User login
Code Status Discussions
Informed consent is one of the ethical, legal, and moral foundations of modern medicine.[1] Key elements of informed consent include: details of the procedure, benefits of the procedure, significant risks involved, likelihood of the outcome if predictable, and alternative therapeutic options.[2] Although rarely identified as such, conversations eliciting patient preferences about cardiopulmonary resuscitation (CPR) are among the most common examples of obtaining informed consent. Nevertheless, discussing CPR preference, often called code status discussions, differs from other examples of obtaining informed consent in 2 important ways. First, they occur well in advance of the potential need for CPR, so that the patient is well enough to participate meaningfully in the discussion. Second, because the default assumption is for patients to undergo the intervention (i.e. CPR), the focus of code status discussions is often on informed refusal, namely a decision about a do not resuscitate(DNR) order.
Since the institution of the Patient Self‐Determination Act in 1990, hospitals are obliged to educate patients about choices regarding end‐of‐life care at the time of hospital admission.[3] In many teaching hospitals, this responsibility falls to the admitting physician, often a trainee, who determines the patient's preferences regarding CPR and documents whether the patient is full code or DNR.
Prior studies have raised concerns about the quality of these conversations, highlighting their superficial nature and revealing trainee dissatisfaction with the results.[4, 5] Importantly, studies have shown that patients are capable of assimilating information about CPR when presented accurately and completely, and that such information can dramatically alter their choices.[6, 7, 8] These findings suggest that patients who are adequately educated will make more informed decisions regarding CPR, and that well‐informed choices about CPR may differ from poorly informed ones.
Although several studies have questioned the quality of code status discussions, none of these studies frames these interactions as examples of informed consent. Therefore, the purpose of the study was to examine the content of code status discussions as reported by internal medicine residents to determine whether they meet the basic tenets of informed consent, thereby facilitating informed decision making.
METHODS
In an iterative, collaborative process, authors A.F.B. and M.K.B. (an internal medicine resident at the time of the study and a board‐certified palliative care specialist/oncologist with experience in survey development, respectively) developed a survey adapted from previously published surveys.[9, 10, 11] The survey solicited respondent demographics, frequency of code status conversations, content of these discussions, and barriers to discussions. The survey instrument can be viewed in the Supporting Information, Appendix A, in the online version of this article. We used a 5‐point frequency scale (almost nevernearly always) for questions regarding: specific aspects of the informed consent related to code status discussions, resident confidence in conducting code status discussions, and barriers to code status discussions. We used a checklist for questions regarding content of code status discussions and patient characteristics influencing code status discussions. Residents provided a numeric percentage answer to 2 knowledge‐based questions of postarrest outcomes: (1) likelihood a patient would survive a witnessed pulseless ventricular tachycardia event and (2) likelihood of survival of a pulseless electrical activity event. The survey was revised by a hospitalist with experience in survey design (G.C.H.). We piloted the survey with 15 residents not part of the subject population and made revisions based on their input.
We sent a link to the online survey over secure email to all 159 internal medicine residents at our urban‐based academic medical center in January 2012. The email described the purpose of the study and stated that participation in the study (or lack thereof) was voluntary, anonymous, and would not have ramifications within the residency program. As part of the recruitment email, we explicitly included the elements of informed consent for the study participants. Not all the questions were mandatory to complete the survey. We sent a reminder e‐mail on a weekly basis for a total of 3 times and closed the survey after 1 month. Our goal was a 60% (N = 95) response rate.
We tabulated the results by question. For analytic purposes, we aligned the content questions with key elements of informed consent as follows: step‐by‐step description of the events (details), patient‐specific likelihood of discharge if resuscitated (benefits), complications of resuscitation (risks), population‐based likelihood of discharge if resuscitated (likelihood), and opportunity for changing code status (alternatives). For the knowledge‐based questions, we deemed the answer correct if it was within 10% (5%) of published statistics from the 2010 national registry of cardiopulmonary resuscitation.[12] We stratified the key elements of informed consent and level of confidence by postgraduate year (PGY), comparing PGY1 residents versus PGY2 and PGY3 residents using 2 tests (or Fisher exact test for observations 5). We performed a univariate logistic regression analysis examining the relationship between confidence and reported use of informed consent elements in code discussions. The dependent variable of confidence in sufficient information having been provided for fully informed decision making was dichotomized as most of the time or nearly always versus other responses, whereas the independent variable was dichotomized as residents who reported using all 5 informed consent elements versus those who did not. We analyzed data using Stata 12 (StataCorp, College Station, TX).
The institutional review board of the Beth Israel Deaconess reviewed the study protocol and determined that it was exempt from institutional review board review.
RESULTS
One hundred of 159 (62.3%) internal medicine residents responded to the survey. Of the respondents 93% (N = 93) completed the survey. The 7% (N = 7) who did not complete the survey omitted the knowledge‐based questions and demographics. Approximately half of participants (54%, N = 50) were male. The majority of residents (85%, N = 79) had either occasional or frequent exposure to palliative care, with 10% (N = 9) having completed a palliative care rotation (Table 1).
| Characteristic | N (%) |
|---|---|
| |
| Sex, male | 50 (54) |
| PGY level | |
| PGY1 | 35 (38) |
| PGY2 | 33 (35) |
| PGY3 | 25 (27) |
| Exposure to palliative care | |
| Very little | 5 (5) |
| Occasional | 55 (59) |
| Frequent | 24 (26) |
| Completed palliative care elective | 9 (10) |
| What type of teaching did you have with code status discussions (check all that apply)? | |
| No teaching | 6 (6) |
| Lectures | 35 (38) |
| Small group teaching sessions | 57 (61) |
| Direct observation and feedback | 50 (54) |
| Exposure to palliative care consultation while rotating on the wards | 54 (58) |
| Other | 4 (4) |
| How much has your previous teaching about resuscitative measures influenced your behavior? | |
| Not at all | 1 (1) |
| Not very much | 15 (16) |
| A little bit | 39 (42) |
| A lot | 38 (41) |
The vast majority of residents (96%, N = 95) discussed code status with more than 40% of patients they admitted to the hospital (Table 2). Two‐thirds (66%, N = 65) of all residents had the conversation with at least 4 out of 5 (81%99% and 100%) patients they admitted to the hospital. Only 1% (N = 1) of residents who responded to the survey reported conducting code status discussions with 20% or fewer of the patients they admitted to the hospital.
| N (%) | |
|---|---|
| Percentage of inpatients with which you discuss code status, n = 99 | |
| 100% | 12 (12) |
| 8199% | 53 (54) |
| 6180% | 19 (19) |
| 4160% | 11 (11) |
| 2140% | 3 (3) |
| 120% | 1 (1) |
| Aspects of resuscitative measures routinely discussed, n = 100 | |
| Intubation/ventilation | 100 (100) |
| Chest compressions | 99 (99) |
| Defibrillation | 86 (86) |
| Surrogate decision maker | 61 (61) |
| Likelihood of success | 35 (35) |
| Quality of life | 32 (32) |
| Vasopressors | 13 (13) |
| Likelihood of discharge | 10 (10) |
| Possible role of depression | 10 (10) |
| Physical states worse than death | 7 (7) |
| Religious beliefs as a factor | 6 (6) |
| Makes recommendations for code status, n = 93 | |
| Never | 19 (20) |
| Rarely | 33 (35) |
| Sometimes | 33 (35) |
| Often | 7 (8) |
| Nearly always | 1 (1) |
Most residents (66%, N = 66) identified the healthcare proxy or surrogate decision maker most of the time or nearly always. In addition, most residents (62%, N = 62) reminded patients that they could reverse their code status at any time. Almost half included a description of step‐by‐step events during resuscitation (45%, N = 45) or factored in patient's comorbidities (43%, N = 43) when discussing resuscitation at least most of the time. Few residents described complications (31%, N = 31) or outcomes (17%, N = 17) of cardiopulmonary arrests to patients most of the time or nearly always. Most residents did not explore factors such as quality of life, role of depression or physical states worse than death, factors that could potentially affect patient decision making (Table 2). Few (9%, N = 8) internal medicine residents (often or nearly always) offered their opinion regarding a patient's code status.
Many factors influenced residents' decisions to have a code status conversation. At least 85% (N = 86) of residents reported that older age, particular admitting diagnoses, and multiple comorbidities made them more likely to have a code status discussion (see Supporting Table 1 in the online version of this article). Patient race/ethnicity did not influence this decision, with only 1 respondent reporting this factor as relevant.
Residents identified lack of time (49%, N = 49 responding often or nearly always) as the most frequent barrier to having a code status discussion, followed by lack of rapport (29%, N = 29). Lack of experience (6%, N = 6), lack of information about the patient's clinical status (11%, N = 11), and lack of knowledge about outcomes (13%, N = 13) did not represent frequent barriers for residents.
Fifty‐five percent (N = 53) of residents often or nearly always felt confident that they provided enough information for patients to make fully informed decisions about code status, and this did not differ by PGY status (PGY1 vs PGY2/3, P = 0.80, 2 test). However, only 8% (N = 8) of residents most of the time or nearly always addressed all 5 key elements of informed consent in reporting the content of their code status discussions. When stratified by training year, PGY2/3 residents were significantly more likely than PGY1 residents to factor in a patient's comorbidities when discussing resuscitation and were also significantly more likely to relay the likelihood of hospital discharge. They were not significantly more likely to discuss other key elements of informed consent (Table 3).
| Elements of Code Status Discussion (Most of the Time or Nearly Always), n = 100 | Elements | Total, N (%) | PGY1, N (%) | PGY2/3, N (%) | P Value |
|---|---|---|---|---|---|
| |||||
| Identify the patient's HCP or surrogate | 66 (66) | N/A | N/A | N/A | |
| Describe the step‐by‐step events that occur during resuscitative measures | Details | 45 (45) | 14 (40) | 28 (33) | 0.437 |
| Describe the complications associated with resuscitative measures | Risks | 31 (31) | 8 (23) | 19 (33) | 0.308 |
| Describe the likelihood the patient will be discharged from the hospital if resuscitated | Likelihood | 17 (17) | 2 (6) | 14 (24) | 0.025 |
| Factor in the patient's comorbidities when discussing the likelihood of discharge from the hospital if resuscitated | Benefits | 43 (43) | 8 (23) | 33 (57) | 0.002 |
| Tell the patient that decisions regarding code status can be changed at any time | Alternatives | 62 (62) | 18 (51) | 38 (66) | 0.179 |
Our subanalysis showed that residents reporting all 5 key elements of informed consent were associated with higher levels of confidence that they had provided enough information to patients for them to make an informed decision (odds ratio of 1.7, 95% confidence interval 1.2‐2.3).
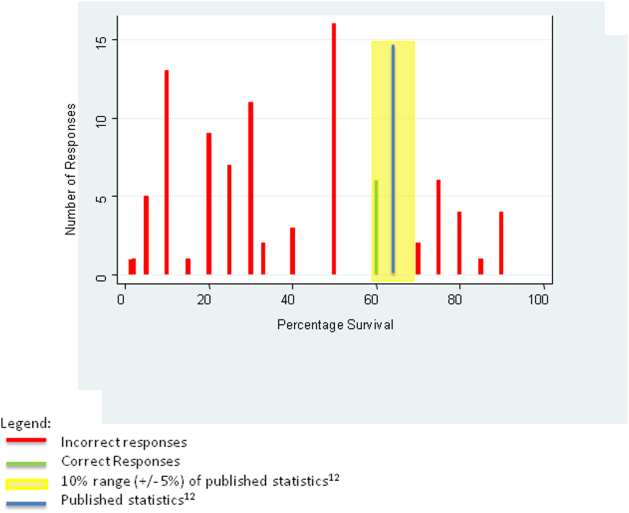
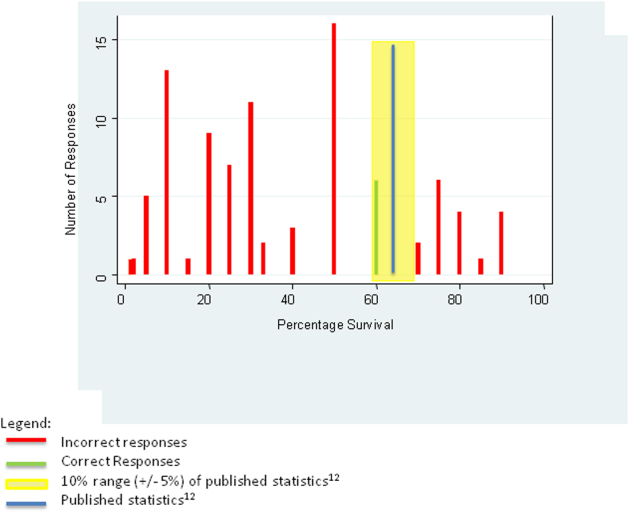
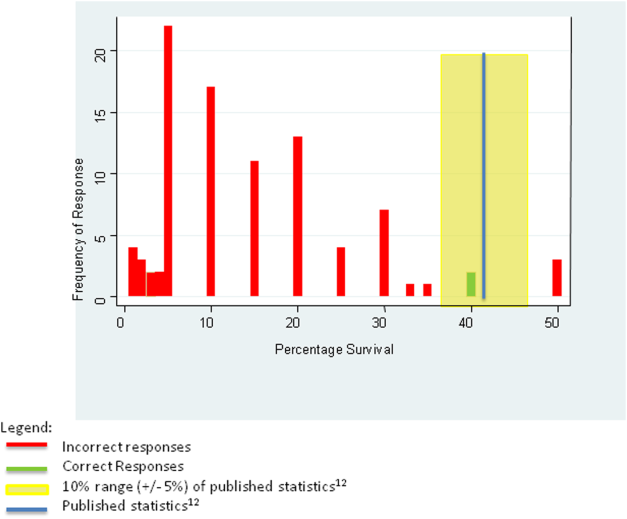
For the first knowledge‐based question about witnessed pulseless ventricular tachycardia, according to the 2010 registry,[12] 64% survived the event (range of responses 1%90%). Six out of 92 (7%) respondents were within 5% of the correct answer. For the second question about survival after unwitnessed pulseless electrical activity, 41.5% survived the event according to the registry (range of responses 1%50%). Three out of 92 (3%) respondents gave estimates within 5% of the correct answer. Figures 1 and 2 display the ranges of responses from residents.
DISCUSSION
We found that although our internal medicine residents frequently have code status discussions with their patients, very few routinely report addressing all 5 key elements of informed consent. Furthermore, residents lack accurate knowledge about the outcomes of CPR, with most tending to underestimate the benefit expected of resuscitation. These deficiencies raise serious concerns about whether patients are receiving all the information essential to making fully informed decisions about their preferences for resuscitation.
The data demonstrate that the residents are routinely discussing code status and regularly discussing some aspects of the procedure itself, such as chest compressions, intubation, or defibrillation; the actual step‐by‐step events of CPR are being described less than half the time. It seems that residents mentally list the possible procedures that may occur in a code without a context for how one intervention would lead to another. Placing CPR into context is important, because studies have shown that more comprehensive discussions or the use of visual aids/videos that depict CPR in more detail improves patients' understanding of CPR and changes their decision about CPR, making them more likely to forego the procedure.[7, 8]
Residents report that they are more likely to have a code status discussion with patient's with multiple comorbidities, suggesting that they take into account information about the patient's clinical condition when deciding with which patients to address code status. They also recognize which patients are at increased risk for an in hospital cardiopulmonary arrest. Additionally, nearly half of residents factor in patient's comorbidities when discussing likelihood of discharge from the hospital, suggesting that they recognize that comorbidities can alter the outcome of CPR. Importantly, however, very few residents describe the likelihood the patient will be discharged from the hospital if resuscitated. Thus, residents in our sample have some insight into the impact of comorbidities on outcomes of CPR, but fail to provide their patients with any information about the outcome of CPR.
One reason residents may not discuss outcomes of CPR is because they do not know the data regarding outcomes. Although few residents reported that lack of knowledge of the risks and outcomes of CPR was a barrier, very few respondents answered the knowledge questions appropriately. Given how few residents gave an accurate estimate of CPR outcomes and simultaneously reported confidence in their code status discussions suggests that many residents fail to recognize their knowledge deficits. This finding corroborates other studies showing that residents don't know what they don't know[10] and may reflect their lack of education on CPR outcomes. Alternatively, some residents who underestimated the outcomes in the examples provided may have done so because, in their experience caring for patients with multiple comorbidites, the outcomes of CPR are in fact poorer than those in the cases described. Outcomes of CPR at our institution might differ from those quoted in the registry. However, given the prevalence of inaccuracy, both for under‐ and overestimation, it seems likely that a true knowledge deficit on the part of the residents still accounts for much of the error and should be a target for education. Understanding CPR outcomes is vital for informed decision making, and studies have shown that when patients have more information, it can substantially affect a patient's decision regarding resuscitation.[7, 13]
Residents are infrequently exploring key determinants that affect a patient's decision‐making process. Only one‐third of residents report discussing quality‐of‐life issues with patients during code status discussions. Understanding an individual patient's values and goals and how he or she describes a good quality of life can help guide the discussion and potential recommendations. For example, some patients may feel it is important to be alive regardless of the physical state, whereas others may feel that if there is not a chance to be independent in their activities of daily living, then they would not want to be resuscitated. By exploring patient's perceptions of what quality of life and physical states worse than death means, residents can better understand and assist in the decision‐making process of their patients.
Our data show that few residents offer a recommendation regarding code status. Thus, residents expect patients to make their own decision with the data provided. At the same time, many residents focus on the details of the procedural components of CPR with little mention of anticipated outcomes or inquiries into key determinants discussed above. Additionally, based on their response to the knowledge‐based questions, residents' estimates of survival, if offered, would be inaccurate. Thus, code status conversations by residents leave patients to make uninformed choices to consent to or refuse resuscitative measures.
