User login
A toxic and fractured political system can breed angst and PTSD
As psychiatrists know, many of our severely traumatized adult patients were victims of abuse during childhood. We routinely ask every new patient about physical, emotional, or sexual abuse when they were growing up because of the well-established, serious neurobiological and mental repercussions.1,2
Perhaps one of the worst experiences for a child is to witness bitterly adversarial parents (their vital role models) who argue viciously, despise each other, and hurl insults (and even punches) at each other. Such a chronically and emotionally traumatic upbringing can haunt kids well into adulthood, disrupting their hypothalamic-pituitary-adrenal axis and triggering anxiety, depression, and even psychosis due to epigenetic changes that ultimately lead to abnormal brain development.3
It often feels that the governance of our country, or the national “political family,” is seriously fractured like a hopelessly dysfunctional family. Could that be negatively impacting the mental health of the citizenry? Having 2 antagonistic political parties expressing visceral hatred and undisguised contempt for each other 24/7 (thanks to the enabling era of cable TV, the internet, and social media) has transformed each party’s fanatic followers from fellow citizens to ideological combatants. In this poisonous societal zeitgeist of bidirectional acrimony and mutual detestation, the opposing parties and their “intellectual militias” label each other as “extremists” or “radicals.” They become completely blind to any redeeming social value in the ideas or principles of their political opponents. They spend enormous time and energy on undermining each other instead of attending to the myriad vital issues involved in the governance of a massive and complex country.
Winston Churchill said, “Democracy is the worst form of government, except for all the others that have been tried.”4 The current toxic cloud of intense “hyperpartisanship” is emblematic of the dark Machiavellian side of democracy. But those who lament the current distorted version of democracy should contemplate living in a dictatorship or totalitarian regime, where a despot would execute any dissenter or invade and destroy an adjacent country at a whim.
Churchill made that statement in 1947. The internet, social media, and smartphones were science fiction back then. Those technological advances have added fuel to the political process and significantly stoked the flames of hyperpartisanship. It’s now democracy on steroids, where freedom of expression goes to extremes, highlighting the warts and pitfalls of the democratic system. Political rivals can now communicate their ferocious disagreements to millions of their disciples instantaneously, triggering immediate rebuttals and counterattacks by their adversaries. This “virtual guerilla warfare” is mentally and emotionally exhausting to all involved, especially to the subset of neutral bystanders who are unaffiliated with either political party, which, due to the “religification” of politics, have become like secular religions.5 Chronic, unremitting, inescapable stress is a sure pathway for anxiety, depression, posttraumatic stress disorder, and even brain atrophy.
Optimists may point out that the United States has weathered and emerged stronger from many serious traumas, including the Civil War (with its lethal divisiveness), World War I, the deadly 1918 influenza pandemic, the Great Depression, Pearl Harbor, World War II, the Cold War, the Vietnam War, the Watergate political scandal, the 9/11 terrorist attacks, the banking collapse and recession, and most recently the COVID-19 pandemic, which brought society to a standstill and induced so much anxiety and uncertainty.
On the other hand, pessimists would insist those sequential crises left indelible scars that cumulatively altered the mindset of political rivals, predisposing them to extreme views of each other. Alienation inevitably leads to fanaticism. It’s perplexing but fascinating how the fierce bidirectional missives of weaponized ideas can be as virulent and destructive as a traditional physical war. Perhaps in this era, the pen is mightier than the sword after all.
Continue to: From a psychiatric perspective...
From a psychiatric perspective, the intransigent groupthink of political partisanship eerily resembles folie en masse, a psychiatric syndrome for which there is no established treatment. It has become a serious threat to our modern democracy. So I decided to ask the “know-it-all” artificial intelligence ChatGPT, whom I previously had “invited” to write a “guest editorial” about myths surrounding psychiatry,6 to answer 3 burning questions:
1. Is there toxic hyperpartisanship in the USA today? (Box 1)
Box 1
Yes, there is toxic hyperpartisanship in the United States today. Political polarization has been on the rise for several decades, and in recent years, it has become increasingly toxic and divisive. This toxic hyperpartisanship has contributed to a breakdown in civil discourse and a lack of cooperation and compromise in government.
The toxic hyperpartisanship is evident in many aspects of American society, including politics, media, and social media. Political leaders often engage in divisive rhetoric and focus on winning at all costs, rather than working towards solutions that benefit the country as a whole. Media outlets often present news in a partisan manner, further fueling political polarization. Social media platforms have also played a role in exacerbating toxic hyperpartisanship by allowing the spread of misinformation and creating echo chambers where people are only exposed to like-minded viewpoints.
The consequences of toxic hyperpartisanship are significant, as it can hinder progress on important issues and erode trust in institutions and government. It also makes it difficult for people to engage in productive dialogue and find common ground, leading to a lack of cooperation and compromise.
It is important for individuals to be aware of the impact of toxic hyperpartisanship and work towards finding ways to bridge the political divide and engage in constructive dialogue.
2. How can severe hyperpartisanship be corrected? (Supplemental Box 1)
Supplemental Box 1
Severe hyperpartisanship, characterized by intense and entrenched divisions between political parties, can be a challenging issue to address, but there are several steps that can be taken to work towards correcting it. Here are some potential approaches:
1. Promote civil discourse: Encourage respectful and constructive conversations between individuals with differing political views. This can be achieved through education programs, media campaigns, and initiatives to foster open dialogue.
2. Electoral reforms: Consider implementing electoral reforms that can reduce the influence of extreme partisanship. For example, ranked-choice voting or nonpartisan primaries may encourage candidates to appeal to a broader base of voters rather than just their party’s base.
3. Campaign finance reform: Limit the influence of money in politics, as it can exacerbate hyperpartisanship by allowing wealthy donors to have undue influence over candidates and parties.
4. Independent redistricting: Ensure that electoral district boundaries are drawn in a fair and nonpartisan manner. Gerrymandering, the manipulation of district lines to favor one party, can contribute to hyperpartisanship by creating “safe” districts where extreme candidates are more likely to be elected.
5. Promote media literacy: Encourage media literacy education to help citizens critically evaluate information sources and avoid echo chambers. A well-informed electorate is less susceptible to extreme partisanship.
6. Encourage bipartisan collaboration: Highlight and support instances of successful bipartisan collaboration. Elected officials who work across the aisle can serve as positive examples and encourage others to follow suit.
7. Address economic inequality: Tackling economic disparities can alleviate some of the underlying factors that contribute to polarization. When people feel economically secure, they may be more willing to seek common ground.
8. Civics education: Improve civics education in schools to foster a deeper understanding of democratic values, the political process, and the importance of civic engagement.
9. Leadership example: Encourage political leaders to model cooperation and compromise rather than relying on divisive rhetoric. Voters often take cues from their leaders’ behavior.
10. Promote third parties: Support and encourage the emergence of viable thirdparty candidates. A more diverse political landscape can provide alternatives to extreme partisanship.
11. Focus on common ground: Identify and prioritize issues where there is potential for bipartisan consensus and progress. Building success on these issues can create momentum for further cooperation.
12. Encourage grassroots activism: Support grassroots movements that seek to bridge divides and foster understanding between individuals and communities with different political perspectives.
It’s essential to recognize that addressing severe hyperpartisanship is a complex and long-term process that requires collective effort from citizens, political leaders, educators, and institutions. It won’t be achieved overnight, but through sustained and committed actions, progress can be made toward a more collaborative and less divisive political climate.
3. What can cause the collapse of a democracy? (Supplemental Box 2).
Supplemental Box 2
The collapse of a democracy can be a complex and multifaceted process, influenced by various factors and circumstances. Some of the common causes that can lead to the erosion or breakdown of a democratic system include:
1. Authoritarianism and power concentration: When leaders or institutions become increasingly authoritarian, centralizing power and weakening checks and balances, it can undermine democratic principles.
2. Corruption and lack of accountability: Rampant corruption among political leaders and institutions can erode public trust in the democratic system, leading to disillusionment and disengagement.
3. Economic instability: Prolonged economic crises or severe inequality can lead to social unrest, increasing the appeal of populist or extremist movements that may challenge democratic norms.
4. Weakening of rule of law: If the rule of law is weakened, and there is a lack of respect for judicial independence and constitutional principles, democratic institutions can become vulnerable to manipulation.
5. Erosion of civil liberties: Suppression of freedom of speech, media, assembly, and association can limit citizens’ ability to express their opinions and participate in the democratic process.
6. Polarization and divisive politics: Extreme political polarization and a breakdown of civil discourse can hinder cooperation and compromise essential for a functioning democracy.
7. External interference: Foreign intervention, propaganda, or cyberattacks can influence electoral processes and undermine democratic institutions.
8. Rise of populism and nationalism: Populist movements that challenge democratic norms, demonize minorities, or advocate for a strongman leadership can pose a threat to democracy.
9. Demographic and social shifts: Demographic changes and social tensions can exacerbate political divisions and weaken the fabric of democratic societies.
10. Lack of civic education and participation: A lack of civic education and low voter turnout can lead to reduced civic engagement and undermine the democratic process.
It is crucial to understand that no democracy is immune to these challenges, and the strength of a democratic system depends on the vigilance and engagement of its citizens, the integrity of its institutions, and the commitment to upholding democratic values. Safeguarding and strengthening democracy requires ongoing efforts to address these issues and protect the principles that underpin democratic governance.
Judge for yourself, but I believe the ChatGPT responses were spot-on.
1. Lippard ETC, Nemeroff CB. The devastating clinical consequences of child abuse and neglect: increased disease vulnerability and poor treatment response in mood disorders. Am J Psychiatry. 2023;180(8):548-564.
2. Nemeroff CB. Paradise lost: the neurobiological and clinical consequences of child abuse and neglect. Neuron. 2016;89(5):892-909.
3. Zhang ZZ, Moeckel C, Mustafa M, et al. The association of epigenetic age acceleration and depressive and anxiety symptom severity among children recently exposed to substantiated maltreatment. J Psychiatr Res. 2023;165:7-13.
4. International Churchill Society. The worst form of government. Accessed August 8, 2023. https://winstonchurchill.org/resources/quotes/the-worst-form-of-government/
5. Nasrallah HA. From ideology to articles of faith: the ‘religification’ of political beliefs. Current Psychiatry. 2021;20(7):4-5,19.
6. Nasrallah HA. A ‘guest editorial’ … generated by ChatGPT? Current Psychiatry. 2023;22(4):22:6-7.
As psychiatrists know, many of our severely traumatized adult patients were victims of abuse during childhood. We routinely ask every new patient about physical, emotional, or sexual abuse when they were growing up because of the well-established, serious neurobiological and mental repercussions.1,2
Perhaps one of the worst experiences for a child is to witness bitterly adversarial parents (their vital role models) who argue viciously, despise each other, and hurl insults (and even punches) at each other. Such a chronically and emotionally traumatic upbringing can haunt kids well into adulthood, disrupting their hypothalamic-pituitary-adrenal axis and triggering anxiety, depression, and even psychosis due to epigenetic changes that ultimately lead to abnormal brain development.3
It often feels that the governance of our country, or the national “political family,” is seriously fractured like a hopelessly dysfunctional family. Could that be negatively impacting the mental health of the citizenry? Having 2 antagonistic political parties expressing visceral hatred and undisguised contempt for each other 24/7 (thanks to the enabling era of cable TV, the internet, and social media) has transformed each party’s fanatic followers from fellow citizens to ideological combatants. In this poisonous societal zeitgeist of bidirectional acrimony and mutual detestation, the opposing parties and their “intellectual militias” label each other as “extremists” or “radicals.” They become completely blind to any redeeming social value in the ideas or principles of their political opponents. They spend enormous time and energy on undermining each other instead of attending to the myriad vital issues involved in the governance of a massive and complex country.
Winston Churchill said, “Democracy is the worst form of government, except for all the others that have been tried.”4 The current toxic cloud of intense “hyperpartisanship” is emblematic of the dark Machiavellian side of democracy. But those who lament the current distorted version of democracy should contemplate living in a dictatorship or totalitarian regime, where a despot would execute any dissenter or invade and destroy an adjacent country at a whim.
Churchill made that statement in 1947. The internet, social media, and smartphones were science fiction back then. Those technological advances have added fuel to the political process and significantly stoked the flames of hyperpartisanship. It’s now democracy on steroids, where freedom of expression goes to extremes, highlighting the warts and pitfalls of the democratic system. Political rivals can now communicate their ferocious disagreements to millions of their disciples instantaneously, triggering immediate rebuttals and counterattacks by their adversaries. This “virtual guerilla warfare” is mentally and emotionally exhausting to all involved, especially to the subset of neutral bystanders who are unaffiliated with either political party, which, due to the “religification” of politics, have become like secular religions.5 Chronic, unremitting, inescapable stress is a sure pathway for anxiety, depression, posttraumatic stress disorder, and even brain atrophy.
Optimists may point out that the United States has weathered and emerged stronger from many serious traumas, including the Civil War (with its lethal divisiveness), World War I, the deadly 1918 influenza pandemic, the Great Depression, Pearl Harbor, World War II, the Cold War, the Vietnam War, the Watergate political scandal, the 9/11 terrorist attacks, the banking collapse and recession, and most recently the COVID-19 pandemic, which brought society to a standstill and induced so much anxiety and uncertainty.
On the other hand, pessimists would insist those sequential crises left indelible scars that cumulatively altered the mindset of political rivals, predisposing them to extreme views of each other. Alienation inevitably leads to fanaticism. It’s perplexing but fascinating how the fierce bidirectional missives of weaponized ideas can be as virulent and destructive as a traditional physical war. Perhaps in this era, the pen is mightier than the sword after all.
Continue to: From a psychiatric perspective...
From a psychiatric perspective, the intransigent groupthink of political partisanship eerily resembles folie en masse, a psychiatric syndrome for which there is no established treatment. It has become a serious threat to our modern democracy. So I decided to ask the “know-it-all” artificial intelligence ChatGPT, whom I previously had “invited” to write a “guest editorial” about myths surrounding psychiatry,6 to answer 3 burning questions:
1. Is there toxic hyperpartisanship in the USA today? (Box 1)
Box 1
Yes, there is toxic hyperpartisanship in the United States today. Political polarization has been on the rise for several decades, and in recent years, it has become increasingly toxic and divisive. This toxic hyperpartisanship has contributed to a breakdown in civil discourse and a lack of cooperation and compromise in government.
The toxic hyperpartisanship is evident in many aspects of American society, including politics, media, and social media. Political leaders often engage in divisive rhetoric and focus on winning at all costs, rather than working towards solutions that benefit the country as a whole. Media outlets often present news in a partisan manner, further fueling political polarization. Social media platforms have also played a role in exacerbating toxic hyperpartisanship by allowing the spread of misinformation and creating echo chambers where people are only exposed to like-minded viewpoints.
The consequences of toxic hyperpartisanship are significant, as it can hinder progress on important issues and erode trust in institutions and government. It also makes it difficult for people to engage in productive dialogue and find common ground, leading to a lack of cooperation and compromise.
It is important for individuals to be aware of the impact of toxic hyperpartisanship and work towards finding ways to bridge the political divide and engage in constructive dialogue.
2. How can severe hyperpartisanship be corrected? (Supplemental Box 1)
Supplemental Box 1
Severe hyperpartisanship, characterized by intense and entrenched divisions between political parties, can be a challenging issue to address, but there are several steps that can be taken to work towards correcting it. Here are some potential approaches:
1. Promote civil discourse: Encourage respectful and constructive conversations between individuals with differing political views. This can be achieved through education programs, media campaigns, and initiatives to foster open dialogue.
2. Electoral reforms: Consider implementing electoral reforms that can reduce the influence of extreme partisanship. For example, ranked-choice voting or nonpartisan primaries may encourage candidates to appeal to a broader base of voters rather than just their party’s base.
3. Campaign finance reform: Limit the influence of money in politics, as it can exacerbate hyperpartisanship by allowing wealthy donors to have undue influence over candidates and parties.
4. Independent redistricting: Ensure that electoral district boundaries are drawn in a fair and nonpartisan manner. Gerrymandering, the manipulation of district lines to favor one party, can contribute to hyperpartisanship by creating “safe” districts where extreme candidates are more likely to be elected.
5. Promote media literacy: Encourage media literacy education to help citizens critically evaluate information sources and avoid echo chambers. A well-informed electorate is less susceptible to extreme partisanship.
6. Encourage bipartisan collaboration: Highlight and support instances of successful bipartisan collaboration. Elected officials who work across the aisle can serve as positive examples and encourage others to follow suit.
7. Address economic inequality: Tackling economic disparities can alleviate some of the underlying factors that contribute to polarization. When people feel economically secure, they may be more willing to seek common ground.
8. Civics education: Improve civics education in schools to foster a deeper understanding of democratic values, the political process, and the importance of civic engagement.
9. Leadership example: Encourage political leaders to model cooperation and compromise rather than relying on divisive rhetoric. Voters often take cues from their leaders’ behavior.
10. Promote third parties: Support and encourage the emergence of viable thirdparty candidates. A more diverse political landscape can provide alternatives to extreme partisanship.
11. Focus on common ground: Identify and prioritize issues where there is potential for bipartisan consensus and progress. Building success on these issues can create momentum for further cooperation.
12. Encourage grassroots activism: Support grassroots movements that seek to bridge divides and foster understanding between individuals and communities with different political perspectives.
It’s essential to recognize that addressing severe hyperpartisanship is a complex and long-term process that requires collective effort from citizens, political leaders, educators, and institutions. It won’t be achieved overnight, but through sustained and committed actions, progress can be made toward a more collaborative and less divisive political climate.
3. What can cause the collapse of a democracy? (Supplemental Box 2).
Supplemental Box 2
The collapse of a democracy can be a complex and multifaceted process, influenced by various factors and circumstances. Some of the common causes that can lead to the erosion or breakdown of a democratic system include:
1. Authoritarianism and power concentration: When leaders or institutions become increasingly authoritarian, centralizing power and weakening checks and balances, it can undermine democratic principles.
2. Corruption and lack of accountability: Rampant corruption among political leaders and institutions can erode public trust in the democratic system, leading to disillusionment and disengagement.
3. Economic instability: Prolonged economic crises or severe inequality can lead to social unrest, increasing the appeal of populist or extremist movements that may challenge democratic norms.
4. Weakening of rule of law: If the rule of law is weakened, and there is a lack of respect for judicial independence and constitutional principles, democratic institutions can become vulnerable to manipulation.
5. Erosion of civil liberties: Suppression of freedom of speech, media, assembly, and association can limit citizens’ ability to express their opinions and participate in the democratic process.
6. Polarization and divisive politics: Extreme political polarization and a breakdown of civil discourse can hinder cooperation and compromise essential for a functioning democracy.
7. External interference: Foreign intervention, propaganda, or cyberattacks can influence electoral processes and undermine democratic institutions.
8. Rise of populism and nationalism: Populist movements that challenge democratic norms, demonize minorities, or advocate for a strongman leadership can pose a threat to democracy.
9. Demographic and social shifts: Demographic changes and social tensions can exacerbate political divisions and weaken the fabric of democratic societies.
10. Lack of civic education and participation: A lack of civic education and low voter turnout can lead to reduced civic engagement and undermine the democratic process.
It is crucial to understand that no democracy is immune to these challenges, and the strength of a democratic system depends on the vigilance and engagement of its citizens, the integrity of its institutions, and the commitment to upholding democratic values. Safeguarding and strengthening democracy requires ongoing efforts to address these issues and protect the principles that underpin democratic governance.
Judge for yourself, but I believe the ChatGPT responses were spot-on.
As psychiatrists know, many of our severely traumatized adult patients were victims of abuse during childhood. We routinely ask every new patient about physical, emotional, or sexual abuse when they were growing up because of the well-established, serious neurobiological and mental repercussions.1,2
Perhaps one of the worst experiences for a child is to witness bitterly adversarial parents (their vital role models) who argue viciously, despise each other, and hurl insults (and even punches) at each other. Such a chronically and emotionally traumatic upbringing can haunt kids well into adulthood, disrupting their hypothalamic-pituitary-adrenal axis and triggering anxiety, depression, and even psychosis due to epigenetic changes that ultimately lead to abnormal brain development.3
It often feels that the governance of our country, or the national “political family,” is seriously fractured like a hopelessly dysfunctional family. Could that be negatively impacting the mental health of the citizenry? Having 2 antagonistic political parties expressing visceral hatred and undisguised contempt for each other 24/7 (thanks to the enabling era of cable TV, the internet, and social media) has transformed each party’s fanatic followers from fellow citizens to ideological combatants. In this poisonous societal zeitgeist of bidirectional acrimony and mutual detestation, the opposing parties and their “intellectual militias” label each other as “extremists” or “radicals.” They become completely blind to any redeeming social value in the ideas or principles of their political opponents. They spend enormous time and energy on undermining each other instead of attending to the myriad vital issues involved in the governance of a massive and complex country.
Winston Churchill said, “Democracy is the worst form of government, except for all the others that have been tried.”4 The current toxic cloud of intense “hyperpartisanship” is emblematic of the dark Machiavellian side of democracy. But those who lament the current distorted version of democracy should contemplate living in a dictatorship or totalitarian regime, where a despot would execute any dissenter or invade and destroy an adjacent country at a whim.
Churchill made that statement in 1947. The internet, social media, and smartphones were science fiction back then. Those technological advances have added fuel to the political process and significantly stoked the flames of hyperpartisanship. It’s now democracy on steroids, where freedom of expression goes to extremes, highlighting the warts and pitfalls of the democratic system. Political rivals can now communicate their ferocious disagreements to millions of their disciples instantaneously, triggering immediate rebuttals and counterattacks by their adversaries. This “virtual guerilla warfare” is mentally and emotionally exhausting to all involved, especially to the subset of neutral bystanders who are unaffiliated with either political party, which, due to the “religification” of politics, have become like secular religions.5 Chronic, unremitting, inescapable stress is a sure pathway for anxiety, depression, posttraumatic stress disorder, and even brain atrophy.
Optimists may point out that the United States has weathered and emerged stronger from many serious traumas, including the Civil War (with its lethal divisiveness), World War I, the deadly 1918 influenza pandemic, the Great Depression, Pearl Harbor, World War II, the Cold War, the Vietnam War, the Watergate political scandal, the 9/11 terrorist attacks, the banking collapse and recession, and most recently the COVID-19 pandemic, which brought society to a standstill and induced so much anxiety and uncertainty.
On the other hand, pessimists would insist those sequential crises left indelible scars that cumulatively altered the mindset of political rivals, predisposing them to extreme views of each other. Alienation inevitably leads to fanaticism. It’s perplexing but fascinating how the fierce bidirectional missives of weaponized ideas can be as virulent and destructive as a traditional physical war. Perhaps in this era, the pen is mightier than the sword after all.
Continue to: From a psychiatric perspective...
From a psychiatric perspective, the intransigent groupthink of political partisanship eerily resembles folie en masse, a psychiatric syndrome for which there is no established treatment. It has become a serious threat to our modern democracy. So I decided to ask the “know-it-all” artificial intelligence ChatGPT, whom I previously had “invited” to write a “guest editorial” about myths surrounding psychiatry,6 to answer 3 burning questions:
1. Is there toxic hyperpartisanship in the USA today? (Box 1)
Box 1
Yes, there is toxic hyperpartisanship in the United States today. Political polarization has been on the rise for several decades, and in recent years, it has become increasingly toxic and divisive. This toxic hyperpartisanship has contributed to a breakdown in civil discourse and a lack of cooperation and compromise in government.
The toxic hyperpartisanship is evident in many aspects of American society, including politics, media, and social media. Political leaders often engage in divisive rhetoric and focus on winning at all costs, rather than working towards solutions that benefit the country as a whole. Media outlets often present news in a partisan manner, further fueling political polarization. Social media platforms have also played a role in exacerbating toxic hyperpartisanship by allowing the spread of misinformation and creating echo chambers where people are only exposed to like-minded viewpoints.
The consequences of toxic hyperpartisanship are significant, as it can hinder progress on important issues and erode trust in institutions and government. It also makes it difficult for people to engage in productive dialogue and find common ground, leading to a lack of cooperation and compromise.
It is important for individuals to be aware of the impact of toxic hyperpartisanship and work towards finding ways to bridge the political divide and engage in constructive dialogue.
2. How can severe hyperpartisanship be corrected? (Supplemental Box 1)
Supplemental Box 1
Severe hyperpartisanship, characterized by intense and entrenched divisions between political parties, can be a challenging issue to address, but there are several steps that can be taken to work towards correcting it. Here are some potential approaches:
1. Promote civil discourse: Encourage respectful and constructive conversations between individuals with differing political views. This can be achieved through education programs, media campaigns, and initiatives to foster open dialogue.
2. Electoral reforms: Consider implementing electoral reforms that can reduce the influence of extreme partisanship. For example, ranked-choice voting or nonpartisan primaries may encourage candidates to appeal to a broader base of voters rather than just their party’s base.
3. Campaign finance reform: Limit the influence of money in politics, as it can exacerbate hyperpartisanship by allowing wealthy donors to have undue influence over candidates and parties.
4. Independent redistricting: Ensure that electoral district boundaries are drawn in a fair and nonpartisan manner. Gerrymandering, the manipulation of district lines to favor one party, can contribute to hyperpartisanship by creating “safe” districts where extreme candidates are more likely to be elected.
5. Promote media literacy: Encourage media literacy education to help citizens critically evaluate information sources and avoid echo chambers. A well-informed electorate is less susceptible to extreme partisanship.
6. Encourage bipartisan collaboration: Highlight and support instances of successful bipartisan collaboration. Elected officials who work across the aisle can serve as positive examples and encourage others to follow suit.
7. Address economic inequality: Tackling economic disparities can alleviate some of the underlying factors that contribute to polarization. When people feel economically secure, they may be more willing to seek common ground.
8. Civics education: Improve civics education in schools to foster a deeper understanding of democratic values, the political process, and the importance of civic engagement.
9. Leadership example: Encourage political leaders to model cooperation and compromise rather than relying on divisive rhetoric. Voters often take cues from their leaders’ behavior.
10. Promote third parties: Support and encourage the emergence of viable thirdparty candidates. A more diverse political landscape can provide alternatives to extreme partisanship.
11. Focus on common ground: Identify and prioritize issues where there is potential for bipartisan consensus and progress. Building success on these issues can create momentum for further cooperation.
12. Encourage grassroots activism: Support grassroots movements that seek to bridge divides and foster understanding between individuals and communities with different political perspectives.
It’s essential to recognize that addressing severe hyperpartisanship is a complex and long-term process that requires collective effort from citizens, political leaders, educators, and institutions. It won’t be achieved overnight, but through sustained and committed actions, progress can be made toward a more collaborative and less divisive political climate.
3. What can cause the collapse of a democracy? (Supplemental Box 2).
Supplemental Box 2
The collapse of a democracy can be a complex and multifaceted process, influenced by various factors and circumstances. Some of the common causes that can lead to the erosion or breakdown of a democratic system include:
1. Authoritarianism and power concentration: When leaders or institutions become increasingly authoritarian, centralizing power and weakening checks and balances, it can undermine democratic principles.
2. Corruption and lack of accountability: Rampant corruption among political leaders and institutions can erode public trust in the democratic system, leading to disillusionment and disengagement.
3. Economic instability: Prolonged economic crises or severe inequality can lead to social unrest, increasing the appeal of populist or extremist movements that may challenge democratic norms.
4. Weakening of rule of law: If the rule of law is weakened, and there is a lack of respect for judicial independence and constitutional principles, democratic institutions can become vulnerable to manipulation.
5. Erosion of civil liberties: Suppression of freedom of speech, media, assembly, and association can limit citizens’ ability to express their opinions and participate in the democratic process.
6. Polarization and divisive politics: Extreme political polarization and a breakdown of civil discourse can hinder cooperation and compromise essential for a functioning democracy.
7. External interference: Foreign intervention, propaganda, or cyberattacks can influence electoral processes and undermine democratic institutions.
8. Rise of populism and nationalism: Populist movements that challenge democratic norms, demonize minorities, or advocate for a strongman leadership can pose a threat to democracy.
9. Demographic and social shifts: Demographic changes and social tensions can exacerbate political divisions and weaken the fabric of democratic societies.
10. Lack of civic education and participation: A lack of civic education and low voter turnout can lead to reduced civic engagement and undermine the democratic process.
It is crucial to understand that no democracy is immune to these challenges, and the strength of a democratic system depends on the vigilance and engagement of its citizens, the integrity of its institutions, and the commitment to upholding democratic values. Safeguarding and strengthening democracy requires ongoing efforts to address these issues and protect the principles that underpin democratic governance.
Judge for yourself, but I believe the ChatGPT responses were spot-on.
1. Lippard ETC, Nemeroff CB. The devastating clinical consequences of child abuse and neglect: increased disease vulnerability and poor treatment response in mood disorders. Am J Psychiatry. 2023;180(8):548-564.
2. Nemeroff CB. Paradise lost: the neurobiological and clinical consequences of child abuse and neglect. Neuron. 2016;89(5):892-909.
