User login
Hyperkeratotic Lesions in a Patient With Hepatitis C Virus
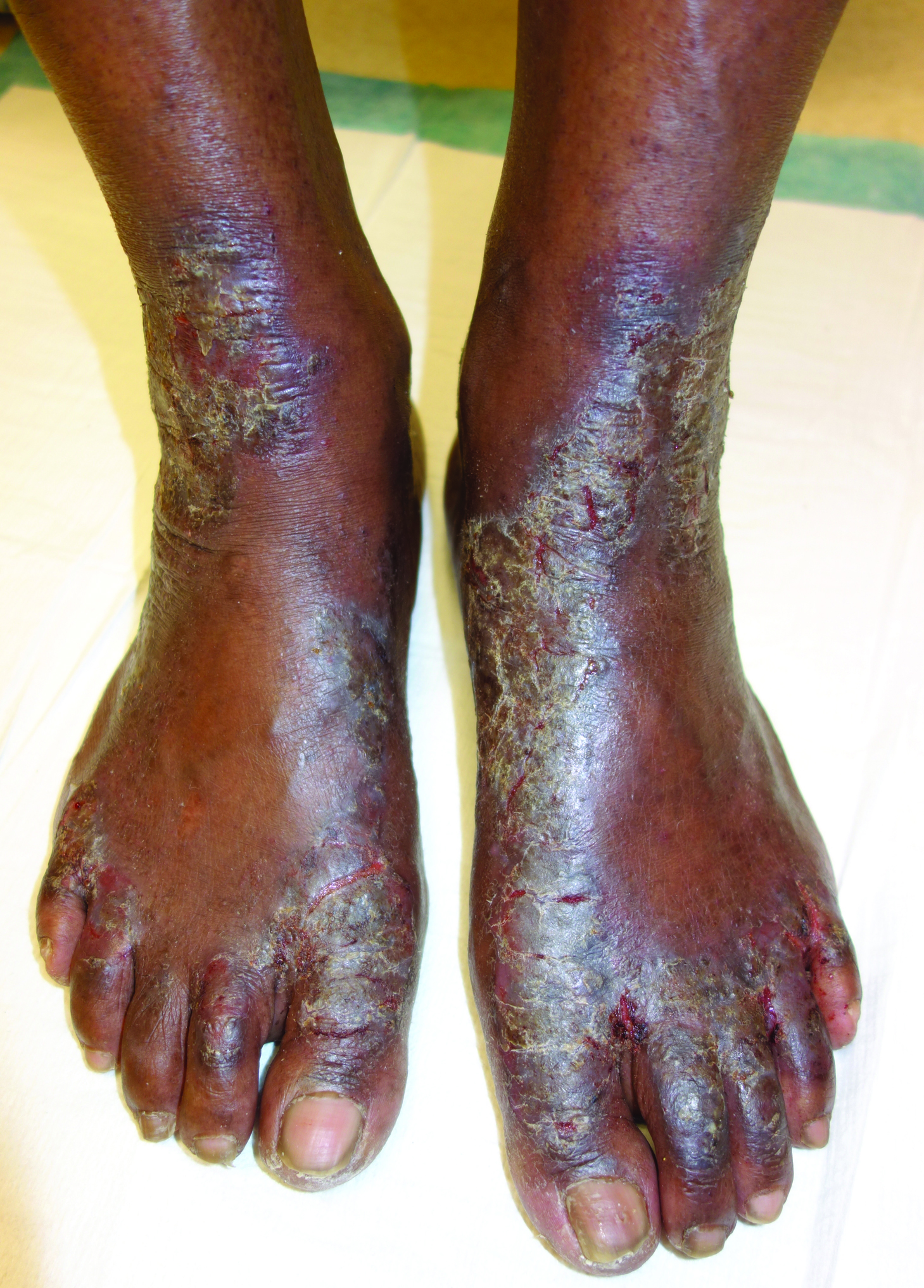
The Diagnosis: Necrolytic Acral Erythema
Histopathologic analysis of a biopsy specimen from the right leg revealed an erosion with parakeratosis containing neutrophils and marked spongiosis favoring the upper layer of the epidermis with focal individual necrotic keratinocytes. In addition, there was a lymphocytic and neutrophilic exocytosis with edema of the papillary dermal papillae, mild papillary dermal fibrosis, and a mild perivascular lymphocytic infiltrate with neutrophils and occasional eosinophils. Clinicopathologic correlation led to a diagnosis of necrolytic acral erythema (NAE).
The patient was prescribed clobetasol propionate ointment 0.05% and oral zinc sulfate 220 mg twice daily but was initially noncompliant with the topical corticosteroid regimen. He did, however, initiate zinc supplementation, which was later increased to 220 mg 3 times daily. At 3-month follow-up, the lesions had nearly completely cleared (Figure) and the serum zinc level was within reference range at 81 μg/dL.
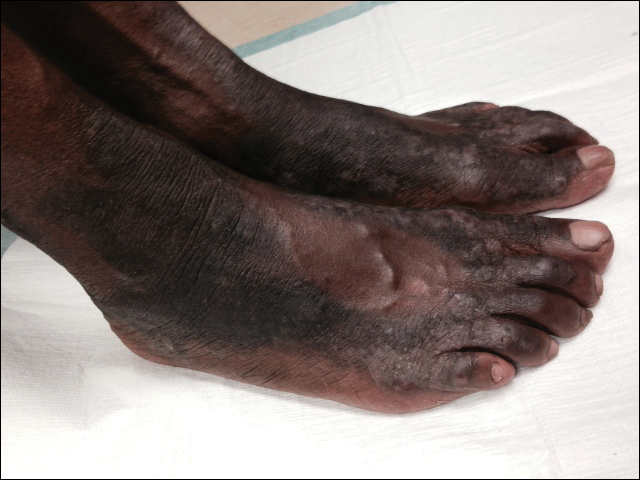
Necrolytic acral erythema is a rare dermatosis that was first described in 1996 by el Darouti and Abu el Ela1 in a series of 7 Egyptian patients. Since then, most of the cases have reported concomitant hepatitis C virus (HCV) infection.2 Necrolytic acral erythema classically presents with symmetric, well-defined, hyperkeratotic plaques in an acral distribution, typically on the dorsal aspect of the feet.2,3 Lesions may involve the dorsal aspect of the toes and the lower legs, with less common involvement of the elbows, hands, and buttocks. Patients often report pruritus and/or burning.3
Abdallah et al4 proposed several stages of NAE development with erythematous papules with dusky eroded centers progressing to marginated, erythematous to violaceous, lichenified plaques. Over time, these lesions tend to thin with progressive hyperpigmentation.
Histologically, early findings of NAE include acanthosis with epidermal spongiosis and upper dermal perivascular dermatitis. Over time, lesions may exhibit psoriasiform hyperplasia with papillomatosis and parakeratosis, epidermal pallor, subcorneal pustules, vascular ectasia, papillary dermal inflammation, and necrotic keratinocytes. Minimal to moderate acanthosis with an inflammatory infiltrate may be observed later in disease progression.5
The differential diagnosis of NAE includes many benign inflammatory skin diseases. Given the acral/extensor distribution of hyperkeratotic lesions and psoriasiform pattern on histopathology, NAE initially may be misdiagnosed as psoriasis. Unlike psoriasis, however, NAE rarely involves palmoplantar skin or nails4 and may respond dramatically to treatment with zinc supplementation.6,7 Necrolytic acral erythema also may be confused with other necrolytic erythemas, including necrolytic migratory erythema, acrodermatitis enteropathica, and pellagra. A deficiency of biotin or essential fatty acids also may mimic NAE. Necrolytic acral erythema can be distinguished from these entities based on its characteristic appearance and distribution, along with comorbid HCV infection.2-4
Several reports of NAE have revealed an associated zinc deficiency.2,8 The underlying pathophysiology of zinc deficiency in NAE has not been elucidated but is thought to be related to HCV infection.2 Clinical improvement has been reported with zinc supplementation in patients with NAE at dosages of 220 mg twice daily, even in those with initial serum zinc levels within reference range.6,7 Our patient was observed to have a low serum zinc level that dramatically improved with oral supplementation.
The recognition of this uncommon entity is critical for dermatologists and dermatopathologists, as NAE has been proposed as an early cutaneous marker of HCV and may prompt the initial diagnosis of HCV.1-10 The severity of NAE has even been linked to HCV severity.1,8 Treatment of HCV has cleared NAE in several cases,4,10 implicating the virus in its pathogenesis. A proper workup for liver dysfunction and follow-up with an appropriate health care provider for HCV treatment is crucial. Our patient was encouraged to follow up with the hepatology department, as he had not been evaluated in several years.
- el Darouti M, Abu el Ela M. Necrolytic acral erythema: a cutaneous marker of viral hepatitis C. Int J Dermatol. 1996;35:252-256.
- Patel U, Loyd A, Patel R, et al. Necrolytic acral erythema. Dermatol Online J. 2010;16:15.
- Geria AN, Holcomb KZ, Scheinfeld NS. Necrolytic acral erythema: a review of the literature. Cutis. 2009;83:309-314.
- Abdallah MA, Ghozzi MY, Monib HA, et al. Necrolytic acral erythema: a cutaneous sign of hepatitis C virus infection. J Am Acad Dermatol. 2005;53:247-251.
- Abdallah MA, Ghozzi MY, Monib HA, et al. Histological study of necrolytic acral erythema. J Ark Med Soc. 2004;100:354-355.
- Khanna VJ, Shieh S, Benjamin J, et al. Necrolytic acral erythema associated with hepatitis C: effective treatment with interferon alfa and zinc. Arch Dermatol. 2000;136:755-757.
- Abdallah MA, Hull C, Horn TD. Necrolytic acral erythema: a patient from the United States successfully treated with oral zinc. Arch Dermatol. 2005;141:85-87.
- Najarian DJ, Lefkowitz I, Balfour E, et al. Zinc deficiency associated with necrolytic acral erythema. J Am Acad Dermatol. 2006;55(5 suppl):S108-S110.
- Nofal AA, Nofal E, Attwa E, et al. Necrolytic acral erythema: a variant of necrolytic migratory erythema or a distinct entity? Int J Dermatol. 2005;44:916-921.
- Hivnor CM, Yan AC, Junkins-Hopkins JM, et al. Necrolytic acral erythema: response to combination therapy with interferon and ribavirin. J Am Acad Dermatol. 2004;50(5 suppl):S121-S124.
The Diagnosis: Necrolytic Acral Erythema
Histopathologic analysis of a biopsy specimen from the right leg revealed an erosion with parakeratosis containing neutrophils and marked spongiosis favoring the upper layer of the epidermis with focal individual necrotic keratinocytes. In addition, there was a lymphocytic and neutrophilic exocytosis with edema of the papillary dermal papillae, mild papillary dermal fibrosis, and a mild perivascular lymphocytic infiltrate with neutrophils and occasional eosinophils. Clinicopathologic correlation led to a diagnosis of necrolytic acral erythema (NAE).
The patient was prescribed clobetasol propionate ointment 0.05% and oral zinc sulfate 220 mg twice daily but was initially noncompliant with the topical corticosteroid regimen. He did, however, initiate zinc supplementation, which was later increased to 220 mg 3 times daily. At 3-month follow-up, the lesions had nearly completely cleared (Figure) and the serum zinc level was within reference range at 81 μg/dL.

Necrolytic acral erythema is a rare dermatosis that was first described in 1996 by el Darouti and Abu el Ela1 in a series of 7 Egyptian patients. Since then, most of the cases have reported concomitant hepatitis C virus (HCV) infection.2 Necrolytic acral erythema classically presents with symmetric, well-defined, hyperkeratotic plaques in an acral distribution, typically on the dorsal aspect of the feet.2,3 Lesions may involve the dorsal aspect of the toes and the lower legs, with less common involvement of the elbows, hands, and buttocks. Patients often report pruritus and/or burning.3
Abdallah et al4 proposed several stages of NAE development with erythematous papules with dusky eroded centers progressing to marginated, erythematous to violaceous, lichenified plaques. Over time, these lesions tend to thin with progressive hyperpigmentation.
Histologically, early findings of NAE include acanthosis with epidermal spongiosis and upper dermal perivascular dermatitis. Over time, lesions may exhibit psoriasiform hyperplasia with papillomatosis and parakeratosis, epidermal pallor, subcorneal pustules, vascular ectasia, papillary dermal inflammation, and necrotic keratinocytes. Minimal to moderate acanthosis with an inflammatory infiltrate may be observed later in disease progression.5
The differential diagnosis of NAE includes many benign inflammatory skin diseases. Given the acral/extensor distribution of hyperkeratotic lesions and psoriasiform pattern on histopathology, NAE initially may be misdiagnosed as psoriasis. Unlike psoriasis, however, NAE rarely involves palmoplantar skin or nails4 and may respond dramatically to treatment with zinc supplementation.6,7 Necrolytic acral erythema also may be confused with other necrolytic erythemas, including necrolytic migratory erythema, acrodermatitis enteropathica, and pellagra. A deficiency of biotin or essential fatty acids also may mimic NAE. Necrolytic acral erythema can be distinguished from these entities based on its characteristic appearance and distribution, along with comorbid HCV infection.2-4
Several reports of NAE have revealed an associated zinc deficiency.2,8 The underlying pathophysiology of zinc deficiency in NAE has not been elucidated but is thought to be related to HCV infection.2 Clinical improvement has been reported with zinc supplementation in patients with NAE at dosages of 220 mg twice daily, even in those with initial serum zinc levels within reference range.6,7 Our patient was observed to have a low serum zinc level that dramatically improved with oral supplementation.
The recognition of this uncommon entity is critical for dermatologists and dermatopathologists, as NAE has been proposed as an early cutaneous marker of HCV and may prompt the initial diagnosis of HCV.1-10 The severity of NAE has even been linked to HCV severity.1,8 Treatment of HCV has cleared NAE in several cases,4,10 implicating the virus in its pathogenesis. A proper workup for liver dysfunction and follow-up with an appropriate health care provider for HCV treatment is crucial. Our patient was encouraged to follow up with the hepatology department, as he had not been evaluated in several years.
The Diagnosis: Necrolytic Acral Erythema
Histopathologic analysis of a biopsy specimen from the right leg revealed an erosion with parakeratosis containing neutrophils and marked spongiosis favoring the upper layer of the epidermis with focal individual necrotic keratinocytes. In addition, there was a lymphocytic and neutrophilic exocytosis with edema of the papillary dermal papillae, mild papillary dermal fibrosis, and a mild perivascular lymphocytic infiltrate with neutrophils and occasional eosinophils. Clinicopathologic correlation led to a diagnosis of necrolytic acral erythema (NAE).
The patient was prescribed clobetasol propionate ointment 0.05% and oral zinc sulfate 220 mg twice daily but was initially noncompliant with the topical corticosteroid regimen. He did, however, initiate zinc supplementation, which was later increased to 220 mg 3 times daily. At 3-month follow-up, the lesions had nearly completely cleared (Figure) and the serum zinc level was within reference range at 81 μg/dL.

Necrolytic acral erythema is a rare dermatosis that was first described in 1996 by el Darouti and Abu el Ela1 in a series of 7 Egyptian patients. Since then, most of the cases have reported concomitant hepatitis C virus (HCV) infection.2 Necrolytic acral erythema classically presents with symmetric, well-defined, hyperkeratotic plaques in an acral distribution, typically on the dorsal aspect of the feet.2,3 Lesions may involve the dorsal aspect of the toes and the lower legs, with less common involvement of the elbows, hands, and buttocks. Patients often report pruritus and/or burning.3
Abdallah et al4 proposed several stages of NAE development with erythematous papules with dusky eroded centers progressing to marginated, erythematous to violaceous, lichenified plaques. Over time, these lesions tend to thin with progressive hyperpigmentation.
Histologically, early findings of NAE include acanthosis with epidermal spongiosis and upper dermal perivascular dermatitis. Over time, lesions may exhibit psoriasiform hyperplasia with papillomatosis and parakeratosis, epidermal pallor, subcorneal pustules, vascular ectasia, papillary dermal inflammation, and necrotic keratinocytes. Minimal to moderate acanthosis with an inflammatory infiltrate may be observed later in disease progression.5
The differential diagnosis of NAE includes many benign inflammatory skin diseases. Given the acral/extensor distribution of hyperkeratotic lesions and psoriasiform pattern on histopathology, NAE initially may be misdiagnosed as psoriasis. Unlike psoriasis, however, NAE rarely involves palmoplantar skin or nails4 and may respond dramatically to treatment with zinc supplementation.6,7 Necrolytic acral erythema also may be confused with other necrolytic erythemas, including necrolytic migratory erythema, acrodermatitis enteropathica, and pellagra. A deficiency of biotin or essential fatty acids also may mimic NAE. Necrolytic acral erythema can be distinguished from these entities based on its characteristic appearance and distribution, along with comorbid HCV infection.2-4
Several reports of NAE have revealed an associated zinc deficiency.2,8 The underlying pathophysiology of zinc deficiency in NAE has not been elucidated but is thought to be related to HCV infection.2 Clinical improvement has been reported with zinc supplementation in patients with NAE at dosages of 220 mg twice daily, even in those with initial serum zinc levels within reference range.6,7 Our patient was observed to have a low serum zinc level that dramatically improved with oral supplementation.
The recognition of this uncommon entity is critical for dermatologists and dermatopathologists, as NAE has been proposed as an early cutaneous marker of HCV and may prompt the initial diagnosis of HCV.1-10 The severity of NAE has even been linked to HCV severity.1,8 Treatment of HCV has cleared NAE in several cases,4,10 implicating the virus in its pathogenesis. A proper workup for liver dysfunction and follow-up with an appropriate health care provider for HCV treatment is crucial. Our patient was encouraged to follow up with the hepatology department, as he had not been evaluated in several years.
- el Darouti M, Abu el Ela M. Necrolytic acral erythema: a cutaneous marker of viral hepatitis C. Int J Dermatol. 1996;35:252-256.
- Patel U, Loyd A, Patel R, et al. Necrolytic acral erythema. Dermatol Online J. 2010;16:15.
- Geria AN, Holcomb KZ, Scheinfeld NS. Necrolytic acral erythema: a review of the literature. Cutis. 2009;83:309-314.
- Abdallah MA, Ghozzi MY, Monib HA, et al. Necrolytic acral erythema: a cutaneous sign of hepatitis C virus infection. J Am Acad Dermatol. 2005;53:247-251.
- Abdallah MA, Ghozzi MY, Monib HA, et al. Histological study of necrolytic acral erythema. J Ark Med Soc. 2004;100:354-355.
- Khanna VJ, Shieh S, Benjamin J, et al. Necrolytic acral erythema associated with hepatitis C: effective treatment with interferon alfa and zinc. Arch Dermatol. 2000;136:755-757.
- Abdallah MA, Hull C, Horn TD. Necrolytic acral erythema: a patient from the United States successfully treated with oral zinc. Arch Dermatol. 2005;141:85-87.
- Najarian DJ, Lefkowitz I, Balfour E, et al. Zinc deficiency associated with necrolytic acral erythema. J Am Acad Dermatol. 2006;55(5 suppl):S108-S110.
- Nofal AA, Nofal E, Attwa E, et al. Necrolytic acral erythema: a variant of necrolytic migratory erythema or a distinct entity? Int J Dermatol. 2005;44:916-921.
- Hivnor CM, Yan AC, Junkins-Hopkins JM, et al. Necrolytic acral erythema: response to combination therapy with interferon and ribavirin. J Am Acad Dermatol. 2004;50(5 suppl):S121-S124.
- el Darouti M, Abu el Ela M. Necrolytic acral erythema: a cutaneous marker of viral hepatitis C. Int J Dermatol. 1996;35:252-256.
- Patel U, Loyd A, Patel R, et al. Necrolytic acral erythema. Dermatol Online J. 2010;16:15.
- Geria AN, Holcomb KZ, Scheinfeld NS. Necrolytic acral erythema: a review of the literature. Cutis. 2009;83:309-314.
- Abdallah MA, Ghozzi MY, Monib HA, et al. Necrolytic acral erythema: a cutaneous sign of hepatitis C virus infection. J Am Acad Dermatol. 2005;53:247-251.
- Abdallah MA, Ghozzi MY, Monib HA, et al. Histological study of necrolytic acral erythema. J Ark Med Soc. 2004;100:354-355.
- Khanna VJ, Shieh S, Benjamin J, et al. Necrolytic acral erythema associated with hepatitis C: effective treatment with interferon alfa and zinc. Arch Dermatol. 2000;136:755-757.
- Abdallah MA, Hull C, Horn TD. Necrolytic acral erythema: a patient from the United States successfully treated with oral zinc. Arch Dermatol. 2005;141:85-87.
- Najarian DJ, Lefkowitz I, Balfour E, et al. Zinc deficiency associated with necrolytic acral erythema. J Am Acad Dermatol. 2006;55(5 suppl):S108-S110.
- Nofal AA, Nofal E, Attwa E, et al. Necrolytic acral erythema: a variant of necrolytic migratory erythema or a distinct entity? Int J Dermatol. 2005;44:916-921.
- Hivnor CM, Yan AC, Junkins-Hopkins JM, et al. Necrolytic acral erythema: response to combination therapy with interferon and ribavirin. J Am Acad Dermatol. 2004;50(5 suppl):S121-S124.

The Arthroscopic Superior Capsular Reconstruction
Rotator cuff tears are very common, and 250,000 to 500,000 rotator cuff repairs are performed in the United States each year.1,2 In most cases, a complete repair of even large or massive tears can be achieved. However, a subset of patients exist in whom the glenohumeral joint has minimal degenerative changes and the rotator cuff tendon is either irreparable or very poor quality and unlikely to heal (ie, failed previous cuff repair). Some authors have advocated for reverse shoulder arthroplasty (RSA) in these patients despite the lack of glenohumeral arthritis. However, due to the permanent destruction of the glenohumeral articular surfaces, complication rates, and concerns about implant longevity with RSA, we believe the superior capsular reconstruction (SCR) is a viable alternative in patients in whom joint preservation is appropriate based on age limitations and/or activity requirements.3
The SCR was first described by Mihata and colleagues4 as a means to reconstruct the superior capsule in shoulders with large, irreparable posterosuperior rotator cuff tears. Originally described using a fascia lata autograft, our technique has been adapted to incorporate a dermal allograft, which limits donor site morbidity and operative time. In most cases, the dermal allograft is fixed to the normal anatomic attachments of the superior glenoid just medial to the superior labrum, laterally to the greater tuberosity, and posteriorly with side-to-side sutures to the remaining rotator cuff. If there is a robust band of “comma” tissue anteriorly, we fix the anterior margin of the dermal graft to this with side-to-side sutures. The comma tissue represents the medial sling of the biceps tendon and connects the upper subscapularis tendon to the anterior supraspinatus. In most cases, this tissue is intact after repair of the subscapularis tendon.
Technique
The patient is positioned in either the lateral decubitus or beach chair position. The arm is positioned in 20° to 30° of abduction and 20° to 30° of forward flexion. A diagnostic arthroscopy is performed through a posterior glenohumeral viewing portal. The subscapularis is visualized and repaired if torn. A biceps tenodesis is performed in most cases, as there is often a tear of the subscapularis, tear or instability of the biceps tendon, and/or a compromised attachment of the biceps root.
Attention is turned to the subacromial space. Posterior viewing and lateral working portals are established. A 10-mm flexible cannula (PassPort; Arthrex) is placed in the lateral portal to aid with suture management and graft passage. A limited subacromial decompression is performed that preserves the coracoacromial arch. The rotator cuff is carefully dissected and freed from the internal deltoid fascia. The scapular spine is identified to visualize the raphé between the supraspinatus and infraspinatus. The infraspinatus is mobilized and repaired as much as possible.
If we think that the tear might be reparable by gaining added excursion from a posterior interval slide, or if it is clearly not reparable but the remaining rim of rotator cuff obscures clear visualization of the superior glenoid, we perform a posterior interval slide. If the additional excursion that is achieved by the posterior slide is adequate for a complete repair, we proceed with the repair. However, if the tear is not reparable even after the posterior interval slide, we have found that the exposure and preparation of the superior glenoid is greatly improved after the posterior slide. After fixation of the dermal graft, we typically perform a partial side-to-side repair of the supraspinatus to the infraspinatus over the top of the graft.
