User login
Drug-induced liver injury: Diagnosing (and treating) it early
› If you suspect your patient may have drug-induced liver injury (DILI), take a careful medication history, assess for risk factors, and investigate other possible causes. B
› Immediately stop any drugs you suspect are causing DILI, especially when the patient’s liver enzymes are rapidly increasing or there is evidence of acute liver failure. A
Strength of recommendation (SOR)
A Good-quality patient-oriented evidence
B Inconsistent or limited-quality patient-oriented evidence
C Consensus, usual practice, opinion, disease-oriented evidence, case series
CASE › James A, age 68, presents to his family physician (FP) with anorexia, nausea, and vague upper abdominal pain that he’s had for 2 weeks. Mr. A has diabetes and hypertension, both of which are well controlled by medications. He also consumes more than 4 alcoholic beverages daily.
On examination, the FP notes icterus and tenderness in the right hypochondrium. Liver function testing reveals elevated liver enzyme levels: aspartate aminotransferase (AST), 864 IU/L (normal range: 10-40 IU/L); alanine aminotransferase (ALT), 1012 IU/L (normal range: 7-56 IU/L); serum bilirubin, 4.8 mg/dL (normal range: 0.3-1.9 mg/dL); and alkaline phosphatase (ALP), 200 IU/L (normal range: 44-147 IU/L). Mr. A’s coagulation profile is slightly abnormal. He is provisionally diagnosed with acute hepatitis. The FP sends blood samples to the lab to assess for viral markers, and starts symptomatic management.
If Mr. A were your patient, how would you proceed?
In the United States, drug-induced liver injury (DILI) is the most common cause of acute liver failure.1,2 It can occur due to ingestion of any therapeutic drug, herbal product, or xenobiotic. Further complicating matters is the fact that it has an unpredictable and heterogeneous course, ranging from an asymptomatic rise in liver enzymes to acute liver failure. This article describes the risk factors, common causative agents, tools for early diagnosis, and effective management of DILI.
Two types of risk factors for DILI
Risk factors for DILI can be classified as drug-related (eg, dose, concomitant medications, polypharmacy) or host-related (eg, age, gender, alcohol intake, concomitant infections).3-5
Drug-related factors. Hundreds of agents can lead to liver injury. In fact, the US National Library of Medicine and the National Institute of Diabetes and Digestive and Kidney Diseases have created LiverTox (http://www.livertox.nih.gov/), an online database that provides detailed information on more than 600 such agents.6 Antibiotics are the most common cause of DILI, followed by neuropsychiatric drugs, immunomodulatory agents, antihypertensives, analgesics, antineoplastic drugs, and lipid-lowering agents.2
Among antibiotics, the specific medication most often responsible for DILI varies by geographical region. Amoxicillin/clavulanic acid is the most common causative antibiotic in the United States, whereas anti-tuberculosis agents such as isoniazid, rifampin, and pyrazinamide are the most common causative drugs in developing countries such as India, where the prevalence of tuberculosis is still high.7,8 Herbal and dietary supplements are emerging as an important cause of DILI.5,9,10
The use of multiple drugs further increases the risk of developing DILI.10 Drugs with a recommended daily dose of <50 mg are rarely associated with DILI.11
Host-related factors. Vulnerability to DILI is influenced by a patient’s age and sex.3,12 Very young and very old patients have an increased risk of developing DILI, and a patient’s age may make him or her particularly susceptible to the effects of certain medications.3,12,13 For example, children are more susceptible to DILI as a result of taking valproate or aspirin, whereas older patients are more likely to experience DILI brought on by amoxicillin/clavulanic acid.13 The pattern of liver injury also varies by age. Younger patients present most commonly with a hepatocellular pattern of injury, whereas older patients mostly present with a cholestatic pattern of liver injury.3
Some studies have found that women have a greater risk of developing DILI than men.13 The presence of chronic liver diseases, alcoholism, and nonalcoholic fatty liver disease (NAFLD) increase the risk of developing DILI.14 Diabetes is an independent risk factor for DILI.3
Clinical presentation of DILI varies widely
Some degree of liver injury may occur in any patient who ingests a drug that is metabolized in the liver. The clinical presentation of a patient with DILI can vary from an asymptomatic rise in liver enzymes to acute liver failure. Unexplained transaminitis should raise the possibility of DILI, especially when the patient has started a new drug in the preceding 3 months. However, in most patients, an asymptomatic rise in liver enzymes is due to hepatic adaptation or tolerance. In such cases, liver enzyme levels tend to normalize even if the patient continues to take the drug in the same dose.15
Apart from nonspecific symptoms such as anorexia, nausea, and vomiting, a patient with DILI may exhibit right upper quadrant pain, skin rash, or itching. A patient with severe DILI might exhibit jaundice, ascites, or encephalopathy.15
A stepwise approach to evaluation
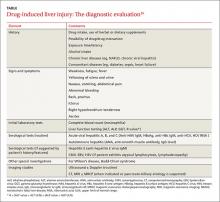
DILI is a diagnosis of exclusion.16 Guidelines from the American College of Gastroenterology (ACG) recommend a stepwise approach to evaluating a patient you suspect may have DILI (TABLE).16 First, take a detailed history regarding the onset of symptoms, time latency, and use of hepatotoxic and other drugs (dosage and duration of use). Also ask the patient about his or her use of herbal products, dietary supplements, and alcohol. Check the patient’s history for the presence of other liver diseases such as NAFLD.
Next, make sure initial laboratory testing includes liver function tests and an eosinophil count. In order to classify the pattern of liver injury as hepatocellular, cholestatic, or mixed, you’ll need to calculate the patient’s R value (ALGORITHM16-18). This value is calculated by dividing the patient’s ALT level by the ALP, using the upper limit of the normal range (ULN) as follows: R = (ALT value ÷ ALT ULN) ÷ (ALP value ÷ ALP ULN).17 A hepatocellular pattern of liver injury is indicated by an R value >5, a cholestatic pattern is an R value <2, and a mixed pattern is suggested by an R value between 2 and 5.17
Quite often, the pattern of liver damage is characteristic of a particular drug or drug class. For example, DILI induced by amoxicillin/clavulanic acid typically will exhibit a cholestatic injury pattern, whereas DILI resulting from a nonsteroidal anti-inflammatory drug typically is associated with a hepatocellular injury pattern.16
Rule out other causes. Further investigations should be directed at ruling out other possible causes of liver injury. If a patient has a hepatocellular pattern of liver injury, order serological tests to rule out acute viral hepatitis (hepatitis A, B, C, and E). Such patients should also be evaluated for autoimmune hepatitis, Budd-Chiari syndrome, Wilson’s disease, and ischemic hepatitis.16 In patients with a predominant cholestatic pattern, imaging studies and other serological tests should be ordered to rule out pancreato-biliary diseases.
