User login
US Behind the World in Telemedicine
NEW YORK - The American College of Physicians (ACP) has issued a baker's dozen of recommendations intended to guide the effective use of telemedicine in primary care settings.
"The recommendations balance the potential benefits and expanded use of telemedicine with the importance of maintaining the patient-physician relationship and patient safety," Hilary Daniel from American College of Physicians, Washington, DC, said by email.
Telemedicine, the use of technology to deliver health care services at a distance, began mainly in rural communities and federal health programs, but now is used in a variety of medical specialties and subspecialties.
Daniel and colleagues on the ACP Health and Public Policy Committee detail pragmatic recommendations on the use of telemedicine in the primary care setting, physician considerations for those who use telemedicine in their practices, and policy recommendations on the practice and reimbursement of telemedicine in their September 8 Annals of Internal Medicine online position paper.
While ACP "supports the expanded role of telemedicine as a method of health care delivery that may enhance patient-physician collaborations," it also recommends that direct-to-patient telemedicine services should be used only as an intermittent alternative to a patient's primary care physician when necessary to meet the patient's immediate care
needs.
These services should take place within the context of a valid patient-physician relationship. Physicians who use telemedicine have an obligation to establish such a relationship based on the standard of care required for an in-person visit or consult with another physician who has such a relationship with the patient.
Physicians should take care to see that financially disadvantaged populations also have access to telemedicine services, where appropriate, as well as ensure that these services comply with federal and state security and privacy regulations while meeting the same standards of practice as if the physician were seeing the patient in person.
ACP supports efforts to reimburse telemedicine communications and telehealth services and supports processes to obtain medical licenses and hospital privileges necessary to support telemedicine across state lines.
The committee also endorses the use of federal funds to support the broadband infrastructure and to establish an evidence base on the safety, efficacy, and cost of telemedicine technologies.
"Telemedicine can be an effective and beneficial tool for physicians and patients to enhance a patient's care," Daniel concluded. "Physicians can take away from this report a greater understanding of the regulatory and payment issues surrounding telemedicine and evaluate how telemedicine may be useful for their patients and practice and augment the care they already provide."
In a related editorial, Dr. David A. Asch, from the University of Pennsylvania's Center for Health Care Innovation, Philadelphia, addressed the hidden economics of telemedicine. He said by email, "I think it is a trap to think that the only promise of telemedicine is the opportunity to do something remotely that used to happen face to face. The real opportunities will come from doing health care in a different way because of this remote technology."
"An important question is, 'If there are so many opportunities from telemedicine, why doesn't more of it happen?'" Dr. Asch said. "I think there are a lot of reasons for that but one important reason is that insurers, in particular, worry that if they make telemedicine payments easy, then they will open up the floodgates of demand."
Dr. Laura Markwick, from Wegmans School of Nursing, St. John Fisher College, Rochester, New York, said by email, "I would like to apply these to all health care providers and not just physicians. Of note, they do mention that they recommend a relationship already be established with the patient, which I do not feel is an absolute must. When we did our telemedicine program, we did not have a previous in-person relationship established and we were able to provide high-quality care that improved access for the patients."
"All health care providers should be reimbursed for telemedicine care, at levels similar to what is reimbursed currently," Dr. Markwick said. "This should be a covered service from the person's insurance, depending upon the care needed. If it is just someone who does not feel like making it to an appointment but otherwise could with little difficulty (social issues included in this decision), then perhaps the patient should contribute toward this reimbursement."
Dr. Manish N. Shah, from the University of Wisconsin School of Medicine and Public Health, Madison, said by email, "It is important to know that the U.S. is behind the rest of the world when it comes to telemedicine. In Canada, the Ontario Telemedicine Network has existed for years. In the UK, telemedicine has been also available."
"Reimbursement for telemedicine is a complex issue, particularly because the entire provider payment system is changing," Dr. Shah said. "However, without funding, telemedicine will not be made available to patients despite increasing evidence showing that patients want to use it, that care can be effectively delivered via telemedicine, and that telemedicine is cost effective."
The authors reported no external funding or disclosures.
NEW YORK - The American College of Physicians (ACP) has issued a baker's dozen of recommendations intended to guide the effective use of telemedicine in primary care settings.
"The recommendations balance the potential benefits and expanded use of telemedicine with the importance of maintaining the patient-physician relationship and patient safety," Hilary Daniel from American College of Physicians, Washington, DC, said by email.
Telemedicine, the use of technology to deliver health care services at a distance, began mainly in rural communities and federal health programs, but now is used in a variety of medical specialties and subspecialties.
Daniel and colleagues on the ACP Health and Public Policy Committee detail pragmatic recommendations on the use of telemedicine in the primary care setting, physician considerations for those who use telemedicine in their practices, and policy recommendations on the practice and reimbursement of telemedicine in their September 8 Annals of Internal Medicine online position paper.
While ACP "supports the expanded role of telemedicine as a method of health care delivery that may enhance patient-physician collaborations," it also recommends that direct-to-patient telemedicine services should be used only as an intermittent alternative to a patient's primary care physician when necessary to meet the patient's immediate care
needs.
These services should take place within the context of a valid patient-physician relationship. Physicians who use telemedicine have an obligation to establish such a relationship based on the standard of care required for an in-person visit or consult with another physician who has such a relationship with the patient.
Physicians should take care to see that financially disadvantaged populations also have access to telemedicine services, where appropriate, as well as ensure that these services comply with federal and state security and privacy regulations while meeting the same standards of practice as if the physician were seeing the patient in person.
ACP supports efforts to reimburse telemedicine communications and telehealth services and supports processes to obtain medical licenses and hospital privileges necessary to support telemedicine across state lines.
The committee also endorses the use of federal funds to support the broadband infrastructure and to establish an evidence base on the safety, efficacy, and cost of telemedicine technologies.
"Telemedicine can be an effective and beneficial tool for physicians and patients to enhance a patient's care," Daniel concluded. "Physicians can take away from this report a greater understanding of the regulatory and payment issues surrounding telemedicine and evaluate how telemedicine may be useful for their patients and practice and augment the care they already provide."
In a related editorial, Dr. David A. Asch, from the University of Pennsylvania's Center for Health Care Innovation, Philadelphia, addressed the hidden economics of telemedicine. He said by email, "I think it is a trap to think that the only promise of telemedicine is the opportunity to do something remotely that used to happen face to face. The real opportunities will come from doing health care in a different way because of this remote technology."
"An important question is, 'If there are so many opportunities from telemedicine, why doesn't more of it happen?'" Dr. Asch said. "I think there are a lot of reasons for that but one important reason is that insurers, in particular, worry that if they make telemedicine payments easy, then they will open up the floodgates of demand."
Dr. Laura Markwick, from Wegmans School of Nursing, St. John Fisher College, Rochester, New York, said by email, "I would like to apply these to all health care providers and not just physicians. Of note, they do mention that they recommend a relationship already be established with the patient, which I do not feel is an absolute must. When we did our telemedicine program, we did not have a previous in-person relationship established and we were able to provide high-quality care that improved access for the patients."
"All health care providers should be reimbursed for telemedicine care, at levels similar to what is reimbursed currently," Dr. Markwick said. "This should be a covered service from the person's insurance, depending upon the care needed. If it is just someone who does not feel like making it to an appointment but otherwise could with little difficulty (social issues included in this decision), then perhaps the patient should contribute toward this reimbursement."
Dr. Manish N. Shah, from the University of Wisconsin School of Medicine and Public Health, Madison, said by email, "It is important to know that the U.S. is behind the rest of the world when it comes to telemedicine. In Canada, the Ontario Telemedicine Network has existed for years. In the UK, telemedicine has been also available."
"Reimbursement for telemedicine is a complex issue, particularly because the entire provider payment system is changing," Dr. Shah said. "However, without funding, telemedicine will not be made available to patients despite increasing evidence showing that patients want to use it, that care can be effectively delivered via telemedicine, and that telemedicine is cost effective."
The authors reported no external funding or disclosures.
NEW YORK - The American College of Physicians (ACP) has issued a baker's dozen of recommendations intended to guide the effective use of telemedicine in primary care settings.
"The recommendations balance the potential benefits and expanded use of telemedicine with the importance of maintaining the patient-physician relationship and patient safety," Hilary Daniel from American College of Physicians, Washington, DC, said by email.
Telemedicine, the use of technology to deliver health care services at a distance, began mainly in rural communities and federal health programs, but now is used in a variety of medical specialties and subspecialties.
Daniel and colleagues on the ACP Health and Public Policy Committee detail pragmatic recommendations on the use of telemedicine in the primary care setting, physician considerations for those who use telemedicine in their practices, and policy recommendations on the practice and reimbursement of telemedicine in their September 8 Annals of Internal Medicine online position paper.
While ACP "supports the expanded role of telemedicine as a method of health care delivery that may enhance patient-physician collaborations," it also recommends that direct-to-patient telemedicine services should be used only as an intermittent alternative to a patient's primary care physician when necessary to meet the patient's immediate care
needs.
These services should take place within the context of a valid patient-physician relationship. Physicians who use telemedicine have an obligation to establish such a relationship based on the standard of care required for an in-person visit or consult with another physician who has such a relationship with the patient.
Physicians should take care to see that financially disadvantaged populations also have access to telemedicine services, where appropriate, as well as ensure that these services comply with federal and state security and privacy regulations while meeting the same standards of practice as if the physician were seeing the patient in person.
ACP supports efforts to reimburse telemedicine communications and telehealth services and supports processes to obtain medical licenses and hospital privileges necessary to support telemedicine across state lines.
The committee also endorses the use of federal funds to support the broadband infrastructure and to establish an evidence base on the safety, efficacy, and cost of telemedicine technologies.
"Telemedicine can be an effective and beneficial tool for physicians and patients to enhance a patient's care," Daniel concluded. "Physicians can take away from this report a greater understanding of the regulatory and payment issues surrounding telemedicine and evaluate how telemedicine may be useful for their patients and practice and augment the care they already provide."
In a related editorial, Dr. David A. Asch, from the University of Pennsylvania's Center for Health Care Innovation, Philadelphia, addressed the hidden economics of telemedicine. He said by email, "I think it is a trap to think that the only promise of telemedicine is the opportunity to do something remotely that used to happen face to face. The real opportunities will come from doing health care in a different way because of this remote technology."
"An important question is, 'If there are so many opportunities from telemedicine, why doesn't more of it happen?'" Dr. Asch said. "I think there are a lot of reasons for that but one important reason is that insurers, in particular, worry that if they make telemedicine payments easy, then they will open up the floodgates of demand."
Dr. Laura Markwick, from Wegmans School of Nursing, St. John Fisher College, Rochester, New York, said by email, "I would like to apply these to all health care providers and not just physicians. Of note, they do mention that they recommend a relationship already be established with the patient, which I do not feel is an absolute must. When we did our telemedicine program, we did not have a previous in-person relationship established and we were able to provide high-quality care that improved access for the patients."
"All health care providers should be reimbursed for telemedicine care, at levels similar to what is reimbursed currently," Dr. Markwick said. "This should be a covered service from the person's insurance, depending upon the care needed. If it is just someone who does not feel like making it to an appointment but otherwise could with little difficulty (social issues included in this decision), then perhaps the patient should contribute toward this reimbursement."
Dr. Manish N. Shah, from the University of Wisconsin School of Medicine and Public Health, Madison, said by email, "It is important to know that the U.S. is behind the rest of the world when it comes to telemedicine. In Canada, the Ontario Telemedicine Network has existed for years. In the UK, telemedicine has been also available."
"Reimbursement for telemedicine is a complex issue, particularly because the entire provider payment system is changing," Dr. Shah said. "However, without funding, telemedicine will not be made available to patients despite increasing evidence showing that patients want to use it, that care can be effectively delivered via telemedicine, and that telemedicine is cost effective."
The authors reported no external funding or disclosures.
Microbiome implicated in sickle cell disease

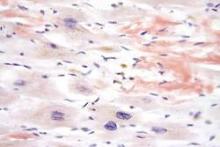
Image by Volker Brinkmann
Preclinical research suggests that usingantibiotics to deplete the body’s microbiome may prevent acute sickle cell crisis in patients with sickle cell disease (SCD) and could potentially offer the first effective strategy for warding off the disease’s long-term complications, such as organ failure.
