User login
New HCV Diagnostic Tests Provide Accuracy and Low Costs
NEW YORK - Several hepatitis C virus core antigen (HCVcAg) tests accurately diagnose hepatitis C virus (HCV) infection and could replace nucleic acid testing (NAT) in settings where HCV is prevalent, according to a systematic review and meta-analysis.
"Overall, several of the tests perform very well and while they are not equal to NAT, the lower costs may improve diagnostic capacity in the appropriate setting," Dr. J. Morgan Freiman from Boston Medical Center in Massachusetts told Reuters Health by email.
The current two-step diagnostic procedure for diagnosing HCV infection -- screening for antibodies to HCV followed by NAT for those with anti-HCV antibodies -- is a major bottleneck for addressing the HCV elimination strategy proposed by the World Health Organization. Currently, there are five tests for HCVcAg commercially available.
Dr. Freiman and colleagues evaluated the accuracy of diagnosis of active HCV infection among adults and children for these five commercially available tests compared with NAT in their systematic review and meta-analysis of 44 published reports.
The pooled sensitivity and specificity were 93.4% and 98.8% for the Abbott ARCHITECT assay, 93.2% and 99.2% for the Ortho HCV Ag ELISA, and 59.5% and 82.9% for the Hunan Jynda HCV Ag ELISA. There was insufficient information for a pooled analysis of the Eiken Lumispot HCV Ag and the Fujirebio Lumipulse Ortho HCV Ag assays.
Three reports showed that the HCVcAg correlated well with RNA when levels were at least 3000 IU/mL when the Abbott ARCHITECT assay was used, according to the June 21 Annals of Internal Medicine report.
"Although even tests with the highest performance are not as sensitive as NAT, well-performing HCVcAg tests with an analytic sensitivity reaching into the femtomolar range (equal to 3000 IU/mL) could replace NAT for HCV detection, particularly if a lower cost per test allows more patients to be served," the researchers conclude. "Therefore, HCVcAg should be explored for point-of-care (POC) testing to increase the number of patients diagnosed and streamline the HCV cascade of care."
"There is much more work to be done to determine at what sensitivity threshold a POC test would be clinically useful," Dr. Freiman said. "In settings with reliable access to centralized laboratory processing and higher diagnostic capacity, a POC test may still prove to be useful as a screening tool, but would be less likely to replace confirmatory nucleic acid testing (NAT)."
"We have the technology to detect circulating HCV RNA down to 15 IU/mL - amazing -- but how clinically relevant is that threshold when access to testing is equally as important as accuracy in resource limited settings?" he wondered.
Dr. Jose-Manuel Echevarria, from Carlos III Health Institute, Madrid, Spain, who recently reported that HCV core-specific antibody may represent occult HCV infection among blood donors, told Reuters Health by email, "Physicians should conclude from the report that HCVcAg testing provides trustful diagnostic results for the characterization of their anti-HCV positive patients as viremic or non-viremic before deciding about antiviral treatment."
"I would add that HCVcAg testing is particularly useful for the purpose of transfusion centers," he said. "Chronically infected blood donors are detected by anti-HCV screening, and HCVcAg will detect efficiently almost every blood unit obtained from donors experiencing the window period of the acute HCV infection, who test negative for anti-HCV."
"At present, high-resource settings will for sure use NAT testing because of its higher sensitivity, and because automatic equipment has reduced the chance for false-positive results because sample-to-sample contamination (is kept) to a minimum," Dr. Echevarria concluded. "However, HCVcAg testing is extremely useful and convenient for low-resource settings, and also for emergency units everywhere."
The National Institutes of Health funded this research. Three coauthors reported disclosures.
SOURCE: http://bit.ly/28LpRcU Ann Intern Med 2016.
NEW YORK - Several hepatitis C virus core antigen (HCVcAg) tests accurately diagnose hepatitis C virus (HCV) infection and could replace nucleic acid testing (NAT) in settings where HCV is prevalent, according to a systematic review and meta-analysis.
"Overall, several of the tests perform very well and while they are not equal to NAT, the lower costs may improve diagnostic capacity in the appropriate setting," Dr. J. Morgan Freiman from Boston Medical Center in Massachusetts told Reuters Health by email.
The current two-step diagnostic procedure for diagnosing HCV infection -- screening for antibodies to HCV followed by NAT for those with anti-HCV antibodies -- is a major bottleneck for addressing the HCV elimination strategy proposed by the World Health Organization. Currently, there are five tests for HCVcAg commercially available.
Dr. Freiman and colleagues evaluated the accuracy of diagnosis of active HCV infection among adults and children for these five commercially available tests compared with NAT in their systematic review and meta-analysis of 44 published reports.
The pooled sensitivity and specificity were 93.4% and 98.8% for the Abbott ARCHITECT assay, 93.2% and 99.2% for the Ortho HCV Ag ELISA, and 59.5% and 82.9% for the Hunan Jynda HCV Ag ELISA. There was insufficient information for a pooled analysis of the Eiken Lumispot HCV Ag and the Fujirebio Lumipulse Ortho HCV Ag assays.
Three reports showed that the HCVcAg correlated well with RNA when levels were at least 3000 IU/mL when the Abbott ARCHITECT assay was used, according to the June 21 Annals of Internal Medicine report.
"Although even tests with the highest performance are not as sensitive as NAT, well-performing HCVcAg tests with an analytic sensitivity reaching into the femtomolar range (equal to 3000 IU/mL) could replace NAT for HCV detection, particularly if a lower cost per test allows more patients to be served," the researchers conclude. "Therefore, HCVcAg should be explored for point-of-care (POC) testing to increase the number of patients diagnosed and streamline the HCV cascade of care."
"There is much more work to be done to determine at what sensitivity threshold a POC test would be clinically useful," Dr. Freiman said. "In settings with reliable access to centralized laboratory processing and higher diagnostic capacity, a POC test may still prove to be useful as a screening tool, but would be less likely to replace confirmatory nucleic acid testing (NAT)."
"We have the technology to detect circulating HCV RNA down to 15 IU/mL - amazing -- but how clinically relevant is that threshold when access to testing is equally as important as accuracy in resource limited settings?" he wondered.
Dr. Jose-Manuel Echevarria, from Carlos III Health Institute, Madrid, Spain, who recently reported that HCV core-specific antibody may represent occult HCV infection among blood donors, told Reuters Health by email, "Physicians should conclude from the report that HCVcAg testing provides trustful diagnostic results for the characterization of their anti-HCV positive patients as viremic or non-viremic before deciding about antiviral treatment."
"I would add that HCVcAg testing is particularly useful for the purpose of transfusion centers," he said. "Chronically infected blood donors are detected by anti-HCV screening, and HCVcAg will detect efficiently almost every blood unit obtained from donors experiencing the window period of the acute HCV infection, who test negative for anti-HCV."
"At present, high-resource settings will for sure use NAT testing because of its higher sensitivity, and because automatic equipment has reduced the chance for false-positive results because sample-to-sample contamination (is kept) to a minimum," Dr. Echevarria concluded. "However, HCVcAg testing is extremely useful and convenient for low-resource settings, and also for emergency units everywhere."
The National Institutes of Health funded this research. Three coauthors reported disclosures.
SOURCE: http://bit.ly/28LpRcU Ann Intern Med 2016.
NEW YORK - Several hepatitis C virus core antigen (HCVcAg) tests accurately diagnose hepatitis C virus (HCV) infection and could replace nucleic acid testing (NAT) in settings where HCV is prevalent, according to a systematic review and meta-analysis.
"Overall, several of the tests perform very well and while they are not equal to NAT, the lower costs may improve diagnostic capacity in the appropriate setting," Dr. J. Morgan Freiman from Boston Medical Center in Massachusetts told Reuters Health by email.
The current two-step diagnostic procedure for diagnosing HCV infection -- screening for antibodies to HCV followed by NAT for those with anti-HCV antibodies -- is a major bottleneck for addressing the HCV elimination strategy proposed by the World Health Organization. Currently, there are five tests for HCVcAg commercially available.
Dr. Freiman and colleagues evaluated the accuracy of diagnosis of active HCV infection among adults and children for these five commercially available tests compared with NAT in their systematic review and meta-analysis of 44 published reports.
The pooled sensitivity and specificity were 93.4% and 98.8% for the Abbott ARCHITECT assay, 93.2% and 99.2% for the Ortho HCV Ag ELISA, and 59.5% and 82.9% for the Hunan Jynda HCV Ag ELISA. There was insufficient information for a pooled analysis of the Eiken Lumispot HCV Ag and the Fujirebio Lumipulse Ortho HCV Ag assays.
Three reports showed that the HCVcAg correlated well with RNA when levels were at least 3000 IU/mL when the Abbott ARCHITECT assay was used, according to the June 21 Annals of Internal Medicine report.
"Although even tests with the highest performance are not as sensitive as NAT, well-performing HCVcAg tests with an analytic sensitivity reaching into the femtomolar range (equal to 3000 IU/mL) could replace NAT for HCV detection, particularly if a lower cost per test allows more patients to be served," the researchers conclude. "Therefore, HCVcAg should be explored for point-of-care (POC) testing to increase the number of patients diagnosed and streamline the HCV cascade of care."
"There is much more work to be done to determine at what sensitivity threshold a POC test would be clinically useful," Dr. Freiman said. "In settings with reliable access to centralized laboratory processing and higher diagnostic capacity, a POC test may still prove to be useful as a screening tool, but would be less likely to replace confirmatory nucleic acid testing (NAT)."
"We have the technology to detect circulating HCV RNA down to 15 IU/mL - amazing -- but how clinically relevant is that threshold when access to testing is equally as important as accuracy in resource limited settings?" he wondered.
Dr. Jose-Manuel Echevarria, from Carlos III Health Institute, Madrid, Spain, who recently reported that HCV core-specific antibody may represent occult HCV infection among blood donors, told Reuters Health by email, "Physicians should conclude from the report that HCVcAg testing provides trustful diagnostic results for the characterization of their anti-HCV positive patients as viremic or non-viremic before deciding about antiviral treatment."
"I would add that HCVcAg testing is particularly useful for the purpose of transfusion centers," he said. "Chronically infected blood donors are detected by anti-HCV screening, and HCVcAg will detect efficiently almost every blood unit obtained from donors experiencing the window period of the acute HCV infection, who test negative for anti-HCV."
"At present, high-resource settings will for sure use NAT testing because of its higher sensitivity, and because automatic equipment has reduced the chance for false-positive results because sample-to-sample contamination (is kept) to a minimum," Dr. Echevarria concluded. "However, HCVcAg testing is extremely useful and convenient for low-resource settings, and also for emergency units everywhere."
The National Institutes of Health funded this research. Three coauthors reported disclosures.
SOURCE: http://bit.ly/28LpRcU Ann Intern Med 2016.
Sign up now for the October Coding and Reimbursement Workshop
The Coding & Reimbursement for Vascular Surgeons Workshop will be held October 21-22, 2016 at the Millennium Knickerbocker Hotel in Chicago.
Vascular surgeons and their support staff need to maintain knowledge and competence for appropriate billing and coding procedures. The Coding and Reimbursement for Vascular Surgeons course is an intensive two-day program designed to address the 2016 updates including changes to endovascular stent placement outside the lower extremity and PQRS as well as coding for intravascular embolization and retrograde intrathoracic carotid stenting.
Additionally, the course will review the proposed updates for 2016 with a focus on reporting standards for interventional and open surgical procedures. The program also includes information about the global surgical package and how it impacts billing and reimbursement, along with the application of modifiers for streamlined reimbursement. This will be accomplished by using a multi-modality educational forum with didactic lectures, panel discussions, case studies, and question-answer sessions.
For further information and to sign up click here.
The Coding & Reimbursement for Vascular Surgeons Workshop will be held October 21-22, 2016 at the Millennium Knickerbocker Hotel in Chicago.
Vascular surgeons and their support staff need to maintain knowledge and competence for appropriate billing and coding procedures. The Coding and Reimbursement for Vascular Surgeons course is an intensive two-day program designed to address the 2016 updates including changes to endovascular stent placement outside the lower extremity and PQRS as well as coding for intravascular embolization and retrograde intrathoracic carotid stenting.
Additionally, the course will review the proposed updates for 2016 with a focus on reporting standards for interventional and open surgical procedures. The program also includes information about the global surgical package and how it impacts billing and reimbursement, along with the application of modifiers for streamlined reimbursement. This will be accomplished by using a multi-modality educational forum with didactic lectures, panel discussions, case studies, and question-answer sessions.
For further information and to sign up click here.
The Coding & Reimbursement for Vascular Surgeons Workshop will be held October 21-22, 2016 at the Millennium Knickerbocker Hotel in Chicago.
Vascular surgeons and their support staff need to maintain knowledge and competence for appropriate billing and coding procedures. The Coding and Reimbursement for Vascular Surgeons course is an intensive two-day program designed to address the 2016 updates including changes to endovascular stent placement outside the lower extremity and PQRS as well as coding for intravascular embolization and retrograde intrathoracic carotid stenting.
Additionally, the course will review the proposed updates for 2016 with a focus on reporting standards for interventional and open surgical procedures. The program also includes information about the global surgical package and how it impacts billing and reimbursement, along with the application of modifiers for streamlined reimbursement. This will be accomplished by using a multi-modality educational forum with didactic lectures, panel discussions, case studies, and question-answer sessions.
For further information and to sign up click here.
Raman Malhotra, MD
The video associated with this article is no longer available on this site. Please view all of our videos on the MDedge YouTube channel
The video associated with this article is no longer available on this site. Please view all of our videos on the MDedge YouTube channel
The video associated with this article is no longer available on this site. Please view all of our videos on the MDedge YouTube channel
Hand compression neuropathy: An assessment guide
› Use provocative testing to confirm a suspected diagnosis in a patient who presents with peripheral entrapment mononeuropathy. B
› Consider electrodiagnostic testing for help in diagnosing a challenging presentation, ruling out a competing diagnosis, or clarifying an atypical clinical picture or vague subjective history. A
› Evaluate any patient who presents with non-anatomic nerve distribution of symptoms—eg, burning, numbness, and tingling of the entire hand—for a metabolic, rather than an entrapment, neuropathy. B
Strength of recommendation (SOR)
A Good-quality patient-oriented evidence
B Inconsistent or limited-quality patient-oriented evidence
C Consensus, usual practice, opinion, disease-oriented evidence, case series
Neuropathic hand complaints—for which patients typically seek medical attention when the pain or paresthesia starts to interfere with their daily routine—are common and diverse. The ability to assess and accurately diagnose upper extremity compression neuropathies is critical for physicians in primary care.
Assessment starts, of course, with a thorough history of the present illness and past medical history, which helps define a broad differential diagnosis and identify comorbidities. Physical examination, including judicious use of provocative testing, allows you to objectively identify the pathologic deficit, evaluate function and coordination of multiple organ systems, and detect nerve dysfunction. The results determine whether additional tools, such as electrodiagnostic testing, are needed.
We’ve created this guide, detailed in the text, tables, and figures that follow, to help you hone your ability to accurately diagnose patients who present with compression neuropathies of the hand.
The medical history: Knowing what to ask
To clearly define a patient’s symptoms and disability, start with a thorough history of the presenting complaint.
Inquire about symptom onset and chronicity. Did the pain or paresthesia begin after an injury? Are the symptoms associated with repetitive use of the extremity? Do they occur at night?
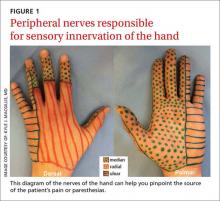
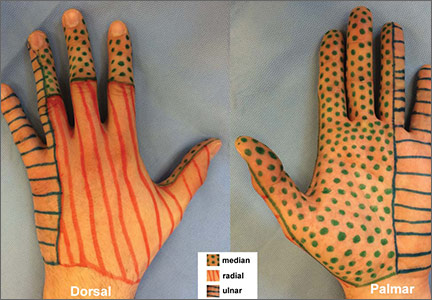
Pinpoint the location or distribution of pain or paresthesia. It is paramount to identify the affected nerve.1,2 Ask patients to complete a hand or upper extremity profile documenting location and/or type of numbness, tingling, or decreased sensation. A diagram of the peripheral nerves responsible for sensory innervation of the hand (FIGURE 1) is an effective way to screen individuals at high risk of carpal tunnel syndrome (CTS) or ulnar tunnel syndrome (UTS).1,2
A patient report such as, “My whole hand is numb,” calls for a follow-up question to determine whether the little finger is affected,3 which would indicate that the ulnar nerve, rather than just the median nerve, is involved. And if a patient reports feeling as if he or she is wearing gloves or mittens, it is essential to consider the possibility of a systemic neuropathy rather than a single peripheral neuropathy.3
Gather basic patient information. Inquire about hand dominance, occupation, and baseline function, any or all of which may be critical in the assessment and initiation of treatment.4,5
Review systemic conditions and medications
A broad range of comorbidities, such as cervical radiculopathy, diabetes, hypothyroidism, and vitamin deficiencies (TABLE 1),6,7 may be responsible for neuropathic hand complaints, and a thorough review of systemic complaints and past medical history is critical. Include a medication history and a review of prior procedures, such as post-traumatic surgeries of the hand or upper extremity or nerve decompression surgeries, which may provide additional insight into disease etiology.
