User login
Group identifies malaria resistance locus

outside of Nairobi, Kenya
Photo by Gabrielle Tenenbaum
Researchers say they have identified genetic variants that protect African children from developing severe malaria, in some cases nearly halving a child’s chance of developing the disease.
The variants are at a locus located next to a cluster of genes that are responsible for creating the receptors the malaria parasite Plasmodium falciparum uses to infect red blood cells.
The researchers described their findings in a letter to Nature.
“The risk of developing severe malaria turns out to be strongly linked to the process by which the malaria parasite gains entry to the human red blood cell,” said Dr Kevin Marsh, of the Kemri-Wellcome Research Programme in Kilifi, Kenya.
“This study strengthens the argument for focusing on the malaria side of the parasite-human interaction in our search for new vaccine candidates.”
For this study, Dr Marsh and his colleagues analyzed data from 8 different African countries: Burkina Faso, Cameroon, Ghana, Kenya, Malawi, Mali, The Gambia, and Tanzania.
They compared the DNA of 5633 children with severe malaria and the DNA of 5919 children without severe malaria. The researchers then replicated their key findings in a further 14,000 children.
The locus the team identified is near a cluster of genes that code for glycophorins, which are involved in P falciparum’s invasion of red blood cells.
The researchers also found an allele that was common among children in Kenya. Having this allele reduced the risk of severe malaria by about 40% in Kenyan children, with a slightly smaller effect across all the other populations studied.
The team said this difference between populations could be due to the genetic features of the local malaria parasite in East Africa.
Balancing selection
The newly identified malaria resistance locus lies within a region of the genome where humans and chimpanzees have been known to share particular combinations of haplotypes.
This indicates that some of the variation seen in contemporary humans has been present for millions of years. The finding also suggests that this region of the genome is the subject of balancing selection.
Balancing selection happens when a particular genetic variant evolves because it confers health benefits, but it is carried by only a proportion of the population because it also has damaging consequences.
“These findings indicate that balancing selection and resistance to malaria are deeply intertwined themes in our ancient evolutionary history,” said Dr Dominic Kwiatkowski, of the Wellcome Trust Sanger Institute in Cambridge, UK.
“This new resistance locus is particularly interesting because it lies so close to genes that are gatekeepers for the malaria parasite’s invasion machinery. We now need to drill down at this locus to characterize these complex patterns of genetic variation more precisely and to understand the molecular mechanisms by which they act.” ![]()

outside of Nairobi, Kenya
Photo by Gabrielle Tenenbaum
Researchers say they have identified genetic variants that protect African children from developing severe malaria, in some cases nearly halving a child’s chance of developing the disease.
The variants are at a locus located next to a cluster of genes that are responsible for creating the receptors the malaria parasite Plasmodium falciparum uses to infect red blood cells.
The researchers described their findings in a letter to Nature.
“The risk of developing severe malaria turns out to be strongly linked to the process by which the malaria parasite gains entry to the human red blood cell,” said Dr Kevin Marsh, of the Kemri-Wellcome Research Programme in Kilifi, Kenya.
“This study strengthens the argument for focusing on the malaria side of the parasite-human interaction in our search for new vaccine candidates.”
For this study, Dr Marsh and his colleagues analyzed data from 8 different African countries: Burkina Faso, Cameroon, Ghana, Kenya, Malawi, Mali, The Gambia, and Tanzania.
They compared the DNA of 5633 children with severe malaria and the DNA of 5919 children without severe malaria. The researchers then replicated their key findings in a further 14,000 children.
The locus the team identified is near a cluster of genes that code for glycophorins, which are involved in P falciparum’s invasion of red blood cells.
The researchers also found an allele that was common among children in Kenya. Having this allele reduced the risk of severe malaria by about 40% in Kenyan children, with a slightly smaller effect across all the other populations studied.
The team said this difference between populations could be due to the genetic features of the local malaria parasite in East Africa.
Balancing selection
The newly identified malaria resistance locus lies within a region of the genome where humans and chimpanzees have been known to share particular combinations of haplotypes.
This indicates that some of the variation seen in contemporary humans has been present for millions of years. The finding also suggests that this region of the genome is the subject of balancing selection.
Balancing selection happens when a particular genetic variant evolves because it confers health benefits, but it is carried by only a proportion of the population because it also has damaging consequences.
“These findings indicate that balancing selection and resistance to malaria are deeply intertwined themes in our ancient evolutionary history,” said Dr Dominic Kwiatkowski, of the Wellcome Trust Sanger Institute in Cambridge, UK.
“This new resistance locus is particularly interesting because it lies so close to genes that are gatekeepers for the malaria parasite’s invasion machinery. We now need to drill down at this locus to characterize these complex patterns of genetic variation more precisely and to understand the molecular mechanisms by which they act.” ![]()

outside of Nairobi, Kenya
Photo by Gabrielle Tenenbaum
Researchers say they have identified genetic variants that protect African children from developing severe malaria, in some cases nearly halving a child’s chance of developing the disease.
The variants are at a locus located next to a cluster of genes that are responsible for creating the receptors the malaria parasite Plasmodium falciparum uses to infect red blood cells.
The researchers described their findings in a letter to Nature.
“The risk of developing severe malaria turns out to be strongly linked to the process by which the malaria parasite gains entry to the human red blood cell,” said Dr Kevin Marsh, of the Kemri-Wellcome Research Programme in Kilifi, Kenya.
“This study strengthens the argument for focusing on the malaria side of the parasite-human interaction in our search for new vaccine candidates.”
For this study, Dr Marsh and his colleagues analyzed data from 8 different African countries: Burkina Faso, Cameroon, Ghana, Kenya, Malawi, Mali, The Gambia, and Tanzania.
They compared the DNA of 5633 children with severe malaria and the DNA of 5919 children without severe malaria. The researchers then replicated their key findings in a further 14,000 children.
The locus the team identified is near a cluster of genes that code for glycophorins, which are involved in P falciparum’s invasion of red blood cells.
The researchers also found an allele that was common among children in Kenya. Having this allele reduced the risk of severe malaria by about 40% in Kenyan children, with a slightly smaller effect across all the other populations studied.
The team said this difference between populations could be due to the genetic features of the local malaria parasite in East Africa.
Balancing selection
The newly identified malaria resistance locus lies within a region of the genome where humans and chimpanzees have been known to share particular combinations of haplotypes.
This indicates that some of the variation seen in contemporary humans has been present for millions of years. The finding also suggests that this region of the genome is the subject of balancing selection.
Balancing selection happens when a particular genetic variant evolves because it confers health benefits, but it is carried by only a proportion of the population because it also has damaging consequences.
“These findings indicate that balancing selection and resistance to malaria are deeply intertwined themes in our ancient evolutionary history,” said Dr Dominic Kwiatkowski, of the Wellcome Trust Sanger Institute in Cambridge, UK.
“This new resistance locus is particularly interesting because it lies so close to genes that are gatekeepers for the malaria parasite’s invasion machinery. We now need to drill down at this locus to characterize these complex patterns of genetic variation more precisely and to understand the molecular mechanisms by which they act.” ![]()
Investigating Unstable Thyroid Function
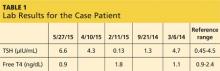
A 43-year-old man presents for his thyroid checkup. He has known hypothyroidism secondary to Hashimoto thyroiditis, also known as chronic lymphocytic thyroiditis. He is taking levothyroxine (LT4) 250 μg (two 125-μg tablets once per day). Review of his prior lab results and notes (see Table 1) reveals frequent dose changes (about every three to six months) and a high dosage of LT4, considering his weight (185 lb).
Patients with little or no residual thyroid function require replacement doses of LT4 at approximately 1.6 μg/kg/d, based on lean body weight.1 Since the case patient weighs 84 kg, the expected LT4 dosage would be around 125 to 150 μg/d.
This patient requires a significantly higher dose than expected, and his thyroid levels are fluctuating. These facts should trigger further investigation.
Important historical questions I consider when patients have frequent or significant fluctuations in TSH include
• Are you consistent in taking your medication?
• How do you take your thyroid medication?
• Are you taking any iron supplements, vitamins with iron, or contraceptive pills containing iron?
• Has there been any change in your other medication regimen(s) or medical condition(s)?
• Did you change pharmacies, or did the shape or color of your pill change?
• Have you experienced significant weight changes?
• Do you have any gastrointestinal complaints (nausea/vomiting/diarrhea/bloating)?
MEDICATION ADHERENCE
It is well known but still puzzling to hear that, overall, patients’ medication adherence is merely 50%.2 It is very important that you verify whether your patient is taking his/her medication consistently. Rather than asking “Are you taking your medications?” (to which they are more likely to answer “yes”), I ask “How many pills do you miss in a given week or month?”
For those who have a hard time remembering to take their medication on a regular basis, I recommend setting up a routine: Keep the medication at their bedside and take it first thing upon awakening, or place it beside the toothpaste so they see it every time they brush their teeth in the morning. Another option is of course to set up an alarm as a reminder.
Continue for rules for taking hypothyroid >>
RULES FOR TAKING HYPOTHYROID MEDICATIONS
Thyroid hormone replacement has a narrow therapeutic index, and a subtle change in dosage can significantly alter the therapeutic target. Hypothyroid medications are absorbed in the jejunum/ileum, and an acidic pH in the stomach is optimal for thyroid absorption.3 Therefore, taking the medication on an empty stomach (fasting) with a full glass of water and waiting at least one hour before breakfast is recommended, if possible. An alternate option is to take it at bedtime, at least three hours after the last meal. Taking medication along with food, especially high-fiber and soy products, can decrease absorption of thyroid hormone, which may result in an unstable thyroid function test.
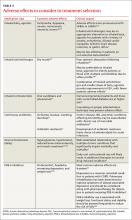
There are supplements and medications that can decrease hypothyroid medication absorption; it is recommended that patients separate these medications by four hours or more in order to minimize this interference. A full list is available in Table 2, but the most commonly encountered are iron supplements, calcium supplements, and proton pump inhibitors.2
In many patients—especially the elderly and those with multiple comorbidities that require polypharmacy—it can be very challenging, if not impossible, to isolate thyroid medication. For these patients, recommend that they be “consistent” with their routine to ensure they achieve a similar absorption rate each time. For example, a patient’s hypothyroid medication absorption might be reduced by 50% by taking it with omeprazole, but as long as the patient consistently takes the medication that way, she can have stable thyroid function.
NEW MEDICATION REGIMEN OR MEDICAL CONDITION
In addition to medications that can interfere with the absorption of thyroid hormone replacement, there are those that affect levels of thyroxine-binding globulin. This affects the bioavailability of thyroid hormones and alters thyroid status.
Thyroid hormones such as thyroxine (T4) and triiodothyronine (T3) are predominantly bound to carrier proteins, and < 1% is unbound (so-called free hormones). Changes in thyroid-binding proteins can alter free hormone levels and thereby change TSH levels. In disease-free euthyroid subjects, the body can compensate by adjusting hormone production for changes in binding proteins to keep the free hormone levels within normal ranges. However, patients who are at or near full replacement doses of hypothyroid medication cannot adjust to the changes.
In patients with hypothyroidism who are taking thyroid hormone replacement, medications or conditions that increase binding proteins will decrease free hormones (by increasing bound hormones) and thereby raise TSH (hypothyroid state). Vice versa, medications and conditions that decrease binding protein will increase free hormones (by decreasing bound hormones) and thereby lower TSH (thyrotoxic state). Table 3 lists commonly encountered medications and conditions associated with altered thyroid-binding proteins.1
It is important to consider pregnancy in women of childbearing age whose TSH has risen for no apparent reason, as their thyroid levels should be maintained in a narrow therapeutic range to prevent fetal complications. Details on thyroid disease during pregnancy can be found in the April 2015 Endocrine Consult, “Managing Thyroid Disease in Pregnancy.”
In women treated for hypothyroidism, starting or discontinuing estrogen-containing medications (birth control pills or hormone replacement therapy) often results in changes in thyroid status. It is a good practice to inform the patient about these changes and to recheck her thyroid labs four to eight weeks after she starts or discontinues estrogen, adjusting the dose if needed.
Continue for changes in manufacturer/brand >>
CHANGES IN MANUFACTURER/BRAND
There are currently multiple brands and generic manufacturers supplying hypothyroid medications and reports that absorption rates and bioavailability vary among them.2 Switching products can result in changes in thyroid status and in TSH levels.
Once a patient has reached euthyroid status, it is imperative to stay on the same dose from the same manufacturer. This may be challenging, as it can be affected by the patient’s insurance carrier, policy changes, or even a change in the pharmacy’s medication supplier. Although patients are supposed to be informed by the pharmacy when the manufacturer is being changed, you may want to educate them to check the shape, color, and dose of their pills and also verify that the manufacturer listed on the bottle is consistent each time they refill their hypothyroid medications. This is especially important for those who require a very narrow TSH target, such as young children, thyroid cancer patients, pregnant women, and frail patients.3
WEIGHT CHANGES
As mentioned, thyroid medications are weight-based, and big changes in weight can lead to changes in thyroid function studies. It is the lean body mass, rather than total body weight, that will affect the thyroid requirement.3 A quick review of the patient’s weight history needs to be done when thyroid function test results have changed.
GASTROINTESTINAL DISTURBANCES
Hypothyroid medications are absorbed in the small intestine, and gastric acidity levels have an impact on absorption. Any acute or chronic conditions that affect these areas can alter medication absorption quite significantly. Commonly encountered diseases and conditions are H pylori–related gastritis, atrophic gastritis, celiac disease, and lactose intolerance. Treating these diseases and conditions can improve medication absorption.
I went through the list with the patient, but there was no applicable scenario. I adjusted his medication but went ahead and tested for tissue transglutaminase antibody IgA to rule out celiac disease; results came back mildly positive. The patient was referred to a gastroenterologist, who performed a small intestine biopsy for definitive diagnosis. This revealed “severe” celiac disease. A strict gluten-free diet was started, and the patient’s LT4 dose was adjusted, with regular monitoring, down to 150 μg/d.
Common symptoms of celiac disease include bloating, abdominal pain, and loose stool after consumption of gluten-containing meals. It should be noted that this patient denied all these symptoms, even though he was asked specifically about them. After he started a gluten-free diet, he reported that he actually felt “very calm” in his abdomen and realized he did have symptoms of celiac disease—but he’d had them for so long that he considered it normal. As is often the case, presence of symptoms would raise suspicion ... but lack of symptoms (or report thereof) does not rule out the disease.
CONCLUSION
Most patients with hypothyroidism are fairly well managed with relatively stable medication dosages, but there are subsets of patients who struggle to maintain euthyroid range. The latter require frequent office visits and dosage changes. Carefully reviewing the list of possible reasons for thyroid level changes can improve stability and patient quality of life, prevent complications of fluctuating thyroid levels, and reduce medical costs, such as repeated labs and frequent clinic visits.
REFERENCES
1. Garber JR, Cobin RH, Gharib H, et al; American Association of Clinical Endocrinologists and American Thyroid Association Taskforce on Hypothyroidism in Adults. Clinical practice guidelines for hypothyroidism in adults [published correction appears in Endocr Pract. 2013;19(1):175]. Endocr Pract. 2012;18(6):988-1028.
2. Sabate E. Adherence to Long-Term Therapies: Evidence for Action. Geneva, Switzerland: World Health Organization; 2003.
3. Jonklaas J, Bianco AC, Bauer AJ, et al; American Thyroid Association Task Force on Thyroid Hormone Replacement. Guidelines for the treatment of hypothyroidism. Thyroid. 2014;24(12):1670-1751.
A 43-year-old man presents for his thyroid checkup. He has known hypothyroidism secondary to Hashimoto thyroiditis, also known as chronic lymphocytic thyroiditis. He is taking levothyroxine (LT4) 250 μg (two 125-μg tablets once per day). Review of his prior lab results and notes (see Table 1) reveals frequent dose changes (about every three to six months) and a high dosage of LT4, considering his weight (185 lb).
Patients with little or no residual thyroid function require replacement doses of LT4 at approximately 1.6 μg/kg/d, based on lean body weight.1 Since the case patient weighs 84 kg, the expected LT4 dosage would be around 125 to 150 μg/d.
This patient requires a significantly higher dose than expected, and his thyroid levels are fluctuating. These facts should trigger further investigation.
Important historical questions I consider when patients have frequent or significant fluctuations in TSH include
• Are you consistent in taking your medication?
• How do you take your thyroid medication?
• Are you taking any iron supplements, vitamins with iron, or contraceptive pills containing iron?
• Has there been any change in your other medication regimen(s) or medical condition(s)?
• Did you change pharmacies, or did the shape or color of your pill change?
• Have you experienced significant weight changes?
• Do you have any gastrointestinal complaints (nausea/vomiting/diarrhea/bloating)?
MEDICATION ADHERENCE
It is well known but still puzzling to hear that, overall, patients’ medication adherence is merely 50%.2 It is very important that you verify whether your patient is taking his/her medication consistently. Rather than asking “Are you taking your medications?” (to which they are more likely to answer “yes”), I ask “How many pills do you miss in a given week or month?”
For those who have a hard time remembering to take their medication on a regular basis, I recommend setting up a routine: Keep the medication at their bedside and take it first thing upon awakening, or place it beside the toothpaste so they see it every time they brush their teeth in the morning. Another option is of course to set up an alarm as a reminder.
Continue for rules for taking hypothyroid >>
RULES FOR TAKING HYPOTHYROID MEDICATIONS
Thyroid hormone replacement has a narrow therapeutic index, and a subtle change in dosage can significantly alter the therapeutic target. Hypothyroid medications are absorbed in the jejunum/ileum, and an acidic pH in the stomach is optimal for thyroid absorption.3 Therefore, taking the medication on an empty stomach (fasting) with a full glass of water and waiting at least one hour before breakfast is recommended, if possible. An alternate option is to take it at bedtime, at least three hours after the last meal. Taking medication along with food, especially high-fiber and soy products, can decrease absorption of thyroid hormone, which may result in an unstable thyroid function test.
There are supplements and medications that can decrease hypothyroid medication absorption; it is recommended that patients separate these medications by four hours or more in order to minimize this interference. A full list is available in Table 2, but the most commonly encountered are iron supplements, calcium supplements, and proton pump inhibitors.2
In many patients—especially the elderly and those with multiple comorbidities that require polypharmacy—it can be very challenging, if not impossible, to isolate thyroid medication. For these patients, recommend that they be “consistent” with their routine to ensure they achieve a similar absorption rate each time. For example, a patient’s hypothyroid medication absorption might be reduced by 50% by taking it with omeprazole, but as long as the patient consistently takes the medication that way, she can have stable thyroid function.
NEW MEDICATION REGIMEN OR MEDICAL CONDITION
In addition to medications that can interfere with the absorption of thyroid hormone replacement, there are those that affect levels of thyroxine-binding globulin. This affects the bioavailability of thyroid hormones and alters thyroid status.
Thyroid hormones such as thyroxine (T4) and triiodothyronine (T3) are predominantly bound to carrier proteins, and < 1% is unbound (so-called free hormones). Changes in thyroid-binding proteins can alter free hormone levels and thereby change TSH levels. In disease-free euthyroid subjects, the body can compensate by adjusting hormone production for changes in binding proteins to keep the free hormone levels within normal ranges. However, patients who are at or near full replacement doses of hypothyroid medication cannot adjust to the changes.
In patients with hypothyroidism who are taking thyroid hormone replacement, medications or conditions that increase binding proteins will decrease free hormones (by increasing bound hormones) and thereby raise TSH (hypothyroid state). Vice versa, medications and conditions that decrease binding protein will increase free hormones (by decreasing bound hormones) and thereby lower TSH (thyrotoxic state). Table 3 lists commonly encountered medications and conditions associated with altered thyroid-binding proteins.1
It is important to consider pregnancy in women of childbearing age whose TSH has risen for no apparent reason, as their thyroid levels should be maintained in a narrow therapeutic range to prevent fetal complications. Details on thyroid disease during pregnancy can be found in the April 2015 Endocrine Consult, “Managing Thyroid Disease in Pregnancy.”
In women treated for hypothyroidism, starting or discontinuing estrogen-containing medications (birth control pills or hormone replacement therapy) often results in changes in thyroid status. It is a good practice to inform the patient about these changes and to recheck her thyroid labs four to eight weeks after she starts or discontinues estrogen, adjusting the dose if needed.
Continue for changes in manufacturer/brand >>
CHANGES IN MANUFACTURER/BRAND
There are currently multiple brands and generic manufacturers supplying hypothyroid medications and reports that absorption rates and bioavailability vary among them.2 Switching products can result in changes in thyroid status and in TSH levels.
Once a patient has reached euthyroid status, it is imperative to stay on the same dose from the same manufacturer. This may be challenging, as it can be affected by the patient’s insurance carrier, policy changes, or even a change in the pharmacy’s medication supplier. Although patients are supposed to be informed by the pharmacy when the manufacturer is being changed, you may want to educate them to check the shape, color, and dose of their pills and also verify that the manufacturer listed on the bottle is consistent each time they refill their hypothyroid medications. This is especially important for those who require a very narrow TSH target, such as young children, thyroid cancer patients, pregnant women, and frail patients.3
WEIGHT CHANGES
As mentioned, thyroid medications are weight-based, and big changes in weight can lead to changes in thyroid function studies. It is the lean body mass, rather than total body weight, that will affect the thyroid requirement.3 A quick review of the patient’s weight history needs to be done when thyroid function test results have changed.
GASTROINTESTINAL DISTURBANCES
Hypothyroid medications are absorbed in the small intestine, and gastric acidity levels have an impact on absorption. Any acute or chronic conditions that affect these areas can alter medication absorption quite significantly. Commonly encountered diseases and conditions are H pylori–related gastritis, atrophic gastritis, celiac disease, and lactose intolerance. Treating these diseases and conditions can improve medication absorption.
I went through the list with the patient, but there was no applicable scenario. I adjusted his medication but went ahead and tested for tissue transglutaminase antibody IgA to rule out celiac disease; results came back mildly positive. The patient was referred to a gastroenterologist, who performed a small intestine biopsy for definitive diagnosis. This revealed “severe” celiac disease. A strict gluten-free diet was started, and the patient’s LT4 dose was adjusted, with regular monitoring, down to 150 μg/d.
Common symptoms of celiac disease include bloating, abdominal pain, and loose stool after consumption of gluten-containing meals. It should be noted that this patient denied all these symptoms, even though he was asked specifically about them. After he started a gluten-free diet, he reported that he actually felt “very calm” in his abdomen and realized he did have symptoms of celiac disease—but he’d had them for so long that he considered it normal. As is often the case, presence of symptoms would raise suspicion ... but lack of symptoms (or report thereof) does not rule out the disease.
CONCLUSION
Most patients with hypothyroidism are fairly well managed with relatively stable medication dosages, but there are subsets of patients who struggle to maintain euthyroid range. The latter require frequent office visits and dosage changes. Carefully reviewing the list of possible reasons for thyroid level changes can improve stability and patient quality of life, prevent complications of fluctuating thyroid levels, and reduce medical costs, such as repeated labs and frequent clinic visits.
REFERENCES
1. Garber JR, Cobin RH, Gharib H, et al; American Association of Clinical Endocrinologists and American Thyroid Association Taskforce on Hypothyroidism in Adults. Clinical practice guidelines for hypothyroidism in adults [published correction appears in Endocr Pract. 2013;19(1):175]. Endocr Pract. 2012;18(6):988-1028.
2. Sabate E. Adherence to Long-Term Therapies: Evidence for Action. Geneva, Switzerland: World Health Organization; 2003.
3. Jonklaas J, Bianco AC, Bauer AJ, et al; American Thyroid Association Task Force on Thyroid Hormone Replacement. Guidelines for the treatment of hypothyroidism. Thyroid. 2014;24(12):1670-1751.
A 43-year-old man presents for his thyroid checkup. He has known hypothyroidism secondary to Hashimoto thyroiditis, also known as chronic lymphocytic thyroiditis. He is taking levothyroxine (LT4) 250 μg (two 125-μg tablets once per day). Review of his prior lab results and notes (see Table 1) reveals frequent dose changes (about every three to six months) and a high dosage of LT4, considering his weight (185 lb).
Patients with little or no residual thyroid function require replacement doses of LT4 at approximately 1.6 μg/kg/d, based on lean body weight.1 Since the case patient weighs 84 kg, the expected LT4 dosage would be around 125 to 150 μg/d.
This patient requires a significantly higher dose than expected, and his thyroid levels are fluctuating. These facts should trigger further investigation.
Important historical questions I consider when patients have frequent or significant fluctuations in TSH include
• Are you consistent in taking your medication?
• How do you take your thyroid medication?
• Are you taking any iron supplements, vitamins with iron, or contraceptive pills containing iron?
• Has there been any change in your other medication regimen(s) or medical condition(s)?
