User login
Osteoarthritis cases projected to balloon over next 30 years
TOPLINE:
METHODOLOGY:
- Researchers estimated the prevalence of osteoarthritis in 204 countries and territories from 1990 to 2020 and projected prevalence levels for the year 2050.
- Population-based surveys offered data from 26 countries for knee osteoarthritis, 23 countries for hip osteoarthritis, and 42 countries for hand osteoarthritis. Researchers used U.S. insurance claims to estimate prevalence for other osteoarthritis types.
- Similar analyses were conducted in 2010 and 2017.
TAKEAWAY:
- Osteoarthritis cases worldwide have grown by an estimated 132.2% since 1990. Population growth and aging were identified as major contributing factors.
- In 2020, an estimated 595 million people had osteoarthritis. From 2020 to 2050, cases of osteoarthritis in the knee are expected to grow by 74.9%, in the hand by 48.6%, in the hip by 78.6%, and in other locations by 95.1%.
- Years lived with disability (age-standardized rate) grew from an estimated 233 per 100,000 in 1990 to 255 per 100,000 in 2020, an increase of 9.5%.
- High body mass index contributed to 20.4% of cases.
IN PRACTICE:
In “a major challenge to health systems,” osteoarthritis may affect nearly 1 billion people in 2050.
SOURCE:
The Global Burden of Disease 2021 Osteoarthritis Collaborators, led by Jaimie D. Steinmetz, PhD, MSc, of the Institute for Health Metrics and Evaluation, Seattle, conducted the study, which was published in The Lancet Rheumatology.
LIMITATIONS:
Limited data, heavy reliance on U.S. insurance data, and other factors may have skewed the results.
DISCLOSURES:
The study was supported by the Bill & Melinda Gates Foundation, the Institute of Bone and Joint Research, and the Global Alliance for Musculoskeletal Health. Multiple authors reported numerous disclosures.
A version of this article first appeared on Medscape.com.
TOPLINE:
METHODOLOGY:
- Researchers estimated the prevalence of osteoarthritis in 204 countries and territories from 1990 to 2020 and projected prevalence levels for the year 2050.
- Population-based surveys offered data from 26 countries for knee osteoarthritis, 23 countries for hip osteoarthritis, and 42 countries for hand osteoarthritis. Researchers used U.S. insurance claims to estimate prevalence for other osteoarthritis types.
- Similar analyses were conducted in 2010 and 2017.
TAKEAWAY:
- Osteoarthritis cases worldwide have grown by an estimated 132.2% since 1990. Population growth and aging were identified as major contributing factors.
- In 2020, an estimated 595 million people had osteoarthritis. From 2020 to 2050, cases of osteoarthritis in the knee are expected to grow by 74.9%, in the hand by 48.6%, in the hip by 78.6%, and in other locations by 95.1%.
- Years lived with disability (age-standardized rate) grew from an estimated 233 per 100,000 in 1990 to 255 per 100,000 in 2020, an increase of 9.5%.
- High body mass index contributed to 20.4% of cases.
IN PRACTICE:
In “a major challenge to health systems,” osteoarthritis may affect nearly 1 billion people in 2050.
SOURCE:
The Global Burden of Disease 2021 Osteoarthritis Collaborators, led by Jaimie D. Steinmetz, PhD, MSc, of the Institute for Health Metrics and Evaluation, Seattle, conducted the study, which was published in The Lancet Rheumatology.
LIMITATIONS:
Limited data, heavy reliance on U.S. insurance data, and other factors may have skewed the results.
DISCLOSURES:
The study was supported by the Bill & Melinda Gates Foundation, the Institute of Bone and Joint Research, and the Global Alliance for Musculoskeletal Health. Multiple authors reported numerous disclosures.
A version of this article first appeared on Medscape.com.
TOPLINE:
METHODOLOGY:
- Researchers estimated the prevalence of osteoarthritis in 204 countries and territories from 1990 to 2020 and projected prevalence levels for the year 2050.
- Population-based surveys offered data from 26 countries for knee osteoarthritis, 23 countries for hip osteoarthritis, and 42 countries for hand osteoarthritis. Researchers used U.S. insurance claims to estimate prevalence for other osteoarthritis types.
- Similar analyses were conducted in 2010 and 2017.
TAKEAWAY:
- Osteoarthritis cases worldwide have grown by an estimated 132.2% since 1990. Population growth and aging were identified as major contributing factors.
- In 2020, an estimated 595 million people had osteoarthritis. From 2020 to 2050, cases of osteoarthritis in the knee are expected to grow by 74.9%, in the hand by 48.6%, in the hip by 78.6%, and in other locations by 95.1%.
- Years lived with disability (age-standardized rate) grew from an estimated 233 per 100,000 in 1990 to 255 per 100,000 in 2020, an increase of 9.5%.
- High body mass index contributed to 20.4% of cases.
IN PRACTICE:
In “a major challenge to health systems,” osteoarthritis may affect nearly 1 billion people in 2050.
SOURCE:
The Global Burden of Disease 2021 Osteoarthritis Collaborators, led by Jaimie D. Steinmetz, PhD, MSc, of the Institute for Health Metrics and Evaluation, Seattle, conducted the study, which was published in The Lancet Rheumatology.
LIMITATIONS:
Limited data, heavy reliance on U.S. insurance data, and other factors may have skewed the results.
DISCLOSURES:
The study was supported by the Bill & Melinda Gates Foundation, the Institute of Bone and Joint Research, and the Global Alliance for Musculoskeletal Health. Multiple authors reported numerous disclosures.
A version of this article first appeared on Medscape.com.
FROM THE LANCET RHEUMATOLOGY
Improving Germline Genetic Testing Among Veterans With High Risk, Very High Risk and Metastatic Prostate Cancer
PURPOSE
To improve germline genetic testing among Veterans with high risk, very high risk and metastatic prostate cancer.
BACKGROUND
During our Commission on Cancer survey in 2021, it was noted that the Detroit VA’s referrals for germline genetic testing and counseling were extremely low. In 2020, only 1 Veteran was referred for prostate germline genetic testing and counseling and only 8 Veterans were referred in 2021. It was felt that the need to refer Veterans outside of the Detroit VA may have contributed to these low numbers. Our Cancer Committee chose prostate cancer as a disease to focus on. We chose a timeline of one year to implement our process.
METHODS
We made testing and counseling locally accessible to Veterans and encouraged medical oncology providers to make it part of the care of Veterans with high risk, very high risk and metastatic prostate cancer. We sought the assistance of the VA’s National Precision Oncology Program and were able to secure financial and logistical support to perform germline molecular prostate panel testing at the Detroit VA. We were also able to identify a cancer genetic specialist at the Ann Arbor VA that would perform genetic counseling among this group of patients based on their test results. Our medical oncology providers identified Veterans meeting the criteria for testing. Education regarding germline testing, its benefits and implications were conducted with Veterans, and performed after obtaining their informed consent in collaboration with our pathology department. The specimen is then sent to a VA central laboratory for processing. Detroit VA providers are alerted by the local laboratory once results are available. Veterans are then referred to the genetic counseling specialist based on the results. Some of these counseling visits are done virtually for the Veteran’s convenience.
DATA ANALYSIS
A retrospective chart analysis was used to collect the data.
RESULTS
After the implementation of our initiative, 97 Veterans with high risk, very high risk or metastatic prostate cancer were educated on the benefits of germline genetic testing, 87 of whom agreed to be tested. As of 4/2/23, 48 tests have already been performed. Pathogenic variants were recorded on 2 Veterans so far. One was for BRCA2 and KDM6A, and the other was for ATM. Data collection and recording is on-going.
IMPLICATIONS
Improving accessibility and incorporating genetic testing and counseling in cancer care can improve their utilization.
PURPOSE
To improve germline genetic testing among Veterans with high risk, very high risk and metastatic prostate cancer.
BACKGROUND
During our Commission on Cancer survey in 2021, it was noted that the Detroit VA’s referrals for germline genetic testing and counseling were extremely low. In 2020, only 1 Veteran was referred for prostate germline genetic testing and counseling and only 8 Veterans were referred in 2021. It was felt that the need to refer Veterans outside of the Detroit VA may have contributed to these low numbers. Our Cancer Committee chose prostate cancer as a disease to focus on. We chose a timeline of one year to implement our process.
METHODS
We made testing and counseling locally accessible to Veterans and encouraged medical oncology providers to make it part of the care of Veterans with high risk, very high risk and metastatic prostate cancer. We sought the assistance of the VA’s National Precision Oncology Program and were able to secure financial and logistical support to perform germline molecular prostate panel testing at the Detroit VA. We were also able to identify a cancer genetic specialist at the Ann Arbor VA that would perform genetic counseling among this group of patients based on their test results. Our medical oncology providers identified Veterans meeting the criteria for testing. Education regarding germline testing, its benefits and implications were conducted with Veterans, and performed after obtaining their informed consent in collaboration with our pathology department. The specimen is then sent to a VA central laboratory for processing. Detroit VA providers are alerted by the local laboratory once results are available. Veterans are then referred to the genetic counseling specialist based on the results. Some of these counseling visits are done virtually for the Veteran’s convenience.
DATA ANALYSIS
A retrospective chart analysis was used to collect the data.
RESULTS
After the implementation of our initiative, 97 Veterans with high risk, very high risk or metastatic prostate cancer were educated on the benefits of germline genetic testing, 87 of whom agreed to be tested. As of 4/2/23, 48 tests have already been performed. Pathogenic variants were recorded on 2 Veterans so far. One was for BRCA2 and KDM6A, and the other was for ATM. Data collection and recording is on-going.
IMPLICATIONS
Improving accessibility and incorporating genetic testing and counseling in cancer care can improve their utilization.
PURPOSE
To improve germline genetic testing among Veterans with high risk, very high risk and metastatic prostate cancer.
BACKGROUND
During our Commission on Cancer survey in 2021, it was noted that the Detroit VA’s referrals for germline genetic testing and counseling were extremely low. In 2020, only 1 Veteran was referred for prostate germline genetic testing and counseling and only 8 Veterans were referred in 2021. It was felt that the need to refer Veterans outside of the Detroit VA may have contributed to these low numbers. Our Cancer Committee chose prostate cancer as a disease to focus on. We chose a timeline of one year to implement our process.
METHODS
We made testing and counseling locally accessible to Veterans and encouraged medical oncology providers to make it part of the care of Veterans with high risk, very high risk and metastatic prostate cancer. We sought the assistance of the VA’s National Precision Oncology Program and were able to secure financial and logistical support to perform germline molecular prostate panel testing at the Detroit VA. We were also able to identify a cancer genetic specialist at the Ann Arbor VA that would perform genetic counseling among this group of patients based on their test results. Our medical oncology providers identified Veterans meeting the criteria for testing. Education regarding germline testing, its benefits and implications were conducted with Veterans, and performed after obtaining their informed consent in collaboration with our pathology department. The specimen is then sent to a VA central laboratory for processing. Detroit VA providers are alerted by the local laboratory once results are available. Veterans are then referred to the genetic counseling specialist based on the results. Some of these counseling visits are done virtually for the Veteran’s convenience.
DATA ANALYSIS
A retrospective chart analysis was used to collect the data.
RESULTS
After the implementation of our initiative, 97 Veterans with high risk, very high risk or metastatic prostate cancer were educated on the benefits of germline genetic testing, 87 of whom agreed to be tested. As of 4/2/23, 48 tests have already been performed. Pathogenic variants were recorded on 2 Veterans so far. One was for BRCA2 and KDM6A, and the other was for ATM. Data collection and recording is on-going.
IMPLICATIONS
Improving accessibility and incorporating genetic testing and counseling in cancer care can improve their utilization.
Minimizing atrial pacing no benefit in sinus node disease: DANPACE II
suggest results of a trial that randomly assigned patients with SND who had received their first pacemaker implant to one of two pacing programs.
Over 2 years of follow-up with remote monitoring, there was no difference in the primary endpoint of time to first device-detected episode of AF lasting more than 6 minutes, reported Max Brix Kronborg, MD, PhD, department of cardiology, Aarhus University Hospital, Denmark.
The study, DANPACE II, excluded patients with permanent or persistent AF or persistent bradycardia prior to or at the time of enrollment.
The findings were presented at annual congress of the European Society of Cardiology and were published online simultaneously in the European Heart Journal.
The 539 participants in the trial were randomly assigned in a 1:1 ratio to a pacing program of 60 beats/minute with rate-adaptive pacing (DDR-60) or 40 beats/minute without rate-adaptive pacing (DDD-40). All patients were equipped with remote monitoring and were followed for 2 years. Tracings were adjudicated for atrial high-rate episodes by experienced device specialists, Dr. Kronborg said.
No difference seen in primary outcome
When graphed, curves for the primary outcome in the two groups were essentially superimposable. For the secondary outcomes of AF lasting more than 6 hours and AF lasting more than 24 hours, there was a modest but progressive separation in the lines favoring the DDR-60 group for both. However, the P value did not approach significance in the first of these endpoints (P = .35) and remained only a trend (P = .08) in the second.
There were no substantial differences in results when patients were stratified by age (> 73 years vs. younger), gender (women represented 50% of patients), PR interval (> 150 milliseconds vs. less), or history of AF prior to study entry; the latter group represented approximately 40% of the trial participants.
There was a between-group difference in the primary composite safety endpoint of syncope and presyncope. By 2 years, 13% of those in the DDR-60 group had experienced one of these safety events, vs. 22% (P = .01) of the DDD-40 group.
The study was not designed to determine a cause for these episodes, but Dr. Kronborg reported that bradycardia was suspected in the majority of cases.
Crossovers more common on minimal pacing
Crossovers were permitted, and 26% of patients did so at some point in the trial. Of these, about one-third were switched to the opposite arm in response to syncope. Almost all of the others crossed over because of chronotropic incompetence. The greater crossover rate in the DDD-40 group (23% vs. 3%; P < .001) was highly significant.
Quality of life was measured with the SF36 tool, and physical function was evaluated with the 6-minute walk distance test (6MWD). Results on these measures did not differ significantly between groups. For 6MWD, the mean gain from baseline was 8 m in both groups.
The results of this study are important because they challenge what has been a widely held perception among electrophysiologists, according to Cecilia Linde, MD, PhD, a professor of cardiology at the Karolinska Institute, Stockholm.
“I think many of us involved in pacing thought for many years that minimizing pacing would be beneficial, and this clearly shows it is not,” said Dr. Linde, who was the moderator of the scientific session in which these results were presented.
Results appear definitive
The ESC-invited discussant, Jose L. Merino, MD, PhD, director of arrhythmia and electrophysiology research, La Paz University Hospital, Madrid, concurred. He said these results are convincing.
On the basis of these findings, which not only failed to show a benefit but showed in the experimental arm a higher incidence of syncope and chronotropic incompetence, Dr. Merino concluded, “Programming intended to minimize atrial pacing should not be used as routine in unselected patients with SND.”
A trend for protection from DDR-60 over DDD-40 from the longest episodes of AF caught Dr. Merino’s attention, leading him to question whether the optimal rate of pacing might be even higher than 60 beats/minute in SND, but he said that is a separate issue. DANPACE was not powered to examine the effect in long duration episodes.
Ultimately, while Dr. Merino characterized the increased risk of syncope with minimized pacing as “an important finding” in regard to dissuading clinicians to pursue this strategy, he said that the underlying question of the DANPACE trial remains unanswered.
Pacing remains “a treatment of choice” in SND, but further investigation is needed “about the optimal pacing rate to minimize AF and syncope” in this population, he said.
Dr. Kronborg reports a financial relationship with Abbott. Dr. Linde reports financial relationships with Cardio 3, Medtronic, St. Jude, and Vifor. Dr. Merino reports financial relationships with Abbott, Medtronic, and Microport.
A version of this article first appeared on Medscape.com.
suggest results of a trial that randomly assigned patients with SND who had received their first pacemaker implant to one of two pacing programs.
Over 2 years of follow-up with remote monitoring, there was no difference in the primary endpoint of time to first device-detected episode of AF lasting more than 6 minutes, reported Max Brix Kronborg, MD, PhD, department of cardiology, Aarhus University Hospital, Denmark.
The study, DANPACE II, excluded patients with permanent or persistent AF or persistent bradycardia prior to or at the time of enrollment.
The findings were presented at annual congress of the European Society of Cardiology and were published online simultaneously in the European Heart Journal.