When stratified by training year, PGY2/3 residents were significantly more likely than PGY1 residents to discuss likelihood of discharge from the hospital as well as factor in patients' comorbidities when discussing outcomes. Although there is a statistically significant improvement between PGY2/3 residents as compared to PGY1 residents, the numbers still show that most PGY2/3 residents and almost all PGY1 residents do not discuss the likelihood of discharge if resuscitated during code status discussions. In addition, there is no difference reported in other key areas of informed consent. Thus, though there is some improvement as housestaff advance in their training, PGY2 and PGY3 residents still do not discuss all 5 key elements of informed consent significantly more than PGY1 residents.
Our findings suggest an opportunity for additional education regarding how to address code status for internal medicine housestaff. Over half of the respondents reported small group teaching sessions, direct observation and feedback, and exposure to palliative care consultation during their clinical rotations; yet, very few of them included all the key elements of informed consent in their discussions. To address this, our institution is developing additional educational initiatives, including a faculty development program for teaching communication skills, using direct observation and feedback. The orientation didactic lecture series for housestaff now includes a lecture on CPR that highlights the data on outcomes and the importance of putting the step‐by‐step procedures of CPR into the context of potential benefits, such as survival to hospital discharge. The curriculum also includes a module on advance care planning for junior and senior residents during their ambulatory block, using simulation and feedback as part of the teaching methods.
There are limitations to this study. Studies based on surveys are subject to recall and selection bias, and we lack objective assessment of actual code status discussions. Furthermore, the nature of the study may lead to an overestimation of the quality of the code status discussions due to social acceptability bias; yet, our data clearly show that the key elements of informed consent are not included during these conversations. Another limitation is that our subjects were residents at a single institution, and our clinical practice may differ from other academic settings in the teaching environment and culture; yet, our findings mirror similar work done in other locations.[10, 14]
In conclusion, our results demonstrate that residents fail to meet standards of informed consent when discussing code status in that they do not provide sufficient information for patients to make an informed decision regarding resuscitation. Residents would benefit from education aimed at improving their knowledge of CPR outcomes as well as training on how to conduct these conversations effectively. Framing code status discussions as an example of an informed consent may help residents recognize the need for the key elements to be included in these conversations. In addition, training should focus on how to conduct these conversations in an efficient yet effective manner. This will require clear simple language, good communication skills, and training with observation and feedback by specialists trained in this field.
Disclosures
This work was presented at the Society of General Internal Medicine New England Regional Meeting, March 8, 2013, Yale Medical Center, New Haven, Connecticut. The authors report no conflicts of interest.
- , , , , Medical informed consent: general considerations for physicians. Mayo Clin Proc. 2008;83(3):313–319.
- Beth Israel Deaconess Medical Center. Policy #PR‐02 45 CFR 46.11679(4):240–243.
- , , , . Medical residents' perspectives on discussions of advanced directives: can prior experience affect how they approach patients? J Palliat Med. 2007;10(3):712–720.
- , , , , . Code status discussions between attending hospitalist physicians and medical patients at hospital admission. J Gen Intern Med. 2010;26(4):359–366.
- , , . The influence of the probability of survival on patient's preferences regarding cardiopulmonary resuscitation. N Engl J Med. 1994;330:545–549.
- , , , , . Using video images to improve the accuracy of surrogate decision‐making: a randomized controlled trial. J Am Med Dir Assoc. 2009;10(8):575–580.
- , , , et al. Use of video to facilitate end‐of‐life discussions with patients with cancer: a randomized controlled trial. J Clin Oncol. 2010;28(2):305–310.
- , , , , , . Resident Approaches to Advance Care Planning on the Day of Hospital Admission. Arch Intern Med. 2006;166:1597–1602.
- , , , . Assessing competence of residents to discuss end‐of‐life issues. J Palliat Med. 2005;8(2):363–371.
- , , , et al. Code status discussions and goals of care among hospitalised adults. J Med Ethics. 2009;35:338–342.
- , , , . Pre‐resuscitation factors associated with mortality in 49,130 cases of in‐hospital cardiac arrest: a report from the National Registry for Cardiopulmonary Resuscitation. Resuscitation. 2010;81:302–311.
- , , , . Resuscitation decision making in the elderly: the value of outcome data. J Gen Intern Med. 1993;8:295–300.
- , , . How do medical residents discuss resuscitation with patients? J Gen Intern Med. 1995;10:436–442.
Informed consent is one of the ethical, legal, and moral foundations of modern medicine.[1] Key elements of informed consent include: details of the procedure, benefits of the procedure, significant risks involved, likelihood of the outcome if predictable, and alternative therapeutic options.[2] Although rarely identified as such, conversations eliciting patient preferences about cardiopulmonary resuscitation (CPR) are among the most common examples of obtaining informed consent. Nevertheless, discussing CPR preference, often called code status discussions, differs from other examples of obtaining informed consent in 2 important ways. First, they occur well in advance of the potential need for CPR, so that the patient is well enough to participate meaningfully in the discussion. Second, because the default assumption is for patients to undergo the intervention (i.e. CPR), the focus of code status discussions is often on informed refusal, namely a decision about a do not resuscitate(DNR) order.
Since the institution of the Patient Self‐Determination Act in 1990, hospitals are obliged to educate patients about choices regarding end‐of‐life care at the time of hospital admission.[3] In many teaching hospitals, this responsibility falls to the admitting physician, often a trainee, who determines the patient's preferences regarding CPR and documents whether the patient is full code or DNR.
Prior studies have raised concerns about the quality of these conversations, highlighting their superficial nature and revealing trainee dissatisfaction with the results.[4, 5] Importantly, studies have shown that patients are capable of assimilating information about CPR when presented accurately and completely, and that such information can dramatically alter their choices.[6, 7, 8] These findings suggest that patients who are adequately educated will make more informed decisions regarding CPR, and that well‐informed choices about CPR may differ from poorly informed ones.
Although several studies have questioned the quality of code status discussions, none of these studies frames these interactions as examples of informed consent. Therefore, the purpose of the study was to examine the content of code status discussions as reported by internal medicine residents to determine whether they meet the basic tenets of informed consent, thereby facilitating informed decision making.
METHODS
In an iterative, collaborative process, authors A.F.B. and M.K.B. (an internal medicine resident at the time of the study and a board‐certified palliative care specialist/oncologist with experience in survey development, respectively) developed a survey adapted from previously published surveys.[9, 10, 11] The survey solicited respondent demographics, frequency of code status conversations, content of these discussions, and barriers to discussions. The survey instrument can be viewed in the Supporting Information, Appendix A, in the online version of this article. We used a 5‐point frequency scale (almost nevernearly always) for questions regarding: specific aspects of the informed consent related to code status discussions, resident confidence in conducting code status discussions, and barriers to code status discussions. We used a checklist for questions regarding content of code status discussions and patient characteristics influencing code status discussions. Residents provided a numeric percentage answer to 2 knowledge‐based questions of postarrest outcomes: (1) likelihood a patient would survive a witnessed pulseless ventricular tachycardia event and (2) likelihood of survival of a pulseless electrical activity event. The survey was revised by a hospitalist with experience in survey design (G.C.H.). We piloted the survey with 15 residents not part of the subject population and made revisions based on their input.
We sent a link to the online survey over secure email to all 159 internal medicine residents at our urban‐based academic medical center in January 2012. The email described the purpose of the study and stated that participation in the study (or lack thereof) was voluntary, anonymous, and would not have ramifications within the residency program. As part of the recruitment email, we explicitly included the elements of informed consent for the study participants. Not all the questions were mandatory to complete the survey. We sent a reminder e‐mail on a weekly basis for a total of 3 times and closed the survey after 1 month. Our goal was a 60% (N = 95) response rate.
We tabulated the results by question. For analytic purposes, we aligned the content questions with key elements of informed consent as follows: step‐by‐step description of the events (details), patient‐specific likelihood of discharge if resuscitated (benefits), complications of resuscitation (risks), population‐based likelihood of discharge if resuscitated (likelihood), and opportunity for changing code status (alternatives). For the knowledge‐based questions, we deemed the answer correct if it was within 10% (5%) of published statistics from the 2010 national registry of cardiopulmonary resuscitation.[12] We stratified the key elements of informed consent and level of confidence by postgraduate year (PGY), comparing PGY1 residents versus PGY2 and PGY3 residents using 2 tests (or Fisher exact test for observations 5). We performed a univariate logistic regression analysis examining the relationship between confidence and reported use of informed consent elements in code discussions. The dependent variable of confidence in sufficient information having been provided for fully informed decision making was dichotomized as most of the time or nearly always versus other responses, whereas the independent variable was dichotomized as residents who reported using all 5 informed consent elements versus those who did not. We analyzed data using Stata 12 (StataCorp, College Station, TX).
The institutional review board of the Beth Israel Deaconess reviewed the study protocol and determined that it was exempt from institutional review board review.
RESULTS
One hundred of 159 (62.3%) internal medicine residents responded to the survey. Of the respondents 93% (N = 93) completed the survey. The 7% (N = 7) who did not complete the survey omitted the knowledge‐based questions and demographics. Approximately half of participants (54%, N = 50) were male. The majority of residents (85%, N = 79) had either occasional or frequent exposure to palliative care, with 10% (N = 9) having completed a palliative care rotation (Table 1).
| Characteristic | N (%) |
|---|---|
| |
| Sex, male | 50 (54) |
| PGY level | |
| PGY1 | 35 (38) |
| PGY2 | 33 (35) |
| PGY3 | 25 (27) |
| Exposure to palliative care | |
| Very little | 5 (5) |
| Occasional | 55 (59) |
| Frequent | 24 (26) |
| Completed palliative care elective | 9 (10) |
| What type of teaching did you have with code status discussions (check all that apply)? | |
| No teaching | 6 (6) |
| Lectures | 35 (38) |
| Small group teaching sessions | 57 (61) |
| Direct observation and feedback | 50 (54) |
| Exposure to palliative care consultation while rotating on the wards | 54 (58) |
| Other | 4 (4) |
| How much has your previous teaching about resuscitative measures influenced your behavior? | |
| Not at all | 1 (1) |
| Not very much | 15 (16) |
| A little bit | 39 (42) |
| A lot | 38 (41) |
The vast majority of residents (96%, N = 95) discussed code status with more than 40% of patients they admitted to the hospital (Table 2). Two‐thirds (66%, N = 65) of all residents had the conversation with at least 4 out of 5 (81%99% and 100%) patients they admitted to the hospital. Only 1% (N = 1) of residents who responded to the survey reported conducting code status discussions with 20% or fewer of the patients they admitted to the hospital.
| N (%) | |
|---|---|
| Percentage of inpatients with which you discuss code status, n = 99 | |
| 100% | 12 (12) |
| 8199% | 53 (54) |
| 6180% | 19 (19) |
| 4160% | 11 (11) |
| 2140% | 3 (3) |
| 120% | 1 (1) |
| Aspects of resuscitative measures routinely discussed, n = 100 | |
| Intubation/ventilation | 100 (100) |
| Chest compressions | 99 (99) |
| Defibrillation | 86 (86) |
| Surrogate decision maker | 61 (61) |
| Likelihood of success | 35 (35) |
| Quality of life | 32 (32) |
| Vasopressors | 13 (13) |
| Likelihood of discharge | 10 (10) |
| Possible role of depression | 10 (10) |
| Physical states worse than death | 7 (7) |
| Religious beliefs as a factor | 6 (6) |
| Makes recommendations for code status, n = 93 | |
| Never | 19 (20) |
| Rarely | 33 (35) |
| Sometimes | 33 (35) |
| Often | 7 (8) |
| Nearly always | 1 (1) |
Most residents (66%, N = 66) identified the healthcare proxy or surrogate decision maker most of the time or nearly always. In addition, most residents (62%, N = 62) reminded patients that they could reverse their code status at any time. Almost half included a description of step‐by‐step events during resuscitation (45%, N = 45) or factored in patient's comorbidities (43%, N = 43) when discussing resuscitation at least most of the time. Few residents described complications (31%, N = 31) or outcomes (17%, N = 17) of cardiopulmonary arrests to patients most of the time or nearly always. Most residents did not explore factors such as quality of life, role of depression or physical states worse than death, factors that could potentially affect patient decision making (Table 2). Few (9%, N = 8) internal medicine residents (often or nearly always) offered their opinion regarding a patient's code status.
Many factors influenced residents' decisions to have a code status conversation. At least 85% (N = 86) of residents reported that older age, particular admitting diagnoses, and multiple comorbidities made them more likely to have a code status discussion (see Supporting Table 1 in the online version of this article). Patient race/ethnicity did not influence this decision, with only 1 respondent reporting this factor as relevant.
Residents identified lack of time (49%, N = 49 responding often or nearly always) as the most frequent barrier to having a code status discussion, followed by lack of rapport (29%, N = 29). Lack of experience (6%, N = 6), lack of information about the patient's clinical status (11%, N = 11), and lack of knowledge about outcomes (13%, N = 13) did not represent frequent barriers for residents.
Fifty‐five percent (N = 53) of residents often or nearly always felt confident that they provided enough information for patients to make fully informed decisions about code status, and this did not differ by PGY status (PGY1 vs PGY2/3, P = 0.80, 2 test). However, only 8% (N = 8) of residents most of the time or nearly always addressed all 5 key elements of informed consent in reporting the content of their code status discussions. When stratified by training year, PGY2/3 residents were significantly more likely than PGY1 residents to factor in a patient's comorbidities when discussing resuscitation and were also significantly more likely to relay the likelihood of hospital discharge. They were not significantly more likely to discuss other key elements of informed consent (Table 3).
| Elements of Code Status Discussion (Most of the Time or Nearly Always), n = 100 | Elements | Total, N (%) | PGY1, N (%) | PGY2/3, N (%) | P Value |
|---|---|---|---|---|---|
| |||||
| Identify the patient's HCP or surrogate | 66 (66) | N/A | N/A | N/A | |
| Describe the step‐by‐step events that occur during resuscitative measures | Details | 45 (45) | 14 (40) | 28 (33) | 0.437 |
| Describe the complications associated with resuscitative measures | Risks | 31 (31) | 8 (23) | 19 (33) | 0.308 |
| Describe the likelihood the patient will be discharged from the hospital if resuscitated | Likelihood | 17 (17) | 2 (6) | 14 (24) | 0.025 |
| Factor in the patient's comorbidities when discussing the likelihood of discharge from the hospital if resuscitated | Benefits | 43 (43) | 8 (23) | 33 (57) | 0.002 |
| Tell the patient that decisions regarding code status can be changed at any time | Alternatives | 62 (62) | 18 (51) | 38 (66) | 0.179 |
Our subanalysis showed that residents reporting all 5 key elements of informed consent were associated with higher levels of confidence that they had provided enough information to patients for them to make an informed decision (odds ratio of 1.7, 95% confidence interval 1.2‐2.3).
For the first knowledge‐based question about witnessed pulseless ventricular tachycardia, according to the 2010 registry,[12] 64% survived the event (range of responses 1%90%). Six out of 92 (7%) respondents were within 5% of the correct answer. For the second question about survival after unwitnessed pulseless electrical activity, 41.5% survived the event according to the registry (range of responses 1%50%). Three out of 92 (3%) respondents gave estimates within 5% of the correct answer. Figures 1 and 2 display the ranges of responses from residents.


DISCUSSION
We found that although our internal medicine residents frequently have code status discussions with their patients, very few routinely report addressing all 5 key elements of informed consent. Furthermore, residents lack accurate knowledge about the outcomes of CPR, with most tending to underestimate the benefit expected of resuscitation. These deficiencies raise serious concerns about whether patients are receiving all the information essential to making fully informed decisions about their preferences for resuscitation.
The data demonstrate that the residents are routinely discussing code status and regularly discussing some aspects of the procedure itself, such as chest compressions, intubation, or defibrillation; the actual step‐by‐step events of CPR are being described less than half the time. It seems that residents mentally list the possible procedures that may occur in a code without a context for how one intervention would lead to another. Placing CPR into context is important, because studies have shown that more comprehensive discussions or the use of visual aids/videos that depict CPR in more detail improves patients' understanding of CPR and changes their decision about CPR, making them more likely to forego the procedure.[7, 8]
Residents report that they are more likely to have a code status discussion with patient's with multiple comorbidities, suggesting that they take into account information about the patient's clinical condition when deciding with which patients to address code status. They also recognize which patients are at increased risk for an in hospital cardiopulmonary arrest. Additionally, nearly half of residents factor in patient's comorbidities when discussing likelihood of discharge from the hospital, suggesting that they recognize that comorbidities can alter the outcome of CPR. Importantly, however, very few residents describe the likelihood the patient will be discharged from the hospital if resuscitated. Thus, residents in our sample have some insight into the impact of comorbidities on outcomes of CPR, but fail to provide their patients with any information about the outcome of CPR.