3. Zhang ZZ, Moeckel C, Mustafa M, et al. The association of epigenetic age acceleration and depressive and anxiety symptom severity among children recently exposed to substantiated maltreatment. J Psychiatr Res. 2023;165:7-13.
4. International Churchill Society. The worst form of government. Accessed August 8, 2023. https://winstonchurchill.org/resources/quotes/the-worst-form-of-government/
5. Nasrallah HA. From ideology to articles of faith: the ‘religification’ of political beliefs. Current Psychiatry. 2021;20(7):4-5,19.
6. Nasrallah HA. A ‘guest editorial’ … generated by ChatGPT? Current Psychiatry. 2023;22(4):22:6-7.
1. Lippard ETC, Nemeroff CB. The devastating clinical consequences of child abuse and neglect: increased disease vulnerability and poor treatment response in mood disorders. Am J Psychiatry. 2023;180(8):548-564.
2. Nemeroff CB. Paradise lost: the neurobiological and clinical consequences of child abuse and neglect. Neuron. 2016;89(5):892-909.
3. Zhang ZZ, Moeckel C, Mustafa M, et al. The association of epigenetic age acceleration and depressive and anxiety symptom severity among children recently exposed to substantiated maltreatment. J Psychiatr Res. 2023;165:7-13.
4. International Churchill Society. The worst form of government. Accessed August 8, 2023. https://winstonchurchill.org/resources/quotes/the-worst-form-of-government/
5. Nasrallah HA. From ideology to articles of faith: the ‘religification’ of political beliefs. Current Psychiatry. 2021;20(7):4-5,19.
6. Nasrallah HA. A ‘guest editorial’ … generated by ChatGPT? Current Psychiatry. 2023;22(4):22:6-7.
Abnormal sexual behaviors in frontotemporal dementia
Mr. S, age 77, is admitted to a long-term care facility due to progressive cognitive impairment and sexually inappropriate behavior. He has a history of sexual assault of medical staff. His medical history includes significant frontotemporal dementia (FTD) with behavioral disturbances, abnormal sexual behaviors, subclinical hypothyroidism, schizoid personality disorder, Parkinson disease, posttraumatic stress disorder, and hyperammonemia.
Upon admission, Mr. S’s vital signs are within normal limits except for an elevated thyroid-stimulating hormone (4.54 mIU/L; reference range 0.40 to 4.50 mIU/L). Prior cognitive testing results and updated ammonia levels are unavailable. Mr. S’s current medications include acetaminophen 650 mg every 4 hours as needed for pain, calcium carbonate/vitamin D twice daily for bone health, carbidopa/levodopa 25/100 mg twice daily for Parkinson disease, melatonin 3 mg/d at bedtime for insomnia, quetiapine 25 mg twice daily for psychosis with disturbance of behavior and 12.5 mg every 4 hours as needed for agitation, and trazodone 50 mg/d at bedtime for insomnia. Before Mr. S was admitted, previous therapy with selective serotonin reuptake inhibitors (SSRIs) had been tapered and discontinued. Mr. S had also started antipsychotic therapy at another facility due to worsening behaviors.
In patients with dementia, the brain is experiencing neurodegeneration. Progressively, neurons may stop functioning, lose connections with other neurons, and ultimately face cell death. The specific dementia diagnosis and its clinical features depend on the type of neurons and region of the brain affected.1,2
FTD occurs in response to damage to the frontal and temporal lobes. The frontal lobe correlates to executive functioning, while the temporal lobe plays a role in speech and comprehension. Damage to these areas may result in loss of movement, trouble speaking, difficulty solving complex problems, and problems with social behavior. Specifically, damage to the orbital frontal cortex may cause disinhibition and abnormal behaviors, including emotional lability, vulgarity, and indifference to social nuances.1 Within an FTD diagnosis, there are 3 disorders: behavioral-variant FTD (bvFTD), semantic dementia, and progressive nonfluent aphasia.1 Specifically, bvFTD can result in abnormal sexual behaviors such as making sexually inappropriate statements, masturbating in public, undressing in public, inappropriately or aggressively touching others, or confusing another individual as an intimate partner. In addition to cognitive impairment, these neurobehavioral symptoms can significantly impact an individual’s quality of life while increasing caregiver burden.2
Occurring at a similar frequency to Alzheimer’s disease in patients age <65, FTD is one of the more common causes of early-onset dementia. The mean age of onset is 58 and onset after age 75 is particularly unusual. Memory may not be affected early in the course of the disease, but social changes are likely. As FTD progresses, symptoms will resemble those of Alzheimer’s disease and patients will require assistance with activities of daily living. In later stages of FTD, patients will exhibit language and behavior symptoms. Due to its unique progression, FTD can be commonly misdiagnosed as other mental illnesses or neurocognitive disorders.1
Approaches to treatment: What to consider
Both nonpharmacologic and pharmacologic interventions are appropriate for addressing FTD. Because nonpharmacologic options improve patient safety and overall physical health, they should be used whenever practical. These interventions include safe driving measures, exercise, speech therapy, redirection, offering simple choices when making decisions, and managing environmental cues for behaviors that should be encouraged or discouraged.3
There are no FDA-approved medications to cure or slow the progression of FTD. Therefore, treatment is focused on alleviating neurobehavioral symptoms. The symptoms depend on the type of FTD the patient has; they include cognitive impairment, anxiety, insomnia or sleep disturbances, compulsive behaviors, speech and language problems, and agitation. While many medications have been commonly used for symptomatic relief, evidence for the efficacy of these treatments in FTD is limited.2
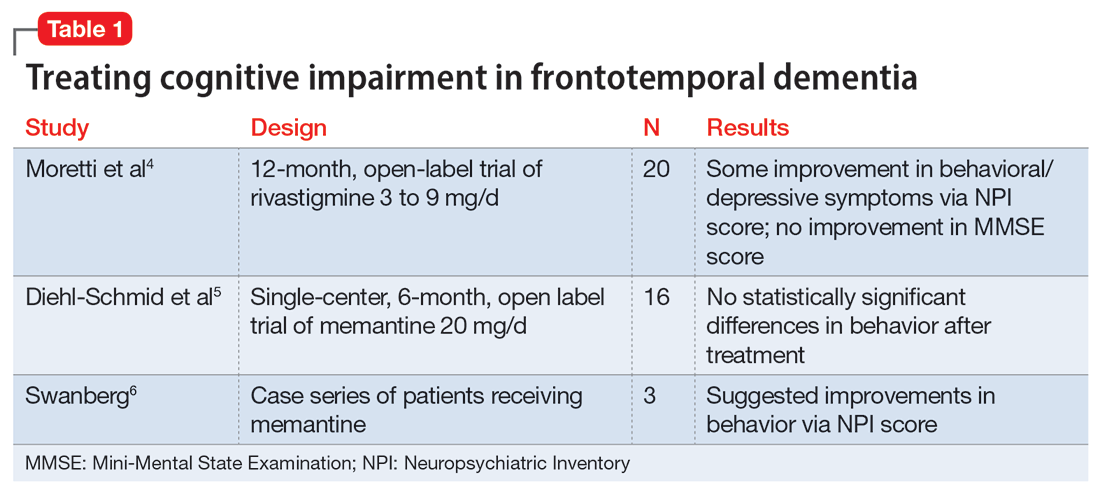
Continue to: A review of the literature...
A review of the literature on potential treatments for cognitive impairment and behavioral symptoms of FTD identified 2 trials and 1 case series (Table 14-6) in addition to a 2014 review article7 of current pharmacologic treatments. These trials evaluated cognitive improvement with rivastigmine, memantine, galantamine, and donepezil. None of the trials found a significant benefit from any of these medications for cognitive improvement in FTD. Data were conflicting on whether these medications improved or worsened behavioral symptoms. For example, the case series of 3 patients by Swanberg6 suggested improvement in behavior with memantine, while an open-label study analyzed in a 2014 review article7 found that donepezil may have worsened behaviors. Use of cholinesterase inhibitors or memantine in FTD is not recommended unless it is not certain if the patient has FTD or Alzheimer’s disease.7
Addressing sexual behaviors. Creating a treatment regimen for FTD behavioral symptoms—specifically for abnormal sexual behaviors—can be challenging. Before starting pharmacotherapy directed at behavioral symptoms secondary to FTD, other causes of symptoms such as delirium, pain, or discomfort should be excluded. Nonpharmacologic approaches should be aimed at the type of sexual behavior and likely underlying environmental cause. For example, patients may inappropriately disrobe themselves. To address this behavior, hospital staff or caregivers should first eliminate environmental causes by ensuring the room is at a comfortable temperature, dressing the patient in light, breathable clothing, or checking if the patient needs to use the bathroom. If no environmental causes are found, a one-piece jumpsuit with closures on the back of the garment could be utilized to increase the difficulty of undressing.
Other nonpharmacologic methods include providing private areas for patients who are behaving inappropriately or removing potentially stimulating television or media from the environment. Another option is to increase the use of positive, pleasant stimuli. One approach that has shown benefit is music therapy, utilizing popular music genres from the patient’s youth.3
Evidence for pharmacotherapy is limited and largely from case reports and case series. A 2020 meta-analysis by Trieu et al8 reviewed 23 studies to expand on current clinical guidance for patients with bvFTD. These studies showed improvements in behavioral symptoms and reductions in caregiver fatigue with citalopram, trazodone, paroxetine, and fluvoxamine. Six of the trials included in this meta-analysis that evaluated these 4 medications are summarized in Table 2.9-14
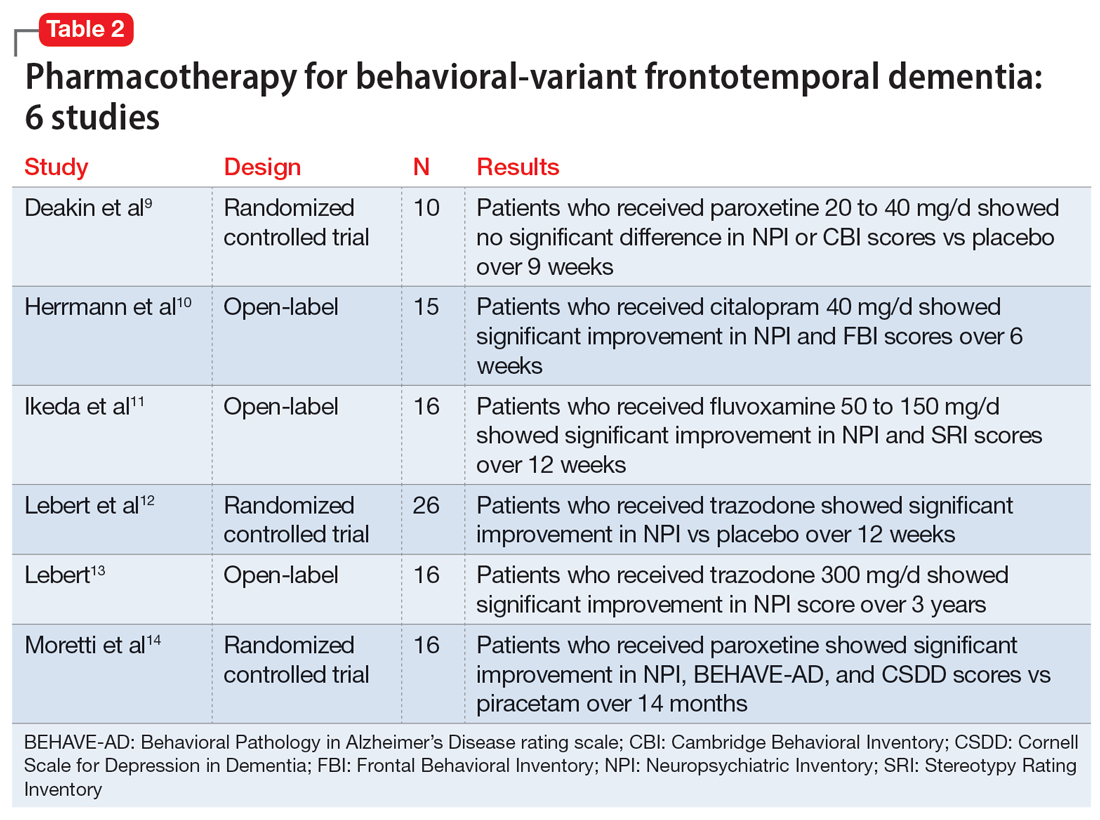
Due to the lower risk of adverse effects and favorable safety profiles, SSRIs and trazodone are considered first-line treatment options. Benefit from these medications is theorized to be a result of their serotonergic effects, because serotonin abnormalities and dysfunction have been linked to FTD symptoms. For example, in a patient experiencing hypersexuality, the common adverse effect of low libido associated with SSRIs can be particularly beneficial.8
Continue to: Other medication classes studied in patients...
Other medication classes studied in patients with FTD include antipsychotics, stimulants, anticonvulsants, benzodiazepines, and hormonal therapies. In addition to a black box warning for increased mortality in older patients with dementia-related psychosis, antipsychotics are associated with other serious adverse effects and should be used with caution.7
FTD is a debilitating disease that has a major impact on quality of life, particularly when behavioral symptoms accompany cognitive decline. Though some therapies may possibly improve behavioral symptoms, their routine use remains controversial due to a lack of clear evidence of benefit. In caring for patients with FTD and behavioral symptoms, a multimodal, team-based approach is vital.1
CASE CONTINUED
The treatment team starts Mr. S on several of the modalities discussed in this article over the span of 2 years, with limited efficacy. Nonpharmacologic methods do not provide much benefit because Mr. S is extremely difficult to redirect. Given Mr. S’s past trials of SSRIs prior to admission, sertraline was retrialed and titrated over 2 years. The highest dose utilized during his admission was 200 mg/d. The team starts estrogen therapy but tapers and discontinues it due to ineffectiveness. Mr. S’s use of carbidopa/levodopa is thought to be contributing to his behavioral abnormalities, so the team tapers it to discontinuation; however, Mr. S’s sexually inappropriate behaviors and agitation continue. The team initiates a plan to reduce the dose of quetiapine and switch to gabapentin, but Mr. S fails gradual dose reduction due to his worsening behaviors. He starts gabapentin. The team gradually increases the dose of gabapentin to decrease libido and agitation, respectively. The increase in sertraline dose and use of nonpharmacologic modalities causes Mr. S’s use of as-needed antipsychotics to decrease.
Related Resources
- Ellison JM. What are the stages of frontotemporal dementia? BrightFocus Foundation. July 5, 2021. Accessed July 7, 2023. https://www.brightfocus.org/alzheimers/article/what-are-stages-frontotemporal-dementia
- Dementia and sexually inappropriate behavior. ReaDementia. January 31, 2022. Accessed July 7, 2023. https://readementia.com/dementia-and-sexually-inappropriate-behavior/
Drug Brand Names
Carbidopa/levodopa • Sinemet
Citalopram • Celexa
Donepezil • Aricept
Fluvoxamine • Luvox
Gabapentin • Neurontin
Galantamine • Razadyne
Memantine • Namenda
Paroxetine • Paxil
Quetiapine • Seroquel
Rivastigmine • Exelon
Sertraline • Zoloft
Trazodone • Desyrel
1. Grossman M. Frontotemporal dementia: a review. J Int Neuropsychol Soc. 2002;8(4):566-583. doi:10.1017/s1355617702814357
2. The Johns Hopkins University. Frontotemporal dementia. Johns Hopkins Medicine. Accessed September 12, 2021. https://www.hopkinsmedicine.org/health/conditions-and-diseases/dementia/frontotemporal-dementia
3. Shinagawa S, Nakajima S, Plitman E, et al. Non-pharmacological management for patients with frontotemporal dementia: a systematic review. J Alzheimers Dis. 2015;45(1):283-293. doi:10.3233/JAD-142109
4. Moretti R, Torre P, Antonello RM, et al. Rivastigmine in frontotemporal dementia: an open-label study. Drugs Aging. 2004;21(14):931-937. doi:10.2165/00002512-200421140-00003
5. Diehl-Schmid J, Förstl H, Perneczky R, et al. A 6-month, open-label study for memantine in patients with frontotemporal dementia. In J Geriatr Psychiatry. 2008;23(7):754-759. doi:10.1002/gps.1973
6. Swanberg MM. Memantine for behavioral disturbances in frontotemporal dementia: a case series. Alzheimer Dis Assoc Disord. 2007;21(2):164-166. doi:10.1097/WAD.0b013e318047df5d
7. Tsai RM, Boxer AL. Treatment of frontotemporal dementia. Curr Treat Options Neurol. 2014;16(11):319. doi:10.1007/s11940-014-0319-0
8. Trieu C, Gossink F, Stek ML, et al. Effectiveness of pharmacological interventions for symptoms of behavioral variant frontotemporal dementia: a systematic review. Cogn Behav Neurol. 2020;33(1):1-15. doi:10.1097/WNN.0000000000000217
9. Deakin JB, Rahman S, Nestor PJ, et al. Paroxetine does not improve symptoms and impairs cognition in frontotemporal dementia: a double-blind randomized controlled trial. Psychopharmacology (Berl). 2004;172(4):400-408. doi:10.1007/s00213-003-1686-5
10. Herrmann N, Black SE, Chow T, et al. Serotonergic function and treatment of behavioral and psychological symptoms of frontotemporal dementia. Am J Geriatr Psychiatry. 2012;20(9):789-797. doi:10.1097/JGP.0b013e31823033f3
11. Ikeda M, Shigenobu K, Fukuhara R, et al. Efficacy of fluvoxamine as a treatment for behavioral symptoms in frontotemporal lobar degeneration patients. Dement Geriatr Cogn Disord. 2004;17(3):117-121. doi:10.1159/000076343
12. Lebert F, Stekke W, Hasenbroekx C, et al. Frontotemporal dementia: a randomised, controlled trial with trazodone. Dement Geriatr Cogn Disord. 2004;17(4):355-359. doi:10.1159/000077171
13. Lebert F. Behavioral benefits of trazodone are sustained for the long term in frontotemporal dementia. Therapy. 2006;3(1):93-96. doi:10.1586/14750708.3.1.93
14. Moretti R, Torre P, Antonello RM, et al. Frontotemporal dementia: paroxetine as a possible treatment of behavior symptoms. A randomized, controlled, open 14-month study. Eur Neurol. 2003;49(1):13-19. doi:10.1159/000067021
Mr. S, age 77, is admitted to a long-term care facility due to progressive cognitive impairment and sexually inappropriate behavior. He has a history of sexual assault of medical staff. His medical history includes significant frontotemporal dementia (FTD) with behavioral disturbances, abnormal sexual behaviors, subclinical hypothyroidism, schizoid personality disorder, Parkinson disease, posttraumatic stress disorder, and hyperammonemia.
Upon admission, Mr. S’s vital signs are within normal limits except for an elevated thyroid-stimulating hormone (4.54 mIU/L; reference range 0.40 to 4.50 mIU/L). Prior cognitive testing results and updated ammonia levels are unavailable. Mr. S’s current medications include acetaminophen 650 mg every 4 hours as needed for pain, calcium carbonate/vitamin D twice daily for bone health, carbidopa/levodopa 25/100 mg twice daily for Parkinson disease, melatonin 3 mg/d at bedtime for insomnia, quetiapine 25 mg twice daily for psychosis with disturbance of behavior and 12.5 mg every 4 hours as needed for agitation, and trazodone 50 mg/d at bedtime for insomnia. Before Mr. S was admitted, previous therapy with selective serotonin reuptake inhibitors (SSRIs) had been tapered and discontinued. Mr. S had also started antipsychotic therapy at another facility due to worsening behaviors.
In patients with dementia, the brain is experiencing neurodegeneration. Progressively, neurons may stop functioning, lose connections with other neurons, and ultimately face cell death. The specific dementia diagnosis and its clinical features depend on the type of neurons and region of the brain affected.1,2
FTD occurs in response to damage to the frontal and temporal lobes. The frontal lobe correlates to executive functioning, while the temporal lobe plays a role in speech and comprehension. Damage to these areas may result in loss of movement, trouble speaking, difficulty solving complex problems, and problems with social behavior. Specifically, damage to the orbital frontal cortex may cause disinhibition and abnormal behaviors, including emotional lability, vulgarity, and indifference to social nuances.1 Within an FTD diagnosis, there are 3 disorders: behavioral-variant FTD (bvFTD), semantic dementia, and progressive nonfluent aphasia.1 Specifically, bvFTD can result in abnormal sexual behaviors such as making sexually inappropriate statements, masturbating in public, undressing in public, inappropriately or aggressively touching others, or confusing another individual as an intimate partner. In addition to cognitive impairment, these neurobehavioral symptoms can significantly impact an individual’s quality of life while increasing caregiver burden.2
Occurring at a similar frequency to Alzheimer’s disease in patients age <65, FTD is one of the more common causes of early-onset dementia. The mean age of onset is 58 and onset after age 75 is particularly unusual. Memory may not be affected early in the course of the disease, but social changes are likely. As FTD progresses, symptoms will resemble those of Alzheimer’s disease and patients will require assistance with activities of daily living. In later stages of FTD, patients will exhibit language and behavior symptoms. Due to its unique progression, FTD can be commonly misdiagnosed as other mental illnesses or neurocognitive disorders.1
Approaches to treatment: What to consider
Both nonpharmacologic and pharmacologic interventions are appropriate for addressing FTD. Because nonpharmacologic options improve patient safety and overall physical health, they should be used whenever practical. These interventions include safe driving measures, exercise, speech therapy, redirection, offering simple choices when making decisions, and managing environmental cues for behaviors that should be encouraged or discouraged.3
There are no FDA-approved medications to cure or slow the progression of FTD. Therefore, treatment is focused on alleviating neurobehavioral symptoms. The symptoms depend on the type of FTD the patient has; they include cognitive impairment, anxiety, insomnia or sleep disturbances, compulsive behaviors, speech and language problems, and agitation. While many medications have been commonly used for symptomatic relief, evidence for the efficacy of these treatments in FTD is limited.2
Continue to: A review of the literature...
A review of the literature on potential treatments for cognitive impairment and behavioral symptoms of FTD identified 2 trials and 1 case series (Table 14-6) in addition to a 2014 review article7 of current pharmacologic treatments. These trials evaluated cognitive improvement with rivastigmine, memantine, galantamine, and donepezil. None of the trials found a significant benefit from any of these medications for cognitive improvement in FTD. Data were conflicting on whether these medications improved or worsened behavioral symptoms. For example, the case series of 3 patients by Swanberg6 suggested improvement in behavior with memantine, while an open-label study analyzed in a 2014 review article7 found that donepezil may have worsened behaviors. Use of cholinesterase inhibitors or memantine in FTD is not recommended unless it is not certain if the patient has FTD or Alzheimer’s disease.7

Addressing sexual behaviors. Creating a treatment regimen for FTD behavioral symptoms—specifically for abnormal sexual behaviors—can be challenging. Before starting pharmacotherapy directed at behavioral symptoms secondary to FTD, other causes of symptoms such as delirium, pain, or discomfort should be excluded. Nonpharmacologic approaches should be aimed at the type of sexual behavior and likely underlying environmental cause. For example, patients may inappropriately disrobe themselves. To address this behavior, hospital staff or caregivers should first eliminate environmental causes by ensuring the room is at a comfortable temperature, dressing the patient in light, breathable clothing, or checking if the patient needs to use the bathroom. If no environmental causes are found, a one-piece jumpsuit with closures on the back of the garment could be utilized to increase the difficulty of undressing.
Other nonpharmacologic methods include providing private areas for patients who are behaving inappropriately or removing potentially stimulating television or media from the environment. Another option is to increase the use of positive, pleasant stimuli. One approach that has shown benefit is music therapy, utilizing popular music genres from the patient’s youth.3
Evidence for pharmacotherapy is limited and largely from case reports and case series. A 2020 meta-analysis by Trieu et al8 reviewed 23 studies to expand on current clinical guidance for patients with bvFTD. These studies showed improvements in behavioral symptoms and reductions in caregiver fatigue with citalopram, trazodone, paroxetine, and fluvoxamine. Six of the trials included in this meta-analysis that evaluated these 4 medications are summarized in Table 2.9-14

Due to the lower risk of adverse effects and favorable safety profiles, SSRIs and trazodone are considered first-line treatment options. Benefit from these medications is theorized to be a result of their serotonergic effects, because serotonin abnormalities and dysfunction have been linked to FTD symptoms. For example, in a patient experiencing hypersexuality, the common adverse effect of low libido associated with SSRIs can be particularly beneficial.8
Continue to: Other medication classes studied in patients...
Other medication classes studied in patients with FTD include antipsychotics, stimulants, anticonvulsants, benzodiazepines, and hormonal therapies. In addition to a black box warning for increased mortality in older patients with dementia-related psychosis, antipsychotics are associated with other serious adverse effects and should be used with caution.7
FTD is a debilitating disease that has a major impact on quality of life, particularly when behavioral symptoms accompany cognitive decline. Though some therapies may possibly improve behavioral symptoms, their routine use remains controversial due to a lack of clear evidence of benefit. In caring for patients with FTD and behavioral symptoms, a multimodal, team-based approach is vital.1
CASE CONTINUED
The treatment team starts Mr. S on several of the modalities discussed in this article over the span of 2 years, with limited efficacy. Nonpharmacologic methods do not provide much benefit because Mr. S is extremely difficult to redirect. Given Mr. S’s past trials of SSRIs prior to admission, sertraline was retrialed and titrated over 2 years. The highest dose utilized during his admission was 200 mg/d. The team starts estrogen therapy but tapers and discontinues it due to ineffectiveness. Mr. S’s use of carbidopa/levodopa is thought to be contributing to his behavioral abnormalities, so the team tapers it to discontinuation; however, Mr. S’s sexually inappropriate behaviors and agitation continue. The team initiates a plan to reduce the dose of quetiapine and switch to gabapentin, but Mr. S fails gradual dose reduction due to his worsening behaviors. He starts gabapentin. The team gradually increases the dose of gabapentin to decrease libido and agitation, respectively. The increase in sertraline dose and use of nonpharmacologic modalities causes Mr. S’s use of as-needed antipsychotics to decrease.
Related Resources
- Ellison JM. What are the stages of frontotemporal dementia? BrightFocus Foundation. July 5, 2021. Accessed July 7, 2023. https://www.brightfocus.org/alzheimers/article/what-are-stages-frontotemporal-dementia
- Dementia and sexually inappropriate behavior. ReaDementia. January 31, 2022. Accessed July 7, 2023. https://readementia.com/dementia-and-sexually-inappropriate-behavior/
Drug Brand Names
Carbidopa/levodopa • Sinemet
Citalopram • Celexa
Donepezil • Aricept
Fluvoxamine • Luvox
Gabapentin • Neurontin
Galantamine • Razadyne
Memantine • Namenda
Paroxetine • Paxil
Quetiapine • Seroquel
Rivastigmine • Exelon
Sertraline • Zoloft
Trazodone • Desyrel
Mr. S, age 77, is admitted to a long-term care facility due to progressive cognitive impairment and sexually inappropriate behavior. He has a history of sexual assault of medical staff. His medical history includes significant frontotemporal dementia (FTD) with behavioral disturbances, abnormal sexual behaviors, subclinical hypothyroidism, schizoid personality disorder, Parkinson disease, posttraumatic stress disorder, and hyperammonemia.
Upon admission, Mr. S’s vital signs are within normal limits except for an elevated thyroid-stimulating hormone (4.54 mIU/L; reference range 0.40 to 4.50 mIU/L). Prior cognitive testing results and updated ammonia levels are unavailable. Mr. S’s current medications include acetaminophen 650 mg every 4 hours as needed for pain, calcium carbonate/vitamin D twice daily for bone health, carbidopa/levodopa 25/100 mg twice daily for Parkinson disease, melatonin 3 mg/d at bedtime for insomnia, quetiapine 25 mg twice daily for psychosis with disturbance of behavior and 12.5 mg every 4 hours as needed for agitation, and trazodone 50 mg/d at bedtime for insomnia. Before Mr. S was admitted, previous therapy with selective serotonin reuptake inhibitors (SSRIs) had been tapered and discontinued. Mr. S had also started antipsychotic therapy at another facility due to worsening behaviors.