The work, published in Nature, may also have implications for other inflammatory blood-vessel disorders, including septic shock.
The study was led by Paul Frenette, MD, of Albert Einstein College of Medicine in Bronx, New York. In 2002, Dr Frenette and his colleagues reported that SCD vessel blockages occur when sickled red cells bind to neutrophils that have adhered to the vessel walls.
“This earlier work indicated that not all neutrophils are the same,” Dr Frenette said. “Some appear to be inert, while others appear overly active in promoting inflammation, which is useful for attacking microbes but causes neutrophils to capture sickled red cells inside vessels.”
“So in the current study, we investigated whether the age of the neutrophils might be influencing whether they become active and pro-inflammatory.”
Neutrophils and SCD
The researchers began by transfusing whole blood into mice and then analyzing young neutrophils (harvested 10 minutes post-transfusion) and aged neutrophils (harvested 6 hours post-transfusion). The neutrophils became more active as they aged, suggesting that neutrophils receive external signals telling them to age.
The investigators were able to trace these “aging” signals to the body’s microbiome. They found the microbiome produces chemicals that cross the intestinal barrier and enter the bloodstream, where they generate the aged, overly active subset of neutrophils that contribute to SCD.
“Since the body’s microbiota seem to ‘educate’ neutrophils to age, we realized that purging those microbes through use of antibiotics might help against SCD,” Dr Frenette said.
To find out, he and his colleagues conducted experiments in a mouse model of SCD. These mice possessed 5 times as many aged neutrophils as healthy control mice.
When the researchers depleted the microbiota of SCD mice using antibiotics, they observed a striking reduction in neutrophils but not in other white blood cells (such as monocytes, T cells, and B cells).
Moreover, giving antibiotics to SCD mice appeared to prevent sickle cell crisis. Interactions between neutrophils and red cells were markedly reduced in microbiota-depleted SCD mice, resulting in improved local blood flow and greatly improved survival in the mice.
“What was most surprising and exciting to us was the effect of antibiotics on chronic tissue damage,” Dr Frenette said.
“We found that the spleen enlargement of SCD mice was significantly reduced in the microbiota-depleted animals, and liver analysis revealed major reductions in liver damage, including inflammation, scarring, and tissue death. This is the first time that anything has been found to have an impact on the organ damage that can be so devastating in SCD.”
Septic shock
The investigators then studied septic shock, another serious blood disorder in which activated, pro-inflammatory neutrophils play a role.
To induce septic shock, the team gave control and microbiota-depleted mice a dose of a bacterial toxin that would normally be lethal.
The control mice had the neutrophil aggregates and clumping of neutrophil DNA that contribute to death from septic shock, but the microbiota-depleted mice were largely free of neutrophil complications and survived.
“Remarkably, we could prevent microbiota-depleted mice from surviving septic shock if we infused them with aged neutrophils but not if we infused the same number of young neutrophils,” Dr Frenette said. “So depleting the microbiota may help against inflammatory blood diseases in addition to SCD.”
SCD patient samples
Finally, the researchers investigated whether their findings in mice might be relevant to humans with SCD.
The team obtained blood samples from 9 healthy children and 34 patients with SCD. Eleven patients were taking penicillin daily to ward off infections, as is recommended for children with SCD who are 5 years of age or younger. The other 23 patients with SCD had been off penicillin for at least 2 months.
Consistent with the findings in mice, children with SCD who were not taking penicillin had many more circulating aged neutrophils than the healthy children.
The investigators then compared neutrophil levels in the 2 groups of children with SCD—those taking penicillin and those not on the drug—and found a much lower number of aged neutrophils in the blood of those who were taking penicillin.
“Daily penicillin for patients with SCD younger than 5 works really well in preventing infections,” Dr Frenette said. “Our study suggests that penicillin and other antibiotics could play an even broader role in potentially benefiting older patients.”
“[W]e hope to carry out a clinical trial to determine whether antibiotics can help patients with SCD by preventing the sickle cell crisis and long-term organ damage associated with the disease.” ![]()

Image by Volker Brinkmann
Preclinical research suggests that usingantibiotics to deplete the body’s microbiome may prevent acute sickle cell crisis in patients with sickle cell disease (SCD) and could potentially offer the first effective strategy for warding off the disease’s long-term complications, such as organ failure.
The work, published in Nature, may also have implications for other inflammatory blood-vessel disorders, including septic shock.
The study was led by Paul Frenette, MD, of Albert Einstein College of Medicine in Bronx, New York. In 2002, Dr Frenette and his colleagues reported that SCD vessel blockages occur when sickled red cells bind to neutrophils that have adhered to the vessel walls.
“This earlier work indicated that not all neutrophils are the same,” Dr Frenette said. “Some appear to be inert, while others appear overly active in promoting inflammation, which is useful for attacking microbes but causes neutrophils to capture sickled red cells inside vessels.”
“So in the current study, we investigated whether the age of the neutrophils might be influencing whether they become active and pro-inflammatory.”
Neutrophils and SCD
The researchers began by transfusing whole blood into mice and then analyzing young neutrophils (harvested 10 minutes post-transfusion) and aged neutrophils (harvested 6 hours post-transfusion). The neutrophils became more active as they aged, suggesting that neutrophils receive external signals telling them to age.
The investigators were able to trace these “aging” signals to the body’s microbiome. They found the microbiome produces chemicals that cross the intestinal barrier and enter the bloodstream, where they generate the aged, overly active subset of neutrophils that contribute to SCD.
“Since the body’s microbiota seem to ‘educate’ neutrophils to age, we realized that purging those microbes through use of antibiotics might help against SCD,” Dr Frenette said.
To find out, he and his colleagues conducted experiments in a mouse model of SCD. These mice possessed 5 times as many aged neutrophils as healthy control mice.
When the researchers depleted the microbiota of SCD mice using antibiotics, they observed a striking reduction in neutrophils but not in other white blood cells (such as monocytes, T cells, and B cells).
Moreover, giving antibiotics to SCD mice appeared to prevent sickle cell crisis. Interactions between neutrophils and red cells were markedly reduced in microbiota-depleted SCD mice, resulting in improved local blood flow and greatly improved survival in the mice.
“What was most surprising and exciting to us was the effect of antibiotics on chronic tissue damage,” Dr Frenette said.
“We found that the spleen enlargement of SCD mice was significantly reduced in the microbiota-depleted animals, and liver analysis revealed major reductions in liver damage, including inflammation, scarring, and tissue death. This is the first time that anything has been found to have an impact on the organ damage that can be so devastating in SCD.”
Septic shock
The investigators then studied septic shock, another serious blood disorder in which activated, pro-inflammatory neutrophils play a role.
To induce septic shock, the team gave control and microbiota-depleted mice a dose of a bacterial toxin that would normally be lethal.
The control mice had the neutrophil aggregates and clumping of neutrophil DNA that contribute to death from septic shock, but the microbiota-depleted mice were largely free of neutrophil complications and survived.
“Remarkably, we could prevent microbiota-depleted mice from surviving septic shock if we infused them with aged neutrophils but not if we infused the same number of young neutrophils,” Dr Frenette said. “So depleting the microbiota may help against inflammatory blood diseases in addition to SCD.”
SCD patient samples
Finally, the researchers investigated whether their findings in mice might be relevant to humans with SCD.
The team obtained blood samples from 9 healthy children and 34 patients with SCD. Eleven patients were taking penicillin daily to ward off infections, as is recommended for children with SCD who are 5 years of age or younger. The other 23 patients with SCD had been off penicillin for at least 2 months.
Consistent with the findings in mice, children with SCD who were not taking penicillin had many more circulating aged neutrophils than the healthy children.
The investigators then compared neutrophil levels in the 2 groups of children with SCD—those taking penicillin and those not on the drug—and found a much lower number of aged neutrophils in the blood of those who were taking penicillin.
“Daily penicillin for patients with SCD younger than 5 works really well in preventing infections,” Dr Frenette said. “Our study suggests that penicillin and other antibiotics could play an even broader role in potentially benefiting older patients.”
“[W]e hope to carry out a clinical trial to determine whether antibiotics can help patients with SCD by preventing the sickle cell crisis and long-term organ damage associated with the disease.” ![]()

Image by Volker Brinkmann
Preclinical research suggests that usingantibiotics to deplete the body’s microbiome may prevent acute sickle cell crisis in patients with sickle cell disease (SCD) and could potentially offer the first effective strategy for warding off the disease’s long-term complications, such as organ failure.
The work, published in Nature, may also have implications for other inflammatory blood-vessel disorders, including septic shock.
The study was led by Paul Frenette, MD, of Albert Einstein College of Medicine in Bronx, New York. In 2002, Dr Frenette and his colleagues reported that SCD vessel blockages occur when sickled red cells bind to neutrophils that have adhered to the vessel walls.
“This earlier work indicated that not all neutrophils are the same,” Dr Frenette said. “Some appear to be inert, while others appear overly active in promoting inflammation, which is useful for attacking microbes but causes neutrophils to capture sickled red cells inside vessels.”
“So in the current study, we investigated whether the age of the neutrophils might be influencing whether they become active and pro-inflammatory.”
Neutrophils and SCD
The researchers began by transfusing whole blood into mice and then analyzing young neutrophils (harvested 10 minutes post-transfusion) and aged neutrophils (harvested 6 hours post-transfusion). The neutrophils became more active as they aged, suggesting that neutrophils receive external signals telling them to age.
The investigators were able to trace these “aging” signals to the body’s microbiome. They found the microbiome produces chemicals that cross the intestinal barrier and enter the bloodstream, where they generate the aged, overly active subset of neutrophils that contribute to SCD.
“Since the body’s microbiota seem to ‘educate’ neutrophils to age, we realized that purging those microbes through use of antibiotics might help against SCD,” Dr Frenette said.
To find out, he and his colleagues conducted experiments in a mouse model of SCD. These mice possessed 5 times as many aged neutrophils as healthy control mice.
When the researchers depleted the microbiota of SCD mice using antibiotics, they observed a striking reduction in neutrophils but not in other white blood cells (such as monocytes, T cells, and B cells).
Moreover, giving antibiotics to SCD mice appeared to prevent sickle cell crisis. Interactions between neutrophils and red cells were markedly reduced in microbiota-depleted SCD mice, resulting in improved local blood flow and greatly improved survival in the mice.
“What was most surprising and exciting to us was the effect of antibiotics on chronic tissue damage,” Dr Frenette said.
“We found that the spleen enlargement of SCD mice was significantly reduced in the microbiota-depleted animals, and liver analysis revealed major reductions in liver damage, including inflammation, scarring, and tissue death. This is the first time that anything has been found to have an impact on the organ damage that can be so devastating in SCD.”
Septic shock
The investigators then studied septic shock, another serious blood disorder in which activated, pro-inflammatory neutrophils play a role.
To induce septic shock, the team gave control and microbiota-depleted mice a dose of a bacterial toxin that would normally be lethal.
The control mice had the neutrophil aggregates and clumping of neutrophil DNA that contribute to death from septic shock, but the microbiota-depleted mice were largely free of neutrophil complications and survived.
“Remarkably, we could prevent microbiota-depleted mice from surviving septic shock if we infused them with aged neutrophils but not if we infused the same number of young neutrophils,” Dr Frenette said. “So depleting the microbiota may help against inflammatory blood diseases in addition to SCD.”
SCD patient samples
Finally, the researchers investigated whether their findings in mice might be relevant to humans with SCD.
The team obtained blood samples from 9 healthy children and 34 patients with SCD. Eleven patients were taking penicillin daily to ward off infections, as is recommended for children with SCD who are 5 years of age or younger. The other 23 patients with SCD had been off penicillin for at least 2 months.
Consistent with the findings in mice, children with SCD who were not taking penicillin had many more circulating aged neutrophils than the healthy children.
The investigators then compared neutrophil levels in the 2 groups of children with SCD—those taking penicillin and those not on the drug—and found a much lower number of aged neutrophils in the blood of those who were taking penicillin.
“Daily penicillin for patients with SCD younger than 5 works really well in preventing infections,” Dr Frenette said. “Our study suggests that penicillin and other antibiotics could play an even broader role in potentially benefiting older patients.”