Symptoms guide physical exam, provocative testing
A physical examination, including provocative testing, follows based on reported symptoms, medical history, and suspected source of nerve compression.
Carpal tunnel syndrome
CTS is the most common peripheral neuropathy.8 Patients often report nocturnal pain or paresthesia in the distal median nerve distribution, comprising the palmar surface of the thumb, index, middle, and radial half of the ring finger.
Researchers have identified 6 standardized clinical criteria for the diagnosis of CTS. Two criteria—numbness mostly in median nerve territory and nocturnal numbness—can be ascertained during the history of present illness.The other 4, detailed below, will be found during the physical exam.9
Thenar weakness or atrophy.9 Begin your evaluation by inspecting the thenar musculature for atrophic changes. Motor exam of intrinsic musculature innervated by the recurrent motor branch of the median nerve includes assessment of thumb abduction strength (assessed by applying resistance to the metacarpophalangeal joint [MCPJ] base towards the palm in the position of maximal abduction) and opposition strength (assessed by applying force to the MCPJ from the ulnar aspect).10
Positive Phalen’s test.9 Provocative testing for CTS includes Phalen’s test (sensitivity 43%-86%, specificity 48%-67%),11 which is an attempt to reproduce the numbness or tingling in the median nerve territory within 60 seconds of full wrist flexion. Ask the patient to hold his or her forearms vertically with elbows resting on the table (allowing gravity to flex the wrists),12 and to tell you if numbness or tingling occurs.
Positive Tinel’s sign.9 Tinel’s sign (sensitivity 45%-75%, specificity 48%-67%)11 is performed by lightly tapping the median nerve from the proximal to distal end over the carpal tunnel. The test is positive if paresthesia results. Provocative testing may also include Durkan’s test, also known as the carpal compression test. Durkan’s test (sensitivity 49%-89%, specificity 54%-96%)11 involves placing your thumb directly over the carpal tunnel and holding light compression for 60 seconds, or until paresthesia is reported.
Positive 2-point discrimination test.9 To assess CTS disease severity, use 2-point discrimination to evaluate the patient’s sensation qualitatively and quantitatively. Two-point discrimination can only be tested, however, if light touch sensation is intact. It is typically performed by lightly applying 2 caliper points at fixed distances sufficient to blanch the skin, but some clinicians have used other tools, such as a modified paperclip.13 The smallest distance at which the patient can detect 2 distinct stimuli is then recorded.
Researchers have reported an average of 3 to 5 mm for 2-point discrimination at the fingertipand a normal 2-point discrimination of 6 to 9 mm in the volar surface of the hand (TABLE 2).14,15
The scratch collapse test (sensitivity 64%, specificity 99%) is a supplemental exam that uses a different outcome measure to diagnose CTS.16 It involves lightly scratching the skin over the compressed carpal tunnel while the patient performs sustained resisted bilateral shoulder external rotation in an adducted position. A momentary loss of muscle resistance to external rotation indicates a positive test.
Pronator syndrome
Pronator syndrome (PS) is a proximal median neuropathy that may present in isolation or in combination with CTS as a double crush syndrome. Clinical symptoms include features of CTS and sensory paresthesias in the palm and distal forearm in the distribution of the palmar cutaneous branch of the median nerve. PS is commonly associated with volar proximal forearm pain exacerbated by repetitive activities involving pronation and supination.
PS is not easy to assess. Palpatory examination of a large supracondylar process at the distal humerus proximal to the medial epicondyle on its anteromedial aspect can be difficult, especially if the patient is overweight. And motor weakness is not a prominent feature. What’s more, power assessment of the pronator teres, flexor carpi radialis, and flexor digitorum superficialis may exacerbate symptoms.
Because the symptoms of PS and CTS may be the same, PS provocation maneuvers should be performed on patients with CTS symptoms and paresthesia involving the palm. Start by testing for Tinel’s sign over the pronator teres muscle, although this has been found to be positive in less than 50% of PS cases.17 Palpate the antecubital fossa and the proximal aspect of the pronator teres muscle to assess for discomfort or tenderness.
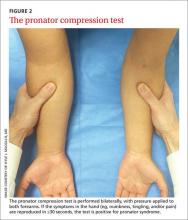
Pronator compression test. The pronator compression test has been found to be the most sensitive way to assess PS.18,19 This test involves direct compression of the proximal and radial edge of the pronator teres muscle belly along the proximal volar forearm with the thumb.20 It is performed bilaterally on supinated upper extremities, with the clinician applying pressure on each forearm simultaneously (FIGURE 2). If the symptoms in the hand are reproduced in ≤30 seconds, the test is positive. In a study of 10 patients with surgically confirmed PS, the pronator compression test was positive in every case.20,21
Resistance testing. You can also evaluate the pronator teres compression site by testing the patient’s ability to resist pronation with his or her elbow extended and the forearm in neutral position. To test for compression from the bicipital aponeurosis, ask the patient to flex the elbow to approximately 120° to 130° and apply active supinated resistance.22 Likewise, resistance of the long finger proximal interphalangeal joint (IPJ) to flexion—a maneuver performed with elbow fully extended—assesses compression from the fibrous arcade of the flexor digitorum superficialis (FDS).21 A positive resistance test will reproduce the reported symptoms.
Ulnar tunnel syndrome
Symptoms of UTS, which is much less common than CTS, include pain in the wrist and hand that is associated with paresthesia or numbness in the small finger and ulnar half of the ring finger. Patients may report difficulty with motor tasks involving grip and pinch strength or fatigue with prolonged action of the intrinsic muscles. Many also report an exacerbation of symptoms associated with increased wrist flexion or at night.
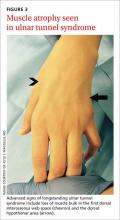
Evaluation of UTS requires a full assessment of the upper extremity, starting with observation of hand posture and muscle bulk to identify signs of chronic nerve compression. The contralateral extremity serves as a control to the neuropathic hand. Classically, chronic ulnar nerve compression leads to intrinsic muscle atrophy, evidenced by loss of topographical soft tissue bulk in the first dorsal web space, the palmar transverse metacarpal arch, and the hypothenar area.23 Ulnar motor nerve dysfunction is limited to the intrinsic muscles of the hand. The inverted pyramid sign, signified by atrophy of the transverse head of the adductor pollicis, is another visual aberrancy,24 as is clawing of the ring finger and small finger. The clawing, which involves hyperextension of the MCPJ and flexion of the proximal and distal IPJ, is commonly known as Duchenne’s sign. FIGURE 3 demonstrates hypothenar atrophy and the loss of muscle bulk in the first dorsal web space.
When you suspect UTS, palpate the wrist and hand in an attempt to locate a mass or area of tenderness. Not all patients with a volar ganglion cyst responsible for UTS present with a palpable mass, but tenderness along the radial aspect of the pisiform or an undefined fullness in this area may be noted.25 Any patient with a palpable mass should be tested for Tinel’s sign over the mass and undergo a thorough vascular assessment. Fracture of the hook of the hamate is indicated by tenderness in the region approximated by the intersection of Kaplan’s line and the proximal extension line from the ring finger.
Perform 2-point discrimination testing at the palmar distal aspect of the small finger and ulnar half of the ring finger. This tests the superficial sensory division of the ulnar nerve that travels within Guyon’s canal. Testing the dorsal ulnar cutaneous nerve involves the skin of the dorso-ulnar hand and dorsum of the long finger proximal to the IPJ. If this area is spared and the palmar distal ulnar digits are affected, compression within Guyon’s canal is likely. If both areas are affected, suspect a more proximal compression site at the cubital tunnel, as the dorsal ulnar cutaneous nerve branches proximal to Guyon’s canal.26
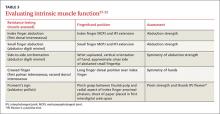
Ulnar motor nerve dysfunction in UTS is limited to the intrinsic muscles of the hand. Assessment of intrinsic muscle function is described in TABLE 3.27-32 It is important to become familiar with the tests and maneuvers described, but also to be aware that a comprehensive evaluation of ulnar nerve motor function requires a combination of tests.
Cubital tunnel syndrome
Cubital tunnel syndrome—the second most common peripheral neuropathy8—involves the proximal site of ulnar nerve compression in the upper extremity. Patients typically report symptoms similar to those of UTS, with sensory paresthesia in the ulnar digits and intrinsic weakness. To learn more about the symptoms, ask if the onset of pain or paresthesia is related to a particular elbow position, such as increased elbow flexion.
Notably, pain is usually not the initial complaint, unless the disease is advanced. This may be the reason atrophic intrinsic changes are 4 times more likely to be seen in patients with cubital tunnel syndrome than in those with CTS.33 You’re more likely to hear about vague motor problems, including hand clumsiness and difficulty with fine coordination of the fingers.34 Thus, it is important to evaluate patients for concurrent UTS and/or CTS, as well as for differentiation.
Focus on the elbow. Whenever you suspect cubital tunnel syndrome, pay special attention to the elbow. Examine the carrying angle of the elbow in relation to the contralateral extremity. Deformity may provide clues to a history of trauma. Assess the ulnar nerve during active flexion and extension to identify a subluxatable nerve at the cubital tunnel. Examine the ulno-humeral joint for crepitus, and palpate the joint line for large osteophytes and/or ganglion cysts.
Motor examination of the ulnar nerve primarily focuses on the intrinsic muscles detailed in TABLE 3,27-32 although the flexor carpi ulnaris (FCU) and flexor digitorum profundus (FDP) to the ring finger and small finger are also innervated by the ulnar nerve. The FCU mediates the power grip, and can be tested by resisted wrist flexion and ulnar deviation. Ring finger and small finger FDP strength should be examined by resistance testing at the distal IPJ.
Provocative testing of cubital tunnel syndrome includes Tinel’s sign (performed over the cubital tunnel), the elbow flexion test (performed with elbow in maximum flexion and wrist at neutral position and held for 60 seconds), and the pressure provocation test (performed by applying pressure to the ulnar nerve just proximal to the cubital tunnel with your index and long fingers for 60 seconds while the patient’s elbow is at 20° flexion with the forearm supinated). For each test, eliciting distal paresthesia in ulnar nerve territory is a positive result. The sensitivity of these tests ranges from 70% (Tinel’s sign) to 98% (combined elbow flexion and pressure); specificity ranges from 95% to 99%.35
The scratch collapse test for cubital tunnel syndrome evaluation is similar to the version used to assess for CTS; the only difference is the location of the site that is scratched, in this case the cubital tunnel. The reported sensitivity and specificity are 69% and 99%, respectively.11
Wartenberg’s syndrome
When a patient reports paresthesia or pain along the radial aspect of the forearm that radiates into the dorsal thumb and index and middle fingers, consider Wartenberg’s syndrome—compression of the superficial radial nerve. The condition, also known as “cheiralgia paresthetica,” may exist anywhere along the course of the nerve in the forearm,36 but the compression typically occurs 9 cm proximal to the radial styloid.
Assess the skin over the forearm and hand for evidence of prior trauma, surgical scars, or external compression sources, such as a watch.37-39 Then use palpation to identify superficial or deep masses along the nerve.40 Perform Tinel’s sign by lightly tapping along the nerve course from proximal to distal in the forearm. The test is positive if paresthesias are provoked distally.41,42
Motor function of the extremity is unaffected in Wartenberg’s syndrome, unless a prior traumatic injury occurred or a comorbidity exists. Comorbidities to consider include de Quervain’s tenosynovitis, cervical radiculopathy, injury to the lateral antebrachial cutaneous nerve, and CTS.
Gross sensory testing usually is negative, but 2-point discrimination may show diminished responses. Testing distribution includes the dorsal radial wrist and hand, dorsal thumb skin proximal to the IPJ, and the dorsal surface of the index, middle, and radial half of the ring finger proximal to the IPJ.
Proceed with caution. Provocative testing with a pronated forearm and a flexed and ulnar-deviated wrist may exacerbate symptoms,12,42 and Finkelstein’s maneuver (isolated ulnar deviation at the wrist to elicit pain over the first dorsal wrist compartment) and Phalen’s test may elicit false-positive results. Upper motor neuron exams (ie, deep tendon reflexes) and Hoffman’s sign (reflexive flexion of the terminal phalanges of the thumb and index finger induced by flicking or tapping the distal phalanx of the long finger) should be symmetric to the contralateral extremity.
In patients with more than one compression injury, Spurling’s sign (neck extension and lateral rotation towards the affected extremity) may induce paresthesia when combined with axial compression, while the shoulder abduction test (shoulder 90° abduction, with external rotation) may diminish reported paresthesia. In those for whom Wartenberg’s syndrome is their only compression injury, however, these provocative maneuvers will be negative.
Anterior interosseous nerve syndrome
A rare clinical entity that affects the anterior interosseous innervated muscles in the forearm, anterior interosseous nerve syndrome (AINS)’s etiology is unclear. But it is likely related to a spontaneous neuritis rather than a compressive etiology.43 Paresis or paralysis of the flexor pollicis longus (FPL) and the flexor digitorum profundus (FDP) of the index and long finger is its hallmark, but patients may report vague forearm pain associated with weakness or absence of function.
Examination begins with a visual assessment and palpation of the forearm and hand for atrophy or a space-occupying lesion. Notably, sensory function of the hand should remain at baseline despite the motor dysfunction, as the anterior interosseous is purely a motor nerve.
Motor testing should focus on the median and anterior interosseous innervated muscles.
The FPL is tested by isolated thumb IPJ flexion and the Kiloh and Nevin sign—the inability to make an “OK” sign with the thumb and index finger.44 Assess pinch grasp with a maximally flexed thumb IPJ and distal IPJ of the index finger. Anterior interosseous nerve dysfunction results in a flattened pinch without IPJ or distal IPJ flexion, which increases the contact of the thumb and index finger pulp.45 In a series of 14 patients, researchers reported complete paralysis of FPL and FDP index finger in 5 patients, isolated paralysis of FPL in 7 patients, and isolated paralysis of FDP index and long fingers in 2 patients. None had isolated paralysis of the FDP long finger.46FIGURE 4 shows complete (A) and incomplete (B) paralysis.
Evaluation of AINS also includes assessment of FPL and FDP tendon integrity with passive tenodesis. The appearance of the hand at rest should reflect the natural digital cascade. In a completely relaxed or anesthetized hand, the forearm is placed in wrist supination and extension, allowing gravity to extend the wrist and placing the thumb MCPJ at 30° flexion, the index finger MCPJ at 40°, and the small finger MCPJ at 70°. The thumb IPJ should approximate the radial fingertip pulp of the index finger. The forearm is then pronated and flexed at the wrist, straightening the thumb MCPJ and producing a mild flexion cascade at the proximal and distal IPJ of the index, long, ring, and small fingers within 20° of full extension. This dynamic exercise is used to confirm that the patient has an intact tenodesis effect, thus excluding tendon lacerations or ruptures from the differential diagnosis. In one study, in 9 of 33 cases of partial or complete isolated index finger FPL or FDP, paralysis was initially diagnosed as tendon rupture.47
When to consider electrodiagnostic testing
Electrodiagnostic testing—a combination of electromyography and nerve conduction studies to assess the status of a peripheral nerve48—provides objective data that can help diagnose a challenging presentation, rule out a competing diagnosis, or clarify an atypical clinical picture or vague subjective history. This type of testing is also used to localize the entrapment site, identify a patient with polyneuropathy or brachial plexopathy, and assess the severity of nerve injury or presence of a double crush syndrome.49,50 Any patient with signs and symptoms of a compression neuropathy and supportive findings on physical exam should be referred for electrodiagnostic testing and/or to a surgeon specializing in treating these conditions.
CORRESPONDENCE
Kyle J. MacGillis, MD, University of Illinois at Chicago, Department of Orthopaedic Surgery, 835 South Wolcott Avenue, M/C 844, Chicago, IL 60612; [email protected].
1. Katz JN, Stirrat CR, Larson MG, et al. A self-administered hand symptom diagram for the diagnosis and epidemiologic study of carpal tunnel syndrome. J Rheumatol. 1990;17:1495-1498.
2. Werner RA, Chiodo T, Spiegelberg T, et al. Use of hand diagrams in screening for ulnar neuropathy: comparison with electrodiagnostic studies. Muscle Nerve. 2012;46:891-894.
3. Dellon AL. Patient evaluation and management considerations in nerve compression. Hand Clin. 1992;8:229-239.
4. Kaji H, Honma H, Usui M, et al. Hypothenar hammer syndrome in workers occupationally exposed to vibrating tools. J Hand Surg Br. 1993;18:761-766.
5. Blum J. Examination and interface with the musician. Hand Clin. 2003;19:223-230.
6. Azhary H, Farooq MU, Bhanushali M, et al. Peripheral neuropathy: differential diagnosis and management. Am Fam Physician. 2010;81:887-892.