Once DILI is confirmed, identify offending agent, grade severity
Which medication is responsible for DILI is determined by the physician based on his or her clinical experience and judgment. Guiding points are improvement of liver function tests after stopping the suspected drug (more on that in a bit), exclusion of other possible causes of liver injury, and the results of liver biopsy. (See “Time for a biopsy?”16)
DILI severity can be graded as mild (1+) to fatal (5+).19 Mild forms of DILI are associated with increased levels of liver enzymes (AST, ALT, or ALP) without raised serum bilirubin or clinical jaundice, whereas moderately severe DILI is associated with clinical jaundice or hyperbilirubinemia (bilirubin >2 mg/dL).19 Severe forms of DILI are associated with features of hepatic failure, such as ascites, encephalopathy, and an elevated international normalized ratio (>1.5), in addition to hyperbilirubinemia or jaundice.
American College of Gastroenterology guidelines recommend liver biopsy if autoimmune hepatitis is suspected, liver enzymes remain elevated for more than 6 months, or liver enzymes continue to rise even after stopping the suspected offending drug.16
Biopsy should also be considered if a patient’s alanine aminotransferase level fails to fall by at least half 60 days after stopping the suspected medication (in a patient with a hepatocellular pattern) or if a patient’s peak alkaline phosphatase level doesn’t fall by at least half at 180 days after stopping the suspected medication (in a patient with a cholestatic pattern).
CASE › Mr. A’s lab results are negative for viral markers. On further questioning, he reveals that he had recovered from a sore throat 3 weeks earlier, for which he had been prescribed an unknown dose of amoxicillin/clavulanic acid. A review of Mr. A’s drug history finds that he is taking metformin, pioglitazone, telmisartan, and atorvastatin for his chronic conditions. The FP suspects DILI , and refers Mr. A to a liver specialist for further investigation.
For many patients, stopping the offending drug will be sufficient
The first step in managing DILI is to stop the medication suspected of causing the liver injury.20 Discontinuing the suspected medication may not always be necessary in patients who have only slightly elevated liver enzymes, but should be strongly considered for a patient who has a considerable increase in liver enzymes levels (ie, an AST, ALT, or serum bilirubin level more than 3 times the ULN or an ALP more than 1.5 times the ULN at any time after initiating a new drug).18 Certain drugs, such as those used to treat tuberculosis, are associated with hepatic adaptation, in which there is spontaneous resolution of the increased liver enzymes level even while the drug is continued in the same dose.
In patients with mild to moderate DILI, stopping the offending drug typically results in normalization of liver enzyme levels.20 Management of patients with moderate to severe DILI is mainly supportive; however, a patient with acute liver failure will require intensive care support.21,22 Consider hospital admission for patients who exhibit severe symptoms, such as intractable vomiting or severe dehydration, those who experience bleeding due to coagulation failure, and those who develop hepatic encephalopathy.21
When more aggressive steps are needed
N-acetylcysteine (NAC) should be considered for all patients with DILI who present with acute liver failure.12,23-25 NAC can be administered either orally or intravenously. The following 3 regimens have been well studied for patients with acetaminophen-induced liver injury:26
• Oral 72-hour regimen: Loading dose of 140 mg/kg followed by 70 mg/kg every 4 hours up to 72 hours
• Intravenous 72-hour regimen: Loading infusion of 150 mg/kg over one hour, followed by 50 mg/kg over 4 hours, followed by 418.75 mg/kg over 67 hours
• Intravenous 21-hour regimen: Loading infusion of 150 mg/kg over one hour, followed by 50 mg/kg over 4 hours, followed by 100 mg/kg over 16 hours.
Of these regimens, the 72-hour IV regimen has been found to be more effective than the 21-hour regimen for patients with acetaminophen-induced liver toxicity.26 A study of NAC administered as continuous infusion for 72 hours in patients with acute liver failure found that the transplant-free survival rate was 40% for NAC in comparison with 27% for placebo.26
L-carnitine can be used to treat valproate-induced hepatotoxicity. In a case-control study of 92 patients with severe, symptomatic, valproate-induced hepatotoxicity, nearly half of 42 patients treated with L-carnitine survived, but only 10% of 50 patients treated solely with aggressive supportive care survived.27 Greater benefit has been found for IV vs oral L-carnitine.27,28
Ursodeoxycholic acid (UDCA), 13 to 15 mg/kg, may be helpful for DILI patients with a cholestatic pattern of liver injury.
Other therapies. Steroids have no defined role in management of DILI except in autoimmune-type DILI. Other drugs, such as silymarin and antioxidants, have been used to treat other forms of hepatic toxicities and might be beneficial for patients with DILI.29,30
Liver transplantation may be necessary to prevent death due to acute liver failure in patients with severe DILI. Various criteria, including Kings College criteria,31 can be used to select which patients may best benefit from liver transplantation.
For most patients, hospitalization will not be necessary
Generally, patients with DILI have a good prognosis.20,30 About 70% of patients with DILI do not require hospitalization, and approximately 90% recover without reaching the threshold of acute liver failure. However, patients with acute liver failure have a poor prognosis; 40% will require liver transplantation.16,20
Traditionally, patients with a cholestatic pattern of liver injury have been considered to have a better prognosis than those with a hepatocellular pattern of liver injury. Patients whose DILI is the result of a hypersensitivity reaction to a drug also have a good prognosis. This may be because features such as skin rash prompt early diagnosis and discontinuation of the offending drugs.7
CASE › A liver specialist evaluates Mr. A and concludes that his liver injury was caused by his long-term heavy alcohol consumption and exacerbated by the amoxicillin/clavulanic acid he had recently been prescribed. After 2 days, Mr. A develops drowsiness and is admitted to the hospital for further management. He is managed in the intensive care unit under supervision of a gastroenterologist. A NAC infusion is started at a loading dose of 150 mg/kg to manage acute liver failure. Unfortunately, however, Mr. A succumbs to his illness.
CORRESPONDENCE
Piyush Ranjan, MD, All India Institute of Medical Sciences, Ansari Nagar, New Delhi, India 110029; [email protected].
1. Khashab M, Tector AJ, Kwo PY. Epidemiology of acute liver failure. Curr Gastroenterol Rep. 2007;9:66-73.
2. Reuben A, Koch DG, Lee WM; Acute Liver Failure Study Group. Drug-induced acute liver failure: results of a U.S. multicenter, prospective study. Hepatology. 2010;52:2065-2076.
3. Chalasani N, Fontana RJ, Bonkovsky HL, et al; Drug Induced Liver Injury Network (DILIN). Causes, clinical features, and outcomes from a prospective study of drug-induced liver injury in the United States. Gastroenterology. 2008;135:1924-1934.
4. Björnsson ES. Epidemiology and risk factors for idiosyncratic drug-induced liver injury. Semin Liver Dis. 2014;34:115-122.
5. Suk KT, Kim DJ, Kim CH, et al. A prospective nationwide study of drug-induced liver injury in Korea. Am J Gastroenterol. 2012;107:1380-1387.
6. United States National Library of Medicine and the National Institute of Diabetes and Digestive and Kidney Diseases. LiverTox. United States National Library of Medicine Web site. Available at: http://livertox.nih.gov/. Accessed April 5, 2015.