“[W]e hope to carry out a clinical trial to determine whether antibiotics can help patients with SCD by preventing the sickle cell crisis and long-term organ damage associated with the disease.” ![]()
Cost of MM treatments too high, survey suggests

Photo by Petr Kratochvil
Results of a small survey suggest patients with multiple myeloma (MM) are vulnerable to “financial toxicity,” due to costly treatments, even if they have health insurance and well-paying jobs.
All of the 100 patients surveyed had health insurance and a median household income above the US average.
Yet 46% of respondents said they tapped into their savings to pay for treatment, and 21% borrowed money to pay for care.
Seventeen percent of patients reported delays in treatment due to costs, and 11% said excessive costs caused them to stop treatment altogether.
Results of this survey appear in The Lancet Haematology.
Risk of financial toxicity
Financial toxicity is described as the burden of out-of-pocket costs that can affect patients’ wellbeing and become an adverse effect of treatment.
Previous studies have suggested that patients frequently employ coping mechanisms to help defray out-of-pocket costs, some of which compromise treatment adherence. Financial toxicity may also negatively impact quality of life, and some reports suggest it may contribute to increased mortality.
“While advances in multiple myeloma therapy have contributed to significant improvements in patient outcomes, the clinical gains have come with rising costs,” said Scott Huntington, MD, of Yale University in New Haven, Connecticut.
Costs of newly approved drugs for hematologic malignancies have increased 10-fold in the past 15 years, with many agents costing $10,000 or more a month.
“And today . . . , most patients are on a new drug, compared to a decade ago when less than 5% were,” Dr Huntington said. “So we’re not talking about a select group of patients faced with this burden. Many are facing the financial challenges of treatment.”
Survey population
To investigate the effects of treatment costs in MM, Dr Huntington and his colleagues surveyed 100 patients treated at the Abramson Cancer Center in Philadelphia, Pennsylvania. The median age of the patients was 64, and 53% were female.
The researchers used the COST (Comprehensive Score for Financial Toxicity) questionnaire and other survey questions. The COST tool measures various aspects of financial circumstances, such as income, education, marital status, ability to work, and overall opinions about additional expenses and a person’s current financial situation.
All survey respondents were insured, and all of the patients with Medicare fee-for-service coverage (39%) had additional supplemental insurance to assist with out-of-pocket costs.
The respondents also had a median household income and education level above the national average. The median annual household income
was reported between $60,000 and $79,999, and 70% of respondents reported having some college education.
At the time of the survey, 62% of respondents were receiving first-line (35%) or second-line (27%) treatment. All patients were receiving or had received at least 1 novel drug for MM.
Seventy-five percent of patients had received both lenalidomide and bortezomib since their diagnosis, and 58% had undergone an autologous stem cell transplant. Forty-four percent of patients had received 3 to 4 treatment regimens, and 25% had received 5 or more.
Survey results
Of the 100 patients surveyed, 59% said MM treatment costs were higher than expected, and 71% indicated at least minor financial burden. Fifty-five percent of patients said they had to reduce spending on basic goods since their diagnosis, and 64% said they had to reduce spending on leisure activities.
Thirty-six percent of respondents reported applying for financial assistance to pay for treatment, including 18% who reported incomes over $100,000. Forty-six percent of respondents said they used their savings to pay for treatment, and 21% borrowed money.
The high cost of MM therapy prompted treatment delays for 17% of patients, caused 12% of patients to fill only part of a prescription, and made 11% of patients stop treatment altogether.
Adding to the financial burden, more than half of respondents said they had to reduce their hours at work or quit their job after being diagnosed with MM.
The researchers said these results suggest a need to “acknowledge the untenable rise in treatment costs and its impact on patients.” And “strengthened collaboration” between patients and healthcare stakeholders is needed to promote reforms that lead to more affordable cancer care. ![]()

Photo by Petr Kratochvil
Results of a small survey suggest patients with multiple myeloma (MM) are vulnerable to “financial toxicity,” due to costly treatments, even if they have health insurance and well-paying jobs.
All of the 100 patients surveyed had health insurance and a median household income above the US average.
Yet 46% of respondents said they tapped into their savings to pay for treatment, and 21% borrowed money to pay for care.
Seventeen percent of patients reported delays in treatment due to costs, and 11% said excessive costs caused them to stop treatment altogether.
Results of this survey appear in The Lancet Haematology.
Risk of financial toxicity
Financial toxicity is described as the burden of out-of-pocket costs that can affect patients’ wellbeing and become an adverse effect of treatment.
Previous studies have suggested that patients frequently employ coping mechanisms to help defray out-of-pocket costs, some of which compromise treatment adherence. Financial toxicity may also negatively impact quality of life, and some reports suggest it may contribute to increased mortality.
“While advances in multiple myeloma therapy have contributed to significant improvements in patient outcomes, the clinical gains have come with rising costs,” said Scott Huntington, MD, of Yale University in New Haven, Connecticut.
Costs of newly approved drugs for hematologic malignancies have increased 10-fold in the past 15 years, with many agents costing $10,000 or more a month.
“And today . . . , most patients are on a new drug, compared to a decade ago when less than 5% were,” Dr Huntington said. “So we’re not talking about a select group of patients faced with this burden. Many are facing the financial challenges of treatment.”
Survey population
To investigate the effects of treatment costs in MM, Dr Huntington and his colleagues surveyed 100 patients treated at the Abramson Cancer Center in Philadelphia, Pennsylvania. The median age of the patients was 64, and 53% were female.
The researchers used the COST (Comprehensive Score for Financial Toxicity) questionnaire and other survey questions. The COST tool measures various aspects of financial circumstances, such as income, education, marital status, ability to work, and overall opinions about additional expenses and a person’s current financial situation.
All survey respondents were insured, and all of the patients with Medicare fee-for-service coverage (39%) had additional supplemental insurance to assist with out-of-pocket costs.
The respondents also had a median household income and education level above the national average. The median annual household income
was reported between $60,000 and $79,999, and 70% of respondents reported having some college education.
At the time of the survey, 62% of respondents were receiving first-line (35%) or second-line (27%) treatment. All patients were receiving or had received at least 1 novel drug for MM.
Seventy-five percent of patients had received both lenalidomide and bortezomib since their diagnosis, and 58% had undergone an autologous stem cell transplant. Forty-four percent of patients had received 3 to 4 treatment regimens, and 25% had received 5 or more.
Survey results
Of the 100 patients surveyed, 59% said MM treatment costs were higher than expected, and 71% indicated at least minor financial burden. Fifty-five percent of patients said they had to reduce spending on basic goods since their diagnosis, and 64% said they had to reduce spending on leisure activities.
Thirty-six percent of respondents reported applying for financial assistance to pay for treatment, including 18% who reported incomes over $100,000. Forty-six percent of respondents said they used their savings to pay for treatment, and 21% borrowed money.
The high cost of MM therapy prompted treatment delays for 17% of patients, caused 12% of patients to fill only part of a prescription, and made 11% of patients stop treatment altogether.
Adding to the financial burden, more than half of respondents said they had to reduce their hours at work or quit their job after being diagnosed with MM.
The researchers said these results suggest a need to “acknowledge the untenable rise in treatment costs and its impact on patients.” And “strengthened collaboration” between patients and healthcare stakeholders is needed to promote reforms that lead to more affordable cancer care. ![]()

Photo by Petr Kratochvil
Results of a small survey suggest patients with multiple myeloma (MM) are vulnerable to “financial toxicity,” due to costly treatments, even if they have health insurance and well-paying jobs.
All of the 100 patients surveyed had health insurance and a median household income above the US average.
Yet 46% of respondents said they tapped into their savings to pay for treatment, and 21% borrowed money to pay for care.
Seventeen percent of patients reported delays in treatment due to costs, and 11% said excessive costs caused them to stop treatment altogether.
Results of this survey appear in The Lancet Haematology.
Risk of financial toxicity
Financial toxicity is described as the burden of out-of-pocket costs that can affect patients’ wellbeing and become an adverse effect of treatment.
Previous studies have suggested that patients frequently employ coping mechanisms to help defray out-of-pocket costs, some of which compromise treatment adherence. Financial toxicity may also negatively impact quality of life, and some reports suggest it may contribute to increased mortality.
“While advances in multiple myeloma therapy have contributed to significant improvements in patient outcomes, the clinical gains have come with rising costs,” said Scott Huntington, MD, of Yale University in New Haven, Connecticut.
Costs of newly approved drugs for hematologic malignancies have increased 10-fold in the past 15 years, with many agents costing $10,000 or more a month.
“And today . . . , most patients are on a new drug, compared to a decade ago when less than 5% were,” Dr Huntington said. “So we’re not talking about a select group of patients faced with this burden. Many are facing the financial challenges of treatment.”
Survey population
To investigate the effects of treatment costs in MM, Dr Huntington and his colleagues surveyed 100 patients treated at the Abramson Cancer Center in Philadelphia, Pennsylvania. The median age of the patients was 64, and 53% were female.
The researchers used the COST (Comprehensive Score for Financial Toxicity) questionnaire and other survey questions. The COST tool measures various aspects of financial circumstances, such as income, education, marital status, ability to work, and overall opinions about additional expenses and a person’s current financial situation.
All survey respondents were insured, and all of the patients with Medicare fee-for-service coverage (39%) had additional supplemental insurance to assist with out-of-pocket costs.
The respondents also had a median household income and education level above the national average. The median annual household income
was reported between $60,000 and $79,999, and 70% of respondents reported having some college education.
At the time of the survey, 62% of respondents were receiving first-line (35%) or second-line (27%) treatment. All patients were receiving or had received at least 1 novel drug for MM.
Seventy-five percent of patients had received both lenalidomide and bortezomib since their diagnosis, and 58% had undergone an autologous stem cell transplant. Forty-four percent of patients had received 3 to 4 treatment regimens, and 25% had received 5 or more.
Survey results
Of the 100 patients surveyed, 59% said MM treatment costs were higher than expected, and 71% indicated at least minor financial burden. Fifty-five percent of patients said they had to reduce spending on basic goods since their diagnosis, and 64% said they had to reduce spending on leisure activities.
Thirty-six percent of respondents reported applying for financial assistance to pay for treatment, including 18% who reported incomes over $100,000. Forty-six percent of respondents said they used their savings to pay for treatment, and 21% borrowed money.
The high cost of MM therapy prompted treatment delays for 17% of patients, caused 12% of patients to fill only part of a prescription, and made 11% of patients stop treatment altogether.
Adding to the financial burden, more than half of respondents said they had to reduce their hours at work or quit their job after being diagnosed with MM.
The researchers said these results suggest a need to “acknowledge the untenable rise in treatment costs and its impact on patients.” And “strengthened collaboration” between patients and healthcare stakeholders is needed to promote reforms that lead to more affordable cancer care. ![]()
Home pesticide exposure linked to childhood cancers

in his garden
Researchers have linked residential pesticide exposure to childhood cancers, but the estimated risks vary according to the cancer type, the type of pesticide, and where it is applied.
The investigators conducted a meta-analysis of published studies and found that childhood exposure to indoor pesticides was associated with a
significantly increased risk of all the cancers analyzed, as well as leukemia and lymphoma individually.
Overall, exposure to outdoor pesticides was not associated with an increased risk of childhood cancers. However, herbicide exposure was linked to an increased risk of leukemia and all cancers combined.
Mei Chen, PhD, of the Harvard T.H. Chan School of Public Health in Boston, Massachusetts, and colleagues conducted this meta-analysis and reported the results in Pediatrics.
The team searched for observational studies published in PubMed before February 2014 and ultimately included 16 studies in their analysis.
They assessed exposure to indoor pesticides and indoor insecticides (a subgroup of indoor pesticides), as well as exposure to outdoor pesticides, which included outdoor insecticides, herbicides, and fungicides.
The cancer types analyzed were leukemia, lymphoma, brain tumors, neuroblastoma, Wilms tumor, and soft tissue sarcoma.
Indoor pesticides
When the investigators analyzed all cancer types together, they found a significantly increased risk of childhood cancers associated with exposure to indoor pesticides (odds ratio [OR]=1.40).
