User login
A Lifetime Of Sunshine
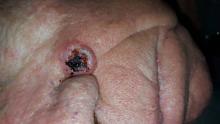
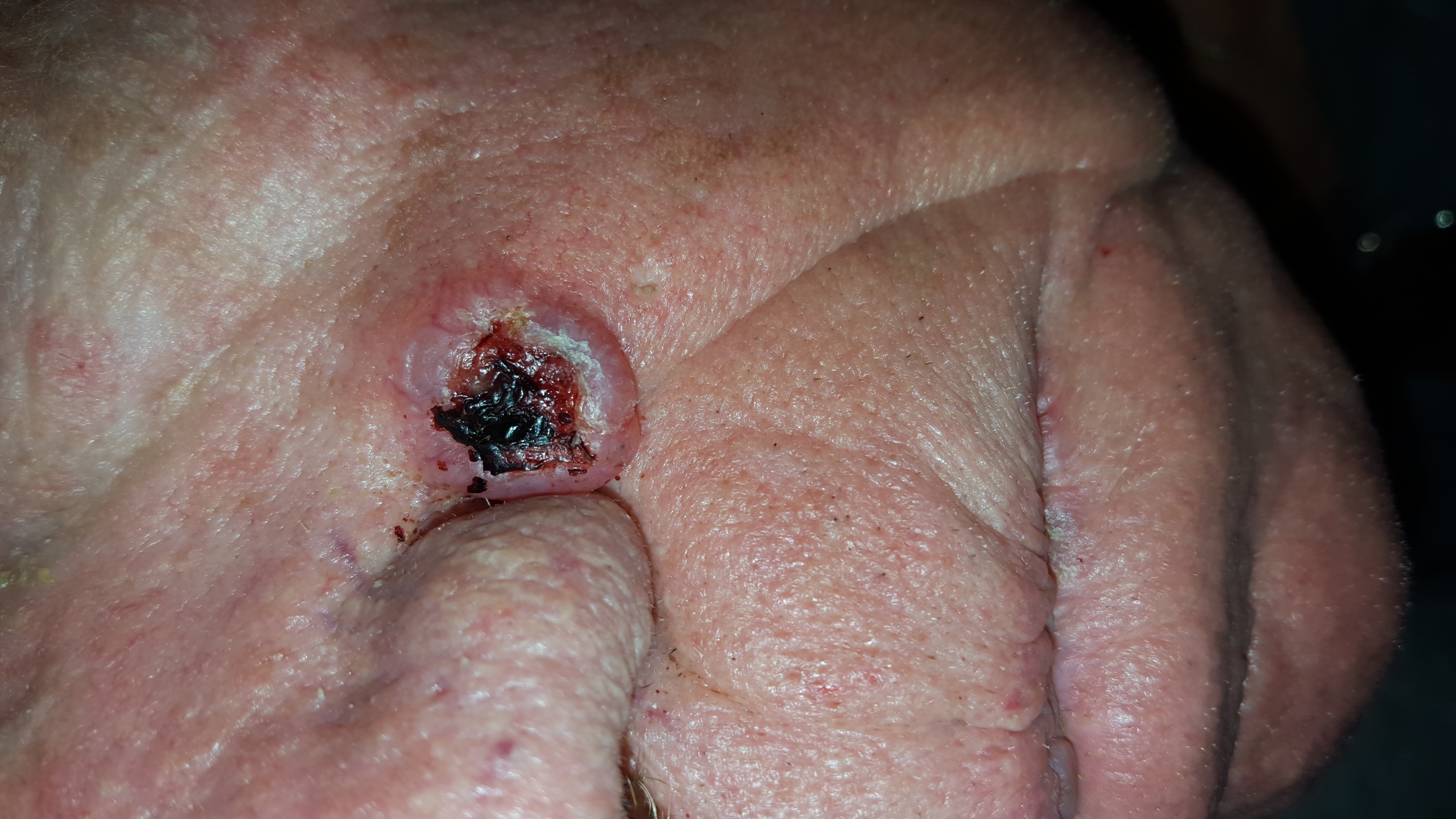
A 68-year-old man is seen for evaluation of a facial sore, which has been present for “at least a month.” It concerns him because, while the lesion is not painful, it won’t heal. The patient denies any preceding trauma to the area but does admit to having several skin cancers removed in his lifetime.
His lesion has been previously diagnosed as “pyoderma” and treated unsuccessfully with topical mupirocin and oral cephalexin.
He has held a number of jobs throughout his life, all of which involved being out in the sun all day—often seven days a week. And it wasn’t until he turned 45 that he finally started wearing a hat.
EXAMINATION
The lesion is an impressive, round, 1.2-cm nodule with extensive central ulceration. It has a rolled, translucent border and is tucked into the upper left nasolabial fold, directly adjacent to the alar bulb. It appears in the context of his fair, sun-damaged skin; he has several scars consistent with his history of skin cancer removal.
His skin elsewhere, while quite sun-damaged, is free of worrisome lesions.
A simple shave biopsy of the lesion is performed.
What is the diagnosis?
DISCUSSION
The biopsy showed changes consistent with basal cell carcinoma (BCC). It would be difficult to find a more typical example of BCC, the most common of all the sun-caused skin cancers (which include squamous cell, melanoma, and Merkel cell carcinoma). And yet, as this case illustrates, classic signs of BCC are often missed or overlooked, leading to “infection” or “pyoderma” diagnoses by providers who are unfamiliar with BCC.
I often tell medical providers that “it will be a rare day when you don’t see a BCC” because they are that common. But what I probably should say is “it will be a rare day when you don’t have a chance to see a BCC.” By that, I mean that you do have to look for them—not all are as obvious as this man’s—and you do need to have an understanding of who is susceptible and what the lesion will look like.
Older, sun-damaged individuals (mostly men) are most at risk for BBCs. Fair skin, freckles, red hair, and blue eyes are additional high-risk characteristics. This varies from melanoma patients, who tend to be much younger and whose lesions look nothing like a basal cell.
For BCC identification, focus on the areas directly and chronically exposed to the sun, such as the tops of the ears, the face, nose, chest, back, and arms.
BCCs can take on many appearances; they look as though they would hurt, but rarely do—a fact that should be noted as significant. Typically, they break down, scab, and bleed as they grow larger. They are usually very slow-growing and often take years to become noticeable. In other words, you have to think of it first, and look for it next. The fact that almost 1.5 million new BCCs are diagnosed each year in the US should heighten your suspicions considerably.
In this case, the patient will need extensive surgery, which may be deforming. Given his history of sun exposure, he will likely develop more skin cancers in the future.
TAKE-HOME LEARNING POINTS
• Basal cell carcinoma (BCC) is by far the most common type of sun-caused skin cancer; it will be seen frequently if properly looked for.
• Identification of BCCs will be more successful if the search is concentrated on the patients most likely to develop one and the areas where they are most likely to appear.
• This patient’s noduloulcerative BCC is the most common type and developed in a classic location. There is no type of infection that presents in this fashion.
• A simple shave biopsy confirms the diagnosis of BCC.
A 68-year-old man is seen for evaluation of a facial sore, which has been present for “at least a month.” It concerns him because, while the lesion is not painful, it won’t heal. The patient denies any preceding trauma to the area but does admit to having several skin cancers removed in his lifetime.
His lesion has been previously diagnosed as “pyoderma” and treated unsuccessfully with topical mupirocin and oral cephalexin.
He has held a number of jobs throughout his life, all of which involved being out in the sun all day—often seven days a week. And it wasn’t until he turned 45 that he finally started wearing a hat.
EXAMINATION
The lesion is an impressive, round, 1.2-cm nodule with extensive central ulceration. It has a rolled, translucent border and is tucked into the upper left nasolabial fold, directly adjacent to the alar bulb. It appears in the context of his fair, sun-damaged skin; he has several scars consistent with his history of skin cancer removal.
His skin elsewhere, while quite sun-damaged, is free of worrisome lesions.
A simple shave biopsy of the lesion is performed.
What is the diagnosis?
DISCUSSION
The biopsy showed changes consistent with basal cell carcinoma (BCC). It would be difficult to find a more typical example of BCC, the most common of all the sun-caused skin cancers (which include squamous cell, melanoma, and Merkel cell carcinoma). And yet, as this case illustrates, classic signs of BCC are often missed or overlooked, leading to “infection” or “pyoderma” diagnoses by providers who are unfamiliar with BCC.
I often tell medical providers that “it will be a rare day when you don’t see a BCC” because they are that common. But what I probably should say is “it will be a rare day when you don’t have a chance to see a BCC.” By that, I mean that you do have to look for them—not all are as obvious as this man’s—and you do need to have an understanding of who is susceptible and what the lesion will look like.
Older, sun-damaged individuals (mostly men) are most at risk for BBCs. Fair skin, freckles, red hair, and blue eyes are additional high-risk characteristics. This varies from melanoma patients, who tend to be much younger and whose lesions look nothing like a basal cell.
For BCC identification, focus on the areas directly and chronically exposed to the sun, such as the tops of the ears, the face, nose, chest, back, and arms.
BCCs can take on many appearances; they look as though they would hurt, but rarely do—a fact that should be noted as significant. Typically, they break down, scab, and bleed as they grow larger. They are usually very slow-growing and often take years to become noticeable. In other words, you have to think of it first, and look for it next. The fact that almost 1.5 million new BCCs are diagnosed each year in the US should heighten your suspicions considerably.
In this case, the patient will need extensive surgery, which may be deforming. Given his history of sun exposure, he will likely develop more skin cancers in the future.
TAKE-HOME LEARNING POINTS
• Basal cell carcinoma (BCC) is by far the most common type of sun-caused skin cancer; it will be seen frequently if properly looked for.
• Identification of BCCs will be more successful if the search is concentrated on the patients most likely to develop one and the areas where they are most likely to appear.
• This patient’s noduloulcerative BCC is the most common type and developed in a classic location. There is no type of infection that presents in this fashion.
• A simple shave biopsy confirms the diagnosis of BCC.
A 68-year-old man is seen for evaluation of a facial sore, which has been present for “at least a month.” It concerns him because, while the lesion is not painful, it won’t heal. The patient denies any preceding trauma to the area but does admit to having several skin cancers removed in his lifetime.
His lesion has been previously diagnosed as “pyoderma” and treated unsuccessfully with topical mupirocin and oral cephalexin.
He has held a number of jobs throughout his life, all of which involved being out in the sun all day—often seven days a week. And it wasn’t until he turned 45 that he finally started wearing a hat.
EXAMINATION
The lesion is an impressive, round, 1.2-cm nodule with extensive central ulceration. It has a rolled, translucent border and is tucked into the upper left nasolabial fold, directly adjacent to the alar bulb. It appears in the context of his fair, sun-damaged skin; he has several scars consistent with his history of skin cancer removal.
His skin elsewhere, while quite sun-damaged, is free of worrisome lesions.
A simple shave biopsy of the lesion is performed.
What is the diagnosis?
DISCUSSION
The biopsy showed changes consistent with basal cell carcinoma (BCC). It would be difficult to find a more typical example of BCC, the most common of all the sun-caused skin cancers (which include squamous cell, melanoma, and Merkel cell carcinoma). And yet, as this case illustrates, classic signs of BCC are often missed or overlooked, leading to “infection” or “pyoderma” diagnoses by providers who are unfamiliar with BCC.
I often tell medical providers that “it will be a rare day when you don’t see a BCC” because they are that common. But what I probably should say is “it will be a rare day when you don’t have a chance to see a BCC.” By that, I mean that you do have to look for them—not all are as obvious as this man’s—and you do need to have an understanding of who is susceptible and what the lesion will look like.
Older, sun-damaged individuals (mostly men) are most at risk for BBCs. Fair skin, freckles, red hair, and blue eyes are additional high-risk characteristics. This varies from melanoma patients, who tend to be much younger and whose lesions look nothing like a basal cell.
For BCC identification, focus on the areas directly and chronically exposed to the sun, such as the tops of the ears, the face, nose, chest, back, and arms.
BCCs can take on many appearances; they look as though they would hurt, but rarely do—a fact that should be noted as significant. Typically, they break down, scab, and bleed as they grow larger. They are usually very slow-growing and often take years to become noticeable. In other words, you have to think of it first, and look for it next. The fact that almost 1.5 million new BCCs are diagnosed each year in the US should heighten your suspicions considerably.
In this case, the patient will need extensive surgery, which may be deforming. Given his history of sun exposure, he will likely develop more skin cancers in the future.
TAKE-HOME LEARNING POINTS
• Basal cell carcinoma (BCC) is by far the most common type of sun-caused skin cancer; it will be seen frequently if properly looked for.
• Identification of BCCs will be more successful if the search is concentrated on the patients most likely to develop one and the areas where they are most likely to appear.