7. Devarbhavi H, Dierkhising R, Kremers WK, et al. Single-center experience with drug-induced liver injury from India: causes, outcome, prognosis, and predictors of mortality. Am J Gastroenterol. 2010;105:2396-2404.
8. Björnsson ES. Drug-induced liver injury: an overview over the most critical compounds. Arch Toxicol. 2015;89:327-334.
9. Suk KT, Kim DJ. Drug-induced liver injury: present and future. Clin Mol Hepatol. 2012;18:249-257.
10. Herbals and dietary supplements. United States National Library of Medicine Web site. Available from: http://livertox.nih.gov/Herbals_and_Dietary_Supplements.htm. Accessed April 4, 2015.
11. Lammert C, Einarsson S, Saha C, et al. Relationship between daily dose of oral medications and idiosyncratic drug-induced liver injury: search for signals. Hepatology. 2008;47:2003-2009.
12. Björnsson ES, Bergmann OM, Björnsson HK, et al. Incidence, presentation, and outcomes in patients with drug-induced liver injury in the general population of Iceland. Gastroenterology. 2013;144:1419-1425.
13. Lucena MI, Andrade RJ, Kaplowitz N, et al; Spanish Group for the Study of Drug-Induced Liver Disease. Phenotypic characterization of idiosyncratic drug-induced liver injury: the influence of age and sex. Hepatology. 2009;49:2001-2009.
14. Tarantino G, Conca P, Basile V, et al. A prospective study of acute drug-induced liver injury in patients suffering from non-alcoholic fatty liver disease. Hepatol Res. 2007;37:410-415.
15. Hayashi PH, Fontana RJ. Clinical features, diagnosis, and natural history of drug-induced liver injury. Semin Liver Dis. 2014;34:134-144.
16. Chalasani NP, Hayashi PH, Bonkovsky HL, et al; Practice Parameters Committee of the American College of Gastroenterology. ACG Clinical Guideline: the diagnosis and management of idiosyncratic drug-induced liver injury. Am J Gastroenterol. 2014;109:950-966.
17. United States National Library of Medicine and the National Institute of Diabetes and Digestive and Kidney Diseases. Roussel Uclaf Causality Assessment Method (RUCAM) in Drug Induced Liver Injury. United States National Library of Medicine Web site. Available at: http://www.livertox.nih.gov/rucam.html. Accessed September 8, 2015.
18. Tajiri K, Shimizu Y. Practical guidelines for diagnosis and early management of drug-induced liver injury. World J Gastroenterol. 2008;14:6774-6785.
19. Fontana RJ, Seeff LB, Andrade RJ, et al. Standardization of nomenclature and causality assessment in drug-induced liver injury: summary of a clinical research workshop. Hepatology. 2010;52:730-742.
20. Leise MD, Poterucha JJ, Talwalkar JA. Drug-induced liver injury. Mayo Clin Proc. 2014;89:95-106.
21. Panackel C, Thomas R, Sebastian B, et al. Recent advances in management of acute liver failure. Indian J Crit Care Med. 2015;19:27-33.
22. Lee WM, Hynan LS, Rossaro L, et al; Acute Liver Failure Study Group. Intravenous N-acetylcysteine improves transplant-free survival in early stage non-acetaminophen acute liver failure. Gastroenterology. 2009;137:856-864.
23. Hu J, Zhang Q, Ren X, et al. Efficacy and safety of acetylcysteine in “non-acetaminophen” acute liver failure: A meta-analysis of prospective clinical trials. Clin Res Hepatol Gastroenterol. 2015.
24. Lancaster EM, Hiatt JR, Zarrinpar A. Acetaminophen hepatotoxicity: an updated review. Arch Toxicol. 2015;89:193-199.
25. Carter BA, Karpen SJ. Intestinal failure-associated liver disease: management and treatment strategies past, present, and future. Semin Liver Dis. 2007;27:251-258.
26. Woodhead JL, Howell BA, Yang Y, et al. An analysis of N-acetylcysteine treatment for acetaminophen overdose using a systems model of drug-induced liver injury. J Pharmacol Exp Ther. 2012;342:529-540.
27. Bohan TP, Helton E, McDonald I, et al. Effect of L-carnitine treatment for valproate-induced hepatotoxicity. Neurology. 2001;56:1405-1409.
28. Russell S. Carnitine as an antidote for acute valproate toxicity in children. Curr Opin Pediatr. 2007;19:206-210.
29. Ghabril M, Chalasani N, Björnsson E. Drug-induced liver injury: a clinical update. Curr Opin Gastroenterol. 2010;26:222-226.
30. Devarbhavi H. An update on drug-induced liver injury. J Clin Exp Hepatol. 2012;2:247-259.
31. Castaldo ET, Chari RS. Liver transplantation for acute hepatic failure. HPB (Oxford). 2006;8:29-34.
› If you suspect your patient may have drug-induced liver injury (DILI), take a careful medication history, assess for risk factors, and investigate other possible causes. B
› Immediately stop any drugs you suspect are causing DILI, especially when the patient’s liver enzymes are rapidly increasing or there is evidence of acute liver failure. A
Strength of recommendation (SOR)
A Good-quality patient-oriented evidence
B Inconsistent or limited-quality patient-oriented evidence
C Consensus, usual practice, opinion, disease-oriented evidence, case series
CASE › James A, age 68, presents to his family physician (FP) with anorexia, nausea, and vague upper abdominal pain that he’s had for 2 weeks. Mr. A has diabetes and hypertension, both of which are well controlled by medications. He also consumes more than 4 alcoholic beverages daily.
On examination, the FP notes icterus and tenderness in the right hypochondrium. Liver function testing reveals elevated liver enzyme levels: aspartate aminotransferase (AST), 864 IU/L (normal range: 10-40 IU/L); alanine aminotransferase (ALT), 1012 IU/L (normal range: 7-56 IU/L); serum bilirubin, 4.8 mg/dL (normal range: 0.3-1.9 mg/dL); and alkaline phosphatase (ALP), 200 IU/L (normal range: 44-147 IU/L). Mr. A’s coagulation profile is slightly abnormal. He is provisionally diagnosed with acute hepatitis. The FP sends blood samples to the lab to assess for viral markers, and starts symptomatic management.
If Mr. A were your patient, how would you proceed?
In the United States, drug-induced liver injury (DILI) is the most common cause of acute liver failure.1,2 It can occur due to ingestion of any therapeutic drug, herbal product, or xenobiotic. Further complicating matters is the fact that it has an unpredictable and heterogeneous course, ranging from an asymptomatic rise in liver enzymes to acute liver failure. This article describes the risk factors, common causative agents, tools for early diagnosis, and effective management of DILI.
