User login
Testing for BRCA1/BRCA2 in the VA
Background: BRCA 1/2 genes have a critical role in DNA repair and genome stability. BRCA mutations are responsible for the majority of hereditary breast and ovarian cancer (HBOC) syndromes, and BRCA mutations increase susceptibility to several cancers, including male breast cancer, ovarian, prostate, pancreatic, and possibly glioblastoma and melanoma. BRCA2 mutations are responsible for about 5% to 10% of all breast cancers. With a growing number of laboratories offering BRCA testing and as clinicians move toward multigene panels for testing, we sought to assess the baseline use of BRCA testing in the VHA.
Methods: Data identifying veterans who underwent BRCA testing were obtained from Ambry, GeneDX, Myriad, and Quest. We merged these data with the VA corporate data warehouse to identify patient and site of characteristics associated with testing.
Results: There were 868 veterans who underwent BRCA testing from January 2012 until December 2013. Of those tested, 141 were male and 727 were female veterans. The age of men tested ranged from 24 years to 80 years; the mean was 62 years. The age of women tested ranged from 21 years to 77 years; the mean was 46 years. Credentials of clinicians ordering the tests included advanced practice nurses (8%), physicians (87%), physician assistants (2%), and genetic counselors (3%). Veterans were tested in 46 of the 52 states. However, the number of veterans per state ranged from a high in California of 43 to just 1 veteran tested in many states. Ongoing analysis is examining clinical characteristics and diagnosis codes of veterans tested to identify whether testing was concordant with guidelines.
Background: BRCA 1/2 genes have a critical role in DNA repair and genome stability. BRCA mutations are responsible for the majority of hereditary breast and ovarian cancer (HBOC) syndromes, and BRCA mutations increase susceptibility to several cancers, including male breast cancer, ovarian, prostate, pancreatic, and possibly glioblastoma and melanoma. BRCA2 mutations are responsible for about 5% to 10% of all breast cancers. With a growing number of laboratories offering BRCA testing and as clinicians move toward multigene panels for testing, we sought to assess the baseline use of BRCA testing in the VHA.
Methods: Data identifying veterans who underwent BRCA testing were obtained from Ambry, GeneDX, Myriad, and Quest. We merged these data with the VA corporate data warehouse to identify patient and site of characteristics associated with testing.
Results: There were 868 veterans who underwent BRCA testing from January 2012 until December 2013. Of those tested, 141 were male and 727 were female veterans. The age of men tested ranged from 24 years to 80 years; the mean was 62 years. The age of women tested ranged from 21 years to 77 years; the mean was 46 years. Credentials of clinicians ordering the tests included advanced practice nurses (8%), physicians (87%), physician assistants (2%), and genetic counselors (3%). Veterans were tested in 46 of the 52 states. However, the number of veterans per state ranged from a high in California of 43 to just 1 veteran tested in many states. Ongoing analysis is examining clinical characteristics and diagnosis codes of veterans tested to identify whether testing was concordant with guidelines.
Background: BRCA 1/2 genes have a critical role in DNA repair and genome stability. BRCA mutations are responsible for the majority of hereditary breast and ovarian cancer (HBOC) syndromes, and BRCA mutations increase susceptibility to several cancers, including male breast cancer, ovarian, prostate, pancreatic, and possibly glioblastoma and melanoma. BRCA2 mutations are responsible for about 5% to 10% of all breast cancers. With a growing number of laboratories offering BRCA testing and as clinicians move toward multigene panels for testing, we sought to assess the baseline use of BRCA testing in the VHA.
Methods: Data identifying veterans who underwent BRCA testing were obtained from Ambry, GeneDX, Myriad, and Quest. We merged these data with the VA corporate data warehouse to identify patient and site of characteristics associated with testing.
Results: There were 868 veterans who underwent BRCA testing from January 2012 until December 2013. Of those tested, 141 were male and 727 were female veterans. The age of men tested ranged from 24 years to 80 years; the mean was 62 years. The age of women tested ranged from 21 years to 77 years; the mean was 46 years. Credentials of clinicians ordering the tests included advanced practice nurses (8%), physicians (87%), physician assistants (2%), and genetic counselors (3%). Veterans were tested in 46 of the 52 states. However, the number of veterans per state ranged from a high in California of 43 to just 1 veteran tested in many states. Ongoing analysis is examining clinical characteristics and diagnosis codes of veterans tested to identify whether testing was concordant with guidelines.
Pilot Study: Is In-Network Care for Veterans Diagnosed With Malignant Pleural Mesothelioma (MPM) Feasible With VAHCS?
Purpose: To assess feasibility of open access and state-of-the-art in-network care for veterans diagnosed with malignant pleural mesothelioma (MPM) in the framework of the VA health care system and according to the patient-centered international mesothelioma program (IMP) care model.
Background: Malignant pleural mesothelioma is a rare disease that disproportionately affects veterans. According to the latest guidelines established by the International Mesothelioma Interest Group, maximal cytoreductive surgery has been shown to be superior to chemotherapy alone in patients with loco-regional MPM. Access to state-of-the-art mesothelioma care for veterans is limited to a handful of VA medical centers, and the West Roxbury VA Campus (WRVA) is uniquely positioned to routinely perform surgery for MPM.
Methods: We welcome all in-network, veteran referrals nationally. After initial phone triage or e-consult, qualified veterans are advised to register at the WRVA. A multidisciplinary team, including experienced MPM thoracic surgeons, pathologists, and radiologists, reviews each case and tailors an individual treatment plan. Controversial cases are further reviewed at the IMP. After thorough evaluation, veterans are advised by the surgeon to send additional documents if needed, come to Boston for an onsite consultation, or to continue local treatment.
Results: Between 2011 and 2015, we phone-triaged 55 veter-ans with suspected MPM. Forty-two patients had confirmed MPM, while 9 (4 unknown) had other thoracic pathology, including desmoplastic mesothelioma, sclerosing pleuritis, poorly differentiated non-small cell lung cancer, and peritoneal mesothelioma. We recommended surgical consultation at our VA for 46 veterans, of which 35 travelled to the WRVA from 21 states, with a median age of 63 years (all male), travelling an average distance of 961 miles. Seventeen veterans flew, 16 drove, and 2 came by train. Ultimately, 29 had MPM and 6 were found to have other diseases or the pathologic diagnosis is pending. The average time from initial contact to arrival at Boston was 15.4 days. Therapeutic recommendations were changed in 22 cases.
Conclusions: A national open-access program for suspected MPM is initially feasible at the WRVA. Treatment choices were altered in 63% of cases. This provides specialty in-network care, which is not locally available, regardless of distance traveled and cost required.
Purpose: To assess feasibility of open access and state-of-the-art in-network care for veterans diagnosed with malignant pleural mesothelioma (MPM) in the framework of the VA health care system and according to the patient-centered international mesothelioma program (IMP) care model.
Background: Malignant pleural mesothelioma is a rare disease that disproportionately affects veterans. According to the latest guidelines established by the International Mesothelioma Interest Group, maximal cytoreductive surgery has been shown to be superior to chemotherapy alone in patients with loco-regional MPM. Access to state-of-the-art mesothelioma care for veterans is limited to a handful of VA medical centers, and the West Roxbury VA Campus (WRVA) is uniquely positioned to routinely perform surgery for MPM.
Methods: We welcome all in-network, veteran referrals nationally. After initial phone triage or e-consult, qualified veterans are advised to register at the WRVA. A multidisciplinary team, including experienced MPM thoracic surgeons, pathologists, and radiologists, reviews each case and tailors an individual treatment plan. Controversial cases are further reviewed at the IMP. After thorough evaluation, veterans are advised by the surgeon to send additional documents if needed, come to Boston for an onsite consultation, or to continue local treatment.