One reason residents may not discuss outcomes of CPR is because they do not know the data regarding outcomes. Although few residents reported that lack of knowledge of the risks and outcomes of CPR was a barrier, very few respondents answered the knowledge questions appropriately. Given how few residents gave an accurate estimate of CPR outcomes and simultaneously reported confidence in their code status discussions suggests that many residents fail to recognize their knowledge deficits. This finding corroborates other studies showing that residents don't know what they don't know[10] and may reflect their lack of education on CPR outcomes. Alternatively, some residents who underestimated the outcomes in the examples provided may have done so because, in their experience caring for patients with multiple comorbidites, the outcomes of CPR are in fact poorer than those in the cases described. Outcomes of CPR at our institution might differ from those quoted in the registry. However, given the prevalence of inaccuracy, both for under‐ and overestimation, it seems likely that a true knowledge deficit on the part of the residents still accounts for much of the error and should be a target for education. Understanding CPR outcomes is vital for informed decision making, and studies have shown that when patients have more information, it can substantially affect a patient's decision regarding resuscitation.[7, 13]
Residents are infrequently exploring key determinants that affect a patient's decision‐making process. Only one‐third of residents report discussing quality‐of‐life issues with patients during code status discussions. Understanding an individual patient's values and goals and how he or she describes a good quality of life can help guide the discussion and potential recommendations. For example, some patients may feel it is important to be alive regardless of the physical state, whereas others may feel that if there is not a chance to be independent in their activities of daily living, then they would not want to be resuscitated. By exploring patient's perceptions of what quality of life and physical states worse than death means, residents can better understand and assist in the decision‐making process of their patients.
Our data show that few residents offer a recommendation regarding code status. Thus, residents expect patients to make their own decision with the data provided. At the same time, many residents focus on the details of the procedural components of CPR with little mention of anticipated outcomes or inquiries into key determinants discussed above. Additionally, based on their response to the knowledge‐based questions, residents' estimates of survival, if offered, would be inaccurate. Thus, code status conversations by residents leave patients to make uninformed choices to consent to or refuse resuscitative measures.
When stratified by training year, PGY2/3 residents were significantly more likely than PGY1 residents to discuss likelihood of discharge from the hospital as well as factor in patients' comorbidities when discussing outcomes. Although there is a statistically significant improvement between PGY2/3 residents as compared to PGY1 residents, the numbers still show that most PGY2/3 residents and almost all PGY1 residents do not discuss the likelihood of discharge if resuscitated during code status discussions. In addition, there is no difference reported in other key areas of informed consent. Thus, though there is some improvement as housestaff advance in their training, PGY2 and PGY3 residents still do not discuss all 5 key elements of informed consent significantly more than PGY1 residents.
Our findings suggest an opportunity for additional education regarding how to address code status for internal medicine housestaff. Over half of the respondents reported small group teaching sessions, direct observation and feedback, and exposure to palliative care consultation during their clinical rotations; yet, very few of them included all the key elements of informed consent in their discussions. To address this, our institution is developing additional educational initiatives, including a faculty development program for teaching communication skills, using direct observation and feedback. The orientation didactic lecture series for housestaff now includes a lecture on CPR that highlights the data on outcomes and the importance of putting the step‐by‐step procedures of CPR into the context of potential benefits, such as survival to hospital discharge. The curriculum also includes a module on advance care planning for junior and senior residents during their ambulatory block, using simulation and feedback as part of the teaching methods.
There are limitations to this study. Studies based on surveys are subject to recall and selection bias, and we lack objective assessment of actual code status discussions. Furthermore, the nature of the study may lead to an overestimation of the quality of the code status discussions due to social acceptability bias; yet, our data clearly show that the key elements of informed consent are not included during these conversations. Another limitation is that our subjects were residents at a single institution, and our clinical practice may differ from other academic settings in the teaching environment and culture; yet, our findings mirror similar work done in other locations.[10, 14]
In conclusion, our results demonstrate that residents fail to meet standards of informed consent when discussing code status in that they do not provide sufficient information for patients to make an informed decision regarding resuscitation. Residents would benefit from education aimed at improving their knowledge of CPR outcomes as well as training on how to conduct these conversations effectively. Framing code status discussions as an example of an informed consent may help residents recognize the need for the key elements to be included in these conversations. In addition, training should focus on how to conduct these conversations in an efficient yet effective manner. This will require clear simple language, good communication skills, and training with observation and feedback by specialists trained in this field.
Disclosures
This work was presented at the Society of General Internal Medicine New England Regional Meeting, March 8, 2013, Yale Medical Center, New Haven, Connecticut. The authors report no conflicts of interest.
Informed consent is one of the ethical, legal, and moral foundations of modern medicine.[1] Key elements of informed consent include: details of the procedure, benefits of the procedure, significant risks involved, likelihood of the outcome if predictable, and alternative therapeutic options.[2] Although rarely identified as such, conversations eliciting patient preferences about cardiopulmonary resuscitation (CPR) are among the most common examples of obtaining informed consent. Nevertheless, discussing CPR preference, often called code status discussions, differs from other examples of obtaining informed consent in 2 important ways. First, they occur well in advance of the potential need for CPR, so that the patient is well enough to participate meaningfully in the discussion. Second, because the default assumption is for patients to undergo the intervention (i.e. CPR), the focus of code status discussions is often on informed refusal, namely a decision about a do not resuscitate(DNR) order.
Since the institution of the Patient Self‐Determination Act in 1990, hospitals are obliged to educate patients about choices regarding end‐of‐life care at the time of hospital admission.[3] In many teaching hospitals, this responsibility falls to the admitting physician, often a trainee, who determines the patient's preferences regarding CPR and documents whether the patient is full code or DNR.
Prior studies have raised concerns about the quality of these conversations, highlighting their superficial nature and revealing trainee dissatisfaction with the results.[4, 5] Importantly, studies have shown that patients are capable of assimilating information about CPR when presented accurately and completely, and that such information can dramatically alter their choices.[6, 7, 8] These findings suggest that patients who are adequately educated will make more informed decisions regarding CPR, and that well‐informed choices about CPR may differ from poorly informed ones.
Although several studies have questioned the quality of code status discussions, none of these studies frames these interactions as examples of informed consent. Therefore, the purpose of the study was to examine the content of code status discussions as reported by internal medicine residents to determine whether they meet the basic tenets of informed consent, thereby facilitating informed decision making.
METHODS
In an iterative, collaborative process, authors A.F.B. and M.K.B. (an internal medicine resident at the time of the study and a board‐certified palliative care specialist/oncologist with experience in survey development, respectively) developed a survey adapted from previously published surveys.[9, 10, 11] The survey solicited respondent demographics, frequency of code status conversations, content of these discussions, and barriers to discussions. The survey instrument can be viewed in the Supporting Information, Appendix A, in the online version of this article. We used a 5‐point frequency scale (almost nevernearly always) for questions regarding: specific aspects of the informed consent related to code status discussions, resident confidence in conducting code status discussions, and barriers to code status discussions. We used a checklist for questions regarding content of code status discussions and patient characteristics influencing code status discussions. Residents provided a numeric percentage answer to 2 knowledge‐based questions of postarrest outcomes: (1) likelihood a patient would survive a witnessed pulseless ventricular tachycardia event and (2) likelihood of survival of a pulseless electrical activity event. The survey was revised by a hospitalist with experience in survey design (G.C.H.). We piloted the survey with 15 residents not part of the subject population and made revisions based on their input.
We sent a link to the online survey over secure email to all 159 internal medicine residents at our urban‐based academic medical center in January 2012. The email described the purpose of the study and stated that participation in the study (or lack thereof) was voluntary, anonymous, and would not have ramifications within the residency program. As part of the recruitment email, we explicitly included the elements of informed consent for the study participants. Not all the questions were mandatory to complete the survey. We sent a reminder e‐mail on a weekly basis for a total of 3 times and closed the survey after 1 month. Our goal was a 60% (N = 95) response rate.
We tabulated the results by question. For analytic purposes, we aligned the content questions with key elements of informed consent as follows: step‐by‐step description of the events (details), patient‐specific likelihood of discharge if resuscitated (benefits), complications of resuscitation (risks), population‐based likelihood of discharge if resuscitated (likelihood), and opportunity for changing code status (alternatives). For the knowledge‐based questions, we deemed the answer correct if it was within 10% (5%) of published statistics from the 2010 national registry of cardiopulmonary resuscitation.[12] We stratified the key elements of informed consent and level of confidence by postgraduate year (PGY), comparing PGY1 residents versus PGY2 and PGY3 residents using 2 tests (or Fisher exact test for observations 5). We performed a univariate logistic regression analysis examining the relationship between confidence and reported use of informed consent elements in code discussions. The dependent variable of confidence in sufficient information having been provided for fully informed decision making was dichotomized as most of the time or nearly always versus other responses, whereas the independent variable was dichotomized as residents who reported using all 5 informed consent elements versus those who did not. We analyzed data using Stata 12 (StataCorp, College Station, TX).
The institutional review board of the Beth Israel Deaconess reviewed the study protocol and determined that it was exempt from institutional review board review.
RESULTS
One hundred of 159 (62.3%) internal medicine residents responded to the survey. Of the respondents 93% (N = 93) completed the survey. The 7% (N = 7) who did not complete the survey omitted the knowledge‐based questions and demographics. Approximately half of participants (54%, N = 50) were male. The majority of residents (85%, N = 79) had either occasional or frequent exposure to palliative care, with 10% (N = 9) having completed a palliative care rotation (Table 1).
| Characteristic | N (%) |
|---|---|
| |
| Sex, male | 50 (54) |
| PGY level | |
| PGY1 | 35 (38) |
| PGY2 | 33 (35) |
| PGY3 | 25 (27) |
| Exposure to palliative care | |
| Very little | 5 (5) |
| Occasional | 55 (59) |
| Frequent | 24 (26) |
| Completed palliative care elective | 9 (10) |
| What type of teaching did you have with code status discussions (check all that apply)? | |
| No teaching | 6 (6) |
| Lectures | 35 (38) |
| Small group teaching sessions | 57 (61) |
| Direct observation and feedback | 50 (54) |
| Exposure to palliative care consultation while rotating on the wards | 54 (58) |
| Other | 4 (4) |
| How much has your previous teaching about resuscitative measures influenced your behavior? | |
| Not at all | 1 (1) |
| Not very much | 15 (16) |
| A little bit | 39 (42) |
| A lot | 38 (41) |
The vast majority of residents (96%, N = 95) discussed code status with more than 40% of patients they admitted to the hospital (Table 2). Two‐thirds (66%, N = 65) of all residents had the conversation with at least 4 out of 5 (81%99% and 100%) patients they admitted to the hospital. Only 1% (N = 1) of residents who responded to the survey reported conducting code status discussions with 20% or fewer of the patients they admitted to the hospital.
| N (%) | |
|---|---|
| Percentage of inpatients with which you discuss code status, n = 99 | |
| 100% | 12 (12) |
| 8199% | 53 (54) |
| 6180% | 19 (19) |
| 4160% | 11 (11) |
| 2140% | 3 (3) |
| 120% | 1 (1) |
| Aspects of resuscitative measures routinely discussed, n = 100 | |
| Intubation/ventilation | 100 (100) |
| Chest compressions | 99 (99) |
| Defibrillation | 86 (86) |
| Surrogate decision maker | 61 (61) |
| Likelihood of success | 35 (35) |
| Quality of life | 32 (32) |
| Vasopressors | 13 (13) |
| Likelihood of discharge | 10 (10) |
| Possible role of depression | 10 (10) |
| Physical states worse than death | 7 (7) |
| Religious beliefs as a factor | 6 (6) |
| Makes recommendations for code status, n = 93 | |
| Never | 19 (20) |
| Rarely | 33 (35) |
| Sometimes | 33 (35) |
| Often | 7 (8) |
| Nearly always | 1 (1) |
Most residents (66%, N = 66) identified the healthcare proxy or surrogate decision maker most of the time or nearly always. In addition, most residents (62%, N = 62) reminded patients that they could reverse their code status at any time. Almost half included a description of step‐by‐step events during resuscitation (45%, N = 45) or factored in patient's comorbidities (43%, N = 43) when discussing resuscitation at least most of the time. Few residents described complications (31%, N = 31) or outcomes (17%, N = 17) of cardiopulmonary arrests to patients most of the time or nearly always. Most residents did not explore factors such as quality of life, role of depression or physical states worse than death, factors that could potentially affect patient decision making (Table 2). Few (9%, N = 8) internal medicine residents (often or nearly always) offered their opinion regarding a patient's code status.
Many factors influenced residents' decisions to have a code status conversation. At least 85% (N = 86) of residents reported that older age, particular admitting diagnoses, and multiple comorbidities made them more likely to have a code status discussion (see Supporting Table 1 in the online version of this article). Patient race/ethnicity did not influence this decision, with only 1 respondent reporting this factor as relevant.
Residents identified lack of time (49%, N = 49 responding often or nearly always) as the most frequent barrier to having a code status discussion, followed by lack of rapport (29%, N = 29). Lack of experience (6%, N = 6), lack of information about the patient's clinical status (11%, N = 11), and lack of knowledge about outcomes (13%, N = 13) did not represent frequent barriers for residents.
Fifty‐five percent (N = 53) of residents often or nearly always felt confident that they provided enough information for patients to make fully informed decisions about code status, and this did not differ by PGY status (PGY1 vs PGY2/3, P = 0.80, 2 test). However, only 8% (N = 8) of residents most of the time or nearly always addressed all 5 key elements of informed consent in reporting the content of their code status discussions. When stratified by training year, PGY2/3 residents were significantly more likely than PGY1 residents to factor in a patient's comorbidities when discussing resuscitation and were also significantly more likely to relay the likelihood of hospital discharge. They were not significantly more likely to discuss other key elements of informed consent (Table 3).
| Elements of Code Status Discussion (Most of the Time or Nearly Always), n = 100 | Elements | Total, N (%) | PGY1, N (%) | PGY2/3, N (%) | P Value |
|---|---|---|---|---|---|
| |||||
| Identify the patient's HCP or surrogate | 66 (66) | N/A | N/A | N/A | |
| Describe the step‐by‐step events that occur during resuscitative measures | Details | 45 (45) | 14 (40) | 28 (33) | 0.437 |
| Describe the complications associated with resuscitative measures | Risks | 31 (31) | 8 (23) | 19 (33) | 0.308 |
| Describe the likelihood the patient will be discharged from the hospital if resuscitated | Likelihood | 17 (17) | 2 (6) | 14 (24) | 0.025 |
| Factor in the patient's comorbidities when discussing the likelihood of discharge from the hospital if resuscitated | Benefits | 43 (43) | 8 (23) | 33 (57) | 0.002 |
| Tell the patient that decisions regarding code status can be changed at any time | Alternatives | 62 (62) | 18 (51) | 38 (66) | 0.179 |
Our subanalysis showed that residents reporting all 5 key elements of informed consent were associated with higher levels of confidence that they had provided enough information to patients for them to make an informed decision (odds ratio of 1.7, 95% confidence interval 1.2‐2.3).
For the first knowledge‐based question about witnessed pulseless ventricular tachycardia, according to the 2010 registry,[12] 64% survived the event (range of responses 1%90%). Six out of 92 (7%) respondents were within 5% of the correct answer. For the second question about survival after unwitnessed pulseless electrical activity, 41.5% survived the event according to the registry (range of responses 1%50%). Three out of 92 (3%) respondents gave estimates within 5% of the correct answer. Figures 1 and 2 display the ranges of responses from residents.


DISCUSSION
We found that although our internal medicine residents frequently have code status discussions with their patients, very few routinely report addressing all 5 key elements of informed consent. Furthermore, residents lack accurate knowledge about the outcomes of CPR, with most tending to underestimate the benefit expected of resuscitation. These deficiencies raise serious concerns about whether patients are receiving all the information essential to making fully informed decisions about their preferences for resuscitation.
The data demonstrate that the residents are routinely discussing code status and regularly discussing some aspects of the procedure itself, such as chest compressions, intubation, or defibrillation; the actual step‐by‐step events of CPR are being described less than half the time. It seems that residents mentally list the possible procedures that may occur in a code without a context for how one intervention would lead to another. Placing CPR into context is important, because studies have shown that more comprehensive discussions or the use of visual aids/videos that depict CPR in more detail improves patients' understanding of CPR and changes their decision about CPR, making them more likely to forego the procedure.[7, 8]
Residents report that they are more likely to have a code status discussion with patient's with multiple comorbidities, suggesting that they take into account information about the patient's clinical condition when deciding with which patients to address code status. They also recognize which patients are at increased risk for an in hospital cardiopulmonary arrest. Additionally, nearly half of residents factor in patient's comorbidities when discussing likelihood of discharge from the hospital, suggesting that they recognize that comorbidities can alter the outcome of CPR. Importantly, however, very few residents describe the likelihood the patient will be discharged from the hospital if resuscitated. Thus, residents in our sample have some insight into the impact of comorbidities on outcomes of CPR, but fail to provide their patients with any information about the outcome of CPR.
One reason residents may not discuss outcomes of CPR is because they do not know the data regarding outcomes. Although few residents reported that lack of knowledge of the risks and outcomes of CPR was a barrier, very few respondents answered the knowledge questions appropriately. Given how few residents gave an accurate estimate of CPR outcomes and simultaneously reported confidence in their code status discussions suggests that many residents fail to recognize their knowledge deficits. This finding corroborates other studies showing that residents don't know what they don't know[10] and may reflect their lack of education on CPR outcomes. Alternatively, some residents who underestimated the outcomes in the examples provided may have done so because, in their experience caring for patients with multiple comorbidites, the outcomes of CPR are in fact poorer than those in the cases described. Outcomes of CPR at our institution might differ from those quoted in the registry. However, given the prevalence of inaccuracy, both for under‐ and overestimation, it seems likely that a true knowledge deficit on the part of the residents still accounts for much of the error and should be a target for education. Understanding CPR outcomes is vital for informed decision making, and studies have shown that when patients have more information, it can substantially affect a patient's decision regarding resuscitation.[7, 13]
Residents are infrequently exploring key determinants that affect a patient's decision‐making process. Only one‐third of residents report discussing quality‐of‐life issues with patients during code status discussions. Understanding an individual patient's values and goals and how he or she describes a good quality of life can help guide the discussion and potential recommendations. For example, some patients may feel it is important to be alive regardless of the physical state, whereas others may feel that if there is not a chance to be independent in their activities of daily living, then they would not want to be resuscitated. By exploring patient's perceptions of what quality of life and physical states worse than death means, residents can better understand and assist in the decision‐making process of their patients.
