User login
Reovirus ready for clinical testing in CLL
Reovirus, a naturally occurring oncolytic virus, appears to exert direct cytotoxic activity against chronic lymphocytic leukemia (CLL) and “phenotypically and functionally” activates patient natural killer cells using a monocyte-derived interferon alpha–dependent mechanism, according to preclinical data published in Leukemia.
Reovirus also enhances antibody-dependent cellular cytotoxicity – mediated killing of CLL cells in combination with anti-CD20 antibodies.
“Reovirus together with anti-CD20 antibodies represents a promising combination strategy for the treatment of CLL … and now warrants clinical evaluation,” wrote Christopher Parrish, Ph.D., of the Leeds (England) Institute of Cancer and Pathology and his colleagues (Leukemia. 2015;29:1799-1810. doi: 10.1038/leu.2015.88).Reovirus is a naturally occurring double-stranded RNA virus, and it exerts its effects against cancer cells by direct oncolysis and activation of antitumor immunity. The researchers investigated the efficacy of reovirus for the treatment of CLL, both as a direct cytotoxic agent and as an immunomodulator, using CLL cell lines and primary CLL cells from 24 patients.
Dr. Parrish and his team treated human CLL cells with live or UV-inactivated reovirus for 7 days. They assessed the ability of reovirus to stimulate immune-mediated killing of CLL using peripheral blood mononuclear cells from healthy volunteers and CLL patients. Reovirus activated natural killer (NK) cells from CLL patients, as well as stimulating innate antitumor immunity.
Rituximab, which is believed to act in part via NK cell–mediated antibody-dependent cellular cytotoxicity, was added to peripheral blood mononuclear cells that were treated with reovirus. Compared with rituximab alone, reovirus treatment significantly increased NK-cell CD107a/b degranulation. Reovirus was then paired with ofatumumab and GA101 to see if the effects observed with reovirus/rituximab could be translated to other anti-CD20 antibodies in which NK cells also play a role. The results were similar.
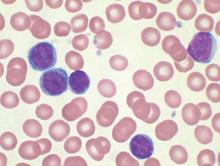
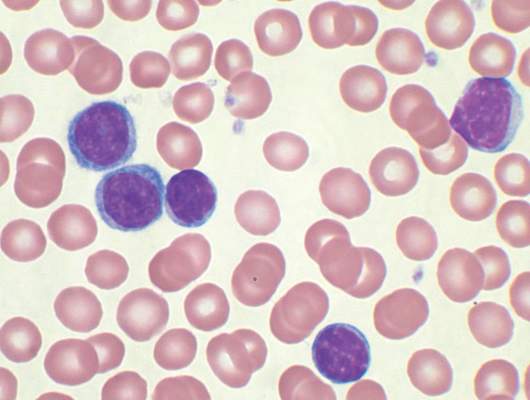
Absolute monocyte count and the type 1 interferon-alpha response could be used to predict the generation of antitumor innate immunity by reovirus. In cells from 24 CLL patients, about 75% responded to reovirus, with NK-cell activation. Further, NK-cell activation correlated with absolute monocyte count. The role of interferon-alpha is further supported by identification of an interferon gene signature within NK cells from reovirus-treated patients, the researchers said.
The study was supported by Yorkshire Cancer Research and Cancer Research UK. One of the investigators is an employee of Oncolytics Biotech and holds company stock and options. All the other authors declared no conflicts of interest.
Reovirus, a naturally occurring oncolytic virus, appears to exert direct cytotoxic activity against chronic lymphocytic leukemia (CLL) and “phenotypically and functionally” activates patient natural killer cells using a monocyte-derived interferon alpha–dependent mechanism, according to preclinical data published in Leukemia.
Reovirus also enhances antibody-dependent cellular cytotoxicity – mediated killing of CLL cells in combination with anti-CD20 antibodies.
“Reovirus together with anti-CD20 antibodies represents a promising combination strategy for the treatment of CLL … and now warrants clinical evaluation,” wrote Christopher Parrish, Ph.D., of the Leeds (England) Institute of Cancer and Pathology and his colleagues (Leukemia. 2015;29:1799-1810. doi: 10.1038/leu.2015.88).Reovirus is a naturally occurring double-stranded RNA virus, and it exerts its effects against cancer cells by direct oncolysis and activation of antitumor immunity. The researchers investigated the efficacy of reovirus for the treatment of CLL, both as a direct cytotoxic agent and as an immunomodulator, using CLL cell lines and primary CLL cells from 24 patients.
Dr. Parrish and his team treated human CLL cells with live or UV-inactivated reovirus for 7 days. They assessed the ability of reovirus to stimulate immune-mediated killing of CLL using peripheral blood mononuclear cells from healthy volunteers and CLL patients. Reovirus activated natural killer (NK) cells from CLL patients, as well as stimulating innate antitumor immunity.
Rituximab, which is believed to act in part via NK cell–mediated antibody-dependent cellular cytotoxicity, was added to peripheral blood mononuclear cells that were treated with reovirus. Compared with rituximab alone, reovirus treatment significantly increased NK-cell CD107a/b degranulation. Reovirus was then paired with ofatumumab and GA101 to see if the effects observed with reovirus/rituximab could be translated to other anti-CD20 antibodies in which NK cells also play a role. The results were similar.
Absolute monocyte count and the type 1 interferon-alpha response could be used to predict the generation of antitumor innate immunity by reovirus. In cells from 24 CLL patients, about 75% responded to reovirus, with NK-cell activation. Further, NK-cell activation correlated with absolute monocyte count. The role of interferon-alpha is further supported by identification of an interferon gene signature within NK cells from reovirus-treated patients, the researchers said.
The study was supported by Yorkshire Cancer Research and Cancer Research UK. One of the investigators is an employee of Oncolytics Biotech and holds company stock and options. All the other authors declared no conflicts of interest.
Reovirus, a naturally occurring oncolytic virus, appears to exert direct cytotoxic activity against chronic lymphocytic leukemia (CLL) and “phenotypically and functionally” activates patient natural killer cells using a monocyte-derived interferon alpha–dependent mechanism, according to preclinical data published in Leukemia.
Reovirus also enhances antibody-dependent cellular cytotoxicity – mediated killing of CLL cells in combination with anti-CD20 antibodies.
“Reovirus together with anti-CD20 antibodies represents a promising combination strategy for the treatment of CLL … and now warrants clinical evaluation,” wrote Christopher Parrish, Ph.D., of the Leeds (England) Institute of Cancer and Pathology and his colleagues (Leukemia. 2015;29:1799-1810. doi: 10.1038/leu.2015.88).Reovirus is a naturally occurring double-stranded RNA virus, and it exerts its effects against cancer cells by direct oncolysis and activation of antitumor immunity. The researchers investigated the efficacy of reovirus for the treatment of CLL, both as a direct cytotoxic agent and as an immunomodulator, using CLL cell lines and primary CLL cells from 24 patients.
Dr. Parrish and his team treated human CLL cells with live or UV-inactivated reovirus for 7 days. They assessed the ability of reovirus to stimulate immune-mediated killing of CLL using peripheral blood mononuclear cells from healthy volunteers and CLL patients. Reovirus activated natural killer (NK) cells from CLL patients, as well as stimulating innate antitumor immunity.
Rituximab, which is believed to act in part via NK cell–mediated antibody-dependent cellular cytotoxicity, was added to peripheral blood mononuclear cells that were treated with reovirus. Compared with rituximab alone, reovirus treatment significantly increased NK-cell CD107a/b degranulation. Reovirus was then paired with ofatumumab and GA101 to see if the effects observed with reovirus/rituximab could be translated to other anti-CD20 antibodies in which NK cells also play a role. The results were similar.
Absolute monocyte count and the type 1 interferon-alpha response could be used to predict the generation of antitumor innate immunity by reovirus. In cells from 24 CLL patients, about 75% responded to reovirus, with NK-cell activation. Further, NK-cell activation correlated with absolute monocyte count. The role of interferon-alpha is further supported by identification of an interferon gene signature within NK cells from reovirus-treated patients, the researchers said.
The study was supported by Yorkshire Cancer Research and Cancer Research UK. One of the investigators is an employee of Oncolytics Biotech and holds company stock and options. All the other authors declared no conflicts of interest.
FROM LEUKEMIA
Key clinical point: Reovirus, a naturally occurring oncolytic virus, in combination with anti-CD20 immunotherapy, may have benefit for the treatment of chronic lymphocytic leukemia.
Major finding: In cells from 24 CLL patients, about 75% responded to reovirus, with natural killer cell activation.
Data source: Preclinical trial that used human CLL lines and murine L929 cells.
Disclosures: The study was supported by Yorkshire Cancer Research and Cancer Research UK. One of the investigators is an employee of Oncolytics Biotech and holds company stock and options. All the other authors declared no conflicts of interest.
CAR T-cell therapy tested in Sweden

NEW YORK—For the first time, according to researchers, chimeric antigen receptor (CAR) T-cell therapy has been tested in a clinical trial in Sweden.
Early results have shown the treatment can produce complete responses (CRs) in leukemia and lymphoma, although most patients ultimately progressed.
Hannah Karlsson, PhD, of Uppsala University in Sweden, presented data from the phase 1/2a trial of the third-generation CD19 CAR T-cell therapy (abstract A041*) at the inaugural CRI-CIMT-EATI-AACR International Cancer Immunotherapy Conference.
The trial is a collaboration between Uppsala University and Baylor College of Medicine and was funded by AFA Insurances AB and the Swedish Cancer Society.
“Third-generation CAR T cells are being tested in clinical trials for leukemia patients in the United States with success,” said senior study author Angelica Loskog, PhD, also of Uppsala University.
“[T]he main purpose of our clinical trial was to evaluate whether we could reproduce the successful results in leukemia patients in Sweden and to also test if patients with lymphoma will also respond to this treatment.”
So the investigators enrolled 13 patients, 11 of whom were evaluable for efficacy at 3 months after CAR T-cell infusion. All patients had relapsed or refractory, CD19-positive, B-cell disease.
Two patients had acute lymphoblastic leukemia (ALL), 2 had chronic lymphocytic leukemia (CLL), and 7 had lymphoma—3 with diffuse large B-cell lymphoma (DLBCL), 2 with mantle cell lymphoma (MCL), 1 with follicular lymphoma (FL)/DLBCL, and 1 with Burkitt lymphoma.
All of the lymphoma patients received chemotherapy before CAR T-cell infusion to shrink their tumors. Seven patients—3 with leukemia and 4 with lymphoma—received pre-conditioning with cyclophosphamide plus fludarabine to reduce their immunosuppressive cell counts.
The investigators used CAR T cells containing signaling domains from both CD28 and 4-1BB and manufactured using a gamma retrovirus.
Patients received a single infusion of the CAR T cells, 2 patients at a dose of 2 x 107 cells/m2, 4 at a dose of 1 x 108 cells/m2, and 5 at 2 x 108 cells/m2.
Response and toxicity
Six patients had achieved a CR at the time of evaluation.
One patient with DLBCL experienced mild cytokine release syndrome (CRS) before achieving CR. However, the patient relapsed after a second CRS occurred (after 3 months).
Another DLBCL patient achieved a CR prior to T-cell infusion and remained in CR for 6 months before progressing.
One CLL patient and another DLBCL patient responded prior to T-cell infusion and remained in CR for more than 3 months. The CLL patient was still in CR at the time of the meeting.
One of the ALL patients achieved a CR after transient central nervous system toxicity but relapsed at 3 months with CD19-negative ALL. The other ALL patient was in CR for more than a month after experiencing CRS but ultimately progressed.
One CLL patient and 2 MCL patients had all progressed by 3 months.
The FL/DLBCL patient progressed after 1 month, with mild CRS. And the patient with Burkitt lymphoma had major CRS and progressive disease.
The investigators noted that 5 of the 6 patients who received pre-conditioning treatment had initial CRs.
The team is now analyzing whether there is any correlation between the level of immunosuppressive cells and patient response. ![]()
*Information presented at the meeting differs from the abstract.

NEW YORK—For the first time, according to researchers, chimeric antigen receptor (CAR) T-cell therapy has been tested in a clinical trial in Sweden.
Early results have shown the treatment can produce complete responses (CRs) in leukemia and lymphoma, although most patients ultimately progressed.
Hannah Karlsson, PhD, of Uppsala University in Sweden, presented data from the phase 1/2a trial of the third-generation CD19 CAR T-cell therapy (abstract A041*) at the inaugural CRI-CIMT-EATI-AACR International Cancer Immunotherapy Conference.
The trial is a collaboration between Uppsala University and Baylor College of Medicine and was funded by AFA Insurances AB and the Swedish Cancer Society.
“Third-generation CAR T cells are being tested in clinical trials for leukemia patients in the United States with success,” said senior study author Angelica Loskog, PhD, also of Uppsala University.
“[T]he main purpose of our clinical trial was to evaluate whether we could reproduce the successful results in leukemia patients in Sweden and to also test if patients with lymphoma will also respond to this treatment.”
So the investigators enrolled 13 patients, 11 of whom were evaluable for efficacy at 3 months after CAR T-cell infusion. All patients had relapsed or refractory, CD19-positive, B-cell disease.
Two patients had acute lymphoblastic leukemia (ALL), 2 had chronic lymphocytic leukemia (CLL), and 7 had lymphoma—3 with diffuse large B-cell lymphoma (DLBCL), 2 with mantle cell lymphoma (MCL), 1 with follicular lymphoma (FL)/DLBCL, and 1 with Burkitt lymphoma.
All of the lymphoma patients received chemotherapy before CAR T-cell infusion to shrink their tumors. Seven patients—3 with leukemia and 4 with lymphoma—received pre-conditioning with cyclophosphamide plus fludarabine to reduce their immunosuppressive cell counts.
The investigators used CAR T cells containing signaling domains from both CD28 and 4-1BB and manufactured using a gamma retrovirus.
Patients received a single infusion of the CAR T cells, 2 patients at a dose of 2 x 107 cells/m2, 4 at a dose of 1 x 108 cells/m2, and 5 at 2 x 108 cells/m2.
Response and toxicity
Six patients had achieved a CR at the time of evaluation.
One patient with DLBCL experienced mild cytokine release syndrome (CRS) before achieving CR. However, the patient relapsed after a second CRS occurred (after 3 months).
Another DLBCL patient achieved a CR prior to T-cell infusion and remained in CR for 6 months before progressing.
One CLL patient and another DLBCL patient responded prior to T-cell infusion and remained in CR for more than 3 months. The CLL patient was still in CR at the time of the meeting.
One of the ALL patients achieved a CR after transient central nervous system toxicity but relapsed at 3 months with CD19-negative ALL. The other ALL patient was in CR for more than a month after experiencing CRS but ultimately progressed.
One CLL patient and 2 MCL patients had all progressed by 3 months.
The FL/DLBCL patient progressed after 1 month, with mild CRS. And the patient with Burkitt lymphoma had major CRS and progressive disease.
The investigators noted that 5 of the 6 patients who received pre-conditioning treatment had initial CRs.
The team is now analyzing whether there is any correlation between the level of immunosuppressive cells and patient response. ![]()
*Information presented at the meeting differs from the abstract.

NEW YORK—For the first time, according to researchers, chimeric antigen receptor (CAR) T-cell therapy has been tested in a clinical trial in Sweden.
Early results have shown the treatment can produce complete responses (CRs) in leukemia and lymphoma, although most patients ultimately progressed.
Hannah Karlsson, PhD, of Uppsala University in Sweden, presented data from the phase 1/2a trial of the third-generation CD19 CAR T-cell therapy (abstract A041*) at the inaugural CRI-CIMT-EATI-AACR International Cancer Immunotherapy Conference.
The trial is a collaboration between Uppsala University and Baylor College of Medicine and was funded by AFA Insurances AB and the Swedish Cancer Society.
“Third-generation CAR T cells are being tested in clinical trials for leukemia patients in the United States with success,” said senior study author Angelica Loskog, PhD, also of Uppsala University.
“[T]he main purpose of our clinical trial was to evaluate whether we could reproduce the successful results in leukemia patients in Sweden and to also test if patients with lymphoma will also respond to this treatment.”
So the investigators enrolled 13 patients, 11 of whom were evaluable for efficacy at 3 months after CAR T-cell infusion. All patients had relapsed or refractory, CD19-positive, B-cell disease.
Two patients had acute lymphoblastic leukemia (ALL), 2 had chronic lymphocytic leukemia (CLL), and 7 had lymphoma—3 with diffuse large B-cell lymphoma (DLBCL), 2 with mantle cell lymphoma (MCL), 1 with follicular lymphoma (FL)/DLBCL, and 1 with Burkitt lymphoma.
All of the lymphoma patients received chemotherapy before CAR T-cell infusion to shrink their tumors. Seven patients—3 with leukemia and 4 with lymphoma—received pre-conditioning with cyclophosphamide plus fludarabine to reduce their immunosuppressive cell counts.
The investigators used CAR T cells containing signaling domains from both CD28 and 4-1BB and manufactured using a gamma retrovirus.
Patients received a single infusion of the CAR T cells, 2 patients at a dose of 2 x 107 cells/m2, 4 at a dose of 1 x 108 cells/m2, and 5 at 2 x 108 cells/m2.
Response and toxicity
Six patients had achieved a CR at the time of evaluation.
One patient with DLBCL experienced mild cytokine release syndrome (CRS) before achieving CR. However, the patient relapsed after a second CRS occurred (after 3 months).
Another DLBCL patient achieved a CR prior to T-cell infusion and remained in CR for 6 months before progressing.
One CLL patient and another DLBCL patient responded prior to T-cell infusion and remained in CR for more than 3 months. The CLL patient was still in CR at the time of the meeting.
One of the ALL patients achieved a CR after transient central nervous system toxicity but relapsed at 3 months with CD19-negative ALL. The other ALL patient was in CR for more than a month after experiencing CRS but ultimately progressed.
One CLL patient and 2 MCL patients had all progressed by 3 months.
The FL/DLBCL patient progressed after 1 month, with mild CRS. And the patient with Burkitt lymphoma had major CRS and progressive disease.
The investigators noted that 5 of the 6 patients who received pre-conditioning treatment had initial CRs.
The team is now analyzing whether there is any correlation between the level of immunosuppressive cells and patient response. ![]()
*Information presented at the meeting differs from the abstract.
Chemo-free transplant can cure SCD, team says

Photo by Chad McNeeley
Chemotherapy-free allogeneic transplant can cure sickle cell disease (SCD) in adults, according to researchers.
In a phase 1/2 trial, the treatment normalized hemoglobin concentrations, reduced SCD-related complications, and improved cardiopulmonary function in 12 of 13 patients.
The single graft failure was due to noncompliance with post-transplant treatment.
There were no deaths and no cases of graft-vs-host disease. However, most patients did experience some form of transplant-related toxicity.
These transplants were performed at the University of Illinois Hospital & Health Sciences System in Chicago. But the chemotherapy-free transplant regimen was developed—and initially tested—at the National Institutes of Health in Bethesda, Maryland.
Physicians there have treated 30 patients with the regimen. An account of that work was published in JAMA last year.
The current study has been published in Biology of Blood & Marrow Transplantation.
“Adults with sickle cell disease can be cured without chemotherapy—the main barrier that has stood in the way for them for so long,” said study author Damiano Rondelli, MD, of the University of Illinois Hospital & Health Sciences System.
“Our data provide more support that this therapy is safe and effective and prevents patients from living shortened lives, condemned to pain and progressive complications.”
Treatment and outcome
The study included 13 patients, ages 17 to 40, who were transplanted between November 2011 and June 2014. Prior to transplant, patients received alemtuzumab and total-body irradiation (300 cGy).
They then received peripheral blood stem cells from matched related donors. All donors were a 10/10 human leukocyte antigen match, but 2 donors had different blood types than the recipients. After transplant, the patients received sirolimus.
All 13 patients initially engrafted, but 1 patient experienced secondary graft failure due to noncompliance with sirolimus.
At a median follow-up of 22 months (range, 12-44), all 13 patients are alive, and 12 have maintained a stable mixed donor/recipient chimerism.
At 1 year after transplant, the 12 patients with stable donor chimerism had significant improvements from baseline in hemoglobin, reticulocyte percentage, lactate dehydrogenase concentration, and cardiopulmonary function.
One of these patients required readmission to the hospital for vaso-occlusive crisis. Before transplant, this patient experienced about 12 crises a year.
No other SCD-related complications have occurred. And 4 patients have been able to stop taking sirolimus without transplant rejection or other complications.
Nine of the engrafted patients completed quality of life assessments before transplant and at 1 year after the procedure. They reported improvements in pain, general health, vitality, and social functioning.
“[W]ith this chemotherapy-free transplant, we are curing adults with sickle cell disease, and we see that their quality of life improves vastly within just 1 month of the transplant,” Dr Rondelli said. “They are able to go back to school, go back to work, and can experience life without pain.”
Toxicity
Four patients did not experience any transplant-related toxicity. And there were no cases of acute or chronic graft-vs-host disease.
One patient developed gram-negative rods in her hip prosthesis after transplant, 1 patient experienced delayed hemolytic transfusion reaction after exchange transfusion, 1 patient developed viral pharyngitis, and 1 patient developed Coxsackie B.
One patient developed a urinary tract infection due to extended spectrum beta-lactamase, Clostridium difficile colitis, and Mycoplasma pneumoniae.
Two patients had grade 2 mucositis, one of whom also developed line-associated deep vein thrombosis and cytomegalovirus (CMV) reactivation. Two other patients had CMV reactivation as well, one of whom also had methicillin-resistant Staphylococcus aureus pneumonia.
All 3 patients with CMV reactivation were successfully treated with valgancyclovir and did not develop CMV disease.
Six patients had arthralgias attributed to sirolimus, and 2 of them required dose reductions.
One patient developed chest pain and a decline in carbon monoxide diffusion capacity by 30% that was attributed to sirolimus. The patient was switched to cyclosporine but developed posterior reversible encephalopathy syndrome and was then put on mycophenolate mofetil.
The patient has since maintained stable donor chimerism, and the carbon monoxide diffusing capacity has increased to near-baseline value. ![]()

Photo by Chad McNeeley
Chemotherapy-free allogeneic transplant can cure sickle cell disease (SCD) in adults, according to researchers.
In a phase 1/2 trial, the treatment normalized hemoglobin concentrations, reduced SCD-related complications, and improved cardiopulmonary function in 12 of 13 patients.
The single graft failure was due to noncompliance with post-transplant treatment.
There were no deaths and no cases of graft-vs-host disease. However, most patients did experience some form of transplant-related toxicity.
These transplants were performed at the University of Illinois Hospital & Health Sciences System in Chicago. But the chemotherapy-free transplant regimen was developed—and initially tested—at the National Institutes of Health in Bethesda, Maryland.
Physicians there have treated 30 patients with the regimen. An account of that work was published in JAMA last year.
The current study has been published in Biology of Blood & Marrow Transplantation.
“Adults with sickle cell disease can be cured without chemotherapy—the main barrier that has stood in the way for them for so long,” said study author Damiano Rondelli, MD, of the University of Illinois Hospital & Health Sciences System.
“Our data provide more support that this therapy is safe and effective and prevents patients from living shortened lives, condemned to pain and progressive complications.”
Treatment and outcome
The study included 13 patients, ages 17 to 40, who were transplanted between November 2011 and June 2014. Prior to transplant, patients received alemtuzumab and total-body irradiation (300 cGy).
They then received peripheral blood stem cells from matched related donors. All donors were a 10/10 human leukocyte antigen match, but 2 donors had different blood types than the recipients. After transplant, the patients received sirolimus.
All 13 patients initially engrafted, but 1 patient experienced secondary graft failure due to noncompliance with sirolimus.
At a median follow-up of 22 months (range, 12-44), all 13 patients are alive, and 12 have maintained a stable mixed donor/recipient chimerism.
At 1 year after transplant, the 12 patients with stable donor chimerism had significant improvements from baseline in hemoglobin, reticulocyte percentage, lactate dehydrogenase concentration, and cardiopulmonary function.
One of these patients required readmission to the hospital for vaso-occlusive crisis. Before transplant, this patient experienced about 12 crises a year.
No other SCD-related complications have occurred. And 4 patients have been able to stop taking sirolimus without transplant rejection or other complications.
Nine of the engrafted patients completed quality of life assessments before transplant and at 1 year after the procedure. They reported improvements in pain, general health, vitality, and social functioning.
“[W]ith this chemotherapy-free transplant, we are curing adults with sickle cell disease, and we see that their quality of life improves vastly within just 1 month of the transplant,” Dr Rondelli said. “They are able to go back to school, go back to work, and can experience life without pain.”
Toxicity
Four patients did not experience any transplant-related toxicity. And there were no cases of acute or chronic graft-vs-host disease.
One patient developed gram-negative rods in her hip prosthesis after transplant, 1 patient experienced delayed hemolytic transfusion reaction after exchange transfusion, 1 patient developed viral pharyngitis, and 1 patient developed Coxsackie B.
One patient developed a urinary tract infection due to extended spectrum beta-lactamase, Clostridium difficile colitis, and Mycoplasma pneumoniae.
Two patients had grade 2 mucositis, one of whom also developed line-associated deep vein thrombosis and cytomegalovirus (CMV) reactivation. Two other patients had CMV reactivation as well, one of whom also had methicillin-resistant Staphylococcus aureus pneumonia.
All 3 patients with CMV reactivation were successfully treated with valgancyclovir and did not develop CMV disease.
Six patients had arthralgias attributed to sirolimus, and 2 of them required dose reductions.
One patient developed chest pain and a decline in carbon monoxide diffusion capacity by 30% that was attributed to sirolimus. The patient was switched to cyclosporine but developed posterior reversible encephalopathy syndrome and was then put on mycophenolate mofetil.
The patient has since maintained stable donor chimerism, and the carbon monoxide diffusing capacity has increased to near-baseline value. ![]()

Photo by Chad McNeeley
Chemotherapy-free allogeneic transplant can cure sickle cell disease (SCD) in adults, according to researchers.
In a phase 1/2 trial, the treatment normalized hemoglobin concentrations, reduced SCD-related complications, and improved cardiopulmonary function in 12 of 13 patients.
The single graft failure was due to noncompliance with post-transplant treatment.
There were no deaths and no cases of graft-vs-host disease. However, most patients did experience some form of transplant-related toxicity.
These transplants were performed at the University of Illinois Hospital & Health Sciences System in Chicago. But the chemotherapy-free transplant regimen was developed—and initially tested—at the National Institutes of Health in Bethesda, Maryland.
Physicians there have treated 30 patients with the regimen. An account of that work was published in JAMA last year.
The current study has been published in Biology of Blood & Marrow Transplantation.
“Adults with sickle cell disease can be cured without chemotherapy—the main barrier that has stood in the way for them for so long,” said study author Damiano Rondelli, MD, of the University of Illinois Hospital & Health Sciences System.
“Our data provide more support that this therapy is safe and effective and prevents patients from living shortened lives, condemned to pain and progressive complications.”
Treatment and outcome
The study included 13 patients, ages 17 to 40, who were transplanted between November 2011 and June 2014. Prior to transplant, patients received alemtuzumab and total-body irradiation (300 cGy).
They then received peripheral blood stem cells from matched related donors. All donors were a 10/10 human leukocyte antigen match, but 2 donors had different blood types than the recipients. After transplant, the patients received sirolimus.
All 13 patients initially engrafted, but 1 patient experienced secondary graft failure due to noncompliance with sirolimus.
At a median follow-up of 22 months (range, 12-44), all 13 patients are alive, and 12 have maintained a stable mixed donor/recipient chimerism.
At 1 year after transplant, the 12 patients with stable donor chimerism had significant improvements from baseline in hemoglobin, reticulocyte percentage, lactate dehydrogenase concentration, and cardiopulmonary function.
One of these patients required readmission to the hospital for vaso-occlusive crisis. Before transplant, this patient experienced about 12 crises a year.
No other SCD-related complications have occurred. And 4 patients have been able to stop taking sirolimus without transplant rejection or other complications.
Nine of the engrafted patients completed quality of life assessments before transplant and at 1 year after the procedure. They reported improvements in pain, general health, vitality, and social functioning.
“[W]ith this chemotherapy-free transplant, we are curing adults with sickle cell disease, and we see that their quality of life improves vastly within just 1 month of the transplant,” Dr Rondelli said. “They are able to go back to school, go back to work, and can experience life without pain.”
Toxicity
Four patients did not experience any transplant-related toxicity. And there were no cases of acute or chronic graft-vs-host disease.
One patient developed gram-negative rods in her hip prosthesis after transplant, 1 patient experienced delayed hemolytic transfusion reaction after exchange transfusion, 1 patient developed viral pharyngitis, and 1 patient developed Coxsackie B.
One patient developed a urinary tract infection due to extended spectrum beta-lactamase, Clostridium difficile colitis, and Mycoplasma pneumoniae.
Two patients had grade 2 mucositis, one of whom also developed line-associated deep vein thrombosis and cytomegalovirus (CMV) reactivation. Two other patients had CMV reactivation as well, one of whom also had methicillin-resistant Staphylococcus aureus pneumonia.
All 3 patients with CMV reactivation were successfully treated with valgancyclovir and did not develop CMV disease.
Six patients had arthralgias attributed to sirolimus, and 2 of them required dose reductions.
One patient developed chest pain and a decline in carbon monoxide diffusion capacity by 30% that was attributed to sirolimus. The patient was switched to cyclosporine but developed posterior reversible encephalopathy syndrome and was then put on mycophenolate mofetil.
The patient has since maintained stable donor chimerism, and the carbon monoxide diffusing capacity has increased to near-baseline value. ![]()
Cancer report highlights progress, makes predictions

Photo courtesy of the FDA
Despite recent progress in the fight against cancers, these diseases continue to exert “an immense toll” in the US, according to the AACR Cancer Progress Report 2015.
The report highlights the recent approval by the US Food and Drug Administration (FDA) of several anticancer therapies, a vaccine, and 2 diagnostic aids.
But the report also includes data suggesting that cancer cases, and costs related to cancer care, are on the rise.
The report states that, between Aug. 1, 2014, and July 31, 2015, the FDA approved 9 anticancer therapies, either for the first time or for new indications.
During the same period, the FDA approved a new cancer vaccine, a new cancer screening test, and a new use for a previously approved imaging agent.
| Cancer-related products approved from Aug. 1, 2014 to July 31, 2015 | |
| Drug | Approved indication |
| bevacizumab (Avastin) | cervical, ovarian, fallopian
tube, and peritoneal cancers |
| blinatumomab (Blincyto) | acute lymphoblastic leukemia |
| denosumab (Xgeva) | potentially lethal complication
of advanced cancers |
| dinutuximab (Unituxin) | neuroblastoma |
| gefitinib (Iressa) | lung cancer |
| ibrutinib (Imbruvica) | Waldenstrom macroglobulinemia |
| lenvatinib (Lenvima) | thyroid cancer |
| nivolumab (Opdivo) | melanoma, lung cancer |
| olaparib (Lynparza) | ovarian cancer |
| palbociclib (Ibrance) | breast cancer |
| panobinostat (Farydak) | multiple myeloma |
| pembrolizumab (Keytruda) | melanoma |
| ramucirumab (Cyramza) | colorectal and lung cancers |
| sonidegib (Odomzo) | skin cancer |
| Imaging agent | Approved indication |
| technetium 99m tilmanocept
(Lymphoseek) |
lymphatic mapping in solid tumors |
| Vaccine | Approved indication |
| human papillomavirus
9-valent vaccine (Gardasil 9) |
cervical, vulvar,
vaginal, and anal cancers |
| Screening test | Approved indication |
| Cologuard (no generic name) | colorectal cancer |
Despite these advances, cancers continue to exert personal and economic tolls, according to the report.
It states that cancer is the number 1 cause of disease-related death among US children. And more than 589,000 people in the US are projected to die from cancer in 2015.
The number of new cancer cases in the US is predicted to rise from 1.7 million in 2015 to 2.4 million in 2035.
In addition, estimates suggest the direct medical costs of cancer care in the US in 2010 were nearly $125 billion, and these costs are predicted to rise to $156 billion in 2020.
These data underscore the need for more research to develop new approaches to cancer prevention and treatment, according to the report.
Its authors call for Congress and the administration to provide the National Institutes of Health, National Cancer Institute, and FDA with annual funding increases.
“We have made spectacular progress against cancer, which has saved the lives of millions of individuals in the United States and around the world,” said Margaret Foti, PhD, MD, chief executive officer of the AACR.
“However, without increased federal funding for cancer research, we will not be able to realize the promise of recent discoveries and technological advances.” ![]()

Photo courtesy of the FDA
Despite recent progress in the fight against cancers, these diseases continue to exert “an immense toll” in the US, according to the AACR Cancer Progress Report 2015.
The report highlights the recent approval by the US Food and Drug Administration (FDA) of several anticancer therapies, a vaccine, and 2 diagnostic aids.
But the report also includes data suggesting that cancer cases, and costs related to cancer care, are on the rise.
The report states that, between Aug. 1, 2014, and July 31, 2015, the FDA approved 9 anticancer therapies, either for the first time or for new indications.
During the same period, the FDA approved a new cancer vaccine, a new cancer screening test, and a new use for a previously approved imaging agent.
| Cancer-related products approved from Aug. 1, 2014 to July 31, 2015 | |
| Drug | Approved indication |
| bevacizumab (Avastin) | cervical, ovarian, fallopian
tube, and peritoneal cancers |
| blinatumomab (Blincyto) | acute lymphoblastic leukemia |
| denosumab (Xgeva) | potentially lethal complication
of advanced cancers |
| dinutuximab (Unituxin) | neuroblastoma |
| gefitinib (Iressa) | lung cancer |
| ibrutinib (Imbruvica) | Waldenstrom macroglobulinemia |
| lenvatinib (Lenvima) | thyroid cancer |
| nivolumab (Opdivo) | melanoma, lung cancer |
| olaparib (Lynparza) | ovarian cancer |
| palbociclib (Ibrance) | breast cancer |
| panobinostat (Farydak) | multiple myeloma |
| pembrolizumab (Keytruda) | melanoma |
| ramucirumab (Cyramza) | colorectal and lung cancers |
| sonidegib (Odomzo) | skin cancer |
| Imaging agent | Approved indication |
| technetium 99m tilmanocept
(Lymphoseek) |
lymphatic mapping in solid tumors |
| Vaccine | Approved indication |
| human papillomavirus
9-valent vaccine (Gardasil 9) |
cervical, vulvar,
vaginal, and anal cancers |
| Screening test | Approved indication |
| Cologuard (no generic name) | colorectal cancer |
Despite these advances, cancers continue to exert personal and economic tolls, according to the report.
It states that cancer is the number 1 cause of disease-related death among US children. And more than 589,000 people in the US are projected to die from cancer in 2015.
The number of new cancer cases in the US is predicted to rise from 1.7 million in 2015 to 2.4 million in 2035.
In addition, estimates suggest the direct medical costs of cancer care in the US in 2010 were nearly $125 billion, and these costs are predicted to rise to $156 billion in 2020.
These data underscore the need for more research to develop new approaches to cancer prevention and treatment, according to the report.
Its authors call for Congress and the administration to provide the National Institutes of Health, National Cancer Institute, and FDA with annual funding increases.
“We have made spectacular progress against cancer, which has saved the lives of millions of individuals in the United States and around the world,” said Margaret Foti, PhD, MD, chief executive officer of the AACR.
“However, without increased federal funding for cancer research, we will not be able to realize the promise of recent discoveries and technological advances.” ![]()