• Did you change pharmacies, or did the shape or color of your pill change?
• Have you experienced significant weight changes?
• Do you have any gastrointestinal complaints (nausea/vomiting/diarrhea/bloating)?
MEDICATION ADHERENCE
It is well known but still puzzling to hear that, overall, patients’ medication adherence is merely 50%.2 It is very important that you verify whether your patient is taking his/her medication consistently. Rather than asking “Are you taking your medications?” (to which they are more likely to answer “yes”), I ask “How many pills do you miss in a given week or month?”
For those who have a hard time remembering to take their medication on a regular basis, I recommend setting up a routine: Keep the medication at their bedside and take it first thing upon awakening, or place it beside the toothpaste so they see it every time they brush their teeth in the morning. Another option is of course to set up an alarm as a reminder.
Continue for rules for taking hypothyroid >>
RULES FOR TAKING HYPOTHYROID MEDICATIONS
Thyroid hormone replacement has a narrow therapeutic index, and a subtle change in dosage can significantly alter the therapeutic target. Hypothyroid medications are absorbed in the jejunum/ileum, and an acidic pH in the stomach is optimal for thyroid absorption.3 Therefore, taking the medication on an empty stomach (fasting) with a full glass of water and waiting at least one hour before breakfast is recommended, if possible. An alternate option is to take it at bedtime, at least three hours after the last meal. Taking medication along with food, especially high-fiber and soy products, can decrease absorption of thyroid hormone, which may result in an unstable thyroid function test.
There are supplements and medications that can decrease hypothyroid medication absorption; it is recommended that patients separate these medications by four hours or more in order to minimize this interference. A full list is available in Table 2, but the most commonly encountered are iron supplements, calcium supplements, and proton pump inhibitors.2
In many patients—especially the elderly and those with multiple comorbidities that require polypharmacy—it can be very challenging, if not impossible, to isolate thyroid medication. For these patients, recommend that they be “consistent” with their routine to ensure they achieve a similar absorption rate each time. For example, a patient’s hypothyroid medication absorption might be reduced by 50% by taking it with omeprazole, but as long as the patient consistently takes the medication that way, she can have stable thyroid function.
NEW MEDICATION REGIMEN OR MEDICAL CONDITION
In addition to medications that can interfere with the absorption of thyroid hormone replacement, there are those that affect levels of thyroxine-binding globulin. This affects the bioavailability of thyroid hormones and alters thyroid status.
Thyroid hormones such as thyroxine (T4) and triiodothyronine (T3) are predominantly bound to carrier proteins, and < 1% is unbound (so-called free hormones). Changes in thyroid-binding proteins can alter free hormone levels and thereby change TSH levels. In disease-free euthyroid subjects, the body can compensate by adjusting hormone production for changes in binding proteins to keep the free hormone levels within normal ranges. However, patients who are at or near full replacement doses of hypothyroid medication cannot adjust to the changes.
In patients with hypothyroidism who are taking thyroid hormone replacement, medications or conditions that increase binding proteins will decrease free hormones (by increasing bound hormones) and thereby raise TSH (hypothyroid state). Vice versa, medications and conditions that decrease binding protein will increase free hormones (by decreasing bound hormones) and thereby lower TSH (thyrotoxic state). Table 3 lists commonly encountered medications and conditions associated with altered thyroid-binding proteins.1
It is important to consider pregnancy in women of childbearing age whose TSH has risen for no apparent reason, as their thyroid levels should be maintained in a narrow therapeutic range to prevent fetal complications. Details on thyroid disease during pregnancy can be found in the April 2015 Endocrine Consult, “Managing Thyroid Disease in Pregnancy.”
In women treated for hypothyroidism, starting or discontinuing estrogen-containing medications (birth control pills or hormone replacement therapy) often results in changes in thyroid status. It is a good practice to inform the patient about these changes and to recheck her thyroid labs four to eight weeks after she starts or discontinues estrogen, adjusting the dose if needed.
Continue for changes in manufacturer/brand >>
CHANGES IN MANUFACTURER/BRAND
There are currently multiple brands and generic manufacturers supplying hypothyroid medications and reports that absorption rates and bioavailability vary among them.2 Switching products can result in changes in thyroid status and in TSH levels.
Once a patient has reached euthyroid status, it is imperative to stay on the same dose from the same manufacturer. This may be challenging, as it can be affected by the patient’s insurance carrier, policy changes, or even a change in the pharmacy’s medication supplier. Although patients are supposed to be informed by the pharmacy when the manufacturer is being changed, you may want to educate them to check the shape, color, and dose of their pills and also verify that the manufacturer listed on the bottle is consistent each time they refill their hypothyroid medications. This is especially important for those who require a very narrow TSH target, such as young children, thyroid cancer patients, pregnant women, and frail patients.3
WEIGHT CHANGES
As mentioned, thyroid medications are weight-based, and big changes in weight can lead to changes in thyroid function studies. It is the lean body mass, rather than total body weight, that will affect the thyroid requirement.3 A quick review of the patient’s weight history needs to be done when thyroid function test results have changed.
GASTROINTESTINAL DISTURBANCES
Hypothyroid medications are absorbed in the small intestine, and gastric acidity levels have an impact on absorption. Any acute or chronic conditions that affect these areas can alter medication absorption quite significantly. Commonly encountered diseases and conditions are H pylori–related gastritis, atrophic gastritis, celiac disease, and lactose intolerance. Treating these diseases and conditions can improve medication absorption.
I went through the list with the patient, but there was no applicable scenario. I adjusted his medication but went ahead and tested for tissue transglutaminase antibody IgA to rule out celiac disease; results came back mildly positive. The patient was referred to a gastroenterologist, who performed a small intestine biopsy for definitive diagnosis. This revealed “severe” celiac disease. A strict gluten-free diet was started, and the patient’s LT4 dose was adjusted, with regular monitoring, down to 150 μg/d.
Common symptoms of celiac disease include bloating, abdominal pain, and loose stool after consumption of gluten-containing meals. It should be noted that this patient denied all these symptoms, even though he was asked specifically about them. After he started a gluten-free diet, he reported that he actually felt “very calm” in his abdomen and realized he did have symptoms of celiac disease—but he’d had them for so long that he considered it normal. As is often the case, presence of symptoms would raise suspicion ... but lack of symptoms (or report thereof) does not rule out the disease.
CONCLUSION
Most patients with hypothyroidism are fairly well managed with relatively stable medication dosages, but there are subsets of patients who struggle to maintain euthyroid range. The latter require frequent office visits and dosage changes. Carefully reviewing the list of possible reasons for thyroid level changes can improve stability and patient quality of life, prevent complications of fluctuating thyroid levels, and reduce medical costs, such as repeated labs and frequent clinic visits.
REFERENCES
1. Garber JR, Cobin RH, Gharib H, et al; American Association of Clinical Endocrinologists and American Thyroid Association Taskforce on Hypothyroidism in Adults. Clinical practice guidelines for hypothyroidism in adults [published correction appears in Endocr Pract. 2013;19(1):175]. Endocr Pract. 2012;18(6):988-1028.
2. Sabate E. Adherence to Long-Term Therapies: Evidence for Action. Geneva, Switzerland: World Health Organization; 2003.
3. Jonklaas J, Bianco AC, Bauer AJ, et al; American Thyroid Association Task Force on Thyroid Hormone Replacement. Guidelines for the treatment of hypothyroidism. Thyroid. 2014;24(12):1670-1751.
COPD: Optimizing treatment
› Individualize treatment regimens based on severity of symptoms and risk for exacerbation, prescribing short-acting beta2-agonists, as needed, for all patients with chronic obstructive pulmonary disease (COPD). A
› Limit use of inhaled long-acting beta2-agonists to the recommended dosage; higher doses do not lead to better outcomes. A
Strength of recommendation (SOR)
A Good-quality patient-oriented evidence
B Inconsistent or limited-quality patient-oriented evidence
C Consensus, usual practice, opinion, disease-oriented evidence, case series
Chronic obstructive pulmonary disease (COPD) carries a high disease burden. In 2012, it was the 4th leading cause of death worldwide.1,2 In 2015, the World Health Organization updated its Global Initiative for Chronic Obstructive Lung Disease (GOLD) guidelines, classifying patients with COPD based on disease burden as determined by symptoms, airflow obstruction, and exacerbation history.3 These revisions, coupled with expanded therapeutic options within established classes of medications and new combination drugs to treat COPD (TABLE 1),3-6 have led to questions about interclass differences and the best treatment regimen for particular patients.
Comparisons of various agents within a therapeutic class and their impact on lung function and rate of exacerbations address many of these concerns. In the text and tables that follow, we present the latest evidence highlighting differences in dosing, safety, and efficacy. We also include the updated GOLD classifications, evidence of efficacy for pulmonary rehabilitation, and practical implications of these findings for the optimal management of patients with COPD.
But first, a word about terminology.
Understanding COPD
COPD is a chronic lung disease characterized by progressive airflow limitation, usually measured by spirometry (TABLE 2),3 and chronic airway inflammation. Emphysema and chronic bronchitis are often used synonymously with COPD. In fact, there are important differences.
Individuals with chronic bronchitis do not necessarily have the airflow limitations found in those with COPD. And patients with COPD develop pathologic lung changes beyond the alveolar damage characteristic of emphysema, including airway fibrosis and inflammation, luminal plugging, and loss of elastic recoil.3
The medications included in this review aim to reduce both the morbidity and mortality associated with COPD. These drugs can also help relieve the symptoms of patients with chronic bronchitis and emphysema, but have limited effect on patient mortality.
Short- and long-acting beta2-agonists
Bronchodilator therapy with beta2-agonists improves forced expiratory volume in one second (FEV1) through relaxation of airway smooth muscle. Beta2-agonists have proven to be safe and effective when used as needed or scheduled for patients with COPD.7
Inhaled short-acting beta2-agonists (SABAs) improve FEV1 and symptoms within 10 minutes, with effects lasting up to 4 to 6 hours; long-acting beta2-agonists (LABAs) have a variable onset, with effects lasting 12 to 24 hours.8 Inhaled levalbuterol, the last SABA to receive US Food and Drug Administration approval, has not proven to be superior to conventional bronchodilators in ambulatory patients with stable COPD.3 In clinical trials, however, the slightly longer half-life of the nebulized formulation of levalbuterol was found to reduce both the frequency of administration and the overall cost of therapy in patients hospitalized with acute exacerbations of COPD.9,10
Recently approved LABAs
Clinical trials have studied the safety and efficacy of newer agents vs older LABAs in patients with moderate to severe COPD. Compared with theophylline, for example, formoterol 12 mcg inhaled every 12 hours for a 12-month period provided a clinically significant increase of >120 ml in FEV1 (P=.026).11 Higher doses of formoterol did not provide any additional improvement.
In a trial comparing indacaterol and tiotropium, an inhaled anticholinergic, both treatment groups had a clinically significant increase in FEV1, but patients receiving indacaterol achieved an additional increase of 40 to 50 mL at 12 weeks.12
Exacerbation rates for all LABAs range from 22% to 44%.5,12,13 In a study of patients receiving formoterol 12 mcg compared with 15-mcg and 25-mcg doses of arformoterol, those taking formoterol had a lower exacerbation rate than those on either strength of arformoterol (22% vs 32% and 31%, respectively).10 In various studies, doses greater than the FDA-approved regimens for indacaterol, arformoterol, and olodaterol did not result in a significant improvement in either FEV1 or exacerbation rates compared with placebo.5,12,14
Studies that assessed the use of rescue medication as well as exacerbation rates in patients taking LABAs reported reductions in the use of the rescue drugs ranging from 0.46 to 1.32 actuations per day, but the findings had limited clinical relevance.5,13 With the exception of indacaterol and olodaterol—both of which may be preferable because of their once-daily dosing regimen—no significant differences in safety and efficacy among LABAs have been found.5,12,13
Long-acting inhaled anticholinergics
Inhaled anticholinergic agents (IACs) can be used in place of, or in conjunction with, LABAs to provide bronchodilation for up to 24 hours.3 The introduction of long-acting IACs dosed once or twice daily has the potential to improve medication adherence over traditional short-acting ipratropium, which requires multiple daily doses for symptom control. Over 4 years, tiotropium has been shown to increase time to first exacerbation by approximately 4 months. It did not, however, significantly reduce the number of exacerbations compared with placebo.15
Long-term use of tiotropium appears to have the potential to preserve lung function. In one trial, it slowed the rate of decline in FEV1 by 5 mL per year, but this finding lacked clinical significance.13 In clinical trials of patients with moderate to severe COPD, however, once-daily tiotropium and umeclidinium provided clinically significant improvements in FEV1 (>120 mL; P<.01), regardless of the dose administered.6,16 In another trial, patients taking aclidinium 200 mcg or 400 mcg every 12 hours did not achieve a clinically significant improvement in FEV1 compared with placebo.17
In patients with moderate to severe COPD, the combination of umeclidinium/vilanterol, a LABA, administered once daily resulted in a clinically significant improvement in FEV1 (167 mL; P<.001) vs placebo—but was not significantly better than treatment with either agent alone.18
Few studies have evaluated time to exacerbation in patients receiving aclidinium or umeclidinium. In comparison to salmeterol, tiotropium reduced the time to first exacerbation by 42 days at one year (hazard ratio=0.83; 95% confidence interval [CI], 0.77-0.9; P<.001).19 The evidence suggests that when used in combination with LABAs, long-acting IACs have a positive impact on FEV1, but their effect on exacerbation rates has not been established.
Combination therapy with steroids and LABAs
The combination of inhaled corticosteroids (ICS) and LABAs has been found to improve FEV1 and symptoms in patients with moderate to severe COPD more than monotherapy with either drug class.20,21 In fact, ICS alone have not been proven to slow the progression of the disease or to lower mortality rates in patients with COPD.22
Fluticasone/salmeterol demonstrated a 25% reduction in exacerbation rates compared with placebo (P<.0001), a greater reduction than that of either drug alone.20 A retrospective observational study comparing fixed dose fluticasone/salmeterol with budesonide/formoterol reported a similar reduction in exacerbation rates, but the number of patients requiring the addition of an IAC was 16% lower in the latter group.23
The combination of fluticasone/vilanterol has the potential to improve adherence, given that it is dosed once daily, unlike other COPD combination drugs. Its clinical efficacy is comparable to that of fluticasone/salmeterol after 12 weeks of therapy, with similar improvements in FEV1,24 but fluticasone/vilanterol is associated with an increased risk of pneumonia.3
Chronic use of oral corticosteroids
Oral corticosteroids (OCS) are clinically indicated in individuals whose symptoms continue despite optimal therapy with inhaled agents that have demonstrated efficacy. Such patients are often referred to as “steroid dependent.”
While OCS are prescribed for both their anti-inflammatory activity and their ability to slow the progression of COPD,25,26 no well-designed studies have investigated their benefits for this patient population. One study concluded that patients who were slowly withdrawn from their OCS regimen had no more frequent exacerbations than those who maintained chronic usage. The withdrawal group did, however, lose weight.27
GOLD guidelines do not recommend OCS for chronic management of COPD due to the risk of toxicity.3 The well-established adverse effects of chronic OCS include hyperglycemia, hypertension, osteoporosis, and myopathy.28,29 A study of muscle function in 21 COPD patients receiving corticosteroids revealed decreases in quadriceps muscle strength and pulmonary function.30 Daily use of OCS will likely result in additional therapies to control drug-induced conditions, as well—another antihypertensive secondary to fluid retention caused by chronic use of OCS in patients with high blood pressure, for example, or additional medication to control elevated blood glucose levels in patients with diabetes.
Phosphodiesterase-4 inhibitors
The recommendation for roflumilast in patients with GOLD Class 2 to 4 symptoms remains unchanged since the introduction of this agent as a treatment option for COPD.3 Phosphodiesterase-4 (PDE-4) inhibitors such as roflumilast reduce inflammation in the lungs and have no activity as a bronchodilator.31,32
Roflumilast has been shown to improve FEV1 in patients concurrently receiving a long-acting bronchodilator and to reduce exacerbations in steroid-dependent patients, a recent systematic review of 29 PDE-4 trials found.33 Patients taking roflumilast, however, suffered from more adverse events (nausea, appetite reduction, diarrhea, weight loss, sleep disturbances, and headache) than those on placebo.33
Antibiotics
GOLD guidelines do not recommend the use of antibiotics for patients with COPD, except to treat acute exacerbations.1 However, recent studies suggest that routine or pulsed dosing of prophylactic antibiotics can reduce the number of exacerbations.34-36 A 2013 review of 7 studies determined that continuous antibiotics, particularly macrolides, reduced the number of COPD exacerbations in patients with a mean age of 66 years (odds ratio [OR]=0.55; 95% CI, 0.39-0.77).37
A more recent trial randomized 92 patients with a history of ≥3 exacerbations in the previous year to receive either prophylactic azithromycin or placebo daily for 12 months. The treatment group experienced a significant decrease in the number of exacerbations (OR=0.58; 95% CI, 0.42-0.79; P=.001).38 This benefit must be weighed against the potential development of antibiotic resistance and adverse effects, so careful patient selection is important.
Pulmonary rehabilitation has proven benefits
GOLD, the American College of Chest Physicians, the American Thoracic Society, and the European Respiratory Society all recommend pulmonary rehabilitation for patients with COPD.39-41 In addition to reducing morbidity and mortality rates—including a reduction in number of hospitalizations and length of stay and improved post-discharge recovery—pulmonary rehabilitation has been shown to have other physical and psychological benefits.42 Specific benefits include improved exercise capacity, greater arm strength and endurance, reduced perception of intensity of breathlessness, and improved overall health-related quality of life.
Key features of rehab programs
Important components of pulmonary rehabilitation include counseling on tobacco cessation, nutrition, education—including correct inhalation technique—and exercise training. There are few contraindications to participation, and patients can derive benefit from both its non-exercise components and upper extremity training regardless of their mobility level.
A 2006 Cochrane review concluded that an effective pulmonary rehabilitation program should be at least 4 weeks in duration,43 and longer programs have been shown to produce greater benefits.44 However, there is no agreement on an optimal time frame. Studies are inconclusive on other specific aspects of pulmonary rehab programs, as well, such as the number of sessions per week, number of hours per session, duration and intensity of exercise regimens, and staff-to-patient ratios.
Home-based exercise training may produce many of the same benefits as a formal pulmonary rehabilitation program. A systematic review found improved quality of life and exercise capacity associated with patient care that lacked formal pulmonary rehabilitation, with no differences between results from home-based training and hospital-based outpatient pulmonary rehabilitation programs.45
Given the lack of availability of formal rehab programs in many communities, homebased training for patients with COPD is important to consider.
Implications for practice
What is the takeaway from this evidence-based review? Overall, it is clear that, with the possible exception of the effect of once-daily dosing on adherence, there is little difference among the therapeutic agents within a particular class of medications—and that more is not necessarily better. Indeed, evidence suggests that higher doses of LABAs may reduce their effectiveness, rendering them no better than placebo. In addition, there is no significant difference in the rate of exacerbations in patients taking ICS/LABA combinations and those receiving IACs alone.
Pulmonary rehabilitation should be recommended for all newly diagnosed patients, while appropriate drug therapies should be individualized based on the GOLD symptoms/risk evaluation categories (TABLE 3).3 While daily OCS and daily antibiotics have the potential to reduce exacerbation rates, for example, the risks of adverse effects and toxicities outweigh the benefits for patients whose condition is stable.
Determining the optimal treatment for a particular patient also requires an assessment of comorbidities, including potential adverse drug effects (TABLE 4).3,27-29,33,46-52 Selection of medication should be driven by patient and physician preference to optimize adherence and clinical outcomes, although cost and accessibility often play a significant role, as well.
CORRESPONDENCE
Nabila Ahmed-Sarwar, PharmD, BCPS, CDE, St. John Fisher College, Wegmans School of Pharmacy, 3690 East Avenue, Rochester, NY 14618; [email protected]
ACKNOWLEDGEMENTS
The authors thank the following people for their assistance in the preparation of this manuscript: Matthew Stryker, PharmD, Timothy Adler, PharmD, and Angela K. Nagel, PharmD, BCPS.
1. World Health Organization. Chronic obstructive pulmonary disease (COPD). Fact Sheet No. 315. World Health Organization Web site. Available at: http://www.who.int/mediacentre/factsheets/fs315/en/. Accessed January 29, 2015.
2. National Heart, Lung, and Blood Institute. Morbidity and mortality: 2012 chart book on cardiovascular, lung, and blood diseases. National Heart, Lung, and Blood Institute Web site. Available at: http://www.nhlbi.nih.gov/files/docs/research/2012_Chart-Book_508.pdf. Accessed January 29, 2015.
3. Global Initiative for Chronic Obstructive Lung Disease. Global strategy for the diagnosis, management, and prevention of chronic obstructive pulmonary disease. Updated 2015. Global Initiative for Chronic Obstructive Lung Disease Web site. Available at: http://www.goldcopd.org/uploads/users/files/GOLD_Report_2015_Sept2.pdf. Accessed July 26, 2015.
4. Hanrahan JP, Hanania NA, Calhoun WJ, et al. Effect of nebulized arformoterol on airway function in COPD: results from two randomized trials. COPD. 2008;5:25-34.
5. Hanania NA, Donohue JF, Nelson H, et al. The safety and efficacy of arformoterol and formoterol in COPD. COPD. 2010;7:17-31.
6. Trivedi R, Richard N, Mehta R, et al. Umeclidinium in patients with COPD: a randomised, placebo-controlled study. Eur Respir J. 2014;43:72-81.
7. Vathenen AS, Britton JR, Ebden P, et al. High-dose inhaled albuterol in severe chronic airflow limitation. Am Rev Respir Dis. 1988;138:850-855.
8. Cazzola M, Matera MG, Santangelo G, et al. Salmeterol and formoterol in partially reversible severe chronic obstructive pulmonary disease: a dose-response study. Respir Med. 1995;89:357-362.
9. Donohue JF, Hanania NA, Ciubotaru RL, et al. Comparison of levalbuterol and racemic albuterol in hospitalized patients with acute asthma or COPD: a 2-week, multicenter, randomized, open-label study. Clin Ther. 2008;30:989-1002.
10. Truitt T, Witko J, Halpern M. Levalbuterol compared to racemic albuterol: efficacy and outcomes in patients hospitalized with COPD or asthma. Chest. 2003;123:128-135.
11. Rossi A, Kristufek P, Levine BE, et al; Formoterol in Chronic Obstructive Pulmonary Disease (FICOPD) II Study Group. Comparison of the efficacy, tolerability, and safety of formoterol dry powder and oral, slow-release theophylline in the treatment of COPD. Chest. 2002;121:1058-1069.
12. Donohue JF, Fogarty C, Lötvall J, et al; INHANCE Study Investigators. Once-daily bronchodilators for chronic obstructive pulmonary disease: indacaterol versus tiotropium. Am J Respir Crit Care Med. 2010;182:155-162.
13. Ferguson GT, Feldman GJ, Hofbauer P, et al. Efficacy and safety of olodaterol once daily delivered via Respimat® in patients with GOLD 2-4 COPD: results from two replicate 48-week studies. Int J Chron Obstruct Pulmon Dis. 2014;9:629-645.
14. Boyd G, Morice AH, Pounsford JC, et al. An evaluation of salmeterol in the treatment of chronic obstructive pulmonary disease (COPD). Eur Respir J. 1997;10:815-821.
15. Tashkin DP, Celli B, Senn S, et al; UPLIFT Study Investigators. A 4-year trial of tiotropium in chronic obstructive pulmonary disease. N Engl J Med. 2008;359:1543-1554.
16. Casaburi R, Mahler DA, Jones PW, et al. A long-term evaluation of once-daily inhaled tiotropium in chronic obstructive pulmonary disease. Eur Respir J. 2002;19:217-224.
17. Jones PW, Singh D, Bateman ED, et al. Efficacy and safety of twice-daily aclidinium bromide in COPD patients: the ATTAIN study. Eur Respir J. 2012;40:830-836.
18. Donohue JF, Maleki-Yazdi MR, Kilbride S, et al. Efficacy and safety of once-daily umeclidinium/vilanterol 62.5/25 mcg in COPD. Respir Med. 2013;107:1538-1546.
19. Vogelmeier C, Hederer B, Glaab T, et al; POET-COPD Investigators. Tiotropium versus salmeterol for the prevention of exacerbations of COPD. N Engl J Med. 2011;364:1093-1103.
20. Calverley P, Pauwels R, Vestbo J, et al; Trial of inhaled steroids and long-acting beta2 agonists study group. Combined salmeterol and fluticasone in the treatment of chronic obstructive pulmonary disease: a randomised controlled trial. Lancet. 2003;361:449-456.