Two types of risk factors for DILI
Risk factors for DILI can be classified as drug-related (eg, dose, concomitant medications, polypharmacy) or host-related (eg, age, gender, alcohol intake, concomitant infections).3-5
Drug-related factors. Hundreds of agents can lead to liver injury. In fact, the US National Library of Medicine and the National Institute of Diabetes and Digestive and Kidney Diseases have created LiverTox (http://www.livertox.nih.gov/), an online database that provides detailed information on more than 600 such agents.6 Antibiotics are the most common cause of DILI, followed by neuropsychiatric drugs, immunomodulatory agents, antihypertensives, analgesics, antineoplastic drugs, and lipid-lowering agents.2
Among antibiotics, the specific medication most often responsible for DILI varies by geographical region. Amoxicillin/clavulanic acid is the most common causative antibiotic in the United States, whereas anti-tuberculosis agents such as isoniazid, rifampin, and pyrazinamide are the most common causative drugs in developing countries such as India, where the prevalence of tuberculosis is still high.7,8 Herbal and dietary supplements are emerging as an important cause of DILI.5,9,10
The use of multiple drugs further increases the risk of developing DILI.10 Drugs with a recommended daily dose of <50 mg are rarely associated with DILI.11
Host-related factors. Vulnerability to DILI is influenced by a patient’s age and sex.3,12 Very young and very old patients have an increased risk of developing DILI, and a patient’s age may make him or her particularly susceptible to the effects of certain medications.3,12,13 For example, children are more susceptible to DILI as a result of taking valproate or aspirin, whereas older patients are more likely to experience DILI brought on by amoxicillin/clavulanic acid.13 The pattern of liver injury also varies by age. Younger patients present most commonly with a hepatocellular pattern of injury, whereas older patients mostly present with a cholestatic pattern of liver injury.3
Some studies have found that women have a greater risk of developing DILI than men.13 The presence of chronic liver diseases, alcoholism, and nonalcoholic fatty liver disease (NAFLD) increase the risk of developing DILI.14 Diabetes is an independent risk factor for DILI.3
Clinical presentation of DILI varies widely
Some degree of liver injury may occur in any patient who ingests a drug that is metabolized in the liver. The clinical presentation of a patient with DILI can vary from an asymptomatic rise in liver enzymes to acute liver failure. Unexplained transaminitis should raise the possibility of DILI, especially when the patient has started a new drug in the preceding 3 months. However, in most patients, an asymptomatic rise in liver enzymes is due to hepatic adaptation or tolerance. In such cases, liver enzyme levels tend to normalize even if the patient continues to take the drug in the same dose.15
Apart from nonspecific symptoms such as anorexia, nausea, and vomiting, a patient with DILI may exhibit right upper quadrant pain, skin rash, or itching. A patient with severe DILI might exhibit jaundice, ascites, or encephalopathy.15
A stepwise approach to evaluation
DILI is a diagnosis of exclusion.16 Guidelines from the American College of Gastroenterology (ACG) recommend a stepwise approach to evaluating a patient you suspect may have DILI (TABLE).16 First, take a detailed history regarding the onset of symptoms, time latency, and use of hepatotoxic and other drugs (dosage and duration of use). Also ask the patient about his or her use of herbal products, dietary supplements, and alcohol. Check the patient’s history for the presence of other liver diseases such as NAFLD.
Next, make sure initial laboratory testing includes liver function tests and an eosinophil count. In order to classify the pattern of liver injury as hepatocellular, cholestatic, or mixed, you’ll need to calculate the patient’s R value (ALGORITHM16-18). This value is calculated by dividing the patient’s ALT level by the ALP, using the upper limit of the normal range (ULN) as follows: R = (ALT value ÷ ALT ULN) ÷ (ALP value ÷ ALP ULN).17 A hepatocellular pattern of liver injury is indicated by an R value >5, a cholestatic pattern is an R value <2, and a mixed pattern is suggested by an R value between 2 and 5.17
Quite often, the pattern of liver damage is characteristic of a particular drug or drug class. For example, DILI induced by amoxicillin/clavulanic acid typically will exhibit a cholestatic injury pattern, whereas DILI resulting from a nonsteroidal anti-inflammatory drug typically is associated with a hepatocellular injury pattern.16
Rule out other causes. Further investigations should be directed at ruling out other possible causes of liver injury. If a patient has a hepatocellular pattern of liver injury, order serological tests to rule out acute viral hepatitis (hepatitis A, B, C, and E). Such patients should also be evaluated for autoimmune hepatitis, Budd-Chiari syndrome, Wilson’s disease, and ischemic hepatitis.16 In patients with a predominant cholestatic pattern, imaging studies and other serological tests should be ordered to rule out pancreato-biliary diseases.
Once DILI is confirmed, identify offending agent, grade severity
Which medication is responsible for DILI is determined by the physician based on his or her clinical experience and judgment. Guiding points are improvement of liver function tests after stopping the suspected drug (more on that in a bit), exclusion of other possible causes of liver injury, and the results of liver biopsy. (See “Time for a biopsy?”16)
DILI severity can be graded as mild (1+) to fatal (5+).19 Mild forms of DILI are associated with increased levels of liver enzymes (AST, ALT, or ALP) without raised serum bilirubin or clinical jaundice, whereas moderately severe DILI is associated with clinical jaundice or hyperbilirubinemia (bilirubin >2 mg/dL).19 Severe forms of DILI are associated with features of hepatic failure, such as ascites, encephalopathy, and an elevated international normalized ratio (>1.5), in addition to hyperbilirubinemia or jaundice.
American College of Gastroenterology guidelines recommend liver biopsy if autoimmune hepatitis is suspected, liver enzymes remain elevated for more than 6 months, or liver enzymes continue to rise even after stopping the suspected offending drug.16
Biopsy should also be considered if a patient’s alanine aminotransferase level fails to fall by at least half 60 days after stopping the suspected medication (in a patient with a hepatocellular pattern) or if a patient’s peak alkaline phosphatase level doesn’t fall by at least half at 180 days after stopping the suspected medication (in a patient with a cholestatic pattern).
CASE › Mr. A’s lab results are negative for viral markers. On further questioning, he reveals that he had recovered from a sore throat 3 weeks earlier, for which he had been prescribed an unknown dose of amoxicillin/clavulanic acid. A review of Mr. A’s drug history finds that he is taking metformin, pioglitazone, telmisartan, and atorvastatin for his chronic conditions. The FP suspects DILI , and refers Mr. A to a liver specialist for further investigation.
For many patients, stopping the offending drug will be sufficient
The first step in managing DILI is to stop the medication suspected of causing the liver injury.20 Discontinuing the suspected medication may not always be necessary in patients who have only slightly elevated liver enzymes, but should be strongly considered for a patient who has a considerable increase in liver enzymes levels (ie, an AST, ALT, or serum bilirubin level more than 3 times the ULN or an ALP more than 1.5 times the ULN at any time after initiating a new drug).18 Certain drugs, such as those used to treat tuberculosis, are associated with hepatic adaptation, in which there is spontaneous resolution of the increased liver enzymes level even while the drug is continued in the same dose.