Photo courtesy of the FDA
Despite recent progress in the fight against cancers, these diseases continue to exert “an immense toll” in the US, according to the AACR Cancer Progress Report 2015.
The report highlights the recent approval by the US Food and Drug Administration (FDA) of several anticancer therapies, a vaccine, and 2 diagnostic aids.
But the report also includes data suggesting that cancer cases, and costs related to cancer care, are on the rise.
The report states that, between Aug. 1, 2014, and July 31, 2015, the FDA approved 9 anticancer therapies, either for the first time or for new indications.
During the same period, the FDA approved a new cancer vaccine, a new cancer screening test, and a new use for a previously approved imaging agent.
| Cancer-related products approved from Aug. 1, 2014 to July 31, 2015 | |
| Drug | Approved indication |
| bevacizumab (Avastin) | cervical, ovarian, fallopian
tube, and peritoneal cancers |
| blinatumomab (Blincyto) | acute lymphoblastic leukemia |
| denosumab (Xgeva) | potentially lethal complication
of advanced cancers |
| dinutuximab (Unituxin) | neuroblastoma |
| gefitinib (Iressa) | lung cancer |
| ibrutinib (Imbruvica) | Waldenstrom macroglobulinemia |
| lenvatinib (Lenvima) | thyroid cancer |
| nivolumab (Opdivo) | melanoma, lung cancer |
| olaparib (Lynparza) | ovarian cancer |
| palbociclib (Ibrance) | breast cancer |
| panobinostat (Farydak) | multiple myeloma |
| pembrolizumab (Keytruda) | melanoma |
| ramucirumab (Cyramza) | colorectal and lung cancers |
| sonidegib (Odomzo) | skin cancer |
| Imaging agent | Approved indication |
| technetium 99m tilmanocept
(Lymphoseek) |
lymphatic mapping in solid tumors |
| Vaccine | Approved indication |
| human papillomavirus
9-valent vaccine (Gardasil 9) |
cervical, vulvar,
vaginal, and anal cancers |
| Screening test | Approved indication |
| Cologuard (no generic name) | colorectal cancer |
Despite these advances, cancers continue to exert personal and economic tolls, according to the report.
It states that cancer is the number 1 cause of disease-related death among US children. And more than 589,000 people in the US are projected to die from cancer in 2015.
The number of new cancer cases in the US is predicted to rise from 1.7 million in 2015 to 2.4 million in 2035.
In addition, estimates suggest the direct medical costs of cancer care in the US in 2010 were nearly $125 billion, and these costs are predicted to rise to $156 billion in 2020.
These data underscore the need for more research to develop new approaches to cancer prevention and treatment, according to the report.
Its authors call for Congress and the administration to provide the National Institutes of Health, National Cancer Institute, and FDA with annual funding increases.
“We have made spectacular progress against cancer, which has saved the lives of millions of individuals in the United States and around the world,” said Margaret Foti, PhD, MD, chief executive officer of the AACR.
“However, without increased federal funding for cancer research, we will not be able to realize the promise of recent discoveries and technological advances.” ![]()
Bringing the B-cell surface into focus

clusters on the cell surface
Image courtesy of V.
Altounian/Science Signaling
New research has provided a clearer picture of the B-cell surface, unearthing new insights regarding antigen receptors.
Mature B cells have 2 classes of antigen receptors on their surface, immunoglobulin M (IgM) and immunoglobulin D (IgD).
Using multiple imaging techniques, researchers studied the spatial relationship of these receptor types in B cells from cell lines, mice, and human blood.
The team described this work in Science Signaling.
Receptors on the surface of resting T cells are thought to reside in preformed clusters known as protein islands, but whether these islands exist on B cells has been unclear.
Palash Maity, PhD, of the University of Freiburg in Germany, and colleagues found these islands do exist on B cells, but their structure changes upon B-cell activation.
Using 2-color direct stochastical optical reconstruction microscopy (dSTORM), the researchers found that IgM and IgD reside on the plasma membrane of resting B cells in separate protein islands of approximately 150 and 240 nanometers, respectively.
The team also observed this class-specific compartmentalization of the antigen receptors using transmission electron microscopy (TEM) and Fab-based proximity-ligation assay (Fab-PLA).
However, the researchers noted a change during B-cell activation. Upon activation, the IgM and IgD protein islands broke up into smaller islands, allowing the 2 types to intermingle, although they did not make direct contact.
The researchers said it is not clear whether this increased affinity between the receptor types is a result of a direct interaction or is mediated by an adaptor protein. The function of the association between the receptors is not clear, either.
But the team believes that one possibility is that, upon B-cell activation, the IgM and IgD protein islands form nanosynapses that allow the islands to exchange proteins and lipids with one another.
The researchers hope these new insights into the nanoscale organization of antigen receptors will support the design of more efficient vaccines or better treatments for B-cell tumors, in which membrane organization is often altered. ![]()

clusters on the cell surface
Image courtesy of V.
Altounian/Science Signaling
New research has provided a clearer picture of the B-cell surface, unearthing new insights regarding antigen receptors.
Mature B cells have 2 classes of antigen receptors on their surface, immunoglobulin M (IgM) and immunoglobulin D (IgD).
Using multiple imaging techniques, researchers studied the spatial relationship of these receptor types in B cells from cell lines, mice, and human blood.
The team described this work in Science Signaling.
Receptors on the surface of resting T cells are thought to reside in preformed clusters known as protein islands, but whether these islands exist on B cells has been unclear.
Palash Maity, PhD, of the University of Freiburg in Germany, and colleagues found these islands do exist on B cells, but their structure changes upon B-cell activation.
Using 2-color direct stochastical optical reconstruction microscopy (dSTORM), the researchers found that IgM and IgD reside on the plasma membrane of resting B cells in separate protein islands of approximately 150 and 240 nanometers, respectively.
The team also observed this class-specific compartmentalization of the antigen receptors using transmission electron microscopy (TEM) and Fab-based proximity-ligation assay (Fab-PLA).
However, the researchers noted a change during B-cell activation. Upon activation, the IgM and IgD protein islands broke up into smaller islands, allowing the 2 types to intermingle, although they did not make direct contact.
The researchers said it is not clear whether this increased affinity between the receptor types is a result of a direct interaction or is mediated by an adaptor protein. The function of the association between the receptors is not clear, either.
But the team believes that one possibility is that, upon B-cell activation, the IgM and IgD protein islands form nanosynapses that allow the islands to exchange proteins and lipids with one another.
The researchers hope these new insights into the nanoscale organization of antigen receptors will support the design of more efficient vaccines or better treatments for B-cell tumors, in which membrane organization is often altered. ![]()

