User login
Drug granted breakthrough, orphan designation for cGVHD

Photo courtesy of Janssen
The US Food and Drug Administration (FDA) has granted breakthrough therapy designation for ibrutinib (Imbruvica), a Bruton’s tyrosine kinase inhibitor, as a potential treatment for chronic graft-versus-host-disease (cGVHD) in patients who have failed 1 or more lines of systemic therapy.
The FDA has also granted ibrutinib orphan drug designation for this indication.
The request for breakthrough therapy designation and orphan designation for ibrutinib in patients with cGVHD was based on preliminary data from a phase 1b/2 study of patients with steroid-dependent or refractory cGVHD.
Results from this trial were presented at the 2015 ASCO Annual Meeting (abstract 7024) and the 2016 EBMT meeting (abstract P124).
About ibrutinib
Ibrutinib is an oral, once-daily therapy that inhibits Bruton’s tyrosine kinase, a signaling molecule in the B-cell receptor signaling complex that plays an important role in the survival and spread of malignant B cells.
Ibrutinib is FDA-approved to treat patients with chronic lymphocytic leukemia (CLL) and small lymphocytic lymphoma (SLL), including those with 17p deletion, patients with mantle cell lymphoma (MCL) who have received at least 1 prior therapy, and patients with Waldenström’s macroglobulinemia.
Accelerated approval was granted for the MCL indication based on overall response rate. Continued approval for this indication may be contingent upon verification of clinical benefit in confirmatory trials.
The FDA previously granted ibrutinib breakthrough designation for the treatment of relapsed or refractory MCL, Waldenström’s macroglobulinemia, and CLL/SLL patients with 17p deletion. The FDA also granted ibrutinib orphan designation for all 3 indications.
Ibrutinib is jointly developed and commercialized by Pharmacyclics LLC, an AbbVie company, and Janssen Biotech, Inc.
About breakthrough designation
The FDA’s breakthrough therapy designation is intended to expedite the development and review of new therapies for serious or life-threatening conditions.
To earn the designation, a treatment must show encouraging early clinical results demonstrating substantial improvement over available therapies with regard to a clinically significant endpoint, or it must fulfill an unmet need.
About orphan designation
The FDA grants orphan designation to drugs and biologics intended to treat, diagnose, or prevent diseases/disorders that affect fewer than 200,000 people in the US.
The designation provides incentives for sponsors to develop products for rare diseases. This may include tax credits toward the cost of clinical trials, prescription drug user fee waivers, and 7 years of market exclusivity if the drug is approved. ![]()

Photo courtesy of Janssen
The US Food and Drug Administration (FDA) has granted breakthrough therapy designation for ibrutinib (Imbruvica), a Bruton’s tyrosine kinase inhibitor, as a potential treatment for chronic graft-versus-host-disease (cGVHD) in patients who have failed 1 or more lines of systemic therapy.
The FDA has also granted ibrutinib orphan drug designation for this indication.
The request for breakthrough therapy designation and orphan designation for ibrutinib in patients with cGVHD was based on preliminary data from a phase 1b/2 study of patients with steroid-dependent or refractory cGVHD.
Results from this trial were presented at the 2015 ASCO Annual Meeting (abstract 7024) and the 2016 EBMT meeting (abstract P124).
About ibrutinib
Ibrutinib is an oral, once-daily therapy that inhibits Bruton’s tyrosine kinase, a signaling molecule in the B-cell receptor signaling complex that plays an important role in the survival and spread of malignant B cells.
Ibrutinib is FDA-approved to treat patients with chronic lymphocytic leukemia (CLL) and small lymphocytic lymphoma (SLL), including those with 17p deletion, patients with mantle cell lymphoma (MCL) who have received at least 1 prior therapy, and patients with Waldenström’s macroglobulinemia.
Accelerated approval was granted for the MCL indication based on overall response rate. Continued approval for this indication may be contingent upon verification of clinical benefit in confirmatory trials.
The FDA previously granted ibrutinib breakthrough designation for the treatment of relapsed or refractory MCL, Waldenström’s macroglobulinemia, and CLL/SLL patients with 17p deletion. The FDA also granted ibrutinib orphan designation for all 3 indications.
Ibrutinib is jointly developed and commercialized by Pharmacyclics LLC, an AbbVie company, and Janssen Biotech, Inc.
About breakthrough designation
The FDA’s breakthrough therapy designation is intended to expedite the development and review of new therapies for serious or life-threatening conditions.
To earn the designation, a treatment must show encouraging early clinical results demonstrating substantial improvement over available therapies with regard to a clinically significant endpoint, or it must fulfill an unmet need.
About orphan designation
The FDA grants orphan designation to drugs and biologics intended to treat, diagnose, or prevent diseases/disorders that affect fewer than 200,000 people in the US.
The designation provides incentives for sponsors to develop products for rare diseases. This may include tax credits toward the cost of clinical trials, prescription drug user fee waivers, and 7 years of market exclusivity if the drug is approved. ![]()

Photo courtesy of Janssen
The US Food and Drug Administration (FDA) has granted breakthrough therapy designation for ibrutinib (Imbruvica), a Bruton’s tyrosine kinase inhibitor, as a potential treatment for chronic graft-versus-host-disease (cGVHD) in patients who have failed 1 or more lines of systemic therapy.
The FDA has also granted ibrutinib orphan drug designation for this indication.
The request for breakthrough therapy designation and orphan designation for ibrutinib in patients with cGVHD was based on preliminary data from a phase 1b/2 study of patients with steroid-dependent or refractory cGVHD.
Results from this trial were presented at the 2015 ASCO Annual Meeting (abstract 7024) and the 2016 EBMT meeting (abstract P124).
About ibrutinib
Ibrutinib is an oral, once-daily therapy that inhibits Bruton’s tyrosine kinase, a signaling molecule in the B-cell receptor signaling complex that plays an important role in the survival and spread of malignant B cells.
Ibrutinib is FDA-approved to treat patients with chronic lymphocytic leukemia (CLL) and small lymphocytic lymphoma (SLL), including those with 17p deletion, patients with mantle cell lymphoma (MCL) who have received at least 1 prior therapy, and patients with Waldenström’s macroglobulinemia.
Accelerated approval was granted for the MCL indication based on overall response rate. Continued approval for this indication may be contingent upon verification of clinical benefit in confirmatory trials.
The FDA previously granted ibrutinib breakthrough designation for the treatment of relapsed or refractory MCL, Waldenström’s macroglobulinemia, and CLL/SLL patients with 17p deletion. The FDA also granted ibrutinib orphan designation for all 3 indications.
Ibrutinib is jointly developed and commercialized by Pharmacyclics LLC, an AbbVie company, and Janssen Biotech, Inc.
About breakthrough designation
The FDA’s breakthrough therapy designation is intended to expedite the development and review of new therapies for serious or life-threatening conditions.
To earn the designation, a treatment must show encouraging early clinical results demonstrating substantial improvement over available therapies with regard to a clinically significant endpoint, or it must fulfill an unmet need.
About orphan designation
The FDA grants orphan designation to drugs and biologics intended to treat, diagnose, or prevent diseases/disorders that affect fewer than 200,000 people in the US.
The designation provides incentives for sponsors to develop products for rare diseases. This may include tax credits toward the cost of clinical trials, prescription drug user fee waivers, and 7 years of market exclusivity if the drug is approved. ![]()
Combo shows promise for treating DLBCL

The mTOR inhibitor everolimus may provide an additional benefit when combined with R-CHOP (rituximab, cyclophosphamide, doxorubicin, vincristine, and prednisone) to treat patients with newly diagnosed diffuse large B-cell lymphoma (DLBCL), according to researchers.
The combination was considered well-tolerated in a phase 1 trial, and 96% of patients responded to the treatment.
“There is an unmet need to develop new therapies based on R-CHOP to try to increase the cure rate for diffuse large B-cell lymphoma,” said Patrick Johnston, MD, PhD, of the Mayo Clinic in Rochester, Minnesota.
“This pilot study suggests that adding mTOR inhibitors to standard therapy could improve outcomes, though it needs to be validated in a larger clinical trial.”
Results from this study were published in The Lancet Haematology.
Patients and treatment
Dr Johnston and his colleagues conducted this study in 24 previously untreated DLBCL patients. Their median age was 58.5 (range, 49.5-71.5), and 58% were male. Most patients had stage IV disease (54%), followed by stage II (25%), and stage III (21%). Five patients (21%) had bulky disease.
The patients received standard R-CHOP-21 (rituximab at 375 mg/m2, cyclophosphamide at 750 mg/m2, doxorubicin at 50 mg/m2, and vincristine at 1.4 mg/m2—all on day 1 of the 21-day cycle—as well as oral prednisone at 100 mg/m2 each day on days 1–5 of the cycle) for 6 cycles, with scheduled pegfilgrastim at 6 mg on day 2 of each cycle.
They also received everolimus at 10 mg/day on 2 different schedules. Nine patients were enrolled initially—3 given everolimus on days 1–10 and 6 receiving it on days 1–14. As there were no dose-limiting toxicities in these patients, another 15 patients went on to receive everolimus on days 1–14.
Results
The median follow-up was 21.5 months. Twenty-three patients (96%) achieved an overall response and a complete metabolic response as assessed by PET. The remaining patient withdrew consent during cycle 1 and achieved a complete response with R-CHOP alone.
The 12-month event-free survival rate was 100%. Nine patients had sufficient follow-up and were event-free at 24 months. At last follow-up (March 30, 2016), no deaths or relapses had occurred.
The most common adverse events were hematologic, such as grade 4 neutropenia (75%) and grade 3 febrile neutropenia (21%).
Three patients experienced “significant” toxicity, according to the researchers. One patient had a treatment delay of 12 days due to grade 3 hypokalemia, which was considered possibly related to everolimus.
A second patient had grade 4 sepsis that was possibly related to treatment, and a third patient had a treatment delay of 10 days due to grade 3 infection that was possibly related to everolimus.
Ten patients (42%) had their dose of everolimus reduced, 2 patients permanently discontinued the drug after cycles 3 and 4, respectively, and 2 patients omitted everolimus for 1 and 2 cycles, respectively, then resumed everolimus for subsequent cycles.
“This study is the first to integrate a P13K-mTOR agent with standard R-CHOP,” Dr Johnston said.
“The encouraging outcome results and toxicity profile of this new regimen, along with the worldwide availability of everolimus, make it potentially applicable to the large population of DLBCL patients.” ![]()

The mTOR inhibitor everolimus may provide an additional benefit when combined with R-CHOP (rituximab, cyclophosphamide, doxorubicin, vincristine, and prednisone) to treat patients with newly diagnosed diffuse large B-cell lymphoma (DLBCL), according to researchers.
The combination was considered well-tolerated in a phase 1 trial, and 96% of patients responded to the treatment.
“There is an unmet need to develop new therapies based on R-CHOP to try to increase the cure rate for diffuse large B-cell lymphoma,” said Patrick Johnston, MD, PhD, of the Mayo Clinic in Rochester, Minnesota.
“This pilot study suggests that adding mTOR inhibitors to standard therapy could improve outcomes, though it needs to be validated in a larger clinical trial.”
Results from this study were published in The Lancet Haematology.
Patients and treatment
Dr Johnston and his colleagues conducted this study in 24 previously untreated DLBCL patients. Their median age was 58.5 (range, 49.5-71.5), and 58% were male. Most patients had stage IV disease (54%), followed by stage II (25%), and stage III (21%). Five patients (21%) had bulky disease.
The patients received standard R-CHOP-21 (rituximab at 375 mg/m2, cyclophosphamide at 750 mg/m2, doxorubicin at 50 mg/m2, and vincristine at 1.4 mg/m2—all on day 1 of the 21-day cycle—as well as oral prednisone at 100 mg/m2 each day on days 1–5 of the cycle) for 6 cycles, with scheduled pegfilgrastim at 6 mg on day 2 of each cycle.
They also received everolimus at 10 mg/day on 2 different schedules. Nine patients were enrolled initially—3 given everolimus on days 1–10 and 6 receiving it on days 1–14. As there were no dose-limiting toxicities in these patients, another 15 patients went on to receive everolimus on days 1–14.
Results
The median follow-up was 21.5 months. Twenty-three patients (96%) achieved an overall response and a complete metabolic response as assessed by PET. The remaining patient withdrew consent during cycle 1 and achieved a complete response with R-CHOP alone.
The 12-month event-free survival rate was 100%. Nine patients had sufficient follow-up and were event-free at 24 months. At last follow-up (March 30, 2016), no deaths or relapses had occurred.
The most common adverse events were hematologic, such as grade 4 neutropenia (75%) and grade 3 febrile neutropenia (21%).
Three patients experienced “significant” toxicity, according to the researchers. One patient had a treatment delay of 12 days due to grade 3 hypokalemia, which was considered possibly related to everolimus.
A second patient had grade 4 sepsis that was possibly related to treatment, and a third patient had a treatment delay of 10 days due to grade 3 infection that was possibly related to everolimus.
Ten patients (42%) had their dose of everolimus reduced, 2 patients permanently discontinued the drug after cycles 3 and 4, respectively, and 2 patients omitted everolimus for 1 and 2 cycles, respectively, then resumed everolimus for subsequent cycles.
“This study is the first to integrate a P13K-mTOR agent with standard R-CHOP,” Dr Johnston said.
“The encouraging outcome results and toxicity profile of this new regimen, along with the worldwide availability of everolimus, make it potentially applicable to the large population of DLBCL patients.” ![]()

The mTOR inhibitor everolimus may provide an additional benefit when combined with R-CHOP (rituximab, cyclophosphamide, doxorubicin, vincristine, and prednisone) to treat patients with newly diagnosed diffuse large B-cell lymphoma (DLBCL), according to researchers.
The combination was considered well-tolerated in a phase 1 trial, and 96% of patients responded to the treatment.