In patients with mild to moderate DILI, stopping the offending drug typically results in normalization of liver enzyme levels.20 Management of patients with moderate to severe DILI is mainly supportive; however, a patient with acute liver failure will require intensive care support.21,22 Consider hospital admission for patients who exhibit severe symptoms, such as intractable vomiting or severe dehydration, those who experience bleeding due to coagulation failure, and those who develop hepatic encephalopathy.21
When more aggressive steps are needed
N-acetylcysteine (NAC) should be considered for all patients with DILI who present with acute liver failure.12,23-25 NAC can be administered either orally or intravenously. The following 3 regimens have been well studied for patients with acetaminophen-induced liver injury:26
• Oral 72-hour regimen: Loading dose of 140 mg/kg followed by 70 mg/kg every 4 hours up to 72 hours
• Intravenous 72-hour regimen: Loading infusion of 150 mg/kg over one hour, followed by 50 mg/kg over 4 hours, followed by 418.75 mg/kg over 67 hours
• Intravenous 21-hour regimen: Loading infusion of 150 mg/kg over one hour, followed by 50 mg/kg over 4 hours, followed by 100 mg/kg over 16 hours.
Of these regimens, the 72-hour IV regimen has been found to be more effective than the 21-hour regimen for patients with acetaminophen-induced liver toxicity.26 A study of NAC administered as continuous infusion for 72 hours in patients with acute liver failure found that the transplant-free survival rate was 40% for NAC in comparison with 27% for placebo.26
L-carnitine can be used to treat valproate-induced hepatotoxicity. In a case-control study of 92 patients with severe, symptomatic, valproate-induced hepatotoxicity, nearly half of 42 patients treated with L-carnitine survived, but only 10% of 50 patients treated solely with aggressive supportive care survived.27 Greater benefit has been found for IV vs oral L-carnitine.27,28
Ursodeoxycholic acid (UDCA), 13 to 15 mg/kg, may be helpful for DILI patients with a cholestatic pattern of liver injury.
Other therapies. Steroids have no defined role in management of DILI except in autoimmune-type DILI. Other drugs, such as silymarin and antioxidants, have been used to treat other forms of hepatic toxicities and might be beneficial for patients with DILI.29,30
Liver transplantation may be necessary to prevent death due to acute liver failure in patients with severe DILI. Various criteria, including Kings College criteria,31 can be used to select which patients may best benefit from liver transplantation.
For most patients, hospitalization will not be necessary
Generally, patients with DILI have a good prognosis.20,30 About 70% of patients with DILI do not require hospitalization, and approximately 90% recover without reaching the threshold of acute liver failure. However, patients with acute liver failure have a poor prognosis; 40% will require liver transplantation.16,20
Traditionally, patients with a cholestatic pattern of liver injury have been considered to have a better prognosis than those with a hepatocellular pattern of liver injury. Patients whose DILI is the result of a hypersensitivity reaction to a drug also have a good prognosis. This may be because features such as skin rash prompt early diagnosis and discontinuation of the offending drugs.7
CASE › A liver specialist evaluates Mr. A and concludes that his liver injury was caused by his long-term heavy alcohol consumption and exacerbated by the amoxicillin/clavulanic acid he had recently been prescribed. After 2 days, Mr. A develops drowsiness and is admitted to the hospital for further management. He is managed in the intensive care unit under supervision of a gastroenterologist. A NAC infusion is started at a loading dose of 150 mg/kg to manage acute liver failure. Unfortunately, however, Mr. A succumbs to his illness.
CORRESPONDENCE
Piyush Ranjan, MD, All India Institute of Medical Sciences, Ansari Nagar, New Delhi, India 110029; [email protected].
› If you suspect your patient may have drug-induced liver injury (DILI), take a careful medication history, assess for risk factors, and investigate other possible causes. B
› Immediately stop any drugs you suspect are causing DILI, especially when the patient’s liver enzymes are rapidly increasing or there is evidence of acute liver failure. A
Strength of recommendation (SOR)
A Good-quality patient-oriented evidence
B Inconsistent or limited-quality patient-oriented evidence
C Consensus, usual practice, opinion, disease-oriented evidence, case series
CASE › James A, age 68, presents to his family physician (FP) with anorexia, nausea, and vague upper abdominal pain that he’s had for 2 weeks. Mr. A has diabetes and hypertension, both of which are well controlled by medications. He also consumes more than 4 alcoholic beverages daily.
On examination, the FP notes icterus and tenderness in the right hypochondrium. Liver function testing reveals elevated liver enzyme levels: aspartate aminotransferase (AST), 864 IU/L (normal range: 10-40 IU/L); alanine aminotransferase (ALT), 1012 IU/L (normal range: 7-56 IU/L); serum bilirubin, 4.8 mg/dL (normal range: 0.3-1.9 mg/dL); and alkaline phosphatase (ALP), 200 IU/L (normal range: 44-147 IU/L). Mr. A’s coagulation profile is slightly abnormal. He is provisionally diagnosed with acute hepatitis. The FP sends blood samples to the lab to assess for viral markers, and starts symptomatic management.
If Mr. A were your patient, how would you proceed?
In the United States, drug-induced liver injury (DILI) is the most common cause of acute liver failure.1,2 It can occur due to ingestion of any therapeutic drug, herbal product, or xenobiotic. Further complicating matters is the fact that it has an unpredictable and heterogeneous course, ranging from an asymptomatic rise in liver enzymes to acute liver failure. This article describes the risk factors, common causative agents, tools for early diagnosis, and effective management of DILI.
Two types of risk factors for DILI
Risk factors for DILI can be classified as drug-related (eg, dose, concomitant medications, polypharmacy) or host-related (eg, age, gender, alcohol intake, concomitant infections).3-5
Drug-related factors. Hundreds of agents can lead to liver injury. In fact, the US National Library of Medicine and the National Institute of Diabetes and Digestive and Kidney Diseases have created LiverTox (http://www.livertox.nih.gov/), an online database that provides detailed information on more than 600 such agents.6 Antibiotics are the most common cause of DILI, followed by neuropsychiatric drugs, immunomodulatory agents, antihypertensives, analgesics, antineoplastic drugs, and lipid-lowering agents.2
Among antibiotics, the specific medication most often responsible for DILI varies by geographical region. Amoxicillin/clavulanic acid is the most common causative antibiotic in the United States, whereas anti-tuberculosis agents such as isoniazid, rifampin, and pyrazinamide are the most common causative drugs in developing countries such as India, where the prevalence of tuberculosis is still high.7,8 Herbal and dietary supplements are emerging as an important cause of DILI.5,9,10
The use of multiple drugs further increases the risk of developing DILI.10 Drugs with a recommended daily dose of <50 mg are rarely associated with DILI.11
Host-related factors. Vulnerability to DILI is influenced by a patient’s age and sex.3,12 Very young and very old patients have an increased risk of developing DILI, and a patient’s age may make him or her particularly susceptible to the effects of certain medications.3,12,13 For example, children are more susceptible to DILI as a result of taking valproate or aspirin, whereas older patients are more likely to experience DILI brought on by amoxicillin/clavulanic acid.13 The pattern of liver injury also varies by age. Younger patients present most commonly with a hepatocellular pattern of injury, whereas older patients mostly present with a cholestatic pattern of liver injury.3
Some studies have found that women have a greater risk of developing DILI than men.13 The presence of chronic liver diseases, alcoholism, and nonalcoholic fatty liver disease (NAFLD) increase the risk of developing DILI.14 Diabetes is an independent risk factor for DILI.3
Clinical presentation of DILI varies widely
Some degree of liver injury may occur in any patient who ingests a drug that is metabolized in the liver. The clinical presentation of a patient with DILI can vary from an asymptomatic rise in liver enzymes to acute liver failure. Unexplained transaminitis should raise the possibility of DILI, especially when the patient has started a new drug in the preceding 3 months. However, in most patients, an asymptomatic rise in liver enzymes is due to hepatic adaptation or tolerance. In such cases, liver enzyme levels tend to normalize even if the patient continues to take the drug in the same dose.15
Apart from nonspecific symptoms such as anorexia, nausea, and vomiting, a patient with DILI may exhibit right upper quadrant pain, skin rash, or itching. A patient with severe DILI might exhibit jaundice, ascites, or encephalopathy.15
A stepwise approach to evaluation
DILI is a diagnosis of exclusion.16 Guidelines from the American College of Gastroenterology (ACG) recommend a stepwise approach to evaluating a patient you suspect may have DILI (TABLE).16 First, take a detailed history regarding the onset of symptoms, time latency, and use of hepatotoxic and other drugs (dosage and duration of use). Also ask the patient about his or her use of herbal products, dietary supplements, and alcohol. Check the patient’s history for the presence of other liver diseases such as NAFLD.
