User login
New “Bone Balance” Index Can Predict Women’s Risk for Rapid Bone Loss
A new index can be used to predict which women will experience faster bone loss while transitioning to menopause, according to a study in the Journal of Clinical Endocrinology & Metabolism.
To create the new index, called the Bone Balance Index, researchers used data from a cohort of 685 women ages 42 to 52 as they went through menopause. The women were either premenopausal or in early perimenopause when they enrolled in the study, and all participants had their final menstrual period during follow-up.
Urine and blood samples were taken from the women to measure bone turnover markers. The women also had their bone mineral density measured every year.
The investigators combined measurements of bone breakdown and bone formation to determine each woman’s net bone balance before their final menstrual period. The study authors found that compared to a measurement of bone breakdown alone, the Bone Balance Index was a stronger predictor of bone loss from 2 years before the final menstrual period to 3 to 4 years later.
Suggested Reading
Shieh A, Han W, Ishii S, et al. Quantifying the balance between total bone formation and total bone resorption: an index of net bone formation. J Clin Endocrinol Metab. 2016 Jun 23:jc20154262. [Epub ahead of print]
A new index can be used to predict which women will experience faster bone loss while transitioning to menopause, according to a study in the Journal of Clinical Endocrinology & Metabolism.
To create the new index, called the Bone Balance Index, researchers used data from a cohort of 685 women ages 42 to 52 as they went through menopause. The women were either premenopausal or in early perimenopause when they enrolled in the study, and all participants had their final menstrual period during follow-up.
Urine and blood samples were taken from the women to measure bone turnover markers. The women also had their bone mineral density measured every year.
The investigators combined measurements of bone breakdown and bone formation to determine each woman’s net bone balance before their final menstrual period. The study authors found that compared to a measurement of bone breakdown alone, the Bone Balance Index was a stronger predictor of bone loss from 2 years before the final menstrual period to 3 to 4 years later.
A new index can be used to predict which women will experience faster bone loss while transitioning to menopause, according to a study in the Journal of Clinical Endocrinology & Metabolism.
To create the new index, called the Bone Balance Index, researchers used data from a cohort of 685 women ages 42 to 52 as they went through menopause. The women were either premenopausal or in early perimenopause when they enrolled in the study, and all participants had their final menstrual period during follow-up.
Urine and blood samples were taken from the women to measure bone turnover markers. The women also had their bone mineral density measured every year.
The investigators combined measurements of bone breakdown and bone formation to determine each woman’s net bone balance before their final menstrual period. The study authors found that compared to a measurement of bone breakdown alone, the Bone Balance Index was a stronger predictor of bone loss from 2 years before the final menstrual period to 3 to 4 years later.
Suggested Reading
Shieh A, Han W, Ishii S, et al. Quantifying the balance between total bone formation and total bone resorption: an index of net bone formation. J Clin Endocrinol Metab. 2016 Jun 23:jc20154262. [Epub ahead of print]
Suggested Reading
Shieh A, Han W, Ishii S, et al. Quantifying the balance between total bone formation and total bone resorption: an index of net bone formation. J Clin Endocrinol Metab. 2016 Jun 23:jc20154262. [Epub ahead of print]
Many Patients Who Take Opioids Before Arthroplasty Continue to Take Them for Months Afterwards
A substantial percentage of patients who receive opioid medications before undergoing arthroplasty continue to take them up to 6 months after surgery, according to a study published in Pain.
Researchers analyzed opioid use in 574 patients who underwent arthroplasty. Patients were followed up at 1, 3, and 6 months after surgery to assess rates of long-term opioid use and risk factors for long-term opioid use. About 30% of patients were taking opioids prior to their joint replacement surgery. Of this group, 53% of knee-replacement patients and 35% of hip replacement patients continued taking opioids 6 months after surgery.
Patients who were not taking opioids prior to surgery were less likely to report persistent opioid use. About 8% in the knee replacement group and 4% in the hip replacement group were still taking opioids at the 6-month follow-up. Patients who were taking the highest doses of opioids before surgery were most likely to continue to take them for 6 months.
Among patients not previously taking opioids, those with higher pain scores the day of surgery were more likely to report persistent opioid use at 6 months. However, improvement in knee or hip pain after arthroplasty did not reduce the likelihood of long-term opioid use.
Suggested Reading
Goesling J, Moser SE, Zaidi B, et al. Trends and predictors of opioid use after total knee and total hip arthroplasty. Pain. 2016;157(6):1259-1265.
A substantial percentage of patients who receive opioid medications before undergoing arthroplasty continue to take them up to 6 months after surgery, according to a study published in Pain.
Researchers analyzed opioid use in 574 patients who underwent arthroplasty. Patients were followed up at 1, 3, and 6 months after surgery to assess rates of long-term opioid use and risk factors for long-term opioid use. About 30% of patients were taking opioids prior to their joint replacement surgery. Of this group, 53% of knee-replacement patients and 35% of hip replacement patients continued taking opioids 6 months after surgery.
Patients who were not taking opioids prior to surgery were less likely to report persistent opioid use. About 8% in the knee replacement group and 4% in the hip replacement group were still taking opioids at the 6-month follow-up. Patients who were taking the highest doses of opioids before surgery were most likely to continue to take them for 6 months.
Among patients not previously taking opioids, those with higher pain scores the day of surgery were more likely to report persistent opioid use at 6 months. However, improvement in knee or hip pain after arthroplasty did not reduce the likelihood of long-term opioid use.
A substantial percentage of patients who receive opioid medications before undergoing arthroplasty continue to take them up to 6 months after surgery, according to a study published in Pain.
Researchers analyzed opioid use in 574 patients who underwent arthroplasty. Patients were followed up at 1, 3, and 6 months after surgery to assess rates of long-term opioid use and risk factors for long-term opioid use. About 30% of patients were taking opioids prior to their joint replacement surgery. Of this group, 53% of knee-replacement patients and 35% of hip replacement patients continued taking opioids 6 months after surgery.
Patients who were not taking opioids prior to surgery were less likely to report persistent opioid use. About 8% in the knee replacement group and 4% in the hip replacement group were still taking opioids at the 6-month follow-up. Patients who were taking the highest doses of opioids before surgery were most likely to continue to take them for 6 months.
Among patients not previously taking opioids, those with higher pain scores the day of surgery were more likely to report persistent opioid use at 6 months. However, improvement in knee or hip pain after arthroplasty did not reduce the likelihood of long-term opioid use.
Suggested Reading
Goesling J, Moser SE, Zaidi B, et al. Trends and predictors of opioid use after total knee and total hip arthroplasty. Pain. 2016;157(6):1259-1265.
Suggested Reading
Goesling J, Moser SE, Zaidi B, et al. Trends and predictors of opioid use after total knee and total hip arthroplasty. Pain. 2016;157(6):1259-1265.
AAOS Introduces New Apps for Patient Education
The American Academy of Orthopedic Surgeons has introduced apps that orthopedic surgeons can use to explain musculoskeletal problems and procedures to their patients. The Guides to Orthopedic Surgery cover total knee replacement, total hip replacement, and ACL reconstruction. These apps can be loaded onto exam room desktops or used on an iPad.
The apps also provide ways to create custom educational information for patients, and may be set up with certain electronic medical records to support Meaningful Use requirements. A free trial of the apps is available until June 30. More information: www.aaosnotice.org/Ortho_App/.
The American Academy of Orthopedic Surgeons has introduced apps that orthopedic surgeons can use to explain musculoskeletal problems and procedures to their patients. The Guides to Orthopedic Surgery cover total knee replacement, total hip replacement, and ACL reconstruction. These apps can be loaded onto exam room desktops or used on an iPad.
The apps also provide ways to create custom educational information for patients, and may be set up with certain electronic medical records to support Meaningful Use requirements. A free trial of the apps is available until June 30. More information: www.aaosnotice.org/Ortho_App/.