21. Szafranski W, Cukier A, Ramirez A, et al. Efficacy and safety of budesonide/formoterol in the management of chronic obstructive pulmonary disease. Eur Respir J. 2003;21:74-81.
22. Calverley PM, Anderson JA, Celli B, et al; TORCH investigators. Salmeterol and fluticasone propionate and survival in chronic obstructive pulmonary disease. N Engl J Med. 2007;356:775-789.
23. Larsson K, Janson C, Lisspers K, et al. Combination of budesonide/formoterol more effective than fluticasone/salmeterol in preventing exacerbations in chronic obstructive pulmonary disease: the PATHOS study. J Intern Med. 2013;273:584-594.
24. Dransfield MT, Feldman G, Korenblat P, et al. Efficacy and safety of once-daily fluticasone furoate/vilanterol (100/25 mcg) versus twice-daily fluticasone propionate/salmeterol (250/50 mcg) in COPD patients. Respir Med. 2014;108:1171-1179.
25. Davies L, Nisar M, Pearson MG, et al. Oral corticosteroid trials in the management of stable chronic obstructive pulmonary disease. QJM. 1999;92:395-400.
26. Walters JA, Walters EH, Wood-Baker R. Oral corticosteroids for stable chronic obstructive pulmonary disease. Cochrane Database Syst Rev. 2005;CD005374.
27. Rice KL, Rubins JB, Lebahn F, et al. Withdrawal of chronic systemic corticosteroids in patients with COPD: a randomized trial. Am J Respir Crit Care Med. 2000;162:174-178.
28. Clore JN, Thurby-Hay L. Glucocorticoid-induced hyperglycemia. Endocr Pract. 2009;15:469-474.
29. McEvoy CE, Ensrud KE, Bender E, et al. Association between corticosteroid use and vertebral fractures in older men with chronic obstructive pulmonary disease. Am J Respir Crit Care Med. 1998;157:704-709.
30. Decramer M, Lacquet LM, Fagard R, et al. Corticosteroids contribute to muscle weakness in chronic airflow obstruction. Am J Respir Crit Care Med. 1994;150:11-16.
31. Fabbri LM, Calverley PM, Izquierdo-Alonso JL, et al; M2-127 and M2-128 study groups. Roflumilast in moderate-to-severe chronic obstructive pulmonary disease treated with longacting bronchodilators: two randomised clinical trials. Lancet. 2009;374:695-703.
32. Calverley PM, Rabe KF, Goehring UM, et al; M2-124 and M2-125 study groups. Roflumilast in symptomatic chronic obstructive pulmonary disease: two randomised clinical trials. Lancet. 2009;374:685-694.
33. Chong J, Leung B, Poole P. Phosphodiesterase 4 inhibitors for chronic obstructive pulmonary disease. Cochrane Database Syst Rev. 2013;11:CD002309.
34. Seemungal TA, Wilkinson TM, Hurst JR, et al. Long-term erythromycin therapy is associated with decreased chronic obstructive pulmonary disease exacerbations. Am J Respir Crit Care Med. 2008;178:1139-1147.
35. Sethi S, Jones PW, Theron MS, et al; PULSE study group. Pulsed moxifloxacin for the prevention of exacerbations of chronic obstructive pulmonary disease: a randomized controlled trial. Respir Res. 2010;11:10.
36. Albert RK, Connett J, Bailey WC, et al; COPD Clinical Research Network. Azithromycin for prevention of exacerbations of COPD. N Engl J Med. 2011;365:689-698.
37. Herath SC, Poole P. Prophylactic antibiotic therapy for chronic obstructive pulmonary disease (COPD). Cochrane Database Syst Rev. 2013;11:CD009764.
38. Uzun S, Djamin RS, Kluytmans JA, et al. Azithromycin maintenance treatment in patients with frequent exacerbations of chronic obstructive pulmonary disease (COLUMBUS): a randomised, double-blind, placebo-controlled trial. Lancet Respir Med. 2014;2:361-368.
39. Ries AL, Bauldoff GS, Carlin BW, et al. Pulmonary rehabilitation: joint ACCP/AACVPR evidence-based clinical practice guidelines. Chest. 2007;131:S4-S42.
40. Spruit MA, Singh SJ, Garvey C, et al; ATS/ERS Task Force on Pulmonary Rehabilitation. An official American Thoracic Society/European Respiratory Society statement: key concepts and advances in pulmonary rehabilitation. Am J Respir Crit Care Med. 2013;188:e13-e64.
41. Qaseem A, Wilt TJ, Weinberger SE, et al; American College of Physicians; American College of Chest Physicians; American Thoracic Society; European Respiratory Society. Diagnosis and management of stable chronic obstructive pulmonary disease: a clinical practice guideline update from the American College of Physicians, American College of Chest Physicians, American Thoracic Society, and European Respiratory Society. Ann Intern Med. 2011;155:179-191.
42. Global Initiative for Chronic Obstructive Lung Disease. Global strategy for the diagnosis, management, and prevention of chronic obstructive pulmonary disease. Updated 2013. Global Initiative for Chronic Obstructive Lung Disease Web site. Available at: http://www.goldcopd.org/uploads/users/files/GOLD_Report_2013_Feb20.pdf. Accessed January 14, 2015.
43. Lacasse Y, Goldstein R, Lasserson TJ, et al. Pulmonary rehabilitation for chronic obstructive pulmonary disease. Cochrane Database Syst Rev. 2006;CD003793.
44. Beauchamp MK, Janaudis-Ferreira T, Goldstein RS, et al. Optimal duration of pulmonary rehabilitation for individuals with chronic obstructive pulmonary disease - a systematic review. Chron Respir Dis. 2011;8:129-140.
45. Vieira DS, Maltais F, Bourbeau J. Home-based pulmonary rehabilitation in chronic obstructive pulmonary disease patients. Curr Opin Pulm Med. 2010;16:134-143.
46. Proair HFM (albuterol sulfate) [package insert]. Miami, FL: IVAX Laboratories; 2005.
47. Foradil (formoterol fumarate) [package insert]. Whitehouse Station, NJ: Merck & Co; 2012.
48. Spiriva (tiotropium bromide) [package insert]. Ridgefield, Conn: Boehringer Ingelheim Pharmaceuticals; 2014.
49. Fried TR, Vaz Fragoso CA, Rabow MW. Caring for the older person with chronic obstructive pulmonary disease. JAMA. 2012;308:1254-1263.
50. Flovent HFA (fluticasone propionate) [package insert]. Research Triangle Park, NC: GlaxoSmithKline; 2014.
51. Zithromax (azithromycin) [package insert]. New York, NY: Pfizer Labs; 2013.
52. Daliresp (roflumilast) [package insert]. St. Louis, Mo: Forest Pharmaceuticals; 2013.
› Individualize treatment regimens based on severity of symptoms and risk for exacerbation, prescribing short-acting beta2-agonists, as needed, for all patients with chronic obstructive pulmonary disease (COPD). A
› Limit use of inhaled long-acting beta2-agonists to the recommended dosage; higher doses do not lead to better outcomes. A
Strength of recommendation (SOR)
A Good-quality patient-oriented evidence
B Inconsistent or limited-quality patient-oriented evidence
C Consensus, usual practice, opinion, disease-oriented evidence, case series
Chronic obstructive pulmonary disease (COPD) carries a high disease burden. In 2012, it was the 4th leading cause of death worldwide.1,2 In 2015, the World Health Organization updated its Global Initiative for Chronic Obstructive Lung Disease (GOLD) guidelines, classifying patients with COPD based on disease burden as determined by symptoms, airflow obstruction, and exacerbation history.3 These revisions, coupled with expanded therapeutic options within established classes of medications and new combination drugs to treat COPD (TABLE 1),3-6 have led to questions about interclass differences and the best treatment regimen for particular patients.
Comparisons of various agents within a therapeutic class and their impact on lung function and rate of exacerbations address many of these concerns. In the text and tables that follow, we present the latest evidence highlighting differences in dosing, safety, and efficacy. We also include the updated GOLD classifications, evidence of efficacy for pulmonary rehabilitation, and practical implications of these findings for the optimal management of patients with COPD.
But first, a word about terminology.
Understanding COPD
COPD is a chronic lung disease characterized by progressive airflow limitation, usually measured by spirometry (TABLE 2),3 and chronic airway inflammation. Emphysema and chronic bronchitis are often used synonymously with COPD. In fact, there are important differences.
Individuals with chronic bronchitis do not necessarily have the airflow limitations found in those with COPD. And patients with COPD develop pathologic lung changes beyond the alveolar damage characteristic of emphysema, including airway fibrosis and inflammation, luminal plugging, and loss of elastic recoil.3
The medications included in this review aim to reduce both the morbidity and mortality associated with COPD. These drugs can also help relieve the symptoms of patients with chronic bronchitis and emphysema, but have limited effect on patient mortality.
Short- and long-acting beta2-agonists
Bronchodilator therapy with beta2-agonists improves forced expiratory volume in one second (FEV1) through relaxation of airway smooth muscle. Beta2-agonists have proven to be safe and effective when used as needed or scheduled for patients with COPD.7
Inhaled short-acting beta2-agonists (SABAs) improve FEV1 and symptoms within 10 minutes, with effects lasting up to 4 to 6 hours; long-acting beta2-agonists (LABAs) have a variable onset, with effects lasting 12 to 24 hours.8 Inhaled levalbuterol, the last SABA to receive US Food and Drug Administration approval, has not proven to be superior to conventional bronchodilators in ambulatory patients with stable COPD.3 In clinical trials, however, the slightly longer half-life of the nebulized formulation of levalbuterol was found to reduce both the frequency of administration and the overall cost of therapy in patients hospitalized with acute exacerbations of COPD.9,10
Recently approved LABAs
Clinical trials have studied the safety and efficacy of newer agents vs older LABAs in patients with moderate to severe COPD. Compared with theophylline, for example, formoterol 12 mcg inhaled every 12 hours for a 12-month period provided a clinically significant increase of >120 ml in FEV1 (P=.026).11 Higher doses of formoterol did not provide any additional improvement.
In a trial comparing indacaterol and tiotropium, an inhaled anticholinergic, both treatment groups had a clinically significant increase in FEV1, but patients receiving indacaterol achieved an additional increase of 40 to 50 mL at 12 weeks.12
Exacerbation rates for all LABAs range from 22% to 44%.5,12,13 In a study of patients receiving formoterol 12 mcg compared with 15-mcg and 25-mcg doses of arformoterol, those taking formoterol had a lower exacerbation rate than those on either strength of arformoterol (22% vs 32% and 31%, respectively).10 In various studies, doses greater than the FDA-approved regimens for indacaterol, arformoterol, and olodaterol did not result in a significant improvement in either FEV1 or exacerbation rates compared with placebo.5,12,14
Studies that assessed the use of rescue medication as well as exacerbation rates in patients taking LABAs reported reductions in the use of the rescue drugs ranging from 0.46 to 1.32 actuations per day, but the findings had limited clinical relevance.5,13 With the exception of indacaterol and olodaterol—both of which may be preferable because of their once-daily dosing regimen—no significant differences in safety and efficacy among LABAs have been found.5,12,13
Long-acting inhaled anticholinergics
Inhaled anticholinergic agents (IACs) can be used in place of, or in conjunction with, LABAs to provide bronchodilation for up to 24 hours.3 The introduction of long-acting IACs dosed once or twice daily has the potential to improve medication adherence over traditional short-acting ipratropium, which requires multiple daily doses for symptom control. Over 4 years, tiotropium has been shown to increase time to first exacerbation by approximately 4 months. It did not, however, significantly reduce the number of exacerbations compared with placebo.15
Long-term use of tiotropium appears to have the potential to preserve lung function. In one trial, it slowed the rate of decline in FEV1 by 5 mL per year, but this finding lacked clinical significance.13 In clinical trials of patients with moderate to severe COPD, however, once-daily tiotropium and umeclidinium provided clinically significant improvements in FEV1 (>120 mL; P<.01), regardless of the dose administered.6,16 In another trial, patients taking aclidinium 200 mcg or 400 mcg every 12 hours did not achieve a clinically significant improvement in FEV1 compared with placebo.17
In patients with moderate to severe COPD, the combination of umeclidinium/vilanterol, a LABA, administered once daily resulted in a clinically significant improvement in FEV1 (167 mL; P<.001) vs placebo—but was not significantly better than treatment with either agent alone.18
Few studies have evaluated time to exacerbation in patients receiving aclidinium or umeclidinium. In comparison to salmeterol, tiotropium reduced the time to first exacerbation by 42 days at one year (hazard ratio=0.83; 95% confidence interval [CI], 0.77-0.9; P<.001).19 The evidence suggests that when used in combination with LABAs, long-acting IACs have a positive impact on FEV1, but their effect on exacerbation rates has not been established.
Combination therapy with steroids and LABAs
The combination of inhaled corticosteroids (ICS) and LABAs has been found to improve FEV1 and symptoms in patients with moderate to severe COPD more than monotherapy with either drug class.20,21 In fact, ICS alone have not been proven to slow the progression of the disease or to lower mortality rates in patients with COPD.22
Fluticasone/salmeterol demonstrated a 25% reduction in exacerbation rates compared with placebo (P<.0001), a greater reduction than that of either drug alone.20 A retrospective observational study comparing fixed dose fluticasone/salmeterol with budesonide/formoterol reported a similar reduction in exacerbation rates, but the number of patients requiring the addition of an IAC was 16% lower in the latter group.23
The combination of fluticasone/vilanterol has the potential to improve adherence, given that it is dosed once daily, unlike other COPD combination drugs. Its clinical efficacy is comparable to that of fluticasone/salmeterol after 12 weeks of therapy, with similar improvements in FEV1,24 but fluticasone/vilanterol is associated with an increased risk of pneumonia.3
Chronic use of oral corticosteroids
Oral corticosteroids (OCS) are clinically indicated in individuals whose symptoms continue despite optimal therapy with inhaled agents that have demonstrated efficacy. Such patients are often referred to as “steroid dependent.”
While OCS are prescribed for both their anti-inflammatory activity and their ability to slow the progression of COPD,25,26 no well-designed studies have investigated their benefits for this patient population. One study concluded that patients who were slowly withdrawn from their OCS regimen had no more frequent exacerbations than those who maintained chronic usage. The withdrawal group did, however, lose weight.27
GOLD guidelines do not recommend OCS for chronic management of COPD due to the risk of toxicity.3 The well-established adverse effects of chronic OCS include hyperglycemia, hypertension, osteoporosis, and myopathy.28,29 A study of muscle function in 21 COPD patients receiving corticosteroids revealed decreases in quadriceps muscle strength and pulmonary function.30 Daily use of OCS will likely result in additional therapies to control drug-induced conditions, as well—another antihypertensive secondary to fluid retention caused by chronic use of OCS in patients with high blood pressure, for example, or additional medication to control elevated blood glucose levels in patients with diabetes.
Phosphodiesterase-4 inhibitors
The recommendation for roflumilast in patients with GOLD Class 2 to 4 symptoms remains unchanged since the introduction of this agent as a treatment option for COPD.3 Phosphodiesterase-4 (PDE-4) inhibitors such as roflumilast reduce inflammation in the lungs and have no activity as a bronchodilator.31,32
Roflumilast has been shown to improve FEV1 in patients concurrently receiving a long-acting bronchodilator and to reduce exacerbations in steroid-dependent patients, a recent systematic review of 29 PDE-4 trials found.33 Patients taking roflumilast, however, suffered from more adverse events (nausea, appetite reduction, diarrhea, weight loss, sleep disturbances, and headache) than those on placebo.33
Antibiotics
GOLD guidelines do not recommend the use of antibiotics for patients with COPD, except to treat acute exacerbations.1 However, recent studies suggest that routine or pulsed dosing of prophylactic antibiotics can reduce the number of exacerbations.34-36 A 2013 review of 7 studies determined that continuous antibiotics, particularly macrolides, reduced the number of COPD exacerbations in patients with a mean age of 66 years (odds ratio [OR]=0.55; 95% CI, 0.39-0.77).37
A more recent trial randomized 92 patients with a history of ≥3 exacerbations in the previous year to receive either prophylactic azithromycin or placebo daily for 12 months. The treatment group experienced a significant decrease in the number of exacerbations (OR=0.58; 95% CI, 0.42-0.79; P=.001).38 This benefit must be weighed against the potential development of antibiotic resistance and adverse effects, so careful patient selection is important.
Pulmonary rehabilitation has proven benefits
GOLD, the American College of Chest Physicians, the American Thoracic Society, and the European Respiratory Society all recommend pulmonary rehabilitation for patients with COPD.39-41 In addition to reducing morbidity and mortality rates—including a reduction in number of hospitalizations and length of stay and improved post-discharge recovery—pulmonary rehabilitation has been shown to have other physical and psychological benefits.42 Specific benefits include improved exercise capacity, greater arm strength and endurance, reduced perception of intensity of breathlessness, and improved overall health-related quality of life.
Key features of rehab programs
Important components of pulmonary rehabilitation include counseling on tobacco cessation, nutrition, education—including correct inhalation technique—and exercise training. There are few contraindications to participation, and patients can derive benefit from both its non-exercise components and upper extremity training regardless of their mobility level.
A 2006 Cochrane review concluded that an effective pulmonary rehabilitation program should be at least 4 weeks in duration,43 and longer programs have been shown to produce greater benefits.44 However, there is no agreement on an optimal time frame. Studies are inconclusive on other specific aspects of pulmonary rehab programs, as well, such as the number of sessions per week, number of hours per session, duration and intensity of exercise regimens, and staff-to-patient ratios.
Home-based exercise training may produce many of the same benefits as a formal pulmonary rehabilitation program. A systematic review found improved quality of life and exercise capacity associated with patient care that lacked formal pulmonary rehabilitation, with no differences between results from home-based training and hospital-based outpatient pulmonary rehabilitation programs.45
Given the lack of availability of formal rehab programs in many communities, homebased training for patients with COPD is important to consider.
Implications for practice
What is the takeaway from this evidence-based review? Overall, it is clear that, with the possible exception of the effect of once-daily dosing on adherence, there is little difference among the therapeutic agents within a particular class of medications—and that more is not necessarily better. Indeed, evidence suggests that higher doses of LABAs may reduce their effectiveness, rendering them no better than placebo. In addition, there is no significant difference in the rate of exacerbations in patients taking ICS/LABA combinations and those receiving IACs alone.
Pulmonary rehabilitation should be recommended for all newly diagnosed patients, while appropriate drug therapies should be individualized based on the GOLD symptoms/risk evaluation categories (TABLE 3).3 While daily OCS and daily antibiotics have the potential to reduce exacerbation rates, for example, the risks of adverse effects and toxicities outweigh the benefits for patients whose condition is stable.
Determining the optimal treatment for a particular patient also requires an assessment of comorbidities, including potential adverse drug effects (TABLE 4).3,27-29,33,46-52 Selection of medication should be driven by patient and physician preference to optimize adherence and clinical outcomes, although cost and accessibility often play a significant role, as well.
CORRESPONDENCE
Nabila Ahmed-Sarwar, PharmD, BCPS, CDE, St. John Fisher College, Wegmans School of Pharmacy, 3690 East Avenue, Rochester, NY 14618; [email protected]
ACKNOWLEDGEMENTS
The authors thank the following people for their assistance in the preparation of this manuscript: Matthew Stryker, PharmD, Timothy Adler, PharmD, and Angela K. Nagel, PharmD, BCPS.
› Individualize treatment regimens based on severity of symptoms and risk for exacerbation, prescribing short-acting beta2-agonists, as needed, for all patients with chronic obstructive pulmonary disease (COPD). A
› Limit use of inhaled long-acting beta2-agonists to the recommended dosage; higher doses do not lead to better outcomes. A
Strength of recommendation (SOR)
A Good-quality patient-oriented evidence
B Inconsistent or limited-quality patient-oriented evidence
C Consensus, usual practice, opinion, disease-oriented evidence, case series
Chronic obstructive pulmonary disease (COPD) carries a high disease burden. In 2012, it was the 4th leading cause of death worldwide.1,2 In 2015, the World Health Organization updated its Global Initiative for Chronic Obstructive Lung Disease (GOLD) guidelines, classifying patients with COPD based on disease burden as determined by symptoms, airflow obstruction, and exacerbation history.3 These revisions, coupled with expanded therapeutic options within established classes of medications and new combination drugs to treat COPD (TABLE 1),3-6 have led to questions about interclass differences and the best treatment regimen for particular patients.
Comparisons of various agents within a therapeutic class and their impact on lung function and rate of exacerbations address many of these concerns. In the text and tables that follow, we present the latest evidence highlighting differences in dosing, safety, and efficacy. We also include the updated GOLD classifications, evidence of efficacy for pulmonary rehabilitation, and practical implications of these findings for the optimal management of patients with COPD.
But first, a word about terminology.
Understanding COPD
COPD is a chronic lung disease characterized by progressive airflow limitation, usually measured by spirometry (TABLE 2),3 and chronic airway inflammation. Emphysema and chronic bronchitis are often used synonymously with COPD. In fact, there are important differences.
Individuals with chronic bronchitis do not necessarily have the airflow limitations found in those with COPD. And patients with COPD develop pathologic lung changes beyond the alveolar damage characteristic of emphysema, including airway fibrosis and inflammation, luminal plugging, and loss of elastic recoil.3
The medications included in this review aim to reduce both the morbidity and mortality associated with COPD. These drugs can also help relieve the symptoms of patients with chronic bronchitis and emphysema, but have limited effect on patient mortality.
Short- and long-acting beta2-agonists
Bronchodilator therapy with beta2-agonists improves forced expiratory volume in one second (FEV1) through relaxation of airway smooth muscle. Beta2-agonists have proven to be safe and effective when used as needed or scheduled for patients with COPD.7
Inhaled short-acting beta2-agonists (SABAs) improve FEV1 and symptoms within 10 minutes, with effects lasting up to 4 to 6 hours; long-acting beta2-agonists (LABAs) have a variable onset, with effects lasting 12 to 24 hours.8 Inhaled levalbuterol, the last SABA to receive US Food and Drug Administration approval, has not proven to be superior to conventional bronchodilators in ambulatory patients with stable COPD.3 In clinical trials, however, the slightly longer half-life of the nebulized formulation of levalbuterol was found to reduce both the frequency of administration and the overall cost of therapy in patients hospitalized with acute exacerbations of COPD.9,10
Recently approved LABAs
Clinical trials have studied the safety and efficacy of newer agents vs older LABAs in patients with moderate to severe COPD. Compared with theophylline, for example, formoterol 12 mcg inhaled every 12 hours for a 12-month period provided a clinically significant increase of >120 ml in FEV1 (P=.026).11 Higher doses of formoterol did not provide any additional improvement.
In a trial comparing indacaterol and tiotropium, an inhaled anticholinergic, both treatment groups had a clinically significant increase in FEV1, but patients receiving indacaterol achieved an additional increase of 40 to 50 mL at 12 weeks.12
Exacerbation rates for all LABAs range from 22% to 44%.5,12,13 In a study of patients receiving formoterol 12 mcg compared with 15-mcg and 25-mcg doses of arformoterol, those taking formoterol had a lower exacerbation rate than those on either strength of arformoterol (22% vs 32% and 31%, respectively).10 In various studies, doses greater than the FDA-approved regimens for indacaterol, arformoterol, and olodaterol did not result in a significant improvement in either FEV1 or exacerbation rates compared with placebo.5,12,14
Studies that assessed the use of rescue medication as well as exacerbation rates in patients taking LABAs reported reductions in the use of the rescue drugs ranging from 0.46 to 1.32 actuations per day, but the findings had limited clinical relevance.5,13 With the exception of indacaterol and olodaterol—both of which may be preferable because of their once-daily dosing regimen—no significant differences in safety and efficacy among LABAs have been found.5,12,13
Long-acting inhaled anticholinergics
Inhaled anticholinergic agents (IACs) can be used in place of, or in conjunction with, LABAs to provide bronchodilation for up to 24 hours.3 The introduction of long-acting IACs dosed once or twice daily has the potential to improve medication adherence over traditional short-acting ipratropium, which requires multiple daily doses for symptom control. Over 4 years, tiotropium has been shown to increase time to first exacerbation by approximately 4 months. It did not, however, significantly reduce the number of exacerbations compared with placebo.15
Long-term use of tiotropium appears to have the potential to preserve lung function. In one trial, it slowed the rate of decline in FEV1 by 5 mL per year, but this finding lacked clinical significance.13 In clinical trials of patients with moderate to severe COPD, however, once-daily tiotropium and umeclidinium provided clinically significant improvements in FEV1 (>120 mL; P<.01), regardless of the dose administered.6,16 In another trial, patients taking aclidinium 200 mcg or 400 mcg every 12 hours did not achieve a clinically significant improvement in FEV1 compared with placebo.17
In patients with moderate to severe COPD, the combination of umeclidinium/vilanterol, a LABA, administered once daily resulted in a clinically significant improvement in FEV1 (167 mL; P<.001) vs placebo—but was not significantly better than treatment with either agent alone.18
Few studies have evaluated time to exacerbation in patients receiving aclidinium or umeclidinium. In comparison to salmeterol, tiotropium reduced the time to first exacerbation by 42 days at one year (hazard ratio=0.83; 95% confidence interval [CI], 0.77-0.9; P<.001).19 The evidence suggests that when used in combination with LABAs, long-acting IACs have a positive impact on FEV1, but their effect on exacerbation rates has not been established.