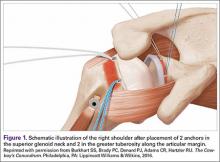
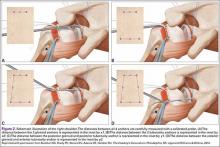
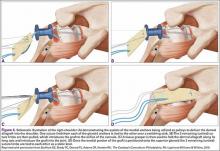
The bone beds of the greater tuberosity and just medial to the superior glenoid labrum are prepared with a shaver and motorized burr. Two anchors (3.0-mm BioComposite SutureTak; Arthrex) are placed in the superior glenoid neck at about the 10 o’clock and 2 o’clock positions approximately 5 mm medial to the superior labrum. Note: the placement medial to the labrum is chosen because this is the normal origin of the superior capsule and because of the angle of approach, these percutaneous portals are often more medial than typical portals for placing anchors during SLAP (superior labral anterior to posterior) repair. Next, 2 threaded anchors (4.75-mm BioComposite SwiveLock; Arthrex) preloaded with suture tape are placed in the greater tuberosity along the articular margin (Figure 1). However, if a biceps tenodesis with an interference screw is placed at the top of the bicipital groove, this anchor preloaded with suture tape can also serve as the anteromedial anchor in the greater tuberosity footprint. The distances between all 4 anchors are carefully measured with a calibrated probe (Figures 2A-2D).
We use a 3.0-mm acellular dermal allograft (ArthroFlex; Arthrex) to reconstruct the superior capsule. The positions of the 4 anchors are carefully marked on the dermal allograft. We routinely add an additional 5 mm of tissue to the medial, anterior, and posterior margins to decrease the risk of suture cut out. An additional 10 mm of tissue is added laterally to cover the greater tuberosity. The final contoured graft is typically trapezoidal in shape.
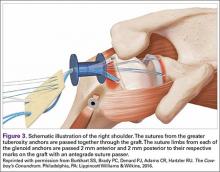
The sutures from the 4 anchors are then sequentially retrieved through the lateral cannula. The sutures from the greater tuberosity anchors are passed through their respective holes in the graft. However, the suture limbs from each of the glenoid anchors are individually passed 2 mm anterior and 2 mm posterior to their respective marks on the graft with an antegrade suture passer (Figure 3). It is important to have an assistant apply tension to each of the sutures after they are passed through the graft to decrease the chance of crossing and tangling the sutures.
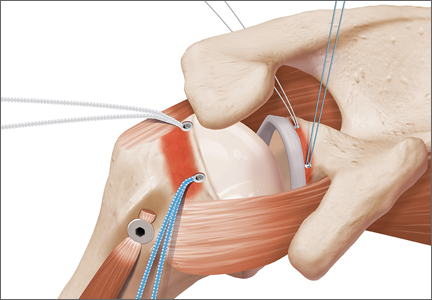
The eyelets of the medial anchors are utilized as pulleys to deliver the dermal allograft into the shoulder. One suture limb from each of the glenoid anchors is tied to the other over a switching stick (Figure 4A). The 2 remaining (untied) suture limbs are then pulled, which introduces the graft to the orifice of the cannula (Figure 4B). A tissue grasper is then used to fold the dermal allograft along its long axis and introduce the graft into the joint (Figure 4C). Once the medial portion of the graft is positioned onto the superior glenoid the 2 remaining (untied) suture limbs are tied to each other as a static knot in the subacromial space (Figure 4D).
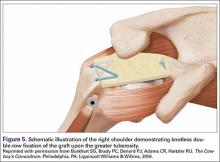
The redundancy in the suture tapes can be removed by sequentially sliding a retriever down each suture and tensioning the suture as the nose of the instrument pushes the dermal graft down to the tuberosity bone bed. The suture tapes are crisscrossed and secured laterally with 2 additional knotless threaded anchors (Figure 5). One may also place cinch stitches at the anterolateral and posterolateral corners of the graft that are incorporated into the lateral anchors. These sutures can be useful for pulling the graft back out of the subacromial space in the event of any suture tangles, and can be used for controlling the lateral aspect of the graft during lateral anchor placement.
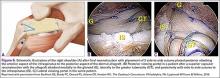
At this point in the procedure, additional glenoid anchors can be placed both anterior and posterior to the superior glenoid anchors if additional glenoid fixation is desired. Finally, 2 to 3 side-to-side sutures are placed posteriorly attaching the anterior aspect of the infraspinatus to the posterior aspect of the dermal allograft (Figures 6A-6C). If rotator interval tissue (comma tissue) is present, anterior side-to-side sutures may be placed. However, we do not recommend placing anterior side-to-side sutures directly from the dermal allograft to the subscapularis as this may deform the graft, over- constrain the shoulder, and restrict motion.
Discussion
Reconstruction of the superior capsule has been shown to restore the normal restraint to superior translation of the humeral head and reestablish a stable fulcrum at the glenohumeral joint.5 It should be mentioned that we do not perform the SCR in patients with advanced glenohumeral arthritis. The short-term results of this novel procedure have been encouraging, including our own series of patients, in which most patients have had a significant reduction in pain, improvement in function, and very few complications (P. J. Denard, MD, S. S. Burkhart, MD, P. C. Brady, MD, J. Tokish, MD, C. R. Adams, MD, unpublished data, May 2016).
The early success of this procedure suggests that a robust superior capsule is necessary, in addition to functional muscle-tendon units, to restore the stable fulcrum and force couples that are necessary for normal shoulder function. Perhaps we have not paid enough attention to the integrity of the superior capsule in the past. In cases of revision cuff repair, we pay special attention to the quality of the capsular layer deep to the cuff tendon. If the capsule is poor quality, we sometimes reconstruct the capsule with a dermal allograft (SCR) and then do a rotator cuff repair (partial or complete) over the top of the SCR to maintain the normal anatomic deep to superficial layering of the capsule and rotator cuff.
We are very conservative with our postoperative rehabilitation program after a SCR. We know that the rate of stiffness with a conservative program after an arthroscopic rotator cuff repair, even in the revision setting, is very low.6 Furthermore, both basic science on healing of soft tissue to bone and radiographic analysis of healing after postoperative rotator cuff repairs support a slow rehabilitation program.7,8 A canine model specifically evaluating acellular dermal allografts in the shoulder suggests that these grafts undergo significant remodeling and become weaker before they get stronger.9 We would rather err on the side of healing of the SCR with potentially a slight increase in the rate of shoulder stiffness than to regain early motion at the expense of graft failure. Therefore, we have the patient wear a sling with no shoulder motion for 6 weeks. Passive motion is started at 6 weeks postoperative and strengthening is delayed until 12 to 16 weeks postoperative.
1. Orr SB, Chainani A, Hippensteel KJ, et al. Aligned multilayered electrospun scaffolds for rotator cuff tendon tissue engineering. Acta Biomater. 2015;24:117-126.
2. Austin L, Black EM, Lombardi NJ, Pepe MD, Lazarus M. Arthroscopic transosseous rotator cuff repair. A prospective study on cost savings, surgical time, and outcomes. Ortho J Sports Med. 2015;3(2 Suppl). doi:10.1177/2325967115S00156.
3. Denard PJ, Lädermann A, Jiwani AZ, Burkhart SS. Functional outcome after arthroscopic repair of massive rotator cuff tears in individuals with pseudoparalysis. Arthroscopy. 2012;28(9):1214-1219.
4. Mihata T, Lee TQ, Watanabe C, et al. Clinical results of arthroscopic superior capsule reconstruction for irreparable rotator cuff tears. Arthroscopy. 2013;29(3):459-470.
5. Mihata T, McGarry MH, Pirolo JM, Kinoshita M, Lee TQ. Superior capsule reconstruction to restore superior stability in irreparable rotator cuff tears: a biomechanical cadaveric study. Am J Sports Med. 2012;40(10):2248-2255.
6. Huberty DP, Schoolfield JD, Brady PC, Vadala AP, Arrigoni P, Burkhart SS. Incidence and treatment of postoperative stiffness following arthroscopic rotator cuff repair. Arthroscopy. 2009;25(8):880-890.
7. Sonnabend DH, Howlett CR, Young AA. Histological evaluation of repair of the rotator cuff in a primate model. J Bone Joint Surg Br. 2010;92(4):586-594.
8. Lee BG, Cho NS, Rhee YG. Effect of two rehabilitation protocols on range of motion and healing rates after arthroscopic rotator cuff repair: aggressive versus limited early passive exercises. Arthroscopy. 2012;28(1):34-42.
9. Adams JE, Zobitz ME, Reach JS Jr, An KN, Steinmann SP. Rotator cuff repair using an acellular dermal matrix graft: an in vivo study in a canine model. Arthroscopy. 2006;22(7):700-709.
Rotator cuff tears are very common, and 250,000 to 500,000 rotator cuff repairs are performed in the United States each year.1,2 In most cases, a complete repair of even large or massive tears can be achieved. However, a subset of patients exist in whom the glenohumeral joint has minimal degenerative changes and the rotator cuff tendon is either irreparable or very poor quality and unlikely to heal (ie, failed previous cuff repair). Some authors have advocated for reverse shoulder arthroplasty (RSA) in these patients despite the lack of glenohumeral arthritis. However, due to the permanent destruction of the glenohumeral articular surfaces, complication rates, and concerns about implant longevity with RSA, we believe the superior capsular reconstruction (SCR) is a viable alternative in patients in whom joint preservation is appropriate based on age limitations and/or activity requirements.3
The SCR was first described by Mihata and colleagues4 as a means to reconstruct the superior capsule in shoulders with large, irreparable posterosuperior rotator cuff tears. Originally described using a fascia lata autograft, our technique has been adapted to incorporate a dermal allograft, which limits donor site morbidity and operative time. In most cases, the dermal allograft is fixed to the normal anatomic attachments of the superior glenoid just medial to the superior labrum, laterally to the greater tuberosity, and posteriorly with side-to-side sutures to the remaining rotator cuff. If there is a robust band of “comma” tissue anteriorly, we fix the anterior margin of the dermal graft to this with side-to-side sutures. The comma tissue represents the medial sling of the biceps tendon and connects the upper subscapularis tendon to the anterior supraspinatus. In most cases, this tissue is intact after repair of the subscapularis tendon.
Technique
The patient is positioned in either the lateral decubitus or beach chair position. The arm is positioned in 20° to 30° of abduction and 20° to 30° of forward flexion. A diagnostic arthroscopy is performed through a posterior glenohumeral viewing portal. The subscapularis is visualized and repaired if torn. A biceps tenodesis is performed in most cases, as there is often a tear of the subscapularis, tear or instability of the biceps tendon, and/or a compromised attachment of the biceps root.
Attention is turned to the subacromial space. Posterior viewing and lateral working portals are established. A 10-mm flexible cannula (PassPort; Arthrex) is placed in the lateral portal to aid with suture management and graft passage. A limited subacromial decompression is performed that preserves the coracoacromial arch. The rotator cuff is carefully dissected and freed from the internal deltoid fascia. The scapular spine is identified to visualize the raphé between the supraspinatus and infraspinatus. The infraspinatus is mobilized and repaired as much as possible.
If we think that the tear might be reparable by gaining added excursion from a posterior interval slide, or if it is clearly not reparable but the remaining rim of rotator cuff obscures clear visualization of the superior glenoid, we perform a posterior interval slide. If the additional excursion that is achieved by the posterior slide is adequate for a complete repair, we proceed with the repair. However, if the tear is not reparable even after the posterior interval slide, we have found that the exposure and preparation of the superior glenoid is greatly improved after the posterior slide. After fixation of the dermal graft, we typically perform a partial side-to-side repair of the supraspinatus to the infraspinatus over the top of the graft.
The bone beds of the greater tuberosity and just medial to the superior glenoid labrum are prepared with a shaver and motorized burr. Two anchors (3.0-mm BioComposite SutureTak; Arthrex) are placed in the superior glenoid neck at about the 10 o’clock and 2 o’clock positions approximately 5 mm medial to the superior labrum. Note: the placement medial to the labrum is chosen because this is the normal origin of the superior capsule and because of the angle of approach, these percutaneous portals are often more medial than typical portals for placing anchors during SLAP (superior labral anterior to posterior) repair. Next, 2 threaded anchors (4.75-mm BioComposite SwiveLock; Arthrex) preloaded with suture tape are placed in the greater tuberosity along the articular margin (Figure 1). However, if a biceps tenodesis with an interference screw is placed at the top of the bicipital groove, this anchor preloaded with suture tape can also serve as the anteromedial anchor in the greater tuberosity footprint. The distances between all 4 anchors are carefully measured with a calibrated probe (Figures 2A-2D).
We use a 3.0-mm acellular dermal allograft (ArthroFlex; Arthrex) to reconstruct the superior capsule. The positions of the 4 anchors are carefully marked on the dermal allograft. We routinely add an additional 5 mm of tissue to the medial, anterior, and posterior margins to decrease the risk of suture cut out. An additional 10 mm of tissue is added laterally to cover the greater tuberosity. The final contoured graft is typically trapezoidal in shape.
The sutures from the 4 anchors are then sequentially retrieved through the lateral cannula. The sutures from the greater tuberosity anchors are passed through their respective holes in the graft. However, the suture limbs from each of the glenoid anchors are individually passed 2 mm anterior and 2 mm posterior to their respective marks on the graft with an antegrade suture passer (Figure 3). It is important to have an assistant apply tension to each of the sutures after they are passed through the graft to decrease the chance of crossing and tangling the sutures.
The eyelets of the medial anchors are utilized as pulleys to deliver the dermal allograft into the shoulder. One suture limb from each of the glenoid anchors is tied to the other over a switching stick (Figure 4A). The 2 remaining (untied) suture limbs are then pulled, which introduces the graft to the orifice of the cannula (Figure 4B). A tissue grasper is then used to fold the dermal allograft along its long axis and introduce the graft into the joint (Figure 4C). Once the medial portion of the graft is positioned onto the superior glenoid the 2 remaining (untied) suture limbs are tied to each other as a static knot in the subacromial space (Figure 4D).
The redundancy in the suture tapes can be removed by sequentially sliding a retriever down each suture and tensioning the suture as the nose of the instrument pushes the dermal graft down to the tuberosity bone bed. The suture tapes are crisscrossed and secured laterally with 2 additional knotless threaded anchors (Figure 5). One may also place cinch stitches at the anterolateral and posterolateral corners of the graft that are incorporated into the lateral anchors. These sutures can be useful for pulling the graft back out of the subacromial space in the event of any suture tangles, and can be used for controlling the lateral aspect of the graft during lateral anchor placement.
At this point in the procedure, additional glenoid anchors can be placed both anterior and posterior to the superior glenoid anchors if additional glenoid fixation is desired. Finally, 2 to 3 side-to-side sutures are placed posteriorly attaching the anterior aspect of the infraspinatus to the posterior aspect of the dermal allograft (Figures 6A-6C). If rotator interval tissue (comma tissue) is present, anterior side-to-side sutures may be placed. However, we do not recommend placing anterior side-to-side sutures directly from the dermal allograft to the subscapularis as this may deform the graft, over- constrain the shoulder, and restrict motion.
Discussion
Reconstruction of the superior capsule has been shown to restore the normal restraint to superior translation of the humeral head and reestablish a stable fulcrum at the glenohumeral joint.5 It should be mentioned that we do not perform the SCR in patients with advanced glenohumeral arthritis. The short-term results of this novel procedure have been encouraging, including our own series of patients, in which most patients have had a significant reduction in pain, improvement in function, and very few complications (P. J. Denard, MD, S. S. Burkhart, MD, P. C. Brady, MD, J. Tokish, MD, C. R. Adams, MD, unpublished data, May 2016).
The early success of this procedure suggests that a robust superior capsule is necessary, in addition to functional muscle-tendon units, to restore the stable fulcrum and force couples that are necessary for normal shoulder function. Perhaps we have not paid enough attention to the integrity of the superior capsule in the past. In cases of revision cuff repair, we pay special attention to the quality of the capsular layer deep to the cuff tendon. If the capsule is poor quality, we sometimes reconstruct the capsule with a dermal allograft (SCR) and then do a rotator cuff repair (partial or complete) over the top of the SCR to maintain the normal anatomic deep to superficial layering of the capsule and rotator cuff.
We are very conservative with our postoperative rehabilitation program after a SCR. We know that the rate of stiffness with a conservative program after an arthroscopic rotator cuff repair, even in the revision setting, is very low.6 Furthermore, both basic science on healing of soft tissue to bone and radiographic analysis of healing after postoperative rotator cuff repairs support a slow rehabilitation program.7,8 A canine model specifically evaluating acellular dermal allografts in the shoulder suggests that these grafts undergo significant remodeling and become weaker before they get stronger.9 We would rather err on the side of healing of the SCR with potentially a slight increase in the rate of shoulder stiffness than to regain early motion at the expense of graft failure. Therefore, we have the patient wear a sling with no shoulder motion for 6 weeks. Passive motion is started at 6 weeks postoperative and strengthening is delayed until 12 to 16 weeks postoperative.
Rotator cuff tears are very common, and 250,000 to 500,000 rotator cuff repairs are performed in the United States each year.1,2 In most cases, a complete repair of even large or massive tears can be achieved. However, a subset of patients exist in whom the glenohumeral joint has minimal degenerative changes and the rotator cuff tendon is either irreparable or very poor quality and unlikely to heal (ie, failed previous cuff repair). Some authors have advocated for reverse shoulder arthroplasty (RSA) in these patients despite the lack of glenohumeral arthritis. However, due to the permanent destruction of the glenohumeral articular surfaces, complication rates, and concerns about implant longevity with RSA, we believe the superior capsular reconstruction (SCR) is a viable alternative in patients in whom joint preservation is appropriate based on age limitations and/or activity requirements.3
The SCR was first described by Mihata and colleagues4 as a means to reconstruct the superior capsule in shoulders with large, irreparable posterosuperior rotator cuff tears. Originally described using a fascia lata autograft, our technique has been adapted to incorporate a dermal allograft, which limits donor site morbidity and operative time. In most cases, the dermal allograft is fixed to the normal anatomic attachments of the superior glenoid just medial to the superior labrum, laterally to the greater tuberosity, and posteriorly with side-to-side sutures to the remaining rotator cuff. If there is a robust band of “comma” tissue anteriorly, we fix the anterior margin of the dermal graft to this with side-to-side sutures. The comma tissue represents the medial sling of the biceps tendon and connects the upper subscapularis tendon to the anterior supraspinatus. In most cases, this tissue is intact after repair of the subscapularis tendon.
Technique
The patient is positioned in either the lateral decubitus or beach chair position. The arm is positioned in 20° to 30° of abduction and 20° to 30° of forward flexion. A diagnostic arthroscopy is performed through a posterior glenohumeral viewing portal. The subscapularis is visualized and repaired if torn. A biceps tenodesis is performed in most cases, as there is often a tear of the subscapularis, tear or instability of the biceps tendon, and/or a compromised attachment of the biceps root.
Attention is turned to the subacromial space. Posterior viewing and lateral working portals are established. A 10-mm flexible cannula (PassPort; Arthrex) is placed in the lateral portal to aid with suture management and graft passage. A limited subacromial decompression is performed that preserves the coracoacromial arch. The rotator cuff is carefully dissected and freed from the internal deltoid fascia. The scapular spine is identified to visualize the raphé between the supraspinatus and infraspinatus. The infraspinatus is mobilized and repaired as much as possible.
If we think that the tear might be reparable by gaining added excursion from a posterior interval slide, or if it is clearly not reparable but the remaining rim of rotator cuff obscures clear visualization of the superior glenoid, we perform a posterior interval slide. If the additional excursion that is achieved by the posterior slide is adequate for a complete repair, we proceed with the repair. However, if the tear is not reparable even after the posterior interval slide, we have found that the exposure and preparation of the superior glenoid is greatly improved after the posterior slide. After fixation of the dermal graft, we typically perform a partial side-to-side repair of the supraspinatus to the infraspinatus over the top of the graft.
The bone beds of the greater tuberosity and just medial to the superior glenoid labrum are prepared with a shaver and motorized burr. Two anchors (3.0-mm BioComposite SutureTak; Arthrex) are placed in the superior glenoid neck at about the 10 o’clock and 2 o’clock positions approximately 5 mm medial to the superior labrum. Note: the placement medial to the labrum is chosen because this is the normal origin of the superior capsule and because of the angle of approach, these percutaneous portals are often more medial than typical portals for placing anchors during SLAP (superior labral anterior to posterior) repair. Next, 2 threaded anchors (4.75-mm BioComposite SwiveLock; Arthrex) preloaded with suture tape are placed in the greater tuberosity along the articular margin (Figure 1). However, if a biceps tenodesis with an interference screw is placed at the top of the bicipital groove, this anchor preloaded with suture tape can also serve as the anteromedial anchor in the greater tuberosity footprint. The distances between all 4 anchors are carefully measured with a calibrated probe (Figures 2A-2D).
We use a 3.0-mm acellular dermal allograft (ArthroFlex; Arthrex) to reconstruct the superior capsule. The positions of the 4 anchors are carefully marked on the dermal allograft. We routinely add an additional 5 mm of tissue to the medial, anterior, and posterior margins to decrease the risk of suture cut out. An additional 10 mm of tissue is added laterally to cover the greater tuberosity. The final contoured graft is typically trapezoidal in shape.
The sutures from the 4 anchors are then sequentially retrieved through the lateral cannula. The sutures from the greater tuberosity anchors are passed through their respective holes in the graft. However, the suture limbs from each of the glenoid anchors are individually passed 2 mm anterior and 2 mm posterior to their respective marks on the graft with an antegrade suture passer (Figure 3). It is important to have an assistant apply tension to each of the sutures after they are passed through the graft to decrease the chance of crossing and tangling the sutures.
The eyelets of the medial anchors are utilized as pulleys to deliver the dermal allograft into the shoulder. One suture limb from each of the glenoid anchors is tied to the other over a switching stick (Figure 4A). The 2 remaining (untied) suture limbs are then pulled, which introduces the graft to the orifice of the cannula (Figure 4B). A tissue grasper is then used to fold the dermal allograft along its long axis and introduce the graft into the joint (Figure 4C). Once the medial portion of the graft is positioned onto the superior glenoid the 2 remaining (untied) suture limbs are tied to each other as a static knot in the subacromial space (Figure 4D).
The redundancy in the suture tapes can be removed by sequentially sliding a retriever down each suture and tensioning the suture as the nose of the instrument pushes the dermal graft down to the tuberosity bone bed. The suture tapes are crisscrossed and secured laterally with 2 additional knotless threaded anchors (Figure 5). One may also place cinch stitches at the anterolateral and posterolateral corners of the graft that are incorporated into the lateral anchors. These sutures can be useful for pulling the graft back out of the subacromial space in the event of any suture tangles, and can be used for controlling the lateral aspect of the graft during lateral anchor placement.
At this point in the procedure, additional glenoid anchors can be placed both anterior and posterior to the superior glenoid anchors if additional glenoid fixation is desired. Finally, 2 to 3 side-to-side sutures are placed posteriorly attaching the anterior aspect of the infraspinatus to the posterior aspect of the dermal allograft (Figures 6A-6C). If rotator interval tissue (comma tissue) is present, anterior side-to-side sutures may be placed. However, we do not recommend placing anterior side-to-side sutures directly from the dermal allograft to the subscapularis as this may deform the graft, over- constrain the shoulder, and restrict motion.
Discussion
Reconstruction of the superior capsule has been shown to restore the normal restraint to superior translation of the humeral head and reestablish a stable fulcrum at the glenohumeral joint.5 It should be mentioned that we do not perform the SCR in patients with advanced glenohumeral arthritis. The short-term results of this novel procedure have been encouraging, including our own series of patients, in which most patients have had a significant reduction in pain, improvement in function, and very few complications (P. J. Denard, MD, S. S. Burkhart, MD, P. C. Brady, MD, J. Tokish, MD, C. R. Adams, MD, unpublished data, May 2016).
The early success of this procedure suggests that a robust superior capsule is necessary, in addition to functional muscle-tendon units, to restore the stable fulcrum and force couples that are necessary for normal shoulder function. Perhaps we have not paid enough attention to the integrity of the superior capsule in the past. In cases of revision cuff repair, we pay special attention to the quality of the capsular layer deep to the cuff tendon. If the capsule is poor quality, we sometimes reconstruct the capsule with a dermal allograft (SCR) and then do a rotator cuff repair (partial or complete) over the top of the SCR to maintain the normal anatomic deep to superficial layering of the capsule and rotator cuff.
