User login
Interdisciplinary Team Interventions Have Little Impact on Traditional Quality Measures
Clinical question: Do interdisciplinary team care interventions affect outcomes for hospitalized patients in general medical wards?
Bottom line: Interdisciplinary team care interventions do not significantly affect oft-used quality measures such as length of stay, readmissions, or mortality. However, some experts question whether these measures are appropriate for assessing the effectiveness of such interventions. A small body of evidence suggests that interdisciplinary interventions may affect complications of care or preventable adverse events. In the future, these and other more appropriate measures should be used when assessing interdisciplinary team care interventions. (LOE = 2a)
Reference: Pannick S, Davis R, Ashrafian H, et al. Effects of interdisciplinary team care interventions on general medical wards. JAMA Intern Med 2015;175(8):1288-1298.
Study design: Systematic review
Funding source: Government
Allocation: Uncertain
Setting: Inpatient (any location)
Synopsis
These investigators searched multiple databases including EMBASE and MEDLINE, as well as reference lists of included studies, to find trials that evaluated the effects of interdisciplinary team care on objective patient outcomes in the general medical wards. Study selection, data extraction, and assessment of bias were performed by independent reviewers.
Thirty studies (randomized controlled trials, cluster studies, and before-after studies) were included in the review. The studies had heterogeneous designs and outcome measures and all of them had a medium or high risk of bias. The majority of the studies, however, reported on complications of care, length of stay, readmission, or mortality.
Out of 10 studies that examined complications of care, five showed a reduction in this outcome by formalizing interdisciplinary rounds or adding specialized clinicians or pharmacists to the interdisciplinary team. Overall, 20% of the studies that looked at length of stay showed a reduction in this measure, but these results may have been confounded by secular trends toward length of stay reduction. No study showed a consistent or persistent effect on readmissions or mortality.
Dr. Kulkarni is an assistant professor of hospital medicine at Northwestern University in Chicago.
Clinical question: Do interdisciplinary team care interventions affect outcomes for hospitalized patients in general medical wards?
Bottom line: Interdisciplinary team care interventions do not significantly affect oft-used quality measures such as length of stay, readmissions, or mortality. However, some experts question whether these measures are appropriate for assessing the effectiveness of such interventions. A small body of evidence suggests that interdisciplinary interventions may affect complications of care or preventable adverse events. In the future, these and other more appropriate measures should be used when assessing interdisciplinary team care interventions. (LOE = 2a)
Reference: Pannick S, Davis R, Ashrafian H, et al. Effects of interdisciplinary team care interventions on general medical wards. JAMA Intern Med 2015;175(8):1288-1298.
Study design: Systematic review
Funding source: Government
Allocation: Uncertain
Setting: Inpatient (any location)
Synopsis
These investigators searched multiple databases including EMBASE and MEDLINE, as well as reference lists of included studies, to find trials that evaluated the effects of interdisciplinary team care on objective patient outcomes in the general medical wards. Study selection, data extraction, and assessment of bias were performed by independent reviewers.
Thirty studies (randomized controlled trials, cluster studies, and before-after studies) were included in the review. The studies had heterogeneous designs and outcome measures and all of them had a medium or high risk of bias. The majority of the studies, however, reported on complications of care, length of stay, readmission, or mortality.
Out of 10 studies that examined complications of care, five showed a reduction in this outcome by formalizing interdisciplinary rounds or adding specialized clinicians or pharmacists to the interdisciplinary team. Overall, 20% of the studies that looked at length of stay showed a reduction in this measure, but these results may have been confounded by secular trends toward length of stay reduction. No study showed a consistent or persistent effect on readmissions or mortality.
Dr. Kulkarni is an assistant professor of hospital medicine at Northwestern University in Chicago.
Clinical question: Do interdisciplinary team care interventions affect outcomes for hospitalized patients in general medical wards?
Bottom line: Interdisciplinary team care interventions do not significantly affect oft-used quality measures such as length of stay, readmissions, or mortality. However, some experts question whether these measures are appropriate for assessing the effectiveness of such interventions. A small body of evidence suggests that interdisciplinary interventions may affect complications of care or preventable adverse events. In the future, these and other more appropriate measures should be used when assessing interdisciplinary team care interventions. (LOE = 2a)
Reference: Pannick S, Davis R, Ashrafian H, et al. Effects of interdisciplinary team care interventions on general medical wards. JAMA Intern Med 2015;175(8):1288-1298.
Study design: Systematic review
Funding source: Government
Allocation: Uncertain
Setting: Inpatient (any location)
Synopsis
These investigators searched multiple databases including EMBASE and MEDLINE, as well as reference lists of included studies, to find trials that evaluated the effects of interdisciplinary team care on objective patient outcomes in the general medical wards. Study selection, data extraction, and assessment of bias were performed by independent reviewers.
Thirty studies (randomized controlled trials, cluster studies, and before-after studies) were included in the review. The studies had heterogeneous designs and outcome measures and all of them had a medium or high risk of bias. The majority of the studies, however, reported on complications of care, length of stay, readmission, or mortality.
Out of 10 studies that examined complications of care, five showed a reduction in this outcome by formalizing interdisciplinary rounds or adding specialized clinicians or pharmacists to the interdisciplinary team. Overall, 20% of the studies that looked at length of stay showed a reduction in this measure, but these results may have been confounded by secular trends toward length of stay reduction. No study showed a consistent or persistent effect on readmissions or mortality.
Dr. Kulkarni is an assistant professor of hospital medicine at Northwestern University in Chicago.
Routine imaging for diffuse large B-cell lymphoma offers no survival benefit
A population-based comparison of patients with diffuse large B-cell lymphoma (DLBCL) in first complete remission indicated that routine imaging surveillance did not improve outcomes, researchers reported.
Overall survival was similar for Danish and Swedish populations who received similar follow-up care, except that routine imaging surveillance is the standard of care in Denmark, but not in Sweden. The 3-year overall survival for Danish and Swedish patients was 92% and 91%, respectively.
Outcomes grouped by international prognostic index (IPI) also showed no significant differences between populations (J Clin Oncol. 2015 Oct 5, doi:10.1200/jco.2015.62.0229.).
“An imaging-based follow-up strategy does not improve postremission [overall survival] for DLBCL,” wrote Dr. Tarec Christoffer El-Galaly, of Aalborg University Hospital, Denmark, and colleagues.
They observed that aside from using IPI as risk stratification, the study “also points to baseline [lactate dehyrogenase] as a single discriminator of patients with high versus low risk of progression,” (Hazard ratio, 3.12; 95% CI, 1.78-5.48; P less than .01).
The retrospective study examined records of patients with DLBCL from Sweden (n=696) and Denmark (n=525) who achieved first complete remission after first-line therapy with R-CHOP (rituximab plus cyclophosphamide, doxorubicin, vincristine, and prednisone) and CHOP-like regimens from 2007 to 2012. The proportion of patients with IPI greater than two were similar for both groups, though more Danish patients received radiotherapy compared with their Swedish counterparts (35% v. 9%).
Standard follow-up care after first complete remission is similar in Denmark and Sweden and typically includes symptom assessment, clinical examination, and blood tests at 3- to 4-month intervals for 2 years, and 6-month intervals in the third year. After 3 years, Swedish patients are seen annually for 2 years and then follow-up is ended for most patients. In Denmark, 6-month checks are continued until 5 years and then follow-up is usually ended. However, in Denmark guidelines support routine computerized tomography (CT) scans of the neck, abdomen, and thorax every 6 months for 2 years, which is not encouraged by guidelines in Sweden.
Early relapse detection aims to improve survival, and although low disease burden is associated with durable survival in patients treated for relapsed DLBCL, most studies show similar outcomes for imaging versus non-imaging detection. Additionally, previous retrospective studies that have reported survival differences based on relapse detection method are prone to lead-time bias, according to the researchers.
Given that a majority of patients with recurrent DLBCL experience symptoms before relapse, that elevated lactate dehyrogenase or abnormal physical examination may raise suspicion, and that exposure to ionizing radiation from medical imaging can lead to radiation-induced cancers, “routine imaging for DLBCL in first [complete remission] is not recommended,” the authors wrote.
The research was supported in part by the North Denmark Region. Dr. El-Galaly and coauthors reported having no financial disclosures.
The best way to determine the effectiveness of surveillance imaging would be a randomized trial including patients with diffuse large B-cell lymphoma (DLBCL) after first complete remission, but it is unlikely that such a study will be done. The study by El-Galaly et al may be the next best approach. Taking advantage of the fact that neighboring countries Denmark and Sweden have opposite policies for surveillance imaging but otherwise similar follow-up visit schedules and testing, the authors identified factors that predicted relapse (e.g., age greater than 60 years and elevated LDH), and they found that routine surveillance imaging had no impact on outcome. The study presents the strongest argument yet published against routine surveillance imaging.
The two other outstanding issues of routine surveillance are long-term safety and cost benefit. The study by El-Galaly et al, in combination with several other reports, suggests that routine surveillance imaging, in the absence of new or suspicious symptoms, physical findings, or change in laboratory results, is unlikely to benefit patients, may add to the patient’s stress, may cause long-term health problems, and incurs substantial economic cost.