7. Craig, AS, Richardson JK. Acquired peripheral neuropathy. Phys Med Rehabil Clin N Am. 2003;14:365-386.
8. Latinovic R, Gulliford MC, Hughes RA. Incidence of common compressive neuropathies in primary care. J Neurol Neurosurg Psychiatry. 2006;77:263-265.
9. Graham B, Regehr G, Naglie G, et al. Development and validation of diagnostic criteria for carpal tunnel syndrome. J Hand Surg Am. 2006;31:919-924.
10. Foucher G, Malizos C, Sammut D, et al. Primary palmaris longus transfer as an opponensplasty in carpal tunnel release: a series of 73 cases. J Hand Surg Br. 1991;16:56-60.
11. Massy-Westropp N, Grimmer K, Bain G. A systematic review of the clinical diagnostic tests for carpal tunnel syndrome. J Hand Surg Am. 2000;25:120-127.
12. Dellon AL, Mackinnon SE. Radial sensory nerve entrapment in the forearm. J Hand Surg Am. 1986;11:199-205.
13. Mackinnon SE, Dellon AL. Two-point discrimination tester. J Hand Surg (Am). 1985;10:906-907.
14. Louis DS, Greene TL, Jacobson KE, et al. Evaluation of normal values for stationary and moving two-point discrimination in the hand. J Hand Surg Am. 1984;9:552-555.
15. Omer GE Jr. Physical diagnosis of peripheral nerve injuries. Orthop Clin North Am. 1981;12:207-228.
16. Cheng CJ, Mackinnon-Patterson B, Beck JL, et al. Scratch collapse test for evaluation of carpal and cubital tunnel syndrome. J Hand Surg Am. 2008;33:1518-1524.
17. Haussmann P, Patel MR. Intraepineurial constriction of nerve fascicles in pronator syndrome and anterior interosseous nerve syndrome. Orthop Clin North Am. 1996;27:339-344.
18. Lee AK, Khorsandi M, Nurbhai N, et al. Endoscopically assisted decompression for pronator syndrome. J Hand Surg Am. 2012;37:1173-1179.
19. Lee MJ, LaStayo PC. Pronator syndrome and other nerve compressions that mimic carpal tunnel syndrome. J Orthop Sports Phys Ther. 2004;34:601-609.
20. Gainor BJ. The pronator compression test revisited: a forgotten physical sign. Orthop Rev. 1990;19:888-892.
21. Rehak DC. Pronator syndrome. Clin Sports Med. 2001;20:531-540.
22. Eversmann WW. Compression and entrapment neuropathies of the upper extremity. J Hand Surg Am. 1983;8:759-766.
23. Masse L. Contribution a l’etude de l’achon des interosseus. J Med (Bordeaux). 1916;46:198-200.
24. Draeger RW, Stern PJ. The inverted pyramid sign and other eponymous signs of ulnar nerve palsy. J Hand Surg Am. 2014;39:2517-2520.
25. Wang B, Zhao Y, Lu A, et al. Ulnar nerve deep branch compression by a ganglion: a review of nine cases. Injury. 2014;45:1126-1130.
26. Polatsch DB, Melone CP, Beldner S, et al. Ulnar nerve anatomy. Hand Clin. 2007;23:283-289.
27. Buschbacher R. Side-to-side confrontational strength-testing for weakness of the intrinsic muscles of the hand. J Bone Joint Surg Am. 1997;79:401-405.
28. Wartenberg R. A sign of ulnar palsy. JAMA. 1939;112:1688.
29. Earle A, Vlastou C. Crossed fingers and other tests of ulnar nerve motor function. J Hand Surg Am. 1980;5:560-565.
30. Tsujino A, Macnicol M. Finger flexion sign for ulnar neuropathy. J Hand Surg Br. 1998;23:240-241.
31. Mannerfelt L. Studies on the hand in ulnar nerve paralysis: a clinical-experimental investigation in normal and anomalous innervations. Acta Orthop Scand. 1966;87(Suppl):S118-S129.
32. Froment J. La prehension dans les paralysies du nerf cubital et Le Signe du Pouce. Presse Med. 1915;23:409.
33. Mallette P, Zhao M, Zurakowski D, et al. Muscle atrophy at diagnosis of carpal and cubital tunnel syndrome. J Hand Surg Am. 2007;32:855-858.
34. Huang JH, Samadani U, Zagar EL. Ulnar nerve entrapment neuropathy at the elbow: simple decompression. Neurosurgery. 2004;55:1150-1153.
35. Novak CB, Lee GW, Mackinnon SE, et al. Provocative testing for cubital tunnel syndrome. J Hand Surg Am. 1994;19:817-820.
36. Auerbach DM, Collins ED, Kunkle KL, et al. The radial sensory nerve. an anatomic study. Clin Orthop Relat Res. 1994;308:241-249.
37. Braidwood AS. Superficial radial neuropathy. J Bone Joint Surg Br. 1975;57:380-383.
38. Bierman HR. Nerve compression due to a tight watchband. N Engl J Med. 1959;261:237-238.
39. Linscheid RL. Injuries to radial nerve at wrist. Arch Surg. 1965;91:942-946.
40. Hermansdorfer JD, Greider JL, Dell PC. A case report of a compressive neuropathy of the radial sensory nerve caused by a ganglion cyst at the elbow. Orthopedics. 1986;9:1005-1006.
41. Poppinchalk SP, Schaffer AA. Physical examination of the upper extremity compressive neuropathies. Orthop Clin N Am. 2012;43:417-430.
42. Posner MA. Compressive neuropathies of the median and radial nerves at the elbow. Clin Sports Med. 1990;9:343-363.
43. Dang AC, Rodner CM. Unusual compression neuropathies of the forearm. Part II: median nerve. J Hand Surg Am. 2009;34A:1915-1920.
44. Kiloh LG, Nevin S. Isolated neuritis of the anterior interosseous nerve. Br Med J. 1952;1:850-851.
45. Park IJ, Roh YT, Jeong C, et al. Spontaneous anterior interosseous nerve syndrome: clinical analysis and eleven surgical cases. J Plast Surg Hand Surg. 2013;47:519-523.
46. Ulrich D, Piatkowski A, Pallua N. Anterior interosseous nerve syndrome: retrospective analysis of 14 patients. Arch Orthop Trauma Surg. 2011;131:1561-1565.
47. Hill NA, Howard FM, Huffer BR. The incomplete anterior interosseous nerve syndrome. J Hand Surg Am. 1985;10:4-16.
48. Bergquist ER, Hammert WC. Timing and appropriate use of electrodiagnostic studies. Hand Clin. 2013;29:363-370.
49. Lo JK, Finestone HM, Gilbert K, et al. Community-based referrals for electrodiagnostic studies in patients with possible carpal tunnel syndrome: what is the diagnosis? Arch Phys Med Rehabil. 2002;83:598-603.
50. Kane NM, Oware A. Nerve conduction and electromyography studies. J Neurol. 2012;259:1502-1508.
› Use provocative testing to confirm a suspected diagnosis in a patient who presents with peripheral entrapment mononeuropathy. B
› Consider electrodiagnostic testing for help in diagnosing a challenging presentation, ruling out a competing diagnosis, or clarifying an atypical clinical picture or vague subjective history. A
› Evaluate any patient who presents with non-anatomic nerve distribution of symptoms—eg, burning, numbness, and tingling of the entire hand—for a metabolic, rather than an entrapment, neuropathy. B
Strength of recommendation (SOR)
A Good-quality patient-oriented evidence
B Inconsistent or limited-quality patient-oriented evidence
C Consensus, usual practice, opinion, disease-oriented evidence, case series
Neuropathic hand complaints—for which patients typically seek medical attention when the pain or paresthesia starts to interfere with their daily routine—are common and diverse. The ability to assess and accurately diagnose upper extremity compression neuropathies is critical for physicians in primary care.
Assessment starts, of course, with a thorough history of the present illness and past medical history, which helps define a broad differential diagnosis and identify comorbidities. Physical examination, including judicious use of provocative testing, allows you to objectively identify the pathologic deficit, evaluate function and coordination of multiple organ systems, and detect nerve dysfunction. The results determine whether additional tools, such as electrodiagnostic testing, are needed.
We’ve created this guide, detailed in the text, tables, and figures that follow, to help you hone your ability to accurately diagnose patients who present with compression neuropathies of the hand.
The medical history: Knowing what to ask
To clearly define a patient’s symptoms and disability, start with a thorough history of the presenting complaint.
Inquire about symptom onset and chronicity. Did the pain or paresthesia begin after an injury? Are the symptoms associated with repetitive use of the extremity? Do they occur at night?
Pinpoint the location or distribution of pain or paresthesia. It is paramount to identify the affected nerve.1,2 Ask patients to complete a hand or upper extremity profile documenting location and/or type of numbness, tingling, or decreased sensation. A diagram of the peripheral nerves responsible for sensory innervation of the hand (FIGURE 1) is an effective way to screen individuals at high risk of carpal tunnel syndrome (CTS) or ulnar tunnel syndrome (UTS).1,2
A patient report such as, “My whole hand is numb,” calls for a follow-up question to determine whether the little finger is affected,3 which would indicate that the ulnar nerve, rather than just the median nerve, is involved. And if a patient reports feeling as if he or she is wearing gloves or mittens, it is essential to consider the possibility of a systemic neuropathy rather than a single peripheral neuropathy.3
Gather basic patient information. Inquire about hand dominance, occupation, and baseline function, any or all of which may be critical in the assessment and initiation of treatment.4,5
Review systemic conditions and medications
A broad range of comorbidities, such as cervical radiculopathy, diabetes, hypothyroidism, and vitamin deficiencies (TABLE 1),6,7 may be responsible for neuropathic hand complaints, and a thorough review of systemic complaints and past medical history is critical. Include a medication history and a review of prior procedures, such as post-traumatic surgeries of the hand or upper extremity or nerve decompression surgeries, which may provide additional insight into disease etiology.
Symptoms guide physical exam, provocative testing
A physical examination, including provocative testing, follows based on reported symptoms, medical history, and suspected source of nerve compression.
Carpal tunnel syndrome
CTS is the most common peripheral neuropathy.8 Patients often report nocturnal pain or paresthesia in the distal median nerve distribution, comprising the palmar surface of the thumb, index, middle, and radial half of the ring finger.
Researchers have identified 6 standardized clinical criteria for the diagnosis of CTS. Two criteria—numbness mostly in median nerve territory and nocturnal numbness—can be ascertained during the history of present illness.The other 4, detailed below, will be found during the physical exam.9
Thenar weakness or atrophy.9 Begin your evaluation by inspecting the thenar musculature for atrophic changes. Motor exam of intrinsic musculature innervated by the recurrent motor branch of the median nerve includes assessment of thumb abduction strength (assessed by applying resistance to the metacarpophalangeal joint [MCPJ] base towards the palm in the position of maximal abduction) and opposition strength (assessed by applying force to the MCPJ from the ulnar aspect).10
Positive Phalen’s test.9 Provocative testing for CTS includes Phalen’s test (sensitivity 43%-86%, specificity 48%-67%),11 which is an attempt to reproduce the numbness or tingling in the median nerve territory within 60 seconds of full wrist flexion. Ask the patient to hold his or her forearms vertically with elbows resting on the table (allowing gravity to flex the wrists),12 and to tell you if numbness or tingling occurs.
Positive Tinel’s sign.9 Tinel’s sign (sensitivity 45%-75%, specificity 48%-67%)11 is performed by lightly tapping the median nerve from the proximal to distal end over the carpal tunnel. The test is positive if paresthesia results. Provocative testing may also include Durkan’s test, also known as the carpal compression test. Durkan’s test (sensitivity 49%-89%, specificity 54%-96%)11 involves placing your thumb directly over the carpal tunnel and holding light compression for 60 seconds, or until paresthesia is reported.
Positive 2-point discrimination test.9 To assess CTS disease severity, use 2-point discrimination to evaluate the patient’s sensation qualitatively and quantitatively. Two-point discrimination can only be tested, however, if light touch sensation is intact. It is typically performed by lightly applying 2 caliper points at fixed distances sufficient to blanch the skin, but some clinicians have used other tools, such as a modified paperclip.13 The smallest distance at which the patient can detect 2 distinct stimuli is then recorded.
Researchers have reported an average of 3 to 5 mm for 2-point discrimination at the fingertipand a normal 2-point discrimination of 6 to 9 mm in the volar surface of the hand (TABLE 2).14,15
The scratch collapse test (sensitivity 64%, specificity 99%) is a supplemental exam that uses a different outcome measure to diagnose CTS.16 It involves lightly scratching the skin over the compressed carpal tunnel while the patient performs sustained resisted bilateral shoulder external rotation in an adducted position. A momentary loss of muscle resistance to external rotation indicates a positive test.
Pronator syndrome
Pronator syndrome (PS) is a proximal median neuropathy that may present in isolation or in combination with CTS as a double crush syndrome. Clinical symptoms include features of CTS and sensory paresthesias in the palm and distal forearm in the distribution of the palmar cutaneous branch of the median nerve. PS is commonly associated with volar proximal forearm pain exacerbated by repetitive activities involving pronation and supination.
PS is not easy to assess. Palpatory examination of a large supracondylar process at the distal humerus proximal to the medial epicondyle on its anteromedial aspect can be difficult, especially if the patient is overweight. And motor weakness is not a prominent feature. What’s more, power assessment of the pronator teres, flexor carpi radialis, and flexor digitorum superficialis may exacerbate symptoms.
Because the symptoms of PS and CTS may be the same, PS provocation maneuvers should be performed on patients with CTS symptoms and paresthesia involving the palm. Start by testing for Tinel’s sign over the pronator teres muscle, although this has been found to be positive in less than 50% of PS cases.17 Palpate the antecubital fossa and the proximal aspect of the pronator teres muscle to assess for discomfort or tenderness.
Pronator compression test. The pronator compression test has been found to be the most sensitive way to assess PS.18,19 This test involves direct compression of the proximal and radial edge of the pronator teres muscle belly along the proximal volar forearm with the thumb.20 It is performed bilaterally on supinated upper extremities, with the clinician applying pressure on each forearm simultaneously (FIGURE 2). If the symptoms in the hand are reproduced in ≤30 seconds, the test is positive. In a study of 10 patients with surgically confirmed PS, the pronator compression test was positive in every case.20,21
Resistance testing. You can also evaluate the pronator teres compression site by testing the patient’s ability to resist pronation with his or her elbow extended and the forearm in neutral position. To test for compression from the bicipital aponeurosis, ask the patient to flex the elbow to approximately 120° to 130° and apply active supinated resistance.22 Likewise, resistance of the long finger proximal interphalangeal joint (IPJ) to flexion—a maneuver performed with elbow fully extended—assesses compression from the fibrous arcade of the flexor digitorum superficialis (FDS).21 A positive resistance test will reproduce the reported symptoms.
Ulnar tunnel syndrome
Symptoms of UTS, which is much less common than CTS, include pain in the wrist and hand that is associated with paresthesia or numbness in the small finger and ulnar half of the ring finger. Patients may report difficulty with motor tasks involving grip and pinch strength or fatigue with prolonged action of the intrinsic muscles. Many also report an exacerbation of symptoms associated with increased wrist flexion or at night.
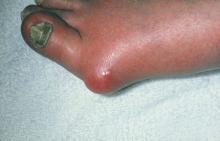
Evaluation of UTS requires a full assessment of the upper extremity, starting with observation of hand posture and muscle bulk to identify signs of chronic nerve compression. The contralateral extremity serves as a control to the neuropathic hand. Classically, chronic ulnar nerve compression leads to intrinsic muscle atrophy, evidenced by loss of topographical soft tissue bulk in the first dorsal web space, the palmar transverse metacarpal arch, and the hypothenar area.23 Ulnar motor nerve dysfunction is limited to the intrinsic muscles of the hand. The inverted pyramid sign, signified by atrophy of the transverse head of the adductor pollicis, is another visual aberrancy,24 as is clawing of the ring finger and small finger. The clawing, which involves hyperextension of the MCPJ and flexion of the proximal and distal IPJ, is commonly known as Duchenne’s sign. FIGURE 3 demonstrates hypothenar atrophy and the loss of muscle bulk in the first dorsal web space.
When you suspect UTS, palpate the wrist and hand in an attempt to locate a mass or area of tenderness. Not all patients with a volar ganglion cyst responsible for UTS present with a palpable mass, but tenderness along the radial aspect of the pisiform or an undefined fullness in this area may be noted.25 Any patient with a palpable mass should be tested for Tinel’s sign over the mass and undergo a thorough vascular assessment. Fracture of the hook of the hamate is indicated by tenderness in the region approximated by the intersection of Kaplan’s line and the proximal extension line from the ring finger.
Perform 2-point discrimination testing at the palmar distal aspect of the small finger and ulnar half of the ring finger. This tests the superficial sensory division of the ulnar nerve that travels within Guyon’s canal. Testing the dorsal ulnar cutaneous nerve involves the skin of the dorso-ulnar hand and dorsum of the long finger proximal to the IPJ. If this area is spared and the palmar distal ulnar digits are affected, compression within Guyon’s canal is likely. If both areas are affected, suspect a more proximal compression site at the cubital tunnel, as the dorsal ulnar cutaneous nerve branches proximal to Guyon’s canal.26
Ulnar motor nerve dysfunction in UTS is limited to the intrinsic muscles of the hand. Assessment of intrinsic muscle function is described in TABLE 3.27-32 It is important to become familiar with the tests and maneuvers described, but also to be aware that a comprehensive evaluation of ulnar nerve motor function requires a combination of tests.
