User login
Gene therapy granted fast track designation for hemophilia B

The US Food and Drug Administration (FDA) has granted fast track designation to a gene therapy product being developed to treat hemophilia B.
The product, DTX101, is designed to deliver factor IX gene expression in a durable fashion to prevent the long-term complications of hemophilia B.
Preclinical studies have indicated that DTX101 has the potential to be a well-tolerated, effective therapy for hemophilia B, according to Dimension Therapeutics, Inc., the company developing DTX101.
The company said it expects to initiate a multicenter, phase 1/2 study to evaluate DTX101 in adults with moderate/severe to severe hemophilia B by the end of this year.
DTX101 also has orphan designation from the FDA.
About fast track designation
The FDA’s fast track program is designed to facilitate and expedite the development and review of new drugs intended to treat serious or life-threatening conditions and address unmet medical need.
Through the fast track program, a product may be eligible for priority review. In addition, the company developing the drug may be allowed to submit sections of the biologic license application or new drug application on a rolling basis as data become available.
Fast track designation also provides the company with opportunities for more frequent meetings with the FDA to discuss the drug’s development plan and ensure collection of the appropriate data needed to support drug approval. And the designation allows for more frequent written communication from the FDA about things such as the design of proposed clinical trials and the use of biomarkers. ![]()

The US Food and Drug Administration (FDA) has granted fast track designation to a gene therapy product being developed to treat hemophilia B.
The product, DTX101, is designed to deliver factor IX gene expression in a durable fashion to prevent the long-term complications of hemophilia B.
Preclinical studies have indicated that DTX101 has the potential to be a well-tolerated, effective therapy for hemophilia B, according to Dimension Therapeutics, Inc., the company developing DTX101.
The company said it expects to initiate a multicenter, phase 1/2 study to evaluate DTX101 in adults with moderate/severe to severe hemophilia B by the end of this year.
DTX101 also has orphan designation from the FDA.
About fast track designation
The FDA’s fast track program is designed to facilitate and expedite the development and review of new drugs intended to treat serious or life-threatening conditions and address unmet medical need.
Through the fast track program, a product may be eligible for priority review. In addition, the company developing the drug may be allowed to submit sections of the biologic license application or new drug application on a rolling basis as data become available.
Fast track designation also provides the company with opportunities for more frequent meetings with the FDA to discuss the drug’s development plan and ensure collection of the appropriate data needed to support drug approval. And the designation allows for more frequent written communication from the FDA about things such as the design of proposed clinical trials and the use of biomarkers. ![]()

The US Food and Drug Administration (FDA) has granted fast track designation to a gene therapy product being developed to treat hemophilia B.
The product, DTX101, is designed to deliver factor IX gene expression in a durable fashion to prevent the long-term complications of hemophilia B.
Preclinical studies have indicated that DTX101 has the potential to be a well-tolerated, effective therapy for hemophilia B, according to Dimension Therapeutics, Inc., the company developing DTX101.
The company said it expects to initiate a multicenter, phase 1/2 study to evaluate DTX101 in adults with moderate/severe to severe hemophilia B by the end of this year.
DTX101 also has orphan designation from the FDA.
About fast track designation
The FDA’s fast track program is designed to facilitate and expedite the development and review of new drugs intended to treat serious or life-threatening conditions and address unmet medical need.
Through the fast track program, a product may be eligible for priority review. In addition, the company developing the drug may be allowed to submit sections of the biologic license application or new drug application on a rolling basis as data become available.
Fast track designation also provides the company with opportunities for more frequent meetings with the FDA to discuss the drug’s development plan and ensure collection of the appropriate data needed to support drug approval. And the designation allows for more frequent written communication from the FDA about things such as the design of proposed clinical trials and the use of biomarkers. ![]()
Using Social Media as a Hospital QI Tool
Patient experience has become a major component of the Center for Medicare and Medicaid Services Value‐Based Purchasing initiative.[1] Hospitals have therefore focused quality improvement (QI) efforts on this area.[2] Hospital performance in the realm of patient experience is generally determined using systematic surveys with closed‐ended questions, but patient‐generated narrative feedback can help hospitals identify the components of care that contribute to patient satisfaction and or are in need of improvement.[3] Online narrative responses posted by patients on rating websites or social media have been criticized because they may not be representative of the population,[4] but they also have some advantages.[5] Any patient may leave a comment, not just those who are selected for a survey. Patients may also experience benefits through the act of sharing their story with others. Moreover, most US hospitals use some form of social media,[6] which they can theoretically use to self‐collect narrative data online. To realize the full potential of patient‐generated online narratives, we need a clearer understanding of the best practices for collecting and using these narratives. We therefore solicited patient feedback on the Facebook page of a large tertiary academic medical center to determine whether it is feasible to use social media platforms for learning about and improving hospital quality.
METHODS
Baystate Medical Center (BMC) is a tertiary care medical center in western Massachusetts. We identified key BMC stakeholders in the areas of QI and public affairs. Noting that patients have expressed interest in leaving comments via social media,[7] the group opted to perform a pilot study to obtain patient narratives via a Facebook prompt (Facebook is a social media site used by an estimated 58% of US adults[8]). The BMC public affairs department delivered a press release to the local media describing a 3‐week period during which patients were invited to leave narrative feedback on the BMC Facebook wall. The BMC Institutional Review Board deemed that this study did not constitute human subjects research.
During March 2014 (March 10, 2014March 24, 2014), we posted (once a week) an open‐ended prompt on BMC's Facebook wall. The prompt was designed to elicit novel descriptions of patient experience that could help to drive QI. It read: We want to hear about your experiences. In the comment section below, please tell us what we do well and how we can improve your care. Because of concerns about the potential reputational risks of allowing open feedback on a public social media page, the prompt also reminded patients of the social media ground rules: there should be no mention of specific physicians, nurses, or other caregivers by name (for liability reasons); and patients should not include details about their medical history (for privacy reasons).
We collected all posts to preserve comments and used directed qualitative content analysis to examine them.[9] Two research team members[3, 10, 11] independently coded the responses. Starting with an a priori codebook that was developed during a previous study,[3] they amended the codebook through an iterative process to incorporate new concepts. After independently coding all blocks of text, the coders reviewed their coding selections and resolved discrepancies through discussion. We then performed second‐level coding, in which codes were organized into major pertinent themes. We reviewed the coded text after applying secondary codes in order to check for accuracy of coding and theme assignment as well as completeness of second‐level coding. We calculated percent agreement, defined as both raters scoring a block of text with the same code divided by total number of codes. We also calculated the Spearman correlation between the 2 reviewers. We used descriptive statistics to assess the frequency of select codes and themes (see Supporting Information, Appendix 1 and Appendix 2, in the online version of this article).[9, 12, 13]
RESULTS
Over a 3‐week study period, 47 comments were submitted by 37 respondents. This yielded 148 codable statements (Table 1). Despite limited information on respondents, we ascertained from Facebook that 32 (86%) were women and 5 (14%) were men.
| Theme | Total Respondents, N (%) | % Positive | Positive Quotation | % Negative | Negative Quotation |
|---|---|---|---|---|---|
| |||||
| Staff | 17 (46) | 45% | The nurses in the pediatric unit, as well as the doctors in radiology and x‐ray department were AMAZING! | 55% | My 24‐year‐old daughter had to go for 5 days of IV treatmentwhile getting her infusion there was a fire alarm. She has a video showing the flashing of the light and the sound of the alarm and the closing of doors and NOT A SINGLE staff member to be found. Her infusions take about 2 hours. They set it and forget it. Luckily there wasn't a fire and someone did finally come to disconnect her. |
| Had a fabulous experience with Wesson women's this week! Had a C section and 3‐day admission. All staff from preoperative to inpatient were so helpful and really anticipated my needs before I could even ask for things. | My mother was hospitalized for at least 3 weeks right after the cardiovascular center openedwhen she went into cardiac arrest and in acute care and the step unit the care was great, very attentive nurses and doctors. When she was starting to recover and moved upstairs, downhill it went. She'd ring for assistance because she wanted to walk to the bathrooms and more times she was left to her own devices because no one would respond. | ||||
| Facility | 9 (24) | 25% | New buildings are beautiful and the new signs are way better. | 75% | The parking situation was disappointing and the waiting room was also very dirty. |
| I really like the individual pods in the ER. | I could have used a single room as my roommate was very annoying and demanding. | ||||
| Departments | 22 (60) | 44% | The NICU was great when my son was in there. The children's unit was great with my daughter and respected my needs. | 56% | Revamp maternity; it needs it desperately. |
| Labor and delivery was a great place. | Love Baystate but hate the ER. | ||||
| Technical aspects of care (eg, errors) | 9 (24) | 0 | 100% | Day 2 of my 24 year old getting her 2‐hour IV infusion....she was set up with her IV. When checked 2 hours later, the staff member was very upset to find that only the saline had run. She never opened the medication clamp. So now they gave her the medication in 1 hour instead of 2. | |
| If I had 1 suggestion it would be to re‐evaluate patient comfort when patients are waiting to be admitted. | |||||
From coded text, several broad themes were identified (see Table 1 for representative quotes): (1) comments about staff (17/37 respondents, 45.9%). These included positive descriptions of efficiency, caring behavior, good training, and good communication, whereas negative comments included descriptions of unfriendliness, apparent lack of caring, inattentiveness, poor training, unprofessional behavior, and poor communication; (2) comments about specific departments (22/37, 59.5%); (3) comments on technical aspects of care, including perceived errors, incorrect diagnoses, and inattention to pain control (9/37, 24.3%); and (4) comments describing the hospital physical plant, parking, and amenities (9/37, 24.3%). There were a few miscellaneous comments that did not fit into these broad themes, such as expressions of gratitude for our solicitation of narratives. Percent agreement between coders was 80% and Spearman's Rho was 0.82 (p0.001).
A small number (n=3) of respondents repeatedly made comments over the 3‐week period, accounting for 30% (45/148) of codes. These repetitive commenters tended to dominate the Facebook conversation, at times describing the same experience more than once.
DISCUSSION
In this study evaluating the potential utility of social media as a hospital QI tool, several broad themes emerged. From these themes, we identified several areas that could be deemed as QI targets, including: training staff to be more responsive and sensitive to patients needs and concerns, improving patient and visitor parking, and reducing emergency department waiting times. However, the insight gained from solicited Facebook comments was similar to feedback gained from more traditional approaches of soliciting patient perspectives on care, such as patient experience surveys.[14]
Our findings should be viewed in the context of prior work focused on patient narratives in healthcare. Greaves et al. used sentiment analysis to describe the content of nearly 200,000 tweets (comments posted on the social networking website Twitter) sent to National Health Service (NHS) hospitals.[15] Themes were similar to those found in our study: (1) interaction with staff, (2) environment and facilities, and (3) issues of access and timeliness of service. Notably, these themes mirrored prior work examining narratives at NHS hospitals[3] and were similar to domains of commonly used surveys of patient experience.[14] The authors noted that there were issues with the signal to noise ratio (only about 10% of tweets were about quality) and the enforced brevity of Twitter (tweets must be 140 characters or less). These limitations suggest that using Twitter to identify QI targets would be difficult.
In contrast to Greaves et al., we chose to solicit feedback on our hospital's Facebook page. Facebook does not have Twitter's enforced brevity, allowing for more detailed narratives. In addition, we did not encounter the signal‐to‐noise problem, because our prompt was designed to request feedback that was relevant to recent experiences of care. However, a few respondents dominated the conversation, supporting the hypothesis that those most likely to comment may be the patients or families who have had the best or worst experiences. In the future, we will attempt to address this limitation and reduce the influence of repeat commenters by changing our prompt (eg, Please tell us about your experience, but please do not post descriptions of the same experience more than once.).
This pilot demonstrated some of the previously described benefits of online narratives.[5] First, there appears to be value in allowing patients to share their experiences and to read the experiences of others (as indicated in a few grateful patients comments). Second, soliciting online narratives offers a way for hospitals to demonstrate a commitment to transparency. Third, in contrast to closed‐ended survey questions, narrative comments help to identify why patients were satisfied or unsatisfied with their care. Although some surveys with closed‐ended questions also allow for narratives, these comments may or may not be carefully reviewed by the hospital. Using social media to solicit and respond to comments enhances existing methods for evaluating patient experience by engaging patients in a public space, which increases the likelihood that hospitals will attempt to improve care in response.
Notably, none of the identified areas for improvement could be considered novel QI targets for BMC. For example, our hospital has been very focused on training staff around patient experience, and emergency department wait times are the focus of a system‐wide improvement effort called Patient Progress.
This study has other limitations. We conducted this study over a 3‐week time period in a single center and on a single social media site whose members may not be representative of the overall patient population at BMC. Although we do not know how generalizable our findings are (in terms of identifying QI targets), we feel that we have demonstrated how using social media to collect data on patient experience is feasible and could be informative for other hospitals in other locations. It is possible that we did not allow the experiment to run long enough; a longer time or broader outreach (eg, a handout given to every discharged patient over a longer period) may be needed to allow patients adequate opportunity to comment. Of note, we did not specifically examine responses by time period, but it does seem, in post hoc analysis, that after 2 weeks of data collection we reached theoretical saturation with no new themes emerging in the third week (eg, third‐week comments included I heart your nurses. and Love Baystate but hate the ER.). More work is also needed that includes a broader range of social media platforms and more participating hospitals.
In conclusion, the opportunity to provide feedback on Facebook has the potential to engage and empower patients, and hospitals can use these online narratives to help to drive improvement efforts. Yet potential benefits must be weighed against reputational risks, a lack of representative respondents, and the paucity of novel QI targets obtained in this study.
Disclosures: Dr. Lagu is supported by the National Heart, Lung, and Blood Institute of the National Institutes of Health under award number K01HL114745. The authors report no conflicts of interest.
- Centers for Medicare 47(2):193–219.
- , , , , . A mixed‐methods analysis of patient reviews of hospital care in England: implications for public reporting of health care quality data in the United States. Jt Comm J Qual Patient Saf. 2013;39(1):7–15.
- , , , , , , , . Taking Patients' Narratives about Clinicians from Anecdote to Science. NEJM. 2015;373(7):675–679.
- , . Putting the public back in public reporting of health care quality. JAMA. 2010;304(15):1711–1712.
- , , , et al. Use of social media across US hospitals: descriptive analysis of adoption and utilization. J Med Internet Res. 2014;16(11):e264.
- , , , , , . Patient use of email, Facebook, and physician websites to communicate with physicians: a national online survey of retail pharmacy users [published online June 24, 2015]. J Gen Intern Med. doi:10.1007/s11606-015-3374-7.
- Pew Research Center. Social networking fact sheet. Available at: http://www.pewinternet.org/fact‐sheets/social‐networking‐fact‐sheet. Accessed March 4, 2015.
- , . Three approaches to qualitative content analysis. Qual Health Res. 2005;15(9):1277–1288.
- , , , . Patients’ evaluations of health care providers in the era of social networking: an analysis of physician‐rating websites. J Gen Intern Med. 2010;25(9):942–946.
- , , , , . Vaccine counseling: a content analysis of patient‐physician discussions regarding human papilloma virus vaccine. Vaccine. 2011;29(43):7343–7349.
- . Qualitative research methods. Int J Qual Health Care. 2002;14(4):329–336.
- , . Doing Qualitative Research. Vol 2. Thousand Oaks, CA: Sage Publications; 1999.
- , , , . Patients’ perception of hospital care in the United States. N Engl J Med. 2008;359(18):1921–1931.
- , , , et al. Tweets about hospital quality: a mixed methods study. BMJ Qual Saf. 2014;23(10):838–846.
Patient experience has become a major component of the Center for Medicare and Medicaid Services Value‐Based Purchasing initiative.[1] Hospitals have therefore focused quality improvement (QI) efforts on this area.[2] Hospital performance in the realm of patient experience is generally determined using systematic surveys with closed‐ended questions, but patient‐generated narrative feedback can help hospitals identify the components of care that contribute to patient satisfaction and or are in need of improvement.[3] Online narrative responses posted by patients on rating websites or social media have been criticized because they may not be representative of the population,[4] but they also have some advantages.[5] Any patient may leave a comment, not just those who are selected for a survey. Patients may also experience benefits through the act of sharing their story with others. Moreover, most US hospitals use some form of social media,[6] which they can theoretically use to self‐collect narrative data online. To realize the full potential of patient‐generated online narratives, we need a clearer understanding of the best practices for collecting and using these narratives. We therefore solicited patient feedback on the Facebook page of a large tertiary academic medical center to determine whether it is feasible to use social media platforms for learning about and improving hospital quality.
METHODS
Baystate Medical Center (BMC) is a tertiary care medical center in western Massachusetts. We identified key BMC stakeholders in the areas of QI and public affairs. Noting that patients have expressed interest in leaving comments via social media,[7] the group opted to perform a pilot study to obtain patient narratives via a Facebook prompt (Facebook is a social media site used by an estimated 58% of US adults[8]). The BMC public affairs department delivered a press release to the local media describing a 3‐week period during which patients were invited to leave narrative feedback on the BMC Facebook wall. The BMC Institutional Review Board deemed that this study did not constitute human subjects research.
During March 2014 (March 10, 2014March 24, 2014), we posted (once a week) an open‐ended prompt on BMC's Facebook wall. The prompt was designed to elicit novel descriptions of patient experience that could help to drive QI. It read: We want to hear about your experiences. In the comment section below, please tell us what we do well and how we can improve your care. Because of concerns about the potential reputational risks of allowing open feedback on a public social media page, the prompt also reminded patients of the social media ground rules: there should be no mention of specific physicians, nurses, or other caregivers by name (for liability reasons); and patients should not include details about their medical history (for privacy reasons).
We collected all posts to preserve comments and used directed qualitative content analysis to examine them.[9] Two research team members[3, 10, 11] independently coded the responses. Starting with an a priori codebook that was developed during a previous study,[3] they amended the codebook through an iterative process to incorporate new concepts. After independently coding all blocks of text, the coders reviewed their coding selections and resolved discrepancies through discussion. We then performed second‐level coding, in which codes were organized into major pertinent themes. We reviewed the coded text after applying secondary codes in order to check for accuracy of coding and theme assignment as well as completeness of second‐level coding. We calculated percent agreement, defined as both raters scoring a block of text with the same code divided by total number of codes. We also calculated the Spearman correlation between the 2 reviewers. We used descriptive statistics to assess the frequency of select codes and themes (see Supporting Information, Appendix 1 and Appendix 2, in the online version of this article).[9, 12, 13]
RESULTS
Over a 3‐week study period, 47 comments were submitted by 37 respondents. This yielded 148 codable statements (Table 1). Despite limited information on respondents, we ascertained from Facebook that 32 (86%) were women and 5 (14%) were men.
| Theme | Total Respondents, N (%) | % Positive | Positive Quotation | % Negative | Negative Quotation |
|---|---|---|---|---|---|
| |||||
| Staff | 17 (46) | 45% | The nurses in the pediatric unit, as well as the doctors in radiology and x‐ray department were AMAZING! | 55% | My 24‐year‐old daughter had to go for 5 days of IV treatmentwhile getting her infusion there was a fire alarm. She has a video showing the flashing of the light and the sound of the alarm and the closing of doors and NOT A SINGLE staff member to be found. Her infusions take about 2 hours. They set it and forget it. Luckily there wasn't a fire and someone did finally come to disconnect her. |
| Had a fabulous experience with Wesson women's this week! Had a C section and 3‐day admission. All staff from preoperative to inpatient were so helpful and really anticipated my needs before I could even ask for things. | My mother was hospitalized for at least 3 weeks right after the cardiovascular center openedwhen she went into cardiac arrest and in acute care and the step unit the care was great, very attentive nurses and doctors. When she was starting to recover and moved upstairs, downhill it went. She'd ring for assistance because she wanted to walk to the bathrooms and more times she was left to her own devices because no one would respond. | ||||
| Facility | 9 (24) | 25% | New buildings are beautiful and the new signs are way better. | 75% | The parking situation was disappointing and the waiting room was also very dirty. |
| I really like the individual pods in the ER. | I could have used a single room as my roommate was very annoying and demanding. | ||||
| Departments | 22 (60) | 44% | The NICU was great when my son was in there. The children's unit was great with my daughter and respected my needs. | 56% | Revamp maternity; it needs it desperately. |
| Labor and delivery was a great place. | Love Baystate but hate the ER. | ||||
| Technical aspects of care (eg, errors) | 9 (24) | 0 | 100% | Day 2 of my 24 year old getting her 2‐hour IV infusion....she was set up with her IV. When checked 2 hours later, the staff member was very upset to find that only the saline had run. She never opened the medication clamp. So now they gave her the medication in 1 hour instead of 2. | |
| If I had 1 suggestion it would be to re‐evaluate patient comfort when patients are waiting to be admitted. | |||||
From coded text, several broad themes were identified (see Table 1 for representative quotes): (1) comments about staff (17/37 respondents, 45.9%). These included positive descriptions of efficiency, caring behavior, good training, and good communication, whereas negative comments included descriptions of unfriendliness, apparent lack of caring, inattentiveness, poor training, unprofessional behavior, and poor communication; (2) comments about specific departments (22/37, 59.5%); (3) comments on technical aspects of care, including perceived errors, incorrect diagnoses, and inattention to pain control (9/37, 24.3%); and (4) comments describing the hospital physical plant, parking, and amenities (9/37, 24.3%). There were a few miscellaneous comments that did not fit into these broad themes, such as expressions of gratitude for our solicitation of narratives. Percent agreement between coders was 80% and Spearman's Rho was 0.82 (p0.001).
