User login
Mandatory prescriber training now available for flibanserin
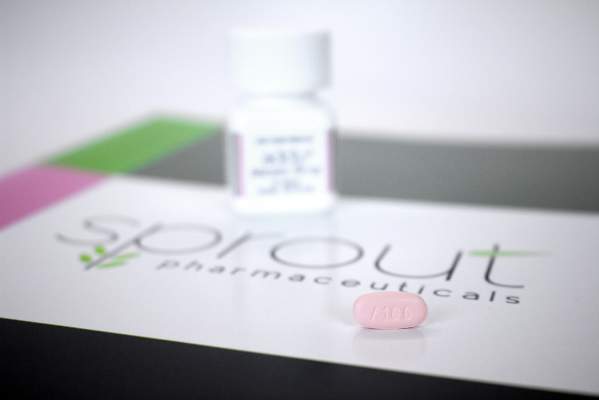
Physicians can now complete the training and paperwork required to prescribe flibanserin (Addyi, Sprout Pharmaceuticals), a new centrally acting, nonhormonal daily medication that treats female hypoactive sexual desire disorder.
The Food and Drug Administration’s August 2015 approval of flibanserin came with a required REMS(Risk Evaluation and Mitigation Strategy ) to address safety concerns.
Flibanserin, which the FDA had twice previously declined to approve, has an increased risk for syncope and hypotension with alcohol and moderate or strong CYP3A4 inhibitors, such as proton pump inhibitors, selective serotonin reuptake inhibitors, benzodiazepines, and antifungals. Flibanserin taken alone also caused hypotension and syncope in a few patients during clinical trials.
The REMS addresses these risks by requiring all prescribers to complete training and a knowledge assessment about flibanserin’s risks and to enroll in a REMS certification program for the drug. Prescribers must also review a patient-provider agreement form with patients and have both parties sign before prescribing flibanserin.
Outpatient pharmacies must designate a representative to complete training and knowledge assessment, train their staff, and counsel every patient receiving flibanserin to abstain from alcohol. Inpatient pharmacies have similar training requirements and may not dispense flibanserin for outpatient use.
Flibanserin is approved for treatment of acquired, generalized hypoactive sexual desire disorder in premenopausal women only. It is a medication that is meant to be taken on a chronic basis, acting as a mixed agonist/antagonist for dopamine and serotonin receptors. In clinical trials, it showed a statistically significant, but modest improvement in reported sexual desire and the number of sexually satisfying events per month.
The certification materials are available online at www.Addyi.com. To complete the certification process, prescribers and pharmacists should fax the completed knowledge assessment and enrollment forms to 844-694-3373 or email scanned copies to [email protected].
On Twitter @karioakes
Physicians can now complete the training and paperwork required to prescribe flibanserin (Addyi, Sprout Pharmaceuticals), a new centrally acting, nonhormonal daily medication that treats female hypoactive sexual desire disorder.
The Food and Drug Administration’s August 2015 approval of flibanserin came with a required REMS(Risk Evaluation and Mitigation Strategy ) to address safety concerns.
Flibanserin, which the FDA had twice previously declined to approve, has an increased risk for syncope and hypotension with alcohol and moderate or strong CYP3A4 inhibitors, such as proton pump inhibitors, selective serotonin reuptake inhibitors, benzodiazepines, and antifungals. Flibanserin taken alone also caused hypotension and syncope in a few patients during clinical trials.
The REMS addresses these risks by requiring all prescribers to complete training and a knowledge assessment about flibanserin’s risks and to enroll in a REMS certification program for the drug. Prescribers must also review a patient-provider agreement form with patients and have both parties sign before prescribing flibanserin.
Outpatient pharmacies must designate a representative to complete training and knowledge assessment, train their staff, and counsel every patient receiving flibanserin to abstain from alcohol. Inpatient pharmacies have similar training requirements and may not dispense flibanserin for outpatient use.
Flibanserin is approved for treatment of acquired, generalized hypoactive sexual desire disorder in premenopausal women only. It is a medication that is meant to be taken on a chronic basis, acting as a mixed agonist/antagonist for dopamine and serotonin receptors. In clinical trials, it showed a statistically significant, but modest improvement in reported sexual desire and the number of sexually satisfying events per month.
The certification materials are available online at www.Addyi.com. To complete the certification process, prescribers and pharmacists should fax the completed knowledge assessment and enrollment forms to 844-694-3373 or email scanned copies to [email protected].
On Twitter @karioakes
Physicians can now complete the training and paperwork required to prescribe flibanserin (Addyi, Sprout Pharmaceuticals), a new centrally acting, nonhormonal daily medication that treats female hypoactive sexual desire disorder.
The Food and Drug Administration’s August 2015 approval of flibanserin came with a required REMS(Risk Evaluation and Mitigation Strategy ) to address safety concerns.
Flibanserin, which the FDA had twice previously declined to approve, has an increased risk for syncope and hypotension with alcohol and moderate or strong CYP3A4 inhibitors, such as proton pump inhibitors, selective serotonin reuptake inhibitors, benzodiazepines, and antifungals. Flibanserin taken alone also caused hypotension and syncope in a few patients during clinical trials.
The REMS addresses these risks by requiring all prescribers to complete training and a knowledge assessment about flibanserin’s risks and to enroll in a REMS certification program for the drug. Prescribers must also review a patient-provider agreement form with patients and have both parties sign before prescribing flibanserin.
Outpatient pharmacies must designate a representative to complete training and knowledge assessment, train their staff, and counsel every patient receiving flibanserin to abstain from alcohol. Inpatient pharmacies have similar training requirements and may not dispense flibanserin for outpatient use.
Flibanserin is approved for treatment of acquired, generalized hypoactive sexual desire disorder in premenopausal women only. It is a medication that is meant to be taken on a chronic basis, acting as a mixed agonist/antagonist for dopamine and serotonin receptors. In clinical trials, it showed a statistically significant, but modest improvement in reported sexual desire and the number of sexually satisfying events per month.
The certification materials are available online at www.Addyi.com. To complete the certification process, prescribers and pharmacists should fax the completed knowledge assessment and enrollment forms to 844-694-3373 or email scanned copies to [email protected].
On Twitter @karioakes
Advances in AML understanding could translate to improved therapy
An understanding of the various molecular differences between acute myeloid leukemia (AML) particles is guiding the search for new drug combinations targeting a variety of AML cellular processes, according to research published in the New England Journal of Medicine.
After a brief overview of disease classification and prognostic factors for AML, the authors, led by Dr. Hartmut Döhner of University Hospital Ulm in Germany, described a number of new treatment strategies. These approaches include directly addressing mutant proteins by targeting mutation-specific dependencies, developing inhibitors that could cut down on the occurrence of mutations in receptor tyrosine kinase genes, and developing new epigenetic therapies based on targeting specific mutant metabolic enzymes such as IDH1 and IDH2.
Other strategies, such as antibody therapy that focuses on the development of new monoclonal antibodies targeting CD33, and new formulations of older cytotoxic agents, also are being developed or are in clinical trial stages; however, many of these developments have not yet been translated into clinical practice, the researchers noted.
“New compounds hold promise to improve treatment outcomes; however, it is unlikely that any of these compounds, when used as single agents, will cure the disease. A major challenge will be to identify predictors for a response to specific agents, which will allow for the rational design of combinatorial therapies,” they wrote.
Dr. Döhner and a coauthor disclosed ties with several pharmaceutical companies.
Read the article here.
An understanding of the various molecular differences between acute myeloid leukemia (AML) particles is guiding the search for new drug combinations targeting a variety of AML cellular processes, according to research published in the New England Journal of Medicine.
After a brief overview of disease classification and prognostic factors for AML, the authors, led by Dr. Hartmut Döhner of University Hospital Ulm in Germany, described a number of new treatment strategies. These approaches include directly addressing mutant proteins by targeting mutation-specific dependencies, developing inhibitors that could cut down on the occurrence of mutations in receptor tyrosine kinase genes, and developing new epigenetic therapies based on targeting specific mutant metabolic enzymes such as IDH1 and IDH2.
Other strategies, such as antibody therapy that focuses on the development of new monoclonal antibodies targeting CD33, and new formulations of older cytotoxic agents, also are being developed or are in clinical trial stages; however, many of these developments have not yet been translated into clinical practice, the researchers noted.
“New compounds hold promise to improve treatment outcomes; however, it is unlikely that any of these compounds, when used as single agents, will cure the disease. A major challenge will be to identify predictors for a response to specific agents, which will allow for the rational design of combinatorial therapies,” they wrote.