clusters on the cell surface
Image courtesy of V.
Altounian/Science Signaling
New research has provided a clearer picture of the B-cell surface, unearthing new insights regarding antigen receptors.
Mature B cells have 2 classes of antigen receptors on their surface, immunoglobulin M (IgM) and immunoglobulin D (IgD).
Using multiple imaging techniques, researchers studied the spatial relationship of these receptor types in B cells from cell lines, mice, and human blood.
The team described this work in Science Signaling.
Receptors on the surface of resting T cells are thought to reside in preformed clusters known as protein islands, but whether these islands exist on B cells has been unclear.
Palash Maity, PhD, of the University of Freiburg in Germany, and colleagues found these islands do exist on B cells, but their structure changes upon B-cell activation.
Using 2-color direct stochastical optical reconstruction microscopy (dSTORM), the researchers found that IgM and IgD reside on the plasma membrane of resting B cells in separate protein islands of approximately 150 and 240 nanometers, respectively.
The team also observed this class-specific compartmentalization of the antigen receptors using transmission electron microscopy (TEM) and Fab-based proximity-ligation assay (Fab-PLA).
However, the researchers noted a change during B-cell activation. Upon activation, the IgM and IgD protein islands broke up into smaller islands, allowing the 2 types to intermingle, although they did not make direct contact.
The researchers said it is not clear whether this increased affinity between the receptor types is a result of a direct interaction or is mediated by an adaptor protein. The function of the association between the receptors is not clear, either.
But the team believes that one possibility is that, upon B-cell activation, the IgM and IgD protein islands form nanosynapses that allow the islands to exchange proteins and lipids with one another.
The researchers hope these new insights into the nanoscale organization of antigen receptors will support the design of more efficient vaccines or better treatments for B-cell tumors, in which membrane organization is often altered. ![]()
Patients with
Staphylococcus aureus is one the most common pathogens isolated in nosocomial and community‐onset bloodstream infections (BSI) in the United States.[1, 2] S aureus bacteremia (SAB) has been reported in the literature to have substantial morbidity and mortality, with rates ranging between 15% and 60% worldwide.[3, 4, 5, 6] In the United States, patients with infections due to S aureus have on average 3 times the length of hospital stay than inpatients without these infections (14.3 days vs 4.5 days; P<0.01).[7] Healthcare costs are negatively impacted by these infections. In a recent meta‐analysis, Zimlichman et al.[8] reported that central‐line BSI (CLABSI) and surgical‐site infection (SSI) caused by methicillin‐resistant S aureus (MRSA) resulted in the highest estimated costs associated with hospital‐acquired infections in the United States ($58,614 [95% CI: $16,760‐$174,755] for CLABSI and $42,300 [95% CI: $4,005‐$82,670] for SSIs).
Appropriate management of SAB includes not only selecting the correct antimicrobial based on susceptibilities but also timely control of the source of infection, appropriate use of ancillary studies when indicated, and pharmacokinetic and pharmacodynamic therapeutic monitoring of antimicrobial therapy when vancomycin is used.[9] Consultation with an infectious diseases (ID) specialist has been associated with increased compliance with evidence‐based strategies in the management of SAB,[10, 11, 12, 13, 14] such as appropriate antibiotic choice, optimized duration of treatment, removal of the source of infection, and better use of cardiac echocardiography, resulting in improved outcomes.[13, 14]
Some, but not all, institutions have adopted bundles,[14] mandatory ID consultation[10] or daily prospective audit and feedback review[15] as part of antimicrobial stewardship program (ASP) interventions aiming to optimize the management of SABs. As part of our ASP quality improvement activities we performed the present study to determine our institutional rate of clinical failure in the treatment of SAB, to identify current practice patterns in the delivery of processes of care, and evaluate their association with clinical outcomes of hospitalized patients with SAB to identify future areas of improvement.
METHODS
A retrospective cohort study was performed at a 1558 licensed‐bed tertiary teaching hospital in Miami, Florida. All hospitalized patients 18 years of age or older with at least 1 positive blood culture with MRSA or methicillin‐susceptible S aureus (MSSA) between January 1, 2012 and April 30, 2013 were included. Patients were identified from the electronic microbiology laboratory database. For the purposes of this study, only the first episode of SAB was included in the analysis. Patients were excluded if aged younger than 18 years or if SAB was detected in an outpatient setting. The primary outcome was clinical failure, defined as a composite endpoint of in‐hospital mortality or persistent bacteremia; persistent bacteremia was defined as bacteremia for 7 or more days after the first positive blood culture. S aureus isolates were identified by standard methods.[16] Species identification was performed by latex agglutination. Antimicrobial susceptibility testing was performed using an automated system (Vitek 2; bioMerieux, Durham, NC) according to standard guidelines.
Data collected included baseline demographics, comorbidities, and treating healthcare provider's service; provider's service was categorized into 1 of 5 groups: internal medicine (academic), internal medicine (hospitalist), surgery, trauma, or neurosurgery. Duration of bacteremia was recorded and defined as the time between first positive and first negative blood culture. The time of first positive culture was defined as the date in which the culture was obtained. Patients who failed to have at least 1 follow‐up blood culture were not counted toward the main outcome. Additionally, presence of a foreign body (cardiac device, orthopedic prosthesis, tunneled catheter, nontunneled catheter) and presumed source of infection as documented in the electronic medical record by the treating service was also collected. Infections were considered community associated when onset of bacteremia occurred within the first 72 hours of admission, and hospital associated if onset of bacteremia occurred after 72 hours of admission.
Based on current practice guidelines,[9] the variables considered processes of care were the time to obtain the first follow‐up blood culture, time from first positive blood culture to initiation of appropriate antibiotic therapy (defined as a loading dose of vancomycin of 15 mg/kg, or a ‐lactam if the organism was susceptible), time to obtain the first vancomycin trough (when indicated), time from first positive blood culture to consultation with ID specialist, appropriate antibiotic de‐escalation (vancomycin to ‐lactam antibiotic if the organism was susceptible and the patient had no allergies or contraindications), and obtaining an echocardiographic study (transthoracic echocardiogram or transesophageal echocardiogram).
Statistical analyses were performed using SAS 9.2 (SAS Institute, Cary, NC). Differences in proportions were analyzed with 2 or Fisher exact test, accordingly. Differences in means among continuous variables were evaluated using independent samples of paired samples t tests as appropriate for the analysis. Continuous variables were dichotomized using a clinically established cutoff to determine relative risk (RR). A univariate analysis of risk factors associated with clinical failure was performed. Multivariable analyses were performed using logistic regression. Models were created using the backward stepwise approach and included all variables found to be statistically significant at less than 0.05 level in the univariate model and those of clinical significance. The study was reviewed and approved by the institutional review boards at the University of Miami and Jackson Memorial Hospital.
RESULTS
During the study period, 241 patients with a first episode of SAB were identified. MRSA and MSSA were isolated in 124 (51.4%) and 117 (48.5%) patients, respectively. Demographic and clinical characteristics of the study population based on isolate are summarized in Table 1. One hundred seventy‐nine (74.3%) patients were under the care of internal medicine services. There was no association between treating service (medical vs surgical) and clinical failure.
| Variable | MRSA, N= 124 (%) | MSSA, N= 117(%) | Overall, N=241 |
|---|---|---|---|
| |||
| Demographics | |||
| Age, y (mean) | 53.915.57 | 53.915.22 | 53.915.3 |
| Age greater than 60 years | 41 (33.1) | 39 (33.3) | 80 (33.2) |
| Male sex | 80 (64.5) | 80 (68.4) | 160 (66.4) |
| White race | 63 (50.8) | 69 (59) | 132 (54.8) |
| Comorbidities | |||
| Diabetes mellitus | 35 (28.2) | 40 (34.2) | 75 (30.7) |
| Hypertension | 56 (45.2) | 40 (34.2) | 96 (39.8) |
| CHF | 6 (4.8) | 9 (7.7) | 15 (6.2) |
| CVD | 8 (6.4) | 6 (5.1) | 14 (5.8) |
| Chronic pulmonary disease | 14 (11.3) | 14 (12) | 28 (11.6) |
| Malignancy | 9 (7.3) | 19 (16.2) | 28 (11.6) |
| Active chemotherapy | 5 (4) | 10 (8.5) | 15 (6.2) |
| HIV | 27 (21.8) | 17 (14.5) | 44 (18.2) |
| Cirrhosis | 6 (4.8) | 8 (6.8) | 14 (5.8) |
| Hepatitis C infection | 7 (5.6) | 11 (9.4) | 18 (7.5) |
| Acute kidney injury | 88 (71) | 80 (68.4) | 168 (69.7) |
| Chronic kidney disease | 29 (23.4) | 24 (20.5) | 53 (22) |
| End‐stage renal disease | 25 (20.2) | 22 (18.8) | 47 (19.5) |
| Connective tissue disease | 3 (2.4) | 3 (2.6) | 6 (2.5) |
| Alcohol abuse | 3 (2.4) | 1 (0.8) | 4 (1.7) |
| IVDU | 4 (3.2) | 5 (4.3) | 9 (3.7) |
| Hemiplegia | 4 (3.2) | 0 | 4 (1.7) |
| Chronic osteomyelitis | 4 (3.2) | 0 | 4 (1.7) |
| History of transplant | 7 (5.6) | 0 | 7 (2.9) |
| Surgery during current admission | 29 (23.4) | 46 (39.3) | 75 (31.1) |
| Surgery during the previous 30 days | 31 (25) | 36 (30.8) | 67 (25.3) |
| Treating service | |||
| Medical service | 89 (71.8) | 90 (76.9) | 179 (74.3) |
| Surgical service | 21 (16.9) | 16 (13.7) | 37 (15.3) |
| Other | 7 (5.6) | 11 (9.4) | 18 (7.5) |
| Presence of foreign body | |||
| PICC line | 24 (19.3) | 34 (29.1) | 58 (24.1) |
| Tunneled CVC | 24 (19.3) | 15 (12.8) | 39 (16.2) |
| Nontunneled CVC | 13 (10.5) | 28 (23.9) | 41 (17) |
| AV fistula | 3 (2.4) | 7 (6) | 10 (4.1) |
| Cardiac device | 8 (6.4) | 9 (7.7) | 17 (7) |
| Other | 4 (3.2) | 11 (9.4) | 15 (6.2) |
| Source of infection | |||
| CLABSI | 32 (25.8) | 21 (17.9) | 53 (22) |
| SSTI | 24 (19.3) | 20 (17.1) | 44 (18.2) |
| Endocarditis | 10 (8.1) | 7 (6) | 17 (7) |
| Thrombophlebitis | 2 (1.6) | 2 (1.7) | 4 (1.7) |
| Prostatic abscess | 3 (2.4) | 1 (0.8) | 4 (1.7) |
| Paravertebral abscess | 2 (1.6) | 2 (1.7) | 4 (1.7) |
| Mediastinal abscess | 2 (1.6) | 1 (0.8) | 3 (1.2) |
| CAP | 4 (3.2) | 4 (3.4) | 8 (3.3) |
| VAP | 3 (2.4) | 2 (1.7) | 5 (2.1) |
| Surgical site infection | 2 (1.6) | 1 (0.8) | 3 (1.2) |
| Ventriculostomy | 0 | 1 (0.8) | 1 (0.4) |
| Bone or joint infection | 2 (1.6) | 3 (2.6) | 5 (2.1) |
| Unknown | 38 (30.6) | 52 (44.4) | 90 (37.3) |
| Onset | |||
| Community onset* | 77 (62.1) | 77 (65.8) | 154 (63.9) |
| Hospital onset | 47 (37.9) | 40 (34.2) | 87 (36.1) |
The onset of infection occurred in the community in 77 (62.1%) patients with MRSA and in 77 (65.8%) patients with MSSA. The documented source of bacteremia was unknown in 30% of patients with MRSA and 44% of those with MSSA BSI. When ID specialists were consulted, patients were more likely to have a source of infection identified (RR: 1.5; 95% confidence interval [CI]: 1.2‐1.8; P<0.0001). The most commonly documented sources of infection were CLABSI, which occurred in 32 (25.8%) patients with MRSA and 21 (17.9%) patients with MSSA, followed by skin and soft tissue infections in 24 (19.3%) patients with MRSA BSI and 20 (17.1%) patients with MSSA BSI. All patients with CLABSI had documentation of catheter removal.
Clinical failure (defined as in‐hospital mortality or persistent bacteremia) occurred in 78 (32.4%) patients. Of these, 50 (20.7%) represented in‐hospital mortality, and 31 (12.9%) had persistent bacteremia. Table 2 summarizes the demographic and clinical characteristics associated with clinical failure. In the univariate analysis, the variables statistically significantly associated with clinical failure were: age greater than 60 years (RR: 1.4; 95% CI: 1.1‐1.8; P=0.001), bacteremia due to MRSA (RR: 1.7; 95% CI: 1.1‐2.5; P=0.008), white race (RR: 0.7; 95% CI: 0.6‐1; P=0.03), acute kidney injury during admission (RR: 2.2; 95% CI: 1.3‐3.7; P=0.004), presence of nontunneled central venous catheters at the onset of bacteremia (RR: 1.9; 95% CI: 1.3‐2.7; P=0.004), and endocarditis (RR: 2.9; 95% CI: 2.1‐3.9; P<0.0001). In the multivariable analysis, age greater than 60 years and endocarditis were found to be independent risk factors for the development of clinical failure.
| Variable | Clinical Failure, N=78 (%) | No Clinical Failure, N=163 (%) | Unadjusted RR (CI) | P Value* | Adjusted OR (CI) | P Value* |
|---|---|---|---|---|---|---|
| ||||||
| Demographics | ||||||
| Age >60 years | 37 (47.4) | 43 (26.4) | 1.4 (1.1‐1.8) | 0.001 | 2.4 (1.2‐4.5) | 0.008 |
| Male | 46 (60) | 114 (69.9) | 0.7 (0.5‐1.04) | 0.09 | ||
| White race | 35 (44.9) | 97 (59.5) | 0.7 (0.6‐1) | 0.03 | 0.5 (0.3‐1.02) | 0.058 |
| Isolate | ||||||
| MRSA | 50 (64.1) | 74 (45.4) | 1.7 (1.1‐2.5) | 0.008 | 1.8 (0.6‐5.2) | 0.3 |
| MSSA | 28 (35.9) | 89 (54.6) | 0.6 (0.4‐0.9) | 0.008 | ||
| Comorbidities | ||||||
| Diabetes mellitus | 21 (26.9) | 54 (33.1) | 0.8 (0.5‐1.2) | 0.34 | ||
| Cirrhosis | 6 (7.7) | 8 (4.9) | 1.3 (0.7‐2.5) | 0.35 | ||
| Acute kidney injury | 65 (83.3) | 103 (63.2) | 2.2 (1.3‐3.7) | 0.004 | 1.6 (0.5‐5.4) | 0.43 |
| Chronic kidney disease | 12 (15.4) | 41 (25.1) | 0.6 (0.4‐1.1) | 0.11 | ||
| End‐stage renal disease | 15 (19.2) | 32 (19.6) | 1 (0.6‐1.5) | 0.94 | ||
| IVDU | 3 (3.8) | 6 (3.7) | 1.03 (0.4‐2.6) | 1 | ||
| Treating service | ||||||
| Medical | 61 (78.2) | 118 (72.4) | 1.3 (0.7‐2.6) | 0.33 | ||
| Surgical | 11 (14.1) | 67 (41.1) | 1 (0.9‐1.1) | 0.71 | ||
| Presence of foreign body | ||||||
| Cardiac device | 6 (7.7) | 11 (6.7) | 1.1 (0.6‐2.1) | 0.78 | ||
| PICC line | 20 (25.6) | 38 (23.3) | 1.1 (0.7‐1.6) | 0.69 | ||
| Nontunneled CVC | 22 (28.2) | 19 (11.7) | 1.9 (1.3‐2.7) | 0.004 | 3.6 (0.7‐17.7) | 0.11 |
| Tunneled CVC | 15 (19.2) | 24 (14.7) | 1.2 (0.8‐1.9) | 0.36 | ||
| AV fistula | 0 | 10 (6.1) | 0.1 (0.09‐2) | 0.15 | ||
| Other | 4 (5.1) | 11 (6.7) | 0.8 (0.3‐1.9) | 0.64 | ||
| Onset | ||||||
| Community onset | 46 (59) | 108 (66.3) | 0.8 (0.6‐1.2) | 0.27 | ||
| Hospital onset | 32 (41) | 55 (33.7) | 1.2 (0.8‐1.8) | 0.27 | ||
| Source | ||||||
| CLABSI | 15 (19.2) | 38 (23.3) | 0.8 (0.5‐1.4) | 0.48 | ||
| SSTI | 12 (15.4) | 32 (19.6) | 0.8 (0.5‐1.4) | 0.44 | ||
| Endocarditis | 14 (17.9) | 3 (1.8) | 2.9 (2.1‐3.9) | <0.0001 | 9.4 (2.2‐1.1) | 0.003 |
| Thrombophlebitis | 0 | 4 (2.4) | 0.3 (0.02‐4.2) | 0.37 | ||
| Prostatic abscess | 1 (1.3) | 3 (1.8) | 0.8 (0.1‐4.2) | 0.76 | ||
| Paravertebral abscess | 0 | 4 (2.4) | 0.3 (0.02‐4.2) | 0.37 | ||
| Mediastinal abscess | 1 (1.3) | 2 (1.2) | 1.03 (0.2‐5.1) | 0.97 | ||
| CAP | 4 (5.1) | 4 (2.4) | 1.5 (0.8‐3.2) | 0.21 | ||
| VAP | 2 (2.6) | 3 (1.8) | 1.2 (0.4‐3.7) | 0.7 | ||
| Surgical site infection | 1 (1.3) | 2 (1.2) | 1.03 (0.2‐5.2) | 0.97 | ||
| Ventriculostomy | 0 | 1 (0.6) | 0.8 (0.1‐8.5) | 0.82 | ||
| Bone or joint infection | 1 (1.3) | 4 (2.4) | 0.6 (0.1‐3.6) | 0.59 | ||
| Unknown | 27 (34.6) | 63 (38.6) | 0.9 (0.6‐1.3) | 0.55 | ||
Performance of Process of Care and Association With Outcomes
The analysis of the performance of the processes of care and outcomes is shown in Table 3. After adjusting for relevant clinical and demographic characteristics, and those with a level of significance of <0.05, obtaining follow‐up blood cultures more than 4 days after the onset of bacteremia independently increased the risk of clinical failure (RR: 6.5; 95% CI: 2.1‐20.5; P=0.001). When consultation with an ID specialist was obtained within the first 6 days from onset of bacteremia, the risk of clinical failure was 0.3 (95% CI: 0.1‐0.9; P=0.03); however, consultation with an ID specialist overall was not associated with clinical failure (RR: 1; 95% CI: 0.7‐1.4; P=0.98).
| Variable | Clinical Failure, n=78 (%) | No Clinical Failure, n=163 (%) | Unadjusted RR (CI) | P Value* | Adjusted OR (CI) | P Value* |
|---|---|---|---|---|---|---|
| ||||||
| Timing of follow‐up blood culture, n=200 | ||||||
| Less than 2 days | 30 (19.2) | 87 (53.4) | 0.7 (0.5‐0.9) | 0.01 | 1.2 (0.5‐2.9) | 0.60 |
| 24 days (ref) | 16 (20.5) | 39 (23.9) | 0.9 (0.8‐1.1) | 0.53 | ||
| More than 4 days | 19 (24.3) | 9 (5.5) | 1.3 (1.1‐1.5) | <0.0001 | 6.6 (2.1‐20.5) | 0.001 |
| Early antibiotic therapy, n=232 | 66 (84.6) | 132 (81) | 1.2 (0.7‐2.3) | 0.45 | ||
| Monitoring of vancomycin levels, n=156 | 37 (20.8) | 97 (59.5) | 0.8 (0.6‐1.03) | 0.09 | ||
| Therapy with ‐lactam, n=103‖ | 7 (8.8) | 49 (30.1) | 0.4 (0.2‐0.8) | 0.01 | 0.1 (0.04‐0.5) | 0.002 |
| Consultation with ID specialist, n=241 | 31 (39.7) | 66 (40.5) | 1 (0.7‐1.4) | 0.98 | ||
| Early consultation with ID specialist, n=97# | 19 (24.3) | 56 (34.3) | 0.5 (0.3‐0.8) | 0.006 | 0.3 (0.1‐0.9) | 0.03 |
| Echocardiography, n=241 | 45 (57.7) | 96 (58.9) | 1 (0.7‐1.4) | 0.86 | ||
| Early echocardiography, n=141** | 35 (44.9) | 91 (55.8) | 0.7 (0.5‐1.07) | 0.11 | ||
A comparison of the average number of days to performance of processes of care is presented in Table 4. Patients with clinical failure had significantly greater elapsed time from the first positive blood culture to the first follow‐up blood culture as compared to those who did not have clinical failure (mean 2.321.3 days vs 3.883.37; P<0.0001). Forty‐one patients (17.1%) failed to have at least 1 follow‐up blood culture.
| Process of Care | Clinical Failure | No Clinical Failure | P Value* |
|---|---|---|---|
| |||
| First follow‐up blood culture, n=200 | 3.883.37 | 2.321.3 | <0.0001 |
| Consultation with infectious diseases, n=97 | 6.96.55 | 4.354.34 | 0.06 |
| First antibiotic dose, n=232 | 0.431.05 | 0.57 1.11 | 0.63 |
| First dose of ‐lactam, n=56 | 4.41.6 | 3.51.4 | 0.1 |
| First vancomycin trough, n=156 | 2.632.04 | 2.552.02 | 0.81 |
| Echocardiography, n=141 | 3.421.74 | 3.312.05 | 0.47 |
Among patients with clinical failure, an ID specialist was consulted at a mean time of 7 days from the onset of bacteremia, compared to patients with no clinical failure in whom a consult was obtained at a mean of 4 days (P=0.06) (Table 4). Overall, ID specialists were only consulted in 97/241 (40.2%) episodes.
Echocardiographic studies were performed in 141/241 (58.5)% of episodes, and they were more likely to be obtained when an ID specialist was consulted (RR: 1.7; 95% CI: 1.4‐2.1; P<0.0001). Lack of performance of these studies was not associated with clinical failure (Table 3).
Antibiotic Administration and De‐escalation of Therapy
There were no significant differences in the average time from the first positive blood culture to the administration of antibiotics between patients who had clinical failure and those who did not (0.571.11 vs 0.431.05; P=0.63) (Table 4).
Patients with MSSA BSI and no documented penicillin allergy were treated with ‐lactam or cephalosporin antibiotics in 56/103(54.3%) episodes. Patients were 2.5 times more likely to receive ‐lactam antibiotics when an ID specialist was consulted (95% CI: 1.8‐3.5; P<0.0001). Among patients with MSSA BSI, treatment with ‐lactams was an independent predictor of decreased risk of clinical failure (RR: 0.2; 95% CI: 0.07‐0.9; P=0.005) (Table 3).
DISCUSSION
Our study showed a significant rate of morbidity associated with S aureus bacteremia and identified processes of care in the management of SAB that impact patient outcomes.
Our results show that early consultation with an ID specialist was associated with a decreased risk of developing clinical failure, increased likelihood of identification of a source of infection, and positively impacted administration of appropriate antibiotic therapy, especially in cases of MSSA BSI, with overall improvement in patient outcomes. However, consultation with an ID specialist was only obtained in 40.2% of our cases, which is consistent with published data.[10, 11, 12, 13] Consultation with an ID specialist itself did not impact clinical failure, but rather timeliness in obtaining expert guidance was associated with better outcomes. As shown in previous studies,[10, 11, 12, 13, 14] compliance with the standards of care and patient prognosis are improved when ID specialists are involved in the management of SAB. Our study reiterates that early consultation with an ID specialist has a positive outcome in patient care, as opposed to delaying consultation once the patient has persistent bacteremia for more than 7 days. This association could be explained by considering that the majority of the standards of care are time sensitive, which include: obtaining surveillance blood cultures 48 to 96 hours after initial detection[10] or initiating therapy,[11, 14] removal of foci of infection,[10, 11, 12, 14] use of parenteral ‐lactams for the treatment of MSSA,[10, 11, 13, 14] performing echocardiography when clinically indicated,[10, 11, 13, 14] and appropriate duration of therapy.[10, 13, 14] Importantly, studies have shown that when ID specialists' recommendations are followed, patients are more likely to be cured,[10, 11, 13] and are less likely to relapse.[10, 11, 12] Given the complexities of treating patients with SAB and high rates of clinical failures, routine guidance could be beneficial to healthcare providers as part of a multidisciplinary structured strategy that is set in motion the moment a patient with SAB is identified by the microbiology laboratory. The processes of care outlined in this this study can serve as quality of care indicators and be integrated into a structured strategy to optimize the management of SAB.
Regarding optimal timing for follow‐up blood cultures, our results show that delays in obtaining follow‐up blood cultures (more than 4 days from onset of bacteremia) was independently associated with increased risk of clinical failure. Timely follow‐up blood cultures have been previously identified as quality of care indicators.[10, 11, 13, 14] Compliance with obtaining follow‐up blood cultures improves when this step is integrated into a bundle of care.[14]
Antimicrobial therapy was promptly initiated in the majority of the patients in our study. However, areas for improvement were identified. Vancomycin was the empirical therapy of choice in most of the cases, but an appropriate dose was only received by 65% of the patients, and vancomycin levels after the fourth dose were obtained in 85.9% of instances when indicated. Although in our cohort these results were not significantly associated with clinical failure, previous studies have described attainment of a target therapeutic vancomycin trough (1520 mg/dL) as a factor for treatment success.[17, 18] This problem could be addressed through physician education on therapeutic drug monitoring,[19] as well as through an ASP intervention, which have successfully led efforts to improve vancomycin utilization and dosing.[20] Among patients with MSSA BSI, therapy with ‐lactams was associated with improved outcomes, and was more likely to be administered when an ID specialist was consulted. This is in accordance with previous studies that have shown that higher rates of appropriate antimicrobial therapy are achieved when ID specialists are involved in management of SAB.[10, 11, 13, 14] The use of ‐lactams for treatment of MSSA BSI has been consistently associated with lower SAB‐related mortality and relapse.[21, 22, 23, 24, 25, 26]
Echocardiographic studies were obtained in only half of the patients in our cohort, and they were twice more likely to be obtained when an ID specialist was consulted. Although we did not evaluate the appropriateness of the echocardiographic study, the increased proportion of studies performed when ID specialists were consulted could indicate a more in‐depth evaluation of the case. Moreover, in our cohort, when ID specialists where involved in direct patient care, a source of infection was more likely to be identified. This is in accordance with previous studies proposing that because evaluation by ID specialists are more detailed, they lead to increased use in ancillary studies and recognition of complicated cases.[10, 12]
Limitations of this study include its retrospective design and the fact that it was performed in a single institution. The source of infection was defined as documented by treating providers and not by independent diagnostic criteria. Antibiotic use was collected throughout duration of admission, and was not followed after patients were discharged, as these data were not available on the electronic medical record for all patients. Deaths that may have occurred after hospital discharge were not included. We did not account for elevated vancomycin minimum inhibitory concentration as a risk factor for the main outcome, and adjustment of vancomycin based on serum levels was not factored in. Acute kidney injury was accounted for anytime during hospitalization, but not in relation to antimicrobial administration. Despite the limitations, our study has strengths that make our results generalizable. Although our institution is a single medical center, it serves a large and diverse population as reflected in our cases. Even though this is a retrospective cohort study, the use of a centralized electronic medical record allowed us to identify each aspect of the management of SAB, as implemented by different treating services (medical and surgical), as continuous variables (days) rather than only in a dichotomous fashion. Moreover, by being a community teaching hospital, we were able to explore aspects of the practice of physicians in training versus practicing clinicians. These results could be extrapolated to other healthcare facilities aiming to improve the management of SAB.
CONCLUSIONS
Our results suggest that obtaining timely follow‐up blood cultures, use of ‐lactams in patients with MSSA BSI, and early consultation with infectious diseases are the processes of care that could serve as quality and patient‐safety indicators for the management of SAB. These results contribute to a growing body of evidence supporting the implementation of structured processes of care to optimize the management and clinical outcomes of hospitalized patients with SAB.
Disclosure: Nothing to report.
- , , , , , . Nosocomial bloodstream infections in US hospitals: analysis of 24,179 cases from a prospective nationwide surveillance study. Clin Infect Dis. 2004;39(3):309–317.
- , , , . Laboratory‐based surveillance of current antimicrobial resistance patterns and trends among Staphylococcus aureus: 2005 status in the United States. Ann Clin Microbiol Antimicrob. 2006;5:2.
- , , , , , . The impact of methicillin resistance in Staphylococcus aureus bacteremia on patient outcomes: mortality, length of stay, and hospital charges. Infect Control Hosp Epidemiol. 2005;26(2):166–174.
- , , , . Staphylococcal bacteremia and altered host resistance. Ann Intern Medicine. 1968;69(5):859–873.
- , , , , , . Comparison of mortality associated with methicillin‐resistant and methicillin‐susceptible Staphylococcus aureus bacteremia: a meta‐analysis. Clin Infect Dis. 2003;36(1):53–59.
- . Unfavourable prognostic factors in Staphylococcus aureus septicemia and endocarditis. Scand J Infect Dis. 1985;17(2):179–187.
- , , , et al. The burden of Staphylococcus aureus infections on hospitals in the United States: an analysis of the 2000 and 2001 Nationwide Inpatient Sample Database. Arch Intern Med. 2005;165(15):1756–1761.
- , , , et al. Health care–associated infections: a meta‐analysis of costs and financial impact on the us health care system. JAMA Intern Med. 2013;173(22):2039–2046.
- , , , et al. Clinical practice guidelines by the Infectious Diseases Society of America for the treatment of methicillin‐resistant Staphylococcus aureus infections in adults and children. Clin Infect Dis. 2011;52(3):e18–e55.
- , , , , . Impact of routine infectious diseases service consultation on the evaluation, management, and outcomes of Staphylococcus aureus bacteremia. Clin Infect Dis. 2008;46(7):1000–1008.
- , , , et al. Outcome of Staphylococcus aureus bacteremia according to compliance with recommendations of infectious diseases specialists: experience with 244 patients. Clin Infect Dis. 1998;27(3):478–486.
- , , , . Infectious disease consultation for Staphylococcus aureus bacteremia improves patient management and outcomes. Infect Dis Clin Pract (Baltim Md). 2012;20(4):261–267.
- , , , , . The value of infectious diseases consultation in Staphylococcus aureus bacteremia. Am J Med. 2010;123(7):631–637.
- , , , et al. Impact of an evidence‐based bundle intervention in the quality‐of‐care management and outcome of Staphylococcus aureus bacteremia. Clin Infect Dis. 2013;57(9):1225–1233.
- , , , et al. Comparison of prior authorization and prospective audit with feedback for antimicrobial stewardship. Infect Control Hosp Epidemiol. 2014;35(9):1092–1099.
- Clinical Laboratory Standards Institute. Performance Standards for Antimicrobial Susceptibility Testing; Twenty‐First Informational Supplement. Wayne, PA: Clinical Laboratory Standards Institute; 2011.
- , , , . Impact of vancomycin exposure on outcomes in patients with methicillin‐resistant Staphylococcus aureus bacteremia: support for consensus guidelines suggested targets. Clin Infect Dis. 2011;52(8):975–981.
- , , , , . High‐dose vancomycin therapy for methicillin‐resistant Staphylococcus aureus infections: efficacy and toxicity. Arch Intern Med. 2006;166(19):2138–2144.
- , , , . Strategies for physician education in therapeutic drug monitoring. Clin Chem. 1998;44(2):401–407.
- , . Impact of antimicrobial stewardship program on vancomycin use in a pediatric teaching hospital. Pediatr Infect Dis J. 2010;29(8):707–711.
- , . Vancomycin for Staphylococcus aureus endocarditis in intravenous drug users. Antimicrob Agents Chemother. 1990;34(6):1227–1231.
- , , , et al. A prospective multicenter study of Staphylococcus aureus bacteremia: incidence of endocarditis, risk factors for mortality, and clinical impact of methicillin resistance. Medicine. 2003;82(5):322–332.
- , , , . Impact of empirical‐therapy selection on outcomes of intravenous drug users with infective endocarditis caused by methicillin‐susceptible Staphylococcus aureus. Antimicrob Agents Chemother. 2007;51(10):3731–3733.
- , , , et al. Use of vancomycin or first‐generation cephalosporins for the treatment of hemodialysis‐dependent patients with methicillin‐susceptible Staphylococcus aureus bacteremia. Clin Infect Dis. 2007;44(2):190–196.
- , , , et al. Outcome of vancomycin treatment in patients with methicillin‐susceptible Staphylococcus aureus bacteremia. Antimicrob Agents Chemother. 2008;52(1):192–197.
- , , , et al. Comparative effectiveness of nafcillin or cefazolin versus vancomycin in methicillin‐susceptible Staphylococcus aureus bacteremia. BMC Infect Dis. 2011;11:279.
Staphylococcus aureus is one the most common pathogens isolated in nosocomial and community‐onset bloodstream infections (BSI) in the United States.[1, 2] S aureus bacteremia (SAB) has been reported in the literature to have substantial morbidity and mortality, with rates ranging between 15% and 60% worldwide.[3, 4, 5, 6] In the United States, patients with infections due to S aureus have on average 3 times the length of hospital stay than inpatients without these infections (14.3 days vs 4.5 days; P<0.01).[7] Healthcare costs are negatively impacted by these infections. In a recent meta‐analysis, Zimlichman et al.[8] reported that central‐line BSI (CLABSI) and surgical‐site infection (SSI) caused by methicillin‐resistant S aureus (MRSA) resulted in the highest estimated costs associated with hospital‐acquired infections in the United States ($58,614 [95% CI: $16,760‐$174,755] for CLABSI and $42,300 [95% CI: $4,005‐$82,670] for SSIs).
Appropriate management of SAB includes not only selecting the correct antimicrobial based on susceptibilities but also timely control of the source of infection, appropriate use of ancillary studies when indicated, and pharmacokinetic and pharmacodynamic therapeutic monitoring of antimicrobial therapy when vancomycin is used.[9] Consultation with an infectious diseases (ID) specialist has been associated with increased compliance with evidence‐based strategies in the management of SAB,[10, 11, 12, 13, 14] such as appropriate antibiotic choice, optimized duration of treatment, removal of the source of infection, and better use of cardiac echocardiography, resulting in improved outcomes.[13, 14]
Some, but not all, institutions have adopted bundles,[14] mandatory ID consultation[10] or daily prospective audit and feedback review[15] as part of antimicrobial stewardship program (ASP) interventions aiming to optimize the management of SABs. As part of our ASP quality improvement activities we performed the present study to determine our institutional rate of clinical failure in the treatment of SAB, to identify current practice patterns in the delivery of processes of care, and evaluate their association with clinical outcomes of hospitalized patients with SAB to identify future areas of improvement.
METHODS
A retrospective cohort study was performed at a 1558 licensed‐bed tertiary teaching hospital in Miami, Florida. All hospitalized patients 18 years of age or older with at least 1 positive blood culture with MRSA or methicillin‐susceptible S aureus (MSSA) between January 1, 2012 and April 30, 2013 were included. Patients were identified from the electronic microbiology laboratory database. For the purposes of this study, only the first episode of SAB was included in the analysis. Patients were excluded if aged younger than 18 years or if SAB was detected in an outpatient setting. The primary outcome was clinical failure, defined as a composite endpoint of in‐hospital mortality or persistent bacteremia; persistent bacteremia was defined as bacteremia for 7 or more days after the first positive blood culture. S aureus isolates were identified by standard methods.[16] Species identification was performed by latex agglutination. Antimicrobial susceptibility testing was performed using an automated system (Vitek 2; bioMerieux, Durham, NC) according to standard guidelines.
Data collected included baseline demographics, comorbidities, and treating healthcare provider's service; provider's service was categorized into 1 of 5 groups: internal medicine (academic), internal medicine (hospitalist), surgery, trauma, or neurosurgery. Duration of bacteremia was recorded and defined as the time between first positive and first negative blood culture. The time of first positive culture was defined as the date in which the culture was obtained. Patients who failed to have at least 1 follow‐up blood culture were not counted toward the main outcome. Additionally, presence of a foreign body (cardiac device, orthopedic prosthesis, tunneled catheter, nontunneled catheter) and presumed source of infection as documented in the electronic medical record by the treating service was also collected. Infections were considered community associated when onset of bacteremia occurred within the first 72 hours of admission, and hospital associated if onset of bacteremia occurred after 72 hours of admission.
Based on current practice guidelines,[9] the variables considered processes of care were the time to obtain the first follow‐up blood culture, time from first positive blood culture to initiation of appropriate antibiotic therapy (defined as a loading dose of vancomycin of 15 mg/kg, or a ‐lactam if the organism was susceptible), time to obtain the first vancomycin trough (when indicated), time from first positive blood culture to consultation with ID specialist, appropriate antibiotic de‐escalation (vancomycin to ‐lactam antibiotic if the organism was susceptible and the patient had no allergies or contraindications), and obtaining an echocardiographic study (transthoracic echocardiogram or transesophageal echocardiogram).
Statistical analyses were performed using SAS 9.2 (SAS Institute, Cary, NC). Differences in proportions were analyzed with 2 or Fisher exact test, accordingly. Differences in means among continuous variables were evaluated using independent samples of paired samples t tests as appropriate for the analysis. Continuous variables were dichotomized using a clinically established cutoff to determine relative risk (RR). A univariate analysis of risk factors associated with clinical failure was performed. Multivariable analyses were performed using logistic regression. Models were created using the backward stepwise approach and included all variables found to be statistically significant at less than 0.05 level in the univariate model and those of clinical significance. The study was reviewed and approved by the institutional review boards at the University of Miami and Jackson Memorial Hospital.
RESULTS
During the study period, 241 patients with a first episode of SAB were identified. MRSA and MSSA were isolated in 124 (51.4%) and 117 (48.5%) patients, respectively. Demographic and clinical characteristics of the study population based on isolate are summarized in Table 1. One hundred seventy‐nine (74.3%) patients were under the care of internal medicine services. There was no association between treating service (medical vs surgical) and clinical failure.
| Variable | MRSA, N= 124 (%) | MSSA, N= 117(%) | Overall, N=241 |
|---|---|---|---|
| |||
| Demographics | |||
| Age, y (mean) | 53.915.57 | 53.915.22 | 53.915.3 |
| Age greater than 60 years | 41 (33.1) | 39 (33.3) | 80 (33.2) |
| Male sex | 80 (64.5) | 80 (68.4) | 160 (66.4) |
| White race | 63 (50.8) | 69 (59) | 132 (54.8) |
| Comorbidities | |||
| Diabetes mellitus | 35 (28.2) | 40 (34.2) | 75 (30.7) |
| Hypertension | 56 (45.2) | 40 (34.2) | 96 (39.8) |
| CHF | 6 (4.8) | 9 (7.7) | 15 (6.2) |
| CVD | 8 (6.4) | 6 (5.1) | 14 (5.8) |
| Chronic pulmonary disease | 14 (11.3) | 14 (12) | 28 (11.6) |
| Malignancy | 9 (7.3) | 19 (16.2) | 28 (11.6) |
| Active chemotherapy | 5 (4) | 10 (8.5) | 15 (6.2) |
| HIV | 27 (21.8) | 17 (14.5) | 44 (18.2) |
| Cirrhosis | 6 (4.8) | 8 (6.8) | 14 (5.8) |
| Hepatitis C infection | 7 (5.6) | 11 (9.4) | 18 (7.5) |
| Acute kidney injury | 88 (71) | 80 (68.4) | 168 (69.7) |
| Chronic kidney disease | 29 (23.4) | 24 (20.5) | 53 (22) |
| End‐stage renal disease | 25 (20.2) | 22 (18.8) | 47 (19.5) |
| Connective tissue disease | 3 (2.4) | 3 (2.6) | 6 (2.5) |
| Alcohol abuse | 3 (2.4) | 1 (0.8) | 4 (1.7) |
| IVDU | 4 (3.2) | 5 (4.3) | 9 (3.7) |
| Hemiplegia | 4 (3.2) | 0 | 4 (1.7) |
| Chronic osteomyelitis | 4 (3.2) | 0 | 4 (1.7) |
| History of transplant | 7 (5.6) | 0 | 7 (2.9) |
| Surgery during current admission | 29 (23.4) | 46 (39.3) | 75 (31.1) |
| Surgery during the previous 30 days | 31 (25) | 36 (30.8) | 67 (25.3) |
| Treating service | |||
| Medical service | 89 (71.8) | 90 (76.9) | 179 (74.3) |
| Surgical service | 21 (16.9) | 16 (13.7) | 37 (15.3) |
| Other | 7 (5.6) | 11 (9.4) | 18 (7.5) |
| Presence of foreign body | |||
| PICC line | 24 (19.3) | 34 (29.1) | 58 (24.1) |
| Tunneled CVC | 24 (19.3) | 15 (12.8) | 39 (16.2) |
| Nontunneled CVC | 13 (10.5) | 28 (23.9) | 41 (17) |
| AV fistula | 3 (2.4) | 7 (6) | 10 (4.1) |
| Cardiac device | 8 (6.4) | 9 (7.7) | 17 (7) |
| Other | 4 (3.2) | 11 (9.4) | 15 (6.2) |
| Source of infection | |||
| CLABSI | 32 (25.8) | 21 (17.9) | 53 (22) |
| SSTI | 24 (19.3) | 20 (17.1) | 44 (18.2) |
| Endocarditis | 10 (8.1) | 7 (6) | 17 (7) |
| Thrombophlebitis | 2 (1.6) | 2 (1.7) | 4 (1.7) |
| Prostatic abscess | 3 (2.4) | 1 (0.8) | 4 (1.7) |
| Paravertebral abscess | 2 (1.6) | 2 (1.7) | 4 (1.7) |
| Mediastinal abscess | 2 (1.6) | 1 (0.8) | 3 (1.2) |
| CAP | 4 (3.2) | 4 (3.4) | 8 (3.3) |
| VAP | 3 (2.4) | 2 (1.7) | 5 (2.1) |
| Surgical site infection | 2 (1.6) | 1 (0.8) | 3 (1.2) |
| Ventriculostomy | 0 | 1 (0.8) | 1 (0.4) |
| Bone or joint infection | 2 (1.6) | 3 (2.6) | 5 (2.1) |
| Unknown | 38 (30.6) | 52 (44.4) | 90 (37.3) |
| Onset | |||
| Community onset* | 77 (62.1) | 77 (65.8) | 154 (63.9) |
| Hospital onset | 47 (37.9) | 40 (34.2) | 87 (36.1) |
The onset of infection occurred in the community in 77 (62.1%) patients with MRSA and in 77 (65.8%) patients with MSSA. The documented source of bacteremia was unknown in 30% of patients with MRSA and 44% of those with MSSA BSI. When ID specialists were consulted, patients were more likely to have a source of infection identified (RR: 1.5; 95% confidence interval [CI]: 1.2‐1.8; P<0.0001). The most commonly documented sources of infection were CLABSI, which occurred in 32 (25.8%) patients with MRSA and 21 (17.9%) patients with MSSA, followed by skin and soft tissue infections in 24 (19.3%) patients with MRSA BSI and 20 (17.1%) patients with MSSA BSI. All patients with CLABSI had documentation of catheter removal.
Clinical failure (defined as in‐hospital mortality or persistent bacteremia) occurred in 78 (32.4%) patients. Of these, 50 (20.7%) represented in‐hospital mortality, and 31 (12.9%) had persistent bacteremia. Table 2 summarizes the demographic and clinical characteristics associated with clinical failure. In the univariate analysis, the variables statistically significantly associated with clinical failure were: age greater than 60 years (RR: 1.4; 95% CI: 1.1‐1.8; P=0.001), bacteremia due to MRSA (RR: 1.7; 95% CI: 1.1‐2.5; P=0.008), white race (RR: 0.7; 95% CI: 0.6‐1; P=0.03), acute kidney injury during admission (RR: 2.2; 95% CI: 1.3‐3.7; P=0.004), presence of nontunneled central venous catheters at the onset of bacteremia (RR: 1.9; 95% CI: 1.3‐2.7; P=0.004), and endocarditis (RR: 2.9; 95% CI: 2.1‐3.9; P<0.0001). In the multivariable analysis, age greater than 60 years and endocarditis were found to be independent risk factors for the development of clinical failure.
| Variable | Clinical Failure, N=78 (%) | No Clinical Failure, N=163 (%) | Unadjusted RR (CI) | P Value* | Adjusted OR (CI) | P Value* |
|---|---|---|---|---|---|---|
| ||||||
| Demographics | ||||||
| Age >60 years | 37 (47.4) | 43 (26.4) | 1.4 (1.1‐1.8) | 0.001 | 2.4 (1.2‐4.5) | 0.008 |
| Male | 46 (60) | 114 (69.9) | 0.7 (0.5‐1.04) | 0.09 | ||
| White race | 35 (44.9) | 97 (59.5) | 0.7 (0.6‐1) | 0.03 | 0.5 (0.3‐1.02) | 0.058 |
| Isolate | ||||||
| MRSA | 50 (64.1) | 74 (45.4) | 1.7 (1.1‐2.5) | 0.008 | 1.8 (0.6‐5.2) | 0.3 |
| MSSA | 28 (35.9) | 89 (54.6) | 0.6 (0.4‐0.9) | 0.008 | ||
| Comorbidities | ||||||
| Diabetes mellitus | 21 (26.9) | 54 (33.1) | 0.8 (0.5‐1.2) | 0.34 | ||
| Cirrhosis | 6 (7.7) | 8 (4.9) | 1.3 (0.7‐2.5) | 0.35 | ||
| Acute kidney injury | 65 (83.3) | 103 (63.2) | 2.2 (1.3‐3.7) | 0.004 | 1.6 (0.5‐5.4) | 0.43 |
| Chronic kidney disease | 12 (15.4) | 41 (25.1) | 0.6 (0.4‐1.1) | 0.11 | ||
| End‐stage renal disease | 15 (19.2) | 32 (19.6) | 1 (0.6‐1.5) | 0.94 | ||
| IVDU | 3 (3.8) | 6 (3.7) | 1.03 (0.4‐2.6) | 1 | ||
| Treating service | ||||||
| Medical | 61 (78.2) | 118 (72.4) | 1.3 (0.7‐2.6) | 0.33 | ||
| Surgical | 11 (14.1) | 67 (41.1) | 1 (0.9‐1.1) | 0.71 | ||
| Presence of foreign body | ||||||
| Cardiac device | 6 (7.7) | 11 (6.7) | 1.1 (0.6‐2.1) | 0.78 | ||
| PICC line | 20 (25.6) | 38 (23.3) | 1.1 (0.7‐1.6) | 0.69 | ||
| Nontunneled CVC | 22 (28.2) | 19 (11.7) | 1.9 (1.3‐2.7) | 0.004 | 3.6 (0.7‐17.7) | 0.11 |
| Tunneled CVC | 15 (19.2) | 24 (14.7) | 1.2 (0.8‐1.9) | 0.36 | ||
| AV fistula | 0 | 10 (6.1) | 0.1 (0.09‐2) | 0.15 | ||
| Other | 4 (5.1) | 11 (6.7) | 0.8 (0.3‐1.9) | 0.64 | ||
| Onset | ||||||
| Community onset | 46 (59) | 108 (66.3) | 0.8 (0.6‐1.2) | 0.27 | ||
| Hospital onset | 32 (41) | 55 (33.7) | 1.2 (0.8‐1.8) | 0.27 | ||
| Source | ||||||
| CLABSI | 15 (19.2) | 38 (23.3) | 0.8 (0.5‐1.4) | 0.48 | ||
| SSTI | 12 (15.4) | 32 (19.6) | 0.8 (0.5‐1.4) | 0.44 | ||
| Endocarditis | 14 (17.9) | 3 (1.8) | 2.9 (2.1‐3.9) | <0.0001 | 9.4 (2.2‐1.1) | 0.003 |
| Thrombophlebitis | 0 | 4 (2.4) | 0.3 (0.02‐4.2) | 0.37 | ||
| Prostatic abscess | 1 (1.3) | 3 (1.8) | 0.8 (0.1‐4.2) | 0.76 | ||
| Paravertebral abscess | 0 | 4 (2.4) | 0.3 (0.02‐4.2) | 0.37 | ||
| Mediastinal abscess | 1 (1.3) | 2 (1.2) | 1.03 (0.2‐5.1) | 0.97 | ||
| CAP | 4 (5.1) | 4 (2.4) | 1.5 (0.8‐3.2) | 0.21 | ||
| VAP | 2 (2.6) | 3 (1.8) | 1.2 (0.4‐3.7) | 0.7 | ||
| Surgical site infection | 1 (1.3) | 2 (1.2) | 1.03 (0.2‐5.2) | 0.97 | ||
| Ventriculostomy | 0 | 1 (0.6) | 0.8 (0.1‐8.5) | 0.82 | ||
| Bone or joint infection | 1 (1.3) | 4 (2.4) | 0.6 (0.1‐3.6) | 0.59 | ||
| Unknown | 27 (34.6) | 63 (38.6) | 0.9 (0.6‐1.3) | 0.55 | ||
Performance of Process of Care and Association With Outcomes
The analysis of the performance of the processes of care and outcomes is shown in Table 3. After adjusting for relevant clinical and demographic characteristics, and those with a level of significance of <0.05, obtaining follow‐up blood cultures more than 4 days after the onset of bacteremia independently increased the risk of clinical failure (RR: 6.5; 95% CI: 2.1‐20.5; P=0.001). When consultation with an ID specialist was obtained within the first 6 days from onset of bacteremia, the risk of clinical failure was 0.3 (95% CI: 0.1‐0.9; P=0.03); however, consultation with an ID specialist overall was not associated with clinical failure (RR: 1; 95% CI: 0.7‐1.4; P=0.98).
| Variable | Clinical Failure, n=78 (%) | No Clinical Failure, n=163 (%) | Unadjusted RR (CI) | P Value* | Adjusted OR (CI) | P Value* |
|---|---|---|---|---|---|---|
| ||||||
| Timing of follow‐up blood culture, n=200 | ||||||
| Less than 2 days | 30 (19.2) | 87 (53.4) | 0.7 (0.5‐0.9) | 0.01 | 1.2 (0.5‐2.9) | 0.60 |
| 24 days (ref) | 16 (20.5) | 39 (23.9) | 0.9 (0.8‐1.1) | 0.53 | ||
| More than 4 days | 19 (24.3) | 9 (5.5) | 1.3 (1.1‐1.5) | <0.0001 | 6.6 (2.1‐20.5) | 0.001 |
| Early antibiotic therapy, n=232 | 66 (84.6) | 132 (81) | 1.2 (0.7‐2.3) | 0.45 | ||
| Monitoring of vancomycin levels, n=156 | 37 (20.8) | 97 (59.5) | 0.8 (0.6‐1.03) | 0.09 | ||
| Therapy with ‐lactam, n=103‖ | 7 (8.8) | 49 (30.1) | 0.4 (0.2‐0.8) | 0.01 | 0.1 (0.04‐0.5) | 0.002 |
| Consultation with ID specialist, n=241 | 31 (39.7) | 66 (40.5) | 1 (0.7‐1.4) | 0.98 | ||
| Early consultation with ID specialist, n=97# | 19 (24.3) | 56 (34.3) | 0.5 (0.3‐0.8) | 0.006 | 0.3 (0.1‐0.9) | 0.03 |
| Echocardiography, n=241 | 45 (57.7) | 96 (58.9) | 1 (0.7‐1.4) | 0.86 | ||
| Early echocardiography, n=141** | 35 (44.9) | 91 (55.8) | 0.7 (0.5‐1.07) | 0.11 | ||
A comparison of the average number of days to performance of processes of care is presented in Table 4. Patients with clinical failure had significantly greater elapsed time from the first positive blood culture to the first follow‐up blood culture as compared to those who did not have clinical failure (mean 2.321.3 days vs 3.883.37; P<0.0001). Forty‐one patients (17.1%) failed to have at least 1 follow‐up blood culture.
| Process of Care | Clinical Failure | No Clinical Failure | P Value* |
|---|---|---|---|
| |||
| First follow‐up blood culture, n=200 | 3.883.37 | 2.321.3 | <0.0001 |
| Consultation with infectious diseases, n=97 | 6.96.55 | 4.354.34 | 0.06 |
| First antibiotic dose, n=232 | 0.431.05 | 0.57 1.11 | 0.63 |
| First dose of ‐lactam, n=56 | 4.41.6 | 3.51.4 | 0.1 |
| First vancomycin trough, n=156 | 2.632.04 | 2.552.02 | 0.81 |
| Echocardiography, n=141 | 3.421.74 | 3.312.05 | 0.47 |
Among patients with clinical failure, an ID specialist was consulted at a mean time of 7 days from the onset of bacteremia, compared to patients with no clinical failure in whom a consult was obtained at a mean of 4 days (P=0.06) (Table 4). Overall, ID specialists were only consulted in 97/241 (40.2%) episodes.
Echocardiographic studies were performed in 141/241 (58.5)% of episodes, and they were more likely to be obtained when an ID specialist was consulted (RR: 1.7; 95% CI: 1.4‐2.1; P<0.0001). Lack of performance of these studies was not associated with clinical failure (Table 3).
Antibiotic Administration and De‐escalation of Therapy
There were no significant differences in the average time from the first positive blood culture to the administration of antibiotics between patients who had clinical failure and those who did not (0.571.11 vs 0.431.05; P=0.63) (Table 4).
Patients with MSSA BSI and no documented penicillin allergy were treated with ‐lactam or cephalosporin antibiotics in 56/103(54.3%) episodes. Patients were 2.5 times more likely to receive ‐lactam antibiotics when an ID specialist was consulted (95% CI: 1.8‐3.5; P<0.0001). Among patients with MSSA BSI, treatment with ‐lactams was an independent predictor of decreased risk of clinical failure (RR: 0.2; 95% CI: 0.07‐0.9; P=0.005) (Table 3).
DISCUSSION
Our study showed a significant rate of morbidity associated with S aureus bacteremia and identified processes of care in the management of SAB that impact patient outcomes.
Our results show that early consultation with an ID specialist was associated with a decreased risk of developing clinical failure, increased likelihood of identification of a source of infection, and positively impacted administration of appropriate antibiotic therapy, especially in cases of MSSA BSI, with overall improvement in patient outcomes. However, consultation with an ID specialist was only obtained in 40.2% of our cases, which is consistent with published data.[10, 11, 12, 13] Consultation with an ID specialist itself did not impact clinical failure, but rather timeliness in obtaining expert guidance was associated with better outcomes. As shown in previous studies,[10, 11, 12, 13, 14] compliance with the standards of care and patient prognosis are improved when ID specialists are involved in the management of SAB. Our study reiterates that early consultation with an ID specialist has a positive outcome in patient care, as opposed to delaying consultation once the patient has persistent bacteremia for more than 7 days. This association could be explained by considering that the majority of the standards of care are time sensitive, which include: obtaining surveillance blood cultures 48 to 96 hours after initial detection[10] or initiating therapy,[11, 14] removal of foci of infection,[10, 11, 12, 14] use of parenteral ‐lactams for the treatment of MSSA,[10, 11, 13, 14] performing echocardiography when clinically indicated,[10, 11, 13, 14] and appropriate duration of therapy.[10, 13, 14] Importantly, studies have shown that when ID specialists' recommendations are followed, patients are more likely to be cured,[10, 11, 13] and are less likely to relapse.[10, 11, 12] Given the complexities of treating patients with SAB and high rates of clinical failures, routine guidance could be beneficial to healthcare providers as part of a multidisciplinary structured strategy that is set in motion the moment a patient with SAB is identified by the microbiology laboratory. The processes of care outlined in this this study can serve as quality of care indicators and be integrated into a structured strategy to optimize the management of SAB.
