stress, and cognitive state may play into alarm response is likewise essential, if we are to design appropriate alarm systems that function effectively in the busy 24‐hour environment of healthcare. Ongoing work suggests that smart alarms, using algorithms that integrate data from multiple vital sign readings over time, may reduce the frequency of false alarms and better identify clinically significant events.[10] Replacing existing range‐limit monitors with these types of smart alarms has the potential to greatly improve both the sensitivity and specificity of hospital alarms, but further work in this area is needed.
Ultimately, if we can better separate out the signal, we will be better poised to respond to the true emergencies that arise that are currently obscured by the ever‐present noise. Better trust in the alarm systems we have would help all of us focus our energies on the problems that matter most. Doing so, we could better care for our patients, and better identify the system failures that cause them harm in our hospitals.
Disclosures: Dr. Landrigan is supported in part by the Children's Hospital Association for his work as an Executive Council Member of the Pediatric Research in Inpatient Settings network. Dr. Landrigan serves as a consultant to Virgin Pulse regarding sleep, safety, and health. In addition, Dr. Landrigan has received monetary awards, honoraria, and travel reimbursement from multiple academic and professional organizations for delivering lectures on sleep deprivation, physician performance, handoffs, and patient safety, and has served as an expert witness in cases regarding patient safety.
- Office of the Inspector General. Adverse events in hospitals: national incidence among Medicare beneficiaries. OEI‐06‐09‐00090. Available at: https://oig.hhs.gov/oei/reports/oei‐06‐09‐00090.pdf. Published November 2010. Accessed February 27, 2015.
- , , , et al. An intervention to decrease catheter‐related bloodstream infections in the ICU. N Engl J Med. 2006;355:2725–2732.
- , , , et al. A surgical safety checklist to reduce morbidity and mortality in a global population. N Engl J Med. 2009;360:491–499.
- , , , et al. Changes in medical errors after implementation of a resident handoff program. New Engl J Med. 2014;371:1803–1812.
- , , , et al. National trends in patient safety for four common conditions, 2005–2011. N Engl J Med. 2014;370:341–351.
- , , , et al. Temporal trends in rates of patient harm due to medical care. New Engl J Med. 2010;363:2124–2134.
- , , , et al. Association between exposure to nonactionable physiologic monitor alarms and response time in a children's hospital. J Hosp Med. 2015;10(6):345–351.
- . Why are people turning off our alarms? J Acoust Soc Am. 1988;84:1107–1108.
- 1987 Maryland train collision. Wikipedia. Available at: http://en.wikipedia.org/wiki/1987_Maryland_train_collision. Accessed February 27, 2015.
- , , , et al. Collection of annotated data in a clinical validation study for alarm algorithms in intensive care—a methodologic framework. J Crit Care. 2010;25:128–135.
Despite 15 years of national and local investment in improving the safety of hospital care, patient safety remains a leading problem in both adult and pediatric hospitals. A 2010 study found that 180,000 Medicare beneficiaries likely die each year due to harm suffered as a result of medical care,[1] a death toll surpassed only by deaths due to cardiovascular disease and cancer. Even though initial efforts in the field have shown great promise for stemming the tide of healthcare‐associated infections,[2] surgical errors,[3] handoff failures,[4] and errors in the care of adults hospitalized for myocardial infarction and congestive heart failure,[5] much work remains to be done.[6] The root causes of many adverse events are poorly understood and unaddressed. Resultant tragedies remain all too common.
In the current issue of the Journal of Hospital Medicine, Bonafide and colleagues report the results of an innovative observational pilot study designed to assess the role of an inadequately addressed root cause of serious errors: alarm fatigue.[7] Alarm fatigue is the phenomenon of desensitization to alarms, particularly in the context of excessive false alarms. In a videotaped observational assessment of nurse response times to 5070 alarms on a pediatric ward and intensive care unit (ICU), the authors found that nurses responded significantly more slowly as the number of nonactionable alarms in the preceding 2 hours increased. Although a substantial majority of these alarms were technically valid (ie, representing true deviations of vital signs outside of the normal range rather than sensor or equipment problems), the vast majority required no action to be takenapproximately 7 out of 8 in the ICU and an astonishing 99 out of 100 on the ward.
As any hospitalist, intensivist, or nurse knows well, alarms are rampant throughout hospitals. It is impossible to walk down any hallway on a busy hospital wardnever mind an ICUwithout seeing a flashing light or 2 above a doorway, and hearing the incessant beeping of oxygen saturation and cardiovascular/respiratory monitors, a thousand bits of technology forever crying wolf. The problem, of course, is that sometimes there really is a wolf, but it is hard to take the risk seriously when the false alarms happen not just twice before a true threat materializes, as in Aesop's fable, but 7 times in the ICU, or worse, 99 times in the setting where most hospitalists practice. Moreover, even when the threat is real, in most cases it is caught in time one way or another, and no lasting harm results.
So why not simply shut off the unremitting noise? In 1987, outside of Baltimore, Amtrak experienced what at the time was the deadliest rail crash in its history after 1 of its passenger trains collided with a Conrail freight train. A major root cause of the crash was that the crew on the freight train had placed duct tape over an annoying automated signal alarm.[8, 9] Tragically, on this particular day, the suppressed alarm was all too relevant. Identifying the real alarm, however, can be nearly impossible when it sounds the same as 100 irritating sounds constantly emanating from the environment. It is the challenge of identifying the needle in the haystack, after you have developed an allergy to the hay.
What then to do? More research like that conducted by Bonafide and colleagues is needed to better understand how healthcare providers respond to the onslaught of alarms they encounter, and to inform refinement of these systems. Understanding how alarm fatigue plays out in the context of different clinical settings, with different workloads, varying levels of distraction, and different rates of true and false‐positive alarms will be critical. Furthermore, understanding how individuals' physiologic fatigue, circadian misalignment, mood, stress, and cognitive state may play into alarm response is likewise essential, if we are to design appropriate alarm systems that function effectively in the busy 24‐hour environment of healthcare. Ongoing work suggests that smart alarms, using algorithms that integrate data from multiple vital sign readings over time, may reduce the frequency of false alarms and better identify clinically significant events.[10] Replacing existing range‐limit monitors with these types of smart alarms has the potential to greatly improve both the sensitivity and specificity of hospital alarms, but further work in this area is needed.
Ultimately, if we can better separate out the signal, we will be better poised to respond to the true emergencies that arise that are currently obscured by the ever‐present noise. Better trust in the alarm systems we have would help all of us focus our energies on the problems that matter most. Doing so, we could better care for our patients, and better identify the system failures that cause them harm in our hospitals.
Disclosures: Dr. Landrigan is supported in part by the Children's Hospital Association for his work as an Executive Council Member of the Pediatric Research in Inpatient Settings network. Dr. Landrigan serves as a consultant to Virgin Pulse regarding sleep, safety, and health. In addition, Dr. Landrigan has received monetary awards, honoraria, and travel reimbursement from multiple academic and professional organizations for delivering lectures on sleep deprivation, physician performance, handoffs, and patient safety, and has served as an expert witness in cases regarding patient safety.
Despite 15 years of national and local investment in improving the safety of hospital care, patient safety remains a leading problem in both adult and pediatric hospitals. A 2010 study found that 180,000 Medicare beneficiaries likely die each year due to harm suffered as a result of medical care,[1] a death toll surpassed only by deaths due to cardiovascular disease and cancer. Even though initial efforts in the field have shown great promise for stemming the tide of healthcare‐associated infections,[2] surgical errors,[3] handoff failures,[4] and errors in the care of adults hospitalized for myocardial infarction and congestive heart failure,[5] much work remains to be done.[6] The root causes of many adverse events are poorly understood and unaddressed. Resultant tragedies remain all too common.
In the current issue of the Journal of Hospital Medicine, Bonafide and colleagues report the results of an innovative observational pilot study designed to assess the role of an inadequately addressed root cause of serious errors: alarm fatigue.[7] Alarm fatigue is the phenomenon of desensitization to alarms, particularly in the context of excessive false alarms. In a videotaped observational assessment of nurse response times to 5070 alarms on a pediatric ward and intensive care unit (ICU), the authors found that nurses responded significantly more slowly as the number of nonactionable alarms in the preceding 2 hours increased. Although a substantial majority of these alarms were technically valid (ie, representing true deviations of vital signs outside of the normal range rather than sensor or equipment problems), the vast majority required no action to be takenapproximately 7 out of 8 in the ICU and an astonishing 99 out of 100 on the ward.
As any hospitalist, intensivist, or nurse knows well, alarms are rampant throughout hospitals. It is impossible to walk down any hallway on a busy hospital wardnever mind an ICUwithout seeing a flashing light or 2 above a doorway, and hearing the incessant beeping of oxygen saturation and cardiovascular/respiratory monitors, a thousand bits of technology forever crying wolf. The problem, of course, is that sometimes there really is a wolf, but it is hard to take the risk seriously when the false alarms happen not just twice before a true threat materializes, as in Aesop's fable, but 7 times in the ICU, or worse, 99 times in the setting where most hospitalists practice. Moreover, even when the threat is real, in most cases it is caught in time one way or another, and no lasting harm results.
So why not simply shut off the unremitting noise? In 1987, outside of Baltimore, Amtrak experienced what at the time was the deadliest rail crash in its history after 1 of its passenger trains collided with a Conrail freight train. A major root cause of the crash was that the crew on the freight train had placed duct tape over an annoying automated signal alarm.[8, 9] Tragically, on this particular day, the suppressed alarm was all too relevant. Identifying the real alarm, however, can be nearly impossible when it sounds the same as 100 irritating sounds constantly emanating from the environment. It is the challenge of identifying the needle in the haystack, after you have developed an allergy to the hay.
What then to do? More research like that conducted by Bonafide and colleagues is needed to better understand how healthcare providers respond to the onslaught of alarms they encounter, and to inform refinement of these systems. Understanding how alarm fatigue plays out in the context of different clinical settings, with different workloads, varying levels of distraction, and different rates of true and false‐positive alarms will be critical. Furthermore, understanding how individuals' physiologic fatigue, circadian misalignment, mood, stress, and cognitive state may play into alarm response is likewise essential, if we are to design appropriate alarm systems that function effectively in the busy 24‐hour environment of healthcare. Ongoing work suggests that smart alarms, using algorithms that integrate data from multiple vital sign readings over time, may reduce the frequency of false alarms and better identify clinically significant events.[10] Replacing existing range‐limit monitors with these types of smart alarms has the potential to greatly improve both the sensitivity and specificity of hospital alarms, but further work in this area is needed.
Ultimately, if we can better separate out the signal, we will be better poised to respond to the true emergencies that arise that are currently obscured by the ever‐present noise. Better trust in the alarm systems we have would help all of us focus our energies on the problems that matter most. Doing so, we could better care for our patients, and better identify the system failures that cause them harm in our hospitals.
Disclosures: Dr. Landrigan is supported in part by the Children's Hospital Association for his work as an Executive Council Member of the Pediatric Research in Inpatient Settings network. Dr. Landrigan serves as a consultant to Virgin Pulse regarding sleep, safety, and health. In addition, Dr. Landrigan has received monetary awards, honoraria, and travel reimbursement from multiple academic and professional organizations for delivering lectures on sleep deprivation, physician performance, handoffs, and patient safety, and has served as an expert witness in cases regarding patient safety.
- Office of the Inspector General. Adverse events in hospitals: national incidence among Medicare beneficiaries. OEI‐06‐09‐00090. Available at: https://oig.hhs.gov/oei/reports/oei‐06‐09‐00090.pdf. Published November 2010. Accessed February 27, 2015.
- , , , et al. An intervention to decrease catheter‐related bloodstream infections in the ICU. N Engl J Med. 2006;355:2725–2732.
- , , , et al. A surgical safety checklist to reduce morbidity and mortality in a global population. N Engl J Med. 2009;360:491–499.
- , , , et al. Changes in medical errors after implementation of a resident handoff program. New Engl J Med. 2014;371:1803–1812.
- , , , et al. National trends in patient safety for four common conditions, 2005–2011. N Engl J Med. 2014;370:341–351.
- , , , et al. Temporal trends in rates of patient harm due to medical care. New Engl J Med. 2010;363:2124–2134.
- , , , et al. Association between exposure to nonactionable physiologic monitor alarms and response time in a children's hospital. J Hosp Med. 2015;10(6):345–351.
- . Why are people turning off our alarms? J Acoust Soc Am. 1988;84:1107–1108.
- 1987 Maryland train collision. Wikipedia. Available at: http://en.wikipedia.org/wiki/1987_Maryland_train_collision. Accessed February 27, 2015.
- , , , et al. Collection of annotated data in a clinical validation study for alarm algorithms in intensive care—a methodologic framework. J Crit Care. 2010;25:128–135.
- Office of the Inspector General. Adverse events in hospitals: national incidence among Medicare beneficiaries. OEI‐06‐09‐00090. Available at: https://oig.hhs.gov/oei/reports/oei‐06‐09‐00090.pdf. Published November 2010. Accessed February 27, 2015.
- , , , et al. An intervention to decrease catheter‐related bloodstream infections in the ICU. N Engl J Med. 2006;355:2725–2732.
- , , , et al. A surgical safety checklist to reduce morbidity and mortality in a global population. N Engl J Med. 2009;360:491–499.
- , , , et al. Changes in medical errors after implementation of a resident handoff program. New Engl J Med. 2014;371:1803–1812.
- , , , et al. National trends in patient safety for four common conditions, 2005–2011. N Engl J Med. 2014;370:341–351.
- , , , et al. Temporal trends in rates of patient harm due to medical care. New Engl J Med. 2010;363:2124–2134.
- , , , et al. Association between exposure to nonactionable physiologic monitor alarms and response time in a children's hospital. J Hosp Med. 2015;10(6):345–351.