Dr. Döhner and a coauthor disclosed ties with several pharmaceutical companies.
Read the article here.
An understanding of the various molecular differences between acute myeloid leukemia (AML) particles is guiding the search for new drug combinations targeting a variety of AML cellular processes, according to research published in the New England Journal of Medicine.
After a brief overview of disease classification and prognostic factors for AML, the authors, led by Dr. Hartmut Döhner of University Hospital Ulm in Germany, described a number of new treatment strategies. These approaches include directly addressing mutant proteins by targeting mutation-specific dependencies, developing inhibitors that could cut down on the occurrence of mutations in receptor tyrosine kinase genes, and developing new epigenetic therapies based on targeting specific mutant metabolic enzymes such as IDH1 and IDH2.
Other strategies, such as antibody therapy that focuses on the development of new monoclonal antibodies targeting CD33, and new formulations of older cytotoxic agents, also are being developed or are in clinical trial stages; however, many of these developments have not yet been translated into clinical practice, the researchers noted.
“New compounds hold promise to improve treatment outcomes; however, it is unlikely that any of these compounds, when used as single agents, will cure the disease. A major challenge will be to identify predictors for a response to specific agents, which will allow for the rational design of combinatorial therapies,” they wrote.
Dr. Döhner and a coauthor disclosed ties with several pharmaceutical companies.
Read the article here.
FROM THE NEW ENGLAND JOURNAL OF MEDICINE
Pain Control in Patients With Bone Metastases
Background: Pain due to bone metastases represents a common source of cancer-related morbidity. Not all patients who develop bone metastases develop pain. It is a subjective experience unique to each patient. We conducted a quality assessment project at the Providence VA Medical Center (PVAMC) to characterize the problem of pain due to bone metastases.
Methods: All veterans with bone metastases seen by the oncology service during 2011 to 2013 at the PVAMC were included. Pain scores were assessed from each outpatient visit. Scores ranged from 0 to10 with 0 to 3 considered mild pain, 4 to 6 moderate pain, and 7 to 10 severe pain. If veterans were found to have severe pain, then their charts were reviewed for the interventions undertaken and whether pain was brought to a more acceptable score.
Results: Sixty-nine veterans with bone metastases were included. Fifty-one percent experienced severe pain. Of those experiencing severe pain, 46% had lung cancer and 37% had prostate cancer. Ninety-four percent of veterans with lung cancer and bone metastases experienced severe pain. Of those veterans with severe pain, 94% were prescribed narcotics, 83% enrolled in hospice, 66% received palliative care, and 63% received radiation therapy. Eleven veterans in total were admitted to the hospital for pain control. It took a median of 25 days to reduce pain to a score of 3. Overall the median time from onset of severe pain to death was 80 days. For veterans with lung cancer, the median time to death was 63 days compared with 371 days in veterans with prostate cancer.
Conclusions: Many veterans with bone metastases experince severe pain. This project demonstrated that the majority of lung cancer patients reported extreme pain from their bone metastases, and the median time from severe pain onset to death was about 2 months. Early and aggressive pain control and palliative or hospice care may be particularly beneficial for these patients. In the future, we will seek to shorten the duration of time to adequate pain control through a quality improvement project.
Background: Pain due to bone metastases represents a common source of cancer-related morbidity. Not all patients who develop bone metastases develop pain. It is a subjective experience unique to each patient. We conducted a quality assessment project at the Providence VA Medical Center (PVAMC) to characterize the problem of pain due to bone metastases.
Methods: All veterans with bone metastases seen by the oncology service during 2011 to 2013 at the PVAMC were included. Pain scores were assessed from each outpatient visit. Scores ranged from 0 to10 with 0 to 3 considered mild pain, 4 to 6 moderate pain, and 7 to 10 severe pain. If veterans were found to have severe pain, then their charts were reviewed for the interventions undertaken and whether pain was brought to a more acceptable score.
Results: Sixty-nine veterans with bone metastases were included. Fifty-one percent experienced severe pain. Of those experiencing severe pain, 46% had lung cancer and 37% had prostate cancer. Ninety-four percent of veterans with lung cancer and bone metastases experienced severe pain. Of those veterans with severe pain, 94% were prescribed narcotics, 83% enrolled in hospice, 66% received palliative care, and 63% received radiation therapy. Eleven veterans in total were admitted to the hospital for pain control. It took a median of 25 days to reduce pain to a score of 3. Overall the median time from onset of severe pain to death was 80 days. For veterans with lung cancer, the median time to death was 63 days compared with 371 days in veterans with prostate cancer.
Conclusions: Many veterans with bone metastases experince severe pain. This project demonstrated that the majority of lung cancer patients reported extreme pain from their bone metastases, and the median time from severe pain onset to death was about 2 months. Early and aggressive pain control and palliative or hospice care may be particularly beneficial for these patients. In the future, we will seek to shorten the duration of time to adequate pain control through a quality improvement project.
Background: Pain due to bone metastases represents a common source of cancer-related morbidity. Not all patients who develop bone metastases develop pain. It is a subjective experience unique to each patient. We conducted a quality assessment project at the Providence VA Medical Center (PVAMC) to characterize the problem of pain due to bone metastases.
Methods: All veterans with bone metastases seen by the oncology service during 2011 to 2013 at the PVAMC were included. Pain scores were assessed from each outpatient visit. Scores ranged from 0 to10 with 0 to 3 considered mild pain, 4 to 6 moderate pain, and 7 to 10 severe pain. If veterans were found to have severe pain, then their charts were reviewed for the interventions undertaken and whether pain was brought to a more acceptable score.
Results: Sixty-nine veterans with bone metastases were included. Fifty-one percent experienced severe pain. Of those experiencing severe pain, 46% had lung cancer and 37% had prostate cancer. Ninety-four percent of veterans with lung cancer and bone metastases experienced severe pain. Of those veterans with severe pain, 94% were prescribed narcotics, 83% enrolled in hospice, 66% received palliative care, and 63% received radiation therapy. Eleven veterans in total were admitted to the hospital for pain control. It took a median of 25 days to reduce pain to a score of 3. Overall the median time from onset of severe pain to death was 80 days. For veterans with lung cancer, the median time to death was 63 days compared with 371 days in veterans with prostate cancer.
Conclusions: Many veterans with bone metastases experince severe pain. This project demonstrated that the majority of lung cancer patients reported extreme pain from their bone metastases, and the median time from severe pain onset to death was about 2 months. Early and aggressive pain control and palliative or hospice care may be particularly beneficial for these patients. In the future, we will seek to shorten the duration of time to adequate pain control through a quality improvement project.
Patterns of Failure and Survival Analysis of Advanced Tonsillar Cancer Treated With IMRT Radiation Therapy and Chemotherapy and Implications of HPV-Positive Tumors in Management
Purpose/Objectives: To evaluate the outcomes of patients with tonsillar cancer treated at G.V. Sonny Montgomery VA Medical Center between 2006 and 2014 and to compare survival and patterns of failure between human papillomavirus (HPV)-positive tonsillar cancer and HPV-negative tonsillar cancer.
Methods: There were 70 patients with biopsy proven squamous cell carcinoma of the tonsil in the retrospective review. Sixty-one of 70 patients had stage III/IV disease. Forty-seven of 70 patients had their HPV status evaluated. There were 22 HPV-positive and 25 HPV-negative patients. The majority of patients were treated with concurrent chemoradiotherapy, consisting of either weekly cisplatin 45 mg/m2 or weekly cetuximab 400 mg/m2 loading dose and 250 mg/m2 once a week for 7 weeks. Radiation therapy was given using intensity modulated radiation therapy to 70 Gy at 2 Gy per fraction over 7 weeks. The median radiation dose was 70 Gy. We evaluated the outcomes, including loco-regional failure, distant metastases, and survival. Rates were estimated by Kaplan-Meier method, and comparisons between HPV groups were evaluated using the Fisher exact test for categorical variables and Kruskal-Wallis test. Intermediate risk was defined as having ≥ 10 pack-years smoking history in the HPV-positive group (11 patients) and < 10 pack-years in the HPV-negative group (5 patients).
Results: Follow-up ranged from 14 to 88 months (median 22 mo). Overall survival (OS) for the entire group was 68% at 3 years with a disease-free survival (DFS) rate of 56%. At 3 years, the OS and DFS were 73% and 59% in the HPV-positive group and 50% and 50%, respectively, in the HPV-negative group. In the HPV-positive group, the failure rate was 2/11(16%) in the low-risk group and 8/11 (72%) in the intermediate-risk group. Six of 11 (55%) of the failures in the HPV-positive intermediate-risk group were local. Failure in the HPV-negative group was 3/5 (60%) in the intermediate-risk group and 12/20 (60%) in the high-risk group.