A small number (n=3) of respondents repeatedly made comments over the 3‐week period, accounting for 30% (45/148) of codes. These repetitive commenters tended to dominate the Facebook conversation, at times describing the same experience more than once.
DISCUSSION
In this study evaluating the potential utility of social media as a hospital QI tool, several broad themes emerged. From these themes, we identified several areas that could be deemed as QI targets, including: training staff to be more responsive and sensitive to patients needs and concerns, improving patient and visitor parking, and reducing emergency department waiting times. However, the insight gained from solicited Facebook comments was similar to feedback gained from more traditional approaches of soliciting patient perspectives on care, such as patient experience surveys.[14]
Our findings should be viewed in the context of prior work focused on patient narratives in healthcare. Greaves et al. used sentiment analysis to describe the content of nearly 200,000 tweets (comments posted on the social networking website Twitter) sent to National Health Service (NHS) hospitals.[15] Themes were similar to those found in our study: (1) interaction with staff, (2) environment and facilities, and (3) issues of access and timeliness of service. Notably, these themes mirrored prior work examining narratives at NHS hospitals[3] and were similar to domains of commonly used surveys of patient experience.[14] The authors noted that there were issues with the signal to noise ratio (only about 10% of tweets were about quality) and the enforced brevity of Twitter (tweets must be 140 characters or less). These limitations suggest that using Twitter to identify QI targets would be difficult.
In contrast to Greaves et al., we chose to solicit feedback on our hospital's Facebook page. Facebook does not have Twitter's enforced brevity, allowing for more detailed narratives. In addition, we did not encounter the signal‐to‐noise problem, because our prompt was designed to request feedback that was relevant to recent experiences of care. However, a few respondents dominated the conversation, supporting the hypothesis that those most likely to comment may be the patients or families who have had the best or worst experiences. In the future, we will attempt to address this limitation and reduce the influence of repeat commenters by changing our prompt (eg, Please tell us about your experience, but please do not post descriptions of the same experience more than once.).
This pilot demonstrated some of the previously described benefits of online narratives.[5] First, there appears to be value in allowing patients to share their experiences and to read the experiences of others (as indicated in a few grateful patients comments). Second, soliciting online narratives offers a way for hospitals to demonstrate a commitment to transparency. Third, in contrast to closed‐ended survey questions, narrative comments help to identify why patients were satisfied or unsatisfied with their care. Although some surveys with closed‐ended questions also allow for narratives, these comments may or may not be carefully reviewed by the hospital. Using social media to solicit and respond to comments enhances existing methods for evaluating patient experience by engaging patients in a public space, which increases the likelihood that hospitals will attempt to improve care in response.
Notably, none of the identified areas for improvement could be considered novel QI targets for BMC. For example, our hospital has been very focused on training staff around patient experience, and emergency department wait times are the focus of a system‐wide improvement effort called Patient Progress.
This study has other limitations. We conducted this study over a 3‐week time period in a single center and on a single social media site whose members may not be representative of the overall patient population at BMC. Although we do not know how generalizable our findings are (in terms of identifying QI targets), we feel that we have demonstrated how using social media to collect data on patient experience is feasible and could be informative for other hospitals in other locations. It is possible that we did not allow the experiment to run long enough; a longer time or broader outreach (eg, a handout given to every discharged patient over a longer period) may be needed to allow patients adequate opportunity to comment. Of note, we did not specifically examine responses by time period, but it does seem, in post hoc analysis, that after 2 weeks of data collection we reached theoretical saturation with no new themes emerging in the third week (eg, third‐week comments included I heart your nurses. and Love Baystate but hate the ER.). More work is also needed that includes a broader range of social media platforms and more participating hospitals.
In conclusion, the opportunity to provide feedback on Facebook has the potential to engage and empower patients, and hospitals can use these online narratives to help to drive improvement efforts. Yet potential benefits must be weighed against reputational risks, a lack of representative respondents, and the paucity of novel QI targets obtained in this study.
Disclosures: Dr. Lagu is supported by the National Heart, Lung, and Blood Institute of the National Institutes of Health under award number K01HL114745. The authors report no conflicts of interest.
Patient experience has become a major component of the Center for Medicare and Medicaid Services Value‐Based Purchasing initiative.[1] Hospitals have therefore focused quality improvement (QI) efforts on this area.[2] Hospital performance in the realm of patient experience is generally determined using systematic surveys with closed‐ended questions, but patient‐generated narrative feedback can help hospitals identify the components of care that contribute to patient satisfaction and or are in need of improvement.[3] Online narrative responses posted by patients on rating websites or social media have been criticized because they may not be representative of the population,[4] but they also have some advantages.[5] Any patient may leave a comment, not just those who are selected for a survey. Patients may also experience benefits through the act of sharing their story with others. Moreover, most US hospitals use some form of social media,[6] which they can theoretically use to self‐collect narrative data online. To realize the full potential of patient‐generated online narratives, we need a clearer understanding of the best practices for collecting and using these narratives. We therefore solicited patient feedback on the Facebook page of a large tertiary academic medical center to determine whether it is feasible to use social media platforms for learning about and improving hospital quality.
METHODS
Baystate Medical Center (BMC) is a tertiary care medical center in western Massachusetts. We identified key BMC stakeholders in the areas of QI and public affairs. Noting that patients have expressed interest in leaving comments via social media,[7] the group opted to perform a pilot study to obtain patient narratives via a Facebook prompt (Facebook is a social media site used by an estimated 58% of US adults[8]). The BMC public affairs department delivered a press release to the local media describing a 3‐week period during which patients were invited to leave narrative feedback on the BMC Facebook wall. The BMC Institutional Review Board deemed that this study did not constitute human subjects research.
During March 2014 (March 10, 2014March 24, 2014), we posted (once a week) an open‐ended prompt on BMC's Facebook wall. The prompt was designed to elicit novel descriptions of patient experience that could help to drive QI. It read: We want to hear about your experiences. In the comment section below, please tell us what we do well and how we can improve your care. Because of concerns about the potential reputational risks of allowing open feedback on a public social media page, the prompt also reminded patients of the social media ground rules: there should be no mention of specific physicians, nurses, or other caregivers by name (for liability reasons); and patients should not include details about their medical history (for privacy reasons).
We collected all posts to preserve comments and used directed qualitative content analysis to examine them.[9] Two research team members[3, 10, 11] independently coded the responses. Starting with an a priori codebook that was developed during a previous study,[3] they amended the codebook through an iterative process to incorporate new concepts. After independently coding all blocks of text, the coders reviewed their coding selections and resolved discrepancies through discussion. We then performed second‐level coding, in which codes were organized into major pertinent themes. We reviewed the coded text after applying secondary codes in order to check for accuracy of coding and theme assignment as well as completeness of second‐level coding. We calculated percent agreement, defined as both raters scoring a block of text with the same code divided by total number of codes. We also calculated the Spearman correlation between the 2 reviewers. We used descriptive statistics to assess the frequency of select codes and themes (see Supporting Information, Appendix 1 and Appendix 2, in the online version of this article).[9, 12, 13]
RESULTS
Over a 3‐week study period, 47 comments were submitted by 37 respondents. This yielded 148 codable statements (Table 1). Despite limited information on respondents, we ascertained from Facebook that 32 (86%) were women and 5 (14%) were men.
| Theme | Total Respondents, N (%) | % Positive | Positive Quotation | % Negative | Negative Quotation |
|---|---|---|---|---|---|
| |||||
| Staff | 17 (46) | 45% | The nurses in the pediatric unit, as well as the doctors in radiology and x‐ray department were AMAZING! | 55% | My 24‐year‐old daughter had to go for 5 days of IV treatmentwhile getting her infusion there was a fire alarm. She has a video showing the flashing of the light and the sound of the alarm and the closing of doors and NOT A SINGLE staff member to be found. Her infusions take about 2 hours. They set it and forget it. Luckily there wasn't a fire and someone did finally come to disconnect her. |
| Had a fabulous experience with Wesson women's this week! Had a C section and 3‐day admission. All staff from preoperative to inpatient were so helpful and really anticipated my needs before I could even ask for things. | My mother was hospitalized for at least 3 weeks right after the cardiovascular center openedwhen she went into cardiac arrest and in acute care and the step unit the care was great, very attentive nurses and doctors. When she was starting to recover and moved upstairs, downhill it went. She'd ring for assistance because she wanted to walk to the bathrooms and more times she was left to her own devices because no one would respond. | ||||
| Facility | 9 (24) | 25% | New buildings are beautiful and the new signs are way better. | 75% | The parking situation was disappointing and the waiting room was also very dirty. |
| I really like the individual pods in the ER. | I could have used a single room as my roommate was very annoying and demanding. | ||||
| Departments | 22 (60) | 44% | The NICU was great when my son was in there. The children's unit was great with my daughter and respected my needs. | 56% | Revamp maternity; it needs it desperately. |
| Labor and delivery was a great place. | Love Baystate but hate the ER. | ||||
| Technical aspects of care (eg, errors) | 9 (24) | 0 | 100% | Day 2 of my 24 year old getting her 2‐hour IV infusion....she was set up with her IV. When checked 2 hours later, the staff member was very upset to find that only the saline had run. She never opened the medication clamp. So now they gave her the medication in 1 hour instead of 2. | |
| If I had 1 suggestion it would be to re‐evaluate patient comfort when patients are waiting to be admitted. | |||||
From coded text, several broad themes were identified (see Table 1 for representative quotes): (1) comments about staff (17/37 respondents, 45.9%). These included positive descriptions of efficiency, caring behavior, good training, and good communication, whereas negative comments included descriptions of unfriendliness, apparent lack of caring, inattentiveness, poor training, unprofessional behavior, and poor communication; (2) comments about specific departments (22/37, 59.5%); (3) comments on technical aspects of care, including perceived errors, incorrect diagnoses, and inattention to pain control (9/37, 24.3%); and (4) comments describing the hospital physical plant, parking, and amenities (9/37, 24.3%). There were a few miscellaneous comments that did not fit into these broad themes, such as expressions of gratitude for our solicitation of narratives. Percent agreement between coders was 80% and Spearman's Rho was 0.82 (p0.001).
A small number (n=3) of respondents repeatedly made comments over the 3‐week period, accounting for 30% (45/148) of codes. These repetitive commenters tended to dominate the Facebook conversation, at times describing the same experience more than once.
DISCUSSION
In this study evaluating the potential utility of social media as a hospital QI tool, several broad themes emerged. From these themes, we identified several areas that could be deemed as QI targets, including: training staff to be more responsive and sensitive to patients needs and concerns, improving patient and visitor parking, and reducing emergency department waiting times. However, the insight gained from solicited Facebook comments was similar to feedback gained from more traditional approaches of soliciting patient perspectives on care, such as patient experience surveys.[14]
Our findings should be viewed in the context of prior work focused on patient narratives in healthcare. Greaves et al. used sentiment analysis to describe the content of nearly 200,000 tweets (comments posted on the social networking website Twitter) sent to National Health Service (NHS) hospitals.[15] Themes were similar to those found in our study: (1) interaction with staff, (2) environment and facilities, and (3) issues of access and timeliness of service. Notably, these themes mirrored prior work examining narratives at NHS hospitals[3] and were similar to domains of commonly used surveys of patient experience.[14] The authors noted that there were issues with the signal to noise ratio (only about 10% of tweets were about quality) and the enforced brevity of Twitter (tweets must be 140 characters or less). These limitations suggest that using Twitter to identify QI targets would be difficult.
In contrast to Greaves et al., we chose to solicit feedback on our hospital's Facebook page. Facebook does not have Twitter's enforced brevity, allowing for more detailed narratives. In addition, we did not encounter the signal‐to‐noise problem, because our prompt was designed to request feedback that was relevant to recent experiences of care. However, a few respondents dominated the conversation, supporting the hypothesis that those most likely to comment may be the patients or families who have had the best or worst experiences. In the future, we will attempt to address this limitation and reduce the influence of repeat commenters by changing our prompt (eg, Please tell us about your experience, but please do not post descriptions of the same experience more than once.).
This pilot demonstrated some of the previously described benefits of online narratives.[5] First, there appears to be value in allowing patients to share their experiences and to read the experiences of others (as indicated in a few grateful patients comments). Second, soliciting online narratives offers a way for hospitals to demonstrate a commitment to transparency. Third, in contrast to closed‐ended survey questions, narrative comments help to identify why patients were satisfied or unsatisfied with their care. Although some surveys with closed‐ended questions also allow for narratives, these comments may or may not be carefully reviewed by the hospital. Using social media to solicit and respond to comments enhances existing methods for evaluating patient experience by engaging patients in a public space, which increases the likelihood that hospitals will attempt to improve care in response.
Notably, none of the identified areas for improvement could be considered novel QI targets for BMC. For example, our hospital has been very focused on training staff around patient experience, and emergency department wait times are the focus of a system‐wide improvement effort called Patient Progress.
This study has other limitations. We conducted this study over a 3‐week time period in a single center and on a single social media site whose members may not be representative of the overall patient population at BMC. Although we do not know how generalizable our findings are (in terms of identifying QI targets), we feel that we have demonstrated how using social media to collect data on patient experience is feasible and could be informative for other hospitals in other locations. It is possible that we did not allow the experiment to run long enough; a longer time or broader outreach (eg, a handout given to every discharged patient over a longer period) may be needed to allow patients adequate opportunity to comment. Of note, we did not specifically examine responses by time period, but it does seem, in post hoc analysis, that after 2 weeks of data collection we reached theoretical saturation with no new themes emerging in the third week (eg, third‐week comments included I heart your nurses. and Love Baystate but hate the ER.). More work is also needed that includes a broader range of social media platforms and more participating hospitals.
In conclusion, the opportunity to provide feedback on Facebook has the potential to engage and empower patients, and hospitals can use these online narratives to help to drive improvement efforts. Yet potential benefits must be weighed against reputational risks, a lack of representative respondents, and the paucity of novel QI targets obtained in this study.
Disclosures: Dr. Lagu is supported by the National Heart, Lung, and Blood Institute of the National Institutes of Health under award number K01HL114745. The authors report no conflicts of interest.
- Centers for Medicare 47(2):193–219.
- , , , , . A mixed‐methods analysis of patient reviews of hospital care in England: implications for public reporting of health care quality data in the United States. Jt Comm J Qual Patient Saf. 2013;39(1):7–15.
- , , , , , , , . Taking Patients' Narratives about Clinicians from Anecdote to Science. NEJM. 2015;373(7):675–679.
- , . Putting the public back in public reporting of health care quality. JAMA. 2010;304(15):1711–1712.
- , , , et al. Use of social media across US hospitals: descriptive analysis of adoption and utilization. J Med Internet Res. 2014;16(11):e264.
- , , , , , . Patient use of email, Facebook, and physician websites to communicate with physicians: a national online survey of retail pharmacy users [published online June 24, 2015]. J Gen Intern Med. doi:10.1007/s11606-015-3374-7.
- Pew Research Center. Social networking fact sheet. Available at: http://www.pewinternet.org/fact‐sheets/social‐networking‐fact‐sheet. Accessed March 4, 2015.
- , . Three approaches to qualitative content analysis. Qual Health Res. 2005;15(9):1277–1288.
- , , , . Patients’ evaluations of health care providers in the era of social networking: an analysis of physician‐rating websites. J Gen Intern Med. 2010;25(9):942–946.
- , , , , . Vaccine counseling: a content analysis of patient‐physician discussions regarding human papilloma virus vaccine. Vaccine. 2011;29(43):7343–7349.
- . Qualitative research methods. Int J Qual Health Care. 2002;14(4):329–336.
- , . Doing Qualitative Research. Vol 2. Thousand Oaks, CA: Sage Publications; 1999.
- , , , . Patients’ perception of hospital care in the United States. N Engl J Med. 2008;359(18):1921–1931.
- , , , et al. Tweets about hospital quality: a mixed methods study. BMJ Qual Saf. 2014;23(10):838–846.
- Centers for Medicare 47(2):193–219.
- , , , , . A mixed‐methods analysis of patient reviews of hospital care in England: implications for public reporting of health care quality data in the United States. Jt Comm J Qual Patient Saf. 2013;39(1):7–15.
- , , , , , , , . Taking Patients' Narratives about Clinicians from Anecdote to Science. NEJM. 2015;373(7):675–679.
- , . Putting the public back in public reporting of health care quality. JAMA. 2010;304(15):1711–1712.
- , , , et al. Use of social media across US hospitals: descriptive analysis of adoption and utilization. J Med Internet Res. 2014;16(11):e264.
- , , , , , . Patient use of email, Facebook, and physician websites to communicate with physicians: a national online survey of retail pharmacy users [published online June 24, 2015]. J Gen Intern Med. doi:10.1007/s11606-015-3374-7.
- Pew Research Center. Social networking fact sheet. Available at: http://www.pewinternet.org/fact‐sheets/social‐networking‐fact‐sheet. Accessed March 4, 2015.
- , . Three approaches to qualitative content analysis. Qual Health Res. 2005;15(9):1277–1288.
- , , , . Patients’ evaluations of health care providers in the era of social networking: an analysis of physician‐rating websites. J Gen Intern Med. 2010;25(9):942–946.
- , , , , . Vaccine counseling: a content analysis of patient‐physician discussions regarding human papilloma virus vaccine. Vaccine. 2011;29(43):7343–7349.
- . Qualitative research methods. Int J Qual Health Care. 2002;14(4):329–336.
- , . Doing Qualitative Research. Vol 2. Thousand Oaks, CA: Sage Publications; 1999.
- , , , . Patients’ perception of hospital care in the United States. N Engl J Med. 2008;359(18):1921–1931.
- , , , et al. Tweets about hospital quality: a mixed methods study. BMJ Qual Saf. 2014;23(10):838–846.
