User login
Billing audits: The bane of a small practice
The photo you see below is a reasonably thick pile of paper, roughly 2 inches high. It’s certainly not as bad as some charts I’ve seen, especially at the VA, but still a lot of pages.
What is it?
This is, believe it or not, the stacked copies of charts we had to print in the last 30 days to fax to insurance companies for billing audits. Yeah – just the last 30 days.
Mind you, to date I don’t have any sort of actual complaints or charges against me for fraudulent billing. If anything, I tend to underbill for fear of risking the ire of insurance companies.
On one level, I understand it. The news is replete with stories of physicians who made fraudulent insurance claims, and the insurance companies want to make sure others are playing fair. Just like security cameras and magnetic tags at retailers, they’re doing what they can to avoid losses. I get that.
On the other hand, this irritates me, and it is a pain in the butt. Someone here has to print up the requested notes, organize them, fill out the accompanying forms, and fax them back. I also have to sign each note in the pile. For the number of charts they typically want, this process takes about 30-45 minutes. Then we fax them, and a 100-plus-page document ties up your office fax for a while. Incoming and outgoing faxes, such as medication refills, get put on hold. Overall, it takes maybe an hour of staff time to do this, not to mention the cost of paper and ink used.
About 25% of the time the company calls us after a few days to say they never got them (even though we have a confirmation). For this reason, we always hold onto the print-out for a month so we don’t have to start over again. Then it all has to be shredded.
In a large practice, I’m sure there are dedicated medical records staff members for this. But in my small solo world it means that someone has to let phones go to voicemail, dictations get delayed, and other work piles up, just so the insurance red tape gets done. Then we have to catch up on the more routine issues of patient care.
I can’t really refuse to send them, either. Doing so, in the insurance company’s mind, would be an admission of guilt that I never saw the patient and my claim is bogus. Then they’ll withhold payment, or ask for a refund.
This is, regrettably, a case where a few bad apples – docs filing bogus claims – have spoiled the entire barrel. Now we’re all guilty of fraud until proven innocent by sending these records. Isn’t that the reverse of the American justice system’s ideal?
I also wonder if there’s an intentional drudgery factor here. By making me do something that’s irritatingly time-wasting, is an insurance plan hoping I’ll drop them because I’m sick of this process? Does having fewer contracted neurologists work out to their benefit? It certainly isn’t to the patient’s advantage.
I don’t have an easy answer. I don’t like the wrench these requests throw into the office routine, but I also know that fraud surveillance is a necessary evil. I just wish there was a less time-consuming way of doing it.
Dr. Block has a solo neurology practice in Scottsdale, Ariz.
The photo you see below is a reasonably thick pile of paper, roughly 2 inches high. It’s certainly not as bad as some charts I’ve seen, especially at the VA, but still a lot of pages.
What is it?
This is, believe it or not, the stacked copies of charts we had to print in the last 30 days to fax to insurance companies for billing audits. Yeah – just the last 30 days.
Mind you, to date I don’t have any sort of actual complaints or charges against me for fraudulent billing. If anything, I tend to underbill for fear of risking the ire of insurance companies.
On one level, I understand it. The news is replete with stories of physicians who made fraudulent insurance claims, and the insurance companies want to make sure others are playing fair. Just like security cameras and magnetic tags at retailers, they’re doing what they can to avoid losses. I get that.
On the other hand, this irritates me, and it is a pain in the butt. Someone here has to print up the requested notes, organize them, fill out the accompanying forms, and fax them back. I also have to sign each note in the pile. For the number of charts they typically want, this process takes about 30-45 minutes. Then we fax them, and a 100-plus-page document ties up your office fax for a while. Incoming and outgoing faxes, such as medication refills, get put on hold. Overall, it takes maybe an hour of staff time to do this, not to mention the cost of paper and ink used.
About 25% of the time the company calls us after a few days to say they never got them (even though we have a confirmation). For this reason, we always hold onto the print-out for a month so we don’t have to start over again. Then it all has to be shredded.
In a large practice, I’m sure there are dedicated medical records staff members for this. But in my small solo world it means that someone has to let phones go to voicemail, dictations get delayed, and other work piles up, just so the insurance red tape gets done. Then we have to catch up on the more routine issues of patient care.
I can’t really refuse to send them, either. Doing so, in the insurance company’s mind, would be an admission of guilt that I never saw the patient and my claim is bogus. Then they’ll withhold payment, or ask for a refund.
This is, regrettably, a case where a few bad apples – docs filing bogus claims – have spoiled the entire barrel. Now we’re all guilty of fraud until proven innocent by sending these records. Isn’t that the reverse of the American justice system’s ideal?
I also wonder if there’s an intentional drudgery factor here. By making me do something that’s irritatingly time-wasting, is an insurance plan hoping I’ll drop them because I’m sick of this process? Does having fewer contracted neurologists work out to their benefit? It certainly isn’t to the patient’s advantage.
I don’t have an easy answer. I don’t like the wrench these requests throw into the office routine, but I also know that fraud surveillance is a necessary evil. I just wish there was a less time-consuming way of doing it.
Dr. Block has a solo neurology practice in Scottsdale, Ariz.
The photo you see below is a reasonably thick pile of paper, roughly 2 inches high. It’s certainly not as bad as some charts I’ve seen, especially at the VA, but still a lot of pages.
What is it?
This is, believe it or not, the stacked copies of charts we had to print in the last 30 days to fax to insurance companies for billing audits. Yeah – just the last 30 days.
Mind you, to date I don’t have any sort of actual complaints or charges against me for fraudulent billing. If anything, I tend to underbill for fear of risking the ire of insurance companies.
On one level, I understand it. The news is replete with stories of physicians who made fraudulent insurance claims, and the insurance companies want to make sure others are playing fair. Just like security cameras and magnetic tags at retailers, they’re doing what they can to avoid losses. I get that.
On the other hand, this irritates me, and it is a pain in the butt. Someone here has to print up the requested notes, organize them, fill out the accompanying forms, and fax them back. I also have to sign each note in the pile. For the number of charts they typically want, this process takes about 30-45 minutes. Then we fax them, and a 100-plus-page document ties up your office fax for a while. Incoming and outgoing faxes, such as medication refills, get put on hold. Overall, it takes maybe an hour of staff time to do this, not to mention the cost of paper and ink used.
About 25% of the time the company calls us after a few days to say they never got them (even though we have a confirmation). For this reason, we always hold onto the print-out for a month so we don’t have to start over again. Then it all has to be shredded.
In a large practice, I’m sure there are dedicated medical records staff members for this. But in my small solo world it means that someone has to let phones go to voicemail, dictations get delayed, and other work piles up, just so the insurance red tape gets done. Then we have to catch up on the more routine issues of patient care.
I can’t really refuse to send them, either. Doing so, in the insurance company’s mind, would be an admission of guilt that I never saw the patient and my claim is bogus. Then they’ll withhold payment, or ask for a refund.
This is, regrettably, a case where a few bad apples – docs filing bogus claims – have spoiled the entire barrel. Now we’re all guilty of fraud until proven innocent by sending these records. Isn’t that the reverse of the American justice system’s ideal?
I also wonder if there’s an intentional drudgery factor here. By making me do something that’s irritatingly time-wasting, is an insurance plan hoping I’ll drop them because I’m sick of this process? Does having fewer contracted neurologists work out to their benefit? It certainly isn’t to the patient’s advantage.
I don’t have an easy answer. I don’t like the wrench these requests throw into the office routine, but I also know that fraud surveillance is a necessary evil. I just wish there was a less time-consuming way of doing it.
Dr. Block has a solo neurology practice in Scottsdale, Ariz.
Racial, Economic Disparities in Life Expectancy after Heart Attack
After a heart attack, black patients typically don't live as long as whites - a racial difference that is starkest among the affluent - according to a new U.S. study.
Researchers evaluated data on more than 132,000 white heart attack patients and almost 9,000 black patients covered by Medicare, the government health program for the elderly and disabled. They used postal codes to assess income levels in patients' communities.
After 17 years of follow-up, the overall survival rate was 7.4 percent for white patients and 5.7 percent for black patients, according to the results published in Circulation, the journal of the American Heart Association.
On average, across all ages, white patients in low-income areas lived longer after a heart attack - about 5.6 years compared with 5.4 years for black patients. But in high-income communities, the gap widened to a life expectancy of seven years for white people and 6.3 years for black individuals.
"We found that socioeconomic status did not explain the racial disparities in life expectancy after a heart attack," lead study author Dr. Emily Bucholz of Boston Children's Hospital said by email.
"Contrary to common belief, this suggests that improving socioeconomic standing may improve outcomes for black and white patients globally but is unlikely to eliminate racial disparities in health," Bucholz added.
To see how race and class impact heart attack outcomes, Bucholz and colleagues reviewed health records collected from 1994 to 1996 for patients aged 65 to 90 years.
Just 6.3 percent of the patients were black, and only 6.8 percent lived in low-income communities, based on the typical household income in their postal codes.
Among white patients under 80, life expectancy was longest for patients in the most affluent neighborhoods and it got progressively shorter for middle-income and poor communities, the study found.
By contrast, life expectancy was similar for black patients residing in poor and middle-income communities across all ages. Only black patients under age 75 living in affluent areas had a survival advantage compared with their peers in less wealthy neighborhoods.
One shortcoming of the study is that it included a small proportion of black and poor patients, the authors acknowledge. It's also possible that using postal codes to assess income may have led to some instances where income levels were inflated or underestimated, the authors note.
It's possible that black patients living in affluent areas don't fare as well as white patients because they don't have the same amount of social support from their peers, said Dr. Joaquin Cigarroa, a cardiovascular medicine researcher at Oregon Health & Science University in Portland.
In poor neighborhoods, black patients may face additional challenges to surviving a heart attack, added Cigarroa, who wasn't involved in the study.
"They more often live in low socioeconomic segments of our community that often have less access to health care resources and less access to stores with good nutrition," Cigarroa said by email. "In addition, these segments of our community are often not ideally configured for promoting physical activity with parks, sidewalks, bike lanes, etc."
The study findings highlight a need to improve outcomes among poor and black patients and suggest some differences in heart attack survival may come down to disparities in quality of care, said senior study author Dr. Harlan Krumholz of Yale University School of Medicine in New Haven, Connecticut.
Because black patients have a greater burden of heart disease than white people, doctors may also need to focus more on prevention in this community, Krumholz said by email.
"Healthy heart habits may be even more important for African-Americans, for whom avoiding a heart attack is even more important given their worse outcomes after the event," Krumholz said.
After a heart attack, black patients typically don't live as long as whites - a racial difference that is starkest among the affluent - according to a new U.S. study.
Researchers evaluated data on more than 132,000 white heart attack patients and almost 9,000 black patients covered by Medicare, the government health program for the elderly and disabled. They used postal codes to assess income levels in patients' communities.
After 17 years of follow-up, the overall survival rate was 7.4 percent for white patients and 5.7 percent for black patients, according to the results published in Circulation, the journal of the American Heart Association.
On average, across all ages, white patients in low-income areas lived longer after a heart attack - about 5.6 years compared with 5.4 years for black patients. But in high-income communities, the gap widened to a life expectancy of seven years for white people and 6.3 years for black individuals.
"We found that socioeconomic status did not explain the racial disparities in life expectancy after a heart attack," lead study author Dr. Emily Bucholz of Boston Children's Hospital said by email.
"Contrary to common belief, this suggests that improving socioeconomic standing may improve outcomes for black and white patients globally but is unlikely to eliminate racial disparities in health," Bucholz added.
To see how race and class impact heart attack outcomes, Bucholz and colleagues reviewed health records collected from 1994 to 1996 for patients aged 65 to 90 years.
Just 6.3 percent of the patients were black, and only 6.8 percent lived in low-income communities, based on the typical household income in their postal codes.
Among white patients under 80, life expectancy was longest for patients in the most affluent neighborhoods and it got progressively shorter for middle-income and poor communities, the study found.
By contrast, life expectancy was similar for black patients residing in poor and middle-income communities across all ages. Only black patients under age 75 living in affluent areas had a survival advantage compared with their peers in less wealthy neighborhoods.
One shortcoming of the study is that it included a small proportion of black and poor patients, the authors acknowledge. It's also possible that using postal codes to assess income may have led to some instances where income levels were inflated or underestimated, the authors note.
It's possible that black patients living in affluent areas don't fare as well as white patients because they don't have the same amount of social support from their peers, said Dr. Joaquin Cigarroa, a cardiovascular medicine researcher at Oregon Health & Science University in Portland.
In poor neighborhoods, black patients may face additional challenges to surviving a heart attack, added Cigarroa, who wasn't involved in the study.
"They more often live in low socioeconomic segments of our community that often have less access to health care resources and less access to stores with good nutrition," Cigarroa said by email. "In addition, these segments of our community are often not ideally configured for promoting physical activity with parks, sidewalks, bike lanes, etc."
The study findings highlight a need to improve outcomes among poor and black patients and suggest some differences in heart attack survival may come down to disparities in quality of care, said senior study author Dr. Harlan Krumholz of Yale University School of Medicine in New Haven, Connecticut.
Because black patients have a greater burden of heart disease than white people, doctors may also need to focus more on prevention in this community, Krumholz said by email.
"Healthy heart habits may be even more important for African-Americans, for whom avoiding a heart attack is even more important given their worse outcomes after the event," Krumholz said.
After a heart attack, black patients typically don't live as long as whites - a racial difference that is starkest among the affluent - according to a new U.S. study.
Researchers evaluated data on more than 132,000 white heart attack patients and almost 9,000 black patients covered by Medicare, the government health program for the elderly and disabled. They used postal codes to assess income levels in patients' communities.
After 17 years of follow-up, the overall survival rate was 7.4 percent for white patients and 5.7 percent for black patients, according to the results published in Circulation, the journal of the American Heart Association.
On average, across all ages, white patients in low-income areas lived longer after a heart attack - about 5.6 years compared with 5.4 years for black patients. But in high-income communities, the gap widened to a life expectancy of seven years for white people and 6.3 years for black individuals.
"We found that socioeconomic status did not explain the racial disparities in life expectancy after a heart attack," lead study author Dr. Emily Bucholz of Boston Children's Hospital said by email.
"Contrary to common belief, this suggests that improving socioeconomic standing may improve outcomes for black and white patients globally but is unlikely to eliminate racial disparities in health," Bucholz added.