Conclusions: The results for the entire tonsillar group were comparable with that found in published literature. Patients with HPV-positive tumors had improved OS compared with HPV-negative tonsillar cancer, although not statistically significant due to small numbers.
Purpose/Objectives: To evaluate the outcomes of patients with tonsillar cancer treated at G.V. Sonny Montgomery VA Medical Center between 2006 and 2014 and to compare survival and patterns of failure between human papillomavirus (HPV)-positive tonsillar cancer and HPV-negative tonsillar cancer.
Methods: There were 70 patients with biopsy proven squamous cell carcinoma of the tonsil in the retrospective review. Sixty-one of 70 patients had stage III/IV disease. Forty-seven of 70 patients had their HPV status evaluated. There were 22 HPV-positive and 25 HPV-negative patients. The majority of patients were treated with concurrent chemoradiotherapy, consisting of either weekly cisplatin 45 mg/m2 or weekly cetuximab 400 mg/m2 loading dose and 250 mg/m2 once a week for 7 weeks. Radiation therapy was given using intensity modulated radiation therapy to 70 Gy at 2 Gy per fraction over 7 weeks. The median radiation dose was 70 Gy. We evaluated the outcomes, including loco-regional failure, distant metastases, and survival. Rates were estimated by Kaplan-Meier method, and comparisons between HPV groups were evaluated using the Fisher exact test for categorical variables and Kruskal-Wallis test. Intermediate risk was defined as having ≥ 10 pack-years smoking history in the HPV-positive group (11 patients) and < 10 pack-years in the HPV-negative group (5 patients).
Results: Follow-up ranged from 14 to 88 months (median 22 mo). Overall survival (OS) for the entire group was 68% at 3 years with a disease-free survival (DFS) rate of 56%. At 3 years, the OS and DFS were 73% and 59% in the HPV-positive group and 50% and 50%, respectively, in the HPV-negative group. In the HPV-positive group, the failure rate was 2/11(16%) in the low-risk group and 8/11 (72%) in the intermediate-risk group. Six of 11 (55%) of the failures in the HPV-positive intermediate-risk group were local. Failure in the HPV-negative group was 3/5 (60%) in the intermediate-risk group and 12/20 (60%) in the high-risk group.
Conclusions: The results for the entire tonsillar group were comparable with that found in published literature. Patients with HPV-positive tumors had improved OS compared with HPV-negative tonsillar cancer, although not statistically significant due to small numbers.
Purpose/Objectives: To evaluate the outcomes of patients with tonsillar cancer treated at G.V. Sonny Montgomery VA Medical Center between 2006 and 2014 and to compare survival and patterns of failure between human papillomavirus (HPV)-positive tonsillar cancer and HPV-negative tonsillar cancer.
Methods: There were 70 patients with biopsy proven squamous cell carcinoma of the tonsil in the retrospective review. Sixty-one of 70 patients had stage III/IV disease. Forty-seven of 70 patients had their HPV status evaluated. There were 22 HPV-positive and 25 HPV-negative patients. The majority of patients were treated with concurrent chemoradiotherapy, consisting of either weekly cisplatin 45 mg/m2 or weekly cetuximab 400 mg/m2 loading dose and 250 mg/m2 once a week for 7 weeks. Radiation therapy was given using intensity modulated radiation therapy to 70 Gy at 2 Gy per fraction over 7 weeks. The median radiation dose was 70 Gy. We evaluated the outcomes, including loco-regional failure, distant metastases, and survival. Rates were estimated by Kaplan-Meier method, and comparisons between HPV groups were evaluated using the Fisher exact test for categorical variables and Kruskal-Wallis test. Intermediate risk was defined as having ≥ 10 pack-years smoking history in the HPV-positive group (11 patients) and < 10 pack-years in the HPV-negative group (5 patients).
Results: Follow-up ranged from 14 to 88 months (median 22 mo). Overall survival (OS) for the entire group was 68% at 3 years with a disease-free survival (DFS) rate of 56%. At 3 years, the OS and DFS were 73% and 59% in the HPV-positive group and 50% and 50%, respectively, in the HPV-negative group. In the HPV-positive group, the failure rate was 2/11(16%) in the low-risk group and 8/11 (72%) in the intermediate-risk group. Six of 11 (55%) of the failures in the HPV-positive intermediate-risk group were local. Failure in the HPV-negative group was 3/5 (60%) in the intermediate-risk group and 12/20 (60%) in the high-risk group.
Conclusions: The results for the entire tonsillar group were comparable with that found in published literature. Patients with HPV-positive tumors had improved OS compared with HPV-negative tonsillar cancer, although not statistically significant due to small numbers.
Improving Access to Care for Veterans Referred for Stem Cell Transplant Using Video-Based Telemedicine
Purpose: The primary purpose of this quality improvement project was to decrease the time from referral to evaluation for stem cell transplant patients to < 30 days.
Background: Stem cell transplant patients referred to the VA Tennessee Valley Healthcare System (VATVHS) from throughout the U.S. require complex coordination of care, including travel and lodging at government expense. Coordination of care for this patient population was becoming labor intensive and inefficient. The prospect of travelling far from home for an extended evaluation creates an undue amount of stress and expense for some veterans. As the number of referrals increased, we sought methods to improve both the cost and efficiency associated with the referral process.
Methods: We implemented the videobased telemedicine evaluation system at VATVHS in July 2014. Our method for implementation involved the creation of a pretransplant evaluation template geared toward video-based telemedicine. Criteria for inclusion depended on the home VA’s ability to schedule and conduct video-based telemedicine. The attending physician assigned to the referral made the final decision as to whether the referred veteran was appropriate for video-based telemedicine or required an inperson evaluation. The time to evaluation from referral was our primary goal for improvement. Travel and lodging savings and reduction of clinic days were secondary measures of improvement.
Data Analysis: From July 9, 2014, to April 8, 2015, we evaluated 51 patient referrals using video-based telemedicine.
Results: We were able to decrease patient evaluation times from > 30 days to 19.4 days, a 35% reduction in time from referral to evaluation. Additionally, we were able to reduce air travel costs by $50,000 and lodging costs by $31,000. We were also able to decrease clinic days at VATVHS by 02 (2 patient clinic visits per evaluation).
Implications: Video-based telemedicine is an efficient, costeffective, convenient method of conducting initial evaluations for stem cell transplant patients. Further implementation of video-based telemedicine could serve to improve efficiency of evaluations, reduce the financial burden associated with travel and lodging, and serve to increase patient satisfaction by reducing travel time.
Purpose: The primary purpose of this quality improvement project was to decrease the time from referral to evaluation for stem cell transplant patients to < 30 days.
Background: Stem cell transplant patients referred to the VA Tennessee Valley Healthcare System (VATVHS) from throughout the U.S. require complex coordination of care, including travel and lodging at government expense. Coordination of care for this patient population was becoming labor intensive and inefficient. The prospect of travelling far from home for an extended evaluation creates an undue amount of stress and expense for some veterans. As the number of referrals increased, we sought methods to improve both the cost and efficiency associated with the referral process.
Methods: We implemented the videobased telemedicine evaluation system at VATVHS in July 2014. Our method for implementation involved the creation of a pretransplant evaluation template geared toward video-based telemedicine. Criteria for inclusion depended on the home VA’s ability to schedule and conduct video-based telemedicine. The attending physician assigned to the referral made the final decision as to whether the referred veteran was appropriate for video-based telemedicine or required an inperson evaluation. The time to evaluation from referral was our primary goal for improvement. Travel and lodging savings and reduction of clinic days were secondary measures of improvement.
Data Analysis: From July 9, 2014, to April 8, 2015, we evaluated 51 patient referrals using video-based telemedicine.
Results: We were able to decrease patient evaluation times from > 30 days to 19.4 days, a 35% reduction in time from referral to evaluation. Additionally, we were able to reduce air travel costs by $50,000 and lodging costs by $31,000. We were also able to decrease clinic days at VATVHS by 02 (2 patient clinic visits per evaluation).
Implications: Video-based telemedicine is an efficient, costeffective, convenient method of conducting initial evaluations for stem cell transplant patients. Further implementation of video-based telemedicine could serve to improve efficiency of evaluations, reduce the financial burden associated with travel and lodging, and serve to increase patient satisfaction by reducing travel time.