Results: Between 2011 and 2015, we phone-triaged 55 veter-ans with suspected MPM. Forty-two patients had confirmed MPM, while 9 (4 unknown) had other thoracic pathology, including desmoplastic mesothelioma, sclerosing pleuritis, poorly differentiated non-small cell lung cancer, and peritoneal mesothelioma. We recommended surgical consultation at our VA for 46 veterans, of which 35 travelled to the WRVA from 21 states, with a median age of 63 years (all male), travelling an average distance of 961 miles. Seventeen veterans flew, 16 drove, and 2 came by train. Ultimately, 29 had MPM and 6 were found to have other diseases or the pathologic diagnosis is pending. The average time from initial contact to arrival at Boston was 15.4 days. Therapeutic recommendations were changed in 22 cases.
Conclusions: A national open-access program for suspected MPM is initially feasible at the WRVA. Treatment choices were altered in 63% of cases. This provides specialty in-network care, which is not locally available, regardless of distance traveled and cost required.
Purpose: To assess feasibility of open access and state-of-the-art in-network care for veterans diagnosed with malignant pleural mesothelioma (MPM) in the framework of the VA health care system and according to the patient-centered international mesothelioma program (IMP) care model.
Background: Malignant pleural mesothelioma is a rare disease that disproportionately affects veterans. According to the latest guidelines established by the International Mesothelioma Interest Group, maximal cytoreductive surgery has been shown to be superior to chemotherapy alone in patients with loco-regional MPM. Access to state-of-the-art mesothelioma care for veterans is limited to a handful of VA medical centers, and the West Roxbury VA Campus (WRVA) is uniquely positioned to routinely perform surgery for MPM.
Methods: We welcome all in-network, veteran referrals nationally. After initial phone triage or e-consult, qualified veterans are advised to register at the WRVA. A multidisciplinary team, including experienced MPM thoracic surgeons, pathologists, and radiologists, reviews each case and tailors an individual treatment plan. Controversial cases are further reviewed at the IMP. After thorough evaluation, veterans are advised by the surgeon to send additional documents if needed, come to Boston for an onsite consultation, or to continue local treatment.
Results: Between 2011 and 2015, we phone-triaged 55 veter-ans with suspected MPM. Forty-two patients had confirmed MPM, while 9 (4 unknown) had other thoracic pathology, including desmoplastic mesothelioma, sclerosing pleuritis, poorly differentiated non-small cell lung cancer, and peritoneal mesothelioma. We recommended surgical consultation at our VA for 46 veterans, of which 35 travelled to the WRVA from 21 states, with a median age of 63 years (all male), travelling an average distance of 961 miles. Seventeen veterans flew, 16 drove, and 2 came by train. Ultimately, 29 had MPM and 6 were found to have other diseases or the pathologic diagnosis is pending. The average time from initial contact to arrival at Boston was 15.4 days. Therapeutic recommendations were changed in 22 cases.
Conclusions: A national open-access program for suspected MPM is initially feasible at the WRVA. Treatment choices were altered in 63% of cases. This provides specialty in-network care, which is not locally available, regardless of distance traveled and cost required.
Discrepancy in Histologic Subtypes of Non-Small Cell Lung Cancer Between Veterans and the General Population
Background: Lung cancer is the most common cause of cancer deaths in the U.S. The burden is even more pronounced in veterans due to higher prevalence of smoking in this subset of the population. Since the 1980s, adenocarcinoma has become more common than squamous cell carcinoma (SCC). Some data show that this is not the case among veterans. In this study, we compare the distribution of lung adenocarcinoma and SCC in the veteran population with that of the general population. We also looked at the survival of patients with different histologies in the VA population.
Methods: The electronic charts of 649 patients diagnosed with non-small cell lung cancer (NSCLC) from January 2000 until December 2010 at the Albany Stratton VA Medical Center were retrospectively reviewed. Patient demographics, clinical characteristics, and survival data were collected. Data were entered and analyzed using SPSS (IBM, Armonk, NY). Survival was estimated using Kaplan Meier method. A value of P < .05 was considered statistically significant. Data were compared with data from the Surveillance, Epidemiology, and End Results database. The study was approved by the VA IRB.
Results: A total of 649 patients with NSCLC were included.The proportion of adenocarcinoma was significantly higher in the general population, while that of SCC was significantly higher in the VA population. There were no differences in chemotherapy, radiation therapy, surgery rates, and age at diagnosis among the different histologies in the VA population. Patients with adenocarcinoma presented more frequently with metastatic disease than did patients with SCC (45.4% vs 29.5%, P < .001). The 5-year survival rate tended to be higher in SCC than in adenocarcinoma (15.7% vs 13.0%, P = .325).
Conclusions: Our study showed a significant difference in histologic subtypes of NSCLC between veterans and the general population, with SCC having a higher incidence rate compared with that of adenocarcinoma among veterans. Adenocarcinoma tended to present at a more advanced stage than did SCC. However, this did not translate into a significant survival difference.
Background: Lung cancer is the most common cause of cancer deaths in the U.S. The burden is even more pronounced in veterans due to higher prevalence of smoking in this subset of the population. Since the 1980s, adenocarcinoma has become more common than squamous cell carcinoma (SCC). Some data show that this is not the case among veterans. In this study, we compare the distribution of lung adenocarcinoma and SCC in the veteran population with that of the general population. We also looked at the survival of patients with different histologies in the VA population.
Methods: The electronic charts of 649 patients diagnosed with non-small cell lung cancer (NSCLC) from January 2000 until December 2010 at the Albany Stratton VA Medical Center were retrospectively reviewed. Patient demographics, clinical characteristics, and survival data were collected. Data were entered and analyzed using SPSS (IBM, Armonk, NY). Survival was estimated using Kaplan Meier method. A value of P < .05 was considered statistically significant. Data were compared with data from the Surveillance, Epidemiology, and End Results database. The study was approved by the VA IRB.
Results: A total of 649 patients with NSCLC were included.The proportion of adenocarcinoma was significantly higher in the general population, while that of SCC was significantly higher in the VA population. There were no differences in chemotherapy, radiation therapy, surgery rates, and age at diagnosis among the different histologies in the VA population. Patients with adenocarcinoma presented more frequently with metastatic disease than did patients with SCC (45.4% vs 29.5%, P < .001). The 5-year survival rate tended to be higher in SCC than in adenocarcinoma (15.7% vs 13.0%, P = .325).
Conclusions: Our study showed a significant difference in histologic subtypes of NSCLC between veterans and the general population, with SCC having a higher incidence rate compared with that of adenocarcinoma among veterans. Adenocarcinoma tended to present at a more advanced stage than did SCC. However, this did not translate into a significant survival difference.
Background: Lung cancer is the most common cause of cancer deaths in the U.S. The burden is even more pronounced in veterans due to higher prevalence of smoking in this subset of the population. Since the 1980s, adenocarcinoma has become more common than squamous cell carcinoma (SCC). Some data show that this is not the case among veterans. In this study, we compare the distribution of lung adenocarcinoma and SCC in the veteran population with that of the general population. We also looked at the survival of patients with different histologies in the VA population.
Methods: The electronic charts of 649 patients diagnosed with non-small cell lung cancer (NSCLC) from January 2000 until December 2010 at the Albany Stratton VA Medical Center were retrospectively reviewed. Patient demographics, clinical characteristics, and survival data were collected. Data were entered and analyzed using SPSS (IBM, Armonk, NY). Survival was estimated using Kaplan Meier method. A value of P < .05 was considered statistically significant. Data were compared with data from the Surveillance, Epidemiology, and End Results database. The study was approved by the VA IRB.
Results: A total of 649 patients with NSCLC were included.The proportion of adenocarcinoma was significantly higher in the general population, while that of SCC was significantly higher in the VA population. There were no differences in chemotherapy, radiation therapy, surgery rates, and age at diagnosis among the different histologies in the VA population. Patients with adenocarcinoma presented more frequently with metastatic disease than did patients with SCC (45.4% vs 29.5%, P < .001). The 5-year survival rate tended to be higher in SCC than in adenocarcinoma (15.7% vs 13.0%, P = .325).
Conclusions: Our study showed a significant difference in histologic subtypes of NSCLC between veterans and the general population, with SCC having a higher incidence rate compared with that of adenocarcinoma among veterans. Adenocarcinoma tended to present at a more advanced stage than did SCC. However, this did not translate into a significant survival difference.