Dr. James O. Armitage and Dr. Julie M. Vose are both at the University of Nebraska, Omaha. Dr. Armitage disclosed a leadership role with Tesaro and consulting or advisory roles with GlaxoSmithKline, Roche, Spectrum Pharmaceuticals, ZIOPHARM Oncology, Conatus, and Celgene. Dr. Vose reported honoraria from Sanofi-Aventis and Seattle Genetics; consulting or advisory roles with Bioconnections; and institutional research funding from Spectrum Pharmaceuticals, Bristol-Myers Squibb, Celgene, Genentech, GlaxoSmithKline, Incyte, Janssen Biotech, Pharmacyclics, Acerta, and Kite Pharma. These remarks were adapted from their accompanying editorial (J Clin Oncol. 2015 Oct 5, doi:10.1200/jco.2015.63.5946).
The best way to determine the effectiveness of surveillance imaging would be a randomized trial including patients with diffuse large B-cell lymphoma (DLBCL) after first complete remission, but it is unlikely that such a study will be done. The study by El-Galaly et al may be the next best approach. Taking advantage of the fact that neighboring countries Denmark and Sweden have opposite policies for surveillance imaging but otherwise similar follow-up visit schedules and testing, the authors identified factors that predicted relapse (e.g., age greater than 60 years and elevated LDH), and they found that routine surveillance imaging had no impact on outcome. The study presents the strongest argument yet published against routine surveillance imaging.
The two other outstanding issues of routine surveillance are long-term safety and cost benefit. The study by El-Galaly et al, in combination with several other reports, suggests that routine surveillance imaging, in the absence of new or suspicious symptoms, physical findings, or change in laboratory results, is unlikely to benefit patients, may add to the patient’s stress, may cause long-term health problems, and incurs substantial economic cost.
Dr. James O. Armitage and Dr. Julie M. Vose are both at the University of Nebraska, Omaha. Dr. Armitage disclosed a leadership role with Tesaro and consulting or advisory roles with GlaxoSmithKline, Roche, Spectrum Pharmaceuticals, ZIOPHARM Oncology, Conatus, and Celgene. Dr. Vose reported honoraria from Sanofi-Aventis and Seattle Genetics; consulting or advisory roles with Bioconnections; and institutional research funding from Spectrum Pharmaceuticals, Bristol-Myers Squibb, Celgene, Genentech, GlaxoSmithKline, Incyte, Janssen Biotech, Pharmacyclics, Acerta, and Kite Pharma. These remarks were adapted from their accompanying editorial (J Clin Oncol. 2015 Oct 5, doi:10.1200/jco.2015.63.5946).
The best way to determine the effectiveness of surveillance imaging would be a randomized trial including patients with diffuse large B-cell lymphoma (DLBCL) after first complete remission, but it is unlikely that such a study will be done. The study by El-Galaly et al may be the next best approach. Taking advantage of the fact that neighboring countries Denmark and Sweden have opposite policies for surveillance imaging but otherwise similar follow-up visit schedules and testing, the authors identified factors that predicted relapse (e.g., age greater than 60 years and elevated LDH), and they found that routine surveillance imaging had no impact on outcome. The study presents the strongest argument yet published against routine surveillance imaging.
The two other outstanding issues of routine surveillance are long-term safety and cost benefit. The study by El-Galaly et al, in combination with several other reports, suggests that routine surveillance imaging, in the absence of new or suspicious symptoms, physical findings, or change in laboratory results, is unlikely to benefit patients, may add to the patient’s stress, may cause long-term health problems, and incurs substantial economic cost.
Dr. James O. Armitage and Dr. Julie M. Vose are both at the University of Nebraska, Omaha. Dr. Armitage disclosed a leadership role with Tesaro and consulting or advisory roles with GlaxoSmithKline, Roche, Spectrum Pharmaceuticals, ZIOPHARM Oncology, Conatus, and Celgene. Dr. Vose reported honoraria from Sanofi-Aventis and Seattle Genetics; consulting or advisory roles with Bioconnections; and institutional research funding from Spectrum Pharmaceuticals, Bristol-Myers Squibb, Celgene, Genentech, GlaxoSmithKline, Incyte, Janssen Biotech, Pharmacyclics, Acerta, and Kite Pharma. These remarks were adapted from their accompanying editorial (J Clin Oncol. 2015 Oct 5, doi:10.1200/jco.2015.63.5946).
A population-based comparison of patients with diffuse large B-cell lymphoma (DLBCL) in first complete remission indicated that routine imaging surveillance did not improve outcomes, researchers reported.
Overall survival was similar for Danish and Swedish populations who received similar follow-up care, except that routine imaging surveillance is the standard of care in Denmark, but not in Sweden. The 3-year overall survival for Danish and Swedish patients was 92% and 91%, respectively.
Outcomes grouped by international prognostic index (IPI) also showed no significant differences between populations (J Clin Oncol. 2015 Oct 5, doi:10.1200/jco.2015.62.0229.).
“An imaging-based follow-up strategy does not improve postremission [overall survival] for DLBCL,” wrote Dr. Tarec Christoffer El-Galaly, of Aalborg University Hospital, Denmark, and colleagues.
They observed that aside from using IPI as risk stratification, the study “also points to baseline [lactate dehyrogenase] as a single discriminator of patients with high versus low risk of progression,” (Hazard ratio, 3.12; 95% CI, 1.78-5.48; P less than .01).
The retrospective study examined records of patients with DLBCL from Sweden (n=696) and Denmark (n=525) who achieved first complete remission after first-line therapy with R-CHOP (rituximab plus cyclophosphamide, doxorubicin, vincristine, and prednisone) and CHOP-like regimens from 2007 to 2012. The proportion of patients with IPI greater than two were similar for both groups, though more Danish patients received radiotherapy compared with their Swedish counterparts (35% v. 9%).
Standard follow-up care after first complete remission is similar in Denmark and Sweden and typically includes symptom assessment, clinical examination, and blood tests at 3- to 4-month intervals for 2 years, and 6-month intervals in the third year. After 3 years, Swedish patients are seen annually for 2 years and then follow-up is ended for most patients. In Denmark, 6-month checks are continued until 5 years and then follow-up is usually ended. However, in Denmark guidelines support routine computerized tomography (CT) scans of the neck, abdomen, and thorax every 6 months for 2 years, which is not encouraged by guidelines in Sweden.
Early relapse detection aims to improve survival, and although low disease burden is associated with durable survival in patients treated for relapsed DLBCL, most studies show similar outcomes for imaging versus non-imaging detection. Additionally, previous retrospective studies that have reported survival differences based on relapse detection method are prone to lead-time bias, according to the researchers.
Given that a majority of patients with recurrent DLBCL experience symptoms before relapse, that elevated lactate dehyrogenase or abnormal physical examination may raise suspicion, and that exposure to ionizing radiation from medical imaging can lead to radiation-induced cancers, “routine imaging for DLBCL in first [complete remission] is not recommended,” the authors wrote.
The research was supported in part by the North Denmark Region. Dr. El-Galaly and coauthors reported having no financial disclosures.
A population-based comparison of patients with diffuse large B-cell lymphoma (DLBCL) in first complete remission indicated that routine imaging surveillance did not improve outcomes, researchers reported.
Overall survival was similar for Danish and Swedish populations who received similar follow-up care, except that routine imaging surveillance is the standard of care in Denmark, but not in Sweden. The 3-year overall survival for Danish and Swedish patients was 92% and 91%, respectively.
Outcomes grouped by international prognostic index (IPI) also showed no significant differences between populations (J Clin Oncol. 2015 Oct 5, doi:10.1200/jco.2015.62.0229.).
“An imaging-based follow-up strategy does not improve postremission [overall survival] for DLBCL,” wrote Dr. Tarec Christoffer El-Galaly, of Aalborg University Hospital, Denmark, and colleagues.
They observed that aside from using IPI as risk stratification, the study “also points to baseline [lactate dehyrogenase] as a single discriminator of patients with high versus low risk of progression,” (Hazard ratio, 3.12; 95% CI, 1.78-5.48; P less than .01).
The retrospective study examined records of patients with DLBCL from Sweden (n=696) and Denmark (n=525) who achieved first complete remission after first-line therapy with R-CHOP (rituximab plus cyclophosphamide, doxorubicin, vincristine, and prednisone) and CHOP-like regimens from 2007 to 2012. The proportion of patients with IPI greater than two were similar for both groups, though more Danish patients received radiotherapy compared with their Swedish counterparts (35% v. 9%).
Standard follow-up care after first complete remission is similar in Denmark and Sweden and typically includes symptom assessment, clinical examination, and blood tests at 3- to 4-month intervals for 2 years, and 6-month intervals in the third year. After 3 years, Swedish patients are seen annually for 2 years and then follow-up is ended for most patients. In Denmark, 6-month checks are continued until 5 years and then follow-up is usually ended. However, in Denmark guidelines support routine computerized tomography (CT) scans of the neck, abdomen, and thorax every 6 months for 2 years, which is not encouraged by guidelines in Sweden.
Early relapse detection aims to improve survival, and although low disease burden is associated with durable survival in patients treated for relapsed DLBCL, most studies show similar outcomes for imaging versus non-imaging detection. Additionally, previous retrospective studies that have reported survival differences based on relapse detection method are prone to lead-time bias, according to the researchers.
Given that a majority of patients with recurrent DLBCL experience symptoms before relapse, that elevated lactate dehyrogenase or abnormal physical examination may raise suspicion, and that exposure to ionizing radiation from medical imaging can lead to radiation-induced cancers, “routine imaging for DLBCL in first [complete remission] is not recommended,” the authors wrote.
