User login
Mortality, outcomes good in AAA repair in octogenarians
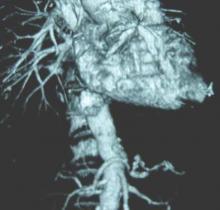
SCOTTSDALE, ARIZ.– Abdominal aortic aneurysm repair in patients 80 years and older can be performed safely and with good medium-term survival rates, a prospective single-site study has shown.
Perioperative mortality in elective and emergent AAA repair for octogenarians was 2% and 35%, respectively, with a median survival rate of 19 months in both groups.
According to these data, “Patients shouldn’t be turned down for aneurysm repair on the basis of their age alone,” Dr. Christopher M. Lamb, a vascular surgery fellow at the University of California Davis Medical Center in Sacramento, said during a presentation at this year’s Southern Association for Vascular Surgery annual meeting.
“However, whether we should be doing these procedures is a different question, and I don’t think these data allow us to answer that question properly.”Dr. Lamb and his colleagues reviewed the records of 847 consecutive patients aged 80 years or older, seen between April 2005 and February 2014 for any type of AAA repair. Cases were sorted according to whether they were elective, ruptured, or urgent but unruptured. A total of 226 patients met the study’s age criteria; there were nearly seven men for every woman, all with a median age of 83 years. Of the elective AAA repair arm of the study, 131 patients (116 men) with a median age of 82 years had an endovascular repair, while the rest underwent open surgical repair. The combined 30-day mortality rate for these patients was 2.3%, with no significant difference between either the endovascular aneurysm repair (EVAR) or the open surgical repair (OSR) patients (1.9% vs. 4.2%; P = .458). The median survival of all elective repair patients was 19 months (interquartile range, 10-35), with no difference seen between the two groups (P = .113)Of the 65 patients (53 men) with ruptured AAA, the median age was 83 years. A third had open repair (32.3%), while the rest had EVAR. The combined 30-day mortality rate was 35.4% but was significantly higher after OSR (52.4% vs. 27.3%; P = .048). The median survival rate was 6 months (IQR, 6-42) when 30-day mortality rates were excluded. The median survival rates in patients who lived longer than 30 days was significantly higher in OSR patients (42.5 months vs. 11 months; P = .019).
Of the 23 men and 7 women with symptomatic but unruptured AAA, all but 1 had EVAR. At 30 days, there was one diverticular perforation–related postoperative death in the EVAR group, which had a median survival rate of 29 months. There being only a single patient in the OSR group obviated a comparative median survival rate analysis.
A subanalysis of the final 20 months of the study showed that 41% of octogenarians seeking any type of AAA repair at the site were rejected (48 rejections vs. 69 repairs). Those who were rejected for repair tended to be older, with a median age of 86 years vs. 83 years for patients who underwent repair (P = .0004).
Dr. Lamb noted that although the findings demonstrate acceptable overall safety rates for the entire cohort, without a control group of patients that did not have AAA repair, it would be hard to draw a definite conclusion about the utility of the findings, and that more data were warranted; however, the potential for limited long-term survival with what previous reports have suggested may include “a reduced quality of life for a good part of it, possibly raises the question that these patients should be treated conservatively, more often.”
The rejection rate data prompted the presentation’s discussant, Dr. William D. Jordan Jr., section chief of vascular surgery at the University of Alabama at Birmingham and the presentation’s discussant, to challenge the findings and asked whether a single surgeon selected the patients.
“You said there is not a selection bias in your study, but I beg to differ. Perhaps all these kinds of studies have a selection bias, and I believe they should. We should select the appropriate patients for the appropriate procedure at the appropriate time, with the appropriate expectation of outcome. Bias in this setting may be seen as good,” Dr. Jordan said.Dr. Lamb responded that the treatment algorithm at the site for all patients with a confirmed AAA of 5.5 cm or greater included CT imaging that is reviewed by a multidisciplinary team comprising vascular surgeons and interventional radiologists, who then evaluated the patients according to their physiology and anatomy, as well as their comorbidities, with the intention that whenever possible, EVAR rather than open repair would be performed.
As to whether there was a bias toward not repairing AAA in older patients, Dr. Lamb said it was incumbent on any health system to evaluate a procedure’s cost-effectiveness, but that, “the life expectancy of a vascular patient is often more limited than I think we’d like to believe ... we don’t know what the natural history of these patients’ life expectancy is. We don’t know from these data what the cause of death was, but anecdotally, we didn’t see hundreds of patients return with ruptured aneurysms after an EVAR.”
“I would truly like to see how many [of these patients] who make it out of the hospital return to normal living within 6 months,” Dr. Samuel R. Money, chair of surgery at the Mayo Clinic in Scottsdale, Ariz., said in an interview following the presentation. “At some point, the question becomes ‘Can we afford to spend $100,000 dollars to keep a 90-year-old patient alive for 6 more months?’ Can this society sustain the cost of that?”
On Twitter @whitneymcknight
This discussion is provocative and raises some interesting points. Obviously cost-effectiveness considerations are important, and our country does not have unlimited funds to spend on medical care. And perhaps there are some elderly and frail individuals who should not have their AAAs repaired electively because the risk of rupture during the patients’ remaining months or years of life is small.
This is particularly true if the patient’s AAA is less than 7 cm and his or her anatomy and condition are unsuitable for an easy repair. However, if the AAA is large and threatening, and the patient has the possibility of living several years, elective repair is justified and reasonable – especially if it can be accomplished endovascularly. As someone who is near 80 [years old], I could not feel more strongly about this, and I would maintain this view if I were near 90 and healthy.

|
Dr. Frank J. Veith |
I hold the same view even more strongly regarding a ruptured AAA. In this setting, the alternative management is nontreatment, which is uniformly fatal. The common term “palliative treatment” for such nontreatment is a misleading misnomer. No sane, reasonably healthy elderly patient would knowingly choose such nontreatment when a good alternative with well over an even chance of living a lot longer is offered. That good alternative – again especially if it can be performed endovascularly – should be offered, and our health system should pay for it and compensate by saving money on unnecessary SFA [superficial femoral artery] stents and carotid procedures.
Dr. Frank J. Veith is professor of surgery at New York University Medical Center and the Cleveland Clinic and is an associate medical editor for Vascular Specialist.
This discussion is provocative and raises some interesting points. Obviously cost-effectiveness considerations are important, and our country does not have unlimited funds to spend on medical care. And perhaps there are some elderly and frail individuals who should not have their AAAs repaired electively because the risk of rupture during the patients’ remaining months or years of life is small.
This is particularly true if the patient’s AAA is less than 7 cm and his or her anatomy and condition are unsuitable for an easy repair. However, if the AAA is large and threatening, and the patient has the possibility of living several years, elective repair is justified and reasonable – especially if it can be accomplished endovascularly. As someone who is near 80 [years old], I could not feel more strongly about this, and I would maintain this view if I were near 90 and healthy.

|
Dr. Frank J. Veith |
I hold the same view even more strongly regarding a ruptured AAA. In this setting, the alternative management is nontreatment, which is uniformly fatal. The common term “palliative treatment” for such nontreatment is a misleading misnomer. No sane, reasonably healthy elderly patient would knowingly choose such nontreatment when a good alternative with well over an even chance of living a lot longer is offered. That good alternative – again especially if it can be performed endovascularly – should be offered, and our health system should pay for it and compensate by saving money on unnecessary SFA [superficial femoral artery] stents and carotid procedures.
Dr. Frank J. Veith is professor of surgery at New York University Medical Center and the Cleveland Clinic and is an associate medical editor for Vascular Specialist.
This discussion is provocative and raises some interesting points. Obviously cost-effectiveness considerations are important, and our country does not have unlimited funds to spend on medical care. And perhaps there are some elderly and frail individuals who should not have their AAAs repaired electively because the risk of rupture during the patients’ remaining months or years of life is small.
This is particularly true if the patient’s AAA is less than 7 cm and his or her anatomy and condition are unsuitable for an easy repair. However, if the AAA is large and threatening, and the patient has the possibility of living several years, elective repair is justified and reasonable – especially if it can be accomplished endovascularly. As someone who is near 80 [years old], I could not feel more strongly about this, and I would maintain this view if I were near 90 and healthy.