In patients with dementia, the brain is experiencing neurodegeneration. Progressively, neurons may stop functioning, lose connections with other neurons, and ultimately face cell death. The specific dementia diagnosis and its clinical features depend on the type of neurons and region of the brain affected.1,2
FTD occurs in response to damage to the frontal and temporal lobes. The frontal lobe correlates to executive functioning, while the temporal lobe plays a role in speech and comprehension. Damage to these areas may result in loss of movement, trouble speaking, difficulty solving complex problems, and problems with social behavior. Specifically, damage to the orbital frontal cortex may cause disinhibition and abnormal behaviors, including emotional lability, vulgarity, and indifference to social nuances.1 Within an FTD diagnosis, there are 3 disorders: behavioral-variant FTD (bvFTD), semantic dementia, and progressive nonfluent aphasia.1 Specifically, bvFTD can result in abnormal sexual behaviors such as making sexually inappropriate statements, masturbating in public, undressing in public, inappropriately or aggressively touching others, or confusing another individual as an intimate partner. In addition to cognitive impairment, these neurobehavioral symptoms can significantly impact an individual’s quality of life while increasing caregiver burden.2
Occurring at a similar frequency to Alzheimer’s disease in patients age <65, FTD is one of the more common causes of early-onset dementia. The mean age of onset is 58 and onset after age 75 is particularly unusual. Memory may not be affected early in the course of the disease, but social changes are likely. As FTD progresses, symptoms will resemble those of Alzheimer’s disease and patients will require assistance with activities of daily living. In later stages of FTD, patients will exhibit language and behavior symptoms. Due to its unique progression, FTD can be commonly misdiagnosed as other mental illnesses or neurocognitive disorders.1
Approaches to treatment: What to consider
Both nonpharmacologic and pharmacologic interventions are appropriate for addressing FTD. Because nonpharmacologic options improve patient safety and overall physical health, they should be used whenever practical. These interventions include safe driving measures, exercise, speech therapy, redirection, offering simple choices when making decisions, and managing environmental cues for behaviors that should be encouraged or discouraged.3
There are no FDA-approved medications to cure or slow the progression of FTD. Therefore, treatment is focused on alleviating neurobehavioral symptoms. The symptoms depend on the type of FTD the patient has; they include cognitive impairment, anxiety, insomnia or sleep disturbances, compulsive behaviors, speech and language problems, and agitation. While many medications have been commonly used for symptomatic relief, evidence for the efficacy of these treatments in FTD is limited.2
Continue to: A review of the literature...
A review of the literature on potential treatments for cognitive impairment and behavioral symptoms of FTD identified 2 trials and 1 case series (Table 14-6) in addition to a 2014 review article7 of current pharmacologic treatments. These trials evaluated cognitive improvement with rivastigmine, memantine, galantamine, and donepezil. None of the trials found a significant benefit from any of these medications for cognitive improvement in FTD. Data were conflicting on whether these medications improved or worsened behavioral symptoms. For example, the case series of 3 patients by Swanberg6 suggested improvement in behavior with memantine, while an open-label study analyzed in a 2014 review article7 found that donepezil may have worsened behaviors. Use of cholinesterase inhibitors or memantine in FTD is not recommended unless it is not certain if the patient has FTD or Alzheimer’s disease.7

Addressing sexual behaviors. Creating a treatment regimen for FTD behavioral symptoms—specifically for abnormal sexual behaviors—can be challenging. Before starting pharmacotherapy directed at behavioral symptoms secondary to FTD, other causes of symptoms such as delirium, pain, or discomfort should be excluded. Nonpharmacologic approaches should be aimed at the type of sexual behavior and likely underlying environmental cause. For example, patients may inappropriately disrobe themselves. To address this behavior, hospital staff or caregivers should first eliminate environmental causes by ensuring the room is at a comfortable temperature, dressing the patient in light, breathable clothing, or checking if the patient needs to use the bathroom. If no environmental causes are found, a one-piece jumpsuit with closures on the back of the garment could be utilized to increase the difficulty of undressing.
Other nonpharmacologic methods include providing private areas for patients who are behaving inappropriately or removing potentially stimulating television or media from the environment. Another option is to increase the use of positive, pleasant stimuli. One approach that has shown benefit is music therapy, utilizing popular music genres from the patient’s youth.3
Evidence for pharmacotherapy is limited and largely from case reports and case series. A 2020 meta-analysis by Trieu et al8 reviewed 23 studies to expand on current clinical guidance for patients with bvFTD. These studies showed improvements in behavioral symptoms and reductions in caregiver fatigue with citalopram, trazodone, paroxetine, and fluvoxamine. Six of the trials included in this meta-analysis that evaluated these 4 medications are summarized in Table 2.9-14

Due to the lower risk of adverse effects and favorable safety profiles, SSRIs and trazodone are considered first-line treatment options. Benefit from these medications is theorized to be a result of their serotonergic effects, because serotonin abnormalities and dysfunction have been linked to FTD symptoms. For example, in a patient experiencing hypersexuality, the common adverse effect of low libido associated with SSRIs can be particularly beneficial.8
Continue to: Other medication classes studied in patients...
Other medication classes studied in patients with FTD include antipsychotics, stimulants, anticonvulsants, benzodiazepines, and hormonal therapies. In addition to a black box warning for increased mortality in older patients with dementia-related psychosis, antipsychotics are associated with other serious adverse effects and should be used with caution.7
FTD is a debilitating disease that has a major impact on quality of life, particularly when behavioral symptoms accompany cognitive decline. Though some therapies may possibly improve behavioral symptoms, their routine use remains controversial due to a lack of clear evidence of benefit. In caring for patients with FTD and behavioral symptoms, a multimodal, team-based approach is vital.1
CASE CONTINUED
The treatment team starts Mr. S on several of the modalities discussed in this article over the span of 2 years, with limited efficacy. Nonpharmacologic methods do not provide much benefit because Mr. S is extremely difficult to redirect. Given Mr. S’s past trials of SSRIs prior to admission, sertraline was retrialed and titrated over 2 years. The highest dose utilized during his admission was 200 mg/d. The team starts estrogen therapy but tapers and discontinues it due to ineffectiveness. Mr. S’s use of carbidopa/levodopa is thought to be contributing to his behavioral abnormalities, so the team tapers it to discontinuation; however, Mr. S’s sexually inappropriate behaviors and agitation continue. The team initiates a plan to reduce the dose of quetiapine and switch to gabapentin, but Mr. S fails gradual dose reduction due to his worsening behaviors. He starts gabapentin. The team gradually increases the dose of gabapentin to decrease libido and agitation, respectively. The increase in sertraline dose and use of nonpharmacologic modalities causes Mr. S’s use of as-needed antipsychotics to decrease.
Related Resources
- Ellison JM. What are the stages of frontotemporal dementia? BrightFocus Foundation. July 5, 2021. Accessed July 7, 2023. https://www.brightfocus.org/alzheimers/article/what-are-stages-frontotemporal-dementia
- Dementia and sexually inappropriate behavior. ReaDementia. January 31, 2022. Accessed July 7, 2023. https://readementia.com/dementia-and-sexually-inappropriate-behavior/
Drug Brand Names
Carbidopa/levodopa • Sinemet
Citalopram • Celexa
Donepezil • Aricept
Fluvoxamine • Luvox
Gabapentin • Neurontin
Galantamine • Razadyne
Memantine • Namenda
Paroxetine • Paxil
Quetiapine • Seroquel
Rivastigmine • Exelon
Sertraline • Zoloft
Trazodone • Desyrel
1. Grossman M. Frontotemporal dementia: a review. J Int Neuropsychol Soc. 2002;8(4):566-583. doi:10.1017/s1355617702814357
2. The Johns Hopkins University. Frontotemporal dementia. Johns Hopkins Medicine. Accessed September 12, 2021. https://www.hopkinsmedicine.org/health/conditions-and-diseases/dementia/frontotemporal-dementia
3. Shinagawa S, Nakajima S, Plitman E, et al. Non-pharmacological management for patients with frontotemporal dementia: a systematic review. J Alzheimers Dis. 2015;45(1):283-293. doi:10.3233/JAD-142109
4. Moretti R, Torre P, Antonello RM, et al. Rivastigmine in frontotemporal dementia: an open-label study. Drugs Aging. 2004;21(14):931-937. doi:10.2165/00002512-200421140-00003
5. Diehl-Schmid J, Förstl H, Perneczky R, et al. A 6-month, open-label study for memantine in patients with frontotemporal dementia. In J Geriatr Psychiatry. 2008;23(7):754-759. doi:10.1002/gps.1973
6. Swanberg MM. Memantine for behavioral disturbances in frontotemporal dementia: a case series. Alzheimer Dis Assoc Disord. 2007;21(2):164-166. doi:10.1097/WAD.0b013e318047df5d
7. Tsai RM, Boxer AL. Treatment of frontotemporal dementia. Curr Treat Options Neurol. 2014;16(11):319. doi:10.1007/s11940-014-0319-0
8. Trieu C, Gossink F, Stek ML, et al. Effectiveness of pharmacological interventions for symptoms of behavioral variant frontotemporal dementia: a systematic review. Cogn Behav Neurol. 2020;33(1):1-15. doi:10.1097/WNN.0000000000000217
9. Deakin JB, Rahman S, Nestor PJ, et al. Paroxetine does not improve symptoms and impairs cognition in frontotemporal dementia: a double-blind randomized controlled trial. Psychopharmacology (Berl). 2004;172(4):400-408. doi:10.1007/s00213-003-1686-5
10. Herrmann N, Black SE, Chow T, et al. Serotonergic function and treatment of behavioral and psychological symptoms of frontotemporal dementia. Am J Geriatr Psychiatry. 2012;20(9):789-797. doi:10.1097/JGP.0b013e31823033f3
11. Ikeda M, Shigenobu K, Fukuhara R, et al. Efficacy of fluvoxamine as a treatment for behavioral symptoms in frontotemporal lobar degeneration patients. Dement Geriatr Cogn Disord. 2004;17(3):117-121. doi:10.1159/000076343
12. Lebert F, Stekke W, Hasenbroekx C, et al. Frontotemporal dementia: a randomised, controlled trial with trazodone. Dement Geriatr Cogn Disord. 2004;17(4):355-359. doi:10.1159/000077171
13. Lebert F. Behavioral benefits of trazodone are sustained for the long term in frontotemporal dementia. Therapy. 2006;3(1):93-96. doi:10.1586/14750708.3.1.93
14. Moretti R, Torre P, Antonello RM, et al. Frontotemporal dementia: paroxetine as a possible treatment of behavior symptoms. A randomized, controlled, open 14-month study. Eur Neurol. 2003;49(1):13-19. doi:10.1159/000067021
1. Grossman M. Frontotemporal dementia: a review. J Int Neuropsychol Soc. 2002;8(4):566-583. doi:10.1017/s1355617702814357
2. The Johns Hopkins University. Frontotemporal dementia. Johns Hopkins Medicine. Accessed September 12, 2021. https://www.hopkinsmedicine.org/health/conditions-and-diseases/dementia/frontotemporal-dementia
3. Shinagawa S, Nakajima S, Plitman E, et al. Non-pharmacological management for patients with frontotemporal dementia: a systematic review. J Alzheimers Dis. 2015;45(1):283-293. doi:10.3233/JAD-142109
4. Moretti R, Torre P, Antonello RM, et al. Rivastigmine in frontotemporal dementia: an open-label study. Drugs Aging. 2004;21(14):931-937. doi:10.2165/00002512-200421140-00003
5. Diehl-Schmid J, Förstl H, Perneczky R, et al. A 6-month, open-label study for memantine in patients with frontotemporal dementia. In J Geriatr Psychiatry. 2008;23(7):754-759. doi:10.1002/gps.1973
6. Swanberg MM. Memantine for behavioral disturbances in frontotemporal dementia: a case series. Alzheimer Dis Assoc Disord. 2007;21(2):164-166. doi:10.1097/WAD.0b013e318047df5d
7. Tsai RM, Boxer AL. Treatment of frontotemporal dementia. Curr Treat Options Neurol. 2014;16(11):319. doi:10.1007/s11940-014-0319-0
8. Trieu C, Gossink F, Stek ML, et al. Effectiveness of pharmacological interventions for symptoms of behavioral variant frontotemporal dementia: a systematic review. Cogn Behav Neurol. 2020;33(1):1-15. doi:10.1097/WNN.0000000000000217
9. Deakin JB, Rahman S, Nestor PJ, et al. Paroxetine does not improve symptoms and impairs cognition in frontotemporal dementia: a double-blind randomized controlled trial. Psychopharmacology (Berl). 2004;172(4):400-408. doi:10.1007/s00213-003-1686-5
10. Herrmann N, Black SE, Chow T, et al. Serotonergic function and treatment of behavioral and psychological symptoms of frontotemporal dementia. Am J Geriatr Psychiatry. 2012;20(9):789-797. doi:10.1097/JGP.0b013e31823033f3
11. Ikeda M, Shigenobu K, Fukuhara R, et al. Efficacy of fluvoxamine as a treatment for behavioral symptoms in frontotemporal lobar degeneration patients. Dement Geriatr Cogn Disord. 2004;17(3):117-121. doi:10.1159/000076343
12. Lebert F, Stekke W, Hasenbroekx C, et al. Frontotemporal dementia: a randomised, controlled trial with trazodone. Dement Geriatr Cogn Disord. 2004;17(4):355-359. doi:10.1159/000077171
13. Lebert F. Behavioral benefits of trazodone are sustained for the long term in frontotemporal dementia. Therapy. 2006;3(1):93-96. doi:10.1586/14750708.3.1.93
14. Moretti R, Torre P, Antonello RM, et al. Frontotemporal dementia: paroxetine as a possible treatment of behavior symptoms. A randomized, controlled, open 14-month study. Eur Neurol. 2003;49(1):13-19. doi:10.1159/000067021
Agitated and depressed with a traumatic brain injury
CASE TBI as a result of self-harm
Mr. N, age 46, presents to the emergency department (ED) after his neighbors report hearing “loud banging sounds” coming from his apartment for approximately 3 days. Emergency medical services found him repeatedly beating his head into a table. Upon admission to the ED, his injuries include a right temporal lobe contusion, right temporal subdural hematoma, facial fractures, bilateral foot fractures, and prevertebral swelling at the C4 vertebrate.
Mr. N is admitted to the surgical intensive care unit for hourly neurology checks. Neurosurgery recommends nonoperative management and for Mr. N to wear a cervical collar for 1 month. He is sedated after he experiences auditory hallucinations and becomes agitated toward the staff, which is later determined to be delirium. The Psychiatry team recommends inpatient psychiatric hospitalization because Mr. N’s self-harming behavior resulted in severe and dangerous injuries.
HISTORY Alcohol use disorder, insomnia, anxiety, and depression
As Mr. N becomes alert and oriented, he reports a history of alcohol use disorder (AUD), insomnia, anxiety, and major depressive disorder (MDD), but no personal or family history of bipolar disorder (BD). He says he has had insomnia and anxiety since age 18, for which he received diazepam and zolpidem for 16 years. He stopped diazepam soon after a recent change in psychiatrists and subsequently had difficulty sleeping. Mr. N started taking mirtazapine, but found minimal relief and stopped it several months ago.
[polldaddy:12704471]
The authors’ observations
The term “agitated depression” refers to a mixed state that includes symptoms of depression plus marked anxiety, restlessness, and delusions. Agitated depression is not a distinct diagnosis in DSM-5, but is classified as depression with mixed features.1 To meet the criteria for the mixed features specifier, a patient who meets the criteria for a major depressive episode needs to have ≥3 of the following manic/hypomanic symptoms1:
- Elevated, expansive mood
- Inflated self-esteem or grandiosity
- More talkative than usual
- Flight of ideas or racing thoughts
- Increase in energy or goal-directed activity
- Increased involvement in activities that have a high potential for painful consequences
- Decreased need for sleep.
The diagnosis for individuals who meet the full criteria for mania or hypomania would be BD I or BD II.1 Additionally, mixed features associated with a major depressive episode are a significant risk factor for BD.1
EVALUATION Agitation and hallucinations
Mr. N recalls multiple falls at home in the weeks prior to hospitalization, but says he does not remember repeatedly hitting his head against a table. He reports sleeping for approximately 2 hours per night since his father’s death 2 months ago, an acute stressor that likely precipitated this depressive episode. Mr. N says he had been experiencing visual hallucinations of his father and a younger version of himself for weeks before presenting to the ED. It is not clear if Mr. N does not recall beating his head on the table due to his traumatic brain injury (TBI) or because it occurred during an acute manic or psychotic episode with hallucinations.
The treatment team assigns Mr. N a working diagnosis of agitated depression with a risk for BD, mixed episode. He meets the criteria for agitated depression (major depressive episode, motor agitation, and psychic agitation), but also has many features of BD; a manic episode may have led to hospitalization. The treating clinicians continue to monitor the progression of Mr. N’s symptoms to clarify his diagnoses. During the course of his hospitalization, Mr. N’s psychiatric diagnoses include delirium (resolved), alcohol withdrawal, catatonia, substance-induced mood disorder, and agitated depression. Mixed episode BD is ruled out.
Continue to: The authors' observations
The authors’ observations
There is significant symptomatic overlap between agitated depression and BD. It can be difficult to differentiate the diagnoses, as psychomotor agitation can be seen in MDD and agitated depression can be seen in BD. Serra et al2 investigated the prevalence of agitated depression in patients with BD and found that agitation accompanied bipolar depression in at least one-third of cases and was associated with concurrent somatic depressive symptoms, which are common features of mixed manic states. Psychomotor agitation was also associated with lifetime experience of mixed mania, comorbid panic disorder, and increased suicidal behavior.2
Though antidepressants are considered a first-line treatment for depression, they should not be used to treat agitated depression because they may increase insomnia, agitation, and suicide risk, and may trigger the onset of psychotic symptoms. In a similar vein, antidepressant monotherapy is contraindicated in BD because it may induce mania or hypomania states.2
TREATMENT Neuroprotective psychotropics
Due to Mr. N’s medical complexity (particularly cervical collar and physical therapy needs), he is not transferred to a psychiatric facility. Instead, the consultation-liaison psychiatry team follows him and provides psychiatric care in the hospital.
Due to concerns for continued self-harm, Mr. N is observed by continuous video monitoring. After initial stabilization, the care team starts valproic acid 250 mg twice daily and titrates it to 500 mg/d in the morning and 1,000 mg/d in the evening for mood stabilization, gabapentin 300 mg 3 times daily, melatonin 3 mg/d at bedtime for insomnia, and lorazepam 1 mg/d at bedtime to rule out catatonia and 1 mg/d as needed for agitation. After starting valproic acid, the care team routinely checks Mr. N’s ammonia levels throughout his hospitalization.
[polldaddy:12704473]
The authors’ observations
Treatment of agitated depression both in isolation and in the context of BD presents a clinical challenge because antidepressants are contraindicated for both agitated depression and BD. In the context of TBI, treatment of agitated depression becomes more complicated because neuroprotection is the priority. Neuroprotection refers to a medication’s ability to prevent neuronal cell death or further injury or damage through neurochemical modulation.
Continue to: To treat agitation associated with MDD...
To treat agitation associated with MDD, second-generation antipsychotics and valproic acid have shown significant neuroprotective effects. The proposed mechanisms for neuroprotection include not only antioxidant effects but 5HT1A agonist properties, with the latter thought to protect against excitotoxic injury that may exacerbate agitation due to brain trauma.3
There is no consensus on which antipsychotics are most efficacious for treating agitation in the setting of an acute TBI. Williamson et al4 reviewed various medications that may treat agitation in the setting of acute TBI with fewer adverse effects.
Though haloperidol is often prescribed to treat agitation in patients with TBI, animal studies have shown it is inferior to second-generation antipsychotics in protecting against excitotoxic/oxidative injury, and haloperidol has been associated with neuronal loss. Haloperidol has been linked to adverse clinical outcomes for patients with aggression after TBI, including prolonged amnesia, which is thought to be linked to haloperidol’s strong and selective dopamine-2 receptor antagonism and the mesocortical and nigrostriatal pathways involved.4
Carbamazepine, phenytoin, and methylphenidate cause oxidative stress and/or apoptosis, and therefore offer no neuroprotection. Data on gabapentin are mixed; a few studies suggest it may block synapse formation or decrease quantities of antioxidant enzymes in the brain, though it’s known to protect against glutamate-induced neuronal injury.3
Additional research is needed to assess which second-generation antipsychotics offer the most neuroprotection. However, based on existing literature, olanzapine and aripiprazole may offer the most benefit because they have the greatest antioxidant—and thus, neuroprotective—activity. Cognitive enhancers such as memantine and donepezil exhibit neuroprotection, particularly in Alzheimer disease. Anticonvulsants such as levetiracetam, lacosamide, and lamotrigine offer neuroprotection and may be considered for seizure prevention.3 The Table3-6 lists psychotropic medications used to treat TBI.
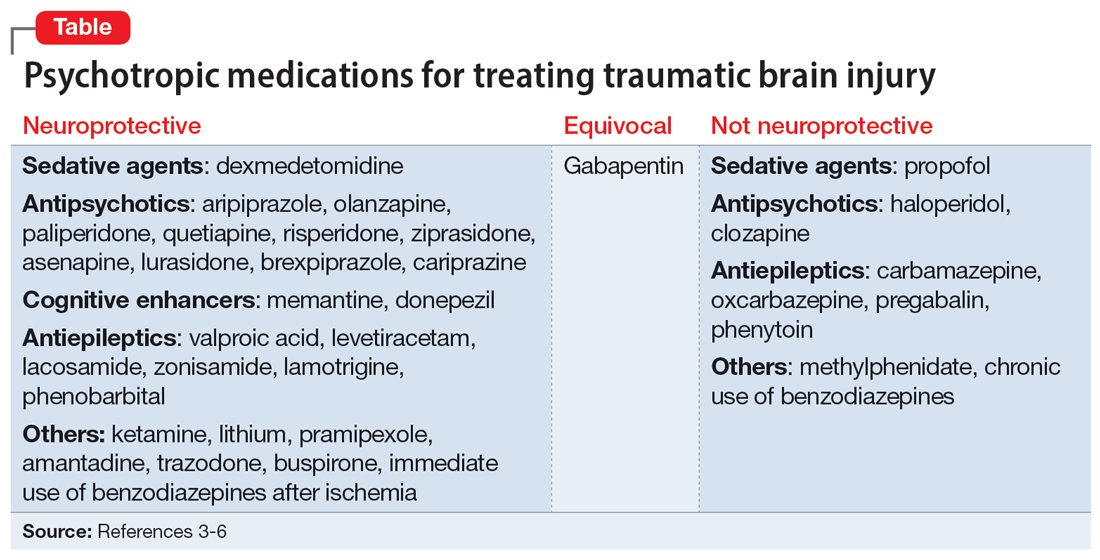
Continue to: Valproic acid stands out among...
Valproic acid stands out among anticonvulsants because its superior antioxidant effects, in combination with its antiepileptic effect in patients with TBI, offer more neuroprotection than other medications.5 It is important to regularly monitor ammonia levels in patients receiving valproic acid because elevated levels can cause hyperammonemic encephalopathy.
A 2005 study by DeBattista et al5 investigated the impact of valproic acid on agitation in 12 adults with MDD who were being treated with antidepressants. Participants were given a low dose of valproic acid for 4 weeks and their agitation, anxiety, and depressed mood were independently assessed by separate rating scales. There was a modest decrease in scores for mood symptoms but a particularly sharp decrease in agitation scores.5
Valproic acid has been shown to be a potentially safe and efficacious treatment for alcohol withdrawal. A clinical trial examining patients with moderate alcohol withdrawal found a faster and more consistent resolution of symptoms in patients given valproic acid detoxification compared to a control group that received the standard benzodiazepine detoxification.6 Additionally, patients who continued maintenance valproic acid following detoxification were completely abstinent at 6-week follow-up compared to patients who did not receive this maintenance therapy.6
Valproic acid was a particularly optimal medication choice for Mr. N due to its neuroprotective properties in the context of TBI, its ability to treat delirium,7 its lack of abuse potential compared with benzodiazepines, and its potential efficacy for managing alcohol withdrawal and AUD.
OUTCOME Improvement and discharge
Mr. N is medically cleared for discharge. Although the psychiatry team initially was concerned about his willingness to attend follow-up appointments and adhere to proper cervical collar use, Mr. N becomes more cooperative with psychiatric care as his stay continues, and he is psychiatrically cleared for discharge 1 month after admission. Discharge plans include attending an intensive outpatient program, continuing the inpatient psychiatric medication regimen, participating in regular outpatient psychiatric follow-up, as well as following up with orthopedic surgery, neurosurgery, podiatry, and ear, nose, and throat for medical conditions.
Bottom Line
Agitated depression is a mixed state that includes features of depression and manic/hypomanic symptoms. Diagnosis and treatment can be challenging because symptoms of agitated depression overlap with bipolar disorder and antidepressants are contraindicated. In a patient with a traumatic brain injury, pharmacotherapy that provides neuroprotection is a priority.
Related Resources
- Ramaswamy S, Driscoll D, Rodriguez A, et al. Nutraceuticals for traumatic brain injury: should you recommend their use? Current Psychiatry. 2017;16(7):34-38,40,41-45.
- Sampogna G, Del Vecchio V, Giallonardo V, et al. Diagnosis, clinical features, and therapeutic implications of agitated depression. Psychiatr Clin North Am. 2020;43(1):47-57. doi: 10.1016/j.psc.2019.10.011
Drug Brand Names
Amantadine • Gocovri
Aripiprazole • Abilify
Asenapine • Saphris
Brexpiprazole • Rexulti
Buspirone • BuSpar
Carbamazepine • Tegretol
Cariprazine • Vraylar
Clozapine • Clozaril
Dexmedetomidine • Igalmi
Diazepam • Valium
Donepezil • Aricept
Gabapentin • Neurontin
Haloperidol • Haldol
Ketamine • Ketalar
Lacosamide • Vimpat
Lamotrigine • Lamictal
Levetiracetam • Keppra
Lithium • Lithobid
Lorazepam • Ativan
Lurasidone • Latuda
Memantine • Namenda
Methylphenidate • Concerta
Mirtazapine • Remeron
Olanzapine • Zyprexa
Oxcarbazepine • Trileptal
Paliperidone • Invega
Phenytoin • Dilantin
Pramipexole • Mirapex
Pregabalin • Lyrica
Quetiapine • Seroquel
Risperidone • Risperdal
Trazodone • Oleptro
Valproic acid • Depakene
Ziprasidone • Geodon
Zolpidem • Ambien
Zonisamide • Zonegran
1. American Psychiatric Association. Diagnostic and Statistical Manual of Mental Disorders. 5th ed, text revision. American Psychiatric Association; 2022.
2. Serra F, Gordon‐Smith K, Perry A, et al. Agitated depression in bipolar disorder. Bipolar Disord. 2019;21(6):547-555. doi:10.1111/bdi.12778
3. Meresh E, Daniels D, Owens JH, et al. Psychotropics and neuroprotection: literature review and case series report. OBM Neurobiol. 2020;4(1). doi:10.21926/obm.neurobiol.2001048
4. Williamson DR, Frenette AJ, Burry L, et al. Pharmacological interventions for agitation in patients with traumatic brain injury: protocol for a systematic review and meta-analysis. Syst Rev. 2016;5(1):193. doi:10.1186/s13643-016-0374-6
5. DeBattista C, Solomon A, Arnow B, et al. The efficacy of divalproex sodium in the treatment of agitation associated with major depression. J Clin Psychopharmacol. 2005;25(5):476-479. doi:10.1097/01.jcp.0000177552.21338.b0
6. Longo LP, Campbell T, Hubatch, S. Divalproex sodium (Depakote) for alcohol withdrawal and relapse prevention. J Addict Dis. 2002;21(2):55-64. doi:10.1300/J069v21n02_05
7. Sher Y, Cramer ACM, Ament A, et al. Valproic acid for treatment of hyperactive or mixed delirium: rationale and literature review. Psychosomatics. 2015;56(6):615-625. doi:10.1016/j.psym.2015.09.008
CASE TBI as a result of self-harm
Mr. N, age 46, presents to the emergency department (ED) after his neighbors report hearing “loud banging sounds” coming from his apartment for approximately 3 days. Emergency medical services found him repeatedly beating his head into a table. Upon admission to the ED, his injuries include a right temporal lobe contusion, right temporal subdural hematoma, facial fractures, bilateral foot fractures, and prevertebral swelling at the C4 vertebrate.
Mr. N is admitted to the surgical intensive care unit for hourly neurology checks. Neurosurgery recommends nonoperative management and for Mr. N to wear a cervical collar for 1 month. He is sedated after he experiences auditory hallucinations and becomes agitated toward the staff, which is later determined to be delirium. The Psychiatry team recommends inpatient psychiatric hospitalization because Mr. N’s self-harming behavior resulted in severe and dangerous injuries.
HISTORY Alcohol use disorder, insomnia, anxiety, and depression
As Mr. N becomes alert and oriented, he reports a history of alcohol use disorder (AUD), insomnia, anxiety, and major depressive disorder (MDD), but no personal or family history of bipolar disorder (BD). He says he has had insomnia and anxiety since age 18, for which he received diazepam and zolpidem for 16 years. He stopped diazepam soon after a recent change in psychiatrists and subsequently had difficulty sleeping. Mr. N started taking mirtazapine, but found minimal relief and stopped it several months ago.
[polldaddy:12704471]
The authors’ observations
The term “agitated depression” refers to a mixed state that includes symptoms of depression plus marked anxiety, restlessness, and delusions. Agitated depression is not a distinct diagnosis in DSM-5, but is classified as depression with mixed features.1 To meet the criteria for the mixed features specifier, a patient who meets the criteria for a major depressive episode needs to have ≥3 of the following manic/hypomanic symptoms1:
- Elevated, expansive mood
- Inflated self-esteem or grandiosity
- More talkative than usual
- Flight of ideas or racing thoughts
- Increase in energy or goal-directed activity
- Increased involvement in activities that have a high potential for painful consequences
- Decreased need for sleep.