“There is an unmet need to develop new therapies based on R-CHOP to try to increase the cure rate for diffuse large B-cell lymphoma,” said Patrick Johnston, MD, PhD, of the Mayo Clinic in Rochester, Minnesota.
“This pilot study suggests that adding mTOR inhibitors to standard therapy could improve outcomes, though it needs to be validated in a larger clinical trial.”
Results from this study were published in The Lancet Haematology.
Patients and treatment
Dr Johnston and his colleagues conducted this study in 24 previously untreated DLBCL patients. Their median age was 58.5 (range, 49.5-71.5), and 58% were male. Most patients had stage IV disease (54%), followed by stage II (25%), and stage III (21%). Five patients (21%) had bulky disease.
The patients received standard R-CHOP-21 (rituximab at 375 mg/m2, cyclophosphamide at 750 mg/m2, doxorubicin at 50 mg/m2, and vincristine at 1.4 mg/m2—all on day 1 of the 21-day cycle—as well as oral prednisone at 100 mg/m2 each day on days 1–5 of the cycle) for 6 cycles, with scheduled pegfilgrastim at 6 mg on day 2 of each cycle.
They also received everolimus at 10 mg/day on 2 different schedules. Nine patients were enrolled initially—3 given everolimus on days 1–10 and 6 receiving it on days 1–14. As there were no dose-limiting toxicities in these patients, another 15 patients went on to receive everolimus on days 1–14.
Results
The median follow-up was 21.5 months. Twenty-three patients (96%) achieved an overall response and a complete metabolic response as assessed by PET. The remaining patient withdrew consent during cycle 1 and achieved a complete response with R-CHOP alone.
The 12-month event-free survival rate was 100%. Nine patients had sufficient follow-up and were event-free at 24 months. At last follow-up (March 30, 2016), no deaths or relapses had occurred.
The most common adverse events were hematologic, such as grade 4 neutropenia (75%) and grade 3 febrile neutropenia (21%).
Three patients experienced “significant” toxicity, according to the researchers. One patient had a treatment delay of 12 days due to grade 3 hypokalemia, which was considered possibly related to everolimus.
A second patient had grade 4 sepsis that was possibly related to treatment, and a third patient had a treatment delay of 10 days due to grade 3 infection that was possibly related to everolimus.
Ten patients (42%) had their dose of everolimus reduced, 2 patients permanently discontinued the drug after cycles 3 and 4, respectively, and 2 patients omitted everolimus for 1 and 2 cycles, respectively, then resumed everolimus for subsequent cycles.
“This study is the first to integrate a P13K-mTOR agent with standard R-CHOP,” Dr Johnston said.
“The encouraging outcome results and toxicity profile of this new regimen, along with the worldwide availability of everolimus, make it potentially applicable to the large population of DLBCL patients.” ![]()
Tonsillectomy for this 35-year-old patient?
THE CASE
A 35-year-old woman sought care for a fever and sore throat that she’d had for 4 days. She denied symptoms of cough, rhinorrhea, or sputum production.
The patient’s medical history included severe recurrent streptococcal pharyngitis as a child and teenager. At the age of 17, she developed a fever of 105° F with associated delirium, dysphagia, nausea, and vomiting, and missed several days of school. She also lost 82 pounds, developed oral thrush, and continued to feel fatigued for approximately a year. After her primary care physician noted a heart murmur on physical exam, she was sent for echocardiography and diagnosed with rheumatic fever secondary to streptococcal pharyngitis.
Eighteen years (and numerous streptococcal infections) later, the patient was at our facility and we were ordering a rapid antigen detection test (RADT) for her current illness. The throat specimen was positive for group A ß-hemolytic streptococcus (GAS). The patient’s 8-year-old daughter also had a sore throat, fever, and positive RADT; her symptoms resolved with oral amoxicillin for 10 days. The patient’s husband was also treated successfully with oral amoxicillin/clavulanate for 10 days for similar symptoms. The patient herself, however, was unsuccessfully treated with oral amoxicillin 500 mg twice daily for 7 days.
She was then given oral amoxicillin/clavulanate 875 mg twice daily for 14 days, but received no relief. Even after receiving clindamycin 600 mg twice daily for 10 days, she had minimal relief and remained positive for GAS on repeat RADT. It was at this point that tonsillectomy was considered as a possible treatment modality for her refractory GAS pharyngitis.
The patient consented to the procedure and underwent a tonsillectomy. She has remained asymptomatic for 2 years and there have been no reported outbreaks of GAS infection in her household.
DISCUSSION
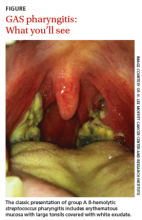
Streptococcal pharyngitis is an infection of the oropharynx and/or nasopharynx that is caused by Streptococcus pyogenes (also known as GAS). It is one of the most frequent illnesses encountered by primary care physicians, and primarily occurs in children ages 5 to 15 years.1,2 The signs and symptoms of GAS pharyngitis include an abrupt onset of a sore throat, tonsillar exudate, tender cervical adenopathy, and fever. (The classic presentation of GAS pharyngitis in a different patient can be seen in the FIGURE.)
Throat cultures are the gold standard for the diagnosis of GAS pharyngitis, but results take 24 to 48 hours, which can delay appropriate treatment. Therefore, the use of the RADT is often preferred clinically.1 RADT is not recommended for children and adults who show clinical symptoms that are highly suggestive of a viral illness, such as cough, rhinorrhea, hoarseness, or oral ulcers. A negative RADT in children and adolescents necessitates a throat culture to confirm the diagnosis.2
The antibiotics of choice are either penicillin 50 mg/kg/d in 4 divided doses or amoxicillin 40 mg/kg/d in 3 divided doses (maximum for both is 2000 mg/d) for 10 days. Options for patients with penicillin allergies include clindamycin or clarithromycin for 10 days or azithromycin for 5 days.2
The Infectious Diseases Society of America (IDSA) does not recommend routine testing or empiric treatment of asymptomatic carriers. However, it does recommend treatment of GAS carriers in certain situations, such as when: 2
- the carrier has acute rheumatic fever
- there is a family or personal history of acute rheumatic fever
- there is a post-streptococcal glomerulonephritis outbreak
- a family has excessive anxiety about GAS infections
- a tonsillectomy is being considered.
When—and for whom—is tonsillectomy beneficial?
Tonsillectomy is a treatment option for patients with recurrent episodes of GAS pharyngitis. Indications include patients with 7 GAS infections in a year, 5 episodes in 2 years, or 3 episodes in 3 years.3,4 In select patient populations, tonsillectomy has been shown to decrease missed work days and medical expenses caused by recurrent pharyngitis.5,6
Alho et al demonstrated that adults with recurrent episodes of GAS pharyngitis benefit from tonsillectomy in terms of fewer repeat infections and more days without throat pain.7 A randomized controlled trial conducted by Koskenkorva et al found that the overall rates of pharyngitis, throat pain, rhinitis, and cough were significantly lower in adults who received a tonsillectomy vs those who did not.5 Still, whether tonsillectomy is worthwhile in adults is debatable; Burton et al found no evidence that tonsillectomy is effective for chronic or recurrent acute tonsillitis in adults.8
Overall meta-analysis results indicate that tonsillectomy results in a 43% reduction in the incidence of pharyngitis in children between the ages of 4 and 16.8,9 One study found that children without tonsillectomy were 3.1 times more likely to develop subsequent GAS pharyngitis than children who underwent tonsillectomy.9 Another study found that children who received tonsillectomy demonstrated a decrease in sore throat episodes by 1.2 episodes per year and a decrease in school absenteeism by 2.8 days per year.6 Tonsillectomy does carry a risk of intraoperative and postoperative bleeding in children and adults, which may make it a less desirable option for some patients.6
THE TAKEAWAY
Recurrent GAS pharyngitis poses a significant challenge for clinicians. When episodes recur, it may be prudent to treat asymptomatic carriers in the patient’s household. Tonsillectomy should be considered in refractory cases since recurrent GAS pharyngitis directly impacts the wellness and productivity of patients. Our patient certainly benefited from the surgery: She has not missed any work days or had to visit her primary care physician because of a GAS infection since her tonsillectomy.
1. Gurol Y, Akan H, Izbirak G, et al. The sensitivity and the specificity of rapid antigen test in streptococcal upper respiratory tract infections. Int J Pediatr Otorhinolaryngol. 2010;74:591-593.
2. Shulman ST, Bisno AL, Clegg HW, et al. Clinical practice guideline for the diagnosis and management of group A streptococcal pharyngitis: 2012 update by the Infectious Diseases Society of America. Clin Infect Dis. 2012;55:1279-1282.
3. Stuck BA, Götte K, Windfuhr JP, et al. Tonsillectomy in children. Dtsch Arztebl Int. 2008;105:852-860.
4. Baugh RF, Archer SM, Mitchell RB, et al; American Academy of Otolaryngology-Head and Neck Surgery Foundation. Clinical practice guideline: tonsillectomy in children. Otolaryngol Head Neck Surg. 2011;144:S1-S30.
5. Koskenkorva T, Koivunen P, Koskela M, et al. Short-term outcomes of tonsillectomy in adult patients with recurrent pharyngitis: a randomized controlled trial. CMAJ. 2013;185:E331-E336.
6. van Staaij BK, van den Akker EH, van der Heijden GJ, et al. Adenotonsillectomy for upper respiratory infections: evidence based? Arch Dis Child. 2005;90:19-25.
7. Alho OP, Koivunen P, Penna T, et al. Tonsillectomy versus watchful waiting in recurrent streptococcal pharyngitis in adults: randomised controlled trial. BMJ. 2007;334:939.
8. Burton MJ, Towler B, Glasziou P. Tonsillectomy versus non-surgical treatment for chronic/recurrent acute tonsillitis. Cochrane Database Syst Rev. 2000;(2):CD001802.
9. Orvidas LJ, St Sauver JL, Weaver AL. Efficacy of tonsillectomy in treatment of recurrent group A beta-hemolytic streptococcal pharyngitis. Laryngoscope. 2006;116:1946-1950.
THE CASE
A 35-year-old woman sought care for a fever and sore throat that she’d had for 4 days. She denied symptoms of cough, rhinorrhea, or sputum production.
The patient’s medical history included severe recurrent streptococcal pharyngitis as a child and teenager. At the age of 17, she developed a fever of 105° F with associated delirium, dysphagia, nausea, and vomiting, and missed several days of school. She also lost 82 pounds, developed oral thrush, and continued to feel fatigued for approximately a year. After her primary care physician noted a heart murmur on physical exam, she was sent for echocardiography and diagnosed with rheumatic fever secondary to streptococcal pharyngitis.
Eighteen years (and numerous streptococcal infections) later, the patient was at our facility and we were ordering a rapid antigen detection test (RADT) for her current illness. The throat specimen was positive for group A ß-hemolytic streptococcus (GAS). The patient’s 8-year-old daughter also had a sore throat, fever, and positive RADT; her symptoms resolved with oral amoxicillin for 10 days. The patient’s husband was also treated successfully with oral amoxicillin/clavulanate for 10 days for similar symptoms. The patient herself, however, was unsuccessfully treated with oral amoxicillin 500 mg twice daily for 7 days.
She was then given oral amoxicillin/clavulanate 875 mg twice daily for 14 days, but received no relief. Even after receiving clindamycin 600 mg twice daily for 10 days, she had minimal relief and remained positive for GAS on repeat RADT. It was at this point that tonsillectomy was considered as a possible treatment modality for her refractory GAS pharyngitis.
The patient consented to the procedure and underwent a tonsillectomy. She has remained asymptomatic for 2 years and there have been no reported outbreaks of GAS infection in her household.
DISCUSSION
Streptococcal pharyngitis is an infection of the oropharynx and/or nasopharynx that is caused by Streptococcus pyogenes (also known as GAS). It is one of the most frequent illnesses encountered by primary care physicians, and primarily occurs in children ages 5 to 15 years.1,2 The signs and symptoms of GAS pharyngitis include an abrupt onset of a sore throat, tonsillar exudate, tender cervical adenopathy, and fever. (The classic presentation of GAS pharyngitis in a different patient can be seen in the FIGURE.)
Throat cultures are the gold standard for the diagnosis of GAS pharyngitis, but results take 24 to 48 hours, which can delay appropriate treatment. Therefore, the use of the RADT is often preferred clinically.1 RADT is not recommended for children and adults who show clinical symptoms that are highly suggestive of a viral illness, such as cough, rhinorrhea, hoarseness, or oral ulcers. A negative RADT in children and adolescents necessitates a throat culture to confirm the diagnosis.2
The antibiotics of choice are either penicillin 50 mg/kg/d in 4 divided doses or amoxicillin 40 mg/kg/d in 3 divided doses (maximum for both is 2000 mg/d) for 10 days. Options for patients with penicillin allergies include clindamycin or clarithromycin for 10 days or azithromycin for 5 days.2
The Infectious Diseases Society of America (IDSA) does not recommend routine testing or empiric treatment of asymptomatic carriers. However, it does recommend treatment of GAS carriers in certain situations, such as when: 2
- the carrier has acute rheumatic fever
- there is a family or personal history of acute rheumatic fever
- there is a post-streptococcal glomerulonephritis outbreak
- a family has excessive anxiety about GAS infections
- a tonsillectomy is being considered.
When—and for whom—is tonsillectomy beneficial?