Purpose: The primary purpose of this quality improvement project was to decrease the time from referral to evaluation for stem cell transplant patients to < 30 days.
Background: Stem cell transplant patients referred to the VA Tennessee Valley Healthcare System (VATVHS) from throughout the U.S. require complex coordination of care, including travel and lodging at government expense. Coordination of care for this patient population was becoming labor intensive and inefficient. The prospect of travelling far from home for an extended evaluation creates an undue amount of stress and expense for some veterans. As the number of referrals increased, we sought methods to improve both the cost and efficiency associated with the referral process.
Methods: We implemented the videobased telemedicine evaluation system at VATVHS in July 2014. Our method for implementation involved the creation of a pretransplant evaluation template geared toward video-based telemedicine. Criteria for inclusion depended on the home VA’s ability to schedule and conduct video-based telemedicine. The attending physician assigned to the referral made the final decision as to whether the referred veteran was appropriate for video-based telemedicine or required an inperson evaluation. The time to evaluation from referral was our primary goal for improvement. Travel and lodging savings and reduction of clinic days were secondary measures of improvement.
Data Analysis: From July 9, 2014, to April 8, 2015, we evaluated 51 patient referrals using video-based telemedicine.
Results: We were able to decrease patient evaluation times from > 30 days to 19.4 days, a 35% reduction in time from referral to evaluation. Additionally, we were able to reduce air travel costs by $50,000 and lodging costs by $31,000. We were also able to decrease clinic days at VATVHS by 02 (2 patient clinic visits per evaluation).
Implications: Video-based telemedicine is an efficient, costeffective, convenient method of conducting initial evaluations for stem cell transplant patients. Further implementation of video-based telemedicine could serve to improve efficiency of evaluations, reduce the financial burden associated with travel and lodging, and serve to increase patient satisfaction by reducing travel time.
Send patients reminders for flu vaccines, study says
ATLANTA – Mobile reminders from health care providers to their patients, notifying them to get influenza vaccinations, significantly increases the likelihood that those patients will get vaccinated, according to a study by Dr. Katherine A. Benedict and coinvestigators in the Enteric Diseases Epidemiology Branch at the Centers for Disease Control and Prevention. Dr. Benedict presented findings of the research at the International Conference on Emerging Infectious Diseases.
The researchers used data from the March 2012 National Flu Survey, a CDC “random digit dial telephone survey” of influenza vaccination trends in adults 18 years of age and older. They analyzed the data to determine whether or not patients received reminders, recommendations, or offers for influenza vaccinations from their health care providers since July 1, 2011. They did a logistic regression analysis to determine if there was any relationship between influenza vaccination rates and receipt of recommendations from health care providers.
Almost 73% of adults reported visiting a physician at least once since July 1, 2011, the start of data collection for the March 2012 study cycle, with 17.2% reporting that they received a reminder from their doctors to get a flu vaccine. Overall, 45.5% of adults ultimately got vaccinated, with 75.6% of those who reported receiving a reminder saying that they also were offered vaccinations by their doctors.
Adults who received recommendations for influenza vaccination from their health care providers were most likely to get vaccinated, and had an unadjusted prevalence ratio of 2.09. Next likely were those who received vaccination offers (PR = 1.90), and those who simply received reminders (PR = 1.33). Furthermore, those between 50 and 64 years old were found more likely to receive a recommendation or offer – PR = 1.23 and PR = 1.09, respectively – than were those in the 18- to 49-year age range (PR = 1.06 for recommendation, PR = 0.99 for offer).
The takeaway from this, according to the investigators, is that reminders should be sent at least once at the beginning of each influenza season. Prevalence ratios were consistently higher for patients with more frequent visits to their health care providers, meaning that continued interaction with and communication from doctors increases the likelihood of vaccination. Dr. Benedict also recommended sending additional reminders throughout the season, and that patients should either seek out or be referred to doctors who offer influenza vaccinations themselves.
Dr. Benedict did not report any relevant financial disclosures.
ATLANTA – Mobile reminders from health care providers to their patients, notifying them to get influenza vaccinations, significantly increases the likelihood that those patients will get vaccinated, according to a study by Dr. Katherine A. Benedict and coinvestigators in the Enteric Diseases Epidemiology Branch at the Centers for Disease Control and Prevention. Dr. Benedict presented findings of the research at the International Conference on Emerging Infectious Diseases.
The researchers used data from the March 2012 National Flu Survey, a CDC “random digit dial telephone survey” of influenza vaccination trends in adults 18 years of age and older. They analyzed the data to determine whether or not patients received reminders, recommendations, or offers for influenza vaccinations from their health care providers since July 1, 2011. They did a logistic regression analysis to determine if there was any relationship between influenza vaccination rates and receipt of recommendations from health care providers.
Almost 73% of adults reported visiting a physician at least once since July 1, 2011, the start of data collection for the March 2012 study cycle, with 17.2% reporting that they received a reminder from their doctors to get a flu vaccine. Overall, 45.5% of adults ultimately got vaccinated, with 75.6% of those who reported receiving a reminder saying that they also were offered vaccinations by their doctors.
Adults who received recommendations for influenza vaccination from their health care providers were most likely to get vaccinated, and had an unadjusted prevalence ratio of 2.09. Next likely were those who received vaccination offers (PR = 1.90), and those who simply received reminders (PR = 1.33). Furthermore, those between 50 and 64 years old were found more likely to receive a recommendation or offer – PR = 1.23 and PR = 1.09, respectively – than were those in the 18- to 49-year age range (PR = 1.06 for recommendation, PR = 0.99 for offer).
The takeaway from this, according to the investigators, is that reminders should be sent at least once at the beginning of each influenza season. Prevalence ratios were consistently higher for patients with more frequent visits to their health care providers, meaning that continued interaction with and communication from doctors increases the likelihood of vaccination. Dr. Benedict also recommended sending additional reminders throughout the season, and that patients should either seek out or be referred to doctors who offer influenza vaccinations themselves.
Dr. Benedict did not report any relevant financial disclosures.
ATLANTA – Mobile reminders from health care providers to their patients, notifying them to get influenza vaccinations, significantly increases the likelihood that those patients will get vaccinated, according to a study by Dr. Katherine A. Benedict and coinvestigators in the Enteric Diseases Epidemiology Branch at the Centers for Disease Control and Prevention. Dr. Benedict presented findings of the research at the International Conference on Emerging Infectious Diseases.
The researchers used data from the March 2012 National Flu Survey, a CDC “random digit dial telephone survey” of influenza vaccination trends in adults 18 years of age and older. They analyzed the data to determine whether or not patients received reminders, recommendations, or offers for influenza vaccinations from their health care providers since July 1, 2011. They did a logistic regression analysis to determine if there was any relationship between influenza vaccination rates and receipt of recommendations from health care providers.
Almost 73% of adults reported visiting a physician at least once since July 1, 2011, the start of data collection for the March 2012 study cycle, with 17.2% reporting that they received a reminder from their doctors to get a flu vaccine. Overall, 45.5% of adults ultimately got vaccinated, with 75.6% of those who reported receiving a reminder saying that they also were offered vaccinations by their doctors.
Adults who received recommendations for influenza vaccination from their health care providers were most likely to get vaccinated, and had an unadjusted prevalence ratio of 2.09. Next likely were those who received vaccination offers (PR = 1.90), and those who simply received reminders (PR = 1.33). Furthermore, those between 50 and 64 years old were found more likely to receive a recommendation or offer – PR = 1.23 and PR = 1.09, respectively – than were those in the 18- to 49-year age range (PR = 1.06 for recommendation, PR = 0.99 for offer).
The takeaway from this, according to the investigators, is that reminders should be sent at least once at the beginning of each influenza season. Prevalence ratios were consistently higher for patients with more frequent visits to their health care providers, meaning that continued interaction with and communication from doctors increases the likelihood of vaccination. Dr. Benedict also recommended sending additional reminders throughout the season, and that patients should either seek out or be referred to doctors who offer influenza vaccinations themselves.
Dr. Benedict did not report any relevant financial disclosures.
AT ICEID 2015
Key clinical point: Sending patients reminders to get their influenza vaccinations can significantly increase the likelihood that these patients will actually go out and get the vaccine.
Major finding: Adults who received a recommendation for influenza vaccination were more likely to get vaccinated, with an adjusted prevalence ratio (APR) of 1.15.
Data source: Retrospective review of adults 18 years and older in the March 2012 National Flu Survey.
Disclosures: Dr. Benedict did not report any relevant financial disclosures.