We are very conservative with our postoperative rehabilitation program after a SCR. We know that the rate of stiffness with a conservative program after an arthroscopic rotator cuff repair, even in the revision setting, is very low.6 Furthermore, both basic science on healing of soft tissue to bone and radiographic analysis of healing after postoperative rotator cuff repairs support a slow rehabilitation program.7,8 A canine model specifically evaluating acellular dermal allografts in the shoulder suggests that these grafts undergo significant remodeling and become weaker before they get stronger.9 We would rather err on the side of healing of the SCR with potentially a slight increase in the rate of shoulder stiffness than to regain early motion at the expense of graft failure. Therefore, we have the patient wear a sling with no shoulder motion for 6 weeks. Passive motion is started at 6 weeks postoperative and strengthening is delayed until 12 to 16 weeks postoperative.
1. Orr SB, Chainani A, Hippensteel KJ, et al. Aligned multilayered electrospun scaffolds for rotator cuff tendon tissue engineering. Acta Biomater. 2015;24:117-126.
2. Austin L, Black EM, Lombardi NJ, Pepe MD, Lazarus M. Arthroscopic transosseous rotator cuff repair. A prospective study on cost savings, surgical time, and outcomes. Ortho J Sports Med. 2015;3(2 Suppl). doi:10.1177/2325967115S00156.
3. Denard PJ, Lädermann A, Jiwani AZ, Burkhart SS. Functional outcome after arthroscopic repair of massive rotator cuff tears in individuals with pseudoparalysis. Arthroscopy. 2012;28(9):1214-1219.
4. Mihata T, Lee TQ, Watanabe C, et al. Clinical results of arthroscopic superior capsule reconstruction for irreparable rotator cuff tears. Arthroscopy. 2013;29(3):459-470.
5. Mihata T, McGarry MH, Pirolo JM, Kinoshita M, Lee TQ. Superior capsule reconstruction to restore superior stability in irreparable rotator cuff tears: a biomechanical cadaveric study. Am J Sports Med. 2012;40(10):2248-2255.
6. Huberty DP, Schoolfield JD, Brady PC, Vadala AP, Arrigoni P, Burkhart SS. Incidence and treatment of postoperative stiffness following arthroscopic rotator cuff repair. Arthroscopy. 2009;25(8):880-890.
7. Sonnabend DH, Howlett CR, Young AA. Histological evaluation of repair of the rotator cuff in a primate model. J Bone Joint Surg Br. 2010;92(4):586-594.
8. Lee BG, Cho NS, Rhee YG. Effect of two rehabilitation protocols on range of motion and healing rates after arthroscopic rotator cuff repair: aggressive versus limited early passive exercises. Arthroscopy. 2012;28(1):34-42.
9. Adams JE, Zobitz ME, Reach JS Jr, An KN, Steinmann SP. Rotator cuff repair using an acellular dermal matrix graft: an in vivo study in a canine model. Arthroscopy. 2006;22(7):700-709.
1. Orr SB, Chainani A, Hippensteel KJ, et al. Aligned multilayered electrospun scaffolds for rotator cuff tendon tissue engineering. Acta Biomater. 2015;24:117-126.
2. Austin L, Black EM, Lombardi NJ, Pepe MD, Lazarus M. Arthroscopic transosseous rotator cuff repair. A prospective study on cost savings, surgical time, and outcomes. Ortho J Sports Med. 2015;3(2 Suppl). doi:10.1177/2325967115S00156.
3. Denard PJ, Lädermann A, Jiwani AZ, Burkhart SS. Functional outcome after arthroscopic repair of massive rotator cuff tears in individuals with pseudoparalysis. Arthroscopy. 2012;28(9):1214-1219.
4. Mihata T, Lee TQ, Watanabe C, et al. Clinical results of arthroscopic superior capsule reconstruction for irreparable rotator cuff tears. Arthroscopy. 2013;29(3):459-470.
5. Mihata T, McGarry MH, Pirolo JM, Kinoshita M, Lee TQ. Superior capsule reconstruction to restore superior stability in irreparable rotator cuff tears: a biomechanical cadaveric study. Am J Sports Med. 2012;40(10):2248-2255.
6. Huberty DP, Schoolfield JD, Brady PC, Vadala AP, Arrigoni P, Burkhart SS. Incidence and treatment of postoperative stiffness following arthroscopic rotator cuff repair. Arthroscopy. 2009;25(8):880-890.
7. Sonnabend DH, Howlett CR, Young AA. Histological evaluation of repair of the rotator cuff in a primate model. J Bone Joint Surg Br. 2010;92(4):586-594.
8. Lee BG, Cho NS, Rhee YG. Effect of two rehabilitation protocols on range of motion and healing rates after arthroscopic rotator cuff repair: aggressive versus limited early passive exercises. Arthroscopy. 2012;28(1):34-42.
9. Adams JE, Zobitz ME, Reach JS Jr, An KN, Steinmann SP. Rotator cuff repair using an acellular dermal matrix graft: an in vivo study in a canine model. Arthroscopy. 2006;22(7):700-709.
Escitalopram falls short in patients with heart failure and depression
Using escitalopram for patients with chronic heart failure and depression for more than 18 months neither significantly reduces mortality or hospitalization, nor improves depression, a study published June 28 involving 372 patients shows.
“To our knowledge, this study was the first to investigate the composite all-cause mortality and hospitalization in a population with heart failure treated with escitalopram,” wrote Dr. Christiane E. Angermann and her associates.
They said the only other randomized trial evaluating selective serotonin reuptake inhibitors in patients with heart failure was the Sertraline Against Depression and Heart Disease in Chronic Heart Failure study, which found that sertraline did not improve depression or cardiovascular status. But the treatment duration in that study was only 12 weeks, reported Dr. Angermann of the Cardiology and Comprehensive Heart Failure Center, Würzburg, Germany.
The current study, called the Effects of Selective Serotonin Re-Uptake Inhibition on Morbidity, Mortality, and Mood in Depressed Heart Failure Patients, was a double-blind, placebo-controlled randomized trial conducted at 16 tertiary medical centers in Germany. Three hundred seventy-two patients (185 in the escitalopram group and 187 in the placebo group) were randomized and given at least one dose of the study medication. The study was supposed to last for 24 months, but the investigators stopped it early.
Primary outcome of death or hospitalization occurred in 116 (63%) patients and 119 (64%) patients, respectively (hazard ratio, 0.99; 95% confidence interval, 0.76-1.27; P = .92).
Read more of the study and findings on the JAMA website (doi: 10.1001/jama.2016.7635).
Using escitalopram for patients with chronic heart failure and depression for more than 18 months neither significantly reduces mortality or hospitalization, nor improves depression, a study published June 28 involving 372 patients shows.
“To our knowledge, this study was the first to investigate the composite all-cause mortality and hospitalization in a population with heart failure treated with escitalopram,” wrote Dr. Christiane E. Angermann and her associates.
They said the only other randomized trial evaluating selective serotonin reuptake inhibitors in patients with heart failure was the Sertraline Against Depression and Heart Disease in Chronic Heart Failure study, which found that sertraline did not improve depression or cardiovascular status. But the treatment duration in that study was only 12 weeks, reported Dr. Angermann of the Cardiology and Comprehensive Heart Failure Center, Würzburg, Germany.
The current study, called the Effects of Selective Serotonin Re-Uptake Inhibition on Morbidity, Mortality, and Mood in Depressed Heart Failure Patients, was a double-blind, placebo-controlled randomized trial conducted at 16 tertiary medical centers in Germany. Three hundred seventy-two patients (185 in the escitalopram group and 187 in the placebo group) were randomized and given at least one dose of the study medication. The study was supposed to last for 24 months, but the investigators stopped it early.
Primary outcome of death or hospitalization occurred in 116 (63%) patients and 119 (64%) patients, respectively (hazard ratio, 0.99; 95% confidence interval, 0.76-1.27; P = .92).
Read more of the study and findings on the JAMA website (doi: 10.1001/jama.2016.7635).
Using escitalopram for patients with chronic heart failure and depression for more than 18 months neither significantly reduces mortality or hospitalization, nor improves depression, a study published June 28 involving 372 patients shows.
“To our knowledge, this study was the first to investigate the composite all-cause mortality and hospitalization in a population with heart failure treated with escitalopram,” wrote Dr. Christiane E. Angermann and her associates.
They said the only other randomized trial evaluating selective serotonin reuptake inhibitors in patients with heart failure was the Sertraline Against Depression and Heart Disease in Chronic Heart Failure study, which found that sertraline did not improve depression or cardiovascular status. But the treatment duration in that study was only 12 weeks, reported Dr. Angermann of the Cardiology and Comprehensive Heart Failure Center, Würzburg, Germany.
The current study, called the Effects of Selective Serotonin Re-Uptake Inhibition on Morbidity, Mortality, and Mood in Depressed Heart Failure Patients, was a double-blind, placebo-controlled randomized trial conducted at 16 tertiary medical centers in Germany. Three hundred seventy-two patients (185 in the escitalopram group and 187 in the placebo group) were randomized and given at least one dose of the study medication. The study was supposed to last for 24 months, but the investigators stopped it early.
Primary outcome of death or hospitalization occurred in 116 (63%) patients and 119 (64%) patients, respectively (hazard ratio, 0.99; 95% confidence interval, 0.76-1.27; P = .92).
Read more of the study and findings on the JAMA website (doi: 10.1001/jama.2016.7635).
FROM JAMA
Product News: 07 2016
Carmex Grant
Carma Labs Inc, the maker of Carmex lip balms, announced a $10,000 grant program to support an entrepreneur with an Unstoppable Dream. Participants can enter through Facebook or Instagram by posting a 30-second video depicting their aspiration related to a business or product. All entries must include the hashtags #InPursuitOf and #Contest to be considered. Entries can be submitted through July 15, and a winner will be selected and announced in August. For more information, visit www.mycarmex.com.
Juvéderm Volbella XC
Allergan announces US Food and Drug Administration approval to market Juvéderm Volbella XC for lip augmentation and for correction of perioral lines in adults older than 21 years. Juvéderm Volbella XC increases lip fullness and softens the appearance of lines around the mouth. It is formulated with Vycross, a proprietary filler technology that contributes to the product’s smoothness. Juvéderm Volbella XC has been customized with a lower hyaluronic acid concentration (15 mg/mL), while still providing the long-lasting results of other Juvéderm products. It will be available to patients in the United States in October 2016. For more information, visit www.juvederm.com.
Teflaro
Allergan announces US Food and Drug Administration approval of a supplemental new drug application for Teflaro (ceftaroline fosamil) to expand the label to include the treatment of children aged 2 months to less than 18 years with acute bacterial skin and skin structure infections (ABSSSI) including infections caused by methicillin-resistant Staphylococcus aureus and community-acquired bacterial pneumonia (CABP) caused by Staphylococcus pneumoniae and other designated susceptible bacteria. Teflaro is a bactericidal cephalosporin with activity against both gram-positive and gram-negative pathogens. Teflaro is already approved for ABSSSI and CABP in adult patients. For more information, visit www.teflaro.com.
If you would like your product included in Product News, please email a press release to the Editorial Office at [email protected].
Carmex Grant
Carma Labs Inc, the maker of Carmex lip balms, announced a $10,000 grant program to support an entrepreneur with an Unstoppable Dream. Participants can enter through Facebook or Instagram by posting a 30-second video depicting their aspiration related to a business or product. All entries must include the hashtags #InPursuitOf and #Contest to be considered. Entries can be submitted through July 15, and a winner will be selected and announced in August. For more information, visit www.mycarmex.com.
Juvéderm Volbella XC
Allergan announces US Food and Drug Administration approval to market Juvéderm Volbella XC for lip augmentation and for correction of perioral lines in adults older than 21 years. Juvéderm Volbella XC increases lip fullness and softens the appearance of lines around the mouth. It is formulated with Vycross, a proprietary filler technology that contributes to the product’s smoothness. Juvéderm Volbella XC has been customized with a lower hyaluronic acid concentration (15 mg/mL), while still providing the long-lasting results of other Juvéderm products. It will be available to patients in the United States in October 2016. For more information, visit www.juvederm.com.
Teflaro
Allergan announces US Food and Drug Administration approval of a supplemental new drug application for Teflaro (ceftaroline fosamil) to expand the label to include the treatment of children aged 2 months to less than 18 years with acute bacterial skin and skin structure infections (ABSSSI) including infections caused by methicillin-resistant Staphylococcus aureus and community-acquired bacterial pneumonia (CABP) caused by Staphylococcus pneumoniae and other designated susceptible bacteria. Teflaro is a bactericidal cephalosporin with activity against both gram-positive and gram-negative pathogens. Teflaro is already approved for ABSSSI and CABP in adult patients. For more information, visit www.teflaro.com.
If you would like your product included in Product News, please email a press release to the Editorial Office at [email protected].
Carmex Grant
Carma Labs Inc, the maker of Carmex lip balms, announced a $10,000 grant program to support an entrepreneur with an Unstoppable Dream. Participants can enter through Facebook or Instagram by posting a 30-second video depicting their aspiration related to a business or product. All entries must include the hashtags #InPursuitOf and #Contest to be considered. Entries can be submitted through July 15, and a winner will be selected and announced in August. For more information, visit www.mycarmex.com.
Juvéderm Volbella XC
Allergan announces US Food and Drug Administration approval to market Juvéderm Volbella XC for lip augmentation and for correction of perioral lines in adults older than 21 years. Juvéderm Volbella XC increases lip fullness and softens the appearance of lines around the mouth. It is formulated with Vycross, a proprietary filler technology that contributes to the product’s smoothness. Juvéderm Volbella XC has been customized with a lower hyaluronic acid concentration (15 mg/mL), while still providing the long-lasting results of other Juvéderm products. It will be available to patients in the United States in October 2016. For more information, visit www.juvederm.com.
Teflaro
Allergan announces US Food and Drug Administration approval of a supplemental new drug application for Teflaro (ceftaroline fosamil) to expand the label to include the treatment of children aged 2 months to less than 18 years with acute bacterial skin and skin structure infections (ABSSSI) including infections caused by methicillin-resistant Staphylococcus aureus and community-acquired bacterial pneumonia (CABP) caused by Staphylococcus pneumoniae and other designated susceptible bacteria. Teflaro is a bactericidal cephalosporin with activity against both gram-positive and gram-negative pathogens. Teflaro is already approved for ABSSSI and CABP in adult patients. For more information, visit www.teflaro.com.
If you would like your product included in Product News, please email a press release to the Editorial Office at [email protected].
Clinical Guidelines: Update in acne treatment
Acne affects 85% of teenagers but can frequently persist into adulthood. It causes significant physical and psychological effects for patients including facial scarring, depression, and decreased self-esteem. The initial approach to acne is determined according to presenting severity, emphasizing topical treatment for milder disease and the addition of oral therapy as disease becomes more severe.
Treatment of mild acne can begin with either benzoyl peroxide (BP) or a topical retinoid (TR). Another option for slightly more severe acne is to start with the initially suggested treatment for moderately severe acne, which is topical combination therapy with BP plus a topical antibiotic; TR plus BP; or TR plus BP in combination with a topical antibiotic. Combination therapy can be given either with separate application of the different medicines or by using fixed combination products that include the separate components in one formulation.
BP is an antibacterial agent, with mild comedolytic properties, and it often is added to topical antibiotic therapy to increase effectiveness and reduce the development of resistance. BP is available in strengths from 2.5% to 10% and in a variety of formulations, which can be used as leave-on or wash-off agents. Common side effects include dose dependent skin irritation and bleaching of fabric.
Topical antibiotics, including clindamycin and erythromycin, work through both their antimicrobial and anti-inflammatory affects. Monotherapy with topical antibiotics is no longer recommended; instead they should be used in combination with BP to prevent bacterial resistance. The preferred topical antibiotic is clindamycin 1% solution or gel. Clindamycin is available in a combination with BP, which may enhance compliance with the treatment regimen.
Topical retinoids are vitamin A derivatives that are the core of treatment. They are effective for all forms of acne and should always be used in the treatment of comedonal acne. There are currently three active agents available: tretinoin (0.025%-0.1% in cream, gel, or microsphere gel vehicles), adapalene (0.1% and 0.3% cream or 0.1% lotion), and tazarotene (0.05% and 0.1% cream, gel, or foam). Combination products are available containing clindamycin and BP. The main side effects of retinoids include dryness, peeling, erythema, and skin irritation. Reducing the frequency of application or potency used may be helpful for limiting these side effects. Topical retinoids increase the risk of photosensitivity, so patients should be counseled on daily sunscreen use, and their use is contraindicated in pregnancy.
Dapsone is an alternative topical treatment for mild acne. Topical dapsone is primarily effective in reducing inflammatory lesions, and seems to be more beneficial for female patients. Dapsone can be combined with topical retinoids if comedonal lesions are present.
Moderate acne can be treated with either topical combination therapy as described above, or systemic antibiotics plus a TR and BP, with or without the addition of a topical antibiotic as well. Female patients may also consider combined oral contraceptives or spironolactone for the treatment of moderate acne.
Systemic antibiotics have been used in the treatment of acne vulgaris for many years, and they are indicated for use in moderate to severe acne. They should always be used in combination with topical therapies, specifically a retinoid or BP. Generally, systemic antibiotics should be used for the shortest possible duration, often 3 months, to prevent the development of bacterial resistance. Tetracyclines and macrolides have the strongest evidence for efficacy. Doxycycline and minocycline are considered equally effective and are the preferred first-line oral antibiotics. Azithromycin has been studied in a variety of pulse dose regimens, and is a good alternative for patients who are not candidates for tetracyclines.
Combined oral contraceptive pills (COCs) are another option for the treatment of acne in female patients. COCs improve acne through their antiandrogenic effects. Spironolactone also has antiandrogen properties, and while it is not FDA approved for the treatment of acne, the AAD guidelines support selective use in women. Spironolactone has been studied at doses from 50 to 200 mg daily and has shown clinically significant improvement in acne. Side effects include diuresis, menstrual irregularities, breast tenderness, and rare hyperkalemia.
Severe acne is treated with an oral antibiotic plus topical combination therapy, or oral isotretinoin, with the addition where appropriate of COCs or oral spironolactone.
Oral isotretinoin, an isomer of retinoic acid, is approved by the FDA for the treatment of severe recalcitrant acne. It causes decreased sebum production, acne lesions, and scarring. It can also be considered in the treatment of moderate acne that is resistant to other treatments, relapses quickly, or produces significant scarring or psychosocial distress. Serum cholesterol, triglycerides, and transaminases can rise during treatment, and should be monitored. Because of the risk of teratogenic effects, the FDA has mandated that all patients receiving isotretinoin must participate in the iPLEDGE risk management program, which requires abstinence or two forms of birth control. While isotretinoin requires monitoring and carries the possibility of significant side effects, it is an effective treatment option for patients with severe recalcitrant acne.
The bottom line
Acne is commonly treated by primary care physicians. A clear approach of graded treatment based on severity of disease yields improvement in outcomes. Mild acne should be treated with benzoyl peroxide, retinoids or a combinations of topical treatments. Systemic antibiotics should be combined with topical therapies for moderate to severe acne. Female patients may also consider using combined oral contraceptives and spironolactone. Oral isotretinoin is an effective option for severe acne, but requires close monitoring.
References
Guidelines of care for the management of acne vulgaris (J Am Acad Dermatol. 2016;74[5]:945-73.e33. doi: 10.1016/j.jaad.2015.12.037. Epub 2016 Feb 17).
Dr. Skolnik is associate director of the family medicine residency program at Abington (Pa.) Memorial Hospital. Dr. Marriott is an attending family physician at Capital Health Primary Care in Hamilton, N.J.
Acne affects 85% of teenagers but can frequently persist into adulthood. It causes significant physical and psychological effects for patients including facial scarring, depression, and decreased self-esteem. The initial approach to acne is determined according to presenting severity, emphasizing topical treatment for milder disease and the addition of oral therapy as disease becomes more severe.
Treatment of mild acne can begin with either benzoyl peroxide (BP) or a topical retinoid (TR). Another option for slightly more severe acne is to start with the initially suggested treatment for moderately severe acne, which is topical combination therapy with BP plus a topical antibiotic; TR plus BP; or TR plus BP in combination with a topical antibiotic. Combination therapy can be given either with separate application of the different medicines or by using fixed combination products that include the separate components in one formulation.
BP is an antibacterial agent, with mild comedolytic properties, and it often is added to topical antibiotic therapy to increase effectiveness and reduce the development of resistance. BP is available in strengths from 2.5% to 10% and in a variety of formulations, which can be used as leave-on or wash-off agents. Common side effects include dose dependent skin irritation and bleaching of fabric.
Topical antibiotics, including clindamycin and erythromycin, work through both their antimicrobial and anti-inflammatory affects. Monotherapy with topical antibiotics is no longer recommended; instead they should be used in combination with BP to prevent bacterial resistance. The preferred topical antibiotic is clindamycin 1% solution or gel. Clindamycin is available in a combination with BP, which may enhance compliance with the treatment regimen.
Topical retinoids are vitamin A derivatives that are the core of treatment. They are effective for all forms of acne and should always be used in the treatment of comedonal acne. There are currently three active agents available: tretinoin (0.025%-0.1% in cream, gel, or microsphere gel vehicles), adapalene (0.1% and 0.3% cream or 0.1% lotion), and tazarotene (0.05% and 0.1% cream, gel, or foam). Combination products are available containing clindamycin and BP. The main side effects of retinoids include dryness, peeling, erythema, and skin irritation. Reducing the frequency of application or potency used may be helpful for limiting these side effects. Topical retinoids increase the risk of photosensitivity, so patients should be counseled on daily sunscreen use, and their use is contraindicated in pregnancy.
Dapsone is an alternative topical treatment for mild acne. Topical dapsone is primarily effective in reducing inflammatory lesions, and seems to be more beneficial for female patients. Dapsone can be combined with topical retinoids if comedonal lesions are present.
Moderate acne can be treated with either topical combination therapy as described above, or systemic antibiotics plus a TR and BP, with or without the addition of a topical antibiotic as well. Female patients may also consider combined oral contraceptives or spironolactone for the treatment of moderate acne.
Systemic antibiotics have been used in the treatment of acne vulgaris for many years, and they are indicated for use in moderate to severe acne. They should always be used in combination with topical therapies, specifically a retinoid or BP. Generally, systemic antibiotics should be used for the shortest possible duration, often 3 months, to prevent the development of bacterial resistance. Tetracyclines and macrolides have the strongest evidence for efficacy. Doxycycline and minocycline are considered equally effective and are the preferred first-line oral antibiotics. Azithromycin has been studied in a variety of pulse dose regimens, and is a good alternative for patients who are not candidates for tetracyclines.
Combined oral contraceptive pills (COCs) are another option for the treatment of acne in female patients. COCs improve acne through their antiandrogenic effects. Spironolactone also has antiandrogen properties, and while it is not FDA approved for the treatment of acne, the AAD guidelines support selective use in women. Spironolactone has been studied at doses from 50 to 200 mg daily and has shown clinically significant improvement in acne. Side effects include diuresis, menstrual irregularities, breast tenderness, and rare hyperkalemia.
Severe acne is treated with an oral antibiotic plus topical combination therapy, or oral isotretinoin, with the addition where appropriate of COCs or oral spironolactone.
Oral isotretinoin, an isomer of retinoic acid, is approved by the FDA for the treatment of severe recalcitrant acne. It causes decreased sebum production, acne lesions, and scarring. It can also be considered in the treatment of moderate acne that is resistant to other treatments, relapses quickly, or produces significant scarring or psychosocial distress. Serum cholesterol, triglycerides, and transaminases can rise during treatment, and should be monitored. Because of the risk of teratogenic effects, the FDA has mandated that all patients receiving isotretinoin must participate in the iPLEDGE risk management program, which requires abstinence or two forms of birth control. While isotretinoin requires monitoring and carries the possibility of significant side effects, it is an effective treatment option for patients with severe recalcitrant acne.
The bottom line
Acne is commonly treated by primary care physicians. A clear approach of graded treatment based on severity of disease yields improvement in outcomes. Mild acne should be treated with benzoyl peroxide, retinoids or a combinations of topical treatments. Systemic antibiotics should be combined with topical therapies for moderate to severe acne. Female patients may also consider using combined oral contraceptives and spironolactone. Oral isotretinoin is an effective option for severe acne, but requires close monitoring.
References
Guidelines of care for the management of acne vulgaris (J Am Acad Dermatol. 2016;74[5]:945-73.e33. doi: 10.1016/j.jaad.2015.12.037. Epub 2016 Feb 17).