Next, make sure initial laboratory testing includes liver function tests and an eosinophil count. In order to classify the pattern of liver injury as hepatocellular, cholestatic, or mixed, you’ll need to calculate the patient’s R value (ALGORITHM16-18). This value is calculated by dividing the patient’s ALT level by the ALP, using the upper limit of the normal range (ULN) as follows: R = (ALT value ÷ ALT ULN) ÷ (ALP value ÷ ALP ULN).17 A hepatocellular pattern of liver injury is indicated by an R value >5, a cholestatic pattern is an R value <2, and a mixed pattern is suggested by an R value between 2 and 5.17
Quite often, the pattern of liver damage is characteristic of a particular drug or drug class. For example, DILI induced by amoxicillin/clavulanic acid typically will exhibit a cholestatic injury pattern, whereas DILI resulting from a nonsteroidal anti-inflammatory drug typically is associated with a hepatocellular injury pattern.16
Rule out other causes. Further investigations should be directed at ruling out other possible causes of liver injury. If a patient has a hepatocellular pattern of liver injury, order serological tests to rule out acute viral hepatitis (hepatitis A, B, C, and E). Such patients should also be evaluated for autoimmune hepatitis, Budd-Chiari syndrome, Wilson’s disease, and ischemic hepatitis.16 In patients with a predominant cholestatic pattern, imaging studies and other serological tests should be ordered to rule out pancreato-biliary diseases.
Once DILI is confirmed, identify offending agent, grade severity
Which medication is responsible for DILI is determined by the physician based on his or her clinical experience and judgment. Guiding points are improvement of liver function tests after stopping the suspected drug (more on that in a bit), exclusion of other possible causes of liver injury, and the results of liver biopsy. (See “Time for a biopsy?”16)
DILI severity can be graded as mild (1+) to fatal (5+).19 Mild forms of DILI are associated with increased levels of liver enzymes (AST, ALT, or ALP) without raised serum bilirubin or clinical jaundice, whereas moderately severe DILI is associated with clinical jaundice or hyperbilirubinemia (bilirubin >2 mg/dL).19 Severe forms of DILI are associated with features of hepatic failure, such as ascites, encephalopathy, and an elevated international normalized ratio (>1.5), in addition to hyperbilirubinemia or jaundice.
American College of Gastroenterology guidelines recommend liver biopsy if autoimmune hepatitis is suspected, liver enzymes remain elevated for more than 6 months, or liver enzymes continue to rise even after stopping the suspected offending drug.16
Biopsy should also be considered if a patient’s alanine aminotransferase level fails to fall by at least half 60 days after stopping the suspected medication (in a patient with a hepatocellular pattern) or if a patient’s peak alkaline phosphatase level doesn’t fall by at least half at 180 days after stopping the suspected medication (in a patient with a cholestatic pattern).
CASE › Mr. A’s lab results are negative for viral markers. On further questioning, he reveals that he had recovered from a sore throat 3 weeks earlier, for which he had been prescribed an unknown dose of amoxicillin/clavulanic acid. A review of Mr. A’s drug history finds that he is taking metformin, pioglitazone, telmisartan, and atorvastatin for his chronic conditions. The FP suspects DILI , and refers Mr. A to a liver specialist for further investigation.
For many patients, stopping the offending drug will be sufficient
The first step in managing DILI is to stop the medication suspected of causing the liver injury.20 Discontinuing the suspected medication may not always be necessary in patients who have only slightly elevated liver enzymes, but should be strongly considered for a patient who has a considerable increase in liver enzymes levels (ie, an AST, ALT, or serum bilirubin level more than 3 times the ULN or an ALP more than 1.5 times the ULN at any time after initiating a new drug).18 Certain drugs, such as those used to treat tuberculosis, are associated with hepatic adaptation, in which there is spontaneous resolution of the increased liver enzymes level even while the drug is continued in the same dose.
In patients with mild to moderate DILI, stopping the offending drug typically results in normalization of liver enzyme levels.20 Management of patients with moderate to severe DILI is mainly supportive; however, a patient with acute liver failure will require intensive care support.21,22 Consider hospital admission for patients who exhibit severe symptoms, such as intractable vomiting or severe dehydration, those who experience bleeding due to coagulation failure, and those who develop hepatic encephalopathy.21
When more aggressive steps are needed
N-acetylcysteine (NAC) should be considered for all patients with DILI who present with acute liver failure.12,23-25 NAC can be administered either orally or intravenously. The following 3 regimens have been well studied for patients with acetaminophen-induced liver injury:26
• Oral 72-hour regimen: Loading dose of 140 mg/kg followed by 70 mg/kg every 4 hours up to 72 hours
• Intravenous 72-hour regimen: Loading infusion of 150 mg/kg over one hour, followed by 50 mg/kg over 4 hours, followed by 418.75 mg/kg over 67 hours
• Intravenous 21-hour regimen: Loading infusion of 150 mg/kg over one hour, followed by 50 mg/kg over 4 hours, followed by 100 mg/kg over 16 hours.