• This patient’s noduloulcerative BCC is the most common type and developed in a classic location. There is no type of infection that presents in this fashion.
• A simple shave biopsy confirms the diagnosis of BCC.
Seasonal variation not seen in C. difficile rates
BOSTON – No winter spike in Clostridium difficile infection (CDI) rates was seen among hospitalized patients after testing methodologies and frequency were accounted for, according to a large multinational study.
A total of 180 hospitals in five European countries had wide variation in CDI testing methods and testing density. However, among the hospitals that used a currently recommended toxin-detecting testing algorithm, there was no significant seasonal variation in cases, defined as mean cases per 10,000 patient bed-days per hospital per month (C/PBDs/H/M). The hospitals using toxin-detecting algorithms had summer C/PBDs/H/M rates of 9.6, compared to 8.0 in winter months (P = .27).
These results, presented at the annual meeting of the American Society for Microbiology by Kerrie Davies, clinical scientist at the University of Leeds (England), stand in contrast to some other studies that have shown a wintertime peak in CDI incidence. The data presented help in “understanding the context in which published reported rate data have been generated,” said Ms. Davies, enabling a better understanding both of how samples are tested, and who gets tested.
The study enrolled 180 hospitals – 38 each in France and Italy, 37 each in Germany and the United Kingdom, and 30 in Spain. Institutions reported patient demographics, as well as CDI testing data and patient bed-days for CDI cases, for 1 year.
Current European and U.K. CDI testing algorithms, said Ms. Davies, begin either with testing for glutamate dehydrogenase (GDH) or with nucleic acid amplification testing (NAAT), and then proceed to enzyme-linked immunosorbent assay (ELISA) testing for C. difficile toxins A and B.
Other algorithms, for example those that begin with toxin testing, are not recommended, said Ms. Davies. Some institutions may diagnose CDI only by toxin detection, GDH testing, or NAAT testing.
For data analysis, Ms. Davies and her collaborators compared CDI-related PBDs and testing density during June, July, and August to data collected in December, January, and February. Testing methods were dichotomized to toxin-detecting CDI testing algorithms (TCTA, using GDH/toxin or NAAT/toxin), or non-TCTA methods, which included all other algorithms or stand-alone testing methods.
Wide variation was seen between countries in testing methodologies. The United Kingdom had the highest rate of TCTA testing at 89%, while Germany had the lowest, at 8%, with 30 of 37 (81%) of participating German hospitals using non–toxin detection methods.
In addition, both testing density and case incidence rates varied between countries. Standardizing test density to mean number of tests per 10,000 PBDs per hospital per month (T/PBDs/H/M), the United Kingdom had the highest density, at 96.0 T/PBDs/H/M, while France had the lowest, at 34.4 T/PBDs/H/M. Overall per-nation case rates ranged from 2.55 C/PBDs/H/M in the United Kingdom to 6.9 C/PBDs/H/M in Spain.
Ms. Davies and her collaborators also analyzed data for all of the hospitals in any country according to testing method. That analysis saw no significant difference in seasonal variation testing rates for TCTA-using hospitals (mean T/PBDs/H/M in summer, 119.2 versus 102.4 in winter, P = .11), and no significant seasonal variation in CDI incidence. However, “the largest variation in CDI rates was seen in those hospitals using toxin-only diagnostic methods,” said Ms. Davies.
By contrast, for hospitals using non-TCTA methods, though testing rates did not change significantly, incidence was significantly higher in winter months, at a mean 13.5 wintertime versus 10.0 summertime C/PBDs/H/M (P = .49).
One country, Italy, stood out for having both higher overall wintertime testing (mean 57.2 summertime versus 78.8 wintertime T/PBDs/H/M, P = .041), and higher incidence (mean 6.6 summertime versus 10.1 wintertime C/PBDs/H/M, P = .017).
“Reported CDI rates only increase in winter if testing rates increase concurrently, or if hospitals use nonrecommended testing methods for diagnosis, especially non–toxin detection methods,” said Ms. Davies.
The study investigators reported receiving financial support from Sanofi Pasteur.
On Twitter @karioakes
BOSTON – No winter spike in Clostridium difficile infection (CDI) rates was seen among hospitalized patients after testing methodologies and frequency were accounted for, according to a large multinational study.
A total of 180 hospitals in five European countries had wide variation in CDI testing methods and testing density. However, among the hospitals that used a currently recommended toxin-detecting testing algorithm, there was no significant seasonal variation in cases, defined as mean cases per 10,000 patient bed-days per hospital per month (C/PBDs/H/M). The hospitals using toxin-detecting algorithms had summer C/PBDs/H/M rates of 9.6, compared to 8.0 in winter months (P = .27).
These results, presented at the annual meeting of the American Society for Microbiology by Kerrie Davies, clinical scientist at the University of Leeds (England), stand in contrast to some other studies that have shown a wintertime peak in CDI incidence. The data presented help in “understanding the context in which published reported rate data have been generated,” said Ms. Davies, enabling a better understanding both of how samples are tested, and who gets tested.
The study enrolled 180 hospitals – 38 each in France and Italy, 37 each in Germany and the United Kingdom, and 30 in Spain. Institutions reported patient demographics, as well as CDI testing data and patient bed-days for CDI cases, for 1 year.
Current European and U.K. CDI testing algorithms, said Ms. Davies, begin either with testing for glutamate dehydrogenase (GDH) or with nucleic acid amplification testing (NAAT), and then proceed to enzyme-linked immunosorbent assay (ELISA) testing for C. difficile toxins A and B.
Other algorithms, for example those that begin with toxin testing, are not recommended, said Ms. Davies. Some institutions may diagnose CDI only by toxin detection, GDH testing, or NAAT testing.
For data analysis, Ms. Davies and her collaborators compared CDI-related PBDs and testing density during June, July, and August to data collected in December, January, and February. Testing methods were dichotomized to toxin-detecting CDI testing algorithms (TCTA, using GDH/toxin or NAAT/toxin), or non-TCTA methods, which included all other algorithms or stand-alone testing methods.
Wide variation was seen between countries in testing methodologies. The United Kingdom had the highest rate of TCTA testing at 89%, while Germany had the lowest, at 8%, with 30 of 37 (81%) of participating German hospitals using non–toxin detection methods.
In addition, both testing density and case incidence rates varied between countries. Standardizing test density to mean number of tests per 10,000 PBDs per hospital per month (T/PBDs/H/M), the United Kingdom had the highest density, at 96.0 T/PBDs/H/M, while France had the lowest, at 34.4 T/PBDs/H/M. Overall per-nation case rates ranged from 2.55 C/PBDs/H/M in the United Kingdom to 6.9 C/PBDs/H/M in Spain.
Ms. Davies and her collaborators also analyzed data for all of the hospitals in any country according to testing method. That analysis saw no significant difference in seasonal variation testing rates for TCTA-using hospitals (mean T/PBDs/H/M in summer, 119.2 versus 102.4 in winter, P = .11), and no significant seasonal variation in CDI incidence. However, “the largest variation in CDI rates was seen in those hospitals using toxin-only diagnostic methods,” said Ms. Davies.
By contrast, for hospitals using non-TCTA methods, though testing rates did not change significantly, incidence was significantly higher in winter months, at a mean 13.5 wintertime versus 10.0 summertime C/PBDs/H/M (P = .49).
One country, Italy, stood out for having both higher overall wintertime testing (mean 57.2 summertime versus 78.8 wintertime T/PBDs/H/M, P = .041), and higher incidence (mean 6.6 summertime versus 10.1 wintertime C/PBDs/H/M, P = .017).
“Reported CDI rates only increase in winter if testing rates increase concurrently, or if hospitals use nonrecommended testing methods for diagnosis, especially non–toxin detection methods,” said Ms. Davies.
The study investigators reported receiving financial support from Sanofi Pasteur.
On Twitter @karioakes
BOSTON – No winter spike in Clostridium difficile infection (CDI) rates was seen among hospitalized patients after testing methodologies and frequency were accounted for, according to a large multinational study.
A total of 180 hospitals in five European countries had wide variation in CDI testing methods and testing density. However, among the hospitals that used a currently recommended toxin-detecting testing algorithm, there was no significant seasonal variation in cases, defined as mean cases per 10,000 patient bed-days per hospital per month (C/PBDs/H/M). The hospitals using toxin-detecting algorithms had summer C/PBDs/H/M rates of 9.6, compared to 8.0 in winter months (P = .27).
These results, presented at the annual meeting of the American Society for Microbiology by Kerrie Davies, clinical scientist at the University of Leeds (England), stand in contrast to some other studies that have shown a wintertime peak in CDI incidence. The data presented help in “understanding the context in which published reported rate data have been generated,” said Ms. Davies, enabling a better understanding both of how samples are tested, and who gets tested.
The study enrolled 180 hospitals – 38 each in France and Italy, 37 each in Germany and the United Kingdom, and 30 in Spain. Institutions reported patient demographics, as well as CDI testing data and patient bed-days for CDI cases, for 1 year.
Current European and U.K. CDI testing algorithms, said Ms. Davies, begin either with testing for glutamate dehydrogenase (GDH) or with nucleic acid amplification testing (NAAT), and then proceed to enzyme-linked immunosorbent assay (ELISA) testing for C. difficile toxins A and B.
Other algorithms, for example those that begin with toxin testing, are not recommended, said Ms. Davies. Some institutions may diagnose CDI only by toxin detection, GDH testing, or NAAT testing.
For data analysis, Ms. Davies and her collaborators compared CDI-related PBDs and testing density during June, July, and August to data collected in December, January, and February. Testing methods were dichotomized to toxin-detecting CDI testing algorithms (TCTA, using GDH/toxin or NAAT/toxin), or non-TCTA methods, which included all other algorithms or stand-alone testing methods.
Wide variation was seen between countries in testing methodologies. The United Kingdom had the highest rate of TCTA testing at 89%, while Germany had the lowest, at 8%, with 30 of 37 (81%) of participating German hospitals using non–toxin detection methods.
In addition, both testing density and case incidence rates varied between countries. Standardizing test density to mean number of tests per 10,000 PBDs per hospital per month (T/PBDs/H/M), the United Kingdom had the highest density, at 96.0 T/PBDs/H/M, while France had the lowest, at 34.4 T/PBDs/H/M. Overall per-nation case rates ranged from 2.55 C/PBDs/H/M in the United Kingdom to 6.9 C/PBDs/H/M in Spain.
Ms. Davies and her collaborators also analyzed data for all of the hospitals in any country according to testing method. That analysis saw no significant difference in seasonal variation testing rates for TCTA-using hospitals (mean T/PBDs/H/M in summer, 119.2 versus 102.4 in winter, P = .11), and no significant seasonal variation in CDI incidence. However, “the largest variation in CDI rates was seen in those hospitals using toxin-only diagnostic methods,” said Ms. Davies.
By contrast, for hospitals using non-TCTA methods, though testing rates did not change significantly, incidence was significantly higher in winter months, at a mean 13.5 wintertime versus 10.0 summertime C/PBDs/H/M (P = .49).
One country, Italy, stood out for having both higher overall wintertime testing (mean 57.2 summertime versus 78.8 wintertime T/PBDs/H/M, P = .041), and higher incidence (mean 6.6 summertime versus 10.1 wintertime C/PBDs/H/M, P = .017).
“Reported CDI rates only increase in winter if testing rates increase concurrently, or if hospitals use nonrecommended testing methods for diagnosis, especially non–toxin detection methods,” said Ms. Davies.
The study investigators reported receiving financial support from Sanofi Pasteur.
On Twitter @karioakes
AT ASM MICROBE 2016
Key clinical point: After researchers accounted for testing frequency and methods, Clostridium difficile infection (CDI) rates were not higher in the winter months.
Major finding: In five European countries, hospitals that used direct toxin-detecting algorithms to test for CDI had no seasonal variation in CDI incidence (mean cases/patient bed-days/hospital/month in summer, 9.6; in winter, 8.0; P = .27).
Data source: Demographic and testing data collection from 180 hospitals in five European countries to ascertain CDI testing methods, rates, cases, and patient bed-days per month.
Disclosures: The study investigators reported financial support from Sanofi Pasteur.
Successfully Quitting Smoking May Take Many Attempts
Though conventional wisdom says it takes five to seven attempts for most smokers to quit, those estimates may be very low, a recent study suggests.
Based on data for more than 1,200 adult smokers in Canada, the real average number of quit attempts before succeeding may be closer to 30.
"For so long we've been talking about five to seven attempts to quit," said lead author Dr. Michael Chaiton of the School of Public Health at the University of Toronto in Canada. "For us (the numbers) were a lot higher."
The lower estimate comes from a few past studies that were based on the lifetime recollections of people who successfully quit, but they didn't include attempts by people who had not yet succeeded, Chaiton and colleagues note in the journal BMJ Open, June 9.