The American Academy of Orthopedic Surgeons has introduced apps that orthopedic surgeons can use to explain musculoskeletal problems and procedures to their patients. The Guides to Orthopedic Surgery cover total knee replacement, total hip replacement, and ACL reconstruction. These apps can be loaded onto exam room desktops or used on an iPad.
The apps also provide ways to create custom educational information for patients, and may be set up with certain electronic medical records to support Meaningful Use requirements. A free trial of the apps is available until June 30. More information: www.aaosnotice.org/Ortho_App/.
Primary care gout patients often discontinue allopurinol
LONDON – Many patients with newly diagnosed gout who are prescribed allopurinol to reduce their uric acid level and prevent recurrent episodes fail to stick with their treatment, according to an analysis of more than 47,000 U.K. gout patients who received prescriptions for allopurinol during the 28-year period of 1987-2014.
One possible contributing factor to this pattern may be physicians who inadequately stress to patients the importance of sticking with allopurinol treatment to improve their long-term health, Lieke E.J.M. Scheepers said at the European Congress of Rheumatology.
“We think that physicians underestimate” the low level of gout patient adherence to allopurinol, said Ms. Scheepers, a PhD student in the department of rheumatology at Maastricht (the Netherlands) University.
“We view gout as a chronic disease, but many physicians and patients believe gout can occur as a single episode, and then it’s over,” explained Dr. Annelies Boonen, the senior investigator on the study, in an interview. “Gout patients often don’t appreciate that they will need to take their medication for many years. We need to convince primary care physicians to follow gout patients closely and not wait [to resume treatment] until the patient has a new episode,” said Dr. Boonen, a professor of rheumatology at Maastricht University.
“Some physicians are not convinced that it harms a patient to have two or three acute gout attacks a year, but there is a subgroup that will have joint damage” from this pattern of recurrence, she noted. However, Dr. Boonen acknowledged that gout patients usually seen in primary care practice often don’t have the same level of disease severity and recurrence as the patients she sees in her referral clinic. “We don’t know which gout patients will develop joint damage,” she admitted.
Another barrier to good adherence with long-term uric acid–lowering treatment is that “patients who don’t have daily symptoms often question why they should continue to take their medication,” added Ms. Scheepers. “Many patients fear the possible adverse effects of their treatment” more than they fear a possible gout recurrence.
Ms. Scheepers and her associates analyzed data from 47,774 patients with newly diagnosed gout receiving treatment exclusively with allopurinol from about 680 primary care U.K. physicians and archived in the Clinical Practice Research Datalink maintained by the U.K. government. The patients averaged 64 years old, and three-quarters were men.
During their first year on treatment, 57% of the patients had at least one 30-day gap in their use of allopurinol, and 38% had at least one 90-day gap in their allopurinol treatment, Ms. Scheepers reported. During an average follow-up of nearly 6 years, 77% of patients had at least one 30-day gap in treatment and 54% had at least one 90-day gap. The median time to a 90-day gap in allopurinol treatment was just under 3 years (1,059 days).
The researchers also assessed patient compliance and adherence to therapy by analyzing the percentage of days during follow-up that they took allopurinol. The overall average percentage of days on treatment was 57%, and 39% of patients received allopurinol on at least 80% of the days when they were followed.
Another analysis focused specifically on 14,808 patients who restarted on allopurinol after they had stopped their use of the drug for at least 90 days. Among these patients, the rate of a new 30-day gap during their first year back on treatment was 72%, with 48% having a new gap of 90 days or more during their first year back on treatment. During total follow-up of this group of patients with an established history of stopping allopurinol, 82% had a new gap in treatment of at least 30 days and 63% had a gap of 90 days or more.
The researchers also examined demographic and clinical variables that significantly linked with either greater or lesser adherence to allopurinol treatment. Two subgroups – women and smokers – showed significantly worse adherence, while older patients, patients who also took other drugs (antihypertensive medications, colchicine, or statins), and patients with various comorbidities (dementia, diabetes, depression, or impaired renal function) all had significantly better adherence. One possible explanation for this pattern is that patients who are older, have comorbidities, or already take other drugs may have a better-established routine and mindset for adhering to medication regimens that helps them remain adherent to allopurinol, Ms. Scheepers said.
Dr. Scheepers and Dr. Boonen had no disclosures.
On Twitter @mitchelzoler
LONDON – Many patients with newly diagnosed gout who are prescribed allopurinol to reduce their uric acid level and prevent recurrent episodes fail to stick with their treatment, according to an analysis of more than 47,000 U.K. gout patients who received prescriptions for allopurinol during the 28-year period of 1987-2014.
One possible contributing factor to this pattern may be physicians who inadequately stress to patients the importance of sticking with allopurinol treatment to improve their long-term health, Lieke E.J.M. Scheepers said at the European Congress of Rheumatology.
“We think that physicians underestimate” the low level of gout patient adherence to allopurinol, said Ms. Scheepers, a PhD student in the department of rheumatology at Maastricht (the Netherlands) University.
“We view gout as a chronic disease, but many physicians and patients believe gout can occur as a single episode, and then it’s over,” explained Dr. Annelies Boonen, the senior investigator on the study, in an interview. “Gout patients often don’t appreciate that they will need to take their medication for many years. We need to convince primary care physicians to follow gout patients closely and not wait [to resume treatment] until the patient has a new episode,” said Dr. Boonen, a professor of rheumatology at Maastricht University.
“Some physicians are not convinced that it harms a patient to have two or three acute gout attacks a year, but there is a subgroup that will have joint damage” from this pattern of recurrence, she noted. However, Dr. Boonen acknowledged that gout patients usually seen in primary care practice often don’t have the same level of disease severity and recurrence as the patients she sees in her referral clinic. “We don’t know which gout patients will develop joint damage,” she admitted.
Another barrier to good adherence with long-term uric acid–lowering treatment is that “patients who don’t have daily symptoms often question why they should continue to take their medication,” added Ms. Scheepers. “Many patients fear the possible adverse effects of their treatment” more than they fear a possible gout recurrence.
Ms. Scheepers and her associates analyzed data from 47,774 patients with newly diagnosed gout receiving treatment exclusively with allopurinol from about 680 primary care U.K. physicians and archived in the Clinical Practice Research Datalink maintained by the U.K. government. The patients averaged 64 years old, and three-quarters were men.
During their first year on treatment, 57% of the patients had at least one 30-day gap in their use of allopurinol, and 38% had at least one 90-day gap in their allopurinol treatment, Ms. Scheepers reported. During an average follow-up of nearly 6 years, 77% of patients had at least one 30-day gap in treatment and 54% had at least one 90-day gap. The median time to a 90-day gap in allopurinol treatment was just under 3 years (1,059 days).
The researchers also assessed patient compliance and adherence to therapy by analyzing the percentage of days during follow-up that they took allopurinol. The overall average percentage of days on treatment was 57%, and 39% of patients received allopurinol on at least 80% of the days when they were followed.
Another analysis focused specifically on 14,808 patients who restarted on allopurinol after they had stopped their use of the drug for at least 90 days. Among these patients, the rate of a new 30-day gap during their first year back on treatment was 72%, with 48% having a new gap of 90 days or more during their first year back on treatment. During total follow-up of this group of patients with an established history of stopping allopurinol, 82% had a new gap in treatment of at least 30 days and 63% had a gap of 90 days or more.
The researchers also examined demographic and clinical variables that significantly linked with either greater or lesser adherence to allopurinol treatment. Two subgroups – women and smokers – showed significantly worse adherence, while older patients, patients who also took other drugs (antihypertensive medications, colchicine, or statins), and patients with various comorbidities (dementia, diabetes, depression, or impaired renal function) all had significantly better adherence. One possible explanation for this pattern is that patients who are older, have comorbidities, or already take other drugs may have a better-established routine and mindset for adhering to medication regimens that helps them remain adherent to allopurinol, Ms. Scheepers said.