Regarding optimal timing for follow‐up blood cultures, our results show that delays in obtaining follow‐up blood cultures (more than 4 days from onset of bacteremia) was independently associated with increased risk of clinical failure. Timely follow‐up blood cultures have been previously identified as quality of care indicators.[10, 11, 13, 14] Compliance with obtaining follow‐up blood cultures improves when this step is integrated into a bundle of care.[14]
Antimicrobial therapy was promptly initiated in the majority of the patients in our study. However, areas for improvement were identified. Vancomycin was the empirical therapy of choice in most of the cases, but an appropriate dose was only received by 65% of the patients, and vancomycin levels after the fourth dose were obtained in 85.9% of instances when indicated. Although in our cohort these results were not significantly associated with clinical failure, previous studies have described attainment of a target therapeutic vancomycin trough (1520 mg/dL) as a factor for treatment success.[17, 18] This problem could be addressed through physician education on therapeutic drug monitoring,[19] as well as through an ASP intervention, which have successfully led efforts to improve vancomycin utilization and dosing.[20] Among patients with MSSA BSI, therapy with ‐lactams was associated with improved outcomes, and was more likely to be administered when an ID specialist was consulted. This is in accordance with previous studies that have shown that higher rates of appropriate antimicrobial therapy are achieved when ID specialists are involved in management of SAB.[10, 11, 13, 14] The use of ‐lactams for treatment of MSSA BSI has been consistently associated with lower SAB‐related mortality and relapse.[21, 22, 23, 24, 25, 26]
Echocardiographic studies were obtained in only half of the patients in our cohort, and they were twice more likely to be obtained when an ID specialist was consulted. Although we did not evaluate the appropriateness of the echocardiographic study, the increased proportion of studies performed when ID specialists were consulted could indicate a more in‐depth evaluation of the case. Moreover, in our cohort, when ID specialists where involved in direct patient care, a source of infection was more likely to be identified. This is in accordance with previous studies proposing that because evaluation by ID specialists are more detailed, they lead to increased use in ancillary studies and recognition of complicated cases.[10, 12]
Limitations of this study include its retrospective design and the fact that it was performed in a single institution. The source of infection was defined as documented by treating providers and not by independent diagnostic criteria. Antibiotic use was collected throughout duration of admission, and was not followed after patients were discharged, as these data were not available on the electronic medical record for all patients. Deaths that may have occurred after hospital discharge were not included. We did not account for elevated vancomycin minimum inhibitory concentration as a risk factor for the main outcome, and adjustment of vancomycin based on serum levels was not factored in. Acute kidney injury was accounted for anytime during hospitalization, but not in relation to antimicrobial administration. Despite the limitations, our study has strengths that make our results generalizable. Although our institution is a single medical center, it serves a large and diverse population as reflected in our cases. Even though this is a retrospective cohort study, the use of a centralized electronic medical record allowed us to identify each aspect of the management of SAB, as implemented by different treating services (medical and surgical), as continuous variables (days) rather than only in a dichotomous fashion. Moreover, by being a community teaching hospital, we were able to explore aspects of the practice of physicians in training versus practicing clinicians. These results could be extrapolated to other healthcare facilities aiming to improve the management of SAB.
CONCLUSIONS
Our results suggest that obtaining timely follow‐up blood cultures, use of ‐lactams in patients with MSSA BSI, and early consultation with infectious diseases are the processes of care that could serve as quality and patient‐safety indicators for the management of SAB. These results contribute to a growing body of evidence supporting the implementation of structured processes of care to optimize the management and clinical outcomes of hospitalized patients with SAB.
Disclosure: Nothing to report.
Staphylococcus aureus is one the most common pathogens isolated in nosocomial and community‐onset bloodstream infections (BSI) in the United States.[1, 2] S aureus bacteremia (SAB) has been reported in the literature to have substantial morbidity and mortality, with rates ranging between 15% and 60% worldwide.[3, 4, 5, 6] In the United States, patients with infections due to S aureus have on average 3 times the length of hospital stay than inpatients without these infections (14.3 days vs 4.5 days; P<0.01).[7] Healthcare costs are negatively impacted by these infections. In a recent meta‐analysis, Zimlichman et al.[8] reported that central‐line BSI (CLABSI) and surgical‐site infection (SSI) caused by methicillin‐resistant S aureus (MRSA) resulted in the highest estimated costs associated with hospital‐acquired infections in the United States ($58,614 [95% CI: $16,760‐$174,755] for CLABSI and $42,300 [95% CI: $4,005‐$82,670] for SSIs).
Appropriate management of SAB includes not only selecting the correct antimicrobial based on susceptibilities but also timely control of the source of infection, appropriate use of ancillary studies when indicated, and pharmacokinetic and pharmacodynamic therapeutic monitoring of antimicrobial therapy when vancomycin is used.[9] Consultation with an infectious diseases (ID) specialist has been associated with increased compliance with evidence‐based strategies in the management of SAB,[10, 11, 12, 13, 14] such as appropriate antibiotic choice, optimized duration of treatment, removal of the source of infection, and better use of cardiac echocardiography, resulting in improved outcomes.[13, 14]
Some, but not all, institutions have adopted bundles,[14] mandatory ID consultation[10] or daily prospective audit and feedback review[15] as part of antimicrobial stewardship program (ASP) interventions aiming to optimize the management of SABs. As part of our ASP quality improvement activities we performed the present study to determine our institutional rate of clinical failure in the treatment of SAB, to identify current practice patterns in the delivery of processes of care, and evaluate their association with clinical outcomes of hospitalized patients with SAB to identify future areas of improvement.
METHODS
A retrospective cohort study was performed at a 1558 licensed‐bed tertiary teaching hospital in Miami, Florida. All hospitalized patients 18 years of age or older with at least 1 positive blood culture with MRSA or methicillin‐susceptible S aureus (MSSA) between January 1, 2012 and April 30, 2013 were included. Patients were identified from the electronic microbiology laboratory database. For the purposes of this study, only the first episode of SAB was included in the analysis. Patients were excluded if aged younger than 18 years or if SAB was detected in an outpatient setting. The primary outcome was clinical failure, defined as a composite endpoint of in‐hospital mortality or persistent bacteremia; persistent bacteremia was defined as bacteremia for 7 or more days after the first positive blood culture. S aureus isolates were identified by standard methods.[16] Species identification was performed by latex agglutination. Antimicrobial susceptibility testing was performed using an automated system (Vitek 2; bioMerieux, Durham, NC) according to standard guidelines.
Data collected included baseline demographics, comorbidities, and treating healthcare provider's service; provider's service was categorized into 1 of 5 groups: internal medicine (academic), internal medicine (hospitalist), surgery, trauma, or neurosurgery. Duration of bacteremia was recorded and defined as the time between first positive and first negative blood culture. The time of first positive culture was defined as the date in which the culture was obtained. Patients who failed to have at least 1 follow‐up blood culture were not counted toward the main outcome. Additionally, presence of a foreign body (cardiac device, orthopedic prosthesis, tunneled catheter, nontunneled catheter) and presumed source of infection as documented in the electronic medical record by the treating service was also collected. Infections were considered community associated when onset of bacteremia occurred within the first 72 hours of admission, and hospital associated if onset of bacteremia occurred after 72 hours of admission.
Based on current practice guidelines,[9] the variables considered processes of care were the time to obtain the first follow‐up blood culture, time from first positive blood culture to initiation of appropriate antibiotic therapy (defined as a loading dose of vancomycin of 15 mg/kg, or a ‐lactam if the organism was susceptible), time to obtain the first vancomycin trough (when indicated), time from first positive blood culture to consultation with ID specialist, appropriate antibiotic de‐escalation (vancomycin to ‐lactam antibiotic if the organism was susceptible and the patient had no allergies or contraindications), and obtaining an echocardiographic study (transthoracic echocardiogram or transesophageal echocardiogram).
Statistical analyses were performed using SAS 9.2 (SAS Institute, Cary, NC). Differences in proportions were analyzed with 2 or Fisher exact test, accordingly. Differences in means among continuous variables were evaluated using independent samples of paired samples t tests as appropriate for the analysis. Continuous variables were dichotomized using a clinically established cutoff to determine relative risk (RR). A univariate analysis of risk factors associated with clinical failure was performed. Multivariable analyses were performed using logistic regression. Models were created using the backward stepwise approach and included all variables found to be statistically significant at less than 0.05 level in the univariate model and those of clinical significance. The study was reviewed and approved by the institutional review boards at the University of Miami and Jackson Memorial Hospital.
RESULTS
During the study period, 241 patients with a first episode of SAB were identified. MRSA and MSSA were isolated in 124 (51.4%) and 117 (48.5%) patients, respectively. Demographic and clinical characteristics of the study population based on isolate are summarized in Table 1. One hundred seventy‐nine (74.3%) patients were under the care of internal medicine services. There was no association between treating service (medical vs surgical) and clinical failure.
| Variable | MRSA, N= 124 (%) | MSSA, N= 117(%) | Overall, N=241 |
|---|---|---|---|
| |||
| Demographics | |||
| Age, y (mean) | 53.915.57 | 53.915.22 | 53.915.3 |
| Age greater than 60 years | 41 (33.1) | 39 (33.3) | 80 (33.2) |
| Male sex | 80 (64.5) | 80 (68.4) | 160 (66.4) |
| White race | 63 (50.8) | 69 (59) | 132 (54.8) |
| Comorbidities | |||
| Diabetes mellitus | 35 (28.2) | 40 (34.2) | 75 (30.7) |
| Hypertension | 56 (45.2) | 40 (34.2) | 96 (39.8) |
| CHF | 6 (4.8) | 9 (7.7) | 15 (6.2) |
| CVD | 8 (6.4) | 6 (5.1) | 14 (5.8) |
| Chronic pulmonary disease | 14 (11.3) | 14 (12) | 28 (11.6) |
| Malignancy | 9 (7.3) | 19 (16.2) | 28 (11.6) |
| Active chemotherapy | 5 (4) | 10 (8.5) | 15 (6.2) |
| HIV | 27 (21.8) | 17 (14.5) | 44 (18.2) |
| Cirrhosis | 6 (4.8) | 8 (6.8) | 14 (5.8) |
| Hepatitis C infection | 7 (5.6) | 11 (9.4) | 18 (7.5) |
| Acute kidney injury | 88 (71) | 80 (68.4) | 168 (69.7) |
| Chronic kidney disease | 29 (23.4) | 24 (20.5) | 53 (22) |
| End‐stage renal disease | 25 (20.2) | 22 (18.8) | 47 (19.5) |
| Connective tissue disease | 3 (2.4) | 3 (2.6) | 6 (2.5) |
| Alcohol abuse | 3 (2.4) | 1 (0.8) | 4 (1.7) |
| IVDU | 4 (3.2) | 5 (4.3) | 9 (3.7) |
| Hemiplegia | 4 (3.2) | 0 | 4 (1.7) |
| Chronic osteomyelitis | 4 (3.2) | 0 | 4 (1.7) |
| History of transplant | 7 (5.6) | 0 | 7 (2.9) |
| Surgery during current admission | 29 (23.4) | 46 (39.3) | 75 (31.1) |
| Surgery during the previous 30 days | 31 (25) | 36 (30.8) | 67 (25.3) |
| Treating service | |||
| Medical service | 89 (71.8) | 90 (76.9) | 179 (74.3) |
| Surgical service | 21 (16.9) | 16 (13.7) | 37 (15.3) |
| Other | 7 (5.6) | 11 (9.4) | 18 (7.5) |
| Presence of foreign body | |||
| PICC line | 24 (19.3) | 34 (29.1) | 58 (24.1) |
| Tunneled CVC | 24 (19.3) | 15 (12.8) | 39 (16.2) |
| Nontunneled CVC | 13 (10.5) | 28 (23.9) | 41 (17) |
| AV fistula | 3 (2.4) | 7 (6) | 10 (4.1) |
| Cardiac device | 8 (6.4) | 9 (7.7) | 17 (7) |
| Other | 4 (3.2) | 11 (9.4) | 15 (6.2) |
| Source of infection | |||
| CLABSI | 32 (25.8) | 21 (17.9) | 53 (22) |
| SSTI | 24 (19.3) | 20 (17.1) | 44 (18.2) |
| Endocarditis | 10 (8.1) | 7 (6) | 17 (7) |
| Thrombophlebitis | 2 (1.6) | 2 (1.7) | 4 (1.7) |
| Prostatic abscess | 3 (2.4) | 1 (0.8) | 4 (1.7) |
| Paravertebral abscess | 2 (1.6) | 2 (1.7) | 4 (1.7) |
| Mediastinal abscess | 2 (1.6) | 1 (0.8) | 3 (1.2) |
| CAP | 4 (3.2) | 4 (3.4) | 8 (3.3) |
| VAP | 3 (2.4) | 2 (1.7) | 5 (2.1) |
| Surgical site infection | 2 (1.6) | 1 (0.8) | 3 (1.2) |
| Ventriculostomy | 0 | 1 (0.8) | 1 (0.4) |
| Bone or joint infection | 2 (1.6) | 3 (2.6) | 5 (2.1) |
| Unknown | 38 (30.6) | 52 (44.4) | 90 (37.3) |
| Onset | |||
| Community onset* | 77 (62.1) | 77 (65.8) | 154 (63.9) |
| Hospital onset | 47 (37.9) | 40 (34.2) | 87 (36.1) |
The onset of infection occurred in the community in 77 (62.1%) patients with MRSA and in 77 (65.8%) patients with MSSA. The documented source of bacteremia was unknown in 30% of patients with MRSA and 44% of those with MSSA BSI. When ID specialists were consulted, patients were more likely to have a source of infection identified (RR: 1.5; 95% confidence interval [CI]: 1.2‐1.8; P<0.0001). The most commonly documented sources of infection were CLABSI, which occurred in 32 (25.8%) patients with MRSA and 21 (17.9%) patients with MSSA, followed by skin and soft tissue infections in 24 (19.3%) patients with MRSA BSI and 20 (17.1%) patients with MSSA BSI. All patients with CLABSI had documentation of catheter removal.
Clinical failure (defined as in‐hospital mortality or persistent bacteremia) occurred in 78 (32.4%) patients. Of these, 50 (20.7%) represented in‐hospital mortality, and 31 (12.9%) had persistent bacteremia. Table 2 summarizes the demographic and clinical characteristics associated with clinical failure. In the univariate analysis, the variables statistically significantly associated with clinical failure were: age greater than 60 years (RR: 1.4; 95% CI: 1.1‐1.8; P=0.001), bacteremia due to MRSA (RR: 1.7; 95% CI: 1.1‐2.5; P=0.008), white race (RR: 0.7; 95% CI: 0.6‐1; P=0.03), acute kidney injury during admission (RR: 2.2; 95% CI: 1.3‐3.7; P=0.004), presence of nontunneled central venous catheters at the onset of bacteremia (RR: 1.9; 95% CI: 1.3‐2.7; P=0.004), and endocarditis (RR: 2.9; 95% CI: 2.1‐3.9; P<0.0001). In the multivariable analysis, age greater than 60 years and endocarditis were found to be independent risk factors for the development of clinical failure.
| Variable | Clinical Failure, N=78 (%) | No Clinical Failure, N=163 (%) | Unadjusted RR (CI) | P Value* | Adjusted OR (CI) | P Value* |
|---|---|---|---|---|---|---|
| ||||||
| Demographics | ||||||
| Age >60 years | 37 (47.4) | 43 (26.4) | 1.4 (1.1‐1.8) | 0.001 | 2.4 (1.2‐4.5) | 0.008 |
| Male | 46 (60) | 114 (69.9) | 0.7 (0.5‐1.04) | 0.09 | ||
| White race | 35 (44.9) | 97 (59.5) | 0.7 (0.6‐1) | 0.03 | 0.5 (0.3‐1.02) | 0.058 |
| Isolate | ||||||
| MRSA | 50 (64.1) | 74 (45.4) | 1.7 (1.1‐2.5) | 0.008 | 1.8 (0.6‐5.2) | 0.3 |
| MSSA | 28 (35.9) | 89 (54.6) | 0.6 (0.4‐0.9) | 0.008 | ||
| Comorbidities | ||||||
| Diabetes mellitus | 21 (26.9) | 54 (33.1) | 0.8 (0.5‐1.2) | 0.34 | ||
| Cirrhosis | 6 (7.7) | 8 (4.9) | 1.3 (0.7‐2.5) | 0.35 | ||
| Acute kidney injury | 65 (83.3) | 103 (63.2) | 2.2 (1.3‐3.7) | 0.004 | 1.6 (0.5‐5.4) | 0.43 |
| Chronic kidney disease | 12 (15.4) | 41 (25.1) | 0.6 (0.4‐1.1) | 0.11 | ||
| End‐stage renal disease | 15 (19.2) | 32 (19.6) | 1 (0.6‐1.5) | 0.94 | ||
| IVDU | 3 (3.8) | 6 (3.7) | 1.03 (0.4‐2.6) | 1 | ||
| Treating service | ||||||
| Medical | 61 (78.2) | 118 (72.4) | 1.3 (0.7‐2.6) | 0.33 | ||
| Surgical | 11 (14.1) | 67 (41.1) | 1 (0.9‐1.1) | 0.71 | ||
| Presence of foreign body | ||||||
| Cardiac device | 6 (7.7) | 11 (6.7) | 1.1 (0.6‐2.1) | 0.78 | ||
| PICC line | 20 (25.6) | 38 (23.3) | 1.1 (0.7‐1.6) | 0.69 | ||
| Nontunneled CVC | 22 (28.2) | 19 (11.7) | 1.9 (1.3‐2.7) | 0.004 | 3.6 (0.7‐17.7) | 0.11 |
| Tunneled CVC | 15 (19.2) | 24 (14.7) | 1.2 (0.8‐1.9) | 0.36 | ||
| AV fistula | 0 | 10 (6.1) | 0.1 (0.09‐2) | 0.15 | ||
| Other | 4 (5.1) | 11 (6.7) | 0.8 (0.3‐1.9) | 0.64 | ||
| Onset | ||||||
| Community onset | 46 (59) | 108 (66.3) | 0.8 (0.6‐1.2) | 0.27 | ||
| Hospital onset | 32 (41) | 55 (33.7) | 1.2 (0.8‐1.8) | 0.27 | ||
| Source | ||||||
| CLABSI | 15 (19.2) | 38 (23.3) | 0.8 (0.5‐1.4) | 0.48 | ||
| SSTI | 12 (15.4) | 32 (19.6) | 0.8 (0.5‐1.4) | 0.44 | ||
| Endocarditis | 14 (17.9) | 3 (1.8) | 2.9 (2.1‐3.9) | <0.0001 | 9.4 (2.2‐1.1) | 0.003 |
| Thrombophlebitis | 0 | 4 (2.4) | 0.3 (0.02‐4.2) | 0.37 | ||
| Prostatic abscess | 1 (1.3) | 3 (1.8) | 0.8 (0.1‐4.2) | 0.76 | ||
| Paravertebral abscess | 0 | 4 (2.4) | 0.3 (0.02‐4.2) | 0.37 | ||
| Mediastinal abscess | 1 (1.3) | 2 (1.2) | 1.03 (0.2‐5.1) | 0.97 | ||
| CAP | 4 (5.1) | 4 (2.4) | 1.5 (0.8‐3.2) | 0.21 | ||
| VAP | 2 (2.6) | 3 (1.8) | 1.2 (0.4‐3.7) | 0.7 | ||
| Surgical site infection | 1 (1.3) | 2 (1.2) | 1.03 (0.2‐5.2) | 0.97 | ||
| Ventriculostomy | 0 | 1 (0.6) | 0.8 (0.1‐8.5) | 0.82 | ||
| Bone or joint infection | 1 (1.3) | 4 (2.4) | 0.6 (0.1‐3.6) | 0.59 | ||
| Unknown | 27 (34.6) | 63 (38.6) | 0.9 (0.6‐1.3) | 0.55 | ||
Performance of Process of Care and Association With Outcomes
The analysis of the performance of the processes of care and outcomes is shown in Table 3. After adjusting for relevant clinical and demographic characteristics, and those with a level of significance of <0.05, obtaining follow‐up blood cultures more than 4 days after the onset of bacteremia independently increased the risk of clinical failure (RR: 6.5; 95% CI: 2.1‐20.5; P=0.001). When consultation with an ID specialist was obtained within the first 6 days from onset of bacteremia, the risk of clinical failure was 0.3 (95% CI: 0.1‐0.9; P=0.03); however, consultation with an ID specialist overall was not associated with clinical failure (RR: 1; 95% CI: 0.7‐1.4; P=0.98).
| Variable | Clinical Failure, n=78 (%) | No Clinical Failure, n=163 (%) | Unadjusted RR (CI) | P Value* | Adjusted OR (CI) | P Value* |
|---|---|---|---|---|---|---|
| ||||||
| Timing of follow‐up blood culture, n=200 | ||||||
| Less than 2 days | 30 (19.2) | 87 (53.4) | 0.7 (0.5‐0.9) | 0.01 | 1.2 (0.5‐2.9) | 0.60 |
| 24 days (ref) | 16 (20.5) | 39 (23.9) | 0.9 (0.8‐1.1) | 0.53 | ||
| More than 4 days | 19 (24.3) | 9 (5.5) | 1.3 (1.1‐1.5) | <0.0001 | 6.6 (2.1‐20.5) | 0.001 |
| Early antibiotic therapy, n=232 | 66 (84.6) | 132 (81) | 1.2 (0.7‐2.3) | 0.45 | ||
| Monitoring of vancomycin levels, n=156 | 37 (20.8) | 97 (59.5) | 0.8 (0.6‐1.03) | 0.09 | ||
| Therapy with ‐lactam, n=103‖ | 7 (8.8) | 49 (30.1) | 0.4 (0.2‐0.8) | 0.01 | 0.1 (0.04‐0.5) | 0.002 |
| Consultation with ID specialist, n=241 | 31 (39.7) | 66 (40.5) | 1 (0.7‐1.4) | 0.98 | ||
| Early consultation with ID specialist, n=97# | 19 (24.3) | 56 (34.3) | 0.5 (0.3‐0.8) | 0.006 | 0.3 (0.1‐0.9) | 0.03 |
| Echocardiography, n=241 | 45 (57.7) | 96 (58.9) | 1 (0.7‐1.4) | 0.86 | ||
| Early echocardiography, n=141** | 35 (44.9) | 91 (55.8) | 0.7 (0.5‐1.07) | 0.11 | ||
A comparison of the average number of days to performance of processes of care is presented in Table 4. Patients with clinical failure had significantly greater elapsed time from the first positive blood culture to the first follow‐up blood culture as compared to those who did not have clinical failure (mean 2.321.3 days vs 3.883.37; P<0.0001). Forty‐one patients (17.1%) failed to have at least 1 follow‐up blood culture.
| Process of Care | Clinical Failure | No Clinical Failure | P Value* |
|---|---|---|---|
| |||
| First follow‐up blood culture, n=200 | 3.883.37 | 2.321.3 | <0.0001 |
| Consultation with infectious diseases, n=97 | 6.96.55 | 4.354.34 | 0.06 |
| First antibiotic dose, n=232 | 0.431.05 | 0.57 1.11 | 0.63 |
| First dose of ‐lactam, n=56 | 4.41.6 | 3.51.4 | 0.1 |
| First vancomycin trough, n=156 | 2.632.04 | 2.552.02 | 0.81 |
| Echocardiography, n=141 | 3.421.74 | 3.312.05 | 0.47 |
Among patients with clinical failure, an ID specialist was consulted at a mean time of 7 days from the onset of bacteremia, compared to patients with no clinical failure in whom a consult was obtained at a mean of 4 days (P=0.06) (Table 4). Overall, ID specialists were only consulted in 97/241 (40.2%) episodes.
Echocardiographic studies were performed in 141/241 (58.5)% of episodes, and they were more likely to be obtained when an ID specialist was consulted (RR: 1.7; 95% CI: 1.4‐2.1; P<0.0001). Lack of performance of these studies was not associated with clinical failure (Table 3).
Antibiotic Administration and De‐escalation of Therapy
There were no significant differences in the average time from the first positive blood culture to the administration of antibiotics between patients who had clinical failure and those who did not (0.571.11 vs 0.431.05; P=0.63) (Table 4).
Patients with MSSA BSI and no documented penicillin allergy were treated with ‐lactam or cephalosporin antibiotics in 56/103(54.3%) episodes. Patients were 2.5 times more likely to receive ‐lactam antibiotics when an ID specialist was consulted (95% CI: 1.8‐3.5; P<0.0001). Among patients with MSSA BSI, treatment with ‐lactams was an independent predictor of decreased risk of clinical failure (RR: 0.2; 95% CI: 0.07‐0.9; P=0.005) (Table 3).
DISCUSSION
Our study showed a significant rate of morbidity associated with S aureus bacteremia and identified processes of care in the management of SAB that impact patient outcomes.
Our results show that early consultation with an ID specialist was associated with a decreased risk of developing clinical failure, increased likelihood of identification of a source of infection, and positively impacted administration of appropriate antibiotic therapy, especially in cases of MSSA BSI, with overall improvement in patient outcomes. However, consultation with an ID specialist was only obtained in 40.2% of our cases, which is consistent with published data.[10, 11, 12, 13] Consultation with an ID specialist itself did not impact clinical failure, but rather timeliness in obtaining expert guidance was associated with better outcomes. As shown in previous studies,[10, 11, 12, 13, 14] compliance with the standards of care and patient prognosis are improved when ID specialists are involved in the management of SAB. Our study reiterates that early consultation with an ID specialist has a positive outcome in patient care, as opposed to delaying consultation once the patient has persistent bacteremia for more than 7 days. This association could be explained by considering that the majority of the standards of care are time sensitive, which include: obtaining surveillance blood cultures 48 to 96 hours after initial detection[10] or initiating therapy,[11, 14] removal of foci of infection,[10, 11, 12, 14] use of parenteral ‐lactams for the treatment of MSSA,[10, 11, 13, 14] performing echocardiography when clinically indicated,[10, 11, 13, 14] and appropriate duration of therapy.[10, 13, 14] Importantly, studies have shown that when ID specialists' recommendations are followed, patients are more likely to be cured,[10, 11, 13] and are less likely to relapse.[10, 11, 12] Given the complexities of treating patients with SAB and high rates of clinical failures, routine guidance could be beneficial to healthcare providers as part of a multidisciplinary structured strategy that is set in motion the moment a patient with SAB is identified by the microbiology laboratory. The processes of care outlined in this this study can serve as quality of care indicators and be integrated into a structured strategy to optimize the management of SAB.
Regarding optimal timing for follow‐up blood cultures, our results show that delays in obtaining follow‐up blood cultures (more than 4 days from onset of bacteremia) was independently associated with increased risk of clinical failure. Timely follow‐up blood cultures have been previously identified as quality of care indicators.[10, 11, 13, 14] Compliance with obtaining follow‐up blood cultures improves when this step is integrated into a bundle of care.[14]
Antimicrobial therapy was promptly initiated in the majority of the patients in our study. However, areas for improvement were identified. Vancomycin was the empirical therapy of choice in most of the cases, but an appropriate dose was only received by 65% of the patients, and vancomycin levels after the fourth dose were obtained in 85.9% of instances when indicated. Although in our cohort these results were not significantly associated with clinical failure, previous studies have described attainment of a target therapeutic vancomycin trough (1520 mg/dL) as a factor for treatment success.[17, 18] This problem could be addressed through physician education on therapeutic drug monitoring,[19] as well as through an ASP intervention, which have successfully led efforts to improve vancomycin utilization and dosing.[20] Among patients with MSSA BSI, therapy with ‐lactams was associated with improved outcomes, and was more likely to be administered when an ID specialist was consulted. This is in accordance with previous studies that have shown that higher rates of appropriate antimicrobial therapy are achieved when ID specialists are involved in management of SAB.[10, 11, 13, 14] The use of ‐lactams for treatment of MSSA BSI has been consistently associated with lower SAB‐related mortality and relapse.[21, 22, 23, 24, 25, 26]
Echocardiographic studies were obtained in only half of the patients in our cohort, and they were twice more likely to be obtained when an ID specialist was consulted. Although we did not evaluate the appropriateness of the echocardiographic study, the increased proportion of studies performed when ID specialists were consulted could indicate a more in‐depth evaluation of the case. Moreover, in our cohort, when ID specialists where involved in direct patient care, a source of infection was more likely to be identified. This is in accordance with previous studies proposing that because evaluation by ID specialists are more detailed, they lead to increased use in ancillary studies and recognition of complicated cases.[10, 12]
Limitations of this study include its retrospective design and the fact that it was performed in a single institution. The source of infection was defined as documented by treating providers and not by independent diagnostic criteria. Antibiotic use was collected throughout duration of admission, and was not followed after patients were discharged, as these data were not available on the electronic medical record for all patients. Deaths that may have occurred after hospital discharge were not included. We did not account for elevated vancomycin minimum inhibitory concentration as a risk factor for the main outcome, and adjustment of vancomycin based on serum levels was not factored in. Acute kidney injury was accounted for anytime during hospitalization, but not in relation to antimicrobial administration. Despite the limitations, our study has strengths that make our results generalizable. Although our institution is a single medical center, it serves a large and diverse population as reflected in our cases. Even though this is a retrospective cohort study, the use of a centralized electronic medical record allowed us to identify each aspect of the management of SAB, as implemented by different treating services (medical and surgical), as continuous variables (days) rather than only in a dichotomous fashion. Moreover, by being a community teaching hospital, we were able to explore aspects of the practice of physicians in training versus practicing clinicians. These results could be extrapolated to other healthcare facilities aiming to improve the management of SAB.
CONCLUSIONS
Our results suggest that obtaining timely follow‐up blood cultures, use of ‐lactams in patients with MSSA BSI, and early consultation with infectious diseases are the processes of care that could serve as quality and patient‐safety indicators for the management of SAB. These results contribute to a growing body of evidence supporting the implementation of structured processes of care to optimize the management and clinical outcomes of hospitalized patients with SAB.
Disclosure: Nothing to report.
- , , , , , . Nosocomial bloodstream infections in US hospitals: analysis of 24,179 cases from a prospective nationwide surveillance study. Clin Infect Dis. 2004;39(3):309–317.
- , , , . Laboratory‐based surveillance of current antimicrobial resistance patterns and trends among Staphylococcus aureus: 2005 status in the United States. Ann Clin Microbiol Antimicrob. 2006;5:2.
- , , , , , . The impact of methicillin resistance in Staphylococcus aureus bacteremia on patient outcomes: mortality, length of stay, and hospital charges. Infect Control Hosp Epidemiol. 2005;26(2):166–174.
- , , , . Staphylococcal bacteremia and altered host resistance. Ann Intern Medicine. 1968;69(5):859–873.
- , , , , , . Comparison of mortality associated with methicillin‐resistant and methicillin‐susceptible Staphylococcus aureus bacteremia: a meta‐analysis. Clin Infect Dis. 2003;36(1):53–59.
- . Unfavourable prognostic factors in Staphylococcus aureus septicemia and endocarditis. Scand J Infect Dis. 1985;17(2):179–187.
- , , , et al. The burden of Staphylococcus aureus infections on hospitals in the United States: an analysis of the 2000 and 2001 Nationwide Inpatient Sample Database. Arch Intern Med. 2005;165(15):1756–1761.
- , , , et al. Health care–associated infections: a meta‐analysis of costs and financial impact on the us health care system. JAMA Intern Med. 2013;173(22):2039–2046.
- , , , et al. Clinical practice guidelines by the Infectious Diseases Society of America for the treatment of methicillin‐resistant Staphylococcus aureus infections in adults and children. Clin Infect Dis. 2011;52(3):e18–e55.
- , , , , . Impact of routine infectious diseases service consultation on the evaluation, management, and outcomes of Staphylococcus aureus bacteremia. Clin Infect Dis. 2008;46(7):1000–1008.
- , , , et al. Outcome of Staphylococcus aureus bacteremia according to compliance with recommendations of infectious diseases specialists: experience with 244 patients. Clin Infect Dis. 1998;27(3):478–486.
- , , , . Infectious disease consultation for Staphylococcus aureus bacteremia improves patient management and outcomes. Infect Dis Clin Pract (Baltim Md). 2012;20(4):261–267.
- , , , , . The value of infectious diseases consultation in Staphylococcus aureus bacteremia. Am J Med. 2010;123(7):631–637.
- , , , et al. Impact of an evidence‐based bundle intervention in the quality‐of‐care management and outcome of Staphylococcus aureus bacteremia. Clin Infect Dis. 2013;57(9):1225–1233.
- , , , et al. Comparison of prior authorization and prospective audit with feedback for antimicrobial stewardship. Infect Control Hosp Epidemiol. 2014;35(9):1092–1099.
- Clinical Laboratory Standards Institute. Performance Standards for Antimicrobial Susceptibility Testing; Twenty‐First Informational Supplement. Wayne, PA: Clinical Laboratory Standards Institute; 2011.
- , , , . Impact of vancomycin exposure on outcomes in patients with methicillin‐resistant Staphylococcus aureus bacteremia: support for consensus guidelines suggested targets. Clin Infect Dis. 2011;52(8):975–981.
- , , , , . High‐dose vancomycin therapy for methicillin‐resistant Staphylococcus aureus infections: efficacy and toxicity. Arch Intern Med. 2006;166(19):2138–2144.
- , , , . Strategies for physician education in therapeutic drug monitoring. Clin Chem. 1998;44(2):401–407.
- , . Impact of antimicrobial stewardship program on vancomycin use in a pediatric teaching hospital. Pediatr Infect Dis J. 2010;29(8):707–711.
- , . Vancomycin for Staphylococcus aureus endocarditis in intravenous drug users. Antimicrob Agents Chemother. 1990;34(6):1227–1231.
- , , , et al. A prospective multicenter study of Staphylococcus aureus bacteremia: incidence of endocarditis, risk factors for mortality, and clinical impact of methicillin resistance. Medicine. 2003;82(5):322–332.
- , , , . Impact of empirical‐therapy selection on outcomes of intravenous drug users with infective endocarditis caused by methicillin‐susceptible Staphylococcus aureus. Antimicrob Agents Chemother. 2007;51(10):3731–3733.
- , , , et al. Use of vancomycin or first‐generation cephalosporins for the treatment of hemodialysis‐dependent patients with methicillin‐susceptible Staphylococcus aureus bacteremia. Clin Infect Dis. 2007;44(2):190–196.
- , , , et al. Outcome of vancomycin treatment in patients with methicillin‐susceptible Staphylococcus aureus bacteremia. Antimicrob Agents Chemother. 2008;52(1):192–197.
- , , , et al. Comparative effectiveness of nafcillin or cefazolin versus vancomycin in methicillin‐susceptible Staphylococcus aureus bacteremia. BMC Infect Dis. 2011;11:279.
- , , , , , . Nosocomial bloodstream infections in US hospitals: analysis of 24,179 cases from a prospective nationwide surveillance study. Clin Infect Dis. 2004;39(3):309–317.
- , , , . Laboratory‐based surveillance of current antimicrobial resistance patterns and trends among Staphylococcus aureus: 2005 status in the United States. Ann Clin Microbiol Antimicrob. 2006;5:2.
- , , , , , . The impact of methicillin resistance in Staphylococcus aureus bacteremia on patient outcomes: mortality, length of stay, and hospital charges. Infect Control Hosp Epidemiol. 2005;26(2):166–174.
- , , , . Staphylococcal bacteremia and altered host resistance. Ann Intern Medicine. 1968;69(5):859–873.
- , , , , , . Comparison of mortality associated with methicillin‐resistant and methicillin‐susceptible Staphylococcus aureus bacteremia: a meta‐analysis. Clin Infect Dis. 2003;36(1):53–59.
- . Unfavourable prognostic factors in Staphylococcus aureus septicemia and endocarditis. Scand J Infect Dis. 1985;17(2):179–187.
- , , , et al. The burden of Staphylococcus aureus infections on hospitals in the United States: an analysis of the 2000 and 2001 Nationwide Inpatient Sample Database. Arch Intern Med. 2005;165(15):1756–1761.
- , , , et al. Health care–associated infections: a meta‐analysis of costs and financial impact on the us health care system. JAMA Intern Med. 2013;173(22):2039–2046.
- , , , et al. Clinical practice guidelines by the Infectious Diseases Society of America for the treatment of methicillin‐resistant Staphylococcus aureus infections in adults and children. Clin Infect Dis. 2011;52(3):e18–e55.
- , , , , . Impact of routine infectious diseases service consultation on the evaluation, management, and outcomes of Staphylococcus aureus bacteremia. Clin Infect Dis. 2008;46(7):1000–1008.
- , , , et al. Outcome of Staphylococcus aureus bacteremia according to compliance with recommendations of infectious diseases specialists: experience with 244 patients. Clin Infect Dis. 1998;27(3):478–486.
- , , , . Infectious disease consultation for Staphylococcus aureus bacteremia improves patient management and outcomes. Infect Dis Clin Pract (Baltim Md). 2012;20(4):261–267.
- , , , , . The value of infectious diseases consultation in Staphylococcus aureus bacteremia. Am J Med. 2010;123(7):631–637.
- , , , et al. Impact of an evidence‐based bundle intervention in the quality‐of‐care management and outcome of Staphylococcus aureus bacteremia. Clin Infect Dis. 2013;57(9):1225–1233.
- , , , et al. Comparison of prior authorization and prospective audit with feedback for antimicrobial stewardship. Infect Control Hosp Epidemiol. 2014;35(9):1092–1099.
- Clinical Laboratory Standards Institute. Performance Standards for Antimicrobial Susceptibility Testing; Twenty‐First Informational Supplement. Wayne, PA: Clinical Laboratory Standards Institute; 2011.
- , , , . Impact of vancomycin exposure on outcomes in patients with methicillin‐resistant Staphylococcus aureus bacteremia: support for consensus guidelines suggested targets. Clin Infect Dis. 2011;52(8):975–981.
- , , , , . High‐dose vancomycin therapy for methicillin‐resistant Staphylococcus aureus infections: efficacy and toxicity. Arch Intern Med. 2006;166(19):2138–2144.
- , , , . Strategies for physician education in therapeutic drug monitoring. Clin Chem. 1998;44(2):401–407.
- , . Impact of antimicrobial stewardship program on vancomycin use in a pediatric teaching hospital. Pediatr Infect Dis J. 2010;29(8):707–711.
- , . Vancomycin for Staphylococcus aureus endocarditis in intravenous drug users. Antimicrob Agents Chemother. 1990;34(6):1227–1231.
- , , , et al. A prospective multicenter study of Staphylococcus aureus bacteremia: incidence of endocarditis, risk factors for mortality, and clinical impact of methicillin resistance. Medicine. 2003;82(5):322–332.
- , , , . Impact of empirical‐therapy selection on outcomes of intravenous drug users with infective endocarditis caused by methicillin‐susceptible Staphylococcus aureus. Antimicrob Agents Chemother. 2007;51(10):3731–3733.
- , , , et al. Use of vancomycin or first‐generation cephalosporins for the treatment of hemodialysis‐dependent patients with methicillin‐susceptible Staphylococcus aureus bacteremia. Clin Infect Dis. 2007;44(2):190–196.
- , , , et al. Outcome of vancomycin treatment in patients with methicillin‐susceptible Staphylococcus aureus bacteremia. Antimicrob Agents Chemother. 2008;52(1):192–197.
- , , , et al. Comparative effectiveness of nafcillin or cefazolin versus vancomycin in methicillin‐susceptible Staphylococcus aureus bacteremia. BMC Infect Dis. 2011;11:279.
© 2015 Society of Hospital Medicine
Identification of Hospitalists
A seminal 1996 New England Journal of Medicine article introduced the term hospitalist to describe the emerging trend of primary care physicians practicing in inpatient hospital settings.[1] Although physicians had practice patterns akin to hospitalists prior to the introduction of the term,[2] the field continues to grow and formalize as a unique specialty in medicine.
There is currently no board certification or specialty billing code associated with hospitalists. In 2009, the American Board of Internal Medicine and American Board of Family Medicine introduced a Focused Practice in Hospital Medicine optional recertification pathway.[3] However, absent a unique identifier, it remains difficult to identify the number of hospitalists practicing today. Issues with identification notwithstanding, published data consistently suggest that the number of hospitalists has grown dramatically over the last 2 decades.[4, 5, 6]
The Centers for Medicare and Medicaid Services (CMS), along with other payers, classify hospitalists based on their board certificationmost commonly internal medicine or family practice. Other approaches for more precise assessment utilized billing data or hospital designation. Saint et al. identified hospital‐based providers practicing in Washington State in 1994 using variable thresholds of billing for inpatient services.[2] In 2011, Welch et al. identified 25,787 hospitalists nationwide, using a 90% threshold of billing inpatient services in Medicare data.[6] That same year, an American Hospital Association survey identified 34,411 hospitalists based on self‐reporting.[4]
Building on the work of previous researchers, we applied an updated threshold of inpatient services in publicly available 2012 Medicare Provider Utilization and Payment Data to identify a range of hospitalists practicing in the United States. We also examine the codes billed by providers identified in different decile billing thresholds to assess the validity of using lower thresholds to identify hospitalists.
METHODS
Approach to Identifying Hospitalists
In April 2014, CMS publicly released Medicare Provider Utilization and Payment data from all 880,000 providers who billed Medicare Part B in 2012. The dataset included services charged for 2012 Medicare Part B fee‐for‐service claims. The data omitted claims billed by a unique National Provider Identifier (NPI) for fewer than 10 Medicare beneficiaries. CMS assigned a specialty designation to each provider in the pay data based on the Medicare specialty billing code listed most frequently on his or her claims.
We explored the number of hospitalists in the 2012 Medicare pay data using specialty designation in combination with patterns of billing data. We first grouped physicians with specialty designations of internal medicine and family practice (IM/FP), the most common board certifications for hospitalists. We then selected 4 Healthcare Common Procedure Coding System (HCPCS) code clusters commonly associated with hospitalist practice: acute inpatient (HCPCS codes 9922199223, 9923199233, and 9923899239), observation (9921899220, 9922499226, and 99217), observation/emnpatient same day (9923499236), and critical care (9929199292). We included observation services codes given the significant role hospitalists play in their use[7, 8] and CMS incorporation of observation services for a threshold to identify and exempt hospital‐based providers in meaningful use.[9]
Analysis of Billing Thresholds and Other Codes Billed by Hospitalists
We examined the numbers of hospitalists who would be identified using a 50%, 60%, 70%, 80%, or 90% threshold, and compared the level of change in the size of the group with each change in decile.
We then analyzed the services billed by hospitalists who billed our threshold codes between 60% and 70% of the time. We looked at all codes billed with a frequency of greater than 0.1%, grouping clusters of similar services to identify patterns of clinical activity performed by these physicians.
RESULTS
The 2012 Medicare pay data included 664,253 physicians with unique NPIs. Of these, 169,317 had IM/FP specialty designations, whereas just under half (46.25%) of those physicians billed any of the inpatient HCPCS codes associated with our threshold.
Table 1 describes the range of number of hospitalists identified by varying the threshold of inpatient services. A total of 28,473 providers bill the threshold‐associated inpatient codes almost exclusively, whereas each descending decile increases in size by an average of 7.29%.
| Threshold (%) | Unique NPIs | % of IM/FP Physicians | % of All Physicians |
|---|---|---|---|
| |||
| 90 | 28,473 | 16.8 | 4.3 |
| 80 | 30,866 | 18.2 | 4.6 |
| 70 | 32,834 | 19.4 | 4.9 |
| 60 | 35,116 | 20.7 | 5.3 |
| 50 | 37,646 | 22.2 | 5.7 |
We also analyzed billing patterns of a subset of physicians who billed our threshold codes between 60% and 70% of the time to better characterize the remainder of clinical work they perform. This group included 2282 physicians and only 56 unique HCPCS codes with frequencies greater than 0.1%. After clustering related codes, we identified 4 common code groups that account for the majority of the remaining billing beyond inpatient threshold codes (Table 2).
| Clinical Service Cluster | HCPCS Codes Included | % |
|---|---|---|
| ||
| Threshold codes | 99217, 99219, 99220, 99221, 99222, 99223, 99231, 99232, 99233, 99238, 99239, 99291 | 64.5 |
| Office visit (new and established) | 99203, 99204, 99205, 99211, 99212, 99213, 99214, 99215 | 15.3 |
| SNF care (initial and subsequent) | 99305, 99306, 99307, 99308, 99309, 99310, 99315 | 7.1 |
| ECG‐related codes | 93000, 93010, 93042 | 2.5 |
| Routine venipuncture | 36415 | 1.0 |
| Other codes with f>0.1%* | 25 codes | 5.1 |
| Codes with f0.1% | 439 codes | 4.5 |
| Total | 495 codes | 100.0 |
DISCUSSION
Hospitalists make up approximately 5% of the practicing physicians nationwide, performing a critical role caring for hospitalized patients. Saint et al. defined a pure hospitalist as a physician who meets a 90% threshold of inpatient services.[2] This approach has been replicated in subsequent studies that used a 90% threshold to identify hospitalists.[5, 6] Our results with the same threshold reveal more than 28,000 hospitalists with nearly uniform practice patterns, a 10% growth in the number of hospitalists from the Welch et al. analysis in 2011.[6]
A threshold is not a perfect tool for identifying groups of practicing physicians, as it creates an arbitrary cutoff within a dataset. Undoubtedly our analysis could include providers who would not consider themselves hospitalists, or alternatively, appear to have a hospital‐based practice when they do not. Our results suggest that a 90% threshold may identify a majority of practicing hospitalists, but excludes providers who likely identify as hospitalists albeit with divergent practice and billing patterns.
A lower threshold may be more inclusive of the current realities of hospitalist practice, accounting for the myriad other services provided during, immediately prior to, or following a hospitalization. With hospitalists commonly practicing in diverse facility settings, rotating through rehabilitation or nursing home facilities, discharge clinics, and preoperative medicine practices, the continued use of a 90% threshold appears to exclude a sizable number of practicing hospitalists.
In the absence of a formal identifier, developing identification methodologies that account for the diversity of hospitalist practice is crucial. As physician payment transitions to value‐based reimbursement, systems must have the ability to account for and allocate the most efficient mix of providers for their patient populations. Because provider alignment and coordination are structural features of these programs, these systems‐based changes in effect require accurate identification of hospitalists, yet currently lack the tools to do so.
Disclosures
The research reported here was supported by the Department of Veterans Affairs, Veterans Health Administration. Investigator salary support is provided through the South Texas Veterans Health Care System. The views expressed in this article are those of the authors and do not necessarily reflect the position or policy of the Department of Veterans Affairs. The authors report no conflicts of interest.
A seminal 1996 New England Journal of Medicine article introduced the term hospitalist to describe the emerging trend of primary care physicians practicing in inpatient hospital settings.[1] Although physicians had practice patterns akin to hospitalists prior to the introduction of the term,[2] the field continues to grow and formalize as a unique specialty in medicine.
There is currently no board certification or specialty billing code associated with hospitalists. In 2009, the American Board of Internal Medicine and American Board of Family Medicine introduced a Focused Practice in Hospital Medicine optional recertification pathway.[3] However, absent a unique identifier, it remains difficult to identify the number of hospitalists practicing today. Issues with identification notwithstanding, published data consistently suggest that the number of hospitalists has grown dramatically over the last 2 decades.[4, 5, 6]
The Centers for Medicare and Medicaid Services (CMS), along with other payers, classify hospitalists based on their board certificationmost commonly internal medicine or family practice. Other approaches for more precise assessment utilized billing data or hospital designation. Saint et al. identified hospital‐based providers practicing in Washington State in 1994 using variable thresholds of billing for inpatient services.[2] In 2011, Welch et al. identified 25,787 hospitalists nationwide, using a 90% threshold of billing inpatient services in Medicare data.[6] That same year, an American Hospital Association survey identified 34,411 hospitalists based on self‐reporting.[4]
Building on the work of previous researchers, we applied an updated threshold of inpatient services in publicly available 2012 Medicare Provider Utilization and Payment Data to identify a range of hospitalists practicing in the United States. We also examine the codes billed by providers identified in different decile billing thresholds to assess the validity of using lower thresholds to identify hospitalists.
METHODS
Approach to Identifying Hospitalists
In April 2014, CMS publicly released Medicare Provider Utilization and Payment data from all 880,000 providers who billed Medicare Part B in 2012. The dataset included services charged for 2012 Medicare Part B fee‐for‐service claims. The data omitted claims billed by a unique National Provider Identifier (NPI) for fewer than 10 Medicare beneficiaries. CMS assigned a specialty designation to each provider in the pay data based on the Medicare specialty billing code listed most frequently on his or her claims.
We explored the number of hospitalists in the 2012 Medicare pay data using specialty designation in combination with patterns of billing data. We first grouped physicians with specialty designations of internal medicine and family practice (IM/FP), the most common board certifications for hospitalists. We then selected 4 Healthcare Common Procedure Coding System (HCPCS) code clusters commonly associated with hospitalist practice: acute inpatient (HCPCS codes 9922199223, 9923199233, and 9923899239), observation (9921899220, 9922499226, and 99217), observation/emnpatient same day (9923499236), and critical care (9929199292). We included observation services codes given the significant role hospitalists play in their use[7, 8] and CMS incorporation of observation services for a threshold to identify and exempt hospital‐based providers in meaningful use.[9]
Analysis of Billing Thresholds and Other Codes Billed by Hospitalists
We examined the numbers of hospitalists who would be identified using a 50%, 60%, 70%, 80%, or 90% threshold, and compared the level of change in the size of the group with each change in decile.
We then analyzed the services billed by hospitalists who billed our threshold codes between 60% and 70% of the time. We looked at all codes billed with a frequency of greater than 0.1%, grouping clusters of similar services to identify patterns of clinical activity performed by these physicians.
RESULTS
The 2012 Medicare pay data included 664,253 physicians with unique NPIs. Of these, 169,317 had IM/FP specialty designations, whereas just under half (46.25%) of those physicians billed any of the inpatient HCPCS codes associated with our threshold.
Table 1 describes the range of number of hospitalists identified by varying the threshold of inpatient services. A total of 28,473 providers bill the threshold‐associated inpatient codes almost exclusively, whereas each descending decile increases in size by an average of 7.29%.
| Threshold (%) | Unique NPIs | % of IM/FP Physicians | % of All Physicians |