Likewise, there was a significantly increased risk for leukemia (OR=1.48), acute leukemia (OR=1.59), lymphoma (OR=1.43), and all hematopoietic malignancies (leukemias and lymphomas, OR=1.47).
But the increased risk of childhood brain tumors was not statistically significant, and the other cancers were not analyzed separately.
Exposure to indoor insecticides was associated with a significant increase in the risk of leukemia (OR=1.47), acute leukemia (OR=1.59), lymphoma (OR=1.43), and all hematopoietic malignancies (OR=1.46).
Outdoor pesticides
There was no significant association between exposure to outdoor pesticides or outdoor insecticides and any of the cancer types. And there were not enough studies on fungicides to assess the risk of cancers associated with their use.
However, there was a significant association between exposure to herbicide and all childhood cancers (OR=1.35) as well as leukemia (OR=1.26).
The investigators said these results suggest cancer risks are related to the type of pesticides used and where they are applied.
And although additional research is needed to confirm the association between pesticide exposure and childhood cancers, steps should be taken to limit exposure to pesticides during childhood. ![]()

in his garden
Researchers have linked residential pesticide exposure to childhood cancers, but the estimated risks vary according to the cancer type, the type of pesticide, and where it is applied.
The investigators conducted a meta-analysis of published studies and found that childhood exposure to indoor pesticides was associated with a
significantly increased risk of all the cancers analyzed, as well as leukemia and lymphoma individually.
Overall, exposure to outdoor pesticides was not associated with an increased risk of childhood cancers. However, herbicide exposure was linked to an increased risk of leukemia and all cancers combined.
Mei Chen, PhD, of the Harvard T.H. Chan School of Public Health in Boston, Massachusetts, and colleagues conducted this meta-analysis and reported the results in Pediatrics.
The team searched for observational studies published in PubMed before February 2014 and ultimately included 16 studies in their analysis.
They assessed exposure to indoor pesticides and indoor insecticides (a subgroup of indoor pesticides), as well as exposure to outdoor pesticides, which included outdoor insecticides, herbicides, and fungicides.
The cancer types analyzed were leukemia, lymphoma, brain tumors, neuroblastoma, Wilms tumor, and soft tissue sarcoma.
Indoor pesticides
When the investigators analyzed all cancer types together, they found a significantly increased risk of childhood cancers associated with exposure to indoor pesticides (odds ratio [OR]=1.40).
Likewise, there was a significantly increased risk for leukemia (OR=1.48), acute leukemia (OR=1.59), lymphoma (OR=1.43), and all hematopoietic malignancies (leukemias and lymphomas, OR=1.47).
But the increased risk of childhood brain tumors was not statistically significant, and the other cancers were not analyzed separately.
Exposure to indoor insecticides was associated with a significant increase in the risk of leukemia (OR=1.47), acute leukemia (OR=1.59), lymphoma (OR=1.43), and all hematopoietic malignancies (OR=1.46).
Outdoor pesticides
There was no significant association between exposure to outdoor pesticides or outdoor insecticides and any of the cancer types. And there were not enough studies on fungicides to assess the risk of cancers associated with their use.
However, there was a significant association between exposure to herbicide and all childhood cancers (OR=1.35) as well as leukemia (OR=1.26).
The investigators said these results suggest cancer risks are related to the type of pesticides used and where they are applied.
And although additional research is needed to confirm the association between pesticide exposure and childhood cancers, steps should be taken to limit exposure to pesticides during childhood. ![]()

in his garden
Researchers have linked residential pesticide exposure to childhood cancers, but the estimated risks vary according to the cancer type, the type of pesticide, and where it is applied.
The investigators conducted a meta-analysis of published studies and found that childhood exposure to indoor pesticides was associated with a
significantly increased risk of all the cancers analyzed, as well as leukemia and lymphoma individually.
Overall, exposure to outdoor pesticides was not associated with an increased risk of childhood cancers. However, herbicide exposure was linked to an increased risk of leukemia and all cancers combined.
Mei Chen, PhD, of the Harvard T.H. Chan School of Public Health in Boston, Massachusetts, and colleagues conducted this meta-analysis and reported the results in Pediatrics.
The team searched for observational studies published in PubMed before February 2014 and ultimately included 16 studies in their analysis.
They assessed exposure to indoor pesticides and indoor insecticides (a subgroup of indoor pesticides), as well as exposure to outdoor pesticides, which included outdoor insecticides, herbicides, and fungicides.
The cancer types analyzed were leukemia, lymphoma, brain tumors, neuroblastoma, Wilms tumor, and soft tissue sarcoma.
Indoor pesticides
When the investigators analyzed all cancer types together, they found a significantly increased risk of childhood cancers associated with exposure to indoor pesticides (odds ratio [OR]=1.40).
Likewise, there was a significantly increased risk for leukemia (OR=1.48), acute leukemia (OR=1.59), lymphoma (OR=1.43), and all hematopoietic malignancies (leukemias and lymphomas, OR=1.47).
But the increased risk of childhood brain tumors was not statistically significant, and the other cancers were not analyzed separately.
Exposure to indoor insecticides was associated with a significant increase in the risk of leukemia (OR=1.47), acute leukemia (OR=1.59), lymphoma (OR=1.43), and all hematopoietic malignancies (OR=1.46).
Outdoor pesticides
There was no significant association between exposure to outdoor pesticides or outdoor insecticides and any of the cancer types. And there were not enough studies on fungicides to assess the risk of cancers associated with their use.
However, there was a significant association between exposure to herbicide and all childhood cancers (OR=1.35) as well as leukemia (OR=1.26).
The investigators said these results suggest cancer risks are related to the type of pesticides used and where they are applied.
And although additional research is needed to confirm the association between pesticide exposure and childhood cancers, steps should be taken to limit exposure to pesticides during childhood. ![]()
Platelet mimics provide targeted drug delivery
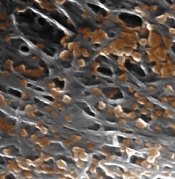
nanoparticles binding to the
lining of a damaged artery
Image courtesy of the
Zhang Research Group
Nanoparticles disguised as human platelets can provide targeted drug delivery, according to research published in Nature.
The nanoparticles are made of a biodegradable polymer coated with human platelet membranes.
This coating allows the nanoparticles to circulate in the bloodstream without being attacked by the immune system and to preferentially bind to damaged blood vessels and certain pathogens.
Murine experiments showed that these platelet-mimicking nanoparticles can deliver drugs to targeted sites, thereby increasing the therapeutic effect.
“Because of their targeting ability, platelet-mimicking nanoparticles can directly provide a much higher dose of medication specifically to diseased areas without saturating the entire body with drugs,” said study author Liangfang Zhang, PhD, of the University of California San Diego.
Creating the platelet mimics
To make the nanoparticles, Dr Zhang and his colleagues first separated platelets from whole blood samples using a centrifuge. The platelets were then processed to isolate the membranes from the platelets.
Next, the platelet membranes were broken up into much smaller pieces and fused to the surface of the nanoparticle cores. The resulting platelet-membrane-coated nanoparticles were approximately 100 nanometers in diameter.
This cloaking technology is based on a strategy Dr Zhang’s group had developed to cloak nanoparticles in red blood cell membranes. The researchers previously demonstrated that nanoparticles disguised as red blood cells are capable of removing toxins from the bloodstream.
With the current work, the researchers were able to produce platelet mimics that contain the complete set of surface receptors, antigens, and proteins naturally present on platelet membranes.
“Our technique takes advantage of the unique natural properties of human platelet membranes, which have a natural preference to bind to certain tissues and organisms in the body,” Dr Zhang said.
This targeting ability, which red blood cell membranes do not have, makes platelet membranes extremely useful for targeted drug delivery, according to the researchers.
Platelet mimics at work
The researchers packed the platelet-mimicking nanoparticles with docetaxel, a drug used to prevent scar tissue formation in the lining of damaged blood vessels, and administered them to rats afflicted with injured arteries.
The docetaxel-containing nanoparticles collected at the damaged sites of arteries and healed them.
The researchers then injected nanoparticles containing one-sixth the clinical dose of the antibiotic vancomycin into a group of mice systemically infected with methicillin-resistant Staphylococcus aureus bacteria.
Bacterial counts in the organs of these mice were up to 1000 times lower than in mice treated with the clinical dose of vancomycin alone.
“Our platelet-mimicking nanoparticles can increase the therapeutic efficacy of antibiotics because they can focus treatment on the bacteria locally without spreading drugs to healthy tissues and organs throughout the rest of the body,” Dr Zhang said. “We hope to develop platelet-mimicking nanoparticles into new treatments for systemic bacterial infections and cardiovascular disease.”
Dr Zhang noted that this drug delivery technique could potentially be used in other diseases as well, including cancers. ![]()

nanoparticles binding to the
lining of a damaged artery
Image courtesy of the
Zhang Research Group
Nanoparticles disguised as human platelets can provide targeted drug delivery, according to research published in Nature.
The nanoparticles are made of a biodegradable polymer coated with human platelet membranes.
This coating allows the nanoparticles to circulate in the bloodstream without being attacked by the immune system and to preferentially bind to damaged blood vessels and certain pathogens.
Murine experiments showed that these platelet-mimicking nanoparticles can deliver drugs to targeted sites, thereby increasing the therapeutic effect.
“Because of their targeting ability, platelet-mimicking nanoparticles can directly provide a much higher dose of medication specifically to diseased areas without saturating the entire body with drugs,” said study author Liangfang Zhang, PhD, of the University of California San Diego.
Creating the platelet mimics
To make the nanoparticles, Dr Zhang and his colleagues first separated platelets from whole blood samples using a centrifuge. The platelets were then processed to isolate the membranes from the platelets.
Next, the platelet membranes were broken up into much smaller pieces and fused to the surface of the nanoparticle cores. The resulting platelet-membrane-coated nanoparticles were approximately 100 nanometers in diameter.
This cloaking technology is based on a strategy Dr Zhang’s group had developed to cloak nanoparticles in red blood cell membranes. The researchers previously demonstrated that nanoparticles disguised as red blood cells are capable of removing toxins from the bloodstream.
With the current work, the researchers were able to produce platelet mimics that contain the complete set of surface receptors, antigens, and proteins naturally present on platelet membranes.
“Our technique takes advantage of the unique natural properties of human platelet membranes, which have a natural preference to bind to certain tissues and organisms in the body,” Dr Zhang said.
This targeting ability, which red blood cell membranes do not have, makes platelet membranes extremely useful for targeted drug delivery, according to the researchers.
Platelet mimics at work
The researchers packed the platelet-mimicking nanoparticles with docetaxel, a drug used to prevent scar tissue formation in the lining of damaged blood vessels, and administered them to rats afflicted with injured arteries.
The docetaxel-containing nanoparticles collected at the damaged sites of arteries and healed them.
The researchers then injected nanoparticles containing one-sixth the clinical dose of the antibiotic vancomycin into a group of mice systemically infected with methicillin-resistant Staphylococcus aureus bacteria.
Bacterial counts in the organs of these mice were up to 1000 times lower than in mice treated with the clinical dose of vancomycin alone.
“Our platelet-mimicking nanoparticles can increase the therapeutic efficacy of antibiotics because they can focus treatment on the bacteria locally without spreading drugs to healthy tissues and organs throughout the rest of the body,” Dr Zhang said. “We hope to develop platelet-mimicking nanoparticles into new treatments for systemic bacterial infections and cardiovascular disease.”
Dr Zhang noted that this drug delivery technique could potentially be used in other diseases as well, including cancers. ![]()

nanoparticles binding to the
lining of a damaged artery
Image courtesy of the
Zhang Research Group
Nanoparticles disguised as human platelets can provide targeted drug delivery, according to research published in Nature.
The nanoparticles are made of a biodegradable polymer coated with human platelet membranes.
This coating allows the nanoparticles to circulate in the bloodstream without being attacked by the immune system and to preferentially bind to damaged blood vessels and certain pathogens.
Murine experiments showed that these platelet-mimicking nanoparticles can deliver drugs to targeted sites, thereby increasing the therapeutic effect.
“Because of their targeting ability, platelet-mimicking nanoparticles can directly provide a much higher dose of medication specifically to diseased areas without saturating the entire body with drugs,” said study author Liangfang Zhang, PhD, of the University of California San Diego.