To see how race and class impact heart attack outcomes, Bucholz and colleagues reviewed health records collected from 1994 to 1996 for patients aged 65 to 90 years.
Just 6.3 percent of the patients were black, and only 6.8 percent lived in low-income communities, based on the typical household income in their postal codes.
Among white patients under 80, life expectancy was longest for patients in the most affluent neighborhoods and it got progressively shorter for middle-income and poor communities, the study found.
By contrast, life expectancy was similar for black patients residing in poor and middle-income communities across all ages. Only black patients under age 75 living in affluent areas had a survival advantage compared with their peers in less wealthy neighborhoods.
One shortcoming of the study is that it included a small proportion of black and poor patients, the authors acknowledge. It's also possible that using postal codes to assess income may have led to some instances where income levels were inflated or underestimated, the authors note.
It's possible that black patients living in affluent areas don't fare as well as white patients because they don't have the same amount of social support from their peers, said Dr. Joaquin Cigarroa, a cardiovascular medicine researcher at Oregon Health & Science University in Portland.
In poor neighborhoods, black patients may face additional challenges to surviving a heart attack, added Cigarroa, who wasn't involved in the study.
"They more often live in low socioeconomic segments of our community that often have less access to health care resources and less access to stores with good nutrition," Cigarroa said by email. "In addition, these segments of our community are often not ideally configured for promoting physical activity with parks, sidewalks, bike lanes, etc."
The study findings highlight a need to improve outcomes among poor and black patients and suggest some differences in heart attack survival may come down to disparities in quality of care, said senior study author Dr. Harlan Krumholz of Yale University School of Medicine in New Haven, Connecticut.
Because black patients have a greater burden of heart disease than white people, doctors may also need to focus more on prevention in this community, Krumholz said by email.
"Healthy heart habits may be even more important for African-Americans, for whom avoiding a heart attack is even more important given their worse outcomes after the event," Krumholz said.
Bipolar phenotype affects present, future self-image
Higher positivity ratings for current self-images were associated with lower depression and anxiety scores among young adults with Bipolar Spectrum Disorder (BPSD), suggesting that BPSD phenotype can shape the relationship between affect and current and future self-images.
As reported in the Journal of Affective Disorders, lead author Dr. Martina Di Simplicio of MRC Cognition and Brain Sciences Unit, Cambridge, England, and her associates assessed a nonclinical sample of 47 participants (66% female; mean age, 23) for hypomanic experiences and BPSD vulnerability, split into two groups based on their Mood Disorders Questionnaire (MDQ) score. Seventy-five percent of the participants in the high MDQ group generated at least one negative current self-image, compared with 48% of participants in the low MDQ group (P = .055).
The investigators noted that, for those with high MDQ scores, the relationship between affect and perception of the stability of negative self-images is different compared with those with low MDQ scores. And while 75% participants in the BPSD phenotype group were likely to endorse negative images of the current self (compared with less than 50% of the group without hypomanic experiences, almost none of the patients from either group had negative images of the future self).
“BPSD phenotype presents with both alterations and resilience in how self-images and mood shape each other. Further investigations could elucidate how this relationship is affected by illness progression and offer targets for early interventions based on experimental cognitive models,” the investigators wrote.
Read the article in Journal of Affective Disorders (doi: 10.1016/j.jad.2015.08.042).
Higher positivity ratings for current self-images were associated with lower depression and anxiety scores among young adults with Bipolar Spectrum Disorder (BPSD), suggesting that BPSD phenotype can shape the relationship between affect and current and future self-images.
As reported in the Journal of Affective Disorders, lead author Dr. Martina Di Simplicio of MRC Cognition and Brain Sciences Unit, Cambridge, England, and her associates assessed a nonclinical sample of 47 participants (66% female; mean age, 23) for hypomanic experiences and BPSD vulnerability, split into two groups based on their Mood Disorders Questionnaire (MDQ) score. Seventy-five percent of the participants in the high MDQ group generated at least one negative current self-image, compared with 48% of participants in the low MDQ group (P = .055).
The investigators noted that, for those with high MDQ scores, the relationship between affect and perception of the stability of negative self-images is different compared with those with low MDQ scores. And while 75% participants in the BPSD phenotype group were likely to endorse negative images of the current self (compared with less than 50% of the group without hypomanic experiences, almost none of the patients from either group had negative images of the future self).
“BPSD phenotype presents with both alterations and resilience in how self-images and mood shape each other. Further investigations could elucidate how this relationship is affected by illness progression and offer targets for early interventions based on experimental cognitive models,” the investigators wrote.
Read the article in Journal of Affective Disorders (doi: 10.1016/j.jad.2015.08.042).
Higher positivity ratings for current self-images were associated with lower depression and anxiety scores among young adults with Bipolar Spectrum Disorder (BPSD), suggesting that BPSD phenotype can shape the relationship between affect and current and future self-images.
As reported in the Journal of Affective Disorders, lead author Dr. Martina Di Simplicio of MRC Cognition and Brain Sciences Unit, Cambridge, England, and her associates assessed a nonclinical sample of 47 participants (66% female; mean age, 23) for hypomanic experiences and BPSD vulnerability, split into two groups based on their Mood Disorders Questionnaire (MDQ) score. Seventy-five percent of the participants in the high MDQ group generated at least one negative current self-image, compared with 48% of participants in the low MDQ group (P = .055).
The investigators noted that, for those with high MDQ scores, the relationship between affect and perception of the stability of negative self-images is different compared with those with low MDQ scores. And while 75% participants in the BPSD phenotype group were likely to endorse negative images of the current self (compared with less than 50% of the group without hypomanic experiences, almost none of the patients from either group had negative images of the future self).
“BPSD phenotype presents with both alterations and resilience in how self-images and mood shape each other. Further investigations could elucidate how this relationship is affected by illness progression and offer targets for early interventions based on experimental cognitive models,” the investigators wrote.
Read the article in Journal of Affective Disorders (doi: 10.1016/j.jad.2015.08.042).
FROM THE JOURNAL OF AFFECTIVE DISORDERS
CBT improves depression but not self-care in heart failure patients
Cognitive behavioral therapy significantly improved major depression but did not improve self-care by heart failure patients, investigators reported online in JAMA Internal Medicine.
“The results suggest that CBT is superior to usual care for depression in patients with heart failure,” said Dr. Kenneth Freedland and his associates at Washington University in St. Louis. They called the findings “especially encouraging” in light of recent negative results from the SADHART-CHF and MOOD-HF trials of selective serotonin reuptake inhibitors in this population.Patients in heart failure often have major depression, which increases their chances of poor self-care, hospitalization, and mortality, the researchers noted. Their single-blind, randomized trial included 158 patients who were in New York Heart Association class I, II, or III heart failure and met criteria for major depression. Patients in the intervention group received standard medical care, plus up to 6 months of CBT designed for cardiac patients.Patients received CBT weekly, then biweekly, and then monthly as they reached their treatment goals, but they also received telephone follow-up to help prevent relapse. The control group received standard medical care plus consultation with a cardiac nurse, written materials on heart failure self-care, and three follow-up phone calls with the nurse (JAMA Intern Med. 2015 Sept. 28. doi:10.1001/jamainternmed.2015.5220). At 6 months, the CBT group scored significantly lower on the BDI-II than did controls (mean score, 12.8 [standard deviation, 10.6] vs. 17.3 [10.7]; P = .008), the researchers said. Remission rates with CBT were 46% based on the BDI-II and 51% based on the Hamilton Depression Scale, both of which significantly exceeded remission rates of 19-20% for controls. The CBT group also improved significantly more than did controls on standard measures for anxiety, heart failure-related quality of life, mental health–related quality of life, fatigue, and social functioning, but not on measures of physical functioning, the researchers reported.
The National Heart, Lung, and Blood Institute partially funded the study. The researchers declared no competing interests.
When depression occurs in patients with heart failure, which is often, the illness burden and management complexity increase multifold. Freedland et al. tested the hypothesis that the effective treatment of comorbid depression with cognitive behavioral therapy (CBT) would also lead to improvements in heart failure self-care and physical functioning and found that it did not. The good news is that CBT did significantly improve emotional health and overall quality of life, and the improvement in depressive symptoms associated with CBT was larger than observed in pharmacotherapy trials for depression in patients with heart disease. This supports evidence for a shift in practice away from so much pharmacotherapy and more use of psychotherapy to achieve better mental health and overall quality of life outcomes in patients with heart failure. In reframing how we think about the management of depression in patients with heart failure, we should be talking more and prescribing less.
Dr. Patrick G. O’Malley is deputy editor of JAMA Internal Medicine. He declared no competing interests. These comments were taken from his accompanying editorial (JAMA Intern Med. 2015 Sept. 28).
When depression occurs in patients with heart failure, which is often, the illness burden and management complexity increase multifold. Freedland et al. tested the hypothesis that the effective treatment of comorbid depression with cognitive behavioral therapy (CBT) would also lead to improvements in heart failure self-care and physical functioning and found that it did not. The good news is that CBT did significantly improve emotional health and overall quality of life, and the improvement in depressive symptoms associated with CBT was larger than observed in pharmacotherapy trials for depression in patients with heart disease. This supports evidence for a shift in practice away from so much pharmacotherapy and more use of psychotherapy to achieve better mental health and overall quality of life outcomes in patients with heart failure. In reframing how we think about the management of depression in patients with heart failure, we should be talking more and prescribing less.
Dr. Patrick G. O’Malley is deputy editor of JAMA Internal Medicine. He declared no competing interests. These comments were taken from his accompanying editorial (JAMA Intern Med. 2015 Sept. 28).
When depression occurs in patients with heart failure, which is often, the illness burden and management complexity increase multifold. Freedland et al. tested the hypothesis that the effective treatment of comorbid depression with cognitive behavioral therapy (CBT) would also lead to improvements in heart failure self-care and physical functioning and found that it did not. The good news is that CBT did significantly improve emotional health and overall quality of life, and the improvement in depressive symptoms associated with CBT was larger than observed in pharmacotherapy trials for depression in patients with heart disease. This supports evidence for a shift in practice away from so much pharmacotherapy and more use of psychotherapy to achieve better mental health and overall quality of life outcomes in patients with heart failure. In reframing how we think about the management of depression in patients with heart failure, we should be talking more and prescribing less.
Dr. Patrick G. O’Malley is deputy editor of JAMA Internal Medicine. He declared no competing interests. These comments were taken from his accompanying editorial (JAMA Intern Med. 2015 Sept. 28).
Cognitive behavioral therapy significantly improved major depression but did not improve self-care by heart failure patients, investigators reported online in JAMA Internal Medicine.
“The results suggest that CBT is superior to usual care for depression in patients with heart failure,” said Dr. Kenneth Freedland and his associates at Washington University in St. Louis. They called the findings “especially encouraging” in light of recent negative results from the SADHART-CHF and MOOD-HF trials of selective serotonin reuptake inhibitors in this population.Patients in heart failure often have major depression, which increases their chances of poor self-care, hospitalization, and mortality, the researchers noted. Their single-blind, randomized trial included 158 patients who were in New York Heart Association class I, II, or III heart failure and met criteria for major depression. Patients in the intervention group received standard medical care, plus up to 6 months of CBT designed for cardiac patients.Patients received CBT weekly, then biweekly, and then monthly as they reached their treatment goals, but they also received telephone follow-up to help prevent relapse. The control group received standard medical care plus consultation with a cardiac nurse, written materials on heart failure self-care, and three follow-up phone calls with the nurse (JAMA Intern Med. 2015 Sept. 28. doi:10.1001/jamainternmed.2015.5220). At 6 months, the CBT group scored significantly lower on the BDI-II than did controls (mean score, 12.8 [standard deviation, 10.6] vs. 17.3 [10.7]; P = .008), the researchers said. Remission rates with CBT were 46% based on the BDI-II and 51% based on the Hamilton Depression Scale, both of which significantly exceeded remission rates of 19-20% for controls. The CBT group also improved significantly more than did controls on standard measures for anxiety, heart failure-related quality of life, mental health–related quality of life, fatigue, and social functioning, but not on measures of physical functioning, the researchers reported.
The National Heart, Lung, and Blood Institute partially funded the study. The researchers declared no competing interests.
Cognitive behavioral therapy significantly improved major depression but did not improve self-care by heart failure patients, investigators reported online in JAMA Internal Medicine.
“The results suggest that CBT is superior to usual care for depression in patients with heart failure,” said Dr. Kenneth Freedland and his associates at Washington University in St. Louis. They called the findings “especially encouraging” in light of recent negative results from the SADHART-CHF and MOOD-HF trials of selective serotonin reuptake inhibitors in this population.Patients in heart failure often have major depression, which increases their chances of poor self-care, hospitalization, and mortality, the researchers noted. Their single-blind, randomized trial included 158 patients who were in New York Heart Association class I, II, or III heart failure and met criteria for major depression. Patients in the intervention group received standard medical care, plus up to 6 months of CBT designed for cardiac patients.Patients received CBT weekly, then biweekly, and then monthly as they reached their treatment goals, but they also received telephone follow-up to help prevent relapse. The control group received standard medical care plus consultation with a cardiac nurse, written materials on heart failure self-care, and three follow-up phone calls with the nurse (JAMA Intern Med. 2015 Sept. 28. doi:10.1001/jamainternmed.2015.5220). At 6 months, the CBT group scored significantly lower on the BDI-II than did controls (mean score, 12.8 [standard deviation, 10.6] vs. 17.3 [10.7]; P = .008), the researchers said. Remission rates with CBT were 46% based on the BDI-II and 51% based on the Hamilton Depression Scale, both of which significantly exceeded remission rates of 19-20% for controls. The CBT group also improved significantly more than did controls on standard measures for anxiety, heart failure-related quality of life, mental health–related quality of life, fatigue, and social functioning, but not on measures of physical functioning, the researchers reported.
The National Heart, Lung, and Blood Institute partially funded the study. The researchers declared no competing interests.
FROM JAMA INTERNAL MEDICINE
Key clinical point: Cognitive behavior therapy significantly improved major depression but not self-care among patients with heart failure.
Major finding: At 6 months, mean BDI-II scores were 12.8 for CBT vs. 17.3 for enhanced usual care (P = .008).
Data source: Single-blind, randomized trial of 158 patients.
Disclosures: The National Heart, Lung, and Blood Institute partially funded the study. The researchers declared no conflicts of interest.