The 539 participants in the trial were randomly assigned in a 1:1 ratio to a pacing program of 60 beats/minute with rate-adaptive pacing (DDR-60) or 40 beats/minute without rate-adaptive pacing (DDD-40). All patients were equipped with remote monitoring and were followed for 2 years. Tracings were adjudicated for atrial high-rate episodes by experienced device specialists, Dr. Kronborg said.
No difference seen in primary outcome
When graphed, curves for the primary outcome in the two groups were essentially superimposable. For the secondary outcomes of AF lasting more than 6 hours and AF lasting more than 24 hours, there was a modest but progressive separation in the lines favoring the DDR-60 group for both. However, the P value did not approach significance in the first of these endpoints (P = .35) and remained only a trend (P = .08) in the second.
There were no substantial differences in results when patients were stratified by age (> 73 years vs. younger), gender (women represented 50% of patients), PR interval (> 150 milliseconds vs. less), or history of AF prior to study entry; the latter group represented approximately 40% of the trial participants.
There was a between-group difference in the primary composite safety endpoint of syncope and presyncope. By 2 years, 13% of those in the DDR-60 group had experienced one of these safety events, vs. 22% (P = .01) of the DDD-40 group.
The study was not designed to determine a cause for these episodes, but Dr. Kronborg reported that bradycardia was suspected in the majority of cases.
Crossovers more common on minimal pacing
Crossovers were permitted, and 26% of patients did so at some point in the trial. Of these, about one-third were switched to the opposite arm in response to syncope. Almost all of the others crossed over because of chronotropic incompetence. The greater crossover rate in the DDD-40 group (23% vs. 3%; P < .001) was highly significant.
Quality of life was measured with the SF36 tool, and physical function was evaluated with the 6-minute walk distance test (6MWD). Results on these measures did not differ significantly between groups. For 6MWD, the mean gain from baseline was 8 m in both groups.
The results of this study are important because they challenge what has been a widely held perception among electrophysiologists, according to Cecilia Linde, MD, PhD, a professor of cardiology at the Karolinska Institute, Stockholm.
“I think many of us involved in pacing thought for many years that minimizing pacing would be beneficial, and this clearly shows it is not,” said Dr. Linde, who was the moderator of the scientific session in which these results were presented.
Results appear definitive
The ESC-invited discussant, Jose L. Merino, MD, PhD, director of arrhythmia and electrophysiology research, La Paz University Hospital, Madrid, concurred. He said these results are convincing.
On the basis of these findings, which not only failed to show a benefit but showed in the experimental arm a higher incidence of syncope and chronotropic incompetence, Dr. Merino concluded, “Programming intended to minimize atrial pacing should not be used as routine in unselected patients with SND.”
A trend for protection from DDR-60 over DDD-40 from the longest episodes of AF caught Dr. Merino’s attention, leading him to question whether the optimal rate of pacing might be even higher than 60 beats/minute in SND, but he said that is a separate issue. DANPACE was not powered to examine the effect in long duration episodes.
Ultimately, while Dr. Merino characterized the increased risk of syncope with minimized pacing as “an important finding” in regard to dissuading clinicians to pursue this strategy, he said that the underlying question of the DANPACE trial remains unanswered.
Pacing remains “a treatment of choice” in SND, but further investigation is needed “about the optimal pacing rate to minimize AF and syncope” in this population, he said.
Dr. Kronborg reports a financial relationship with Abbott. Dr. Linde reports financial relationships with Cardio 3, Medtronic, St. Jude, and Vifor. Dr. Merino reports financial relationships with Abbott, Medtronic, and Microport.
A version of this article first appeared on Medscape.com.
suggest results of a trial that randomly assigned patients with SND who had received their first pacemaker implant to one of two pacing programs.
Over 2 years of follow-up with remote monitoring, there was no difference in the primary endpoint of time to first device-detected episode of AF lasting more than 6 minutes, reported Max Brix Kronborg, MD, PhD, department of cardiology, Aarhus University Hospital, Denmark.
The study, DANPACE II, excluded patients with permanent or persistent AF or persistent bradycardia prior to or at the time of enrollment.
The findings were presented at annual congress of the European Society of Cardiology and were published online simultaneously in the European Heart Journal.
The 539 participants in the trial were randomly assigned in a 1:1 ratio to a pacing program of 60 beats/minute with rate-adaptive pacing (DDR-60) or 40 beats/minute without rate-adaptive pacing (DDD-40). All patients were equipped with remote monitoring and were followed for 2 years. Tracings were adjudicated for atrial high-rate episodes by experienced device specialists, Dr. Kronborg said.
No difference seen in primary outcome
When graphed, curves for the primary outcome in the two groups were essentially superimposable. For the secondary outcomes of AF lasting more than 6 hours and AF lasting more than 24 hours, there was a modest but progressive separation in the lines favoring the DDR-60 group for both. However, the P value did not approach significance in the first of these endpoints (P = .35) and remained only a trend (P = .08) in the second.
There were no substantial differences in results when patients were stratified by age (> 73 years vs. younger), gender (women represented 50% of patients), PR interval (> 150 milliseconds vs. less), or history of AF prior to study entry; the latter group represented approximately 40% of the trial participants.
There was a between-group difference in the primary composite safety endpoint of syncope and presyncope. By 2 years, 13% of those in the DDR-60 group had experienced one of these safety events, vs. 22% (P = .01) of the DDD-40 group.
The study was not designed to determine a cause for these episodes, but Dr. Kronborg reported that bradycardia was suspected in the majority of cases.
Crossovers more common on minimal pacing
Crossovers were permitted, and 26% of patients did so at some point in the trial. Of these, about one-third were switched to the opposite arm in response to syncope. Almost all of the others crossed over because of chronotropic incompetence. The greater crossover rate in the DDD-40 group (23% vs. 3%; P < .001) was highly significant.
Quality of life was measured with the SF36 tool, and physical function was evaluated with the 6-minute walk distance test (6MWD). Results on these measures did not differ significantly between groups. For 6MWD, the mean gain from baseline was 8 m in both groups.
The results of this study are important because they challenge what has been a widely held perception among electrophysiologists, according to Cecilia Linde, MD, PhD, a professor of cardiology at the Karolinska Institute, Stockholm.
“I think many of us involved in pacing thought for many years that minimizing pacing would be beneficial, and this clearly shows it is not,” said Dr. Linde, who was the moderator of the scientific session in which these results were presented.
Results appear definitive
The ESC-invited discussant, Jose L. Merino, MD, PhD, director of arrhythmia and electrophysiology research, La Paz University Hospital, Madrid, concurred. He said these results are convincing.
On the basis of these findings, which not only failed to show a benefit but showed in the experimental arm a higher incidence of syncope and chronotropic incompetence, Dr. Merino concluded, “Programming intended to minimize atrial pacing should not be used as routine in unselected patients with SND.”
A trend for protection from DDR-60 over DDD-40 from the longest episodes of AF caught Dr. Merino’s attention, leading him to question whether the optimal rate of pacing might be even higher than 60 beats/minute in SND, but he said that is a separate issue. DANPACE was not powered to examine the effect in long duration episodes.
Ultimately, while Dr. Merino characterized the increased risk of syncope with minimized pacing as “an important finding” in regard to dissuading clinicians to pursue this strategy, he said that the underlying question of the DANPACE trial remains unanswered.
Pacing remains “a treatment of choice” in SND, but further investigation is needed “about the optimal pacing rate to minimize AF and syncope” in this population, he said.
Dr. Kronborg reports a financial relationship with Abbott. Dr. Linde reports financial relationships with Cardio 3, Medtronic, St. Jude, and Vifor. Dr. Merino reports financial relationships with Abbott, Medtronic, and Microport.
A version of this article first appeared on Medscape.com.
FROM ESC CONGRESS 2023
Abdominal fat linked to lower brain volume in midlife
In a large study of healthy middle-aged adults, greater visceral and subcutaneous abdominal fat on abdominal MRI predicted brain atrophy on imaging, especially in women.
“The study shows that excess fat is bad for the brain and worse in women, including in Alzheimer’s disease risk regions,” lead author Cyrus Raji, MD, PhD, with the Mallinckrodt Institute of Radiology, Washington University, St. Louis, Mo., said in an interview.
The study was published online in the journal Aging and Disease
Modifiable risk factor
Multiple studies have suggested a connection between body fat accumulation and increased dementia risk. But few have examined the relationship between types of fat (visceral and subcutaneous) and brain volume.
For the new study, 10,000 healthy adults aged 20-80 years (mean age, 52.9 years; 53% men) underwent a short whole-body MRI protocol. Regression analyses of abdominal fat types and normalized brain volumes were evaluated, controlling for age and sex.
The research team found that higher amounts of both visceral and subcutaneous abdominal fat predicted lower total gray and white matter volume, as well as lower volume in the hippocampus, frontal cortex, and temporal, parietal, and occipital lobes.
“The findings are quite dramatic,” Dr. Raji told this news organization. “Overall, we found that both subcutaneous and visceral fat has similar levels of negative relationships with brain volumes.”
Women had a higher burden of brain atrophy with increased visceral fat than men. However, it’s difficult to place the sex differences in context because of the lack of prior work specifically investigating visceral fat, brain volume loss, and sex differences, the researchers caution.
They also note that while statistically significant relationships were observed between visceral fat levels and gray matter volume changes, their effect sizes were generally small.
“Thus, the statistical significance of this work is influenced by the large sample size and less so by large effect size in any given set of regions,” the investigators write.
Other limitations include the cross-sectional nature of the study, which precludes conclusions about causality. The analysis also did not account for other lifestyle factors such as physical activity, diet, and genetic variables.
The researchers call for further investigation “to better elucidate underlying mechanisms and discover possible interventions targeting abdominal fat reduction as a strategy to maintain brain health.”
‘Helpful addition to the literature’
In a comment, Claire Sexton, DPhil, Alzheimer’s Association senior director of scientific programs and outreach, noted that “previous studies have linked obesity with cognitive decline and increased risk of dementia. Rather than using BMI as a proxy for body fat, the current study examined visceral and subcutaneous fat directly using imaging techniques.”
Dr. Sexton, who was not associated with this study, said the finding that increased body fat was associated with reduced brain volumes suggests “a possible mechanism to explain the previously reported associations between obesity and cognition.”
“Though some degree of atrophy and brain shrinkage is common with old age, awareness of this association is important because reduced brain volume may be associated with problems with thinking, memory, and performing everyday tasks, and because rates of obesity continue to rise in the United States, along with obesity-related conditions including heart disease, stroke, type 2 diabetes and certain types of cancer,” she added.
“While a helpful addition to the literature, the study does have important limitations. As an observational study, it cannot establish whether higher levels of body fat directly causes reduced brain volumes,” Dr. Sexton cautioned.
In addition, the study did not take into account important related factors like physical activity and diet, which may influence any relationship between body fat and brain volumes, she noted. “Overall, it is not just one factor that is important to consider when considering risk for cognitive decline and dementia, but multiple factors.
“Obesity and the location of body fat must be considered in combination with one’s total lived experience and habits, including physical activity, education, head injury, sleep, mental health, and the health of your heart/cardiovascular system and other key bodily systems,” Dr. Sexton said.
The Alzheimer’s Association is leading a 2-year clinical trial known as U.S. POINTER to see whether combining physical activity, healthy nutrition, social and intellectual challenges, and improved self-management of medical conditions can protect cognitive function in older adults who are at increased risk for cognitive decline.
This work was supported in part by Providence St. Joseph Health in Seattle; Saint John’s Health Center Foundation; Pacific Neuroscience Institute and Foundation; Will and Cary Singleton; and the McLoughlin family. Dr. Raji is a consultant for Brainreader, Apollo Health, Voxelwise, Neurevolution, Pacific Neuroscience Institute Foundation, and Icometrix. Dr. Sexton reports no relevant financial relationships.
A version of this article first appeared on Medscape.com.
In a large study of healthy middle-aged adults, greater visceral and subcutaneous abdominal fat on abdominal MRI predicted brain atrophy on imaging, especially in women.
“The study shows that excess fat is bad for the brain and worse in women, including in Alzheimer’s disease risk regions,” lead author Cyrus Raji, MD, PhD, with the Mallinckrodt Institute of Radiology, Washington University, St. Louis, Mo., said in an interview.
The study was published online in the journal Aging and Disease
Modifiable risk factor
Multiple studies have suggested a connection between body fat accumulation and increased dementia risk. But few have examined the relationship between types of fat (visceral and subcutaneous) and brain volume.
For the new study, 10,000 healthy adults aged 20-80 years (mean age, 52.9 years; 53% men) underwent a short whole-body MRI protocol. Regression analyses of abdominal fat types and normalized brain volumes were evaluated, controlling for age and sex.
The research team found that higher amounts of both visceral and subcutaneous abdominal fat predicted lower total gray and white matter volume, as well as lower volume in the hippocampus, frontal cortex, and temporal, parietal, and occipital lobes.
“The findings are quite dramatic,” Dr. Raji told this news organization. “Overall, we found that both subcutaneous and visceral fat has similar levels of negative relationships with brain volumes.”
Women had a higher burden of brain atrophy with increased visceral fat than men. However, it’s difficult to place the sex differences in context because of the lack of prior work specifically investigating visceral fat, brain volume loss, and sex differences, the researchers caution.
They also note that while statistically significant relationships were observed between visceral fat levels and gray matter volume changes, their effect sizes were generally small.
“Thus, the statistical significance of this work is influenced by the large sample size and less so by large effect size in any given set of regions,” the investigators write.
Other limitations include the cross-sectional nature of the study, which precludes conclusions about causality. The analysis also did not account for other lifestyle factors such as physical activity, diet, and genetic variables.
The researchers call for further investigation “to better elucidate underlying mechanisms and discover possible interventions targeting abdominal fat reduction as a strategy to maintain brain health.”
‘Helpful addition to the literature’
In a comment, Claire Sexton, DPhil, Alzheimer’s Association senior director of scientific programs and outreach, noted that “previous studies have linked obesity with cognitive decline and increased risk of dementia. Rather than using BMI as a proxy for body fat, the current study examined visceral and subcutaneous fat directly using imaging techniques.”
Dr. Sexton, who was not associated with this study, said the finding that increased body fat was associated with reduced brain volumes suggests “a possible mechanism to explain the previously reported associations between obesity and cognition.”
“Though some degree of atrophy and brain shrinkage is common with old age, awareness of this association is important because reduced brain volume may be associated with problems with thinking, memory, and performing everyday tasks, and because rates of obesity continue to rise in the United States, along with obesity-related conditions including heart disease, stroke, type 2 diabetes and certain types of cancer,” she added.
“While a helpful addition to the literature, the study does have important limitations. As an observational study, it cannot establish whether higher levels of body fat directly causes reduced brain volumes,” Dr. Sexton cautioned.
In addition, the study did not take into account important related factors like physical activity and diet, which may influence any relationship between body fat and brain volumes, she noted. “Overall, it is not just one factor that is important to consider when considering risk for cognitive decline and dementia, but multiple factors.
“Obesity and the location of body fat must be considered in combination with one’s total lived experience and habits, including physical activity, education, head injury, sleep, mental health, and the health of your heart/cardiovascular system and other key bodily systems,” Dr. Sexton said.
The Alzheimer’s Association is leading a 2-year clinical trial known as U.S. POINTER to see whether combining physical activity, healthy nutrition, social and intellectual challenges, and improved self-management of medical conditions can protect cognitive function in older adults who are at increased risk for cognitive decline.
This work was supported in part by Providence St. Joseph Health in Seattle; Saint John’s Health Center Foundation; Pacific Neuroscience Institute and Foundation; Will and Cary Singleton; and the McLoughlin family. Dr. Raji is a consultant for Brainreader, Apollo Health, Voxelwise, Neurevolution, Pacific Neuroscience Institute Foundation, and Icometrix. Dr. Sexton reports no relevant financial relationships.
A version of this article first appeared on Medscape.com.
In a large study of healthy middle-aged adults, greater visceral and subcutaneous abdominal fat on abdominal MRI predicted brain atrophy on imaging, especially in women.
“The study shows that excess fat is bad for the brain and worse in women, including in Alzheimer’s disease risk regions,” lead author Cyrus Raji, MD, PhD, with the Mallinckrodt Institute of Radiology, Washington University, St. Louis, Mo., said in an interview.
The study was published online in the journal Aging and Disease
Modifiable risk factor
Multiple studies have suggested a connection between body fat accumulation and increased dementia risk. But few have examined the relationship between types of fat (visceral and subcutaneous) and brain volume.