For their study, the researchers analyzed data from 1,277 people in the Ontario Tobacco Survey who were followed for up to three years. When the study began in 2005, participants reported how many times they recalled ever making a serious attempt to quit smoking, and at each six-month follow-up they reported how many serious quit attempts they had made over the past six months.
A quit attempt was deemed a success when a participant went at least one year without a cigarette.
The researchers used these responses and four different statistical models to estimate how many times the average smoker attempts to quit before succeeding. The most unbiased model suggested an average of 30 quit attempts per smoker.
That's much higher than people tended to report in the previous studies when asked about all their quit attempts since starting smoking, the study team writes.
"People are very bad at remembering over their whole lifetimes," Chaiton told Reuters Health. "The second problem is we were only asking people who have been successful at quitting."
The new study may be a better representation of what most smokers go through over time, but it does only describe their situation rather than predict what will happen to an individual smoker who tries to quit, he cautioned.
"This doesn't mean you hit a magic number and then you can quit," Chaiton said. "There are many people who are able to and do quit on their first attempt or in the first few.
"There are people who are good at many things, some are good at quitting smoking," he added.
Quitting smoking is often a long-term process with many attempts, he said.
"When we talk about trying to reduce the number of smokers, if we try and do that by focusing on one quit attempt at a time we're not going to be very successful," Chaiton said.
A range of smoking cessation medications, policies like smoke-free spaces and plain-pack warnings can all help some smokers quit, he said.
"The main impact of this article is that clinicians should reassure smokers that, just because they have failed 10 times, does not mean they will never quit," said Dr. John Hughes of the University of Vermont School of Medicine in Burlington.
"However, the problem with taking, say, 20 times to quit, is that this may take 10 years and it's not only important to quit but it's important to quit while you are younger," said Hughes, who was not part of the new study.
"So it's important for those who failed several times to seek treatment to increase odds of quitting and we have lots of medication and counseling treatments that work," Hughes told Reuters Health by email.
SOURCE: http://bit.ly/28LH9ED
BMJ Open 2016.
Though conventional wisdom says it takes five to seven attempts for most smokers to quit, those estimates may be very low, a recent study suggests.
Based on data for more than 1,200 adult smokers in Canada, the real average number of quit attempts before succeeding may be closer to 30.
"For so long we've been talking about five to seven attempts to quit," said lead author Dr. Michael Chaiton of the School of Public Health at the University of Toronto in Canada. "For us (the numbers) were a lot higher."
The lower estimate comes from a few past studies that were based on the lifetime recollections of people who successfully quit, but they didn't include attempts by people who had not yet succeeded, Chaiton and colleagues note in the journal BMJ Open, June 9.
For their study, the researchers analyzed data from 1,277 people in the Ontario Tobacco Survey who were followed for up to three years. When the study began in 2005, participants reported how many times they recalled ever making a serious attempt to quit smoking, and at each six-month follow-up they reported how many serious quit attempts they had made over the past six months.
A quit attempt was deemed a success when a participant went at least one year without a cigarette.
The researchers used these responses and four different statistical models to estimate how many times the average smoker attempts to quit before succeeding. The most unbiased model suggested an average of 30 quit attempts per smoker.
That's much higher than people tended to report in the previous studies when asked about all their quit attempts since starting smoking, the study team writes.
"People are very bad at remembering over their whole lifetimes," Chaiton told Reuters Health. "The second problem is we were only asking people who have been successful at quitting."
The new study may be a better representation of what most smokers go through over time, but it does only describe their situation rather than predict what will happen to an individual smoker who tries to quit, he cautioned.
"This doesn't mean you hit a magic number and then you can quit," Chaiton said. "There are many people who are able to and do quit on their first attempt or in the first few.
"There are people who are good at many things, some are good at quitting smoking," he added.
Quitting smoking is often a long-term process with many attempts, he said.
"When we talk about trying to reduce the number of smokers, if we try and do that by focusing on one quit attempt at a time we're not going to be very successful," Chaiton said.
A range of smoking cessation medications, policies like smoke-free spaces and plain-pack warnings can all help some smokers quit, he said.
"The main impact of this article is that clinicians should reassure smokers that, just because they have failed 10 times, does not mean they will never quit," said Dr. John Hughes of the University of Vermont School of Medicine in Burlington.
"However, the problem with taking, say, 20 times to quit, is that this may take 10 years and it's not only important to quit but it's important to quit while you are younger," said Hughes, who was not part of the new study.
"So it's important for those who failed several times to seek treatment to increase odds of quitting and we have lots of medication and counseling treatments that work," Hughes told Reuters Health by email.
SOURCE: http://bit.ly/28LH9ED
BMJ Open 2016.
Though conventional wisdom says it takes five to seven attempts for most smokers to quit, those estimates may be very low, a recent study suggests.
Based on data for more than 1,200 adult smokers in Canada, the real average number of quit attempts before succeeding may be closer to 30.
"For so long we've been talking about five to seven attempts to quit," said lead author Dr. Michael Chaiton of the School of Public Health at the University of Toronto in Canada. "For us (the numbers) were a lot higher."
The lower estimate comes from a few past studies that were based on the lifetime recollections of people who successfully quit, but they didn't include attempts by people who had not yet succeeded, Chaiton and colleagues note in the journal BMJ Open, June 9.
For their study, the researchers analyzed data from 1,277 people in the Ontario Tobacco Survey who were followed for up to three years. When the study began in 2005, participants reported how many times they recalled ever making a serious attempt to quit smoking, and at each six-month follow-up they reported how many serious quit attempts they had made over the past six months.
A quit attempt was deemed a success when a participant went at least one year without a cigarette.
The researchers used these responses and four different statistical models to estimate how many times the average smoker attempts to quit before succeeding. The most unbiased model suggested an average of 30 quit attempts per smoker.
That's much higher than people tended to report in the previous studies when asked about all their quit attempts since starting smoking, the study team writes.
"People are very bad at remembering over their whole lifetimes," Chaiton told Reuters Health. "The second problem is we were only asking people who have been successful at quitting."
The new study may be a better representation of what most smokers go through over time, but it does only describe their situation rather than predict what will happen to an individual smoker who tries to quit, he cautioned.
"This doesn't mean you hit a magic number and then you can quit," Chaiton said. "There are many people who are able to and do quit on their first attempt or in the first few.
"There are people who are good at many things, some are good at quitting smoking," he added.
Quitting smoking is often a long-term process with many attempts, he said.
"When we talk about trying to reduce the number of smokers, if we try and do that by focusing on one quit attempt at a time we're not going to be very successful," Chaiton said.
A range of smoking cessation medications, policies like smoke-free spaces and plain-pack warnings can all help some smokers quit, he said.
"The main impact of this article is that clinicians should reassure smokers that, just because they have failed 10 times, does not mean they will never quit," said Dr. John Hughes of the University of Vermont School of Medicine in Burlington.
"However, the problem with taking, say, 20 times to quit, is that this may take 10 years and it's not only important to quit but it's important to quit while you are younger," said Hughes, who was not part of the new study.
"So it's important for those who failed several times to seek treatment to increase odds of quitting and we have lots of medication and counseling treatments that work," Hughes told Reuters Health by email.
SOURCE: http://bit.ly/28LH9ED
BMJ Open 2016.
Initiative aims to improve transition from pediatric to adult care
Gregg Michael Talente, MD, remembers well a young patient who nearly fell through the gap between pediatric an adult health care.
The woman was treated for lupus by her pediatrician until age 19 when the doctor moved out of town. When she landed in front of Dr. Talente, it was clear the patient lacked the knowledge and skills to self-manage her condition, he recalled. Dr. Talente and his team, including pharmacists, helped the young woman understand how to administer her medications, provided refill reminders, and counseled her about reproductive health and how other medications could interact with lupus treatment.
“I look at her as a near-miss case,” said Dr. Talente, director of the internal medicine resident clinic at the University of South Carolina, Columbia, who specializes in pediatric-adolescent medicine. “A lot of bad things could have happened to her because her transition was delayed, and she wasn’t prepared. Fortunately, she landed in a place with more resources than a typical adult clinic so we were able to catch up.”
A new national initiative is designed to aid patients such as this during the move from pediatric to adult health care. The Pediatric to Adult Care Transitions Initiative is a collaborative effort by various specialty groups to facilitate more effective transition and transfer of young adults, while providing a framework for pediatricians and adult care providers. The project is under the direction of the American College of Physicians’ (ACP) Council of Subspecialty Societies in conjunction with the Got Transition (GT)/Center for Health Care Transition Improvement, the Society of General Internal Medicine (SGIM), and the Society for Adolescent Health and Medicine. Got Transition is a cooperative project by the Maternal and Child Health Bureau and The National Alliance to Advance Adolescent Health to improve pediatric-to-adult-care transitions through innovative strategies.
Since the Pediatric to Adult Care Transitions Initiative launched in spring 2015, project leaders have designed a series of disease-specific tools to enable smoother transition of patients. The downloadable tools include a transition readiness assessment, a medical summary/transfer record tool, and a self-care assessment. The guides were adapted from Got Transition’s six core elements of health care transition, developed from joint clinical recommendations by the ACP, the American Academy of Pediatrics, and the American Academy of Family Physicians.
The disease-specific tools are just the beginning, said Carol Greenlee, MD, chair of the Pediatric to Adult Care Transitions Initiative and chair of the ACP’s Council of Subspecialty Societies.
“We don’t want to just have tools on a website, we want to improve the whole process,” she said. “One of our goals is education and implementation. Part of implementation has to include collaboration because you don’t care-coordinate in isolation. You have to care-coordinate not just with the patient and family, but the pediatric and adult care providers need to collaborate.”
A need for better transition
Data show that knowledge and resources are lacking on both the pediatric and adult care side when it comes to transitioning patients from one realm to the other. A 2009 survey by the AAP found that most pediatric practices neither initiate transition planning early in adolescence nor offer transition-support service (AAP News 2009 Nov. Vol. 30). Another study in the Journal of General Internal Medicine found that many adult providers feel unprepared to care for young adults with complex chronic conditions and that in some cases, there is no identified adult primary care or specialty provider to whom care can be transitioned (J Gen Intern Med. 2008 Oct;23[10]:1621-7). Lack of time, inadequate payment, and poor training also have been cited as barriers to successful transition (Pediatrics. 2001 Jul 1. doi: 10.1542/peds.2011-0969).
Poor transitions often lead to negative health outcomes for young adults, said Dr. Patience White, codirector of Got Transition and professor of medicine and pediatrics at George Washington University, Washington. She has co-led the Pediatric to Adult Care Transitions Initiative.
“The quality of care goes down; many [patients] are lost from their care, and they don’t get the kind of care they need,” Dr. White said. “Therefore, they have poor outcomes, and then of course, the cost goes up because they are utilizing emergency rooms or tests are repeated. You’re looking at poor patient experience, poor quality, and increased cost.”
Enter the Pediatric to Adult Care Transitions Initiative. The project was designed with the busy practices of pediatricians and adult care providers in mind, said Dr. Talente, who is past chair of the SGIM Adults with Complex Conditions Originating in Childhood Task Force.
“Everyone is busy, and asking each individual practitioner to develop the systems they need to do this right is not really practical,” he said. “That’s where this project is really helpful, in the sense that it’s attempting to deliver tools and systems to providers that they can use and just adapt, without doing all the work themselves when they’re trying to run their busy practices.”
The initiative’s readiness assessment is a first step toward improving early transition planning, Dr. Greenlee said. The tool allows pediatricians to measure the knowledge and skill level of patients in the years leading to transition age, and enables doctors to fill any gaps before the transfer occurs.
The tool helps physicians gauge “what this young adult needs to know before they go out into the adult world and take on self-management,” she said. “Making sure they know how to fill a prescription, how to take their medications, know signs and symptoms of a crisis – that sort of thing.”
To enable better communication between providers, initiative leaders created the transfer summary, a hand-off outline that includes critical items the receiving clinician should know about the patients, such as information about their conditions, personal interests, or special needs. The third tool launched by the initiative – a self-management assessment – is a resource for adult care providers to measure the patients’ skills and knowledge once they begin adult care.
Overcoming obstacles to transition
The path to smoother patient transitions is not without bumps in the road. Adding time and new tools to physicians’ already heavy workloads can be challenging, Dr. Greenlee said.
“One of the biggest challenges is the time it takes on both sides,” she said. “Here we’re saying, ‘Here’s more to do.’ As a pediatrician, you’re gong to be preparing and educating [patients] in self-management and trying to get the parents engaged. There’s extra work.”
But helping pediatricians understand the bigger picture results – better outcomes, improved quality, lower health care costs – is key to acceptance, according to initiative leaders.
Strengthening communication between pediatric and adult practices also is critical to making the transition tools effective, added Dr. White. Pediatric practices cannot make successful transfers alone.