Tool helps patients, clinicians choose depression meds
A new tool, the Depression Medication Choice decision aid, helped adults with moderate to severe depression and their primary care physicians choose appropriate medications together, according to a report published online Sept. 28 in JAMA Internal Medicine.
Researchers developed the Depression Medication Choice (DMC) tool to enhance patient involvement in the decision-making process, in the hope that taking their preferences and circumstances into account would improve adherence and stave off premature discontinuation of antidepressants. The investigators then performed a cluster-randomized trial to assess the usefulness of the decision aid in real-world practice, said Annie LeBlanc, Ph.D., of the Knowledge and Evaluation Research Unit, Mayo Clinic, Rochester ,Minn.
The study involved 297 adults treated during a 2-year period by 117 clinicians in 10 rural, urban, and suburban private practices across Minnesota and Wisconsin. These demographically diverse patients had moderate to severe depression as measured by scores of 10 or higher on the Patent Health Questionnaire–9 and were considering antidepressant therapy. They were randomly assigned to clinicians who chose antidepressant therapy in the usual manner (139 patients in the control group) or to clinicians who used the DMC to choose antidepressant therapy together (158 patients in the intervention group).
The DMC tool comprised several laminated 10-by-25-cm cards that presented general information about antidepressant efficacy and adverse effects “in terms that matter to patients: weight change, sleep, libido, discontinuation, and cost,” as well as a leaflet for patients to take home, Dr. LeBlanc and her associates wrote.
Participating clinicians received training in using these cards to prompt discussion during a regular office consultation. Use of the decision aid did not add to the duration of office visits, which is key to routine implementation, the investigators said.
At 3- and 6-month follow-up, patients in the intervention group reported significantly greater comfort with the choice of antidepressant, with a mean difference between the two study groups of 5.3 out of a possible 100 points on a “comfort” scale. Patients in the intervention group also were more knowledgeable about antidepressants (OR, 9.5) and satisfied with their health care (RR, 1.25-2.40), compared with the control group.
Clinicians also were more comfortable with treatment decisions, with a mean difference between the two study groups of 11.4 out of 100 possible points. And clinicians who used the DMC tool reported being more satisfied with the decision-making process (RR, 1.64).
However, there were no significant differences between patients in the two groups regarding control of depression symptoms, remission rate, or rate of response to treatment, as measured by mean PHQ-9 scores. There also was no significant difference in medication adherence. Since most of the clinicians in this study used the DMC tool with very few patients, “it is possible that our trial underestimates the efficacy of the decision aid when used repeatedly and expertly,” Dr. LeBlanc and her associates noted (JAMA Intern Med. 2015 Sep 28. doi:10.10001/jamainternmed.2015.5214).
“Policy makers will have to decide whether the value of decision aids as promoters of patient-centered care and informed patient engagement, as demonstrated in this trial, argue on their own merit for priority,” they noted.
A new tool, the Depression Medication Choice decision aid, helped adults with moderate to severe depression and their primary care physicians choose appropriate medications together, according to a report published online Sept. 28 in JAMA Internal Medicine.
Researchers developed the Depression Medication Choice (DMC) tool to enhance patient involvement in the decision-making process, in the hope that taking their preferences and circumstances into account would improve adherence and stave off premature discontinuation of antidepressants. The investigators then performed a cluster-randomized trial to assess the usefulness of the decision aid in real-world practice, said Annie LeBlanc, Ph.D., of the Knowledge and Evaluation Research Unit, Mayo Clinic, Rochester ,Minn.
The study involved 297 adults treated during a 2-year period by 117 clinicians in 10 rural, urban, and suburban private practices across Minnesota and Wisconsin. These demographically diverse patients had moderate to severe depression as measured by scores of 10 or higher on the Patent Health Questionnaire–9 and were considering antidepressant therapy. They were randomly assigned to clinicians who chose antidepressant therapy in the usual manner (139 patients in the control group) or to clinicians who used the DMC to choose antidepressant therapy together (158 patients in the intervention group).
The DMC tool comprised several laminated 10-by-25-cm cards that presented general information about antidepressant efficacy and adverse effects “in terms that matter to patients: weight change, sleep, libido, discontinuation, and cost,” as well as a leaflet for patients to take home, Dr. LeBlanc and her associates wrote.
Participating clinicians received training in using these cards to prompt discussion during a regular office consultation. Use of the decision aid did not add to the duration of office visits, which is key to routine implementation, the investigators said.
At 3- and 6-month follow-up, patients in the intervention group reported significantly greater comfort with the choice of antidepressant, with a mean difference between the two study groups of 5.3 out of a possible 100 points on a “comfort” scale. Patients in the intervention group also were more knowledgeable about antidepressants (OR, 9.5) and satisfied with their health care (RR, 1.25-2.40), compared with the control group.
Clinicians also were more comfortable with treatment decisions, with a mean difference between the two study groups of 11.4 out of 100 possible points. And clinicians who used the DMC tool reported being more satisfied with the decision-making process (RR, 1.64).
However, there were no significant differences between patients in the two groups regarding control of depression symptoms, remission rate, or rate of response to treatment, as measured by mean PHQ-9 scores. There also was no significant difference in medication adherence. Since most of the clinicians in this study used the DMC tool with very few patients, “it is possible that our trial underestimates the efficacy of the decision aid when used repeatedly and expertly,” Dr. LeBlanc and her associates noted (JAMA Intern Med. 2015 Sep 28. doi:10.10001/jamainternmed.2015.5214).
“Policy makers will have to decide whether the value of decision aids as promoters of patient-centered care and informed patient engagement, as demonstrated in this trial, argue on their own merit for priority,” they noted.
A new tool, the Depression Medication Choice decision aid, helped adults with moderate to severe depression and their primary care physicians choose appropriate medications together, according to a report published online Sept. 28 in JAMA Internal Medicine.
Researchers developed the Depression Medication Choice (DMC) tool to enhance patient involvement in the decision-making process, in the hope that taking their preferences and circumstances into account would improve adherence and stave off premature discontinuation of antidepressants. The investigators then performed a cluster-randomized trial to assess the usefulness of the decision aid in real-world practice, said Annie LeBlanc, Ph.D., of the Knowledge and Evaluation Research Unit, Mayo Clinic, Rochester ,Minn.
The study involved 297 adults treated during a 2-year period by 117 clinicians in 10 rural, urban, and suburban private practices across Minnesota and Wisconsin. These demographically diverse patients had moderate to severe depression as measured by scores of 10 or higher on the Patent Health Questionnaire–9 and were considering antidepressant therapy. They were randomly assigned to clinicians who chose antidepressant therapy in the usual manner (139 patients in the control group) or to clinicians who used the DMC to choose antidepressant therapy together (158 patients in the intervention group).
The DMC tool comprised several laminated 10-by-25-cm cards that presented general information about antidepressant efficacy and adverse effects “in terms that matter to patients: weight change, sleep, libido, discontinuation, and cost,” as well as a leaflet for patients to take home, Dr. LeBlanc and her associates wrote.
Participating clinicians received training in using these cards to prompt discussion during a regular office consultation. Use of the decision aid did not add to the duration of office visits, which is key to routine implementation, the investigators said.
At 3- and 6-month follow-up, patients in the intervention group reported significantly greater comfort with the choice of antidepressant, with a mean difference between the two study groups of 5.3 out of a possible 100 points on a “comfort” scale. Patients in the intervention group also were more knowledgeable about antidepressants (OR, 9.5) and satisfied with their health care (RR, 1.25-2.40), compared with the control group.
Clinicians also were more comfortable with treatment decisions, with a mean difference between the two study groups of 11.4 out of 100 possible points. And clinicians who used the DMC tool reported being more satisfied with the decision-making process (RR, 1.64).
However, there were no significant differences between patients in the two groups regarding control of depression symptoms, remission rate, or rate of response to treatment, as measured by mean PHQ-9 scores. There also was no significant difference in medication adherence. Since most of the clinicians in this study used the DMC tool with very few patients, “it is possible that our trial underestimates the efficacy of the decision aid when used repeatedly and expertly,” Dr. LeBlanc and her associates noted (JAMA Intern Med. 2015 Sep 28. doi:10.10001/jamainternmed.2015.5214).
“Policy makers will have to decide whether the value of decision aids as promoters of patient-centered care and informed patient engagement, as demonstrated in this trial, argue on their own merit for priority,” they noted.
FROM JAMA INTERNAL MEDICINE
Key clinical point: The Depression Medication Choice decision aid helps primary care physicians choose appropriate medication together with patients who have moderate to severe depression.
Major finding: Patients in the intervention group reported significantly greater comfort with the choice of antidepressant, were more knowledgeable about antidepressants (OR, 9.5), and satisfied with their health care (RR, 1.25-2.40), compared with the control group.
Data source: A cluster-randomized trial involving 117 primary care clinicians in 10 private practices who chose antidepressant therapy for and with 297 adult patients.
Disclosures: The Agency for Healthcare and Quality Research funded the study. Dr. LeBlanc and her associates reported having no relevant disclosures.
Is There a Link Between Diabetes and Bone Health?
Diabetes can pose serious complications to bone health. “Clinical trials have revealed a startling elevation in fracture risk in diabetic patients,” says Liyun Wang, PhD, Associate Professor of Mechanical Engineering at the University of Delaware in Newark, Delaware. “Bone fractures can be life threatening — nearly 1 in 6 hip fracture patients dies within a year of injury.”
Because physical exercise is proven to improve bone properties and reduce fracture risk in non-diabetic people, Dr. Wang and colleagues tested its efficacy in type 1 diabetes. Their findings were published online ahead of print July 13 in Bone.
The researchers hypothesized that diabetic bone’s response to anabolic mechanical loading would be attenuated, partially due to impaired mechanosensing of osteocytes under hyperglycemia. For their study, heterozygous male and female diabetic mice and their age- and gender-matched wild-type controls were subjected to unilateral axial ulnar loading with a peak strain of 3500 με at 2 Hz and 3 minutes per day for 5 days.
Overall, the study demonstrated that exercise-induced bone formation was maintained in mildly diabetic mice at a similar level as non-diabetic controls, while the positive effects of exercise were nearly abolished in severely diabetic mice. At the cellular level, the researchers found that hyperglycemia reduced the sensitivity of osteocytes to mechanical stimulation and suppressed osteocytes’ secretion of proteins and signaling molecules that help build stronger bone.
“Our work demonstrates that diabetic bone can respond to exercise when the hyperglycemia is not severe, which suggests that mechanical interventions may be useful to improve bone health and reduce fracture risk in mildly affected diabetic patients,” said Dr. Wang. These results, along with previous findings showing adverse effects of hyperglycemia on osteoblasts and mesenchymal stem cells, suggest that failure to maintain normal glucose levels may impair bone’s responses to mechanical loading in diabetics.
To translate the findings of the study to patient care, Ms. Wang’s team has begun to collaborate with M. James Lenhard, MD, Director of the Center for Diabetes and Metabolic Diseases at Christiana Care Health System in Wilmington, Delaware.
“The plan for collaboration between the University of Delaware and Christiana Care is to evaluate these research findings in humans and expand the research to include other complications of diabetes, such as cardiovascular disease.
Suggested Reading
Parajuli A, Liu C, Wen L, et al. Bone’s responses to mechanical loading are impaired in type 1 diabetes. Bone. 2015 July 13 [Epub ahead of print].
Diabetes can pose serious complications to bone health. “Clinical trials have revealed a startling elevation in fracture risk in diabetic patients,” says Liyun Wang, PhD, Associate Professor of Mechanical Engineering at the University of Delaware in Newark, Delaware. “Bone fractures can be life threatening — nearly 1 in 6 hip fracture patients dies within a year of injury.”
Because physical exercise is proven to improve bone properties and reduce fracture risk in non-diabetic people, Dr. Wang and colleagues tested its efficacy in type 1 diabetes. Their findings were published online ahead of print July 13 in Bone.
The researchers hypothesized that diabetic bone’s response to anabolic mechanical loading would be attenuated, partially due to impaired mechanosensing of osteocytes under hyperglycemia. For their study, heterozygous male and female diabetic mice and their age- and gender-matched wild-type controls were subjected to unilateral axial ulnar loading with a peak strain of 3500 με at 2 Hz and 3 minutes per day for 5 days.
Overall, the study demonstrated that exercise-induced bone formation was maintained in mildly diabetic mice at a similar level as non-diabetic controls, while the positive effects of exercise were nearly abolished in severely diabetic mice. At the cellular level, the researchers found that hyperglycemia reduced the sensitivity of osteocytes to mechanical stimulation and suppressed osteocytes’ secretion of proteins and signaling molecules that help build stronger bone.
“Our work demonstrates that diabetic bone can respond to exercise when the hyperglycemia is not severe, which suggests that mechanical interventions may be useful to improve bone health and reduce fracture risk in mildly affected diabetic patients,” said Dr. Wang. These results, along with previous findings showing adverse effects of hyperglycemia on osteoblasts and mesenchymal stem cells, suggest that failure to maintain normal glucose levels may impair bone’s responses to mechanical loading in diabetics.
To translate the findings of the study to patient care, Ms. Wang’s team has begun to collaborate with M. James Lenhard, MD, Director of the Center for Diabetes and Metabolic Diseases at Christiana Care Health System in Wilmington, Delaware.
“The plan for collaboration between the University of Delaware and Christiana Care is to evaluate these research findings in humans and expand the research to include other complications of diabetes, such as cardiovascular disease.
Diabetes can pose serious complications to bone health. “Clinical trials have revealed a startling elevation in fracture risk in diabetic patients,” says Liyun Wang, PhD, Associate Professor of Mechanical Engineering at the University of Delaware in Newark, Delaware. “Bone fractures can be life threatening — nearly 1 in 6 hip fracture patients dies within a year of injury.”
Because physical exercise is proven to improve bone properties and reduce fracture risk in non-diabetic people, Dr. Wang and colleagues tested its efficacy in type 1 diabetes. Their findings were published online ahead of print July 13 in Bone.
The researchers hypothesized that diabetic bone’s response to anabolic mechanical loading would be attenuated, partially due to impaired mechanosensing of osteocytes under hyperglycemia. For their study, heterozygous male and female diabetic mice and their age- and gender-matched wild-type controls were subjected to unilateral axial ulnar loading with a peak strain of 3500 με at 2 Hz and 3 minutes per day for 5 days.