The diagnosis for individuals who meet the full criteria for mania or hypomania would be BD I or BD II.1 Additionally, mixed features associated with a major depressive episode are a significant risk factor for BD.1
EVALUATION Agitation and hallucinations
Mr. N recalls multiple falls at home in the weeks prior to hospitalization, but says he does not remember repeatedly hitting his head against a table. He reports sleeping for approximately 2 hours per night since his father’s death 2 months ago, an acute stressor that likely precipitated this depressive episode. Mr. N says he had been experiencing visual hallucinations of his father and a younger version of himself for weeks before presenting to the ED. It is not clear if Mr. N does not recall beating his head on the table due to his traumatic brain injury (TBI) or because it occurred during an acute manic or psychotic episode with hallucinations.
The treatment team assigns Mr. N a working diagnosis of agitated depression with a risk for BD, mixed episode. He meets the criteria for agitated depression (major depressive episode, motor agitation, and psychic agitation), but also has many features of BD; a manic episode may have led to hospitalization. The treating clinicians continue to monitor the progression of Mr. N’s symptoms to clarify his diagnoses. During the course of his hospitalization, Mr. N’s psychiatric diagnoses include delirium (resolved), alcohol withdrawal, catatonia, substance-induced mood disorder, and agitated depression. Mixed episode BD is ruled out.
Continue to: The authors' observations
The authors’ observations
There is significant symptomatic overlap between agitated depression and BD. It can be difficult to differentiate the diagnoses, as psychomotor agitation can be seen in MDD and agitated depression can be seen in BD. Serra et al2 investigated the prevalence of agitated depression in patients with BD and found that agitation accompanied bipolar depression in at least one-third of cases and was associated with concurrent somatic depressive symptoms, which are common features of mixed manic states. Psychomotor agitation was also associated with lifetime experience of mixed mania, comorbid panic disorder, and increased suicidal behavior.2
Though antidepressants are considered a first-line treatment for depression, they should not be used to treat agitated depression because they may increase insomnia, agitation, and suicide risk, and may trigger the onset of psychotic symptoms. In a similar vein, antidepressant monotherapy is contraindicated in BD because it may induce mania or hypomania states.2
TREATMENT Neuroprotective psychotropics
Due to Mr. N’s medical complexity (particularly cervical collar and physical therapy needs), he is not transferred to a psychiatric facility. Instead, the consultation-liaison psychiatry team follows him and provides psychiatric care in the hospital.
Due to concerns for continued self-harm, Mr. N is observed by continuous video monitoring. After initial stabilization, the care team starts valproic acid 250 mg twice daily and titrates it to 500 mg/d in the morning and 1,000 mg/d in the evening for mood stabilization, gabapentin 300 mg 3 times daily, melatonin 3 mg/d at bedtime for insomnia, and lorazepam 1 mg/d at bedtime to rule out catatonia and 1 mg/d as needed for agitation. After starting valproic acid, the care team routinely checks Mr. N’s ammonia levels throughout his hospitalization.
[polldaddy:12704473]
The authors’ observations
Treatment of agitated depression both in isolation and in the context of BD presents a clinical challenge because antidepressants are contraindicated for both agitated depression and BD. In the context of TBI, treatment of agitated depression becomes more complicated because neuroprotection is the priority. Neuroprotection refers to a medication’s ability to prevent neuronal cell death or further injury or damage through neurochemical modulation.
Continue to: To treat agitation associated with MDD...
To treat agitation associated with MDD, second-generation antipsychotics and valproic acid have shown significant neuroprotective effects. The proposed mechanisms for neuroprotection include not only antioxidant effects but 5HT1A agonist properties, with the latter thought to protect against excitotoxic injury that may exacerbate agitation due to brain trauma.3
There is no consensus on which antipsychotics are most efficacious for treating agitation in the setting of an acute TBI. Williamson et al4 reviewed various medications that may treat agitation in the setting of acute TBI with fewer adverse effects.
Though haloperidol is often prescribed to treat agitation in patients with TBI, animal studies have shown it is inferior to second-generation antipsychotics in protecting against excitotoxic/oxidative injury, and haloperidol has been associated with neuronal loss. Haloperidol has been linked to adverse clinical outcomes for patients with aggression after TBI, including prolonged amnesia, which is thought to be linked to haloperidol’s strong and selective dopamine-2 receptor antagonism and the mesocortical and nigrostriatal pathways involved.4
Carbamazepine, phenytoin, and methylphenidate cause oxidative stress and/or apoptosis, and therefore offer no neuroprotection. Data on gabapentin are mixed; a few studies suggest it may block synapse formation or decrease quantities of antioxidant enzymes in the brain, though it’s known to protect against glutamate-induced neuronal injury.3
Additional research is needed to assess which second-generation antipsychotics offer the most neuroprotection. However, based on existing literature, olanzapine and aripiprazole may offer the most benefit because they have the greatest antioxidant—and thus, neuroprotective—activity. Cognitive enhancers such as memantine and donepezil exhibit neuroprotection, particularly in Alzheimer disease. Anticonvulsants such as levetiracetam, lacosamide, and lamotrigine offer neuroprotection and may be considered for seizure prevention.3 The Table3-6 lists psychotropic medications used to treat TBI.

Continue to: Valproic acid stands out among...
Valproic acid stands out among anticonvulsants because its superior antioxidant effects, in combination with its antiepileptic effect in patients with TBI, offer more neuroprotection than other medications.5 It is important to regularly monitor ammonia levels in patients receiving valproic acid because elevated levels can cause hyperammonemic encephalopathy.
A 2005 study by DeBattista et al5 investigated the impact of valproic acid on agitation in 12 adults with MDD who were being treated with antidepressants. Participants were given a low dose of valproic acid for 4 weeks and their agitation, anxiety, and depressed mood were independently assessed by separate rating scales. There was a modest decrease in scores for mood symptoms but a particularly sharp decrease in agitation scores.5
Valproic acid has been shown to be a potentially safe and efficacious treatment for alcohol withdrawal. A clinical trial examining patients with moderate alcohol withdrawal found a faster and more consistent resolution of symptoms in patients given valproic acid detoxification compared to a control group that received the standard benzodiazepine detoxification.6 Additionally, patients who continued maintenance valproic acid following detoxification were completely abstinent at 6-week follow-up compared to patients who did not receive this maintenance therapy.6
Valproic acid was a particularly optimal medication choice for Mr. N due to its neuroprotective properties in the context of TBI, its ability to treat delirium,7 its lack of abuse potential compared with benzodiazepines, and its potential efficacy for managing alcohol withdrawal and AUD.
OUTCOME Improvement and discharge
Mr. N is medically cleared for discharge. Although the psychiatry team initially was concerned about his willingness to attend follow-up appointments and adhere to proper cervical collar use, Mr. N becomes more cooperative with psychiatric care as his stay continues, and he is psychiatrically cleared for discharge 1 month after admission. Discharge plans include attending an intensive outpatient program, continuing the inpatient psychiatric medication regimen, participating in regular outpatient psychiatric follow-up, as well as following up with orthopedic surgery, neurosurgery, podiatry, and ear, nose, and throat for medical conditions.
Bottom Line
Agitated depression is a mixed state that includes features of depression and manic/hypomanic symptoms. Diagnosis and treatment can be challenging because symptoms of agitated depression overlap with bipolar disorder and antidepressants are contraindicated. In a patient with a traumatic brain injury, pharmacotherapy that provides neuroprotection is a priority.
Related Resources
- Ramaswamy S, Driscoll D, Rodriguez A, et al. Nutraceuticals for traumatic brain injury: should you recommend their use? Current Psychiatry. 2017;16(7):34-38,40,41-45.
- Sampogna G, Del Vecchio V, Giallonardo V, et al. Diagnosis, clinical features, and therapeutic implications of agitated depression. Psychiatr Clin North Am. 2020;43(1):47-57. doi: 10.1016/j.psc.2019.10.011
Drug Brand Names
Amantadine • Gocovri
Aripiprazole • Abilify
Asenapine • Saphris
Brexpiprazole • Rexulti
Buspirone • BuSpar
Carbamazepine • Tegretol
Cariprazine • Vraylar
Clozapine • Clozaril
Dexmedetomidine • Igalmi
Diazepam • Valium
Donepezil • Aricept
Gabapentin • Neurontin
Haloperidol • Haldol
Ketamine • Ketalar
Lacosamide • Vimpat
Lamotrigine • Lamictal
Levetiracetam • Keppra
Lithium • Lithobid
Lorazepam • Ativan
Lurasidone • Latuda
Memantine • Namenda
Methylphenidate • Concerta
Mirtazapine • Remeron
Olanzapine • Zyprexa
Oxcarbazepine • Trileptal
Paliperidone • Invega
Phenytoin • Dilantin
Pramipexole • Mirapex
Pregabalin • Lyrica
Quetiapine • Seroquel
Risperidone • Risperdal
Trazodone • Oleptro
Valproic acid • Depakene
Ziprasidone • Geodon
Zolpidem • Ambien
Zonisamide • Zonegran
CASE TBI as a result of self-harm
Mr. N, age 46, presents to the emergency department (ED) after his neighbors report hearing “loud banging sounds” coming from his apartment for approximately 3 days. Emergency medical services found him repeatedly beating his head into a table. Upon admission to the ED, his injuries include a right temporal lobe contusion, right temporal subdural hematoma, facial fractures, bilateral foot fractures, and prevertebral swelling at the C4 vertebrate.
Mr. N is admitted to the surgical intensive care unit for hourly neurology checks. Neurosurgery recommends nonoperative management and for Mr. N to wear a cervical collar for 1 month. He is sedated after he experiences auditory hallucinations and becomes agitated toward the staff, which is later determined to be delirium. The Psychiatry team recommends inpatient psychiatric hospitalization because Mr. N’s self-harming behavior resulted in severe and dangerous injuries.
HISTORY Alcohol use disorder, insomnia, anxiety, and depression
As Mr. N becomes alert and oriented, he reports a history of alcohol use disorder (AUD), insomnia, anxiety, and major depressive disorder (MDD), but no personal or family history of bipolar disorder (BD). He says he has had insomnia and anxiety since age 18, for which he received diazepam and zolpidem for 16 years. He stopped diazepam soon after a recent change in psychiatrists and subsequently had difficulty sleeping. Mr. N started taking mirtazapine, but found minimal relief and stopped it several months ago.
[polldaddy:12704471]
The authors’ observations
The term “agitated depression” refers to a mixed state that includes symptoms of depression plus marked anxiety, restlessness, and delusions. Agitated depression is not a distinct diagnosis in DSM-5, but is classified as depression with mixed features.1 To meet the criteria for the mixed features specifier, a patient who meets the criteria for a major depressive episode needs to have ≥3 of the following manic/hypomanic symptoms1:
- Elevated, expansive mood
- Inflated self-esteem or grandiosity
- More talkative than usual
- Flight of ideas or racing thoughts
- Increase in energy or goal-directed activity
- Increased involvement in activities that have a high potential for painful consequences
- Decreased need for sleep.
The diagnosis for individuals who meet the full criteria for mania or hypomania would be BD I or BD II.1 Additionally, mixed features associated with a major depressive episode are a significant risk factor for BD.1
EVALUATION Agitation and hallucinations
Mr. N recalls multiple falls at home in the weeks prior to hospitalization, but says he does not remember repeatedly hitting his head against a table. He reports sleeping for approximately 2 hours per night since his father’s death 2 months ago, an acute stressor that likely precipitated this depressive episode. Mr. N says he had been experiencing visual hallucinations of his father and a younger version of himself for weeks before presenting to the ED. It is not clear if Mr. N does not recall beating his head on the table due to his traumatic brain injury (TBI) or because it occurred during an acute manic or psychotic episode with hallucinations.
The treatment team assigns Mr. N a working diagnosis of agitated depression with a risk for BD, mixed episode. He meets the criteria for agitated depression (major depressive episode, motor agitation, and psychic agitation), but also has many features of BD; a manic episode may have led to hospitalization. The treating clinicians continue to monitor the progression of Mr. N’s symptoms to clarify his diagnoses. During the course of his hospitalization, Mr. N’s psychiatric diagnoses include delirium (resolved), alcohol withdrawal, catatonia, substance-induced mood disorder, and agitated depression. Mixed episode BD is ruled out.
Continue to: The authors' observations
The authors’ observations
There is significant symptomatic overlap between agitated depression and BD. It can be difficult to differentiate the diagnoses, as psychomotor agitation can be seen in MDD and agitated depression can be seen in BD. Serra et al2 investigated the prevalence of agitated depression in patients with BD and found that agitation accompanied bipolar depression in at least one-third of cases and was associated with concurrent somatic depressive symptoms, which are common features of mixed manic states. Psychomotor agitation was also associated with lifetime experience of mixed mania, comorbid panic disorder, and increased suicidal behavior.2
Though antidepressants are considered a first-line treatment for depression, they should not be used to treat agitated depression because they may increase insomnia, agitation, and suicide risk, and may trigger the onset of psychotic symptoms. In a similar vein, antidepressant monotherapy is contraindicated in BD because it may induce mania or hypomania states.2
TREATMENT Neuroprotective psychotropics
Due to Mr. N’s medical complexity (particularly cervical collar and physical therapy needs), he is not transferred to a psychiatric facility. Instead, the consultation-liaison psychiatry team follows him and provides psychiatric care in the hospital.
Due to concerns for continued self-harm, Mr. N is observed by continuous video monitoring. After initial stabilization, the care team starts valproic acid 250 mg twice daily and titrates it to 500 mg/d in the morning and 1,000 mg/d in the evening for mood stabilization, gabapentin 300 mg 3 times daily, melatonin 3 mg/d at bedtime for insomnia, and lorazepam 1 mg/d at bedtime to rule out catatonia and 1 mg/d as needed for agitation. After starting valproic acid, the care team routinely checks Mr. N’s ammonia levels throughout his hospitalization.
[polldaddy:12704473]
The authors’ observations
Treatment of agitated depression both in isolation and in the context of BD presents a clinical challenge because antidepressants are contraindicated for both agitated depression and BD. In the context of TBI, treatment of agitated depression becomes more complicated because neuroprotection is the priority. Neuroprotection refers to a medication’s ability to prevent neuronal cell death or further injury or damage through neurochemical modulation.
Continue to: To treat agitation associated with MDD...
To treat agitation associated with MDD, second-generation antipsychotics and valproic acid have shown significant neuroprotective effects. The proposed mechanisms for neuroprotection include not only antioxidant effects but 5HT1A agonist properties, with the latter thought to protect against excitotoxic injury that may exacerbate agitation due to brain trauma.3
There is no consensus on which antipsychotics are most efficacious for treating agitation in the setting of an acute TBI. Williamson et al4 reviewed various medications that may treat agitation in the setting of acute TBI with fewer adverse effects.
Though haloperidol is often prescribed to treat agitation in patients with TBI, animal studies have shown it is inferior to second-generation antipsychotics in protecting against excitotoxic/oxidative injury, and haloperidol has been associated with neuronal loss. Haloperidol has been linked to adverse clinical outcomes for patients with aggression after TBI, including prolonged amnesia, which is thought to be linked to haloperidol’s strong and selective dopamine-2 receptor antagonism and the mesocortical and nigrostriatal pathways involved.4
Carbamazepine, phenytoin, and methylphenidate cause oxidative stress and/or apoptosis, and therefore offer no neuroprotection. Data on gabapentin are mixed; a few studies suggest it may block synapse formation or decrease quantities of antioxidant enzymes in the brain, though it’s known to protect against glutamate-induced neuronal injury.3
Additional research is needed to assess which second-generation antipsychotics offer the most neuroprotection. However, based on existing literature, olanzapine and aripiprazole may offer the most benefit because they have the greatest antioxidant—and thus, neuroprotective—activity. Cognitive enhancers such as memantine and donepezil exhibit neuroprotection, particularly in Alzheimer disease. Anticonvulsants such as levetiracetam, lacosamide, and lamotrigine offer neuroprotection and may be considered for seizure prevention.3 The Table3-6 lists psychotropic medications used to treat TBI.

Continue to: Valproic acid stands out among...
Valproic acid stands out among anticonvulsants because its superior antioxidant effects, in combination with its antiepileptic effect in patients with TBI, offer more neuroprotection than other medications.5 It is important to regularly monitor ammonia levels in patients receiving valproic acid because elevated levels can cause hyperammonemic encephalopathy.
A 2005 study by DeBattista et al5 investigated the impact of valproic acid on agitation in 12 adults with MDD who were being treated with antidepressants. Participants were given a low dose of valproic acid for 4 weeks and their agitation, anxiety, and depressed mood were independently assessed by separate rating scales. There was a modest decrease in scores for mood symptoms but a particularly sharp decrease in agitation scores.5
Valproic acid has been shown to be a potentially safe and efficacious treatment for alcohol withdrawal. A clinical trial examining patients with moderate alcohol withdrawal found a faster and more consistent resolution of symptoms in patients given valproic acid detoxification compared to a control group that received the standard benzodiazepine detoxification.6 Additionally, patients who continued maintenance valproic acid following detoxification were completely abstinent at 6-week follow-up compared to patients who did not receive this maintenance therapy.6
Valproic acid was a particularly optimal medication choice for Mr. N due to its neuroprotective properties in the context of TBI, its ability to treat delirium,7 its lack of abuse potential compared with benzodiazepines, and its potential efficacy for managing alcohol withdrawal and AUD.
OUTCOME Improvement and discharge
Mr. N is medically cleared for discharge. Although the psychiatry team initially was concerned about his willingness to attend follow-up appointments and adhere to proper cervical collar use, Mr. N becomes more cooperative with psychiatric care as his stay continues, and he is psychiatrically cleared for discharge 1 month after admission. Discharge plans include attending an intensive outpatient program, continuing the inpatient psychiatric medication regimen, participating in regular outpatient psychiatric follow-up, as well as following up with orthopedic surgery, neurosurgery, podiatry, and ear, nose, and throat for medical conditions.
Bottom Line
Agitated depression is a mixed state that includes features of depression and manic/hypomanic symptoms. Diagnosis and treatment can be challenging because symptoms of agitated depression overlap with bipolar disorder and antidepressants are contraindicated. In a patient with a traumatic brain injury, pharmacotherapy that provides neuroprotection is a priority.
Related Resources
- Ramaswamy S, Driscoll D, Rodriguez A, et al. Nutraceuticals for traumatic brain injury: should you recommend their use? Current Psychiatry. 2017;16(7):34-38,40,41-45.
- Sampogna G, Del Vecchio V, Giallonardo V, et al. Diagnosis, clinical features, and therapeutic implications of agitated depression. Psychiatr Clin North Am. 2020;43(1):47-57. doi: 10.1016/j.psc.2019.10.011
Drug Brand Names
Amantadine • Gocovri
Aripiprazole • Abilify
Asenapine • Saphris
Brexpiprazole • Rexulti
Buspirone • BuSpar
Carbamazepine • Tegretol
Cariprazine • Vraylar
Clozapine • Clozaril
Dexmedetomidine • Igalmi
Diazepam • Valium
Donepezil • Aricept
Gabapentin • Neurontin
Haloperidol • Haldol
Ketamine • Ketalar
Lacosamide • Vimpat
Lamotrigine • Lamictal
Levetiracetam • Keppra
Lithium • Lithobid
Lorazepam • Ativan
Lurasidone • Latuda
Memantine • Namenda
Methylphenidate • Concerta
Mirtazapine • Remeron
Olanzapine • Zyprexa
Oxcarbazepine • Trileptal
Paliperidone • Invega
Phenytoin • Dilantin
Pramipexole • Mirapex
Pregabalin • Lyrica
Quetiapine • Seroquel
Risperidone • Risperdal
Trazodone • Oleptro
Valproic acid • Depakene
Ziprasidone • Geodon
Zolpidem • Ambien
Zonisamide • Zonegran
1. American Psychiatric Association. Diagnostic and Statistical Manual of Mental Disorders. 5th ed, text revision. American Psychiatric Association; 2022.
2. Serra F, Gordon‐Smith K, Perry A, et al. Agitated depression in bipolar disorder. Bipolar Disord. 2019;21(6):547-555. doi:10.1111/bdi.12778
3. Meresh E, Daniels D, Owens JH, et al. Psychotropics and neuroprotection: literature review and case series report. OBM Neurobiol. 2020;4(1). doi:10.21926/obm.neurobiol.2001048
4. Williamson DR, Frenette AJ, Burry L, et al. Pharmacological interventions for agitation in patients with traumatic brain injury: protocol for a systematic review and meta-analysis. Syst Rev. 2016;5(1):193. doi:10.1186/s13643-016-0374-6
5. DeBattista C, Solomon A, Arnow B, et al. The efficacy of divalproex sodium in the treatment of agitation associated with major depression. J Clin Psychopharmacol. 2005;25(5):476-479. doi:10.1097/01.jcp.0000177552.21338.b0
6. Longo LP, Campbell T, Hubatch, S. Divalproex sodium (Depakote) for alcohol withdrawal and relapse prevention. J Addict Dis. 2002;21(2):55-64. doi:10.1300/J069v21n02_05
7. Sher Y, Cramer ACM, Ament A, et al. Valproic acid for treatment of hyperactive or mixed delirium: rationale and literature review. Psychosomatics. 2015;56(6):615-625. doi:10.1016/j.psym.2015.09.008
1. American Psychiatric Association. Diagnostic and Statistical Manual of Mental Disorders. 5th ed, text revision. American Psychiatric Association; 2022.
2. Serra F, Gordon‐Smith K, Perry A, et al. Agitated depression in bipolar disorder. Bipolar Disord. 2019;21(6):547-555. doi:10.1111/bdi.12778
3. Meresh E, Daniels D, Owens JH, et al. Psychotropics and neuroprotection: literature review and case series report. OBM Neurobiol. 2020;4(1). doi:10.21926/obm.neurobiol.2001048
4. Williamson DR, Frenette AJ, Burry L, et al. Pharmacological interventions for agitation in patients with traumatic brain injury: protocol for a systematic review and meta-analysis. Syst Rev. 2016;5(1):193. doi:10.1186/s13643-016-0374-6
5. DeBattista C, Solomon A, Arnow B, et al. The efficacy of divalproex sodium in the treatment of agitation associated with major depression. J Clin Psychopharmacol. 2005;25(5):476-479. doi:10.1097/01.jcp.0000177552.21338.b0
6. Longo LP, Campbell T, Hubatch, S. Divalproex sodium (Depakote) for alcohol withdrawal and relapse prevention. J Addict Dis. 2002;21(2):55-64. doi:10.1300/J069v21n02_05
7. Sher Y, Cramer ACM, Ament A, et al. Valproic acid for treatment of hyperactive or mixed delirium: rationale and literature review. Psychosomatics. 2015;56(6):615-625. doi:10.1016/j.psym.2015.09.008
How to avoid abandonment claims when terminating care
Psychiatric clinicians may unilaterally decide to end a treatment relationship with a patient when the relationship is no longer therapeutic, such as when the patient does not adhere to treatment, repeatedly misses appointments, exhibits abusive behaviors, or fails to pay for treatment.1 Claims of abandonment can arise if ending the treatment relationship is not executed properly. Abandonment is the termination of a treatment relationship with a patient who remains in need of treatment, has no suitable substitute treatment, and subsequently experiences damages as a result of the termination.2 When a patient terminates a treatment relationship, there are no legal bases for abandonment claims.3 In this article, I provide a few practical tips for properly terminating the doctor-patient relationship to limit the likelihood of claims of abandonment.
Know your jurisdiction’s requirements for terminating the relationship. Each state has its own legal definition of a doctor-patient relationship as well as requirements for ending it. Abandonment claims are unfounded in the absence of a doctor-patient relationship.3 Contact the appropriate licensing board to determine what your state’s regulatory requirements are. If necessary, consult with your attorney or a risk management professional for guidance.4
Communicate clearly. Communicate with your patient about the end of the treatment relationship in a clear and consistent manner, both verbally and in writing, because a termination should be viewed as a formal, documented event.3 Except in situations requiring immediate termination, psychiatric clinicians should inform the patient about the reason(s) for termination,4 the need for continued treatment,3 and the type of recommended treatment.3 This discussion should be summarized in a termination letter given to the patient that includes termination language, referral sources, the end date of treatment, and a request for authorization to release a copy of the patient’s medical records to their new clinician.3,4
Give adequate time, set boundaries, and document. Thirty days is generally considered adequate time for a patient to find a new clinician,5 unless the patient lives in an area where there is a shortage of psychiatric clinicians, in which case a longer time period would be appropriate.3 Ensure your patient has a sufficient supply of medication(s) until they establish care with a new clinician.4 Offer to provide emergency care for a reasonable period of time during the termination process unless a safety concern requires immediate termination.4 Avoid situations in which the patient attempts to re-enter your care. Document the reason for the termination in your progress notes and keep a copy of the termination letter in the patient’s medical record.4
1. Mossman D. ‘Firing’ a patient: may a psychiatrist unilaterally terminate care? Current Psychiatry. 2010;9(12):18,20,22,29.
2. Van Susteren L. Psychiatric abandonment: pitfalls and prevention. Psychiatric Times. 2001;18(8). Accessed April 30, 2023. https://www.psychiatrictimes.com/view/psychiatric-abandonment-pitfalls-and-prevention
3. Stankowski J, Sorrentino R. Abandonment and unnecessary commitment. In: Ash P, Frierson RL, Hatters Friedman S, eds. Malpractice and Liability in Psychiatry. Springer Nature Publishing; 2022:129-135.
4. Funicelli A. Avoiding abandonment claim: how to properly terminate patients from your practice. Psychiatric News. 2022;57(12):13,41. https://psychnews.psychiatryonline.org/doi/10.1176/appi.pn.2022.12.12.23
5. American Psychiatric Association. APA Quick Practice Guide: Ending the Physician/Patient Relationship. 2014. Accessed April 30, 2023. https://www.psychiatry.org/File%20Library/Psychiatrists/Practice/Practice-Management/Practice-Management-Guides/GeneralIssues-terminating-patient-relationships.pdf
Psychiatric clinicians may unilaterally decide to end a treatment relationship with a patient when the relationship is no longer therapeutic, such as when the patient does not adhere to treatment, repeatedly misses appointments, exhibits abusive behaviors, or fails to pay for treatment.1 Claims of abandonment can arise if ending the treatment relationship is not executed properly. Abandonment is the termination of a treatment relationship with a patient who remains in need of treatment, has no suitable substitute treatment, and subsequently experiences damages as a result of the termination.2 When a patient terminates a treatment relationship, there are no legal bases for abandonment claims.3 In this article, I provide a few practical tips for properly terminating the doctor-patient relationship to limit the likelihood of claims of abandonment.
Know your jurisdiction’s requirements for terminating the relationship. Each state has its own legal definition of a doctor-patient relationship as well as requirements for ending it. Abandonment claims are unfounded in the absence of a doctor-patient relationship.3 Contact the appropriate licensing board to determine what your state’s regulatory requirements are. If necessary, consult with your attorney or a risk management professional for guidance.4
Communicate clearly. Communicate with your patient about the end of the treatment relationship in a clear and consistent manner, both verbally and in writing, because a termination should be viewed as a formal, documented event.3 Except in situations requiring immediate termination, psychiatric clinicians should inform the patient about the reason(s) for termination,4 the need for continued treatment,3 and the type of recommended treatment.3 This discussion should be summarized in a termination letter given to the patient that includes termination language, referral sources, the end date of treatment, and a request for authorization to release a copy of the patient’s medical records to their new clinician.3,4
Give adequate time, set boundaries, and document. Thirty days is generally considered adequate time for a patient to find a new clinician,5 unless the patient lives in an area where there is a shortage of psychiatric clinicians, in which case a longer time period would be appropriate.3 Ensure your patient has a sufficient supply of medication(s) until they establish care with a new clinician.4 Offer to provide emergency care for a reasonable period of time during the termination process unless a safety concern requires immediate termination.4 Avoid situations in which the patient attempts to re-enter your care. Document the reason for the termination in your progress notes and keep a copy of the termination letter in the patient’s medical record.4
Psychiatric clinicians may unilaterally decide to end a treatment relationship with a patient when the relationship is no longer therapeutic, such as when the patient does not adhere to treatment, repeatedly misses appointments, exhibits abusive behaviors, or fails to pay for treatment.1 Claims of abandonment can arise if ending the treatment relationship is not executed properly. Abandonment is the termination of a treatment relationship with a patient who remains in need of treatment, has no suitable substitute treatment, and subsequently experiences damages as a result of the termination.2 When a patient terminates a treatment relationship, there are no legal bases for abandonment claims.3 In this article, I provide a few practical tips for properly terminating the doctor-patient relationship to limit the likelihood of claims of abandonment.