Dr. Skolnik is associate director of the family medicine residency program at Abington (Pa.) Memorial Hospital. Dr. Marriott is an attending family physician at Capital Health Primary Care in Hamilton, N.J.
Acne affects 85% of teenagers but can frequently persist into adulthood. It causes significant physical and psychological effects for patients including facial scarring, depression, and decreased self-esteem. The initial approach to acne is determined according to presenting severity, emphasizing topical treatment for milder disease and the addition of oral therapy as disease becomes more severe.
Treatment of mild acne can begin with either benzoyl peroxide (BP) or a topical retinoid (TR). Another option for slightly more severe acne is to start with the initially suggested treatment for moderately severe acne, which is topical combination therapy with BP plus a topical antibiotic; TR plus BP; or TR plus BP in combination with a topical antibiotic. Combination therapy can be given either with separate application of the different medicines or by using fixed combination products that include the separate components in one formulation.
BP is an antibacterial agent, with mild comedolytic properties, and it often is added to topical antibiotic therapy to increase effectiveness and reduce the development of resistance. BP is available in strengths from 2.5% to 10% and in a variety of formulations, which can be used as leave-on or wash-off agents. Common side effects include dose dependent skin irritation and bleaching of fabric.
Topical antibiotics, including clindamycin and erythromycin, work through both their antimicrobial and anti-inflammatory affects. Monotherapy with topical antibiotics is no longer recommended; instead they should be used in combination with BP to prevent bacterial resistance. The preferred topical antibiotic is clindamycin 1% solution or gel. Clindamycin is available in a combination with BP, which may enhance compliance with the treatment regimen.
Topical retinoids are vitamin A derivatives that are the core of treatment. They are effective for all forms of acne and should always be used in the treatment of comedonal acne. There are currently three active agents available: tretinoin (0.025%-0.1% in cream, gel, or microsphere gel vehicles), adapalene (0.1% and 0.3% cream or 0.1% lotion), and tazarotene (0.05% and 0.1% cream, gel, or foam). Combination products are available containing clindamycin and BP. The main side effects of retinoids include dryness, peeling, erythema, and skin irritation. Reducing the frequency of application or potency used may be helpful for limiting these side effects. Topical retinoids increase the risk of photosensitivity, so patients should be counseled on daily sunscreen use, and their use is contraindicated in pregnancy.
Dapsone is an alternative topical treatment for mild acne. Topical dapsone is primarily effective in reducing inflammatory lesions, and seems to be more beneficial for female patients. Dapsone can be combined with topical retinoids if comedonal lesions are present.
Moderate acne can be treated with either topical combination therapy as described above, or systemic antibiotics plus a TR and BP, with or without the addition of a topical antibiotic as well. Female patients may also consider combined oral contraceptives or spironolactone for the treatment of moderate acne.
Systemic antibiotics have been used in the treatment of acne vulgaris for many years, and they are indicated for use in moderate to severe acne. They should always be used in combination with topical therapies, specifically a retinoid or BP. Generally, systemic antibiotics should be used for the shortest possible duration, often 3 months, to prevent the development of bacterial resistance. Tetracyclines and macrolides have the strongest evidence for efficacy. Doxycycline and minocycline are considered equally effective and are the preferred first-line oral antibiotics. Azithromycin has been studied in a variety of pulse dose regimens, and is a good alternative for patients who are not candidates for tetracyclines.
Combined oral contraceptive pills (COCs) are another option for the treatment of acne in female patients. COCs improve acne through their antiandrogenic effects. Spironolactone also has antiandrogen properties, and while it is not FDA approved for the treatment of acne, the AAD guidelines support selective use in women. Spironolactone has been studied at doses from 50 to 200 mg daily and has shown clinically significant improvement in acne. Side effects include diuresis, menstrual irregularities, breast tenderness, and rare hyperkalemia.
Severe acne is treated with an oral antibiotic plus topical combination therapy, or oral isotretinoin, with the addition where appropriate of COCs or oral spironolactone.
Oral isotretinoin, an isomer of retinoic acid, is approved by the FDA for the treatment of severe recalcitrant acne. It causes decreased sebum production, acne lesions, and scarring. It can also be considered in the treatment of moderate acne that is resistant to other treatments, relapses quickly, or produces significant scarring or psychosocial distress. Serum cholesterol, triglycerides, and transaminases can rise during treatment, and should be monitored. Because of the risk of teratogenic effects, the FDA has mandated that all patients receiving isotretinoin must participate in the iPLEDGE risk management program, which requires abstinence or two forms of birth control. While isotretinoin requires monitoring and carries the possibility of significant side effects, it is an effective treatment option for patients with severe recalcitrant acne.
The bottom line
Acne is commonly treated by primary care physicians. A clear approach of graded treatment based on severity of disease yields improvement in outcomes. Mild acne should be treated with benzoyl peroxide, retinoids or a combinations of topical treatments. Systemic antibiotics should be combined with topical therapies for moderate to severe acne. Female patients may also consider using combined oral contraceptives and spironolactone. Oral isotretinoin is an effective option for severe acne, but requires close monitoring.
References
Guidelines of care for the management of acne vulgaris (J Am Acad Dermatol. 2016;74[5]:945-73.e33. doi: 10.1016/j.jaad.2015.12.037. Epub 2016 Feb 17).
Dr. Skolnik is associate director of the family medicine residency program at Abington (Pa.) Memorial Hospital. Dr. Marriott is an attending family physician at Capital Health Primary Care in Hamilton, N.J.
Clinical Guidelines: Update in acne treatment
Acne affects 85% of teenagers but can frequently persist into adulthood. It causes significant physical and psychological effects for patients including facial scarring, depression, and decreased self-esteem. The initial approach to acne is determined according to presenting severity, emphasizing topical treatment for milder disease and the addition of oral therapy as disease becomes more severe.
Treatment of mild acne can begin with either benzoyl peroxide (BP) or a topical retinoid (TR). Another option for slightly more severe acne is to start with the initially suggested treatment for moderately severe acne, which is topical combination therapy with BP plus a topical antibiotic; TR plus BP; or TR plus BP in combination with a topical antibiotic. Combination therapy can be given either with separate application of the different medicines or by using fixed combination products that include the separate components in one formulation.
BP is an antibacterial agent, with mild comedolytic properties, and it often is added to topical antibiotic therapy to increase effectiveness and reduce the development of resistance. BP is available in strengths from 2.5% to 10% and in a variety of formulations, which can be used as leave-on or wash-off agents. Common side effects include dose dependent skin irritation and bleaching of fabric.
Topical antibiotics, including clindamycin and erythromycin, work through both their antimicrobial and anti-inflammatory affects. Monotherapy with topical antibiotics is no longer recommended; instead they should be used in combination with BP to prevent bacterial resistance. The preferred topical antibiotic is clindamycin 1% solution or gel. Clindamycin is available in a combination with BP, which may enhance compliance with the treatment regimen.
Topical retinoids are vitamin A derivatives that are the core of treatment. They are effective for all forms of acne and should always be used in the treatment of comedonal acne. There are currently three active agents available: tretinoin (0.025%-0.1% in cream, gel, or microsphere gel vehicles), adapalene (0.1% and 0.3% cream or 0.1% lotion), and tazarotene (0.05% and 0.1% cream, gel, or foam). Combination products are available containing clindamycin and BP. The main side effects of retinoids include dryness, peeling, erythema, and skin irritation. Reducing the frequency of application or potency used may be helpful for limiting these side effects. Topical retinoids increase the risk of photosensitivity, so patients should be counseled on daily sunscreen use, and their use is contraindicated in pregnancy.
Dapsone is an alternative topical treatment for mild acne. Topical dapsone is primarily effective in reducing inflammatory lesions, and seems to be more beneficial for female patients. Dapsone can be combined with topical retinoids if comedonal lesions are present.
Moderate acne can be treated with either topical combination therapy as described above, or systemic antibiotics plus a TR and BP, with or without the addition of a topical antibiotic as well. Female patients may also consider combined oral contraceptives or spironolactone for the treatment of moderate acne.
Systemic antibiotics have been used in the treatment of acne vulgaris for many years, and they are indicated for use in moderate to severe acne. They should always be used in combination with topical therapies, specifically a retinoid or BP. Generally, systemic antibiotics should be used for the shortest possible duration, often 3 months, to prevent the development of bacterial resistance. Tetracyclines and macrolides have the strongest evidence for efficacy. Doxycycline and minocycline are considered equally effective and are the preferred first-line oral antibiotics. Azithromycin has been studied in a variety of pulse dose regimens, and is a good alternative for patients who are not candidates for tetracyclines.
Combined oral contraceptive pills (COCs) are another option for the treatment of acne in female patients. COCs improve acne through their antiandrogenic effects. Spironolactone also has antiandrogen properties, and while it is not FDA approved for the treatment of acne, the AAD guidelines support selective use in women. Spironolactone has been studied at doses from 50 to 200 mg daily and has shown clinically significant improvement in acne. Side effects include diuresis, menstrual irregularities, breast tenderness, and rare hyperkalemia.
Severe acne is treated with an oral antibiotic plus topical combination therapy, or oral isotretinoin, with the addition where appropriate of COCs or oral spironolactone.
Oral isotretinoin, an isomer of retinoic acid, is approved by the FDA for the treatment of severe recalcitrant acne. It causes decreased sebum production, acne lesions, and scarring. It can also be considered in the treatment of moderate acne that is resistant to other treatments, relapses quickly, or produces significant scarring or psychosocial distress. Serum cholesterol, triglycerides, and transaminases can rise during treatment, and should be monitored. Because of the risk of teratogenic effects, the FDA has mandated that all patients receiving isotretinoin must participate in the iPLEDGE risk management program, which requires abstinence or two forms of birth control. While isotretinoin requires monitoring and carries the possibility of significant side effects, it is an effective treatment option for patients with severe recalcitrant acne.
The bottom line
Acne is commonly treated by primary care physicians. A clear approach of graded treatment based on severity of disease yields improvement in outcomes. Mild acne should be treated with benzoyl peroxide, retinoids or a combinations of topical treatments. Systemic antibiotics should be combined with topical therapies for moderate to severe acne. Female patients may also consider using combined oral contraceptives and spironolactone. Oral isotretinoin is an effective option for severe acne, but requires close monitoring.
References
Guidelines of care for the management of acne vulgaris (J Am Acad Dermatol. 2016;74[5]:945-73.e33. doi: 10.1016/j.jaad.2015.12.037. Epub 2016 Feb 17).
Dr. Skolnik is associate director of the family medicine residency program at Abington (Pa.) Memorial Hospital. Dr. Marriott is an attending family physician at Capital Health Primary Care in Hamilton, N.J.
Acne affects 85% of teenagers but can frequently persist into adulthood. It causes significant physical and psychological effects for patients including facial scarring, depression, and decreased self-esteem. The initial approach to acne is determined according to presenting severity, emphasizing topical treatment for milder disease and the addition of oral therapy as disease becomes more severe.
Treatment of mild acne can begin with either benzoyl peroxide (BP) or a topical retinoid (TR). Another option for slightly more severe acne is to start with the initially suggested treatment for moderately severe acne, which is topical combination therapy with BP plus a topical antibiotic; TR plus BP; or TR plus BP in combination with a topical antibiotic. Combination therapy can be given either with separate application of the different medicines or by using fixed combination products that include the separate components in one formulation.
BP is an antibacterial agent, with mild comedolytic properties, and it often is added to topical antibiotic therapy to increase effectiveness and reduce the development of resistance. BP is available in strengths from 2.5% to 10% and in a variety of formulations, which can be used as leave-on or wash-off agents. Common side effects include dose dependent skin irritation and bleaching of fabric.
Topical antibiotics, including clindamycin and erythromycin, work through both their antimicrobial and anti-inflammatory affects. Monotherapy with topical antibiotics is no longer recommended; instead they should be used in combination with BP to prevent bacterial resistance. The preferred topical antibiotic is clindamycin 1% solution or gel. Clindamycin is available in a combination with BP, which may enhance compliance with the treatment regimen.
Topical retinoids are vitamin A derivatives that are the core of treatment. They are effective for all forms of acne and should always be used in the treatment of comedonal acne. There are currently three active agents available: tretinoin (0.025%-0.1% in cream, gel, or microsphere gel vehicles), adapalene (0.1% and 0.3% cream or 0.1% lotion), and tazarotene (0.05% and 0.1% cream, gel, or foam). Combination products are available containing clindamycin and BP. The main side effects of retinoids include dryness, peeling, erythema, and skin irritation. Reducing the frequency of application or potency used may be helpful for limiting these side effects. Topical retinoids increase the risk of photosensitivity, so patients should be counseled on daily sunscreen use, and their use is contraindicated in pregnancy.
Dapsone is an alternative topical treatment for mild acne. Topical dapsone is primarily effective in reducing inflammatory lesions, and seems to be more beneficial for female patients. Dapsone can be combined with topical retinoids if comedonal lesions are present.
Moderate acne can be treated with either topical combination therapy as described above, or systemic antibiotics plus a TR and BP, with or without the addition of a topical antibiotic as well. Female patients may also consider combined oral contraceptives or spironolactone for the treatment of moderate acne.
Systemic antibiotics have been used in the treatment of acne vulgaris for many years, and they are indicated for use in moderate to severe acne. They should always be used in combination with topical therapies, specifically a retinoid or BP. Generally, systemic antibiotics should be used for the shortest possible duration, often 3 months, to prevent the development of bacterial resistance. Tetracyclines and macrolides have the strongest evidence for efficacy. Doxycycline and minocycline are considered equally effective and are the preferred first-line oral antibiotics. Azithromycin has been studied in a variety of pulse dose regimens, and is a good alternative for patients who are not candidates for tetracyclines.
Combined oral contraceptive pills (COCs) are another option for the treatment of acne in female patients. COCs improve acne through their antiandrogenic effects. Spironolactone also has antiandrogen properties, and while it is not FDA approved for the treatment of acne, the AAD guidelines support selective use in women. Spironolactone has been studied at doses from 50 to 200 mg daily and has shown clinically significant improvement in acne. Side effects include diuresis, menstrual irregularities, breast tenderness, and rare hyperkalemia.
Severe acne is treated with an oral antibiotic plus topical combination therapy, or oral isotretinoin, with the addition where appropriate of COCs or oral spironolactone.
Oral isotretinoin, an isomer of retinoic acid, is approved by the FDA for the treatment of severe recalcitrant acne. It causes decreased sebum production, acne lesions, and scarring. It can also be considered in the treatment of moderate acne that is resistant to other treatments, relapses quickly, or produces significant scarring or psychosocial distress. Serum cholesterol, triglycerides, and transaminases can rise during treatment, and should be monitored. Because of the risk of teratogenic effects, the FDA has mandated that all patients receiving isotretinoin must participate in the iPLEDGE risk management program, which requires abstinence or two forms of birth control. While isotretinoin requires monitoring and carries the possibility of significant side effects, it is an effective treatment option for patients with severe recalcitrant acne.
The bottom line
Acne is commonly treated by primary care physicians. A clear approach of graded treatment based on severity of disease yields improvement in outcomes. Mild acne should be treated with benzoyl peroxide, retinoids or a combinations of topical treatments. Systemic antibiotics should be combined with topical therapies for moderate to severe acne. Female patients may also consider using combined oral contraceptives and spironolactone. Oral isotretinoin is an effective option for severe acne, but requires close monitoring.
References
Guidelines of care for the management of acne vulgaris (J Am Acad Dermatol. 2016;74[5]:945-73.e33. doi: 10.1016/j.jaad.2015.12.037. Epub 2016 Feb 17).
Dr. Skolnik is associate director of the family medicine residency program at Abington (Pa.) Memorial Hospital. Dr. Marriott is an attending family physician at Capital Health Primary Care in Hamilton, N.J.
Acne affects 85% of teenagers but can frequently persist into adulthood. It causes significant physical and psychological effects for patients including facial scarring, depression, and decreased self-esteem. The initial approach to acne is determined according to presenting severity, emphasizing topical treatment for milder disease and the addition of oral therapy as disease becomes more severe.
Treatment of mild acne can begin with either benzoyl peroxide (BP) or a topical retinoid (TR). Another option for slightly more severe acne is to start with the initially suggested treatment for moderately severe acne, which is topical combination therapy with BP plus a topical antibiotic; TR plus BP; or TR plus BP in combination with a topical antibiotic. Combination therapy can be given either with separate application of the different medicines or by using fixed combination products that include the separate components in one formulation.
BP is an antibacterial agent, with mild comedolytic properties, and it often is added to topical antibiotic therapy to increase effectiveness and reduce the development of resistance. BP is available in strengths from 2.5% to 10% and in a variety of formulations, which can be used as leave-on or wash-off agents. Common side effects include dose dependent skin irritation and bleaching of fabric.
Topical antibiotics, including clindamycin and erythromycin, work through both their antimicrobial and anti-inflammatory affects. Monotherapy with topical antibiotics is no longer recommended; instead they should be used in combination with BP to prevent bacterial resistance. The preferred topical antibiotic is clindamycin 1% solution or gel. Clindamycin is available in a combination with BP, which may enhance compliance with the treatment regimen.
Topical retinoids are vitamin A derivatives that are the core of treatment. They are effective for all forms of acne and should always be used in the treatment of comedonal acne. There are currently three active agents available: tretinoin (0.025%-0.1% in cream, gel, or microsphere gel vehicles), adapalene (0.1% and 0.3% cream or 0.1% lotion), and tazarotene (0.05% and 0.1% cream, gel, or foam). Combination products are available containing clindamycin and BP. The main side effects of retinoids include dryness, peeling, erythema, and skin irritation. Reducing the frequency of application or potency used may be helpful for limiting these side effects. Topical retinoids increase the risk of photosensitivity, so patients should be counseled on daily sunscreen use, and their use is contraindicated in pregnancy.
Dapsone is an alternative topical treatment for mild acne. Topical dapsone is primarily effective in reducing inflammatory lesions, and seems to be more beneficial for female patients. Dapsone can be combined with topical retinoids if comedonal lesions are present.
Moderate acne can be treated with either topical combination therapy as described above, or systemic antibiotics plus a TR and BP, with or without the addition of a topical antibiotic as well. Female patients may also consider combined oral contraceptives or spironolactone for the treatment of moderate acne.
Systemic antibiotics have been used in the treatment of acne vulgaris for many years, and they are indicated for use in moderate to severe acne. They should always be used in combination with topical therapies, specifically a retinoid or BP. Generally, systemic antibiotics should be used for the shortest possible duration, often 3 months, to prevent the development of bacterial resistance. Tetracyclines and macrolides have the strongest evidence for efficacy. Doxycycline and minocycline are considered equally effective and are the preferred first-line oral antibiotics. Azithromycin has been studied in a variety of pulse dose regimens, and is a good alternative for patients who are not candidates for tetracyclines.
Combined oral contraceptive pills (COCs) are another option for the treatment of acne in female patients. COCs improve acne through their antiandrogenic effects. Spironolactone also has antiandrogen properties, and while it is not FDA approved for the treatment of acne, the AAD guidelines support selective use in women. Spironolactone has been studied at doses from 50 to 200 mg daily and has shown clinically significant improvement in acne. Side effects include diuresis, menstrual irregularities, breast tenderness, and rare hyperkalemia.
Severe acne is treated with an oral antibiotic plus topical combination therapy, or oral isotretinoin, with the addition where appropriate of COCs or oral spironolactone.
Oral isotretinoin, an isomer of retinoic acid, is approved by the FDA for the treatment of severe recalcitrant acne. It causes decreased sebum production, acne lesions, and scarring. It can also be considered in the treatment of moderate acne that is resistant to other treatments, relapses quickly, or produces significant scarring or psychosocial distress. Serum cholesterol, triglycerides, and transaminases can rise during treatment, and should be monitored. Because of the risk of teratogenic effects, the FDA has mandated that all patients receiving isotretinoin must participate in the iPLEDGE risk management program, which requires abstinence or two forms of birth control. While isotretinoin requires monitoring and carries the possibility of significant side effects, it is an effective treatment option for patients with severe recalcitrant acne.
The bottom line
Acne is commonly treated by primary care physicians. A clear approach of graded treatment based on severity of disease yields improvement in outcomes. Mild acne should be treated with benzoyl peroxide, retinoids or a combinations of topical treatments. Systemic antibiotics should be combined with topical therapies for moderate to severe acne. Female patients may also consider using combined oral contraceptives and spironolactone. Oral isotretinoin is an effective option for severe acne, but requires close monitoring.
References
Guidelines of care for the management of acne vulgaris (J Am Acad Dermatol. 2016;74[5]:945-73.e33. doi: 10.1016/j.jaad.2015.12.037. Epub 2016 Feb 17).
Dr. Skolnik is associate director of the family medicine residency program at Abington (Pa.) Memorial Hospital. Dr. Marriott is an attending family physician at Capital Health Primary Care in Hamilton, N.J.
Effective Applications of Medication-Assisted Opioid Dependence Counseling
NEW YORK - Patients dependent on prescription opioids are likely to quit if they get individual drug counseling with their prescribed medications, according to long-term follow-up results.
"Medication-assisted treatment for opioid dependence benefited people who were dependent on prescription opioids. Good standard medical management, medically based counseling, can be effective for these people if given in conjunction with buprenorphine treatment," said lead author Dr. Roger D. Weiss, professor of psychiatry at Harvard Medical School in Boston and chief of the Division of Alcohol and Drug Abuse at McLean Hospital in Belmont, Massachusetts.
"Over time, with treatment, these patients have relatively optimistic outcomes, but there are a number of risks involved, including people shifting from prescription opioids to heroin who had never injected drugs, injecting them, and a higher death rate," he told Reuters Health by phone.
Dr. Weiss and colleagues examined the outcomes over a 42-month follow-up period among adult participants in POATS (the Prescription Opioid Addiction Treatment Study), the first long-term multisite randomized trial to investigate whether outcomes for patients dependent on prescription opioid analgesics can be improved with standard medical management by adding individual drug counseling to prescribed buprenorphine (combined with naloxone to prevent withdrawal symptoms).
The researchers enrolled 653 participants at 12 medical centers in nine states through the National Institute on Drug Abuse (NIDA) Clinical Trials Network. They examined the results at four and 12 weeks and conducted follow-up assessments of opioid and other substance abuse through telephone interviews with 375 patients at 1.5 years, 2.5 years, and 3.5 years from study entry.
At 1.5 years, most follow-up participants no longer depended on opioids, and at 3.5 years, fewer than 10% of them were opioid dependent. Patients who reported a lifetime history of heroin use at study entry were more likely to be opioid dependent at 3.5 years (odds ratio 4.56, p<0.05).
At 2.5 years, 61% of patients reported that they had abstained from opioids for one month. The roughly one-third of the sample who received opioid agonist treatment during follow-up were more likely to be abstinent at month 42. Even so, 27 (8%) of 338 patients used heroin for the first time during follow-up and 10.1% reported that they had injected heroin for the first time.
The results were presented in a poster May 16 at the Annual Meeting of the American Psychiatric Association (APA) in Atlanta, Georgia.
"Prior to this study, there had never been a study of treatment in people dependent on prescription opioids. There had been many opioid-dependence treatment studies, but they had all focused on people who exclusively or primarily used heroin, and most were done in methadone maintenance programs. This was the first study done with people receiving office-based buprenorphine treatment," Dr. Weiss told Reuters Health.
"A real strength of this study is that it was very large and included a mixture of urban, suburban, and rural sites. No other study has looked at even 100 people," he added.
Regarding future related research, Dr. Weiss told Reuters Health that it would be good to learn exactly which people do well over time and how to increase their success rate.
The authors reported no funding or disclosures.
SOURCE: http://bit.ly/28MTqd6 APA 2016.
NEW YORK - Patients dependent on prescription opioids are likely to quit if they get individual drug counseling with their prescribed medications, according to long-term follow-up results.
"Medication-assisted treatment for opioid dependence benefited people who were dependent on prescription opioids. Good standard medical management, medically based counseling, can be effective for these people if given in conjunction with buprenorphine treatment," said lead author Dr. Roger D. Weiss, professor of psychiatry at Harvard Medical School in Boston and chief of the Division of Alcohol and Drug Abuse at McLean Hospital in Belmont, Massachusetts.
"Over time, with treatment, these patients have relatively optimistic outcomes, but there are a number of risks involved, including people shifting from prescription opioids to heroin who had never injected drugs, injecting them, and a higher death rate," he told Reuters Health by phone.