Overall, the study demonstrated that exercise-induced bone formation was maintained in mildly diabetic mice at a similar level as non-diabetic controls, while the positive effects of exercise were nearly abolished in severely diabetic mice. At the cellular level, the researchers found that hyperglycemia reduced the sensitivity of osteocytes to mechanical stimulation and suppressed osteocytes’ secretion of proteins and signaling molecules that help build stronger bone.
“Our work demonstrates that diabetic bone can respond to exercise when the hyperglycemia is not severe, which suggests that mechanical interventions may be useful to improve bone health and reduce fracture risk in mildly affected diabetic patients,” said Dr. Wang. These results, along with previous findings showing adverse effects of hyperglycemia on osteoblasts and mesenchymal stem cells, suggest that failure to maintain normal glucose levels may impair bone’s responses to mechanical loading in diabetics.
To translate the findings of the study to patient care, Ms. Wang’s team has begun to collaborate with M. James Lenhard, MD, Director of the Center for Diabetes and Metabolic Diseases at Christiana Care Health System in Wilmington, Delaware.
“The plan for collaboration between the University of Delaware and Christiana Care is to evaluate these research findings in humans and expand the research to include other complications of diabetes, such as cardiovascular disease.
Suggested Reading
Parajuli A, Liu C, Wen L, et al. Bone’s responses to mechanical loading are impaired in type 1 diabetes. Bone. 2015 July 13 [Epub ahead of print].
Suggested Reading
Parajuli A, Liu C, Wen L, et al. Bone’s responses to mechanical loading are impaired in type 1 diabetes. Bone. 2015 July 13 [Epub ahead of print].
Generalized, well-dispersed rash • wheal development after tactile irritation • normal vital signs • Dx?
THE CASE
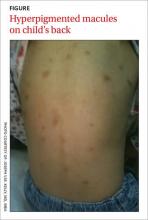
A 3-year-old girl was brought to our clinic with a generalized rash over her scalp, face, neck, chest, abdomen, back, perianal area, extremities, and the plantar surface of her right foot. On physical examination, we noted many round, hyperpigmented, brown and reddish pink, well-circumscribed macules on her body (FIGURE). Only a few of these macules had appeared on the girl’s trunk within the first 3 months of her life, but since then they’d increased in number and spread to other parts of her body as she’d aged. The lesions became edematous and erythematous with tactile irritation. Darier’s sign (the development of a hive or wheal when a lesion is stroked) was present.
The patient’s vitals at the time of examination included a temperature of 98.2°F, respiratory rate of 17 breaths/min, heart rate of 92 beats/min, blood pressure of 100/66 mm Hg, and oxygen saturation level of 100% on room air. The girl’s parents said they hadn’t traveled. There was no mucosal involvement and no systemic involvement. The patient had no past surgical or medical history, was not taking any medications, and had no significant birth history. A skin biopsy was performed.
THE DIAGNOSIS
Based on the presence of a positive Darier’s sign and the results of the skin biopsy (which showed increased mast cells), we diagnosed urticaria pigmentosa (UP), which is the most common form of cutaneous mastocytosis.1
The diagnosis had been delayed for almost 3 years because of several factors. For one thing, there had been few lesions present early in the child’s life, and as a result, the parents chalked them up to “beauty marks.” Then, as the lesions started to increase in number, the parents thought bed bugs were to blame.
As time went on, the parents attempted to treat their daughter’s hives with homeopathic remedies suggested by family members. When the lesions didn’t resolve with homeopathic remedies, the parents tried over-the-counter H1 and H2 antihistamines such as diphenhydramine, loratadine, and ranitidine. When these treatments failed, the parents brought their child to our office for medical evaluation.
DISCUSSION
UP is a chronic skin disorder in which there is an abnormal proliferation of mast cells in the dermis of the skin. It is considered an orphan disease.1 UP that presents in children is most often benign. Approximately 50% of cases occur before 6 months of age and 25% occur before puberty.2 The lesions are often self-limited and completely resolve in approximately 50% of patients by puberty.3 By adulthood, the lesions either resolve or some lightly colored non-urticating macules remain; however, some patients will continue to have a positive Darier’s sign.4
Dermatologic symptoms. A patient with UP may present with brown or reddish maculopapules, papules, nodules, pruritus, and flushing of the face. Darier’s sign is usually seen in cases of UP; in a study of mastocytosis in children, Darier’s sign was present in 94% of cases.5 Lesions are more prominent in areas where clothes can rub the skin, and they often vary in size and appearance. The presence of lesions can vary from localized and scant to hundreds located over the entire body. UP can be difficult to identify when the lesions are limited, which can lead to delayed diagnosis and treatment.6
Systemic involvement. UP also can affect the skeleton, bone marrow, liver, spleen, lymph nodes, gastrointestinal tract, kidneys, cardiovascular system, and/or central nervous system. Skeletal involvement may manifest as osteoporosis or bone pain in 10% to 20% of patients with UP.7 Bone marrow involvement may progress to anemia or mast cell leukemia. UP can result in hepatomegaly or enlarged lymph nodes. Patients may experience nausea, diarrhea, or abdominal pain if the gastrointestinal tract is affected. Cardiovascular involvement may manifest as tachycardia and shock.8
Diagnosis of UP is made based on the physical exam findings noted earlier, as well as skin biopsy laboratory results. Skin biopsy will reveal increased mast cells. In up to two-thirds of patients who have systemic involvement, laboratory testing will show elevated urine histamine levels, as well as elevated serum concentrations of tryptase.9
UP can appear similar to many other skin conditions
The differential diagnosis of UP can include urticaria, atopic dermatitis, contact dermatitis, pityriasis rosea, an allergic reaction/drug eruption, Henoch-Schonlein purpura, erythema multiforme, fifth disease, folliculitis, guttate psoriasis, miliaria rubra, insect bites, viral exanthem, lichen planus, and scabies.10,11 In addition to the clinical appearance of the rash, these conditions can be distinguished from UP by skin biopsy and other relevant tests, as well as a thorough history.
Treatment options include antihistamines, corticosteroids, PUVA
Patients with UP should be instructed to avoid precipitating factors such as temperature changes, friction, alcohol ingestion, aspirin, physical exertion, or opiates. Treatment options include H1 and H2 antihistamines, cromolyn sodium, topical corticosteroids, and PUVA (psoralen plus ultraviolet A photochemotherapy).3 PUVA is normally avoided in pediatric patients because it is associated with an increased risk of skin cancer later in life.12
Our patient. We prescribed a topical corticosteroid, 0.05% betamethasone dipropionate cream, and oral cromolyn sodium 100 mg qid for our patient, but this failed to significantly improve the macules. The patient and parents grew increasingly anxious. Ultimately, the parents decided to have their daughter treated with PUVA in limited amounts. Topical psoralen was also used. After 2 months of treatment, the patient’s lesions substantially improved and many of them disappeared. In addition, the parents were educated on the importance of sunscreen and limiting their daughter’s exposure to the sun, when possible.
THE TAKEAWAY
UP can be diagnosed by taking a thorough history and conducting a physical examination; a skin biopsy that reveals increased mast cells will confirm the diagnosis. UP is usually self-limited and resolves in about one-half of patients by puberty. Treatment options include H1 and H2 antihistamines, cromolyn sodium, topical corticosteroids, and PUVA. Patients should be referred to a specialist if their symptoms become severe, systemic UP is suspected, or they do not respond to therapy.
1. Lain EL, Hsu S. Photo quiz. Chronic, papular rash that develops a wheal when rubbed. Am Fam Physician. 2004;69:1493-1494.
2. Jain S. Dermatology: Illustrated Study Guide and Comprehensive Board Review. New York: Springer. 2012;47.
3. Soter NA. The skin in mastocytosis. J Invest Dermatol. 1991;96:32S-38S; discussion 38S-39S.
4. Caplan RM. Urticaria pigmentosa and systemic mastocytosis. JAMA. 1965;194:1077-1080.
5. Kiszewski AE, Durán-Mckinster C, Orozco-Covarrubias L, et al. Cutaneous mastocytosis in children: a clinical analysis of 71 cases. J Eur Acad Dermatol Venereol. 2004;18:285-290.
6. Alto WA, Clarcq L. Cutaneous and systemic manifestations of mastocytosis. Am Fam Physician. 1999;59:3047-3054, 3059-3060.
7. Borenstein DG, Wiesel SW, Boden SD, eds. Low Back and Neck Pain: Comprehensive Diagnosis and Management. 3rd ed. Philadelphia, Pa: Elsevier; 2004.
8. Vigorita VJ. Metabolic bone disease: Part II. In: Vigorita VJ, Ghelman B, Mintz D, eds. Orthopaedic Pathology. 2nd ed. Philadelphia, PA: Walter Kluwer Lippincott Williams & Wilkins. 2008;197.
9. Rosenbaum RC, Frieri M, Metcalfe DD. Patterns of skeletal scintigraphy and their relationship to plasma and urinary histamine levels in systemic mastocytosis. J Nucl Med. 1984;25:859-864.
10. Islas AA, Penaranda E. Generalized brownish macules in infancy. Urticaria pigmentosa. Am Fam Physician. 2009;80:987.
11. Ely JW, Seabury Stone M. The generalized rash: part I. Differential diagnosis. Am Fam Physician. 2010;81:726-734.
12. Archier E, Devaux S, Castela E, et al. Carcinogenic risks of psoralen UV-A therapy and narrowband UV-B therapy in chronic plaque psoriasis: a systematic literature review. J Eur Acad Dermatol Venereol. 2012;26 Suppl 3:22-31.
THE CASE
A 3-year-old girl was brought to our clinic with a generalized rash over her scalp, face, neck, chest, abdomen, back, perianal area, extremities, and the plantar surface of her right foot. On physical examination, we noted many round, hyperpigmented, brown and reddish pink, well-circumscribed macules on her body (FIGURE). Only a few of these macules had appeared on the girl’s trunk within the first 3 months of her life, but since then they’d increased in number and spread to other parts of her body as she’d aged. The lesions became edematous and erythematous with tactile irritation. Darier’s sign (the development of a hive or wheal when a lesion is stroked) was present.
The patient’s vitals at the time of examination included a temperature of 98.2°F, respiratory rate of 17 breaths/min, heart rate of 92 beats/min, blood pressure of 100/66 mm Hg, and oxygen saturation level of 100% on room air. The girl’s parents said they hadn’t traveled. There was no mucosal involvement and no systemic involvement. The patient had no past surgical or medical history, was not taking any medications, and had no significant birth history. A skin biopsy was performed.
THE DIAGNOSIS
Based on the presence of a positive Darier’s sign and the results of the skin biopsy (which showed increased mast cells), we diagnosed urticaria pigmentosa (UP), which is the most common form of cutaneous mastocytosis.1
The diagnosis had been delayed for almost 3 years because of several factors. For one thing, there had been few lesions present early in the child’s life, and as a result, the parents chalked them up to “beauty marks.” Then, as the lesions started to increase in number, the parents thought bed bugs were to blame.
As time went on, the parents attempted to treat their daughter’s hives with homeopathic remedies suggested by family members. When the lesions didn’t resolve with homeopathic remedies, the parents tried over-the-counter H1 and H2 antihistamines such as diphenhydramine, loratadine, and ranitidine. When these treatments failed, the parents brought their child to our office for medical evaluation.
DISCUSSION
UP is a chronic skin disorder in which there is an abnormal proliferation of mast cells in the dermis of the skin. It is considered an orphan disease.1 UP that presents in children is most often benign. Approximately 50% of cases occur before 6 months of age and 25% occur before puberty.2 The lesions are often self-limited and completely resolve in approximately 50% of patients by puberty.3 By adulthood, the lesions either resolve or some lightly colored non-urticating macules remain; however, some patients will continue to have a positive Darier’s sign.4
Dermatologic symptoms. A patient with UP may present with brown or reddish maculopapules, papules, nodules, pruritus, and flushing of the face. Darier’s sign is usually seen in cases of UP; in a study of mastocytosis in children, Darier’s sign was present in 94% of cases.5 Lesions are more prominent in areas where clothes can rub the skin, and they often vary in size and appearance. The presence of lesions can vary from localized and scant to hundreds located over the entire body. UP can be difficult to identify when the lesions are limited, which can lead to delayed diagnosis and treatment.6
Systemic involvement. UP also can affect the skeleton, bone marrow, liver, spleen, lymph nodes, gastrointestinal tract, kidneys, cardiovascular system, and/or central nervous system. Skeletal involvement may manifest as osteoporosis or bone pain in 10% to 20% of patients with UP.7 Bone marrow involvement may progress to anemia or mast cell leukemia. UP can result in hepatomegaly or enlarged lymph nodes. Patients may experience nausea, diarrhea, or abdominal pain if the gastrointestinal tract is affected. Cardiovascular involvement may manifest as tachycardia and shock.8
Diagnosis of UP is made based on the physical exam findings noted earlier, as well as skin biopsy laboratory results. Skin biopsy will reveal increased mast cells. In up to two-thirds of patients who have systemic involvement, laboratory testing will show elevated urine histamine levels, as well as elevated serum concentrations of tryptase.9
UP can appear similar to many other skin conditions
The differential diagnosis of UP can include urticaria, atopic dermatitis, contact dermatitis, pityriasis rosea, an allergic reaction/drug eruption, Henoch-Schonlein purpura, erythema multiforme, fifth disease, folliculitis, guttate psoriasis, miliaria rubra, insect bites, viral exanthem, lichen planus, and scabies.10,11 In addition to the clinical appearance of the rash, these conditions can be distinguished from UP by skin biopsy and other relevant tests, as well as a thorough history.
Treatment options include antihistamines, corticosteroids, PUVA
Patients with UP should be instructed to avoid precipitating factors such as temperature changes, friction, alcohol ingestion, aspirin, physical exertion, or opiates. Treatment options include H1 and H2 antihistamines, cromolyn sodium, topical corticosteroids, and PUVA (psoralen plus ultraviolet A photochemotherapy).3 PUVA is normally avoided in pediatric patients because it is associated with an increased risk of skin cancer later in life.12
Our patient. We prescribed a topical corticosteroid, 0.05% betamethasone dipropionate cream, and oral cromolyn sodium 100 mg qid for our patient, but this failed to significantly improve the macules. The patient and parents grew increasingly anxious. Ultimately, the parents decided to have their daughter treated with PUVA in limited amounts. Topical psoralen was also used. After 2 months of treatment, the patient’s lesions substantially improved and many of them disappeared. In addition, the parents were educated on the importance of sunscreen and limiting their daughter’s exposure to the sun, when possible.