Of these regimens, the 72-hour IV regimen has been found to be more effective than the 21-hour regimen for patients with acetaminophen-induced liver toxicity.26 A study of NAC administered as continuous infusion for 72 hours in patients with acute liver failure found that the transplant-free survival rate was 40% for NAC in comparison with 27% for placebo.26
L-carnitine can be used to treat valproate-induced hepatotoxicity. In a case-control study of 92 patients with severe, symptomatic, valproate-induced hepatotoxicity, nearly half of 42 patients treated with L-carnitine survived, but only 10% of 50 patients treated solely with aggressive supportive care survived.27 Greater benefit has been found for IV vs oral L-carnitine.27,28
Ursodeoxycholic acid (UDCA), 13 to 15 mg/kg, may be helpful for DILI patients with a cholestatic pattern of liver injury.
Other therapies. Steroids have no defined role in management of DILI except in autoimmune-type DILI. Other drugs, such as silymarin and antioxidants, have been used to treat other forms of hepatic toxicities and might be beneficial for patients with DILI.29,30
Liver transplantation may be necessary to prevent death due to acute liver failure in patients with severe DILI. Various criteria, including Kings College criteria,31 can be used to select which patients may best benefit from liver transplantation.
For most patients, hospitalization will not be necessary
Generally, patients with DILI have a good prognosis.20,30 About 70% of patients with DILI do not require hospitalization, and approximately 90% recover without reaching the threshold of acute liver failure. However, patients with acute liver failure have a poor prognosis; 40% will require liver transplantation.16,20
Traditionally, patients with a cholestatic pattern of liver injury have been considered to have a better prognosis than those with a hepatocellular pattern of liver injury. Patients whose DILI is the result of a hypersensitivity reaction to a drug also have a good prognosis. This may be because features such as skin rash prompt early diagnosis and discontinuation of the offending drugs.7
CASE › A liver specialist evaluates Mr. A and concludes that his liver injury was caused by his long-term heavy alcohol consumption and exacerbated by the amoxicillin/clavulanic acid he had recently been prescribed. After 2 days, Mr. A develops drowsiness and is admitted to the hospital for further management. He is managed in the intensive care unit under supervision of a gastroenterologist. A NAC infusion is started at a loading dose of 150 mg/kg to manage acute liver failure. Unfortunately, however, Mr. A succumbs to his illness.
CORRESPONDENCE
Piyush Ranjan, MD, All India Institute of Medical Sciences, Ansari Nagar, New Delhi, India 110029; [email protected].
1. Khashab M, Tector AJ, Kwo PY. Epidemiology of acute liver failure. Curr Gastroenterol Rep. 2007;9:66-73.
2. Reuben A, Koch DG, Lee WM; Acute Liver Failure Study Group. Drug-induced acute liver failure: results of a U.S. multicenter, prospective study. Hepatology. 2010;52:2065-2076.
3. Chalasani N, Fontana RJ, Bonkovsky HL, et al; Drug Induced Liver Injury Network (DILIN). Causes, clinical features, and outcomes from a prospective study of drug-induced liver injury in the United States. Gastroenterology. 2008;135:1924-1934.
4. Björnsson ES. Epidemiology and risk factors for idiosyncratic drug-induced liver injury. Semin Liver Dis. 2014;34:115-122.
5. Suk KT, Kim DJ, Kim CH, et al. A prospective nationwide study of drug-induced liver injury in Korea. Am J Gastroenterol. 2012;107:1380-1387.
6. United States National Library of Medicine and the National Institute of Diabetes and Digestive and Kidney Diseases. LiverTox. United States National Library of Medicine Web site. Available at: http://livertox.nih.gov/. Accessed April 5, 2015.
7. Devarbhavi H, Dierkhising R, Kremers WK, et al. Single-center experience with drug-induced liver injury from India: causes, outcome, prognosis, and predictors of mortality. Am J Gastroenterol. 2010;105:2396-2404.
8. Björnsson ES. Drug-induced liver injury: an overview over the most critical compounds. Arch Toxicol. 2015;89:327-334.
9. Suk KT, Kim DJ. Drug-induced liver injury: present and future. Clin Mol Hepatol. 2012;18:249-257.
10. Herbals and dietary supplements. United States National Library of Medicine Web site. Available from: http://livertox.nih.gov/Herbals_and_Dietary_Supplements.htm. Accessed April 4, 2015.
11. Lammert C, Einarsson S, Saha C, et al. Relationship between daily dose of oral medications and idiosyncratic drug-induced liver injury: search for signals. Hepatology. 2008;47:2003-2009.
12. Björnsson ES, Bergmann OM, Björnsson HK, et al. Incidence, presentation, and outcomes in patients with drug-induced liver injury in the general population of Iceland. Gastroenterology. 2013;144:1419-1425.
13. Lucena MI, Andrade RJ, Kaplowitz N, et al; Spanish Group for the Study of Drug-Induced Liver Disease. Phenotypic characterization of idiosyncratic drug-induced liver injury: the influence of age and sex. Hepatology. 2009;49:2001-2009.
14. Tarantino G, Conca P, Basile V, et al. A prospective study of acute drug-induced liver injury in patients suffering from non-alcoholic fatty liver disease. Hepatol Res. 2007;37:410-415.
15. Hayashi PH, Fontana RJ. Clinical features, diagnosis, and natural history of drug-induced liver injury. Semin Liver Dis. 2014;34:134-144.
16. Chalasani NP, Hayashi PH, Bonkovsky HL, et al; Practice Parameters Committee of the American College of Gastroenterology. ACG Clinical Guideline: the diagnosis and management of idiosyncratic drug-induced liver injury. Am J Gastroenterol. 2014;109:950-966.
17. United States National Library of Medicine and the National Institute of Diabetes and Digestive and Kidney Diseases. Roussel Uclaf Causality Assessment Method (RUCAM) in Drug Induced Liver Injury. United States National Library of Medicine Web site. Available at: http://www.livertox.nih.gov/rucam.html. Accessed September 8, 2015.
18. Tajiri K, Shimizu Y. Practical guidelines for diagnosis and early management of drug-induced liver injury. World J Gastroenterol. 2008;14:6774-6785.
19. Fontana RJ, Seeff LB, Andrade RJ, et al. Standardization of nomenclature and causality assessment in drug-induced liver injury: summary of a clinical research workshop. Hepatology. 2010;52:730-742.
20. Leise MD, Poterucha JJ, Talwalkar JA. Drug-induced liver injury. Mayo Clin Proc. 2014;89:95-106.
21. Panackel C, Thomas R, Sebastian B, et al. Recent advances in management of acute liver failure. Indian J Crit Care Med. 2015;19:27-33.
22. Lee WM, Hynan LS, Rossaro L, et al; Acute Liver Failure Study Group. Intravenous N-acetylcysteine improves transplant-free survival in early stage non-acetaminophen acute liver failure. Gastroenterology. 2009;137:856-864.
23. Hu J, Zhang Q, Ren X, et al. Efficacy and safety of acetylcysteine in “non-acetaminophen” acute liver failure: A meta-analysis of prospective clinical trials. Clin Res Hepatol Gastroenterol. 2015.
24. Lancaster EM, Hiatt JR, Zarrinpar A. Acetaminophen hepatotoxicity: an updated review. Arch Toxicol. 2015;89:193-199.
25. Carter BA, Karpen SJ. Intestinal failure-associated liver disease: management and treatment strategies past, present, and future. Semin Liver Dis. 2007;27:251-258.