Our data show that few residents offer a recommendation regarding code status. Thus, residents expect patients to make their own decision with the data provided. At the same time, many residents focus on the details of the procedural components of CPR with little mention of anticipated outcomes or inquiries into key determinants discussed above. Additionally, based on their response to the knowledge‐based questions, residents' estimates of survival, if offered, would be inaccurate. Thus, code status conversations by residents leave patients to make uninformed choices to consent to or refuse resuscitative measures.
When stratified by training year, PGY2/3 residents were significantly more likely than PGY1 residents to discuss likelihood of discharge from the hospital as well as factor in patients' comorbidities when discussing outcomes. Although there is a statistically significant improvement between PGY2/3 residents as compared to PGY1 residents, the numbers still show that most PGY2/3 residents and almost all PGY1 residents do not discuss the likelihood of discharge if resuscitated during code status discussions. In addition, there is no difference reported in other key areas of informed consent. Thus, though there is some improvement as housestaff advance in their training, PGY2 and PGY3 residents still do not discuss all 5 key elements of informed consent significantly more than PGY1 residents.
Our findings suggest an opportunity for additional education regarding how to address code status for internal medicine housestaff. Over half of the respondents reported small group teaching sessions, direct observation and feedback, and exposure to palliative care consultation during their clinical rotations; yet, very few of them included all the key elements of informed consent in their discussions. To address this, our institution is developing additional educational initiatives, including a faculty development program for teaching communication skills, using direct observation and feedback. The orientation didactic lecture series for housestaff now includes a lecture on CPR that highlights the data on outcomes and the importance of putting the step‐by‐step procedures of CPR into the context of potential benefits, such as survival to hospital discharge. The curriculum also includes a module on advance care planning for junior and senior residents during their ambulatory block, using simulation and feedback as part of the teaching methods.
There are limitations to this study. Studies based on surveys are subject to recall and selection bias, and we lack objective assessment of actual code status discussions. Furthermore, the nature of the study may lead to an overestimation of the quality of the code status discussions due to social acceptability bias; yet, our data clearly show that the key elements of informed consent are not included during these conversations. Another limitation is that our subjects were residents at a single institution, and our clinical practice may differ from other academic settings in the teaching environment and culture; yet, our findings mirror similar work done in other locations.[10, 14]
In conclusion, our results demonstrate that residents fail to meet standards of informed consent when discussing code status in that they do not provide sufficient information for patients to make an informed decision regarding resuscitation. Residents would benefit from education aimed at improving their knowledge of CPR outcomes as well as training on how to conduct these conversations effectively. Framing code status discussions as an example of an informed consent may help residents recognize the need for the key elements to be included in these conversations. In addition, training should focus on how to conduct these conversations in an efficient yet effective manner. This will require clear simple language, good communication skills, and training with observation and feedback by specialists trained in this field.
Disclosures
This work was presented at the Society of General Internal Medicine New England Regional Meeting, March 8, 2013, Yale Medical Center, New Haven, Connecticut. The authors report no conflicts of interest.
- , , , , Medical informed consent: general considerations for physicians. Mayo Clin Proc. 2008;83(3):313–319.
- Beth Israel Deaconess Medical Center. Policy #PR‐02 45 CFR 46.11679(4):240–243.
- , , , . Medical residents' perspectives on discussions of advanced directives: can prior experience affect how they approach patients? J Palliat Med. 2007;10(3):712–720.
- , , , , . Code status discussions between attending hospitalist physicians and medical patients at hospital admission. J Gen Intern Med. 2010;26(4):359–366.
- , , . The influence of the probability of survival on patient's preferences regarding cardiopulmonary resuscitation. N Engl J Med. 1994;330:545–549.
- , , , , . Using video images to improve the accuracy of surrogate decision‐making: a randomized controlled trial. J Am Med Dir Assoc. 2009;10(8):575–580.
- , , , et al. Use of video to facilitate end‐of‐life discussions with patients with cancer: a randomized controlled trial. J Clin Oncol. 2010;28(2):305–310.
- , , , , , . Resident Approaches to Advance Care Planning on the Day of Hospital Admission. Arch Intern Med. 2006;166:1597–1602.
- , , , . Assessing competence of residents to discuss end‐of‐life issues. J Palliat Med. 2005;8(2):363–371.
- , , , et al. Code status discussions and goals of care among hospitalised adults. J Med Ethics. 2009;35:338–342.
- , , , . Pre‐resuscitation factors associated with mortality in 49,130 cases of in‐hospital cardiac arrest: a report from the National Registry for Cardiopulmonary Resuscitation. Resuscitation. 2010;81:302–311.
- , , , . Resuscitation decision making in the elderly: the value of outcome data. J Gen Intern Med. 1993;8:295–300.
- , , . How do medical residents discuss resuscitation with patients? J Gen Intern Med. 1995;10:436–442.
- , , , , Medical informed consent: general considerations for physicians. Mayo Clin Proc. 2008;83(3):313–319.
- Beth Israel Deaconess Medical Center. Policy #PR‐02 45 CFR 46.11679(4):240–243.
- , , , . Medical residents' perspectives on discussions of advanced directives: can prior experience affect how they approach patients? J Palliat Med. 2007;10(3):712–720.
- , , , , . Code status discussions between attending hospitalist physicians and medical patients at hospital admission. J Gen Intern Med. 2010;26(4):359–366.
- , , . The influence of the probability of survival on patient's preferences regarding cardiopulmonary resuscitation. N Engl J Med. 1994;330:545–549.
- , , , , . Using video images to improve the accuracy of surrogate decision‐making: a randomized controlled trial. J Am Med Dir Assoc. 2009;10(8):575–580.
- , , , et al. Use of video to facilitate end‐of‐life discussions with patients with cancer: a randomized controlled trial. J Clin Oncol. 2010;28(2):305–310.
- , , , , , . Resident Approaches to Advance Care Planning on the Day of Hospital Admission. Arch Intern Med. 2006;166:1597–1602.
- , , , . Assessing competence of residents to discuss end‐of‐life issues. J Palliat Med. 2005;8(2):363–371.
- , , , et al. Code status discussions and goals of care among hospitalised adults. J Med Ethics. 2009;35:338–342.
- , , , . Pre‐resuscitation factors associated with mortality in 49,130 cases of in‐hospital cardiac arrest: a report from the National Registry for Cardiopulmonary Resuscitation. Resuscitation. 2010;81:302–311.
- , , , . Resuscitation decision making in the elderly: the value of outcome data. J Gen Intern Med. 1993;8:295–300.
- , , . How do medical residents discuss resuscitation with patients? J Gen Intern Med. 1995;10:436–442.
© 2015 Society of Hospital Medicine
Small nucleolar RNA signals colorectal cancer outcomes
Expression of a set of small nucleolar RNAs (snoRNAs) was found to be significantly elevated in cancer tissue, compared with normal tissue, and expression of a specific snoRNA, SNORA42, was associated with poor overall and disease-free survival in patients with colorectal cancer (CRC).
Elevated expression of the specific snoRNA SNORA42 was an independent prognostic indicator for overall survival (hazard ratio, 2.11; P = .021), disease-free survival (HR, 3.17; P = .011), and distant metastasis (HR, 2.66; P .023). In a subset of patients with stage II CRC, the significant association between high expression of SNORA42 and poor prognosis remained.
The majority of patients with stage II CRC are cured by surgery alone, but a significant proportion experience disease progression. According to Dr. Yoshinaga Okugawa of the Center for Gastrointestinal Cancer Research at the Baylor University Medical Center, Dallas, and colleagues, adjuvant chemotherapy treatment for all patients with stage II cancer is controversial.
“Therefore, identification of such high-risk patients with CRC using molecular biomarkers such as SNORA42 expression will allow use of adjuvant chemotherapy after surgery only in a select subgroup of high-risk patients to improve their prognosis,” they wrote (Gut 2015 Oct 15. doi: 10.1136/gutjnl-2015.309359).
The research examined expression levels of RNA extracted from formalin-fixed paraffin-embedded (FFPE) tissue samples from 250 patients with colorectal cancer and 24 matched controls. The initial focus was on four snoRNAs that were reported to be dysregulated in other cancers. The study found that all four of the snoRNAs were significantly upregulated in CRC tissue, compared with normal tissue. Receiver operating characteristic (ROC) curves showed that expression levels of all four snoRNAs distinguished between cancer and noncancer tissue; area under the ROC curves values ranged from 0.75 to 0.88.
SnoRNAs are single-stranded, noncoding RNAs that have recently emerged as important components in the control of cell fate and oncogenesis in various cancers. In addition, studies have suggested the potential of snoRNAs as prognostic indicators.
While the expression levels of all four snoRNAs distinguished cancer tissue from normal tissue, only one of the snoRNAs, SNORA42, had expression levels that correlated with disease outcomes (overall survival, disease-free survival, and distant metastasis).
To characterize the biologic role of SNORN42 in CRC progression, the research team investigated its expression and impact on cancer cell lines and animal models. Overexpressed SNORN42 enhanced cell proliferation and tumorigenicity in cultured cells and in an animal model of CRC.
Taken together, the results suggest potential clinical usefulness of SNORA42 as a diagnostic and predictive biomarker in patients with CRC.
Dr. Okugawa and coauthors reported having no disclosures.
Expression of a set of small nucleolar RNAs (snoRNAs) was found to be significantly elevated in cancer tissue, compared with normal tissue, and expression of a specific snoRNA, SNORA42, was associated with poor overall and disease-free survival in patients with colorectal cancer (CRC).
Elevated expression of the specific snoRNA SNORA42 was an independent prognostic indicator for overall survival (hazard ratio, 2.11; P = .021), disease-free survival (HR, 3.17; P = .011), and distant metastasis (HR, 2.66; P .023). In a subset of patients with stage II CRC, the significant association between high expression of SNORA42 and poor prognosis remained.
The majority of patients with stage II CRC are cured by surgery alone, but a significant proportion experience disease progression. According to Dr. Yoshinaga Okugawa of the Center for Gastrointestinal Cancer Research at the Baylor University Medical Center, Dallas, and colleagues, adjuvant chemotherapy treatment for all patients with stage II cancer is controversial.
“Therefore, identification of such high-risk patients with CRC using molecular biomarkers such as SNORA42 expression will allow use of adjuvant chemotherapy after surgery only in a select subgroup of high-risk patients to improve their prognosis,” they wrote (Gut 2015 Oct 15. doi: 10.1136/gutjnl-2015.309359).
The research examined expression levels of RNA extracted from formalin-fixed paraffin-embedded (FFPE) tissue samples from 250 patients with colorectal cancer and 24 matched controls. The initial focus was on four snoRNAs that were reported to be dysregulated in other cancers. The study found that all four of the snoRNAs were significantly upregulated in CRC tissue, compared with normal tissue. Receiver operating characteristic (ROC) curves showed that expression levels of all four snoRNAs distinguished between cancer and noncancer tissue; area under the ROC curves values ranged from 0.75 to 0.88.
SnoRNAs are single-stranded, noncoding RNAs that have recently emerged as important components in the control of cell fate and oncogenesis in various cancers. In addition, studies have suggested the potential of snoRNAs as prognostic indicators.
While the expression levels of all four snoRNAs distinguished cancer tissue from normal tissue, only one of the snoRNAs, SNORA42, had expression levels that correlated with disease outcomes (overall survival, disease-free survival, and distant metastasis).
To characterize the biologic role of SNORN42 in CRC progression, the research team investigated its expression and impact on cancer cell lines and animal models. Overexpressed SNORN42 enhanced cell proliferation and tumorigenicity in cultured cells and in an animal model of CRC.
Taken together, the results suggest potential clinical usefulness of SNORA42 as a diagnostic and predictive biomarker in patients with CRC.
Dr. Okugawa and coauthors reported having no disclosures.
Expression of a set of small nucleolar RNAs (snoRNAs) was found to be significantly elevated in cancer tissue, compared with normal tissue, and expression of a specific snoRNA, SNORA42, was associated with poor overall and disease-free survival in patients with colorectal cancer (CRC).
Elevated expression of the specific snoRNA SNORA42 was an independent prognostic indicator for overall survival (hazard ratio, 2.11; P = .021), disease-free survival (HR, 3.17; P = .011), and distant metastasis (HR, 2.66; P .023). In a subset of patients with stage II CRC, the significant association between high expression of SNORA42 and poor prognosis remained.
The majority of patients with stage II CRC are cured by surgery alone, but a significant proportion experience disease progression. According to Dr. Yoshinaga Okugawa of the Center for Gastrointestinal Cancer Research at the Baylor University Medical Center, Dallas, and colleagues, adjuvant chemotherapy treatment for all patients with stage II cancer is controversial.
“Therefore, identification of such high-risk patients with CRC using molecular biomarkers such as SNORA42 expression will allow use of adjuvant chemotherapy after surgery only in a select subgroup of high-risk patients to improve their prognosis,” they wrote (Gut 2015 Oct 15. doi: 10.1136/gutjnl-2015.309359).
The research examined expression levels of RNA extracted from formalin-fixed paraffin-embedded (FFPE) tissue samples from 250 patients with colorectal cancer and 24 matched controls. The initial focus was on four snoRNAs that were reported to be dysregulated in other cancers. The study found that all four of the snoRNAs were significantly upregulated in CRC tissue, compared with normal tissue. Receiver operating characteristic (ROC) curves showed that expression levels of all four snoRNAs distinguished between cancer and noncancer tissue; area under the ROC curves values ranged from 0.75 to 0.88.
SnoRNAs are single-stranded, noncoding RNAs that have recently emerged as important components in the control of cell fate and oncogenesis in various cancers. In addition, studies have suggested the potential of snoRNAs as prognostic indicators.
While the expression levels of all four snoRNAs distinguished cancer tissue from normal tissue, only one of the snoRNAs, SNORA42, had expression levels that correlated with disease outcomes (overall survival, disease-free survival, and distant metastasis).
To characterize the biologic role of SNORN42 in CRC progression, the research team investigated its expression and impact on cancer cell lines and animal models. Overexpressed SNORN42 enhanced cell proliferation and tumorigenicity in cultured cells and in an animal model of CRC.
Taken together, the results suggest potential clinical usefulness of SNORA42 as a diagnostic and predictive biomarker in patients with CRC.
Dr. Okugawa and coauthors reported having no disclosures.
FROM GUT
Key clinical point: Elevated expression of a small nucleolar RNA was associated with decreased overall and disease-free survival in patients with colorectal cancer.
Major finding: Elevated expression of the specific snoRNA SNORA42 was an independent prognostic indicator for overall survival (HR, 2.11; P = .021), disease-free survival (HR, 3.17; P = .011), and distant metastasis (HR, 2.66; P = .023).
Data source: RNA was extracted from FFPE tissue samples from 250 patients with colorectal cancer and 24 samples from matched controls.
Disclosures: Dr. Okugawa and coauthors reported having no disclosures.
ESC: Air quality level linked to STEMI risk in men
LONDON – The risk for ST-elevation myocardial infarction (STEMI) increased in men when air quality dipped below acceptable levels as designated by the World Health Organization (WHO), based on the results of an observational study in Belgian.
The relative risk for a STEMI increased by 2.8% (1.026; 95% confidence interval, 1.005-1.048) for every 10 mcg/cm3 rise in fine particulate air pollution and by 5.1% (RR, 1.051; 95% CI, 1.018-1.084) for a comparable rise in nitric oxide air pollution. Ozone level was not associated with the risk for STEMI. Air pollution levels did not affect in-hospital mortality due to STEMI and had no clear effect on patients with coronary artery disease, diabetes or hypertension.
“Of course, it is very interesting that the results are only observed in men. This may be due to a statistical issue, because in our study population, the female group represents less than 25% of the population,” Dr. Jean-François Argacha, a study investigator, said during a press briefing at the annual congress of the European Society of Cardiology.
Subgroup analysis suggested that patients who were aged 75 years and older were more likely to develop STEMI in relation to exposure to particulate matter (RR, 1.046; 95% CI, 1.002-1.092, P = .041). Those aged 54 years and younger seemed more susceptible to nitric oxide levels (RR, 1.071; 95% CI, 1.010-1.136; P = .021).“The detrimental impact of nitric oxide exceeded that of fine particles and may be of particular concern in the younger population,” said Dr. Argacha, a cardiologist at University Hospital Brussels.
Previous research had shown that particulate matter is associated with an increased risk for acute myocardial infarction, but there had been no specific assessments on air pollution’s potential effects on STEMI, according to Dr. Argacha.
The study considered information on 11,428 patients in the Belgian Interdisciplinary Working Group on Acute Cardiology STEMI Registry between 2009 and 2013. Data on national air pollution parameters – particulate matter with an aerodynamic diameter of less than 10 (PM10) or 2.5 (PM2.5) mcm, nitric oxide and ozone – and air temperature were obtained from the Belgian Environment Agency database and adjusted for population density.
A case crossover analysis of STEMI risk was then performed, with risk being adjusted for ambient temperature, day of the week, and the season. The study’s case crossover design ensured that the effects were limited to air pollution and excluded other confounders including respiratory disease and air temperature.
Worldwide, WHO has estimated that poor urban air quality is responsible for around 1.3 million deaths per year, Dr. Argacha observed, noting that air pollution consists of fine (PM2.5) and larger (PM10) particles, nitric oxide, and ozone as well as sulfur dioxide and carbon monoxide.