“The challenges are to find the partnerships that you need to start it, and the next big challenge is the buy-in,” she said. “You’ve got to get your leadership and senior physicians in a practice to agree that this is something they’re going to do. What’s frustrating for families is when different physicians use different modes of this whole process.”
Reimbursement for transition-related care is an ongoing climb, added Dr. Talente. In the past, physicians have struggled to receive payment for certain nonvisit time needed for transitions, he said.
However, Got Transition recently made headway toward improved payment, Dr. White said. Code 99420 now can be used to bill for transition readiness assessments conducted with youth and self-care assessments conducted with young adults. Got Transition and several physician specialty organizations also have developed a payment work group to address transition care codes. Got Transition offers a coding and reimbursement tip sheet to aid doctors in billing for pediatric to adult transitions.
Dr. Greenlee said she hopes that more pediatricians will start using the tools developed by the initiative, and she recommends reviewing the Got Transition website (www.gottransition.org) and considering how to incorporate transition efforts into practices.
“Start with a policy,” she said. “Start thinking about your approach and then make that approach intentional. My advice ... is to just start with one step at a time.”
On Twitter @legal_med
Gregg Michael Talente, MD, remembers well a young patient who nearly fell through the gap between pediatric an adult health care.
The woman was treated for lupus by her pediatrician until age 19 when the doctor moved out of town. When she landed in front of Dr. Talente, it was clear the patient lacked the knowledge and skills to self-manage her condition, he recalled. Dr. Talente and his team, including pharmacists, helped the young woman understand how to administer her medications, provided refill reminders, and counseled her about reproductive health and how other medications could interact with lupus treatment.
“I look at her as a near-miss case,” said Dr. Talente, director of the internal medicine resident clinic at the University of South Carolina, Columbia, who specializes in pediatric-adolescent medicine. “A lot of bad things could have happened to her because her transition was delayed, and she wasn’t prepared. Fortunately, she landed in a place with more resources than a typical adult clinic so we were able to catch up.”
A new national initiative is designed to aid patients such as this during the move from pediatric to adult health care. The Pediatric to Adult Care Transitions Initiative is a collaborative effort by various specialty groups to facilitate more effective transition and transfer of young adults, while providing a framework for pediatricians and adult care providers. The project is under the direction of the American College of Physicians’ (ACP) Council of Subspecialty Societies in conjunction with the Got Transition (GT)/Center for Health Care Transition Improvement, the Society of General Internal Medicine (SGIM), and the Society for Adolescent Health and Medicine. Got Transition is a cooperative project by the Maternal and Child Health Bureau and The National Alliance to Advance Adolescent Health to improve pediatric-to-adult-care transitions through innovative strategies.
Since the Pediatric to Adult Care Transitions Initiative launched in spring 2015, project leaders have designed a series of disease-specific tools to enable smoother transition of patients. The downloadable tools include a transition readiness assessment, a medical summary/transfer record tool, and a self-care assessment. The guides were adapted from Got Transition’s six core elements of health care transition, developed from joint clinical recommendations by the ACP, the American Academy of Pediatrics, and the American Academy of Family Physicians.
The disease-specific tools are just the beginning, said Carol Greenlee, MD, chair of the Pediatric to Adult Care Transitions Initiative and chair of the ACP’s Council of Subspecialty Societies.
“We don’t want to just have tools on a website, we want to improve the whole process,” she said. “One of our goals is education and implementation. Part of implementation has to include collaboration because you don’t care-coordinate in isolation. You have to care-coordinate not just with the patient and family, but the pediatric and adult care providers need to collaborate.”
A need for better transition
Data show that knowledge and resources are lacking on both the pediatric and adult care side when it comes to transitioning patients from one realm to the other. A 2009 survey by the AAP found that most pediatric practices neither initiate transition planning early in adolescence nor offer transition-support service (AAP News 2009 Nov. Vol. 30). Another study in the Journal of General Internal Medicine found that many adult providers feel unprepared to care for young adults with complex chronic conditions and that in some cases, there is no identified adult primary care or specialty provider to whom care can be transitioned (J Gen Intern Med. 2008 Oct;23[10]:1621-7). Lack of time, inadequate payment, and poor training also have been cited as barriers to successful transition (Pediatrics. 2001 Jul 1. doi: 10.1542/peds.2011-0969).
Poor transitions often lead to negative health outcomes for young adults, said Dr. Patience White, codirector of Got Transition and professor of medicine and pediatrics at George Washington University, Washington. She has co-led the Pediatric to Adult Care Transitions Initiative.
“The quality of care goes down; many [patients] are lost from their care, and they don’t get the kind of care they need,” Dr. White said. “Therefore, they have poor outcomes, and then of course, the cost goes up because they are utilizing emergency rooms or tests are repeated. You’re looking at poor patient experience, poor quality, and increased cost.”
Enter the Pediatric to Adult Care Transitions Initiative. The project was designed with the busy practices of pediatricians and adult care providers in mind, said Dr. Talente, who is past chair of the SGIM Adults with Complex Conditions Originating in Childhood Task Force.
“Everyone is busy, and asking each individual practitioner to develop the systems they need to do this right is not really practical,” he said. “That’s where this project is really helpful, in the sense that it’s attempting to deliver tools and systems to providers that they can use and just adapt, without doing all the work themselves when they’re trying to run their busy practices.”
The initiative’s readiness assessment is a first step toward improving early transition planning, Dr. Greenlee said. The tool allows pediatricians to measure the knowledge and skill level of patients in the years leading to transition age, and enables doctors to fill any gaps before the transfer occurs.
The tool helps physicians gauge “what this young adult needs to know before they go out into the adult world and take on self-management,” she said. “Making sure they know how to fill a prescription, how to take their medications, know signs and symptoms of a crisis – that sort of thing.”
To enable better communication between providers, initiative leaders created the transfer summary, a hand-off outline that includes critical items the receiving clinician should know about the patients, such as information about their conditions, personal interests, or special needs. The third tool launched by the initiative – a self-management assessment – is a resource for adult care providers to measure the patients’ skills and knowledge once they begin adult care.
Overcoming obstacles to transition
The path to smoother patient transitions is not without bumps in the road. Adding time and new tools to physicians’ already heavy workloads can be challenging, Dr. Greenlee said.
“One of the biggest challenges is the time it takes on both sides,” she said. “Here we’re saying, ‘Here’s more to do.’ As a pediatrician, you’re gong to be preparing and educating [patients] in self-management and trying to get the parents engaged. There’s extra work.”
But helping pediatricians understand the bigger picture results – better outcomes, improved quality, lower health care costs – is key to acceptance, according to initiative leaders.
Strengthening communication between pediatric and adult practices also is critical to making the transition tools effective, added Dr. White. Pediatric practices cannot make successful transfers alone.
“The challenges are to find the partnerships that you need to start it, and the next big challenge is the buy-in,” she said. “You’ve got to get your leadership and senior physicians in a practice to agree that this is something they’re going to do. What’s frustrating for families is when different physicians use different modes of this whole process.”
Reimbursement for transition-related care is an ongoing climb, added Dr. Talente. In the past, physicians have struggled to receive payment for certain nonvisit time needed for transitions, he said.
However, Got Transition recently made headway toward improved payment, Dr. White said. Code 99420 now can be used to bill for transition readiness assessments conducted with youth and self-care assessments conducted with young adults. Got Transition and several physician specialty organizations also have developed a payment work group to address transition care codes. Got Transition offers a coding and reimbursement tip sheet to aid doctors in billing for pediatric to adult transitions.
Dr. Greenlee said she hopes that more pediatricians will start using the tools developed by the initiative, and she recommends reviewing the Got Transition website (www.gottransition.org) and considering how to incorporate transition efforts into practices.
“Start with a policy,” she said. “Start thinking about your approach and then make that approach intentional. My advice ... is to just start with one step at a time.”
On Twitter @legal_med
Gregg Michael Talente, MD, remembers well a young patient who nearly fell through the gap between pediatric an adult health care.
The woman was treated for lupus by her pediatrician until age 19 when the doctor moved out of town. When she landed in front of Dr. Talente, it was clear the patient lacked the knowledge and skills to self-manage her condition, he recalled. Dr. Talente and his team, including pharmacists, helped the young woman understand how to administer her medications, provided refill reminders, and counseled her about reproductive health and how other medications could interact with lupus treatment.
“I look at her as a near-miss case,” said Dr. Talente, director of the internal medicine resident clinic at the University of South Carolina, Columbia, who specializes in pediatric-adolescent medicine. “A lot of bad things could have happened to her because her transition was delayed, and she wasn’t prepared. Fortunately, she landed in a place with more resources than a typical adult clinic so we were able to catch up.”
A new national initiative is designed to aid patients such as this during the move from pediatric to adult health care. The Pediatric to Adult Care Transitions Initiative is a collaborative effort by various specialty groups to facilitate more effective transition and transfer of young adults, while providing a framework for pediatricians and adult care providers. The project is under the direction of the American College of Physicians’ (ACP) Council of Subspecialty Societies in conjunction with the Got Transition (GT)/Center for Health Care Transition Improvement, the Society of General Internal Medicine (SGIM), and the Society for Adolescent Health and Medicine. Got Transition is a cooperative project by the Maternal and Child Health Bureau and The National Alliance to Advance Adolescent Health to improve pediatric-to-adult-care transitions through innovative strategies.
Since the Pediatric to Adult Care Transitions Initiative launched in spring 2015, project leaders have designed a series of disease-specific tools to enable smoother transition of patients. The downloadable tools include a transition readiness assessment, a medical summary/transfer record tool, and a self-care assessment. The guides were adapted from Got Transition’s six core elements of health care transition, developed from joint clinical recommendations by the ACP, the American Academy of Pediatrics, and the American Academy of Family Physicians.
The disease-specific tools are just the beginning, said Carol Greenlee, MD, chair of the Pediatric to Adult Care Transitions Initiative and chair of the ACP’s Council of Subspecialty Societies.
“We don’t want to just have tools on a website, we want to improve the whole process,” she said. “One of our goals is education and implementation. Part of implementation has to include collaboration because you don’t care-coordinate in isolation. You have to care-coordinate not just with the patient and family, but the pediatric and adult care providers need to collaborate.”
A need for better transition
Data show that knowledge and resources are lacking on both the pediatric and adult care side when it comes to transitioning patients from one realm to the other. A 2009 survey by the AAP found that most pediatric practices neither initiate transition planning early in adolescence nor offer transition-support service (AAP News 2009 Nov. Vol. 30). Another study in the Journal of General Internal Medicine found that many adult providers feel unprepared to care for young adults with complex chronic conditions and that in some cases, there is no identified adult primary care or specialty provider to whom care can be transitioned (J Gen Intern Med. 2008 Oct;23[10]:1621-7). Lack of time, inadequate payment, and poor training also have been cited as barriers to successful transition (Pediatrics. 2001 Jul 1. doi: 10.1542/peds.2011-0969).
Poor transitions often lead to negative health outcomes for young adults, said Dr. Patience White, codirector of Got Transition and professor of medicine and pediatrics at George Washington University, Washington. She has co-led the Pediatric to Adult Care Transitions Initiative.
“The quality of care goes down; many [patients] are lost from their care, and they don’t get the kind of care they need,” Dr. White said. “Therefore, they have poor outcomes, and then of course, the cost goes up because they are utilizing emergency rooms or tests are repeated. You’re looking at poor patient experience, poor quality, and increased cost.”
Enter the Pediatric to Adult Care Transitions Initiative. The project was designed with the busy practices of pediatricians and adult care providers in mind, said Dr. Talente, who is past chair of the SGIM Adults with Complex Conditions Originating in Childhood Task Force.
“Everyone is busy, and asking each individual practitioner to develop the systems they need to do this right is not really practical,” he said. “That’s where this project is really helpful, in the sense that it’s attempting to deliver tools and systems to providers that they can use and just adapt, without doing all the work themselves when they’re trying to run their busy practices.”
The initiative’s readiness assessment is a first step toward improving early transition planning, Dr. Greenlee said. The tool allows pediatricians to measure the knowledge and skill level of patients in the years leading to transition age, and enables doctors to fill any gaps before the transfer occurs.
The tool helps physicians gauge “what this young adult needs to know before they go out into the adult world and take on self-management,” she said. “Making sure they know how to fill a prescription, how to take their medications, know signs and symptoms of a crisis – that sort of thing.”
To enable better communication between providers, initiative leaders created the transfer summary, a hand-off outline that includes critical items the receiving clinician should know about the patients, such as information about their conditions, personal interests, or special needs. The third tool launched by the initiative – a self-management assessment – is a resource for adult care providers to measure the patients’ skills and knowledge once they begin adult care.
Overcoming obstacles to transition
The path to smoother patient transitions is not without bumps in the road. Adding time and new tools to physicians’ already heavy workloads can be challenging, Dr. Greenlee said.