Combination therapy with steroids and LABAs
The combination of inhaled corticosteroids (ICS) and LABAs has been found to improve FEV1 and symptoms in patients with moderate to severe COPD more than monotherapy with either drug class.20,21 In fact, ICS alone have not been proven to slow the progression of the disease or to lower mortality rates in patients with COPD.22
Fluticasone/salmeterol demonstrated a 25% reduction in exacerbation rates compared with placebo (P<.0001), a greater reduction than that of either drug alone.20 A retrospective observational study comparing fixed dose fluticasone/salmeterol with budesonide/formoterol reported a similar reduction in exacerbation rates, but the number of patients requiring the addition of an IAC was 16% lower in the latter group.23
The combination of fluticasone/vilanterol has the potential to improve adherence, given that it is dosed once daily, unlike other COPD combination drugs. Its clinical efficacy is comparable to that of fluticasone/salmeterol after 12 weeks of therapy, with similar improvements in FEV1,24 but fluticasone/vilanterol is associated with an increased risk of pneumonia.3
Chronic use of oral corticosteroids
Oral corticosteroids (OCS) are clinically indicated in individuals whose symptoms continue despite optimal therapy with inhaled agents that have demonstrated efficacy. Such patients are often referred to as “steroid dependent.”
While OCS are prescribed for both their anti-inflammatory activity and their ability to slow the progression of COPD,25,26 no well-designed studies have investigated their benefits for this patient population. One study concluded that patients who were slowly withdrawn from their OCS regimen had no more frequent exacerbations than those who maintained chronic usage. The withdrawal group did, however, lose weight.27
GOLD guidelines do not recommend OCS for chronic management of COPD due to the risk of toxicity.3 The well-established adverse effects of chronic OCS include hyperglycemia, hypertension, osteoporosis, and myopathy.28,29 A study of muscle function in 21 COPD patients receiving corticosteroids revealed decreases in quadriceps muscle strength and pulmonary function.30 Daily use of OCS will likely result in additional therapies to control drug-induced conditions, as well—another antihypertensive secondary to fluid retention caused by chronic use of OCS in patients with high blood pressure, for example, or additional medication to control elevated blood glucose levels in patients with diabetes.
Phosphodiesterase-4 inhibitors
The recommendation for roflumilast in patients with GOLD Class 2 to 4 symptoms remains unchanged since the introduction of this agent as a treatment option for COPD.3 Phosphodiesterase-4 (PDE-4) inhibitors such as roflumilast reduce inflammation in the lungs and have no activity as a bronchodilator.31,32
Roflumilast has been shown to improve FEV1 in patients concurrently receiving a long-acting bronchodilator and to reduce exacerbations in steroid-dependent patients, a recent systematic review of 29 PDE-4 trials found.33 Patients taking roflumilast, however, suffered from more adverse events (nausea, appetite reduction, diarrhea, weight loss, sleep disturbances, and headache) than those on placebo.33
Antibiotics
GOLD guidelines do not recommend the use of antibiotics for patients with COPD, except to treat acute exacerbations.1 However, recent studies suggest that routine or pulsed dosing of prophylactic antibiotics can reduce the number of exacerbations.34-36 A 2013 review of 7 studies determined that continuous antibiotics, particularly macrolides, reduced the number of COPD exacerbations in patients with a mean age of 66 years (odds ratio [OR]=0.55; 95% CI, 0.39-0.77).37
A more recent trial randomized 92 patients with a history of ≥3 exacerbations in the previous year to receive either prophylactic azithromycin or placebo daily for 12 months. The treatment group experienced a significant decrease in the number of exacerbations (OR=0.58; 95% CI, 0.42-0.79; P=.001).38 This benefit must be weighed against the potential development of antibiotic resistance and adverse effects, so careful patient selection is important.
Pulmonary rehabilitation has proven benefits
GOLD, the American College of Chest Physicians, the American Thoracic Society, and the European Respiratory Society all recommend pulmonary rehabilitation for patients with COPD.39-41 In addition to reducing morbidity and mortality rates—including a reduction in number of hospitalizations and length of stay and improved post-discharge recovery—pulmonary rehabilitation has been shown to have other physical and psychological benefits.42 Specific benefits include improved exercise capacity, greater arm strength and endurance, reduced perception of intensity of breathlessness, and improved overall health-related quality of life.
Key features of rehab programs
Important components of pulmonary rehabilitation include counseling on tobacco cessation, nutrition, education—including correct inhalation technique—and exercise training. There are few contraindications to participation, and patients can derive benefit from both its non-exercise components and upper extremity training regardless of their mobility level.
A 2006 Cochrane review concluded that an effective pulmonary rehabilitation program should be at least 4 weeks in duration,43 and longer programs have been shown to produce greater benefits.44 However, there is no agreement on an optimal time frame. Studies are inconclusive on other specific aspects of pulmonary rehab programs, as well, such as the number of sessions per week, number of hours per session, duration and intensity of exercise regimens, and staff-to-patient ratios.
Home-based exercise training may produce many of the same benefits as a formal pulmonary rehabilitation program. A systematic review found improved quality of life and exercise capacity associated with patient care that lacked formal pulmonary rehabilitation, with no differences between results from home-based training and hospital-based outpatient pulmonary rehabilitation programs.45
Given the lack of availability of formal rehab programs in many communities, homebased training for patients with COPD is important to consider.
Implications for practice
What is the takeaway from this evidence-based review? Overall, it is clear that, with the possible exception of the effect of once-daily dosing on adherence, there is little difference among the therapeutic agents within a particular class of medications—and that more is not necessarily better. Indeed, evidence suggests that higher doses of LABAs may reduce their effectiveness, rendering them no better than placebo. In addition, there is no significant difference in the rate of exacerbations in patients taking ICS/LABA combinations and those receiving IACs alone.
Pulmonary rehabilitation should be recommended for all newly diagnosed patients, while appropriate drug therapies should be individualized based on the GOLD symptoms/risk evaluation categories (TABLE 3).3 While daily OCS and daily antibiotics have the potential to reduce exacerbation rates, for example, the risks of adverse effects and toxicities outweigh the benefits for patients whose condition is stable.
Determining the optimal treatment for a particular patient also requires an assessment of comorbidities, including potential adverse drug effects (TABLE 4).3,27-29,33,46-52 Selection of medication should be driven by patient and physician preference to optimize adherence and clinical outcomes, although cost and accessibility often play a significant role, as well.
CORRESPONDENCE
Nabila Ahmed-Sarwar, PharmD, BCPS, CDE, St. John Fisher College, Wegmans School of Pharmacy, 3690 East Avenue, Rochester, NY 14618; [email protected]
ACKNOWLEDGEMENTS
The authors thank the following people for their assistance in the preparation of this manuscript: Matthew Stryker, PharmD, Timothy Adler, PharmD, and Angela K. Nagel, PharmD, BCPS.
1. World Health Organization. Chronic obstructive pulmonary disease (COPD). Fact Sheet No. 315. World Health Organization Web site. Available at: http://www.who.int/mediacentre/factsheets/fs315/en/. Accessed January 29, 2015.
2. National Heart, Lung, and Blood Institute. Morbidity and mortality: 2012 chart book on cardiovascular, lung, and blood diseases. National Heart, Lung, and Blood Institute Web site. Available at: http://www.nhlbi.nih.gov/files/docs/research/2012_Chart-Book_508.pdf. Accessed January 29, 2015.
3. Global Initiative for Chronic Obstructive Lung Disease. Global strategy for the diagnosis, management, and prevention of chronic obstructive pulmonary disease. Updated 2015. Global Initiative for Chronic Obstructive Lung Disease Web site. Available at: http://www.goldcopd.org/uploads/users/files/GOLD_Report_2015_Sept2.pdf. Accessed July 26, 2015.
4. Hanrahan JP, Hanania NA, Calhoun WJ, et al. Effect of nebulized arformoterol on airway function in COPD: results from two randomized trials. COPD. 2008;5:25-34.
5. Hanania NA, Donohue JF, Nelson H, et al. The safety and efficacy of arformoterol and formoterol in COPD. COPD. 2010;7:17-31.
6. Trivedi R, Richard N, Mehta R, et al. Umeclidinium in patients with COPD: a randomised, placebo-controlled study. Eur Respir J. 2014;43:72-81.
7. Vathenen AS, Britton JR, Ebden P, et al. High-dose inhaled albuterol in severe chronic airflow limitation. Am Rev Respir Dis. 1988;138:850-855.
8. Cazzola M, Matera MG, Santangelo G, et al. Salmeterol and formoterol in partially reversible severe chronic obstructive pulmonary disease: a dose-response study. Respir Med. 1995;89:357-362.
9. Donohue JF, Hanania NA, Ciubotaru RL, et al. Comparison of levalbuterol and racemic albuterol in hospitalized patients with acute asthma or COPD: a 2-week, multicenter, randomized, open-label study. Clin Ther. 2008;30:989-1002.
10. Truitt T, Witko J, Halpern M. Levalbuterol compared to racemic albuterol: efficacy and outcomes in patients hospitalized with COPD or asthma. Chest. 2003;123:128-135.
11. Rossi A, Kristufek P, Levine BE, et al; Formoterol in Chronic Obstructive Pulmonary Disease (FICOPD) II Study Group. Comparison of the efficacy, tolerability, and safety of formoterol dry powder and oral, slow-release theophylline in the treatment of COPD. Chest. 2002;121:1058-1069.
12. Donohue JF, Fogarty C, Lötvall J, et al; INHANCE Study Investigators. Once-daily bronchodilators for chronic obstructive pulmonary disease: indacaterol versus tiotropium. Am J Respir Crit Care Med. 2010;182:155-162.
13. Ferguson GT, Feldman GJ, Hofbauer P, et al. Efficacy and safety of olodaterol once daily delivered via Respimat® in patients with GOLD 2-4 COPD: results from two replicate 48-week studies. Int J Chron Obstruct Pulmon Dis. 2014;9:629-645.
14. Boyd G, Morice AH, Pounsford JC, et al. An evaluation of salmeterol in the treatment of chronic obstructive pulmonary disease (COPD). Eur Respir J. 1997;10:815-821.
15. Tashkin DP, Celli B, Senn S, et al; UPLIFT Study Investigators. A 4-year trial of tiotropium in chronic obstructive pulmonary disease. N Engl J Med. 2008;359:1543-1554.
16. Casaburi R, Mahler DA, Jones PW, et al. A long-term evaluation of once-daily inhaled tiotropium in chronic obstructive pulmonary disease. Eur Respir J. 2002;19:217-224.
17. Jones PW, Singh D, Bateman ED, et al. Efficacy and safety of twice-daily aclidinium bromide in COPD patients: the ATTAIN study. Eur Respir J. 2012;40:830-836.
18. Donohue JF, Maleki-Yazdi MR, Kilbride S, et al. Efficacy and safety of once-daily umeclidinium/vilanterol 62.5/25 mcg in COPD. Respir Med. 2013;107:1538-1546.
19. Vogelmeier C, Hederer B, Glaab T, et al; POET-COPD Investigators. Tiotropium versus salmeterol for the prevention of exacerbations of COPD. N Engl J Med. 2011;364:1093-1103.
20. Calverley P, Pauwels R, Vestbo J, et al; Trial of inhaled steroids and long-acting beta2 agonists study group. Combined salmeterol and fluticasone in the treatment of chronic obstructive pulmonary disease: a randomised controlled trial. Lancet. 2003;361:449-456.
21. Szafranski W, Cukier A, Ramirez A, et al. Efficacy and safety of budesonide/formoterol in the management of chronic obstructive pulmonary disease. Eur Respir J. 2003;21:74-81.
22. Calverley PM, Anderson JA, Celli B, et al; TORCH investigators. Salmeterol and fluticasone propionate and survival in chronic obstructive pulmonary disease. N Engl J Med. 2007;356:775-789.
23. Larsson K, Janson C, Lisspers K, et al. Combination of budesonide/formoterol more effective than fluticasone/salmeterol in preventing exacerbations in chronic obstructive pulmonary disease: the PATHOS study. J Intern Med. 2013;273:584-594.
24. Dransfield MT, Feldman G, Korenblat P, et al. Efficacy and safety of once-daily fluticasone furoate/vilanterol (100/25 mcg) versus twice-daily fluticasone propionate/salmeterol (250/50 mcg) in COPD patients. Respir Med. 2014;108:1171-1179.
25. Davies L, Nisar M, Pearson MG, et al. Oral corticosteroid trials in the management of stable chronic obstructive pulmonary disease. QJM. 1999;92:395-400.
26. Walters JA, Walters EH, Wood-Baker R. Oral corticosteroids for stable chronic obstructive pulmonary disease. Cochrane Database Syst Rev. 2005;CD005374.
27. Rice KL, Rubins JB, Lebahn F, et al. Withdrawal of chronic systemic corticosteroids in patients with COPD: a randomized trial. Am J Respir Crit Care Med. 2000;162:174-178.
28. Clore JN, Thurby-Hay L. Glucocorticoid-induced hyperglycemia. Endocr Pract. 2009;15:469-474.
29. McEvoy CE, Ensrud KE, Bender E, et al. Association between corticosteroid use and vertebral fractures in older men with chronic obstructive pulmonary disease. Am J Respir Crit Care Med. 1998;157:704-709.
30. Decramer M, Lacquet LM, Fagard R, et al. Corticosteroids contribute to muscle weakness in chronic airflow obstruction. Am J Respir Crit Care Med. 1994;150:11-16.
31. Fabbri LM, Calverley PM, Izquierdo-Alonso JL, et al; M2-127 and M2-128 study groups. Roflumilast in moderate-to-severe chronic obstructive pulmonary disease treated with longacting bronchodilators: two randomised clinical trials. Lancet. 2009;374:695-703.
32. Calverley PM, Rabe KF, Goehring UM, et al; M2-124 and M2-125 study groups. Roflumilast in symptomatic chronic obstructive pulmonary disease: two randomised clinical trials. Lancet. 2009;374:685-694.
33. Chong J, Leung B, Poole P. Phosphodiesterase 4 inhibitors for chronic obstructive pulmonary disease. Cochrane Database Syst Rev. 2013;11:CD002309.
34. Seemungal TA, Wilkinson TM, Hurst JR, et al. Long-term erythromycin therapy is associated with decreased chronic obstructive pulmonary disease exacerbations. Am J Respir Crit Care Med. 2008;178:1139-1147.
35. Sethi S, Jones PW, Theron MS, et al; PULSE study group. Pulsed moxifloxacin for the prevention of exacerbations of chronic obstructive pulmonary disease: a randomized controlled trial. Respir Res. 2010;11:10.
36. Albert RK, Connett J, Bailey WC, et al; COPD Clinical Research Network. Azithromycin for prevention of exacerbations of COPD. N Engl J Med. 2011;365:689-698.
37. Herath SC, Poole P. Prophylactic antibiotic therapy for chronic obstructive pulmonary disease (COPD). Cochrane Database Syst Rev. 2013;11:CD009764.
38. Uzun S, Djamin RS, Kluytmans JA, et al. Azithromycin maintenance treatment in patients with frequent exacerbations of chronic obstructive pulmonary disease (COLUMBUS): a randomised, double-blind, placebo-controlled trial. Lancet Respir Med. 2014;2:361-368.
39. Ries AL, Bauldoff GS, Carlin BW, et al. Pulmonary rehabilitation: joint ACCP/AACVPR evidence-based clinical practice guidelines. Chest. 2007;131:S4-S42.
40. Spruit MA, Singh SJ, Garvey C, et al; ATS/ERS Task Force on Pulmonary Rehabilitation. An official American Thoracic Society/European Respiratory Society statement: key concepts and advances in pulmonary rehabilitation. Am J Respir Crit Care Med. 2013;188:e13-e64.
41. Qaseem A, Wilt TJ, Weinberger SE, et al; American College of Physicians; American College of Chest Physicians; American Thoracic Society; European Respiratory Society. Diagnosis and management of stable chronic obstructive pulmonary disease: a clinical practice guideline update from the American College of Physicians, American College of Chest Physicians, American Thoracic Society, and European Respiratory Society. Ann Intern Med. 2011;155:179-191.
42. Global Initiative for Chronic Obstructive Lung Disease. Global strategy for the diagnosis, management, and prevention of chronic obstructive pulmonary disease. Updated 2013. Global Initiative for Chronic Obstructive Lung Disease Web site. Available at: http://www.goldcopd.org/uploads/users/files/GOLD_Report_2013_Feb20.pdf. Accessed January 14, 2015.
43. Lacasse Y, Goldstein R, Lasserson TJ, et al. Pulmonary rehabilitation for chronic obstructive pulmonary disease. Cochrane Database Syst Rev. 2006;CD003793.
44. Beauchamp MK, Janaudis-Ferreira T, Goldstein RS, et al. Optimal duration of pulmonary rehabilitation for individuals with chronic obstructive pulmonary disease - a systematic review. Chron Respir Dis. 2011;8:129-140.
45. Vieira DS, Maltais F, Bourbeau J. Home-based pulmonary rehabilitation in chronic obstructive pulmonary disease patients. Curr Opin Pulm Med. 2010;16:134-143.
46. Proair HFM (albuterol sulfate) [package insert]. Miami, FL: IVAX Laboratories; 2005.
47. Foradil (formoterol fumarate) [package insert]. Whitehouse Station, NJ: Merck & Co; 2012.
48. Spiriva (tiotropium bromide) [package insert]. Ridgefield, Conn: Boehringer Ingelheim Pharmaceuticals; 2014.
49. Fried TR, Vaz Fragoso CA, Rabow MW. Caring for the older person with chronic obstructive pulmonary disease. JAMA. 2012;308:1254-1263.
50. Flovent HFA (fluticasone propionate) [package insert]. Research Triangle Park, NC: GlaxoSmithKline; 2014.
51. Zithromax (azithromycin) [package insert]. New York, NY: Pfizer Labs; 2013.
52. Daliresp (roflumilast) [package insert]. St. Louis, Mo: Forest Pharmaceuticals; 2013.
1. World Health Organization. Chronic obstructive pulmonary disease (COPD). Fact Sheet No. 315. World Health Organization Web site. Available at: http://www.who.int/mediacentre/factsheets/fs315/en/. Accessed January 29, 2015.
2. National Heart, Lung, and Blood Institute. Morbidity and mortality: 2012 chart book on cardiovascular, lung, and blood diseases. National Heart, Lung, and Blood Institute Web site. Available at: http://www.nhlbi.nih.gov/files/docs/research/2012_Chart-Book_508.pdf. Accessed January 29, 2015.
3. Global Initiative for Chronic Obstructive Lung Disease. Global strategy for the diagnosis, management, and prevention of chronic obstructive pulmonary disease. Updated 2015. Global Initiative for Chronic Obstructive Lung Disease Web site. Available at: http://www.goldcopd.org/uploads/users/files/GOLD_Report_2015_Sept2.pdf. Accessed July 26, 2015.
4. Hanrahan JP, Hanania NA, Calhoun WJ, et al. Effect of nebulized arformoterol on airway function in COPD: results from two randomized trials. COPD. 2008;5:25-34.
5. Hanania NA, Donohue JF, Nelson H, et al. The safety and efficacy of arformoterol and formoterol in COPD. COPD. 2010;7:17-31.
6. Trivedi R, Richard N, Mehta R, et al. Umeclidinium in patients with COPD: a randomised, placebo-controlled study. Eur Respir J. 2014;43:72-81.
7. Vathenen AS, Britton JR, Ebden P, et al. High-dose inhaled albuterol in severe chronic airflow limitation. Am Rev Respir Dis. 1988;138:850-855.
8. Cazzola M, Matera MG, Santangelo G, et al. Salmeterol and formoterol in partially reversible severe chronic obstructive pulmonary disease: a dose-response study. Respir Med. 1995;89:357-362.
9. Donohue JF, Hanania NA, Ciubotaru RL, et al. Comparison of levalbuterol and racemic albuterol in hospitalized patients with acute asthma or COPD: a 2-week, multicenter, randomized, open-label study. Clin Ther. 2008;30:989-1002.
10. Truitt T, Witko J, Halpern M. Levalbuterol compared to racemic albuterol: efficacy and outcomes in patients hospitalized with COPD or asthma. Chest. 2003;123:128-135.
11. Rossi A, Kristufek P, Levine BE, et al; Formoterol in Chronic Obstructive Pulmonary Disease (FICOPD) II Study Group. Comparison of the efficacy, tolerability, and safety of formoterol dry powder and oral, slow-release theophylline in the treatment of COPD. Chest. 2002;121:1058-1069.
12. Donohue JF, Fogarty C, Lötvall J, et al; INHANCE Study Investigators. Once-daily bronchodilators for chronic obstructive pulmonary disease: indacaterol versus tiotropium. Am J Respir Crit Care Med. 2010;182:155-162.
13. Ferguson GT, Feldman GJ, Hofbauer P, et al. Efficacy and safety of olodaterol once daily delivered via Respimat® in patients with GOLD 2-4 COPD: results from two replicate 48-week studies. Int J Chron Obstruct Pulmon Dis. 2014;9:629-645.
14. Boyd G, Morice AH, Pounsford JC, et al. An evaluation of salmeterol in the treatment of chronic obstructive pulmonary disease (COPD). Eur Respir J. 1997;10:815-821.
15. Tashkin DP, Celli B, Senn S, et al; UPLIFT Study Investigators. A 4-year trial of tiotropium in chronic obstructive pulmonary disease. N Engl J Med. 2008;359:1543-1554.
16. Casaburi R, Mahler DA, Jones PW, et al. A long-term evaluation of once-daily inhaled tiotropium in chronic obstructive pulmonary disease. Eur Respir J. 2002;19:217-224.
17. Jones PW, Singh D, Bateman ED, et al. Efficacy and safety of twice-daily aclidinium bromide in COPD patients: the ATTAIN study. Eur Respir J. 2012;40:830-836.
18. Donohue JF, Maleki-Yazdi MR, Kilbride S, et al. Efficacy and safety of once-daily umeclidinium/vilanterol 62.5/25 mcg in COPD. Respir Med. 2013;107:1538-1546.
19. Vogelmeier C, Hederer B, Glaab T, et al; POET-COPD Investigators. Tiotropium versus salmeterol for the prevention of exacerbations of COPD. N Engl J Med. 2011;364:1093-1103.
20. Calverley P, Pauwels R, Vestbo J, et al; Trial of inhaled steroids and long-acting beta2 agonists study group. Combined salmeterol and fluticasone in the treatment of chronic obstructive pulmonary disease: a randomised controlled trial. Lancet. 2003;361:449-456.
21. Szafranski W, Cukier A, Ramirez A, et al. Efficacy and safety of budesonide/formoterol in the management of chronic obstructive pulmonary disease. Eur Respir J. 2003;21:74-81.
22. Calverley PM, Anderson JA, Celli B, et al; TORCH investigators. Salmeterol and fluticasone propionate and survival in chronic obstructive pulmonary disease. N Engl J Med. 2007;356:775-789.
23. Larsson K, Janson C, Lisspers K, et al. Combination of budesonide/formoterol more effective than fluticasone/salmeterol in preventing exacerbations in chronic obstructive pulmonary disease: the PATHOS study. J Intern Med. 2013;273:584-594.
24. Dransfield MT, Feldman G, Korenblat P, et al. Efficacy and safety of once-daily fluticasone furoate/vilanterol (100/25 mcg) versus twice-daily fluticasone propionate/salmeterol (250/50 mcg) in COPD patients. Respir Med. 2014;108:1171-1179.
25. Davies L, Nisar M, Pearson MG, et al. Oral corticosteroid trials in the management of stable chronic obstructive pulmonary disease. QJM. 1999;92:395-400.
26. Walters JA, Walters EH, Wood-Baker R. Oral corticosteroids for stable chronic obstructive pulmonary disease. Cochrane Database Syst Rev. 2005;CD005374.
27. Rice KL, Rubins JB, Lebahn F, et al. Withdrawal of chronic systemic corticosteroids in patients with COPD: a randomized trial. Am J Respir Crit Care Med. 2000;162:174-178.
28. Clore JN, Thurby-Hay L. Glucocorticoid-induced hyperglycemia. Endocr Pract. 2009;15:469-474.
29. McEvoy CE, Ensrud KE, Bender E, et al. Association between corticosteroid use and vertebral fractures in older men with chronic obstructive pulmonary disease. Am J Respir Crit Care Med. 1998;157:704-709.