Epidemiology of Stage IV Thyroid Cancer Patients: A Review of the National Cancer Database, 2000-2012
Background: Patients with thyroid cancer often have distinctive characteristics that change as the cancer progresses to stage IV and warrants varied treatment. A Surveillance, Epidemiology, and End Results-based study reported that men with thyroid cancer of follicular cell origin are more likely to present with advanced disease compared with that of female patients. This is the largest study to evaluate stage IV thyroid cancer.
Methods: A population-based study was conducted using the National Cancer Database (NCDB), which captures nearly 70% of incident cancers in the U.S. For the accession years 2000 to 2012, NCDB took epidemiologic information for a sample of 343,386 thyroid cancer cases and used data from the 2012 U.S. census. The diagnosis of stage IV disease represented 6.88% (23,613) of the total patient population. The demographics of stage IV patients were compared with patients with all other stages using the chi-square test.
Results: There was an increased incidence of stage IV thyroid cancer in Medicare, lower high school graduation rates, annual median household income < $44,000, aged ≥ 70 years, male, more comorbidities, and further distance from a treatment facility (P < .0001). Ethnicity/race had little impact on the incidence of stage IV disease. Stage IV cancer incidence is higher in males (12.14%) compared with that of females (5.15%), and stage IV patients are more likely have Medicare (14.60%) or be uninsured (8.72%) than have private insurance (4.63%). Patients with ≥ 2 comorbidities (14.23%) are more than twice as likely to have stage IV as those without comorbidities (6.77%). Medullary and anaplastic cancers (20.08%) are much more likely to be stage IV than papillary (5.76%) or follicular cancers (3.94%, P < .0001).
Conclusions: Patients with the following characteristics are more likely to present with stage IV thyroid cancer: Medicare, less education, low income, older age, male, comorbidities, far from a treatment facility, and medullary or anaplastic thyroid cancer.
Background: Patients with thyroid cancer often have distinctive characteristics that change as the cancer progresses to stage IV and warrants varied treatment. A Surveillance, Epidemiology, and End Results-based study reported that men with thyroid cancer of follicular cell origin are more likely to present with advanced disease compared with that of female patients. This is the largest study to evaluate stage IV thyroid cancer.
Methods: A population-based study was conducted using the National Cancer Database (NCDB), which captures nearly 70% of incident cancers in the U.S. For the accession years 2000 to 2012, NCDB took epidemiologic information for a sample of 343,386 thyroid cancer cases and used data from the 2012 U.S. census. The diagnosis of stage IV disease represented 6.88% (23,613) of the total patient population. The demographics of stage IV patients were compared with patients with all other stages using the chi-square test.
Results: There was an increased incidence of stage IV thyroid cancer in Medicare, lower high school graduation rates, annual median household income < $44,000, aged ≥ 70 years, male, more comorbidities, and further distance from a treatment facility (P < .0001). Ethnicity/race had little impact on the incidence of stage IV disease. Stage IV cancer incidence is higher in males (12.14%) compared with that of females (5.15%), and stage IV patients are more likely have Medicare (14.60%) or be uninsured (8.72%) than have private insurance (4.63%). Patients with ≥ 2 comorbidities (14.23%) are more than twice as likely to have stage IV as those without comorbidities (6.77%). Medullary and anaplastic cancers (20.08%) are much more likely to be stage IV than papillary (5.76%) or follicular cancers (3.94%, P < .0001).
Conclusions: Patients with the following characteristics are more likely to present with stage IV thyroid cancer: Medicare, less education, low income, older age, male, comorbidities, far from a treatment facility, and medullary or anaplastic thyroid cancer.
Background: Patients with thyroid cancer often have distinctive characteristics that change as the cancer progresses to stage IV and warrants varied treatment. A Surveillance, Epidemiology, and End Results-based study reported that men with thyroid cancer of follicular cell origin are more likely to present with advanced disease compared with that of female patients. This is the largest study to evaluate stage IV thyroid cancer.
Methods: A population-based study was conducted using the National Cancer Database (NCDB), which captures nearly 70% of incident cancers in the U.S. For the accession years 2000 to 2012, NCDB took epidemiologic information for a sample of 343,386 thyroid cancer cases and used data from the 2012 U.S. census. The diagnosis of stage IV disease represented 6.88% (23,613) of the total patient population. The demographics of stage IV patients were compared with patients with all other stages using the chi-square test.
Results: There was an increased incidence of stage IV thyroid cancer in Medicare, lower high school graduation rates, annual median household income < $44,000, aged ≥ 70 years, male, more comorbidities, and further distance from a treatment facility (P < .0001). Ethnicity/race had little impact on the incidence of stage IV disease. Stage IV cancer incidence is higher in males (12.14%) compared with that of females (5.15%), and stage IV patients are more likely have Medicare (14.60%) or be uninsured (8.72%) than have private insurance (4.63%). Patients with ≥ 2 comorbidities (14.23%) are more than twice as likely to have stage IV as those without comorbidities (6.77%). Medullary and anaplastic cancers (20.08%) are much more likely to be stage IV than papillary (5.76%) or follicular cancers (3.94%, P < .0001).
Conclusions: Patients with the following characteristics are more likely to present with stage IV thyroid cancer: Medicare, less education, low income, older age, male, comorbidities, far from a treatment facility, and medullary or anaplastic thyroid cancer.
PsA, PsC do not affect total hip replacement outcomes
Neither psoriatic arthritis (PsA) nor cutaneous psoriasis (PsC) is an independent predictor of poor postoperative pain or function following a total hip arthroplasty, according to the results of a case-control study by Dr. Lisa A. Mandl and her colleagues.
The study’s participants underwent surgery between May 1, 2007, and Dec. 31, 2010, in a center that performs more than 4,300 THAs annually. All subjects lived for at least 2 years after their operations. The researchers compared pre- and postoperative data from patients in the following three categories: those with PsA, those with PsC without evidence of inflammatory arthritis, and those with osteoarthritis (OA). Patients with OA comprised the control group, which excluded any patient who self-reported a history of PsA, rheumatoid arthritis, lupus erythematosus, or any other systematic rheumatic disease, or who had documentation of skin psoriasis. The researchers acquired postoperative self-report data from 47 PsA patients, 106 PsC patients, and 864 OA patients. Seventeen percent of patients submitted information on their status at 1 year, 69% at 2 years, and 14% at 3-5 years.
The primary outcomes of interest were postoperative pain and function, which were assessed via the Hip Osteoarthritis Outcome Score (HOOS), from which the Western Ontario and McMaster Universities Osteoarthritis Index (WOMAC) was derived.
There were no statistically significant differences in postoperative WOMAC pain or function scores between the three groups of patients (P = .78 and .96, respectively). The mean pain scores were 14.9, 6.1, and 15.8 for patients with PsA, PsC, and OA, respectively. These patients’ mean function scores were 16.3, 19.6, and 18.8 for the PsA, PsC and OA groups, respectively.
Overall levels of satisfaction with the surgery were similar among the three groups (P = .54). Ninety-three percent of the PsA patients, 79% of the PsC patients, and 84% of the OA patients were “very satisfied” with their total hip arthroplasty. Between 1% and 3% of each group reported being “very dissatisfied” with their surgery. The researchers found that extent of skin disease was not associated with worse postoperative pain or function.
“Further work needs to be done to better understand the interplay of disease activity and quality of life on the outcomes of [total hip arthroplasty] in PsA and PsC,” they wrote.
Read the report in Arthritis & Rheumatology (doi: 10.1002/art.39431).
Neither psoriatic arthritis (PsA) nor cutaneous psoriasis (PsC) is an independent predictor of poor postoperative pain or function following a total hip arthroplasty, according to the results of a case-control study by Dr. Lisa A. Mandl and her colleagues.