For the new study, 10,000 healthy adults aged 20-80 years (mean age, 52.9 years; 53% men) underwent a short whole-body MRI protocol. Regression analyses of abdominal fat types and normalized brain volumes were evaluated, controlling for age and sex.
The research team found that higher amounts of both visceral and subcutaneous abdominal fat predicted lower total gray and white matter volume, as well as lower volume in the hippocampus, frontal cortex, and temporal, parietal, and occipital lobes.
“The findings are quite dramatic,” Dr. Raji told this news organization. “Overall, we found that both subcutaneous and visceral fat has similar levels of negative relationships with brain volumes.”
Women had a higher burden of brain atrophy with increased visceral fat than men. However, it’s difficult to place the sex differences in context because of the lack of prior work specifically investigating visceral fat, brain volume loss, and sex differences, the researchers caution.
They also note that while statistically significant relationships were observed between visceral fat levels and gray matter volume changes, their effect sizes were generally small.
“Thus, the statistical significance of this work is influenced by the large sample size and less so by large effect size in any given set of regions,” the investigators write.
Other limitations include the cross-sectional nature of the study, which precludes conclusions about causality. The analysis also did not account for other lifestyle factors such as physical activity, diet, and genetic variables.
The researchers call for further investigation “to better elucidate underlying mechanisms and discover possible interventions targeting abdominal fat reduction as a strategy to maintain brain health.”
‘Helpful addition to the literature’
In a comment, Claire Sexton, DPhil, Alzheimer’s Association senior director of scientific programs and outreach, noted that “previous studies have linked obesity with cognitive decline and increased risk of dementia. Rather than using BMI as a proxy for body fat, the current study examined visceral and subcutaneous fat directly using imaging techniques.”
Dr. Sexton, who was not associated with this study, said the finding that increased body fat was associated with reduced brain volumes suggests “a possible mechanism to explain the previously reported associations between obesity and cognition.”
“Though some degree of atrophy and brain shrinkage is common with old age, awareness of this association is important because reduced brain volume may be associated with problems with thinking, memory, and performing everyday tasks, and because rates of obesity continue to rise in the United States, along with obesity-related conditions including heart disease, stroke, type 2 diabetes and certain types of cancer,” she added.
“While a helpful addition to the literature, the study does have important limitations. As an observational study, it cannot establish whether higher levels of body fat directly causes reduced brain volumes,” Dr. Sexton cautioned.
In addition, the study did not take into account important related factors like physical activity and diet, which may influence any relationship between body fat and brain volumes, she noted. “Overall, it is not just one factor that is important to consider when considering risk for cognitive decline and dementia, but multiple factors.
“Obesity and the location of body fat must be considered in combination with one’s total lived experience and habits, including physical activity, education, head injury, sleep, mental health, and the health of your heart/cardiovascular system and other key bodily systems,” Dr. Sexton said.
The Alzheimer’s Association is leading a 2-year clinical trial known as U.S. POINTER to see whether combining physical activity, healthy nutrition, social and intellectual challenges, and improved self-management of medical conditions can protect cognitive function in older adults who are at increased risk for cognitive decline.
This work was supported in part by Providence St. Joseph Health in Seattle; Saint John’s Health Center Foundation; Pacific Neuroscience Institute and Foundation; Will and Cary Singleton; and the McLoughlin family. Dr. Raji is a consultant for Brainreader, Apollo Health, Voxelwise, Neurevolution, Pacific Neuroscience Institute Foundation, and Icometrix. Dr. Sexton reports no relevant financial relationships.
A version of this article first appeared on Medscape.com.
FROM AGING AND DISEASES
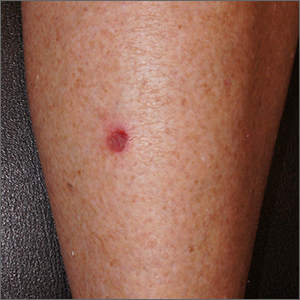
Small persistent leg wound

A leg ulcer may have many causes, including venous stasis, trauma, vasculitis, infection, or (as in this case) squamous cell carcinoma in situ (SCCis), aka Bowen’s Disease.
SCC and SCCis are common skin cancers that occur less frequently than basal cell carcinomas (BCCs).1 SCCis is normally scaly and hyperkeratotic, but it can manifest in rare cases as a chronic ulcer. Fair skin, long history of sun damage, and immunosuppression are significant risk factors for both SCCis and SCC.
While history and other clinical features may help narrow the diagnosis, a wound that does not heal despite treatments should be biopsied. Shave and punch biopsies are both excellent ways to diagnose an SCCis that has a classic appearance. However, ulcers and blisters can be caused by inflammatory processes (as in pyoderma gangrenosum or a fixed drug eruption) with characteristic findings deeper in the dermis; these lesions are better assessed with a punch biopsy.
In this case, a 4-mm punch biopsy was performed at the tissue edge and showed atypical keratinocytes limited to the epidermis. These atypical keratinocytes are associated with vesicle formation and ulcer, consistent with SCCis.
SCCis transforms into invasive disease in 3% to 5% of cases.2 Surgical treatment includes fusiform excision and electrodessication and curettage, both with cure rates that often exceed 90%.2,3 Nonsurgical options include topical 5-fluorouracil (67%-92% effective), topical imiquimod (75%-93%), and photodynamic therapy (52%-98%).4
Treatment choices depend on patient preference and provider capabilities. With surgical options there is the risk of bleeding and the need to care for a healing wound. Nonsurgical treatments can last longer and require topical treatment regimens and medications.
This patient opted for a fusiform excision and linear closure. She will continue to undergo serial skin evaluations twice a year for at least 2 years.
Photos and text for Photo Rounds Friday courtesy of Jonathan Karnes, MD (copyright retained). Dr. Karnes is the medical director of MDFMR Dermatology Services, Augusta, Maine.
1. Lukowiak TM, Aizman L, Perz A, et al. Association of age, sex, race, and geographic region with variation of the ratio of basal cell to cutaneous squamous cell carcinomas in the United States. JAMA Dermatol. 2020;156:1192-1198. doi:10.1001/jamadermatol.2020.2571
2. Morton CA, Birnie AJ, Eedy DJ. British Association of Dermatologists’ guidelines for the management of squamous cell carcinoma in situ (Bowen's disease). Br J Dermatol. 2014;170:245-246. doi: 10.1111/bjd.12766
3. Veverka KK, Stratman EJ. Electrodesiccation and curettage for squamous cell carcinoma in situ: the effect of anatomic location on local recurrence. Dermatol Surg. 2023;49:821-824. doi: 10.1097/DSS.0000000000003855
4. Algarin, YA, Jambusaria-Pahlajani A. Ruiz E, et al. Advances in topical treatments of cutaneous malignancies. Am J Clin Dermatol. 2023;24:69-80. doi: 10.1007/s40257-022-00731-x

A leg ulcer may have many causes, including venous stasis, trauma, vasculitis, infection, or (as in this case) squamous cell carcinoma in situ (SCCis), aka Bowen’s Disease.
SCC and SCCis are common skin cancers that occur less frequently than basal cell carcinomas (BCCs).1 SCCis is normally scaly and hyperkeratotic, but it can manifest in rare cases as a chronic ulcer. Fair skin, long history of sun damage, and immunosuppression are significant risk factors for both SCCis and SCC.
While history and other clinical features may help narrow the diagnosis, a wound that does not heal despite treatments should be biopsied. Shave and punch biopsies are both excellent ways to diagnose an SCCis that has a classic appearance. However, ulcers and blisters can be caused by inflammatory processes (as in pyoderma gangrenosum or a fixed drug eruption) with characteristic findings deeper in the dermis; these lesions are better assessed with a punch biopsy.
In this case, a 4-mm punch biopsy was performed at the tissue edge and showed atypical keratinocytes limited to the epidermis. These atypical keratinocytes are associated with vesicle formation and ulcer, consistent with SCCis.
SCCis transforms into invasive disease in 3% to 5% of cases.2 Surgical treatment includes fusiform excision and electrodessication and curettage, both with cure rates that often exceed 90%.2,3 Nonsurgical options include topical 5-fluorouracil (67%-92% effective), topical imiquimod (75%-93%), and photodynamic therapy (52%-98%).4
Treatment choices depend on patient preference and provider capabilities. With surgical options there is the risk of bleeding and the need to care for a healing wound. Nonsurgical treatments can last longer and require topical treatment regimens and medications.
This patient opted for a fusiform excision and linear closure. She will continue to undergo serial skin evaluations twice a year for at least 2 years.
Photos and text for Photo Rounds Friday courtesy of Jonathan Karnes, MD (copyright retained). Dr. Karnes is the medical director of MDFMR Dermatology Services, Augusta, Maine.

A leg ulcer may have many causes, including venous stasis, trauma, vasculitis, infection, or (as in this case) squamous cell carcinoma in situ (SCCis), aka Bowen’s Disease.
SCC and SCCis are common skin cancers that occur less frequently than basal cell carcinomas (BCCs).1 SCCis is normally scaly and hyperkeratotic, but it can manifest in rare cases as a chronic ulcer. Fair skin, long history of sun damage, and immunosuppression are significant risk factors for both SCCis and SCC.
While history and other clinical features may help narrow the diagnosis, a wound that does not heal despite treatments should be biopsied. Shave and punch biopsies are both excellent ways to diagnose an SCCis that has a classic appearance. However, ulcers and blisters can be caused by inflammatory processes (as in pyoderma gangrenosum or a fixed drug eruption) with characteristic findings deeper in the dermis; these lesions are better assessed with a punch biopsy.
In this case, a 4-mm punch biopsy was performed at the tissue edge and showed atypical keratinocytes limited to the epidermis. These atypical keratinocytes are associated with vesicle formation and ulcer, consistent with SCCis.
SCCis transforms into invasive disease in 3% to 5% of cases.2 Surgical treatment includes fusiform excision and electrodessication and curettage, both with cure rates that often exceed 90%.2,3 Nonsurgical options include topical 5-fluorouracil (67%-92% effective), topical imiquimod (75%-93%), and photodynamic therapy (52%-98%).4
Treatment choices depend on patient preference and provider capabilities. With surgical options there is the risk of bleeding and the need to care for a healing wound. Nonsurgical treatments can last longer and require topical treatment regimens and medications.
This patient opted for a fusiform excision and linear closure. She will continue to undergo serial skin evaluations twice a year for at least 2 years.
Photos and text for Photo Rounds Friday courtesy of Jonathan Karnes, MD (copyright retained). Dr. Karnes is the medical director of MDFMR Dermatology Services, Augusta, Maine.
1. Lukowiak TM, Aizman L, Perz A, et al. Association of age, sex, race, and geographic region with variation of the ratio of basal cell to cutaneous squamous cell carcinomas in the United States. JAMA Dermatol. 2020;156:1192-1198. doi:10.1001/jamadermatol.2020.2571
2. Morton CA, Birnie AJ, Eedy DJ. British Association of Dermatologists’ guidelines for the management of squamous cell carcinoma in situ (Bowen's disease). Br J Dermatol. 2014;170:245-246. doi: 10.1111/bjd.12766
3. Veverka KK, Stratman EJ. Electrodesiccation and curettage for squamous cell carcinoma in situ: the effect of anatomic location on local recurrence. Dermatol Surg. 2023;49:821-824. doi: 10.1097/DSS.0000000000003855
4. Algarin, YA, Jambusaria-Pahlajani A. Ruiz E, et al. Advances in topical treatments of cutaneous malignancies. Am J Clin Dermatol. 2023;24:69-80. doi: 10.1007/s40257-022-00731-x
1. Lukowiak TM, Aizman L, Perz A, et al. Association of age, sex, race, and geographic region with variation of the ratio of basal cell to cutaneous squamous cell carcinomas in the United States. JAMA Dermatol. 2020;156:1192-1198. doi:10.1001/jamadermatol.2020.2571
2. Morton CA, Birnie AJ, Eedy DJ. British Association of Dermatologists’ guidelines for the management of squamous cell carcinoma in situ (Bowen's disease). Br J Dermatol. 2014;170:245-246. doi: 10.1111/bjd.12766
3. Veverka KK, Stratman EJ. Electrodesiccation and curettage for squamous cell carcinoma in situ: the effect of anatomic location on local recurrence. Dermatol Surg. 2023;49:821-824. doi: 10.1097/DSS.0000000000003855
4. Algarin, YA, Jambusaria-Pahlajani A. Ruiz E, et al. Advances in topical treatments of cutaneous malignancies. Am J Clin Dermatol. 2023;24:69-80. doi: 10.1007/s40257-022-00731-x
Sepsis too often neglected in hospitals
according to a recent survey by the Centers for Disease Control and Prevention.
For the hospitals that do have sepsis teams, only 55% of them report that their team leaders get dedicated time to manage their sepsis programs.
“One in three people who dies in a hospital has sepsis during that hospitalization,” CDC Director Mandy Cohen, MD, MPH, noted in a statement. “That’s why CDC is calling on all U.S. hospitals to have a sepsis program and raise the bar on sepsis care by incorporating seven core elements.”
The sepsis seven
- Leadership: Dedicating the necessary human, financial, and information technology resources.
- Accountability: Appointing a leader responsible for program outcomes and setting concrete goals.
- Multiprofessional: Engaging key partners throughout the organization.
- Action: Implementing structures and processes to improve the identification, management, and recovery from sepsis.
- Tracking: Measuring sepsis epidemiology, outcomes, and progress toward program goals and the impact of sepsis initiatives.
- Reporting: Providing usable information on sepsis treatment and outcomes to relevant partners.
- Education: Providing sepsis education to health care professionals during onboarding and annually.
Craig Weinert, MD, MPH, a pulmonologist and critical care physician and professor of medicine at the University of Minnesota, Minneapolis, says the point the CDC is making with the announcement is that when these sepsis programs have been implemented at hospitals, they have been successful at reducing mortality. And now, the agency is urging all hospitals to implement them and support them properly.
“It’s not asking hospitals to develop new, innovative kinds of sepsis programs. This is not about new drugs or new antibiotics or new devices,” Dr. Weinert says. “This is about having hospitals dedicate organizational resources to implementing sepsis programs.”
The CDC’s announcement is aimed toward hospital administrators, Dr. Weinert adds. The agency is making the case that sepsis needs more funding in hospitals that either don’t have the programs or aren’t supporting them with dedicated resources.
There’s another message as well, Dr. Weinert says.
“COVID basically obliterated sepsis programs for two and a half years,” he says. Now the CDC is saying it’s time to divert staff back to sepsis care.
Stepping up sepsis care
Raymund Dantes, MD, assistant professor of medicine at Emory University, Atlanta, one of the developers of the core elements, says this is like a recipe for sepsis care.
Dr. Dantes compares the instructions for hospitals with getting a good restaurant up and running. And in the restaurant business, “you need more than the recipes. You need a leader or manager to ensure you have the right people working together, with the right supplies, getting the right feedback on their work to continuously improve,” he explains.
Dr. Dantes, who is also the physician lead for the Emory Healthcare Sepsis Program, says the approach is meant to be flexible to the size of the hospital, population served, and available resources.
He points out that a well-run sepsis program at a 25-bed rural hospital will look very different from the program at a 1,000-bed tertiary care hospital.
Some hospitals, Dr. Dantes says, will be starting from scratch when getting a sepsis program, and for those hospitals, the developers included a “Getting Started” section as part of the detailed, 29-page full report.
In September, Sepsis Awareness Month, the CDC will provide educational information to health care professionals, patients, families, and caregivers about preventing infections that can lead to sepsis through its ongoing Get Ahead of Sepsis campaign.
A version of this article first appeared on Medscape.com.
according to a recent survey by the Centers for Disease Control and Prevention.
For the hospitals that do have sepsis teams, only 55% of them report that their team leaders get dedicated time to manage their sepsis programs.
“One in three people who dies in a hospital has sepsis during that hospitalization,” CDC Director Mandy Cohen, MD, MPH, noted in a statement. “That’s why CDC is calling on all U.S. hospitals to have a sepsis program and raise the bar on sepsis care by incorporating seven core elements.”
The sepsis seven
- Leadership: Dedicating the necessary human, financial, and information technology resources.
- Accountability: Appointing a leader responsible for program outcomes and setting concrete goals.
- Multiprofessional: Engaging key partners throughout the organization.
- Action: Implementing structures and processes to improve the identification, management, and recovery from sepsis.
- Tracking: Measuring sepsis epidemiology, outcomes, and progress toward program goals and the impact of sepsis initiatives.
- Reporting: Providing usable information on sepsis treatment and outcomes to relevant partners.