Know your jurisdiction’s requirements for terminating the relationship. Each state has its own legal definition of a doctor-patient relationship as well as requirements for ending it. Abandonment claims are unfounded in the absence of a doctor-patient relationship.3 Contact the appropriate licensing board to determine what your state’s regulatory requirements are. If necessary, consult with your attorney or a risk management professional for guidance.4
Communicate clearly. Communicate with your patient about the end of the treatment relationship in a clear and consistent manner, both verbally and in writing, because a termination should be viewed as a formal, documented event.3 Except in situations requiring immediate termination, psychiatric clinicians should inform the patient about the reason(s) for termination,4 the need for continued treatment,3 and the type of recommended treatment.3 This discussion should be summarized in a termination letter given to the patient that includes termination language, referral sources, the end date of treatment, and a request for authorization to release a copy of the patient’s medical records to their new clinician.3,4
Give adequate time, set boundaries, and document. Thirty days is generally considered adequate time for a patient to find a new clinician,5 unless the patient lives in an area where there is a shortage of psychiatric clinicians, in which case a longer time period would be appropriate.3 Ensure your patient has a sufficient supply of medication(s) until they establish care with a new clinician.4 Offer to provide emergency care for a reasonable period of time during the termination process unless a safety concern requires immediate termination.4 Avoid situations in which the patient attempts to re-enter your care. Document the reason for the termination in your progress notes and keep a copy of the termination letter in the patient’s medical record.4
1. Mossman D. ‘Firing’ a patient: may a psychiatrist unilaterally terminate care? Current Psychiatry. 2010;9(12):18,20,22,29.
2. Van Susteren L. Psychiatric abandonment: pitfalls and prevention. Psychiatric Times. 2001;18(8). Accessed April 30, 2023. https://www.psychiatrictimes.com/view/psychiatric-abandonment-pitfalls-and-prevention
3. Stankowski J, Sorrentino R. Abandonment and unnecessary commitment. In: Ash P, Frierson RL, Hatters Friedman S, eds. Malpractice and Liability in Psychiatry. Springer Nature Publishing; 2022:129-135.
4. Funicelli A. Avoiding abandonment claim: how to properly terminate patients from your practice. Psychiatric News. 2022;57(12):13,41. https://psychnews.psychiatryonline.org/doi/10.1176/appi.pn.2022.12.12.23
5. American Psychiatric Association. APA Quick Practice Guide: Ending the Physician/Patient Relationship. 2014. Accessed April 30, 2023. https://www.psychiatry.org/File%20Library/Psychiatrists/Practice/Practice-Management/Practice-Management-Guides/GeneralIssues-terminating-patient-relationships.pdf
1. Mossman D. ‘Firing’ a patient: may a psychiatrist unilaterally terminate care? Current Psychiatry. 2010;9(12):18,20,22,29.
2. Van Susteren L. Psychiatric abandonment: pitfalls and prevention. Psychiatric Times. 2001;18(8). Accessed April 30, 2023. https://www.psychiatrictimes.com/view/psychiatric-abandonment-pitfalls-and-prevention
3. Stankowski J, Sorrentino R. Abandonment and unnecessary commitment. In: Ash P, Frierson RL, Hatters Friedman S, eds. Malpractice and Liability in Psychiatry. Springer Nature Publishing; 2022:129-135.
4. Funicelli A. Avoiding abandonment claim: how to properly terminate patients from your practice. Psychiatric News. 2022;57(12):13,41. https://psychnews.psychiatryonline.org/doi/10.1176/appi.pn.2022.12.12.23
5. American Psychiatric Association. APA Quick Practice Guide: Ending the Physician/Patient Relationship. 2014. Accessed April 30, 2023. https://www.psychiatry.org/File%20Library/Psychiatrists/Practice/Practice-Management/Practice-Management-Guides/GeneralIssues-terminating-patient-relationships.pdf
Crafting a dynamic learning environment during psychiatry clerkships
Editor’s note: Readers’ Forum is a department for correspondence from readers that is not in response to articles published in
Creating an optimal learning environment for medical students studying psychiatry is essential for their growth and development. Over the last 25 years, I have worked with hundreds of medical students in a busy urban emergency department (ED), and I have developed a style that has worked well for them and for me. A supportive, engaging atmosphere can significantly enhance students’ understanding of psychiatric conditions, therapeutic approaches, and patient care. To ensure a productive and inspiring learning experience, educators should consider several key factors.
The educators
Faculty physicians should invest themselves in the students’ individual growth and aspirations by providing personalized guidance that caters to each student’s goals and challenges.1 Educators must also embody a passion for psychiatry. I’ve found that integrating a lighthearted and humorous approach to my teaching style can relieve stress and enhance learning. I’ve also found it crucial to demonstrate empathy and effective communication skills that students can emulate in their professional development.2 Encourage students to take an active role in their learning process by engaging in clinical discussions and decision-making. Lastly, providing regular assessments and constructive feedback in a supportive manner allows students to better understand their strengths and weaknesses, and to continually improve their knowledge and skills.3
The students
Encourage students to fully express their unique personalities, perspectives, and learning styles. This diversity can fuel creativity and promote an atmosphere of inclusivity and enhanced learning. Teach students to recognize the value in each patient encounter, because each offers a unique opportunity to deepen their understanding of psychiatric conditions.4 Instead of being mere observers, students should actively participate in their education by involving themselves in clinical discussions, treatment planning, and decision-making.
The environment
A supportive, inclusive learning environment should foster diversity, inclusivity, and collaborative learning by creating an engaging atmosphere in which students can express themselves. In my experience, a sense of relaxed focus can help alleviate stress and enhance creativity. Emphasize a patient-centered approach to instill empathy and compassion in students and enrich their understanding of psychiatric conditions.4
The peers
Encourage students to engage in peer feedback, which will provide their fellow trainees additional perspective on their performance and offer an avenue for constructive criticism and improvement.3 Promoting collaborative learning will foster a sense of camaraderie, help students share their diverse perspectives, and enhance the learning experience. Peers also play a crucial role in reinforcing positive behaviors and attitudes.
My extensive experience educating medical students studying psychiatry in a busy ED has taught me that creating an exceptional learning environment requires understanding the role of educators, students, the environment, and peers. By implementing these principles, educators can contribute to their students’ professional growth, equipping them with the skills and mindset necessary to become a compassionate, competent, effective physician.
1. Sutkin G, Wager E, Harris I, et al. What makes a good clinical teacher in medicine? A review of the literature. Acad Med. 2008;83(5):452-466. doi:10.1097/ACM.0b013e31816bee61
2. Passi V, Johnson S, Peile E, et al. Doctor role modelling in medical education: BEME Guide No. 27. Med Teach. 2013;35(9):e1422-e1436. doi:10.3109/0142159X.2013.806982
3. Lerchenfeldt S, Mi M, Eng M. The utilization of peer feedback during collaborative learning in undergraduate medical education: a systematic review. BMC Med Educ. 2019;19(1):321. doi:10.1186/s12909-019-1755-z
4. Bleakley A, Bligh J. Students learning from patients: let’s get real in medical education. Adv Health Sci Educ Theory Pract. 2008;13(1):89-107. doi:10.1007/s10459-006-9028-0
Editor’s note: Readers’ Forum is a department for correspondence from readers that is not in response to articles published in
Creating an optimal learning environment for medical students studying psychiatry is essential for their growth and development. Over the last 25 years, I have worked with hundreds of medical students in a busy urban emergency department (ED), and I have developed a style that has worked well for them and for me. A supportive, engaging atmosphere can significantly enhance students’ understanding of psychiatric conditions, therapeutic approaches, and patient care. To ensure a productive and inspiring learning experience, educators should consider several key factors.
The educators
Faculty physicians should invest themselves in the students’ individual growth and aspirations by providing personalized guidance that caters to each student’s goals and challenges.1 Educators must also embody a passion for psychiatry. I’ve found that integrating a lighthearted and humorous approach to my teaching style can relieve stress and enhance learning. I’ve also found it crucial to demonstrate empathy and effective communication skills that students can emulate in their professional development.2 Encourage students to take an active role in their learning process by engaging in clinical discussions and decision-making. Lastly, providing regular assessments and constructive feedback in a supportive manner allows students to better understand their strengths and weaknesses, and to continually improve their knowledge and skills.3
The students
Encourage students to fully express their unique personalities, perspectives, and learning styles. This diversity can fuel creativity and promote an atmosphere of inclusivity and enhanced learning. Teach students to recognize the value in each patient encounter, because each offers a unique opportunity to deepen their understanding of psychiatric conditions.4 Instead of being mere observers, students should actively participate in their education by involving themselves in clinical discussions, treatment planning, and decision-making.
The environment
A supportive, inclusive learning environment should foster diversity, inclusivity, and collaborative learning by creating an engaging atmosphere in which students can express themselves. In my experience, a sense of relaxed focus can help alleviate stress and enhance creativity. Emphasize a patient-centered approach to instill empathy and compassion in students and enrich their understanding of psychiatric conditions.4
The peers
Encourage students to engage in peer feedback, which will provide their fellow trainees additional perspective on their performance and offer an avenue for constructive criticism and improvement.3 Promoting collaborative learning will foster a sense of camaraderie, help students share their diverse perspectives, and enhance the learning experience. Peers also play a crucial role in reinforcing positive behaviors and attitudes.
My extensive experience educating medical students studying psychiatry in a busy ED has taught me that creating an exceptional learning environment requires understanding the role of educators, students, the environment, and peers. By implementing these principles, educators can contribute to their students’ professional growth, equipping them with the skills and mindset necessary to become a compassionate, competent, effective physician.
Editor’s note: Readers’ Forum is a department for correspondence from readers that is not in response to articles published in
Creating an optimal learning environment for medical students studying psychiatry is essential for their growth and development. Over the last 25 years, I have worked with hundreds of medical students in a busy urban emergency department (ED), and I have developed a style that has worked well for them and for me. A supportive, engaging atmosphere can significantly enhance students’ understanding of psychiatric conditions, therapeutic approaches, and patient care. To ensure a productive and inspiring learning experience, educators should consider several key factors.
The educators
Faculty physicians should invest themselves in the students’ individual growth and aspirations by providing personalized guidance that caters to each student’s goals and challenges.1 Educators must also embody a passion for psychiatry. I’ve found that integrating a lighthearted and humorous approach to my teaching style can relieve stress and enhance learning. I’ve also found it crucial to demonstrate empathy and effective communication skills that students can emulate in their professional development.2 Encourage students to take an active role in their learning process by engaging in clinical discussions and decision-making. Lastly, providing regular assessments and constructive feedback in a supportive manner allows students to better understand their strengths and weaknesses, and to continually improve their knowledge and skills.3
The students
Encourage students to fully express their unique personalities, perspectives, and learning styles. This diversity can fuel creativity and promote an atmosphere of inclusivity and enhanced learning. Teach students to recognize the value in each patient encounter, because each offers a unique opportunity to deepen their understanding of psychiatric conditions.4 Instead of being mere observers, students should actively participate in their education by involving themselves in clinical discussions, treatment planning, and decision-making.
The environment
A supportive, inclusive learning environment should foster diversity, inclusivity, and collaborative learning by creating an engaging atmosphere in which students can express themselves. In my experience, a sense of relaxed focus can help alleviate stress and enhance creativity. Emphasize a patient-centered approach to instill empathy and compassion in students and enrich their understanding of psychiatric conditions.4
The peers
Encourage students to engage in peer feedback, which will provide their fellow trainees additional perspective on their performance and offer an avenue for constructive criticism and improvement.3 Promoting collaborative learning will foster a sense of camaraderie, help students share their diverse perspectives, and enhance the learning experience. Peers also play a crucial role in reinforcing positive behaviors and attitudes.
My extensive experience educating medical students studying psychiatry in a busy ED has taught me that creating an exceptional learning environment requires understanding the role of educators, students, the environment, and peers. By implementing these principles, educators can contribute to their students’ professional growth, equipping them with the skills and mindset necessary to become a compassionate, competent, effective physician.
1. Sutkin G, Wager E, Harris I, et al. What makes a good clinical teacher in medicine? A review of the literature. Acad Med. 2008;83(5):452-466. doi:10.1097/ACM.0b013e31816bee61
2. Passi V, Johnson S, Peile E, et al. Doctor role modelling in medical education: BEME Guide No. 27. Med Teach. 2013;35(9):e1422-e1436. doi:10.3109/0142159X.2013.806982
3. Lerchenfeldt S, Mi M, Eng M. The utilization of peer feedback during collaborative learning in undergraduate medical education: a systematic review. BMC Med Educ. 2019;19(1):321. doi:10.1186/s12909-019-1755-z
4. Bleakley A, Bligh J. Students learning from patients: let’s get real in medical education. Adv Health Sci Educ Theory Pract. 2008;13(1):89-107. doi:10.1007/s10459-006-9028-0
1. Sutkin G, Wager E, Harris I, et al. What makes a good clinical teacher in medicine? A review of the literature. Acad Med. 2008;83(5):452-466. doi:10.1097/ACM.0b013e31816bee61
2. Passi V, Johnson S, Peile E, et al. Doctor role modelling in medical education: BEME Guide No. 27. Med Teach. 2013;35(9):e1422-e1436. doi:10.3109/0142159X.2013.806982
3. Lerchenfeldt S, Mi M, Eng M. The utilization of peer feedback during collaborative learning in undergraduate medical education: a systematic review. BMC Med Educ. 2019;19(1):321. doi:10.1186/s12909-019-1755-z
4. Bleakley A, Bligh J. Students learning from patients: let’s get real in medical education. Adv Health Sci Educ Theory Pract. 2008;13(1):89-107. doi:10.1007/s10459-006-9028-0
More on prescribing controlled substances
I was disheartened with the June 2023 issue of
The benzodiazepine pharmacology discussed in this article is interesting, but it would be helpful if it had been integrated within a much more extensive discussion of careful prescribing practices. In 2020, the FDA updated the boxed warning to alert prescribers to the serious risks of abuse, addiction, physical dependence, and withdrawal reactions associated with benzodiazepines.2 I would hope that an article on benzodiazepines would provide more discussion and guidance surrounding these important issues.
The June 2023 issue also included “High-dose stimulants for adult ADHD” (p. 34-39, doi:10.12788/cp.0366). This article provided esoteric advice on managing stimulant therapy in the setting of Roux-en-Y gastric bypass surgery, yet I would regard stimulant misuse as a far more common and pressing issue.3,4 The recent Drug Enforcement Administration investigation of telehealth stimulant prescribing is a notable example of this problem.5
The patient discussed in this article was receiving large doses of stimulants for a purported case of refractory attention-deficit/hyperactivity disorder (ADHD). The article provided a sparse differential diagnosis for the patient’s intractable symptoms. While rapid metabolism may be an explanation, I would also like to know how the authors ruled out physiological dependence and/or addiction to a controlled substance. How was misuse excluded? Was urine drug testing (UDS) performed? UDS is highly irregular among prescribers,6 which suggests that practices for detecting covert substance abuse and stimulant misuse are inadequate. Wouldn’t such investigations be fundamental to ethical stimulant prescribing?
Jeff Sanders, MD, PhD
Atlanta, Georgia
References
1. Centers for Disease Control and Prevention. Trends in nonfatal and fatal overdoses involving benzodiazepines—38 states and the District of Columbia, 2019-2020. Accessed August 9, 2023. https://www.cdc.gov/mmwr/volumes/70/wr/mm7034a2.htm
2. US Food & Drug Administration. FDA requiring boxed warning updated to improve safe use of benzodiazepine drug class. Accessed August 14, 2023. https://www.fda.gov/drugs/drug-safety-and-availability/fda-requiring-boxed-warning-updated-improve-safe-use-benzodiazepine-drug-class
3. McCabe SE, Schulenberg JE, Wilens TE, et al. Prescription stimulant medical and nonmedical use among US secondary school students, 2005 to 2020. JAMA Netw Open. 2023;6(4):e238707. doi:10.1001/jamanetworkopen.2023.8707
4. US Food & Drug Administration. FDA updating warnings to improve safe use of prescription stimulants used to treat ADHD and other conditions. Accessed August 14, 2023. https://www.fda.gov/safety/medical-product-safety-information/fda-updating-warnings-improve-safe-use-prescription-stimulants-used-treat-adhd-and-other-conditions
5. Vaidya A. Report: telehealth company’s prescribing practices come under DEA scrutiny. September 16, 2022. Accessed August 9, 2023. https://mhealthintelligence.com/news/report-telehealth-company-dones-prescribing-practices-come-under-dea-scrutiny
6. Zionts A. Some ADHD patients are drug-tested often, while others are never asked. Kaiser Health News. March 25, 2023. Accessed August 9, 2023. https://www.nbcnews.com/news/amp/rcna76330
Continue to: Drs. Stimpfl and Strawn respond
Drs. Stimpfl and Strawn respond
We thank Dr. Sanders for highlighting the need for clinical equipoise in considering the risks and benefits of medications—something that is true for benzodiazepines, antipsychotics, antidepressants, and in fact all medications. He reminds us that the risks of misuse, dependence, and withdrawal associated with benzodiazepines led to a boxed warning in September 2020 and highlights recent trends of fatal and nonfatal benzodiazepine overdose, especially when combined with opiates.
Our article, which aimed to educate clinicians on benzodiazepine pharmacology and patient-specific factors influencing benzodiazepine selection and dosing, did not focus significantly on the risks associated with benzodiazepines. We do encourage careful and individualized benzodiazepine prescribing. However, we wish to remind our colleagues that benzodiazepines, while associated with risks, continue to have utility in acute and periprocedural settings, and remain an important treatment option for patients with panic disorder, generalized anxiety disorder (especially while waiting for other medications to take effect), catatonia, seizure disorders, and alcohol withdrawal.
We agree that patient-specific risk assessment is essential, as some patients benefit from benzodiazepines despite the risks. However, we also acknowledge that some individuals are at higher risk for adverse outcomes, including those with concurrent opiate use or who are prescribed other sedative-hypnotics; older adults and those with neurocognitive disorders; and patients susceptible to respiratory depression due to other medical reasons (eg, myasthenia gravis, sleep apnea, and chronic obstructive pulmonary disease). Further, we agree that benzodiazepine use during pregnancy is generally not advised due to the risks of neonatal hypotonia and neonatal withdrawal syndrome1 as well as a possible risk of cleft palate that has been reported in some studies.2 Finally, paradoxical reactions may be more common at the extremes of age and in patients with intellectual disability or personality disorders.3,4
Patient characteristics that have been associated with a higher risk of benzodiazepine use disorder include lower education/income, unemployment, having another substance use disorder, and severe psychopathology.5 In some studies, using benzodiazepines for prolonged periods at high doses as well as using those with a rapid onset of action was associated with an increased risk of benzodiazepine use disorder.5-7
Ultimately, we concur with Dr. Sanders on the perils of the “irresponsible use” of medication and emphasize the need for discernment when choosing treatments to avoid rashly discarding an effective remedy while attempting to mitigate all conceivable risks.
Julia Stimpfl, MD
Jeffrey R. Strawn, MD
Cincinnati, Ohio
References
1. McElhatton PR. The effects of benzodiazepine use during pregnancy and lactation. Reprod Toxicol. 1994;8(6):461-475. doi:10.1016/0890-6238(94)90029-9
2. Enato E, Moretti M, Koren G. The fetal safety of benzodiazepines: an updated meta-analysis. J Obstet Gynaecol Can. 2011;33(1):46-48. doi:10.1016/S1701-2163(16)34772-7 Erratum in: J Obstet Gynaecol Can. 2011;33(4):319.
3. Hakimi Y, Petitpain N, Pinzani V, et al. Paradoxical adverse drug reactions: descriptive analysis of French reports. Eur J Clin Pharmacol. 2020;76(8):1169-1174. doi:10.1007/s00228-020-02892-2
4. Paton C. Benzodiazepines and disinhibition: a review. Psychiatric Bulletin. 2002;26(12):460-462. doi:10.1192/pb.26.12.460
5. Fride Tvete I, Bjørner T, Skomedal T. Risk factors for excessive benzodiazepine use in a working age population: a nationwide 5-year survey in Norway. Scand J Prim Health Care. 2015;33(4):252-259. doi:10.3109/02813432.2015.1117282
6. Griffiths RR, Johnson MW. Relative abuse liability of hypnotic drugs: a conceptual framework and algorithm for differentiating among compounds. J Clin Psychiatry. 2005;66 Suppl 9:31-41.
7. Kan CC, Hilberink SR, Breteler MH. Determination of the main risk factors for benzodiazepine dependence using a multivariate and multidimensional approach. Compr Psychiatry. 2004;45(2):88-94. doi:10.1016/j.comppsych.2003.12.007
Continue to: Drs. Sarma and Grady respond
Drs. Sarma and Grady respond
Dr. Sanders’ letter highlights the potential caveats associated with prescribing controlled substances. We agree that our short case summary includes numerous interesting elements, each of which would be worthy of further exploration and discussion. Our choice was to highlight the patient history of bariatric surgery and use this as a springboard into a review of stimulants, including the newest formulations for ADHD. For more than 1 year, many generic stimulants have been in short supply, and patients and clinicians have been seeking other therapeutic options. Given this background and with newer, branded stimulant use becoming more commonplace, we believe our article was useful and timely.
Our original intent had been to include an example of a controlled substance agreement. Regrettably, there was simply not enough space for this document or the additional discussion that its inclusion would deem necessary. Nevertheless, had the May 2023 FDA requirement for manufacturers to update the labeling of prescription stimulants1 to clarify misuse and abuse been published before our article’s final revision, we would have mentioned it and provided the appropriate link.
Subbu J. Sarma, MD, FAPA
Kansas City, Missouri
Sarah E. Grady, PharmD, BCPS, BCPP
Des Moines, Iowa
References
1. US Food & Drug Administration. FDA requires updates to clarify labeling of prescription stimulants used to treat ADHD and other conditions. Accessed August 9, 2023. https://www.fda.gov/drugs/drug-safety-and-availability/fda-requires-updates-clarify-labeling-prescription-stimulants-used-treat-adhd-and-other-conditions
I was disheartened with the June 2023 issue of
The benzodiazepine pharmacology discussed in this article is interesting, but it would be helpful if it had been integrated within a much more extensive discussion of careful prescribing practices. In 2020, the FDA updated the boxed warning to alert prescribers to the serious risks of abuse, addiction, physical dependence, and withdrawal reactions associated with benzodiazepines.2 I would hope that an article on benzodiazepines would provide more discussion and guidance surrounding these important issues.
The June 2023 issue also included “High-dose stimulants for adult ADHD” (p. 34-39, doi:10.12788/cp.0366). This article provided esoteric advice on managing stimulant therapy in the setting of Roux-en-Y gastric bypass surgery, yet I would regard stimulant misuse as a far more common and pressing issue.3,4 The recent Drug Enforcement Administration investigation of telehealth stimulant prescribing is a notable example of this problem.5
The patient discussed in this article was receiving large doses of stimulants for a purported case of refractory attention-deficit/hyperactivity disorder (ADHD). The article provided a sparse differential diagnosis for the patient’s intractable symptoms. While rapid metabolism may be an explanation, I would also like to know how the authors ruled out physiological dependence and/or addiction to a controlled substance. How was misuse excluded? Was urine drug testing (UDS) performed? UDS is highly irregular among prescribers,6 which suggests that practices for detecting covert substance abuse and stimulant misuse are inadequate. Wouldn’t such investigations be fundamental to ethical stimulant prescribing?
Jeff Sanders, MD, PhD
Atlanta, Georgia
References
1. Centers for Disease Control and Prevention. Trends in nonfatal and fatal overdoses involving benzodiazepines—38 states and the District of Columbia, 2019-2020. Accessed August 9, 2023. https://www.cdc.gov/mmwr/volumes/70/wr/mm7034a2.htm
2. US Food & Drug Administration. FDA requiring boxed warning updated to improve safe use of benzodiazepine drug class. Accessed August 14, 2023. https://www.fda.gov/drugs/drug-safety-and-availability/fda-requiring-boxed-warning-updated-improve-safe-use-benzodiazepine-drug-class
3. McCabe SE, Schulenberg JE, Wilens TE, et al. Prescription stimulant medical and nonmedical use among US secondary school students, 2005 to 2020. JAMA Netw Open. 2023;6(4):e238707. doi:10.1001/jamanetworkopen.2023.8707
4. US Food & Drug Administration. FDA updating warnings to improve safe use of prescription stimulants used to treat ADHD and other conditions. Accessed August 14, 2023. https://www.fda.gov/safety/medical-product-safety-information/fda-updating-warnings-improve-safe-use-prescription-stimulants-used-treat-adhd-and-other-conditions
5. Vaidya A. Report: telehealth company’s prescribing practices come under DEA scrutiny. September 16, 2022. Accessed August 9, 2023. https://mhealthintelligence.com/news/report-telehealth-company-dones-prescribing-practices-come-under-dea-scrutiny
6. Zionts A. Some ADHD patients are drug-tested often, while others are never asked. Kaiser Health News. March 25, 2023. Accessed August 9, 2023. https://www.nbcnews.com/news/amp/rcna76330
Continue to: Drs. Stimpfl and Strawn respond
Drs. Stimpfl and Strawn respond
We thank Dr. Sanders for highlighting the need for clinical equipoise in considering the risks and benefits of medications—something that is true for benzodiazepines, antipsychotics, antidepressants, and in fact all medications. He reminds us that the risks of misuse, dependence, and withdrawal associated with benzodiazepines led to a boxed warning in September 2020 and highlights recent trends of fatal and nonfatal benzodiazepine overdose, especially when combined with opiates.
Our article, which aimed to educate clinicians on benzodiazepine pharmacology and patient-specific factors influencing benzodiazepine selection and dosing, did not focus significantly on the risks associated with benzodiazepines. We do encourage careful and individualized benzodiazepine prescribing. However, we wish to remind our colleagues that benzodiazepines, while associated with risks, continue to have utility in acute and periprocedural settings, and remain an important treatment option for patients with panic disorder, generalized anxiety disorder (especially while waiting for other medications to take effect), catatonia, seizure disorders, and alcohol withdrawal.
We agree that patient-specific risk assessment is essential, as some patients benefit from benzodiazepines despite the risks. However, we also acknowledge that some individuals are at higher risk for adverse outcomes, including those with concurrent opiate use or who are prescribed other sedative-hypnotics; older adults and those with neurocognitive disorders; and patients susceptible to respiratory depression due to other medical reasons (eg, myasthenia gravis, sleep apnea, and chronic obstructive pulmonary disease). Further, we agree that benzodiazepine use during pregnancy is generally not advised due to the risks of neonatal hypotonia and neonatal withdrawal syndrome1 as well as a possible risk of cleft palate that has been reported in some studies.2 Finally, paradoxical reactions may be more common at the extremes of age and in patients with intellectual disability or personality disorders.3,4
Patient characteristics that have been associated with a higher risk of benzodiazepine use disorder include lower education/income, unemployment, having another substance use disorder, and severe psychopathology.5 In some studies, using benzodiazepines for prolonged periods at high doses as well as using those with a rapid onset of action was associated with an increased risk of benzodiazepine use disorder.5-7
Ultimately, we concur with Dr. Sanders on the perils of the “irresponsible use” of medication and emphasize the need for discernment when choosing treatments to avoid rashly discarding an effective remedy while attempting to mitigate all conceivable risks.
Julia Stimpfl, MD
Jeffrey R. Strawn, MD
Cincinnati, Ohio
References
1. McElhatton PR. The effects of benzodiazepine use during pregnancy and lactation. Reprod Toxicol. 1994;8(6):461-475. doi:10.1016/0890-6238(94)90029-9
2. Enato E, Moretti M, Koren G. The fetal safety of benzodiazepines: an updated meta-analysis. J Obstet Gynaecol Can. 2011;33(1):46-48. doi:10.1016/S1701-2163(16)34772-7 Erratum in: J Obstet Gynaecol Can. 2011;33(4):319.
3. Hakimi Y, Petitpain N, Pinzani V, et al. Paradoxical adverse drug reactions: descriptive analysis of French reports. Eur J Clin Pharmacol. 2020;76(8):1169-1174. doi:10.1007/s00228-020-02892-2
4. Paton C. Benzodiazepines and disinhibition: a review. Psychiatric Bulletin. 2002;26(12):460-462. doi:10.1192/pb.26.12.460
5. Fride Tvete I, Bjørner T, Skomedal T. Risk factors for excessive benzodiazepine use in a working age population: a nationwide 5-year survey in Norway. Scand J Prim Health Care. 2015;33(4):252-259. doi:10.3109/02813432.2015.1117282
6. Griffiths RR, Johnson MW. Relative abuse liability of hypnotic drugs: a conceptual framework and algorithm for differentiating among compounds. J Clin Psychiatry. 2005;66 Suppl 9:31-41.