- . Why are people turning off our alarms? J Acoust Soc Am. 1988;84:1107–1108.
- 1987 Maryland train collision. Wikipedia. Available at: http://en.wikipedia.org/wiki/1987_Maryland_train_collision. Accessed February 27, 2015.
- , , , et al. Collection of annotated data in a clinical validation study for alarm algorithms in intensive care—a methodologic framework. J Crit Care. 2010;25:128–135.
Monitor Alarms and Response Time
Hospital physiologic monitors can alert clinicians to early signs of physiologic deterioration, and thus have great potential to save lives. However, monitors generate frequent alarms,[1, 2, 3, 4, 5, 6, 7, 8] and most are not relevant to the patient's safety (over 90% of pediatric intensive care unit (PICU)[1, 2] and over 70% of adult intensive care alarms).[5, 6] In psychology experiments, humans rapidly learn to ignore or respond more slowly to alarms when exposed to high false‐alarm rates, exhibiting alarm fatigue.[9, 10] In 2013, The Joint Commission named alarm fatigue the most common contributing factor to alarm‐related sentinel events in hospitals.[11, 12]
Although alarm fatigue has been implicated as a major threat to patient safety, little empirical data support its existence in hospitals. In this study, we aimed to determine if there was an association between nurses' recent exposure to nonactionable physiologic monitor alarms and their response time to future alarms for the same patients. This exploratory work was designed to inform future research in this area, acknowledging that the sample size would be too small for multivariable modeling.
METHODS
Study Definitions
The alarm classification scheme is shown in Figure 1. Note that, for clarity, we have intentionally avoided using the terms true and false alarms because their interpretations vary across studies and can be misleading.
Potentially Critical Alarm
A potentially critical alarm is any alarm for a clinical condition for which a timely response is important to determine if the alarm requires intervention to save the patient's life. This is based on the alarm type alone, including alarms for life‐threatening arrhythmias such as asystole and ventricular tachycardia, as well as alarms for vital signs outside the set limits. Supporting Table 1 in the online version of this article lists the breakdown of alarm types that we defined a priori as potentially and not potentially critical.
| PICU | Ward | |||||||
|---|---|---|---|---|---|---|---|---|
| Alarm type | No. | % of Total | % Valid | % Actionable | No. | % of Total | % Valid | % Actionable |
| ||||||||
| Oxygen saturation | 197 | 19.4 | 82.7 | 38.6 | 590 | 41.2 | 24.4 | 1.9 |
| Heart rate | 194 | 19.1 | 95.4 | 1.0 | 266 | 18.6 | 87.2 | 0.0 |
| Respiratory rate | 229 | 22.6 | 80.8 | 13.5 | 316 | 22.1 | 48.1 | 1.0 |
| Blood pressure | 259 | 25.5 | 83.8 | 5.8 | 11 | 0.8 | 72.7 | 0.0 |
| Critical arrhythmia | 1 | 0.1 | 0.0 | 0.0 | 4 | 0.3 | 0.0 | 0.0 |
| Noncritical arrhythmia | 71 | 7.0 | 2.8 | 0.0 | 244 | 17.1 | 8.6 | 0.0 |
| Central venous pressure | 49 | 4.8 | 0.0 | 0.0 | 0 | 0.0 | N/A | N/A |
| Exhaled carbon dioxide | 14 | 1.4 | 92.9 | 50.0 | 0 | 0.0 | N/A | N/A |
| Total | 1014 | 100.0 | 75.6 | 12.9 | 1,431 | 100.0 | 38.9 | 1.0 |
Valid Alarm
A valid alarm is any alarm that correctly identifies the physiologic status of the patient. Validity was based on waveform quality, lead signal strength indicators, and artifact conditions, referencing each monitor's operator's manual.
Actionable Alarm
An actionable alarm is any valid alarm for a clinical condition that either: (1) leads to a clinical intervention; (2) leads to a consultation with another clinician at the bedside (and thus visible on camera); or (3) is a situation that should have led to intervention or consultation, but the alarm was unwitnessed or misinterpreted by the staff at the bedside.
Nonactionable Alarm
An unactionable alarm is any alarm that does not meet the actionable definition above, including invalid alarms such as those caused by motion artifact, equipment/technical alarms, and alarms that are valid but nonactionable (nuisance alarms).[13]
Response Time
The response time is the time elapsed from when the alarm fired at the bedside to when the nurse entered the room or peered through a window or door, measured in seconds.
Setting and Subjects
We performed this study between August 2012 and July 2013 at a freestanding children's hospital. We evaluated nurses caring for 2 populations: (1) PICU patients with heart and/or lung failure (requiring inotropic support and/or invasive mechanical ventilation), and (2) medical patients on a general inpatient ward. Nurses caring for heart and/or lung failure patients in the PICU typically were assigned 1 to 2 total patients. Nurses on the medical ward typically were assigned 2 to 4 patients. We identified subjects from the population of nurses caring for eligible patients with parents available to provide in‐person consent in each setting. Our primary interest was to evaluate the association between nonactionable alarms and response time, and not to study the epidemiology of alarms in a random sample. Therefore, when alarm data were available prior to screening, we first approached nurses caring for patients in the top 25% of alarm rates for that unit over the preceding 4 hours. We identified preceding alarm rates using BedMasterEx (Excel Medical Electronics, Jupiter, FL).
Human Subjects Protection
This study was approved by the institutional review board of The Children's Hospital of Philadelphia. We obtained written in‐person consent from the patient's parent and the nurse subject. We obtained a Certificate of Confidentiality from the National Institutes of Health to further protect study participants.[14]
Monitoring Equipment
All patients in the PICU were monitored continuously using General Electric (GE) (Fairfield, CT) solar devices. All bed spaces on the wards include GE Dash monitors that are used if ordered. On the ward we studied, 30% to 50% of patients are typically monitored at any given time. In addition to alarming at the bedside, most clinical alarms also generated a text message sent to the nurse's wireless phone listing the room number and the word monitor. Messages did not provide any clinical information about the alarm or patient's status. There were no technicians reviewing alarms centrally.
Physicians used an order set to order monitoring, selecting 1 of 4 available preconfigured profiles: infant <6 months, infant 6 months to 1 year, child, and adult. The parameters for each age group are in Supporting Figure 1, available in the online version of this article. A physician order is required for a nurse to change the parameters. Participating in the study did not affect this workflow.
Primary Outcome
The primary outcome was the nurse's response time to potentially critical monitor alarms that occurred while neither they nor any other clinicians were in the patient's room.
Primary Exposure and Alarm Classification
The primary exposure was the number of nonactionable alarms in the same patient over the preceding 120 minutes (rolling and updated each minute). The alarm classification scheme is shown in Figure 1.
Due to technical limitations with obtaining time‐stamped alarm data from the different ventilators in use during the study period, we were unable to identify the causes of all ventilator alarms. Therefore, we included ventilator alarms that did not lead to clinical interventions as nonactionable alarm exposures, but we did not evaluate the response time to any ventilator alarms.
Data Collection
We combined video recordings with monitor time‐stamp data to evaluate the association between nonactionable alarms and the nurse's response time. Our detailed video recording and annotation methods have been published separately.[15] Briefly, we mounted up to 6 small video cameras in patients' rooms and recorded up to 6 hours per session. The cameras captured the monitor display, a wide view of the room, a close‐up view of the patient, and all windows and doors through which staff could visually assess the patient without entering the room.
Video Processing, Review, and Annotation
The first 5 video sessions were reviewed in a group training setting. Research assistants received instruction on how to determine alarm validity and actionability in accordance with the study definitions. Following the training period, the review workflow was as follows. First, a research assistant entered basic information and a preliminary assessment of the alarm's clinical validity and actionability into a REDCap (Research Electronic Data Capture; Vanderbilt University, Nashville, TN) database.[16] Later, a physician investigator secondarily reviewed all alarms and confirmed the assessments of the research assistants or, when disagreements occurred, discussed and reconciled the database. Alarms that remained unresolved after secondary review were flagged for review with an additional physician or nurse investigator in a team meeting.
Data Analysis
We summarized the patient and nurse subjects, the distributions of alarms, and the response times to potentially critical monitor alarms that occurred while neither the nurse nor any other clinicians were in the patient's room. We explored the data using plots of alarms and response times occurring within individual video sessions as well as with simple linear regression. Hypothesizing that any alarm fatigue effect would be strongest in the highest alarm patients, and having observed that alarms are distributed very unevenly across patients in both the PICU and ward, we made the decision not to use quartiles, but rather to form clinically meaningful categories. We also hypothesized that nurses might not exhibit alarm fatigue unless they were inundated with alarms. We thus divided the nonactionable alarm counts over the preceding 120 minutes into 3 categories: 0 to 29 alarms to represent a low to average alarm rate exhibited by the bottom 50% of the patients, 30 to 79 alarms to represent an elevated alarm rate, and 80+ alarms to represent an extremely high alarm rate exhibited by the top 5%. Because the exposure time was 120 minutes, we conducted the analysis on the alarms occurring after a nurse had been video recorded for at least 120 minutes.
We further evaluated the relationship between nonactionable alarms and nurse response time with Kaplan‐Meier plots by nonactionable alarm count category using the observed response‐time data. The Kaplan‐Meier plots compared response time across the nonactionable alarm exposure group, without any statistical modeling. A log‐rank test stratified by nurse evaluated whether the distributions of response time in the Kaplan‐Meier plots differed across the 3 alarm exposure groups, accounting for within‐nurse clustering.
Accelerated failure‐time regression based on the Weibull distribution then allowed us to compare response time across each alarm exposure group and provided confidence intervals. Accelerated failure‐time models are comparable to Cox models, but emphasize time to event rather than hazards.[17, 18] We determined that the Weibull distribution was suitable by evaluating smoothed hazard and log‐hazard plots, the confidence intervals of the shape parameters in the Weibull models that did not include 1, and by demonstrating that the Weibull model had better fit than an alternative (exponential) model using the likelihood‐ratio test (P<0.0001 for PICU, P=0.02 for ward). Due to the small sample size of nurses and patients, we could not adjust for nurse‐ or patient‐level covariates in the model. When comparing the nonactionable alarm exposure groups in the regression model (029 vs 3079, 3079 vs 80+, and 029 vs 80+), we Bonferroni corrected the critical P value for the 3 comparisons, for a critical P value of 0.05/3=0.0167.
Nurse Questionnaire
At the session's conclusion, nurses completed a questionnaire that included demographics and asked, Did you respond more quickly to monitor alarms during this study because you knew you were being filmed? to measure if nurses would report experiencing a Hawthorne‐like effect.[19, 20, 21]
RESULTS
We performed 40 sessions among 40 patients and 36 nurses over 210 hours. We performed 20 sessions in children with heart and/or lung failure in the PICU and 20 sessions in children on a general ward. Sessions took place on weekdays between 9:00 am and 6:00 pm. There were 3 occasions when we filmed 2 patients cared for by the same nurse at the same time.
Nurses were mostly female (94.4%) and had between 2 months and 28 years of experience (median, 4.8 years). Patients on the ward ranged from 5 days to 5.4 years old (median, 6 months). Patients in the PICU ranged from 5 months to 16 years old (median, 2.5 years). Among the PICU patients, 14 (70%) were receiving mechanical ventilation only, 3 (15%) were receiving vasopressors only, and 3 (15%) were receiving mechanical ventilation and vasopressors.
We observed 5070 alarms during the 40 sessions. We excluded 108 (2.1%) that occurred at the end of video recording sessions with the nurse absent from the room because the nurse's response could not be determined. Alarms per session ranged from 10 to 1430 (median, 75; interquartile range [IQR], 35138). We excluded the outlier PICU patient with 1430 alarms in 5 hours from the analysis to avoid the potential for biasing the results. Figure 2 depicts the data flow.
Following the 5 training sessions, research assistants independently reviewed and made preliminary assessments on 4674 alarms; these alarms were all secondarily reviewed by a physician. Using the physician reviewer as the gold standard, the research assistant's sensitivity (assess alarm as actionable when physician also assesses as actionable) was 96.8% and specificity (assess alarm as nonactionable when physician also assesses as nonactionable) was 96.9%. We had to review 54 of 4674 alarms (1.2%) with an additional physician or nurse investigator to achieve consensus.
Characteristics of the 2445 alarms for clinical conditions are shown in Table 1. Only 12.9% of alarms in heart‐ and/or lung‐failure patients in the PICU were actionable, and only 1.0% of alarms in medical patients on a general inpatient ward were actionable.
Overall Response Times for Out‐of‐Room Alarms
We first evaluated response times without excluding alarms occurring prior to the 120‐minute mark. Of the 2445 clinical condition alarms, we excluded the 315 noncritical arrhythmia types from analysis of response time because they did not meet our definition of potentially critical alarms. Of the 2130 potentially critical alarms, 1185 (55.6%) occurred while neither the nurse nor any other clinician was in the patient's room. We proceeded to analyze the response time to these 1185 alarms (307 in the PICU and 878 on the ward). In the PICU, median response time was 3.3 minutes (IQR, 0.814.4). On the ward, median response time was 9.8 minutes (IQR, 3.222.4).