Ischemic Stroke Workup
After entertaining the possibility of acute intervention, the majority of hospitalists efforts in the management of patients with ischemic stroke involve identifying an etiology and initiating secondary prevention strategies. Other than evaluating stroke risk factors, workup has traditionally involved extracranial and intracranial vessel imaging, cardiac telemetry, and echocardiography. Even after exhaustive searches, no cause for stroke is found in nearly 25% of cases, leading to a recent focus on determining why these so‐called cryptogenic strokes happen and how to prevent their recurrence.[1, 2]
Echocardiography is commonly obtained in most patients with ischemic stroke, but its yield is probably modest at best. Although transesophageal echocardiography (TEE) may be superior to transthoracic echocardiography (TTE) for determining an etiology of stroke, whether these findings substantially change management remains debatable.[3, 4] In this issue of the Journal of Hospital Medicine, Marino and colleagues examined the yield of TEE in patients without a known cause of ischemic stroke following a normal TTE.[5] A possible cause of stroke was identified in 42%, including aortic plaques and patent foramen ovale (PFO), but in only 1 patient did this discovery change management.
Secondary prevention strategies in ischemic stroke outside of atrial fibrillation now almost exclusively involve antiplatelet medications.[6] Studies of secondary prevention in aortic arch atheromas, patients with depressed systolic function, and those with PFO have failed to demonstrate any strategy that is superior to antiplatelets, and therefore the bar is high to show that any TEE findings impact treatment other than obvious and rare smoking guns such as a rare valvular lesion, cardiac tumor, or atrial thrombus.[7, 8, 9]
What is more of a recent headline in stroke workup is the increasing emphasis on long‐term cardiac monitoring following discharge to detect those with atrial fibrillation, which likely comprise between 15% and 20% of cryptogenic stroke patients.[10] Finding atrial fibrillation clearly changes management and therefore has a higher yield than the vast majority of possible findings on echocardiography. Perhaps in patients in whom a TEE is being considered, extended monitoring should happen first as an outpatient, followed by TEE if the stroke etiology remains obscure. On the other hand, severe left atrial enlargement, thrombus in the atrium, or atrial spontaneous echo contrast (smoke) are features on echocardiography that might raise the suspicion of atrial fibrillation so high that anticoagulation could be considered while long‐term monitoring is being used to definitively prove an atrial arrhythmia.
The current study does have some limitations other than those inherent to its retrospective design. Patients were only included if they were older than 50 years. Some have advocated using TEE as the echocardiogram of choice in young stroke patients due to its perhaps higher yield in these individuals; this study does not address this strategy. At institutions such as ours, an abnormal TTE in a cryptogenic stroke patient is followed by a TEE, and this study again does not alter this approach, because only those with a normal TTE were included. The definition of a normal TTE used in the study was so narrow, including normal left ventricular systolic function, that a majority of stroke patients with vascular risk factors such as hypertension would have likely been excluded. Determining what features and quality of a TTE are so definitive that a TEE is not necessary will be an important thrust of additional research. However, because TEE shows a better view of the left atrial appendage, the aortic arch, and is probably a better shunt study compared with TTE, it is not clear if a normal TTE will ever be adequate to prevent this second more invasive study in selected patients.
At the heart of the matter for health systems is the cost‐effectiveness of any screening approach used to determine the etiology of acute ischemic stroke. Studies are underway that will likely demonstrate that long‐term monitoring for atrial fibrillation will be worth it. Although it is dubious that TEE would ever fall into the same category due to its low yield, one might imagine a scenario, as our workup for cryptogenic stroke becomes more and more complicated, where obtaining a TEE is cost‐effective simply because it gives an answer and therefore can halt further testing. Perhaps at the end of the day, a TEE will just allow us to say to our stroke patients that enough is enough.
Disclosures: Dr. Josephson receives personal compensation as Editor‐in‐Chief of the New England Journal of Medicine Journal Watch Neurology and in an editorial capacity for Continuum Audio.
- , , , et al. Embolic strokes of undetermined source: the case for a new clinical construct. Lancet Neurol. 2014;13:429–438.
- , , , et al. Incidence, outcome, risk factors, and long‐term prognosis of cryptogenic transient ischaemic attack and ischaemic stroke: a population‐based study. Lancet Neurol. 2015;14:903–913.
- , , , et al. Transesophageal echocardiography is superior to transthoracic echocardiography in management of patients of any age with transient ischemic attack or stroke. Stroke. 2006;37:2531–2534.
- , , , , , . Transesophageal echocardiography in patients with cryptogenic ischemic stroke: a systematic review. Am Heart J. 2014;168:706–712.
- , , , , . Impact of transesophageal echocardiography on clinical management of patients over age 50 with cryptogenic stroke and normal transthoracic echocardiogram. J Hosp Med. 2016;11(2):95–98.
- , , , et al. Guidelines for the prevention of stroke in patients with stroke and transient ischemic attack: a guideline for healthcare professionals from the American Heart Association/American Stroke Association. Stroke. 2014;45:2160–2236.
- , , , et al. Clopidogrel plus aspirin versus warfarin in patients with stroke and aortic arch plaques. Stroke. 2014;45:1248–1257.
- , , , et al. Warfarin and aspirin in patients with heart failure and sinus rhythm. N Engl J Med. 2012;366:1859–1869.
- , , , et al. Closure or medical therapy for cryptogenic stroke with patent foramen ovale. N Engl J Med. 2012;366:991–999.
- , , , et al. Atrial fibrillation in patients with cryptogenic stroke. N Engl J Med. 2014;370:2467–2477.
After entertaining the possibility of acute intervention, the majority of hospitalists efforts in the management of patients with ischemic stroke involve identifying an etiology and initiating secondary prevention strategies. Other than evaluating stroke risk factors, workup has traditionally involved extracranial and intracranial vessel imaging, cardiac telemetry, and echocardiography. Even after exhaustive searches, no cause for stroke is found in nearly 25% of cases, leading to a recent focus on determining why these so‐called cryptogenic strokes happen and how to prevent their recurrence.[1, 2]
Echocardiography is commonly obtained in most patients with ischemic stroke, but its yield is probably modest at best. Although transesophageal echocardiography (TEE) may be superior to transthoracic echocardiography (TTE) for determining an etiology of stroke, whether these findings substantially change management remains debatable.[3, 4] In this issue of the Journal of Hospital Medicine, Marino and colleagues examined the yield of TEE in patients without a known cause of ischemic stroke following a normal TTE.[5] A possible cause of stroke was identified in 42%, including aortic plaques and patent foramen ovale (PFO), but in only 1 patient did this discovery change management.
Secondary prevention strategies in ischemic stroke outside of atrial fibrillation now almost exclusively involve antiplatelet medications.[6] Studies of secondary prevention in aortic arch atheromas, patients with depressed systolic function, and those with PFO have failed to demonstrate any strategy that is superior to antiplatelets, and therefore the bar is high to show that any TEE findings impact treatment other than obvious and rare smoking guns such as a rare valvular lesion, cardiac tumor, or atrial thrombus.[7, 8, 9]
What is more of a recent headline in stroke workup is the increasing emphasis on long‐term cardiac monitoring following discharge to detect those with atrial fibrillation, which likely comprise between 15% and 20% of cryptogenic stroke patients.[10] Finding atrial fibrillation clearly changes management and therefore has a higher yield than the vast majority of possible findings on echocardiography. Perhaps in patients in whom a TEE is being considered, extended monitoring should happen first as an outpatient, followed by TEE if the stroke etiology remains obscure. On the other hand, severe left atrial enlargement, thrombus in the atrium, or atrial spontaneous echo contrast (smoke) are features on echocardiography that might raise the suspicion of atrial fibrillation so high that anticoagulation could be considered while long‐term monitoring is being used to definitively prove an atrial arrhythmia.
The current study does have some limitations other than those inherent to its retrospective design. Patients were only included if they were older than 50 years. Some have advocated using TEE as the echocardiogram of choice in young stroke patients due to its perhaps higher yield in these individuals; this study does not address this strategy. At institutions such as ours, an abnormal TTE in a cryptogenic stroke patient is followed by a TEE, and this study again does not alter this approach, because only those with a normal TTE were included. The definition of a normal TTE used in the study was so narrow, including normal left ventricular systolic function, that a majority of stroke patients with vascular risk factors such as hypertension would have likely been excluded. Determining what features and quality of a TTE are so definitive that a TEE is not necessary will be an important thrust of additional research. However, because TEE shows a better view of the left atrial appendage, the aortic arch, and is probably a better shunt study compared with TTE, it is not clear if a normal TTE will ever be adequate to prevent this second more invasive study in selected patients.
At the heart of the matter for health systems is the cost‐effectiveness of any screening approach used to determine the etiology of acute ischemic stroke. Studies are underway that will likely demonstrate that long‐term monitoring for atrial fibrillation will be worth it. Although it is dubious that TEE would ever fall into the same category due to its low yield, one might imagine a scenario, as our workup for cryptogenic stroke becomes more and more complicated, where obtaining a TEE is cost‐effective simply because it gives an answer and therefore can halt further testing. Perhaps at the end of the day, a TEE will just allow us to say to our stroke patients that enough is enough.
Disclosures: Dr. Josephson receives personal compensation as Editor‐in‐Chief of the New England Journal of Medicine Journal Watch Neurology and in an editorial capacity for Continuum Audio.
After entertaining the possibility of acute intervention, the majority of hospitalists efforts in the management of patients with ischemic stroke involve identifying an etiology and initiating secondary prevention strategies. Other than evaluating stroke risk factors, workup has traditionally involved extracranial and intracranial vessel imaging, cardiac telemetry, and echocardiography. Even after exhaustive searches, no cause for stroke is found in nearly 25% of cases, leading to a recent focus on determining why these so‐called cryptogenic strokes happen and how to prevent their recurrence.[1, 2]
Echocardiography is commonly obtained in most patients with ischemic stroke, but its yield is probably modest at best. Although transesophageal echocardiography (TEE) may be superior to transthoracic echocardiography (TTE) for determining an etiology of stroke, whether these findings substantially change management remains debatable.[3, 4] In this issue of the Journal of Hospital Medicine, Marino and colleagues examined the yield of TEE in patients without a known cause of ischemic stroke following a normal TTE.[5] A possible cause of stroke was identified in 42%, including aortic plaques and patent foramen ovale (PFO), but in only 1 patient did this discovery change management.
Secondary prevention strategies in ischemic stroke outside of atrial fibrillation now almost exclusively involve antiplatelet medications.[6] Studies of secondary prevention in aortic arch atheromas, patients with depressed systolic function, and those with PFO have failed to demonstrate any strategy that is superior to antiplatelets, and therefore the bar is high to show that any TEE findings impact treatment other than obvious and rare smoking guns such as a rare valvular lesion, cardiac tumor, or atrial thrombus.[7, 8, 9]
What is more of a recent headline in stroke workup is the increasing emphasis on long‐term cardiac monitoring following discharge to detect those with atrial fibrillation, which likely comprise between 15% and 20% of cryptogenic stroke patients.[10] Finding atrial fibrillation clearly changes management and therefore has a higher yield than the vast majority of possible findings on echocardiography. Perhaps in patients in whom a TEE is being considered, extended monitoring should happen first as an outpatient, followed by TEE if the stroke etiology remains obscure. On the other hand, severe left atrial enlargement, thrombus in the atrium, or atrial spontaneous echo contrast (smoke) are features on echocardiography that might raise the suspicion of atrial fibrillation so high that anticoagulation could be considered while long‐term monitoring is being used to definitively prove an atrial arrhythmia.
The current study does have some limitations other than those inherent to its retrospective design. Patients were only included if they were older than 50 years. Some have advocated using TEE as the echocardiogram of choice in young stroke patients due to its perhaps higher yield in these individuals; this study does not address this strategy. At institutions such as ours, an abnormal TTE in a cryptogenic stroke patient is followed by a TEE, and this study again does not alter this approach, because only those with a normal TTE were included. The definition of a normal TTE used in the study was so narrow, including normal left ventricular systolic function, that a majority of stroke patients with vascular risk factors such as hypertension would have likely been excluded. Determining what features and quality of a TTE are so definitive that a TEE is not necessary will be an important thrust of additional research. However, because TEE shows a better view of the left atrial appendage, the aortic arch, and is probably a better shunt study compared with TTE, it is not clear if a normal TTE will ever be adequate to prevent this second more invasive study in selected patients.
At the heart of the matter for health systems is the cost‐effectiveness of any screening approach used to determine the etiology of acute ischemic stroke. Studies are underway that will likely demonstrate that long‐term monitoring for atrial fibrillation will be worth it. Although it is dubious that TEE would ever fall into the same category due to its low yield, one might imagine a scenario, as our workup for cryptogenic stroke becomes more and more complicated, where obtaining a TEE is cost‐effective simply because it gives an answer and therefore can halt further testing. Perhaps at the end of the day, a TEE will just allow us to say to our stroke patients that enough is enough.
Disclosures: Dr. Josephson receives personal compensation as Editor‐in‐Chief of the New England Journal of Medicine Journal Watch Neurology and in an editorial capacity for Continuum Audio.
- , , , et al. Embolic strokes of undetermined source: the case for a new clinical construct. Lancet Neurol. 2014;13:429–438.
- , , , et al. Incidence, outcome, risk factors, and long‐term prognosis of cryptogenic transient ischaemic attack and ischaemic stroke: a population‐based study. Lancet Neurol. 2015;14:903–913.
- , , , et al. Transesophageal echocardiography is superior to transthoracic echocardiography in management of patients of any age with transient ischemic attack or stroke. Stroke. 2006;37:2531–2534.
- , , , , , . Transesophageal echocardiography in patients with cryptogenic ischemic stroke: a systematic review. Am Heart J. 2014;168:706–712.
- , , , , . Impact of transesophageal echocardiography on clinical management of patients over age 50 with cryptogenic stroke and normal transthoracic echocardiogram. J Hosp Med. 2016;11(2):95–98.
- , , , et al. Guidelines for the prevention of stroke in patients with stroke and transient ischemic attack: a guideline for healthcare professionals from the American Heart Association/American Stroke Association. Stroke. 2014;45:2160–2236.
- , , , et al. Clopidogrel plus aspirin versus warfarin in patients with stroke and aortic arch plaques. Stroke. 2014;45:1248–1257.
- , , , et al. Warfarin and aspirin in patients with heart failure and sinus rhythm. N Engl J Med. 2012;366:1859–1869.
- , , , et al. Closure or medical therapy for cryptogenic stroke with patent foramen ovale. N Engl J Med. 2012;366:991–999.
- , , , et al. Atrial fibrillation in patients with cryptogenic stroke. N Engl J Med. 2014;370:2467–2477.
- , , , et al. Embolic strokes of undetermined source: the case for a new clinical construct. Lancet Neurol. 2014;13:429–438.
- , , , et al. Incidence, outcome, risk factors, and long‐term prognosis of cryptogenic transient ischaemic attack and ischaemic stroke: a population‐based study. Lancet Neurol. 2015;14:903–913.
- , , , et al. Transesophageal echocardiography is superior to transthoracic echocardiography in management of patients of any age with transient ischemic attack or stroke. Stroke. 2006;37:2531–2534.
- , , , , , . Transesophageal echocardiography in patients with cryptogenic ischemic stroke: a systematic review. Am Heart J. 2014;168:706–712.
- , , , , . Impact of transesophageal echocardiography on clinical management of patients over age 50 with cryptogenic stroke and normal transthoracic echocardiogram. J Hosp Med. 2016;11(2):95–98.
- , , , et al. Guidelines for the prevention of stroke in patients with stroke and transient ischemic attack: a guideline for healthcare professionals from the American Heart Association/American Stroke Association. Stroke. 2014;45:2160–2236.
- , , , et al. Clopidogrel plus aspirin versus warfarin in patients with stroke and aortic arch plaques. Stroke. 2014;45:1248–1257.
- , , , et al. Warfarin and aspirin in patients with heart failure and sinus rhythm. N Engl J Med. 2012;366:1859–1869.
- , , , et al. Closure or medical therapy for cryptogenic stroke with patent foramen ovale. N Engl J Med. 2012;366:991–999.
- , , , et al. Atrial fibrillation in patients with cryptogenic stroke. N Engl J Med. 2014;370:2467–2477.
TEE Impact on Managing Stroke Patients
Specific transesophageal echocardiography (TEE) findings associated with stroke include cardiac thrombi (particularly left atrial appendage [LAA]), left atrial spontaneous echo contrast, interatrial septal anomalies (particularly patent foramen ovale [PFO]), and atheromatous disease of the aorta. In younger patients (aged <50 years) with stroke of uncertain etiology, TEE is often recommended because of reported higher yield than transthoracic echocardiogram (TTE), particularly in detecting PFO or atrial septal aneurysm (ASA).[1]
Aside from oral anticoagulation in patients with an intracardiac thrombus, current guidelines and scientific evidence do not support specific therapeutic interventions for the other TEE findings. For example, the most effective therapy for stroke prevention with findings of aortic arch plaque remains uncertain. In addition, the very rare patient presenting with stroke from a cardiac tumor, which is generally visible on TTE, might benefit from surgical removal.[2]
We sought to examine the benefit of performing TEE after a normal TTE in patients over age 50 years admitted with a stroke of uncertain etiology. We hypothesized that there would be minimal change in management based on TEE findings after a normal TTE in older patients hospitalized with an unexplained stroke.
METHODS
Over a 4‐year period from 2009 to 2012, all patients over the age of 50 years admitted to our community‐based teaching hospital with a primary diagnosis of ischemic stroke were identified and retrospectively screened by review of our institutional echocardiography database during this time period. Stroke diagnosis had to be confirmed with acute or subacute ischemia on brain magnetic resonance imaging. Patients with an indication for anticoagulation or who had a known history of atrial fibrillation or flutter were excluded. Patients were monitored with continuous telemetry during hospital admission and were also excluded if they developed atrial fibrillation or flutter after admission. Additionally, patients were excluded if a neurologist‐directed evaluation revealed another etiology for the stroke.
A TTE acquired in all patients was performed according to Intersocietal Commission for the Accreditation of Echocardiography Laboratories standards and included 2‐dimensional, color Doppler, continuous wave, and pulse wave data. Images were obtained in the parasternal long and short axis, apical 4‐chamber, 2‐chamber, and long axis views. An abnormal TTE was defined as a study with a prosthetic valve, abnormal left ventricular (LV) systolic function, an intracardiac mass, intracardiac shunt, or severe valvular heart disease, as these significant findings may explain stroke.
Standardized TEE images were obtained with midesophageal 4‐chamber, mitral commissural, 2‐chamber, long axis, ascending aorta long axis, aortic valve short axis, right ventricular inflow‐outflow, and bicaval views. Detailed multiplanar evaluation of the LAA was performed. If no interatrial shunt was visualized with color flow Doppler in the bicaval view, agitated intravenous saline was administered for further evaluation. Additional standard images were obtained of the descending aorta and aortic arch in the short and long axis. Transgastric images were obtained when feasible or necessary.
The study was submitted to our institutional review board. As no patient identifiers were stored, and we used previously existing data from an institutional echocardiography database to conduct the study, it was determined to be exempt.
Statistical analysis was performed by recording the prevalence of each potential cardiac source of embolism.
RESULTS
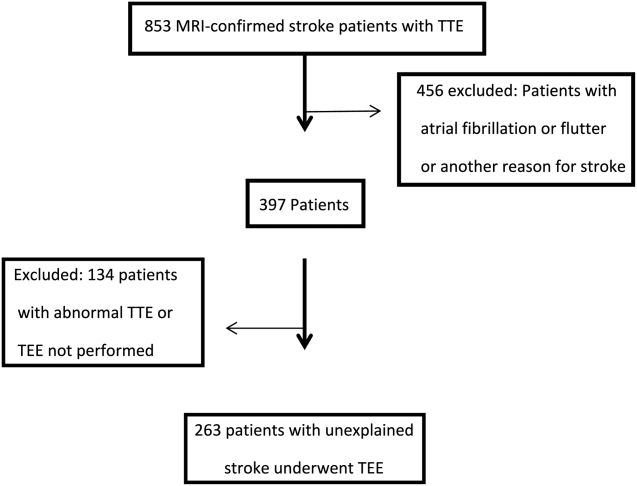
Of the 853 consecutive patients screened, 456 were excluded because of atrial fibrillation, atrial flutter, or another etiology of stroke. An additional 134 patients were excluded with an abnormal TTE or if a TEE was not performed. The remaining 263 patients were analyzed based on TEE findings (Figure 1).