Cubital tunnel syndrome
Cubital tunnel syndrome—the second most common peripheral neuropathy8—involves the proximal site of ulnar nerve compression in the upper extremity. Patients typically report symptoms similar to those of UTS, with sensory paresthesia in the ulnar digits and intrinsic weakness. To learn more about the symptoms, ask if the onset of pain or paresthesia is related to a particular elbow position, such as increased elbow flexion.
Notably, pain is usually not the initial complaint, unless the disease is advanced. This may be the reason atrophic intrinsic changes are 4 times more likely to be seen in patients with cubital tunnel syndrome than in those with CTS.33 You’re more likely to hear about vague motor problems, including hand clumsiness and difficulty with fine coordination of the fingers.34 Thus, it is important to evaluate patients for concurrent UTS and/or CTS, as well as for differentiation.
Focus on the elbow. Whenever you suspect cubital tunnel syndrome, pay special attention to the elbow. Examine the carrying angle of the elbow in relation to the contralateral extremity. Deformity may provide clues to a history of trauma. Assess the ulnar nerve during active flexion and extension to identify a subluxatable nerve at the cubital tunnel. Examine the ulno-humeral joint for crepitus, and palpate the joint line for large osteophytes and/or ganglion cysts.
Motor examination of the ulnar nerve primarily focuses on the intrinsic muscles detailed in TABLE 3,27-32 although the flexor carpi ulnaris (FCU) and flexor digitorum profundus (FDP) to the ring finger and small finger are also innervated by the ulnar nerve. The FCU mediates the power grip, and can be tested by resisted wrist flexion and ulnar deviation. Ring finger and small finger FDP strength should be examined by resistance testing at the distal IPJ.
Provocative testing of cubital tunnel syndrome includes Tinel’s sign (performed over the cubital tunnel), the elbow flexion test (performed with elbow in maximum flexion and wrist at neutral position and held for 60 seconds), and the pressure provocation test (performed by applying pressure to the ulnar nerve just proximal to the cubital tunnel with your index and long fingers for 60 seconds while the patient’s elbow is at 20° flexion with the forearm supinated). For each test, eliciting distal paresthesia in ulnar nerve territory is a positive result. The sensitivity of these tests ranges from 70% (Tinel’s sign) to 98% (combined elbow flexion and pressure); specificity ranges from 95% to 99%.35
The scratch collapse test for cubital tunnel syndrome evaluation is similar to the version used to assess for CTS; the only difference is the location of the site that is scratched, in this case the cubital tunnel. The reported sensitivity and specificity are 69% and 99%, respectively.11
Wartenberg’s syndrome
When a patient reports paresthesia or pain along the radial aspect of the forearm that radiates into the dorsal thumb and index and middle fingers, consider Wartenberg’s syndrome—compression of the superficial radial nerve. The condition, also known as “cheiralgia paresthetica,” may exist anywhere along the course of the nerve in the forearm,36 but the compression typically occurs 9 cm proximal to the radial styloid.
Assess the skin over the forearm and hand for evidence of prior trauma, surgical scars, or external compression sources, such as a watch.37-39 Then use palpation to identify superficial or deep masses along the nerve.40 Perform Tinel’s sign by lightly tapping along the nerve course from proximal to distal in the forearm. The test is positive if paresthesias are provoked distally.41,42
Motor function of the extremity is unaffected in Wartenberg’s syndrome, unless a prior traumatic injury occurred or a comorbidity exists. Comorbidities to consider include de Quervain’s tenosynovitis, cervical radiculopathy, injury to the lateral antebrachial cutaneous nerve, and CTS.
Gross sensory testing usually is negative, but 2-point discrimination may show diminished responses. Testing distribution includes the dorsal radial wrist and hand, dorsal thumb skin proximal to the IPJ, and the dorsal surface of the index, middle, and radial half of the ring finger proximal to the IPJ.
Proceed with caution. Provocative testing with a pronated forearm and a flexed and ulnar-deviated wrist may exacerbate symptoms,12,42 and Finkelstein’s maneuver (isolated ulnar deviation at the wrist to elicit pain over the first dorsal wrist compartment) and Phalen’s test may elicit false-positive results. Upper motor neuron exams (ie, deep tendon reflexes) and Hoffman’s sign (reflexive flexion of the terminal phalanges of the thumb and index finger induced by flicking or tapping the distal phalanx of the long finger) should be symmetric to the contralateral extremity.
In patients with more than one compression injury, Spurling’s sign (neck extension and lateral rotation towards the affected extremity) may induce paresthesia when combined with axial compression, while the shoulder abduction test (shoulder 90° abduction, with external rotation) may diminish reported paresthesia. In those for whom Wartenberg’s syndrome is their only compression injury, however, these provocative maneuvers will be negative.
Anterior interosseous nerve syndrome
A rare clinical entity that affects the anterior interosseous innervated muscles in the forearm, anterior interosseous nerve syndrome (AINS)’s etiology is unclear. But it is likely related to a spontaneous neuritis rather than a compressive etiology.43 Paresis or paralysis of the flexor pollicis longus (FPL) and the flexor digitorum profundus (FDP) of the index and long finger is its hallmark, but patients may report vague forearm pain associated with weakness or absence of function.
Examination begins with a visual assessment and palpation of the forearm and hand for atrophy or a space-occupying lesion. Notably, sensory function of the hand should remain at baseline despite the motor dysfunction, as the anterior interosseous is purely a motor nerve.
Motor testing should focus on the median and anterior interosseous innervated muscles.
The FPL is tested by isolated thumb IPJ flexion and the Kiloh and Nevin sign—the inability to make an “OK” sign with the thumb and index finger.44 Assess pinch grasp with a maximally flexed thumb IPJ and distal IPJ of the index finger. Anterior interosseous nerve dysfunction results in a flattened pinch without IPJ or distal IPJ flexion, which increases the contact of the thumb and index finger pulp.45 In a series of 14 patients, researchers reported complete paralysis of FPL and FDP index finger in 5 patients, isolated paralysis of FPL in 7 patients, and isolated paralysis of FDP index and long fingers in 2 patients. None had isolated paralysis of the FDP long finger.46FIGURE 4 shows complete (A) and incomplete (B) paralysis.
Evaluation of AINS also includes assessment of FPL and FDP tendon integrity with passive tenodesis. The appearance of the hand at rest should reflect the natural digital cascade. In a completely relaxed or anesthetized hand, the forearm is placed in wrist supination and extension, allowing gravity to extend the wrist and placing the thumb MCPJ at 30° flexion, the index finger MCPJ at 40°, and the small finger MCPJ at 70°. The thumb IPJ should approximate the radial fingertip pulp of the index finger. The forearm is then pronated and flexed at the wrist, straightening the thumb MCPJ and producing a mild flexion cascade at the proximal and distal IPJ of the index, long, ring, and small fingers within 20° of full extension. This dynamic exercise is used to confirm that the patient has an intact tenodesis effect, thus excluding tendon lacerations or ruptures from the differential diagnosis. In one study, in 9 of 33 cases of partial or complete isolated index finger FPL or FDP, paralysis was initially diagnosed as tendon rupture.47
When to consider electrodiagnostic testing
Electrodiagnostic testing—a combination of electromyography and nerve conduction studies to assess the status of a peripheral nerve48—provides objective data that can help diagnose a challenging presentation, rule out a competing diagnosis, or clarify an atypical clinical picture or vague subjective history. This type of testing is also used to localize the entrapment site, identify a patient with polyneuropathy or brachial plexopathy, and assess the severity of nerve injury or presence of a double crush syndrome.49,50 Any patient with signs and symptoms of a compression neuropathy and supportive findings on physical exam should be referred for electrodiagnostic testing and/or to a surgeon specializing in treating these conditions.
CORRESPONDENCE
Kyle J. MacGillis, MD, University of Illinois at Chicago, Department of Orthopaedic Surgery, 835 South Wolcott Avenue, M/C 844, Chicago, IL 60612; [email protected].
› Use provocative testing to confirm a suspected diagnosis in a patient who presents with peripheral entrapment mononeuropathy. B
› Consider electrodiagnostic testing for help in diagnosing a challenging presentation, ruling out a competing diagnosis, or clarifying an atypical clinical picture or vague subjective history. A
› Evaluate any patient who presents with non-anatomic nerve distribution of symptoms—eg, burning, numbness, and tingling of the entire hand—for a metabolic, rather than an entrapment, neuropathy. B
Strength of recommendation (SOR)
A Good-quality patient-oriented evidence
B Inconsistent or limited-quality patient-oriented evidence
C Consensus, usual practice, opinion, disease-oriented evidence, case series
Neuropathic hand complaints—for which patients typically seek medical attention when the pain or paresthesia starts to interfere with their daily routine—are common and diverse. The ability to assess and accurately diagnose upper extremity compression neuropathies is critical for physicians in primary care.
Assessment starts, of course, with a thorough history of the present illness and past medical history, which helps define a broad differential diagnosis and identify comorbidities. Physical examination, including judicious use of provocative testing, allows you to objectively identify the pathologic deficit, evaluate function and coordination of multiple organ systems, and detect nerve dysfunction. The results determine whether additional tools, such as electrodiagnostic testing, are needed.
We’ve created this guide, detailed in the text, tables, and figures that follow, to help you hone your ability to accurately diagnose patients who present with compression neuropathies of the hand.
The medical history: Knowing what to ask
To clearly define a patient’s symptoms and disability, start with a thorough history of the presenting complaint.
Inquire about symptom onset and chronicity. Did the pain or paresthesia begin after an injury? Are the symptoms associated with repetitive use of the extremity? Do they occur at night?
Pinpoint the location or distribution of pain or paresthesia. It is paramount to identify the affected nerve.1,2 Ask patients to complete a hand or upper extremity profile documenting location and/or type of numbness, tingling, or decreased sensation. A diagram of the peripheral nerves responsible for sensory innervation of the hand (FIGURE 1) is an effective way to screen individuals at high risk of carpal tunnel syndrome (CTS) or ulnar tunnel syndrome (UTS).1,2
A patient report such as, “My whole hand is numb,” calls for a follow-up question to determine whether the little finger is affected,3 which would indicate that the ulnar nerve, rather than just the median nerve, is involved. And if a patient reports feeling as if he or she is wearing gloves or mittens, it is essential to consider the possibility of a systemic neuropathy rather than a single peripheral neuropathy.3
Gather basic patient information. Inquire about hand dominance, occupation, and baseline function, any or all of which may be critical in the assessment and initiation of treatment.4,5
Review systemic conditions and medications
A broad range of comorbidities, such as cervical radiculopathy, diabetes, hypothyroidism, and vitamin deficiencies (TABLE 1),6,7 may be responsible for neuropathic hand complaints, and a thorough review of systemic complaints and past medical history is critical. Include a medication history and a review of prior procedures, such as post-traumatic surgeries of the hand or upper extremity or nerve decompression surgeries, which may provide additional insight into disease etiology.
Symptoms guide physical exam, provocative testing
A physical examination, including provocative testing, follows based on reported symptoms, medical history, and suspected source of nerve compression.
Carpal tunnel syndrome
CTS is the most common peripheral neuropathy.8 Patients often report nocturnal pain or paresthesia in the distal median nerve distribution, comprising the palmar surface of the thumb, index, middle, and radial half of the ring finger.
Researchers have identified 6 standardized clinical criteria for the diagnosis of CTS. Two criteria—numbness mostly in median nerve territory and nocturnal numbness—can be ascertained during the history of present illness.The other 4, detailed below, will be found during the physical exam.9
Thenar weakness or atrophy.9 Begin your evaluation by inspecting the thenar musculature for atrophic changes. Motor exam of intrinsic musculature innervated by the recurrent motor branch of the median nerve includes assessment of thumb abduction strength (assessed by applying resistance to the metacarpophalangeal joint [MCPJ] base towards the palm in the position of maximal abduction) and opposition strength (assessed by applying force to the MCPJ from the ulnar aspect).10
Positive Phalen’s test.9 Provocative testing for CTS includes Phalen’s test (sensitivity 43%-86%, specificity 48%-67%),11 which is an attempt to reproduce the numbness or tingling in the median nerve territory within 60 seconds of full wrist flexion. Ask the patient to hold his or her forearms vertically with elbows resting on the table (allowing gravity to flex the wrists),12 and to tell you if numbness or tingling occurs.
Positive Tinel’s sign.9 Tinel’s sign (sensitivity 45%-75%, specificity 48%-67%)11 is performed by lightly tapping the median nerve from the proximal to distal end over the carpal tunnel. The test is positive if paresthesia results. Provocative testing may also include Durkan’s test, also known as the carpal compression test. Durkan’s test (sensitivity 49%-89%, specificity 54%-96%)11 involves placing your thumb directly over the carpal tunnel and holding light compression for 60 seconds, or until paresthesia is reported.
Positive 2-point discrimination test.9 To assess CTS disease severity, use 2-point discrimination to evaluate the patient’s sensation qualitatively and quantitatively. Two-point discrimination can only be tested, however, if light touch sensation is intact. It is typically performed by lightly applying 2 caliper points at fixed distances sufficient to blanch the skin, but some clinicians have used other tools, such as a modified paperclip.13 The smallest distance at which the patient can detect 2 distinct stimuli is then recorded.
Researchers have reported an average of 3 to 5 mm for 2-point discrimination at the fingertipand a normal 2-point discrimination of 6 to 9 mm in the volar surface of the hand (TABLE 2).14,15
The scratch collapse test (sensitivity 64%, specificity 99%) is a supplemental exam that uses a different outcome measure to diagnose CTS.16 It involves lightly scratching the skin over the compressed carpal tunnel while the patient performs sustained resisted bilateral shoulder external rotation in an adducted position. A momentary loss of muscle resistance to external rotation indicates a positive test.
Pronator syndrome
Pronator syndrome (PS) is a proximal median neuropathy that may present in isolation or in combination with CTS as a double crush syndrome. Clinical symptoms include features of CTS and sensory paresthesias in the palm and distal forearm in the distribution of the palmar cutaneous branch of the median nerve. PS is commonly associated with volar proximal forearm pain exacerbated by repetitive activities involving pronation and supination.
PS is not easy to assess. Palpatory examination of a large supracondylar process at the distal humerus proximal to the medial epicondyle on its anteromedial aspect can be difficult, especially if the patient is overweight. And motor weakness is not a prominent feature. What’s more, power assessment of the pronator teres, flexor carpi radialis, and flexor digitorum superficialis may exacerbate symptoms.
Because the symptoms of PS and CTS may be the same, PS provocation maneuvers should be performed on patients with CTS symptoms and paresthesia involving the palm. Start by testing for Tinel’s sign over the pronator teres muscle, although this has been found to be positive in less than 50% of PS cases.17 Palpate the antecubital fossa and the proximal aspect of the pronator teres muscle to assess for discomfort or tenderness.
Pronator compression test. The pronator compression test has been found to be the most sensitive way to assess PS.18,19 This test involves direct compression of the proximal and radial edge of the pronator teres muscle belly along the proximal volar forearm with the thumb.20 It is performed bilaterally on supinated upper extremities, with the clinician applying pressure on each forearm simultaneously (FIGURE 2). If the symptoms in the hand are reproduced in ≤30 seconds, the test is positive. In a study of 10 patients with surgically confirmed PS, the pronator compression test was positive in every case.20,21
Resistance testing. You can also evaluate the pronator teres compression site by testing the patient’s ability to resist pronation with his or her elbow extended and the forearm in neutral position. To test for compression from the bicipital aponeurosis, ask the patient to flex the elbow to approximately 120° to 130° and apply active supinated resistance.22 Likewise, resistance of the long finger proximal interphalangeal joint (IPJ) to flexion—a maneuver performed with elbow fully extended—assesses compression from the fibrous arcade of the flexor digitorum superficialis (FDS).21 A positive resistance test will reproduce the reported symptoms.
Ulnar tunnel syndrome
Symptoms of UTS, which is much less common than CTS, include pain in the wrist and hand that is associated with paresthesia or numbness in the small finger and ulnar half of the ring finger. Patients may report difficulty with motor tasks involving grip and pinch strength or fatigue with prolonged action of the intrinsic muscles. Many also report an exacerbation of symptoms associated with increased wrist flexion or at night.
Evaluation of UTS requires a full assessment of the upper extremity, starting with observation of hand posture and muscle bulk to identify signs of chronic nerve compression. The contralateral extremity serves as a control to the neuropathic hand. Classically, chronic ulnar nerve compression leads to intrinsic muscle atrophy, evidenced by loss of topographical soft tissue bulk in the first dorsal web space, the palmar transverse metacarpal arch, and the hypothenar area.23 Ulnar motor nerve dysfunction is limited to the intrinsic muscles of the hand. The inverted pyramid sign, signified by atrophy of the transverse head of the adductor pollicis, is another visual aberrancy,24 as is clawing of the ring finger and small finger. The clawing, which involves hyperextension of the MCPJ and flexion of the proximal and distal IPJ, is commonly known as Duchenne’s sign. FIGURE 3 demonstrates hypothenar atrophy and the loss of muscle bulk in the first dorsal web space.