The research was supported in part by the North Denmark Region. Dr. El-Galaly and coauthors reported having no financial disclosures.
FROM THE JOURNAL OF CLINICAL ONCOLOGY
Key clinical point:Danish patients with diffuse large B-cell lymphoma (DLBCL) who received routine imaging during follow up had similar survival to Swedish patients who did not undergo routine imaging surveillance.
Major finding: After first complete remission, the 3-year overall survival for Danish and Swedish patients was 92% and 91%, respectively.
Data source: Population-based study of 525 Danish patients and 696 Swedish patients with DLBCL who achieved first complete remission after R-CHOP/CHOP-like first-line therapies from 2007 to 2012.
Disclosures: The research was supported in part by the North Denmark Region. Dr. El-Galaly and coauthors reported having no financial disclosures.
Pearls on the Use of Tape in Dermatology: Report From the AAD Meeting
At the recent Summer Meeting of the American Academy of Dermatology, Dr. Stone discussed the use of tapes in dermatology. He provides highlights from this session, including the diagnosis of tinea versicolor with plastic tape, suturing in patients with fragile skin to add strength to skin, and the diagnosis of scabies.
At the recent Summer Meeting of the American Academy of Dermatology, Dr. Stone discussed the use of tapes in dermatology. He provides highlights from this session, including the diagnosis of tinea versicolor with plastic tape, suturing in patients with fragile skin to add strength to skin, and the diagnosis of scabies.
At the recent Summer Meeting of the American Academy of Dermatology, Dr. Stone discussed the use of tapes in dermatology. He provides highlights from this session, including the diagnosis of tinea versicolor with plastic tape, suturing in patients with fragile skin to add strength to skin, and the diagnosis of scabies.
Women dogged by unplanned readmissions after aortic surgery
CHICAGO – Women undergoing aortic surgery have a 30% higher chance of unplanned readmission within 30 days than men.
This occurs despite a significantly longer length of stay (6.4 vs. 4.8 days; P < .001), Dr. Benjamin Flink said at the annual meeting of the Midwestern Vascular Surgical Society.
Women undergoing aortic surgery are known to have higher morbidity and mortality with respect to cardiovascular events and infections, but no studies have specifically looked at sex disparities in readmission following aortic surgery, he said.
“We feel gender disparities are an understudied area of surgical care and there is a lot of work to be done in reducing these differences,” principal investigator Dr. Shipra Arya said in an interview.
To better examine this issue, Dr. Arya and Dr. Flink, both of Emory University in Atlanta, and investigators at the University of Michigan identified all patients undergoing open or endovascular abdominal aortic aneurysm (AAA), thoracic aortic aneurysm (TAA), and thoracoabdominal aortic aneurysm (TAAA) repair from 2011 to 2013 who were in the American College of Surgeons National Surgical Quality Improvement Program (ACS/NSQIP) database. Of the 18,977 patients, 23% were women.
Use of endovascular procedures varied significantly by sex, with women having significantly fewer endovascular AAA (68.8% vs. 77.1%; P less than .001) and TAAA (43.2% vs. 65.2%; P < .001) repairs than men. Endovascular TAA repairs were similar in women and men (96.1% vs. 95.6%; P = .8), Dr. Flink said.
Overall, 1,541 patients (8.1%) experienced the primary outcome of an unplanned readmission within 30 days, with a significantly higher risk observed in women than men (10.1% vs. 7.6%; P less than .001).
This risk persisted for most aneurysm types, with women having a higher risk of readmission for AAA (9.4% vs. 7.3%; P less than .001) and TAAA (13.7% vs. 8.3%; P = .03) aneurysms, but not TAAs (13% vs. 12.5%; P = .8), he said.
The overall length of stay was 5.2 days. Women stayed 1.6 days longer than men (data above), readmitted patients stayed 1 day longer during their index hospitalization than patients who avoided readmission (5.1 days vs. 4.1 days; P less than .001), and open-repair patients stayed more than twice as long as endovascular patients (10.3 days vs. 3.7 days; P less than .001).
Patients discharged to home, however, had less than one-third the length of stay as those discharged to a facility other than home (4 days vs. 12.8 days; P less than .001).
Notably, women were discharged to a facility other than home nearly twice as often as men (20.4% vs. 10.6%; P less than .001), Dr. Flink said.
In multivariate analysis, the odds of an unplanned readmission were 30% higher for women than men after controlling for 13 variables (odds ratio, 1.3; 95% confidence interval, 1.14-1.48).
When the analysis was stratified by discharge destination, the higher odds of readmission among women remained for those discharged home (OR, 1.3; 95% CI, 1.12-1.51), but not when discharged to a skilled or rehabilitation facility (OR, 1.1; 95% CI, 0.83-1.45).
“Further study into the discharge planning process, social factors, and the use of rehabilitation is needed,” Dr. Flink said. “For example, why are we keeping women longer? Are we missing opportunities to better utilize rehabilitation in hospital? And what gender-specific social factors might be influencing unplanned readmissions that we’re currently not measuring?”
Dr. John Blebea of the University of Oklahoma, Tulsa, asked whether marital status was examined as an independent variable, “because I would suspect that’s the answer to the question. More women are widowed than men and therefore are less likely to have a spouse at home to take care of them, which would also explain why they’d be in the hospital longer.”
Unfortunately, that information is not available in the ACS/NSQIP database, but “I do agree that home-social factors are likely playing a role,” Dr. Flink responded.
Along the same vein, another attendee questioned whether the study accounted for frailty index scores. They were not, but the analysis included patients’ functional status as well as comorbidities such as congestive heart failure, stroke, peripheral arterial disease, and dialysis dependence that would limit their physical independence, Dr. Flink said.
Dr. Flink reported having no financial disclosures. Principal investigator Dr. Shipra Arya is funded by a research grant from the American Heart Association.
On Twitter @pwendl
CHICAGO – Women undergoing aortic surgery have a 30% higher chance of unplanned readmission within 30 days than men.
This occurs despite a significantly longer length of stay (6.4 vs. 4.8 days; P < .001), Dr. Benjamin Flink said at the annual meeting of the Midwestern Vascular Surgical Society.
Women undergoing aortic surgery are known to have higher morbidity and mortality with respect to cardiovascular events and infections, but no studies have specifically looked at sex disparities in readmission following aortic surgery, he said.
“We feel gender disparities are an understudied area of surgical care and there is a lot of work to be done in reducing these differences,” principal investigator Dr. Shipra Arya said in an interview.
To better examine this issue, Dr. Arya and Dr. Flink, both of Emory University in Atlanta, and investigators at the University of Michigan identified all patients undergoing open or endovascular abdominal aortic aneurysm (AAA), thoracic aortic aneurysm (TAA), and thoracoabdominal aortic aneurysm (TAAA) repair from 2011 to 2013 who were in the American College of Surgeons National Surgical Quality Improvement Program (ACS/NSQIP) database. Of the 18,977 patients, 23% were women.
Use of endovascular procedures varied significantly by sex, with women having significantly fewer endovascular AAA (68.8% vs. 77.1%; P less than .001) and TAAA (43.2% vs. 65.2%; P < .001) repairs than men. Endovascular TAA repairs were similar in women and men (96.1% vs. 95.6%; P = .8), Dr. Flink said.
Overall, 1,541 patients (8.1%) experienced the primary outcome of an unplanned readmission within 30 days, with a significantly higher risk observed in women than men (10.1% vs. 7.6%; P less than .001).
This risk persisted for most aneurysm types, with women having a higher risk of readmission for AAA (9.4% vs. 7.3%; P less than .001) and TAAA (13.7% vs. 8.3%; P = .03) aneurysms, but not TAAs (13% vs. 12.5%; P = .8), he said.
The overall length of stay was 5.2 days. Women stayed 1.6 days longer than men (data above), readmitted patients stayed 1 day longer during their index hospitalization than patients who avoided readmission (5.1 days vs. 4.1 days; P less than .001), and open-repair patients stayed more than twice as long as endovascular patients (10.3 days vs. 3.7 days; P less than .001).
Patients discharged to home, however, had less than one-third the length of stay as those discharged to a facility other than home (4 days vs. 12.8 days; P less than .001).
Notably, women were discharged to a facility other than home nearly twice as often as men (20.4% vs. 10.6%; P less than .001), Dr. Flink said.
In multivariate analysis, the odds of an unplanned readmission were 30% higher for women than men after controlling for 13 variables (odds ratio, 1.3; 95% confidence interval, 1.14-1.48).
When the analysis was stratified by discharge destination, the higher odds of readmission among women remained for those discharged home (OR, 1.3; 95% CI, 1.12-1.51), but not when discharged to a skilled or rehabilitation facility (OR, 1.1; 95% CI, 0.83-1.45).
“Further study into the discharge planning process, social factors, and the use of rehabilitation is needed,” Dr. Flink said. “For example, why are we keeping women longer? Are we missing opportunities to better utilize rehabilitation in hospital? And what gender-specific social factors might be influencing unplanned readmissions that we’re currently not measuring?”
Dr. John Blebea of the University of Oklahoma, Tulsa, asked whether marital status was examined as an independent variable, “because I would suspect that’s the answer to the question. More women are widowed than men and therefore are less likely to have a spouse at home to take care of them, which would also explain why they’d be in the hospital longer.”
Unfortunately, that information is not available in the ACS/NSQIP database, but “I do agree that home-social factors are likely playing a role,” Dr. Flink responded.