During the observation period, the mean air pollution levels over the course of 1 year in Belgium were 23.9 mcg/cm3 for PM10, 16.1 mcg/cm3 for PM2.5, and 23.7 mcg/cm3 for nitric oxide. WHO guidelines set a daily limit of 25 mcg/cm3 for PM2.5. Dr. Argacha noted that this limit was exceeded in Belgium on 17.5% of days in the study.
Dr. Oscar Franco, professor of preventative medicine at the Erasmus University Medical Center in Rotterdam, the Netherlands, who cochaired the press conference noted that the European Society of Cardiology recently published a position paper on air pollution on cardiovascular disease (Eur Heart J. 2015;36:83-93) and has launched a major campaign to raise awareness of the detrimental effects that the environment can have on the heart.
The campaign notes that air and noise pollution are modifiable risk factors for the prevention and control of cardiovascular diseases, and advocated for acceptable limits that reflect WHO levels. Individuals “need to take action,” Dr. Franco urged. The decisions that people make every day – how they get to work for example – could have a potentially huge impact on the environment and health.
Dr. Argacha and Dr. Franco had no disclosures to report.
LONDON – The risk for ST-elevation myocardial infarction (STEMI) increased in men when air quality dipped below acceptable levels as designated by the World Health Organization (WHO), based on the results of an observational study in Belgian.
The relative risk for a STEMI increased by 2.8% (1.026; 95% confidence interval, 1.005-1.048) for every 10 mcg/cm3 rise in fine particulate air pollution and by 5.1% (RR, 1.051; 95% CI, 1.018-1.084) for a comparable rise in nitric oxide air pollution. Ozone level was not associated with the risk for STEMI. Air pollution levels did not affect in-hospital mortality due to STEMI and had no clear effect on patients with coronary artery disease, diabetes or hypertension.
“Of course, it is very interesting that the results are only observed in men. This may be due to a statistical issue, because in our study population, the female group represents less than 25% of the population,” Dr. Jean-François Argacha, a study investigator, said during a press briefing at the annual congress of the European Society of Cardiology.
Subgroup analysis suggested that patients who were aged 75 years and older were more likely to develop STEMI in relation to exposure to particulate matter (RR, 1.046; 95% CI, 1.002-1.092, P = .041). Those aged 54 years and younger seemed more susceptible to nitric oxide levels (RR, 1.071; 95% CI, 1.010-1.136; P = .021).“The detrimental impact of nitric oxide exceeded that of fine particles and may be of particular concern in the younger population,” said Dr. Argacha, a cardiologist at University Hospital Brussels.
Previous research had shown that particulate matter is associated with an increased risk for acute myocardial infarction, but there had been no specific assessments on air pollution’s potential effects on STEMI, according to Dr. Argacha.
The study considered information on 11,428 patients in the Belgian Interdisciplinary Working Group on Acute Cardiology STEMI Registry between 2009 and 2013. Data on national air pollution parameters – particulate matter with an aerodynamic diameter of less than 10 (PM10) or 2.5 (PM2.5) mcm, nitric oxide and ozone – and air temperature were obtained from the Belgian Environment Agency database and adjusted for population density.
A case crossover analysis of STEMI risk was then performed, with risk being adjusted for ambient temperature, day of the week, and the season. The study’s case crossover design ensured that the effects were limited to air pollution and excluded other confounders including respiratory disease and air temperature.
Worldwide, WHO has estimated that poor urban air quality is responsible for around 1.3 million deaths per year, Dr. Argacha observed, noting that air pollution consists of fine (PM2.5) and larger (PM10) particles, nitric oxide, and ozone as well as sulfur dioxide and carbon monoxide.
During the observation period, the mean air pollution levels over the course of 1 year in Belgium were 23.9 mcg/cm3 for PM10, 16.1 mcg/cm3 for PM2.5, and 23.7 mcg/cm3 for nitric oxide. WHO guidelines set a daily limit of 25 mcg/cm3 for PM2.5. Dr. Argacha noted that this limit was exceeded in Belgium on 17.5% of days in the study.
Dr. Oscar Franco, professor of preventative medicine at the Erasmus University Medical Center in Rotterdam, the Netherlands, who cochaired the press conference noted that the European Society of Cardiology recently published a position paper on air pollution on cardiovascular disease (Eur Heart J. 2015;36:83-93) and has launched a major campaign to raise awareness of the detrimental effects that the environment can have on the heart.
The campaign notes that air and noise pollution are modifiable risk factors for the prevention and control of cardiovascular diseases, and advocated for acceptable limits that reflect WHO levels. Individuals “need to take action,” Dr. Franco urged. The decisions that people make every day – how they get to work for example – could have a potentially huge impact on the environment and health.
Dr. Argacha and Dr. Franco had no disclosures to report.
LONDON – The risk for ST-elevation myocardial infarction (STEMI) increased in men when air quality dipped below acceptable levels as designated by the World Health Organization (WHO), based on the results of an observational study in Belgian.
The relative risk for a STEMI increased by 2.8% (1.026; 95% confidence interval, 1.005-1.048) for every 10 mcg/cm3 rise in fine particulate air pollution and by 5.1% (RR, 1.051; 95% CI, 1.018-1.084) for a comparable rise in nitric oxide air pollution. Ozone level was not associated with the risk for STEMI. Air pollution levels did not affect in-hospital mortality due to STEMI and had no clear effect on patients with coronary artery disease, diabetes or hypertension.
“Of course, it is very interesting that the results are only observed in men. This may be due to a statistical issue, because in our study population, the female group represents less than 25% of the population,” Dr. Jean-François Argacha, a study investigator, said during a press briefing at the annual congress of the European Society of Cardiology.
Subgroup analysis suggested that patients who were aged 75 years and older were more likely to develop STEMI in relation to exposure to particulate matter (RR, 1.046; 95% CI, 1.002-1.092, P = .041). Those aged 54 years and younger seemed more susceptible to nitric oxide levels (RR, 1.071; 95% CI, 1.010-1.136; P = .021).“The detrimental impact of nitric oxide exceeded that of fine particles and may be of particular concern in the younger population,” said Dr. Argacha, a cardiologist at University Hospital Brussels.
Previous research had shown that particulate matter is associated with an increased risk for acute myocardial infarction, but there had been no specific assessments on air pollution’s potential effects on STEMI, according to Dr. Argacha.
The study considered information on 11,428 patients in the Belgian Interdisciplinary Working Group on Acute Cardiology STEMI Registry between 2009 and 2013. Data on national air pollution parameters – particulate matter with an aerodynamic diameter of less than 10 (PM10) or 2.5 (PM2.5) mcm, nitric oxide and ozone – and air temperature were obtained from the Belgian Environment Agency database and adjusted for population density.
A case crossover analysis of STEMI risk was then performed, with risk being adjusted for ambient temperature, day of the week, and the season. The study’s case crossover design ensured that the effects were limited to air pollution and excluded other confounders including respiratory disease and air temperature.
Worldwide, WHO has estimated that poor urban air quality is responsible for around 1.3 million deaths per year, Dr. Argacha observed, noting that air pollution consists of fine (PM2.5) and larger (PM10) particles, nitric oxide, and ozone as well as sulfur dioxide and carbon monoxide.
During the observation period, the mean air pollution levels over the course of 1 year in Belgium were 23.9 mcg/cm3 for PM10, 16.1 mcg/cm3 for PM2.5, and 23.7 mcg/cm3 for nitric oxide. WHO guidelines set a daily limit of 25 mcg/cm3 for PM2.5. Dr. Argacha noted that this limit was exceeded in Belgium on 17.5% of days in the study.
Dr. Oscar Franco, professor of preventative medicine at the Erasmus University Medical Center in Rotterdam, the Netherlands, who cochaired the press conference noted that the European Society of Cardiology recently published a position paper on air pollution on cardiovascular disease (Eur Heart J. 2015;36:83-93) and has launched a major campaign to raise awareness of the detrimental effects that the environment can have on the heart.
The campaign notes that air and noise pollution are modifiable risk factors for the prevention and control of cardiovascular diseases, and advocated for acceptable limits that reflect WHO levels. Individuals “need to take action,” Dr. Franco urged. The decisions that people make every day – how they get to work for example – could have a potentially huge impact on the environment and health.
Dr. Argacha and Dr. Franco had no disclosures to report.
AT THE ESC CONGRESS 2015
Key clinical point: Air pollution may be a potentially modifiable risk factor for STEMI in men.
Major finding: The relative risk for a STEMI increased by 2.8% for a 10 mcg/cm3 rise in fine particulate air pollution and by 5.1% for a comparable rise in nitric oxide air pollution.
Data source: 11,428 patients logged in the Belgian Interdisciplinary Working Group on Acute Cardiology STEMI Registry between 2009 and 2013.
Disclosures: Dr. Argacha and Dr. Franco had no disclosures to report.
Study identifies possible bipolar I subtype with externalizing features
Bipolar disorder might include a distinct subphenotype characterized by externalizing symptoms and specific genetic variations, report Shanker Swaminathan, Ph.D., and his colleagues.
A study of 2,505 bipolar I disorder patients categorized participants into two groups: externalizing (those with comorbid externalizing disorders, plus a subgroup of patients with externalizing symptoms before 15 years of age) and nonexternalizing, wrote Dr. Swaminathan, formerly with the department of medical and molecular genetics at Indiana University, Indianapolis, and now with Baylor College of Medicine, Houston.
Patients in the externalizing group showed significant differences in clinical features from the nonexternalizing group in multiple areas, including earlier age of onset of bipolar disorder, depression, and mania (P less than .001).
Additionally, those in the externalizing group had a higher frequency of self-harm incidents (P less than .001) and suicide attempts (P less than .008). These patients also were more likely to report a history of rapid switching and cycling (P less than .001), the authors reported.
Genetic analysis found numerous “nominally associated” SNPs, though none reached genome-wide significance, Dr. Swaminathan and his colleagues reported.
The findings “suggest the presence of an externalizing disorder subphenotype within [bipolar I disorder] warranting further investigation,” they concluded.
Read the full study here: J Affect Disord. 2015 Jun;178:206-14.
Bipolar disorder might include a distinct subphenotype characterized by externalizing symptoms and specific genetic variations, report Shanker Swaminathan, Ph.D., and his colleagues.
A study of 2,505 bipolar I disorder patients categorized participants into two groups: externalizing (those with comorbid externalizing disorders, plus a subgroup of patients with externalizing symptoms before 15 years of age) and nonexternalizing, wrote Dr. Swaminathan, formerly with the department of medical and molecular genetics at Indiana University, Indianapolis, and now with Baylor College of Medicine, Houston.
Patients in the externalizing group showed significant differences in clinical features from the nonexternalizing group in multiple areas, including earlier age of onset of bipolar disorder, depression, and mania (P less than .001).
Additionally, those in the externalizing group had a higher frequency of self-harm incidents (P less than .001) and suicide attempts (P less than .008). These patients also were more likely to report a history of rapid switching and cycling (P less than .001), the authors reported.
Genetic analysis found numerous “nominally associated” SNPs, though none reached genome-wide significance, Dr. Swaminathan and his colleagues reported.
The findings “suggest the presence of an externalizing disorder subphenotype within [bipolar I disorder] warranting further investigation,” they concluded.
Read the full study here: J Affect Disord. 2015 Jun;178:206-14.
Bipolar disorder might include a distinct subphenotype characterized by externalizing symptoms and specific genetic variations, report Shanker Swaminathan, Ph.D., and his colleagues.
A study of 2,505 bipolar I disorder patients categorized participants into two groups: externalizing (those with comorbid externalizing disorders, plus a subgroup of patients with externalizing symptoms before 15 years of age) and nonexternalizing, wrote Dr. Swaminathan, formerly with the department of medical and molecular genetics at Indiana University, Indianapolis, and now with Baylor College of Medicine, Houston.
Patients in the externalizing group showed significant differences in clinical features from the nonexternalizing group in multiple areas, including earlier age of onset of bipolar disorder, depression, and mania (P less than .001).
Additionally, those in the externalizing group had a higher frequency of self-harm incidents (P less than .001) and suicide attempts (P less than .008). These patients also were more likely to report a history of rapid switching and cycling (P less than .001), the authors reported.
Genetic analysis found numerous “nominally associated” SNPs, though none reached genome-wide significance, Dr. Swaminathan and his colleagues reported.
The findings “suggest the presence of an externalizing disorder subphenotype within [bipolar I disorder] warranting further investigation,” they concluded.
Read the full study here: J Affect Disord. 2015 Jun;178:206-14.
Mental health apps deemed ineffective
Several of the National Health Service’s mental health apps should be removed from the NHS Health Apps Library, because the apps lack evidence of their effectiveness, an article suggests.
The authors, researchers Simon Leigh of the Management School at the University of Liverpool, England, and Steve Flatt of the Liverpool Psychological Therapies Unit Community Interest Company, specifically fire shots at most of the NHS apps for the management of depression and anxiety. After assessing the metrics used to evaluate mental health apps currently in the library, they found that only four of the apps dedicated to the management of depression and anxiety “provide any evidence of patient-reported outcomes to substantiate claims of effectiveness” and only two apply validated metrics, including the generalized anxiety disorder 7-item scale (GAD-7) and 9-item patient health questionnaire (PHQ-9) to assess clinical performance.
“As such, confidence in, and the validity of the claims made by apps that fail to apply such metrics must be considered as low at best, suggesting that the true clinical value of over 85% of NHS accredited mental health apps is at present impossible to determine,” they wrote.
The authors criticized many mental health apps sponsored by the NHS and other organizations, but they also advocate for the use of mental health apps that meet certain specifications, including those that “demonstrate evidence of real-world clinical effectiveness prior to receiving a seal of approval from a world-leading health care system and [be] recommended for patients in need of high-quality psychological interventions.”
Among the reasons the article’s authors list for supporting the idea of mental health apps is the possibility of such tools helping to decrease the wait time for receiving treatment, citing grim expected outcomes for patients currently waiting for mental health services in the United Kingdom. For example, 1 in 6 patients on waiting lists for mental health are expected to attempt suicide.
The writers also laud app-based psychological interventions’ potential to remove financial barriers to treatment and the fact that the use of some mental health apps has led to symptom improvement among patients with mental health issues.
“Given the ever increasing demands and limited supply of NHS mental health services, coupled with barriers to care, including a desire for anonymity, indirect financial costs, and impaired access to treatment centers, the use of apps may not only promote health service efficiency, but also support the NHS in returning to its seminal promise of equal access for equal need,” the authors wrote.
They added: “However, if this is to be an effective venture, this space clearly requires more stringent regulation, vetting, and quality control,”
Reacting to the article, a spokesperson for NHS England said: “This study illustrates that digital tools can act as powerful psychological interventions. It’s vital that patients know which apps to choose and that’s why we are working to upgrade the Health Apps Library, which launched as a pilot site in 2013 and reviews and recommends apps against a defined set of criteria. Earlier this year, we launched the Mental Health Apps Library, which features apps and digital tools that are compliant with IAPT [increasing access to psychological therapies] quality standards and offer National Institute of Health and Care Excellence approved treatments that can demonstrate effectiveness in treating mild and moderate depression and anxiety.”
Read the full article in Evidence-Based Mental Health (doi: 10.1136/eb-20150102203).
Several of the National Health Service’s mental health apps should be removed from the NHS Health Apps Library, because the apps lack evidence of their effectiveness, an article suggests.
The authors, researchers Simon Leigh of the Management School at the University of Liverpool, England, and Steve Flatt of the Liverpool Psychological Therapies Unit Community Interest Company, specifically fire shots at most of the NHS apps for the management of depression and anxiety. After assessing the metrics used to evaluate mental health apps currently in the library, they found that only four of the apps dedicated to the management of depression and anxiety “provide any evidence of patient-reported outcomes to substantiate claims of effectiveness” and only two apply validated metrics, including the generalized anxiety disorder 7-item scale (GAD-7) and 9-item patient health questionnaire (PHQ-9) to assess clinical performance.
“As such, confidence in, and the validity of the claims made by apps that fail to apply such metrics must be considered as low at best, suggesting that the true clinical value of over 85% of NHS accredited mental health apps is at present impossible to determine,” they wrote.
The authors criticized many mental health apps sponsored by the NHS and other organizations, but they also advocate for the use of mental health apps that meet certain specifications, including those that “demonstrate evidence of real-world clinical effectiveness prior to receiving a seal of approval from a world-leading health care system and [be] recommended for patients in need of high-quality psychological interventions.”
Among the reasons the article’s authors list for supporting the idea of mental health apps is the possibility of such tools helping to decrease the wait time for receiving treatment, citing grim expected outcomes for patients currently waiting for mental health services in the United Kingdom. For example, 1 in 6 patients on waiting lists for mental health are expected to attempt suicide.
The writers also laud app-based psychological interventions’ potential to remove financial barriers to treatment and the fact that the use of some mental health apps has led to symptom improvement among patients with mental health issues.
“Given the ever increasing demands and limited supply of NHS mental health services, coupled with barriers to care, including a desire for anonymity, indirect financial costs, and impaired access to treatment centers, the use of apps may not only promote health service efficiency, but also support the NHS in returning to its seminal promise of equal access for equal need,” the authors wrote.
They added: “However, if this is to be an effective venture, this space clearly requires more stringent regulation, vetting, and quality control,”
Reacting to the article, a spokesperson for NHS England said: “This study illustrates that digital tools can act as powerful psychological interventions. It’s vital that patients know which apps to choose and that’s why we are working to upgrade the Health Apps Library, which launched as a pilot site in 2013 and reviews and recommends apps against a defined set of criteria. Earlier this year, we launched the Mental Health Apps Library, which features apps and digital tools that are compliant with IAPT [increasing access to psychological therapies] quality standards and offer National Institute of Health and Care Excellence approved treatments that can demonstrate effectiveness in treating mild and moderate depression and anxiety.”