Tonsillectomy is a treatment option for patients with recurrent episodes of GAS pharyngitis. Indications include patients with 7 GAS infections in a year, 5 episodes in 2 years, or 3 episodes in 3 years.3,4 In select patient populations, tonsillectomy has been shown to decrease missed work days and medical expenses caused by recurrent pharyngitis.5,6
Alho et al demonstrated that adults with recurrent episodes of GAS pharyngitis benefit from tonsillectomy in terms of fewer repeat infections and more days without throat pain.7 A randomized controlled trial conducted by Koskenkorva et al found that the overall rates of pharyngitis, throat pain, rhinitis, and cough were significantly lower in adults who received a tonsillectomy vs those who did not.5 Still, whether tonsillectomy is worthwhile in adults is debatable; Burton et al found no evidence that tonsillectomy is effective for chronic or recurrent acute tonsillitis in adults.8
Overall meta-analysis results indicate that tonsillectomy results in a 43% reduction in the incidence of pharyngitis in children between the ages of 4 and 16.8,9 One study found that children without tonsillectomy were 3.1 times more likely to develop subsequent GAS pharyngitis than children who underwent tonsillectomy.9 Another study found that children who received tonsillectomy demonstrated a decrease in sore throat episodes by 1.2 episodes per year and a decrease in school absenteeism by 2.8 days per year.6 Tonsillectomy does carry a risk of intraoperative and postoperative bleeding in children and adults, which may make it a less desirable option for some patients.6
THE TAKEAWAY
Recurrent GAS pharyngitis poses a significant challenge for clinicians. When episodes recur, it may be prudent to treat asymptomatic carriers in the patient’s household. Tonsillectomy should be considered in refractory cases since recurrent GAS pharyngitis directly impacts the wellness and productivity of patients. Our patient certainly benefited from the surgery: She has not missed any work days or had to visit her primary care physician because of a GAS infection since her tonsillectomy.
THE CASE
A 35-year-old woman sought care for a fever and sore throat that she’d had for 4 days. She denied symptoms of cough, rhinorrhea, or sputum production.
The patient’s medical history included severe recurrent streptococcal pharyngitis as a child and teenager. At the age of 17, she developed a fever of 105° F with associated delirium, dysphagia, nausea, and vomiting, and missed several days of school. She also lost 82 pounds, developed oral thrush, and continued to feel fatigued for approximately a year. After her primary care physician noted a heart murmur on physical exam, she was sent for echocardiography and diagnosed with rheumatic fever secondary to streptococcal pharyngitis.
Eighteen years (and numerous streptococcal infections) later, the patient was at our facility and we were ordering a rapid antigen detection test (RADT) for her current illness. The throat specimen was positive for group A ß-hemolytic streptococcus (GAS). The patient’s 8-year-old daughter also had a sore throat, fever, and positive RADT; her symptoms resolved with oral amoxicillin for 10 days. The patient’s husband was also treated successfully with oral amoxicillin/clavulanate for 10 days for similar symptoms. The patient herself, however, was unsuccessfully treated with oral amoxicillin 500 mg twice daily for 7 days.
She was then given oral amoxicillin/clavulanate 875 mg twice daily for 14 days, but received no relief. Even after receiving clindamycin 600 mg twice daily for 10 days, she had minimal relief and remained positive for GAS on repeat RADT. It was at this point that tonsillectomy was considered as a possible treatment modality for her refractory GAS pharyngitis.
The patient consented to the procedure and underwent a tonsillectomy. She has remained asymptomatic for 2 years and there have been no reported outbreaks of GAS infection in her household.
DISCUSSION
Streptococcal pharyngitis is an infection of the oropharynx and/or nasopharynx that is caused by Streptococcus pyogenes (also known as GAS). It is one of the most frequent illnesses encountered by primary care physicians, and primarily occurs in children ages 5 to 15 years.1,2 The signs and symptoms of GAS pharyngitis include an abrupt onset of a sore throat, tonsillar exudate, tender cervical adenopathy, and fever. (The classic presentation of GAS pharyngitis in a different patient can be seen in the FIGURE.)
Throat cultures are the gold standard for the diagnosis of GAS pharyngitis, but results take 24 to 48 hours, which can delay appropriate treatment. Therefore, the use of the RADT is often preferred clinically.1 RADT is not recommended for children and adults who show clinical symptoms that are highly suggestive of a viral illness, such as cough, rhinorrhea, hoarseness, or oral ulcers. A negative RADT in children and adolescents necessitates a throat culture to confirm the diagnosis.2
The antibiotics of choice are either penicillin 50 mg/kg/d in 4 divided doses or amoxicillin 40 mg/kg/d in 3 divided doses (maximum for both is 2000 mg/d) for 10 days. Options for patients with penicillin allergies include clindamycin or clarithromycin for 10 days or azithromycin for 5 days.2
The Infectious Diseases Society of America (IDSA) does not recommend routine testing or empiric treatment of asymptomatic carriers. However, it does recommend treatment of GAS carriers in certain situations, such as when: 2
- the carrier has acute rheumatic fever
- there is a family or personal history of acute rheumatic fever
- there is a post-streptococcal glomerulonephritis outbreak
- a family has excessive anxiety about GAS infections
- a tonsillectomy is being considered.
When—and for whom—is tonsillectomy beneficial?
Tonsillectomy is a treatment option for patients with recurrent episodes of GAS pharyngitis. Indications include patients with 7 GAS infections in a year, 5 episodes in 2 years, or 3 episodes in 3 years.3,4 In select patient populations, tonsillectomy has been shown to decrease missed work days and medical expenses caused by recurrent pharyngitis.5,6
Alho et al demonstrated that adults with recurrent episodes of GAS pharyngitis benefit from tonsillectomy in terms of fewer repeat infections and more days without throat pain.7 A randomized controlled trial conducted by Koskenkorva et al found that the overall rates of pharyngitis, throat pain, rhinitis, and cough were significantly lower in adults who received a tonsillectomy vs those who did not.5 Still, whether tonsillectomy is worthwhile in adults is debatable; Burton et al found no evidence that tonsillectomy is effective for chronic or recurrent acute tonsillitis in adults.8
Overall meta-analysis results indicate that tonsillectomy results in a 43% reduction in the incidence of pharyngitis in children between the ages of 4 and 16.8,9 One study found that children without tonsillectomy were 3.1 times more likely to develop subsequent GAS pharyngitis than children who underwent tonsillectomy.9 Another study found that children who received tonsillectomy demonstrated a decrease in sore throat episodes by 1.2 episodes per year and a decrease in school absenteeism by 2.8 days per year.6 Tonsillectomy does carry a risk of intraoperative and postoperative bleeding in children and adults, which may make it a less desirable option for some patients.6
THE TAKEAWAY
Recurrent GAS pharyngitis poses a significant challenge for clinicians. When episodes recur, it may be prudent to treat asymptomatic carriers in the patient’s household. Tonsillectomy should be considered in refractory cases since recurrent GAS pharyngitis directly impacts the wellness and productivity of patients. Our patient certainly benefited from the surgery: She has not missed any work days or had to visit her primary care physician because of a GAS infection since her tonsillectomy.
1. Gurol Y, Akan H, Izbirak G, et al. The sensitivity and the specificity of rapid antigen test in streptococcal upper respiratory tract infections. Int J Pediatr Otorhinolaryngol. 2010;74:591-593.
2. Shulman ST, Bisno AL, Clegg HW, et al. Clinical practice guideline for the diagnosis and management of group A streptococcal pharyngitis: 2012 update by the Infectious Diseases Society of America. Clin Infect Dis. 2012;55:1279-1282.
3. Stuck BA, Götte K, Windfuhr JP, et al. Tonsillectomy in children. Dtsch Arztebl Int. 2008;105:852-860.
4. Baugh RF, Archer SM, Mitchell RB, et al; American Academy of Otolaryngology-Head and Neck Surgery Foundation. Clinical practice guideline: tonsillectomy in children. Otolaryngol Head Neck Surg. 2011;144:S1-S30.
5. Koskenkorva T, Koivunen P, Koskela M, et al. Short-term outcomes of tonsillectomy in adult patients with recurrent pharyngitis: a randomized controlled trial. CMAJ. 2013;185:E331-E336.
6. van Staaij BK, van den Akker EH, van der Heijden GJ, et al. Adenotonsillectomy for upper respiratory infections: evidence based? Arch Dis Child. 2005;90:19-25.
7. Alho OP, Koivunen P, Penna T, et al. Tonsillectomy versus watchful waiting in recurrent streptococcal pharyngitis in adults: randomised controlled trial. BMJ. 2007;334:939.
8. Burton MJ, Towler B, Glasziou P. Tonsillectomy versus non-surgical treatment for chronic/recurrent acute tonsillitis. Cochrane Database Syst Rev. 2000;(2):CD001802.
9. Orvidas LJ, St Sauver JL, Weaver AL. Efficacy of tonsillectomy in treatment of recurrent group A beta-hemolytic streptococcal pharyngitis. Laryngoscope. 2006;116:1946-1950.
1. Gurol Y, Akan H, Izbirak G, et al. The sensitivity and the specificity of rapid antigen test in streptococcal upper respiratory tract infections. Int J Pediatr Otorhinolaryngol. 2010;74:591-593.
2. Shulman ST, Bisno AL, Clegg HW, et al. Clinical practice guideline for the diagnosis and management of group A streptococcal pharyngitis: 2012 update by the Infectious Diseases Society of America. Clin Infect Dis. 2012;55:1279-1282.
3. Stuck BA, Götte K, Windfuhr JP, et al. Tonsillectomy in children. Dtsch Arztebl Int. 2008;105:852-860.
4. Baugh RF, Archer SM, Mitchell RB, et al; American Academy of Otolaryngology-Head and Neck Surgery Foundation. Clinical practice guideline: tonsillectomy in children. Otolaryngol Head Neck Surg. 2011;144:S1-S30.
5. Koskenkorva T, Koivunen P, Koskela M, et al. Short-term outcomes of tonsillectomy in adult patients with recurrent pharyngitis: a randomized controlled trial. CMAJ. 2013;185:E331-E336.
6. van Staaij BK, van den Akker EH, van der Heijden GJ, et al. Adenotonsillectomy for upper respiratory infections: evidence based? Arch Dis Child. 2005;90:19-25.
7. Alho OP, Koivunen P, Penna T, et al. Tonsillectomy versus watchful waiting in recurrent streptococcal pharyngitis in adults: randomised controlled trial. BMJ. 2007;334:939.
8. Burton MJ, Towler B, Glasziou P. Tonsillectomy versus non-surgical treatment for chronic/recurrent acute tonsillitis. Cochrane Database Syst Rev. 2000;(2):CD001802.
9. Orvidas LJ, St Sauver JL, Weaver AL. Efficacy of tonsillectomy in treatment of recurrent group A beta-hemolytic streptococcal pharyngitis. Laryngoscope. 2006;116:1946-1950.
Children’s environmental health: An updated resource
In 1996, 2 exposure incidents sparked a movement to better understand children’s environmental health. In both incidents, children were exposed to significant toxicants in unexpected ways. In one, the organophosphate insecticide methyl parathion was applied illegally in indoor settings.1 In another, elemental mercury residue was detected in apartments converted from a fluorescent bulb facility.2 These incidents, and others like them, alerted physicians and government agencies to the collective lack of training and experience in the field of pediatric environmental health.
To address the situation, the Agency for Toxic Substances and Disease Registry and the Environmental Protection Agency created the Pediatric Environmental Health Specialty Unit (PEHSU) program. The program, which is now jointly operated by the American College of Medical Toxicology and the American Academy of Pediatrics, maintains sites in 10 regions3 and seeks to enhance education and promote consultation and referral related to reproductive and children’s environmental health.
This past fall, PEHSU updated its Web site at www.pehsu.net, which provides information, training, and resources for health professionals and the general public. The Web site provides news, fact sheets, and online education regarding environment-related pediatric and reproductive health issues. It also provides a tool for finding a local expert in the PEHSU national network, should a family physician need to refer a patient for more extensive assistance.
We believe that family physicians will find the PEHSU program resources informative, educational, and relevant to their practice.
Carl R. Baum, MD, FAAP, FACMT, Medical Director
Dana Turner, MPH, CHES
Amanda Allen, MS
PEHSU Program
National Office—West
Phoenix, Ariz
References
1. Esteban E, Rubin C, Hill R, et al. Association between indoor residential contamination with methyl parathion and urinary para-nitrophenol. J Expo Anal Environ Epidemiol. 1996;6:375-387.
2. Centers for Disease Control and Prevention (CDC). Mercury exposure among residents of a building formerly used for industrial purposes—New Jersey, 1995. MMWR Morb Mortal Wkly Rep. 1996;45:422-424.
3. Wilborne-Davis P, Kirkland KH, Mulloy KB. A model for physician education and consultation in pediatric environmental health—the Pediatric Environmental Health Specialty Units (PEHSU) program. Pediatr Clin North Am. 2007;54:1-13.
In 1996, 2 exposure incidents sparked a movement to better understand children’s environmental health. In both incidents, children were exposed to significant toxicants in unexpected ways. In one, the organophosphate insecticide methyl parathion was applied illegally in indoor settings.1 In another, elemental mercury residue was detected in apartments converted from a fluorescent bulb facility.2 These incidents, and others like them, alerted physicians and government agencies to the collective lack of training and experience in the field of pediatric environmental health.
To address the situation, the Agency for Toxic Substances and Disease Registry and the Environmental Protection Agency created the Pediatric Environmental Health Specialty Unit (PEHSU) program. The program, which is now jointly operated by the American College of Medical Toxicology and the American Academy of Pediatrics, maintains sites in 10 regions3 and seeks to enhance education and promote consultation and referral related to reproductive and children’s environmental health.
This past fall, PEHSU updated its Web site at www.pehsu.net, which provides information, training, and resources for health professionals and the general public. The Web site provides news, fact sheets, and online education regarding environment-related pediatric and reproductive health issues. It also provides a tool for finding a local expert in the PEHSU national network, should a family physician need to refer a patient for more extensive assistance.
We believe that family physicians will find the PEHSU program resources informative, educational, and relevant to their practice.
Carl R. Baum, MD, FAAP, FACMT, Medical Director
Dana Turner, MPH, CHES
Amanda Allen, MS
PEHSU Program
National Office—West
Phoenix, Ariz
References
1. Esteban E, Rubin C, Hill R, et al. Association between indoor residential contamination with methyl parathion and urinary para-nitrophenol. J Expo Anal Environ Epidemiol. 1996;6:375-387.