The study’s participants underwent surgery between May 1, 2007, and Dec. 31, 2010, in a center that performs more than 4,300 THAs annually. All subjects lived for at least 2 years after their operations. The researchers compared pre- and postoperative data from patients in the following three categories: those with PsA, those with PsC without evidence of inflammatory arthritis, and those with osteoarthritis (OA). Patients with OA comprised the control group, which excluded any patient who self-reported a history of PsA, rheumatoid arthritis, lupus erythematosus, or any other systematic rheumatic disease, or who had documentation of skin psoriasis. The researchers acquired postoperative self-report data from 47 PsA patients, 106 PsC patients, and 864 OA patients. Seventeen percent of patients submitted information on their status at 1 year, 69% at 2 years, and 14% at 3-5 years.
The primary outcomes of interest were postoperative pain and function, which were assessed via the Hip Osteoarthritis Outcome Score (HOOS), from which the Western Ontario and McMaster Universities Osteoarthritis Index (WOMAC) was derived.
There were no statistically significant differences in postoperative WOMAC pain or function scores between the three groups of patients (P = .78 and .96, respectively). The mean pain scores were 14.9, 6.1, and 15.8 for patients with PsA, PsC, and OA, respectively. These patients’ mean function scores were 16.3, 19.6, and 18.8 for the PsA, PsC and OA groups, respectively.
Overall levels of satisfaction with the surgery were similar among the three groups (P = .54). Ninety-three percent of the PsA patients, 79% of the PsC patients, and 84% of the OA patients were “very satisfied” with their total hip arthroplasty. Between 1% and 3% of each group reported being “very dissatisfied” with their surgery. The researchers found that extent of skin disease was not associated with worse postoperative pain or function.
“Further work needs to be done to better understand the interplay of disease activity and quality of life on the outcomes of [total hip arthroplasty] in PsA and PsC,” they wrote.
Read the report in Arthritis & Rheumatology (doi: 10.1002/art.39431).
Neither psoriatic arthritis (PsA) nor cutaneous psoriasis (PsC) is an independent predictor of poor postoperative pain or function following a total hip arthroplasty, according to the results of a case-control study by Dr. Lisa A. Mandl and her colleagues.
The study’s participants underwent surgery between May 1, 2007, and Dec. 31, 2010, in a center that performs more than 4,300 THAs annually. All subjects lived for at least 2 years after their operations. The researchers compared pre- and postoperative data from patients in the following three categories: those with PsA, those with PsC without evidence of inflammatory arthritis, and those with osteoarthritis (OA). Patients with OA comprised the control group, which excluded any patient who self-reported a history of PsA, rheumatoid arthritis, lupus erythematosus, or any other systematic rheumatic disease, or who had documentation of skin psoriasis. The researchers acquired postoperative self-report data from 47 PsA patients, 106 PsC patients, and 864 OA patients. Seventeen percent of patients submitted information on their status at 1 year, 69% at 2 years, and 14% at 3-5 years.
The primary outcomes of interest were postoperative pain and function, which were assessed via the Hip Osteoarthritis Outcome Score (HOOS), from which the Western Ontario and McMaster Universities Osteoarthritis Index (WOMAC) was derived.
There were no statistically significant differences in postoperative WOMAC pain or function scores between the three groups of patients (P = .78 and .96, respectively). The mean pain scores were 14.9, 6.1, and 15.8 for patients with PsA, PsC, and OA, respectively. These patients’ mean function scores were 16.3, 19.6, and 18.8 for the PsA, PsC and OA groups, respectively.
Overall levels of satisfaction with the surgery were similar among the three groups (P = .54). Ninety-three percent of the PsA patients, 79% of the PsC patients, and 84% of the OA patients were “very satisfied” with their total hip arthroplasty. Between 1% and 3% of each group reported being “very dissatisfied” with their surgery. The researchers found that extent of skin disease was not associated with worse postoperative pain or function.
“Further work needs to be done to better understand the interplay of disease activity and quality of life on the outcomes of [total hip arthroplasty] in PsA and PsC,” they wrote.
Read the report in Arthritis & Rheumatology (doi: 10.1002/art.39431).
FROM ARTHRITIS & RHEUMATOLOGY
Memantine for pain control in fibromyalgia
The prevalence of fibromyalgia in our clinical practices is about 2%-3%. But it may feel much higher, because some of these patients use a lot of staff and office time. Vacillations in our certainty about the diagnosis, inadequate time to differentiate new problems from the underlying one, failing to engage the patient in disease self-management, and inadequately exhausting the plethora of pharmacologic management options (however weak the data may be for each one) make care of patients with fibromyalgia challenging. Unfortunately, many of these patients are treated with chronic opioids.
So, maybe you have tried every pharmacologic option under the sun. But have you tried memantine?
Dr. Bárbara Olivan-Blázquez of the University of Zaragoza, Spain, and colleagues evaluated the efficacy of memantine for pain symptom control in fibromyalgia (Pain. 2014 Dec;155[12]:2517-25).
In this study, 63 patients with fibromyalgia were randomized to 20 mg/day of memantine or matching placebo for a total of 6 months, including an initial 1-month up-titration period. Follow-up occurred at 3 months and 6 months.
Compared with placebo, memantine decreased pain and depression at 3 months and 6 months. It also increased cognitive function and overall perceptions of function at 3 months and 6 months. More than 80% of patients completed the trial. Dizziness and headaches were the most commonly reported symptoms.
Why does it work? Memantine blocks glutamate, and glutamate may be a player in perpetuating the pain cycle for patients with fibromyalgia. Previously, memantine had been shown to be effective in other pain conditions such as complex regional pain syndrome and phantom limb pain. Memantine is well tolerated and is generically available. In my location, I was able to locate it for less than $1 per day of therapy.
So, before giving up hope or reaching for the opioid du jour, memantine may be worth a trial to decrease pain and improve function in our patients with fibromyalgia.
Dr. Ebbert is professor of medicine, a general internist at the Mayo Clinic in Rochester, Minn., and a diplomate of the American Board of Addiction Medicine. The opinions expressed are those of the author and do not necessarily represent the views and opinions of the Mayo Clinic. The opinions expressed in this article should not be used to diagnose or treat any medical condition nor should they be used as a substitute for medical advice from a qualified, board-certified practicing clinician. Dr. Ebbert has no relevant financial disclosures about this article. Follow him on Twitter @jonebbert.
The prevalence of fibromyalgia in our clinical practices is about 2%-3%. But it may feel much higher, because some of these patients use a lot of staff and office time. Vacillations in our certainty about the diagnosis, inadequate time to differentiate new problems from the underlying one, failing to engage the patient in disease self-management, and inadequately exhausting the plethora of pharmacologic management options (however weak the data may be for each one) make care of patients with fibromyalgia challenging. Unfortunately, many of these patients are treated with chronic opioids.
So, maybe you have tried every pharmacologic option under the sun. But have you tried memantine?
Dr. Bárbara Olivan-Blázquez of the University of Zaragoza, Spain, and colleagues evaluated the efficacy of memantine for pain symptom control in fibromyalgia (Pain. 2014 Dec;155[12]:2517-25).
In this study, 63 patients with fibromyalgia were randomized to 20 mg/day of memantine or matching placebo for a total of 6 months, including an initial 1-month up-titration period. Follow-up occurred at 3 months and 6 months.
Compared with placebo, memantine decreased pain and depression at 3 months and 6 months. It also increased cognitive function and overall perceptions of function at 3 months and 6 months. More than 80% of patients completed the trial. Dizziness and headaches were the most commonly reported symptoms.
Why does it work? Memantine blocks glutamate, and glutamate may be a player in perpetuating the pain cycle for patients with fibromyalgia. Previously, memantine had been shown to be effective in other pain conditions such as complex regional pain syndrome and phantom limb pain. Memantine is well tolerated and is generically available. In my location, I was able to locate it for less than $1 per day of therapy.
So, before giving up hope or reaching for the opioid du jour, memantine may be worth a trial to decrease pain and improve function in our patients with fibromyalgia.