HELIOS trial: Ibrutinib safely boosts survival in CLL/SLL
CHICAGO – Adding ibrutinib to bendamustine and rituximab improved outcomes without significantly reducing safety in patients with chronic lymphocytic leukemia/small lymphocytic lymphoma (CLL/SLL) in the randomized, placebo-controlled, phase III HELIOS trial.
Efficacy results from the double-blind HELIOS trial, as reported by Dr. Asher Alban Chanan-Khan at the 2015 meeting of the American Society of Clinical Oncology, showed that adding ibrutinib to bendamustine and rituximab (BR) significantly extended progression-free survival, compared with BR plus placebo, in patients with CLL/SLL; the risk of progression and death was reduced by 80%.
The current findings, reported by Dr. Chanan-Khan at the American Society of Hematology Meeting on Hematologic Malignancies, demonstrate that this improvement was achieved without sacrificing safety, and they characterize the management of adverse events.
In 578 patients with active chronic CLL/SLL following at least one prior line of systemic therapy who were randomized to receive 420 mg of ibrutinib plus BR or placebo plus BR for six cycles, exposure was 14.7 months and 12.8 months, respectively. Infection rates were similar in the two groups, but exposure-adjusted analysis showed an overall lower infection rate in the ibrutinib group, compared with the placebo group (10.3/100 vs. 11.2/100 patient months), and the rates of grade 3 or higher infections was similar in the groups, said Dr. Chanan-Khan of the Mayo Clinic, Jacksonville, Fla.
The rates of all-grade and grade 3/4 anemia were 22.3% and 3.5%, respectively, in the ibrutinib group, and 28.9% and 8.0%, respectively, in the BR group. The ibrutinib patients also required fewer transfusions – most often red blood cell transfusions (23% vs. 29% in the BR group).This may have been a reflection of restoration of the hematopoietic system in the ibrutinib group, said Dr. Chanan-Khan.
Grade 3/4 neutropenia was similar in the groups (53.7% and 50.5%), but fewer patients discontinued treatment due to treatment-related neutropenia with ibrutinib (1% vs. 2.8%), he noted.
Thrombocytopenia occurred slightly more often in the ibrutinib group (30.7% vs. 24%), but grade 3/4 events occurred in 15% of patients in each group.
Atrial fibrillation (AF) occurred in a small number of patients, but was observed more often with ibrutinib (7.3% vs. 2.8% overall, and 2.8% vs. 0.7% for grade 3/4 AF). Only seven patients required dose interruption – for a median duration of 7 days – to manage AF.
“No dose reductions were required,” said Dr. Chanan-Khan, adding that four patients, all with grade 3/4 AF and all in the ibrutinib group, discontinued therapy because of AF.
“We then analyzed our data to identify potential risk factors for predisposition to AF ... no one baseline risk factor could be identified as causative. However, most patients who developed AF had a known risk factor,” he said.
He added that among those with a prior history of AF, 28% on the ibrutinib arm, and only 9% on the placebo arm, developed AF.
Baseline cardiac comorbidities also were found to have no effect on progression-free survival in either arm.
“We therefore concluded that the risk of AF is low at around 5%, it does not impact progression-free survival, prior history of AF is not a contraindication in the absence of any great freak event, ibrutinib dose interruption or reduction is not warranted, and you should treat CLL patients first for CLL and manage AF second,” he said.
Another important factor that often impacts clinical decision making is the use of anticoagulants or antiplatelet agents and the bleeding risk with ibrutinib, he said, noting that more than 40% of patients in the ibrutinib arm were using such agents.
“We did not see any impact on the progression-free survival outcomes on either of the arms in patients who were on anticoagulant or antiplatelet therapy,” he said.
Bleeding occurred in 31% and 14.6% of patients in the ibrutinib and placebo groups, respectively, and most cases involved grade 1 bruises and contusions. Only four patients discontinued therapy because of bleeding.
The rates of grade 3/4 major bleeding and major hemorrhage events were low in both groups, at less than 4%, and two patients discontinued therapy because of major bleeding. Two patients in the ibrutinib arm died because of major bleeding, including one who had a large preexisting abdominal aortic aneurysm, and one who experienced a large postsurgical intestinal perforation.
“Overall, these data support the use of ibrutinib in patients on concurrent anticoagulant or antiplatelet therapy, with no significantly increased major risk of bleeding with ibrutinib vs. placebo, and most bleeding events being grade 1 in nature,” said Dr. Chanan-Khan.
The rate of treatment-related lymphocytosis – a known pharmacodynamic effect of ibrutinib – occurred in 7% and 5.9% of the ibrutinib and placebo group patients, and most cases resolved within 2 weeks.
Based on the results of the 2014 phase III RESONATE trial and others looking at ibrutinib as a single-agent treatment for CLL, the agent is considered a new standard of care in patients with previously treated CLL/SLL. HELIOS was the first study to investigate ibrutinib in combination with BR.
“Considering the significant improvement in progression-free survival and overall survival, ibrutinib has a strong overall risk-benefit profile,” Dr. Chanan-Khan concluded.
The HELIOS study was sponsored by Janssen Pharmaceuticals. Dr. Chanan-Khan reported having no disclosures.
CHICAGO – Adding ibrutinib to bendamustine and rituximab improved outcomes without significantly reducing safety in patients with chronic lymphocytic leukemia/small lymphocytic lymphoma (CLL/SLL) in the randomized, placebo-controlled, phase III HELIOS trial.
Efficacy results from the double-blind HELIOS trial, as reported by Dr. Asher Alban Chanan-Khan at the 2015 meeting of the American Society of Clinical Oncology, showed that adding ibrutinib to bendamustine and rituximab (BR) significantly extended progression-free survival, compared with BR plus placebo, in patients with CLL/SLL; the risk of progression and death was reduced by 80%.
The current findings, reported by Dr. Chanan-Khan at the American Society of Hematology Meeting on Hematologic Malignancies, demonstrate that this improvement was achieved without sacrificing safety, and they characterize the management of adverse events.
In 578 patients with active chronic CLL/SLL following at least one prior line of systemic therapy who were randomized to receive 420 mg of ibrutinib plus BR or placebo plus BR for six cycles, exposure was 14.7 months and 12.8 months, respectively. Infection rates were similar in the two groups, but exposure-adjusted analysis showed an overall lower infection rate in the ibrutinib group, compared with the placebo group (10.3/100 vs. 11.2/100 patient months), and the rates of grade 3 or higher infections was similar in the groups, said Dr. Chanan-Khan of the Mayo Clinic, Jacksonville, Fla.
The rates of all-grade and grade 3/4 anemia were 22.3% and 3.5%, respectively, in the ibrutinib group, and 28.9% and 8.0%, respectively, in the BR group. The ibrutinib patients also required fewer transfusions – most often red blood cell transfusions (23% vs. 29% in the BR group).This may have been a reflection of restoration of the hematopoietic system in the ibrutinib group, said Dr. Chanan-Khan.
Grade 3/4 neutropenia was similar in the groups (53.7% and 50.5%), but fewer patients discontinued treatment due to treatment-related neutropenia with ibrutinib (1% vs. 2.8%), he noted.
Thrombocytopenia occurred slightly more often in the ibrutinib group (30.7% vs. 24%), but grade 3/4 events occurred in 15% of patients in each group.
Atrial fibrillation (AF) occurred in a small number of patients, but was observed more often with ibrutinib (7.3% vs. 2.8% overall, and 2.8% vs. 0.7% for grade 3/4 AF). Only seven patients required dose interruption – for a median duration of 7 days – to manage AF.
“No dose reductions were required,” said Dr. Chanan-Khan, adding that four patients, all with grade 3/4 AF and all in the ibrutinib group, discontinued therapy because of AF.
“We then analyzed our data to identify potential risk factors for predisposition to AF ... no one baseline risk factor could be identified as causative. However, most patients who developed AF had a known risk factor,” he said.
He added that among those with a prior history of AF, 28% on the ibrutinib arm, and only 9% on the placebo arm, developed AF.
Baseline cardiac comorbidities also were found to have no effect on progression-free survival in either arm.
“We therefore concluded that the risk of AF is low at around 5%, it does not impact progression-free survival, prior history of AF is not a contraindication in the absence of any great freak event, ibrutinib dose interruption or reduction is not warranted, and you should treat CLL patients first for CLL and manage AF second,” he said.
Another important factor that often impacts clinical decision making is the use of anticoagulants or antiplatelet agents and the bleeding risk with ibrutinib, he said, noting that more than 40% of patients in the ibrutinib arm were using such agents.