Response‐Time Association With Nonactionable Alarm Exposure
Next, we analyzed the association between response time to potentially critical alarms that occurred when the nurse was not in the patient's room and the number of nonactionable alarms occurring over the preceding 120‐minute window. This required excluding the alarms that occurred in the first 120 minutes of each session, leaving 647 alarms with eligible response times to evaluate the exposure between prior nonactionable alarm exposure and response time: 219 in the PICU and 428 on the ward. Kaplan‐Meier plots and tabulated response times demonstrated the incremental relationships between each nonactionable alarm exposure category in the observed data, with the effects most prominent as the Kaplan‐Meier plots diverged beyond the median (Figure 3 and Table 2). Excluding the extreme outlier patient had no effect on the results, because 1378 of the 1430 alarms occurred with the nurse present at the bedside, and only 2 of the remaining alarms were potentially critical.
| Observed Data | Accelerated Failure‐Time Model | |||||||
|---|---|---|---|---|---|---|---|---|
| Number of Potentially Critical Alarms | Minutes Elapsed Until This Percentage of Alarms Was Responded to | Modeled Response Time, min | 95% CI, min | P Value* | ||||
| 50% (Median) | 75% | 90% | 95% | |||||
| ||||||||
| PICU | ||||||||
| 029 nonactionable alarms | 70 | 1.6 | 8.0 | 18.6 | 25.1 | 2.8 | 1.9‐3.8 | Reference |
| 3079 nonactionable alarms | 122 | 6.3 | 17.8 | 22.5 | 26.0 | 5.3 | 4.06.7 | 0.001 (vs 029) |
| 80+ nonactionable alarms | 27 | 16.0 | 28.4 | 32.0 | 33.1 | 8.5 | 4.312.7 | 0.009 (vs 029), 0.15 (vs 3079) |
| Ward | ||||||||
| 029 nonactionable alarms | 159 | 9.8 | 17.8 | 25.0 | 28.9 | 7.7 | 6.39.1 | Reference |
| 3079 nonactionable alarms | 211 | 11.6 | 22.4 | 44.6 | 63.2 | 11.5 | 9.613.3 | 0.001 (vs 029) |
| 80+ nonactionable alarms | 58 | 8.3 | 57.6 | 63.8 | 69.5 | 15.6 | 11.020.1 | 0.001 (vs 029), 0.09 (vs 3079) |
Accelerated failure‐time regressions revealed significant incremental increases in the modeled response time as the number of preceding nonactionable alarms increased in both the PICU and ward settings (Table 2).
Hawthorne‐like Effects
Four of the 36 nurses reported that they responded more quickly to monitor alarms because they knew they were being filmed.
DISCUSSION
Alarm fatigue has recently generated interest among nurses,[22] physicians,[23] regulatory bodies,[24] patient safety organizations,[25] and even attorneys,[26] despite a lack of prior evidence linking nonactionable alarm exposure to response time or other adverse patient‐relevant outcomes. This study's main findings were that (1) the vast majority of alarms were nonactionable, (2) response time to alarms occurring while the nurse was out of the room increased as the number of nonactionable alarms over the preceding 120 minutes increased. These findings may be explained by alarm fatigue.
Our results build upon the findings of other related studies. The nonactionable alarm proportions we found were similar to other pediatric studies, reporting greater than 90% nonactionable alarms.[1, 2] One other study has reported a relationship between alarm exposure and response time. In that study, Voepel‐Lewis and colleagues evaluated nurse responses to pulse oximetry desaturation alarms in adult orthopedic surgery patients using time‐stamp data from their monitor notification system.[27] They found that alarm response time was significantly longer for patients in the highest quartile of alarms compared to those in lower quartiles. Our study provides new data suggesting a relationship between nonactionable alarm exposure and nurse response time.
Our study has several limitations. First, as a preliminary study to investigate feasibility and possible association, the sample of patients and nurses was necessarily limited and did not permit adjustment for nurse‐ or patient‐level covariates. A multivariable analysis with a larger sample might provide insight into alternate explanations for these findings other than alarm fatigue, including measures of nurse workload and patient factors (such as age and illness severity). Additional factors that are not as easily measured can also contribute to the complex decision of when and how to respond to alarms.[28, 29] Second, nurses were aware that they were being video recorded as part of a study of nonactionable alarms, although they did not know the specific details of measurement. Although this lack of blinding might lead to a Hawthorne‐like effect, our positive results suggest that this effect, if present, did not fully obscure the association. Third, all sessions took place on weekdays during daytime hours, but effects of nonactionable alarms might vary by time and day. Finally, we suspect that when nurses experience critical alarms that require them to intervene and rescue a patient, their response times to that patient's alarms that occur later in their shift will be quicker due to a heightened concern for the alarm being actionable. We were unable to explore that relationship in this analysis because the number of critical alarms requiring intervention was very small. This is a topic of future study.
CONCLUSIONS
We identified an association between a nurse's prior exposure to nonactionable alarms and response time to future alarms. This finding is consistent with alarm fatigue, but requires further study to more clearly delineate other factors that might confound or modify that relationship.
Disclosures
This project was funded by the Health Research Formula Fund Grant 4100050891 from the Pennsylvania Department of Public Health Commonwealth Universal Research Enhancement Program (awarded to Drs. Keren and Bonafide). Dr. Bonafide is also supported by the National Heart, Lung, and Blood Institute of the National Institutes of Health under award number K23HL116427. The content is solely the responsibility of the authors and does not necessarily represent the official views of the National Institutes of Health. The authors have no financial relationships or conflicts of interest relevant to this article to disclose.
- . Crying wolf: false alarms in a pediatric intensive care unit. Crit Care Med. 1994;22(6):981–985.
- , . Poor prognosis for existing monitors in the intensive care unit. Crit Care Med. 1997;25(4):614–619.
- , , , , . Clinical evaluation of alarm efficiency in intensive care [in French]. Ann Fr Anesth Reanim. 2000;19:459–466.
- , , , . Reducing false alarms of intensive care online‐monitoring systems: an evaluation of two signal extraction algorithms. Comput Math Methods Med. 2011;2011:143480.
- , , , , , . Multicentric study of monitoring alarms in the adult intensive care unit (ICU): a descriptive analysis. Intensive Care Med. 1999;25:1360–1366.
- , , . Improving alarm performance in the medical intensive care unit using delays and clinical context. Anesth Analg. 2009;108:1546–1552.
- , . Monitor alarm fatigue: standardizing use of physiological monitors and decreasing nuisance alarms. Am J Crit Care. 2010;19:28–34.
- , , , , , . Intensive care unit alarms—how many do we need? Crit Care Med. 2010;38:451–456.
- , , , . System operator response to warnings of danger: a laboratory investigation of the effects of the predictive value of a warning on human response time. J Exp Psychol Appl. 1995;1:19–33.
- , , . Human probability matching behaviour in response to alarms of varying reliability. Ergonomics. 1995;38:2300–2312.
- The Joint Commission. Sentinel event alert: medical device alarm safety in hospitals. 2013. Available at: http://www.jointcommission.org/sea_issue_50/. Accessed October 9, 2014.
- . Joint commission warns of alarm fatigue: multitude of alarms from monitoring devices problematic. JAMA. 2013;309(22):2315–2316.
- . Monitor alarm fatigue: an integrative review. Biomed Instrum Technol. 2012;46(4):268–277.
- NIH Certificates of Confidentiality Kiosk. Available at: http://grants.nih.gov/grants/policy/coc/. Accessed April 21, 2014.
- , , , et al. Video methods for evaluating physiologic monitor alarms and alarm responses. Biomed Instrum Technol. 2014;48(3):220–230.
- , , , , , . Research electronic data capture (REDCap)—a metadata‐driven methodology and workflow process for providing translational research informatics support. J Biomed Inf. 2009;42:377–381.
- . Accelerated failure time and other parametric models. In: Modelling Survival Data in Medical Research. 2nd ed. Boca Raton, FL: Chapman 2003:197–229.
- , , , . Parametric models. In: An Introduction to Survival Analysis Using Stata, 3rd ed. College Station, TX: Stata Press; 2010:229–244.
- , . Management and the Worker. Cambridge, MA: Harvard University Press; 1939.
- . What happened at Hawthorne? Science. 1974;183(4128):922–932.
- , , , , . Validation of the Work Observation Method By Activity Timing (WOMBAT) method of conducting time‐motion observations in critical care settings: an observational study. BMC Med Inf Decis Mak. 2011;11:32.
- , . Alarm fatigue: a patient safety concern. AACN Adv Crit Care. 2013;24(4):378–386.
- , . Redesigning hospital alarms for patient safety: alarmed and potentially dangerous. JAMA. 2014;311(12):1199–1200.
- The Joint Commission. The Joint Commission announces 2014 National Patient Safety Goal. Jt Comm Perspect. 2013;33:1–4.
- Top 10 health technology hazards for 2014. Health Devices. 2013;42(11):354–380.
- My Philly Lawyer. Medical malpractice: alarm fatigue threatens patient safety. 2014. Available at: http://www.myphillylawyer.com/Resources/Legal-Articles/Medical-Malpractice-Alarm-Fatigue-Threatens-Patient-Safety.shtml. Accessed April 4, 2014.
- , , , et al. Pulse oximetry desaturation alarms on a general postoperative adult unit: a prospective observational study of nurse response time. Int J Nurs Stud. 2013;50(10):1351–1358.
- , , , , . A description of nurses' decision‐making in managing electrocardiographic monitor alarms [published online ahead of print May 10, 2014]. J Clin Nurs. doi:10.1111/jocn.12625.
- . Nurses' response to frequency and types of electrocardiography alarms in a non‐critical care setting: a descriptive study. Int J Nurs Stud. 2014;51(2):190–197.
Hospital physiologic monitors can alert clinicians to early signs of physiologic deterioration, and thus have great potential to save lives. However, monitors generate frequent alarms,[1, 2, 3, 4, 5, 6, 7, 8] and most are not relevant to the patient's safety (over 90% of pediatric intensive care unit (PICU)[1, 2] and over 70% of adult intensive care alarms).[5, 6] In psychology experiments, humans rapidly learn to ignore or respond more slowly to alarms when exposed to high false‐alarm rates, exhibiting alarm fatigue.[9, 10] In 2013, The Joint Commission named alarm fatigue the most common contributing factor to alarm‐related sentinel events in hospitals.[11, 12]
Although alarm fatigue has been implicated as a major threat to patient safety, little empirical data support its existence in hospitals. In this study, we aimed to determine if there was an association between nurses' recent exposure to nonactionable physiologic monitor alarms and their response time to future alarms for the same patients. This exploratory work was designed to inform future research in this area, acknowledging that the sample size would be too small for multivariable modeling.
METHODS
Study Definitions
The alarm classification scheme is shown in Figure 1. Note that, for clarity, we have intentionally avoided using the terms true and false alarms because their interpretations vary across studies and can be misleading.

Potentially Critical Alarm
A potentially critical alarm is any alarm for a clinical condition for which a timely response is important to determine if the alarm requires intervention to save the patient's life. This is based on the alarm type alone, including alarms for life‐threatening arrhythmias such as asystole and ventricular tachycardia, as well as alarms for vital signs outside the set limits. Supporting Table 1 in the online version of this article lists the breakdown of alarm types that we defined a priori as potentially and not potentially critical.
| PICU | Ward | |||||||
|---|---|---|---|---|---|---|---|---|
| Alarm type | No. | % of Total | % Valid | % Actionable | No. | % of Total | % Valid | % Actionable |
| ||||||||
| Oxygen saturation | 197 | 19.4 | 82.7 | 38.6 | 590 | 41.2 | 24.4 | 1.9 |
| Heart rate | 194 | 19.1 | 95.4 | 1.0 | 266 | 18.6 | 87.2 | 0.0 |
| Respiratory rate | 229 | 22.6 | 80.8 | 13.5 | 316 | 22.1 | 48.1 | 1.0 |
| Blood pressure | 259 | 25.5 | 83.8 | 5.8 | 11 | 0.8 | 72.7 | 0.0 |
| Critical arrhythmia | 1 | 0.1 | 0.0 | 0.0 | 4 | 0.3 | 0.0 | 0.0 |
| Noncritical arrhythmia | 71 | 7.0 | 2.8 | 0.0 | 244 | 17.1 | 8.6 | 0.0 |
| Central venous pressure | 49 | 4.8 | 0.0 | 0.0 | 0 | 0.0 | N/A | N/A |
| Exhaled carbon dioxide | 14 | 1.4 | 92.9 | 50.0 | 0 | 0.0 | N/A | N/A |
| Total | 1014 | 100.0 | 75.6 | 12.9 | 1,431 | 100.0 | 38.9 | 1.0 |
Valid Alarm
A valid alarm is any alarm that correctly identifies the physiologic status of the patient. Validity was based on waveform quality, lead signal strength indicators, and artifact conditions, referencing each monitor's operator's manual.
Actionable Alarm
An actionable alarm is any valid alarm for a clinical condition that either: (1) leads to a clinical intervention; (2) leads to a consultation with another clinician at the bedside (and thus visible on camera); or (3) is a situation that should have led to intervention or consultation, but the alarm was unwitnessed or misinterpreted by the staff at the bedside.
Nonactionable Alarm
An unactionable alarm is any alarm that does not meet the actionable definition above, including invalid alarms such as those caused by motion artifact, equipment/technical alarms, and alarms that are valid but nonactionable (nuisance alarms).[13]
Response Time
The response time is the time elapsed from when the alarm fired at the bedside to when the nurse entered the room or peered through a window or door, measured in seconds.
Setting and Subjects
We performed this study between August 2012 and July 2013 at a freestanding children's hospital. We evaluated nurses caring for 2 populations: (1) PICU patients with heart and/or lung failure (requiring inotropic support and/or invasive mechanical ventilation), and (2) medical patients on a general inpatient ward. Nurses caring for heart and/or lung failure patients in the PICU typically were assigned 1 to 2 total patients. Nurses on the medical ward typically were assigned 2 to 4 patients. We identified subjects from the population of nurses caring for eligible patients with parents available to provide in‐person consent in each setting. Our primary interest was to evaluate the association between nonactionable alarms and response time, and not to study the epidemiology of alarms in a random sample. Therefore, when alarm data were available prior to screening, we first approached nurses caring for patients in the top 25% of alarm rates for that unit over the preceding 4 hours. We identified preceding alarm rates using BedMasterEx (Excel Medical Electronics, Jupiter, FL).