Along the same vein, another attendee questioned whether the study accounted for frailty index scores. They were not, but the analysis included patients’ functional status as well as comorbidities such as congestive heart failure, stroke, peripheral arterial disease, and dialysis dependence that would limit their physical independence, Dr. Flink said.
Dr. Flink reported having no financial disclosures. Principal investigator Dr. Shipra Arya is funded by a research grant from the American Heart Association.
On Twitter @pwendl
CHICAGO – Women undergoing aortic surgery have a 30% higher chance of unplanned readmission within 30 days than men.
This occurs despite a significantly longer length of stay (6.4 vs. 4.8 days; P < .001), Dr. Benjamin Flink said at the annual meeting of the Midwestern Vascular Surgical Society.
Women undergoing aortic surgery are known to have higher morbidity and mortality with respect to cardiovascular events and infections, but no studies have specifically looked at sex disparities in readmission following aortic surgery, he said.
“We feel gender disparities are an understudied area of surgical care and there is a lot of work to be done in reducing these differences,” principal investigator Dr. Shipra Arya said in an interview.
To better examine this issue, Dr. Arya and Dr. Flink, both of Emory University in Atlanta, and investigators at the University of Michigan identified all patients undergoing open or endovascular abdominal aortic aneurysm (AAA), thoracic aortic aneurysm (TAA), and thoracoabdominal aortic aneurysm (TAAA) repair from 2011 to 2013 who were in the American College of Surgeons National Surgical Quality Improvement Program (ACS/NSQIP) database. Of the 18,977 patients, 23% were women.
Use of endovascular procedures varied significantly by sex, with women having significantly fewer endovascular AAA (68.8% vs. 77.1%; P less than .001) and TAAA (43.2% vs. 65.2%; P < .001) repairs than men. Endovascular TAA repairs were similar in women and men (96.1% vs. 95.6%; P = .8), Dr. Flink said.
Overall, 1,541 patients (8.1%) experienced the primary outcome of an unplanned readmission within 30 days, with a significantly higher risk observed in women than men (10.1% vs. 7.6%; P less than .001).
This risk persisted for most aneurysm types, with women having a higher risk of readmission for AAA (9.4% vs. 7.3%; P less than .001) and TAAA (13.7% vs. 8.3%; P = .03) aneurysms, but not TAAs (13% vs. 12.5%; P = .8), he said.
The overall length of stay was 5.2 days. Women stayed 1.6 days longer than men (data above), readmitted patients stayed 1 day longer during their index hospitalization than patients who avoided readmission (5.1 days vs. 4.1 days; P less than .001), and open-repair patients stayed more than twice as long as endovascular patients (10.3 days vs. 3.7 days; P less than .001).
Patients discharged to home, however, had less than one-third the length of stay as those discharged to a facility other than home (4 days vs. 12.8 days; P less than .001).
Notably, women were discharged to a facility other than home nearly twice as often as men (20.4% vs. 10.6%; P less than .001), Dr. Flink said.
In multivariate analysis, the odds of an unplanned readmission were 30% higher for women than men after controlling for 13 variables (odds ratio, 1.3; 95% confidence interval, 1.14-1.48).
When the analysis was stratified by discharge destination, the higher odds of readmission among women remained for those discharged home (OR, 1.3; 95% CI, 1.12-1.51), but not when discharged to a skilled or rehabilitation facility (OR, 1.1; 95% CI, 0.83-1.45).
“Further study into the discharge planning process, social factors, and the use of rehabilitation is needed,” Dr. Flink said. “For example, why are we keeping women longer? Are we missing opportunities to better utilize rehabilitation in hospital? And what gender-specific social factors might be influencing unplanned readmissions that we’re currently not measuring?”
Dr. John Blebea of the University of Oklahoma, Tulsa, asked whether marital status was examined as an independent variable, “because I would suspect that’s the answer to the question. More women are widowed than men and therefore are less likely to have a spouse at home to take care of them, which would also explain why they’d be in the hospital longer.”
Unfortunately, that information is not available in the ACS/NSQIP database, but “I do agree that home-social factors are likely playing a role,” Dr. Flink responded.
Along the same vein, another attendee questioned whether the study accounted for frailty index scores. They were not, but the analysis included patients’ functional status as well as comorbidities such as congestive heart failure, stroke, peripheral arterial disease, and dialysis dependence that would limit their physical independence, Dr. Flink said.
Dr. Flink reported having no financial disclosures. Principal investigator Dr. Shipra Arya is funded by a research grant from the American Heart Association.
On Twitter @pwendl
AT MIDWESTERN VASCULAR 2015
Key clinical point: Women undergoing aortic surgery are at higher risk for unplanned readmissions, compared with men, especially when discharged to home.
Major finding: The odds of an unplanned readmission at 30 days were 30% higher for women than men.
Data source: Retrospective study of 18,977 patients undergoing aortic aneurysm repair in the ACS/NSQIP database.
Disclosures: Dr. Flink reported having no financial disclosures. Principal investigator Dr. Shipra Arya is funded by a research grant from the American Heart Association.
Hospital Medicine Administrator Amanda Trask Values Hospitalists, HM Role in Healthcare
Most people think a career in hospital medicine means a medical degree that confers those two ubiquitous letters after your name.
Amanda Trask blazed her own path.
She got to her job—vice president of the national hospitalist service line for Catholic Health Initiatives of Englewood, Colo.—by following a slightly different path. In her case, it was a master’s of business administration (MBA), a master’s in health administration (MHA), and a few fellowships to boot.
“Many years ago I chose to move forward in my education and attain advanced degrees,” says Trask, MBA, MHA, SFHM, FACHE, CMPE. “Through that, you get a really broad perspective of healthcare and the business of healthcare.”
It’s a perspective Dr. Trask is bringing to Team Hospitalist, as one of seven new members of The Hospitalist’s volunteer editorial advisory board. She sees HM as a vital specialty in a changing healthcare landscape.
“Hospital medicine is uniquely positioned to truly impact a very large breadth of patients and improve the continuum of care,” she says.
Question: Tell me about your role at Catholic Health Initiatives.
Answer: CHI operates in 19 states and 105 hospitals. We have a variety of hospitalist models in our hospitals, everything from direct employed with our local medical groups to contracted with hospitalist companies to independent groups that provide hospitalist services to their patients. At CHI, my role is to coordinate hospitalist efforts to improve clinical and efficiency outcomes in our hospitals and in other pre- and post-acute care settings where hospitalists play a role.
Q: People like to say, “If you’ve seen one hospitalist group, you’ve seen one hospitalist group.” How difficult is it to replicate commonalities in different buildings?
A: I definitely agree that “if you’ve seen one hospitalist group, you’ve seen one hospitalist group”; however, a great percentage of the work is common among hospitalists. We have a national hospital medicine leadership team composed of our divisional medical directors and dyad administrative partners to oversee the efforts of hospital medicine at CHI. That leadership team identifies the commonalities of opportunities across our hospital medicine markets. How can we support local innovation while maximizing the opportunity for standardization? What are the things that are fairly consistent no matter where you practice? What are those things that might have a substantial amount of difference? The focus of CHI’s national hospital medicine service line is to align standards that improve the practice of hospital medicine across CHI.

—Dr. Trask
Q: How important is it to find those commonalities?
A: In this day and age of healthcare, as we consider new payment models, we look at population health and what that means in a future state for healthcare. In the future, hospitalists are a critical component of ensuring we deliver higher clinical quality outcomes and better efficiencies to care for our population as a whole. As opposed to having each of our practices continuing to work individually and, in many cases, on many of the same exact issues, we identified the opportunity to bring those efforts together and try to do so in a more efficient fashion.
I’ll give an example: When we look at clinical documentation, much of that is related to electronic health records. How can we work together to identify opportunities to improve the use of our electronic health record when we have the same health record in different divisions?
Q: Where do you see yourself in five years, 10 years?
A: It’s funny you ask that question, as that is the question I always ask people I’m interviewing. My answer to that is not always as concrete as others’ answers.
I look at what doors might open, and I look at what opportunities present themselves. I think, looking at opportunities like we have in hospital medicine and looking at opportunities to really expand beyond current state, many of my experiences have led me to realize that I like to be involved in improvements, change in evolving the healthcare industry, and bringing teams together to improve the status quo.
Q: You work with some 900 hospitalists. What’s your favorite thing about working with them and the role they play?
A: Every single hospitalist I’ve encountered has demonstrated such a strong desire to make improvements in the patients that they’re caring for. What’s great about hospitalists is that they have an innate desire to improve the health and well-being of our communities. Hospitalists are truly passionate about improving the way we care for patients, advancing quality outcomes, and leading the shift from volume to value for the communities we serve. For me, this role is not simply a task or a job that I do, but it really is a passion.
Q: The flip side of that is nobody’s job is perfect. What’s the toughest thing about working with hospitalists?
A: One of the toughest things in this particular industry is the fact that it has been the fastest growing of all of time [in medicine], and there are times when supply and demand are not well balanced. There are a lot of demands placed on hospitalists, and there are a lot of expectations by hospital leaders and health system leaders, that hospitalists can solve many of the problems that may exist. Because of that, sometimes the supply of hospitalists, or the ability to have top talent, is really challenging.
The balance is not yet perfect between the availability of top talent and the ability to meet the needs of the organization and community.
Richard Quinn is a freelance writer in New Jersey.
Most people think a career in hospital medicine means a medical degree that confers those two ubiquitous letters after your name.