7. Kan CC, Hilberink SR, Breteler MH. Determination of the main risk factors for benzodiazepine dependence using a multivariate and multidimensional approach. Compr Psychiatry. 2004;45(2):88-94. doi:10.1016/j.comppsych.2003.12.007
Continue to: Drs. Sarma and Grady respond
Drs. Sarma and Grady respond
Dr. Sanders’ letter highlights the potential caveats associated with prescribing controlled substances. We agree that our short case summary includes numerous interesting elements, each of which would be worthy of further exploration and discussion. Our choice was to highlight the patient history of bariatric surgery and use this as a springboard into a review of stimulants, including the newest formulations for ADHD. For more than 1 year, many generic stimulants have been in short supply, and patients and clinicians have been seeking other therapeutic options. Given this background and with newer, branded stimulant use becoming more commonplace, we believe our article was useful and timely.
Our original intent had been to include an example of a controlled substance agreement. Regrettably, there was simply not enough space for this document or the additional discussion that its inclusion would deem necessary. Nevertheless, had the May 2023 FDA requirement for manufacturers to update the labeling of prescription stimulants1 to clarify misuse and abuse been published before our article’s final revision, we would have mentioned it and provided the appropriate link.
Subbu J. Sarma, MD, FAPA
Kansas City, Missouri
Sarah E. Grady, PharmD, BCPS, BCPP
Des Moines, Iowa
References
1. US Food & Drug Administration. FDA requires updates to clarify labeling of prescription stimulants used to treat ADHD and other conditions. Accessed August 9, 2023. https://www.fda.gov/drugs/drug-safety-and-availability/fda-requires-updates-clarify-labeling-prescription-stimulants-used-treat-adhd-and-other-conditions
I was disheartened with the June 2023 issue of
The benzodiazepine pharmacology discussed in this article is interesting, but it would be helpful if it had been integrated within a much more extensive discussion of careful prescribing practices. In 2020, the FDA updated the boxed warning to alert prescribers to the serious risks of abuse, addiction, physical dependence, and withdrawal reactions associated with benzodiazepines.2 I would hope that an article on benzodiazepines would provide more discussion and guidance surrounding these important issues.
The June 2023 issue also included “High-dose stimulants for adult ADHD” (p. 34-39, doi:10.12788/cp.0366). This article provided esoteric advice on managing stimulant therapy in the setting of Roux-en-Y gastric bypass surgery, yet I would regard stimulant misuse as a far more common and pressing issue.3,4 The recent Drug Enforcement Administration investigation of telehealth stimulant prescribing is a notable example of this problem.5
The patient discussed in this article was receiving large doses of stimulants for a purported case of refractory attention-deficit/hyperactivity disorder (ADHD). The article provided a sparse differential diagnosis for the patient’s intractable symptoms. While rapid metabolism may be an explanation, I would also like to know how the authors ruled out physiological dependence and/or addiction to a controlled substance. How was misuse excluded? Was urine drug testing (UDS) performed? UDS is highly irregular among prescribers,6 which suggests that practices for detecting covert substance abuse and stimulant misuse are inadequate. Wouldn’t such investigations be fundamental to ethical stimulant prescribing?
Jeff Sanders, MD, PhD
Atlanta, Georgia
References
1. Centers for Disease Control and Prevention. Trends in nonfatal and fatal overdoses involving benzodiazepines—38 states and the District of Columbia, 2019-2020. Accessed August 9, 2023. https://www.cdc.gov/mmwr/volumes/70/wr/mm7034a2.htm
2. US Food & Drug Administration. FDA requiring boxed warning updated to improve safe use of benzodiazepine drug class. Accessed August 14, 2023. https://www.fda.gov/drugs/drug-safety-and-availability/fda-requiring-boxed-warning-updated-improve-safe-use-benzodiazepine-drug-class
3. McCabe SE, Schulenberg JE, Wilens TE, et al. Prescription stimulant medical and nonmedical use among US secondary school students, 2005 to 2020. JAMA Netw Open. 2023;6(4):e238707. doi:10.1001/jamanetworkopen.2023.8707
4. US Food & Drug Administration. FDA updating warnings to improve safe use of prescription stimulants used to treat ADHD and other conditions. Accessed August 14, 2023. https://www.fda.gov/safety/medical-product-safety-information/fda-updating-warnings-improve-safe-use-prescription-stimulants-used-treat-adhd-and-other-conditions
5. Vaidya A. Report: telehealth company’s prescribing practices come under DEA scrutiny. September 16, 2022. Accessed August 9, 2023. https://mhealthintelligence.com/news/report-telehealth-company-dones-prescribing-practices-come-under-dea-scrutiny
6. Zionts A. Some ADHD patients are drug-tested often, while others are never asked. Kaiser Health News. March 25, 2023. Accessed August 9, 2023. https://www.nbcnews.com/news/amp/rcna76330
Continue to: Drs. Stimpfl and Strawn respond
Drs. Stimpfl and Strawn respond
We thank Dr. Sanders for highlighting the need for clinical equipoise in considering the risks and benefits of medications—something that is true for benzodiazepines, antipsychotics, antidepressants, and in fact all medications. He reminds us that the risks of misuse, dependence, and withdrawal associated with benzodiazepines led to a boxed warning in September 2020 and highlights recent trends of fatal and nonfatal benzodiazepine overdose, especially when combined with opiates.
Our article, which aimed to educate clinicians on benzodiazepine pharmacology and patient-specific factors influencing benzodiazepine selection and dosing, did not focus significantly on the risks associated with benzodiazepines. We do encourage careful and individualized benzodiazepine prescribing. However, we wish to remind our colleagues that benzodiazepines, while associated with risks, continue to have utility in acute and periprocedural settings, and remain an important treatment option for patients with panic disorder, generalized anxiety disorder (especially while waiting for other medications to take effect), catatonia, seizure disorders, and alcohol withdrawal.
We agree that patient-specific risk assessment is essential, as some patients benefit from benzodiazepines despite the risks. However, we also acknowledge that some individuals are at higher risk for adverse outcomes, including those with concurrent opiate use or who are prescribed other sedative-hypnotics; older adults and those with neurocognitive disorders; and patients susceptible to respiratory depression due to other medical reasons (eg, myasthenia gravis, sleep apnea, and chronic obstructive pulmonary disease). Further, we agree that benzodiazepine use during pregnancy is generally not advised due to the risks of neonatal hypotonia and neonatal withdrawal syndrome1 as well as a possible risk of cleft palate that has been reported in some studies.2 Finally, paradoxical reactions may be more common at the extremes of age and in patients with intellectual disability or personality disorders.3,4
Patient characteristics that have been associated with a higher risk of benzodiazepine use disorder include lower education/income, unemployment, having another substance use disorder, and severe psychopathology.5 In some studies, using benzodiazepines for prolonged periods at high doses as well as using those with a rapid onset of action was associated with an increased risk of benzodiazepine use disorder.5-7
Ultimately, we concur with Dr. Sanders on the perils of the “irresponsible use” of medication and emphasize the need for discernment when choosing treatments to avoid rashly discarding an effective remedy while attempting to mitigate all conceivable risks.
Julia Stimpfl, MD
Jeffrey R. Strawn, MD
Cincinnati, Ohio
References
1. McElhatton PR. The effects of benzodiazepine use during pregnancy and lactation. Reprod Toxicol. 1994;8(6):461-475. doi:10.1016/0890-6238(94)90029-9
2. Enato E, Moretti M, Koren G. The fetal safety of benzodiazepines: an updated meta-analysis. J Obstet Gynaecol Can. 2011;33(1):46-48. doi:10.1016/S1701-2163(16)34772-7 Erratum in: J Obstet Gynaecol Can. 2011;33(4):319.
3. Hakimi Y, Petitpain N, Pinzani V, et al. Paradoxical adverse drug reactions: descriptive analysis of French reports. Eur J Clin Pharmacol. 2020;76(8):1169-1174. doi:10.1007/s00228-020-02892-2
4. Paton C. Benzodiazepines and disinhibition: a review. Psychiatric Bulletin. 2002;26(12):460-462. doi:10.1192/pb.26.12.460
5. Fride Tvete I, Bjørner T, Skomedal T. Risk factors for excessive benzodiazepine use in a working age population: a nationwide 5-year survey in Norway. Scand J Prim Health Care. 2015;33(4):252-259. doi:10.3109/02813432.2015.1117282
6. Griffiths RR, Johnson MW. Relative abuse liability of hypnotic drugs: a conceptual framework and algorithm for differentiating among compounds. J Clin Psychiatry. 2005;66 Suppl 9:31-41.
7. Kan CC, Hilberink SR, Breteler MH. Determination of the main risk factors for benzodiazepine dependence using a multivariate and multidimensional approach. Compr Psychiatry. 2004;45(2):88-94. doi:10.1016/j.comppsych.2003.12.007
Continue to: Drs. Sarma and Grady respond
Drs. Sarma and Grady respond
Dr. Sanders’ letter highlights the potential caveats associated with prescribing controlled substances. We agree that our short case summary includes numerous interesting elements, each of which would be worthy of further exploration and discussion. Our choice was to highlight the patient history of bariatric surgery and use this as a springboard into a review of stimulants, including the newest formulations for ADHD. For more than 1 year, many generic stimulants have been in short supply, and patients and clinicians have been seeking other therapeutic options. Given this background and with newer, branded stimulant use becoming more commonplace, we believe our article was useful and timely.
Our original intent had been to include an example of a controlled substance agreement. Regrettably, there was simply not enough space for this document or the additional discussion that its inclusion would deem necessary. Nevertheless, had the May 2023 FDA requirement for manufacturers to update the labeling of prescription stimulants1 to clarify misuse and abuse been published before our article’s final revision, we would have mentioned it and provided the appropriate link.
Subbu J. Sarma, MD, FAPA
Kansas City, Missouri
Sarah E. Grady, PharmD, BCPS, BCPP
Des Moines, Iowa
References
1. US Food & Drug Administration. FDA requires updates to clarify labeling of prescription stimulants used to treat ADHD and other conditions. Accessed August 9, 2023. https://www.fda.gov/drugs/drug-safety-and-availability/fda-requires-updates-clarify-labeling-prescription-stimulants-used-treat-adhd-and-other-conditions
Diversity in Multiple Sclerosis Care: How the Field of Underrepresented Minorities Has Evolved, and Where We Still See Areas for Improvement
The persistent notion that multiple sclerosis (MS) is predominantly a White patient’s disease has been challenged by scientific data and our clinical experience in the field. Recent research has shown a higher risk of MS in non-White populations than originally thought. This may be surprising, but new data are influencing the way we now approach MS in under-represented minorities, bringing this topic to the forefront of scientific interest.
The early conviction that “there is no MS in minorities” led to underdiagnosis and misdiagnosis of MS in those patients, which in turn deepened these patients’ distrust of physicians and reluctance to seek further medical care, very often delivered by non-minority providers. Inequities in social determinants of health, low health literacy, and lack of private insurance, along with structural racism in healthcare, has further hindered active engagement with an already marginalized patient population in their MS care. This lack of engagement and lack of minorities in scientific research has proved to be unfavorable for MS research as well, creating large and persistent knowledge gaps in understanding MS course, severity, and response to treatment specific to this group. A 2014 PubMed search found 52,000 publications on MS in English, but in only 136 of those were minority patients with MS (Black or Hispanic/Latino) the primary research focus. In 2019, the same search indicated that the subsequent 5 years produced only 30 more articles focusing solely on minority patients.
Research participation of underrepresented minorities is another area where we, as a field, continue to fail these patients. A review of participant enrollment in MS clinical trials that took place between 1993 and 2006 showed a significant decrease in the percentage of enrolled Black patients (from 7% to about 4%). This trend did not improve by the DEFINE treatment trial (2012), in which only 2% of enrolled patients were Black. Of the 1246 participants in the 2019 SUNBEAM MS study, only 2 were Black. Low numbers of minority patients in trials prevent us from drawing any reasonable conclusion as to the efficacy of disease-modifying agents in those patients and make the goal of personalized medicine for this group impossible.
The results of the research conducted on these groups are compelling and should be prompting further work. Not only do Black patients have a higher risk of MS, but there is also now convincing evidence that MS in minorities is more severe overall, causing early progression of disability and necessitating assistive gait devices such as a cane or wheelchair. Minority patients tend to have more extensive involvement of spinal cord and infra-tentorial brain structures during the disease, which could explain the increased likelihood of more severe disease and earlier disability. Minority patients were admitted to nursing homes at a younger age, with greater physical and cognitive impairment than nonminority patients. A study looking at MS mortality between 1999 and 2015 found that Black males with MS had the highest mortality rate before age 45, and Black females before age 53. MS mortality increased with age but peaked at age 55 to 64 for Black patients and 65 to 74 for White patients. Underrepresented minorities are also less likely to use community resources, case management, medical equipment, and home nursing services. When looking at other measures of disease impact on these patients, studies evaluating magnetic resonance imaging (MRI) data showed higher lesion volume in Black patients with MS, as well as a higher degree of brain demyelination and atrophy when compared with White patients.
Treatment strategies currently used for underrepresented minority patients, as well as estimations of medication efficacy, treatment responses, and adverse-event profiles are largely driven by data from clinical trials with only minimal representation of those patients. How can we propose a patient-tailored and individualized treatment plan without these crucial data? Given that, to this day, not a single trial has focused solely on underrepresented minorities, we are left with either post hoc exploratory subgroup analyses of existing trials or pragmatic, observational, and very often retrospective studies using chart analysis. Notwithstanding the methodological flaws of either approach, prior studies did suggest worse response to platform therapies in Black patients, but equal response to high-efficacy therapies when compared with White patients.
Definitive biological underpinnings of disparities in disease severity have not been identified. In recent years, the field of health outcomes research has suggested we move away from considering racial categories as biologically distinct and instead focus on long-overlooked sociodemographic and modifiable lifestyle
factors. The role of diet, exercise, body mass index, smoking, and vascular comorbidities as risk factors associated with worse MS outcomes has been previously shown; however, these factors have not been rigorously assessed in underrepresented populations with MS. Recent studies focused on uncovering what drives the differences in MS severity in underrepresented populations disagree on the role biological differences, socioeconomic disparities, and structural racism in both healthcare settings and society play in answering this question. While it is plausible that a combination of these factors might explain our observations, more research on larger, underserved patient populations and better-defined measures of socioeconomic differences are needed to answer this complex question.
The path of recognizing and correcting our mistakes is not simple but must be done, and our underrepresented minority patients depend on our swift action. There are many places where we as a field of experts can and must do better—in communities, healthcare systems, and society in general.
Increasing community health literacy around MS, rebuilding trust, and addressing structural racism on every level is important. Outreach and educational programs that include in-person meetings and leverage social media platforms can help empower patients and their families—and hopefully increase trust in healthcare providers. Devising targeted interventions focusing on modifiable factors of a healthy lifestyle such as diet and exercise can increase community engagement and strengthen the support system for our patients. Increasing diversity in our own field of physicians, nurses, and other healthcare providers can also aid in strengthening mutual relationships.
Improving access to comprehensive MS care for underrepresented minorities who very often also lack robust insurance coverage is paramount. Recipients of comprehensive care are more likely to participate in research, as these patients receive more well-rounded care and have a lower risk of mismanaged comorbidities. Their involvement in the treatment plan is higher, which also improves compliance with treatment. Patients in comprehensive care centers are more likely to receive newer treatment agents with better efficacy without hindrance of monitoring barriers, and they are likely to benefit from treatment strategies using newly approved agents soon after US Food and Drug Administration approval.
Increasing research participation and, ideally, conducting a clinical trial devoted solely to studying MS in underrepresented minorities is something for which we should actively strive. Identifying the main factors prohibiting enrollment and retention of a high number of minority participants in trials is critical to success. Multiple deterrents in day-to-day life, very often directly connected to economic hardship and racism, pose a very real threat to equitable trial participation. To even consider a successful trial for underrepresented minorities, we must do better in devising strategies and accommodations to help overcome those barriers.
The underserved minorities with MS deserve and need our attention and focus. These patients have largely been neglected and forgotten, but now are emerging at the forefront of our attention—where they belong.
The persistent notion that multiple sclerosis (MS) is predominantly a White patient’s disease has been challenged by scientific data and our clinical experience in the field. Recent research has shown a higher risk of MS in non-White populations than originally thought. This may be surprising, but new data are influencing the way we now approach MS in under-represented minorities, bringing this topic to the forefront of scientific interest.
The early conviction that “there is no MS in minorities” led to underdiagnosis and misdiagnosis of MS in those patients, which in turn deepened these patients’ distrust of physicians and reluctance to seek further medical care, very often delivered by non-minority providers. Inequities in social determinants of health, low health literacy, and lack of private insurance, along with structural racism in healthcare, has further hindered active engagement with an already marginalized patient population in their MS care. This lack of engagement and lack of minorities in scientific research has proved to be unfavorable for MS research as well, creating large and persistent knowledge gaps in understanding MS course, severity, and response to treatment specific to this group. A 2014 PubMed search found 52,000 publications on MS in English, but in only 136 of those were minority patients with MS (Black or Hispanic/Latino) the primary research focus. In 2019, the same search indicated that the subsequent 5 years produced only 30 more articles focusing solely on minority patients.
Research participation of underrepresented minorities is another area where we, as a field, continue to fail these patients. A review of participant enrollment in MS clinical trials that took place between 1993 and 2006 showed a significant decrease in the percentage of enrolled Black patients (from 7% to about 4%). This trend did not improve by the DEFINE treatment trial (2012), in which only 2% of enrolled patients were Black. Of the 1246 participants in the 2019 SUNBEAM MS study, only 2 were Black. Low numbers of minority patients in trials prevent us from drawing any reasonable conclusion as to the efficacy of disease-modifying agents in those patients and make the goal of personalized medicine for this group impossible.
The results of the research conducted on these groups are compelling and should be prompting further work. Not only do Black patients have a higher risk of MS, but there is also now convincing evidence that MS in minorities is more severe overall, causing early progression of disability and necessitating assistive gait devices such as a cane or wheelchair. Minority patients tend to have more extensive involvement of spinal cord and infra-tentorial brain structures during the disease, which could explain the increased likelihood of more severe disease and earlier disability. Minority patients were admitted to nursing homes at a younger age, with greater physical and cognitive impairment than nonminority patients. A study looking at MS mortality between 1999 and 2015 found that Black males with MS had the highest mortality rate before age 45, and Black females before age 53. MS mortality increased with age but peaked at age 55 to 64 for Black patients and 65 to 74 for White patients. Underrepresented minorities are also less likely to use community resources, case management, medical equipment, and home nursing services. When looking at other measures of disease impact on these patients, studies evaluating magnetic resonance imaging (MRI) data showed higher lesion volume in Black patients with MS, as well as a higher degree of brain demyelination and atrophy when compared with White patients.
Treatment strategies currently used for underrepresented minority patients, as well as estimations of medication efficacy, treatment responses, and adverse-event profiles are largely driven by data from clinical trials with only minimal representation of those patients. How can we propose a patient-tailored and individualized treatment plan without these crucial data? Given that, to this day, not a single trial has focused solely on underrepresented minorities, we are left with either post hoc exploratory subgroup analyses of existing trials or pragmatic, observational, and very often retrospective studies using chart analysis. Notwithstanding the methodological flaws of either approach, prior studies did suggest worse response to platform therapies in Black patients, but equal response to high-efficacy therapies when compared with White patients.
Definitive biological underpinnings of disparities in disease severity have not been identified. In recent years, the field of health outcomes research has suggested we move away from considering racial categories as biologically distinct and instead focus on long-overlooked sociodemographic and modifiable lifestyle
factors. The role of diet, exercise, body mass index, smoking, and vascular comorbidities as risk factors associated with worse MS outcomes has been previously shown; however, these factors have not been rigorously assessed in underrepresented populations with MS. Recent studies focused on uncovering what drives the differences in MS severity in underrepresented populations disagree on the role biological differences, socioeconomic disparities, and structural racism in both healthcare settings and society play in answering this question. While it is plausible that a combination of these factors might explain our observations, more research on larger, underserved patient populations and better-defined measures of socioeconomic differences are needed to answer this complex question.
The path of recognizing and correcting our mistakes is not simple but must be done, and our underrepresented minority patients depend on our swift action. There are many places where we as a field of experts can and must do better—in communities, healthcare systems, and society in general.
Increasing community health literacy around MS, rebuilding trust, and addressing structural racism on every level is important. Outreach and educational programs that include in-person meetings and leverage social media platforms can help empower patients and their families—and hopefully increase trust in healthcare providers. Devising targeted interventions focusing on modifiable factors of a healthy lifestyle such as diet and exercise can increase community engagement and strengthen the support system for our patients. Increasing diversity in our own field of physicians, nurses, and other healthcare providers can also aid in strengthening mutual relationships.
Improving access to comprehensive MS care for underrepresented minorities who very often also lack robust insurance coverage is paramount. Recipients of comprehensive care are more likely to participate in research, as these patients receive more well-rounded care and have a lower risk of mismanaged comorbidities. Their involvement in the treatment plan is higher, which also improves compliance with treatment. Patients in comprehensive care centers are more likely to receive newer treatment agents with better efficacy without hindrance of monitoring barriers, and they are likely to benefit from treatment strategies using newly approved agents soon after US Food and Drug Administration approval.
Increasing research participation and, ideally, conducting a clinical trial devoted solely to studying MS in underrepresented minorities is something for which we should actively strive. Identifying the main factors prohibiting enrollment and retention of a high number of minority participants in trials is critical to success. Multiple deterrents in day-to-day life, very often directly connected to economic hardship and racism, pose a very real threat to equitable trial participation. To even consider a successful trial for underrepresented minorities, we must do better in devising strategies and accommodations to help overcome those barriers.
The underserved minorities with MS deserve and need our attention and focus. These patients have largely been neglected and forgotten, but now are emerging at the forefront of our attention—where they belong.
The persistent notion that multiple sclerosis (MS) is predominantly a White patient’s disease has been challenged by scientific data and our clinical experience in the field. Recent research has shown a higher risk of MS in non-White populations than originally thought. This may be surprising, but new data are influencing the way we now approach MS in under-represented minorities, bringing this topic to the forefront of scientific interest.
The early conviction that “there is no MS in minorities” led to underdiagnosis and misdiagnosis of MS in those patients, which in turn deepened these patients’ distrust of physicians and reluctance to seek further medical care, very often delivered by non-minority providers. Inequities in social determinants of health, low health literacy, and lack of private insurance, along with structural racism in healthcare, has further hindered active engagement with an already marginalized patient population in their MS care. This lack of engagement and lack of minorities in scientific research has proved to be unfavorable for MS research as well, creating large and persistent knowledge gaps in understanding MS course, severity, and response to treatment specific to this group. A 2014 PubMed search found 52,000 publications on MS in English, but in only 136 of those were minority patients with MS (Black or Hispanic/Latino) the primary research focus. In 2019, the same search indicated that the subsequent 5 years produced only 30 more articles focusing solely on minority patients.
Research participation of underrepresented minorities is another area where we, as a field, continue to fail these patients. A review of participant enrollment in MS clinical trials that took place between 1993 and 2006 showed a significant decrease in the percentage of enrolled Black patients (from 7% to about 4%). This trend did not improve by the DEFINE treatment trial (2012), in which only 2% of enrolled patients were Black. Of the 1246 participants in the 2019 SUNBEAM MS study, only 2 were Black. Low numbers of minority patients in trials prevent us from drawing any reasonable conclusion as to the efficacy of disease-modifying agents in those patients and make the goal of personalized medicine for this group impossible.
The results of the research conducted on these groups are compelling and should be prompting further work. Not only do Black patients have a higher risk of MS, but there is also now convincing evidence that MS in minorities is more severe overall, causing early progression of disability and necessitating assistive gait devices such as a cane or wheelchair. Minority patients tend to have more extensive involvement of spinal cord and infra-tentorial brain structures during the disease, which could explain the increased likelihood of more severe disease and earlier disability. Minority patients were admitted to nursing homes at a younger age, with greater physical and cognitive impairment than nonminority patients. A study looking at MS mortality between 1999 and 2015 found that Black males with MS had the highest mortality rate before age 45, and Black females before age 53. MS mortality increased with age but peaked at age 55 to 64 for Black patients and 65 to 74 for White patients. Underrepresented minorities are also less likely to use community resources, case management, medical equipment, and home nursing services. When looking at other measures of disease impact on these patients, studies evaluating magnetic resonance imaging (MRI) data showed higher lesion volume in Black patients with MS, as well as a higher degree of brain demyelination and atrophy when compared with White patients.
Treatment strategies currently used for underrepresented minority patients, as well as estimations of medication efficacy, treatment responses, and adverse-event profiles are largely driven by data from clinical trials with only minimal representation of those patients. How can we propose a patient-tailored and individualized treatment plan without these crucial data? Given that, to this day, not a single trial has focused solely on underrepresented minorities, we are left with either post hoc exploratory subgroup analyses of existing trials or pragmatic, observational, and very often retrospective studies using chart analysis. Notwithstanding the methodological flaws of either approach, prior studies did suggest worse response to platform therapies in Black patients, but equal response to high-efficacy therapies when compared with White patients.
Definitive biological underpinnings of disparities in disease severity have not been identified. In recent years, the field of health outcomes research has suggested we move away from considering racial categories as biologically distinct and instead focus on long-overlooked sociodemographic and modifiable lifestyle
factors. The role of diet, exercise, body mass index, smoking, and vascular comorbidities as risk factors associated with worse MS outcomes has been previously shown; however, these factors have not been rigorously assessed in underrepresented populations with MS. Recent studies focused on uncovering what drives the differences in MS severity in underrepresented populations disagree on the role biological differences, socioeconomic disparities, and structural racism in both healthcare settings and society play in answering this question. While it is plausible that a combination of these factors might explain our observations, more research on larger, underserved patient populations and better-defined measures of socioeconomic differences are needed to answer this complex question.
The path of recognizing and correcting our mistakes is not simple but must be done, and our underrepresented minority patients depend on our swift action. There are many places where we as a field of experts can and must do better—in communities, healthcare systems, and society in general.
Increasing community health literacy around MS, rebuilding trust, and addressing structural racism on every level is important. Outreach and educational programs that include in-person meetings and leverage social media platforms can help empower patients and their families—and hopefully increase trust in healthcare providers. Devising targeted interventions focusing on modifiable factors of a healthy lifestyle such as diet and exercise can increase community engagement and strengthen the support system for our patients. Increasing diversity in our own field of physicians, nurses, and other healthcare providers can also aid in strengthening mutual relationships.
Improving access to comprehensive MS care for underrepresented minorities who very often also lack robust insurance coverage is paramount. Recipients of comprehensive care are more likely to participate in research, as these patients receive more well-rounded care and have a lower risk of mismanaged comorbidities. Their involvement in the treatment plan is higher, which also improves compliance with treatment. Patients in comprehensive care centers are more likely to receive newer treatment agents with better efficacy without hindrance of monitoring barriers, and they are likely to benefit from treatment strategies using newly approved agents soon after US Food and Drug Administration approval.
Increasing research participation and, ideally, conducting a clinical trial devoted solely to studying MS in underrepresented minorities is something for which we should actively strive. Identifying the main factors prohibiting enrollment and retention of a high number of minority participants in trials is critical to success. Multiple deterrents in day-to-day life, very often directly connected to economic hardship and racism, pose a very real threat to equitable trial participation. To even consider a successful trial for underrepresented minorities, we must do better in devising strategies and accommodations to help overcome those barriers.
The underserved minorities with MS deserve and need our attention and focus. These patients have largely been neglected and forgotten, but now are emerging at the forefront of our attention—where they belong.
Introducing the ‘Ethics Corner’
In addition to reporting on scientific advances from our GI journals that can inform frontline clinical care of patients with gastrointestinal conditions, we have launched several new columns over the past year, including our monthly Member Spotlight and quarterly Health Policy and Advocacy columns, to diversify our content.
In our September 2023 issue, in addition to debuting a new cover design, we are pleased to introduce yet another new offering – the “Ethics Corner” column. It is intended to highlight important ethical considerations and challenges arising in GI practice and offer practical guidance on how to navigate them. In our inaugural Ethics Corner, AGA Ethics Committee members Dr. Sheldon Sloan and Dr. David Drew discuss the “good, the bad, and the ugly” of direct-to-consumer advertising (DTCA). They highlight the pros and cons of DTCA from an ethical perspective, illustrate how DTCA can impact everyday clinical interactions with patients, and provide insight into how to navigate these challenging conversations. We hope you enjoy this new addition to the newspaper and welcome your ideas for future Ethics Corner columns and other content.
Also in this month’s issue, we update you on AGA’s response to the American College of Physicians’ recent decision to recommend against initiating colon cancer screening at age 45, contrary to the recommendation of the GI community. We also present a story on Humira biosimilars and how they are likely to impact clinical practice. Finally, our September Member Spotlight features GI dietitian Renee Euler, MS, RD, LD, who discusses the intersection between diet and GI disorders, the importance of a team approach to GI care, and her work as a liaison between the AGA and Academy of Nutrition and Dietetics.
We hope you enjoy these, and all the stories featured in our September issue.