“We did not see any impact on the progression-free survival outcomes on either of the arms in patients who were on anticoagulant or antiplatelet therapy,” he said.
Bleeding occurred in 31% and 14.6% of patients in the ibrutinib and placebo groups, respectively, and most cases involved grade 1 bruises and contusions. Only four patients discontinued therapy because of bleeding.
The rates of grade 3/4 major bleeding and major hemorrhage events were low in both groups, at less than 4%, and two patients discontinued therapy because of major bleeding. Two patients in the ibrutinib arm died because of major bleeding, including one who had a large preexisting abdominal aortic aneurysm, and one who experienced a large postsurgical intestinal perforation.
“Overall, these data support the use of ibrutinib in patients on concurrent anticoagulant or antiplatelet therapy, with no significantly increased major risk of bleeding with ibrutinib vs. placebo, and most bleeding events being grade 1 in nature,” said Dr. Chanan-Khan.
The rate of treatment-related lymphocytosis – a known pharmacodynamic effect of ibrutinib – occurred in 7% and 5.9% of the ibrutinib and placebo group patients, and most cases resolved within 2 weeks.
Based on the results of the 2014 phase III RESONATE trial and others looking at ibrutinib as a single-agent treatment for CLL, the agent is considered a new standard of care in patients with previously treated CLL/SLL. HELIOS was the first study to investigate ibrutinib in combination with BR.
“Considering the significant improvement in progression-free survival and overall survival, ibrutinib has a strong overall risk-benefit profile,” Dr. Chanan-Khan concluded.
The HELIOS study was sponsored by Janssen Pharmaceuticals. Dr. Chanan-Khan reported having no disclosures.
CHICAGO – Adding ibrutinib to bendamustine and rituximab improved outcomes without significantly reducing safety in patients with chronic lymphocytic leukemia/small lymphocytic lymphoma (CLL/SLL) in the randomized, placebo-controlled, phase III HELIOS trial.
Efficacy results from the double-blind HELIOS trial, as reported by Dr. Asher Alban Chanan-Khan at the 2015 meeting of the American Society of Clinical Oncology, showed that adding ibrutinib to bendamustine and rituximab (BR) significantly extended progression-free survival, compared with BR plus placebo, in patients with CLL/SLL; the risk of progression and death was reduced by 80%.
The current findings, reported by Dr. Chanan-Khan at the American Society of Hematology Meeting on Hematologic Malignancies, demonstrate that this improvement was achieved without sacrificing safety, and they characterize the management of adverse events.
In 578 patients with active chronic CLL/SLL following at least one prior line of systemic therapy who were randomized to receive 420 mg of ibrutinib plus BR or placebo plus BR for six cycles, exposure was 14.7 months and 12.8 months, respectively. Infection rates were similar in the two groups, but exposure-adjusted analysis showed an overall lower infection rate in the ibrutinib group, compared with the placebo group (10.3/100 vs. 11.2/100 patient months), and the rates of grade 3 or higher infections was similar in the groups, said Dr. Chanan-Khan of the Mayo Clinic, Jacksonville, Fla.
The rates of all-grade and grade 3/4 anemia were 22.3% and 3.5%, respectively, in the ibrutinib group, and 28.9% and 8.0%, respectively, in the BR group. The ibrutinib patients also required fewer transfusions – most often red blood cell transfusions (23% vs. 29% in the BR group).This may have been a reflection of restoration of the hematopoietic system in the ibrutinib group, said Dr. Chanan-Khan.
Grade 3/4 neutropenia was similar in the groups (53.7% and 50.5%), but fewer patients discontinued treatment due to treatment-related neutropenia with ibrutinib (1% vs. 2.8%), he noted.
Thrombocytopenia occurred slightly more often in the ibrutinib group (30.7% vs. 24%), but grade 3/4 events occurred in 15% of patients in each group.
Atrial fibrillation (AF) occurred in a small number of patients, but was observed more often with ibrutinib (7.3% vs. 2.8% overall, and 2.8% vs. 0.7% for grade 3/4 AF). Only seven patients required dose interruption – for a median duration of 7 days – to manage AF.
“No dose reductions were required,” said Dr. Chanan-Khan, adding that four patients, all with grade 3/4 AF and all in the ibrutinib group, discontinued therapy because of AF.
“We then analyzed our data to identify potential risk factors for predisposition to AF ... no one baseline risk factor could be identified as causative. However, most patients who developed AF had a known risk factor,” he said.
He added that among those with a prior history of AF, 28% on the ibrutinib arm, and only 9% on the placebo arm, developed AF.
Baseline cardiac comorbidities also were found to have no effect on progression-free survival in either arm.
“We therefore concluded that the risk of AF is low at around 5%, it does not impact progression-free survival, prior history of AF is not a contraindication in the absence of any great freak event, ibrutinib dose interruption or reduction is not warranted, and you should treat CLL patients first for CLL and manage AF second,” he said.
Another important factor that often impacts clinical decision making is the use of anticoagulants or antiplatelet agents and the bleeding risk with ibrutinib, he said, noting that more than 40% of patients in the ibrutinib arm were using such agents.
“We did not see any impact on the progression-free survival outcomes on either of the arms in patients who were on anticoagulant or antiplatelet therapy,” he said.
Bleeding occurred in 31% and 14.6% of patients in the ibrutinib and placebo groups, respectively, and most cases involved grade 1 bruises and contusions. Only four patients discontinued therapy because of bleeding.
The rates of grade 3/4 major bleeding and major hemorrhage events were low in both groups, at less than 4%, and two patients discontinued therapy because of major bleeding. Two patients in the ibrutinib arm died because of major bleeding, including one who had a large preexisting abdominal aortic aneurysm, and one who experienced a large postsurgical intestinal perforation.
“Overall, these data support the use of ibrutinib in patients on concurrent anticoagulant or antiplatelet therapy, with no significantly increased major risk of bleeding with ibrutinib vs. placebo, and most bleeding events being grade 1 in nature,” said Dr. Chanan-Khan.
The rate of treatment-related lymphocytosis – a known pharmacodynamic effect of ibrutinib – occurred in 7% and 5.9% of the ibrutinib and placebo group patients, and most cases resolved within 2 weeks.
Based on the results of the 2014 phase III RESONATE trial and others looking at ibrutinib as a single-agent treatment for CLL, the agent is considered a new standard of care in patients with previously treated CLL/SLL. HELIOS was the first study to investigate ibrutinib in combination with BR.
“Considering the significant improvement in progression-free survival and overall survival, ibrutinib has a strong overall risk-benefit profile,” Dr. Chanan-Khan concluded.
The HELIOS study was sponsored by Janssen Pharmaceuticals. Dr. Chanan-Khan reported having no disclosures.
AT MHM 2015
Key clinical point: Adding ibrutinib to bendamustine and rituximab improved outcomes without significantly reducing safety in patients with chronic lymphocytic leukemia/small lymphocytic lymphoma (CLL/SLL).
Major finding: The overall infection rate was lower in the ibrutinib group than in the placebo group (10.3/100 vs. 11.2/100 patient months).
Data source: The phase III HELIOS study involving 578 patients.
Disclosures: Janssen Pharmaceuticals sponsored the study. Dr. Chanan-Khan reported having no disclosures.
Assessment of Body Weight After Completion of Radiotherapy With or Without Chemotherapy and With or Without Prophylactic Feeding Tube Placement in Head and Neck Cancer
Purpose: A majority of the patients with a diagnosis of locally advanced head and neck cancer receiving combined chemoradiotherapy experience mucositis, odynophagia, and dysphagia resulting in reduced oral intake and weight loss during the treatment. A prophylactic feeding tube is generally recommended for these patients to maintain body weight during the treatment. The purpose of this retrospective study is to understand the change in body weight from baseline to last follow-up after completion of radiotherapy or combined chemoradiotherapy and to assess the role of prophylactic feeding tube placement in maintaining body weight.
Methods: Thirty-seven patients with a diagnosis of locally advanced head and neck cancers were treated either with adjuvant or definitive radiotherapy with or without chemotherapy at Kansas City VA Medical Center during 2013. Eleven patients did not receive chemotherapy, and a majority of the patients did not receive prophylactic percutaneous endoscopic gastrostomy tube placement. Twenty-six patient received cisplatin-based chemotherapy during radio-therapy; of these, 9 patients had no feeding tube and 17 patients received a prophylactic feeding tube. The radiation dose ranged from 60 to 70 Gy in 30 to 35 fractions. All these patients were followed on a regular basis, and weights were recorded on each visit. The follow-up period ranged from a minimum of 6 months to a maximum of 18 months. Results: Five patients died either from locoregional recurrence or distant metastases. The average weight loss for patients with combined modality treatments was 9.7% vs 6.3% for patients receiving no chemotherapy. The average weight loss for patients receiving concurrent chemotherapy and with prophylactic feeding tube placement was 7.4% com-pared with average weight loss of 16% for patients receiving chemotherapy and no prophylactic feeding tube placement. The majority of these patients both in the chemotherapy and the no chemotherapy groups never regained their baseline weight.