THE TAKEAWAY
UP can be diagnosed by taking a thorough history and conducting a physical examination; a skin biopsy that reveals increased mast cells will confirm the diagnosis. UP is usually self-limited and resolves in about one-half of patients by puberty. Treatment options include H1 and H2 antihistamines, cromolyn sodium, topical corticosteroids, and PUVA. Patients should be referred to a specialist if their symptoms become severe, systemic UP is suspected, or they do not respond to therapy.
THE CASE
A 3-year-old girl was brought to our clinic with a generalized rash over her scalp, face, neck, chest, abdomen, back, perianal area, extremities, and the plantar surface of her right foot. On physical examination, we noted many round, hyperpigmented, brown and reddish pink, well-circumscribed macules on her body (FIGURE). Only a few of these macules had appeared on the girl’s trunk within the first 3 months of her life, but since then they’d increased in number and spread to other parts of her body as she’d aged. The lesions became edematous and erythematous with tactile irritation. Darier’s sign (the development of a hive or wheal when a lesion is stroked) was present.
The patient’s vitals at the time of examination included a temperature of 98.2°F, respiratory rate of 17 breaths/min, heart rate of 92 beats/min, blood pressure of 100/66 mm Hg, and oxygen saturation level of 100% on room air. The girl’s parents said they hadn’t traveled. There was no mucosal involvement and no systemic involvement. The patient had no past surgical or medical history, was not taking any medications, and had no significant birth history. A skin biopsy was performed.
THE DIAGNOSIS
Based on the presence of a positive Darier’s sign and the results of the skin biopsy (which showed increased mast cells), we diagnosed urticaria pigmentosa (UP), which is the most common form of cutaneous mastocytosis.1
The diagnosis had been delayed for almost 3 years because of several factors. For one thing, there had been few lesions present early in the child’s life, and as a result, the parents chalked them up to “beauty marks.” Then, as the lesions started to increase in number, the parents thought bed bugs were to blame.
As time went on, the parents attempted to treat their daughter’s hives with homeopathic remedies suggested by family members. When the lesions didn’t resolve with homeopathic remedies, the parents tried over-the-counter H1 and H2 antihistamines such as diphenhydramine, loratadine, and ranitidine. When these treatments failed, the parents brought their child to our office for medical evaluation.
DISCUSSION
UP is a chronic skin disorder in which there is an abnormal proliferation of mast cells in the dermis of the skin. It is considered an orphan disease.1 UP that presents in children is most often benign. Approximately 50% of cases occur before 6 months of age and 25% occur before puberty.2 The lesions are often self-limited and completely resolve in approximately 50% of patients by puberty.3 By adulthood, the lesions either resolve or some lightly colored non-urticating macules remain; however, some patients will continue to have a positive Darier’s sign.4
Dermatologic symptoms. A patient with UP may present with brown or reddish maculopapules, papules, nodules, pruritus, and flushing of the face. Darier’s sign is usually seen in cases of UP; in a study of mastocytosis in children, Darier’s sign was present in 94% of cases.5 Lesions are more prominent in areas where clothes can rub the skin, and they often vary in size and appearance. The presence of lesions can vary from localized and scant to hundreds located over the entire body. UP can be difficult to identify when the lesions are limited, which can lead to delayed diagnosis and treatment.6
Systemic involvement. UP also can affect the skeleton, bone marrow, liver, spleen, lymph nodes, gastrointestinal tract, kidneys, cardiovascular system, and/or central nervous system. Skeletal involvement may manifest as osteoporosis or bone pain in 10% to 20% of patients with UP.7 Bone marrow involvement may progress to anemia or mast cell leukemia. UP can result in hepatomegaly or enlarged lymph nodes. Patients may experience nausea, diarrhea, or abdominal pain if the gastrointestinal tract is affected. Cardiovascular involvement may manifest as tachycardia and shock.8
Diagnosis of UP is made based on the physical exam findings noted earlier, as well as skin biopsy laboratory results. Skin biopsy will reveal increased mast cells. In up to two-thirds of patients who have systemic involvement, laboratory testing will show elevated urine histamine levels, as well as elevated serum concentrations of tryptase.9
UP can appear similar to many other skin conditions
The differential diagnosis of UP can include urticaria, atopic dermatitis, contact dermatitis, pityriasis rosea, an allergic reaction/drug eruption, Henoch-Schonlein purpura, erythema multiforme, fifth disease, folliculitis, guttate psoriasis, miliaria rubra, insect bites, viral exanthem, lichen planus, and scabies.10,11 In addition to the clinical appearance of the rash, these conditions can be distinguished from UP by skin biopsy and other relevant tests, as well as a thorough history.
Treatment options include antihistamines, corticosteroids, PUVA
Patients with UP should be instructed to avoid precipitating factors such as temperature changes, friction, alcohol ingestion, aspirin, physical exertion, or opiates. Treatment options include H1 and H2 antihistamines, cromolyn sodium, topical corticosteroids, and PUVA (psoralen plus ultraviolet A photochemotherapy).3 PUVA is normally avoided in pediatric patients because it is associated with an increased risk of skin cancer later in life.12
Our patient. We prescribed a topical corticosteroid, 0.05% betamethasone dipropionate cream, and oral cromolyn sodium 100 mg qid for our patient, but this failed to significantly improve the macules. The patient and parents grew increasingly anxious. Ultimately, the parents decided to have their daughter treated with PUVA in limited amounts. Topical psoralen was also used. After 2 months of treatment, the patient’s lesions substantially improved and many of them disappeared. In addition, the parents were educated on the importance of sunscreen and limiting their daughter’s exposure to the sun, when possible.
THE TAKEAWAY
UP can be diagnosed by taking a thorough history and conducting a physical examination; a skin biopsy that reveals increased mast cells will confirm the diagnosis. UP is usually self-limited and resolves in about one-half of patients by puberty. Treatment options include H1 and H2 antihistamines, cromolyn sodium, topical corticosteroids, and PUVA. Patients should be referred to a specialist if their symptoms become severe, systemic UP is suspected, or they do not respond to therapy.
1. Lain EL, Hsu S. Photo quiz. Chronic, papular rash that develops a wheal when rubbed. Am Fam Physician. 2004;69:1493-1494.
2. Jain S. Dermatology: Illustrated Study Guide and Comprehensive Board Review. New York: Springer. 2012;47.
3. Soter NA. The skin in mastocytosis. J Invest Dermatol. 1991;96:32S-38S; discussion 38S-39S.
4. Caplan RM. Urticaria pigmentosa and systemic mastocytosis. JAMA. 1965;194:1077-1080.
5. Kiszewski AE, Durán-Mckinster C, Orozco-Covarrubias L, et al. Cutaneous mastocytosis in children: a clinical analysis of 71 cases. J Eur Acad Dermatol Venereol. 2004;18:285-290.
6. Alto WA, Clarcq L. Cutaneous and systemic manifestations of mastocytosis. Am Fam Physician. 1999;59:3047-3054, 3059-3060.
7. Borenstein DG, Wiesel SW, Boden SD, eds. Low Back and Neck Pain: Comprehensive Diagnosis and Management. 3rd ed. Philadelphia, Pa: Elsevier; 2004.
8. Vigorita VJ. Metabolic bone disease: Part II. In: Vigorita VJ, Ghelman B, Mintz D, eds. Orthopaedic Pathology. 2nd ed. Philadelphia, PA: Walter Kluwer Lippincott Williams & Wilkins. 2008;197.
9. Rosenbaum RC, Frieri M, Metcalfe DD. Patterns of skeletal scintigraphy and their relationship to plasma and urinary histamine levels in systemic mastocytosis. J Nucl Med. 1984;25:859-864.
10. Islas AA, Penaranda E. Generalized brownish macules in infancy. Urticaria pigmentosa. Am Fam Physician. 2009;80:987.
11. Ely JW, Seabury Stone M. The generalized rash: part I. Differential diagnosis. Am Fam Physician. 2010;81:726-734.
12. Archier E, Devaux S, Castela E, et al. Carcinogenic risks of psoralen UV-A therapy and narrowband UV-B therapy in chronic plaque psoriasis: a systematic literature review. J Eur Acad Dermatol Venereol. 2012;26 Suppl 3:22-31.
1. Lain EL, Hsu S. Photo quiz. Chronic, papular rash that develops a wheal when rubbed. Am Fam Physician. 2004;69:1493-1494.
2. Jain S. Dermatology: Illustrated Study Guide and Comprehensive Board Review. New York: Springer. 2012;47.
3. Soter NA. The skin in mastocytosis. J Invest Dermatol. 1991;96:32S-38S; discussion 38S-39S.
4. Caplan RM. Urticaria pigmentosa and systemic mastocytosis. JAMA. 1965;194:1077-1080.
5. Kiszewski AE, Durán-Mckinster C, Orozco-Covarrubias L, et al. Cutaneous mastocytosis in children: a clinical analysis of 71 cases. J Eur Acad Dermatol Venereol. 2004;18:285-290.
6. Alto WA, Clarcq L. Cutaneous and systemic manifestations of mastocytosis. Am Fam Physician. 1999;59:3047-3054, 3059-3060.
7. Borenstein DG, Wiesel SW, Boden SD, eds. Low Back and Neck Pain: Comprehensive Diagnosis and Management. 3rd ed. Philadelphia, Pa: Elsevier; 2004.
8. Vigorita VJ. Metabolic bone disease: Part II. In: Vigorita VJ, Ghelman B, Mintz D, eds. Orthopaedic Pathology. 2nd ed. Philadelphia, PA: Walter Kluwer Lippincott Williams & Wilkins. 2008;197.
9. Rosenbaum RC, Frieri M, Metcalfe DD. Patterns of skeletal scintigraphy and their relationship to plasma and urinary histamine levels in systemic mastocytosis. J Nucl Med. 1984;25:859-864.
10. Islas AA, Penaranda E. Generalized brownish macules in infancy. Urticaria pigmentosa. Am Fam Physician. 2009;80:987.
11. Ely JW, Seabury Stone M. The generalized rash: part I. Differential diagnosis. Am Fam Physician. 2010;81:726-734.
12. Archier E, Devaux S, Castela E, et al. Carcinogenic risks of psoralen UV-A therapy and narrowband UV-B therapy in chronic plaque psoriasis: a systematic literature review. J Eur Acad Dermatol Venereol. 2012;26 Suppl 3:22-31.
Infection prevention
Not a long ago, I received a call from a friend working in a local pediatric clinic. One of her partners had just seen a young child with an unusual rash. The diagnosis? Crusted scabies.
Sarcoptes scabiei var. hominis, the mite that causes typical scabies, also causes crusted or Norwegian scabies. These terms refer to severe infestations that occur in individuals who are immune compromised or debilitated. The rash is characterized by vesicles and thick crusts and may or may not be itchy. Because patients with crusted scabies can be infested with as many as 2 million mites, transmission from very brief skin-to-skin contact is possible, and outbreaks have occurred in health care facilities and other institutional settings.
That was the reason for my friend’s call. “What do we do for the doctors and nurses in the clinic who saw the patient?” she wanted to know.
“Everyone wore gloves, right?” I asked. There was silence on the other end of the phone.
After a quick consultation with our health department, every health care provider (HCP) who touched the patient without gloves was treated preemptively with topical permethrin. None went on to develop scabies. The experience prompted me to think about the challenges of infection prevention in ambulatory care.
Both the American Academy of Pediatrics (AAP Committee on Infectious Diseases, “Infection prevention and control in pediatric ambulatory settings,” Pediatrics 2007;20[3]:650-65) and the Centers for Disease Control and Prevention (Guide to Infection Prevention for Outpatient Settings: Minimum Expectations for Safe Care) have published recommendations for infection prevention in outpatient settings. Both organizations emphasize the importance of standard precautions. According to the CDC, standard precautions “are the minimum infection prevention practices that apply to all patient care, regardless of suspected or confirmed infection status of the patient, in any setting where health care is delivered.” They are designed to protect HCPs, as well as prevent us from spreading infections among patients. Standard precautions include:
• Hand hygiene.
• Use of personal protective equipment (gloves, gowns, masks).
• Safe injection practices.
• Safe handling of potentially contaminated equipment or surfaces in the patient environment.
• Respiratory hygiene/cough etiquette.
Some of these elements are likely second nature to office-based pediatricians. Hands must be cleaned before and after every patient encounter or an encounter with the patient’s immediate environment. “Cover your cough” signs have become ubiquitous in ambulatory care waiting rooms, even as we acknowledge the difficulties associated with expecting toddlers to wear masks or use a tissue to contain their coughs and sneezes.
Other elements of standard precautions may receive increased attention because the consequences of noncompliance are perceived to be dangerous or severe. For example, we know that failure to reliably employ safe injection practices (see table) has resulted in transmission of blood-borne pathogens, including hepatitis B and C, in ambulatory settings.
In my experience, the use of personal protective equipment (PPE) in the ambulatory setting is the element of standard precautions that is the least understood and perhaps the most underutilized. It’s certainly easier in the inpatient setting, where we use transmission-based precautions, and colorful isolation signs instruct us to put on gown and gloves when we visit the patient with viral gastroenteritis, or gown, gloves, and mask for the child with acute viral respiratory tract infection. In the office, we expect the HCP to anticipate what kind of contact with blood or body fluids is likely and choose PPE accordingly.