Human Subjects Protection
This study was approved by the institutional review board of The Children's Hospital of Philadelphia. We obtained written in‐person consent from the patient's parent and the nurse subject. We obtained a Certificate of Confidentiality from the National Institutes of Health to further protect study participants.[14]
Monitoring Equipment
All patients in the PICU were monitored continuously using General Electric (GE) (Fairfield, CT) solar devices. All bed spaces on the wards include GE Dash monitors that are used if ordered. On the ward we studied, 30% to 50% of patients are typically monitored at any given time. In addition to alarming at the bedside, most clinical alarms also generated a text message sent to the nurse's wireless phone listing the room number and the word monitor. Messages did not provide any clinical information about the alarm or patient's status. There were no technicians reviewing alarms centrally.
Physicians used an order set to order monitoring, selecting 1 of 4 available preconfigured profiles: infant <6 months, infant 6 months to 1 year, child, and adult. The parameters for each age group are in Supporting Figure 1, available in the online version of this article. A physician order is required for a nurse to change the parameters. Participating in the study did not affect this workflow.
Primary Outcome
The primary outcome was the nurse's response time to potentially critical monitor alarms that occurred while neither they nor any other clinicians were in the patient's room.
Primary Exposure and Alarm Classification
The primary exposure was the number of nonactionable alarms in the same patient over the preceding 120 minutes (rolling and updated each minute). The alarm classification scheme is shown in Figure 1.
Due to technical limitations with obtaining time‐stamped alarm data from the different ventilators in use during the study period, we were unable to identify the causes of all ventilator alarms. Therefore, we included ventilator alarms that did not lead to clinical interventions as nonactionable alarm exposures, but we did not evaluate the response time to any ventilator alarms.
Data Collection
We combined video recordings with monitor time‐stamp data to evaluate the association between nonactionable alarms and the nurse's response time. Our detailed video recording and annotation methods have been published separately.[15] Briefly, we mounted up to 6 small video cameras in patients' rooms and recorded up to 6 hours per session. The cameras captured the monitor display, a wide view of the room, a close‐up view of the patient, and all windows and doors through which staff could visually assess the patient without entering the room.
Video Processing, Review, and Annotation
The first 5 video sessions were reviewed in a group training setting. Research assistants received instruction on how to determine alarm validity and actionability in accordance with the study definitions. Following the training period, the review workflow was as follows. First, a research assistant entered basic information and a preliminary assessment of the alarm's clinical validity and actionability into a REDCap (Research Electronic Data Capture; Vanderbilt University, Nashville, TN) database.[16] Later, a physician investigator secondarily reviewed all alarms and confirmed the assessments of the research assistants or, when disagreements occurred, discussed and reconciled the database. Alarms that remained unresolved after secondary review were flagged for review with an additional physician or nurse investigator in a team meeting.
Data Analysis
We summarized the patient and nurse subjects, the distributions of alarms, and the response times to potentially critical monitor alarms that occurred while neither the nurse nor any other clinicians were in the patient's room. We explored the data using plots of alarms and response times occurring within individual video sessions as well as with simple linear regression. Hypothesizing that any alarm fatigue effect would be strongest in the highest alarm patients, and having observed that alarms are distributed very unevenly across patients in both the PICU and ward, we made the decision not to use quartiles, but rather to form clinically meaningful categories. We also hypothesized that nurses might not exhibit alarm fatigue unless they were inundated with alarms. We thus divided the nonactionable alarm counts over the preceding 120 minutes into 3 categories: 0 to 29 alarms to represent a low to average alarm rate exhibited by the bottom 50% of the patients, 30 to 79 alarms to represent an elevated alarm rate, and 80+ alarms to represent an extremely high alarm rate exhibited by the top 5%. Because the exposure time was 120 minutes, we conducted the analysis on the alarms occurring after a nurse had been video recorded for at least 120 minutes.
We further evaluated the relationship between nonactionable alarms and nurse response time with Kaplan‐Meier plots by nonactionable alarm count category using the observed response‐time data. The Kaplan‐Meier plots compared response time across the nonactionable alarm exposure group, without any statistical modeling. A log‐rank test stratified by nurse evaluated whether the distributions of response time in the Kaplan‐Meier plots differed across the 3 alarm exposure groups, accounting for within‐nurse clustering.
Accelerated failure‐time regression based on the Weibull distribution then allowed us to compare response time across each alarm exposure group and provided confidence intervals. Accelerated failure‐time models are comparable to Cox models, but emphasize time to event rather than hazards.[17, 18] We determined that the Weibull distribution was suitable by evaluating smoothed hazard and log‐hazard plots, the confidence intervals of the shape parameters in the Weibull models that did not include 1, and by demonstrating that the Weibull model had better fit than an alternative (exponential) model using the likelihood‐ratio test (P<0.0001 for PICU, P=0.02 for ward). Due to the small sample size of nurses and patients, we could not adjust for nurse‐ or patient‐level covariates in the model. When comparing the nonactionable alarm exposure groups in the regression model (029 vs 3079, 3079 vs 80+, and 029 vs 80+), we Bonferroni corrected the critical P value for the 3 comparisons, for a critical P value of 0.05/3=0.0167.
Nurse Questionnaire
At the session's conclusion, nurses completed a questionnaire that included demographics and asked, Did you respond more quickly to monitor alarms during this study because you knew you were being filmed? to measure if nurses would report experiencing a Hawthorne‐like effect.[19, 20, 21]
RESULTS
We performed 40 sessions among 40 patients and 36 nurses over 210 hours. We performed 20 sessions in children with heart and/or lung failure in the PICU and 20 sessions in children on a general ward. Sessions took place on weekdays between 9:00 am and 6:00 pm. There were 3 occasions when we filmed 2 patients cared for by the same nurse at the same time.
Nurses were mostly female (94.4%) and had between 2 months and 28 years of experience (median, 4.8 years). Patients on the ward ranged from 5 days to 5.4 years old (median, 6 months). Patients in the PICU ranged from 5 months to 16 years old (median, 2.5 years). Among the PICU patients, 14 (70%) were receiving mechanical ventilation only, 3 (15%) were receiving vasopressors only, and 3 (15%) were receiving mechanical ventilation and vasopressors.
We observed 5070 alarms during the 40 sessions. We excluded 108 (2.1%) that occurred at the end of video recording sessions with the nurse absent from the room because the nurse's response could not be determined. Alarms per session ranged from 10 to 1430 (median, 75; interquartile range [IQR], 35138). We excluded the outlier PICU patient with 1430 alarms in 5 hours from the analysis to avoid the potential for biasing the results. Figure 2 depicts the data flow.

Following the 5 training sessions, research assistants independently reviewed and made preliminary assessments on 4674 alarms; these alarms were all secondarily reviewed by a physician. Using the physician reviewer as the gold standard, the research assistant's sensitivity (assess alarm as actionable when physician also assesses as actionable) was 96.8% and specificity (assess alarm as nonactionable when physician also assesses as nonactionable) was 96.9%. We had to review 54 of 4674 alarms (1.2%) with an additional physician or nurse investigator to achieve consensus.
Characteristics of the 2445 alarms for clinical conditions are shown in Table 1. Only 12.9% of alarms in heart‐ and/or lung‐failure patients in the PICU were actionable, and only 1.0% of alarms in medical patients on a general inpatient ward were actionable.
Overall Response Times for Out‐of‐Room Alarms
We first evaluated response times without excluding alarms occurring prior to the 120‐minute mark. Of the 2445 clinical condition alarms, we excluded the 315 noncritical arrhythmia types from analysis of response time because they did not meet our definition of potentially critical alarms. Of the 2130 potentially critical alarms, 1185 (55.6%) occurred while neither the nurse nor any other clinician was in the patient's room. We proceeded to analyze the response time to these 1185 alarms (307 in the PICU and 878 on the ward). In the PICU, median response time was 3.3 minutes (IQR, 0.814.4). On the ward, median response time was 9.8 minutes (IQR, 3.222.4).
Response‐Time Association With Nonactionable Alarm Exposure
Next, we analyzed the association between response time to potentially critical alarms that occurred when the nurse was not in the patient's room and the number of nonactionable alarms occurring over the preceding 120‐minute window. This required excluding the alarms that occurred in the first 120 minutes of each session, leaving 647 alarms with eligible response times to evaluate the exposure between prior nonactionable alarm exposure and response time: 219 in the PICU and 428 on the ward. Kaplan‐Meier plots and tabulated response times demonstrated the incremental relationships between each nonactionable alarm exposure category in the observed data, with the effects most prominent as the Kaplan‐Meier plots diverged beyond the median (Figure 3 and Table 2). Excluding the extreme outlier patient had no effect on the results, because 1378 of the 1430 alarms occurred with the nurse present at the bedside, and only 2 of the remaining alarms were potentially critical.

| Observed Data | Accelerated Failure‐Time Model | |||||||
|---|---|---|---|---|---|---|---|---|
| Number of Potentially Critical Alarms | Minutes Elapsed Until This Percentage of Alarms Was Responded to | Modeled Response Time, min | 95% CI, min | P Value* | ||||
| 50% (Median) | 75% | 90% | 95% | |||||
| ||||||||
| PICU | ||||||||
| 029 nonactionable alarms | 70 | 1.6 | 8.0 | 18.6 | 25.1 | 2.8 | 1.9‐3.8 | Reference |
| 3079 nonactionable alarms | 122 | 6.3 | 17.8 | 22.5 | 26.0 | 5.3 | 4.06.7 | 0.001 (vs 029) |
| 80+ nonactionable alarms | 27 | 16.0 | 28.4 | 32.0 | 33.1 | 8.5 | 4.312.7 | 0.009 (vs 029), 0.15 (vs 3079) |
| Ward | ||||||||
| 029 nonactionable alarms | 159 | 9.8 | 17.8 | 25.0 | 28.9 | 7.7 | 6.39.1 | Reference |
| 3079 nonactionable alarms | 211 | 11.6 | 22.4 | 44.6 | 63.2 | 11.5 | 9.613.3 | 0.001 (vs 029) |
| 80+ nonactionable alarms | 58 | 8.3 | 57.6 | 63.8 | 69.5 | 15.6 | 11.020.1 | 0.001 (vs 029), 0.09 (vs 3079) |
Accelerated failure‐time regressions revealed significant incremental increases in the modeled response time as the number of preceding nonactionable alarms increased in both the PICU and ward settings (Table 2).
Hawthorne‐like Effects
Four of the 36 nurses reported that they responded more quickly to monitor alarms because they knew they were being filmed.
DISCUSSION
Alarm fatigue has recently generated interest among nurses,[22] physicians,[23] regulatory bodies,[24] patient safety organizations,[25] and even attorneys,[26] despite a lack of prior evidence linking nonactionable alarm exposure to response time or other adverse patient‐relevant outcomes. This study's main findings were that (1) the vast majority of alarms were nonactionable, (2) response time to alarms occurring while the nurse was out of the room increased as the number of nonactionable alarms over the preceding 120 minutes increased. These findings may be explained by alarm fatigue.
Our results build upon the findings of other related studies. The nonactionable alarm proportions we found were similar to other pediatric studies, reporting greater than 90% nonactionable alarms.[1, 2] One other study has reported a relationship between alarm exposure and response time. In that study, Voepel‐Lewis and colleagues evaluated nurse responses to pulse oximetry desaturation alarms in adult orthopedic surgery patients using time‐stamp data from their monitor notification system.[27] They found that alarm response time was significantly longer for patients in the highest quartile of alarms compared to those in lower quartiles. Our study provides new data suggesting a relationship between nonactionable alarm exposure and nurse response time.
Our study has several limitations. First, as a preliminary study to investigate feasibility and possible association, the sample of patients and nurses was necessarily limited and did not permit adjustment for nurse‐ or patient‐level covariates. A multivariable analysis with a larger sample might provide insight into alternate explanations for these findings other than alarm fatigue, including measures of nurse workload and patient factors (such as age and illness severity). Additional factors that are not as easily measured can also contribute to the complex decision of when and how to respond to alarms.[28, 29] Second, nurses were aware that they were being video recorded as part of a study of nonactionable alarms, although they did not know the specific details of measurement. Although this lack of blinding might lead to a Hawthorne‐like effect, our positive results suggest that this effect, if present, did not fully obscure the association. Third, all sessions took place on weekdays during daytime hours, but effects of nonactionable alarms might vary by time and day. Finally, we suspect that when nurses experience critical alarms that require them to intervene and rescue a patient, their response times to that patient's alarms that occur later in their shift will be quicker due to a heightened concern for the alarm being actionable. We were unable to explore that relationship in this analysis because the number of critical alarms requiring intervention was very small. This is a topic of future study.
CONCLUSIONS
We identified an association between a nurse's prior exposure to nonactionable alarms and response time to future alarms. This finding is consistent with alarm fatigue, but requires further study to more clearly delineate other factors that might confound or modify that relationship.
Disclosures
This project was funded by the Health Research Formula Fund Grant 4100050891 from the Pennsylvania Department of Public Health Commonwealth Universal Research Enhancement Program (awarded to Drs. Keren and Bonafide). Dr. Bonafide is also supported by the National Heart, Lung, and Blood Institute of the National Institutes of Health under award number K23HL116427. The content is solely the responsibility of the authors and does not necessarily represent the official views of the National Institutes of Health. The authors have no financial relationships or conflicts of interest relevant to this article to disclose.
Hospital physiologic monitors can alert clinicians to early signs of physiologic deterioration, and thus have great potential to save lives. However, monitors generate frequent alarms,[1, 2, 3, 4, 5, 6, 7, 8] and most are not relevant to the patient's safety (over 90% of pediatric intensive care unit (PICU)[1, 2] and over 70% of adult intensive care alarms).[5, 6] In psychology experiments, humans rapidly learn to ignore or respond more slowly to alarms when exposed to high false‐alarm rates, exhibiting alarm fatigue.[9, 10] In 2013, The Joint Commission named alarm fatigue the most common contributing factor to alarm‐related sentinel events in hospitals.[11, 12]
Although alarm fatigue has been implicated as a major threat to patient safety, little empirical data support its existence in hospitals. In this study, we aimed to determine if there was an association between nurses' recent exposure to nonactionable physiologic monitor alarms and their response time to future alarms for the same patients. This exploratory work was designed to inform future research in this area, acknowledging that the sample size would be too small for multivariable modeling.