|---|---|---|---|
| |||
| 90 | 28,473 | 16.8 | 4.3 |
| 80 | 30,866 | 18.2 | 4.6 |
| 70 | 32,834 | 19.4 | 4.9 |
| 60 | 35,116 | 20.7 | 5.3 |
| 50 | 37,646 | 22.2 | 5.7 |
We also analyzed billing patterns of a subset of physicians who billed our threshold codes between 60% and 70% of the time to better characterize the remainder of clinical work they perform. This group included 2282 physicians and only 56 unique HCPCS codes with frequencies greater than 0.1%. After clustering related codes, we identified 4 common code groups that account for the majority of the remaining billing beyond inpatient threshold codes (Table 2).
| Clinical Service Cluster | HCPCS Codes Included | % |
|---|---|---|
| ||
| Threshold codes | 99217, 99219, 99220, 99221, 99222, 99223, 99231, 99232, 99233, 99238, 99239, 99291 | 64.5 |
| Office visit (new and established) | 99203, 99204, 99205, 99211, 99212, 99213, 99214, 99215 | 15.3 |
| SNF care (initial and subsequent) | 99305, 99306, 99307, 99308, 99309, 99310, 99315 | 7.1 |
| ECG‐related codes | 93000, 93010, 93042 | 2.5 |
| Routine venipuncture | 36415 | 1.0 |
| Other codes with f>0.1%* | 25 codes | 5.1 |
| Codes with f0.1% | 439 codes | 4.5 |
| Total | 495 codes | 100.0 |
DISCUSSION
Hospitalists make up approximately 5% of the practicing physicians nationwide, performing a critical role caring for hospitalized patients. Saint et al. defined a pure hospitalist as a physician who meets a 90% threshold of inpatient services.[2] This approach has been replicated in subsequent studies that used a 90% threshold to identify hospitalists.[5, 6] Our results with the same threshold reveal more than 28,000 hospitalists with nearly uniform practice patterns, a 10% growth in the number of hospitalists from the Welch et al. analysis in 2011.[6]
A threshold is not a perfect tool for identifying groups of practicing physicians, as it creates an arbitrary cutoff within a dataset. Undoubtedly our analysis could include providers who would not consider themselves hospitalists, or alternatively, appear to have a hospital‐based practice when they do not. Our results suggest that a 90% threshold may identify a majority of practicing hospitalists, but excludes providers who likely identify as hospitalists albeit with divergent practice and billing patterns.
A lower threshold may be more inclusive of the current realities of hospitalist practice, accounting for the myriad other services provided during, immediately prior to, or following a hospitalization. With hospitalists commonly practicing in diverse facility settings, rotating through rehabilitation or nursing home facilities, discharge clinics, and preoperative medicine practices, the continued use of a 90% threshold appears to exclude a sizable number of practicing hospitalists.
In the absence of a formal identifier, developing identification methodologies that account for the diversity of hospitalist practice is crucial. As physician payment transitions to value‐based reimbursement, systems must have the ability to account for and allocate the most efficient mix of providers for their patient populations. Because provider alignment and coordination are structural features of these programs, these systems‐based changes in effect require accurate identification of hospitalists, yet currently lack the tools to do so.
Disclosures
The research reported here was supported by the Department of Veterans Affairs, Veterans Health Administration. Investigator salary support is provided through the South Texas Veterans Health Care System. The views expressed in this article are those of the authors and do not necessarily reflect the position or policy of the Department of Veterans Affairs. The authors report no conflicts of interest.
A seminal 1996 New England Journal of Medicine article introduced the term hospitalist to describe the emerging trend of primary care physicians practicing in inpatient hospital settings.[1] Although physicians had practice patterns akin to hospitalists prior to the introduction of the term,[2] the field continues to grow and formalize as a unique specialty in medicine.
There is currently no board certification or specialty billing code associated with hospitalists. In 2009, the American Board of Internal Medicine and American Board of Family Medicine introduced a Focused Practice in Hospital Medicine optional recertification pathway.[3] However, absent a unique identifier, it remains difficult to identify the number of hospitalists practicing today. Issues with identification notwithstanding, published data consistently suggest that the number of hospitalists has grown dramatically over the last 2 decades.[4, 5, 6]
The Centers for Medicare and Medicaid Services (CMS), along with other payers, classify hospitalists based on their board certificationmost commonly internal medicine or family practice. Other approaches for more precise assessment utilized billing data or hospital designation. Saint et al. identified hospital‐based providers practicing in Washington State in 1994 using variable thresholds of billing for inpatient services.[2] In 2011, Welch et al. identified 25,787 hospitalists nationwide, using a 90% threshold of billing inpatient services in Medicare data.[6] That same year, an American Hospital Association survey identified 34,411 hospitalists based on self‐reporting.[4]
Building on the work of previous researchers, we applied an updated threshold of inpatient services in publicly available 2012 Medicare Provider Utilization and Payment Data to identify a range of hospitalists practicing in the United States. We also examine the codes billed by providers identified in different decile billing thresholds to assess the validity of using lower thresholds to identify hospitalists.
METHODS
Approach to Identifying Hospitalists
In April 2014, CMS publicly released Medicare Provider Utilization and Payment data from all 880,000 providers who billed Medicare Part B in 2012. The dataset included services charged for 2012 Medicare Part B fee‐for‐service claims. The data omitted claims billed by a unique National Provider Identifier (NPI) for fewer than 10 Medicare beneficiaries. CMS assigned a specialty designation to each provider in the pay data based on the Medicare specialty billing code listed most frequently on his or her claims.
We explored the number of hospitalists in the 2012 Medicare pay data using specialty designation in combination with patterns of billing data. We first grouped physicians with specialty designations of internal medicine and family practice (IM/FP), the most common board certifications for hospitalists. We then selected 4 Healthcare Common Procedure Coding System (HCPCS) code clusters commonly associated with hospitalist practice: acute inpatient (HCPCS codes 9922199223, 9923199233, and 9923899239), observation (9921899220, 9922499226, and 99217), observation/emnpatient same day (9923499236), and critical care (9929199292). We included observation services codes given the significant role hospitalists play in their use[7, 8] and CMS incorporation of observation services for a threshold to identify and exempt hospital‐based providers in meaningful use.[9]
Analysis of Billing Thresholds and Other Codes Billed by Hospitalists
We examined the numbers of hospitalists who would be identified using a 50%, 60%, 70%, 80%, or 90% threshold, and compared the level of change in the size of the group with each change in decile.
We then analyzed the services billed by hospitalists who billed our threshold codes between 60% and 70% of the time. We looked at all codes billed with a frequency of greater than 0.1%, grouping clusters of similar services to identify patterns of clinical activity performed by these physicians.
RESULTS
The 2012 Medicare pay data included 664,253 physicians with unique NPIs. Of these, 169,317 had IM/FP specialty designations, whereas just under half (46.25%) of those physicians billed any of the inpatient HCPCS codes associated with our threshold.
Table 1 describes the range of number of hospitalists identified by varying the threshold of inpatient services. A total of 28,473 providers bill the threshold‐associated inpatient codes almost exclusively, whereas each descending decile increases in size by an average of 7.29%.
| Threshold (%) | Unique NPIs | % of IM/FP Physicians | % of All Physicians |
|---|---|---|---|
| |||
| 90 | 28,473 | 16.8 | 4.3 |
| 80 | 30,866 | 18.2 | 4.6 |
| 70 | 32,834 | 19.4 | 4.9 |
| 60 | 35,116 | 20.7 | 5.3 |
| 50 | 37,646 | 22.2 | 5.7 |
We also analyzed billing patterns of a subset of physicians who billed our threshold codes between 60% and 70% of the time to better characterize the remainder of clinical work they perform. This group included 2282 physicians and only 56 unique HCPCS codes with frequencies greater than 0.1%. After clustering related codes, we identified 4 common code groups that account for the majority of the remaining billing beyond inpatient threshold codes (Table 2).
| Clinical Service Cluster | HCPCS Codes Included | % |
|---|---|---|
| ||
| Threshold codes | 99217, 99219, 99220, 99221, 99222, 99223, 99231, 99232, 99233, 99238, 99239, 99291 | 64.5 |
| Office visit (new and established) | 99203, 99204, 99205, 99211, 99212, 99213, 99214, 99215 | 15.3 |
| SNF care (initial and subsequent) | 99305, 99306, 99307, 99308, 99309, 99310, 99315 | 7.1 |
| ECG‐related codes | 93000, 93010, 93042 | 2.5 |
| Routine venipuncture | 36415 | 1.0 |
| Other codes with f>0.1%* | 25 codes | 5.1 |
| Codes with f0.1% | 439 codes | 4.5 |
| Total | 495 codes | 100.0 |
DISCUSSION
Hospitalists make up approximately 5% of the practicing physicians nationwide, performing a critical role caring for hospitalized patients. Saint et al. defined a pure hospitalist as a physician who meets a 90% threshold of inpatient services.[2] This approach has been replicated in subsequent studies that used a 90% threshold to identify hospitalists.[5, 6] Our results with the same threshold reveal more than 28,000 hospitalists with nearly uniform practice patterns, a 10% growth in the number of hospitalists from the Welch et al. analysis in 2011.[6]
A threshold is not a perfect tool for identifying groups of practicing physicians, as it creates an arbitrary cutoff within a dataset. Undoubtedly our analysis could include providers who would not consider themselves hospitalists, or alternatively, appear to have a hospital‐based practice when they do not. Our results suggest that a 90% threshold may identify a majority of practicing hospitalists, but excludes providers who likely identify as hospitalists albeit with divergent practice and billing patterns.
A lower threshold may be more inclusive of the current realities of hospitalist practice, accounting for the myriad other services provided during, immediately prior to, or following a hospitalization. With hospitalists commonly practicing in diverse facility settings, rotating through rehabilitation or nursing home facilities, discharge clinics, and preoperative medicine practices, the continued use of a 90% threshold appears to exclude a sizable number of practicing hospitalists.
In the absence of a formal identifier, developing identification methodologies that account for the diversity of hospitalist practice is crucial. As physician payment transitions to value‐based reimbursement, systems must have the ability to account for and allocate the most efficient mix of providers for their patient populations. Because provider alignment and coordination are structural features of these programs, these systems‐based changes in effect require accurate identification of hospitalists, yet currently lack the tools to do so.
Disclosures
The research reported here was supported by the Department of Veterans Affairs, Veterans Health Administration. Investigator salary support is provided through the South Texas Veterans Health Care System. The views expressed in this article are those of the authors and do not necessarily reflect the position or policy of the Department of Veterans Affairs. The authors report no conflicts of interest.
Measuring Patient Experiences
The hospitalized patient experience has become an area of increased focus for hospitals given the recent coupling of patient satisfaction to reimbursement rates for Medicare patients.[1] Although patient experiences are multifactorial, 1 component is the relationship that hospitalized patients develop with their inpatient physicians. In recognition of the importance of this relationship, several organizations including the Society of Hospital Medicine, Society of General Internal Medicine, American College of Physicians, the American College of Emergency Physicians, and the Accreditation Council for Graduate Medical Education have recommended that patients know and understand who is guiding their care at all times during their hospitalization.[2, 3] Unfortunately, previous studies have shown that hospitalized patients often lack the ability to identify[4, 5] and understand their course of care.[6, 7] This may be due to numerous clinical factors including lack of a prior relationship, rapid pace of clinical care, and the frequent transitions of care found in both hospitalists and general medicine teaching services.[5, 8, 9] Regardless of the cause, one could hypothesize that patients who are unable to identify or understand the role of their physician may be less informed about their hospitalization, which may lead to further confusion, dissatisfaction, and ultimately a poor experience.
Given the proliferation of nonteaching hospitalist services in teaching hospitals, it is important to understand if patient experiences differ between general medicine teaching and hospitalist services. Several reasons could explain why patient experiences may vary on these services. For example, patients on a hospitalist service will likely interact with a single physician caretaker, which may give a feeling of more personalized care. In contrast, patients on general medicine teaching services are cared for by larger teams of residents under the supervision of an attending physician. Residents are also subjected to duty‐hour restrictions, clinic responsibilities, and other educational requirements that may impede the continuity of care for hospitalized patients.[10, 11, 12] Although 1 study has shown that hospitalist‐intensive hospitals perform better on patient satisfaction measures,[13] no study to date has compared patient‐reported experiences on general medicine teaching and nonteaching hospitalist services. This study aimed to evaluate the hospitalized patient experience on both teaching and nonteaching hospitalist services by assessing several patient‐reported measures of their experience, namely their confidence in their ability to identify their physician(s), understand their roles, and their rating of both the coordination and overall care.
METHODS
Study Design
We performed a retrospective cohort analysis at the University of Chicago Medical Center between July 2007 and June 2013. Data were acquired as part of the Hospitalist Project, an ongoing study that is used to evaluate the impact of hospitalists, and now serves as infrastructure to continue research related to hospital care at University of Chicago.[14] Patients were cared for by either the general medicine teaching service or the nonteaching hospitalist service. General medicine teaching services were composed of an attending physician who rotates for 2 weeks at a time, a second‐ or third‐year medicine resident, 1 to 2 medicine interns, and 1 to 2 medical students.[15] The attending physician assigned to the patient's hospitalization was the attending listed on the first day of hospitalization, regardless of the length of hospitalization. Nonteaching hospitalist services consisted of a single hospitalist who worked 7‐day shifts, and were assisted by a nurse practitioner/physician's assistant (NPA). The majority of attendings on the hospitalist service were less than 5 years out of residency. Both services admitted 7 days a week, with patients initially admitted to the general medicine teaching service until resident caps were met, after which all subsequent admissions were admitted to the hospitalist service. In addition, the hospitalist service is also responsible for specific patient subpopulations, such as lung and renal transplants, and oncologic patients who have previously established care with our institution.
Data Collection
During a 30‐day posthospitalization follow‐up questionnaire, patients were surveyed regarding their confidence in their ability to identify and understand the roles of their physician(s) and their perceptions of the overall coordination of care and their overall care, using a 5‐point Likert scale (1 = poor understanding to 5 = excellent understanding). Questions related to satisfaction with care and coordination were derived from the Picker‐Commonwealth Survey, a previously validated survey meant to evaluate patient‐centered care.[16] Patients were also asked to report their race, level of education, comorbid diseases, and whether they had any prior hospitalizations within 1 year. Chart review was performed to obtain patient age, gender, and hospital length of stay (LOS), and calculated Charlson Comorbidity Index (CCI).[17] Patients with missing data or responses to survey questions were excluded from final analysis. The University of Chicago Institutional Review Board approved the study protocol, and all patients provided written consented prior to participation.
Data Analysis
After initial analysis noted that outcomes were skewed, the decision was made to dichotomize the data and use logistic rather than linear regression models. Patient responses to the follow‐up phone questionnaire were dichotomized to reflect the top 2 categories (excellent and very good). Pearson 2 analysis was used to assess for any differences in demographic characteristics, disease severity, and measures of patient experience between the 2 services. To assess if service type was associated with differences in our 4 measures of patient experience, we created a 3‐level mixed‐effects logistic regression using a logit function while controlling for age, gender, race, CCI, LOS, previous hospitalizations within 1 year, level of education, and academic year. These models studied the longitudinal association between teaching service and the 4 outcome measures, while also controlling for the cluster effect of time nested within individual patients who were clustered within physicians. The model included random intercepts at both the patient and physician level and also included a random effect of service (teaching vs nonteaching) at the patient level. A Hausman test was used to determine if these random‐effects models improved fit over a fixed‐effects model, and the intraclass correlations were compared using likelihood ratio tests to determine the appropriateness of a 3‐level versus 2‐level model. Data management and 2 analyses were performed using Stata version 13.0 (StataCorp, College Station, TX), and mixed‐effects regression models were done in SuperMix (Scientific Software International, Skokie, IL).
RESULTS
In total, 14,855 patients were enrolled during their hospitalization with 57% and 61% completing the 30‐day follow‐up survey on the hospitalist and general medicine teaching service, respectively. In total, 4131 (69%) and 4322 (48%) of the hospitalist and general medicine services, respectively, either did not answer all survey questions, or were missing basic demographic data, and thus were excluded. Data from 4591 patients on the general medicine teaching (52% of those enrolled at hospitalization) and 1811 on the hospitalist service (31% of those enrolled at hospitalization) were used for final analysis (Figure 1). Respondents were predominantly female (61% and 56%), African American (75% and 63%), with a mean age of 56.2 (19.4) and 57.1 (16.1) years, for the general medicine teaching and hospitalist services, respectively. A majority of patients (71% and 66%) had a CCI of 0 to 3 on both services. There were differences in self‐reported comorbidities between the 2 groups, with hospitalist services having a higher prevalence of cancer (20% vs 7%), renal disease (25% vs 18%), and liver disease (23% vs 7%). Patients on the hospitalist service had a longer mean LOS (5.5 vs 4.8 days), a greater percentage of a hospitalization within 1 year (58% vs 52%), and a larger proportion who were admitted in 2011 to 2013 compared to 2007 to 2010 (75% vs 39%), when compared to the general medicine teaching services. Median LOS and interquartile ranges were similar between both groups. Although most baseline demographics were statistically different between the 2 groups (Table 1), these differences were likely clinically insignificant. Compared to those who responded to the follow‐up survey, nonresponders were more likely to be African American (73% and 64%, P < 0.001) and female (60% and 56%, P < 0.01). The nonresponders were more likely to be hospitalized in the past 1 year (62% and 53%, P < 0.001) and have a lower CCI (CCI 03 [75% and 80%, P < 0.001]) compared to responders. Demographics between responders and nonresponders were also statistically different from one another.
| Variable | General Medicine Teaching | Nonteaching Hospitalist | P Value |
|---|---|---|---|
| |||
| Total (n) | 4,591 | 1,811 | <0.001 |
| Attending classification, hospitalist, n (%) | 1,147 (25) | 1,811 (100) | |
| Response rate, % | 61 | 57 | <0.01 |
| Age, y, mean SD | 56.2 19.4 | 57.1 16.1 | <0.01 |
| Gender, n (%) | <0.01 | ||
| Male | 1,796 (39) | 805 (44) | |
| Female | 2,795 (61) | 1,004 (56) | |
| Race, n (%) | <0.01 | ||
| African American | 3,440 (75) | 1,092 (63) | |
| White | 900 (20) | 571 (32) | |
| Asian/Pacific | 38 (1) | 17 (1) | |
| Other | 20 (1) | 10 (1) | |
| Unknown | 134 (3) | 52 (3) | |
| Charlson Comorbidity Index, n (%) | <0.001 | ||
| 0 | 1,635 (36) | 532 (29) | |
| 12 | 1,590 (35) | 675 (37) | |
| 39 | 1,366 (30) | 602 (33) | |
| Self‐reported comorbidities | |||
| Anemia/sickle cell disease | 1,201 (26) | 408 (23) | 0.003 |
| Asthma/COPD | 1,251 (28) | 432 (24) | 0.006 |
| Cancer* | 300 (7) | 371 (20) | <0.001 |
| Depression | 1,035 (23) | 411 (23) | 0.887 |
| Diabetes | 1,381 (30) | 584 (32) | 0.087 |
| Gastrointestinal | 1,140 (25) | 485 (27) | 0.104 |
| Cardiac | 1,336 (29) | 520 (29) | 0.770 |
| Hypertension | 2,566 (56) | 1,042 (58) | 0.222 |
| HIV/AIDS | 151 (3) | 40 (2) | 0.022 |
| Kidney disease | 828 (18) | 459 (25) | <0.001 |
| Liver disease | 313 (7) | 417 (23) | <0.001 |
| Stroke | 543 (12) | 201 (11) | 0.417 |
| Education level | 0.066 | ||
| High school | 2,248 (49) | 832 (46) | |
| Junior college/college | 1,878 (41) | 781 (43) | |
| Postgraduate | 388 (8) | 173 (10) | |
| Don't know | 77 (2) | 23 (1) | |
| Academic year, n (%) | <0.001 | ||
| July 2007 June 2008 | 938 (20) | 90 (5) | |
| July 2008 June 2009 | 702 (15) | 148 (8) | |
| July 2009 June 2010 | 576(13) | 85 (5) | |
| July 2010 June 2011 | 602 (13) | 138 (8) | |
| July 2011 June 2012 | 769 (17) | 574 (32) | |
| July 2012 June 2013 | 1,004 (22) | 774 (43) | |
| Length of stay, d, mean SD | 4.8 7.3 | 5.5 6.4 | <0.01 |
| Prior hospitalization (within 1 year), yes, n (%) | 2,379 (52) | 1,039 (58) | <0.01 |
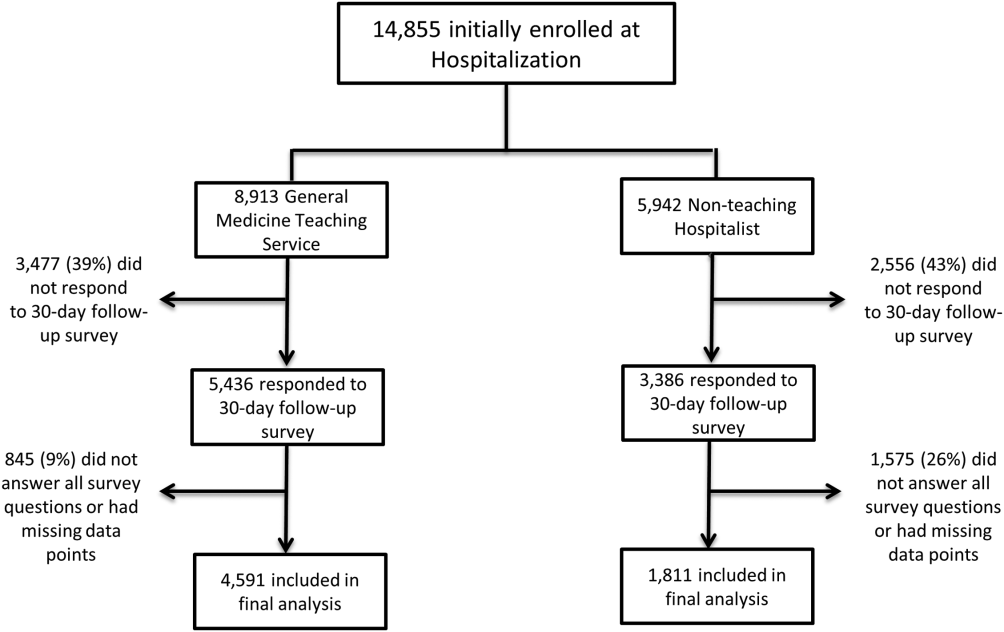
Unadjusted results revealed that patients on the hospitalist service were more confident in their abilities to identify their physician(s) (50% vs 45%, P < 0.001), perceived greater ability in understanding the role of their physician(s) (54% vs 50%, P < 0.001), and reported greater satisfaction with coordination and teamwork (68% vs 64%, P = 0.006) and with overall care (73% vs 67%, P < 0.001) (Figure 2).
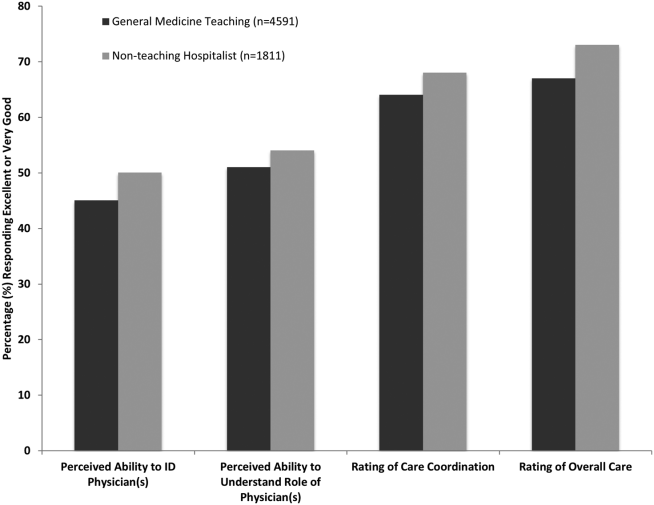
From the mixed‐effects regression models it was discovered that admission to the hospitalist service was associated with a higher odds ratio (OR) of reporting overall care as excellent or very good (OR: 1.33; 95% confidence interval [CI]: 1.15‐1.47). There was no difference between services in patients' ability to identify their physician(s) (OR: 0.89; 95% CI: 0.61‐1.11), in patients reporting a better understanding of the role of their physician(s) (OR: 1.09; 95% CI: 0.94‐1.23), or in their rating of overall coordination and teamwork (OR: 0.71; 95% CI: 0.42‐1.89).
A subgroup analysis was performed on the 25% of hospitalist attendings in the general medicine teaching service comparing this cohort to the hospitalist services, and it was found that patients perceived better overall care on the hospitalist service (OR: 1.17; 95% CI: 1.01‐ 1.31) than on the general medicine service (Table 2). All other domains in the subgroup analysis were not statistically significant. Finally, an ordinal logistic regression was performed for each of these outcomes, but it did not show any major differences compared to the logistic regression of dichotomous outcomes.
| Domains in Patient Experience* | Odds Ratio (95% CI) | P Value |
|---|---|---|
| ||
| How would you rate your ability to identify the physicians and trainees on your general medicine team during the hospitalization? | ||
| Model 1 | 0.89 (0.611.11) | 0.32 |
| Model 2 | 0.98 (0.671.22) | 0.86 |
| How would you rate your understanding of the roles of the physicians and trainees on your general medicine team? | ||
| Model 1 | 1.09 (0.941.23) | 0.25 |
| Model 2 | 1.19 (0.981.36) | 0.08 |
| How would you rate the overall coordination and teamwork among the doctors and nurses who care for you during your hospital stay? | ||
| Model 1 | 0.71 (0.421.89) | 0.18 |
| Model 2 | 0.82 (0.651.20) | 0.23 |
| Overall, how would you rate the care you received at the hospital? | ||
| Model 1 | 1.33 (1.151.47) | 0.001 |
| Model 2 | 1.17 (1.011.31) | 0.04 |
DISCUSSION
This study is the first to directly compare measures of patient experience on hospitalist and general medicine teaching services in a large, multiyear comparison across multiple domains. In adjusted analysis, we found that patients on nonteaching hospitalist services rated their overall care better than those on general medicine teaching services, whereas no differences in patients' ability to identify their physician(s), understand their role in their care, or rating of coordination of care were found. Although the magnitude of the differences in rating of overall care may appear small, it remains noteworthy because of the recent focus on patient experience at the reimbursement level, where small differences in performance can lead to large changes in payment. Because of the observational design of this study, it is important to consider mechanisms that could account for our findings.
The first are the structural differences between the 2 services. Our subgroup analysis comparing patients rating of overall care on a general medicine service with a hospitalist attending to a pure hospitalist cohort found a significant difference between the groups, indicating that the structural differences between the 2 groups may be a significant contributor to patient satisfaction ratings. Under the care of a hospitalist service, a patient would only interact with a single physician on a daily basis, possibly leading to a more meaningful relationship and improved communication between patient and provider. Alternatively, while on a general medicine teaching service, patients would likely interact with multiple physicians, as a result making their confidence in their ability to identify and perception at understanding physicians' roles more challenging.[18] This dilemma is further compounded by duty hour restrictions, which have subsequently led to increased fragmentation in housestaff scheduling. The patient experience on the general medicine teaching service may be further complicated by recent data that show residents spend a minority of time in direct patient care,[19, 20] which could additionally contribute to patients' inability to understand who their physicians are and to the decreased satisfaction with their care. This combination of structural complexity, duty hour reform, and reduced direct patient interaction would likely decrease the chance a patient will interact with the same resident on a consistent basis,[5, 21] thus making the ability to truly understand who their caretakers are, and the role they play, more difficult.
Another contributing factor could be the use of NPAs on our hospitalist service. Given that these providers often see the patient on a more continual basis, hospitalized patients' exposure to a single, continuous caretaker may be a factor in our findings.[22] Furthermore, with studies showing that hospitalists also spend a small fraction of their day in direct patient care,[23, 24, 25] the use of NPAs may allow our hospitalists to spend greater amounts of time with their patients, thus improving patients' rating of their overall care and influencing their perceived ability to understand their physician's role.
Although there was no difference between general medicine teaching and hospitalist services with respect to patient understanding of their roles, our data suggest that both groups would benefit from interventions to target this area. Focused attempts at improving patient's ability to identify and explain the roles of their inpatient physician(s) have been performed. For example, previous studies have attempted to improve a patient's ability to identify their physician through physician facecards[8, 9] or the use of other simple interventions (ie, bedside whiteboards).[4, 26] Results from such interventions are mixed, as they have demonstrated the capacity to improve patients' ability to identify who their physician is, whereas few have shown any appreciable improvement in patient satisfaction.[26]
Although our findings suggest that structural differences in team composition may be a possible explanation, it is also important to consider how the quality of care a patient receives affects their experience. For instance, hospitalists have been shown to produce moderate improvements in patient‐centered outcomes such as 30‐day readmission[27] and hospital length of stay[14, 28, 29, 30, 31] when compared to other care providers, which in turn could be reflected in the patient's perception of their overall care. In a large national study of acute care hospitals using the Hospital Consumer Assessment of Healthcare Providers and Systems survey, Chen and colleagues found that for most measures of patient satisfaction, hospitals with greater use of hospitalist care were associated with better patient‐centered care.[13] These outcomes were in part driven by patient‐centered domains such as discharge planning, pain control, and medication management. It is possible that patients are sensitive to the improved outcomes that are associated with hospitalist services, and reflect this in their measures of patient satisfaction.
Last, because this is an observational study and not a randomized trial, it is possible that the clinical differences in the patients cared for by these services could have led to our findings. Although the clinical significance of the differences in patient demographics were small, patients seen on the hospitalist service were more likely to be older white males, with a slightly longer LOS, greater comorbidities, and more hospitalizations in the previous year than those seen on the general medicine teaching service. Additionally, our hospitalist service frequently cares for highly specific subpopulations (ie, liver and renal transplant patients, and oncology patients), which could have influenced our results. For example, transplant patients who may be very grateful for their second chance, are preferentially admitted to the hospitalist service, which could have biased our results in favor of hospitalists.[32] Unfortunately, we were unable to control for all such factors.
Although we hope that multivariable analysis can adjust for many of these differences, we are not able to account for possible unmeasured confounders such as time of day of admission, health literacy, personality differences, physician turnover, or nursing and other ancillary care that could contribute to these findings. In addition to its observational study design, our study has several other limitations. First, our study was performed at a single institution, thus limiting its generalizability. Second, as a retrospective study based on observational data, no definitive conclusions regarding causality can be made. Third, although our response rate was low, it is comparable to other studies that have examined underserved populations.[33, 34] Fourth, because our survey was performed 30 days after hospitalization, this may impart imprecision on our outcomes measures. Finally, we were not able to mitigate selection bias through imputation for missing data .
All together, given the small absolute differences between the groups in patients' ratings of their overall care compared to large differences in possible confounders, these findings call for further exploration into the significance and possible mechanisms of these outcomes. Our study raises the potential possibility that the structural component of a care team may play a role in overall patient satisfaction. If this is the case, future studies of team structure could help inform how best to optimize this component for the patient experience. On the other hand, if process differences are to explain our findings, it is important to distill the types of processes hospitalists are using to improve the patient experience and potentially export this to resident services.
Finally, if similar results were found in other institutions, these findings could have implications on how hospitals respond to new payment models that are linked to patient‐experience measures. For example, the Hospital Value‐Based Purchasing Program currently links the Centers for Medicare and Medicaid Services payments to a set of quality measures that consist of (1) clinical processes of care (70%) and (2) the patient experience (30%).[1] Given this linkage, any small changes in the domain of patient satisfaction could have large payment implications on a national level.
CONCLUSION
In summary, in this large‐scale multiyear study, patients cared for by a nonteaching hospitalist service reported greater satisfaction with their overall care than patients cared for by a general medicine teaching service. This difference could be mediated by the structural differences between these 2 services. As hospitals seek to optimize patient experiences in an era where reimbursement models are now being linked to patient‐experience measures, future work should focus on further understanding the mechanisms for these findings.
Disclosures
Financial support for this work was provided by the Robert Wood Johnson Investigator Program (RWJF Grant ID 63910 PI Meltzer), a Midcareer Career Development Award from the National Institute of Aging (1 K24 AG031326‐01, PI Meltzer), and a Clinical and Translational Science Award (NIH/NCATS 2UL1TR000430‐08, PI Solway, Meltzer Core Leader). The authors report no conflicts of interest.
- Hospital Consumer Assessment of Healthcare Providers and Systems. HCAHPS fact sheet. CAHPS hospital survey August 2013. Available at: http://www.hcahpsonline.org/files/August_2013_HCAHPS_Fact_Sheet3.pdf. Accessed February 2, 2015.
- , , , et al. Transitions of Care Consensus policy statement: American College of Physicians, Society of General Internal Medicine, Society of Hospital Medicine, American Geriatrics Society, American College Of Emergency Physicians, and Society for Academic Emergency Medicine. J Hosp Med. 2009;4(6):364–370.
- Accreditation Council for Graduate Medical Education. Common program requirements. Available at: http://www.acgme.org/acgmeweb/Portals/0/PFAssets/ProgramRequirements/CPRs2013.pdf. Accessed January 15, 2015.
- , , . Increasing a patient's ability to identify his or her attending physician using a patient room display. Arch Intern Med. 2010;170(12):1084–1085.
- , , , , , . Ability of hospitalized patients to identify their in‐hospital physicians. Arch Intern Med. 2009;169(2):199–201.
- , , , et al. Hospitalized patients' understanding of their plan of care. Mayo Clin Proc. 2010;85(1):47–52.
- , , , et al. Patient‐physician communication at hospital discharge and patients' understanding of the postdischarge treatment plan. Arch Intern Med. 1997;157(9):1026–1030.
- , , , et al. Improving inpatients' identification of their doctors: use of FACE cards. Jt Comm J Qual Patient Saf. 2009;35(12):613–619.
- , , , , , . The impact of facecards on patients' knowledge, satisfaction, trust, and agreement with hospital physicians: a pilot study. J Hosp Med. 2014;9(3):137–141.
- , , . Restructuring an inpatient resident service to improve outcomes for residents, students, and patients. Acad Med. 2011;86(12):1500–1507.
- , , . Residency training in the modern era: the pipe dream of less time to learn more, care better, and be more professional. Arch Intern Med. 2005;165(22):2561–2562.
- , , , , . Managing discontinuity in academic medical centers: strategies for a safe and effective resident sign‐out. J Hosp Med. 2006;1(4):257–266.
- , , , . Hospitalist staffing and patient satisfaction in the national Medicare population. J Hosp Med. 2013;8(3):126–131.
- , , , et al. Effects of physician experience on costs and outcomes on an academic general medicine service: results of a trial of hospitalists. Ann Intern Med. 2002;137(11):866–874.
- , , , , , . The Effects of on‐duty napping on intern sleep time and fatigue. Ann Intern Med. 2006;144(11):792–798.
- , , , et al. Patients evaluate their hospital care: a national survey. Health Aff (Millwood). 1991;10(4):254–267.
- , , , . A new method of classifying prognostic comorbidity in longitudinal studies: development and validation. J Chronic Dis. 1987;40(5):373–383.
- Agency for Healthcare Research and Quality. Welcome to HCUPnet. Available at: http://hcupnet.ahrq.gov/HCUPnet.jsp?Id=F70FC59C286BADCB371(4):293–295.
- , , , et al. In the wake of the 2003 and 2011 duty hours regulations, how do internal medicine interns spend their time? J Gen Intern Med. 2013;28(8):1042–1047.
- , , , , , . The composition of intern work while on call. J Gen Intern Med. 2012;27(11):1432–1437.
- , , , et al. Effect of the 2011 vs 2003 duty hour regulation‐compliant models on sleep duration, trainee education, and continuity of patient care among internal medicine house staff: a randomized trial. JAMA Intern Med. 2013;173(8):649–655.
- , , , et al. The impact of hospitalist discontinuity on hospital cost, readmissions, and patient satisfaction. J Gen Intern Med. 2014;29(7):1004–1008.
- , , , , . Hospitalist time usage and cyclicality: opportunities to improve efficiency. J Hosp Med. 2010;5(6):329–334.
- , , , et al. Where did the day go?—a time‐motion study of hospitalists. J Hosp Med. 2010;5(6):323–328.
- , , . How hospitalists spend their time: insights on efficiency and safety. J Hosp Med. 2006;1(2):88–93.
- , , . Patient satisfaction associated with correct identification of physician's photographs. Mayo Clin Proc. 2001;76(6):604–608.
- , , , . Comparing patient outcomes of academician‐preceptors, hospitalist‐preceptors, and hospitalists on internal medicine services in an academic medical center. J Gen Intern Med. 2014;29(12):1672–1678.
- , , , . Comparison of processes and outcomes of pneumonia care between hospitalists and community‐based primary care physicians. Mayo Clin Proc. 2002;77(10):1053–1058.
- , , , , , . Outcomes of care by hospitalists, general internists, and family physicians. N Engl J Med. 2007;357(25):2589–2600.
- . A systematic review of outcomes and quality measures in adult patients cared for by hospitalists vs nonhospitalists. Mayo Clin Proc. 2009;84(3):248–254.
- , . Do hospitalist physicians improve the quality of inpatient care delivery? A systematic review of process, efficiency and outcome measures. BMC Med. 2011;9(1):58.
- , . Patients' experiences of everyday life after lung transplantation. J Clin Nurs. 2009;18(24):3472–3479.
- , , , et al. Optimal design features for surveying low‐income populations. J Health Care Poor Underserved. 2005;16(4):677–690.
The hospitalized patient experience has become an area of increased focus for hospitals given the recent coupling of patient satisfaction to reimbursement rates for Medicare patients.[1] Although patient experiences are multifactorial, 1 component is the relationship that hospitalized patients develop with their inpatient physicians. In recognition of the importance of this relationship, several organizations including the Society of Hospital Medicine, Society of General Internal Medicine, American College of Physicians, the American College of Emergency Physicians, and the Accreditation Council for Graduate Medical Education have recommended that patients know and understand who is guiding their care at all times during their hospitalization.[2, 3] Unfortunately, previous studies have shown that hospitalized patients often lack the ability to identify[4, 5] and understand their course of care.[6, 7] This may be due to numerous clinical factors including lack of a prior relationship, rapid pace of clinical care, and the frequent transitions of care found in both hospitalists and general medicine teaching services.[5, 8, 9] Regardless of the cause, one could hypothesize that patients who are unable to identify or understand the role of their physician may be less informed about their hospitalization, which may lead to further confusion, dissatisfaction, and ultimately a poor experience.
Given the proliferation of nonteaching hospitalist services in teaching hospitals, it is important to understand if patient experiences differ between general medicine teaching and hospitalist services. Several reasons could explain why patient experiences may vary on these services. For example, patients on a hospitalist service will likely interact with a single physician caretaker, which may give a feeling of more personalized care. In contrast, patients on general medicine teaching services are cared for by larger teams of residents under the supervision of an attending physician. Residents are also subjected to duty‐hour restrictions, clinic responsibilities, and other educational requirements that may impede the continuity of care for hospitalized patients.[10, 11, 12] Although 1 study has shown that hospitalist‐intensive hospitals perform better on patient satisfaction measures,[13] no study to date has compared patient‐reported experiences on general medicine teaching and nonteaching hospitalist services. This study aimed to evaluate the hospitalized patient experience on both teaching and nonteaching hospitalist services by assessing several patient‐reported measures of their experience, namely their confidence in their ability to identify their physician(s), understand their roles, and their rating of both the coordination and overall care.
METHODS
Study Design
We performed a retrospective cohort analysis at the University of Chicago Medical Center between July 2007 and June 2013. Data were acquired as part of the Hospitalist Project, an ongoing study that is used to evaluate the impact of hospitalists, and now serves as infrastructure to continue research related to hospital care at University of Chicago.[14] Patients were cared for by either the general medicine teaching service or the nonteaching hospitalist service. General medicine teaching services were composed of an attending physician who rotates for 2 weeks at a time, a second‐ or third‐year medicine resident, 1 to 2 medicine interns, and 1 to 2 medical students.[15] The attending physician assigned to the patient's hospitalization was the attending listed on the first day of hospitalization, regardless of the length of hospitalization. Nonteaching hospitalist services consisted of a single hospitalist who worked 7‐day shifts, and were assisted by a nurse practitioner/physician's assistant (NPA). The majority of attendings on the hospitalist service were less than 5 years out of residency. Both services admitted 7 days a week, with patients initially admitted to the general medicine teaching service until resident caps were met, after which all subsequent admissions were admitted to the hospitalist service. In addition, the hospitalist service is also responsible for specific patient subpopulations, such as lung and renal transplants, and oncologic patients who have previously established care with our institution.
Data Collection
During a 30‐day posthospitalization follow‐up questionnaire, patients were surveyed regarding their confidence in their ability to identify and understand the roles of their physician(s) and their perceptions of the overall coordination of care and their overall care, using a 5‐point Likert scale (1 = poor understanding to 5 = excellent understanding). Questions related to satisfaction with care and coordination were derived from the Picker‐Commonwealth Survey, a previously validated survey meant to evaluate patient‐centered care.[16] Patients were also asked to report their race, level of education, comorbid diseases, and whether they had any prior hospitalizations within 1 year. Chart review was performed to obtain patient age, gender, and hospital length of stay (LOS), and calculated Charlson Comorbidity Index (CCI).[17] Patients with missing data or responses to survey questions were excluded from final analysis. The University of Chicago Institutional Review Board approved the study protocol, and all patients provided written consented prior to participation.
Data Analysis
After initial analysis noted that outcomes were skewed, the decision was made to dichotomize the data and use logistic rather than linear regression models. Patient responses to the follow‐up phone questionnaire were dichotomized to reflect the top 2 categories (excellent and very good). Pearson 2 analysis was used to assess for any differences in demographic characteristics, disease severity, and measures of patient experience between the 2 services. To assess if service type was associated with differences in our 4 measures of patient experience, we created a 3‐level mixed‐effects logistic regression using a logit function while controlling for age, gender, race, CCI, LOS, previous hospitalizations within 1 year, level of education, and academic year. These models studied the longitudinal association between teaching service and the 4 outcome measures, while also controlling for the cluster effect of time nested within individual patients who were clustered within physicians. The model included random intercepts at both the patient and physician level and also included a random effect of service (teaching vs nonteaching) at the patient level. A Hausman test was used to determine if these random‐effects models improved fit over a fixed‐effects model, and the intraclass correlations were compared using likelihood ratio tests to determine the appropriateness of a 3‐level versus 2‐level model. Data management and 2 analyses were performed using Stata version 13.0 (StataCorp, College Station, TX), and mixed‐effects regression models were done in SuperMix (Scientific Software International, Skokie, IL).
RESULTS
In total, 14,855 patients were enrolled during their hospitalization with 57% and 61% completing the 30‐day follow‐up survey on the hospitalist and general medicine teaching service, respectively. In total, 4131 (69%) and 4322 (48%) of the hospitalist and general medicine services, respectively, either did not answer all survey questions, or were missing basic demographic data, and thus were excluded. Data from 4591 patients on the general medicine teaching (52% of those enrolled at hospitalization) and 1811 on the hospitalist service (31% of those enrolled at hospitalization) were used for final analysis (Figure 1). Respondents were predominantly female (61% and 56%), African American (75% and 63%), with a mean age of 56.2 (19.4) and 57.1 (16.1) years, for the general medicine teaching and hospitalist services, respectively. A majority of patients (71% and 66%) had a CCI of 0 to 3 on both services. There were differences in self‐reported comorbidities between the 2 groups, with hospitalist services having a higher prevalence of cancer (20% vs 7%), renal disease (25% vs 18%), and liver disease (23% vs 7%). Patients on the hospitalist service had a longer mean LOS (5.5 vs 4.8 days), a greater percentage of a hospitalization within 1 year (58% vs 52%), and a larger proportion who were admitted in 2011 to 2013 compared to 2007 to 2010 (75% vs 39%), when compared to the general medicine teaching services. Median LOS and interquartile ranges were similar between both groups. Although most baseline demographics were statistically different between the 2 groups (Table 1), these differences were likely clinically insignificant. Compared to those who responded to the follow‐up survey, nonresponders were more likely to be African American (73% and 64%, P < 0.001) and female (60% and 56%, P < 0.01). The nonresponders were more likely to be hospitalized in the past 1 year (62% and 53%, P < 0.001) and have a lower CCI (CCI 03 [75% and 80%, P < 0.001]) compared to responders. Demographics between responders and nonresponders were also statistically different from one another.
| Variable | General Medicine Teaching | Nonteaching Hospitalist | P Value |
|---|---|---|---|
| |||
| Total (n) | 4,591 | 1,811 | <0.001 |
| Attending classification, hospitalist, n (%) | 1,147 (25) | 1,811 (100) | |
| Response rate, % | 61 | 57 | <0.01 |
| Age, y, mean SD | 56.2 19.4 | 57.1 16.1 | <0.01 |
| Gender, n (%) | <0.01 | ||
| Male | 1,796 (39) | 805 (44) | |
| Female | 2,795 (61) | 1,004 (56) | |
| Race, n (%) | <0.01 | ||
| African American | 3,440 (75) | 1,092 (63) | |
| White | 900 (20) | 571 (32) | |
| Asian/Pacific | 38 (1) | 17 (1) | |
| Other | 20 (1) | 10 (1) | |
| Unknown | 134 (3) | 52 (3) | |
| Charlson Comorbidity Index, n (%) | <0.001 | ||
| 0 | 1,635 (36) | 532 (29) | |
| 12 | 1,590 (35) | 675 (37) | |
| 39 | 1,366 (30) | 602 (33) | |
| Self‐reported comorbidities | |||
| Anemia/sickle cell disease | 1,201 (26) | 408 (23) | 0.003 |
| Asthma/COPD | 1,251 (28) | 432 (24) | 0.006 |
| Cancer* | 300 (7) | 371 (20) | <0.001 |
| Depression | 1,035 (23) | 411 (23) | 0.887 |
| Diabetes | 1,381 (30) | 584 (32) | 0.087 |
| Gastrointestinal | 1,140 (25) | 485 (27) | 0.104 |
| Cardiac | 1,336 (29) | 520 (29) | 0.770 |
| Hypertension | 2,566 (56) | 1,042 (58) | 0.222 |
| HIV/AIDS | 151 (3) | 40 (2) | 0.022 |
| Kidney disease | 828 (18) | 459 (25) | <0.001 |
| Liver disease | 313 (7) | 417 (23) | <0.001 |
| Stroke | 543 (12) | 201 (11) | 0.417 |
| Education level | 0.066 | ||
| High school | 2,248 (49) | 832 (46) | |
| Junior college/college | 1,878 (41) | 781 (43) | |
| Postgraduate | 388 (8) | 173 (10) | |
| Don't know | 77 (2) | 23 (1) | |
| Academic year, n (%) | <0.001 | ||
| July 2007 June 2008 | 938 (20) | 90 (5) | |
| July 2008 June 2009 | 702 (15) | 148 (8) | |
| July 2009 June 2010 | 576(13) | 85 (5) | |
| July 2010 June 2011 | 602 (13) | 138 (8) | |
| July 2011 June 2012 | 769 (17) | 574 (32) | |
| July 2012 June 2013 | 1,004 (22) | 774 (43) | |
| Length of stay, d, mean SD | 4.8 7.3 | 5.5 6.4 | <0.01 |
| Prior hospitalization (within 1 year), yes, n (%) | 2,379 (52) | 1,039 (58) | <0.01 |