Read the full article in Evidence-Based Mental Health (doi: 10.1136/eb-20150102203).
Several of the National Health Service’s mental health apps should be removed from the NHS Health Apps Library, because the apps lack evidence of their effectiveness, an article suggests.
The authors, researchers Simon Leigh of the Management School at the University of Liverpool, England, and Steve Flatt of the Liverpool Psychological Therapies Unit Community Interest Company, specifically fire shots at most of the NHS apps for the management of depression and anxiety. After assessing the metrics used to evaluate mental health apps currently in the library, they found that only four of the apps dedicated to the management of depression and anxiety “provide any evidence of patient-reported outcomes to substantiate claims of effectiveness” and only two apply validated metrics, including the generalized anxiety disorder 7-item scale (GAD-7) and 9-item patient health questionnaire (PHQ-9) to assess clinical performance.
“As such, confidence in, and the validity of the claims made by apps that fail to apply such metrics must be considered as low at best, suggesting that the true clinical value of over 85% of NHS accredited mental health apps is at present impossible to determine,” they wrote.
The authors criticized many mental health apps sponsored by the NHS and other organizations, but they also advocate for the use of mental health apps that meet certain specifications, including those that “demonstrate evidence of real-world clinical effectiveness prior to receiving a seal of approval from a world-leading health care system and [be] recommended for patients in need of high-quality psychological interventions.”
Among the reasons the article’s authors list for supporting the idea of mental health apps is the possibility of such tools helping to decrease the wait time for receiving treatment, citing grim expected outcomes for patients currently waiting for mental health services in the United Kingdom. For example, 1 in 6 patients on waiting lists for mental health are expected to attempt suicide.
The writers also laud app-based psychological interventions’ potential to remove financial barriers to treatment and the fact that the use of some mental health apps has led to symptom improvement among patients with mental health issues.
“Given the ever increasing demands and limited supply of NHS mental health services, coupled with barriers to care, including a desire for anonymity, indirect financial costs, and impaired access to treatment centers, the use of apps may not only promote health service efficiency, but also support the NHS in returning to its seminal promise of equal access for equal need,” the authors wrote.
They added: “However, if this is to be an effective venture, this space clearly requires more stringent regulation, vetting, and quality control,”
Reacting to the article, a spokesperson for NHS England said: “This study illustrates that digital tools can act as powerful psychological interventions. It’s vital that patients know which apps to choose and that’s why we are working to upgrade the Health Apps Library, which launched as a pilot site in 2013 and reviews and recommends apps against a defined set of criteria. Earlier this year, we launched the Mental Health Apps Library, which features apps and digital tools that are compliant with IAPT [increasing access to psychological therapies] quality standards and offer National Institute of Health and Care Excellence approved treatments that can demonstrate effectiveness in treating mild and moderate depression and anxiety.”
Read the full article in Evidence-Based Mental Health (doi: 10.1136/eb-20150102203).
FROM EVIDENCE-BASED MENTAL HEALTH
By the numbers
An old joke about old jokes:
Three men have been friends for so long that to save time they tell jokes by number.
“38,” says one. Laughter.
“82,” says another. “That’s a good one!” say the others.
A puzzled onlooker decides to join in. “14!” he says. Stony silence. “What’s the matter?” he asks.
“You told it wrong,” they say.
Numbers are on my mind these days. ICD-10 is here. So many numbers. So little time.
As you recall, the ICD-10 rolled out on Oct. 1 after a year of postponement. Just before that date, a government spokesman sternly announced that doctors hoping for another reprieve were pipe-dreaming. “There will be no further delays,” he said. “Our ability to track Ebola and other epidemics depends on ICD-10.”
Ebola? Google helped me to understand. In the words of one health care consultant, ICD-9 has no specific code for Ebola, forcing doctors to use code 078.89: Other specified diseases due to viruses. This gave U.S. doctors no way to report and track Ebola. People were dying from inadequate classification.
I told this to a nonphysician friend, who asked, “Couldn’t they just make up a code for Ebola?” But that cannot be a good question, because no one of importance has asked it.
Now we have what we need: A98.4, Ebola virus disease, nestled between A98.1, Omsk hemorrhagic fever, and A98.8, Other specified viral hemorrhagic fevers. Note that these “Others” are specified. You must specify.
Now we can code for Ebola. And we have ICD-10, installed at a cost of untold billions of dollars spent by doctors, hospitals, billing services, and insurers. Armies of consultants stand ready to help all parties deal with the conversion. Things are bound to be better, though, for health care and for patients.
It is easy to make fun of ICD-10 by citing absurdities: V91.00XA, Burn due to merchant ship on fire, initial encounter. V97.33XD, Sucked into jet engine, subsequent encounter. (When will the silly fellow learn not to stand so close to jet engines?)
A truer flavor of dealing with the new classification system, however, comes from the degree of specificity – what the business-school types like to call granularity – that we now have to provide for the ordinary problems we clinicians encounter every day:
D23.10 Benign neoplasm, skin of eyelid.
D23.11 Other benign neoplasm of skin of right eyelid.
D23.12 Other benign neoplasm of skin of left eyelid.
Ditto for the ear, including external auditory canal, right or left (D23.21 and D23.22), unspecified parts of the face (D23.30), scalp and neck (D23.4), trunk (D23.5), right and left upper limb including shoulder, (D23.61 and D23.62), right and left lower limb, including hip (D23.71 and D23.72.) If you don’t know what side the lesion is on, you can use D23.70, Other benign neoplasm of skin of unspecified lower limb, including hip. But don’t use an unspecified code. We will be paid less if we don’t specify. Or so they say. Who knows, really? Even the payers don’t seem to know yet. We will find out.
I have a pain in an unspecified upper limb. I won’t say which. You will have to guess.
The same goes not just for skin cancers but for furuncles, lipomas, and so on. Furuncle of foot: L02.629. Furuncle of neck: L02.12. Furuncle of perineum: L02.225. There is also L02.229, furuncle of trunk, unspecified. Don’t go there. Specify. It is vital that we collect data on precisely which body parts furunculize.
In a current film, Matt Damon plays a man on Mars. Were he to return, he might look at all of this coding granularity and think the world has gone mad.
But that cannot be true, since no one of importance thinks so. And then of course there is Ebola.
Jokes by the numbers. Diseases by the numbers. Patients by the numbers. That’s why we became doctors, isn’t it? I don’t recall. It’s been a long time.
I end with a reverie:
The three men who tell jokes by numbers are sitting at tables. Each faces a rectangular card covered with white squares bordered in black. Red counters fill some of the squares.
The interloper who can’t tell a joke stands before them. “Toenail fungus,” he says.
One of the men leaps up.
“B35.1!” he cries.
“BINGO!”
Dr. Rockoff practices dermatology in Brookline, Mass., and is a longtime contributor to Dermatology News. He serves on the clinical faculty at Tufts University, Boston, and has taught senior medical students and other trainees for 30 years. Write to him at [email protected].
An old joke about old jokes:
Three men have been friends for so long that to save time they tell jokes by number.
“38,” says one. Laughter.
“82,” says another. “That’s a good one!” say the others.
A puzzled onlooker decides to join in. “14!” he says. Stony silence. “What’s the matter?” he asks.
“You told it wrong,” they say.
Numbers are on my mind these days. ICD-10 is here. So many numbers. So little time.
As you recall, the ICD-10 rolled out on Oct. 1 after a year of postponement. Just before that date, a government spokesman sternly announced that doctors hoping for another reprieve were pipe-dreaming. “There will be no further delays,” he said. “Our ability to track Ebola and other epidemics depends on ICD-10.”
Ebola? Google helped me to understand. In the words of one health care consultant, ICD-9 has no specific code for Ebola, forcing doctors to use code 078.89: Other specified diseases due to viruses. This gave U.S. doctors no way to report and track Ebola. People were dying from inadequate classification.
I told this to a nonphysician friend, who asked, “Couldn’t they just make up a code for Ebola?” But that cannot be a good question, because no one of importance has asked it.
Now we have what we need: A98.4, Ebola virus disease, nestled between A98.1, Omsk hemorrhagic fever, and A98.8, Other specified viral hemorrhagic fevers. Note that these “Others” are specified. You must specify.
Now we can code for Ebola. And we have ICD-10, installed at a cost of untold billions of dollars spent by doctors, hospitals, billing services, and insurers. Armies of consultants stand ready to help all parties deal with the conversion. Things are bound to be better, though, for health care and for patients.
It is easy to make fun of ICD-10 by citing absurdities: V91.00XA, Burn due to merchant ship on fire, initial encounter. V97.33XD, Sucked into jet engine, subsequent encounter. (When will the silly fellow learn not to stand so close to jet engines?)
A truer flavor of dealing with the new classification system, however, comes from the degree of specificity – what the business-school types like to call granularity – that we now have to provide for the ordinary problems we clinicians encounter every day:
D23.10 Benign neoplasm, skin of eyelid.
D23.11 Other benign neoplasm of skin of right eyelid.
D23.12 Other benign neoplasm of skin of left eyelid.
Ditto for the ear, including external auditory canal, right or left (D23.21 and D23.22), unspecified parts of the face (D23.30), scalp and neck (D23.4), trunk (D23.5), right and left upper limb including shoulder, (D23.61 and D23.62), right and left lower limb, including hip (D23.71 and D23.72.) If you don’t know what side the lesion is on, you can use D23.70, Other benign neoplasm of skin of unspecified lower limb, including hip. But don’t use an unspecified code. We will be paid less if we don’t specify. Or so they say. Who knows, really? Even the payers don’t seem to know yet. We will find out.
I have a pain in an unspecified upper limb. I won’t say which. You will have to guess.
The same goes not just for skin cancers but for furuncles, lipomas, and so on. Furuncle of foot: L02.629. Furuncle of neck: L02.12. Furuncle of perineum: L02.225. There is also L02.229, furuncle of trunk, unspecified. Don’t go there. Specify. It is vital that we collect data on precisely which body parts furunculize.
In a current film, Matt Damon plays a man on Mars. Were he to return, he might look at all of this coding granularity and think the world has gone mad.
But that cannot be true, since no one of importance thinks so. And then of course there is Ebola.
Jokes by the numbers. Diseases by the numbers. Patients by the numbers. That’s why we became doctors, isn’t it? I don’t recall. It’s been a long time.
I end with a reverie:
The three men who tell jokes by numbers are sitting at tables. Each faces a rectangular card covered with white squares bordered in black. Red counters fill some of the squares.
The interloper who can’t tell a joke stands before them. “Toenail fungus,” he says.
One of the men leaps up.
“B35.1!” he cries.
“BINGO!”
Dr. Rockoff practices dermatology in Brookline, Mass., and is a longtime contributor to Dermatology News. He serves on the clinical faculty at Tufts University, Boston, and has taught senior medical students and other trainees for 30 years. Write to him at [email protected].
An old joke about old jokes:
Three men have been friends for so long that to save time they tell jokes by number.
“38,” says one. Laughter.
“82,” says another. “That’s a good one!” say the others.
A puzzled onlooker decides to join in. “14!” he says. Stony silence. “What’s the matter?” he asks.
“You told it wrong,” they say.
Numbers are on my mind these days. ICD-10 is here. So many numbers. So little time.
As you recall, the ICD-10 rolled out on Oct. 1 after a year of postponement. Just before that date, a government spokesman sternly announced that doctors hoping for another reprieve were pipe-dreaming. “There will be no further delays,” he said. “Our ability to track Ebola and other epidemics depends on ICD-10.”
Ebola? Google helped me to understand. In the words of one health care consultant, ICD-9 has no specific code for Ebola, forcing doctors to use code 078.89: Other specified diseases due to viruses. This gave U.S. doctors no way to report and track Ebola. People were dying from inadequate classification.
I told this to a nonphysician friend, who asked, “Couldn’t they just make up a code for Ebola?” But that cannot be a good question, because no one of importance has asked it.
Now we have what we need: A98.4, Ebola virus disease, nestled between A98.1, Omsk hemorrhagic fever, and A98.8, Other specified viral hemorrhagic fevers. Note that these “Others” are specified. You must specify.
Now we can code for Ebola. And we have ICD-10, installed at a cost of untold billions of dollars spent by doctors, hospitals, billing services, and insurers. Armies of consultants stand ready to help all parties deal with the conversion. Things are bound to be better, though, for health care and for patients.
It is easy to make fun of ICD-10 by citing absurdities: V91.00XA, Burn due to merchant ship on fire, initial encounter. V97.33XD, Sucked into jet engine, subsequent encounter. (When will the silly fellow learn not to stand so close to jet engines?)
A truer flavor of dealing with the new classification system, however, comes from the degree of specificity – what the business-school types like to call granularity – that we now have to provide for the ordinary problems we clinicians encounter every day:
D23.10 Benign neoplasm, skin of eyelid.
D23.11 Other benign neoplasm of skin of right eyelid.
D23.12 Other benign neoplasm of skin of left eyelid.
Ditto for the ear, including external auditory canal, right or left (D23.21 and D23.22), unspecified parts of the face (D23.30), scalp and neck (D23.4), trunk (D23.5), right and left upper limb including shoulder, (D23.61 and D23.62), right and left lower limb, including hip (D23.71 and D23.72.) If you don’t know what side the lesion is on, you can use D23.70, Other benign neoplasm of skin of unspecified lower limb, including hip. But don’t use an unspecified code. We will be paid less if we don’t specify. Or so they say. Who knows, really? Even the payers don’t seem to know yet. We will find out.
I have a pain in an unspecified upper limb. I won’t say which. You will have to guess.
The same goes not just for skin cancers but for furuncles, lipomas, and so on. Furuncle of foot: L02.629. Furuncle of neck: L02.12. Furuncle of perineum: L02.225. There is also L02.229, furuncle of trunk, unspecified. Don’t go there. Specify. It is vital that we collect data on precisely which body parts furunculize.
In a current film, Matt Damon plays a man on Mars. Were he to return, he might look at all of this coding granularity and think the world has gone mad.
But that cannot be true, since no one of importance thinks so. And then of course there is Ebola.
Jokes by the numbers. Diseases by the numbers. Patients by the numbers. That’s why we became doctors, isn’t it? I don’t recall. It’s been a long time.
I end with a reverie:
The three men who tell jokes by numbers are sitting at tables. Each faces a rectangular card covered with white squares bordered in black. Red counters fill some of the squares.
The interloper who can’t tell a joke stands before them. “Toenail fungus,” he says.
One of the men leaps up.
“B35.1!” he cries.
“BINGO!”
Dr. Rockoff practices dermatology in Brookline, Mass., and is a longtime contributor to Dermatology News. He serves on the clinical faculty at Tufts University, Boston, and has taught senior medical students and other trainees for 30 years. Write to him at [email protected].
For subacute STEMI, thrombectomy adds no benefit to PCI
The addition of thrombectomy to percutaneous coronary intervention didn’t improve cardiac flow or clinical endpoints any more than PCI alone in patients with a subacute ST-elevation myocardial infarction.
Thrombosis aspiration decreased microvascular obstruction less than 1% more than did PCI alone – 2.5% vs. 3% of the left ventricular mass, Dr. Steffan Desch said at the Transcatheter Cardiovascular Therapeutics annual meeting. There were also no significant differences in infarct size, myocardial salvage, or left ventricular ejection fraction, he said at the meeting sponsored by the Cardiovascular Research Foundation.
“Routine manual thrombectomy and aspiration didn’t show any significant reduction in microvascular obstruction on imaging,” said Dr. Desch of University Heart Center, Lübeck, Germany. “This finding was supported by a variety of secondary endpoints, none of them significantly beneficial.”
The study, which was simultaneously published online (JACC Cardiovasc Interv. 2015. doi: 10.1016/j.jcin.2015.09.010), gives the first firm evidence that clot removal is not particularly helpful to patients who present late after symptom onset. With a mean development time of 28 hours, thrombi in this cohort were more mature, with higher fibrin content than the typically soft material seen in patients with acute ST-elevation myocardial infarction (STEMI). When the clot becomes denser and more organized, it is likely less suitable for aspiration, he said.
The cohort comprised 152 patients who presented in a 12- to 48-hour window after onset of symptoms. They were randomized to either standard PCI or to clot aspiration followed by PCI. The primary outcome was the extent of major vessel occlusion on magnetic resonance imaging conducted 1-4 days after the intervention. Secondary outcomes included infarct size, myocardial salvage, and left ventricular volume and ejection fraction.
Patients were a mean of 66 years old, with typical baseline characteristics. Most were men; hypertension was common (about 70%). About half had signs of ongoing ischemia at admission. The door-to-balloon time was 78 minutes in the combination therapy group and 62 minutes in the PCI-only group. Most (62.5%) had a complete occlusion of the culprit vessel.
In addition to providing no benefit in microvascular occlusion, aspiration did not significantly improve TIMI flow grade above that achieved with PCI only. After the intervention, 78% of those in the thrombectomy group and 69% of those in the PCI group achieved a TIMI flow grade 3. Nor was there a significant difference in myocardial blush grade (70% vs. 65%). When troponin T values were used to assess enzymatic infarct size, they were similar in both groups at 24 and 48 hours.
Clinical outcomes were similar as well. All-cause mortality was 3% in the aspiration group and 5% in the PCI-only group; cardiovascular death occurred in 3% and 4%, respectively. There were no reinfarctions and no stent thromboses. One stroke occurred in a patient who underwent PCI alone.