30. Decramer M, Lacquet LM, Fagard R, et al. Corticosteroids contribute to muscle weakness in chronic airflow obstruction. Am J Respir Crit Care Med. 1994;150:11-16.
31. Fabbri LM, Calverley PM, Izquierdo-Alonso JL, et al; M2-127 and M2-128 study groups. Roflumilast in moderate-to-severe chronic obstructive pulmonary disease treated with longacting bronchodilators: two randomised clinical trials. Lancet. 2009;374:695-703.
32. Calverley PM, Rabe KF, Goehring UM, et al; M2-124 and M2-125 study groups. Roflumilast in symptomatic chronic obstructive pulmonary disease: two randomised clinical trials. Lancet. 2009;374:685-694.
33. Chong J, Leung B, Poole P. Phosphodiesterase 4 inhibitors for chronic obstructive pulmonary disease. Cochrane Database Syst Rev. 2013;11:CD002309.
34. Seemungal TA, Wilkinson TM, Hurst JR, et al. Long-term erythromycin therapy is associated with decreased chronic obstructive pulmonary disease exacerbations. Am J Respir Crit Care Med. 2008;178:1139-1147.
35. Sethi S, Jones PW, Theron MS, et al; PULSE study group. Pulsed moxifloxacin for the prevention of exacerbations of chronic obstructive pulmonary disease: a randomized controlled trial. Respir Res. 2010;11:10.
36. Albert RK, Connett J, Bailey WC, et al; COPD Clinical Research Network. Azithromycin for prevention of exacerbations of COPD. N Engl J Med. 2011;365:689-698.
37. Herath SC, Poole P. Prophylactic antibiotic therapy for chronic obstructive pulmonary disease (COPD). Cochrane Database Syst Rev. 2013;11:CD009764.
38. Uzun S, Djamin RS, Kluytmans JA, et al. Azithromycin maintenance treatment in patients with frequent exacerbations of chronic obstructive pulmonary disease (COLUMBUS): a randomised, double-blind, placebo-controlled trial. Lancet Respir Med. 2014;2:361-368.
39. Ries AL, Bauldoff GS, Carlin BW, et al. Pulmonary rehabilitation: joint ACCP/AACVPR evidence-based clinical practice guidelines. Chest. 2007;131:S4-S42.
40. Spruit MA, Singh SJ, Garvey C, et al; ATS/ERS Task Force on Pulmonary Rehabilitation. An official American Thoracic Society/European Respiratory Society statement: key concepts and advances in pulmonary rehabilitation. Am J Respir Crit Care Med. 2013;188:e13-e64.
41. Qaseem A, Wilt TJ, Weinberger SE, et al; American College of Physicians; American College of Chest Physicians; American Thoracic Society; European Respiratory Society. Diagnosis and management of stable chronic obstructive pulmonary disease: a clinical practice guideline update from the American College of Physicians, American College of Chest Physicians, American Thoracic Society, and European Respiratory Society. Ann Intern Med. 2011;155:179-191.
42. Global Initiative for Chronic Obstructive Lung Disease. Global strategy for the diagnosis, management, and prevention of chronic obstructive pulmonary disease. Updated 2013. Global Initiative for Chronic Obstructive Lung Disease Web site. Available at: http://www.goldcopd.org/uploads/users/files/GOLD_Report_2013_Feb20.pdf. Accessed January 14, 2015.
43. Lacasse Y, Goldstein R, Lasserson TJ, et al. Pulmonary rehabilitation for chronic obstructive pulmonary disease. Cochrane Database Syst Rev. 2006;CD003793.
44. Beauchamp MK, Janaudis-Ferreira T, Goldstein RS, et al. Optimal duration of pulmonary rehabilitation for individuals with chronic obstructive pulmonary disease - a systematic review. Chron Respir Dis. 2011;8:129-140.
45. Vieira DS, Maltais F, Bourbeau J. Home-based pulmonary rehabilitation in chronic obstructive pulmonary disease patients. Curr Opin Pulm Med. 2010;16:134-143.
46. Proair HFM (albuterol sulfate) [package insert]. Miami, FL: IVAX Laboratories; 2005.
47. Foradil (formoterol fumarate) [package insert]. Whitehouse Station, NJ: Merck & Co; 2012.
48. Spiriva (tiotropium bromide) [package insert]. Ridgefield, Conn: Boehringer Ingelheim Pharmaceuticals; 2014.
49. Fried TR, Vaz Fragoso CA, Rabow MW. Caring for the older person with chronic obstructive pulmonary disease. JAMA. 2012;308:1254-1263.
50. Flovent HFA (fluticasone propionate) [package insert]. Research Triangle Park, NC: GlaxoSmithKline; 2014.
51. Zithromax (azithromycin) [package insert]. New York, NY: Pfizer Labs; 2013.
52. Daliresp (roflumilast) [package insert]. St. Louis, Mo: Forest Pharmaceuticals; 2013.
This adjunct medication can speed CAP recovery
Prescribe oral prednisone 50 mg/d to hospitalized patients with mild to moderate community-acquired pneumonia. It decreases time to clinical stability and length of hospital stay.1
Strength of recommendation
A: Based on a single good-quality randomized controlled trial and meta-analysis.
Blum CA, Nigro N, Briel M, et al. Adjunct prednisone therapy for patients with community-acquired pneumonia: a multicentre, double-blind, randomized, placebo-controlled trial. Lancet. 2015;385:1511-1518.
Illustrative case
A 75-year-old woman with hypertension and diabetes mellitus presents to the emergency department with shortness of breath, cough, and fever that she’s had for 4 days. On examination, her temperature is 38.2°C (100.7°F), heart rate is 110 beats/min, respiratory rate is 28 breaths/min, oxygen saturation is 91%, and rhonchi are heard in her right lower lung field. A chest x-ray reveals an infiltrate in her right lower lobe. The patient is admitted and started on intravenous (IV) antibiotics, IV fluids, acetaminophen for fever, and oxygen. Can anything else be done to speed her recovery?
Community-acquired pneumonia (CAP) is responsible for more than one million hospitalizations annually in the United States, and is the 8th leading cause of death.2,3 Treatment of CAP typically consists of antibiotics and supportive measures such as IV fluids and antipyretics. Because the disease process of CAP involves extensive inflammation, adjunct treatment with corticosteroids may be beneficial.
Multiple studies have shown that treatment with corticosteroids can help patients with severe CAP, but the potential benefit in patients with less severe CAP has been uncertain.4,5 A Cochrane systematic review published in 2011 identified 6 small randomized controlled trials (RCTs) that evaluated the impact of corticosteroids on recovery from CAP.4 It suggested that corticosteroids may decrease time to recovery, but the studies that included patients with less severe CAP had a relatively high risk of bias.
Subsequently, a 2012 meta-analysis of 9 RCTs explored whether corticosteroids affected mortality in CAP; no benefit was observed in patients with less severe CAP.5 Most recently, a 2013 meta-analysis of 8 moderate-quality RCTs showed that corticosteroid use was associated with shorter hospital stays, but no change in mortality.6
The synthesis of small or moderate-quality studies suggests some potential benefit in treating less severe CAP with corticosteroids, but there has been a need for a large, definitive, high-quality RCT. This study investigated the impact of a short course of oral steroids on inpatients with less severe CAP.
STUDY SUMMARY: Prednisone hastens clinical stabilization, cuts length of hospital stay
In a multicenter, double-blind RCT, Blum et al1 enrolled 785 patients with CAP admitted to 7 tertiary care hospitals in Switzerland from 2009 to 2014. Patients were eligible for the study if they were ≥18 years old, had a new infiltrate on chest x-ray, and had at least one additional sign or symptom of respiratory illness (eg, cough, dyspnea, fever, abnormal breathing signs or rales, or elevated or decreased white blood cell count). Patients were excluded if they had one of several possible contraindications to corticosteroids, cystic fibrosis, or active tuberculosis.
Patients were randomized to receive either prednisone 50 mg/d or placebo for 7 days. They were treated with antibiotics according to accepted local guidelines; most patients received either amoxicillin/clavulanic acid or ceftriaxone. Antibiotic treatment was adjusted according to susceptibility whenever a specific pathogen was identified. Nurses assessed all patients every 12 hours during hospitalization, and laboratory tests were obtained on hospital Days 1, 3, 5, and 7, and before discharge. Follow-up telephone interviews were conducted on Day 30.
The primary outcome was length of time to clinical stability, which was defined as at least 24 hours of stable vital signs. Stable vital signs was a composite endpoint that required all of the following: temperature ≤37.8°C (≤100°F), heart rate ≤100 beats/min, spontaneous respiratory rate ≤24 breaths/min, systolic blood pressure ≥90 mm Hg (≥100 mm Hg for patients diagnosed with hypertension) without vasopressor support, mental status back to baseline, ability to take food by mouth, and adequate oxygenation on room air.
Secondary outcomes included length of hospital stay, pneumonia recurrence, hospital readmission, intensive care unit (ICU) admission, all-cause mortality, and duration of antibiotic treatment. Researchers also explored whether the rates of complications from pneumonia or corticosteroid use differed between the prednisone and placebo groups.
In an intention-to-treat analysis, the median time to clinical stability was shorter for the prednisone group at 3 days (interquartile range [IQR]=2.5-3.4) compared to the placebo group at 4.4 days (IQR=4-5; hazard ratio [HR]=1.33; 95% confidence interval [CI], 1.15-1.50; P<.0001). Median time to hospital discharge was also shorter for the prednisone group (6 days vs 7 days; HR=1.19; 95% CI, 1.04-1.38; P=.012) as was duration of IV antibiotic treatment (4 days vs 5 days, difference=-0.89 days; 95% CI, -1.57 to -0.20; P=.011).
There were no statistically significant differences in pneumonia recurrence, hospital readmission, ICU admission, or all-cause mortality. Patients treated with prednisone were more likely to experience hyperglycemia that required insulin treatment during admission (19% vs 11%; odds ratio=1.96; 95% CI, 1.31-2.93; P=.001).
WHAT'S NEW: This large, good-quality study reinforces previous evidence
This is the largest good-quality RCT to explore the impact of corticosteroid treatment on less severe CAP. Previous studies suggested that corticosteroids may decrease the duration of illness, but this is the first rigorous study to show a clear decrease in both time to clinical stability and length of hospital stay.
Also, this study used an easy-to-administer dose of oral steroids, instead of the several-day course of IV steroids used in most other studies. The findings from this study were incorporated into a 2015 meta-analysis that confirmed that corticosteroid treatment in patients with less severe CAP results in a shorter length of hospital stay and decreased time to clinical stability.7
CAVEATS: It's unclear whether steroids can benefit nonhospitalized patients
Because this study included hospitalized patients only, it’s not clear whether corticosteroids have a role in outpatient treatment of CAP. Additionally, while this was a large, well performed study, it did not have a sufficient number of patients to examine whether corticosteroids impact mortality among patients with CAP. Finally, the average length of hospital stay reported in this study was approximately 1.5 days longer than the typical length of stay in the United States.2 The average length of stay has varied widely in studies examining corticosteroids in CAP, but good-quality studies have consistently shown a median reduction in length of stay of one day.7
CHALLENGES TO IMPLEMENTATION: Steroids carry a risk of adverse events, including hyperglycemia
Treatment with prednisone increases the risk of corticosteroid-related adverse events, primarily hyperglycemia and the need for insulin. This may not be well received by patients or providers. However, these adverse effects appear to resolve quickly after treatment, and do not impact the overall time to clinical stability.
ACKNOWLEDGEMENT
The PURLs Surveillance System was supported in part by Grant Number UL1RR024999 from the National Center For Research Resources, a Clinical Translational Science Award to the University of Chicago. The content is solely the responsibility of the authors and does not necessarily represent the official views of the National Center For Research Resources or the National Institutes of Health.
1. Blum CA, Nigro N, Briel M, et al. Adjunct prednisone therapy for patients with community-acquired pneumonia: a multicentre, double-blind, randomized, placebo-controlled trial. Lancet. 2015;385:1511-1518.
2. Centers for Disease Control and Prevention (CDC). FastStats: Pneumonia. Centers for Disease Control and Prevention Web site. Available at: http://www.cdc.gov/nchs/fastats/pneumonia.htm. Accessed July 15, 2015.
3. Tejada-Vera B, Chong Y, Lu L, et al. Top 10 leading causes of death: United States, 1999–2013. Centers for Disease Control and Prevention National Center for Health Statistics Web site. Available at: http://blogs.cdc.gov/nchs-data-visualization/2015/06/01/leading-causes-of-death. Accessed September 10, 2015.
4. Chen Y, Li K, Pu H, et al. Corticosteroids for pneumonia. Cochrane Database Syst Rev. 2011;3:CD007720.
5. Nie W, Zhang Y, Cheng J, et al. Corticosteroids in the treatment of community-acquired pneumonia in adults: a meta-analysis. PLoS One. 2012;7:e47926.
6. Shafiq M, Mansoor MS, Khan AA, et al. Adjuvant steroid therapy in community-acquired pneumonia: a systematic review and meta-analysis. J Hosp Med. 2013;8:68-75.
7. Siemieniuk RA, Meade MO, Alonso-Coello P, et al. Corticosteroid therapy for patients hospitalized with community-acquired pneumonia: a systematic review and meta-analysis. Ann Intern Med. 2015. [Epub ahead of print].
Prescribe oral prednisone 50 mg/d to hospitalized patients with mild to moderate community-acquired pneumonia. It decreases time to clinical stability and length of hospital stay.1
Strength of recommendation
A: Based on a single good-quality randomized controlled trial and meta-analysis.
Blum CA, Nigro N, Briel M, et al. Adjunct prednisone therapy for patients with community-acquired pneumonia: a multicentre, double-blind, randomized, placebo-controlled trial. Lancet. 2015;385:1511-1518.
Illustrative case
A 75-year-old woman with hypertension and diabetes mellitus presents to the emergency department with shortness of breath, cough, and fever that she’s had for 4 days. On examination, her temperature is 38.2°C (100.7°F), heart rate is 110 beats/min, respiratory rate is 28 breaths/min, oxygen saturation is 91%, and rhonchi are heard in her right lower lung field. A chest x-ray reveals an infiltrate in her right lower lobe. The patient is admitted and started on intravenous (IV) antibiotics, IV fluids, acetaminophen for fever, and oxygen. Can anything else be done to speed her recovery?
Community-acquired pneumonia (CAP) is responsible for more than one million hospitalizations annually in the United States, and is the 8th leading cause of death.2,3 Treatment of CAP typically consists of antibiotics and supportive measures such as IV fluids and antipyretics. Because the disease process of CAP involves extensive inflammation, adjunct treatment with corticosteroids may be beneficial.
Multiple studies have shown that treatment with corticosteroids can help patients with severe CAP, but the potential benefit in patients with less severe CAP has been uncertain.4,5 A Cochrane systematic review published in 2011 identified 6 small randomized controlled trials (RCTs) that evaluated the impact of corticosteroids on recovery from CAP.4 It suggested that corticosteroids may decrease time to recovery, but the studies that included patients with less severe CAP had a relatively high risk of bias.
Subsequently, a 2012 meta-analysis of 9 RCTs explored whether corticosteroids affected mortality in CAP; no benefit was observed in patients with less severe CAP.5 Most recently, a 2013 meta-analysis of 8 moderate-quality RCTs showed that corticosteroid use was associated with shorter hospital stays, but no change in mortality.6
The synthesis of small or moderate-quality studies suggests some potential benefit in treating less severe CAP with corticosteroids, but there has been a need for a large, definitive, high-quality RCT. This study investigated the impact of a short course of oral steroids on inpatients with less severe CAP.
STUDY SUMMARY: Prednisone hastens clinical stabilization, cuts length of hospital stay
In a multicenter, double-blind RCT, Blum et al1 enrolled 785 patients with CAP admitted to 7 tertiary care hospitals in Switzerland from 2009 to 2014. Patients were eligible for the study if they were ≥18 years old, had a new infiltrate on chest x-ray, and had at least one additional sign or symptom of respiratory illness (eg, cough, dyspnea, fever, abnormal breathing signs or rales, or elevated or decreased white blood cell count). Patients were excluded if they had one of several possible contraindications to corticosteroids, cystic fibrosis, or active tuberculosis.
Patients were randomized to receive either prednisone 50 mg/d or placebo for 7 days. They were treated with antibiotics according to accepted local guidelines; most patients received either amoxicillin/clavulanic acid or ceftriaxone. Antibiotic treatment was adjusted according to susceptibility whenever a specific pathogen was identified. Nurses assessed all patients every 12 hours during hospitalization, and laboratory tests were obtained on hospital Days 1, 3, 5, and 7, and before discharge. Follow-up telephone interviews were conducted on Day 30.
The primary outcome was length of time to clinical stability, which was defined as at least 24 hours of stable vital signs. Stable vital signs was a composite endpoint that required all of the following: temperature ≤37.8°C (≤100°F), heart rate ≤100 beats/min, spontaneous respiratory rate ≤24 breaths/min, systolic blood pressure ≥90 mm Hg (≥100 mm Hg for patients diagnosed with hypertension) without vasopressor support, mental status back to baseline, ability to take food by mouth, and adequate oxygenation on room air.
Secondary outcomes included length of hospital stay, pneumonia recurrence, hospital readmission, intensive care unit (ICU) admission, all-cause mortality, and duration of antibiotic treatment. Researchers also explored whether the rates of complications from pneumonia or corticosteroid use differed between the prednisone and placebo groups.
In an intention-to-treat analysis, the median time to clinical stability was shorter for the prednisone group at 3 days (interquartile range [IQR]=2.5-3.4) compared to the placebo group at 4.4 days (IQR=4-5; hazard ratio [HR]=1.33; 95% confidence interval [CI], 1.15-1.50; P<.0001). Median time to hospital discharge was also shorter for the prednisone group (6 days vs 7 days; HR=1.19; 95% CI, 1.04-1.38; P=.012) as was duration of IV antibiotic treatment (4 days vs 5 days, difference=-0.89 days; 95% CI, -1.57 to -0.20; P=.011).
There were no statistically significant differences in pneumonia recurrence, hospital readmission, ICU admission, or all-cause mortality. Patients treated with prednisone were more likely to experience hyperglycemia that required insulin treatment during admission (19% vs 11%; odds ratio=1.96; 95% CI, 1.31-2.93; P=.001).
WHAT'S NEW: This large, good-quality study reinforces previous evidence
This is the largest good-quality RCT to explore the impact of corticosteroid treatment on less severe CAP. Previous studies suggested that corticosteroids may decrease the duration of illness, but this is the first rigorous study to show a clear decrease in both time to clinical stability and length of hospital stay.
Also, this study used an easy-to-administer dose of oral steroids, instead of the several-day course of IV steroids used in most other studies. The findings from this study were incorporated into a 2015 meta-analysis that confirmed that corticosteroid treatment in patients with less severe CAP results in a shorter length of hospital stay and decreased time to clinical stability.7
CAVEATS: It's unclear whether steroids can benefit nonhospitalized patients
Because this study included hospitalized patients only, it’s not clear whether corticosteroids have a role in outpatient treatment of CAP. Additionally, while this was a large, well performed study, it did not have a sufficient number of patients to examine whether corticosteroids impact mortality among patients with CAP. Finally, the average length of hospital stay reported in this study was approximately 1.5 days longer than the typical length of stay in the United States.2 The average length of stay has varied widely in studies examining corticosteroids in CAP, but good-quality studies have consistently shown a median reduction in length of stay of one day.7
CHALLENGES TO IMPLEMENTATION: Steroids carry a risk of adverse events, including hyperglycemia
Treatment with prednisone increases the risk of corticosteroid-related adverse events, primarily hyperglycemia and the need for insulin. This may not be well received by patients or providers. However, these adverse effects appear to resolve quickly after treatment, and do not impact the overall time to clinical stability.
ACKNOWLEDGEMENT
The PURLs Surveillance System was supported in part by Grant Number UL1RR024999 from the National Center For Research Resources, a Clinical Translational Science Award to the University of Chicago. The content is solely the responsibility of the authors and does not necessarily represent the official views of the National Center For Research Resources or the National Institutes of Health.
Prescribe oral prednisone 50 mg/d to hospitalized patients with mild to moderate community-acquired pneumonia. It decreases time to clinical stability and length of hospital stay.1
Strength of recommendation
A: Based on a single good-quality randomized controlled trial and meta-analysis.
Blum CA, Nigro N, Briel M, et al. Adjunct prednisone therapy for patients with community-acquired pneumonia: a multicentre, double-blind, randomized, placebo-controlled trial. Lancet. 2015;385:1511-1518.
Illustrative case
A 75-year-old woman with hypertension and diabetes mellitus presents to the emergency department with shortness of breath, cough, and fever that she’s had for 4 days. On examination, her temperature is 38.2°C (100.7°F), heart rate is 110 beats/min, respiratory rate is 28 breaths/min, oxygen saturation is 91%, and rhonchi are heard in her right lower lung field. A chest x-ray reveals an infiltrate in her right lower lobe. The patient is admitted and started on intravenous (IV) antibiotics, IV fluids, acetaminophen for fever, and oxygen. Can anything else be done to speed her recovery?
Community-acquired pneumonia (CAP) is responsible for more than one million hospitalizations annually in the United States, and is the 8th leading cause of death.2,3 Treatment of CAP typically consists of antibiotics and supportive measures such as IV fluids and antipyretics. Because the disease process of CAP involves extensive inflammation, adjunct treatment with corticosteroids may be beneficial.
Multiple studies have shown that treatment with corticosteroids can help patients with severe CAP, but the potential benefit in patients with less severe CAP has been uncertain.4,5 A Cochrane systematic review published in 2011 identified 6 small randomized controlled trials (RCTs) that evaluated the impact of corticosteroids on recovery from CAP.4 It suggested that corticosteroids may decrease time to recovery, but the studies that included patients with less severe CAP had a relatively high risk of bias.
Subsequently, a 2012 meta-analysis of 9 RCTs explored whether corticosteroids affected mortality in CAP; no benefit was observed in patients with less severe CAP.5 Most recently, a 2013 meta-analysis of 8 moderate-quality RCTs showed that corticosteroid use was associated with shorter hospital stays, but no change in mortality.6
The synthesis of small or moderate-quality studies suggests some potential benefit in treating less severe CAP with corticosteroids, but there has been a need for a large, definitive, high-quality RCT. This study investigated the impact of a short course of oral steroids on inpatients with less severe CAP.
STUDY SUMMARY: Prednisone hastens clinical stabilization, cuts length of hospital stay
In a multicenter, double-blind RCT, Blum et al1 enrolled 785 patients with CAP admitted to 7 tertiary care hospitals in Switzerland from 2009 to 2014. Patients were eligible for the study if they were ≥18 years old, had a new infiltrate on chest x-ray, and had at least one additional sign or symptom of respiratory illness (eg, cough, dyspnea, fever, abnormal breathing signs or rales, or elevated or decreased white blood cell count). Patients were excluded if they had one of several possible contraindications to corticosteroids, cystic fibrosis, or active tuberculosis.
Patients were randomized to receive either prednisone 50 mg/d or placebo for 7 days. They were treated with antibiotics according to accepted local guidelines; most patients received either amoxicillin/clavulanic acid or ceftriaxone. Antibiotic treatment was adjusted according to susceptibility whenever a specific pathogen was identified. Nurses assessed all patients every 12 hours during hospitalization, and laboratory tests were obtained on hospital Days 1, 3, 5, and 7, and before discharge. Follow-up telephone interviews were conducted on Day 30.
The primary outcome was length of time to clinical stability, which was defined as at least 24 hours of stable vital signs. Stable vital signs was a composite endpoint that required all of the following: temperature ≤37.8°C (≤100°F), heart rate ≤100 beats/min, spontaneous respiratory rate ≤24 breaths/min, systolic blood pressure ≥90 mm Hg (≥100 mm Hg for patients diagnosed with hypertension) without vasopressor support, mental status back to baseline, ability to take food by mouth, and adequate oxygenation on room air.
Secondary outcomes included length of hospital stay, pneumonia recurrence, hospital readmission, intensive care unit (ICU) admission, all-cause mortality, and duration of antibiotic treatment. Researchers also explored whether the rates of complications from pneumonia or corticosteroid use differed between the prednisone and placebo groups.
In an intention-to-treat analysis, the median time to clinical stability was shorter for the prednisone group at 3 days (interquartile range [IQR]=2.5-3.4) compared to the placebo group at 4.4 days (IQR=4-5; hazard ratio [HR]=1.33; 95% confidence interval [CI], 1.15-1.50; P<.0001). Median time to hospital discharge was also shorter for the prednisone group (6 days vs 7 days; HR=1.19; 95% CI, 1.04-1.38; P=.012) as was duration of IV antibiotic treatment (4 days vs 5 days, difference=-0.89 days; 95% CI, -1.57 to -0.20; P=.011).