Unadjusted results revealed that patients on the hospitalist service were more confident in their abilities to identify their physician(s) (50% vs 45%, P < 0.001), perceived greater ability in understanding the role of their physician(s) (54% vs 50%, P < 0.001), and reported greater satisfaction with coordination and teamwork (68% vs 64%, P = 0.006) and with overall care (73% vs 67%, P < 0.001) (Figure 2).

From the mixed‐effects regression models it was discovered that admission to the hospitalist service was associated with a higher odds ratio (OR) of reporting overall care as excellent or very good (OR: 1.33; 95% confidence interval [CI]: 1.15‐1.47). There was no difference between services in patients' ability to identify their physician(s) (OR: 0.89; 95% CI: 0.61‐1.11), in patients reporting a better understanding of the role of their physician(s) (OR: 1.09; 95% CI: 0.94‐1.23), or in their rating of overall coordination and teamwork (OR: 0.71; 95% CI: 0.42‐1.89).
A subgroup analysis was performed on the 25% of hospitalist attendings in the general medicine teaching service comparing this cohort to the hospitalist services, and it was found that patients perceived better overall care on the hospitalist service (OR: 1.17; 95% CI: 1.01‐ 1.31) than on the general medicine service (Table 2). All other domains in the subgroup analysis were not statistically significant. Finally, an ordinal logistic regression was performed for each of these outcomes, but it did not show any major differences compared to the logistic regression of dichotomous outcomes.
| Domains in Patient Experience* | Odds Ratio (95% CI) | P Value |
|---|---|---|
| ||
| How would you rate your ability to identify the physicians and trainees on your general medicine team during the hospitalization? | ||
| Model 1 | 0.89 (0.611.11) | 0.32 |
| Model 2 | 0.98 (0.671.22) | 0.86 |
| How would you rate your understanding of the roles of the physicians and trainees on your general medicine team? | ||
| Model 1 | 1.09 (0.941.23) | 0.25 |
| Model 2 | 1.19 (0.981.36) | 0.08 |
| How would you rate the overall coordination and teamwork among the doctors and nurses who care for you during your hospital stay? | ||
| Model 1 | 0.71 (0.421.89) | 0.18 |
| Model 2 | 0.82 (0.651.20) | 0.23 |
| Overall, how would you rate the care you received at the hospital? | ||
| Model 1 | 1.33 (1.151.47) | 0.001 |
| Model 2 | 1.17 (1.011.31) | 0.04 |
DISCUSSION
This study is the first to directly compare measures of patient experience on hospitalist and general medicine teaching services in a large, multiyear comparison across multiple domains. In adjusted analysis, we found that patients on nonteaching hospitalist services rated their overall care better than those on general medicine teaching services, whereas no differences in patients' ability to identify their physician(s), understand their role in their care, or rating of coordination of care were found. Although the magnitude of the differences in rating of overall care may appear small, it remains noteworthy because of the recent focus on patient experience at the reimbursement level, where small differences in performance can lead to large changes in payment. Because of the observational design of this study, it is important to consider mechanisms that could account for our findings.
The first are the structural differences between the 2 services. Our subgroup analysis comparing patients rating of overall care on a general medicine service with a hospitalist attending to a pure hospitalist cohort found a significant difference between the groups, indicating that the structural differences between the 2 groups may be a significant contributor to patient satisfaction ratings. Under the care of a hospitalist service, a patient would only interact with a single physician on a daily basis, possibly leading to a more meaningful relationship and improved communication between patient and provider. Alternatively, while on a general medicine teaching service, patients would likely interact with multiple physicians, as a result making their confidence in their ability to identify and perception at understanding physicians' roles more challenging.[18] This dilemma is further compounded by duty hour restrictions, which have subsequently led to increased fragmentation in housestaff scheduling. The patient experience on the general medicine teaching service may be further complicated by recent data that show residents spend a minority of time in direct patient care,[19, 20] which could additionally contribute to patients' inability to understand who their physicians are and to the decreased satisfaction with their care. This combination of structural complexity, duty hour reform, and reduced direct patient interaction would likely decrease the chance a patient will interact with the same resident on a consistent basis,[5, 21] thus making the ability to truly understand who their caretakers are, and the role they play, more difficult.
Another contributing factor could be the use of NPAs on our hospitalist service. Given that these providers often see the patient on a more continual basis, hospitalized patients' exposure to a single, continuous caretaker may be a factor in our findings.[22] Furthermore, with studies showing that hospitalists also spend a small fraction of their day in direct patient care,[23, 24, 25] the use of NPAs may allow our hospitalists to spend greater amounts of time with their patients, thus improving patients' rating of their overall care and influencing their perceived ability to understand their physician's role.
Although there was no difference between general medicine teaching and hospitalist services with respect to patient understanding of their roles, our data suggest that both groups would benefit from interventions to target this area. Focused attempts at improving patient's ability to identify and explain the roles of their inpatient physician(s) have been performed. For example, previous studies have attempted to improve a patient's ability to identify their physician through physician facecards[8, 9] or the use of other simple interventions (ie, bedside whiteboards).[4, 26] Results from such interventions are mixed, as they have demonstrated the capacity to improve patients' ability to identify who their physician is, whereas few have shown any appreciable improvement in patient satisfaction.[26]
Although our findings suggest that structural differences in team composition may be a possible explanation, it is also important to consider how the quality of care a patient receives affects their experience. For instance, hospitalists have been shown to produce moderate improvements in patient‐centered outcomes such as 30‐day readmission[27] and hospital length of stay[14, 28, 29, 30, 31] when compared to other care providers, which in turn could be reflected in the patient's perception of their overall care. In a large national study of acute care hospitals using the Hospital Consumer Assessment of Healthcare Providers and Systems survey, Chen and colleagues found that for most measures of patient satisfaction, hospitals with greater use of hospitalist care were associated with better patient‐centered care.[13] These outcomes were in part driven by patient‐centered domains such as discharge planning, pain control, and medication management. It is possible that patients are sensitive to the improved outcomes that are associated with hospitalist services, and reflect this in their measures of patient satisfaction.
Last, because this is an observational study and not a randomized trial, it is possible that the clinical differences in the patients cared for by these services could have led to our findings. Although the clinical significance of the differences in patient demographics were small, patients seen on the hospitalist service were more likely to be older white males, with a slightly longer LOS, greater comorbidities, and more hospitalizations in the previous year than those seen on the general medicine teaching service. Additionally, our hospitalist service frequently cares for highly specific subpopulations (ie, liver and renal transplant patients, and oncology patients), which could have influenced our results. For example, transplant patients who may be very grateful for their second chance, are preferentially admitted to the hospitalist service, which could have biased our results in favor of hospitalists.[32] Unfortunately, we were unable to control for all such factors.
Although we hope that multivariable analysis can adjust for many of these differences, we are not able to account for possible unmeasured confounders such as time of day of admission, health literacy, personality differences, physician turnover, or nursing and other ancillary care that could contribute to these findings. In addition to its observational study design, our study has several other limitations. First, our study was performed at a single institution, thus limiting its generalizability. Second, as a retrospective study based on observational data, no definitive conclusions regarding causality can be made. Third, although our response rate was low, it is comparable to other studies that have examined underserved populations.[33, 34] Fourth, because our survey was performed 30 days after hospitalization, this may impart imprecision on our outcomes measures. Finally, we were not able to mitigate selection bias through imputation for missing data .
All together, given the small absolute differences between the groups in patients' ratings of their overall care compared to large differences in possible confounders, these findings call for further exploration into the significance and possible mechanisms of these outcomes. Our study raises the potential possibility that the structural component of a care team may play a role in overall patient satisfaction. If this is the case, future studies of team structure could help inform how best to optimize this component for the patient experience. On the other hand, if process differences are to explain our findings, it is important to distill the types of processes hospitalists are using to improve the patient experience and potentially export this to resident services.
Finally, if similar results were found in other institutions, these findings could have implications on how hospitals respond to new payment models that are linked to patient‐experience measures. For example, the Hospital Value‐Based Purchasing Program currently links the Centers for Medicare and Medicaid Services payments to a set of quality measures that consist of (1) clinical processes of care (70%) and (2) the patient experience (30%).[1] Given this linkage, any small changes in the domain of patient satisfaction could have large payment implications on a national level.
CONCLUSION
In summary, in this large‐scale multiyear study, patients cared for by a nonteaching hospitalist service reported greater satisfaction with their overall care than patients cared for by a general medicine teaching service. This difference could be mediated by the structural differences between these 2 services. As hospitals seek to optimize patient experiences in an era where reimbursement models are now being linked to patient‐experience measures, future work should focus on further understanding the mechanisms for these findings.
Disclosures
Financial support for this work was provided by the Robert Wood Johnson Investigator Program (RWJF Grant ID 63910 PI Meltzer), a Midcareer Career Development Award from the National Institute of Aging (1 K24 AG031326‐01, PI Meltzer), and a Clinical and Translational Science Award (NIH/NCATS 2UL1TR000430‐08, PI Solway, Meltzer Core Leader). The authors report no conflicts of interest.
The hospitalized patient experience has become an area of increased focus for hospitals given the recent coupling of patient satisfaction to reimbursement rates for Medicare patients.[1] Although patient experiences are multifactorial, 1 component is the relationship that hospitalized patients develop with their inpatient physicians. In recognition of the importance of this relationship, several organizations including the Society of Hospital Medicine, Society of General Internal Medicine, American College of Physicians, the American College of Emergency Physicians, and the Accreditation Council for Graduate Medical Education have recommended that patients know and understand who is guiding their care at all times during their hospitalization.[2, 3] Unfortunately, previous studies have shown that hospitalized patients often lack the ability to identify[4, 5] and understand their course of care.[6, 7] This may be due to numerous clinical factors including lack of a prior relationship, rapid pace of clinical care, and the frequent transitions of care found in both hospitalists and general medicine teaching services.[5, 8, 9] Regardless of the cause, one could hypothesize that patients who are unable to identify or understand the role of their physician may be less informed about their hospitalization, which may lead to further confusion, dissatisfaction, and ultimately a poor experience.
Given the proliferation of nonteaching hospitalist services in teaching hospitals, it is important to understand if patient experiences differ between general medicine teaching and hospitalist services. Several reasons could explain why patient experiences may vary on these services. For example, patients on a hospitalist service will likely interact with a single physician caretaker, which may give a feeling of more personalized care. In contrast, patients on general medicine teaching services are cared for by larger teams of residents under the supervision of an attending physician. Residents are also subjected to duty‐hour restrictions, clinic responsibilities, and other educational requirements that may impede the continuity of care for hospitalized patients.[10, 11, 12] Although 1 study has shown that hospitalist‐intensive hospitals perform better on patient satisfaction measures,[13] no study to date has compared patient‐reported experiences on general medicine teaching and nonteaching hospitalist services. This study aimed to evaluate the hospitalized patient experience on both teaching and nonteaching hospitalist services by assessing several patient‐reported measures of their experience, namely their confidence in their ability to identify their physician(s), understand their roles, and their rating of both the coordination and overall care.
METHODS
Study Design
We performed a retrospective cohort analysis at the University of Chicago Medical Center between July 2007 and June 2013. Data were acquired as part of the Hospitalist Project, an ongoing study that is used to evaluate the impact of hospitalists, and now serves as infrastructure to continue research related to hospital care at University of Chicago.[14] Patients were cared for by either the general medicine teaching service or the nonteaching hospitalist service. General medicine teaching services were composed of an attending physician who rotates for 2 weeks at a time, a second‐ or third‐year medicine resident, 1 to 2 medicine interns, and 1 to 2 medical students.[15] The attending physician assigned to the patient's hospitalization was the attending listed on the first day of hospitalization, regardless of the length of hospitalization. Nonteaching hospitalist services consisted of a single hospitalist who worked 7‐day shifts, and were assisted by a nurse practitioner/physician's assistant (NPA). The majority of attendings on the hospitalist service were less than 5 years out of residency. Both services admitted 7 days a week, with patients initially admitted to the general medicine teaching service until resident caps were met, after which all subsequent admissions were admitted to the hospitalist service. In addition, the hospitalist service is also responsible for specific patient subpopulations, such as lung and renal transplants, and oncologic patients who have previously established care with our institution.
Data Collection
During a 30‐day posthospitalization follow‐up questionnaire, patients were surveyed regarding their confidence in their ability to identify and understand the roles of their physician(s) and their perceptions of the overall coordination of care and their overall care, using a 5‐point Likert scale (1 = poor understanding to 5 = excellent understanding). Questions related to satisfaction with care and coordination were derived from the Picker‐Commonwealth Survey, a previously validated survey meant to evaluate patient‐centered care.[16] Patients were also asked to report their race, level of education, comorbid diseases, and whether they had any prior hospitalizations within 1 year. Chart review was performed to obtain patient age, gender, and hospital length of stay (LOS), and calculated Charlson Comorbidity Index (CCI).[17] Patients with missing data or responses to survey questions were excluded from final analysis. The University of Chicago Institutional Review Board approved the study protocol, and all patients provided written consented prior to participation.
Data Analysis
After initial analysis noted that outcomes were skewed, the decision was made to dichotomize the data and use logistic rather than linear regression models. Patient responses to the follow‐up phone questionnaire were dichotomized to reflect the top 2 categories (excellent and very good). Pearson 2 analysis was used to assess for any differences in demographic characteristics, disease severity, and measures of patient experience between the 2 services. To assess if service type was associated with differences in our 4 measures of patient experience, we created a 3‐level mixed‐effects logistic regression using a logit function while controlling for age, gender, race, CCI, LOS, previous hospitalizations within 1 year, level of education, and academic year. These models studied the longitudinal association between teaching service and the 4 outcome measures, while also controlling for the cluster effect of time nested within individual patients who were clustered within physicians. The model included random intercepts at both the patient and physician level and also included a random effect of service (teaching vs nonteaching) at the patient level. A Hausman test was used to determine if these random‐effects models improved fit over a fixed‐effects model, and the intraclass correlations were compared using likelihood ratio tests to determine the appropriateness of a 3‐level versus 2‐level model. Data management and 2 analyses were performed using Stata version 13.0 (StataCorp, College Station, TX), and mixed‐effects regression models were done in SuperMix (Scientific Software International, Skokie, IL).
RESULTS
In total, 14,855 patients were enrolled during their hospitalization with 57% and 61% completing the 30‐day follow‐up survey on the hospitalist and general medicine teaching service, respectively. In total, 4131 (69%) and 4322 (48%) of the hospitalist and general medicine services, respectively, either did not answer all survey questions, or were missing basic demographic data, and thus were excluded. Data from 4591 patients on the general medicine teaching (52% of those enrolled at hospitalization) and 1811 on the hospitalist service (31% of those enrolled at hospitalization) were used for final analysis (Figure 1). Respondents were predominantly female (61% and 56%), African American (75% and 63%), with a mean age of 56.2 (19.4) and 57.1 (16.1) years, for the general medicine teaching and hospitalist services, respectively. A majority of patients (71% and 66%) had a CCI of 0 to 3 on both services. There were differences in self‐reported comorbidities between the 2 groups, with hospitalist services having a higher prevalence of cancer (20% vs 7%), renal disease (25% vs 18%), and liver disease (23% vs 7%). Patients on the hospitalist service had a longer mean LOS (5.5 vs 4.8 days), a greater percentage of a hospitalization within 1 year (58% vs 52%), and a larger proportion who were admitted in 2011 to 2013 compared to 2007 to 2010 (75% vs 39%), when compared to the general medicine teaching services. Median LOS and interquartile ranges were similar between both groups. Although most baseline demographics were statistically different between the 2 groups (Table 1), these differences were likely clinically insignificant. Compared to those who responded to the follow‐up survey, nonresponders were more likely to be African American (73% and 64%, P < 0.001) and female (60% and 56%, P < 0.01). The nonresponders were more likely to be hospitalized in the past 1 year (62% and 53%, P < 0.001) and have a lower CCI (CCI 03 [75% and 80%, P < 0.001]) compared to responders. Demographics between responders and nonresponders were also statistically different from one another.
| Variable | General Medicine Teaching | Nonteaching Hospitalist | P Value |
|---|---|---|---|
| |||
| Total (n) | 4,591 | 1,811 | <0.001 |
| Attending classification, hospitalist, n (%) | 1,147 (25) | 1,811 (100) | |
| Response rate, % | 61 | 57 | <0.01 |
| Age, y, mean SD | 56.2 19.4 | 57.1 16.1 | <0.01 |
| Gender, n (%) | <0.01 | ||
| Male | 1,796 (39) | 805 (44) | |
| Female | 2,795 (61) | 1,004 (56) | |
| Race, n (%) | <0.01 | ||
| African American | 3,440 (75) | 1,092 (63) | |
| White | 900 (20) | 571 (32) | |
| Asian/Pacific | 38 (1) | 17 (1) | |
| Other | 20 (1) | 10 (1) | |
| Unknown | 134 (3) | 52 (3) | |
| Charlson Comorbidity Index, n (%) | <0.001 | ||
| 0 | 1,635 (36) | 532 (29) | |
| 12 | 1,590 (35) | 675 (37) | |
| 39 | 1,366 (30) | 602 (33) | |
| Self‐reported comorbidities | |||
| Anemia/sickle cell disease | 1,201 (26) | 408 (23) | 0.003 |
| Asthma/COPD | 1,251 (28) | 432 (24) | 0.006 |
| Cancer* | 300 (7) | 371 (20) | <0.001 |
| Depression | 1,035 (23) | 411 (23) | 0.887 |
| Diabetes | 1,381 (30) | 584 (32) | 0.087 |
| Gastrointestinal | 1,140 (25) | 485 (27) | 0.104 |
| Cardiac | 1,336 (29) | 520 (29) | 0.770 |
| Hypertension | 2,566 (56) | 1,042 (58) | 0.222 |
| HIV/AIDS | 151 (3) | 40 (2) | 0.022 |
| Kidney disease | 828 (18) | 459 (25) | <0.001 |
| Liver disease | 313 (7) | 417 (23) | <0.001 |
| Stroke | 543 (12) | 201 (11) | 0.417 |
| Education level | 0.066 | ||
| High school | 2,248 (49) | 832 (46) | |
| Junior college/college | 1,878 (41) | 781 (43) | |
| Postgraduate | 388 (8) | 173 (10) | |
| Don't know | 77 (2) | 23 (1) | |
| Academic year, n (%) | <0.001 | ||
| July 2007 June 2008 | 938 (20) | 90 (5) | |
| July 2008 June 2009 | 702 (15) | 148 (8) | |
| July 2009 June 2010 | 576(13) | 85 (5) | |
| July 2010 June 2011 | 602 (13) | 138 (8) | |
| July 2011 June 2012 | 769 (17) | 574 (32) | |
| July 2012 June 2013 | 1,004 (22) | 774 (43) | |
| Length of stay, d, mean SD | 4.8 7.3 | 5.5 6.4 | <0.01 |
| Prior hospitalization (within 1 year), yes, n (%) | 2,379 (52) | 1,039 (58) | <0.01 |