Megan A. Adams, MD, JD, MSc
In addition to reporting on scientific advances from our GI journals that can inform frontline clinical care of patients with gastrointestinal conditions, we have launched several new columns over the past year, including our monthly Member Spotlight and quarterly Health Policy and Advocacy columns, to diversify our content.
In our September 2023 issue, in addition to debuting a new cover design, we are pleased to introduce yet another new offering – the “Ethics Corner” column. It is intended to highlight important ethical considerations and challenges arising in GI practice and offer practical guidance on how to navigate them. In our inaugural Ethics Corner, AGA Ethics Committee members Dr. Sheldon Sloan and Dr. David Drew discuss the “good, the bad, and the ugly” of direct-to-consumer advertising (DTCA). They highlight the pros and cons of DTCA from an ethical perspective, illustrate how DTCA can impact everyday clinical interactions with patients, and provide insight into how to navigate these challenging conversations. We hope you enjoy this new addition to the newspaper and welcome your ideas for future Ethics Corner columns and other content.
Also in this month’s issue, we update you on AGA’s response to the American College of Physicians’ recent decision to recommend against initiating colon cancer screening at age 45, contrary to the recommendation of the GI community. We also present a story on Humira biosimilars and how they are likely to impact clinical practice. Finally, our September Member Spotlight features GI dietitian Renee Euler, MS, RD, LD, who discusses the intersection between diet and GI disorders, the importance of a team approach to GI care, and her work as a liaison between the AGA and Academy of Nutrition and Dietetics.
We hope you enjoy these, and all the stories featured in our September issue.
Megan A. Adams, MD, JD, MSc
In addition to reporting on scientific advances from our GI journals that can inform frontline clinical care of patients with gastrointestinal conditions, we have launched several new columns over the past year, including our monthly Member Spotlight and quarterly Health Policy and Advocacy columns, to diversify our content.
In our September 2023 issue, in addition to debuting a new cover design, we are pleased to introduce yet another new offering – the “Ethics Corner” column. It is intended to highlight important ethical considerations and challenges arising in GI practice and offer practical guidance on how to navigate them. In our inaugural Ethics Corner, AGA Ethics Committee members Dr. Sheldon Sloan and Dr. David Drew discuss the “good, the bad, and the ugly” of direct-to-consumer advertising (DTCA). They highlight the pros and cons of DTCA from an ethical perspective, illustrate how DTCA can impact everyday clinical interactions with patients, and provide insight into how to navigate these challenging conversations. We hope you enjoy this new addition to the newspaper and welcome your ideas for future Ethics Corner columns and other content.
Also in this month’s issue, we update you on AGA’s response to the American College of Physicians’ recent decision to recommend against initiating colon cancer screening at age 45, contrary to the recommendation of the GI community. We also present a story on Humira biosimilars and how they are likely to impact clinical practice. Finally, our September Member Spotlight features GI dietitian Renee Euler, MS, RD, LD, who discusses the intersection between diet and GI disorders, the importance of a team approach to GI care, and her work as a liaison between the AGA and Academy of Nutrition and Dietetics.
We hope you enjoy these, and all the stories featured in our September issue.
Megan A. Adams, MD, JD, MSc
Coping with burnout and repetitive injuries
Dear colleagues,
We are all part of one of the most exciting and varied fields of medicine and hope to have long and productive careers. In this month’s AGA Perspectives we explore two different impediments to longevity as a gastroenterologist: work-related disability and burnout.
Physician burnout has reached almost epidemic levels in medicine and is best approached in a multimodal manner, incorporating both institutional and individual changes. Dr. Sumeet Tewani discusses ways in which groups and institutions can foster physician wellness to reduce burnout. In particular, he will explore how flexibility in work schedules, among other initiatives, can improve workplace morale. In an accompanying perspective, Dr. Anna Lipowska and Dr. Amandeep Shergill explore how to incorporate ergonomics in endoscopy to prevent injury. Endoscopic practice, with its repetitive tasks and physical demands, can predispose to injury at all levels of training and experience. Ergonomics is thus a critical topic that is unfortunately covered too little, if at all, in our endoscopy training.
We hope these essays will help your medical practice and welcome your thoughts on these important issues at @AGA_GIHN.
Gyanprakash A. Ketwaroo, MD, MSc, is associate professor of medicine, Yale University, New Haven, Conn., and chief of endoscopy at West Haven (Conn.) VA Medical Center. He is an associate editor for GI & Hepatology News.
Fostering physician wellness to prevent burnout
BY SUMEET TEWANI, MD
Gastroenterology can be a challenging field, both professionally and personally, as it requires providers to have high clinical knowledge, expertise, and emotional intelligence. Burnout is a state of emotional, physical, and mental exhaustion, depersonalization, and a reduced sense of personal accomplishment, caused by prolonged or excessive stress. Burnout can be a serious and progressive chronic condition that negatively impacts the provider and patient experience, with serious consequences on the provider’s health, job performance, patient satisfaction, and personal relationships.
Adopting strategies to combat burnout are of vital importance to promoting provider wellness, a healthy work environment, and positive interactions with colleagues, staff, and patients. This also positively impacts providers’ out-of-work experiences and relationships. on an institutional level and on an individual level.
Multiple factors affecting burnout have been identified, including individual factors, work volume, professional risk and responsibility, resources, and relationships with colleagues and patients. Practicing gastroenterology frequently requires long and irregular work hours, heavy workloads, large panels of complex patients, invasive procedures, and high amounts of stress. Additional stressors may include an inefficient work environment, inadequate support, and loss of value and meaning in work. Nearly 50% of physicians meet criteria for burnout, citing such reasons as excessive bureaucratic tasks, lack of control, flexibility and autonomy, lack of peer respect, increasing computerization of practice, and lack of respect from patients.
Preventing burnout
When coping with burnout, many providers choose positive mechanisms such as exercise, listening to music, meditation, and talking with family and friends. Others become more isolated, eat junk food or binge eat, or turn to drug or alcohol abuse. Our primary approach to preventing burnout at an individual level is to ensure providers have access to self-care techniques such as stress management and mindfulness, and to encourage wellness with regular exercise and healthy habits. Promoting a culture of work-life balance requires providing adequate time for personal activities and hobbies, rest, relaxation, and spending time with family and friends. Allowing providers to personally shape their career paths aligns their personal and professional goals, leading to greater satisfaction. To this end, providers may become involved in clinical research, medical education, and clinical and administrative committees through the practice, local medical school and hospitals, and local and national societies. We provide ample vacation time and CME opportunities for our providers. Vacation time is flexible and can be taken in half-day or full-day increments, or on an hourly basis for personal time as necessary. This allows for enhanced flexibility with scheduling time off from work.
On an institutional level, leadership plays a prime role in creating a healthy work environment. Having good leaders influences the well-being and satisfaction of everyone within the organization. Leaders can have a positive impact by aligning values and work culture, using incentives in a productive manner, and promoting strategies to reduce burnout. Involving physician partners in leadership on a rotating basis allows them to better understand the roles of the leaders in the organization and empowers them to have a voice in changing policies to reduce administrative burdens and foster wellness.
We promote the concept of working together as a team for the success of the practice. All partners have an equal say in the management of the group. We eliminated the stress of competition within the group, equalizing pay across all physician partners, while maintaining equal exposure to work and equal time off from work. This levels the playing field between physicians who have varied interests and expertise, so that everyone is working towards the success of the practice and not individual compensation. To that end, our providers do not have individual offices, but work out of a “bullpen” with an open concept where we have individual workspaces and interact with each other continuously throughout the day. This promotes cohesion and teamwork between the providers for all our patients and promotes professional relationships and peer support. Efforts to promote workplace morale include access to a fully stocked deli and a newly installed espresso machine.
The concept of teamwork
The concept of teamwork also needs to pervade through the entire organization. To manage the demands of a busy workday, we have directly trained advanced practice nurses in the clinic and inpatient settings, allowing our physicians to increase throughput and procedures while maintaining a high level of patient care, satisfaction, and efficiency. Providers report excessive administrative tasks and frustration with the electronic health record as major factors contributing to burnout. Delegating tasks to staff, commensurate with their training and scope of practice, alleviates some of this burden. Each of the providers in our practice has a triage nurse who functions in a key capacity to ensure the appropriate clinical and administrative tasks are complete. Medical scribes, medical assistants, nurses, and physician assistants can be utilized for data entry and other tasks. We have developed templates within the electronic health record that can be standardized across the practice. Promoting teamwork with staff also means respecting the staff and understanding their needs. A highly functioning health care team can provide comprehensive care proactively and efficiently, with improved professional satisfaction.
In summary, I identify several ways to promote physician wellness. Every GI practice should strive to implement local approaches to prevent physician burnout and help maintain a happy and productive workforce.
Dr. Tewani is a gastroenterologist with Rockford Gastroenterology Associates in Rockford, Ill. He has no relevant disclosures.
References
1. Koval ML. Medscape gastroenterologist lifestyle, happiness & burnout report 2023: Contentment amid stress. Medscape. 24 Feb 2023.
2. Ong J et al. The prevalence of burnout, risk factors, and job-related stressors in gastroenterologists: A systematic review. J Gastroenterol Hepatol. 2021 Sep;36(9):2338-48.
3. Anderson J et al. Strategies to combat physician burnout in gastroenterology. Am J Gastroenterol. 2017 Sep;112(9):1356-9.
4. Keswani R et al. Burnout in gastroenterologists and how to prevent it. Gastroenterology. 2014 Jul;147(1):11-4.
The hazards of endoscopy: Ergonomics guide the way
BY ANNA LIPOWSKA, MD, AND AMANDEEP SHERGILL, MD, MS
Preventing disability and promoting a long and successful endoscopic career involves proactive measures to support well-being, and ergonomics plays a key role. Ergonomics is the science of fitting a job to the worker, with a primary goal of working smarter and safer. When hazards are identified, mitigation measures, guided by a hierarchy of controls, must be implemented that improve the fit of the tool, task and job to the worker in order to reduce the risk of endoscopy-related injury (ERI). As more women enter the field and as the overall GI physician population ages, ensuring that endoscopy is designed to be safely performed within the capacity of a diverse group of workers will be critical to creating an inclusive and equitable work environment.
Ergonomic education is foundational: Awareness of ERI risk factors allows endoscopists to identify hazards and advocate for effective control solutions. Ergonomic education materials are available through all of the major GI societies. A road map for implementing an endoscopy ergonomics program has been previously published and provides guidance on risk assessment and mitigation measures.
Respect pain
Overuse injuries occur when the physical demands of a job are greater than tissue tolerances, leading to cumulative microtrauma. The first sign of microtraumatic injury is discomfort and pain. Studies have demonstrated that an estimated three-quarters of gastroenterologists experience ERI, with 20% requiring time off work and 12% requiring surgery. Gastroenterology trainees are also at risk, with 20% of surveyed fellows endorsing overuse injury, some even requiring work-related leaves of absence. In the early stages of ERI, aching and tiredness occur during the work shift only. In the intermediate stage, aching and tiredness occur early in the work shift and persist at night, and may be associated with a reduced capacity for repetitive work. In the late stages, aching, fatigue and weakness persist at rest and may be associated with inability to sleep and to perform light duties. Pain is an important signal indicating mitigation measures are required to control exposures.
Utilize the hierarchy of controls
The responsibility for adoption of ergonomically friendly practices does not lie solely with the physician; both institutional and industry-level support are key to its success. The hierarchy of controls defines which actions will best mitigate exposures to hazards in the workplace, highlighting that modifications to personal practice have the smallest impact. Current endoscope design does not accommodate the full range of hand strengths and sizes and contributes to ERI.
Advancements at the industry level by eliminating or substituting hazards, or designing engineering controls to reduce exposure, will be most effective at preventing distal upper extremity ERI. The next most effective controls are at the institutional level, with endoscopy units ensuring access to engineering controls and implementing effective administrative controls. For example, institutional support and investment in adjustable workstations is imperative to accommodate a range of anthropometric dimensions of the population. Support for ergonomic education, scheduling changes and a culture where safety is a priority can help reduce exposure to hazards and injury risk.
Adjust the endoscopy suite to achieve a comfortable position before every procedure
Neutral body posture is our position of greatest comfort and maximum strength, and any deviation from neutral posture decreases the amount of force the muscles can produce and causes the muscles to fatigue sooner. The most important factor affecting the endoscopists’ overall posture is the monitor position and height. Monitors must be adjustable. Place the monitor directly in front of you, with the center of the screen 15-25 degrees below eye height for a neutral neck position and resting eye position. Procedure bed height should be adjusted 0-10 cm below elbow height to allow for neutral elbow postures and relaxed shoulders. Antifatigue mats and shoes with supportive insoles can reduce fatigue. Two-piece lead aprons distribute a portion of the static load to the hips and decrease back strain. Incorporate a preprocedure ergonomic time-out, to assess proper room set up, body mechanics, equipment and team preparedness.
Give yourself a break
Breaks should be built into the endoscopy schedule, especially for a full day of endoscopy. At a minimum, incorporate microbreaks during procedures, which have been found to alleviate pain and improve performance. Exercises and stretching can be incorporated between cases, including routines designed specifically for endoscopists.
Getting older isn’t for the weak
Currently, 50% of our gastroenterologists are over 55 years old. The aging process leads to a distinct muscle mass and strength loss. Women are already at a disadvantage because, on average, they have less muscle mass than men in all age groups. Muscle starts to deteriorate when we reach our 30s, and after age 40, we lose on average 8% of our muscle mass every decade which accelerates at an even faster rate after age 60. Both resistance and aerobic exercise can be very useful to counteract sarcopenia and maintain strength. Given the physical demands of endoscopy, exercise can help safeguard career longevity and maintain overall wellness. Multiple resources are available to tailor a program that fits your time, budget and needs.
Optimize all of your workstations
Prolonged computer use and desk work is also a significant part of a gastroenterologist’s profession. If using a sitting desk, chair height should allow for 90-degree flexion at the hips and knees and for feet to rest flatly on the floor. The chair should also provide adequate back support for a relaxed upright position. Similar to endoscopy, place the monitor directly in front with the center of the screen slightly below eye level. For mouse and keyboard placement, aim to have the elbows at or slightly below 90 degrees and one’s wrists and fingers in neutral position.
Endoscopy can be hazardous to the endoscopists’ health. Incorporating ergonomic principles creates a safer and more efficient work environment. At the individual level, ergonomically optimized postures during endoscopy as well as during computer-related tasks, room set up, inclusion of microbreaks, and protective exercises can help decrease the risk of repetitive strain injury and prevent disability. Importantly, change at the industry and institutional level has the greatest potential for positive impact. Adoption of ergonomic practices promotes career longevity and ensures that gastroenterologists can continue successful and long careers and provide quality care to their patients without compromising their own health.
Dr. Lipowska is an assistant professor in the division of gastroenterology and hepatology, University of Illinois at Chicago. She disclosed no conflicts. Dr. Shergill is chief of gastroenterology for the San Francisco VA Health Care System. She disclosed consulting work for Boston Scientific and Neptune Medical, honoraria for visiting professorship with Intuitive Surgical, and a research gift from Pentax.
References
Shergill AK. Top tips for implementing an endoscopy ergonomics program. Gastrointest Endosc. 2023 Feb;97(2):361-4.
Pawa S et al. Are all endoscopy-related musculoskeletal injuries created equal? Results of a national gender-based survey. Am J Gastroenterol. 2021;116(3):530-8.
Austin K et al. Musculoskeletal injuries are commonly reported among gastroenterology trainees: Results of a national survey. Dig Dis Sci. 2019;64(6):1439-47.
Lipowska A et al. Ergonomics in the unit: Modeling the environment around the endoscopist. Tech Innov Gastrointest Endosc. 2021;23(3):256-62.
Park A et al. Intraoperative “Micro Breaks” with a targeted stretching enhance surgeon physical function and mental focus: A multicenter cohort study. Ann Surg. 2017;265(2):340-6.
Shergill A et al. Ergonomic endoscopy: An oxymoron or realistic goal? Gastrointest Endosc. 2019;90(6):966-70.
Dear colleagues,
We are all part of one of the most exciting and varied fields of medicine and hope to have long and productive careers. In this month’s AGA Perspectives we explore two different impediments to longevity as a gastroenterologist: work-related disability and burnout.
Physician burnout has reached almost epidemic levels in medicine and is best approached in a multimodal manner, incorporating both institutional and individual changes. Dr. Sumeet Tewani discusses ways in which groups and institutions can foster physician wellness to reduce burnout. In particular, he will explore how flexibility in work schedules, among other initiatives, can improve workplace morale. In an accompanying perspective, Dr. Anna Lipowska and Dr. Amandeep Shergill explore how to incorporate ergonomics in endoscopy to prevent injury. Endoscopic practice, with its repetitive tasks and physical demands, can predispose to injury at all levels of training and experience. Ergonomics is thus a critical topic that is unfortunately covered too little, if at all, in our endoscopy training.
We hope these essays will help your medical practice and welcome your thoughts on these important issues at @AGA_GIHN.
Gyanprakash A. Ketwaroo, MD, MSc, is associate professor of medicine, Yale University, New Haven, Conn., and chief of endoscopy at West Haven (Conn.) VA Medical Center. He is an associate editor for GI & Hepatology News.
Fostering physician wellness to prevent burnout
BY SUMEET TEWANI, MD
Gastroenterology can be a challenging field, both professionally and personally, as it requires providers to have high clinical knowledge, expertise, and emotional intelligence. Burnout is a state of emotional, physical, and mental exhaustion, depersonalization, and a reduced sense of personal accomplishment, caused by prolonged or excessive stress. Burnout can be a serious and progressive chronic condition that negatively impacts the provider and patient experience, with serious consequences on the provider’s health, job performance, patient satisfaction, and personal relationships.
Adopting strategies to combat burnout are of vital importance to promoting provider wellness, a healthy work environment, and positive interactions with colleagues, staff, and patients. This also positively impacts providers’ out-of-work experiences and relationships. on an institutional level and on an individual level.
Multiple factors affecting burnout have been identified, including individual factors, work volume, professional risk and responsibility, resources, and relationships with colleagues and patients. Practicing gastroenterology frequently requires long and irregular work hours, heavy workloads, large panels of complex patients, invasive procedures, and high amounts of stress. Additional stressors may include an inefficient work environment, inadequate support, and loss of value and meaning in work. Nearly 50% of physicians meet criteria for burnout, citing such reasons as excessive bureaucratic tasks, lack of control, flexibility and autonomy, lack of peer respect, increasing computerization of practice, and lack of respect from patients.
Preventing burnout
When coping with burnout, many providers choose positive mechanisms such as exercise, listening to music, meditation, and talking with family and friends. Others become more isolated, eat junk food or binge eat, or turn to drug or alcohol abuse. Our primary approach to preventing burnout at an individual level is to ensure providers have access to self-care techniques such as stress management and mindfulness, and to encourage wellness with regular exercise and healthy habits. Promoting a culture of work-life balance requires providing adequate time for personal activities and hobbies, rest, relaxation, and spending time with family and friends. Allowing providers to personally shape their career paths aligns their personal and professional goals, leading to greater satisfaction. To this end, providers may become involved in clinical research, medical education, and clinical and administrative committees through the practice, local medical school and hospitals, and local and national societies. We provide ample vacation time and CME opportunities for our providers. Vacation time is flexible and can be taken in half-day or full-day increments, or on an hourly basis for personal time as necessary. This allows for enhanced flexibility with scheduling time off from work.
On an institutional level, leadership plays a prime role in creating a healthy work environment. Having good leaders influences the well-being and satisfaction of everyone within the organization. Leaders can have a positive impact by aligning values and work culture, using incentives in a productive manner, and promoting strategies to reduce burnout. Involving physician partners in leadership on a rotating basis allows them to better understand the roles of the leaders in the organization and empowers them to have a voice in changing policies to reduce administrative burdens and foster wellness.
We promote the concept of working together as a team for the success of the practice. All partners have an equal say in the management of the group. We eliminated the stress of competition within the group, equalizing pay across all physician partners, while maintaining equal exposure to work and equal time off from work. This levels the playing field between physicians who have varied interests and expertise, so that everyone is working towards the success of the practice and not individual compensation. To that end, our providers do not have individual offices, but work out of a “bullpen” with an open concept where we have individual workspaces and interact with each other continuously throughout the day. This promotes cohesion and teamwork between the providers for all our patients and promotes professional relationships and peer support. Efforts to promote workplace morale include access to a fully stocked deli and a newly installed espresso machine.
The concept of teamwork
The concept of teamwork also needs to pervade through the entire organization. To manage the demands of a busy workday, we have directly trained advanced practice nurses in the clinic and inpatient settings, allowing our physicians to increase throughput and procedures while maintaining a high level of patient care, satisfaction, and efficiency. Providers report excessive administrative tasks and frustration with the electronic health record as major factors contributing to burnout. Delegating tasks to staff, commensurate with their training and scope of practice, alleviates some of this burden. Each of the providers in our practice has a triage nurse who functions in a key capacity to ensure the appropriate clinical and administrative tasks are complete. Medical scribes, medical assistants, nurses, and physician assistants can be utilized for data entry and other tasks. We have developed templates within the electronic health record that can be standardized across the practice. Promoting teamwork with staff also means respecting the staff and understanding their needs. A highly functioning health care team can provide comprehensive care proactively and efficiently, with improved professional satisfaction.
In summary, I identify several ways to promote physician wellness. Every GI practice should strive to implement local approaches to prevent physician burnout and help maintain a happy and productive workforce.
Dr. Tewani is a gastroenterologist with Rockford Gastroenterology Associates in Rockford, Ill. He has no relevant disclosures.
References
1. Koval ML. Medscape gastroenterologist lifestyle, happiness & burnout report 2023: Contentment amid stress. Medscape. 24 Feb 2023.
2. Ong J et al. The prevalence of burnout, risk factors, and job-related stressors in gastroenterologists: A systematic review. J Gastroenterol Hepatol. 2021 Sep;36(9):2338-48.
3. Anderson J et al. Strategies to combat physician burnout in gastroenterology. Am J Gastroenterol. 2017 Sep;112(9):1356-9.
4. Keswani R et al. Burnout in gastroenterologists and how to prevent it. Gastroenterology. 2014 Jul;147(1):11-4.
The hazards of endoscopy: Ergonomics guide the way
BY ANNA LIPOWSKA, MD, AND AMANDEEP SHERGILL, MD, MS
Preventing disability and promoting a long and successful endoscopic career involves proactive measures to support well-being, and ergonomics plays a key role. Ergonomics is the science of fitting a job to the worker, with a primary goal of working smarter and safer. When hazards are identified, mitigation measures, guided by a hierarchy of controls, must be implemented that improve the fit of the tool, task and job to the worker in order to reduce the risk of endoscopy-related injury (ERI). As more women enter the field and as the overall GI physician population ages, ensuring that endoscopy is designed to be safely performed within the capacity of a diverse group of workers will be critical to creating an inclusive and equitable work environment.
Ergonomic education is foundational: Awareness of ERI risk factors allows endoscopists to identify hazards and advocate for effective control solutions. Ergonomic education materials are available through all of the major GI societies. A road map for implementing an endoscopy ergonomics program has been previously published and provides guidance on risk assessment and mitigation measures.
Respect pain
Overuse injuries occur when the physical demands of a job are greater than tissue tolerances, leading to cumulative microtrauma. The first sign of microtraumatic injury is discomfort and pain. Studies have demonstrated that an estimated three-quarters of gastroenterologists experience ERI, with 20% requiring time off work and 12% requiring surgery. Gastroenterology trainees are also at risk, with 20% of surveyed fellows endorsing overuse injury, some even requiring work-related leaves of absence. In the early stages of ERI, aching and tiredness occur during the work shift only. In the intermediate stage, aching and tiredness occur early in the work shift and persist at night, and may be associated with a reduced capacity for repetitive work. In the late stages, aching, fatigue and weakness persist at rest and may be associated with inability to sleep and to perform light duties. Pain is an important signal indicating mitigation measures are required to control exposures.
Utilize the hierarchy of controls
The responsibility for adoption of ergonomically friendly practices does not lie solely with the physician; both institutional and industry-level support are key to its success. The hierarchy of controls defines which actions will best mitigate exposures to hazards in the workplace, highlighting that modifications to personal practice have the smallest impact. Current endoscope design does not accommodate the full range of hand strengths and sizes and contributes to ERI.
Advancements at the industry level by eliminating or substituting hazards, or designing engineering controls to reduce exposure, will be most effective at preventing distal upper extremity ERI. The next most effective controls are at the institutional level, with endoscopy units ensuring access to engineering controls and implementing effective administrative controls. For example, institutional support and investment in adjustable workstations is imperative to accommodate a range of anthropometric dimensions of the population. Support for ergonomic education, scheduling changes and a culture where safety is a priority can help reduce exposure to hazards and injury risk.
Adjust the endoscopy suite to achieve a comfortable position before every procedure
Neutral body posture is our position of greatest comfort and maximum strength, and any deviation from neutral posture decreases the amount of force the muscles can produce and causes the muscles to fatigue sooner. The most important factor affecting the endoscopists’ overall posture is the monitor position and height. Monitors must be adjustable. Place the monitor directly in front of you, with the center of the screen 15-25 degrees below eye height for a neutral neck position and resting eye position. Procedure bed height should be adjusted 0-10 cm below elbow height to allow for neutral elbow postures and relaxed shoulders. Antifatigue mats and shoes with supportive insoles can reduce fatigue. Two-piece lead aprons distribute a portion of the static load to the hips and decrease back strain. Incorporate a preprocedure ergonomic time-out, to assess proper room set up, body mechanics, equipment and team preparedness.
Give yourself a break
Breaks should be built into the endoscopy schedule, especially for a full day of endoscopy. At a minimum, incorporate microbreaks during procedures, which have been found to alleviate pain and improve performance. Exercises and stretching can be incorporated between cases, including routines designed specifically for endoscopists.
Getting older isn’t for the weak
Currently, 50% of our gastroenterologists are over 55 years old. The aging process leads to a distinct muscle mass and strength loss. Women are already at a disadvantage because, on average, they have less muscle mass than men in all age groups. Muscle starts to deteriorate when we reach our 30s, and after age 40, we lose on average 8% of our muscle mass every decade which accelerates at an even faster rate after age 60. Both resistance and aerobic exercise can be very useful to counteract sarcopenia and maintain strength. Given the physical demands of endoscopy, exercise can help safeguard career longevity and maintain overall wellness. Multiple resources are available to tailor a program that fits your time, budget and needs.
Optimize all of your workstations
Prolonged computer use and desk work is also a significant part of a gastroenterologist’s profession. If using a sitting desk, chair height should allow for 90-degree flexion at the hips and knees and for feet to rest flatly on the floor. The chair should also provide adequate back support for a relaxed upright position. Similar to endoscopy, place the monitor directly in front with the center of the screen slightly below eye level. For mouse and keyboard placement, aim to have the elbows at or slightly below 90 degrees and one’s wrists and fingers in neutral position.
Endoscopy can be hazardous to the endoscopists’ health. Incorporating ergonomic principles creates a safer and more efficient work environment. At the individual level, ergonomically optimized postures during endoscopy as well as during computer-related tasks, room set up, inclusion of microbreaks, and protective exercises can help decrease the risk of repetitive strain injury and prevent disability. Importantly, change at the industry and institutional level has the greatest potential for positive impact. Adoption of ergonomic practices promotes career longevity and ensures that gastroenterologists can continue successful and long careers and provide quality care to their patients without compromising their own health.
Dr. Lipowska is an assistant professor in the division of gastroenterology and hepatology, University of Illinois at Chicago. She disclosed no conflicts. Dr. Shergill is chief of gastroenterology for the San Francisco VA Health Care System. She disclosed consulting work for Boston Scientific and Neptune Medical, honoraria for visiting professorship with Intuitive Surgical, and a research gift from Pentax.
References
Shergill AK. Top tips for implementing an endoscopy ergonomics program. Gastrointest Endosc. 2023 Feb;97(2):361-4.
Pawa S et al. Are all endoscopy-related musculoskeletal injuries created equal? Results of a national gender-based survey. Am J Gastroenterol. 2021;116(3):530-8.
Austin K et al. Musculoskeletal injuries are commonly reported among gastroenterology trainees: Results of a national survey. Dig Dis Sci. 2019;64(6):1439-47.
Lipowska A et al. Ergonomics in the unit: Modeling the environment around the endoscopist. Tech Innov Gastrointest Endosc. 2021;23(3):256-62.
Park A et al. Intraoperative “Micro Breaks” with a targeted stretching enhance surgeon physical function and mental focus: A multicenter cohort study. Ann Surg. 2017;265(2):340-6.
Shergill A et al. Ergonomic endoscopy: An oxymoron or realistic goal? Gastrointest Endosc. 2019;90(6):966-70.
Dear colleagues,
We are all part of one of the most exciting and varied fields of medicine and hope to have long and productive careers. In this month’s AGA Perspectives we explore two different impediments to longevity as a gastroenterologist: work-related disability and burnout.