Dr. Desch noted in the published article that the study took all comers, rather than selecting for specific patient characteristics. Therefore, he said “it is possible that thrombus aspiration might only be advantageous in specific subsets of patients such as those with large thrombus burden, total occlusion or reduced flow.”
The study was funded by a research grant from Medtronic. Dr. Desch reported grant/research support from Medtronic.
The addition of thrombectomy to percutaneous coronary intervention didn’t improve cardiac flow or clinical endpoints any more than PCI alone in patients with a subacute ST-elevation myocardial infarction.
Thrombosis aspiration decreased microvascular obstruction less than 1% more than did PCI alone – 2.5% vs. 3% of the left ventricular mass, Dr. Steffan Desch said at the Transcatheter Cardiovascular Therapeutics annual meeting. There were also no significant differences in infarct size, myocardial salvage, or left ventricular ejection fraction, he said at the meeting sponsored by the Cardiovascular Research Foundation.
“Routine manual thrombectomy and aspiration didn’t show any significant reduction in microvascular obstruction on imaging,” said Dr. Desch of University Heart Center, Lübeck, Germany. “This finding was supported by a variety of secondary endpoints, none of them significantly beneficial.”
The study, which was simultaneously published online (JACC Cardiovasc Interv. 2015. doi: 10.1016/j.jcin.2015.09.010), gives the first firm evidence that clot removal is not particularly helpful to patients who present late after symptom onset. With a mean development time of 28 hours, thrombi in this cohort were more mature, with higher fibrin content than the typically soft material seen in patients with acute ST-elevation myocardial infarction (STEMI). When the clot becomes denser and more organized, it is likely less suitable for aspiration, he said.
The cohort comprised 152 patients who presented in a 12- to 48-hour window after onset of symptoms. They were randomized to either standard PCI or to clot aspiration followed by PCI. The primary outcome was the extent of major vessel occlusion on magnetic resonance imaging conducted 1-4 days after the intervention. Secondary outcomes included infarct size, myocardial salvage, and left ventricular volume and ejection fraction.
Patients were a mean of 66 years old, with typical baseline characteristics. Most were men; hypertension was common (about 70%). About half had signs of ongoing ischemia at admission. The door-to-balloon time was 78 minutes in the combination therapy group and 62 minutes in the PCI-only group. Most (62.5%) had a complete occlusion of the culprit vessel.
In addition to providing no benefit in microvascular occlusion, aspiration did not significantly improve TIMI flow grade above that achieved with PCI only. After the intervention, 78% of those in the thrombectomy group and 69% of those in the PCI group achieved a TIMI flow grade 3. Nor was there a significant difference in myocardial blush grade (70% vs. 65%). When troponin T values were used to assess enzymatic infarct size, they were similar in both groups at 24 and 48 hours.
Clinical outcomes were similar as well. All-cause mortality was 3% in the aspiration group and 5% in the PCI-only group; cardiovascular death occurred in 3% and 4%, respectively. There were no reinfarctions and no stent thromboses. One stroke occurred in a patient who underwent PCI alone.
Dr. Desch noted in the published article that the study took all comers, rather than selecting for specific patient characteristics. Therefore, he said “it is possible that thrombus aspiration might only be advantageous in specific subsets of patients such as those with large thrombus burden, total occlusion or reduced flow.”
The study was funded by a research grant from Medtronic. Dr. Desch reported grant/research support from Medtronic.
The addition of thrombectomy to percutaneous coronary intervention didn’t improve cardiac flow or clinical endpoints any more than PCI alone in patients with a subacute ST-elevation myocardial infarction.
Thrombosis aspiration decreased microvascular obstruction less than 1% more than did PCI alone – 2.5% vs. 3% of the left ventricular mass, Dr. Steffan Desch said at the Transcatheter Cardiovascular Therapeutics annual meeting. There were also no significant differences in infarct size, myocardial salvage, or left ventricular ejection fraction, he said at the meeting sponsored by the Cardiovascular Research Foundation.
“Routine manual thrombectomy and aspiration didn’t show any significant reduction in microvascular obstruction on imaging,” said Dr. Desch of University Heart Center, Lübeck, Germany. “This finding was supported by a variety of secondary endpoints, none of them significantly beneficial.”
The study, which was simultaneously published online (JACC Cardiovasc Interv. 2015. doi: 10.1016/j.jcin.2015.09.010), gives the first firm evidence that clot removal is not particularly helpful to patients who present late after symptom onset. With a mean development time of 28 hours, thrombi in this cohort were more mature, with higher fibrin content than the typically soft material seen in patients with acute ST-elevation myocardial infarction (STEMI). When the clot becomes denser and more organized, it is likely less suitable for aspiration, he said.
The cohort comprised 152 patients who presented in a 12- to 48-hour window after onset of symptoms. They were randomized to either standard PCI or to clot aspiration followed by PCI. The primary outcome was the extent of major vessel occlusion on magnetic resonance imaging conducted 1-4 days after the intervention. Secondary outcomes included infarct size, myocardial salvage, and left ventricular volume and ejection fraction.
Patients were a mean of 66 years old, with typical baseline characteristics. Most were men; hypertension was common (about 70%). About half had signs of ongoing ischemia at admission. The door-to-balloon time was 78 minutes in the combination therapy group and 62 minutes in the PCI-only group. Most (62.5%) had a complete occlusion of the culprit vessel.
In addition to providing no benefit in microvascular occlusion, aspiration did not significantly improve TIMI flow grade above that achieved with PCI only. After the intervention, 78% of those in the thrombectomy group and 69% of those in the PCI group achieved a TIMI flow grade 3. Nor was there a significant difference in myocardial blush grade (70% vs. 65%). When troponin T values were used to assess enzymatic infarct size, they were similar in both groups at 24 and 48 hours.
Clinical outcomes were similar as well. All-cause mortality was 3% in the aspiration group and 5% in the PCI-only group; cardiovascular death occurred in 3% and 4%, respectively. There were no reinfarctions and no stent thromboses. One stroke occurred in a patient who underwent PCI alone.
Dr. Desch noted in the published article that the study took all comers, rather than selecting for specific patient characteristics. Therefore, he said “it is possible that thrombus aspiration might only be advantageous in specific subsets of patients such as those with large thrombus burden, total occlusion or reduced flow.”
The study was funded by a research grant from Medtronic. Dr. Desch reported grant/research support from Medtronic.
AT TCT 2015
Key clinical point: Thrombus aspiration doesn’t reduce microvascular obstruction in subacute STEMI patients undergoing PCI late after symptom onset.
Major finding: Thrombosis aspiration improved microvascular obstruction less than 1% more than did PCI alone – 2.5% vs. 3% of the left ventricular mass – and conferred no other indications of clinical benefit.
Data source: The study randomized 152 patients with late-presentation STEMI to PCI alone or to thrombectomy plus PCI.
Disclosures: The study was funded by a research grant from Medtronic. Dr. Desch reported grant/research support from Medtronic.
Refining confinement
You probably first heard the acronym EDC in medical school, and it replaced what you had been referring to as a “due date.” Of course, you remember the “C” is the first letter of “confinement.” Or is it? You would be forgiven if you thought EDC stood for Estimated Date of Cesarean.
While the practice of keeping new mothers cooped up in their homes for month and placed on dietary, activity, and even hygienic restrictions has all but disappeared in this country, the tradition persists in China. Believing that the process of even a normal delivery renders a woman vulnerable to all sorts of maladies, for 2,000 years Chinese grandmothers have been confining their daughters at home for the first month post partum.
In a recent article in the New York Times, I learned that while confinement continues post partum in China, it has changed among some affluent families so that it is more like spending a month in a high-end spa (“A Tradition for New Mothers in China, Now $27,000 a month” By Dan Levin, Oct. 1, 2015). The new confinement includes breastfeeding instruction, and dietary and activity choices that purport to be more scientifically based than the traditional restrictions. It has become popular with women who can afford it, while in the past confinement could be a month filled with tension between grandmothers and their daughters taking care of their new babies.
I can’t see the new Chinese version of confinement catching on here in North America, but the New York Times article did get me thinking about how we could do a better job helping mothers navigate the choppy waters of those first 30 days post partum. The Chinese are correct that a delivery is an assault on the body of even a previously healthy young woman. Even as one who hasn’t had the experience, I can only imagine it is like pulling an all-nighter (or two) and then running a marathon. Oh, and along the way losing a pint or two of blood.
There are a few families in North America who can afford to hire trained personnel (doulas), but for the most part we aren’t doing a very good job of helping women transition into motherhood. Of course, universal and more liberal family leave policies could make things easier. But simply lessening some of the tension associated with the inevitable return to the workplace isn’t enough. It is unlikely that we have the political will to make the changes to see those policies enacted.
However, there are things that we as pediatricians can do to make the postpartum period safer, healthier, and more comfortable for struggling families. First, we can encourage expectant mothers to make prenatal visits in our offices. While these visits are often little more than doctor shopping, we can ask the families who have committed to our practices to make a second appointment with more educational content. Would we get paid for it? Maybe not, but these second visits could pay for themselves in fewer after-hours calls.
We should do a better job of getting to know a new mother before she goes home from the hospital. What is her discharge hemoglobin? Does she have a history of depression and/or anxiety? Anemia and psychiatric issues can dramatically increase the risk that breastfeeding won’t go well and that post partum depression is more likely to ensue.
Are our offices and lactation consultants really available 24/7? Are we all on the same page when it comes to post partum advice? Do we return calls promptly and make follow-up calls? Are our offices and schedules truly new-mother friendly? Have we made use of all the available home health services that might be required?
The first postpartum month is critical, and new mothers need to be treated as our highest priority, but not confined.
Dr. Wilkoff practiced primary care pediatrics in Brunswick, Maine, for nearly 40 years. He has authored several books on behavioral pediatrics, including “Coping With a Picky Eater.” Email him at [email protected].
You probably first heard the acronym EDC in medical school, and it replaced what you had been referring to as a “due date.” Of course, you remember the “C” is the first letter of “confinement.” Or is it? You would be forgiven if you thought EDC stood for Estimated Date of Cesarean.
While the practice of keeping new mothers cooped up in their homes for month and placed on dietary, activity, and even hygienic restrictions has all but disappeared in this country, the tradition persists in China. Believing that the process of even a normal delivery renders a woman vulnerable to all sorts of maladies, for 2,000 years Chinese grandmothers have been confining their daughters at home for the first month post partum.
In a recent article in the New York Times, I learned that while confinement continues post partum in China, it has changed among some affluent families so that it is more like spending a month in a high-end spa (“A Tradition for New Mothers in China, Now $27,000 a month” By Dan Levin, Oct. 1, 2015). The new confinement includes breastfeeding instruction, and dietary and activity choices that purport to be more scientifically based than the traditional restrictions. It has become popular with women who can afford it, while in the past confinement could be a month filled with tension between grandmothers and their daughters taking care of their new babies.
I can’t see the new Chinese version of confinement catching on here in North America, but the New York Times article did get me thinking about how we could do a better job helping mothers navigate the choppy waters of those first 30 days post partum. The Chinese are correct that a delivery is an assault on the body of even a previously healthy young woman. Even as one who hasn’t had the experience, I can only imagine it is like pulling an all-nighter (or two) and then running a marathon. Oh, and along the way losing a pint or two of blood.
There are a few families in North America who can afford to hire trained personnel (doulas), but for the most part we aren’t doing a very good job of helping women transition into motherhood. Of course, universal and more liberal family leave policies could make things easier. But simply lessening some of the tension associated with the inevitable return to the workplace isn’t enough. It is unlikely that we have the political will to make the changes to see those policies enacted.
However, there are things that we as pediatricians can do to make the postpartum period safer, healthier, and more comfortable for struggling families. First, we can encourage expectant mothers to make prenatal visits in our offices. While these visits are often little more than doctor shopping, we can ask the families who have committed to our practices to make a second appointment with more educational content. Would we get paid for it? Maybe not, but these second visits could pay for themselves in fewer after-hours calls.
We should do a better job of getting to know a new mother before she goes home from the hospital. What is her discharge hemoglobin? Does she have a history of depression and/or anxiety? Anemia and psychiatric issues can dramatically increase the risk that breastfeeding won’t go well and that post partum depression is more likely to ensue.
Are our offices and lactation consultants really available 24/7? Are we all on the same page when it comes to post partum advice? Do we return calls promptly and make follow-up calls? Are our offices and schedules truly new-mother friendly? Have we made use of all the available home health services that might be required?
The first postpartum month is critical, and new mothers need to be treated as our highest priority, but not confined.
Dr. Wilkoff practiced primary care pediatrics in Brunswick, Maine, for nearly 40 years. He has authored several books on behavioral pediatrics, including “Coping With a Picky Eater.” Email him at [email protected].
You probably first heard the acronym EDC in medical school, and it replaced what you had been referring to as a “due date.” Of course, you remember the “C” is the first letter of “confinement.” Or is it? You would be forgiven if you thought EDC stood for Estimated Date of Cesarean.
While the practice of keeping new mothers cooped up in their homes for month and placed on dietary, activity, and even hygienic restrictions has all but disappeared in this country, the tradition persists in China. Believing that the process of even a normal delivery renders a woman vulnerable to all sorts of maladies, for 2,000 years Chinese grandmothers have been confining their daughters at home for the first month post partum.
In a recent article in the New York Times, I learned that while confinement continues post partum in China, it has changed among some affluent families so that it is more like spending a month in a high-end spa (“A Tradition for New Mothers in China, Now $27,000 a month” By Dan Levin, Oct. 1, 2015). The new confinement includes breastfeeding instruction, and dietary and activity choices that purport to be more scientifically based than the traditional restrictions. It has become popular with women who can afford it, while in the past confinement could be a month filled with tension between grandmothers and their daughters taking care of their new babies.
I can’t see the new Chinese version of confinement catching on here in North America, but the New York Times article did get me thinking about how we could do a better job helping mothers navigate the choppy waters of those first 30 days post partum. The Chinese are correct that a delivery is an assault on the body of even a previously healthy young woman. Even as one who hasn’t had the experience, I can only imagine it is like pulling an all-nighter (or two) and then running a marathon. Oh, and along the way losing a pint or two of blood.
There are a few families in North America who can afford to hire trained personnel (doulas), but for the most part we aren’t doing a very good job of helping women transition into motherhood. Of course, universal and more liberal family leave policies could make things easier. But simply lessening some of the tension associated with the inevitable return to the workplace isn’t enough. It is unlikely that we have the political will to make the changes to see those policies enacted.
However, there are things that we as pediatricians can do to make the postpartum period safer, healthier, and more comfortable for struggling families. First, we can encourage expectant mothers to make prenatal visits in our offices. While these visits are often little more than doctor shopping, we can ask the families who have committed to our practices to make a second appointment with more educational content. Would we get paid for it? Maybe not, but these second visits could pay for themselves in fewer after-hours calls.
We should do a better job of getting to know a new mother before she goes home from the hospital. What is her discharge hemoglobin? Does she have a history of depression and/or anxiety? Anemia and psychiatric issues can dramatically increase the risk that breastfeeding won’t go well and that post partum depression is more likely to ensue.
Are our offices and lactation consultants really available 24/7? Are we all on the same page when it comes to post partum advice? Do we return calls promptly and make follow-up calls? Are our offices and schedules truly new-mother friendly? Have we made use of all the available home health services that might be required?
The first postpartum month is critical, and new mothers need to be treated as our highest priority, but not confined.
Dr. Wilkoff practiced primary care pediatrics in Brunswick, Maine, for nearly 40 years. He has authored several books on behavioral pediatrics, including “Coping With a Picky Eater.” Email him at [email protected].
CML outcomes in the age of TKIs
CHICAGO – Major molecular response rates at 12 months were better among chronic myeloid leukemia patients treated with dasatinib/nilotinib vs. imatinib for first-line therapy, and among patients enrolled vs. not enrolled in clinical trials, in a retrospective review of patients treated with first-line tyrosine kinase inhibitors between 2002 and 2014.
The overall rates of major molecular response (MMR) at 12 and 24 months in the 51 patients in the study were 23.5% (12 patients) and 44.9% (22 patients), which was comparable to historical data, Dr. Isabelle Phuong Le reported in a poster at the American Society of Hematology Meeting on Hematologic Malignancies.
The MMR rate among those treated with dasatinib/nilotinib first line was 53%, compared with 10% for those treated with imatinib, said Dr. Le of the University of Texas Health Science Center, San Antonio.
Further the MMR rates at 12 and 24 months among those enrolled vs. not enrolled in clinical trials were 83% vs 15% and 100% vs. 45%, respectively, she noted.
Patients in the analysis were adults with a mean age of 44.6 years who had chronic phase chronic myeloid leukemia (CML). Almost half (49%) were Hispanic, and 56.9% were women. Two-thirds (66.7%) received imatinib first line, and 33.3% received dasatinib/nilotinib first line. More than a third (37.2%) were younger than age 40 years, 15% had a language barrier, and 11.7% were enrolled in clinical trials.
No differences in the MMR rates at 12 and 24 months were seen between Hispanics and non-Hispanics, those with and without language barrier, or men and women, nor were any differences seen between those with more than three lines of therapy and those with three or fewer lines of therapy, or between patients older vs. younger than 40 years, although there was a trend toward improved response rates in older patients, she noted.
“Our patients have similar MMR rates at 12 months and 24 months compared to historical data,” Dr. Le wrote, noting that the findings regarding improved MMR rates with dasatinib/nilotinib were expected.
The findings regarding improved MMR rates among those enrolled in clinical trials could be due to “selective patients as clinical trial patients, more controlled and monitored treatment, frequent follow-ups, and better education regarding disease and treatment,” she said, concluding that “ we can improve treatment response rates in our patients with better disease education.”