- Education: Providing sepsis education to health care professionals during onboarding and annually.
Craig Weinert, MD, MPH, a pulmonologist and critical care physician and professor of medicine at the University of Minnesota, Minneapolis, says the point the CDC is making with the announcement is that when these sepsis programs have been implemented at hospitals, they have been successful at reducing mortality. And now, the agency is urging all hospitals to implement them and support them properly.
“It’s not asking hospitals to develop new, innovative kinds of sepsis programs. This is not about new drugs or new antibiotics or new devices,” Dr. Weinert says. “This is about having hospitals dedicate organizational resources to implementing sepsis programs.”
The CDC’s announcement is aimed toward hospital administrators, Dr. Weinert adds. The agency is making the case that sepsis needs more funding in hospitals that either don’t have the programs or aren’t supporting them with dedicated resources.
There’s another message as well, Dr. Weinert says.
“COVID basically obliterated sepsis programs for two and a half years,” he says. Now the CDC is saying it’s time to divert staff back to sepsis care.
Stepping up sepsis care
Raymund Dantes, MD, assistant professor of medicine at Emory University, Atlanta, one of the developers of the core elements, says this is like a recipe for sepsis care.
Dr. Dantes compares the instructions for hospitals with getting a good restaurant up and running. And in the restaurant business, “you need more than the recipes. You need a leader or manager to ensure you have the right people working together, with the right supplies, getting the right feedback on their work to continuously improve,” he explains.
Dr. Dantes, who is also the physician lead for the Emory Healthcare Sepsis Program, says the approach is meant to be flexible to the size of the hospital, population served, and available resources.
He points out that a well-run sepsis program at a 25-bed rural hospital will look very different from the program at a 1,000-bed tertiary care hospital.
Some hospitals, Dr. Dantes says, will be starting from scratch when getting a sepsis program, and for those hospitals, the developers included a “Getting Started” section as part of the detailed, 29-page full report.
In September, Sepsis Awareness Month, the CDC will provide educational information to health care professionals, patients, families, and caregivers about preventing infections that can lead to sepsis through its ongoing Get Ahead of Sepsis campaign.
A version of this article first appeared on Medscape.com.
according to a recent survey by the Centers for Disease Control and Prevention.
For the hospitals that do have sepsis teams, only 55% of them report that their team leaders get dedicated time to manage their sepsis programs.
“One in three people who dies in a hospital has sepsis during that hospitalization,” CDC Director Mandy Cohen, MD, MPH, noted in a statement. “That’s why CDC is calling on all U.S. hospitals to have a sepsis program and raise the bar on sepsis care by incorporating seven core elements.”
The sepsis seven
- Leadership: Dedicating the necessary human, financial, and information technology resources.
- Accountability: Appointing a leader responsible for program outcomes and setting concrete goals.
- Multiprofessional: Engaging key partners throughout the organization.
- Action: Implementing structures and processes to improve the identification, management, and recovery from sepsis.
- Tracking: Measuring sepsis epidemiology, outcomes, and progress toward program goals and the impact of sepsis initiatives.
- Reporting: Providing usable information on sepsis treatment and outcomes to relevant partners.
- Education: Providing sepsis education to health care professionals during onboarding and annually.
Craig Weinert, MD, MPH, a pulmonologist and critical care physician and professor of medicine at the University of Minnesota, Minneapolis, says the point the CDC is making with the announcement is that when these sepsis programs have been implemented at hospitals, they have been successful at reducing mortality. And now, the agency is urging all hospitals to implement them and support them properly.
“It’s not asking hospitals to develop new, innovative kinds of sepsis programs. This is not about new drugs or new antibiotics or new devices,” Dr. Weinert says. “This is about having hospitals dedicate organizational resources to implementing sepsis programs.”
The CDC’s announcement is aimed toward hospital administrators, Dr. Weinert adds. The agency is making the case that sepsis needs more funding in hospitals that either don’t have the programs or aren’t supporting them with dedicated resources.
There’s another message as well, Dr. Weinert says.
“COVID basically obliterated sepsis programs for two and a half years,” he says. Now the CDC is saying it’s time to divert staff back to sepsis care.
Stepping up sepsis care
Raymund Dantes, MD, assistant professor of medicine at Emory University, Atlanta, one of the developers of the core elements, says this is like a recipe for sepsis care.
Dr. Dantes compares the instructions for hospitals with getting a good restaurant up and running. And in the restaurant business, “you need more than the recipes. You need a leader or manager to ensure you have the right people working together, with the right supplies, getting the right feedback on their work to continuously improve,” he explains.
Dr. Dantes, who is also the physician lead for the Emory Healthcare Sepsis Program, says the approach is meant to be flexible to the size of the hospital, population served, and available resources.
He points out that a well-run sepsis program at a 25-bed rural hospital will look very different from the program at a 1,000-bed tertiary care hospital.
Some hospitals, Dr. Dantes says, will be starting from scratch when getting a sepsis program, and for those hospitals, the developers included a “Getting Started” section as part of the detailed, 29-page full report.
In September, Sepsis Awareness Month, the CDC will provide educational information to health care professionals, patients, families, and caregivers about preventing infections that can lead to sepsis through its ongoing Get Ahead of Sepsis campaign.
A version of this article first appeared on Medscape.com.
The magic of music
I’m really going to miss Jimmy Buffett.
I’ve liked his music as far back as I can remember, and was lucky enough to see him in person in the mid-90s.
I’ve written about music before, but its affect on us never fails to amaze me. Songs can be background noise conducive to getting things done. They can also be in the foreground, serving as a mental vacation (or accompanying a real one). They can transport you to another place, briefly clearing your head from the daily goings-on around you. Even if it’s just during the drive home, it’s a welcome escape to a virtual beach and tropical drink.
Songs can bring back memories of certain events or people that we link them to. My dad loved anything by Neil Diamond, and nothing brings back thoughts of Dad more than when my iTunes randomly picks “I Am ... I Said.” Or John Williams’ Star Wars theme, taking me back to the summer of 1977 when I sat, spellbound, by this incredible movie whose magic is still going strong two generations later.
It’s amazing how our brain tries to make music out of nothing. Even in silence we have ear worms, the songs stuck in our head for hours to days (recently I’ve had “I Sing the Body Electric” from the 1980 movie Fame playing in there).
My office is over an MRI scanner, so I can always hear the chiller pumps softly running in the background. Sometimes my brain will turn their rhythmic chirping into a song, altering the pace of the song to fit them. The soft clicking of the ceiling fan, in my home office, does the same thing (for some reason my brain usually tries to fit “Yellow Submarine” to that one, no idea why).
Music is a part of that mysterious essence that makes us human. It touches all of us in some way, which varies between people, songs, and artists.
Jimmy Buffet’s music has a vacation vibe. Songs of the Caribbean & Keys, beaches, bars, boats, and tropical drinks. The 4:12 running time of his most well-known song, “Margaritaville,” gives a brief respite from my day when it comes on.
He passes into the beyond, to the sadness of his family, friends, and fans. But, unlike people, music can be immortal, and so he lives on through his creations. Like, Bach, Lennon, Bowie, Joplin, Sousa, and too many others to count, his work – and the enjoyment we get from it – are a gift left behind for the future.
Tight lines, Jimmy.
Dr. Block has a solo neurology practice in Scottsdale, Ariz.
I’m really going to miss Jimmy Buffett.
I’ve liked his music as far back as I can remember, and was lucky enough to see him in person in the mid-90s.
I’ve written about music before, but its affect on us never fails to amaze me. Songs can be background noise conducive to getting things done. They can also be in the foreground, serving as a mental vacation (or accompanying a real one). They can transport you to another place, briefly clearing your head from the daily goings-on around you. Even if it’s just during the drive home, it’s a welcome escape to a virtual beach and tropical drink.
Songs can bring back memories of certain events or people that we link them to. My dad loved anything by Neil Diamond, and nothing brings back thoughts of Dad more than when my iTunes randomly picks “I Am ... I Said.” Or John Williams’ Star Wars theme, taking me back to the summer of 1977 when I sat, spellbound, by this incredible movie whose magic is still going strong two generations later.
It’s amazing how our brain tries to make music out of nothing. Even in silence we have ear worms, the songs stuck in our head for hours to days (recently I’ve had “I Sing the Body Electric” from the 1980 movie Fame playing in there).
My office is over an MRI scanner, so I can always hear the chiller pumps softly running in the background. Sometimes my brain will turn their rhythmic chirping into a song, altering the pace of the song to fit them. The soft clicking of the ceiling fan, in my home office, does the same thing (for some reason my brain usually tries to fit “Yellow Submarine” to that one, no idea why).
Music is a part of that mysterious essence that makes us human. It touches all of us in some way, which varies between people, songs, and artists.
Jimmy Buffet’s music has a vacation vibe. Songs of the Caribbean & Keys, beaches, bars, boats, and tropical drinks. The 4:12 running time of his most well-known song, “Margaritaville,” gives a brief respite from my day when it comes on.
He passes into the beyond, to the sadness of his family, friends, and fans. But, unlike people, music can be immortal, and so he lives on through his creations. Like, Bach, Lennon, Bowie, Joplin, Sousa, and too many others to count, his work – and the enjoyment we get from it – are a gift left behind for the future.
Tight lines, Jimmy.
Dr. Block has a solo neurology practice in Scottsdale, Ariz.
I’m really going to miss Jimmy Buffett.
I’ve liked his music as far back as I can remember, and was lucky enough to see him in person in the mid-90s.
I’ve written about music before, but its affect on us never fails to amaze me. Songs can be background noise conducive to getting things done. They can also be in the foreground, serving as a mental vacation (or accompanying a real one). They can transport you to another place, briefly clearing your head from the daily goings-on around you. Even if it’s just during the drive home, it’s a welcome escape to a virtual beach and tropical drink.
Songs can bring back memories of certain events or people that we link them to. My dad loved anything by Neil Diamond, and nothing brings back thoughts of Dad more than when my iTunes randomly picks “I Am ... I Said.” Or John Williams’ Star Wars theme, taking me back to the summer of 1977 when I sat, spellbound, by this incredible movie whose magic is still going strong two generations later.
It’s amazing how our brain tries to make music out of nothing. Even in silence we have ear worms, the songs stuck in our head for hours to days (recently I’ve had “I Sing the Body Electric” from the 1980 movie Fame playing in there).
My office is over an MRI scanner, so I can always hear the chiller pumps softly running in the background. Sometimes my brain will turn their rhythmic chirping into a song, altering the pace of the song to fit them. The soft clicking of the ceiling fan, in my home office, does the same thing (for some reason my brain usually tries to fit “Yellow Submarine” to that one, no idea why).
Music is a part of that mysterious essence that makes us human. It touches all of us in some way, which varies between people, songs, and artists.
Jimmy Buffet’s music has a vacation vibe. Songs of the Caribbean & Keys, beaches, bars, boats, and tropical drinks. The 4:12 running time of his most well-known song, “Margaritaville,” gives a brief respite from my day when it comes on.
He passes into the beyond, to the sadness of his family, friends, and fans. But, unlike people, music can be immortal, and so he lives on through his creations. Like, Bach, Lennon, Bowie, Joplin, Sousa, and too many others to count, his work – and the enjoyment we get from it – are a gift left behind for the future.
Tight lines, Jimmy.
Dr. Block has a solo neurology practice in Scottsdale, Ariz.
MS drugs during pregnancy show no safety signals
AURORA, COLO. – Several drugs for multiple sclerosis (MS) that are contraindicated during pregnancy nevertheless have not shown concerning safety signals in a series of small studies presented as posters at the annual meeting of the Consortium of Multiple Sclerosis Centers. The industry-sponsored research included an assessment of pregnancy and infant outcomes for cladribine, ocrelizumab, ofatumumab, and ozanimod, all of which are not recommended during pregnancy based primarily on minimal data that suggests, but does not confirm, possible teratogenicity.
“When these new medications hit the market, maternal-fetal medicine physicians and obstetricians are left with very scant data on how to counsel patients, and it’s often based on theory, case reports, or animal studies,” said Teodora Kolarova, MD, a maternal-fetal medicine physician at the University of Washington, Seattle, who was not involved in any of the research. “Although these sample sizes seem small, the population they are sampling from – patients with MS who take immunomodulators who then experience a pregnancy – is much smaller than all pregnant patients.”
Taken together, the findings suggest no increased risk of miscarriage or congenital malformation, compared with baseline risk, Dr. Kolarova said.
“As a whole, these studies are overall reassuring with, of course, some caveats, including timing of medication exposure, limited sample size, and limited outcome data,” Dr. Kolarova said. She noted that embryonic organ formation is complete by 10 weeks gestation, by which time an unplanned pregnancy may not have been recognized yet. “In the subset of patients in the studies that were exposed during the first trimester, there was no increase in congenital malformations from a baseline risk of about 2%-3% in the general population, which is helpful for patient counseling.”
Counseling during the childbearing years
That kind of counseling is important yet absent for many people capable of pregnancy, suggests separate research also presented at the conference by Suma Shah, MD, an associate professor of neurology at Duke University, Durham, N.C. Dr. Shah gave 13-question surveys to female MS patients of all ages at her institution and presented an analysis of data from 38 completed surveys. Among those taking disease-modifying therapies, their medications included ocrelizumab, rituximab, teriflunomide, fingolimod, fumarates, interferons, natalizumab, and cladribine.
“MS disproportionately impacts women among 20 to 40 years, and that’s a really big part of their childbearing years when there are big decisions being made about whether they’re going to choose to grow family or not,” said Dr. Shah. The average age of those who completed the survey was 44. Dr. Shah noted that a lot of research has looked at the safety of older disease-modifying agents in pregnancy, but that information doesn’t appear to be filtering down to patients. “What I really wanted to look at is what do our parent patients understand about whether or not they can even think about pregnancy – and there’s a lot of work to be done.”
Just under a third of survey respondents said they did not have as many children as they would like, and a quarter said they were told they couldn’t have children if they had a diagnosis of MS.
“That was a little heartbreaking to hear because that’s not the truth,” Dr. Shah said. She said it’s necessary to have a more detailed conversation looking at tailored decisions for patients. “Both of those things – patients not being able to grow their family to the number that they desire, and not feeling like they can grow a family – I would think in 2023 we would have come farther than that, and there’s still a lot of room there to improve.”
She advised clinicians not to assume that MS patients know what their options are regarding family planning. “There’s still a lot of room for conversations,” she said. She also explicitly recommends discussing family planning and pregnancy planning with every patient, no matter their gender, early and often.
Cladribine shows no miscarriage, malformations
Dr. Kolarova noted that one of the studies, on cladribine, had a fairly robust sample size with its 180 pregnancy exposures. In that study, led by Kerstin Hellwig, MD, of Ruhr University in Bochum, Germany, data came from the global surveillance program MAPLE-MS, established to assess cladribine effects on pregnancy and infant outcomes. The researchers analyzed data from 76 mothers and 9 fathers who, at any time from 2017 to 2022, were taking cladribine during pregnancy or up to 6 months before pregnancy. Outcomes included live birth, miscarriage, stillbirth, elective abortion, ectopic pregnancy, and major congenital anomalies.
Just over half the mothers (53.9%) were exposed before pregnancy, and about a quarter (26.3%) were exposed during the first trimester. The timing was unknown for most of the other mothers (18.4%). Among the fathers, two-thirds (66.7%) were exposed before pregnancy, and one-third had unknown timing.
Among the 180 pregnancies in the maternal cohort, 42.2% had known outcomes. Nearly half the women (48.7%) taking cladribine had live births, 28.9% had elective abortions, and 21.1% had miscarriages. Only 9 of the 22 pregnancies in the paternal cohort had known outcomes, which included 88.9% live births and 11.1% miscarriages. None of the pregnancies resulted in stillbirth or in a live birth with major congenital anomalies.
”Robust conclusions cannot be made about the risks of adverse pregnancy outcomes with cladribine tablets, but no increase has been signaled thus far,” the researchers reported. ”It is necessary to counsel patients to prevent pregnancy and to use effective contraception during cladribine tablets intake and for at least 6 months after the last cladribine tablet intake in each treatment year.”
Emily Evans, MD, MBE, medical director at U.S. Neurology and Immunology in Rockland, Mass., speaking on behalf of the findings, said they were fairly encouraging.
“Of course, we don’t encourage patients to get pregnant within 6 months of their last dose of cladribine tablets,” Dr. Evans said, but “within those individuals who have gotten pregnant within 6 months of their last dose of cladribine, or who have fathered a child within 6 months of their last dose of cladribine tablets, we’re seeing overall encouraging outcomes. We’re specifically not seeing any differences in the rates of spontaneous abortions, and we’re not seeing any differences in the rates of congenital malformations.”