Physician burnout has reached almost epidemic levels in medicine and is best approached in a multimodal manner, incorporating both institutional and individual changes. Dr. Sumeet Tewani discusses ways in which groups and institutions can foster physician wellness to reduce burnout. In particular, he will explore how flexibility in work schedules, among other initiatives, can improve workplace morale. In an accompanying perspective, Dr. Anna Lipowska and Dr. Amandeep Shergill explore how to incorporate ergonomics in endoscopy to prevent injury. Endoscopic practice, with its repetitive tasks and physical demands, can predispose to injury at all levels of training and experience. Ergonomics is thus a critical topic that is unfortunately covered too little, if at all, in our endoscopy training.
We hope these essays will help your medical practice and welcome your thoughts on these important issues at @AGA_GIHN.
Gyanprakash A. Ketwaroo, MD, MSc, is associate professor of medicine, Yale University, New Haven, Conn., and chief of endoscopy at West Haven (Conn.) VA Medical Center. He is an associate editor for GI & Hepatology News.
Fostering physician wellness to prevent burnout
BY SUMEET TEWANI, MD
Gastroenterology can be a challenging field, both professionally and personally, as it requires providers to have high clinical knowledge, expertise, and emotional intelligence. Burnout is a state of emotional, physical, and mental exhaustion, depersonalization, and a reduced sense of personal accomplishment, caused by prolonged or excessive stress. Burnout can be a serious and progressive chronic condition that negatively impacts the provider and patient experience, with serious consequences on the provider’s health, job performance, patient satisfaction, and personal relationships.
Adopting strategies to combat burnout are of vital importance to promoting provider wellness, a healthy work environment, and positive interactions with colleagues, staff, and patients. This also positively impacts providers’ out-of-work experiences and relationships. on an institutional level and on an individual level.
Multiple factors affecting burnout have been identified, including individual factors, work volume, professional risk and responsibility, resources, and relationships with colleagues and patients. Practicing gastroenterology frequently requires long and irregular work hours, heavy workloads, large panels of complex patients, invasive procedures, and high amounts of stress. Additional stressors may include an inefficient work environment, inadequate support, and loss of value and meaning in work. Nearly 50% of physicians meet criteria for burnout, citing such reasons as excessive bureaucratic tasks, lack of control, flexibility and autonomy, lack of peer respect, increasing computerization of practice, and lack of respect from patients.
Preventing burnout
When coping with burnout, many providers choose positive mechanisms such as exercise, listening to music, meditation, and talking with family and friends. Others become more isolated, eat junk food or binge eat, or turn to drug or alcohol abuse. Our primary approach to preventing burnout at an individual level is to ensure providers have access to self-care techniques such as stress management and mindfulness, and to encourage wellness with regular exercise and healthy habits. Promoting a culture of work-life balance requires providing adequate time for personal activities and hobbies, rest, relaxation, and spending time with family and friends. Allowing providers to personally shape their career paths aligns their personal and professional goals, leading to greater satisfaction. To this end, providers may become involved in clinical research, medical education, and clinical and administrative committees through the practice, local medical school and hospitals, and local and national societies. We provide ample vacation time and CME opportunities for our providers. Vacation time is flexible and can be taken in half-day or full-day increments, or on an hourly basis for personal time as necessary. This allows for enhanced flexibility with scheduling time off from work.
On an institutional level, leadership plays a prime role in creating a healthy work environment. Having good leaders influences the well-being and satisfaction of everyone within the organization. Leaders can have a positive impact by aligning values and work culture, using incentives in a productive manner, and promoting strategies to reduce burnout. Involving physician partners in leadership on a rotating basis allows them to better understand the roles of the leaders in the organization and empowers them to have a voice in changing policies to reduce administrative burdens and foster wellness.
We promote the concept of working together as a team for the success of the practice. All partners have an equal say in the management of the group. We eliminated the stress of competition within the group, equalizing pay across all physician partners, while maintaining equal exposure to work and equal time off from work. This levels the playing field between physicians who have varied interests and expertise, so that everyone is working towards the success of the practice and not individual compensation. To that end, our providers do not have individual offices, but work out of a “bullpen” with an open concept where we have individual workspaces and interact with each other continuously throughout the day. This promotes cohesion and teamwork between the providers for all our patients and promotes professional relationships and peer support. Efforts to promote workplace morale include access to a fully stocked deli and a newly installed espresso machine.
The concept of teamwork
The concept of teamwork also needs to pervade through the entire organization. To manage the demands of a busy workday, we have directly trained advanced practice nurses in the clinic and inpatient settings, allowing our physicians to increase throughput and procedures while maintaining a high level of patient care, satisfaction, and efficiency. Providers report excessive administrative tasks and frustration with the electronic health record as major factors contributing to burnout. Delegating tasks to staff, commensurate with their training and scope of practice, alleviates some of this burden. Each of the providers in our practice has a triage nurse who functions in a key capacity to ensure the appropriate clinical and administrative tasks are complete. Medical scribes, medical assistants, nurses, and physician assistants can be utilized for data entry and other tasks. We have developed templates within the electronic health record that can be standardized across the practice. Promoting teamwork with staff also means respecting the staff and understanding their needs. A highly functioning health care team can provide comprehensive care proactively and efficiently, with improved professional satisfaction.
In summary, I identify several ways to promote physician wellness. Every GI practice should strive to implement local approaches to prevent physician burnout and help maintain a happy and productive workforce.
Dr. Tewani is a gastroenterologist with Rockford Gastroenterology Associates in Rockford, Ill. He has no relevant disclosures.
References
1. Koval ML. Medscape gastroenterologist lifestyle, happiness & burnout report 2023: Contentment amid stress. Medscape. 24 Feb 2023.
2. Ong J et al. The prevalence of burnout, risk factors, and job-related stressors in gastroenterologists: A systematic review. J Gastroenterol Hepatol. 2021 Sep;36(9):2338-48.
3. Anderson J et al. Strategies to combat physician burnout in gastroenterology. Am J Gastroenterol. 2017 Sep;112(9):1356-9.
4. Keswani R et al. Burnout in gastroenterologists and how to prevent it. Gastroenterology. 2014 Jul;147(1):11-4.
The hazards of endoscopy: Ergonomics guide the way
BY ANNA LIPOWSKA, MD, AND AMANDEEP SHERGILL, MD, MS
Preventing disability and promoting a long and successful endoscopic career involves proactive measures to support well-being, and ergonomics plays a key role. Ergonomics is the science of fitting a job to the worker, with a primary goal of working smarter and safer. When hazards are identified, mitigation measures, guided by a hierarchy of controls, must be implemented that improve the fit of the tool, task and job to the worker in order to reduce the risk of endoscopy-related injury (ERI). As more women enter the field and as the overall GI physician population ages, ensuring that endoscopy is designed to be safely performed within the capacity of a diverse group of workers will be critical to creating an inclusive and equitable work environment.
Ergonomic education is foundational: Awareness of ERI risk factors allows endoscopists to identify hazards and advocate for effective control solutions. Ergonomic education materials are available through all of the major GI societies. A road map for implementing an endoscopy ergonomics program has been previously published and provides guidance on risk assessment and mitigation measures.
Respect pain
Overuse injuries occur when the physical demands of a job are greater than tissue tolerances, leading to cumulative microtrauma. The first sign of microtraumatic injury is discomfort and pain. Studies have demonstrated that an estimated three-quarters of gastroenterologists experience ERI, with 20% requiring time off work and 12% requiring surgery. Gastroenterology trainees are also at risk, with 20% of surveyed fellows endorsing overuse injury, some even requiring work-related leaves of absence. In the early stages of ERI, aching and tiredness occur during the work shift only. In the intermediate stage, aching and tiredness occur early in the work shift and persist at night, and may be associated with a reduced capacity for repetitive work. In the late stages, aching, fatigue and weakness persist at rest and may be associated with inability to sleep and to perform light duties. Pain is an important signal indicating mitigation measures are required to control exposures.
Utilize the hierarchy of controls
The responsibility for adoption of ergonomically friendly practices does not lie solely with the physician; both institutional and industry-level support are key to its success. The hierarchy of controls defines which actions will best mitigate exposures to hazards in the workplace, highlighting that modifications to personal practice have the smallest impact. Current endoscope design does not accommodate the full range of hand strengths and sizes and contributes to ERI.
Advancements at the industry level by eliminating or substituting hazards, or designing engineering controls to reduce exposure, will be most effective at preventing distal upper extremity ERI. The next most effective controls are at the institutional level, with endoscopy units ensuring access to engineering controls and implementing effective administrative controls. For example, institutional support and investment in adjustable workstations is imperative to accommodate a range of anthropometric dimensions of the population. Support for ergonomic education, scheduling changes and a culture where safety is a priority can help reduce exposure to hazards and injury risk.
Adjust the endoscopy suite to achieve a comfortable position before every procedure
Neutral body posture is our position of greatest comfort and maximum strength, and any deviation from neutral posture decreases the amount of force the muscles can produce and causes the muscles to fatigue sooner. The most important factor affecting the endoscopists’ overall posture is the monitor position and height. Monitors must be adjustable. Place the monitor directly in front of you, with the center of the screen 15-25 degrees below eye height for a neutral neck position and resting eye position. Procedure bed height should be adjusted 0-10 cm below elbow height to allow for neutral elbow postures and relaxed shoulders. Antifatigue mats and shoes with supportive insoles can reduce fatigue. Two-piece lead aprons distribute a portion of the static load to the hips and decrease back strain. Incorporate a preprocedure ergonomic time-out, to assess proper room set up, body mechanics, equipment and team preparedness.
Give yourself a break
Breaks should be built into the endoscopy schedule, especially for a full day of endoscopy. At a minimum, incorporate microbreaks during procedures, which have been found to alleviate pain and improve performance. Exercises and stretching can be incorporated between cases, including routines designed specifically for endoscopists.
Getting older isn’t for the weak
Currently, 50% of our gastroenterologists are over 55 years old. The aging process leads to a distinct muscle mass and strength loss. Women are already at a disadvantage because, on average, they have less muscle mass than men in all age groups. Muscle starts to deteriorate when we reach our 30s, and after age 40, we lose on average 8% of our muscle mass every decade which accelerates at an even faster rate after age 60. Both resistance and aerobic exercise can be very useful to counteract sarcopenia and maintain strength. Given the physical demands of endoscopy, exercise can help safeguard career longevity and maintain overall wellness. Multiple resources are available to tailor a program that fits your time, budget and needs.
Optimize all of your workstations
Prolonged computer use and desk work is also a significant part of a gastroenterologist’s profession. If using a sitting desk, chair height should allow for 90-degree flexion at the hips and knees and for feet to rest flatly on the floor. The chair should also provide adequate back support for a relaxed upright position. Similar to endoscopy, place the monitor directly in front with the center of the screen slightly below eye level. For mouse and keyboard placement, aim to have the elbows at or slightly below 90 degrees and one’s wrists and fingers in neutral position.
Endoscopy can be hazardous to the endoscopists’ health. Incorporating ergonomic principles creates a safer and more efficient work environment. At the individual level, ergonomically optimized postures during endoscopy as well as during computer-related tasks, room set up, inclusion of microbreaks, and protective exercises can help decrease the risk of repetitive strain injury and prevent disability. Importantly, change at the industry and institutional level has the greatest potential for positive impact. Adoption of ergonomic practices promotes career longevity and ensures that gastroenterologists can continue successful and long careers and provide quality care to their patients without compromising their own health.
Dr. Lipowska is an assistant professor in the division of gastroenterology and hepatology, University of Illinois at Chicago. She disclosed no conflicts. Dr. Shergill is chief of gastroenterology for the San Francisco VA Health Care System. She disclosed consulting work for Boston Scientific and Neptune Medical, honoraria for visiting professorship with Intuitive Surgical, and a research gift from Pentax.
References
Shergill AK. Top tips for implementing an endoscopy ergonomics program. Gastrointest Endosc. 2023 Feb;97(2):361-4.
Pawa S et al. Are all endoscopy-related musculoskeletal injuries created equal? Results of a national gender-based survey. Am J Gastroenterol. 2021;116(3):530-8.
Austin K et al. Musculoskeletal injuries are commonly reported among gastroenterology trainees: Results of a national survey. Dig Dis Sci. 2019;64(6):1439-47.
Lipowska A et al. Ergonomics in the unit: Modeling the environment around the endoscopist. Tech Innov Gastrointest Endosc. 2021;23(3):256-62.
Park A et al. Intraoperative “Micro Breaks” with a targeted stretching enhance surgeon physical function and mental focus: A multicenter cohort study. Ann Surg. 2017;265(2):340-6.
Shergill A et al. Ergonomic endoscopy: An oxymoron or realistic goal? Gastrointest Endosc. 2019;90(6):966-70.
Bridging the gap between GI disorders and nutrition
The gluten-free section in the grocery store didn’t exist when Renee Euler, MS, RD, LD, was diagnosed with celiac disease 30 years ago. A physician handed her a fax about the gluten-free diet from a national support group and said: “Here, read this.”
There was no Google to inform decisions. Patients had to rely on fact sheets or a book from the library.

“I didn’t realize how much nutrition was going to change my world,” said Ms. Euler, who worked as a landscape architect for 15 years before making a pivotal decision to go back to school and train as a dietitian.
Volunteering as a support group leader, and volunteering with the University of Chicago Celiac Disease Center guided this important career change. Ms. Euler discovered she enjoyed teaching people how to live a gluten-free life and that they could enjoy travel and social functions while adhering to dietary restrictions.
she emphasized.
Ms. Euler has made it her life’s work to navigate GI disorders with physicians and patients alike.
She runs her own business, Nutrition Redefined, in Albuquerque and is the chair of the National Celiac Association Celiac/Gluten Intolerance Support Group in Albuquerque. Previously, she chaired the Dietitians in Medical Nutrition Therapy Dietetic Practice Group, a part of the Academy of Nutrition and Dietetics.
In an interview, she talked about the unique dietary struggles people with celiac and other gastrointestinal conditions face, and the strategies she uses to help these patients overcome hurdles and live a more normal life.
Q: What fears did you have to push past to get to where you are in your career?
Ms. Euler: Leaving a successful career as a landscape architect and going back to school was definitely a huge hurdle. When I started my practice in 2017, in my area there were no outpatient GI dietitians providing specialized care for adults with conditions like celiac disease, irritable bowel syndrome (IBS), and inflammatory bowel disease (IBD). I was starting out with no real support.
Realizing that I was going to start a private practice of my own to help the people I wanted to help, was another big fear. “Am I going to succeed? Am I going to fail? What’s going to happen?” But over the years, my practice has grown as I learned to bill insurance and started receiving referrals from a large local GI practice, both of which have been the keys to my success. I have also limited my practice to GI clients so that I can focus my attention on this specialized area of nutrition and stay up to date on the latest developments.
Q: What interests you about the intersection between diet and GI disorders?
Ms. Euler: It’s not just about diet. We’re learning so much about how the gut microbiome can have a potential impact [on other parts of our health]. It’s interesting in terms of how we respond to certain foods, for instance, could affect our mental health. This especially applies to IBS and how the microbiome might be connected to these conditions.
It’s very challenging. There is so much information out there that is not super accurate, or it’s misleading.
Q: You serve as a liaison between the American Gastroenterological Association and the Academy of Nutrition and Dietetics. As a nutritionist with a focus on GI, how do you work with gastroenterologists to manage GI disorders?
Ms. Euler: Some of the dietary therapies that GI doctors recommend don’t provide sufficient guidance. They hand out that two-page fact sheet about diet and send the patient on their way. A lot of these diets have more nuance than what can be expressed in a two-page handout.
Many times, the physician doesn’t know the nuance, or they don’t have time to go over it. That’s where we can really help.
Patients often want diet to be the answer. They want to be told: “You need to eat this and only this, and everything will be fine, and diet’s going to change your world, and you won’t have to take medication.”
What they often don’t realize and understand, is a lot of these dietary therapies are not black and white. Celiac disease means a gluten-free diet for life. But a lot of these dietary therapies that get thrown out to patients as a possibility, like low FODMAPs (fermentable oligosaccharides, disaccharides, monosaccharides, and polyols), are not lifetime diets. They’re tools for us to use to find out what the offending foods are for this person, and what can we do to get their symptoms under control.
Q: What is the biggest practice-related challenge in getting patients to alter their diet to improve their symptoms?
Ms. Euler: A lot of patients that come to me already have over restricted diets. They’re trying to solve things themselves. Rightfully so, a lot of them have a lot of food fears because they have been living with very uncomfortable symptoms for years, and they’re trying to find answers. Those food fears unfortunately are reinforced by social media and the news.
One of my biggest challenges with those clients is working through that process of building their confidence to broaden their diets and add foods back in, without causing their symptoms to flare up. The goal is to get them back on track to having a nutritious diet while trying to manage symptoms.
Q: Can you give me an anecdotal example of a case that wasn’t easy, and you ended up helping that person?
Ms. Euler: I had a patient who had been listening to all the wellness gurus. She was overrestricted to the point of eating just 10 different foods due to allergic and GI symptoms. Patients like this are definitely a challenge because you have to reorient them to the fact that what they’re doing isn’t necessarily working,
My initial assessments are 90 minutes long, so I have a lot of time to sit with a patient and hear their story and understand their background.
I suggested to the patient: “Why don’t we try adding these foods back in, but eliminating these other types of foods and see whether that would help?” 48 hours later, she sent me an email, telling me that she and her husband had talked this through, and they thought I hit the nail on the head: She was focusing on the wrong foods which were causing problems. Those are always great messages to get from patients, when they say: “Oh my gosh, I hadn’t even considered that.”
Q: Describe how you would spend a free Saturday afternoon.
Ms. Euler: They’re so rare – those free Saturday afternoons, but it would probably be a good book that would turn into a nap on the couch.
LIGHTNING ROUND
Do you prefer texting or talking?
Talking in person
What’s your favorite breakfast?
Greek yogurt with fiber, flax seeds, and berries
What’s your favorite junk food?
Ice cream
What’s your favorite fruit?
Garden grown strawberries
What’s your favorite holiday?
Thanksgiving
What’s your favorite type of music?
Jazz
If you weren’t a GI nutritionist, what would you be?
Probably a landscape architect.
The gluten-free section in the grocery store didn’t exist when Renee Euler, MS, RD, LD, was diagnosed with celiac disease 30 years ago. A physician handed her a fax about the gluten-free diet from a national support group and said: “Here, read this.”
There was no Google to inform decisions. Patients had to rely on fact sheets or a book from the library.

“I didn’t realize how much nutrition was going to change my world,” said Ms. Euler, who worked as a landscape architect for 15 years before making a pivotal decision to go back to school and train as a dietitian.
Volunteering as a support group leader, and volunteering with the University of Chicago Celiac Disease Center guided this important career change. Ms. Euler discovered she enjoyed teaching people how to live a gluten-free life and that they could enjoy travel and social functions while adhering to dietary restrictions.
she emphasized.
Ms. Euler has made it her life’s work to navigate GI disorders with physicians and patients alike.
She runs her own business, Nutrition Redefined, in Albuquerque and is the chair of the National Celiac Association Celiac/Gluten Intolerance Support Group in Albuquerque. Previously, she chaired the Dietitians in Medical Nutrition Therapy Dietetic Practice Group, a part of the Academy of Nutrition and Dietetics.
In an interview, she talked about the unique dietary struggles people with celiac and other gastrointestinal conditions face, and the strategies she uses to help these patients overcome hurdles and live a more normal life.
Q: What fears did you have to push past to get to where you are in your career?
Ms. Euler: Leaving a successful career as a landscape architect and going back to school was definitely a huge hurdle. When I started my practice in 2017, in my area there were no outpatient GI dietitians providing specialized care for adults with conditions like celiac disease, irritable bowel syndrome (IBS), and inflammatory bowel disease (IBD). I was starting out with no real support.
Realizing that I was going to start a private practice of my own to help the people I wanted to help, was another big fear. “Am I going to succeed? Am I going to fail? What’s going to happen?” But over the years, my practice has grown as I learned to bill insurance and started receiving referrals from a large local GI practice, both of which have been the keys to my success. I have also limited my practice to GI clients so that I can focus my attention on this specialized area of nutrition and stay up to date on the latest developments.
Q: What interests you about the intersection between diet and GI disorders?
Ms. Euler: It’s not just about diet. We’re learning so much about how the gut microbiome can have a potential impact [on other parts of our health]. It’s interesting in terms of how we respond to certain foods, for instance, could affect our mental health. This especially applies to IBS and how the microbiome might be connected to these conditions.
It’s very challenging. There is so much information out there that is not super accurate, or it’s misleading.
Q: You serve as a liaison between the American Gastroenterological Association and the Academy of Nutrition and Dietetics. As a nutritionist with a focus on GI, how do you work with gastroenterologists to manage GI disorders?
Ms. Euler: Some of the dietary therapies that GI doctors recommend don’t provide sufficient guidance. They hand out that two-page fact sheet about diet and send the patient on their way. A lot of these diets have more nuance than what can be expressed in a two-page handout.
Many times, the physician doesn’t know the nuance, or they don’t have time to go over it. That’s where we can really help.
Patients often want diet to be the answer. They want to be told: “You need to eat this and only this, and everything will be fine, and diet’s going to change your world, and you won’t have to take medication.”
What they often don’t realize and understand, is a lot of these dietary therapies are not black and white. Celiac disease means a gluten-free diet for life. But a lot of these dietary therapies that get thrown out to patients as a possibility, like low FODMAPs (fermentable oligosaccharides, disaccharides, monosaccharides, and polyols), are not lifetime diets. They’re tools for us to use to find out what the offending foods are for this person, and what can we do to get their symptoms under control.
Q: What is the biggest practice-related challenge in getting patients to alter their diet to improve their symptoms?
Ms. Euler: A lot of patients that come to me already have over restricted diets. They’re trying to solve things themselves. Rightfully so, a lot of them have a lot of food fears because they have been living with very uncomfortable symptoms for years, and they’re trying to find answers. Those food fears unfortunately are reinforced by social media and the news.
One of my biggest challenges with those clients is working through that process of building their confidence to broaden their diets and add foods back in, without causing their symptoms to flare up. The goal is to get them back on track to having a nutritious diet while trying to manage symptoms.
Q: Can you give me an anecdotal example of a case that wasn’t easy, and you ended up helping that person?
Ms. Euler: I had a patient who had been listening to all the wellness gurus. She was overrestricted to the point of eating just 10 different foods due to allergic and GI symptoms. Patients like this are definitely a challenge because you have to reorient them to the fact that what they’re doing isn’t necessarily working,
My initial assessments are 90 minutes long, so I have a lot of time to sit with a patient and hear their story and understand their background.
I suggested to the patient: “Why don’t we try adding these foods back in, but eliminating these other types of foods and see whether that would help?” 48 hours later, she sent me an email, telling me that she and her husband had talked this through, and they thought I hit the nail on the head: She was focusing on the wrong foods which were causing problems. Those are always great messages to get from patients, when they say: “Oh my gosh, I hadn’t even considered that.”
Q: Describe how you would spend a free Saturday afternoon.
Ms. Euler: They’re so rare – those free Saturday afternoons, but it would probably be a good book that would turn into a nap on the couch.
LIGHTNING ROUND
Do you prefer texting or talking?
Talking in person
What’s your favorite breakfast?
Greek yogurt with fiber, flax seeds, and berries
What’s your favorite junk food?
Ice cream
What’s your favorite fruit?
Garden grown strawberries
What’s your favorite holiday?
Thanksgiving
What’s your favorite type of music?
Jazz
If you weren’t a GI nutritionist, what would you be?
Probably a landscape architect.
The gluten-free section in the grocery store didn’t exist when Renee Euler, MS, RD, LD, was diagnosed with celiac disease 30 years ago. A physician handed her a fax about the gluten-free diet from a national support group and said: “Here, read this.”
There was no Google to inform decisions. Patients had to rely on fact sheets or a book from the library.

“I didn’t realize how much nutrition was going to change my world,” said Ms. Euler, who worked as a landscape architect for 15 years before making a pivotal decision to go back to school and train as a dietitian.
Volunteering as a support group leader, and volunteering with the University of Chicago Celiac Disease Center guided this important career change. Ms. Euler discovered she enjoyed teaching people how to live a gluten-free life and that they could enjoy travel and social functions while adhering to dietary restrictions.
she emphasized.
Ms. Euler has made it her life’s work to navigate GI disorders with physicians and patients alike.
She runs her own business, Nutrition Redefined, in Albuquerque and is the chair of the National Celiac Association Celiac/Gluten Intolerance Support Group in Albuquerque. Previously, she chaired the Dietitians in Medical Nutrition Therapy Dietetic Practice Group, a part of the Academy of Nutrition and Dietetics.
In an interview, she talked about the unique dietary struggles people with celiac and other gastrointestinal conditions face, and the strategies she uses to help these patients overcome hurdles and live a more normal life.
Q: What fears did you have to push past to get to where you are in your career?
Ms. Euler: Leaving a successful career as a landscape architect and going back to school was definitely a huge hurdle. When I started my practice in 2017, in my area there were no outpatient GI dietitians providing specialized care for adults with conditions like celiac disease, irritable bowel syndrome (IBS), and inflammatory bowel disease (IBD). I was starting out with no real support.
Realizing that I was going to start a private practice of my own to help the people I wanted to help, was another big fear. “Am I going to succeed? Am I going to fail? What’s going to happen?” But over the years, my practice has grown as I learned to bill insurance and started receiving referrals from a large local GI practice, both of which have been the keys to my success. I have also limited my practice to GI clients so that I can focus my attention on this specialized area of nutrition and stay up to date on the latest developments.
Q: What interests you about the intersection between diet and GI disorders?
Ms. Euler: It’s not just about diet. We’re learning so much about how the gut microbiome can have a potential impact [on other parts of our health]. It’s interesting in terms of how we respond to certain foods, for instance, could affect our mental health. This especially applies to IBS and how the microbiome might be connected to these conditions.
It’s very challenging. There is so much information out there that is not super accurate, or it’s misleading.
Q: You serve as a liaison between the American Gastroenterological Association and the Academy of Nutrition and Dietetics. As a nutritionist with a focus on GI, how do you work with gastroenterologists to manage GI disorders?
Ms. Euler: Some of the dietary therapies that GI doctors recommend don’t provide sufficient guidance. They hand out that two-page fact sheet about diet and send the patient on their way. A lot of these diets have more nuance than what can be expressed in a two-page handout.
Many times, the physician doesn’t know the nuance, or they don’t have time to go over it. That’s where we can really help.
Patients often want diet to be the answer. They want to be told: “You need to eat this and only this, and everything will be fine, and diet’s going to change your world, and you won’t have to take medication.”
What they often don’t realize and understand, is a lot of these dietary therapies are not black and white. Celiac disease means a gluten-free diet for life. But a lot of these dietary therapies that get thrown out to patients as a possibility, like low FODMAPs (fermentable oligosaccharides, disaccharides, monosaccharides, and polyols), are not lifetime diets. They’re tools for us to use to find out what the offending foods are for this person, and what can we do to get their symptoms under control.
Q: What is the biggest practice-related challenge in getting patients to alter their diet to improve their symptoms?
Ms. Euler: A lot of patients that come to me already have over restricted diets. They’re trying to solve things themselves. Rightfully so, a lot of them have a lot of food fears because they have been living with very uncomfortable symptoms for years, and they’re trying to find answers. Those food fears unfortunately are reinforced by social media and the news.
One of my biggest challenges with those clients is working through that process of building their confidence to broaden their diets and add foods back in, without causing their symptoms to flare up. The goal is to get them back on track to having a nutritious diet while trying to manage symptoms.
Q: Can you give me an anecdotal example of a case that wasn’t easy, and you ended up helping that person?
Ms. Euler: I had a patient who had been listening to all the wellness gurus. She was overrestricted to the point of eating just 10 different foods due to allergic and GI symptoms. Patients like this are definitely a challenge because you have to reorient them to the fact that what they’re doing isn’t necessarily working,
My initial assessments are 90 minutes long, so I have a lot of time to sit with a patient and hear their story and understand their background.
I suggested to the patient: “Why don’t we try adding these foods back in, but eliminating these other types of foods and see whether that would help?” 48 hours later, she sent me an email, telling me that she and her husband had talked this through, and they thought I hit the nail on the head: She was focusing on the wrong foods which were causing problems. Those are always great messages to get from patients, when they say: “Oh my gosh, I hadn’t even considered that.”
Q: Describe how you would spend a free Saturday afternoon.
Ms. Euler: They’re so rare – those free Saturday afternoons, but it would probably be a good book that would turn into a nap on the couch.
LIGHTNING ROUND
Do you prefer texting or talking?
Talking in person
What’s your favorite breakfast?
Greek yogurt with fiber, flax seeds, and berries
What’s your favorite junk food?
Ice cream
What’s your favorite fruit?
Garden grown strawberries
What’s your favorite holiday?
Thanksgiving
What’s your favorite type of music?
Jazz
If you weren’t a GI nutritionist, what would you be?
Probably a landscape architect.