Dr. Ebbert is professor of medicine, a general internist at the Mayo Clinic in Rochester, Minn., and a diplomate of the American Board of Addiction Medicine. The opinions expressed are those of the author and do not necessarily represent the views and opinions of the Mayo Clinic. The opinions expressed in this article should not be used to diagnose or treat any medical condition nor should they be used as a substitute for medical advice from a qualified, board-certified practicing clinician. Dr. Ebbert has no relevant financial disclosures about this article. Follow him on Twitter @jonebbert.
The prevalence of fibromyalgia in our clinical practices is about 2%-3%. But it may feel much higher, because some of these patients use a lot of staff and office time. Vacillations in our certainty about the diagnosis, inadequate time to differentiate new problems from the underlying one, failing to engage the patient in disease self-management, and inadequately exhausting the plethora of pharmacologic management options (however weak the data may be for each one) make care of patients with fibromyalgia challenging. Unfortunately, many of these patients are treated with chronic opioids.
So, maybe you have tried every pharmacologic option under the sun. But have you tried memantine?
Dr. Bárbara Olivan-Blázquez of the University of Zaragoza, Spain, and colleagues evaluated the efficacy of memantine for pain symptom control in fibromyalgia (Pain. 2014 Dec;155[12]:2517-25).
In this study, 63 patients with fibromyalgia were randomized to 20 mg/day of memantine or matching placebo for a total of 6 months, including an initial 1-month up-titration period. Follow-up occurred at 3 months and 6 months.
Compared with placebo, memantine decreased pain and depression at 3 months and 6 months. It also increased cognitive function and overall perceptions of function at 3 months and 6 months. More than 80% of patients completed the trial. Dizziness and headaches were the most commonly reported symptoms.
Why does it work? Memantine blocks glutamate, and glutamate may be a player in perpetuating the pain cycle for patients with fibromyalgia. Previously, memantine had been shown to be effective in other pain conditions such as complex regional pain syndrome and phantom limb pain. Memantine is well tolerated and is generically available. In my location, I was able to locate it for less than $1 per day of therapy.
So, before giving up hope or reaching for the opioid du jour, memantine may be worth a trial to decrease pain and improve function in our patients with fibromyalgia.
Dr. Ebbert is professor of medicine, a general internist at the Mayo Clinic in Rochester, Minn., and a diplomate of the American Board of Addiction Medicine. The opinions expressed are those of the author and do not necessarily represent the views and opinions of the Mayo Clinic. The opinions expressed in this article should not be used to diagnose or treat any medical condition nor should they be used as a substitute for medical advice from a qualified, board-certified practicing clinician. Dr. Ebbert has no relevant financial disclosures about this article. Follow him on Twitter @jonebbert.
Noninvasive Cosmetic Procedures: Delegation Among Dermatologists
Noninvasive cosmetic surgery procedures continue to increase in number, according to the American Society of Plastic Surgeons. Furthermore, these procedures are being performed in the offices of a growing number of physician specialties. As demand increases and offices become busy, there is increased delegation of these procedures to nurses, physician assistants, aestheticians, and medical assistants.
Austin et al (Dermatol Surg. 2015;41:827-832.) performed an Internet-based survey of physician members of the American Society for Dermatologic Surgery, the American Society for Laser Medicine and Surgery, and the American Society for Aesthetic Plastic Surgery to evaluate the delegation practices among the various specialties. The survey asked for physician training, age, gender, residency, and geographic location, as well as a breakdown of how much of their practice involved cosmetic procedures. Respondents were asked if they delegate procedures, to whom do they delegate (eg, registered nurse, physician assistant, MAs), and which procedures they delegate. A point system was used to give a score based on the perceived level of education and/or training of the person delegated to perform a procedure: do not delegate (1); physician assistant or nurse practitioner (2); registered nurse (3); and MA, aesthetician, or other (4).
Respondents included 823 physicians: 521 dermatologists and 302 nondermatologists. Of the respondents, 291 of dermatologists (55.9%) and 223 of nondermatologist physicians (73.8%) delegated cosmetic procedures. Procedures most often delegated by dermatologists compared to nondermatologists included chemical peels, neuromodulator and filler injections, laser hair removal, pulsed dye laser, tattoo removal, intense pulsed light, nonablative factional laser, and sclerotherapy.
The outcome of this survey shows that delegating certain cosmetic procedures is common, with more than half of all survey respondents delegating at least 1 cosmetic procedure. Dermatologists, as a whole, delegated less frequently than nondermatologist physicians, and when they did, dermatologists delegated to higher-level providers compared to nondermatologists. Medical practices in which the focus is primarily cosmetic and in those in which the physician is older also were more likely to delegate procedures.
What’s the issue?
There is tremendous variability between states about who is competent to perform many of the noninvasive cosmetic procedures. Some state medical boards specify the type of provider and the procedures allowed, while other states have no policy. However, this rule is changing, and there are more regulations being brought before the state medical boards with the goal of ensuring patient safety. As the demand for noninvasive procedures continues to grow, there will be an increase in the number of nonphysician providers performing these procedures. Patient safety should be at the forefront of all legislation, but at the same time there should be training courses and oversight boards to allow for safe delegation. Do you see yourself delegating more of these noninvasive procedures in the future?
Noninvasive cosmetic surgery procedures continue to increase in number, according to the American Society of Plastic Surgeons. Furthermore, these procedures are being performed in the offices of a growing number of physician specialties. As demand increases and offices become busy, there is increased delegation of these procedures to nurses, physician assistants, aestheticians, and medical assistants.
Austin et al (Dermatol Surg. 2015;41:827-832.) performed an Internet-based survey of physician members of the American Society for Dermatologic Surgery, the American Society for Laser Medicine and Surgery, and the American Society for Aesthetic Plastic Surgery to evaluate the delegation practices among the various specialties. The survey asked for physician training, age, gender, residency, and geographic location, as well as a breakdown of how much of their practice involved cosmetic procedures. Respondents were asked if they delegate procedures, to whom do they delegate (eg, registered nurse, physician assistant, MAs), and which procedures they delegate. A point system was used to give a score based on the perceived level of education and/or training of the person delegated to perform a procedure: do not delegate (1); physician assistant or nurse practitioner (2); registered nurse (3); and MA, aesthetician, or other (4).
Respondents included 823 physicians: 521 dermatologists and 302 nondermatologists. Of the respondents, 291 of dermatologists (55.9%) and 223 of nondermatologist physicians (73.8%) delegated cosmetic procedures. Procedures most often delegated by dermatologists compared to nondermatologists included chemical peels, neuromodulator and filler injections, laser hair removal, pulsed dye laser, tattoo removal, intense pulsed light, nonablative factional laser, and sclerotherapy.
The outcome of this survey shows that delegating certain cosmetic procedures is common, with more than half of all survey respondents delegating at least 1 cosmetic procedure. Dermatologists, as a whole, delegated less frequently than nondermatologist physicians, and when they did, dermatologists delegated to higher-level providers compared to nondermatologists. Medical practices in which the focus is primarily cosmetic and in those in which the physician is older also were more likely to delegate procedures.
What’s the issue?
There is tremendous variability between states about who is competent to perform many of the noninvasive cosmetic procedures. Some state medical boards specify the type of provider and the procedures allowed, while other states have no policy. However, this rule is changing, and there are more regulations being brought before the state medical boards with the goal of ensuring patient safety. As the demand for noninvasive procedures continues to grow, there will be an increase in the number of nonphysician providers performing these procedures. Patient safety should be at the forefront of all legislation, but at the same time there should be training courses and oversight boards to allow for safe delegation. Do you see yourself delegating more of these noninvasive procedures in the future?
Noninvasive cosmetic surgery procedures continue to increase in number, according to the American Society of Plastic Surgeons. Furthermore, these procedures are being performed in the offices of a growing number of physician specialties. As demand increases and offices become busy, there is increased delegation of these procedures to nurses, physician assistants, aestheticians, and medical assistants.