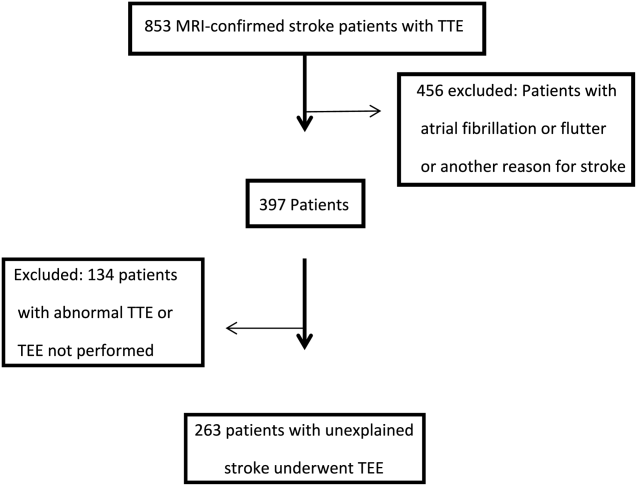
The mean age was 66.7 years (range, 5091 years), and 42.5% were female. A possible etiology of stroke (Table 1) discovered included complex plaque of the ascending aorta or arch 44/263 (16.7%), PFO 18/263 (6.8%), atrial septal aneurysm 25/263 (9.5%), and both ASA and PFO in 11/263 (4.2%), and spontaneous contrast was seen in the left atrium or LAA in 13/263 (4.9%) patients. One patient had a thrombus in the LAA for which anticoagulation was prescribed. No other intracardiac masses were identified.
| Potential Source | No. (%) |
|---|---|
| |
| Atrial septal aneurysm | 25 (5.3%) |
| Patent foramen ovale | 18 (2.7%) |
| Atrial septal aneurysm and patent foramen ovale | 11 (4.2%) |
| Complex aortic plaque | 44 (16.7%) |
| Spontaneous contrast | 13 (4.9%) |
| Left atrial appendage thrombus* | 1 (0.4%) |
| Total | 112 (42.6%) |
Overall, 42.6% of patients had a TEE finding which could explain the etiology of stroke or transient ischemic attack (TIA), but only 1 patient (0.4%) had a finding that changed therapy. Follow‐up was available at 6 months for 85 patients, and 13 (15%) of these patients had been discovered to develop atrial fibrillation in the interim.
DISCUSSION
Our study retrospectively analyzed the utility of TEE in patients over age 50 years admitted with ischemic stroke without a clear etiology. We found that TEE provides significant incremental diagnostic benefit as compared to TTE in identifying a possible etiology of stroke in these patients. This is consistent with prior studies showing a high diagnostic yield of TEE in patient with ischemic stroke of uncertain etiology.[3] However, in our study, based on current guidelines, virtually none of these findings directly altered patient management.
The 2014 guidelines for secondary stroke prevention recommend antiplatelet and statin therapy (in addition to lifestyle modification, smoking cessation, and blood glucose and blood pressure control) as a standard medical regimen in patients with stroke or TIA of uncertain etiology. The finding of aortic arch atheroma does not warrant supplementary treatment in addition to an antiplatelet and statin according to current guidelines. Atherosclerosis of the aortic arch is an important source of cerebral embolism, particularly in cases where plaque is >4 mm in size.[4] A recent study by Amarenco et al., comparing efficacy of combined antiplatelet therapy (clopidogrel and aspirin) to warfarin in recurrent stroke prevention in patients with >4 mm aortic arch plaque, showed nonsignificant reduction in rate of recurrent stroke with dual antiplatelet therapy.[5] However, optimal therapy for these patients still remains uncertain beyond standard stroke‐prevention treatment. Although there are emerging data on therapeutic options in patients with complex atheroma, there is currently no specific guideline‐recommended therapy or consensus among stroke neurologists. Potentially, if an individual practitioner had a strong feeling on therapeutic modifications based on the presence of complex aortic arch atheroma, the TEE would have value to their patient. However, in our study, which had a prevalence of 16.8% of complex plaque of the ascending aorta or arch, there were no therapeutic changes based on this finding. This reinforces the limited value of this test that we observed in our study population.
Anticoagulation has not been shown to be superior to aspirin in patients with PFO (with or without ASA), and recent studies showed no benefit of procedural PFO closure compared to best medical management for stroke prevention (Randomized Evaluation of Recurrent Stroke Comparing PFO Closure to Established Current Standard of Care Treatment [RESPECT], Evaluation of the STARFlex Septal Closure System in Patients with a Stroke and/or Transient Ischemic Attack due to Presumed Paradoxical Embolism through a Patent Foramen Ovale [CLOSURE I]).[6, 7] However, a patient with a PFO and deep vein thrombosis would benefit from anticoagulation and consideration of PFO closure.[8] This rare entity could be excluded with a simple lower extremity duplex without the need for a TEE, which does come with a small risk of complications related to anesthesia and local oropharyngeal trauma as well as discomfort to the patient and increased cost. Spontaneous echo contrast is not an independent indication for anticoagulation. If spontaneous contrast were associated with mitral stenosis and an embolic event, then anticoagulation would be indicated.[9] Mitral stenosis is easily diagnosed with TTE.
LAA or left atrial thrombus is the predominant finding exclusive to TEE that would change management for secondary stroke prevention, specifically anticoagulation. Fifteen studies representing over 3000 patients in a 2014 meta‐analysis reported the prevalence of left atrial or LAA thrombus in patients aged 55 years with a cryptogenic stroke to be 4%, with a range in the studies of 0% to 21.2%.[3] The wide range of prevalence of this finding is likely related to the prevalence of known atrial arrhythmias or structural heart disease in the population of patients included in the analysis. Left atrial or LAA thrombus in the absence of systolic dysfunction, severe valve disease, or known atrial fibrillation is exceedingly uncommon (0.3%).[10] It is likely that the few patients with left atrial or LAA thrombus without 1 of these conditions probably has undiagnosed paroxysmal atrial fibrillation. In previous studies that showed a high prevalence of left atrial or LAA thrombus, there was no mention of the presence or absence of LV dysfunction or severe valve disease in patients with left atrial or LAA thrombus. Additionally, these studies only required a 12‐lead electrocardiogram or did not specify the presence or duration of continuous rhythm monitoring.[11, 12, 13, 14] Several of the studies with high incidence of left atrial or LAA thrombus specifically stated that some of these patients were known to have atrial fibrillation.[11, 13]
Approximately 8% of patients admitted with stroke are found to have atrial fibrillation only after admission with continuous electrocardiogram monitoring. The detection rate is nearly half if monitoring is limited to 24 hours instead of several days. Overall, detection rates of atrial fibrillation following stroke are relatively low during initial hospitalization.[15] More intense monitoring for atrial fibrillation in patients with a stroke of uncertain etiology with the use of a subcutaneous implantable cardiac monitor increases the detection rate to 12.4% at 1 year, and increases with longer monitoring time.[16] Therefore, identification of older stroke patients without significant stroke risk factors may be candidates for longer‐term cardiac monitoring to increase yield for detection of atrial fibrillation. Currently, continuous electrocardiographic monitoring of patients for the duration of their hospitalization and up to 30 days afterward is recommended.[8]
Our study differs from prior studies that showed a much higher prevalence of LAA or left atrial thrombus in 2 important ways. Patients with severe valve disease or LV dysfunction were excluded on the basis of TTE. Additionally, our patients underwent continuous electrocardiographic monitoring for the duration of their hospitalization and were excluded with a prior history or newly discovered atrial fibrillation or flutter. Our intention was to examine the value of adding TEE when no other etiology of stroke was identified. Value can be defined as healthcare outcomes achieved per dollar spent. Our study was not designed to look at long‐term outcomes; rather, we used immediate change in patient management as a surrogate.
There are several limitations to our study that must be noted. This was a single‐center study potentially creating a bias as less stringent selection of patients undergoing TEE may be the practice at other institutions. This analysis was retrospective; therefore, there may have been bias as to which patients were selected to undergo TEE. Additionally, stroke subtype was not specified, and the pretest probability of a cardioembolic source differs based on subtype. Last, we focused this study on immediate changes in clinical management prompted by TEE results, and did not assess patient perceptions of TEE value related to enhanced knowledge about the etiology of their stroke; this area represents an opportunity for further research.
CONCLUSIONS
TEE provides a substantial increase in possible explanation of stroke etiology in patients over age 50 years admitted with a stroke of uncertain cause and a normal TTE. However, there is minimal incremental value in regard to change in therapeutic management in these patients. In a time of increased focus on providing cost effective healthcare, our findings suggest that the need for TEE in this stroke population should be more closely examined.
Disclosure: Nothing to report.
- , , . Influence of transesophageal echocardiogram on therapy and prognosis in young patients with TIA or ischemic stroke. Neth Heart J. 2009;17:373–377.
- , , , et al. Diagnosis of Heart Tumors by Transesophageal Echocardiography: a multicentre study in 154 patients. Eur Heart J. 1993;14:1223–1228.
- , , , , , . Transesophageal echocardiography in patients with cryptogenic ischemic stroke: a systematic review. Am Heart J. 2014;168:706–712.
- , , . Protruding atheromas in the thoracic aorta and systemic embolization. Ann Intern Med. 1991;115:423–427.
- , , , et al.; The Aortic Arch Related Cerebral Hazard Trial Investigators. Clopidogrel plus aspirin versus warfarin in patients with stroke and aortic arch plaques. Stroke. 2014;45:1248–1257.
- , , , et al.; RESPECT Investigators. Closure of patent foramen ovale versus medical therapy after cryptogenic stroke. N Engl J Med. 2013;368:1092–1100.
- , , , et al.; CLOSURE I Investigators. Closure or medical therapy for cryptogenic stroke with patent foramen ovale. N Engl J Med. 2012;366:991–999.
- , , , et al. Guidelines for the prevention of stroke in patients with stroke and transient ischemic attack: a guideline for healthcare professionals from the American Heart Association/American Stroke Association. Stroke. 2014;45(7):2160–2236.
- , , , et al. 2014 AHA/ACA guideline for the management of patients with valvular heart disease. J Am Coll Cardiol. 2014; 63:e57–e185.
- , , , . Clinical and echocardiographic characteristics of patients with left atrial thrombus and sinus rhythm: experience in 20 643 consecutive transesophageal echocardiographic examinations. Circulation. 2002;105(1):27–31.
- , , , et al. Usefulness of transesophageal echocardiography in unexplained cerebral ischemia. Am J Cardiol. 1993;72:1448–1452.
- , , , , , . Transesophageal echocardiography in patients with recent stroke and normal carotid arteries. Am J Cardiol. 2001;88:820–823.
- , , , et al. Transesophageal echocardiography is superior to transthoracic echocardiography in management of patients of any age with transient ischemic attack or stroke. Stroke. 2006;37:2531–2534.
- , , , , , . Age‐dependent prevalence of cardioembolic sources detected by TEE: diagnostic and therapeutic implications. Echocardiography. 1997;14:597–606.
- , , , et al. Continuous stroke unit electrocardiographic monitoring versus 24‐hour Holter electrocardiography for detection of paroxysmal atrial fibrillation after stroke. Stroke. 2012;43:2689–2694.
- , , , et al.; CRYSTAL AF Investigators. Cryptogenic stroke and underlying atrial fibrillation. N Engl J Med. 2014;370(26):2478–2486.
Specific transesophageal echocardiography (TEE) findings associated with stroke include cardiac thrombi (particularly left atrial appendage [LAA]), left atrial spontaneous echo contrast, interatrial septal anomalies (particularly patent foramen ovale [PFO]), and atheromatous disease of the aorta. In younger patients (aged <50 years) with stroke of uncertain etiology, TEE is often recommended because of reported higher yield than transthoracic echocardiogram (TTE), particularly in detecting PFO or atrial septal aneurysm (ASA).[1]
Aside from oral anticoagulation in patients with an intracardiac thrombus, current guidelines and scientific evidence do not support specific therapeutic interventions for the other TEE findings. For example, the most effective therapy for stroke prevention with findings of aortic arch plaque remains uncertain. In addition, the very rare patient presenting with stroke from a cardiac tumor, which is generally visible on TTE, might benefit from surgical removal.[2]
We sought to examine the benefit of performing TEE after a normal TTE in patients over age 50 years admitted with a stroke of uncertain etiology. We hypothesized that there would be minimal change in management based on TEE findings after a normal TTE in older patients hospitalized with an unexplained stroke.
METHODS
Over a 4‐year period from 2009 to 2012, all patients over the age of 50 years admitted to our community‐based teaching hospital with a primary diagnosis of ischemic stroke were identified and retrospectively screened by review of our institutional echocardiography database during this time period. Stroke diagnosis had to be confirmed with acute or subacute ischemia on brain magnetic resonance imaging. Patients with an indication for anticoagulation or who had a known history of atrial fibrillation or flutter were excluded. Patients were monitored with continuous telemetry during hospital admission and were also excluded if they developed atrial fibrillation or flutter after admission. Additionally, patients were excluded if a neurologist‐directed evaluation revealed another etiology for the stroke.
A TTE acquired in all patients was performed according to Intersocietal Commission for the Accreditation of Echocardiography Laboratories standards and included 2‐dimensional, color Doppler, continuous wave, and pulse wave data. Images were obtained in the parasternal long and short axis, apical 4‐chamber, 2‐chamber, and long axis views. An abnormal TTE was defined as a study with a prosthetic valve, abnormal left ventricular (LV) systolic function, an intracardiac mass, intracardiac shunt, or severe valvular heart disease, as these significant findings may explain stroke.
Standardized TEE images were obtained with midesophageal 4‐chamber, mitral commissural, 2‐chamber, long axis, ascending aorta long axis, aortic valve short axis, right ventricular inflow‐outflow, and bicaval views. Detailed multiplanar evaluation of the LAA was performed. If no interatrial shunt was visualized with color flow Doppler in the bicaval view, agitated intravenous saline was administered for further evaluation. Additional standard images were obtained of the descending aorta and aortic arch in the short and long axis. Transgastric images were obtained when feasible or necessary.
The study was submitted to our institutional review board. As no patient identifiers were stored, and we used previously existing data from an institutional echocardiography database to conduct the study, it was determined to be exempt.
Statistical analysis was performed by recording the prevalence of each potential cardiac source of embolism.
RESULTS
Of the 853 consecutive patients screened, 456 were excluded because of atrial fibrillation, atrial flutter, or another etiology of stroke. An additional 134 patients were excluded with an abnormal TTE or if a TEE was not performed. The remaining 263 patients were analyzed based on TEE findings (Figure 1).

The mean age was 66.7 years (range, 5091 years), and 42.5% were female. A possible etiology of stroke (Table 1) discovered included complex plaque of the ascending aorta or arch 44/263 (16.7%), PFO 18/263 (6.8%), atrial septal aneurysm 25/263 (9.5%), and both ASA and PFO in 11/263 (4.2%), and spontaneous contrast was seen in the left atrium or LAA in 13/263 (4.9%) patients. One patient had a thrombus in the LAA for which anticoagulation was prescribed. No other intracardiac masses were identified.
| Potential Source | No. (%) |
|---|---|
| |
| Atrial septal aneurysm | 25 (5.3%) |
| Patent foramen ovale | 18 (2.7%) |
| Atrial septal aneurysm and patent foramen ovale | 11 (4.2%) |
| Complex aortic plaque | 44 (16.7%) |
| Spontaneous contrast | 13 (4.9%) |
| Left atrial appendage thrombus* | 1 (0.4%) |
| Total | 112 (42.6%) |
Overall, 42.6% of patients had a TEE finding which could explain the etiology of stroke or transient ischemic attack (TIA), but only 1 patient (0.4%) had a finding that changed therapy. Follow‐up was available at 6 months for 85 patients, and 13 (15%) of these patients had been discovered to develop atrial fibrillation in the interim.
DISCUSSION
Our study retrospectively analyzed the utility of TEE in patients over age 50 years admitted with ischemic stroke without a clear etiology. We found that TEE provides significant incremental diagnostic benefit as compared to TTE in identifying a possible etiology of stroke in these patients. This is consistent with prior studies showing a high diagnostic yield of TEE in patient with ischemic stroke of uncertain etiology.[3] However, in our study, based on current guidelines, virtually none of these findings directly altered patient management.
The 2014 guidelines for secondary stroke prevention recommend antiplatelet and statin therapy (in addition to lifestyle modification, smoking cessation, and blood glucose and blood pressure control) as a standard medical regimen in patients with stroke or TIA of uncertain etiology. The finding of aortic arch atheroma does not warrant supplementary treatment in addition to an antiplatelet and statin according to current guidelines. Atherosclerosis of the aortic arch is an important source of cerebral embolism, particularly in cases where plaque is >4 mm in size.[4] A recent study by Amarenco et al., comparing efficacy of combined antiplatelet therapy (clopidogrel and aspirin) to warfarin in recurrent stroke prevention in patients with >4 mm aortic arch plaque, showed nonsignificant reduction in rate of recurrent stroke with dual antiplatelet therapy.[5] However, optimal therapy for these patients still remains uncertain beyond standard stroke‐prevention treatment. Although there are emerging data on therapeutic options in patients with complex atheroma, there is currently no specific guideline‐recommended therapy or consensus among stroke neurologists. Potentially, if an individual practitioner had a strong feeling on therapeutic modifications based on the presence of complex aortic arch atheroma, the TEE would have value to their patient. However, in our study, which had a prevalence of 16.8% of complex plaque of the ascending aorta or arch, there were no therapeutic changes based on this finding. This reinforces the limited value of this test that we observed in our study population.
Anticoagulation has not been shown to be superior to aspirin in patients with PFO (with or without ASA), and recent studies showed no benefit of procedural PFO closure compared to best medical management for stroke prevention (Randomized Evaluation of Recurrent Stroke Comparing PFO Closure to Established Current Standard of Care Treatment [RESPECT], Evaluation of the STARFlex Septal Closure System in Patients with a Stroke and/or Transient Ischemic Attack due to Presumed Paradoxical Embolism through a Patent Foramen Ovale [CLOSURE I]).[6, 7] However, a patient with a PFO and deep vein thrombosis would benefit from anticoagulation and consideration of PFO closure.[8] This rare entity could be excluded with a simple lower extremity duplex without the need for a TEE, which does come with a small risk of complications related to anesthesia and local oropharyngeal trauma as well as discomfort to the patient and increased cost. Spontaneous echo contrast is not an independent indication for anticoagulation. If spontaneous contrast were associated with mitral stenosis and an embolic event, then anticoagulation would be indicated.[9] Mitral stenosis is easily diagnosed with TTE.