When you suspect UTS, palpate the wrist and hand in an attempt to locate a mass or area of tenderness. Not all patients with a volar ganglion cyst responsible for UTS present with a palpable mass, but tenderness along the radial aspect of the pisiform or an undefined fullness in this area may be noted.25 Any patient with a palpable mass should be tested for Tinel’s sign over the mass and undergo a thorough vascular assessment. Fracture of the hook of the hamate is indicated by tenderness in the region approximated by the intersection of Kaplan’s line and the proximal extension line from the ring finger.
Perform 2-point discrimination testing at the palmar distal aspect of the small finger and ulnar half of the ring finger. This tests the superficial sensory division of the ulnar nerve that travels within Guyon’s canal. Testing the dorsal ulnar cutaneous nerve involves the skin of the dorso-ulnar hand and dorsum of the long finger proximal to the IPJ. If this area is spared and the palmar distal ulnar digits are affected, compression within Guyon’s canal is likely. If both areas are affected, suspect a more proximal compression site at the cubital tunnel, as the dorsal ulnar cutaneous nerve branches proximal to Guyon’s canal.26
Ulnar motor nerve dysfunction in UTS is limited to the intrinsic muscles of the hand. Assessment of intrinsic muscle function is described in TABLE 3.27-32 It is important to become familiar with the tests and maneuvers described, but also to be aware that a comprehensive evaluation of ulnar nerve motor function requires a combination of tests.
Cubital tunnel syndrome
Cubital tunnel syndrome—the second most common peripheral neuropathy8—involves the proximal site of ulnar nerve compression in the upper extremity. Patients typically report symptoms similar to those of UTS, with sensory paresthesia in the ulnar digits and intrinsic weakness. To learn more about the symptoms, ask if the onset of pain or paresthesia is related to a particular elbow position, such as increased elbow flexion.
Notably, pain is usually not the initial complaint, unless the disease is advanced. This may be the reason atrophic intrinsic changes are 4 times more likely to be seen in patients with cubital tunnel syndrome than in those with CTS.33 You’re more likely to hear about vague motor problems, including hand clumsiness and difficulty with fine coordination of the fingers.34 Thus, it is important to evaluate patients for concurrent UTS and/or CTS, as well as for differentiation.
Focus on the elbow. Whenever you suspect cubital tunnel syndrome, pay special attention to the elbow. Examine the carrying angle of the elbow in relation to the contralateral extremity. Deformity may provide clues to a history of trauma. Assess the ulnar nerve during active flexion and extension to identify a subluxatable nerve at the cubital tunnel. Examine the ulno-humeral joint for crepitus, and palpate the joint line for large osteophytes and/or ganglion cysts.
Motor examination of the ulnar nerve primarily focuses on the intrinsic muscles detailed in TABLE 3,27-32 although the flexor carpi ulnaris (FCU) and flexor digitorum profundus (FDP) to the ring finger and small finger are also innervated by the ulnar nerve. The FCU mediates the power grip, and can be tested by resisted wrist flexion and ulnar deviation. Ring finger and small finger FDP strength should be examined by resistance testing at the distal IPJ.
Provocative testing of cubital tunnel syndrome includes Tinel’s sign (performed over the cubital tunnel), the elbow flexion test (performed with elbow in maximum flexion and wrist at neutral position and held for 60 seconds), and the pressure provocation test (performed by applying pressure to the ulnar nerve just proximal to the cubital tunnel with your index and long fingers for 60 seconds while the patient’s elbow is at 20° flexion with the forearm supinated). For each test, eliciting distal paresthesia in ulnar nerve territory is a positive result. The sensitivity of these tests ranges from 70% (Tinel’s sign) to 98% (combined elbow flexion and pressure); specificity ranges from 95% to 99%.35
The scratch collapse test for cubital tunnel syndrome evaluation is similar to the version used to assess for CTS; the only difference is the location of the site that is scratched, in this case the cubital tunnel. The reported sensitivity and specificity are 69% and 99%, respectively.11
Wartenberg’s syndrome
When a patient reports paresthesia or pain along the radial aspect of the forearm that radiates into the dorsal thumb and index and middle fingers, consider Wartenberg’s syndrome—compression of the superficial radial nerve. The condition, also known as “cheiralgia paresthetica,” may exist anywhere along the course of the nerve in the forearm,36 but the compression typically occurs 9 cm proximal to the radial styloid.
Assess the skin over the forearm and hand for evidence of prior trauma, surgical scars, or external compression sources, such as a watch.37-39 Then use palpation to identify superficial or deep masses along the nerve.40 Perform Tinel’s sign by lightly tapping along the nerve course from proximal to distal in the forearm. The test is positive if paresthesias are provoked distally.41,42
Motor function of the extremity is unaffected in Wartenberg’s syndrome, unless a prior traumatic injury occurred or a comorbidity exists. Comorbidities to consider include de Quervain’s tenosynovitis, cervical radiculopathy, injury to the lateral antebrachial cutaneous nerve, and CTS.
Gross sensory testing usually is negative, but 2-point discrimination may show diminished responses. Testing distribution includes the dorsal radial wrist and hand, dorsal thumb skin proximal to the IPJ, and the dorsal surface of the index, middle, and radial half of the ring finger proximal to the IPJ.
Proceed with caution. Provocative testing with a pronated forearm and a flexed and ulnar-deviated wrist may exacerbate symptoms,12,42 and Finkelstein’s maneuver (isolated ulnar deviation at the wrist to elicit pain over the first dorsal wrist compartment) and Phalen’s test may elicit false-positive results. Upper motor neuron exams (ie, deep tendon reflexes) and Hoffman’s sign (reflexive flexion of the terminal phalanges of the thumb and index finger induced by flicking or tapping the distal phalanx of the long finger) should be symmetric to the contralateral extremity.
In patients with more than one compression injury, Spurling’s sign (neck extension and lateral rotation towards the affected extremity) may induce paresthesia when combined with axial compression, while the shoulder abduction test (shoulder 90° abduction, with external rotation) may diminish reported paresthesia. In those for whom Wartenberg’s syndrome is their only compression injury, however, these provocative maneuvers will be negative.
Anterior interosseous nerve syndrome
A rare clinical entity that affects the anterior interosseous innervated muscles in the forearm, anterior interosseous nerve syndrome (AINS)’s etiology is unclear. But it is likely related to a spontaneous neuritis rather than a compressive etiology.43 Paresis or paralysis of the flexor pollicis longus (FPL) and the flexor digitorum profundus (FDP) of the index and long finger is its hallmark, but patients may report vague forearm pain associated with weakness or absence of function.
Examination begins with a visual assessment and palpation of the forearm and hand for atrophy or a space-occupying lesion. Notably, sensory function of the hand should remain at baseline despite the motor dysfunction, as the anterior interosseous is purely a motor nerve.
Motor testing should focus on the median and anterior interosseous innervated muscles.
The FPL is tested by isolated thumb IPJ flexion and the Kiloh and Nevin sign—the inability to make an “OK” sign with the thumb and index finger.44 Assess pinch grasp with a maximally flexed thumb IPJ and distal IPJ of the index finger. Anterior interosseous nerve dysfunction results in a flattened pinch without IPJ or distal IPJ flexion, which increases the contact of the thumb and index finger pulp.45 In a series of 14 patients, researchers reported complete paralysis of FPL and FDP index finger in 5 patients, isolated paralysis of FPL in 7 patients, and isolated paralysis of FDP index and long fingers in 2 patients. None had isolated paralysis of the FDP long finger.46FIGURE 4 shows complete (A) and incomplete (B) paralysis.
Evaluation of AINS also includes assessment of FPL and FDP tendon integrity with passive tenodesis. The appearance of the hand at rest should reflect the natural digital cascade. In a completely relaxed or anesthetized hand, the forearm is placed in wrist supination and extension, allowing gravity to extend the wrist and placing the thumb MCPJ at 30° flexion, the index finger MCPJ at 40°, and the small finger MCPJ at 70°. The thumb IPJ should approximate the radial fingertip pulp of the index finger. The forearm is then pronated and flexed at the wrist, straightening the thumb MCPJ and producing a mild flexion cascade at the proximal and distal IPJ of the index, long, ring, and small fingers within 20° of full extension. This dynamic exercise is used to confirm that the patient has an intact tenodesis effect, thus excluding tendon lacerations or ruptures from the differential diagnosis. In one study, in 9 of 33 cases of partial or complete isolated index finger FPL or FDP, paralysis was initially diagnosed as tendon rupture.47
When to consider electrodiagnostic testing
Electrodiagnostic testing—a combination of electromyography and nerve conduction studies to assess the status of a peripheral nerve48—provides objective data that can help diagnose a challenging presentation, rule out a competing diagnosis, or clarify an atypical clinical picture or vague subjective history. This type of testing is also used to localize the entrapment site, identify a patient with polyneuropathy or brachial plexopathy, and assess the severity of nerve injury or presence of a double crush syndrome.49,50 Any patient with signs and symptoms of a compression neuropathy and supportive findings on physical exam should be referred for electrodiagnostic testing and/or to a surgeon specializing in treating these conditions.
CORRESPONDENCE
Kyle J. MacGillis, MD, University of Illinois at Chicago, Department of Orthopaedic Surgery, 835 South Wolcott Avenue, M/C 844, Chicago, IL 60612; [email protected].
1. Katz JN, Stirrat CR, Larson MG, et al. A self-administered hand symptom diagram for the diagnosis and epidemiologic study of carpal tunnel syndrome. J Rheumatol. 1990;17:1495-1498.
2. Werner RA, Chiodo T, Spiegelberg T, et al. Use of hand diagrams in screening for ulnar neuropathy: comparison with electrodiagnostic studies. Muscle Nerve. 2012;46:891-894.
3. Dellon AL. Patient evaluation and management considerations in nerve compression. Hand Clin. 1992;8:229-239.
4. Kaji H, Honma H, Usui M, et al. Hypothenar hammer syndrome in workers occupationally exposed to vibrating tools. J Hand Surg Br. 1993;18:761-766.
5. Blum J. Examination and interface with the musician. Hand Clin. 2003;19:223-230.
6. Azhary H, Farooq MU, Bhanushali M, et al. Peripheral neuropathy: differential diagnosis and management. Am Fam Physician. 2010;81:887-892.
7. Craig, AS, Richardson JK. Acquired peripheral neuropathy. Phys Med Rehabil Clin N Am. 2003;14:365-386.
8. Latinovic R, Gulliford MC, Hughes RA. Incidence of common compressive neuropathies in primary care. J Neurol Neurosurg Psychiatry. 2006;77:263-265.
9. Graham B, Regehr G, Naglie G, et al. Development and validation of diagnostic criteria for carpal tunnel syndrome. J Hand Surg Am. 2006;31:919-924.
10. Foucher G, Malizos C, Sammut D, et al. Primary palmaris longus transfer as an opponensplasty in carpal tunnel release: a series of 73 cases. J Hand Surg Br. 1991;16:56-60.
11. Massy-Westropp N, Grimmer K, Bain G. A systematic review of the clinical diagnostic tests for carpal tunnel syndrome. J Hand Surg Am. 2000;25:120-127.
12. Dellon AL, Mackinnon SE. Radial sensory nerve entrapment in the forearm. J Hand Surg Am. 1986;11:199-205.
13. Mackinnon SE, Dellon AL. Two-point discrimination tester. J Hand Surg (Am). 1985;10:906-907.
14. Louis DS, Greene TL, Jacobson KE, et al. Evaluation of normal values for stationary and moving two-point discrimination in the hand. J Hand Surg Am. 1984;9:552-555.
15. Omer GE Jr. Physical diagnosis of peripheral nerve injuries. Orthop Clin North Am. 1981;12:207-228.
16. Cheng CJ, Mackinnon-Patterson B, Beck JL, et al. Scratch collapse test for evaluation of carpal and cubital tunnel syndrome. J Hand Surg Am. 2008;33:1518-1524.
17. Haussmann P, Patel MR. Intraepineurial constriction of nerve fascicles in pronator syndrome and anterior interosseous nerve syndrome. Orthop Clin North Am. 1996;27:339-344.
18. Lee AK, Khorsandi M, Nurbhai N, et al. Endoscopically assisted decompression for pronator syndrome. J Hand Surg Am. 2012;37:1173-1179.
19. Lee MJ, LaStayo PC. Pronator syndrome and other nerve compressions that mimic carpal tunnel syndrome. J Orthop Sports Phys Ther. 2004;34:601-609.
20. Gainor BJ. The pronator compression test revisited: a forgotten physical sign. Orthop Rev. 1990;19:888-892.
21. Rehak DC. Pronator syndrome. Clin Sports Med. 2001;20:531-540.
22. Eversmann WW. Compression and entrapment neuropathies of the upper extremity. J Hand Surg Am. 1983;8:759-766.
23. Masse L. Contribution a l’etude de l’achon des interosseus. J Med (Bordeaux). 1916;46:198-200.
24. Draeger RW, Stern PJ. The inverted pyramid sign and other eponymous signs of ulnar nerve palsy. J Hand Surg Am. 2014;39:2517-2520.
25. Wang B, Zhao Y, Lu A, et al. Ulnar nerve deep branch compression by a ganglion: a review of nine cases. Injury. 2014;45:1126-1130.
26. Polatsch DB, Melone CP, Beldner S, et al. Ulnar nerve anatomy. Hand Clin. 2007;23:283-289.
27. Buschbacher R. Side-to-side confrontational strength-testing for weakness of the intrinsic muscles of the hand. J Bone Joint Surg Am. 1997;79:401-405.
28. Wartenberg R. A sign of ulnar palsy. JAMA. 1939;112:1688.
29. Earle A, Vlastou C. Crossed fingers and other tests of ulnar nerve motor function. J Hand Surg Am. 1980;5:560-565.
30. Tsujino A, Macnicol M. Finger flexion sign for ulnar neuropathy. J Hand Surg Br. 1998;23:240-241.
31. Mannerfelt L. Studies on the hand in ulnar nerve paralysis: a clinical-experimental investigation in normal and anomalous innervations. Acta Orthop Scand. 1966;87(Suppl):S118-S129.
32. Froment J. La prehension dans les paralysies du nerf cubital et Le Signe du Pouce. Presse Med. 1915;23:409.
33. Mallette P, Zhao M, Zurakowski D, et al. Muscle atrophy at diagnosis of carpal and cubital tunnel syndrome. J Hand Surg Am. 2007;32:855-858.
34. Huang JH, Samadani U, Zagar EL. Ulnar nerve entrapment neuropathy at the elbow: simple decompression. Neurosurgery. 2004;55:1150-1153.
35. Novak CB, Lee GW, Mackinnon SE, et al. Provocative testing for cubital tunnel syndrome. J Hand Surg Am. 1994;19:817-820.
36. Auerbach DM, Collins ED, Kunkle KL, et al. The radial sensory nerve. an anatomic study. Clin Orthop Relat Res. 1994;308:241-249.
37. Braidwood AS. Superficial radial neuropathy. J Bone Joint Surg Br. 1975;57:380-383.
38. Bierman HR. Nerve compression due to a tight watchband. N Engl J Med. 1959;261:237-238.
39. Linscheid RL. Injuries to radial nerve at wrist. Arch Surg. 1965;91:942-946.
40. Hermansdorfer JD, Greider JL, Dell PC. A case report of a compressive neuropathy of the radial sensory nerve caused by a ganglion cyst at the elbow. Orthopedics. 1986;9:1005-1006.
41. Poppinchalk SP, Schaffer AA. Physical examination of the upper extremity compressive neuropathies. Orthop Clin N Am. 2012;43:417-430.
42. Posner MA. Compressive neuropathies of the median and radial nerves at the elbow. Clin Sports Med. 1990;9:343-363.
43. Dang AC, Rodner CM. Unusual compression neuropathies of the forearm. Part II: median nerve. J Hand Surg Am. 2009;34A:1915-1920.
44. Kiloh LG, Nevin S. Isolated neuritis of the anterior interosseous nerve. Br Med J. 1952;1:850-851.
45. Park IJ, Roh YT, Jeong C, et al. Spontaneous anterior interosseous nerve syndrome: clinical analysis and eleven surgical cases. J Plast Surg Hand Surg. 2013;47:519-523.
46. Ulrich D, Piatkowski A, Pallua N. Anterior interosseous nerve syndrome: retrospective analysis of 14 patients. Arch Orthop Trauma Surg. 2011;131:1561-1565.
47. Hill NA, Howard FM, Huffer BR. The incomplete anterior interosseous nerve syndrome. J Hand Surg Am. 1985;10:4-16.
48. Bergquist ER, Hammert WC. Timing and appropriate use of electrodiagnostic studies. Hand Clin. 2013;29:363-370.
49. Lo JK, Finestone HM, Gilbert K, et al. Community-based referrals for electrodiagnostic studies in patients with possible carpal tunnel syndrome: what is the diagnosis? Arch Phys Med Rehabil. 2002;83:598-603.