Dr. Scheepers and Dr. Boonen had no disclosures.
On Twitter @mitchelzoler
LONDON – Many patients with newly diagnosed gout who are prescribed allopurinol to reduce their uric acid level and prevent recurrent episodes fail to stick with their treatment, according to an analysis of more than 47,000 U.K. gout patients who received prescriptions for allopurinol during the 28-year period of 1987-2014.
One possible contributing factor to this pattern may be physicians who inadequately stress to patients the importance of sticking with allopurinol treatment to improve their long-term health, Lieke E.J.M. Scheepers said at the European Congress of Rheumatology.
“We think that physicians underestimate” the low level of gout patient adherence to allopurinol, said Ms. Scheepers, a PhD student in the department of rheumatology at Maastricht (the Netherlands) University.
“We view gout as a chronic disease, but many physicians and patients believe gout can occur as a single episode, and then it’s over,” explained Dr. Annelies Boonen, the senior investigator on the study, in an interview. “Gout patients often don’t appreciate that they will need to take their medication for many years. We need to convince primary care physicians to follow gout patients closely and not wait [to resume treatment] until the patient has a new episode,” said Dr. Boonen, a professor of rheumatology at Maastricht University.
“Some physicians are not convinced that it harms a patient to have two or three acute gout attacks a year, but there is a subgroup that will have joint damage” from this pattern of recurrence, she noted. However, Dr. Boonen acknowledged that gout patients usually seen in primary care practice often don’t have the same level of disease severity and recurrence as the patients she sees in her referral clinic. “We don’t know which gout patients will develop joint damage,” she admitted.
Another barrier to good adherence with long-term uric acid–lowering treatment is that “patients who don’t have daily symptoms often question why they should continue to take their medication,” added Ms. Scheepers. “Many patients fear the possible adverse effects of their treatment” more than they fear a possible gout recurrence.
Ms. Scheepers and her associates analyzed data from 47,774 patients with newly diagnosed gout receiving treatment exclusively with allopurinol from about 680 primary care U.K. physicians and archived in the Clinical Practice Research Datalink maintained by the U.K. government. The patients averaged 64 years old, and three-quarters were men.
During their first year on treatment, 57% of the patients had at least one 30-day gap in their use of allopurinol, and 38% had at least one 90-day gap in their allopurinol treatment, Ms. Scheepers reported. During an average follow-up of nearly 6 years, 77% of patients had at least one 30-day gap in treatment and 54% had at least one 90-day gap. The median time to a 90-day gap in allopurinol treatment was just under 3 years (1,059 days).
The researchers also assessed patient compliance and adherence to therapy by analyzing the percentage of days during follow-up that they took allopurinol. The overall average percentage of days on treatment was 57%, and 39% of patients received allopurinol on at least 80% of the days when they were followed.
Another analysis focused specifically on 14,808 patients who restarted on allopurinol after they had stopped their use of the drug for at least 90 days. Among these patients, the rate of a new 30-day gap during their first year back on treatment was 72%, with 48% having a new gap of 90 days or more during their first year back on treatment. During total follow-up of this group of patients with an established history of stopping allopurinol, 82% had a new gap in treatment of at least 30 days and 63% had a gap of 90 days or more.
The researchers also examined demographic and clinical variables that significantly linked with either greater or lesser adherence to allopurinol treatment. Two subgroups – women and smokers – showed significantly worse adherence, while older patients, patients who also took other drugs (antihypertensive medications, colchicine, or statins), and patients with various comorbidities (dementia, diabetes, depression, or impaired renal function) all had significantly better adherence. One possible explanation for this pattern is that patients who are older, have comorbidities, or already take other drugs may have a better-established routine and mindset for adhering to medication regimens that helps them remain adherent to allopurinol, Ms. Scheepers said.
Dr. Scheepers and Dr. Boonen had no disclosures.
On Twitter @mitchelzoler
AT THE EULAR 2016 CONGRESS
Key clinical point: A majority of newly diagnosed gout patients in the U.K. have significant gaps in treatment and only a minority show good treatment adherence.
Major finding: During their first year of allopurinol treatment, 57% of gout patients had a treatment gap of 30 days or longer.
Data source: A database of about 680 U.K. general practice physicians maintained by the Clinical Practice Research Datalink that included 47,774 patients with incident gout treated exclusively with allopurinol during 1987-2014.
Disclosures: Dr. Scheepers and Dr. Boonen had no disclosures.
Empagliflozin surpasses glimepiride as metformin add-on
NEW ORLEANS – When used as an add-on therapy to metformin in patients with type 2 diabetes mellitus, empagliflozin has sustained safety and efficacy in reducing in hemoglobin A1c (HbA1c) and other key metabolic measures for up to 4 years, according to results of the EMPA-REG H2H-SU trial presented at the annual scientific sessions of the American Diabetes Association.
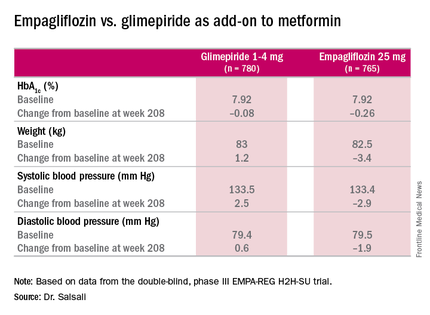
The latest results of the double-blind, phase III trial extended out to 4 years the previously published 2-year results (Lancet Diabetes Endocrinol. 2014;2:691-700) in comparing empagliflozin, a sodium glucose cotransporter inhibitor, 25 mg daily, and the sulfonylurea glimepiride, 1-4 mg daily, as add-on therapy to metformin. “As previously reported, after 2 years there was a modest difference in HbA1c between empagliflozin and glimepiride,” said Dr. Afshin Salsali, reporting for the trial group. “This difference continued for the remainder of the study, although there was a slight rebound in each group,” according to Dr. Salsali, executive director of global clinical development at Boehringer Ingelheim, Ridgefield, Conn.
The 4-year results involved more than 73% of the 1,545 patients who participated in the 2-year study, Dr. Salsali said. The majority of patients were white, with average HbA1c levels of 7.92% and weight of 82.5 kg at the outset of the 2-year study. After 4 years, average HbA1c levels declined 0.08% in those on glimepiride vs. 0.26% for empagliflozin, Dr. Salsali said, achieving the primary study endpoint of noninferiority to glimepiride. However, he noted that rates of hypoglycemia varied dramatically between the two therapies. “This was achieved at the rate of much lower hypoglycemia on empagliflozin, compared with glimepiride, 3% vs. 25% (P less than .001),” he said.
Dr. Salsali pointed out that patients in the empagliflozin group were less likely to need rescue therapy, with an odds ratio of 0.56 (P less than.001), and a much later need for intervention. The advantage empagliflozin showed in both weight loss and blood pressure reduction in the 2-year study also held up for 4 years, Dr. Salsali said. “The pattern of blood pressure reduction achieved at initiation in the first few weeks after using empagliflozin more or less remained at the same level throughout this study,” he said.
One of most important messages from the study, Dr. Salsali said, is the impact empagliflozin had on the estimated glomerular filtration rate (eGFR). “Previously in the pivotal diabetes trial that we had, we showed a reduction in eGFR with a slight rebound, but we didn’t have enough time to see the full picture,” he said. “Now we have the luxury of looking into the full eGFR change over time up to 4 years. After the initial reduction in eGFR, there is a gradual increase and return of eGFR to the baseline level which remained stable for the duration of this study.” However, the glimepiride arm showed the “expected” average rate of eGFR reduction of 2 mL/min per year for type 2 diabetes, he said.