Dr. Weiss and colleagues examined the outcomes over a 42-month follow-up period among adult participants in POATS (the Prescription Opioid Addiction Treatment Study), the first long-term multisite randomized trial to investigate whether outcomes for patients dependent on prescription opioid analgesics can be improved with standard medical management by adding individual drug counseling to prescribed buprenorphine (combined with naloxone to prevent withdrawal symptoms).
The researchers enrolled 653 participants at 12 medical centers in nine states through the National Institute on Drug Abuse (NIDA) Clinical Trials Network. They examined the results at four and 12 weeks and conducted follow-up assessments of opioid and other substance abuse through telephone interviews with 375 patients at 1.5 years, 2.5 years, and 3.5 years from study entry.
At 1.5 years, most follow-up participants no longer depended on opioids, and at 3.5 years, fewer than 10% of them were opioid dependent. Patients who reported a lifetime history of heroin use at study entry were more likely to be opioid dependent at 3.5 years (odds ratio 4.56, p<0.05).
At 2.5 years, 61% of patients reported that they had abstained from opioids for one month. The roughly one-third of the sample who received opioid agonist treatment during follow-up were more likely to be abstinent at month 42. Even so, 27 (8%) of 338 patients used heroin for the first time during follow-up and 10.1% reported that they had injected heroin for the first time.
The results were presented in a poster May 16 at the Annual Meeting of the American Psychiatric Association (APA) in Atlanta, Georgia.
"Prior to this study, there had never been a study of treatment in people dependent on prescription opioids. There had been many opioid-dependence treatment studies, but they had all focused on people who exclusively or primarily used heroin, and most were done in methadone maintenance programs. This was the first study done with people receiving office-based buprenorphine treatment," Dr. Weiss told Reuters Health.
"A real strength of this study is that it was very large and included a mixture of urban, suburban, and rural sites. No other study has looked at even 100 people," he added.
Regarding future related research, Dr. Weiss told Reuters Health that it would be good to learn exactly which people do well over time and how to increase their success rate.
The authors reported no funding or disclosures.
SOURCE: http://bit.ly/28MTqd6 APA 2016.
NEW YORK - Patients dependent on prescription opioids are likely to quit if they get individual drug counseling with their prescribed medications, according to long-term follow-up results.
"Medication-assisted treatment for opioid dependence benefited people who were dependent on prescription opioids. Good standard medical management, medically based counseling, can be effective for these people if given in conjunction with buprenorphine treatment," said lead author Dr. Roger D. Weiss, professor of psychiatry at Harvard Medical School in Boston and chief of the Division of Alcohol and Drug Abuse at McLean Hospital in Belmont, Massachusetts.
"Over time, with treatment, these patients have relatively optimistic outcomes, but there are a number of risks involved, including people shifting from prescription opioids to heroin who had never injected drugs, injecting them, and a higher death rate," he told Reuters Health by phone.
Dr. Weiss and colleagues examined the outcomes over a 42-month follow-up period among adult participants in POATS (the Prescription Opioid Addiction Treatment Study), the first long-term multisite randomized trial to investigate whether outcomes for patients dependent on prescription opioid analgesics can be improved with standard medical management by adding individual drug counseling to prescribed buprenorphine (combined with naloxone to prevent withdrawal symptoms).
The researchers enrolled 653 participants at 12 medical centers in nine states through the National Institute on Drug Abuse (NIDA) Clinical Trials Network. They examined the results at four and 12 weeks and conducted follow-up assessments of opioid and other substance abuse through telephone interviews with 375 patients at 1.5 years, 2.5 years, and 3.5 years from study entry.
At 1.5 years, most follow-up participants no longer depended on opioids, and at 3.5 years, fewer than 10% of them were opioid dependent. Patients who reported a lifetime history of heroin use at study entry were more likely to be opioid dependent at 3.5 years (odds ratio 4.56, p<0.05).
At 2.5 years, 61% of patients reported that they had abstained from opioids for one month. The roughly one-third of the sample who received opioid agonist treatment during follow-up were more likely to be abstinent at month 42. Even so, 27 (8%) of 338 patients used heroin for the first time during follow-up and 10.1% reported that they had injected heroin for the first time.
The results were presented in a poster May 16 at the Annual Meeting of the American Psychiatric Association (APA) in Atlanta, Georgia.
"Prior to this study, there had never been a study of treatment in people dependent on prescription opioids. There had been many opioid-dependence treatment studies, but they had all focused on people who exclusively or primarily used heroin, and most were done in methadone maintenance programs. This was the first study done with people receiving office-based buprenorphine treatment," Dr. Weiss told Reuters Health.
"A real strength of this study is that it was very large and included a mixture of urban, suburban, and rural sites. No other study has looked at even 100 people," he added.
Regarding future related research, Dr. Weiss told Reuters Health that it would be good to learn exactly which people do well over time and how to increase their success rate.
The authors reported no funding or disclosures.
SOURCE: http://bit.ly/28MTqd6 APA 2016.
New Antiepileptic Medications Expand Patients’ Options
VANCOUVER—None of the seven new antiepileptic drugs (AEDs) approved by the FDA during the past 10 years greatly increase the likelihood of seizure control, relative to previously existing therapies, according to an overview presented at the 68th Annual Meeting of the American Academy of Neurology. The broader array of choices is facilitating the individualization of therapy, however. Several of the newer agents are chemically related to previously existing AEDs, but all of the drugs have distinguishing features.
“We do have more options for the treatment of epilepsy,” confirmed Carl W. Bazil, MD, PhD, Director of the Comprehensive Epilepsy Center at Columbia University College of Physicians and Surgeons in New York City. “Although we do not yet have the perfect therapy, more are coming.”
In his evaluation of how newer agents fit into current clinical practice, Dr. Bazil indicated that relative efficacy among the newer agents has not been well defined in the absence of head-to-head trials. Treatment choice is instead based on their approved indications, side effect profiles, and half-lives.
Yet the emergence of more choices was characterized as a positive development. Since pregabalin received regulatory approval in 2005, the newer therapies have included lacosamide (2008), rufinamide (2009), clobazam (2010), ezogabine (2011), perampanel (2012), eslicarbazepine (2013), and brivaracetam (2016). Of these, Dr. Bazil focused primarily on the latter five and how they fit into routine care. Overall, these agents “have not been all that different” in terms of their associated rates of total seizure control or other meaningful end points.
This similarity does not suggest that these agents are interchangeable. The clinical profiles differ by characteristics such as dosing frequency, relative risk of somnolence and other common side effects, and the potential to ameliorate accompanying symptoms, such as anxiety. Moreover, due to differences in trial design and entry criteria, not all of the newer drugs have been granted the same indication, said Dr. Bazil.
Clobazam for Lennox-Gastaut Syndrome
Clobazam is a benzodiazepine receptor agonist that shares features with other benzodiazepines. One of the key features of this agent is a half-life that approaches 80 hours. This characteristic makes clobazam a “forgiving” drug for those who miss a dose, said Dr. Bazil.
Clobazam’s FDA-approved indication is for the adjunctive treatment of seizures associated with Lennox-Gastaut syndrome for individuals age 2 or older. Although this Schedule IV drug is not approved in the United States for partial onset seizures, it is widely used for this indication elsewhere, including in Europe and Canada. Clobazam, like other benzodiazepine-related agents, could have a favorable effect on anxiety or sleep problems, even though it is not indicated for these uses, said Dr. Bazil.
Relative to other commonly used AEDs, the rate of rash associated with clobazam has been relatively low, but dizziness and somnolence are sufficiently common that the once-daily dose should be taken before bedtime, according to Dr. Bazil. Relative to other benzodiazepines, clobazam appears to have a low potential for tolerance and dependence. Initial daily doses of 5 mg to 10 mg are standard, but lower doses should be considered in older patients or those with low body weight, said Dr. Bazil. Titration is performed on a weekly basis to a maximum recommended dose of 60 mg.
Perampanel Has a Long Half-Life
Perampanel, which is a noncompetitive AMPA-receptor inhibitor, has an even longer half-life of 100 hours. The drug’s FDA approval is for the adjunctive treatment of partial epilepsy and primary generalized tonic-clonic seizures in patients age 12 and older. Prudent initial dosing begins at 2 mg daily with a recommended maximum dose of 12 mg, according to Dr. Bazil. Due to its reliance on hepatic metabolism, a relatively slow upward titration of this Schedule III drug is appropriate in patients with impaired liver function, he added.
Dizziness and somnolence are commonly reported side effects for perampanel, as they are for most AEDs, and the drug also has a black box warning about its association with homicidal ideation, irritability and aggression. Yet the drug’s relative efficacy may compensate for these risks in selected patients. Even though researchers have not compared perampanel directly with another AED, the drug had an “impressive” seizure-free rate in a placebo-controlled trial, said Dr. Bazil. In this multicenter study of patients with drug-resistant, primary generalized tonic-clonic seizures, 30.9% of participants on perampanel, versus 12.3% of those on placebo, remained seizure-free during maintenance.
Ezogabine and Risk of Discoloration
Ezogabine, which is FDA-approved as adjunctive therapy for refractory partial epilepsy, is a K-channel opener. Due to a relatively short half-life of seven to 11 hours, thrice-daily dosing is required. In addition, bluish discoloration, primarily of the lips and fingertips, commonly occurs due to an interaction of a drug metabolite with sun exposure, according to Dr. Bazil. Concern that this drug may lead to retinal pigmentation means that eye examinations are recommended at baseline and every six months.
“Clinical use of ezogabine has been limited by the pigmentation and by tid dosing, which patients do not like,” Dr. Bazil said. Although the bluish color resolves over time, patients are likely to discontinue this Schedule V drug, which also is associated with dizziness and somnolence, before this occurs.
Eslicarbazepine, an Unscheduled Agent
Eslicarbazepine, a sodium channel blocker, has been approved as adjunctive and monotherapy for partial epilepsy. Although monotherapy trials with a placebo control are no longer considered ethical in patients with epilepsy, eslicarbazepine and lacosamide have been evaluated and found effective in trials in which other active agents were withdrawn over time to allow response on monotherapy to be monitored, Dr. Bazil explained.
Eslicarbazepine is also distinguished from other newer agents by the fact that it is not a scheduled agent. It is primarily metabolized by the liver and has a half-life of 13 to 20 hours. Due to its structural similarity to carbamazepine and oxcarbazepine, these agents should not be combined, said Dr. Bazil. Eslicarbazepine, like clobazam, also could interfere with the bioavailability of oral contraceptives.
“Eslicarbazepine is essentially a longer-acting carbamazepine, a drug with which we are all familiar,” said Dr. Bazil. Although eslicarbazepine also may cause the dizziness, somnolence, and nausea common to carbamazepine, its longer half-life has the potential to diminish peak effects to improve tolerability.
New and Emerging Therapies
Brivaracetam, which was approved for add-on therapy of refractory partial seizures for patients age 16 or older, “is structurally and functionally related to levetiracetam, but approximately 20 times more potent at the SV2A receptor,” said Dr. Bazil. As this recently approved drug is not yet widely used, its relative clinical utility within the context of other choices is not well established, but the side effects common to AEDs, such as dizziness and somnolence, have been relatively mild on this agent in the clinical trials.
Of treatment strategies on the horizon, Dr. Bazil drew attention to SAGE-547, an experimental agent now in a phase III study that has demonstrated uncommon efficacy for refractory status epilepticus, a condition for which there is a large unmet need for reliably effective treatments. In addition, researchers are conducting ongoing efforts to individualize therapy by identifying targetable underlying genetic mutations responsible for seizure activity.
Overall, the therapies approved during the past 10 years have expanded choice in a way that may increase the likelihood of providing a patient with adequate efficacy and acceptable tolerability. Although newer pharmacologic options have not yet generated a major leap in seizure control, coming therapies or strategies to better match existing therapies to the underlying seizure mechanism may yield greater rates of seizure freedom, said Dr. Bazil.
—Ted Bosworth
Suggested Reading
French JA, Krauss GL, Wechsler RT, et al. Perampanel for tonic-clonic seizures in idiopathic generalized epilepsy A randomized trial. Neurology. 2015;85(11):950-957.
VANCOUVER—None of the seven new antiepileptic drugs (AEDs) approved by the FDA during the past 10 years greatly increase the likelihood of seizure control, relative to previously existing therapies, according to an overview presented at the 68th Annual Meeting of the American Academy of Neurology. The broader array of choices is facilitating the individualization of therapy, however. Several of the newer agents are chemically related to previously existing AEDs, but all of the drugs have distinguishing features.
“We do have more options for the treatment of epilepsy,” confirmed Carl W. Bazil, MD, PhD, Director of the Comprehensive Epilepsy Center at Columbia University College of Physicians and Surgeons in New York City. “Although we do not yet have the perfect therapy, more are coming.”
In his evaluation of how newer agents fit into current clinical practice, Dr. Bazil indicated that relative efficacy among the newer agents has not been well defined in the absence of head-to-head trials. Treatment choice is instead based on their approved indications, side effect profiles, and half-lives.
Yet the emergence of more choices was characterized as a positive development. Since pregabalin received regulatory approval in 2005, the newer therapies have included lacosamide (2008), rufinamide (2009), clobazam (2010), ezogabine (2011), perampanel (2012), eslicarbazepine (2013), and brivaracetam (2016). Of these, Dr. Bazil focused primarily on the latter five and how they fit into routine care. Overall, these agents “have not been all that different” in terms of their associated rates of total seizure control or other meaningful end points.
This similarity does not suggest that these agents are interchangeable. The clinical profiles differ by characteristics such as dosing frequency, relative risk of somnolence and other common side effects, and the potential to ameliorate accompanying symptoms, such as anxiety. Moreover, due to differences in trial design and entry criteria, not all of the newer drugs have been granted the same indication, said Dr. Bazil.
Clobazam for Lennox-Gastaut Syndrome
Clobazam is a benzodiazepine receptor agonist that shares features with other benzodiazepines. One of the key features of this agent is a half-life that approaches 80 hours. This characteristic makes clobazam a “forgiving” drug for those who miss a dose, said Dr. Bazil.
Clobazam’s FDA-approved indication is for the adjunctive treatment of seizures associated with Lennox-Gastaut syndrome for individuals age 2 or older. Although this Schedule IV drug is not approved in the United States for partial onset seizures, it is widely used for this indication elsewhere, including in Europe and Canada. Clobazam, like other benzodiazepine-related agents, could have a favorable effect on anxiety or sleep problems, even though it is not indicated for these uses, said Dr. Bazil.
Relative to other commonly used AEDs, the rate of rash associated with clobazam has been relatively low, but dizziness and somnolence are sufficiently common that the once-daily dose should be taken before bedtime, according to Dr. Bazil. Relative to other benzodiazepines, clobazam appears to have a low potential for tolerance and dependence. Initial daily doses of 5 mg to 10 mg are standard, but lower doses should be considered in older patients or those with low body weight, said Dr. Bazil. Titration is performed on a weekly basis to a maximum recommended dose of 60 mg.
Perampanel Has a Long Half-Life
Perampanel, which is a noncompetitive AMPA-receptor inhibitor, has an even longer half-life of 100 hours. The drug’s FDA approval is for the adjunctive treatment of partial epilepsy and primary generalized tonic-clonic seizures in patients age 12 and older. Prudent initial dosing begins at 2 mg daily with a recommended maximum dose of 12 mg, according to Dr. Bazil. Due to its reliance on hepatic metabolism, a relatively slow upward titration of this Schedule III drug is appropriate in patients with impaired liver function, he added.
Dizziness and somnolence are commonly reported side effects for perampanel, as they are for most AEDs, and the drug also has a black box warning about its association with homicidal ideation, irritability and aggression. Yet the drug’s relative efficacy may compensate for these risks in selected patients. Even though researchers have not compared perampanel directly with another AED, the drug had an “impressive” seizure-free rate in a placebo-controlled trial, said Dr. Bazil. In this multicenter study of patients with drug-resistant, primary generalized tonic-clonic seizures, 30.9% of participants on perampanel, versus 12.3% of those on placebo, remained seizure-free during maintenance.
Ezogabine and Risk of Discoloration
Ezogabine, which is FDA-approved as adjunctive therapy for refractory partial epilepsy, is a K-channel opener. Due to a relatively short half-life of seven to 11 hours, thrice-daily dosing is required. In addition, bluish discoloration, primarily of the lips and fingertips, commonly occurs due to an interaction of a drug metabolite with sun exposure, according to Dr. Bazil. Concern that this drug may lead to retinal pigmentation means that eye examinations are recommended at baseline and every six months.
“Clinical use of ezogabine has been limited by the pigmentation and by tid dosing, which patients do not like,” Dr. Bazil said. Although the bluish color resolves over time, patients are likely to discontinue this Schedule V drug, which also is associated with dizziness and somnolence, before this occurs.
Eslicarbazepine, an Unscheduled Agent
Eslicarbazepine, a sodium channel blocker, has been approved as adjunctive and monotherapy for partial epilepsy. Although monotherapy trials with a placebo control are no longer considered ethical in patients with epilepsy, eslicarbazepine and lacosamide have been evaluated and found effective in trials in which other active agents were withdrawn over time to allow response on monotherapy to be monitored, Dr. Bazil explained.
Eslicarbazepine is also distinguished from other newer agents by the fact that it is not a scheduled agent. It is primarily metabolized by the liver and has a half-life of 13 to 20 hours. Due to its structural similarity to carbamazepine and oxcarbazepine, these agents should not be combined, said Dr. Bazil. Eslicarbazepine, like clobazam, also could interfere with the bioavailability of oral contraceptives.
“Eslicarbazepine is essentially a longer-acting carbamazepine, a drug with which we are all familiar,” said Dr. Bazil. Although eslicarbazepine also may cause the dizziness, somnolence, and nausea common to carbamazepine, its longer half-life has the potential to diminish peak effects to improve tolerability.
New and Emerging Therapies
Brivaracetam, which was approved for add-on therapy of refractory partial seizures for patients age 16 or older, “is structurally and functionally related to levetiracetam, but approximately 20 times more potent at the SV2A receptor,” said Dr. Bazil. As this recently approved drug is not yet widely used, its relative clinical utility within the context of other choices is not well established, but the side effects common to AEDs, such as dizziness and somnolence, have been relatively mild on this agent in the clinical trials.
Of treatment strategies on the horizon, Dr. Bazil drew attention to SAGE-547, an experimental agent now in a phase III study that has demonstrated uncommon efficacy for refractory status epilepticus, a condition for which there is a large unmet need for reliably effective treatments. In addition, researchers are conducting ongoing efforts to individualize therapy by identifying targetable underlying genetic mutations responsible for seizure activity.
Overall, the therapies approved during the past 10 years have expanded choice in a way that may increase the likelihood of providing a patient with adequate efficacy and acceptable tolerability. Although newer pharmacologic options have not yet generated a major leap in seizure control, coming therapies or strategies to better match existing therapies to the underlying seizure mechanism may yield greater rates of seizure freedom, said Dr. Bazil.
—Ted Bosworth
VANCOUVER—None of the seven new antiepileptic drugs (AEDs) approved by the FDA during the past 10 years greatly increase the likelihood of seizure control, relative to previously existing therapies, according to an overview presented at the 68th Annual Meeting of the American Academy of Neurology. The broader array of choices is facilitating the individualization of therapy, however. Several of the newer agents are chemically related to previously existing AEDs, but all of the drugs have distinguishing features.
“We do have more options for the treatment of epilepsy,” confirmed Carl W. Bazil, MD, PhD, Director of the Comprehensive Epilepsy Center at Columbia University College of Physicians and Surgeons in New York City. “Although we do not yet have the perfect therapy, more are coming.”
In his evaluation of how newer agents fit into current clinical practice, Dr. Bazil indicated that relative efficacy among the newer agents has not been well defined in the absence of head-to-head trials. Treatment choice is instead based on their approved indications, side effect profiles, and half-lives.
Yet the emergence of more choices was characterized as a positive development. Since pregabalin received regulatory approval in 2005, the newer therapies have included lacosamide (2008), rufinamide (2009), clobazam (2010), ezogabine (2011), perampanel (2012), eslicarbazepine (2013), and brivaracetam (2016). Of these, Dr. Bazil focused primarily on the latter five and how they fit into routine care. Overall, these agents “have not been all that different” in terms of their associated rates of total seizure control or other meaningful end points.
This similarity does not suggest that these agents are interchangeable. The clinical profiles differ by characteristics such as dosing frequency, relative risk of somnolence and other common side effects, and the potential to ameliorate accompanying symptoms, such as anxiety. Moreover, due to differences in trial design and entry criteria, not all of the newer drugs have been granted the same indication, said Dr. Bazil.
Clobazam for Lennox-Gastaut Syndrome
Clobazam is a benzodiazepine receptor agonist that shares features with other benzodiazepines. One of the key features of this agent is a half-life that approaches 80 hours. This characteristic makes clobazam a “forgiving” drug for those who miss a dose, said Dr. Bazil.
Clobazam’s FDA-approved indication is for the adjunctive treatment of seizures associated with Lennox-Gastaut syndrome for individuals age 2 or older. Although this Schedule IV drug is not approved in the United States for partial onset seizures, it is widely used for this indication elsewhere, including in Europe and Canada. Clobazam, like other benzodiazepine-related agents, could have a favorable effect on anxiety or sleep problems, even though it is not indicated for these uses, said Dr. Bazil.
Relative to other commonly used AEDs, the rate of rash associated with clobazam has been relatively low, but dizziness and somnolence are sufficiently common that the once-daily dose should be taken before bedtime, according to Dr. Bazil. Relative to other benzodiazepines, clobazam appears to have a low potential for tolerance and dependence. Initial daily doses of 5 mg to 10 mg are standard, but lower doses should be considered in older patients or those with low body weight, said Dr. Bazil. Titration is performed on a weekly basis to a maximum recommended dose of 60 mg.
Perampanel Has a Long Half-Life
Perampanel, which is a noncompetitive AMPA-receptor inhibitor, has an even longer half-life of 100 hours. The drug’s FDA approval is for the adjunctive treatment of partial epilepsy and primary generalized tonic-clonic seizures in patients age 12 and older. Prudent initial dosing begins at 2 mg daily with a recommended maximum dose of 12 mg, according to Dr. Bazil. Due to its reliance on hepatic metabolism, a relatively slow upward titration of this Schedule III drug is appropriate in patients with impaired liver function, he added.
Dizziness and somnolence are commonly reported side effects for perampanel, as they are for most AEDs, and the drug also has a black box warning about its association with homicidal ideation, irritability and aggression. Yet the drug’s relative efficacy may compensate for these risks in selected patients. Even though researchers have not compared perampanel directly with another AED, the drug had an “impressive” seizure-free rate in a placebo-controlled trial, said Dr. Bazil. In this multicenter study of patients with drug-resistant, primary generalized tonic-clonic seizures, 30.9% of participants on perampanel, versus 12.3% of those on placebo, remained seizure-free during maintenance.
Ezogabine and Risk of Discoloration
Ezogabine, which is FDA-approved as adjunctive therapy for refractory partial epilepsy, is a K-channel opener. Due to a relatively short half-life of seven to 11 hours, thrice-daily dosing is required. In addition, bluish discoloration, primarily of the lips and fingertips, commonly occurs due to an interaction of a drug metabolite with sun exposure, according to Dr. Bazil. Concern that this drug may lead to retinal pigmentation means that eye examinations are recommended at baseline and every six months.
“Clinical use of ezogabine has been limited by the pigmentation and by tid dosing, which patients do not like,” Dr. Bazil said. Although the bluish color resolves over time, patients are likely to discontinue this Schedule V drug, which also is associated with dizziness and somnolence, before this occurs.
Eslicarbazepine, an Unscheduled Agent
Eslicarbazepine, a sodium channel blocker, has been approved as adjunctive and monotherapy for partial epilepsy. Although monotherapy trials with a placebo control are no longer considered ethical in patients with epilepsy, eslicarbazepine and lacosamide have been evaluated and found effective in trials in which other active agents were withdrawn over time to allow response on monotherapy to be monitored, Dr. Bazil explained.
Eslicarbazepine is also distinguished from other newer agents by the fact that it is not a scheduled agent. It is primarily metabolized by the liver and has a half-life of 13 to 20 hours. Due to its structural similarity to carbamazepine and oxcarbazepine, these agents should not be combined, said Dr. Bazil. Eslicarbazepine, like clobazam, also could interfere with the bioavailability of oral contraceptives.