Ocrelizumab and ofatumumab: No infections so far
Current recommendations for ocrelizumab are to avoid pregnancy for 6 months after the last infusion and stop any breastfeeding during therapy. Yet these recommendations are only because of insufficient data rather than evidence of risk, according to Lana Zhovtis Ryerson, MD, of the NYU Multiple Sclerosis Comprehensive Care Center in New York. She and her colleagues identified all women of childbearing age who had received ocrelizumab within 1 year of pregnancy at their NYU institution. A retrospective chart review found 18 women, with an average age of 35, an average 11 years of an MS diagnosis, and an average 11 months taking ocrelizumab.
Among the 18 pregnancies, four women had a first trimester miscarriage, one had a second trimester miscarriage, and one had an abortion. The miscarriage rate could have been partly influenced by the older maternal population, the authors noted. Of the remaining 12 live births, one infant was premature at 34 weeks, and three infants stayed in NICU but were discharged within 2 weeks.
One patient experienced an MS relapse postpartum, despite receiving ocrelizumab within 45 days of delivery. Of the 16 women who agreed to participate in a Pregnancy Assessment Monitoring System (PRAMS) developed by the CDC, two women chose to breastfeed, and seven said their neurologist recommended against breastfeeding. None of the children’s pediatricians advised delaying vaccinations.
“This small sample observational study has not identified a potential additional risk with ocrelizumab for an adverse pregnancy outcome,” the authors concluded, but they added that ongoing studies, MINORE and SOPRANINO, can help guide future recommendations.
Though still limited, slightly more data exists on ofatumumab during pregnancy, including transient B-cell depletion and lymphopenia in infants whose mothers received anti-CD20 antibodies during pregnancy. However, research has found minimal IgG transfer in the first trimester, though it begins rising in the second trimester, and in utero ofatumumab exposure did not lead to any maternal toxicity or adverse prenatal or postnatal developmental effects in cynomolgus monkeys.
Riley Love, MD, of the University of California, San Francisco, Weill Institute for Neuroscience, and her colleagues both prospectively and retrospectively examined pregnancy and infant outcomes for up to 1 year post partum in women with MS who took ofatumumab during pregnancy or in the 6 months leading up to pregnancy. Their population included 104 prospective cases, most of which (84%) included first trimester exposure, and 14 retrospective cases. One in five of the prospective cases occurred during a clinical trial, while the remaining 80% occurred in postmarketing surveillance.
The prospectively followed women were an average 32 years old and were an average 7 weeks pregnant at the time of reporting. Among the 106 fetuses (including two twin pregnancies), only 30 outcomes had data at the cutoff time, including 16 live births, 9 abortions, and 5 miscarriages. None of the live births had congenital anomalies or serious infections. Another 30 pregnancies were lost to follow-up, and 46 were ongoing.
In the 14 retrospective cases, 57% of women were exposed in the first trimester, and 43% were exposed leading up to pregnancy. Half the cases occurred during clinical trials, and half in postmarketing surveillance. The women were an average 32 years old and were an average 10 weeks pregnant at reporting. Among the 14 pregnancies, nine were miscarriages, one was aborted, and four were born live with no congenital anomalies.
The authors did not draw any conclusions from the findings; they cited too little data and an ongoing study by Novartis to investigate ofatumumab in pregnancy.
“Therapies such as ofatumumab and ocrelizumab can lead to increased risk of infection due to transient B-cell depletion in neonates, but the two studies looking at this did not demonstrate increased infectious morbidity for these infants,” Dr. Kolarova said. “As with all poster presentations, I look forward to reading the full papers once they are published as they will often include a lot more detail about when during pregnancy medication exposure occurred and more detailed outcome data that was assessed.”
Ozanimod outcomes within general population’s ‘expected ranges’
The final study looked at outcomes of pregnancies in people taking ozanimod and in the partners of people taking ozanimod in a clinical trial setting. The findings show low rates of miscarriage, preterm birth, and congenital anomalies that the authors concluded were within the typical range expected for the general population.
“While pregnancy should be avoided when taking and for 3 months after stopping ozanimod to allow for drug elimination, there is no evidence to date of increased occurrence of adverse pregnancy outcomes with ozanimod exposure during early pregnancy,” wrote Anthony Krakovich, of Bristol Myers Squibb in Princeton, N.J., and his associates.
Ozanimod is an oral sphingosine 1-phosphate (S1P) receptor 1 and 5 modulator whose therapeutic mechanism is not fully understood “but may involve the reduction of lymphocyte migration into the central nervous system and intestine,” the authors wrote. S1P receptors are involved in vascular formation during embryogenesis, and animal studies in rats and rabbits have shown toxicity to the embryo and fetus from S1P receptor modulators, including death and malformations. S1P receptor modulator labels therefore note potential fetal risk and the need for effective contraception while taking the drug.
The study prospectively tracked clinical trial participants taking ozanimod as healthy volunteers or for relapsing MS, ulcerative colitis, or Crohn’s disease. Most of the participants who became pregnant (73%) had relapsing MS, while 18% had ulcerative colitis and 8% had Crohn’s disease.
In female patients receiving ozanimod, 78 pregnancies resulted in 12 miscarriages (including one twin), 15 abortions, and 42 live births, with 6 pregnancies ongoing at the time of reporting and no data available for the remaining 4 pregnancies. Among the 42 live births, 4 were premature but otherwise healthy, 1 had a duplex kidney, and the other 37 infants were typical with no apparent health concerns. These rates of miscarriage, preterm birth, and congenital anomalies were within the expected ranges for the general population, the researchers wrote.
The researchers also assessed pregnancy outcomes for partners of male participants taking ozanimod. The 29 partner pregnancies resulted in 21 live births and one miscarriage, with one pregnancy ongoing and no information available for the other seven. The live births included 5 premature infants (including twins), 13 typical and healthy infants, 1 with Hirschsprung’s disease, 1 with a congenital hydrocele, and 1 with a partial atrioventricular septal defect. Again, the researchers concluded that these rates were within the typical range for the general population and that “no teratogenicity was observed.”
“We often encourage patients with MS, regardless of disease activity and therapies, to seek preconception evaluations with Maternal-Fetal Medicine and their neurologists in order to make plans for pregnancy and postpartum care,” Dr. Kolarova said. “That being said, access to subspecialized health care is not available to all, and pregnancy prior to such consultation does occur. These studies provide novel information that we have not had access to in the past and can improve patient counseling regarding their risks and options.”
The study on cladribine was funded by Merck KGaA, at which two authors are employed. Dr. Hellwig reported consulting, speaker, and/or research support from Bayer, Biogen, Teva, Novartis, Roche, Sanofi, Schering Healthcare, Serono, and Merck, and one author is a former employee of EMD Serono. The study on ocrelizumab was funded by Genentech. Dr. Zhovtis Ryerson reported personal fees from Biogen, Genentech, and Novartis, and research grants from Biogen, Genentech, and CMSC. The other authors had no disclosures. The study on ofatumumab was funded by Novartis. Dr. Bove has received research funds from Biogen, Novartis, and Roche Genentech, and consulting fees from EMD Serono, Horizon, Janssen, and TG Therapeutics; she has an ownership interest in Global Consult MD. Five authors are Novartis employees. Her coauthors, including Dr. Hellwig, reported advisory, consulting, research, speaking, or traveling fees from Alexion, Bayer, Biogen, Celgene BMS, EMD Serono, Horizon, Janssen, Lundbeck, Merck, Pfizer, Roche Genentech, Sanofi Genzyme, Schering Healthcare, Teva, TG Therapeutics, and Novartis. The study on ozanimod was funded by Bristol Myers Squibb. Dr. Krakovich and another author are employees and/or shareholders of Bristol Myers Squibb. The other authors reported consulting, speaking, advisory board, and/or research fees from AbbVie, Almirall, Arena, Biogen, Boehringer Ingelhei, Celgene, Celltrion, EXCEMED, Falk Benelux, Ferring, Forward Pharma, Genentech, Genzyme, Gilead, Janssen, Lilly, Merck, Novartis, Ono Pharma, Pfizer, Prometheus Labs, Protagonist, Roche, Sanofi, Synthon, Takeda, and Teva. Dr. Kolarova had no disclosures. Dr. Shah has received research support from Biogen and VeraSci.
AURORA, COLO. – Several drugs for multiple sclerosis (MS) that are contraindicated during pregnancy nevertheless have not shown concerning safety signals in a series of small studies presented as posters at the annual meeting of the Consortium of Multiple Sclerosis Centers. The industry-sponsored research included an assessment of pregnancy and infant outcomes for cladribine, ocrelizumab, ofatumumab, and ozanimod, all of which are not recommended during pregnancy based primarily on minimal data that suggests, but does not confirm, possible teratogenicity.
“When these new medications hit the market, maternal-fetal medicine physicians and obstetricians are left with very scant data on how to counsel patients, and it’s often based on theory, case reports, or animal studies,” said Teodora Kolarova, MD, a maternal-fetal medicine physician at the University of Washington, Seattle, who was not involved in any of the research. “Although these sample sizes seem small, the population they are sampling from – patients with MS who take immunomodulators who then experience a pregnancy – is much smaller than all pregnant patients.”
Taken together, the findings suggest no increased risk of miscarriage or congenital malformation, compared with baseline risk, Dr. Kolarova said.
“As a whole, these studies are overall reassuring with, of course, some caveats, including timing of medication exposure, limited sample size, and limited outcome data,” Dr. Kolarova said. She noted that embryonic organ formation is complete by 10 weeks gestation, by which time an unplanned pregnancy may not have been recognized yet. “In the subset of patients in the studies that were exposed during the first trimester, there was no increase in congenital malformations from a baseline risk of about 2%-3% in the general population, which is helpful for patient counseling.”
Counseling during the childbearing years
That kind of counseling is important yet absent for many people capable of pregnancy, suggests separate research also presented at the conference by Suma Shah, MD, an associate professor of neurology at Duke University, Durham, N.C. Dr. Shah gave 13-question surveys to female MS patients of all ages at her institution and presented an analysis of data from 38 completed surveys. Among those taking disease-modifying therapies, their medications included ocrelizumab, rituximab, teriflunomide, fingolimod, fumarates, interferons, natalizumab, and cladribine.
“MS disproportionately impacts women among 20 to 40 years, and that’s a really big part of their childbearing years when there are big decisions being made about whether they’re going to choose to grow family or not,” said Dr. Shah. The average age of those who completed the survey was 44. Dr. Shah noted that a lot of research has looked at the safety of older disease-modifying agents in pregnancy, but that information doesn’t appear to be filtering down to patients. “What I really wanted to look at is what do our parent patients understand about whether or not they can even think about pregnancy – and there’s a lot of work to be done.”
Just under a third of survey respondents said they did not have as many children as they would like, and a quarter said they were told they couldn’t have children if they had a diagnosis of MS.
“That was a little heartbreaking to hear because that’s not the truth,” Dr. Shah said. She said it’s necessary to have a more detailed conversation looking at tailored decisions for patients. “Both of those things – patients not being able to grow their family to the number that they desire, and not feeling like they can grow a family – I would think in 2023 we would have come farther than that, and there’s still a lot of room there to improve.”
She advised clinicians not to assume that MS patients know what their options are regarding family planning. “There’s still a lot of room for conversations,” she said. She also explicitly recommends discussing family planning and pregnancy planning with every patient, no matter their gender, early and often.
Cladribine shows no miscarriage, malformations
Dr. Kolarova noted that one of the studies, on cladribine, had a fairly robust sample size with its 180 pregnancy exposures. In that study, led by Kerstin Hellwig, MD, of Ruhr University in Bochum, Germany, data came from the global surveillance program MAPLE-MS, established to assess cladribine effects on pregnancy and infant outcomes. The researchers analyzed data from 76 mothers and 9 fathers who, at any time from 2017 to 2022, were taking cladribine during pregnancy or up to 6 months before pregnancy. Outcomes included live birth, miscarriage, stillbirth, elective abortion, ectopic pregnancy, and major congenital anomalies.
Just over half the mothers (53.9%) were exposed before pregnancy, and about a quarter (26.3%) were exposed during the first trimester. The timing was unknown for most of the other mothers (18.4%). Among the fathers, two-thirds (66.7%) were exposed before pregnancy, and one-third had unknown timing.
Among the 180 pregnancies in the maternal cohort, 42.2% had known outcomes. Nearly half the women (48.7%) taking cladribine had live births, 28.9% had elective abortions, and 21.1% had miscarriages. Only 9 of the 22 pregnancies in the paternal cohort had known outcomes, which included 88.9% live births and 11.1% miscarriages. None of the pregnancies resulted in stillbirth or in a live birth with major congenital anomalies.
”Robust conclusions cannot be made about the risks of adverse pregnancy outcomes with cladribine tablets, but no increase has been signaled thus far,” the researchers reported. ”It is necessary to counsel patients to prevent pregnancy and to use effective contraception during cladribine tablets intake and for at least 6 months after the last cladribine tablet intake in each treatment year.”
Emily Evans, MD, MBE, medical director at U.S. Neurology and Immunology in Rockland, Mass., speaking on behalf of the findings, said they were fairly encouraging.
“Of course, we don’t encourage patients to get pregnant within 6 months of their last dose of cladribine tablets,” Dr. Evans said, but “within those individuals who have gotten pregnant within 6 months of their last dose of cladribine, or who have fathered a child within 6 months of their last dose of cladribine tablets, we’re seeing overall encouraging outcomes. We’re specifically not seeing any differences in the rates of spontaneous abortions, and we’re not seeing any differences in the rates of congenital malformations.”
Ocrelizumab and ofatumumab: No infections so far
Current recommendations for ocrelizumab are to avoid pregnancy for 6 months after the last infusion and stop any breastfeeding during therapy. Yet these recommendations are only because of insufficient data rather than evidence of risk, according to Lana Zhovtis Ryerson, MD, of the NYU Multiple Sclerosis Comprehensive Care Center in New York. She and her colleagues identified all women of childbearing age who had received ocrelizumab within 1 year of pregnancy at their NYU institution. A retrospective chart review found 18 women, with an average age of 35, an average 11 years of an MS diagnosis, and an average 11 months taking ocrelizumab.
Among the 18 pregnancies, four women had a first trimester miscarriage, one had a second trimester miscarriage, and one had an abortion. The miscarriage rate could have been partly influenced by the older maternal population, the authors noted. Of the remaining 12 live births, one infant was premature at 34 weeks, and three infants stayed in NICU but were discharged within 2 weeks.
One patient experienced an MS relapse postpartum, despite receiving ocrelizumab within 45 days of delivery. Of the 16 women who agreed to participate in a Pregnancy Assessment Monitoring System (PRAMS) developed by the CDC, two women chose to breastfeed, and seven said their neurologist recommended against breastfeeding. None of the children’s pediatricians advised delaying vaccinations.
“This small sample observational study has not identified a potential additional risk with ocrelizumab for an adverse pregnancy outcome,” the authors concluded, but they added that ongoing studies, MINORE and SOPRANINO, can help guide future recommendations.
Though still limited, slightly more data exists on ofatumumab during pregnancy, including transient B-cell depletion and lymphopenia in infants whose mothers received anti-CD20 antibodies during pregnancy. However, research has found minimal IgG transfer in the first trimester, though it begins rising in the second trimester, and in utero ofatumumab exposure did not lead to any maternal toxicity or adverse prenatal or postnatal developmental effects in cynomolgus monkeys.
Riley Love, MD, of the University of California, San Francisco, Weill Institute for Neuroscience, and her colleagues both prospectively and retrospectively examined pregnancy and infant outcomes for up to 1 year post partum in women with MS who took ofatumumab during pregnancy or in the 6 months leading up to pregnancy. Their population included 104 prospective cases, most of which (84%) included first trimester exposure, and 14 retrospective cases. One in five of the prospective cases occurred during a clinical trial, while the remaining 80% occurred in postmarketing surveillance.
The prospectively followed women were an average 32 years old and were an average 7 weeks pregnant at the time of reporting. Among the 106 fetuses (including two twin pregnancies), only 30 outcomes had data at the cutoff time, including 16 live births, 9 abortions, and 5 miscarriages. None of the live births had congenital anomalies or serious infections. Another 30 pregnancies were lost to follow-up, and 46 were ongoing.