The reporting of adverse events was about the same between both groups, but serious adverse events were slightly higher among those on empagliflozin: 7.36/100 patient years vs. 7.06/100 patient years on glimepiride. The former had higher rates of urinary tract infections (6.96 vs. 5.82 per 100 patient-years) and volume depletion (0.82 vs. 0.63 per 100 patient years), but rates of bone fractures were almost identical (4.1% vs. 4.2%). There was no reported diabetic ketoacidosis in either group.
“Empagliflozin 25 mg after 208 weeks of treatment as an add-on to metformin led to modest numerical advantage in mean HbA1c change from baseline and clinically relevant reduction in weight, systolic blood pressure, and diastolic blood pressure,” Dr. Salsali said. “The difference in changes in HbA1c between empagliflozin and glimepiride were small, but empagliflozin was associated with a significant lower risk of hypoglycemia and significantly fewer patients required rescue therapy.”
Besides Dr. Salsali’s disclosure, coauthors disclosed relationships with Novo Nordisk, Medtronic, and the Steno Diabetes Center. Three other coauthors were employees of Boehringer Ingelheim.
NEW ORLEANS – When used as an add-on therapy to metformin in patients with type 2 diabetes mellitus, empagliflozin has sustained safety and efficacy in reducing in hemoglobin A1c (HbA1c) and other key metabolic measures for up to 4 years, according to results of the EMPA-REG H2H-SU trial presented at the annual scientific sessions of the American Diabetes Association.
The latest results of the double-blind, phase III trial extended out to 4 years the previously published 2-year results (Lancet Diabetes Endocrinol. 2014;2:691-700) in comparing empagliflozin, a sodium glucose cotransporter inhibitor, 25 mg daily, and the sulfonylurea glimepiride, 1-4 mg daily, as add-on therapy to metformin. “As previously reported, after 2 years there was a modest difference in HbA1c between empagliflozin and glimepiride,” said Dr. Afshin Salsali, reporting for the trial group. “This difference continued for the remainder of the study, although there was a slight rebound in each group,” according to Dr. Salsali, executive director of global clinical development at Boehringer Ingelheim, Ridgefield, Conn.
The 4-year results involved more than 73% of the 1,545 patients who participated in the 2-year study, Dr. Salsali said. The majority of patients were white, with average HbA1c levels of 7.92% and weight of 82.5 kg at the outset of the 2-year study. After 4 years, average HbA1c levels declined 0.08% in those on glimepiride vs. 0.26% for empagliflozin, Dr. Salsali said, achieving the primary study endpoint of noninferiority to glimepiride. However, he noted that rates of hypoglycemia varied dramatically between the two therapies. “This was achieved at the rate of much lower hypoglycemia on empagliflozin, compared with glimepiride, 3% vs. 25% (P less than .001),” he said.
Dr. Salsali pointed out that patients in the empagliflozin group were less likely to need rescue therapy, with an odds ratio of 0.56 (P less than.001), and a much later need for intervention. The advantage empagliflozin showed in both weight loss and blood pressure reduction in the 2-year study also held up for 4 years, Dr. Salsali said. “The pattern of blood pressure reduction achieved at initiation in the first few weeks after using empagliflozin more or less remained at the same level throughout this study,” he said.

One of most important messages from the study, Dr. Salsali said, is the impact empagliflozin had on the estimated glomerular filtration rate (eGFR). “Previously in the pivotal diabetes trial that we had, we showed a reduction in eGFR with a slight rebound, but we didn’t have enough time to see the full picture,” he said. “Now we have the luxury of looking into the full eGFR change over time up to 4 years. After the initial reduction in eGFR, there is a gradual increase and return of eGFR to the baseline level which remained stable for the duration of this study.” However, the glimepiride arm showed the “expected” average rate of eGFR reduction of 2 mL/min per year for type 2 diabetes, he said.
The reporting of adverse events was about the same between both groups, but serious adverse events were slightly higher among those on empagliflozin: 7.36/100 patient years vs. 7.06/100 patient years on glimepiride. The former had higher rates of urinary tract infections (6.96 vs. 5.82 per 100 patient-years) and volume depletion (0.82 vs. 0.63 per 100 patient years), but rates of bone fractures were almost identical (4.1% vs. 4.2%). There was no reported diabetic ketoacidosis in either group.
“Empagliflozin 25 mg after 208 weeks of treatment as an add-on to metformin led to modest numerical advantage in mean HbA1c change from baseline and clinically relevant reduction in weight, systolic blood pressure, and diastolic blood pressure,” Dr. Salsali said. “The difference in changes in HbA1c between empagliflozin and glimepiride were small, but empagliflozin was associated with a significant lower risk of hypoglycemia and significantly fewer patients required rescue therapy.”
Besides Dr. Salsali’s disclosure, coauthors disclosed relationships with Novo Nordisk, Medtronic, and the Steno Diabetes Center. Three other coauthors were employees of Boehringer Ingelheim.
NEW ORLEANS – When used as an add-on therapy to metformin in patients with type 2 diabetes mellitus, empagliflozin has sustained safety and efficacy in reducing in hemoglobin A1c (HbA1c) and other key metabolic measures for up to 4 years, according to results of the EMPA-REG H2H-SU trial presented at the annual scientific sessions of the American Diabetes Association.
The latest results of the double-blind, phase III trial extended out to 4 years the previously published 2-year results (Lancet Diabetes Endocrinol. 2014;2:691-700) in comparing empagliflozin, a sodium glucose cotransporter inhibitor, 25 mg daily, and the sulfonylurea glimepiride, 1-4 mg daily, as add-on therapy to metformin. “As previously reported, after 2 years there was a modest difference in HbA1c between empagliflozin and glimepiride,” said Dr. Afshin Salsali, reporting for the trial group. “This difference continued for the remainder of the study, although there was a slight rebound in each group,” according to Dr. Salsali, executive director of global clinical development at Boehringer Ingelheim, Ridgefield, Conn.
The 4-year results involved more than 73% of the 1,545 patients who participated in the 2-year study, Dr. Salsali said. The majority of patients were white, with average HbA1c levels of 7.92% and weight of 82.5 kg at the outset of the 2-year study. After 4 years, average HbA1c levels declined 0.08% in those on glimepiride vs. 0.26% for empagliflozin, Dr. Salsali said, achieving the primary study endpoint of noninferiority to glimepiride. However, he noted that rates of hypoglycemia varied dramatically between the two therapies. “This was achieved at the rate of much lower hypoglycemia on empagliflozin, compared with glimepiride, 3% vs. 25% (P less than .001),” he said.
Dr. Salsali pointed out that patients in the empagliflozin group were less likely to need rescue therapy, with an odds ratio of 0.56 (P less than.001), and a much later need for intervention. The advantage empagliflozin showed in both weight loss and blood pressure reduction in the 2-year study also held up for 4 years, Dr. Salsali said. “The pattern of blood pressure reduction achieved at initiation in the first few weeks after using empagliflozin more or less remained at the same level throughout this study,” he said.

One of most important messages from the study, Dr. Salsali said, is the impact empagliflozin had on the estimated glomerular filtration rate (eGFR). “Previously in the pivotal diabetes trial that we had, we showed a reduction in eGFR with a slight rebound, but we didn’t have enough time to see the full picture,” he said. “Now we have the luxury of looking into the full eGFR change over time up to 4 years. After the initial reduction in eGFR, there is a gradual increase and return of eGFR to the baseline level which remained stable for the duration of this study.” However, the glimepiride arm showed the “expected” average rate of eGFR reduction of 2 mL/min per year for type 2 diabetes, he said.
The reporting of adverse events was about the same between both groups, but serious adverse events were slightly higher among those on empagliflozin: 7.36/100 patient years vs. 7.06/100 patient years on glimepiride. The former had higher rates of urinary tract infections (6.96 vs. 5.82 per 100 patient-years) and volume depletion (0.82 vs. 0.63 per 100 patient years), but rates of bone fractures were almost identical (4.1% vs. 4.2%). There was no reported diabetic ketoacidosis in either group.