“Eslicarbazepine is essentially a longer-acting carbamazepine, a drug with which we are all familiar,” said Dr. Bazil. Although eslicarbazepine also may cause the dizziness, somnolence, and nausea common to carbamazepine, its longer half-life has the potential to diminish peak effects to improve tolerability.
New and Emerging Therapies
Brivaracetam, which was approved for add-on therapy of refractory partial seizures for patients age 16 or older, “is structurally and functionally related to levetiracetam, but approximately 20 times more potent at the SV2A receptor,” said Dr. Bazil. As this recently approved drug is not yet widely used, its relative clinical utility within the context of other choices is not well established, but the side effects common to AEDs, such as dizziness and somnolence, have been relatively mild on this agent in the clinical trials.
Of treatment strategies on the horizon, Dr. Bazil drew attention to SAGE-547, an experimental agent now in a phase III study that has demonstrated uncommon efficacy for refractory status epilepticus, a condition for which there is a large unmet need for reliably effective treatments. In addition, researchers are conducting ongoing efforts to individualize therapy by identifying targetable underlying genetic mutations responsible for seizure activity.
Overall, the therapies approved during the past 10 years have expanded choice in a way that may increase the likelihood of providing a patient with adequate efficacy and acceptable tolerability. Although newer pharmacologic options have not yet generated a major leap in seizure control, coming therapies or strategies to better match existing therapies to the underlying seizure mechanism may yield greater rates of seizure freedom, said Dr. Bazil.
—Ted Bosworth
Suggested Reading
French JA, Krauss GL, Wechsler RT, et al. Perampanel for tonic-clonic seizures in idiopathic generalized epilepsy A randomized trial. Neurology. 2015;85(11):950-957.
Suggested Reading
French JA, Krauss GL, Wechsler RT, et al. Perampanel for tonic-clonic seizures in idiopathic generalized epilepsy A randomized trial. Neurology. 2015;85(11):950-957.
Clinical Challenges - June 2016: Diffuse idiopathic skeletal hyperostosis
What's Your Diagnosis?
The diagnosis
The radiograph (Figure B) and CT scan (Figure C) of the neck revealed severe calcification of soft tissue along the anterior portion of cervical spine starting from C2 to T1. The calcification is pressing on the esophagus and also displaces the trachea forward.
These findings are consistent with diffuse idiopathic skeletal hyperostosis (DISH). Initially, the patient was treated with partial liquid diet and nutritional supplement. Subsequently, the patient underwent orthopedic consultation for surgical consideration.
DISH is a noninflammatory ossification and calcification of ligaments and tendons anterior to the spine. Even though this condition was first described by Forestier et al. in 1950,1 the etiology and pathogenesis of DISH remain unknown. Many risk factors have been postulated to be associated with DISH, such as mechanical factors, diet, and drugs. DISH is more prevalent among the elderly, especially, males; however, it is a rare cause of dysphagia.2 The clinical manifestation varies depending on the location of calcification. There have been case reports of DISH causing dysphagia, difficult airway management, and spinal cord root compression.3
For esophageal involvement, the severity of symptoms is correlated with degree of compression. Initially, DISH may cause only an isolated globus sensation without any significant dysphagia. However, as the compression progresses, it can cause significant progressive dysphagia that mimics the presentation of esophageal cancer. Thus, the effort to rule out esophageal cancer or other structural diseases is needed before the diagnosis of DISH can be established, such as an upper endoscopy or CT of the neck.
Because DISH can resemble benign degenerative osteophytes on a plain neck radiograph, it is sometimes overlooked by physicians. DISH is an uncommon condition; it should be considered in the differential diagnosis of dysphagia, especially in the elderly population.References
1. Forestier, J., Rotes-Querol, J. Senile ankylosing hyperostosis of the spine. Ann Rheum Dis. 1950;9:321-30.
2. Schlapbach, P., Beyeler, C., Gerber, N.J., et al. Diffuse idiopathic skeletal hyperostosis (DISH) of the spine: a cause of back pain? (A controlled study). Br J Rheumatol. 1989;28:299-303.
3. Verlaan, J.J., Boswijk, P.F., de Ru, J.A., et al. Diffuse idiopathic skeletal hyperostosis of the cervical spine: an underestimated cause of dysphagia and airway obstruction. Spine J. 2011;11:1058-67.
The diagnosis
The radiograph (Figure B) and CT scan (Figure C) of the neck revealed severe calcification of soft tissue along the anterior portion of cervical spine starting from C2 to T1. The calcification is pressing on the esophagus and also displaces the trachea forward.
These findings are consistent with diffuse idiopathic skeletal hyperostosis (DISH). Initially, the patient was treated with partial liquid diet and nutritional supplement. Subsequently, the patient underwent orthopedic consultation for surgical consideration.
DISH is a noninflammatory ossification and calcification of ligaments and tendons anterior to the spine. Even though this condition was first described by Forestier et al. in 1950,1 the etiology and pathogenesis of DISH remain unknown. Many risk factors have been postulated to be associated with DISH, such as mechanical factors, diet, and drugs. DISH is more prevalent among the elderly, especially, males; however, it is a rare cause of dysphagia.2 The clinical manifestation varies depending on the location of calcification. There have been case reports of DISH causing dysphagia, difficult airway management, and spinal cord root compression.3
For esophageal involvement, the severity of symptoms is correlated with degree of compression. Initially, DISH may cause only an isolated globus sensation without any significant dysphagia. However, as the compression progresses, it can cause significant progressive dysphagia that mimics the presentation of esophageal cancer. Thus, the effort to rule out esophageal cancer or other structural diseases is needed before the diagnosis of DISH can be established, such as an upper endoscopy or CT of the neck.
Because DISH can resemble benign degenerative osteophytes on a plain neck radiograph, it is sometimes overlooked by physicians. DISH is an uncommon condition; it should be considered in the differential diagnosis of dysphagia, especially in the elderly population.References
1. Forestier, J., Rotes-Querol, J. Senile ankylosing hyperostosis of the spine. Ann Rheum Dis. 1950;9:321-30.
2. Schlapbach, P., Beyeler, C., Gerber, N.J., et al. Diffuse idiopathic skeletal hyperostosis (DISH) of the spine: a cause of back pain? (A controlled study). Br J Rheumatol. 1989;28:299-303.
3. Verlaan, J.J., Boswijk, P.F., de Ru, J.A., et al. Diffuse idiopathic skeletal hyperostosis of the cervical spine: an underestimated cause of dysphagia and airway obstruction. Spine J. 2011;11:1058-67.
The diagnosis
The radiograph (Figure B) and CT scan (Figure C) of the neck revealed severe calcification of soft tissue along the anterior portion of cervical spine starting from C2 to T1. The calcification is pressing on the esophagus and also displaces the trachea forward.
These findings are consistent with diffuse idiopathic skeletal hyperostosis (DISH). Initially, the patient was treated with partial liquid diet and nutritional supplement. Subsequently, the patient underwent orthopedic consultation for surgical consideration.
DISH is a noninflammatory ossification and calcification of ligaments and tendons anterior to the spine. Even though this condition was first described by Forestier et al. in 1950,1 the etiology and pathogenesis of DISH remain unknown. Many risk factors have been postulated to be associated with DISH, such as mechanical factors, diet, and drugs. DISH is more prevalent among the elderly, especially, males; however, it is a rare cause of dysphagia.2 The clinical manifestation varies depending on the location of calcification. There have been case reports of DISH causing dysphagia, difficult airway management, and spinal cord root compression.3
For esophageal involvement, the severity of symptoms is correlated with degree of compression. Initially, DISH may cause only an isolated globus sensation without any significant dysphagia. However, as the compression progresses, it can cause significant progressive dysphagia that mimics the presentation of esophageal cancer. Thus, the effort to rule out esophageal cancer or other structural diseases is needed before the diagnosis of DISH can be established, such as an upper endoscopy or CT of the neck.
Because DISH can resemble benign degenerative osteophytes on a plain neck radiograph, it is sometimes overlooked by physicians. DISH is an uncommon condition; it should be considered in the differential diagnosis of dysphagia, especially in the elderly population.References
1. Forestier, J., Rotes-Querol, J. Senile ankylosing hyperostosis of the spine. Ann Rheum Dis. 1950;9:321-30.
2. Schlapbach, P., Beyeler, C., Gerber, N.J., et al. Diffuse idiopathic skeletal hyperostosis (DISH) of the spine: a cause of back pain? (A controlled study). Br J Rheumatol. 1989;28:299-303.
3. Verlaan, J.J., Boswijk, P.F., de Ru, J.A., et al. Diffuse idiopathic skeletal hyperostosis of the cervical spine: an underestimated cause of dysphagia and airway obstruction. Spine J. 2011;11:1058-67.
What's Your Diagnosis?
What's Your Diagnosis?
What's Your Diagnosis?
Upon arrival, his vital signs were stable. Physical examination was unremarkable. No cervical lymphadenopathy or masses were appreciated in his neck. Hemoglobin, platelets, and white blood cell count were within normal reference ranges. An esophagogram was initially performed, illustrating mildly thickened folds of distal esophagus with no obstruction or ulcer (Figure A).
Upper endoscopy was subsequently performed, revealing normal esophageal mucosa without any strictures. An ENT specialist was consulted because of the concern of oropharyngeal dysphagia. Laryngoscope and video swallowing evaluations were performed; the results were normal. Ultimately, a neck radiograph and computed tomography (CT) of the neck were performed (Figures B and C).
Antibiotic interactions: Answers to 4 common questions
› Avoid preemptive warfarin dose reductions unless you are prescribing trimethoprim/sulfamethoxazole (TMP/SMX) or metronidazole. B
› Recommend a back-up contraceptive method to a woman who is taking a broad-spectrum antibiotic and low-dose OCs—especially if the woman is overweight. C
› Consider using the macrolide, clarithromycin, or the fluoroquinolone, ciprofloxacin, in patients taking medications that prolong QT interval or who are at higher risk for torsades de pointes (TdP). B
› Refrain from cautioning patients taking metronidazole against consuming alcohol. A
Strength of recommendation (SOR)
A Good-quality patient-oriented evidence
B Inconsistent or limited-quality patient-oriented evidence
C Consensus, usual practice, opinion, disease-oriented evidence, case series
Despite encouraging data that antibiotic prescribing is on the decline, patients are still prescribed antibiotics frequently, making these agents the 12th most frequently used drug class.1 At the same time, prescribers are caring for patients with increasingly complex drug regimens that provide fertile ground for drug interactions with these antibiotics. And, of course, lifestyle factors such as alcohol consumption are a consideration when any prescription is written.
As pharmacists, we find that certain questions about antibiotic prescribing and interactions come up with frequency. These questions often pertain to the use of warfarin, oral contraceptives, drugs that prolong the QT interval, and alcohol. But conflicting reports about issues such as monitoring international normalized ratio (INR) in patients taking warfarin and antibiotics, and whether (or which) antibiotics decrease the efficacy of oral contraceptives (OCs) can make decision-making challenging.
This review provides evidence-based answers to questions you may have. It also details some reliable sources of information you can consult (TABLE 12-7) when discussing treatment options with other members of the health care team.
1. Which antibiotics are preferable when a patient is taking warfarin, and are preemptive warfarin dose reductions advisable?
The simple answer is that agents with a lower likelihood of affecting the INR, such as penicillin G, clindamycin, and 1st- and 4th-generation cephalosporins, are a good place to start, and whether to preemptively reduce the warfarin dose hinges on the antibiotic being prescribed.
The more detailed answer. The fundamental mechanisms of interaction between warfarin and antibiotics are two-fold:8
- Antimicrobial agents disrupt gastrointestinal flora that synthesize vitamin K.
- Antimicrobials inhibit cytochrome p450 (CYP450) enzymes (primarily CYP2C9 and 3A4), which are responsible for the metabolism of warfarin.
The antibiotics most likely to interfere with warfarin are TMP/SMX, ciprofloxacin, levofloxacin, metronidazole, fluconazole, azithromycin, and clarithromycin (TABLE 2).9,10 Low-risk agents include clindamycin, cephalexin, and penicillin G. When prescribing an antibiotic for a patient taking warfarin, it is important not only to be aware of the agents that should be avoided, but also the agents that do not require more frequent monitoring of INR.
Preemptive warfarin dose reductions? Some physicians make preemptive warfarin dose reductions in an attempt to avoid supratherapeutic INRs in patients being prescribed antibiotics. But the evidence suggests that this step should be considered only in the presence of the antibiotics TMP/SMX and metronidazole.9,11
A 2008 study investigated the anticoagulation effects of a 10% to 20% preemptive warfarin dose reduction vs no dosing change in patients taking TMP/SMX or levofloxacin. The investigators found that the preemptive warfarin dose reduction (intervention) significantly decreased the number of supratherapeutic INR values above 4 when compared to controls (2 of 8 vs 8 of 9).12
In the dose-reduction group, no patients receiving TMP/SMX developed a subtherapeutic INR, whereas 40% (4 of 10 patients) who received levofloxacin developed a subtherapeutic INR.12 The authors of the study concluded that a prophylactic warfarin dose reduction of 10% to 20% is effective in maintaining therapeutic anticoagulation in patients receiving TMP/SMX. They added that while no change in warfarin dosing is necessary with levofloxacin, short-term INR follow-up is a prudent approach to prevent subtherapeutic INRs. Others recommend INR monitoring when antibiotic therapy is started and stopped and whenever the dose is changed.9
A 2010 retrospective, single-center, cohort study looked at patients who were taking metronidazole and warfarin. Researchers compared those who received a preemptive dose reduction of warfarin (mean reduction was 34.6% ± 13.4%) to those who did not and found a statistically significant mean difference in INR of 1.28 (P=.01).13
Almost half (46%) of the patients who did not receive a warfarin dose reduction had an INR >4, whereas none of the patients in the warfarin dose reduction group did (P=.05). Although this secondary outcome was not statistically significant (most likely due to the small sample population [N=20]), the implication is clinically significant. Two patients who reduced their dose had a subtherapeutic INR compared to none of the patients in the control group, which was also not a statistically significant difference.
The authors concluded that a 30% to 35% reduction in mean daily warfarin dose is effective in maintaining therapeutic anticoagulation in patients started on metronidazole.
Significant bleeding events. A retrospective cohort study of slightly more than 22,000 veterans who were prescribed warfarin for ≥30 uninterrupted days and given antibiotics with either a high or low risk for interaction with warfarin were studied for significant bleeding events for one month.10 Ninety-three significant bleeding events occurred in the high-risk group and 36 occurred in the low-risk group over the course of the study. The agent associated with the greatest increased risk of bleeding was TMP/SMX (hazard ratio [HR]=2.09; 95% CI, 1.45-3.02). Of note, metronidazole was not included in this study endpoint.
The study’s secondary endpoint of INR >4 found that 10% of patients taking metronidazole and 8% of patients taking TMP/SMX in addition to warfarin had INRs >4. Almost 10% (9.7%) of patients prescribed fluconazole had a peak INR value >6. Patients taking low-risk antibiotics (clindamycin or cephalexin) had no increased risk of bleeding. Monitoring INR within 3 to 14 days of starting patients on antibiotics was found to decrease the risk of serious bleeding events (HR=0.61; 95% CI, 0.42-0.88). More frequent INR monitoring by itself (without preemptive warfarin dose reductions) is appropriate for other antibiotics, including macrolides, tetracyclines, and some cephalosporins (2nd and 3rd generation).9
THE BOTTOM LINE When prescribing antibiotics for patients taking warfarin, try to choose agents with a lower likelihood of affecting INR such as penicillin G, clindamycin, and 1st- and 4th-generation cephalosporins. With these agents, there is no need for more frequent INR testing or preemptive reductions in warfarin dose. In patients for whom the use of TMP/SMX or metronidazole can’t be avoided, consider reducing the patient’s warfarin dose by 10% to 35% and rechecking the INR 5 days after starting the antibiotic.9,11,12 When prescribing agents such as fluoroquinolones, macrolides, and tetracyclines, do not reduce the patient’s warfarin dose preemptively and recheck INR 5 days after starting therapy.
2. Do antibiotics decrease the efficacy of oral contraceptives?
It’s unlikely, but antibiotics may reduce the efficacy of OCs.
There have been few, but well documented, reports of women using OCs who became pregnant after taking antimicrobials.14 It is recognized that rifampin, an inducer of enzymes that metabolize estrogens, decreases the efficacy of OCs.15 Ketoconazole’s interaction seems less well documented, but combining the agent with low-estrogen (low-dose) OCs warrants caution.16 What is not well understood is whether more common or broad-spectrum antibiotics also increase the risk of OC failure.
Three mechanisms have been proposed:16
- Antimicrobials affect hepatic enzyme induction, which increases metabolism of hormones.
- Broad-spectrum antibiotics reduce gut bacteria, which alters enterohepatic circulation and reduces plasma hormone concentrations.
- Antibiotics increase gastrointestinal motility, which decreases absorption (and reabsorption) of OCs.
A 2007 study found that when physicians and pharmacists were surveyed and asked if broad-spectrum antibiotics have a clinically significant interaction with OCs, 83% of physicians and 89% of pharmacists answered “Yes;”17 however, a large epidemiologic study performed in the United States showed no association between antibiotic use and OC failure.18
After this report, investigators in the Netherlands completed a similar cross-over analysis and found that there was a relationship between the use of antibiotics and breakthrough pregnancy in a population-based prescription database, but that the results didn’t hold for broad-spectrum antibiotics or in a sensitivity analysis.19 Pharmacokinetic studies are also conflicting, as some have shown an effect on serum hormone levels, while others have not.15,20-22
High- vs low-risk agents. Ciprofloxacin did not affect hormone levels in 2 studies.20,21 Rifampin and voriconazole may enhance systemic exposure to OCs.15,22 And erythromycin and azithromycin may interact with OCs, but the clinical significance of this interaction is still unknown.16
Short-courses of TMP/SMX are generally thought to be safe;16 a small study looked at cotrimoxazole 1 g twice daily in 9 women taking long-term OC steroids and found that short courses of the drug were unlikely to cause any adverse effects on contraceptive control.23 Tetracyclines and penicillins were the antibiotics most frequently involved in case reports of pregnancy from the United Kingdom (TABLE 32).16
It is hypothesized that some women may have a higher risk of OC failure than others due to how they metabolize ethinyl estradiol.24 Another hypothesis is that some women have gut flora that is more susceptible to the antibiotic being used. And still another possibility is that lower doses of hormones are being used in OCs than were studied for this interaction.15 Anything that decreases the concentration of these lower-dose OCs is concerning, especially in patients with a higher body mass index (BMI). The few pharmacokinetic studies that have been conducted show that it takes longer for OCs to reach a steady state in obese women and that they have a lower area under the curve (AUC) and maximum estrogen concentration than women with a normal BMI.25
THE BOTTOM LINE Because the degree of variability between patients is unknown and obesity rates are increasing, concern that low-dose OCs may lose efficacy when combined with antibiotics is warranted. While the absolute risk of breakthrough pregnancy seems small, the most conservative approach is to advise patients to use a back-up method of contraception during times of antibiotic use.
3. Which drugs prolong QT intervals?
Macrolides and fluoroquinolones are 2 classes of antibiotics associated with prolonged QT intervals, but other drugs and risk factors are important to consider, as well.
Physicians often receive phone calls from pharmacists warning about drug-drug interactions when they prescribe macrolides or fluoroquinolones for patients already taking medications known to prolong QT intervals or inhibit cytochrome P450 enzymes. Long QT syndrome increases the risk of TdP, a life-threatening arrhythmia. While TdP is rare, its severity warrants a discussion of risk factors and the likelihood of occurrence.
Two QT interval prolonging medications used together in healthy individuals does not warrant a change in therapy. TdP is most likely to occur when 2 or more QT interval prolonging medications are used in a patient who is already at high risk for arrhythmia because of risk factors such as prolonged QT interval at baseline, family history of prolonged QT intervals, female gender, age >60 years, electrolyte abnormalities (hypokalemia, hypomagnesemia, hypocalcemia), underlying comorbid diseases (eg, chronic heart failure, left ventricular hypertrophy, atrial fibrillation), hypertension, bradycardia, and genetic (ion channel) polymorphisms.26,27
Antiarrhythmics and antipsychotics are most commonly associated with drug-induced prolonged QT interval, with most case reports and research being linked to antiarrhythmics (TABLE 42).28 But macrolide and fluoroquinolone antibiotics also have been associated with TdP, although to a lesser extent. In a retrospective analysis of case reports of TdP involving macrolides, erythromycin was present (with or without other medications thought to prolong QT) in 53% of the cases and clarithromycin was involved in 36% of the reports.29
An analysis of 2 studies by the US Food and Drug Administration estimated an occurrence rate of serious cardiac arrhythmias of 46 to 85 per 100,000 users with cardiovascular disease, compared to 5 to 44 per 100,000 users without cardiovascular disease.30 And this may underestimate the actual incidence because spontaneous reporting of adverse effects declines the longer a drug is on the market. Ciprofloxacin is associated with less risk than levofloxacin and gatifloxacin (the latter of which is no longer available in the United States).26
A recent population-based study using data on over 10.6 million people from the Taiwan National Health Insurance Database examined the risk of cardiovascular death among patients using new-generation macrolides, fluoroquinolones, and β-lactam/β-lactamase inhibitors.31 The absolute risk of cardiovascular death per 1000 individuals was 0.06 for clarithromycin, 0.12 for ciprofloxacin, 0.13 for amoxicillin-clavulanate, 0.36 for azithromycin, 0.39 for levofloxacin, and 0.46 for moxifloxacin. The mean interval between first antibiotic use and the adverse cardiac event was <4 days. Not surprisingly, the highest risk was seen in patients with underlying cardiovascular disease.
Another population-based study, this time conducted in Hong Kong, evaluated the cardiovascular safety of clarithromycin compared to that of amoxicillin. Clarithromycin was found to increase the incidence of myocardial infarction, arrhythmia, and cardiac mortality in the short term, with the risk returning to baseline after treatment concluded.32 A binational cohort study of Danish and Swedish adults confirmed that fluoroquinolones (especially ciprofloxacin) do not increase the risk of a serious arrhythmia compared to penicillins.33
THE BOTTOM LINE For patients taking other QT interval prolonging medications or who are at a higher risk for TdP, consider using clarithromycin over erythromycin or azithromycin for a macrolide antibiotic or ciprofloxacin over levofloxacin or moxifloxacin if a fluoroquinolone is warranted. Using 2 drugs that may increase the QT interval is likely safe in the absence of certain risk factors.
4. Should patients avoid alcohol while taking metronidazole?
Probably not.
Warning patients against drinking alcohol while taking metronidazole has been a common practice for years. The mechanism for this theorized interaction was thought to be similar to the interaction between disulfiram and ethanol.34 Disulfiram inhibits hepatic aldehyde dehydrogenase (ALDH) when combined with alcohol, which leads to increased levels of acetaldehyde in the blood and symptoms of flushing, palpitations, nausea, vomiting, headache, and visual disturbances.35 However, multiple studies using rats have found that metronidazole does not inhibit ALDH or increase acetaldehyde concentrations like disulfiram does.34
A 2000 review article discussed 6 cases involving serious metronidazole-ethanol interactions. Ethanol alone was found to explain the reaction in 2 of the cases, and the remaining 4 could be linked to the use of other drugs or disease states.35 A 2002 Finnish study found no statistically significant differences in objective or subjective signs of a disulfiram-like interaction.34 When considering the symptoms associated with the interaction, it is important to remember that many of the symptoms can result from metronidazole therapy alone, regardless of whether other medications or alcohol are used.35
THE BOTTOM LINE Researchers have failed to identify a clinically significant interaction between metronidazole and alcohol. Avoiding alcohol while taking metronidazole does not appear to be necessary.
CORRESPONDENCE
Mary Onysko, PharmD, BCPS, University of Wyoming, School of Pharmacy Health Sciences Center, Room 292, 1000 E. University Avenue, Laramie, WY 82071; [email protected].
1. Kantor ED, Rehm CD, Haas JS, et al. Trends in prescription drug use among adults in the United States from 1999-2012. JAMA. 2015;314:1818-1831.
2. Lexicomp Online. Clinical Drug Information. Available at: http://www.wolterskluwercdi.com/lexicomp-online/. Accessed December 8, 2015.