Austin et al (Dermatol Surg. 2015;41:827-832.) performed an Internet-based survey of physician members of the American Society for Dermatologic Surgery, the American Society for Laser Medicine and Surgery, and the American Society for Aesthetic Plastic Surgery to evaluate the delegation practices among the various specialties. The survey asked for physician training, age, gender, residency, and geographic location, as well as a breakdown of how much of their practice involved cosmetic procedures. Respondents were asked if they delegate procedures, to whom do they delegate (eg, registered nurse, physician assistant, MAs), and which procedures they delegate. A point system was used to give a score based on the perceived level of education and/or training of the person delegated to perform a procedure: do not delegate (1); physician assistant or nurse practitioner (2); registered nurse (3); and MA, aesthetician, or other (4).
Respondents included 823 physicians: 521 dermatologists and 302 nondermatologists. Of the respondents, 291 of dermatologists (55.9%) and 223 of nondermatologist physicians (73.8%) delegated cosmetic procedures. Procedures most often delegated by dermatologists compared to nondermatologists included chemical peels, neuromodulator and filler injections, laser hair removal, pulsed dye laser, tattoo removal, intense pulsed light, nonablative factional laser, and sclerotherapy.
The outcome of this survey shows that delegating certain cosmetic procedures is common, with more than half of all survey respondents delegating at least 1 cosmetic procedure. Dermatologists, as a whole, delegated less frequently than nondermatologist physicians, and when they did, dermatologists delegated to higher-level providers compared to nondermatologists. Medical practices in which the focus is primarily cosmetic and in those in which the physician is older also were more likely to delegate procedures.
What’s the issue?
There is tremendous variability between states about who is competent to perform many of the noninvasive cosmetic procedures. Some state medical boards specify the type of provider and the procedures allowed, while other states have no policy. However, this rule is changing, and there are more regulations being brought before the state medical boards with the goal of ensuring patient safety. As the demand for noninvasive procedures continues to grow, there will be an increase in the number of nonphysician providers performing these procedures. Patient safety should be at the forefront of all legislation, but at the same time there should be training courses and oversight boards to allow for safe delegation. Do you see yourself delegating more of these noninvasive procedures in the future?
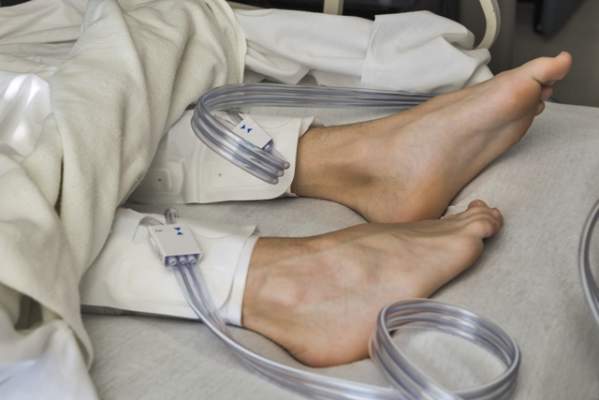
IPC maintains first-line status in preventing DVT in most surgical patients
Intermittent pneumatic compression remains the consensus choice as the sole prophylactic agent for deep vein thrombosis in low- or moderate-risk surgical patients, according to a literature analysis published online ahead of print in September in the Journal of Vascular Surgery: Venous and Lymphatic Disorders.
Dr. Nirvana Sadaghianloo of the University of Nice (France) Sophia Antipolis and Dr. Alan Dardik of Yale University in New Haven, Conn., used the MEDLINE and Cochrane libraries to find individual studies and meta-analyses published in English since 2011 assessing the efficacy of intermittent pneumatic compression (IPC) in preventing deep vein thrombosis (DVT), which included the American College of Chest Physicians ninth edition guidelines (2012). They stated that, although the overall quality of studies regarding the use of IPC was low, IPC showed efficacy in prevention of DVT for more than 30 years (J Vasc Surg Venous Lymphat Disord. 2015 doi: 10.1016/j.jvsv.2015.07.006).
“IPC represents a good alternative to pharmacologic agents when the risk of thrombosis is moderate or low or when the risk of bleeding is high or may have serious consequences for the patient,” they stated.
However, they also found that most recommendations suggested that, in high-risk patients, IPC plays a role primarily as an additional modality to provide additional benefit in preventing DVT when is used in combination with pharmacologic therapy. They highlighted how the choice of any thromboprophylactic agent required a systematic risk assessment as a critical prerequisite.
Overall, risk stratification was most frequently assessed by the Caprini or Rogers score for most general, abdominal-pelvic, bariatric, vascular, plastic, and gynecologic surgery patients and by the Padua Prediction Score for hospitalized medical patients. In addition, major orthopedic surgery patients and stroke patients with restricted mobility were usually considered high risk, Dr. Sadaghianloo and Dr. Dardik said.
From their assessment of the literature, they determined that “further studies are needed to assess practical clinical questions that remain unanswered, including optimal cuff length and location, sequence and duration of pressure, and whether use of IPC in an outpatient setting can be effective and achieve good compliance.”
The authors reported that they had no conflicts of interest.
Intermittent pneumatic compression remains the consensus choice as the sole prophylactic agent for deep vein thrombosis in low- or moderate-risk surgical patients, according to a literature analysis published online ahead of print in September in the Journal of Vascular Surgery: Venous and Lymphatic Disorders.
Dr. Nirvana Sadaghianloo of the University of Nice (France) Sophia Antipolis and Dr. Alan Dardik of Yale University in New Haven, Conn., used the MEDLINE and Cochrane libraries to find individual studies and meta-analyses published in English since 2011 assessing the efficacy of intermittent pneumatic compression (IPC) in preventing deep vein thrombosis (DVT), which included the American College of Chest Physicians ninth edition guidelines (2012). They stated that, although the overall quality of studies regarding the use of IPC was low, IPC showed efficacy in prevention of DVT for more than 30 years (J Vasc Surg Venous Lymphat Disord. 2015 doi: 10.1016/j.jvsv.2015.07.006).
“IPC represents a good alternative to pharmacologic agents when the risk of thrombosis is moderate or low or when the risk of bleeding is high or may have serious consequences for the patient,” they stated.
However, they also found that most recommendations suggested that, in high-risk patients, IPC plays a role primarily as an additional modality to provide additional benefit in preventing DVT when is used in combination with pharmacologic therapy. They highlighted how the choice of any thromboprophylactic agent required a systematic risk assessment as a critical prerequisite.
Overall, risk stratification was most frequently assessed by the Caprini or Rogers score for most general, abdominal-pelvic, bariatric, vascular, plastic, and gynecologic surgery patients and by the Padua Prediction Score for hospitalized medical patients. In addition, major orthopedic surgery patients and stroke patients with restricted mobility were usually considered high risk, Dr. Sadaghianloo and Dr. Dardik said.
From their assessment of the literature, they determined that “further studies are needed to assess practical clinical questions that remain unanswered, including optimal cuff length and location, sequence and duration of pressure, and whether use of IPC in an outpatient setting can be effective and achieve good compliance.”
The authors reported that they had no conflicts of interest.
Intermittent pneumatic compression remains the consensus choice as the sole prophylactic agent for deep vein thrombosis in low- or moderate-risk surgical patients, according to a literature analysis published online ahead of print in September in the Journal of Vascular Surgery: Venous and Lymphatic Disorders.
Dr. Nirvana Sadaghianloo of the University of Nice (France) Sophia Antipolis and Dr. Alan Dardik of Yale University in New Haven, Conn., used the MEDLINE and Cochrane libraries to find individual studies and meta-analyses published in English since 2011 assessing the efficacy of intermittent pneumatic compression (IPC) in preventing deep vein thrombosis (DVT), which included the American College of Chest Physicians ninth edition guidelines (2012). They stated that, although the overall quality of studies regarding the use of IPC was low, IPC showed efficacy in prevention of DVT for more than 30 years (J Vasc Surg Venous Lymphat Disord. 2015 doi: 10.1016/j.jvsv.2015.07.006).
“IPC represents a good alternative to pharmacologic agents when the risk of thrombosis is moderate or low or when the risk of bleeding is high or may have serious consequences for the patient,” they stated.