“Empagliflozin 25 mg after 208 weeks of treatment as an add-on to metformin led to modest numerical advantage in mean HbA1c change from baseline and clinically relevant reduction in weight, systolic blood pressure, and diastolic blood pressure,” Dr. Salsali said. “The difference in changes in HbA1c between empagliflozin and glimepiride were small, but empagliflozin was associated with a significant lower risk of hypoglycemia and significantly fewer patients required rescue therapy.”
Besides Dr. Salsali’s disclosure, coauthors disclosed relationships with Novo Nordisk, Medtronic, and the Steno Diabetes Center. Three other coauthors were employees of Boehringer Ingelheim.
AT THE ADA ANNUAL SCIENTIFIC SESSIONS
Key clinical point: Clinical trial results show the HBA1c-lowering effects of empagliflozin endure at least 4 years.
Major finding: Average HbA1c levels declined 0.26% for those on empagliflozin vs. 0.08% for people taking glimepiride as add-on therapy to metformin.
Data source: Double-blind, phase III clinical trial involving 1,545 patients.
Disclosures: Dr. Salsali is an employee of Boehringer Ingelheim, as are three other coauthors. Other coauthors disclosed relationships with Novo Nordisk, Medtronic, and the Steno Diabetes Center.
Apixaban Reduces Risks for AF Patients with Renal Dysfunction
NEW YORK - In patients with atrial fibrillation (AF) and a wide range of renal function, compared to warfarin, treatment with apixaban reduces the risk of cardiovascular events, according to multinational investigators.
As Dr. Ziad Hijazi told Reuters Health by email, "Renal dysfunction is a complex issue in patients with atrial fibrillation when balancing the risk of stroke versus the risk of bleeding."
"This study," he added, "shows that apixaban, compared with warfarin, was associated with a lower risk of stroke, death, and major bleeding, regardless of changes in renal function over time. These findings may aid clinicians in the treatment decision."
In a June 15 online paper in JAMA Cardiology, Dr. Hijazi, of Uppsala University Hospital, Sweden, and colleagues report that they came to this conclusion after examining data from a clinical trial (ARISTOTLE) on more than 16,800 AF patients randomized to apixaban or warfarin.
Over the course of a year, about a quarter (26%) maintained good renal function. Renal function declined in the others, and 13.6% showed a drop of more than 20%. The decline in renal function was more rapid in patients who were older or had comorbidities.
Overall, the risks of stroke or systemic embolism, major bleeding, and mortality were greater in patients with worsening renal function (hazard ratio, 1.53 for stroke or systemic embolism, 1.56 for major bleeding, and 2.31 for mortality).
However, such patients on apixaban, compared with warfarin, consistently demonstrated a lower relative risk of stroke or systemic embolism (HR 0.80), ischemic or unspecified stroke (HR 0.88), and major bleeding (HR 0.76).
In fact, as well as showing benefit in this group of patients, the researchers conclude, "The superior efficacy and safety of apixaban as compared with warfarin were similar in patients with normal, poor, and worsening renal function."
Commenting on the findings by email, cardiologist Dr. Anil Pandit of Scottsdale, Arizona, told Reuters Health, "The study by Hijazi et al answers very important clinical questions regarding safety and efficacy of apixaban in situations of declining renal function, a common phenomenon in a real world scenario."
An earlier meta-analysis, in which Dr. Pandit was involved, found decreased risk of major bleeding with apixaban in mild to moderate renal impairment when compared with other anticoagulants (warfarin, aspirin, and Lovenox) as a group.
"The main criticism of the findings of our meta-analysis was inapplicability in the real world scenario, where subclinical episodes of acute kidney injury and worsening renal failure, may lead to increased anticoagulant effect and bleeding," Dr. Pandit said. This new study "exactly answers this question in a large patient population, providing sustained evidence that apixaban is safe and effective in mild to moderate renal impairment patients."
"However," Dr. Pandit concluded, "one should keep in mind limitations of the retrospective data." He also pointed out that "the efficacy and safety of apixaban is not established in patients with severe renal failure, ... as this group of patients was not studied in the ARISTOTLE trial."
Bristol Myers Squibb and Pfizer funded the ARISTOTLE trial. Ten coauthors reported disclosures.
SOURCE: http://bit.ly/28LbKlt JAMA Cardiol 2016.
NEW YORK - In patients with atrial fibrillation (AF) and a wide range of renal function, compared to warfarin, treatment with apixaban reduces the risk of cardiovascular events, according to multinational investigators.
As Dr. Ziad Hijazi told Reuters Health by email, "Renal dysfunction is a complex issue in patients with atrial fibrillation when balancing the risk of stroke versus the risk of bleeding."
"This study," he added, "shows that apixaban, compared with warfarin, was associated with a lower risk of stroke, death, and major bleeding, regardless of changes in renal function over time. These findings may aid clinicians in the treatment decision."
In a June 15 online paper in JAMA Cardiology, Dr. Hijazi, of Uppsala University Hospital, Sweden, and colleagues report that they came to this conclusion after examining data from a clinical trial (ARISTOTLE) on more than 16,800 AF patients randomized to apixaban or warfarin.
Over the course of a year, about a quarter (26%) maintained good renal function. Renal function declined in the others, and 13.6% showed a drop of more than 20%. The decline in renal function was more rapid in patients who were older or had comorbidities.
Overall, the risks of stroke or systemic embolism, major bleeding, and mortality were greater in patients with worsening renal function (hazard ratio, 1.53 for stroke or systemic embolism, 1.56 for major bleeding, and 2.31 for mortality).
However, such patients on apixaban, compared with warfarin, consistently demonstrated a lower relative risk of stroke or systemic embolism (HR 0.80), ischemic or unspecified stroke (HR 0.88), and major bleeding (HR 0.76).
In fact, as well as showing benefit in this group of patients, the researchers conclude, "The superior efficacy and safety of apixaban as compared with warfarin were similar in patients with normal, poor, and worsening renal function."
Commenting on the findings by email, cardiologist Dr. Anil Pandit of Scottsdale, Arizona, told Reuters Health, "The study by Hijazi et al answers very important clinical questions regarding safety and efficacy of apixaban in situations of declining renal function, a common phenomenon in a real world scenario."
An earlier meta-analysis, in which Dr. Pandit was involved, found decreased risk of major bleeding with apixaban in mild to moderate renal impairment when compared with other anticoagulants (warfarin, aspirin, and Lovenox) as a group.
"The main criticism of the findings of our meta-analysis was inapplicability in the real world scenario, where subclinical episodes of acute kidney injury and worsening renal failure, may lead to increased anticoagulant effect and bleeding," Dr. Pandit said. This new study "exactly answers this question in a large patient population, providing sustained evidence that apixaban is safe and effective in mild to moderate renal impairment patients."
"However," Dr. Pandit concluded, "one should keep in mind limitations of the retrospective data." He also pointed out that "the efficacy and safety of apixaban is not established in patients with severe renal failure, ... as this group of patients was not studied in the ARISTOTLE trial."
Bristol Myers Squibb and Pfizer funded the ARISTOTLE trial. Ten coauthors reported disclosures.
SOURCE: http://bit.ly/28LbKlt JAMA Cardiol 2016.
NEW YORK - In patients with atrial fibrillation (AF) and a wide range of renal function, compared to warfarin, treatment with apixaban reduces the risk of cardiovascular events, according to multinational investigators.
As Dr. Ziad Hijazi told Reuters Health by email, "Renal dysfunction is a complex issue in patients with atrial fibrillation when balancing the risk of stroke versus the risk of bleeding."
"This study," he added, "shows that apixaban, compared with warfarin, was associated with a lower risk of stroke, death, and major bleeding, regardless of changes in renal function over time. These findings may aid clinicians in the treatment decision."