2. Centers for Disease Control and Prevention (CDC). Mercury exposure among residents of a building formerly used for industrial purposes—New Jersey, 1995. MMWR Morb Mortal Wkly Rep. 1996;45:422-424.
3. Wilborne-Davis P, Kirkland KH, Mulloy KB. A model for physician education and consultation in pediatric environmental health—the Pediatric Environmental Health Specialty Units (PEHSU) program. Pediatr Clin North Am. 2007;54:1-13.
In 1996, 2 exposure incidents sparked a movement to better understand children’s environmental health. In both incidents, children were exposed to significant toxicants in unexpected ways. In one, the organophosphate insecticide methyl parathion was applied illegally in indoor settings.1 In another, elemental mercury residue was detected in apartments converted from a fluorescent bulb facility.2 These incidents, and others like them, alerted physicians and government agencies to the collective lack of training and experience in the field of pediatric environmental health.
To address the situation, the Agency for Toxic Substances and Disease Registry and the Environmental Protection Agency created the Pediatric Environmental Health Specialty Unit (PEHSU) program. The program, which is now jointly operated by the American College of Medical Toxicology and the American Academy of Pediatrics, maintains sites in 10 regions3 and seeks to enhance education and promote consultation and referral related to reproductive and children’s environmental health.
This past fall, PEHSU updated its Web site at www.pehsu.net, which provides information, training, and resources for health professionals and the general public. The Web site provides news, fact sheets, and online education regarding environment-related pediatric and reproductive health issues. It also provides a tool for finding a local expert in the PEHSU national network, should a family physician need to refer a patient for more extensive assistance.
We believe that family physicians will find the PEHSU program resources informative, educational, and relevant to their practice.
Carl R. Baum, MD, FAAP, FACMT, Medical Director
Dana Turner, MPH, CHES
Amanda Allen, MS
PEHSU Program
National Office—West
Phoenix, Ariz
References
1. Esteban E, Rubin C, Hill R, et al. Association between indoor residential contamination with methyl parathion and urinary para-nitrophenol. J Expo Anal Environ Epidemiol. 1996;6:375-387.
2. Centers for Disease Control and Prevention (CDC). Mercury exposure among residents of a building formerly used for industrial purposes—New Jersey, 1995. MMWR Morb Mortal Wkly Rep. 1996;45:422-424.
3. Wilborne-Davis P, Kirkland KH, Mulloy KB. A model for physician education and consultation in pediatric environmental health—the Pediatric Environmental Health Specialty Units (PEHSU) program. Pediatr Clin North Am. 2007;54:1-13.
Vesicular eruption in a 2-year-old boy
A 2-year-old boy with atopic dermatitis developed a flare of his eczema after having a bath with mint-scented soap. His mother treated the flare with over-the-counter topical hydrocortisone cream. Two to 3 days later, he developed grouped vesicles on the right side of his neck. Three days after that, he developed a painful generalized vesicular eruption all over his body.
The boy was admitted to a hospital for supportive care and empiric antibiotics, but was discharged when no bacterial infection was found. The patient’s mother was instructed to follow up with his primary care provider in the next 2 weeks.
Three days after his hospitalization, the eruption on the young boy’s body spread and he was uncomfortable. He was brought to our hospital’s pediatric clinic, where physicians examined him and decided to transfer him to the university hospital for further evaluation.
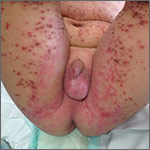
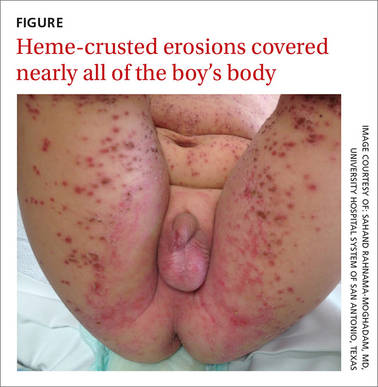
On exam, the boy was afebrile, but uncomfortable and irritable. Diffuse heme-crusted and punched-out erosions covered about 90% of his body (FIGURE). His mucous membranes were not involved. Underneath the heme-crusted erosions, there were lichenified pink plaques on the antecubital fossae, popliteal fossae, periocular face, and buttocks. The patient’s right dorsal foot had a small vesicle; all other vesicles on his body had crusted over.
The patient’s family indicated that the child had received the varicella vaccine without incident at 12 months of age. He had no history of travel, no contact with sick individuals, and no exposure to pets or other animals.
WHAT IS YOUR DIAGNOSIS?
HOW WOULD YOU TREAT THIS PATIENT?
Diagnosis: Eczema herpeticum
Eczema herpeticum (EH) was suspected based on the appearance of the lesions. A Tzanck smear came back positive for multinucleated giant cells and a herpes simplex virus (HSV) amplified probe came back positive for HSV-1—confirming the diagnosis.
EH—also known as Kaposi varicelliform eruption—is a superficial generalized viral infection (typically caused by HSV-1; HSV-2 is less common). The infection commonly occurs in patients with underlying atopic dermatitis, but may also occur in those with Darier disease, pemphigus, burns, and other conditions that disrupt the skin barrier. Other viruses, such as Coxsackie virus, can also cause EH. Eczema vaccinatum is a variant that may occur after smallpox vaccination.1 EH occurs more often in infants and children than in adults,2 and is a potentially life-threatening dermatologic emergency.
Who’s at risk? Patients with underlying chronic skin conditions such as eczema may have impaired cell-mediated immunity, making them more susceptible to a viral infection like EH.1 In addition, treatment of underlying chronic skin conditions with immunosuppressive therapies often increases susceptibility to superimposed infection.1 (In this case, the patient’s parents had treated an eczema flare with a topical hydrocortisone cream.) Lastly, increased risk may be associated with mutations in the gene encoding filaggrin.2
Areas affected. EH typically appears in areas of pre-existing dermatitis as monomorphic, discrete, 2- to 3-mm, punched-out, heme-crusted erosions with scalloped borders.2 The erosions initially appear as vesicles or pustules, which may appear concurrently with the erosions. The erosions can coalesce to form larger lesions.3 Fever, malaise, and lymphadenopathy may also be present.2,3
4 factors differentiate EH from other conditions
The differential for eczema herpeticum includes impetigo, bullous impetigo, shingles, chicken pox, scabies, pustular psoriasis, bullous pemphigoid, drug hypersensitivity reactions, and exacerbation of a primary dermatosis or skin condition.1,4
EH may be differentiated from these by its location, its development in the setting of pre-existing dermatitis, its response to antiviral medications, and the results of laboratory testing. Because of the vast differential, physicians must maintain a high index of suspicion for EH, particularly when a patient with a pre-existing skin condition presents with acute onset cutaneous pain.3
Perform a Tzanck smear to diagnose the underlying infection
If EH is suspected, treatment must be initiated immediately.3 (In our patient’s case, he was started on intravenous acyclovir 10 mg/kg every 8 hours.)
Once treatment is underway, a Tzanck smear of the vesicle base can be performed at the patient’s bedside to narrow the cause of the infection to HSV or varicella zoster virus (VZV). Multinucleated giant keratinocytes (as in our patient’s case) are diagnostic for one of the herpes viruses; concurrent inflammatory cells are also to be expected in an inflammatory skin condition but by themselves are not diagnostic of herpes.
If available in the laboratory, direct fluorescent antibody testing can differentiate between HSV and VZV. Alternatively, a nucleic acid amplified probe test may be used to provide a quick and specific result. The most specific test is a viral culture, but it lacks sensitivity and usually requires 2 to 5 daysfor results.2 A bacterial skin swab and blood culture should also be considered to direct antibiotic therapy if superinfection has occurred.
Antivirals and antibiotics should be given until lesions heal
Patients with EH should be admitted to the hospital for at least 24 to 48 hours of intravenous acyclovir.4 Antivirals—oral or intravenous—should be given for 10 to 14 days or until all mucocutaneous lesions are healed. Recommended dosing for acyclovir is 15 mg/kg (up to 400 mg) by mouth 3 to 5 times per day or, if severe, 5 mg/kg (if ≥12 years of age) to 10 mg/kg (if <12 years of age) intravenously every 8 hours.2 Patients should also receive a 3- to 6-month suppressive course of oral acyclovir, valacyclovir, or famciclovir.4
Intravenous antibiotics should also be considered, pending the results of bacterial skin swabs and a blood culture, as the skin of patients with atopic dermatitis is colonized with staphylococcus 90% of the time.4
Potential complications. Bacterial sepsis resulting from superinfection and disseminated HSV, although extremely rare, is the main cause of death associated with EH.3 One case in the literature described a 43-year-old woman with extensive EH superimposed on atopic dermatitis, disseminated HSV, and Pseudomonas aeruginosa septicemia. Despite treatment with intravenous acyclovir and antibiotics in a burn center intensive care unit, the patient experienced septic shock and disseminated intravascular coagulation with progression to multiorgan failure and death.3
Our patient’s antiviral regimen was transitioned to a 14-day course of oral acyclovir, which he completed. Topical steroids and an immunosuppressant (tacrolimus ointment) were applied concurrently. He was subsequently prescribed a 6-month suppressive course of acyclovir and was scheduled for follow-up at an outpatient dermatology clinic to discuss resuming therapy for atopic dermatitis.
CORRESPONDENCE
Sahand Rahnama-Moghadam, MD, 7323 Snowden Road #1205, San Antonio, TX 78240; [email protected].
1. Studdiford JS, Valko GP, Belin LJ, et al. Eczema herpeticum: making the diagnosis in the emergency department. J Emerg Med. 2011;40:167-169.
2. Mendoza N, Madkan V, Sra K, et al. Human herpesviruses. In: Bolognia JL, Jorizzo JL, Schaffer JV, et al, eds. Dermatology. 3rd ed. Philadelphia, PA: Elsevier Saunders; 2012:1321-1343.
3. Mackool BT, Goverman J, Nazarian RM. Case records of the Massachusetts General Hospital. Case 14-2012. A 43-year-old woman with fever and a generalized rash. N Engl J Med. 2012;366:1825-1834.
4. Kress DW. Pediatric dermatology emergencies. Curr Opin Pediatr. 2011;23:403-406.
A 2-year-old boy with atopic dermatitis developed a flare of his eczema after having a bath with mint-scented soap. His mother treated the flare with over-the-counter topical hydrocortisone cream. Two to 3 days later, he developed grouped vesicles on the right side of his neck. Three days after that, he developed a painful generalized vesicular eruption all over his body.
The boy was admitted to a hospital for supportive care and empiric antibiotics, but was discharged when no bacterial infection was found. The patient’s mother was instructed to follow up with his primary care provider in the next 2 weeks.
Three days after his hospitalization, the eruption on the young boy’s body spread and he was uncomfortable. He was brought to our hospital’s pediatric clinic, where physicians examined him and decided to transfer him to the university hospital for further evaluation.
On exam, the boy was afebrile, but uncomfortable and irritable. Diffuse heme-crusted and punched-out erosions covered about 90% of his body (FIGURE). His mucous membranes were not involved. Underneath the heme-crusted erosions, there were lichenified pink plaques on the antecubital fossae, popliteal fossae, periocular face, and buttocks. The patient’s right dorsal foot had a small vesicle; all other vesicles on his body had crusted over.
The patient’s family indicated that the child had received the varicella vaccine without incident at 12 months of age. He had no history of travel, no contact with sick individuals, and no exposure to pets or other animals.

WHAT IS YOUR DIAGNOSIS?
HOW WOULD YOU TREAT THIS PATIENT?
Diagnosis: Eczema herpeticum
Eczema herpeticum (EH) was suspected based on the appearance of the lesions. A Tzanck smear came back positive for multinucleated giant cells and a herpes simplex virus (HSV) amplified probe came back positive for HSV-1—confirming the diagnosis.
EH—also known as Kaposi varicelliform eruption—is a superficial generalized viral infection (typically caused by HSV-1; HSV-2 is less common). The infection commonly occurs in patients with underlying atopic dermatitis, but may also occur in those with Darier disease, pemphigus, burns, and other conditions that disrupt the skin barrier. Other viruses, such as Coxsackie virus, can also cause EH. Eczema vaccinatum is a variant that may occur after smallpox vaccination.1 EH occurs more often in infants and children than in adults,2 and is a potentially life-threatening dermatologic emergency.
Who’s at risk? Patients with underlying chronic skin conditions such as eczema may have impaired cell-mediated immunity, making them more susceptible to a viral infection like EH.1 In addition, treatment of underlying chronic skin conditions with immunosuppressive therapies often increases susceptibility to superimposed infection.1 (In this case, the patient’s parents had treated an eczema flare with a topical hydrocortisone cream.) Lastly, increased risk may be associated with mutations in the gene encoding filaggrin.2
Areas affected. EH typically appears in areas of pre-existing dermatitis as monomorphic, discrete, 2- to 3-mm, punched-out, heme-crusted erosions with scalloped borders.2 The erosions initially appear as vesicles or pustules, which may appear concurrently with the erosions. The erosions can coalesce to form larger lesions.3 Fever, malaise, and lymphadenopathy may also be present.2,3
4 factors differentiate EH from other conditions
The differential for eczema herpeticum includes impetigo, bullous impetigo, shingles, chicken pox, scabies, pustular psoriasis, bullous pemphigoid, drug hypersensitivity reactions, and exacerbation of a primary dermatosis or skin condition.1,4
EH may be differentiated from these by its location, its development in the setting of pre-existing dermatitis, its response to antiviral medications, and the results of laboratory testing. Because of the vast differential, physicians must maintain a high index of suspicion for EH, particularly when a patient with a pre-existing skin condition presents with acute onset cutaneous pain.3
Perform a Tzanck smear to diagnose the underlying infection
If EH is suspected, treatment must be initiated immediately.3 (In our patient’s case, he was started on intravenous acyclovir 10 mg/kg every 8 hours.)