3. GlobalRPh: The Clinician’s Ultimate Reference. Available at: http://www.globalrph.com/. Accessed December 8, 2015.
4. Medscape Apps. Available at: http://www.medscape.com/public/applanding. Accessed December 8, 2015.
5. Micromedex Solutions. Academic Institutions. Available at: http://micromedex.com/academic. Accessed December 8, 2015.
6. Patel A. Clinical Pharmacology Mobile-A mobile web app compatible on all smart phones [review] May 31, 2010. Available at: http://www.imedicalapps.com/2010/05/clinical-pharmocology-app-review/. Accessed December 8, 2015.
7. Epocrates. Available at: http://www.epocrates.com/. Accessed December 8, 2015.
8. Baillargeon J, Holmes HM, Lin Y, et al. Concurrent use of warfarin and antibiotics and the risk of bleeding in older adults. Am J Med. 2012;125:183-189.
9. PL Detail-Document #280806. Antimicrobial drug interactions with warfarin. Pharmacist’s Letter/Prescriber’s Letter. August 2012.
10. Lane M, Zeringue A, McDonald J. Serious bleeding events due to warfarin and antibiotic co-prescription in a cohort of veterans. Am J Med. 2014;127:657-663.e2.
11. Hale SF, Lesar TS. Interaction of vitamin K antagonists and trimethoprim-sulfamethoxazole: ignore at your patient’s risk. Drug Metab Drug Interact. 2014;29:53-60.
12. Ahmed A, Stephens JC, Kaus CA, et al. Impact of preemptive warfarin dose reduction on anticoagulation after initiation of trimethoprim-sulfamethoxazole or levofloxacin. J Thromb Thrombolysis. 2008;26:44-48.
13. Holt RK, Anderson EA, Cantrell MA, et al. Preemptive dose reduction of warfarin in patients initiating metronidazole. Drug Metabol Drug Interact. 2010;25:35-39.
14. Hughes BR, Cunliffe WJ. Interactions between the oral contraceptive pill and antibiotics. Br J Dermatol. 1990;122:717-718.
15. Bolt HM. Interactions between clinically used drugs and oral contraceptives. Environ Health Perspect. 1994;102:35-38.
16. Aronson JK. Meyler’s Side Effects of Drugs. 16th ed. The International Encyclopedia of Adverse Drug Reactions and Interactions. Amsterdam, Netherlands: Elsevier; 2016. Available at: http://ac.els-cdn.com/B978044453717101009X/3-s2.0-B978044453717101009X-main.pdf?_tid=b33f6564-9deb-11e5-a8f0-00000aab0f01&acdnat=1449607315_83f5068fc5105226fcc6d7279c083516. Accessed December 8, 2015.
17. Masters KP, Carr BM. Survey of pharmacists and physicians on drug interactions between combined oral contraceptives and broad-spectrum antibiotics. Pharm Pract (Granada). 2009;7:139-144.
18. Toh S, Mitchell AA, Anderka M, et al; National Birth Defects Prevention Study. Antibiotics and oral contraceptive failure—a case-crossover study. Contraception. 2011;83:418-425.
19. Koopmans PC, Bos JH, de Jong van den Berg LT. Are antibiotics related to oral combination contraceptive failures in the Netherlands? A case-crossover study. Pharmacoepidemiol Drug Saf. 2012;21:865-871.
20. Archer JS, Archer DF. Oral contraceptive efficacy and antibiotic interaction: A myth debunked. J Am Acad Dermatol. 2002;46:917–923.
21. Scholten PC, Droppert RM, Zwinkels MGJ, et al. No interaction between ciprofloxacin and an oral contraceptive. Antimicrob Agents Chemother. 1998;42:3266-3268.
22. Andrews E, Damle BD, Fang A, et al. Pharmacokinetics and tolerability of voriconazole and a combination oral contraceptive co-administered in healthy female subjects. Br J Clin Pharmacol. 2008;65:531-539.
23. Grimmer SF, Allen WL, Back DJ, et al. The effect of cotrimoxazole on oral contraceptive steroids in women. Contraception. 1983;28:53-59.
24. Dickinson BD, Altman RD, Nielsen NH, et al; Council on Scientific Affairs, American Medical Association. Drug interactions between oral contraceptives and antibiotics. Obstet Gynecol. 2001;98:853-860.
25. Edelman AB, Cherala G, Stanczyk FZ. Metabolism and pharmacokinetics of contraceptive steroids in obese women: a review. Contraception. 2010;82:314-323.
26. Owens RC Jr, Ambrose PG. Torsades de pointes associated with fluoroquinolones. Pharmacotherapy. 2002;22:663-668.
27. Letsas KP, Efremidis M, Kounas SP, et al. Clinical characteristics of patients with drug-induced QT interval prolongation and torsade de pointes: identification of risk factors. Clin Res Cardiol. 2009;98:208-212.
28. Yap YG, Camm AJ. Drug induced QT prolongation and torsades de pointes. Heart. 2003;89:1363-1372.
29. Shaffer D, Singer S, Korvick J, et al. Concomitant risk factors in reports of torsades de pointes associated with macrolide use: review of the United States Food and Drug Administration adverse event reporting system. Clin Infect Dis. 2002;35:197-200.
30. FDA Briefing Document. Joint Meeting of the Antimicrobial Drugs Advisory Committee and the Drug Safety and Risk Management Advisory Committee. November 5, 2015. Available at: http://www.fda.gov/downloads/AdvisoryCommittees/CommitteesMeetingMaterials/Drugs/Anti-InfectiveDrugsAdvisoryCommittee/UCM467383.pdf. Accessed June 11, 2016.
31. Chou HW, Wang JL, Chang CH, et al. Risks of cardiac arrhythmia and mortality among patients using new-generation macrolides, fluoroquinolones, and β-lactam/β-lactamase inhibitors: a Taiwanese nationwide study. Clin Infect Dis. 2015;60:566-577.
32. Wong AY, Root A, Douglas IJ, et al. Cardiovascular outcomes associated with use of clarithromycin: population based study. BMJ. 2016;352:h6926.
33. Inghammar M, Svanström H, Melbye M, et al. Oral fluoroquinolone use and serious arrhythmia: bi-national cohort study. BMJ. 2016;352:i843.
34. Visapää JP, Tillonen JS, Kaihovaara PS, et al. Lack of disulfiram-like reaction with metronidazole and ethanol. Ann Pharmacother. 2002;36:971-974. 35. Fjeld H, Raknes G. Is combining metronidazole and alcohol really hazardous? Tidsskr Nor Laegeforen. 2014;134:1661-1663.
› Avoid preemptive warfarin dose reductions unless you are prescribing trimethoprim/sulfamethoxazole (TMP/SMX) or metronidazole. B
› Recommend a back-up contraceptive method to a woman who is taking a broad-spectrum antibiotic and low-dose OCs—especially if the woman is overweight. C
› Consider using the macrolide, clarithromycin, or the fluoroquinolone, ciprofloxacin, in patients taking medications that prolong QT interval or who are at higher risk for torsades de pointes (TdP). B
› Refrain from cautioning patients taking metronidazole against consuming alcohol. A
Strength of recommendation (SOR)
A Good-quality patient-oriented evidence
B Inconsistent or limited-quality patient-oriented evidence
C Consensus, usual practice, opinion, disease-oriented evidence, case series
Despite encouraging data that antibiotic prescribing is on the decline, patients are still prescribed antibiotics frequently, making these agents the 12th most frequently used drug class.1 At the same time, prescribers are caring for patients with increasingly complex drug regimens that provide fertile ground for drug interactions with these antibiotics. And, of course, lifestyle factors such as alcohol consumption are a consideration when any prescription is written.
As pharmacists, we find that certain questions about antibiotic prescribing and interactions come up with frequency. These questions often pertain to the use of warfarin, oral contraceptives, drugs that prolong the QT interval, and alcohol. But conflicting reports about issues such as monitoring international normalized ratio (INR) in patients taking warfarin and antibiotics, and whether (or which) antibiotics decrease the efficacy of oral contraceptives (OCs) can make decision-making challenging.
This review provides evidence-based answers to questions you may have. It also details some reliable sources of information you can consult (TABLE 12-7) when discussing treatment options with other members of the health care team.
1. Which antibiotics are preferable when a patient is taking warfarin, and are preemptive warfarin dose reductions advisable?
The simple answer is that agents with a lower likelihood of affecting the INR, such as penicillin G, clindamycin, and 1st- and 4th-generation cephalosporins, are a good place to start, and whether to preemptively reduce the warfarin dose hinges on the antibiotic being prescribed.
The more detailed answer. The fundamental mechanisms of interaction between warfarin and antibiotics are two-fold:8
- Antimicrobial agents disrupt gastrointestinal flora that synthesize vitamin K.
- Antimicrobials inhibit cytochrome p450 (CYP450) enzymes (primarily CYP2C9 and 3A4), which are responsible for the metabolism of warfarin.
The antibiotics most likely to interfere with warfarin are TMP/SMX, ciprofloxacin, levofloxacin, metronidazole, fluconazole, azithromycin, and clarithromycin (TABLE 2).9,10 Low-risk agents include clindamycin, cephalexin, and penicillin G. When prescribing an antibiotic for a patient taking warfarin, it is important not only to be aware of the agents that should be avoided, but also the agents that do not require more frequent monitoring of INR.
Preemptive warfarin dose reductions? Some physicians make preemptive warfarin dose reductions in an attempt to avoid supratherapeutic INRs in patients being prescribed antibiotics. But the evidence suggests that this step should be considered only in the presence of the antibiotics TMP/SMX and metronidazole.9,11
A 2008 study investigated the anticoagulation effects of a 10% to 20% preemptive warfarin dose reduction vs no dosing change in patients taking TMP/SMX or levofloxacin. The investigators found that the preemptive warfarin dose reduction (intervention) significantly decreased the number of supratherapeutic INR values above 4 when compared to controls (2 of 8 vs 8 of 9).12
In the dose-reduction group, no patients receiving TMP/SMX developed a subtherapeutic INR, whereas 40% (4 of 10 patients) who received levofloxacin developed a subtherapeutic INR.12 The authors of the study concluded that a prophylactic warfarin dose reduction of 10% to 20% is effective in maintaining therapeutic anticoagulation in patients receiving TMP/SMX. They added that while no change in warfarin dosing is necessary with levofloxacin, short-term INR follow-up is a prudent approach to prevent subtherapeutic INRs. Others recommend INR monitoring when antibiotic therapy is started and stopped and whenever the dose is changed.9
A 2010 retrospective, single-center, cohort study looked at patients who were taking metronidazole and warfarin. Researchers compared those who received a preemptive dose reduction of warfarin (mean reduction was 34.6% ± 13.4%) to those who did not and found a statistically significant mean difference in INR of 1.28 (P=.01).13
Almost half (46%) of the patients who did not receive a warfarin dose reduction had an INR >4, whereas none of the patients in the warfarin dose reduction group did (P=.05). Although this secondary outcome was not statistically significant (most likely due to the small sample population [N=20]), the implication is clinically significant. Two patients who reduced their dose had a subtherapeutic INR compared to none of the patients in the control group, which was also not a statistically significant difference.
The authors concluded that a 30% to 35% reduction in mean daily warfarin dose is effective in maintaining therapeutic anticoagulation in patients started on metronidazole.
Significant bleeding events. A retrospective cohort study of slightly more than 22,000 veterans who were prescribed warfarin for ≥30 uninterrupted days and given antibiotics with either a high or low risk for interaction with warfarin were studied for significant bleeding events for one month.10 Ninety-three significant bleeding events occurred in the high-risk group and 36 occurred in the low-risk group over the course of the study. The agent associated with the greatest increased risk of bleeding was TMP/SMX (hazard ratio [HR]=2.09; 95% CI, 1.45-3.02). Of note, metronidazole was not included in this study endpoint.
The study’s secondary endpoint of INR >4 found that 10% of patients taking metronidazole and 8% of patients taking TMP/SMX in addition to warfarin had INRs >4. Almost 10% (9.7%) of patients prescribed fluconazole had a peak INR value >6. Patients taking low-risk antibiotics (clindamycin or cephalexin) had no increased risk of bleeding. Monitoring INR within 3 to 14 days of starting patients on antibiotics was found to decrease the risk of serious bleeding events (HR=0.61; 95% CI, 0.42-0.88). More frequent INR monitoring by itself (without preemptive warfarin dose reductions) is appropriate for other antibiotics, including macrolides, tetracyclines, and some cephalosporins (2nd and 3rd generation).9
THE BOTTOM LINE When prescribing antibiotics for patients taking warfarin, try to choose agents with a lower likelihood of affecting INR such as penicillin G, clindamycin, and 1st- and 4th-generation cephalosporins. With these agents, there is no need for more frequent INR testing or preemptive reductions in warfarin dose. In patients for whom the use of TMP/SMX or metronidazole can’t be avoided, consider reducing the patient’s warfarin dose by 10% to 35% and rechecking the INR 5 days after starting the antibiotic.9,11,12 When prescribing agents such as fluoroquinolones, macrolides, and tetracyclines, do not reduce the patient’s warfarin dose preemptively and recheck INR 5 days after starting therapy.
2. Do antibiotics decrease the efficacy of oral contraceptives?
It’s unlikely, but antibiotics may reduce the efficacy of OCs.
There have been few, but well documented, reports of women using OCs who became pregnant after taking antimicrobials.14 It is recognized that rifampin, an inducer of enzymes that metabolize estrogens, decreases the efficacy of OCs.15 Ketoconazole’s interaction seems less well documented, but combining the agent with low-estrogen (low-dose) OCs warrants caution.16 What is not well understood is whether more common or broad-spectrum antibiotics also increase the risk of OC failure.
Three mechanisms have been proposed:16
- Antimicrobials affect hepatic enzyme induction, which increases metabolism of hormones.
- Broad-spectrum antibiotics reduce gut bacteria, which alters enterohepatic circulation and reduces plasma hormone concentrations.
- Antibiotics increase gastrointestinal motility, which decreases absorption (and reabsorption) of OCs.
A 2007 study found that when physicians and pharmacists were surveyed and asked if broad-spectrum antibiotics have a clinically significant interaction with OCs, 83% of physicians and 89% of pharmacists answered “Yes;”17 however, a large epidemiologic study performed in the United States showed no association between antibiotic use and OC failure.18
After this report, investigators in the Netherlands completed a similar cross-over analysis and found that there was a relationship between the use of antibiotics and breakthrough pregnancy in a population-based prescription database, but that the results didn’t hold for broad-spectrum antibiotics or in a sensitivity analysis.19 Pharmacokinetic studies are also conflicting, as some have shown an effect on serum hormone levels, while others have not.15,20-22
High- vs low-risk agents. Ciprofloxacin did not affect hormone levels in 2 studies.20,21 Rifampin and voriconazole may enhance systemic exposure to OCs.15,22 And erythromycin and azithromycin may interact with OCs, but the clinical significance of this interaction is still unknown.16
Short-courses of TMP/SMX are generally thought to be safe;16 a small study looked at cotrimoxazole 1 g twice daily in 9 women taking long-term OC steroids and found that short courses of the drug were unlikely to cause any adverse effects on contraceptive control.23 Tetracyclines and penicillins were the antibiotics most frequently involved in case reports of pregnancy from the United Kingdom (TABLE 32).16
It is hypothesized that some women may have a higher risk of OC failure than others due to how they metabolize ethinyl estradiol.24 Another hypothesis is that some women have gut flora that is more susceptible to the antibiotic being used. And still another possibility is that lower doses of hormones are being used in OCs than were studied for this interaction.15 Anything that decreases the concentration of these lower-dose OCs is concerning, especially in patients with a higher body mass index (BMI). The few pharmacokinetic studies that have been conducted show that it takes longer for OCs to reach a steady state in obese women and that they have a lower area under the curve (AUC) and maximum estrogen concentration than women with a normal BMI.25
THE BOTTOM LINE Because the degree of variability between patients is unknown and obesity rates are increasing, concern that low-dose OCs may lose efficacy when combined with antibiotics is warranted. While the absolute risk of breakthrough pregnancy seems small, the most conservative approach is to advise patients to use a back-up method of contraception during times of antibiotic use.
3. Which drugs prolong QT intervals?
Macrolides and fluoroquinolones are 2 classes of antibiotics associated with prolonged QT intervals, but other drugs and risk factors are important to consider, as well.
Physicians often receive phone calls from pharmacists warning about drug-drug interactions when they prescribe macrolides or fluoroquinolones for patients already taking medications known to prolong QT intervals or inhibit cytochrome P450 enzymes. Long QT syndrome increases the risk of TdP, a life-threatening arrhythmia. While TdP is rare, its severity warrants a discussion of risk factors and the likelihood of occurrence.
Two QT interval prolonging medications used together in healthy individuals does not warrant a change in therapy. TdP is most likely to occur when 2 or more QT interval prolonging medications are used in a patient who is already at high risk for arrhythmia because of risk factors such as prolonged QT interval at baseline, family history of prolonged QT intervals, female gender, age >60 years, electrolyte abnormalities (hypokalemia, hypomagnesemia, hypocalcemia), underlying comorbid diseases (eg, chronic heart failure, left ventricular hypertrophy, atrial fibrillation), hypertension, bradycardia, and genetic (ion channel) polymorphisms.26,27
Antiarrhythmics and antipsychotics are most commonly associated with drug-induced prolonged QT interval, with most case reports and research being linked to antiarrhythmics (TABLE 42).28 But macrolide and fluoroquinolone antibiotics also have been associated with TdP, although to a lesser extent. In a retrospective analysis of case reports of TdP involving macrolides, erythromycin was present (with or without other medications thought to prolong QT) in 53% of the cases and clarithromycin was involved in 36% of the reports.29
An analysis of 2 studies by the US Food and Drug Administration estimated an occurrence rate of serious cardiac arrhythmias of 46 to 85 per 100,000 users with cardiovascular disease, compared to 5 to 44 per 100,000 users without cardiovascular disease.30 And this may underestimate the actual incidence because spontaneous reporting of adverse effects declines the longer a drug is on the market. Ciprofloxacin is associated with less risk than levofloxacin and gatifloxacin (the latter of which is no longer available in the United States).26
A recent population-based study using data on over 10.6 million people from the Taiwan National Health Insurance Database examined the risk of cardiovascular death among patients using new-generation macrolides, fluoroquinolones, and β-lactam/β-lactamase inhibitors.31 The absolute risk of cardiovascular death per 1000 individuals was 0.06 for clarithromycin, 0.12 for ciprofloxacin, 0.13 for amoxicillin-clavulanate, 0.36 for azithromycin, 0.39 for levofloxacin, and 0.46 for moxifloxacin. The mean interval between first antibiotic use and the adverse cardiac event was <4 days. Not surprisingly, the highest risk was seen in patients with underlying cardiovascular disease.
Another population-based study, this time conducted in Hong Kong, evaluated the cardiovascular safety of clarithromycin compared to that of amoxicillin. Clarithromycin was found to increase the incidence of myocardial infarction, arrhythmia, and cardiac mortality in the short term, with the risk returning to baseline after treatment concluded.32 A binational cohort study of Danish and Swedish adults confirmed that fluoroquinolones (especially ciprofloxacin) do not increase the risk of a serious arrhythmia compared to penicillins.33
THE BOTTOM LINE For patients taking other QT interval prolonging medications or who are at a higher risk for TdP, consider using clarithromycin over erythromycin or azithromycin for a macrolide antibiotic or ciprofloxacin over levofloxacin or moxifloxacin if a fluoroquinolone is warranted. Using 2 drugs that may increase the QT interval is likely safe in the absence of certain risk factors.
4. Should patients avoid alcohol while taking metronidazole?
Probably not.
Warning patients against drinking alcohol while taking metronidazole has been a common practice for years. The mechanism for this theorized interaction was thought to be similar to the interaction between disulfiram and ethanol.34 Disulfiram inhibits hepatic aldehyde dehydrogenase (ALDH) when combined with alcohol, which leads to increased levels of acetaldehyde in the blood and symptoms of flushing, palpitations, nausea, vomiting, headache, and visual disturbances.35 However, multiple studies using rats have found that metronidazole does not inhibit ALDH or increase acetaldehyde concentrations like disulfiram does.34
A 2000 review article discussed 6 cases involving serious metronidazole-ethanol interactions. Ethanol alone was found to explain the reaction in 2 of the cases, and the remaining 4 could be linked to the use of other drugs or disease states.35 A 2002 Finnish study found no statistically significant differences in objective or subjective signs of a disulfiram-like interaction.34 When considering the symptoms associated with the interaction, it is important to remember that many of the symptoms can result from metronidazole therapy alone, regardless of whether other medications or alcohol are used.35
THE BOTTOM LINE Researchers have failed to identify a clinically significant interaction between metronidazole and alcohol. Avoiding alcohol while taking metronidazole does not appear to be necessary.
CORRESPONDENCE
Mary Onysko, PharmD, BCPS, University of Wyoming, School of Pharmacy Health Sciences Center, Room 292, 1000 E. University Avenue, Laramie, WY 82071; [email protected].
› Avoid preemptive warfarin dose reductions unless you are prescribing trimethoprim/sulfamethoxazole (TMP/SMX) or metronidazole. B
› Recommend a back-up contraceptive method to a woman who is taking a broad-spectrum antibiotic and low-dose OCs—especially if the woman is overweight. C
› Consider using the macrolide, clarithromycin, or the fluoroquinolone, ciprofloxacin, in patients taking medications that prolong QT interval or who are at higher risk for torsades de pointes (TdP). B
› Refrain from cautioning patients taking metronidazole against consuming alcohol. A
Strength of recommendation (SOR)
A Good-quality patient-oriented evidence
B Inconsistent or limited-quality patient-oriented evidence
C Consensus, usual practice, opinion, disease-oriented evidence, case series
Despite encouraging data that antibiotic prescribing is on the decline, patients are still prescribed antibiotics frequently, making these agents the 12th most frequently used drug class.1 At the same time, prescribers are caring for patients with increasingly complex drug regimens that provide fertile ground for drug interactions with these antibiotics. And, of course, lifestyle factors such as alcohol consumption are a consideration when any prescription is written.
As pharmacists, we find that certain questions about antibiotic prescribing and interactions come up with frequency. These questions often pertain to the use of warfarin, oral contraceptives, drugs that prolong the QT interval, and alcohol. But conflicting reports about issues such as monitoring international normalized ratio (INR) in patients taking warfarin and antibiotics, and whether (or which) antibiotics decrease the efficacy of oral contraceptives (OCs) can make decision-making challenging.
This review provides evidence-based answers to questions you may have. It also details some reliable sources of information you can consult (TABLE 12-7) when discussing treatment options with other members of the health care team.
1. Which antibiotics are preferable when a patient is taking warfarin, and are preemptive warfarin dose reductions advisable?
The simple answer is that agents with a lower likelihood of affecting the INR, such as penicillin G, clindamycin, and 1st- and 4th-generation cephalosporins, are a good place to start, and whether to preemptively reduce the warfarin dose hinges on the antibiotic being prescribed.
The more detailed answer. The fundamental mechanisms of interaction between warfarin and antibiotics are two-fold:8
- Antimicrobial agents disrupt gastrointestinal flora that synthesize vitamin K.
- Antimicrobials inhibit cytochrome p450 (CYP450) enzymes (primarily CYP2C9 and 3A4), which are responsible for the metabolism of warfarin.
The antibiotics most likely to interfere with warfarin are TMP/SMX, ciprofloxacin, levofloxacin, metronidazole, fluconazole, azithromycin, and clarithromycin (TABLE 2).9,10 Low-risk agents include clindamycin, cephalexin, and penicillin G. When prescribing an antibiotic for a patient taking warfarin, it is important not only to be aware of the agents that should be avoided, but also the agents that do not require more frequent monitoring of INR.