|
Dr. Frank J. Veith |
I hold the same view even more strongly regarding a ruptured AAA. In this setting, the alternative management is nontreatment, which is uniformly fatal. The common term “palliative treatment” for such nontreatment is a misleading misnomer. No sane, reasonably healthy elderly patient would knowingly choose such nontreatment when a good alternative with well over an even chance of living a lot longer is offered. That good alternative – again especially if it can be performed endovascularly – should be offered, and our health system should pay for it and compensate by saving money on unnecessary SFA [superficial femoral artery] stents and carotid procedures.
Dr. Frank J. Veith is professor of surgery at New York University Medical Center and the Cleveland Clinic and is an associate medical editor for Vascular Specialist.
SCOTTSDALE, ARIZ.– Abdominal aortic aneurysm repair in patients 80 years and older can be performed safely and with good medium-term survival rates, a prospective single-site study has shown.
Perioperative mortality in elective and emergent AAA repair for octogenarians was 2% and 35%, respectively, with a median survival rate of 19 months in both groups.
According to these data, “Patients shouldn’t be turned down for aneurysm repair on the basis of their age alone,” Dr. Christopher M. Lamb, a vascular surgery fellow at the University of California Davis Medical Center in Sacramento, said during a presentation at this year’s Southern Association for Vascular Surgery annual meeting.
“However, whether we should be doing these procedures is a different question, and I don’t think these data allow us to answer that question properly.”Dr. Lamb and his colleagues reviewed the records of 847 consecutive patients aged 80 years or older, seen between April 2005 and February 2014 for any type of AAA repair. Cases were sorted according to whether they were elective, ruptured, or urgent but unruptured. A total of 226 patients met the study’s age criteria; there were nearly seven men for every woman, all with a median age of 83 years. Of the elective AAA repair arm of the study, 131 patients (116 men) with a median age of 82 years had an endovascular repair, while the rest underwent open surgical repair. The combined 30-day mortality rate for these patients was 2.3%, with no significant difference between either the endovascular aneurysm repair (EVAR) or the open surgical repair (OSR) patients (1.9% vs. 4.2%; P = .458). The median survival of all elective repair patients was 19 months (interquartile range, 10-35), with no difference seen between the two groups (P = .113)Of the 65 patients (53 men) with ruptured AAA, the median age was 83 years. A third had open repair (32.3%), while the rest had EVAR. The combined 30-day mortality rate was 35.4% but was significantly higher after OSR (52.4% vs. 27.3%; P = .048). The median survival rate was 6 months (IQR, 6-42) when 30-day mortality rates were excluded. The median survival rates in patients who lived longer than 30 days was significantly higher in OSR patients (42.5 months vs. 11 months; P = .019).
Of the 23 men and 7 women with symptomatic but unruptured AAA, all but 1 had EVAR. At 30 days, there was one diverticular perforation–related postoperative death in the EVAR group, which had a median survival rate of 29 months. There being only a single patient in the OSR group obviated a comparative median survival rate analysis.
A subanalysis of the final 20 months of the study showed that 41% of octogenarians seeking any type of AAA repair at the site were rejected (48 rejections vs. 69 repairs). Those who were rejected for repair tended to be older, with a median age of 86 years vs. 83 years for patients who underwent repair (P = .0004).
Dr. Lamb noted that although the findings demonstrate acceptable overall safety rates for the entire cohort, without a control group of patients that did not have AAA repair, it would be hard to draw a definite conclusion about the utility of the findings, and that more data were warranted; however, the potential for limited long-term survival with what previous reports have suggested may include “a reduced quality of life for a good part of it, possibly raises the question that these patients should be treated conservatively, more often.”
The rejection rate data prompted the presentation’s discussant, Dr. William D. Jordan Jr., section chief of vascular surgery at the University of Alabama at Birmingham and the presentation’s discussant, to challenge the findings and asked whether a single surgeon selected the patients.
“You said there is not a selection bias in your study, but I beg to differ. Perhaps all these kinds of studies have a selection bias, and I believe they should. We should select the appropriate patients for the appropriate procedure at the appropriate time, with the appropriate expectation of outcome. Bias in this setting may be seen as good,” Dr. Jordan said.Dr. Lamb responded that the treatment algorithm at the site for all patients with a confirmed AAA of 5.5 cm or greater included CT imaging that is reviewed by a multidisciplinary team comprising vascular surgeons and interventional radiologists, who then evaluated the patients according to their physiology and anatomy, as well as their comorbidities, with the intention that whenever possible, EVAR rather than open repair would be performed.
As to whether there was a bias toward not repairing AAA in older patients, Dr. Lamb said it was incumbent on any health system to evaluate a procedure’s cost-effectiveness, but that, “the life expectancy of a vascular patient is often more limited than I think we’d like to believe ... we don’t know what the natural history of these patients’ life expectancy is. We don’t know from these data what the cause of death was, but anecdotally, we didn’t see hundreds of patients return with ruptured aneurysms after an EVAR.”
“I would truly like to see how many [of these patients] who make it out of the hospital return to normal living within 6 months,” Dr. Samuel R. Money, chair of surgery at the Mayo Clinic in Scottsdale, Ariz., said in an interview following the presentation. “At some point, the question becomes ‘Can we afford to spend $100,000 dollars to keep a 90-year-old patient alive for 6 more months?’ Can this society sustain the cost of that?”
On Twitter @whitneymcknight
SCOTTSDALE, ARIZ.– Abdominal aortic aneurysm repair in patients 80 years and older can be performed safely and with good medium-term survival rates, a prospective single-site study has shown.
Perioperative mortality in elective and emergent AAA repair for octogenarians was 2% and 35%, respectively, with a median survival rate of 19 months in both groups.
According to these data, “Patients shouldn’t be turned down for aneurysm repair on the basis of their age alone,” Dr. Christopher M. Lamb, a vascular surgery fellow at the University of California Davis Medical Center in Sacramento, said during a presentation at this year’s Southern Association for Vascular Surgery annual meeting.
“However, whether we should be doing these procedures is a different question, and I don’t think these data allow us to answer that question properly.”Dr. Lamb and his colleagues reviewed the records of 847 consecutive patients aged 80 years or older, seen between April 2005 and February 2014 for any type of AAA repair. Cases were sorted according to whether they were elective, ruptured, or urgent but unruptured. A total of 226 patients met the study’s age criteria; there were nearly seven men for every woman, all with a median age of 83 years. Of the elective AAA repair arm of the study, 131 patients (116 men) with a median age of 82 years had an endovascular repair, while the rest underwent open surgical repair. The combined 30-day mortality rate for these patients was 2.3%, with no significant difference between either the endovascular aneurysm repair (EVAR) or the open surgical repair (OSR) patients (1.9% vs. 4.2%; P = .458). The median survival of all elective repair patients was 19 months (interquartile range, 10-35), with no difference seen between the two groups (P = .113)Of the 65 patients (53 men) with ruptured AAA, the median age was 83 years. A third had open repair (32.3%), while the rest had EVAR. The combined 30-day mortality rate was 35.4% but was significantly higher after OSR (52.4% vs. 27.3%; P = .048). The median survival rate was 6 months (IQR, 6-42) when 30-day mortality rates were excluded. The median survival rates in patients who lived longer than 30 days was significantly higher in OSR patients (42.5 months vs. 11 months; P = .019).
Of the 23 men and 7 women with symptomatic but unruptured AAA, all but 1 had EVAR. At 30 days, there was one diverticular perforation–related postoperative death in the EVAR group, which had a median survival rate of 29 months. There being only a single patient in the OSR group obviated a comparative median survival rate analysis.
A subanalysis of the final 20 months of the study showed that 41% of octogenarians seeking any type of AAA repair at the site were rejected (48 rejections vs. 69 repairs). Those who were rejected for repair tended to be older, with a median age of 86 years vs. 83 years for patients who underwent repair (P = .0004).
Dr. Lamb noted that although the findings demonstrate acceptable overall safety rates for the entire cohort, without a control group of patients that did not have AAA repair, it would be hard to draw a definite conclusion about the utility of the findings, and that more data were warranted; however, the potential for limited long-term survival with what previous reports have suggested may include “a reduced quality of life for a good part of it, possibly raises the question that these patients should be treated conservatively, more often.”
The rejection rate data prompted the presentation’s discussant, Dr. William D. Jordan Jr., section chief of vascular surgery at the University of Alabama at Birmingham and the presentation’s discussant, to challenge the findings and asked whether a single surgeon selected the patients.
“You said there is not a selection bias in your study, but I beg to differ. Perhaps all these kinds of studies have a selection bias, and I believe they should. We should select the appropriate patients for the appropriate procedure at the appropriate time, with the appropriate expectation of outcome. Bias in this setting may be seen as good,” Dr. Jordan said.Dr. Lamb responded that the treatment algorithm at the site for all patients with a confirmed AAA of 5.5 cm or greater included CT imaging that is reviewed by a multidisciplinary team comprising vascular surgeons and interventional radiologists, who then evaluated the patients according to their physiology and anatomy, as well as their comorbidities, with the intention that whenever possible, EVAR rather than open repair would be performed.
As to whether there was a bias toward not repairing AAA in older patients, Dr. Lamb said it was incumbent on any health system to evaluate a procedure’s cost-effectiveness, but that, “the life expectancy of a vascular patient is often more limited than I think we’d like to believe ... we don’t know what the natural history of these patients’ life expectancy is. We don’t know from these data what the cause of death was, but anecdotally, we didn’t see hundreds of patients return with ruptured aneurysms after an EVAR.”
“I would truly like to see how many [of these patients] who make it out of the hospital return to normal living within 6 months,” Dr. Samuel R. Money, chair of surgery at the Mayo Clinic in Scottsdale, Ariz., said in an interview following the presentation. “At some point, the question becomes ‘Can we afford to spend $100,000 dollars to keep a 90-year-old patient alive for 6 more months?’ Can this society sustain the cost of that?”
On Twitter @whitneymcknight
Helping parents manage rules across two homes
A major challenge faced by parents is the task of setting basic ground rules and expectations for their children, and then enforcing these with limits, rewards, and consequences. This task is made far more difficult when parents are separated or divorced. Agreeing upon and enforcing rules in separate homes often becomes burdened by the angry baggage that led to the divorce. When a family in your practice is going through a divorce, you have an opportunity to provide the parents with valuable strategies to manage rules effectively so that conflict is minimized.
Many happily married parents who communicate very well on most matters struggle to get on the same page when negotiating rules and limits. One parent’s sense of what is an appropriate bedtime, how children should help with chores, or even how often they can have sweets can become a deeply held belief and might be very different than their spouse’s opinions. Sometimes, a parent has old anger about how they were raised and finds it hard to distinguish what might have been better for them, compared with what is best for their own child. Cultural and family differences on how much choice children should have at different ages, criteria and severity of any consequences for misbehavior, and opportunities for redemption or amnesty all add complexity to the discussion. Once they have found common ground on what makes sense for their joint rules, values, and needs of their child, they have to manage enforcing rules and limits, agreeing upon appropriate rewards and punishments, and bearing the inevitable distress of their children when facing a limit or consequence. And, of course, once parents think they have it all figured out, their children react and grow, and they must reset the rules, expectations, and consequences.
When parents get separated or divorced, this process becomes considerably more difficult. Negotiating new rules or limits is very difficult when communication is hampered by conflict. Parental guilt about the divorce itself, anger at old hurts or disputes about money and custody, missing the child between visits, and remarriages all add baggage to the discussion of a reasonable bedtime or consequences for a poor grade at school. If the divorce required aggressive negotiation between lawyers, appointment of a guardian ad Litem to manage ongoing disputes involving the children, or a court case to reach resolution, the tensions between parents can be intense, enduring, and with no issue too small to add fuel to the arguments. Enforcing limits is much harder for a single parent than when there are two parents doing the enforcement. And divorced parents, already feeling guilty and insecure, are more likely to suspend rules or limits so that they don’t have to be the “bad parent.” For the child or children, the stress and disruptions that come with divorce can cause an increase in regressed or disrespectful behavior. While it can be a time when limits are increasingly tested, being reasonable and consistent in enforcing limits becomes more important, as it provides reassuring steadiness in the midst of turbulent change.
Let’s take the example of a 12-year-old coming home from school with poor grades. One parent may see the need for a tutor, but might be using that approach as part of a financial attack if the other parent has to pay for it. The other parent may want to limit the use of computer games or access to television until the grades go up. And one may expect movement from a D to a C average while the other may expect A’s, period. Is the poor grade based on lack of ability, effort, an attempt to get attention, a reaction to the divorce, or preoccupation with ongoing parental discord? What is the impact on the child if in one home there is a tutor and a C expectation, and in the other there is no tutor, no computer use, no TV... and these change every time the child moves from one home to the other? A child striving to overcome a poor grade needs calm, consistent, patient, and optimistic support, rather than managing the increased tension across two homes or feeling like the cause of increased conflict. Virtually any reasonable approach is better for the child than each parent doing something different as a reflection of ongoing tension. Pediatricians can be extraordinarily helpful to their patient if they can get divorced parents to agree on a single approach that is based on their child’s needs rather than past and ongoing angers. The emotional damage of ongoing discord is far worse than any C average.
As the pediatrician to a family managing divorce, you may be one of the few authority figures whom both parents and the children all still respect and trust. You are in a strong position to ask a parent during an appointment how rules and limits are being managed across two homes. Find out if they have a clear plan for handling routine communication about the children, whether about summer camps or a new curfew, so that they don’t default to communicating only once there is a crisis. See if rules are a vehicle for ongoing parental fighting so that a minor difference (an 8 o’clock bedtime in one house versus 9 o’clock in the other) carries a high emotional charge. Find out if there are certain rules that have become very hard to enforce, or if their child has been testing limits more. Ask if there has been a consequence enforced in one home, but not in another. Often simply providing a calm affirmation that increased limit testing is normal in children after a divorce is very reassuring for parents. Remind them that providing reasonably consistent rules and limits will be very helpful to their children during this period, the opposite of making them a “bad parent.”
Some divorced parents will become more rigid about rules, managing any infraction or extenuating circumstance more like a contract negotiation. These parents might benefit from a suggestion that consistency and simplicity are the keys to effective rules across two households. Rules also provide an opportunity to listen to their children’s thoughts and feelings and share the family’s values that are the basis for the rules. Parents should be curious about their children’s opinions and be ready to show thoughtful flexibility when rules become outdated or special circumstances exist.
You can suggest a rule the parents should follow. While they can talk honestly about what each parent may struggle with or acknowledge clear differences in style or personality, they should strive to never vilify the other parent. Even in circumstances in which it is very difficult for two parents to collaborate, sharing grievances with the children will only be painful and confusing for them.
Lastly, pediatricians can discuss the long-term goals that all parents, even those alienated from each other, share. Children will do best when they have a positive, honest, warm relationship with each parent, and do not carry responsibility for negotiating conflict between their parents. Ultimately, more autonomy and fewer rules will be an important part of the child’s adolescence. Discord between parents, sabotaging of rules and consequences, and explicit contempt for their children’s other parent all will lead to children feeling burdened, having lower self-esteem, and being at greater risk for serious problems in school, emotionally or with substances as they grow into adolescents and young adults. If you are frustrated in your effort to protect children from ongoing discord, suggest a referral to a mental health clinician with expertise helping parents after a divorce.
Dr. Swick is an attending psychiatrist in the division of child psychiatry at Massachusetts General Hospital, Boston, and director of the Parenting at a Challenging Time (PACT) Program at the Vernon Cancer Center at Newton Wellesley Hospital, also in Boston. Dr. Jellinek is professor of psychiatry and of pediatrics at Harvard Medical School, Boston. E-mail them at [email protected].
A major challenge faced by parents is the task of setting basic ground rules and expectations for their children, and then enforcing these with limits, rewards, and consequences. This task is made far more difficult when parents are separated or divorced. Agreeing upon and enforcing rules in separate homes often becomes burdened by the angry baggage that led to the divorce. When a family in your practice is going through a divorce, you have an opportunity to provide the parents with valuable strategies to manage rules effectively so that conflict is minimized.
Many happily married parents who communicate very well on most matters struggle to get on the same page when negotiating rules and limits. One parent’s sense of what is an appropriate bedtime, how children should help with chores, or even how often they can have sweets can become a deeply held belief and might be very different than their spouse’s opinions. Sometimes, a parent has old anger about how they were raised and finds it hard to distinguish what might have been better for them, compared with what is best for their own child. Cultural and family differences on how much choice children should have at different ages, criteria and severity of any consequences for misbehavior, and opportunities for redemption or amnesty all add complexity to the discussion. Once they have found common ground on what makes sense for their joint rules, values, and needs of their child, they have to manage enforcing rules and limits, agreeing upon appropriate rewards and punishments, and bearing the inevitable distress of their children when facing a limit or consequence. And, of course, once parents think they have it all figured out, their children react and grow, and they must reset the rules, expectations, and consequences.
When parents get separated or divorced, this process becomes considerably more difficult. Negotiating new rules or limits is very difficult when communication is hampered by conflict. Parental guilt about the divorce itself, anger at old hurts or disputes about money and custody, missing the child between visits, and remarriages all add baggage to the discussion of a reasonable bedtime or consequences for a poor grade at school. If the divorce required aggressive negotiation between lawyers, appointment of a guardian ad Litem to manage ongoing disputes involving the children, or a court case to reach resolution, the tensions between parents can be intense, enduring, and with no issue too small to add fuel to the arguments. Enforcing limits is much harder for a single parent than when there are two parents doing the enforcement. And divorced parents, already feeling guilty and insecure, are more likely to suspend rules or limits so that they don’t have to be the “bad parent.” For the child or children, the stress and disruptions that come with divorce can cause an increase in regressed or disrespectful behavior. While it can be a time when limits are increasingly tested, being reasonable and consistent in enforcing limits becomes more important, as it provides reassuring steadiness in the midst of turbulent change.
Let’s take the example of a 12-year-old coming home from school with poor grades. One parent may see the need for a tutor, but might be using that approach as part of a financial attack if the other parent has to pay for it. The other parent may want to limit the use of computer games or access to television until the grades go up. And one may expect movement from a D to a C average while the other may expect A’s, period. Is the poor grade based on lack of ability, effort, an attempt to get attention, a reaction to the divorce, or preoccupation with ongoing parental discord? What is the impact on the child if in one home there is a tutor and a C expectation, and in the other there is no tutor, no computer use, no TV... and these change every time the child moves from one home to the other? A child striving to overcome a poor grade needs calm, consistent, patient, and optimistic support, rather than managing the increased tension across two homes or feeling like the cause of increased conflict. Virtually any reasonable approach is better for the child than each parent doing something different as a reflection of ongoing tension. Pediatricians can be extraordinarily helpful to their patient if they can get divorced parents to agree on a single approach that is based on their child’s needs rather than past and ongoing angers. The emotional damage of ongoing discord is far worse than any C average.
As the pediatrician to a family managing divorce, you may be one of the few authority figures whom both parents and the children all still respect and trust. You are in a strong position to ask a parent during an appointment how rules and limits are being managed across two homes. Find out if they have a clear plan for handling routine communication about the children, whether about summer camps or a new curfew, so that they don’t default to communicating only once there is a crisis. See if rules are a vehicle for ongoing parental fighting so that a minor difference (an 8 o’clock bedtime in one house versus 9 o’clock in the other) carries a high emotional charge. Find out if there are certain rules that have become very hard to enforce, or if their child has been testing limits more. Ask if there has been a consequence enforced in one home, but not in another. Often simply providing a calm affirmation that increased limit testing is normal in children after a divorce is very reassuring for parents. Remind them that providing reasonably consistent rules and limits will be very helpful to their children during this period, the opposite of making them a “bad parent.”
Some divorced parents will become more rigid about rules, managing any infraction or extenuating circumstance more like a contract negotiation. These parents might benefit from a suggestion that consistency and simplicity are the keys to effective rules across two households. Rules also provide an opportunity to listen to their children’s thoughts and feelings and share the family’s values that are the basis for the rules. Parents should be curious about their children’s opinions and be ready to show thoughtful flexibility when rules become outdated or special circumstances exist.
You can suggest a rule the parents should follow. While they can talk honestly about what each parent may struggle with or acknowledge clear differences in style or personality, they should strive to never vilify the other parent. Even in circumstances in which it is very difficult for two parents to collaborate, sharing grievances with the children will only be painful and confusing for them.
Lastly, pediatricians can discuss the long-term goals that all parents, even those alienated from each other, share. Children will do best when they have a positive, honest, warm relationship with each parent, and do not carry responsibility for negotiating conflict between their parents. Ultimately, more autonomy and fewer rules will be an important part of the child’s adolescence. Discord between parents, sabotaging of rules and consequences, and explicit contempt for their children’s other parent all will lead to children feeling burdened, having lower self-esteem, and being at greater risk for serious problems in school, emotionally or with substances as they grow into adolescents and young adults. If you are frustrated in your effort to protect children from ongoing discord, suggest a referral to a mental health clinician with expertise helping parents after a divorce.
Dr. Swick is an attending psychiatrist in the division of child psychiatry at Massachusetts General Hospital, Boston, and director of the Parenting at a Challenging Time (PACT) Program at the Vernon Cancer Center at Newton Wellesley Hospital, also in Boston. Dr. Jellinek is professor of psychiatry and of pediatrics at Harvard Medical School, Boston. E-mail them at [email protected].
A major challenge faced by parents is the task of setting basic ground rules and expectations for their children, and then enforcing these with limits, rewards, and consequences. This task is made far more difficult when parents are separated or divorced. Agreeing upon and enforcing rules in separate homes often becomes burdened by the angry baggage that led to the divorce. When a family in your practice is going through a divorce, you have an opportunity to provide the parents with valuable strategies to manage rules effectively so that conflict is minimized.
Many happily married parents who communicate very well on most matters struggle to get on the same page when negotiating rules and limits. One parent’s sense of what is an appropriate bedtime, how children should help with chores, or even how often they can have sweets can become a deeply held belief and might be very different than their spouse’s opinions. Sometimes, a parent has old anger about how they were raised and finds it hard to distinguish what might have been better for them, compared with what is best for their own child. Cultural and family differences on how much choice children should have at different ages, criteria and severity of any consequences for misbehavior, and opportunities for redemption or amnesty all add complexity to the discussion. Once they have found common ground on what makes sense for their joint rules, values, and needs of their child, they have to manage enforcing rules and limits, agreeing upon appropriate rewards and punishments, and bearing the inevitable distress of their children when facing a limit or consequence. And, of course, once parents think they have it all figured out, their children react and grow, and they must reset the rules, expectations, and consequences.
When parents get separated or divorced, this process becomes considerably more difficult. Negotiating new rules or limits is very difficult when communication is hampered by conflict. Parental guilt about the divorce itself, anger at old hurts or disputes about money and custody, missing the child between visits, and remarriages all add baggage to the discussion of a reasonable bedtime or consequences for a poor grade at school. If the divorce required aggressive negotiation between lawyers, appointment of a guardian ad Litem to manage ongoing disputes involving the children, or a court case to reach resolution, the tensions between parents can be intense, enduring, and with no issue too small to add fuel to the arguments. Enforcing limits is much harder for a single parent than when there are two parents doing the enforcement. And divorced parents, already feeling guilty and insecure, are more likely to suspend rules or limits so that they don’t have to be the “bad parent.” For the child or children, the stress and disruptions that come with divorce can cause an increase in regressed or disrespectful behavior. While it can be a time when limits are increasingly tested, being reasonable and consistent in enforcing limits becomes more important, as it provides reassuring steadiness in the midst of turbulent change.
Let’s take the example of a 12-year-old coming home from school with poor grades. One parent may see the need for a tutor, but might be using that approach as part of a financial attack if the other parent has to pay for it. The other parent may want to limit the use of computer games or access to television until the grades go up. And one may expect movement from a D to a C average while the other may expect A’s, period. Is the poor grade based on lack of ability, effort, an attempt to get attention, a reaction to the divorce, or preoccupation with ongoing parental discord? What is the impact on the child if in one home there is a tutor and a C expectation, and in the other there is no tutor, no computer use, no TV... and these change every time the child moves from one home to the other? A child striving to overcome a poor grade needs calm, consistent, patient, and optimistic support, rather than managing the increased tension across two homes or feeling like the cause of increased conflict. Virtually any reasonable approach is better for the child than each parent doing something different as a reflection of ongoing tension. Pediatricians can be extraordinarily helpful to their patient if they can get divorced parents to agree on a single approach that is based on their child’s needs rather than past and ongoing angers. The emotional damage of ongoing discord is far worse than any C average.