Once treatment is underway, a Tzanck smear of the vesicle base can be performed at the patient’s bedside to narrow the cause of the infection to HSV or varicella zoster virus (VZV). Multinucleated giant keratinocytes (as in our patient’s case) are diagnostic for one of the herpes viruses; concurrent inflammatory cells are also to be expected in an inflammatory skin condition but by themselves are not diagnostic of herpes.
If available in the laboratory, direct fluorescent antibody testing can differentiate between HSV and VZV. Alternatively, a nucleic acid amplified probe test may be used to provide a quick and specific result. The most specific test is a viral culture, but it lacks sensitivity and usually requires 2 to 5 daysfor results.2 A bacterial skin swab and blood culture should also be considered to direct antibiotic therapy if superinfection has occurred.
Antivirals and antibiotics should be given until lesions heal
Patients with EH should be admitted to the hospital for at least 24 to 48 hours of intravenous acyclovir.4 Antivirals—oral or intravenous—should be given for 10 to 14 days or until all mucocutaneous lesions are healed. Recommended dosing for acyclovir is 15 mg/kg (up to 400 mg) by mouth 3 to 5 times per day or, if severe, 5 mg/kg (if ≥12 years of age) to 10 mg/kg (if <12 years of age) intravenously every 8 hours.2 Patients should also receive a 3- to 6-month suppressive course of oral acyclovir, valacyclovir, or famciclovir.4
Intravenous antibiotics should also be considered, pending the results of bacterial skin swabs and a blood culture, as the skin of patients with atopic dermatitis is colonized with staphylococcus 90% of the time.4
Potential complications. Bacterial sepsis resulting from superinfection and disseminated HSV, although extremely rare, is the main cause of death associated with EH.3 One case in the literature described a 43-year-old woman with extensive EH superimposed on atopic dermatitis, disseminated HSV, and Pseudomonas aeruginosa septicemia. Despite treatment with intravenous acyclovir and antibiotics in a burn center intensive care unit, the patient experienced septic shock and disseminated intravascular coagulation with progression to multiorgan failure and death.3
Our patient’s antiviral regimen was transitioned to a 14-day course of oral acyclovir, which he completed. Topical steroids and an immunosuppressant (tacrolimus ointment) were applied concurrently. He was subsequently prescribed a 6-month suppressive course of acyclovir and was scheduled for follow-up at an outpatient dermatology clinic to discuss resuming therapy for atopic dermatitis.
CORRESPONDENCE
Sahand Rahnama-Moghadam, MD, 7323 Snowden Road #1205, San Antonio, TX 78240; [email protected].
A 2-year-old boy with atopic dermatitis developed a flare of his eczema after having a bath with mint-scented soap. His mother treated the flare with over-the-counter topical hydrocortisone cream. Two to 3 days later, he developed grouped vesicles on the right side of his neck. Three days after that, he developed a painful generalized vesicular eruption all over his body.
The boy was admitted to a hospital for supportive care and empiric antibiotics, but was discharged when no bacterial infection was found. The patient’s mother was instructed to follow up with his primary care provider in the next 2 weeks.
Three days after his hospitalization, the eruption on the young boy’s body spread and he was uncomfortable. He was brought to our hospital’s pediatric clinic, where physicians examined him and decided to transfer him to the university hospital for further evaluation.
On exam, the boy was afebrile, but uncomfortable and irritable. Diffuse heme-crusted and punched-out erosions covered about 90% of his body (FIGURE). His mucous membranes were not involved. Underneath the heme-crusted erosions, there were lichenified pink plaques on the antecubital fossae, popliteal fossae, periocular face, and buttocks. The patient’s right dorsal foot had a small vesicle; all other vesicles on his body had crusted over.
The patient’s family indicated that the child had received the varicella vaccine without incident at 12 months of age. He had no history of travel, no contact with sick individuals, and no exposure to pets or other animals.

WHAT IS YOUR DIAGNOSIS?
HOW WOULD YOU TREAT THIS PATIENT?
Diagnosis: Eczema herpeticum
Eczema herpeticum (EH) was suspected based on the appearance of the lesions. A Tzanck smear came back positive for multinucleated giant cells and a herpes simplex virus (HSV) amplified probe came back positive for HSV-1—confirming the diagnosis.
EH—also known as Kaposi varicelliform eruption—is a superficial generalized viral infection (typically caused by HSV-1; HSV-2 is less common). The infection commonly occurs in patients with underlying atopic dermatitis, but may also occur in those with Darier disease, pemphigus, burns, and other conditions that disrupt the skin barrier. Other viruses, such as Coxsackie virus, can also cause EH. Eczema vaccinatum is a variant that may occur after smallpox vaccination.1 EH occurs more often in infants and children than in adults,2 and is a potentially life-threatening dermatologic emergency.
Who’s at risk? Patients with underlying chronic skin conditions such as eczema may have impaired cell-mediated immunity, making them more susceptible to a viral infection like EH.1 In addition, treatment of underlying chronic skin conditions with immunosuppressive therapies often increases susceptibility to superimposed infection.1 (In this case, the patient’s parents had treated an eczema flare with a topical hydrocortisone cream.) Lastly, increased risk may be associated with mutations in the gene encoding filaggrin.2
Areas affected. EH typically appears in areas of pre-existing dermatitis as monomorphic, discrete, 2- to 3-mm, punched-out, heme-crusted erosions with scalloped borders.2 The erosions initially appear as vesicles or pustules, which may appear concurrently with the erosions. The erosions can coalesce to form larger lesions.3 Fever, malaise, and lymphadenopathy may also be present.2,3
4 factors differentiate EH from other conditions
The differential for eczema herpeticum includes impetigo, bullous impetigo, shingles, chicken pox, scabies, pustular psoriasis, bullous pemphigoid, drug hypersensitivity reactions, and exacerbation of a primary dermatosis or skin condition.1,4
EH may be differentiated from these by its location, its development in the setting of pre-existing dermatitis, its response to antiviral medications, and the results of laboratory testing. Because of the vast differential, physicians must maintain a high index of suspicion for EH, particularly when a patient with a pre-existing skin condition presents with acute onset cutaneous pain.3
Perform a Tzanck smear to diagnose the underlying infection
If EH is suspected, treatment must be initiated immediately.3 (In our patient’s case, he was started on intravenous acyclovir 10 mg/kg every 8 hours.)
Once treatment is underway, a Tzanck smear of the vesicle base can be performed at the patient’s bedside to narrow the cause of the infection to HSV or varicella zoster virus (VZV). Multinucleated giant keratinocytes (as in our patient’s case) are diagnostic for one of the herpes viruses; concurrent inflammatory cells are also to be expected in an inflammatory skin condition but by themselves are not diagnostic of herpes.
If available in the laboratory, direct fluorescent antibody testing can differentiate between HSV and VZV. Alternatively, a nucleic acid amplified probe test may be used to provide a quick and specific result. The most specific test is a viral culture, but it lacks sensitivity and usually requires 2 to 5 daysfor results.2 A bacterial skin swab and blood culture should also be considered to direct antibiotic therapy if superinfection has occurred.
Antivirals and antibiotics should be given until lesions heal
Patients with EH should be admitted to the hospital for at least 24 to 48 hours of intravenous acyclovir.4 Antivirals—oral or intravenous—should be given for 10 to 14 days or until all mucocutaneous lesions are healed. Recommended dosing for acyclovir is 15 mg/kg (up to 400 mg) by mouth 3 to 5 times per day or, if severe, 5 mg/kg (if ≥12 years of age) to 10 mg/kg (if <12 years of age) intravenously every 8 hours.2 Patients should also receive a 3- to 6-month suppressive course of oral acyclovir, valacyclovir, or famciclovir.4
Intravenous antibiotics should also be considered, pending the results of bacterial skin swabs and a blood culture, as the skin of patients with atopic dermatitis is colonized with staphylococcus 90% of the time.4
Potential complications. Bacterial sepsis resulting from superinfection and disseminated HSV, although extremely rare, is the main cause of death associated with EH.3 One case in the literature described a 43-year-old woman with extensive EH superimposed on atopic dermatitis, disseminated HSV, and Pseudomonas aeruginosa septicemia. Despite treatment with intravenous acyclovir and antibiotics in a burn center intensive care unit, the patient experienced septic shock and disseminated intravascular coagulation with progression to multiorgan failure and death.3
Our patient’s antiviral regimen was transitioned to a 14-day course of oral acyclovir, which he completed. Topical steroids and an immunosuppressant (tacrolimus ointment) were applied concurrently. He was subsequently prescribed a 6-month suppressive course of acyclovir and was scheduled for follow-up at an outpatient dermatology clinic to discuss resuming therapy for atopic dermatitis.
CORRESPONDENCE
Sahand Rahnama-Moghadam, MD, 7323 Snowden Road #1205, San Antonio, TX 78240; [email protected].
1. Studdiford JS, Valko GP, Belin LJ, et al. Eczema herpeticum: making the diagnosis in the emergency department. J Emerg Med. 2011;40:167-169.
2. Mendoza N, Madkan V, Sra K, et al. Human herpesviruses. In: Bolognia JL, Jorizzo JL, Schaffer JV, et al, eds. Dermatology. 3rd ed. Philadelphia, PA: Elsevier Saunders; 2012:1321-1343.
3. Mackool BT, Goverman J, Nazarian RM. Case records of the Massachusetts General Hospital. Case 14-2012. A 43-year-old woman with fever and a generalized rash. N Engl J Med. 2012;366:1825-1834.
4. Kress DW. Pediatric dermatology emergencies. Curr Opin Pediatr. 2011;23:403-406.
1. Studdiford JS, Valko GP, Belin LJ, et al. Eczema herpeticum: making the diagnosis in the emergency department. J Emerg Med. 2011;40:167-169.
2. Mendoza N, Madkan V, Sra K, et al. Human herpesviruses. In: Bolognia JL, Jorizzo JL, Schaffer JV, et al, eds. Dermatology. 3rd ed. Philadelphia, PA: Elsevier Saunders; 2012:1321-1343.
3. Mackool BT, Goverman J, Nazarian RM. Case records of the Massachusetts General Hospital. Case 14-2012. A 43-year-old woman with fever and a generalized rash. N Engl J Med. 2012;366:1825-1834.
4. Kress DW. Pediatric dermatology emergencies. Curr Opin Pediatr. 2011;23:403-406.
Newer St. Jude leads last as long as Medtronic Sprint Quattro
SAN FRANCISCO – St. Jude Medical’s Durata and Riata ST Optim defibrillator leads performed comparably to Medtronic’s Sprint Quattro out to 7 years in a Veterans Affairs analysis of almost 18,000 patients in the VA National Cardiac Device Surveillance Program.
The “highly satisfactory electrical survival” of the Optim leads, at least until year 5, should be of some reassurance to cardiologists, especially since the findings come from the VA, not a device company, investigator Seema Pursnani, MD, said.
The investigators combined Durata and Riata ST Optim leads together in their analysis, since they are similar; both are 7 Fr leads with St. Jude’s silicone/polyurethane Optim coating. After a mean follow-up of 3.4 years in 4,091 Durata patients and 351 Riata ST Optim patients, there were 26 electrical lead failures, which translated to 0.17% failures per device-year.
The investigators compared those results with Medtronic’s Sprint Quattro, which “is sort of a gold standard. It’s been around for quite a long time, and people have confidence in it,” said Dr. Pursnani. After a mean follow-up of 3.8 years in 13,254 patients, there were 57 failures, translating to 0.11% failures per device-year.
Seven-year lead survival was 97.7% with St. Jude’s products, and 98.9% with Medtronic’s. Although the difference was not statistically significant, “we need a little more follow-up to see why the curves are diverging at years 6 and 7,” said Dr. Pursnani, a cardiologist at the San Francisco Veterans Affairs Medical Center when the study was done, but now with the Kaiser Permanente San Leandro (Calif.) Medical Center.
There’s been lingering concern about St. Jude leads ever since the recall of earlier versions of Riata – with silicone-only insulation – in 2011 because of lead abrasion and subsequent safety problems. Optim was developed to address the issue.
“One of the most common modes of lead failure that we saw” with all three leads “was a rise in the pace-sense conductor impedance from the baseline impedance. Sometimes, there is nonphysiologic noise that can also be a sign of early failure,” she said.
There was no industry funding for the work, and the investigators have no disclosures.
SAN FRANCISCO – St. Jude Medical’s Durata and Riata ST Optim defibrillator leads performed comparably to Medtronic’s Sprint Quattro out to 7 years in a Veterans Affairs analysis of almost 18,000 patients in the VA National Cardiac Device Surveillance Program.
The “highly satisfactory electrical survival” of the Optim leads, at least until year 5, should be of some reassurance to cardiologists, especially since the findings come from the VA, not a device company, investigator Seema Pursnani, MD, said.
The investigators combined Durata and Riata ST Optim leads together in their analysis, since they are similar; both are 7 Fr leads with St. Jude’s silicone/polyurethane Optim coating. After a mean follow-up of 3.4 years in 4,091 Durata patients and 351 Riata ST Optim patients, there were 26 electrical lead failures, which translated to 0.17% failures per device-year.
The investigators compared those results with Medtronic’s Sprint Quattro, which “is sort of a gold standard. It’s been around for quite a long time, and people have confidence in it,” said Dr. Pursnani. After a mean follow-up of 3.8 years in 13,254 patients, there were 57 failures, translating to 0.11% failures per device-year.
Seven-year lead survival was 97.7% with St. Jude’s products, and 98.9% with Medtronic’s. Although the difference was not statistically significant, “we need a little more follow-up to see why the curves are diverging at years 6 and 7,” said Dr. Pursnani, a cardiologist at the San Francisco Veterans Affairs Medical Center when the study was done, but now with the Kaiser Permanente San Leandro (Calif.) Medical Center.
There’s been lingering concern about St. Jude leads ever since the recall of earlier versions of Riata – with silicone-only insulation – in 2011 because of lead abrasion and subsequent safety problems. Optim was developed to address the issue.