In a June 15 online paper in JAMA Cardiology, Dr. Hijazi, of Uppsala University Hospital, Sweden, and colleagues report that they came to this conclusion after examining data from a clinical trial (ARISTOTLE) on more than 16,800 AF patients randomized to apixaban or warfarin.
Over the course of a year, about a quarter (26%) maintained good renal function. Renal function declined in the others, and 13.6% showed a drop of more than 20%. The decline in renal function was more rapid in patients who were older or had comorbidities.
Overall, the risks of stroke or systemic embolism, major bleeding, and mortality were greater in patients with worsening renal function (hazard ratio, 1.53 for stroke or systemic embolism, 1.56 for major bleeding, and 2.31 for mortality).
However, such patients on apixaban, compared with warfarin, consistently demonstrated a lower relative risk of stroke or systemic embolism (HR 0.80), ischemic or unspecified stroke (HR 0.88), and major bleeding (HR 0.76).
In fact, as well as showing benefit in this group of patients, the researchers conclude, "The superior efficacy and safety of apixaban as compared with warfarin were similar in patients with normal, poor, and worsening renal function."
Commenting on the findings by email, cardiologist Dr. Anil Pandit of Scottsdale, Arizona, told Reuters Health, "The study by Hijazi et al answers very important clinical questions regarding safety and efficacy of apixaban in situations of declining renal function, a common phenomenon in a real world scenario."
An earlier meta-analysis, in which Dr. Pandit was involved, found decreased risk of major bleeding with apixaban in mild to moderate renal impairment when compared with other anticoagulants (warfarin, aspirin, and Lovenox) as a group.
"The main criticism of the findings of our meta-analysis was inapplicability in the real world scenario, where subclinical episodes of acute kidney injury and worsening renal failure, may lead to increased anticoagulant effect and bleeding," Dr. Pandit said. This new study "exactly answers this question in a large patient population, providing sustained evidence that apixaban is safe and effective in mild to moderate renal impairment patients."
"However," Dr. Pandit concluded, "one should keep in mind limitations of the retrospective data." He also pointed out that "the efficacy and safety of apixaban is not established in patients with severe renal failure, ... as this group of patients was not studied in the ARISTOTLE trial."
Bristol Myers Squibb and Pfizer funded the ARISTOTLE trial. Ten coauthors reported disclosures.
SOURCE: http://bit.ly/28LbKlt JAMA Cardiol 2016.
ACIP hints at move from three-dose to two-dose HPV vaccination schedule for youth
A work group for the Centers for Disease Control and Prevention’s Advisory Committee on Immunization Practices is leaning toward recommending a change from three to two doses of the human papillomavirus (HPV) vaccine in boys and girls aged 11-12 years.
Members of the ACIP Human Papillomavirus Work Group told the entire committee at ACIP’s June meeting that a review of all available data showed that, regardless of whether the HPV vaccine were bivalent, quadrivalent, or nine-valent, two doses were found to be noninferior, compared with three doses. A two-dose schedule, therefore, could possibly be recommended at the next ACIP meeting later this year.
The work group said it also could recommend that HPV vaccine–naive women up to age 26 years and HPV vaccine-naive men up to age 21 years also receive the vaccine. For persons who initiated but did not complete vaccination before age 15 years, or for persons who initiate the schedule after their 15th birthday – the same schedule as is currently recommended for 11- and 12-year-olds – a similar schedule is likely to be recommended again.
The recommendation for immunocompromised persons of any age would be to receive the three-dose schedule.
If these recommendations are put forth officially, the question of whether families also should be given a three-dose option will need to be decided, work group members said.
Although studies are ongoing to determine antibody persistence and long-term effectiveness after two doses, existing data indicate that waning antibody responses to HPV18 in persons vaccinated with three doses of quadrivalent HPV were not associated with loss of protection. This could mean that protective levels are actually lower than the minimum levels detected by assays, or that antibodies against other epitopes also are protective.
Predictive modeling showed that, if a two-dose schedule can provide more than 20 years of protection, over $118,000 per quality adjusted life year could be realized without sacrificing population health benefits.
The current CDC vaccination schedule for HPV in adolescents is for a three-dose series of the vaccine on a schedule of 0, 1-2, and 6 months. The same vaccination schedule is recommended for “catch-up” of previously unvaccinated adolescents aged 13-18 years.
On Twitter @whitneymcknight
A work group for the Centers for Disease Control and Prevention’s Advisory Committee on Immunization Practices is leaning toward recommending a change from three to two doses of the human papillomavirus (HPV) vaccine in boys and girls aged 11-12 years.
Members of the ACIP Human Papillomavirus Work Group told the entire committee at ACIP’s June meeting that a review of all available data showed that, regardless of whether the HPV vaccine were bivalent, quadrivalent, or nine-valent, two doses were found to be noninferior, compared with three doses. A two-dose schedule, therefore, could possibly be recommended at the next ACIP meeting later this year.
The work group said it also could recommend that HPV vaccine–naive women up to age 26 years and HPV vaccine-naive men up to age 21 years also receive the vaccine. For persons who initiated but did not complete vaccination before age 15 years, or for persons who initiate the schedule after their 15th birthday – the same schedule as is currently recommended for 11- and 12-year-olds – a similar schedule is likely to be recommended again.
The recommendation for immunocompromised persons of any age would be to receive the three-dose schedule.
If these recommendations are put forth officially, the question of whether families also should be given a three-dose option will need to be decided, work group members said.
Although studies are ongoing to determine antibody persistence and long-term effectiveness after two doses, existing data indicate that waning antibody responses to HPV18 in persons vaccinated with three doses of quadrivalent HPV were not associated with loss of protection. This could mean that protective levels are actually lower than the minimum levels detected by assays, or that antibodies against other epitopes also are protective.
Predictive modeling showed that, if a two-dose schedule can provide more than 20 years of protection, over $118,000 per quality adjusted life year could be realized without sacrificing population health benefits.
The current CDC vaccination schedule for HPV in adolescents is for a three-dose series of the vaccine on a schedule of 0, 1-2, and 6 months. The same vaccination schedule is recommended for “catch-up” of previously unvaccinated adolescents aged 13-18 years.
On Twitter @whitneymcknight
A work group for the Centers for Disease Control and Prevention’s Advisory Committee on Immunization Practices is leaning toward recommending a change from three to two doses of the human papillomavirus (HPV) vaccine in boys and girls aged 11-12 years.
Members of the ACIP Human Papillomavirus Work Group told the entire committee at ACIP’s June meeting that a review of all available data showed that, regardless of whether the HPV vaccine were bivalent, quadrivalent, or nine-valent, two doses were found to be noninferior, compared with three doses. A two-dose schedule, therefore, could possibly be recommended at the next ACIP meeting later this year.
The work group said it also could recommend that HPV vaccine–naive women up to age 26 years and HPV vaccine-naive men up to age 21 years also receive the vaccine. For persons who initiated but did not complete vaccination before age 15 years, or for persons who initiate the schedule after their 15th birthday – the same schedule as is currently recommended for 11- and 12-year-olds – a similar schedule is likely to be recommended again.
The recommendation for immunocompromised persons of any age would be to receive the three-dose schedule.
If these recommendations are put forth officially, the question of whether families also should be given a three-dose option will need to be decided, work group members said.
Although studies are ongoing to determine antibody persistence and long-term effectiveness after two doses, existing data indicate that waning antibody responses to HPV18 in persons vaccinated with three doses of quadrivalent HPV were not associated with loss of protection. This could mean that protective levels are actually lower than the minimum levels detected by assays, or that antibodies against other epitopes also are protective.
Predictive modeling showed that, if a two-dose schedule can provide more than 20 years of protection, over $118,000 per quality adjusted life year could be realized without sacrificing population health benefits.