LAA or left atrial thrombus is the predominant finding exclusive to TEE that would change management for secondary stroke prevention, specifically anticoagulation. Fifteen studies representing over 3000 patients in a 2014 meta‐analysis reported the prevalence of left atrial or LAA thrombus in patients aged 55 years with a cryptogenic stroke to be 4%, with a range in the studies of 0% to 21.2%.[3] The wide range of prevalence of this finding is likely related to the prevalence of known atrial arrhythmias or structural heart disease in the population of patients included in the analysis. Left atrial or LAA thrombus in the absence of systolic dysfunction, severe valve disease, or known atrial fibrillation is exceedingly uncommon (0.3%).[10] It is likely that the few patients with left atrial or LAA thrombus without 1 of these conditions probably has undiagnosed paroxysmal atrial fibrillation. In previous studies that showed a high prevalence of left atrial or LAA thrombus, there was no mention of the presence or absence of LV dysfunction or severe valve disease in patients with left atrial or LAA thrombus. Additionally, these studies only required a 12‐lead electrocardiogram or did not specify the presence or duration of continuous rhythm monitoring.[11, 12, 13, 14] Several of the studies with high incidence of left atrial or LAA thrombus specifically stated that some of these patients were known to have atrial fibrillation.[11, 13]
Approximately 8% of patients admitted with stroke are found to have atrial fibrillation only after admission with continuous electrocardiogram monitoring. The detection rate is nearly half if monitoring is limited to 24 hours instead of several days. Overall, detection rates of atrial fibrillation following stroke are relatively low during initial hospitalization.[15] More intense monitoring for atrial fibrillation in patients with a stroke of uncertain etiology with the use of a subcutaneous implantable cardiac monitor increases the detection rate to 12.4% at 1 year, and increases with longer monitoring time.[16] Therefore, identification of older stroke patients without significant stroke risk factors may be candidates for longer‐term cardiac monitoring to increase yield for detection of atrial fibrillation. Currently, continuous electrocardiographic monitoring of patients for the duration of their hospitalization and up to 30 days afterward is recommended.[8]
Our study differs from prior studies that showed a much higher prevalence of LAA or left atrial thrombus in 2 important ways. Patients with severe valve disease or LV dysfunction were excluded on the basis of TTE. Additionally, our patients underwent continuous electrocardiographic monitoring for the duration of their hospitalization and were excluded with a prior history or newly discovered atrial fibrillation or flutter. Our intention was to examine the value of adding TEE when no other etiology of stroke was identified. Value can be defined as healthcare outcomes achieved per dollar spent. Our study was not designed to look at long‐term outcomes; rather, we used immediate change in patient management as a surrogate.
There are several limitations to our study that must be noted. This was a single‐center study potentially creating a bias as less stringent selection of patients undergoing TEE may be the practice at other institutions. This analysis was retrospective; therefore, there may have been bias as to which patients were selected to undergo TEE. Additionally, stroke subtype was not specified, and the pretest probability of a cardioembolic source differs based on subtype. Last, we focused this study on immediate changes in clinical management prompted by TEE results, and did not assess patient perceptions of TEE value related to enhanced knowledge about the etiology of their stroke; this area represents an opportunity for further research.
CONCLUSIONS
TEE provides a substantial increase in possible explanation of stroke etiology in patients over age 50 years admitted with a stroke of uncertain cause and a normal TTE. However, there is minimal incremental value in regard to change in therapeutic management in these patients. In a time of increased focus on providing cost effective healthcare, our findings suggest that the need for TEE in this stroke population should be more closely examined.
Disclosure: Nothing to report.
Specific transesophageal echocardiography (TEE) findings associated with stroke include cardiac thrombi (particularly left atrial appendage [LAA]), left atrial spontaneous echo contrast, interatrial septal anomalies (particularly patent foramen ovale [PFO]), and atheromatous disease of the aorta. In younger patients (aged <50 years) with stroke of uncertain etiology, TEE is often recommended because of reported higher yield than transthoracic echocardiogram (TTE), particularly in detecting PFO or atrial septal aneurysm (ASA).[1]
Aside from oral anticoagulation in patients with an intracardiac thrombus, current guidelines and scientific evidence do not support specific therapeutic interventions for the other TEE findings. For example, the most effective therapy for stroke prevention with findings of aortic arch plaque remains uncertain. In addition, the very rare patient presenting with stroke from a cardiac tumor, which is generally visible on TTE, might benefit from surgical removal.[2]
We sought to examine the benefit of performing TEE after a normal TTE in patients over age 50 years admitted with a stroke of uncertain etiology. We hypothesized that there would be minimal change in management based on TEE findings after a normal TTE in older patients hospitalized with an unexplained stroke.
METHODS
Over a 4‐year period from 2009 to 2012, all patients over the age of 50 years admitted to our community‐based teaching hospital with a primary diagnosis of ischemic stroke were identified and retrospectively screened by review of our institutional echocardiography database during this time period. Stroke diagnosis had to be confirmed with acute or subacute ischemia on brain magnetic resonance imaging. Patients with an indication for anticoagulation or who had a known history of atrial fibrillation or flutter were excluded. Patients were monitored with continuous telemetry during hospital admission and were also excluded if they developed atrial fibrillation or flutter after admission. Additionally, patients were excluded if a neurologist‐directed evaluation revealed another etiology for the stroke.
A TTE acquired in all patients was performed according to Intersocietal Commission for the Accreditation of Echocardiography Laboratories standards and included 2‐dimensional, color Doppler, continuous wave, and pulse wave data. Images were obtained in the parasternal long and short axis, apical 4‐chamber, 2‐chamber, and long axis views. An abnormal TTE was defined as a study with a prosthetic valve, abnormal left ventricular (LV) systolic function, an intracardiac mass, intracardiac shunt, or severe valvular heart disease, as these significant findings may explain stroke.
Standardized TEE images were obtained with midesophageal 4‐chamber, mitral commissural, 2‐chamber, long axis, ascending aorta long axis, aortic valve short axis, right ventricular inflow‐outflow, and bicaval views. Detailed multiplanar evaluation of the LAA was performed. If no interatrial shunt was visualized with color flow Doppler in the bicaval view, agitated intravenous saline was administered for further evaluation. Additional standard images were obtained of the descending aorta and aortic arch in the short and long axis. Transgastric images were obtained when feasible or necessary.
The study was submitted to our institutional review board. As no patient identifiers were stored, and we used previously existing data from an institutional echocardiography database to conduct the study, it was determined to be exempt.
Statistical analysis was performed by recording the prevalence of each potential cardiac source of embolism.
RESULTS
Of the 853 consecutive patients screened, 456 were excluded because of atrial fibrillation, atrial flutter, or another etiology of stroke. An additional 134 patients were excluded with an abnormal TTE or if a TEE was not performed. The remaining 263 patients were analyzed based on TEE findings (Figure 1).

The mean age was 66.7 years (range, 5091 years), and 42.5% were female. A possible etiology of stroke (Table 1) discovered included complex plaque of the ascending aorta or arch 44/263 (16.7%), PFO 18/263 (6.8%), atrial septal aneurysm 25/263 (9.5%), and both ASA and PFO in 11/263 (4.2%), and spontaneous contrast was seen in the left atrium or LAA in 13/263 (4.9%) patients. One patient had a thrombus in the LAA for which anticoagulation was prescribed. No other intracardiac masses were identified.
| Potential Source | No. (%) |
|---|---|
| |
| Atrial septal aneurysm | 25 (5.3%) |
| Patent foramen ovale | 18 (2.7%) |
| Atrial septal aneurysm and patent foramen ovale | 11 (4.2%) |
| Complex aortic plaque | 44 (16.7%) |
| Spontaneous contrast | 13 (4.9%) |
| Left atrial appendage thrombus* | 1 (0.4%) |
| Total | 112 (42.6%) |
Overall, 42.6% of patients had a TEE finding which could explain the etiology of stroke or transient ischemic attack (TIA), but only 1 patient (0.4%) had a finding that changed therapy. Follow‐up was available at 6 months for 85 patients, and 13 (15%) of these patients had been discovered to develop atrial fibrillation in the interim.
DISCUSSION
Our study retrospectively analyzed the utility of TEE in patients over age 50 years admitted with ischemic stroke without a clear etiology. We found that TEE provides significant incremental diagnostic benefit as compared to TTE in identifying a possible etiology of stroke in these patients. This is consistent with prior studies showing a high diagnostic yield of TEE in patient with ischemic stroke of uncertain etiology.[3] However, in our study, based on current guidelines, virtually none of these findings directly altered patient management.
The 2014 guidelines for secondary stroke prevention recommend antiplatelet and statin therapy (in addition to lifestyle modification, smoking cessation, and blood glucose and blood pressure control) as a standard medical regimen in patients with stroke or TIA of uncertain etiology. The finding of aortic arch atheroma does not warrant supplementary treatment in addition to an antiplatelet and statin according to current guidelines. Atherosclerosis of the aortic arch is an important source of cerebral embolism, particularly in cases where plaque is >4 mm in size.[4] A recent study by Amarenco et al., comparing efficacy of combined antiplatelet therapy (clopidogrel and aspirin) to warfarin in recurrent stroke prevention in patients with >4 mm aortic arch plaque, showed nonsignificant reduction in rate of recurrent stroke with dual antiplatelet therapy.[5] However, optimal therapy for these patients still remains uncertain beyond standard stroke‐prevention treatment. Although there are emerging data on therapeutic options in patients with complex atheroma, there is currently no specific guideline‐recommended therapy or consensus among stroke neurologists. Potentially, if an individual practitioner had a strong feeling on therapeutic modifications based on the presence of complex aortic arch atheroma, the TEE would have value to their patient. However, in our study, which had a prevalence of 16.8% of complex plaque of the ascending aorta or arch, there were no therapeutic changes based on this finding. This reinforces the limited value of this test that we observed in our study population.
Anticoagulation has not been shown to be superior to aspirin in patients with PFO (with or without ASA), and recent studies showed no benefit of procedural PFO closure compared to best medical management for stroke prevention (Randomized Evaluation of Recurrent Stroke Comparing PFO Closure to Established Current Standard of Care Treatment [RESPECT], Evaluation of the STARFlex Septal Closure System in Patients with a Stroke and/or Transient Ischemic Attack due to Presumed Paradoxical Embolism through a Patent Foramen Ovale [CLOSURE I]).[6, 7] However, a patient with a PFO and deep vein thrombosis would benefit from anticoagulation and consideration of PFO closure.[8] This rare entity could be excluded with a simple lower extremity duplex without the need for a TEE, which does come with a small risk of complications related to anesthesia and local oropharyngeal trauma as well as discomfort to the patient and increased cost. Spontaneous echo contrast is not an independent indication for anticoagulation. If spontaneous contrast were associated with mitral stenosis and an embolic event, then anticoagulation would be indicated.[9] Mitral stenosis is easily diagnosed with TTE.
LAA or left atrial thrombus is the predominant finding exclusive to TEE that would change management for secondary stroke prevention, specifically anticoagulation. Fifteen studies representing over 3000 patients in a 2014 meta‐analysis reported the prevalence of left atrial or LAA thrombus in patients aged 55 years with a cryptogenic stroke to be 4%, with a range in the studies of 0% to 21.2%.[3] The wide range of prevalence of this finding is likely related to the prevalence of known atrial arrhythmias or structural heart disease in the population of patients included in the analysis. Left atrial or LAA thrombus in the absence of systolic dysfunction, severe valve disease, or known atrial fibrillation is exceedingly uncommon (0.3%).[10] It is likely that the few patients with left atrial or LAA thrombus without 1 of these conditions probably has undiagnosed paroxysmal atrial fibrillation. In previous studies that showed a high prevalence of left atrial or LAA thrombus, there was no mention of the presence or absence of LV dysfunction or severe valve disease in patients with left atrial or LAA thrombus. Additionally, these studies only required a 12‐lead electrocardiogram or did not specify the presence or duration of continuous rhythm monitoring.[11, 12, 13, 14] Several of the studies with high incidence of left atrial or LAA thrombus specifically stated that some of these patients were known to have atrial fibrillation.[11, 13]
Approximately 8% of patients admitted with stroke are found to have atrial fibrillation only after admission with continuous electrocardiogram monitoring. The detection rate is nearly half if monitoring is limited to 24 hours instead of several days. Overall, detection rates of atrial fibrillation following stroke are relatively low during initial hospitalization.[15] More intense monitoring for atrial fibrillation in patients with a stroke of uncertain etiology with the use of a subcutaneous implantable cardiac monitor increases the detection rate to 12.4% at 1 year, and increases with longer monitoring time.[16] Therefore, identification of older stroke patients without significant stroke risk factors may be candidates for longer‐term cardiac monitoring to increase yield for detection of atrial fibrillation. Currently, continuous electrocardiographic monitoring of patients for the duration of their hospitalization and up to 30 days afterward is recommended.[8]
Our study differs from prior studies that showed a much higher prevalence of LAA or left atrial thrombus in 2 important ways. Patients with severe valve disease or LV dysfunction were excluded on the basis of TTE. Additionally, our patients underwent continuous electrocardiographic monitoring for the duration of their hospitalization and were excluded with a prior history or newly discovered atrial fibrillation or flutter. Our intention was to examine the value of adding TEE when no other etiology of stroke was identified. Value can be defined as healthcare outcomes achieved per dollar spent. Our study was not designed to look at long‐term outcomes; rather, we used immediate change in patient management as a surrogate.
There are several limitations to our study that must be noted. This was a single‐center study potentially creating a bias as less stringent selection of patients undergoing TEE may be the practice at other institutions. This analysis was retrospective; therefore, there may have been bias as to which patients were selected to undergo TEE. Additionally, stroke subtype was not specified, and the pretest probability of a cardioembolic source differs based on subtype. Last, we focused this study on immediate changes in clinical management prompted by TEE results, and did not assess patient perceptions of TEE value related to enhanced knowledge about the etiology of their stroke; this area represents an opportunity for further research.
CONCLUSIONS
TEE provides a substantial increase in possible explanation of stroke etiology in patients over age 50 years admitted with a stroke of uncertain cause and a normal TTE. However, there is minimal incremental value in regard to change in therapeutic management in these patients. In a time of increased focus on providing cost effective healthcare, our findings suggest that the need for TEE in this stroke population should be more closely examined.
Disclosure: Nothing to report.
- , , . Influence of transesophageal echocardiogram on therapy and prognosis in young patients with TIA or ischemic stroke. Neth Heart J. 2009;17:373–377.
- , , , et al. Diagnosis of Heart Tumors by Transesophageal Echocardiography: a multicentre study in 154 patients. Eur Heart J. 1993;14:1223–1228.
- , , , , , . Transesophageal echocardiography in patients with cryptogenic ischemic stroke: a systematic review. Am Heart J. 2014;168:706–712.
- , , . Protruding atheromas in the thoracic aorta and systemic embolization. Ann Intern Med. 1991;115:423–427.
- , , , et al.; The Aortic Arch Related Cerebral Hazard Trial Investigators. Clopidogrel plus aspirin versus warfarin in patients with stroke and aortic arch plaques. Stroke. 2014;45:1248–1257.
- , , , et al.; RESPECT Investigators. Closure of patent foramen ovale versus medical therapy after cryptogenic stroke. N Engl J Med. 2013;368:1092–1100.
- , , , et al.; CLOSURE I Investigators. Closure or medical therapy for cryptogenic stroke with patent foramen ovale. N Engl J Med. 2012;366:991–999.
- , , , et al. Guidelines for the prevention of stroke in patients with stroke and transient ischemic attack: a guideline for healthcare professionals from the American Heart Association/American Stroke Association. Stroke. 2014;45(7):2160–2236.
- , , , et al. 2014 AHA/ACA guideline for the management of patients with valvular heart disease. J Am Coll Cardiol. 2014; 63:e57–e185.
- , , , . Clinical and echocardiographic characteristics of patients with left atrial thrombus and sinus rhythm: experience in 20 643 consecutive transesophageal echocardiographic examinations. Circulation. 2002;105(1):27–31.
- , , , et al. Usefulness of transesophageal echocardiography in unexplained cerebral ischemia. Am J Cardiol. 1993;72:1448–1452.
- , , , , , . Transesophageal echocardiography in patients with recent stroke and normal carotid arteries. Am J Cardiol. 2001;88:820–823.
- , , , et al. Transesophageal echocardiography is superior to transthoracic echocardiography in management of patients of any age with transient ischemic attack or stroke. Stroke. 2006;37:2531–2534.
- , , , , , . Age‐dependent prevalence of cardioembolic sources detected by TEE: diagnostic and therapeutic implications. Echocardiography. 1997;14:597–606.
- , , , et al. Continuous stroke unit electrocardiographic monitoring versus 24‐hour Holter electrocardiography for detection of paroxysmal atrial fibrillation after stroke. Stroke. 2012;43:2689–2694.
- , , , et al.; CRYSTAL AF Investigators. Cryptogenic stroke and underlying atrial fibrillation. N Engl J Med. 2014;370(26):2478–2486.
- , , . Influence of transesophageal echocardiogram on therapy and prognosis in young patients with TIA or ischemic stroke. Neth Heart J. 2009;17:373–377.
- , , , et al. Diagnosis of Heart Tumors by Transesophageal Echocardiography: a multicentre study in 154 patients. Eur Heart J. 1993;14:1223–1228.
- , , , , , . Transesophageal echocardiography in patients with cryptogenic ischemic stroke: a systematic review. Am Heart J. 2014;168:706–712.
- , , . Protruding atheromas in the thoracic aorta and systemic embolization. Ann Intern Med. 1991;115:423–427.
- , , , et al.; The Aortic Arch Related Cerebral Hazard Trial Investigators. Clopidogrel plus aspirin versus warfarin in patients with stroke and aortic arch plaques. Stroke. 2014;45:1248–1257.
- , , , et al.; RESPECT Investigators. Closure of patent foramen ovale versus medical therapy after cryptogenic stroke. N Engl J Med. 2013;368:1092–1100.
- , , , et al.; CLOSURE I Investigators. Closure or medical therapy for cryptogenic stroke with patent foramen ovale. N Engl J Med. 2012;366:991–999.
- , , , et al. Guidelines for the prevention of stroke in patients with stroke and transient ischemic attack: a guideline for healthcare professionals from the American Heart Association/American Stroke Association. Stroke. 2014;45(7):2160–2236.
- , , , et al. 2014 AHA/ACA guideline for the management of patients with valvular heart disease. J Am Coll Cardiol. 2014; 63:e57–e185.
- , , , . Clinical and echocardiographic characteristics of patients with left atrial thrombus and sinus rhythm: experience in 20 643 consecutive transesophageal echocardiographic examinations. Circulation. 2002;105(1):27–31.
- , , , et al. Usefulness of transesophageal echocardiography in unexplained cerebral ischemia. Am J Cardiol. 1993;72:1448–1452.
- , , , , , . Transesophageal echocardiography in patients with recent stroke and normal carotid arteries. Am J Cardiol. 2001;88:820–823.
- , , , et al. Transesophageal echocardiography is superior to transthoracic echocardiography in management of patients of any age with transient ischemic attack or stroke. Stroke. 2006;37:2531–2534.
- , , , , , . Age‐dependent prevalence of cardioembolic sources detected by TEE: diagnostic and therapeutic implications. Echocardiography. 1997;14:597–606.
- , , , et al. Continuous stroke unit electrocardiographic monitoring versus 24‐hour Holter electrocardiography for detection of paroxysmal atrial fibrillation after stroke. Stroke. 2012;43:2689–2694.
- , , , et al.; CRYSTAL AF Investigators. Cryptogenic stroke and underlying atrial fibrillation. N Engl J Med. 2014;370(26):2478–2486.
© 2015 Society of Hospital Medicine
Individualizing treatment of menopausal symptoms
Menopause experts Andrew M. Kaunitz, MD, and JoAnn E. Manson, MD, DrPH, provide a comprehensive review of various treatments for menopausal symptoms in an article recently published ahead of print in Obstetrics and Gynecology.1 They discuss hormonal and nonhormonal options to treat vasomotor symptoms, genitourinary syndrome of menopause (GSM), and considerations for the use of hormone therapy in special populations: women with early menopause, women with a history of breast cancer and those who carry the BRCA gene mutation, and women with a history of venous thrombosis.1
The authors write that, “given the lower rates of adverse events on HT among women close to menopause onset and at lower baseline risk of cardiovascular disease, risk stratification and personalized risk assessment appear to represent a sound strategy for optimizing the benefit–risk profile and safety of HT.”1 They suggest that instead of stopping systemic HT at age 65 years, the length of treatment be individualized based on a woman’s risk profile and preferences. The authors encourage gynecologists and other clinicians to use benefit–risk profile tools for both hormonal and nonhormonal options to help women make sound decisions on treating menopausal symptoms.1
Readthe full Clinical Expert Series here.