50. Kane NM, Oware A. Nerve conduction and electromyography studies. J Neurol. 2012;259:1502-1508.
1. Katz JN, Stirrat CR, Larson MG, et al. A self-administered hand symptom diagram for the diagnosis and epidemiologic study of carpal tunnel syndrome. J Rheumatol. 1990;17:1495-1498.
2. Werner RA, Chiodo T, Spiegelberg T, et al. Use of hand diagrams in screening for ulnar neuropathy: comparison with electrodiagnostic studies. Muscle Nerve. 2012;46:891-894.
3. Dellon AL. Patient evaluation and management considerations in nerve compression. Hand Clin. 1992;8:229-239.
4. Kaji H, Honma H, Usui M, et al. Hypothenar hammer syndrome in workers occupationally exposed to vibrating tools. J Hand Surg Br. 1993;18:761-766.
5. Blum J. Examination and interface with the musician. Hand Clin. 2003;19:223-230.
6. Azhary H, Farooq MU, Bhanushali M, et al. Peripheral neuropathy: differential diagnosis and management. Am Fam Physician. 2010;81:887-892.
7. Craig, AS, Richardson JK. Acquired peripheral neuropathy. Phys Med Rehabil Clin N Am. 2003;14:365-386.
8. Latinovic R, Gulliford MC, Hughes RA. Incidence of common compressive neuropathies in primary care. J Neurol Neurosurg Psychiatry. 2006;77:263-265.
9. Graham B, Regehr G, Naglie G, et al. Development and validation of diagnostic criteria for carpal tunnel syndrome. J Hand Surg Am. 2006;31:919-924.
10. Foucher G, Malizos C, Sammut D, et al. Primary palmaris longus transfer as an opponensplasty in carpal tunnel release: a series of 73 cases. J Hand Surg Br. 1991;16:56-60.
11. Massy-Westropp N, Grimmer K, Bain G. A systematic review of the clinical diagnostic tests for carpal tunnel syndrome. J Hand Surg Am. 2000;25:120-127.
12. Dellon AL, Mackinnon SE. Radial sensory nerve entrapment in the forearm. J Hand Surg Am. 1986;11:199-205.
13. Mackinnon SE, Dellon AL. Two-point discrimination tester. J Hand Surg (Am). 1985;10:906-907.
14. Louis DS, Greene TL, Jacobson KE, et al. Evaluation of normal values for stationary and moving two-point discrimination in the hand. J Hand Surg Am. 1984;9:552-555.
15. Omer GE Jr. Physical diagnosis of peripheral nerve injuries. Orthop Clin North Am. 1981;12:207-228.
16. Cheng CJ, Mackinnon-Patterson B, Beck JL, et al. Scratch collapse test for evaluation of carpal and cubital tunnel syndrome. J Hand Surg Am. 2008;33:1518-1524.
17. Haussmann P, Patel MR. Intraepineurial constriction of nerve fascicles in pronator syndrome and anterior interosseous nerve syndrome. Orthop Clin North Am. 1996;27:339-344.
18. Lee AK, Khorsandi M, Nurbhai N, et al. Endoscopically assisted decompression for pronator syndrome. J Hand Surg Am. 2012;37:1173-1179.
19. Lee MJ, LaStayo PC. Pronator syndrome and other nerve compressions that mimic carpal tunnel syndrome. J Orthop Sports Phys Ther. 2004;34:601-609.
20. Gainor BJ. The pronator compression test revisited: a forgotten physical sign. Orthop Rev. 1990;19:888-892.
21. Rehak DC. Pronator syndrome. Clin Sports Med. 2001;20:531-540.
22. Eversmann WW. Compression and entrapment neuropathies of the upper extremity. J Hand Surg Am. 1983;8:759-766.
23. Masse L. Contribution a l’etude de l’achon des interosseus. J Med (Bordeaux). 1916;46:198-200.
24. Draeger RW, Stern PJ. The inverted pyramid sign and other eponymous signs of ulnar nerve palsy. J Hand Surg Am. 2014;39:2517-2520.
25. Wang B, Zhao Y, Lu A, et al. Ulnar nerve deep branch compression by a ganglion: a review of nine cases. Injury. 2014;45:1126-1130.
26. Polatsch DB, Melone CP, Beldner S, et al. Ulnar nerve anatomy. Hand Clin. 2007;23:283-289.
27. Buschbacher R. Side-to-side confrontational strength-testing for weakness of the intrinsic muscles of the hand. J Bone Joint Surg Am. 1997;79:401-405.
28. Wartenberg R. A sign of ulnar palsy. JAMA. 1939;112:1688.
29. Earle A, Vlastou C. Crossed fingers and other tests of ulnar nerve motor function. J Hand Surg Am. 1980;5:560-565.
30. Tsujino A, Macnicol M. Finger flexion sign for ulnar neuropathy. J Hand Surg Br. 1998;23:240-241.
31. Mannerfelt L. Studies on the hand in ulnar nerve paralysis: a clinical-experimental investigation in normal and anomalous innervations. Acta Orthop Scand. 1966;87(Suppl):S118-S129.
32. Froment J. La prehension dans les paralysies du nerf cubital et Le Signe du Pouce. Presse Med. 1915;23:409.
33. Mallette P, Zhao M, Zurakowski D, et al. Muscle atrophy at diagnosis of carpal and cubital tunnel syndrome. J Hand Surg Am. 2007;32:855-858.
34. Huang JH, Samadani U, Zagar EL. Ulnar nerve entrapment neuropathy at the elbow: simple decompression. Neurosurgery. 2004;55:1150-1153.
35. Novak CB, Lee GW, Mackinnon SE, et al. Provocative testing for cubital tunnel syndrome. J Hand Surg Am. 1994;19:817-820.
36. Auerbach DM, Collins ED, Kunkle KL, et al. The radial sensory nerve. an anatomic study. Clin Orthop Relat Res. 1994;308:241-249.
37. Braidwood AS. Superficial radial neuropathy. J Bone Joint Surg Br. 1975;57:380-383.
38. Bierman HR. Nerve compression due to a tight watchband. N Engl J Med. 1959;261:237-238.
39. Linscheid RL. Injuries to radial nerve at wrist. Arch Surg. 1965;91:942-946.
40. Hermansdorfer JD, Greider JL, Dell PC. A case report of a compressive neuropathy of the radial sensory nerve caused by a ganglion cyst at the elbow. Orthopedics. 1986;9:1005-1006.
41. Poppinchalk SP, Schaffer AA. Physical examination of the upper extremity compressive neuropathies. Orthop Clin N Am. 2012;43:417-430.
42. Posner MA. Compressive neuropathies of the median and radial nerves at the elbow. Clin Sports Med. 1990;9:343-363.
43. Dang AC, Rodner CM. Unusual compression neuropathies of the forearm. Part II: median nerve. J Hand Surg Am. 2009;34A:1915-1920.
44. Kiloh LG, Nevin S. Isolated neuritis of the anterior interosseous nerve. Br Med J. 1952;1:850-851.
45. Park IJ, Roh YT, Jeong C, et al. Spontaneous anterior interosseous nerve syndrome: clinical analysis and eleven surgical cases. J Plast Surg Hand Surg. 2013;47:519-523.
46. Ulrich D, Piatkowski A, Pallua N. Anterior interosseous nerve syndrome: retrospective analysis of 14 patients. Arch Orthop Trauma Surg. 2011;131:1561-1565.
47. Hill NA, Howard FM, Huffer BR. The incomplete anterior interosseous nerve syndrome. J Hand Surg Am. 1985;10:4-16.
48. Bergquist ER, Hammert WC. Timing and appropriate use of electrodiagnostic studies. Hand Clin. 2013;29:363-370.
49. Lo JK, Finestone HM, Gilbert K, et al. Community-based referrals for electrodiagnostic studies in patients with possible carpal tunnel syndrome: what is the diagnosis? Arch Phys Med Rehabil. 2002;83:598-603.
50. Kane NM, Oware A. Nerve conduction and electromyography studies. J Neurol. 2012;259:1502-1508.
Richard Bogan, MD
The video associated with this article is no longer available on this site. Please view all of our videos on the MDedge YouTube channel
The video associated with this article is no longer available on this site. Please view all of our videos on the MDedge YouTube channel
The video associated with this article is no longer available on this site. Please view all of our videos on the MDedge YouTube channel
Lifetime Achievement Award Presented
The Society for Vascular Surgery’s highest honor, the Lifetime Achievement Award, was presented during the Vascular Annual Meeting to Dr. Jack Cronenwett.
This honor recognizes an individual’s outstanding and sustained contributions to the profession and SVS, as well as his or her exemplary professional practice and leadership.
Dr. Cronenwett’s achievements include building the vascular surgery division at Dartmouth-Hitchcock Center into a “powerhouse, a division of vascular surgery that has national and international recognition,” as one fellow SVS member wrote in his nomination. In addition, he was largely responsible for the development at Dartmouth of a new vascular surgery training paradigm – the 0 + 5 residency. He spearheaded the Vascular Study Group of New England, which became the model for the future Vascular Quality Initiative; with Dr. Tom Riles, then president of the American Association for Vascular Surgery, led the merger of AAVS with SVS, creating today’s more inclusive SVS; was senior co-editor of the Journal of Vascular Surgery, was co-editor of the 7th and 8th editions of Rutherford’s Vascular Surgery textbook and helped launch the SVS-PSO as an official new entity and served as its medical director.
View the video of him receiving this award and a story of Dr. Cronenwett’s accomplishments.
The Society for Vascular Surgery’s highest honor, the Lifetime Achievement Award, was presented during the Vascular Annual Meeting to Dr. Jack Cronenwett.
This honor recognizes an individual’s outstanding and sustained contributions to the profession and SVS, as well as his or her exemplary professional practice and leadership.
Dr. Cronenwett’s achievements include building the vascular surgery division at Dartmouth-Hitchcock Center into a “powerhouse, a division of vascular surgery that has national and international recognition,” as one fellow SVS member wrote in his nomination. In addition, he was largely responsible for the development at Dartmouth of a new vascular surgery training paradigm – the 0 + 5 residency. He spearheaded the Vascular Study Group of New England, which became the model for the future Vascular Quality Initiative; with Dr. Tom Riles, then president of the American Association for Vascular Surgery, led the merger of AAVS with SVS, creating today’s more inclusive SVS; was senior co-editor of the Journal of Vascular Surgery, was co-editor of the 7th and 8th editions of Rutherford’s Vascular Surgery textbook and helped launch the SVS-PSO as an official new entity and served as its medical director.
View the video of him receiving this award and a story of Dr. Cronenwett’s accomplishments.
The Society for Vascular Surgery’s highest honor, the Lifetime Achievement Award, was presented during the Vascular Annual Meeting to Dr. Jack Cronenwett.
This honor recognizes an individual’s outstanding and sustained contributions to the profession and SVS, as well as his or her exemplary professional practice and leadership.
Dr. Cronenwett’s achievements include building the vascular surgery division at Dartmouth-Hitchcock Center into a “powerhouse, a division of vascular surgery that has national and international recognition,” as one fellow SVS member wrote in his nomination. In addition, he was largely responsible for the development at Dartmouth of a new vascular surgery training paradigm – the 0 + 5 residency. He spearheaded the Vascular Study Group of New England, which became the model for the future Vascular Quality Initiative; with Dr. Tom Riles, then president of the American Association for Vascular Surgery, led the merger of AAVS with SVS, creating today’s more inclusive SVS; was senior co-editor of the Journal of Vascular Surgery, was co-editor of the 7th and 8th editions of Rutherford’s Vascular Surgery textbook and helped launch the SVS-PSO as an official new entity and served as its medical director.
View the video of him receiving this award and a story of Dr. Cronenwett’s accomplishments.
Bone Ache and Stiffness
1. This patient presented with rapid onset of severe pain in the MTP joint of the great toe accompanied swelling, redness, and fever. The pain was most severe in the first two to 24 hours and peaked within 24 hours. The history was remarkable for a trauma to the toe and a first-degree relative who had experienced the same symptoms. The patient admitted to drinking alcohol regularly.
Diagnosis: A presumptive diagnosis of gout may be acceptable in a patient with the classic presentation: rapid onset of severe pain in a swollen, erythematous joint (classically described in Latin as calor, rubor, dolor, et tumor) and symptoms peaking within 24 hours. The presence of tophi is pathognomonic for chronic tophaceous gout. In cases of questionable or unusual manifestation of gout, however, various imaging techniques and crystal visualization may be indicated.
For more information, see “Gout: A Clinical Overview.” Clin Rev. 2011;21(7):29-35.
For the next photograph, proceed to the next page >>
2. A 75-year-old woman presented to her family physician (FP) with pain in both hands. She said that she’d had the pain for a year and that it got worse with cold weather and better with ibuprofen.
Diagnosis: Osteoarthritis (OA) was diagnosed after noting Heberden’s nodes and Bouchard’s nodes at the distal and proximal interphalangeal joints, respectively. Arthritis is the most common cause of disability in the United States. Twenty-one million adults have functional limitations because of arthritis. Fifty percent of adults ages 65 years or older have been given an arthritis diagnosis. The most commonly affected joints are the knees, hips, hands (distal and proximal interphalangeal joints), and spine. If the diagnosis is uncertain, plain x-rays can often differentiate between OA and other forms of arthritis.
For more information, read “Hand pain.” J Fam Pract. 2015.
Photo courtesy of Dr. Richard Usatine, MD. The case was adapted with permission from Chumley H, Usatine R. Arthritis overview. In: Usatine R, Smith M, Mayeaux EJ, et al, eds. Color Atlas of Family Medicine. 2nd ed. New York, NY: McGraw-Hill; 2013:562-568.
To learn more about the Color Atlas of Family Medicine, see: www.amazon.com/Color-Family-Medicine-Richard-Usatine/dp/0071769641/
You can now get the second edition of the Color Atlas of Family Medicine as an app by clicking on this link: usatinemedia.com
For the next photograph, proceed to the next page >>
3. A 45-year-old man with longstanding psoriasis began experiencing finger pain and swelling. The patient, who had a history of hepatitis C, noted that his fingernails had recently begun crumbling and his fingers were stiff in the morning.
Diagnosis: Psoriatic arthritis (with dactylitis and significant distal interphalangeal joint [DIP] involvement) was diagnosed in both hands, but the left hand was worse. The condition of the patient’s nails was not surprising, given that almost all patients with psoriatic arthritis have nail involvement. Radiographs of the patient’s hands showed periarticular erosions and new bone formation. There was also telescoping of the third DIP joint.
For more information, read “Finger pain and swelling.” J Fam Pract. 2015.
Photo courtesy of Ricardo Zuniga-Montes, MD; text courtesy of Richard P. Usatine, MD. The case was adapted with permission from Chumley H, Usatine R. Arthritis overview. In: Usatine R, Smith M, Mayeaux EJ, et al, eds. Color Atlas of Family Medicine. 2nd ed. New York, NY: McGraw-Hill; 2013:562-568.
To learn more about the Color Atlas of Family Medicine, see: www.amazon.com/Color-Family-Medicine-Richard-Usatine/dp/0071769641/
You can now get the second edition of the Color Atlas of Family Medicine as an app by clicking on this link: usatinemedia.com
For the next photograph, proceed to the next page >>
4. A 62-year-old woman was concerned about her fingers, which weren’t straight. She’d had increasing pain in both hands (specifically the metacarpophalangeal [MCP] joints) over the past 2 years. Her fingers were stiff in the morning, but she was able to gain some relief with ibuprofen and naproxen. On physical exam, her fingers had an ulnar deviation and there was swelling of the MCP joints on both hands.
Diagnosis: Rheumatoid arthritis (RA) was diagnosed, based on the patient’s bilateral MCP joint swelling, ulnar deviation, and morning stiffness. Patients with RA initially experience swelling and stiffness in their wrists, as well as their MCP and metatarsophalangeal joints. Later, the larger joints are affected. When RA is advanced, severe destruction and subluxation occur.
“Crooked fingers.” J Fam Pract. 2015.
Photo courtesy of Dr. Richard Usatine, MD. The case was adapted with permission from Chumley H, Usatine R. Arthritis overview. In: Usatine R, Smith M, Mayeaux EJ, et al, eds. Color Atlas of Family Medicine. 2nd ed. New York, NY: McGraw-Hill; 2013:562-568.
To learn more about the Color Atlas of Family Medicine, see: www.amazon.com/Color-Family-Medicine-Richard-Usatine/dp/0071769641/
You can now get the second edition of the Color Atlas of Family Medicine as an app by clicking on this link: usatinemedia.com
1. This patient presented with rapid onset of severe pain in the MTP joint of the great toe accompanied swelling, redness, and fever. The pain was most severe in the first two to 24 hours and peaked within 24 hours. The history was remarkable for a trauma to the toe and a first-degree relative who had experienced the same symptoms. The patient admitted to drinking alcohol regularly.