The current CDC vaccination schedule for HPV in adolescents is for a three-dose series of the vaccine on a schedule of 0, 1-2, and 6 months. The same vaccination schedule is recommended for “catch-up” of previously unvaccinated adolescents aged 13-18 years.
On Twitter @whitneymcknight
FROM AN ACIP MEETING
AATS Focus on Thoracic Surgery: Current and Future Challenges
The preliminary program and registration information is now available for AATS Focus on Thoracic Surgery: Current and Future Challenges.
October 28-29, 2016
Westin Boston Waterfront Hotel, Boston, MA
Program Directors
G. Alexander Patterson
David J. Sugarbaker
Program Committee
Thomas A. D’Amico
Shaf Keshavjee
James D. Luketich
Bryan F. Meyers
Scott J. Swanson
Overview
Currently practicing surgeons will be able to improve patient outcomes by enriching their knowledge and technical skills in the definition, diagnosis and resolution of thoracic surgical difficulties and post-operative complications. Expert faculty will provide state-of-the-art solutions to challenges in the field. Attendees will augment their overall understanding of thoracic diseases, upgrade their competency and ability to formulate new clinical strategies, and enhance their diagnosis and surgical treatment of thoracic diseases.
More information: http://aats.org/focus/
The preliminary program and registration information is now available for AATS Focus on Thoracic Surgery: Current and Future Challenges.
October 28-29, 2016
Westin Boston Waterfront Hotel, Boston, MA
Program Directors
G. Alexander Patterson
David J. Sugarbaker
Program Committee
Thomas A. D’Amico
Shaf Keshavjee
James D. Luketich
Bryan F. Meyers
Scott J. Swanson
Overview
Currently practicing surgeons will be able to improve patient outcomes by enriching their knowledge and technical skills in the definition, diagnosis and resolution of thoracic surgical difficulties and post-operative complications. Expert faculty will provide state-of-the-art solutions to challenges in the field. Attendees will augment their overall understanding of thoracic diseases, upgrade their competency and ability to formulate new clinical strategies, and enhance their diagnosis and surgical treatment of thoracic diseases.
More information: http://aats.org/focus/
The preliminary program and registration information is now available for AATS Focus on Thoracic Surgery: Current and Future Challenges.
October 28-29, 2016
Westin Boston Waterfront Hotel, Boston, MA
Program Directors
G. Alexander Patterson
David J. Sugarbaker
Program Committee
Thomas A. D’Amico
Shaf Keshavjee
James D. Luketich
Bryan F. Meyers
Scott J. Swanson
Overview
Currently practicing surgeons will be able to improve patient outcomes by enriching their knowledge and technical skills in the definition, diagnosis and resolution of thoracic surgical difficulties and post-operative complications. Expert faculty will provide state-of-the-art solutions to challenges in the field. Attendees will augment their overall understanding of thoracic diseases, upgrade their competency and ability to formulate new clinical strategies, and enhance their diagnosis and surgical treatment of thoracic diseases.
More information: http://aats.org/focus/
AATS Focus on Thoracic Surgery: Current and Future Challenges
The preliminary program and registration information is now available for AATS Focus on Thoracic Surgery: Current and Future Challenges.
October 28-29, 2016
Westin Boston Waterfront Hotel
Boston, MA
Program Directors
G. Alexander Patterson
David J. Sugarbaker
Program Committee
Thomas A. D’Amico
Shaf Keshavjee
James D. Luketich
Bryan F. Meyers
Scott J. Swanson
Overview
Currently practicing surgeons will be able to improve patient outcomes by enriching their knowledge and technical skills in the definition, diagnosis and resolution of thoracic surgical difficulties and post-operative complications. Expert faculty will provide state-of-the-art solutions to challenges in the field. Attendees will augment their overall understanding of thoracic diseases, upgrade their competency and ability to formulate new clinical strategies, and enhance their diagnosis and surgical treatment of thoracic diseases.
The preliminary program and registration information is now available for AATS Focus on Thoracic Surgery: Current and Future Challenges.
October 28-29, 2016
Westin Boston Waterfront Hotel
Boston, MA
Program Directors
G. Alexander Patterson
David J. Sugarbaker
Program Committee
Thomas A. D’Amico
Shaf Keshavjee
James D. Luketich
Bryan F. Meyers
Scott J. Swanson
Overview
Currently practicing surgeons will be able to improve patient outcomes by enriching their knowledge and technical skills in the definition, diagnosis and resolution of thoracic surgical difficulties and post-operative complications. Expert faculty will provide state-of-the-art solutions to challenges in the field. Attendees will augment their overall understanding of thoracic diseases, upgrade their competency and ability to formulate new clinical strategies, and enhance their diagnosis and surgical treatment of thoracic diseases.
The preliminary program and registration information is now available for AATS Focus on Thoracic Surgery: Current and Future Challenges.
October 28-29, 2016
Westin Boston Waterfront Hotel
Boston, MA
Program Directors
G. Alexander Patterson
David J. Sugarbaker
Program Committee
Thomas A. D’Amico
Shaf Keshavjee
James D. Luketich
Bryan F. Meyers
Scott J. Swanson
Overview
Currently practicing surgeons will be able to improve patient outcomes by enriching their knowledge and technical skills in the definition, diagnosis and resolution of thoracic surgical difficulties and post-operative complications. Expert faculty will provide state-of-the-art solutions to challenges in the field. Attendees will augment their overall understanding of thoracic diseases, upgrade their competency and ability to formulate new clinical strategies, and enhance their diagnosis and surgical treatment of thoracic diseases.
Long-haul travel turbulent for many with type 1 diabetes
NEW ORLEANS – For many patients with type 1 diabetes mellitus, crossing multiple time zones by airplane upset their disease self-management. In fact, results from a new survey found that 74% reported more hyperglycemia and/or hypoglycemia while traveling overseas, and 9% avoided international travel altogether because of their disease.
“Travel has become a ubiquitous feature of modern day life. Every day around the world approximately 8 million people board an aircraft,” Benjamen E. Schoenberg said at the annual scientific sessions of the American Diabetes Association. “Based on known rates of diabetes and how often people travel, the Centers for Disease Control and Prevention and the U.S. Travel Association estimate that annually in the United States alone approximately 17 million individuals with diabetes travel for leisure, and approximately 5.6 million travel for business, and these numbers are rising by the year.”
Noting limited existing medical literature on the topic, Mr. Schoenberg of the William Sansum Diabetes Center, Santa Barbara, Calif., and his associates set out to examine the real-life experiences of individuals with type 1 diabetes traveling long-haul by airplane – defined as crossing multiple time zones. They invited members of an online community of the T1D Exchange known as Glu to complete a 45-question online survey that collected information on respondents’ diabetes history, use of technology in self-management of their diabetes, and overall travel experiences.
Of the 503 survey respondents, 71% were female, their average age was 44 years, and 75% had type 1 diabetes for longer than 10 years. The majority of respondents (81%) reported currently having a passport, and 71% reported flying long-haul at least once in the past 5 years. Nearly three-quarters of respondents (74%) experienced increased levels of hyperglycemia and/or hypoglycemia while traveling overseas, 22% ran out of insulin at some point during a trip in the past 5 years, and 9% reported avoiding international travel altogether because of problems related to diabetes management.
Mr. Schoenberg, who is an incoming first-year medical student at Thomas Jefferson University, Philadelphia, went on to report that about one in three participants indicated that their insulin “does not work the same” in flight, compared with when they’re not flying. At the same time, 5% believe that their continuous glucose monitors are less reliable in flight, and about 10% believe that their insulin pumps are less reliable in flight.
When asked what practical difficulties they faced while traveling with type 1 diabetes, the top three responses from survey participants were airport security, taking enough supplies, and crossing time zones. “Furthermore, we found that pump users experienced more difficulty with airport security than non–pump users; 42% of pump users wore their device through body scanners, and 44% reported having at least one negative experience with airport security in the past,” he said.