“One of the biggest challenges is the time it takes on both sides,” she said. “Here we’re saying, ‘Here’s more to do.’ As a pediatrician, you’re gong to be preparing and educating [patients] in self-management and trying to get the parents engaged. There’s extra work.”
But helping pediatricians understand the bigger picture results – better outcomes, improved quality, lower health care costs – is key to acceptance, according to initiative leaders.
Strengthening communication between pediatric and adult practices also is critical to making the transition tools effective, added Dr. White. Pediatric practices cannot make successful transfers alone.
“The challenges are to find the partnerships that you need to start it, and the next big challenge is the buy-in,” she said. “You’ve got to get your leadership and senior physicians in a practice to agree that this is something they’re going to do. What’s frustrating for families is when different physicians use different modes of this whole process.”
Reimbursement for transition-related care is an ongoing climb, added Dr. Talente. In the past, physicians have struggled to receive payment for certain nonvisit time needed for transitions, he said.
However, Got Transition recently made headway toward improved payment, Dr. White said. Code 99420 now can be used to bill for transition readiness assessments conducted with youth and self-care assessments conducted with young adults. Got Transition and several physician specialty organizations also have developed a payment work group to address transition care codes. Got Transition offers a coding and reimbursement tip sheet to aid doctors in billing for pediatric to adult transitions.
Dr. Greenlee said she hopes that more pediatricians will start using the tools developed by the initiative, and she recommends reviewing the Got Transition website (www.gottransition.org) and considering how to incorporate transition efforts into practices.
“Start with a policy,” she said. “Start thinking about your approach and then make that approach intentional. My advice ... is to just start with one step at a time.”
On Twitter @legal_med
MDS Task Force Launches First-Ever Genetic Mutation Database for Movement Disorders
BERLIN—The International Parkinson and Movement Disorder Society (MDS) Task Force on Genetic Nomenclature in Movement Disorders has constructed an online database that provides a comprehensive overview of movement disorders phenotypes linked to causative gene mutations. The new MDS Genetic Mutation Database, MDSGene, was introduced at the 20th International Congress of Parkinson’s Disease and Movement Disorders.
Christine Klein, MD
Led by Christine Klein, MD, at the University of Lübeck and Connie Marras, MD, PhD, at the University of Toronto, the MDS Task Force is comprised of a global team of experienced movement disorder specialists and geneticists. Christina Lill, MD, MS, in conjunction with Lars Bertram, MD, both at the University of Lübeck in Germany, spearheaded the development of this innovative tool, which uses genetic and phenotypic/clinical data extracted from relevant literature. MDSGene displays extensive data on mutations in Parkinson’s disease, paroxysmal movement disorders, and familial brain calcification. The database also provides a comprehensive list of the available literature and an extensive summary of patients’ characteristics for each gene of interest using graphic and tabular data summaries. Primarily, MDSGene can be used as a resource to assist clinical diagnosis and guide research in the field of hereditary movement disorders.
Connie Marras, MD, PhD
José A. Obeso, MD, PhD, Professor of Neurology at the University of Navarra, Pamplona, Spain, and Editor-in-Chief of Movement Disorders, stated, “This new database is based on a formal analysis of the different clinical presentation of movement disorders and the associated genetic mutations published in the May issue of Movement Disorders. This very valuable effort will eventually lead to a comprehensive account of most types of movement disorders and their genetic origin, thus allowing and facilitating many newer studies and also serving as a diagnostic tool to clinicians worldwide.” Dr. Obeso added, “In sum, MDSGene is a major contribution to a better definition, classification, and understanding of Parkinson’s disease and several other movement disorders.”
The MDSGene database is available at www.mdsgene.org.
BERLIN—The International Parkinson and Movement Disorder Society (MDS) Task Force on Genetic Nomenclature in Movement Disorders has constructed an online database that provides a comprehensive overview of movement disorders phenotypes linked to causative gene mutations. The new MDS Genetic Mutation Database, MDSGene, was introduced at the 20th International Congress of Parkinson’s Disease and Movement Disorders.
Christine Klein, MD
Led by Christine Klein, MD, at the University of Lübeck and Connie Marras, MD, PhD, at the University of Toronto, the MDS Task Force is comprised of a global team of experienced movement disorder specialists and geneticists. Christina Lill, MD, MS, in conjunction with Lars Bertram, MD, both at the University of Lübeck in Germany, spearheaded the development of this innovative tool, which uses genetic and phenotypic/clinical data extracted from relevant literature. MDSGene displays extensive data on mutations in Parkinson’s disease, paroxysmal movement disorders, and familial brain calcification. The database also provides a comprehensive list of the available literature and an extensive summary of patients’ characteristics for each gene of interest using graphic and tabular data summaries. Primarily, MDSGene can be used as a resource to assist clinical diagnosis and guide research in the field of hereditary movement disorders.
Connie Marras, MD, PhD
José A. Obeso, MD, PhD, Professor of Neurology at the University of Navarra, Pamplona, Spain, and Editor-in-Chief of Movement Disorders, stated, “This new database is based on a formal analysis of the different clinical presentation of movement disorders and the associated genetic mutations published in the May issue of Movement Disorders. This very valuable effort will eventually lead to a comprehensive account of most types of movement disorders and their genetic origin, thus allowing and facilitating many newer studies and also serving as a diagnostic tool to clinicians worldwide.” Dr. Obeso added, “In sum, MDSGene is a major contribution to a better definition, classification, and understanding of Parkinson’s disease and several other movement disorders.”
The MDSGene database is available at www.mdsgene.org.
BERLIN—The International Parkinson and Movement Disorder Society (MDS) Task Force on Genetic Nomenclature in Movement Disorders has constructed an online database that provides a comprehensive overview of movement disorders phenotypes linked to causative gene mutations. The new MDS Genetic Mutation Database, MDSGene, was introduced at the 20th International Congress of Parkinson’s Disease and Movement Disorders.
Christine Klein, MD
Led by Christine Klein, MD, at the University of Lübeck and Connie Marras, MD, PhD, at the University of Toronto, the MDS Task Force is comprised of a global team of experienced movement disorder specialists and geneticists. Christina Lill, MD, MS, in conjunction with Lars Bertram, MD, both at the University of Lübeck in Germany, spearheaded the development of this innovative tool, which uses genetic and phenotypic/clinical data extracted from relevant literature. MDSGene displays extensive data on mutations in Parkinson’s disease, paroxysmal movement disorders, and familial brain calcification. The database also provides a comprehensive list of the available literature and an extensive summary of patients’ characteristics for each gene of interest using graphic and tabular data summaries. Primarily, MDSGene can be used as a resource to assist clinical diagnosis and guide research in the field of hereditary movement disorders.
Connie Marras, MD, PhD
José A. Obeso, MD, PhD, Professor of Neurology at the University of Navarra, Pamplona, Spain, and Editor-in-Chief of Movement Disorders, stated, “This new database is based on a formal analysis of the different clinical presentation of movement disorders and the associated genetic mutations published in the May issue of Movement Disorders. This very valuable effort will eventually lead to a comprehensive account of most types of movement disorders and their genetic origin, thus allowing and facilitating many newer studies and also serving as a diagnostic tool to clinicians worldwide.” Dr. Obeso added, “In sum, MDSGene is a major contribution to a better definition, classification, and understanding of Parkinson’s disease and several other movement disorders.”
The MDSGene database is available at www.mdsgene.org.
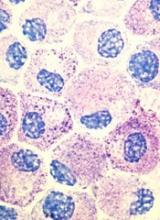
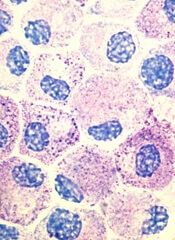
Inhibitor produces responses in advanced SM
Results of a phase 2 trial suggest the multikinase inhibitor midostaurin can repair organ damage in patients with advanced systemic mastocytosis (SM).
The drug produced a 60% response rate among patients with mastocytosis-related organ damage, and the median duration of response was 24.1 months.
Fifty-six percent of patients required dose reductions due to toxic effects, but 32% of these patients were able to return to the starting dose.
Jason Gotlib, MD, of the Stanford University School of Medicine in California, and his colleagues conducted this study and reported the results in NEJM.
The study was funded by Novartis Inc., which manufactures midostaurin, also known as PKC412.
The researchers noted that roughly 90% of patients with advanced SM have a mutation known as D816V in the gene that encodes the protein KIT, which controls the growth of mast cells.
Unfortunately, the only drug approved to treat advanced SM in the US is the tyrosine kinase inhibitor imatinib, and this drug is not active against the mutated KIT D816V protein. Midostaurin, on the other hand, does inhibit KIT D816V.
With this in mind, Dr Gotlib and his colleagues set out to test midostaurin (given at 100 mg twice daily until disease progression or unacceptable toxicity) in 116 patients.
Eighty-nine of the patients had mastocytosis-related organ damage, 16 had aggressive SM, 57 had SM with an associated hematologic neoplasm, and 16 had mast cell leukemia.
Response
The median follow-up was 26 months (range, 12 to 54), and the study’s primary outcome was the best overall response.
The overall response rate for the primary efficacy population (the 89 patients with mastocytosis-related organ damage) was 60%. Forty-five percent of the patients had a major response, which was defined as complete resolution of at least one type of mastocytosis-related organ damage.
The overall response rate was 75% for patients with aggressive SM, 58% for SM patients with an associated hematologic neoplasm, and 50% for patients with mast-cell leukemia.
Responses occurred in all subgroups, which included patients who were positive for KIT D816V.
The researchers noted that responding patients were less likely to need red blood cell or platelet transfusions, experienced improvements in liver function, and had fewer signs of malabsorption such as weight loss.
The median duration of response for all responders in the primary efficacy population (n=89) was 24.1 months.
Survival
The median overall survival was 28.7 months in the primary efficacy population (n=89) and 33.9 months in the intention-to-treat population (n=116). The median progression-free survival was 14.1 months in the primary efficacy population.
The survival benefit among patients with mast cell leukemia was particularly striking, according to Dr Gotlib. Although most people succumb to this form of the disease within 6 months of diagnosis, the median overall survival of midostaurin-treated patients with mast cell leukemia was 9.4 months.
The median overall survival was not reached among patients with aggressive SM and was 20.7 months among patients who had SM with an associated hematologic neoplasm.
Safety
The most common nonhematologic adverse events were nausea (79%), vomiting (66%), and diarrhea (54%). The most common grade 3/4 nonhematologic adverse events were fatigue (9%) and diarrhea (8%).
Grade 3/4 hematologic adverse events included new or worsening neutropenia (24%), anemia (41%), and thrombocytopenia (29%).
Researchers reduced the dose of midostaurin in 65 patients (56%), mostly due to adverse events (n=48). Twenty-one of these patients (32%) were later able to return to the initial dose.
Eighty-four patients (72%) discontinued treatment, and 32 (28%) were receiving ongoing treatment at the time of data cutoff. The most frequent reasons for treatment discontinuation were disease progression (33%) and adverse events (22%).
Compassionate use
The phase 2 study results are reinforced by a letter published in the same issue of NEJM, which describes a compassionate use program for midostaurin in advanced SM.
The letter includes results in 28 patients. After a median follow-up of 18.5 months, the overall response rate was 71%. The overall survival rate was 42.7%.
The most frequent adverse events were nausea/vomiting in 89% of patients (leading to failure/discontinuation in 18%), lymphocytopenia in 61% (without opportunistic infection), and photosensitivity in 25%. ![]()
Results of a phase 2 trial suggest the multikinase inhibitor midostaurin can repair organ damage in patients with advanced systemic mastocytosis (SM).
The drug produced a 60% response rate among patients with mastocytosis-related organ damage, and the median duration of response was 24.1 months.
Fifty-six percent of patients required dose reductions due to toxic effects, but 32% of these patients were able to return to the starting dose.
Jason Gotlib, MD, of the Stanford University School of Medicine in California, and his colleagues conducted this study and reported the results in NEJM.
The study was funded by Novartis Inc., which manufactures midostaurin, also known as PKC412.
The researchers noted that roughly 90% of patients with advanced SM have a mutation known as D816V in the gene that encodes the protein KIT, which controls the growth of mast cells.
Unfortunately, the only drug approved to treat advanced SM in the US is the tyrosine kinase inhibitor imatinib, and this drug is not active against the mutated KIT D816V protein. Midostaurin, on the other hand, does inhibit KIT D816V.
With this in mind, Dr Gotlib and his colleagues set out to test midostaurin (given at 100 mg twice daily until disease progression or unacceptable toxicity) in 116 patients.
Eighty-nine of the patients had mastocytosis-related organ damage, 16 had aggressive SM, 57 had SM with an associated hematologic neoplasm, and 16 had mast cell leukemia.