Diagnosis: A presumptive diagnosis of gout may be acceptable in a patient with the classic presentation: rapid onset of severe pain in a swollen, erythematous joint (classically described in Latin as calor, rubor, dolor, et tumor) and symptoms peaking within 24 hours. The presence of tophi is pathognomonic for chronic tophaceous gout. In cases of questionable or unusual manifestation of gout, however, various imaging techniques and crystal visualization may be indicated.
For more information, see “Gout: A Clinical Overview.” Clin Rev. 2011;21(7):29-35.
For the next photograph, proceed to the next page >>
2. A 75-year-old woman presented to her family physician (FP) with pain in both hands. She said that she’d had the pain for a year and that it got worse with cold weather and better with ibuprofen.
Diagnosis: Osteoarthritis (OA) was diagnosed after noting Heberden’s nodes and Bouchard’s nodes at the distal and proximal interphalangeal joints, respectively. Arthritis is the most common cause of disability in the United States. Twenty-one million adults have functional limitations because of arthritis. Fifty percent of adults ages 65 years or older have been given an arthritis diagnosis. The most commonly affected joints are the knees, hips, hands (distal and proximal interphalangeal joints), and spine. If the diagnosis is uncertain, plain x-rays can often differentiate between OA and other forms of arthritis.
For more information, read “Hand pain.” J Fam Pract. 2015.
Photo courtesy of Dr. Richard Usatine, MD. The case was adapted with permission from Chumley H, Usatine R. Arthritis overview. In: Usatine R, Smith M, Mayeaux EJ, et al, eds. Color Atlas of Family Medicine. 2nd ed. New York, NY: McGraw-Hill; 2013:562-568.
To learn more about the Color Atlas of Family Medicine, see: www.amazon.com/Color-Family-Medicine-Richard-Usatine/dp/0071769641/
You can now get the second edition of the Color Atlas of Family Medicine as an app by clicking on this link: usatinemedia.com
For the next photograph, proceed to the next page >>
3. A 45-year-old man with longstanding psoriasis began experiencing finger pain and swelling. The patient, who had a history of hepatitis C, noted that his fingernails had recently begun crumbling and his fingers were stiff in the morning.
Diagnosis: Psoriatic arthritis (with dactylitis and significant distal interphalangeal joint [DIP] involvement) was diagnosed in both hands, but the left hand was worse. The condition of the patient’s nails was not surprising, given that almost all patients with psoriatic arthritis have nail involvement. Radiographs of the patient’s hands showed periarticular erosions and new bone formation. There was also telescoping of the third DIP joint.
For more information, read “Finger pain and swelling.” J Fam Pract. 2015.
Photo courtesy of Ricardo Zuniga-Montes, MD; text courtesy of Richard P. Usatine, MD. The case was adapted with permission from Chumley H, Usatine R. Arthritis overview. In: Usatine R, Smith M, Mayeaux EJ, et al, eds. Color Atlas of Family Medicine. 2nd ed. New York, NY: McGraw-Hill; 2013:562-568.
To learn more about the Color Atlas of Family Medicine, see: www.amazon.com/Color-Family-Medicine-Richard-Usatine/dp/0071769641/
You can now get the second edition of the Color Atlas of Family Medicine as an app by clicking on this link: usatinemedia.com
For the next photograph, proceed to the next page >>
4. A 62-year-old woman was concerned about her fingers, which weren’t straight. She’d had increasing pain in both hands (specifically the metacarpophalangeal [MCP] joints) over the past 2 years. Her fingers were stiff in the morning, but she was able to gain some relief with ibuprofen and naproxen. On physical exam, her fingers had an ulnar deviation and there was swelling of the MCP joints on both hands.
Diagnosis: Rheumatoid arthritis (RA) was diagnosed, based on the patient’s bilateral MCP joint swelling, ulnar deviation, and morning stiffness. Patients with RA initially experience swelling and stiffness in their wrists, as well as their MCP and metatarsophalangeal joints. Later, the larger joints are affected. When RA is advanced, severe destruction and subluxation occur.
“Crooked fingers.” J Fam Pract. 2015.
Photo courtesy of Dr. Richard Usatine, MD. The case was adapted with permission from Chumley H, Usatine R. Arthritis overview. In: Usatine R, Smith M, Mayeaux EJ, et al, eds. Color Atlas of Family Medicine. 2nd ed. New York, NY: McGraw-Hill; 2013:562-568.
To learn more about the Color Atlas of Family Medicine, see: www.amazon.com/Color-Family-Medicine-Richard-Usatine/dp/0071769641/
You can now get the second edition of the Color Atlas of Family Medicine as an app by clicking on this link: usatinemedia.com
1. This patient presented with rapid onset of severe pain in the MTP joint of the great toe accompanied swelling, redness, and fever. The pain was most severe in the first two to 24 hours and peaked within 24 hours. The history was remarkable for a trauma to the toe and a first-degree relative who had experienced the same symptoms. The patient admitted to drinking alcohol regularly.
Diagnosis: A presumptive diagnosis of gout may be acceptable in a patient with the classic presentation: rapid onset of severe pain in a swollen, erythematous joint (classically described in Latin as calor, rubor, dolor, et tumor) and symptoms peaking within 24 hours. The presence of tophi is pathognomonic for chronic tophaceous gout. In cases of questionable or unusual manifestation of gout, however, various imaging techniques and crystal visualization may be indicated.
For more information, see “Gout: A Clinical Overview.” Clin Rev. 2011;21(7):29-35.
For the next photograph, proceed to the next page >>
2. A 75-year-old woman presented to her family physician (FP) with pain in both hands. She said that she’d had the pain for a year and that it got worse with cold weather and better with ibuprofen.
Diagnosis: Osteoarthritis (OA) was diagnosed after noting Heberden’s nodes and Bouchard’s nodes at the distal and proximal interphalangeal joints, respectively. Arthritis is the most common cause of disability in the United States. Twenty-one million adults have functional limitations because of arthritis. Fifty percent of adults ages 65 years or older have been given an arthritis diagnosis. The most commonly affected joints are the knees, hips, hands (distal and proximal interphalangeal joints), and spine. If the diagnosis is uncertain, plain x-rays can often differentiate between OA and other forms of arthritis.
For more information, read “Hand pain.” J Fam Pract. 2015.
Photo courtesy of Dr. Richard Usatine, MD. The case was adapted with permission from Chumley H, Usatine R. Arthritis overview. In: Usatine R, Smith M, Mayeaux EJ, et al, eds. Color Atlas of Family Medicine. 2nd ed. New York, NY: McGraw-Hill; 2013:562-568.
To learn more about the Color Atlas of Family Medicine, see: www.amazon.com/Color-Family-Medicine-Richard-Usatine/dp/0071769641/
You can now get the second edition of the Color Atlas of Family Medicine as an app by clicking on this link: usatinemedia.com
For the next photograph, proceed to the next page >>
3. A 45-year-old man with longstanding psoriasis began experiencing finger pain and swelling. The patient, who had a history of hepatitis C, noted that his fingernails had recently begun crumbling and his fingers were stiff in the morning.
Diagnosis: Psoriatic arthritis (with dactylitis and significant distal interphalangeal joint [DIP] involvement) was diagnosed in both hands, but the left hand was worse. The condition of the patient’s nails was not surprising, given that almost all patients with psoriatic arthritis have nail involvement. Radiographs of the patient’s hands showed periarticular erosions and new bone formation. There was also telescoping of the third DIP joint.
For more information, read “Finger pain and swelling.” J Fam Pract. 2015.
Photo courtesy of Ricardo Zuniga-Montes, MD; text courtesy of Richard P. Usatine, MD. The case was adapted with permission from Chumley H, Usatine R. Arthritis overview. In: Usatine R, Smith M, Mayeaux EJ, et al, eds. Color Atlas of Family Medicine. 2nd ed. New York, NY: McGraw-Hill; 2013:562-568.
To learn more about the Color Atlas of Family Medicine, see: www.amazon.com/Color-Family-Medicine-Richard-Usatine/dp/0071769641/
You can now get the second edition of the Color Atlas of Family Medicine as an app by clicking on this link: usatinemedia.com
For the next photograph, proceed to the next page >>
4. A 62-year-old woman was concerned about her fingers, which weren’t straight. She’d had increasing pain in both hands (specifically the metacarpophalangeal [MCP] joints) over the past 2 years. Her fingers were stiff in the morning, but she was able to gain some relief with ibuprofen and naproxen. On physical exam, her fingers had an ulnar deviation and there was swelling of the MCP joints on both hands.
Diagnosis: Rheumatoid arthritis (RA) was diagnosed, based on the patient’s bilateral MCP joint swelling, ulnar deviation, and morning stiffness. Patients with RA initially experience swelling and stiffness in their wrists, as well as their MCP and metatarsophalangeal joints. Later, the larger joints are affected. When RA is advanced, severe destruction and subluxation occur.
“Crooked fingers.” J Fam Pract. 2015.
Photo courtesy of Dr. Richard Usatine, MD. The case was adapted with permission from Chumley H, Usatine R. Arthritis overview. In: Usatine R, Smith M, Mayeaux EJ, et al, eds. Color Atlas of Family Medicine. 2nd ed. New York, NY: McGraw-Hill; 2013:562-568.
To learn more about the Color Atlas of Family Medicine, see: www.amazon.com/Color-Family-Medicine-Richard-Usatine/dp/0071769641/
You can now get the second edition of the Color Atlas of Family Medicine as an app by clicking on this link: usatinemedia.com
Lisinopril tied to 19% drop in conduction system disease
Compared with chlorthalidone, the angiotensin-converting enzyme inhibitor lisinopril was associated with a 19% drop in new-onset conduction system disease among high-risk patients with hypertension, based on a secondary analysis of the randomized, double-blind Antihypertensive and Lipid-Lowering Treatment to Prevent Heart Attack Trial (ALLHAT).
Amlodipine besylate and pravastatin did not affect rates of conduction defects, added Dr. Thomas A. Dewland of Oregon Health and Science University, Portland, and his associates, who reported the findings online June 27 in JAMA Internal Medicine.
ALLHAT was a randomized, double-blind trial comparing chlorthalidone, amlodipine, lisinopril, or doxazosin mesylate in patients with hypertension and at least one other cardiac risk factor. Patients with elevated fasting low-density lipoprotein levels also were randomized to pravastatin sodium or usual care. Most (56%) patients were men, and the average age was about 67 years (standard deviation, 7.3 years). Twelve-lead electrocardiograms were obtained every 2 years, enabling the current analysis, the researchers said. In all, 21,004 patients without a baseline pacemaker or conduction system disease were followed for an average of 5 years (JAMA Intern Med. 2016 Jun 27. doi: 10.1001/jamainternmed.2016.250).
A total of 1,114 patients developed new-onset conduction system disease (3 events per 1,000 person-years), including 389 patients with left bundle branch block (4.5 events per 1,000 person-years), 570 patients with right bundle branch block (6.6 events per 1,000 person-years), and 155 patients with intraventricular conduction delay. The risk of conduction system disease was 19% lower with lisinopril than chlorthalidone (hazard ratio, 0.81; 95% confidence interval, 0.69-0.95; P = .01). Neither amlodipine nor pravastatin significantly affected the chances of conduction system disease, but patients who were older, white, or had diabetes or left ventricular hypertrophy were at significantly greater risk than the overall cohort.
“The antifibrotic effects of angiotensin-converting enzyme inhibitors may prevent or slow the development of conduction system disease, and future research is warranted to understand whether this treatment affects other conduction-related clinical outcomes,” including the need for pacemaker implantation, the researchers concluded.
The study was supported by the American Heart Association, the Joseph Drown Foundation, the National Heart, Lung, and Blood Institute of the National Institutes of Health, and by Pfizer. The investigators had no disclosures.
To our knowledge, this is the first report that incident conduction system disease may be prevented by drug therapy, in this case lisinopril. This important observation is consistent with established knowledge that angiotensin-converting enzyme inhibition has salutary effects on inflammation, hypertrophy, and fibrosis. We look forward to future studies to confirm that ECG conduction disease can indeed be prevented or progression slowed by relatively simple interventions, to define those individuals most likely to benefit, and to assess whether such preventive strategies can indeed ward off the need for invasive procedures such as pacemakers in selected patient subsets. This is indeed an exciting prospect!
Dr. Sanjiv M. Narayan, Dr. Tina Baykaner, and Dr. David J. Maron are at Falk Cardiovascular Research Center, Stanford University, Palo Alto, California. Dr. Narayan reported receiving research support from the National Institutes of Health; being coinventor of intellectual property owned by the University of California and licensed to Topera, in which he held equity; and receiving consulting fees from the American College of Cardiology and Uptodate.com and speaking fees from St. Jude Medical and Medtronic. The other authors had no disclosures. These comments are from their editorial (JAMA Intern Med. 2016 Jun 27. doi: 10.1001 /jamainternmed.2016.2502).
To our knowledge, this is the first report that incident conduction system disease may be prevented by drug therapy, in this case lisinopril. This important observation is consistent with established knowledge that angiotensin-converting enzyme inhibition has salutary effects on inflammation, hypertrophy, and fibrosis. We look forward to future studies to confirm that ECG conduction disease can indeed be prevented or progression slowed by relatively simple interventions, to define those individuals most likely to benefit, and to assess whether such preventive strategies can indeed ward off the need for invasive procedures such as pacemakers in selected patient subsets. This is indeed an exciting prospect!
Dr. Sanjiv M. Narayan, Dr. Tina Baykaner, and Dr. David J. Maron are at Falk Cardiovascular Research Center, Stanford University, Palo Alto, California. Dr. Narayan reported receiving research support from the National Institutes of Health; being coinventor of intellectual property owned by the University of California and licensed to Topera, in which he held equity; and receiving consulting fees from the American College of Cardiology and Uptodate.com and speaking fees from St. Jude Medical and Medtronic. The other authors had no disclosures. These comments are from their editorial (JAMA Intern Med. 2016 Jun 27. doi: 10.1001 /jamainternmed.2016.2502).
To our knowledge, this is the first report that incident conduction system disease may be prevented by drug therapy, in this case lisinopril. This important observation is consistent with established knowledge that angiotensin-converting enzyme inhibition has salutary effects on inflammation, hypertrophy, and fibrosis. We look forward to future studies to confirm that ECG conduction disease can indeed be prevented or progression slowed by relatively simple interventions, to define those individuals most likely to benefit, and to assess whether such preventive strategies can indeed ward off the need for invasive procedures such as pacemakers in selected patient subsets. This is indeed an exciting prospect!
Dr. Sanjiv M. Narayan, Dr. Tina Baykaner, and Dr. David J. Maron are at Falk Cardiovascular Research Center, Stanford University, Palo Alto, California. Dr. Narayan reported receiving research support from the National Institutes of Health; being coinventor of intellectual property owned by the University of California and licensed to Topera, in which he held equity; and receiving consulting fees from the American College of Cardiology and Uptodate.com and speaking fees from St. Jude Medical and Medtronic. The other authors had no disclosures. These comments are from their editorial (JAMA Intern Med. 2016 Jun 27. doi: 10.1001 /jamainternmed.2016.2502).
Compared with chlorthalidone, the angiotensin-converting enzyme inhibitor lisinopril was associated with a 19% drop in new-onset conduction system disease among high-risk patients with hypertension, based on a secondary analysis of the randomized, double-blind Antihypertensive and Lipid-Lowering Treatment to Prevent Heart Attack Trial (ALLHAT).
Amlodipine besylate and pravastatin did not affect rates of conduction defects, added Dr. Thomas A. Dewland of Oregon Health and Science University, Portland, and his associates, who reported the findings online June 27 in JAMA Internal Medicine.
ALLHAT was a randomized, double-blind trial comparing chlorthalidone, amlodipine, lisinopril, or doxazosin mesylate in patients with hypertension and at least one other cardiac risk factor. Patients with elevated fasting low-density lipoprotein levels also were randomized to pravastatin sodium or usual care. Most (56%) patients were men, and the average age was about 67 years (standard deviation, 7.3 years). Twelve-lead electrocardiograms were obtained every 2 years, enabling the current analysis, the researchers said. In all, 21,004 patients without a baseline pacemaker or conduction system disease were followed for an average of 5 years (JAMA Intern Med. 2016 Jun 27. doi: 10.1001/jamainternmed.2016.250).
A total of 1,114 patients developed new-onset conduction system disease (3 events per 1,000 person-years), including 389 patients with left bundle branch block (4.5 events per 1,000 person-years), 570 patients with right bundle branch block (6.6 events per 1,000 person-years), and 155 patients with intraventricular conduction delay. The risk of conduction system disease was 19% lower with lisinopril than chlorthalidone (hazard ratio, 0.81; 95% confidence interval, 0.69-0.95; P = .01). Neither amlodipine nor pravastatin significantly affected the chances of conduction system disease, but patients who were older, white, or had diabetes or left ventricular hypertrophy were at significantly greater risk than the overall cohort.
“The antifibrotic effects of angiotensin-converting enzyme inhibitors may prevent or slow the development of conduction system disease, and future research is warranted to understand whether this treatment affects other conduction-related clinical outcomes,” including the need for pacemaker implantation, the researchers concluded.