In the 14 retrospective cases, 57% of women were exposed in the first trimester, and 43% were exposed leading up to pregnancy. Half the cases occurred during clinical trials, and half in postmarketing surveillance. The women were an average 32 years old and were an average 10 weeks pregnant at reporting. Among the 14 pregnancies, nine were miscarriages, one was aborted, and four were born live with no congenital anomalies.
The authors did not draw any conclusions from the findings; they cited too little data and an ongoing study by Novartis to investigate ofatumumab in pregnancy.
“Therapies such as ofatumumab and ocrelizumab can lead to increased risk of infection due to transient B-cell depletion in neonates, but the two studies looking at this did not demonstrate increased infectious morbidity for these infants,” Dr. Kolarova said. “As with all poster presentations, I look forward to reading the full papers once they are published as they will often include a lot more detail about when during pregnancy medication exposure occurred and more detailed outcome data that was assessed.”
Ozanimod outcomes within general population’s ‘expected ranges’
The final study looked at outcomes of pregnancies in people taking ozanimod and in the partners of people taking ozanimod in a clinical trial setting. The findings show low rates of miscarriage, preterm birth, and congenital anomalies that the authors concluded were within the typical range expected for the general population.
“While pregnancy should be avoided when taking and for 3 months after stopping ozanimod to allow for drug elimination, there is no evidence to date of increased occurrence of adverse pregnancy outcomes with ozanimod exposure during early pregnancy,” wrote Anthony Krakovich, of Bristol Myers Squibb in Princeton, N.J., and his associates.
Ozanimod is an oral sphingosine 1-phosphate (S1P) receptor 1 and 5 modulator whose therapeutic mechanism is not fully understood “but may involve the reduction of lymphocyte migration into the central nervous system and intestine,” the authors wrote. S1P receptors are involved in vascular formation during embryogenesis, and animal studies in rats and rabbits have shown toxicity to the embryo and fetus from S1P receptor modulators, including death and malformations. S1P receptor modulator labels therefore note potential fetal risk and the need for effective contraception while taking the drug.
The study prospectively tracked clinical trial participants taking ozanimod as healthy volunteers or for relapsing MS, ulcerative colitis, or Crohn’s disease. Most of the participants who became pregnant (73%) had relapsing MS, while 18% had ulcerative colitis and 8% had Crohn’s disease.
In female patients receiving ozanimod, 78 pregnancies resulted in 12 miscarriages (including one twin), 15 abortions, and 42 live births, with 6 pregnancies ongoing at the time of reporting and no data available for the remaining 4 pregnancies. Among the 42 live births, 4 were premature but otherwise healthy, 1 had a duplex kidney, and the other 37 infants were typical with no apparent health concerns. These rates of miscarriage, preterm birth, and congenital anomalies were within the expected ranges for the general population, the researchers wrote.
The researchers also assessed pregnancy outcomes for partners of male participants taking ozanimod. The 29 partner pregnancies resulted in 21 live births and one miscarriage, with one pregnancy ongoing and no information available for the other seven. The live births included 5 premature infants (including twins), 13 typical and healthy infants, 1 with Hirschsprung’s disease, 1 with a congenital hydrocele, and 1 with a partial atrioventricular septal defect. Again, the researchers concluded that these rates were within the typical range for the general population and that “no teratogenicity was observed.”
“We often encourage patients with MS, regardless of disease activity and therapies, to seek preconception evaluations with Maternal-Fetal Medicine and their neurologists in order to make plans for pregnancy and postpartum care,” Dr. Kolarova said. “That being said, access to subspecialized health care is not available to all, and pregnancy prior to such consultation does occur. These studies provide novel information that we have not had access to in the past and can improve patient counseling regarding their risks and options.”
The study on cladribine was funded by Merck KGaA, at which two authors are employed. Dr. Hellwig reported consulting, speaker, and/or research support from Bayer, Biogen, Teva, Novartis, Roche, Sanofi, Schering Healthcare, Serono, and Merck, and one author is a former employee of EMD Serono. The study on ocrelizumab was funded by Genentech. Dr. Zhovtis Ryerson reported personal fees from Biogen, Genentech, and Novartis, and research grants from Biogen, Genentech, and CMSC. The other authors had no disclosures. The study on ofatumumab was funded by Novartis. Dr. Bove has received research funds from Biogen, Novartis, and Roche Genentech, and consulting fees from EMD Serono, Horizon, Janssen, and TG Therapeutics; she has an ownership interest in Global Consult MD. Five authors are Novartis employees. Her coauthors, including Dr. Hellwig, reported advisory, consulting, research, speaking, or traveling fees from Alexion, Bayer, Biogen, Celgene BMS, EMD Serono, Horizon, Janssen, Lundbeck, Merck, Pfizer, Roche Genentech, Sanofi Genzyme, Schering Healthcare, Teva, TG Therapeutics, and Novartis. The study on ozanimod was funded by Bristol Myers Squibb. Dr. Krakovich and another author are employees and/or shareholders of Bristol Myers Squibb. The other authors reported consulting, speaking, advisory board, and/or research fees from AbbVie, Almirall, Arena, Biogen, Boehringer Ingelhei, Celgene, Celltrion, EXCEMED, Falk Benelux, Ferring, Forward Pharma, Genentech, Genzyme, Gilead, Janssen, Lilly, Merck, Novartis, Ono Pharma, Pfizer, Prometheus Labs, Protagonist, Roche, Sanofi, Synthon, Takeda, and Teva. Dr. Kolarova had no disclosures. Dr. Shah has received research support from Biogen and VeraSci.
AURORA, COLO. – Several drugs for multiple sclerosis (MS) that are contraindicated during pregnancy nevertheless have not shown concerning safety signals in a series of small studies presented as posters at the annual meeting of the Consortium of Multiple Sclerosis Centers. The industry-sponsored research included an assessment of pregnancy and infant outcomes for cladribine, ocrelizumab, ofatumumab, and ozanimod, all of which are not recommended during pregnancy based primarily on minimal data that suggests, but does not confirm, possible teratogenicity.
“When these new medications hit the market, maternal-fetal medicine physicians and obstetricians are left with very scant data on how to counsel patients, and it’s often based on theory, case reports, or animal studies,” said Teodora Kolarova, MD, a maternal-fetal medicine physician at the University of Washington, Seattle, who was not involved in any of the research. “Although these sample sizes seem small, the population they are sampling from – patients with MS who take immunomodulators who then experience a pregnancy – is much smaller than all pregnant patients.”
Taken together, the findings suggest no increased risk of miscarriage or congenital malformation, compared with baseline risk, Dr. Kolarova said.
“As a whole, these studies are overall reassuring with, of course, some caveats, including timing of medication exposure, limited sample size, and limited outcome data,” Dr. Kolarova said. She noted that embryonic organ formation is complete by 10 weeks gestation, by which time an unplanned pregnancy may not have been recognized yet. “In the subset of patients in the studies that were exposed during the first trimester, there was no increase in congenital malformations from a baseline risk of about 2%-3% in the general population, which is helpful for patient counseling.”
Counseling during the childbearing years
That kind of counseling is important yet absent for many people capable of pregnancy, suggests separate research also presented at the conference by Suma Shah, MD, an associate professor of neurology at Duke University, Durham, N.C. Dr. Shah gave 13-question surveys to female MS patients of all ages at her institution and presented an analysis of data from 38 completed surveys. Among those taking disease-modifying therapies, their medications included ocrelizumab, rituximab, teriflunomide, fingolimod, fumarates, interferons, natalizumab, and cladribine.
“MS disproportionately impacts women among 20 to 40 years, and that’s a really big part of their childbearing years when there are big decisions being made about whether they’re going to choose to grow family or not,” said Dr. Shah. The average age of those who completed the survey was 44. Dr. Shah noted that a lot of research has looked at the safety of older disease-modifying agents in pregnancy, but that information doesn’t appear to be filtering down to patients. “What I really wanted to look at is what do our parent patients understand about whether or not they can even think about pregnancy – and there’s a lot of work to be done.”
Just under a third of survey respondents said they did not have as many children as they would like, and a quarter said they were told they couldn’t have children if they had a diagnosis of MS.
“That was a little heartbreaking to hear because that’s not the truth,” Dr. Shah said. She said it’s necessary to have a more detailed conversation looking at tailored decisions for patients. “Both of those things – patients not being able to grow their family to the number that they desire, and not feeling like they can grow a family – I would think in 2023 we would have come farther than that, and there’s still a lot of room there to improve.”
She advised clinicians not to assume that MS patients know what their options are regarding family planning. “There’s still a lot of room for conversations,” she said. She also explicitly recommends discussing family planning and pregnancy planning with every patient, no matter their gender, early and often.
Cladribine shows no miscarriage, malformations
Dr. Kolarova noted that one of the studies, on cladribine, had a fairly robust sample size with its 180 pregnancy exposures. In that study, led by Kerstin Hellwig, MD, of Ruhr University in Bochum, Germany, data came from the global surveillance program MAPLE-MS, established to assess cladribine effects on pregnancy and infant outcomes. The researchers analyzed data from 76 mothers and 9 fathers who, at any time from 2017 to 2022, were taking cladribine during pregnancy or up to 6 months before pregnancy. Outcomes included live birth, miscarriage, stillbirth, elective abortion, ectopic pregnancy, and major congenital anomalies.
Just over half the mothers (53.9%) were exposed before pregnancy, and about a quarter (26.3%) were exposed during the first trimester. The timing was unknown for most of the other mothers (18.4%). Among the fathers, two-thirds (66.7%) were exposed before pregnancy, and one-third had unknown timing.
Among the 180 pregnancies in the maternal cohort, 42.2% had known outcomes. Nearly half the women (48.7%) taking cladribine had live births, 28.9% had elective abortions, and 21.1% had miscarriages. Only 9 of the 22 pregnancies in the paternal cohort had known outcomes, which included 88.9% live births and 11.1% miscarriages. None of the pregnancies resulted in stillbirth or in a live birth with major congenital anomalies.
”Robust conclusions cannot be made about the risks of adverse pregnancy outcomes with cladribine tablets, but no increase has been signaled thus far,” the researchers reported. ”It is necessary to counsel patients to prevent pregnancy and to use effective contraception during cladribine tablets intake and for at least 6 months after the last cladribine tablet intake in each treatment year.”
Emily Evans, MD, MBE, medical director at U.S. Neurology and Immunology in Rockland, Mass., speaking on behalf of the findings, said they were fairly encouraging.
“Of course, we don’t encourage patients to get pregnant within 6 months of their last dose of cladribine tablets,” Dr. Evans said, but “within those individuals who have gotten pregnant within 6 months of their last dose of cladribine, or who have fathered a child within 6 months of their last dose of cladribine tablets, we’re seeing overall encouraging outcomes. We’re specifically not seeing any differences in the rates of spontaneous abortions, and we’re not seeing any differences in the rates of congenital malformations.”
Ocrelizumab and ofatumumab: No infections so far
Current recommendations for ocrelizumab are to avoid pregnancy for 6 months after the last infusion and stop any breastfeeding during therapy. Yet these recommendations are only because of insufficient data rather than evidence of risk, according to Lana Zhovtis Ryerson, MD, of the NYU Multiple Sclerosis Comprehensive Care Center in New York. She and her colleagues identified all women of childbearing age who had received ocrelizumab within 1 year of pregnancy at their NYU institution. A retrospective chart review found 18 women, with an average age of 35, an average 11 years of an MS diagnosis, and an average 11 months taking ocrelizumab.
Among the 18 pregnancies, four women had a first trimester miscarriage, one had a second trimester miscarriage, and one had an abortion. The miscarriage rate could have been partly influenced by the older maternal population, the authors noted. Of the remaining 12 live births, one infant was premature at 34 weeks, and three infants stayed in NICU but were discharged within 2 weeks.
One patient experienced an MS relapse postpartum, despite receiving ocrelizumab within 45 days of delivery. Of the 16 women who agreed to participate in a Pregnancy Assessment Monitoring System (PRAMS) developed by the CDC, two women chose to breastfeed, and seven said their neurologist recommended against breastfeeding. None of the children’s pediatricians advised delaying vaccinations.
“This small sample observational study has not identified a potential additional risk with ocrelizumab for an adverse pregnancy outcome,” the authors concluded, but they added that ongoing studies, MINORE and SOPRANINO, can help guide future recommendations.
Though still limited, slightly more data exists on ofatumumab during pregnancy, including transient B-cell depletion and lymphopenia in infants whose mothers received anti-CD20 antibodies during pregnancy. However, research has found minimal IgG transfer in the first trimester, though it begins rising in the second trimester, and in utero ofatumumab exposure did not lead to any maternal toxicity or adverse prenatal or postnatal developmental effects in cynomolgus monkeys.
Riley Love, MD, of the University of California, San Francisco, Weill Institute for Neuroscience, and her colleagues both prospectively and retrospectively examined pregnancy and infant outcomes for up to 1 year post partum in women with MS who took ofatumumab during pregnancy or in the 6 months leading up to pregnancy. Their population included 104 prospective cases, most of which (84%) included first trimester exposure, and 14 retrospective cases. One in five of the prospective cases occurred during a clinical trial, while the remaining 80% occurred in postmarketing surveillance.
The prospectively followed women were an average 32 years old and were an average 7 weeks pregnant at the time of reporting. Among the 106 fetuses (including two twin pregnancies), only 30 outcomes had data at the cutoff time, including 16 live births, 9 abortions, and 5 miscarriages. None of the live births had congenital anomalies or serious infections. Another 30 pregnancies were lost to follow-up, and 46 were ongoing.
In the 14 retrospective cases, 57% of women were exposed in the first trimester, and 43% were exposed leading up to pregnancy. Half the cases occurred during clinical trials, and half in postmarketing surveillance. The women were an average 32 years old and were an average 10 weeks pregnant at reporting. Among the 14 pregnancies, nine were miscarriages, one was aborted, and four were born live with no congenital anomalies.
The authors did not draw any conclusions from the findings; they cited too little data and an ongoing study by Novartis to investigate ofatumumab in pregnancy.
“Therapies such as ofatumumab and ocrelizumab can lead to increased risk of infection due to transient B-cell depletion in neonates, but the two studies looking at this did not demonstrate increased infectious morbidity for these infants,” Dr. Kolarova said. “As with all poster presentations, I look forward to reading the full papers once they are published as they will often include a lot more detail about when during pregnancy medication exposure occurred and more detailed outcome data that was assessed.”
Ozanimod outcomes within general population’s ‘expected ranges’
The final study looked at outcomes of pregnancies in people taking ozanimod and in the partners of people taking ozanimod in a clinical trial setting. The findings show low rates of miscarriage, preterm birth, and congenital anomalies that the authors concluded were within the typical range expected for the general population.
“While pregnancy should be avoided when taking and for 3 months after stopping ozanimod to allow for drug elimination, there is no evidence to date of increased occurrence of adverse pregnancy outcomes with ozanimod exposure during early pregnancy,” wrote Anthony Krakovich, of Bristol Myers Squibb in Princeton, N.J., and his associates.
Ozanimod is an oral sphingosine 1-phosphate (S1P) receptor 1 and 5 modulator whose therapeutic mechanism is not fully understood “but may involve the reduction of lymphocyte migration into the central nervous system and intestine,” the authors wrote. S1P receptors are involved in vascular formation during embryogenesis, and animal studies in rats and rabbits have shown toxicity to the embryo and fetus from S1P receptor modulators, including death and malformations. S1P receptor modulator labels therefore note potential fetal risk and the need for effective contraception while taking the drug.
The study prospectively tracked clinical trial participants taking ozanimod as healthy volunteers or for relapsing MS, ulcerative colitis, or Crohn’s disease. Most of the participants who became pregnant (73%) had relapsing MS, while 18% had ulcerative colitis and 8% had Crohn’s disease.
In female patients receiving ozanimod, 78 pregnancies resulted in 12 miscarriages (including one twin), 15 abortions, and 42 live births, with 6 pregnancies ongoing at the time of reporting and no data available for the remaining 4 pregnancies. Among the 42 live births, 4 were premature but otherwise healthy, 1 had a duplex kidney, and the other 37 infants were typical with no apparent health concerns. These rates of miscarriage, preterm birth, and congenital anomalies were within the expected ranges for the general population, the researchers wrote.
The researchers also assessed pregnancy outcomes for partners of male participants taking ozanimod. The 29 partner pregnancies resulted in 21 live births and one miscarriage, with one pregnancy ongoing and no information available for the other seven. The live births included 5 premature infants (including twins), 13 typical and healthy infants, 1 with Hirschsprung’s disease, 1 with a congenital hydrocele, and 1 with a partial atrioventricular septal defect. Again, the researchers concluded that these rates were within the typical range for the general population and that “no teratogenicity was observed.”