Reference
- Kaunitz AM, Manson JE. Management of menopausal symptoms [published online ahead of print September 3, 2015]. Obstet Gynecol. doi: 10.1097/AOG.0000000000001058. Accessed September 18, 2015.
Menopause experts Andrew M. Kaunitz, MD, and JoAnn E. Manson, MD, DrPH, provide a comprehensive review of various treatments for menopausal symptoms in an article recently published ahead of print in Obstetrics and Gynecology.1 They discuss hormonal and nonhormonal options to treat vasomotor symptoms, genitourinary syndrome of menopause (GSM), and considerations for the use of hormone therapy in special populations: women with early menopause, women with a history of breast cancer and those who carry the BRCA gene mutation, and women with a history of venous thrombosis.1
The authors write that, “given the lower rates of adverse events on HT among women close to menopause onset and at lower baseline risk of cardiovascular disease, risk stratification and personalized risk assessment appear to represent a sound strategy for optimizing the benefit–risk profile and safety of HT.”1 They suggest that instead of stopping systemic HT at age 65 years, the length of treatment be individualized based on a woman’s risk profile and preferences. The authors encourage gynecologists and other clinicians to use benefit–risk profile tools for both hormonal and nonhormonal options to help women make sound decisions on treating menopausal symptoms.1
Readthe full Clinical Expert Series here.
Menopause experts Andrew M. Kaunitz, MD, and JoAnn E. Manson, MD, DrPH, provide a comprehensive review of various treatments for menopausal symptoms in an article recently published ahead of print in Obstetrics and Gynecology.1 They discuss hormonal and nonhormonal options to treat vasomotor symptoms, genitourinary syndrome of menopause (GSM), and considerations for the use of hormone therapy in special populations: women with early menopause, women with a history of breast cancer and those who carry the BRCA gene mutation, and women with a history of venous thrombosis.1
The authors write that, “given the lower rates of adverse events on HT among women close to menopause onset and at lower baseline risk of cardiovascular disease, risk stratification and personalized risk assessment appear to represent a sound strategy for optimizing the benefit–risk profile and safety of HT.”1 They suggest that instead of stopping systemic HT at age 65 years, the length of treatment be individualized based on a woman’s risk profile and preferences. The authors encourage gynecologists and other clinicians to use benefit–risk profile tools for both hormonal and nonhormonal options to help women make sound decisions on treating menopausal symptoms.1
Readthe full Clinical Expert Series here.
Reference
- Kaunitz AM, Manson JE. Management of menopausal symptoms [published online ahead of print September 3, 2015]. Obstet Gynecol. doi: 10.1097/AOG.0000000000001058. Accessed September 18, 2015.
Reference
- Kaunitz AM, Manson JE. Management of menopausal symptoms [published online ahead of print September 3, 2015]. Obstet Gynecol. doi: 10.1097/AOG.0000000000001058. Accessed September 18, 2015.
Vegetative Sacral Plaque in a Patient With Human Immunodeficiency Virus
The Diagnosis: Herpes Simplex Vegetans
Histopathologic examination using hematoxylin and eosin stain demonstrated marked pseudoepitheliomatous hyperplasia with granulation tissue, ulceration, and abundant exudate joined by a dense mixed inflammatory cell infiltrate that included a myriad of eosinophils (Figure, A). At higher power (Figure, B), many single and multinucleate acantholytic keratinocytes showed ground-glass nuclei and peripheral margination of chromatin within zones of ulceration and crust. Viral culture and direct fluorescent antibody assay identified herpes simplex virus (HSV) type 2. Based on the clinical and histopathologic findings, the patient was diagnosed with herpes simplex vegetans. He was initially treated with oral acyclovir and then oral famciclovir but showed minimal improvement. Eventually, he was referred to surgery and the mass was totally excised with clear margins and no evidence of underlying malignancy.

|
| Histopathology revealed marked pseudoepitheliomatous hyperplasia, ulceration, and a dense mixed inflammatory cell infiltrate (A)(H&E, original magnification ×20). Many multinucleate acantholytic keratinocytes with ground-glass nuclei and peripheral margination of chromatin were shown (B)(H&E, original magnification ×400). |
Herpes simplex virus is one of the most common sexually transmitted infections, with a notably increased incidence and prevalence among individuals with human immunodeficiency virus (HIV) infection.1 Although typical HSV manifestation in immunocompetent patients includes clustered vesicles and/or ulcerations, immunocompromised patients may have unusual presentations, such as persistent and extensive ulcerations or nodular hyperkeratotic lesions.2,3 Herpes vegetans, a term used to describe these atypical exophytic lesions, rarely has been reported in literature, but its presence should raise suspicion for possible underlying immunocompromise. The pathogenesis behind the hypertrophic nature of these lesions is not well understood, but it is postulated that the immune dysregulation from concomitant HIV and HSV infection plays a role.2 Overproduction of tumor necrosis factor and IL-6 by HIV-infected dermal dendritic cells causes an increase in antiapoptotic factors within the epidermis, resulting in enhanced keratinocyte proliferation and clinical hyperkeratosis.2,4
The differential diagnosis for herpes vegetans is somewhat broad, owing to the verrucous and often eroded appearance of the lesions. Biopsy and cultures can be obtained to differentiate from condyloma acuminatum, condyloma latum (secondary syphilis), pyoderma vegetans, pemphigus vegetans, granuloma inguinale, extraintestinal Crohn disease, deep fungal infections, cutaneous tuberculosis, and malignancy.2-4 Histopathology shows epithelial hyperplasia and ulceration with scattered multinucleate keratinocytes, usually at the periphery of the ulcer, and intranuclear inclusions typical of HSV. In addition, a dense dermal infiltrate of lymphocytes, histiocytes, plasma cells, and eosinophils is usually present beneath the base of the ulcer.2,4
Treatment options for herpes vegetans are limited due to the high prevalence of acyclovir-resistant (ACV-R) HSV-2 strains in HIV patients. Valacyclovir and penciclovir have been largely ineffective against ACV-R HSV due to their dependence on the same enzyme—thymidine kinase—involved in the mechanism of acyclovir resistance. Intravenous foscarnet and cidofovir have shown efficacy against ACV-R virus, but concerns of nephrotoxicity have limited their use over prolonged intervals.5 Castelo-Soccio et al6 reported promising results with intralesional cidofovir. This route of administration provides the advantage of increased bioavailability with reduced risk for nephrotoxicity.6 Finally, surgical resection may be considered for refractory lesions to circumvent the toxicity from systemically administered drugs.3
- Severson JL, Tyring SK. Relation between herpes simplex viruses and human immunodeficiency virus infections. Arch Dermatol. 1999;135:1393-1397.
- Patel AB, Rosen T. Herpes vegetans as a sign of HIV infection. Dermatol Online J. 2008;14:6.
- Chung VQ, Parker DC, Parker SR. Surgical excision for vegetative herpes simplex virus infection. Dermatol Surg. 2007;33:1374-1379.
- Beasley KL, Cooley GE, Kao GF, et al. Herpes simplex vegetans: atypical genital herpes infection in a patient with common variable immunodeficiency. J Am Acad Dermatol. 1997;37(5, pt 2):860-863.
- Chilukuri S, Rosen T. Management of acyclovir-resistant herpes simplex virus. Dermatol Clin. 2003;21:311-320.
- Castelo-Soccio L, Bernardin R, Stern J, et al. Successful treatment of acyclovir-resistant herpes simplex virus with intralesional cidofovir. Arch Dermatol. 2010;146:124-126.
The Diagnosis: Herpes Simplex Vegetans
Histopathologic examination using hematoxylin and eosin stain demonstrated marked pseudoepitheliomatous hyperplasia with granulation tissue, ulceration, and abundant exudate joined by a dense mixed inflammatory cell infiltrate that included a myriad of eosinophils (Figure, A). At higher power (Figure, B), many single and multinucleate acantholytic keratinocytes showed ground-glass nuclei and peripheral margination of chromatin within zones of ulceration and crust. Viral culture and direct fluorescent antibody assay identified herpes simplex virus (HSV) type 2. Based on the clinical and histopathologic findings, the patient was diagnosed with herpes simplex vegetans. He was initially treated with oral acyclovir and then oral famciclovir but showed minimal improvement. Eventually, he was referred to surgery and the mass was totally excised with clear margins and no evidence of underlying malignancy.


|
| Histopathology revealed marked pseudoepitheliomatous hyperplasia, ulceration, and a dense mixed inflammatory cell infiltrate (A)(H&E, original magnification ×20). Many multinucleate acantholytic keratinocytes with ground-glass nuclei and peripheral margination of chromatin were shown (B)(H&E, original magnification ×400). |
Herpes simplex virus is one of the most common sexually transmitted infections, with a notably increased incidence and prevalence among individuals with human immunodeficiency virus (HIV) infection.1 Although typical HSV manifestation in immunocompetent patients includes clustered vesicles and/or ulcerations, immunocompromised patients may have unusual presentations, such as persistent and extensive ulcerations or nodular hyperkeratotic lesions.2,3 Herpes vegetans, a term used to describe these atypical exophytic lesions, rarely has been reported in literature, but its presence should raise suspicion for possible underlying immunocompromise. The pathogenesis behind the hypertrophic nature of these lesions is not well understood, but it is postulated that the immune dysregulation from concomitant HIV and HSV infection plays a role.2 Overproduction of tumor necrosis factor and IL-6 by HIV-infected dermal dendritic cells causes an increase in antiapoptotic factors within the epidermis, resulting in enhanced keratinocyte proliferation and clinical hyperkeratosis.2,4
The differential diagnosis for herpes vegetans is somewhat broad, owing to the verrucous and often eroded appearance of the lesions. Biopsy and cultures can be obtained to differentiate from condyloma acuminatum, condyloma latum (secondary syphilis), pyoderma vegetans, pemphigus vegetans, granuloma inguinale, extraintestinal Crohn disease, deep fungal infections, cutaneous tuberculosis, and malignancy.2-4 Histopathology shows epithelial hyperplasia and ulceration with scattered multinucleate keratinocytes, usually at the periphery of the ulcer, and intranuclear inclusions typical of HSV. In addition, a dense dermal infiltrate of lymphocytes, histiocytes, plasma cells, and eosinophils is usually present beneath the base of the ulcer.2,4
Treatment options for herpes vegetans are limited due to the high prevalence of acyclovir-resistant (ACV-R) HSV-2 strains in HIV patients. Valacyclovir and penciclovir have been largely ineffective against ACV-R HSV due to their dependence on the same enzyme—thymidine kinase—involved in the mechanism of acyclovir resistance. Intravenous foscarnet and cidofovir have shown efficacy against ACV-R virus, but concerns of nephrotoxicity have limited their use over prolonged intervals.5 Castelo-Soccio et al6 reported promising results with intralesional cidofovir. This route of administration provides the advantage of increased bioavailability with reduced risk for nephrotoxicity.6 Finally, surgical resection may be considered for refractory lesions to circumvent the toxicity from systemically administered drugs.3
The Diagnosis: Herpes Simplex Vegetans
Histopathologic examination using hematoxylin and eosin stain demonstrated marked pseudoepitheliomatous hyperplasia with granulation tissue, ulceration, and abundant exudate joined by a dense mixed inflammatory cell infiltrate that included a myriad of eosinophils (Figure, A). At higher power (Figure, B), many single and multinucleate acantholytic keratinocytes showed ground-glass nuclei and peripheral margination of chromatin within zones of ulceration and crust. Viral culture and direct fluorescent antibody assay identified herpes simplex virus (HSV) type 2. Based on the clinical and histopathologic findings, the patient was diagnosed with herpes simplex vegetans. He was initially treated with oral acyclovir and then oral famciclovir but showed minimal improvement. Eventually, he was referred to surgery and the mass was totally excised with clear margins and no evidence of underlying malignancy.


|
| Histopathology revealed marked pseudoepitheliomatous hyperplasia, ulceration, and a dense mixed inflammatory cell infiltrate (A)(H&E, original magnification ×20). Many multinucleate acantholytic keratinocytes with ground-glass nuclei and peripheral margination of chromatin were shown (B)(H&E, original magnification ×400). |
Herpes simplex virus is one of the most common sexually transmitted infections, with a notably increased incidence and prevalence among individuals with human immunodeficiency virus (HIV) infection.1 Although typical HSV manifestation in immunocompetent patients includes clustered vesicles and/or ulcerations, immunocompromised patients may have unusual presentations, such as persistent and extensive ulcerations or nodular hyperkeratotic lesions.2,3 Herpes vegetans, a term used to describe these atypical exophytic lesions, rarely has been reported in literature, but its presence should raise suspicion for possible underlying immunocompromise. The pathogenesis behind the hypertrophic nature of these lesions is not well understood, but it is postulated that the immune dysregulation from concomitant HIV and HSV infection plays a role.2 Overproduction of tumor necrosis factor and IL-6 by HIV-infected dermal dendritic cells causes an increase in antiapoptotic factors within the epidermis, resulting in enhanced keratinocyte proliferation and clinical hyperkeratosis.2,4
The differential diagnosis for herpes vegetans is somewhat broad, owing to the verrucous and often eroded appearance of the lesions. Biopsy and cultures can be obtained to differentiate from condyloma acuminatum, condyloma latum (secondary syphilis), pyoderma vegetans, pemphigus vegetans, granuloma inguinale, extraintestinal Crohn disease, deep fungal infections, cutaneous tuberculosis, and malignancy.2-4 Histopathology shows epithelial hyperplasia and ulceration with scattered multinucleate keratinocytes, usually at the periphery of the ulcer, and intranuclear inclusions typical of HSV. In addition, a dense dermal infiltrate of lymphocytes, histiocytes, plasma cells, and eosinophils is usually present beneath the base of the ulcer.2,4
Treatment options for herpes vegetans are limited due to the high prevalence of acyclovir-resistant (ACV-R) HSV-2 strains in HIV patients. Valacyclovir and penciclovir have been largely ineffective against ACV-R HSV due to their dependence on the same enzyme—thymidine kinase—involved in the mechanism of acyclovir resistance. Intravenous foscarnet and cidofovir have shown efficacy against ACV-R virus, but concerns of nephrotoxicity have limited their use over prolonged intervals.5 Castelo-Soccio et al6 reported promising results with intralesional cidofovir. This route of administration provides the advantage of increased bioavailability with reduced risk for nephrotoxicity.6 Finally, surgical resection may be considered for refractory lesions to circumvent the toxicity from systemically administered drugs.3
- Severson JL, Tyring SK. Relation between herpes simplex viruses and human immunodeficiency virus infections. Arch Dermatol. 1999;135:1393-1397.
- Patel AB, Rosen T. Herpes vegetans as a sign of HIV infection. Dermatol Online J. 2008;14:6.
- Chung VQ, Parker DC, Parker SR. Surgical excision for vegetative herpes simplex virus infection. Dermatol Surg. 2007;33:1374-1379.
- Beasley KL, Cooley GE, Kao GF, et al. Herpes simplex vegetans: atypical genital herpes infection in a patient with common variable immunodeficiency. J Am Acad Dermatol. 1997;37(5, pt 2):860-863.
- Chilukuri S, Rosen T. Management of acyclovir-resistant herpes simplex virus. Dermatol Clin. 2003;21:311-320.
- Castelo-Soccio L, Bernardin R, Stern J, et al. Successful treatment of acyclovir-resistant herpes simplex virus with intralesional cidofovir. Arch Dermatol. 2010;146:124-126.
- Severson JL, Tyring SK. Relation between herpes simplex viruses and human immunodeficiency virus infections. Arch Dermatol. 1999;135:1393-1397.
- Patel AB, Rosen T. Herpes vegetans as a sign of HIV infection. Dermatol Online J. 2008;14:6.
- Chung VQ, Parker DC, Parker SR. Surgical excision for vegetative herpes simplex virus infection. Dermatol Surg. 2007;33:1374-1379.
- Beasley KL, Cooley GE, Kao GF, et al. Herpes simplex vegetans: atypical genital herpes infection in a patient with common variable immunodeficiency. J Am Acad Dermatol. 1997;37(5, pt 2):860-863.
- Chilukuri S, Rosen T. Management of acyclovir-resistant herpes simplex virus. Dermatol Clin. 2003;21:311-320.
- Castelo-Soccio L, Bernardin R, Stern J, et al. Successful treatment of acyclovir-resistant herpes simplex virus with intralesional cidofovir. Arch Dermatol. 2010;146:124-126.
A 53-year-old man presented to our clinic with a sacral mass that had progressively enlarged over 2 years. The patient reported occasional oozing from the mass as well as pain when laying flat but denied fever or other symptoms. His medical history was remarkable for human immunodeficiency virus infection with variable adherence to a highly active antiretroviral therapy regimen. At the time of presentation, the patient had a CD4 lymphocyte count of 78 cells/mm3 (reference range, 500–1400 cells/mm3) and a viral load of 290 copies/mL (reference range, 0 copies/mL). Physical examination revealed a 10-cm discrete, moist and pink, exophytic plaque on the sacrum with superficial erosions. The plaque was nontender and without associated lymphadenopathy. The skin and mucous membranes were otherwise clear. A cutaneous biopsy specimen was obtained from the tumor and sent for histopathologic analysis.
Readmissions rise with endovascular lower limb procedures
CHICAGO – Endovascular lower-extremity procedures were not associated with lower 30-day readmission rates compared with open surgery in a retrospective review of 7,089 patients.
All-cause, 30-day readmissions were actually higher with an endovascular approach at 12.3% vs. 9.6% for open procedures (Relative risk, 1.28; P = .0003).
Among all patients, an index diagnosis of gangrene was most predictive of readmission (RR, 1.89; P less than .0001), Dr. Todd R. Vogel said at the annual meeting of the Midwestern Vascular Surgical Society.
The data were compiled from 7,089 patients in the Cerner Health Facts database who were admitted for peripheral artery disease and elective lower extremity procedures (3,615 open; 3,474 endo) between September 2008 and March 2014. Their average age was 67.7 years, 44.7% were aged 70 years or older, 60% were men, and 21% were African American.
Older patients and men were significantly more likely to receive endovascular procedures (P less than .0001), said Dr. Vogel, chief of vascular surgery, University of Missouri Health System in Columbia.
Overall, 767 patients (11%) were readmitted (344 open; 423 endo), with gangrene accounting for 21.7% of readmissions.
Other index diagnoses associated with higher 30-day readmissions for all lower extremity procedures were fluid and electrolyte disorders, chronic anemia, lower extremity infection, heart failure, chronic kidney disease, and chronic pulmonary disease.
When stratified by procedure type, the reasons for readmission were very different within the same population of patients based on procedure type, Dr. Vogel said.
Patients who underwent an open procedure were more likely to be readmitted if they had heart failure (RR, 1.78; P less than .0001) or posthemorrhagic anemia (RR, 1.54: P = .006).
Infections – be they lower extremity infection, other infection, postoperative infection, or sepsis – were not predictive of readmission when documented at the index admission for the open cohort.
In contrast, chronic conditions were the major predictors of readmission for patients undergoing endovascular procedures, he said. They included chronic anemia (RR, 1.58; P less than .0001), chronic airway obstruction (RR, 1.36; P = .0095), chronic heart disease (RR, 1.33; P = .0019), chronic kidney disease (RR, 1.37; P = .0013), diabetes (RR, 1.34; P = .0012), and hypertension (RR, 1.27; P = .023).
Fluid and electrolyte disorders (RR, 1.65, P less than .0001) and lower extremity infections (RR, 1.57, P = .0016) were also significant predictors of readmission in the endovascular group.
To ensure there were no disparities between index and readmission diagnoses, a final analysis was performed by procedure type in the 767 readmissions. It confirmed that for the endovascular procedures, chronic problems are bringing patients back to the hospital and not necessarily complications from the procedure, whereas infections, device complications, and hemorrhage are the reasons open surgery patients return, Dr. Vogel said.
“The question is are chronic conditions associated with readmissions the fault of the intervention? As physicians can we hope to curb this in patients who have chronic problems and are then readmitted?” he said.