Creating the platelet mimics
To make the nanoparticles, Dr Zhang and his colleagues first separated platelets from whole blood samples using a centrifuge. The platelets were then processed to isolate the membranes from the platelets.
Next, the platelet membranes were broken up into much smaller pieces and fused to the surface of the nanoparticle cores. The resulting platelet-membrane-coated nanoparticles were approximately 100 nanometers in diameter.
This cloaking technology is based on a strategy Dr Zhang’s group had developed to cloak nanoparticles in red blood cell membranes. The researchers previously demonstrated that nanoparticles disguised as red blood cells are capable of removing toxins from the bloodstream.
With the current work, the researchers were able to produce platelet mimics that contain the complete set of surface receptors, antigens, and proteins naturally present on platelet membranes.
“Our technique takes advantage of the unique natural properties of human platelet membranes, which have a natural preference to bind to certain tissues and organisms in the body,” Dr Zhang said.
This targeting ability, which red blood cell membranes do not have, makes platelet membranes extremely useful for targeted drug delivery, according to the researchers.
Platelet mimics at work
The researchers packed the platelet-mimicking nanoparticles with docetaxel, a drug used to prevent scar tissue formation in the lining of damaged blood vessels, and administered them to rats afflicted with injured arteries.
The docetaxel-containing nanoparticles collected at the damaged sites of arteries and healed them.
The researchers then injected nanoparticles containing one-sixth the clinical dose of the antibiotic vancomycin into a group of mice systemically infected with methicillin-resistant Staphylococcus aureus bacteria.
Bacterial counts in the organs of these mice were up to 1000 times lower than in mice treated with the clinical dose of vancomycin alone.
“Our platelet-mimicking nanoparticles can increase the therapeutic efficacy of antibiotics because they can focus treatment on the bacteria locally without spreading drugs to healthy tissues and organs throughout the rest of the body,” Dr Zhang said. “We hope to develop platelet-mimicking nanoparticles into new treatments for systemic bacterial infections and cardiovascular disease.”
Dr Zhang noted that this drug delivery technique could potentially be used in other diseases as well, including cancers. ![]()
Excellent survival after ASCT for light-chain amyloidosis
Early mortality after autologous hematopoietic stem cell transplantation (ASCT) for light-chain amyloidosis has declined dramatically in recent years, and 5-year survival is now deemed “excellent” at 77%, according to a report published online Sept. 14 in Journal of Clinical Oncology.
Light-chain amyloidosis results in deposition of insoluble amyloid fibers in many tissues, particularly the heart and kidneys, and can lead to organ failure and death. While medical therapies target the plasma cell clone that is the source of the amyloid, treatments have minimal effect on amyloid that has already accumulated in tissues. Alternatively, ASCT can produce durable hematologic responses and has resulted in improved function of affected organs, based on several single-center studies.
However, the only large, prospective randomized clinical trial to compare ASCT and medical therapy was done in 2007. That study showed autotransplantation carried a high (24%) early mortality, which contributed heavily to inferior overall survival, said Dr. Anita D’Souza of the division of hematology and oncology, Medical College of Wisconsin, Milwaukee, and her associates.
Since that study, supportive care during the peritransplantation period has greatly improved. Large U.S. transplant centers now report that early mortality is less than 5%.
To assess time trends in autotransplantation mortality, Dr. D’Souza and her associates analyzed data from the Center for International Blood & Marrow Transplant Research, a registry that collects transplant data from 320 centers worldwide and captures information concerning most U.S. procedures. They focused on 1,536 North American patients with light-chain amyloidosis who underwent ASCT during three successive 5-year periods: 1995-2000, 2001-2006, and 2007-2012. The median follow-up was 56 months.
Over time, 30-day mortality declined from 11% to 5% to 3%, respectively; and 100-day mortality declined from 20% to 11% to 5%. One-year overall survival rose from 75% to 85% to 90%, and 5-year overall survival improved from 55% to 61% to 77%.
Even among patients with renal amyloidosis, 3-year overall survival improved over time from 78% during the earliest study period to 89% during the most recent time period. However, mortality did not improve significantly over time among patients with cardiac involvement, which continues to be the single most-important variable associated with poor outcomes, the researchers said (J Clin Oncol. 2015 Sep 14 [doi:10.1200/JCO.2015.62.4015]).
A transplant center’s experience with this procedure proved to be of crucial importance to early patient mortality. Centers that performed a low volume of ASCT for light-chain amyloidosis, defined as fewer than four transplants per year, had a 30-day mortality of 5% and a 100-day mortality of 7%, while centers that performed more than four procedures per year had significantly lower mortalities of 1% and 3%, respectively. There were no significant differences between low- and high-volume centers regarding patient, organ, or medication factors, which “led us to believe that high-volume centers do not necessarily select fitter patients for transplantation. Rather, they may be more experienced in supporting and treating these patients in the early posttransplantation period,” Dr. D’Souza and her associates said.
These “reassuring” findings, together with the recent development of novel plasma-cell–targeting agents such as bortezomib, suggest that it is time to consider performing a more current multicenter prospective study comparing autotransplantation against medical therapy, they added.
It is important to place these study findings in context, and note that approximately 20% of patients seen at most transplant centers are considered good candidates for autologous hematopoietic cell transplantation. The outcomes of the procedure remain unsatisfactory for most patients.
Delayed diagnosis appears to be a major obstacle to effective treatment. Many patients experience significant organ dysfunction before a diagnosis is established, even when the underlying plasma cell dyscrasia is being actively followed by a hematologist.
Dr. Noffar Bar, Dr. Terri L. Parker, and Dr. Madhav V. Dhodapkar are at Yale Cancer Center, New Haven Conn. They made these remarks in an editorial accompanying Dr. D’Souza’s report (J Clin Oncol. Sep 14 [doi:10.1200/HCO.2015.63.2224]). Their financial disclosures are available at www.jco.org.
It is important to place these study findings in context, and note that approximately 20% of patients seen at most transplant centers are considered good candidates for autologous hematopoietic cell transplantation. The outcomes of the procedure remain unsatisfactory for most patients.
Delayed diagnosis appears to be a major obstacle to effective treatment. Many patients experience significant organ dysfunction before a diagnosis is established, even when the underlying plasma cell dyscrasia is being actively followed by a hematologist.
Dr. Noffar Bar, Dr. Terri L. Parker, and Dr. Madhav V. Dhodapkar are at Yale Cancer Center, New Haven Conn. They made these remarks in an editorial accompanying Dr. D’Souza’s report (J Clin Oncol. Sep 14 [doi:10.1200/HCO.2015.63.2224]). Their financial disclosures are available at www.jco.org.
It is important to place these study findings in context, and note that approximately 20% of patients seen at most transplant centers are considered good candidates for autologous hematopoietic cell transplantation. The outcomes of the procedure remain unsatisfactory for most patients.
Delayed diagnosis appears to be a major obstacle to effective treatment. Many patients experience significant organ dysfunction before a diagnosis is established, even when the underlying plasma cell dyscrasia is being actively followed by a hematologist.
Dr. Noffar Bar, Dr. Terri L. Parker, and Dr. Madhav V. Dhodapkar are at Yale Cancer Center, New Haven Conn. They made these remarks in an editorial accompanying Dr. D’Souza’s report (J Clin Oncol. Sep 14 [doi:10.1200/HCO.2015.63.2224]). Their financial disclosures are available at www.jco.org.
Early mortality after autologous hematopoietic stem cell transplantation (ASCT) for light-chain amyloidosis has declined dramatically in recent years, and 5-year survival is now deemed “excellent” at 77%, according to a report published online Sept. 14 in Journal of Clinical Oncology.
Light-chain amyloidosis results in deposition of insoluble amyloid fibers in many tissues, particularly the heart and kidneys, and can lead to organ failure and death. While medical therapies target the plasma cell clone that is the source of the amyloid, treatments have minimal effect on amyloid that has already accumulated in tissues. Alternatively, ASCT can produce durable hematologic responses and has resulted in improved function of affected organs, based on several single-center studies.
However, the only large, prospective randomized clinical trial to compare ASCT and medical therapy was done in 2007. That study showed autotransplantation carried a high (24%) early mortality, which contributed heavily to inferior overall survival, said Dr. Anita D’Souza of the division of hematology and oncology, Medical College of Wisconsin, Milwaukee, and her associates.
Since that study, supportive care during the peritransplantation period has greatly improved. Large U.S. transplant centers now report that early mortality is less than 5%.
To assess time trends in autotransplantation mortality, Dr. D’Souza and her associates analyzed data from the Center for International Blood & Marrow Transplant Research, a registry that collects transplant data from 320 centers worldwide and captures information concerning most U.S. procedures. They focused on 1,536 North American patients with light-chain amyloidosis who underwent ASCT during three successive 5-year periods: 1995-2000, 2001-2006, and 2007-2012. The median follow-up was 56 months.
Over time, 30-day mortality declined from 11% to 5% to 3%, respectively; and 100-day mortality declined from 20% to 11% to 5%. One-year overall survival rose from 75% to 85% to 90%, and 5-year overall survival improved from 55% to 61% to 77%.
Even among patients with renal amyloidosis, 3-year overall survival improved over time from 78% during the earliest study period to 89% during the most recent time period. However, mortality did not improve significantly over time among patients with cardiac involvement, which continues to be the single most-important variable associated with poor outcomes, the researchers said (J Clin Oncol. 2015 Sep 14 [doi:10.1200/JCO.2015.62.4015]).
A transplant center’s experience with this procedure proved to be of crucial importance to early patient mortality. Centers that performed a low volume of ASCT for light-chain amyloidosis, defined as fewer than four transplants per year, had a 30-day mortality of 5% and a 100-day mortality of 7%, while centers that performed more than four procedures per year had significantly lower mortalities of 1% and 3%, respectively. There were no significant differences between low- and high-volume centers regarding patient, organ, or medication factors, which “led us to believe that high-volume centers do not necessarily select fitter patients for transplantation. Rather, they may be more experienced in supporting and treating these patients in the early posttransplantation period,” Dr. D’Souza and her associates said.
These “reassuring” findings, together with the recent development of novel plasma-cell–targeting agents such as bortezomib, suggest that it is time to consider performing a more current multicenter prospective study comparing autotransplantation against medical therapy, they added.
Early mortality after autologous hematopoietic stem cell transplantation (ASCT) for light-chain amyloidosis has declined dramatically in recent years, and 5-year survival is now deemed “excellent” at 77%, according to a report published online Sept. 14 in Journal of Clinical Oncology.
Light-chain amyloidosis results in deposition of insoluble amyloid fibers in many tissues, particularly the heart and kidneys, and can lead to organ failure and death. While medical therapies target the plasma cell clone that is the source of the amyloid, treatments have minimal effect on amyloid that has already accumulated in tissues. Alternatively, ASCT can produce durable hematologic responses and has resulted in improved function of affected organs, based on several single-center studies.
However, the only large, prospective randomized clinical trial to compare ASCT and medical therapy was done in 2007. That study showed autotransplantation carried a high (24%) early mortality, which contributed heavily to inferior overall survival, said Dr. Anita D’Souza of the division of hematology and oncology, Medical College of Wisconsin, Milwaukee, and her associates.
Since that study, supportive care during the peritransplantation period has greatly improved. Large U.S. transplant centers now report that early mortality is less than 5%.
To assess time trends in autotransplantation mortality, Dr. D’Souza and her associates analyzed data from the Center for International Blood & Marrow Transplant Research, a registry that collects transplant data from 320 centers worldwide and captures information concerning most U.S. procedures. They focused on 1,536 North American patients with light-chain amyloidosis who underwent ASCT during three successive 5-year periods: 1995-2000, 2001-2006, and 2007-2012. The median follow-up was 56 months.
Over time, 30-day mortality declined from 11% to 5% to 3%, respectively; and 100-day mortality declined from 20% to 11% to 5%. One-year overall survival rose from 75% to 85% to 90%, and 5-year overall survival improved from 55% to 61% to 77%.
Even among patients with renal amyloidosis, 3-year overall survival improved over time from 78% during the earliest study period to 89% during the most recent time period. However, mortality did not improve significantly over time among patients with cardiac involvement, which continues to be the single most-important variable associated with poor outcomes, the researchers said (J Clin Oncol. 2015 Sep 14 [doi:10.1200/JCO.2015.62.4015]).