Conclusions: Patients receiving chemotherapy benefited from prophylactic feeding tube placement in maintaining body weight similar to patients receiving no chemotherapy.
Purpose: A majority of the patients with a diagnosis of locally advanced head and neck cancer receiving combined chemoradiotherapy experience mucositis, odynophagia, and dysphagia resulting in reduced oral intake and weight loss during the treatment. A prophylactic feeding tube is generally recommended for these patients to maintain body weight during the treatment. The purpose of this retrospective study is to understand the change in body weight from baseline to last follow-up after completion of radiotherapy or combined chemoradiotherapy and to assess the role of prophylactic feeding tube placement in maintaining body weight.
Methods: Thirty-seven patients with a diagnosis of locally advanced head and neck cancers were treated either with adjuvant or definitive radiotherapy with or without chemotherapy at Kansas City VA Medical Center during 2013. Eleven patients did not receive chemotherapy, and a majority of the patients did not receive prophylactic percutaneous endoscopic gastrostomy tube placement. Twenty-six patient received cisplatin-based chemotherapy during radio-therapy; of these, 9 patients had no feeding tube and 17 patients received a prophylactic feeding tube. The radiation dose ranged from 60 to 70 Gy in 30 to 35 fractions. All these patients were followed on a regular basis, and weights were recorded on each visit. The follow-up period ranged from a minimum of 6 months to a maximum of 18 months. Results: Five patients died either from locoregional recurrence or distant metastases. The average weight loss for patients with combined modality treatments was 9.7% vs 6.3% for patients receiving no chemotherapy. The average weight loss for patients receiving concurrent chemotherapy and with prophylactic feeding tube placement was 7.4% com-pared with average weight loss of 16% for patients receiving chemotherapy and no prophylactic feeding tube placement. The majority of these patients both in the chemotherapy and the no chemotherapy groups never regained their baseline weight.
Conclusions: Patients receiving chemotherapy benefited from prophylactic feeding tube placement in maintaining body weight similar to patients receiving no chemotherapy.
Purpose: A majority of the patients with a diagnosis of locally advanced head and neck cancer receiving combined chemoradiotherapy experience mucositis, odynophagia, and dysphagia resulting in reduced oral intake and weight loss during the treatment. A prophylactic feeding tube is generally recommended for these patients to maintain body weight during the treatment. The purpose of this retrospective study is to understand the change in body weight from baseline to last follow-up after completion of radiotherapy or combined chemoradiotherapy and to assess the role of prophylactic feeding tube placement in maintaining body weight.
Methods: Thirty-seven patients with a diagnosis of locally advanced head and neck cancers were treated either with adjuvant or definitive radiotherapy with or without chemotherapy at Kansas City VA Medical Center during 2013. Eleven patients did not receive chemotherapy, and a majority of the patients did not receive prophylactic percutaneous endoscopic gastrostomy tube placement. Twenty-six patient received cisplatin-based chemotherapy during radio-therapy; of these, 9 patients had no feeding tube and 17 patients received a prophylactic feeding tube. The radiation dose ranged from 60 to 70 Gy in 30 to 35 fractions. All these patients were followed on a regular basis, and weights were recorded on each visit. The follow-up period ranged from a minimum of 6 months to a maximum of 18 months. Results: Five patients died either from locoregional recurrence or distant metastases. The average weight loss for patients with combined modality treatments was 9.7% vs 6.3% for patients receiving no chemotherapy. The average weight loss for patients receiving concurrent chemotherapy and with prophylactic feeding tube placement was 7.4% com-pared with average weight loss of 16% for patients receiving chemotherapy and no prophylactic feeding tube placement. The majority of these patients both in the chemotherapy and the no chemotherapy groups never regained their baseline weight.
Conclusions: Patients receiving chemotherapy benefited from prophylactic feeding tube placement in maintaining body weight similar to patients receiving no chemotherapy.
Cancer Cachexia and Survival in Patients With Lung Cancer by Histology: Who Is Most at Risk?
Background: Cancer cachexia is a syndrome characterized by wasting of lean body mass and fat, resulting in decreased treatment tolerance and quality of life. Approximately 50% to 60% of patients with lung cancer develop cancer cachexia. Knowledge of cancer cachexia trends and outcomes in different types of lung cancers can help target patients most at risk of decreased survival. In this study, we examined weight loss trends in patients with lung cancer and survival according to cancer histology.
Methods: We conducted a chart review of patients diagnosed with lung cancer from 2007 to 2013 at a medical center in a large metropolitan area. Data collected included demographics, date of diagnosis, treatment received, type of cancer by histology, presence of metastasis, preexisting conditions, and other medications used. Body mass index and weight before, during, and after treatments were recorded, and percentage weight loss and survival days were calculated. Patients were also categorized according to the extent of weight loss (< 5%, 5%-10%, > 10%) through their disease course. Descriptive statistics, ANOVA, t tests, and regression analysis were used for data analysis to assess differences and relationships between percentage of weight loss and survival for all groups.
Results: Data from 197 patients were available for the study. All patients were males with an average age of 68 years; 55% of the patients were white and 35% were African American. Of the total patients, 47.7% had adenocarcinoma, 8.1% had small cell lung cancer (SCLC), and 44.2% had squamous cell cancer (SCC). Percentage weight loss trends for patients with adenocarcinoma showed a 9% loss from baseline till time of diagnosis and a total of 15% loss posttreatments. Patients with SCLC similarly showed a decline from 13% to 22%, and patients with SCC showed a 14% to 17% weight loss. Of the total patients with > 10% weight loss over time, 48% were patients with adenocarcinoma, compared with 10% of patients with SCLC, and 42% of patients with SCC. Average survival for patients with adenocarcinoma was 420 days, for patients with SCLC 321 days, and 492 days for patients with SCC. Weight loss percentage was significantly related to survival in all patients (r = -0.19, P < .01) and patient groups with adenocarcinoma (r = -0.25, P < .01) but not in patients with SCC or SCLC.
Conclusions: Cancer cachexia trends in patients with lung cancer are similar for patients with adenocarcinoma, SCLC, and SCC; however, the percentage of weight loss may adversely impact survival in patients with SCLC.
Background: Cancer cachexia is a syndrome characterized by wasting of lean body mass and fat, resulting in decreased treatment tolerance and quality of life. Approximately 50% to 60% of patients with lung cancer develop cancer cachexia. Knowledge of cancer cachexia trends and outcomes in different types of lung cancers can help target patients most at risk of decreased survival. In this study, we examined weight loss trends in patients with lung cancer and survival according to cancer histology.
Methods: We conducted a chart review of patients diagnosed with lung cancer from 2007 to 2013 at a medical center in a large metropolitan area. Data collected included demographics, date of diagnosis, treatment received, type of cancer by histology, presence of metastasis, preexisting conditions, and other medications used. Body mass index and weight before, during, and after treatments were recorded, and percentage weight loss and survival days were calculated. Patients were also categorized according to the extent of weight loss (< 5%, 5%-10%, > 10%) through their disease course. Descriptive statistics, ANOVA, t tests, and regression analysis were used for data analysis to assess differences and relationships between percentage of weight loss and survival for all groups.
Results: Data from 197 patients were available for the study. All patients were males with an average age of 68 years; 55% of the patients were white and 35% were African American. Of the total patients, 47.7% had adenocarcinoma, 8.1% had small cell lung cancer (SCLC), and 44.2% had squamous cell cancer (SCC). Percentage weight loss trends for patients with adenocarcinoma showed a 9% loss from baseline till time of diagnosis and a total of 15% loss posttreatments. Patients with SCLC similarly showed a decline from 13% to 22%, and patients with SCC showed a 14% to 17% weight loss. Of the total patients with > 10% weight loss over time, 48% were patients with adenocarcinoma, compared with 10% of patients with SCLC, and 42% of patients with SCC. Average survival for patients with adenocarcinoma was 420 days, for patients with SCLC 321 days, and 492 days for patients with SCC. Weight loss percentage was significantly related to survival in all patients (r = -0.19, P < .01) and patient groups with adenocarcinoma (r = -0.25, P < .01) but not in patients with SCC or SCLC.