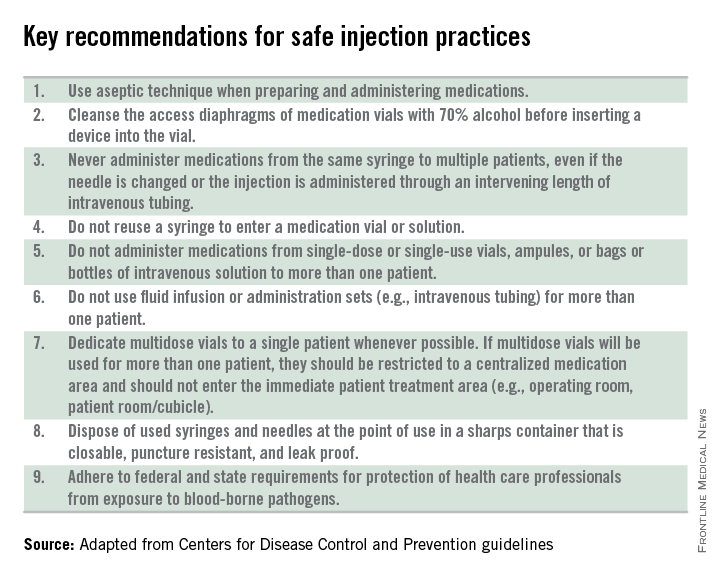
Of course, anticipation can be tricky. Gowns, for example, are only required during procedures or activities when contact with blood and body fluids is likely. In routine office-based care, these sorts of procedures are uncommon. Incision and drainage of an abscess is one example of a procedure that might warrant protection of one’s clothing with a gown. Conversely, the need for a mask might arise several times a day, as these are worn to protect the mouth, nose, and eyes “during procedures that are likely to generate splashes or sprays of blood or other body fluids.” Examination of a coughing patient is a common “procedure” likely to results in sprays of saliva. Use of a mask can protect the examiner from potential exposures to Bordetella pertussis, Mycoplasma pneumoniae, and a host of respiratory viruses.
While the AAP has been careful to point out that gloves are not needed for the routine care of well children, they should be used when “there is the potential to contact blood, body fluids, mucous membranes, nonintact skin, or potentially infectious material.” In our world, potentially infectious material might include a cluster of vesicles thought to be herpes simplex, the honey-crusted lesions of impetigo, or the weeping, crusted rash of Norwegian scabies.
My own office had a powerful reminder about the importance of standard precautions last year when we were referred a young infant with recurrent fevers and a mostly dry, peeling rash. As we learned in medical school, the mucocutanous lesions of congenital syphilis can be highly contagious. In accordance with AAP recommendations, all HCPs who examined this child without the protection of gloves underwent serologic testing for syphilis. Fortunately, there were no transmissions!
Published data about infectious disease exposures and the transmission of infectious diseases in the outpatient setting, either from patients to health care workers or among patients, are largely limited to outbreak or case reports. A 1991 review identified 53 reports of infectious disease transmission in outpatient settings between 1961 and 1990 (JAMA 1991;265(18): 2377-81). Transmission occurred in medical and dental offices, clinics, emergency departments, ophthalmology offices, and alternative care settings that included chiropractic clinics and an acupuncture practice. A variety of pathogens were involved, including measles, adenovirus, hepatitis B, atypical mycobacteria, and Streptococcus pyogenes. The authors concluded that many of the outbreaks and episodes of transmission could have been prevented “if existing infection control guidelines,” including what we now consider standard precautions, had been utilized. Many reports published in the intervening 25 years have come to similar conclusions.
So why don’t HCPs yet follow standard precautions, including appropriate use of PPE? The reasons are complex and multifactorial. We’re all busy and lack of time is a common complaint. Gowns, gloves, masks, and alcohol hand gel aren’t always readily available. Some HCPs may not be knowledgeable about the elements of standard precautions while others may not understand the risks to themselves and their patients associated with nonadherence. Finally, some organizations have not established clear expectations related to infection prevention and compliance with AAP and CDC recommendations.
Several years ago, at the very beginning of the H1N1 influenza epidemic, a colleague of mine working in a pediatric practice saw a patient complaining of fever, lethargy, and myalgia. Not surprisingly, the patient’s rapid influenza test was positive. My colleague recalls that she was handed the result before she ever walked into the room – without any PPE – to see the patient.
“This was different than my usual routine at the hospital,” she told me. The expectation at the hospital was gown, gloves, and masks for any patient with influenza or influenzalike illness. At the office though, there was no such expectation, and providers did not routinely wear masks, even when seeing patients with respiratory symptoms. My colleague wasn’t reckless or rebellious. She was simply conforming to the culture in that office, and following the behavioral cues of more senior physicians in the practice. Subsequently, she developed severe influenza infection requiring a prolonged hospital stay.
It’s time to change the culture. As a first step, perform a quick audit in the office, using the AAP’s “Infection prevention and control in pediatric ambulatory settings” as a guide.
Dr. Bryant is a pediatrician specializing in infectious diseases at the University of Louisville (Ky.) and Kosair Children’s Hospital, also in Louisville. She had no relevant financial disclosures.
Not a long ago, I received a call from a friend working in a local pediatric clinic. One of her partners had just seen a young child with an unusual rash. The diagnosis? Crusted scabies.
Sarcoptes scabiei var. hominis, the mite that causes typical scabies, also causes crusted or Norwegian scabies. These terms refer to severe infestations that occur in individuals who are immune compromised or debilitated. The rash is characterized by vesicles and thick crusts and may or may not be itchy. Because patients with crusted scabies can be infested with as many as 2 million mites, transmission from very brief skin-to-skin contact is possible, and outbreaks have occurred in health care facilities and other institutional settings.
That was the reason for my friend’s call. “What do we do for the doctors and nurses in the clinic who saw the patient?” she wanted to know.
“Everyone wore gloves, right?” I asked. There was silence on the other end of the phone.
After a quick consultation with our health department, every health care provider (HCP) who touched the patient without gloves was treated preemptively with topical permethrin. None went on to develop scabies. The experience prompted me to think about the challenges of infection prevention in ambulatory care.
Both the American Academy of Pediatrics (AAP Committee on Infectious Diseases, “Infection prevention and control in pediatric ambulatory settings,” Pediatrics 2007;20[3]:650-65) and the Centers for Disease Control and Prevention (Guide to Infection Prevention for Outpatient Settings: Minimum Expectations for Safe Care) have published recommendations for infection prevention in outpatient settings. Both organizations emphasize the importance of standard precautions. According to the CDC, standard precautions “are the minimum infection prevention practices that apply to all patient care, regardless of suspected or confirmed infection status of the patient, in any setting where health care is delivered.” They are designed to protect HCPs, as well as prevent us from spreading infections among patients. Standard precautions include:
• Hand hygiene.
• Use of personal protective equipment (gloves, gowns, masks).
• Safe injection practices.
• Safe handling of potentially contaminated equipment or surfaces in the patient environment.
• Respiratory hygiene/cough etiquette.
Some of these elements are likely second nature to office-based pediatricians. Hands must be cleaned before and after every patient encounter or an encounter with the patient’s immediate environment. “Cover your cough” signs have become ubiquitous in ambulatory care waiting rooms, even as we acknowledge the difficulties associated with expecting toddlers to wear masks or use a tissue to contain their coughs and sneezes.
Other elements of standard precautions may receive increased attention because the consequences of noncompliance are perceived to be dangerous or severe. For example, we know that failure to reliably employ safe injection practices (see table) has resulted in transmission of blood-borne pathogens, including hepatitis B and C, in ambulatory settings.
In my experience, the use of personal protective equipment (PPE) in the ambulatory setting is the element of standard precautions that is the least understood and perhaps the most underutilized. It’s certainly easier in the inpatient setting, where we use transmission-based precautions, and colorful isolation signs instruct us to put on gown and gloves when we visit the patient with viral gastroenteritis, or gown, gloves, and mask for the child with acute viral respiratory tract infection. In the office, we expect the HCP to anticipate what kind of contact with blood or body fluids is likely and choose PPE accordingly.

Of course, anticipation can be tricky. Gowns, for example, are only required during procedures or activities when contact with blood and body fluids is likely. In routine office-based care, these sorts of procedures are uncommon. Incision and drainage of an abscess is one example of a procedure that might warrant protection of one’s clothing with a gown. Conversely, the need for a mask might arise several times a day, as these are worn to protect the mouth, nose, and eyes “during procedures that are likely to generate splashes or sprays of blood or other body fluids.” Examination of a coughing patient is a common “procedure” likely to results in sprays of saliva. Use of a mask can protect the examiner from potential exposures to Bordetella pertussis, Mycoplasma pneumoniae, and a host of respiratory viruses.
While the AAP has been careful to point out that gloves are not needed for the routine care of well children, they should be used when “there is the potential to contact blood, body fluids, mucous membranes, nonintact skin, or potentially infectious material.” In our world, potentially infectious material might include a cluster of vesicles thought to be herpes simplex, the honey-crusted lesions of impetigo, or the weeping, crusted rash of Norwegian scabies.
My own office had a powerful reminder about the importance of standard precautions last year when we were referred a young infant with recurrent fevers and a mostly dry, peeling rash. As we learned in medical school, the mucocutanous lesions of congenital syphilis can be highly contagious. In accordance with AAP recommendations, all HCPs who examined this child without the protection of gloves underwent serologic testing for syphilis. Fortunately, there were no transmissions!
Published data about infectious disease exposures and the transmission of infectious diseases in the outpatient setting, either from patients to health care workers or among patients, are largely limited to outbreak or case reports. A 1991 review identified 53 reports of infectious disease transmission in outpatient settings between 1961 and 1990 (JAMA 1991;265(18): 2377-81). Transmission occurred in medical and dental offices, clinics, emergency departments, ophthalmology offices, and alternative care settings that included chiropractic clinics and an acupuncture practice. A variety of pathogens were involved, including measles, adenovirus, hepatitis B, atypical mycobacteria, and Streptococcus pyogenes. The authors concluded that many of the outbreaks and episodes of transmission could have been prevented “if existing infection control guidelines,” including what we now consider standard precautions, had been utilized. Many reports published in the intervening 25 years have come to similar conclusions.
So why don’t HCPs yet follow standard precautions, including appropriate use of PPE? The reasons are complex and multifactorial. We’re all busy and lack of time is a common complaint. Gowns, gloves, masks, and alcohol hand gel aren’t always readily available. Some HCPs may not be knowledgeable about the elements of standard precautions while others may not understand the risks to themselves and their patients associated with nonadherence. Finally, some organizations have not established clear expectations related to infection prevention and compliance with AAP and CDC recommendations.
Several years ago, at the very beginning of the H1N1 influenza epidemic, a colleague of mine working in a pediatric practice saw a patient complaining of fever, lethargy, and myalgia. Not surprisingly, the patient’s rapid influenza test was positive. My colleague recalls that she was handed the result before she ever walked into the room – without any PPE – to see the patient.
“This was different than my usual routine at the hospital,” she told me. The expectation at the hospital was gown, gloves, and masks for any patient with influenza or influenzalike illness. At the office though, there was no such expectation, and providers did not routinely wear masks, even when seeing patients with respiratory symptoms. My colleague wasn’t reckless or rebellious. She was simply conforming to the culture in that office, and following the behavioral cues of more senior physicians in the practice. Subsequently, she developed severe influenza infection requiring a prolonged hospital stay.
It’s time to change the culture. As a first step, perform a quick audit in the office, using the AAP’s “Infection prevention and control in pediatric ambulatory settings” as a guide.
Dr. Bryant is a pediatrician specializing in infectious diseases at the University of Louisville (Ky.) and Kosair Children’s Hospital, also in Louisville. She had no relevant financial disclosures.
Not a long ago, I received a call from a friend working in a local pediatric clinic. One of her partners had just seen a young child with an unusual rash. The diagnosis? Crusted scabies.
Sarcoptes scabiei var. hominis, the mite that causes typical scabies, also causes crusted or Norwegian scabies. These terms refer to severe infestations that occur in individuals who are immune compromised or debilitated. The rash is characterized by vesicles and thick crusts and may or may not be itchy. Because patients with crusted scabies can be infested with as many as 2 million mites, transmission from very brief skin-to-skin contact is possible, and outbreaks have occurred in health care facilities and other institutional settings.
That was the reason for my friend’s call. “What do we do for the doctors and nurses in the clinic who saw the patient?” she wanted to know.
“Everyone wore gloves, right?” I asked. There was silence on the other end of the phone.
After a quick consultation with our health department, every health care provider (HCP) who touched the patient without gloves was treated preemptively with topical permethrin. None went on to develop scabies. The experience prompted me to think about the challenges of infection prevention in ambulatory care.
Both the American Academy of Pediatrics (AAP Committee on Infectious Diseases, “Infection prevention and control in pediatric ambulatory settings,” Pediatrics 2007;20[3]:650-65) and the Centers for Disease Control and Prevention (Guide to Infection Prevention for Outpatient Settings: Minimum Expectations for Safe Care) have published recommendations for infection prevention in outpatient settings. Both organizations emphasize the importance of standard precautions. According to the CDC, standard precautions “are the minimum infection prevention practices that apply to all patient care, regardless of suspected or confirmed infection status of the patient, in any setting where health care is delivered.” They are designed to protect HCPs, as well as prevent us from spreading infections among patients. Standard precautions include:
• Hand hygiene.
• Use of personal protective equipment (gloves, gowns, masks).
• Safe injection practices.
• Safe handling of potentially contaminated equipment or surfaces in the patient environment.
• Respiratory hygiene/cough etiquette.
Some of these elements are likely second nature to office-based pediatricians. Hands must be cleaned before and after every patient encounter or an encounter with the patient’s immediate environment. “Cover your cough” signs have become ubiquitous in ambulatory care waiting rooms, even as we acknowledge the difficulties associated with expecting toddlers to wear masks or use a tissue to contain their coughs and sneezes.
Other elements of standard precautions may receive increased attention because the consequences of noncompliance are perceived to be dangerous or severe. For example, we know that failure to reliably employ safe injection practices (see table) has resulted in transmission of blood-borne pathogens, including hepatitis B and C, in ambulatory settings.
In my experience, the use of personal protective equipment (PPE) in the ambulatory setting is the element of standard precautions that is the least understood and perhaps the most underutilized. It’s certainly easier in the inpatient setting, where we use transmission-based precautions, and colorful isolation signs instruct us to put on gown and gloves when we visit the patient with viral gastroenteritis, or gown, gloves, and mask for the child with acute viral respiratory tract infection. In the office, we expect the HCP to anticipate what kind of contact with blood or body fluids is likely and choose PPE accordingly.

Of course, anticipation can be tricky. Gowns, for example, are only required during procedures or activities when contact with blood and body fluids is likely. In routine office-based care, these sorts of procedures are uncommon. Incision and drainage of an abscess is one example of a procedure that might warrant protection of one’s clothing with a gown. Conversely, the need for a mask might arise several times a day, as these are worn to protect the mouth, nose, and eyes “during procedures that are likely to generate splashes or sprays of blood or other body fluids.” Examination of a coughing patient is a common “procedure” likely to results in sprays of saliva. Use of a mask can protect the examiner from potential exposures to Bordetella pertussis, Mycoplasma pneumoniae, and a host of respiratory viruses.