METHODS
Study Definitions
The alarm classification scheme is shown in Figure 1. Note that, for clarity, we have intentionally avoided using the terms true and false alarms because their interpretations vary across studies and can be misleading.

Potentially Critical Alarm
A potentially critical alarm is any alarm for a clinical condition for which a timely response is important to determine if the alarm requires intervention to save the patient's life. This is based on the alarm type alone, including alarms for life‐threatening arrhythmias such as asystole and ventricular tachycardia, as well as alarms for vital signs outside the set limits. Supporting Table 1 in the online version of this article lists the breakdown of alarm types that we defined a priori as potentially and not potentially critical.
| PICU | Ward | |||||||
|---|---|---|---|---|---|---|---|---|
| Alarm type | No. | % of Total | % Valid | % Actionable | No. | % of Total | % Valid | % Actionable |
| ||||||||
| Oxygen saturation | 197 | 19.4 | 82.7 | 38.6 | 590 | 41.2 | 24.4 | 1.9 |
| Heart rate | 194 | 19.1 | 95.4 | 1.0 | 266 | 18.6 | 87.2 | 0.0 |
| Respiratory rate | 229 | 22.6 | 80.8 | 13.5 | 316 | 22.1 | 48.1 | 1.0 |
| Blood pressure | 259 | 25.5 | 83.8 | 5.8 | 11 | 0.8 | 72.7 | 0.0 |
| Critical arrhythmia | 1 | 0.1 | 0.0 | 0.0 | 4 | 0.3 | 0.0 | 0.0 |
| Noncritical arrhythmia | 71 | 7.0 | 2.8 | 0.0 | 244 | 17.1 | 8.6 | 0.0 |
| Central venous pressure | 49 | 4.8 | 0.0 | 0.0 | 0 | 0.0 | N/A | N/A |
| Exhaled carbon dioxide | 14 | 1.4 | 92.9 | 50.0 | 0 | 0.0 | N/A | N/A |
| Total | 1014 | 100.0 | 75.6 | 12.9 | 1,431 | 100.0 | 38.9 | 1.0 |
Valid Alarm
A valid alarm is any alarm that correctly identifies the physiologic status of the patient. Validity was based on waveform quality, lead signal strength indicators, and artifact conditions, referencing each monitor's operator's manual.
Actionable Alarm
An actionable alarm is any valid alarm for a clinical condition that either: (1) leads to a clinical intervention; (2) leads to a consultation with another clinician at the bedside (and thus visible on camera); or (3) is a situation that should have led to intervention or consultation, but the alarm was unwitnessed or misinterpreted by the staff at the bedside.
Nonactionable Alarm
An unactionable alarm is any alarm that does not meet the actionable definition above, including invalid alarms such as those caused by motion artifact, equipment/technical alarms, and alarms that are valid but nonactionable (nuisance alarms).[13]
Response Time
The response time is the time elapsed from when the alarm fired at the bedside to when the nurse entered the room or peered through a window or door, measured in seconds.
Setting and Subjects
We performed this study between August 2012 and July 2013 at a freestanding children's hospital. We evaluated nurses caring for 2 populations: (1) PICU patients with heart and/or lung failure (requiring inotropic support and/or invasive mechanical ventilation), and (2) medical patients on a general inpatient ward. Nurses caring for heart and/or lung failure patients in the PICU typically were assigned 1 to 2 total patients. Nurses on the medical ward typically were assigned 2 to 4 patients. We identified subjects from the population of nurses caring for eligible patients with parents available to provide in‐person consent in each setting. Our primary interest was to evaluate the association between nonactionable alarms and response time, and not to study the epidemiology of alarms in a random sample. Therefore, when alarm data were available prior to screening, we first approached nurses caring for patients in the top 25% of alarm rates for that unit over the preceding 4 hours. We identified preceding alarm rates using BedMasterEx (Excel Medical Electronics, Jupiter, FL).
Human Subjects Protection
This study was approved by the institutional review board of The Children's Hospital of Philadelphia. We obtained written in‐person consent from the patient's parent and the nurse subject. We obtained a Certificate of Confidentiality from the National Institutes of Health to further protect study participants.[14]
Monitoring Equipment
All patients in the PICU were monitored continuously using General Electric (GE) (Fairfield, CT) solar devices. All bed spaces on the wards include GE Dash monitors that are used if ordered. On the ward we studied, 30% to 50% of patients are typically monitored at any given time. In addition to alarming at the bedside, most clinical alarms also generated a text message sent to the nurse's wireless phone listing the room number and the word monitor. Messages did not provide any clinical information about the alarm or patient's status. There were no technicians reviewing alarms centrally.
Physicians used an order set to order monitoring, selecting 1 of 4 available preconfigured profiles: infant <6 months, infant 6 months to 1 year, child, and adult. The parameters for each age group are in Supporting Figure 1, available in the online version of this article. A physician order is required for a nurse to change the parameters. Participating in the study did not affect this workflow.
Primary Outcome
The primary outcome was the nurse's response time to potentially critical monitor alarms that occurred while neither they nor any other clinicians were in the patient's room.
Primary Exposure and Alarm Classification
The primary exposure was the number of nonactionable alarms in the same patient over the preceding 120 minutes (rolling and updated each minute). The alarm classification scheme is shown in Figure 1.
Due to technical limitations with obtaining time‐stamped alarm data from the different ventilators in use during the study period, we were unable to identify the causes of all ventilator alarms. Therefore, we included ventilator alarms that did not lead to clinical interventions as nonactionable alarm exposures, but we did not evaluate the response time to any ventilator alarms.
Data Collection
We combined video recordings with monitor time‐stamp data to evaluate the association between nonactionable alarms and the nurse's response time. Our detailed video recording and annotation methods have been published separately.[15] Briefly, we mounted up to 6 small video cameras in patients' rooms and recorded up to 6 hours per session. The cameras captured the monitor display, a wide view of the room, a close‐up view of the patient, and all windows and doors through which staff could visually assess the patient without entering the room.
Video Processing, Review, and Annotation
The first 5 video sessions were reviewed in a group training setting. Research assistants received instruction on how to determine alarm validity and actionability in accordance with the study definitions. Following the training period, the review workflow was as follows. First, a research assistant entered basic information and a preliminary assessment of the alarm's clinical validity and actionability into a REDCap (Research Electronic Data Capture; Vanderbilt University, Nashville, TN) database.[16] Later, a physician investigator secondarily reviewed all alarms and confirmed the assessments of the research assistants or, when disagreements occurred, discussed and reconciled the database. Alarms that remained unresolved after secondary review were flagged for review with an additional physician or nurse investigator in a team meeting.
Data Analysis
We summarized the patient and nurse subjects, the distributions of alarms, and the response times to potentially critical monitor alarms that occurred while neither the nurse nor any other clinicians were in the patient's room. We explored the data using plots of alarms and response times occurring within individual video sessions as well as with simple linear regression. Hypothesizing that any alarm fatigue effect would be strongest in the highest alarm patients, and having observed that alarms are distributed very unevenly across patients in both the PICU and ward, we made the decision not to use quartiles, but rather to form clinically meaningful categories. We also hypothesized that nurses might not exhibit alarm fatigue unless they were inundated with alarms. We thus divided the nonactionable alarm counts over the preceding 120 minutes into 3 categories: 0 to 29 alarms to represent a low to average alarm rate exhibited by the bottom 50% of the patients, 30 to 79 alarms to represent an elevated alarm rate, and 80+ alarms to represent an extremely high alarm rate exhibited by the top 5%. Because the exposure time was 120 minutes, we conducted the analysis on the alarms occurring after a nurse had been video recorded for at least 120 minutes.
We further evaluated the relationship between nonactionable alarms and nurse response time with Kaplan‐Meier plots by nonactionable alarm count category using the observed response‐time data. The Kaplan‐Meier plots compared response time across the nonactionable alarm exposure group, without any statistical modeling. A log‐rank test stratified by nurse evaluated whether the distributions of response time in the Kaplan‐Meier plots differed across the 3 alarm exposure groups, accounting for within‐nurse clustering.
Accelerated failure‐time regression based on the Weibull distribution then allowed us to compare response time across each alarm exposure group and provided confidence intervals. Accelerated failure‐time models are comparable to Cox models, but emphasize time to event rather than hazards.[17, 18] We determined that the Weibull distribution was suitable by evaluating smoothed hazard and log‐hazard plots, the confidence intervals of the shape parameters in the Weibull models that did not include 1, and by demonstrating that the Weibull model had better fit than an alternative (exponential) model using the likelihood‐ratio test (P<0.0001 for PICU, P=0.02 for ward). Due to the small sample size of nurses and patients, we could not adjust for nurse‐ or patient‐level covariates in the model. When comparing the nonactionable alarm exposure groups in the regression model (029 vs 3079, 3079 vs 80+, and 029 vs 80+), we Bonferroni corrected the critical P value for the 3 comparisons, for a critical P value of 0.05/3=0.0167.
Nurse Questionnaire
At the session's conclusion, nurses completed a questionnaire that included demographics and asked, Did you respond more quickly to monitor alarms during this study because you knew you were being filmed? to measure if nurses would report experiencing a Hawthorne‐like effect.[19, 20, 21]
RESULTS
We performed 40 sessions among 40 patients and 36 nurses over 210 hours. We performed 20 sessions in children with heart and/or lung failure in the PICU and 20 sessions in children on a general ward. Sessions took place on weekdays between 9:00 am and 6:00 pm. There were 3 occasions when we filmed 2 patients cared for by the same nurse at the same time.
Nurses were mostly female (94.4%) and had between 2 months and 28 years of experience (median, 4.8 years). Patients on the ward ranged from 5 days to 5.4 years old (median, 6 months). Patients in the PICU ranged from 5 months to 16 years old (median, 2.5 years). Among the PICU patients, 14 (70%) were receiving mechanical ventilation only, 3 (15%) were receiving vasopressors only, and 3 (15%) were receiving mechanical ventilation and vasopressors.
We observed 5070 alarms during the 40 sessions. We excluded 108 (2.1%) that occurred at the end of video recording sessions with the nurse absent from the room because the nurse's response could not be determined. Alarms per session ranged from 10 to 1430 (median, 75; interquartile range [IQR], 35138). We excluded the outlier PICU patient with 1430 alarms in 5 hours from the analysis to avoid the potential for biasing the results. Figure 2 depicts the data flow.

Following the 5 training sessions, research assistants independently reviewed and made preliminary assessments on 4674 alarms; these alarms were all secondarily reviewed by a physician. Using the physician reviewer as the gold standard, the research assistant's sensitivity (assess alarm as actionable when physician also assesses as actionable) was 96.8% and specificity (assess alarm as nonactionable when physician also assesses as nonactionable) was 96.9%. We had to review 54 of 4674 alarms (1.2%) with an additional physician or nurse investigator to achieve consensus.
Characteristics of the 2445 alarms for clinical conditions are shown in Table 1. Only 12.9% of alarms in heart‐ and/or lung‐failure patients in the PICU were actionable, and only 1.0% of alarms in medical patients on a general inpatient ward were actionable.
Overall Response Times for Out‐of‐Room Alarms
We first evaluated response times without excluding alarms occurring prior to the 120‐minute mark. Of the 2445 clinical condition alarms, we excluded the 315 noncritical arrhythmia types from analysis of response time because they did not meet our definition of potentially critical alarms. Of the 2130 potentially critical alarms, 1185 (55.6%) occurred while neither the nurse nor any other clinician was in the patient's room. We proceeded to analyze the response time to these 1185 alarms (307 in the PICU and 878 on the ward). In the PICU, median response time was 3.3 minutes (IQR, 0.814.4). On the ward, median response time was 9.8 minutes (IQR, 3.222.4).
Response‐Time Association With Nonactionable Alarm Exposure
Next, we analyzed the association between response time to potentially critical alarms that occurred when the nurse was not in the patient's room and the number of nonactionable alarms occurring over the preceding 120‐minute window. This required excluding the alarms that occurred in the first 120 minutes of each session, leaving 647 alarms with eligible response times to evaluate the exposure between prior nonactionable alarm exposure and response time: 219 in the PICU and 428 on the ward. Kaplan‐Meier plots and tabulated response times demonstrated the incremental relationships between each nonactionable alarm exposure category in the observed data, with the effects most prominent as the Kaplan‐Meier plots diverged beyond the median (Figure 3 and Table 2). Excluding the extreme outlier patient had no effect on the results, because 1378 of the 1430 alarms occurred with the nurse present at the bedside, and only 2 of the remaining alarms were potentially critical.