“One of the most common modes of lead failure that we saw” with all three leads “was a rise in the pace-sense conductor impedance from the baseline impedance. Sometimes, there is nonphysiologic noise that can also be a sign of early failure,” she said.
There was no industry funding for the work, and the investigators have no disclosures.
SAN FRANCISCO – St. Jude Medical’s Durata and Riata ST Optim defibrillator leads performed comparably to Medtronic’s Sprint Quattro out to 7 years in a Veterans Affairs analysis of almost 18,000 patients in the VA National Cardiac Device Surveillance Program.
The “highly satisfactory electrical survival” of the Optim leads, at least until year 5, should be of some reassurance to cardiologists, especially since the findings come from the VA, not a device company, investigator Seema Pursnani, MD, said.
The investigators combined Durata and Riata ST Optim leads together in their analysis, since they are similar; both are 7 Fr leads with St. Jude’s silicone/polyurethane Optim coating. After a mean follow-up of 3.4 years in 4,091 Durata patients and 351 Riata ST Optim patients, there were 26 electrical lead failures, which translated to 0.17% failures per device-year.
The investigators compared those results with Medtronic’s Sprint Quattro, which “is sort of a gold standard. It’s been around for quite a long time, and people have confidence in it,” said Dr. Pursnani. After a mean follow-up of 3.8 years in 13,254 patients, there were 57 failures, translating to 0.11% failures per device-year.
Seven-year lead survival was 97.7% with St. Jude’s products, and 98.9% with Medtronic’s. Although the difference was not statistically significant, “we need a little more follow-up to see why the curves are diverging at years 6 and 7,” said Dr. Pursnani, a cardiologist at the San Francisco Veterans Affairs Medical Center when the study was done, but now with the Kaiser Permanente San Leandro (Calif.) Medical Center.
There’s been lingering concern about St. Jude leads ever since the recall of earlier versions of Riata – with silicone-only insulation – in 2011 because of lead abrasion and subsequent safety problems. Optim was developed to address the issue.
“One of the most common modes of lead failure that we saw” with all three leads “was a rise in the pace-sense conductor impedance from the baseline impedance. Sometimes, there is nonphysiologic noise that can also be a sign of early failure,” she said.
There was no industry funding for the work, and the investigators have no disclosures.
AT HEART RHYTHM 2016
Key clinical point: St. Jude may have solved the Riata lead problem.
Major finding: Seven-year lead survival was 97.7% with St. Jude’s products, and 98.9% with Medtronic’s; the difference was not statistically significant.
Data source: Veterans Affairs analysis of almost 18,000 patients
Disclosures: There was no industry funding for the work, and the investigators have no disclosures.
Midostaurin cut organ damage in systemic mastocytosis
Midostaurin completely resolved at least one type of organ damage for 45% of patients with advanced systemic mastocytosis, based on a multicenter, open-label, phase II, industry-sponsored trial.
“Response rates were similar regardless of the subtype of advanced systemic mastocytosis, KIT mutation status, or exposure to previous therapy,” reported Jason R. Gotlib, MD, of Stanford (Calif.) University, and his associates. Adverse effects led to dose reductions for 41% of patients, however, and caused 22% of patients to stop treatment, the researchers wrote online June 29 in the New England Journal of Medicine.
Systemic mastocytosis is related to a constitutively activated receptor tyrosine kinase encoded by the KIT D816V mutation. As neoplastic mast cells infiltrate and damage organs, patients develop cytopenias, hypoalbuminemia, osteolytic bone lesions, abnormal liver function, ascites, and weight loss. Mastocytosis lacks effective treatments, and patients with aggressive disease tend to live about 3.5 years, the researchers noted (N Engl J Med. 2016 Jun 29;374:2530-40).
Of 116 patients with advanced systemic mastocytosis, 27 lacked measurable signs of disease or had unrelated signs and symptoms. The remaining 89 patients included 16 with aggressive systemic disease, 57 with systemic disease and an associated hematologic neoplasm, and 16 with mast cell leukemia. Patients received 100 mg oral midostaurin twice daily in continuous 4-week cycles for a median of 11.4 months, with a median follow-up of 26 months.
In all, 53 (60%) patients experienced at least 50% improvement in one type of organ damage or improvement in more than one type of organ damage, said the researchers. These responders included 12 patients with aggressive systemic mastocytosis, 33 patients with systemic mastocytosis and a hematologic neoplasm, and eight patients with mast-cell leukemia. No one achieved complete remission, but after six treatment cycles, 45% of patients had complete resolution of at least one type of organ damage.
Patients typically experienced, at best, a nearly 60% drop in bone marrow mast cell burden and serum tryptase. The median duration of response was 24 months, median overall survival was 28.7 months, and median progression-free survival was 14.1 months. Mast cell leukemia and a history of treatment for mastocytosis were tied to shorter survival, while a 50% decrease in mast cell burden significantly improved survival (hazard ratio, 0.33; P = .01).
Grade 3 or 4 hematologic abnormalities included neutropenia (24% of patients), anemia (41%), and thrombocytopenia (29%). Marked myelosuppression was associated with baseline cytopenia and may have reflected either treatment-related effects or disease progression, the researchers said. The most common grade 3/4 nonhematologic adverse effects were fatigue (9% of patients) and diarrhea (8%).
Novartis Pharmaceuticals sponsored the study, and designed it and collected the data with the authors. Dr. Gotlib disclosed travel reimbursement from Novartis. Ten coinvestigators disclosed financial ties to Novartis and to several other pharmaceutical companies. Two coinvestigators disclosed direct research support from Novartis. The remaining four coinvestigators had no disclosures.
Midostaurin completely resolved at least one type of organ damage for 45% of patients with advanced systemic mastocytosis, based on a multicenter, open-label, phase II, industry-sponsored trial.
“Response rates were similar regardless of the subtype of advanced systemic mastocytosis, KIT mutation status, or exposure to previous therapy,” reported Jason R. Gotlib, MD, of Stanford (Calif.) University, and his associates. Adverse effects led to dose reductions for 41% of patients, however, and caused 22% of patients to stop treatment, the researchers wrote online June 29 in the New England Journal of Medicine.
Systemic mastocytosis is related to a constitutively activated receptor tyrosine kinase encoded by the KIT D816V mutation. As neoplastic mast cells infiltrate and damage organs, patients develop cytopenias, hypoalbuminemia, osteolytic bone lesions, abnormal liver function, ascites, and weight loss. Mastocytosis lacks effective treatments, and patients with aggressive disease tend to live about 3.5 years, the researchers noted (N Engl J Med. 2016 Jun 29;374:2530-40).
Of 116 patients with advanced systemic mastocytosis, 27 lacked measurable signs of disease or had unrelated signs and symptoms. The remaining 89 patients included 16 with aggressive systemic disease, 57 with systemic disease and an associated hematologic neoplasm, and 16 with mast cell leukemia. Patients received 100 mg oral midostaurin twice daily in continuous 4-week cycles for a median of 11.4 months, with a median follow-up of 26 months.
In all, 53 (60%) patients experienced at least 50% improvement in one type of organ damage or improvement in more than one type of organ damage, said the researchers. These responders included 12 patients with aggressive systemic mastocytosis, 33 patients with systemic mastocytosis and a hematologic neoplasm, and eight patients with mast-cell leukemia. No one achieved complete remission, but after six treatment cycles, 45% of patients had complete resolution of at least one type of organ damage.
Patients typically experienced, at best, a nearly 60% drop in bone marrow mast cell burden and serum tryptase. The median duration of response was 24 months, median overall survival was 28.7 months, and median progression-free survival was 14.1 months. Mast cell leukemia and a history of treatment for mastocytosis were tied to shorter survival, while a 50% decrease in mast cell burden significantly improved survival (hazard ratio, 0.33; P = .01).
Grade 3 or 4 hematologic abnormalities included neutropenia (24% of patients), anemia (41%), and thrombocytopenia (29%). Marked myelosuppression was associated with baseline cytopenia and may have reflected either treatment-related effects or disease progression, the researchers said. The most common grade 3/4 nonhematologic adverse effects were fatigue (9% of patients) and diarrhea (8%).
Novartis Pharmaceuticals sponsored the study, and designed it and collected the data with the authors. Dr. Gotlib disclosed travel reimbursement from Novartis. Ten coinvestigators disclosed financial ties to Novartis and to several other pharmaceutical companies. Two coinvestigators disclosed direct research support from Novartis. The remaining four coinvestigators had no disclosures.
Midostaurin completely resolved at least one type of organ damage for 45% of patients with advanced systemic mastocytosis, based on a multicenter, open-label, phase II, industry-sponsored trial.
“Response rates were similar regardless of the subtype of advanced systemic mastocytosis, KIT mutation status, or exposure to previous therapy,” reported Jason R. Gotlib, MD, of Stanford (Calif.) University, and his associates. Adverse effects led to dose reductions for 41% of patients, however, and caused 22% of patients to stop treatment, the researchers wrote online June 29 in the New England Journal of Medicine.
Systemic mastocytosis is related to a constitutively activated receptor tyrosine kinase encoded by the KIT D816V mutation. As neoplastic mast cells infiltrate and damage organs, patients develop cytopenias, hypoalbuminemia, osteolytic bone lesions, abnormal liver function, ascites, and weight loss. Mastocytosis lacks effective treatments, and patients with aggressive disease tend to live about 3.5 years, the researchers noted (N Engl J Med. 2016 Jun 29;374:2530-40).
Of 116 patients with advanced systemic mastocytosis, 27 lacked measurable signs of disease or had unrelated signs and symptoms. The remaining 89 patients included 16 with aggressive systemic disease, 57 with systemic disease and an associated hematologic neoplasm, and 16 with mast cell leukemia. Patients received 100 mg oral midostaurin twice daily in continuous 4-week cycles for a median of 11.4 months, with a median follow-up of 26 months.
In all, 53 (60%) patients experienced at least 50% improvement in one type of organ damage or improvement in more than one type of organ damage, said the researchers. These responders included 12 patients with aggressive systemic mastocytosis, 33 patients with systemic mastocytosis and a hematologic neoplasm, and eight patients with mast-cell leukemia. No one achieved complete remission, but after six treatment cycles, 45% of patients had complete resolution of at least one type of organ damage.
Patients typically experienced, at best, a nearly 60% drop in bone marrow mast cell burden and serum tryptase. The median duration of response was 24 months, median overall survival was 28.7 months, and median progression-free survival was 14.1 months. Mast cell leukemia and a history of treatment for mastocytosis were tied to shorter survival, while a 50% decrease in mast cell burden significantly improved survival (hazard ratio, 0.33; P = .01).
Grade 3 or 4 hematologic abnormalities included neutropenia (24% of patients), anemia (41%), and thrombocytopenia (29%). Marked myelosuppression was associated with baseline cytopenia and may have reflected either treatment-related effects or disease progression, the researchers said. The most common grade 3/4 nonhematologic adverse effects were fatigue (9% of patients) and diarrhea (8%).
Novartis Pharmaceuticals sponsored the study, and designed it and collected the data with the authors. Dr. Gotlib disclosed travel reimbursement from Novartis. Ten coinvestigators disclosed financial ties to Novartis and to several other pharmaceutical companies. Two coinvestigators disclosed direct research support from Novartis. The remaining four coinvestigators had no disclosures.
FROM THE NEW ENGLAND JOURNAL OF MEDICINE
Key clinical point: Midostaurin helped to resolve organ damage related to mastocytosis.
Major finding: In all, 45% of patients had complete resolution of at least one type of organ damage within six, 4-week treatment cycles.
Data source: An international, open-label, phase II study of 116 patients given 100 mg oral midostaurin twice daily.
Disclosures: Novartis Pharmaceuticals sponsored the study, designed the study, and collected the data together with the authors. Dr. Gotlib disclosed travel reimbursement from Novartis. Ten coinvestigators disclosed financial ties to Novartis and to several other pharmaceutical companies. Two coinvestigators disclosed direct research support from Novartis. Four coinvestigators had no disclosures.
Staffing, work environment drive VAP risk in the ICU
SAN FRANCISCO – The work environment for nurses and the physician staffing model in the intensive care unit influence patients’ likelihood of acquiring ventilator-associated pneumonia (VAP), based on a cohort study of 25 ICUs.
Overall, each 1-point increase in the score for the nurse work environment – indicating that nurses had a greater sense of playing an important role in patient care – was unexpectedly associated with a roughly sixfold higher rate of VAP among the ICU’s patients, according to data reported in a session and press briefing at an international conference of the American Thoracic Society. However, additional analyses showed that the rate of VAP was higher in closed units where a board-certified critical care physician (intensivist) managed and led care rather than an open unit where care is shared.
“We think that the organization of the ICU is actually influencing nursing practice, which is a really novel finding,” commented first author Deena Kelly Costa, PhD, RN, of the University of Michigan School of Nursing in Ann Arbor. “In closed ICUs, when you have a board-certified physician and an ICU team managing and leading care, even if the work environment is better, nurses may not feel as empowered to standardize their care or practice.”
“ICU nurses are the ones who are primarily responsible for VAP preventive practices: they keep the head of the bed higher than 45 degrees, they conduct oral care, they conduct (patient) surveillance. ICU physicians are involved with writing the orders and ventilator setting management. So how these providers work together could theoretically influence the risk for patients developing VAP,” Dr. Costa said.
“We need to be thinking a little bit more critically about not only the care that’s happening at the bedside... but also at an organizational level. How are these providers organized, and can we work together to improve patient outcomes?”
“I’m not suggesting that we get rid of all closed ICUs because I don’t think that’s the solution,” Dr. Costa maintained. “I think from an administrative perspective, we need to be considering what’s the organization of these clinicians and this unit, and [in a context-specific manner], how can we improve it for better patient outcomes? That may be both working on improving the work environment and making the nurses feel more empowered, or it could be potentially considering other staffing models.”