26. Woodhead JL, Howell BA, Yang Y, et al. An analysis of N-acetylcysteine treatment for acetaminophen overdose using a systems model of drug-induced liver injury. J Pharmacol Exp Ther. 2012;342:529-540.
27. Bohan TP, Helton E, McDonald I, et al. Effect of L-carnitine treatment for valproate-induced hepatotoxicity. Neurology. 2001;56:1405-1409.
28. Russell S. Carnitine as an antidote for acute valproate toxicity in children. Curr Opin Pediatr. 2007;19:206-210.
29. Ghabril M, Chalasani N, Björnsson E. Drug-induced liver injury: a clinical update. Curr Opin Gastroenterol. 2010;26:222-226.
30. Devarbhavi H. An update on drug-induced liver injury. J Clin Exp Hepatol. 2012;2:247-259.
31. Castaldo ET, Chari RS. Liver transplantation for acute hepatic failure. HPB (Oxford). 2006;8:29-34.
1. Khashab M, Tector AJ, Kwo PY. Epidemiology of acute liver failure. Curr Gastroenterol Rep. 2007;9:66-73.
2. Reuben A, Koch DG, Lee WM; Acute Liver Failure Study Group. Drug-induced acute liver failure: results of a U.S. multicenter, prospective study. Hepatology. 2010;52:2065-2076.
3. Chalasani N, Fontana RJ, Bonkovsky HL, et al; Drug Induced Liver Injury Network (DILIN). Causes, clinical features, and outcomes from a prospective study of drug-induced liver injury in the United States. Gastroenterology. 2008;135:1924-1934.
4. Björnsson ES. Epidemiology and risk factors for idiosyncratic drug-induced liver injury. Semin Liver Dis. 2014;34:115-122.
5. Suk KT, Kim DJ, Kim CH, et al. A prospective nationwide study of drug-induced liver injury in Korea. Am J Gastroenterol. 2012;107:1380-1387.
6. United States National Library of Medicine and the National Institute of Diabetes and Digestive and Kidney Diseases. LiverTox. United States National Library of Medicine Web site. Available at: http://livertox.nih.gov/. Accessed April 5, 2015.
7. Devarbhavi H, Dierkhising R, Kremers WK, et al. Single-center experience with drug-induced liver injury from India: causes, outcome, prognosis, and predictors of mortality. Am J Gastroenterol. 2010;105:2396-2404.
8. Björnsson ES. Drug-induced liver injury: an overview over the most critical compounds. Arch Toxicol. 2015;89:327-334.
9. Suk KT, Kim DJ. Drug-induced liver injury: present and future. Clin Mol Hepatol. 2012;18:249-257.
10. Herbals and dietary supplements. United States National Library of Medicine Web site. Available from: http://livertox.nih.gov/Herbals_and_Dietary_Supplements.htm. Accessed April 4, 2015.
11. Lammert C, Einarsson S, Saha C, et al. Relationship between daily dose of oral medications and idiosyncratic drug-induced liver injury: search for signals. Hepatology. 2008;47:2003-2009.
12. Björnsson ES, Bergmann OM, Björnsson HK, et al. Incidence, presentation, and outcomes in patients with drug-induced liver injury in the general population of Iceland. Gastroenterology. 2013;144:1419-1425.
13. Lucena MI, Andrade RJ, Kaplowitz N, et al; Spanish Group for the Study of Drug-Induced Liver Disease. Phenotypic characterization of idiosyncratic drug-induced liver injury: the influence of age and sex. Hepatology. 2009;49:2001-2009.
14. Tarantino G, Conca P, Basile V, et al. A prospective study of acute drug-induced liver injury in patients suffering from non-alcoholic fatty liver disease. Hepatol Res. 2007;37:410-415.
15. Hayashi PH, Fontana RJ. Clinical features, diagnosis, and natural history of drug-induced liver injury. Semin Liver Dis. 2014;34:134-144.
16. Chalasani NP, Hayashi PH, Bonkovsky HL, et al; Practice Parameters Committee of the American College of Gastroenterology. ACG Clinical Guideline: the diagnosis and management of idiosyncratic drug-induced liver injury. Am J Gastroenterol. 2014;109:950-966.
17. United States National Library of Medicine and the National Institute of Diabetes and Digestive and Kidney Diseases. Roussel Uclaf Causality Assessment Method (RUCAM) in Drug Induced Liver Injury. United States National Library of Medicine Web site. Available at: http://www.livertox.nih.gov/rucam.html. Accessed September 8, 2015.
18. Tajiri K, Shimizu Y. Practical guidelines for diagnosis and early management of drug-induced liver injury. World J Gastroenterol. 2008;14:6774-6785.
19. Fontana RJ, Seeff LB, Andrade RJ, et al. Standardization of nomenclature and causality assessment in drug-induced liver injury: summary of a clinical research workshop. Hepatology. 2010;52:730-742.
20. Leise MD, Poterucha JJ, Talwalkar JA. Drug-induced liver injury. Mayo Clin Proc. 2014;89:95-106.
21. Panackel C, Thomas R, Sebastian B, et al. Recent advances in management of acute liver failure. Indian J Crit Care Med. 2015;19:27-33.
22. Lee WM, Hynan LS, Rossaro L, et al; Acute Liver Failure Study Group. Intravenous N-acetylcysteine improves transplant-free survival in early stage non-acetaminophen acute liver failure. Gastroenterology. 2009;137:856-864.
23. Hu J, Zhang Q, Ren X, et al. Efficacy and safety of acetylcysteine in “non-acetaminophen” acute liver failure: A meta-analysis of prospective clinical trials. Clin Res Hepatol Gastroenterol. 2015.
24. Lancaster EM, Hiatt JR, Zarrinpar A. Acetaminophen hepatotoxicity: an updated review. Arch Toxicol. 2015;89:193-199.
25. Carter BA, Karpen SJ. Intestinal failure-associated liver disease: management and treatment strategies past, present, and future. Semin Liver Dis. 2007;27:251-258.
26. Woodhead JL, Howell BA, Yang Y, et al. An analysis of N-acetylcysteine treatment for acetaminophen overdose using a systems model of drug-induced liver injury. J Pharmacol Exp Ther. 2012;342:529-540.
27. Bohan TP, Helton E, McDonald I, et al. Effect of L-carnitine treatment for valproate-induced hepatotoxicity. Neurology. 2001;56:1405-1409.
28. Russell S. Carnitine as an antidote for acute valproate toxicity in children. Curr Opin Pediatr. 2007;19:206-210.
29. Ghabril M, Chalasani N, Björnsson E. Drug-induced liver injury: a clinical update. Curr Opin Gastroenterol. 2010;26:222-226.
30. Devarbhavi H. An update on drug-induced liver injury. J Clin Exp Hepatol. 2012;2:247-259.
31. Castaldo ET, Chari RS. Liver transplantation for acute hepatic failure. HPB (Oxford). 2006;8:29-34.