Amanda Trask blazed her own path.
She got to her job—vice president of the national hospitalist service line for Catholic Health Initiatives of Englewood, Colo.—by following a slightly different path. In her case, it was a master’s of business administration (MBA), a master’s in health administration (MHA), and a few fellowships to boot.
“Many years ago I chose to move forward in my education and attain advanced degrees,” says Trask, MBA, MHA, SFHM, FACHE, CMPE. “Through that, you get a really broad perspective of healthcare and the business of healthcare.”
It’s a perspective Dr. Trask is bringing to Team Hospitalist, as one of seven new members of The Hospitalist’s volunteer editorial advisory board. She sees HM as a vital specialty in a changing healthcare landscape.
“Hospital medicine is uniquely positioned to truly impact a very large breadth of patients and improve the continuum of care,” she says.
Question: Tell me about your role at Catholic Health Initiatives.
Answer: CHI operates in 19 states and 105 hospitals. We have a variety of hospitalist models in our hospitals, everything from direct employed with our local medical groups to contracted with hospitalist companies to independent groups that provide hospitalist services to their patients. At CHI, my role is to coordinate hospitalist efforts to improve clinical and efficiency outcomes in our hospitals and in other pre- and post-acute care settings where hospitalists play a role.
Q: People like to say, “If you’ve seen one hospitalist group, you’ve seen one hospitalist group.” How difficult is it to replicate commonalities in different buildings?
A: I definitely agree that “if you’ve seen one hospitalist group, you’ve seen one hospitalist group”; however, a great percentage of the work is common among hospitalists. We have a national hospital medicine leadership team composed of our divisional medical directors and dyad administrative partners to oversee the efforts of hospital medicine at CHI. That leadership team identifies the commonalities of opportunities across our hospital medicine markets. How can we support local innovation while maximizing the opportunity for standardization? What are the things that are fairly consistent no matter where you practice? What are those things that might have a substantial amount of difference? The focus of CHI’s national hospital medicine service line is to align standards that improve the practice of hospital medicine across CHI.

—Dr. Trask
Q: How important is it to find those commonalities?
A: In this day and age of healthcare, as we consider new payment models, we look at population health and what that means in a future state for healthcare. In the future, hospitalists are a critical component of ensuring we deliver higher clinical quality outcomes and better efficiencies to care for our population as a whole. As opposed to having each of our practices continuing to work individually and, in many cases, on many of the same exact issues, we identified the opportunity to bring those efforts together and try to do so in a more efficient fashion.
I’ll give an example: When we look at clinical documentation, much of that is related to electronic health records. How can we work together to identify opportunities to improve the use of our electronic health record when we have the same health record in different divisions?
Q: Where do you see yourself in five years, 10 years?
A: It’s funny you ask that question, as that is the question I always ask people I’m interviewing. My answer to that is not always as concrete as others’ answers.
I look at what doors might open, and I look at what opportunities present themselves. I think, looking at opportunities like we have in hospital medicine and looking at opportunities to really expand beyond current state, many of my experiences have led me to realize that I like to be involved in improvements, change in evolving the healthcare industry, and bringing teams together to improve the status quo.
Q: You work with some 900 hospitalists. What’s your favorite thing about working with them and the role they play?
A: Every single hospitalist I’ve encountered has demonstrated such a strong desire to make improvements in the patients that they’re caring for. What’s great about hospitalists is that they have an innate desire to improve the health and well-being of our communities. Hospitalists are truly passionate about improving the way we care for patients, advancing quality outcomes, and leading the shift from volume to value for the communities we serve. For me, this role is not simply a task or a job that I do, but it really is a passion.
Q: The flip side of that is nobody’s job is perfect. What’s the toughest thing about working with hospitalists?
A: One of the toughest things in this particular industry is the fact that it has been the fastest growing of all of time [in medicine], and there are times when supply and demand are not well balanced. There are a lot of demands placed on hospitalists, and there are a lot of expectations by hospital leaders and health system leaders, that hospitalists can solve many of the problems that may exist. Because of that, sometimes the supply of hospitalists, or the ability to have top talent, is really challenging.
The balance is not yet perfect between the availability of top talent and the ability to meet the needs of the organization and community.
Richard Quinn is a freelance writer in New Jersey.
Most people think a career in hospital medicine means a medical degree that confers those two ubiquitous letters after your name.
Amanda Trask blazed her own path.
She got to her job—vice president of the national hospitalist service line for Catholic Health Initiatives of Englewood, Colo.—by following a slightly different path. In her case, it was a master’s of business administration (MBA), a master’s in health administration (MHA), and a few fellowships to boot.
“Many years ago I chose to move forward in my education and attain advanced degrees,” says Trask, MBA, MHA, SFHM, FACHE, CMPE. “Through that, you get a really broad perspective of healthcare and the business of healthcare.”
It’s a perspective Dr. Trask is bringing to Team Hospitalist, as one of seven new members of The Hospitalist’s volunteer editorial advisory board. She sees HM as a vital specialty in a changing healthcare landscape.
“Hospital medicine is uniquely positioned to truly impact a very large breadth of patients and improve the continuum of care,” she says.
Question: Tell me about your role at Catholic Health Initiatives.
Answer: CHI operates in 19 states and 105 hospitals. We have a variety of hospitalist models in our hospitals, everything from direct employed with our local medical groups to contracted with hospitalist companies to independent groups that provide hospitalist services to their patients. At CHI, my role is to coordinate hospitalist efforts to improve clinical and efficiency outcomes in our hospitals and in other pre- and post-acute care settings where hospitalists play a role.
Q: People like to say, “If you’ve seen one hospitalist group, you’ve seen one hospitalist group.” How difficult is it to replicate commonalities in different buildings?
A: I definitely agree that “if you’ve seen one hospitalist group, you’ve seen one hospitalist group”; however, a great percentage of the work is common among hospitalists. We have a national hospital medicine leadership team composed of our divisional medical directors and dyad administrative partners to oversee the efforts of hospital medicine at CHI. That leadership team identifies the commonalities of opportunities across our hospital medicine markets. How can we support local innovation while maximizing the opportunity for standardization? What are the things that are fairly consistent no matter where you practice? What are those things that might have a substantial amount of difference? The focus of CHI’s national hospital medicine service line is to align standards that improve the practice of hospital medicine across CHI.

—Dr. Trask
Q: How important is it to find those commonalities?
A: In this day and age of healthcare, as we consider new payment models, we look at population health and what that means in a future state for healthcare. In the future, hospitalists are a critical component of ensuring we deliver higher clinical quality outcomes and better efficiencies to care for our population as a whole. As opposed to having each of our practices continuing to work individually and, in many cases, on many of the same exact issues, we identified the opportunity to bring those efforts together and try to do so in a more efficient fashion.
I’ll give an example: When we look at clinical documentation, much of that is related to electronic health records. How can we work together to identify opportunities to improve the use of our electronic health record when we have the same health record in different divisions?
Q: Where do you see yourself in five years, 10 years?
A: It’s funny you ask that question, as that is the question I always ask people I’m interviewing. My answer to that is not always as concrete as others’ answers.
I look at what doors might open, and I look at what opportunities present themselves. I think, looking at opportunities like we have in hospital medicine and looking at opportunities to really expand beyond current state, many of my experiences have led me to realize that I like to be involved in improvements, change in evolving the healthcare industry, and bringing teams together to improve the status quo.
Q: You work with some 900 hospitalists. What’s your favorite thing about working with them and the role they play?
A: Every single hospitalist I’ve encountered has demonstrated such a strong desire to make improvements in the patients that they’re caring for. What’s great about hospitalists is that they have an innate desire to improve the health and well-being of our communities. Hospitalists are truly passionate about improving the way we care for patients, advancing quality outcomes, and leading the shift from volume to value for the communities we serve. For me, this role is not simply a task or a job that I do, but it really is a passion.
Q: The flip side of that is nobody’s job is perfect. What’s the toughest thing about working with hospitalists?
A: One of the toughest things in this particular industry is the fact that it has been the fastest growing of all of time [in medicine], and there are times when supply and demand are not well balanced. There are a lot of demands placed on hospitalists, and there are a lot of expectations by hospital leaders and health system leaders, that hospitalists can solve many of the problems that may exist. Because of that, sometimes the supply of hospitalists, or the ability to have top talent, is really challenging.
The balance is not yet perfect between the availability of top talent and the ability to meet the needs of the organization and community.
Richard Quinn is a freelance writer in New Jersey.
Hospitals Save Estimated $67 Million by Tracking Energy Consumption
Estimated savings in energy costs posted by hospitals participating in the American Hospital Association’s affiliated American Society for Healthcare Engineering (ASHE) Energy to Care Program. Twenty participating hospitals received Energy to Care awards from ASHE in July for reducing their energy consumption by 10% or more. ASHE’s free program includes a benchmarking dashboard hospitals can use to track their own energy consumption, thereby saving energy and reducing costs.
Larry Beresford is a freelance writer in Alameda, Calif.
Estimated savings in energy costs posted by hospitals participating in the American Hospital Association’s affiliated American Society for Healthcare Engineering (ASHE) Energy to Care Program. Twenty participating hospitals received Energy to Care awards from ASHE in July for reducing their energy consumption by 10% or more. ASHE’s free program includes a benchmarking dashboard hospitals can use to track their own energy consumption, thereby saving energy and reducing costs.
Larry Beresford is a freelance writer in Alameda, Calif.