| Observed Data | Accelerated Failure‐Time Model | |||||||
|---|---|---|---|---|---|---|---|---|
| Number of Potentially Critical Alarms | Minutes Elapsed Until This Percentage of Alarms Was Responded to | Modeled Response Time, min | 95% CI, min | P Value* | ||||
| 50% (Median) | 75% | 90% | 95% | |||||
| ||||||||
| PICU | ||||||||
| 029 nonactionable alarms | 70 | 1.6 | 8.0 | 18.6 | 25.1 | 2.8 | 1.9‐3.8 | Reference |
| 3079 nonactionable alarms | 122 | 6.3 | 17.8 | 22.5 | 26.0 | 5.3 | 4.06.7 | 0.001 (vs 029) |
| 80+ nonactionable alarms | 27 | 16.0 | 28.4 | 32.0 | 33.1 | 8.5 | 4.312.7 | 0.009 (vs 029), 0.15 (vs 3079) |
| Ward | ||||||||
| 029 nonactionable alarms | 159 | 9.8 | 17.8 | 25.0 | 28.9 | 7.7 | 6.39.1 | Reference |
| 3079 nonactionable alarms | 211 | 11.6 | 22.4 | 44.6 | 63.2 | 11.5 | 9.613.3 | 0.001 (vs 029) |
| 80+ nonactionable alarms | 58 | 8.3 | 57.6 | 63.8 | 69.5 | 15.6 | 11.020.1 | 0.001 (vs 029), 0.09 (vs 3079) |
Accelerated failure‐time regressions revealed significant incremental increases in the modeled response time as the number of preceding nonactionable alarms increased in both the PICU and ward settings (Table 2).
Hawthorne‐like Effects
Four of the 36 nurses reported that they responded more quickly to monitor alarms because they knew they were being filmed.
DISCUSSION
Alarm fatigue has recently generated interest among nurses,[22] physicians,[23] regulatory bodies,[24] patient safety organizations,[25] and even attorneys,[26] despite a lack of prior evidence linking nonactionable alarm exposure to response time or other adverse patient‐relevant outcomes. This study's main findings were that (1) the vast majority of alarms were nonactionable, (2) response time to alarms occurring while the nurse was out of the room increased as the number of nonactionable alarms over the preceding 120 minutes increased. These findings may be explained by alarm fatigue.
Our results build upon the findings of other related studies. The nonactionable alarm proportions we found were similar to other pediatric studies, reporting greater than 90% nonactionable alarms.[1, 2] One other study has reported a relationship between alarm exposure and response time. In that study, Voepel‐Lewis and colleagues evaluated nurse responses to pulse oximetry desaturation alarms in adult orthopedic surgery patients using time‐stamp data from their monitor notification system.[27] They found that alarm response time was significantly longer for patients in the highest quartile of alarms compared to those in lower quartiles. Our study provides new data suggesting a relationship between nonactionable alarm exposure and nurse response time.
Our study has several limitations. First, as a preliminary study to investigate feasibility and possible association, the sample of patients and nurses was necessarily limited and did not permit adjustment for nurse‐ or patient‐level covariates. A multivariable analysis with a larger sample might provide insight into alternate explanations for these findings other than alarm fatigue, including measures of nurse workload and patient factors (such as age and illness severity). Additional factors that are not as easily measured can also contribute to the complex decision of when and how to respond to alarms.[28, 29] Second, nurses were aware that they were being video recorded as part of a study of nonactionable alarms, although they did not know the specific details of measurement. Although this lack of blinding might lead to a Hawthorne‐like effect, our positive results suggest that this effect, if present, did not fully obscure the association. Third, all sessions took place on weekdays during daytime hours, but effects of nonactionable alarms might vary by time and day. Finally, we suspect that when nurses experience critical alarms that require them to intervene and rescue a patient, their response times to that patient's alarms that occur later in their shift will be quicker due to a heightened concern for the alarm being actionable. We were unable to explore that relationship in this analysis because the number of critical alarms requiring intervention was very small. This is a topic of future study.
CONCLUSIONS
We identified an association between a nurse's prior exposure to nonactionable alarms and response time to future alarms. This finding is consistent with alarm fatigue, but requires further study to more clearly delineate other factors that might confound or modify that relationship.
Disclosures
This project was funded by the Health Research Formula Fund Grant 4100050891 from the Pennsylvania Department of Public Health Commonwealth Universal Research Enhancement Program (awarded to Drs. Keren and Bonafide). Dr. Bonafide is also supported by the National Heart, Lung, and Blood Institute of the National Institutes of Health under award number K23HL116427. The content is solely the responsibility of the authors and does not necessarily represent the official views of the National Institutes of Health. The authors have no financial relationships or conflicts of interest relevant to this article to disclose.
- . Crying wolf: false alarms in a pediatric intensive care unit. Crit Care Med. 1994;22(6):981–985.
- , . Poor prognosis for existing monitors in the intensive care unit. Crit Care Med. 1997;25(4):614–619.
- , , , , . Clinical evaluation of alarm efficiency in intensive care [in French]. Ann Fr Anesth Reanim. 2000;19:459–466.
- , , , . Reducing false alarms of intensive care online‐monitoring systems: an evaluation of two signal extraction algorithms. Comput Math Methods Med. 2011;2011:143480.
- , , , , , . Multicentric study of monitoring alarms in the adult intensive care unit (ICU): a descriptive analysis. Intensive Care Med. 1999;25:1360–1366.
- , , . Improving alarm performance in the medical intensive care unit using delays and clinical context. Anesth Analg. 2009;108:1546–1552.
- , . Monitor alarm fatigue: standardizing use of physiological monitors and decreasing nuisance alarms. Am J Crit Care. 2010;19:28–34.
- , , , , , . Intensive care unit alarms—how many do we need? Crit Care Med. 2010;38:451–456.
- , , , . System operator response to warnings of danger: a laboratory investigation of the effects of the predictive value of a warning on human response time. J Exp Psychol Appl. 1995;1:19–33.
- , , . Human probability matching behaviour in response to alarms of varying reliability. Ergonomics. 1995;38:2300–2312.
- The Joint Commission. Sentinel event alert: medical device alarm safety in hospitals. 2013. Available at: http://www.jointcommission.org/sea_issue_50/. Accessed October 9, 2014.
- . Joint commission warns of alarm fatigue: multitude of alarms from monitoring devices problematic. JAMA. 2013;309(22):2315–2316.
- . Monitor alarm fatigue: an integrative review. Biomed Instrum Technol. 2012;46(4):268–277.
- NIH Certificates of Confidentiality Kiosk. Available at: http://grants.nih.gov/grants/policy/coc/. Accessed April 21, 2014.
- , , , et al. Video methods for evaluating physiologic monitor alarms and alarm responses. Biomed Instrum Technol. 2014;48(3):220–230.
- , , , , , . Research electronic data capture (REDCap)—a metadata‐driven methodology and workflow process for providing translational research informatics support. J Biomed Inf. 2009;42:377–381.
- . Accelerated failure time and other parametric models. In: Modelling Survival Data in Medical Research. 2nd ed. Boca Raton, FL: Chapman 2003:197–229.
- , , , . Parametric models. In: An Introduction to Survival Analysis Using Stata, 3rd ed. College Station, TX: Stata Press; 2010:229–244.
- , . Management and the Worker. Cambridge, MA: Harvard University Press; 1939.
- . What happened at Hawthorne? Science. 1974;183(4128):922–932.
- , , , , . Validation of the Work Observation Method By Activity Timing (WOMBAT) method of conducting time‐motion observations in critical care settings: an observational study. BMC Med Inf Decis Mak. 2011;11:32.
- , . Alarm fatigue: a patient safety concern. AACN Adv Crit Care. 2013;24(4):378–386.
- , . Redesigning hospital alarms for patient safety: alarmed and potentially dangerous. JAMA. 2014;311(12):1199–1200.
- The Joint Commission. The Joint Commission announces 2014 National Patient Safety Goal. Jt Comm Perspect. 2013;33:1–4.
- Top 10 health technology hazards for 2014. Health Devices. 2013;42(11):354–380.
- My Philly Lawyer. Medical malpractice: alarm fatigue threatens patient safety. 2014. Available at: http://www.myphillylawyer.com/Resources/Legal-Articles/Medical-Malpractice-Alarm-Fatigue-Threatens-Patient-Safety.shtml. Accessed April 4, 2014.
- , , , et al. Pulse oximetry desaturation alarms on a general postoperative adult unit: a prospective observational study of nurse response time. Int J Nurs Stud. 2013;50(10):1351–1358.
- , , , , . A description of nurses' decision‐making in managing electrocardiographic monitor alarms [published online ahead of print May 10, 2014]. J Clin Nurs. doi:10.1111/jocn.12625.
- . Nurses' response to frequency and types of electrocardiography alarms in a non‐critical care setting: a descriptive study. Int J Nurs Stud. 2014;51(2):190–197.
- . Crying wolf: false alarms in a pediatric intensive care unit. Crit Care Med. 1994;22(6):981–985.
- , . Poor prognosis for existing monitors in the intensive care unit. Crit Care Med. 1997;25(4):614–619.
- , , , , . Clinical evaluation of alarm efficiency in intensive care [in French]. Ann Fr Anesth Reanim. 2000;19:459–466.
- , , , . Reducing false alarms of intensive care online‐monitoring systems: an evaluation of two signal extraction algorithms. Comput Math Methods Med. 2011;2011:143480.
- , , , , , . Multicentric study of monitoring alarms in the adult intensive care unit (ICU): a descriptive analysis. Intensive Care Med. 1999;25:1360–1366.
- , , . Improving alarm performance in the medical intensive care unit using delays and clinical context. Anesth Analg. 2009;108:1546–1552.
- , . Monitor alarm fatigue: standardizing use of physiological monitors and decreasing nuisance alarms. Am J Crit Care. 2010;19:28–34.
- , , , , , . Intensive care unit alarms—how many do we need? Crit Care Med. 2010;38:451–456.
- , , , . System operator response to warnings of danger: a laboratory investigation of the effects of the predictive value of a warning on human response time. J Exp Psychol Appl. 1995;1:19–33.
- , , . Human probability matching behaviour in response to alarms of varying reliability. Ergonomics. 1995;38:2300–2312.
- The Joint Commission. Sentinel event alert: medical device alarm safety in hospitals. 2013. Available at: http://www.jointcommission.org/sea_issue_50/. Accessed October 9, 2014.
- . Joint commission warns of alarm fatigue: multitude of alarms from monitoring devices problematic. JAMA. 2013;309(22):2315–2316.
- . Monitor alarm fatigue: an integrative review. Biomed Instrum Technol. 2012;46(4):268–277.
- NIH Certificates of Confidentiality Kiosk. Available at: http://grants.nih.gov/grants/policy/coc/. Accessed April 21, 2014.
- , , , et al. Video methods for evaluating physiologic monitor alarms and alarm responses. Biomed Instrum Technol. 2014;48(3):220–230.
- , , , , , . Research electronic data capture (REDCap)—a metadata‐driven methodology and workflow process for providing translational research informatics support. J Biomed Inf. 2009;42:377–381.
- . Accelerated failure time and other parametric models. In: Modelling Survival Data in Medical Research. 2nd ed. Boca Raton, FL: Chapman 2003:197–229.
- , , , . Parametric models. In: An Introduction to Survival Analysis Using Stata, 3rd ed. College Station, TX: Stata Press; 2010:229–244.
- , . Management and the Worker. Cambridge, MA: Harvard University Press; 1939.
- . What happened at Hawthorne? Science. 1974;183(4128):922–932.
- , , , , . Validation of the Work Observation Method By Activity Timing (WOMBAT) method of conducting time‐motion observations in critical care settings: an observational study. BMC Med Inf Decis Mak. 2011;11:32.
- , . Alarm fatigue: a patient safety concern. AACN Adv Crit Care. 2013;24(4):378–386.
- , . Redesigning hospital alarms for patient safety: alarmed and potentially dangerous. JAMA. 2014;311(12):1199–1200.
- The Joint Commission. The Joint Commission announces 2014 National Patient Safety Goal. Jt Comm Perspect. 2013;33:1–4.
- Top 10 health technology hazards for 2014. Health Devices. 2013;42(11):354–380.
- My Philly Lawyer. Medical malpractice: alarm fatigue threatens patient safety. 2014. Available at: http://www.myphillylawyer.com/Resources/Legal-Articles/Medical-Malpractice-Alarm-Fatigue-Threatens-Patient-Safety.shtml. Accessed April 4, 2014.
- , , , et al. Pulse oximetry desaturation alarms on a general postoperative adult unit: a prospective observational study of nurse response time. Int J Nurs Stud. 2013;50(10):1351–1358.
- , , , , . A description of nurses' decision‐making in managing electrocardiographic monitor alarms [published online ahead of print May 10, 2014]. J Clin Nurs. doi:10.1111/jocn.12625.
- . Nurses' response to frequency and types of electrocardiography alarms in a non‐critical care setting: a descriptive study. Int J Nurs Stud. 2014;51(2):190–197.
© 2015 Society of Hospital Medicine
Code Status Documentation
In the hospital, cardiopulmonary resuscitation (CPR) is the default treatment for a patient who suffers a cardiac arrest. Clinician assessment of patient preferences regarding resuscitation, with appropriate documentation in the medical record, is therefore essential for patients who do not wish to be resuscitated.[1] In addition, given frequent patient handoffs between physicians, consistent documentation of patient preferences is critical.[2] Unfortunately, multiple deficiencies in the quality of code status documentation have been identified in prior work.[3, 4] In this issue of the Journal of Hospital Medicine, Weinerman and colleagues[5] build on this literature by not only evaluating the completeness of code status documentation in multiple documentation sites, but also its consistency.
In this Canadian multihospital study, the authors found that only 38 of the 187 patients (20%) admitted to 1 of 4 medicine services had complete and consistent documentation of code status. Even more worrisome is that two‐thirds of the patients had inconsistent code status documentation. Although most of these inconsistencies involved missing information in 1 of the 5 sites of documentation (progress note, physician order, electronic resident sign‐out lists, nursing‐care plan, and do‐not‐resuscitate [DNR] face sheet), 31% were deemed clinically significant (eg, DNR in 1 source and full code in another). Such inconsistent documentation represents a serious threat to patient safety, and highlights the need for interventions aimed at improving the quality and reliability of code status documentation.