As the pediatrician to a family managing divorce, you may be one of the few authority figures whom both parents and the children all still respect and trust. You are in a strong position to ask a parent during an appointment how rules and limits are being managed across two homes. Find out if they have a clear plan for handling routine communication about the children, whether about summer camps or a new curfew, so that they don’t default to communicating only once there is a crisis. See if rules are a vehicle for ongoing parental fighting so that a minor difference (an 8 o’clock bedtime in one house versus 9 o’clock in the other) carries a high emotional charge. Find out if there are certain rules that have become very hard to enforce, or if their child has been testing limits more. Ask if there has been a consequence enforced in one home, but not in another. Often simply providing a calm affirmation that increased limit testing is normal in children after a divorce is very reassuring for parents. Remind them that providing reasonably consistent rules and limits will be very helpful to their children during this period, the opposite of making them a “bad parent.”
Some divorced parents will become more rigid about rules, managing any infraction or extenuating circumstance more like a contract negotiation. These parents might benefit from a suggestion that consistency and simplicity are the keys to effective rules across two households. Rules also provide an opportunity to listen to their children’s thoughts and feelings and share the family’s values that are the basis for the rules. Parents should be curious about their children’s opinions and be ready to show thoughtful flexibility when rules become outdated or special circumstances exist.
You can suggest a rule the parents should follow. While they can talk honestly about what each parent may struggle with or acknowledge clear differences in style or personality, they should strive to never vilify the other parent. Even in circumstances in which it is very difficult for two parents to collaborate, sharing grievances with the children will only be painful and confusing for them.
Lastly, pediatricians can discuss the long-term goals that all parents, even those alienated from each other, share. Children will do best when they have a positive, honest, warm relationship with each parent, and do not carry responsibility for negotiating conflict between their parents. Ultimately, more autonomy and fewer rules will be an important part of the child’s adolescence. Discord between parents, sabotaging of rules and consequences, and explicit contempt for their children’s other parent all will lead to children feeling burdened, having lower self-esteem, and being at greater risk for serious problems in school, emotionally or with substances as they grow into adolescents and young adults. If you are frustrated in your effort to protect children from ongoing discord, suggest a referral to a mental health clinician with expertise helping parents after a divorce.
Dr. Swick is an attending psychiatrist in the division of child psychiatry at Massachusetts General Hospital, Boston, and director of the Parenting at a Challenging Time (PACT) Program at the Vernon Cancer Center at Newton Wellesley Hospital, also in Boston. Dr. Jellinek is professor of psychiatry and of pediatrics at Harvard Medical School, Boston. E-mail them at [email protected].
Is the wedding still on?
“So tell me, Kathy,” I asked as I walked in. “Is the wedding still on?”
“Yes!” she said.
I was kidding, of course. I wanted to defuse the tension every bride feels as her big day approaches. With her nuptials 2 months off, Kathy was here for an acne tune-up.
Good news: no new pimples. Though Kathy had stopped squeezing her old ones, their marks were fading slowly. Brides don’t want to depend on makeup or Photoshop.
Her current regimen was a topical antibiotic in the morning and a retinoid at night. The question was whether to add or change anything.
“Maybe we should consider adding an oral medicine to help speed healing?” I asked. Then I watched her eyes. Her frown gave me my answer.
“I’d prefer to avoid oral medications unless I absolutely need them,” Kathy said.
“No problem,” I said. “You’re doing well, and we still have 2 months for the marks you have to fade.” I arranged to see her again shortly before the wedding, for any last-minute adjustments.
Outside the exam room, I took my student aside. “That’s how you negotiate,” I told her.
“Some young women approach their weddings in a kind of panic. They want to do whatever it takes to speed healing. If Kathy had felt that way, and I told her things were fine as they were, she would have been upset. ‘Isn’t there something else we can do, maybe something to take by mouth?’ she’d have asked.
“Instead, Kathy felt the opposite,” I told my student. “When patients have a specific problem, you can make a shrewd guess about how aggressive they want to be in addressing it. But you can’t be sure. That means watching their eyes and body language when making suggestions.
“Of course, not every medical condition is negotiable. Sometimes, the matter is so urgent or dire that there really is only one thing to do. Then you have to be more direct. But many situations are not so clear cut. You and the patient will have choices. Which is best may depend less on the medical condition than on the patient’s mindset and circumstances.
“Your job is to know the options, watch their eyes, and negotiate,” I said.
My student nodded, probably noticing that this is not standard clinical advice. In school, they teach you to make the right diagnosis and prescribe the treatment of choice. Anything else would be substandard care, a dereliction of professional duty.
Nowadays, teachers – and insurers – go in for algorithms, cookbook medicine. If the patient has this, do this. If that, do that. “How do you treat acne?” students often ask at the start of their rotation. “Can you give me a decision tree?”
These days more and more doctors spend their visit time clicking tablets or laptops. If the patient has acne, they are checking off vital data points like:
• Are there pimples, pustules, whiteheads, blackheads, cysts?
• How many of each?
• Where they are – face, chest, back?
This information is supposed to objectively describe and grade the patient’s acne. You click what is important: what you can count and measure.
Here is what electronic medical records do not have you click off:
• Is the patient getting married soon?
• Is she afraid of oral antibiotics because she’s heard they wreck your immune system and make you sick?
• Have her friends recommended an acne cream they are sure is the best thing since sliced tretinoin?
They don’t make boxes for what goes on inside people’s brains. You can’t count or measure that, and if you can’t count it, it doesn’t count.
So doctors click what they tell us to. As we click the keyboard, we are not looking at the patient’s face. So we don’t know whether the patient is buying what we have to offer.
More medical treatment than we care to admit is – or should be – a process of negotiation. Negotiating means looking people in the eye and hearing what they say and the way they say it. That way you know not only what they have, but what they want. In Kathy’s case, that would be a wedding to remember.
As she proceeds in her career, my student may do more than counting pimples and grading acne. At any rate, I hope so.
Dr. Rockoff practices dermatology in Brookline, Mass., and is a longtime contributor to Dermatology News. He serves on the clinical faculty at Tufts University, Boston, and has taught senior medical students and other trainees for 30 years.
“So tell me, Kathy,” I asked as I walked in. “Is the wedding still on?”
“Yes!” she said.
I was kidding, of course. I wanted to defuse the tension every bride feels as her big day approaches. With her nuptials 2 months off, Kathy was here for an acne tune-up.
Good news: no new pimples. Though Kathy had stopped squeezing her old ones, their marks were fading slowly. Brides don’t want to depend on makeup or Photoshop.
Her current regimen was a topical antibiotic in the morning and a retinoid at night. The question was whether to add or change anything.
“Maybe we should consider adding an oral medicine to help speed healing?” I asked. Then I watched her eyes. Her frown gave me my answer.
“I’d prefer to avoid oral medications unless I absolutely need them,” Kathy said.
“No problem,” I said. “You’re doing well, and we still have 2 months for the marks you have to fade.” I arranged to see her again shortly before the wedding, for any last-minute adjustments.
Outside the exam room, I took my student aside. “That’s how you negotiate,” I told her.
“Some young women approach their weddings in a kind of panic. They want to do whatever it takes to speed healing. If Kathy had felt that way, and I told her things were fine as they were, she would have been upset. ‘Isn’t there something else we can do, maybe something to take by mouth?’ she’d have asked.
“Instead, Kathy felt the opposite,” I told my student. “When patients have a specific problem, you can make a shrewd guess about how aggressive they want to be in addressing it. But you can’t be sure. That means watching their eyes and body language when making suggestions.
“Of course, not every medical condition is negotiable. Sometimes, the matter is so urgent or dire that there really is only one thing to do. Then you have to be more direct. But many situations are not so clear cut. You and the patient will have choices. Which is best may depend less on the medical condition than on the patient’s mindset and circumstances.
“Your job is to know the options, watch their eyes, and negotiate,” I said.
My student nodded, probably noticing that this is not standard clinical advice. In school, they teach you to make the right diagnosis and prescribe the treatment of choice. Anything else would be substandard care, a dereliction of professional duty.
Nowadays, teachers – and insurers – go in for algorithms, cookbook medicine. If the patient has this, do this. If that, do that. “How do you treat acne?” students often ask at the start of their rotation. “Can you give me a decision tree?”
These days more and more doctors spend their visit time clicking tablets or laptops. If the patient has acne, they are checking off vital data points like:
• Are there pimples, pustules, whiteheads, blackheads, cysts?
• How many of each?
• Where they are – face, chest, back?
This information is supposed to objectively describe and grade the patient’s acne. You click what is important: what you can count and measure.
Here is what electronic medical records do not have you click off:
• Is the patient getting married soon?
• Is she afraid of oral antibiotics because she’s heard they wreck your immune system and make you sick?
• Have her friends recommended an acne cream they are sure is the best thing since sliced tretinoin?
They don’t make boxes for what goes on inside people’s brains. You can’t count or measure that, and if you can’t count it, it doesn’t count.
So doctors click what they tell us to. As we click the keyboard, we are not looking at the patient’s face. So we don’t know whether the patient is buying what we have to offer.
More medical treatment than we care to admit is – or should be – a process of negotiation. Negotiating means looking people in the eye and hearing what they say and the way they say it. That way you know not only what they have, but what they want. In Kathy’s case, that would be a wedding to remember.
As she proceeds in her career, my student may do more than counting pimples and grading acne. At any rate, I hope so.
Dr. Rockoff practices dermatology in Brookline, Mass., and is a longtime contributor to Dermatology News. He serves on the clinical faculty at Tufts University, Boston, and has taught senior medical students and other trainees for 30 years.
“So tell me, Kathy,” I asked as I walked in. “Is the wedding still on?”
“Yes!” she said.
I was kidding, of course. I wanted to defuse the tension every bride feels as her big day approaches. With her nuptials 2 months off, Kathy was here for an acne tune-up.
Good news: no new pimples. Though Kathy had stopped squeezing her old ones, their marks were fading slowly. Brides don’t want to depend on makeup or Photoshop.
Her current regimen was a topical antibiotic in the morning and a retinoid at night. The question was whether to add or change anything.
“Maybe we should consider adding an oral medicine to help speed healing?” I asked. Then I watched her eyes. Her frown gave me my answer.
“I’d prefer to avoid oral medications unless I absolutely need them,” Kathy said.
“No problem,” I said. “You’re doing well, and we still have 2 months for the marks you have to fade.” I arranged to see her again shortly before the wedding, for any last-minute adjustments.
Outside the exam room, I took my student aside. “That’s how you negotiate,” I told her.
“Some young women approach their weddings in a kind of panic. They want to do whatever it takes to speed healing. If Kathy had felt that way, and I told her things were fine as they were, she would have been upset. ‘Isn’t there something else we can do, maybe something to take by mouth?’ she’d have asked.
“Instead, Kathy felt the opposite,” I told my student. “When patients have a specific problem, you can make a shrewd guess about how aggressive they want to be in addressing it. But you can’t be sure. That means watching their eyes and body language when making suggestions.
“Of course, not every medical condition is negotiable. Sometimes, the matter is so urgent or dire that there really is only one thing to do. Then you have to be more direct. But many situations are not so clear cut. You and the patient will have choices. Which is best may depend less on the medical condition than on the patient’s mindset and circumstances.
“Your job is to know the options, watch their eyes, and negotiate,” I said.
My student nodded, probably noticing that this is not standard clinical advice. In school, they teach you to make the right diagnosis and prescribe the treatment of choice. Anything else would be substandard care, a dereliction of professional duty.
Nowadays, teachers – and insurers – go in for algorithms, cookbook medicine. If the patient has this, do this. If that, do that. “How do you treat acne?” students often ask at the start of their rotation. “Can you give me a decision tree?”
These days more and more doctors spend their visit time clicking tablets or laptops. If the patient has acne, they are checking off vital data points like:
• Are there pimples, pustules, whiteheads, blackheads, cysts?
• How many of each?
• Where they are – face, chest, back?
This information is supposed to objectively describe and grade the patient’s acne. You click what is important: what you can count and measure.
Here is what electronic medical records do not have you click off:
• Is the patient getting married soon?
• Is she afraid of oral antibiotics because she’s heard they wreck your immune system and make you sick?
• Have her friends recommended an acne cream they are sure is the best thing since sliced tretinoin?
They don’t make boxes for what goes on inside people’s brains. You can’t count or measure that, and if you can’t count it, it doesn’t count.
So doctors click what they tell us to. As we click the keyboard, we are not looking at the patient’s face. So we don’t know whether the patient is buying what we have to offer.
More medical treatment than we care to admit is – or should be – a process of negotiation. Negotiating means looking people in the eye and hearing what they say and the way they say it. That way you know not only what they have, but what they want. In Kathy’s case, that would be a wedding to remember.
As she proceeds in her career, my student may do more than counting pimples and grading acne. At any rate, I hope so.
Dr. Rockoff practices dermatology in Brookline, Mass., and is a longtime contributor to Dermatology News. He serves on the clinical faculty at Tufts University, Boston, and has taught senior medical students and other trainees for 30 years.
Plantago major
For centuries, the leaves of Plantago major have been used in most regions of the world in traditional medical treatment of wounds and various diseases, including cutaneous conditions (J. Ethnopharmacol. 2000;71:1-21). P. major, also known as broadleaf plantain or greater plantain, is a member of the Plantaginaceae family, which is now widely dispersed throughout the world, though native to much of Europe as well as northern and central Asia. The Norwegian and Swedish name for the plant, groblad, means “healing leaves” (J. Ethnopharmacol. 2000;71:1-21). It was brought to the Americas by Europeans during the colonial period. Native Americans referred to it as the “white man’s footprint,” which inspired the genus name Plantago from the Latin planta (foot) (J. Ethnopharmacol. 2000;71:1-21).
Among the biologically active constituents of P. major are polysaccharides, lipids, caffeic acid derivatives, flavonoids (apigenin, luteolin, scutellarin, baicalein, nepetin, hispidulin, plantagoside), iridoid glycosides (aucubin, catalpol), terpenoids, and alkaloids (J. Ethnopharmacol. 2000;71:1-21; Medical Herbalism: The Science and Practice of Herbal Medicine, 2003, Healing Arts Press). In an ethnopharmacologic and folk medicine survey study of 1,225 residents of the Atlantic coast of Colombia completed in 2011, Gómez-Estrada et al. found that P. major was one of the plants traditionally used to treat inflammation; it also was used to treat kidney pain and eye injuries (J. Ethnobiol. Ethnomed. 2011;7:27). P. major also is traditionally used as a mucilage and bulk laxative (Principles and Practice of Phytotherapy: Modern Herbal Medicine, 2013, Churchill Livingstone).
Extracts of the plant have been associated with myriad biologic activities, including wound healing, anti-inflammatory, antimicrobial, analgesic, antioxidant, immunomodulating, and antiulcerogenic action, which Samuelsen suggested may account for the use of the botanical in traditional medicine (J. Ethnopharmacol. 2000;71:1-21; Medical Herbalism: The Science and Practice of Herbal Medicine, 2003, Healing Arts Press). In fact, the range of biologic properties attributed to P. major also includes astringent, anesthetic, antihelminthic, analeptic, antihistaminic, antirheumatic, antiviral, antitumor, antiulcer, diuretic, hypotensive, and expectorant activity (Exp. Biol. Med. [Maywood] 2012;237:1379-86). Though not the most popular botanical for this indication, P. major is among the plants used in the treatment of cutaneous leishmanial ulcers in Bahia, Brazil, where Leishmania brazilenesis is endemic (Rev. Soc. Bras. Med. Trop. 1996;29:229-32). Other dermatologic uses in traditional medicine include eczema, cuts, hemorrhoids, ulcerations, and wounds (Medical Herbalism: The Science and Practice of Herbal Medicine, 2003, Healing Arts Press).
Wound healing
The use of P. major for wound healing dates back to the first century, as described by the Greek physician Dioscorides in “De Materia Medica” (J. Ethnopharmacol. 2000;71:1-21).
In 2011, Krasnov et al. developed an experimental model for characterizing proteins and showed that a newly discovered group of tissue-specific biogregulating proteins found previously in animal tissues was also present in P. major and responsible for the wound-healing activity associated with the plant (Prikl Biokhim. Mikrobiol. 2011;47:146-53).
The next year, Thomé et al. investigated and compared the wound-healing effects of P. major and Siparuna guianensis with a commercial product used in Brazil. Mice with cervical dorsal area wounds were treated with the botanical ingredients and the commercial product. Decreases in the wound area occurred earliest in mice treated with P. major, with complete closure (by day 15) seen only in this group. The investigators concluded that their findings support the traditional application of P. major, which shows potential as a viable wound-healing agent (Exp. Biol. Med. [Maywood] 2012;237:1379-86).
Conclusion
The numerous biologic properties of P. major are well established. In addition, use of the plant in traditional medicine for some cutaneous indications warrants consideration for modern therapeutic usage. Much more research is necessary, however, to elucidate the potential incorporation of this botanical into standard topical preparations for any of various skin conditions.
Dr. Baumann is chief executive officer of the Baumann Cosmetic & Research Institute in the Design District in Miami. She founded the Cosmetic Dermatology Center at the University of Miami in 1997. Dr. Baumann wrote the textbook “Cosmetic Dermatology: Principles and Practice” (New York: McGraw-Hill, 2002), and a book for consumers, “The Skin Type Solution” (New York: Bantam Dell, 2006). She has contributed to the Cosmeceutical Critique column in Dermatology News since January 2001. Her latest book, “Cosmeceuticals and Cosmetic Ingredients,” was published in November 2014. Dr. Baumann has received funding for clinical grants from Allergan, Aveeno, Avon Products, Evolus, Galderma, GlaxoSmithKline, Kythera Biopharmaceuticals, Mary Kay, Medicis Pharmaceuticals, Neutrogena, Philosophy, Topix Pharmaceuticals, and Unilever.
For centuries, the leaves of Plantago major have been used in most regions of the world in traditional medical treatment of wounds and various diseases, including cutaneous conditions (J. Ethnopharmacol. 2000;71:1-21). P. major, also known as broadleaf plantain or greater plantain, is a member of the Plantaginaceae family, which is now widely dispersed throughout the world, though native to much of Europe as well as northern and central Asia. The Norwegian and Swedish name for the plant, groblad, means “healing leaves” (J. Ethnopharmacol. 2000;71:1-21). It was brought to the Americas by Europeans during the colonial period. Native Americans referred to it as the “white man’s footprint,” which inspired the genus name Plantago from the Latin planta (foot) (J. Ethnopharmacol. 2000;71:1-21).
Among the biologically active constituents of P. major are polysaccharides, lipids, caffeic acid derivatives, flavonoids (apigenin, luteolin, scutellarin, baicalein, nepetin, hispidulin, plantagoside), iridoid glycosides (aucubin, catalpol), terpenoids, and alkaloids (J. Ethnopharmacol. 2000;71:1-21; Medical Herbalism: The Science and Practice of Herbal Medicine, 2003, Healing Arts Press). In an ethnopharmacologic and folk medicine survey study of 1,225 residents of the Atlantic coast of Colombia completed in 2011, Gómez-Estrada et al. found that P. major was one of the plants traditionally used to treat inflammation; it also was used to treat kidney pain and eye injuries (J. Ethnobiol. Ethnomed. 2011;7:27). P. major also is traditionally used as a mucilage and bulk laxative (Principles and Practice of Phytotherapy: Modern Herbal Medicine, 2013, Churchill Livingstone).
Extracts of the plant have been associated with myriad biologic activities, including wound healing, anti-inflammatory, antimicrobial, analgesic, antioxidant, immunomodulating, and antiulcerogenic action, which Samuelsen suggested may account for the use of the botanical in traditional medicine (J. Ethnopharmacol. 2000;71:1-21; Medical Herbalism: The Science and Practice of Herbal Medicine, 2003, Healing Arts Press). In fact, the range of biologic properties attributed to P. major also includes astringent, anesthetic, antihelminthic, analeptic, antihistaminic, antirheumatic, antiviral, antitumor, antiulcer, diuretic, hypotensive, and expectorant activity (Exp. Biol. Med. [Maywood] 2012;237:1379-86). Though not the most popular botanical for this indication, P. major is among the plants used in the treatment of cutaneous leishmanial ulcers in Bahia, Brazil, where Leishmania brazilenesis is endemic (Rev. Soc. Bras. Med. Trop. 1996;29:229-32). Other dermatologic uses in traditional medicine include eczema, cuts, hemorrhoids, ulcerations, and wounds (Medical Herbalism: The Science and Practice of Herbal Medicine, 2003, Healing Arts Press).
Wound healing
The use of P. major for wound healing dates back to the first century, as described by the Greek physician Dioscorides in “De Materia Medica” (J. Ethnopharmacol. 2000;71:1-21).
In 2011, Krasnov et al. developed an experimental model for characterizing proteins and showed that a newly discovered group of tissue-specific biogregulating proteins found previously in animal tissues was also present in P. major and responsible for the wound-healing activity associated with the plant (Prikl Biokhim. Mikrobiol. 2011;47:146-53).
The next year, Thomé et al. investigated and compared the wound-healing effects of P. major and Siparuna guianensis with a commercial product used in Brazil. Mice with cervical dorsal area wounds were treated with the botanical ingredients and the commercial product. Decreases in the wound area occurred earliest in mice treated with P. major, with complete closure (by day 15) seen only in this group. The investigators concluded that their findings support the traditional application of P. major, which shows potential as a viable wound-healing agent (Exp. Biol. Med. [Maywood] 2012;237:1379-86).
Conclusion
The numerous biologic properties of P. major are well established. In addition, use of the plant in traditional medicine for some cutaneous indications warrants consideration for modern therapeutic usage. Much more research is necessary, however, to elucidate the potential incorporation of this botanical into standard topical preparations for any of various skin conditions.
Dr. Baumann is chief executive officer of the Baumann Cosmetic & Research Institute in the Design District in Miami. She founded the Cosmetic Dermatology Center at the University of Miami in 1997. Dr. Baumann wrote the textbook “Cosmetic Dermatology: Principles and Practice” (New York: McGraw-Hill, 2002), and a book for consumers, “The Skin Type Solution” (New York: Bantam Dell, 2006). She has contributed to the Cosmeceutical Critique column in Dermatology News since January 2001. Her latest book, “Cosmeceuticals and Cosmetic Ingredients,” was published in November 2014. Dr. Baumann has received funding for clinical grants from Allergan, Aveeno, Avon Products, Evolus, Galderma, GlaxoSmithKline, Kythera Biopharmaceuticals, Mary Kay, Medicis Pharmaceuticals, Neutrogena, Philosophy, Topix Pharmaceuticals, and Unilever.
For centuries, the leaves of Plantago major have been used in most regions of the world in traditional medical treatment of wounds and various diseases, including cutaneous conditions (J. Ethnopharmacol. 2000;71:1-21). P. major, also known as broadleaf plantain or greater plantain, is a member of the Plantaginaceae family, which is now widely dispersed throughout the world, though native to much of Europe as well as northern and central Asia. The Norwegian and Swedish name for the plant, groblad, means “healing leaves” (J. Ethnopharmacol. 2000;71:1-21). It was brought to the Americas by Europeans during the colonial period. Native Americans referred to it as the “white man’s footprint,” which inspired the genus name Plantago from the Latin planta (foot) (J. Ethnopharmacol. 2000;71:1-21).
Among the biologically active constituents of P. major are polysaccharides, lipids, caffeic acid derivatives, flavonoids (apigenin, luteolin, scutellarin, baicalein, nepetin, hispidulin, plantagoside), iridoid glycosides (aucubin, catalpol), terpenoids, and alkaloids (J. Ethnopharmacol. 2000;71:1-21; Medical Herbalism: The Science and Practice of Herbal Medicine, 2003, Healing Arts Press). In an ethnopharmacologic and folk medicine survey study of 1,225 residents of the Atlantic coast of Colombia completed in 2011, Gómez-Estrada et al. found that P. major was one of the plants traditionally used to treat inflammation; it also was used to treat kidney pain and eye injuries (J. Ethnobiol. Ethnomed. 2011;7:27). P. major also is traditionally used as a mucilage and bulk laxative (Principles and Practice of Phytotherapy: Modern Herbal Medicine, 2013, Churchill Livingstone).
Extracts of the plant have been associated with myriad biologic activities, including wound healing, anti-inflammatory, antimicrobial, analgesic, antioxidant, immunomodulating, and antiulcerogenic action, which Samuelsen suggested may account for the use of the botanical in traditional medicine (J. Ethnopharmacol. 2000;71:1-21; Medical Herbalism: The Science and Practice of Herbal Medicine, 2003, Healing Arts Press). In fact, the range of biologic properties attributed to P. major also includes astringent, anesthetic, antihelminthic, analeptic, antihistaminic, antirheumatic, antiviral, antitumor, antiulcer, diuretic, hypotensive, and expectorant activity (Exp. Biol. Med. [Maywood] 2012;237:1379-86). Though not the most popular botanical for this indication, P. major is among the plants used in the treatment of cutaneous leishmanial ulcers in Bahia, Brazil, where Leishmania brazilenesis is endemic (Rev. Soc. Bras. Med. Trop. 1996;29:229-32). Other dermatologic uses in traditional medicine include eczema, cuts, hemorrhoids, ulcerations, and wounds (Medical Herbalism: The Science and Practice of Herbal Medicine, 2003, Healing Arts Press).
Wound healing
The use of P. major for wound healing dates back to the first century, as described by the Greek physician Dioscorides in “De Materia Medica” (J. Ethnopharmacol. 2000;71:1-21).
In 2011, Krasnov et al. developed an experimental model for characterizing proteins and showed that a newly discovered group of tissue-specific biogregulating proteins found previously in animal tissues was also present in P. major and responsible for the wound-healing activity associated with the plant (Prikl Biokhim. Mikrobiol. 2011;47:146-53).
The next year, Thomé et al. investigated and compared the wound-healing effects of P. major and Siparuna guianensis with a commercial product used in Brazil. Mice with cervical dorsal area wounds were treated with the botanical ingredients and the commercial product. Decreases in the wound area occurred earliest in mice treated with P. major, with complete closure (by day 15) seen only in this group. The investigators concluded that their findings support the traditional application of P. major, which shows potential as a viable wound-healing agent (Exp. Biol. Med. [Maywood] 2012;237:1379-86).
Conclusion
The numerous biologic properties of P. major are well established. In addition, use of the plant in traditional medicine for some cutaneous indications warrants consideration for modern therapeutic usage. Much more research is necessary, however, to elucidate the potential incorporation of this botanical into standard topical preparations for any of various skin conditions.
Dr. Baumann is chief executive officer of the Baumann Cosmetic & Research Institute in the Design District in Miami. She founded the Cosmetic Dermatology Center at the University of Miami in 1997. Dr. Baumann wrote the textbook “Cosmetic Dermatology: Principles and Practice” (New York: McGraw-Hill, 2002), and a book for consumers, “The Skin Type Solution” (New York: Bantam Dell, 2006). She has contributed to the Cosmeceutical Critique column in Dermatology News since January 2001. Her latest book, “Cosmeceuticals and Cosmetic Ingredients,” was published in November 2014. Dr. Baumann has received funding for clinical grants from Allergan, Aveeno, Avon Products, Evolus, Galderma, GlaxoSmithKline, Kythera Biopharmaceuticals, Mary Kay, Medicis Pharmaceuticals, Neutrogena, Philosophy, Topix Pharmaceuticals, and Unilever.
DIAGNOSING CTD IN CLINICAL PRACTICE
This educational video is sponsored by Exagen Diagnostics, Inc.
For more information, please read the supplement below.
This educational supplement to Rheumatology News is supported by Exagen Diagnostics, Inc.
Nimesh Dayal, MD
Arthritis Center of Orlando
Ocoee, FL
John Goldman, MD, MACR, FACP, CCD
Chief of Rheumatology
Emory St. Joseph’s Hospital
Atlanta, GA
Richard Haddad, MD, FACR
Clinical Assistant Professor of Medicine
Rutgers/Robert Wood Johnson
Red Bank, NJ
Patricia Hopkins, MD
Private Practice
Quincy, MA
James Mossell, DO
Tift Regional Medical Center
Tifton, GA
This educational video is sponsored by Exagen Diagnostics, Inc.
For more information, please read the supplement below.
This educational supplement to Rheumatology News is supported by Exagen Diagnostics, Inc.
Nimesh Dayal, MD
Arthritis Center of Orlando
Ocoee, FL
John Goldman, MD, MACR, FACP, CCD
Chief of Rheumatology
Emory St. Joseph’s Hospital
Atlanta, GA
Richard Haddad, MD, FACR
Clinical Assistant Professor of Medicine
Rutgers/Robert Wood Johnson
Red Bank, NJ
Patricia Hopkins, MD
Private Practice
Quincy, MA
James Mossell, DO
Tift Regional Medical Center
Tifton, GA
This educational video is sponsored by Exagen Diagnostics, Inc.
For more information, please read the supplement below.
This educational supplement to Rheumatology News is supported by Exagen Diagnostics, Inc.
Nimesh Dayal, MD
Arthritis Center of Orlando
Ocoee, FL
John Goldman, MD, MACR, FACP, CCD
Chief of Rheumatology
Emory St. Joseph’s Hospital
Atlanta, GA
Richard Haddad, MD, FACR
Clinical Assistant Professor of Medicine
Rutgers/Robert Wood Johnson
Red Bank, NJ
Patricia Hopkins, MD
Private Practice
Quincy, MA
James Mossell, DO
Tift Regional Medical Center
Tifton, GA
A new treatment approach for ALK- ALCL?