Estimated savings in energy costs posted by hospitals participating in the American Hospital Association’s affiliated American Society for Healthcare Engineering (ASHE) Energy to Care Program. Twenty participating hospitals received Energy to Care awards from ASHE in July for reducing their energy consumption by 10% or more. ASHE’s free program includes a benchmarking dashboard hospitals can use to track their own energy consumption, thereby saving energy and reducing costs.
Larry Beresford is a freelance writer in Alameda, Calif.
How to Develop a Comprehensive Pediatric Palliative Care Program
For Ami Doshi, MD, FAAP, a hospitalist at Rady Children’s Hospital San Diego, the path to establishing a comprehensive pediatric palliative care program began with her realization during medical training that doctors didn’t always adequately address the suffering of young patients with advanced disease and their families. Then, in a hospice rotation, she saw that the palliative approach could offer a better way.
During a pediatric hospital medicine fellowship at the University of California at San Diego, Dr. Doshi conducted an educational needs assessment and then created a palliative care curriculum for residents. Rady administrators supported her attending the Palliative Care Leadership Center training at UC San Francisco, with a team from Rady and Harvard Medical School’s program in Palliative Care Education and Practice.
After five years of development, the program Dr. Doshi helped to launch at Rady has grown into a division of palliative medicine, with a medical director, an inpatient consultation service, a palliative home care program coordinated by a health navigator, and a variety of models in the outpatient clinics.
“The goal is to be seamless and to treat patients across the continuum of care,” says Dr. Doshi, who is now board certified in hospice and palliative. Although she is based in the division of hospital medicine, she leads sit-down rounds with the full palliative care team and bioethics consultants every other week.
“Finding time for this work is always a challenge,” she says, adding that administrative support for physicians’ protected time is growing and that the program is ramping up its data collection to document outcomes resulting from palliative care.
For more information on the program, email her at [email protected].
Larry Beresford is a freelance writer in Alameda, Calif.
For Ami Doshi, MD, FAAP, a hospitalist at Rady Children’s Hospital San Diego, the path to establishing a comprehensive pediatric palliative care program began with her realization during medical training that doctors didn’t always adequately address the suffering of young patients with advanced disease and their families. Then, in a hospice rotation, she saw that the palliative approach could offer a better way.
During a pediatric hospital medicine fellowship at the University of California at San Diego, Dr. Doshi conducted an educational needs assessment and then created a palliative care curriculum for residents. Rady administrators supported her attending the Palliative Care Leadership Center training at UC San Francisco, with a team from Rady and Harvard Medical School’s program in Palliative Care Education and Practice.
After five years of development, the program Dr. Doshi helped to launch at Rady has grown into a division of palliative medicine, with a medical director, an inpatient consultation service, a palliative home care program coordinated by a health navigator, and a variety of models in the outpatient clinics.
“The goal is to be seamless and to treat patients across the continuum of care,” says Dr. Doshi, who is now board certified in hospice and palliative. Although she is based in the division of hospital medicine, she leads sit-down rounds with the full palliative care team and bioethics consultants every other week.
“Finding time for this work is always a challenge,” she says, adding that administrative support for physicians’ protected time is growing and that the program is ramping up its data collection to document outcomes resulting from palliative care.
For more information on the program, email her at [email protected].
Larry Beresford is a freelance writer in Alameda, Calif.
For Ami Doshi, MD, FAAP, a hospitalist at Rady Children’s Hospital San Diego, the path to establishing a comprehensive pediatric palliative care program began with her realization during medical training that doctors didn’t always adequately address the suffering of young patients with advanced disease and their families. Then, in a hospice rotation, she saw that the palliative approach could offer a better way.
During a pediatric hospital medicine fellowship at the University of California at San Diego, Dr. Doshi conducted an educational needs assessment and then created a palliative care curriculum for residents. Rady administrators supported her attending the Palliative Care Leadership Center training at UC San Francisco, with a team from Rady and Harvard Medical School’s program in Palliative Care Education and Practice.
After five years of development, the program Dr. Doshi helped to launch at Rady has grown into a division of palliative medicine, with a medical director, an inpatient consultation service, a palliative home care program coordinated by a health navigator, and a variety of models in the outpatient clinics.
“The goal is to be seamless and to treat patients across the continuum of care,” says Dr. Doshi, who is now board certified in hospice and palliative. Although she is based in the division of hospital medicine, she leads sit-down rounds with the full palliative care team and bioethics consultants every other week.
“Finding time for this work is always a challenge,” she says, adding that administrative support for physicians’ protected time is growing and that the program is ramping up its data collection to document outcomes resulting from palliative care.
For more information on the program, email her at [email protected].
Larry Beresford is a freelance writer in Alameda, Calif.
Consumer Reports Rates Hospitals on Infection Control, Prevention
Consumer Reports included for the first time in its national hospital quality ratings a ranking of how well 3,000 hospitals are controlling common deadly infections such as methicillin-resistant Staphylococcus aureus (MRSA) and Clostridium difficile.
The How Your Hospital Can Make You Sick report is based on information provided to the CDC between October 2013 and September 2014. The CDC found that 105 hospitals distinguished themselves by earning high ratings against both infections. Nine hospitals received top ratings for having no infections from MRSA, C. diff, or other measured infections, although none of the country’s highest-profile hospitals are on that list. Only 6% of hospitals scored well against both infections in the new ratings. The CDC estimates that 648,000 people develop infections during their hospital stay, with 75,000 dying from them; many of the deaths can be traced back to widespread, inappropriate use of antibiotics.
“High rates for MRSA and C. diff can be a red flag that a hospital isn’t following the best practices in preventing infections and prescribing antibiotics,” notes Doris Peter, PhD, director of Consumer Reports’ Health Ratings Center, in a prepared statement. “The data show that it is possible to keep infection rates down and in some cases avoid them altogether.”
Among Consumer Reports’ recommendations for hospitals:
- Consistently follow established protocols for managing superbug infections;
- Accurately track how many infections patients get; and
- Promptly report outbreaks to patients and health authorities.
Reference
- Consumer Reports. America’s antibiotic crisis: how your hospital can make you sick. July 29, 2015. Accessed September 12, 2015.
Consumer Reports included for the first time in its national hospital quality ratings a ranking of how well 3,000 hospitals are controlling common deadly infections such as methicillin-resistant Staphylococcus aureus (MRSA) and Clostridium difficile.
The How Your Hospital Can Make You Sick report is based on information provided to the CDC between October 2013 and September 2014. The CDC found that 105 hospitals distinguished themselves by earning high ratings against both infections. Nine hospitals received top ratings for having no infections from MRSA, C. diff, or other measured infections, although none of the country’s highest-profile hospitals are on that list. Only 6% of hospitals scored well against both infections in the new ratings. The CDC estimates that 648,000 people develop infections during their hospital stay, with 75,000 dying from them; many of the deaths can be traced back to widespread, inappropriate use of antibiotics.
“High rates for MRSA and C. diff can be a red flag that a hospital isn’t following the best practices in preventing infections and prescribing antibiotics,” notes Doris Peter, PhD, director of Consumer Reports’ Health Ratings Center, in a prepared statement. “The data show that it is possible to keep infection rates down and in some cases avoid them altogether.”
Among Consumer Reports’ recommendations for hospitals:
- Consistently follow established protocols for managing superbug infections;
- Accurately track how many infections patients get; and
- Promptly report outbreaks to patients and health authorities.
Reference
- Consumer Reports. America’s antibiotic crisis: how your hospital can make you sick. July 29, 2015. Accessed September 12, 2015.
Consumer Reports included for the first time in its national hospital quality ratings a ranking of how well 3,000 hospitals are controlling common deadly infections such as methicillin-resistant Staphylococcus aureus (MRSA) and Clostridium difficile.
The How Your Hospital Can Make You Sick report is based on information provided to the CDC between October 2013 and September 2014. The CDC found that 105 hospitals distinguished themselves by earning high ratings against both infections. Nine hospitals received top ratings for having no infections from MRSA, C. diff, or other measured infections, although none of the country’s highest-profile hospitals are on that list. Only 6% of hospitals scored well against both infections in the new ratings. The CDC estimates that 648,000 people develop infections during their hospital stay, with 75,000 dying from them; many of the deaths can be traced back to widespread, inappropriate use of antibiotics.
“High rates for MRSA and C. diff can be a red flag that a hospital isn’t following the best practices in preventing infections and prescribing antibiotics,” notes Doris Peter, PhD, director of Consumer Reports’ Health Ratings Center, in a prepared statement. “The data show that it is possible to keep infection rates down and in some cases avoid them altogether.”
Among Consumer Reports’ recommendations for hospitals:
- Consistently follow established protocols for managing superbug infections;
- Accurately track how many infections patients get; and
- Promptly report outbreaks to patients and health authorities.
Reference
- Consumer Reports. America’s antibiotic crisis: how your hospital can make you sick. July 29, 2015. Accessed September 12, 2015.
Joint Commission Offers Resource to Prevent Hospital Falls
The Joint Commission’s Center for Transforming Healthcare has released its Targeted Solutions Tool for preventing hospital inpatient falls and falls with injuries. This step-by-step, online resource helps hospitals measure their fall rates and identify barriers to fall prevention and the specific contributing factors that lead to falls. A systematic approach enables the organization to assess each patient’s risk for falling and then implement specific targeted solutions to address the contributing factors, which will vary from one organization to the next.