“We often encourage patients with MS, regardless of disease activity and therapies, to seek preconception evaluations with Maternal-Fetal Medicine and their neurologists in order to make plans for pregnancy and postpartum care,” Dr. Kolarova said. “That being said, access to subspecialized health care is not available to all, and pregnancy prior to such consultation does occur. These studies provide novel information that we have not had access to in the past and can improve patient counseling regarding their risks and options.”
The study on cladribine was funded by Merck KGaA, at which two authors are employed. Dr. Hellwig reported consulting, speaker, and/or research support from Bayer, Biogen, Teva, Novartis, Roche, Sanofi, Schering Healthcare, Serono, and Merck, and one author is a former employee of EMD Serono. The study on ocrelizumab was funded by Genentech. Dr. Zhovtis Ryerson reported personal fees from Biogen, Genentech, and Novartis, and research grants from Biogen, Genentech, and CMSC. The other authors had no disclosures. The study on ofatumumab was funded by Novartis. Dr. Bove has received research funds from Biogen, Novartis, and Roche Genentech, and consulting fees from EMD Serono, Horizon, Janssen, and TG Therapeutics; she has an ownership interest in Global Consult MD. Five authors are Novartis employees. Her coauthors, including Dr. Hellwig, reported advisory, consulting, research, speaking, or traveling fees from Alexion, Bayer, Biogen, Celgene BMS, EMD Serono, Horizon, Janssen, Lundbeck, Merck, Pfizer, Roche Genentech, Sanofi Genzyme, Schering Healthcare, Teva, TG Therapeutics, and Novartis. The study on ozanimod was funded by Bristol Myers Squibb. Dr. Krakovich and another author are employees and/or shareholders of Bristol Myers Squibb. The other authors reported consulting, speaking, advisory board, and/or research fees from AbbVie, Almirall, Arena, Biogen, Boehringer Ingelhei, Celgene, Celltrion, EXCEMED, Falk Benelux, Ferring, Forward Pharma, Genentech, Genzyme, Gilead, Janssen, Lilly, Merck, Novartis, Ono Pharma, Pfizer, Prometheus Labs, Protagonist, Roche, Sanofi, Synthon, Takeda, and Teva. Dr. Kolarova had no disclosures. Dr. Shah has received research support from Biogen and VeraSci.
FROM CMSC 2023
Underprescribed menopause relief: Women suffer needlessly
The result: Countless women grapple with the physical and emotional toll of this life transition.
These shortcomings have led to an influx of doctors moving from traditional practice to virtual startups that focus on women’s health issues, treating patients who come to them desperate and frustrated after years of unresolved issues.
The solution is often so simple it is almost maddening, specialists say: vaginal creams containing low-dose estrogen which can address the symptoms of menopause, from vaginal dryness to recurrent urinary tract infections.
“Hands down, this is one of the most meaningful interventions I’ve ever offered to a patient and yet it is underutilized,” said Ashley Winter, MD, chief medical officer and urologist at Odela Health, a digital women’s health clinic. “A lot of companies are blossoming in this menopause space because it is underserved by traditional health care – your gynecologist typically deals with reproduction, and typically when women are done with child-bearing, they’re kind of discharged from the care of their gynecologist.”
More than 1 million women in the United States go through menopause each year. According to a 2022 survey, 4 in 10 women report menopause symptoms that have been disruptive enough to interfere with their work performance on at least a weekly basis.
And yet, many women are not getting appropriate treatment.
Partially to blame is the harmful legacy of faulty data, doctors say. The early results of the federally funded Women’s Health Initiative, released in 2002, showed that hormone therapy (HT) led to increased risk for heart attacks, strokes, and breast cancer. But further analysis showed the opposite: Hormonal therapies have a helpful effect on cardiovascular and bone health and generally reduce risk of death in younger women or those in the early postmenopausal period.
Hormone therapy delivers estrogen, sometimes with progesterone, to the body through gels, creams, patches, pills, suppositories, or a device fitted inside the uterus. Systemic HT sends hormones into the bloodstream, while local HT – like vaginal estrogen cream – specifically treats vaginal symptoms of menopause.
Myths about the health risks linked to systemic and topical HT have long been debunked, and research on topical HT in particular shows it poses no risk for cancer or other chronic diseases.
Yet while 2 decades have passed since the misinformation first started to spread, people remain woefully uninformed about hormone treatments.
The FDA still requires that estrogen products carry a black-box warning on the early data, even though it has since been proven false.
“This is one of the most damaging PR misadventures of modern medicine in my opinion,” Dr. Winter said. “It has literally killed women, and it’s made them miserable.”
The public has a glaring lack of knowledge about menopause management, said Stephanie Faubion, MD, medical director for the North American Menopause Society and director of Mayo Clinic’s Center for Women’s Health.
Treating with low-dose estrogen isn’t a radical approach – in fact, it is the standard of care for women experiencing many menopause symptoms, Dr. Faubion said. But the topic does have nuance, and some people get lost in the specifics.
“I don’t think there’s a lot of knowledge on the risk-benefits of hormone therapy in general,” Dr. Faubion said. “New information comes out so frequently it’s difficult to keep track of. The answer is complicated and depends on dose, duration of treatment, what formulation you’re on. It’s difficult for a lot of people to understand.”
But Dr. Winter said the lack of public knowledge reflects a bigger problem: Knowledge gaps exist among doctors, too, stemming from insufficient training on menopause-related issues.
During her 6-year urology residency, she never learned the role of vaginal estrogen on urinary problems, Dr. Winter said. Only during a 1-year fellowship on sexual dysfunction did she hear about the treatment.
“Despite dealing with urinary issues, incontinence, blood in the urine – training to manage all those concerns – the role of local hormones in the vagina for managing all them was never taught, never discussed,” Dr. Winter said. “I never prescribed any of it.”
A year ago, Dr. Winter left her job at Kaiser Permanente to join Odela. After years of prescribing medications for overactive bladder with little to no results, she said, she now uses the knowledge she gained during her fellowship by helping women who have spent years battling debilitating symptoms.
Urologists are not the only clinicians who lack appropriate training. Obstetrics and gynecology residencies offer little knowledge on menopause treatments, said Ghazaleh Moayedi, DO, an ob.gyn. and complex family planning specialist for Texas-based Pegasus Health Justice Center.
The problem is partly a systems-based one, she said. Training programs often direct patients who are uninsured, or covered through public insurance, to medical residents. Patients who qualify for Medicaid or Medicare are often either pregnant or over 65, Dr. Moayedi said, so women actively going through the transition can slip through the cracks.
“What that means in a state like Texas where I’m based, where it is difficult to qualify for Medicaid, is that the people we see who do qualify are pregnant,” she said. “And you’re not on Medicare until you’re 65. So most ob.gyn. residents don’t graduate with expansive experience in menopause.”
According to Medicaid.gov, 80% of the national population covered by Medicaid is age 45 and younger.
When doctors have proper training and prescribe local hormones, patients don’t always follow the treatment plan, said Andrea Rapkin, MD, professor of obstetrics and gynecology at David Geffen School of Medicine at UCLA.
That failure to follow treatment is yet another example of remaining doubts from the misinformation spread through early research, Dr. Rapkin said.
“I’ll prescribe an estrogen product, and I’ll find out they didn’t take it even though I’ll reassure them,” she said. “I do think there are some lingering concerns, but I’m glad to see there is a growing interest in vaginal hormones.”
A version of this article first appeared on WebMD.com.
The result: Countless women grapple with the physical and emotional toll of this life transition.
These shortcomings have led to an influx of doctors moving from traditional practice to virtual startups that focus on women’s health issues, treating patients who come to them desperate and frustrated after years of unresolved issues.
The solution is often so simple it is almost maddening, specialists say: vaginal creams containing low-dose estrogen which can address the symptoms of menopause, from vaginal dryness to recurrent urinary tract infections.
“Hands down, this is one of the most meaningful interventions I’ve ever offered to a patient and yet it is underutilized,” said Ashley Winter, MD, chief medical officer and urologist at Odela Health, a digital women’s health clinic. “A lot of companies are blossoming in this menopause space because it is underserved by traditional health care – your gynecologist typically deals with reproduction, and typically when women are done with child-bearing, they’re kind of discharged from the care of their gynecologist.”
More than 1 million women in the United States go through menopause each year. According to a 2022 survey, 4 in 10 women report menopause symptoms that have been disruptive enough to interfere with their work performance on at least a weekly basis.
And yet, many women are not getting appropriate treatment.
Partially to blame is the harmful legacy of faulty data, doctors say. The early results of the federally funded Women’s Health Initiative, released in 2002, showed that hormone therapy (HT) led to increased risk for heart attacks, strokes, and breast cancer. But further analysis showed the opposite: Hormonal therapies have a helpful effect on cardiovascular and bone health and generally reduce risk of death in younger women or those in the early postmenopausal period.
Hormone therapy delivers estrogen, sometimes with progesterone, to the body through gels, creams, patches, pills, suppositories, or a device fitted inside the uterus. Systemic HT sends hormones into the bloodstream, while local HT – like vaginal estrogen cream – specifically treats vaginal symptoms of menopause.
Myths about the health risks linked to systemic and topical HT have long been debunked, and research on topical HT in particular shows it poses no risk for cancer or other chronic diseases.
Yet while 2 decades have passed since the misinformation first started to spread, people remain woefully uninformed about hormone treatments.
The FDA still requires that estrogen products carry a black-box warning on the early data, even though it has since been proven false.
“This is one of the most damaging PR misadventures of modern medicine in my opinion,” Dr. Winter said. “It has literally killed women, and it’s made them miserable.”
The public has a glaring lack of knowledge about menopause management, said Stephanie Faubion, MD, medical director for the North American Menopause Society and director of Mayo Clinic’s Center for Women’s Health.
Treating with low-dose estrogen isn’t a radical approach – in fact, it is the standard of care for women experiencing many menopause symptoms, Dr. Faubion said. But the topic does have nuance, and some people get lost in the specifics.
“I don’t think there’s a lot of knowledge on the risk-benefits of hormone therapy in general,” Dr. Faubion said. “New information comes out so frequently it’s difficult to keep track of. The answer is complicated and depends on dose, duration of treatment, what formulation you’re on. It’s difficult for a lot of people to understand.”
But Dr. Winter said the lack of public knowledge reflects a bigger problem: Knowledge gaps exist among doctors, too, stemming from insufficient training on menopause-related issues.
During her 6-year urology residency, she never learned the role of vaginal estrogen on urinary problems, Dr. Winter said. Only during a 1-year fellowship on sexual dysfunction did she hear about the treatment.
“Despite dealing with urinary issues, incontinence, blood in the urine – training to manage all those concerns – the role of local hormones in the vagina for managing all them was never taught, never discussed,” Dr. Winter said. “I never prescribed any of it.”
A year ago, Dr. Winter left her job at Kaiser Permanente to join Odela. After years of prescribing medications for overactive bladder with little to no results, she said, she now uses the knowledge she gained during her fellowship by helping women who have spent years battling debilitating symptoms.
Urologists are not the only clinicians who lack appropriate training. Obstetrics and gynecology residencies offer little knowledge on menopause treatments, said Ghazaleh Moayedi, DO, an ob.gyn. and complex family planning specialist for Texas-based Pegasus Health Justice Center.
The problem is partly a systems-based one, she said. Training programs often direct patients who are uninsured, or covered through public insurance, to medical residents. Patients who qualify for Medicaid or Medicare are often either pregnant or over 65, Dr. Moayedi said, so women actively going through the transition can slip through the cracks.
“What that means in a state like Texas where I’m based, where it is difficult to qualify for Medicaid, is that the people we see who do qualify are pregnant,” she said. “And you’re not on Medicare until you’re 65. So most ob.gyn. residents don’t graduate with expansive experience in menopause.”
According to Medicaid.gov, 80% of the national population covered by Medicaid is age 45 and younger.
When doctors have proper training and prescribe local hormones, patients don’t always follow the treatment plan, said Andrea Rapkin, MD, professor of obstetrics and gynecology at David Geffen School of Medicine at UCLA.
That failure to follow treatment is yet another example of remaining doubts from the misinformation spread through early research, Dr. Rapkin said.
“I’ll prescribe an estrogen product, and I’ll find out they didn’t take it even though I’ll reassure them,” she said. “I do think there are some lingering concerns, but I’m glad to see there is a growing interest in vaginal hormones.”
A version of this article first appeared on WebMD.com.
The result: Countless women grapple with the physical and emotional toll of this life transition.
These shortcomings have led to an influx of doctors moving from traditional practice to virtual startups that focus on women’s health issues, treating patients who come to them desperate and frustrated after years of unresolved issues.
The solution is often so simple it is almost maddening, specialists say: vaginal creams containing low-dose estrogen which can address the symptoms of menopause, from vaginal dryness to recurrent urinary tract infections.
“Hands down, this is one of the most meaningful interventions I’ve ever offered to a patient and yet it is underutilized,” said Ashley Winter, MD, chief medical officer and urologist at Odela Health, a digital women’s health clinic. “A lot of companies are blossoming in this menopause space because it is underserved by traditional health care – your gynecologist typically deals with reproduction, and typically when women are done with child-bearing, they’re kind of discharged from the care of their gynecologist.”
More than 1 million women in the United States go through menopause each year. According to a 2022 survey, 4 in 10 women report menopause symptoms that have been disruptive enough to interfere with their work performance on at least a weekly basis.
And yet, many women are not getting appropriate treatment.
Partially to blame is the harmful legacy of faulty data, doctors say. The early results of the federally funded Women’s Health Initiative, released in 2002, showed that hormone therapy (HT) led to increased risk for heart attacks, strokes, and breast cancer. But further analysis showed the opposite: Hormonal therapies have a helpful effect on cardiovascular and bone health and generally reduce risk of death in younger women or those in the early postmenopausal period.
Hormone therapy delivers estrogen, sometimes with progesterone, to the body through gels, creams, patches, pills, suppositories, or a device fitted inside the uterus. Systemic HT sends hormones into the bloodstream, while local HT – like vaginal estrogen cream – specifically treats vaginal symptoms of menopause.
Myths about the health risks linked to systemic and topical HT have long been debunked, and research on topical HT in particular shows it poses no risk for cancer or other chronic diseases.
Yet while 2 decades have passed since the misinformation first started to spread, people remain woefully uninformed about hormone treatments.
The FDA still requires that estrogen products carry a black-box warning on the early data, even though it has since been proven false.
“This is one of the most damaging PR misadventures of modern medicine in my opinion,” Dr. Winter said. “It has literally killed women, and it’s made them miserable.”
The public has a glaring lack of knowledge about menopause management, said Stephanie Faubion, MD, medical director for the North American Menopause Society and director of Mayo Clinic’s Center for Women’s Health.
Treating with low-dose estrogen isn’t a radical approach – in fact, it is the standard of care for women experiencing many menopause symptoms, Dr. Faubion said. But the topic does have nuance, and some people get lost in the specifics.
“I don’t think there’s a lot of knowledge on the risk-benefits of hormone therapy in general,” Dr. Faubion said. “New information comes out so frequently it’s difficult to keep track of. The answer is complicated and depends on dose, duration of treatment, what formulation you’re on. It’s difficult for a lot of people to understand.”
But Dr. Winter said the lack of public knowledge reflects a bigger problem: Knowledge gaps exist among doctors, too, stemming from insufficient training on menopause-related issues.
During her 6-year urology residency, she never learned the role of vaginal estrogen on urinary problems, Dr. Winter said. Only during a 1-year fellowship on sexual dysfunction did she hear about the treatment.
“Despite dealing with urinary issues, incontinence, blood in the urine – training to manage all those concerns – the role of local hormones in the vagina for managing all them was never taught, never discussed,” Dr. Winter said. “I never prescribed any of it.”
A year ago, Dr. Winter left her job at Kaiser Permanente to join Odela. After years of prescribing medications for overactive bladder with little to no results, she said, she now uses the knowledge she gained during her fellowship by helping women who have spent years battling debilitating symptoms.
Urologists are not the only clinicians who lack appropriate training. Obstetrics and gynecology residencies offer little knowledge on menopause treatments, said Ghazaleh Moayedi, DO, an ob.gyn. and complex family planning specialist for Texas-based Pegasus Health Justice Center.