Investigators say they have uncovered a new approach for treating ALK-negative anaplastic large cell lymphoma (ALCL).
Results of genomic analyses indicated that many cases of ALK-negative ALCL may be driven by alterations in the JAK/STAT3 pathway, and in vivo
experiments showed the disease can be inhibited by compounds that target this pathway.
The investigators believe these compounds could be more effective than current therapies.
“Current therapies for this form of lymphoma fail to work in the majority of cases,” said Raul Rabadan, PhD, of Columbia University in New York, New York.
“However, now that we know the mutations that drive a significant percentage of cases, we can envision a new, personalized genomic approach to the treatment of ALK-negative ALCL.”
Dr Rabadan and his colleagues recounted their discovery of the mutations in Cancer Cell.
The team had sequenced the exomes and RNA of cancer cells from 155 patients with ALCL and 74 control subjects with other types of lymphoma.
Results revealed mutations in either JAK1 or STAT3 in about 20% of the 88 patients with ALK-negative ALCL. Of that 20%, 38% had mutations in both genes.
The investigators also detected the presence of several novel gene fusions (NFκB2-ROS1, NFκB2-TYK2, NCOC2-ROS1, and PABPC4-TYK2), some of which appear to activate the JAK/STAT3 pathway (NFκB2-ROS1 and NFκB2-TYK2).
Patients with these fusions did not have JAK1 or STAT3 mutations, which suggests the fusions are an independent cause of ALK-negative ALCL.
To confirm whether JAK1 and STAT3 mutations can cause ALK-negative ALCL, the investigators induced these mutations in normal human cells. The mutations did, in fact, lead to diseased cells.
Finally, the team tested JAK/STAT3 pathway inhibitors—ruxolitinib and PUH71—in mouse models of ALK-negative ALCL. And they found that both drugs significantly inhibited tumor growth.
“Our findings demonstrate that drugs targeting the JAK/STAT3 pathway offer a viable therapeutic strategy in a subset of patients with ALCL,” Dr Rabadan said.
“A couple of JAK/STAT3 inhibitors have been approved by the FDA for the treatment of psoriasis and rheumatoid arthritis, and several more are currently in clinical trials. These could be tested in patients whose genetic profile matches those we identified in our study.” ![]()

Investigators say they have uncovered a new approach for treating ALK-negative anaplastic large cell lymphoma (ALCL).
Results of genomic analyses indicated that many cases of ALK-negative ALCL may be driven by alterations in the JAK/STAT3 pathway, and in vivo
experiments showed the disease can be inhibited by compounds that target this pathway.
The investigators believe these compounds could be more effective than current therapies.
“Current therapies for this form of lymphoma fail to work in the majority of cases,” said Raul Rabadan, PhD, of Columbia University in New York, New York.
“However, now that we know the mutations that drive a significant percentage of cases, we can envision a new, personalized genomic approach to the treatment of ALK-negative ALCL.”
Dr Rabadan and his colleagues recounted their discovery of the mutations in Cancer Cell.
The team had sequenced the exomes and RNA of cancer cells from 155 patients with ALCL and 74 control subjects with other types of lymphoma.
Results revealed mutations in either JAK1 or STAT3 in about 20% of the 88 patients with ALK-negative ALCL. Of that 20%, 38% had mutations in both genes.
The investigators also detected the presence of several novel gene fusions (NFκB2-ROS1, NFκB2-TYK2, NCOC2-ROS1, and PABPC4-TYK2), some of which appear to activate the JAK/STAT3 pathway (NFκB2-ROS1 and NFκB2-TYK2).
Patients with these fusions did not have JAK1 or STAT3 mutations, which suggests the fusions are an independent cause of ALK-negative ALCL.
To confirm whether JAK1 and STAT3 mutations can cause ALK-negative ALCL, the investigators induced these mutations in normal human cells. The mutations did, in fact, lead to diseased cells.
Finally, the team tested JAK/STAT3 pathway inhibitors—ruxolitinib and PUH71—in mouse models of ALK-negative ALCL. And they found that both drugs significantly inhibited tumor growth.
“Our findings demonstrate that drugs targeting the JAK/STAT3 pathway offer a viable therapeutic strategy in a subset of patients with ALCL,” Dr Rabadan said.
“A couple of JAK/STAT3 inhibitors have been approved by the FDA for the treatment of psoriasis and rheumatoid arthritis, and several more are currently in clinical trials. These could be tested in patients whose genetic profile matches those we identified in our study.” ![]()

Investigators say they have uncovered a new approach for treating ALK-negative anaplastic large cell lymphoma (ALCL).
Results of genomic analyses indicated that many cases of ALK-negative ALCL may be driven by alterations in the JAK/STAT3 pathway, and in vivo
experiments showed the disease can be inhibited by compounds that target this pathway.
The investigators believe these compounds could be more effective than current therapies.
“Current therapies for this form of lymphoma fail to work in the majority of cases,” said Raul Rabadan, PhD, of Columbia University in New York, New York.
“However, now that we know the mutations that drive a significant percentage of cases, we can envision a new, personalized genomic approach to the treatment of ALK-negative ALCL.”
Dr Rabadan and his colleagues recounted their discovery of the mutations in Cancer Cell.
The team had sequenced the exomes and RNA of cancer cells from 155 patients with ALCL and 74 control subjects with other types of lymphoma.
Results revealed mutations in either JAK1 or STAT3 in about 20% of the 88 patients with ALK-negative ALCL. Of that 20%, 38% had mutations in both genes.
The investigators also detected the presence of several novel gene fusions (NFκB2-ROS1, NFκB2-TYK2, NCOC2-ROS1, and PABPC4-TYK2), some of which appear to activate the JAK/STAT3 pathway (NFκB2-ROS1 and NFκB2-TYK2).
Patients with these fusions did not have JAK1 or STAT3 mutations, which suggests the fusions are an independent cause of ALK-negative ALCL.
To confirm whether JAK1 and STAT3 mutations can cause ALK-negative ALCL, the investigators induced these mutations in normal human cells. The mutations did, in fact, lead to diseased cells.
Finally, the team tested JAK/STAT3 pathway inhibitors—ruxolitinib and PUH71—in mouse models of ALK-negative ALCL. And they found that both drugs significantly inhibited tumor growth.
“Our findings demonstrate that drugs targeting the JAK/STAT3 pathway offer a viable therapeutic strategy in a subset of patients with ALCL,” Dr Rabadan said.
“A couple of JAK/STAT3 inhibitors have been approved by the FDA for the treatment of psoriasis and rheumatoid arthritis, and several more are currently in clinical trials. These could be tested in patients whose genetic profile matches those we identified in our study.” ![]()
Combo improves PFS in untreated CLL

Results of a phase 3 study suggest that adding ofatumumab to chlorambucil can improve progression-free survival (PFS) in treatment-naïve patients with chronic lymphocytic leukemia (CLL).
Ofatumumab plus chlorambucil improved the median PFS by 71% compared to chlorambucil alone.
The combination also improved the overall response rate, duration of response, and time to next treatment.
However, patients in the combination arm had a higher rate of grade 3 or greater adverse events (AEs).
Researchers reported these results in The Lancet. The study, known as COMPLEMENT 1, was funded by GlaxoSmithKline and Genmab A/S.
The study included 447 patients with previously untreated CLL for whom fludarabine-based therapy was considered inappropriate. Patients were randomized to treatment with up to 12 cycles of ofatumumab in combination with chlorambucil (n=221) or up to 12 cycles of chlorambucil alone (n=226).
The study’s primary endpoint was the median PFS, which was 22.4 months in the combination arm and 13.1 months in the chlorambucil arm (hazard ratio [HR]=0.57, P<0.0001). This improvement in PFS was observed in most subgroups, irrespective of age, gender, disease stage, and prognostic factors.
As for secondary endpoints, patients in the combination arm had a higher overall response rate than patients in the chlorambucil arm—82% and 69%, respectively (odds ratio=2.16, P=0.001).
And combination treatment increased the duration of response compared to chlorambucil alone—22.1 months and 13.2 months, respectively (HR=0.56, P<0.001).
Patients in the combination arm also experienced a significantly longer time to next therapy compared to the chlorambucil arm—39.8 months and 24.7 months, respectively (HR=0.49, P<0.0001).
Safety data
The most common AEs (occurring in at least 2% of patients) were neutropenia, thrombocytopenia, anemia, infections, and infusion-related reactions.
Neutropenia occurred more frequently in the combination arm (27% vs 18%), as did infusion-related reactions (67% vs 0%) and infections (46% vs 42%). But thrombocytopenia and anemia were more frequent in the chlorambucil arm (26% vs 14% and 13% vs 9%, respectively).
The incidence of grade 3 or greater AEs was higher in the combination arm than the chlorambucil arm—50% and 43%, respectively.
Grade 3/4 infusion-related reactions occurred in 10% of patients in the combination arm, leading to drug withdrawal in 3% of patients and hospitalization in 2% of patients. No fatal infusion-related reactions were reported.
The most common infections were respiratory tract infections, with an incidence of 27% in the combination arm and 31% in the chlorambucil arm. There were similar frequencies of sepsis (3% and 2%, respectively) and opportunistic infections between the arms (4% and 5%, respectively).
The incidence of AEs leading to treatment withdrawal was 13% in both arms. And the incidence of death during treatment or within 60 days after the last dose was 3% in both arms.
These data formed the basis for regulatory approvals of ofatumumab (Arzerra) in the US and European Union, as well as the recent inclusion of ofatumumab plus chlorambucil in the National Comprehensive Cancer Network treatment guidelines. ![]()

Results of a phase 3 study suggest that adding ofatumumab to chlorambucil can improve progression-free survival (PFS) in treatment-naïve patients with chronic lymphocytic leukemia (CLL).
Ofatumumab plus chlorambucil improved the median PFS by 71% compared to chlorambucil alone.
The combination also improved the overall response rate, duration of response, and time to next treatment.
However, patients in the combination arm had a higher rate of grade 3 or greater adverse events (AEs).
Researchers reported these results in The Lancet. The study, known as COMPLEMENT 1, was funded by GlaxoSmithKline and Genmab A/S.
The study included 447 patients with previously untreated CLL for whom fludarabine-based therapy was considered inappropriate. Patients were randomized to treatment with up to 12 cycles of ofatumumab in combination with chlorambucil (n=221) or up to 12 cycles of chlorambucil alone (n=226).
The study’s primary endpoint was the median PFS, which was 22.4 months in the combination arm and 13.1 months in the chlorambucil arm (hazard ratio [HR]=0.57, P<0.0001). This improvement in PFS was observed in most subgroups, irrespective of age, gender, disease stage, and prognostic factors.
As for secondary endpoints, patients in the combination arm had a higher overall response rate than patients in the chlorambucil arm—82% and 69%, respectively (odds ratio=2.16, P=0.001).
And combination treatment increased the duration of response compared to chlorambucil alone—22.1 months and 13.2 months, respectively (HR=0.56, P<0.001).
Patients in the combination arm also experienced a significantly longer time to next therapy compared to the chlorambucil arm—39.8 months and 24.7 months, respectively (HR=0.49, P<0.0001).
Safety data
The most common AEs (occurring in at least 2% of patients) were neutropenia, thrombocytopenia, anemia, infections, and infusion-related reactions.
Neutropenia occurred more frequently in the combination arm (27% vs 18%), as did infusion-related reactions (67% vs 0%) and infections (46% vs 42%). But thrombocytopenia and anemia were more frequent in the chlorambucil arm (26% vs 14% and 13% vs 9%, respectively).
The incidence of grade 3 or greater AEs was higher in the combination arm than the chlorambucil arm—50% and 43%, respectively.
Grade 3/4 infusion-related reactions occurred in 10% of patients in the combination arm, leading to drug withdrawal in 3% of patients and hospitalization in 2% of patients. No fatal infusion-related reactions were reported.
The most common infections were respiratory tract infections, with an incidence of 27% in the combination arm and 31% in the chlorambucil arm. There were similar frequencies of sepsis (3% and 2%, respectively) and opportunistic infections between the arms (4% and 5%, respectively).
The incidence of AEs leading to treatment withdrawal was 13% in both arms. And the incidence of death during treatment or within 60 days after the last dose was 3% in both arms.
These data formed the basis for regulatory approvals of ofatumumab (Arzerra) in the US and European Union, as well as the recent inclusion of ofatumumab plus chlorambucil in the National Comprehensive Cancer Network treatment guidelines. ![]()

Results of a phase 3 study suggest that adding ofatumumab to chlorambucil can improve progression-free survival (PFS) in treatment-naïve patients with chronic lymphocytic leukemia (CLL).
Ofatumumab plus chlorambucil improved the median PFS by 71% compared to chlorambucil alone.
The combination also improved the overall response rate, duration of response, and time to next treatment.
However, patients in the combination arm had a higher rate of grade 3 or greater adverse events (AEs).
Researchers reported these results in The Lancet. The study, known as COMPLEMENT 1, was funded by GlaxoSmithKline and Genmab A/S.
The study included 447 patients with previously untreated CLL for whom fludarabine-based therapy was considered inappropriate. Patients were randomized to treatment with up to 12 cycles of ofatumumab in combination with chlorambucil (n=221) or up to 12 cycles of chlorambucil alone (n=226).
The study’s primary endpoint was the median PFS, which was 22.4 months in the combination arm and 13.1 months in the chlorambucil arm (hazard ratio [HR]=0.57, P<0.0001). This improvement in PFS was observed in most subgroups, irrespective of age, gender, disease stage, and prognostic factors.
As for secondary endpoints, patients in the combination arm had a higher overall response rate than patients in the chlorambucil arm—82% and 69%, respectively (odds ratio=2.16, P=0.001).
And combination treatment increased the duration of response compared to chlorambucil alone—22.1 months and 13.2 months, respectively (HR=0.56, P<0.001).
Patients in the combination arm also experienced a significantly longer time to next therapy compared to the chlorambucil arm—39.8 months and 24.7 months, respectively (HR=0.49, P<0.0001).
Safety data
The most common AEs (occurring in at least 2% of patients) were neutropenia, thrombocytopenia, anemia, infections, and infusion-related reactions.
Neutropenia occurred more frequently in the combination arm (27% vs 18%), as did infusion-related reactions (67% vs 0%) and infections (46% vs 42%). But thrombocytopenia and anemia were more frequent in the chlorambucil arm (26% vs 14% and 13% vs 9%, respectively).
The incidence of grade 3 or greater AEs was higher in the combination arm than the chlorambucil arm—50% and 43%, respectively.
Grade 3/4 infusion-related reactions occurred in 10% of patients in the combination arm, leading to drug withdrawal in 3% of patients and hospitalization in 2% of patients. No fatal infusion-related reactions were reported.
The most common infections were respiratory tract infections, with an incidence of 27% in the combination arm and 31% in the chlorambucil arm. There were similar frequencies of sepsis (3% and 2%, respectively) and opportunistic infections between the arms (4% and 5%, respectively).
The incidence of AEs leading to treatment withdrawal was 13% in both arms. And the incidence of death during treatment or within 60 days after the last dose was 3% in both arms.
These data formed the basis for regulatory approvals of ofatumumab (Arzerra) in the US and European Union, as well as the recent inclusion of ofatumumab plus chlorambucil in the National Comprehensive Cancer Network treatment guidelines. ![]()
Study illustrates challenges of parsing genetic data

Photo courtesy of NIGMS
A study of genes associated with bleeding disorders suggests it can be challenging to gauge the clinical significance of novel gene variants.
Researchers analyzed genomic data from more than 16,000 subjects, looking for missense variants in the integrin aIIbß3 receptor subunit genes ITGA2B and ITGB3.
Although the team identified close to 200 novel variants in these genes, they found it difficult to determine the functional impact of the variants.
Barry S. Coller, MD, of The Rockefeller University in New York, New York, and his colleagues described this research in PNAS.
The team analyzed genomic data from 16,108 people and found that about 1.3% of the individuals had missense variants in ITGA2B, ITGB3, or both genes.
To determine which of these missense variants were newly discovered, the researchers looked for 111 variants that were previously reported as being associated with Glanzmann thrombasthenia, 20 variants that were associated with alloimmune thrombocytopenia, and 5 variants that were associated with aniso/macrothrombocytopenia.
The results suggested the team had discovered 114 novel missense variants in ITGA2B and 68 novel variants in ITGB3. And they did not find any of the previously reported variants in the 2 genes.
“This means the disease-causing mutations previously reported are very rare and, thus, probably first appeared relatively recently—that is, in the past several hundred years,” Dr Coller said.
The researchers then used 3 different algorithms to try to predict the proportion of the missense variants that would likely have a negative impact on a person’s health by causing excess bleeding.
They got a wide range of results. Depending on the algorithm and other variables, between 27% and 71% of variants were predicted to be harmful.
To test the validity of the predictions, the team took a closer look at 3 of the variants that affect amino acids previously associated with Glanzmann thrombasthenia.
They found that 2 of the variants—aIIb P176H and ß3 C547G—severely reduced aIIbß3 expression. The third variant—aIIb P943A—partially reduced aIIbß3 expression but had no effect on fibrinogen binding.
These results show how challenging it can be to interpret genetic data, Dr Coller said.
“Some variants of these 2 genes will likely be obviously deleterious, but it may be impossible to predict whether others are deleterious,” he noted. “In those cases, we will need to use additional information to judge the likelihood of a mutation being deleterious, and, in many cases, there will be residual uncertainty.” ![]()

Photo courtesy of NIGMS
A study of genes associated with bleeding disorders suggests it can be challenging to gauge the clinical significance of novel gene variants.
Researchers analyzed genomic data from more than 16,000 subjects, looking for missense variants in the integrin aIIbß3 receptor subunit genes ITGA2B and ITGB3.
Although the team identified close to 200 novel variants in these genes, they found it difficult to determine the functional impact of the variants.
Barry S. Coller, MD, of The Rockefeller University in New York, New York, and his colleagues described this research in PNAS.
The team analyzed genomic data from 16,108 people and found that about 1.3% of the individuals had missense variants in ITGA2B, ITGB3, or both genes.
To determine which of these missense variants were newly discovered, the researchers looked for 111 variants that were previously reported as being associated with Glanzmann thrombasthenia, 20 variants that were associated with alloimmune thrombocytopenia, and 5 variants that were associated with aniso/macrothrombocytopenia.
The results suggested the team had discovered 114 novel missense variants in ITGA2B and 68 novel variants in ITGB3. And they did not find any of the previously reported variants in the 2 genes.
“This means the disease-causing mutations previously reported are very rare and, thus, probably first appeared relatively recently—that is, in the past several hundred years,” Dr Coller said.
The researchers then used 3 different algorithms to try to predict the proportion of the missense variants that would likely have a negative impact on a person’s health by causing excess bleeding.
They got a wide range of results. Depending on the algorithm and other variables, between 27% and 71% of variants were predicted to be harmful.
To test the validity of the predictions, the team took a closer look at 3 of the variants that affect amino acids previously associated with Glanzmann thrombasthenia.
They found that 2 of the variants—aIIb P176H and ß3 C547G—severely reduced aIIbß3 expression. The third variant—aIIb P943A—partially reduced aIIbß3 expression but had no effect on fibrinogen binding.
These results show how challenging it can be to interpret genetic data, Dr Coller said.
“Some variants of these 2 genes will likely be obviously deleterious, but it may be impossible to predict whether others are deleterious,” he noted. “In those cases, we will need to use additional information to judge the likelihood of a mutation being deleterious, and, in many cases, there will be residual uncertainty.” ![]()