Hospital falls total between 700,000 and one million per year, according to the Agency for Healthcare Research and Quality; since 2008, the Centers for Medicare and Medicaid Services has not paid hospitals for the costs of extra care related to falls.
The Joint Commission calculates, based on average baseline and improvement figures from its Preventing Falls with Injury Project, that a typical 200-bed hospital could reduce its number of patients injured by falls annually from 117 to 45. Key elements of a program achieving that kind of success include consistent messaging focused on operational and cultural change, staff engagement, and an “all hands on deck” approach that involves hospitalists and other physicians in helping to prevent falls by hospitalized patients.
The Joint Commission’s Center for Transforming Healthcare has released its Targeted Solutions Tool for preventing hospital inpatient falls and falls with injuries. This step-by-step, online resource helps hospitals measure their fall rates and identify barriers to fall prevention and the specific contributing factors that lead to falls. A systematic approach enables the organization to assess each patient’s risk for falling and then implement specific targeted solutions to address the contributing factors, which will vary from one organization to the next.
Hospital falls total between 700,000 and one million per year, according to the Agency for Healthcare Research and Quality; since 2008, the Centers for Medicare and Medicaid Services has not paid hospitals for the costs of extra care related to falls.
The Joint Commission calculates, based on average baseline and improvement figures from its Preventing Falls with Injury Project, that a typical 200-bed hospital could reduce its number of patients injured by falls annually from 117 to 45. Key elements of a program achieving that kind of success include consistent messaging focused on operational and cultural change, staff engagement, and an “all hands on deck” approach that involves hospitalists and other physicians in helping to prevent falls by hospitalized patients.
The Joint Commission’s Center for Transforming Healthcare has released its Targeted Solutions Tool for preventing hospital inpatient falls and falls with injuries. This step-by-step, online resource helps hospitals measure their fall rates and identify barriers to fall prevention and the specific contributing factors that lead to falls. A systematic approach enables the organization to assess each patient’s risk for falling and then implement specific targeted solutions to address the contributing factors, which will vary from one organization to the next.
Hospital falls total between 700,000 and one million per year, according to the Agency for Healthcare Research and Quality; since 2008, the Centers for Medicare and Medicaid Services has not paid hospitals for the costs of extra care related to falls.
The Joint Commission calculates, based on average baseline and improvement figures from its Preventing Falls with Injury Project, that a typical 200-bed hospital could reduce its number of patients injured by falls annually from 117 to 45. Key elements of a program achieving that kind of success include consistent messaging focused on operational and cultural change, staff engagement, and an “all hands on deck” approach that involves hospitalists and other physicians in helping to prevent falls by hospitalized patients.
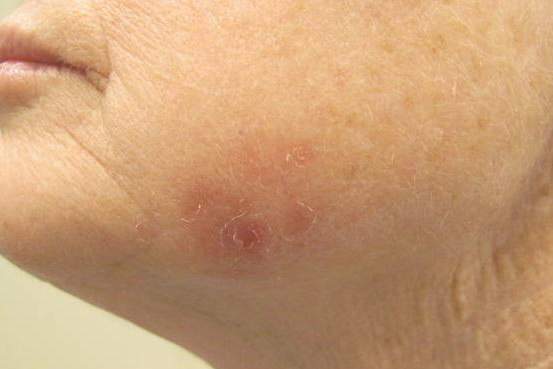
Erythematous Scaly Patch on the Jawline
The Diagnosis: Amelanotic Melanoma In Situ
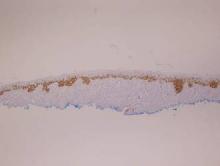
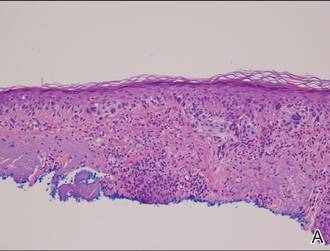
Histopathology revealed a broad asymmetric melanocytic proliferation at the dermoepidermal junction, consisting both of singly dispersed cells as well as randomly positioned nests (Figure 1). The single cells demonstrated junctional confluence and extension along adnexal structures highlighted by melan-A stain (Figure 2). The melanocytes were markedly atypical with enlarged and hyperchromatic nuclei containing multiple nucleoli. No dermal involvement was seen. There was papillary dermal fibrosis and an active host lymphocytic response. Based on these findings, a diagnosis of amelanotic melanoma in situ was made.
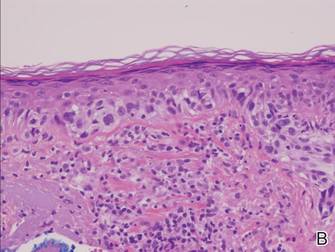
|
Figure 1. Histopathology revealed confluence of atypical melanocytes at the dermoepidermal junction and pagetoid scatter of melanocytes to the spinous layer (A)(H&E, original magnification ×4). Higher-power magnification highlighted the atypia of the individual melanocytes (B)(H&E, original magnification ×10). |
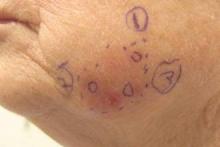
Subsequent scouting punch biopsies at the superior, anterior, and posterior aspects of the lesion were performed (Figure 3). All 3 revealed a similar nested and single cell proliferation at the dermoepidermal junction, confirming residual amelanotic melanoma in situ. The patient was referred to the otolaryngology department and underwent wide local excision with 5-mm margins and reconstructive repair.
Amelanotic melanoma comprises 2% to 8% of cutaneous melanomas. It is more common in fair-skinned elderly women with an average age of diagnosis of 61.8 years. Because features typically associated with melanoma such as asymmetry, border irregularity, and color variegation often are absent, amelanotic melanoma represents a notable diagnostic challenge for clinicians. Lesions can present nonspecifically as erythematous macules, papules, patches, or plaques and can have associated pruritus and scale.1,2
Clinical misdiagnoses for amelanotic melanoma include Bowen disease, basal cell carcinoma, actinic keratosis, lichenoid keratosis, intradermal nevus, dermatofibroma, inflamed seborrheic keratosis, nummular dermatitis, pyogenic granuloma, and granuloma annulare.1-6 There have been few case reports of amelanotic melanoma in situ, with most being the lentigo maligna variant that were initially clinically diagnosed as superficial basal cell carcinoma, Bowen disease, or dermatitis.7,8 In one case report, an amelanotic lentigo maligna was incidentally discovered after performing a mapping shave biopsy on what was normal-appearing skin.9
Dermoscopic evidence of vascular structures in lesions, including the presence of dotted vessels, milky red areas, and/or serpentine (linear irregular) vessels, may be the only clues to suggest amelanotic melanoma before biopsy. However, these findings are nonspecific and can be seen in other benign and malignant skin conditions.2
Complete surgical excision is the standard treatment of amelanotic melanoma in situ given its potential for invasion. However, the lack of pigment can make margins difficult to define. Because of its ability to detect disease beyond visual margins, Mohs micrographic surgery may have better cure rates than conventional excision.4 Prognosis for amelanotic melanoma is the same as other melanomas of equal thickness and location, though delay in diagnosis can adversely affect outcomes. Furthermore, amelanotic melanoma in situ can rapidly progress to invasive melanoma.3,5 Thus it is important to maintain clinical suspicion for amelanotic melanoma in fair-skinned elderly women presenting with a persistent or recurring erythematous scaly lesion on sun-exposed skin.
- Rahbari H, Nabai H, Mehregan AH, et al. Amelanotic lentigo maligna melanoma: a diagnostic conundrum— presentation of four new cases. Cancer. 1996;77:2052-2057.
- Jaimes N, Braun RP, Thomas L, et al. Clinical and dermoscopic characteristics of amelanotic melanomas that are not of the nodular subtype. J Eur Acad Dermatol Venereol. 2012;26:591-596.
- Koch SE, Lange JR. Amelanotic melanoma: the great masquerader. J Am Acad Dermatol. 2000;42:731-734.
- Conrad N, Jackson B, Goldberg L. Amelanotic lentigo maligna melanoma: a unique case presentation. Dermatol Surg. 1999;25:408-411.
- Cliff S, Otter M, Holden CA. Amelanotic lentigo maligna melanoma of the face: a case report and review of the literature. Clin Exp Dermatol. 1997;22:177-179.
- Dalton SR, Fillman EP, Altman CE, et al. Atypical junctional melanocytic proliferations in benign lichenoid keratosis. Hum Pathol. 2003;34:706-709.
- Paver K, Stewart M, Kossard S, et al. Amelanotic lentigo maligna. Australas J Dermatol. 1981;22:106-108.
- Lewis JE. Lentigo maligna presenting as an eczematous lesion. Cutis. 1987;40:357-359.
- Perera E, Mellick N, Teng P, et al. A clinically invisible melanoma. Australas J Dermatol. 2014;55:e58-e59.
The Diagnosis: Amelanotic Melanoma In Situ
Histopathology revealed a broad asymmetric melanocytic proliferation at the dermoepidermal junction, consisting both of singly dispersed cells as well as randomly positioned nests (Figure 1). The single cells demonstrated junctional confluence and extension along adnexal structures highlighted by melan-A stain (Figure 2). The melanocytes were markedly atypical with enlarged and hyperchromatic nuclei containing multiple nucleoli. No dermal involvement was seen. There was papillary dermal fibrosis and an active host lymphocytic response. Based on these findings, a diagnosis of amelanotic melanoma in situ was made.