Unadjusted results revealed that patients on the hospitalist service were more confident in their abilities to identify their physician(s) (50% vs 45%, P < 0.001), perceived greater ability in understanding the role of their physician(s) (54% vs 50%, P < 0.001), and reported greater satisfaction with coordination and teamwork (68% vs 64%, P = 0.006) and with overall care (73% vs 67%, P < 0.001) (Figure 2).

From the mixed‐effects regression models it was discovered that admission to the hospitalist service was associated with a higher odds ratio (OR) of reporting overall care as excellent or very good (OR: 1.33; 95% confidence interval [CI]: 1.15‐1.47). There was no difference between services in patients' ability to identify their physician(s) (OR: 0.89; 95% CI: 0.61‐1.11), in patients reporting a better understanding of the role of their physician(s) (OR: 1.09; 95% CI: 0.94‐1.23), or in their rating of overall coordination and teamwork (OR: 0.71; 95% CI: 0.42‐1.89).
A subgroup analysis was performed on the 25% of hospitalist attendings in the general medicine teaching service comparing this cohort to the hospitalist services, and it was found that patients perceived better overall care on the hospitalist service (OR: 1.17; 95% CI: 1.01‐ 1.31) than on the general medicine service (Table 2). All other domains in the subgroup analysis were not statistically significant. Finally, an ordinal logistic regression was performed for each of these outcomes, but it did not show any major differences compared to the logistic regression of dichotomous outcomes.
| Domains in Patient Experience* | Odds Ratio (95% CI) | P Value |
|---|---|---|
| ||
| How would you rate your ability to identify the physicians and trainees on your general medicine team during the hospitalization? | ||
| Model 1 | 0.89 (0.611.11) | 0.32 |
| Model 2 | 0.98 (0.671.22) | 0.86 |
| How would you rate your understanding of the roles of the physicians and trainees on your general medicine team? | ||
| Model 1 | 1.09 (0.941.23) | 0.25 |
| Model 2 | 1.19 (0.981.36) | 0.08 |
| How would you rate the overall coordination and teamwork among the doctors and nurses who care for you during your hospital stay? | ||
| Model 1 | 0.71 (0.421.89) | 0.18 |
| Model 2 | 0.82 (0.651.20) | 0.23 |
| Overall, how would you rate the care you received at the hospital? | ||
| Model 1 | 1.33 (1.151.47) | 0.001 |
| Model 2 | 1.17 (1.011.31) | 0.04 |
DISCUSSION
This study is the first to directly compare measures of patient experience on hospitalist and general medicine teaching services in a large, multiyear comparison across multiple domains. In adjusted analysis, we found that patients on nonteaching hospitalist services rated their overall care better than those on general medicine teaching services, whereas no differences in patients' ability to identify their physician(s), understand their role in their care, or rating of coordination of care were found. Although the magnitude of the differences in rating of overall care may appear small, it remains noteworthy because of the recent focus on patient experience at the reimbursement level, where small differences in performance can lead to large changes in payment. Because of the observational design of this study, it is important to consider mechanisms that could account for our findings.
The first are the structural differences between the 2 services. Our subgroup analysis comparing patients rating of overall care on a general medicine service with a hospitalist attending to a pure hospitalist cohort found a significant difference between the groups, indicating that the structural differences between the 2 groups may be a significant contributor to patient satisfaction ratings. Under the care of a hospitalist service, a patient would only interact with a single physician on a daily basis, possibly leading to a more meaningful relationship and improved communication between patient and provider. Alternatively, while on a general medicine teaching service, patients would likely interact with multiple physicians, as a result making their confidence in their ability to identify and perception at understanding physicians' roles more challenging.[18] This dilemma is further compounded by duty hour restrictions, which have subsequently led to increased fragmentation in housestaff scheduling. The patient experience on the general medicine teaching service may be further complicated by recent data that show residents spend a minority of time in direct patient care,[19, 20] which could additionally contribute to patients' inability to understand who their physicians are and to the decreased satisfaction with their care. This combination of structural complexity, duty hour reform, and reduced direct patient interaction would likely decrease the chance a patient will interact with the same resident on a consistent basis,[5, 21] thus making the ability to truly understand who their caretakers are, and the role they play, more difficult.
Another contributing factor could be the use of NPAs on our hospitalist service. Given that these providers often see the patient on a more continual basis, hospitalized patients' exposure to a single, continuous caretaker may be a factor in our findings.[22] Furthermore, with studies showing that hospitalists also spend a small fraction of their day in direct patient care,[23, 24, 25] the use of NPAs may allow our hospitalists to spend greater amounts of time with their patients, thus improving patients' rating of their overall care and influencing their perceived ability to understand their physician's role.
Although there was no difference between general medicine teaching and hospitalist services with respect to patient understanding of their roles, our data suggest that both groups would benefit from interventions to target this area. Focused attempts at improving patient's ability to identify and explain the roles of their inpatient physician(s) have been performed. For example, previous studies have attempted to improve a patient's ability to identify their physician through physician facecards[8, 9] or the use of other simple interventions (ie, bedside whiteboards).[4, 26] Results from such interventions are mixed, as they have demonstrated the capacity to improve patients' ability to identify who their physician is, whereas few have shown any appreciable improvement in patient satisfaction.[26]
Although our findings suggest that structural differences in team composition may be a possible explanation, it is also important to consider how the quality of care a patient receives affects their experience. For instance, hospitalists have been shown to produce moderate improvements in patient‐centered outcomes such as 30‐day readmission[27] and hospital length of stay[14, 28, 29, 30, 31] when compared to other care providers, which in turn could be reflected in the patient's perception of their overall care. In a large national study of acute care hospitals using the Hospital Consumer Assessment of Healthcare Providers and Systems survey, Chen and colleagues found that for most measures of patient satisfaction, hospitals with greater use of hospitalist care were associated with better patient‐centered care.[13] These outcomes were in part driven by patient‐centered domains such as discharge planning, pain control, and medication management. It is possible that patients are sensitive to the improved outcomes that are associated with hospitalist services, and reflect this in their measures of patient satisfaction.
Last, because this is an observational study and not a randomized trial, it is possible that the clinical differences in the patients cared for by these services could have led to our findings. Although the clinical significance of the differences in patient demographics were small, patients seen on the hospitalist service were more likely to be older white males, with a slightly longer LOS, greater comorbidities, and more hospitalizations in the previous year than those seen on the general medicine teaching service. Additionally, our hospitalist service frequently cares for highly specific subpopulations (ie, liver and renal transplant patients, and oncology patients), which could have influenced our results. For example, transplant patients who may be very grateful for their second chance, are preferentially admitted to the hospitalist service, which could have biased our results in favor of hospitalists.[32] Unfortunately, we were unable to control for all such factors.
Although we hope that multivariable analysis can adjust for many of these differences, we are not able to account for possible unmeasured confounders such as time of day of admission, health literacy, personality differences, physician turnover, or nursing and other ancillary care that could contribute to these findings. In addition to its observational study design, our study has several other limitations. First, our study was performed at a single institution, thus limiting its generalizability. Second, as a retrospective study based on observational data, no definitive conclusions regarding causality can be made. Third, although our response rate was low, it is comparable to other studies that have examined underserved populations.[33, 34] Fourth, because our survey was performed 30 days after hospitalization, this may impart imprecision on our outcomes measures. Finally, we were not able to mitigate selection bias through imputation for missing data .
All together, given the small absolute differences between the groups in patients' ratings of their overall care compared to large differences in possible confounders, these findings call for further exploration into the significance and possible mechanisms of these outcomes. Our study raises the potential possibility that the structural component of a care team may play a role in overall patient satisfaction. If this is the case, future studies of team structure could help inform how best to optimize this component for the patient experience. On the other hand, if process differences are to explain our findings, it is important to distill the types of processes hospitalists are using to improve the patient experience and potentially export this to resident services.
Finally, if similar results were found in other institutions, these findings could have implications on how hospitals respond to new payment models that are linked to patient‐experience measures. For example, the Hospital Value‐Based Purchasing Program currently links the Centers for Medicare and Medicaid Services payments to a set of quality measures that consist of (1) clinical processes of care (70%) and (2) the patient experience (30%).[1] Given this linkage, any small changes in the domain of patient satisfaction could have large payment implications on a national level.
CONCLUSION
In summary, in this large‐scale multiyear study, patients cared for by a nonteaching hospitalist service reported greater satisfaction with their overall care than patients cared for by a general medicine teaching service. This difference could be mediated by the structural differences between these 2 services. As hospitals seek to optimize patient experiences in an era where reimbursement models are now being linked to patient‐experience measures, future work should focus on further understanding the mechanisms for these findings.
Disclosures
Financial support for this work was provided by the Robert Wood Johnson Investigator Program (RWJF Grant ID 63910 PI Meltzer), a Midcareer Career Development Award from the National Institute of Aging (1 K24 AG031326‐01, PI Meltzer), and a Clinical and Translational Science Award (NIH/NCATS 2UL1TR000430‐08, PI Solway, Meltzer Core Leader). The authors report no conflicts of interest.
- Hospital Consumer Assessment of Healthcare Providers and Systems. HCAHPS fact sheet. CAHPS hospital survey August 2013. Available at: http://www.hcahpsonline.org/files/August_2013_HCAHPS_Fact_Sheet3.pdf. Accessed February 2, 2015.
- , , , et al. Transitions of Care Consensus policy statement: American College of Physicians, Society of General Internal Medicine, Society of Hospital Medicine, American Geriatrics Society, American College Of Emergency Physicians, and Society for Academic Emergency Medicine. J Hosp Med. 2009;4(6):364–370.
- Accreditation Council for Graduate Medical Education. Common program requirements. Available at: http://www.acgme.org/acgmeweb/Portals/0/PFAssets/ProgramRequirements/CPRs2013.pdf. Accessed January 15, 2015.
- , , . Increasing a patient's ability to identify his or her attending physician using a patient room display. Arch Intern Med. 2010;170(12):1084–1085.
- , , , , , . Ability of hospitalized patients to identify their in‐hospital physicians. Arch Intern Med. 2009;169(2):199–201.
- , , , et al. Hospitalized patients' understanding of their plan of care. Mayo Clin Proc. 2010;85(1):47–52.
- , , , et al. Patient‐physician communication at hospital discharge and patients' understanding of the postdischarge treatment plan. Arch Intern Med. 1997;157(9):1026–1030.
- , , , et al. Improving inpatients' identification of their doctors: use of FACE cards. Jt Comm J Qual Patient Saf. 2009;35(12):613–619.
- , , , , , . The impact of facecards on patients' knowledge, satisfaction, trust, and agreement with hospital physicians: a pilot study. J Hosp Med. 2014;9(3):137–141.
- , , . Restructuring an inpatient resident service to improve outcomes for residents, students, and patients. Acad Med. 2011;86(12):1500–1507.
- , , . Residency training in the modern era: the pipe dream of less time to learn more, care better, and be more professional. Arch Intern Med. 2005;165(22):2561–2562.
- , , , , . Managing discontinuity in academic medical centers: strategies for a safe and effective resident sign‐out. J Hosp Med. 2006;1(4):257–266.
- , , , . Hospitalist staffing and patient satisfaction in the national Medicare population. J Hosp Med. 2013;8(3):126–131.
- , , , et al. Effects of physician experience on costs and outcomes on an academic general medicine service: results of a trial of hospitalists. Ann Intern Med. 2002;137(11):866–874.
- , , , , , . The Effects of on‐duty napping on intern sleep time and fatigue. Ann Intern Med. 2006;144(11):792–798.
- , , , et al. Patients evaluate their hospital care: a national survey. Health Aff (Millwood). 1991;10(4):254–267.
- , , , . A new method of classifying prognostic comorbidity in longitudinal studies: development and validation. J Chronic Dis. 1987;40(5):373–383.
- Agency for Healthcare Research and Quality. Welcome to HCUPnet. Available at: http://hcupnet.ahrq.gov/HCUPnet.jsp?Id=F70FC59C286BADCB371(4):293–295.
- , , , et al. In the wake of the 2003 and 2011 duty hours regulations, how do internal medicine interns spend their time? J Gen Intern Med. 2013;28(8):1042–1047.
- , , , , , . The composition of intern work while on call. J Gen Intern Med. 2012;27(11):1432–1437.
- , , , et al. Effect of the 2011 vs 2003 duty hour regulation‐compliant models on sleep duration, trainee education, and continuity of patient care among internal medicine house staff: a randomized trial. JAMA Intern Med. 2013;173(8):649–655.
- , , , et al. The impact of hospitalist discontinuity on hospital cost, readmissions, and patient satisfaction. J Gen Intern Med. 2014;29(7):1004–1008.
- , , , , . Hospitalist time usage and cyclicality: opportunities to improve efficiency. J Hosp Med. 2010;5(6):329–334.
- , , , et al. Where did the day go?—a time‐motion study of hospitalists. J Hosp Med. 2010;5(6):323–328.
- , , . How hospitalists spend their time: insights on efficiency and safety. J Hosp Med. 2006;1(2):88–93.
- , , . Patient satisfaction associated with correct identification of physician's photographs. Mayo Clin Proc. 2001;76(6):604–608.
- , , , . Comparing patient outcomes of academician‐preceptors, hospitalist‐preceptors, and hospitalists on internal medicine services in an academic medical center. J Gen Intern Med. 2014;29(12):1672–1678.
- , , , . Comparison of processes and outcomes of pneumonia care between hospitalists and community‐based primary care physicians. Mayo Clin Proc. 2002;77(10):1053–1058.
- , , , , , . Outcomes of care by hospitalists, general internists, and family physicians. N Engl J Med. 2007;357(25):2589–2600.
- . A systematic review of outcomes and quality measures in adult patients cared for by hospitalists vs nonhospitalists. Mayo Clin Proc. 2009;84(3):248–254.
- , . Do hospitalist physicians improve the quality of inpatient care delivery? A systematic review of process, efficiency and outcome measures. BMC Med. 2011;9(1):58.
- , . Patients' experiences of everyday life after lung transplantation. J Clin Nurs. 2009;18(24):3472–3479.
- , , , et al. Optimal design features for surveying low‐income populations. J Health Care Poor Underserved. 2005;16(4):677–690.
- Hospital Consumer Assessment of Healthcare Providers and Systems. HCAHPS fact sheet. CAHPS hospital survey August 2013. Available at: http://www.hcahpsonline.org/files/August_2013_HCAHPS_Fact_Sheet3.pdf. Accessed February 2, 2015.
- , , , et al. Transitions of Care Consensus policy statement: American College of Physicians, Society of General Internal Medicine, Society of Hospital Medicine, American Geriatrics Society, American College Of Emergency Physicians, and Society for Academic Emergency Medicine. J Hosp Med. 2009;4(6):364–370.
- Accreditation Council for Graduate Medical Education. Common program requirements. Available at: http://www.acgme.org/acgmeweb/Portals/0/PFAssets/ProgramRequirements/CPRs2013.pdf. Accessed January 15, 2015.
- , , . Increasing a patient's ability to identify his or her attending physician using a patient room display. Arch Intern Med. 2010;170(12):1084–1085.
- , , , , , . Ability of hospitalized patients to identify their in‐hospital physicians. Arch Intern Med. 2009;169(2):199–201.
- , , , et al. Hospitalized patients' understanding of their plan of care. Mayo Clin Proc. 2010;85(1):47–52.
- , , , et al. Patient‐physician communication at hospital discharge and patients' understanding of the postdischarge treatment plan. Arch Intern Med. 1997;157(9):1026–1030.
- , , , et al. Improving inpatients' identification of their doctors: use of FACE cards. Jt Comm J Qual Patient Saf. 2009;35(12):613–619.
- , , , , , . The impact of facecards on patients' knowledge, satisfaction, trust, and agreement with hospital physicians: a pilot study. J Hosp Med. 2014;9(3):137–141.
- , , . Restructuring an inpatient resident service to improve outcomes for residents, students, and patients. Acad Med. 2011;86(12):1500–1507.
- , , . Residency training in the modern era: the pipe dream of less time to learn more, care better, and be more professional. Arch Intern Med. 2005;165(22):2561–2562.
- , , , , . Managing discontinuity in academic medical centers: strategies for a safe and effective resident sign‐out. J Hosp Med. 2006;1(4):257–266.
- , , , . Hospitalist staffing and patient satisfaction in the national Medicare population. J Hosp Med. 2013;8(3):126–131.
- , , , et al. Effects of physician experience on costs and outcomes on an academic general medicine service: results of a trial of hospitalists. Ann Intern Med. 2002;137(11):866–874.
- , , , , , . The Effects of on‐duty napping on intern sleep time and fatigue. Ann Intern Med. 2006;144(11):792–798.
- , , , et al. Patients evaluate their hospital care: a national survey. Health Aff (Millwood). 1991;10(4):254–267.
- , , , . A new method of classifying prognostic comorbidity in longitudinal studies: development and validation. J Chronic Dis. 1987;40(5):373–383.
- Agency for Healthcare Research and Quality. Welcome to HCUPnet. Available at: http://hcupnet.ahrq.gov/HCUPnet.jsp?Id=F70FC59C286BADCB371(4):293–295.
- , , , et al. In the wake of the 2003 and 2011 duty hours regulations, how do internal medicine interns spend their time? J Gen Intern Med. 2013;28(8):1042–1047.
- , , , , , . The composition of intern work while on call. J Gen Intern Med. 2012;27(11):1432–1437.
- , , , et al. Effect of the 2011 vs 2003 duty hour regulation‐compliant models on sleep duration, trainee education, and continuity of patient care among internal medicine house staff: a randomized trial. JAMA Intern Med. 2013;173(8):649–655.
- , , , et al. The impact of hospitalist discontinuity on hospital cost, readmissions, and patient satisfaction. J Gen Intern Med. 2014;29(7):1004–1008.
- , , , , . Hospitalist time usage and cyclicality: opportunities to improve efficiency. J Hosp Med. 2010;5(6):329–334.
- , , , et al. Where did the day go?—a time‐motion study of hospitalists. J Hosp Med. 2010;5(6):323–328.
- , , . How hospitalists spend their time: insights on efficiency and safety. J Hosp Med. 2006;1(2):88–93.
- , , . Patient satisfaction associated with correct identification of physician's photographs. Mayo Clin Proc. 2001;76(6):604–608.
- , , , . Comparing patient outcomes of academician‐preceptors, hospitalist‐preceptors, and hospitalists on internal medicine services in an academic medical center. J Gen Intern Med. 2014;29(12):1672–1678.
- , , , . Comparison of processes and outcomes of pneumonia care between hospitalists and community‐based primary care physicians. Mayo Clin Proc. 2002;77(10):1053–1058.
- , , , , , . Outcomes of care by hospitalists, general internists, and family physicians. N Engl J Med. 2007;357(25):2589–2600.
- . A systematic review of outcomes and quality measures in adult patients cared for by hospitalists vs nonhospitalists. Mayo Clin Proc. 2009;84(3):248–254.
- , . Do hospitalist physicians improve the quality of inpatient care delivery? A systematic review of process, efficiency and outcome measures. BMC Med. 2011;9(1):58.
- , . Patients' experiences of everyday life after lung transplantation. J Clin Nurs. 2009;18(24):3472–3479.
- , , , et al. Optimal design features for surveying low‐income populations. J Health Care Poor Underserved. 2005;16(4):677–690.
© 2015 Society of Hospital Medicine
Updates in Perioperative Medicine
Given the rapid expansion of the field of perioperative medicine, clinicians need to remain apprised of the current evidence to ensure optimization of patient care. In this update, we review 10 key articles from the perioperative literature, with the goal of summarizing the most clinically important evidence over the past year. This summary of recent literature in perioperative medicine is derived from the Update in Perioperative Medicine sessions presented at the 10th Annual Perioperative Medicine Summit and the Society of General Internal Medicine 38th Annual Meeting. A systematic search strategy was used to identify pertinent articles, and the following were selected by the authors based on their relevance to the clinical practice of perioperative medicine.
PERIOPERATIVE CARDIOVASCULAR CARE
Fleisher LA, Fleischmann KE, Auerbach AD, et al. 2014 ACC/AHA guideline on perioperative cardiovascular evaluation and management of patients undergoing noncardiac surgery: a report of the American College of Cardiology/American Heart Association Task Force on practice guidelines. Circulation. 2014;130:e278e333.
Background
The American College of Cardiology/American Heart Association (ACC/AHA) perioperative guideline provides recommendations for the evaluation and management of cardiovascular disease in patients undergoing noncardiac surgery.
Findings
The new guideline combines the evaluation of surgery‐ and patient‐specific risk in the algorithm for preoperative cardiovascular evaluation into a single step and recommends the use of 1 of 3 tools: the Revised Cardiac Risk Index (RCRI),[1] National Surgical Quality Improvement Program (NSQIP) Surgical Risk Calculator,[2] or the NSQIP‐derived myocardial infarction and cardiac arrest calculator.[3] Estimation of risk is also simplified by stratification into only 2 groups: low risk (risk of major adverse cardiac event 1%) and elevated risk (1% risk). Coronary evaluation can be considered for patients with elevated cardiac risk and poor functional capacity, but is advised only if the results would alter perioperative management. For example, a patient with very high risk who has evidence of ischemia on stress testing may choose to forego surgery. Preoperative coronary revascularization is only indicated for patients meeting criteria in the nonsurgical setting.
For patients with previous percutaneous coronary intervention, the ACC/AHA has not changed its recommendations to optimally delay surgery for at least 30 days after bare‐metal stenting and at least 1 year after drug‐eluting stent (DES) placement. However, in patients with a DES placed 6 to 12 months previously, surgery can be performed if the risks of surgical delay outweigh the risks of DES thrombosis. After any type of coronary stenting, dual antiplatelet therapy should be continued uninterrupted through the first 4 to 6 weeks and even later whenever feasible. If not possible, aspirin therapy should be maintained through surgery unless bleeding risk is too high.
The guideline recommends perioperative continuation of ‐blockers in patients taking them chronically. Preoperative initiation of ‐blocker therapy may be considered for patients with myocardial ischemia on stress testing or 3 RCRI factors and should be started far enough in advance to allow determination of patient's tolerance prior to surgery.
Cautions
Many recommendations are based on data from nonrandomized trials or expert opinion, and the data in areas such as perioperative ‐blockade continue to evolve.
Implications
The ACC/AHA guideline continues to be a critically valuable resource for hospitalists providing perioperative care to noncardiac surgery patients.
Wijeysundera DN, Duncan D, Nkonde‐Price C, et al. Perioperative beta blockade in noncardiac surgery: a systematic review for the 2014 ACC/AHA guideline on perioperative cardiovascular evaluation and management of patients undergoing noncardiac surgery: a report of the American College of Cardiology/American Heart Association Task Force on practice guidelines.
J Am Coll Cardiol. 2014;64(22):24062425.
Background
Various clinical trials have reported conflicting results regarding the efficacy and safety of perioperative ‐blockers resulting in guideline committees changing their recommendations. Because of questions raised regarding the scientific integrity of the DECREASE (Dutch Echocardiographic Cardiac Risk Evaluation Applying Stress Echocardiography)‐I[4] and DECREASE‐IV[5] trials as well as the dosing of ‐blockers in POISE (PeriOperative Ischemic Evaluation) study,[6] this systematic review was performed in conjunction with the ACC/AHA guideline update[7] to evaluate the data with and without these trials.
Findings
Sixteen randomized control trials (RCTs) (n=12,043) and 1 cohort study (n=348) were included in the analysis. Perioperative ‐blockers were associated with a reduction in nonfatal myocardial infarction (MI) (relative risk [RR]: 0.69; 95% confidence interval [CI]: 0.58‐0.82; P0.001) but an increase in bradycardia (RR: 2.61; 95% CI: 2.18‐3.12), hypotension (RR: 1.47; 95% CI: 1.34‐1.6), and nonfatal strokes (RR: 1.76; 95% CI: 1.07‐2.91; P=0.02). The POISE trial was the only one demonstrating a statistically significant increase in stroke.
The major discrepancy between the DECREASE trials and the other RCTs was related to mortalitya reduction in both cardiovascular and all‐cause death in DECREASE but an increased risk of all‐cause death in the other trials.
Cautions
Because of its size, the POISE trial heavily influences the results, particularly for mortality and stroke. Including the DECREASE trials reduces the otherwise increased risk for death to a null effect. Exclusion of the POISE and DECREASE trials leaves few data to make conclusions about safety and efficacy of perioperative ‐blockade. Several cohort studies have found metoprolol to be associated with worse outcomes than with atenolol or bisoprolol (which were preferred by the European Society of Cardiology guidelines).[8]
Implications
Perioperative ‐blockade started within 1 day of noncardiac surgery was associated with fewer nonfatal MIs but at the cost of an increase in hypotension, bradycardia, and a possible increase in stroke and death. Long‐term ‐blockade should be continued perioperatively, whereas the decision to initiate a ‐blocker should be individualized. If starting a ‐blocker perioperatively, it should be done 2 days before surgery.
Botto F, Alonso‐Coello P, Chan MT, et al.; on behalf of The Vascular events In noncardiac Surgery patIents cOhort evaluatioN (VISION) Investigators. Myocardial injury after noncardiac surgery: a large, international, prospective cohort study establishing diagnostic criteria, characteristics, predictors, and 30‐day outcomes. Anesthesiology. 2014;120(3):564578.
Background
Many patients sustain myocardial injury in the perioperative period as evidenced by troponin elevations, but most do not meet diagnostic criteria for MI. Myocardial injury after noncardiac surgery (MINS) is defined as prognostically relevant myocardial injury due to ischemia that occurs within 30 days after noncardiac surgery. This international, prospective cohort study of 15,065 patients 45 years old who underwent in‐patient noncardiac surgery determined diagnostic criteria, characteristics, predictors, and 30‐day outcomes of MINS.
Findings
The diagnostic criterion for MINS was a peak troponin T level 0.03 ng/mL judged to be due to an ischemic etiology. Twelve independent predictors of MINS were identified including age 75 years, known cardiovascular disease or risk factors, and surgical factors. MINS was an independent predictor of 30‐day mortality (adjusted hazard ratio [HR]: 3.87; 95% CI: 2.96‐5.08). Age >75 years, ST elevation, or new left bundle branch block, and anterior ischemic findings were independent predictors of 30‐day mortality among patients with MINS.
Cautions
Although screening high‐risk surgical patients without signs or symptoms of ischemia with postoperative troponins will increase the frequency of diagnosing MINS, evidence for an effective treatment has not yet been established. The ACC/AHA guidelines state that routine screening is of uncertain benefit for this reason.
Implications
Because MINS is common and carries a poor 30‐day prognosis, clinical trials are needed to determine when to obtain postoperative troponins and how to prevent and treat this complication.[9] Some observational data from POISE suggest that aspirin and statins can reduce the risk of 30‐day mortality in patients with postoperative MIs.
Devereaux PJ, Mrkobrada M, Sessler DI, et al. for the POISE‐2 Investigators. Aspirin in patients undergoing noncardiac surgery. N Engl J Med. 2014; 370(16):14941503.
Devereaux PJ, Sessler DI, Leslie K, et al. for the POISE‐2 Investigators. Clonidine in patients undergoing noncardiac surgery. N Engl J Med. 2014; 370(16):15041513.
Background
Medical risk reduction with aspirin and other agents in perioperative patients remains controversial. The POISE‐2 trial is a blinded RCT examining the effects of aspirin and clonidine on outcomes in >10,000 noncardiac surgery patients at risk of cardiovascular complications. The aspirin arm of the study included the initiation group and the continuation stratum, as well as placebo. Patients in the clonidine portion of the trial received 0.2 mg of clonidine or placebo daily for the same time periods.
Findings
The primary outcome was a composite of death or nonfatal MI within 30 days of surgery. Outcomes were similar in patients initiated or continued on aspirin. No difference was seen between aspirin or placebo in the primary outcome (7.0% vs 7.1%; HR: 0.86; 95% CI: 0.86‐1.15; P=0.92). There were no differences in rates of MI, venous thromboembolism, or stroke. Major bleeding rates were higher in aspirin versus placebo‐treated patients (4.6% vs 3.8%; HR: 1.23; 95% CI: 1.01‐1.49; P=0.04).
Clonidine did not alter the composite outcome of death or nonfatal MI (7.3% vs 6.8%; HR: 1.08; 95% CI: 0.93‐1.26; P=0.29). Clinically significant hypotension, bradycardia, and nonfatal cardiac arrest were more common in clonidine‐treated patients, although no difference was detected in stroke rates.
Cautions
Although patients in the trial had cardiovascular risk factors, 24% of patients had known coronary artery disease, and 5% had coronary stents. Conclusions based on this trial regarding perioperative management of antiplatelet therapy should not include patients with coronary artery stents.
Implications
Aspirin started before surgery and continued perioperatively did not decrease the rate of death or nonfatal MI but increased the risk of major bleeding. Perioperative management of aspirin needs to be undertaken in the context of cardiac and bleeding risks. Clonidine also did not improve outcomes and increased the risk of bradycardia and hypotension. Current guidelines recommend against using alpha‐2 agonists for prevention of perioperative cardiac events7; however, patients already on alpha‐2 agonists should not stop them abruptly.
PERIOPERATIVE PULMONARY CARE
Mutter TC, Chateau D, Moffatt M, et al. A matched cohort study of postoperative outcomes in obstructive sleep apnea: could preoperative diagnosis and treatment prevent complications? Anesthesiology. 2014;121(4):707718.
Background
An increasing body of literature associates obstructive sleep apnea (OSA) with an increased risk of postoperative complications. Despite evidence of risk, potential benefits of preoperative diagnosis and treatment of OSA remain unclear.
Findings
Using databases to identify patients prescribed continuous positive airway pressure (CPAP) therapy, the study compared postoperative outcomes of patients who underwent surgery any time after polysomnography (PSG) and CPAP prescription (diagnosed OSA [DOSA]) and those who had surgery during the 5 years preceding their PSG (undiagnosed OSA [UOSA]). These patients were matched with patients who underwent the same procedure for the same indication and had no insurance claims for PSG or diagnosis of sleep‐disordered breathing.
After multivariate analysis, OSA of any type was associated with increased pulmonary complications (odds ratio [OR]: 2.08; 95% CI: 1.35‐2.19). However, no significant differences in respiratory outcomes were noted between DOSA patients (N=2640) and those with UOSA (N=1571). DOSA patients did have fewer cardiovascular complications than UOSA patients (OR: 0.34; 95% CI: 0.15‐0.77). Only severe OSA (apnea‐hypopnea index >30) was associated with increased pulmonary and cardiovascular complications.
Cautions
Although this study suggests an association between preoperative diagnosis and treatment of OSA and reduced cardiovascular complications, the results are not definitive due to the inability to control for all confounding variables in a retrospective study utilizing an administrative database.
Implications
OSA is an important risk factor for postoperative complications, and this study suggests that preoperative treatment with CPAP is associated with reduced risk of cardiovascular complications, particularly in patients with severe OSA. Future controlled trials should focus on the risk‐reduction potential of preoperative diagnosis and treatment of OSA.
Mazo V, Sabat S, Canet J, et al. Prospective external validation of a predictive score for postoperative pulmonary complications. Anesthesiology. 2014;121:219231.
Background
In 2010, Canet et al. published a novel risk index, the Assess Respiratory Risk in Surgical Patients in Catalonia (ARISCAT) index, to provide a quantitative estimate of the risk of postoperative pulmonary complications (PPCs).[10]
In the current report, Mazo and colleagues studied the ARISCAT index in a broader sample to characterize its accuracy in predicting PPC risk. The ARISCAT index is derived from clinical risk factors: (1) age, (2) preoperative oxygen saturation, (3) respiratory infection in the prior month, (4) anemia, (5) surgical site, (6) duration of surgery, and (7) emergency surgery, with varying weights based on the strength of the association in a multivariable analysis. This score can be calculated via addition of these weighted risk factors, with a score>45 equal to high risk for PPC.
Findings
Examining 5099 patients from 63 European hospitals, the authors definition of PPC included respiratory failure, pulmonary infection, pleural effusion, atelectasis, pneumothorax, bronchospasm, and aspiration pneumonitis. PPC rates were as follows: low risk (3.39%), intermediate risk (12.98%), and high risk (38.01%). The positive likelihood ratio for PPC among the highest risk group was 7.12. The C statistic for fit was 0.80. Observed PPC rates were higher than predicted for the low (3.39% vs 0.87%) and intermediate (12.98% vs 7.82%) risk groups.
Cautions
The calibration slopes were less than ideal in all subsamples, with the Western European sample performing better than the other geographic areas; suggesting that the coefficients on the ARISCAT index may benefit from recalibration to match specific populations.
Implications
This is the first major pulmonary risk index that has been externally validated. Its use of readily available clinical information, simplicity, and accuracy in estimating PPC risk make it an important addition to the toolkit during a preoperative evaluation.
PERIOPERATIVE ATRIAL FIBRILLATION/ANTICOAGULATION
Gialdini G, Nearing K, Bhave P, et al. Perioperative atrial fibrillation and the long term risk of ischemic stroke. JAMA. 2014;312(6):616622.
Background
New‐onset atrial fibrillation (AF) is the most common perioperative arrhythmia.[11] However, little is known regarding the long‐term risks of ischemic stroke in patients who develop perioperative AF. This retrospective cohort study examined adults with no preexisting history of AF, hospitalized for surgery, and discharged free of cerebrovascular disease between 2007 and 2011 (n=1,729,360).
Findings
Of the eligible patients, 1.43% (95% CI: 1.41%‐1.45%) developed perioperative AF, and 0.81% (95% CI: 0.79%‐0.82%) had a stroke up to 1 year after discharge. Perioperative AF was associated with subsequent stroke after both cardiac (HR: 1.3; 95% CI: 1.1‐1.6) and noncardiac surgery (HR: 2; 95% CI: 1.7‐2.3). The association with stroke was stronger for perioperative AF after noncardiac versus cardiac surgery (P0.001 for interaction).
Cautions
This is a retrospective cohort study, using claims data to identify AF and stroke. Data on duration of the perioperative AF episodes or use of antithrombotic therapies were not available.
Implications
The association found between perioperative AF and long‐term risk of ischemic stroke may suggest that perioperative AF, especially after noncardiac surgery, should be treated aggressively in terms of thromboembolic risk; however, further data will be required to validate this association.
Van Diepen S, Youngson E, Ezekowitz J, McAlister F. Which risk score best predicts perioperative outcomes in nonvalvular atrial fibrillation patients undergoing noncardiac surgery? Am Heart J. 2014;168(1):6067.
Background
Patients with nonvalvular AF (NVAF) are at increased risk for adverse perioperative outcomes after noncardiac surgery.[12] The RCRI is commonly used to predict perioperative cardiovascular events for all patients, including those with NVAF, though AF is not part of this risk assessment. The goal of this retrospective cohort study was to examine the prognostic utility of already existing NVAF risk indices, including the CHADS2 (Congestive heart failure, Hypertension, Age 75 years, Diabetes mellitus, prior stroke or transient ischemic attack), CHA2DS2‐VASc (Congestive heart failure; Hypertension; Age 75 years; Diabetes mellitus; Stroke, TIA, or thromboembolism [TE]; Vascular disease; Age 65 to 74 years; Sex category [female]), and R2CHADS2 (Renal dysfunction, Congestive heart failure, Hypertension, Age, Diabetes, Stroke/TIA) for perioperative outcomes in patients undergoing noncardiac surgery.
Findings
A population dataset of NVAF patients (n=32,160) who underwent noncardiac surgery was examined, with outcome measures including 30‐day mortality, stroke, TIA, or systemic embolism. The incidence of the 30‐day composite outcome was 4.2% and the C indices were 0.65 for the RCRI, 0.67 for CHADS2, 0.67 for CHA2DS2‐VASc, and 0.68 for R2CHADS2. The Net Reclassification Index (NRI), a measure evaluating the improvement in prediction performance gained by adding a marker to a set of baseline predictors, was calculated. All NVAF scores performed better than the RCRI for predicting mortality risk (NRI: 12.3%, 8.4%, and 13.3% respectively, all P0.01).
Cautions
Patients in the highest risk category by RCRI appear to have an unadjusted higher 30‐day mortality risk (8%) than that predicted by the other 3 scores (5%, 5.6%, and 5%), indicating that these risk scores should not completely supplant the RCRI for risk stratification in this population. In addition, the overall improvement in predictive capacity of the CHADS2, CHA2DS2‐VASc, and R2CHADS2, although superior to the RCRI, is modest.
Implications
These findings indicate that the preoperative risk stratification for patients with NVAF can be improved by utilizing the CHADS2, CHA2DS2‐VASc, or R2CHADS2 scores when undergoing noncardiac surgery. For patients with NVAF identified as high risk for adverse outcomes, this assessment can be integrated into the preoperative discussion on the risks/benefits of surgery.
Steinberg BA, Peterson ED, Kim S, et al. Use and outcomes associated with bridging during anticoagulation interruptions in patients with atrial fibrillation: findings from the Outcomes Registry for Better Informed Treatment of Atrial Fibrillation (ORBIT‐AF). Circulation. 2015;131:488494
Background
Oral anticoagulation (OAC) significantly reduces the risk of stroke in patients with AF. Many AF patients on long‐term anticoagulation undergo procedures requiring temporary interruption of OAC. Although guidelines have been published on when and how to initiate bridging therapy, they are based on observational data. Thus, it remains unclear which patients should receive bridging anticoagulation.
Findings
This is a US registry of outpatients with AF with temporary interruptions of OAC for a procedure. Of 7372 patients treated with OAC, 2803 overall interruption events occurred in 2200 patients (30%). Bridging anticoagulants were used in 24% (n=665). Bleeding events were more common in bridged than nonbridged patients (5.0% vs 1.3%; adjusted OR: 3.84; P0.0001). The overall composite end point of myocardial infarction, stroke or systemic embolism, major bleeding, hospitalization, or death within 30 days was significantly higher in patients receiving bridging (13% vs 6.3%; adjusted OR: 1.94; P=0.0001). This statistically significant increase in the composite outcome, which includes cardiovascular events, is most likely in part secondary to inclusion of bleeding events. The recently published BRIDGE (Bridging Anticoagulation in Patients who Require Temporary Interruption of Warfarin Therapy for an Elective Invasive Procedure or Surgery) trial did not find a statistically significant difference in cardiovascular events between bridged and nonbridged patients.[13]
Cautions
Although patients who were bridged appear to have had more comorbidities and a higher mean CHADS2 score than patients who were not bridged, it is difficult to determine which population of patients may be high risk enough to warrant bridging, as indicated by current American College of Chest Physicians guidelines, as this was not evaluated in this study
Implications
The use of bridging anticoagulation was significantly associated with higher overall bleeding and adverse event rates. The BRIDGE trial also found that forgoing bridging anticoagulation decreased the risk of major bleeding in patients with AF and was noninferior to bridging for the prevention of arterial TE.[13]
- , , , et al. Derivation and prospective evaluation of a simple index for prediction of cardiac risk of major noncardiac surgery. Circulation. 1999;100:1043–1049.
- , , , et al. Development and evaluation of the universal ACS NSQIP surgical risk calculator: a decision aid and informed consent tool for patients and surgeons. J Am Coll Surg. 2013;217(5):833–842.
- , , , et al. Development and validation of a risk calculator for prediction of cardiac risk after surgery. Circulation. 2011;124:381–387.
- , , , et al. The effect of bisoprolol on perioperative mortality and myocardial infarction in high‐risk patients undergoing vascular surgery. Dutch Echocardiographic Cardiac Risk Evaluation Applying Stress Echocardiography Study Group. N Engl J Med. 1999;341(24):1789–1794.
- , , , et al; Dutch Echocardiographic Cardiac Risk Evaluation Applying Stress Echocardiography Study Group. Bisoprolol and fluvastatin for the reduction of perioperative cardiac mortality and myocardial infarction in intermediate‐risk patients undergoing noncardiovascular surgery: a randomized controlled trial (DECREASE‐IV). Ann Surg. 2009;249(6):921–926.
- POISE Study Group, , , , et al. Effects of extended‐release metoprolol succinate in patients undergoing non‐cardiac surgery (POISE trial): a randomised controlled trial. Lancet. 2008;371(9627):1839–1847.
- , , , et al. American College of Cardiology; American Heart Association. 2014 ACC/AHA guideline on perioperative cardiovascular evaluation and management of patients undergoing noncardiac surgery: a report of the American College of Cardiology/American Heart Association Task Force on practice guidelines. J Am Coll Cardiol. 2014;64(22):e77–e137.
- , , , et al. 2014 ESC/ESA Guidelines on non‐cardiac surgery: cardiovascular assessment and management: The Joint Task Force on non‐cardiac surgery: cardiovascular assessment and management of the European Society of Cardiology (ESC) and the European Society of Anaesthesiology (ESA). Eur Heart J. 2014;35(35):2383–431.
- , , , et al. The long‐term impact of early cardiovascular therapy intensification for postoperative troponin elevation after major vascular surgery. Anesth Analg. 2014;119(5):1053–1063.
- , , , et al. ARISCAT Group: Prediction of postoperative pulmonary complications in a population‐based surgical cohort. Anesthesiology. 2010;113:1338–1350.
- , . Noncardiac surgery: postoperative arrhythmias. Crit Care Med. 2000;28(10 suppl):N145–N150.
- , , , et al. Incidence, predictors, and outcomes associated with postoperative atrial fibrillation after major cardiac surgery. Am Heart J. 2012;164(6):918–924.
- , , , et al. Perioperative bridging anticoagulation in patients with atrial fibrillation. N Engl J Med. 2015;373(9):823–833.
Given the rapid expansion of the field of perioperative medicine, clinicians need to remain apprised of the current evidence to ensure optimization of patient care. In this update, we review 10 key articles from the perioperative literature, with the goal of summarizing the most clinically important evidence over the past year. This summary of recent literature in perioperative medicine is derived from the Update in Perioperative Medicine sessions presented at the 10th Annual Perioperative Medicine Summit and the Society of General Internal Medicine 38th Annual Meeting. A systematic search strategy was used to identify pertinent articles, and the following were selected by the authors based on their relevance to the clinical practice of perioperative medicine.
PERIOPERATIVE CARDIOVASCULAR CARE
Fleisher LA, Fleischmann KE, Auerbach AD, et al. 2014 ACC/AHA guideline on perioperative cardiovascular evaluation and management of patients undergoing noncardiac surgery: a report of the American College of Cardiology/American Heart Association Task Force on practice guidelines. Circulation. 2014;130:e278e333.
Background
The American College of Cardiology/American Heart Association (ACC/AHA) perioperative guideline provides recommendations for the evaluation and management of cardiovascular disease in patients undergoing noncardiac surgery.
Findings
The new guideline combines the evaluation of surgery‐ and patient‐specific risk in the algorithm for preoperative cardiovascular evaluation into a single step and recommends the use of 1 of 3 tools: the Revised Cardiac Risk Index (RCRI),[1] National Surgical Quality Improvement Program (NSQIP) Surgical Risk Calculator,[2] or the NSQIP‐derived myocardial infarction and cardiac arrest calculator.[3] Estimation of risk is also simplified by stratification into only 2 groups: low risk (risk of major adverse cardiac event 1%) and elevated risk (1% risk). Coronary evaluation can be considered for patients with elevated cardiac risk and poor functional capacity, but is advised only if the results would alter perioperative management. For example, a patient with very high risk who has evidence of ischemia on stress testing may choose to forego surgery. Preoperative coronary revascularization is only indicated for patients meeting criteria in the nonsurgical setting.
For patients with previous percutaneous coronary intervention, the ACC/AHA has not changed its recommendations to optimally delay surgery for at least 30 days after bare‐metal stenting and at least 1 year after drug‐eluting stent (DES) placement. However, in patients with a DES placed 6 to 12 months previously, surgery can be performed if the risks of surgical delay outweigh the risks of DES thrombosis. After any type of coronary stenting, dual antiplatelet therapy should be continued uninterrupted through the first 4 to 6 weeks and even later whenever feasible. If not possible, aspirin therapy should be maintained through surgery unless bleeding risk is too high.
The guideline recommends perioperative continuation of ‐blockers in patients taking them chronically. Preoperative initiation of ‐blocker therapy may be considered for patients with myocardial ischemia on stress testing or 3 RCRI factors and should be started far enough in advance to allow determination of patient's tolerance prior to surgery.
Cautions
Many recommendations are based on data from nonrandomized trials or expert opinion, and the data in areas such as perioperative ‐blockade continue to evolve.
Implications
The ACC/AHA guideline continues to be a critically valuable resource for hospitalists providing perioperative care to noncardiac surgery patients.
Wijeysundera DN, Duncan D, Nkonde‐Price C, et al. Perioperative beta blockade in noncardiac surgery: a systematic review for the 2014 ACC/AHA guideline on perioperative cardiovascular evaluation and management of patients undergoing noncardiac surgery: a report of the American College of Cardiology/American Heart Association Task Force on practice guidelines.
J Am Coll Cardiol. 2014;64(22):24062425.
Background
Various clinical trials have reported conflicting results regarding the efficacy and safety of perioperative ‐blockers resulting in guideline committees changing their recommendations. Because of questions raised regarding the scientific integrity of the DECREASE (Dutch Echocardiographic Cardiac Risk Evaluation Applying Stress Echocardiography)‐I[4] and DECREASE‐IV[5] trials as well as the dosing of ‐blockers in POISE (PeriOperative Ischemic Evaluation) study,[6] this systematic review was performed in conjunction with the ACC/AHA guideline update[7] to evaluate the data with and without these trials.
Findings
Sixteen randomized control trials (RCTs) (n=12,043) and 1 cohort study (n=348) were included in the analysis. Perioperative ‐blockers were associated with a reduction in nonfatal myocardial infarction (MI) (relative risk [RR]: 0.69; 95% confidence interval [CI]: 0.58‐0.82; P0.001) but an increase in bradycardia (RR: 2.61; 95% CI: 2.18‐3.12), hypotension (RR: 1.47; 95% CI: 1.34‐1.6), and nonfatal strokes (RR: 1.76; 95% CI: 1.07‐2.91; P=0.02). The POISE trial was the only one demonstrating a statistically significant increase in stroke.
The major discrepancy between the DECREASE trials and the other RCTs was related to mortalitya reduction in both cardiovascular and all‐cause death in DECREASE but an increased risk of all‐cause death in the other trials.
Cautions
Because of its size, the POISE trial heavily influences the results, particularly for mortality and stroke. Including the DECREASE trials reduces the otherwise increased risk for death to a null effect. Exclusion of the POISE and DECREASE trials leaves few data to make conclusions about safety and efficacy of perioperative ‐blockade. Several cohort studies have found metoprolol to be associated with worse outcomes than with atenolol or bisoprolol (which were preferred by the European Society of Cardiology guidelines).[8]
Implications
Perioperative ‐blockade started within 1 day of noncardiac surgery was associated with fewer nonfatal MIs but at the cost of an increase in hypotension, bradycardia, and a possible increase in stroke and death. Long‐term ‐blockade should be continued perioperatively, whereas the decision to initiate a ‐blocker should be individualized. If starting a ‐blocker perioperatively, it should be done 2 days before surgery.
Botto F, Alonso‐Coello P, Chan MT, et al.; on behalf of The Vascular events In noncardiac Surgery patIents cOhort evaluatioN (VISION) Investigators. Myocardial injury after noncardiac surgery: a large, international, prospective cohort study establishing diagnostic criteria, characteristics, predictors, and 30‐day outcomes. Anesthesiology. 2014;120(3):564578.
Background
Many patients sustain myocardial injury in the perioperative period as evidenced by troponin elevations, but most do not meet diagnostic criteria for MI. Myocardial injury after noncardiac surgery (MINS) is defined as prognostically relevant myocardial injury due to ischemia that occurs within 30 days after noncardiac surgery. This international, prospective cohort study of 15,065 patients 45 years old who underwent in‐patient noncardiac surgery determined diagnostic criteria, characteristics, predictors, and 30‐day outcomes of MINS.
Findings
The diagnostic criterion for MINS was a peak troponin T level 0.03 ng/mL judged to be due to an ischemic etiology. Twelve independent predictors of MINS were identified including age 75 years, known cardiovascular disease or risk factors, and surgical factors. MINS was an independent predictor of 30‐day mortality (adjusted hazard ratio [HR]: 3.87; 95% CI: 2.96‐5.08). Age >75 years, ST elevation, or new left bundle branch block, and anterior ischemic findings were independent predictors of 30‐day mortality among patients with MINS.
Cautions
Although screening high‐risk surgical patients without signs or symptoms of ischemia with postoperative troponins will increase the frequency of diagnosing MINS, evidence for an effective treatment has not yet been established. The ACC/AHA guidelines state that routine screening is of uncertain benefit for this reason.
Implications
Because MINS is common and carries a poor 30‐day prognosis, clinical trials are needed to determine when to obtain postoperative troponins and how to prevent and treat this complication.[9] Some observational data from POISE suggest that aspirin and statins can reduce the risk of 30‐day mortality in patients with postoperative MIs.
Devereaux PJ, Mrkobrada M, Sessler DI, et al. for the POISE‐2 Investigators. Aspirin in patients undergoing noncardiac surgery. N Engl J Med. 2014; 370(16):14941503.
Devereaux PJ, Sessler DI, Leslie K, et al. for the POISE‐2 Investigators. Clonidine in patients undergoing noncardiac surgery. N Engl J Med. 2014; 370(16):15041513.
Background
Medical risk reduction with aspirin and other agents in perioperative patients remains controversial. The POISE‐2 trial is a blinded RCT examining the effects of aspirin and clonidine on outcomes in >10,000 noncardiac surgery patients at risk of cardiovascular complications. The aspirin arm of the study included the initiation group and the continuation stratum, as well as placebo. Patients in the clonidine portion of the trial received 0.2 mg of clonidine or placebo daily for the same time periods.
Findings
The primary outcome was a composite of death or nonfatal MI within 30 days of surgery. Outcomes were similar in patients initiated or continued on aspirin. No difference was seen between aspirin or placebo in the primary outcome (7.0% vs 7.1%; HR: 0.86; 95% CI: 0.86‐1.15; P=0.92). There were no differences in rates of MI, venous thromboembolism, or stroke. Major bleeding rates were higher in aspirin versus placebo‐treated patients (4.6% vs 3.8%; HR: 1.23; 95% CI: 1.01‐1.49; P=0.04).
Clonidine did not alter the composite outcome of death or nonfatal MI (7.3% vs 6.8%; HR: 1.08; 95% CI: 0.93‐1.26; P=0.29). Clinically significant hypotension, bradycardia, and nonfatal cardiac arrest were more common in clonidine‐treated patients, although no difference was detected in stroke rates.
Cautions
Although patients in the trial had cardiovascular risk factors, 24% of patients had known coronary artery disease, and 5% had coronary stents. Conclusions based on this trial regarding perioperative management of antiplatelet therapy should not include patients with coronary artery stents.
Implications
Aspirin started before surgery and continued perioperatively did not decrease the rate of death or nonfatal MI but increased the risk of major bleeding. Perioperative management of aspirin needs to be undertaken in the context of cardiac and bleeding risks. Clonidine also did not improve outcomes and increased the risk of bradycardia and hypotension. Current guidelines recommend against using alpha‐2 agonists for prevention of perioperative cardiac events7; however, patients already on alpha‐2 agonists should not stop them abruptly.
PERIOPERATIVE PULMONARY CARE
Mutter TC, Chateau D, Moffatt M, et al. A matched cohort study of postoperative outcomes in obstructive sleep apnea: could preoperative diagnosis and treatment prevent complications? Anesthesiology. 2014;121(4):707718.
Background
An increasing body of literature associates obstructive sleep apnea (OSA) with an increased risk of postoperative complications. Despite evidence of risk, potential benefits of preoperative diagnosis and treatment of OSA remain unclear.
Findings
Using databases to identify patients prescribed continuous positive airway pressure (CPAP) therapy, the study compared postoperative outcomes of patients who underwent surgery any time after polysomnography (PSG) and CPAP prescription (diagnosed OSA [DOSA]) and those who had surgery during the 5 years preceding their PSG (undiagnosed OSA [UOSA]). These patients were matched with patients who underwent the same procedure for the same indication and had no insurance claims for PSG or diagnosis of sleep‐disordered breathing.
After multivariate analysis, OSA of any type was associated with increased pulmonary complications (odds ratio [OR]: 2.08; 95% CI: 1.35‐2.19). However, no significant differences in respiratory outcomes were noted between DOSA patients (N=2640) and those with UOSA (N=1571). DOSA patients did have fewer cardiovascular complications than UOSA patients (OR: 0.34; 95% CI: 0.15‐0.77). Only severe OSA (apnea‐hypopnea index >30) was associated with increased pulmonary and cardiovascular complications.
Cautions
Although this study suggests an association between preoperative diagnosis and treatment of OSA and reduced cardiovascular complications, the results are not definitive due to the inability to control for all confounding variables in a retrospective study utilizing an administrative database.
Implications
OSA is an important risk factor for postoperative complications, and this study suggests that preoperative treatment with CPAP is associated with reduced risk of cardiovascular complications, particularly in patients with severe OSA. Future controlled trials should focus on the risk‐reduction potential of preoperative diagnosis and treatment of OSA.
Mazo V, Sabat S, Canet J, et al. Prospective external validation of a predictive score for postoperative pulmonary complications. Anesthesiology. 2014;121:219231.
Background
In 2010, Canet et al. published a novel risk index, the Assess Respiratory Risk in Surgical Patients in Catalonia (ARISCAT) index, to provide a quantitative estimate of the risk of postoperative pulmonary complications (PPCs).[10]
In the current report, Mazo and colleagues studied the ARISCAT index in a broader sample to characterize its accuracy in predicting PPC risk. The ARISCAT index is derived from clinical risk factors: (1) age, (2) preoperative oxygen saturation, (3) respiratory infection in the prior month, (4) anemia, (5) surgical site, (6) duration of surgery, and (7) emergency surgery, with varying weights based on the strength of the association in a multivariable analysis. This score can be calculated via addition of these weighted risk factors, with a score>45 equal to high risk for PPC.
Findings
Examining 5099 patients from 63 European hospitals, the authors definition of PPC included respiratory failure, pulmonary infection, pleural effusion, atelectasis, pneumothorax, bronchospasm, and aspiration pneumonitis. PPC rates were as follows: low risk (3.39%), intermediate risk (12.98%), and high risk (38.01%). The positive likelihood ratio for PPC among the highest risk group was 7.12. The C statistic for fit was 0.80. Observed PPC rates were higher than predicted for the low (3.39% vs 0.87%) and intermediate (12.98% vs 7.82%) risk groups.
Cautions
The calibration slopes were less than ideal in all subsamples, with the Western European sample performing better than the other geographic areas; suggesting that the coefficients on the ARISCAT index may benefit from recalibration to match specific populations.
Implications
This is the first major pulmonary risk index that has been externally validated. Its use of readily available clinical information, simplicity, and accuracy in estimating PPC risk make it an important addition to the toolkit during a preoperative evaluation.
PERIOPERATIVE ATRIAL FIBRILLATION/ANTICOAGULATION
Gialdini G, Nearing K, Bhave P, et al. Perioperative atrial fibrillation and the long term risk of ischemic stroke. JAMA. 2014;312(6):616622.
Background
New‐onset atrial fibrillation (AF) is the most common perioperative arrhythmia.[11] However, little is known regarding the long‐term risks of ischemic stroke in patients who develop perioperative AF. This retrospective cohort study examined adults with no preexisting history of AF, hospitalized for surgery, and discharged free of cerebrovascular disease between 2007 and 2011 (n=1,729,360).
Findings
Of the eligible patients, 1.43% (95% CI: 1.41%‐1.45%) developed perioperative AF, and 0.81% (95% CI: 0.79%‐0.82%) had a stroke up to 1 year after discharge. Perioperative AF was associated with subsequent stroke after both cardiac (HR: 1.3; 95% CI: 1.1‐1.6) and noncardiac surgery (HR: 2; 95% CI: 1.7‐2.3). The association with stroke was stronger for perioperative AF after noncardiac versus cardiac surgery (P0.001 for interaction).
Cautions
This is a retrospective cohort study, using claims data to identify AF and stroke. Data on duration of the perioperative AF episodes or use of antithrombotic therapies were not available.
Implications
The association found between perioperative AF and long‐term risk of ischemic stroke may suggest that perioperative AF, especially after noncardiac surgery, should be treated aggressively in terms of thromboembolic risk; however, further data will be required to validate this association.
Van Diepen S, Youngson E, Ezekowitz J, McAlister F. Which risk score best predicts perioperative outcomes in nonvalvular atrial fibrillation patients undergoing noncardiac surgery? Am Heart J. 2014;168(1):6067.
Background
Patients with nonvalvular AF (NVAF) are at increased risk for adverse perioperative outcomes after noncardiac surgery.[12] The RCRI is commonly used to predict perioperative cardiovascular events for all patients, including those with NVAF, though AF is not part of this risk assessment. The goal of this retrospective cohort study was to examine the prognostic utility of already existing NVAF risk indices, including the CHADS2 (Congestive heart failure, Hypertension, Age 75 years, Diabetes mellitus, prior stroke or transient ischemic attack), CHA2DS2‐VASc (Congestive heart failure; Hypertension; Age 75 years; Diabetes mellitus; Stroke, TIA, or thromboembolism [TE]; Vascular disease; Age 65 to 74 years; Sex category [female]), and R2CHADS2 (Renal dysfunction, Congestive heart failure, Hypertension, Age, Diabetes, Stroke/TIA) for perioperative outcomes in patients undergoing noncardiac surgery.
Findings
A population dataset of NVAF patients (n=32,160) who underwent noncardiac surgery was examined, with outcome measures including 30‐day mortality, stroke, TIA, or systemic embolism. The incidence of the 30‐day composite outcome was 4.2% and the C indices were 0.65 for the RCRI, 0.67 for CHADS2, 0.67 for CHA2DS2‐VASc, and 0.68 for R2CHADS2. The Net Reclassification Index (NRI), a measure evaluating the improvement in prediction performance gained by adding a marker to a set of baseline predictors, was calculated. All NVAF scores performed better than the RCRI for predicting mortality risk (NRI: 12.3%, 8.4%, and 13.3% respectively, all P0.01).
Cautions
Patients in the highest risk category by RCRI appear to have an unadjusted higher 30‐day mortality risk (8%) than that predicted by the other 3 scores (5%, 5.6%, and 5%), indicating that these risk scores should not completely supplant the RCRI for risk stratification in this population. In addition, the overall improvement in predictive capacity of the CHADS2, CHA2DS2‐VASc, and R2CHADS2, although superior to the RCRI, is modest.
Implications
These findings indicate that the preoperative risk stratification for patients with NVAF can be improved by utilizing the CHADS2, CHA2DS2‐VASc, or R2CHADS2 scores when undergoing noncardiac surgery. For patients with NVAF identified as high risk for adverse outcomes, this assessment can be integrated into the preoperative discussion on the risks/benefits of surgery.
Steinberg BA, Peterson ED, Kim S, et al. Use and outcomes associated with bridging during anticoagulation interruptions in patients with atrial fibrillation: findings from the Outcomes Registry for Better Informed Treatment of Atrial Fibrillation (ORBIT‐AF). Circulation. 2015;131:488494
Background
Oral anticoagulation (OAC) significantly reduces the risk of stroke in patients with AF. Many AF patients on long‐term anticoagulation undergo procedures requiring temporary interruption of OAC. Although guidelines have been published on when and how to initiate bridging therapy, they are based on observational data. Thus, it remains unclear which patients should receive bridging anticoagulation.
Findings
This is a US registry of outpatients with AF with temporary interruptions of OAC for a procedure. Of 7372 patients treated with OAC, 2803 overall interruption events occurred in 2200 patients (30%). Bridging anticoagulants were used in 24% (n=665). Bleeding events were more common in bridged than nonbridged patients (5.0% vs 1.3%; adjusted OR: 3.84; P0.0001). The overall composite end point of myocardial infarction, stroke or systemic embolism, major bleeding, hospitalization, or death within 30 days was significantly higher in patients receiving bridging (13% vs 6.3%; adjusted OR: 1.94; P=0.0001). This statistically significant increase in the composite outcome, which includes cardiovascular events, is most likely in part secondary to inclusion of bleeding events. The recently published BRIDGE (Bridging Anticoagulation in Patients who Require Temporary Interruption of Warfarin Therapy for an Elective Invasive Procedure or Surgery) trial did not find a statistically significant difference in cardiovascular events between bridged and nonbridged patients.[13]
Cautions
Although patients who were bridged appear to have had more comorbidities and a higher mean CHADS2 score than patients who were not bridged, it is difficult to determine which population of patients may be high risk enough to warrant bridging, as indicated by current American College of Chest Physicians guidelines, as this was not evaluated in this study
Implications
The use of bridging anticoagulation was significantly associated with higher overall bleeding and adverse event rates. The BRIDGE trial also found that forgoing bridging anticoagulation decreased the risk of major bleeding in patients with AF and was noninferior to bridging for the prevention of arterial TE.[13]
Given the rapid expansion of the field of perioperative medicine, clinicians need to remain apprised of the current evidence to ensure optimization of patient care. In this update, we review 10 key articles from the perioperative literature, with the goal of summarizing the most clinically important evidence over the past year. This summary of recent literature in perioperative medicine is derived from the Update in Perioperative Medicine sessions presented at the 10th Annual Perioperative Medicine Summit and the Society of General Internal Medicine 38th Annual Meeting. A systematic search strategy was used to identify pertinent articles, and the following were selected by the authors based on their relevance to the clinical practice of perioperative medicine.
PERIOPERATIVE CARDIOVASCULAR CARE
Fleisher LA, Fleischmann KE, Auerbach AD, et al. 2014 ACC/AHA guideline on perioperative cardiovascular evaluation and management of patients undergoing noncardiac surgery: a report of the American College of Cardiology/American Heart Association Task Force on practice guidelines. Circulation. 2014;130:e278e333.
Background
The American College of Cardiology/American Heart Association (ACC/AHA) perioperative guideline provides recommendations for the evaluation and management of cardiovascular disease in patients undergoing noncardiac surgery.
Findings
The new guideline combines the evaluation of surgery‐ and patient‐specific risk in the algorithm for preoperative cardiovascular evaluation into a single step and recommends the use of 1 of 3 tools: the Revised Cardiac Risk Index (RCRI),[1] National Surgical Quality Improvement Program (NSQIP) Surgical Risk Calculator,[2] or the NSQIP‐derived myocardial infarction and cardiac arrest calculator.[3] Estimation of risk is also simplified by stratification into only 2 groups: low risk (risk of major adverse cardiac event 1%) and elevated risk (1% risk). Coronary evaluation can be considered for patients with elevated cardiac risk and poor functional capacity, but is advised only if the results would alter perioperative management. For example, a patient with very high risk who has evidence of ischemia on stress testing may choose to forego surgery. Preoperative coronary revascularization is only indicated for patients meeting criteria in the nonsurgical setting.
For patients with previous percutaneous coronary intervention, the ACC/AHA has not changed its recommendations to optimally delay surgery for at least 30 days after bare‐metal stenting and at least 1 year after drug‐eluting stent (DES) placement. However, in patients with a DES placed 6 to 12 months previously, surgery can be performed if the risks of surgical delay outweigh the risks of DES thrombosis. After any type of coronary stenting, dual antiplatelet therapy should be continued uninterrupted through the first 4 to 6 weeks and even later whenever feasible. If not possible, aspirin therapy should be maintained through surgery unless bleeding risk is too high.
The guideline recommends perioperative continuation of ‐blockers in patients taking them chronically. Preoperative initiation of ‐blocker therapy may be considered for patients with myocardial ischemia on stress testing or 3 RCRI factors and should be started far enough in advance to allow determination of patient's tolerance prior to surgery.
Cautions
Many recommendations are based on data from nonrandomized trials or expert opinion, and the data in areas such as perioperative ‐blockade continue to evolve.
Implications
The ACC/AHA guideline continues to be a critically valuable resource for hospitalists providing perioperative care to noncardiac surgery patients.
Wijeysundera DN, Duncan D, Nkonde‐Price C, et al. Perioperative beta blockade in noncardiac surgery: a systematic review for the 2014 ACC/AHA guideline on perioperative cardiovascular evaluation and management of patients undergoing noncardiac surgery: a report of the American College of Cardiology/American Heart Association Task Force on practice guidelines.
J Am Coll Cardiol. 2014;64(22):24062425.
Background
Various clinical trials have reported conflicting results regarding the efficacy and safety of perioperative ‐blockers resulting in guideline committees changing their recommendations. Because of questions raised regarding the scientific integrity of the DECREASE (Dutch Echocardiographic Cardiac Risk Evaluation Applying Stress Echocardiography)‐I[4] and DECREASE‐IV[5] trials as well as the dosing of ‐blockers in POISE (PeriOperative Ischemic Evaluation) study,[6] this systematic review was performed in conjunction with the ACC/AHA guideline update[7] to evaluate the data with and without these trials.
Findings
Sixteen randomized control trials (RCTs) (n=12,043) and 1 cohort study (n=348) were included in the analysis. Perioperative ‐blockers were associated with a reduction in nonfatal myocardial infarction (MI) (relative risk [RR]: 0.69; 95% confidence interval [CI]: 0.58‐0.82; P0.001) but an increase in bradycardia (RR: 2.61; 95% CI: 2.18‐3.12), hypotension (RR: 1.47; 95% CI: 1.34‐1.6), and nonfatal strokes (RR: 1.76; 95% CI: 1.07‐2.91; P=0.02). The POISE trial was the only one demonstrating a statistically significant increase in stroke.
The major discrepancy between the DECREASE trials and the other RCTs was related to mortalitya reduction in both cardiovascular and all‐cause death in DECREASE but an increased risk of all‐cause death in the other trials.
Cautions
Because of its size, the POISE trial heavily influences the results, particularly for mortality and stroke. Including the DECREASE trials reduces the otherwise increased risk for death to a null effect. Exclusion of the POISE and DECREASE trials leaves few data to make conclusions about safety and efficacy of perioperative ‐blockade. Several cohort studies have found metoprolol to be associated with worse outcomes than with atenolol or bisoprolol (which were preferred by the European Society of Cardiology guidelines).[8]
Implications
Perioperative ‐blockade started within 1 day of noncardiac surgery was associated with fewer nonfatal MIs but at the cost of an increase in hypotension, bradycardia, and a possible increase in stroke and death. Long‐term ‐blockade should be continued perioperatively, whereas the decision to initiate a ‐blocker should be individualized. If starting a ‐blocker perioperatively, it should be done 2 days before surgery.
Botto F, Alonso‐Coello P, Chan MT, et al.; on behalf of The Vascular events In noncardiac Surgery patIents cOhort evaluatioN (VISION) Investigators. Myocardial injury after noncardiac surgery: a large, international, prospective cohort study establishing diagnostic criteria, characteristics, predictors, and 30‐day outcomes. Anesthesiology. 2014;120(3):564578.
Background
Many patients sustain myocardial injury in the perioperative period as evidenced by troponin elevations, but most do not meet diagnostic criteria for MI. Myocardial injury after noncardiac surgery (MINS) is defined as prognostically relevant myocardial injury due to ischemia that occurs within 30 days after noncardiac surgery. This international, prospective cohort study of 15,065 patients 45 years old who underwent in‐patient noncardiac surgery determined diagnostic criteria, characteristics, predictors, and 30‐day outcomes of MINS.
Findings
The diagnostic criterion for MINS was a peak troponin T level 0.03 ng/mL judged to be due to an ischemic etiology. Twelve independent predictors of MINS were identified including age 75 years, known cardiovascular disease or risk factors, and surgical factors. MINS was an independent predictor of 30‐day mortality (adjusted hazard ratio [HR]: 3.87; 95% CI: 2.96‐5.08). Age >75 years, ST elevation, or new left bundle branch block, and anterior ischemic findings were independent predictors of 30‐day mortality among patients with MINS.
Cautions
Although screening high‐risk surgical patients without signs or symptoms of ischemia with postoperative troponins will increase the frequency of diagnosing MINS, evidence for an effective treatment has not yet been established. The ACC/AHA guidelines state that routine screening is of uncertain benefit for this reason.
Implications
Because MINS is common and carries a poor 30‐day prognosis, clinical trials are needed to determine when to obtain postoperative troponins and how to prevent and treat this complication.[9] Some observational data from POISE suggest that aspirin and statins can reduce the risk of 30‐day mortality in patients with postoperative MIs.
Devereaux PJ, Mrkobrada M, Sessler DI, et al. for the POISE‐2 Investigators. Aspirin in patients undergoing noncardiac surgery. N Engl J Med. 2014; 370(16):14941503.
Devereaux PJ, Sessler DI, Leslie K, et al. for the POISE‐2 Investigators. Clonidine in patients undergoing noncardiac surgery. N Engl J Med. 2014; 370(16):15041513.
Background
Medical risk reduction with aspirin and other agents in perioperative patients remains controversial. The POISE‐2 trial is a blinded RCT examining the effects of aspirin and clonidine on outcomes in >10,000 noncardiac surgery patients at risk of cardiovascular complications. The aspirin arm of the study included the initiation group and the continuation stratum, as well as placebo. Patients in the clonidine portion of the trial received 0.2 mg of clonidine or placebo daily for the same time periods.
Findings
The primary outcome was a composite of death or nonfatal MI within 30 days of surgery. Outcomes were similar in patients initiated or continued on aspirin. No difference was seen between aspirin or placebo in the primary outcome (7.0% vs 7.1%; HR: 0.86; 95% CI: 0.86‐1.15; P=0.92). There were no differences in rates of MI, venous thromboembolism, or stroke. Major bleeding rates were higher in aspirin versus placebo‐treated patients (4.6% vs 3.8%; HR: 1.23; 95% CI: 1.01‐1.49; P=0.04).
Clonidine did not alter the composite outcome of death or nonfatal MI (7.3% vs 6.8%; HR: 1.08; 95% CI: 0.93‐1.26; P=0.29). Clinically significant hypotension, bradycardia, and nonfatal cardiac arrest were more common in clonidine‐treated patients, although no difference was detected in stroke rates.
Cautions
Although patients in the trial had cardiovascular risk factors, 24% of patients had known coronary artery disease, and 5% had coronary stents. Conclusions based on this trial regarding perioperative management of antiplatelet therapy should not include patients with coronary artery stents.
Implications
Aspirin started before surgery and continued perioperatively did not decrease the rate of death or nonfatal MI but increased the risk of major bleeding. Perioperative management of aspirin needs to be undertaken in the context of cardiac and bleeding risks. Clonidine also did not improve outcomes and increased the risk of bradycardia and hypotension. Current guidelines recommend against using alpha‐2 agonists for prevention of perioperative cardiac events7; however, patients already on alpha‐2 agonists should not stop them abruptly.
PERIOPERATIVE PULMONARY CARE
Mutter TC, Chateau D, Moffatt M, et al. A matched cohort study of postoperative outcomes in obstructive sleep apnea: could preoperative diagnosis and treatment prevent complications? Anesthesiology. 2014;121(4):707718.
Background
An increasing body of literature associates obstructive sleep apnea (OSA) with an increased risk of postoperative complications. Despite evidence of risk, potential benefits of preoperative diagnosis and treatment of OSA remain unclear.
Findings
Using databases to identify patients prescribed continuous positive airway pressure (CPAP) therapy, the study compared postoperative outcomes of patients who underwent surgery any time after polysomnography (PSG) and CPAP prescription (diagnosed OSA [DOSA]) and those who had surgery during the 5 years preceding their PSG (undiagnosed OSA [UOSA]). These patients were matched with patients who underwent the same procedure for the same indication and had no insurance claims for PSG or diagnosis of sleep‐disordered breathing.
After multivariate analysis, OSA of any type was associated with increased pulmonary complications (odds ratio [OR]: 2.08; 95% CI: 1.35‐2.19). However, no significant differences in respiratory outcomes were noted between DOSA patients (N=2640) and those with UOSA (N=1571). DOSA patients did have fewer cardiovascular complications than UOSA patients (OR: 0.34; 95% CI: 0.15‐0.77). Only severe OSA (apnea‐hypopnea index >30) was associated with increased pulmonary and cardiovascular complications.
Cautions
Although this study suggests an association between preoperative diagnosis and treatment of OSA and reduced cardiovascular complications, the results are not definitive due to the inability to control for all confounding variables in a retrospective study utilizing an administrative database.
Implications
OSA is an important risk factor for postoperative complications, and this study suggests that preoperative treatment with CPAP is associated with reduced risk of cardiovascular complications, particularly in patients with severe OSA. Future controlled trials should focus on the risk‐reduction potential of preoperative diagnosis and treatment of OSA.
Mazo V, Sabat S, Canet J, et al. Prospective external validation of a predictive score for postoperative pulmonary complications. Anesthesiology. 2014;121:219231.
Background
In 2010, Canet et al. published a novel risk index, the Assess Respiratory Risk in Surgical Patients in Catalonia (ARISCAT) index, to provide a quantitative estimate of the risk of postoperative pulmonary complications (PPCs).[10]
In the current report, Mazo and colleagues studied the ARISCAT index in a broader sample to characterize its accuracy in predicting PPC risk. The ARISCAT index is derived from clinical risk factors: (1) age, (2) preoperative oxygen saturation, (3) respiratory infection in the prior month, (4) anemia, (5) surgical site, (6) duration of surgery, and (7) emergency surgery, with varying weights based on the strength of the association in a multivariable analysis. This score can be calculated via addition of these weighted risk factors, with a score>45 equal to high risk for PPC.
Findings
Examining 5099 patients from 63 European hospitals, the authors definition of PPC included respiratory failure, pulmonary infection, pleural effusion, atelectasis, pneumothorax, bronchospasm, and aspiration pneumonitis. PPC rates were as follows: low risk (3.39%), intermediate risk (12.98%), and high risk (38.01%). The positive likelihood ratio for PPC among the highest risk group was 7.12. The C statistic for fit was 0.80. Observed PPC rates were higher than predicted for the low (3.39% vs 0.87%) and intermediate (12.98% vs 7.82%) risk groups.
Cautions
The calibration slopes were less than ideal in all subsamples, with the Western European sample performing better than the other geographic areas; suggesting that the coefficients on the ARISCAT index may benefit from recalibration to match specific populations.
Implications
This is the first major pulmonary risk index that has been externally validated. Its use of readily available clinical information, simplicity, and accuracy in estimating PPC risk make it an important addition to the toolkit during a preoperative evaluation.
PERIOPERATIVE ATRIAL FIBRILLATION/ANTICOAGULATION
Gialdini G, Nearing K, Bhave P, et al. Perioperative atrial fibrillation and the long term risk of ischemic stroke. JAMA. 2014;312(6):616622.
Background
New‐onset atrial fibrillation (AF) is the most common perioperative arrhythmia.[11] However, little is known regarding the long‐term risks of ischemic stroke in patients who develop perioperative AF. This retrospective cohort study examined adults with no preexisting history of AF, hospitalized for surgery, and discharged free of cerebrovascular disease between 2007 and 2011 (n=1,729,360).
Findings
Of the eligible patients, 1.43% (95% CI: 1.41%‐1.45%) developed perioperative AF, and 0.81% (95% CI: 0.79%‐0.82%) had a stroke up to 1 year after discharge. Perioperative AF was associated with subsequent stroke after both cardiac (HR: 1.3; 95% CI: 1.1‐1.6) and noncardiac surgery (HR: 2; 95% CI: 1.7‐2.3). The association with stroke was stronger for perioperative AF after noncardiac versus cardiac surgery (P0.001 for interaction).
Cautions
This is a retrospective cohort study, using claims data to identify AF and stroke. Data on duration of the perioperative AF episodes or use of antithrombotic therapies were not available.
Implications
The association found between perioperative AF and long‐term risk of ischemic stroke may suggest that perioperative AF, especially after noncardiac surgery, should be treated aggressively in terms of thromboembolic risk; however, further data will be required to validate this association.
Van Diepen S, Youngson E, Ezekowitz J, McAlister F. Which risk score best predicts perioperative outcomes in nonvalvular atrial fibrillation patients undergoing noncardiac surgery? Am Heart J. 2014;168(1):6067.
Background
Patients with nonvalvular AF (NVAF) are at increased risk for adverse perioperative outcomes after noncardiac surgery.[12] The RCRI is commonly used to predict perioperative cardiovascular events for all patients, including those with NVAF, though AF is not part of this risk assessment. The goal of this retrospective cohort study was to examine the prognostic utility of already existing NVAF risk indices, including the CHADS2 (Congestive heart failure, Hypertension, Age 75 years, Diabetes mellitus, prior stroke or transient ischemic attack), CHA2DS2‐VASc (Congestive heart failure; Hypertension; Age 75 years; Diabetes mellitus; Stroke, TIA, or thromboembolism [TE]; Vascular disease; Age 65 to 74 years; Sex category [female]), and R2CHADS2 (Renal dysfunction, Congestive heart failure, Hypertension, Age, Diabetes, Stroke/TIA) for perioperative outcomes in patients undergoing noncardiac surgery.
Findings
A population dataset of NVAF patients (n=32,160) who underwent noncardiac surgery was examined, with outcome measures including 30‐day mortality, stroke, TIA, or systemic embolism. The incidence of the 30‐day composite outcome was 4.2% and the C indices were 0.65 for the RCRI, 0.67 for CHADS2, 0.67 for CHA2DS2‐VASc, and 0.68 for R2CHADS2. The Net Reclassification Index (NRI), a measure evaluating the improvement in prediction performance gained by adding a marker to a set of baseline predictors, was calculated. All NVAF scores performed better than the RCRI for predicting mortality risk (NRI: 12.3%, 8.4%, and 13.3% respectively, all P0.01).
Cautions
Patients in the highest risk category by RCRI appear to have an unadjusted higher 30‐day mortality risk (8%) than that predicted by the other 3 scores (5%, 5.6%, and 5%), indicating that these risk scores should not completely supplant the RCRI for risk stratification in this population. In addition, the overall improvement in predictive capacity of the CHADS2, CHA2DS2‐VASc, and R2CHADS2, although superior to the RCRI, is modest.
Implications
These findings indicate that the preoperative risk stratification for patients with NVAF can be improved by utilizing the CHADS2, CHA2DS2‐VASc, or R2CHADS2 scores when undergoing noncardiac surgery. For patients with NVAF identified as high risk for adverse outcomes, this assessment can be integrated into the preoperative discussion on the risks/benefits of surgery.
Steinberg BA, Peterson ED, Kim S, et al. Use and outcomes associated with bridging during anticoagulation interruptions in patients with atrial fibrillation: findings from the Outcomes Registry for Better Informed Treatment of Atrial Fibrillation (ORBIT‐AF). Circulation. 2015;131:488494
Background
Oral anticoagulation (OAC) significantly reduces the risk of stroke in patients with AF. Many AF patients on long‐term anticoagulation undergo procedures requiring temporary interruption of OAC. Although guidelines have been published on when and how to initiate bridging therapy, they are based on observational data. Thus, it remains unclear which patients should receive bridging anticoagulation.
Findings
This is a US registry of outpatients with AF with temporary interruptions of OAC for a procedure. Of 7372 patients treated with OAC, 2803 overall interruption events occurred in 2200 patients (30%). Bridging anticoagulants were used in 24% (n=665). Bleeding events were more common in bridged than nonbridged patients (5.0% vs 1.3%; adjusted OR: 3.84; P0.0001). The overall composite end point of myocardial infarction, stroke or systemic embolism, major bleeding, hospitalization, or death within 30 days was significantly higher in patients receiving bridging (13% vs 6.3%; adjusted OR: 1.94; P=0.0001). This statistically significant increase in the composite outcome, which includes cardiovascular events, is most likely in part secondary to inclusion of bleeding events. The recently published BRIDGE (Bridging Anticoagulation in Patients who Require Temporary Interruption of Warfarin Therapy for an Elective Invasive Procedure or Surgery) trial did not find a statistically significant difference in cardiovascular events between bridged and nonbridged patients.[13]
Cautions
Although patients who were bridged appear to have had more comorbidities and a higher mean CHADS2 score than patients who were not bridged, it is difficult to determine which population of patients may be high risk enough to warrant bridging, as indicated by current American College of Chest Physicians guidelines, as this was not evaluated in this study
Implications
The use of bridging anticoagulation was significantly associated with higher overall bleeding and adverse event rates. The BRIDGE trial also found that forgoing bridging anticoagulation decreased the risk of major bleeding in patients with AF and was noninferior to bridging for the prevention of arterial TE.[13]
- , , , et al. Derivation and prospective evaluation of a simple index for prediction of cardiac risk of major noncardiac surgery. Circulation. 1999;100:1043–1049.
- , , , et al. Development and evaluation of the universal ACS NSQIP surgical risk calculator: a decision aid and informed consent tool for patients and surgeons. J Am Coll Surg. 2013;217(5):833–842.
- , , , et al. Development and validation of a risk calculator for prediction of cardiac risk after surgery. Circulation. 2011;124:381–387.
- , , , et al. The effect of bisoprolol on perioperative mortality and myocardial infarction in high‐risk patients undergoing vascular surgery. Dutch Echocardiographic Cardiac Risk Evaluation Applying Stress Echocardiography Study Group. N Engl J Med. 1999;341(24):1789–1794.
- , , , et al; Dutch Echocardiographic Cardiac Risk Evaluation Applying Stress Echocardiography Study Group. Bisoprolol and fluvastatin for the reduction of perioperative cardiac mortality and myocardial infarction in intermediate‐risk patients undergoing noncardiovascular surgery: a randomized controlled trial (DECREASE‐IV). Ann Surg. 2009;249(6):921–926.
- POISE Study Group, , , , et al. Effects of extended‐release metoprolol succinate in patients undergoing non‐cardiac surgery (POISE trial): a randomised controlled trial. Lancet. 2008;371(9627):1839–1847.
- , , , et al. American College of Cardiology; American Heart Association. 2014 ACC/AHA guideline on perioperative cardiovascular evaluation and management of patients undergoing noncardiac surgery: a report of the American College of Cardiology/American Heart Association Task Force on practice guidelines. J Am Coll Cardiol. 2014;64(22):e77–e137.
- , , , et al. 2014 ESC/ESA Guidelines on non‐cardiac surgery: cardiovascular assessment and management: The Joint Task Force on non‐cardiac surgery: cardiovascular assessment and management of the European Society of Cardiology (ESC) and the European Society of Anaesthesiology (ESA). Eur Heart J. 2014;35(35):2383–431.
- , , , et al. The long‐term impact of early cardiovascular therapy intensification for postoperative troponin elevation after major vascular surgery. Anesth Analg. 2014;119(5):1053–1063.
- , , , et al. ARISCAT Group: Prediction of postoperative pulmonary complications in a population‐based surgical cohort. Anesthesiology. 2010;113:1338–1350.
- , . Noncardiac surgery: postoperative arrhythmias. Crit Care Med. 2000;28(10 suppl):N145–N150.
- , , , et al. Incidence, predictors, and outcomes associated with postoperative atrial fibrillation after major cardiac surgery. Am Heart J. 2012;164(6):918–924.
- , , , et al. Perioperative bridging anticoagulation in patients with atrial fibrillation. N Engl J Med. 2015;373(9):823–833.
- , , , et al. Derivation and prospective evaluation of a simple index for prediction of cardiac risk of major noncardiac surgery. Circulation. 1999;100:1043–1049.
- , , , et al. Development and evaluation of the universal ACS NSQIP surgical risk calculator: a decision aid and informed consent tool for patients and surgeons. J Am Coll Surg. 2013;217(5):833–842.
- , , , et al. Development and validation of a risk calculator for prediction of cardiac risk after surgery. Circulation. 2011;124:381–387.
- , , , et al. The effect of bisoprolol on perioperative mortality and myocardial infarction in high‐risk patients undergoing vascular surgery. Dutch Echocardiographic Cardiac Risk Evaluation Applying Stress Echocardiography Study Group. N Engl J Med. 1999;341(24):1789–1794.
- , , , et al; Dutch Echocardiographic Cardiac Risk Evaluation Applying Stress Echocardiography Study Group. Bisoprolol and fluvastatin for the reduction of perioperative cardiac mortality and myocardial infarction in intermediate‐risk patients undergoing noncardiovascular surgery: a randomized controlled trial (DECREASE‐IV). Ann Surg. 2009;249(6):921–926.
- POISE Study Group, , , , et al. Effects of extended‐release metoprolol succinate in patients undergoing non‐cardiac surgery (POISE trial): a randomised controlled trial. Lancet. 2008;371(9627):1839–1847.
- , , , et al. American College of Cardiology; American Heart Association. 2014 ACC/AHA guideline on perioperative cardiovascular evaluation and management of patients undergoing noncardiac surgery: a report of the American College of Cardiology/American Heart Association Task Force on practice guidelines. J Am Coll Cardiol. 2014;64(22):e77–e137.
- , , , et al. 2014 ESC/ESA Guidelines on non‐cardiac surgery: cardiovascular assessment and management: The Joint Task Force on non‐cardiac surgery: cardiovascular assessment and management of the European Society of Cardiology (ESC) and the European Society of Anaesthesiology (ESA). Eur Heart J. 2014;35(35):2383–431.
- , , , et al. The long‐term impact of early cardiovascular therapy intensification for postoperative troponin elevation after major vascular surgery. Anesth Analg. 2014;119(5):1053–1063.
- , , , et al. ARISCAT Group: Prediction of postoperative pulmonary complications in a population‐based surgical cohort. Anesthesiology. 2010;113:1338–1350.
- , . Noncardiac surgery: postoperative arrhythmias. Crit Care Med. 2000;28(10 suppl):N145–N150.
- , , , et al. Incidence, predictors, and outcomes associated with postoperative atrial fibrillation after major cardiac surgery. Am Heart J. 2012;164(6):918–924.
- , , , et al. Perioperative bridging anticoagulation in patients with atrial fibrillation. N Engl J Med. 2015;373(9):823–833.
Pediatric Admission and Readmission
Patient outcomes tend to be worse for adults admitted on the weekend compared to the weekday.[1, 2, 3, 4] In pediatric populations, urgent surgeries on weekends are associated with increased morbidity and mortality[5]; however, studies of mortality and admission timing in the pediatric critical care setting are mixed.[6, 7] Hospital readmission is considered a potential marker of hospital quality. We hypothesized that (1) being admitted and (2) being discharged on the weekend would adversely affect 30‐day unplanned readmission for pediatric patients.
METHODS
Population
All discharges from January 1, 2006 through December 31, 2012 from C. S. Mott Children's Hospital were initially eligible. All hospitalizations were considered potential index admissions; therefore, children may contribute more than 1 hospitalization to the dataset. We excluded hospitalizations in which the patient died, was transferred to another institution, was discharged against medical advice, or was discharged to hospice. Newborns admitted to a normal newborn service were also excluded, as they do not represent a typical hospitalization for illness. Among newborns admitted to a higher‐intensity clinical service (eg, special care nursery or neonatal intensive care), we also excluded newborns with a length of stay 5 days, given the typical length of stay of up to 4 days for uncomplicated delivery via Cesarean section that would indicate infants for whom precautionary measures had been taken but there was low estimated health risk. We used International Classification of Diseases, Ninth Revision codes to identify children with complex chronic conditions (CCCs) and technology dependency.[8]
Outcome
We examined unplanned readmission within 30 days of discharge. We defined unplanned readmission as a readmission that was not entered into the hospital registration system at least 24 hours before discharge.[9] Additionally, we performed sensitive analyses examining any 30‐day readmissions.
Statistical Analysis
We fit multivariable logistic regression models for 30‐day unplanned readmission, with the primary predictor of either weekend (Saturday or Sunday) admission or weekend discharge (in separate models). We adjusted for patient age, gender, race/ethnicity, source of admission, insurance, and length of stay. We also adjusted for patient chronic illness complexity using the number of CCCs and technology dependency (yes/no). Variance in all analyses was clustered on individual patients.
RESULTS
We included a total of 55,383 hospitalizations from 32,112 patients (see Supporting Appendix Figure in the online version of this article for cohort derivation). All‐cause 30‐day readmissions occurred in 14.9% of hospital discharges; the 30‐day unplanned readmission rate was 10.3% (see the Supporting Appendix Table in the online version of this article for demographic characteristics).
Weekend Admission
Overall, 82% of admissions occurred during the week, with Tuesday as the highest admitting volume day (Figure 1). Children admitted on the weekend had higher odds of unplanned readmission compared to children admitted on weekdays (unadjusted odds ratio [OR]=1.15 [95% confidence interval {CI}: 1.07‐1.24]). Adjusting the analysis for age, gender, race/ethnicity, insurance, length of stay, CCCs, and technology dependency, higher odds of readmission remains significantly higher than weekday admission (adjusted OR=1.09 [95% CI: 1.004‐1.18]) (Table 1). Age, admission source, payer, length of stay, number of complex chronic conditions, and technology dependency were also significantly associated with readmission in the weekend admission model (see the Supporting Appendix Table in the online version of this article). A sensitivity analysis examining the association of weekend admission and readmission within different subpopulations of children with varying numbers of CCCs (ie, among children without CCCs, with 1 CCC, 2 CCCs, and 3+ CCCs) showed that the association remains the same in each subgroup. Further, a sensitivity analysis examining odds of any 30‐day readmission was similar to the primary analysis with higher odds of readmission in adjusted analysis (adjusted OR=1.09 [95% CI: 1.02‐1.18]).
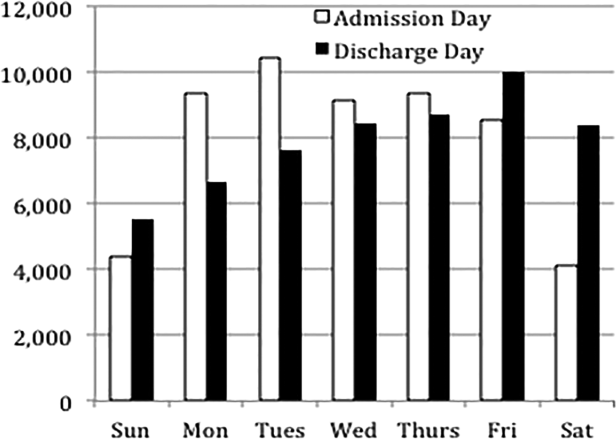
| 30‐Day Unplanned Readmission Rate | Unadjusted Odds of Unplanned Readmission (95% CI) | Weekend Admission Model: Adjusted Odds of Unplanned Readmission (95% CI) | Weekend Discharge Model: Adjusted Odds of Unplanned Readmission (95% CI) | |
|---|---|---|---|---|
| ||||
| Weekend admission, n=7,533 | 11.4%, n=973 | 1.15 (1.07‐1.24)* | 1.09 (1.004‐1.18)* | |
| Weekend discharge, n=13,911 | 9.7%, n=1,344 | 0.91 (0.85‐0.97)* | 0.97 (0.91‐1.04) | |
Weekend Discharge
Weekend discharges accounted for 34% of all discharges. Fridays had the highest discharge volumes, with lowest discharge volumes on Sunday (Figure 1). Children discharged on the weekend had lower odds of unplanned readmission compared to children discharged on weekdays in bivariate analysis (unadjusted OR=0.91 [95% CI: 0.85‐0.97]). However, when adjusting for important confounders, the relationship was no longer statistically significant (adjusted OR=0.97 [95% CI: 0.91‐1.03]) (Table 1). Age, admission source, payer, length of stay, and number of complex chronic conditions were associated with readmission in the weekend discharge model (see the Supporting Appendix Table in the online version of this article). In a sensitivity analysis examining any 30‐day readmission, weekend discharge was not associated with readmission in adjusted analysis.
DISCUSSION
Although the so‐called weekend effect has been established in adults,[1, 2, 3, 4] evidence is mixed for children. In this sample, where the 30‐day pediatric readmission rate is consistent with national pediatric rates,[10] pediatric patients admitted on the weekend have higher odds of readmission compared to children admitted during the week, even when accounting for patient characteristics and hospital length of stay. In contrast, weekend discharge was not associated with readmission.
The association of weekend admission and subsequent readmission is intriguing and may be interpreted in 1 of 2 ways: either patients admitted on the weekend are fundamentally different and thus have higher readmission rates, or care on the weekend is different. It is important to note that we adjusted the analysis for patient characteristics including number of CCCs and technology dependency to account for differences in chronic illness. We also accounted for length of stay as a marker of severity of illness in the hospital. Yet even accounting for these known differences, we cannot discern from these data if the different outcomes for children admitted on the weekend are related to residual population differences (eg, lack of access to primary care or walk‐in clinics) or differences in initial clinical management on the weekend.
Initial clinical management on weekend may be different due to differences in physician, nursing, and other ancillary staffing affecting availability of diagnostic and therapeutic interventions. Additionally, smaller staff size on the weekend may lead to increased workload. Although we are unable to directly measure resident workload in our study, prior studies suggest higher workload is associated with worse outcomes for adult patients,[11] including readmission.[12] Additionally, nurse staffing, which may vary based on day of week, has been associated with pediatric readmission.[13]
Discharge timing in our population is consistent with prior literature, with Friday being the most common discharge day of week.[14] Prior literature has shown no difference in readmission rates between Friday discharge and midweek discharge for pediatric patients.[14] Our work builds on this existing literature, demonstrating no association with weekend discharge and readmission. There were lower discharge volumes on the weekends, particularly in patients with more CCCs, suggesting that physicians avoid complicated discharges on Saturday and Sunday.
This study should be interpreted in the context of several limitations. First, this study was conducted at a single tertiary care pediatric institution. Our patient population had a high rate of children with CCCs, potentially limiting generalizability to other pediatric institutions. Ideally, we would adjust our model for clusters at the clinical service or attending physician level; however, the heterogeneity of our services and data limits prohibited these analyses. Readmissions that may have occurred at other institutions are not observable in this dataset; however, there is no reason to believe patients admitted or discharged on the weekend would have different rates of other hospital readmissions than patients admitted or discharged on weekdays. Additionally, early readmissions may be particularly affected by in‐hospital and discharge factors.[15] However, the very low rate of early readmission prohibited limiting the analyses to early readmission. Finally, we relied on administrative data to adjust for patient severity using typical methods such as CCCs; however, other patient differences may have existed beyond those that could be captured with administrative data.
CONCLUSION
Children admitted to the hospital on the weekend have higher rates of 30‐day unplanned readmission than children admitted during the week, suggesting differences of care in initial management on the weekend. Understanding this difference from the perspectives of multiple stakeholders may illuminate potential reasons for this disparity.
Disclosures
Dr. Auger received salary support from the Robert Wood Johnson Foundation Clinical Scholars program during work on this project. The hospital database was assembled with funds from a grant from the Blue Cross Blue Shield of Michigan Foundation. The authors report no conflicts of interest.
- , , , . A comparison of in‐hospital mortality risk conferred by high hospital occupancy, differences in nurse staffing levels, weekend admission, and seasonal influenza. Med Care. 2010;48(3):224–232.
- , . Mortality among patients admitted to hospitals on weekends as compared with weekdays. N Engl J Med. 2001;345(9):663–668.
- , , , . Effects of weekend admission and hospital teaching status on in‐hospital mortality. Am J Med. 2004;117(3):151–157.
- , , . Weekend admission for myocardial infarction. N Engl J Med. 2007;357(1):86–87; author reply 87–88.
- , , , et al. The "weekend effect" in pediatric surgery—increased mortality for children undergoing urgent surgery during the weekend. J Pediatr Surg. 2014;49(7):1087–1091.
- , , , , , Paediatric Intensive Care Audit Network (PICANet). Effects of out‐of‐hours and winter admissions and number of patients per unit on mortality in pediatric intensive care. J Pediatr. 2013;163(4):1039–1044.e1035.
- , , , . Do weekends or evenings matter in a pediatric intensive care unit? Pediatr Crit Care Med. 2005;6(5):523–530.
- , , , , . Pediatric complex chronic conditions classification system version 2: updated for ICD‐10 and complex medical technology dependence and transplantation. BMC Pediatr. 2014;14:199.
- , , , et al. Using hospital designation to identify unplanned pediatric readmissions [abstract]. J Hosp Med. Available at: http://www.shmabstracts.com/abstract/using‐hospital‐designation‐to‐identify‐unplanned‐pediatric‐readmissions. Accessed July 15, 2015.
- , , , et al. Rates and impact of potentially preventable readmissions at children's hospitals. J Pediatr. 2015;166(3):613–619.e615.
- , , , , . House staff team workload and organization effects on patient outcomes in an academic general internal medicine inpatient service. Arch Intern Med. 2007;167(1):47–52.
- , . A "reverse july effect": association between timing of admission, medical team workload, and 30‐day readmission rate. J Grad Med Educ. 2014;6(1):65–70.
- , , , , . An observational study of nurse staffing ratios and hospital readmission among children admitted for common conditions. BMJ Qual Saf. 2013;22(9):735–742.
- , , , , . Day of discharge and hospital readmission rates within 30 days in children: a population‐based study. Paediatr Child Health. 2006;11(7):409–412.
- , , , , . Differences between early and late readmissions among patients: a cohort study. Ann Intern Med. 2015;162(11):741–749.
Patient outcomes tend to be worse for adults admitted on the weekend compared to the weekday.[1, 2, 3, 4] In pediatric populations, urgent surgeries on weekends are associated with increased morbidity and mortality[5]; however, studies of mortality and admission timing in the pediatric critical care setting are mixed.[6, 7] Hospital readmission is considered a potential marker of hospital quality. We hypothesized that (1) being admitted and (2) being discharged on the weekend would adversely affect 30‐day unplanned readmission for pediatric patients.
METHODS
Population
All discharges from January 1, 2006 through December 31, 2012 from C. S. Mott Children's Hospital were initially eligible. All hospitalizations were considered potential index admissions; therefore, children may contribute more than 1 hospitalization to the dataset. We excluded hospitalizations in which the patient died, was transferred to another institution, was discharged against medical advice, or was discharged to hospice. Newborns admitted to a normal newborn service were also excluded, as they do not represent a typical hospitalization for illness. Among newborns admitted to a higher‐intensity clinical service (eg, special care nursery or neonatal intensive care), we also excluded newborns with a length of stay 5 days, given the typical length of stay of up to 4 days for uncomplicated delivery via Cesarean section that would indicate infants for whom precautionary measures had been taken but there was low estimated health risk. We used International Classification of Diseases, Ninth Revision codes to identify children with complex chronic conditions (CCCs) and technology dependency.[8]
Outcome
We examined unplanned readmission within 30 days of discharge. We defined unplanned readmission as a readmission that was not entered into the hospital registration system at least 24 hours before discharge.[9] Additionally, we performed sensitive analyses examining any 30‐day readmissions.
Statistical Analysis
We fit multivariable logistic regression models for 30‐day unplanned readmission, with the primary predictor of either weekend (Saturday or Sunday) admission or weekend discharge (in separate models). We adjusted for patient age, gender, race/ethnicity, source of admission, insurance, and length of stay. We also adjusted for patient chronic illness complexity using the number of CCCs and technology dependency (yes/no). Variance in all analyses was clustered on individual patients.
RESULTS
We included a total of 55,383 hospitalizations from 32,112 patients (see Supporting Appendix Figure in the online version of this article for cohort derivation). All‐cause 30‐day readmissions occurred in 14.9% of hospital discharges; the 30‐day unplanned readmission rate was 10.3% (see the Supporting Appendix Table in the online version of this article for demographic characteristics).
Weekend Admission
Overall, 82% of admissions occurred during the week, with Tuesday as the highest admitting volume day (Figure 1). Children admitted on the weekend had higher odds of unplanned readmission compared to children admitted on weekdays (unadjusted odds ratio [OR]=1.15 [95% confidence interval {CI}: 1.07‐1.24]). Adjusting the analysis for age, gender, race/ethnicity, insurance, length of stay, CCCs, and technology dependency, higher odds of readmission remains significantly higher than weekday admission (adjusted OR=1.09 [95% CI: 1.004‐1.18]) (Table 1). Age, admission source, payer, length of stay, number of complex chronic conditions, and technology dependency were also significantly associated with readmission in the weekend admission model (see the Supporting Appendix Table in the online version of this article). A sensitivity analysis examining the association of weekend admission and readmission within different subpopulations of children with varying numbers of CCCs (ie, among children without CCCs, with 1 CCC, 2 CCCs, and 3+ CCCs) showed that the association remains the same in each subgroup. Further, a sensitivity analysis examining odds of any 30‐day readmission was similar to the primary analysis with higher odds of readmission in adjusted analysis (adjusted OR=1.09 [95% CI: 1.02‐1.18]).