The authors identified 71 cases where code status documentation in progress notes was missing or inconsistent with documentation in other sites. Sixty of these notes lacked mention of a preference for full code status, 10 lacked documentation of DNR status, and 1 note incorrectly documented full code rather than DNR status. Interpretation of these findings requires consensus on whether the progress note is an appropriate location for code status documentation. With the evolution of the electronic medical record, the role of the progress note has changed, and unfortunately, these notes have become a lengthy chronicle of a patient's hospital course that includes all clinical data, medical problems, and an array of bottom‐of‐the‐list items such as code status. Information is easily added, but rarely removed, and what remains often goes unedited even for high‐stakes issues such as code status. Given the potential for copying and pasting of progress notes day after day, it is critical that clinicians periodically review the code status documented in the patient's notes and update this information as those preferences change. One solution that may minimize the potential for inaccurate documentation in progress notes is for institutions to utilize a separate note for code status documentation that the clinician fills out following any code status discussion. Having this note clearly labeled (eg, Code Status Note) and in a universal place within the electronic record may provide a reliable and efficient way for both physicians and nurses to identify a patient's preferences, while minimizing the inclusion of repetitive information in daily notes. Furthermore, if entered into a discrete field within the electronic record, this information could then autopopulate other sites (eg, sign‐out, nursing forms), thereby maintaining consistency. Use of note templates can provide a way to then help standardize the quality of information that is included in this type of code status note.
An alternate solution that may minimize the potential for inaccurate implementation of code status preferences is to focus on the fact that they are orders. As this study highlights, there is a need to improve both the completeness and consistency of code status documentation and, to this end, orders such as the Medical Orders for Life‐Sustaining Treatment (MOLST) or Physician Orders for Life‐Sustaining Treatment (POLST) may help.[6] Not only do these orders expand upon resuscitation preferences to include broader preferences for treatment in the context of serious illness, but they are also meant to serve as a standard way to document patient care preferences across healthcare settings. Although the MOLST and POLST primarily aim to translate patient preferences into medical orders to be followed outside of the hospital, their implementation into the electronic medical record may provide a more consistent way to document patient preferences in the hospital as well.
Although many studies have identified the need to improve the quality of code status discussions,[7, 8, 9, 10] the work by Weinerman and colleagues reminds us that attention to documentation is also critical. Ensuring that the electronic medical record contains documentation of the patient's resuscitation preferences and overall goals of care, and that this information can be found easily and reliably by physicians and nurses, should drive future quality improvement and research in this area.
Disclosure
Nothing to report.
- , , , et al. Physician understanding of patient resuscitation preferences: insights and clinical implications. J Am Geriatr Soc. 2000;48(5 suppl):S44–S51.
- , , , et al. The patient handoff: a comprehensive curricular blueprint for resident education to improve continuity of care. Acad Med. 2012;87(4):411–418.
- , , , . Documentation quality of inpatient code status discussions. J Pain Symptom Manage. 2014;48(4):632–638.
- , , , et al. Factors associated with discussion of care plans and code status at the time of hospital admission: results from the Multicenter Hospitalist Study. J Hosp Med. 2008;3(6):437–445.
- , , , , , . Frequency and clinical relevance of inconsistent code status documentation. J Hosp Med. 2015;10(8):491–496.
- , , . Use of the Physician Orders for Life‐Sustaining Treatment Program in the clinical setting: a systematic review of the literature. J Am Geriatr Soc. 2015;63(2):341–350.
- The SUPPORT Principal Investigators. A controlled trial to improve care for seriously ill hospitalized patients. JAMA. 1995;274(20):1591–1598.
- , , . How do medical residents discuss resuscitation with patients? J Gen Intern Med. 1995;10(8):436–442.
- , , , , . Code status discussions between attending hospitalist physicians and medical patients at hospital admission. J Gen Intern Med. 2011;26(4):359–366.
- , , , . Code status discussions: agreement between internal medicine residents and hospitalized patients. Teach Learn Med. 2010;22(4):251–256.
In the hospital, cardiopulmonary resuscitation (CPR) is the default treatment for a patient who suffers a cardiac arrest. Clinician assessment of patient preferences regarding resuscitation, with appropriate documentation in the medical record, is therefore essential for patients who do not wish to be resuscitated.[1] In addition, given frequent patient handoffs between physicians, consistent documentation of patient preferences is critical.[2] Unfortunately, multiple deficiencies in the quality of code status documentation have been identified in prior work.[3, 4] In this issue of the Journal of Hospital Medicine, Weinerman and colleagues[5] build on this literature by not only evaluating the completeness of code status documentation in multiple documentation sites, but also its consistency.
In this Canadian multihospital study, the authors found that only 38 of the 187 patients (20%) admitted to 1 of 4 medicine services had complete and consistent documentation of code status. Even more worrisome is that two‐thirds of the patients had inconsistent code status documentation. Although most of these inconsistencies involved missing information in 1 of the 5 sites of documentation (progress note, physician order, electronic resident sign‐out lists, nursing‐care plan, and do‐not‐resuscitate [DNR] face sheet), 31% were deemed clinically significant (eg, DNR in 1 source and full code in another). Such inconsistent documentation represents a serious threat to patient safety, and highlights the need for interventions aimed at improving the quality and reliability of code status documentation.
The authors identified 71 cases where code status documentation in progress notes was missing or inconsistent with documentation in other sites. Sixty of these notes lacked mention of a preference for full code status, 10 lacked documentation of DNR status, and 1 note incorrectly documented full code rather than DNR status. Interpretation of these findings requires consensus on whether the progress note is an appropriate location for code status documentation. With the evolution of the electronic medical record, the role of the progress note has changed, and unfortunately, these notes have become a lengthy chronicle of a patient's hospital course that includes all clinical data, medical problems, and an array of bottom‐of‐the‐list items such as code status. Information is easily added, but rarely removed, and what remains often goes unedited even for high‐stakes issues such as code status. Given the potential for copying and pasting of progress notes day after day, it is critical that clinicians periodically review the code status documented in the patient's notes and update this information as those preferences change. One solution that may minimize the potential for inaccurate documentation in progress notes is for institutions to utilize a separate note for code status documentation that the clinician fills out following any code status discussion. Having this note clearly labeled (eg, Code Status Note) and in a universal place within the electronic record may provide a reliable and efficient way for both physicians and nurses to identify a patient's preferences, while minimizing the inclusion of repetitive information in daily notes. Furthermore, if entered into a discrete field within the electronic record, this information could then autopopulate other sites (eg, sign‐out, nursing forms), thereby maintaining consistency. Use of note templates can provide a way to then help standardize the quality of information that is included in this type of code status note.
An alternate solution that may minimize the potential for inaccurate implementation of code status preferences is to focus on the fact that they are orders. As this study highlights, there is a need to improve both the completeness and consistency of code status documentation and, to this end, orders such as the Medical Orders for Life‐Sustaining Treatment (MOLST) or Physician Orders for Life‐Sustaining Treatment (POLST) may help.[6] Not only do these orders expand upon resuscitation preferences to include broader preferences for treatment in the context of serious illness, but they are also meant to serve as a standard way to document patient care preferences across healthcare settings. Although the MOLST and POLST primarily aim to translate patient preferences into medical orders to be followed outside of the hospital, their implementation into the electronic medical record may provide a more consistent way to document patient preferences in the hospital as well.
Although many studies have identified the need to improve the quality of code status discussions,[7, 8, 9, 10] the work by Weinerman and colleagues reminds us that attention to documentation is also critical. Ensuring that the electronic medical record contains documentation of the patient's resuscitation preferences and overall goals of care, and that this information can be found easily and reliably by physicians and nurses, should drive future quality improvement and research in this area.
Disclosure
Nothing to report.
In the hospital, cardiopulmonary resuscitation (CPR) is the default treatment for a patient who suffers a cardiac arrest. Clinician assessment of patient preferences regarding resuscitation, with appropriate documentation in the medical record, is therefore essential for patients who do not wish to be resuscitated.[1] In addition, given frequent patient handoffs between physicians, consistent documentation of patient preferences is critical.[2] Unfortunately, multiple deficiencies in the quality of code status documentation have been identified in prior work.[3, 4] In this issue of the Journal of Hospital Medicine, Weinerman and colleagues[5] build on this literature by not only evaluating the completeness of code status documentation in multiple documentation sites, but also its consistency.
In this Canadian multihospital study, the authors found that only 38 of the 187 patients (20%) admitted to 1 of 4 medicine services had complete and consistent documentation of code status. Even more worrisome is that two‐thirds of the patients had inconsistent code status documentation. Although most of these inconsistencies involved missing information in 1 of the 5 sites of documentation (progress note, physician order, electronic resident sign‐out lists, nursing‐care plan, and do‐not‐resuscitate [DNR] face sheet), 31% were deemed clinically significant (eg, DNR in 1 source and full code in another). Such inconsistent documentation represents a serious threat to patient safety, and highlights the need for interventions aimed at improving the quality and reliability of code status documentation.
The authors identified 71 cases where code status documentation in progress notes was missing or inconsistent with documentation in other sites. Sixty of these notes lacked mention of a preference for full code status, 10 lacked documentation of DNR status, and 1 note incorrectly documented full code rather than DNR status. Interpretation of these findings requires consensus on whether the progress note is an appropriate location for code status documentation. With the evolution of the electronic medical record, the role of the progress note has changed, and unfortunately, these notes have become a lengthy chronicle of a patient's hospital course that includes all clinical data, medical problems, and an array of bottom‐of‐the‐list items such as code status. Information is easily added, but rarely removed, and what remains often goes unedited even for high‐stakes issues such as code status. Given the potential for copying and pasting of progress notes day after day, it is critical that clinicians periodically review the code status documented in the patient's notes and update this information as those preferences change. One solution that may minimize the potential for inaccurate documentation in progress notes is for institutions to utilize a separate note for code status documentation that the clinician fills out following any code status discussion. Having this note clearly labeled (eg, Code Status Note) and in a universal place within the electronic record may provide a reliable and efficient way for both physicians and nurses to identify a patient's preferences, while minimizing the inclusion of repetitive information in daily notes. Furthermore, if entered into a discrete field within the electronic record, this information could then autopopulate other sites (eg, sign‐out, nursing forms), thereby maintaining consistency. Use of note templates can provide a way to then help standardize the quality of information that is included in this type of code status note.
An alternate solution that may minimize the potential for inaccurate implementation of code status preferences is to focus on the fact that they are orders. As this study highlights, there is a need to improve both the completeness and consistency of code status documentation and, to this end, orders such as the Medical Orders for Life‐Sustaining Treatment (MOLST) or Physician Orders for Life‐Sustaining Treatment (POLST) may help.[6] Not only do these orders expand upon resuscitation preferences to include broader preferences for treatment in the context of serious illness, but they are also meant to serve as a standard way to document patient care preferences across healthcare settings. Although the MOLST and POLST primarily aim to translate patient preferences into medical orders to be followed outside of the hospital, their implementation into the electronic medical record may provide a more consistent way to document patient preferences in the hospital as well.
Although many studies have identified the need to improve the quality of code status discussions,[7, 8, 9, 10] the work by Weinerman and colleagues reminds us that attention to documentation is also critical. Ensuring that the electronic medical record contains documentation of the patient's resuscitation preferences and overall goals of care, and that this information can be found easily and reliably by physicians and nurses, should drive future quality improvement and research in this area.
Disclosure
Nothing to report.
- , , , et al. Physician understanding of patient resuscitation preferences: insights and clinical implications. J Am Geriatr Soc. 2000;48(5 suppl):S44–S51.
- , , , et al. The patient handoff: a comprehensive curricular blueprint for resident education to improve continuity of care. Acad Med. 2012;87(4):411–418.
- , , , . Documentation quality of inpatient code status discussions. J Pain Symptom Manage. 2014;48(4):632–638.
- , , , et al. Factors associated with discussion of care plans and code status at the time of hospital admission: results from the Multicenter Hospitalist Study. J Hosp Med. 2008;3(6):437–445.
- , , , , , . Frequency and clinical relevance of inconsistent code status documentation. J Hosp Med. 2015;10(8):491–496.
- , , . Use of the Physician Orders for Life‐Sustaining Treatment Program in the clinical setting: a systematic review of the literature. J Am Geriatr Soc. 2015;63(2):341–350.
- The SUPPORT Principal Investigators. A controlled trial to improve care for seriously ill hospitalized patients. JAMA. 1995;274(20):1591–1598.
- , , . How do medical residents discuss resuscitation with patients? J Gen Intern Med. 1995;10(8):436–442.
- , , , , . Code status discussions between attending hospitalist physicians and medical patients at hospital admission. J Gen Intern Med. 2011;26(4):359–366.
- , , , . Code status discussions: agreement between internal medicine residents and hospitalized patients. Teach Learn Med. 2010;22(4):251–256.
- , , , et al. Physician understanding of patient resuscitation preferences: insights and clinical implications. J Am Geriatr Soc. 2000;48(5 suppl):S44–S51.
- , , , et al. The patient handoff: a comprehensive curricular blueprint for resident education to improve continuity of care. Acad Med. 2012;87(4):411–418.
- , , , . Documentation quality of inpatient code status discussions. J Pain Symptom Manage. 2014;48(4):632–638.
- , , , et al. Factors associated with discussion of care plans and code status at the time of hospital admission: results from the Multicenter Hospitalist Study. J Hosp Med. 2008;3(6):437–445.
- , , , , , . Frequency and clinical relevance of inconsistent code status documentation. J Hosp Med. 2015;10(8):491–496.
- , , . Use of the Physician Orders for Life‐Sustaining Treatment Program in the clinical setting: a systematic review of the literature. J Am Geriatr Soc. 2015;63(2):341–350.
- The SUPPORT Principal Investigators. A controlled trial to improve care for seriously ill hospitalized patients. JAMA. 1995;274(20):1591–1598.
- , , . How do medical residents discuss resuscitation with patients? J Gen Intern Med. 1995;10(8):436–442.
- , , , , . Code status discussions between attending hospitalist physicians and medical patients at hospital admission. J Gen Intern Med. 2011;26(4):359–366.