|
Figure 1. Histopathology revealed confluence of atypical melanocytes at the dermoepidermal junction and pagetoid scatter of melanocytes to the spinous layer (A)(H&E, original magnification ×4). Higher-power magnification highlighted the atypia of the individual melanocytes (B)(H&E, original magnification ×10). |
Subsequent scouting punch biopsies at the superior, anterior, and posterior aspects of the lesion were performed (Figure 3). All 3 revealed a similar nested and single cell proliferation at the dermoepidermal junction, confirming residual amelanotic melanoma in situ. The patient was referred to the otolaryngology department and underwent wide local excision with 5-mm margins and reconstructive repair.
Amelanotic melanoma comprises 2% to 8% of cutaneous melanomas. It is more common in fair-skinned elderly women with an average age of diagnosis of 61.8 years. Because features typically associated with melanoma such as asymmetry, border irregularity, and color variegation often are absent, amelanotic melanoma represents a notable diagnostic challenge for clinicians. Lesions can present nonspecifically as erythematous macules, papules, patches, or plaques and can have associated pruritus and scale.1,2
Clinical misdiagnoses for amelanotic melanoma include Bowen disease, basal cell carcinoma, actinic keratosis, lichenoid keratosis, intradermal nevus, dermatofibroma, inflamed seborrheic keratosis, nummular dermatitis, pyogenic granuloma, and granuloma annulare.1-6 There have been few case reports of amelanotic melanoma in situ, with most being the lentigo maligna variant that were initially clinically diagnosed as superficial basal cell carcinoma, Bowen disease, or dermatitis.7,8 In one case report, an amelanotic lentigo maligna was incidentally discovered after performing a mapping shave biopsy on what was normal-appearing skin.9
Dermoscopic evidence of vascular structures in lesions, including the presence of dotted vessels, milky red areas, and/or serpentine (linear irregular) vessels, may be the only clues to suggest amelanotic melanoma before biopsy. However, these findings are nonspecific and can be seen in other benign and malignant skin conditions.2
Complete surgical excision is the standard treatment of amelanotic melanoma in situ given its potential for invasion. However, the lack of pigment can make margins difficult to define. Because of its ability to detect disease beyond visual margins, Mohs micrographic surgery may have better cure rates than conventional excision.4 Prognosis for amelanotic melanoma is the same as other melanomas of equal thickness and location, though delay in diagnosis can adversely affect outcomes. Furthermore, amelanotic melanoma in situ can rapidly progress to invasive melanoma.3,5 Thus it is important to maintain clinical suspicion for amelanotic melanoma in fair-skinned elderly women presenting with a persistent or recurring erythematous scaly lesion on sun-exposed skin.
The Diagnosis: Amelanotic Melanoma In Situ
Histopathology revealed a broad asymmetric melanocytic proliferation at the dermoepidermal junction, consisting both of singly dispersed cells as well as randomly positioned nests (Figure 1). The single cells demonstrated junctional confluence and extension along adnexal structures highlighted by melan-A stain (Figure 2). The melanocytes were markedly atypical with enlarged and hyperchromatic nuclei containing multiple nucleoli. No dermal involvement was seen. There was papillary dermal fibrosis and an active host lymphocytic response. Based on these findings, a diagnosis of amelanotic melanoma in situ was made.


|
Figure 1. Histopathology revealed confluence of atypical melanocytes at the dermoepidermal junction and pagetoid scatter of melanocytes to the spinous layer (A)(H&E, original magnification ×4). Higher-power magnification highlighted the atypia of the individual melanocytes (B)(H&E, original magnification ×10). |
Subsequent scouting punch biopsies at the superior, anterior, and posterior aspects of the lesion were performed (Figure 3). All 3 revealed a similar nested and single cell proliferation at the dermoepidermal junction, confirming residual amelanotic melanoma in situ. The patient was referred to the otolaryngology department and underwent wide local excision with 5-mm margins and reconstructive repair.
Amelanotic melanoma comprises 2% to 8% of cutaneous melanomas. It is more common in fair-skinned elderly women with an average age of diagnosis of 61.8 years. Because features typically associated with melanoma such as asymmetry, border irregularity, and color variegation often are absent, amelanotic melanoma represents a notable diagnostic challenge for clinicians. Lesions can present nonspecifically as erythematous macules, papules, patches, or plaques and can have associated pruritus and scale.1,2
Clinical misdiagnoses for amelanotic melanoma include Bowen disease, basal cell carcinoma, actinic keratosis, lichenoid keratosis, intradermal nevus, dermatofibroma, inflamed seborrheic keratosis, nummular dermatitis, pyogenic granuloma, and granuloma annulare.1-6 There have been few case reports of amelanotic melanoma in situ, with most being the lentigo maligna variant that were initially clinically diagnosed as superficial basal cell carcinoma, Bowen disease, or dermatitis.7,8 In one case report, an amelanotic lentigo maligna was incidentally discovered after performing a mapping shave biopsy on what was normal-appearing skin.9
Dermoscopic evidence of vascular structures in lesions, including the presence of dotted vessels, milky red areas, and/or serpentine (linear irregular) vessels, may be the only clues to suggest amelanotic melanoma before biopsy. However, these findings are nonspecific and can be seen in other benign and malignant skin conditions.2
Complete surgical excision is the standard treatment of amelanotic melanoma in situ given its potential for invasion. However, the lack of pigment can make margins difficult to define. Because of its ability to detect disease beyond visual margins, Mohs micrographic surgery may have better cure rates than conventional excision.4 Prognosis for amelanotic melanoma is the same as other melanomas of equal thickness and location, though delay in diagnosis can adversely affect outcomes. Furthermore, amelanotic melanoma in situ can rapidly progress to invasive melanoma.3,5 Thus it is important to maintain clinical suspicion for amelanotic melanoma in fair-skinned elderly women presenting with a persistent or recurring erythematous scaly lesion on sun-exposed skin.
- Rahbari H, Nabai H, Mehregan AH, et al. Amelanotic lentigo maligna melanoma: a diagnostic conundrum— presentation of four new cases. Cancer. 1996;77:2052-2057.
- Jaimes N, Braun RP, Thomas L, et al. Clinical and dermoscopic characteristics of amelanotic melanomas that are not of the nodular subtype. J Eur Acad Dermatol Venereol. 2012;26:591-596.
- Koch SE, Lange JR. Amelanotic melanoma: the great masquerader. J Am Acad Dermatol. 2000;42:731-734.
- Conrad N, Jackson B, Goldberg L. Amelanotic lentigo maligna melanoma: a unique case presentation. Dermatol Surg. 1999;25:408-411.
- Cliff S, Otter M, Holden CA. Amelanotic lentigo maligna melanoma of the face: a case report and review of the literature. Clin Exp Dermatol. 1997;22:177-179.
- Dalton SR, Fillman EP, Altman CE, et al. Atypical junctional melanocytic proliferations in benign lichenoid keratosis. Hum Pathol. 2003;34:706-709.
- Paver K, Stewart M, Kossard S, et al. Amelanotic lentigo maligna. Australas J Dermatol. 1981;22:106-108.
- Lewis JE. Lentigo maligna presenting as an eczematous lesion. Cutis. 1987;40:357-359.
- Perera E, Mellick N, Teng P, et al. A clinically invisible melanoma. Australas J Dermatol. 2014;55:e58-e59.
- Rahbari H, Nabai H, Mehregan AH, et al. Amelanotic lentigo maligna melanoma: a diagnostic conundrum— presentation of four new cases. Cancer. 1996;77:2052-2057.
- Jaimes N, Braun RP, Thomas L, et al. Clinical and dermoscopic characteristics of amelanotic melanomas that are not of the nodular subtype. J Eur Acad Dermatol Venereol. 2012;26:591-596.
- Koch SE, Lange JR. Amelanotic melanoma: the great masquerader. J Am Acad Dermatol. 2000;42:731-734.
- Conrad N, Jackson B, Goldberg L. Amelanotic lentigo maligna melanoma: a unique case presentation. Dermatol Surg. 1999;25:408-411.
- Cliff S, Otter M, Holden CA. Amelanotic lentigo maligna melanoma of the face: a case report and review of the literature. Clin Exp Dermatol. 1997;22:177-179.
- Dalton SR, Fillman EP, Altman CE, et al. Atypical junctional melanocytic proliferations in benign lichenoid keratosis. Hum Pathol. 2003;34:706-709.
- Paver K, Stewart M, Kossard S, et al. Amelanotic lentigo maligna. Australas J Dermatol. 1981;22:106-108.
- Lewis JE. Lentigo maligna presenting as an eczematous lesion. Cutis. 1987;40:357-359.
- Perera E, Mellick N, Teng P, et al. A clinically invisible melanoma. Australas J Dermatol. 2014;55:e58-e59.

Atypia of the individual melanocytes.
Melan-A stain highlighted the density and confluence of melanocytes within the epidermis.
Three scouting punch biopsies were performed along the periphery of the lesion.
A 70-year-old white woman with a history of basal cell carcinoma presented with a 2.7×1.9-cm ill-defined, erythematous, scaly patch along the left side of the jawline. Ten months prior to presentation, the lesion appeared as a grayish macule that was clinically diagnosed as a pigmented actinic keratosis and was treated with cryotherapy with resolution noted at 6-month follow-up. Differential diagnosis of the current lesion included actinic keratosis, lichenoid keratosis, and superficial basal cell carcinoma. A shave biopsy was performed.