Conclusions: Cancer cachexia trends in patients with lung cancer are similar for patients with adenocarcinoma, SCLC, and SCC; however, the percentage of weight loss may adversely impact survival in patients with SCLC.
Background: Cancer cachexia is a syndrome characterized by wasting of lean body mass and fat, resulting in decreased treatment tolerance and quality of life. Approximately 50% to 60% of patients with lung cancer develop cancer cachexia. Knowledge of cancer cachexia trends and outcomes in different types of lung cancers can help target patients most at risk of decreased survival. In this study, we examined weight loss trends in patients with lung cancer and survival according to cancer histology.
Methods: We conducted a chart review of patients diagnosed with lung cancer from 2007 to 2013 at a medical center in a large metropolitan area. Data collected included demographics, date of diagnosis, treatment received, type of cancer by histology, presence of metastasis, preexisting conditions, and other medications used. Body mass index and weight before, during, and after treatments were recorded, and percentage weight loss and survival days were calculated. Patients were also categorized according to the extent of weight loss (< 5%, 5%-10%, > 10%) through their disease course. Descriptive statistics, ANOVA, t tests, and regression analysis were used for data analysis to assess differences and relationships between percentage of weight loss and survival for all groups.
Results: Data from 197 patients were available for the study. All patients were males with an average age of 68 years; 55% of the patients were white and 35% were African American. Of the total patients, 47.7% had adenocarcinoma, 8.1% had small cell lung cancer (SCLC), and 44.2% had squamous cell cancer (SCC). Percentage weight loss trends for patients with adenocarcinoma showed a 9% loss from baseline till time of diagnosis and a total of 15% loss posttreatments. Patients with SCLC similarly showed a decline from 13% to 22%, and patients with SCC showed a 14% to 17% weight loss. Of the total patients with > 10% weight loss over time, 48% were patients with adenocarcinoma, compared with 10% of patients with SCLC, and 42% of patients with SCC. Average survival for patients with adenocarcinoma was 420 days, for patients with SCLC 321 days, and 492 days for patients with SCC. Weight loss percentage was significantly related to survival in all patients (r = -0.19, P < .01) and patient groups with adenocarcinoma (r = -0.25, P < .01) but not in patients with SCC or SCLC.
Conclusions: Cancer cachexia trends in patients with lung cancer are similar for patients with adenocarcinoma, SCLC, and SCC; however, the percentage of weight loss may adversely impact survival in patients with SCLC.
Frequency of EGFR Mutations and ALK Rearrangements in Pulmonary Carcinomas at the Minneapolis VA Medical Center
Purpose: To evaluate the frequency of molecular testing in pulmonary carcinomas.
Background: Pulmonary carcinoma represents the second most common type of new malignancies in males and females. Although its incidence is plateauing, it represents the most common cause of cancer-related death in both genders. The 5-year survival rate is only 18%. The main risk factor in both genders is smoking. Carcinogenesis and tumor progression in some tumors involve mutations in relevant genes that further cell survival and proliferation and provide advantage over neighboring cells. Although therapy has traditionally been guided by tumor histology, drugs have been developed that specifically target driver mutation metabolic pathways. These drugs promise higher efficacy and lower toxicity; however, their use must be guided by the genetic makeup of individual tumors. One such mutation occurs in the EGFR gene and is more common in women with no smoking history. Another, mutually exclusive mutation consists of rearrangements in the ALK gene. These alterations render the cells sensitive to targeted therapy with tyrosine kinase inhibitors.
Data Analysis/Results: At the Minneapolis VA Medical Center, these tests are performed when ordered by the oncologists. We analyzed the frequency of mutations in exons 18-21 of the EGFR gene by PCR-based essay and ALK rearrangements by FISH technique in lung carcinomas. One hundred eighty-three biopsy and cytology specimens collected between January 2009 and June 2015 were evaluated. Of these, 11 (6%) were positive for EGFR mutations; none was positive for ALK rearrangements. All positive cases were morphologically adenocarcinomas; pulmonary origin was supported by clinical, radiologic and immunohistochemical criteria (positivity for cytokeratin 7 and for TTF-1). Specimens consisted of cell blocks (pleural effusion, fine needle aspirates) and needle biopsy cores of primary tumors or metastases or lobectomy specimen.
Implications: Compared with the literature, our frequency of mutant cases for the 2 targets analyzed is lower. This may be due to the fact that our patient population consisted predominantly of men with a history of smoking. It is important to analyze local statistics and compare them with the VA systemwide data for establishing guidelines and cost analysis.
Purpose: To evaluate the frequency of molecular testing in pulmonary carcinomas.
Background: Pulmonary carcinoma represents the second most common type of new malignancies in males and females. Although its incidence is plateauing, it represents the most common cause of cancer-related death in both genders. The 5-year survival rate is only 18%. The main risk factor in both genders is smoking. Carcinogenesis and tumor progression in some tumors involve mutations in relevant genes that further cell survival and proliferation and provide advantage over neighboring cells. Although therapy has traditionally been guided by tumor histology, drugs have been developed that specifically target driver mutation metabolic pathways. These drugs promise higher efficacy and lower toxicity; however, their use must be guided by the genetic makeup of individual tumors. One such mutation occurs in the EGFR gene and is more common in women with no smoking history. Another, mutually exclusive mutation consists of rearrangements in the ALK gene. These alterations render the cells sensitive to targeted therapy with tyrosine kinase inhibitors.
Data Analysis/Results: At the Minneapolis VA Medical Center, these tests are performed when ordered by the oncologists. We analyzed the frequency of mutations in exons 18-21 of the EGFR gene by PCR-based essay and ALK rearrangements by FISH technique in lung carcinomas. One hundred eighty-three biopsy and cytology specimens collected between January 2009 and June 2015 were evaluated. Of these, 11 (6%) were positive for EGFR mutations; none was positive for ALK rearrangements. All positive cases were morphologically adenocarcinomas; pulmonary origin was supported by clinical, radiologic and immunohistochemical criteria (positivity for cytokeratin 7 and for TTF-1). Specimens consisted of cell blocks (pleural effusion, fine needle aspirates) and needle biopsy cores of primary tumors or metastases or lobectomy specimen.
Implications: Compared with the literature, our frequency of mutant cases for the 2 targets analyzed is lower. This may be due to the fact that our patient population consisted predominantly of men with a history of smoking. It is important to analyze local statistics and compare them with the VA systemwide data for establishing guidelines and cost analysis.
Purpose: To evaluate the frequency of molecular testing in pulmonary carcinomas.
Background: Pulmonary carcinoma represents the second most common type of new malignancies in males and females. Although its incidence is plateauing, it represents the most common cause of cancer-related death in both genders. The 5-year survival rate is only 18%. The main risk factor in both genders is smoking. Carcinogenesis and tumor progression in some tumors involve mutations in relevant genes that further cell survival and proliferation and provide advantage over neighboring cells. Although therapy has traditionally been guided by tumor histology, drugs have been developed that specifically target driver mutation metabolic pathways. These drugs promise higher efficacy and lower toxicity; however, their use must be guided by the genetic makeup of individual tumors. One such mutation occurs in the EGFR gene and is more common in women with no smoking history. Another, mutually exclusive mutation consists of rearrangements in the ALK gene. These alterations render the cells sensitive to targeted therapy with tyrosine kinase inhibitors.
Data Analysis/Results: At the Minneapolis VA Medical Center, these tests are performed when ordered by the oncologists. We analyzed the frequency of mutations in exons 18-21 of the EGFR gene by PCR-based essay and ALK rearrangements by FISH technique in lung carcinomas. One hundred eighty-three biopsy and cytology specimens collected between January 2009 and June 2015 were evaluated. Of these, 11 (6%) were positive for EGFR mutations; none was positive for ALK rearrangements. All positive cases were morphologically adenocarcinomas; pulmonary origin was supported by clinical, radiologic and immunohistochemical criteria (positivity for cytokeratin 7 and for TTF-1). Specimens consisted of cell blocks (pleural effusion, fine needle aspirates) and needle biopsy cores of primary tumors or metastases or lobectomy specimen.
Implications: Compared with the literature, our frequency of mutant cases for the 2 targets analyzed is lower. This may be due to the fact that our patient population consisted predominantly of men with a history of smoking. It is important to analyze local statistics and compare them with the VA systemwide data for establishing guidelines and cost analysis.