Photo courtesy of NIGMS
A study of genes associated with bleeding disorders suggests it can be challenging to gauge the clinical significance of novel gene variants.
Researchers analyzed genomic data from more than 16,000 subjects, looking for missense variants in the integrin aIIbß3 receptor subunit genes ITGA2B and ITGB3.
Although the team identified close to 200 novel variants in these genes, they found it difficult to determine the functional impact of the variants.
Barry S. Coller, MD, of The Rockefeller University in New York, New York, and his colleagues described this research in PNAS.
The team analyzed genomic data from 16,108 people and found that about 1.3% of the individuals had missense variants in ITGA2B, ITGB3, or both genes.
To determine which of these missense variants were newly discovered, the researchers looked for 111 variants that were previously reported as being associated with Glanzmann thrombasthenia, 20 variants that were associated with alloimmune thrombocytopenia, and 5 variants that were associated with aniso/macrothrombocytopenia.
The results suggested the team had discovered 114 novel missense variants in ITGA2B and 68 novel variants in ITGB3. And they did not find any of the previously reported variants in the 2 genes.
“This means the disease-causing mutations previously reported are very rare and, thus, probably first appeared relatively recently—that is, in the past several hundred years,” Dr Coller said.
The researchers then used 3 different algorithms to try to predict the proportion of the missense variants that would likely have a negative impact on a person’s health by causing excess bleeding.
They got a wide range of results. Depending on the algorithm and other variables, between 27% and 71% of variants were predicted to be harmful.
To test the validity of the predictions, the team took a closer look at 3 of the variants that affect amino acids previously associated with Glanzmann thrombasthenia.
They found that 2 of the variants—aIIb P176H and ß3 C547G—severely reduced aIIbß3 expression. The third variant—aIIb P943A—partially reduced aIIbß3 expression but had no effect on fibrinogen binding.
These results show how challenging it can be to interpret genetic data, Dr Coller said.
“Some variants of these 2 genes will likely be obviously deleterious, but it may be impossible to predict whether others are deleterious,” he noted. “In those cases, we will need to use additional information to judge the likelihood of a mutation being deleterious, and, in many cases, there will be residual uncertainty.” ![]()
SGR repeal dubbed ‘victory’ for cancer patients

chemotherapy
Photo by Rhoda Baer
A bill that repeals the sustainable growth rate (SGR) formula for physician reimbursement under Medicare is a victory for cancer patients, according to oncologist groups.
The bill—known as H.R.2—passed both the US House of Representatives and the Senate with an overwhelming majority. It must still be signed into law by President Obama, but he has indicated he will sign it.
By repealing the SGR payment methodology, the bill will prevent a 21.2% reduction in physician reimbursement rates.
“Today’s courageous vote by the US Senate to finally end the sustainable growth rate formula is a vote for the millions of patients with cancer who depend on Medicare to help them fight their disease,” said Peter Paul Yu, MD, president of the American Society of Clinical Oncology.
“With Congress passing this historic legislation to finally end the 13-year SGR roller coaster ride, Medicare beneficiaries and their physicians can breathe easier knowing that they will no longer face the perennial threat of payment cuts that risk disruption of care and cause anxiety among patients.”
Under H.R. 2, Medicare’s physician reimbursements will increase by 0.5% in the second half of 2015, then an additional 0.5% annually from 2016 through the end of 2019. The 2019 rates will be maintained through 2025 with no additional increases.
The bill also includes comprehensive structural changes to Medicare’s reimbursement model that aim to promote physician participation in clinical quality improvement activities and value-based care that will take full effect in 2019.
Current Medicare programs that reward electronic health records, quality reporting, the value-based modifier, and meaningful use will be merged by 2019 to encourage participation and to reduce the administrative burden.
The bill also ensures the Children’s Health Insurance Program will receive funding for 2 more years and allocates $7.2 billion for community health centers.
“[P]assage of this legislation represents a long-awaited, historic victory for our patients,” said Bruce G. Haffty, MD, chair of the American Society for Radiation Oncology’s board of directors.
“Permanently repealing the SGR and replacing it with a stabilized reimbursement plan focused on quality will strengthen Medicare and allow us to enhance cancer care for the more than 1 million patients treated with radiation therapy each year.” ![]()

chemotherapy
Photo by Rhoda Baer
A bill that repeals the sustainable growth rate (SGR) formula for physician reimbursement under Medicare is a victory for cancer patients, according to oncologist groups.
The bill—known as H.R.2—passed both the US House of Representatives and the Senate with an overwhelming majority. It must still be signed into law by President Obama, but he has indicated he will sign it.
By repealing the SGR payment methodology, the bill will prevent a 21.2% reduction in physician reimbursement rates.
“Today’s courageous vote by the US Senate to finally end the sustainable growth rate formula is a vote for the millions of patients with cancer who depend on Medicare to help them fight their disease,” said Peter Paul Yu, MD, president of the American Society of Clinical Oncology.
“With Congress passing this historic legislation to finally end the 13-year SGR roller coaster ride, Medicare beneficiaries and their physicians can breathe easier knowing that they will no longer face the perennial threat of payment cuts that risk disruption of care and cause anxiety among patients.”
Under H.R. 2, Medicare’s physician reimbursements will increase by 0.5% in the second half of 2015, then an additional 0.5% annually from 2016 through the end of 2019. The 2019 rates will be maintained through 2025 with no additional increases.
The bill also includes comprehensive structural changes to Medicare’s reimbursement model that aim to promote physician participation in clinical quality improvement activities and value-based care that will take full effect in 2019.
Current Medicare programs that reward electronic health records, quality reporting, the value-based modifier, and meaningful use will be merged by 2019 to encourage participation and to reduce the administrative burden.
The bill also ensures the Children’s Health Insurance Program will receive funding for 2 more years and allocates $7.2 billion for community health centers.
“[P]assage of this legislation represents a long-awaited, historic victory for our patients,” said Bruce G. Haffty, MD, chair of the American Society for Radiation Oncology’s board of directors.
“Permanently repealing the SGR and replacing it with a stabilized reimbursement plan focused on quality will strengthen Medicare and allow us to enhance cancer care for the more than 1 million patients treated with radiation therapy each year.” ![]()