However, they also found that most recommendations suggested that, in high-risk patients, IPC plays a role primarily as an additional modality to provide additional benefit in preventing DVT when is used in combination with pharmacologic therapy. They highlighted how the choice of any thromboprophylactic agent required a systematic risk assessment as a critical prerequisite.
Overall, risk stratification was most frequently assessed by the Caprini or Rogers score for most general, abdominal-pelvic, bariatric, vascular, plastic, and gynecologic surgery patients and by the Padua Prediction Score for hospitalized medical patients. In addition, major orthopedic surgery patients and stroke patients with restricted mobility were usually considered high risk, Dr. Sadaghianloo and Dr. Dardik said.
From their assessment of the literature, they determined that “further studies are needed to assess practical clinical questions that remain unanswered, including optimal cuff length and location, sequence and duration of pressure, and whether use of IPC in an outpatient setting can be effective and achieve good compliance.”
The authors reported that they had no conflicts of interest.
FROM JOURNAL OF VASCULAR SURGERY: VENOUS AND LYMPHATIC DISORDERS
Key clinical point: IPC is efficacious as the sole prophylactic agent in low- or moderate-risk surgical patients and in patients with high risk of bleeding with drug therapy.
Major finding: In high-risk patients, IPC is an added modality for preventing DVT in combination with pharmacologic prophylaxis.
Data source: Researchers performed an assessment of the literature in English since 2011 in MEDLINE and the Cochrane libraries.
Disclosures: The authors reported that they had no conflicts of interest.
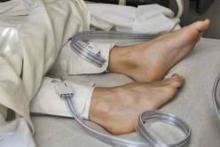
Low incidence of DVT seen in routine MRI of damaged knees
The incidence of deep vein thrombosis (DVT) in knees undergoing routine assessment of damage was found to be low but suggests the need to interrogate the popliteal vein for evidence of thrombosis, according to Dr. Ryan M. Shulman of the joint department of medical imaging of University Health Network and Mount Sinai Hospital, Toronto, and his colleagues.
They documented the appearance and determined the prevalence of findings suspicious for popliteal vein thrombosis on magnetic resonance imaging (MRI) assessment of the knee joint by retrospectively reviewing 2,888 MRI examinations.
MRI images were classified as showing either normal appearing popliteal vein or findings suspicious for popliteal vein thrombosis. They found 2,879 of the MRI studies as having a normal appearing popliteal vein, with 9 showing findings suspicious for popliteal vein thrombosis.
“Although the prevalence of MR findings is low (0.3%), our findings reiterate the need to interrogate the popliteal vein for evidence of thrombosis,” Dr. Shulman and his colleagues concluded.
Find the full study online the Journal of Clinical Imaging 2015 (doi: 10.1016/j.clinimag.2015.09.008).
The incidence of deep vein thrombosis (DVT) in knees undergoing routine assessment of damage was found to be low but suggests the need to interrogate the popliteal vein for evidence of thrombosis, according to Dr. Ryan M. Shulman of the joint department of medical imaging of University Health Network and Mount Sinai Hospital, Toronto, and his colleagues.
They documented the appearance and determined the prevalence of findings suspicious for popliteal vein thrombosis on magnetic resonance imaging (MRI) assessment of the knee joint by retrospectively reviewing 2,888 MRI examinations.
MRI images were classified as showing either normal appearing popliteal vein or findings suspicious for popliteal vein thrombosis. They found 2,879 of the MRI studies as having a normal appearing popliteal vein, with 9 showing findings suspicious for popliteal vein thrombosis.
“Although the prevalence of MR findings is low (0.3%), our findings reiterate the need to interrogate the popliteal vein for evidence of thrombosis,” Dr. Shulman and his colleagues concluded.
Find the full study online the Journal of Clinical Imaging 2015 (doi: 10.1016/j.clinimag.2015.09.008).
The incidence of deep vein thrombosis (DVT) in knees undergoing routine assessment of damage was found to be low but suggests the need to interrogate the popliteal vein for evidence of thrombosis, according to Dr. Ryan M. Shulman of the joint department of medical imaging of University Health Network and Mount Sinai Hospital, Toronto, and his colleagues.
They documented the appearance and determined the prevalence of findings suspicious for popliteal vein thrombosis on magnetic resonance imaging (MRI) assessment of the knee joint by retrospectively reviewing 2,888 MRI examinations.
MRI images were classified as showing either normal appearing popliteal vein or findings suspicious for popliteal vein thrombosis. They found 2,879 of the MRI studies as having a normal appearing popliteal vein, with 9 showing findings suspicious for popliteal vein thrombosis.
“Although the prevalence of MR findings is low (0.3%), our findings reiterate the need to interrogate the popliteal vein for evidence of thrombosis,” Dr. Shulman and his colleagues concluded.
Find the full study online the Journal of Clinical Imaging 2015 (doi: 10.1016/j.clinimag.2015.09.008).
FROM JOURNAL OF CLINICAL IMAGING
Immunotherapy: Inject locally, treat globally?
NEW YORK – “Inject locally, treat globally” may become a new mantra for cancer immunotherapy. Dr. Ronald Levy, professor and chief of the department of oncology at Stanford (Calif.) University, discussed impressive and durable systemic results from local treatment of tumors, reviewing his group’s recent work and discussing ongoing early stage clinical trials.
In the hope of triggering an immune response that induces a systemic CD8 T-cell response, Dr. Levy and other investigators began experimenting with intralesional injections of immunotherapies, with and without adjunctive radiation or chemotherapy. The principle has been evaluated in a mouse model, in which up to 80% of mice receiving intratumoral immunotherapy were cured. Now, human trials are underway for solid tumors as well as lymphoma.
An early clinical trial involving 15 patients with recurrent low-grade B-cell lymphoma combined targeted low-dose radiation of a single tumor site with injection of CpG, immune-boosting snippets of DNA, into the same site. Looking for partial or complete regression, investigators saw promising results, though some patients who were responders didn’t see full effect until 24 weeks after injection (J Clin Oncol. 2010 Oct 1;28[28]:4324-32). The overall objective response rate was 27%, although 80% of patients (12 of 15) had stable disease or partial or complete response through a median follow-up of 33.7 months. Some individual patients had marked regression or disappearance of bulky tumors at distant sites.
CpG – motifs of cytosines and guanines – were strung together, said Dr. Levy, with a sulfur rather than a phosphate backbone to make them more stable for injection. These bits of DNA, which are present in both bacteria and vertebrates, are an agonist for toll-like receptor 9, activating B cells and dendritic cells, and then tumor-specific T-cells.
Dr. Levy and his collaborators used a two-tumor mouse model to track intralesional injection effectiveness for a variety of immunotherapies. Mice seeded with tumor cells bilaterally over the abdomen were injected with CpG and two other immune therapies at a single abdominal tumor site, and bilateral regression, if any, was tracked in comparison to systemic immunotherapy (J Clin Invest. 2013;123[6]:2447-63).
Though both treatment arms had good initial response, 70% of the systemically treated mice relapsed by 150 days after injection, compared with just 10% of those receiving intratumoral therapy (P = .002).
Of the 23 mice whose therapy consisted of intratumoral administration of CpG together with anti-CTLA4 and anti-OX40 antibodies (aCTLA4 and aOX40), 21 (91%) were alive 50 days after treatment. These mice fared better than did those receiving any other intratumoral treatment combination (P = .004, compared with CpG+aOX40; P = .03, compared with aCTLA4), suggesting a synergistic benefit to the triple combination.
Somewhat surprisingly, even murine models that also had tumor seeding into brain tissue saw marked reduction or even resolution of brain tumors, showing that the blood-brain barrier does not impede the effect within the CNS.
What didn’t work? PD-1 inhibitors were not particularly effective at provoking a systemic effect when injected into tumors. Peritumoral injection, though theoretically taking advantage of some aspects of the tumor microenvironment, also did not show global effect.