Some data have already linked a more favorable nurse work environment and the presence of a board-certified critical care physician independently with better patient outcomes in the ICU. But studies of their joint impact are lacking.
The investigators performed a secondary, unit-level analysis of nurse survey data collected during 2005 and 2006 in ICUs in southern Michigan.
In all, 462 nurses working in 25 ICUs completed the Practice Environment Scale of the Nursing Work Index, on which averaged summary scores range between 1 (unfavorable) and 4 (favorable). The scale captures environmental factors such as the adequacy of resources for nurses, support from their managers, and their level of involvement in hospital policy decisions.
The rate of VAP during the same period was assessed using data from more than 1,000 patients from each ICU.
The summary nurse work environment score averaged 2.69 points in the 21 ICUs that had a closed physician staffing model and 2.62 points in the 4 ICUs that had an open physician staffing model. The respective rates of VAP were 7.5% and 2.5%.
In adjusted analysis among all 25 ICUs, each 1-point increase in an ICU’s Practice Environment Scale score was associated with a sharply higher rate of VAP on the unit (adjusted incidence rate ratio, 5.76; P = .02).
However, there was a strong interaction between the score and physician staffing model (P less than .001). In open ICUs, as the score rose, the rate of VAP fell (from about 16% to 5%), whereas in closed ICUs, as the score rose, so did the rate of VAP (from about 3% to 14%).
Dr. Costa disclosed that she had no relevant conflicts of interest. The parent survey was funded by the Blue Cross Blue Shield Foundation of Michigan.
SAN FRANCISCO – The work environment for nurses and the physician staffing model in the intensive care unit influence patients’ likelihood of acquiring ventilator-associated pneumonia (VAP), based on a cohort study of 25 ICUs.
Overall, each 1-point increase in the score for the nurse work environment – indicating that nurses had a greater sense of playing an important role in patient care – was unexpectedly associated with a roughly sixfold higher rate of VAP among the ICU’s patients, according to data reported in a session and press briefing at an international conference of the American Thoracic Society. However, additional analyses showed that the rate of VAP was higher in closed units where a board-certified critical care physician (intensivist) managed and led care rather than an open unit where care is shared.
“We think that the organization of the ICU is actually influencing nursing practice, which is a really novel finding,” commented first author Deena Kelly Costa, PhD, RN, of the University of Michigan School of Nursing in Ann Arbor. “In closed ICUs, when you have a board-certified physician and an ICU team managing and leading care, even if the work environment is better, nurses may not feel as empowered to standardize their care or practice.”
“ICU nurses are the ones who are primarily responsible for VAP preventive practices: they keep the head of the bed higher than 45 degrees, they conduct oral care, they conduct (patient) surveillance. ICU physicians are involved with writing the orders and ventilator setting management. So how these providers work together could theoretically influence the risk for patients developing VAP,” Dr. Costa said.
“We need to be thinking a little bit more critically about not only the care that’s happening at the bedside... but also at an organizational level. How are these providers organized, and can we work together to improve patient outcomes?”
“I’m not suggesting that we get rid of all closed ICUs because I don’t think that’s the solution,” Dr. Costa maintained. “I think from an administrative perspective, we need to be considering what’s the organization of these clinicians and this unit, and [in a context-specific manner], how can we improve it for better patient outcomes? That may be both working on improving the work environment and making the nurses feel more empowered, or it could be potentially considering other staffing models.”
Some data have already linked a more favorable nurse work environment and the presence of a board-certified critical care physician independently with better patient outcomes in the ICU. But studies of their joint impact are lacking.
The investigators performed a secondary, unit-level analysis of nurse survey data collected during 2005 and 2006 in ICUs in southern Michigan.
In all, 462 nurses working in 25 ICUs completed the Practice Environment Scale of the Nursing Work Index, on which averaged summary scores range between 1 (unfavorable) and 4 (favorable). The scale captures environmental factors such as the adequacy of resources for nurses, support from their managers, and their level of involvement in hospital policy decisions.
The rate of VAP during the same period was assessed using data from more than 1,000 patients from each ICU.
The summary nurse work environment score averaged 2.69 points in the 21 ICUs that had a closed physician staffing model and 2.62 points in the 4 ICUs that had an open physician staffing model. The respective rates of VAP were 7.5% and 2.5%.
In adjusted analysis among all 25 ICUs, each 1-point increase in an ICU’s Practice Environment Scale score was associated with a sharply higher rate of VAP on the unit (adjusted incidence rate ratio, 5.76; P = .02).
However, there was a strong interaction between the score and physician staffing model (P less than .001). In open ICUs, as the score rose, the rate of VAP fell (from about 16% to 5%), whereas in closed ICUs, as the score rose, so did the rate of VAP (from about 3% to 14%).
Dr. Costa disclosed that she had no relevant conflicts of interest. The parent survey was funded by the Blue Cross Blue Shield Foundation of Michigan.
SAN FRANCISCO – The work environment for nurses and the physician staffing model in the intensive care unit influence patients’ likelihood of acquiring ventilator-associated pneumonia (VAP), based on a cohort study of 25 ICUs.
Overall, each 1-point increase in the score for the nurse work environment – indicating that nurses had a greater sense of playing an important role in patient care – was unexpectedly associated with a roughly sixfold higher rate of VAP among the ICU’s patients, according to data reported in a session and press briefing at an international conference of the American Thoracic Society. However, additional analyses showed that the rate of VAP was higher in closed units where a board-certified critical care physician (intensivist) managed and led care rather than an open unit where care is shared.
“We think that the organization of the ICU is actually influencing nursing practice, which is a really novel finding,” commented first author Deena Kelly Costa, PhD, RN, of the University of Michigan School of Nursing in Ann Arbor. “In closed ICUs, when you have a board-certified physician and an ICU team managing and leading care, even if the work environment is better, nurses may not feel as empowered to standardize their care or practice.”
“ICU nurses are the ones who are primarily responsible for VAP preventive practices: they keep the head of the bed higher than 45 degrees, they conduct oral care, they conduct (patient) surveillance. ICU physicians are involved with writing the orders and ventilator setting management. So how these providers work together could theoretically influence the risk for patients developing VAP,” Dr. Costa said.
“We need to be thinking a little bit more critically about not only the care that’s happening at the bedside... but also at an organizational level. How are these providers organized, and can we work together to improve patient outcomes?”
“I’m not suggesting that we get rid of all closed ICUs because I don’t think that’s the solution,” Dr. Costa maintained. “I think from an administrative perspective, we need to be considering what’s the organization of these clinicians and this unit, and [in a context-specific manner], how can we improve it for better patient outcomes? That may be both working on improving the work environment and making the nurses feel more empowered, or it could be potentially considering other staffing models.”
Some data have already linked a more favorable nurse work environment and the presence of a board-certified critical care physician independently with better patient outcomes in the ICU. But studies of their joint impact are lacking.
The investigators performed a secondary, unit-level analysis of nurse survey data collected during 2005 and 2006 in ICUs in southern Michigan.
In all, 462 nurses working in 25 ICUs completed the Practice Environment Scale of the Nursing Work Index, on which averaged summary scores range between 1 (unfavorable) and 4 (favorable). The scale captures environmental factors such as the adequacy of resources for nurses, support from their managers, and their level of involvement in hospital policy decisions.
The rate of VAP during the same period was assessed using data from more than 1,000 patients from each ICU.
The summary nurse work environment score averaged 2.69 points in the 21 ICUs that had a closed physician staffing model and 2.62 points in the 4 ICUs that had an open physician staffing model. The respective rates of VAP were 7.5% and 2.5%.
In adjusted analysis among all 25 ICUs, each 1-point increase in an ICU’s Practice Environment Scale score was associated with a sharply higher rate of VAP on the unit (adjusted incidence rate ratio, 5.76; P = .02).
However, there was a strong interaction between the score and physician staffing model (P less than .001). In open ICUs, as the score rose, the rate of VAP fell (from about 16% to 5%), whereas in closed ICUs, as the score rose, so did the rate of VAP (from about 3% to 14%).
Dr. Costa disclosed that she had no relevant conflicts of interest. The parent survey was funded by the Blue Cross Blue Shield Foundation of Michigan.
AT ATS 2016
Key clinical point: The impact of nurse work environment on risk of VAP in the ICU depends on the unit’s physician staffing model.
Major finding: A better nurse work environment was associated with a higher rate of VAP overall (incidence rate ratio, 5.76), but there was an interaction whereby it was positively associated with rate in closed units but negatively so in open units.
Data source: A cohort study of 25 ICUs, 462 nurses, and more than 25,000 patients in southern Michigan between 2005 and 2006.
Disclosures: Dr. Costa disclosed that she had no relevant conflicts of interest. The parent study was funded by the Blue Cross Blue Shield Foundation of Michigan.
In T1D, quality of life measures fall short
NEW ORLEANS – Quality of life measures are sorely lacking for patients with type 1 diabetes, but researchers are attempting to identify measures of patient well-being that go beyond the hemoglobin A1c (HbA1c) level.
At a symposium at the annual scientific sessions of the American Diabetes Association, moderator Kimberly A. Driscoll, PhD, of the University of Colorado, said that “measuring quality of life [should become] a standard part of the routine diabetes clinic visit, just like taking blood pressure.” Parents, partners, and caregivers also need to be involved in deciding what quality of life measures matter, as they are the patients’ sources of support.
Marisa E. Hilliard, PhD, reported on her research team’s efforts to glean from questionnaires the measures that would matter to those with type 1 diabetes and their caregivers. The goal is to create a tool to measure diabetes-related quality of life.
“We want to be able to track quality of life over time and understand how it’s different for different people, and there is not great research on that data,” said Dr. Hilliard of Baylor College of Medicine/Texas Children’s Hospital, Houston.
Lawrence Fisher, PhD, concurred that a quality of life research gap exists for those with type 1 diabetes. A PubMed search indicates that 1,273 papers were published in 2015 with the key words “diabetes” and “quality of life,” yet few of these papers had designated quality of life as a primary outcome. In type 1 diabetes, quality of life “is comprised of one gigantic bucket into which we have thrown all kinds of things, and it’s mired by dozens of different kinds of measures that are all called ‘quality of life’ but define and measure it in very different ways,” said Dr. Fisher of the Behavioral Diabetes Research Group at the University of California, San Francisco.
Patient management in diabetes is a three-legged stool comprised of equal components of glycemic control, behavioral change, and quality of life. Patients are living longer and have healthier lives, but “I don’t think we’re doing as good a job with the happy” aspect of patients’ lives, he said “We spend far too much time treating glucose numbers and not enough time treating people.”
Without measuring quality of life, “you never evaluate the actual cost to individuals of achieving a gain in improved HbA1c or improved behavioral change.”
“I’m told that today, at this point, there are between 15 and 20 diabetes-specific quality of life scales,” he said. “It’s a real hodgepodge. … and one shouldn’t “just go in and pull the measure off the shelf because it has ‘quality of life’ in the title.” The measure must also take into account factors such as patient age, gender, and ethnicity.
Dr. Hilliard and her team are trying to address the “major lack of developmentally tailored measurement instruments. When you have diabetes, you have it for life, and the issues relevant to your quality of life change from age 8 to 18 to 40 and so on.”
Based on interviews with 81 people with type 1 diabetes and their caregivers, the research team developed 14 different measures specifically designed for seven different age bands of people with diabetes – from age 8 years and younger through age 60 years and older. Each age band, with the exception of age 8 years and younger, involves self-reporting by the patient and either the parent, partner, or caregiver. Only the parent self-reports in the youngest age band.
To validate the measures, the researchers plan to enroll 3,600 participants at six sites from the Type 1 Diabetes Exchange and hope to have results in about 18 months. “Our goal is to get the questionnaire to less than 30 items so it takes less than 5 minutes to complete, and to develop a scoring system,” Dr. Hilliard said.
Besides patient care, the measures could be used for clinical trials across the lifespan of people with type 1 diabetes and quality improvement initiatives, she said. Once established, an expert committee would review the measures every 5 years and update them as needed.
Dr. Fisher disclosed he is a consultant to Abbott, Eli Lilly and Company, and Roche Diagnostics.
Dr. Hilliard had no financial disclosures. The Leona M. and Harry B. Helmsley Charitable Trust and the National Institutes of Diabetes and Digestive and Kidney Disease have provided funding for her study.
NEW ORLEANS – Quality of life measures are sorely lacking for patients with type 1 diabetes, but researchers are attempting to identify measures of patient well-being that go beyond the hemoglobin A1c (HbA1c) level.
At a symposium at the annual scientific sessions of the American Diabetes Association, moderator Kimberly A. Driscoll, PhD, of the University of Colorado, said that “measuring quality of life [should become] a standard part of the routine diabetes clinic visit, just like taking blood pressure.” Parents, partners, and caregivers also need to be involved in deciding what quality of life measures matter, as they are the patients’ sources of support.
Marisa E. Hilliard, PhD, reported on her research team’s efforts to glean from questionnaires the measures that would matter to those with type 1 diabetes and their caregivers. The goal is to create a tool to measure diabetes-related quality of life.
“We want to be able to track quality of life over time and understand how it’s different for different people, and there is not great research on that data,” said Dr. Hilliard of Baylor College of Medicine/Texas Children’s Hospital, Houston.
Lawrence Fisher, PhD, concurred that a quality of life research gap exists for those with type 1 diabetes. A PubMed search indicates that 1,273 papers were published in 2015 with the key words “diabetes” and “quality of life,” yet few of these papers had designated quality of life as a primary outcome. In type 1 diabetes, quality of life “is comprised of one gigantic bucket into which we have thrown all kinds of things, and it’s mired by dozens of different kinds of measures that are all called ‘quality of life’ but define and measure it in very different ways,” said Dr. Fisher of the Behavioral Diabetes Research Group at the University of California, San Francisco.
Patient management in diabetes is a three-legged stool comprised of equal components of glycemic control, behavioral change, and quality of life. Patients are living longer and have healthier lives, but “I don’t think we’re doing as good a job with the happy” aspect of patients’ lives, he said “We spend far too much time treating glucose numbers and not enough time treating people.”