chemotherapy
Photo by Rhoda Baer
A bill that repeals the sustainable growth rate (SGR) formula for physician reimbursement under Medicare is a victory for cancer patients, according to oncologist groups.
The bill—known as H.R.2—passed both the US House of Representatives and the Senate with an overwhelming majority. It must still be signed into law by President Obama, but he has indicated he will sign it.
By repealing the SGR payment methodology, the bill will prevent a 21.2% reduction in physician reimbursement rates.
“Today’s courageous vote by the US Senate to finally end the sustainable growth rate formula is a vote for the millions of patients with cancer who depend on Medicare to help them fight their disease,” said Peter Paul Yu, MD, president of the American Society of Clinical Oncology.
“With Congress passing this historic legislation to finally end the 13-year SGR roller coaster ride, Medicare beneficiaries and their physicians can breathe easier knowing that they will no longer face the perennial threat of payment cuts that risk disruption of care and cause anxiety among patients.”
Under H.R. 2, Medicare’s physician reimbursements will increase by 0.5% in the second half of 2015, then an additional 0.5% annually from 2016 through the end of 2019. The 2019 rates will be maintained through 2025 with no additional increases.
The bill also includes comprehensive structural changes to Medicare’s reimbursement model that aim to promote physician participation in clinical quality improvement activities and value-based care that will take full effect in 2019.
Current Medicare programs that reward electronic health records, quality reporting, the value-based modifier, and meaningful use will be merged by 2019 to encourage participation and to reduce the administrative burden.
The bill also ensures the Children’s Health Insurance Program will receive funding for 2 more years and allocates $7.2 billion for community health centers.
“[P]assage of this legislation represents a long-awaited, historic victory for our patients,” said Bruce G. Haffty, MD, chair of the American Society for Radiation Oncology’s board of directors.
“Permanently repealing the SGR and replacing it with a stabilized reimbursement plan focused on quality will strengthen Medicare and allow us to enhance cancer care for the more than 1 million patients treated with radiation therapy each year.” ![]()
Research Agenda for Older Patient Care
Older adults with high levels of medical complexity occupy an increasing fraction of beds in acute‐care hospitals in the United States.[1, 2] By 2007, patients age 65 years and older accounted for nearly half of adult inpatient days of care.[1] These patients are commonly cared for by hospitalists who number more than 40,000.[3] Although hospitalists are most often trained in internal medicine, they have typically received limited formal geriatrics training. Increasingly, access to experts in geriatric medicine is limited.[4] Further, hospitalists and others who practice in acute care are limited by the lack of research to address the needs of the older adult population, specifically in the diagnosis and management of conditions encountered during acute illness.
To better support hospitalists in providing acute inpatient geriatric care, the Society of Hospital Medicine (SHM) partnered with the Association of Specialty Professors to develop a research agenda to bridge this gap. Using methodology from the James Lind Alliance (JLA) and the Patient Centered Outcomes Research Institute (PCORI), the SHM joined with older adult advocacy groups, professional societies of providers, and funders to create a geriatric‐focused acute‐care research agenda, highlighting 10 key research questions.[5, 6, 7] The goal of this approach was to produce and promote high integrity, evidence‐based information that comes from research guided by patients, caregivers, and the broader healthcare community.[8] In this article, we describe the methodology and results of this agenda‐setting process, referred to as the Acute Care of Older Patients (ACOP) Priority Setting Partnership.
METHODS
Overview
This project focused on topic generation, the first step in the PCORI framework for identification and prioritization of research areas.[5] We employed a specific and defined methodology to elicit and prioritize potential research topics incorporating input from representatives of older patients, family caregivers, and healthcare providers.[6]
To elicit this input, we chose a collaborative and consultative approach to stakeholder engagement, drawing heavily from the published work of the JLA, an initiative promoting patient‐clinician partnerships in health research developed in the United Kingdom.[6] We previously described the approach elsewhere.[7]
The ACOP process for determining the research agenda consisted of 4 steps: (1) convene, (2) consult, (3) collate, and (4) prioritize.[6] Through these steps, detailed below, we were able to obtain input from a broad group of stakeholders and engage the stakeholders in a process of reducing and refining our research questions.
Convene
The steering committee (the article's authors) convened a stakeholder partnership group that included stakeholders representing patients and caregivers, advocacy organizations for the elderly, organizations that address diseases and conditions common among hospitalized older patients, provider professional societies (eg, hospitalists, subspecialists, and nurses and social workers), payers, and funders. Patient, caregiver, and advocacy organizations were identified based on their engagement in aging and health policy advocacy by SHM staff and 1 author who had completed a Health and Aging Policy Fellowship (H.L.W.).
The steering committee issued e‐mail invitations to stakeholder organizations, making initial inquiries through professional staff and relevant committee chairs. Second inquiries were made via e‐mail to each organization's volunteer leadership. We developed a webinar that outlined the overall research agenda setting process and distributed the webinar to all stakeholders. The stakeholder organizations were asked to commit to (1) surveying their memberships and (2) participating actively in prioritization by e‐mail and at a 1‐day meeting in Washington DC.
Consult
Each stakeholder organization conducted a survey of its membership via an Internet‐based survey in the summer of 2013 (see Supporting Information, Appendix A, in the online version of this article). Stakeholder organizations were asked to provide up to 75 survey responses each. Though a standard survey was used, the steering committee was not prescriptive in the methodology of survey distribution to accommodate the structure and communication methods of the individual stakeholder organizations. Survey respondents were asked to identify up to 5 unanswered questions relevant to the acute care of older persons and also provide demographic information.
Collate
In the collating process, we clarified and categorized the unanswered questions submitted in the individual surveys. Each question was initially reviewed by a member of the steering committee, using explicit criteria (see Supporting Information, Appendix B, in the online version of this article). Questions that did not meet all 4 criteria were removed. For questions that met all criteria, we clarified language, combined similar questions, and categorized each question. Categories were created in a grounded process, in which individual reviewers assigned categories based on the content of the questions. Each question could be assigned to up to 2 categories. Each question was then reviewed by a second member of the steering committee using the same 4 criteria. As part of this review, similar questions were consolidated, and when possible, questions were rewritten in a standard format.[6]
Finally, the steering committee reviewed previously published research agendas looking for additional relevant unanswered questions, specifically the New Frontiers Research Agenda created by the American Geriatrics Society in conjunction with participating subspecialty societies,[9] the Cochrane Library, and other systematic reviews identified in the literature via PubMed search.[10, 11, 12, 13, 14, 15]
Prioritize
The resulting list of unanswered questions was prioritized in 2 phases. First, the list was e‐mailed to all stakeholder organizations. The organizations were asked to vote on their top 10 priorities from this list using an online ballot, assigning 10 points to their highest priority down to 1 point for their lowest priority. In so doing, they were asked to consider explicit criteria (see Supporting Information, Appendix B, in the online version of this article). Each organization had only 1 ballot and could arrive at their top 10 list in any manner they wished. The balloting from this phase was used to develop a list of unanswered questions for the second round of in‐person prioritization. Each priority's scores were totaled across all voting organizations. The 29 priorities with the highest point totals were brought to the final prioritization round because of a natural cut point at priority number 29, rather than number 30.
For the final prioritization round, the steering committee facilitated an in‐person meeting in Washington, DC in October 2013 using nominal group technique (NGT) methodologies to arrive at consensus.[16] During this process stakeholders were asked to consider additional criteria (see Supporting Information, Appendix B, in the online version of this article).
RESULTS
Table 1 lists the organizations who engaged in 1 or more parts of the topic generation process. Eighteen stakeholder organizations agreed to participate in the convening process. Ten organizations did not respond to our solicitation and 1 declined to participate.
| Organization (N=18) | Consultation % of Survey Responses (N=580) | Prioritization Round 1 | Prioritization Round 2 |
|---|---|---|---|
| Alzheimer's Association | 7.0% | Yes | Yes |
| American Academy of Neurology | 3.4% | Yes | Yes |
| American Association of Retired Persons | 0.8% | No | No |
| American College of Cardiology | 11.4% | Yes | Yes |
| American College of Emergency Physicians | 1.3% | No | No |
| American College of Surgeons | 1.0% | Yes | Yes |
| American Geriatrics Society | 7.6% | Yes | Yes |
| American Hospital Association | 1.7% | Yes | No |
| Centers for Medicare & Medicaid Services | 0.8% | Yes | Yes |
| Gerontological Society of America | 18.9% | Yes | Yes |
| National Alliance for Caregiving | 1.0% | Yes | Yes |
| National Association of Social Workers | 5.9% | Yes | Yes |
| National Coalition for Healthcare | 0.6% | No | No |
| National Institute on Aging | 2.1% | Yes | Yes |
| National Partnership for Women and Families | 0.0% | Yes | Yes |
| Nursing Improving Care for Healthsystem Elders | 28.6% | Yes | No |
| Society of Critical Care Medicine | 12.0% | Yes | Yes |
| Society of Hospital Medicine | 4.6% | Yes | Yes |
Seventeen stakeholder organizations obtained survey responses from a total of 580 individuals (range, 3150 per organization), who were asked to identify important unanswered questions in the acute care of older persons. Survey respondents were typically female (77%), white (85%), aged 45 to 65 years (65%), and identified themselves as health professionals (90%). Twenty‐six percent of respondents also identified as patients or family caregivers. Their surveys included 1299 individual questions.
Figure 1 summarizes our collation and prioritization process and reports the numbers of questions resulting at each stage. Nine hundred nineteen questions were removed during the first review conducted by steering committee members, and 31 question categories were identified. An additional 305 questions were removed in the second review, with 75 questions remaining. As the final step of the collating process, literature review identified 39 relevant questions not already suggested or moved forward through our consultation and collation process. These questions were added to the list of unanswered questions.
In the first round of prioritization, this list of 114 questions was emailed to each stakeholder organization (Table 1). After the stakeholder voting process was completed, 29 unanswered questions remained (see Supporting Information, Appendix C, in the online version of this article). These questions were refined and prioritized in the in‐person meeting to create the final list of 10 questions. The stakeholders present in the meeting represented 13 organizations (Table 1). Using the NGT with several rounds of small group breakouts and large group deliberation, 9 of the top 10 questions were selected from the list of 29. One additional highly relevant question that had been removed earlier in the collation process regarding workforce was added back by the stakeholder group.
This prioritized research agenda appears in Table 2 and below, organized alphabetically by topic.
- Advanced care planning: What approaches for determining and communicating goals of care across and within healthcare settings are most effective in promoting goal‐concordant care for hospitalized older patients?
- Care transitions: What is the comparative effectiveness of transitional care models on patient‐centered outcomes for hospitalized older adults?
- Delirium: What practices are most effective for consistent recognition, prevention, and treatment of delirium subtypes among hospitalized older adults?
- Dementia: Does universal assessment of hospitalized older adults for cognitive impairment (eg, at presentation and/or discharge) lead to more appropriate application of geriatric care principles and improve patient‐centered outcomes?
- Depression: Does identifying depressive symptoms during a hospital stay and initiating a therapeutic plan prior to discharge improve patient‐centered and/or disease‐specific outcomes?
- Medications: What systems interventions improve medication management for older adults (ie, appropriateness of medication choices and dosing, compliance, cost) in the hospital and postacute care?
- Models of care: For which populations of hospitalized older adults does systematic implementation of geriatric care principles/processes improve patient‐centered outcomes?
- Physical function: What is the comparative effectiveness of interventions that promote in‐hospital mobility, improve and preserve physical function, and reduce falls among older hospitalized patients?
- Surgery: What perioperative strategies can be used to optimize care processes and improve outcomes in older surgical patients?
- Training: What is the most effective approach to training hospital‐based providers in geriatric and palliative care competencies?
| Topic | Scope of Problem | What Is known | Unanswered Question | Proposed Dimensions |
|---|---|---|---|---|
| ||||
| Advanced‐care planning | Older persons who lack decision‐making capacity often do not have surrogates or clear goals of care documented.[19] Advanced‐care directives are associated with an increase in patient autonomy and empowerment, and although 15% to 25% of adults completed the documentation in 2004,[20] a recent study found completion rates have increased to 72%.[21] | Nursing home residents with advanced directives are less likely to be hospitalized.[22, 23] Advanced directive tools, such as POLST, work to translate patient preferences to medical order.[24] standardized patient transfer tools may help to improve transitions between nursing homes and hospitals.[25] However, advanced care planning fails to integrate into courses of care if providers are unwilling or unskilled in using advanced care documentation.[26] | What approaches for determining and communicating goals of care across and within healthcare settings are most effective in promoting goal‐concordant care for hospitalized older patients? | Potential interventions: |
| Decision aids | ||||
| Standard interdisciplinary advanced care planning approach | ||||
| Patient advocates | ||||
| Potential outcomes might include: | ||||
| Completion of advanced directives and healthcare power of attorney | ||||
| Patient‐centered outcomesa | ||||
| Care transitions | Hospital readmission from home and skilled nursing facilities occurs within 30 days in up to a quarter of patients.[27, 28] The discharge of complex older hospitalized patients is fraught with challenges. The quality of the hospital discharge process can influence outcomes for vulnerable older patients.[29, 30, 31, 32] Studies measuring the quality of hospital discharge frequently find deficits in documentation of assessment of geriatric syndromes,[33] poor patient/caregiver understanding,[34, 35] and poor communication and follow‐up with postacute providers.[35, 36, 37, 38] | As many as 10 separate domains may influence the success of a discharge.[39] There is limited evidence, regarding quality‐of‐care transitions for hospitalized older patients. The Coordinated‐Transitional Care Program found that follow‐up with telecommunication decreased readmission rates and improved transitional care for a high‐risk condition veteran population.[40] There is modest evidence for single interventions,[41] whereas the most effective hospital‐to‐community care interventions address multiple processes in nongeriatric populations.[39, 42, 43] | What is the comparative effectiveness of the transitional care models on patient‐centered outcomes for hospitalized older adults? | Possible models: |
| Established vs novel care‐transition models | ||||
| Disease‐specific vs general approaches | ||||
| Accountable care models | ||||
| Caregiver and family engagement | ||||
| Community engagement | ||||
| Populations of interest: | ||||
| Patients with dementia | ||||
| Patients with multimorbidity | ||||
| Patients with geriatric syndromes | ||||
| Patients with psychiatric disease | ||||
| Racially and ethnically diverse patients | ||||
| Outcomes: | ||||
| Readmission | ||||
| Other adverse events | ||||
| Cost and healthcare utilization | ||||
| Patient‐centered outcomesa | ||||
| Delirium | Among older inpatients, the prevalence of delirium varies with severity of illness. Among general medical patients, in‐hospital prevalence ranges from 10% to 25 %.[44, 45] In the ICU, prevalence estimates are higher, ranging from 25% to as high as 80%.[46, 47] Delirium independently predicts increased length of stay,[48, 49] long‐term cognitive impairment,[50, 51] functional decline,[51] institutionalization,[52] and short‐ and long‐term mortality.[52, 53, 54] | Multicomponent strategies have been shown to be effective in preventing delirium. A systematic review of 19 such interventions identified the most commonly included such as[55]: early mobilization, nutrition supplements, medication review, pain management, sleep enhancement, vision/hearing protocols, and specialized geriatric care. Studies have included general medical patients, postoperative patients, and patients in the ICU. The majority of these studies found reductions in either delirium incidence (including postoperative), delirium prevalence, or delirium duration. Although medications have not been effective in treating delirium in general medical patients,[48] the choice and dose of sedative agents has been shown to impact delirium in the ICU.[56, 57, 58] | What practices are most effective for consistent recognition, prevention, and treatment of delirium subtypes (hypoactive, hyperactive, and mixed) among hospitalized older adults? | Outcomes to examine: |
| Delirium incidence (including postoperative) | ||||
| Delirium duration | ||||
| Delirium‐/coma‐free days | ||||
| Delirium prevalence at discharge | ||||
| Subsyndromal delirium | ||||
| Potential prevention and treatment modalities: | ||||
| Family education or psychosocial interventions | ||||
| Pharmacologic interventions | ||||
| Environmental modifications | ||||
| Possible areas of focus: | ||||
| Special populations | ||||
| Patients with varying stages of dementia | ||||
| Patients with multimorbidity | ||||
| Patients with geriatric syndromes | ||||
| Observation patients | ||||
| Diverse settings | ||||
| Emergency department | ||||
| Perioperative | ||||
| Skilled nursing/rehab/long‐term acute‐care facilities | ||||
| Dementia | 13% to 63% of older persons in the hospital have dementia.[59] Dementia is often unrecognized among hospitalized patients.[60] The presence of dementia is associated with a more rapid functional decline during admission and delayed hospital discharge.[59] Patients with dementia require more nursing hours, and are more likely to have complications[61] or die in care homes rather than in their preferred site.[59] | Several tools have been validated to screen for dementia in the hospital setting.[62] Studies have assessed approaches to diagnosing delirium in hospitalized patients with dementia.[63] Cognitive and functional stimulation interventions may have a positive impact on reducing behavioral issues.[64, 65] | Does universal assessment of hospitalized older adults for cognitive impairment (eg, at presentation and/or discharge) lead to more appropriate application of geriatric care principles and improve patient centered outcomes? | Potential interventions: |
| Dementia or delirium care | ||||
| Patient/family communication and engagement strategies | ||||
| Maintenance/recovery of independent functional status | ||||
| Potential outcomes: | ||||
| Patient‐centered outcomesa | ||||
| Length of stay, cost, and healthcare utilization (including palliative care) | ||||
| Immediate invasive vs early conservative treatments pursued | ||||
| Depression | Depression is a common geriatric syndrome among acutely ill older patients, occurring in up to 45% of patients.[66, 67] Rates of depression are similar among patients discharged following a critical illness, with somatic, rather than cognitive‐affective complaints being the most prevalent.[68] Depression among inpatients or immediately following hospitalization independently predicts worse functional outcomes,[69] cognitive decline,[70] hospital readmission,[71, 72] and long‐term mortality.[69, 73] Finally, geriatric patients are known to respond differently to medical treatment.[74, 75] | Although highly prevalent, depression is poorly recognized and managed in the inpatient setting. Depression is recognized in only 50% of patients, with previously undiagnosed or untreated depression being at highest risk for being missed.[76] The role of treatment of depression in the inpatient setting is poorly understood, particularly for those with newly recognized depression or depressive symptoms. Some novel collaborative care and telephone outreach programs have led to increases in depression treatment in patients with specific medical and surgical conditions, resulting in early promising mental health and comorbid outcomes.[77, 78] The efficacy of such programs for older patients is unknown. | Does identifying depressive symptoms during a hospital stay and initiating a therapeutic plan prior to discharge improve patient‐centered and/or disease‐specific outcomes? | Possible areas of focus: |
| Comprehensive geriatric and psychosocial assessment; | ||||
| Inpatient vs outpatient initiation of pharmacological therapy | ||||
| Integration of confusion assessment method into therapeutic approaches | ||||
| Linkages with outpatient mental health resources | ||||
| Medications | Medication exposure, particularly potentially inappropriate medications, is common in hospitalized elders.[79] Medication errorsof dosage, type, and discrepancy between what a patient takes at home and what is known to his/her prescribing physicianare common and adversely affects patient safety.[80] Geriatric populations are disproportionately affected, especially those taking more than 5 prescription medications per day.[81] | Numerous strategies including electronic alerts, screening protocols, and potentially inappropriate medication lists (Beers list, STOPP) exist, though the optimal strategies to limit the use of potentially inappropriate medications is not yet known.[82, 83, 84] | What systems interventions improve medication management for older adults (ie, appropriateness of medication choices and dosing, compliance, cost) in hospital and post‐acute care? | Possible areas of focus: |
| Use of healthcare information technology | ||||
| Communication across sites of care | ||||
| Reducing medication‐related adverse events | ||||
| Engagement of family caregivers | ||||
| Patient‐centered strategies to simplify regimens | ||||
| Models of care | Hospitalization marks a time of high risk for older patients. Up to half die during hospitalization or within the year following the hospitalization. There is high risk of nosocomial events, and more than a third experience a decline in health resulting in longer hospitalizations and/or placement in extended‐care facilities.[73, 85, 86] | Comprehensive inpatient care for older adults (acute care for elders units, geriatric evaluation and management units, geriatric consultation services) were studied in 2 meta‐analyses, 5 RCTs, and 1 quasiexperimental study and summarized in a systematic review.[87] The studies reported improved quality of care (1 of 1 article), quality of life (3 of 4), functional autonomy (5 of 6), survival (3 of 6), and equal or lower healthcare utilization (7 of 8). | For which populations of hospitalized older adults does systematic implementation of geriatric care principles/processes improve patient‐centered outcomes? | Potential populations: |
| Patients of the emergency department, critical care, perioperative, and targeted medical/surgical units | ||||
| Examples of care principles: | ||||
| Geriatric assessment, early mobility, medication management, delirium prevention, advanced‐care planning, risk‐factor modification, caregiver engagement | ||||
| Potential outcomes: | ||||
| Patient‐centered outcomesa | ||||
| Cost | ||||
| Physical function | Half of older patients will lose functional capacity during hospitalization.[88] Loss of physical function, particularly of lower extremities, is a risk factor for nursing home placement.[89, 90] Older hospitalized patients spend the majority (up to 80%) of their time lying in bed, even when they are capable of walking independently.[91] | Loss of independences with ADL capabilities is associated with longer hospital stays, higher readmission rates, and higher mortality risk.[92] Excessive time in bed during a hospital stay is also associated with falls.[93] Often, hospital nursing protocols and physician orders increase in‐hospital immobility in patients.[91, 94] However, nursing‐driven mobility protocols can improve functional outcomes of older hospitalized patients.[95, 96] | What is the comparative effectiveness of interventions that promote in‐hospital mobility, improve and preserve physical function, and reduce falls among older hospitalized patients? | Potential interventions: |
| Intensive physical therapy | ||||
| Incidental functional training | ||||
| Restraint reduction | ||||
| Medication management | ||||
| Potential outcomes: | ||||
| Discharge location | ||||
| Delirium, pressure ulcers, and falls | ||||
| Surgery | An increasing number of persons over age 65 years are undergoing surgical procedures.[97] These persons are at increased risk for developing delirium/cogitative dysfunction,[98] loss of functional status,[99] and exacerbations of chronic illness.[97] Additionally, pain management may be harder to address in this population.[100] Current outcomes may not reflect the clinical needs of elder surgical patients.[101] | Tailored drug selection and nursing protocols may prevent delirium.[98] Postoperative cognitive dysfunction may require weeks for resolution. Identifying frail patients preoperatively may lead to more appropriate risk stratification and improved surgical outcomes.[99] Pain management strategies focused on mitigating cognitive impact and other effects may also be beneficial.[100] Development of risk‐adjustment tools specific to older populations, as well as measures of frailty and patient‐centered care, have been proposed.[101] | What perioperative strategies can be used to optimize care processes and improve outcomes in older surgical patients? | Potential strategies: |
| Preoperative risk assessment and optimization for frail or multimorbid older patients | ||||
| Perioperative management protocols for frail or multimorbid older patients | ||||
| Potential outcomes: | ||||
| Postoperative patient centered outcomesa | ||||
| Perioperative cost, healthcare utilization | ||||
| Training | Adults over age 65 years comprise 13.2 % of the US population, but account for >30% of hospital discharges and 50% of hospital days.[86, 102, 103] By 2030, there will only be 1 geriatrician for every 3798 Americans >75 years.[4] Between 1997 and 2006, the odds that a hospitalist would treat a hospitalized Medicare patient rose 29% per year.[3] | Train the trainer programs for physicians include the CHAMP, the AGESP, and the PAGE. Education for nurses include the NICHE. Outcomes include improved self‐confidence, attitudes, teaching skills, and geriatric care environment.[104, 105, 106] | What is the most effective approach to training hospital‐based providers in geriatric and palliative care competencies? | Potential interventions: |
| Mentored implementation | ||||
| Train the trainer | ||||
| Technical support | ||||
Table 2 also contains a capsule summary of the scope of the problem addressed by each research priority, a capsule summary of related work in the content area (what is known) not intended as a systematic review, and proposed dimensions or subquestions suggested by the stakeholders at the final prioritization meeting
DISCUSSION
Older hospitalized patients account for an increasing number and proportion of hospitalized patients,[1, 2] and hospitalists increasingly are responsible for inpatient care for this population.[3] The knowledge required for hospitalists to deliver optimal care and improve outcomes has not kept pace with the rapid growth of either hospitalists or hospitalized elders. Through a rigorous prioritization process, we identified 10 areas that deserve the highest priority in directing future research efforts to improve care for the older hospitalized patient. Assessment, prevention, and treatment of geriatric syndromes in the hospital account for almost half of the priority areas. Additional research is needed to improve advanced care planning, develop new care models, and develop training models for future hospitalists competent in geriatric and palliative care competencies.
A decade ago, the American Geriatric Society and the John A. Hartford Foundation embarked upon a research agenda aimed at improving the care of hospitalized elders cared for by specialists (ie, New Frontiers in Geriatrics Research: An Agenda for Surgical and Related Medical Specialties).[9] This effort differed in many important ways from the current priortization process. First, the New Frontiers agenda focused upon specific diseases, whereas the ACOP agenda addresses geriatric syndromes that cut across multiple diseases. Second, the New Frontiers agenda was made by researchers and based upon published literature, whereas the ACOP agenda involved the input of multiple stakeholders. Finally, the New Frontiers prioritized a research agenda across a number of surgical specialties, emergency medicine, and geriatric rehabilitation. Hospital medicine, however, was still early in its development and was not considered a unique specialty. Since that time, hospital medicine has matured into a unique specialty, with increased numbers of hospitalists,[3] increased research in hospital medicine,[17] and a separate recertification pathway for internal medicine licensure.[18] To date, there has not been a similar effort performed to direct geriatric research efforts for hospital medicine.
For researchers working in the field of hospital medicine, this list of topics has several implications. First, as hospitalists are commonly generalists, hospitalist researchers may be particularly well‐suited to study syndromes that cut across specialties. However, this does raise concerns about funding sources, as most National Institutes of Health institutes are disease‐focused. Funders that are not disease‐focused such as PCORI, National Institute on Aging, National Institute of Nursing Research, and Agency for Healthcare Research and Quality, and private foundations (Hartford, Robert Wood Johnson, and Commonwealth) may be more fruitful sources of funding for this work, but funding may be challenging. Nonetheless, the increased focus on patient‐centered work may increase funders' interest in such work. Second, the topics on this list would suggest that interventions will not be pharmacologic, but will focus on nonpharmacologic, behavioral, and social interventions. Similarly, outcomes of interest must expand beyond utilization metrics such as length of stay and mortality, to include functional status and symptom management, and goal‐concordant care. Therefore, research in geriatric acute care will necessarily be multidisciplinary.
Although these 10 high‐priority areas have been selected, this prioritized list is inherently limited by our methodology. First, our survey question was not focused on a disease state, and this wording may have resulted in the list favoring geriatric syndromes rather than common disease processes. Additionally, the resulting questions encompass large research areas and not specific questions about discrete interventions. Our results may also have been skewed by the types of engaged respondents who participated in the consultation, collating, and prioritization phases. In particular, we had a large response from geriatric medicine nurses, whereas some stakeholder groups provided no survey responses. Thus, these respondents were not representative of all possible stakeholders, nor were the survey respondents necessarily representative of each of their organizations. Nonetheless, the participants self‐identified as representative of diverse viewpoints that included patients, caregivers, and advocacy groups, with the majority of stakeholder organizations remaining engaged through the completion of the process. Thus, the general nature of this agenda helps us focus upon larger areas of importance, leaving researchers the flexibility to choose to narrow the focus on a specific research question that may include potential interventions and unique outcomes. Finally, our methodology may have inadvertently limited the number of patient and family caregiver voices in the process given our approach to large advocacy groups, our desire to be inclusive of healthcare professional organizations, and our survey methodology. Other methodologies may have reached more patients and caregivers, yet many healthcare professionals have served as family caregivers to frail elders requiring hospitalization and may have been in an ideal position to answer the survey.
In conclusion, several forces are shaping the future of acute inpatient care. These include the changing demographics of the hospitalized patient population, a rapid increase in the proportion of multimorbid hospitalized older adults, an inpatient workforce (hospitalists, generalists, and subspecialists) with potentially limited geriatrics training, and gaps in evidence‐based guidance to inform diagnostic and therapeutic decision making for acutely ill older patients. Training programs in hospital medicine should be aware of and could benefit from the resulting list of unanswered questions. Our findings also have implications for training to enrich education in geriatrics. Moreover, there is growing recognition that patients and other stakeholders deserve a greater voice in determining the direction of research. In addition to efforts to improve patient‐centeredness of research, these areas have been uniquely identified by stakeholders as important, and therefore are in line with newer priorities of PCORI. This project followed a road map resulting in a patient‐centered research agenda at the intersection of hospital medicine and geriatric medicine.[7] In creating this agenda, we relied heavily on the framework proposed by PCORI. We propose to pursue a dissemination and evaluation strategy for this research agenda as well as additional prioritization steps. We believe the adoption of this methodology will create a knowledge base that is rigorously derived and most relevant to the care of hospitalized older adults and their families. Its application will ultimately result in improved outcomes for hospitalized older adults.
Acknowledgements
The authors acknowledge Claudia Stahl, Society of Hospital Medicine; Cynthia Drake, University of Colorado; and the ACOP stakeholder organizations.
Disclosures: This work was supported by the Association of Specialty Professors/American Society of Internal Medicine and the John A. Hartford Foundation. Dr. Vasilevskis was supported by the National Institute on Aging of the National Institutes of Health under award number K23AG040157 and the Veterans Affairs Clinical Research Center of Excellence, and the Geriatric Research, Education and Clinical Center (GRECC). Dr. Vasilevskis' institution receives grant funding for an aspect of submitted work. Dr. Meltzer is a PCORI Methodology Committee member. The content is solely the responsibility of the authors and does not necessarily represent the official views of the National Institutes of Health or the Department of Veterans' Affairs. The authors report no conflicts of interest.
- , , , , . National Hospital Discharge Survey: 2007 summary. Natl Health Stat Report. 2010(29):1–20, 24.
- Centers for Medicare 2012. Available at: http://www.cms.gov/Research‐Statistics‐Data‐and‐Systems/Statistics‐Trends‐and‐Reports/Chronic‐Conditions/Downloads/2012Chartbook.pdf. Accessed December 12, 2014.
- , , , . Growth in the care of older patients by hospitalists in the United States. N Engl J Med. 2009;360(11):1102–1112.
- , . A revelation of numbers: will America's eldercare workforce be ready to care for an aging America? Generations. 2010;34(4):11–19.
- Patient‐Centered Outcomes Research Institute Methodology Committee. The PCORI methodology report. Available at: http://www.pcori.org/assets/2013/11/PCORI‐Methodology‐Report.pdf. Published November 2013. Accessed December 19, 2013.
- The James Lind Alliance. JLA method. Available at: http://www.lindalliance.org/JLA_Method.asp. Accessed December 19, 2013.
- , , , , . Road map to a patient‐centered research agenda at the intersection of hospital medicine and geriatric medicine. J Gen Intern Med. 2014;29(6):926–931.
- Patient‐Centered Outcomes Research Institute. About us. Available at: http://www.pcori.org/about‐us. Accessed February 23, 2015.
- , , . New Frontiers of Geriatrics Research: An Agenda for Surgical and Related Medical Specialties. New York, NY: American Geriatrics Society; 2004.
- , , , . Linking the NIH strategic plan to the research agenda for social workers in health and aging. J Gerontol Soc Work. 2010;53(1):77–93.
- , . Assessing the capacity to make everyday decisions: a guide for clinicians and an agenda for future research. Am J Geriatr Psychiatry. 2007;15(2):101–111.
- , , , et al. Practitioners' views on elder mistreatment research priorities: recommendations from a Research‐to‐Practice Consensus conference. J Elder Abuse Negl. 2011;23(2):115–126.
- , . The intersection between geriatrics and palliative care: a call for a new research agenda. J Am Geriatr Soc. 2005;53(9):1593–1598.
- . The cancer aging interface: a research agenda. J Clin Oncol. 2007;25(14):1945–1948.
- , , , . Clinical care of persons with dementia in the emergency department: a review of the literature and agenda for research. J Am Geriatr Soc. 2012;60(9):1742–1748.
- , . A group process model for problem identification and program planning. J Appl Behav Sci. 1971;7(4):466–492.
- , , , , . Research and publication trends in hospital medicine. J Hosp Med. 2014;9(3):148–154.
- . ABFM and ABIM to jointly participate in recognition of focused practice (rfp) in hospital medicine pilot approved by abms. Ann Fam Med. 2010;8(1):87.
- , , , . Medical decision‐making for older adults without family. J Am Geriatr Soc. 2012;60(11):2144–2150.
- , , , , , . Promoting advance directives among elderly primary care patients. J Gen Intern Med. 2004;19(9):944–951.
- , , . Advance directive completion by elderly Americans: a decade of change. J Am Geriatr Soc. 2014;62(4):706–710.
- , . Care transitions by older adults from nursing homes to hospitals: implications for long‐term care practice, geriatrics education, and research. J Am Med Dir Assoc. 2010;11(4):231–238.
- , , , . Decisions to hospitalize nursing home residents dying with advanced dementia. J Am Geriatr Soc. 2005;53(8):1396–1401.
- , , , , , . A comparison of methods to communicate treatment preferences in nursing facilities: traditional practices versus the physician orders for life‐sustaining treatment program. J Am Geriatr Soc. 2010;58(7):1241–1248.
- , , , , . Interventions to improve transitional care between nursing homes and hospitals: a systematic review. J Am Geriatr Soc. 2010;58(4):777–782.
- , , . Opening end‐of‐life discussions: how to introduce Voicing My CHOiCES, an advance care planning guide for adolescents and young adults [published online ahead of print March 13, 2014]. Palliat Support Care. doi: 10.1017/S1478951514000054.
- , , , . The revolving door of rehospitalization from skilled nursing facilities. Health Aff (Millwood). 2010;29(1):57–64.
- , , . Rehospitalizations among patients in the Medicare fee‐for‐service program. N Engl J Med. 2009;360(14):1418–1428.
- , , , et al. Predictors of rehospitalization among elderly patients admitted to a rehabilitation hospital: the role of polypharmacy, functional status, and length of stay. J Am Med Dir Assoc. 2013;14(10):761–767.
- , , , et al. Mobility after hospital discharge as a marker for 30‐day readmission. J Gerontol A Biol Sci Med Sci. 2013;68(7):805–810.
- , , , , . The association between the quality of inpatient care and early readmission: a meta‐analysis of the evidence. Med Care. 1997;35(10):1044–1059.
- , , , , , . Association of impaired functional status at hospital discharge and subsequent rehospitalization. J Hosp Med. May 2014;9(5):277–282.
- , , , , , . A prospective cohort study of geriatric syndromes among older medical patients admitted to acute care hospitals. J Am Geriatr Soc. 2011;59(11):2001–2008.
- , , , et al. Hospital discharge instructions: comprehension and compliance among older adults. J Gen Intern Med. 2014;29(11):1491–1498.
- , , , et al. Problems after discharge and understanding of communication with their primary care physicians among hospitalized seniors: a mixed methods study. J Hosp Med. 2010;5(7):385–391.
- , , , et al. Communication and information deficits in patients discharged to rehabilitation facilities: an evaluation of five acute care hospitals. J Hosp Med. 2009;4(8):E28–E33.
- , , , , , . The consequences of poor communication during transitions from hospital to skilled nursing facility: a qualitative study. J Am Geriatr Soc. 2013;61(7):1095–1102.
- , , , , , . Deficits in communication and information transfer between hospital‐based and primary care physicians: implications for patient safety and continuity of care. JAMA. 2007;297(8):831–841.
- , , , . Moving beyond readmission penalties: creating an ideal process to improve transitional care. J Hosp Med. 2013;8(2):102–109.
- , , , et al. Low‐cost transitional care with nurse managers making mostly phone contact with patients cut rehospitalization at a VA hospital. Health Aff (Millwood). 2012;31(12):2659–2668.
- , , , et al. Transitional care interventions to prevent readmissions for persons with heart failure: a systematic review and meta‐analysis. Ann Intern Med. 2014;160(11):774–784.
- , , , , . Interventions to reduce 30‐day rehospitalization: a systematic review. Ann Intern Med. 2011;155(8):520–528.
- , , , , , . Hospital‐initiated transitional care interventions as a patient safety strategy: a systematic review. Ann Intern Med. 2013;158(5 pt 2):433–440.
- , , , . Epidemiology and risk factors for delirium across hospital settings. Best Pract Res Clin Anaesthesiol. 2012;26(3):277–287.
- , , , , . Does delirium contribute to poor hospital outcomes? A three‐site epidemiologic study. J Gen Intern Med. 1998;13(4):234–242.
- , , , . Incidence, risk factors and consequences of ICU delirium. Intensive Care Med. 2007;33(1):66–73.
- , , , et al. Delirium in mechanically ventilated patients: validity and reliability of the confusion assessment method for the intensive care unit (CAM‐ICU). JAMA. 2001;286(21):2703–2710.
- , , , et al. Impact and recognition of cognitive impairment among hospitalized elders. J Hosp Med. 2010;5(2):69–75.
- , , , et al. The impact of delirium in the intensive care unit on hospital length of stay. Intensive Care Med. 2001;27(12):1892–1900.
- , , . Long‐term cognitive impairment after critical illness. N Engl J Med. 2014;370(2):185–186.
- , , , et al. Delirium in the ICU and subsequent long‐term disability among survivors of mechanical ventilation. Crit Care Med. 2014;42(2):369–377.
- , , , , , . Delirium in elderly patients and the risk of postdischarge mortality, institutionalization, and dementia: a meta‐analysis. JAMA. 2010;304(4):443–451.
- , , , et al. Delirium as a predictor of mortality in mechanically ventilated patients in the intensive care unit. JAMA. 2004;291(14):1753–1762.
- , , , , , . Days of delirium are associated with 1‐year mortality in an older intensive care unit population. Am J Respir Crit Care Med. 2009;180(11):1092–1097.
- , . In‐facility delirium prevention programs as a patient safety strategy: a systematic review. Ann Intern Med. 2013;158(5 pt 2):375–380.
- , , , et al. Dexmedetomidine vs midazolam for sedation of critically ill patients: a randomized trial. JAMA. 2009;301(5):489–499.
- , , , et al. Effect of sedation with dexmedetomidine vs lorazepam on acute brain dysfunction in mechanically ventilated patients: the MENDS randomized controlled trial. JAMA. 2007;298(22):2644–2653.
- , , , , , . Protocolized intensive care unit management of analgesia, sedation, and delirium improves analgesia and subsyndromal delirium rates. Anesth Analg. 2010;111(2):451–463.
- , . A systematic review of the prevalence, associations and outcomes of dementia in older general hospital inpatients. Int Psychogeriatr. 2011;23(3):344–355.
- , , , . Cognitive impairment is undetected in medical inpatients: a study of mortality and recognition amongst healthcare professionals. BMC Geriatr. 2012;12:47.
- , , . How can we keep patients with dementia safe in our acute hospitals? A review of challenges and solutions. J R Soc Med. 2013;106(9):355–361.
- , , . Screening for dementia in general hospital inpatients: a systematic review and meta‐analysis of available instruments. Age Ageing. 2013;42(6):689–695.
- , , , et al. Tools to detect delirium superimposed on dementia: a systematic review. J Am Geriatr Soc. 2012;60(11):2005–2013.
- , , , , , . Functional analysis‐based interventions for challenging behaviour in dementia. Cochrane Database Syst Rev. 2012;2:CD006929.
- , , , . Cognitive stimulation to improve cognitive functioning in people with dementia. Cochrane Database Syst Rev. 2012;2:CD005562.
- , , , et al. The prevalence and correlates of major and minor depression in older medical inpatients. J Am Geriatr Soc. 2005;53(8):1344–1353.
- , , , , , . Major depressive disorder in hospitalized medically ill patients: an examination of young and elderly male veterans. J Am Geriatr Soc. 1991;39(9):881–890.
- , , , et al. Depression, post‐traumatic stress disorder, and functional disability in survivors of critical illness in the BRAIN‐ICU study: a longitudinal cohort study. Lancet Respir Med. 2014;2(5):369–379.
- , , , et al. Depressive symptoms after hospitalization in older adults: function and mortality outcomes. J Am Geriatr Soc. 2012;60(12):2254–2262.
- , , , , . 12‐month cognitive outcomes of major and minor depression in older medical patients. Am J Geriatr Psychiatry. 2008;16(9):742–751.
- , , , et al. Depression is a risk factor for rehospitalization in medical inpatients. Prim Care Companion J Clin Psychiatry. 2007;9(4):256–262.
- , , , , , . Dose‐response relationship between depressive symptoms and hospital readmission. J Hosp Med. 2014;9(6):358–364.
- , , , , , . Depressive symptoms and 3‐year mortality in older hospitalized medical patients. Ann Intern Med. 1999;130(7):563–569.
- , , , et al. Support for the vascular depression hypothesis in late‐life depression: results of a 2‐site, prospective, antidepressant treatment trial. Arch Gen Psychiatry. 2010;67(3):277–285.
- , , , , , . Executive dysfunction and the course of geriatric depression. Biol Psychiatry. 2005;58(3):204–210.
- , , , , . Recognition of depression in older medical inpatients. J Gen Intern Med. 2007;22(5):559–564.
- , , , , , . A collaborative care depression management program for cardiac inpatients: depression characteristics and in‐hospital outcomes. Psychosomatics. 2011;52(1):26–33.
- , , , , , . Impact of a depression care management program for hospitalized cardiac patients. Circ Cardiovasc Qual Outcomes. 2011;4(2):198–205.
- , , , et al. Potentially inappropriate medication use in hospitalized elders. J Hosp Med. 2008;3(2):91–102.
- , , , , , . Prevalence, incidence and nature of prescribing errors in hospital inpatients: a systematic review. Drug Saf. 2009;32(5):379–389.
- , . Minimizing adverse drug events in older patients. Am Fam Physician. 2007;76(12):1837–1844.
- , , , , . STOPP (Screening Tool of Older Person's Prescriptions) and START (Screening Tool to Alert doctors to Right Treatment). Consensus validation. Int J Clin Pharmacol Ther. 2008;46(2):72–83.
- American Geriatrics Society 2012 Beers Criteria Update Expert Panel. American Geriatrics Society updated Beers Criteria for potentially inappropriate medication use in older adults. J Am Geriatr Soc. 2012;60(4):616–631.
- , , , . Preventing potentially inappropriate medication use in hospitalized older patients with a computerized provider order entry warning system. Arch Intern Med. 2010;170(15):1331–1336.
- . Hazards of Hospitalization of the Elderly. Ann Intern Med. 1993;118(3):219–223.
- . Improving health care for older persons. Ann Intern Med. 2003;139(5 part 2):421–424.
- , , , , , . Successful models of comprehensive care for older adults with chronic conditions: evidence for the Institute of Medicine's "retooling for an aging America" report. J Am Geriatr Soc. 2009;57(12):2328–2337.
- , , , et al. Recovery of activities of daily living in older adults after hospitalization for acute medical illness. J Am Geriatr Soc. 2008;56(12):2171–2179.
- , , , . Changes in functional status and the risks of subsequent nursing home placement and death. J Gerontol. 1993;48(3):S94–S101.
- , , . Risk factors for nursing home placement in a population‐based dementia cohort. J Am Geriatr Soc. 2000;48(5):519–525.
- , , , . The underrecognized epidemic of low mobility during hospitalization of older adults. J Am Geriatr Soc. 2009;57(9):1660–1665.
- , , , , . A systematic review of predictors and screening instruments to identify older hospitalized patients at risk for functional decline. J Clin Nurs. 2007;16(1):46–57.
- . Immobility and falls. Clin Geriatr Med. 1998;14(4):699–726.
- , , . Prevalence and outcomes of low mobility in hospitalized older patients. J Am Geriatr Soc. 2004;52(8):1263–1270.
- , , . Impact of a nurse‐driven mobility protocol on functional decline in hospitalized older adults. J Nurs Care Qual. 2009;24(4):325–331.
- , . Impact of early mobilization protocol on the medical‐surgical inpatient population: an integrated review of literature. Clin Nurse Spec. 2012;26(2):87–94.
- , , , . The aging population and its impact on the surgery workforce. Ann Surg. 2003;238(2):170–177.
- . Perioperative care of the elderly patient: an update. Cleve Clin J Med. 2009;76(suppl 4):S16–S21.
- , , . Frailty in the older surgical patient: a review. Age Ageing. 2012;41(2):142–147.
- . The assessment and management of peri‐operative pain in older adults. Anaesthesia. 2014;69(suppl 1):54–60.
- , . National Research Strategies: what outcomes are important in peri‐operative elderly care? Anaesthesia. 2014;69(suppl 1):61–69.
- , . 2002 National Hospital Discharge Survey. Adv Data. 2004;342:1–30.
- , . Hospitalization in the United States, 2002. Rockville, MD: Agency for Healthcare Research and Quality; 2005.
- , , , et al. The Curriculum for the Hospitalized Aging Medical Patient program: a collaborative faculty development program for hospitalists, general internists, and geriatricians. J Hosp Med. 2008;3(5):384–393.
- , . Advancement of geriatrics education. J Hosp Med. 2011;6(6):370.
- , , , , , . Advancing geriatrics education: an efficient faculty development program for academic hospitalists increases geriatric teaching. J Hosp Med. 2010;5(9):541–546.
Older adults with high levels of medical complexity occupy an increasing fraction of beds in acute‐care hospitals in the United States.[1, 2] By 2007, patients age 65 years and older accounted for nearly half of adult inpatient days of care.[1] These patients are commonly cared for by hospitalists who number more than 40,000.[3] Although hospitalists are most often trained in internal medicine, they have typically received limited formal geriatrics training. Increasingly, access to experts in geriatric medicine is limited.[4] Further, hospitalists and others who practice in acute care are limited by the lack of research to address the needs of the older adult population, specifically in the diagnosis and management of conditions encountered during acute illness.
To better support hospitalists in providing acute inpatient geriatric care, the Society of Hospital Medicine (SHM) partnered with the Association of Specialty Professors to develop a research agenda to bridge this gap. Using methodology from the James Lind Alliance (JLA) and the Patient Centered Outcomes Research Institute (PCORI), the SHM joined with older adult advocacy groups, professional societies of providers, and funders to create a geriatric‐focused acute‐care research agenda, highlighting 10 key research questions.[5, 6, 7] The goal of this approach was to produce and promote high integrity, evidence‐based information that comes from research guided by patients, caregivers, and the broader healthcare community.[8] In this article, we describe the methodology and results of this agenda‐setting process, referred to as the Acute Care of Older Patients (ACOP) Priority Setting Partnership.
METHODS
Overview
This project focused on topic generation, the first step in the PCORI framework for identification and prioritization of research areas.[5] We employed a specific and defined methodology to elicit and prioritize potential research topics incorporating input from representatives of older patients, family caregivers, and healthcare providers.[6]
To elicit this input, we chose a collaborative and consultative approach to stakeholder engagement, drawing heavily from the published work of the JLA, an initiative promoting patient‐clinician partnerships in health research developed in the United Kingdom.[6] We previously described the approach elsewhere.[7]
The ACOP process for determining the research agenda consisted of 4 steps: (1) convene, (2) consult, (3) collate, and (4) prioritize.[6] Through these steps, detailed below, we were able to obtain input from a broad group of stakeholders and engage the stakeholders in a process of reducing and refining our research questions.
Convene
The steering committee (the article's authors) convened a stakeholder partnership group that included stakeholders representing patients and caregivers, advocacy organizations for the elderly, organizations that address diseases and conditions common among hospitalized older patients, provider professional societies (eg, hospitalists, subspecialists, and nurses and social workers), payers, and funders. Patient, caregiver, and advocacy organizations were identified based on their engagement in aging and health policy advocacy by SHM staff and 1 author who had completed a Health and Aging Policy Fellowship (H.L.W.).
The steering committee issued e‐mail invitations to stakeholder organizations, making initial inquiries through professional staff and relevant committee chairs. Second inquiries were made via e‐mail to each organization's volunteer leadership. We developed a webinar that outlined the overall research agenda setting process and distributed the webinar to all stakeholders. The stakeholder organizations were asked to commit to (1) surveying their memberships and (2) participating actively in prioritization by e‐mail and at a 1‐day meeting in Washington DC.
Consult
Each stakeholder organization conducted a survey of its membership via an Internet‐based survey in the summer of 2013 (see Supporting Information, Appendix A, in the online version of this article). Stakeholder organizations were asked to provide up to 75 survey responses each. Though a standard survey was used, the steering committee was not prescriptive in the methodology of survey distribution to accommodate the structure and communication methods of the individual stakeholder organizations. Survey respondents were asked to identify up to 5 unanswered questions relevant to the acute care of older persons and also provide demographic information.
Collate
In the collating process, we clarified and categorized the unanswered questions submitted in the individual surveys. Each question was initially reviewed by a member of the steering committee, using explicit criteria (see Supporting Information, Appendix B, in the online version of this article). Questions that did not meet all 4 criteria were removed. For questions that met all criteria, we clarified language, combined similar questions, and categorized each question. Categories were created in a grounded process, in which individual reviewers assigned categories based on the content of the questions. Each question could be assigned to up to 2 categories. Each question was then reviewed by a second member of the steering committee using the same 4 criteria. As part of this review, similar questions were consolidated, and when possible, questions were rewritten in a standard format.[6]
Finally, the steering committee reviewed previously published research agendas looking for additional relevant unanswered questions, specifically the New Frontiers Research Agenda created by the American Geriatrics Society in conjunction with participating subspecialty societies,[9] the Cochrane Library, and other systematic reviews identified in the literature via PubMed search.[10, 11, 12, 13, 14, 15]
Prioritize
The resulting list of unanswered questions was prioritized in 2 phases. First, the list was e‐mailed to all stakeholder organizations. The organizations were asked to vote on their top 10 priorities from this list using an online ballot, assigning 10 points to their highest priority down to 1 point for their lowest priority. In so doing, they were asked to consider explicit criteria (see Supporting Information, Appendix B, in the online version of this article). Each organization had only 1 ballot and could arrive at their top 10 list in any manner they wished. The balloting from this phase was used to develop a list of unanswered questions for the second round of in‐person prioritization. Each priority's scores were totaled across all voting organizations. The 29 priorities with the highest point totals were brought to the final prioritization round because of a natural cut point at priority number 29, rather than number 30.
For the final prioritization round, the steering committee facilitated an in‐person meeting in Washington, DC in October 2013 using nominal group technique (NGT) methodologies to arrive at consensus.[16] During this process stakeholders were asked to consider additional criteria (see Supporting Information, Appendix B, in the online version of this article).
RESULTS
Table 1 lists the organizations who engaged in 1 or more parts of the topic generation process. Eighteen stakeholder organizations agreed to participate in the convening process. Ten organizations did not respond to our solicitation and 1 declined to participate.
| Organization (N=18) | Consultation % of Survey Responses (N=580) | Prioritization Round 1 | Prioritization Round 2 |
|---|---|---|---|
| Alzheimer's Association | 7.0% | Yes | Yes |
| American Academy of Neurology | 3.4% | Yes | Yes |
| American Association of Retired Persons | 0.8% | No | No |
| American College of Cardiology | 11.4% | Yes | Yes |
| American College of Emergency Physicians | 1.3% | No | No |
| American College of Surgeons | 1.0% | Yes | Yes |
| American Geriatrics Society | 7.6% | Yes | Yes |
| American Hospital Association | 1.7% | Yes | No |
| Centers for Medicare & Medicaid Services | 0.8% | Yes | Yes |
| Gerontological Society of America | 18.9% | Yes | Yes |
| National Alliance for Caregiving | 1.0% | Yes | Yes |
| National Association of Social Workers | 5.9% | Yes | Yes |
| National Coalition for Healthcare | 0.6% | No | No |
| National Institute on Aging | 2.1% | Yes | Yes |
| National Partnership for Women and Families | 0.0% | Yes | Yes |
| Nursing Improving Care for Healthsystem Elders | 28.6% | Yes | No |
| Society of Critical Care Medicine | 12.0% | Yes | Yes |
| Society of Hospital Medicine | 4.6% | Yes | Yes |
Seventeen stakeholder organizations obtained survey responses from a total of 580 individuals (range, 3150 per organization), who were asked to identify important unanswered questions in the acute care of older persons. Survey respondents were typically female (77%), white (85%), aged 45 to 65 years (65%), and identified themselves as health professionals (90%). Twenty‐six percent of respondents also identified as patients or family caregivers. Their surveys included 1299 individual questions.
Figure 1 summarizes our collation and prioritization process and reports the numbers of questions resulting at each stage. Nine hundred nineteen questions were removed during the first review conducted by steering committee members, and 31 question categories were identified. An additional 305 questions were removed in the second review, with 75 questions remaining. As the final step of the collating process, literature review identified 39 relevant questions not already suggested or moved forward through our consultation and collation process. These questions were added to the list of unanswered questions.