Based on the human CpG + radiation trials and the mouse experiments, the research group is proceeding with phase I and II clinical trials of CpG in combinations with aCTLA4 and aOX40. These, Dr. Levy said, were more likely to be available clinically, and human intratumoral T regulatory cells are known to express both CTLA4 and OX40.
All agents and combinations had an enhancing effect, said Dr. Levy. “We like this treatment and trial design,” he said, noting that everyone sees benefit at the local injection site, and some see global results.
Dr. Levy discussed multiple studies, and he disclosed receiving grant support from Pfizer, Dynavax, and Bristol-Myers Squibb. He also has served as a consultant to Five Prime, Kite, BeiGene, Innate Pharma, Bullet Biotech, and Immune Design.
NEW YORK – “Inject locally, treat globally” may become a new mantra for cancer immunotherapy. Dr. Ronald Levy, professor and chief of the department of oncology at Stanford (Calif.) University, discussed impressive and durable systemic results from local treatment of tumors, reviewing his group’s recent work and discussing ongoing early stage clinical trials.
In the hope of triggering an immune response that induces a systemic CD8 T-cell response, Dr. Levy and other investigators began experimenting with intralesional injections of immunotherapies, with and without adjunctive radiation or chemotherapy. The principle has been evaluated in a mouse model, in which up to 80% of mice receiving intratumoral immunotherapy were cured. Now, human trials are underway for solid tumors as well as lymphoma.
An early clinical trial involving 15 patients with recurrent low-grade B-cell lymphoma combined targeted low-dose radiation of a single tumor site with injection of CpG, immune-boosting snippets of DNA, into the same site. Looking for partial or complete regression, investigators saw promising results, though some patients who were responders didn’t see full effect until 24 weeks after injection (J Clin Oncol. 2010 Oct 1;28[28]:4324-32). The overall objective response rate was 27%, although 80% of patients (12 of 15) had stable disease or partial or complete response through a median follow-up of 33.7 months. Some individual patients had marked regression or disappearance of bulky tumors at distant sites.
CpG – motifs of cytosines and guanines – were strung together, said Dr. Levy, with a sulfur rather than a phosphate backbone to make them more stable for injection. These bits of DNA, which are present in both bacteria and vertebrates, are an agonist for toll-like receptor 9, activating B cells and dendritic cells, and then tumor-specific T-cells.
Dr. Levy and his collaborators used a two-tumor mouse model to track intralesional injection effectiveness for a variety of immunotherapies. Mice seeded with tumor cells bilaterally over the abdomen were injected with CpG and two other immune therapies at a single abdominal tumor site, and bilateral regression, if any, was tracked in comparison to systemic immunotherapy (J Clin Invest. 2013;123[6]:2447-63).
Though both treatment arms had good initial response, 70% of the systemically treated mice relapsed by 150 days after injection, compared with just 10% of those receiving intratumoral therapy (P = .002).
Of the 23 mice whose therapy consisted of intratumoral administration of CpG together with anti-CTLA4 and anti-OX40 antibodies (aCTLA4 and aOX40), 21 (91%) were alive 50 days after treatment. These mice fared better than did those receiving any other intratumoral treatment combination (P = .004, compared with CpG+aOX40; P = .03, compared with aCTLA4), suggesting a synergistic benefit to the triple combination.
Somewhat surprisingly, even murine models that also had tumor seeding into brain tissue saw marked reduction or even resolution of brain tumors, showing that the blood-brain barrier does not impede the effect within the CNS.
What didn’t work? PD-1 inhibitors were not particularly effective at provoking a systemic effect when injected into tumors. Peritumoral injection, though theoretically taking advantage of some aspects of the tumor microenvironment, also did not show global effect.
Based on the human CpG + radiation trials and the mouse experiments, the research group is proceeding with phase I and II clinical trials of CpG in combinations with aCTLA4 and aOX40. These, Dr. Levy said, were more likely to be available clinically, and human intratumoral T regulatory cells are known to express both CTLA4 and OX40.
All agents and combinations had an enhancing effect, said Dr. Levy. “We like this treatment and trial design,” he said, noting that everyone sees benefit at the local injection site, and some see global results.
Dr. Levy discussed multiple studies, and he disclosed receiving grant support from Pfizer, Dynavax, and Bristol-Myers Squibb. He also has served as a consultant to Five Prime, Kite, BeiGene, Innate Pharma, Bullet Biotech, and Immune Design.
NEW YORK – “Inject locally, treat globally” may become a new mantra for cancer immunotherapy. Dr. Ronald Levy, professor and chief of the department of oncology at Stanford (Calif.) University, discussed impressive and durable systemic results from local treatment of tumors, reviewing his group’s recent work and discussing ongoing early stage clinical trials.
In the hope of triggering an immune response that induces a systemic CD8 T-cell response, Dr. Levy and other investigators began experimenting with intralesional injections of immunotherapies, with and without adjunctive radiation or chemotherapy. The principle has been evaluated in a mouse model, in which up to 80% of mice receiving intratumoral immunotherapy were cured. Now, human trials are underway for solid tumors as well as lymphoma.
An early clinical trial involving 15 patients with recurrent low-grade B-cell lymphoma combined targeted low-dose radiation of a single tumor site with injection of CpG, immune-boosting snippets of DNA, into the same site. Looking for partial or complete regression, investigators saw promising results, though some patients who were responders didn’t see full effect until 24 weeks after injection (J Clin Oncol. 2010 Oct 1;28[28]:4324-32). The overall objective response rate was 27%, although 80% of patients (12 of 15) had stable disease or partial or complete response through a median follow-up of 33.7 months. Some individual patients had marked regression or disappearance of bulky tumors at distant sites.
CpG – motifs of cytosines and guanines – were strung together, said Dr. Levy, with a sulfur rather than a phosphate backbone to make them more stable for injection. These bits of DNA, which are present in both bacteria and vertebrates, are an agonist for toll-like receptor 9, activating B cells and dendritic cells, and then tumor-specific T-cells.
Dr. Levy and his collaborators used a two-tumor mouse model to track intralesional injection effectiveness for a variety of immunotherapies. Mice seeded with tumor cells bilaterally over the abdomen were injected with CpG and two other immune therapies at a single abdominal tumor site, and bilateral regression, if any, was tracked in comparison to systemic immunotherapy (J Clin Invest. 2013;123[6]:2447-63).
Though both treatment arms had good initial response, 70% of the systemically treated mice relapsed by 150 days after injection, compared with just 10% of those receiving intratumoral therapy (P = .002).
Of the 23 mice whose therapy consisted of intratumoral administration of CpG together with anti-CTLA4 and anti-OX40 antibodies (aCTLA4 and aOX40), 21 (91%) were alive 50 days after treatment. These mice fared better than did those receiving any other intratumoral treatment combination (P = .004, compared with CpG+aOX40; P = .03, compared with aCTLA4), suggesting a synergistic benefit to the triple combination.
Somewhat surprisingly, even murine models that also had tumor seeding into brain tissue saw marked reduction or even resolution of brain tumors, showing that the blood-brain barrier does not impede the effect within the CNS.
What didn’t work? PD-1 inhibitors were not particularly effective at provoking a systemic effect when injected into tumors. Peritumoral injection, though theoretically taking advantage of some aspects of the tumor microenvironment, also did not show global effect.
Based on the human CpG + radiation trials and the mouse experiments, the research group is proceeding with phase I and II clinical trials of CpG in combinations with aCTLA4 and aOX40. These, Dr. Levy said, were more likely to be available clinically, and human intratumoral T regulatory cells are known to express both CTLA4 and OX40.
All agents and combinations had an enhancing effect, said Dr. Levy. “We like this treatment and trial design,” he said, noting that everyone sees benefit at the local injection site, and some see global results.
Dr. Levy discussed multiple studies, and he disclosed receiving grant support from Pfizer, Dynavax, and Bristol-Myers Squibb. He also has served as a consultant to Five Prime, Kite, BeiGene, Innate Pharma, Bullet Biotech, and Immune Design.
EXPERT ANALYSIS FROM THE FIRST INTERNATIONAL CANCER IMMUNOTHERAPY CONFERENCE