A transplant center’s experience with this procedure proved to be of crucial importance to early patient mortality. Centers that performed a low volume of ASCT for light-chain amyloidosis, defined as fewer than four transplants per year, had a 30-day mortality of 5% and a 100-day mortality of 7%, while centers that performed more than four procedures per year had significantly lower mortalities of 1% and 3%, respectively. There were no significant differences between low- and high-volume centers regarding patient, organ, or medication factors, which “led us to believe that high-volume centers do not necessarily select fitter patients for transplantation. Rather, they may be more experienced in supporting and treating these patients in the early posttransplantation period,” Dr. D’Souza and her associates said.
These “reassuring” findings, together with the recent development of novel plasma-cell–targeting agents such as bortezomib, suggest that it is time to consider performing a more current multicenter prospective study comparing autotransplantation against medical therapy, they added.
FROM THE JOURNAL OF CLINICAL ONCOLOGY
Key clinical point: Early mortality after autologous hematopoietic stem cell transplantation for light-chain amyloidosis has declined dramatically in recent years.
Major finding: 30-day mortality declined from 11% to 5% to 3% in three successive time periods, and 100-day mortality declined from 20% to 11% to 5%.
Data source: A retrospective international cohort study of mortality outcomes in 1,536 patients with light-chain amyloidosis treated during 1995-2012 and followed for a median of 56 months.
Disclosures: This study was supported by the National Cancer Institute; the National Heart, Lung, and Blood Institute; the Health Resources and Services Administration; the Department of the Navy, the Department of Defense, other government groups, several private organizations; and numerous industry sources. Dr. D’Souza reported having no relevant financial disclosures; her associates reported ties to numerous pharmaceutical and biomedical companies.
Nail psoriasis therapies lack supporting evidence
VANCOUVER – Evidence-based therapy for nail psoriasis is in a sorry state because of a lack of consensus on a reliable nail psoriasis scoring system for use in clinical trials, according to a coauthor of the Cochrane systematic review of interventions for nail psoriasis.
“The last 12 randomized clinical trials used 21 ways of scoring the results of treatment, so comparing the studies means comparing apples to oranges. Which is the most effective treatment? What should we advise our patients? We don’t know. Comparison is impossible,” Dr. Marcel C. Pasch said at the World Congress of Dermatology.
The Cochrane report (Cochrane Database Syst Rev. 2013 Jan 31;1:CD007633) deemed the evidence for topical therapies as “inconclusive and weak,” even though topicals are the treatment mainstay for this localized expression of psoriasis. Indeed, Dr. Pasch and his coauthors found that no topical therapy has been shown effective in improving nail psoriasis. The Cochrane group concluded that just five therapies rise to the standard of being evidence based in terms of efficacy: the tumor necrosis factor (TNF) inhibitors infliximab (Remicade) and golimumab (Simponi), superficial radiation therapy, Grenz rays, and electron beam therapy. All five are strikingly impractical for use in clinical practice.
“The findings are quite disappointing because nobody sends a patient with psoriasis to the radiotherapist, and while giving an anti-TNF biologic only for the nails will be effective, at least in my country it won’t be reimbursed,” wrote Dr. Pasch, a dermatologist at Radboud University Nijmegen (the Netherlands) Medical Centre.
The presence and severity of nail psoriasis is unrelated to the severity of cutaneous psoriasis. Moreover, nail psoriasis without cutaneous involvement occurs in 5%-10% of psoriasis patients.
Since publication of the Cochrane systematic review, 12 new randomized controlled trials of treatments for nail psoriasis have appeared. Six focused on biologics: the anti-TNF agents certolizumab (Cimzia), etanercept (Enbrel), and adalimumab (Humira); the anti–interleukin-12/23 agent ustekinumab (Stelara); and the interleukin-17A inhibitor secukiumab (Cosentyx). Dr. Pasch said in his opinion all five biologics were supported by convincing studies and now can be added to the short list of evidence-based nail psoriasis therapies.
Of the six recent studies of topical therapies, two provided persuasive evidence of efficacy, in his view: tacrolimus ointment and indigo naturalis extract in oil (Lindioil), a variant of a traditional Chinese medicine therapy, which at this time isn’t commercially available.
In contrast, studies of clobetasol nail lacquer, pulsed dye laser therapy, a nail lacquer based upon chitin from crab shells, and a study of calcitriol ointment versus betamethasone dipropionate ointment failed to be convincing either because of methodologic problems or lack of efficacy, he continued.
These 12 recent randomized clinical trials utilized 21 different nail psoriasis scoring systems.
“Which scoring system is best? The answer is, we don’t know,” Dr. Pasch said.
He and his coinvestigators compared eight different scoring systems in a prospective study and concluded that the Nijmegen–Nail Psoriasis Activity Index Tool (N-NAIL), which Dr. Pasch helped develop, best reflected the clinical severity of nail psoriasis (J Am Acad Dermatol. 2014 Jun;70[6]:1061-6).
However, he added that at present there is no validated scoring system for nail psoriasis. And creation of a single validated scoring system that researchers can agree on as the standard is a prerequisite for making major advances in the treatment of nail psoriasis, in Dr. Pasch’s view.
He is so convinced of this that he has created an organization whose goal is to achieve consensus on one reliable, validated nail psoriasis scoring system for use in clinical trials. At the World Congress of Dermatology, he invited stakeholders – including academic and community dermatologists, patient organizations, and the pharmaceutical industry – to join (www.nailinitiative.org).
Session chair Dr. Peter van de Kerkhof, chairman of dermatology at Radboud University, said he sees the NAPSI (Nail Psoriasis Severity Index) being used in lots of clinical trials in psoriasis. What’s wrong with building a consensus around NAPSI? he asked.
“The problem is not the NAPSI score,” Dr. Pasch replied. “The problem is that in each trial a modified NAPSI score is used, but they are all modified in different ways. We have the single-hand NAPSI, the eight-finger NAPSI, the 10-finger NAPSI, the target NAPSI. The NAPSI doesn’t exist anymore.”
He reported receiving research grants from Pfizer and Janssen-Cilag.
VANCOUVER – Evidence-based therapy for nail psoriasis is in a sorry state because of a lack of consensus on a reliable nail psoriasis scoring system for use in clinical trials, according to a coauthor of the Cochrane systematic review of interventions for nail psoriasis.
“The last 12 randomized clinical trials used 21 ways of scoring the results of treatment, so comparing the studies means comparing apples to oranges. Which is the most effective treatment? What should we advise our patients? We don’t know. Comparison is impossible,” Dr. Marcel C. Pasch said at the World Congress of Dermatology.
The Cochrane report (Cochrane Database Syst Rev. 2013 Jan 31;1:CD007633) deemed the evidence for topical therapies as “inconclusive and weak,” even though topicals are the treatment mainstay for this localized expression of psoriasis. Indeed, Dr. Pasch and his coauthors found that no topical therapy has been shown effective in improving nail psoriasis. The Cochrane group concluded that just five therapies rise to the standard of being evidence based in terms of efficacy: the tumor necrosis factor (TNF) inhibitors infliximab (Remicade) and golimumab (Simponi), superficial radiation therapy, Grenz rays, and electron beam therapy. All five are strikingly impractical for use in clinical practice.
“The findings are quite disappointing because nobody sends a patient with psoriasis to the radiotherapist, and while giving an anti-TNF biologic only for the nails will be effective, at least in my country it won’t be reimbursed,” wrote Dr. Pasch, a dermatologist at Radboud University Nijmegen (the Netherlands) Medical Centre.
The presence and severity of nail psoriasis is unrelated to the severity of cutaneous psoriasis. Moreover, nail psoriasis without cutaneous involvement occurs in 5%-10% of psoriasis patients.
Since publication of the Cochrane systematic review, 12 new randomized controlled trials of treatments for nail psoriasis have appeared. Six focused on biologics: the anti-TNF agents certolizumab (Cimzia), etanercept (Enbrel), and adalimumab (Humira); the anti–interleukin-12/23 agent ustekinumab (Stelara); and the interleukin-17A inhibitor secukiumab (Cosentyx). Dr. Pasch said in his opinion all five biologics were supported by convincing studies and now can be added to the short list of evidence-based nail psoriasis therapies.
Of the six recent studies of topical therapies, two provided persuasive evidence of efficacy, in his view: tacrolimus ointment and indigo naturalis extract in oil (Lindioil), a variant of a traditional Chinese medicine therapy, which at this time isn’t commercially available.
In contrast, studies of clobetasol nail lacquer, pulsed dye laser therapy, a nail lacquer based upon chitin from crab shells, and a study of calcitriol ointment versus betamethasone dipropionate ointment failed to be convincing either because of methodologic problems or lack of efficacy, he continued.
These 12 recent randomized clinical trials utilized 21 different nail psoriasis scoring systems.
“Which scoring system is best? The answer is, we don’t know,” Dr. Pasch said.
He and his coinvestigators compared eight different scoring systems in a prospective study and concluded that the Nijmegen–Nail Psoriasis Activity Index Tool (N-NAIL), which Dr. Pasch helped develop, best reflected the clinical severity of nail psoriasis (J Am Acad Dermatol. 2014 Jun;70[6]:1061-6).
However, he added that at present there is no validated scoring system for nail psoriasis. And creation of a single validated scoring system that researchers can agree on as the standard is a prerequisite for making major advances in the treatment of nail psoriasis, in Dr. Pasch’s view.
He is so convinced of this that he has created an organization whose goal is to achieve consensus on one reliable, validated nail psoriasis scoring system for use in clinical trials. At the World Congress of Dermatology, he invited stakeholders – including academic and community dermatologists, patient organizations, and the pharmaceutical industry – to join (www.nailinitiative.org).
Session chair Dr. Peter van de Kerkhof, chairman of dermatology at Radboud University, said he sees the NAPSI (Nail Psoriasis Severity Index) being used in lots of clinical trials in psoriasis. What’s wrong with building a consensus around NAPSI? he asked.
“The problem is not the NAPSI score,” Dr. Pasch replied. “The problem is that in each trial a modified NAPSI score is used, but they are all modified in different ways. We have the single-hand NAPSI, the eight-finger NAPSI, the 10-finger NAPSI, the target NAPSI. The NAPSI doesn’t exist anymore.”
He reported receiving research grants from Pfizer and Janssen-Cilag.
VANCOUVER – Evidence-based therapy for nail psoriasis is in a sorry state because of a lack of consensus on a reliable nail psoriasis scoring system for use in clinical trials, according to a coauthor of the Cochrane systematic review of interventions for nail psoriasis.
“The last 12 randomized clinical trials used 21 ways of scoring the results of treatment, so comparing the studies means comparing apples to oranges. Which is the most effective treatment? What should we advise our patients? We don’t know. Comparison is impossible,” Dr. Marcel C. Pasch said at the World Congress of Dermatology.
The Cochrane report (Cochrane Database Syst Rev. 2013 Jan 31;1:CD007633) deemed the evidence for topical therapies as “inconclusive and weak,” even though topicals are the treatment mainstay for this localized expression of psoriasis. Indeed, Dr. Pasch and his coauthors found that no topical therapy has been shown effective in improving nail psoriasis. The Cochrane group concluded that just five therapies rise to the standard of being evidence based in terms of efficacy: the tumor necrosis factor (TNF) inhibitors infliximab (Remicade) and golimumab (Simponi), superficial radiation therapy, Grenz rays, and electron beam therapy. All five are strikingly impractical for use in clinical practice.
“The findings are quite disappointing because nobody sends a patient with psoriasis to the radiotherapist, and while giving an anti-TNF biologic only for the nails will be effective, at least in my country it won’t be reimbursed,” wrote Dr. Pasch, a dermatologist at Radboud University Nijmegen (the Netherlands) Medical Centre.
The presence and severity of nail psoriasis is unrelated to the severity of cutaneous psoriasis. Moreover, nail psoriasis without cutaneous involvement occurs in 5%-10% of psoriasis patients.