Response
The median follow-up was 26 months (range, 12 to 54), and the study’s primary outcome was the best overall response.
The overall response rate for the primary efficacy population (the 89 patients with mastocytosis-related organ damage) was 60%. Forty-five percent of the patients had a major response, which was defined as complete resolution of at least one type of mastocytosis-related organ damage.
The overall response rate was 75% for patients with aggressive SM, 58% for SM patients with an associated hematologic neoplasm, and 50% for patients with mast-cell leukemia.
Responses occurred in all subgroups, which included patients who were positive for KIT D816V.
The researchers noted that responding patients were less likely to need red blood cell or platelet transfusions, experienced improvements in liver function, and had fewer signs of malabsorption such as weight loss.
The median duration of response for all responders in the primary efficacy population (n=89) was 24.1 months.
Survival
The median overall survival was 28.7 months in the primary efficacy population (n=89) and 33.9 months in the intention-to-treat population (n=116). The median progression-free survival was 14.1 months in the primary efficacy population.
The survival benefit among patients with mast cell leukemia was particularly striking, according to Dr Gotlib. Although most people succumb to this form of the disease within 6 months of diagnosis, the median overall survival of midostaurin-treated patients with mast cell leukemia was 9.4 months.
The median overall survival was not reached among patients with aggressive SM and was 20.7 months among patients who had SM with an associated hematologic neoplasm.
Safety
The most common nonhematologic adverse events were nausea (79%), vomiting (66%), and diarrhea (54%). The most common grade 3/4 nonhematologic adverse events were fatigue (9%) and diarrhea (8%).
Grade 3/4 hematologic adverse events included new or worsening neutropenia (24%), anemia (41%), and thrombocytopenia (29%).
Researchers reduced the dose of midostaurin in 65 patients (56%), mostly due to adverse events (n=48). Twenty-one of these patients (32%) were later able to return to the initial dose.
Eighty-four patients (72%) discontinued treatment, and 32 (28%) were receiving ongoing treatment at the time of data cutoff. The most frequent reasons for treatment discontinuation were disease progression (33%) and adverse events (22%).
Compassionate use
The phase 2 study results are reinforced by a letter published in the same issue of NEJM, which describes a compassionate use program for midostaurin in advanced SM.
The letter includes results in 28 patients. After a median follow-up of 18.5 months, the overall response rate was 71%. The overall survival rate was 42.7%.
The most frequent adverse events were nausea/vomiting in 89% of patients (leading to failure/discontinuation in 18%), lymphocytopenia in 61% (without opportunistic infection), and photosensitivity in 25%. ![]()
Results of a phase 2 trial suggest the multikinase inhibitor midostaurin can repair organ damage in patients with advanced systemic mastocytosis (SM).
The drug produced a 60% response rate among patients with mastocytosis-related organ damage, and the median duration of response was 24.1 months.
Fifty-six percent of patients required dose reductions due to toxic effects, but 32% of these patients were able to return to the starting dose.
Jason Gotlib, MD, of the Stanford University School of Medicine in California, and his colleagues conducted this study and reported the results in NEJM.
The study was funded by Novartis Inc., which manufactures midostaurin, also known as PKC412.
The researchers noted that roughly 90% of patients with advanced SM have a mutation known as D816V in the gene that encodes the protein KIT, which controls the growth of mast cells.
Unfortunately, the only drug approved to treat advanced SM in the US is the tyrosine kinase inhibitor imatinib, and this drug is not active against the mutated KIT D816V protein. Midostaurin, on the other hand, does inhibit KIT D816V.
With this in mind, Dr Gotlib and his colleagues set out to test midostaurin (given at 100 mg twice daily until disease progression or unacceptable toxicity) in 116 patients.
Eighty-nine of the patients had mastocytosis-related organ damage, 16 had aggressive SM, 57 had SM with an associated hematologic neoplasm, and 16 had mast cell leukemia.
Response
The median follow-up was 26 months (range, 12 to 54), and the study’s primary outcome was the best overall response.
The overall response rate for the primary efficacy population (the 89 patients with mastocytosis-related organ damage) was 60%. Forty-five percent of the patients had a major response, which was defined as complete resolution of at least one type of mastocytosis-related organ damage.
The overall response rate was 75% for patients with aggressive SM, 58% for SM patients with an associated hematologic neoplasm, and 50% for patients with mast-cell leukemia.
Responses occurred in all subgroups, which included patients who were positive for KIT D816V.
The researchers noted that responding patients were less likely to need red blood cell or platelet transfusions, experienced improvements in liver function, and had fewer signs of malabsorption such as weight loss.
The median duration of response for all responders in the primary efficacy population (n=89) was 24.1 months.
Survival
The median overall survival was 28.7 months in the primary efficacy population (n=89) and 33.9 months in the intention-to-treat population (n=116). The median progression-free survival was 14.1 months in the primary efficacy population.
The survival benefit among patients with mast cell leukemia was particularly striking, according to Dr Gotlib. Although most people succumb to this form of the disease within 6 months of diagnosis, the median overall survival of midostaurin-treated patients with mast cell leukemia was 9.4 months.
The median overall survival was not reached among patients with aggressive SM and was 20.7 months among patients who had SM with an associated hematologic neoplasm.
Safety
The most common nonhematologic adverse events were nausea (79%), vomiting (66%), and diarrhea (54%). The most common grade 3/4 nonhematologic adverse events were fatigue (9%) and diarrhea (8%).
Grade 3/4 hematologic adverse events included new or worsening neutropenia (24%), anemia (41%), and thrombocytopenia (29%).
Researchers reduced the dose of midostaurin in 65 patients (56%), mostly due to adverse events (n=48). Twenty-one of these patients (32%) were later able to return to the initial dose.
Eighty-four patients (72%) discontinued treatment, and 32 (28%) were receiving ongoing treatment at the time of data cutoff. The most frequent reasons for treatment discontinuation were disease progression (33%) and adverse events (22%).
Compassionate use
The phase 2 study results are reinforced by a letter published in the same issue of NEJM, which describes a compassionate use program for midostaurin in advanced SM.
The letter includes results in 28 patients. After a median follow-up of 18.5 months, the overall response rate was 71%. The overall survival rate was 42.7%.
The most frequent adverse events were nausea/vomiting in 89% of patients (leading to failure/discontinuation in 18%), lymphocytopenia in 61% (without opportunistic infection), and photosensitivity in 25%. ![]()
Account for all medications, even if they’re banned
I recently saw a 73-year-old Hispanic woman in the emergency department who said (through her daughter, who was translating) that she was experiencing mild headache, shortness of breath, and throat swelling. She had previously sought care for these complaints, but her condition had not improved. Her past medical and surgical history was otherwise unremarkable.
On examination, her voice was hoarse and she had bilateral 1+ pitting edema of her lower extremities. Her vital signs were stable, her lungs were clear, her throat appeared normal, and she didn’t have any skin rashes. However, her lab results included a white blood cell count of 2100/mcL, platelet count of 73,000/mcL, and an absolute neutrophil count of 1000/mm3. Her b-type natriuretic peptide, cardiac marker, and thyroid-stimulating hormone levels were normal.
The diagnosis was clear—neutropenia and agranulocytosis—although the cause was not.
I gathered a more detailed history and learned that the patient had been living in the United States for years, but she occasionally returned to Mexico for visits and routine medical care. During one of these trips, she’d obtained metamizole—a drug banned in the United States—and was taking it for her headaches.
A Web search revealed rare adverse effects of agranulocytosis, neutropenia, and anaphylaxis from metamizole. It is highly probable that the metamizole caused my patient’s symptoms and abnormal labs findings. I advised her of my suspicions and recommended that she stop taking the medication. A hospitalist then took over her care.
The key takeaway from this case is to account for all medications when gathering a patient’s history, including those that may be obtained outside of the United States.
Nick Ly, DO
Lillington, NC
I recently saw a 73-year-old Hispanic woman in the emergency department who said (through her daughter, who was translating) that she was experiencing mild headache, shortness of breath, and throat swelling. She had previously sought care for these complaints, but her condition had not improved. Her past medical and surgical history was otherwise unremarkable.
On examination, her voice was hoarse and she had bilateral 1+ pitting edema of her lower extremities. Her vital signs were stable, her lungs were clear, her throat appeared normal, and she didn’t have any skin rashes. However, her lab results included a white blood cell count of 2100/mcL, platelet count of 73,000/mcL, and an absolute neutrophil count of 1000/mm3. Her b-type natriuretic peptide, cardiac marker, and thyroid-stimulating hormone levels were normal.
The diagnosis was clear—neutropenia and agranulocytosis—although the cause was not.
I gathered a more detailed history and learned that the patient had been living in the United States for years, but she occasionally returned to Mexico for visits and routine medical care. During one of these trips, she’d obtained metamizole—a drug banned in the United States—and was taking it for her headaches.
A Web search revealed rare adverse effects of agranulocytosis, neutropenia, and anaphylaxis from metamizole. It is highly probable that the metamizole caused my patient’s symptoms and abnormal labs findings. I advised her of my suspicions and recommended that she stop taking the medication. A hospitalist then took over her care.
The key takeaway from this case is to account for all medications when gathering a patient’s history, including those that may be obtained outside of the United States.
Nick Ly, DO
Lillington, NC
I recently saw a 73-year-old Hispanic woman in the emergency department who said (through her daughter, who was translating) that she was experiencing mild headache, shortness of breath, and throat swelling. She had previously sought care for these complaints, but her condition had not improved. Her past medical and surgical history was otherwise unremarkable.
On examination, her voice was hoarse and she had bilateral 1+ pitting edema of her lower extremities. Her vital signs were stable, her lungs were clear, her throat appeared normal, and she didn’t have any skin rashes. However, her lab results included a white blood cell count of 2100/mcL, platelet count of 73,000/mcL, and an absolute neutrophil count of 1000/mm3. Her b-type natriuretic peptide, cardiac marker, and thyroid-stimulating hormone levels were normal.
The diagnosis was clear—neutropenia and agranulocytosis—although the cause was not.
I gathered a more detailed history and learned that the patient had been living in the United States for years, but she occasionally returned to Mexico for visits and routine medical care. During one of these trips, she’d obtained metamizole—a drug banned in the United States—and was taking it for her headaches.
A Web search revealed rare adverse effects of agranulocytosis, neutropenia, and anaphylaxis from metamizole. It is highly probable that the metamizole caused my patient’s symptoms and abnormal labs findings. I advised her of my suspicions and recommended that she stop taking the medication. A hospitalist then took over her care.
The key takeaway from this case is to account for all medications when gathering a patient’s history, including those that may be obtained outside of the United States.
Nick Ly, DO
Lillington, NC
Does it matter who serves as the scribe?
As a scribe and accepted future medical student, I was quite interested in the article, “Medical scribes: How do their notes stack up?” by Misra-Hebert, et al (J Fam Pract. 2016;65:155-159) and the editorial, “Working with scribes—the good, the surprising” (J Fam Pract. 2016;65:154,176) in the March issue. I was struck, however, that both pieces implied that only medical assistants (MAs) are scribes.
I am familiar with practices where MAs assume a “documentation support” function in addition to their traditional role, and I currently work in a practice with professional scribes. Professional scribes are often recent college graduates who are working before beginning their studies to become a physician or physician assistant.
In my experience, MAs want to do the work they were hired and trained to do and are not enthusiastic about extensive charting. However, professional scribes apply to the position expecting to do this very task, with the goals of learning from the doctor-patient interaction, deepening their medical knowledge, and becoming comfortable in a clinical setting.
Although I am glad to see that MAs improve documentation quality per the original research mentioned above, it would be beneficial to compare the outcomes of professional scribes to those of cross-trained MAs or to traditional providers who do their own note-writing.
D. Brendan Johnson, BA
Minneapolis, Minn
As a scribe and accepted future medical student, I was quite interested in the article, “Medical scribes: How do their notes stack up?” by Misra-Hebert, et al (J Fam Pract. 2016;65:155-159) and the editorial, “Working with scribes—the good, the surprising” (J Fam Pract. 2016;65:154,176) in the March issue. I was struck, however, that both pieces implied that only medical assistants (MAs) are scribes.
I am familiar with practices where MAs assume a “documentation support” function in addition to their traditional role, and I currently work in a practice with professional scribes. Professional scribes are often recent college graduates who are working before beginning their studies to become a physician or physician assistant.
In my experience, MAs want to do the work they were hired and trained to do and are not enthusiastic about extensive charting. However, professional scribes apply to the position expecting to do this very task, with the goals of learning from the doctor-patient interaction, deepening their medical knowledge, and becoming comfortable in a clinical setting.
Although I am glad to see that MAs improve documentation quality per the original research mentioned above, it would be beneficial to compare the outcomes of professional scribes to those of cross-trained MAs or to traditional providers who do their own note-writing.