In the first round of prioritization, this list of 114 questions was emailed to each stakeholder organization (Table 1). After the stakeholder voting process was completed, 29 unanswered questions remained (see Supporting Information, Appendix C, in the online version of this article). These questions were refined and prioritized in the in‐person meeting to create the final list of 10 questions. The stakeholders present in the meeting represented 13 organizations (Table 1). Using the NGT with several rounds of small group breakouts and large group deliberation, 9 of the top 10 questions were selected from the list of 29. One additional highly relevant question that had been removed earlier in the collation process regarding workforce was added back by the stakeholder group.
This prioritized research agenda appears in Table 2 and below, organized alphabetically by topic.
- Advanced care planning: What approaches for determining and communicating goals of care across and within healthcare settings are most effective in promoting goal‐concordant care for hospitalized older patients?
- Care transitions: What is the comparative effectiveness of transitional care models on patient‐centered outcomes for hospitalized older adults?
- Delirium: What practices are most effective for consistent recognition, prevention, and treatment of delirium subtypes among hospitalized older adults?
- Dementia: Does universal assessment of hospitalized older adults for cognitive impairment (eg, at presentation and/or discharge) lead to more appropriate application of geriatric care principles and improve patient‐centered outcomes?
- Depression: Does identifying depressive symptoms during a hospital stay and initiating a therapeutic plan prior to discharge improve patient‐centered and/or disease‐specific outcomes?
- Medications: What systems interventions improve medication management for older adults (ie, appropriateness of medication choices and dosing, compliance, cost) in the hospital and postacute care?
- Models of care: For which populations of hospitalized older adults does systematic implementation of geriatric care principles/processes improve patient‐centered outcomes?
- Physical function: What is the comparative effectiveness of interventions that promote in‐hospital mobility, improve and preserve physical function, and reduce falls among older hospitalized patients?
- Surgery: What perioperative strategies can be used to optimize care processes and improve outcomes in older surgical patients?
- Training: What is the most effective approach to training hospital‐based providers in geriatric and palliative care competencies?
| Topic | Scope of Problem | What Is known | Unanswered Question | Proposed Dimensions |
|---|---|---|---|---|
| ||||
| Advanced‐care planning | Older persons who lack decision‐making capacity often do not have surrogates or clear goals of care documented.[19] Advanced‐care directives are associated with an increase in patient autonomy and empowerment, and although 15% to 25% of adults completed the documentation in 2004,[20] a recent study found completion rates have increased to 72%.[21] | Nursing home residents with advanced directives are less likely to be hospitalized.[22, 23] Advanced directive tools, such as POLST, work to translate patient preferences to medical order.[24] standardized patient transfer tools may help to improve transitions between nursing homes and hospitals.[25] However, advanced care planning fails to integrate into courses of care if providers are unwilling or unskilled in using advanced care documentation.[26] | What approaches for determining and communicating goals of care across and within healthcare settings are most effective in promoting goal‐concordant care for hospitalized older patients? | Potential interventions: |
| Decision aids | ||||
| Standard interdisciplinary advanced care planning approach | ||||
| Patient advocates | ||||
| Potential outcomes might include: | ||||
| Completion of advanced directives and healthcare power of attorney | ||||
| Patient‐centered outcomesa | ||||
| Care transitions | Hospital readmission from home and skilled nursing facilities occurs within 30 days in up to a quarter of patients.[27, 28] The discharge of complex older hospitalized patients is fraught with challenges. The quality of the hospital discharge process can influence outcomes for vulnerable older patients.[29, 30, 31, 32] Studies measuring the quality of hospital discharge frequently find deficits in documentation of assessment of geriatric syndromes,[33] poor patient/caregiver understanding,[34, 35] and poor communication and follow‐up with postacute providers.[35, 36, 37, 38] | As many as 10 separate domains may influence the success of a discharge.[39] There is limited evidence, regarding quality‐of‐care transitions for hospitalized older patients. The Coordinated‐Transitional Care Program found that follow‐up with telecommunication decreased readmission rates and improved transitional care for a high‐risk condition veteran population.[40] There is modest evidence for single interventions,[41] whereas the most effective hospital‐to‐community care interventions address multiple processes in nongeriatric populations.[39, 42, 43] | What is the comparative effectiveness of the transitional care models on patient‐centered outcomes for hospitalized older adults? | Possible models: |
| Established vs novel care‐transition models | ||||
| Disease‐specific vs general approaches | ||||
| Accountable care models | ||||
| Caregiver and family engagement | ||||
| Community engagement | ||||
| Populations of interest: | ||||
| Patients with dementia | ||||
| Patients with multimorbidity | ||||
| Patients with geriatric syndromes | ||||
| Patients with psychiatric disease | ||||
| Racially and ethnically diverse patients | ||||
| Outcomes: | ||||
| Readmission | ||||
| Other adverse events | ||||
| Cost and healthcare utilization | ||||
| Patient‐centered outcomesa | ||||
| Delirium | Among older inpatients, the prevalence of delirium varies with severity of illness. Among general medical patients, in‐hospital prevalence ranges from 10% to 25 %.[44, 45] In the ICU, prevalence estimates are higher, ranging from 25% to as high as 80%.[46, 47] Delirium independently predicts increased length of stay,[48, 49] long‐term cognitive impairment,[50, 51] functional decline,[51] institutionalization,[52] and short‐ and long‐term mortality.[52, 53, 54] | Multicomponent strategies have been shown to be effective in preventing delirium. A systematic review of 19 such interventions identified the most commonly included such as[55]: early mobilization, nutrition supplements, medication review, pain management, sleep enhancement, vision/hearing protocols, and specialized geriatric care. Studies have included general medical patients, postoperative patients, and patients in the ICU. The majority of these studies found reductions in either delirium incidence (including postoperative), delirium prevalence, or delirium duration. Although medications have not been effective in treating delirium in general medical patients,[48] the choice and dose of sedative agents has been shown to impact delirium in the ICU.[56, 57, 58] | What practices are most effective for consistent recognition, prevention, and treatment of delirium subtypes (hypoactive, hyperactive, and mixed) among hospitalized older adults? | Outcomes to examine: |
| Delirium incidence (including postoperative) | ||||
| Delirium duration | ||||
| Delirium‐/coma‐free days | ||||
| Delirium prevalence at discharge | ||||
| Subsyndromal delirium | ||||
| Potential prevention and treatment modalities: | ||||
| Family education or psychosocial interventions | ||||
| Pharmacologic interventions | ||||
| Environmental modifications | ||||
| Possible areas of focus: | ||||
| Special populations | ||||
| Patients with varying stages of dementia | ||||
| Patients with multimorbidity | ||||
| Patients with geriatric syndromes | ||||
| Observation patients | ||||
| Diverse settings | ||||
| Emergency department | ||||
| Perioperative | ||||
| Skilled nursing/rehab/long‐term acute‐care facilities | ||||
| Dementia | 13% to 63% of older persons in the hospital have dementia.[59] Dementia is often unrecognized among hospitalized patients.[60] The presence of dementia is associated with a more rapid functional decline during admission and delayed hospital discharge.[59] Patients with dementia require more nursing hours, and are more likely to have complications[61] or die in care homes rather than in their preferred site.[59] | Several tools have been validated to screen for dementia in the hospital setting.[62] Studies have assessed approaches to diagnosing delirium in hospitalized patients with dementia.[63] Cognitive and functional stimulation interventions may have a positive impact on reducing behavioral issues.[64, 65] | Does universal assessment of hospitalized older adults for cognitive impairment (eg, at presentation and/or discharge) lead to more appropriate application of geriatric care principles and improve patient centered outcomes? | Potential interventions: |
| Dementia or delirium care | ||||
| Patient/family communication and engagement strategies | ||||
| Maintenance/recovery of independent functional status | ||||
| Potential outcomes: | ||||
| Patient‐centered outcomesa | ||||
| Length of stay, cost, and healthcare utilization (including palliative care) | ||||
| Immediate invasive vs early conservative treatments pursued | ||||
| Depression | Depression is a common geriatric syndrome among acutely ill older patients, occurring in up to 45% of patients.[66, 67] Rates of depression are similar among patients discharged following a critical illness, with somatic, rather than cognitive‐affective complaints being the most prevalent.[68] Depression among inpatients or immediately following hospitalization independently predicts worse functional outcomes,[69] cognitive decline,[70] hospital readmission,[71, 72] and long‐term mortality.[69, 73] Finally, geriatric patients are known to respond differently to medical treatment.[74, 75] | Although highly prevalent, depression is poorly recognized and managed in the inpatient setting. Depression is recognized in only 50% of patients, with previously undiagnosed or untreated depression being at highest risk for being missed.[76] The role of treatment of depression in the inpatient setting is poorly understood, particularly for those with newly recognized depression or depressive symptoms. Some novel collaborative care and telephone outreach programs have led to increases in depression treatment in patients with specific medical and surgical conditions, resulting in early promising mental health and comorbid outcomes.[77, 78] The efficacy of such programs for older patients is unknown. | Does identifying depressive symptoms during a hospital stay and initiating a therapeutic plan prior to discharge improve patient‐centered and/or disease‐specific outcomes? | Possible areas of focus: |
| Comprehensive geriatric and psychosocial assessment; | ||||
| Inpatient vs outpatient initiation of pharmacological therapy | ||||
| Integration of confusion assessment method into therapeutic approaches | ||||
| Linkages with outpatient mental health resources | ||||
| Medications | Medication exposure, particularly potentially inappropriate medications, is common in hospitalized elders.[79] Medication errorsof dosage, type, and discrepancy between what a patient takes at home and what is known to his/her prescribing physicianare common and adversely affects patient safety.[80] Geriatric populations are disproportionately affected, especially those taking more than 5 prescription medications per day.[81] | Numerous strategies including electronic alerts, screening protocols, and potentially inappropriate medication lists (Beers list, STOPP) exist, though the optimal strategies to limit the use of potentially inappropriate medications is not yet known.[82, 83, 84] | What systems interventions improve medication management for older adults (ie, appropriateness of medication choices and dosing, compliance, cost) in hospital and post‐acute care? | Possible areas of focus: |
| Use of healthcare information technology | ||||
| Communication across sites of care | ||||
| Reducing medication‐related adverse events | ||||
| Engagement of family caregivers | ||||
| Patient‐centered strategies to simplify regimens | ||||
| Models of care | Hospitalization marks a time of high risk for older patients. Up to half die during hospitalization or within the year following the hospitalization. There is high risk of nosocomial events, and more than a third experience a decline in health resulting in longer hospitalizations and/or placement in extended‐care facilities.[73, 85, 86] | Comprehensive inpatient care for older adults (acute care for elders units, geriatric evaluation and management units, geriatric consultation services) were studied in 2 meta‐analyses, 5 RCTs, and 1 quasiexperimental study and summarized in a systematic review.[87] The studies reported improved quality of care (1 of 1 article), quality of life (3 of 4), functional autonomy (5 of 6), survival (3 of 6), and equal or lower healthcare utilization (7 of 8). | For which populations of hospitalized older adults does systematic implementation of geriatric care principles/processes improve patient‐centered outcomes? | Potential populations: |
| Patients of the emergency department, critical care, perioperative, and targeted medical/surgical units | ||||
| Examples of care principles: | ||||
| Geriatric assessment, early mobility, medication management, delirium prevention, advanced‐care planning, risk‐factor modification, caregiver engagement | ||||
| Potential outcomes: | ||||
| Patient‐centered outcomesa | ||||
| Cost | ||||
| Physical function | Half of older patients will lose functional capacity during hospitalization.[88] Loss of physical function, particularly of lower extremities, is a risk factor for nursing home placement.[89, 90] Older hospitalized patients spend the majority (up to 80%) of their time lying in bed, even when they are capable of walking independently.[91] | Loss of independences with ADL capabilities is associated with longer hospital stays, higher readmission rates, and higher mortality risk.[92] Excessive time in bed during a hospital stay is also associated with falls.[93] Often, hospital nursing protocols and physician orders increase in‐hospital immobility in patients.[91, 94] However, nursing‐driven mobility protocols can improve functional outcomes of older hospitalized patients.[95, 96] | What is the comparative effectiveness of interventions that promote in‐hospital mobility, improve and preserve physical function, and reduce falls among older hospitalized patients? | Potential interventions: |
| Intensive physical therapy | ||||
| Incidental functional training | ||||
| Restraint reduction | ||||
| Medication management | ||||
| Potential outcomes: | ||||
| Discharge location | ||||
| Delirium, pressure ulcers, and falls | ||||
| Surgery | An increasing number of persons over age 65 years are undergoing surgical procedures.[97] These persons are at increased risk for developing delirium/cogitative dysfunction,[98] loss of functional status,[99] and exacerbations of chronic illness.[97] Additionally, pain management may be harder to address in this population.[100] Current outcomes may not reflect the clinical needs of elder surgical patients.[101] | Tailored drug selection and nursing protocols may prevent delirium.[98] Postoperative cognitive dysfunction may require weeks for resolution. Identifying frail patients preoperatively may lead to more appropriate risk stratification and improved surgical outcomes.[99] Pain management strategies focused on mitigating cognitive impact and other effects may also be beneficial.[100] Development of risk‐adjustment tools specific to older populations, as well as measures of frailty and patient‐centered care, have been proposed.[101] | What perioperative strategies can be used to optimize care processes and improve outcomes in older surgical patients? | Potential strategies: |
| Preoperative risk assessment and optimization for frail or multimorbid older patients | ||||
| Perioperative management protocols for frail or multimorbid older patients | ||||
| Potential outcomes: | ||||
| Postoperative patient centered outcomesa | ||||
| Perioperative cost, healthcare utilization | ||||
| Training | Adults over age 65 years comprise 13.2 % of the US population, but account for >30% of hospital discharges and 50% of hospital days.[86, 102, 103] By 2030, there will only be 1 geriatrician for every 3798 Americans >75 years.[4] Between 1997 and 2006, the odds that a hospitalist would treat a hospitalized Medicare patient rose 29% per year.[3] | Train the trainer programs for physicians include the CHAMP, the AGESP, and the PAGE. Education for nurses include the NICHE. Outcomes include improved self‐confidence, attitudes, teaching skills, and geriatric care environment.[104, 105, 106] | What is the most effective approach to training hospital‐based providers in geriatric and palliative care competencies? | Potential interventions: |
| Mentored implementation | ||||
| Train the trainer | ||||
| Technical support | ||||
Table 2 also contains a capsule summary of the scope of the problem addressed by each research priority, a capsule summary of related work in the content area (what is known) not intended as a systematic review, and proposed dimensions or subquestions suggested by the stakeholders at the final prioritization meeting
DISCUSSION
Older hospitalized patients account for an increasing number and proportion of hospitalized patients,[1, 2] and hospitalists increasingly are responsible for inpatient care for this population.[3] The knowledge required for hospitalists to deliver optimal care and improve outcomes has not kept pace with the rapid growth of either hospitalists or hospitalized elders. Through a rigorous prioritization process, we identified 10 areas that deserve the highest priority in directing future research efforts to improve care for the older hospitalized patient. Assessment, prevention, and treatment of geriatric syndromes in the hospital account for almost half of the priority areas. Additional research is needed to improve advanced care planning, develop new care models, and develop training models for future hospitalists competent in geriatric and palliative care competencies.
A decade ago, the American Geriatric Society and the John A. Hartford Foundation embarked upon a research agenda aimed at improving the care of hospitalized elders cared for by specialists (ie, New Frontiers in Geriatrics Research: An Agenda for Surgical and Related Medical Specialties).[9] This effort differed in many important ways from the current priortization process. First, the New Frontiers agenda focused upon specific diseases, whereas the ACOP agenda addresses geriatric syndromes that cut across multiple diseases. Second, the New Frontiers agenda was made by researchers and based upon published literature, whereas the ACOP agenda involved the input of multiple stakeholders. Finally, the New Frontiers prioritized a research agenda across a number of surgical specialties, emergency medicine, and geriatric rehabilitation. Hospital medicine, however, was still early in its development and was not considered a unique specialty. Since that time, hospital medicine has matured into a unique specialty, with increased numbers of hospitalists,[3] increased research in hospital medicine,[17] and a separate recertification pathway for internal medicine licensure.[18] To date, there has not been a similar effort performed to direct geriatric research efforts for hospital medicine.
For researchers working in the field of hospital medicine, this list of topics has several implications. First, as hospitalists are commonly generalists, hospitalist researchers may be particularly well‐suited to study syndromes that cut across specialties. However, this does raise concerns about funding sources, as most National Institutes of Health institutes are disease‐focused. Funders that are not disease‐focused such as PCORI, National Institute on Aging, National Institute of Nursing Research, and Agency for Healthcare Research and Quality, and private foundations (Hartford, Robert Wood Johnson, and Commonwealth) may be more fruitful sources of funding for this work, but funding may be challenging. Nonetheless, the increased focus on patient‐centered work may increase funders' interest in such work. Second, the topics on this list would suggest that interventions will not be pharmacologic, but will focus on nonpharmacologic, behavioral, and social interventions. Similarly, outcomes of interest must expand beyond utilization metrics such as length of stay and mortality, to include functional status and symptom management, and goal‐concordant care. Therefore, research in geriatric acute care will necessarily be multidisciplinary.
Although these 10 high‐priority areas have been selected, this prioritized list is inherently limited by our methodology. First, our survey question was not focused on a disease state, and this wording may have resulted in the list favoring geriatric syndromes rather than common disease processes. Additionally, the resulting questions encompass large research areas and not specific questions about discrete interventions. Our results may also have been skewed by the types of engaged respondents who participated in the consultation, collating, and prioritization phases. In particular, we had a large response from geriatric medicine nurses, whereas some stakeholder groups provided no survey responses. Thus, these respondents were not representative of all possible stakeholders, nor were the survey respondents necessarily representative of each of their organizations. Nonetheless, the participants self‐identified as representative of diverse viewpoints that included patients, caregivers, and advocacy groups, with the majority of stakeholder organizations remaining engaged through the completion of the process. Thus, the general nature of this agenda helps us focus upon larger areas of importance, leaving researchers the flexibility to choose to narrow the focus on a specific research question that may include potential interventions and unique outcomes. Finally, our methodology may have inadvertently limited the number of patient and family caregiver voices in the process given our approach to large advocacy groups, our desire to be inclusive of healthcare professional organizations, and our survey methodology. Other methodologies may have reached more patients and caregivers, yet many healthcare professionals have served as family caregivers to frail elders requiring hospitalization and may have been in an ideal position to answer the survey.
In conclusion, several forces are shaping the future of acute inpatient care. These include the changing demographics of the hospitalized patient population, a rapid increase in the proportion of multimorbid hospitalized older adults, an inpatient workforce (hospitalists, generalists, and subspecialists) with potentially limited geriatrics training, and gaps in evidence‐based guidance to inform diagnostic and therapeutic decision making for acutely ill older patients. Training programs in hospital medicine should be aware of and could benefit from the resulting list of unanswered questions. Our findings also have implications for training to enrich education in geriatrics. Moreover, there is growing recognition that patients and other stakeholders deserve a greater voice in determining the direction of research. In addition to efforts to improve patient‐centeredness of research, these areas have been uniquely identified by stakeholders as important, and therefore are in line with newer priorities of PCORI. This project followed a road map resulting in a patient‐centered research agenda at the intersection of hospital medicine and geriatric medicine.[7] In creating this agenda, we relied heavily on the framework proposed by PCORI. We propose to pursue a dissemination and evaluation strategy for this research agenda as well as additional prioritization steps. We believe the adoption of this methodology will create a knowledge base that is rigorously derived and most relevant to the care of hospitalized older adults and their families. Its application will ultimately result in improved outcomes for hospitalized older adults.
Acknowledgements
The authors acknowledge Claudia Stahl, Society of Hospital Medicine; Cynthia Drake, University of Colorado; and the ACOP stakeholder organizations.
Disclosures: This work was supported by the Association of Specialty Professors/American Society of Internal Medicine and the John A. Hartford Foundation. Dr. Vasilevskis was supported by the National Institute on Aging of the National Institutes of Health under award number K23AG040157 and the Veterans Affairs Clinical Research Center of Excellence, and the Geriatric Research, Education and Clinical Center (GRECC). Dr. Vasilevskis' institution receives grant funding for an aspect of submitted work. Dr. Meltzer is a PCORI Methodology Committee member. The content is solely the responsibility of the authors and does not necessarily represent the official views of the National Institutes of Health or the Department of Veterans' Affairs. The authors report no conflicts of interest.
Older adults with high levels of medical complexity occupy an increasing fraction of beds in acute‐care hospitals in the United States.[1, 2] By 2007, patients age 65 years and older accounted for nearly half of adult inpatient days of care.[1] These patients are commonly cared for by hospitalists who number more than 40,000.[3] Although hospitalists are most often trained in internal medicine, they have typically received limited formal geriatrics training. Increasingly, access to experts in geriatric medicine is limited.[4] Further, hospitalists and others who practice in acute care are limited by the lack of research to address the needs of the older adult population, specifically in the diagnosis and management of conditions encountered during acute illness.
To better support hospitalists in providing acute inpatient geriatric care, the Society of Hospital Medicine (SHM) partnered with the Association of Specialty Professors to develop a research agenda to bridge this gap. Using methodology from the James Lind Alliance (JLA) and the Patient Centered Outcomes Research Institute (PCORI), the SHM joined with older adult advocacy groups, professional societies of providers, and funders to create a geriatric‐focused acute‐care research agenda, highlighting 10 key research questions.[5, 6, 7] The goal of this approach was to produce and promote high integrity, evidence‐based information that comes from research guided by patients, caregivers, and the broader healthcare community.[8] In this article, we describe the methodology and results of this agenda‐setting process, referred to as the Acute Care of Older Patients (ACOP) Priority Setting Partnership.
METHODS
Overview
This project focused on topic generation, the first step in the PCORI framework for identification and prioritization of research areas.[5] We employed a specific and defined methodology to elicit and prioritize potential research topics incorporating input from representatives of older patients, family caregivers, and healthcare providers.[6]
To elicit this input, we chose a collaborative and consultative approach to stakeholder engagement, drawing heavily from the published work of the JLA, an initiative promoting patient‐clinician partnerships in health research developed in the United Kingdom.[6] We previously described the approach elsewhere.[7]
The ACOP process for determining the research agenda consisted of 4 steps: (1) convene, (2) consult, (3) collate, and (4) prioritize.[6] Through these steps, detailed below, we were able to obtain input from a broad group of stakeholders and engage the stakeholders in a process of reducing and refining our research questions.
Convene
The steering committee (the article's authors) convened a stakeholder partnership group that included stakeholders representing patients and caregivers, advocacy organizations for the elderly, organizations that address diseases and conditions common among hospitalized older patients, provider professional societies (eg, hospitalists, subspecialists, and nurses and social workers), payers, and funders. Patient, caregiver, and advocacy organizations were identified based on their engagement in aging and health policy advocacy by SHM staff and 1 author who had completed a Health and Aging Policy Fellowship (H.L.W.).
The steering committee issued e‐mail invitations to stakeholder organizations, making initial inquiries through professional staff and relevant committee chairs. Second inquiries were made via e‐mail to each organization's volunteer leadership. We developed a webinar that outlined the overall research agenda setting process and distributed the webinar to all stakeholders. The stakeholder organizations were asked to commit to (1) surveying their memberships and (2) participating actively in prioritization by e‐mail and at a 1‐day meeting in Washington DC.
Consult
Each stakeholder organization conducted a survey of its membership via an Internet‐based survey in the summer of 2013 (see Supporting Information, Appendix A, in the online version of this article). Stakeholder organizations were asked to provide up to 75 survey responses each. Though a standard survey was used, the steering committee was not prescriptive in the methodology of survey distribution to accommodate the structure and communication methods of the individual stakeholder organizations. Survey respondents were asked to identify up to 5 unanswered questions relevant to the acute care of older persons and also provide demographic information.
Collate
In the collating process, we clarified and categorized the unanswered questions submitted in the individual surveys. Each question was initially reviewed by a member of the steering committee, using explicit criteria (see Supporting Information, Appendix B, in the online version of this article). Questions that did not meet all 4 criteria were removed. For questions that met all criteria, we clarified language, combined similar questions, and categorized each question. Categories were created in a grounded process, in which individual reviewers assigned categories based on the content of the questions. Each question could be assigned to up to 2 categories. Each question was then reviewed by a second member of the steering committee using the same 4 criteria. As part of this review, similar questions were consolidated, and when possible, questions were rewritten in a standard format.[6]
Finally, the steering committee reviewed previously published research agendas looking for additional relevant unanswered questions, specifically the New Frontiers Research Agenda created by the American Geriatrics Society in conjunction with participating subspecialty societies,[9] the Cochrane Library, and other systematic reviews identified in the literature via PubMed search.[10, 11, 12, 13, 14, 15]
Prioritize
The resulting list of unanswered questions was prioritized in 2 phases. First, the list was e‐mailed to all stakeholder organizations. The organizations were asked to vote on their top 10 priorities from this list using an online ballot, assigning 10 points to their highest priority down to 1 point for their lowest priority. In so doing, they were asked to consider explicit criteria (see Supporting Information, Appendix B, in the online version of this article). Each organization had only 1 ballot and could arrive at their top 10 list in any manner they wished. The balloting from this phase was used to develop a list of unanswered questions for the second round of in‐person prioritization. Each priority's scores were totaled across all voting organizations. The 29 priorities with the highest point totals were brought to the final prioritization round because of a natural cut point at priority number 29, rather than number 30.
For the final prioritization round, the steering committee facilitated an in‐person meeting in Washington, DC in October 2013 using nominal group technique (NGT) methodologies to arrive at consensus.[16] During this process stakeholders were asked to consider additional criteria (see Supporting Information, Appendix B, in the online version of this article).
RESULTS
Table 1 lists the organizations who engaged in 1 or more parts of the topic generation process. Eighteen stakeholder organizations agreed to participate in the convening process. Ten organizations did not respond to our solicitation and 1 declined to participate.
| Organization (N=18) | Consultation % of Survey Responses (N=580) | Prioritization Round 1 | Prioritization Round 2 |
|---|---|---|---|
| Alzheimer's Association | 7.0% | Yes | Yes |
| American Academy of Neurology | 3.4% | Yes | Yes |
| American Association of Retired Persons | 0.8% | No | No |
| American College of Cardiology | 11.4% | Yes | Yes |
| American College of Emergency Physicians | 1.3% | No | No |
| American College of Surgeons | 1.0% | Yes | Yes |
| American Geriatrics Society | 7.6% | Yes | Yes |
| American Hospital Association | 1.7% | Yes | No |
| Centers for Medicare & Medicaid Services | 0.8% | Yes | Yes |
| Gerontological Society of America | 18.9% | Yes | Yes |
| National Alliance for Caregiving | 1.0% | Yes | Yes |
| National Association of Social Workers | 5.9% | Yes | Yes |
| National Coalition for Healthcare | 0.6% | No | No |
| National Institute on Aging | 2.1% | Yes | Yes |
| National Partnership for Women and Families | 0.0% | Yes | Yes |
| Nursing Improving Care for Healthsystem Elders | 28.6% | Yes | No |
| Society of Critical Care Medicine | 12.0% | Yes | Yes |
| Society of Hospital Medicine | 4.6% | Yes | Yes |
Seventeen stakeholder organizations obtained survey responses from a total of 580 individuals (range, 3150 per organization), who were asked to identify important unanswered questions in the acute care of older persons. Survey respondents were typically female (77%), white (85%), aged 45 to 65 years (65%), and identified themselves as health professionals (90%). Twenty‐six percent of respondents also identified as patients or family caregivers. Their surveys included 1299 individual questions.
Figure 1 summarizes our collation and prioritization process and reports the numbers of questions resulting at each stage. Nine hundred nineteen questions were removed during the first review conducted by steering committee members, and 31 question categories were identified. An additional 305 questions were removed in the second review, with 75 questions remaining. As the final step of the collating process, literature review identified 39 relevant questions not already suggested or moved forward through our consultation and collation process. These questions were added to the list of unanswered questions.