| 30‐Day Unplanned Readmission Rate | Unadjusted Odds of Unplanned Readmission (95% CI) | Weekend Admission Model: Adjusted Odds of Unplanned Readmission (95% CI) | Weekend Discharge Model: Adjusted Odds of Unplanned Readmission (95% CI) | |
|---|---|---|---|---|
| ||||
| Weekend admission, n=7,533 | 11.4%, n=973 | 1.15 (1.07‐1.24)* | 1.09 (1.004‐1.18)* | |
| Weekend discharge, n=13,911 | 9.7%, n=1,344 | 0.91 (0.85‐0.97)* | 0.97 (0.91‐1.04) | |
Weekend Discharge
Weekend discharges accounted for 34% of all discharges. Fridays had the highest discharge volumes, with lowest discharge volumes on Sunday (Figure 1). Children discharged on the weekend had lower odds of unplanned readmission compared to children discharged on weekdays in bivariate analysis (unadjusted OR=0.91 [95% CI: 0.85‐0.97]). However, when adjusting for important confounders, the relationship was no longer statistically significant (adjusted OR=0.97 [95% CI: 0.91‐1.03]) (Table 1). Age, admission source, payer, length of stay, and number of complex chronic conditions were associated with readmission in the weekend discharge model (see the Supporting Appendix Table in the online version of this article). In a sensitivity analysis examining any 30‐day readmission, weekend discharge was not associated with readmission in adjusted analysis.
DISCUSSION
Although the so‐called weekend effect has been established in adults,[1, 2, 3, 4] evidence is mixed for children. In this sample, where the 30‐day pediatric readmission rate is consistent with national pediatric rates,[10] pediatric patients admitted on the weekend have higher odds of readmission compared to children admitted during the week, even when accounting for patient characteristics and hospital length of stay. In contrast, weekend discharge was not associated with readmission.
The association of weekend admission and subsequent readmission is intriguing and may be interpreted in 1 of 2 ways: either patients admitted on the weekend are fundamentally different and thus have higher readmission rates, or care on the weekend is different. It is important to note that we adjusted the analysis for patient characteristics including number of CCCs and technology dependency to account for differences in chronic illness. We also accounted for length of stay as a marker of severity of illness in the hospital. Yet even accounting for these known differences, we cannot discern from these data if the different outcomes for children admitted on the weekend are related to residual population differences (eg, lack of access to primary care or walk‐in clinics) or differences in initial clinical management on the weekend.
Initial clinical management on weekend may be different due to differences in physician, nursing, and other ancillary staffing affecting availability of diagnostic and therapeutic interventions. Additionally, smaller staff size on the weekend may lead to increased workload. Although we are unable to directly measure resident workload in our study, prior studies suggest higher workload is associated with worse outcomes for adult patients,[11] including readmission.[12] Additionally, nurse staffing, which may vary based on day of week, has been associated with pediatric readmission.[13]
Discharge timing in our population is consistent with prior literature, with Friday being the most common discharge day of week.[14] Prior literature has shown no difference in readmission rates between Friday discharge and midweek discharge for pediatric patients.[14] Our work builds on this existing literature, demonstrating no association with weekend discharge and readmission. There were lower discharge volumes on the weekends, particularly in patients with more CCCs, suggesting that physicians avoid complicated discharges on Saturday and Sunday.
This study should be interpreted in the context of several limitations. First, this study was conducted at a single tertiary care pediatric institution. Our patient population had a high rate of children with CCCs, potentially limiting generalizability to other pediatric institutions. Ideally, we would adjust our model for clusters at the clinical service or attending physician level; however, the heterogeneity of our services and data limits prohibited these analyses. Readmissions that may have occurred at other institutions are not observable in this dataset; however, there is no reason to believe patients admitted or discharged on the weekend would have different rates of other hospital readmissions than patients admitted or discharged on weekdays. Additionally, early readmissions may be particularly affected by in‐hospital and discharge factors.[15] However, the very low rate of early readmission prohibited limiting the analyses to early readmission. Finally, we relied on administrative data to adjust for patient severity using typical methods such as CCCs; however, other patient differences may have existed beyond those that could be captured with administrative data.
CONCLUSION
Children admitted to the hospital on the weekend have higher rates of 30‐day unplanned readmission than children admitted during the week, suggesting differences of care in initial management on the weekend. Understanding this difference from the perspectives of multiple stakeholders may illuminate potential reasons for this disparity.
Disclosures
Dr. Auger received salary support from the Robert Wood Johnson Foundation Clinical Scholars program during work on this project. The hospital database was assembled with funds from a grant from the Blue Cross Blue Shield of Michigan Foundation. The authors report no conflicts of interest.
Patient outcomes tend to be worse for adults admitted on the weekend compared to the weekday.[1, 2, 3, 4] In pediatric populations, urgent surgeries on weekends are associated with increased morbidity and mortality[5]; however, studies of mortality and admission timing in the pediatric critical care setting are mixed.[6, 7] Hospital readmission is considered a potential marker of hospital quality. We hypothesized that (1) being admitted and (2) being discharged on the weekend would adversely affect 30‐day unplanned readmission for pediatric patients.
METHODS
Population
All discharges from January 1, 2006 through December 31, 2012 from C. S. Mott Children's Hospital were initially eligible. All hospitalizations were considered potential index admissions; therefore, children may contribute more than 1 hospitalization to the dataset. We excluded hospitalizations in which the patient died, was transferred to another institution, was discharged against medical advice, or was discharged to hospice. Newborns admitted to a normal newborn service were also excluded, as they do not represent a typical hospitalization for illness. Among newborns admitted to a higher‐intensity clinical service (eg, special care nursery or neonatal intensive care), we also excluded newborns with a length of stay 5 days, given the typical length of stay of up to 4 days for uncomplicated delivery via Cesarean section that would indicate infants for whom precautionary measures had been taken but there was low estimated health risk. We used International Classification of Diseases, Ninth Revision codes to identify children with complex chronic conditions (CCCs) and technology dependency.[8]
Outcome
We examined unplanned readmission within 30 days of discharge. We defined unplanned readmission as a readmission that was not entered into the hospital registration system at least 24 hours before discharge.[9] Additionally, we performed sensitive analyses examining any 30‐day readmissions.
Statistical Analysis
We fit multivariable logistic regression models for 30‐day unplanned readmission, with the primary predictor of either weekend (Saturday or Sunday) admission or weekend discharge (in separate models). We adjusted for patient age, gender, race/ethnicity, source of admission, insurance, and length of stay. We also adjusted for patient chronic illness complexity using the number of CCCs and technology dependency (yes/no). Variance in all analyses was clustered on individual patients.
RESULTS
We included a total of 55,383 hospitalizations from 32,112 patients (see Supporting Appendix Figure in the online version of this article for cohort derivation). All‐cause 30‐day readmissions occurred in 14.9% of hospital discharges; the 30‐day unplanned readmission rate was 10.3% (see the Supporting Appendix Table in the online version of this article for demographic characteristics).
Weekend Admission
Overall, 82% of admissions occurred during the week, with Tuesday as the highest admitting volume day (Figure 1). Children admitted on the weekend had higher odds of unplanned readmission compared to children admitted on weekdays (unadjusted odds ratio [OR]=1.15 [95% confidence interval {CI}: 1.07‐1.24]). Adjusting the analysis for age, gender, race/ethnicity, insurance, length of stay, CCCs, and technology dependency, higher odds of readmission remains significantly higher than weekday admission (adjusted OR=1.09 [95% CI: 1.004‐1.18]) (Table 1). Age, admission source, payer, length of stay, number of complex chronic conditions, and technology dependency were also significantly associated with readmission in the weekend admission model (see the Supporting Appendix Table in the online version of this article). A sensitivity analysis examining the association of weekend admission and readmission within different subpopulations of children with varying numbers of CCCs (ie, among children without CCCs, with 1 CCC, 2 CCCs, and 3+ CCCs) showed that the association remains the same in each subgroup. Further, a sensitivity analysis examining odds of any 30‐day readmission was similar to the primary analysis with higher odds of readmission in adjusted analysis (adjusted OR=1.09 [95% CI: 1.02‐1.18]).

| 30‐Day Unplanned Readmission Rate | Unadjusted Odds of Unplanned Readmission (95% CI) | Weekend Admission Model: Adjusted Odds of Unplanned Readmission (95% CI) | Weekend Discharge Model: Adjusted Odds of Unplanned Readmission (95% CI) | |
|---|---|---|---|---|
| ||||
| Weekend admission, n=7,533 | 11.4%, n=973 | 1.15 (1.07‐1.24)* | 1.09 (1.004‐1.18)* | |
| Weekend discharge, n=13,911 | 9.7%, n=1,344 | 0.91 (0.85‐0.97)* | 0.97 (0.91‐1.04) | |
Weekend Discharge
Weekend discharges accounted for 34% of all discharges. Fridays had the highest discharge volumes, with lowest discharge volumes on Sunday (Figure 1). Children discharged on the weekend had lower odds of unplanned readmission compared to children discharged on weekdays in bivariate analysis (unadjusted OR=0.91 [95% CI: 0.85‐0.97]). However, when adjusting for important confounders, the relationship was no longer statistically significant (adjusted OR=0.97 [95% CI: 0.91‐1.03]) (Table 1). Age, admission source, payer, length of stay, and number of complex chronic conditions were associated with readmission in the weekend discharge model (see the Supporting Appendix Table in the online version of this article). In a sensitivity analysis examining any 30‐day readmission, weekend discharge was not associated with readmission in adjusted analysis.
DISCUSSION
Although the so‐called weekend effect has been established in adults,[1, 2, 3, 4] evidence is mixed for children. In this sample, where the 30‐day pediatric readmission rate is consistent with national pediatric rates,[10] pediatric patients admitted on the weekend have higher odds of readmission compared to children admitted during the week, even when accounting for patient characteristics and hospital length of stay. In contrast, weekend discharge was not associated with readmission.
The association of weekend admission and subsequent readmission is intriguing and may be interpreted in 1 of 2 ways: either patients admitted on the weekend are fundamentally different and thus have higher readmission rates, or care on the weekend is different. It is important to note that we adjusted the analysis for patient characteristics including number of CCCs and technology dependency to account for differences in chronic illness. We also accounted for length of stay as a marker of severity of illness in the hospital. Yet even accounting for these known differences, we cannot discern from these data if the different outcomes for children admitted on the weekend are related to residual population differences (eg, lack of access to primary care or walk‐in clinics) or differences in initial clinical management on the weekend.
Initial clinical management on weekend may be different due to differences in physician, nursing, and other ancillary staffing affecting availability of diagnostic and therapeutic interventions. Additionally, smaller staff size on the weekend may lead to increased workload. Although we are unable to directly measure resident workload in our study, prior studies suggest higher workload is associated with worse outcomes for adult patients,[11] including readmission.[12] Additionally, nurse staffing, which may vary based on day of week, has been associated with pediatric readmission.[13]
Discharge timing in our population is consistent with prior literature, with Friday being the most common discharge day of week.[14] Prior literature has shown no difference in readmission rates between Friday discharge and midweek discharge for pediatric patients.[14] Our work builds on this existing literature, demonstrating no association with weekend discharge and readmission. There were lower discharge volumes on the weekends, particularly in patients with more CCCs, suggesting that physicians avoid complicated discharges on Saturday and Sunday.
This study should be interpreted in the context of several limitations. First, this study was conducted at a single tertiary care pediatric institution. Our patient population had a high rate of children with CCCs, potentially limiting generalizability to other pediatric institutions. Ideally, we would adjust our model for clusters at the clinical service or attending physician level; however, the heterogeneity of our services and data limits prohibited these analyses. Readmissions that may have occurred at other institutions are not observable in this dataset; however, there is no reason to believe patients admitted or discharged on the weekend would have different rates of other hospital readmissions than patients admitted or discharged on weekdays. Additionally, early readmissions may be particularly affected by in‐hospital and discharge factors.[15] However, the very low rate of early readmission prohibited limiting the analyses to early readmission. Finally, we relied on administrative data to adjust for patient severity using typical methods such as CCCs; however, other patient differences may have existed beyond those that could be captured with administrative data.
CONCLUSION
Children admitted to the hospital on the weekend have higher rates of 30‐day unplanned readmission than children admitted during the week, suggesting differences of care in initial management on the weekend. Understanding this difference from the perspectives of multiple stakeholders may illuminate potential reasons for this disparity.
Disclosures
Dr. Auger received salary support from the Robert Wood Johnson Foundation Clinical Scholars program during work on this project. The hospital database was assembled with funds from a grant from the Blue Cross Blue Shield of Michigan Foundation. The authors report no conflicts of interest.
- , , , . A comparison of in‐hospital mortality risk conferred by high hospital occupancy, differences in nurse staffing levels, weekend admission, and seasonal influenza. Med Care. 2010;48(3):224–232.
- , . Mortality among patients admitted to hospitals on weekends as compared with weekdays. N Engl J Med. 2001;345(9):663–668.
- , , , . Effects of weekend admission and hospital teaching status on in‐hospital mortality. Am J Med. 2004;117(3):151–157.
- , , . Weekend admission for myocardial infarction. N Engl J Med. 2007;357(1):86–87; author reply 87–88.
- , , , et al. The "weekend effect" in pediatric surgery—increased mortality for children undergoing urgent surgery during the weekend. J Pediatr Surg. 2014;49(7):1087–1091.
- , , , , , Paediatric Intensive Care Audit Network (PICANet). Effects of out‐of‐hours and winter admissions and number of patients per unit on mortality in pediatric intensive care. J Pediatr. 2013;163(4):1039–1044.e1035.
- , , , . Do weekends or evenings matter in a pediatric intensive care unit? Pediatr Crit Care Med. 2005;6(5):523–530.
- , , , , . Pediatric complex chronic conditions classification system version 2: updated for ICD‐10 and complex medical technology dependence and transplantation. BMC Pediatr. 2014;14:199.
- , , , et al. Using hospital designation to identify unplanned pediatric readmissions [abstract]. J Hosp Med. Available at: http://www.shmabstracts.com/abstract/using‐hospital‐designation‐to‐identify‐unplanned‐pediatric‐readmissions. Accessed July 15, 2015.
- , , , et al. Rates and impact of potentially preventable readmissions at children's hospitals. J Pediatr. 2015;166(3):613–619.e615.
- , , , , . House staff team workload and organization effects on patient outcomes in an academic general internal medicine inpatient service. Arch Intern Med. 2007;167(1):47–52.
- , . A "reverse july effect": association between timing of admission, medical team workload, and 30‐day readmission rate. J Grad Med Educ. 2014;6(1):65–70.
- , , , , . An observational study of nurse staffing ratios and hospital readmission among children admitted for common conditions. BMJ Qual Saf. 2013;22(9):735–742.
- , , , , . Day of discharge and hospital readmission rates within 30 days in children: a population‐based study. Paediatr Child Health. 2006;11(7):409–412.
- , , , , . Differences between early and late readmissions among patients: a cohort study. Ann Intern Med. 2015;162(11):741–749.
- , , , . A comparison of in‐hospital mortality risk conferred by high hospital occupancy, differences in nurse staffing levels, weekend admission, and seasonal influenza. Med Care. 2010;48(3):224–232.
- , . Mortality among patients admitted to hospitals on weekends as compared with weekdays. N Engl J Med. 2001;345(9):663–668.
- , , , . Effects of weekend admission and hospital teaching status on in‐hospital mortality. Am J Med. 2004;117(3):151–157.
- , , . Weekend admission for myocardial infarction. N Engl J Med. 2007;357(1):86–87; author reply 87–88.
- , , , et al. The "weekend effect" in pediatric surgery—increased mortality for children undergoing urgent surgery during the weekend. J Pediatr Surg. 2014;49(7):1087–1091.
- , , , , , Paediatric Intensive Care Audit Network (PICANet). Effects of out‐of‐hours and winter admissions and number of patients per unit on mortality in pediatric intensive care. J Pediatr. 2013;163(4):1039–1044.e1035.
- , , , . Do weekends or evenings matter in a pediatric intensive care unit? Pediatr Crit Care Med. 2005;6(5):523–530.
- , , , , . Pediatric complex chronic conditions classification system version 2: updated for ICD‐10 and complex medical technology dependence and transplantation. BMC Pediatr. 2014;14:199.
- , , , et al. Using hospital designation to identify unplanned pediatric readmissions [abstract]. J Hosp Med. Available at: http://www.shmabstracts.com/abstract/using‐hospital‐designation‐to‐identify‐unplanned‐pediatric‐readmissions. Accessed July 15, 2015.
- , , , et al. Rates and impact of potentially preventable readmissions at children's hospitals. J Pediatr. 2015;166(3):613–619.e615.
- , , , , . House staff team workload and organization effects on patient outcomes in an academic general internal medicine inpatient service. Arch Intern Med. 2007;167(1):47–52.
- , . A "reverse july effect": association between timing of admission, medical team workload, and 30‐day readmission rate. J Grad Med Educ. 2014;6(1):65–70.
- , , , , . An observational study of nurse staffing ratios and hospital readmission among children admitted for common conditions. BMJ Qual Saf. 2013;22(9):735–742.
- , , , , . Day of discharge and hospital readmission rates within 30 days in children: a population‐based study. Paediatr Child Health. 2006;11(7):409–412.
- , , , , . Differences between early and late readmissions among patients: a cohort study. Ann Intern Med. 2015;162(11):741–749.