The study was supported by the American Heart Association, the Joseph Drown Foundation, the National Heart, Lung, and Blood Institute of the National Institutes of Health, and by Pfizer. The investigators had no disclosures.
Compared with chlorthalidone, the angiotensin-converting enzyme inhibitor lisinopril was associated with a 19% drop in new-onset conduction system disease among high-risk patients with hypertension, based on a secondary analysis of the randomized, double-blind Antihypertensive and Lipid-Lowering Treatment to Prevent Heart Attack Trial (ALLHAT).
Amlodipine besylate and pravastatin did not affect rates of conduction defects, added Dr. Thomas A. Dewland of Oregon Health and Science University, Portland, and his associates, who reported the findings online June 27 in JAMA Internal Medicine.
ALLHAT was a randomized, double-blind trial comparing chlorthalidone, amlodipine, lisinopril, or doxazosin mesylate in patients with hypertension and at least one other cardiac risk factor. Patients with elevated fasting low-density lipoprotein levels also were randomized to pravastatin sodium or usual care. Most (56%) patients were men, and the average age was about 67 years (standard deviation, 7.3 years). Twelve-lead electrocardiograms were obtained every 2 years, enabling the current analysis, the researchers said. In all, 21,004 patients without a baseline pacemaker or conduction system disease were followed for an average of 5 years (JAMA Intern Med. 2016 Jun 27. doi: 10.1001/jamainternmed.2016.250).
A total of 1,114 patients developed new-onset conduction system disease (3 events per 1,000 person-years), including 389 patients with left bundle branch block (4.5 events per 1,000 person-years), 570 patients with right bundle branch block (6.6 events per 1,000 person-years), and 155 patients with intraventricular conduction delay. The risk of conduction system disease was 19% lower with lisinopril than chlorthalidone (hazard ratio, 0.81; 95% confidence interval, 0.69-0.95; P = .01). Neither amlodipine nor pravastatin significantly affected the chances of conduction system disease, but patients who were older, white, or had diabetes or left ventricular hypertrophy were at significantly greater risk than the overall cohort.
“The antifibrotic effects of angiotensin-converting enzyme inhibitors may prevent or slow the development of conduction system disease, and future research is warranted to understand whether this treatment affects other conduction-related clinical outcomes,” including the need for pacemaker implantation, the researchers concluded.
The study was supported by the American Heart Association, the Joseph Drown Foundation, the National Heart, Lung, and Blood Institute of the National Institutes of Health, and by Pfizer. The investigators had no disclosures.
FROM JAMA INTERNAL MEDICINE
Key clinical point: Compared with chlorthalidone, the angiotensin-converting enzyme inhibitor lisinopril was associated with a significant drop in new-onset conduction system disease among high-risk patients with hypertension.
Major finding: The risk of conduction system disease was 19% lower with lisinopril than chlorthalidone (hazard ratio, 0.81; 95% CI, 0.69 to 0.95; P = .01).
Data source: Serial 12-lead electrocardiograms of 21,004 patients with hypertension and at least one other cardiac risk factor, with an average of 5 years of follow-up.
Disclosures: The study was supported by the American Heart Association, the Joseph Drown Foundation, the National Heart, Lung, and Blood Institute of the National Institutes of Health, and by Pfizer, Inc. The investigators had no disclosures.
RT + ADT linked with improved survival in mPC
The addition of radiotherapy (RT) to androgen deprivation therapy (ADT) appears to boost overall survival in men with metastatic prostate cancer. However, it is more common for men to receive ADT alone than ADT plus RT.
“In this large contemporary analysis, men receiving prostate RT plus ADT lived substantially longer than men treated with ADT alone,” noted Dr. Chad Rusthoven of the University of Colorado, Aurora, and his associates (J Clin Oncol. 2016 June doi: 10.1200/JCO.2016.67.4788).
Investigators reviewed the National Cancer Data Base and identified 6,382 men with metastatic prostate cancer who received ADT as first-line therapy. Of those men, 5,844 (91.6%) received ADT alone, and the remaining 538 (8.4%) men received ADT plus prostate RT. The median age of the study population was 69, 75% were white, the most common T stage was T2, N0 was the most common N stage, and 6% of the entire cohort received chemotherapy. The median follow-up time was 5.1 years, and the median time from diagnosis to initiation of RT was 101 days. Patients were excluded from the study if they died within a month of diagnosis or if they were receiving prostatectomy, cryotherapy, or brachytherapy.
Among men receiving ADT plus RT, 48% were on Medicare and 41% were privately insured. Among men receiving ADT alone, 56% were on Medicare and 27% were privately insured.
Univariate analysis revealed that, compared with ADT alone, RT plus ADT was associated with longer median overall survival (29 vs. 53 months) as well as improved 3-year (43% vs. 62%), 5-year (25% vs. 49%), and 8-year (13% vs. 33%) overall survival estimates.
Multivariate analysis also found an independent association between the addition of radiotherapy with improved overall survival (HR, 0.624; 95% confidence interval, 0.551-0.706; P less than .001). In addition, RT to the prostate only and RT to the prostate and pelvis were both associated with longer overall survival times compared with ADT alone.
The funding source for this study was not listed. Six investigators reported serving in advisory roles for, receiving honoraria or financial compensation from, or holding patents in accordance with multiple companies.
On Twitter @jessnicolecraig
The addition of radiotherapy (RT) to androgen deprivation therapy (ADT) appears to boost overall survival in men with metastatic prostate cancer. However, it is more common for men to receive ADT alone than ADT plus RT.
“In this large contemporary analysis, men receiving prostate RT plus ADT lived substantially longer than men treated with ADT alone,” noted Dr. Chad Rusthoven of the University of Colorado, Aurora, and his associates (J Clin Oncol. 2016 June doi: 10.1200/JCO.2016.67.4788).
Investigators reviewed the National Cancer Data Base and identified 6,382 men with metastatic prostate cancer who received ADT as first-line therapy. Of those men, 5,844 (91.6%) received ADT alone, and the remaining 538 (8.4%) men received ADT plus prostate RT. The median age of the study population was 69, 75% were white, the most common T stage was T2, N0 was the most common N stage, and 6% of the entire cohort received chemotherapy. The median follow-up time was 5.1 years, and the median time from diagnosis to initiation of RT was 101 days. Patients were excluded from the study if they died within a month of diagnosis or if they were receiving prostatectomy, cryotherapy, or brachytherapy.
Among men receiving ADT plus RT, 48% were on Medicare and 41% were privately insured. Among men receiving ADT alone, 56% were on Medicare and 27% were privately insured.
Univariate analysis revealed that, compared with ADT alone, RT plus ADT was associated with longer median overall survival (29 vs. 53 months) as well as improved 3-year (43% vs. 62%), 5-year (25% vs. 49%), and 8-year (13% vs. 33%) overall survival estimates.
Multivariate analysis also found an independent association between the addition of radiotherapy with improved overall survival (HR, 0.624; 95% confidence interval, 0.551-0.706; P less than .001). In addition, RT to the prostate only and RT to the prostate and pelvis were both associated with longer overall survival times compared with ADT alone.
The funding source for this study was not listed. Six investigators reported serving in advisory roles for, receiving honoraria or financial compensation from, or holding patents in accordance with multiple companies.
On Twitter @jessnicolecraig
The addition of radiotherapy (RT) to androgen deprivation therapy (ADT) appears to boost overall survival in men with metastatic prostate cancer. However, it is more common for men to receive ADT alone than ADT plus RT.
“In this large contemporary analysis, men receiving prostate RT plus ADT lived substantially longer than men treated with ADT alone,” noted Dr. Chad Rusthoven of the University of Colorado, Aurora, and his associates (J Clin Oncol. 2016 June doi: 10.1200/JCO.2016.67.4788).
Investigators reviewed the National Cancer Data Base and identified 6,382 men with metastatic prostate cancer who received ADT as first-line therapy. Of those men, 5,844 (91.6%) received ADT alone, and the remaining 538 (8.4%) men received ADT plus prostate RT. The median age of the study population was 69, 75% were white, the most common T stage was T2, N0 was the most common N stage, and 6% of the entire cohort received chemotherapy. The median follow-up time was 5.1 years, and the median time from diagnosis to initiation of RT was 101 days. Patients were excluded from the study if they died within a month of diagnosis or if they were receiving prostatectomy, cryotherapy, or brachytherapy.
Among men receiving ADT plus RT, 48% were on Medicare and 41% were privately insured. Among men receiving ADT alone, 56% were on Medicare and 27% were privately insured.
Univariate analysis revealed that, compared with ADT alone, RT plus ADT was associated with longer median overall survival (29 vs. 53 months) as well as improved 3-year (43% vs. 62%), 5-year (25% vs. 49%), and 8-year (13% vs. 33%) overall survival estimates.
Multivariate analysis also found an independent association between the addition of radiotherapy with improved overall survival (HR, 0.624; 95% confidence interval, 0.551-0.706; P less than .001). In addition, RT to the prostate only and RT to the prostate and pelvis were both associated with longer overall survival times compared with ADT alone.
The funding source for this study was not listed. Six investigators reported serving in advisory roles for, receiving honoraria or financial compensation from, or holding patents in accordance with multiple companies.
On Twitter @jessnicolecraig
FROM THE JOURNAL OF CLINICAL ONCOLOGY
Key clinical point: The addition of radiotherapy (RT) to androgen deprivation therapy (ADT) was associated with improved survival in men with prostate cancer.
Major finding: The addition of radiotherapy to androgen deprivation therapy was independently associated with improved overall survival (HR, 0.624; 95% confidence interval, 0.551-0.706; P less than .001).
Data source: A review of the National Cancer Data Base on 6,382 men with metastatic prostate cancer.
Disclosures: The funding source for this study was not listed. Six investigators reported serving in advisory roles for, receiving honoraria or financial compensation from, or holding patents in accordance with multiple companies.
Single Dose of Dexamethasone Not an Alternative to ‘Steroid Burst’ for Acute Asthma Treatment
Clinical question: Is one dose of dexamethasone comparable to five days of prednisone for treating mild-to-moderate asthma exacerbations?
Background: Corticosteroids are the mainstay of initial treatment for asthma exacerbations. The National Heart, Lung, and Blood Institute recommends a minimum of five days of prednisone, though studies have shown incomplete adherence to prolonged therapies. Dexamethasone has a longer duration of action than prednisone.
Study design: Randomized, controlled, double-blinded trial.
Setting: Urban, safety-net, teaching hospital.
Synopsis: The study included 376 adults ages 18–55 presenting to the emergency department for a mild-to-moderate asthma exacerbation who were randomized to two treatment courses of corticosteroids: one 12 mg dose of oral dexamethasone followed by four days of placebo versus five days of 60 mg of oral prednisone. Two weeks later, a telephone survey asked if they had relapsed and had to seek medical attention. This study did not show noninferiority of the dexamethasone option compared to the standard of care. Specifically, it showed a 12.1% relapse rate in the dexamethasone group versus a 9.8% relapse rate for prednisone (95% CI, -4.1% to 8.6%).
This was a small study looking at adults without other chronic lung diseases or diabetes. The authors did not include those patients who were either lost to follow-up (20% of those initially randomized) or ultimately admitted after their emergency department course.
Hospitalists who care for patients with asthma should look to the current standards of corticosteroid selection and duration to minimize clinical relapses and possibly readmissions.
Bottom line: One large dose of dexamethasone is inferior to the standard five days of prednisone for treating acute asthma exacerbations in adults.
Citation: Rehrer MW, Liu B, Rodriguez M, Lam J, Alter HJ. A randomized controlled noninferiority trial of single dose of oral dexamethasone versus 5 days of oral prednisone in acute adult asthma [published online ahead of print April 14, 2016]. Ann Emerg Med. doi:10.1016/j.annemergmed.2016.03.017.
Short Take
Guideline Recommends ED Asthma Management Associated with Shorter Inpatient Stay
Observational study found ED treatment concordance with four guideline-based processes for acute asthma treatment (inhaled beta-agonists, inhaled anticholinergics, systemic corticosteroids, and avoidance of methylxanthines) is associated with a 17% shorter hospital length of stay.
Citation: Hasegawa K, Brenner BE, Nowak RM, et al. Association of guideline-concordant acute asthma care in the emergency department with shorter hospital length of stay: a multicenter observational study. Acad Emerg Med. 2016;23(5):616-622.
Clinical question: Is one dose of dexamethasone comparable to five days of prednisone for treating mild-to-moderate asthma exacerbations?
Background: Corticosteroids are the mainstay of initial treatment for asthma exacerbations. The National Heart, Lung, and Blood Institute recommends a minimum of five days of prednisone, though studies have shown incomplete adherence to prolonged therapies. Dexamethasone has a longer duration of action than prednisone.
Study design: Randomized, controlled, double-blinded trial.
Setting: Urban, safety-net, teaching hospital.
Synopsis: The study included 376 adults ages 18–55 presenting to the emergency department for a mild-to-moderate asthma exacerbation who were randomized to two treatment courses of corticosteroids: one 12 mg dose of oral dexamethasone followed by four days of placebo versus five days of 60 mg of oral prednisone. Two weeks later, a telephone survey asked if they had relapsed and had to seek medical attention. This study did not show noninferiority of the dexamethasone option compared to the standard of care. Specifically, it showed a 12.1% relapse rate in the dexamethasone group versus a 9.8% relapse rate for prednisone (95% CI, -4.1% to 8.6%).
This was a small study looking at adults without other chronic lung diseases or diabetes. The authors did not include those patients who were either lost to follow-up (20% of those initially randomized) or ultimately admitted after their emergency department course.
Hospitalists who care for patients with asthma should look to the current standards of corticosteroid selection and duration to minimize clinical relapses and possibly readmissions.
Bottom line: One large dose of dexamethasone is inferior to the standard five days of prednisone for treating acute asthma exacerbations in adults.
Citation: Rehrer MW, Liu B, Rodriguez M, Lam J, Alter HJ. A randomized controlled noninferiority trial of single dose of oral dexamethasone versus 5 days of oral prednisone in acute adult asthma [published online ahead of print April 14, 2016]. Ann Emerg Med. doi:10.1016/j.annemergmed.2016.03.017.
Short Take
Guideline Recommends ED Asthma Management Associated with Shorter Inpatient Stay
Observational study found ED treatment concordance with four guideline-based processes for acute asthma treatment (inhaled beta-agonists, inhaled anticholinergics, systemic corticosteroids, and avoidance of methylxanthines) is associated with a 17% shorter hospital length of stay.
Citation: Hasegawa K, Brenner BE, Nowak RM, et al. Association of guideline-concordant acute asthma care in the emergency department with shorter hospital length of stay: a multicenter observational study. Acad Emerg Med. 2016;23(5):616-622.
Clinical question: Is one dose of dexamethasone comparable to five days of prednisone for treating mild-to-moderate asthma exacerbations?
Background: Corticosteroids are the mainstay of initial treatment for asthma exacerbations. The National Heart, Lung, and Blood Institute recommends a minimum of five days of prednisone, though studies have shown incomplete adherence to prolonged therapies. Dexamethasone has a longer duration of action than prednisone.
Study design: Randomized, controlled, double-blinded trial.
Setting: Urban, safety-net, teaching hospital.
Synopsis: The study included 376 adults ages 18–55 presenting to the emergency department for a mild-to-moderate asthma exacerbation who were randomized to two treatment courses of corticosteroids: one 12 mg dose of oral dexamethasone followed by four days of placebo versus five days of 60 mg of oral prednisone. Two weeks later, a telephone survey asked if they had relapsed and had to seek medical attention. This study did not show noninferiority of the dexamethasone option compared to the standard of care. Specifically, it showed a 12.1% relapse rate in the dexamethasone group versus a 9.8% relapse rate for prednisone (95% CI, -4.1% to 8.6%).
This was a small study looking at adults without other chronic lung diseases or diabetes. The authors did not include those patients who were either lost to follow-up (20% of those initially randomized) or ultimately admitted after their emergency department course.
Hospitalists who care for patients with asthma should look to the current standards of corticosteroid selection and duration to minimize clinical relapses and possibly readmissions.
Bottom line: One large dose of dexamethasone is inferior to the standard five days of prednisone for treating acute asthma exacerbations in adults.
Citation: Rehrer MW, Liu B, Rodriguez M, Lam J, Alter HJ. A randomized controlled noninferiority trial of single dose of oral dexamethasone versus 5 days of oral prednisone in acute adult asthma [published online ahead of print April 14, 2016]. Ann Emerg Med. doi:10.1016/j.annemergmed.2016.03.017.
Short Take
Guideline Recommends ED Asthma Management Associated with Shorter Inpatient Stay
Observational study found ED treatment concordance with four guideline-based processes for acute asthma treatment (inhaled beta-agonists, inhaled anticholinergics, systemic corticosteroids, and avoidance of methylxanthines) is associated with a 17% shorter hospital length of stay.
Citation: Hasegawa K, Brenner BE, Nowak RM, et al. Association of guideline-concordant acute asthma care in the emergency department with shorter hospital length of stay: a multicenter observational study. Acad Emerg Med. 2016;23(5):616-622.