When asked about their greatest fear of flying long-haul, the top four responses were more hyperglycemia and/or hypoglycemia, problems with airport security, losing supplies, and glucose variability. In addition, only about 30% of respondents reported being satisfied with currently available resources intended to help people manage their disease while traveling. “There is a call to action for providers and nonprofits to continue to develop information to better suit the needs of individuals traveling with [type 1 diabetes],” Mr. Schoenberg said. “Furthermore, 55% reported that they use the Internet as their primary source and about one in four use a smart phone regularly for their self-management.”
He concluded that long-haul air travel with type 1 diabetes “is neither straightforward nor easy. Real-life experiences suggest an unmet need for personalized information. There also may be a need to evaluate insulin and devices at altitude and across time zones. Finally, the impacts of long-haul travel become more relevant as we move toward a commercial artificial pancreas system.” The researchers reported having no financial conflicts.
NEW ORLEANS – For many patients with type 1 diabetes mellitus, crossing multiple time zones by airplane upset their disease self-management. In fact, results from a new survey found that 74% reported more hyperglycemia and/or hypoglycemia while traveling overseas, and 9% avoided international travel altogether because of their disease.
“Travel has become a ubiquitous feature of modern day life. Every day around the world approximately 8 million people board an aircraft,” Benjamen E. Schoenberg said at the annual scientific sessions of the American Diabetes Association. “Based on known rates of diabetes and how often people travel, the Centers for Disease Control and Prevention and the U.S. Travel Association estimate that annually in the United States alone approximately 17 million individuals with diabetes travel for leisure, and approximately 5.6 million travel for business, and these numbers are rising by the year.”
Noting limited existing medical literature on the topic, Mr. Schoenberg of the William Sansum Diabetes Center, Santa Barbara, Calif., and his associates set out to examine the real-life experiences of individuals with type 1 diabetes traveling long-haul by airplane – defined as crossing multiple time zones. They invited members of an online community of the T1D Exchange known as Glu to complete a 45-question online survey that collected information on respondents’ diabetes history, use of technology in self-management of their diabetes, and overall travel experiences.
Of the 503 survey respondents, 71% were female, their average age was 44 years, and 75% had type 1 diabetes for longer than 10 years. The majority of respondents (81%) reported currently having a passport, and 71% reported flying long-haul at least once in the past 5 years. Nearly three-quarters of respondents (74%) experienced increased levels of hyperglycemia and/or hypoglycemia while traveling overseas, 22% ran out of insulin at some point during a trip in the past 5 years, and 9% reported avoiding international travel altogether because of problems related to diabetes management.
Mr. Schoenberg, who is an incoming first-year medical student at Thomas Jefferson University, Philadelphia, went on to report that about one in three participants indicated that their insulin “does not work the same” in flight, compared with when they’re not flying. At the same time, 5% believe that their continuous glucose monitors are less reliable in flight, and about 10% believe that their insulin pumps are less reliable in flight.
When asked what practical difficulties they faced while traveling with type 1 diabetes, the top three responses from survey participants were airport security, taking enough supplies, and crossing time zones. “Furthermore, we found that pump users experienced more difficulty with airport security than non–pump users; 42% of pump users wore their device through body scanners, and 44% reported having at least one negative experience with airport security in the past,” he said.
When asked about their greatest fear of flying long-haul, the top four responses were more hyperglycemia and/or hypoglycemia, problems with airport security, losing supplies, and glucose variability. In addition, only about 30% of respondents reported being satisfied with currently available resources intended to help people manage their disease while traveling. “There is a call to action for providers and nonprofits to continue to develop information to better suit the needs of individuals traveling with [type 1 diabetes],” Mr. Schoenberg said. “Furthermore, 55% reported that they use the Internet as their primary source and about one in four use a smart phone regularly for their self-management.”
He concluded that long-haul air travel with type 1 diabetes “is neither straightforward nor easy. Real-life experiences suggest an unmet need for personalized information. There also may be a need to evaluate insulin and devices at altitude and across time zones. Finally, the impacts of long-haul travel become more relevant as we move toward a commercial artificial pancreas system.” The researchers reported having no financial conflicts.
NEW ORLEANS – For many patients with type 1 diabetes mellitus, crossing multiple time zones by airplane upset their disease self-management. In fact, results from a new survey found that 74% reported more hyperglycemia and/or hypoglycemia while traveling overseas, and 9% avoided international travel altogether because of their disease.
“Travel has become a ubiquitous feature of modern day life. Every day around the world approximately 8 million people board an aircraft,” Benjamen E. Schoenberg said at the annual scientific sessions of the American Diabetes Association. “Based on known rates of diabetes and how often people travel, the Centers for Disease Control and Prevention and the U.S. Travel Association estimate that annually in the United States alone approximately 17 million individuals with diabetes travel for leisure, and approximately 5.6 million travel for business, and these numbers are rising by the year.”
Noting limited existing medical literature on the topic, Mr. Schoenberg of the William Sansum Diabetes Center, Santa Barbara, Calif., and his associates set out to examine the real-life experiences of individuals with type 1 diabetes traveling long-haul by airplane – defined as crossing multiple time zones. They invited members of an online community of the T1D Exchange known as Glu to complete a 45-question online survey that collected information on respondents’ diabetes history, use of technology in self-management of their diabetes, and overall travel experiences.
Of the 503 survey respondents, 71% were female, their average age was 44 years, and 75% had type 1 diabetes for longer than 10 years. The majority of respondents (81%) reported currently having a passport, and 71% reported flying long-haul at least once in the past 5 years. Nearly three-quarters of respondents (74%) experienced increased levels of hyperglycemia and/or hypoglycemia while traveling overseas, 22% ran out of insulin at some point during a trip in the past 5 years, and 9% reported avoiding international travel altogether because of problems related to diabetes management.
Mr. Schoenberg, who is an incoming first-year medical student at Thomas Jefferson University, Philadelphia, went on to report that about one in three participants indicated that their insulin “does not work the same” in flight, compared with when they’re not flying. At the same time, 5% believe that their continuous glucose monitors are less reliable in flight, and about 10% believe that their insulin pumps are less reliable in flight.
When asked what practical difficulties they faced while traveling with type 1 diabetes, the top three responses from survey participants were airport security, taking enough supplies, and crossing time zones. “Furthermore, we found that pump users experienced more difficulty with airport security than non–pump users; 42% of pump users wore their device through body scanners, and 44% reported having at least one negative experience with airport security in the past,” he said.
When asked about their greatest fear of flying long-haul, the top four responses were more hyperglycemia and/or hypoglycemia, problems with airport security, losing supplies, and glucose variability. In addition, only about 30% of respondents reported being satisfied with currently available resources intended to help people manage their disease while traveling. “There is a call to action for providers and nonprofits to continue to develop information to better suit the needs of individuals traveling with [type 1 diabetes],” Mr. Schoenberg said. “Furthermore, 55% reported that they use the Internet as their primary source and about one in four use a smart phone regularly for their self-management.”
He concluded that long-haul air travel with type 1 diabetes “is neither straightforward nor easy. Real-life experiences suggest an unmet need for personalized information. There also may be a need to evaluate insulin and devices at altitude and across time zones. Finally, the impacts of long-haul travel become more relevant as we move toward a commercial artificial pancreas system.” The researchers reported having no financial conflicts.
AT THE ADA SCIENTIFIC SESSIONS
Key clinical point: Long-haul air travel poses certain challenges for patients with type 1 diabetes.
Major finding: Nearly three-quarters of respondents (74%) experienced increased levels of hyperglycemia and/or hypoglycemia while traveling overseas, and 22% ran out of insulin at some point during a trip in the past 5 years.
Data source: A online survey of 503 individuals with type 1 diabetes who were asked about their real-life experiences while traveling across multiple time zones.
Disclosures: The researchers reported having no financial disclosures.