In the first round of prioritization, this list of 114 questions was emailed to each stakeholder organization (Table 1). After the stakeholder voting process was completed, 29 unanswered questions remained (see Supporting Information, Appendix C, in the online version of this article). These questions were refined and prioritized in the in‐person meeting to create the final list of 10 questions. The stakeholders present in the meeting represented 13 organizations (Table 1). Using the NGT with several rounds of small group breakouts and large group deliberation, 9 of the top 10 questions were selected from the list of 29. One additional highly relevant question that had been removed earlier in the collation process regarding workforce was added back by the stakeholder group.
This prioritized research agenda appears in Table 2 and below, organized alphabetically by topic.
- Advanced care planning: What approaches for determining and communicating goals of care across and within healthcare settings are most effective in promoting goal‐concordant care for hospitalized older patients?
- Care transitions: What is the comparative effectiveness of transitional care models on patient‐centered outcomes for hospitalized older adults?
- Delirium: What practices are most effective for consistent recognition, prevention, and treatment of delirium subtypes among hospitalized older adults?
- Dementia: Does universal assessment of hospitalized older adults for cognitive impairment (eg, at presentation and/or discharge) lead to more appropriate application of geriatric care principles and improve patient‐centered outcomes?
- Depression: Does identifying depressive symptoms during a hospital stay and initiating a therapeutic plan prior to discharge improve patient‐centered and/or disease‐specific outcomes?
- Medications: What systems interventions improve medication management for older adults (ie, appropriateness of medication choices and dosing, compliance, cost) in the hospital and postacute care?
- Models of care: For which populations of hospitalized older adults does systematic implementation of geriatric care principles/processes improve patient‐centered outcomes?
- Physical function: What is the comparative effectiveness of interventions that promote in‐hospital mobility, improve and preserve physical function, and reduce falls among older hospitalized patients?
- Surgery: What perioperative strategies can be used to optimize care processes and improve outcomes in older surgical patients?
- Training: What is the most effective approach to training hospital‐based providers in geriatric and palliative care competencies?
| Topic | Scope of Problem | What Is known | Unanswered Question | Proposed Dimensions |
|---|---|---|---|---|
| ||||
| Advanced‐care planning | Older persons who lack decision‐making capacity often do not have surrogates or clear goals of care documented.[19] Advanced‐care directives are associated with an increase in patient autonomy and empowerment, and although 15% to 25% of adults completed the documentation in 2004,[20] a recent study found completion rates have increased to 72%.[21] | Nursing home residents with advanced directives are less likely to be hospitalized.[22, 23] Advanced directive tools, such as POLST, work to translate patient preferences to medical order.[24] standardized patient transfer tools may help to improve transitions between nursing homes and hospitals.[25] However, advanced care planning fails to integrate into courses of care if providers are unwilling or unskilled in using advanced care documentation.[26] | What approaches for determining and communicating goals of care across and within healthcare settings are most effective in promoting goal‐concordant care for hospitalized older patients? | Potential interventions: |
| Decision aids | ||||
| Standard interdisciplinary advanced care planning approach | ||||
| Patient advocates | ||||
| Potential outcomes might include: | ||||
| Completion of advanced directives and healthcare power of attorney | ||||
| Patient‐centered outcomesa | ||||
| Care transitions | Hospital readmission from home and skilled nursing facilities occurs within 30 days in up to a quarter of patients.[27, 28] The discharge of complex older hospitalized patients is fraught with challenges. The quality of the hospital discharge process can influence outcomes for vulnerable older patients.[29, 30, 31, 32] Studies measuring the quality of hospital discharge frequently find deficits in documentation of assessment of geriatric syndromes,[33] poor patient/caregiver understanding,[34, 35] and poor communication and follow‐up with postacute providers.[35, 36, 37, 38] | As many as 10 separate domains may influence the success of a discharge.[39] There is limited evidence, regarding quality‐of‐care transitions for hospitalized older patients. The Coordinated‐Transitional Care Program found that follow‐up with telecommunication decreased readmission rates and improved transitional care for a high‐risk condition veteran population.[40] There is modest evidence for single interventions,[41] whereas the most effective hospital‐to‐community care interventions address multiple processes in nongeriatric populations.[39, 42, 43] | What is the comparative effectiveness of the transitional care models on patient‐centered outcomes for hospitalized older adults? | Possible models: |
| Established vs novel care‐transition models | ||||
| Disease‐specific vs general approaches | ||||
| Accountable care models | ||||
| Caregiver and family engagement | ||||
| Community engagement | ||||
| Populations of interest: | ||||
| Patients with dementia | ||||
| Patients with multimorbidity | ||||
| Patients with geriatric syndromes | ||||
| Patients with psychiatric disease | ||||
| Racially and ethnically diverse patients | ||||
| Outcomes: | ||||
| Readmission | ||||
| Other adverse events | ||||
| Cost and healthcare utilization | ||||
| Patient‐centered outcomesa | ||||
| Delirium | Among older inpatients, the prevalence of delirium varies with severity of illness. Among general medical patients, in‐hospital prevalence ranges from 10% to 25 %.[44, 45] In the ICU, prevalence estimates are higher, ranging from 25% to as high as 80%.[46, 47] Delirium independently predicts increased length of stay,[48, 49] long‐term cognitive impairment,[50, 51] functional decline,[51] institutionalization,[52] and short‐ and long‐term mortality.[52, 53, 54] | Multicomponent strategies have been shown to be effective in preventing delirium. A systematic review of 19 such interventions identified the most commonly included such as[55]: early mobilization, nutrition supplements, medication review, pain management, sleep enhancement, vision/hearing protocols, and specialized geriatric care. Studies have included general medical patients, postoperative patients, and patients in the ICU. The majority of these studies found reductions in either delirium incidence (including postoperative), delirium prevalence, or delirium duration. Although medications have not been effective in treating delirium in general medical patients,[48] the choice and dose of sedative agents has been shown to impact delirium in the ICU.[56, 57, 58] | What practices are most effective for consistent recognition, prevention, and treatment of delirium subtypes (hypoactive, hyperactive, and mixed) among hospitalized older adults? | Outcomes to examine: |
| Delirium incidence (including postoperative) | ||||
| Delirium duration | ||||
| Delirium‐/coma‐free days | ||||
| Delirium prevalence at discharge | ||||
| Subsyndromal delirium | ||||
| Potential prevention and treatment modalities: | ||||
| Family education or psychosocial interventions | ||||
| Pharmacologic interventions | ||||
| Environmental modifications | ||||
| Possible areas of focus: | ||||
| Special populations | ||||
| Patients with varying stages of dementia | ||||
| Patients with multimorbidity | ||||
| Patients with geriatric syndromes | ||||
| Observation patients | ||||
| Diverse settings | ||||
| Emergency department | ||||
| Perioperative | ||||
| Skilled nursing/rehab/long‐term acute‐care facilities | ||||
| Dementia | 13% to 63% of older persons in the hospital have dementia.[59] Dementia is often unrecognized among hospitalized patients.[60] The presence of dementia is associated with a more rapid functional decline during admission and delayed hospital discharge.[59] Patients with dementia require more nursing hours, and are more likely to have complications[61] or die in care homes rather than in their preferred site.[59] | Several tools have been validated to screen for dementia in the hospital setting.[62] Studies have assessed approaches to diagnosing delirium in hospitalized patients with dementia.[63] Cognitive and functional stimulation interventions may have a positive impact on reducing behavioral issues.[64, 65] | Does universal assessment of hospitalized older adults for cognitive impairment (eg, at presentation and/or discharge) lead to more appropriate application of geriatric care principles and improve patient centered outcomes? | Potential interventions: |
| Dementia or delirium care | ||||
| Patient/family communication and engagement strategies | ||||
| Maintenance/recovery of independent functional status | ||||
| Potential outcomes: | ||||
| Patient‐centered outcomesa | ||||
| Length of stay, cost, and healthcare utilization (including palliative care) | ||||
| Immediate invasive vs early conservative treatments pursued | ||||
| Depression | Depression is a common geriatric syndrome among acutely ill older patients, occurring in up to 45% of patients.[66, 67] Rates of depression are similar among patients discharged following a critical illness, with somatic, rather than cognitive‐affective complaints being the most prevalent.[68] Depression among inpatients or immediately following hospitalization independently predicts worse functional outcomes,[69] cognitive decline,[70] hospital readmission,[71, 72] and long‐term mortality.[69, 73] Finally, geriatric patients are known to respond differently to medical treatment.[74, 75] | Although highly prevalent, depression is poorly recognized and managed in the inpatient setting. Depression is recognized in only 50% of patients, with previously undiagnosed or untreated depression being at highest risk for being missed.[76] The role of treatment of depression in the inpatient setting is poorly understood, particularly for those with newly recognized depression or depressive symptoms. Some novel collaborative care and telephone outreach programs have led to increases in depression treatment in patients with specific medical and surgical conditions, resulting in early promising mental health and comorbid outcomes.[77, 78] The efficacy of such programs for older patients is unknown. | Does identifying depressive symptoms during a hospital stay and initiating a therapeutic plan prior to discharge improve patient‐centered and/or disease‐specific outcomes? | Possible areas of focus: |
| Comprehensive geriatric and psychosocial assessment; | ||||
| Inpatient vs outpatient initiation of pharmacological therapy | ||||
| Integration of confusion assessment method into therapeutic approaches | ||||
| Linkages with outpatient mental health resources | ||||
| Medications | Medication exposure, particularly potentially inappropriate medications, is common in hospitalized elders.[79] Medication errorsof dosage, type, and discrepancy between what a patient takes at home and what is known to his/her prescribing physicianare common and adversely affects patient safety.[80] Geriatric populations are disproportionately affected, especially those taking more than 5 prescription medications per day.[81] | Numerous strategies including electronic alerts, screening protocols, and potentially inappropriate medication lists (Beers list, STOPP) exist, though the optimal strategies to limit the use of potentially inappropriate medications is not yet known.[82, 83, 84] | What systems interventions improve medication management for older adults (ie, appropriateness of medication choices and dosing, compliance, cost) in hospital and post‐acute care? | Possible areas of focus: |
| Use of healthcare information technology | ||||
| Communication across sites of care | ||||
| Reducing medication‐related adverse events | ||||
| Engagement of family caregivers | ||||
| Patient‐centered strategies to simplify regimens | ||||
| Models of care | Hospitalization marks a time of high risk for older patients. Up to half die during hospitalization or within the year following the hospitalization. There is high risk of nosocomial events, and more than a third experience a decline in health resulting in longer hospitalizations and/or placement in extended‐care facilities.[73, 85, 86] | Comprehensive inpatient care for older adults (acute care for elders units, geriatric evaluation and management units, geriatric consultation services) were studied in 2 meta‐analyses, 5 RCTs, and 1 quasiexperimental study and summarized in a systematic review.[87] The studies reported improved quality of care (1 of 1 article), quality of life (3 of 4), functional autonomy (5 of 6), survival (3 of 6), and equal or lower healthcare utilization (7 of 8). | For which populations of hospitalized older adults does systematic implementation of geriatric care principles/processes improve patient‐centered outcomes? | Potential populations: |
| Patients of the emergency department, critical care, perioperative, and targeted medical/surgical units | ||||
| Examples of care principles: | ||||
| Geriatric assessment, early mobility, medication management, delirium prevention, advanced‐care planning, risk‐factor modification, caregiver engagement | ||||
| Potential outcomes: | ||||
| Patient‐centered outcomesa | ||||
| Cost | ||||
| Physical function | Half of older patients will lose functional capacity during hospitalization.[88] Loss of physical function, particularly of lower extremities, is a risk factor for nursing home placement.[89, 90] Older hospitalized patients spend the majority (up to 80%) of their time lying in bed, even when they are capable of walking independently.[91] | Loss of independences with ADL capabilities is associated with longer hospital stays, higher readmission rates, and higher mortality risk.[92] Excessive time in bed during a hospital stay is also associated with falls.[93] Often, hospital nursing protocols and physician orders increase in‐hospital immobility in patients.[91, 94] However, nursing‐driven mobility protocols can improve functional outcomes of older hospitalized patients.[95, 96] | What is the comparative effectiveness of interventions that promote in‐hospital mobility, improve and preserve physical function, and reduce falls among older hospitalized patients? | Potential interventions: |
| Intensive physical therapy | ||||
| Incidental functional training | ||||
| Restraint reduction | ||||
| Medication management | ||||
| Potential outcomes: | ||||
| Discharge location | ||||
| Delirium, pressure ulcers, and falls | ||||
| Surgery | An increasing number of persons over age 65 years are undergoing surgical procedures.[97] These persons are at increased risk for developing delirium/cogitative dysfunction,[98] loss of functional status,[99] and exacerbations of chronic illness.[97] Additionally, pain management may be harder to address in this population.[100] Current outcomes may not reflect the clinical needs of elder surgical patients.[101] | Tailored drug selection and nursing protocols may prevent delirium.[98] Postoperative cognitive dysfunction may require weeks for resolution. Identifying frail patients preoperatively may lead to more appropriate risk stratification and improved surgical outcomes.[99] Pain management strategies focused on mitigating cognitive impact and other effects may also be beneficial.[100] Development of risk‐adjustment tools specific to older populations, as well as measures of frailty and patient‐centered care, have been proposed.[101] | What perioperative strategies can be used to optimize care processes and improve outcomes in older surgical patients? | Potential strategies: |
| Preoperative risk assessment and optimization for frail or multimorbid older patients | ||||
| Perioperative management protocols for frail or multimorbid older patients | ||||
| Potential outcomes: | ||||
| Postoperative patient centered outcomesa | ||||
| Perioperative cost, healthcare utilization | ||||
| Training | Adults over age 65 years comprise 13.2 % of the US population, but account for >30% of hospital discharges and 50% of hospital days.[86, 102, 103] By 2030, there will only be 1 geriatrician for every 3798 Americans >75 years.[4] Between 1997 and 2006, the odds that a hospitalist would treat a hospitalized Medicare patient rose 29% per year.[3] | Train the trainer programs for physicians include the CHAMP, the AGESP, and the PAGE. Education for nurses include the NICHE. Outcomes include improved self‐confidence, attitudes, teaching skills, and geriatric care environment.[104, 105, 106] | What is the most effective approach to training hospital‐based providers in geriatric and palliative care competencies? | Potential interventions: |
| Mentored implementation | ||||
| Train the trainer | ||||
| Technical support | ||||
Table 2 also contains a capsule summary of the scope of the problem addressed by each research priority, a capsule summary of related work in the content area (what is known) not intended as a systematic review, and proposed dimensions or subquestions suggested by the stakeholders at the final prioritization meeting
DISCUSSION
Older hospitalized patients account for an increasing number and proportion of hospitalized patients,[1, 2] and hospitalists increasingly are responsible for inpatient care for this population.[3] The knowledge required for hospitalists to deliver optimal care and improve outcomes has not kept pace with the rapid growth of either hospitalists or hospitalized elders. Through a rigorous prioritization process, we identified 10 areas that deserve the highest priority in directing future research efforts to improve care for the older hospitalized patient. Assessment, prevention, and treatment of geriatric syndromes in the hospital account for almost half of the priority areas. Additional research is needed to improve advanced care planning, develop new care models, and develop training models for future hospitalists competent in geriatric and palliative care competencies.
A decade ago, the American Geriatric Society and the John A. Hartford Foundation embarked upon a research agenda aimed at improving the care of hospitalized elders cared for by specialists (ie, New Frontiers in Geriatrics Research: An Agenda for Surgical and Related Medical Specialties).[9] This effort differed in many important ways from the current priortization process. First, the New Frontiers agenda focused upon specific diseases, whereas the ACOP agenda addresses geriatric syndromes that cut across multiple diseases. Second, the New Frontiers agenda was made by researchers and based upon published literature, whereas the ACOP agenda involved the input of multiple stakeholders. Finally, the New Frontiers prioritized a research agenda across a number of surgical specialties, emergency medicine, and geriatric rehabilitation. Hospital medicine, however, was still early in its development and was not considered a unique specialty. Since that time, hospital medicine has matured into a unique specialty, with increased numbers of hospitalists,[3] increased research in hospital medicine,[17] and a separate recertification pathway for internal medicine licensure.[18] To date, there has not been a similar effort performed to direct geriatric research efforts for hospital medicine.
For researchers working in the field of hospital medicine, this list of topics has several implications. First, as hospitalists are commonly generalists, hospitalist researchers may be particularly well‐suited to study syndromes that cut across specialties. However, this does raise concerns about funding sources, as most National Institutes of Health institutes are disease‐focused. Funders that are not disease‐focused such as PCORI, National Institute on Aging, National Institute of Nursing Research, and Agency for Healthcare Research and Quality, and private foundations (Hartford, Robert Wood Johnson, and Commonwealth) may be more fruitful sources of funding for this work, but funding may be challenging. Nonetheless, the increased focus on patient‐centered work may increase funders' interest in such work. Second, the topics on this list would suggest that interventions will not be pharmacologic, but will focus on nonpharmacologic, behavioral, and social interventions. Similarly, outcomes of interest must expand beyond utilization metrics such as length of stay and mortality, to include functional status and symptom management, and goal‐concordant care. Therefore, research in geriatric acute care will necessarily be multidisciplinary.
Although these 10 high‐priority areas have been selected, this prioritized list is inherently limited by our methodology. First, our survey question was not focused on a disease state, and this wording may have resulted in the list favoring geriatric syndromes rather than common disease processes. Additionally, the resulting questions encompass large research areas and not specific questions about discrete interventions. Our results may also have been skewed by the types of engaged respondents who participated in the consultation, collating, and prioritization phases. In particular, we had a large response from geriatric medicine nurses, whereas some stakeholder groups provided no survey responses. Thus, these respondents were not representative of all possible stakeholders, nor were the survey respondents necessarily representative of each of their organizations. Nonetheless, the participants self‐identified as representative of diverse viewpoints that included patients, caregivers, and advocacy groups, with the majority of stakeholder organizations remaining engaged through the completion of the process. Thus, the general nature of this agenda helps us focus upon larger areas of importance, leaving researchers the flexibility to choose to narrow the focus on a specific research question that may include potential interventions and unique outcomes. Finally, our methodology may have inadvertently limited the number of patient and family caregiver voices in the process given our approach to large advocacy groups, our desire to be inclusive of healthcare professional organizations, and our survey methodology. Other methodologies may have reached more patients and caregivers, yet many healthcare professionals have served as family caregivers to frail elders requiring hospitalization and may have been in an ideal position to answer the survey.
In conclusion, several forces are shaping the future of acute inpatient care. These include the changing demographics of the hospitalized patient population, a rapid increase in the proportion of multimorbid hospitalized older adults, an inpatient workforce (hospitalists, generalists, and subspecialists) with potentially limited geriatrics training, and gaps in evidence‐based guidance to inform diagnostic and therapeutic decision making for acutely ill older patients. Training programs in hospital medicine should be aware of and could benefit from the resulting list of unanswered questions. Our findings also have implications for training to enrich education in geriatrics. Moreover, there is growing recognition that patients and other stakeholders deserve a greater voice in determining the direction of research. In addition to efforts to improve patient‐centeredness of research, these areas have been uniquely identified by stakeholders as important, and therefore are in line with newer priorities of PCORI. This project followed a road map resulting in a patient‐centered research agenda at the intersection of hospital medicine and geriatric medicine.[7] In creating this agenda, we relied heavily on the framework proposed by PCORI. We propose to pursue a dissemination and evaluation strategy for this research agenda as well as additional prioritization steps. We believe the adoption of this methodology will create a knowledge base that is rigorously derived and most relevant to the care of hospitalized older adults and their families. Its application will ultimately result in improved outcomes for hospitalized older adults.
Acknowledgements
The authors acknowledge Claudia Stahl, Society of Hospital Medicine; Cynthia Drake, University of Colorado; and the ACOP stakeholder organizations.
Disclosures: This work was supported by the Association of Specialty Professors/American Society of Internal Medicine and the John A. Hartford Foundation. Dr. Vasilevskis was supported by the National Institute on Aging of the National Institutes of Health under award number K23AG040157 and the Veterans Affairs Clinical Research Center of Excellence, and the Geriatric Research, Education and Clinical Center (GRECC). Dr. Vasilevskis' institution receives grant funding for an aspect of submitted work. Dr. Meltzer is a PCORI Methodology Committee member. The content is solely the responsibility of the authors and does not necessarily represent the official views of the National Institutes of Health or the Department of Veterans' Affairs. The authors report no conflicts of interest.
- , , , , . National Hospital Discharge Survey: 2007 summary. Natl Health Stat Report. 2010(29):1–20, 24.
- Centers for Medicare 2012. Available at: http://www.cms.gov/Research‐Statistics‐Data‐and‐Systems/Statistics‐Trends‐and‐Reports/Chronic‐Conditions/Downloads/2012Chartbook.pdf. Accessed December 12, 2014.
- , , , . Growth in the care of older patients by hospitalists in the United States. N Engl J Med. 2009;360(11):1102–1112.
- , . A revelation of numbers: will America's eldercare workforce be ready to care for an aging America? Generations. 2010;34(4):11–19.
- Patient‐Centered Outcomes Research Institute Methodology Committee. The PCORI methodology report. Available at: http://www.pcori.org/assets/2013/11/PCORI‐Methodology‐Report.pdf. Published November 2013. Accessed December 19, 2013.
- The James Lind Alliance. JLA method. Available at: http://www.lindalliance.org/JLA_Method.asp. Accessed December 19, 2013.
- , , , , . Road map to a patient‐centered research agenda at the intersection of hospital medicine and geriatric medicine. J Gen Intern Med. 2014;29(6):926–931.
- Patient‐Centered Outcomes Research Institute. About us. Available at: http://www.pcori.org/about‐us. Accessed February 23, 2015.
- , , . New Frontiers of Geriatrics Research: An Agenda for Surgical and Related Medical Specialties. New York, NY: American Geriatrics Society; 2004.
- , , , . Linking the NIH strategic plan to the research agenda for social workers in health and aging. J Gerontol Soc Work. 2010;53(1):77–93.
- , . Assessing the capacity to make everyday decisions: a guide for clinicians and an agenda for future research. Am J Geriatr Psychiatry. 2007;15(2):101–111.
- , , , et al. Practitioners' views on elder mistreatment research priorities: recommendations from a Research‐to‐Practice Consensus conference. J Elder Abuse Negl. 2011;23(2):115–126.
- , . The intersection between geriatrics and palliative care: a call for a new research agenda. J Am Geriatr Soc. 2005;53(9):1593–1598.
- . The cancer aging interface: a research agenda. J Clin Oncol. 2007;25(14):1945–1948.
- , , , . Clinical care of persons with dementia in the emergency department: a review of the literature and agenda for research. J Am Geriatr Soc. 2012;60(9):1742–1748.
- , . A group process model for problem identification and program planning. J Appl Behav Sci. 1971;7(4):466–492.
- , , , , . Research and publication trends in hospital medicine. J Hosp Med. 2014;9(3):148–154.
- . ABFM and ABIM to jointly participate in recognition of focused practice (rfp) in hospital medicine pilot approved by abms. Ann Fam Med. 2010;8(1):87.
- , , , . Medical decision‐making for older adults without family. J Am Geriatr Soc. 2012;60(11):2144–2150.
- , , , , , . Promoting advance directives among elderly primary care patients. J Gen Intern Med. 2004;19(9):944–951.
- , , . Advance directive completion by elderly Americans: a decade of change. J Am Geriatr Soc. 2014;62(4):706–710.
- , . Care transitions by older adults from nursing homes to hospitals: implications for long‐term care practice, geriatrics education, and research. J Am Med Dir Assoc. 2010;11(4):231–238.
- , , , . Decisions to hospitalize nursing home residents dying with advanced dementia. J Am Geriatr Soc. 2005;53(8):1396–1401.
- , , , , , . A comparison of methods to communicate treatment preferences in nursing facilities: traditional practices versus the physician orders for life‐sustaining treatment program. J Am Geriatr Soc. 2010;58(7):1241–1248.
- , , , , . Interventions to improve transitional care between nursing homes and hospitals: a systematic review. J Am Geriatr Soc. 2010;58(4):777–782.
- , , . Opening end‐of‐life discussions: how to introduce Voicing My CHOiCES, an advance care planning guide for adolescents and young adults [published online ahead of print March 13, 2014]. Palliat Support Care. doi: 10.1017/S1478951514000054.
- , , , . The revolving door of rehospitalization from skilled nursing facilities. Health Aff (Millwood). 2010;29(1):57–64.
- , , . Rehospitalizations among patients in the Medicare fee‐for‐service program. N Engl J Med. 2009;360(14):1418–1428.
- , , , et al. Predictors of rehospitalization among elderly patients admitted to a rehabilitation hospital: the role of polypharmacy, functional status, and length of stay. J Am Med Dir Assoc. 2013;14(10):761–767.
- , , , et al. Mobility after hospital discharge as a marker for 30‐day readmission. J Gerontol A Biol Sci Med Sci. 2013;68(7):805–810.
- , , , , . The association between the quality of inpatient care and early readmission: a meta‐analysis of the evidence. Med Care. 1997;35(10):1044–1059.
- , , , , , . Association of impaired functional status at hospital discharge and subsequent rehospitalization. J Hosp Med. May 2014;9(5):277–282.
- , , , , , . A prospective cohort study of geriatric syndromes among older medical patients admitted to acute care hospitals. J Am Geriatr Soc. 2011;59(11):2001–2008.
- , , , et al. Hospital discharge instructions: comprehension and compliance among older adults. J Gen Intern Med. 2014;29(11):1491–1498.
- , , , et al. Problems after discharge and understanding of communication with their primary care physicians among hospitalized seniors: a mixed methods study. J Hosp Med. 2010;5(7):385–391.
- , , , et al. Communication and information deficits in patients discharged to rehabilitation facilities: an evaluation of five acute care hospitals. J Hosp Med. 2009;4(8):E28–E33.
- , , , , , . The consequences of poor communication during transitions from hospital to skilled nursing facility: a qualitative study. J Am Geriatr Soc. 2013;61(7):1095–1102.
- , , , , , . Deficits in communication and information transfer between hospital‐based and primary care physicians: implications for patient safety and continuity of care. JAMA. 2007;297(8):831–841.
- , , , . Moving beyond readmission penalties: creating an ideal process to improve transitional care. J Hosp Med. 2013;8(2):102–109.
- , , , et al. Low‐cost transitional care with nurse managers making mostly phone contact with patients cut rehospitalization at a VA hospital. Health Aff (Millwood). 2012;31(12):2659–2668.
- , , , et al. Transitional care interventions to prevent readmissions for persons with heart failure: a systematic review and meta‐analysis. Ann Intern Med. 2014;160(11):774–784.
- , , , , . Interventions to reduce 30‐day rehospitalization: a systematic review. Ann Intern Med. 2011;155(8):520–528.
- , , , , , . Hospital‐initiated transitional care interventions as a patient safety strategy: a systematic review. Ann Intern Med. 2013;158(5 pt 2):433–440.
- , , , . Epidemiology and risk factors for delirium across hospital settings. Best Pract Res Clin Anaesthesiol. 2012;26(3):277–287.
- , , , , . Does delirium contribute to poor hospital outcomes? A three‐site epidemiologic study. J Gen Intern Med. 1998;13(4):234–242.
- , , , . Incidence, risk factors and consequences of ICU delirium. Intensive Care Med. 2007;33(1):66–73.
- , , , et al. Delirium in mechanically ventilated patients: validity and reliability of the confusion assessment method for the intensive care unit (CAM‐ICU). JAMA. 2001;286(21):2703–2710.
- , , , et al. Impact and recognition of cognitive impairment among hospitalized elders. J Hosp Med. 2010;5(2):69–75.
- , , , et al. The impact of delirium in the intensive care unit on hospital length of stay. Intensive Care Med. 2001;27(12):1892–1900.
- , , . Long‐term cognitive impairment after critical illness. N Engl J Med. 2014;370(2):185–186.
- , , , et al. Delirium in the ICU and subsequent long‐term disability among survivors of mechanical ventilation. Crit Care Med. 2014;42(2):369–377.
- , , , , , . Delirium in elderly patients and the risk of postdischarge mortality, institutionalization, and dementia: a meta‐analysis. JAMA. 2010;304(4):443–451.
- , , , et al. Delirium as a predictor of mortality in mechanically ventilated patients in the intensive care unit. JAMA. 2004;291(14):1753–1762.
- , , , , , . Days of delirium are associated with 1‐year mortality in an older intensive care unit population. Am J Respir Crit Care Med. 2009;180(11):1092–1097.
- , . In‐facility delirium prevention programs as a patient safety strategy: a systematic review. Ann Intern Med. 2013;158(5 pt 2):375–380.
- , , , et al. Dexmedetomidine vs midazolam for sedation of critically ill patients: a randomized trial. JAMA. 2009;301(5):489–499.
- , , , et al. Effect of sedation with dexmedetomidine vs lorazepam on acute brain dysfunction in mechanically ventilated patients: the MENDS randomized controlled trial. JAMA. 2007;298(22):2644–2653.
- , , , , , . Protocolized intensive care unit management of analgesia, sedation, and delirium improves analgesia and subsyndromal delirium rates. Anesth Analg. 2010;111(2):451–463.
- , . A systematic review of the prevalence, associations and outcomes of dementia in older general hospital inpatients. Int Psychogeriatr. 2011;23(3):344–355.
- , , , . Cognitive impairment is undetected in medical inpatients: a study of mortality and recognition amongst healthcare professionals. BMC Geriatr. 2012;12:47.
- , , . How can we keep patients with dementia safe in our acute hospitals? A review of challenges and solutions. J R Soc Med. 2013;106(9):355–361.
- , , . Screening for dementia in general hospital inpatients: a systematic review and meta‐analysis of available instruments. Age Ageing. 2013;42(6):689–695.
- , , , et al. Tools to detect delirium superimposed on dementia: a systematic review. J Am Geriatr Soc. 2012;60(11):2005–2013.
- , , , , , . Functional analysis‐based interventions for challenging behaviour in dementia. Cochrane Database Syst Rev. 2012;2:CD006929.
- , , , . Cognitive stimulation to improve cognitive functioning in people with dementia. Cochrane Database Syst Rev. 2012;2:CD005562.
- , , , et al. The prevalence and correlates of major and minor depression in older medical inpatients. J Am Geriatr Soc. 2005;53(8):1344–1353.
- , , , , , . Major depressive disorder in hospitalized medically ill patients: an examination of young and elderly male veterans. J Am Geriatr Soc. 1991;39(9):881–890.
- , , , et al. Depression, post‐traumatic stress disorder, and functional disability in survivors of critical illness in the BRAIN‐ICU study: a longitudinal cohort study. Lancet Respir Med. 2014;2(5):369–379.
- , , , et al. Depressive symptoms after hospitalization in older adults: function and mortality outcomes. J Am Geriatr Soc. 2012;60(12):2254–2262.
- , , , , . 12‐month cognitive outcomes of major and minor depression in older medical patients. Am J Geriatr Psychiatry. 2008;16(9):742–751.
- , , , et al. Depression is a risk factor for rehospitalization in medical inpatients. Prim Care Companion J Clin Psychiatry. 2007;9(4):256–262.
- , , , , , . Dose‐response relationship between depressive symptoms and hospital readmission. J Hosp Med. 2014;9(6):358–364.
- , , , , , . Depressive symptoms and 3‐year mortality in older hospitalized medical patients. Ann Intern Med. 1999;130(7):563–569.
- , , , et al. Support for the vascular depression hypothesis in late‐life depression: results of a 2‐site, prospective, antidepressant treatment trial. Arch Gen Psychiatry. 2010;67(3):277–285.
- , , , , , . Executive dysfunction and the course of geriatric depression. Biol Psychiatry. 2005;58(3):204–210.
- , , , , . Recognition of depression in older medical inpatients. J Gen Intern Med. 2007;22(5):559–564.
- , , , , , . A collaborative care depression management program for cardiac inpatients: depression characteristics and in‐hospital outcomes. Psychosomatics. 2011;52(1):26–33.
- , , , , , . Impact of a depression care management program for hospitalized cardiac patients. Circ Cardiovasc Qual Outcomes. 2011;4(2):198–205.
- , , , et al. Potentially inappropriate medication use in hospitalized elders. J Hosp Med. 2008;3(2):91–102.
- , , , , , . Prevalence, incidence and nature of prescribing errors in hospital inpatients: a systematic review. Drug Saf. 2009;32(5):379–389.
- , . Minimizing adverse drug events in older patients. Am Fam Physician. 2007;76(12):1837–1844.
- , , , , . STOPP (Screening Tool of Older Person's Prescriptions) and START (Screening Tool to Alert doctors to Right Treatment). Consensus validation. Int J Clin Pharmacol Ther. 2008;46(2):72–83.
- American Geriatrics Society 2012 Beers Criteria Update Expert Panel. American Geriatrics Society updated Beers Criteria for potentially inappropriate medication use in older adults. J Am Geriatr Soc. 2012;60(4):616–631.
- , , , . Preventing potentially inappropriate medication use in hospitalized older patients with a computerized provider order entry warning system. Arch Intern Med. 2010;170(15):1331–1336.
- . Hazards of Hospitalization of the Elderly. Ann Intern Med. 1993;118(3):219–223.
- . Improving health care for older persons. Ann Intern Med. 2003;139(5 part 2):421–424.
- , , , , , . Successful models of comprehensive care for older adults with chronic conditions: evidence for the Institute of Medicine's "retooling for an aging America" report. J Am Geriatr Soc. 2009;57(12):2328–2337.
- , , , et al. Recovery of activities of daily living in older adults after hospitalization for acute medical illness. J Am Geriatr Soc. 2008;56(12):2171–2179.
- , , , . Changes in functional status and the risks of subsequent nursing home placement and death. J Gerontol. 1993;48(3):S94–S101.
- , , . Risk factors for nursing home placement in a population‐based dementia cohort. J Am Geriatr Soc. 2000;48(5):519–525.
- , , , . The underrecognized epidemic of low mobility during hospitalization of older adults. J Am Geriatr Soc. 2009;57(9):1660–1665.
- , , , , . A systematic review of predictors and screening instruments to identify older hospitalized patients at risk for functional decline. J Clin Nurs. 2007;16(1):46–57.
- . Immobility and falls. Clin Geriatr Med. 1998;14(4):699–726.
- , , . Prevalence and outcomes of low mobility in hospitalized older patients. J Am Geriatr Soc. 2004;52(8):1263–1270.
- , , . Impact of a nurse‐driven mobility protocol on functional decline in hospitalized older adults. J Nurs Care Qual. 2009;24(4):325–331.
- , . Impact of early mobilization protocol on the medical‐surgical inpatient population: an integrated review of literature. Clin Nurse Spec. 2012;26(2):87–94.
- , , , . The aging population and its impact on the surgery workforce. Ann Surg. 2003;238(2):170–177.
- . Perioperative care of the elderly patient: an update. Cleve Clin J Med. 2009;76(suppl 4):S16–S21.
- , , . Frailty in the older surgical patient: a review. Age Ageing. 2012;41(2):142–147.
- . The assessment and management of peri‐operative pain in older adults. Anaesthesia. 2014;69(suppl 1):54–60.
- , . National Research Strategies: what outcomes are important in peri‐operative elderly care? Anaesthesia. 2014;69(suppl 1):61–69.
- , . 2002 National Hospital Discharge Survey. Adv Data. 2004;342:1–30.
- , . Hospitalization in the United States, 2002. Rockville, MD: Agency for Healthcare Research and Quality; 2005.
- , , , et al. The Curriculum for the Hospitalized Aging Medical Patient program: a collaborative faculty development program for hospitalists, general internists, and geriatricians. J Hosp Med. 2008;3(5):384–393.
- , . Advancement of geriatrics education. J Hosp Med. 2011;6(6):370.
- , , , , , . Advancing geriatrics education: an efficient faculty development program for academic hospitalists increases geriatric teaching. J Hosp Med. 2010;5(9):541–546.
- , , , , . National Hospital Discharge Survey: 2007 summary. Natl Health Stat Report. 2010(29):1–20, 24.
- Centers for Medicare 2012. Available at: http://www.cms.gov/Research‐Statistics‐Data‐and‐Systems/Statistics‐Trends‐and‐Reports/Chronic‐Conditions/Downloads/2012Chartbook.pdf. Accessed December 12, 2014.
- , , , . Growth in the care of older patients by hospitalists in the United States. N Engl J Med. 2009;360(11):1102–1112.
- , . A revelation of numbers: will America's eldercare workforce be ready to care for an aging America? Generations. 2010;34(4):11–19.
- Patient‐Centered Outcomes Research Institute Methodology Committee. The PCORI methodology report. Available at: http://www.pcori.org/assets/2013/11/PCORI‐Methodology‐Report.pdf. Published November 2013. Accessed December 19, 2013.
- The James Lind Alliance. JLA method. Available at: http://www.lindalliance.org/JLA_Method.asp. Accessed December 19, 2013.
- , , , , . Road map to a patient‐centered research agenda at the intersection of hospital medicine and geriatric medicine. J Gen Intern Med. 2014;29(6):926–931.
- Patient‐Centered Outcomes Research Institute. About us. Available at: http://www.pcori.org/about‐us. Accessed February 23, 2015.
- , , . New Frontiers of Geriatrics Research: An Agenda for Surgical and Related Medical Specialties. New York, NY: American Geriatrics Society; 2004.
- , , , . Linking the NIH strategic plan to the research agenda for social workers in health and aging. J Gerontol Soc Work. 2010;53(1):77–93.
- , . Assessing the capacity to make everyday decisions: a guide for clinicians and an agenda for future research. Am J Geriatr Psychiatry. 2007;15(2):101–111.
- , , , et al. Practitioners' views on elder mistreatment research priorities: recommendations from a Research‐to‐Practice Consensus conference. J Elder Abuse Negl. 2011;23(2):115–126.
- , . The intersection between geriatrics and palliative care: a call for a new research agenda. J Am Geriatr Soc. 2005;53(9):1593–1598.
- . The cancer aging interface: a research agenda. J Clin Oncol. 2007;25(14):1945–1948.
- , , , . Clinical care of persons with dementia in the emergency department: a review of the literature and agenda for research. J Am Geriatr Soc. 2012;60(9):1742–1748.
- , . A group process model for problem identification and program planning. J Appl Behav Sci. 1971;7(4):466–492.
- , , , , . Research and publication trends in hospital medicine. J Hosp Med. 2014;9(3):148–154.
- . ABFM and ABIM to jointly participate in recognition of focused practice (rfp) in hospital medicine pilot approved by abms. Ann Fam Med. 2010;8(1):87.
- , , , . Medical decision‐making for older adults without family. J Am Geriatr Soc. 2012;60(11):2144–2150.
- , , , , , . Promoting advance directives among elderly primary care patients. J Gen Intern Med. 2004;19(9):944–951.
- , , . Advance directive completion by elderly Americans: a decade of change. J Am Geriatr Soc. 2014;62(4):706–710.
- , . Care transitions by older adults from nursing homes to hospitals: implications for long‐term care practice, geriatrics education, and research. J Am Med Dir Assoc. 2010;11(4):231–238.
- , , , . Decisions to hospitalize nursing home residents dying with advanced dementia. J Am Geriatr Soc. 2005;53(8):1396–1401.
- , , , , , . A comparison of methods to communicate treatment preferences in nursing facilities: traditional practices versus the physician orders for life‐sustaining treatment program. J Am Geriatr Soc. 2010;58(7):1241–1248.
- , , , , . Interventions to improve transitional care between nursing homes and hospitals: a systematic review. J Am Geriatr Soc. 2010;58(4):777–782.
- , , . Opening end‐of‐life discussions: how to introduce Voicing My CHOiCES, an advance care planning guide for adolescents and young adults [published online ahead of print March 13, 2014]. Palliat Support Care. doi: 10.1017/S1478951514000054.
- , , , . The revolving door of rehospitalization from skilled nursing facilities. Health Aff (Millwood). 2010;29(1):57–64.
- , , . Rehospitalizations among patients in the Medicare fee‐for‐service program. N Engl J Med. 2009;360(14):1418–1428.
- , , , et al. Predictors of rehospitalization among elderly patients admitted to a rehabilitation hospital: the role of polypharmacy, functional status, and length of stay. J Am Med Dir Assoc. 2013;14(10):761–767.
- , , , et al. Mobility after hospital discharge as a marker for 30‐day readmission. J Gerontol A Biol Sci Med Sci. 2013;68(7):805–810.
- , , , , . The association between the quality of inpatient care and early readmission: a meta‐analysis of the evidence. Med Care. 1997;35(10):1044–1059.
- , , , , , . Association of impaired functional status at hospital discharge and subsequent rehospitalization. J Hosp Med. May 2014;9(5):277–282.
- , , , , , . A prospective cohort study of geriatric syndromes among older medical patients admitted to acute care hospitals. J Am Geriatr Soc. 2011;59(11):2001–2008.
- , , , et al. Hospital discharge instructions: comprehension and compliance among older adults. J Gen Intern Med. 2014;29(11):1491–1498.
- , , , et al. Problems after discharge and understanding of communication with their primary care physicians among hospitalized seniors: a mixed methods study. J Hosp Med. 2010;5(7):385–391.
- , , , et al. Communication and information deficits in patients discharged to rehabilitation facilities: an evaluation of five acute care hospitals. J Hosp Med. 2009;4(8):E28–E33.
- , , , , , . The consequences of poor communication during transitions from hospital to skilled nursing facility: a qualitative study. J Am Geriatr Soc. 2013;61(7):1095–1102.
- , , , , , . Deficits in communication and information transfer between hospital‐based and primary care physicians: implications for patient safety and continuity of care. JAMA. 2007;297(8):831–841.
- , , , . Moving beyond readmission penalties: creating an ideal process to improve transitional care. J Hosp Med. 2013;8(2):102–109.
- , , , et al. Low‐cost transitional care with nurse managers making mostly phone contact with patients cut rehospitalization at a VA hospital. Health Aff (Millwood). 2012;31(12):2659–2668.
- , , , et al. Transitional care interventions to prevent readmissions for persons with heart failure: a systematic review and meta‐analysis. Ann Intern Med. 2014;160(11):774–784.
- , , , , . Interventions to reduce 30‐day rehospitalization: a systematic review. Ann Intern Med. 2011;155(8):520–528.
- , , , , , . Hospital‐initiated transitional care interventions as a patient safety strategy: a systematic review. Ann Intern Med. 2013;158(5 pt 2):433–440.
- , , , . Epidemiology and risk factors for delirium across hospital settings. Best Pract Res Clin Anaesthesiol. 2012;26(3):277–287.
- , , , , . Does delirium contribute to poor hospital outcomes? A three‐site epidemiologic study. J Gen Intern Med. 1998;13(4):234–242.
- , , , . Incidence, risk factors and consequences of ICU delirium. Intensive Care Med. 2007;33(1):66–73.
- , , , et al. Delirium in mechanically ventilated patients: validity and reliability of the confusion assessment method for the intensive care unit (CAM‐ICU). JAMA. 2001;286(21):2703–2710.
- , , , et al. Impact and recognition of cognitive impairment among hospitalized elders. J Hosp Med. 2010;5(2):69–75.
- , , , et al. The impact of delirium in the intensive care unit on hospital length of stay. Intensive Care Med. 2001;27(12):1892–1900.
- , , . Long‐term cognitive impairment after critical illness. N Engl J Med. 2014;370(2):185–186.
- , , , et al. Delirium in the ICU and subsequent long‐term disability among survivors of mechanical ventilation. Crit Care Med. 2014;42(2):369–377.
- , , , , , . Delirium in elderly patients and the risk of postdischarge mortality, institutionalization, and dementia: a meta‐analysis. JAMA. 2010;304(4):443–451.
- , , , et al. Delirium as a predictor of mortality in mechanically ventilated patients in the intensive care unit. JAMA. 2004;291(14):1753–1762.
- , , , , , . Days of delirium are associated with 1‐year mortality in an older intensive care unit population. Am J Respir Crit Care Med. 2009;180(11):1092–1097.
- , . In‐facility delirium prevention programs as a patient safety strategy: a systematic review. Ann Intern Med. 2013;158(5 pt 2):375–380.
- , , , et al. Dexmedetomidine vs midazolam for sedation of critically ill patients: a randomized trial. JAMA. 2009;301(5):489–499.
- , , , et al. Effect of sedation with dexmedetomidine vs lorazepam on acute brain dysfunction in mechanically ventilated patients: the MENDS randomized controlled trial. JAMA. 2007;298(22):2644–2653.
- , , , , , . Protocolized intensive care unit management of analgesia, sedation, and delirium improves analgesia and subsyndromal delirium rates. Anesth Analg. 2010;111(2):451–463.
- , . A systematic review of the prevalence, associations and outcomes of dementia in older general hospital inpatients. Int Psychogeriatr. 2011;23(3):344–355.
- , , , . Cognitive impairment is undetected in medical inpatients: a study of mortality and recognition amongst healthcare professionals. BMC Geriatr. 2012;12:47.
- , , . How can we keep patients with dementia safe in our acute hospitals? A review of challenges and solutions. J R Soc Med. 2013;106(9):355–361.
- , , . Screening for dementia in general hospital inpatients: a systematic review and meta‐analysis of available instruments. Age Ageing. 2013;42(6):689–695.
- , , , et al. Tools to detect delirium superimposed on dementia: a systematic review. J Am Geriatr Soc. 2012;60(11):2005–2013.
- , , , , , . Functional analysis‐based interventions for challenging behaviour in dementia. Cochrane Database Syst Rev. 2012;2:CD006929.
- , , , . Cognitive stimulation to improve cognitive functioning in people with dementia. Cochrane Database Syst Rev. 2012;2:CD005562.
- , , , et al. The prevalence and correlates of major and minor depression in older medical inpatients. J Am Geriatr Soc. 2005;53(8):1344–1353.
- , , , , , . Major depressive disorder in hospitalized medically ill patients: an examination of young and elderly male veterans. J Am Geriatr Soc. 1991;39(9):881–890.
- , , , et al. Depression, post‐traumatic stress disorder, and functional disability in survivors of critical illness in the BRAIN‐ICU study: a longitudinal cohort study. Lancet Respir Med. 2014;2(5):369–379.
- , , , et al. Depressive symptoms after hospitalization in older adults: function and mortality outcomes. J Am Geriatr Soc. 2012;60(12):2254–2262.
- , , , , . 12‐month cognitive outcomes of major and minor depression in older medical patients. Am J Geriatr Psychiatry. 2008;16(9):742–751.
- , , , et al. Depression is a risk factor for rehospitalization in medical inpatients. Prim Care Companion J Clin Psychiatry. 2007;9(4):256–262.
- , , , , , . Dose‐response relationship between depressive symptoms and hospital readmission. J Hosp Med. 2014;9(6):358–364.
- , , , , , . Depressive symptoms and 3‐year mortality in older hospitalized medical patients. Ann Intern Med. 1999;130(7):563–569.
- , , , et al. Support for the vascular depression hypothesis in late‐life depression: results of a 2‐site, prospective, antidepressant treatment trial. Arch Gen Psychiatry. 2010;67(3):277–285.
- , , , , , . Executive dysfunction and the course of geriatric depression. Biol Psychiatry. 2005;58(3):204–210.
- , , , , . Recognition of depression in older medical inpatients. J Gen Intern Med. 2007;22(5):559–564.
- , , , , , . A collaborative care depression management program for cardiac inpatients: depression characteristics and in‐hospital outcomes. Psychosomatics. 2011;52(1):26–33.
- , , , , , . Impact of a depression care management program for hospitalized cardiac patients. Circ Cardiovasc Qual Outcomes. 2011;4(2):198–205.
- , , , et al. Potentially inappropriate medication use in hospitalized elders. J Hosp Med. 2008;3(2):91–102.
- , , , , , . Prevalence, incidence and nature of prescribing errors in hospital inpatients: a systematic review. Drug Saf. 2009;32(5):379–389.
- , . Minimizing adverse drug events in older patients. Am Fam Physician. 2007;76(12):1837–1844.
- , , , , . STOPP (Screening Tool of Older Person's Prescriptions) and START (Screening Tool to Alert doctors to Right Treatment). Consensus validation. Int J Clin Pharmacol Ther. 2008;46(2):72–83.
- American Geriatrics Society 2012 Beers Criteria Update Expert Panel. American Geriatrics Society updated Beers Criteria for potentially inappropriate medication use in older adults. J Am Geriatr Soc. 2012;60(4):616–631.
- , , , . Preventing potentially inappropriate medication use in hospitalized older patients with a computerized provider order entry warning system. Arch Intern Med. 2010;170(15):1331–1336.
- . Hazards of Hospitalization of the Elderly. Ann Intern Med. 1993;118(3):219–223.
- . Improving health care for older persons. Ann Intern Med. 2003;139(5 part 2):421–424.
- , , , , , . Successful models of comprehensive care for older adults with chronic conditions: evidence for the Institute of Medicine's "retooling for an aging America" report. J Am Geriatr Soc. 2009;57(12):2328–2337.
- , , , et al. Recovery of activities of daily living in older adults after hospitalization for acute medical illness. J Am Geriatr Soc. 2008;56(12):2171–2179.
- , , , . Changes in functional status and the risks of subsequent nursing home placement and death. J Gerontol. 1993;48(3):S94–S101.
- , , . Risk factors for nursing home placement in a population‐based dementia cohort. J Am Geriatr Soc. 2000;48(5):519–525.
- , , , . The underrecognized epidemic of low mobility during hospitalization of older adults. J Am Geriatr Soc. 2009;57(9):1660–1665.
- , , , , . A systematic review of predictors and screening instruments to identify older hospitalized patients at risk for functional decline. J Clin Nurs. 2007;16(1):46–57.
- . Immobility and falls. Clin Geriatr Med. 1998;14(4):699–726.
- , , . Prevalence and outcomes of low mobility in hospitalized older patients. J Am Geriatr Soc. 2004;52(8):1263–1270.
- , , . Impact of a nurse‐driven mobility protocol on functional decline in hospitalized older adults. J Nurs Care Qual. 2009;24(4):325–331.
- , . Impact of early mobilization protocol on the medical‐surgical inpatient population: an integrated review of literature. Clin Nurse Spec. 2012;26(2):87–94.
- , , , . The aging population and its impact on the surgery workforce. Ann Surg. 2003;238(2):170–177.
- . Perioperative care of the elderly patient: an update. Cleve Clin J Med. 2009;76(suppl 4):S16–S21.
- , , . Frailty in the older surgical patient: a review. Age Ageing. 2012;41(2):142–147.
- . The assessment and management of peri‐operative pain in older adults. Anaesthesia. 2014;69(suppl 1):54–60.
- , . National Research Strategies: what outcomes are important in peri‐operative elderly care? Anaesthesia. 2014;69(suppl 1):61–69.
- , . 2002 National Hospital Discharge Survey. Adv Data. 2004;342:1–30.
- , . Hospitalization in the United States, 2002. Rockville, MD: Agency for Healthcare Research and Quality; 2005.
- , , , et al. The Curriculum for the Hospitalized Aging Medical Patient program: a collaborative faculty development program for hospitalists, general internists, and geriatricians. J Hosp Med. 2008;3(5):384–393.
- , . Advancement of geriatrics education. J Hosp Med. 2011;6(6):370.
- , , , , , . Advancing geriatrics education: an efficient faculty development program for academic hospitalists increases geriatric teaching. J Hosp Med. 2010;5(9):541–546.