While the AAP has been careful to point out that gloves are not needed for the routine care of well children, they should be used when “there is the potential to contact blood, body fluids, mucous membranes, nonintact skin, or potentially infectious material.” In our world, potentially infectious material might include a cluster of vesicles thought to be herpes simplex, the honey-crusted lesions of impetigo, or the weeping, crusted rash of Norwegian scabies.
My own office had a powerful reminder about the importance of standard precautions last year when we were referred a young infant with recurrent fevers and a mostly dry, peeling rash. As we learned in medical school, the mucocutanous lesions of congenital syphilis can be highly contagious. In accordance with AAP recommendations, all HCPs who examined this child without the protection of gloves underwent serologic testing for syphilis. Fortunately, there were no transmissions!
Published data about infectious disease exposures and the transmission of infectious diseases in the outpatient setting, either from patients to health care workers or among patients, are largely limited to outbreak or case reports. A 1991 review identified 53 reports of infectious disease transmission in outpatient settings between 1961 and 1990 (JAMA 1991;265(18): 2377-81). Transmission occurred in medical and dental offices, clinics, emergency departments, ophthalmology offices, and alternative care settings that included chiropractic clinics and an acupuncture practice. A variety of pathogens were involved, including measles, adenovirus, hepatitis B, atypical mycobacteria, and Streptococcus pyogenes. The authors concluded that many of the outbreaks and episodes of transmission could have been prevented “if existing infection control guidelines,” including what we now consider standard precautions, had been utilized. Many reports published in the intervening 25 years have come to similar conclusions.
So why don’t HCPs yet follow standard precautions, including appropriate use of PPE? The reasons are complex and multifactorial. We’re all busy and lack of time is a common complaint. Gowns, gloves, masks, and alcohol hand gel aren’t always readily available. Some HCPs may not be knowledgeable about the elements of standard precautions while others may not understand the risks to themselves and their patients associated with nonadherence. Finally, some organizations have not established clear expectations related to infection prevention and compliance with AAP and CDC recommendations.
Several years ago, at the very beginning of the H1N1 influenza epidemic, a colleague of mine working in a pediatric practice saw a patient complaining of fever, lethargy, and myalgia. Not surprisingly, the patient’s rapid influenza test was positive. My colleague recalls that she was handed the result before she ever walked into the room – without any PPE – to see the patient.
“This was different than my usual routine at the hospital,” she told me. The expectation at the hospital was gown, gloves, and masks for any patient with influenza or influenzalike illness. At the office though, there was no such expectation, and providers did not routinely wear masks, even when seeing patients with respiratory symptoms. My colleague wasn’t reckless or rebellious. She was simply conforming to the culture in that office, and following the behavioral cues of more senior physicians in the practice. Subsequently, she developed severe influenza infection requiring a prolonged hospital stay.
It’s time to change the culture. As a first step, perform a quick audit in the office, using the AAP’s “Infection prevention and control in pediatric ambulatory settings” as a guide.
Dr. Bryant is a pediatrician specializing in infectious diseases at the University of Louisville (Ky.) and Kosair Children’s Hospital, also in Louisville. She had no relevant financial disclosures.
Variations in blood cancer survival across Europe

chemotherapy
Photo by Rhoda Baer
VIENNA—Results of the EUROCARE-5 study have revealed regional differences in survival for European patients with hematologic malignancies.
The data showed regional variations in 5-year relative survival rates for a number of cancers.
But the differences were particularly pronounced for leukemias, non-Hodgkin lymphomas (NHLs), and plasma cell neoplasms (PCNs).
Milena Sant, MD, of the Fondazione IRCCS Istituto Nazionale dei Tumori in Milan, Italy, presented these results at the 2015 European Cancer Congress (LBA 1).
Data from this study have also been published in several articles in the October 2015 issue of the European Journal of Cancer.
EUROCARE-5 includes records from 22 million cancer patients diagnosed between 1978 and 2007. The latest data encompass more than 10 million patients (ages 15 and older) diagnosed from 1995 to 2007 and followed up to 2008.
The data came from 107 cancer registries in 29 countries. The researchers estimated 5-year relative survival and trends from 1999 to 2007 according to region—Ireland/UK, Northern Europe, Central Europe, Southern Europe, and Eastern Europe.
“In general, 5-year relative survival—survival that is adjusted for causes of death other than cancer—increased steadily over time in Europe, particularly in Eastern Europe, for most cancers,” Dr Sant said.
“However, the most dramatic geographical variations were observed for cancers of the blood where there have been recent advances in treatment, such as chronic myeloid and lymphocytic leukemias, non-Hodgkin lymphoma and 2 of its subtypes (follicular and diffuse large B-cell lymphoma), and multiple myeloma. Hodgkin lymphoma was the exception, with smaller regional variations and a fairly good prognosis in most countries.”
Hodgkin lymphoma and NHL
Of all the hematologic malignancies, 5-year relative survival was highest for Hodgkin lymphoma, at 80.8% (40,625 cases). Five-year survival was 79.4% in Ireland and the UK, 85% in Northern countries, and 74.3% in Eastern Europe, which was significantly below the European average (P<0.0001).
For NHL, the 5-year relative survival was 59.4% (329,204 cases). Survival rates for NHL patients ranged from 49.7% in Eastern Europe to 63.3% in Northern Europe.
CLL/SLL
For chronic lymphocytic leukemia/small lymphocytic lymphoma (CLL/SLL), the 5-year relative survival was 70.4% (81,914 cases). CLL/SLL survival ranged from 58% in Eastern Europe to about 74% in Central and Northern Europe.
The researchers noted that between-country variations in CLL/SLL survival were high in all regions. Outliers that were significantly below the regional average were Austria (67%), Croatia (52%), and Bulgaria (45.5%).
PCNs
PCNs included multiple myeloma, plasmacytoma, and plasma cell leukemias. The 5-year relative survival for all PCNs was 39.2% (94,024 cases).
PCN survival rates were lowest in Eastern Europe (31.7%), slightly higher in the UK/Ireland (35.9%), and between 39.1% and 42% in the rest of Europe.
Myeloid leukemias
Of all the hematologic malignancies, 5-year relative survival was poorest for patients with acute myeloid leukemia (AML), at 17.1% (57,026 cases).
AML survival rates in Ireland/UK (15.0%) and Eastern Europe (13.0%) were significantly below the European average. But AML survival in Sweden, Belgium, France, and Germany was significantly higher than the average (P<0.005).
Five-year relative survival for chronic myeloid leukemia (CML) was 52.9% (17,713 cases).
Of all the hematologic malignancies, the survival gap between Eastern Europe and the rest of Europe was highest for CML. Five-year survival for CML patients was 33% in Eastern Europe and ranged from 51% to 58% in the rest of Europe.
The researchers also said there were striking survival variations by country in all areas. They found significant deviations from the regional average in Sweden (69.7%), Scotland (64.6%), France (71.7%), Austria (48.2%), Croatia (37.8%), Estonia (48.9%), Czech Republic (45.2%), and Latvia (22.1%).
“Results from EUROCARE can help to identify regions of low survival where action is needed to improve patients’ outcomes,” Dr Sant noted.
“Population-based survival information is essential for physicians, policy-makers, administrators, researchers, and patient organizations who deal with the needs of cancer patients, as well as with the issue of the growing expenditure on healthcare.” ![]()

chemotherapy
Photo by Rhoda Baer
VIENNA—Results of the EUROCARE-5 study have revealed regional differences in survival for European patients with hematologic malignancies.
The data showed regional variations in 5-year relative survival rates for a number of cancers.
But the differences were particularly pronounced for leukemias, non-Hodgkin lymphomas (NHLs), and plasma cell neoplasms (PCNs).
Milena Sant, MD, of the Fondazione IRCCS Istituto Nazionale dei Tumori in Milan, Italy, presented these results at the 2015 European Cancer Congress (LBA 1).
Data from this study have also been published in several articles in the October 2015 issue of the European Journal of Cancer.
EUROCARE-5 includes records from 22 million cancer patients diagnosed between 1978 and 2007. The latest data encompass more than 10 million patients (ages 15 and older) diagnosed from 1995 to 2007 and followed up to 2008.
The data came from 107 cancer registries in 29 countries. The researchers estimated 5-year relative survival and trends from 1999 to 2007 according to region—Ireland/UK, Northern Europe, Central Europe, Southern Europe, and Eastern Europe.
“In general, 5-year relative survival—survival that is adjusted for causes of death other than cancer—increased steadily over time in Europe, particularly in Eastern Europe, for most cancers,” Dr Sant said.
“However, the most dramatic geographical variations were observed for cancers of the blood where there have been recent advances in treatment, such as chronic myeloid and lymphocytic leukemias, non-Hodgkin lymphoma and 2 of its subtypes (follicular and diffuse large B-cell lymphoma), and multiple myeloma. Hodgkin lymphoma was the exception, with smaller regional variations and a fairly good prognosis in most countries.”
Hodgkin lymphoma and NHL
Of all the hematologic malignancies, 5-year relative survival was highest for Hodgkin lymphoma, at 80.8% (40,625 cases). Five-year survival was 79.4% in Ireland and the UK, 85% in Northern countries, and 74.3% in Eastern Europe, which was significantly below the European average (P<0.0001).
For NHL, the 5-year relative survival was 59.4% (329,204 cases). Survival rates for NHL patients ranged from 49.7% in Eastern Europe to 63.3% in Northern Europe.
CLL/SLL
For chronic lymphocytic leukemia/small lymphocytic lymphoma (CLL/SLL), the 5-year relative survival was 70.4% (81,914 cases). CLL/SLL survival ranged from 58% in Eastern Europe to about 74% in Central and Northern Europe.
The researchers noted that between-country variations in CLL/SLL survival were high in all regions. Outliers that were significantly below the regional average were Austria (67%), Croatia (52%), and Bulgaria (45.5%).
PCNs
PCNs included multiple myeloma, plasmacytoma, and plasma cell leukemias. The 5-year relative survival for all PCNs was 39.2% (94,024 cases).
PCN survival rates were lowest in Eastern Europe (31.7%), slightly higher in the UK/Ireland (35.9%), and between 39.1% and 42% in the rest of Europe.
Myeloid leukemias
Of all the hematologic malignancies, 5-year relative survival was poorest for patients with acute myeloid leukemia (AML), at 17.1% (57,026 cases).
AML survival rates in Ireland/UK (15.0%) and Eastern Europe (13.0%) were significantly below the European average. But AML survival in Sweden, Belgium, France, and Germany was significantly higher than the average (P<0.005).
Five-year relative survival for chronic myeloid leukemia (CML) was 52.9% (17,713 cases).
Of all the hematologic malignancies, the survival gap between Eastern Europe and the rest of Europe was highest for CML. Five-year survival for CML patients was 33% in Eastern Europe and ranged from 51% to 58% in the rest of Europe.
The researchers also said there were striking survival variations by country in all areas. They found significant deviations from the regional average in Sweden (69.7%), Scotland (64.6%), France (71.7%), Austria (48.2%), Croatia (37.8%), Estonia (48.9%), Czech Republic (45.2%), and Latvia (22.1%).
“Results from EUROCARE can help to identify regions of low survival where action is needed to improve patients’ outcomes,” Dr Sant noted.
“Population-based survival information is essential for physicians, policy-makers, administrators, researchers, and patient organizations who deal with the needs of cancer patients, as well as with the issue of the growing expenditure on healthcare.” ![]()

chemotherapy
Photo by Rhoda Baer
VIENNA—Results of the EUROCARE-5 study have revealed regional differences in survival for European patients with hematologic malignancies.
The data showed regional variations in 5-year relative survival rates for a number of cancers.
But the differences were particularly pronounced for leukemias, non-Hodgkin lymphomas (NHLs), and plasma cell neoplasms (PCNs).
Milena Sant, MD, of the Fondazione IRCCS Istituto Nazionale dei Tumori in Milan, Italy, presented these results at the 2015 European Cancer Congress (LBA 1).
Data from this study have also been published in several articles in the October 2015 issue of the European Journal of Cancer.
EUROCARE-5 includes records from 22 million cancer patients diagnosed between 1978 and 2007. The latest data encompass more than 10 million patients (ages 15 and older) diagnosed from 1995 to 2007 and followed up to 2008.
The data came from 107 cancer registries in 29 countries. The researchers estimated 5-year relative survival and trends from 1999 to 2007 according to region—Ireland/UK, Northern Europe, Central Europe, Southern Europe, and Eastern Europe.
“In general, 5-year relative survival—survival that is adjusted for causes of death other than cancer—increased steadily over time in Europe, particularly in Eastern Europe, for most cancers,” Dr Sant said.
“However, the most dramatic geographical variations were observed for cancers of the blood where there have been recent advances in treatment, such as chronic myeloid and lymphocytic leukemias, non-Hodgkin lymphoma and 2 of its subtypes (follicular and diffuse large B-cell lymphoma), and multiple myeloma. Hodgkin lymphoma was the exception, with smaller regional variations and a fairly good prognosis in most countries.”
Hodgkin lymphoma and NHL
Of all the hematologic malignancies, 5-year relative survival was highest for Hodgkin lymphoma, at 80.8% (40,625 cases). Five-year survival was 79.4% in Ireland and the UK, 85% in Northern countries, and 74.3% in Eastern Europe, which was significantly below the European average (P<0.0001).
For NHL, the 5-year relative survival was 59.4% (329,204 cases). Survival rates for NHL patients ranged from 49.7% in Eastern Europe to 63.3% in Northern Europe.
CLL/SLL
For chronic lymphocytic leukemia/small lymphocytic lymphoma (CLL/SLL), the 5-year relative survival was 70.4% (81,914 cases). CLL/SLL survival ranged from 58% in Eastern Europe to about 74% in Central and Northern Europe.
The researchers noted that between-country variations in CLL/SLL survival were high in all regions. Outliers that were significantly below the regional average were Austria (67%), Croatia (52%), and Bulgaria (45.5%).
PCNs
PCNs included multiple myeloma, plasmacytoma, and plasma cell leukemias. The 5-year relative survival for all PCNs was 39.2% (94,024 cases).