There were no statistically significant differences in pneumonia recurrence, hospital readmission, ICU admission, or all-cause mortality. Patients treated with prednisone were more likely to experience hyperglycemia that required insulin treatment during admission (19% vs 11%; odds ratio=1.96; 95% CI, 1.31-2.93; P=.001).
WHAT'S NEW: This large, good-quality study reinforces previous evidence
This is the largest good-quality RCT to explore the impact of corticosteroid treatment on less severe CAP. Previous studies suggested that corticosteroids may decrease the duration of illness, but this is the first rigorous study to show a clear decrease in both time to clinical stability and length of hospital stay.
Also, this study used an easy-to-administer dose of oral steroids, instead of the several-day course of IV steroids used in most other studies. The findings from this study were incorporated into a 2015 meta-analysis that confirmed that corticosteroid treatment in patients with less severe CAP results in a shorter length of hospital stay and decreased time to clinical stability.7
CAVEATS: It's unclear whether steroids can benefit nonhospitalized patients
Because this study included hospitalized patients only, it’s not clear whether corticosteroids have a role in outpatient treatment of CAP. Additionally, while this was a large, well performed study, it did not have a sufficient number of patients to examine whether corticosteroids impact mortality among patients with CAP. Finally, the average length of hospital stay reported in this study was approximately 1.5 days longer than the typical length of stay in the United States.2 The average length of stay has varied widely in studies examining corticosteroids in CAP, but good-quality studies have consistently shown a median reduction in length of stay of one day.7
CHALLENGES TO IMPLEMENTATION: Steroids carry a risk of adverse events, including hyperglycemia
Treatment with prednisone increases the risk of corticosteroid-related adverse events, primarily hyperglycemia and the need for insulin. This may not be well received by patients or providers. However, these adverse effects appear to resolve quickly after treatment, and do not impact the overall time to clinical stability.
ACKNOWLEDGEMENT
The PURLs Surveillance System was supported in part by Grant Number UL1RR024999 from the National Center For Research Resources, a Clinical Translational Science Award to the University of Chicago. The content is solely the responsibility of the authors and does not necessarily represent the official views of the National Center For Research Resources or the National Institutes of Health.
1. Blum CA, Nigro N, Briel M, et al. Adjunct prednisone therapy for patients with community-acquired pneumonia: a multicentre, double-blind, randomized, placebo-controlled trial. Lancet. 2015;385:1511-1518.
2. Centers for Disease Control and Prevention (CDC). FastStats: Pneumonia. Centers for Disease Control and Prevention Web site. Available at: http://www.cdc.gov/nchs/fastats/pneumonia.htm. Accessed July 15, 2015.
3. Tejada-Vera B, Chong Y, Lu L, et al. Top 10 leading causes of death: United States, 1999–2013. Centers for Disease Control and Prevention National Center for Health Statistics Web site. Available at: http://blogs.cdc.gov/nchs-data-visualization/2015/06/01/leading-causes-of-death. Accessed September 10, 2015.
4. Chen Y, Li K, Pu H, et al. Corticosteroids for pneumonia. Cochrane Database Syst Rev. 2011;3:CD007720.
5. Nie W, Zhang Y, Cheng J, et al. Corticosteroids in the treatment of community-acquired pneumonia in adults: a meta-analysis. PLoS One. 2012;7:e47926.
6. Shafiq M, Mansoor MS, Khan AA, et al. Adjuvant steroid therapy in community-acquired pneumonia: a systematic review and meta-analysis. J Hosp Med. 2013;8:68-75.
7. Siemieniuk RA, Meade MO, Alonso-Coello P, et al. Corticosteroid therapy for patients hospitalized with community-acquired pneumonia: a systematic review and meta-analysis. Ann Intern Med. 2015. [Epub ahead of print].
1. Blum CA, Nigro N, Briel M, et al. Adjunct prednisone therapy for patients with community-acquired pneumonia: a multicentre, double-blind, randomized, placebo-controlled trial. Lancet. 2015;385:1511-1518.
2. Centers for Disease Control and Prevention (CDC). FastStats: Pneumonia. Centers for Disease Control and Prevention Web site. Available at: http://www.cdc.gov/nchs/fastats/pneumonia.htm. Accessed July 15, 2015.
3. Tejada-Vera B, Chong Y, Lu L, et al. Top 10 leading causes of death: United States, 1999–2013. Centers for Disease Control and Prevention National Center for Health Statistics Web site. Available at: http://blogs.cdc.gov/nchs-data-visualization/2015/06/01/leading-causes-of-death. Accessed September 10, 2015.
4. Chen Y, Li K, Pu H, et al. Corticosteroids for pneumonia. Cochrane Database Syst Rev. 2011;3:CD007720.
5. Nie W, Zhang Y, Cheng J, et al. Corticosteroids in the treatment of community-acquired pneumonia in adults: a meta-analysis. PLoS One. 2012;7:e47926.
6. Shafiq M, Mansoor MS, Khan AA, et al. Adjuvant steroid therapy in community-acquired pneumonia: a systematic review and meta-analysis. J Hosp Med. 2013;8:68-75.
7. Siemieniuk RA, Meade MO, Alonso-Coello P, et al. Corticosteroid therapy for patients hospitalized with community-acquired pneumonia: a systematic review and meta-analysis. Ann Intern Med. 2015. [Epub ahead of print].
Copyright © 2015. The Family Physicians Inquiries Network. All rights reserved.
When in Doubt, Blame the Drug
A 54-year-old woman with chronic renal disease was diagnosed with gout and prescribed allopurinol. Two days later, she was evaluated by her nephrologist, whom she informed about her new medication.
Subsequently, the patient developed fever and rash. Laboratory analysis indicated elevated transaminase levels and eosinophilia. She was admitted to the hospital.
During her stay, an infectious disease consultation was obtained, and the allopurinol was discontinued. When the patient’s condition improved, she was discharged.
Following discharge, the patient resumed taking allopurinol, and her rash returned. Eleven days later, she returned to the hospital, where she was diagnosed with toxic epidermal necrolysis. She was found to have a desquamating rash covering 62% of her body. The patient was transferred to a burn center but eventually succumbed to multi-organ failure.
The patient’s estate filed a medical malpractice lawsuit against the nephrologist alleging negligence—specifically, failure to diagnose toxic epidermal necrolysis and failure to review her medications more carefully.
Continue for the outcome >>
OUTCOME
A $5.1 million verdict was returned against the nephrologist.
COMMENT
Many medications cause rash and are subsequently withdrawn; in a few cases, the effects are life threatening. Toxic epidermal necrolysis (TEN) and Stevens-Johnson syndrome (SJS) are relatively uncommon but potentially fatal examples.
From the limited facts presented, we know that a 54-year-old woman with established renal disease of unknown magnitude was prescribed allopurinol for gout and consulted the nephrologist two days later. It is unclear if the patient had the rash during the first visit with her nephrologist. But we do know that she was eventually admitted and maintained on allopurinol while she had the rash, pending infectious disease consultation. At some point, the allopurinol was apparently stopped and the rash improved. After discharge, the patient resumed taking allopurinol. The rash not only returned but also worsened, necessitating her readmission to a burn center.
TEN, like SJS, is often induced by certain medications, including sulfonamides, macrolides, penicillins, and quinolones. Allopurinol, phenobarbital, phenytoin, carbamazepine, valproic acid, and lamotrigine are frequently implicated as well.
TEN is rare but serious. The initial presentation may be subtle, with influenza-like symptoms such as malaise, fever, cough, rhinitis, headache, and arthralgia—and the most discriminating sign: rash.
The rash begins as a poorly defined, erythematous macular rash with purpuric centers. The lesions predominate on the torso and face, sparing the scalp. Mucosal membranes are involved in more than 90% of cases.1 Pain at the site of the skin lesions is often the predominate symptom and is often out of proportion to physical findings. Over a period of hours to days, the rash coalesces to form flaccid blisters and sheetlike epidermal detachment.2 In established cases, patients will nearly universally demonstrate Nikolsky’s sign: Mild frictional contact with the skin results in epithelial desquamation and immediate blistering.
Management involves immediate withdrawal of the offending agent and hospitalization for aggressive management. The mortality rate is high (30% to 60%3) and generally attributed to sepsis or multi-organ failure.
As clinicians, we are sometimes hesitant to label a rash allergic—thereby forever disqualifying an entire class of useful agents from that patient. However, in this case, the fact that the rash occurred simultaneously with a constellation of signs and symptoms perhaps made the rash appear to be part of an infectious process and not a drug-induced reaction. That is the challenge with TEN and SJS: The symptoms are subtle, flu-like, and confounding.
Here, the nephrologist apparently did not take action to stop the allopurinol after the patient first developed the rash. The jury was persuaded that a reasonably prudent clinician would have recognized the clinical presentation and stopped the allopurinol—and certainly not restarted it following discharge (especially after the allopurinol was stopped in the hospital and the rash began to improve).
This case brings to mind two physicians from my training who made an impression. The first was a second-year internal medicine resident. I remember quietly remarking to another student during rounds, “He is really good.” Overhearing, an attending physician answered, “He is really good because in his workup he always considers a presentation as a function of an underlying process, and walks through each of those processes in formulating his differential.”
“Walking through” various disease categories forces the clinician to consider them all: infectious, autoimmune, neoplastic, environmental/toxic, vascular, traumatic, metabolic, inflammatory. In challenging cases, I’ve found it helpful to step backward into those broad basic categories of disease and reconsider the clinical picture.
Here, doing so may have allowed the clinician to reconsider inflammatory and autoimmune processes and revisit the possibility of iatrogenic toxic/environmental causes (ie, the allopurinol). Perhaps the outcome of this case would have been different.
The second physician was a nephrology fellow, who left me with this piece of wisdom: “When in doubt, blame the drug.” Since nephrologists are expert drug-blamers, I suspect the early stages of this unfortunate case presented a clinical challenge.
IN SUM
Before you “missile lock” onto a diagnosis, take a mental step back to consider broad categories of disease. —DML
REFERENCES
1. Letko E, Papaliodis DN, Papaliodis GN, et al. Stevens-Johnson syndrome and toxic epidermal necrolysis: a review of the literature. Ann Allergy Asthma Immunol. 2005;94(4):419-436.
2. Cohen V, Jellinek SP, Schwartz RA, et al. Toxic epidermal necrolysis. Medscape; 2013. emedicine.medscape.com/article/229698-overview. Accessed September 16, 2015.
3. Schulz JT, Sheridan RL, Ryan CM, et al. A 10-year experience with toxic epidermal necrolysis. J Burn Care Rehabil. 2000;21(3): 199-204.
A 54-year-old woman with chronic renal disease was diagnosed with gout and prescribed allopurinol. Two days later, she was evaluated by her nephrologist, whom she informed about her new medication.
Subsequently, the patient developed fever and rash. Laboratory analysis indicated elevated transaminase levels and eosinophilia. She was admitted to the hospital.
During her stay, an infectious disease consultation was obtained, and the allopurinol was discontinued. When the patient’s condition improved, she was discharged.
Following discharge, the patient resumed taking allopurinol, and her rash returned. Eleven days later, she returned to the hospital, where she was diagnosed with toxic epidermal necrolysis. She was found to have a desquamating rash covering 62% of her body. The patient was transferred to a burn center but eventually succumbed to multi-organ failure.
The patient’s estate filed a medical malpractice lawsuit against the nephrologist alleging negligence—specifically, failure to diagnose toxic epidermal necrolysis and failure to review her medications more carefully.
Continue for the outcome >>
OUTCOME
A $5.1 million verdict was returned against the nephrologist.
COMMENT
Many medications cause rash and are subsequently withdrawn; in a few cases, the effects are life threatening. Toxic epidermal necrolysis (TEN) and Stevens-Johnson syndrome (SJS) are relatively uncommon but potentially fatal examples.
From the limited facts presented, we know that a 54-year-old woman with established renal disease of unknown magnitude was prescribed allopurinol for gout and consulted the nephrologist two days later. It is unclear if the patient had the rash during the first visit with her nephrologist. But we do know that she was eventually admitted and maintained on allopurinol while she had the rash, pending infectious disease consultation. At some point, the allopurinol was apparently stopped and the rash improved. After discharge, the patient resumed taking allopurinol. The rash not only returned but also worsened, necessitating her readmission to a burn center.
TEN, like SJS, is often induced by certain medications, including sulfonamides, macrolides, penicillins, and quinolones. Allopurinol, phenobarbital, phenytoin, carbamazepine, valproic acid, and lamotrigine are frequently implicated as well.
TEN is rare but serious. The initial presentation may be subtle, with influenza-like symptoms such as malaise, fever, cough, rhinitis, headache, and arthralgia—and the most discriminating sign: rash.
The rash begins as a poorly defined, erythematous macular rash with purpuric centers. The lesions predominate on the torso and face, sparing the scalp. Mucosal membranes are involved in more than 90% of cases.1 Pain at the site of the skin lesions is often the predominate symptom and is often out of proportion to physical findings. Over a period of hours to days, the rash coalesces to form flaccid blisters and sheetlike epidermal detachment.2 In established cases, patients will nearly universally demonstrate Nikolsky’s sign: Mild frictional contact with the skin results in epithelial desquamation and immediate blistering.
Management involves immediate withdrawal of the offending agent and hospitalization for aggressive management. The mortality rate is high (30% to 60%3) and generally attributed to sepsis or multi-organ failure.
As clinicians, we are sometimes hesitant to label a rash allergic—thereby forever disqualifying an entire class of useful agents from that patient. However, in this case, the fact that the rash occurred simultaneously with a constellation of signs and symptoms perhaps made the rash appear to be part of an infectious process and not a drug-induced reaction. That is the challenge with TEN and SJS: The symptoms are subtle, flu-like, and confounding.
Here, the nephrologist apparently did not take action to stop the allopurinol after the patient first developed the rash. The jury was persuaded that a reasonably prudent clinician would have recognized the clinical presentation and stopped the allopurinol—and certainly not restarted it following discharge (especially after the allopurinol was stopped in the hospital and the rash began to improve).
This case brings to mind two physicians from my training who made an impression. The first was a second-year internal medicine resident. I remember quietly remarking to another student during rounds, “He is really good.” Overhearing, an attending physician answered, “He is really good because in his workup he always considers a presentation as a function of an underlying process, and walks through each of those processes in formulating his differential.”
“Walking through” various disease categories forces the clinician to consider them all: infectious, autoimmune, neoplastic, environmental/toxic, vascular, traumatic, metabolic, inflammatory. In challenging cases, I’ve found it helpful to step backward into those broad basic categories of disease and reconsider the clinical picture.
Here, doing so may have allowed the clinician to reconsider inflammatory and autoimmune processes and revisit the possibility of iatrogenic toxic/environmental causes (ie, the allopurinol). Perhaps the outcome of this case would have been different.
The second physician was a nephrology fellow, who left me with this piece of wisdom: “When in doubt, blame the drug.” Since nephrologists are expert drug-blamers, I suspect the early stages of this unfortunate case presented a clinical challenge.
IN SUM
Before you “missile lock” onto a diagnosis, take a mental step back to consider broad categories of disease. —DML
REFERENCES
1. Letko E, Papaliodis DN, Papaliodis GN, et al. Stevens-Johnson syndrome and toxic epidermal necrolysis: a review of the literature. Ann Allergy Asthma Immunol. 2005;94(4):419-436.
2. Cohen V, Jellinek SP, Schwartz RA, et al. Toxic epidermal necrolysis. Medscape; 2013. emedicine.medscape.com/article/229698-overview. Accessed September 16, 2015.
3. Schulz JT, Sheridan RL, Ryan CM, et al. A 10-year experience with toxic epidermal necrolysis. J Burn Care Rehabil. 2000;21(3): 199-204.
A 54-year-old woman with chronic renal disease was diagnosed with gout and prescribed allopurinol. Two days later, she was evaluated by her nephrologist, whom she informed about her new medication.
Subsequently, the patient developed fever and rash. Laboratory analysis indicated elevated transaminase levels and eosinophilia. She was admitted to the hospital.
During her stay, an infectious disease consultation was obtained, and the allopurinol was discontinued. When the patient’s condition improved, she was discharged.
Following discharge, the patient resumed taking allopurinol, and her rash returned. Eleven days later, she returned to the hospital, where she was diagnosed with toxic epidermal necrolysis. She was found to have a desquamating rash covering 62% of her body. The patient was transferred to a burn center but eventually succumbed to multi-organ failure.
The patient’s estate filed a medical malpractice lawsuit against the nephrologist alleging negligence—specifically, failure to diagnose toxic epidermal necrolysis and failure to review her medications more carefully.
Continue for the outcome >>
OUTCOME
A $5.1 million verdict was returned against the nephrologist.
COMMENT
Many medications cause rash and are subsequently withdrawn; in a few cases, the effects are life threatening. Toxic epidermal necrolysis (TEN) and Stevens-Johnson syndrome (SJS) are relatively uncommon but potentially fatal examples.
From the limited facts presented, we know that a 54-year-old woman with established renal disease of unknown magnitude was prescribed allopurinol for gout and consulted the nephrologist two days later. It is unclear if the patient had the rash during the first visit with her nephrologist. But we do know that she was eventually admitted and maintained on allopurinol while she had the rash, pending infectious disease consultation. At some point, the allopurinol was apparently stopped and the rash improved. After discharge, the patient resumed taking allopurinol. The rash not only returned but also worsened, necessitating her readmission to a burn center.
TEN, like SJS, is often induced by certain medications, including sulfonamides, macrolides, penicillins, and quinolones. Allopurinol, phenobarbital, phenytoin, carbamazepine, valproic acid, and lamotrigine are frequently implicated as well.
TEN is rare but serious. The initial presentation may be subtle, with influenza-like symptoms such as malaise, fever, cough, rhinitis, headache, and arthralgia—and the most discriminating sign: rash.
The rash begins as a poorly defined, erythematous macular rash with purpuric centers. The lesions predominate on the torso and face, sparing the scalp. Mucosal membranes are involved in more than 90% of cases.1 Pain at the site of the skin lesions is often the predominate symptom and is often out of proportion to physical findings. Over a period of hours to days, the rash coalesces to form flaccid blisters and sheetlike epidermal detachment.2 In established cases, patients will nearly universally demonstrate Nikolsky’s sign: Mild frictional contact with the skin results in epithelial desquamation and immediate blistering.
Management involves immediate withdrawal of the offending agent and hospitalization for aggressive management. The mortality rate is high (30% to 60%3) and generally attributed to sepsis or multi-organ failure.
As clinicians, we are sometimes hesitant to label a rash allergic—thereby forever disqualifying an entire class of useful agents from that patient. However, in this case, the fact that the rash occurred simultaneously with a constellation of signs and symptoms perhaps made the rash appear to be part of an infectious process and not a drug-induced reaction. That is the challenge with TEN and SJS: The symptoms are subtle, flu-like, and confounding.
Here, the nephrologist apparently did not take action to stop the allopurinol after the patient first developed the rash. The jury was persuaded that a reasonably prudent clinician would have recognized the clinical presentation and stopped the allopurinol—and certainly not restarted it following discharge (especially after the allopurinol was stopped in the hospital and the rash began to improve).
This case brings to mind two physicians from my training who made an impression. The first was a second-year internal medicine resident. I remember quietly remarking to another student during rounds, “He is really good.” Overhearing, an attending physician answered, “He is really good because in his workup he always considers a presentation as a function of an underlying process, and walks through each of those processes in formulating his differential.”
“Walking through” various disease categories forces the clinician to consider them all: infectious, autoimmune, neoplastic, environmental/toxic, vascular, traumatic, metabolic, inflammatory. In challenging cases, I’ve found it helpful to step backward into those broad basic categories of disease and reconsider the clinical picture.
Here, doing so may have allowed the clinician to reconsider inflammatory and autoimmune processes and revisit the possibility of iatrogenic toxic/environmental causes (ie, the allopurinol). Perhaps the outcome of this case would have been different.
The second physician was a nephrology fellow, who left me with this piece of wisdom: “When in doubt, blame the drug.” Since nephrologists are expert drug-blamers, I suspect the early stages of this unfortunate case presented a clinical challenge.
IN SUM
Before you “missile lock” onto a diagnosis, take a mental step back to consider broad categories of disease. —DML
REFERENCES
1. Letko E, Papaliodis DN, Papaliodis GN, et al. Stevens-Johnson syndrome and toxic epidermal necrolysis: a review of the literature. Ann Allergy Asthma Immunol. 2005;94(4):419-436.
2. Cohen V, Jellinek SP, Schwartz RA, et al. Toxic epidermal necrolysis. Medscape; 2013. emedicine.medscape.com/article/229698-overview. Accessed September 16, 2015.
3. Schulz JT, Sheridan RL, Ryan CM, et al. A 10-year experience with toxic epidermal necrolysis. J Burn Care Rehabil. 2000;21(3): 199-204.
Ask, listen, help: Pearls to treat sexual dysfunction in menopause
LAS VEGAS – The first step is to ask. A menopausal woman may be struggling with a female sexual disorder (FSD), but unless her clinician asks, the patient may never volunteer information about her sexual health.
Susan Kellogg Spadt, Ph.D., offered that advice, along with a toolkit of tips, treatments, and pearls for physicians and others caring for the menopausal woman’s sexual health.
Dr. Kellogg Spadt, a certified sexual counselor and professor of obstetrics and gynecology at Drexel University, Philadelphia, addressed FSD in the context of the complicated psychosocial landscape of midlife.
Women at this stage of life may be experiencing life stress as children move out, retirement looms, and aging parents require time and attention. Also, as women age, they are more likely to require medications that can negatively affect sexual health. Body image issues, depression, anxiety, and discrepancy with partner desire levels can all be prevalent in women aged 45-64 years, the group most likely to experience distress from sexual problems, she said at the NAMS 2015 annual meeting.
This is important in the context of a relationship, said Dr. Kellogg Spadt. She pointed out that “when sex is good, it adds a little bit – like icing on the cupcake – to a good relationship.” But when sex is bad or nonexistent, she said, it plays an inordinately negative role, reducing the quality of the relationship by 50%-70% in some studies.
Dr. Kellogg Spadt said a good opening approach should confirm the ubiquity of sexual problems in midlife and normalize concerns. Clinicians can ask: “With menopause, many women have changes in their sexual response. These concerns are very common. Tell me – are you feeling well and complete in your sex life?”
If questioning reveals unsatisfying or nonexistent sex, many problems can be addressed in the office. First, careful questioning and an exam can tease out the extent to which dyspareunia and vaginal dryness may be limiting sexual pleasure. In that case, lubricants, moisturizers, and topical estrogen can be considered.
Office sessions with physical therapists certified in pelvic issues, combined with home use of dilators, can help overcome physical contributors to an uncomfortable sexual experience, she said.
Clinicians can also provide brief office-based counseling using the “PLISSIT” model, which. gives permission for the patient to speak openly about sexual issues; provides limited information to educate the patient about her anatomy and resources available; offers specific suggestions, for example, positioning tips or moisturizer recommendations; and offers intensive therapy, when indicated, such as referring for adjunctive psychotherapy.
Dr. Kellogg Stadt concluded with her top clinical pearls for sexual health in menopausal women:
• Add moisture daily. Using a water-based, bioadhesive lubricant several times a week regardless of sexual frequency can significantly ease comfort and satisfaction with sex and make it easier to have an orgasm.
• Nourish. A Mediterranean diet has been shown to promote sexual function, and regular exercise improves mood and overall health.
• Talk. Partners can use “I” language to talk about sex honestly and in a nonaccusatory way. Clinicians can help provide the vocabulary and communication tips to facilitate this.
• Prioritize pleasure. Intimate time together won’t just happen; even a 20-minute block of time, scheduled weekly, for touching and intimate conversation can clear the way to better sex.
• Think. Reading or watching erotica, being mindful of erotic thoughts as they occur, and focusing on sensation rather than distractions during arousal are all important.
• Stimulate. After menopause, some women need more intense stimulation to reach orgasm, so vibrators can be incorporated into sex play. Women who are uncomfortable with this can use the “doctor’s orders” approach with their partners.
• Try. Just opening up and talking about sex problems shows that a woman is committed to her partner, and taking action shows her level of care and concern for the relationship.
Dr. Kellogg Stadt reported being a consultant or on the advisory board of Neogyn and Nuelle, and on the speakers bureau of Novo Nordisk and Shionogi.
On Twitter @karioakes
LAS VEGAS – The first step is to ask. A menopausal woman may be struggling with a female sexual disorder (FSD), but unless her clinician asks, the patient may never volunteer information about her sexual health.
Susan Kellogg Spadt, Ph.D., offered that advice, along with a toolkit of tips, treatments, and pearls for physicians and others caring for the menopausal woman’s sexual health.