Some audience members argued that no matter if the patient had a chronic condition or not preoperatively, the responsibility rests with the surgeon because he or she opted to put the patient through an elective endovascular procedure and now they’re returning with chronic heart failure, for example.
Dr. Vogel said this was the first pass at the data and trying to understand what drives readmissions and that it’s possible an endovascular procedure could exacerbate a chronic condition, but that surgeons should take steps to mitigate readmission risk in those with known chronic conditions.
Other attendees questioned how many of the readmissions were planned, hinting that the readmissions may not be directly related to the endovascular technique.
Dr. Vogel said it was difficult using only the ICD-9 codes in the database to determine exactly how many readmissions were planned, but noted that further analyses are intended.
“Reasons for readmission can be exacerbation of chronic patient issues, as seen in the endovascular group, or may be secondary to later complications of the procedure such as wound infections and device complications, as seen after open bypass procedures,” he said in an interview. “Identifying patients with increased risk for readmission after vascular procedures may lead to more effective and higher quality care during the index hospitalization. Our future studies will focus on a more detailed, granular evaluation of these high-risk diagnoses groups through use of the electronic medical record.”
Dr. Vogel reported having no financial disclosures.
On Twitter @pwendl
CHICAGO – Endovascular lower-extremity procedures were not associated with lower 30-day readmission rates compared with open surgery in a retrospective review of 7,089 patients.
All-cause, 30-day readmissions were actually higher with an endovascular approach at 12.3% vs. 9.6% for open procedures (Relative risk, 1.28; P = .0003).
Among all patients, an index diagnosis of gangrene was most predictive of readmission (RR, 1.89; P less than .0001), Dr. Todd R. Vogel said at the annual meeting of the Midwestern Vascular Surgical Society.
The data were compiled from 7,089 patients in the Cerner Health Facts database who were admitted for peripheral artery disease and elective lower extremity procedures (3,615 open; 3,474 endo) between September 2008 and March 2014. Their average age was 67.7 years, 44.7% were aged 70 years or older, 60% were men, and 21% were African American.
Older patients and men were significantly more likely to receive endovascular procedures (P less than .0001), said Dr. Vogel, chief of vascular surgery, University of Missouri Health System in Columbia.
Overall, 767 patients (11%) were readmitted (344 open; 423 endo), with gangrene accounting for 21.7% of readmissions.
Other index diagnoses associated with higher 30-day readmissions for all lower extremity procedures were fluid and electrolyte disorders, chronic anemia, lower extremity infection, heart failure, chronic kidney disease, and chronic pulmonary disease.
When stratified by procedure type, the reasons for readmission were very different within the same population of patients based on procedure type, Dr. Vogel said.
Patients who underwent an open procedure were more likely to be readmitted if they had heart failure (RR, 1.78; P less than .0001) or posthemorrhagic anemia (RR, 1.54: P = .006).
Infections – be they lower extremity infection, other infection, postoperative infection, or sepsis – were not predictive of readmission when documented at the index admission for the open cohort.
In contrast, chronic conditions were the major predictors of readmission for patients undergoing endovascular procedures, he said. They included chronic anemia (RR, 1.58; P less than .0001), chronic airway obstruction (RR, 1.36; P = .0095), chronic heart disease (RR, 1.33; P = .0019), chronic kidney disease (RR, 1.37; P = .0013), diabetes (RR, 1.34; P = .0012), and hypertension (RR, 1.27; P = .023).
Fluid and electrolyte disorders (RR, 1.65, P less than .0001) and lower extremity infections (RR, 1.57, P = .0016) were also significant predictors of readmission in the endovascular group.
To ensure there were no disparities between index and readmission diagnoses, a final analysis was performed by procedure type in the 767 readmissions. It confirmed that for the endovascular procedures, chronic problems are bringing patients back to the hospital and not necessarily complications from the procedure, whereas infections, device complications, and hemorrhage are the reasons open surgery patients return, Dr. Vogel said.
“The question is are chronic conditions associated with readmissions the fault of the intervention? As physicians can we hope to curb this in patients who have chronic problems and are then readmitted?” he said.
Some audience members argued that no matter if the patient had a chronic condition or not preoperatively, the responsibility rests with the surgeon because he or she opted to put the patient through an elective endovascular procedure and now they’re returning with chronic heart failure, for example.
Dr. Vogel said this was the first pass at the data and trying to understand what drives readmissions and that it’s possible an endovascular procedure could exacerbate a chronic condition, but that surgeons should take steps to mitigate readmission risk in those with known chronic conditions.
Other attendees questioned how many of the readmissions were planned, hinting that the readmissions may not be directly related to the endovascular technique.
Dr. Vogel said it was difficult using only the ICD-9 codes in the database to determine exactly how many readmissions were planned, but noted that further analyses are intended.
“Reasons for readmission can be exacerbation of chronic patient issues, as seen in the endovascular group, or may be secondary to later complications of the procedure such as wound infections and device complications, as seen after open bypass procedures,” he said in an interview. “Identifying patients with increased risk for readmission after vascular procedures may lead to more effective and higher quality care during the index hospitalization. Our future studies will focus on a more detailed, granular evaluation of these high-risk diagnoses groups through use of the electronic medical record.”
Dr. Vogel reported having no financial disclosures.
On Twitter @pwendl
CHICAGO – Endovascular lower-extremity procedures were not associated with lower 30-day readmission rates compared with open surgery in a retrospective review of 7,089 patients.
All-cause, 30-day readmissions were actually higher with an endovascular approach at 12.3% vs. 9.6% for open procedures (Relative risk, 1.28; P = .0003).
Among all patients, an index diagnosis of gangrene was most predictive of readmission (RR, 1.89; P less than .0001), Dr. Todd R. Vogel said at the annual meeting of the Midwestern Vascular Surgical Society.
The data were compiled from 7,089 patients in the Cerner Health Facts database who were admitted for peripheral artery disease and elective lower extremity procedures (3,615 open; 3,474 endo) between September 2008 and March 2014. Their average age was 67.7 years, 44.7% were aged 70 years or older, 60% were men, and 21% were African American.
Older patients and men were significantly more likely to receive endovascular procedures (P less than .0001), said Dr. Vogel, chief of vascular surgery, University of Missouri Health System in Columbia.
Overall, 767 patients (11%) were readmitted (344 open; 423 endo), with gangrene accounting for 21.7% of readmissions.
Other index diagnoses associated with higher 30-day readmissions for all lower extremity procedures were fluid and electrolyte disorders, chronic anemia, lower extremity infection, heart failure, chronic kidney disease, and chronic pulmonary disease.
When stratified by procedure type, the reasons for readmission were very different within the same population of patients based on procedure type, Dr. Vogel said.
Patients who underwent an open procedure were more likely to be readmitted if they had heart failure (RR, 1.78; P less than .0001) or posthemorrhagic anemia (RR, 1.54: P = .006).
Infections – be they lower extremity infection, other infection, postoperative infection, or sepsis – were not predictive of readmission when documented at the index admission for the open cohort.
In contrast, chronic conditions were the major predictors of readmission for patients undergoing endovascular procedures, he said. They included chronic anemia (RR, 1.58; P less than .0001), chronic airway obstruction (RR, 1.36; P = .0095), chronic heart disease (RR, 1.33; P = .0019), chronic kidney disease (RR, 1.37; P = .0013), diabetes (RR, 1.34; P = .0012), and hypertension (RR, 1.27; P = .023).
Fluid and electrolyte disorders (RR, 1.65, P less than .0001) and lower extremity infections (RR, 1.57, P = .0016) were also significant predictors of readmission in the endovascular group.
To ensure there were no disparities between index and readmission diagnoses, a final analysis was performed by procedure type in the 767 readmissions. It confirmed that for the endovascular procedures, chronic problems are bringing patients back to the hospital and not necessarily complications from the procedure, whereas infections, device complications, and hemorrhage are the reasons open surgery patients return, Dr. Vogel said.
“The question is are chronic conditions associated with readmissions the fault of the intervention? As physicians can we hope to curb this in patients who have chronic problems and are then readmitted?” he said.
Some audience members argued that no matter if the patient had a chronic condition or not preoperatively, the responsibility rests with the surgeon because he or she opted to put the patient through an elective endovascular procedure and now they’re returning with chronic heart failure, for example.
Dr. Vogel said this was the first pass at the data and trying to understand what drives readmissions and that it’s possible an endovascular procedure could exacerbate a chronic condition, but that surgeons should take steps to mitigate readmission risk in those with known chronic conditions.
Other attendees questioned how many of the readmissions were planned, hinting that the readmissions may not be directly related to the endovascular technique.
Dr. Vogel said it was difficult using only the ICD-9 codes in the database to determine exactly how many readmissions were planned, but noted that further analyses are intended.
“Reasons for readmission can be exacerbation of chronic patient issues, as seen in the endovascular group, or may be secondary to later complications of the procedure such as wound infections and device complications, as seen after open bypass procedures,” he said in an interview. “Identifying patients with increased risk for readmission after vascular procedures may lead to more effective and higher quality care during the index hospitalization. Our future studies will focus on a more detailed, granular evaluation of these high-risk diagnoses groups through use of the electronic medical record.”
Dr. Vogel reported having no financial disclosures.
On Twitter @pwendl
AT MIDWESTERN VASCULAR 2015
Key clinical point: Endovascular procedures were not superior to open surgery in reducing 30-day readmissions in patients undergoing lower extremity procedures.
Major finding: All-cause 30-day readmissions were 12.3% for endovascular and 9.6% for open (P = .0003).
Data source: Retrospective study in 7,089 patients undergoing elective lower extremity procedures.
Disclosures: The research was supported by an award from the Agency for Healthcare Research and Quality. Dr. Vogel reported having no conflicts of interest.
MM drugs granted priority review

multiple myeloma
The US Food and Drug Administration (FDA) has granted priority review for 4 drugs intended to treat multiple myeloma (MM): elotuzumab (Empliciti), daratumumab, ixazomib (MLN9708), and carfilzomib (Kyprolis).
The FDA grants priority review to investigational therapies that, if approved, may offer significant improvements in the treatment, prevention, or diagnosis of a serious condition.
The designation shortens the review period from 10 months to 6 months.
Carfilzomib
Carfilzomib, an injectable proteasome inhibitor, is currently under review for use in combination with dexamethasone to treat patients with relapsed MM who have received at least 1 prior therapy.
Carfilzomib is already FDA-approved for use in combination with lenalidomide and dexamethasone to treat patients with relapsed MM who have received 1 to 3 prior lines of therapy.
Carfilzomib also has accelerated approval from the FDA as monotherapy for MM patients who have received at least 2 prior therapies, including bortezomib and an immunomodulatory agent (IMiD), and have demonstrated disease progression on or within 60 days of completing their last treatment.
The new regulatory submission for carfilzomib is based on data from the phase 3 ENDEAVOR trial, which were presented at ASCO 2015. In this trial, relapsed MM patients who received carfilzomib and low-dose dexamethasone had significantly longer progression-free survival (PFS) than patients who received bortezomib and low-dose dexamethasone.
Carfilzomib is under development by Onyx Pharmaceuticals, Inc., an Amgen subsidiary.
Ixazomib
Ixazomib, an oral proteasome inhibitor, is under review for the treatment of relapsed and/or refractory MM. Ixazomib has orphan drug designation from the FDA for this patient population.
The regulatory submission for ixazomib is primarily based on results of the first interim analysis of the phase 3 TOURMALINE-MM1 trial.
In this trial, patients with relapsed and/or refractory MM who received ixazomib plus lenalidomide and dexamethasone had superior PFS to patients who received placebo plus lenalidomide and dexamethasone, according to Takeda Pharmaceutical Company Limited, the company developing ixazomib. Detailed data from this study have not yet been released.
Ixazomib is currently under investigation in 3 other phase 3 trials of MM patients:
- TOURMALINE-MM2, investigating ixazomib vs placebo, both in combination with lenalidomide and dexamethasone in patients with newly diagnosed MM
- TOURMALINE-MM3, investigating ixazomib vs placebo as maintenance therapy in patients with newly diagnosed MM following induction therapy and autologous stem cell transplant
- TOURMALINE-MM4, investigating ixazomib vs placebo as maintenance therapy in patients with newly diagnosed MM who have not undergone autologous stem cell transplant.
Daratumumab
Daratumumab, an investigational human anti-CD38 monoclonal antibody, is under review as monotherapy for MM patients who are refractory to both a proteasome inhibitor and an IMiD, or who have received 3 or more prior lines of therapy, including a proteasome inhibitor and an IMiD.
Daratumumab already has fast track, breakthrough therapy, and orphan drug designations from the FDA.
The regulatory submission for daratumumab is primarily supported by data from the phase 2 MMY2002 (SIRIUS) monotherapy study, which were presented at ASCO 2015. Results from this study indicated that daratumumab can produce responses in heavily pretreated MM patients.
Additional data from 4 other studies, including the phase 1/2 GEN501 monotherapy study, which was recently published in NEJM, also support the submission.
Daratumumab is under development by Janssen Research & Development, LLC.
Elotuzumab
Elotuzumab, a signaling lymphocyte activation molecule (SLAMF7)-directed immunostimulatory antibody, is under review for use in combination with lenalidomide and dexamethasone to treat MM patients who have received at least 1 prior treatment.
The FDA has already granted elotuzumab breakthrough therapy and orphan drug designations.
The regulatory submission for elotuzumab is primarily supported by data from the ELOQUENT-2 trial, which were presented at ASCO 2015. In this phase 3 study, researchers evaluated elotuzumab in combination with lenalidomide and dexamethasone, compared to lenalidomide and dexamethasone alone.
The submission is also supported by data from the CA204-009 trial, which were presented at EHA 2015. In this phase 2 study, researchers evaluated elotuzumab in combination with bortezomib and dexamethasone compared to bortezomib and dexamethasone alone.
Results from both trials suggested that adding elotuzumab to combination treatment can prolong PFS in MM patients.
Bristol-Myers Squibb and AbbVie are co-developing elotuzumab, with Bristol-Myers Squibb solely responsible for commercial activities. ![]()

multiple myeloma
The US Food and Drug Administration (FDA) has granted priority review for 4 drugs intended to treat multiple myeloma (MM): elotuzumab (Empliciti), daratumumab, ixazomib (MLN9708), and carfilzomib (Kyprolis).
The FDA grants priority review to investigational therapies that, if approved, may offer significant improvements in the treatment, prevention, or diagnosis of a serious condition.
The designation shortens the review period from 10 months to 6 months.
Carfilzomib
Carfilzomib, an injectable proteasome inhibitor, is currently under review for use in combination with dexamethasone to treat patients with relapsed MM who have received at least 1 prior therapy.
Carfilzomib is already FDA-approved for use in combination with lenalidomide and dexamethasone to treat patients with relapsed MM who have received 1 to 3 prior lines of therapy.
Carfilzomib also has accelerated approval from the FDA as monotherapy for MM patients who have received at least 2 prior therapies, including bortezomib and an immunomodulatory agent (IMiD), and have demonstrated disease progression on or within 60 days of completing their last treatment.
The new regulatory submission for carfilzomib is based on data from the phase 3 ENDEAVOR trial, which were presented at ASCO 2015. In this trial, relapsed MM patients who received carfilzomib and low-dose dexamethasone had significantly longer progression-free survival (PFS) than patients who received bortezomib and low-dose dexamethasone.
Carfilzomib is under development by Onyx Pharmaceuticals, Inc., an Amgen subsidiary.
Ixazomib
Ixazomib, an oral proteasome inhibitor, is under review for the treatment of relapsed and/or refractory MM. Ixazomib has orphan drug designation from the FDA for this patient population.
The regulatory submission for ixazomib is primarily based on results of the first interim analysis of the phase 3 TOURMALINE-MM1 trial.
In this trial, patients with relapsed and/or refractory MM who received ixazomib plus lenalidomide and dexamethasone had superior PFS to patients who received placebo plus lenalidomide and dexamethasone, according to Takeda Pharmaceutical Company Limited, the company developing ixazomib. Detailed data from this study have not yet been released.
Ixazomib is currently under investigation in 3 other phase 3 trials of MM patients:
- TOURMALINE-MM2, investigating ixazomib vs placebo, both in combination with lenalidomide and dexamethasone in patients with newly diagnosed MM
- TOURMALINE-MM3, investigating ixazomib vs placebo as maintenance therapy in patients with newly diagnosed MM following induction therapy and autologous stem cell transplant
- TOURMALINE-MM4, investigating ixazomib vs placebo as maintenance therapy in patients with newly diagnosed MM who have not undergone autologous stem cell transplant.
Daratumumab
Daratumumab, an investigational human anti-CD38 monoclonal antibody, is under review as monotherapy for MM patients who are refractory to both a proteasome inhibitor and an IMiD, or who have received 3 or more prior lines of therapy, including a proteasome inhibitor and an IMiD.
Daratumumab already has fast track, breakthrough therapy, and orphan drug designations from the FDA.
The regulatory submission for daratumumab is primarily supported by data from the phase 2 MMY2002 (SIRIUS) monotherapy study, which were presented at ASCO 2015. Results from this study indicated that daratumumab can produce responses in heavily pretreated MM patients.
Additional data from 4 other studies, including the phase 1/2 GEN501 monotherapy study, which was recently published in NEJM, also support the submission.
Daratumumab is under development by Janssen Research & Development, LLC.
Elotuzumab
Elotuzumab, a signaling lymphocyte activation molecule (SLAMF7)-directed immunostimulatory antibody, is under review for use in combination with lenalidomide and dexamethasone to treat MM patients who have received at least 1 prior treatment.
The FDA has already granted elotuzumab breakthrough therapy and orphan drug designations.
The regulatory submission for elotuzumab is primarily supported by data from the ELOQUENT-2 trial, which were presented at ASCO 2015. In this phase 3 study, researchers evaluated elotuzumab in combination with lenalidomide and dexamethasone, compared to lenalidomide and dexamethasone alone.
The submission is also supported by data from the CA204-009 trial, which were presented at EHA 2015. In this phase 2 study, researchers evaluated elotuzumab in combination with bortezomib and dexamethasone compared to bortezomib and dexamethasone alone.
Results from both trials suggested that adding elotuzumab to combination treatment can prolong PFS in MM patients.
Bristol-Myers Squibb and AbbVie are co-developing elotuzumab, with Bristol-Myers Squibb solely responsible for commercial activities. ![]()

multiple myeloma
The US Food and Drug Administration (FDA) has granted priority review for 4 drugs intended to treat multiple myeloma (MM): elotuzumab (Empliciti), daratumumab, ixazomib (MLN9708), and carfilzomib (Kyprolis).
The FDA grants priority review to investigational therapies that, if approved, may offer significant improvements in the treatment, prevention, or diagnosis of a serious condition.
The designation shortens the review period from 10 months to 6 months.
Carfilzomib
Carfilzomib, an injectable proteasome inhibitor, is currently under review for use in combination with dexamethasone to treat patients with relapsed MM who have received at least 1 prior therapy.
Carfilzomib is already FDA-approved for use in combination with lenalidomide and dexamethasone to treat patients with relapsed MM who have received 1 to 3 prior lines of therapy.
Carfilzomib also has accelerated approval from the FDA as monotherapy for MM patients who have received at least 2 prior therapies, including bortezomib and an immunomodulatory agent (IMiD), and have demonstrated disease progression on or within 60 days of completing their last treatment.
The new regulatory submission for carfilzomib is based on data from the phase 3 ENDEAVOR trial, which were presented at ASCO 2015. In this trial, relapsed MM patients who received carfilzomib and low-dose dexamethasone had significantly longer progression-free survival (PFS) than patients who received bortezomib and low-dose dexamethasone.
Carfilzomib is under development by Onyx Pharmaceuticals, Inc., an Amgen subsidiary.