Since publication of the Cochrane systematic review, 12 new randomized controlled trials of treatments for nail psoriasis have appeared. Six focused on biologics: the anti-TNF agents certolizumab (Cimzia), etanercept (Enbrel), and adalimumab (Humira); the anti–interleukin-12/23 agent ustekinumab (Stelara); and the interleukin-17A inhibitor secukiumab (Cosentyx). Dr. Pasch said in his opinion all five biologics were supported by convincing studies and now can be added to the short list of evidence-based nail psoriasis therapies.
Of the six recent studies of topical therapies, two provided persuasive evidence of efficacy, in his view: tacrolimus ointment and indigo naturalis extract in oil (Lindioil), a variant of a traditional Chinese medicine therapy, which at this time isn’t commercially available.
In contrast, studies of clobetasol nail lacquer, pulsed dye laser therapy, a nail lacquer based upon chitin from crab shells, and a study of calcitriol ointment versus betamethasone dipropionate ointment failed to be convincing either because of methodologic problems or lack of efficacy, he continued.
These 12 recent randomized clinical trials utilized 21 different nail psoriasis scoring systems.
“Which scoring system is best? The answer is, we don’t know,” Dr. Pasch said.
He and his coinvestigators compared eight different scoring systems in a prospective study and concluded that the Nijmegen–Nail Psoriasis Activity Index Tool (N-NAIL), which Dr. Pasch helped develop, best reflected the clinical severity of nail psoriasis (J Am Acad Dermatol. 2014 Jun;70[6]:1061-6).
However, he added that at present there is no validated scoring system for nail psoriasis. And creation of a single validated scoring system that researchers can agree on as the standard is a prerequisite for making major advances in the treatment of nail psoriasis, in Dr. Pasch’s view.
He is so convinced of this that he has created an organization whose goal is to achieve consensus on one reliable, validated nail psoriasis scoring system for use in clinical trials. At the World Congress of Dermatology, he invited stakeholders – including academic and community dermatologists, patient organizations, and the pharmaceutical industry – to join (www.nailinitiative.org).
Session chair Dr. Peter van de Kerkhof, chairman of dermatology at Radboud University, said he sees the NAPSI (Nail Psoriasis Severity Index) being used in lots of clinical trials in psoriasis. What’s wrong with building a consensus around NAPSI? he asked.
“The problem is not the NAPSI score,” Dr. Pasch replied. “The problem is that in each trial a modified NAPSI score is used, but they are all modified in different ways. We have the single-hand NAPSI, the eight-finger NAPSI, the 10-finger NAPSI, the target NAPSI. The NAPSI doesn’t exist anymore.”
He reported receiving research grants from Pfizer and Janssen-Cilag.
EXPERT ANALYSIS FROM WCD 2015
FDA approves product for hemophilia A
The US Food and Drug Administration (FDA) has approved the recombinant factor VIII product simoctocog alfa (Nuwiq) for adults and children with hemophilia A.
The approval includes on-demand treatment and control of bleeding episodes, routine prophylaxis to reduce the frequency of bleeding episodes, and perioperative management of bleeding.
Simoctocog alfa is the first B-domain-deleted recombinant factor VIII product derived from a human cell line—not chemically modified or fused with another protein—designed to treat hemophilia A.
Simoctocog alfa is already approved for use in the European Union, Argentina, Australia, and Canada.
In the US, simoctocog alfa is being developed by Octapharma USA, a subsidiary of Octapharma AG.
According to Octapharma USA, simoctocog alfa should be available in the US by early 2016. The company plans to offer hemophilia A patients educational and support services in connection with the product.
Trials of simoctocog alfa
Simoctocog alfa has been evaluated for safety in 5 prospective trials and for efficacy in 3 prospective studies.
A total of 135 previously treated patients with severe hemophilia A have received simoctocog alfa across all the studies. This includes 74 adults, 3 adolescents between ages 12 and 17, and 58 pediatric patients between ages 2 and 11.
The patients were treated with a total of 16,134 infusions over 15,950 exposure days.
In a study of adults, the overall prophylactic efficacy of simoctocog alfa for spontaneous bleeds was rated “excellent” or “good” in 92% of patients. In a study of children, prophylactic efficacy for spontaneous bleeds was rated “excellent” or “good” in 97% of patients.
The mean annualized bleeding rates for spontaneous bleeds during prophylaxis were approximately 1.5 in children and 1.2 in adults.
For hemophilia A patients receiving simoctocog alfa prophylaxis compared to on-demand treatment, the annualized bleeding rates were reduced 96% for adults and 93% for children.
Treatment of breakthrough bleeds during simoctocog alfa prophylaxis was rated as “excellent” or “good” in 100% of bleeds (30/30) in adults and 82% of bleeds (89/108) in children.
For on-demand treatment with simoctocog alfa in 20 adults and 2 adolescents, efficacy for the treatment of bleeds was considered “excellent” or “good” in 94% of bleeds (931/986).
The overall efficacy in surgical prophylaxis was rated “excellent” or “good” in 97% of procedures using simoctocog alfa (32/33).
For all the trials of simoctocog alfa, there were 7 adverse events reported. Each of these events occurred once, with a rate of 0.7% across all 135 patients. The events were paresthesia, headache, injection site inflammation, injection site pain, back pain, vertigo, and dry mouth.
Non-neutralizing anti-factor VIII antibodies (without inhibitory activity as measured by the modified Bethesda assay) were reported in 4 patients (3%). Three of the 4 patients had pre-existing non-neutralizing antibodies prior to simoctocog alfa exposure.
For more details on simoctocog alfa, see the full prescribing information, available at www.octapharmausa.com. ![]()
The US Food and Drug Administration (FDA) has approved the recombinant factor VIII product simoctocog alfa (Nuwiq) for adults and children with hemophilia A.
The approval includes on-demand treatment and control of bleeding episodes, routine prophylaxis to reduce the frequency of bleeding episodes, and perioperative management of bleeding.
Simoctocog alfa is the first B-domain-deleted recombinant factor VIII product derived from a human cell line—not chemically modified or fused with another protein—designed to treat hemophilia A.
Simoctocog alfa is already approved for use in the European Union, Argentina, Australia, and Canada.
In the US, simoctocog alfa is being developed by Octapharma USA, a subsidiary of Octapharma AG.
According to Octapharma USA, simoctocog alfa should be available in the US by early 2016. The company plans to offer hemophilia A patients educational and support services in connection with the product.
Trials of simoctocog alfa
Simoctocog alfa has been evaluated for safety in 5 prospective trials and for efficacy in 3 prospective studies.
A total of 135 previously treated patients with severe hemophilia A have received simoctocog alfa across all the studies. This includes 74 adults, 3 adolescents between ages 12 and 17, and 58 pediatric patients between ages 2 and 11.
The patients were treated with a total of 16,134 infusions over 15,950 exposure days.
In a study of adults, the overall prophylactic efficacy of simoctocog alfa for spontaneous bleeds was rated “excellent” or “good” in 92% of patients. In a study of children, prophylactic efficacy for spontaneous bleeds was rated “excellent” or “good” in 97% of patients.
The mean annualized bleeding rates for spontaneous bleeds during prophylaxis were approximately 1.5 in children and 1.2 in adults.
For hemophilia A patients receiving simoctocog alfa prophylaxis compared to on-demand treatment, the annualized bleeding rates were reduced 96% for adults and 93% for children.
Treatment of breakthrough bleeds during simoctocog alfa prophylaxis was rated as “excellent” or “good” in 100% of bleeds (30/30) in adults and 82% of bleeds (89/108) in children.
For on-demand treatment with simoctocog alfa in 20 adults and 2 adolescents, efficacy for the treatment of bleeds was considered “excellent” or “good” in 94% of bleeds (931/986).
The overall efficacy in surgical prophylaxis was rated “excellent” or “good” in 97% of procedures using simoctocog alfa (32/33).
For all the trials of simoctocog alfa, there were 7 adverse events reported. Each of these events occurred once, with a rate of 0.7% across all 135 patients. The events were paresthesia, headache, injection site inflammation, injection site pain, back pain, vertigo, and dry mouth.
Non-neutralizing anti-factor VIII antibodies (without inhibitory activity as measured by the modified Bethesda assay) were reported in 4 patients (3%). Three of the 4 patients had pre-existing non-neutralizing antibodies prior to simoctocog alfa exposure.
For more details on simoctocog alfa, see the full prescribing information, available at www.octapharmausa.com. ![]()
The US Food and Drug Administration (FDA) has approved the recombinant factor VIII product simoctocog alfa (Nuwiq) for adults and children with hemophilia A.
The approval includes on-demand treatment and control of bleeding episodes, routine prophylaxis to reduce the frequency of bleeding episodes, and perioperative management of bleeding.
Simoctocog alfa is the first B-domain-deleted recombinant factor VIII product derived from a human cell line—not chemically modified or fused with another protein—designed to treat hemophilia A.
Simoctocog alfa is already approved for use in the European Union, Argentina, Australia, and Canada.
In the US, simoctocog alfa is being developed by Octapharma USA, a subsidiary of Octapharma AG.
According to Octapharma USA, simoctocog alfa should be available in the US by early 2016. The company plans to offer hemophilia A patients educational and support services in connection with the product.
Trials of simoctocog alfa
Simoctocog alfa has been evaluated for safety in 5 prospective trials and for efficacy in 3 prospective studies.
A total of 135 previously treated patients with severe hemophilia A have received simoctocog alfa across all the studies. This includes 74 adults, 3 adolescents between ages 12 and 17, and 58 pediatric patients between ages 2 and 11.
The patients were treated with a total of 16,134 infusions over 15,950 exposure days.
In a study of adults, the overall prophylactic efficacy of simoctocog alfa for spontaneous bleeds was rated “excellent” or “good” in 92% of patients. In a study of children, prophylactic efficacy for spontaneous bleeds was rated “excellent” or “good” in 97% of patients.
The mean annualized bleeding rates for spontaneous bleeds during prophylaxis were approximately 1.5 in children and 1.2 in adults.
For hemophilia A patients receiving simoctocog alfa prophylaxis compared to on-demand treatment, the annualized bleeding rates were reduced 96% for adults and 93% for children.
Treatment of breakthrough bleeds during simoctocog alfa prophylaxis was rated as “excellent” or “good” in 100% of bleeds (30/30) in adults and 82% of bleeds (89/108) in children.
For on-demand treatment with simoctocog alfa in 20 adults and 2 adolescents, efficacy for the treatment of bleeds was considered “excellent” or “good” in 94% of bleeds (931/986).
The overall efficacy in surgical prophylaxis was rated “excellent” or “good” in 97% of procedures using simoctocog alfa (32/33).
For all the trials of simoctocog alfa, there were 7 adverse events reported. Each of these events occurred once, with a rate of 0.7% across all 135 patients. The events were paresthesia, headache, injection site inflammation, injection site pain, back pain, vertigo, and dry mouth.
Non-neutralizing anti-factor VIII antibodies (without inhibitory activity as measured by the modified Bethesda assay) were reported in 4 patients (3%). Three of the 4 patients had pre-existing non-neutralizing antibodies prior to simoctocog alfa exposure.
For more details on simoctocog alfa, see the full prescribing information, available at www.octapharmausa.com. ![]()
Acne and Melanoma: What to Do With the Reported Connection?
Dermatologists have become accustomed to reading about the associations of dermatologic disease with extracutaneous comorbidities (psoriasis certainly takes the lead). One may see the headline “Study finds increased risk for melanoma in female acne patients” and say “Sure, why not?” However, before we all jump on the association bandwagon, let’s better appreciate this finding.
A study published online January 8 in Cancer by Zhang et al followed 99,128 female nurses in the Nurses’ Health Study II cohort for 20 years. This cohort has been utilized for numerous prospective studies over the year. Even after adjusting for known risk factors, investigators discovered that women with a history of severe cystic teenage acne had a hazard ratio of 1.44 for melanoma. The authors replicated the association with an independent melanoma case-control study of 930 cases and 1026 controls, finding an odds ratio of 1.27. They also found that individuals with teenage acne were more likely to have nevi (52.7% vs 50.1% in the cohort study; 55.2% vs 45.1% in the control study).
These data points ultimately led the team to conclude that acne may serve as an independent risk factor for melanoma, attributing androgens in female acne as a possible and plausible explanation due to their known effect on telomere elongation; melanocytes with longer telomere lengths have more opportunity to develop mutations, which could lead to malignant transformation, as the extended length ultimately delays initiation of cellular senescence. The longer these cells are “awake,” more moles can form, which means more room for trouble.