The problem is partly a systems-based one, she said. Training programs often direct patients who are uninsured, or covered through public insurance, to medical residents. Patients who qualify for Medicaid or Medicare are often either pregnant or over 65, Dr. Moayedi said, so women actively going through the transition can slip through the cracks.
“What that means in a state like Texas where I’m based, where it is difficult to qualify for Medicaid, is that the people we see who do qualify are pregnant,” she said. “And you’re not on Medicare until you’re 65. So most ob.gyn. residents don’t graduate with expansive experience in menopause.”
According to Medicaid.gov, 80% of the national population covered by Medicaid is age 45 and younger.
When doctors have proper training and prescribe local hormones, patients don’t always follow the treatment plan, said Andrea Rapkin, MD, professor of obstetrics and gynecology at David Geffen School of Medicine at UCLA.
That failure to follow treatment is yet another example of remaining doubts from the misinformation spread through early research, Dr. Rapkin said.
“I’ll prescribe an estrogen product, and I’ll find out they didn’t take it even though I’ll reassure them,” she said. “I do think there are some lingering concerns, but I’m glad to see there is a growing interest in vaginal hormones.”
A version of this article first appeared on WebMD.com.
The most important study from ESC: FRAIL-AF
One of the hardest tasks of a clinician is applying evidence from trials to the person in your office. At the annual congress of the European Society of Cardiology, the surprising and unexpected results of the FRAIL-AF trial confirm the massive challenge of evidence translation.
FRAIL-AF investigators set out to study the question of whether frail, elderly patients with atrial fibrillation who were doing well with vitamin K antagonists (VKA) should be switched to direct-acting oral anticoagulants (DOAC).
Senior author Geert-Jan Geersing, MD, PhD, from the University Medical Center Utrecht (the Netherlands), told me that frustration led him to design this study. He was frustrated that colleagues assumed that evidence in nonfrail patients can always be translated to frail patients.
Dr. Geersing offered two reasons why common wisdom may be wrong. First was that the large DOAC versus warfarin trials included few elderly patients with frailty. Second, first author Linda Joosten, MD, made it clear in her presentation that frailty is a lot more than aging. It is a clinical syndrome, which entails a “high burden of comorbidities, dependency on others, and a reduced ability to resist stressors.”
The FRAIL-AF trial
The investigators recruited elderly, frail patients with fibrillation who were treated with VKAs and had stable international normalized ratios from outpatient clinics throughout the Netherlands. They screened about 2,600 patients and enrolled nearly 1,400. Most were excluded for not being frail.
Half the group was randomized to switching to a DOAC – drug choice was left to the treating clinician – and the other half remained on VKAs. Patients were 83 years of age on average with a mean CHA2DS2-VASc score of 4. All four classes of DOAC were used in the switching arm.
The primary endpoint was major or clinically relevant nonmajor bleeding, whichever came first, accounting for death as a competing risk. Follow-up was 1 year.
The results for switching to DOAC vs. VKA
Dr. Joosten started her presentation with this: “The results turned out to be different than we expected.” The authors designed the trial with the idea that switching to DOACs would be superior in safety to remaining on VKAs.
But the trial was halted after an interim analysis found a rate of major bleeding in the switching arm of 15.3% versus 9.4% in the arm staying on VKA (hazard ratio, 1.69; 95% confidence interval, 1.23-2.32; P = .0012).
The Kaplan-Meier event curves reveal that the excess risk of bleeding occurred after 100 days and increased with time. This argued against an early effect from transitioning the drugs.
An analysis looking at specific DOAC drugs revealed similar hazards for the two most common ones used – apixaban and rivaroxaban.
Thrombotic events were a secondary endpoint and were low in absolute numbers, 2.4% versus 2.0%, for remaining on VKA and switching to DOAC, respectively (HR, 1.26; 95% CI, 0.60-2.61).
The time in therapeutic range in FRAIL-AF was similar to that in the seminal DOAC trials.
Comments
Three reasons lead me to choose FRAIL-AF as the most important study from the 2023 ESC congress.
First is the specific lesson about switching drugs. Note that FRAIL-AF did not address the question of starting anticoagulation. The trial results show that if you have a frail older patient who is doing well on VKA, don’t change to a DOAC. That is important to know, but it is not what gives this study its heft.
The second reason centers on the investigators choice to do this trial. Dr. Geersing had a feeling that common wisdom was wrong. He did not try to persuade colleagues with anecdote or plausibility or meta-analyses of observational studies. He set out to answer a question in the correct way – with a randomized trial.
This is the path forward in medicine. I’ve often heard proponents of observational research declare that many topics in medicine cannot be studied with trials. I could hear people arguing that it’s not feasible to study mostly home-bound, elderly frail patients. And the fact that there exist so few trials in this space would support that argument.
But the FRAIL-AF authors showed that it is possible. This is the kind of science that medicine should celebrate. There were no soft endpoints, financial conflicts, or spin. If medical science had science as its incentive, rather than attention, FRAIL-AF easily wins top honors.
The third reason FRAIL-AF is so important is that it teaches us the humility required in translating evidence in our clinics. I like to say evidence is what separates doctors from palm readers. But using this evidence requires thinking hard about how average effects in trial environments apply to our patient.
Yes, of course, there is clear evidence from tens of thousands of patients in the DOAC versus warfarin trials, that, for those patients, on average, DOACs compare favorably with VKA. The average age of patients in these trials was 70-73 years; the average age in FRAIL-AF was 83 years. And that is just age. A substudy of the ENGAGE AF-TIMI 48 trial found that only 360 of more than 20,000 patients in the trial had severe frailty.
That lesson extends to nearly every common therapy in medicine today. It also casts great doubt on the soft-thinking idea of using evidence from trials to derive quality metrics. As if the nuance of evidence translation can be captured in an electronic health record.
The skillful use of evidence will be one of the main challenges of the next generation of clinicians. Thanks to advances in medical science, more patients will live long enough to become frail. And the so-called “guideline-directed” therapies may not apply to them.
Dr. Joosten, Dr. Geersing, and the FRAIL-AF team have taught us specific lessons about anticoagulation, but their greatest contribution has been to demonstrate the value of humility in science and the practice of evidence-based medicine.
If you treat patients, no trial at this meeting is more important.
Dr. Mandrola is a clinical electrophysiologist at Baptist Medical Associates, Louisville, Ky. He reported no relevant conflicts of interest.
A version of this article first appeared on Medscape.com.
One of the hardest tasks of a clinician is applying evidence from trials to the person in your office. At the annual congress of the European Society of Cardiology, the surprising and unexpected results of the FRAIL-AF trial confirm the massive challenge of evidence translation.
FRAIL-AF investigators set out to study the question of whether frail, elderly patients with atrial fibrillation who were doing well with vitamin K antagonists (VKA) should be switched to direct-acting oral anticoagulants (DOAC).
Senior author Geert-Jan Geersing, MD, PhD, from the University Medical Center Utrecht (the Netherlands), told me that frustration led him to design this study. He was frustrated that colleagues assumed that evidence in nonfrail patients can always be translated to frail patients.
Dr. Geersing offered two reasons why common wisdom may be wrong. First was that the large DOAC versus warfarin trials included few elderly patients with frailty. Second, first author Linda Joosten, MD, made it clear in her presentation that frailty is a lot more than aging. It is a clinical syndrome, which entails a “high burden of comorbidities, dependency on others, and a reduced ability to resist stressors.”
The FRAIL-AF trial
The investigators recruited elderly, frail patients with fibrillation who were treated with VKAs and had stable international normalized ratios from outpatient clinics throughout the Netherlands. They screened about 2,600 patients and enrolled nearly 1,400. Most were excluded for not being frail.
Half the group was randomized to switching to a DOAC – drug choice was left to the treating clinician – and the other half remained on VKAs. Patients were 83 years of age on average with a mean CHA2DS2-VASc score of 4. All four classes of DOAC were used in the switching arm.
The primary endpoint was major or clinically relevant nonmajor bleeding, whichever came first, accounting for death as a competing risk. Follow-up was 1 year.
The results for switching to DOAC vs. VKA
Dr. Joosten started her presentation with this: “The results turned out to be different than we expected.” The authors designed the trial with the idea that switching to DOACs would be superior in safety to remaining on VKAs.
But the trial was halted after an interim analysis found a rate of major bleeding in the switching arm of 15.3% versus 9.4% in the arm staying on VKA (hazard ratio, 1.69; 95% confidence interval, 1.23-2.32; P = .0012).
The Kaplan-Meier event curves reveal that the excess risk of bleeding occurred after 100 days and increased with time. This argued against an early effect from transitioning the drugs.
An analysis looking at specific DOAC drugs revealed similar hazards for the two most common ones used – apixaban and rivaroxaban.
Thrombotic events were a secondary endpoint and were low in absolute numbers, 2.4% versus 2.0%, for remaining on VKA and switching to DOAC, respectively (HR, 1.26; 95% CI, 0.60-2.61).
The time in therapeutic range in FRAIL-AF was similar to that in the seminal DOAC trials.
Comments
Three reasons lead me to choose FRAIL-AF as the most important study from the 2023 ESC congress.
First is the specific lesson about switching drugs. Note that FRAIL-AF did not address the question of starting anticoagulation. The trial results show that if you have a frail older patient who is doing well on VKA, don’t change to a DOAC. That is important to know, but it is not what gives this study its heft.
The second reason centers on the investigators choice to do this trial. Dr. Geersing had a feeling that common wisdom was wrong. He did not try to persuade colleagues with anecdote or plausibility or meta-analyses of observational studies. He set out to answer a question in the correct way – with a randomized trial.
This is the path forward in medicine. I’ve often heard proponents of observational research declare that many topics in medicine cannot be studied with trials. I could hear people arguing that it’s not feasible to study mostly home-bound, elderly frail patients. And the fact that there exist so few trials in this space would support that argument.
But the FRAIL-AF authors showed that it is possible. This is the kind of science that medicine should celebrate. There were no soft endpoints, financial conflicts, or spin. If medical science had science as its incentive, rather than attention, FRAIL-AF easily wins top honors.
The third reason FRAIL-AF is so important is that it teaches us the humility required in translating evidence in our clinics. I like to say evidence is what separates doctors from palm readers. But using this evidence requires thinking hard about how average effects in trial environments apply to our patient.
Yes, of course, there is clear evidence from tens of thousands of patients in the DOAC versus warfarin trials, that, for those patients, on average, DOACs compare favorably with VKA. The average age of patients in these trials was 70-73 years; the average age in FRAIL-AF was 83 years. And that is just age. A substudy of the ENGAGE AF-TIMI 48 trial found that only 360 of more than 20,000 patients in the trial had severe frailty.
That lesson extends to nearly every common therapy in medicine today. It also casts great doubt on the soft-thinking idea of using evidence from trials to derive quality metrics. As if the nuance of evidence translation can be captured in an electronic health record.
The skillful use of evidence will be one of the main challenges of the next generation of clinicians. Thanks to advances in medical science, more patients will live long enough to become frail. And the so-called “guideline-directed” therapies may not apply to them.
Dr. Joosten, Dr. Geersing, and the FRAIL-AF team have taught us specific lessons about anticoagulation, but their greatest contribution has been to demonstrate the value of humility in science and the practice of evidence-based medicine.
If you treat patients, no trial at this meeting is more important.
Dr. Mandrola is a clinical electrophysiologist at Baptist Medical Associates, Louisville, Ky. He reported no relevant conflicts of interest.
A version of this article first appeared on Medscape.com.
One of the hardest tasks of a clinician is applying evidence from trials to the person in your office. At the annual congress of the European Society of Cardiology, the surprising and unexpected results of the FRAIL-AF trial confirm the massive challenge of evidence translation.
FRAIL-AF investigators set out to study the question of whether frail, elderly patients with atrial fibrillation who were doing well with vitamin K antagonists (VKA) should be switched to direct-acting oral anticoagulants (DOAC).
Senior author Geert-Jan Geersing, MD, PhD, from the University Medical Center Utrecht (the Netherlands), told me that frustration led him to design this study. He was frustrated that colleagues assumed that evidence in nonfrail patients can always be translated to frail patients.
Dr. Geersing offered two reasons why common wisdom may be wrong. First was that the large DOAC versus warfarin trials included few elderly patients with frailty. Second, first author Linda Joosten, MD, made it clear in her presentation that frailty is a lot more than aging. It is a clinical syndrome, which entails a “high burden of comorbidities, dependency on others, and a reduced ability to resist stressors.”
The FRAIL-AF trial
The investigators recruited elderly, frail patients with fibrillation who were treated with VKAs and had stable international normalized ratios from outpatient clinics throughout the Netherlands. They screened about 2,600 patients and enrolled nearly 1,400. Most were excluded for not being frail.
Half the group was randomized to switching to a DOAC – drug choice was left to the treating clinician – and the other half remained on VKAs. Patients were 83 years of age on average with a mean CHA2DS2-VASc score of 4. All four classes of DOAC were used in the switching arm.
The primary endpoint was major or clinically relevant nonmajor bleeding, whichever came first, accounting for death as a competing risk. Follow-up was 1 year.
The results for switching to DOAC vs. VKA
Dr. Joosten started her presentation with this: “The results turned out to be different than we expected.” The authors designed the trial with the idea that switching to DOACs would be superior in safety to remaining on VKAs.
But the trial was halted after an interim analysis found a rate of major bleeding in the switching arm of 15.3% versus 9.4% in the arm staying on VKA (hazard ratio, 1.69; 95% confidence interval, 1.23-2.32; P = .0012).
The Kaplan-Meier event curves reveal that the excess risk of bleeding occurred after 100 days and increased with time. This argued against an early effect from transitioning the drugs.
An analysis looking at specific DOAC drugs revealed similar hazards for the two most common ones used – apixaban and rivaroxaban.
Thrombotic events were a secondary endpoint and were low in absolute numbers, 2.4% versus 2.0%, for remaining on VKA and switching to DOAC, respectively (HR, 1.26; 95% CI, 0.60-2.61).
The time in therapeutic range in FRAIL-AF was similar to that in the seminal DOAC trials.
Comments
Three reasons lead me to choose FRAIL-AF as the most important study from the 2023 ESC congress.
First is the specific lesson about switching drugs. Note that FRAIL-AF did not address the question of starting anticoagulation. The trial results show that if you have a frail older patient who is doing well on VKA, don’t change to a DOAC. That is important to know, but it is not what gives this study its heft.
The second reason centers on the investigators choice to do this trial. Dr. Geersing had a feeling that common wisdom was wrong. He did not try to persuade colleagues with anecdote or plausibility or meta-analyses of observational studies. He set out to answer a question in the correct way – with a randomized trial.
This is the path forward in medicine. I’ve often heard proponents of observational research declare that many topics in medicine cannot be studied with trials. I could hear people arguing that it’s not feasible to study mostly home-bound, elderly frail patients. And the fact that there exist so few trials in this space would support that argument.
But the FRAIL-AF authors showed that it is possible. This is the kind of science that medicine should celebrate. There were no soft endpoints, financial conflicts, or spin. If medical science had science as its incentive, rather than attention, FRAIL-AF easily wins top honors.
The third reason FRAIL-AF is so important is that it teaches us the humility required in translating evidence in our clinics. I like to say evidence is what separates doctors from palm readers. But using this evidence requires thinking hard about how average effects in trial environments apply to our patient.
Yes, of course, there is clear evidence from tens of thousands of patients in the DOAC versus warfarin trials, that, for those patients, on average, DOACs compare favorably with VKA. The average age of patients in these trials was 70-73 years; the average age in FRAIL-AF was 83 years. And that is just age. A substudy of the ENGAGE AF-TIMI 48 trial found that only 360 of more than 20,000 patients in the trial had severe frailty.
That lesson extends to nearly every common therapy in medicine today. It also casts great doubt on the soft-thinking idea of using evidence from trials to derive quality metrics. As if the nuance of evidence translation can be captured in an electronic health record.
The skillful use of evidence will be one of the main challenges of the next generation of clinicians. Thanks to advances in medical science, more patients will live long enough to become frail. And the so-called “guideline-directed” therapies may not apply to them.
Dr. Joosten, Dr. Geersing, and the FRAIL-AF team have taught us specific lessons about anticoagulation, but their greatest contribution has been to demonstrate the value of humility in science and the practice of evidence-based medicine.
If you treat patients, no trial at this meeting is more important.
Dr. Mandrola is a clinical electrophysiologist at Baptist Medical Associates, Louisville, Ky. He reported no relevant conflicts of interest.
A version of this article first appeared on Medscape.com.