Dr. Kellogg Spadt, a certified sexual counselor and professor of obstetrics and gynecology at Drexel University, Philadelphia, addressed FSD in the context of the complicated psychosocial landscape of midlife.
Women at this stage of life may be experiencing life stress as children move out, retirement looms, and aging parents require time and attention. Also, as women age, they are more likely to require medications that can negatively affect sexual health. Body image issues, depression, anxiety, and discrepancy with partner desire levels can all be prevalent in women aged 45-64 years, the group most likely to experience distress from sexual problems, she said at the NAMS 2015 annual meeting.
This is important in the context of a relationship, said Dr. Kellogg Spadt. She pointed out that “when sex is good, it adds a little bit – like icing on the cupcake – to a good relationship.” But when sex is bad or nonexistent, she said, it plays an inordinately negative role, reducing the quality of the relationship by 50%-70% in some studies.
Dr. Kellogg Spadt said a good opening approach should confirm the ubiquity of sexual problems in midlife and normalize concerns. Clinicians can ask: “With menopause, many women have changes in their sexual response. These concerns are very common. Tell me – are you feeling well and complete in your sex life?”
If questioning reveals unsatisfying or nonexistent sex, many problems can be addressed in the office. First, careful questioning and an exam can tease out the extent to which dyspareunia and vaginal dryness may be limiting sexual pleasure. In that case, lubricants, moisturizers, and topical estrogen can be considered.
Office sessions with physical therapists certified in pelvic issues, combined with home use of dilators, can help overcome physical contributors to an uncomfortable sexual experience, she said.
Clinicians can also provide brief office-based counseling using the “PLISSIT” model, which. gives permission for the patient to speak openly about sexual issues; provides limited information to educate the patient about her anatomy and resources available; offers specific suggestions, for example, positioning tips or moisturizer recommendations; and offers intensive therapy, when indicated, such as referring for adjunctive psychotherapy.
Dr. Kellogg Stadt concluded with her top clinical pearls for sexual health in menopausal women:
• Add moisture daily. Using a water-based, bioadhesive lubricant several times a week regardless of sexual frequency can significantly ease comfort and satisfaction with sex and make it easier to have an orgasm.
• Nourish. A Mediterranean diet has been shown to promote sexual function, and regular exercise improves mood and overall health.
• Talk. Partners can use “I” language to talk about sex honestly and in a nonaccusatory way. Clinicians can help provide the vocabulary and communication tips to facilitate this.
• Prioritize pleasure. Intimate time together won’t just happen; even a 20-minute block of time, scheduled weekly, for touching and intimate conversation can clear the way to better sex.
• Think. Reading or watching erotica, being mindful of erotic thoughts as they occur, and focusing on sensation rather than distractions during arousal are all important.
• Stimulate. After menopause, some women need more intense stimulation to reach orgasm, so vibrators can be incorporated into sex play. Women who are uncomfortable with this can use the “doctor’s orders” approach with their partners.
• Try. Just opening up and talking about sex problems shows that a woman is committed to her partner, and taking action shows her level of care and concern for the relationship.
Dr. Kellogg Stadt reported being a consultant or on the advisory board of Neogyn and Nuelle, and on the speakers bureau of Novo Nordisk and Shionogi.
On Twitter @karioakes
LAS VEGAS – The first step is to ask. A menopausal woman may be struggling with a female sexual disorder (FSD), but unless her clinician asks, the patient may never volunteer information about her sexual health.
Susan Kellogg Spadt, Ph.D., offered that advice, along with a toolkit of tips, treatments, and pearls for physicians and others caring for the menopausal woman’s sexual health.
Dr. Kellogg Spadt, a certified sexual counselor and professor of obstetrics and gynecology at Drexel University, Philadelphia, addressed FSD in the context of the complicated psychosocial landscape of midlife.
Women at this stage of life may be experiencing life stress as children move out, retirement looms, and aging parents require time and attention. Also, as women age, they are more likely to require medications that can negatively affect sexual health. Body image issues, depression, anxiety, and discrepancy with partner desire levels can all be prevalent in women aged 45-64 years, the group most likely to experience distress from sexual problems, she said at the NAMS 2015 annual meeting.
This is important in the context of a relationship, said Dr. Kellogg Spadt. She pointed out that “when sex is good, it adds a little bit – like icing on the cupcake – to a good relationship.” But when sex is bad or nonexistent, she said, it plays an inordinately negative role, reducing the quality of the relationship by 50%-70% in some studies.
Dr. Kellogg Spadt said a good opening approach should confirm the ubiquity of sexual problems in midlife and normalize concerns. Clinicians can ask: “With menopause, many women have changes in their sexual response. These concerns are very common. Tell me – are you feeling well and complete in your sex life?”
If questioning reveals unsatisfying or nonexistent sex, many problems can be addressed in the office. First, careful questioning and an exam can tease out the extent to which dyspareunia and vaginal dryness may be limiting sexual pleasure. In that case, lubricants, moisturizers, and topical estrogen can be considered.
Office sessions with physical therapists certified in pelvic issues, combined with home use of dilators, can help overcome physical contributors to an uncomfortable sexual experience, she said.
Clinicians can also provide brief office-based counseling using the “PLISSIT” model, which. gives permission for the patient to speak openly about sexual issues; provides limited information to educate the patient about her anatomy and resources available; offers specific suggestions, for example, positioning tips or moisturizer recommendations; and offers intensive therapy, when indicated, such as referring for adjunctive psychotherapy.
Dr. Kellogg Stadt concluded with her top clinical pearls for sexual health in menopausal women:
• Add moisture daily. Using a water-based, bioadhesive lubricant several times a week regardless of sexual frequency can significantly ease comfort and satisfaction with sex and make it easier to have an orgasm.
• Nourish. A Mediterranean diet has been shown to promote sexual function, and regular exercise improves mood and overall health.
• Talk. Partners can use “I” language to talk about sex honestly and in a nonaccusatory way. Clinicians can help provide the vocabulary and communication tips to facilitate this.
• Prioritize pleasure. Intimate time together won’t just happen; even a 20-minute block of time, scheduled weekly, for touching and intimate conversation can clear the way to better sex.
• Think. Reading or watching erotica, being mindful of erotic thoughts as they occur, and focusing on sensation rather than distractions during arousal are all important.
• Stimulate. After menopause, some women need more intense stimulation to reach orgasm, so vibrators can be incorporated into sex play. Women who are uncomfortable with this can use the “doctor’s orders” approach with their partners.
• Try. Just opening up and talking about sex problems shows that a woman is committed to her partner, and taking action shows her level of care and concern for the relationship.
Dr. Kellogg Stadt reported being a consultant or on the advisory board of Neogyn and Nuelle, and on the speakers bureau of Novo Nordisk and Shionogi.
On Twitter @karioakes
EXPERT ANALYSIS FROM THE NAMS 2015 ANNUAL MEETING
Colon cancer, colonoscopy, and intestinal issues
Hereditary syndromes in gastroenterology have emerged from being esoteric to being common issues that all of us must deal with, according to Dr. Sapna Syngal of Dana-Farber/Brigham and Women’s Cancer Center, Boston. Screening, surveillance, and treatment of hereditary cancers are all different, compared with sporadic cancer. For colorectal cancers, an excellent resource is http://www.nccn.org/professionals/physician_gls/pdf/genetics_colon.pdf, and there are recent society summaries (Am. J. Gastroenterol. 2015;110:223-62). These recommend a family history of cancer be recorded for all patients, including type of cancer, age at diagnosis, and relationship to patient. Lynch syndrome testing should now be done on all newly diagnosed colorectal cancers by testing of mismatch repair proteins or microsatellite instability. Genetic testing should be done when these are detected and on all family members of affected individuals. Familial adenomatous polyposis testing, including the APC and MUTYH genes, should be done for individuals with more than 10 cumulative adenomas, or a family history of any familial adenomatous condition. Peutz-Jeghers syndrome should be tested for in individuals with perioral/buccal pigmentation or those with two or more hamartomatous polyps. Juvenile polyposis syndrome should be tested for in individuals with five or more juvenile polyps in the colorectum or any juvenile polyps in other parts of the GI tract. Simple clinical questions can help identify those who should be tested (Am. J. Gastroenterol. 2009;104:1508-18).
Modern evidence has refuted several long-held beliefs about diverticulosis and diverticulitis, said Dr. Neil Stollman of the University of California, San Francisco. Evidence now shows that diverticulosis is not associated with a low-fiber diet and that high-fiber diets are associated with higher rates of diverticular complications, although this may be due to confounding. Nonsteroidal anti-inflammatory drugs are associated with increased risk of diverticular bleeding. The risk of diverticulitis is much lower than previously thought. Prior estimates of 10%-25% have now been revised downward to 1%-4%. Nuts and seeds do not cause acute diverticulitis and do not need to be avoided. Diverticulitis is a spectrum of diseases from asymptomatic to symptomatic uncomplicated diverticular disease (SUDD) to acute diverticulitis. Mesalamine may be effective in SUDD and acute diverticulitis but evidence is mixed. Cyclic rifaximin and probiotics may also be helpful in SUDD. Antibiotics, previously thought to be mandatory in acute diverticulitis, have not been clearly beneficial in large randomized trials. Surgery, which was also previously recommended after a second attack of acute diverticulitis, is now considered optional and should be individualized.
Dr. Douglas K. Rex of Indiana University Health, Indianapolis, reviewed key measures of quality in colonoscopy and how to achieve these, even under difficult circumstances. Key measures include adenoma detection rate, which can be improved through careful attention to good technique (looking behind folds, adequate distention, and washing) and recognition of flat and sessile polyps. New technologies include high definition, special endoscope caps that flatten folds, and new wide-angle colonoscopes. Water immersion and CO2 insufflation have also improved quality through easier insertion and less gas distention and pain after the procedure. For removal of large, lateral spreading polyps, the technique of endoscopic mucosal resection has been highly effective. Use of a “bulking agent” for submucosal injection such as hydroxyethyl starch, injectable contrast agent, underwater endoscopic mucosal resection, and avulsion (with cold or hot forceps) of fibrotic/scarred polyps have all been demonstrated to be effective in expert hands.
Dr. Charlene M. Prather of Saint Louis University summarized the increasing prevalence of constipation and reviewed risk factors (decreased activity, low fiber consumption, and use of multiple constipating medications). Constipation can be divided into three types: normal transit, slow transit, and pelvic floor dysfunction (PFD). Physical exam, particularly a high-quality rectal exam, can be very effective with high negative predictive value for PFD. Treatment includes fiber supplementation (most effective for mild/moderate constipation, less helpful in slow transit or PFD), laxatives (best data are for osmotic laxatives and bisacodyl), and newer agents that stimulate chloride secretion. Testing is appropriate for refractory constipation and includes transit time studies, balloon expulsion, and anorectal manometry.
Dr. Brennan Spiegel of the Cedars-Sinai Center for Outcomes Research and Education, Los Angeles, provided a summary of current national efforts to measure, report, and improve quality in gastroenterology. He highlighted several reports of high variability in quality such as adenoma detection, testing in patients with uncomplicated inflammatory bowel disease, and approaches to diagnosis of irritable bowel syndrome. A general approach to achieving quality was outlined, including: 1. standardization, 2. measurement, 3. reporting, 4. incentivizing (getting paid for performance), and 5. improving. U.S. Medicare oversight groups (PQRS) have chosen four general areas of quality (polyp surveillance, gastroesophageal reflux disease, hepatitis C, and IBD) measures. Gastroenterology societies have provided effective tools to measure and report these, including GIQUIC and the AGA Digestive Health Recognition Program.
Dr. Wallace is professor of medicine and director, digestive diseases research program, Mayo Clinic, Jacksonville, Fla. He has received grants from BSCI and Cosmo and has consulted for Olympus and iLumen. This is a summary provided by the moderator of one of the spring postgraduate course sessions held at DDW 2015.
Hereditary syndromes in gastroenterology have emerged from being esoteric to being common issues that all of us must deal with, according to Dr. Sapna Syngal of Dana-Farber/Brigham and Women’s Cancer Center, Boston. Screening, surveillance, and treatment of hereditary cancers are all different, compared with sporadic cancer. For colorectal cancers, an excellent resource is http://www.nccn.org/professionals/physician_gls/pdf/genetics_colon.pdf, and there are recent society summaries (Am. J. Gastroenterol. 2015;110:223-62). These recommend a family history of cancer be recorded for all patients, including type of cancer, age at diagnosis, and relationship to patient. Lynch syndrome testing should now be done on all newly diagnosed colorectal cancers by testing of mismatch repair proteins or microsatellite instability. Genetic testing should be done when these are detected and on all family members of affected individuals. Familial adenomatous polyposis testing, including the APC and MUTYH genes, should be done for individuals with more than 10 cumulative adenomas, or a family history of any familial adenomatous condition. Peutz-Jeghers syndrome should be tested for in individuals with perioral/buccal pigmentation or those with two or more hamartomatous polyps. Juvenile polyposis syndrome should be tested for in individuals with five or more juvenile polyps in the colorectum or any juvenile polyps in other parts of the GI tract. Simple clinical questions can help identify those who should be tested (Am. J. Gastroenterol. 2009;104:1508-18).
Modern evidence has refuted several long-held beliefs about diverticulosis and diverticulitis, said Dr. Neil Stollman of the University of California, San Francisco. Evidence now shows that diverticulosis is not associated with a low-fiber diet and that high-fiber diets are associated with higher rates of diverticular complications, although this may be due to confounding. Nonsteroidal anti-inflammatory drugs are associated with increased risk of diverticular bleeding. The risk of diverticulitis is much lower than previously thought. Prior estimates of 10%-25% have now been revised downward to 1%-4%. Nuts and seeds do not cause acute diverticulitis and do not need to be avoided. Diverticulitis is a spectrum of diseases from asymptomatic to symptomatic uncomplicated diverticular disease (SUDD) to acute diverticulitis. Mesalamine may be effective in SUDD and acute diverticulitis but evidence is mixed. Cyclic rifaximin and probiotics may also be helpful in SUDD. Antibiotics, previously thought to be mandatory in acute diverticulitis, have not been clearly beneficial in large randomized trials. Surgery, which was also previously recommended after a second attack of acute diverticulitis, is now considered optional and should be individualized.
Dr. Douglas K. Rex of Indiana University Health, Indianapolis, reviewed key measures of quality in colonoscopy and how to achieve these, even under difficult circumstances. Key measures include adenoma detection rate, which can be improved through careful attention to good technique (looking behind folds, adequate distention, and washing) and recognition of flat and sessile polyps. New technologies include high definition, special endoscope caps that flatten folds, and new wide-angle colonoscopes. Water immersion and CO2 insufflation have also improved quality through easier insertion and less gas distention and pain after the procedure. For removal of large, lateral spreading polyps, the technique of endoscopic mucosal resection has been highly effective. Use of a “bulking agent” for submucosal injection such as hydroxyethyl starch, injectable contrast agent, underwater endoscopic mucosal resection, and avulsion (with cold or hot forceps) of fibrotic/scarred polyps have all been demonstrated to be effective in expert hands.
Dr. Charlene M. Prather of Saint Louis University summarized the increasing prevalence of constipation and reviewed risk factors (decreased activity, low fiber consumption, and use of multiple constipating medications). Constipation can be divided into three types: normal transit, slow transit, and pelvic floor dysfunction (PFD). Physical exam, particularly a high-quality rectal exam, can be very effective with high negative predictive value for PFD. Treatment includes fiber supplementation (most effective for mild/moderate constipation, less helpful in slow transit or PFD), laxatives (best data are for osmotic laxatives and bisacodyl), and newer agents that stimulate chloride secretion. Testing is appropriate for refractory constipation and includes transit time studies, balloon expulsion, and anorectal manometry.
Dr. Brennan Spiegel of the Cedars-Sinai Center for Outcomes Research and Education, Los Angeles, provided a summary of current national efforts to measure, report, and improve quality in gastroenterology. He highlighted several reports of high variability in quality such as adenoma detection, testing in patients with uncomplicated inflammatory bowel disease, and approaches to diagnosis of irritable bowel syndrome. A general approach to achieving quality was outlined, including: 1. standardization, 2. measurement, 3. reporting, 4. incentivizing (getting paid for performance), and 5. improving. U.S. Medicare oversight groups (PQRS) have chosen four general areas of quality (polyp surveillance, gastroesophageal reflux disease, hepatitis C, and IBD) measures. Gastroenterology societies have provided effective tools to measure and report these, including GIQUIC and the AGA Digestive Health Recognition Program.
Dr. Wallace is professor of medicine and director, digestive diseases research program, Mayo Clinic, Jacksonville, Fla. He has received grants from BSCI and Cosmo and has consulted for Olympus and iLumen. This is a summary provided by the moderator of one of the spring postgraduate course sessions held at DDW 2015.
Hereditary syndromes in gastroenterology have emerged from being esoteric to being common issues that all of us must deal with, according to Dr. Sapna Syngal of Dana-Farber/Brigham and Women’s Cancer Center, Boston. Screening, surveillance, and treatment of hereditary cancers are all different, compared with sporadic cancer. For colorectal cancers, an excellent resource is http://www.nccn.org/professionals/physician_gls/pdf/genetics_colon.pdf, and there are recent society summaries (Am. J. Gastroenterol. 2015;110:223-62). These recommend a family history of cancer be recorded for all patients, including type of cancer, age at diagnosis, and relationship to patient. Lynch syndrome testing should now be done on all newly diagnosed colorectal cancers by testing of mismatch repair proteins or microsatellite instability. Genetic testing should be done when these are detected and on all family members of affected individuals. Familial adenomatous polyposis testing, including the APC and MUTYH genes, should be done for individuals with more than 10 cumulative adenomas, or a family history of any familial adenomatous condition. Peutz-Jeghers syndrome should be tested for in individuals with perioral/buccal pigmentation or those with two or more hamartomatous polyps. Juvenile polyposis syndrome should be tested for in individuals with five or more juvenile polyps in the colorectum or any juvenile polyps in other parts of the GI tract. Simple clinical questions can help identify those who should be tested (Am. J. Gastroenterol. 2009;104:1508-18).
Modern evidence has refuted several long-held beliefs about diverticulosis and diverticulitis, said Dr. Neil Stollman of the University of California, San Francisco. Evidence now shows that diverticulosis is not associated with a low-fiber diet and that high-fiber diets are associated with higher rates of diverticular complications, although this may be due to confounding. Nonsteroidal anti-inflammatory drugs are associated with increased risk of diverticular bleeding. The risk of diverticulitis is much lower than previously thought. Prior estimates of 10%-25% have now been revised downward to 1%-4%. Nuts and seeds do not cause acute diverticulitis and do not need to be avoided. Diverticulitis is a spectrum of diseases from asymptomatic to symptomatic uncomplicated diverticular disease (SUDD) to acute diverticulitis. Mesalamine may be effective in SUDD and acute diverticulitis but evidence is mixed. Cyclic rifaximin and probiotics may also be helpful in SUDD. Antibiotics, previously thought to be mandatory in acute diverticulitis, have not been clearly beneficial in large randomized trials. Surgery, which was also previously recommended after a second attack of acute diverticulitis, is now considered optional and should be individualized.
Dr. Douglas K. Rex of Indiana University Health, Indianapolis, reviewed key measures of quality in colonoscopy and how to achieve these, even under difficult circumstances. Key measures include adenoma detection rate, which can be improved through careful attention to good technique (looking behind folds, adequate distention, and washing) and recognition of flat and sessile polyps. New technologies include high definition, special endoscope caps that flatten folds, and new wide-angle colonoscopes. Water immersion and CO2 insufflation have also improved quality through easier insertion and less gas distention and pain after the procedure. For removal of large, lateral spreading polyps, the technique of endoscopic mucosal resection has been highly effective. Use of a “bulking agent” for submucosal injection such as hydroxyethyl starch, injectable contrast agent, underwater endoscopic mucosal resection, and avulsion (with cold or hot forceps) of fibrotic/scarred polyps have all been demonstrated to be effective in expert hands.
Dr. Charlene M. Prather of Saint Louis University summarized the increasing prevalence of constipation and reviewed risk factors (decreased activity, low fiber consumption, and use of multiple constipating medications). Constipation can be divided into three types: normal transit, slow transit, and pelvic floor dysfunction (PFD). Physical exam, particularly a high-quality rectal exam, can be very effective with high negative predictive value for PFD. Treatment includes fiber supplementation (most effective for mild/moderate constipation, less helpful in slow transit or PFD), laxatives (best data are for osmotic laxatives and bisacodyl), and newer agents that stimulate chloride secretion. Testing is appropriate for refractory constipation and includes transit time studies, balloon expulsion, and anorectal manometry.
Dr. Brennan Spiegel of the Cedars-Sinai Center for Outcomes Research and Education, Los Angeles, provided a summary of current national efforts to measure, report, and improve quality in gastroenterology. He highlighted several reports of high variability in quality such as adenoma detection, testing in patients with uncomplicated inflammatory bowel disease, and approaches to diagnosis of irritable bowel syndrome. A general approach to achieving quality was outlined, including: 1. standardization, 2. measurement, 3. reporting, 4. incentivizing (getting paid for performance), and 5. improving. U.S. Medicare oversight groups (PQRS) have chosen four general areas of quality (polyp surveillance, gastroesophageal reflux disease, hepatitis C, and IBD) measures. Gastroenterology societies have provided effective tools to measure and report these, including GIQUIC and the AGA Digestive Health Recognition Program.
Dr. Wallace is professor of medicine and director, digestive diseases research program, Mayo Clinic, Jacksonville, Fla. He has received grants from BSCI and Cosmo and has consulted for Olympus and iLumen. This is a summary provided by the moderator of one of the spring postgraduate course sessions held at DDW 2015.
Upper GI tract
This year’s session on esophagus/upper GI at the AGA Spring Postgraduate Course was packed with pragmatic, useful information for the evaluation of patients with upper GI disorders. The session began with a talk on the manifestations of extraesophageal reflux disease. The take-home message of this talk was that putative extraesophageal manifestations of gastroesophageal reflux disease rarely respond to high-dose therapy with PPIs (proton-pump inhibitors), in the absence of concurrent esophageal symptoms such as heartburn or regurgitation. In such situations, investigation of other etiologies of patients’ symptoms, including occult postnasal drainage or cough-variant asthma, may be more rewarding than escalating anti-acid therapy.
Dr. John E. Pandolfino, AGAF, of Northwestern University, Chicago, discussed the utilization of high-resolution manometry in the evaluation of dysphagia. A central focus of this discussion was the use of the Chicago classification of motility abnormalities in assessing these patients. In the future, it is likely that the care of these patients will be dictated by the type of abnormality the patient has in this classification scheme. Especially in the setting of achalasia, data are emerging that some subtypes of achalasia are less likely to respond to some therapies. For instance, type 3 achalasia is unlikely to respond to pneumatic balloon dilatation.
Dr. Rhonda Souza of the University of Texas Southwestern Medical Center, Dallas, taught us that a diagnosis of eosinophilic esophagitis requires an 8-week trial of PPI therapy with no resolution of the eosinophilia. Esophageal dilation is generally safe in the setting of a dominant stricture, but it can be delayed prior to medical therapy if the patient is tolerating oral intake well. Although the most commonly used therapies for EoE now involve either swallowed steroids or dietary elimination therapy, several new agents are on the horizon that may give us new treatment options.
Finally, Dr. Amitabh Chak of Case Western Reserve University, Cleveland, reviewed the care of patients with Barrett’s esophagus and low-grade dysplasia. This is an especially difficult group of patients to care for, due in part to the low reproducibility in the diagnosis of low-grade dysplasia, as well as the highly variable reported cancer outcomes in this patient population. Level 1 evidence now exists demonstrating that treatment with radiofrequency ablation decreases the incidence of cancer in patients with low-grade dysplasia, but most patients with this finding will not progress. Therefore, the field would benefit from better risk stratification of these patients.
Dr. Shaheen is professor of medicine and epidemiology and chief of the division of gastroenterology & hepatology, University of North Carolina School of Medicine, Chapel Hill. This is a summary provided by the moderator of one of the spring postgraduate course sessions held at DDW 2015.
This year’s session on esophagus/upper GI at the AGA Spring Postgraduate Course was packed with pragmatic, useful information for the evaluation of patients with upper GI disorders. The session began with a talk on the manifestations of extraesophageal reflux disease. The take-home message of this talk was that putative extraesophageal manifestations of gastroesophageal reflux disease rarely respond to high-dose therapy with PPIs (proton-pump inhibitors), in the absence of concurrent esophageal symptoms such as heartburn or regurgitation. In such situations, investigation of other etiologies of patients’ symptoms, including occult postnasal drainage or cough-variant asthma, may be more rewarding than escalating anti-acid therapy.