Ixazomib
Ixazomib, an oral proteasome inhibitor, is under review for the treatment of relapsed and/or refractory MM. Ixazomib has orphan drug designation from the FDA for this patient population.
The regulatory submission for ixazomib is primarily based on results of the first interim analysis of the phase 3 TOURMALINE-MM1 trial.
In this trial, patients with relapsed and/or refractory MM who received ixazomib plus lenalidomide and dexamethasone had superior PFS to patients who received placebo plus lenalidomide and dexamethasone, according to Takeda Pharmaceutical Company Limited, the company developing ixazomib. Detailed data from this study have not yet been released.
Ixazomib is currently under investigation in 3 other phase 3 trials of MM patients:
- TOURMALINE-MM2, investigating ixazomib vs placebo, both in combination with lenalidomide and dexamethasone in patients with newly diagnosed MM
- TOURMALINE-MM3, investigating ixazomib vs placebo as maintenance therapy in patients with newly diagnosed MM following induction therapy and autologous stem cell transplant
- TOURMALINE-MM4, investigating ixazomib vs placebo as maintenance therapy in patients with newly diagnosed MM who have not undergone autologous stem cell transplant.
Daratumumab
Daratumumab, an investigational human anti-CD38 monoclonal antibody, is under review as monotherapy for MM patients who are refractory to both a proteasome inhibitor and an IMiD, or who have received 3 or more prior lines of therapy, including a proteasome inhibitor and an IMiD.
Daratumumab already has fast track, breakthrough therapy, and orphan drug designations from the FDA.
The regulatory submission for daratumumab is primarily supported by data from the phase 2 MMY2002 (SIRIUS) monotherapy study, which were presented at ASCO 2015. Results from this study indicated that daratumumab can produce responses in heavily pretreated MM patients.
Additional data from 4 other studies, including the phase 1/2 GEN501 monotherapy study, which was recently published in NEJM, also support the submission.
Daratumumab is under development by Janssen Research & Development, LLC.
Elotuzumab
Elotuzumab, a signaling lymphocyte activation molecule (SLAMF7)-directed immunostimulatory antibody, is under review for use in combination with lenalidomide and dexamethasone to treat MM patients who have received at least 1 prior treatment.
The FDA has already granted elotuzumab breakthrough therapy and orphan drug designations.
The regulatory submission for elotuzumab is primarily supported by data from the ELOQUENT-2 trial, which were presented at ASCO 2015. In this phase 3 study, researchers evaluated elotuzumab in combination with lenalidomide and dexamethasone, compared to lenalidomide and dexamethasone alone.
The submission is also supported by data from the CA204-009 trial, which were presented at EHA 2015. In this phase 2 study, researchers evaluated elotuzumab in combination with bortezomib and dexamethasone compared to bortezomib and dexamethasone alone.
Results from both trials suggested that adding elotuzumab to combination treatment can prolong PFS in MM patients.
Bristol-Myers Squibb and AbbVie are co-developing elotuzumab, with Bristol-Myers Squibb solely responsible for commercial activities. ![]()
Aspirin, hydrochlorothiazide okay in gout
It’s okay in most cases for gout patients to be on thiazide diuretics or low-dose aspirin, so long as hypouricemic therapy is adjusted as needed, according to rheumatologist and Cleveland Clinic professor Dr. Brian F. Mandell.
Coronary artery disease is common in gout, so patients benefit from inexpensive, well-tolerated, and effective treatments like thiazides for hypertension and 81 mg aspirin daily for cardioprotection, Dr. Mandell said at the annual Perspectives in Rheumatic Diseases conference held by Global Academy for Medical Education.
The concern, however, is that both can elevate serum uric acid. Observational and survey data suggest that the drugs may be associated with an increase in attacks, so some shy away from them in gout.
To make sense of the issue, the first thing to remember is that the drugs cause only “minimal elevations in serum uric acid. Serum urate can still be lowered to less than 6 mg/dL with appropriate therapy without stopping them,” Dr. Mandell said.
Low-dose aspirin raises serum urate by about 0.3 mg/dL. At 12.5 or 25 mg once a day – common hypertension doses – hydrochlorothiazide increases serum urate by 0.8 mg/dL or less in patients with normal renal function (Arthritis Rheum. 2012 Jan;64[1]:121-9).
“In patients with chronic gout treated with a xanthine oxidase inhibitor (allopurinol or febuxostat) to lower the serum urate” to recommended target levels below 6.0 mg/dL, “the small elevation in serum urate is unlikely to negate the clinical efficacy of these drugs when dosing is optimized,” Dr. Mandell wrote in an article that expands upon his presentation points (Cleve Clin J Med. 2014 Feb;81[2]:83-6).
Based on those considerations, “my practice in most patients is to use a thiazide if it helps to control the blood pressure and to adjust the dose of the hypouricemic therapy as needed to reduce the serum urate to the desired level. ... Continuing thiazide therapy and, if necessary, adjusting hypouricemic therapy will not worsen the control of the serum urate level or gouty arthritis, and in most patients will not complicate the management of gout.” In general, “when I add a thiazide to a patient’s regimen, I do not usually need to increase the dose of allopurinol significantly to keep the serum urate level below the desired target,” he said.
The approach is similar with low-dose aspirin. Because of its negligible effect on serum uric acid levels, it does not need to be stopped in hyperuricemia or gout. “Since patients with gout have a higher risk of having cardiovascular disease, metabolic syndrome, and chronic kidney disease, many will benefit from low-dose aspirin therapy,” he said.
Occasionally, it makes sense to switch from a thiazide to another hypertensive, such as losartan, in chronic, hypertensive gout patients with serum urate levels marginally above the precipitation threshold of 6.7 mg/dL. “Losartan is a weak uricosuric and can lower the serum urate level slightly, possibly making the addition of another hypouricemic agent unnecessary, while still controlling the blood pressure with a single pill,” Dr. Mandell said.
The decision “must be individualized, taking into consideration the efficacy and cost of the alternative antihypertensive drug, as well as the potential but as yet unproven cardiovascular and renal benefits of lowering the serum urate with a more potent hypouricemic to a degree not likely to be attained with losartan alone,” he said.
Dr. Mandell has been a consultant for Savient/Crealta, AstraZeneca, Regeneron, and Novartis. Global Academy for Medical Education and this news organization are owned by the same parent company.
It’s okay in most cases for gout patients to be on thiazide diuretics or low-dose aspirin, so long as hypouricemic therapy is adjusted as needed, according to rheumatologist and Cleveland Clinic professor Dr. Brian F. Mandell.
Coronary artery disease is common in gout, so patients benefit from inexpensive, well-tolerated, and effective treatments like thiazides for hypertension and 81 mg aspirin daily for cardioprotection, Dr. Mandell said at the annual Perspectives in Rheumatic Diseases conference held by Global Academy for Medical Education.
The concern, however, is that both can elevate serum uric acid. Observational and survey data suggest that the drugs may be associated with an increase in attacks, so some shy away from them in gout.
To make sense of the issue, the first thing to remember is that the drugs cause only “minimal elevations in serum uric acid. Serum urate can still be lowered to less than 6 mg/dL with appropriate therapy without stopping them,” Dr. Mandell said.
Low-dose aspirin raises serum urate by about 0.3 mg/dL. At 12.5 or 25 mg once a day – common hypertension doses – hydrochlorothiazide increases serum urate by 0.8 mg/dL or less in patients with normal renal function (Arthritis Rheum. 2012 Jan;64[1]:121-9).
“In patients with chronic gout treated with a xanthine oxidase inhibitor (allopurinol or febuxostat) to lower the serum urate” to recommended target levels below 6.0 mg/dL, “the small elevation in serum urate is unlikely to negate the clinical efficacy of these drugs when dosing is optimized,” Dr. Mandell wrote in an article that expands upon his presentation points (Cleve Clin J Med. 2014 Feb;81[2]:83-6).
Based on those considerations, “my practice in most patients is to use a thiazide if it helps to control the blood pressure and to adjust the dose of the hypouricemic therapy as needed to reduce the serum urate to the desired level. ... Continuing thiazide therapy and, if necessary, adjusting hypouricemic therapy will not worsen the control of the serum urate level or gouty arthritis, and in most patients will not complicate the management of gout.” In general, “when I add a thiazide to a patient’s regimen, I do not usually need to increase the dose of allopurinol significantly to keep the serum urate level below the desired target,” he said.
The approach is similar with low-dose aspirin. Because of its negligible effect on serum uric acid levels, it does not need to be stopped in hyperuricemia or gout. “Since patients with gout have a higher risk of having cardiovascular disease, metabolic syndrome, and chronic kidney disease, many will benefit from low-dose aspirin therapy,” he said.
Occasionally, it makes sense to switch from a thiazide to another hypertensive, such as losartan, in chronic, hypertensive gout patients with serum urate levels marginally above the precipitation threshold of 6.7 mg/dL. “Losartan is a weak uricosuric and can lower the serum urate level slightly, possibly making the addition of another hypouricemic agent unnecessary, while still controlling the blood pressure with a single pill,” Dr. Mandell said.
The decision “must be individualized, taking into consideration the efficacy and cost of the alternative antihypertensive drug, as well as the potential but as yet unproven cardiovascular and renal benefits of lowering the serum urate with a more potent hypouricemic to a degree not likely to be attained with losartan alone,” he said.
Dr. Mandell has been a consultant for Savient/Crealta, AstraZeneca, Regeneron, and Novartis. Global Academy for Medical Education and this news organization are owned by the same parent company.
It’s okay in most cases for gout patients to be on thiazide diuretics or low-dose aspirin, so long as hypouricemic therapy is adjusted as needed, according to rheumatologist and Cleveland Clinic professor Dr. Brian F. Mandell.
Coronary artery disease is common in gout, so patients benefit from inexpensive, well-tolerated, and effective treatments like thiazides for hypertension and 81 mg aspirin daily for cardioprotection, Dr. Mandell said at the annual Perspectives in Rheumatic Diseases conference held by Global Academy for Medical Education.
The concern, however, is that both can elevate serum uric acid. Observational and survey data suggest that the drugs may be associated with an increase in attacks, so some shy away from them in gout.
To make sense of the issue, the first thing to remember is that the drugs cause only “minimal elevations in serum uric acid. Serum urate can still be lowered to less than 6 mg/dL with appropriate therapy without stopping them,” Dr. Mandell said.
Low-dose aspirin raises serum urate by about 0.3 mg/dL. At 12.5 or 25 mg once a day – common hypertension doses – hydrochlorothiazide increases serum urate by 0.8 mg/dL or less in patients with normal renal function (Arthritis Rheum. 2012 Jan;64[1]:121-9).
“In patients with chronic gout treated with a xanthine oxidase inhibitor (allopurinol or febuxostat) to lower the serum urate” to recommended target levels below 6.0 mg/dL, “the small elevation in serum urate is unlikely to negate the clinical efficacy of these drugs when dosing is optimized,” Dr. Mandell wrote in an article that expands upon his presentation points (Cleve Clin J Med. 2014 Feb;81[2]:83-6).
Based on those considerations, “my practice in most patients is to use a thiazide if it helps to control the blood pressure and to adjust the dose of the hypouricemic therapy as needed to reduce the serum urate to the desired level. ... Continuing thiazide therapy and, if necessary, adjusting hypouricemic therapy will not worsen the control of the serum urate level or gouty arthritis, and in most patients will not complicate the management of gout.” In general, “when I add a thiazide to a patient’s regimen, I do not usually need to increase the dose of allopurinol significantly to keep the serum urate level below the desired target,” he said.
The approach is similar with low-dose aspirin. Because of its negligible effect on serum uric acid levels, it does not need to be stopped in hyperuricemia or gout. “Since patients with gout have a higher risk of having cardiovascular disease, metabolic syndrome, and chronic kidney disease, many will benefit from low-dose aspirin therapy,” he said.
Occasionally, it makes sense to switch from a thiazide to another hypertensive, such as losartan, in chronic, hypertensive gout patients with serum urate levels marginally above the precipitation threshold of 6.7 mg/dL. “Losartan is a weak uricosuric and can lower the serum urate level slightly, possibly making the addition of another hypouricemic agent unnecessary, while still controlling the blood pressure with a single pill,” Dr. Mandell said.
The decision “must be individualized, taking into consideration the efficacy and cost of the alternative antihypertensive drug, as well as the potential but as yet unproven cardiovascular and renal benefits of lowering the serum urate with a more potent hypouricemic to a degree not likely to be attained with losartan alone,” he said.
Dr. Mandell has been a consultant for Savient/Crealta, AstraZeneca, Regeneron, and Novartis. Global Academy for Medical Education and this news organization are owned by the same parent company.
EXPERT ANALYSIS FROM THE ANNUAL PERSPECTIVES IN RHEUMATIC DISEASES
Traffic-related pollution linked to AML, not ALL, in kids

A French study has revealed an increased incidence of acute myeloid leukemia (AML) among children living close to heavily used roads.
The incidence of AML was 30% higher among children who lived within 150 m of heavily used roads and where the combined length of road sections
within this radius exceeded 260 m.
The researchers believe the association between AML and road proximity may be driven by traffic-related benzene exposure.
Previous research has shown an increased risk of leukemia among adults with a history of occupational exposure to benzene.
The current study did not suggest an increased risk of acute lymphoblastic leukemia (ALL) among children living closed to heavily used roads.
Jacqueline Clavel, MD, PhD, of INSERM in Paris, France, and her colleagues reported these findings in the American Journal of Epidemiology.
The team analyzed 2760 cases of leukemia diagnosed in children younger than 15 years of age in metropolitan France between 2002 and 2007, including 418 cases of AML and 2275 cases of ALL.
The researchers compared these cases to a contemporary sample of 30,000 control children representative of the metropolitan population.
The data showed that neither distance from the nearest major road(s) nor the length of major roads within 150 m of a child’s residence was associated with ALL.
However, there was an association for AML. For children whose home was less than 150 m from the nearest major road(s), the odds ratio (OR) was 1.2.
When the total length of major road(s) within 150 m from the child’s residence was 257-308 m (second tertile) or 309 m or greater (third tertile), the OR was 1.3. When the total length of major roads was 1-256 m (first tertile), the OR was 0.90.
The researchers noted that traffic-related nitrogen dioxide concentration was not associated with ALL or AML. But their data indicated that benzene concentration was associated with AML.
To assess this potential association, the team studied the Île-de-France region of Paris, the most urbanized region, for which the mean annual concentration of benzene, mainly from road traffic, was estimated in the vicinity of each residence.
The median estimated benzene concentration for controls living in the Île-de-France region was 1.3 μg/m3 (range, 0.3 to 8.5 μg/m3). And the length of major roads within 150 m of a child’s residence was positively and significantly correlated with log benzene concentration (r=0.3, P<0.001).
So it followed that exposure to an estimated benzene concentration greater than the median was associated with AML (OR=1.6).
The researchers also used a composite variable based on the estimated benzene concentration and the length of major roads around a child’s residence.
The association with AML was largest among children with at least 309 m of major roads within 150 m of their residence and estimated benzene concentrations of 1.3 μg/m3 or greater (OR=2.2).
The researchers said these results support a role for traffic-related benzene exposure in the etiology of childhood AML. ![]()

A French study has revealed an increased incidence of acute myeloid leukemia (AML) among children living close to heavily used roads.
The incidence of AML was 30% higher among children who lived within 150 m of heavily used roads and where the combined length of road sections
within this radius exceeded 260 m.
The researchers believe the association between AML and road proximity may be driven by traffic-related benzene exposure.
Previous research has shown an increased risk of leukemia among adults with a history of occupational exposure to benzene.
The current study did not suggest an increased risk of acute lymphoblastic leukemia (ALL) among children living closed to heavily used roads.
Jacqueline Clavel, MD, PhD, of INSERM in Paris, France, and her colleagues reported these findings in the American Journal of Epidemiology.
The team analyzed 2760 cases of leukemia diagnosed in children younger than 15 years of age in metropolitan France between 2002 and 2007, including 418 cases of AML and 2275 cases of ALL.
The researchers compared these cases to a contemporary sample of 30,000 control children representative of the metropolitan population.
The data showed that neither distance from the nearest major road(s) nor the length of major roads within 150 m of a child’s residence was associated with ALL.
However, there was an association for AML. For children whose home was less than 150 m from the nearest major road(s), the odds ratio (OR) was 1.2.
When the total length of major road(s) within 150 m from the child’s residence was 257-308 m (second tertile) or 309 m or greater (third tertile), the OR was 1.3. When the total length of major roads was 1-256 m (first tertile), the OR was 0.90.
The researchers noted that traffic-related nitrogen dioxide concentration was not associated with ALL or AML. But their data indicated that benzene concentration was associated with AML.
To assess this potential association, the team studied the Île-de-France region of Paris, the most urbanized region, for which the mean annual concentration of benzene, mainly from road traffic, was estimated in the vicinity of each residence.
The median estimated benzene concentration for controls living in the Île-de-France region was 1.3 μg/m3 (range, 0.3 to 8.5 μg/m3). And the length of major roads within 150 m of a child’s residence was positively and significantly correlated with log benzene concentration (r=0.3, P<0.001).
So it followed that exposure to an estimated benzene concentration greater than the median was associated with AML (OR=1.6).
The researchers also used a composite variable based on the estimated benzene concentration and the length of major roads around a child’s residence.
The association with AML was largest among children with at least 309 m of major roads within 150 m of their residence and estimated benzene concentrations of 1.3 μg/m3 or greater (OR=2.2).
The researchers said these results support a role for traffic-related benzene exposure in the etiology of childhood AML. ![]()

A French study has revealed an increased incidence of acute myeloid leukemia (AML) among children living close to heavily used roads.
The incidence of AML was 30% higher among children who lived within 150 m of heavily used roads and where the combined length of road sections
within this radius exceeded 260 m.
The researchers believe the association between AML and road proximity may be driven by traffic-related benzene exposure.
Previous research has shown an increased risk of leukemia among adults with a history of occupational exposure to benzene.
The current study did not suggest an increased risk of acute lymphoblastic leukemia (ALL) among children living closed to heavily used roads.
Jacqueline Clavel, MD, PhD, of INSERM in Paris, France, and her colleagues reported these findings in the American Journal of Epidemiology.
The team analyzed 2760 cases of leukemia diagnosed in children younger than 15 years of age in metropolitan France between 2002 and 2007, including 418 cases of AML and 2275 cases of ALL.
The researchers compared these cases to a contemporary sample of 30,000 control children representative of the metropolitan population.
The data showed that neither distance from the nearest major road(s) nor the length of major roads within 150 m of a child’s residence was associated with ALL.
However, there was an association for AML. For children whose home was less than 150 m from the nearest major road(s), the odds ratio (OR) was 1.2.
When the total length of major road(s) within 150 m from the child’s residence was 257-308 m (second tertile) or 309 m or greater (third tertile), the OR was 1.3. When the total length of major roads was 1-256 m (first tertile), the OR was 0.90.
The researchers noted that traffic-related nitrogen dioxide concentration was not associated with ALL or AML. But their data indicated that benzene concentration was associated with AML.
To assess this potential association, the team studied the Île-de-France region of Paris, the most urbanized region, for which the mean annual concentration of benzene, mainly from road traffic, was estimated in the vicinity of each residence.
The median estimated benzene concentration for controls living in the Île-de-France region was 1.3 μg/m3 (range, 0.3 to 8.5 μg/m3). And the length of major roads within 150 m of a child’s residence was positively and significantly correlated with log benzene concentration (r=0.3, P<0.001).
So it followed that exposure to an estimated benzene concentration greater than the median was associated with AML (OR=1.6).
The researchers also used a composite variable based on the estimated benzene concentration and the length of major roads around a child’s residence.
The association with AML was largest among children with at least 309 m of major roads within 150 m of their residence and estimated benzene concentrations of 1.3 μg/m3 or greater (OR=2.2).
The researchers said these results support a role for traffic-related benzene exposure in the etiology of childhood AML. ![]()