D. Brendan Johnson, BA
Minneapolis, Minn
As a scribe and accepted future medical student, I was quite interested in the article, “Medical scribes: How do their notes stack up?” by Misra-Hebert, et al (J Fam Pract. 2016;65:155-159) and the editorial, “Working with scribes—the good, the surprising” (J Fam Pract. 2016;65:154,176) in the March issue. I was struck, however, that both pieces implied that only medical assistants (MAs) are scribes.
I am familiar with practices where MAs assume a “documentation support” function in addition to their traditional role, and I currently work in a practice with professional scribes. Professional scribes are often recent college graduates who are working before beginning their studies to become a physician or physician assistant.
In my experience, MAs want to do the work they were hired and trained to do and are not enthusiastic about extensive charting. However, professional scribes apply to the position expecting to do this very task, with the goals of learning from the doctor-patient interaction, deepening their medical knowledge, and becoming comfortable in a clinical setting.
Although I am glad to see that MAs improve documentation quality per the original research mentioned above, it would be beneficial to compare the outcomes of professional scribes to those of cross-trained MAs or to traditional providers who do their own note-writing.
D. Brendan Johnson, BA
Minneapolis, Minn
Women with BRCA1 mutations at higher risk for endometrial cancers
Women with BRCA1 mutations who undergo risk-reducing ovary and fallopian tube removal without concomitant hysterectomy appear to be at increased risk for serous or serous-like endometrial carcinoma, results of a long-term prospective study suggest.
Among 1,083 women with deleterious mutations in BRCA1, BRCA2, or both who underwent risk-reducing salpingo-oophorectomy (RRSO) without hysterectomy, there was no overall increase in the incidence of uterine corpus cancers, compared with the background population. But among women with BRCA1 mutations, however, there was increased risk for serous/serous-like endometrial carcinomas, which comprise only 10% of endometrial cancers, but account for about 40% of endometrial cancer deaths, reported Catherine A. Shu, MD, of Columbia University, New York, and her colleagues (JAMA Oncol. 2016 Jun 30. doi: 10.1001/jamaoncol.2016.1820).
“Our results suggest that BRCA1+ women are at increased risk for serous/serous-like endometrial carcinoma. Although instability in the estimated magnitude of this risk remains, we believe that the possibility of this cancer should be considered when discussing the advantages and risks of hysterectomy at the time of RRSO in BRCA1+ women,” they wrote.
Salpingo-oophorectomy to reduce risk for breast, ovarian, and fallopian-tube cancers is a standard option for women with BRCA mutations, but it’s unclear whether concomitant hysterectomy offers additional benefit, or adds only morbidity.
To help clarify the issue, investigators at nine comprehensive cancer centers enrolled 627 women with BRCA1 mutations, 453 with BRCA2 mutations, and 3 with mutations in both genes who underwent RRSO without either prior or concomitant hysterectomy. They then followed the patients for a median of 5.1 years after the date of ascertainment, receipt of BRCA testing results, or RRSO.
There were a total of 8 incident uterine cancers over the course of follow-up, compared with 4.3 that would be expected based on Surveillance, Epidemiology, and End Results (SEER) data. The observed-to-expected incidence ratio was 1.9, and was not statistically significant.
In addition, when the investigators stratified by subtype, there was no increased risk for endometrioid endometrial carcinoma or sarcoma.
However, there were five cases of serous/serous-like endometrial carcinomas occurring from 7.2 to 12, 9 years after surgery. The patients included four women positive for BRCA1 mutations, and one positive for BRCA2.
In the SEER population, 0.18 incident cases would be expected, translating into an observed-to-expected ratio for women with BRCA1 of 22.2 (P less than .001). For BRCA2, however, the ratio was 6.4, and was not statistically significant.
In the three serous/serous-like tumors from women positive for BRCA1, tumor analysis showed loss of the wild-type BRCA1 gene and/or loss of protein expression.
Finally to see whether the results could have been confounded by a history of breast cancer or exposure to tamoxifen, which is associated with a small but significant increase in risk for endometrial cancer, the investigators looked at the serous/serous-like subtype tumors, 4 of which occurred among 727 women with history of breast cancer, compared with 0.26 expected (observed-to-expected ratio 15.5, P less than .001). Among 356 women with no history of breast cancer, the expected incidence was 0.08, and the observed-to-expected ratio was 12.6 (not significant).
There were 3 serous/serous-like carcinomas among 273 women with tamoxifen exposure (expected rate 0.12, observed-to-expected ratio, 24.4, P less than .001) and two occurring in 655 women without tamoxifen exposure (expected 0.18, observed-to-expected ratio 11.3, P = .01).
The authors noted that with minimally invasive approaches, the additional surgical risks, mortality rates, and costs of adding concomitant hysterectomy to RRSO are relatively modest and may be an acceptable trade-off for some high-risk patients.
“[I]f the present results are confirmed by future studies, hysterectomy with bilateral salpingo-oophorectomy may become the preferred risk-reducing surgical approach for BRCA1+ women. However, even if these results are confirmed, RRSO alone may still have a role for BRCA1+ women if strong reasons exist for uterine retention, such as dense pelvic adhesions or desire for future pregnancy using assisted reproductive approaches,” they wrote.
Is the addition of concomitant hysterectomy during RRSO [risk-reducing salpingo-oophorectomy] to reduce the risk of uterine cancer justifiable? It’s difficult to fully define the additional risk and morbidity associated with combining a hysterectomy with RRSO; however, most surgeons would agree that this additional procedure does add some additional risk to women. Along these same lines, overall mortality associated with hysterectomy is rare but not nonexistent. Recent studies suggest less intraoperative blood loss, lower wound complications, shorter hospitalization, and faster return to normal activity when this procedure is accomplished with a minimally invasive surgical approach when compared with an open laparotomy approach.
This morbidity risk and rare but potential mortality risk must be weighed against the risk for recurrence and death for women with a BRCA mutation who are diagnosed with uterine cancer. This is particularly notable for those women diagnosed with serous carcinoma, who are well recognized to harbor worse outcomes, even when they present at stage I disease. In this particular study, two of the five women with serous adenocarcinoma developed a recurrence and one of these two died despite having stage IA disease. Serous carcinomas are biologically and clinically different from most endometrioid adenocarcinomas of the uterus and the risk is essentially eradicated with hysterectomy.
Charles A. Leath, MD, MSPH, Warner K. Huh, MD, and Ronald D. Alvarez, MD, MBA, are in the Division of Gynecologic Oncology, University of Alabama at Birmingham. These comments were taken from an editorial they authored accompanying the report by Shu et al. (JAMA Oncol. 2016 Jun 30. doi: 10.1001/jamaoncol.2016.1773).
Is the addition of concomitant hysterectomy during RRSO [risk-reducing salpingo-oophorectomy] to reduce the risk of uterine cancer justifiable? It’s difficult to fully define the additional risk and morbidity associated with combining a hysterectomy with RRSO; however, most surgeons would agree that this additional procedure does add some additional risk to women. Along these same lines, overall mortality associated with hysterectomy is rare but not nonexistent. Recent studies suggest less intraoperative blood loss, lower wound complications, shorter hospitalization, and faster return to normal activity when this procedure is accomplished with a minimally invasive surgical approach when compared with an open laparotomy approach.
This morbidity risk and rare but potential mortality risk must be weighed against the risk for recurrence and death for women with a BRCA mutation who are diagnosed with uterine cancer. This is particularly notable for those women diagnosed with serous carcinoma, who are well recognized to harbor worse outcomes, even when they present at stage I disease. In this particular study, two of the five women with serous adenocarcinoma developed a recurrence and one of these two died despite having stage IA disease. Serous carcinomas are biologically and clinically different from most endometrioid adenocarcinomas of the uterus and the risk is essentially eradicated with hysterectomy.
Charles A. Leath, MD, MSPH, Warner K. Huh, MD, and Ronald D. Alvarez, MD, MBA, are in the Division of Gynecologic Oncology, University of Alabama at Birmingham. These comments were taken from an editorial they authored accompanying the report by Shu et al. (JAMA Oncol. 2016 Jun 30. doi: 10.1001/jamaoncol.2016.1773).
Is the addition of concomitant hysterectomy during RRSO [risk-reducing salpingo-oophorectomy] to reduce the risk of uterine cancer justifiable? It’s difficult to fully define the additional risk and morbidity associated with combining a hysterectomy with RRSO; however, most surgeons would agree that this additional procedure does add some additional risk to women. Along these same lines, overall mortality associated with hysterectomy is rare but not nonexistent. Recent studies suggest less intraoperative blood loss, lower wound complications, shorter hospitalization, and faster return to normal activity when this procedure is accomplished with a minimally invasive surgical approach when compared with an open laparotomy approach.
This morbidity risk and rare but potential mortality risk must be weighed against the risk for recurrence and death for women with a BRCA mutation who are diagnosed with uterine cancer. This is particularly notable for those women diagnosed with serous carcinoma, who are well recognized to harbor worse outcomes, even when they present at stage I disease. In this particular study, two of the five women with serous adenocarcinoma developed a recurrence and one of these two died despite having stage IA disease. Serous carcinomas are biologically and clinically different from most endometrioid adenocarcinomas of the uterus and the risk is essentially eradicated with hysterectomy.
Charles A. Leath, MD, MSPH, Warner K. Huh, MD, and Ronald D. Alvarez, MD, MBA, are in the Division of Gynecologic Oncology, University of Alabama at Birmingham. These comments were taken from an editorial they authored accompanying the report by Shu et al. (JAMA Oncol. 2016 Jun 30. doi: 10.1001/jamaoncol.2016.1773).
Women with BRCA1 mutations who undergo risk-reducing ovary and fallopian tube removal without concomitant hysterectomy appear to be at increased risk for serous or serous-like endometrial carcinoma, results of a long-term prospective study suggest.
Among 1,083 women with deleterious mutations in BRCA1, BRCA2, or both who underwent risk-reducing salpingo-oophorectomy (RRSO) without hysterectomy, there was no overall increase in the incidence of uterine corpus cancers, compared with the background population. But among women with BRCA1 mutations, however, there was increased risk for serous/serous-like endometrial carcinomas, which comprise only 10% of endometrial cancers, but account for about 40% of endometrial cancer deaths, reported Catherine A. Shu, MD, of Columbia University, New York, and her colleagues (JAMA Oncol. 2016 Jun 30. doi: 10.1001/jamaoncol.2016.1820).
“Our results suggest that BRCA1+ women are at increased risk for serous/serous-like endometrial carcinoma. Although instability in the estimated magnitude of this risk remains, we believe that the possibility of this cancer should be considered when discussing the advantages and risks of hysterectomy at the time of RRSO in BRCA1+ women,” they wrote.
Salpingo-oophorectomy to reduce risk for breast, ovarian, and fallopian-tube cancers is a standard option for women with BRCA mutations, but it’s unclear whether concomitant hysterectomy offers additional benefit, or adds only morbidity.
To help clarify the issue, investigators at nine comprehensive cancer centers enrolled 627 women with BRCA1 mutations, 453 with BRCA2 mutations, and 3 with mutations in both genes who underwent RRSO without either prior or concomitant hysterectomy. They then followed the patients for a median of 5.1 years after the date of ascertainment, receipt of BRCA testing results, or RRSO.
There were a total of 8 incident uterine cancers over the course of follow-up, compared with 4.3 that would be expected based on Surveillance, Epidemiology, and End Results (SEER) data. The observed-to-expected incidence ratio was 1.9, and was not statistically significant.
In addition, when the investigators stratified by subtype, there was no increased risk for endometrioid endometrial carcinoma or sarcoma.
However, there were five cases of serous/serous-like endometrial carcinomas occurring from 7.2 to 12, 9 years after surgery. The patients included four women positive for BRCA1 mutations, and one positive for BRCA2.
In the SEER population, 0.18 incident cases would be expected, translating into an observed-to-expected ratio for women with BRCA1 of 22.2 (P less than .001). For BRCA2, however, the ratio was 6.4, and was not statistically significant.
In the three serous/serous-like tumors from women positive for BRCA1, tumor analysis showed loss of the wild-type BRCA1 gene and/or loss of protein expression.
Finally to see whether the results could have been confounded by a history of breast cancer or exposure to tamoxifen, which is associated with a small but significant increase in risk for endometrial cancer, the investigators looked at the serous/serous-like subtype tumors, 4 of which occurred among 727 women with history of breast cancer, compared with 0.26 expected (observed-to-expected ratio 15.5, P less than .001). Among 356 women with no history of breast cancer, the expected incidence was 0.08, and the observed-to-expected ratio was 12.6 (not significant).
There were 3 serous/serous-like carcinomas among 273 women with tamoxifen exposure (expected rate 0.12, observed-to-expected ratio, 24.4, P less than .001) and two occurring in 655 women without tamoxifen exposure (expected 0.18, observed-to-expected ratio 11.3, P = .01).