- , , , . Code status discussions: agreement between internal medicine residents and hospitalized patients. Teach Learn Med. 2010;22(4):251–256.
HHS secretary tells how to combat drug abuse
More people are dying of drug overdoses in the United States than in car crashes, Nicholas Garlow writes in a blog entry for the U.S. Department of Health & Human Services.
Sylvia Mathews Burwell, secretary of the HHS, announced ways to combat opioid abuse during a speech at the 4th annual National Rx Drug Abuse Summit in Atlanta.
The strategies Ms. Burwell cited are:
• Provide the training, tools, and educational resources that health care professionals need to make more informed prescribing decisions.
• Increase the use of naloxone, a drug that can reverse opioid overdose*.
• Use medication-assisted treatment to help lift people from opioid addiction.
Drug overdose is the leading cause of injury death in the country. In fact, the number of drug overdoses resulting in deaths has increased fivefold since 1980, Mr. Garlow writes.
*Correction, 4/16/2015: An earlier version of this article misstated naloxone's indication.
More people are dying of drug overdoses in the United States than in car crashes, Nicholas Garlow writes in a blog entry for the U.S. Department of Health & Human Services.
Sylvia Mathews Burwell, secretary of the HHS, announced ways to combat opioid abuse during a speech at the 4th annual National Rx Drug Abuse Summit in Atlanta.
The strategies Ms. Burwell cited are:
• Provide the training, tools, and educational resources that health care professionals need to make more informed prescribing decisions.
• Increase the use of naloxone, a drug that can reverse opioid overdose*.
• Use medication-assisted treatment to help lift people from opioid addiction.
Drug overdose is the leading cause of injury death in the country. In fact, the number of drug overdoses resulting in deaths has increased fivefold since 1980, Mr. Garlow writes.
*Correction, 4/16/2015: An earlier version of this article misstated naloxone's indication.
More people are dying of drug overdoses in the United States than in car crashes, Nicholas Garlow writes in a blog entry for the U.S. Department of Health & Human Services.
Sylvia Mathews Burwell, secretary of the HHS, announced ways to combat opioid abuse during a speech at the 4th annual National Rx Drug Abuse Summit in Atlanta.
The strategies Ms. Burwell cited are:
• Provide the training, tools, and educational resources that health care professionals need to make more informed prescribing decisions.
• Increase the use of naloxone, a drug that can reverse opioid overdose*.
• Use medication-assisted treatment to help lift people from opioid addiction.
Drug overdose is the leading cause of injury death in the country. In fact, the number of drug overdoses resulting in deaths has increased fivefold since 1980, Mr. Garlow writes.
*Correction, 4/16/2015: An earlier version of this article misstated naloxone's indication.
Ablation during mitral valve surgery offers up mixed results
SAN DIEGO – Surgical ablation of atrial fibrillation at the time of mitral valve surgery provides significantly greater rhythm control than mitral valve surgery alone, a study showed.
Freedom from atrial fibrillation (AF) at both 6 months and 1 year was 63% in patients undergoing mitral valve surgery (MVS) plus ablation and 29% in those undergoing MVS alone, a statistically significant difference.
However, patients who had ablation plus MVS were 2.5 times more likely to have a permanent pacemaker implanted than were those who had MVS alone, at 21.5% and 8.1%, respectively, also a significant difference.
Ablation did not increase mortality or major adverse cardiac or cerebrovascular events, Dr. A. Marc Gillinov said at the annual meeting of the American College of Cardiology.
Preoperative AF is present in up to 50% of patients undergoing mitral valve operations and is associated with an increased risk of death and stroke.
The study enrolled 260 relatively elderly patients (mean age 69 years) with AF that was persistent (non–self-terminating for at least 7 days) or long-standing persistent (continuous for at least a year), in addition to mitral valve disease. A total of 133 patients were randomly assigned to MVS plus ablation and 127 to MVS alone. The ablation group was further randomized to pulmonary vein isolation or a biatrial maze procedure; all underwent closure of the left atrial appendage.
There was no significant difference in freedom from AF at 6 months and 1 year between patients who had pulmonary vein isolation or a biatrial maze procedure, at 61% and 66%, respectively, said Dr. Gillinov, a cardiac surgeon at Cleveland Clinic.
One-year mortality was similar among all patients undergoing MVS plus ablation vs. MVS alone, at 6.8% and 8.7%.
The two groups also had similar Short Form-12 questionnaire scores for physical function and mental function, although AF occurring at least once daily was significantly less common with ablation, at 19.8%, compared with 45.2% in the MVS-alone patients, he said.
The heart rhythm endpoint was “stringent,” with 3-day Holter monitors obtained at both 6 and 12 months and repeat ablation procedures and death considered treatment failures, Dr. Gillinov said.
He acknowledged that 20% of patients did not have data for the primary endpoint and that the endpoint was not a clinical one, but said a trial with mortality or stroke as the endpoint would require more than 1,000 patients and many years follow-up.
Regarding whether ablation should now be performed routinely, “the glass is half full or half empty,” remarked discussant Dr. Bernard Gersh of Mayo Clinic in Rochester, Minn. “On one hand, you have shown less atrial fibrillation [with ablation], but no effect on quality of life, and the price to be paid was a higher rate of pacemaker implantation,” he said.
The pacemaker implantation rate was higher than expected – 17% in-hospital – and does represent a potential cost, but he would routinely do a maze procedure, Dr. Gillinov said.
Discussant Dr. Alice Jacobs of the Cardiovascular Center at Boston Medical Center, said she expected Dr. Gillinov to say the procedure should not be used in everyone given the lack of benefit in stroke, probably because they tied off the left atrium appendage, and the increase in pacemaker implantations.
About half of the pacemaker implantations were due to atrioventricular block, possibly a consequence of the valve surgery, and one-third to sinus-node dysfunction, which is common in elderly patients, Dr. Gillinov explained.
The study was funded by the National Institutes of Health and the Canadian Institutes of Health Research. Dr. Gillinov reported serving as a consultant/speaker for AtriCure, Medtronic, On-X, Edwards, and Tendyne; research funding from St. Jude Medical; an equity interest in Clear Catheter; and that his institution receives royalties from AtriCure for a left atrial appendage occlusion device.
SAN DIEGO – Surgical ablation of atrial fibrillation at the time of mitral valve surgery provides significantly greater rhythm control than mitral valve surgery alone, a study showed.
Freedom from atrial fibrillation (AF) at both 6 months and 1 year was 63% in patients undergoing mitral valve surgery (MVS) plus ablation and 29% in those undergoing MVS alone, a statistically significant difference.
However, patients who had ablation plus MVS were 2.5 times more likely to have a permanent pacemaker implanted than were those who had MVS alone, at 21.5% and 8.1%, respectively, also a significant difference.
Ablation did not increase mortality or major adverse cardiac or cerebrovascular events, Dr. A. Marc Gillinov said at the annual meeting of the American College of Cardiology.
Preoperative AF is present in up to 50% of patients undergoing mitral valve operations and is associated with an increased risk of death and stroke.
The study enrolled 260 relatively elderly patients (mean age 69 years) with AF that was persistent (non–self-terminating for at least 7 days) or long-standing persistent (continuous for at least a year), in addition to mitral valve disease. A total of 133 patients were randomly assigned to MVS plus ablation and 127 to MVS alone. The ablation group was further randomized to pulmonary vein isolation or a biatrial maze procedure; all underwent closure of the left atrial appendage.
There was no significant difference in freedom from AF at 6 months and 1 year between patients who had pulmonary vein isolation or a biatrial maze procedure, at 61% and 66%, respectively, said Dr. Gillinov, a cardiac surgeon at Cleveland Clinic.
One-year mortality was similar among all patients undergoing MVS plus ablation vs. MVS alone, at 6.8% and 8.7%.
The two groups also had similar Short Form-12 questionnaire scores for physical function and mental function, although AF occurring at least once daily was significantly less common with ablation, at 19.8%, compared with 45.2% in the MVS-alone patients, he said.
The heart rhythm endpoint was “stringent,” with 3-day Holter monitors obtained at both 6 and 12 months and repeat ablation procedures and death considered treatment failures, Dr. Gillinov said.
He acknowledged that 20% of patients did not have data for the primary endpoint and that the endpoint was not a clinical one, but said a trial with mortality or stroke as the endpoint would require more than 1,000 patients and many years follow-up.
Regarding whether ablation should now be performed routinely, “the glass is half full or half empty,” remarked discussant Dr. Bernard Gersh of Mayo Clinic in Rochester, Minn. “On one hand, you have shown less atrial fibrillation [with ablation], but no effect on quality of life, and the price to be paid was a higher rate of pacemaker implantation,” he said.
The pacemaker implantation rate was higher than expected – 17% in-hospital – and does represent a potential cost, but he would routinely do a maze procedure, Dr. Gillinov said.
Discussant Dr. Alice Jacobs of the Cardiovascular Center at Boston Medical Center, said she expected Dr. Gillinov to say the procedure should not be used in everyone given the lack of benefit in stroke, probably because they tied off the left atrium appendage, and the increase in pacemaker implantations.
About half of the pacemaker implantations were due to atrioventricular block, possibly a consequence of the valve surgery, and one-third to sinus-node dysfunction, which is common in elderly patients, Dr. Gillinov explained.
The study was funded by the National Institutes of Health and the Canadian Institutes of Health Research. Dr. Gillinov reported serving as a consultant/speaker for AtriCure, Medtronic, On-X, Edwards, and Tendyne; research funding from St. Jude Medical; an equity interest in Clear Catheter; and that his institution receives royalties from AtriCure for a left atrial appendage occlusion device.
SAN DIEGO – Surgical ablation of atrial fibrillation at the time of mitral valve surgery provides significantly greater rhythm control than mitral valve surgery alone, a study showed.
Freedom from atrial fibrillation (AF) at both 6 months and 1 year was 63% in patients undergoing mitral valve surgery (MVS) plus ablation and 29% in those undergoing MVS alone, a statistically significant difference.
However, patients who had ablation plus MVS were 2.5 times more likely to have a permanent pacemaker implanted than were those who had MVS alone, at 21.5% and 8.1%, respectively, also a significant difference.
Ablation did not increase mortality or major adverse cardiac or cerebrovascular events, Dr. A. Marc Gillinov said at the annual meeting of the American College of Cardiology.
Preoperative AF is present in up to 50% of patients undergoing mitral valve operations and is associated with an increased risk of death and stroke.
The study enrolled 260 relatively elderly patients (mean age 69 years) with AF that was persistent (non–self-terminating for at least 7 days) or long-standing persistent (continuous for at least a year), in addition to mitral valve disease. A total of 133 patients were randomly assigned to MVS plus ablation and 127 to MVS alone. The ablation group was further randomized to pulmonary vein isolation or a biatrial maze procedure; all underwent closure of the left atrial appendage.
There was no significant difference in freedom from AF at 6 months and 1 year between patients who had pulmonary vein isolation or a biatrial maze procedure, at 61% and 66%, respectively, said Dr. Gillinov, a cardiac surgeon at Cleveland Clinic.
One-year mortality was similar among all patients undergoing MVS plus ablation vs. MVS alone, at 6.8% and 8.7%.
The two groups also had similar Short Form-12 questionnaire scores for physical function and mental function, although AF occurring at least once daily was significantly less common with ablation, at 19.8%, compared with 45.2% in the MVS-alone patients, he said.
The heart rhythm endpoint was “stringent,” with 3-day Holter monitors obtained at both 6 and 12 months and repeat ablation procedures and death considered treatment failures, Dr. Gillinov said.
He acknowledged that 20% of patients did not have data for the primary endpoint and that the endpoint was not a clinical one, but said a trial with mortality or stroke as the endpoint would require more than 1,000 patients and many years follow-up.
Regarding whether ablation should now be performed routinely, “the glass is half full or half empty,” remarked discussant Dr. Bernard Gersh of Mayo Clinic in Rochester, Minn. “On one hand, you have shown less atrial fibrillation [with ablation], but no effect on quality of life, and the price to be paid was a higher rate of pacemaker implantation,” he said.
The pacemaker implantation rate was higher than expected – 17% in-hospital – and does represent a potential cost, but he would routinely do a maze procedure, Dr. Gillinov said.
Discussant Dr. Alice Jacobs of the Cardiovascular Center at Boston Medical Center, said she expected Dr. Gillinov to say the procedure should not be used in everyone given the lack of benefit in stroke, probably because they tied off the left atrium appendage, and the increase in pacemaker implantations.
About half of the pacemaker implantations were due to atrioventricular block, possibly a consequence of the valve surgery, and one-third to sinus-node dysfunction, which is common in elderly patients, Dr. Gillinov explained.
The study was funded by the National Institutes of Health and the Canadian Institutes of Health Research. Dr. Gillinov reported serving as a consultant/speaker for AtriCure, Medtronic, On-X, Edwards, and Tendyne; research funding from St. Jude Medical; an equity interest in Clear Catheter; and that his institution receives royalties from AtriCure for a left atrial appendage occlusion device.
AT ACC 15
Key clinical point: Surgical ablation of atrial fibrillation during mitral valve surgery decreases AF at 6 months and 1 year, but increases pacemaker implantations.
Major finding: Freedom from AF at both 6 months and 1 year was 63% with mitral valve surgery plus ablation and 29% for MVS alone.
Data source: Prospective, randomized study in 260 patients with persistent or longstanding persistent AF who required mitral valve surgery.
Disclosures: The study was funded by the National Institutes of Health and the Canadian Institutes of Health Research. Dr. Gillinov reported serving as a consultant/speaker for AtriCure, Medtronic, On-X, Edwards, and Tendyne; research funding from St. Jude Medical; an equity interest in Clear Catheter; and that his institution receives royalties from AtriCure for a left atrial appendage occlusion device.