Without measuring quality of life, “you never evaluate the actual cost to individuals of achieving a gain in improved HbA1c or improved behavioral change.”
“I’m told that today, at this point, there are between 15 and 20 diabetes-specific quality of life scales,” he said. “It’s a real hodgepodge. … and one shouldn’t “just go in and pull the measure off the shelf because it has ‘quality of life’ in the title.” The measure must also take into account factors such as patient age, gender, and ethnicity.
Dr. Hilliard and her team are trying to address the “major lack of developmentally tailored measurement instruments. When you have diabetes, you have it for life, and the issues relevant to your quality of life change from age 8 to 18 to 40 and so on.”
Based on interviews with 81 people with type 1 diabetes and their caregivers, the research team developed 14 different measures specifically designed for seven different age bands of people with diabetes – from age 8 years and younger through age 60 years and older. Each age band, with the exception of age 8 years and younger, involves self-reporting by the patient and either the parent, partner, or caregiver. Only the parent self-reports in the youngest age band.
To validate the measures, the researchers plan to enroll 3,600 participants at six sites from the Type 1 Diabetes Exchange and hope to have results in about 18 months. “Our goal is to get the questionnaire to less than 30 items so it takes less than 5 minutes to complete, and to develop a scoring system,” Dr. Hilliard said.
Besides patient care, the measures could be used for clinical trials across the lifespan of people with type 1 diabetes and quality improvement initiatives, she said. Once established, an expert committee would review the measures every 5 years and update them as needed.
Dr. Fisher disclosed he is a consultant to Abbott, Eli Lilly and Company, and Roche Diagnostics.
Dr. Hilliard had no financial disclosures. The Leona M. and Harry B. Helmsley Charitable Trust and the National Institutes of Diabetes and Digestive and Kidney Disease have provided funding for her study.
NEW ORLEANS – Quality of life measures are sorely lacking for patients with type 1 diabetes, but researchers are attempting to identify measures of patient well-being that go beyond the hemoglobin A1c (HbA1c) level.
At a symposium at the annual scientific sessions of the American Diabetes Association, moderator Kimberly A. Driscoll, PhD, of the University of Colorado, said that “measuring quality of life [should become] a standard part of the routine diabetes clinic visit, just like taking blood pressure.” Parents, partners, and caregivers also need to be involved in deciding what quality of life measures matter, as they are the patients’ sources of support.
Marisa E. Hilliard, PhD, reported on her research team’s efforts to glean from questionnaires the measures that would matter to those with type 1 diabetes and their caregivers. The goal is to create a tool to measure diabetes-related quality of life.
“We want to be able to track quality of life over time and understand how it’s different for different people, and there is not great research on that data,” said Dr. Hilliard of Baylor College of Medicine/Texas Children’s Hospital, Houston.
Lawrence Fisher, PhD, concurred that a quality of life research gap exists for those with type 1 diabetes. A PubMed search indicates that 1,273 papers were published in 2015 with the key words “diabetes” and “quality of life,” yet few of these papers had designated quality of life as a primary outcome. In type 1 diabetes, quality of life “is comprised of one gigantic bucket into which we have thrown all kinds of things, and it’s mired by dozens of different kinds of measures that are all called ‘quality of life’ but define and measure it in very different ways,” said Dr. Fisher of the Behavioral Diabetes Research Group at the University of California, San Francisco.
Patient management in diabetes is a three-legged stool comprised of equal components of glycemic control, behavioral change, and quality of life. Patients are living longer and have healthier lives, but “I don’t think we’re doing as good a job with the happy” aspect of patients’ lives, he said “We spend far too much time treating glucose numbers and not enough time treating people.”
Without measuring quality of life, “you never evaluate the actual cost to individuals of achieving a gain in improved HbA1c or improved behavioral change.”
“I’m told that today, at this point, there are between 15 and 20 diabetes-specific quality of life scales,” he said. “It’s a real hodgepodge. … and one shouldn’t “just go in and pull the measure off the shelf because it has ‘quality of life’ in the title.” The measure must also take into account factors such as patient age, gender, and ethnicity.
Dr. Hilliard and her team are trying to address the “major lack of developmentally tailored measurement instruments. When you have diabetes, you have it for life, and the issues relevant to your quality of life change from age 8 to 18 to 40 and so on.”
Based on interviews with 81 people with type 1 diabetes and their caregivers, the research team developed 14 different measures specifically designed for seven different age bands of people with diabetes – from age 8 years and younger through age 60 years and older. Each age band, with the exception of age 8 years and younger, involves self-reporting by the patient and either the parent, partner, or caregiver. Only the parent self-reports in the youngest age band.
To validate the measures, the researchers plan to enroll 3,600 participants at six sites from the Type 1 Diabetes Exchange and hope to have results in about 18 months. “Our goal is to get the questionnaire to less than 30 items so it takes less than 5 minutes to complete, and to develop a scoring system,” Dr. Hilliard said.
Besides patient care, the measures could be used for clinical trials across the lifespan of people with type 1 diabetes and quality improvement initiatives, she said. Once established, an expert committee would review the measures every 5 years and update them as needed.
Dr. Fisher disclosed he is a consultant to Abbott, Eli Lilly and Company, and Roche Diagnostics.
Dr. Hilliard had no financial disclosures. The Leona M. and Harry B. Helmsley Charitable Trust and the National Institutes of Diabetes and Digestive and Kidney Disease have provided funding for her study.
AT THE ADA ANNUAL SCIENTIFIC SESSIONS
Key clinical point: Physicians have inadequate tools for measuring quality of life in people with type 1 diabetes.
Major finding: Very few PubMed citations on “diabetes” and “quality of life” designated “quality of life” as a primary outcome.
Data source: Review of 1,273 PubMed citations, and interviews with 81 people with type 1 diabetes and caregivers to inform involvement of 3,600 participants at six Type 1 Diabetes Exchange sites in the development of a quality of life questionnaire.
Disclosures: Dr. Fisher disclosed he is a consultant to Abbott, Eli Lilly and Company, and Roche Diagnostics. Dr. Hilliard had no financial disclosures. The Leona M. and Harry B. Helmsley Charitable Trust and the National Institutes of Diabetes and Digestive and Kidney Disease have provided funding for her study.
Nearly 200 practices expected to participate in Oncology Care Model
The Department of Health and Human Services has announced that nearly 200 physician group practices – more than double the number expected – and 17 health insurance companies will be participating in the Oncology Care Model, a voluntary payment and care delivery model developed by the CMS Innovation Center and advanced by the Affordable Care Act. The 5-year program, designed to improve cancer care by providing financial incentives to physician practices that provide effective treatment, is set to begin July 1, 2016.
The Medicare arm of the Oncology Care Model (OCM) will include more than 3,200 oncologists and will cover approximately 155,000 Medicare beneficiaries nationwide, according to a written statement from the HHS.
“CMS is thrilled with how many physician groups chose to be a part of the Oncology Care Model,” Patrick Conway, MD, CMS principal deputy administrator and chief medical officer, said in the statement.
Physician practices from 31 states will be participating, with the highest levels of provider participation in Alabama, California, Illinois, New Jersey, New York, Ohio, Pennsylvania, and Virginia, according to an analysis conducted by Avalere Health, a Washington, DC–based health care consulting firm.
The CMS first announced the OCM project in February 2015 and originally aimed to have 100 physician practices participating in the first-ever oncology-specific payment reform model.
“Based on feedback from the medical, consumer, and business communities, we are launching this new model of care to support clinicians’ work with their patients,” HHS Secretary Sylvia M. Burwell said in a written statement in February 2015.
“We aim to provide Medicare beneficiaries struggling with cancer with high-quality care around the clock and to reward doctors for the value, not volume, of care they provide. Improving the way we pay providers and deliver care to patients will result in healthier people,” she said.
The OCM encourages practices to improve care and lower costs through episodic and performance-based payments that reward high-quality patient care. It is a multipayer model that includes Medicare’s fee-for-service (OCM-FFS) and commercial payers.
OCM participants will receive regular OCM-FFS payments during the model. To create incentives to improve the quality of care, reimbursement will include a monthly payment of $160 per beneficiary for delivery of OCM enhanced services, and a performance-based payment for OCM episodes, according to a CMS fact sheet.
An OCM-FFS episode begins on the date of initial Part B or D chemotherapy claim and includes all Medicare Part A and B (and some Part D) services received during the episode period which lasts 6 months. Beneficiaries who receive chemotherapy after the end of an episode will begin a new episode.
Enhanced services include patient navigation, a care plan based on the Institute of Medicine care management report, patient access 24 hours a day, 7 days a week, and treatment with therapies that are consistent with nationally recognized clinical guidelines.
View the complete list of participating practices at https://innovation.cms.gov/initiatives/Oncology-Care.
On Twitter @jessnicolecraig
The Department of Health and Human Services has announced that nearly 200 physician group practices – more than double the number expected – and 17 health insurance companies will be participating in the Oncology Care Model, a voluntary payment and care delivery model developed by the CMS Innovation Center and advanced by the Affordable Care Act. The 5-year program, designed to improve cancer care by providing financial incentives to physician practices that provide effective treatment, is set to begin July 1, 2016.
The Medicare arm of the Oncology Care Model (OCM) will include more than 3,200 oncologists and will cover approximately 155,000 Medicare beneficiaries nationwide, according to a written statement from the HHS.
“CMS is thrilled with how many physician groups chose to be a part of the Oncology Care Model,” Patrick Conway, MD, CMS principal deputy administrator and chief medical officer, said in the statement.
Physician practices from 31 states will be participating, with the highest levels of provider participation in Alabama, California, Illinois, New Jersey, New York, Ohio, Pennsylvania, and Virginia, according to an analysis conducted by Avalere Health, a Washington, DC–based health care consulting firm.
The CMS first announced the OCM project in February 2015 and originally aimed to have 100 physician practices participating in the first-ever oncology-specific payment reform model.
“Based on feedback from the medical, consumer, and business communities, we are launching this new model of care to support clinicians’ work with their patients,” HHS Secretary Sylvia M. Burwell said in a written statement in February 2015.
“We aim to provide Medicare beneficiaries struggling with cancer with high-quality care around the clock and to reward doctors for the value, not volume, of care they provide. Improving the way we pay providers and deliver care to patients will result in healthier people,” she said.
The OCM encourages practices to improve care and lower costs through episodic and performance-based payments that reward high-quality patient care. It is a multipayer model that includes Medicare’s fee-for-service (OCM-FFS) and commercial payers.
OCM participants will receive regular OCM-FFS payments during the model. To create incentives to improve the quality of care, reimbursement will include a monthly payment of $160 per beneficiary for delivery of OCM enhanced services, and a performance-based payment for OCM episodes, according to a CMS fact sheet.
An OCM-FFS episode begins on the date of initial Part B or D chemotherapy claim and includes all Medicare Part A and B (and some Part D) services received during the episode period which lasts 6 months. Beneficiaries who receive chemotherapy after the end of an episode will begin a new episode.
Enhanced services include patient navigation, a care plan based on the Institute of Medicine care management report, patient access 24 hours a day, 7 days a week, and treatment with therapies that are consistent with nationally recognized clinical guidelines.
View the complete list of participating practices at https://innovation.cms.gov/initiatives/Oncology-Care.
On Twitter @jessnicolecraig
The Department of Health and Human Services has announced that nearly 200 physician group practices – more than double the number expected – and 17 health insurance companies will be participating in the Oncology Care Model, a voluntary payment and care delivery model developed by the CMS Innovation Center and advanced by the Affordable Care Act. The 5-year program, designed to improve cancer care by providing financial incentives to physician practices that provide effective treatment, is set to begin July 1, 2016.
The Medicare arm of the Oncology Care Model (OCM) will include more than 3,200 oncologists and will cover approximately 155,000 Medicare beneficiaries nationwide, according to a written statement from the HHS.
“CMS is thrilled with how many physician groups chose to be a part of the Oncology Care Model,” Patrick Conway, MD, CMS principal deputy administrator and chief medical officer, said in the statement.
Physician practices from 31 states will be participating, with the highest levels of provider participation in Alabama, California, Illinois, New Jersey, New York, Ohio, Pennsylvania, and Virginia, according to an analysis conducted by Avalere Health, a Washington, DC–based health care consulting firm.
The CMS first announced the OCM project in February 2015 and originally aimed to have 100 physician practices participating in the first-ever oncology-specific payment reform model.
“Based on feedback from the medical, consumer, and business communities, we are launching this new model of care to support clinicians’ work with their patients,” HHS Secretary Sylvia M. Burwell said in a written statement in February 2015.
“We aim to provide Medicare beneficiaries struggling with cancer with high-quality care around the clock and to reward doctors for the value, not volume, of care they provide. Improving the way we pay providers and deliver care to patients will result in healthier people,” she said.
The OCM encourages practices to improve care and lower costs through episodic and performance-based payments that reward high-quality patient care. It is a multipayer model that includes Medicare’s fee-for-service (OCM-FFS) and commercial payers.
OCM participants will receive regular OCM-FFS payments during the model. To create incentives to improve the quality of care, reimbursement will include a monthly payment of $160 per beneficiary for delivery of OCM enhanced services, and a performance-based payment for OCM episodes, according to a CMS fact sheet.
An OCM-FFS episode begins on the date of initial Part B or D chemotherapy claim and includes all Medicare Part A and B (and some Part D) services received during the episode period which lasts 6 months. Beneficiaries who receive chemotherapy after the end of an episode will begin a new episode.
Enhanced services include patient navigation, a care plan based on the Institute of Medicine care management report, patient access 24 hours a day, 7 days a week, and treatment with therapies that are consistent with nationally recognized clinical guidelines.
View the complete list of participating practices at https://innovation.cms.gov/initiatives/Oncology-Care.
On Twitter @jessnicolecraig