What’s the issue?
The size of this cohort certainly gives credibility to the data and statistics presented. Although the study is powered very well by the numbers, it is a unique cohort because all participants were nurses, narrowing down the demographics to some degree given general patterns, behaviors, and backgrounds when it comes to this group, an issue that has been previously raised with using this cohort. That said, more research is certainly warranted to elucidate the proposed mechanism and further clarify the association.
From a purely clinical standpoint, this paper is powerful ammo that can be used in our war against skin cancer. This very large cohort probably does not follow the American Academy of Dermatology guidelines for sun protection, skin cancer prevention, and surveillance. It could be a nice tidbit for patients at the end of your spiel on acne and then work in the photoprotection discussion, something we haven’t been the best at according to a recent study published in JAMA Dermatology (JAMA Dermatol. 2014;150:51-55)! Would it be such a bad thing if this paper helped us encourage all women with moderate to severe acne to undertake more effective sun-safe behaviors and to visit their dermatologist every year for total-body skin examinations?
Dermatologists have become accustomed to reading about the associations of dermatologic disease with extracutaneous comorbidities (psoriasis certainly takes the lead). One may see the headline “Study finds increased risk for melanoma in female acne patients” and say “Sure, why not?” However, before we all jump on the association bandwagon, let’s better appreciate this finding.
A study published online January 8 in Cancer by Zhang et al followed 99,128 female nurses in the Nurses’ Health Study II cohort for 20 years. This cohort has been utilized for numerous prospective studies over the year. Even after adjusting for known risk factors, investigators discovered that women with a history of severe cystic teenage acne had a hazard ratio of 1.44 for melanoma. The authors replicated the association with an independent melanoma case-control study of 930 cases and 1026 controls, finding an odds ratio of 1.27. They also found that individuals with teenage acne were more likely to have nevi (52.7% vs 50.1% in the cohort study; 55.2% vs 45.1% in the control study).
These data points ultimately led the team to conclude that acne may serve as an independent risk factor for melanoma, attributing androgens in female acne as a possible and plausible explanation due to their known effect on telomere elongation; melanocytes with longer telomere lengths have more opportunity to develop mutations, which could lead to malignant transformation, as the extended length ultimately delays initiation of cellular senescence. The longer these cells are “awake,” more moles can form, which means more room for trouble.
What’s the issue?
The size of this cohort certainly gives credibility to the data and statistics presented. Although the study is powered very well by the numbers, it is a unique cohort because all participants were nurses, narrowing down the demographics to some degree given general patterns, behaviors, and backgrounds when it comes to this group, an issue that has been previously raised with using this cohort. That said, more research is certainly warranted to elucidate the proposed mechanism and further clarify the association.
From a purely clinical standpoint, this paper is powerful ammo that can be used in our war against skin cancer. This very large cohort probably does not follow the American Academy of Dermatology guidelines for sun protection, skin cancer prevention, and surveillance. It could be a nice tidbit for patients at the end of your spiel on acne and then work in the photoprotection discussion, something we haven’t been the best at according to a recent study published in JAMA Dermatology (JAMA Dermatol. 2014;150:51-55)! Would it be such a bad thing if this paper helped us encourage all women with moderate to severe acne to undertake more effective sun-safe behaviors and to visit their dermatologist every year for total-body skin examinations?
Dermatologists have become accustomed to reading about the associations of dermatologic disease with extracutaneous comorbidities (psoriasis certainly takes the lead). One may see the headline “Study finds increased risk for melanoma in female acne patients” and say “Sure, why not?” However, before we all jump on the association bandwagon, let’s better appreciate this finding.
A study published online January 8 in Cancer by Zhang et al followed 99,128 female nurses in the Nurses’ Health Study II cohort for 20 years. This cohort has been utilized for numerous prospective studies over the year. Even after adjusting for known risk factors, investigators discovered that women with a history of severe cystic teenage acne had a hazard ratio of 1.44 for melanoma. The authors replicated the association with an independent melanoma case-control study of 930 cases and 1026 controls, finding an odds ratio of 1.27. They also found that individuals with teenage acne were more likely to have nevi (52.7% vs 50.1% in the cohort study; 55.2% vs 45.1% in the control study).
These data points ultimately led the team to conclude that acne may serve as an independent risk factor for melanoma, attributing androgens in female acne as a possible and plausible explanation due to their known effect on telomere elongation; melanocytes with longer telomere lengths have more opportunity to develop mutations, which could lead to malignant transformation, as the extended length ultimately delays initiation of cellular senescence. The longer these cells are “awake,” more moles can form, which means more room for trouble.
What’s the issue?
The size of this cohort certainly gives credibility to the data and statistics presented. Although the study is powered very well by the numbers, it is a unique cohort because all participants were nurses, narrowing down the demographics to some degree given general patterns, behaviors, and backgrounds when it comes to this group, an issue that has been previously raised with using this cohort. That said, more research is certainly warranted to elucidate the proposed mechanism and further clarify the association.
From a purely clinical standpoint, this paper is powerful ammo that can be used in our war against skin cancer. This very large cohort probably does not follow the American Academy of Dermatology guidelines for sun protection, skin cancer prevention, and surveillance. It could be a nice tidbit for patients at the end of your spiel on acne and then work in the photoprotection discussion, something we haven’t been the best at according to a recent study published in JAMA Dermatology (JAMA Dermatol. 2014;150:51-55)! Would it be such a bad thing if this paper helped us encourage all women with moderate to severe acne to undertake more effective sun-safe behaviors and to visit their dermatologist every year for total-body skin examinations?
Coating on Endovascular Devices Could Cause Stroke or Death
NEW YORK - Coating on endovascular devices is associated with embolization and microvascular occlusion leading to purpura or livedo racemosa, according to a new report.
Dr. Alina Bridges, of the Department of Dermatology at Mayo Clinic in Rochester, Minnesota, said by email that the study was conducted "to make clinicians and pathologists aware of this underrecognized phenomenon of iatrogenic hydrophilic polymer gel embolization that can involve the skin and present with purpura."
The phenomenon "has distinctive microscopic morphology and potential for internal organ involvement," she added.
Endovascular devices commonly are coated with hydrophilic polymer gels to improve maneuverability and prevent vasospasm. However, there are reports of the coating embolizing, resulting
in severe reactions such as stroke, pulmonary infarction, and death.
Dr. Bridges and colleagues presented a case study of eight patients with livedo racemosa and purpura after an endovascular procedure. The patients had punch biopsies obtained with hematoxylin-eosin-stained sections.
The study subjects were between 58 and 81 years old, most were men and most had previous endovascular procedures and multiple comorbidities, according to an article online August 11 in the Journal of the American Academy of Dermatology.
In all but one patient, the cutaneous lesions were unilateral and all but two were asymptomatic. Six patients presented with livedo racemosa and two with purpura.
All cases demonstrated pauci-inflammatory occlusion in the mid-dermal and small superficial vessels. Likewise, histopathologic evidence was consistent with previously reported cases of emboli secondary to hydrophilic gel polymer.
There was no evidence of embolic sequela to the organs in three patients. However, one patient died of unknown reasons and four patients experienced postoperative complications including spinal cord ischemia, acute kidney injury, and cerebral infarction. In all cases, the cutaneous manifestations resolved without intervention.
The authors say they suspect the incidence of this type of embolization is underrecognized, especially with the common use of hydrophilic polymer gel coatings.
"This report highlights the importance of awareness of this rare iatrogenic complication and the importance of investigating a patient's clinical history to determine if there had been recent exposure to an intravascular device with a hydrophilic coating," Dr. Bridges said.
"While the use of polymer-coated devices offers several advantages, clinicians must be aware of their potential complications, including stroke, myocardial and pulmonary infarction, gangrene, and/or death," she said.
The authors reported no funding or conflicts of interest.
NEW YORK - Coating on endovascular devices is associated with embolization and microvascular occlusion leading to purpura or livedo racemosa, according to a new report.
Dr. Alina Bridges, of the Department of Dermatology at Mayo Clinic in Rochester, Minnesota, said by email that the study was conducted "to make clinicians and pathologists aware of this underrecognized phenomenon of iatrogenic hydrophilic polymer gel embolization that can involve the skin and present with purpura."
The phenomenon "has distinctive microscopic morphology and potential for internal organ involvement," she added.
Endovascular devices commonly are coated with hydrophilic polymer gels to improve maneuverability and prevent vasospasm. However, there are reports of the coating embolizing, resulting
in severe reactions such as stroke, pulmonary infarction, and death.
Dr. Bridges and colleagues presented a case study of eight patients with livedo racemosa and purpura after an endovascular procedure. The patients had punch biopsies obtained with hematoxylin-eosin-stained sections.
The study subjects were between 58 and 81 years old, most were men and most had previous endovascular procedures and multiple comorbidities, according to an article online August 11 in the Journal of the American Academy of Dermatology.
In all but one patient, the cutaneous lesions were unilateral and all but two were asymptomatic. Six patients presented with livedo racemosa and two with purpura.
All cases demonstrated pauci-inflammatory occlusion in the mid-dermal and small superficial vessels. Likewise, histopathologic evidence was consistent with previously reported cases of emboli secondary to hydrophilic gel polymer.
There was no evidence of embolic sequela to the organs in three patients. However, one patient died of unknown reasons and four patients experienced postoperative complications including spinal cord ischemia, acute kidney injury, and cerebral infarction. In all cases, the cutaneous manifestations resolved without intervention.
The authors say they suspect the incidence of this type of embolization is underrecognized, especially with the common use of hydrophilic polymer gel coatings.
"This report highlights the importance of awareness of this rare iatrogenic complication and the importance of investigating a patient's clinical history to determine if there had been recent exposure to an intravascular device with a hydrophilic coating," Dr. Bridges said.
"While the use of polymer-coated devices offers several advantages, clinicians must be aware of their potential complications, including stroke, myocardial and pulmonary infarction, gangrene, and/or death," she said.
The authors reported no funding or conflicts of interest.
NEW YORK - Coating on endovascular devices is associated with embolization and microvascular occlusion leading to purpura or livedo racemosa, according to a new report.
Dr. Alina Bridges, of the Department of Dermatology at Mayo Clinic in Rochester, Minnesota, said by email that the study was conducted "to make clinicians and pathologists aware of this underrecognized phenomenon of iatrogenic hydrophilic polymer gel embolization that can involve the skin and present with purpura."
The phenomenon "has distinctive microscopic morphology and potential for internal organ involvement," she added.
Endovascular devices commonly are coated with hydrophilic polymer gels to improve maneuverability and prevent vasospasm. However, there are reports of the coating embolizing, resulting
in severe reactions such as stroke, pulmonary infarction, and death.
Dr. Bridges and colleagues presented a case study of eight patients with livedo racemosa and purpura after an endovascular procedure. The patients had punch biopsies obtained with hematoxylin-eosin-stained sections.
The study subjects were between 58 and 81 years old, most were men and most had previous endovascular procedures and multiple comorbidities, according to an article online August 11 in the Journal of the American Academy of Dermatology.
In all but one patient, the cutaneous lesions were unilateral and all but two were asymptomatic. Six patients presented with livedo racemosa and two with purpura.
All cases demonstrated pauci-inflammatory occlusion in the mid-dermal and small superficial vessels. Likewise, histopathologic evidence was consistent with previously reported cases of emboli secondary to hydrophilic gel polymer.
There was no evidence of embolic sequela to the organs in three patients. However, one patient died of unknown reasons and four patients experienced postoperative complications including spinal cord ischemia, acute kidney injury, and cerebral infarction. In all cases, the cutaneous manifestations resolved without intervention.
The authors say they suspect the incidence of this type of embolization is underrecognized, especially with the common use of hydrophilic polymer gel coatings.
"This report highlights the importance of awareness of this rare iatrogenic complication and the importance of investigating a patient's clinical history to determine if there had been recent exposure to an intravascular device with a hydrophilic coating," Dr. Bridges said.
"While the use of polymer-coated devices offers several advantages, clinicians must be aware of their potential complications, including stroke, myocardial and pulmonary infarction, gangrene, and/or death," she said.
The authors reported no funding or conflicts of interest.