The authors noted that with minimally invasive approaches, the additional surgical risks, mortality rates, and costs of adding concomitant hysterectomy to RRSO are relatively modest and may be an acceptable trade-off for some high-risk patients.
“[I]f the present results are confirmed by future studies, hysterectomy with bilateral salpingo-oophorectomy may become the preferred risk-reducing surgical approach for BRCA1+ women. However, even if these results are confirmed, RRSO alone may still have a role for BRCA1+ women if strong reasons exist for uterine retention, such as dense pelvic adhesions or desire for future pregnancy using assisted reproductive approaches,” they wrote.
Women with BRCA1 mutations who undergo risk-reducing ovary and fallopian tube removal without concomitant hysterectomy appear to be at increased risk for serous or serous-like endometrial carcinoma, results of a long-term prospective study suggest.
Among 1,083 women with deleterious mutations in BRCA1, BRCA2, or both who underwent risk-reducing salpingo-oophorectomy (RRSO) without hysterectomy, there was no overall increase in the incidence of uterine corpus cancers, compared with the background population. But among women with BRCA1 mutations, however, there was increased risk for serous/serous-like endometrial carcinomas, which comprise only 10% of endometrial cancers, but account for about 40% of endometrial cancer deaths, reported Catherine A. Shu, MD, of Columbia University, New York, and her colleagues (JAMA Oncol. 2016 Jun 30. doi: 10.1001/jamaoncol.2016.1820).
“Our results suggest that BRCA1+ women are at increased risk for serous/serous-like endometrial carcinoma. Although instability in the estimated magnitude of this risk remains, we believe that the possibility of this cancer should be considered when discussing the advantages and risks of hysterectomy at the time of RRSO in BRCA1+ women,” they wrote.
Salpingo-oophorectomy to reduce risk for breast, ovarian, and fallopian-tube cancers is a standard option for women with BRCA mutations, but it’s unclear whether concomitant hysterectomy offers additional benefit, or adds only morbidity.
To help clarify the issue, investigators at nine comprehensive cancer centers enrolled 627 women with BRCA1 mutations, 453 with BRCA2 mutations, and 3 with mutations in both genes who underwent RRSO without either prior or concomitant hysterectomy. They then followed the patients for a median of 5.1 years after the date of ascertainment, receipt of BRCA testing results, or RRSO.
There were a total of 8 incident uterine cancers over the course of follow-up, compared with 4.3 that would be expected based on Surveillance, Epidemiology, and End Results (SEER) data. The observed-to-expected incidence ratio was 1.9, and was not statistically significant.
In addition, when the investigators stratified by subtype, there was no increased risk for endometrioid endometrial carcinoma or sarcoma.
However, there were five cases of serous/serous-like endometrial carcinomas occurring from 7.2 to 12, 9 years after surgery. The patients included four women positive for BRCA1 mutations, and one positive for BRCA2.
In the SEER population, 0.18 incident cases would be expected, translating into an observed-to-expected ratio for women with BRCA1 of 22.2 (P less than .001). For BRCA2, however, the ratio was 6.4, and was not statistically significant.
In the three serous/serous-like tumors from women positive for BRCA1, tumor analysis showed loss of the wild-type BRCA1 gene and/or loss of protein expression.
Finally to see whether the results could have been confounded by a history of breast cancer or exposure to tamoxifen, which is associated with a small but significant increase in risk for endometrial cancer, the investigators looked at the serous/serous-like subtype tumors, 4 of which occurred among 727 women with history of breast cancer, compared with 0.26 expected (observed-to-expected ratio 15.5, P less than .001). Among 356 women with no history of breast cancer, the expected incidence was 0.08, and the observed-to-expected ratio was 12.6 (not significant).
There were 3 serous/serous-like carcinomas among 273 women with tamoxifen exposure (expected rate 0.12, observed-to-expected ratio, 24.4, P less than .001) and two occurring in 655 women without tamoxifen exposure (expected 0.18, observed-to-expected ratio 11.3, P = .01).
The authors noted that with minimally invasive approaches, the additional surgical risks, mortality rates, and costs of adding concomitant hysterectomy to RRSO are relatively modest and may be an acceptable trade-off for some high-risk patients.
“[I]f the present results are confirmed by future studies, hysterectomy with bilateral salpingo-oophorectomy may become the preferred risk-reducing surgical approach for BRCA1+ women. However, even if these results are confirmed, RRSO alone may still have a role for BRCA1+ women if strong reasons exist for uterine retention, such as dense pelvic adhesions or desire for future pregnancy using assisted reproductive approaches,” they wrote.
FROM JAMA ONCOLOGY
Key clinical point: Clinicians may wish to discuss the option of hysterectomy at the time of salpingo-oophorectomy in women with deleterious BRCA1 mutations.
Major finding: Among women with BRCA1 but not BRCA2 mutations there was increased risk for serous/serous-like endometrial carcinomas.
Data source: Prospective multicenter follow-up study of 1,083 women with BRCA mutations who underwent salpingo-oophorectomy without hysterectomy.
Disclosures: The study was supported by grants from the Department of Defense, National Institutes of Health, and public and private foundations. Coauthor Robert Soslow, MD, disclosed consulting for EMD Serono. No others reported conflicts of interest. The editorialists reported no conflicts of interest related to the study.
Common Medication Provides Insight Into Brain Abnormalities in Dystonia
BERLIN—A common medication used for the symptomatic treatment of dystonia has been shown to target brain abnormalities in the cerebral cortex in patients with cervical dystonia, according to a study presented at the 20th International Congress of Parkinson’s Disease and Movement Disorders.
Roxana G. Burciu, PhD, a postdoctoral fellow, and a team of researchers at the University of Florida, Gainesville, aimed to assess with fMRI task-related brain activity in patients with cervical dystonia with and without a single-dose administration of trihexyphenidyl, an anticholinergic medication. For decades, anticholinergic medications have been commonly prescribed for patients with varying types of dystonia, but their mechanism of action has not been determined. Although it was previously thought that anticholinergic medications primarily affect the basal ganglia, the results of this study are evidence that they are effective in other areas of the brain, particularly in the cerebral cortex.
Roxana G. Burciu, PhD
Sixteen patients with idiopathic cervical dystonia were compared using a 3T MRI scanner with 16 age- and gender-matched healthy individuals. Patients with cervical dystonia were scanned twice, both off-medication and on average two hours after a single dose of trihexyphenidyl. Control subjects did not receive the medication and were only scanned once. While off medication, the patients had reduced motor activity compared with the healthy subjects. After administration of trihexyphenidyl, there was an increase in motor-related activity in middle frontal gyrus and primary somatosensory cortex. The results suggest that somatosensory processing in cervical dystonia can be acutely changed through trihexyphenidyl administration.
Hyder A. Jinnah, MD, PhD, Professor of Neurosurgery, Human Genetics, and Pediatrics at Emory University School of Medicine in Atlanta, said, “The study by Burciu and colleagues is the first attempt to determine what part of the brain is influenced by anticholinergic drugs in dystonia. Before treatment, the patients with cervical dystonia showed abnormal activity in multiple brain regions. After treatment, the abnormal brain activity was at least partly corrected in two regions. Both of these regions were in the cerebral cortex, not the basal ganglia. This study provides clues towards which regions of the brain might be abnormal, and how trihexyphenidyl might correct these abnormalities.”
Dr. Jinnah added, “Like any good study, the findings from this study lead to many more questions than answers. Do the brain abnormalities found reflect a cause for dystonia, or a consequence of it? Does the result mean that these medications work in the cortex, not the basal ganglia, as previously believed? Why do patients with cervical dystonia have brain abnormalities that show up when they use their hands, which are not affected? Does the drug affect the brains of normal people who do not have dystonia in the same way? How can we exploit this new information to improve the value of anticholinergics for patients with dystonia?”
BERLIN—A common medication used for the symptomatic treatment of dystonia has been shown to target brain abnormalities in the cerebral cortex in patients with cervical dystonia, according to a study presented at the 20th International Congress of Parkinson’s Disease and Movement Disorders.
Roxana G. Burciu, PhD, a postdoctoral fellow, and a team of researchers at the University of Florida, Gainesville, aimed to assess with fMRI task-related brain activity in patients with cervical dystonia with and without a single-dose administration of trihexyphenidyl, an anticholinergic medication. For decades, anticholinergic medications have been commonly prescribed for patients with varying types of dystonia, but their mechanism of action has not been determined. Although it was previously thought that anticholinergic medications primarily affect the basal ganglia, the results of this study are evidence that they are effective in other areas of the brain, particularly in the cerebral cortex.
Roxana G. Burciu, PhD
Sixteen patients with idiopathic cervical dystonia were compared using a 3T MRI scanner with 16 age- and gender-matched healthy individuals. Patients with cervical dystonia were scanned twice, both off-medication and on average two hours after a single dose of trihexyphenidyl. Control subjects did not receive the medication and were only scanned once. While off medication, the patients had reduced motor activity compared with the healthy subjects. After administration of trihexyphenidyl, there was an increase in motor-related activity in middle frontal gyrus and primary somatosensory cortex. The results suggest that somatosensory processing in cervical dystonia can be acutely changed through trihexyphenidyl administration.
Hyder A. Jinnah, MD, PhD, Professor of Neurosurgery, Human Genetics, and Pediatrics at Emory University School of Medicine in Atlanta, said, “The study by Burciu and colleagues is the first attempt to determine what part of the brain is influenced by anticholinergic drugs in dystonia. Before treatment, the patients with cervical dystonia showed abnormal activity in multiple brain regions. After treatment, the abnormal brain activity was at least partly corrected in two regions. Both of these regions were in the cerebral cortex, not the basal ganglia. This study provides clues towards which regions of the brain might be abnormal, and how trihexyphenidyl might correct these abnormalities.”
Dr. Jinnah added, “Like any good study, the findings from this study lead to many more questions than answers. Do the brain abnormalities found reflect a cause for dystonia, or a consequence of it? Does the result mean that these medications work in the cortex, not the basal ganglia, as previously believed? Why do patients with cervical dystonia have brain abnormalities that show up when they use their hands, which are not affected? Does the drug affect the brains of normal people who do not have dystonia in the same way? How can we exploit this new information to improve the value of anticholinergics for patients with dystonia?”
BERLIN—A common medication used for the symptomatic treatment of dystonia has been shown to target brain abnormalities in the cerebral cortex in patients with cervical dystonia, according to a study presented at the 20th International Congress of Parkinson’s Disease and Movement Disorders.
Roxana G. Burciu, PhD, a postdoctoral fellow, and a team of researchers at the University of Florida, Gainesville, aimed to assess with fMRI task-related brain activity in patients with cervical dystonia with and without a single-dose administration of trihexyphenidyl, an anticholinergic medication. For decades, anticholinergic medications have been commonly prescribed for patients with varying types of dystonia, but their mechanism of action has not been determined. Although it was previously thought that anticholinergic medications primarily affect the basal ganglia, the results of this study are evidence that they are effective in other areas of the brain, particularly in the cerebral cortex.
Roxana G. Burciu, PhD
Sixteen patients with idiopathic cervical dystonia were compared using a 3T MRI scanner with 16 age- and gender-matched healthy individuals. Patients with cervical dystonia were scanned twice, both off-medication and on average two hours after a single dose of trihexyphenidyl. Control subjects did not receive the medication and were only scanned once. While off medication, the patients had reduced motor activity compared with the healthy subjects. After administration of trihexyphenidyl, there was an increase in motor-related activity in middle frontal gyrus and primary somatosensory cortex. The results suggest that somatosensory processing in cervical dystonia can be acutely changed through trihexyphenidyl administration.
Hyder A. Jinnah, MD, PhD, Professor of Neurosurgery, Human Genetics, and Pediatrics at Emory University School of Medicine in Atlanta, said, “The study by Burciu and colleagues is the first attempt to determine what part of the brain is influenced by anticholinergic drugs in dystonia. Before treatment, the patients with cervical dystonia showed abnormal activity in multiple brain regions. After treatment, the abnormal brain activity was at least partly corrected in two regions. Both of these regions were in the cerebral cortex, not the basal ganglia. This study provides clues towards which regions of the brain might be abnormal, and how trihexyphenidyl might correct these abnormalities.”
Dr. Jinnah added, “Like any good study, the findings from this study lead to many more questions than answers. Do the brain abnormalities found reflect a cause for dystonia, or a consequence of it? Does the result mean that these medications work in the cortex, not the basal ganglia, as previously believed? Why do patients with cervical dystonia have brain abnormalities that show up when they use their hands, which are not affected? Does the drug affect the brains of normal people who do not have dystonia in the same way? How can we exploit this new information to improve the value of anticholinergics for patients with dystonia?”