Preemptive warfarin dose reductions? Some physicians make preemptive warfarin dose reductions in an attempt to avoid supratherapeutic INRs in patients being prescribed antibiotics. But the evidence suggests that this step should be considered only in the presence of the antibiotics TMP/SMX and metronidazole.9,11
A 2008 study investigated the anticoagulation effects of a 10% to 20% preemptive warfarin dose reduction vs no dosing change in patients taking TMP/SMX or levofloxacin. The investigators found that the preemptive warfarin dose reduction (intervention) significantly decreased the number of supratherapeutic INR values above 4 when compared to controls (2 of 8 vs 8 of 9).12
In the dose-reduction group, no patients receiving TMP/SMX developed a subtherapeutic INR, whereas 40% (4 of 10 patients) who received levofloxacin developed a subtherapeutic INR.12 The authors of the study concluded that a prophylactic warfarin dose reduction of 10% to 20% is effective in maintaining therapeutic anticoagulation in patients receiving TMP/SMX. They added that while no change in warfarin dosing is necessary with levofloxacin, short-term INR follow-up is a prudent approach to prevent subtherapeutic INRs. Others recommend INR monitoring when antibiotic therapy is started and stopped and whenever the dose is changed.9
A 2010 retrospective, single-center, cohort study looked at patients who were taking metronidazole and warfarin. Researchers compared those who received a preemptive dose reduction of warfarin (mean reduction was 34.6% ± 13.4%) to those who did not and found a statistically significant mean difference in INR of 1.28 (P=.01).13
Almost half (46%) of the patients who did not receive a warfarin dose reduction had an INR >4, whereas none of the patients in the warfarin dose reduction group did (P=.05). Although this secondary outcome was not statistically significant (most likely due to the small sample population [N=20]), the implication is clinically significant. Two patients who reduced their dose had a subtherapeutic INR compared to none of the patients in the control group, which was also not a statistically significant difference.
The authors concluded that a 30% to 35% reduction in mean daily warfarin dose is effective in maintaining therapeutic anticoagulation in patients started on metronidazole.
Significant bleeding events. A retrospective cohort study of slightly more than 22,000 veterans who were prescribed warfarin for ≥30 uninterrupted days and given antibiotics with either a high or low risk for interaction with warfarin were studied for significant bleeding events for one month.10 Ninety-three significant bleeding events occurred in the high-risk group and 36 occurred in the low-risk group over the course of the study. The agent associated with the greatest increased risk of bleeding was TMP/SMX (hazard ratio [HR]=2.09; 95% CI, 1.45-3.02). Of note, metronidazole was not included in this study endpoint.
The study’s secondary endpoint of INR >4 found that 10% of patients taking metronidazole and 8% of patients taking TMP/SMX in addition to warfarin had INRs >4. Almost 10% (9.7%) of patients prescribed fluconazole had a peak INR value >6. Patients taking low-risk antibiotics (clindamycin or cephalexin) had no increased risk of bleeding. Monitoring INR within 3 to 14 days of starting patients on antibiotics was found to decrease the risk of serious bleeding events (HR=0.61; 95% CI, 0.42-0.88). More frequent INR monitoring by itself (without preemptive warfarin dose reductions) is appropriate for other antibiotics, including macrolides, tetracyclines, and some cephalosporins (2nd and 3rd generation).9
THE BOTTOM LINE When prescribing antibiotics for patients taking warfarin, try to choose agents with a lower likelihood of affecting INR such as penicillin G, clindamycin, and 1st- and 4th-generation cephalosporins. With these agents, there is no need for more frequent INR testing or preemptive reductions in warfarin dose. In patients for whom the use of TMP/SMX or metronidazole can’t be avoided, consider reducing the patient’s warfarin dose by 10% to 35% and rechecking the INR 5 days after starting the antibiotic.9,11,12 When prescribing agents such as fluoroquinolones, macrolides, and tetracyclines, do not reduce the patient’s warfarin dose preemptively and recheck INR 5 days after starting therapy.
2. Do antibiotics decrease the efficacy of oral contraceptives?
It’s unlikely, but antibiotics may reduce the efficacy of OCs.
There have been few, but well documented, reports of women using OCs who became pregnant after taking antimicrobials.14 It is recognized that rifampin, an inducer of enzymes that metabolize estrogens, decreases the efficacy of OCs.15 Ketoconazole’s interaction seems less well documented, but combining the agent with low-estrogen (low-dose) OCs warrants caution.16 What is not well understood is whether more common or broad-spectrum antibiotics also increase the risk of OC failure.
Three mechanisms have been proposed:16
- Antimicrobials affect hepatic enzyme induction, which increases metabolism of hormones.
- Broad-spectrum antibiotics reduce gut bacteria, which alters enterohepatic circulation and reduces plasma hormone concentrations.
- Antibiotics increase gastrointestinal motility, which decreases absorption (and reabsorption) of OCs.
A 2007 study found that when physicians and pharmacists were surveyed and asked if broad-spectrum antibiotics have a clinically significant interaction with OCs, 83% of physicians and 89% of pharmacists answered “Yes;”17 however, a large epidemiologic study performed in the United States showed no association between antibiotic use and OC failure.18
After this report, investigators in the Netherlands completed a similar cross-over analysis and found that there was a relationship between the use of antibiotics and breakthrough pregnancy in a population-based prescription database, but that the results didn’t hold for broad-spectrum antibiotics or in a sensitivity analysis.19 Pharmacokinetic studies are also conflicting, as some have shown an effect on serum hormone levels, while others have not.15,20-22
High- vs low-risk agents. Ciprofloxacin did not affect hormone levels in 2 studies.20,21 Rifampin and voriconazole may enhance systemic exposure to OCs.15,22 And erythromycin and azithromycin may interact with OCs, but the clinical significance of this interaction is still unknown.16
Short-courses of TMP/SMX are generally thought to be safe;16 a small study looked at cotrimoxazole 1 g twice daily in 9 women taking long-term OC steroids and found that short courses of the drug were unlikely to cause any adverse effects on contraceptive control.23 Tetracyclines and penicillins were the antibiotics most frequently involved in case reports of pregnancy from the United Kingdom (TABLE 32).16
It is hypothesized that some women may have a higher risk of OC failure than others due to how they metabolize ethinyl estradiol.24 Another hypothesis is that some women have gut flora that is more susceptible to the antibiotic being used. And still another possibility is that lower doses of hormones are being used in OCs than were studied for this interaction.15 Anything that decreases the concentration of these lower-dose OCs is concerning, especially in patients with a higher body mass index (BMI). The few pharmacokinetic studies that have been conducted show that it takes longer for OCs to reach a steady state in obese women and that they have a lower area under the curve (AUC) and maximum estrogen concentration than women with a normal BMI.25
THE BOTTOM LINE Because the degree of variability between patients is unknown and obesity rates are increasing, concern that low-dose OCs may lose efficacy when combined with antibiotics is warranted. While the absolute risk of breakthrough pregnancy seems small, the most conservative approach is to advise patients to use a back-up method of contraception during times of antibiotic use.
3. Which drugs prolong QT intervals?
Macrolides and fluoroquinolones are 2 classes of antibiotics associated with prolonged QT intervals, but other drugs and risk factors are important to consider, as well.
Physicians often receive phone calls from pharmacists warning about drug-drug interactions when they prescribe macrolides or fluoroquinolones for patients already taking medications known to prolong QT intervals or inhibit cytochrome P450 enzymes. Long QT syndrome increases the risk of TdP, a life-threatening arrhythmia. While TdP is rare, its severity warrants a discussion of risk factors and the likelihood of occurrence.
Two QT interval prolonging medications used together in healthy individuals does not warrant a change in therapy. TdP is most likely to occur when 2 or more QT interval prolonging medications are used in a patient who is already at high risk for arrhythmia because of risk factors such as prolonged QT interval at baseline, family history of prolonged QT intervals, female gender, age >60 years, electrolyte abnormalities (hypokalemia, hypomagnesemia, hypocalcemia), underlying comorbid diseases (eg, chronic heart failure, left ventricular hypertrophy, atrial fibrillation), hypertension, bradycardia, and genetic (ion channel) polymorphisms.26,27
Antiarrhythmics and antipsychotics are most commonly associated with drug-induced prolonged QT interval, with most case reports and research being linked to antiarrhythmics (TABLE 42).28 But macrolide and fluoroquinolone antibiotics also have been associated with TdP, although to a lesser extent. In a retrospective analysis of case reports of TdP involving macrolides, erythromycin was present (with or without other medications thought to prolong QT) in 53% of the cases and clarithromycin was involved in 36% of the reports.29
An analysis of 2 studies by the US Food and Drug Administration estimated an occurrence rate of serious cardiac arrhythmias of 46 to 85 per 100,000 users with cardiovascular disease, compared to 5 to 44 per 100,000 users without cardiovascular disease.30 And this may underestimate the actual incidence because spontaneous reporting of adverse effects declines the longer a drug is on the market. Ciprofloxacin is associated with less risk than levofloxacin and gatifloxacin (the latter of which is no longer available in the United States).26
A recent population-based study using data on over 10.6 million people from the Taiwan National Health Insurance Database examined the risk of cardiovascular death among patients using new-generation macrolides, fluoroquinolones, and β-lactam/β-lactamase inhibitors.31 The absolute risk of cardiovascular death per 1000 individuals was 0.06 for clarithromycin, 0.12 for ciprofloxacin, 0.13 for amoxicillin-clavulanate, 0.36 for azithromycin, 0.39 for levofloxacin, and 0.46 for moxifloxacin. The mean interval between first antibiotic use and the adverse cardiac event was <4 days. Not surprisingly, the highest risk was seen in patients with underlying cardiovascular disease.
Another population-based study, this time conducted in Hong Kong, evaluated the cardiovascular safety of clarithromycin compared to that of amoxicillin. Clarithromycin was found to increase the incidence of myocardial infarction, arrhythmia, and cardiac mortality in the short term, with the risk returning to baseline after treatment concluded.32 A binational cohort study of Danish and Swedish adults confirmed that fluoroquinolones (especially ciprofloxacin) do not increase the risk of a serious arrhythmia compared to penicillins.33
THE BOTTOM LINE For patients taking other QT interval prolonging medications or who are at a higher risk for TdP, consider using clarithromycin over erythromycin or azithromycin for a macrolide antibiotic or ciprofloxacin over levofloxacin or moxifloxacin if a fluoroquinolone is warranted. Using 2 drugs that may increase the QT interval is likely safe in the absence of certain risk factors.
4. Should patients avoid alcohol while taking metronidazole?
Probably not.
Warning patients against drinking alcohol while taking metronidazole has been a common practice for years. The mechanism for this theorized interaction was thought to be similar to the interaction between disulfiram and ethanol.34 Disulfiram inhibits hepatic aldehyde dehydrogenase (ALDH) when combined with alcohol, which leads to increased levels of acetaldehyde in the blood and symptoms of flushing, palpitations, nausea, vomiting, headache, and visual disturbances.35 However, multiple studies using rats have found that metronidazole does not inhibit ALDH or increase acetaldehyde concentrations like disulfiram does.34
A 2000 review article discussed 6 cases involving serious metronidazole-ethanol interactions. Ethanol alone was found to explain the reaction in 2 of the cases, and the remaining 4 could be linked to the use of other drugs or disease states.35 A 2002 Finnish study found no statistically significant differences in objective or subjective signs of a disulfiram-like interaction.34 When considering the symptoms associated with the interaction, it is important to remember that many of the symptoms can result from metronidazole therapy alone, regardless of whether other medications or alcohol are used.35
THE BOTTOM LINE Researchers have failed to identify a clinically significant interaction between metronidazole and alcohol. Avoiding alcohol while taking metronidazole does not appear to be necessary.
CORRESPONDENCE
Mary Onysko, PharmD, BCPS, University of Wyoming, School of Pharmacy Health Sciences Center, Room 292, 1000 E. University Avenue, Laramie, WY 82071; [email protected].
1. Kantor ED, Rehm CD, Haas JS, et al. Trends in prescription drug use among adults in the United States from 1999-2012. JAMA. 2015;314:1818-1831.
2. Lexicomp Online. Clinical Drug Information. Available at: http://www.wolterskluwercdi.com/lexicomp-online/. Accessed December 8, 2015.
3. GlobalRPh: The Clinician’s Ultimate Reference. Available at: http://www.globalrph.com/. Accessed December 8, 2015.
4. Medscape Apps. Available at: http://www.medscape.com/public/applanding. Accessed December 8, 2015.
5. Micromedex Solutions. Academic Institutions. Available at: http://micromedex.com/academic. Accessed December 8, 2015.
6. Patel A. Clinical Pharmacology Mobile-A mobile web app compatible on all smart phones [review] May 31, 2010. Available at: http://www.imedicalapps.com/2010/05/clinical-pharmocology-app-review/. Accessed December 8, 2015.
7. Epocrates. Available at: http://www.epocrates.com/. Accessed December 8, 2015.
8. Baillargeon J, Holmes HM, Lin Y, et al. Concurrent use of warfarin and antibiotics and the risk of bleeding in older adults. Am J Med. 2012;125:183-189.
9. PL Detail-Document #280806. Antimicrobial drug interactions with warfarin. Pharmacist’s Letter/Prescriber’s Letter. August 2012.
10. Lane M, Zeringue A, McDonald J. Serious bleeding events due to warfarin and antibiotic co-prescription in a cohort of veterans. Am J Med. 2014;127:657-663.e2.
11. Hale SF, Lesar TS. Interaction of vitamin K antagonists and trimethoprim-sulfamethoxazole: ignore at your patient’s risk. Drug Metab Drug Interact. 2014;29:53-60.
12. Ahmed A, Stephens JC, Kaus CA, et al. Impact of preemptive warfarin dose reduction on anticoagulation after initiation of trimethoprim-sulfamethoxazole or levofloxacin. J Thromb Thrombolysis. 2008;26:44-48.
13. Holt RK, Anderson EA, Cantrell MA, et al. Preemptive dose reduction of warfarin in patients initiating metronidazole. Drug Metabol Drug Interact. 2010;25:35-39.
14. Hughes BR, Cunliffe WJ. Interactions between the oral contraceptive pill and antibiotics. Br J Dermatol. 1990;122:717-718.
15. Bolt HM. Interactions between clinically used drugs and oral contraceptives. Environ Health Perspect. 1994;102:35-38.
16. Aronson JK. Meyler’s Side Effects of Drugs. 16th ed. The International Encyclopedia of Adverse Drug Reactions and Interactions. Amsterdam, Netherlands: Elsevier; 2016. Available at: http://ac.els-cdn.com/B978044453717101009X/3-s2.0-B978044453717101009X-main.pdf?_tid=b33f6564-9deb-11e5-a8f0-00000aab0f01&acdnat=1449607315_83f5068fc5105226fcc6d7279c083516. Accessed December 8, 2015.
17. Masters KP, Carr BM. Survey of pharmacists and physicians on drug interactions between combined oral contraceptives and broad-spectrum antibiotics. Pharm Pract (Granada). 2009;7:139-144.
18. Toh S, Mitchell AA, Anderka M, et al; National Birth Defects Prevention Study. Antibiotics and oral contraceptive failure—a case-crossover study. Contraception. 2011;83:418-425.
19. Koopmans PC, Bos JH, de Jong van den Berg LT. Are antibiotics related to oral combination contraceptive failures in the Netherlands? A case-crossover study. Pharmacoepidemiol Drug Saf. 2012;21:865-871.
20. Archer JS, Archer DF. Oral contraceptive efficacy and antibiotic interaction: A myth debunked. J Am Acad Dermatol. 2002;46:917–923.
21. Scholten PC, Droppert RM, Zwinkels MGJ, et al. No interaction between ciprofloxacin and an oral contraceptive. Antimicrob Agents Chemother. 1998;42:3266-3268.
22. Andrews E, Damle BD, Fang A, et al. Pharmacokinetics and tolerability of voriconazole and a combination oral contraceptive co-administered in healthy female subjects. Br J Clin Pharmacol. 2008;65:531-539.
23. Grimmer SF, Allen WL, Back DJ, et al. The effect of cotrimoxazole on oral contraceptive steroids in women. Contraception. 1983;28:53-59.
24. Dickinson BD, Altman RD, Nielsen NH, et al; Council on Scientific Affairs, American Medical Association. Drug interactions between oral contraceptives and antibiotics. Obstet Gynecol. 2001;98:853-860.
25. Edelman AB, Cherala G, Stanczyk FZ. Metabolism and pharmacokinetics of contraceptive steroids in obese women: a review. Contraception. 2010;82:314-323.
26. Owens RC Jr, Ambrose PG. Torsades de pointes associated with fluoroquinolones. Pharmacotherapy. 2002;22:663-668.
27. Letsas KP, Efremidis M, Kounas SP, et al. Clinical characteristics of patients with drug-induced QT interval prolongation and torsade de pointes: identification of risk factors. Clin Res Cardiol. 2009;98:208-212.
28. Yap YG, Camm AJ. Drug induced QT prolongation and torsades de pointes. Heart. 2003;89:1363-1372.
29. Shaffer D, Singer S, Korvick J, et al. Concomitant risk factors in reports of torsades de pointes associated with macrolide use: review of the United States Food and Drug Administration adverse event reporting system. Clin Infect Dis. 2002;35:197-200.
30. FDA Briefing Document. Joint Meeting of the Antimicrobial Drugs Advisory Committee and the Drug Safety and Risk Management Advisory Committee. November 5, 2015. Available at: http://www.fda.gov/downloads/AdvisoryCommittees/CommitteesMeetingMaterials/Drugs/Anti-InfectiveDrugsAdvisoryCommittee/UCM467383.pdf. Accessed June 11, 2016.
31. Chou HW, Wang JL, Chang CH, et al. Risks of cardiac arrhythmia and mortality among patients using new-generation macrolides, fluoroquinolones, and β-lactam/β-lactamase inhibitors: a Taiwanese nationwide study. Clin Infect Dis. 2015;60:566-577.
32. Wong AY, Root A, Douglas IJ, et al. Cardiovascular outcomes associated with use of clarithromycin: population based study. BMJ. 2016;352:h6926.
33. Inghammar M, Svanström H, Melbye M, et al. Oral fluoroquinolone use and serious arrhythmia: bi-national cohort study. BMJ. 2016;352:i843.
34. Visapää JP, Tillonen JS, Kaihovaara PS, et al. Lack of disulfiram-like reaction with metronidazole and ethanol. Ann Pharmacother. 2002;36:971-974. 35. Fjeld H, Raknes G. Is combining metronidazole and alcohol really hazardous? Tidsskr Nor Laegeforen. 2014;134:1661-1663.
1. Kantor ED, Rehm CD, Haas JS, et al. Trends in prescription drug use among adults in the United States from 1999-2012. JAMA. 2015;314:1818-1831.
2. Lexicomp Online. Clinical Drug Information. Available at: http://www.wolterskluwercdi.com/lexicomp-online/. Accessed December 8, 2015.
3. GlobalRPh: The Clinician’s Ultimate Reference. Available at: http://www.globalrph.com/. Accessed December 8, 2015.
4. Medscape Apps. Available at: http://www.medscape.com/public/applanding. Accessed December 8, 2015.
5. Micromedex Solutions. Academic Institutions. Available at: http://micromedex.com/academic. Accessed December 8, 2015.
6. Patel A. Clinical Pharmacology Mobile-A mobile web app compatible on all smart phones [review] May 31, 2010. Available at: http://www.imedicalapps.com/2010/05/clinical-pharmocology-app-review/. Accessed December 8, 2015.
7. Epocrates. Available at: http://www.epocrates.com/. Accessed December 8, 2015.
8. Baillargeon J, Holmes HM, Lin Y, et al. Concurrent use of warfarin and antibiotics and the risk of bleeding in older adults. Am J Med. 2012;125:183-189.
9. PL Detail-Document #280806. Antimicrobial drug interactions with warfarin. Pharmacist’s Letter/Prescriber’s Letter. August 2012.
10. Lane M, Zeringue A, McDonald J. Serious bleeding events due to warfarin and antibiotic co-prescription in a cohort of veterans. Am J Med. 2014;127:657-663.e2.
11. Hale SF, Lesar TS. Interaction of vitamin K antagonists and trimethoprim-sulfamethoxazole: ignore at your patient’s risk. Drug Metab Drug Interact. 2014;29:53-60.
12. Ahmed A, Stephens JC, Kaus CA, et al. Impact of preemptive warfarin dose reduction on anticoagulation after initiation of trimethoprim-sulfamethoxazole or levofloxacin. J Thromb Thrombolysis. 2008;26:44-48.
13. Holt RK, Anderson EA, Cantrell MA, et al. Preemptive dose reduction of warfarin in patients initiating metronidazole. Drug Metabol Drug Interact. 2010;25:35-39.
14. Hughes BR, Cunliffe WJ. Interactions between the oral contraceptive pill and antibiotics. Br J Dermatol. 1990;122:717-718.
15. Bolt HM. Interactions between clinically used drugs and oral contraceptives. Environ Health Perspect. 1994;102:35-38.
16. Aronson JK. Meyler’s Side Effects of Drugs. 16th ed. The International Encyclopedia of Adverse Drug Reactions and Interactions. Amsterdam, Netherlands: Elsevier; 2016. Available at: http://ac.els-cdn.com/B978044453717101009X/3-s2.0-B978044453717101009X-main.pdf?_tid=b33f6564-9deb-11e5-a8f0-00000aab0f01&acdnat=1449607315_83f5068fc5105226fcc6d7279c083516. Accessed December 8, 2015.
17. Masters KP, Carr BM. Survey of pharmacists and physicians on drug interactions between combined oral contraceptives and broad-spectrum antibiotics. Pharm Pract (Granada). 2009;7:139-144.
18. Toh S, Mitchell AA, Anderka M, et al; National Birth Defects Prevention Study. Antibiotics and oral contraceptive failure—a case-crossover study. Contraception. 2011;83:418-425.
19. Koopmans PC, Bos JH, de Jong van den Berg LT. Are antibiotics related to oral combination contraceptive failures in the Netherlands? A case-crossover study. Pharmacoepidemiol Drug Saf. 2012;21:865-871.
20. Archer JS, Archer DF. Oral contraceptive efficacy and antibiotic interaction: A myth debunked. J Am Acad Dermatol. 2002;46:917–923.
21. Scholten PC, Droppert RM, Zwinkels MGJ, et al. No interaction between ciprofloxacin and an oral contraceptive. Antimicrob Agents Chemother. 1998;42:3266-3268.
22. Andrews E, Damle BD, Fang A, et al. Pharmacokinetics and tolerability of voriconazole and a combination oral contraceptive co-administered in healthy female subjects. Br J Clin Pharmacol. 2008;65:531-539.
23. Grimmer SF, Allen WL, Back DJ, et al. The effect of cotrimoxazole on oral contraceptive steroids in women. Contraception. 1983;28:53-59.
24. Dickinson BD, Altman RD, Nielsen NH, et al; Council on Scientific Affairs, American Medical Association. Drug interactions between oral contraceptives and antibiotics. Obstet Gynecol. 2001;98:853-860.
25. Edelman AB, Cherala G, Stanczyk FZ. Metabolism and pharmacokinetics of contraceptive steroids in obese women: a review. Contraception. 2010;82:314-323.
26. Owens RC Jr, Ambrose PG. Torsades de pointes associated with fluoroquinolones. Pharmacotherapy. 2002;22:663-668.
27. Letsas KP, Efremidis M, Kounas SP, et al. Clinical characteristics of patients with drug-induced QT interval prolongation and torsade de pointes: identification of risk factors. Clin Res Cardiol. 2009;98:208-212.
28. Yap YG, Camm AJ. Drug induced QT prolongation and torsades de pointes. Heart. 2003;89:1363-1372.
29. Shaffer D, Singer S, Korvick J, et al. Concomitant risk factors in reports of torsades de pointes associated with macrolide use: review of the United States Food and Drug Administration adverse event reporting system. Clin Infect Dis. 2002;35:197-200.
30. FDA Briefing Document. Joint Meeting of the Antimicrobial Drugs Advisory Committee and the Drug Safety and Risk Management Advisory Committee. November 5, 2015. Available at: http://www.fda.gov/downloads/AdvisoryCommittees/CommitteesMeetingMaterials/Drugs/Anti-InfectiveDrugsAdvisoryCommittee/UCM467383.pdf. Accessed June 11, 2016.
31. Chou HW, Wang JL, Chang CH, et al. Risks of cardiac arrhythmia and mortality among patients using new-generation macrolides, fluoroquinolones, and β-lactam/β-lactamase inhibitors: a Taiwanese nationwide study. Clin Infect Dis. 2015;60:566-577.
32. Wong AY, Root A, Douglas IJ, et al. Cardiovascular outcomes associated with use of clarithromycin: population based study. BMJ. 2016;352:h6926.
33. Inghammar M, Svanström H, Melbye M, et al. Oral fluoroquinolone use and serious arrhythmia: bi-national cohort study. BMJ. 2016;352:i843.
34. Visapää JP, Tillonen JS, Kaihovaara PS, et al. Lack of disulfiram-like reaction with metronidazole and ethanol. Ann Pharmacother. 2002;36:971-974. 35. Fjeld H, Raknes G. Is combining metronidazole and alcohol really hazardous? Tidsskr Nor Laegeforen. 2014;134:1661-1663.