Dr. Le reported having no disclosures.
CHICAGO – Major molecular response rates at 12 months were better among chronic myeloid leukemia patients treated with dasatinib/nilotinib vs. imatinib for first-line therapy, and among patients enrolled vs. not enrolled in clinical trials, in a retrospective review of patients treated with first-line tyrosine kinase inhibitors between 2002 and 2014.
The overall rates of major molecular response (MMR) at 12 and 24 months in the 51 patients in the study were 23.5% (12 patients) and 44.9% (22 patients), which was comparable to historical data, Dr. Isabelle Phuong Le reported in a poster at the American Society of Hematology Meeting on Hematologic Malignancies.
The MMR rate among those treated with dasatinib/nilotinib first line was 53%, compared with 10% for those treated with imatinib, said Dr. Le of the University of Texas Health Science Center, San Antonio.
Further the MMR rates at 12 and 24 months among those enrolled vs. not enrolled in clinical trials were 83% vs 15% and 100% vs. 45%, respectively, she noted.
Patients in the analysis were adults with a mean age of 44.6 years who had chronic phase chronic myeloid leukemia (CML). Almost half (49%) were Hispanic, and 56.9% were women. Two-thirds (66.7%) received imatinib first line, and 33.3% received dasatinib/nilotinib first line. More than a third (37.2%) were younger than age 40 years, 15% had a language barrier, and 11.7% were enrolled in clinical trials.
No differences in the MMR rates at 12 and 24 months were seen between Hispanics and non-Hispanics, those with and without language barrier, or men and women, nor were any differences seen between those with more than three lines of therapy and those with three or fewer lines of therapy, or between patients older vs. younger than 40 years, although there was a trend toward improved response rates in older patients, she noted.
“Our patients have similar MMR rates at 12 months and 24 months compared to historical data,” Dr. Le wrote, noting that the findings regarding improved MMR rates with dasatinib/nilotinib were expected.
The findings regarding improved MMR rates among those enrolled in clinical trials could be due to “selective patients as clinical trial patients, more controlled and monitored treatment, frequent follow-ups, and better education regarding disease and treatment,” she said, concluding that “ we can improve treatment response rates in our patients with better disease education.”
Dr. Le reported having no disclosures.
CHICAGO – Major molecular response rates at 12 months were better among chronic myeloid leukemia patients treated with dasatinib/nilotinib vs. imatinib for first-line therapy, and among patients enrolled vs. not enrolled in clinical trials, in a retrospective review of patients treated with first-line tyrosine kinase inhibitors between 2002 and 2014.
The overall rates of major molecular response (MMR) at 12 and 24 months in the 51 patients in the study were 23.5% (12 patients) and 44.9% (22 patients), which was comparable to historical data, Dr. Isabelle Phuong Le reported in a poster at the American Society of Hematology Meeting on Hematologic Malignancies.
The MMR rate among those treated with dasatinib/nilotinib first line was 53%, compared with 10% for those treated with imatinib, said Dr. Le of the University of Texas Health Science Center, San Antonio.
Further the MMR rates at 12 and 24 months among those enrolled vs. not enrolled in clinical trials were 83% vs 15% and 100% vs. 45%, respectively, she noted.
Patients in the analysis were adults with a mean age of 44.6 years who had chronic phase chronic myeloid leukemia (CML). Almost half (49%) were Hispanic, and 56.9% were women. Two-thirds (66.7%) received imatinib first line, and 33.3% received dasatinib/nilotinib first line. More than a third (37.2%) were younger than age 40 years, 15% had a language barrier, and 11.7% were enrolled in clinical trials.
No differences in the MMR rates at 12 and 24 months were seen between Hispanics and non-Hispanics, those with and without language barrier, or men and women, nor were any differences seen between those with more than three lines of therapy and those with three or fewer lines of therapy, or between patients older vs. younger than 40 years, although there was a trend toward improved response rates in older patients, she noted.
“Our patients have similar MMR rates at 12 months and 24 months compared to historical data,” Dr. Le wrote, noting that the findings regarding improved MMR rates with dasatinib/nilotinib were expected.
The findings regarding improved MMR rates among those enrolled in clinical trials could be due to “selective patients as clinical trial patients, more controlled and monitored treatment, frequent follow-ups, and better education regarding disease and treatment,” she said, concluding that “ we can improve treatment response rates in our patients with better disease education.”
Dr. Le reported having no disclosures.
AT MHM 2015
Key clinical point: Major molecular response rates at 12 months were better among CML patients treated with dasatinib/nilotinib vs. imatinib for first-line therapy, and among patients enrolled vs. not enrolled in clinical trials.
Major finding: The major molecular response rates at 12 and 24 months were 23.5% and 44.9%, which is comparable to historical data.
Data source: A retrospective review of 51 cases.
Disclosures: Dr. Le reported having no disclosures.
New CPR guide sets compression limits, scratches vasopressin
New guidelines on cardiopulmonary resuscitation (CPR) and emergency cardiovascular care (ECC) set upper limits on chest compression rate and depth, add naloxone to the care of suspected opioid abusers, and remove vasopressin from the advanced cardiac life support (ACLS) algorithm.
The American Heart Association published its revised guidelines Oct. 15 in Circulation. The AHA released its previous guidelines in 2010.
“Everyone has a role to play in the chain of survival – from bystanders to dispatchers, emergency responders to health care providers,” Dr. Mark Creager said in a statement. “When everyone knows their role, knows CPR, and works together, we can dramatically improve cardiac arrest victims’ chances of survival,” said Dr. Creager, AHA president and director of the Heart and Vascular Center at Dartmouth-Hitchcock Medical Center, Lebanon, N.H.
The 2015 guidelines’ new recommendations include the following:
• Resuscitation pathways. The guidelines note that the resuscitation pathways are very different for patients who experience cardiac arrest present in either a hospital setting (IHCA) or out-of-hospital setting (OHCA). In OHCA, the patient depends on lay rescuers to not only recognize the situation but also call for help, initiate CPR, and, if available, administer defibrillation until emergency medical personnel arrive. However, IHCA involves prevention of cardiac arrest and smooth delivery of care in a multidisciplinary setting.
• Layperson CPR. Untrained lay rescuers should provide compression-only CPR for OHCA. Trained lay rescuers who are able to provide rescue breaths should begin CPR with compressions followed by breaths at a ratio of 30 compressions to two breaths. Compression-only CPR is easier to perform for untrained lay rescuers, the guidelines note, and survival rates are similar using CPR with or without rescue breaths in adult cardiac arrest with a cardiac etiology.
• Compression rate and depth. The new guidelines set upper limits on chest compression depth and heart rate, recommending a compression rate of 100-120 compressions per minute with a depth of at least 2 inches, not to exceed 2.4 inches in adults.
• Social media dispatching. Despite limited evidence, the guideline authors said that it may be reasonable for communities to use social media technologies to alert lay rescuers with mobile phones about nearby OHCA cases.
• Naloxone and opioid addiction. Also new to the guidelines is the recommended use of naloxone for patients with suspected or known opioid addiction by appropriately trained lay rescuers or basic life support (BLS) providers.
• CPR training. The guidelines highlight several changes to simplify health care provider training in CPR. For example, trained rescuers can simultaneously perform some tasks to reduce the time to initiate chest compressions. Likewise, in a team of trained rescuers, multiple steps such as activating the emergency response system, chest compression, ventilation, and defibrillator retrieval can be accomplished simultaneously.
• High-quality CPR. Finally, the guidelines focus on emphasizing high-quality CPR with adequate compression rate and depth, complete chest recoil, few interruptions to compressions, and appropriate ventilation.
The guidelines offer several changes to advanced cardiac life support (ACLS). The algorithm was simplified by removing vasopressin, because the authors note that “the combined use of vasopressin and epinephrine offers no advantage to using standard-dose epinephrine in cardiac arrest.”
Likewise, the guidelines note conflicting studies to support the use of lidocaine after return of spontaneous circulation (ROSC). “However, the initiation or continuation of lidocaine may be considered immediately after ROSC from VF/pulseless ventricular tachycardia cardiac arrest,” the guideline authors wrote. Finally, the guidelines highlight updates in post–cardiac arrest care, including a wider range of target temperatures, between 32° C and 36° C, to be maintained for at least 24 hours in comatose adults with ROSC after cardiac arrest. In comparison, the 2010 guidelines called for a target temperature range of 32° C to 34° C for 12-24 hours. The guidelines also detail new updates for acute coronary syndrome, pediatric BLS, pediatric ACLS, and neonatal resuscitation.
As the AHA updates its CPR guidelines, it’s also important for lay rescuers and health providers to update their own training, noted Dr. Clifton Callaway, chair of the AHA’s Emergency Cardiovascular Care (ECC) committee.
“Research shows resuscitation skills can decline within a few months after training – far before the 2-year period in which basic and advanced life support skills are currently evaluated,” cautioned Dr. Callaway, professor of emergency medicine at the University of Pittsburgh. “Frequent training with shorter intervals of basic and advanced cardiovascular life support skills may be helpful for providers who are likely to encounter a cardiac arrest to ensure the patient receives high-quality CPR,” he added.
New guidelines on cardiopulmonary resuscitation (CPR) and emergency cardiovascular care (ECC) set upper limits on chest compression rate and depth, add naloxone to the care of suspected opioid abusers, and remove vasopressin from the advanced cardiac life support (ACLS) algorithm.
The American Heart Association published its revised guidelines Oct. 15 in Circulation. The AHA released its previous guidelines in 2010.
“Everyone has a role to play in the chain of survival – from bystanders to dispatchers, emergency responders to health care providers,” Dr. Mark Creager said in a statement. “When everyone knows their role, knows CPR, and works together, we can dramatically improve cardiac arrest victims’ chances of survival,” said Dr. Creager, AHA president and director of the Heart and Vascular Center at Dartmouth-Hitchcock Medical Center, Lebanon, N.H.
The 2015 guidelines’ new recommendations include the following:
• Resuscitation pathways. The guidelines note that the resuscitation pathways are very different for patients who experience cardiac arrest present in either a hospital setting (IHCA) or out-of-hospital setting (OHCA). In OHCA, the patient depends on lay rescuers to not only recognize the situation but also call for help, initiate CPR, and, if available, administer defibrillation until emergency medical personnel arrive. However, IHCA involves prevention of cardiac arrest and smooth delivery of care in a multidisciplinary setting.
• Layperson CPR. Untrained lay rescuers should provide compression-only CPR for OHCA. Trained lay rescuers who are able to provide rescue breaths should begin CPR with compressions followed by breaths at a ratio of 30 compressions to two breaths. Compression-only CPR is easier to perform for untrained lay rescuers, the guidelines note, and survival rates are similar using CPR with or without rescue breaths in adult cardiac arrest with a cardiac etiology.
• Compression rate and depth. The new guidelines set upper limits on chest compression depth and heart rate, recommending a compression rate of 100-120 compressions per minute with a depth of at least 2 inches, not to exceed 2.4 inches in adults.
• Social media dispatching. Despite limited evidence, the guideline authors said that it may be reasonable for communities to use social media technologies to alert lay rescuers with mobile phones about nearby OHCA cases.
• Naloxone and opioid addiction. Also new to the guidelines is the recommended use of naloxone for patients with suspected or known opioid addiction by appropriately trained lay rescuers or basic life support (BLS) providers.
• CPR training. The guidelines highlight several changes to simplify health care provider training in CPR. For example, trained rescuers can simultaneously perform some tasks to reduce the time to initiate chest compressions. Likewise, in a team of trained rescuers, multiple steps such as activating the emergency response system, chest compression, ventilation, and defibrillator retrieval can be accomplished simultaneously.
• High-quality CPR. Finally, the guidelines focus on emphasizing high-quality CPR with adequate compression rate and depth, complete chest recoil, few interruptions to compressions, and appropriate ventilation.
The guidelines offer several changes to advanced cardiac life support (ACLS). The algorithm was simplified by removing vasopressin, because the authors note that “the combined use of vasopressin and epinephrine offers no advantage to using standard-dose epinephrine in cardiac arrest.”
Likewise, the guidelines note conflicting studies to support the use of lidocaine after return of spontaneous circulation (ROSC). “However, the initiation or continuation of lidocaine may be considered immediately after ROSC from VF/pulseless ventricular tachycardia cardiac arrest,” the guideline authors wrote. Finally, the guidelines highlight updates in post–cardiac arrest care, including a wider range of target temperatures, between 32° C and 36° C, to be maintained for at least 24 hours in comatose adults with ROSC after cardiac arrest. In comparison, the 2010 guidelines called for a target temperature range of 32° C to 34° C for 12-24 hours. The guidelines also detail new updates for acute coronary syndrome, pediatric BLS, pediatric ACLS, and neonatal resuscitation.
As the AHA updates its CPR guidelines, it’s also important for lay rescuers and health providers to update their own training, noted Dr. Clifton Callaway, chair of the AHA’s Emergency Cardiovascular Care (ECC) committee.
“Research shows resuscitation skills can decline within a few months after training – far before the 2-year period in which basic and advanced life support skills are currently evaluated,” cautioned Dr. Callaway, professor of emergency medicine at the University of Pittsburgh. “Frequent training with shorter intervals of basic and advanced cardiovascular life support skills may be helpful for providers who are likely to encounter a cardiac arrest to ensure the patient receives high-quality CPR,” he added.
New guidelines on cardiopulmonary resuscitation (CPR) and emergency cardiovascular care (ECC) set upper limits on chest compression rate and depth, add naloxone to the care of suspected opioid abusers, and remove vasopressin from the advanced cardiac life support (ACLS) algorithm.
The American Heart Association published its revised guidelines Oct. 15 in Circulation. The AHA released its previous guidelines in 2010.
“Everyone has a role to play in the chain of survival – from bystanders to dispatchers, emergency responders to health care providers,” Dr. Mark Creager said in a statement. “When everyone knows their role, knows CPR, and works together, we can dramatically improve cardiac arrest victims’ chances of survival,” said Dr. Creager, AHA president and director of the Heart and Vascular Center at Dartmouth-Hitchcock Medical Center, Lebanon, N.H.
The 2015 guidelines’ new recommendations include the following:
• Resuscitation pathways. The guidelines note that the resuscitation pathways are very different for patients who experience cardiac arrest present in either a hospital setting (IHCA) or out-of-hospital setting (OHCA). In OHCA, the patient depends on lay rescuers to not only recognize the situation but also call for help, initiate CPR, and, if available, administer defibrillation until emergency medical personnel arrive. However, IHCA involves prevention of cardiac arrest and smooth delivery of care in a multidisciplinary setting.
• Layperson CPR. Untrained lay rescuers should provide compression-only CPR for OHCA. Trained lay rescuers who are able to provide rescue breaths should begin CPR with compressions followed by breaths at a ratio of 30 compressions to two breaths. Compression-only CPR is easier to perform for untrained lay rescuers, the guidelines note, and survival rates are similar using CPR with or without rescue breaths in adult cardiac arrest with a cardiac etiology.
• Compression rate and depth. The new guidelines set upper limits on chest compression depth and heart rate, recommending a compression rate of 100-120 compressions per minute with a depth of at least 2 inches, not to exceed 2.4 inches in adults.
• Social media dispatching. Despite limited evidence, the guideline authors said that it may be reasonable for communities to use social media technologies to alert lay rescuers with mobile phones about nearby OHCA cases.
• Naloxone and opioid addiction. Also new to the guidelines is the recommended use of naloxone for patients with suspected or known opioid addiction by appropriately trained lay rescuers or basic life support (BLS) providers.
• CPR training. The guidelines highlight several changes to simplify health care provider training in CPR. For example, trained rescuers can simultaneously perform some tasks to reduce the time to initiate chest compressions. Likewise, in a team of trained rescuers, multiple steps such as activating the emergency response system, chest compression, ventilation, and defibrillator retrieval can be accomplished simultaneously.
• High-quality CPR. Finally, the guidelines focus on emphasizing high-quality CPR with adequate compression rate and depth, complete chest recoil, few interruptions to compressions, and appropriate ventilation.
The guidelines offer several changes to advanced cardiac life support (ACLS). The algorithm was simplified by removing vasopressin, because the authors note that “the combined use of vasopressin and epinephrine offers no advantage to using standard-dose epinephrine in cardiac arrest.”
Likewise, the guidelines note conflicting studies to support the use of lidocaine after return of spontaneous circulation (ROSC). “However, the initiation or continuation of lidocaine may be considered immediately after ROSC from VF/pulseless ventricular tachycardia cardiac arrest,” the guideline authors wrote. Finally, the guidelines highlight updates in post–cardiac arrest care, including a wider range of target temperatures, between 32° C and 36° C, to be maintained for at least 24 hours in comatose adults with ROSC after cardiac arrest. In comparison, the 2010 guidelines called for a target temperature range of 32° C to 34° C for 12-24 hours. The guidelines also detail new updates for acute coronary syndrome, pediatric BLS, pediatric ACLS, and neonatal resuscitation.
As the AHA updates its CPR guidelines, it’s also important for lay rescuers and health providers to update their own training, noted Dr. Clifton Callaway, chair of the AHA’s Emergency Cardiovascular Care (ECC) committee.
“Research shows resuscitation skills can decline within a few months after training – far before the 2-year period in which basic and advanced life support skills are currently evaluated,” cautioned Dr. Callaway, professor of emergency medicine at the University of Pittsburgh. “Frequent training with shorter intervals of basic and advanced cardiovascular life support skills may be helpful for providers who are likely to encounter a cardiac arrest to ensure the patient receives high-quality CPR,” he added.
FROM CIRCULATION