Dr. John E. Pandolfino, AGAF, of Northwestern University, Chicago, discussed the utilization of high-resolution manometry in the evaluation of dysphagia. A central focus of this discussion was the use of the Chicago classification of motility abnormalities in assessing these patients. In the future, it is likely that the care of these patients will be dictated by the type of abnormality the patient has in this classification scheme. Especially in the setting of achalasia, data are emerging that some subtypes of achalasia are less likely to respond to some therapies. For instance, type 3 achalasia is unlikely to respond to pneumatic balloon dilatation.
Dr. Rhonda Souza of the University of Texas Southwestern Medical Center, Dallas, taught us that a diagnosis of eosinophilic esophagitis requires an 8-week trial of PPI therapy with no resolution of the eosinophilia. Esophageal dilation is generally safe in the setting of a dominant stricture, but it can be delayed prior to medical therapy if the patient is tolerating oral intake well. Although the most commonly used therapies for EoE now involve either swallowed steroids or dietary elimination therapy, several new agents are on the horizon that may give us new treatment options.
Finally, Dr. Amitabh Chak of Case Western Reserve University, Cleveland, reviewed the care of patients with Barrett’s esophagus and low-grade dysplasia. This is an especially difficult group of patients to care for, due in part to the low reproducibility in the diagnosis of low-grade dysplasia, as well as the highly variable reported cancer outcomes in this patient population. Level 1 evidence now exists demonstrating that treatment with radiofrequency ablation decreases the incidence of cancer in patients with low-grade dysplasia, but most patients with this finding will not progress. Therefore, the field would benefit from better risk stratification of these patients.
Dr. Shaheen is professor of medicine and epidemiology and chief of the division of gastroenterology & hepatology, University of North Carolina School of Medicine, Chapel Hill. This is a summary provided by the moderator of one of the spring postgraduate course sessions held at DDW 2015.
This year’s session on esophagus/upper GI at the AGA Spring Postgraduate Course was packed with pragmatic, useful information for the evaluation of patients with upper GI disorders. The session began with a talk on the manifestations of extraesophageal reflux disease. The take-home message of this talk was that putative extraesophageal manifestations of gastroesophageal reflux disease rarely respond to high-dose therapy with PPIs (proton-pump inhibitors), in the absence of concurrent esophageal symptoms such as heartburn or regurgitation. In such situations, investigation of other etiologies of patients’ symptoms, including occult postnasal drainage or cough-variant asthma, may be more rewarding than escalating anti-acid therapy.
Dr. John E. Pandolfino, AGAF, of Northwestern University, Chicago, discussed the utilization of high-resolution manometry in the evaluation of dysphagia. A central focus of this discussion was the use of the Chicago classification of motility abnormalities in assessing these patients. In the future, it is likely that the care of these patients will be dictated by the type of abnormality the patient has in this classification scheme. Especially in the setting of achalasia, data are emerging that some subtypes of achalasia are less likely to respond to some therapies. For instance, type 3 achalasia is unlikely to respond to pneumatic balloon dilatation.
Dr. Rhonda Souza of the University of Texas Southwestern Medical Center, Dallas, taught us that a diagnosis of eosinophilic esophagitis requires an 8-week trial of PPI therapy with no resolution of the eosinophilia. Esophageal dilation is generally safe in the setting of a dominant stricture, but it can be delayed prior to medical therapy if the patient is tolerating oral intake well. Although the most commonly used therapies for EoE now involve either swallowed steroids or dietary elimination therapy, several new agents are on the horizon that may give us new treatment options.
Finally, Dr. Amitabh Chak of Case Western Reserve University, Cleveland, reviewed the care of patients with Barrett’s esophagus and low-grade dysplasia. This is an especially difficult group of patients to care for, due in part to the low reproducibility in the diagnosis of low-grade dysplasia, as well as the highly variable reported cancer outcomes in this patient population. Level 1 evidence now exists demonstrating that treatment with radiofrequency ablation decreases the incidence of cancer in patients with low-grade dysplasia, but most patients with this finding will not progress. Therefore, the field would benefit from better risk stratification of these patients.
Dr. Shaheen is professor of medicine and epidemiology and chief of the division of gastroenterology & hepatology, University of North Carolina School of Medicine, Chapel Hill. This is a summary provided by the moderator of one of the spring postgraduate course sessions held at DDW 2015.
EASD: Diabetes doubles death risk from many causes
STOCKHOLM – Having diabetes doubles the risk for death from a multitude of causes and shortens the life span by 5-7 years, judging from evidence from the United Kingdom Prospective Studies Collaboration presented at the annual meeting of the European Association for the Study of Diabetes.
Patients with diabetes were two and a half times more likely than those without it to die from ischemic heart disease (odds ratio, 2.37; 95% confidence interval, 2.23-2.52). They were also more likely to die from ischemic stroke (OR, 2.75; 95% CI, 2.29-3.31) and “a surprising association with” cardiac arrhythmia (OR, 2.47; 95% CI, 1.80-3.38) was found, said the presenting author, Dr. Louisa Gnatiuc of the clinical trial service unit and epidemiological studies unit at Oxford (England) University. She noted, however, that diabetes was more weakly associated with death from nonischemic causes (OR, 1.57; 95% CI, 1.34-1.85).
In addition, diabetic individuals were almost twice as likely as those with no diabetes to die from renal (OR, 1.90; 95% CI, 1.40-2.58) or hepatic (OR, 1.75; 95% CI, 1.36-2.26) causes, and there was a 13% increase in the overall risk for death from cancer (OR, 1.13; 95% CI, 1.06-1.21). The small increased risk for cancer death was largely due to deaths from oral (OR, 1.81; 95% CI, 1.17-2.81), liver (OR, 1.80; 95% CI, 1.26-2.58), and pancreatic (OR, 1.57; 95% CI, 1.21-2.03) cancers, Dr. Gnatiuc observed. Diabetic individuals were also more likely to die from respiratory causes than their nondiabetic counterparts (OR, 1.59; 95% CI, 1.37-1.83).
These data provide further insight into the risk for death from a multitude of causes in patients with diabetes, said Dr. Naveed Sattar, who was not involved in the analysis, in an interview. “While it is pretty much a replication of the Emerging Risk Factor Paper that was published in the New England Journal of Medicine, there is a little bit of new information here, such as the relative risks in women versus men and by age as well,” he observed.
The odds ratio for death from ischemic heart disease was higher among women aged 35-39 years with diabetes versus those with no diabetes than in men aged 35-39 years with diabetes versus no diabetes, at 5.61 (95% CI, 4.08-7.72) and 2.49 (95% CI, 2.16-2.85), respectively. Similar finding were seen at other age ranges, with around a twofold increase in men aged 60-69 years (OR, 2.26; 95% CI, 2.01-2.53) and 70-89 years (OR, 1.89; 95% CI, 1.70-2.11), but a two- to fourfold increase in women aged 60-69 years (OR, 4.15; 95% CI, 3.38-5.10) and 70-79 years (OR, 2.45, 95% CI, 2.11-2.84).
“So it looks like if you develop diabetes younger, your risk for mortality’s higher,” said Dr. Sattar, professor of metabolic medicine at the Institute of Cardiovascular and Medical Sciences, University of Glasgow (Scotland). “That makes sense,” he added, “because if you develop type 2 diabetes at the age of 30, it must be a much more malignant type of diabetes than, say, if you developed diabetes at age 80, and there are other data that now confirm that is the case as well.”
The analysis was based on data from 44 prospective studies that recorded 55,855 deaths in 690,700 adults who had no prior vascular or other chronic diseases at baseline. Men accounted for 60% of the study population, with a mean age of 48 years. During the postpresentation discussion, Dr. Gnatiuc noted that it was difficult to standardize the definition of diabetes used in the study and that all 25,000 cases of diabetes included in the study had been self-reported. It is likely that most cases were type 2. No information on the duration of diabetes was available, but she noted that this should not matter given the prospective nature of the analysis.
During 13 million patient-years of follow up the most common cause of death was ischemic heart disease, accounting for 17,218 deaths, followed by cancer (18,658 deaths). There were 5,466 strokes of which 1,353 were ischemic (OR, 2.75; 95% CI 2.29-3.31), 1,124 hemorrhagic (OR, 1.59; 95% CI 1.26-2.01), 2,563 that were unspecified (OR, 2.30; 95% CI 1.99-2.66), and 626 classed as subarachnoid hemorrhage (OR, 0.62; 95% CI, 0.36-1.08). Vascular causes of death included inflammatory heart disease, sudden death, and atherosclerosis, among others, which were all increased in diabetic versus nondiabetic subjects.
Dr. Gnatiuc noted that the risk for ischemic heart disease increased with increasing age, systolic blood pressure, and total cholesterol in diabetics and in nondiabetics, although it was much higher in those with diabetes than in those without. “Controlling blood pressure, cholesterol, and obesity are particularly important to reduce absolute mortality rates in those with diabetes, she suggested.
The differences between the sexes in terms of ischemic heart disease need further study, she suggested, noting that improved understanding of the sex differences may help better understand and thus reduce the excess mortality seen.
Premature mortality of up to 5 years in men and 7 years in women was observed. “At the age of 70 a man with diabetes would have about 10% less probability to survive up to the age of 90 as compared to their nondiabetic counterparts,” Dr. Gnatiuc said. Women would have about a 30% decrease in survival probability from age 70-90, she added.
STOCKHOLM – Having diabetes doubles the risk for death from a multitude of causes and shortens the life span by 5-7 years, judging from evidence from the United Kingdom Prospective Studies Collaboration presented at the annual meeting of the European Association for the Study of Diabetes.
Patients with diabetes were two and a half times more likely than those without it to die from ischemic heart disease (odds ratio, 2.37; 95% confidence interval, 2.23-2.52). They were also more likely to die from ischemic stroke (OR, 2.75; 95% CI, 2.29-3.31) and “a surprising association with” cardiac arrhythmia (OR, 2.47; 95% CI, 1.80-3.38) was found, said the presenting author, Dr. Louisa Gnatiuc of the clinical trial service unit and epidemiological studies unit at Oxford (England) University. She noted, however, that diabetes was more weakly associated with death from nonischemic causes (OR, 1.57; 95% CI, 1.34-1.85).
In addition, diabetic individuals were almost twice as likely as those with no diabetes to die from renal (OR, 1.90; 95% CI, 1.40-2.58) or hepatic (OR, 1.75; 95% CI, 1.36-2.26) causes, and there was a 13% increase in the overall risk for death from cancer (OR, 1.13; 95% CI, 1.06-1.21). The small increased risk for cancer death was largely due to deaths from oral (OR, 1.81; 95% CI, 1.17-2.81), liver (OR, 1.80; 95% CI, 1.26-2.58), and pancreatic (OR, 1.57; 95% CI, 1.21-2.03) cancers, Dr. Gnatiuc observed. Diabetic individuals were also more likely to die from respiratory causes than their nondiabetic counterparts (OR, 1.59; 95% CI, 1.37-1.83).
These data provide further insight into the risk for death from a multitude of causes in patients with diabetes, said Dr. Naveed Sattar, who was not involved in the analysis, in an interview. “While it is pretty much a replication of the Emerging Risk Factor Paper that was published in the New England Journal of Medicine, there is a little bit of new information here, such as the relative risks in women versus men and by age as well,” he observed.
The odds ratio for death from ischemic heart disease was higher among women aged 35-39 years with diabetes versus those with no diabetes than in men aged 35-39 years with diabetes versus no diabetes, at 5.61 (95% CI, 4.08-7.72) and 2.49 (95% CI, 2.16-2.85), respectively. Similar finding were seen at other age ranges, with around a twofold increase in men aged 60-69 years (OR, 2.26; 95% CI, 2.01-2.53) and 70-89 years (OR, 1.89; 95% CI, 1.70-2.11), but a two- to fourfold increase in women aged 60-69 years (OR, 4.15; 95% CI, 3.38-5.10) and 70-79 years (OR, 2.45, 95% CI, 2.11-2.84).
“So it looks like if you develop diabetes younger, your risk for mortality’s higher,” said Dr. Sattar, professor of metabolic medicine at the Institute of Cardiovascular and Medical Sciences, University of Glasgow (Scotland). “That makes sense,” he added, “because if you develop type 2 diabetes at the age of 30, it must be a much more malignant type of diabetes than, say, if you developed diabetes at age 80, and there are other data that now confirm that is the case as well.”
The analysis was based on data from 44 prospective studies that recorded 55,855 deaths in 690,700 adults who had no prior vascular or other chronic diseases at baseline. Men accounted for 60% of the study population, with a mean age of 48 years. During the postpresentation discussion, Dr. Gnatiuc noted that it was difficult to standardize the definition of diabetes used in the study and that all 25,000 cases of diabetes included in the study had been self-reported. It is likely that most cases were type 2. No information on the duration of diabetes was available, but she noted that this should not matter given the prospective nature of the analysis.
During 13 million patient-years of follow up the most common cause of death was ischemic heart disease, accounting for 17,218 deaths, followed by cancer (18,658 deaths). There were 5,466 strokes of which 1,353 were ischemic (OR, 2.75; 95% CI 2.29-3.31), 1,124 hemorrhagic (OR, 1.59; 95% CI 1.26-2.01), 2,563 that were unspecified (OR, 2.30; 95% CI 1.99-2.66), and 626 classed as subarachnoid hemorrhage (OR, 0.62; 95% CI, 0.36-1.08). Vascular causes of death included inflammatory heart disease, sudden death, and atherosclerosis, among others, which were all increased in diabetic versus nondiabetic subjects.
Dr. Gnatiuc noted that the risk for ischemic heart disease increased with increasing age, systolic blood pressure, and total cholesterol in diabetics and in nondiabetics, although it was much higher in those with diabetes than in those without. “Controlling blood pressure, cholesterol, and obesity are particularly important to reduce absolute mortality rates in those with diabetes, she suggested.
The differences between the sexes in terms of ischemic heart disease need further study, she suggested, noting that improved understanding of the sex differences may help better understand and thus reduce the excess mortality seen.
Premature mortality of up to 5 years in men and 7 years in women was observed. “At the age of 70 a man with diabetes would have about 10% less probability to survive up to the age of 90 as compared to their nondiabetic counterparts,” Dr. Gnatiuc said. Women would have about a 30% decrease in survival probability from age 70-90, she added.
STOCKHOLM – Having diabetes doubles the risk for death from a multitude of causes and shortens the life span by 5-7 years, judging from evidence from the United Kingdom Prospective Studies Collaboration presented at the annual meeting of the European Association for the Study of Diabetes.
Patients with diabetes were two and a half times more likely than those without it to die from ischemic heart disease (odds ratio, 2.37; 95% confidence interval, 2.23-2.52). They were also more likely to die from ischemic stroke (OR, 2.75; 95% CI, 2.29-3.31) and “a surprising association with” cardiac arrhythmia (OR, 2.47; 95% CI, 1.80-3.38) was found, said the presenting author, Dr. Louisa Gnatiuc of the clinical trial service unit and epidemiological studies unit at Oxford (England) University. She noted, however, that diabetes was more weakly associated with death from nonischemic causes (OR, 1.57; 95% CI, 1.34-1.85).
In addition, diabetic individuals were almost twice as likely as those with no diabetes to die from renal (OR, 1.90; 95% CI, 1.40-2.58) or hepatic (OR, 1.75; 95% CI, 1.36-2.26) causes, and there was a 13% increase in the overall risk for death from cancer (OR, 1.13; 95% CI, 1.06-1.21). The small increased risk for cancer death was largely due to deaths from oral (OR, 1.81; 95% CI, 1.17-2.81), liver (OR, 1.80; 95% CI, 1.26-2.58), and pancreatic (OR, 1.57; 95% CI, 1.21-2.03) cancers, Dr. Gnatiuc observed. Diabetic individuals were also more likely to die from respiratory causes than their nondiabetic counterparts (OR, 1.59; 95% CI, 1.37-1.83).
These data provide further insight into the risk for death from a multitude of causes in patients with diabetes, said Dr. Naveed Sattar, who was not involved in the analysis, in an interview. “While it is pretty much a replication of the Emerging Risk Factor Paper that was published in the New England Journal of Medicine, there is a little bit of new information here, such as the relative risks in women versus men and by age as well,” he observed.
The odds ratio for death from ischemic heart disease was higher among women aged 35-39 years with diabetes versus those with no diabetes than in men aged 35-39 years with diabetes versus no diabetes, at 5.61 (95% CI, 4.08-7.72) and 2.49 (95% CI, 2.16-2.85), respectively. Similar finding were seen at other age ranges, with around a twofold increase in men aged 60-69 years (OR, 2.26; 95% CI, 2.01-2.53) and 70-89 years (OR, 1.89; 95% CI, 1.70-2.11), but a two- to fourfold increase in women aged 60-69 years (OR, 4.15; 95% CI, 3.38-5.10) and 70-79 years (OR, 2.45, 95% CI, 2.11-2.84).
“So it looks like if you develop diabetes younger, your risk for mortality’s higher,” said Dr. Sattar, professor of metabolic medicine at the Institute of Cardiovascular and Medical Sciences, University of Glasgow (Scotland). “That makes sense,” he added, “because if you develop type 2 diabetes at the age of 30, it must be a much more malignant type of diabetes than, say, if you developed diabetes at age 80, and there are other data that now confirm that is the case as well.”
The analysis was based on data from 44 prospective studies that recorded 55,855 deaths in 690,700 adults who had no prior vascular or other chronic diseases at baseline. Men accounted for 60% of the study population, with a mean age of 48 years. During the postpresentation discussion, Dr. Gnatiuc noted that it was difficult to standardize the definition of diabetes used in the study and that all 25,000 cases of diabetes included in the study had been self-reported. It is likely that most cases were type 2. No information on the duration of diabetes was available, but she noted that this should not matter given the prospective nature of the analysis.
During 13 million patient-years of follow up the most common cause of death was ischemic heart disease, accounting for 17,218 deaths, followed by cancer (18,658 deaths). There were 5,466 strokes of which 1,353 were ischemic (OR, 2.75; 95% CI 2.29-3.31), 1,124 hemorrhagic (OR, 1.59; 95% CI 1.26-2.01), 2,563 that were unspecified (OR, 2.30; 95% CI 1.99-2.66), and 626 classed as subarachnoid hemorrhage (OR, 0.62; 95% CI, 0.36-1.08). Vascular causes of death included inflammatory heart disease, sudden death, and atherosclerosis, among others, which were all increased in diabetic versus nondiabetic subjects.
Dr. Gnatiuc noted that the risk for ischemic heart disease increased with increasing age, systolic blood pressure, and total cholesterol in diabetics and in nondiabetics, although it was much higher in those with diabetes than in those without. “Controlling blood pressure, cholesterol, and obesity are particularly important to reduce absolute mortality rates in those with diabetes, she suggested.
The differences between the sexes in terms of ischemic heart disease need further study, she suggested, noting that improved understanding of the sex differences may help better understand and thus reduce the excess mortality seen.
Premature mortality of up to 5 years in men and 7 years in women was observed. “At the age of 70 a man with diabetes would have about 10% less probability to survive up to the age of 90 as compared to their nondiabetic counterparts,” Dr. Gnatiuc said. Women would have about a 30% decrease in survival probability from age 70-90, she added.
AT EASD 2015
Key clinical point:Women with diabetes were more likely to die from ischemic heart disease than men with diabetes when compared to those without diabetes.
Major finding: Women with diabetes were more likely to die from ischemic heart disease than men with diabetes when compared to those without diabetes.
Data source: United Kingdom Prospective Studies Collaboration analysis of 44 prospective studies that recorded 55,855 deaths in 690,700 adults who had no prior vascular or other chronic diseases at baseline.
Disclosures: The study was funded by research grants from the British Heart Foundation, UK Medical Research Council, The National Institutes of Health (UK), Cancer Research UK, and the Clinical Trial Service Unit and Epidemiological Studies Unit at Oxford University, UK. Dr. Gnatiuc did not report any personal disclosures.
October 2015 Quiz 2
ANSWER: C
Critique
The first step in managing this patient should not be to stop the tube feedings. Enteral feedings are providing much-needed nutrition to this patient. There is no evidence that either continuous or bolus feedings are superior. Diarrhea has been shown to occur in up to 18% of patients receiving enteral nutrition. Clostridium difficile is the leading cause of health care–associated diarrhea. Antibiotic therapy is the most common risk factor associated with acquisition of C. difficile infection. Internally fed patients are more likely than non–tube fed patients to acquire C. difficile infection and, therefore, it is recommended to check for C. difficile in tube-fed patients, especially when they are receiving antibiotics. The patient has no comorbid conditions that would require elemental formula, such as short bowel syndrome or malabsorption. In addition, it is not clear that elemental formulas are superior to standard formulas in patients with malabsorption or maldigestion. Finally, standard polymeric formulas are lactose free. Other considerations in a patient on enteral feedings with diarrhea include non–C. difficile antibiotic-associated diarrhea, formula composition, sorbitol-containing medication preparations, and side effects of medications as well as hypoalbuminemia.
References
1. Loo V.G., Bourgault A.M., Poirier L., et al. Host and pathogen factors for Clostridium difficile infection and colonization. N Engl J Med. 2011;365:1693-703.
2. Bliss D.Z., Johnson S., Savik K. Acquisition of Clostridium difficile and Clostridium difficile–associated diarrhea in hospitalized patients receiving tube feeding. Ann Intern Med. 1998;129:1012.
3. Macleod J.B., Lefton J., Houghton D., et al. Prospective randomized control trial of intermittent versus continuous gastric feeds for critically ill trauma patients. J Trauma. 2007;63:57.
ANSWER: C
Critique
The first step in managing this patient should not be to stop the tube feedings. Enteral feedings are providing much-needed nutrition to this patient. There is no evidence that either continuous or bolus feedings are superior. Diarrhea has been shown to occur in up to 18% of patients receiving enteral nutrition. Clostridium difficile is the leading cause of health care–associated diarrhea. Antibiotic therapy is the most common risk factor associated with acquisition of C. difficile infection. Internally fed patients are more likely than non–tube fed patients to acquire C. difficile infection and, therefore, it is recommended to check for C. difficile in tube-fed patients, especially when they are receiving antibiotics. The patient has no comorbid conditions that would require elemental formula, such as short bowel syndrome or malabsorption. In addition, it is not clear that elemental formulas are superior to standard formulas in patients with malabsorption or maldigestion. Finally, standard polymeric formulas are lactose free. Other considerations in a patient on enteral feedings with diarrhea include non–C. difficile antibiotic-associated diarrhea, formula composition, sorbitol-containing medication preparations, and side effects of medications as well as hypoalbuminemia.
References
1. Loo V.G., Bourgault A.M., Poirier L., et al. Host and pathogen factors for Clostridium difficile infection and colonization. N Engl J Med. 2011;365:1693-703.
2. Bliss D.Z., Johnson S., Savik K. Acquisition of Clostridium difficile and Clostridium difficile–associated diarrhea in hospitalized patients receiving tube feeding. Ann Intern Med. 1998;129:1012.
3. Macleod J.B., Lefton J., Houghton D., et al. Prospective randomized control trial of intermittent versus continuous gastric feeds for critically ill trauma patients. J Trauma. 2007;63:57.
ANSWER: C
Critique
The first step in managing this patient should not be to stop the tube feedings. Enteral feedings are providing much-needed nutrition to this patient. There is no evidence that either continuous or bolus feedings are superior. Diarrhea has been shown to occur in up to 18% of patients receiving enteral nutrition. Clostridium difficile is the leading cause of health care–associated diarrhea. Antibiotic therapy is the most common risk factor associated with acquisition of C. difficile infection. Internally fed patients are more likely than non–tube fed patients to acquire C. difficile infection and, therefore, it is recommended to check for C. difficile in tube-fed patients, especially when they are receiving antibiotics. The patient has no comorbid conditions that would require elemental formula, such as short bowel syndrome or malabsorption. In addition, it is not clear that elemental formulas are superior to standard formulas in patients with malabsorption or maldigestion. Finally, standard polymeric formulas are lactose free. Other considerations in a patient on enteral feedings with diarrhea include non–C. difficile antibiotic-associated diarrhea, formula composition, sorbitol-containing medication preparations, and side effects of medications as well as hypoalbuminemia.
References
1. Loo V.G., Bourgault A.M., Poirier L., et al. Host and pathogen factors for Clostridium difficile infection and colonization. N Engl J Med. 2011;365:1693-703.
2. Bliss D.Z., Johnson S., Savik K. Acquisition of Clostridium difficile and Clostridium difficile–associated diarrhea in hospitalized patients receiving tube feeding. Ann Intern Med. 1998;129:1012.
3. Macleod J.B., Lefton J., Houghton D., et al. Prospective randomized control trial of intermittent versus continuous gastric feeds for critically ill trauma patients. J Trauma. 2007;63:57.