PCN survival rates were lowest in Eastern Europe (31.7%), slightly higher in the UK/Ireland (35.9%), and between 39.1% and 42% in the rest of Europe.
Myeloid leukemias
Of all the hematologic malignancies, 5-year relative survival was poorest for patients with acute myeloid leukemia (AML), at 17.1% (57,026 cases).
AML survival rates in Ireland/UK (15.0%) and Eastern Europe (13.0%) were significantly below the European average. But AML survival in Sweden, Belgium, France, and Germany was significantly higher than the average (P<0.005).
Five-year relative survival for chronic myeloid leukemia (CML) was 52.9% (17,713 cases).
Of all the hematologic malignancies, the survival gap between Eastern Europe and the rest of Europe was highest for CML. Five-year survival for CML patients was 33% in Eastern Europe and ranged from 51% to 58% in the rest of Europe.
The researchers also said there were striking survival variations by country in all areas. They found significant deviations from the regional average in Sweden (69.7%), Scotland (64.6%), France (71.7%), Austria (48.2%), Croatia (37.8%), Estonia (48.9%), Czech Republic (45.2%), and Latvia (22.1%).
“Results from EUROCARE can help to identify regions of low survival where action is needed to improve patients’ outcomes,” Dr Sant noted.
“Population-based survival information is essential for physicians, policy-makers, administrators, researchers, and patient organizations who deal with the needs of cancer patients, as well as with the issue of the growing expenditure on healthcare.” ![]()
mAb produces ‘encouraging’ results in MM

multiple myeloma
ROME—Combination therapy incorporating a novel monoclonal antibody (mAb) can provide “encouraging, long-lasting tumor control” in heavily pretreated patients with relapsed/refractory multiple myeloma (MM), according to investigators.
The mAb, MOR202, was also considered to be well-tolerated in this ongoing phase 1/2a study.
Early results from this study were presented at the 15th International Myeloma Workshop (poster #156). The study was sponsored by MorphoSys AG, makers of MOR202. The poster is available on the company’s website.
MOR202 is a HuCAL-derived mAb directed against CD38. In the phase 1/2a study, 50 MM patients have received the drug thus far.
At baseline, the patients’ median age was 67. They had received a median of 4 prior therapies, including bortezomib (98%), lenalidomide (92%), melphalan (92%), cyclophosphamide (76%), doxorubicin (60%), thalidomide (32%), pomalidomide (14%), carfilzomib (6%), elotuzumab (4%), and panobinostat (4%). Seventy-six percent had received a stem cell transplant.
Study design
The study consists of several parts and dosing cohorts in which the investigators are assessing MOR202 alone or in combination with other agents.
Treatment in Part A consists of a 2-hour intravenous infusion of MOR202 once every 2 weeks at several different doses: 0.01 mg/kg , 0.04 mg/kg, 0.15 mg/kg, 0.5 mg/kg, 1.5 mg/kg, 4.0 mg/kg, 8.0 mg/kg, or 16.0 mg/kg.
Part B is a 2-hour intravenous infusion of MOR202 once a week at 4 mg/kg, 8 mg/kg, or 16 mg/kg.
Part C is dexamethasone plus MOR202 once a week at 4 mg/kg, 8 mg/kg, or 16 mg/kg.
Part D is pomalidomide, dexamethasone, and MOR202 once a week at 8 mg/kg or 16 mg/kg.
Part E is lenalidomide, dexamethasone, and MOR202 once a week at 8 mg/kg or 16 mg/kg.
In the confirmatory cohorts, patients receive MOR202 with or without dexamethasone once a week or once every 2 weeks, MOR202 with pomalidomide and dexamethasone once a week, or MOR202 with lenalidomide and dexamethasone once a week.
Efficacy
Of the 50 patients treated thus far, 27 were evaluable for efficacy. One patient achieved a very good partial response, 2 had a partial response, and 2 had a minor response. Eleven patients had stable disease, and 11 progressed.
The very good partial response occurred in a patient receiving weekly MOR202 at 4 mg/kg plus dexamethasone.
One partial response occurred in a patient receiving weekly MOR202 at 8 mg/kg plus dexamethasone. The other occurred in a patient receiving weekly MOR202 at 8 mg/kg plus dexamethasone and pomalidomide.
One minor response occurred in a patient receiving weekly MOR202 at 8 mg/kg plus dexamethasone and lenalidomide. The other occurred in a patient receiving weekly MOR202 at 16 mg/kg plus dexamethasone.
“[T]he preliminary efficacy seen so far with MOR202 as single agent and in combinations is promising,” said investigator Marc-Steffen Raab, MD, of Heidelberg University Hospital and the German Cancer Research Center DKFZ in Heidelberg, Germany.
Safety
All 50 patients were evaluable for safety. Ninety-eight percent experienced an adverse event (AE), 66% of which were grade 3 or higher.
The most frequent AEs (overall and grade 3 or higher) were anemia (34%, 6%), leukopenia (30%, 10%), neutropenia (20%, 10%), thrombocytopenia (18%, 8%), fatigue (30%, 0%), nausea (22%, 0%), diarrhea (20%, 0%), and headache (16%, 0%).
Thirty-six percent of patients discontinued MOR202 due to treatment-emergent AEs. However, only 6% (n=3) of these AEs were considered possibly related to MOR202.
Infusion-related reactions occurred in 15 patients (30%). One of these patients received dexamethasone as well and experienced an infusion-related reaction (grade 1).
In the absence of dexamethasone, nearly all infusion reactions were grade 1-2. The exception was 1 patient with a grade 3 reaction that was mainly limited to the first infusion.
The maximum tolerated dose of MOR202 has not been reached.
“Considering the low rate of infusion reactions, even in cohorts without dexamethasone, the short infusion time, and other aspects, MOR202 may turn out to be an excellent choice in terms of safety and tolerability,” Dr Raab concluded. ![]()

multiple myeloma
ROME—Combination therapy incorporating a novel monoclonal antibody (mAb) can provide “encouraging, long-lasting tumor control” in heavily pretreated patients with relapsed/refractory multiple myeloma (MM), according to investigators.
The mAb, MOR202, was also considered to be well-tolerated in this ongoing phase 1/2a study.
Early results from this study were presented at the 15th International Myeloma Workshop (poster #156). The study was sponsored by MorphoSys AG, makers of MOR202. The poster is available on the company’s website.
MOR202 is a HuCAL-derived mAb directed against CD38. In the phase 1/2a study, 50 MM patients have received the drug thus far.
At baseline, the patients’ median age was 67. They had received a median of 4 prior therapies, including bortezomib (98%), lenalidomide (92%), melphalan (92%), cyclophosphamide (76%), doxorubicin (60%), thalidomide (32%), pomalidomide (14%), carfilzomib (6%), elotuzumab (4%), and panobinostat (4%). Seventy-six percent had received a stem cell transplant.
Study design
The study consists of several parts and dosing cohorts in which the investigators are assessing MOR202 alone or in combination with other agents.
Treatment in Part A consists of a 2-hour intravenous infusion of MOR202 once every 2 weeks at several different doses: 0.01 mg/kg , 0.04 mg/kg, 0.15 mg/kg, 0.5 mg/kg, 1.5 mg/kg, 4.0 mg/kg, 8.0 mg/kg, or 16.0 mg/kg.
Part B is a 2-hour intravenous infusion of MOR202 once a week at 4 mg/kg, 8 mg/kg, or 16 mg/kg.
Part C is dexamethasone plus MOR202 once a week at 4 mg/kg, 8 mg/kg, or 16 mg/kg.
Part D is pomalidomide, dexamethasone, and MOR202 once a week at 8 mg/kg or 16 mg/kg.
Part E is lenalidomide, dexamethasone, and MOR202 once a week at 8 mg/kg or 16 mg/kg.
In the confirmatory cohorts, patients receive MOR202 with or without dexamethasone once a week or once every 2 weeks, MOR202 with pomalidomide and dexamethasone once a week, or MOR202 with lenalidomide and dexamethasone once a week.
Efficacy
Of the 50 patients treated thus far, 27 were evaluable for efficacy. One patient achieved a very good partial response, 2 had a partial response, and 2 had a minor response. Eleven patients had stable disease, and 11 progressed.
The very good partial response occurred in a patient receiving weekly MOR202 at 4 mg/kg plus dexamethasone.
One partial response occurred in a patient receiving weekly MOR202 at 8 mg/kg plus dexamethasone. The other occurred in a patient receiving weekly MOR202 at 8 mg/kg plus dexamethasone and pomalidomide.
One minor response occurred in a patient receiving weekly MOR202 at 8 mg/kg plus dexamethasone and lenalidomide. The other occurred in a patient receiving weekly MOR202 at 16 mg/kg plus dexamethasone.
“[T]he preliminary efficacy seen so far with MOR202 as single agent and in combinations is promising,” said investigator Marc-Steffen Raab, MD, of Heidelberg University Hospital and the German Cancer Research Center DKFZ in Heidelberg, Germany.
Safety
All 50 patients were evaluable for safety. Ninety-eight percent experienced an adverse event (AE), 66% of which were grade 3 or higher.
The most frequent AEs (overall and grade 3 or higher) were anemia (34%, 6%), leukopenia (30%, 10%), neutropenia (20%, 10%), thrombocytopenia (18%, 8%), fatigue (30%, 0%), nausea (22%, 0%), diarrhea (20%, 0%), and headache (16%, 0%).
Thirty-six percent of patients discontinued MOR202 due to treatment-emergent AEs. However, only 6% (n=3) of these AEs were considered possibly related to MOR202.
Infusion-related reactions occurred in 15 patients (30%). One of these patients received dexamethasone as well and experienced an infusion-related reaction (grade 1).
In the absence of dexamethasone, nearly all infusion reactions were grade 1-2. The exception was 1 patient with a grade 3 reaction that was mainly limited to the first infusion.
The maximum tolerated dose of MOR202 has not been reached.
“Considering the low rate of infusion reactions, even in cohorts without dexamethasone, the short infusion time, and other aspects, MOR202 may turn out to be an excellent choice in terms of safety and tolerability,” Dr Raab concluded. ![]()

multiple myeloma
ROME—Combination therapy incorporating a novel monoclonal antibody (mAb) can provide “encouraging, long-lasting tumor control” in heavily pretreated patients with relapsed/refractory multiple myeloma (MM), according to investigators.
The mAb, MOR202, was also considered to be well-tolerated in this ongoing phase 1/2a study.
Early results from this study were presented at the 15th International Myeloma Workshop (poster #156). The study was sponsored by MorphoSys AG, makers of MOR202. The poster is available on the company’s website.
MOR202 is a HuCAL-derived mAb directed against CD38. In the phase 1/2a study, 50 MM patients have received the drug thus far.
At baseline, the patients’ median age was 67. They had received a median of 4 prior therapies, including bortezomib (98%), lenalidomide (92%), melphalan (92%), cyclophosphamide (76%), doxorubicin (60%), thalidomide (32%), pomalidomide (14%), carfilzomib (6%), elotuzumab (4%), and panobinostat (4%). Seventy-six percent had received a stem cell transplant.
Study design
The study consists of several parts and dosing cohorts in which the investigators are assessing MOR202 alone or in combination with other agents.
Treatment in Part A consists of a 2-hour intravenous infusion of MOR202 once every 2 weeks at several different doses: 0.01 mg/kg , 0.04 mg/kg, 0.15 mg/kg, 0.5 mg/kg, 1.5 mg/kg, 4.0 mg/kg, 8.0 mg/kg, or 16.0 mg/kg.
Part B is a 2-hour intravenous infusion of MOR202 once a week at 4 mg/kg, 8 mg/kg, or 16 mg/kg.
Part C is dexamethasone plus MOR202 once a week at 4 mg/kg, 8 mg/kg, or 16 mg/kg.
Part D is pomalidomide, dexamethasone, and MOR202 once a week at 8 mg/kg or 16 mg/kg.
Part E is lenalidomide, dexamethasone, and MOR202 once a week at 8 mg/kg or 16 mg/kg.
In the confirmatory cohorts, patients receive MOR202 with or without dexamethasone once a week or once every 2 weeks, MOR202 with pomalidomide and dexamethasone once a week, or MOR202 with lenalidomide and dexamethasone once a week.
Efficacy
Of the 50 patients treated thus far, 27 were evaluable for efficacy. One patient achieved a very good partial response, 2 had a partial response, and 2 had a minor response. Eleven patients had stable disease, and 11 progressed.
The very good partial response occurred in a patient receiving weekly MOR202 at 4 mg/kg plus dexamethasone.
One partial response occurred in a patient receiving weekly MOR202 at 8 mg/kg plus dexamethasone. The other occurred in a patient receiving weekly MOR202 at 8 mg/kg plus dexamethasone and pomalidomide.
One minor response occurred in a patient receiving weekly MOR202 at 8 mg/kg plus dexamethasone and lenalidomide. The other occurred in a patient receiving weekly MOR202 at 16 mg/kg plus dexamethasone.
“[T]he preliminary efficacy seen so far with MOR202 as single agent and in combinations is promising,” said investigator Marc-Steffen Raab, MD, of Heidelberg University Hospital and the German Cancer Research Center DKFZ in Heidelberg, Germany.
Safety
All 50 patients were evaluable for safety. Ninety-eight percent experienced an adverse event (AE), 66% of which were grade 3 or higher.
The most frequent AEs (overall and grade 3 or higher) were anemia (34%, 6%), leukopenia (30%, 10%), neutropenia (20%, 10%), thrombocytopenia (18%, 8%), fatigue (30%, 0%), nausea (22%, 0%), diarrhea (20%, 0%), and headache (16%, 0%).
Thirty-six percent of patients discontinued MOR202 due to treatment-emergent AEs. However, only 6% (n=3) of these AEs were considered possibly related to MOR202.
Infusion-related reactions occurred in 15 patients (30%). One of these patients received dexamethasone as well and experienced an infusion-related reaction (grade 1).
In the absence of dexamethasone, nearly all infusion reactions were grade 1-2. The exception was 1 patient with a grade 3 reaction that was mainly limited to the first infusion.
The maximum tolerated dose of MOR202 has not been reached.
“Considering the low rate of infusion reactions, even in cohorts without dexamethasone, the short infusion time, and other aspects, MOR202 may turn out to be an excellent choice in terms of safety and tolerability,” Dr Raab concluded. ![]()